Form 8-K PhaseBio Pharmaceuticals For: Aug 12
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
___________________________________
FORM 8-K
___________________________________
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): August 12, 2021
___________________________________
PhaseBio Pharmaceuticals, Inc.
(Exact name of registrant as specified in its Charter)
___________________________________
Delaware | 001-38697 | 03-0375697 | ||||||
(State or Other Jurisdiction of Incorporation) | (Commission File Number) | (IRS Employer Identification No.) | ||||||
1 Great Valley Parkway, Suite 30 Malvern, Pennsylvania | 19355 | |||||||
(Address of Principal Executive Offices) | (Zip Code) | |||||||
(610) 981-6500
(Registrant’s Telephone Number, Including Area Code)
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
___________________________________
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instructions A.2. below):
☐ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act.
Title of each class | Trading Symbol(s) | Name of exchange on which registered | ||||||
| Common Stock, par value $0.001 per share | PHAS | The Nasdaq Stock Market LLC | ||||||
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company x
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. x
Item 2.02. Results of Operations and Financial Condition.
On August 12, 2021, PhaseBio Pharmaceuticals, Inc. (the “Company”) reported financial results for the second quarter ended June 30, 2021. A copy of this press release (“Earnings Press Release”) is furnished as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated by reference.
The information in this Item 2.02 of this Current Report on Form 8-K, including Exhibit 99.1, is being furnished pursuant to Item 2.02 and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended (the “Securities Act”), or the Exchange Act, except as expressly set forth by specific reference in such filing to this item of this report.
Item 7.01. Regulation FD Disclosure.
On August 12, 2021, the Company updated its corporate presentation for use in meetings with investors, analysts and others. The presentation is available through the Company’s website and a copy is attached as Exhibit 99.2 to this Current Report on Form 8-K.
The information in this Item 7.01 of this Current Report on Form 8-K, including Exhibit 99.2, is being furnished pursuant to Item 7.01 and shall not be deemed “filed” for purposes of Section 18 of the Exchange Act or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act or the Exchange Act, whether made before or after the date hereof, except as expressly set forth by specific reference in such filing to this item of this report.
Item 8.01. Other Events.
On August 12, 2021, the Company issued a press release entitled “PhaseBio Pharmaceuticals Achieves Enrollment Milestones Supporting Interim Analysis of REVERSE-IT Global Phase 3 Trial, Enabling Preparation of a BLA submission for Bentracimab for Reversal of Antiplatelet Effects of Ticagrelor.” The full text of the press release (“Interim Analysis Press Release”) is attached as Exhibit 99.3 to this Current Report on Form 8-K.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
| Exhibit No. | Description | |||||||
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| PhaseBio Pharmaceuticals, Inc. | ||||||||||||||
| Dated: August 12, 2021 | By: | /s/ John P. Sharp | ||||||||||||
| John P. Sharp | ||||||||||||||
| Chief Financial Officer | ||||||||||||||

PhaseBio Reports Second-Quarter 2021 Financial Results and Recent Business Highlights
Entered exclusive licensing agreement with Alfasigma S.p.A with up to $245 million in milestone payments and tiered royalty payments for development and commercialization of bentracimab in European and other key markets
Achieved interim enrollment milestone with first 143 bentracimab patients enrolled in the REVERSE-IT pivotal Phase 3 trial, with top-line results from interim analysis expected later this year
Bentracimab Phase 2b trial enrollment completed; safety and efficacy data will supplement Phase 3 interim results, with combined data package planned to serve as the basis for a Biologics License Application (BLA) submission in mid-2022
Cash and Equivalents of $64.5 million as of June 30, 2021
Malvern, PA and San Diego, CA, August 12, 2021 — PhaseBio Pharmaceuticals, Inc. (Nasdaq: PHAS), a clinical-stage biopharmaceutical company focused on the development and commercialization of novel therapies for cardiopulmonary diseases, today provided an update on corporate activities and reported second-quarter 2021 financial results.
“Since the end of the first quarter, the PhaseBio team has made excellent progress with our lead program, bentracimab, including completing enrollment of the first 143 patients in our pivotal Phase 3 REVERSE-IT trial, completing enrollment in the Phase 2b bentracimab trial and signing an exclusive licensing agreement with Alfasigma S.p.A for commercialization in nearly 50 countries across Europe and the Commonwealth of Independent States,” said Jonathan P. Mow, Chief Executive Officer, PhaseBio Pharmaceuticals. “We believe these clinical and strategic milestones position PhaseBio to finish 2021 with significant momentum. The bentracimab program remains on track, and with Alfasigma taking the commercial lead across Europe and other key markets, we believe PhaseBio is well positioned to focus on the BLA submission for bentracimab and prepare for expected commercial launch in the United States.”
Program Highlights and Corporate Updates
•Achieved Enrollment Milestones Supporting Interim Analysis of REVERSE-IT Global Phase 3 Trial of Bentracimab for Reversal of Antiplatelet Effects of Ticagrelor: In August 2021, PhaseBio announced that it had completed enrollment of the first 143 patients in its pivotal Phase 3 REVERSE-IT trial for its lead product candidate bentracimab, 138 of whom required urgent surgery or an invasive procedure and five of whom experienced uncontrolled major or life-threatening bleeding. In total, the REVERSE-IT trial is expected to enroll approximately 200 major bleeding or urgent surgery patients at sites in the United States, Canada, European Union and China. Based on prior guidance following the End of Phase 1 Meeting with the U.S. Food and Drug Administration (FDA) to balance the two patient populations, the REVERSE-IT trial does not
allow enrollment of more than approximately two thirds of either the uncontrolled major or life-threatening bleeding population or urgent surgery or invasive procedure population. Because the total number of patients enrolled to date includes 138 patients who required urgent surgery or an invasive procedure, the surgery cohort of the trial has been fully enrolled. With the successful completion of enrollment in this surgery cohort, REVERSE-IT trial sites have shifted focus to enrolling patients with uncontrolled major or life-threatening bleeding events. The Company is continuing to attempt to accelerate enrollment of patients with uncontrolled major or life-threatening bleeding, including by working to increase the number of enrolling clinical trial sites in the United States, Canada and the European Union, as it believes that a broader site footprint will increase the probability of enrolling these patients. The FDA also previously indicated that an interim analysis of the first approximately 100 patients enrolled in the REVERSE-IT trial would be sufficient to support the submission of a BLA for accelerated approval. The FDA recommended that the 100 patients comprising the interim analysis include approximately 50 patients from each of the uncontrolled major or life-threatening bleeding population and the urgent surgery or invasive procedure population, although the FDA noted that whether there are an adequate number of patients from either cohort would be a review issue and considered in the context of other data submitted with the BLA. The Company is commencing preparation of the BLA and targeting a BLA submission to the FDA in mid-2022.
•Completed Enrollment in Bentracimab Phase 2b trial: In August 2021, PhaseBio announced the completion of enrollment in the randomized, double-blind, placebo-controlled Phase 2b trial of bentracimab. The Phase 2b trial enrolled 200 healthy older and elderly (ages 50 to 80) subjects on dual antiplatelet therapy of ticagrelor and low-dose aspirin; 150 subjects were randomized to receive bentracimab, with reversal of the antiplatelet effects of ticagrelor, as measured by the VerifyNow® PRUTest biomarker, serving as the primary endpoint for the trial. Top-line results from the Phase 2b trial are expected later this year. The Phase 2b trial was designed to supplement the safety and efficacy results that will be included in the BLA submission.
•Announced Approval of Bentracimab IND in China: In August 2021, PhaseBio announced that the Investigational New Drug (IND) application for bentracimab submitted to the Center for Drug Evaluation (CDE) of the China National Medical Products Administration (NMPA) in collaboration with development partner, SFJ Pharmaceuticals (SFJ), has been approved. PhaseBio and SFJ anticipate enrolling the first patients at sites in China later in 2021. Patients enrolled in China are expected to contribute to the completion of full enrollment of the trial, post interim analysis. In January 2020, PhaseBio announced a financing and co-development partnership with SFJ Pharmaceuticals, and since this time, SFJ has been leading clinical development efforts in China. PhaseBio retains commercial rights to bentracimab in China and is pursuing prospective commercial partners to license the marketing rights in China and other countries in the Asia-Pacific region.
•Entered European Licensing Agreement with Alfasigma for Commercialization of Bentracimab: In June 2021, PhaseBio announced an exclusive licensing agreement with Alfasigma, a privately owned specialty pharmaceutical company focused on commercializing medicines in Europe and other key markets, for the commercialization of bentracimab. Under the terms of the license agreement, PhaseBio received a $20 million upfront payment and will be eligible to receive up to $35 million in pre-revenue regulatory milestones and up to $190 million in payments contingent upon the achievement of certain sales milestones. PhaseBio will also receive tiered
royalties on net sales, with percentages starting in the low double digits and escalating up to the mid-twenties.
•Presented Real-World Healthcare Cost and Bleeding Cost Data Featured at the International Society for Pharmacoeconomic and Outcomes Research (ISPOR) Virtual 2021 Conference: In May 2021, PhaseBio presented a poster analysis of the IBM® MarketScan® Commercial and Medicare Supplemental claims databases and focused on patients newly initiating a P2Y12 inhibitor, factor Xa inhibitor or dabigatran between 2014 and 2018. Among other things, results of the analyses demonstrated that, in the year prior to initiating therapy, total healthcare costs were higher among P2Y12 inhibitor patients compared to factor Xa and dabigatran patients. These conference proceedings present compelling evidence of an unmet medical and pharmacoeconomic need for an effective reversal agent for patients treated with P2Y12 inhibitors.
•Presented Real-World Bleeding and Surgery Data Featured at The American College of Cardiology's 70th Annual Scientific Session: In May 2021, PhaseBio presented a poster summarizing an analysis of the IBM® MarketScan® Commercial and Medicare Supplemental claims databases and focused on patients newly initiating a P2Y12 inhibitor, factor Xa inhibitor or dabigatran between 2014 and 2018. The results of the analysis demonstrated that patients receiving P2Y12 inhibitors presented a significantly higher burden of baseline comorbid conditions than factor Xa and dabigatran patients. Additionally, the results indicate that bleeding complications and medical procedures are common in patients taking antithrombotic medications. PhaseBio and the authors believe this study demonstrates an unmet need for an effective reversal agent for patients prescribed P2Y12 inhibitors.
•SFJ Financing and Co-Development Agreement Update: From execution of the co-development agreement through June 30, 2021, SFJ has funded or reimbursed $77.5 million of clinical trial costs and other expenses of the initial $90.0 million commitment under the agreement, leaving $12.5 million of funding remaining available to support the bentracimab Phase 3 program. PhaseBio is eligible to receive up to an additional $30 million of funding if specific, pre-defined clinical development milestones for bentracimab are met.
Second-Quarter 2021 Financial Results
•Cash and cash equivalents at June 30, 2021 were $64.5 million, compared to $28.1 million at December 31, 2020. The increase reflects proceeds from the March 2021 offering of common stock, partially offset by cash used in operating activities.
•Sublicense revenue for the quarter was $10.3 million and reflects recognition of a portion of the upfront milestone payment received as part of the Alfasigma licensing agreement.
•Net loss for the quarters ended June 30, 2021 and 2020 was $28.7 million.
•Research and development expense increased to $27.4 million for the quarter ended June 30, 2021, as compared to $20.9 million for the same period in 2020, driven by an increase in manufacturing, clinical and nonclinical development activities related to bentracimab.
•General and administrative expense increased to $4.0 million for the quarter ended June 30, 2021, compared to $3.2 million for the same period in 2020.
About PhaseBio
PhaseBio Pharmaceuticals, Inc. is a clinical-stage biopharmaceutical company focused on the development and commercialization of novel therapies for cardiovascular and cardiopulmonary diseases. The company’s pipeline includes: bentracimab (PB2452), a novel reversal agent for the antiplatelet therapy ticagrelor; pemziviptadil (PB1046), a once-weekly vasoactive intestinal peptide (VIP) receptor agonist for the treatment of pulmonary arterial hypertension; and PB6440, an oral agent for the treatment of resistant hypertension. PhaseBio’s proprietary elastin-like polypeptide technology platform enables the development of therapies with potential for less-frequent dosing and improved pharmacokinetics, including pemziviptadil, and drives both internal and partnership drug-development opportunities.
PhaseBio is located in Malvern, PA, and San Diego, CA. For more information, please visit www.phasebio.com, and follow us on Twitter @PhaseBio and LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as “anticipates,” “believes,” “expects,” “intends,” “potential,” “projects,” “target,” “will,” “would” and “future” or similar expressions are intended to identify forward-looking statements.
Forward-looking statements include statements concerning or implying the conduct or timing of our clinical trials and our research, development and regulatory plans for our product candidates, the timing of availability or disclosure of data from those clinical trials and the timing of planned regulatory submissions, the potential for these product candidates to receive regulatory approval from the FDA or equivalent foreign regulatory agencies, and whether, if approved, these product candidates will be successfully distributed and marketed, including through our partnerships with Alfasigma and SFJ. Forward-looking statements are based on management's current expectations and are subject to various risks and uncertainties that could cause actual results to differ materially and adversely from those expressed or implied by such forward-looking statements. Accordingly, these forward-looking statements do not constitute guarantees of future performance, and you are cautioned not to place undue reliance on these forward-looking statements.
Risks regarding our business are described in detail in our Securities and Exchange Commission filings, including in our Quarterly Report on Form 10-Q for the quarter ended June 30, 2021. These forward-looking statements speak only as of the date hereof, and PhaseBio Pharmaceuticals, Inc. disclaims any obligation to update these statements except as may be required by law.
| PhaseBio Pharmaceuticals, Inc. | ||||||||||||||
| Condensed Balance Sheets | ||||||||||||||
| (in thousands) | ||||||||||||||
| (unaudited) | ||||||||||||||
| June 30, 2021 | December 31, 2020 | |||||||||||||
| Assets: | ||||||||||||||
| Cash and cash equivalents | $ 64,456 | $ 28,122 | ||||||||||||
| Receivable from sublicense | 18,400 | — | ||||||||||||
| Prepaid expenses and other current assets | 5,644 | 12,027 | ||||||||||||
| Property and equipment, net | 10,379 | 8,224 | ||||||||||||
| Operating lease right-of-use assets | 1,701 | 1,927 | ||||||||||||
| Other non-current assets | 57 | 57 | ||||||||||||
| Total assets | $ 100,637 | $ 50,357 | ||||||||||||
| Liabilities and stockholders' deficit: | ||||||||||||||
| Current portion of long-term debt | $ 5,384 | $ 5,355 | ||||||||||||
| Current portion of deferred sublicense revenue | 1,424 | — | ||||||||||||
| Accounts payable, accrued expenses and other current liabilities | 9,366 | 9,605 | ||||||||||||
| Long-term debt, net | 4,073 | 6,773 | ||||||||||||
| Operating lease liabilities, net | 1,306 | 1,548 | ||||||||||||
| Deferred sublicense revenue, net | 8,238 | — | ||||||||||||
| Development derivative liability | 89,329 | 51,719 | ||||||||||||
| Other long-term liabilities | 692 | 559 | ||||||||||||
| Stockholders’ deficit | (19,175) | (25,202) | ||||||||||||
| Total liabilities and stockholders' deficit | $ 100,637 | $ 50,357 | ||||||||||||
PhaseBio Pharmaceuticals, Inc. | ||||||||||||||||||||||||||
| Condensed Statements of Operations | ||||||||||||||||||||||||||
| (in thousands, except share and per share amounts) | ||||||||||||||||||||||||||
| (unaudited) | ||||||||||||||||||||||||||
| Three Months Ended June 30, | Six Months Ended June 30, | |||||||||||||||||||||||||
| 2021 | 2020 | 2021 | 2020 | |||||||||||||||||||||||
| Revenue: | ||||||||||||||||||||||||||
Sublicense revenue | $ 10,337 | $ — | $ 10,337 | $ — | ||||||||||||||||||||||
Grant revenue | — | — | — | 320 | ||||||||||||||||||||||
| Total revenue | 10,337 | — | 10,337 | 320 | ||||||||||||||||||||||
| Operating expenses: | ||||||||||||||||||||||||||
Research and development | 27,366 | 20,856 | 49,686 | 32,305 | ||||||||||||||||||||||
General and administrative | 4,024 | 3,242 | 7,351 | 6,401 | ||||||||||||||||||||||
| Total operating expenses | 31,390 | 24,098 | 57,037 | 38,706 | ||||||||||||||||||||||
| Loss from operations | (21,053) | (24,098) | (46,700) | (38,386) | ||||||||||||||||||||||
| Other (expense) income | (6,026) | (4,044) | (7,737) | (4,661) | ||||||||||||||||||||||
| Net loss before income taxes | (27,079) | (28,142) | (54,437) | (43,047) | ||||||||||||||||||||||
Provision for income taxes | 1,600 | — | 1,600 | — | ||||||||||||||||||||||
| Net loss | $ (28,679) | $ (28,142) | $ (56,037) | $ (43,047) | ||||||||||||||||||||||
| Net loss per common share, basic and diluted | $ (0.60) | $ (0.98) | $ (1.41) | $ (1.50) | ||||||||||||||||||||||
| Weighted average common shares outstanding, basic and diluted | 47,985,871 | 28,805,238 | 39,680,408 | 28,789,256 | ||||||||||||||||||||||
Investor Contact:
John Sharp
PhaseBio Pharmaceuticals, Inc.
Chief Financial Officer
(610) 981-6506
john.sharp@phasebio.com
Media Contact:
Will Zasadny
Canale Communications, Inc.
(619) 961-8848
will.zasadny@canalecomm.com

Corporate Overview August 2021

This presentation includes forward-looking statements. All statements contained in this presentation other than statements of historical facts, including statements regarding future results of operations and financial position of PhaseBio Pharmaceuticals, Inc. (“we,” “us” or “our”), our business strategy and plans, the preclinical and clinical development of our product candidates and our objectives for future operations, are forward-looking statements. The words “anticipate,” believe,” “continue,” “estimate,” “expect,” “intend,” “may,” “will” and similar expressions are intended to identify forward-looking statements. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy, clinical development, short-term and long-term business operations and objectives and financial needs. These forward-looking statements are subject to a number of risks, uncertainties and assumptions. Risks regarding our business are described in detail in our Securities and Exchange Commission filings, including in our Quarterly Report on Form 10-Q for the quarter ended June 30, 2021. Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the future events and trends discussed in this presentation may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance, achievements or events and circumstances reflected in the forward-looking statements will occur. We are under no duty to update any of these forward-looking statements after the date of this presentation to conform these statements to actual results or revised expectations, except as required by law. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this presentation. Moreover, except as required by law, neither we nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements contained in this presentation. Legal Disclaimer 2
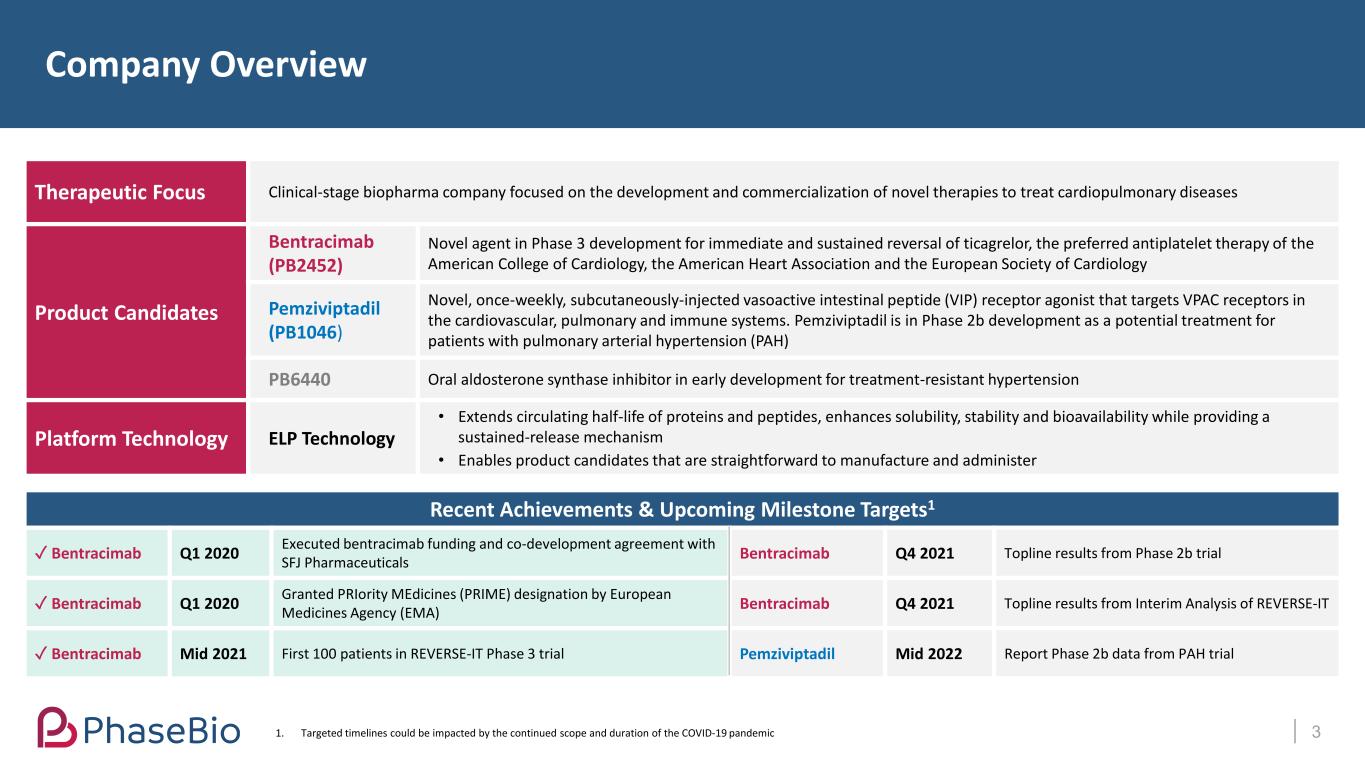
Therapeutic Focus Clinical-stage biopharma company focused on the development and commercialization of novel therapies to treat cardiopulmonary diseases Product Candidates Bentracimab (PB2452) Novel agent in Phase 3 development for immediate and sustained reversal of ticagrelor, the preferred antiplatelet therapy of the American College of Cardiology, the American Heart Association and the European Society of Cardiology Pemziviptadil (PB1046) Novel, once-weekly, subcutaneously-injected vasoactive intestinal peptide (VIP) receptor agonist that targets VPAC receptors in the cardiovascular, pulmonary and immune systems. Pemziviptadil is in Phase 2b development as a potential treatment for patients with pulmonary arterial hypertension (PAH) PB6440 Oral aldosterone synthase inhibitor in early development for treatment-resistant hypertension Platform Technology ELP Technology • Extends circulating half-life of proteins and peptides, enhances solubility, stability and bioavailability while providing a sustained-release mechanism • Enables product candidates that are straightforward to manufacture and administer Recent Achievements & Upcoming Milestone Targets1 ✓ Bentracimab Q1 2020 Executed bentracimab funding and co-development agreement with SFJ Pharmaceuticals Bentracimab Q4 2021 Topline results from Phase 2b trial ✓ Bentracimab Q1 2020 Granted PRIority MEdicines (PRIME) designation by European Medicines Agency (EMA) Bentracimab Q4 2021 Topline results from Interim Analysis of REVERSE-IT ✓ Bentracimab Mid 2021 First 100 patients in REVERSE-IT Phase 3 trial Pemziviptadil Mid 2022 Report Phase 2b data from PAH trial Company Overview 31. Targeted timelines could be impacted by the continued scope and duration of the COVID-19 pandemic

Program Pre-Clinical Phase 1 Phase 2 Phase 3 Commercial Rights Upcoming Milestone Target2 Bentracimab Reversal of Ticagrelor Antiplatelet Activity Q4 2021 Topline results from Interim Analysis of REVERSE-IT and Phase 2b trial of bentracimab Pemziviptadil Pulmonary Arterial Hypertension (PAH) Mid 2022 Report Phase 2b data PB6440 Resistant Hypertension 2022 Submit IND and initiate first-in-human clinical trial Partnering Opportunities GLP2-ELP Short Bowel Syndrome CNP-ELP Achondroplasia Early Programs PROPRIETARY LONG-ACTING INJECTABLE RECOMBINANT BIOPOLYMERS (Elastin-like Polypeptides – ELPs) A Clinical Stage, Cardiopulmonary Focused Biopharmaceutical Company 4 REVERSE-IT1 Phase 3 ongoing Targeting to submit BLA in Mid 20222 Phase 2b ongoing2 Late research Late research 1. REVERSE-IT: Rapid and SustainEd ReVERSal of TicagrElor – Intervention Trial 2. Targeted timeline could be impacted by the continued scope and duration of the COVID-19 pandemic Pre-Clinical

Corporate

6 Experienced Management Team JONATHAN MOW Chief Executive Officer SUSAN ARNOLD, PhD VP of Preclinical & CMC GLEN BURKHARDT VP Human Resources KRIS HANSON VP Legal JOHN LEE, MD, PhD, FACC Chief Medical Officer JOHN SHARP Chief Financial Officer MICHAEL YORK VP Corp Development & Commercial Strategy Despite the unprecedented challenges posed by the ongoing COVID-19 pandemic, 2020 has been a year of significant progress for PhaseBio. In addition to refining our mission, advancing our pipeline programs and kicking off the REVERSE-IT Phase 3 clinical trial for our lead program, bentracimab, we have evolved our corporate logo and the overall look and feel of our website, drawing inspiration for the PhaseBio brand from our prospective patients, healthcare providers and our people. The new PhaseBio logo is defined by a patient-friendly representation of the heart composed of the letters ‘P’ and ‘B’ from the PhaseBio name. This shows that cardiovascular disease is not just what PhaseBio does – it is who we are. Dedicated to transforming patients’ lives through science and excellence LAUREN RICHARDSON Assistant VP Regulatory & Quality

• Rapid pace of development and execution in 2020, sets the stage for success in 2021 Announced innovative funding and co-development collaboration for bentracimab with SFJ Pharmaceuticals Bolstered pipeline with acquisition of resistant hypertension asset, PB6440 Granted EMA PRIME designation for bentracimab Initiated pivotal REVERSE-IT Phase 3 trial for bentracimab in March 2020 Launched international expansion of REVERSE-IT Phase 3 trial, enrolling patients in Canada in October 2020 Made significant progress enrolling REVERSE-IT Phase 3 trial despite pandemic headwinds • Lead program (bentracimab) funded through potential approvals in key global markets • Novel program for PAH (pemziviptadil) being developed using ELP platform technology • Evolving focus on high-unmet need areas of cardiovascular disease with new pipeline asset (PB6440) 2020 Was A Year of Substantial Progress 7

• Sublicense revenue*: $10.3M • Operating expense: $31.4M ⎯ R&D: $27.4M ⎯ SG&A: $4.0M • Loss from Operations: ($21.1M) • Net Loss of ($28.7M) or ($0.60) per share, basic and diluted ⎯ 48.0M shares used for computing Q2 2021 net loss per share • Cash and cash equivalents as of 06/3/2021: $64.5M ⎯ Cash balance does not include $20.0M upfront payment related to Alfasigma sublicense agreement received in July 2021 • Available SFJ funding as of 06/30/2021: $12.5M ⎯ Additional up to $30 million of SFJ funding available if specific, pre-defined clinical development milestones for bentracimab are met Q2 2021 Financial Highlights 8*Sublicense revenue for the quarter was $10.3 million and reflects recognition of a portion of the upfront milestone payment received as part of the Alfasigma licensing agreement.

Bentracimab (PB2452) Reversal Agent for Ticagrelor
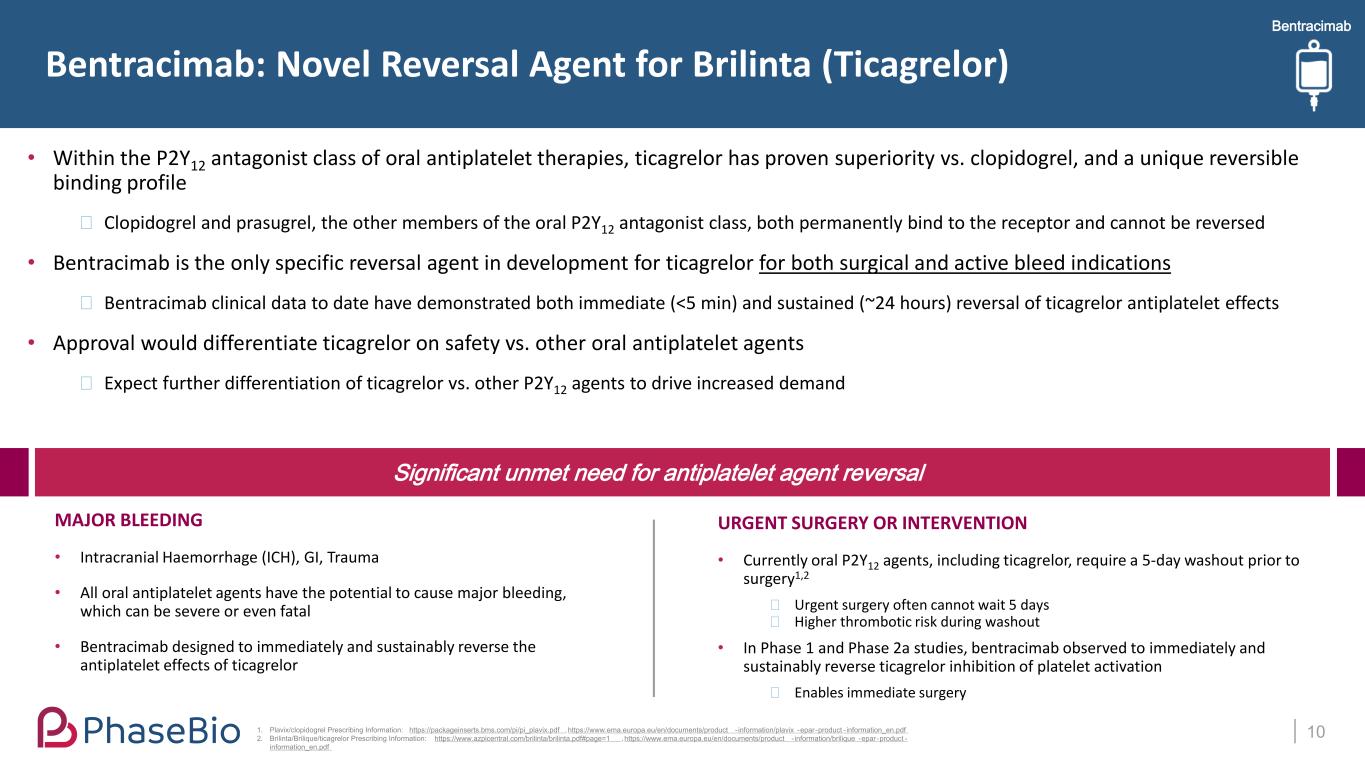
Bentracimab 10 URGENT SURGERY OR INTERVENTION • Currently oral P2Y12 agents, including ticagrelor, require a 5-day washout prior to surgery1,2 ⎯ Urgent surgery often cannot wait 5 days ⎯ Higher thrombotic risk during washout • In Phase 1 and Phase 2a studies, bentracimab observed to immediately and sustainably reverse ticagrelor inhibition of platelet activation ⎯ Enables immediate surgery • Within the P2Y12 antagonist class of oral antiplatelet therapies, ticagrelor has proven superiority vs. clopidogrel, and a unique reversible binding profile ⎯ Clopidogrel and prasugrel, the other members of the oral P2Y12 antagonist class, both permanently bind to the receptor and cannot be reversed • Bentracimab is the only specific reversal agent in development for ticagrelor for both surgical and active bleed indications ⎯ Bentracimab clinical data to date have demonstrated both immediate (<5 min) and sustained (~24 hours) reversal of ticagrelor antiplatelet effects • Approval would differentiate ticagrelor on safety vs. other oral antiplatelet agents ⎯ Expect further differentiation of ticagrelor vs. other P2Y12 agents to drive increased demand Bentracimab: Novel Reversal Agent for Brilinta (Ticagrelor) 1. Plavix/clopidogrel Prescribing Information: https://packageinserts.bms.com/pi/pi_plavix.pdf , https://www.ema.europa.eu/en/documents/product - information/plavix -epar-product - information_en.pdf 2. Brilinta/Brilique/ticagrelor Prescribing Information: https://www.azpicentral.com/brilinta/brilinta.pdf#page=1 , https://www.ema.europa.eu/en/documents/product - information/brilique -epar-product - information_en.pdf MAJOR BLEEDING • Intracranial Haemorrhage (ICH), GI, Trauma • All oral antiplatelet agents have the potential to cause major bleeding, which can be severe or even fatal • Bentracimab designed to immediately and sustainably reverse the antiplatelet effects of ticagrelor Significant unmet need for antiplatelet agent reversal
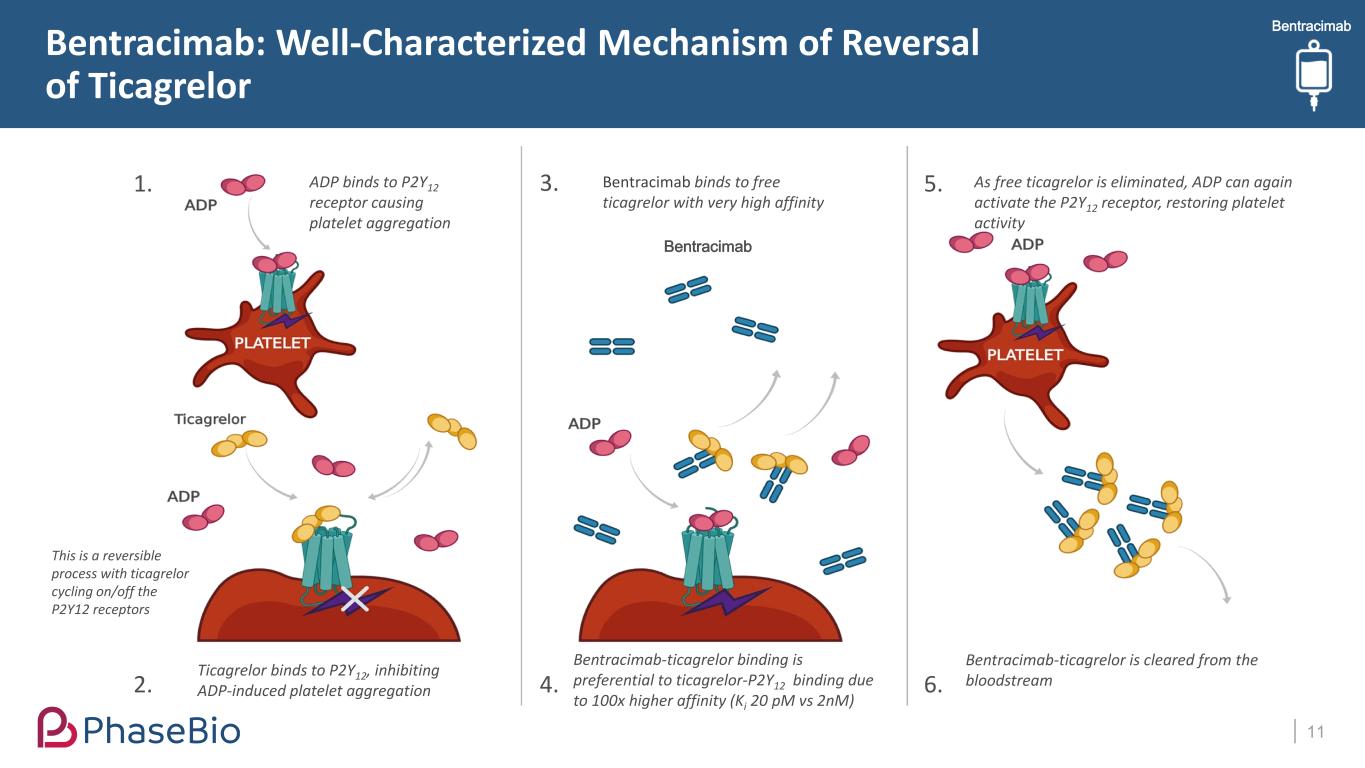
BentracimabBentracimab: Well-Characterized Mechanism of Reversal of Ticagrelor 11 1. 2. 3. 4. 5. 6. ADP binds to P2Y12 receptor causing platelet aggregation Ticagrelor binds to P2Y12, inhibiting ADP-induced platelet aggregation Bentracimab binds to free ticagrelor with very high affinity This is a reversible process with ticagrelor cycling on/off the P2Y12 receptors Bentracimab-ticagrelor binding is preferential to ticagrelor-P2Y12 binding due to 100x higher affinity (Ki 20 pM vs 2nM) As free ticagrelor is eliminated, ADP can again activate the P2Y12 receptor, restoring platelet activity Bentracimab-ticagrelor is cleared from the bloodstream Bentracimab

Bentracimab Bentracimab Phase 1 Proof-of-Concept Study in Healthy Subjects • Randomized, double-blind, placebo-controlled, single ascending dose, sequential group study (n=64) • Platelet function evaluated using three well established and commonly used assays: LTA, VerifyNow PRUTest® and VASP ⎯ Results from all three assays were highly correlated • Onset of reversal occurred within 5 minutes and was sustained for over 20 hours • Well tolerated with no drug-related serious adverse events • Importantly, no evidence of rebound in platelet activity after drug cessation Selected as late-breaking oral presentation during featured clinical research session at the American College of Cardiology’s Annual Scientific Session – March 17, 20191 Simultaneously published in the New England Journal of Medicine2 12 LTA = light transmittance aggregometry, VASP = vasodilator stimulated phosphoprotein phosphorylation immunoassay 1. https://www.acc.org/latest - in-cardiology/clinical -trials/2019/03/15/21/37/ticagrelor - reversal -agent 2. Bhatt DL, Pollack CV, Weitz JI, et al. Antibody -Based Ticagrelor Reversal Agent in Healthy Volunteers. https://www.nejm.org/doi/full/10.1056/NEJMoa1901778 ORIGINAL ARTICLE
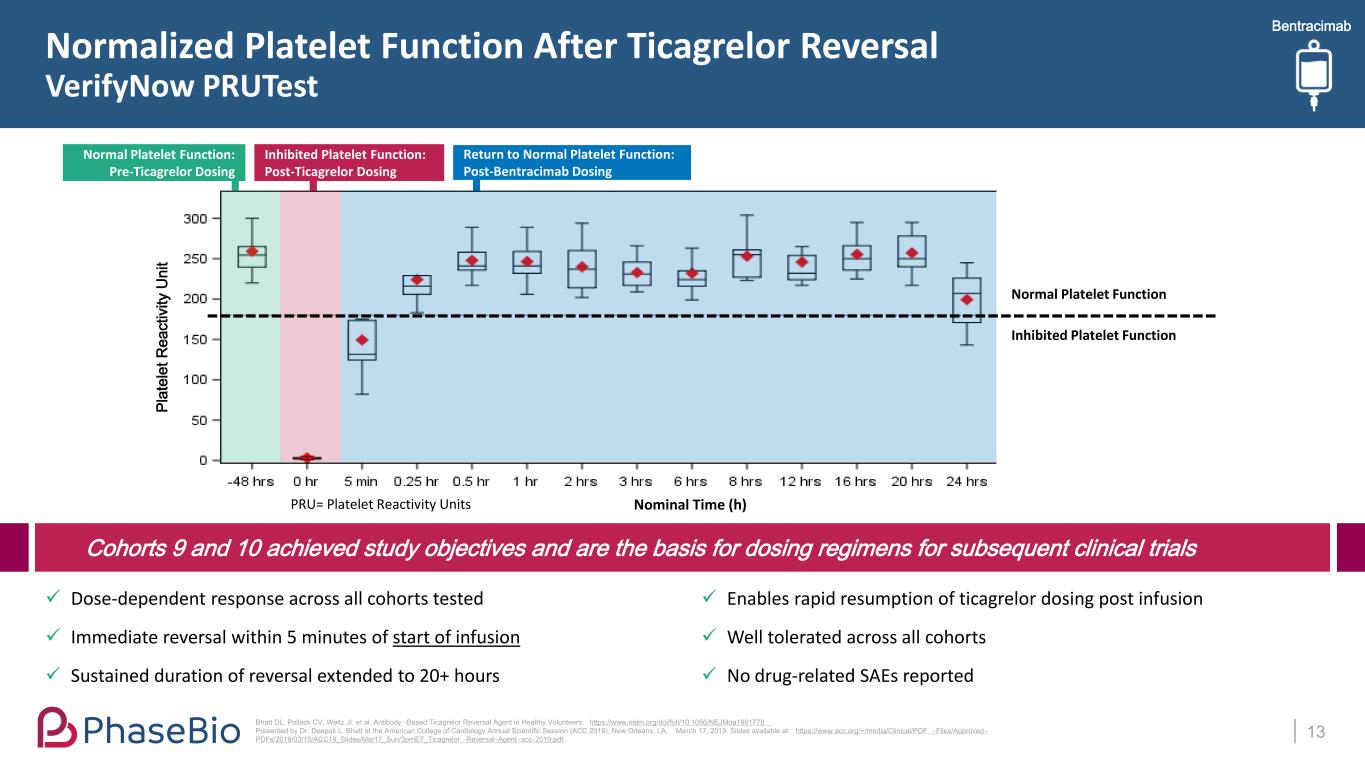
Bentracimab Dose-dependent response across all cohorts tested Immediate reversal within 5 minutes of start of infusion Sustained duration of reversal extended to 20+ hours Enables rapid resumption of ticagrelor dosing post infusion Well tolerated across all cohorts No drug-related SAEs reported Normalized Platelet Function After Ticagrelor Reversal VerifyNow PRUTest 13 Bhatt DL, Pollack CV, Weitz JI, et al. Antibody -Based Ticagrelor Reversal Agent in Healthy Volunteers. https://www.nejm.org/doi/full/10.1056/NEJMoa1901778 Presented by Dr. Deepak L. Bhatt at the American College of Cardiology Annual Scientific Session (ACC 2019), New Orleans, LA, March 17, 2019. Slides available at: https://www.acc.org/~/media/Clinical/PDF -Files/Approved - PDFs/2019/03/15/ACC19_Slides/Mar17_Sun/3pmET_Ticagrelor -Reversal-Agent -acc-2019.pdf Cohorts 9 and 10 achieved study objectives and are the basis for dosing regimens for subsequent clinical trials PRU= Platelet Reactivity Units Normal Platelet Function Inhibited Platelet Function Nominal Time (h) Pl at el et R ea ct iv ity U ni t Return to Normal Platelet Function: Post-Bentracimab Dosing Inhibited Platelet Function: Post-Ticagrelor Dosing Normal Platelet Function: Pre-Ticagrelor Dosing

Bentracimab Positive Preliminary Bentracimab Phase 2a Results • Positive preliminary results from the bentracimab Phase 2a trial were disclosed via press release1 ⎯ First trial of bentracimab to include older and elderly subjects (ages 50-80) on dual antiplatelet therapy (DAPT) of ticagrelor and low-dose aspirin ⎯ Subjects in the trial resembled the patient population most likely to be treated with ticagrelor and potentially benefit from bentracimab, if approved • Per FDA request, the Phase 2a trial also explored reversal of supratherapeutic blood levels of ticagrelor that could result from ticagrelor overdosage or drug-drug interactions ⎯ PhaseBio believes an appropriate bentracimab regimen has been identified for these patients • Confirmed dosing regimen to be used in the Phase 2b and Phase 3 trials • Results to be published and presented at an upcoming medical congress 141. https://investors.phasebio.com/news -releases/news -release-details/phasebio -announces -completion -phase-2a-clinical -trial -pb2452 2. Bhatt, D. L. et al. Antibody -based ticagrelor reversal agent in healthy volunteers. N. Engl. J. Med. ht tps ://doi.org /10 .1056 /NEJMoa 19 017 78 Bentracimab Phase 2a Trial Older & Elderly Subjects on DAPT Supratherapeutic Dose of Ticagrelor Immediate and sustained reversal of the antiplatelet effects of ticagrelor ✓ ✓ Efficacy demonstrated using same three assays used in Phase 1 trial2 ✓ ✓ Results highly correlated across assays ✓ ✓ Generally well tolerated with only minor adverse events reported ✓ ✓

Bentracimab REVERSE-IT: Bentracimab Pivotal Phase 3 Trial Overview • REVERSE-IT: Rapid and SustainEd ReVERSal of TicagrElor – Intervention Trial • Phase 3 trial is a key element of development plan that has garnered FDA Breakthrough Therapy and EMA PRIME designations • Open-label, single-arm study of reversal of the antiplatelet effects of ticagrelor with bentracimab in patients who present with uncontrolled major or life-threatening bleeding or who require urgent surgery or invasive procedure • Total of 200 patients targeted for enrollment ⎯First 143 patients expected to form the basis of accelerated BLA filing in US and MAA in EU ⎯Prior FDA guidance following the End of Phase 1 Meeting in 2019 recommended the following: • Interim analysis of first 100 enrolled would be sufficient to support submission of a BLA for Accelerated Approval • Composition of the 100 patients comprising the interim analysis include approximately 50 subjects from each of the major bleeding and surgical populations • Whether there are an adequate number of patients from either cohort, FDA indicated that this would be a review issue and and considered in the context of other data submitted with the BLA • Accelerated BLA endpoint is restoration of platelet function based on VerifyNow® PRUTest® platelet function assay ⎯VerifyNow has been used in the Phase 1, Phase 2a and Phase 2b trials of bentracimab with consistent results across studies completed to date • Additional endpoints related to hemostasis will be captured as part of the primary outcome analysis • Trial posted online at ClinicalTrials.gov and initiated in March 2020 15

bentracimab REVERSE-IT Enrollment for Interim Analysis Complete 16 As of August 9, 2021, REVERSE-IT had enrolled 143 patients for the Interim Analysis • Enrollment includes 138 patients requiring urgent surgery and five patients with uncontrolled major bleeding • Surgery cohort enrollment complete; trial protocol capped enrollment from either cohort at approximately 2/3rds of the 200 total • Focus shifted to enrolling patients with uncontrolled major or life-threatening bleeding events • Attempting to accelerate enrollment of patients with uncontrolled major or life-threatening bleeding • BLA submission expected in mid-2022 REVERSE-IT patients enrolled from all recruitment regions (US, Canada, Europe) • Surgical patient population includes cardiac surgery, orthopedic surgery, and urgent procedure patients • Smaller pool of bleeding patients observed includes ICH, GI bleed, and procedure-related bleeds REVERSE-IT trial in ticagrelor patients with major bleeding or who need urgent surgery (N=100 for Interim Analysis, N=200 total) Screening (pre-dose) Central Adjudica tion • Inclus ion criteria • Hemosta s is • Thrombotic Events
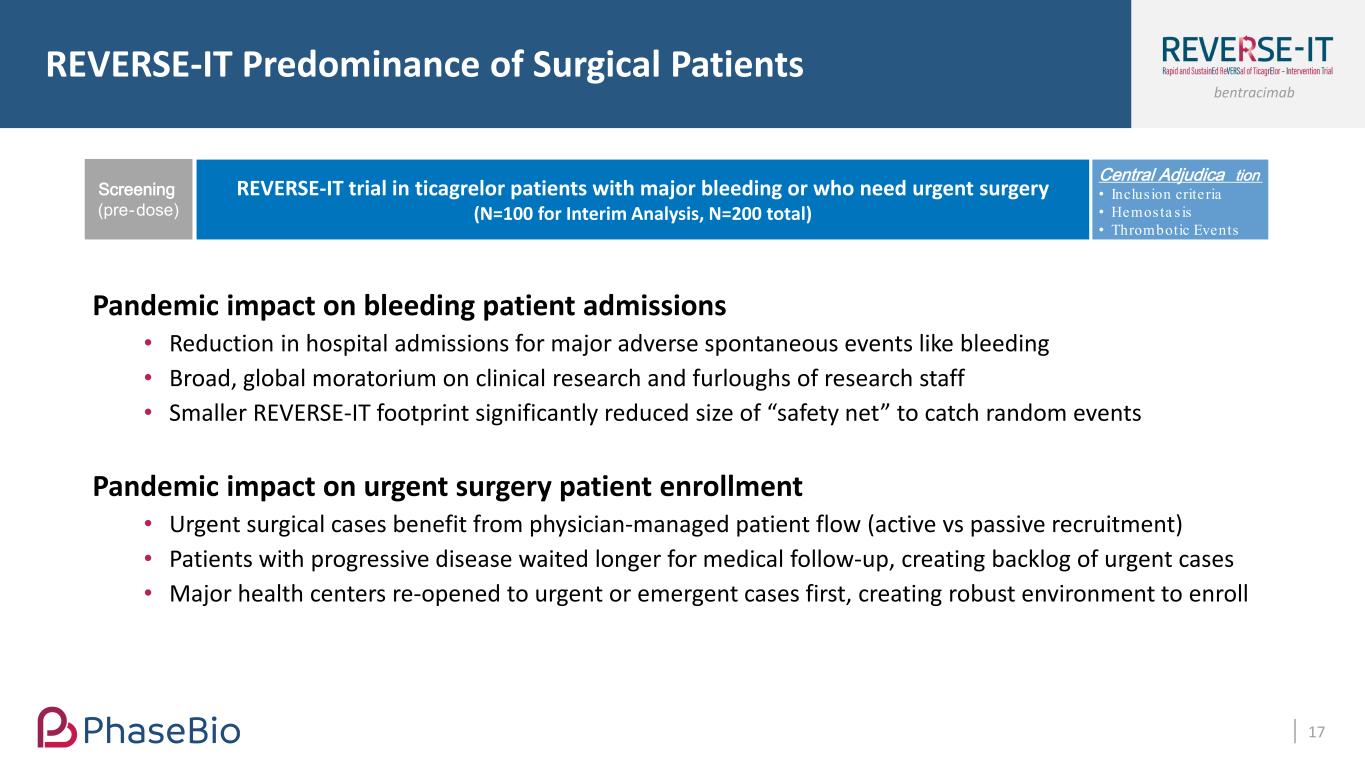
bentracimab REVERSE-IT Predominance of Surgical Patients 17 Pandemic impact on bleeding patient admissions • Reduction in hospital admissions for major adverse spontaneous events like bleeding • Broad, global moratorium on clinical research and furloughs of research staff • Smaller REVERSE-IT footprint significantly reduced size of “safety net” to catch random events Pandemic impact on urgent surgery patient enrollment • Urgent surgical cases benefit from physician-managed patient flow (active vs passive recruitment) • Patients with progressive disease waited longer for medical follow-up, creating backlog of urgent cases • Major health centers re-opened to urgent or emergent cases first, creating robust environment to enroll REVERSE-IT trial in ticagrelor patients with major bleeding or who need urgent surgery (N=100 for Interim Analysis, N=200 total) Screening (pre-dose) Central Adjudica tion • Inclus ion criteria • Hemosta s is • Thrombotic Events
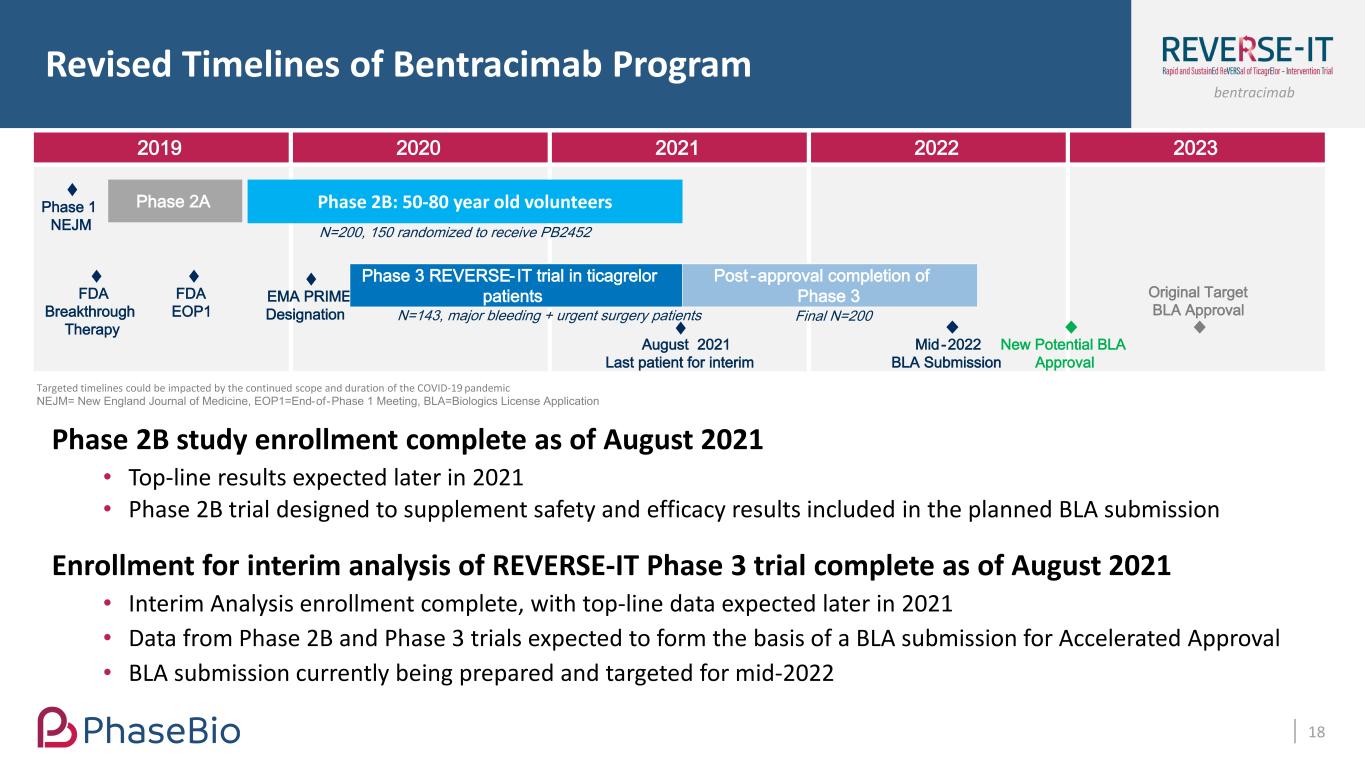
bentracimab Revised Timelines of Bentracimab Program 18 2019 2020 2021 2022 2023 Phase 2B: 50-80 year old volunteersPhase 2APhase 1 NEJM FDA Breakthrough Therapy FDA EOP1 EMA PRIME Designation N=200, 150 randomized to receive PB2452 N=143, major bleeding + urgent surgery patients Final N=200 Post -approval completion of Phase 3 Phase 3 REVERSE-IT trial in ticagrelor patients Mid-2022 BLA Submission New Potential BLA Approval August 2021 Last patient for interim Targeted timelines could be impacted by the continued scope and duration of the COVID-19 pandemic NEJM= New England Journal of Medicine, EOP1=End-of-Phase 1 Meeting, BLA=Biologics License Application Phase 2B study enrollment complete as of August 2021 • Top-line results expected later in 2021 • Phase 2B trial designed to supplement safety and efficacy results included in the planned BLA submission Enrollment for interim analysis of REVERSE-IT Phase 3 trial complete as of August 2021 • Interim Analysis enrollment complete, with top-line data expected later in 2021 • Data from Phase 2B and Phase 3 trials expected to form the basis of a BLA submission for Accelerated Approval • BLA submission currently being prepared and targeted for mid-2022 Original Target BLA Approval

bentracimab EMA: • Scientific Advice ⎯ Face-to-face interaction and written guidance in general agreement with proposed development plan for bentracimab • PRIME designation1 ⎯ Granted to support medicines that demonstrate the potential to address substantial unmet medical need ⎯ Potentially expedites the review and approval process • Multi-disciplinary PRIME Meeting ⎯ Ongoing interactions to meet MAA expectations FDA: • End-of-Phase 1 meeting ⎯ Alignment on development plan and Accelerated approval regulatory path • Breakthrough Therapy designation2 ⎯ Granted to drugs intended to treat a serious condition with potential to address substantial improvement over currently available therapies ⎯ Potentially expedites the development and review of promising new drugs • Breakthrough Therapy Multi-disciplinary Meeting ⎯ Final Phase 3 protocol agreement of study design, endpoints and statistical analysis Bentracimab Regulatory Correspondence Development plan for bentracimab designed with objective to broadly support global regulatory filings 191. The U.S. Food and Drug Administration. “Expedited Programs for Serious Conditions – Drugs and Biologics.” Available at: https://www.fda.gov/downloads/Drugs/Guidances/UCM358301.pdf . Accessed February 2020 2. The European Medicines Agency. “PRIME: Priority Medicines” Available at: https://www.ema.europa.eu/en/human -regulatory/research -development/prime -priority -medicines# . Accessed February 2020 Separate written guidance from FDA and EMA indicates that REVERSE-IT, a single, non-randomized, open-label Phase 3 trial of bentracimab in both surgical and major bleeding populations, has the potential to support regulatory filings in the United States and the European Union Bentracimab
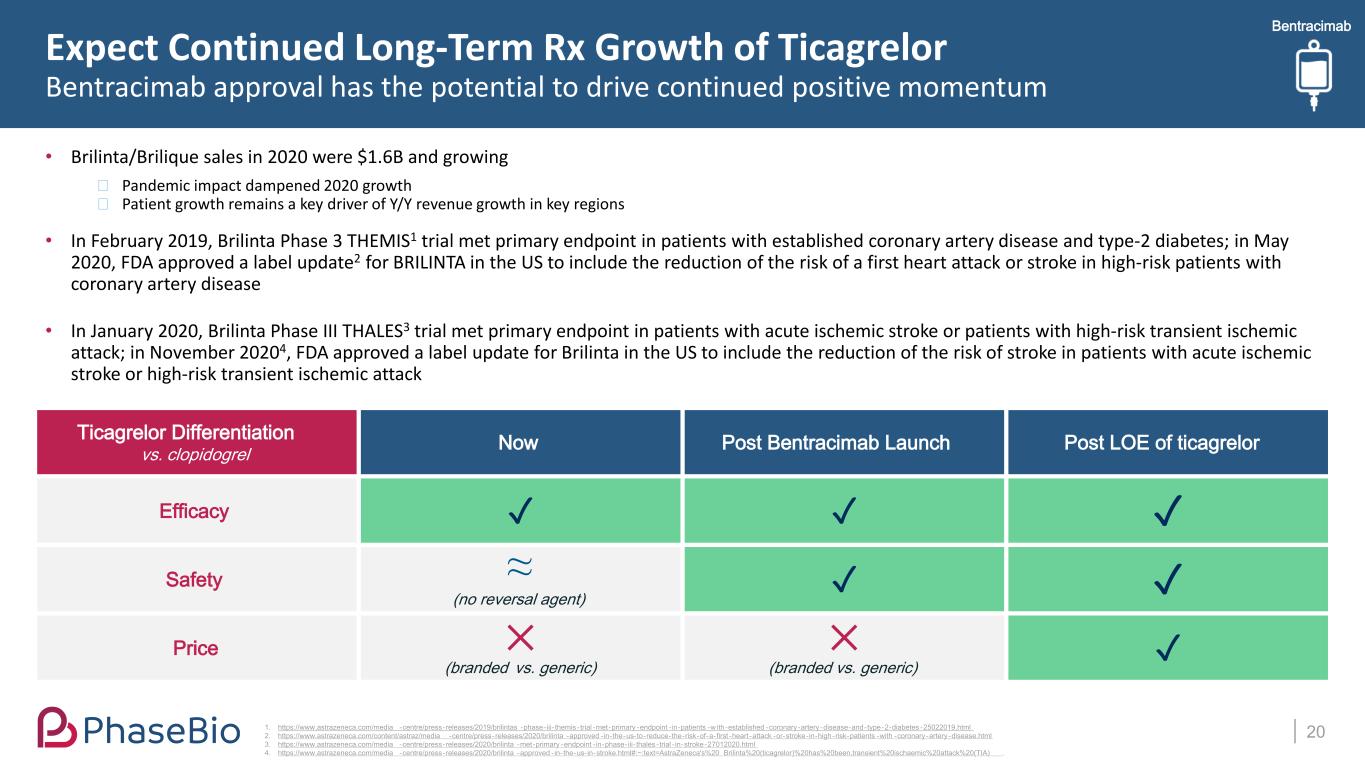
Bentracimab Expect Continued Long-Term Rx Growth of Ticagrelor Bentracimab approval has the potential to drive continued positive momentum • Brilinta/Brilique sales in 2020 were $1.6B and growing ⎯ Pandemic impact dampened 2020 growth ⎯ Patient growth remains a key driver of Y/Y revenue growth in key regions • In February 2019, Brilinta Phase 3 THEMIS1 trial met primary endpoint in patients with established coronary artery disease and type-2 diabetes; in May 2020, FDA approved a label update2 for BRILINTA in the US to include the reduction of the risk of a first heart attack or stroke in high-risk patients with coronary artery disease • In January 2020, Brilinta Phase III THALES3 trial met primary endpoint in patients with acute ischemic stroke or patients with high-risk transient ischemic attack; in November 20204, FDA approved a label update for Brilinta in the US to include the reduction of the risk of stroke in patients with acute ischemic stroke or high-risk transient ischemic attack 201. https://www.astrazeneca.com/media -centre/press-releases/2019/brilintas -phase-iii- themis -trial -met-primary -endpoint -in-patients -w ith-established -coronary -artery -disease-and-type-2-diabetes -25022019.html 2. https://www.astrazeneca.com/content/astraz/media -centre/press-releases/2020/brilinta -approved -in-the-us-to-reduce-the-risk-of-a-first -heart -attack -or-stroke-in-high-risk-patients -with -coronary -artery -disease.html 3. https://www.astrazeneca.com/media -centre/press-releases/2020/brilinta -met-primary -endpoint -in-phase-iii- thales -trial - in-stroke-27012020.html 4. https://www.astrazeneca.com/media -centre/press-releases/2020/brilinta -approved -in-the-us-in-stroke.html#:~:text=AstraZeneca's%20 Brilinta%20(ticagrelor)%20has%20been,transient%20ischaemic%20attack%20(TIA) . Ticagrelor Differentiation vs. clopidogrel Now Post Bentracimab Launch Post LOE of ticagrelor Efficacy ✓ ✓ ✓ Safety ≈ (no reversal agent) ✓ ✓ Price ✕ (branded vs. generic) ✕ (branded vs. generic) ✓
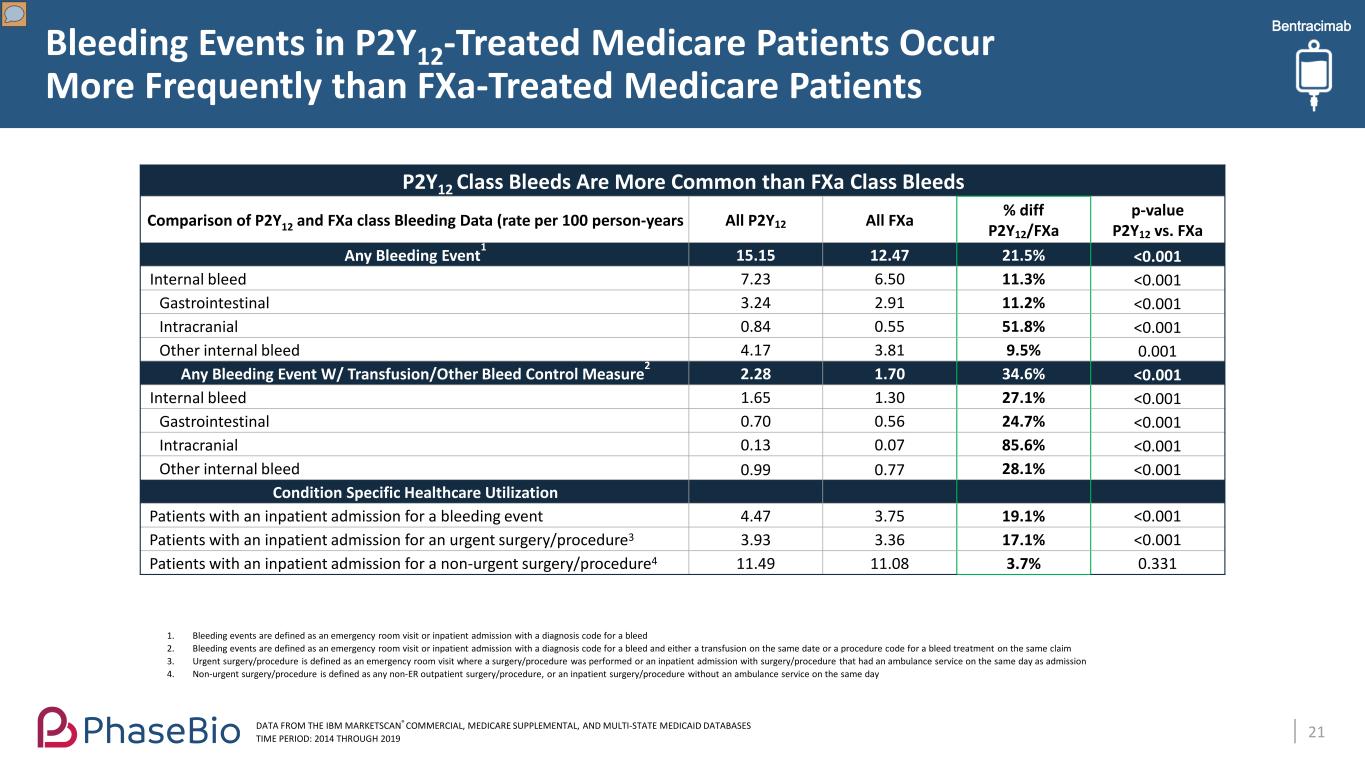
Bleeding Events in P2Y12-Treated Medicare Patients Occur More Frequently than FXa-Treated Medicare Patients 21 P2Y12 Class Bleeds Are More Common than FXa Class Bleeds Comparison of P2Y12 and FXa class Bleeding Data (rate per 100 person-years All P2Y12 All FXa % diff P2Y12/FXa p-value P2Y12 vs. FXa Any Bleeding Event1 15.15 12.47 21.5% <0.001 Internal bleed 7.23 6.50 11.3% <0.001 Gastrointestinal 3.24 2.91 11.2% <0.001 Intracranial 0.84 0.55 51.8% <0.001 Other internal bleed 4.17 3.81 9.5% 0.001 Any Bleeding Event W/ Transfusion/Other Bleed Control Measure2 2.28 1.70 34.6% <0.001 Internal bleed 1.65 1.30 27.1% <0.001 Gastrointestinal 0.70 0.56 24.7% <0.001 Intracranial 0.13 0.07 85.6% <0.001 Other internal bleed 0.99 0.77 28.1% <0.001 Condition Specific Healthcare Utilization Patients with an inpatient admission for a bleeding event 4.47 3.75 19.1% <0.001 Patients with an inpatient admission for an urgent surgery/procedure3 3.93 3.36 17.1% <0.001 Patients with an inpatient admission for a non-urgent surgery/procedure4 11.49 11.08 3.7% 0.331 DATA FROM THE IBM MARKETSCAN® COMMERCIAL, MEDICARE SUPPLEMENTAL, AND MULTI-STATE MEDICAID DATABASES TIME PERIOD: 2014 THROUGH 2019 1. Bleeding events are defined as an emergency room visit or inpatient admission with a diagnosis code for a bleed 2. Bleeding events are defined as an emergency room visit or inpatient admission with a diagnosis code for a bleed and either a transfusion on the same date or a procedure code for a bleed treatment on the same claim 3. Urgent surgery/procedure is defined as an emergency room visit where a surgery/procedure was performed or an inpatient admission with surgery/procedure that had an ambulance service on the same day as admission 4. Non-urgent surgery/procedure is defined as any non-ER outpatient surgery/procedure, or an inpatient surgery/procedure without an ambulance service on the same day Bentracimab
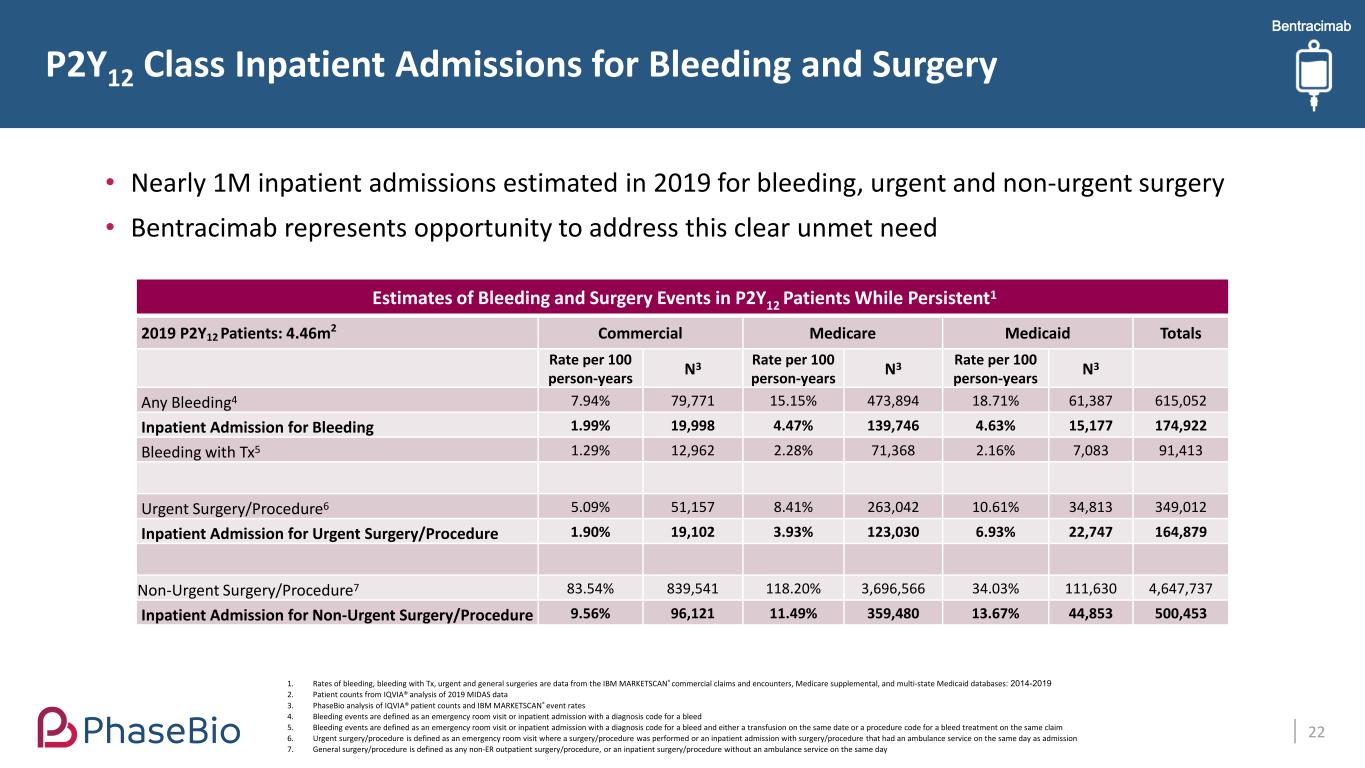
P2Y12 Class Inpatient Admissions for Bleeding and Surgery 22 Estimates of Bleeding and Surgery Events in P2Y12 Patients While Persistent1 2019 P2Y12 Patients: 4.46m2 Commercial Medicare Medicaid Totals Rate per 100 person-years N3 Rate per 100 person-years N3 Rate per 100 person-years N3 Any Bleeding4 7.94% 79,771 15.15% 473,894 18.71% 61,387 615,052 Inpatient Admission for Bleeding 1.99% 19,998 4.47% 139,746 4.63% 15,177 174,922 Bleeding with Tx5 1.29% 12,962 2.28% 71,368 2.16% 7,083 91,413 Urgent Surgery/Procedure6 5.09% 51,157 8.41% 263,042 10.61% 34,813 349,012 Inpatient Admission for Urgent Surgery/Procedure 1.90% 19,102 3.93% 123,030 6.93% 22,747 164,879 Non-Urgent Surgery/Procedure7 83.54% 839,541 118.20% 3,696,566 34.03% 111,630 4,647,737 Inpatient Admission for Non-Urgent Surgery/Procedure 9.56% 96,121 11.49% 359,480 13.67% 44,853 500,453 • Nearly 1M inpatient admissions estimated in 2019 for bleeding, urgent and non-urgent surgery • Bentracimab represents opportunity to address this clear unmet need 1. Rates of bleeding, bleeding with Tx, urgent and general surgeries are data from the IBM MARKETSCAN® commercial claims and encounters, Medicare supplemental, and multi-state Medicaid databases: 2014-2019 2. Patient counts from IQVIA® analysis of 2019 MIDAS data 3. PhaseBio analysis of IQVIA® patient counts and IBM MARKETSCAN® event rates 4. Bleeding events are defined as an emergency room visit or inpatient admission with a diagnosis code for a bleed 5. Bleeding events are defined as an emergency room visit or inpatient admission with a diagnosis code for a bleed and either a transfusion on the same date or a procedure code for a bleed treatment on the same claim 6. Urgent surgery/procedure is defined as an emergency room visit where a surgery/procedure was performed or an inpatient admission with surgery/procedure that had an ambulance service on the same day as admission 7. General surgery/procedure is defined as any non-ER outpatient surgery/procedure, or an inpatient surgery/procedure without an ambulance service on the same day Bentracimab
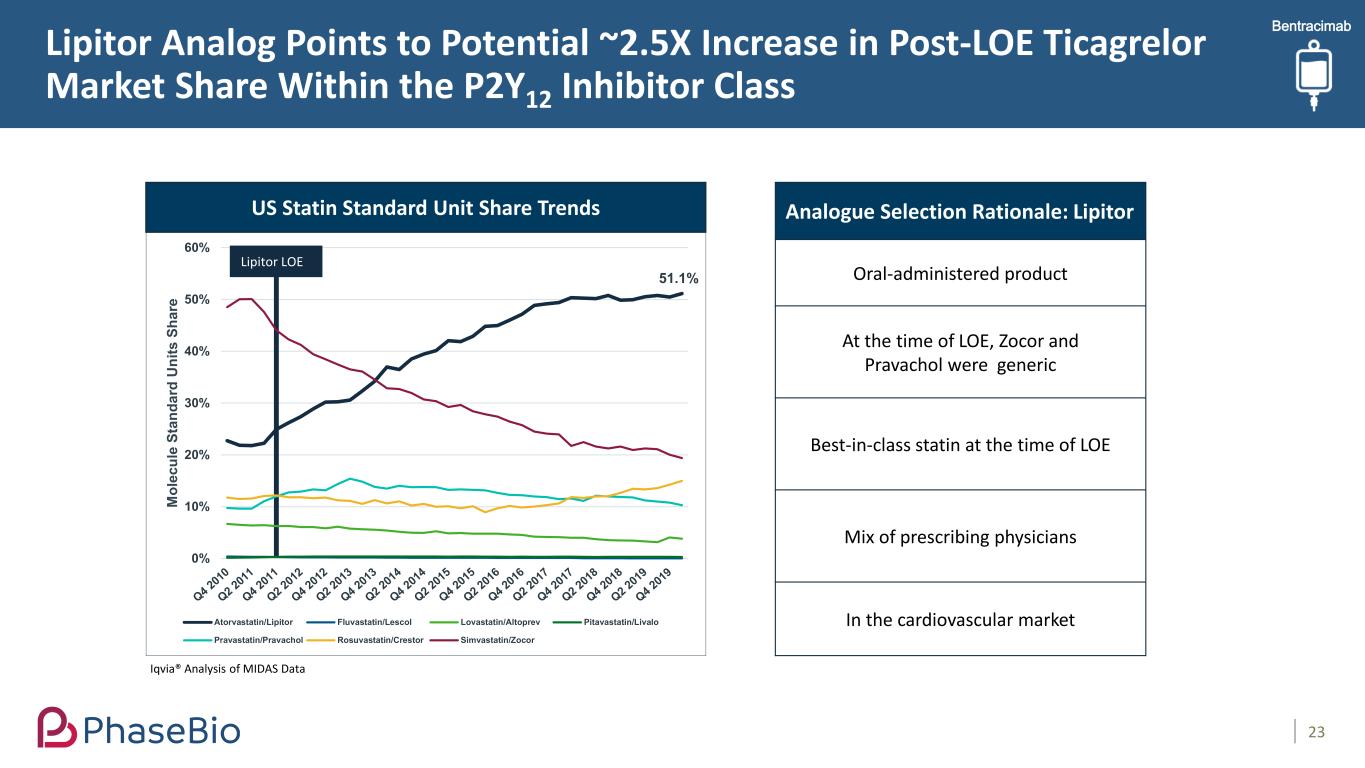
Lipitor Analog Points to Potential ~2.5X Increase in Post-LOE Ticagrelor Market Share Within the P2Y12 Inhibitor Class 23 51.1% 0% 10% 20% 30% 40% 50% 60% M ol ec ul e St an da rd U ni ts S ha re Atorvastatin/Lipitor Fluvastatin/Lescol Lovastatin/Altoprev Pitavastatin/Livalo Pravastatin/Pravachol Rosuvastatin/Crestor Simvastatin/Zocor Lipitor LOE Analogue Selection Rationale: Lipitor Oral-administered product At the time of LOE, Zocor and Pravachol were generic Best-in-class statin at the time of LOE Mix of prescribing physicians In the cardiovascular market Iqvia® Analysis of MIDAS Data US Statin Standard Unit Share Trends Bentracimab
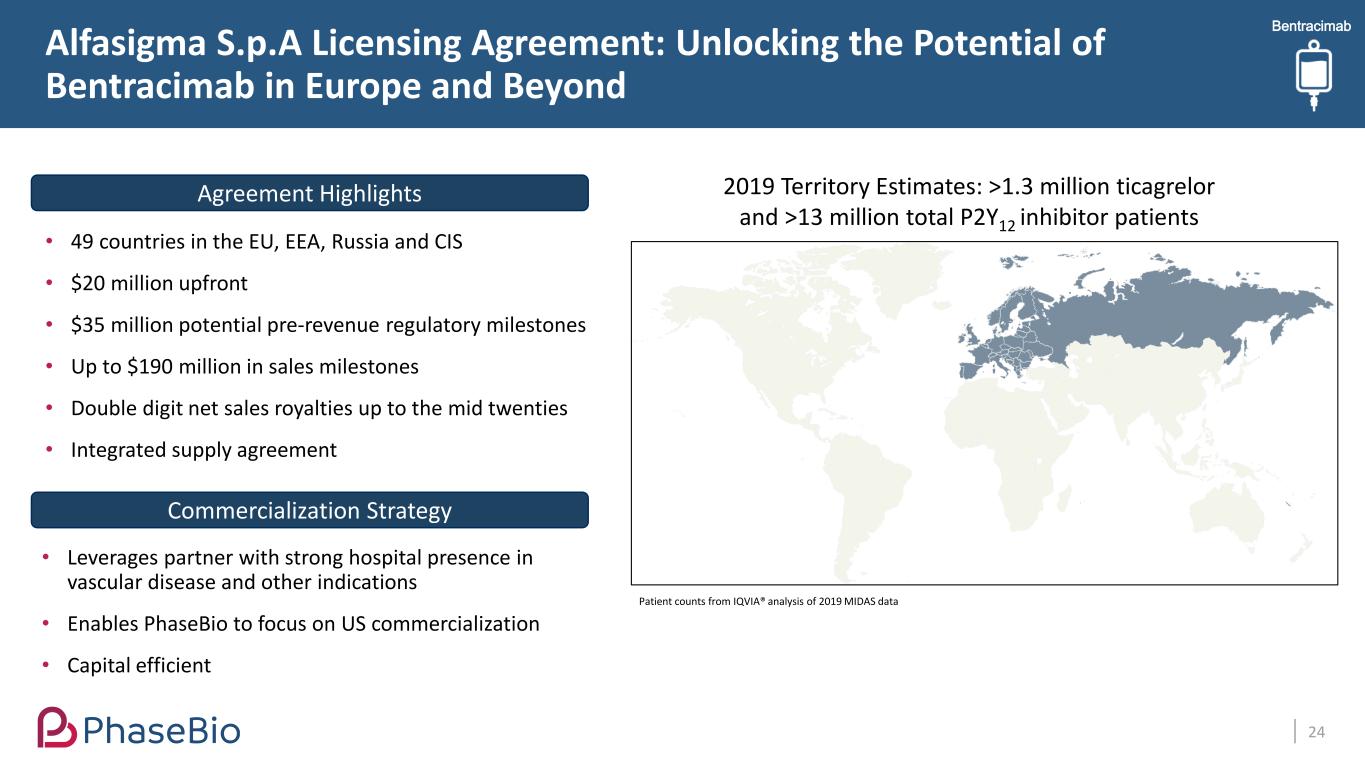
Alfasigma S.p.A Licensing Agreement: Unlocking the Potential of Bentracimab in Europe and Beyond 24 • 49 countries in the EU, EEA, Russia and CIS • $20 million upfront • $35 million potential pre-revenue regulatory milestones • Up to $190 million in sales milestones • Double digit net sales royalties up to the mid twenties • Integrated supply agreement Agreement Highlights Commercialization Strategy • Leverages partner with strong hospital presence in vascular disease and other indications • Enables PhaseBio to focus on US commercialization • Capital efficient Bentracimab 2019 Territory Estimates: >1.3 million ticagrelor and >13 million total P2Y12 inhibitor patients Patient counts from IQVIA® analysis of 2019 MIDAS data

Bentracimab SFJ Pharmaceuticals Funding and Co-Development Collaboration • Innovative collaboration between SFJ and PhaseBio to support the global development of bentracimab • SFJ will fund up to $120 million to support the clinical development of bentracimab ⎯ SFJ has extensive experience in the global clinical development and regulatory approval of numerous pharmaceutical products across multiple indications and therapeutic areas • SFJ providing central role in global development and regulatory activities for bentracimab outside the United States ⎯ SFJ will lead development and regulatory activities in China and Japan ⎯ PhaseBio and SFJ working closely on clinical program in EU ⎯ PhaseBio is conducting REVERSE-IT Phase 3 trial in North America • PhaseBio retains exclusive worldwide commercial rights to bentracimab 25

Pemziviptadil (PB1046) Once Weekly Vasoactive Intestinal Peptide (VIP) Analog

Pemziviptadil PRECLINICAL STUDIES Support Clinical Development for: • Pulmonary Arterial Hypertension • DMD Cardiomyopathy • Heart Failure (Chemotherapy-Induced HF or HFpEF) • Cystic Fibrosis MECHANISM OF ACTION VIA VPAC2 RECEPTOR • Potent vasodilator and immunomodulator • Anti-inflammatory and anti-fibrotic • Cardiac support through increased inotropy and lusitropy Pemziviptadil: Harnessing VIP to Create a Stable, Long-Acting Drug 27 VIP as a THERAPEUTIC AGENT PemziviptadilEndogenous VIP Pemziviptadil
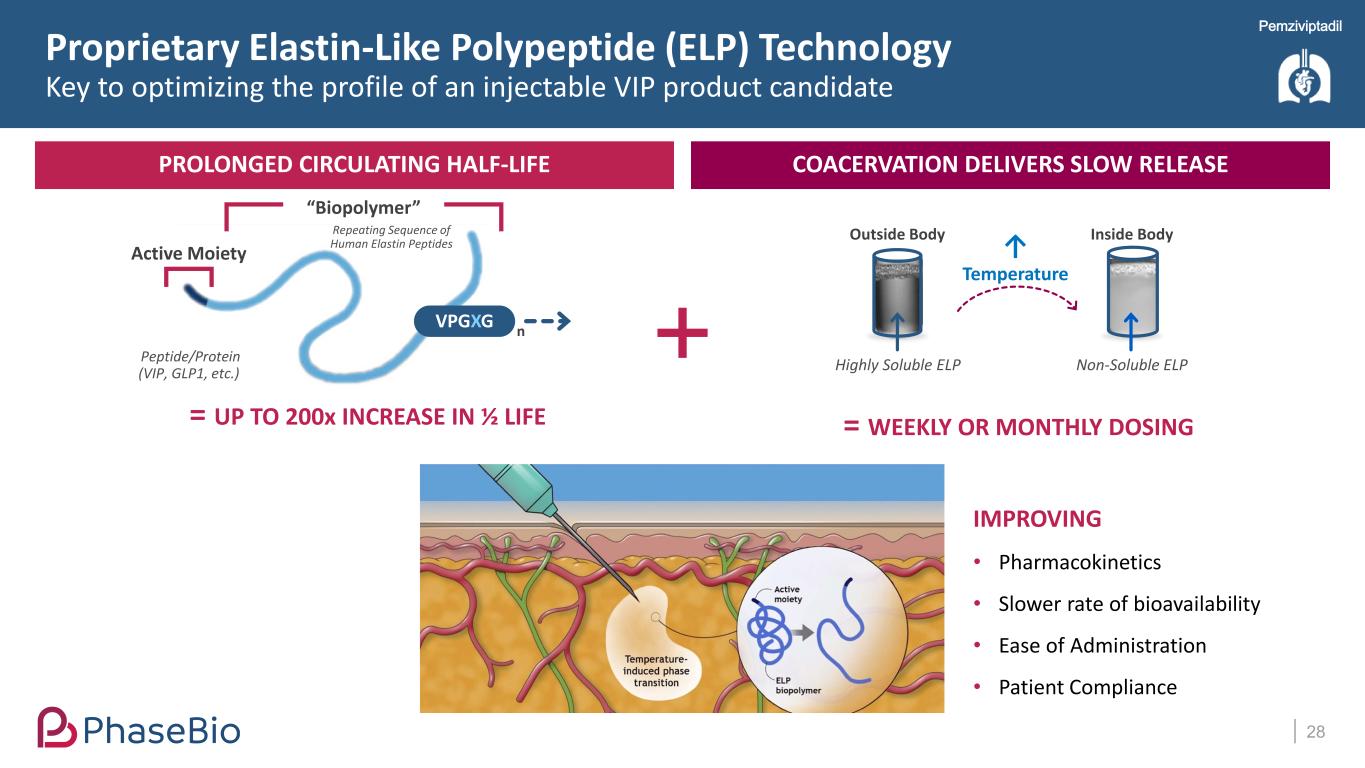
Pemziviptadil PROLONGED CIRCULATING HALF-LIFE COACERVATION DELIVERS SLOW RELEASE Proprietary Elastin-Like Polypeptide (ELP) Technology Key to optimizing the profile of an injectable VIP product candidate 28 Repeating Sequence of Human Elastin Peptides VPGXG Peptide/Protein (VIP, GLP1, etc.) Active Moiety “Biopolymer” n = UP TO 200x INCREASE IN ½ LIFE ↑ Temperature Inside Body Non-Soluble ELP Outside Body Highly Soluble ELP = WEEKLY OR MONTHLY DOSING IMPROVING • Pharmacokinetics • Slower rate of bioavailability • Ease of Administration • Patient Compliance

Pemziviptadil High Unmet Need in an Orphan Disease Primary Pulmonary Arterial Hypertension (PAH, WHO Group 1 PH) • High unmet need for novel disease-modifying PAH therapies for greater efficacy ⎯ All 3 approved drug classes in PAH are vasodilators: prostacyclin, endothelin, and nitric oxide pathways ⎯ Patients inevitably continue to decline and die on current standard of care • VIP addresses PAH vasoconstriction, progressive vascular remodeling, and right heart failure 29

PemziviptadilPemziviptadil Clinical Development Activities to Date Have Supported Advancement into Phase 2 Efficacy Studies 30 PHASE 1 Studies Completed PHASE 2 PAH Studies • Well tolerated for a week over broad range of exposure; no drug-related SAEs reported • Prolonged PK/PD profile over 1 week • VIP activity confirmed (Systolic and Diastolic BP lowering) • Well tolerated across dose range; no drug-related SAEs reported • Replicated PK/PD from SAD over 4 weekly SC injections • VIP activity reproduced in HFrEF patients on SOC CardioMEMS Open-Label PAH Study • N = 3 patients, dosed weekly for 8 wks, followed by extension – No longer enrolling patients • Real-time PA pressure and other hemodynamic monitoring • Initial data show improved hemodynamics • One drug-related SAE reported in extension portion of open-label pilot study Phase 2B PAH 16 wk randomized, controlled study • N = ~60 NYHA class II/III PAH patients, dosed weekly x 16 weeks – Individual dose titration to MTD • Efficacy endpoints PVR via RHC, 6MWD • Extension study to follow COMPLETED COMPLETED COMPLETED Phase 2B ongoing SAD study in hypertensive patients washed off meds 4-week MAD study in HFrEF patients on SOC Open-Label Phase 2A CardioMEMS in PAH study: Safety and Hemodynamics Phase 2B PAH Efficacy 16-Wk Randomized, Controlled Study

PB6440 Aldosterone Synthase Inhibitor for Resistant Hypertension

PB6440 PB6440 for Resistant Hypertension • Upwards of 10 million patients in the United States have resistant hypertension and are at risk for serious, costly medical consequences (stroke, heart attack, kidney failure, etc.)1 • Physicians currently prescribe numerous combinations of antihypertensives to lower blood pressure and diminish risk • Blocking aldosterone has been shown to be an effective mechanism for treating resistant hypertension ⎯ Currently available aldosterone blockers suffer from poor potency and pharmacokinetics (eplerenone) or poor tolerability (spironolactone) and thus are rarely used • Recent draft guidance from the FDA outlines a streamlined regulatory path for novel drugs to treat resistant hypertension without the need for large outcomes studies2 • Market research indicates that payors aware of high medical costs associated with resistant hypertension 32 Large, growing patient population, coupled with a high unmet need, creates an attractive opportunity for a novel therapy to help patients and care-providers better manage blood pressure 1. Carey RM, Sakhuja S, Calhoun DA, Whelton PK, Muntner P. Prevalence of apparent treatment‐resistant hypertension in the United Sta tes : compa rison of the 2008 a nd 2018 America n hea rt a ssocia tion scientific s ta tements on res is ta nt hypertens ion. Hypertens ion. 2019; 73: 424‐ 431. Ava ila ble a t: https ://w w w .a ha journa ls .org/doi/full/10.1161/HYPERTENSIONAHA.118.12191?url_ver=Z39.88-2003&rfr_id=ori:rid:cross ref.org&rfr_da t=cr_pub%3dpubmed. Accessed Februa ry 2020 2. U. S. Food a nd Drug Adminis tra tion. Center for Drug Eva lua tion a nd Resea rch. (2018) Hypertens ion: Conducting Studies of Drugs to Trea t Pa tients on a Ba ckground of Multiple Antihypertens ive Drugs Guida nce for Indus try. Ava ila ble a t: https ://w w w .fda .gov/regula tory-informa tion/sea rch-fda -guida nce-documents/hypertens ion-conducting-s tudies -drugs-trea t-pa tients -ba ckground-multiple-a ntihypertens ive-drugs . Accessed Februa ry 2020

PB6440 CYP11B Potency and Selectivity (IC50, µM) Human Monkey CYP11B2 CYP11B1# Selectivity CYP11B2 CYP11B1 Selectivity PB6440 0.024 4.859 202 0.016 5.802 363 LCI699* 0.0007 0.013 19 0.016 0.059 3.7 PB6440 Is Highly Selective for Aldosterone Synthase (CYP11B2) Selectivity and Potency Demonstrated in Primate Chronic Oral Dosing Model 33 In a primate model, oral PB6440 demonstrated a sustainable reduction in aldosterone without a significant increase in steroids upstream of CYP11B1, suggesting no significant inhibition of CYP11B1 in vivo # Steroid 11β-hydroxylase *Discontinued Novartis compound; active in Phase 2 studies, but blocked cortisol production, likely due to inadequate selectivity


PhaseBio Pharmaceuticals Achieves Enrollment Milestones Supporting Interim Analysis of REVERSE-IT Global Phase 3 Trial, Enabling Preparation of a BLA submission for Bentracimab for Reversal of Antiplatelet Effects of Ticagrelor
First 143 patients enrolled in the pivotal Phase 3 trial, with top-line results from interim analysis expected later this year
Enrollment of patients in the REVERSE-IT trial requiring urgent surgery or invasive procedure now complete, with trial focus shifting to enrollment of patients with uncontrolled major or life-threatening bleeding
Phase 2b bentracimab trial enrollment also completed; safety and efficacy data will supplement Phase 3 interim results, with combined data package planned to serve as the basis for a Biologics License Application (BLA) submission in mid-2022
MALVERN, PA & SAN DIEGO, CA – August 12, 2021-- PhaseBio Pharmaceuticals, Inc. (Nasdaq: PHAS), a clinical-stage biopharmaceutical company focused on the development and commercialization of novel therapies for cardiopulmonary diseases, today announced that it has enrolled the first 143 patients in its pivotal Phase 3 REVERSE-IT trial for its lead product candidate bentracimab, 138 of whom required urgent surgery or an invasive procedure and five of whom experienced uncontrolled major or life-threatening bleeding. The Company is commencing preparation of the BLA and targeting a BLA submission to the U.S. Food and Drug Administration (FDA) in mid-2022.
The REVERSE-IT trial is expected to enroll approximately 200 major bleeding or urgent surgery patients at sites in the United States, Canada, the European Union and China. Based on prior guidance following the End of Phase 1 Meeting with the FDA to balance the two patient populations, the REVERSE-IT trial does not allow enrollment of more than approximately two thirds of either the uncontrolled major or life-threatening bleeding population or urgent surgery or invasive procedure population. Because the total number of patients enrolled to date includes 138 patients who required urgent surgery or an invasive procedure, the surgery cohort of the trial has been fully enrolled. With the successful completion of enrollment in this surgery cohort, REVERSE-IT trial sites have shifted focus to enrolling patients with uncontrolled major or life-threatening bleeding events. The Company is continuing to attempt to accelerate enrollment of patients with uncontrolled major or life-threatening bleeding, including by working to increase the number of enrolling clinical trial sites in the United States, Canada, and the European Union, as it believes that a broader site footprint will increase the probability of enrolling these patients. Additionally, the Company expects to begin enrolling the first patients at sites in China later in 2021.
The FDA also previously indicated that an interim analysis of the first approximately 100 patients enrolled in the REVERSE-IT trial would be sufficient to support the submission of a BLA for accelerated approval. The FDA recommended that the 100 patients comprising the interim analysis include approximately 50 patients from each of the uncontrolled major or life-threatening bleeding population and the urgent surgery or invasive procedure population, although the FDA noted that whether there are an adequate number of patients from either cohort would be a review issue and considered in the context of other data submitted with the BLA.
All of the patients enrolled in the REVERSE-IT trial, both in the urgent surgery and major bleeding cohorts as described above, will be measured against the same VerifyNow® PRUTest biomarker, which is the primary endpoint for the interim analysis and a co-primary endpoint for the full trial. Hemostasis is the other co-primary endpoint for the REVERSE-IT trial. Following a 35-day safety follow-up period from the date of last patient enrollment, PhaseBio will lock the trial database and analyze the data, with the expectation of providing top-line safety and efficacy results from the interim analysis later this year. Full results from the interim analysis are planned to be presented at an upcoming medical congress and published.
In conjunction with the updates regarding the Phase 3 REVERSE-IT trial, PhaseBio also announced today the completion of enrollment in the randomized, double-blind, placebo-controlled Phase 2b trial of bentracimab. The Phase 2b trial enrolled 200 healthy older and elderly (ages 50 to 80) subjects on dual antiplatelet therapy of ticagrelor and low-dose aspirin; 150 subjects were randomized to receive bentracimab, with reversal of the antiplatelet effects of ticagrelor, as measured by the VerifyNow® PRUTest biomarker, serving as the primary endpoint for the trial. Top-line results from the Phase 2b trial are expected later this year. The Phase 2b trial was designed to supplement the safety and efficacy results that will be included in the planned BLA submission.
“We’re pleased to announce that we’ve completed enrollment for interim analysis of the REVERSE-IT global Phase 3 trial and fully enrolled the required Phase 2b trial, both of which are key milestones that set in motion a series of important events that move us one step closer to potentially commercializing the first ticagrelor reversal agent,” said Jonathan Mow, Chief Executive Officer of PhaseBio. “Achieving these enrollment milestones in a timely manner to support our planned BLA submission are major achievements that speak to the efforts of the PhaseBio team and the unmet need for bentracimab. The unmet need for a reversal agent for patients treated with P2Y12 inhibitors is clear. There are currently no effective therapeutic interventions to restore platelet function, and we believe that bentracimab, if approved, has the potential to give patients and physicians an important option for reversing the antiplatelet activity of ticagrelor in major bleeding and urgent surgery situations.”
John Lee, M.D., Ph.D., Chief Medical Officer of PhaseBio added, “The decision to continue enrollment of patients in the Phase 3 REVERSE-IT trial beyond the initial 100 recommended by regulatory authorities was driven by our success in the rapid pace of surgical enrollment in the trial, as well as uncertainty regarding the potential impact of the COVID-19 pandemic on future enrollment. Additionally, with a larger pool of patients available, our ability to assess the clinical
hemostasis co-primary endpoint is improved.” Dr. Lee continued, “Based on our original outlook for the trial, we had expected the hemostasis endpoint to be part of the final analysis of the completed 200 patient study. I’d like to thank the PhaseBio team, the patients enrolled in the trial and the clinical investigators who worked tirelessly and under extraordinarily challenging conditions to advance the trial to this juncture.”
Bentracimab is currently in late-stage clinical development in the REVERSE-IT (Rapid and SustainEd ReVERSal of TicagrElor – Intervention Trial) trial. REVERSE-IT is a Phase 3, multi-center, open-label, prospective single-arm trial designed to study reversal of the antiplatelet effects of ticagrelor with bentracimab in patients who present with uncontrolled major or life-threatening bleeding or who require urgent surgery or an invasive procedure. Learn more about the trial at clinicaltrials.gov.
About Bentracimab (PB2452)
Bentracimab is a novel, recombinant, human monoclonal antibody antigen-binding fragment designed to reverse the antiplatelet activity of ticagrelor in major bleeding and urgent surgery situations. In a Phase 1 clinical trial, bentracimab demonstrated the potential to bring life-saving therapeutic benefit through immediate and sustained reversal of ticagrelor’s antiplatelet activity, mitigating concerns regarding bleeding risks associated with the use of this antiplatelet drug. The Phase 1 clinical trial of bentracimab in healthy volunteers was published in the New England Journal of Medicine in March 2019. In April 2019, bentracimab received Breakthrough Therapy Designation from the U.S. Food and Drug Administration (FDA). Breakthrough Therapy Designation may be granted by the FDA when preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapy. In September 2019, PhaseBio completed a Phase 2a trial in which bentracimab was investigated in older and elderly subjects on dual antiplatelet therapy of ticagrelor and low-dose aspirin. Additionally, the Phase 2a trial investigated a bentracimab regimen for the reversal of supratherapeutic doses of ticagrelor in healthy younger subjects. In both arms of the trial, bentracimab achieved immediate and sustained reversal of the antiplatelet effects of ticagrelor and was generally well-tolerated, with only minor adverse events reported. These results are consistent with the results observed in healthy younger subjects treated with ticagrelor in the previously published Phase 1 trial. PhaseBio initiated the REVERSE-IT trial, a pivotal Phase 3 clinical trial of bentracimab, in March 2020 to support a Biologics License Application for bentracimab in both major bleeding and urgent surgery indications.
About PhaseBio
PhaseBio Pharmaceuticals, Inc. is a clinical-stage biopharmaceutical company focused on the development and commercialization of novel therapies for cardiovascular and cardiopulmonary diseases. The company’s pipeline includes: bentracimab (PB2452), a novel reversal agent for the antiplatelet therapy ticagrelor; pemziviptadil (PB1046), a once-weekly vasoactive intestinal peptide (VIP) receptor agonist for the treatment of pulmonary arterial hypertension; and PB6440, an oral agent for the treatment of resistant hypertension. PhaseBio’s proprietary elastin-like polypeptide technology platform enables the development of therapies with potential for less-frequent dosing and improved pharmacokinetics, including pemziviptadil, and drives both internal and partnership drug-development opportunities.
PhaseBio is located in Malvern, PA, and San Diego, CA. For more information, please visit www.phasebio.com, and follow us on Twitter @PhaseBio and LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as “anticipates,” “believes,” “expects,” “intends,” “potential,” “projects,” “target,” “will,” “would” and “future” or similar expressions are intended to identify forward-looking statements.
Forward-looking statements include statements concerning or implying the conduct or timing of our clinical trials and our research, development and regulatory plans for our product candidates, the timing of availability or disclosure of data from those clinical trials and the timing of planned regulatory submissions, the potential for these product candidates to receive regulatory approval from the FDA or equivalent foreign regulatory agencies, and whether, if approved, these product candidates will be successfully distributed and marketed. Forward-looking statements are based on management's current expectations and are subject to various risks and uncertainties that could cause actual results to differ materially and adversely from those expressed or implied by such forward-looking statements. Accordingly, these forward-looking statements do not constitute guarantees of future performance, and you are cautioned not to place undue reliance on these forward-looking statements.
Risks regarding our business are described in detail in our Securities and Exchange Commission filings, including in our Quarterly Report on Form 10-Q for the quarter ended June 30, 2021. These forward-looking statements speak only as of the date hereof, and PhaseBio Pharmaceuticals, Inc. disclaims any obligation to update these statements except as may be required by law.

PhaseBio Investor Contact:
John Sharp
PhaseBio Pharmaceuticals, Inc.
Chief Financial Officer
(610) 981-6506
john.sharp@phasebio.com
PhaseBio Media Contact:
Will Zasadny
Canale Communications, Inc.
(619) 961-8848
will.zasadny@canalecomm.com
John Sharp
PhaseBio Pharmaceuticals, Inc.
Chief Financial Officer
(610) 981-6506
john.sharp@phasebio.com
PhaseBio Media Contact:
Will Zasadny
Canale Communications, Inc.
(619) 961-8848
will.zasadny@canalecomm.com
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Vincerx Pharma (VINC) Announces Proposed Share and Warrant Offering
- Gryphon Digital Mining (GRYP) Enters $70M ATM Agreement
- AZZ, Inc. (AZZ) Announces Proposed 4M Share Offering
Create E-mail Alert Related Categories
SEC FilingsRelated Entities
S3Sign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share