Form 8-K AQUINOX PHARMACEUTICALS, For: Aug 05
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): August 5, 2019
Aquinox Pharmaceuticals, Inc.
(Exact name of Registrant as specified in its charter)
| Delaware | 001-36327 | 98-0542593 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
450 - 887 Great Northern Way,
Vancouver, B.C.
Canada, V5T 4T5
(Address of principal executive offices, including zip code)
Registrant’s telephone number, including area code: (604) 629-9223
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered | ||
| Common Stock, par value $0.000001 | AQXP | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
Item 1.01 Entry into a Material Definitive Agreement.
On August 5, 2019, Aquinox Pharmaceuticals, Inc. (“Aquinox”) entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Neoleukin Therapeutics, Inc., a Delaware corporation (“Neoleukin”), and Apollo Sub, Inc., a Delaware corporation and wholly-owned subsidiary of Aquinox (“Merger Sub”). Upon the terms and subject to the satisfaction of the conditions described in the Merger Agreement, Merger Sub will be merged with and into Neoleukin (the “Merger”), with Neoleukin surviving the Merger as a wholly-owned subsidiary of Aquinox. The Merger is intended to qualify as a tax-free reorganization for U.S. federal income tax purposes.
Subject to the terms and conditions of the Merger Agreement, at the effective time of the Merger (the “Effective Time”), Aquinox will issue to the former holders of Neoleukin capital stock (i) shares of Aquinox common stock representing approximately 19.5% of Aquinox’s issued and outstanding shares of common stock (calculated prior to the issuance of those new shares of common stock) and (ii) shares of a newly created Aquinox non-voting convertible preferred stock that, following approval of Aquinox’s stockholders, will be convertible (the “Preferred Stock Conversion”) into a number of additional shares of Aquinox common stock such that following such conversion, the former holders of Neoleukin capital stock will, together with the shares of common stock issued at the Effective Time, hold in aggregate approximately 38.58% of the fully diluted outstanding shares of Aquinox (taking into account currently outstanding Aquinox stock options and a portion of the currently granted Aquinox options that may be in the money at closing, but excluding equity incentive awards covering shares of Aquinox common stock that are expected to be granted to continuing employees, including members of management, of Neoleukin). Any outstanding shares of Neoleukin common stock that are unvested or subject to repurchase or forfeiture restrictions will become fully vested and any repurchase or forfeiture restrictions thereon will lapse immediately prior to the Effective Time.
A certificate of designation creating the new convertible preferred stock was adopted by the board of directors of Aquinox (the “Aquinox Board”) at the time the Merger Agreement was signed and will be filed and be effective as of the time of closing (the “Certificate of Designation”).
Following the closing, Dr. Jonathan G. Drachman will serve as Chief Executive Officer of the combined company and Kamran Alam will serve as interim Chief Financial Officer of the combined company. Additionally, following the closing, the Aquinox Board will consist of seven directors, including three designees of Neoleukin, three designees of Aquinox and an independent director mutually selected by Neoleukin and Aquinox, and is expected to be comprised of Dr. Drachman, Ms. Sarah B. Noonberg, Dr. Lewis T. “Rusty” Williams, Mr. Todd Simpson, Mr. Sean Nolan and Ms. M. Cantey Boyd with the independent director seat deemed vacant until it is filled after the closing.
The Merger Agreement contains customary representations, warranties and covenants made by Aquinox and Neoleukin, including covenants relating to obtaining the requisite approvals of the stockholders of Aquinox and Neoleukin, indemnification of directors and officers, Aquinox’s and Neoleukin’s conduct of their respective businesses between the date of signing of the Merger Agreement and the closing.
In connection with the Merger, Aquinox will prepare and file with the U.S. Securities and Exchange Commission (“SEC”) a proxy statement, and will seek the approval of Aquinox’s stockholders with respect to certain actions, including the following (collectively, the “Aquinox Stockholder Matters”):
| • | the approval of the Preferred Stock Conversion; and |
| • | the amendment of Aquinox’s restated certificate of incorporation to increase the number of authorized shares of common stock to an amount as determined by the Aquinox Board following the closing. |
The closing is subject to satisfaction or waiver of certain conditions including, among other things, (i) the required approvals by the Neoleukin stockholders, (ii) the accuracy of the representations and warranties, (iii) compliance by the parties with their respective covenants, (iv) no law or order preventing the Merger and related transactions, (v) Nasdaq approval for listing of the shares of common stock to be issued at closing, and (vi) the filing of the Certificate of Designation.
Support Agreements
In connection with the execution of the Merger Agreement, the executive officers and directors of Aquinox, and certain other stockholders of Aquinox entered into support agreements with Neoleukin and Aquinox relating to the Merger covering approximately 47% of the outstanding capital stock of Aquinox, as of date of the Merger Agreement (the “Aquinox Support Agreements”). The Aquinox Support Agreements provide, among other things, that the stockholders who are parties to the Aquinox Support Agreements will vote all of the shares held by them in favor of Aquinox Stockholder Matters. The Aquinox Support Agreements also place certain restrictions on the transfer of the shares of Aquinox held by the respective signatories thereto.
2
Lock-Up Agreements
Concurrently with the execution of the Merger Agreement, certain officers, directors and stockholders of Aquinox and Neoleukin, respectively, entered into lock-up agreements (the “Lock-Up Agreements”) pursuant to which they accepted certain restrictions on transfers of any shares of Aquinox’s common stock for up to 130 days following the Effective Time.
The foregoing descriptions of the Merger Agreement, the Aquinox Support Agreements, and the Lock-Up Agreements, are not complete and are qualified in their entirety by reference to those agreements or the forms thereof, as applicable, which are attached hereto as Exhibit 2.1, 2.2, and 2.3, respectively, to this report and incorporated herein by reference.
The Merger Agreement (and the foregoing description of the Merger Agreement and the transactions contemplated thereby) has been included to provide investors and stockholders with information regarding the terms of the Merger Agreement and the transactions contemplated thereby. It is not intended to provide any other factual information about Aquinox or Neoleukin. The representations, warranties and covenants contained in the Merger Agreement were made only as of specified dates for the purposes of the Merger Agreement, were solely for the benefit of the parties to the Merger Agreement and may be subject to qualifications and limitations agreed upon by such parties. In particular, in reviewing the representations, warranties and covenants contained in the Merger Agreement and discussed in the foregoing description, it is important to bear in mind that such representations, warranties and covenants were negotiated with the principal purpose of allocating risk between the parties, rather than establishing matters as facts. Such representations, warranties and covenants may also be subject to a contractual standard of materiality different from those generally applicable to stockholders and reports and documents filed with the SEC. Investors and stockholders are not third-party beneficiaries under the Merger Agreement. Accordingly, investors and stockholders should not rely on such representations, warranties and covenants as characterizations of the actual state of facts or circumstances described therein. Information concerning the subject matter of such representations, warranties and covenants may change after the date of the Merger Agreement, which subsequent information may or may not be fully reflected in the parties’ public disclosures.
Item 5.02 Departure of Directors or Certain Officers; Election of Directors; Appointment of Certain Officers; Compensatory Arrangements of Certain Officers.
(b) Resignation of Directors; Resignation of Executive Officer
In connection with the Merger, the Aquinox Board accepted the resignations of Gary Bridger, Ph.D., Daniel Levitt, M.D., Ph.D., Richard S. Levy, M.D., David J. Main, Kevin Neu, M.D., and Robert E. Pelzer from the Aquinox Board, including the resignations of Mr. Main as Chairman of the Aquinox Board and as President and Chief Executive Officer of Aquinox, Mr. Pelzer from the Audit Committee of the Aquinox Board (the “Audit Committee”), Dr. Levitt and Dr. Levy from the Compensation Committee of the Aquinox Board (“Compensation Committee”), and Dr. Bridger and Dr. Neu from the Nominating and Corporate Governance Committee of the Aquinox Board (“Governance Committee”), each to be effective as of the closing date.
Separation Agreement with David J. Main
In connection with the Merger, Aquinox also entered into a separation agreement with Mr. Main. The separation agreement provides that Mr. Main’s employment will end effective as of the closing date. Pursuant to the separation agreement and consistent with the terms of Mr. Main’s employment agreement as amended through May 8, 2019, Mr. Main will be entitled to: (i) a lump sum payment equal to 18 months of his current base salary; (ii) a lump sum payment equal to 18 months bonus pay, that will be calculated and based on Mr. Main’s bonus payment average from the previous three years; (iii) the premiums necessary to continue Mr. Main’s health and dental benefits until the earliest of (A) 18 months from the separation date or (B) the date on which Mr. Main obtains other benefit coverage; (iv) a lump sum payment of $2,000 CDN on the separation date to cover the differential cost in coverage amounts between the group health benefits program and the individual health benefits plan; and (v) a transaction bonus of $401,250 upon the closing of the Merger. Aquinox will also cover the cost of Mr. Main’s U.S. and Canadian tax filing support services for the 2019 tax year. In addition, all unvested options held by Mr. Main will immediately vest and will remain exercisable for a period of 90 days following the separation date, at which time any vested but unexercised options will expire and be forfeit, with the exception of the options granted to Mr. Main in August of 2018 which provides for three years to exercise from the separation date.
The foregoing description of the terms of Mr. Main’s separation agreement is qualified in its entirety by reference to the full text of the separation agreement, a copy of which is filed as Exhibit 10.1 attached hereto, and the terms of which are incorporated by reference herein.
(c) Appointment of Executive Officer
In connection with the Merger, Dr. Drachman entered into an employment agreement pursuant to which he will be appointed Chief Executive Officer of Aquinox, effective as of the closing of the Merger.
3
Jonathan G. Drachman, M.D., age 57, has served as a director and the Chief Executive Officer of Neoleukin since November 2018. From November 2004 to May 2018, Dr. Drachman held several positions at Seattle Genetics, Inc., culminating in the position of Chief Medical Officer and Executive Vice President of Research and Development. From 1998 to 2004, he was a faculty member in the Division of Hematology at the University of Washington, and a Senior Investigator in the Division of Research and Education at Puget Sound Blood Center. He currently serves on the board of directors of Harpoon Therapeutics, Inc. and Calithera Biosciences, Inc. Dr. Drachman received his M.D. at Harvard Medical School and his A.B. in Biochemistry from Harvard College. He completed his residency in internal medicine and a fellowship in medical oncology at the University of Washington.
Dr. Drachman has no family relationships with any member of the Aquinox Board or any executive officer of Aquinox, and has not been involved in any related person transactions within the meaning of Item 404(a) of Regulation S-K promulgated by the SEC required to be disclosed herein.
Compensatory Arrangement with Jonathan G. Drachman
Pursuant to his employment agreement, Dr. Drachman will receive an annual base salary of $350,000 and will be eligible to receive an annual bonus with a target level of 50% of his base salary. As an inducement to enter into the employment agreement, subject to the approval of the Aquinox Board, Dr. Drachman will also be granted an option to purchase 1,650,000 shares of Aquinox’s common stock with an exercise price to be equal to the fair market value of Aquinox’s common stock on the date of grant, as determined by the Aquinox Board. The option will vest and become exercisable with respect to (i) 1/4th of the total underlying shares on the first anniversary of the grant date and (ii) with respect to 1/48th of the total underlying shares on a monthly basis thereafter such that the option will be fully vested and exercisable on the fourth anniversary of the grant date, subject to Dr. Drachman’s continuous service through each applicable vesting date.
In the event Dr. Drachman experiences a termination of his employment without “cause” or he resigns for “good reason” (each as defined in Dr. Drachman’s employment agreement), provided that he executes and makes effective a release of claims against Aquinox and its affiliates, Dr. Drachman will become entitled to (i) continued base salary for nine months, payable in accordance with Aquinox’s standard payroll practices; (ii) a pro-rated portion of his annual bonus, payable in a single lump-sum; (iii) premium payments for continued healthcare coverage for up to nine months; and (iv) accelerated vesting of the portion of his then-outstanding equity awards that would have vested and become exercisable, as applicable, if he had remained in service for an additional 12 months following his date of termination. In the event Dr. Drachman experiences a termination without “cause” or he resigns for “good reason” during the 12-month period following a change in control of Aquinox, then in lieu of the foregoing, Dr. Drachman would become entitled to (a) continued base salary for 12 months, payable in accordance with Aquinox’s standard payroll practices; (b) 100% of his annual target bonus, payable in a single lump-sum; (c) premium payments for continued healthcare coverage for up to 12 months; and (d) accelerated vesting his then-outstanding equity awards.
As previously disclosed in Aquinox’s proxy statement for the 2018 Annual Meeting of Stockholders, employee directors are not compensated for services on the Aquinox Board in addition to their regular employee compensation.
Aquinox expects to enter into Aquinox’s standard form of indemnification agreement for directors and executive officers with Dr. Drachman, which requires us to indemnify our directors and executive officers for certain expenses, including attorneys’ fees, judgments, penalties, fines and settlement amounts incurred by a director or executive officer in any action or proceeding arising out of their services as one of our directors or executive officers or as a director or executive officer of any other company or enterprise to which the person provides services at our request.
The foregoing description of Dr. Drachman’s employment agreement is qualified in its entirety by reference to the full text of his employment agreement, a copy of which is filed as Exhibit 10.2 attached hereto, and the terms of which are incorporated by reference herein.
(d) Election of Directors; Appointment of Committee Members
In connection with the Merger and effective as of the time of closing of the Merger, the Aquinox Board elected Ms. M. Cantey Boyd, Dr. Drachman, Dr. Sarah B. Noonberg, and Dr. Lewis T. “Rusty” Williams, to the Aquinox Board. Dr. Williams was designated as a Class I member of the Aquinox Board, to serve until the 2021 annual meeting of the stockholders of Aquinox; each of Dr. Drachman and Dr. Noonberg was designated as a Class II member of the Aquinox Board, to serve until the 2022 annual meeting of the stockholders of Aquinox; and Ms. Boyd designated as a Class III member of the Aquinox Board, to serve until the 2020 annual meeting of the stockholders of Aquinox. Ms. Boyd, Dr. Drachman, Dr. Noonberg, and Dr. Williams will serve until his or her respective terms expire and until his or her successor is duly elected and qualified or until his or her death. Mr. Sean Nolan will remain as a Class I member of the Aquinox Board and Mr. Todd Simpson will remain as a Class III member of the Aquinox Board.
4
In addition, to fill in the vacancies created by the departures of Dr. Bridger, Dr. Levitt, Dr. Levy, Mr. Main, Dr. Neu, and Mr. Pelzer, the Aquinox Board appointed (i) Dr. Drachman to serve as the interim Chairman of the Aquinox Board; (ii) Dr. Williams to serve as a member of the Audit Committee, with Mr. Nolan remaining as a member of the Audit Committee and Mr. Simpson remaining as Chair of the Audit Committee; (iii) Dr. Noonberg and Mr. Simpson to serve as members of the Compensation Committee, and Mr. Nolan to serve as the Chair of the Compensation Committee; and (iv) Ms. Boyd and Dr. Noonberg to serve as members of the Governance Committee, and Dr. Williams to serve as the Chair of the Governance Committee. Mr. Nolan and Mr. Simpson will both step down from the Governance Committee.
Aquinox expects to enter into Aquinox’s standard form of indemnification agreement for directors and executive officers with Ms. Boyd, Dr. Noonberg, and Dr. Williams, which requires us to indemnify our directors and executive officers for certain expenses, including attorneys’ fees, judgments, penalties, fines and settlement amounts incurred by a director or executive officer in any action or proceeding arising out of their services as one of our directors or executive officers or as a director or executive officer of any other company or enterprise to which the person provides services at our request.
M. Cantey Boyd
M. Cantey Boyd, age 39, has served as a Managing Director of Baker Bros. Advisors LP, a registered investment adviser focused on long-term investments in life-sciences companies, since March 2005. Prior to joining Baker Bros., Ms. Boyd served as an Analyst in the healthcare investment banking group of Deutsche Bank Securities from June 2002 to September 2004. Ms. Boyd received her A.B. in Business Economics from Brown University.
As a Managing Director of Baker Bros. Advisors LP, Ms. Boyd may be deemed to hold shared and voting dispositive power held by entities affiliated with Baker Bros. Advisors LP (the “Baker Entities”). After the Merger, the Baker Entities will be the beneficial owner of approximately 23% of Aquinox’s common stock. Pursuant to the Registration Rights Agreement, dated September 19, 2016 by and between Aquinox and the other parties thereto, which will remain in effect after the Merger, Aquinox has granted certain registration rights to the Baker Entities and Aquinox will, among other things, prepare and file with the SEC a registration statement to register for resale all shares now held or hereafter acquired by the Baker Entities, for up to ten years, and would include our obligation to facilitate certain underwritten public offerings of our common stock by the Baker Entities in the future.
Sarah B. Noonberg, M.D., Ph.D.
Sarah B. Noonberg, M.D., Ph.D., age 51, served as the Chief Medical Officer of Nohla Therapeutics Inc., a developer of universal, off-the-shelf cell therapies for patients with hematologic malignancies and other critical diseases, from May 2018 to May 2019. Prior to joining Nohla Therapeutics, she served as the Chief Medical Officer of Prothena Corporation plc, a biotechnology company, from May 2017 to March 2018. Prior to joining Prothena, Dr. Noonberg served as Group Vice President and Head of Global Clinical Development at BioMarin Pharmaceuticals Inc., a biotechnology company, from August 2015 to March 2017. From May 2007 to August 2015, she held several positions at Medivation, Inc., a biopharmaceutical company, culminating in the position of Senior Vice President of Early Development. She currently serves on the board of directors of Protagonist Therapeutics, Inc. Dr. Noonberg received her M.D. at the University of California, San Francisco, her Ph.D. in Bioengineering at the University of California, Berkeley, and her B.S. in Engineering at Dartmouth College. She is a board-certified internist and completed her residency at Johns Hopkins Hospital.
There are no related person transactions within the meaning of Item 404(a) of Regulation S-K promulgated by the SEC between Dr. Noonberg and Aquinox required to be disclosed herein.
Lewis T. “Rusty” Williams, M.D., Ph.D.
Lewis T. “Rusty” Williams, M.D., Ph.D., age 70, has served as the Chairman and Chief Executive Officer of Walking Fish Therapeutics, Inc., a biotechnology start-up company, since February 2019. Dr. Williams has also served as a venture partner of Quan Capital, LLP, a healthcare-focused venture capital firm, since October 2018. From January 2002 to December 2017, Dr. Williams founded and held several positions at Five Prime Therapeutics, Inc., culminating in the position of President and Chief Executive Officer from April 2011 to December 2017. From September 1992 to December 2001, Dr. Williams held several positions at Chiron Corporation, culminating in the position as Chief Scientific Officer and as a member of the board of directors. Prior to joining Chiron, Dr. Williams was a professor of medicine at the University of California, San Francisco from August 1984 to June 1993, where he served as the director of the Cardiovascular Research Institution and Daiichi Research Center. Prior to UCSF, Dr. Williams co-founded and serve on the board of directors of COR Therapeutics, Inc., a biotechnology company focused on cardiovascular disease, from June 1989 to September 1994, and served on the faculties of Harvard Medical School and Massachusetts General Hospital from July 1982 to July 1984. He is a member of the National Academy of Sciences and a fellow of the American Academy of Arts and Sciences. He currently serves on the board of directors of Five Prime Therapeutics. Dr. Williams received his B.S. in Chemistry from Rice University, and his M.D. and Ph.D. in Pharmacology / Biochemistry from Duke University.
5
There are no related person transactions within the meaning of Item 404(a) of Regulation S-K promulgated by the SEC between Dr. Williams and Aquinox required to be disclosed herein.
(e) Transition Agreement with Kamram Alam
In connection with the Merger, Aquinox also entered into a fixed-term transition agreement with Mr. Alam, who will serve as our Interim Chief Financial Officer following the closing of the Merger until May 31, 2020 (the “Transition Period”), unless terminated in compliance with the transition agreement.
Pursuant to his transition agreement, Mr. Alam will receive an annual base salary of $380,000 and will be eligible to receive a rentention bonus of $332,500. The retention bonus will be payable upon the earlier of Mr. Alam’s termination without cause and May 31, 2010. Mr. Alam will not be eligible for any additional equity awards during the Transition Period, but any equity awards granted prior to the transition retention agreement will continue to vest during the Transition Period. During the Transition Period, Mr. Alam will be eligible to participate in an individual extended health benefits plan paid for by Aquinox until August 8, 2020. In addition, Aquinox will pay Mr. Alam a lump sum payment of $2,000 CDN on August 8, 2019 to cover the differential cost in coverage amounts between the group health benefits program and the individual health benefits plan.
Consistent with the terms of his employment agreement, as amended, in connection with his separation and effective upon closing, Mr. Alam will be entitled to: (i) a lump sum payment equal to 12 months of his current base salary; (ii) a lump sum payment equal to 12 months bonus pay, that will be calculated and based on Mr. Alam’s bonus payment average from the previous three years; (iii) the premiums necessary to continue Alam’s health and dental benefits until the earliest of (A) 12 months from the separation date or (B) the date on which Mr. Alam obtains other benefit coverage; and (iv) a transaction bonus of $319,375 upon the closing of the Merger. In addition, all unvested options held by Mr. Alam will immediately vest and will remain exercisable for a period of 90 days following the separation date, at which time any vested but unexercised options will expire and be forfeit, with the exception of the options granted to Mr. Alam in August of 2018 which provides for three years to exercise from the separation date.
The foregoing description is qualified in its entirety by reference to the full text ofMr. Alam’s employment agreement, as amended, a copy of which was filed as Exhibit 10.2 with the Company’s Form 10-Q on August 1, 2019, and Mr. Alam’s transition agreement, a copy of which is filed as Exhibit 10.3 attached hereto, and each of which are incorporated by reference herein.
Item 7.01
On March 7, 2019, Aquinox and Neoleukin issued a joint press release announcing the execution of the Merger Agreement. The press release is attached hereto as Exhibit 99.1 and is incorporated herein by reference.
Aquinox and Neoleukin prepared investor presentation materials with information about the combined company, which it intends to use as part of investor presentations. A copy of the investor presentation materials to be used by management for presentations is attached hereto as Exhibit 99.2 and is incorporated herein by reference.
This information contained in Item 7.01 of this Current Report on Form 8-K, including the attached Exhibit 99.1 and Exhibit 99.2, shall be deemed “furnished” and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference to such filing.
Forward Looking Statements
This report, including the exhibits attached hereto, contain forward-looking statements based upon Aquinox’s and Neoleukin’s current expectations. Forward-looking statements involve risks and uncertainties, and include, but are not limited to, statements about the structure, timing and completion of the proposed Merger; the combined company’s listing on Nasdaq after closing of the proposed Merger; expectations regarding the ownership structure of the combined company; the expected executive officers and directors of the combined company; the combined company’s expected cash position at the closing of the proposed Merger; the future operations of the combined company; the nature, strategy and focus of the combined company; the development and commercial potential and potential benefits of any product candidates of the combined company; the executive and board structure of the combined company; the location of the combined company’s corporate headquarters; anticipated preclinical and clinical drug development activities and related timelines, including the expected timing for data and other clinical and preclinical results; Neoleukin having sufficient resources to advance its pipeline; and other statements that are not historical fact. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of these risks and uncertainties, which include, without limitation: (i) the risk that the conditions to the closing of the proposed Merger are not satisfied, including the failure to timely
6
obtain stockholder approval for the transaction, if at all; (ii) uncertainties as to the timing of the consummation of the proposed Merger and the ability of each of Aquinox and Neoleukin to consummate the proposed Merger; (iii) risks related to Aquinox’s ability to manage its operating expenses and its expenses associated with the proposed Merger pending closing; (iv) risks related to the failure or delay in obtaining required approvals from any governmental or quasi-governmental entity necessary to consummate the proposed Merger; (v) the risk that as a result of adjustments to the exchange ratio, Aquinox stockholders and Neoleukin stockholders could own more or less of the combined company than is currently anticipated; (vi) risks related to the market price of Aquinox’s common stock relative to the exchange ratio; (vii) unexpected costs, charges or expenses resulting from the transaction; (viii) potential adverse reactions or changes to business relationships resulting from the announcement or completion of the proposed Merger; (ix) the uncertainties associated with the clinical development and regulatory approval of product candidates; (x) risks related to the inability of the combined company to obtain sufficient additional capital to continue to advance these product candidates and its preclinical programs; (xi) uncertainties in obtaining successful clinical results for product candidates and unexpected costs that may result therefrom; (xii) risks related to the failure to realize any value from product candidates and preclinical programs being developed and anticipated to be developed in light of inherent risks and difficulties involved in successfully bringing product candidates to market; and (xiii) risks associated with the possible failure to realize certain anticipated benefits of the proposed Merger, including with respect to future financial and operating results. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of these risks and uncertainties. These and other risks and uncertainties are more fully described in periodic filings with the SEC, including the factors described in the section entitled “Risk Factors” in Aquinox’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2019 filed with the SEC, and in other filings that Aquinox makes and will make with the SEC. You should not place undue reliance on these forward-looking statements, which are made only as of the date hereof or as of the dates indicated in the forward-looking statements. Aquinox expressly disclaims any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in its expectations with regard thereto or any change in events, conditions or circumstances on which any such statements are based.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits
| * | Schedules and exhibits have been omitted pursuant to Item 601(b)(2) of Regulation S-K. A copy of any omitted schedule and/or exhibit will be furnished to the SEC upon request. |
7
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| Aquinox Pharmaceuticals, Inc. | ||
| By: | /s/ Kamran Alam | |
| Name: | Kamran Alam | |
| Title: | Chief Financial Officer | |
Date: August 6, 2019
8
Exhibit 2.1
AGREEMENT AND PLAN OF MERGER
among:
AQUINOX PHARMACEUTICALS, INC.,
a Delaware corporation;
APOLLO SUB, INC.,
a Delaware corporation;
and
NEOLEUKIN THERAPEUTICS, INC.,
a Delaware corporation
Dated as of August 5, 2019
TABLE OF CONTENTS
| Page | ||||||
| SECTION 1. DESCRIPTION OF TRANSACTION |
2 | |||||
| 1.1 |
The Merger | 2 | ||||
| 1.2 |
Effects of the Merger | 2 | ||||
| 1.3 |
Closing; Effective Time | 2 | ||||
| 1.4 |
Certificate of Incorporation and Bylaws; Directors and Officers | 3 | ||||
| 1.5 |
Merger Consideration; Effect of Merger on Company Common Stock | 3 | ||||
| 1.6 |
Convertible Note Conversion | 3 | ||||
| 1.7 |
Conversion of Shares | 4 | ||||
| 1.8 |
Closing of the Company’s Transfer Books | 5 | ||||
| 1.9 |
Exchange of Shares | 5 | ||||
| 1.10 |
Appraisal Rights | 6 | ||||
| 1.11 |
UW Equity Right | 7 | ||||
| 1.12 |
Further Action | 7 | ||||
| 1.13 |
Withholding | 7 | ||||
| SECTION 2. REPRESENTATIONS AND WARRANTIES OF THE COMPANY |
7 | |||||
| 2.1 |
Due Organization; Subsidiaries | 7 | ||||
| 2.2 |
Organizational Documents | 8 | ||||
| 2.3 |
Authority; Binding Nature of Agreement | 8 | ||||
| 2.4 |
Vote Required | 9 | ||||
| 2.5 |
Non-Contravention; Consents | 9 | ||||
| 2.6 |
Capitalization | 10 | ||||
| 2.7 |
Financial Statements | 11 | ||||
| 2.8 |
Absence of Changes | 12 | ||||
| 2.9 |
Absence of Undisclosed Liabilities | 13 | ||||
| 2.10 |
Title to Assets | 14 | ||||
| 2.11 |
Real Property; Leasehold | 14 | ||||
| 2.12 |
Intellectual Property | 14 | ||||
| 2.13 |
Agreements, Contracts and Commitments | 16 | ||||
| 2.14 |
Compliance; Permits; Restrictions | 17 | ||||
| 2.15 |
Legal Proceedings; Orders | 19 | ||||
| 2.16 |
Tax Matters | 20 | ||||
| 2.17 |
Employee and Labor Matters; Benefit Plans | 22 | ||||
| 2.18 |
Environmental Matters | 25 | ||||
| 2.19 |
Insurance | 26 | ||||
| 2.20 |
No Financial Advisors | 26 | ||||
| 2.21 |
Transactions with Affiliates | 26 | ||||
| 2.22 |
Anti-Bribery | 26 | ||||
| 2.23 |
Disclaimer of Other Representations or Warranties | 26 | ||||
| SECTION 3. REPRESENTATIONS AND WARRANTIES OF PARENT AND MERGER SUB |
27 | |||||
| 3.1 |
Due Organization; Subsidiaries | 27 | ||||
| 3.2 |
Organizational Documents | 28 | ||||
| 3.3 |
Authority; Binding Nature of Agreement | 28 | ||||
i
TABLE OF CONTENTS
(continued)
| Page | ||||||
| 3.4 |
Vote Required | 28 | ||||
| 3.5 |
Non-Contravention; Consents | 29 | ||||
| 3.6 |
Capitalization | 30 | ||||
| 3.7 |
SEC Filings; Financial Statements | 31 | ||||
| 3.8 |
Absence of Changes | 33 | ||||
| 3.9 |
Absence of Undisclosed Liabilities | 34 | ||||
| 3.10 |
Title to Assets | 34 | ||||
| 3.11 |
Real Property; Leasehold | 34 | ||||
| 3.12 |
Intellectual Property | 35 | ||||
| 3.13 |
Agreements, Contracts and Commitments | 36 | ||||
| 3.14 |
Compliance; Permits | 38 | ||||
| 3.15 |
Legal Proceedings; Orders | 39 | ||||
| 3.16 |
Tax Matters | 40 | ||||
| 3.17 |
Employee and Labor Matters; Benefit Plans | 42 | ||||
| 3.18 |
Environmental Matters | 45 | ||||
| 3.19 |
Transactions with Affiliates | 45 | ||||
| 3.20 |
Insurance | 46 | ||||
| 3.21 |
No Financial Advisors | 46 | ||||
| 3.22 |
Anti-Bribery | 46 | ||||
| 3.23 |
Valid Issuance | 46 | ||||
| 3.24 |
Opinion of Financial Advisor | 46 | ||||
| 3.25 |
Disclaimer of Other Representations or Warranties | 46 | ||||
| SECTION 4. |
COVENANTS RELATING TO CONDUCT OF BUSINESS PENDING THE MERGER | 47 | ||||
| 4.1 |
Operation of Parent’s Business. | 47 | ||||
| 4.2 |
Operation of the Company’s Business | 48 | ||||
| SECTION 5. |
ADDITIONAL AGREEMENTS OF THE PARTIES | 50 | ||||
| 5.1 |
Company Information Statement; Stockholder Written Consent; Section 280G Approval | 50 | ||||
| 5.2 |
Parent Stockholders’ Meeting | 52 | ||||
| 5.3 |
Reservation of Parent Common Stock; Issuance of Shares of Parent Common Stock | 52 | ||||
| 5.4 |
Employee Benefits | 53 | ||||
| 5.5 |
Indemnification of Officers and Directors | 53 | ||||
| 5.6 |
Additional Agreements | 55 | ||||
| 5.7 |
Access and Investigation | 55 | ||||
| 5.8 |
Notification of Certain Matters | 55 | ||||
| 5.9 |
Disclosure | 56 | ||||
| 5.10 |
Listing | 56 | ||||
| 5.11 |
Tax Matters | 57 | ||||
| 5.12 |
Legends | 57 | ||||
ii
TABLE OF CONTENTS
(continued)
| Page | ||||||
| 5.13 |
Directors and Officers | 57 | ||||
| 5.14 |
Section 16 Matters | 58 | ||||
| 5.15 |
Cooperation | 58 | ||||
| 5.16 |
Closing Certificates | 58 | ||||
| 5.17 |
Takeover Statutes | 59 | ||||
| 5.18 |
Stockholder Litigation | 59 | ||||
| 5.19 |
Parent Options | 59 | ||||
| 5.20 |
Private Placement | 59 | ||||
| SECTION 6. |
CONDITIONS PRECEDENT TO OBLIGATIONS OF EACH PARTY | 60 | ||||
| 6.1 |
No Restraints | 60 | ||||
| 6.2 |
Stockholder Approval | 60 | ||||
| 6.3 |
Listing | 60 | ||||
| 6.4 |
Certificate of Designation | 60 | ||||
| SECTION 7. |
ADDITIONAL CONDITIONS PRECEDENT TO OBLIGATIONS OF PARENT AND MERGER SUB | 60 | ||||
| 7.1 |
Accuracy of Representations | 60 | ||||
| 7.2 |
Performance of Covenants | 61 | ||||
| 7.3 |
Documents | 61 | ||||
| 7.4 |
FIRPTA Certificate | 61 | ||||
| 7.5 |
No Company Material Adverse Effect | 61 | ||||
| 7.6 |
Company Lock-Up Agreements | 61 | ||||
| SECTION 8. |
ADDITIONAL CONDITIONS PRECEDENT TO OBLIGATION OF THE COMPANY | 61 | ||||
| 8.1 |
Accuracy of Representations | 61 | ||||
| 8.2 |
Performance of Covenants | 62 | ||||
| 8.3 |
Documents | 62 | ||||
| 8.4 |
No Parent Material Adverse Effect | 62 | ||||
| 8.5 |
Parent Lock-Up Agreements | 62 | ||||
| 8.6 |
Company Employee Agreements | 62 | ||||
| 8.7 |
Retention Agreements | 62 | ||||
| SECTION 9. |
TERMINATION | 62 | ||||
| 9.1 |
Termination | 62 | ||||
| 9.2 |
Effect of Termination | 63 | ||||
| 9.3 |
Expenses | 64 | ||||
| SECTION 10. |
MISCELLANEOUS PROVISIONS | 64 | ||||
| 10.1 |
Non-Survival of Representations and Warranties | 64 | ||||
| 10.2 |
Amendment | 64 | ||||
iii
TABLE OF CONTENTS
(continued)
| Page | ||||||
| 10.3 |
Waiver | 64 | ||||
| 10.4 |
Entire Agreement; Counterparts; Exchanges by Electronic Transmission | 64 | ||||
| 10.5 |
Applicable Law; Jurisdiction | 65 | ||||
| 10.6 |
Attorneys’ Fees | 65 | ||||
| 10.7 |
Assignability | 65 | ||||
| 10.8 |
Notices | 65 | ||||
| 10.9 |
Cooperation | 66 | ||||
| 10.10 |
Severability | 66 | ||||
| 10.11 |
Other Remedies; Specific Performance | 66 | ||||
| 10.12 |
No Third Party Beneficiaries | 67 | ||||
| 10.13 |
Construction | 67 | ||||
iv
Exhibits:
| Exhibit A | Definitions | |
| Exhibit B-1 | Form of Parent Stockholder Support Agreement | |
| Exhibit B-2 | Form of Lock-Up Agreement | |
| Exhibit C | Form of Company Stockholder Written Consent | |
| Exhibit D | Form of Certificate of Designation | |
| Exhibit E-1 | Company Tax Representation Letter | |
| Exhibit E-2 | Parent Tax Representation Letter | |
| Exhibit F-1 | Company Tax Opinion | |
| Exhibit F-2 | Parent Tax Opinion | |
| Exhibit G | Post-Closing Directors and Officers |
v
AGREEMENT AND PLAN OF MERGER
THIS AGREEMENT AND PLAN OF MERGER (this “Agreement”) is made and entered into as of August 5, 2019, by and among AQUINOX PHARMACEUTICALS, INC., a Delaware corporation (“Parent”), APOLLO SUB, INC., a Delaware corporation and wholly owned subsidiary of Parent (“Merger Sub”), and NEOLEUKIN THERAPEUTICS, INC., a Delaware corporation (the “Company”). Certain capitalized terms used in this Agreement are defined in Exhibit A.
RECITALS
A. Parent and the Company intend to effect a merger of Merger Sub with and into the Company (the “Merger”) in accordance with this Agreement and the DGCL. Upon consummation of the Merger, Merger Sub will cease to exist and the Company will become a wholly owned subsidiary of Parent.
B. The Parties intend that the Merger qualify as a “reorganization” within the meaning of Section 368(a) of the Code, and by executing this Agreement, the Parties intend to adopt a plan of reorganization within the meaning of Treasury Regulations Sections 1.368-2(g) and 1.368-3.
C. The Parent Board has (i) determined that the Contemplated Transactions are fair to, advisable and in the best interests of Parent and its stockholders, (ii) approved and declared advisable this Agreement and the Contemplated Transactions, including the issuance of the Parent Common Stock Payment Shares and the Parent Preferred Stock Payment Shares to the stockholders of the Company pursuant to the terms of this Agreement, and (iii) determined to recommend, upon the terms and subject to the conditions set forth in this Agreement, that the stockholders of the Parent vote to approve the Parent Stockholder Matters at the Parent Stockholders Meeting to be convened following the Closing.
D. The Merger Sub Board has (i) determined that the Contemplated Transactions are fair to, advisable, and in the best interests of Merger Sub and its sole stockholder, (ii) approved and declared advisable this Agreement and the Contemplated Transactions and (iii) determined to recommend, upon the terms and subject to the conditions set forth in this Agreement, that the stockholder of Merger Sub votes to adopt this Agreement and thereby approve the Contemplated Transactions.
E. The Company Board has (i) determined that the Contemplated Transactions are fair to, advisable and in the best interests of the Company and its stockholders, (ii) approved and declared advisable this Agreement and the Contemplated Transactions and (iii) determined to recommend, upon the terms and subject to the conditions set forth in this Agreement, that the stockholders of the Company vote to approve the Company Stockholder Matters.
F. Concurrently with the execution and delivery of this Agreement and as a condition and inducement to the Company’s willingness to enter into this Agreement, certain stockholders of Parent listed on Section A of the Parent Disclosure Schedule (solely in their capacity as stockholders of Parent) are executing support agreements in favor of the Company in substantially the form attached hereto as Exhibit B-1 (the “Parent Stockholder Support Agreement”), pursuant to which such Persons (the “Parent Signatories”) have, subject to the terms and conditions set forth therein, agreed to vote all of their shares of Parent Common Stock in favor of the Parent Stockholder Matters.
G. Concurrently with the execution and delivery of this Agreement and as a condition and inducement to each of Parent and the Company’s willingness to enter into this Agreement, the stockholders of Parent listed on Section A of the Parent Disclosure Schedule (solely in their capacity as stockholders of Parent) and the stockholders of the Company listed on Section A of the Company Disclosure Schedule, (the “Company Signatories”) (solely in their capacity as stockholders of the Company) are executing lock-up agreements in substantially the form attached as Exhibit B-2 (each, a “Lock-Up Agreement”).
H. It is expected that promptly after execution of this Agreement, the Company Signatories will execute and deliver an action by written consent in the form attached hereto as Exhibit C adopting the Company Stockholder Matters (each, a “Company Stockholder Written Consent”).
I. Concurrently with the execution and delivery of this Agreement and as a condition and inducement of the Company’s willingness to enter into this Agreement, certain employees of the Company identified in Section B of the Company Disclosure Schedule (each, a “Company Named Employee”) have each executed employee agreements with Parent (the “Company Employee Agreements”), each to become effective upon the Closing (as defined herein).
J. Concurrently with the execution and delivery of this Agreement and as a condition and inducement of the Company’s willingness to enter into this Agreement, certain employees of Parent identified on Section C of the Company Disclosure Schedule (each, a “Parent Named Employee”) have each executed transition retention agreements with the Canadian subsidiary of Parent (the “Retention Agreements”), each to become effective upon the Closing (as defined herein).
AGREEMENT
The Parties, intending to be legally bound, agree as follows:
Section 1. DESCRIPTION OF TRANSACTION
1.1 The Merger. Upon the terms and subject to the conditions set forth in this Agreement, at the Effective Time, Merger Sub shall be merged with and into the Company, and the separate existence of Merger Sub shall cease. The Company will continue as the surviving corporation in the Merger (the “Surviving Corporation”).
1.2 Effects of the Merger. The Merger shall have the effects set forth in this Agreement, the Certificate of Merger and in the applicable provisions of the DGCL. As a result of the Merger, the Company will become a wholly owned subsidiary of Parent.
1.3 Closing; Effective Time. Subject to the satisfaction or waiver of the conditions set forth in Sections 6, 7 and 8, the consummation of the Merger (the “Closing”) shall take place electronically as promptly as practicable (but in no event later than the second Business Day following the satisfaction or waiver of the last to be satisfied or waived of the conditions set forth in Sections 6, 7 and 8, other than those conditions that by their nature are to be satisfied at the Closing, but subject to the satisfaction or waiver of each of such conditions), or at such other time, date and place as Parent and the Company may mutually agree in writing. The date on which the Closing actually takes place is referred to as the “Closing Date.” At the Closing, the Parties shall cause the Merger to be consummated by executing and filing with the Secretary of State of the State of Delaware a certificate of merger with respect to the Merger, satisfying the applicable requirements of the DGCL and in a form reasonably acceptable to Parent and the Company (the “Certificate of Merger”). The Merger shall become effective at the time of the filing of such Certificate of Merger with the Secretary of State of the State of Delaware or at such later time as may be specified in such Certificate of Merger with the consent of Parent and the Company (the time as of which the Merger becomes effective being referred to as the “Effective Time”).
2
1.4 Certificate of Incorporation and Bylaws; Directors and Officers. At the Effective Time:
(a) the certificate of incorporation of the Surviving Corporation shall be amended and restated in its entirety to read identically to the certificate of incorporation of Merger Sub as in effect immediately prior to the Effective Time, until thereafter amended as provided by the DGCL and such certificate of incorporation; provided, however, that at the Effective Time (as part of the Certificate of Merger), the certificate of incorporation shall be amended to (i) change the name of the Surviving Corporation to “Neoleukin Corporation” and (ii) make such other changes as are mutually agreed to by Parent and the Company;
(b) the certificate of incorporation of Parent shall be identical to the certificate of incorporation of Parent immediately prior to the Effective Time, until thereafter amended as provided by the DGCL and such certificate of incorporation, provided, however, that following the Effective Time but as soon thereafter as practicable the corporate name of Parent shall be changed to “Neoleukin Therapeutics, Inc.”;
(c) the bylaws of the Surviving Corporation shall be amended and restated in their entirety to read identically to the bylaws of Merger Sub as in effect immediately prior to the Effective Time, until thereafter amended as provided by the DGCL and such bylaws;
(d) the directors and officers of Parent, each to hold office in accordance with the certificate of incorporation and bylaws of Parent, shall be as set forth in Section 5.13; and
(e) the directors and officers of the Surviving Corporation, each to hold office in accordance with the certificate of incorporation and bylaws of the Surviving Corporation, shall be the persons in such roles as set forth in Section 5.13, or such other persons as shall be mutually agreed upon by Parent and the Company.
1.5 Merger Consideration; Effect of Merger on Company Common Stock. The aggregate merger consideration (the “Merger Consideration”) to be paid by Parent for all of the outstanding shares of Company Common Stock at the Closing shall be (i) 4,589,787 shares of Parent Common Stock (“Parent Common Stock Payment Shares”), which shares shall represent a number of shares equal to no more than 19.5% of the outstanding shares of Parent Common Stock as of immediately before the Effective Time, and (ii) 101,948 shares of Parent Convertible Preferred Stock (“Parent Preferred Stock Payment Shares”). Each Parent Preferred Stock Payment Share shall be convertible into 100 shares of Parent Common Stock, subject to and contingent upon the affirmative vote of a majority of the Parent Common Stock present or represented and entitled to vote at a meeting of stockholders of Parent to approve, for purposes of the Nasdaq Stock Market Rules, the issuance of shares of Parent Common Stock to the stockholders of the Company upon conversion of the Parent Preferred Stock Payment Shares in accordance with the terms of the Certificate of Designation in substantially the form attached hereto as Exhibit D (the “Preferred Stock Conversion Proposal”).
1.6 Convertible Note Conversion. Immediately prior to the Effective Time, without any action on the part of Parent, Merger Sub, the Company or the holders of Company Convertible Notes, each Company Convertible Note outstanding immediately prior shall have immediately been cancelled and converted automatically into the right of the holder of such Company Convertible Note to receive, upon and subject to the terms set forth in the Company Convertible Note, Company Common Stock. Upon conversion of the Company Convertible Notes, such Company Common Stock will be treated in accordance with Section 1.7.
3
1.7 Conversion of Shares.
(a) At the Effective Time, by virtue of the Merger and without any further action on the part of Parent, Merger Sub, the Company or any stockholder of the Company or Parent:
(i) any shares of Company Common Stock held as treasury stock or held or owned by the Company or any wholly owned Subsidiary of the Company immediately prior to the Effective Time shall be canceled and retired and shall cease to exist, and no consideration shall be delivered in exchange therefor; and
(ii) subject to Section 1.7(c), each share of Company Common Stock outstanding immediately prior to the Effective Time (excluding shares to be canceled pursuant to Section 1.7(a)(i) and excluding Dissenting Shares) shall be automatically converted solely into the right to receive (A) a number of Parent Common Stock Payment Shares equal to the Common Stock Exchange Ratio and (B) a number of Parent Preferred Stock Payment Shares equal to the Preferred Stock Exchange Ratio.
(b) If any shares of Company Common Stock outstanding immediately prior to the Effective Time are unvested or are subject to a repurchase option or a risk of forfeiture under any applicable restricted stock purchase agreement or other similar agreement with the Company, the vesting of such shares of Company Common Stock shall be accelerated immediately prior to the Effective Time, and such shares of Company Common Stock shall no longer be subject to any further vesting, right of repurchase, risk of forfeiture or other such conditions.
(c) No fractional shares of Parent Common Stock and Parent Convertible Preferred Stock shall be issued in connection with the Merger, and no certificates or scrip for any such fractional shares shall be issued. Any holder of Company Common Stock who would otherwise be entitled to receive a fraction of a share of Parent Common Stock (after aggregating all fractional shares of Parent Common Stock issuable to such holder) or a fraction of a share of Parent Convertible Preferred Stock (after aggregating all fractional shares of Parent Convertible Preferred Stock issuable to such holder) shall, in lieu of such fraction of a share and upon surrender by such holder of a letter of transmittal in accordance with Section 1.9 and any accompanying documents as required therein, be paid in cash the dollar amount (rounded to the nearest whole cent), without interest, determined by multiplying such fraction by (i) the Parent Closing Price in respect of shares of Parent Common Stock or (ii)(A) the Parent Closing Price multiplied by (B) 100, in respect of shares of Parent Convertible Preferred Stock.
(d) Each share of common stock, $0.0001 par value per share, of Merger Sub issued and outstanding immediately prior to the Effective Time shall be converted into and exchanged for one validly issued, fully paid and nonassessable share of common stock, $0.0001 par value per share, of the Surviving Corporation. Each stock certificate of Merger Sub evidencing ownership of any such shares shall, as of the Effective Time, evidence ownership of such shares of common stock of the Surviving Corporation.
(e) If, between the date of this Agreement and the Effective Time, the outstanding shares of Company Common Stock or Parent Common Stock or Parent Convertible Preferred Stock shall have been changed into, or exchanged for, a different number of shares or a different class, by reason of any stock dividend, subdivision, reclassification, recapitalization, split, combination or exchange of shares or other like change, the Common Stock Exchange Ratio or the Preferred Stock Exchange Ratio, as the case may be, shall, to the extent necessary, be equitably adjusted to reflect such change to the extent necessary to provide the holders of Company Common Stock and Parent Common Stock and Parent Convertible Preferred Stock, with the same economic effect as contemplated by this Agreement prior to such stock dividend, subdivision, reclassification, recapitalization, split, combination or exchange of shares or other like change; provided, however, that nothing herein will be construed to permit the Company or Parent to take any action with respect to Company Common Stock or Parent Common Stock or Parent Convertible Preferred Stock, respectively, that is prohibited or not expressly permitted by the terms of this Agreement.
4
1.8 Closing of the Company’s Transfer Books. At the Effective Time: (a) all holders of certificates representing shares of Company Common Stock that were outstanding immediately prior to the Effective Time shall cease to have any rights as stockholders of the Company; and (b) the stock transfer books of the Company shall be closed with respect to all shares of Company Common Stock outstanding immediately prior to the Effective Time. No further transfer of any such shares of Company Common Stock shall be made on such stock transfer books after the Effective Time. If, after the Effective Time, a valid certificate previously representing any shares of Company Common Stock outstanding immediately prior to the Effective Time (a “Company Stock Certificate”) is presented to the Exchange Agent or to the Surviving Corporation, such Company Stock Certificate shall be canceled and shall be exchanged as provided in Sections 1.7 and 1.9.
1.9 Exchange of Shares.
(a) On or prior to the Closing Date, Parent appoint American Stock Transfer & Trust Company, LLC to act as exchange agent in the Merger (the “Exchange Agent”). At the Effective Time, Parent shall deposit with the Exchange Agent: (i) certificates or evidence of book-entry shares representing the Parent Common Stock and Parent Convertible Preferred Stock issuable pursuant to Section 1.7(a) and (ii) cash sufficient to make payments in lieu of fractional shares in accordance with Section 1.7(c). The Parent Common Stock, Parent Convertible Preferred Stock and cash amounts so deposited with the Exchange Agent, together with any dividends or distributions received by the Exchange Agent with respect to such shares, are referred to collectively as the “Exchange Fund.”
(b) Promptly after the Effective Time, the Parties shall cause the Exchange Agent to mail to the Persons who were record holders of shares of Company Common Stock that were converted into the right to receive the Merger Consideration: (i) a letter of transmittal in customary form and containing such provisions as Parent may reasonably specify (including a provision confirming that delivery of Company Stock Certificates shall be effected, and risk of loss and title to Company Stock Certificates shall pass, only upon proper delivery of such Company Stock Certificates to the Exchange Agent, all to the extent applicable); and (ii) instructions for effecting the surrender of Company Stock Certificates in exchange for shares of Parent Common Stock. Upon surrender of a Company Stock Certificate to the Exchange Agent for exchange, together with a duly executed letter of transmittal and such other documents as may be reasonably required by the Exchange Agent or Parent: (A) the holder of such Company Stock Certificate shall be entitled to receive in exchange therefor a certificate or certificates or book-entry shares representing the Merger Consideration (in a number of whole shares of Parent Common Stock and Parent Convertible Preferred Stock) that such holder has the right to receive pursuant to the provisions of Section 1.7(a) (and cash in lieu of any fractional share of Parent Common Stock or Parent Convertible Preferred Stock pursuant to the provisions of Section 1.7(c)); and (B) the Company Stock Certificate so surrendered shall be canceled. Until surrendered as contemplated by this Section 1.9(b), each Company Stock Certificate shall be deemed, from and after the Effective Time, to represent only the right to receive a certificate or certificates or book-entry shares of Parent Common Stock and Parent Convertible Preferred Stock representing the Merger Consideration (and cash in lieu of any fractional share of Parent Common Stock or Parent Convertible Preferred Stock). If any Company Stock Certificate shall have been lost, stolen or destroyed, Parent may, in its discretion and as a condition precedent to the delivery of any shares of Parent Common Stock, require the owner of such lost, stolen or destroyed Company Stock Certificate to provide an applicable affidavit with respect to such Company Stock Certificate that includes an obligation of such owners to indemnify Parent against any claim suffered by Parent related to the lost, stolen or destroyed Company Stock Certificate as Parent may reasonably request. In the event of a transfer
5
of ownership of a Company Stock Certificate that is not registered in the transfer records of the Company, payment of the Merger Consideration in respect of such Company Stock Certificate may be made to a Person other than the Person in whose name such Company Stock Certificate so surrendered is registered if such Company Stock Certificate shall be properly endorsed or otherwise be in proper form for transfer and the Person requesting such payment shall pay any transfer or other Taxes required by reason of the transfer or establish to the reasonable satisfaction of Parent that such Taxes have been paid or are not applicable. The Merger Consideration and any dividends or other distributions as are payable pursuant to Section 1.9(c) shall be deemed to have been in full satisfaction of all rights pertaining to Company Common Stock formerly represented by such Company Stock Certificates.
(c) No dividends or other distributions declared or made with respect to Parent Common Stock or Parent Convertible Preferred Stock with a record date on or after the Effective Time shall be paid to the holder of any unsurrendered Company Stock Certificate with respect to the shares of Parent Common Stock and/or Parent Convertible Preferred Stock that such holder has the right to receive in the Merger until such holder surrenders such Company Stock Certificate or provides an affidavit of loss or destruction in lieu thereof in accordance with this Section 1.9 (at which time (or, if later, on the applicable payment date) such holder shall be entitled, subject to the effect of applicable abandoned property, escheat or similar Laws, to receive all such dividends and distributions, without interest).
(d) Any portion of the Exchange Fund that remains undistributed to holders of Company Stock Certificates as of the date that is one year after the Closing Date shall be delivered to Parent upon demand, and any holders of Company Stock Certificates who have not theretofore surrendered their Company Stock Certificates in accordance with this Section 1.9 shall thereafter look only to Parent for satisfaction of their claims for Parent Common Stock and Parent Convertible Preferred Stock, cash in lieu of fractional shares of Parent Common Stock and Parent Convertible Preferred Stock and any dividends or distributions with respect to shares of Parent Common Stock and Parent Convertible Preferred Stock.
(e) No party to this Agreement shall be liable to any holder of any Company Stock Certificate or to any other Person with respect to any shares of Parent Common Stock or Parent Convertible Preferred Stock (or dividends or distributions with respect thereto) or for any cash amounts delivered to any public official pursuant to any applicable abandoned property Law, escheat Law or similar Law.
1.10 Appraisal Rights.
(a) Notwithstanding any provision of this Agreement to the contrary, shares of Company Common Stock that are outstanding immediately prior to the Effective Time and which are held by stockholders who have exercised and perfected appraisal rights for such shares of Company Common Stock in accordance with the DGCL (collectively, the “Dissenting Shares”) shall not be converted into or represent the right to receive the Merger Consideration described in Section 1.5 attributable to such Dissenting Shares. Such stockholders shall be entitled to receive payment of the appraised value of such shares of Company Common Stock held by them in accordance with the DGCL unless and until such stockholders fail to perfect or effectively withdraw or otherwise lose their appraisal rights under the DGCL. All Dissenting Shares held by stockholders who shall have failed to perfect or shall have effectively withdrawn or lost their right to appraisal of such shares of Company Common Stock under the DGCL (whether occurring before, at or after the Effective Time) shall thereupon be deemed to be converted into and to have become exchangeable for, as of the Effective Time, the right to receive the Merger Consideration, without interest, attributable to such Dissenting Shares upon their surrender in the manner provided in Sections 1.7 and 1.9.
6
(b) The Company shall give Parent prompt written notice of any demands by dissenting stockholders received by the Company, withdrawals of such demands and any other instruments served on the Company and any material correspondence received by the Company in connection with such demands, and the Company shall have the right to direct all negotiations and proceedings with respect to such demands; provided that the Parent shall have the right to participate in such negotiations and proceedings. Neither the Parent nor the Company shall, except with the other party’s prior written consent, voluntarily make any payment with respect to, or settle or offer to settle, any such demands, or approve any withdrawal of any such demands or agree to do any of the foregoing.
1.11 UW Equity Right. At and after the Effective Time, if UW is or becomes entitled to receive Company Common Stock under the UW Equity Right, the UW Equity Right shall be converted into a right to receive Parent Common Stock and Parent Convertible Preferred Stock, and Parent shall assume the UW Equity Right in accordance with the terms (as in effect as of the date of this Agreement) of the UW Equity Right. All rights with respect to Company Common Stock under the UW Equity Right assumed by Parent shall thereupon be converted into rights with respect to Parent Common Stock and Parent Convertible Preferred Stock. Accordingly, from and after the Effective Time: (i) the UW Equity Right assumed by Parent may be exercised solely for shares of Parent Common Stock and Parent Convertible Preferred Stock; (ii) the number of shares of Parent Common Stock and Parent Convertible Preferred Stock subject to the UW Equity Right assumed by Parent shall be determined by multiplying (A) the number of shares of Company Common Stock that were subject to such UW Equity Right, by (B) the Common Stock Exchange Ratio and Preferred Stock Exchange Ratio, respectively, and rounding the resulting number down to the nearest whole number of shares of Parent Common Stock and Parent Convertible Preferred Stock.
1.12 Further Action. If, at any time after the Effective Time, any further action is determined by the Surviving Corporation to be necessary or desirable to carry out the purposes of this Agreement or to vest the Surviving Corporation with full right, title and possession of and to all rights and property of the Company, then the officers and directors of the Surviving Corporation shall be fully authorized, and shall use their and its reasonable best efforts (in the name of the Company, in the name of Merger Sub, in the name of the Surviving Corporation and otherwise) to take such action.
1.13 Withholding. The Parties and the Exchange Agent shall be entitled to deduct and withhold from the consideration otherwise payable pursuant to this Agreement to any holder of Company Common Stock or any other Person such amounts as such Party or the Exchange Agent is required to deduct and withhold under the Code or any other Law with respect to the making of such payment. To the extent that amounts are so withheld and paid over to the appropriate Governmental Body, such withheld amounts shall be treated for all purposes of this Agreement as having been paid to the Person in respect of whom such deduction and withholding was made.
Section 2. REPRESENTATIONS AND WARRANTIES OF THE COMPANY
Subject to Section 10.13(h), except as set forth in the disclosure schedule delivered by the Company to Parent (the “Company Disclosure Schedule”), the Company represents and warrants to Parent and Merger Sub as follows:
2.1 Due Organization; Subsidiaries.
(a) The Company is a corporation or other legal entity duly incorporated or otherwise organized, validly existing and in good standing under the Laws of Delaware and has all necessary corporate power and authority: (i) to conduct its business in the manner in which its business is currently being conducted; (ii) to own or lease and use its property and assets in the manner in which its
7
property and assets are currently owned or leased and used; and (iii) to perform its obligations under all Contracts by which it is bound, in each case, except where the failure to have such power or authority would not reasonably be expected to prevent or materially delay the ability of the Company to consummate the Contemplated Transactions.
(b) The Company is duly licensed and qualified to do business, and is in good standing (to the extent applicable in such jurisdiction), under the Laws of all jurisdictions where the nature of its business requires such licensing or qualification other than in jurisdictions where the failure to be so qualified individually or in the aggregate would not be reasonably expected to have a Company Material Adverse Effect.
(c) The Company has no Subsidiaries, except for the Entities identified in Section 2.1(c) of the Company Disclosure Schedule; and neither the Company nor any of the Entities identified in Section 2.1(c) of the Company Disclosure Schedule owns any capital stock of, or any equity, ownership or profit sharing interest of any nature in, or controls directly or indirectly, any other Entity other than the Entities identified in Section 2.1(c) of the Company Disclosure Schedule. Each of the Company’s Subsidiaries is a corporation or other legal entity duly organized, validly existing and, if applicable, in good standing under the Laws of the jurisdiction of its organization and has all necessary corporate or other power and authority to conduct its business in the manner in which its business is currently being conducted and to own or lease and use its property and assets in the manner in which its property and assets are currently owned or leased and used, except where the failure to have such power or authority would not be reasonably expected to have a Company Material Adverse Effect.
(d) Neither the Company nor any of its Subsidiaries is or has otherwise been, directly or indirectly, a party to, member of or participant in any partnership, joint venture or similar business entity. Neither the Company nor any of its Subsidiaries has agreed or is obligated to make, or is bound by any Contract under which it may become obligated to make, any future investment in or capital contribution to any other Entity. Neither the Company nor any of its Subsidiaries has, at any time, been a general partner of, or has otherwise been liable for any of the debts or other obligations of, any general partnership, limited partnership or other Entity.
2.2 Organizational Documents. The Company has made available to Parent accurate and complete copies of the Organizational Documents of the Company and each of its Subsidiaries in effect as of the date of this Agreement. Neither the Company nor any of its Subsidiaries is in breach or violation of its respective Organizational Documents.
2.3 Authority; Binding Nature of Agreement.
(a) The Company and each of its Subsidiaries have all necessary corporate power and authority to enter into and to perform its obligations under this Agreement and, subject to receipt of the Required Company Stockholder Vote, to consummate the Contemplated Transactions. The Company Board (at meetings duly called and held) has (i) determined that the Contemplated Transactions are fair to, advisable and in the best interests of the Company and its stockholders, (ii) approved and declared advisable this Agreement and the Contemplated Transactions and (iii) determined to recommend, upon the terms and subject to the conditions set forth in this Agreement, that the stockholders of the Company vote in favor of the Company Stockholder Matters.
(b) This Agreement has been duly executed and delivered by the Company and assuming the due authorization, execution and delivery by Parent and Merger Sub, constitutes the legal, valid and binding obligation of the Company, enforceable against the Company in accordance with its terms, subject to the Enforceability Exceptions.
8
2.4 Vote Required. The affirmative vote (or written consent) of the holders of a majority of the shares of Company Common Stock each outstanding on the record date for the Company Stockholder Written Consent and entitled to vote thereon, voting as a single class (collectively, the “Required Company Stockholder Vote”), is the only vote (or written consent) of the holders of any class or series of Company Common Stock necessary to adopt and approve this Agreement and approve the Contemplated Transactions.
2.5 Non-Contravention; Consents. Subject to obtaining the Required Company Stockholder Vote, the filing of the Certificate of Merger required by the DGCL, and the filing of the Certificate of Designation, neither (x) the execution, delivery or performance of this Agreement by the Company, nor (y) the consummation of the Contemplated Transactions, will directly or indirectly (with or without notice or lapse of time):
(a) contravene, conflict with or result in a violation of any of the provisions of the Company’s Organizational Documents;
(b) contravene, conflict with or result in a violation of, or give any Governmental Body or other Person the right to challenge the Contemplated Transactions or to exercise any remedy or obtain any relief under, any Law or any order, writ, injunction, judgment or decree to which the Company or its Subsidiaries, or any of the assets owned or used by the Company or its Subsidiaries, is subject, except as would not reasonably be expected to be material to the Company or its business;
(c) contravene, conflict with or result in a violation of any of the terms or requirements of, or give any Governmental Body the right to revoke, withdraw, suspend, cancel, terminate or modify, any Governmental Authorization that is held by the Company or its Subsidiaries, except as would not reasonably be expected to be material to the Company or its business;
(d) contravene, conflict with or result in a violation or breach of, or result in a default under, any provision of any Company Material Contract, or give any Person the right to: (i) declare a default or exercise any remedy under any Company Material Contract; (ii) any material payment, rebate, chargeback, penalty or change in delivery schedule under any Company Material Contract; (iii) accelerate the maturity or performance of any Company Material Contract; or (iv) cancel, terminate or modify any term of any Company Material Contract, except in the case of any non-material breach, default, penalty or modification; or
(e) result in the imposition or creation of any Encumbrance upon or with respect to any asset owned or used by the Company or its Subsidiaries (except for Permitted Encumbrances).
Except for (i) any Consent set forth on Section 2.5 of the Company Disclosure Schedule under any Company Contract, (ii) the Required Company Stockholder Vote, (iii) the filing of the Certificate of Merger with the Secretary of State of the State of Delaware pursuant to the DGCL, (iv) the filing of the Certificate of Designation with the Secretary of State of the State of Delaware and (v) such consents, waivers, approvals, orders, authorizations, registrations, declarations and filings as may be required under applicable federal and state securities Laws, neither the Company nor any of its Subsidiaries is or will be required to make any filing with or give any notice to, or to obtain any Consent from, any Person in connection with (A) the execution, delivery or performance of this Agreement, or (B) the consummation of the Contemplated Transactions. The Company Board has taken and will take all actions necessary to ensure that the restrictions applicable to business combinations contained in Section 203 of the DGCL are, and will be, inapplicable to the execution, delivery and performance of this Agreement, the Lock-Up Agreements and to the consummation of the Contemplated Transactions. No other state takeover statute or similar Law applies or purports to apply to the Merger, this Agreement, the Lock-Up Agreements or any of the Contemplated Transactions.
9
2.6 Capitalization.
(a) The authorized capital stock of the Company as of the date of this Agreement consists of (i) 20,000,000 shares of Company Common Stock, of which 10,031,579 shares have been issued and are outstanding as of the date of this Agreement (including Company RSA Awards). 2,300,522 shares of Company Common Stock are issuable upon conversion of the Company Convertible Notes and will be outstanding at the Effective Time. The Company does not hold any shares of its capital stock in its treasury. Section 2.6(a) of the Company Disclosure Schedule lists, as of the date of this Agreement, each record holder of issued and outstanding Company Common Stock and the number and type of shares of Company Common Stock held by such holder. Accurate and complete copies of the Company Convertible Notes, as amended, have been provided to Parent.
(b) All of the outstanding shares of Company Common Stock have been duly authorized and validly issued, and are fully paid and nonassessable. None of the outstanding shares of Company Common Stock is entitled or subject to any preemptive right, right of participation, right of maintenance or any similar right and none of the outstanding shares of Company Common Stock is subject to any right of first refusal in favor of the Company. Except as contemplated herein and in the Company Plan (and related award agreements), there is no Company Contract relating to the voting or registration of, or restricting any Person from purchasing, selling, pledging or otherwise disposing of (or granting any option or similar right with respect to), any shares of Company Common Stock. The Company is not under any obligation, nor is it bound by any Contract pursuant to which it may become obligated, to repurchase, redeem or otherwise acquire any outstanding shares of Company Common Stock or other securities. Section 2.6(b) of the Company Disclosure Schedule accurately and completely lists all repurchase rights held by the Company with respect to shares of Company Common Stock and specifies which of those repurchase rights are currently exercisable and whether the holder of such shares of Company Common Stock timely filed an election with the relevant Governmental Bodies under Section 83(b) of the Code with respect to such shares.
(c) Except for the Company’s 2018 Equity Incentive Plan, as adopted on November 16, 2018 (the “Company Plan”), the Company does not have any stock option plan or any other plan, program, agreement or arrangement providing for any equity-based compensation for any Person. As of the date of this Agreement, the Company has reserved 500,000 shares of Company Common Stock for issuance under the Company Plan, of which no shares have been issued and are currently outstanding, 6,660,640 shares are subject to outstanding awards of restricted Company Common Stock (such awards, the “Company RSA Awards”), and 500,000 shares of Company Common Stock remain available for future issuance of awards pursuant to the Company Plan. Section 2.6(c) of the Company Disclosure Schedule sets forth the following information with respect to each Company RSA Award outstanding as of the date of this Agreement: (i) the name of the holder of each RSA Award; (ii) the number of shares of Company Common Stock subject to such Company RSA Award at the time of purchase; (iii) the number of shares of Company Common Stock subject to such Company RSA Award as of the date of this Agreement; (iv) the purchase price of such Company RSA Award; (v) the date on which such Company RSA Award was purchased; and (vi) the applicable vesting schedule, including the number of vested and unvested shares as of the date of this Agreement and any acceleration provisions. The Company has made available to Parent an accurate and complete copy of the Company Plan and all restricted stock purchase agreements evidencing outstanding Company RSA Awards.
10
(d) Except for Company RSA Awards set forth on Section 2.6(c) of the Company Disclosure Schedule, the UW Equity Right and the Company Convertible Notes, there is no: (i) outstanding subscription, option, call, warrant or right (whether or not currently exercisable) to acquire any shares of the capital stock or other securities of the Company or any of its Subsidiaries; (ii) outstanding security, instrument or obligation that is or may become convertible into or exchangeable for any shares of the capital stock or other securities of the Company or any of its Subsidiaries; or (iii) condition or circumstance that is reasonably likely to give rise to or provide a basis for the assertion of a claim by any Person to the effect that such Person is entitled to acquire or receive any shares of capital stock or other securities of the Company or any of its Subsidiaries. There are no outstanding or authorized stock appreciation, phantom stock, profit participation or other similar rights with respect to the Company or any of its Subsidiaries.
(e) All outstanding shares of Company Common Stock, Company Convertible Notes and other securities of the Company have been issued and granted in material compliance with (i) all applicable securities Laws and other applicable Law, and (ii) all requirements set forth in applicable Contracts.
2.7 Financial Statements.
(a) Concurrently with the execution hereof, the Company has provided to Parent true and complete copies of (i) the Company’s audited balance sheet at December 31, 2018, together with related audited statements of income, stockholders’ equity and cash flows, and notes thereto, of the Company for the fiscal period then ended and (ii) the Company Unaudited Interim Balance Sheet, together with the unaudited statements of income, stockholders’ equity and cash flows of the Company for the period reflected in the Company Unaudited Interim Balance Sheet (collectively, the “Company Financials”). The Company Financials were prepared in accordance with GAAP (except as may be indicated in the notes to such financial statements and except that the unaudited financial statements may not contain footnotes and are subject to normal and recurring year-end adjustments, none of which are material) and fairly present, in all material respects, the financial position and operating results of the Company and its consolidated Subsidiaries as of the dates and for the periods indicated therein.
(b) Each of the Company and its Subsidiaries maintains accurate books and records reflecting their assets and liabilities and maintains a system of internal accounting controls designed to provide reasonable assurance that: (i) transactions are executed in accordance with management’s general or specific authorizations; (ii) transactions are recorded as necessary to permit preparation of the financial statements of the Company and its Subsidiaries and to maintain accountability of the Company’s and its Subsidiaries’ assets; (iii) access to the Company’s and its Subsidiaries’ assets is permitted only in accordance with management’s general or specific authorization; (iv) the recorded accountability for the Company’s and its Subsidiaries’ assets is compared with the existing assets at regular intervals and appropriate action is taken with respect to any differences; and (v) accounts, notes and other receivables and inventory are recorded accurately, and proper and adequate procedures are implemented which are designed to effect the collection thereof on a current and timely basis. The Company and each of its Subsidiaries maintains internal control over financial reporting that provides reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes.
(c) Section 2.7(c) of the Company Disclosure Schedule lists, and the Company has delivered to Parent accurate and complete copies of the documentation creating or governing, all securitization transactions and “off-balance sheet arrangements” (as defined in Item 303(c) of Regulation S-K under the Exchange Act) effected by the Company or any of its Subsidiaries since the Company’s inception.
11
(d) Since the Company’s inception, there have been no formal internal investigations regarding financial reporting or accounting policies and practices discussed with, reviewed by or initiated at the direction of the chief executive officer, chief financial officer or general counsel of the Company, the Company Board or any committee thereof. Since the Company’s inception, neither the Company nor its independent auditors have identified (i) any significant deficiency or material weakness in the design or operation of the system of internal accounting controls utilized by the Company and its Subsidiaries, (ii) any fraud, whether or not material, that involves the Company, any of its Subsidiaries, the Company’s management or other employees who have a role in the preparation of financial statements or the internal accounting controls utilized by the Company and its Subsidiaries or (iii) any claim or allegation regarding any of the foregoing.
2.8 Absence of Changes. Except as set forth on Section 2.8 of the Company Disclosure Schedule, after the date of the Company Unaudited Interim Balance Sheet, the Company has conducted its business only in the Ordinary Course of Business (except for the execution and performance of this Agreement and the discussions, negotiations and transactions related thereto) and there has not been any (a) Company Material Adverse Effect and (b) the Company has not done any of the following:
(a) declared, accrued, set aside or paid any dividend or made any other distribution in respect of any shares of its capital stock; or repurchased, redeemed or otherwise reacquired any shares of its capital stock or other securities (except for shares of Company Common Stock from terminated employees, directors or consultants of the Company or in connection with the payment of the exercise price and/or withholding Taxes incurred upon the exercise, settlement or vesting of any award granted under the Company Plan in accordance with the terms of such award in effect on the date of this Agreement);
(b) sold, issued, granted, pledged or otherwise disposed of or encumbered or authorized any of the foregoing with respect to: (A) any capital stock or other security of the Company or any of its Subsidiaries; (B) any option, warrant or right to acquire any capital stock or any other security, other than option grants to employees and service providers in the Ordinary Course of Business; or (C) any instrument convertible into or exchangeable for any capital stock or other security of the Company or any of its Subsidiaries;
(c) except as required to give effect to anything in contemplation of the Closing, amended any of its or its Subsidiaries’ Organizational Documents, or effected or be a party to any merger, consolidation, share exchange, business combination, recapitalization, reclassification of shares, stock split, reverse stock split or similar transaction except, for the avoidance of doubt, the Contemplated Transactions;
(d) formed any Subsidiary or acquired any equity interest or other interest in any other Entity or entered into a joint venture with any other Entity;
(e) (A) lend money to any Person (except for the advance of reasonable business expenses to employees, directors and consultants in the Ordinary Course of Business), (B) incurred or guaranteed any indebtedness for borrowed money, or (C) guaranteed any debt securities of others;
(f) other than as required by applicable Law or the terms of any Company Benefit Plan as in effect on the date of this Agreement: (A) adopted, terminated, established or entered into any Company Benefit Plan; (B) caused or permitted any Company Benefit Plan to be amended in any material respect; (C) paid any bonus or make any profit-sharing or similar payment to, or increased the amount of the wages, salary, commissions, benefits or other compensation or remuneration payable to, any of its directors, officers or employees; (D) increased the severance or change of control benefits offered to any current, former or new employees, directors or consultants or (E) hired, terminated or gave notice of termination (other than for cause) to, any (x) officer or (y) employee whose annual base salary is or is expected to be more than $125,000 per year;
12
(g) recognized any labor union, labor organization, or similar Person, except as otherwise required by law and after advance notice to the Parent;
(h) entered into any material transaction other than in the Ordinary Course of Business;
(i) acquired any material asset or sold, leased or otherwise irrevocably disposed of any of its assets or properties, or granted any Encumbrance with respect to such assets or properties, except in the Ordinary Course of Business;
(j) sold, assigned, transferred, licensed, sublicensed or otherwise disposed of any material Company IP (other than pursuant to non-exclusive licenses in the Ordinary Course of Business);
(k) made, changed or revoked any material Tax election, failed to pay any income or other material Tax as such Tax becomes due and payable, filed any amendment making any material change to any Tax Return, settled or compromised any income or other material Tax liability, entered into any Tax allocation, sharing, indemnification or other similar agreement or arrangement (other than customary commercial contracts entered into in the Ordinary Course of Business the principal subject matter of which is not Taxes), requested or consented to any extension or waiver of any limitation period with respect to any claim or assessment for any income or other material Taxes (other than pursuant to an extension of time to file any Tax Return granted in the Ordinary Course of Business of not more than six months), or adopted or changed any material accounting method in respect of Taxes;
(l) made any expenditures, incurred any Liabilities or discharged or satisfied any Liabilities, in each case, in amounts that exceed $300,000;
(m) other than as required by Law or GAAP, taken any action to change accounting policies or procedures;
(n) initiated or settled any Legal Proceeding; or
(o) agreed, resolved or committed to do any of the foregoing.
2.9 Absence of Undisclosed Liabilities. As of the date hereof, neither the Company nor any of its Subsidiaries has any liability, indebtedness, obligation or expense of any kind, whether accrued, absolute, contingent, matured or unmatured (whether or not required to be reflected in the financial statements in accordance with GAAP) (each a “Liability”), individually or in the aggregate, of a type required to be recorded or reflected on a balance sheet or disclosed in the footnotes thereto under GAAP, except for: (a) Liabilities disclosed, reflected or reserved against in the Company Unaudited Interim Balance Sheet; (b) Liabilities that have been incurred by the Company or its Subsidiaries since the date of the Company Unaudited Interim Balance Sheet in the Ordinary Course of Business; (c) Liabilities for performance of obligations of the Company or any of its Subsidiaries under Company Contracts; (d) Liabilities incurred in connection with the Contemplated Transactions; (e) Liabilities which would not, individually or in the aggregate, reasonably be expected to be material to the Company; and (f) Liabilities described in Section 2.9 of the Company Disclosure Schedule.
13
2.10 Title to Assets. Each of the Company and its Subsidiaries owns, and has good and valid title to, or, in the case of leased properties and assets, valid leasehold interests in, all tangible properties or tangible assets and equipment used or held for use in its business or operations or purported to be owned by it, including: (a) all tangible assets reflected on the Company Unaudited Interim Balance Sheet; and (b) all other tangible assets reflected in the books and records of the Company or any of its Subsidiaries as being owned by the Company or such Subsidiary, in each case except as would not reasonably be expected to be material to the Company. All of such assets are owned or, in the case of leased assets, leased by the Company or any of its Subsidiaries free and clear of any Encumbrances, other than Permitted Encumbrances.
2.11 Real Property; Leasehold. Neither the Company nor any of its Subsidiaries owns or has ever owned any real property. The Company has made available to Parent (a) an accurate and complete list of all real properties with respect to which the Company directly or indirectly holds a valid leasehold interest as well as any other real estate that is in the possession of or leased by the Company or any of its Subsidiaries, and (b) copies of all leases under which any such real property is possessed (the “Company Real Estate Leases”), each of which is in full force and effect, with no existing material default thereunder. The Company’s use and operation of each such leased property conforms to all applicable Laws in all material respects, and the Company has exclusive possession of each such leased property and has not granted any occupancy rights to tenants or licensees with respect to such leased property. In addition, each such leased property is free and clear of all Encumbrances other than Permitted Encumbrances. The Company has not received written notice from its landlords or any Governmental Body that: (i) relates to violations of building, zoning, safety or fire ordinances or regulations; (ii) claims any defect or deficiency with respect to any of such properties; or (iii) requests the performance of any repairs, alterations or other work to such properties.
2.12 Intellectual Property.
(a) Section 2.12(a) of the Company Disclosure Schedule identifies each item of material Registered IP owned in whole or in part by the Company or its Subsidiaries, including, with respect to each registration and application: (i) the name of the applicant/registrant, (ii) the jurisdiction of application/registration, (iii) the application or registration number and (iv) any other co-owners. To the Knowledge of the Company, each of the patents and patent applications included in the material Registered IP properly identifies by name each and every inventor of the inventions claimed therein as determined in accordance with applicable Laws of the United States. As of the date of this Agreement, no cancellation, interference, opposition, reissue, reexamination or other proceeding of any nature (other than office actions or similar communications issued by any Governmental Body in the ordinary course of prosecution of any pending applications for registration) is pending or, to the Knowledge of the Company, threatened in writing, in which the scope, validity, enforceability or ownership of any material Registered IP owned in whole or in part by the Company or its Subsidiaries is being or has been contested or challenged.
(b) Except as has not had and would not reasonably be expected to have, individually or in the aggregate, a Company Material Adverse Effect, the Company or its Subsidiaries owns all right, title and interest in and to all material Company IP (other than as disclosed on Section 2.12(b) of the Company Disclosure Schedule), free and clear of all Encumbrances other than Permitted Encumbrances. To the Knowledge of the Company, each Company Associate involved in the creation or development of any material Company IP, pursuant to such Company Associate’s activities on behalf of the Company or its Subsidiaries, has signed a written agreement containing an assignment of such Company Associate’s rights in such Company IP to the Company or its Subsidiaries and confidentiality provisions protecting the Company IP.
14
(c) To the Knowledge of the Company, no funding, facilities or personnel of any Governmental Body or any university, college, research institute or other educational institution has been used to create Company IP, except for any such funding or use of facilities or personnel that does not result in such Governmental Body or institution obtaining ownership rights to such Company IP or the right to receive royalties for the practice of such Company IP.
(d) Section 2.12(d) of the Company Disclosure Schedule sets forth each license agreement pursuant to which the Company (i) is granted a license under any material Intellectual Property Right owned by any third party that is used by the Company or its Subsidiaries in its business as currently conducted (each a “Company In-bound License”) or (ii) grants to any third party a license under any material Company IP or material Intellectual Property Right licensed to the Company or its Subsidiaries under a Company In-bound License (each a “Company Out-bound License”) (provided, that, Company In-bound Licenses shall not include, when entered into in the Ordinary Course of Business, material transfer agreements, clinical trial agreements, agreements with Company Associates, services agreements, non-disclosure agreements, commercially available Software-as-a-Service offerings, off-the-shelf software licenses or generally available patent license agreements; and Company Out-bound Licenses shall not include, when entered into in the Ordinary Course of Business, material transfer agreements, clinical trial agreements, services agreements, non-disclosure agreements, or non-exclusive outbound licenses).
(e) To the Knowledge of the Company: (i) the operation of the businesses of the Company and its Subsidiaries as currently conducted does not infringe, misappropriate or otherwise violate any valid and enforceable Registered IP or any other Intellectual Property Right owned by any other Person and (ii) no other Person is infringing, misappropriating or otherwise violating any Company IP or any Intellectual Property Rights exclusively licensed to the Company or its Subsidiaries. As of the date of this Agreement, no Legal Proceeding is pending (or, to the Knowledge of the Company, is threatened in writing) (A) against the Company or its Subsidiaries alleging that the operation of the business of the Company or its Subsidiaries infringes or constitutes the misappropriation or other violation of any Intellectual Property Rights of another Person or (B) by the Company or its Subsidiaries alleging that another Person has infringed, misappropriated or otherwise violated any of the Company IP or any Intellectual Property Rights exclusively licensed to the Company or its Subsidiaries. Since the Company’s inception, neither the Company nor its Subsidiaries has received any written notice or other written communication alleging that the operation of the business of the Company or its Subsidiaries infringes or constitutes the misappropriation or other violation of any Intellectual Property Right of another Person.
(f) None of the Company IP or, to the Knowledge of the Company, any material Intellectual Property Rights exclusively licensed to the Company or its Subsidiaries is subject to any pending or outstanding injunction, directive, order, judgment or other disposition of dispute that adversely and materially restricts the use, transfer, registration or licensing by the Company or its Subsidiaries of any such Company IP or material Intellectual Property Rights exclusively licensed to the Company or its Subsidiaries.
(g) To the Knowledge of the Company, the Company, its Subsidiaries and the operation of the Company’s and its Subsidiaries’ business are in substantial compliance with all Laws pertaining to data privacy and data security of any personally identifiable information and sensitive business information (collectively, “Sensitive Data”) except to the extent that such noncompliance has not and would not reasonably be expected to have a Company Material Adverse Effect. To the Knowledge of the Company, since the Company’s inception, there have been (i) no material losses or thefts of data or security breaches relating to Sensitive Data used in the business of the Company or its Subsidiaries, (ii) no violations of any security policy of the Company regarding any such Sensitive Data used in the business of the Company or its Subsidiaries, and (iii) no unauthorized access, unauthorized use or unintended or improper disclosure of any Sensitive Data used in the business of the Company or its Subsidiaries, in each case of (i) through (iii), except as would not reasonably be expected to, individually or in the aggregate, have a Company Material Adverse Effect.
15
2.13 Agreements, Contracts and Commitments.
(a) Section 2.13(a) of the Company Disclosure Schedule lists the following Company Contracts in effect as of the date of this Agreement other than any Benefit Plans (each, a “Company Material Contract” and collectively, the “Company Material Contracts”):
(i) each Company Contract relating to any agreement of indemnification or guaranty not entered into in the Ordinary Course of Business;
(ii) each Company Contract containing (A) any covenant limiting the freedom of the Company, its Subsidiaries or the Surviving Corporation to engage in any line of business or compete with any Person, (B) any most-favored pricing arrangement, (C) any exclusivity provision, or (D) any non-solicitation provision;
(iii) each Company Contract relating to capital expenditures and requiring payments after the date of this Agreement in excess of $100,000 pursuant to its express terms and not cancelable without penalty;
(iv) each Company Contract relating to the disposition or acquisition of material assets or any ownership interest in any Entity;
(v) each Company Contract relating to any mortgages, indentures, loans, notes or credit agreements, security agreements or other agreements or instruments relating to the borrowing of money or extension of credit or creating any material Encumbrances with respect to any assets of the Company or any of its Subsidiaries or any loans or debt obligations with officers or directors of the Company;
(vi) each Company Contract requiring payment by or to the Company after the date of this Agreement in excess of $100,000 pursuant to its express terms relating to: (A) any distribution agreement (identifying any that contain exclusivity provisions); (B) any agreement involving provision of services or products with respect to any pre-clinical or clinical development activities of the Company; (C) any dealer, distributor, joint marketing, alliance, joint venture, cooperation, development or other agreement currently in force under which the Company has continuing obligations to develop or market any product, technology or service, or any agreement pursuant to which the Company has continuing obligations to develop any Intellectual Property Rights that will not be owned, in whole or in part, by the Company; or (D) any Contract to license any third party to manufacture or produce any product, service or technology of the Company or any Contract to sell, distribute or commercialize any products or service of the Company, in each case, except for Company Contracts entered into in the Ordinary Course of Business;
(vii) each Company Contract with any Person, including any financial advisor, broker, finder, investment banker or other Person, providing financial advisory services to the Company in connection with the Contemplated Transactions;
(viii) each Company Real Estate Lease;
(ix) each Company Contract with any Governmental Body;
16
(x) each Company Out-bound License and Company In-bound License;
(xi) each Company Contract containing any royalty, dividend or similar arrangement based on the revenues or profits of the Company or any of its Subsidiaries;
(xii) each Company Contract, offer letter, or employment agreement, or independent contractor agreement with any employee or service provider that (A) is not immediately terminable at-will by the Company without notice, severance or other cost or payment, except as required under applicable Law, or (B) provides for retention payments, change of control payments, severance, accelerated vesting, or any similar payment or benefit that may or will become due as a result of the Merger; or
(xiii) any other Company Contract that is not terminable at will (with no penalty or payment or requirement for prior notice) by the Company or its Subsidiaries, as applicable, and (A) which involves payment or receipt by the Company or its Subsidiaries after the date of this Agreement under any such agreement, contract or commitment of more than $100,000 in the aggregate, or obligations after the date of this Agreement in excess of $100,000 in the aggregate, or (B) that is material to the business or operations of the Company and its Subsidiaries, taken as a whole.
(b) The Company has delivered or made available to Parent accurate and complete copies of all Company Material Contracts, including all amendments thereto. Except as set forth in Section 2.13(b) of the Company Disclosure Schedule, there are no Company Material Contracts that are not in written form. Neither the Company nor any of its Subsidiaries has, nor to the Company’s Knowledge, as of the date of this Agreement has any other party to a Company Material Contract, breached, violated or defaulted under, or received notice that it breached, violated or defaulted under, any of the terms or conditions of any Company Material Contract in such manner as would permit any other party to cancel or terminate any such Company Material Contract, or would permit any other party to seek damages which would reasonably be expected to be material to the Company or its business. As to the Company and its Subsidiaries, as of the date of this Agreement, each Company Material Contract is valid, binding, enforceable and in full force and effect, subject to the Enforceability Exceptions. No Person is renegotiating, or has a right pursuant to the terms of any Company Material Contract to change, any material amount paid or payable to the Company under any Company Material Contract or any other material term or provision of any Company Material Contract.
2.14 Compliance; Permits; Restrictions.
(a) The Company and each of its Subsidiaries are, and since the Company’s inception, have been, in compliance in all material respects with all applicable Laws, including the Federal Food, Drug, and Cosmetic Act (“FDCA”), the Food and Drug Administration (“FDA”) regulations adopted thereunder, the Public Health Service Act and any other similar Law administered or promulgated by the FDA or other comparable Governmental Body responsible for regulation of the development, clinical testing, manufacturing, sale, marketing, distribution and importation or exportation of drug and biopharmaceutical products (each, a “Drug Regulatory Agency”), except for any noncompliance, either individually or in the aggregate, which would not be material to the Company. No investigation, claim, suit, proceeding, audit or other action by any Governmental Body is pending or, to the Knowledge of the Company, threatened against the Company or any of its Subsidiaries. There is no agreement, judgment, injunction, order or decree binding upon the Company or any of its Subsidiaries which (i) has or would reasonably be expected to have the effect of prohibiting or materially impairing any business practice of the Company or any of its Subsidiaries, any acquisition of material property by the Company or any of its Subsidiaries or the conduct of business by the Company or any of its Subsidiaries as currently conducted, (ii) is reasonably likely to have an adverse effect on the Company’s ability to comply with or perform any covenant or obligation under this Agreement, or (iii) is reasonably likely to have the effect of preventing, delaying, making illegal or otherwise interfering with the Contemplated Transactions.
17
(b) The Company and its Subsidiaries hold all required Governmental Authorizations which are material to the operation of the business of the Company and its Subsidiaries as currently conducted (the “Company Permits”). Section 2.14(b) of the Company Disclosure Schedule identifies each Company Permit. Each of the Company and its Subsidiaries is in material compliance with the terms of the Company Permits. No Legal Proceeding is pending or, to the Knowledge of the Company, threatened, which seeks to revoke, limit, suspend, or materially modify any Company Permit. The rights and benefits of each Company Permit will be available to the Surviving Corporation or its Subsidiaries, as applicable, immediately after the Effective Time on terms substantially identical to those enjoyed by the Company and its Subsidiaries as of the date of this Agreement and immediately prior to the Effective Time.
(c) There are no proceedings pending or, to the Knowledge of the Company, threatened with respect to an alleged material violation by the Company or any of its Subsidiaries of the FDCA, FDA regulations adopted thereunder, the Public Health Service Act or any other similar Law administered or promulgated by any Drug Regulatory Agency.
(d) The Company and each of its Subsidiaries holds all required Governmental Authorizations issuable by any Drug Regulatory Agency necessary or material to the conduct of the business of the Company or such Subsidiary as currently conducted (collectively, the “Company Regulatory Permits”) and no such Company Regulatory Permit has been (i) revoked, withdrawn, suspended, cancelled or terminated or (ii) modified in any adverse manner. The Company and each of its Subsidiaries are in compliance in all material respects with the Company Regulatory Permits and have not received any written notice or other written communication, or to the Knowledge of the Company, any other communication from any Drug Regulatory Agency regarding (A) any material violation of or failure to comply materially with any term or requirement of any Company Regulatory Permit or (B) any revocation, withdrawal, suspension, cancellation, termination or material modification of any Company Regulatory Permit. The Company and each of its Subsidiaries have complied in all material respects with the ICH E9 Guidance for Industry: Statistical Principles for Clinical Trials in the management of the clinical data that have been presented to the Company. To the Knowledge of the Company, there are no facts that would be reasonably likely to result in any warning, untitled or notice of violation letter or Form FDA-483 from the FDA.
(e) All clinical, pre-clinical and other studies and tests conducted by or on behalf of, or sponsored by, the Company or its Subsidiaries, or in which the Company or its Subsidiaries or their respective current products or product candidates have participated, were and, if still pending, are being conducted in all material respects in accordance with standard medical and scientific research procedures and in compliance in all material respects with the applicable regulations of any applicable Drug Regulatory Agency and other applicable Law, including 21 C.F.R. Parts 50, 54, 56, 58 and 312. No preclinical or clinical trial conducted by or on behalf of the Company or any of its Subsidiaries has been terminated or suspended prior to completion for safety or non-compliance reasons. Since their inception, neither the Company nor any of its Subsidiaries has received any notices, correspondence, or other communications from any Drug Regulatory Agency requiring, or to the Knowledge of the Company threatening to initiate, the termination or suspension of any clinical studies conducted by or on behalf of, or sponsored by, the Company or any of its Subsidiaries or in which the Company or any of its Subsidiaries or their respective current products or product candidates have participated.
18
(f) Neither the Company nor any of its Subsidiaries is the subject of any pending or, to the Knowledge of the Company, threatened investigation in respect of its business or products by the FDA pursuant to its “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal Gratuities” Final Policy set forth in 56 Fed. Reg. 46191 (September 10, 1991) and any amendments thereto. To the Knowledge of the Company, neither the Company nor any of its Subsidiaries has committed any acts, made any statement, or failed to make any statement, in each case in respect of its business or products that would violate the FDA’s “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal Gratuities” Final Policy, and any amendments thereto. None of the Company, any of its Subsidiaries or any of their respective officers, employees or, to the Knowledge of the Company, agents has been convicted of any crime or engaged in any conduct that could result in a debarment or exclusion (i) under 21 U.S.C. Section 335a or (ii) any similar applicable Law. No debarment or exclusionary claims, actions, proceedings or investigations in respect of their business or products are pending or, to the Knowledge of the Company, threatened against the Company, any of its Subsidiaries or any of their respective officers, employees or, to the Knowledge of the Company, agents.
(g) The Company and its Subsidiaries have complied with all Laws relating to patient, medical or individual health information, including the Health Insurance Portability and Accountability Act of 1996 and its implementing regulations promulgated thereunder, all as amended from time to time (collectively “HIPAA”), including the standards for the privacy of Individually Identifiable Health Information at 45 C.F.R. Parts 160 and 164, Subparts A and E, the standards for the protection of Electronic Protected Health Information set forth at 45 C.F.R. Part 160 and 45 C.F.R. Part 164, Subpart A and Subpart C, the standards for transactions and code sets used in electronic transactions at 45 C.F.R. Part 160, Subpart A and Part 162, and the standards for Breach Notification for Unsecured Protected Health Information at 45 C.F.R. Part 164, Subpart D, all as amended from time to time. The Company and its Subsidiaries have entered into, where required, and are in compliance in all material respects with the terms of all Business Associate (as defined in HIPAA) agreements (“Business Associate Agreements”) to which the Company or a Subsidiary is a party or otherwise bound. The Company and its Subsidiaries have created and maintained written policies and procedures to protect the privacy of all protected health information, provide training to all employees and agents as required under HIPAA, and have implemented security procedures, including physical, technical and administrative safeguards, to protect all personal information and Protected Health Information stored or transmitted in electronic form. Neither the Company nor its Subsidiaries have received written notice from the Office for Civil Rights for the U.S. Department of Health and Human Services or any other Governmental Body of any allegation regarding its failure to comply with HIPAA or any other state law or regulation applicable to the protection of individually identifiable health information or personally identifiable information. No successful Security Incident, Breach of Unsecured Protected Health Information or breach of personally identifiable information under applicable state or federal laws have occurred with respect to information maintained or transmitted to the Company, any of its Subsidiaries, or an agent or third party subject to a Business Associate Agreement with the Company or a Subsidiary of the Company. The Company is currently submitting, receiving and handling or is capable of submitting receiving and handling transactions in accordance with the Standard Transaction Rule. All capitalized terms in this Section 2.14(g) not otherwise defined in this Agreement shall have the meanings set forth under HIPAA.
2.15 Legal Proceedings; Orders.
(a) As of the date of this Agreement, there is no material pending Legal Proceeding and, to the Knowledge of the Company, no Person has threatened in writing to commence any Legal Proceeding: (i) that involves (A) the Company, (B) any of its Subsidiaries, (C) any Company Associate (in his or her capacity as such) or (D) any of the material assets owned or used by the Company or its Subsidiaries; or (ii) that challenges, or that may have the effect of preventing, delaying, making illegal or otherwise interfering with, the Contemplated Transactions.
19
(b) Except as set forth in Section 2.15(b) of the Company Disclosure Schedule, since the Company’s inception through the date of this Agreement, no Legal Proceeding has been pending against the Company that resulted in material liability to the Company.
(c) There is no order, writ, injunction, judgment or decree to which the Company or any of its Subsidiaries, or any of the material assets owned or used by the Company or any of its Subsidiaries, is subject. To the Knowledge of the Company, no officer of the Company or any of its Subsidiaries is subject to any order, writ, injunction, judgment or decree that prohibits such officer or employee from engaging in or continuing any conduct, activity or practice relating to the business of the Company or any of its Subsidiaries or to any material assets owned or used by the Company or any of its Subsidiaries.
2.16 Tax Matters.
(a) The Company and each of its Subsidiaries have timely filed all income Tax Returns and other material Tax Returns that they were required to file under applicable Law. All such Tax Returns are correct and complete in all material respects and have been prepared in compliance with all applicable Law. No claim has ever been made by any Governmental Body in any jurisdiction where the Company or any of its Subsidiaries does not file a particular Tax Return or pay a particular Tax that the Company or such Subsidiary is subject to taxation by that jurisdiction.
(b) All income and other material Taxes due and owing by the Company or any of its Subsidiaries on or before the date hereof (whether or not shown on any Tax Return) have been fully paid. The unpaid Taxes of the Company and its Subsidiaries did not, as of the date of the Company Unaudited Interim Balance Sheet, materially exceed the reserve for Tax liability (excluding any reserve for deferred Taxes established to reflect timing differences between book and Tax items) set forth on the face of the Company Unaudited Interim Balance Sheet. Since the date of the Company Unaudited Interim Balance Sheet, neither the Company nor any of its Subsidiaries has incurred any material Liability for Taxes outside the Ordinary Course of Business.
(c) All Taxes that the Company or any of its Subsidiaries are or were required by Law to withhold or collect have been duly and timely withheld or collected in all material respects on behalf of its respective employees, independent contractors, stockholders, lenders, customers or other third parties and, have been timely paid to the proper Governmental Body or other Person or properly set aside in accounts for this purpose.
(d) There are no Encumbrances for material Taxes (other than Taxes not yet due and payable) upon any of the assets of the Company or any of its Subsidiaries.
(e) No deficiencies for income or other material Taxes with respect to the Company or any of its Subsidiaries have been claimed, proposed or assessed by any Governmental Body in writing. There are no pending or ongoing, and to the Knowledge of the Company, threatened audits, assessments or other actions for or relating to any liability in respect of a material amount of Taxes of the Company or any of its Subsidiaries. Neither the Company nor any of its Subsidiaries (or any of their predecessors) has waived any statute of limitations in respect of any income or other material Taxes or agreed to any extension of time with respect to any income or other material Tax assessment or deficiency.
(f) Neither the Company nor any of its Subsidiaries has been a United States real property holding corporation within the meaning of Section 897(c)(2) of the Code during the applicable period specified in Section 897(c)(1)(A)(ii) of the Code.
20
(g) Neither the Company nor any of its Subsidiaries is a party to any Tax allocation agreement, Tax sharing agreement, Tax indemnity agreement, or similar agreement or arrangement, other than customary commercial contracts entered into in the Ordinary Course of Business the principal subject matter of which is not Taxes.
(h) Neither the Company nor any of its Subsidiaries will be required to include any material item of income in, or exclude any material item of deduction from, taxable income for any Tax period (or portion thereof) ending after the Closing Date as a result of any: (i) change in method of accounting for Tax purposes; (ii) use of an improper method of accounting for a Tax period ending on or prior to the Closing Date; (iii) “closing agreement” as described in Section 7121 of the Code (or any similar provision of state, local or foreign Law) executed on or prior to the Closing Date; (iv) intercompany transaction or excess loss account described in Treasury Regulations under Section 1502 of the Code (or any similar provision of state, local or foreign Law); (v) installment sale or open transaction disposition made on or prior to the Closing Date; (vi) prepaid amount received or deferred revenue accrued on or prior to the Closing Date; (vii) application of Section 367(d) of the Code to any transfer of intangible property on or prior to the Closing Date; (viii) application of Sections 951 or 951A of the Code (or any similar provision of state, local or foreign Law) to any income received or accrued on or prior to the Closing Date; or (ix) election under Section 108(i) of the Code (or any similar provision of state, local or foreign Law). The Company has not made any election under Section 965(h) of the Code.
(i) Neither the Company nor any of its Subsidiaries has ever been (i) a member of a consolidated, combined or unitary Tax group (other than such a group the common parent of which is the Company) or (ii) a party to any joint venture, partnership, or other arrangement that is treated as a partnership for U.S. federal income Tax purposes. Neither the Company nor any of its Subsidiaries has any Liability for any material Taxes of any Person (other than the Company and any of its Subsidiaries) under Treasury Regulations Section 1.1502-6 (or any similar provision of state, local, or foreign Law), or as a transferee or successor.
(j) Neither the Company nor any of its Subsidiaries has ever distributed stock of another Person, or had its stock distributed by another Person, in a transaction that was purported or intended to be governed in whole or in part by Section 355 of the Code or Section 361 of the Code (or any similar provisions of state, local or foreign Law).
(k) Neither the Company nor any of its Subsidiaries (i) is a “controlled foreign corporation” as defined in Section 957 of the Code, (ii) is a “passive foreign investment company” within the meaning of Section 1297 of the Code, or (iii) has ever had a permanent establishment (within the meaning of an applicable Tax treaty) or otherwise had an office or fixed place of business in a country other than the country in which it is organized.
(l) Neither the Company nor any of its Subsidiaries has participated in or been a party to a transaction that, as of the date of this Agreement, constitutes a “listed transaction” that is required to be reported to the IRS pursuant to Section 6011 of the Code and applicable Treasury Regulations thereunder.
(m) Neither the Company nor any of its Subsidiaries has taken any action or knows of any fact that would reasonably be expected to prevent the Merger from qualifying for the Intended Tax Treatment.
For purposes of this Section 2.16, each reference to the Company or any of its Subsidiaries shall be deemed to include any Person that was liquidated into, merged with, or is otherwise a predecessor to, the Company.
21
2.17 Employee and Labor Matters; Benefit Plans.
(a) Section 2.17(a) of the Company Disclosure Schedule is a list of all material Company Benefit Plans, including, without limitation, each Company Benefit Plan that provides for retirement, change in control, stay or retention, deferred compensation, incentive compensation, severance or retiree medical or life insurance benefits. “Company Benefit Plan” means each (i) “employee benefit plan” as defined in Section 3(3) of ERISA and (ii) other pension, retirement, deferred compensation, excess benefit, profit sharing, bonus, incentive, equity or equity-based, phantom equity, employment (other than at-will employment offer letters on the Company’s standard form and other than individual compensatory equity award agreements made pursuant to the Company’s standard forms, in which case only representative standard forms of such agreements shall be scheduled), consulting, severance, change-of-control, retention, health, life, disability, group insurance, paid-time off, holiday, welfare and fringe benefit plan, program, agreement, contract, or arrangement (whether written or unwritten, qualified or nonqualified, funded or unfunded and including any that have been frozen), in any case, maintained, contributed to, or required to be contributed to, by the Company or any of its Subsidiaries or Company ERISA Affiliates for the benefit of any current or former employee, director, officer or independent contractor of the Company or any of its Subsidiaries or under which the Company or any of its Subsidiaries has any actual or contingent liability (including, without limitation, as to the result of it being treated as a single employer under Code Section 414 with any other person).
(b) As applicable with respect to each material Company Benefit Plan, the Company has made available to Parent, true and complete copies of (i) each material Company Benefit Plan, including all amendments thereto, and in the case of an unwritten material Company Benefit Plan, a written description thereof, (ii) all current trust documents, investment management contracts, custodial agreements, administrative services agreements and insurance and annuity contracts relating thereto, (iii) the current summary plan description and each summary of material modifications thereto, (iv) the most recently filed annual reports with any Governmental Body (e.g., Form 5500 and all schedules thereto), (v) the most recent IRS determination, opinion or advisory letter, (vi) the most recent summary annual reports, nondiscrimination testing reports, actuarial reports, financial statements and trustee reports, (vii) all records, notices and filings concerning IRS or Department of Labor or other Governmental Body audits or investigations, or “prohibited transactions” within the meaning of Section 406 of ERISA or Section 4975 of the Code, (viii) all policies and procedures established to comply with the privacy and security rules of HIPAA and (ix) any written reports constituting a valuation of the Company’s capital stock for purposes of Sections 409A or 422 of the Code, whether prepared internally by the Company or by an outside, third-party valuation firm.
(c) Each Company Benefit Plan has been maintained, operated and administered in compliance in all material respects with its terms and any related documents or agreements and the applicable provisions of ERISA, the Code and all other Laws.
(d) The Company Benefit Plans which are “employee pension benefit plans” within the meaning of Section 3(2) of ERISA and which are intended to meet the qualification requirements of Section 401(a) of the Code have received determination or opinion letters from the IRS on which they may currently rely to the effect that such plans are qualified under Section 401(a) of the Code and the related trusts are exempt from federal income Taxes under Section 501(a) of the Code, respectively, and to the Knowledge of the Company, nothing has occurred that would reasonably be expected to materially adversely affect the qualification of such Company Benefit Plan or the tax exempt status of the related trust.
22
(e) Neither the Company, any of its Subsidiaries nor any Company ERISA Affiliate maintains, contributes to, is required to contribute to, or has any actual or contingent liability with respect to, (i) any “employee pension benefit plan” (within the meaning of Section 3(2) of ERISA) that is subject to Title IV or Section 302 of ERISA or Section 412 of the Code, (ii) any “multiemployer plan” (within the meaning of Section 3(37) of ERISA), (iii) any “multiple employer plan” (within the meaning of Section 413 of the Code) or (iv) any “multiple employer welfare arrangement” (within the meaning of Section 3(40) of ERISA).
(f) There are no pending audits or investigations by any Governmental Body involving any Company Benefit Plan, and no pending or, to the Knowledge of the Company, threatened claims (except for individual claims for benefits payable in the normal operation of the Company Benefit Plans), suits or proceedings involving any Company Benefit Plan, or, to the Knowledge of the Company, any fiduciary thereof or service provider thereto, in any case except as would not be reasonably expected to result in material liability to the Company or any of its Subsidiaries. All contributions and premium payments required to have been made under any of the Company Benefit Plans or by applicable Law (without regard to any waivers granted under Section 412 of the Code), have been timely made and neither the Company nor any Company ERISA Affiliate has any liability for any unpaid contributions with respect to any Company Benefit Plan.
(g) Neither the Company, any of its Subsidiaries or Company ERISA Affiliates, nor to the Knowledge of the Company, any fiduciary, trustee or administrator of any Company Benefit Plan, has engaged in, or in connection with the Contemplated Transactions will engage in, any transaction with respect to any Company Benefit Plan which would subject any such Company Benefit Plan, the Company, any of its Subsidiaries or Company ERISA Affiliates or Parent to a material Tax, material penalty or material liability for a “prohibited transaction” under Section 406 of ERISA or Section 4975 of the Code.
(h) No Company Benefit Plan provides death, medical, dental, vision, life insurance or other welfare benefits beyond termination of service or retirement other than coverage mandated by Law and to the Knowledge of the Company, neither the Company nor any of its Subsidiaries or Company ERISA Affiliates has made a written or oral representation promising the same.
(i) Neither the execution of, nor the performance of the Contemplated Transactions (either alone or when combined with the occurrence of any other event, including without limitation, a termination of employment), will: (i) result in any payment becoming due to any current or former employee, director, officer, or independent contractor of the Company or any Subsidiary thereof, pursuant to any Company Benefit Plan (ii) increase any amount of compensation or benefits otherwise payable under any Company Benefit Plan, (iii) result in the acceleration of the time of payment, funding or vesting of any benefits under any Company Benefit Plan, (iv) require any contribution or payment to fund any obligation under any Company Benefit Plan or (v) limit the right to merge, amend or terminate any Company Benefit Plan.
(j) Except as set forth in Section 2.17(a) of the Company Disclosure Schedule, neither the execution of, nor the consummation of the Contemplated Transactions (either alone or when combined with the occurrence of any other event, including without limitation, a termination of employment) will result in the receipt or retention by any person who is a “disqualified individual” (within the meaning of Code Section 280G) with respect to the Company and its Subsidiaries of any payment or benefit that is or could be characterized as a “parachute payment” (within the meaning of Code Section 280G), determined without regard to the application of Code Section 280G(b)(5).
23
(k) Each Company Benefit Plan providing for deferred compensation that constitutes a “nonqualified deferred compensation plan” (as defined in Section 409A(d)(1) of the Code and the regulations promulgated thereunder) is, and has been, established, administered and maintained in compliance with the requirements of Section 409A of the Code and the regulations promulgated thereunder in all material respects.
(l) No current or former employee, officer, director or independent contractor of the Company or any of its Subsidiaries has any “gross up” agreements with the Company or any of its Subsidiaries or other assurance of reimbursement by the Company or any of its Subsidiaries for any Taxes imposed under Code Section 409A or Code Section 4999.
(m) The Company does not maintain any Company Benefit Plan outside of the United States.
(n) The Company has provided to Parent a true and correct list, as of the date of this Agreement, containing the names of all current full-time, part-time or temporary employees and independent contractors (and indication as such), and, as applicable: (i) the annual dollar amount of all compensation (including wages, salary or fees, commissions, director’s fees, material fringe benefits, bonuses, profit sharing payments, and other compensatory payments or benefits of any type) payable to each person; (ii), dates of employment or service; (iii) title and, with respect to independent contractors, a current written description of such person’s contracting services; (iv) any eligibility to receive severance, notice of termination, retention payment, change of control payment, or other similar compensation; (v) visa status, if applicable; and (vi) with respect to employees, a designation of whether they are classified as exempt or non-exempt for purposes of the Fair Labor Standards Act, as amended (“FLSA”) and any similar state law.
(o) Neither the Company nor any of its Subsidiaries is or has ever been a party to, bound by, or has a duty to bargain under, any collective bargaining agreement or other Contract with a labor union, labor organization, or similar Person representing any of its employees, and there is no labor union, labor organization, or similar Person representing or, to the Knowledge of the Company, purporting to represent or seeking to represent any employees of the Company or its Subsidiaries, including through the filing of a petition for representation election. There is not and has not been since the Company’s inception, nor is there or has there been since the Company’s inception any threat of, any strike, slowdown, work stoppage, lockout, union election petition, demand for recognition, or any similar activity or dispute, or, to the Knowledge of the Company, any union organizing activity, against the Company or any of its Subsidiaries. To the Knowledge of the Company, no event has occurred, and no condition or circumstance exists, that might directly or indirectly be likely to give rise to or provide a basis for the commencement of any such strike, slowdown, work stoppage, lockout, union election petition, demand for recognition, any similar activity or dispute, or any union organizing activity.
(p) The Company and each of its Subsidiaries is, and since the Company’s inception has been, in material compliance with all applicable Laws respecting labor, employment, employment practices, and terms and conditions of employment, including worker classification, discrimination, harassment and retaliation, equal employment opportunities, fair employment practices, meal and rest periods, immigration, employee safety and health, payment of wages (including overtime wages), unemployment and workers’ compensation, leaves of absence, and hours of work. Except as would not be reasonably likely to result in a material liability to the Company or any of its Subsidiaries, with respect to employees of the Company and its Subsidiaries, each of the Company and its Subsidiaries, since the Company’s inception: (i) has withheld and reported all amounts required by Law or by agreement to be withheld and reported with respect to wages, salaries and other payments, benefits, or compensation to employees, (ii) is not liable for any arrears of wages (including overtime wages), severance pay or any Taxes or any penalty for failure to comply with any of the foregoing, and (iii) is not liable for any payment to any trust or other fund governed by or maintained by or on behalf of any Governmental Body, with respect to unemployment compensation benefits, disability, social security or other benefits or obligations
24
for employees (other than routine payments to be made in the Ordinary Course of Business). There are no actions, suits, claims, charges, lawsuits, investigations, audits or administrative matters pending or, to the Knowledge of the Company, threatened or reasonably anticipated against the Company or any of its Subsidiaries relating to any employee, applicant for employment, consultant, employment agreement or Company Benefit Plan (other than routine claims for benefits).
(q) Except as would not be reasonably likely to result in a material liability to the Company or any of its Subsidiaries, with respect to each individual who currently renders services to the Company or any of its Subsidiaries, the Company and each of its Subsidiaries has accurately classified each such individual as an employee, independent contractor, or otherwise under all applicable Laws and, for each individual classified as an employee, the Company and each of its Subsidiaries has accurately classified him or her as exempt from or ineligible for overtime under all applicable Laws. Neither the Company nor any of its Subsidiaries has any material liability with respect to any misclassification of: (a) any Person as an independent contractor rather than as an employee, (b) any employee leased from another employer, or (c) any employee currently or formerly classified as exempt from or ineligible for overtime under all applicable Laws.
(r) Since the Company’s inception, the Company has not implemented any “plant closing” or “mass layoff” of employees that would reasonably be expected to require notification under the WARN Act or any similar state or local Law, no such “plant closing” or “mass layoff” will be implemented before the Closing Date without advance notification to and approval of Parent, and there has been no “employment loss” as defined by the WARN Act within the ninety (90) days prior to the date of this Agreement.
2.18 Environmental Matters. The Company and each of its Subsidiaries are and since the Company’s inception have complied with all applicable Environmental Laws, which compliance includes the possession by the Company of all permits and other Governmental Authorizations required under applicable Environmental Laws and compliance with the terms and conditions thereof, except for any failure to be in such compliance that, either individually or in the aggregate, would not reasonably be expected to be material to the Company or its business. Neither the Company nor any of its Subsidiaries has received since the Company’s inception (or prior to that time, which is pending and unresolved), any written notice or other communication (in writing or otherwise), whether from a Governmental Body or other Person, that alleges that the Company or any of its Subsidiaries is not in compliance with or has liability pursuant to any Environmental Law and, to the Knowledge of the Company, there are no circumstances that would reasonably be expected to prevent or interfere with the Company’s or any of its Subsidiaries’ compliance in any material respects with any Environmental Law, except where such failure to comply would not reasonably be expected to be material to the Company or its business. No current or (during the time a prior property was leased or controlled by the Company or any of its Subsidiaries) prior property leased or controlled by the Company or any of its Subsidiaries has had a release of or exposure to Hazardous Materials in material violation of or as would reasonably be expected to result in any material liability of the Company or any of its Subsidiaries pursuant to Environmental Law. No consent, approval or Governmental Authorization of or registration or filing with any Governmental Body is required by Environmental Laws in connection with the execution and delivery of this Agreement or the Contemplated Transactions. Prior to the date hereof, the Company has provided or otherwise made available to Parent true and correct copies of all material environmental reports, assessments, studies and audits in the possession or control of the Company or any of its Subsidiaries with respect to any property leased or controlled by the Company or any of its Subsidiaries or any business operated by them.
25
2.19 Insurance. The Company has delivered or made available to Parent accurate and complete copies of all material insurance policies and all material self-insurance programs and arrangements relating to the business, assets, liabilities and operations of the Company and each of its Subsidiaries. Each of such insurance policies is in full force and effect and the Company and each of its Subsidiaries are in compliance in all material respects with the terms thereof. Other than customary end of policy notifications from insurance carriers, since the Company’s inception, neither the Company nor any of its Subsidiaries has received any notice or other communication regarding any actual or possible: (i) cancellation or invalidation of any insurance policy; or (ii) refusal or denial of any coverage, reservation of rights or rejection of any material claim under any insurance policy. The Company and each of its Subsidiaries have provided timely written notice to the appropriate insurance carrier(s) of each Legal Proceeding that is currently pending against the Company or any of its Subsidiaries for which the Company or such Subsidiary has insurance coverage, and no such carrier has issued a denial of coverage or a reservation of rights with respect to any such Legal Proceeding, or informed the Company or any of its Subsidiaries of its intent to do so.
2.20 No Financial Advisors. Except as set forth on Section 2.20 of the Company Disclosure Schedule, no broker, finder or investment banker is entitled to any brokerage fee, finder’s fee, opinion fee, success fee, transaction fee or other fee or commission in connection with the Contemplated Transactions based upon arrangements made by or on behalf of the Company or any of its Subsidiaries.
2.21 Transactions with Affiliates.
(a) Section 2.21(a) of the Company Disclosure Schedule describes any material transactions or relationships, since the Company’s inception, between, on one hand, the Company or any of its Subsidiaries and, on the other hand, any (i) executive officer or director of the Company or, to the Knowledge of the Company, any of its Subsidiaries or any of such executive officer’s or director’s immediate family members, (ii) owner of more than 5% of the voting power of the outstanding Company Common Stock or (iii) to the Knowledge of the Company, any “related person” (within the meaning of Item 404 of Regulation S-K under the Securities Act) of any such officer, director or owner (other than the Company or its Subsidiaries) in the case of each of (i), (ii) or (iii) that is of the type that would be required to be disclosed under Item 404 of Regulation S-K under the Securities Act.
(b) There is no stockholders agreement, voting agreement, registration rights agreement, co-sale agreement or other similar Contract between the Company and any holders of Company Common Stock, including any such Contract granting any Person investor rights, rights of first refusal, rights of first offer, registration rights, director designation rights or similar rights.
2.22 Anti-Bribery. None of the Company or any of its Subsidiaries or any of their respective directors, officers, employees or, to the Company’s Knowledge, agents or any other Person acting on their behalf (in each in their respective capacities as such) has directly or indirectly made any bribes, rebates, payoffs, influence payments, kickbacks, illegal payments, illegal political contributions, or other payments, in the form of cash, gifts, or otherwise, or taken any other action, in violation of the Foreign Corrupt Practices Act of 1977, the UK Bribery Act of 2010 or any other anti-bribery or anti-corruption Law (collectively, the “Anti-Bribery Laws”). Neither the Company nor any of its Subsidiaries is or has been the subject of any investigation or inquiry by any Governmental Body with respect to potential violations of Anti-Bribery Laws.
2.23 Disclaimer of Other Representations or Warranties.
(a) Except as previously set forth in this Section 2 or in any certificate delivered by the Company to Parent and/or Merger Sub pursuant to this Agreement, the Company makes no representation or warranty, express or implied, at law or in equity, with respect to it or any of its assets, liabilities or operations, and any such other representations or warranties are hereby expressly disclaimed.
26
(b) The Company acknowledges and agrees that, except for the representations and warranties of Parent and Merger Sub set forth in Section 3, none of Parent, Merger Sub or any of their respective Representatives is relying on any other representation or warranty of Parent or any other Person made outside of Section 3, including regarding the accuracy or completeness of any such other representations or warranties or the omission of any material information, whether express or implied, in each case, with respect to the Contemplated Transactions.
Section 3. REPRESENTATIONS AND WARRANTIES OF PARENT AND MERGER SUB
Subject to Section 10.13(h), except (a) as set forth in the disclosure schedule delivered by Parent to the Company (the “Parent Disclosure Schedule”) or (b) as disclosed in the Parent SEC Documents filed with the SEC after December 31, 2018 and prior to the date hereof and publicly available on the SEC’s Electronic Data Gathering Analysis and Retrieval system (but (i) without giving effect to any amendment thereof filed with, or furnished to the SEC on or after the date hereof and (ii) excluding any disclosures contained under the heading “Risk Factors” and any disclosure of risks included in any “forward-looking statements” disclaimer or in any other section to the extent they are forward-looking statements or cautionary, predictive or forward-looking in nature), it being understood that any matter disclosed in the Parent SEC Documents (x) shall not be deemed disclosed for purposes of Section 3.1, Section 3.2, Section 3.3, Section 3.4, Section 3.5, Section 3.6, and Section 3.7 and (y) shall be deemed to be disclosed in a section of the Parent Disclosure Schedule only to the extent that it is readily apparent from a reading of such Parent SEC Documents that is applicable to such section of the Parent Disclosure Schedule, Parent and Merger Sub represent and warrant to the Company as follows:
3.1 Due Organization; Subsidiaries.
(a) Each of Parent and Merger Sub is a corporation duly incorporated, validly existing and in good standing under the Laws of the jurisdiction of its incorporation, and has all necessary corporate power and authority to conduct its business in the manner in which its business is currently being conducted and to own or lease and use its property and assets in the manner in which its property and assets are currently owned or leased and used, except where the failure to have such power or authority would not reasonably be expected to prevent or materially delay the ability of Parent and Merger Sub to consummate the Contemplated Transactions. Since the date of its incorporation, Merger Sub has not engaged in any activities other than activities incident to its formation or in connection with or as contemplated by this Agreement.
(b) Parent is duly licensed and qualified to do business, and is in good standing (to the extent applicable in such jurisdiction), under the Laws of all jurisdictions where the nature of its business requires such licensing or qualification other than in jurisdictions where the failure to be so qualified individually or in the aggregate would not be reasonably expected to have a Parent Material Adverse Effect.
(c) Parent has no Subsidiaries, except for the Entities identified in Section 3.1(c) of the Parent Disclosure Schedule; and neither Parent nor any of the Entities identified in Section 3.1(c) of the Company Disclosure Schedule owns any capital stock of, or any equity, ownership or profit sharing interest of any nature in, or controls directly or indirectly, any other Entity other than the Entities identified in Section 3.1(c) of the Company Disclosure Schedule. Each of Parent’s Subsidiaries is a corporation or other legal entity duly organized, validly existing and, if applicable, in good standing under the Laws of the jurisdiction of its organization and has all necessary corporate or other power and authority to conduct its business in the manner in which its business is currently being conducted and to own or lease and use its property and assets in the manner in which its property and assets are currently owned or leased and used, except where the failure to have such power or authority would not be reasonably expected to have a Parent Material Adverse Effect.
27
(d) Neither the Parent nor any of its Subsidiaries is or has otherwise been, directly or indirectly, a party to, member of or participant in any partnership, joint venture or similar business entity. Neither the Parent nor any of its Subsidiaries has agreed or is obligated to make, or is bound by any Contract under which it may become obligated to make, any future investment in or capital contribution to any other Entity. Neither the Parent nor any of its Subsidiaries has, at any time, been a general partner of, or has otherwise been liable for any of the debts or other obligations of, any general partnership, limited partnership or other Entity.
3.2 Organizational Documents. Parent has made available to the Company accurate and complete copies of the Organizational Documents or Parent and each of its Subsidiaries in effect as of the date of this Agreement. Neither Parent nor any of its Subsidiaries is in breach or violation of its respective Organizational Documents.
3.3 Authority; Binding Nature of Agreement.
(a) The Parent and each of its Subsidiaries have all necessary corporate power and authority to enter into and to perform its obligations under this Agreement and, subject, with respect to Merger Sub, the adoption of this Agreement by Parent in its capacity as sole stockholder of Merger Sub, to perform its obligations hereunder and to consummate the Contemplated Transactions. The Parent Board (at meetings duly called and held) has: (i) determined that the Contemplated Transactions are fair to, advisable and in the best interests of Parent and its stockholders; (ii) authorized, approved and declared advisable this Agreement and the Contemplated Transactions, including the issuance of Parent Common Stock Payment Shares and Parent Preferred Stock Payment Shares to the stockholders of the Company pursuant to the terms of this Agreement; and (iii) determined to recommend, upon the terms and subject to the conditions set forth in this Agreement, that the stockholders of Parent vote to approve the Parent Stockholder Matters. The Merger Sub Board (by unanimous written consent) has: (A) determined that the Contemplated Transactions are fair to, advisable, and in the best interests of Merger Sub and its sole stockholder; (B) deemed advisable and approved this Agreement and the Contemplated Transactions; and (C) determined to recommend, upon the terms and subject to the conditions set forth in this Agreement, that the stockholder of Merger Sub vote to adopt this Agreement and thereby approve the Contemplated Transactions.
(b) This Agreement has been duly executed and delivered by Parent and Merger Sub and, assuming the due authorization, execution and delivery by the Company, constitutes the legal, valid and binding obligation of Parent and Merger Sub, enforceable against each of Parent and Merger Sub in accordance with its terms, subject to the Enforceability Exceptions. Prior to the execution of the Parent Stockholder Support Agreements, the Parent Board approved the Parent Stockholder Support Agreements and the transactions contemplated thereby.
3.4 Vote Required. The affirmative vote of a majority of the votes cast at the Parent Stockholders’ Meeting by the holders of Parent Common Stock (other than the Parent Common Stock Payment Shares to be issued at Closing pursuant to this Agreement) are the only vote of the holders of any class or series of Parent’s capital stock necessary to approve the proposal described in Section 5.2(a)(i) (“Required Parent Stockholder Vote”). The approval of holders of Parent Common Stock is not required in order to approve this Agreement or, except with respect to Parent Stockholder Matters, the transactions contemplated hereby.
28
3.5 Non-Contravention; Consents. Subject to the filing of the Certificate of Merger required by the DGCL and the filing of the Certificate of Designation, neither (x) the execution, delivery or performance of this Agreement by Parent or Merger Sub, nor (y) the consummation of the Contemplated Transactions, will directly or indirectly (with or without notice or lapse of time):
(a) contravene, conflict with or result in a violation of any of the provisions of the Organizational Documents of Parent or Merger Sub;
(b) contravene, conflict with or result in a violation of, give any Governmental Body or other Person the right to challenge the Contemplated Transactions or to exercise any remedy or obtain any relief under, any Law or any order, writ, injunction, judgment or decree to which Parent or its Subsidiaries, or any of the assets owned or used by Parent or its Subsidiaries, is subject, except as would not reasonably be expected to be material to Parent or its business;
(c) contravene, conflict with or result in a violation of any of the terms or requirements of, or give any Governmental Body the right to revoke, withdraw, suspend, cancel, terminate or modify, any Governmental Authorization that is held by Parent, except as would not reasonably be expected to be material to Parent or its business;
(d) contravene, conflict with or result in a violation or breach of, or result in a default under, any provision of any Parent Material Contract, or give any Person the right to: (i) declare a default or exercise any remedy under any Parent Material Contract; (ii) any material payment, rebate, chargeback, penalty or change in delivery schedule under any Parent Material Contract; (iii) accelerate the maturity or performance of any Parent Material Contract; or (iv) cancel, terminate or modify any term of any Parent Material Contract, except in the case of any non-material breach, default, penalty or modification; or
(e) result in the imposition or creation of any Encumbrance upon or with respect to any asset owned or used by Parent (except for Permitted Encumbrances).
Except for (i) any Consent set forth on Section 3.5 of the Parent Disclosure Schedule under any Parent Contract, (ii) the filing of the Certificate of Merger with the Secretary of State of the State of Delaware pursuant to the DGCL, (iii) the filing of the Certificate of Designation with the Secretary of State of the State of Delaware and (iv) such consents, waivers, approvals, orders, authorizations, registrations, declarations and filings as may be required under applicable federal and state securities Laws, neither Parent nor any of its Subsidiaries is or will be required to make any filing with or give any notice to, or to obtain any Consent from, any Person in connection with (A) the execution, delivery or performance of this Agreement and the Parent Stockholder Support Agreements, or (B) the consummation of the Contemplated Transactions. The Parent Board and the Merger Sub Board have taken and will take all actions necessary to ensure that the restrictions applicable to business combinations contained in Section 203 of the DGCL are, and will be, inapplicable to the execution, delivery and performance of this Agreement, the Parent Stockholder Support Agreements, the Lock-Up Agreements and to the consummation of the Contemplated Transactions. No other state takeover statute or similar Law applies or purports to apply to the Merger, this Agreement or any of the other Contemplated Transactions.
29
3.6 Capitalization.
(a) The authorized capital stock of Parent as of the date of this Agreement consists of (i) 50,000,000 shares of Parent Common Stock, of which 23,537,368 shares were issued and outstanding as of the close of business on the Reference Date and (ii) 5,000,000 shares of preferred stock of Parent, par value $0.000001 per share, of which no shares have been issued and are outstanding as of the date of this Agreement. Parent does not hold any shares of its capital stock in its treasury.
(b) All of the outstanding shares of Parent Common Stock have been duly authorized and validly issued, and are fully paid and nonassessable. None of the outstanding shares of Parent Common Stock is entitled or subject to any preemptive right, right of participation, right of maintenance or any similar right and none of the outstanding shares of Parent Common Stock is subject to any right of first refusal in favor of Parent. Except as contemplated herein, there is no Parent Contract relating to the voting or registration of, or restricting any Person from purchasing, selling, pledging or otherwise disposing of (or granting any option or similar right with respect to), any shares of Parent Common Stock. Parent is not under any obligation, nor is it bound by any Contract pursuant to which it may become obligated, to repurchase, redeem or otherwise acquire any outstanding shares of Parent Common Stock or other securities. Section 3.6(b) of the Parent Disclosure Schedule accurately and completely lists all repurchase rights held by Parent with respect to shares of capital stock of Parent (including shares issued pursuant to the exercise of stock options) and specifies which of those repurchase rights are currently exercisable and whether the holder of such shares of capital stock of Parent timely filed an election with the relevant Governmental Bodies under Section 83(b) of the Code with respect to such shares.
(c) Except for the Parent Stock Plan, and except as set forth on Section 3.6(c) of the Parent Disclosure Schedule, Parent does not have any stock option plan or any other plan, program, agreement or arrangement providing for any equity-based compensation for any Person. As of the close of business on the Reference Date, 2,631,097 shares were reserved for issuance upon exercise of Parent Options granted under the Parent Stock Plan that are outstanding as of the date of this Agreement, and 2,364,069 shares remain available for future issuance pursuant to the Parent Stock Plan. Section 3.6(c) of the Parent Disclosure Schedule sets forth the following information with respect to each Parent Option outstanding as of the Reference Date: (i) the name of the optionee; (ii) the number of shares of Parent Common Stock subject to such Parent Option at the time of grant; (iii) the number of shares of Parent Common Stock subject to such Parent Option as of the Reference Date; (iv) the exercise price of such Parent Option; (v) the date on which such Parent Option was granted; (vi) the applicable vesting schedule, including the number of vested and unvested shares as of the Reference Date and any acceleration provisions; and (vii) whether such Parent Option is intended to constitute an “incentive stock option” (as defined in the Code) or a non-qualified stock option. Parent has made available to the Company an accurate and complete copy of the Parent Plan and all stock option agreements evidencing outstanding options granted thereunder. No vesting of Parent Options will be accelerated in connection with the closing of the Contemplated Transactions other than as set forth on such Section 3.6(c) of the Parent Disclosure Schedule.
(d) Except for the Parent Options, and as otherwise set forth on Section 3.6(d) of the Parent Disclosure Schedule, there is no: (i) outstanding subscription, option, call, warrant or right (whether or not currently exercisable) to acquire any shares of the capital stock or other securities of Parent or any of its Subsidiaries; (ii) outstanding security, instrument or obligation that is or may become convertible into or exchangeable for any shares of the capital stock or other securities of Parent or any of its Subsidiaries; or (iii) condition or circumstance that is reasonably likely to give rise to or provide a basis for the assertion of a claim by any Person to the effect that such Person is entitled to acquire or receive any shares of capital stock or other securities of Parent or any of its Subsidiaries (it being understood that Parent intends to issue prior to (but contingent upon) the Closing restricted stock units to certain employees of the Company identified in the Company Disclosure Schedule). There are no outstanding or authorized stock appreciation, phantom stock, profit participation or other similar rights with respect to Parent or any of its Subsidiaries.
30
(e) All outstanding shares of Parent Common Stock, Parent Options, and other securities of Parent have been issued and granted in material compliance with (i) all applicable securities Laws and other applicable Law, and (ii) all requirements set forth in applicable Contracts.
3.7 SEC Filings; Financial Statements.
(a) Parent has delivered or made available to the Company accurate and complete copies of all registration statements, proxy statements, Certifications (as defined below) and other statements, reports, schedules, forms and other documents filed by Parent with the SEC since January 1, 2017 (the “Parent SEC Documents”), other than such documents that can be obtained on the SEC’s website at www.sec.gov. Since January 1, 2017, all material statements, reports, schedules, forms and other documents required to have been filed by Parent or its officers with the SEC have been so filed on a timely basis. As of the time it was filed with the SEC (or, if amended or superseded by a filing prior to the date of this Agreement, then on the date of such filing), each of the Parent SEC Documents complied in all material respects with the applicable requirements of the Securities Act or the Exchange Act (as the case may be) and, as of the time they were filed, none of the Parent SEC Documents contained any untrue statement of a material fact or omitted to state a material fact required to be stated therein or necessary in order to make the statements therein, in light of the circumstances under which they were made, not misleading. The certifications and statements required by (i) Rule 13a-14 under the Exchange Act and (ii) 18 U.S.C. §1350 (Section 906 of the Sarbanes-Oxley Act) relating to the Parent SEC Documents (collectively, the “Certifications”) are accurate and complete and comply as to form and content with all applicable Laws. As used in this Section 3.7, the term “file” and variations thereof shall be broadly construed to include any manner in which a document or information is furnished, supplied or otherwise made available to the SEC.
(b) The financial statements (including any related notes) contained or incorporated by reference in the Parent SEC Documents: (i) complied as to form in all material respects with the published rules and regulations of the SEC applicable thereto; (ii) were prepared in accordance with GAAP (except as may be indicated in the notes to such financial statements or, in the case of unaudited financial statements, except as permitted by Form 10-Q of the SEC, and except that the unaudited financial statements may not contain footnotes and are subject to normal and recurring year-end adjustments) applied on a consistent basis unless otherwise noted therein throughout the periods indicated; and (iii) fairly present, in all material respects, the financial position of Parent and its consolidated Subsidiaries as of the respective dates thereof and the results of operations and cash flows of Parent for the periods covered thereby. Other than as expressly disclosed in the Parent SEC Documents filed prior to the date hereof, there has been no material change in Parent’s accounting methods or principles that would be required to be disclosed in Parent’s financial statements in accordance with GAAP. Parent’s auditor has at all times since the date of enactment of the Sarbanes-Oxley Act been: (i) a registered public accounting firm (as defined in Section 2(a)(12) of the Sarbanes-Oxley Act); (ii) to the Knowledge of Parent, “independent” with respect to Parent within the meaning of Regulation S-X under the Exchange Act; and (iii) to the Knowledge of Parent, in compliance with subsections (g) through (l) of Section 10A of the Exchange Act and the rules and regulations promulgated by the SEC and the Public Company Accounting Oversight Board thereunder.
(c) Since January 1, 2017, through the date of this Agreement, Parent has not received any comment letter from the SEC or the staff thereof or any correspondence from officials of Nasdaq or the staff thereof relating to the delisting or maintenance of listing of the Parent Common Stock on Nasdaq. Parent has not disclosed any unresolved comments in the Parent SEC Documents.
(d) Since January 1, 2017, there have been no formal investigations regarding financial report or accounting policies and practices discussed with, reviewed by or initiated at the direction of the chief executive officer, chief financial officer, principal accounting officer or general counsel of Parent, the Parent Board or any committee thereof, other than ordinary course audit or reviews of accounting policies and practices or internal controls required by the Sarbanes Oxley Act.
31
(e) Parent is and since January 1, 2017 has been, in compliance in all material respects with the applicable current listing and governance rules and regulations of Nasdaq.
(f) Parent maintains, and at all times since January 1, 2017, has maintained, a system of internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act) that is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with GAAP and to provide reasonable assurance (i) that transactions are recorded as necessary to permit preparation of financial statements in accordance with GAAP, (ii) that receipts and expenditures are made only in accordance with authorizations of management and the Parent Board, (iii) regarding prevention or timely detection of the unauthorized acquisition, use or disposition of Parent’s assets that could have a material effect on Parent’s financial statements and (iv) that Parent maintains records in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of Parent and any of its Subsidiaries. Parent has evaluated the effectiveness of Parent’s internal control over financial reporting as of December 31, 2018, and, to the extent required by applicable Law, presented in any applicable Parent SEC Document that is a report on Form 10-K or Form 10-Q (or any amendment thereto) its conclusions about the effectiveness of the internal control over financial reporting as of the end of the period covered by such report or amendment based on such evaluation. Parent has disclosed, based on its most recent evaluation of internal control over financial reporting, to Parent’s auditors and audit committee (and has described in Section 3.7(f)of the Parent Disclosure Schedule) (A) all material weaknesses and all significant deficiencies, if any, in the design or operation of internal control over financial reporting that are reasonably likely to adversely affect Parent’s ability to record, process, summarize and report financial information and (B) any fraud, whether or not material, that involves Parent, any of its Subsidiaries, Parent’s management or other employees who have a role in the preparation of financial statements or the internal accounting controls utilized by the Parent and its Subsidiaries or (C) any claim or allegation regarding any of the foregoing,. Parent has not identified, based on its most recent evaluation of internal control over financial reporting, any significant deficiencies or material weaknesses in the design or operation of Parent’s internal control over financial reporting or (iii) any claim or allegation regarding any of the foregoing.
(g) Parent maintains “disclosure controls and procedures” (as defined in Rules 13a-15(e) and 15d-15(e) of the Exchange Act) that are reasonably designed to ensure that information required to be disclosed by Parent in the periodic reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported within the required time periods, and that all such information is accumulated and communicated to Parent’s management as appropriate to allow timely decisions regarding required disclosure and to make the Certifications.
(h) Section 3.7(h) to the Parent Disclosure Schedule sets forth an accurate statement of Parent’s cash and cash equivalents as of the close of business on the Business Day preceding the date of this Agreement, and there has been no material change in the amount thereof from such statement through the date of this Agreement. The cash forecast set forth on Section 3.7(h) to the Parent Disclosure Schedule (i) has been prepared by Parent in good faith, (ii) is based on assumptions that Parent considers to be reasonable, and (iii) fairly reflect Parent’s reasonably anticipated rate of cash usage for the periods covered therein.
32
3.8 Absence of Changes. Except as set forth on Section 3.8 of the Parent Disclosure Schedule, after the date of the Parent Balance Sheet, Parent and its Subsidiaries have conducted its business only in the Ordinary Course of Business (except for the execution and performance of this Agreement and the discussions, negotiations and transactions related thereto) and there has not been any (a) Parent Material Adverse Effect and (b) neither Parent nor any of its Subsidiaries has done any of the following:
(a) declared, accrued, set aside or paid any dividend or made any other distribution in respect of any shares of its capital stock or repurchased, redeemed or otherwise reacquired any shares of its capital stock or other securities (except in connection with the payment of the exercise price and/or withholding Taxes incurred upon the exercise, settlement or vesting of any award granted under the Parent Stock Plan in accordance with the terms of such award in effect on the date of this Agreement);
(b) sold, issued, granted, pledged or otherwise disposed of or encumbered or authorized any of the foregoing with respect to: (A) any capital stock or other security of Parent (except for Parent Common Stock issued upon the valid exercise of outstanding Parent Options); (B) any option, warrant or right to acquire any capital stock or any other security, other than option grants to employees in the Ordinary Course of Business; or (C) any instrument convertible into or exchangeable for any capital stock or other security of Parent;
(c) except as required to give effect to anything in contemplation of the Closing, amended any of its Organizational Documents, or effected or be a party to any merger, consolidation, share exchange, business combination, recapitalization, reclassification of shares, stock split, reverse stock split or similar transaction except, for the avoidance of doubt, the Contemplated Transactions;
(d) formed any Subsidiary or acquired any equity interest or other interest in any other Entity or enter into a joint venture with any other Entity;
(e) (A) lend money to any Person (except for the advance of reasonable business expenses to employees, directors and consultants in the Ordinary Course of Business), (B) incurred or guaranteed any indebtedness for borrowed money, or (C) guaranteed any debt securities of others;
(f) other than as required by applicable Law or the terms of any Parent Benefit Plan as in effect on the date of this Agreement: (A) adopted, terminated, established or entered into any Parent Benefit Plan; (B) caused or permitted any Parent Benefit Plan to be amended in any material respect; (C) paid any bonus or make any profit-sharing or similar payment to, or increased the amount of the wages, salary, commissions, benefits or other compensation or remuneration payable to, any of its directors, officers or employees; (D) increased the severance or change of control benefits offered to any current, former or new employees, directors or consultants or (E) hired, terminated or gave notice of termination (other than for cause) to any (x) officer or (y) employee whose annual base salary is or is expected to be more than $125,000 per year;
(g) recognized any labor union, labor organization, or similar Person except as otherwise required by law and after advance notice to the Company;
(h) entered into any material transaction other than in the Ordinary Course of Business;
(i) acquired any material asset or sold, leased or otherwise irrevocably disposed of any of its assets or properties, or granted any Encumbrance with respect to such assets or properties, except in the Ordinary Course of Business;
(j) sold, assigned, transferred, licensed, sublicensed or otherwise disposed of any material Parent IP (other than pursuant to non-exclusive licenses in the Ordinary Course of Business);
33
(k) made, changed or revoked any material Tax election, failed to pay any income or other material Tax as such Tax becomes due and payable, filed any amendment making any material change to any Tax Return, settled or compromised any income or other material Tax liability, entered into any Tax allocation, sharing, indemnification or other similar agreement or arrangement (other than customary commercial contracts entered into in the Ordinary Course of Business the principal subject matter of which is not Taxes), requested or consented to any extension or waiver of any limitation period with respect to any claim or assessment for any income or other material Taxes (other than pursuant to an extension of time to file any Tax Return granted in the Ordinary Course of Business of not more than six months), or adopted or changed any material accounting method in respect of Taxes;
(l) made any expenditures, incurred any Liabilities or discharged or satisfied any Liabilities, in each case, in amounts that exceed the aggregate amount of $300,000;
(m) other than as required by Law or GAAP, took any action to change accounting policies or procedures;
(n) initiated or settled any Legal Proceeding; or
(o) agreed, resolved or committed to do any of the foregoing.
3.9 Absence of Undisclosed Liabilities. As of the date hereof, neither Parent nor any of its Subsidiaries has any Liability, individually or in the aggregate, of a type required to be recorded or reflected on a balance sheet or disclosed in the footnotes thereto under GAAP except for: (a) Liabilities disclosed, reflected or reserved against in the Parent Balance Sheet; (b) Liabilities that have been incurred by Parent or its Subsidiaries since the date of the Parent Balance Sheet in the Ordinary Course of Business; (c) Liabilities for performance of obligations of Parent or any of its Subsidiaries under Parent Contracts; (d) Liabilities incurred in connection with the Contemplated Transactions; (e) Liabilities which would not, individually or in the aggregate, reasonably be expected to be material to the Parent; and (f) Liabilities described in Section 3.9 of the Parent Disclosure Schedule.
3.10 Title to Assets. Each of Parent and its Subsidiaries owns, and has good and valid title to, or, in the case of leased properties and assets, valid leasehold interests in, all tangible properties or tangible assets and equipment used or held for use in its business or operations or purported to be owned by it, including: (a) all tangible assets reflected on the Parent Balance Sheet; and (b) all other tangible assets reflected in the books and records of Parent or any of its Subsidiaries as being owned by Parent or such Subsidiary. All of such assets are owned or, in the case of leased assets, leased by Parent or its Subsidiaries free and clear of any Encumbrances, other than Permitted Encumbrances.
3.11 Real Property; Leasehold. Neither Parent nor any of its Subsidiaries own or ever have owned any real property. Parent has made available to the Company (a) an accurate and complete list of all real properties with respect to which Parent directly or indirectly holds a valid leasehold interest as well as any other real estate that is in the possession of or leased by Parent or any of its Subsidiaries, and (b) copies of all leases under which any such real property is possessed (the “Parent Real Estate Leases”), each of which is in full force and effect, with no existing material default thereunder. Parent’s use and operation of each such leased property conforms to all applicable Laws in all material respects, and Parent has exclusive possession of each such leased property and has not granted any occupancy rights to tenants or licensees with respect to such leased property. In addition, each such leased property is free and clear of all Encumbrances other than Permitted Encumbrances. Parent has not received written notice from its landlords or any Governmental Body that: (i) relates to violations of building, zoning, safety or fire ordinances or regulations; (ii) claims any defect or deficiency with respect to any of such properties; or (iii) requests the performance of any repairs, alterations or other work to such properties.
34
3.12 Intellectual Property.
(a) Section 3.12(a) of the Parent Disclosure Schedule identifies each item of material Registered IP owned in whole or in part by the Parent or its Subsidiaries, including, with respect to each registration and application: (i) the name of the applicant/registrant, (ii) the jurisdiction of application/registration, (iii) the application or registration number and (iv) any other co-owners. To the Knowledge of Parent, each of the patents and patent applications included in such material Registered IP properly identifies by name each and every inventor of the inventions claimed therein as determined in accordance with applicable Laws of the United States. As of the date of this Agreement, no cancellation, interference, opposition, reissue, reexamination or other proceeding of any nature (other than office actions or similar communications issued by any Governmental Body in the ordinary course of prosecution of any pending applications for registration) is pending or, to the Knowledge of Parent, threatened in writing, in which the scope, validity, enforceability or ownership of any material Registered IP owned in whole or in part by the Parent or its Subsidiaries is being or has been contested or challenged.
(b) Except as has not had and would not reasonably be expected to have, individually or in the aggregate, a Parent Material Adverse Effect, Parent or its Subsidiaries owns all right, title and interest in and to all material Parent IP (other than as disclosed on Section 3.12(b) of the Parent Disclosure Schedule), free and clear of all Encumbrances other than Permitted Encumbrances. To the Knowledge of Parent, each Parent Associate involved in the creation or development of any material Parent IP, pursuant to such Parent Associate’s activities on behalf of Parent or its Subsidiaries, has signed a written agreement containing an assignment of such Parent Associate’s rights in such Parent IP to Parent or its Subsidiaries and confidentiality provisions protecting the Parent IP.
(c) To the Knowledge of Parent, no funding, facilities or personnel of any Governmental Body or any university, college, research institute or other educational institution has been used to create Parent IP, except for any such funding or use of facilities or personnel that does not result in such Governmental Body or institution obtaining ownership rights to such Parent IP or the right to receive royalties for the practice of such Parent IP.
(d) Section 3.12(d) of Parent Disclosure Schedule sets forth each license agreement pursuant to which the Parent (i) is granted a license under any material Intellectual Property Right owned by any third party that is used by Parent or its Subsidiaries in its business as currently conducted (each a “Parent In-bound License”) or (ii) grants to any third party a license under any material Parent IP or material Intellectual Property Right licensed to the Parent or its Subsidiaries under a Parent In-bound License (each a “Parent Out-bound License”) (provided, that, Parent In-bound Licenses shall not include, when entered into in the Ordinary Course of Business, material transfer agreements, services agreements, clinical trial agreements, agreements with Parent Associates, non-disclosure agreements, commercially available Software-as-a-Service offerings, off-the-shelf software licenses or generally available patent license agreements; and Parent Out-bound Licenses shall not include, when entered into in the Ordinary Course of Business, material transfer agreements, clinical trial agreements, services agreements, non-disclosure agreements, or non-exclusive outbound licenses).
(e) To the Knowledge of Parent: (i) the operation of the business of Parent and its Subsidiaries as currently conducted does not infringe, misappropriate or otherwise violate any valid and enforceable Registered IP or any other Intellectual Property Right owned by any other Person and (ii) no other Person is infringing, misappropriating or otherwise violating any Parent IP or any Intellectual Property Rights exclusively licensed to Parent or its Subsidiaries. As of the date of this Agreement, no Legal Proceeding is pending (or, to the Knowledge of Parent, is threatened in writing) (A) against Parent or its Subsidiaries alleging that the operation of the business of Parent or its Subsidiaries infringes or constitutes the misappropriation or other violation of any Intellectual Property Rights of another Person or
35
(B) by Parent or its Subsidiaries alleging that another Person has infringed, misappropriated or otherwise violated any of Parent IP or any Intellectual Property Rights exclusively licensed to Parent or its Subsidiaries. Since January 1, 2017, neither Parent nor its Subsidiaries has received any written notice or other written communication alleging that the operation of the business of Parent or its Subsidiaries infringes or constitutes the misappropriation or other violation of any Intellectual Property Right of another Person.
(f) None of Parent IP or, to the Knowledge of Parent, any material Intellectual Property Rights exclusively licensed to Parent or its Subsidiaries is subject to any pending or outstanding injunction, directive, order, judgment or other disposition of dispute that adversely and materially restricts the use, transfer, registration or licensing by Parent or its Subsidiaries of any such Parent IP or material Intellectual Property Rights exclusively licensed to Parent or its Subsidiaries.
(g) To the Knowledge of Parent, the operation of Parent’s and its Subsidiaries’ business are in substantial compliance with all Laws pertaining to data privacy and data security of Sensitive Data, except to the extent that such noncompliance has not and would not reasonably be expected to have a Parent Material Adverse Effect. To the Knowledge of Parent, since January 1, 2017, there have been (i) no material losses or thefts of data or security breaches relating to Sensitive Data used in the business of Parent or its Subsidiaries, (ii) no violations of any security policy of Parent regarding any such Sensitive Data used in the business of Parent or its Subsidiaries, (iii) no unauthorized access, unauthorized use or unintended or improper disclosure of any Sensitive Data used in the business of Parent or its Subsidiaries, in each case of (i) through (iii), except as would not reasonably be expected to, individually or in the aggregate, have a Parent Material Adverse Effect.
3.13 Agreements, Contracts and Commitments. Section 3.13 of the Parent Disclosure Schedule identifies each Parent Contract that is in effect as of the date of this Agreement (other than any Parent Benefit Plan) and is:
(a) a material contract as defined in Item 601(b)(10) of Regulation S-K as promulgated under the Securities Act;
(b) each Contract relating to any agreement of indemnification or guaranty not entered into in the Ordinary Course of Business;
(c) each Contract containing (A) any covenant limiting the freedom of Parent or its Subsidiaries to engage in any line of business or compete with any Person, (B) any most-favored pricing arrangement, (C) any exclusivity provision, or (D) any non-solicitation provision;
(d) each Contract relating to capital expenditures and requiring payments after the date of this Agreement in excess of $100,000 pursuant to its express terms and not cancelable without penalty;
(e) each Contract relating to the disposition or acquisition of material assets or any ownership interest in any Entity;
(f) each Contract relating to any mortgages, indentures, loans, notes or credit agreements, security agreements or other agreements or instruments relating to the borrowing of money or extension of credit or creating any material Encumbrances with respect to any assets of Parent or its Subsidiaries or any loans or debt obligations with officers or directors of Parent;
36
(g) each Contract requiring payment by or to Parent after the date of this Agreement in excess of $100,000 pursuant to its express terms relating to: (A) any distribution agreement (identifying any that contain exclusivity provisions); (B) any agreement involving provision of services or products with respect to any pre-clinical or clinical development activities of Parent; (C) any dealer, distributor, joint marketing, alliance, joint venture, cooperation, development or other agreement currently in force under which Parent has continuing obligations to develop or market any product, technology or service, or any agreement pursuant to which Parent has continuing obligations to develop any Intellectual Property Rights that will not be owned, in whole or in part, by Parent; or (D) any Contract to license any third party to manufacture or produce any product, service or technology of Parent or any Contract to sell, distribute or commercialize any products or service of Parent, in each case, except for Contracts entered into in the Ordinary Course of Business;
(h) each Contract with any Person, including any financial advisor, broker, finder, investment banker or other Person, providing financial advisory services to Parent in connection with the Contemplated Transactions;
(i) each Parent Real Estate Lease;
(j) each Contract with any Governmental Body;
(k) each Parent Out-bound License and Parent In-bound License;
(l) each Contract containing any royalty, dividend or similar arrangement based on the revenues or profits of Parent or its Subsidiaries;
(m) each Parent Contract, offer letter, employment agreement, or independent contractor agreement with any employee or service provider that (A) is not immediately terminable by Parent without notice, severance, or other cost or liability, except as required under applicable Law, or (B) provides for retention payments, change of control payments, severance, accelerated vesting, or any similar payment or benefit that may or will become due as a result of the Merger; or
(n) any other Contract that is not terminable at will (with no penalty or payment or requirement for prior notice) by Parent or its Subsidiaries, as applicable, and (A) which involves payment or receipt by Parent or its Subsidiaries after the date of this Agreement under any such agreement, contract or commitment of more than $100,000 in the aggregate, or obligations after the date of this Agreement in excess of $100,000 in the aggregate, or (B) that is material to the business or operations of Parent and its Subsidiaries, taken as a whole.
Parent has delivered or made available to the Company accurate and complete copies of all Contracts to which Parent is a party or by which it is bound of the type described in the foregoing clauses (a)-(m) (any such Contract, a “Parent Material Contract”). There are no Parent Material Contracts that are not in written form. Neither Parent nor any of its Subsidiaries has not nor, to Parent’s Knowledge, as of the date of this Agreement, has any other party to a Parent Material Contract, breached, violated or defaulted under, or received notice that it breached, violated or defaulted under, any of the terms or conditions of any Parent Material Contract in such manner as would permit any other party to cancel or terminate any such Parent Material Contract, or would permit any other party to seek damages which would reasonably be expected to be material to Parent or its business. As to Parent and its Subsidiaries, as of the date of this Agreement, each Parent Material Contract is valid, binding, enforceable and in full force and effect, subject to the Enforceability Exceptions. No Person is renegotiating, or has a right pursuant to the terms of any Parent Material Contract to change, any material amount paid or payable to Parent under any Parent Material Contract or any other material term or provision of any Parent Material Contract.
37
3.14 Compliance; Permits.
(a) Parent and its Subsidiaries are, and since January 1, 2016 have been, in compliance in all material respects with all applicable Laws, including the FDCA, the FDA regulations adopted thereunder, the Public Health Services Act and any other similar Law administered or promulgated by the FDA or other Drug Regulatory Agency, except for any noncompliance, either individually or in the aggregate, which would not be material to Parent. No investigation, claim, suit, proceeding, audit or other action by any Governmental Body is pending or, to the Knowledge of Parent, threatened against Parent or any Subsidiary. There is no agreement, judgment, injunction, order or decree binding upon Parent or any Subsidiary which (i) has or would reasonably be expected to have the effect of prohibiting or materially impairing any business practice of Parent or any Subsidiary, any acquisition of material property by Parent or any Subsidiary or the conduct of business by Parent or any Subsidiary as currently conducted, (ii) is reasonably likely to have an adverse effect on Parent’s or any Subsidiary’s ability to comply with or perform any covenant or obligation under this Agreement, or (iii) is reasonably likely to have the effect of preventing, delaying, making illegal or otherwise interfering with the Contemplated Transactions.
(b) Parent or its Subsidiaries hold all required Governmental Authorizations which are material to the operation of the business of Parent or such Subsidiary as currently conducted (the “Parent Permits”). Section 3.14(b) of the Parent Disclosure Schedule identifies each Parent Permit. Parent is in material compliance with the terms of the Parent Permits. No Legal Proceeding is pending or, to the Knowledge of Parent, threatened, which seeks to revoke, limit, suspend, or materially modify any Parent Permit.
(c) There are no proceedings pending or, to the Knowledge of Parent, threatened with respect to an alleged material violation by Parent or any of its Subsidiaries of the FDCA, FDA regulations adopted thereunder, the Public Health Service Act or any other similar Law administered or promulgated by any Drug Regulatory Agency.
(d) Parent and each of its Subsidiaries holds all required Governmental Authorizations issuable by any Drug Regulatory Agency necessary or material to the conduct of the business of Parent or such Subsidiary as currently conducted (collectively, the “Parent Regulatory Permits”) and no such Parent Regulatory Permit has been (i) revoked, withdrawn, suspended, cancelled or terminated or (ii) modified in any adverse manner. Parent and each of its Subsidiaries are in compliance in all material respects with the Parent Regulatory Permits and have not received any written notice or other written communication, or to the Knowledge of Parent, any other communication from any Drug Regulatory Agency regarding (A) any material violation of or failure to comply materially with any term or requirement of any Parent Regulatory Permit or (B) any revocation, withdrawal, suspension, cancellation, termination or material modification of any Parent Regulatory Permit. Parent and each of its Subsidiaries have complied in all material respects with the ICH E9 Guidance for Industry: Statistical Principles for Clinical Trials in the management of the clinical data that have been presented to the Company. To the Knowledge of Parent, there are no facts that would be reasonably likely to result in any warning, untitled or notice of violation letter or Form FDA-483 from the FDA.
(e) All clinical, pre-clinical and other studies and tests conducted by or on behalf of, or sponsored by, Parent or its Subsidiaries, or in which Parent or its Subsidiaries or their respective current products or product candidates have participated, were and, if still pending, are being conducted in all material respects in accordance with standard medical and scientific research procedures and in compliance in all material respects with the applicable regulations of any applicable Drug Regulatory Agency and other applicable Law, including 21 C.F.R. Parts 50, 54, 56, 58 and 312. No preclinical or clinical trial conducted by or on behalf of Parent or any of its Subsidiaries has been terminated or suspended prior to completion for safety or non-compliance reasons. Since January 1, 2016, neither Parent nor any of
38
its Subsidiaries has received any notices, correspondence, or other communications from any Drug Regulatory Agency requiring, or to the Knowledge of the Parent threatening to initiate, the termination or suspension of any clinical studies conducted by or on behalf of, or sponsored by, Parent or any of its Subsidiaries or in which Parent or any of its Subsidiaries or their respective current products or product candidates have participated.
(f) Neither Parent nor any of its Subsidiaries is the subject of any pending or, to the Knowledge of Parent, threatened investigation in respect of their respective businesses or products by the FDA pursuant to its “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal Gratuities” Final Policy set forth in 56 Fed. Reg. 46191 (September 10, 1991) and any amendments thereto. To the Knowledge of Parent, neither Parent nor any of its Subsidiaries has committed any acts, made any statement, or has not failed to make any statement, in each case in respect of its business or products that would violate the FDA’s “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal Gratuities” Final Policy, and any amendments thereto. Neither Parent, nor any of its Subsidiaries, nor any of their respective officers, employees or agents has been convicted of any crime or engaged in any conduct that could result in a debarment or exclusion (i) under 21 U.S.C. Section 335a or (ii) any similar applicable Law. No debarment or exclusionary claims, actions, proceedings or investigations in respect of their business or products are pending or, to the Knowledge of Parent, threatened against Parent, or any of its Subsidiaries or any of their respective officers, employees or agents.
(g) Parent and each of its Subsidiaries has complied with all Laws relating to patient, medical or individual health information, including HIPAA, including the standards for the privacy of Individually Identifiable Health Information at 45 C.F.R. Parts 160 and 164, Subparts A and E, the standards for the protection of Electronic Protected Health Information set forth at 45 C.F.R. Part 160 and 45 C.F.R. Part 164, Subpart A and Subpart C, the standards for transactions and code sets used in electronic transactions at 45 C.F.R. Part 160, Subpart A and Part 162, and the standards for Breach Notification for Unsecured Protected Health Information at 45 C.F.R. Part 164, Subpart D, all as amended from time to time. Parent or its Subsidiaries has entered into, where required, and is in compliance in all material respects with the terms of all Business Associate Agreements to which Parent or any of its Subsidiaries is a party or otherwise bound. Parent has created and maintained written policies and procedures to protect the privacy of all protected health information, provide training to all employees and agents as required under HIPAA, and has implemented security procedures, including physical, technical and administrative safeguards, to protect all personal information and Protected Health Information stored or transmitted in electronic form. Neither Parent nor any of its Subsidiaries has received written notice from the Office for Civil Rights for the U.S. Department of Health and Human Services or any other Governmental Body of any allegation regarding its failure to comply with HIPAA or any other state law or regulation applicable to the protection of individually identifiable health information or personally identifiable information. No successful Security Incident, Breach of Unsecured Protected Health Information or breach of personally identifiable information under applicable state or federal laws have occurred with respect to information maintained or transmitted to Parent or any of its Subsidiaries or an agent or third party subject to a Business Associate Agreement with Parent or such Subsidiary. Parent or its Subsidiaries is currently submitting, receiving and handling or is capable of submitting receiving and handling transactions in accordance with the Standard Transaction Rule. All capitalized terms in this Section 3.14(g) not otherwise defined in this Agreement shall have the meanings set forth under HIPAA.
3.15 Legal Proceedings; Orders.
(a) As of the date of this Agreement, there is no material pending Legal Proceeding and, to the Knowledge of Parent, no Person has threatened in writing to commence any Legal Proceeding: (i) that involves (A) Parent, (B) any of its Subsidiaries, (C) any Parent Associate (in his or her capacity as such) or (D) any of the material assets owned or used by Parent or its Subsidiaries; or (ii) that challenges, or that may have the effect of preventing, delaying, making illegal or otherwise interfering with, the Contemplated Transactions.
39
(b) Except as set forth in Section 3.15(b) of the Parent Disclosure Schedule, since January 1, 2016 through the date of this Agreement, no Legal Proceeding has been pending against Parent that resulted in material liability to Parent.
(c) There is no order, writ, injunction, judgment or decree to which Parent or any of its Subsidiaries, or any of the material assets owned or used by Parent or any of its Subsidiaries, is subject. To the Knowledge of Parent, no officer of Parent or any of its Subsidiaries is subject to any order, writ, injunction, judgment or decree that prohibits such officer or employee from engaging in or continuing any conduct, activity or practice relating to the business of Parent or any of its Subsidiaries or to any material assets owned or used by Parent or any of its Subsidiaries.
3.16 Tax Matters.
(a) Parent and each of its Subsidiaries has timely filed all income Tax Returns and other material Tax Returns that they were required to file under applicable Law. All such Tax Returns are correct and complete in all material respects and have been prepared in compliance with all applicable Law. No claim has ever been made by any Governmental Body in any jurisdiction where Parent or any of its Subsidiaries does not file a particular Tax Return or pay a particular Tax that Parent or such Subsidiary is subject to taxation by that jurisdiction.
(b) All income and other material Taxes due and owing by Parent or any of its Subsidiaries on or before the date hereof (whether or not shown on any Tax Return) have been fully paid. The unpaid Taxes of Parent and its Subsidiaries did not, as of the date of the Parent Balance Sheet, materially exceed the reserve for Tax liability (excluding any reserve for deferred Taxes established to reflect timing differences between book and Tax items) set forth on the face of the Parent Balance Sheet. Since the Parent Balance Sheet Date, neither Parent nor any of its Subsidiaries has not incurred any material Liability for Taxes outside the Ordinary Course of Business.
(c) All Taxes that Parent is or was required by Law to withhold or collect have been duly and timely withheld or collected in all material respects on behalf of its respective employees, independent contractors, stockholders, lenders, customers or other third parties and, have been timely paid to the proper Governmental Body or other Person or properly set aside in accounts for this purpose.
(d) There are no Encumbrances for material Taxes (other than Taxes not yet due and payable) upon any of the assets of Parent.
(e) No deficiencies for income or other material Taxes with respect to Parent have been claimed, proposed or assessed by any Governmental Body in writing. There are no pending or ongoing, and to the Knowledge of Parent, threatened audits, assessments or other actions for or relating to any liability in respect of a material amount of Taxes of Parent. Neither Parent nor any of its predecessors has waived any statute of limitations in respect of any income or other material Taxes or agreed to any extension of time with respect to any income or other material Tax assessment or deficiency.
(f) Neither Parent nor any of its Subsidiaries has been a United States real property holding corporation within the meaning of Section 897(c)(2) of the Code during the applicable period specified in Section 897(c)(1)(A)(ii) of the Code.
40
(g) Neither Parent nor any of its Subsidiaries is a party to any Tax allocation agreement, Tax sharing agreement, Tax indemnity agreement, or similar agreement or arrangement, other than customary commercial contracts entered into in the Ordinary Course of Business the principal subject matter of which is not Taxes.
(h) Neither Parent nor any of its Subsidiaries will be required to include any material item of income in, or exclude any material item of deduction from, taxable income for any Tax period (or portion thereof) ending after the Closing Date as a result of any: (i) change in method of accounting for Tax purposes; (ii) use of an improper method of accounting for a Tax period ending on or prior to the Closing Date; (iii) “closing agreement” as described in Section 7121 of the Code (or any similar provision of state, local or foreign Law) executed on or prior to the Closing Date; (iv) intercompany transaction or excess loss account described in Treasury Regulations under Section 1502 of the Code (or any similar provision of state, local or foreign Law); (v) installment sale or open transaction disposition made on or prior to the Closing Date; (vi) prepaid amount received or deferred revenue accrued on or prior to the Closing Date; (vii) application of Section 367(d) of the Code to any transfer of intangible property on or prior to the Closing Date; (viii) application of Sections 951 or 951A of the Code (or any similar provision of state, local or foreign Law) to any income received or accrued on or prior to the Closing Date; or (ix) election under Section 108(i) of the Code (or any similar provision of state, local or foreign Law). Parent has not made any election under Section 965(h) of the Code.
(i) Neither Parent nor any of its Subsidiaries has ever been (i) a member of a consolidated, combined or unitary Tax group (other than such a group the common parent of which is Parent) or (ii) a party to any joint venture, partnership, or other arrangement that is treated as a partnership for U.S. federal income Tax purposes. Parent has no Liability for any material Taxes of any Person (other than Parent and any of its Subsidiaries) under Treasury Regulations Section 1.1502-6 (or any similar provision of state, local, or foreign Law), or as a transferee or successor.
(j) Neither Parent nor any of its Subsidiaries has distributed stock of another Person, or had its stock distributed by another Person, in a transaction that was purported or intended to be governed in whole or in part by Section 355 of the Code or Section 361 of the Code (or any similar provisions of state, local or foreign Law).
(k) Parent (i) is not a “controlled foreign corporation” as defined in Section 957 of the Code; (ii) is not a “passive foreign investment company” within the meaning of Section 1297 of the Code; or (iii) has ever had a permanent establishment (within the meaning of an applicable Tax treaty) or otherwise had an office or fixed place of business in a country other than the country in which it is organized.
(l) Neither Parent nor any of its Subsidiaries has participated in or been a party to a transaction that, as of the date of this Agreement, constitutes a “listed transaction” that is required to be reported to the IRS pursuant to Section 6011 of the Code and applicable Treasury Regulations thereunder.
(m) Neither Parent nor any of its Subsidiaries has taken any action nor knows of any fact that would reasonably be expected to prevent the Merger from qualifying for the Intended Tax Treatment.
For purposes of this Section 3.16, each reference to Parent or any of its Subsidiaries shall be deemed to include any Person that was liquidated into, merged with, or is otherwise a predecessor to, Parent.
41
3.17 Employee and Labor Matters; Benefit Plans.
(a) Section 3.17(a) of the Parent Disclosure Schedule is a list of all material Parent Benefit Plans, including, without limitation, each Parent Benefit Plan that provides for retirement, change in control, stay or retention deferred compensation, incentive compensation, severance or retiree medical or life insurance benefits. “Parent Benefit Plan” means each (i) “employee benefit plan” as defined in Section 3(3) of ERISA and (ii) other pension, retirement, deferred compensation, excess benefit, profit sharing, bonus, incentive, equity or equity-based, phantom equity, employment (other than employment offer letters on Parent’s standard form and other than individual Parent Options or other compensatory equity award agreements made pursuant to the Parent’s standard forms, in which case only representative standard forms of such agreements shall be scheduled), consulting, severance, change-of-control, retention, health, life, disability, group insurance, paid-time off, holiday, welfare and fringe benefit plan, program, contract, or arrangement (whether written or unwritten, qualified or nonqualified, funded or unfunded and including any that have been frozen), in any case, maintained, contributed to, or required to be contributed to, by Parent or any of its Subsidiaries or Parent ERISA Affiliates for the benefit of any current or former employee, director, officer or independent contractor of Parent or any of its Subsidiaries or under which Parent or any of its Subsidiaries has any actual or contingent liability (including, without limitation, as to the result of it being treated as a single employer under Code Section 414 with any other person).
(b) As applicable with respect to each material Parent Benefit Plan, Parent has made available to the Company, true and complete copies of (i) each material Parent Benefit Plan, including all amendments thereto, and in the case of an unwritten material Parent Benefit Plan, a written description thereof, (ii) all current trust documents, investment management contracts, custodial agreements, administrative services agreements and insurance and annuity contracts relating thereto, (iii) the current summary plan description and each summary of material modifications thereto, (iv) the most recently filed annual reports with any Governmental Body (e.g., Form 5500 and all schedules thereto), (v) the most recent IRS determination, opinion or advisory letter, (vi) the most recent summary annual reports, nondiscrimination testing reports, actuarial reports, financial statements and trustee reports, (vii) all records, notices and filings concerning IRS or Department of Labor or other Governmental Body audits or investigations, or “prohibited transactions” within the meaning of Section 406 of ERISA or Section 4975 of the Code and (viii) all policies and procedures established to comply with the privacy and security rules of HIPAA.
(c) Each Parent Benefit Plan has been maintained, operated and administered in compliance in all material respects with its terms and any related documents or agreements and the applicable provisions of ERISA, the Code and all other Laws.
(d) The Parent Benefit Plans which are “employee pension benefit plans” within the meaning of Section 3(2) of ERISA and which are intended to meet the qualification requirements of Section 401(a) of the Code have received determination or opinion letters from the IRS on which they may currently rely to the effect that such plans are qualified under Section 401(a) of the Code and the related trusts are exempt from federal income Taxes under Section 501(a) of the Code, respectively, and to the Knowledge of Parent nothing has occurred that would reasonably be expected to materially adversely affect the qualification of such Parent Benefit Plan or the tax exempt status of the related trust.
(e) Neither Parent, any of its Subsidiaries nor any Parent ERISA Affiliate maintains, contributes to, is required to contribute to, or has any actual or contingent liability with respect to, (i) any “employee pension benefit plan” (within the meaning of Section 3(2) of ERISA) that is subject to Title IV or Section 302 of ERISA or Section 412 of the Code, (ii) any “multiemployer plan” (within the meaning of Section 3(37) of ERISA), (iii) any “multiple employer plan” (within the meaning of Section 413 of the Code) or (iv) any “multiple employer welfare arrangement” (within the meaning of Section 3(40) of ERISA).
42
(f) There are no pending audits or investigations by any Governmental Body involving any Parent Benefit Plan, and no pending or, to the Knowledge of Parent, threatened claims (except for individual claims for benefits payable in the normal operation of the Parent Benefit Plans), suits or proceedings involving any Parent Benefit Plan, or, to the Knowledge of Parent, any fiduciary thereof or service provider thereto, in any case except as would not be reasonably expected to result in material liability to Parent or any of its Subsidiaries. All contributions and premium payments required to have been made under any of the Parent Benefit Plans or by applicable Law (without regard to any waivers granted under Section 412 of the Code), have been timely made and neither Parent nor any Parent ERISA Affiliate has any liability for any unpaid contributions with respect to any Parent Benefit Plan.
(g) Neither Parent, any of its Subsidiaries or any Parent ERISA Affiliates, nor to the Knowledge of Parent, any fiduciary, trustee or administrator of any Parent Benefit Plan, has engaged in, or in connection with the Contemplated Transactions will engage in, any transaction with respect to any Parent Benefit Plan which would subject any such Parent Benefit Plan, Parent, any of its Subsidiaries or Parent ERISA Affiliates to a material Tax, material penalty or material liability for a “prohibited transaction” under Section 406 of ERISA or Section 4975 of the Code.
(h) No Parent Benefit Plan provides death, medical, dental, vision, life insurance or other welfare benefits beyond termination of service or retirement other than coverage mandated by Law and to the Knowledge of Parent, neither Parent nor any of its Subsidiaries or any Parent ERISA Affiliates has made a written or oral representation promising the same.
(i) Except as set forth on Section 3.17(i) of the Parent Disclosure Schedule, neither the execution of, nor the performance of the transactions contemplated by, this Agreement (either alone or when combined with the occurrence of any other event, including without limitation, a termination of employment) will: (i) result in any payment becoming due to any current or former employee, director, officer, or independent contractor of Parent or any Subsidiary thereof pursuant to any Parent Benefit Plan, (ii) increase any amount of compensation or benefits otherwise payable under any Parent Benefit Plan, (iii) result in the acceleration of the time of payment, funding or vesting of any benefits under any Parent Benefit Plan, (iv) require any contribution or payment to fund any obligation under any Parent Benefit Plan or (v) limit the right to merge, amend or terminate any Parent Benefit Plan.
(j) Neither the execution of, nor the consummation of the transactions contemplated by this Agreement (either alone or when combined with the occurrence of any other event, including without limitation, a termination of employment) will result in the receipt or retention by any person who is a “disqualified individual” (within the meaning of Code Section 280G) with respect to Parent and its Subsidiaries of any payment or benefit that is or could be characterized as a “parachute payment” (within the meaning of Code Section 280G), determined without regard to the application of Code Section 280G(b)(5).
(k) Each Parent Benefit Plan providing for deferred compensation that constitutes a “nonqualified deferred compensation plan” (as defined in Section 409A(d)(1) of the Code and the regulations promulgated thereunder) is, and has been, established, administered and maintained in compliance with the requirements of Section 409A of the Code and the regulations promulgated thereunder in all material respects.
(l) No current or former employee, officer, director or independent contractor of Parent or any of its Subsidiaries has any “gross up” agreements with the Parent or any of its Subsidiaries or other assurance of reimbursement by the Parent or any of its Subsidiaries for any Taxes imposed under Code Section 409A or Code Section 4999.
43
(m) Each Parent Benefit Plan maintained outside of the United States (each, a “Parent Foreign Plan”) has obtained from the Governmental Body having jurisdiction with respect to such plan any required determinations that such plan is in compliance with the Laws of any such Governmental Body.
(n) The assets of each of the Parent Foreign Plans (which is an employee pension benefit plan as defined in Section 3(2) of ERISA (whether or not subject to ERISA) or otherwise provides retirement, medical or life insurance benefits following retirement or other termination of service or employment) are at least equal to the liabilities of such plans.
(o) Parent has provided to the Company a true and correct list, as of the date of this Agreement, containing the names of all current full-time, part-time or temporary employees and independent contractors (and indication as such), and, as applicable: (i) the annual dollar amount of all compensation (including wages, salary or fees, commissions, director’s fees, material fringe benefits, bonuses, profit sharing payments, and other compensatory payments or benefits of any type) payable to each person; (ii), dates of employment or service; (iii) title and, with respect to independent contractors, a current written description of such person’s contracting services; (iv) any eligibility to receive severance, notice of termination, retention payment, change of control payment, or other similar compensation; (v) visa status, if applicable; and (vi) with respect to employees, a designation of whether they are classified as eligible or ineligible for overtime for purposes of FLSA and any similar state, federal or Foreign law.
(p) Neither Parent nor any of its Subsidiaries is or has ever been a party to, bound by, or has a duty to bargain under, any collective bargaining agreement or other Contract with a labor union, labor organization, or similar Person representing any of its employees, and there is no labor union, labor organization, or similar Person representing or, to the Knowledge of Parent, purporting to represent or seeking to represent any employees of Parent or its Subsidiaries, including through the filing of a petition for representation election. There is not and has not been in the past three years, nor is there or has there been in the past three years any threat of, any strike, slowdown, work stoppage, lockout, union election petition, demand for recognition, or any similar activity or dispute, or, to the Knowledge of Parent, any union organizing activity, against Parent or any of its Subsidiaries. To the Knowledge of the Parent, no event has occurred, and no condition or circumstance exists, that might directly or indirectly be likely to give rise to or provide a basis for the commencement of any such strike, slowdown, work stoppage, lockout, union election petition, demand for recognition, any similar activity or dispute, or any union organizing activity.
(q) Parent and each of its Subsidiaries is, and since January 1, 2016 has been, in material compliance with all applicable Laws respecting labor, employment, employment practices, and terms and conditions of employment, including worker classification, discrimination, harassment and retaliation, equal employment opportunities, fair employment practices, meal and rest periods, immigration, employee safety and health, payment of wages (including overtime wages), unemployment and workers’ compensation, leaves of absence, and hours of work. Except as would not be reasonably likely to result in a material liability to Parent or any of its Subsidiaries, with respect to employees of Parent and its Subsidiaries, each of Parent and its Subsidiaries, since January 1, 2016: (i) has withheld and reported all amounts required by Law or by agreement to be withheld and reported with respect to wages, salaries and other payments, benefits, or compensation to employees, (ii) is not liable for any arrears of wages (including overtime wages), severance pay or any Taxes or any penalty for failure to comply with any of the foregoing, and (iii) is not liable for any payment to any trust or other fund governed by or maintained by or on behalf of any Governmental Body, with respect to unemployment compensation benefits, disability, social security
44
or other benefits or obligations for employees (other than routine payments to be made in the Ordinary Course of Business). There are no actions, suits, claims, charges, lawsuits, investigations, audits or administrative matters pending or, to the Knowledge of Parent, threatened or reasonably anticipated against Parent or any of its Subsidiaries relating to any employee, applicant for employment, consultant, employment agreement or Parent Benefit Plan (other than routine claims for benefits).
(r) Except as would not be reasonably likely to result in a material liability to Parent or any of its Subsidiaries, with respect to each individual who currently renders services to Parent or any of its Subsidiaries, Parent and each of its Subsidiaries has accurately classified each such individual as an employee, independent contractor, or otherwise under all applicable Laws and, for each individual classified as an employee, Parent has accurately classified him or her as exempt from or ineligible for overtime under all applicable Laws. Neither Parent nor any of its Subsidiaries has any material liability with respect to any misclassification of: (i) any Person as an independent contractor rather than as an employee, (ii) any employee leased from another employer, or (iii) any employee currently or formerly classified as exempt from or ineligible for overtime under all applicable Laws.
(s) Within the preceding five years, Parent has not implemented any “plant closing” or “mass layoff” of employees that would reasonably be expected to require notification under the WARN Act or any similar state or local Law, no such “plant closing” or “mass layoff” will be implemented before the Closing Date without advance notification to and approval of the Company, and there has been no “employment loss” as defined by the WARN Act within the 90 days prior the date of this Agreement.
3.18 Environmental Matters. Parent and each of its Subsidiaries are and since January 1, 2016 have complied with all applicable Environmental Laws, which compliance includes the possession by Parent of all permits and other Governmental Authorizations required under applicable Environmental Laws and compliance with the terms and conditions thereof, except for any failure to be in such compliance that, either individually or in the aggregate, would not reasonably be expected to be material to Parent or its business. Neither Parent nor any of its Subsidiaries has received since January 1, 2016 (or prior to that time, which is pending and unresolved), any written notice or other communication (in writing or otherwise), whether from a Governmental Body or other Person, that alleges that Parent or any of its Subsidiaries is not in compliance with or has liability pursuant to any Environmental Law and, to the Knowledge of Parent, there are no circumstances that would reasonably be expected to prevent or interfere with Parent’s or any of its Subsidiaries’ compliance in any material respects with any Environmental Law, except where such failure to comply would not reasonably be expected to be material to Parent or its business. No current or (during the time a prior property was leased or controlled by Parent or any of its Subsidiaries) prior property leased or controlled by Parent or any of its Subsidiaries has had a release of or exposure to Hazardous Materials in material violation of or as would reasonably be expected to result in any material liability of Parent or any of its Subsidiaries pursuant to Environmental Law. No consent, approval or Governmental Authorization of or registration or filing with any Governmental Body is required by Environmental Laws in connection with the execution and delivery of this Agreement or the consummation of Contemplated Transactions. Prior to the date hereof, Parent has provided or otherwise made available to the Company true and correct copies of all material environmental reports, assessments, studies and audits in the possession or control of Parent or any of its Subsidiaries with respect to any property leased or controlled by Parent or any of its Subsidiaries or any business operated by it.
3.19 Transactions with Affiliates. Except as set forth in the Parent SEC Documents filed prior to the date of this Agreement, since the date of Parent’s last proxy statement filed in March 2018 with the SEC, no event has occurred that would be required to be reported by Parent pursuant to Item 404 of Regulation S-K. Section 3.19 of the Parent Disclosure Schedule identifies each Person who is (or who may be deemed to be) an Affiliate of Parent as of the date of this Agreement.
45
3.20 Insurance. Parent has delivered or made available to the Company accurate and complete copies of all material insurance policies and all material self-insurance programs and arrangements relating to the business, assets, liabilities and operations of Parent and each of its Subsidiaries. Each of such insurance policies is in full force and effect and Parent and each of its Subsidiaries is in compliance in all material respects with the terms thereof. Other than customary end of policy notifications from insurance carriers, since January 1, 2016, neither Parent nor any of its Subsidiaries has received any notice or other communication regarding any actual or possible: (a) cancellation or invalidation of any insurance policy; or (b) refusal or denial of any coverage, reservation of rights or rejection of any material claim under any insurance policy. Parent and each of its Subsidiaries has provided timely written notice to the appropriate insurance carrier(s) of each Legal Proceeding that is currently pending against Parent or any of its Subsidiaries for which Parent or such Subsidiary has insurance coverage, and no such carrier has issued a denial of coverage or a reservation of rights with respect to any such Legal Proceeding, or informed Parent or any of its Subsidiaries of its intent to do so.
3.21 No Financial Advisors. No broker, finder or investment banker, other than Leerink Partners LLC, is entitled to any brokerage fee, finder’s fee, opinion fee, success fee, transaction fee or other fee or commission in connection with the Contemplated Transactions based upon arrangements made by or on behalf of Parent or any of its Subsidiaries.
3.22 Anti-Bribery. None of Parent or any of its Subsidiaries nor any of their respective directors, officers, employees or, to Parent’s Knowledge, agents or any other Person acting on its behalf has directly or indirectly made any bribes, rebates, payoffs, influence payments, kickbacks, illegal payments, illegal political contributions, or other payments, in the form of cash, gifts, or otherwise, or taken any other action, in violation of Anti-Bribery Laws. Neither Parent nor any of its Subsidiaries is or has been the subject of any investigation or inquiry by any Governmental Body with respect to potential violations of Anti-Bribery Laws.
3.23 Valid Issuance. The Parent Common Stock and Parent Convertible Preferred Stock to be issued in the Merger will, when issued in accordance with the provisions of this Agreement, be validly issued, fully paid and nonassessable.
3.24 Opinion of Financial Advisor. The Parent Board has received an opinion of Leerink Partners LLC to the effect that, as of the date of this Agreement and subject to the assumptions, qualifications, limitations and other matters set forth therein, the Merger Consideration to be issued to the stockholder of the Company under this Agreement is fair, from a financial point of view, to Parent. It is agreed and understood that such opinion is for the benefit of the Parent Board and may not be relied upon by the Company.
3.25 Disclaimer of Other Representations or Warranties.
(a) Except as previously set forth in this Section 3 or in any certificate delivered by Parent or Merger Sub to the Company pursuant to this Agreement, neither Parent nor Merger Sub makes any representation or warranty, express or implied, at law or in equity, with respect to it or any of its assets, liabilities or operations, and any such other representations or warranties are hereby expressly disclaimed.
(b) Each of Parent and Merger Sub acknowledges and agrees that, except for the representations and warranties of the Company set forth in Section 2, none of the Company or any of their respective Representatives is relying on any other representation or warranty of the Company or any other Person made outside of Section 2, including regarding the accuracy or completeness of any such other representations or warranties or the omission of any material information, whether express or implied, in each case, with respect to the Contemplated Transactions.
46
Section 4. COVENANTS RELATING TO CONDUCT OF BUSINESS PENDING THE MERGER
4.1 Operation of Parent’s Business.
(a) Except as set forth on Section 4.1(a) of the Parent Disclosure Schedule, as expressly permitted by this Agreement, as required by applicable Law or unless the Company shall otherwise consent in writing (which consent shall not be unreasonably withheld, delayed or conditioned), during the period commencing on the date of this Agreement and continuing until the earlier to occur of the termination of this Agreement pursuant to Section 9 and the Effective Time (the “Pre-Closing Period”): each of Parent and its Subsidiaries shall conduct their respective business and operations in the Ordinary Course of Business and in compliance in all material respects with all applicable Laws and the requirements of all Contracts that constitute Parent Material Contracts.
(b) Except (i) as expressly permitted by this Agreement, (ii) as set forth in Section 4.1(b) of the Parent Disclosure Schedule, (iii) as required by applicable Law or (iv) with the prior written consent of the Company (which consent shall not be unreasonably withheld, delayed or conditioned), at all times during the Pre-Closing Period, Parent shall not, nor shall its cause or permit any of its Subsidiaries to, do any of the following:
(i) declare, accrue, set aside or pay any dividend or make any other distribution in respect of any shares of its capital stock or repurchase, redeem or otherwise reacquire any shares of its capital stock or other securities (except in connection with the payment of withholding Taxes incurred upon the exercise, settlement or vesting of any award granted under the Parent Stock Plans in accordance with the terms of such award in effect on the date of this Agreement);
(ii) sell, issue, grant, pledge or otherwise dispose of or encumber or authorize any of the foregoing with respect to: (A) any capital stock or other security of Parent or of any of its Subsidiaries (except for Parent Common Stock issued upon the valid exercise of outstanding Parent Options); (B) any option, warrant or right to acquire any capital stock or any other security; or (C) any instrument convertible into or exchangeable for any capital stock or other security of Parent or of any of its Subsidiaries;
(iii) amend any of its Organizational Documents, or effect or be a party to any merger, consolidation, share exchange, business combination, recapitalization, reclassification of shares, stock split, reverse stock split or similar transaction except, for the avoidance of doubt, the Contemplated Transactions;
(iv) form any Subsidiary or acquire any equity interest or other interest in any other Entity or enter into a joint venture with any other Entity;
(v) (A) lend money to any Person, (B) incur or guarantee any indebtedness for borrowed money, (C) guarantee any debt securities of others;
(vi) other than as required by applicable Law or the terms of any Parent Benefit Plan as in effect on the date of this Agreement: (A) adopt, terminate, establish or enter into any Parent Benefit Plan; (B) cause or permit any Parent Benefit Plan to be amended in any material respect; (C) pay any bonus or make any profit-sharing or similar payment to, or increase the amount of the wages,
47
salary, commissions, benefits or other compensation or remuneration payable to, any of its directors, officers or employees; (D) increase the severance, retention or change of control benefits offered to any current or former or new employees, directors or consultants; (E) hire or retain any officer, employee or consultant; or (F) terminate or give notice of termination to any officer or employee, other than any termination for cause;
(vii) recognize any labor union, labor organization, or similar Person except as otherwise required by law and after advance notice to the Company;
(viii) acquire any material asset or sell, lease or otherwise irrevocably dispose of any of its material assets or properties, or grant any Encumbrance with respect to such assets or properties;
(ix) sell, assign, transfer, license, sublicense or otherwise dispose of any material Parent IP (other than pursuant to non-exclusive licenses in the Ordinary Course of Business);
(x) make, change or revoke any material Tax election, fail to pay any income or other material Tax as such Tax becomes due and payable, file any amendment making any material change to any Tax Return, settle or compromise any income or other material Tax liability, enter into any Tax allocation, sharing, indemnification or other similar agreement or arrangement (other than customary commercial contracts entered into in the Ordinary Course of Business the principal subject matter of which is not Taxes), request or consent to any extension or waiver of any limitation period with respect to any claim or assessment for any income or other material Taxes (other than pursuant to an extension of time to file any Tax Return granted in the Ordinary Course of Business of not more than six months), or adopt or change any material accounting method in respect of Taxes;
(xi) enter into, materially amend or terminate any Parent Material Contract;
(xii) Make any expenditures, incur any Liabilities or discharge or satisfy any Liabilities, in each case, in amounts that exceed $50,000 in the aggregate;other than as required by Law or GAAP, take any action to change accounting policies or procedures;
(xiii) initiate or settle any Legal Proceeding; or
(xiv) agree, resolve or commit to do any of the foregoing.
Nothing contained in this Agreement shall give the Company, directly or indirectly, the right to control or direct the operations of Parent prior to the Effective Time. Prior to the Effective Time, Parent shall exercise, consistent with the terms and conditions of this Agreement, complete unilateral control and supervision over its business operations.
4.2 Operation of the Company’s Business.
(a) Except as set forth on Section 4.2(a) of the Company Disclosure Schedule, as expressly permitted by this Agreement, as required by applicable Law or unless Parent shall otherwise consent in writing (which consent shall not be unreasonably withheld, delayed or conditioned), during the Pre-Closing Period: the Company shall conduct its business and operations in the Ordinary Course of Business and in compliance in all material respects with all applicable Laws and the requirements of all Contracts that constitute Company Material Contracts.
48
(b) Except (i) as expressly permitted by this Agreement, (ii) as set forth in Section 4.2(b) of the Company Disclosure Schedule, (iii) as required by applicable Law or (iv) with the prior written consent of Parent (which consent shall not be unreasonably withheld, delayed or conditioned), at all times during the Pre-Closing Period, the Company shall not do any of the following:
(i) declare, accrue, set aside or pay any dividend or make any other distribution in respect of any shares of its capital stock or repurchase, redeem or otherwise reacquire any shares of its capital stock or other securities (except for shares of Company Common Stock from terminated employees, directors or consultants of the Company), in each case, other than in connection with the UW Equity Right;
(ii) sell, issue, grant, pledge or otherwise dispose of or encumber or authorize any of the foregoing with respect to: (A) any capital stock or other security of the Company; (B) any option, warrant, right to acquire any capital stock or any other security; or (C) any other instrument convertible into or exchangeable for any capital stock or other security of the Company, in each case, other than in connection with the UW Equity Right;
(iii) except as required to give effect to anything in contemplation of the Closing, amend any of its or its Subsidiaries’ Organizational Documents, or effect or be a party to any merger, consolidation, share exchange, business combination, recapitalization, reclassification of shares, stock split, reverse stock split or similar transaction except, for the avoidance of doubt, the Contemplated Transactions;
(iv) form any Subsidiary or acquire any equity interest or other interest in any other Entity or enter into a joint venture with any other Entity, in each case, other than in connection with the UW Equity Right;
(v) (A) lend money to any Person, (B) incur or guarantee any indebtedness for borrowed money, or (C) guarantee any debt securities of others;
(vi) other than in the Ordinary Course of Business by the Company, as required by applicable Law or the terms of any Company Benefit Plan as in effect on the date of this Agreement: (A) adopt, terminate, establish or enter into any Company Benefit Plan; (B) cause or permit any Company Benefit Plan to be amended in any material respect; (C) pay any bonus or make any profit-sharing or similar payment to, or increase the amount of the wages, salary, commissions, benefits or other compensation or remuneration payable to, any of its directors, officers or employees; (D) increase the severance or change of control benefits offered to any current or new employees, directors or consultants or (E) terminate or give notice of termination to any (x) officer or (y) employee whose annual base salary is or is expected to be more than $125,000 per year, other than any termination for cause;
(vii) recognize any labor union, labor organization, or similar Person, except as otherwise required by law and after advance notice to the Parent;
(viii) acquire any material asset or sell, lease or otherwise irrevocably dispose of any of its material assets or properties, or grant any Encumbrance with respect to such assets or properties;
(ix) sell, assign, transfer, license, sublicense or otherwise dispose of any material Company IP (other than pursuant to non-exclusive licenses in the Ordinary Course of Business);
49
(x) make, change or revoke any material Tax election, fail to pay any income or other material Tax as such Tax becomes due and payable, file any amendment making any material change to any Tax Return, settle or compromise any income or other material Tax liability, enter into any Tax allocation, sharing, indemnification or other similar agreement or arrangement (other than customary commercial contracts entered into in the Ordinary Course of Business the principal subject matter of which is not Taxes), request or consent to any extension or waiver of any limitation period with respect to any claim or assessment for any income or other material Taxes (other than pursuant to an extension of time to file any Tax Return granted in the Ordinary Course of Business of not more than six months), or adopt or change any material accounting method in respect of Taxes;
(xi) enter into, materially amend or terminate any Company Material Contract other than in the Ordinary Course of Business by the Company;
(xii) Make any expenditures, incur any Liabilities or discharge or satisfy any Liabilities, in each case, in amounts that exceed $50,000 in the aggregate, other than in the Ordinary Course of Business by the Company;
(xiii) other than as required by Law or GAAP, take any action to change accounting policies or procedures;
(xiv) initiate or settle any Legal Proceeding; or
(xv) agree, resolve or commit to do any of the foregoing.
Section 5. ADDITIONAL AGREEMENTS OF THE PARTIES
5.1 Company Information Statement; Stockholder Written Consent; Section 280G Approval.
(a) Immediately following the execution of this Agreement, the Company shall solicit the Company Stockholder Written Consent evidencing the Required Company Stockholder Vote, for purposes of (i) adopting and approving this Agreement and the Contemplated Transactions, (ii) acknowledging that the approval given thereby is irrevocable and that such stockholder is aware of its rights to demand appraisal for its shares pursuant to Section 262 of the DGCL, a true and correct copy of which will be attached thereto, and that such stockholder has received and read a copy of Section 262 of the DGCL, and (iii) acknowledging that by its approval of the Merger it is not entitled to appraisal rights with respect to its shares in connection with the Merger and thereby waives any rights to receive payment of the fair value of its capital stock under the DGCL (collectively, the “Company Stockholder Matters”). The Company has prepared, with the cooperation of Parent, and immediately following the date hereof,will cause to be mailed to its stockholders an information statement (the “Information Statement”) describing the Company Stockholder Matters. Under no circumstances shall the Company assert that any other approval or consent is necessary by its stockholders to approve this Agreement and the Contemplated Transactions. All materials (including any amendments thereto) submitted to the stockholders of the Company in accordance with this Section 5.1(a) shall be subject to Parent’s advance review and reasonable approval.
(b) The Company covenants and agrees that the Information Statement (and the letter to stockholders and form of Company Stockholder Written Consent included therewith), will not, at the time that the Information Statement or any amendment or supplement thereto is first mailed to the stockholders of the Company, at the time of receipt of the Required Company Stockholder Vote and at the Effective Time, contain any untrue statement of a material fact or omit to state any material fact required
50
to be stated therein or necessary in order to make the statements made therein, in light of the circumstances under which they were made, not misleading. Notwithstanding the foregoing, the Company makes no covenant, representation or warranty with respect to statements made in the Information Statement (and the letter to the stockholders and form of Company Stockholder Written Consent included therewith), if any, based on information furnished in writing by Parent specifically for inclusion therein. Each of the Parties shall use reasonable best efforts to cause the Information Statement to comply with the applicable rules and regulations promulgated by the SEC in all material respects.
(c) Promptly following receipt of the Required Company Stockholder Vote, the Company shall prepare and mail a notice (the “Stockholder Notice”) to every stockholder of the Company that did not execute the Company Stockholder Written Consent. The Stockholder Notice shall (i) be a statement to the effect that the Company Board determined that the Merger is advisable in accordance with Section 251(b) of the DGCL and in the best interests of the stockholders of the Company and approved and adopted this Agreement, the Merger and the other Contemplated Transactions, (ii) provide the stockholders of the Company to whom it is sent with notice of the actions taken in the Company Stockholder Written Consent, including the adoption and approval of this Agreement, the Merger and the other Contemplated Transactions in accordance with Section 228(e) of the DGCL and the certificate of incorporation and bylaws of the Company and (iii) include a description of the appraisal rights of the Company’s stockholders available under the DGCL, along with such other information as is required thereunder and pursuant to applicable Law. All materials (including any amendments thereto) submitted to the stockholders of the Company in accordance with this Section 5.1(c) shall be subject to Parent’s advance review and reasonable approval.
(d) The Company agrees that the Company Board shall recommend that the Company’s stockholders vote to approve the Company Stockholder Matters and shall use reasonable best efforts to solicit such approval from each of the Company stockholders necessary to deliver the Company Stockholder Written Consent evidencing the Required Company Stockholder Vote within the time set forth within the time set forth in Section 5.1(a).
(e) As soon as practicable following the execution of this Agreement, the Company shall obtain, prior to the initiation of the 280G Approval (as defined below), a waiver (a “Parachute Payment Waiver”), in a form reasonably acceptable to Parent, from each Person who, with respect to the Company, would reasonably be expected to be a “disqualified individual” (within the meaning of Section 280G of the Code and the regulations promulgated thereunder), as determined immediately prior to the initiation of the 280G Approval, and who, with respect to the Company, reasonably might otherwise receive, have received, or have the right or entitlement to receive any parachute payment under Section 280G of the Code, pursuant to which Parachute Payment Waiver each such Person will agree, unless the 280G Approval has been obtained in a manner which satisfies all applicable requirements of Section 280G(b)(5)(B) of the Code and the regulations promulgated thereunder, to waive any and all right or entitlement to the payments, acceleration of vesting and/or other benefits referred to in this Section 5.1(e) to the extent the value thereof exceeds 2.99 times such Person’s base amount determined in accordance with Section 280G of the Code and the regulations promulgated thereunder.
(f) As soon as practicable following the execution of this Agreement, the Company shall submit to its stockholders for a vote all such waived payments in a manner such that, if such vote is adopted by the stockholders in a manner which satisfies Section 280G(b)(5)(B) of the Code and the Treasury Regulations thereunder, including Q-7 of Section 1.280G-1 of such Treasury Regulations, no payment or benefit received by such “disqualified individual” would be a “parachute payment” for purposes of Section 280G of the Code (the “280G Approval”). The taking of any such vote to obtain the 280G Approval, including all materials and information that are provided to stockholders in connection with such vote, shall comply with applicable Laws and prior to the Closing Date, the Company shall deliver to Parent
51
evidence that a vote of the stockholders was solicited in accordance with the foregoing provisions of this Section 5.1(f) and that either (i) 280G Approval was obtained, or (ii) 280G Approval was not obtained, and as a consequence, the parachute payments subject to the Parachute Payment Waivers shall not be made or provided.
5.2 Parent Stockholders’ Meeting.
(a) As promptly as practicable following the execution of this Agreement, Parent shall take all action necessary under applicable Law to call and give notice of a meeting of the holders of Parent Common Stock for the purpose of seeking:
(i) approval of the Preferred Stock Conversion Proposal; and
(ii) approval of an amendment to Parent’s certificate of incorporation to increase the number of authorizes shares of Parent Common Stock to such amount as determined by the Parent Board following the Closing (the matters contemplated by the clauses 5.2(a)(i) – (ii) are referred to as the “Parent Stockholder Matters,” and such meeting, the “Parent Stockholders’ Meeting”).
(b) Parent agrees to use reasonable best efforts to call and hold the Parent Stockholders’ Meeting as soon as practicable after the date hereof, and in any event on or before the date 100 days after the date hereof. If the approval of the Parent Stockholder Matters is not obtained at the Parent Stockholders’ Meeting, then Parent will use its reasonable best efforts to adjourn the Parent Stockholders’ Meeting one or more times to a date or dates no more than 30 days after the scheduled date for such meeting, and to obtain such approvals at such time. If the Parent Stockholders’ Meeting is not so adjourned, and/or if the approval of the Parent Stockholder Matters is not then obtained, Parent will use its reasonable best efforts to obtain such approvals as soon as practicable thereafter, and in any event to obtain such approvals at the next occurring annual meeting of the stockholders of Parent or, if such annual meeting is not scheduled to be held within six months after the Parent Stockholders’ Meeting, a special meeting of the stockholders of Parent to be held within six months after the Parent Stockholders’ Meeting. Parent will hold an annual meeting or special meeting of its stockholders, at which a vote of the stockholders of Parent to approve the Parent Stockholder Matters will be solicited and taken, at least once every six months until Parent obtains approval of the Parent Stockholder Matters.
(c) Parent agrees that: (i) the Parent Board shall, subject in all cases to compliance with its fiduciary duties under applicable Law, recommend that the holders of Parent Common Stock vote to approve the Parent Stockholder Matters and shall use its reasonable best efforts to solicit and obtain such approval. The Company and Parent acknowledge that, under the Nasdaq Stock Market Rules, the Parent Common Stock Payment Shares and the Parent Preferred Stock Payment Shares will not be entitled to vote on the Preferred Stock Conversion Proposal.
5.3 Reservation of Parent Common Stock; Issuance of Shares of Parent Common Stock. For as long as any Parent Preferred Stock Payment Shares remain outstanding, Parent shall at all times reserve and keep available, free from preemptive rights, out of its authorized but unissued Parent Common Stock or shares of Parent Common Stock held in treasury by Parent, for the purpose of effecting the conversion of the Parent Preferred Stock Payment Shares, the full number of shares of Parent Common Stock then issuable upon the conversion of all Parent Preferred Stock Payment Shares then outstanding. All shares of Parent Common Stock delivered upon conversion of the Parent Preferred Stock Payment Shares shall be newly issued shares or shares held in treasury by Parent, shall have been duly authorized and validly issued and shall be fully paid and nonassessable, and shall be free from preemptive rights and free of any Encumbrance.
52
5.4 Employee Benefits.
(a) For purposes of vesting, eligibility to participate, and level of benefits (other than for purposes of determining awards under an equity incentive plan or accrued benefits under any defined benefit pension plan) under the benefit plans, programs, contracts or arrangements of Parent or any of its Subsidiaries (including, following the Closing, the Surviving Corporation and its Subsidiaries) (the “Post-Closing Plans”), Parent shall use reasonable best efforts to cause each employee who remains employed by Parent or the Surviving Corporation, or any of their respective Subsidiaries following the Closing, (together, the “Continuing Employees”) to be credited with his or her years of service with Parent, the Company or any of their respective Subsidiaries and their respective predecessors; provided that the foregoing shall not apply to the extent that its application would result in a duplication of benefits. In addition, and without limiting the generality of the foregoing, for purposes of each Post-Closing Plan providing medical, dental, pharmaceutical and/or vision benefits to a Continuing Employee, Parent shall use reasonable best efforts to cause all pre-existing condition exclusions and actively-at-work requirements of such Post-Closing Plan to be waived for such Continuing Employee and his or her covered dependents to the extent and unless such conditions would have been waived or satisfied under the employee benefit plan whose coverage is being replaced under the Post-Closing Plan, and Parent shall use reasonable best efforts to cause any eligible expenses incurred by a Continuing Employee and his or her covered dependents during the portion of such plan year in which coverage is replaced with coverage under a Post-Closing Plan to be taken into account under such Post-Closing Plan with respect to the plan year in which participation in such Post-Closing Plan begins for purposes of satisfying all deductible, coinsurance and maximum out-of-pocket requirements applicable to such Continuing Employee and his or her covered dependents for such plan year as if such amounts had been paid in accordance with such Post-Closing Plan. For employees of Parent who are not Continuing Employees and who are entitled to severance under their applicable employment agreements, Parent shall prepay for medical, dental, pharmaceutical and/or vision benefits for such employees, if the provider of such benefits is under an individual plan.
(b) As soon as practicable following the Closing, Parent shall grant to each Continuing Employee set forth on Section 5.4(b) of the Parent Disclosure Schedule an option to purchase the number of shares of Parent Common Stock and/or restricted stock units opposite each such Continuing Employee’s name therein, with a per share exercise price (in the case of the options) equal to fair market value of a share of Parent Common Stock on the date of grant (the “Retention Equity Awards”). Each Retention Equity Award shall vest as set forth on Section 5.4(b) of the Parent Disclosure Schedule.
(c) The provisions of this Section 5.4 are for the sole benefit of Parent and the Company and no provision of this Agreement shall (i) create any third-party beneficiary or other rights in any Person, including rights in respect of any benefits that may be provided, directly or indirectly, under any Company Benefit Plan, Parent Benefit Plan or Post-Closing Plan or rights to continued employment or service with the Company or the Parent (or any Subsidiary thereof), (ii) be construed as an amendment, waiver or creation of or limitation on the ability to terminate any Company Benefit Plan, Parent Benefit Plan or Post-Closing Plan, or (iii) limit the ability of the Parent to terminate the employment of any Continuing Employee.
5.5 Indemnification of Officers and Directors.
(a) From the Effective Time through the sixth anniversary of the date on which the Effective Time occurs, each of Parent and the Surviving Corporation shall indemnify and hold harmless each person who is now, or has been at any time prior to the date hereof, or who becomes prior to the Effective Time, a director or officer of Parent or the Company or any of their respective Subsidiaries, respectively (the “D&O Indemnified Parties”), against all claims, losses, liabilities, damages, judgments, fines and reasonable fees, costs and expenses, including attorneys’ fees and disbursements (collectively,
53
“Costs”), incurred in connection with any claim, action, suit, proceeding or investigation, whether civil, criminal, administrative or investigative, arising out of or pertaining to the fact that the D&O Indemnified Party is or was a director or officer of Parent or of the Company, or any Subsidiary thereof,asserted or claimed prior to the Effective Time, in each case, to the fullest extent permitted under applicable Law. Each D&O Indemnified Party will be entitled to advancement of expenses incurred in the defense of any such claim, action, suit, proceeding or investigation from each of Parent and the Surviving Corporation, jointly and severally, upon receipt by Parent or the Surviving Corporation from the D&O Indemnified Party of a request therefor; provided that any such person to whom expenses are advanced provides an undertaking to Parent, to the extent then required by the DGCL, to repay such advances if it is ultimately determined that such person is not entitled to indemnification.
(b) The provisions of the certificate of incorporation and bylaws of Parent with respect to indemnification, advancement of expenses and exculpation of present and former directors and officers of Parent that are presently set forth in the certificate of incorporation and bylaws of Parent shall not be amended, modified or repealed for a period of six years from the Effective Time in a manner that would adversely affect the rights thereunder of individuals who, at or prior to the Effective Time, were officers or directors of Parent. The certificate of incorporation and bylaws of the Surviving Corporation shall contain, and Parent shall cause the certificate of incorporation and bylaws of the Surviving Corporation to so contain, provisions no less favorable with respect to indemnification, advancement of expenses and exculpation of present and former directors and officers as those presently set forth in the certificate of incorporation and bylaws of Parent.
(c) From and after the Effective Time, (i) the Surviving Corporation shall fulfill and honor in all respects the obligations of the Company to its D&O Indemnified Parties as of immediately prior to the Closing pursuant to any indemnification provisions under the Company’s Organizational Documents and pursuant to any indemnification agreements between the Company and such D&O Indemnified Parties, with respect to claims arising out of matters occurring at or prior to the Effective Time and (ii) Parent shall fulfill and honor in all respects the obligations of Parent to its D&O Indemnified Parties as of immediately prior to the Closing pursuant to any indemnification provisions under Parent’s Organizational Documents and pursuant to any indemnification agreements between Parent and such D&O Indemnified Parties, with respect to claims arising out of matters occurring at or prior to the Effective Time.
(d) From and after the Effective Time, Parent shall continue to maintain directors’ and officers’ liability insurance policies, with an effective date as of the Closing Date, on commercially available terms and conditions and with coverage limits customary for U.S. public companies similarly situated to Parent. From and after the Effective Time, Parent shall pay all expenses, including reasonable attorneys’ fees, that are incurred by the persons referred to in this Section 5.5 in connection with their successful enforcement of the rights provided to such persons in this Section 5.5.
(e) The provisions of this Section 5.5 are intended to be in addition to the rights otherwise available to the current and former officers and directors of Parent and the Company by Law, charter, statute, bylaw or agreement, and shall operate for the benefit of, and shall be enforceable by, each of the D&O Indemnified Parties, their heirs and their representatives.
(f) In the event Parent or the Surviving Corporation or any of their respective successors or assigns (i) consolidates with or merges into any other Person and shall not be the continuing or surviving corporation or entity of such consolidation or merger, or (ii) transfers all or substantially all of its properties and assets to any Person, then, and in each such case, proper provision shall be made so that the successors and assigns of Parent or the Surviving Corporation, as the case may be, shall succeed to the obligations set forth in this Section 5.5. Parent shall cause the Surviving Corporation to perform all of the obligations of the Surviving Corporation under this Section 5.5.
54
5.6 Additional Agreements. The Parties shall use reasonable best efforts to cause to be taken all actions necessary to consummate the Contemplated Transactions. Without limiting the generality of the foregoing, each Party to this Agreement: (a) shall make all filings and other submissions (if any) and give all notices (if any) required to be made and given by such Party in connection with the Contemplated Transactions; (b) shall use reasonable best efforts to obtain each Consent (if any) reasonably required to be obtained (pursuant to any applicable Law or Contract, or otherwise) by such Party in connection with the Contemplated Transactions or for such Contract to remain in full force and effect; (c) shall use reasonable best efforts to lift any injunction prohibiting, or any other legal bar to, the Contemplated Transactions; and (d) shall use reasonable best efforts to satisfy the conditions precedent to the consummation of this Agreement.
5.7 Access and Investigation.
(a) Subject to the terms of the Confidentiality Agreement, during the Pre-Closing Period, upon reasonable notice, Parent, on the one hand, and the Company, on the other hand, shall and shall use reasonable best efforts to cause such Party’s Representatives to: (i) provide the other Party and such other Party’s Representatives with reasonable access during normal business hours to such Party’s Representatives, personnel, property and assets and to all existing books, records, Tax Returns, work papers and other documents and information relating to such Party and its Subsidiaries; (ii) provide the other Party and such other Party’s Representatives with such copies of the existing books, records, Tax Returns, work papers, product data, and other documents and information relating to such Party and its Subsidiaries, and with such additional financial, operating and other data and information regarding such Party and its Subsidiaries as the other Party may reasonably request; (iii) permit the other Party’s officers and other employees to meet, upon reasonable notice and during normal business hours, with the chief financial officer and other officers and managers of such Party responsible for such Party’s financial statements and the internal controls of such Party to discuss such matters as the other Party may deem necessary or appropriate and; (iv) make available to the other Party copies of unaudited financial statements, material operating and financial reports prepared for senior management or the board of directors of such Party, and any material notice, report or other document filed with or sent to or received from any Governmental Body in connection with the Contemplated Transactions. Any investigation conducted by either Parent or the Company pursuant to this Section 5.7 shall be conducted in such manner as not to interfere unreasonably with the conduct of the business of the other Party.
(b) Notwithstanding the foregoing, any Party may restrict the foregoing access to the extent that any Law applicable to such Party requires such Party to restrict or prohibit access to any such properties or information.
5.8 Notification of Certain Matters.
(a) During the Pre-Closing Period, the Company shall promptly notify Parent (and, if in writing, furnish copies of) if any of the following occurs: (i) any notice or other communication is received from any Person alleging that the Consent of such Person is or may be required in connection with any of the Contemplated Transactions; (ii) any Legal Proceeding against or involving or otherwise affecting the Company is commenced, or, to the Knowledge of the Company, threatened against the Company or, to the Knowledge of the Company, any director or officer of the Company; (iii) the Company becomes aware of any inaccuracy in any representation or warranty made by it in this Agreement; or (iv) the failure of the Company to comply with any covenant or obligation of the Company; in the case of (iii) and (iv) that could reasonably be expected to make the timely satisfaction of any of the conditions set forth in Sections 6 or 7, as applicable, impossible or materially less likely. No notification given to Parent pursuant to this Section 5.8(a) shall change, limit or otherwise affect any of the representations, warranties, covenants or obligations of the Company contained in this Agreement or the Company Disclosure Schedule for purposes of Sections 6 and 7, as applicable.
55
(b) During the Pre-Closing Period, Parent shall promptly notify the Company (and, if in writing, furnish copies of) if any of the following occurs: (i) any notice or other communication is received from any Person alleging that the Consent of such Person is or may be required in connection with any of the Contemplated Transactions; (ii) any Legal Proceeding against or involving or otherwise affecting Parent is commenced, or, to the Knowledge of Parent, threatened against Parent or, to the Knowledge of Parent, any director or officer of Parent; (iii) Parent becomes aware of any inaccuracy in any representation or warranty made by it in this Agreement; or (iv) the failure of Parent to comply with any covenant or obligation of Parent or Merger Sub; in the case of (iii) and (iv) that could reasonably be expected to make the timely satisfaction of any of the conditions set forth in Sections 6 or 8, as applicable, impossible or materially less likely. No notification given to the Company pursuant to this Section 5.8(b) shall change, limit or otherwise affect any of the representations, warranties, covenants or obligations of Parent contained in this Agreement or the Parent Disclosure Schedule for purposes of Sections 6 and 8, as applicable.
5.9 Disclosure. The initial press release relating to this Agreement shall be a joint press release issued by the Company and Parent and thereafter, between the date of this Agreement and the earlier to occur of (i) the Closing Date and (ii) the termination of this Agreement in accordance with its terms, Parent and the Company shall consult with each other before issuing any further press release(s) or otherwise making any public statement or making any announcement to Parent Associates or Company Associates (to the extent not previously issued or made in accordance with this Agreement) with respect to the Contemplated Transactions and shall not issue any such press release, public statement or announcement to Parent Associates or Company Associates without the other Party’s written consent (which shall not be unreasonably withheld, conditioned or delayed). Notwithstanding the foregoing: (a) each Party may, without such consultation or consent, make any public statement in response to questions from the press, analysts, investors or those attending industry conferences, make internal announcements to employees and make disclosures in Parent SEC Documents, so long as such statements are consistent with previous press releases, public disclosures or public statements made jointly by the parties (or individually, if approved by the other Party) and (b) a Party may, without the prior consent of the other Party hereto but subject to giving advance notice to the other Party and allowing reasonable time for comment by the other Party, issue any such press release or make any such public announcement or statement as may be required by any Law.
5.10 Listing. Parent shall use its reasonable best efforts to prepare and submit to Nasdaq a notification form for the listing of the shares of Parent Common Stock Payment Shares and the Parent Common Stock to be issued upon conversion of the Parent Preferred Stock Payment Shares to be issued in connection with the Contemplated Transactions, and to cause such shares to be approved for listing (subject to official notice of issuance) (the “Nasdaq Listing Application”) and to cause such Nasdaq Listing Application to be conditionally approved prior to the Effective Time. The Parties will use reasonable best efforts to coordinate with respect to compliance with Nasdaq rules and regulations. Each Party will promptly inform the other Party of all verbal or written communications between Nasdaq and such Party or its representatives. Parent and the Company agree to evenly split all Nasdaq fees associated with the Nasdaq Listing Application. The Company will cooperate with Parent as reasonably requested by Parent with respect to the Nasdaq Listing Application and promptly furnish to Parent all information concerning the Company and its stockholders that may be required or reasonably requested in connection with any action contemplated by this Section 5.10.
56
5.11 Tax Matters.
(a) For United States federal income Tax purposes, (i) the Parties intend that the Merger qualify as a “reorganization” within the meaning of Section 368(a) of the Code (the “Intended Tax Treatment”), and (ii) this Agreement is intended to be, and is hereby adopted as, a “plan of reorganization” for purposes of Section 354 and 361 of the Code and Treasury Regulations Section 1.368-2(g) and 1.368-3(a), to which the Parent, Merger Sub and the Company are parties under Section 368(b) of the Code. The Parties shall treat and shall not take any tax reporting position inconsistent with the treatment of the Merger as a reorganization within the meaning of Section 368(a) of the Code for U.S. federal, state and other relevant Tax purposes, unless otherwise required pursuant to a “determination” within the meaning of Section 1313(a) of the Code.
(b) The Parties shall use their respective reasonable best efforts to cause the Merger to qualify, and will not take any action or cause any action to be taken which action would reasonably be expected to prevent the Merger from qualifying, for the Intended Tax Treatment.
(c) Parent shall use reasonable best efforts to deliver to Fenwick & West LLP (“Fenwick”) and Cooley LLP (“Cooley”) a “Tax Representation Letter,” dated as of the Closing Date and signed by an officer of Parent, containing representations of Parent and Merger Sub, substantially in the form attached hereto as Exhibit E-1, and the Company shall use reasonable best efforts to deliver to Fenwick and Cooley a “Tax Representation Letter,” dated as of the Closing Date and signed by an officer of the Company, containing representations of the Company, substantially in the form attached hereto as Exhibit E-2, in each case in order to enable Fenwick to render the opinion described in Section 5.11(d) of this Agreement and Cooley to render the opinion described in Section 5.11(e) of this Agreement.
(d) The Company shall use reasonable best efforts to obtain an opinion of Fenwick, substantially in the form attached hereto as Exhibit F-1, to the effect that the Merger will qualify for the Intended Tax Treatment, dated as of the Closing. In rendering such opinion, Fenwick may require and rely upon (and may incorporate by reference) reasonable and customary representations and covenants, including the Tax Representation Letters described in Section 5.11(c) of this Agreement.
(e) Parent shall use reasonable best efforts to obtain an opinion of Cooley, substantially in the form attached hereto as Exhibit F-2, to the effect that the Merger will qualify for the Intended Tax Treatment, dated as of the Closing. In rendering such opinion, Cooley may require and rely upon (and may incorporate by reference) reasonable and customary representations and covenants, including those contained in the Tax Representation Letters described in Section 5.11(c) of this Agreement.
5.12 Legends. Parent shall be entitled to place appropriate legends, including the legend noted in Section 5.20, on the book entries and/or certificates evidencing any shares of Parent Common Stock or Parent Convertible Preferred Stock to be received in the Merger by equity holders of the Company who may be considered “affiliates” of Parent for purposes of Rules 144 and 145 under the Securities Act reflecting the restrictions set forth in Rules 144 and 145 and to issue appropriate stop transfer instructions to the transfer agent for Parent Common Stock and Parent Convertible Preferred Stock.
5.13 Directors and Officers. The Parties shall use reasonable best efforts and take all necessary action so that immediately after the Effective Time, (a) the Parent Board is comprised of seven members, with three such members designated by Parent (one of whom shall be an independent board member acceptable to the Company, such acceptance not to be unreasonably withheld), and three such members designated by the Company (two of whom shall be independent board member acceptable to Parent, such acceptance not to be unreasonably withheld), and one nominee to be mutually selected by Parent and the Company as an independent board member following the Closing (with the understanding
57
that until such seat shall be filled it will be deemed vacant), and (b) the Persons listed in Exhibit G under the heading “Officers” are elected or appointed, as applicable, to the positions of officers of Parent and the Surviving Corporation, as set forth therein, to serve in such positions effective as of the Effective Time until successors are duly appointed and qualified in accordance with applicable Law. If any Person listed in Exhibit G is unable or unwilling to serve as a director or an officer, as the case may be, of Parent or the Surviving Corporation, as set forth therein, as of the Effective Time, the Parties shall mutually agree upon a successor. The Persons listed in Exhibit G under the heading “Board Designees – Parent” shall be Parent’s designees pursuant to clause (a) of this Section 5.13 (which list may be changed by Parent at any time prior to the Closing by written notice to the Company to include different board designees who are reasonably acceptable to the Company). The Persons listed in Exhibit G under the heading “Board Designees – Company” shall be the Company’s designees pursuant to clause (a) of this Section 5.13 (which list may be changed by the Company at any time prior to the Closing by written notice to Parent to include different board designees who are reasonably acceptable to Parent). All independent board members must qualify as “independent directors” under applicable SEC rules. All newly appointed directors pursuant to the foregoing shall be appointed to applicable “class” in Parent’s classified board structure as noted on such Exhibit G. Concurrently with the Closing, the newly constituted Parent Board shall ensure that the various committees of the Parent Board are constituted in the manner set forth on such Exhibit G.
5.14 Section 16 Matters. Prior to the Effective Time, Parent and the Company shall take all such steps as may be required (to the extent permitted under applicable Laws) to cause any acquisitions of Parent Common Stock, restricted stock awards to acquire Parent Common Stock and any Parent Stock Options to purchase Parent Common Stock in connection with the Contemplated Transactions, by each individual who is reasonably expected to become subject to the reporting requirements of Section 16(a) of the Exchange Act with respect to Parent, to be exempt under Rule 16b-3 promulgated under the Exchange Act. Promptly following the date of this Agreement and at least three (3) Business Days prior to the Closing Date, the Company shall furnish the following information to Parent for each individual who, immediately after the Effective Time, will become subject to the reporting requirements of Section 16(a) of the Exchange Act with respect to Parent: (a) the number of shares of Company Common Stock owned by such individual and expected to be exchanged for shares of Parent Common Stock pursuant to the Merger, and (b) the number of other derivative securities (if any) with respect to Company Common Stock owned by such individual and expected to be converted into shares of Parent Common Stock, restricted stock awards to acquire Parent Common Stock or derivative securities with respect to Parent Common Stock in connection with the Merger.
5.15 Cooperation. Each Party shall cooperate reasonably with the other Party and shall provide the other Party with such assistance as may be reasonably requested for the purpose of facilitating the performance by each Party of its respective obligations under this Agreement and to enable the combined entity to continue to meet its obligations following the Effective Time.
5.16 Closing Certificates.
(a) The Company will prepare and deliver to Parent at least two (2) Business Days prior to the Closing Date a certificate signed by the Chief Executive Officer of the Company in a form reasonably acceptable to Parent setting forth, as of immediately prior to the Effective Time, after giving effect to the conversion of the Company Convertible Notes contemplated by Section 6.7: (i) each holder of Company Common Stock and Company RSA Awards, (ii) such holder’s name and address; (iii) the number and type of Company Common Stock held and/or underlying the Company RSA Awards as of the immediately prior to the Effective Time for each such holder; and (iv) the number of Parent Common Stock Payment Shares or Parent Preferred Stock Payment Shares to be issued to such holder, or to underlie any Parent Option to be issued to such holder, pursuant to this Agreement in respect of the Company Common Stock or Company RSA Awards held by such holder as of immediately prior to the Effective Time (the “Allocation Certificate”).
58
(b) Parent will prepare and deliver to the Company at least two (2) Business Days prior to the Closing Date a certificate signed by the Chief Financial Officer of Parent in a form reasonably acceptable to the Company, setting forth, as of immediately prior to the Reference Date (i) each record holder of Parent Common Stock or Parent Options, and (ii) the number of shares of Parent Common Stock held and/or underlying the Parent Options for such record holder or holder of Parent Stock Options, as the case may be (the “Parent Outstanding Shares Certificate”).
5.17 Takeover Statutes. If any Takeover Statute is or may become applicable to the Contemplated Transactions, each of the Company, the Company Board, Parent and the Parent Board, as applicable, shall grant such approvals and take such actions as are necessary so that the Contemplated Transactions may be consummated as promptly as practicable on the terms contemplated by this Agreement and otherwise act to eliminate or minimize the effects of such statute or regulation on the Contemplated Transactions.
5.18 Stockholder Litigation. Parent shall keep the Company reasonably informed regarding and conduct and control the settlement and defense of any stockholder litigation against Parent or any of its directors relating to this Agreement or the Contemplated Transactions; provided that any settlement or other resolution of any such stockholder litigation agreed to by Parent after the Closing shall be approved in advance by a majority of the post-Closing Parent Board. Without limiting the foregoing, prior to the Closing, Parent shall give the Company the opportunity to consult with Parent in connection with the defense and settlement of any such stockholder litigation. Parent shall promptly advise the Company orally and in writing of the initiation of, and Parent shall keep the Company reasonably apprised of any material developments in connection with, any such stockholder litigation.
5.19 Parent Options. Each unexpired and unexercised Parent Option, whether vested or unvested, shall remain outstanding immediately after the Effective Time in accordance with its current terms; provided that the foregoing shall not affect any Parent Options that accelerate pursuant to their terms.
5.20 Private Placement. Each of the Company and Parent shall take all reasonably necessary action on its part such that the issuance of Parent Common Stock Payment Shares and Parent Preferred Stock Payment Shares pursuant to this Agreement constitutes a transaction exempt from registration under the Securities Act in compliance with Rule 506 of Regulation D promulgated thereunder. Each certificate representing Parent Common Stock Payment Shares and the Parent Preferred Stock Payment Shares comprising Merger Consideration shall, until such time that such shares are not so restricted under the Securities Act, bear a legend identical or similar in effect to the following legend (together with any other legend or legends required by applicable state securities Applicable Law or otherwise, if any):
“THE SHARES REPRESENTED BY THIS CERTIFICATE HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933 (THE “ACT”) AND MAY NOT BE OFFERED, SOLD OR OTHERWISE TRANSFERRED, ASSIGNED, PLEDGED OR HYPOTHECATED UNLESS REGISTERED UNDER THE ACT OR UNLESS AN EXEMPTION FROM THE REGISTRATION REQUIREMENTS OF THE ACT IS AVAILABLE.”
59
Section 6. CONDITIONS PRECEDENT TO OBLIGATIONS OF EACH PARTY
The obligations of each Party to effect the Merger and otherwise consummate the Contemplated Transactions to be consummated at the Closing are subject to the satisfaction or, to the extent permitted by applicable Law, the written waiver by each of the Parties, at or prior to the Closing, of each of the following conditions:
6.1 No Restraints. No temporary restraining order, preliminary or permanent injunction or other order preventing the consummation of the Contemplated Transactions shall have been issued by any court of competent jurisdiction or other Governmental Body of competent jurisdiction and remain in effect and there shall not be any Law which has the effect of making the consummation of the Contemplated Transactions illegal.
6.2 Stockholder Approval. The Company shall have obtained the Required Company Stockholder Vote.
6.3 Listing. The shares of Parent Common Stock to be issued in the Merger pursuant to this Agreement and the shares of Parent Common Stock to be issued in connection with the conversion of the Parent Convertible Preferred Stock issued pursuant to Sections 1.5 and 1.7, subject to prior receipt of Parent stockholder approval in respect of such conversion, shall have been approved for listing (subject to official notice of issuance) on Nasdaq as of the Closing.
6.4 Certificate of Designation. Parent shall have filed the Certificate of Designation with the Secretary of State of the State of Delaware.
Section 7. ADDITIONAL CONDITIONS PRECEDENT TO OBLIGATIONS OF PARENT AND MERGER SUB
The obligations of Parent and Merger Sub to effect the Merger and otherwise consummate the transactions to be consummated at the Closing are subject to the satisfaction or the written waiver by Parent, at or prior to the Closing, of each of the following conditions:
7.1 Accuracy of Representations. The Company Fundamental Representations shall have been true and correct in all respects as of the date of this Agreement and shall be true and correct in all respects on and as of the Closing Date with the same force and effect as if made on and as of such date (except to the extent such representations and warranties are specifically made as of a particular date, in which case such representations and warranties shall be true and correct in all respects as of such date and it being understood that, for purposes of determining the accuracy of such representations and warranties, any update of or modification to the Company Disclosure Schedule made or purported to have been made after the date of this Agreement shall be disregarded). The representations and warranties of the Company contained in this Agreement (other than the Company Fundamental Representations) shall have been true and correct in all respects as of the date of this Agreement and shall be true and correct in all respects on and as of the Closing Date with the same force and effect as if made on the Closing Date (except to the extent such representations and warranties are specifically made as of a particular date, in which case such representations and warranties shall be true and correct in all respects as of such date and it being understood that, for purposes of determining the accuracy of such representations and warranties, any update of or modification to the Company Disclosure Schedule made or purported to have been made after the date of this Agreement shall be disregarded), except in each case where the failure of such representations to be true and correct would not have a Company Material Adverse Effect.
60
7.2 Performance of Covenants. The Company shall have performed or complied with in all material respects all agreements and covenants required to be performed or complied with by it under this Agreement at or prior to the Effective Time.
7.3 Documents. Parent shall have received the following documents, each of which shall be in full force and effect:
(a) a certificate executed by the Chief Executive Officer or Chief Financial Officer of the Company certifying (i) that the conditions set forth in Sections 7.1, 7.2, and 7.5 have been duly satisfied and (ii) that the information set forth in the Allocation Certificate delivered by the Company in accordance with Section 5.16(a) is true and accurate in all respects as of the Closing Date;
(b) a written resignation, in a form reasonably satisfactory to Parent, dated as of the Closing Date and effective as of the Closing, executed by each of the directors of the Company listed in Section 8.3(b) of the Company Disclosure Schedule; and
(c) the Allocation Certificate.
7.4 FIRPTA Certificate. Parent shall have received (i) an original signed statement from the Company that the Company is not, and has not been at any time during the applicable period specified in Section 897(c)(1)(A)(ii) of the Code, a “United States real property holding corporation,” as defined in Section 897(c)(2) of the Code, conforming to the requirements of Treasury Regulations Section 1.1445-2(c)(3) and 1.897-2(h), and (ii) an original signed notice to be delivered to the IRS in accordance with the provisions of Treasury Regulations Section 1.897-2(h)(2), together with written authorization for Parent to deliver such notice to the IRS on behalf of the Company following the Closing, each dated as of the Closing Date, duly executed by an authorized officer of the Company, and in form and substance reasonably acceptable to Parent.
7.5 No Company Material Adverse Effect. Since the date of this Agreement, there shall not have occurred any Company Material Adverse Effect that is continuing.
7.6 Company Lock-Up Agreements. Parent shall have received the Lock-Up Agreements duly executed by each of the Company Signatories, each of which shall be in full force and effect.
Section 8. ADDITIONAL CONDITIONS PRECEDENT TO OBLIGATION OF THE COMPANY
The obligations of the Company to effect the Merger and otherwise consummate the transactions to be consummated at the Closing are subject to the satisfaction or the written waiver by the Company, at or prior to the Closing, of each of the following conditions:
8.1 Accuracy of Representations. The Parent Fundamental Representations shall have been true and correct in all respects as of the date of this Agreement and shall be true and correct in all respects on and as of the Closing Date with the same force and effect as if made on and as of such date (except to the extent such representations and warranties are specifically made as of a particular date, in which case such representations and warranties shall be true and correct in all respects as of such date and it being understood that, for purposes of determining the accuracy of such representations and warranties, any update of or modification to the Parent Disclosure Schedule made or purported to have been made after the date of this Agreement shall be disregarded). The representations and warranties of Parent and Merger Sub contained in this Agreement (other than the Parent Fundamental Representations) shall have been true
61
and correct in all respects as of the date of this Agreement and shall be true and correct in all respects on and as of the Closing Date with the same force and effect as if made on the Closing Date (except to the extent such representations and warranties are specifically made as of a particular date, in which case such representations and warranties shall be true and correct in all respects as of such date and it being understood that, for purposes of determining the accuracy of such representations and warranties, any update of or modification to the Parent Disclosure Schedule made or purported to have been made after the date of this Agreement shall be disregarded); except in each case where the failure of such representations to be true and correct would not have a Parent Material Adverse Effect.
8.2 Performance of Covenants. Parent and Merger Sub shall have performed or complied with in all material respects all of their agreements and covenants required to be performed or complied with by each of them under this Agreement at or prior to the Effective Time.
8.3 Documents. The Company shall have received the following documents, each of which shall be in full force and effect:
(a) a certificate executed by the Chief Executive Officer or Chief Financial Officer of Parent confirming that the conditions set forth in Sections 8.1, 8.2, and 8.4 have been duly satisfied;
(b) the Parent Outstanding Shares Certificate;
(c) a written resignation, in a form reasonably satisfactory to the Company, dated as of the Closing Date and effective as of the Closing, executed by each of the officers and directors of Parent who are not to continue as officers or directors, as the case may be, of Parent after the Closing pursuant to Section 5.13 hereof; and
(d) certified copies of the resolutions duly adopted by the Parent Board and in full force and effect as of the Closing authorizing the appointment of the directors and officers set forth on Exhibit G.
8.4 No Parent Material Adverse Effect. Since the date of this Agreement, there shall not have occurred any Parent Material Adverse Effect that is continuing.
8.5 Parent Lock-Up Agreements. The Company shall have received the Lock-Up Agreements duly executed by each of the Parent Signatories each of which shall be in full force and effect.
8.6 Company Employee Agreements. The Company shall have received the Company Employee Agreements duly executed by Parent and each Company Named Employee.
8.7 Retention Agreements. The Company shall have received Retention Agreements duly executed by Parent (or one of its wholly owned Subsidiaries) and each Parent Named Employee.
Section 9. TERMINATION
9.1 Termination. This Agreement may be terminated and the Contemplated Transactions may be abandoned at any time prior to the Effective Time (whether before or after adoption of this Agreement by the Company’s stockholders, unless otherwise specified below):
(a) by mutual written consent of Parent and the Company;
62
(b) by either Parent or the Company if the Contemplated Transactions shall not have been consummated by August 31, 2019 (the “End Date”); provided, however, that the right to terminate this Agreement under this Section 9.1(b) shall not be available to the Company, on the one hand, or to Parent, on the other hand, if such Party’s action or failure to act has been a principal cause of the failure of the Contemplated Transactions to occur on or before the End Date and such action or failure to act constitutes a breach of this Agreement;
(c) by either Parent or the Company if a court of competent jurisdiction or other Governmental Body shall have issued a final and nonappealable order, decree or ruling, or shall have taken any other action, having the effect of permanently restraining, enjoining or otherwise prohibiting the Contemplated Transactions;
(d) by Parent if the Company Stockholder Written Consent evidencing the Required Company Stockholder Vote shall not have been obtained within 24 hours of the time of the execution and delivery of this Agreement;
(e) by the Company, upon a breach of any representation, warranty, covenant or agreement set forth in this Agreement by Parent or Merger Sub or if any representation or warranty of Parent or Merger Sub shall have become inaccurate, in either case, such that the conditions set forth in Section 8.1 or Section 8.2 would not be satisfied as of the time of such breach or as of the time such representation or warranty shall have become inaccurate; provided that the Company is not then in material breach of any representation, warranty, covenant or agreement under this Agreement; provided, further, that if such inaccuracy in Parent’s or Merger Sub’s representations and warranties or breach by Parent or Merger Sub is curable by the End Date by Parent or Merger Sub, then this Agreement shall not terminate pursuant to this Section 9.1(e) as a result of such particular breach or inaccuracy until the expiration of the earlier of (i) a 30-day period commencing upon delivery of written notice from the Company to Parent or Merger Sub of such breach or inaccuracy and its intention to terminate pursuant to this Section 9.1(e) or (ii) three (3) Business Days before the End Date (it being understood that this Agreement shall not terminate pursuant to this Section 9.1(e) as a result of such particular breach or inaccuracy if such breach by Parent or Merger Sub is cured prior to such termination becoming effective); or
(f) by Parent, upon a breach of any representation, warranty, covenant or agreement set forth in this Agreement by the Company or if any representation or warranty of the Company shall have become inaccurate, in either case, such that the conditions set forth in Section 7.1 or Section 7.2 would not be satisfied as of the time of such breach or as of the time such representation or warranty shall have become inaccurate; provided that Parent is not then in material breach of any representation, warranty, covenant or agreement under this Agreement; provided, further, that if such inaccuracy in the Company’s representations and warranties or breach by the Company is curable by the End Date by the Company then this Agreement shall not terminate pursuant to this Section 9.1(f) as a result of such particular breach or inaccuracy until the expiration of the earlier of (i) a 30-day period commencing upon delivery of written notice from Parent to the Company of such breach or inaccuracy and its intention to terminate pursuant to this Section 9.1(f) and (ii) three (3) Business Days before the End Date (it being understood that this Agreement shall not terminate pursuant to this Section 9.1(f) as a result of such particular breach or inaccuracy if such breach by the Company is cured prior to such termination becoming effective).
9.2 Effect of Termination. In the event of the termination of this Agreement as provided in Section 9.1, this Agreement shall be of no further force or effect; provided, however, that (a) this Section 9.2, Section 9.3, Section 10 and the definitions of the defined terms in such Sections shall survive the termination of this Agreement and shall remain in full force and effect, (b) the termination of this Agreement and the provisions of Section 9.3 shall not relieve any Party of any liability for fraud or for any willful and material breach of any representation, warranty, covenant, obligation or other provision contained in this Agreement.
63
9.3 Expenses.
Except as otherwise expressly provided in this Agreement, all expenses incurred in connection with this Agreement and the Contemplated Transactions will be paid by the Party incurring such expenses.
Section 10. MISCELLANEOUS PROVISIONS
10.1 Non-Survival of Representations and Warranties. The representations and warranties of the Company, Parent and Merger Sub contained in this Agreement or any certificate or instrument delivered pursuant to this Agreement shall terminate at the Effective Time, and only the covenants that by their terms survive the Effective Time and this Section 10 shall survive the Effective Time.
10.2 Amendment. This Agreement may be amended with the approval of the respective boards of directors of the Company, Merger Sub and Parent at any time (whether before or after the adoption and approval of this Agreement by the Company’s stockholders); provided, however, that after any such approval of this Agreement by a Party’s stockholders, no amendment shall be made which by Law requires further approval of such stockholders without the further approval of such stockholders. This Agreement may not be amended except by an instrument in writing signed on behalf of each of the Company, Merger Sub and Parent.
10.3 Waiver.
(a) No failure on the part of any Party to exercise any power, right, privilege or remedy under this Agreement, and no delay on the part of any Party in exercising any power, right, privilege or remedy under this Agreement, shall operate as a waiver of such power, right, privilege or remedy; and no single or partial exercise of any such power, right, privilege or remedy shall preclude any other or further exercise thereof or of any other power, right, privilege or remedy.
(b) No Party shall be deemed to have waived any claim arising out of this Agreement, or any power, right, privilege or remedy under this Agreement, unless the waiver of such claim, power, right, privilege or remedy is expressly set forth in a written instrument duly executed and delivered on behalf of such Party and any such waiver shall not be applicable or have any effect except in the specific instance in which it is given.
10.4 Entire Agreement; Counterparts; Exchanges by Electronic Transmission. This Agreement and the other agreements referred to in this Agreement constitute the entire agreement and supersede all prior agreements and understandings, both written and oral, among or between any of the Parties with respect to the subject matter hereof and thereof; provided, however, that the Confidentiality Agreement shall not be superseded and shall remain in full force and effect in accordance with its terms. This Agreement may be executed in several counterparts, each of which shall be deemed an original and all of which shall constitute one and the same instrument. The exchange of a fully executed Agreement (in counterparts or otherwise) by all Parties by electronic transmission in .PDF format shall be sufficient to bind the Parties to the terms and conditions of this Agreement.
64
10.5 Applicable Law; Jurisdiction. This Agreement shall be governed by, and construed in accordance with, the Laws of the State of Delaware, regardless of the Laws that might otherwise govern under applicable principles of conflicts of laws. In any action or proceeding between any of the Parties arising out of or relating to this Agreement or any of the Contemplated Transactions, each of the Parties: (a) irrevocably and unconditionally consents and submits to the exclusive jurisdiction and venue of the Court of Chancery of the State of Delaware or, to the extent such court does not have subject matter jurisdiction, the United States District Court for the District of Delaware or, to the extent that neither of the foregoing courts has jurisdiction, the Superior Court of the State of Delaware; (b) agrees that all claims in respect of such action or proceeding shall be heard and determined exclusively in accordance with clause (a) of this Section 10.5; (c) waives any objection to laying venue in any such action or proceeding in such courts; (d) waives any objection that such courts are an inconvenient forum or do not have jurisdiction over any Party; (e) agrees that service of process upon such Party in any such action or proceeding shall be effective if notice is given in accordance with Section 10.8 of this Agreement; and (f) irrevocably and unconditionally waives the right to trial by jury.
10.6 Attorneys’ Fees. In any action at law or suit in equity to enforce this Agreement or the rights of any of the Parties, the prevailing Party in such action or suit (as determined by a court of competent jurisdiction) shall be entitled to recover its reasonable out-of-pocket attorneys’ fees and all other reasonable costs and expenses incurred in such action or suit.
10.7 Assignability. This Agreement shall be binding upon, and shall be enforceable by and inure solely to the benefit of, the Parties and their respective successors and permitted assigns; provided, however, that neither this Agreement nor any of a Party’s rights or obligations hereunder may be assigned or delegated by such Party without the prior written consent of the other Party, and any attempted assignment or delegation of this Agreement or any of such rights or obligations by such Party without the other Party’s prior written consent shall be void and of no effect.
10.8 Notices. All notices and other communications hereunder shall be in writing and shall be deemed to have been duly delivered and received hereunder (a) one Business Day after being sent for next Business Day delivery, fees prepaid, via a reputable international overnight courier service, (b) upon delivery in the case of delivery by hand, or (c) on the date delivered in the place of delivery if sent by email (with a written or electronic confirmation of delivery) prior to 5:00 p.m. Pacific Time, otherwise on the next succeeding Business Day, in each case to the intended recipient as set forth below:
if to Parent or Merger Sub:
Aquinox Pharmaceuticals, Inc.
450-887 Great Northern Way
Vancouver, British Columbia
Canada V5T 4TS
Attention: Kamran Alam, Chief Financial Officer
Email: [email protected]
with a copy to (which shall not constitute notice):
Cooley LLP
3175 Hanover Street
Palo Alto, CA 94304-1130
Attention: Michael E. Tenta
Email: [email protected]
65
if to the Company:
Neoleukin Therapeutics, Inc.
401 Terry Avenue North
Seattle, Washington 98109
Attention: Jonathan G. Drachman, Chief Executive Officer
Email: [email protected]
with a copy to (which shall not constitute notice):
Fenwick & West LLP
Silicon Valley Center
801 California Street
Mountain View, CA 94041
Attention: David K. Michaels
Email: [email protected]
10.9 Cooperation. Each Party agrees to cooperate fully with the other Party and to execute and deliver such further documents, certificates, agreements and instruments and to take such other actions as may be reasonably requested by the other Party to evidence or reflect the Contemplated Transactions and to carry out the intent and purposes of this Agreement.
10.10 Severability. Any term or provision of this Agreement that is invalid or unenforceable in any situation in any jurisdiction shall not affect the validity or enforceability of the remaining terms and provisions of this Agreement or the validity or enforceability of the offending term or provision in any other situation or in any other jurisdiction. If a final judgment of a court of competent jurisdiction declares that any term or provision of this Agreement is invalid or unenforceable, the Parties agree that the court making such determination shall have the power to limit such term or provision, to delete specific words or phrases or to replace such term or provision with a term or provision that is valid and enforceable and that comes closest to expressing the intention of the invalid or unenforceable term or provision, and this Agreement shall be valid and enforceable as so modified. In the event such court does not exercise the power granted to it in the prior sentence, the Parties agree to replace such invalid or unenforceable term or provision with a valid and enforceable term or provision that will achieve, to the extent possible, the economic, business and other purposes of such invalid or unenforceable term or provision.
10.11 Other Remedies; Specific Performance. Except as otherwise provided herein, any and all remedies herein expressly conferred upon a Party will be deemed cumulative with and not exclusive of any other remedy conferred hereby, or by law or equity upon such Party, and the exercise by a Party of any one remedy will not preclude the exercise of any other remedy. The Parties agree that irreparable damage for which monetary damages, even if available, would not be an adequate remedy, would occur in the event that any Party does not perform the provisions of this Agreement (including failing to take such actions as are required of it hereunder to consummate this Agreement) in accordance with its specified terms or otherwise breaches such provisions. Accordingly, the Parties acknowledge and agree that the Parties shall be entitled to an injunction, specific performance and other equitable relief to prevent breaches of this Agreement and to enforce specifically the terms and provisions hereof, in addition to any other remedy to which they are entitled at law or in equity. Each of the Parties agrees that it will not oppose the granting of an injunction, specific performance or other equitable relief on the basis that any other Party has an adequate remedy at law or that any award of specific performance is not an appropriate remedy for any reason at law or in equity. Any Party seeking an injunction or injunctions to prevent breaches of this Agreement shall not be required to provide any bond or other security in connection with any such order or injunction.
66
10.12 No Third Party Beneficiaries. Nothing in this Agreement, express or implied, is intended to or shall confer upon any Person (other than the Parties and the D&O Indemnified Parties to the extent of their respective rights pursuant to Section 5.5) any right, benefit or remedy of any nature whatsoever under or by reason of this Agreement.
10.13 Construction.
(a) References to “cash,” “dollars” or “$” are to U.S. dollars.
(b) For purposes of this Agreement, whenever the context requires: the singular number shall include the plural, and vice versa; the masculine gender shall include the feminine and neuter genders; the feminine gender shall include the masculine and neuter genders; and the neuter gender shall include masculine and feminine genders.
(c) The Parties have participated jointly in the negotiating and drafting of this Agreement and agree that any rule of construction to the effect that ambiguities are to be resolved against the drafting Party shall not be applied in the construction or interpretation of this Agreement, and no presumption or burden of proof shall arise favoring or disfavoring any Party by virtue of the authorship of any provision of this Agreement.
(d) As used in this Agreement, the words “include” and “including,” and variations thereof, shall not be deemed to be terms of limitation, but rather shall be deemed to be followed by the words “without limitation.”
(e) Except as otherwise indicated, all references in this Agreement to “Sections,” “Exhibits” and “Schedules” are intended to refer to Sections of this Agreement and Exhibits and Schedules to this Agreement, respectively.
(f) Any reference to legislation or to any provision of any legislation shall include any modification, amendment, re-enactment thereof, any legislative provision substituted therefore and all rules, regulations, and statutory instruments issued or related to such legislations.
(g) The bold-faced headings and table of contents contained in this Agreement are for convenience of reference only, shall not be deemed to be a part of this Agreement and shall not be referred to in connection with the construction or interpretation of this Agreement.
(h) The Parties agree that each of the Company Disclosure Schedule and the Parent Disclosure Schedule shall be arranged in sections and subsections corresponding to the numbered and lettered sections and subsections contained in this Agreement. The disclosures in any section or subsection of the Company Disclosure Schedule or the Parent Disclosure Schedule shall qualify other sections and subsections in this Agreement to the extent it is readily apparent on its face from a reading of the disclosure that such disclosure is applicable to such other sections and subsections.
(i) Each of “delivered” or “made available” means, with respect to any documentation, that prior to 11:59 p.m. (Pacific Time) on the date that is two Business Days prior to the date of this Agreement (i) a copy of such material has been posted to and made available by a Party to the other Party and its Representatives in the electronic data room maintained by such disclosing Party or (ii) such material is disclosed in the Parent SEC Documents filed with the SEC prior to the date hereof and publicly made available on the SEC’s Electronic Data Gathering Analysis and Retrieval system.
67
(j) Whenever the last day for the exercise of any privilege or the discharge of any duty hereunder shall fall upon a Saturday, Sunday, or any date on which banks in San Francisco, California are authorized or obligated by Law to be closed, the Party having such privilege or duty may exercise such privilege or discharge such duty on the next succeeding day which is a regular Business Day.
(Remainder of page intentionally left blank)
68
IN WITNESS WHEREOF, the Parties have caused this Agreement to be executed as of the date first above written.
| AQUINOX PHARMACEUTICALS, INC. | ||
| By: | /s/ David Main | |
| Name: | David J. Main | |
| Title: | President and Chief Executive Officer | |
| APOLLO SUB, INC. | ||
| By: | /s/ David Main | |
| Name: | David J. Main | |
| Title: | President and Chief Executive Officer | |
| NEOLEUKIN THERAPEUTICS, INC. | ||
| By: | /s/ Jonathan Drachman | |
| Name: | Jonathan G. Drachman, MD | |
| Title: | Chief Executive Officer and President |
EXHIBIT A
CERTAIN DEFINITIONS
a) For purposes of this Agreement (including this Exhibit A):
“Affiliate” of a Person means any other Person that directly or indirectly, through one or more intermediaries, controls, is controlled by, or is under common control with, such Person. The term “control” (including the terms “controlled by” and “under common control with”) means the possession, directly or indirectly, of the power to direct or cause the direction of the management and policies of a Person, whether through the ownership of voting securities, by contract or otherwise.
“Agreement” means the Agreement and Plan of Merger to which this Exhibit A is attached, as it may be amended from time to time.
“Business Day” means any day other than a Saturday, Sunday or other day on which banks in Vancouver, British Columbia or Seattle, Washington are authorized or obligated by Law to be closed.
“Certificate of Designation” means the Certificate of Designation of Preferences, Rights and Limitations of Parent Convertible Preferred Stock in the form attached hereto as Exhibit F.
“Code” means the Internal Revenue Code of 1986, as amended.
“Common Stock Exchange Ratio” means 0.3520299, subject to adjustment pursuant to Section 1.7(e).
“Company Affiliate” means any Person that is (or at any relevant time was) under common control with the Company within the meaning of Sections 414(b), (c), (m) and (o) of the Code, and the regulations issued thereunder.
“Company Associate” means any current or former employee, independent contractor, officer or director of the Company.
“Company Board” means the board of directors of the Company.
“Company Common Stock” means the Common Stock, $0.0001 par value per share, of the Company.
“Company Convertible Notes” shall mean those Convertible Promissory Notes issued by the Company pursuant to that certain Note Purchase Agreement, dated as of December 14, 2018, by and among the Company and each of the investors party thereto, each as amended by Amendment Number One thereto dated as of July 31, 2019.
“Company Contract” means any Contract: (a) to which the Company or any of its Subsidiaries is a Party; (b) by which the Company or any of its Subsidiaries or any Company IP or any other asset of the Company or its Subsidiaries is or may become bound or under which the Company or any of its Subsidiaries has, or may become subject to, any obligation; or (c) under which the Company or any of its Subsidiaries has or may acquire any right or interest.
A-1
“Company ERISA Affiliate” means any corporation or trade or business (whether or not incorporated) which is (or at any relevant time was) treated with the Company or any of its Subsidiaries as a single employer within the meaning of Section 414 of the Code.
“Company Fundamental Representations” means the representations and warranties of the Company set forth in Sections 2.1 (Due Organization; Subsidiaries), 2.3 (Authority; Binding Nature of Agreement), 2.6(a) and (c) (Capitalization) and 2.20 (No Financial Advisors).
“Company IP” means all Intellectual Property Rights that are owned or purported to be owned by the Company or its Subsidiaries.
“Company Material Adverse Effect” means any Effect that, considered together with all other Effects that have occurred prior to the date of determination of the occurrence of a Company Material Adverse Effect, has or would reasonably be expected to have a material adverse effect on the business, condition (financial or otherwise), assets, liabilities or results of operations of the Company or its Subsidiaries, taken as a whole; provided, however, that Effects arising or resulting from the following shall not be taken into account in determining whether there has been a Company Material Adverse Effect: (a) general business or economic conditions affecting the industry in which the Company and its Subsidiaries operate, (b) acts of war, armed hostilities or terrorism, (c) changes in financial, banking or securities markets, (d) the failure of the Company to meet internal or analysts’ expectations or projections or the results of operations of the Company, (e) any clinical trial programs or studies, including any adverse data, event or outcome arising out of or relating to any such programs or studies, (f) any change in, or any compliance with or action taken for the purpose of complying with, any Law or GAAP (or interpretations of any Law or GAAP), (g) resulting from the announcement of this Agreement or the pendency of the Contemplated Transactions, or (h) resulting from the taking of any action required to be taken by this Agreement; except in each case with respect to clauses (a) through (c), to the extent disproportionately affecting the Company and its Subsidiaries, taken as a whole, relative to other similarly situated companies in the industries in which the Company and its Subsidiaries operate.
“Company Unaudited Interim Balance Sheet” means the unaudited balance sheet of the Company for the period ended June 30, 2019 provided to Parent prior to the date of this Agreement.
“Confidentiality Agreement” means the Mutual Non-Disclosure Agreement, dated as of March 18, 2019, between the Company and Parent.
“Consent” means any approval, consent, ratification, permission, waiver or authorization (including any Governmental Authorization).
“Contemplated Transactions” means the Merger and the other transactions and actions contemplated by this Agreement to be consummated at or prior to the Closing (but not, for the avoidance of doubt, the actions proposed to be taken as the Parent Stockholders’ Meeting following the Closing pursuant to Section 5.2).
“Contract” means, with respect to any Person, any written or oral agreement, contract, subcontract, lease (whether for real or personal property), mortgage, license, sublicense or other legally binding commitment or undertaking of any nature to which such Person is a party or by which such Person or any of its assets are bound or affected under applicable Law.
“DGCL” means the General Corporation Law of the State of Delaware.
“Effect” means any effect, change, event, circumstance, or development.
A-2
“Encumbrance” means any lien, pledge, hypothecation, charge, mortgage, security interest, lease, license, option, easement, reservation, servitude, adverse title, claim, infringement, interference, option, right of first refusal, preemptive right, community property interest or restriction or encumbrance of any nature (including any restriction on the voting of any security, any restriction on the transfer of any security or other asset, any restriction on the receipt of any income derived from any asset, any restriction on the use of any asset and any restriction on the possession, exercise or transfer of any other attribute of ownership of any asset).
“Enforceability Exceptions” means the (a) Laws of general application relating to bankruptcy, insolvency and the relief of debtors; and (b) rules of law governing specific performance, injunctive relief and other equitable remedies.
“Entity” means any corporation (including any non-profit corporation), partnership (including any general partnership, limited partnership or limited liability partnership), joint venture, estate, trust, company (including any company limited by shares, limited liability company or joint stock company), firm, society or other enterprise, association, organization or entity, and each of its successors.
“Environmental Law” means any federal, state, local or foreign Law relating to pollution or protection of human health or the environment (including ambient air, surface water, ground water, land surface or subsurface strata), including any Law or regulation relating to emissions, discharges, releases or threatened releases of Hazardous Materials, or otherwise relating to the manufacture, processing, distribution, use, treatment, storage, disposal, transport or handling of Hazardous Materials.
“ERISA” means the Employee Retirement Income Security Act of 1974, as amended.
“Exchange Act” means the Securities Exchange Act of 1934.
“GAAP” means generally accepted accounting principles and practices in effect from time to time within the United States applied consistently throughout the period involved.
“Governmental Authorization” means any: (a) permit, license, certificate, franchise, permission, variance, exception, approval, exemption, order, clearance, registration, qualification or authorization issued, granted, given or otherwise made available by or under the authority of any Governmental Body or pursuant to any Law; or (b) right under any Contract with any Governmental Body.
“Governmental Body” means any: (a) nation, state, commonwealth, province, territory, county, municipality, district or other jurisdiction of any nature; (b) federal, state, local, municipal, foreign or other government; (c) governmental or quasi-governmental authority of any nature (including any governmental division, department, agency, commission, bureau, instrumentality, official, ministry, fund, foundation, center, organization, unit, body or Entity and any court or other tribunal, and for the avoidance of doubt, any taxing authority); or (d) self-regulatory organization (including Nasdaq).
“Hazardous Materials” means any pollutant, chemical, substance and any toxic, infectious, carcinogenic, reactive, corrosive, ignitable or flammable chemical, or chemical compound, or hazardous substance, material or waste, whether solid, liquid or gas, that is subject to regulation, control or remediation under any Environmental Law, including without limitation, crude oil or any fraction thereof, and petroleum products or by-products.
“Intellectual Property Rights” means and includes all past, present, and future rights of the following types, which may exist or be created under the laws of any jurisdiction in the world: (a) rights associated with works of authorship, including exclusive exploitation rights, copyrights, moral rights,
A-3
software, databases, and mask works; (b) trademarks, service marks, trade dress, logos, trade names and other source identifiers, domain names and URLs and similar rights and any goodwill associated therewith; (c) rights associated with trade secrets, know how, inventions, invention disclosures, methods, processes, protocols, specifications, techniques and other forms of technology; (d) patents and industrial property rights; and (e) other similar proprietary rights in intellectual property of every kind and nature; (f) rights of privacy and publicity; and (g) all registrations, renewals, extensions, statutory invention registrations, provisionals, continuations, continuations-in-part, provisionals, divisions, or reissues of, and applications for, any of the rights referred to in clauses “(a)” through “(f)” above (whether or not in tangible form and including all tangible embodiments of any of the foregoing, such as samples, studies and summaries), along with all rights to prosecute and perfect the same through administrative prosecution, registration, recordation or other administrative proceeding, and all causes of action and rights to sue or seek other remedies arising from or relating to the foregoing.
“IRS” means the United States Internal Revenue Service.
“Knowledge” means, with respect to an individual, that such individual is actually aware of the relevant fact or such individual would reasonably be expected to know such fact in the ordinary course of the performance of such individual’s employment responsibilities. Any Person that is an Entity shall have Knowledge if any officer or director of such Person as of the date such knowledge is imputed has Knowledge of such fact or other matter.
“Law” means any federal, state, national, foreign, material local or municipal or other law, statute, constitution, principle of common law, resolution, ordinance, code, edict, decree, rule, regulation, ruling or requirement issued, enacted, adopted, promulgated, implemented or otherwise put into effect by or under the authority of any Governmental Body (including under the authority of Nasdaq or the Financial Industry Regulatory Authority).
“Legal Proceeding” means any action, suit, litigation, arbitration, proceeding (including any civil, criminal, administrative, investigative or appellate proceeding), hearing, inquiry, audit, examination or investigation commenced, brought, conducted or heard by or before, or otherwise involving, any court or other Governmental Body or any arbitrator or arbitration panel.
“Merger Sub Board” means the board of directors of Merger Sub.
“Nasdaq” means the Nasdaq Stock Market, including the Nasdaq Global Select Market or such other Nasdaq market on which shares of Parent Common Stock are then listed.
“Ordinary Course of Business” means, in the case of each of the Company and Parent, such actions taken in the ordinary course of its normal operations and consistent with its past practices.
“Organizational Documents” means, with respect to any Person (other than an individual), (a) the certificate or articles of association or incorporation or organization or limited partnership or limited liability company, and any joint venture, limited liability company, operating or partnership agreement and other similar documents adopted or filed in connection with the creation, formation or organization of such Person and (b) all bylaws, regulations and similar documents or agreements relating to the organization or governance of such Person, in each case, as amended or supplemented.
“Parent Associate” means any current or former employee, independent contractor, officer or director of Parent.
A-4
“Parent Balance Sheet” means the unaudited balance sheet of Parent as of March 31, 2019 (the “Parent Balance Sheet Date”), included in Parent’s Report on Form 10-Q for the quarterly period ended March 31, 2019, as filed with the SEC.
“Parent Board” means the board of directors of Parent.
“Parent Closing Price” means the volume weighted average closing trading price of a share of Parent Common Stock on Nasdaq for the five consecutive trading days ending five trading days immediately prior to the date upon which the Merger becomes effective.
“Parent Common Stock” means the Common Stock, $0.000001 par value per share, of Parent.
“Parent Contract” means any Contract: (a) to which Parent or any of its Subsidiaries is a party; (b) by which Parent or any of its Subsidiaries or any Parent IP or any other asset of Parent or any of its Subsidiaries is or may become bound or under which Parent or any of its Subsidiaries has, or may become subject to, any obligation; or (c) under which Parent or any of its Subsidiaries has or may acquire any right or interest.
“Parent Convertible Preferred Stock” means Parent’s series A convertible preferred stock, par value $0.000001 per share, with the rights, preferences, powers and privileges specified in the Certificate of Designation.
“Parent ERISA Affiliate” means any corporation or trade or business (whether or not incorporated) which is (or at any relevant time was) treated with Parent or any of its Subsidiaries as a single employer within the meaning of Section 414 of the Code.
“Parent Fundamental Representations” means the representations and warranties of Parent and Merger Sub set forth in Sections 3.1(a) (Due Organization; Subsidiaries), 3.3 (Authority; Binding Nature of Agreement), 3.6(a) and (c) (Capitalization) and 3.21 (No Financial Advisors).
“Parent IP” means all Intellectual Property Rights that are owned or purported to be owned by Parent or its Subsidiaries.
“Parent Material Adverse Effect” means any Effect that, considered together with all other Effects that have occurred prior to the date of determination of the occurrence of a Parent Material Adverse Effect, has or would reasonably be expected to have a material adverse effect on the business, condition (financial or otherwise), assets, liabilities or results of operations of Parent; provided, however, that Effects arising or resulting from the following shall not be taken into account in determining whether there has been a Parent Material Adverse Effect: (a) general business or economic conditions affecting the industry in which Parent operates, (b) acts of war, armed hostilities or terrorism, (c) changes in financial, banking or securities markets, (d) the taking of any action required to be taken by this Agreement, (e) any change in the stock price or trading volume of Parent Common Stock (it being understood, however, that any Effect causing or contributing to any change in stock price or trading volume of Parent Common Stock may be taken into account in determining whether a Parent Material Adverse Effect has occurred, unless such Effects are otherwise excepted from this definition), (f) the failure of Parent to meet internal or analysts’ expectations or projections or the results of operations of Parent; (g) any clinical trial programs or studies, including any adverse data, event or outcome arising out of or related to any such programs or studies; (h) any change in, or any compliance with or action taken for the purpose of complying with, any Law or GAAP (or interpretations of any Law or GAAP); (i) resulting from the announcement of this Agreement or the pendency of the Contemplated Transactions; or (j) resulting from the taking of any action or the failure to take any action, by Parent that is required to be taken by this Agreement, except in each case with respect to clauses (a) through (c), to the extent disproportionately affecting Parent relative to other similarly situated companies in the industries in which Parent operates.
A-5
“Parent Options” means options or other rights to purchase shares of Parent Common Stock issued by Parent.
“Parent Stock Plan” means the Parent 2014 Equity Incentive Plan, as may be amended from time to time.
“Party” or “Parties” means the Company, Merger Sub and Parent.
“Permitted Encumbrance” means: (a) any liens for current Taxes not yet due and payable or for Taxes that are being contested in good faith and for which adequate reserves have been made on the Company Unaudited Interim Balance Sheet or the Parent Balance Sheet, as applicable; (b) minor liens that have arisen in the Ordinary Course of Business and that do not (in any case or in the aggregate) materially detract from the value of the assets or properties subject thereto or materially impair the operations of the Company or any of its Subsidiaries or Parent, as applicable; (c) statutory liens to secure obligations to landlords, lessors or renters under leases or rental agreements; (d) deposits or pledges made in connection with, or to secure payment of, workers’ compensation, unemployment insurance or similar programs mandated by Law; (e) non-exclusive licenses of Intellectual Property Rights granted by the Company or any of its Subsidiaries or Parent, as applicable, in the Ordinary Course of Business and that do not (in any case or in the aggregate) materially detract from the value of the Intellectual Property Rights subject thereto; and (f) statutory liens in favor of carriers, warehousemen, mechanics and materialmen, to secure claims for labor, materials or supplies.
“Person” means any individual, Entity or Governmental Body.
“Preferred Stock Exchange Ratio” means 0.007819, subject to adjustment pursuant to Section 1.7(e).
“Reference Date” means July 23, 2019.
“Registered IP” means all Intellectual Property Rights that are registered or issued under the authority of any Governmental Body, including all patents, registered copyrights, registered mask works, and registered trademarks, service marks and trade dress, and all applications for any of the foregoing.
“Representatives” means directors, officers, employees, agents, attorneys, accountants, investment bankers, advisors and representatives.
“Sarbanes-Oxley Act” means the Sarbanes-Oxley Act of 2002.
“SEC” means the United States Securities and Exchange Commission.
“Securities Act” means the Securities Act of 1933, as amended.
A-6
An entity shall be deemed to be a “Subsidiary” of a Person if such Person directly or indirectly owns or purports to own, beneficially or of record, (a) an amount of voting securities or other interests in such entity that is sufficient to enable such Person to elect at least a majority of the members of such entity’s board of directors or other governing body, or (b) at least 50% of the outstanding equity, voting, beneficial or financial interests in such Entity.
“Takeover Statute” means any “fair price,” “moratorium,” “control share acquisition” or other similar anti-takeover Law.
“Tax” means any federal, state, local, foreign or other tax, including any income, capital gain, gross receipts, capital stock, profits, transfer, estimated, registration, stamp, premium, escheat, unclaimed property, customs duty, ad valorem, occupancy, occupation, alternative, add-on, windfall profits, value added, severance, property, business, production, sales, use, license, excise, franchise, employment, payroll, social security, disability, unemployment, workers’ compensation, national health insurance, withholding or other taxes, duties, fees, assessments or governmental charges, surtaxes or deficiencies thereof in the nature of a tax, however denominated, and including any fine, penalty, addition to tax or interest imposed by a Governmental Body with respect thereto.
“Tax Return” means any return (including any information return), report, statement, declaration, estimate, schedule, notice, notification, form, election, certificate or other document, and any amendment or supplement to any of the foregoing, filed with or submitted to, or required to be filed with or submitted to, any Governmental Body in connection with the determination, assessment, collection or payment of any Tax or in connection with the administration, implementation or enforcement of or compliance with any Law relating to any Tax.
“Treasury Regulations” means the United States Treasury regulations promulgated under the Code.
“UW” means the University of Washington.
“UW Equity Right” means all rights of the UW to acquire up to 100,000 shares of Company Common Stock pursuant to that certain contemplated Neoleukin Therapeautics, Inc. Common Stock Donation Agreement, by and between the UW and the Company.
“WARN Act” means the Worker Adjustment Retraining and Notification Act of 1988, as amended, or any similar state or local plant closing mass layoff statute, rule or regulation.
A-7
b) Each of the following terms is defined in the Section set forth opposite such term:
| Term |
Section | |
| 280G Approval | 5.1(f) | |
| Allocation Certificate | 5.16(a) | |
| Anti-Bribery Laws | 2.22 | |
| Business Associate Agreement | 2.14(g) | |
| Certificate of Merger | 1.3 | |
| Certifications | 3.7(a) | |
| Closing | 1.3 | |
| Closing Date | 1.3 | |
| Company | Preamble | |
| Company Benefit Plan | 2.17(a) | |
| Company Disclosure Schedule | Section 2 | |
| Company Employee Agreement | Recitals | |
| Company Financials | 2.7(a) | |
| Company Foreign Plans | 2.17(m) | |
| Company In-bound Licenses | 2.12(d) | |
| Company Material Contract | 2.13(a) | |
| Company Named Employee | Recitals | |
| Company Out-bound Licenses | 2.12(d) | |
| Company Permits | 2.14(b) | |
| Company Plan | 2.6(c) | |
| Company Real Estate Leases | 2.11 | |
| Company RSA Awards | 2.6(c) | |
| Company Signatories | Recitals | |
| Company Stock Certificate | 1.8 | |
| Company Stockholder Matters | 5.1 | |
| Company Stockholder Written Consent | Recitals | |
| Continuing Employees | 5.4(a) | |
| Cooley | 5.10(c) | |
| Costs | 5.5(a) | |
| D&O Indemnified Parties | 5.5(a) | |
| Dissenting Shares | 1.10(a) | |
| Drug Regulatory Agency | 2.14(a) | |
| Effective Time | 1.3 | |
| Exchange Agent | 1.9(a) | |
| Exchange Fund | 1.9(a) | |
| FDA | 2.14(a) | |
| FDCA | 2.14(a) | |
| FLSA | 2.17(p) | |
| HIPAA | 2.14(g) | |
| Information Statement | 5.1(a) | |
| Intended Tax Treatment | 5.11(a) | |
| Investor Agreements | 2.21(b) | |
| Liability | 2.9 | |
| Lock-Up Agreement | Recitals | |
| Merger | Recitals | |
| Merger Consideration | 1.7(ii) | |
| Merger Sub | Preamble |
A-8
| Term |
Section | |
| Nasdaq Listing Application | 5.10 | |
| Other Parent Stockholder Matter | 5.2(a)(i) | |
| Parachute Payment Waiver | 5.1(e) | |
| Parent | Preamble | |
| Parent Benefit Plan | 3.17(a) | |
| Parent Disclosure Schedule | Section 3 | |
| Parent Foreign Plans | 3.17(m) | |
| Parent In-bound License | 3.12(d) | |
| Parent Material Contract | 3.13 | |
| Parent Named Employee | Recitals | |
| Parent Out-bound License | 3.12(d) | |
| Parent Permits | 3.14(b) | |
| Parent Real Estate Leases | 3.11 | |
| Parent SEC Documents | 3.7(a) | |
| Parent Stockholder Matters | 5.2(a)(iv) | |
| Parent Stockholder Support Agreement | Recitals | |
| Parent Stockholders’ Meeting | 5.2(a)(iv) | |
| Post-Closing Plans | 5.4(a) | |
| Required Company Stockholder Vote | 2.4 | |
| Retention Agreement | Recitals | |
| Retention Equity Awards | 5.4(b) | |
| Sensitive Data | 2.12(g) | |
| Stockholder Notice | 5.1(c) | |
| Surviving Corporation | 1.1 |
A-9
Exhibit 2.2
FORM OF STOCKHOLDER SUPPORT AGREEMENT
This Stockholder Support Agreement (this “Agreement”), dated as of August 5, 2019, is by and between Aquinox Pharmaceuticals, Inc., a Delaware corporation (“Parent”), Neoleukin Therapeutics, Inc., a Delaware corporation (the “Company”) and the undersigned (“Stockholder”).
A. Parent, the Company and Apollo Sub, Inc., a Delaware corporation and wholly-owned subsidiary of Parent (“Merger Sub”) are contemporaneously entering into an Agreement and Plan of Merger (as amended from time to time, the “Merger Agreement”), dated as of August 5, 2019, pursuant to which, among other things, the Merger Sub is to merge with and into the Company, with the Company surviving as a wholly-owned subsidiary of Parent (the “Merger”).
B. As of the date hereof, Stockholder is the Beneficial Owner (as defined below) of and has the sole power to vote (or to direct the voting of) that number of shares of common stock, par value $0.000001 per share, of Parent (“Parent Common Stock”) as set forth beside Stockholder’s name on Schedule A hereto.
C. Parent and the Company have required that Stockholder enter into this Agreement as a condition and inducement to the willingness of Parent and the Company to enter into the Merger Agreement and consummate the transactions contemplated thereby, transactions from which the Stockholder believes it will derive substantial benefits through its ownership interest in the combined company, the Stockholder is entering into this Agreement.
Accordingly, and in consideration of the foregoing and the mutual representations, warranties, covenants and agreements contained herein, the parties hereto, intending to be legally bound, hereby agree as follows:
ARTICLE 1
VOTING AGREEMENT
1.1 Agreement to Vote.
(a) From the date of this Agreement until the Expiration Date (as defined below), Stockholder will (and, if applicable, will cause any of its Affiliates who have the right to vote or direct the voting of any Subject Shares (as defined below) to) (i) appear at any meeting of stockholders or otherwise cause any Subject Shares to be counted as present thereat for purposes of calculating a quorum and (ii) vote (or consent pursuant to an action by written consent of the stockholders, if applicable) all of its Subject Shares (a) in favor of any proposal to adopt and approve or reapprove, or that would reasonably be expected to further, implement or carry into effect the purposes and intent of, the Parent Stockholder Matters and the Contemplated Transactions, and against (b) any action, proposal, transaction or agreement that, to the knowledge of the Stockholder, would reasonably be expected to result in a material breach of any covenant, representation or warranty or any other obligation or agreement of the Stockholder under this Agreement.
(b) Stockholder will not enter into any agreement with any Person (other than Parent) prior to the Expiration Date (with respect to periods prior to the Expiration Date) directly or indirectly to vote, grant any proxy or give instructions with respect to the voting of, the Subject Shares, the effect of which would be inconsistent with or violate any provision contained in this Section 1.1. Any vote or consent (or withholding of a vote or consent or otherwise abstaining from voting or consenting) by Stockholder that is not in accordance with this Section 1.1 will be considered null and void.
(c) The Parent may, in its sole discretion, waive the provisions of this Section 1.1 as to any matter brought to the stockholders of Parent for a vote (or consent pursuant to an action by written consent of the stockholders, if applicable).
(d) Notwithstanding anything in this Section 1 to the contrary, (i) Stockholder shall not be required to vote (or cause to be voted) any of its Subject Shares to: (I) amend the Merger Agreement (including any schedule or Exhibit thereto), or take any action that would reasonably be expected to result in the amendment or modification, or a waiver of a provision therein in a manner that (A) alters or changes the amount or kind of consideration to be paid to the Company’s stockholders in connection with the Merger, (B) adversely affects the tax consequences of the Merger to Stockholder or (C) impedes or delays the consummation of the Merger or (II) amend the terms of the terms of the Parent Convertible Preferred Stock (together, an “Adverse Amendment”) and (ii) Stockholder shall remain free to vote (or execute proxies with respect to) the Subject Shares with respect to any matter not covered by clauses (a) and (b) of Section 1.2(a)(ii) in any manner Stockholder deems appropriate.
1.2 Revocation of Proxies. Stockholder hereby represents and warrants that any proxies heretofore given in respect of the Subject Shares are not irrevocable, and Stockholder hereby revokes any and all prior proxies with respect to the Subject Shares. Prior to the Expiration Date, Stockholder will not directly or indirectly grant any proxies or powers of attorney (other than to Parent), deposit any of the Subject Shares into a voting trust or enter into a voting agreement (other than this Agreement) with respect to any of the Subject Shares.
ARTICLE 2
DEFINITIONS
Capitalized terms used but not defined in this Agreement are used in this Agreement with the meanings given to such terms in the Merger Agreement. In addition, for purposes of this Agreement:
“Affiliate” means, with respect to any specified Person, a Person who, at the time of determination, directly or indirectly through one or more intermediaries, controls, or is controlled by, or is under common control with, such specified Person. For purposes of this Agreement, with respect to Stockholder, “Affiliate” does not include Parent and the Persons that directly or indirectly through one or more intermediaries are controlled by Parent.
“Beneficially Owned” or “Beneficial Ownership” with respect to any securities means having beneficial ownership of such securities (as determined pursuant to Rule 13d-3 under the Exchange Act, disregarding the phrase “within 60 days” in paragraph (d)(1)(i) thereof), including pursuant to any agreement, arrangement or understanding, whether or not in writing. Without duplicative counting of the same securities, securities Beneficially Owned by a Person include securities Beneficially Owned by (i) all Affiliates of such Person, and (ii) all other Persons with whom such Person would constitute a “group” within the meaning of Section 13(d) of the Exchange Act and the rules promulgated thereunder.
“Beneficial Owner” with respect to any securities means a Person that has Beneficial Ownership of such securities.
“Person” has the meaning ascribed thereto in the Merger Agreement.
“Series A Certificate of Designation” means Parent’s Certificate of Designation of Preferences, Rights and Limitations of Series A Convertible Preferred Stock, dated as of the date hereof (as amended from time to time).
“Subject Shares” means, with respect to Stockholder, without duplication, and subject to the Lock-Up Agreement, the shares of Parent Common Stock held by Stockholder on the date of any Parent Stockholders’ Meeting (as defined herein), which as of the date hereof, are reflected on Schedule A.
ARTICLE 3
REPRESENTATIONS, WARRANTIES AND ADDITIONAL COVENANTS OF STOCKHOLDER
The Stockholder represents, warrants and covenants to Parent that:
3.1 Ownership. Stockholder is the sole Beneficial Owner and the record and legal owner of the Parent Common Stock identified on Schedule A, subject to Parent’s due authorization and valid issuance thereof, and such shares constitute all of the capital stock of Parent that are Beneficially Owned by Stockholder. Subject to Parent’s due authorization and valid issuance thereof, Stockholder has good and valid title to all of the Parent Common Stock, free and clear of all liens, claims, options, proxies, voting agreements and security interests and has the sole right to such Parent Common Stock and there are no restrictions on rights of disposition or other liens or encumbrances pertaining to such Parent Common Stock (other than pursuant to this Agreement and compliance with applicable securities laws) that could adversely affect the Merger, the Merger Agreement, or the exercise or fulfillment of the rights and obligations of Stockholder under this Agreement. None of the Parent Common Stock are subject to any voting trust or other contract with respect to the voting thereof, and no proxy, power of attorney or other authorization has been granted with respect to any of such Parent Common Stock
3.2 Authority and Non-Contravention.
(a) Stockholder is a natural person, corporation, limited partnership or limited liability company, duly organized, validly existing and in good standing under the laws of the jurisdiction in which it is incorporated, organized or constituted. Stockholder has all necessary power and authority to execute and deliver this Agreement, to perform its obligations hereunder
and to consummate the transactions contemplated hereby. The execution and delivery of this Agreement by Stockholder and the consummation by Stockholder of the transactions contemplated hereby have been duly and validly authorized by all necessary corporate action, and no other corporate proceedings on the part of Stockholder are necessary to authorize this Agreement or to consummate the transactions contemplated hereby.
(b) This Agreement has been duly and validly executed and delivered by Stockholder and constitutes the legal, valid and binding obligation of Stockholder, enforceable against Stockholder in accordance with its terms except (i) to the extent limited by applicable bankruptcy, insolvency or similar laws affecting creditors’ rights and (ii) the remedy of specific performance and injunctive and other forms of equitable relief may be subject to equitable defenses and to the discretion of the court before which any proceeding therefor may be brought.
(c) Stockholder is not nor will it be required to make any filing with or give any notice to, or to obtain any consent from, any Person in connection with the execution, delivery or performance of this Agreement or obtain any permit or approval from any government authority for any of the transactions contemplated hereby, except to the extent required by Section 13 or Section 16 of the Exchange Act and the rules promulgated thereunder.
(d) Neither the execution and delivery of this Agreement by Stockholder nor the consummation of the transactions contemplated hereby will directly or indirectly (whether with notice or lapse of time or both) (i) conflict with, result in any violation of or constitute a default by Stockholder under any mortgage, bond, indenture, agreement, instrument or obligation to which Stockholder is a party or by which it or any of the Parent Common Stock are bound, or violate any permit of any government authority, or any applicable law or order to which Stockholder, or any of the Parent Common Stock, may be subject, or (ii) result in the imposition or creation of any lien or encumbrance upon or with respect to any of the Parent Common Stock; except, in each case, for conflicts, violations, defaults or liens or encumbrances that would not individually or in the aggregate be reasonably expected to prevent or materially impair or delay the performance by the Stockholder of its obligations hereunder.
(e) Stockholder has requisite voting power and requisite power to issue instructions with respect to the matters set forth in Article 1 hereof and requisite power to agree to all of the matters set forth in this Agreement, in each case with respect to all of the Parent Common Stock, with no limitations, qualifications or restrictions on such rights, in each case, to the extent permitted by applicable law.
3.3 Total Shares. Except as set forth on Schedule A, Stockholder is not the Beneficial Owner of, and does not have (whether currently, upon lapse of time, following the satisfaction of any conditions, upon the occurrence of any event or any combination of the foregoing) any right to acquire, and has no other interest in or voting rights with respect to, any Parent Common Stock or any securities convertible into or exchangeable or exercisable for Parent Common Stock.
3.4 Reliance. Stockholder understands and acknowledges that Parent and the Company are each entering into the Merger Agreement in reliance upon Stockholder’s execution, delivery and performance of this Agreement.
3.5 No Litigation. As of the date of this Agreement, there is no Legal Proceeding pending or, to the knowledge of the Stockholder, threatened against the Stockholder that would reasonably be expected to impair the ability of the Stockholder to perform its obligations hereunder or consummate the transactions contemplated hereby
ARTICLE 4
REPRESENTATIONS, WARRANTIES AND COVENANTS OF PARENT
4.1 Certain Representations, Warranties and Covenants of Parent
Parent represents, warrants and covenants to Stockholder that this Agreement constitutes the legal, valid and binding obligation of Parent, enforceable against Parent in accordance with its terms, except (i) to the extent limited by applicable bankruptcy, insolvency or similar laws affecting creditors’ rights and (ii) the remedy of specific performance and injunctive and other forms of equitable relief may be subject to equitable defenses and to the discretion of the court before which any proceeding therefor may be brought. Parent has the corporate power and authority to execute and deliver this Agreement and to perform its obligations hereunder. The execution and delivery by Parent of this Agreement and the consummation by Parent of the transactions contemplated hereby have been duly and validly authorized by Parent and no other corporate proceedings on the part of Parent are necessary to authorize this Agreement or to consummate the transactions contemplated hereby. This Agreement has been duly and validly executed and delivered by Parent.
4.2 Parent Stockholders’ Meeting
(a) As promptly as practicable following the date hereof, Parent shall take all action necessary under applicable Law to call and give notice of a meeting of the holders of Parent Common Stock for the purpose of seeking:
(i) approval the issuance of shares of Parent Common Stock to the stockholders of the Company upon conversion of the Parent Preferred Stock Payment Shares in accordance with the terms of the Series A Certificate of Designation; and
(ii) approval of an amendment to Parent’s certificate of incorporation to increase the number of authorizes shares of Parent Common Stock to such amount as determined by the board of directors of Parent following the Closing (the matters contemplated by the Sections 4.2(a)(i) – (ii) are referred to as the “Parent Stockholder Matters,” and such meeting, the “Parent Stockholders’ Meeting”).
(b) Parent agrees to use reasonable best efforts to call and hold the Parent Stockholders’ Meeting as soon as practicable after the date hereof, and in any event on or before the date 100 days after the date hereof. If the approval of the Parent Stockholder Matters is not obtained at the Parent Stockholders’ Meeting, then Parent will use its reasonable best efforts to adjourn the Parent Stockholders’ Meeting one or more times to a date or dates no more than 30 days after the scheduled date for such meeting, and to obtain such approvals at such time. If the
Parent Stockholder Meeting is not so adjourned, and/or if the approval of the Parent Stockholder Matters is not then obtained, Parent will use its reasonable best efforts to obtain such approvals as soon as practicable thereafter, and in any event to obtain such approvals at the next occurring annual meeting of the stockholders of Parent or, if such annual meeting is not scheduled to be held within six months after the Parent Stockholders’ Meeting, a special meeting of the stockholders of Parent to be held within six months after the Parent Stockholders’ Meeting. Parent will hold an annual meeting or special meeting of its stockholders, at which a vote of the stockholders of Parent to approve the Parent Stockholder Matters will be solicited and taken, at least once every six months until Parent obtains approval of the Parent Stockholder Matters.
ARTICLE 5
TERM AND TERMINATION
This Agreement will become effective as of the date hereof. This Agreement will terminate upon the earlier to occur of (i) the approval of the Parent Stockholder Matters, (ii) the date of any Adverse Amendment, (iii) the date of termination of the Merger Agreement, or (vi) the mutual agreement of Parent and Stockholder to terminate this Agreement (any such date under clauses (i) through (iii) being referred to herein as the “Expiration Date”. The parties acknowledge that upon termination of this Agreement as permitted under and in accordance with the terms of this Article 5, no party to this Agreement shall have the right to recover any claim with respect to any losses suffered by such party in connection with such termination, except that the termination of this Agreement will not relieve Stockholder from any liability for any inaccuracy in or breach of any representation, warranty or covenant contained in this Agreement. Notwithstanding anything to the contrary herein, Article 6 of this Agreement will survive any termination of this Agreement.
ARTICLE 6
GENERAL PROVISIONS
6.1 Action in Stockholder Capacity Only. Stockholder is entering into this Agreement solely in Stockholder’s capacity as a record holder and beneficial owner, as applicable, of the Parent Common Stock and not in Stockholder’s capacity as a director or officer of the Company. Nothing herein will limit or affect Stockholder’s ability to act as an officer or director of the Company.
6.2 No Ownership Interest. Nothing contained in this Agreement will be deemed to vest in Parent or any of its Affiliates any direct or indirect ownership or incidents of ownership of or with respect to the Subject Shares.
6.3 Notices. All notices and other communications hereunder must be in writing and will be deemed given if delivered personally or by commercial messenger or courier service, or mailed by registered or certified mail (return receipt requested) or sent via facsimile (with acknowledgment of complete transmission) or e-mail to the parties at the following addresses (or at such other address for a party as specified by like notice or, if specifically provided for elsewhere in this Agreement, by email); provided, however, that notices sent by mail will not be deemed given until as follows:
if to Parent or Merger Sub, to
| Aquinox Pharmaceuticals, Inc. 450-887 Great Northern Way Vancouver, British Columbia Canada V5T 4TS Attention: Kamran Alam, Chief Financial Officer Email: [email protected] |
with a copy to (which shall not constitute notice) to:
| Cooley LLP 3175 Hanover Street Palo Alto, CA 94304-1130 Attention: Michael E. Tenta Email: [email protected] |
If to Stockholder, to Stockholder’s address set forth on Schedule A.
6.4 Publicity. Unless required by applicable law or permitted by the Merger Agreement, Stockholder will not, and will not authorize or direct any of its Affiliates or representatives to, make any press release or public announcement with respect to this Agreement or the Merger Agreement or the transactions contemplated hereby or thereby, without the prior written consent of Parent in each instance.
6.5 Further Actions. Upon the request of any party to this Agreement, the other party will (a) furnish to the requesting party any additional information, (b) execute and deliver, at their own expense, any other documents and (c) take any other actions as the requesting party may reasonably require to more effectively carry out the intent of this Agreement. Stockholder hereby agrees that Parent may publish and disclose in any filing made by Parent with the SEC, the Nasdaq Stock Market or other applicable regulatory authority, the Stockholder’s identity and ownership of any Parent Common Stock and the nature of such Stockholder’s commitments, arrangements, and understandings under this Agreement and may further file this Agreement as an exhibit to any other filing made by Parent with the SEC. Stockholder agrees to (x) provide any information reasonably requested by Parent for any such regulatory application or filing and (y) notify Parent promptly of any additional shares of capital stock of Parent of which Stockholder becomes the record or beneficial owner after the date of this Agreement.
6.6 Entire Agreement and Modification. This Agreement, and any other documents delivered by the parties in connection herewith constitute the entire agreement between the parties with respect to the subject matter hereof and supersede all prior agreements and understandings, both written and oral, between the parties with respect to its subject matter and constitute (along with the documents delivered pursuant to this Agreement) a complete and exclusive statement of the terms of the agreement between the parties with respect to its subject matter. This Agreement may not be amended, supplemented or otherwise modified except by a written document executed by the party against whose interest the modification will operate. The parties will not enter into any other agreement inconsistent with the terms and conditions of this Agreement, or that addresses any of the subject matters addressed in this Agreement.
6.7 Severability. Any provision of this Agreement which is prohibited or unenforceable in any jurisdiction will, as to such jurisdiction, be ineffective to the extent of such prohibition or unenforceability without affecting the validity or enforceability of the remaining provisions hereof. Any such prohibition or unenforceability in any jurisdiction will not invalidate or render unenforceable such provision in any other jurisdiction. If any provision of this Agreement is so broad as to be unenforceable, the provision will be interpreted to be only so broad as is enforceable.
6.8 No Third-Party Rights. Stockholder may not assign any of its rights or delegate any of its obligations under this Agreement without the prior written consent of Parent. This Agreement will apply to, be binding in all respects upon, and inure to the benefit of each of the respective successors, personal or legal representatives, heirs, distributes, devisees, legatees, executors, administrators and permitted assigns of Stockholder and the successors and permitted assigns of Parent. Nothing expressed or referred to in this Agreement will be construed to give any Person, other than the parties to this Agreement, any legal or equitable right, remedy or claim under or with respect to this Agreement or any provision of this Agreement except such rights as may inure to a successor or permitted assignee under this Section 6.8.
6.9 Enforcement of Agreement. Stockholder acknowledges and agrees that Parent could be damaged irreparably if any of the provisions of this Agreement are not performed in accordance with their specific terms and that any breach of this Agreement by Stockholder could not be adequately compensated by monetary damages. Accordingly, Stockholder agrees that, (a) it will waive, in any action for specific performance, the defense of adequacy of a remedy at law, and (b) in addition to any other right or remedy to which Parent may be entitled, at law or in equity, Parent will be entitled to enforce any provision of this Agreement by a decree of specific performance and to temporary, preliminary and permanent injunctive relief to prevent breaches or threatened breaches of any of the provisions of this Agreement, without posting any bond or other undertaking.
6.10 Waiver. The rights and remedies of the parties to this Agreement are cumulative and not alternative. Neither any failure nor any delay by a party in exercising any right, power or privilege under this Agreement, or any of the documents referred to in this Agreement will operate as a waiver of such right, power or privilege, and no single or partial exercise of any such right, power or privilege will preclude any other or further exercise of such right, power or privilege or the exercise of any other right, power or privilege. To the maximum extent permitted by applicable law, (a) no claim or right arising out of this Agreement, or any of the documents referred to in this Agreement can be discharged by one party, in whole or in part, by a waiver or renunciation of the claim or right unless in a written document signed by the other party, (b) no waiver that may be given by a party will be applicable except in the specific instance for which it is given, and (c) no notice to or demand on one party will be deemed to be a waiver of any obligation of that party or of the right of the party giving such notice or demand to take further action without notice or demand as provided in this Agreement, or the documents referred to in this Agreement.
6.11 Governing Law; Jurisdiction. This Agreement shall be governed by, and construed in accordance with, the laws of the State of Delaware, regardless of the laws that might otherwise govern under applicable principles of conflicts of laws. In any action or proceeding between any of the parties arising out of or relating to this Agreement, the Merger or any of the Contemplated Transactions, each of the parties: (a) irrevocably and unconditionally consents and submits to the exclusive jurisdiction and venue of the Court of Chancery of the State of Delaware or, to the extent such court does not have subject matter jurisdiction, the United States District Court for the District of Delaware or, to the extent that neither of the foregoing courts has jurisdiction, the Superior Court of the State of Delaware; (b) agrees that all claims in respect of such action or proceeding shall be heard and determined exclusively in accordance with clause (a) of this Section 6.11; (c) waives any objection to laying venue in any such action or proceeding in such courts; (d) waives any objection that such courts are an inconvenient forum or do not have jurisdiction over any party; (e) agrees that service of process upon such party in any such action or proceeding shall be effective if notice is given in accordance with Section 6.3 of this Agreement; and (f) irrevocably and unconditionally waives the right to trial by jury.
6.12 Counterparts. This Agreement may be executed in one or more counterparts, each of which will be deemed to be an original, but all of which, taken together, will constitute one and the same instrument. This Agreement may be executed by facsimile signature (including signatures in Adobe PDF or similar format).
6.13 Expenses. Except as otherwise provided in this Agreement, all costs and expenses incurred in connection with this Agreement and the transactions contemplated hereby will be paid by the party incurring such expenses.
6.14 Interpretation and Construction. When a reference is made in this Agreement to a Section, such reference is to a Section of this Agreement, unless otherwise indicated. The headings contained in this Agreement are for reference purposes only and will not affect in any way the meaning or interpretation of this Agreement. Whenever the words “include,” “includes” and “including” are used in this Agreement, they will be deemed to be followed by the words “without limitation.” The words “hereof, “herein” and “hereunder” and words of similar import when used in this Agreement will refer to this Agreement as a whole and not to any particular provision of this Agreement. The word “will” shall be construed to have the same meaning as the word “shall.” The words “dates hereof” will refer to the date of this Agreement. The word “or” is used in the inclusive sense of “and/or.” The terms “or,” “any” and “either” are not exclusive. When used herein, the words “to the extent” shall be deemed to be followed by the words “but only to the extent.” The definitions contained in this Agreement are applicable to the singular as well as the plural forms of such terms. Any agreement, instrument, law, rule or statute defined or referred to herein means, unless otherwise indicated, such agreement, instrument, law, rule or statute as from time to time amended, modified or supplemented. Each of the parties hereto acknowledges that it has been represented by counsel of its choice throughout all negotiations that have preceded the execution of this Agreement, and that it has executed the same with the advice of said independent counsel. Each party cooperated and participated in the drafting and preparation of this Agreement and the documents referred to herein, and any and all drafts relating thereto exchanged among the parties will be deemed the work product of all of the parties and may not be construed against any party by reason of its drafting or preparation. Accordingly, any rule of law or any legal decision that would require interpretation of any ambiguities in this Agreement against any party that drafted or prepared it is of no application and is hereby expressly waived by each of the parties hereto, and any controversy over interpretations of this Agreement will be decided without regards to events of drafting or preparation.
IN WITNESS WHEREOF, the parties hereto have caused this Stockholder Support Agreement to be duly executed as of the day and year first above written.
| PARENT: | AQUINOX PHARMACEUTICALS, INC. | |||
| By: |
| |||
| Name: David Main | ||||
| Title: Chief Executive Officer | ||||
| COMPANY: | NEOLEUKIN THERAPEUTICS, INC. | |||
| By: |
| |||
| Name: | ||||
| Title: | ||||
| STOCKHOLDER: | [ENTITY NAME] | |||
| By: |
| |||
| Name: Title: | ||||
| [NATURAL PERSON NAME] | ||||
|
| ||||
| Additional Signature (if held jointly): | ||||
|
| ||||
| (If held jointly) | ||||
|
| ||||
| (Printed Full Name) | ||||
SCHEDULE A
| NAME AND | PARENT COMMON STOCK | |
| ADDRESS OF STOCKHOLDER |
BENEFICIALLY OWNED | |
| [Name] |
[•] shares of common stock | |
| [Address 1] |
||
| [Address 2] |
||
| [E-mail] |
Exhibit 2.3
FORM OF LOCK-UP AGREEMENT
AUGUST 5, 2019
This Lock-Up Agreement (this “Agreement”) is executed in connection with the Agreement and Plan of Merger (the “Merger Agreement”) by and among Aquinox Pharmaceuticals, Inc., a Delaware corporation (the “Parent”), Apollo Sub, Inc., a Delaware corporation and wholly owned subsidiary of Parent (“Merger Sub”), and Neoleukin Therapeutics, Inc., a Delaware corporation (the “Company”), dated as of August 5, 2019. Capitalized terms used herein but not defined shall have the meanings ascribed to such terms in the Merger Agreement.
In connection with, and as an inducement to, the parties entering into the Merger Agreement and for other good and valuable consideration the receipt and sufficiency of which is hereby acknowledged, the undersigned, by executing this Agreement, agrees that, without the prior written consent of the Parent and the Company, during the period commencing on the date hereof and continuing until the end of the Lock-Up Period (as hereinafter defined), the undersigned will not: (1) offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, make any short sale or otherwise transfer or dispose of or lend, directly or indirectly, any shares of Common Stock, $0.000001 par value per share, of Parent (the “Parent Common Stock”) or any securities convertible into, exercisable or exchangeable for or that represent the right to receive Parent Common Stock (including without limitation, Parent Common Stock which may be deemed to be beneficially owned by the undersigned in accordance with the rules and regulations of the Securities and Exchange Commission and securities which may be issued upon exercise of a stock option or warrant) whether now owned or hereafter acquired (the “Securities”); (2) enter into any swap or other agreement that transfers, in whole or in part, any of the economic consequences of ownership of the Securities, whether any such transaction described in clause (1) or (2) above is to be settled by delivery of Parent Common Stock or such other securities, in cash or otherwise; (3) make any demand for or exercise any right with respect to, the registration of any Parent Common Stock or any security convertible into or exercisable or exchangeable for Parent Common Stock; (4) except for the Parent Stockholder Support Agreement, grant any proxies or powers of attorney with respect to any Securities, deposit any Securities into a voting trust or enter into a voting agreement or similar arrangement or commitment with respect to any Securities; or (5) publicly disclose the intention to do any of the foregoing (each of the foregoing restrictions, the “Lock- Up Restrictions”).
Notwithstanding the terms of the foregoing paragraph, this Agreement and the Lock-Up Restrictions shall automatically terminate and cease to be effective on the date that is the earlier to occur of (i) the date of approval of the Parent Stockholder Matters by the stockholders of Parent, (ii) November 13, 2019 (such date, as the same may be extended, the “Lock-Up Outside Date”), provided, however, that such date may be extended one time by 30 calendar days if: (1) despite Parent’s good faith efforts to obtain the approval of Parent’s stockholders to the Parent Stockholder Matters, Parent has not obtained such approval of the Parent Stockholders’ Matters by the original Lock-Up Outside Date, and (2) Parent determines in good faith (and confirms in writing to the undersigned that it has so determined in a certificate executed on behalf of the Company by either the Chief Executive Officer or the Chief Financial
Officer thereof) that there is a reasonable prospect for obtaining such approval during such 30-day extension period; provided, further, that in no event shall the Lock-Up Outside Date, as extended, be later than December 13, 2019, after which this agreement shall automatically terminate and cease to be effective, (iii) the date of any amendment to the Merger Agreement (including any schedule or Exhibit thereto), or any action that would reasonably be expected to result in the amendment or modification, or a waiver of a provision therein in a manner that (A) alters or changes the amount or kind of consideration to be paid to the Company’s stockholders in connection with the Merger, (B) adversely affects the tax consequences of the Merger to Stockholder or (C) impedes or delays the consummation of the Merger, (iv) the date of any amendment to the terms of the Parent Convertible Preferred Stock, (v) the date of termination of the Parent Stockholder Support Agreement to which the undersigned is a party, (vi) the date of termination of the Merger Agreement or (vii) the mutual agreement of Parent and the undersigned to terminate this Agreement. The period during which the Lock-Up Restrictions apply to the Securities shall be deemed the “Lock-Up Period” with respect thereto.
The undersigned agrees that the Lock-Up Restrictions preclude the undersigned from engaging in any hedging or other transaction with respect to any then-subject Securities which is designed to or which reasonably could be expected to lead to or result in a sale or disposition of such Securities even if such Securities would be disposed of by someone other than the undersigned. Such prohibited hedging or other transactions would include without limitation any short sale or any purchase, sale or grant of any right (including without limitation any put or call option) with respect to such Securities or with respect to any security that includes, relates to, or derives any significant part of its value from such Securities.
Notwithstanding the foregoing, the undersigned may transfer any of the Securities (i) as a bona fide gift or charitable contribution, (ii) to any trust for the direct or indirect benefit of the undersigned or the immediate family of the undersigned, (iii) if the undersigned is a corporation, partnership, limited liability company, trust or other business entity (1) to another corporation, partnership, limited liability company, trust or other business entity that is a direct or indirect affiliate (as defined in Rule 405 promulgated under the Securities Act of 1933, as amended) of the undersigned or (2) as distributions or dividends of shares of Parent Common Stock or any security convertible into or exercisable for Parent Common Stock to limited partners, limited liability company members or stockholders of the undersigned or holders of similar equity interests in the undersigned, (iv) if the undersigned is a trust, to the beneficiary of such trust, (v) by testate succession or intestate succession, (vi) to any immediate family member, any investment fund, family partnership, family limited liability company or other entity controlled or managed by the undersigned, (vii) to a nominee or custodian of a person or entity to whom a disposition or transfer would be permissible under clauses (i) through (vi), (viii) to Parent in a transaction exempt from Section 16(b) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) upon a vesting event of the Securities or upon the exercise of options to purchase Parent Common Stock on a “cashless” or “net exercise” basis or to cover tax withholding obligations of the undersigned in connection with such vesting or exercise (but for the avoidance of doubt, excluding all manners of exercise that would involve a sale in the open market of any securities relating to such options, whether to cover the applicable aggregate exercise price, withholding tax obligations or otherwise), (ix) to Parent in connection with the termination of employment or other termination of a service provider and pursuant to agreements in effect as of the Effective Time whereby Parent has the option to repurchase such shares or securities, (x) acquired by the undersigned in open market transactions after the Effective Time, (xi) pursuant to a bona fide third party tender offer, merger, consolidation, share purchase, business combination or other similar
2
transaction made to all holders of the Parent’s capital stock involving a change of control of the Parent, provided that in the event that such tender offer, merger, consolidation, share purchase, business combination or other such transaction is not completed, the Securities shall remain subject to the restrictions contained in this Agreement, or (xii) pursuant to an order of a court or regulatory agency; provided, in the case of clauses (i)-(vii), that (A) such transfer shall not involve a disposition for value and (B) the transferee agrees in writing with Parent to be bound by the terms of this Agreement; and provided, further, in the case of clauses (i)-(x), no filing by any party under Section 16(a) of the Exchange Act shall be required or shall be made voluntarily in connection with such transfer. For purposes of this Agreement, “immediate family” shall mean any relationship by blood, marriage or adoption, not more remote than first cousin.
In addition, the foregoing restrictions shall not apply to (i) the exercise of stock options granted pursuant to equity incentive plans existing immediately following the Effective Time, including the “net” exercise of such options in accordance with their terms and the surrender of Parent Common Stock in lieu of payment in cash of the exercise price and any tax withholding obligations due as a result of such exercise (but for the avoidance of doubt, excluding all manners of exercise that would involve a sale in the open market of any securities relating to such options, whether to cover the applicable aggregate exercise price, withholding tax obligations or otherwise); provided that it shall apply to any of the Securities issued upon such exercise, or (ii) the establishment of any contract, instruction or plan (a “Plan”) that satisfies all of the requirements of Rule 10b5-1(c)(1)(i)(B) under the Exchange Act; provided that no sales of the Securities shall be made pursuant to such a Plan prior to the expiration of the Lock-Up Period, and such a Plan may only be established if no public announcement of the establishment or existence thereof and no filing with the Securities and Exchange Commission or other regulatory authority in respect thereof or transactions thereunder or contemplated thereby, by the undersigned, Parent or any other person, shall be required, and no such announcement or filing is made voluntarily, by the undersigned, Parent or any other person, prior to the expiration of the applicable Lock-Up Period.
Any attempted transfer in violation of this Agreement will be of no effect and null and void, regardless of whether the purported transferee has any actual or constructive knowledge of the transfer restrictions set forth in this Agreement, and will not be recorded on the share register of Parent. In furtherance of the foregoing, Parent and its transfer agent and registrar are hereby authorized to decline to make any transfer of shares of Parent Common Stock if such transfer would constitute a violation or breach of this Agreement.
The undersigned hereby represents and warrants that the undersigned has full power and authority to enter into this Agreement and that upon request, the undersigned will execute any additional documents reasonably necessary to ensure the validity or enforcement of this Agreement. All authority herein conferred or agreed to be conferred and any obligations of the undersigned shall be binding upon the successors, assigns, heirs or personal representatives of the undersigned.
The undersigned understands that Parent, the Merger Sub and the Company are entering into the Merger Agreement in reliance upon this Agreement. The undersigned further understands that this letter agreement is irrevocable and shall be binding upon the undersigned’s heirs, legal representatives, successors and assigns.
3
This Agreement and any claim, controversy or dispute arising under or related to this Agreement shall be governed by, and construed in accordance with, the laws of the State of Delaware, without regard to the conflict of laws principles thereof.
(Signature Page Follows)
4
This Agreement, and any certificates, documents, instruments and writings that are delivered pursuant hereto, constitutes the entire agreement and understanding of the Parent, the Company and the undersigned in respect of the subject matter hereof and supersedes all prior understandings, agreements or representations by or among the Parent, the Company and the undersigned, written or oral, to the extent they relate in any way to the subject matter hereof. The delivery of a fully executed Agreement (in counterparts or otherwise) by the undersigned by facsimile or electronic transmission in .pdf format shall be sufficient to bind the undersigned to the terms and conditions of this Agreement.
| Very truly yours, | ||
|
| ||
| Printed Name of Holder | ||
| By: |
| |
| Signature | ||
|
| ||
| Printed Name of Person Signing | ||
| (and indicate capacity of person signing if signing as custodian, trustee, or on behalf of an entity) | ||
Exhibit 10.1
SEPARATION AGREEMENT AND RELEASE
THIS AGREEMENT made as of August 5, 2019
BETWEEN:
AQUINOX PHARMACEUTICALS (CANADA) INC. of 450-887 Great Northern Way, Vancouver, BC, V5T 4T5
(“Aquinox” or the “Company”)
AND:
DAVID MAIN
(“Main”)
WHEREAS:
| A. | Main is employed pursuant to an employment agreement dated January 1, 2014, and amended on November 6, 2018 (the “Employment Agreement”); |
| B. | The Company is merging with Neoleukin Therapeutics Inc. on or around August 8, 2019 (the “Transaction”). As a result of this restructuring, Main’s employment with the Company will terminate; and |
| C. | The parties wish to resolve all matters relating to Main’s employment and departure from the Company. |
IN CONSIDERATION of the conditions set out herein and other good and valuable consideration, the receipt and sufficiency of which is hereby expressly acknowledged by each of the Parties hereto, the Parties agree as follows:
| 1. | Main will resign from all Director, Officer and employee positions held with the Company and any related or associated entities effective August 8, 2019 (the “Date of Separation”). For greater clarity, Main’s employment with the Company will end on the Date of Separation. |
| 2. | The Company will continue to compensate Main as required under the Employment Agreement up to the Date of Separation. |
| 3. | The Company will pay Main any accrued and unpaid vacation earned up to the Date of Separation. |
| 4. | The parties agree that the Company will provide Main with: |
| (a) | a lump sum payment equivalent to eighteen (18) months’ of his Base Salary (as defined in the Employment Agreement) in effect at the time of the Date of Separation; |
| (b) | a lump sum payment equivalent to eighteen (18) months’ bonus pay, that will be calculated and based on the bonus payment average from the previous three (3) years from the Date of Separation; and |
| (c) | continuance of health and dental benefits coverage under an individual extended health benefits plan paid for by the Company, subject to the terms of the plan, for eighteen (18) months from the Date of Separation (the “Notice Period”), or until Main obtains other benefit coverage, whichever occurs first. In the event that the plan does not provide continuation of coverage after termination of Main’s employment, the Company will provide, or reimburse him for the cost of, equivalent replacement coverage until the earlier of Main obtaining coverage from a new employer or the expiry of the Notice Period; and |
| (d) | a lump sum payment of $2,000 CDN on the Date of Separation to cover the differential cost in coverage amounts between the group health benefits program which Main may have participated in previously with the Company, and the individual health benefits plan. |
| 5. | The Company will also provide Main with, and cover the cost of, U.S. and Canadian tax filing support services for the 2019 tax year. |
| 6. | All unvested options will immediately vest and will remain exercisable for a period of ninety (90) days following the Date of Separation, at which time any vested but unexercised options will expire and be forfeit, with the exception of the August 2018 Option Grant which provides for three (3) years to exercise from the Date of Separation. |
| 7. | Additionally, the Company will provide Main with a transaction bonus of $401,250 USD equivalent to 1.5x his annual target bonus upon close of the Transaction. |
| 8. | Main shall execute the Full and Final Release attached as Appendix “A”, which releases the Company, including its respective parent, subsidiaries, affiliates and its respective employees, officers, directors, servants, agents, and their successors and assigns from any and all claims related to Main’s employment of any nature whatsoever. |
| 9. | Main will immediately return any and all material, equipment, credit cards, identification cards, keys, documentation, etc. belonging to the Company in his or her possession or control as requested by the Company. |
| 10. | Main acknowledges and agrees to comply with the obligations around confidential information contained in Article 3 of the Employment Agreement. |
- 2 -
| 11. | Main agrees that the terms of this Agreement shall not be disclosed, in who or in part, to any person, except his spouse, and legal and financial advisor(s), or as otherwise required by law. |
| 12. | Main agrees and acknowledges that this Agreement and Release are governed by the laws of Canada and British Columbia. |
| 13. | Main expressly acknowledges that the content, terms and effect of this Agreement are fully understood and that he has had the opportunity to obtain independent legal counsel to review the contents of this Agreement. Main confirms that he is executing this Agreement voluntarily and without duress. |
| 14. | This Agreement may be executed in counterpart and each counterpart when executed shall have the efficacy of a signed original. Photographic copies of such signed counterparts may be used in lieu of the originals for any purpose. |
[Signature Page Follows]
- 3 -
IN WITNESS WHEREOF the Parties have executed this Separation Agreement as of the date appearing above.
| SIGNED, SEALED AND DELIVERED by DAVID MAIN in the presence of:
/s/ Darcie Stuart |
|
) ) ) ) |
|
/s/ David Main | ||||
| Witness Darcie Stuart |
|
) ) |
|
DAVID MAIN | ||||
| Name 450-887 Great Northern Way |
|
) ) |
|
|||||
| Address Vancouver, BC VC3 549 |
|
) ) |
|
|||||
| AQUINOX PHARMACEUTICALS (CANADA) INC. |
|
) ) |
|
|||||
| Per: |
/s/ Kamran Alam |
|
) ) ) |
|
||||
| Authorized Signatory |
) | |||||||
[Signature Page to Separation Agreement]
FULL AND FINAL RELEASE
(“Release”)
I, DAVID MAIN, in consideration of the payment set forth in the attached Separation Agreement dated August 5, 2019 (the “Consideration”), the receipt and sufficiency of which is hereby acknowledged, agree:
1. I hereby release and forever discharge AQUINOX PHARMACEUTICALS (CANADA) INC. (the “Company”), and their successors, assigns, directors, officers, servants, employees, any affiliated companies and agents, (collectively referred to as the “Releasees”) from any and all proceedings, claims, demands, any and all actions, causes of action, claims, suits, debts, contracts, complaints, damages, interest, costs, expenses and compensation of whatsoever kind and howsoever arising, whether in law or in equity, contract or tort law, whether known or unknown which I have or may have now or in the future against the Releasee (except for the requirement to provide the Consideration), which arise out of or are in any manner whatsoever, directly or indirectly connected to either or both of my employment and the cessation of such employment with the Company, including without limitation any claim or rights under the Employment Standards Act (BC) and the Human Rights Code (BC), any claims for severance pay or pay in lieu of notice of termination, any outstanding wages, bonuses, salary, overtime pay, any vacation pay, holiday pay, pension or other employment benefit entitlements, including long and short term disability benefits, life insurance, claims for physical or mental injury or loss of dignity and any other type of damages, or claims for benefits.
2. I agree not to initiate any proceeding or claim against the Releasees in respect of any matters which are the subject matter of this Release. I agree that, if I violate this covenant not to sue, I will pay all costs and expenses of defending against the proceeding or claim incurred by the Releasee, including lawyers’ reasonable fees and disbursements.
3. I will not take any action detrimental to the Releasees, nor disparage the Releasees to any third party.
4. In my employment with the Company I had obligations to protect the Company’s’ confidential information. I agree that such obligations survive the termination of my employment and that the Releasees will suffer irreparable harm if the Company’s confidential information is disclosed or used by me other than strictly for the benefit of the Company or without their express written authorization.
5. The Company will withhold income tax and other statutory deductions from the Consideration and I will indemnify and hold harmless the Releasees from any liability for tax, penalty, interest or any other amount of any kind arising under statutory authority including but not limited to Canada Pension Plan and Employment Insurance obligations and income tax obligations as required by the Canada Revenue Agency with respect to the Consideration, my employment with the Company, or its termination.
6. It is a condition of this Release that the circumstances leading to the termination of my employment and the terms of this Release are strictly confidential and shall not be disclosed by me, except as required by law or to my spouse or partner, financial or legal advisors, all of whom shall be advised on the confidential nature of the terms and be required to maintain confidentiality over the terms.
7. The provision of the Consideration shall not be deemed nor construed as an admission of liability on the part of the Releasee, by whom liability is expressly denied.
I represent and warrant that:
| (a) | I have returned all property of the Company including all originals or copies of the Company’s records, files, financial documents, client list, or any other material that in any way touches upon the business, customers, personnel or operations of the Company. |
| (b) | I have read and fully understand the contents, terms and effect of this Release. The Consideration is accepted voluntarily for the purpose of making a full and final settlement of all claims that I have or may have against the Company and that I have had the opportunity to obtain independent legal advice as to its terms and I acknowledge that the Company relies on this representation and declaration. |
| (c) | I have been advised that my eligibility for coverage for benefits paid for by the Company will cease, subject to the terms of the benefits plans, at the end of Notice Period, or until I obtain other benefit coverage, whichever occurs first. |
This Release will be governed by and construed and interpreted in accordance with the laws of the Province of British Columbia without regard to principles of conflict of laws.
Should any provision of this Release be declared or determined by any court of competent jurisdiction to be illegal, invalid or unenforceable, all remaining provisions of this Release shall otherwise remain in full force and effect and be construed as if said illegal, invalid or unenforceable provision had not been included herein.
[Signature Page Follows]
IN WITNESS WHEREOF I have set my hand this 5th day of August 2019.
| /s/ Darcie Stuart | ) | |||
| Witness Signature
Darcie Stuart |
) )
) |
|||
| Witness Name
Director, Contracts & Legal |
) ) ) |
/s/ David Main | ||
| Occupation | ) | DAVID MAIN | ||
| 450-887 Great Northern Way | ) ) |
|||
| Address
Vancouver, BC VC3 549 |
) ) ) |
[Signature Page to Full and Final Release]
Exhibit 10.2
EXECUTIVE EMPLOYMENT AGREEMENT
for
JONATHAN G. DRACHMAN, MD
This Executive Employment Agreement (the “Agreement”), made between Aquinox Pharmaceuticals, Inc., a Delaware corporation (the “Company”), and Jonathan G. Drachman, MD (the “Executive” and, collectively with the Company, the “Parties”), is entered into as of August 5, 2019, to be effective as of the Effective Date (as defined below).
WHEREAS, Executive is the Chief Executive Officer of Neoleukin Therapeutics, Inc. (“Neoleukin”);
WHEREAS, the Company is entering into an Agreement and Plan of Merger with Neoleukin (the “Merger Agreement”), pursuant to which the Company will acquire Neoleukin pursuant to a merger (the “Merger”) of a wholly owned subsidiary of the Company with and into Neoleukin, with Neoleukin surviving the Merger;
WHEREAS, the Company desires for Executive to provide services to the Company following the closing of the Merger (the “Closing”, and the date of the closing, the “Effective Date”), and wishes to provide Executive with certain compensation and benefits in return for such employment services; and
WHEREAS, Executive wishes to be employed by the Company and to provide personal services to the Company in return for certain compensation and benefits.
NOW, THEREFORE, in consideration of the mutual promises and covenants contained herein and for other good and valuable consideration, the receipt and sufficiency of which is hereby acknowledged, the Parties hereto agree as follows:
1. Employment by the Company.
1.1 Employment. This Agreement shall govern the terms of Executive’s employment with the Company, effective as of the Effective Date; provided that this Agreement shall automatically terminate and shall have no force or effect if the Merger Agreement is terminated in accordance with its terms and the Closing does not occur.
1.2 Position. Executive shall serve as the Company’s Chief Executive Officer. During the term of Executive’s employment with the Company, Executive will devote Executive’s best efforts and substantially all of Executive’s business time and attention to the business of the Company.
1.2 Duties and Location. Executive shall perform such duties as are typically performed by a Chief Executive Officer. Executive will report to the Company’s Board of Directors. Executive’s primary office location shall be the Company’s office located in Seattle, Washington.
1.
1.3 Policies and Procedures. The employment relationship between the Parties shall be governed by the general employment policies and practices of the Company, except that when the terms of this Agreement differ from or are in conflict with the Company’s general employment policies or practices, this Agreement shall control.
2. Compensation.
2.1 Salary. For services to be rendered hereunder, Executive shall receive a base salary at the rate of Three Hundred and Fifty Thousand U.S. Dollars ($350,000) per year (such base salary, as may be increased (but decreased) from time to time, the “Base Salary”), subject to standard payroll deductions and withholdings and payable in accordance with the Company’s regular payroll schedule.
2.2 Bonus. Executive will be eligible for an annual discretionary bonus of up to 50% of Executive’s Base Salary (the “Annual Bonus”). Whether Executive receives an Annual Bonus for any given year, and the amount of any such Annual Bonus, will be determined by the Company’s Board of Directors (the “Board”) in its sole discretion based upon the Company’s and Executive’s achievement of objectives and milestones to be determined on an annual basis by the Board. Annual Bonuses are typically paid no later than March 15th of the year following the applicable bonus year. Executive will not be eligible for, and will not earn, any Annual Bonus (including a prorated bonus) if Executive’s employment terminates for any reason before any Annual Bonus is paid, except as otherwise expressly provided in Section 6.2 or Section 6.3 below.
3. Standard Company Benefits. Executive shall be entitled to participate in all employee benefit programs for which Executive is eligible under the terms and conditions of the benefit plans that may be in effect from time to time and provided by the Company to its employees.
4. Expenses. The Company will reimburse Executive for reasonable travel, entertainment or other expenses incurred by Executive in furtherance or in connection with the performance of Executive’s duties hereunder, in accordance with the Company’s expense reimbursement policy as in effect from time to time.
5. Equity. On or as soon as practicable following the Effective Date, as an inducement to enter into this Agreement, the Company will grant Executive an option (the “Stock Option”) to purchase 1,650,000 shares of the Company’s common stock with a per-share exercise price equal to the fair market value of a share of the Company’s common stock on the date of grant, as determined by the Board. 1/4 of the shares underlying the Stock Option will vest and become exercisable on the one-year anniversary of the grant date, and 1/48th of the shares underlying the Stock Option will vest and become exercisable on a monthly basis thereafter, such that 100% of the shares underlying the Stock Option shall be vested and exercisable as of the four-year anniversary of the grant date, in each case so long as Executive remains employed by the Company through each applicable vesting date. The Stock Option will be subject to terms and conditions consistent with those provided in the Company’s 2014 Equity Incentive Plan, and will be governed in all respects by the terms of the stock option agreement to be entered into between Executive and the Company. Further details regarding the Stock Option will be provided to Executive upon approval of such grant by the Board.
2.
6. Termination of Employment; Severance.
6.1 At-Will Employment. Executive’s employment relationship is at-will. Either Executive or the Company may terminate the employment relationship at any time, with or without Cause or advance notice. In the event Executive’s employment relationship is terminated for any reason, Executive shall be entitled to receive Executive’s earned but unpaid Base Salary, unreimbursed business expenses properly incurred by Executive pursuant to Section 4 and any other compensation or benefit earned by or owed to (but not yet paid to) Executive through and including the date of termination, payable in a lump sum on the next regularly scheduled payroll date following the date on which Executive’s employment terminated, or at such other date as shall be specified under the terms of the employee benefit plan pursuant to which such compensation or benefit is payable.
6.2 Severance Benefits for Termination Without Cause or Resignation with Good Reason Unrelated to a Change of Control. In the event Executive’s employment with the Company is terminated by the Company without Cause or Executive resigns for Good Reason prior to a Change of Control (as defined below) or more than twelve (12) months following a Change of Control, provided that Executive remains in compliance with the terms of this Agreement and subject to Section 7 below, the Company shall provide Executive with the following severance benefits:
(i) The Company shall pay Executive, as severance, the equivalent of nine (9) months of Executive’s Base Salary in effect as of the date of Executive’s employment termination. This severance will be paid in the form of salary continuation, payable on the Company’s regular payroll dates, subject to standard payroll deductions and withholdings, starting on the 60th day after Executive’s termination date, with the first payment to include those payments that would have occurred earlier but for the 60-day delay.
(ii) Provided that Executive is then eligible for and timely elects continued coverage under COBRA, the Company shall pay Executive’s COBRA premiums to continue Executive’s coverage (including coverage for eligible dependents, if applicable) through the period starting on Executive’s termination date and ending on the earliest to occur of: (a) nine (9) months following Executive’s termination date; (b) the date Executive becomes eligible for group health insurance coverage through a new employer; or (c) the date Executive ceases to be eligible for COBRA continuation coverage for any reason, including plan termination. In the event Executive becomes covered under another employer’s group health plan or otherwise ceases to be eligible for COBRA during this time period, Executive must immediately notify the Company of such event. Notwithstanding the foregoing, if the Company determines, in its sole discretion, that it cannot pay the COBRA premiums without a substantial risk of violating applicable law, the Company instead shall pay to Executive, on the first day of each calendar month, a fully taxable cash payment equal to the applicable COBRA premiums for that month, subject to applicable tax withholdings, for the remainder of the COBRA premium period. Executive may, but is not obligated to, use such payments toward the cost of COBRA premiums.
3.
(iii) The Company shall pay Executive an amount equal to one year of bonus pay, to be calculated at target, pro-rated based on the portion of the plan year elapsed as of the date of termination, payable in a lump sum, less deductions and withholdings, at the same time as the first severance payment in Section 6.2(i) above.
(iv) The unvested equity-based incentive compensation awards then held by Executive that would have vested and become exercisable in the twelve (12) month period immediately following the date of termination shall be accelerated and shall be deemed immediately vested and exercisable as of Executive’s last day of employment; provided that, in the case of any unvested equity-based incentive compensation awards that are subject to performance-based vesting terms as of the date of such termination (whether prior to or following a Change in Control), the treatment of such performance-based vesting conditions shall be governed by the applicable equity plan and award agreement.
6.3 Severance Benefits for Termination Without Cause or Resignation with Good Reason Related to a Change of Control. In the event Executive’s employment with the Company is terminated by the Company without Cause or Executive resigns for Good Reason during the twelve (12) month period immediately following a Change of Control, and provided that Executive remains in compliance with the terms of this Agreement and subject to Section 7 below, the Company shall provide Executive with the following severance benefits:
(i) The Company shall pay Executive, as severance, the equivalent of twelve (12) months of Executive’s base salary in effect as of the date of Executive’s employment termination. This severance will be paid in the form of salary continuation, payable on the Company’s regular payroll dates, subject to standard payroll deductions and withholdings, starting on the 60th day after Executive’s termination date, with the first payment to include those payments that would have occurred earlier but for the 60-day delay.
(ii) Provided that Executive is then eligible for and timely elects continued coverage under COBRA, the Company shall pay Executive’s COBRA premiums to continue Executive’s coverage (including coverage for eligible dependents, if applicable) through the period starting on Executive’s termination date and ending on the earliest to occur of: (a) twelve (12) months following Executive’s termination date; (b) the date Executive becomes eligible for group health insurance coverage through a new employer; or (c) the date Executive ceases to be eligible for COBRA continuation coverage for any reason, including plan termination. In the event Executive becomes covered under another employer’s group health plan or otherwise ceases to be eligible for COBRA during this time period, Executive must immediately notify the Company of such event. Notwithstanding the foregoing, if the Company determines, in its sole discretion, that it cannot pay the COBRA premiums without a substantial risk of violating applicable
4.
law, the Company instead shall pay to Executive, on the first day of each calendar month, a fully taxable cash payment equal to the applicable COBRA premiums for that month, subject to applicable tax withholdings, for the remainder of the COBRA premium period. Executive may, but is not obligated to, use such payments toward the cost of COBRA premiums.
(iii) The Company shall pay Executive an amount equal to one year of bonus pay, to be calculated at target, payable in a lump sum, less deductions and withholdings, at the same time as the first severance payment in Section 6.3(i) above. For the avoidance of doubt, the amount payable pursuant to this Section 6.3(iii) shall not be subject to proration based on the portion of the year elapsed as of the date of termination.
(iv) The vesting of all unvested equity-based incentive compensation awards then held by Executive shall be accelerated such 100% of the shares underlying such awards shall be deemed immediately vested and exercisable; provided that, in the case of any unvested equity-based incentive compensation awards that are subject to performance-based vesting terms as of the date of such termination, the treatment of such performance-based vesting conditions shall be governed by the applicable equity plan and award agreement.
6.4 Termination for Cause; Resignation Without Good Reason; Death or Disability.
(i) If Executive resigns without Good Reason or the Company terminates Executive’s employment for Cause, Executive shall not be entitled to receive any payments or benefits under this Agreement, other than as set forth in Section 6.1. In addition, Executive shall resign from all positions and terminate any relationships as an employee, advisor, officer or director with the Company and any of its affiliates, each effective on the date of termination.
(ii) Executive’s employment shall terminate automatically upon the death or Total Disability of Executive. “Total Disability” shall mean Executive’s inability, with reasonable accommodation, to perform the duties of his position for a period or periods aggregating ninety (90) calendar days in any period of one hundred eighty days (180) consecutive days as a result of physical or mental illness, loss of legal capacity or any other cause beyond Executive’s control. Executive and the Company hereby acknowledge that Executive’s ability to perform the duties specified in Section 1 is the essence of this Agreement. Termination hereunder shall be deemed to be effective (a) at the end of the calendar month in which Executive’s death occurs or (b) immediately upon a determination by the Board (or the Compensation Committee thereof) of Executive’s Total Disability. In the case of termination of employment under this Section 6.3(ii), Executive shall not be entitled to receive any payments or benefits under this Agreement, other than as set forth in Section 6.1.
7. Conditions to Receipt of Severance Benefits. The receipt of the severance benefits set forth in Section 6.2 and Section 6.3 above will be subject to Executive signing and not revoking a separation agreement and release of claims in a form reasonably satisfactory to the Company (the “Separation Agreement”) no later than 60
5.
days following the date of termination. No severance benefits will be paid or provided unless and until the Separation Agreement becomes effective. Executive shall also resign from all positions and terminate any relationships as an employee, advisor, officer or director with the Company and any of its affiliates, each effective on the date of termination.
8. Section 409A. It is intended that all of the severance benefits and other payments payable under this Agreement satisfy, to the greatest extent possible, the exemptions from the application of Code Section 409A provided under Treasury Regulations 1.409A-1(b)(4), 1.409A-1(b)(5) and 1.409A-1(b)(9), and this Agreement will be construed to the greatest extent possible as consistent with those provisions, and to the extent no so exempt, this Agreement (and any definitions hereunder) will be construed in a manner that complies with Section 409A. All payments and benefits that are payable upon a termination of employment hereunder shall be paid or provided only upon the Executive’s “separation from service” from the Company (within the meaning of Code Section 409A). For purposes of Code Section 409A (including, without limitation, for purposes of Treasury Regulation Section 1.409A-2(b)(2)(iii)), Executive’s right to receive any installment payments under this Agreement (whether severance payments, reimbursements or otherwise) shall be treated as a right to receive a series of separate payments and, accordingly, each installment payment hereunder shall at all times be considered a separate and distinct payment. Notwithstanding any provision to the contrary in this Agreement, if Executive is deemed by the Company at the time of Executive’s termination to be a “specified employee” for purposes of Code Section 409A(a)(2)(B)(i), and if any of the payments upon termination set forth herein and/or under any other agreement with the Company are deemed to be “deferred compensation”, then to the extent delayed commencement of any portion of such payments is required in order to avoid a prohibited distribution under Code Section 409A(a)(2)(B)(i) and the related adverse taxation under Section 409A, such payments shall not be provided to Executive prior to the earliest of (i) the expiration of the six-month period measured from the date of Executive’s termination with the Company, (ii) the date of Executive’s death or (iii) such earlier date as permitted under Section 409A without the imposition of adverse taxation. Upon the first business day following the expiration of such applicable Code Section 409A(a)(2)(B)(i) period, all payments deferred pursuant to this Paragraph shall be paid in a lump sum to Executive, and any remaining payments due shall be paid as otherwise provided herein or in the applicable agreement. No interest shall be due on any amounts so deferred.
9. Definitions.
9.1 Cause. For purposes of this Agreement, “Cause” for termination will mean: (a) a material breach of any of Executive’s obligations or duties pursuant to this Agreement, which remains uncured seven days after Executive becomes aware of the breach by formal written notification by the Company; (b) gross negligence or willful misconduct in the course of employment; (c) any action or activity that is contrary to applicable insider trading rules or any other applicable securities rules or legislation; or (d) a material act or omission involving substantial dishonesty or fraud that harms or would reasonably be expected to harm the Company.
6.
9.2 Good Reason. For purposes of this Agreement, Executive shall have “Good Reason” for resignation from employment with the Company if any of the following actions are taken by the Company without Executive’s prior written consent: (a) any material and adverse change to Executive’s position, authority, responsibilities, or job location in effect under this Agreement; (b) any material reduction in base salary or bonus opportunity as provided under this Agreement; (c) an assignment to Executive of any duties materially inconsistent with Executive’s status as Chief Executive Officer; or (d) any failure to secure the agreement of any successor entity to fully assume the Company’s obligations under this Agreement. In order to resign for Good Reason, Executive must provide written notice to the Board within 60 days after the first occurrence of the event giving rise to Good Reason setting forth the basis for Executive’s resignation, allow the Company at least 30 days from receipt of such written notice to cure such event, and if such event is not reasonably cured within such period, Executive must resign from all positions Executive then holds with the Company not later than 90 days after the expiration of the cure period.
9.3 Change of Control. For purposes of this Agreement, “Change of Control” means the occurrence of one or more of the following: (a) a merger, a consolidation, a reorganization or an arrangement that results in a transfer of more than fifty percent (50%) of the total voting power of the Company’s outstanding securities to a person or a group of persons different from a person or a group of persons holding those securities immediately prior to such transaction (other than the Company or a person that directly or indirectly controls, is controlled by, or is under common control with, the Company); (b) a direct or indirect sale or other transfer of beneficial ownership of securities of the Company possessing more than fifty percent (50%) of the total combined voting power of the Company’s outstanding securities to a person or a group of persons different from a person or a group of persons holding those securities immediately prior to such transaction (other than the Company or a person that directly or indirectly controls, is controlled by, or is under common control with, the Company); (c) a direct or indirect sale or other transfer of the right to appoint more than fifty percent (50%) of the directors of the Board or otherwise directly or indirectly control the management, affairs and business of the Company to a person or a group of persons different from a person or a group of persons holding this right immediately prior to such transaction (other than the Company or a person that directly or indirectly controls, is controlled by, or is under common control with, the Company); (d) a direct or indirect sale or other transfer of all or substantially all of the assets of the Company to a person or a group of persons different from a person or a group of persons holding those assets immediately prior to such transaction (other than the Company or a person that directly or indirectly controls, is controlled by, or is under common control with, the Company); or (e) a complete liquidation, dissolution or winding-up of the Company; provided, however, that a Change in Control will not be deemed to have occurred if such Change in Control results solely from the issuance, in connection with a bona fide financing or series of financings by the Company, of voting securities of the Company or any rights to acquire voting securities of the Company which are convertible into voting securities.
7.
10. Proprietary Information Obligations. As a condition of employment, Executive has previously executed and shall continue to abide by the Company’s standard form of Confidential Information, Invention Assignment Agreement (the “Confidentiality Agreement”).
11. Outside Activities During Employment.
11.1 Non-Company Business. Except with the prior written consent of the Board, Executive will not during the term of Executive’s employment with the Company undertake or engage in any other employment, occupation or business enterprise, other than ones in which Executive is a passive investor. Executive may engage in civic and not-for-profit activities so long as such activities do not materially interfere with the performance of Executive’s duties hereunder.
11.2 No Adverse Interests. Executive agrees not to acquire, assume or participate in, directly or indirectly, any position, investment or interest known to be adverse or antagonistic to the Company, its business or prospects, financial or otherwise.
12. Dispute Resolution. To ensure the timely and economical resolution of disputes that may arise in connection with Executive’s employment with the Company, Executive and the Company agree that any and all disputes, claims, or causes of action arising from or relating to the enforcement, breach, performance, negotiation, execution, or interpretation of this Agreement, Executive’s employment, or the termination of Executive’s employment, including but not limited to statutory claims, shall be resolved to the fullest extent permitted by law by final, binding and confidential arbitration, by a single arbitrator, in Seattle, Washington conducted by JAMS, Inc. (“JAMS”) under the then applicable JAMS rules or by another arbitration company if mutually agreed upon by Executive and Board. By agreeing to this arbitration procedure, both Executive and the Company waive the right to resolve any such dispute through a trial by jury or judge or administrative proceeding. The Company acknowledges that Executive will have the right to be represented by legal counsel at any arbitration proceeding. The arbitrator shall: (a) have the authority to compel adequate discovery for the resolution of the dispute and to award such relief as would otherwise be permitted by law; and (b) issue a written arbitration decision, to include the arbitrator’s essential findings and conclusions and a statement of the award. The arbitrator shall be authorized to award any or all remedies that Executive or the Company would be entitled to seek in a court of law. The Company shall pay all JAMS’ arbitration fees in excess of the amount of court fees that would be required of Executive if the dispute were decided in a court of law. Nothing in this Agreement is intended to prevent either Executive or the Company from obtaining injunctive relief in court to prevent irreparable harm pending the conclusion of any such arbitration. Any awards or orders in such arbitrations may be entered and enforced as judgments in the federal and state courts of any competent jurisdiction.
8.
13. General Provisions.
13.1 Notices. Any notices provided must be in writing and will be deemed effective upon the earlier of personal delivery (including personal delivery by fax) or the next day after sending by overnight carrier, to the Company at its primary office location and to Executive at the address as listed on the Company payroll.
13.2 Severability. Whenever possible, each provision of this Agreement will be interpreted in such manner as to be effective and valid under applicable law, but if any provision of this Agreement is held to be invalid, illegal or unenforceable in any respect under any applicable law or rule in any jurisdiction, such invalidity, illegality or unenforceability will not affect any other provision or any other jurisdiction, but this Agreement will be reformed, construed and enforced in such jurisdiction to the extent possible in keeping with the intent of the parties.
13.3 Waiver. Any waiver of any breach of any provisions of this Agreement must be in writing to be effective, and it shall not thereby be deemed to have waived any preceding or succeeding breach of the same or any other provision of this Agreement.
13.4 Complete Agreement. This Agreement, together with the Confidentiality Agreement, constitutes the entire agreement between Executive and the Company with regard to this subject matter and is the complete, final, and exclusive embodiment of the Parties’ agreement with regard to this subject matter. This Agreement is entered into without reliance on any promise or representation, written or oral, other than those expressly contained herein, and it supersedes any other such promises, warranties or representations. It is entered into without reliance on any promise or representation other than those expressly contained herein, and it cannot be modified or amended except in a writing signed by a duly authorized officer of the Company.
13.5 Counterparts. This Agreement may be executed in separate counterparts, any one of which need not contain signatures of more than one party, but all of which taken together will constitute one and the same Agreement.
13.6 Headings. The headings of the paragraphs hereof are inserted for convenience only and shall not be deemed to constitute a part hereof nor to affect the meaning thereof.
13.7 Successors and Assigns. This Agreement is intended to bind and inure to the benefit of and be enforceable by Executive and the Company, and their respective successors, assigns, heirs, executors and administrators, except that Executive may not assign any of his duties hereunder and he may not assign any of his rights hereunder without the written consent of the Company, which shall not be withheld unreasonably.
9.
13.8 Tax Withholding and Indemnification. All payments and awards contemplated or made pursuant to this Agreement will be subject to withholdings of applicable taxes in compliance with all relevant laws and regulations of all appropriate government authorities. Executive acknowledges and agrees that the Company has neither made any assurances nor any guarantees concerning the tax treatment of any payments or awards contemplated by or made pursuant to this Agreement. Executive has had the opportunity to retain a tax and financial advisor and fully understands the tax and economic consequences of all payments and awards made pursuant to the Agreement.
13.9 Choice of Law. All questions concerning the construction, validity and interpretation of this Agreement will be governed by the laws of the State of Washington.
[Remainder of Page Intentionally Left Blank]
10.
IN WITNESS WHEREOF, the Parties have executed this Agreement on the day and year first written above.
| AQUINOX PHARMACEUTICALS, INC, INC. | ||
| By: | /s/ Kamran Alam | |
| Name: | Kamran Alam | |
| Title: | Chief Financial Officer | |
| JONATHAN G. DRACHMAN, MD | ||
| /s/ Jonathan G. Drachman, M.D. | ||
[Signature Page to J. Drachman Employment Agreement]
Exhibit 10.3
|
|
AQUINOX PHARMACEUTICALS (CANADA) INC. |
|||
| 450 – 887 Great Northern Way |
Tel 604.629.9223 | |||
| Vancouver, BC, Canada V5T 4T5 |
Fax 778-331-4486 | |||
| Web www.aqxpharma.com |
Private & Confidential
August 5, 2019
Kamran Alam
Dear Kamran:
Re: Fixed-Term Employment Agreement
As part of ongoing restructuring, in which Aquinox Pharmaceuticals, Inc. is merging with Neoleukin Therapeutics, Inc. on or around August 8, 2019, we are offering you a further term contract with Aquinox Pharmaceuticals (Canada) Inc. (“Aquinox” or the “Company”), in return for which we are offering a Retention Bonus. As your employment with Aquinox is set to end on August 8, 2019 in accordance with section 5(d) of your Employment Agreement, and amendments thereto, dated May 13, 2014. As a result, pursuant to good and valuable consideration, this new term of employment will be treated as fresh employment, for all purposes.
The following Agreement contains the terms and conditions of your employment with Aquinox commencing on August 9, 2019 and continuing until May 31, 2020 unless terminated in accordance with the provisions of this Agreement. Therefore, in consideration of your fresh employment with the Company and the promises, the mutual covenants and agreements set forth in this Agreement and other good and valuable consideration, the receipt and sufficiency of which you hereby acknowledge, you agree as follows:
| 1. | Definitions |
In this Agreement:
| (a) | “Affiliate” has the same meaning as in the Canada Business Corporations Act or any successor legislation, as amended from time to time. |
| (b) | “Agreement” means this agreement and schedules attached to this agreement, as amended or supplemented from time to time by mutual written consent of both Parties. |
| (c) | “approved by the Company” or words of similar import means approved by an authorized representative of the Company other than you. |
| (d) | “Base Salary” means the base compensation paid to you on a semi-monthly basis and does not include benefits or other incentive compensation. |
| (e) | “Board” means the board of directors of the Company. |
| (f) | “Business” means the business of investigating, discovering, developing, evaluating, or commercializing pharmaceutical compositions for which the Company has initiated a plan or program of investigation, discovery, development, evaluation or commercialization prior to or during your employment with the Company. |
| (g) | “Cause” means any one or more of the following: |
| (i) | A material breach of any of your obligations or duties pursuant to this Agreement, which remains uncured seven days from you becoming aware of the breach; |
| (ii) | Gross negligence or willful misconduct in the course of employment; |
| (iii) | Any action or activity that is contrary to applicable insider trading rules or any other applicable securities rules or legislation; |
| (iv) | An act or omission involving dishonesty or fraud; |
| (v) | Substantial and repeated failure to perform the duties reasonably expected of an employee in the biotechnology industry, or to perform certain duties as reasonably directed by management or the Board, or |
| (vi) | Any other act, omission or conduct constituting cause at common law or under the laws of British Columbia. |
| (h) | “Commencement Date” means your first day of employment of August 9, 2019. |
| (i) | “Company” means Aquinox Pharmaceuticals (Canada) Inc., a corporation continued under the laws of Canada having a business address at Suite 450 – 887 Great Northern Way, Vancouver, British Columbia V5T 4T5, and includes subsidiaries or affiliates of the Company where used in the context of Confidential Information or intellectual property rights or protection. |
| (j) | “Confidential Information” means trade secrets and other information, in whatever form or media, in the possession or control of the Company, which is owned by the Company or by one of its clients or suppliers or a third party with whom the Company has a business relationship (collectively, the “Associates”), and which is not generally known to the public and has been specifically identified as confidential or proprietary by the Company, or its nature is such that it would generally be considered confidential in the industry in which the Company or its Associates operate, or which the Company is obligated to treat as confidential or proprietary. |
Confidential Information includes, without limitation, the following: (i) the Products and confidential or proprietary facts, data, techniques, materials and other information related to the Products or the Business of the Company, including all related developmental or experimental work or research, related documentation owned or marketed by the Company and related formulas, algorithms, patent applications, concepts, designs, flowcharts, ideas, programming techniques, specifications and software programs (including source code listings), methods, processes, inventions, sources, drawings, prototypes and patterns; (ii) all Developments; (iii) information regarding the Company’s business operations, methods and practices, including market strategies, product pricing,
margins and hourly rates for staff and information regarding the financial, legal and corporate affairs of the Company; (iv) the names of the Company’s Associates and the nature of the Company’s relationships with such Associates; and (v) technical and business information of or regarding the Company’s Associates. Confidential Information does not include information that is or becomes generally available to the public without your fault or that you can establish, through written records, was in your possession prior to its disclosure to you in connection with your employment.
| (k) | “Developments” includes, without limitation, all: |
| (i) | Products, software, documentation, research, data, designs, reports, flowcharts, trade-marks, specifications and source code listings, and any related works, including any enhancements, modifications or additions to the Products owned, licensed, sold, marketed or used by the Company; |
| (ii) | copyrightable works of authorship including, without limitation, any technical descriptions for Products, user guides, illustrations and advertising materials; and |
| (iii) | inventions, devices, integrated circuit topographies, discoveries, concepts, ideas, algorithms, formulae, know-how, processes, techniques, systems, methods, operating capabilities and improvements, whether patentable or not, |
developed, created, generated or reduced to practice by you, alone or jointly with others, as a result of your employment, which result from your employment or which result from the use of the premises or property (including equipment, supplies or Confidential Information) owned, leased or licensed by the Company or which reasonably relate to the Business of the Company.
| (l) | “Parties” means, collectively, you and the Company and, for clarity, a “Party” means any one of the Parties. |
| (m) | “Person” means any individual, partnership, limited partnership, joint venture, syndicate, sole proprietorship, company or corporation with or without share capital, unincorporated association, trust, trustee, executor, administrator or other legal personal representative, regulatory body or agency, government or governmental agency or entity however designated or constituted. |
| (n) | “Products” means (i) therapies, approaches, screening methodologies, diagnostic assays, therapeutic molecules, compounds, and any other products derived from the discovery or development of molecular compounds that can be used to treat human inflammatory diseases, autoimmune disorders and cancer for which a research program has been initiated by the Company and disclosed to you; (ii) any intellectual property or assets owned, licensed, sold, marketed or used by the Company in connection with the Business, including enhancements, modifications, additions or other improvements to such intellectual property; and (iii) any other products or technologies that the Company discovers or develops during the employment relationship. |
| (o) | “Termination Date” means your last day of employment of May 31, 2020. |
| (p) | Use of the defined terms will include both the singular and the plural of each such term, and such use will not be interpreted as changing the meaning first given thereto. |
| 2. | Employment |
The terms of your employment will be as follows:
| (a) | Term: You will be employed for a fixed-term commencing on the Commencement Date, and ending without any further action or notice by either party on the Termination Date, subject to earlier termination in accordance with this Agreement. |
| (b) | Position and Responsibilities: You will be employed in the position of Chief Financial Officer reporting to Jonathan Drachman, Chief Executive Officer. You will perform or fulfil the duties and responsibilities as set out in Schedule A. |
| (c) | Scope of Duties: During your employment you will devote the whole of your working time, attention and abilities to your duties. You agree to give the Company the full benefit of your knowledge, expertise, skill and ingenuity. |
| (d) | Position May Evolve or Vary: You understand and agree that the above noted duties and responsibilities, and your position, may evolve or vary from time to time over the course of your employment to deal with changing business conditions, expansion or reorganization, and/or an evolving regulatory environment, and you consent to such reasonable variations in such duties and responsibilities, and in the position, as may reasonably be required by the Company from time to time as a result. The company shall not be deemed to have waived the right to require you to perform any duties hereunder by assigning you to any other duties or services or by assigning another individual to perform your duties. |
| (e) | Base Salary: You will receive an annual Base Salary of USD $380,000 for all hours worked, exclusive of bonuses, benefits and other compensation, payable in equal bi-monthly instalments, on the fifteenth day and last day of each month; this amount will be pro-rated for any partial period of employment or less than full-time work. Should the fifteenth or last day of any month not be a business day, then your salary otherwise due on such date shall be paid to you on the immediately preceding business day and will be subject to source deductions and other deductions required to be deducted and remitted under applicable laws. |
| (f) | Retention Bonus: You will be eligible for a one-time retention bonus of USD $332,500 less statutory deductions, to be payable upon the earlier of (i) either the termination of this Agreement by the Company on a without cause basis in accordance with Article 5(c) of this Agreement and (ii) the Termination Date; provided in each case that you execute a general release of claims in a form provided by the Company. If you resign prior to the Termination Date or this Agreement is otherwise terminated prior to the Termination Date under any circumstances other than in accordance with Article 5(c), you will not be entitled to the Retention Bonus. |
| (g) | Excess Hours: You agree that as a manager or high technology professional as defined in the Employment Standards Act of British Columbia, your hours of work will vary and may be irregular and will be those hours required to meet the objectives of your employment. You agree that the compensation described in this Agreement compensates for you all hours worked. |
| (h) | Stock Options: No new Stock Options will be granted during the term of this Agreement. Any Stock Options that were granted prior to this Agreement will become fully vested as of the consummation of the contemplated merger. For the avoidance of doubt, notwithstanding any other provision hereof, the Company acknowledges that your termination of employment and subsequent commencement of employment pursuant to this Agreement does not represent a break in or termination of “Continuous Service” as defined in the 2014 Equity Incentive Plan for purposes of the ability to exercise any such Stock Options granted prior to the date of this Agreement. |
| (i) | Vacation Entitlement: You will be entitled to twenty-five (25) days’ paid vacation, pro-rated for any partial year of employment. Your vacation must be taken in accordance with the Company’s vacation policy in effect from time to time. |
| (j) | Benefits: |
(i) Subject to your insurability, you will be eligible to participate in an individual extended health benefits plan paid for by the Company, covering MSP premiums, employee health, medical or other related benefits which the Company shall from time to time provide to its employees, subject to the policies and procedures as set out by the Company and the insurer, until August 8, 2020. For clarity, you will not be eligible to participate in a group health benefits program provided by the Company.
(ii) The Company will provide you with a lump sum payment of CDN $2,000 on August 8, 2019 to cover the differential cost in coverage amounts between the group health benefits program which you may have participated in previously with the Company, and the individual health benefit plan, for the period from August 8, 2019 until August 8, 2020. Such payment shall be treated as a taxable benefit.
| (k) | Business Equipment and Other Expenses: The Company will provide you with a computer for business use. You acknowledge that during the term of your employment and thereafter this equipment remains the sole property of the Company. The Company will reimburse you for all reasonable travelling and out-of-pocket expenses actually and properly incurred by you in connection with your duties under this Agreement and in accordance with Company policy and Board approval, provided that you first furnish statements, and receipts or vouchers for all such expenses to the Company. |
| (l) | Policies. You will be required to comply with the Company’s policies which are in effect as are implemented from time to time during the course of employment. In particular, in compliance with our obligations as a public company, you will be expected to comply with the Company’s Code of Business Conduct and Ethics, Insider Trading and Trading Window, Whistleblower, Confidential Information and Disclosure, Communications, Media and IT Policies. |
| (m) | Tax Filing Support Services. The Company will provide you with, and cover the cost of, U.S. and Canadian tax filing support services for the 2019 and 2020 tax years. |
| 3. | Confidential Information |
As consideration for your promotion and continued employment with the Company, you covenant and agree as follows:
| (a) | General Obligation of Confidentiality: You acknowledge that the Confidential Information is the exclusive property of the Company or Persons from whom the Company has obtained its rights. You will treat the Confidential Information in strict confidence and will not directly or indirectly, either during or subsequent to your employment with the Company, disclose, allow access to, transmit or transfer the Confidential Information to a third party (other than the Company’s directors, officers, bankers, legal and financial advisors in the ordinary course of business) unless otherwise required by law or by a regulatory authority having jurisdiction over the Company, or except as previously approved in writing by the Company. You will protect such Confidential Information from disclosure by exercising a standard of care as may reasonably be expected to preserve its secret and confidential nature. You acknowledge and agree that nothing contained in this Agreement will be construed as an assignment to you of any right, title or interest in the Confidential Information. All right, title and interest relating to the Confidential Information is expressly reserved by the Company. All documents containing Confidential Information are the property of the Company. Without limiting the generality of the foregoing, you hereby transfer to the Company the property rights in all documents that now or hereafter may contain the Confidential Information. |
| (b) | Use of Confidential Information: You agree that at all times during and subsequent to your employment with the Company, you will not use any of the Confidential Information in any manner except as reasonably required for you to perform your duties for the Company. Without limiting the generality of the foregoing, you agree that at all times during and subsequent to your employment, you will not use or take advantage of the Confidential Information for creating, maintaining or marketing, or aiding in the creation, maintenance or marketing, of any product that is competitive with any of the Products. |
| (c) | Prohibition on Copying: You will not copy or reproduce the Confidential Information except in the course of your employment with and for the benefit of the Company or with the written approval of the Company. All such copies remain the property of the Company. |
| (d) | Injunctive Relief: You acknowledge that irreparable harm may result to the Company if you breach your obligations under this Article or under subsections 4(d) and 4(e). You acknowledge that such a breach may not properly be compensated by an award of damages. Accordingly, the Company’s remedy for any such breach may include, in addition to other available remedies and damages, injunctive relief or other equitable relief enjoining such breach at the earliest possible date. |
| (e) | Assignment: You agree to make full disclosure to the Company of each Development promptly after its creation. You hereby irrevocably assign and transfer to the Company, and agree that the Company will be the exclusive owner of, all of your right, title and interest in and to each Development throughout the world, including all trade secrets, patent rights, copyrights trademarks, industrial designs and all other intellectual property rights therein, whether realized within or beyond the scope of your employment, and regardless of the true purpose of the employment relationship, and you irrevocably waive |
| all moral rights you may have in these Developments. You further agree to cooperate fully at all times during and subsequent to your employment with respect to signing further documents and doing such acts and other things reasonably requested by the Company, at the Company’s expense, to confirm such transfer of ownership of rights, including intellectual property rights, effective at or after the time the Development is created and to apply for and obtain patents or copyrights, industrial designs trademarks, other intellectual property registrations or other similar rights covering the Development. The Company will be exclusively entitled to make applications for registration of all such rights, in the Company’s sole and unfettered discretion, in any jurisdictions that the company deems necessary. Should the Company be unable to secure your signature on any document necessary to apply for, prosecute, obtain, or enforce any patent, copyright or other right or protection relating to any Development, due to your incapacity or any other cause, you hereby irrevocably designate and appoint the Company and each of its duly authorized officers and agents as your agent and attorney-in-fact to do all lawfully permitted acts to further the prosecution, issuance, and enforcement of patents, copyrights, or other rights or protection with the same force and effect as if executed and delivered by you. You agree that the obligations in this subsection will continue beyond the termination of your employment with respect to any and all Developments created during your employment. For purposes of the copyright laws of the United States of America and other jurisdictions, to the extent, if any, that such laws are applicable to any Confidential Information, it will be considered a work made for hire and the Company will be considered the author thereof. |
| 4. | Obligations of Employment |
You further covenant and agree as follows:
| (a) | Performance and Duty to the Company: Throughout your employment you will well and faithfully serve the Company and use your best efforts to promote the Business of the Company. You will act honestly and in good faith in what you reasonably believe to be in the best interests of the Company. You will adhere to all applicable policies of the Company and exercise the degree of care, diligence and skill that a reasonably prudent Chief Financial Officer would exercise in comparable circumstances. |
| (b) | Business of the Company: You will not, during your employment with the Company, engage in any business, enterprise or activity that is contrary to or detracts from the due performance of the Business of the Company. |
| (d) | No Personal Benefit: You will not receive or accept for your own benefit, either directly or indirectly, any commission, rebate, discount, financial gratuity or profit from any Person having or proposing to have one or more business transactions with the Company, without the prior approval of the Board, except that you may accept dinners, event tickets and other customary gifts with values of less than US$200, as long as there is no frequent pattern of such customary gifts from any person or entity, or related group of persons or entities, that would give rise to the perception of a conflict of interest. |
| (e) | Business Contacts: During your employment you will communicate and channel to the Company all knowledge, business and customer contacts and any other information that could concern or be in any way beneficial to the Business of the Company. Any such information communicated to the Company as aforesaid will be and remain the property of the Company notwithstanding the subsequent termination of your employment. |
| (f) | Return of Company Property: Upon termination of your employment, you will promptly return to the Company all Company property including all written information, tapes, discs or memory devices and copies thereof, and any other material on any medium in your possession or control pertaining to the Business of the Company, without retaining any copies or records of any Confidential Information whatsoever. You will also return any keys, pass cards, identification cards, equipment or other property belonging to the Company. You will be permitted to keep your laptop, cell phone and computer desk monitor upon termination of your employment. |
| (g) | Pre-existing Obligations: You are hereby requested and directed by the Company to comply with any existing common law, contractual or statutory obligations to any former employers and to any other Person. The Company is not employing you to obtain the confidential information or business opportunities of any former employers or any other Person. |
| 5. | Termination |
| (a) | Resignation: If for any reason you should wish to leave the Company, you will provide the Company with three weeks’ prior written notice of your intention (the “Resignation Period”). The Parties hereby agree that in order to protect the Company’s interests, the Company may, in its sole and unfettered discretion, waive the Resignation Period or any part thereof, and end your employment by delivering to you a written notice accompanied by payment of your Base Salary due to you during the remainder of the Resignation Period. |
| (b) | Termination for Cause: The Company may terminate your employment at any time for Cause, effective upon delivery by the Company to you of a written notice of termination of your employment for Cause. You will not be entitled to receive any further pay or compensation (except for pay, if any, accrued and owing under this Agreement up to the date of termination of your employment), severance pay, notice, payment in lieu of notice, benefits or damages of any kind, and for clarity, without limiting the foregoing, you will not be entitled to any bonus or pro rata bonus payment that has not already been awarded by the Board. |
| (c) | Termination Without Cause: The Company may terminate your employment at any time without Cause in accordance with the B.C. Employment Standards Act (“ESA”). You acknowledge, agree and accept the incorporation of the termination provisions of the ESA into this Agreement and expressly waive any right to reasonable notice at common law. You agree that for the purposes of determining any entitlement under the ESA, your start date with the Company is August 1, 2019. |
| 6. | Agreement Voluntary and Equitable |
The Parties agree that they each have carefully considered and understand the terms of employment contained in this Agreement, that the terms are mutually fair and equitable, and that they each have executed this Agreement voluntarily and of their own free will.
| 7. | Assignment and Enurement |
You may not assign this Agreement, any part of this Agreement or any of your rights under this Agreement without the prior written consent of the Company. This Agreement enures to the benefit of and is binding upon you and the Company and your respective heirs, executors, administrators, successors and permitted assigns.
| 8. | Severability |
If any part, article, section, clause, paragraph or subparagraph of this Agreement is held to be indefinite, invalid, illegal or otherwise voidable or unenforceable, the entire Agreement will not fail on the account thereof and the validity, legality and enforceability of the remaining provisions will in no way be affected or impaired thereby. Further, if any provision of this Agreement is held by a court of competent jurisdiction to be excessively broad as to duration, activity, geography, or subject, it shall be deemed to extend only over the maximum duration, activity, geographic extent, and subject as to which such provision shall be valid and enforceable under applicable law.
| 9. | Entire Agreement |
This Agreement constitutes the entire agreement between you and the Company with respect to the subject matter herein and cancels and supersedes all previous invitations, proposals, letters, correspondence, negotiations, promises, agreements, covenants, conditions, representations and warranties with respect to the subject matter of this Agreement. There is no representation, warranty, collateral term or condition affecting this Agreement for which any Party can be held responsible in any way, other than as expressed in writing in this Agreement. No change or modification of this Agreement will be valid unless it is in writing and signed by both Parties.
| 10. | Notice |
Any notice required or permitted to be given hereunder must be in writing and will be sufficiently given or made if delivered by hand to you or to the Chair of the Board, as appropriate, or delivered or sent by registered mail, fax or e-mail to the address of the Parties set out below. Any notice so given will be deemed to have been given and to have been received on the day of delivery if it is a business day and otherwise on the next succeeding business day or, if mailed, on the third business day following the mailing thereof (excluding each day during which there exists any interruption of postal services due to strike, lockout or other cause). Addresses for notice may be changed by giving notice in accordance with this section.
| Aquinox Pharmaceuticals (Canada) Inc. 450 – 887 Great Northern Way, V5T 4T5 Vancouver, British Columbia Attn: President & CEO Fax: 604-295-4748 |
Kamran Alam
|
| 11. | Non-waiver |
No failure or delay by you or the Company in exercising any power or right under this Agreement will operate as a waiver of such power or right. Any consent or waiver by any Party to this Agreement to any breach or default under this Agreement will be effective only in the specific instance and for the specific purpose for which it was given.
| 12. | Survival of Terms |
The provisions of sections 1, 3, 8, 9, 12, 13, 14 and 17, and of subsections 4(d) and 4(e) of this Agreement will survive the termination of your employment.
| 13. | Further Assistance |
The Parties will execute and deliver any documents and perform any acts necessary to carry out the intent of this Agreement.
| 14. | Equitable Remedies |
You hereby acknowledge and agree that a breach of your obligations under this Agreement would result in damages to the Company that could not be adequately compensated for by monetary award. Accordingly, in the event of any such breach by you, in addition to all other remedies available to the Company at law or in equity, the Company shall be entitled as a matter of right to apply to a court of competent jurisdiction for such relief by way of restraining order, injunction, decree or otherwise, as may be appropriate to ensure compliance with the provisions of this Agreement. The Company hereby acknowledges that any material unilateral change or modification to this Agreement or a material adverse change to your position, duties or compensation, without your prior written consent, except as provided for in section 5, may constitute constructive dismissal or breach of contract and, in addition to all other remedies available to you at law or in equity, you shall be entitled as a matter of right to apply to a court of competent jurisdiction for compensation, relief or other award as may be determined appropriate in the circumstances to ensure compliance with the provisions of this Agreement.
| 15. | Conflict |
In the event of any conflict between the terms and conditions of this agreement and any other agreement, the terms of this agreement shall prevail.
| 16. | Time |
Time is of the essence of this Agreement.
| 17. | Governing Laws |
This Agreement will be governed by and construed in accordance with the laws of British Columbia and the laws of Canada applicable in British Columbia. Each Party attorns to the non-exclusive jurisdiction of courts of British Columbia.
| 18. | Independent Legal Advice |
You acknowledge that you have been given an opportunity to seek independent legal advice with respect to the terms of this Agreement prior to its execution and have been advised to do so by the Company and that you understand the terms and rights and obligations under this Agreement.
| 19. | Counterparts |
This Agreement may be executed in two or more counterparts, each of which will be deemed to be an original and all of which will constitute one Agreement.
Aquinox Pharmaceuticals (Canada) Inc.
| By: |
/s/ David J. Main | |
| Name: |
David J. Main | |
| Title: |
President and Chief Executive Officer | |
| Date: | August 5, 2019 | |
I acknowledge and accept the terms and conditions of my employment with the Company as set out above. I agree that because I received good and valuable consideration in the termination of my employment from the Company on August 8, 2019, that I am commencing this Term of employment as a new employee for all purposes:
| SIGNED, SEALED AND DELIVERED by Kamran Alam in the presence of: |
)
) |
|||
| ) | ||||
| /s/ Sean Barnicle | ) | |||
| Signature of Witness | ) ) |
|||
| Sean Barnicle | ) | /s/ Kamran Alam | ||
| Name of Witness | ) ) |
Kamran Alam | ||
| ) | Date: August 5, 2019 | |||
| Address of Witness | ) | |||
| Vancouver, BC V6G 2PS | ) ) |
|||
| ) | ||||
| ) | ||||
| Accountant | ) | |||
| Occupation of Witness | ) |
SCHEDULE A
Responsibilities and Duties
Job responsibilities include, but are not limited to, the following activities:
JOB DESCRIPTION (Brief Listing of Major Job Duties)
The Vice President Finance and CFO is responsible for all company finances, financial reporting (internal & external), business development, and the financing and business strategies for the Company.
The CFO reports to the CEO and is responsible (directly or indirectly) for all of the members of the finance team and business development team.
ESSENTIAL FUNCTIONS (Essential Functions & Responsibilities)
| Description |
% | |||
| 1. | Corporate Finance & Corporate Strategy
• Strategic Plan – work in conjunction with the executive team to implement and carry out strategic plan.
• Financial Strategy – works with the CEO to achieve financing goals for the company.
• Build/manage relationships with current and potential future Investment bankers to optimize financings/M&A opportunities and timing.
• Budgeting & Cash management -plan, implement, and report on internal budgets/investment in programs/projects/infrastructure to optimize shareholder value/return on investment.
• Cash Flow Projections -short-term-long-term -create 3-year plan & maintain to incorporate ongoing business strategy and financing strategy. |
35% | ||
| 2. | Financial Accounting/Reporting & Compliance
• Manage all aspects of public company requirements, such as SEC reporting, SOX internal control assessment, stock exchange rules, board and committee activities, shareholder reporting, and annual meetings:
• Financial reporting to Board & Management-present management reports to Board and Management as per pre-set reporting schedule and provide verbal updates as required.
• Financial Statement Review-review of all statements for external publication and internal analyses of revenues, expenditures, assets & liabilities.
• Responsible for all Canadian and US regulatory filing requirements including Sarbanes Oxley in line with securities guidelines, ensuring continued compliance.
• MD&A & Shareholder Messages -update & maintain MD&A to current reporting. period, as applicable.
• Supervise Finance Team.
• Liaison with solicitors and auditors -with respect to reporting requirements, legal documents and filings. |
25% | ||
| Description |
% | |||
| 3. | Investor Relations
• Develop and maintain relationships with investors in order to maximize the value of the corporation and raise capital as appropriate and necessary:
• Shareholder Relations – answer shareholder questions when appropriate (re finances, budge, etc).
• Banker Conferences and NDRs- participate in meetings with current and potential future investors as needed.
• Analyst relations - Meet with analysts & maintain dialogue re company’s progress (in conjunction with CEO & Investor Relations Team). |
15% | ||
| 4. | Corporate Secretary/Governance
• Establish credibility throughout the organization and with the Board as an effective developer of solutions to business challenges:
• Manage dissemination of Board materials, and capture minutes of meetings
• Maintain good Corporate Governance practices at the Board level and within the organization
• Participate in strategic and finance-related discussions at the Board and Audit Committee level |
10% | ||
| 5. | Business Development & Business Operations
• Business Development- in conjunction with CEO develop and implement strategy and plan for %partnering/M&A. Responsible for overseeing licensing/partnering and due diligence process.
• Supervise BD team
• Risk management & assessment, insurance, treasury management.
• Contract Review & Negotiation - review all material corporate contracts, related financial information & negotiable terms, before finalization.
• Enhance and/or develop, implement and enforce policies and procedures of the organization by way of systems that will improve the overall performance and effectiveness of the corporation. |
25% | ||
JOB SPECIFICATIONS
| SP |
RD | |||||
| 1. | Designated CMA, CGA or CA with min. ten years’ experience. | X | ||||
| 2. | Demonstrated US GAAP reporting requirements. | X | X | |||
| 3. | Experience achieving public equity financing and alternate sources of capital. | X | X | |||
| 4. | Working experience with Sarbanes/Oxley reporting requirements & Corporate Governance compliance. | X | X | |||
| 5. | Knowledge of Industry/Competition. | X | ||||
| 6. | Public Speaking. | X | X | |||
| 7. | Demonstrated ability to build collaborative and strategic relationships to support the requirements of the role. | X | ||||
| 8. | Demonstrated strategic thinking and leadership ability. | X | ||||
| 9. | Proactive and forward thinking. | X | ||||
| 10. | Ability to motivate and challenge peers and direct reports. | X | ||||
| 11. | Ability to quickly adapt and support changing business needs. | X | ||||
| 12. | Ability to wear many hats, be productive and deliver on responsibilities. | X | ||||
| 13. | A “make it happen” attitude, long with a strong commitment to teamwork, and initiative. | X | ||||
Exhibit 99.1

|

|
Aquinox Pharmaceuticals and Neoleukin Therapeutics
announce merger agreement
| • | Combined company capitalized with approximately $65M to develop novel immunotherapies |
| • | Sophisticated computational platform enables new class of de novo protein therapeutics |
| • | Lead program, NL-201, is a potent IL-2/IL-15 agonist with favorable therapeutic index |
| • | Conference call on August 6, 2019 at 9 a.m. EDT / 6 a.m. PDT |
VANCOUVER, British Columbia and SEATTLE, Washington, August 6, 2019 Aquinox Pharmaceuticals, Inc. (“Aquinox”) (NASDAQ: AQXP) and Neoleukin Therapeutics, Inc. (“Neoleukin”), a privately held biopharmaceutical company utilizing sophisticated computational methods to design de novo protein therapeutics, today announced that the two companies entered into a definitive merger agreement under which Aquinox agreed to the acquisition of Neoleukin, which is expected to close on or about August 8th, 2019. Pursuant to the merger agreement, Aquinox will acquire all of the outstanding capital stock of Neoleukin in exchange for a combination of common and preferred shares. In connection with the merger, Aquinox will be renamed as Neoleukin Therapeutics, Inc., and is expected to trade on the Nasdaq Global Market under the new ticker symbol “NLTX”, concurrent with closing.
The combined company will focus on the development and commercialization of computationally-designed protein therapeutics to address significant unmet medical needs in immuno-oncology, inflammation, and autoimmunity. Neoleukin’s lead product candidate, NL-201, is a de novo protein designed to mimic the therapeutic activity of the cytokines interleukin-2 and interleukin-15 for the treatment of various types of cancer by activating both T-cells and NK-cells to fight cancer, while limiting toxicity with minimal loss of activity.
“Neoleukin Therapeutics is a new company based on sophisticated computational technology licensed from the Institute for Protein Design and the University of Washington that enables us to design and create de novo proteins as therapeutic candidates,” said Jonathan G. Drachman, M.D., CEO of Neoleukin Therapeutics. “In January, our scientific founders published their seminal findings in the journal, Nature. Since then, we have been advancing our lead program, NL-201, toward IND-enabling studies. The merger with Aquinox is transformational for our company, providing additional capital to prepare an IND submission, generate clinical data, develop additional preclinical programs, and advance our computational technology. We believe that cytokine mimetics, or NeoleukinsTM, have the potential to offer enhanced therapeutic effects with fewer toxic side effects.”
“Since announcing our plans to seek and consider strategic alternatives for Aquinox, our priority has been to identify a merger candidate we believe has the potential to continue our mission to help patients and provide meaningful value to our stockholders,” said David J. Main, President & CEO of Aquinox. “Following an extensive evaluation and diligence process, the Aquinox Board of Directors concluded that a merger with Neoleukin, with a strong platform technology, seasoned leadership team, and compelling clinical development plan, offered an excellent opportunity to create such value. We believe Neoleukin represents an attractive merger partner for Aquinox, offering a novel approach to creating de novo proteins for patients with unmet medical need.”
About the Transaction
Pursuant to the merger agreement, Aquinox will acquire all of the outstanding capital stock of Neoleukin in exchange for the issuance of 4,589,787 newly issued shares of Aquinox common stock, which represented approximately 19.5% of the voting power of Aquinox as of immediately prior to the issuance of such shares, and shares of Aquinox convertible preferred stock convertible into a total of 10,194,838 shares of Aquinox common stock upon receipt of the required approval of the Aquinox stockholders under Nasdaq rules. Following completion of the merger and on an as-converted basis, the former Aquinox stockholders will own approximately 61.42% of the combined company’s capital stock and the former Neoleukin stockholders will own 38.58% of the combined company’s capital stock. The shares of convertible preferred stock are not tradeable by the holders of the shares.
In connection with the merger, Aquinox will be renamed as Neoleukin Therapeutics, Inc. and is expected to trade on the Nasdaq under the new ticker symbol NLTX at the time of closing, on or about August 8th, 2019, subject to customary legal and regulatory clearances and procedures. The corporate headquarters for the combined company will be located in Seattle, Washington at Neoleukin’s existing facility.
SVB Leerink is acting as exclusive financial advisor and Cooley LLP is serving as legal counsel to Aquinox. MTS Health Partners is acting as exclusive financial advisor and Fenwick & West LLP is serving as legal counsel to Neoleukin.
Management and Organization
Effective as of the closing of the transaction, Jonathan G. Drachman, M.D. will be the President and Chief Executive Officer of the combined company with Kamran Alam continuing as the interim Chief Financial Officer. Senior leadership of the combined company will also include Daniel Silva, Ph.D. as VP, Head of Research; Umut Ulge, M.D., Ph.D. as VP, Translational Medicine; and Carl Walkey, Ph.D. as VP, Corporate Development. In connection with the merger, David J. Main, President and Chief Executive Officer of Aquinox, will be stepping down.
Additionally, effective as of the closing of the transaction, Gary Bridger, Ph.D., Daniel Levitt, M.D., Ph.D., Richard S. Levy, M.D., David J. Main, Kevin Neu, M.D., and Robert E. Pelzer will resign from the Aquinox’s board of directors and the board of directors of the combined company will be comprised of six directors: Todd Simpson and Sean Nolan will be continuing Board members and will be joined by Cantey Boyd, Managing Director at Baker Brothers Advisors; Jonathan G.
Drachman, CEO, Neoleukin Therapeutics; Sarah B. Noonberg, former Chief Medical Officer at Nohla Therapeutics; and Lewis “Rusty” Williams, former CEO of FivePrime Therapeutics.
Conference Call and Webcast
Management will host a conference call later today for investors regarding this announcement with details as follows:
Conference Call and Webcast Details:
Date: August 6, 2019
Time: 9:00 AM EDT, 6:00 AM PDT
Toll-free: (866) 357-7878
International: (315) 625-3088
Audience Passcode 5457714
Webcast URL: https://edge.media-server.com/mmc/p/ktdhvpdd
The archived webcast will be available on the Investor Relations section of the Aquinox website and the News section of the Neoleukin website approximately two hours after the event and will be available for replay for at least 30 days after the event.
About Aquinox Pharmaceuticals, Inc.
Aquinox Pharmaceuticals, Inc. (NASDAQ: AQXP) is a pharmaceutical company discovering and developing novel therapeutics for conditions marked by inflammation, inflammatory pain, and blood cancers.
About Neoleukin Therapeutics, Inc.
Neoleukin is a privately-held biopharmaceutical company creating next generation immunotherapies using de novo protein design technology. Neoleukin uses sophisticated computational methods to design proteins that demonstrate specific pharmaceutical properties that provide potentially superior therapeutic benefit over native proteins. Neoleukin’s lead product candidate, NL-201, is a combined IL-2 and IL-15 agonist designed to eliminate alpha receptor binding. For more information, please visit the Neoleukin website: www.neoleukin.com.
Cautionary Note on Forward-Looking Statements
Certain of the statements made in this press release are forward looking, including those relating to the benefits of the merger, future management of the Company, Neoleukin’s business, the strategy of the combined company, future operations, advancement of its product candidates and product pipeline, clinical development of the combined company’s product candidates, including expectations regarding timing of regulatory submissions and initiation of clinical trials, regulatory requirements for initiation of clinical trials and registration of product candidates, the sufficiency of its cash resources and other statements containing the words “anticipate,” “believe,” “expect,” “may,” “plan,” “project,” “potential,” “will,” “would,” “could,” “continue,” and similar expressions. These statements are subject to risks and uncertainties that could cause actual results and events to differ materially from those anticipated, including, but not limited to, risks and uncertainties related to: whether results of early clinical trials or preclinical studies will be indicative of the results of future trials, the adequacy of any clinical models, uncertainties associated with regulatory review of clinical trials; our ability to identify or acquire additional clinical candidates, our ability to obtain and maintain regulatory approval for any product candidates and the potential safety, efficacy or clinical utility of or any product
candidates, and other factors discussed in the “Risk Factors” section of the Aquinox’s report on Form 10-Q for the quarter ended June 30, 2019 as filed with the Securities and Exchange Commission. Actual results or developments may differ materially from those projected or implied in these forward-looking statements. More information about the risks and uncertainties faced by Aquinox is contained in the company’s Quarterly Report on Form 10-Q for the year ended June 30, 2019, and subsequent reports, filed with the Securities and Exchange Commission. The Company disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
Contacts:
Media
Julie Rathbun
206-769-9219
Investors
Solebury Trout
Brian Korb
646-378-2923

Conference Call August 6, 2019 Exhibit 99.2

Forward Looking Statements Certain of the statements made in these slides and the accompanying oral presentation are forward looking, including those relating to the benefits of the merger, future management of Neoleukin Therapeutics, following its combination with Aquinox Pharmaceutical, Inc., Neoleukin’s business, the strategy of the combined company, future operations, advancement of its product candidates and product pipeline, clinical development of the combined company’s product candidates, including expectations regarding timing of regulatory submissions and initiation of clinical trials, regulatory requirements for initiation of clinical trials and registration of product candidates, the sufficiency of its cash resources and other statements containing the words “anticipate,” “believe,” “expect,” “may,” “plan,” “project,” “potential,” “will,” “would,” “could,” “continue,” and similar expressions. These statements are subject to risks and uncertainties that could cause actual results and events to differ materially from those anticipated, including, but not limited to, risks and uncertainties related to: whether results of early clinical trials or preclinical studies will be indicative of the results of future trials, the adequacy of any clinical models, uncertainties associated with regulatory review of clinical trials; our ability to identify or acquire additional clinical candidates, our ability to obtain and maintain regulatory approval for any product candidates and the potential safety, efficacy or clinical utility of or any product candidates, and other factors discussed in the “Risk Factors” section of the Aquinox, Inc.’s report on Form 10-Q for the quarter ended June 30, 2019 as filed with the Securities and Exchange Commission. Actual results or developments may differ materially from those projected or implied in these forward-looking statements. More information about the risks and uncertainties faced by Aquinox is contained in the company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2019, and subsequent reports, filed with the Securities and Exchange Commission. The Company disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
Announced August 6, 2019; Close expected on or about August 8, 2019 After giving effect to conversion of preferred shares subject to shareholder vote: 14,784,625 shares to Neoleukin shareholders 38,321,993 total shares outstanding NASDAQ listing expected to change from AQXP to NLTX Post-merger entity will have approximately $65M in cash and cash equivalents, net of transaction-related expenses Aquinox CFO and financial team retained during transition Neoleukin CEO and senior management to lead merged entity Aquinox/Neoleukin Merger

Functional De Novo Proteins: Better Immunotherapies by Design Computational algorithms invented by Daniel-Adriano Silva at UW IPD NL-201: IL-2/IL-15 cytokine mimetic (Neoleukin™) for cancer immunotherapy Discovery published in Nature January 2019 Company formed to advance technology platform and lead asset

Neoleukin Therapeutics: Pioneers in De Novo Protein Design Technology originated at University of Washington’s Institute for Protein Design, led by David Baker, PhD Scientific founders are world leaders in de novo protein design Exclusive license obtained for commercialization of NL-201 and other de novo protein assets Investigating additional Neoleukins for cancer, inflammation, and autoimmunity

The Good and Bad of Interleukin-2 (IL-2) Immunotherapy IL-2 one of the few immunology drugs proven to work as single-agent Stimulates T cells to fight cancer rhIL-2 (Proleukin®) approved for RCC and melanoma Durable remissions in 5-8% of patients Vascular leak syndrome, cytokine storm are frequent side effects Treatment administered in hospital due to significant toxicity Low doses generally insufficient for activity
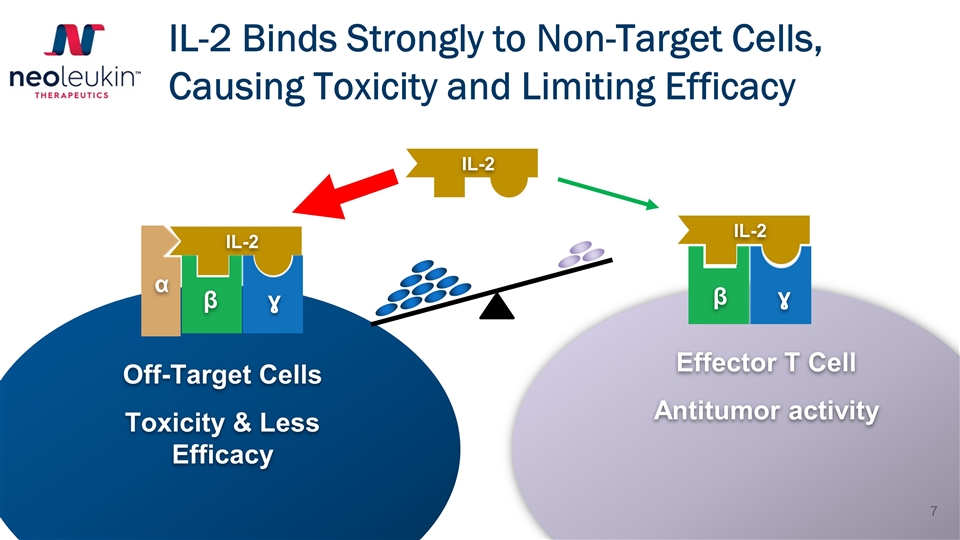
Off-Target Cells Toxicity & Less Efficacy β ɣ α IL-2 IL-2 Binds Strongly to Non-Target Cells, Causing Toxicity and Limiting Efficacy Effector T Cell Antitumor activity β ɣ IL-2 IL-2
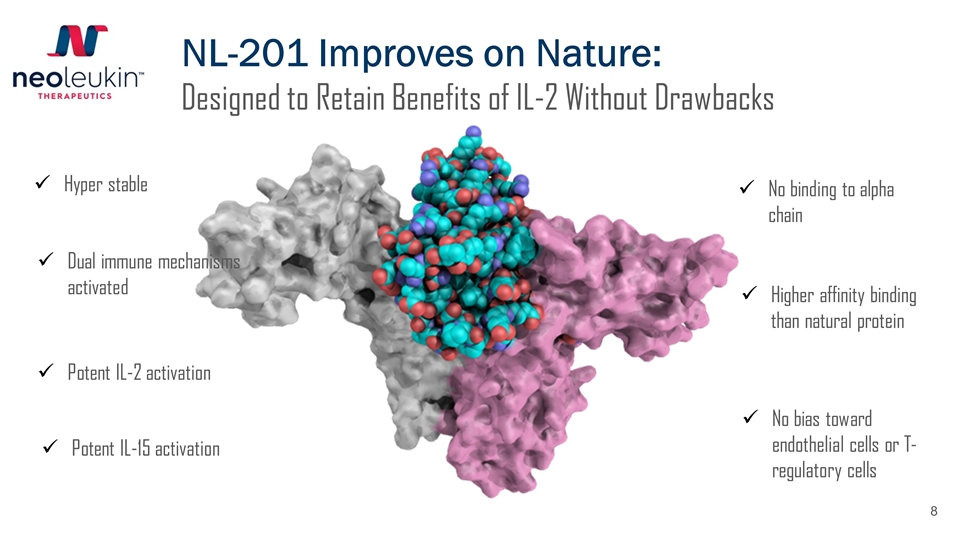
NL-201 Improves on Nature: Designed to Retain Benefits of IL-2 Without Drawbacks No binding to alpha chain No bias toward endothelial cells or T-regulatory cells Potent IL-2 activation Potent IL-15 activation Hyper stable Higher affinity binding than natural protein Dual immune mechanisms activated
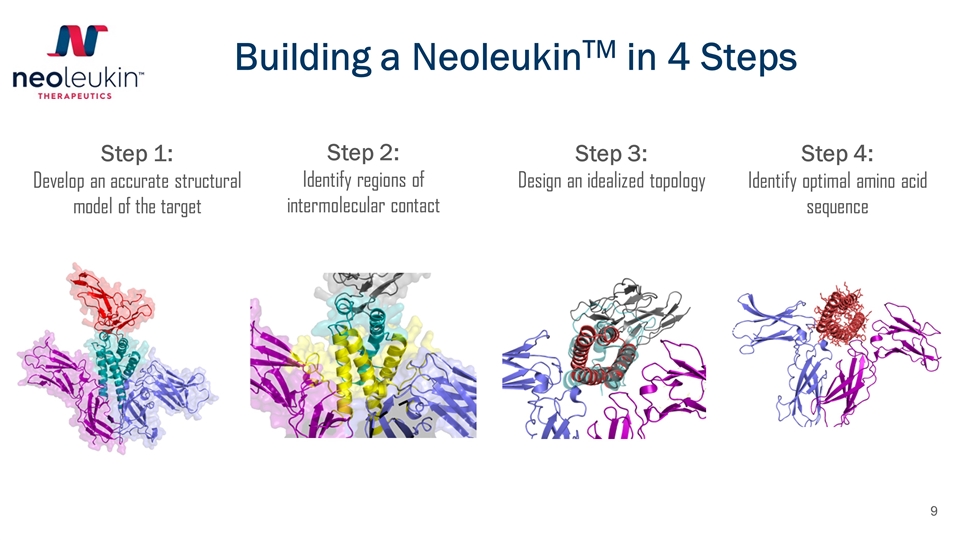
Building a NeoleukinTM in 4 Steps Step 1: Develop an accurate structural model of the target Step 2: Identify regions of intermolecular contact Step 3: Design an idealized topology Step 4: Identify optimal amino acid sequence

IL-2/IL-15 Competitive Landscape Other IL-2/IL-15 immunotherapies in development are modified versions of native IL-2 or IL-15 IL-2 modifications often result in less potent, less stable molecules, with some residual binding to the alpha subunit We believe that NL-201 is the only IL-2 agonist specifically designed with no alpha-binding region NL-201 potently agonizes both IL-2 and IL-15, stimulating complementary cancer-fighting pathways
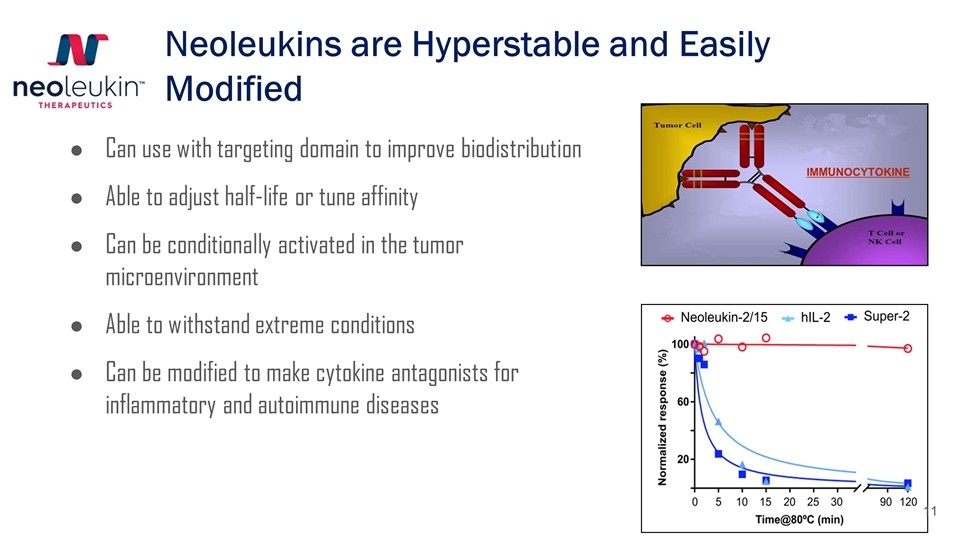
Neoleukins are Hyperstable and Easily Modified Can use with targeting domain to improve biodistribution Able to adjust half-life or tune affinity Can be conditionally activated in the tumor microenvironment Able to withstand extreme conditions Can be modified to make cytokine antagonists for inflammatory and autoimmune diseases

Carl Walkey, Ph.D. Vice President Corporate Development Previous: Postdoctoral Fellow, IPD, UW Daniel-Adriano Silva, Ph.D. Vice President, Head of Research Previous: Translational Investigator, IPD, UW Umut Ulge, M.D., Ph.D. V.P. Translational Medicine Previous: Postdoctoral Fellow, UW Jonathan Drachman, M.D. Chief Executive Officer Previous: CMO, EVP R&D, Seattle Genetics Leadership Team Kamran Alam Interim Chief Financial Officer Previous: CFO Aquinox

Board of Directors Lewis T. Williams, M.D., Ph.D. Director CEO Walking Fish Therapeutics Previous: CEO, FivePrime Therapeutics Todd Simpson Director CFO Seattle Genetics Cantey Boyd Director Managing Director, Baker Brothers Advisors Sarah Noonberg, M.D. Director Previous: CMO, Nohla Therapeutics Sean Nolan Director Previous: CEO, AveXis Jonathan G. Drachman, M.D. Chair of the Board CEO and President, Neoleukin Therapeutics

Merger expected to close on or about August 8th, 2019 Present preclinical data in first half of 2020 Plan to submit IND for NL-201 by end of 2020 Cash and cash equivalents expected to fund operations through 2021 Upcoming Events/Milestones

Improving on nature. Designing for life.
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- HSBC Continental Europe: Post Stabilisation Notice
- Konsolidator maintains its financial expectations for 2024
- Riber: 2024 first quarter revenues: +20%
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share
