Form 6-K TAKEDA PHARMACEUTICAL For: May 30
Table of Contents
FORM 6-K
U.S. SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Report of Foreign Private Issuer
Pursuant to Rule 13a-16 or 15d-16 of
the Securities Exchange Act of 1934
Commission File Number: 001-38757
For the month of May 2019
TAKEDA PHARMACEUTICAL COMPANY LIMITED
(Translation of registrant’s name into English)
1-1, Nihonbashi-Honcho 2-Chome
Chuo-ku, Tokyo 103-8668
Japan
(Address of principal executive offices)
Indicate by check mark whether the registrant files or will file annual reports under cover Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): ☐
Table of Contents
Information furnished on this form:
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| TAKEDA PHARMACEUTICAL COMPANY LIMITED | ||||||
| Date: May 30, 2019 | By: | /s/ Takashi Okubo | ||||
| Takashi Okubo | ||||||
| Global Head of Investor Relations | ||||||
Table of Contents

Dear Takeda Shareholder,
It is my great pleasure to invite you, on behalf of the Takeda Board of Directors, to our 2019 Annual Shareholders Meeting, to be held on June 27th 2019. Exceptionally this year, the meeting will take place outside of Osaka, as a consequence of the G20 Summit meeting being hosted for the first time by the Japanese government in Osaka at the end of June. We apologize to any shareholders inconvenienced by this change in location.
During our shareholders meeting, we look forward to updating you on the integration following the Shire acquisition, and the transformation of Takeda into a very competitive values-based, R&D-driven, global biopharmaceutical leader headquartered in Japan.
Since January 8th 2019, we have been working relentlessly to execute the integration, in order for the New Takeda to operate efficiently as soon as possible. We are pleased to inform you that it is progressing with significant pace. As an example, Day One, when all sites became Takeda and all employees could communicate by e-mail, was executed perfectly without business disruption.
Our strong business momentum was also clearly demonstrated in our excellent FY2018 results, which we disclosed on May 14th 1, along with FY2019 guidance and our mid-term outlook for the combined company. We are confident about the growth prospect for the company, driven by key products in our focus business areas, a lean and innovative R&D engine, and financial strength to realize top-tier margins and quickly reduce our debt level.
Takeda’s values, Takeda-ism: Integrity-Honesty-Fairness-Perseverance, combined with a specific focus on Patient-Trust-Reputation-Business is attractive to and embraced by our employees. As part of the integration, we have been conducting numerous training sessions globally using case studies to ensure a common values-based culture. In fact, our first employee integration survey showed that 79% of our employees are excited by the company’s future, a particularly high score versus benchmarks, in spite of being during a period of uncertainty for many people.
The difficult and sensitive selection process for management positions is on-going with speed, but also with fairness and transparency. This is extremely important as it not only answers our employees’ expectations, but is also very much aligned with our values.
1 FY2018 Full Year Results (released on May 14th, 2019)
1
Table of Contents
We are more confident than ever before that the integration is being executed in a way that preserves our long-established identity while creating a more competitive company in a challenging environment.
Indeed, while the pace of innovation is stronger than ever, many healthcare systems around the world are facing significant financial challenges. As a consequence, payers are becoming increasingly selective in determining which innovation will be reimbursed and when to be prescribed. They are also more actively promoting generic and biosimilar alternatives, and are more and more inclined to reduce drug prices.
The new Takeda is well positioned among the large pharmaceutical companies to prevail in this environment.
Our current portfolio is primarily composed of life-saving or life-transforming, highly differentiated medicines which we are aiming to make available to patients throughout the world. Across our five key business areas (GI, Rare Diseases, Plasma-Derived Therapies, Oncology, and Neuroscience), fourteen global innovative medicines will drive the majority of our future growth. The distribution of growth across these innovative therapies allows us to better withstand market uncertainty. Furthermore, our business footprint is now truly global with a leading position now and in the future in Japan, a formidable presence in the U.S.A, and a competitive status in many other countries including China. Indeed, Takeda is now capable of effectively launching our medicines in 80 countries.
We are an R&D-driven company with the aim to discover and develop highly innovative medicines for patients in four therapy areas (Oncology, Gastroenterology, Rare Diseases and Neuroscience). Our willingness and ability to collaborate with innovative partners (e.g. biotech, start-ups, academia, etc.) in these therapy areas is yielding tangible results with approximately 200 partnerships now on-going. Our transformed R&D engine is enriched in key skill sets such as expertise in our core therapeutic areas, in translational medicine and in data sciences. We have an exciting pipeline with multiple innovative new therapies beginning to emerge, some of which offer life-saving potential to patients such as those in our growing cell and gene therapy portfolio. Our R&D organization has been optimized to maximize output by combining internal scientific strength with innovation from our partner network. This unique model will improve our productivity and increase our efficiency. With an extremely lean and agile organization compared to other top 10 pharmaceutical companies, we have the ability to provide new innovative medicines and vaccines to patients and to deliver significantly improved returns on our R&D investment. We indeed aspire to be one of the most productive R&D engines in the industry. We will share with you more details during an R&D day before the end of this calendar year.
2
Table of Contents
Beyond our core therapeutic areas of Oncology, Gastroenterology, Rare Diseases and Neuroscience, we are dedicated to serving patients with a wide range of rare diseases with our innovative plasma-derived therapies, and hope to soon file regulatory submissions for a dengue vaccine that will serve a massive public health need. The Plasma-Derived Therapy and Vaccine Business Units will ensure dedicated and targeted long-term focus on these specific areas in order to serve patient needs.
Thanks to substantial integration cost synergies and continued cost discipline, we are driving towards margins among the top-tier in the industry. This combined with our growing portfolio will allow us to rapidly reduce the debt on our balance sheet, continue to invest in our growth drivers, and maintain our well-established dividend policy of 180 yen per share annually as a key component of shareholder return. We are committed to deleverage within three to five years driven by strong cash flows, and helped also by a disposal program of non-core assets such as Xiidra® (lifitegrast ophthalmic solution) and TachoSil® Fibrin Sealant Patch, announced in early May2. Once deleveraged, our financial resilience will be significant and highly competitive which will open further opportunities.
The Takeda Executive Team is comprised of twenty strong, diverse (e.g. eleven nationalities, seven females), extremely experienced leaders, and is poised to lead Takeda through its next stage of growth. Our corporate governance is extremely robust against global standards, with a board of fifteen directors including eleven external directors, an Audit and Supervisory Committee, a Nomination Committee and a Compensation Committee. Every committee is chaired by an external director and is comprised of a majority of external directors. The board meeting is also chaired by an external director.
This is an exciting time for Takeda, the health care providers and their patients treated by our medicines, for our employees and for you our shareholders.
We hope that you will share with us our enthusiasm for the future, and are looking forward to seeing you on June 27th.
With my best regards,

Christophe Weber
President & CEO
Takeda Pharmaceutical Company Limited
2 Press Release: TAKEDA SIMPLIFIES PORTFOLIO AND ACCELERATES DELEVERAGING THROUGH TWO DIVESTITURES (released on May 9th, 2019).
3
Table of Contents
Please note that the following is an English translation of the original Japanese version, prepared only for the convenience of shareholders residing outside Japan. In case of any discrepancy between the translation and the Japanese original, the latter shall prevail.
TAKEDA PHARMACEUTICAL COMPANY LIMITED (the “Company” or “TAKEDA”) HEREBY DISCLAIMS ALL REPRESENTATIONS AND WARRANTIES WITH RESPECT TO THIS TRANSLATION, WHETHER EXPRESS OR IMPLIED INCLUDING, BUT WITHOUT LIMITATION TO, ANY REPRESENTATIONS OR WARRANTIES WITH RESPECT TO ACCURACY, RELIABILITY OR COMPLETENESS OF THIS TRANSLATION. IN NO EVENT SHALL TAKEDA BE LIABLE FOR ANY DAMAGES OF ANY KIND OR NATURE INCLUDING, BUT WITHOUT LIMITATION TO, DIRECT, INDIRECT, SPECIAL, PUNITIVE, CONSEQUENTIAL OR INCIDENTAL DAMAGES ARISING FROM OR IN CONNECTION WITH THIS TRANSLATION.
Better Health, Brighter Future
Notice of Convocation of the 143rd Ordinary General Meeting of Shareholders
| Date | : June 27, 2019 (Thursday), 10:00 a.m. (The reception is scheduled to open at 8:50 a.m.) |
| Venue | : PACIFICO Yokohama, National Convention Hall |
| Notice of Convocation of the 143rd Ordinary General Meeting of Shareholders |
1 | |||
| 5 | ||||
| Documents Enclosed with the Notice of Convocation of the 143rd Ordinary General Meeting of Shareholders |
||||
| 51 | ||||
| 101 | ||||
| 104 | ||||
| 106 | ||||
| Guidance Notes on the Exercise of Voting Rights via Electronic Means (e.g., the Internet, etc.) |
112 | |||
| (Reference) |
||||
| 113 | ||||
Venue of the General Meeting of Shareholders
Please note that the venue for this General Meeting of Shareholders is different from that of last year. Please refer to the map at the end of this notice and ensure that you attend the correct venue. (The map is omitted in this translation.)
No Gifts for Attendees of the General Meeting of Shareholders
Please kindly note that no gifts will be given to attendees at this General Meeting of Shareholders. Thank you very much for your kind understanding.
| Takeda Pharmaceutical Company Limited | Securities Code: 4502 |
Table of Contents
| Securities Code: 4502 | ||
| June 5, 2019 | ||
Dear Shareholders
Notice of Convocation of the 143rd Ordinary General Meeting of Shareholders
This is to inform you that the Company will be holding its 143rd Ordinary General Meeting of Shareholders (the “Meeting”) as follows and invite you to attend.
If you are unable to attend the Meeting, you may exercise your voting rights in writing or via electronic means (e.g., the Internet, etc.). Please kindly go through the Reference Document for the General Meeting of Shareholders and exercise your voting rights no later than 5:30 p.m. on June 26, 2019 (Wednesday).
Details
|
1. |
Date: |
June 27, 2019 (Thursday), 10:00 a.m. | ||||
| (The reception is scheduled to open at 8:50 a.m.) | ||||||
| 2. | Venue: | PACIFICO Yokohama, National Convention Hall | ||||
| 1-1-1, Minato Mirai, Nishi-ku, Yokohama, Japan | ||||||
|
(Please refer to the map at the end of this notice and ensure that you attend the correct venue, since the venue for this General Meeting of Shareholders is different from that of last year.) | ||||||
| (The map is omitted in this translation.) | ||||||
| 3. Objectives of the Meeting: | ||||||
| Matters to be reported: | ||||||
| 1. | Reports on the Business Report, Consolidated Financial Statements and Unconsolidated Financial Statements for the 142nd fiscal year (from April 1, 2018 to March 31, 2019) | |||||
| 2. | Reports on the Audit Reports on the Consolidated Financial Statements for the 142nd fiscal year by the Accounting Auditors and Audit and Supervisory Committee | |||||
| Matters to be resolved: | ||||||
| <The Company’s proposals (First to Sixth Proposals)> | ||||||
| First Proposal: | Appropriation of Surplus | |||||
| Second Proposal: | Election of Twelve (12) Directors who are not Audit and Supervisory Committee Members | |||||
| Third Proposal: | Election of Two (2) Directors who are Audit and Supervisory Committee Members | |||||
| Fourth Proposal: | Revisions Pertaining to the Amount and the Contents of Stock Compensation, etc. for Directors who are not Audit and Supervisory Committee Members | |||||
| Fifth Proposal: | Revisions Pertaining to the Contents of Stock Compensation, etc. for Directors who are Audit and Supervisory Committee Members | |||||
| Sixth Proposal: | Payment of Bonuses to Directors who are not Audit and Supervisory Committee Members | |||||
| <Shareholders’ proposal (Seventh Proposal and Eighth Proposal)> | ||||||
| Seventh Proposal: | Partial Amendment to the Articles of Incorporation (Individual disclosure of the directors’ compensation) | |||||
| Eighth Proposal: | Partial Amendment to the Articles of Incorporation (Adoption of a clawback clause) | |||||
|
The contents of the proposals above are described in the Reference Document for the General Meeting of Shareholders below (pages 5 to 50 herein).
| ||||||
1
Table of Contents
Please note that the Company decided to hold the Meeting on June 27, 2019 in Yokohama since the Company prioritized the retention of a venue with a large capacity in other area, as it is expected that the G20 Summit (Summit on Financial Markets and the World Economy) is being held in Osaka in late June.
2
Table of Contents
Guidance Notes on the Exercise of Voting Rights
| • | Exercise of Voting Rights by Attending the Meeting |
Please be so kind as to submit the enclosed Voting Right Exercise Form to a receptionist at the venue as evidence of your attendance. We also ask that you bring this Notice of Convocation with you to the venue. (The Voting Right Exercise Form is omitted in this translation.)
Date: June 27, 2019 (Thursday), 10:00 a.m. (The reception is scheduled to open at 8:50 a.m.)
| • | Exercise of Voting Rights in Writing |
Please indicate your approval or disapproval of the proposals on the enclosed “Voting Right Exercise Form” and send it back to reach us before the deadline below. (The Voting Right Exercise Form is omitted in this translation.)
Deadline for Exercise (arrival): 5:30 p.m. on June 26, 2019 (Wednesday)
| • | Exercise of Voting Rights via Electronic Means (e.g.: the Internet, etc.) |
Please refer to the “Guidance Notes on the Exercise of Voting Rights via Electronic Means (e.g., the Internet, etc.)” on page 112, and complete the entry of your approval or disapproval of the proposals in accordance with the instructions on the screen on or before the deadline below.
Deadline for Exercise (completion of entry): 5:30 p.m. on June 26, 2019 (Wednesday)
Guidance Notes on the Treatment of Exercise of Voting Rights
| (1) | If you exercise your voting rights both in writing and via electronic means (e.g., the Internet, etc.), the Company will regard only the vote cast via electronic means (e.g., the Internet, etc.) as valid, regardless of the time and date the votes are received. |
| (2) | If you exercise your voting rights more than once via electronic means (e.g., the Internet, etc.), the Company will regard only your last vote as valid. |
| (3) | If you exercise your voting rights by proxy, you may delegate your voting rights to one shareholder who holds voting rights in the Company. However, please note that you are required to submit a document certifying the authority of such proxy. |
| (4) | If neither “for” nor “against” is marked on the submitted Voting Right Exercise Form, with regard to the Company’s proposals, it will be treated as a consent for the relevant proposal(s), and with regard to the Shareholders’ proposals, it will be treated as a dissent for the relevant proposal(s). |
Disclosure of information via the Internet
| • | The documents listed below have been posted on the Company’s website based on laws and regulations and Article 14 of the Company’s Articles of Incorporation and have not been included in this Notice of Convocation. |
| 1. | Consolidated Statement of Changes in Equity on the Consolidated Financial Statements |
| 2. | Notes on the Consolidated Financial Statements |
| 3. | Unconsolidated Statement of Changes in Net Assets on the Unconsolidated Accounts |
| 4. | Notes on the Unconsolidated Accounts |
3
Table of Contents
The Consolidated Financial Statements and Unconsolidated Financial Statements that the Accounting Auditors and Audit and Supervisory Committee audited include, apart from the documents stated in the list of documents enclosed with the Notice of Convocation of the 143rd Ordinary General Meeting of Shareholders, the Consolidated Statement of Changes in Equity, the Notes on the Consolidated Financial Statements, the Unconsolidated Statement of Changes in Net Assets and the Notes on the Unconsolidated Accounts posted on the Company’s website.
| • | Any modification made to the Reference Document for the General Meeting of Shareholders and the Business Report, Unconsolidated Financial Statements and Consolidated Financial Statements shall be communicated by posting the modified information on the Company’s website. |
| Company’s website | https://www.takeda.com/investors/reports/shareholders-meetings/ |
| Yours faithfully, |
| Christophe Weber President and Representative Director Takeda Pharmaceutical Company Limited 1-1, Doshomachi 4-chome Chuo-ku, Osaka 540-8645, Japan |
END OF DOCUMENT
4
Table of Contents
Reference Document for the General Meeting of Shareholders
Proposals and Reference Matters:
<Company’s proposals (First to Sixth Proposals)>
First Proposal: Appropriation of Surplus
The Company’s policy in the allocation of capital is as follows:
| • | Short-range Deleveraging; |
| • | Investing in the Growth Driver; |
| • | Return to the Shareholders. |
With regard to “Short-range Deleveraging,” the Company is committed to keep the investment-grade rating with the target lowering the net interest-bearing debt adjusted EBITDA to x2 within 3 to 5 years.
With regard to “Investing in the Growth Driver,” the Company strategically makes investments in the internal R&D and new product launches and conducts R&D collaborations focused on the therapeutic areas with discipline.
With regard to “Return to the Shareholders,” the Company keeps annual 180 yen dividends per share as the established policy.
Based on the policy above, the Company submits the following proposal with respect to the appropriation of surplus for this fiscal year:
Year-end dividends
| (1) | Type of dividend asset |
Cash
| (2) | Allocation of dividend asset to shareholders and total amount of allocation |
90 JPY per share of common stock;
Total amount: 140,835,668,220 JPY
(Reference)
Combined with the interim dividend of 90 JPY per share, the annual dividend will be 180 JPY per share (the same amount as in the previous fiscal year).
| (3) | Effective date of distribution of the dividend |
June 28, 2019
Second Proposal: Election of twelve (12) Directors who are not Audit and Supervisory Committee Members
The term of office of the eleven (11) Directors who are not Audit and Supervisory Committee (ASC) Members, namely, Christophe Weber, Masato Iwasaki, Andrew Plump, Masahiro Sakane, Olivier Bohuon, Ian Clark, Yoshiaki Fujimori, Steven Gillis, Emiko Higashi, Michel Orsinger and Toshiyuki Shiga, will expire at the close of this General Meeting of Shareholders. The Company therefore proposes that the number of Directors be increased by one in order to further reinforce and enhance the Company’s management structure, and proposes the election of these twelve (12) Directors who are not ASC Members, including the eight (8) External Directors.
The candidates for Directors who are not ASC Members are as follows. (The photographs of the candidates are omitted in this translation.):
5
Table of Contents
| Candidate No. |
Name |
Current position and responsibilities |
Tenure as |
Number of Board of attended | ||||||
| 1 |
Christophe Weber | To be reelected | President and Representative Director Chief Executive Officer | 5 years | 12/12 (100%) | |||||
| 2 |
Masato Iwasaki | To be reelected | Director President, Japan Pharma Business Unit |
7 years | 12/12 (100%) | |||||
| 3 |
Andrew Plump | To be reelected | Director President, Research and Development | 4 years | 12/12 (100%) | |||||
| 4 |
Constantine Saroukos | To be newly elected | Corporate Officer Chief Financial Officer | — | — | |||||
| 5 |
Masahiro Sakane | To be reelected as External Director Independent Director | Director Chair of the Board of Directors meeting |
5 years | 12/12 (100%) | |||||
| 6 |
Olivier Bohuon | To be reelected as External Director Independent Director | Director | 6 months | 1/2(-) | |||||
| 7 |
Ian Clark | To be reelected as External Director Independent Director | Director | 6 months | 1/2(-) | |||||
6
Table of Contents
| 8 |
Yoshiaki Fujimori | To be reelected as External Director Independent Director | Director | 3 years | 12/12 (100%) | |||||
| 9 |
Steven Gillis | To be reelected as External Director Independent Director | Director | 6 months | 2/2 (-) | |||||
| 10 |
Toshiyuki Shiga | To be reelected as External Director Independent Director | Director | 3 years | 12/12 (100%) | |||||
| 11 |
Jean-Luc Butel | To be newly elected as External Director Independent Director | Director ASC Member |
3 years | 9/10 (90%) | |||||
| 12 |
Shiro Kuniya | To be newly elected as External Director Independent Director | Director Head of the ASC |
3 years | 12/12 (100%) | |||||
| (Notes) |
1 | Directors Olivier Bohuon, Ian Clark and Steven Gillis were elected at the Extraordinary General Meeting of Shareholders held on December 5, 2018 and took office as of January 8, 2019. Accordingly, the Board of Directors meetings where their attendance was sought were the meetings held thereafter. |
| 2 | With regard to the Director Jean-Luc Butel’s “Number of Board of Directors meeting attended” in the table above, 2 Extraordinary meetings of Board of Directors are excluded therefrom because they were held only for discussing the acquisition of Shire and he didn’t join in order to avoid a conflict of interest as he was a shareholder of Shire. |
7
Table of Contents
| Candidate No.1 |
Christophe Weber |
Number of Company Shares Owned (of which, number of Company Shares to be provided |
293,592 shares (145,392 shares) | |||
| (Photo)
|
Profile, Position and Responsibilities at the Company, and Important Duties Concurrently Held | |||||
|
May 2008 |
Senior Vice President & Regional Director, Asia Pacific, GlaxoSmithKline | |||||
|
April 2012 |
President & General Manager, GlaxoSmithKline Vaccines | |||||
|
April 2012 |
CEO, GlaxoSmithKline Biologicals | |||||
|
April 2012 |
Member of GlaxoSmithKline Corporate Executive Team | |||||
|
Born on November 14, 1966 (52 years old)
To be Reelected as Internal Director
Tenure as Director: 5 years
Attended 12 of the 12 meetings (100%) of the Board of Directors |
April 2014 |
Chief Operating Officer of the Company | ||||
|
April 2014 |
Corporate Officer of the Company | |||||
|
June 2014 |
President and Representative Director of the Company (to present) | |||||
|
April 2015 |
Chief Executive Officer of the Company (to present) | |||||
[Reason for Election as Director]
Showed strong leadership in transforming Takeda into a sustainable and profitable organization that always puts patients at the center by implementing mid-term key priorities: focusing on key products of Growth Drivers, reinforcing specialty capabilities and pursuing opportunities to divest or acquire assets. Strongly committed to talent development and succession planning; lead the Takeda Executive Team to meet several times per year to discuss talent development and succession planning, launched an international cross-divisional development program to train high potential talents at an early stage of their careers.
The Company believes his competency and experience as CEO are necessary for its success.
8
Table of Contents
| Candidate No.2 |
Masato Iwasaki |
Number of Company Shares Owned (of which, number of Company Shares to be provided |
21,830 shares (8,634 shares) | |||
| (Photo)
|
Profile, Position and Responsibilities at the Company, and Important Duties Concurrently Held | |||||
|
April 1985 |
Joined the Company | |||||
|
April 2008 |
Senior Vice President, Strategic Product Planning Department of the Company | |||||
|
June 2010 |
Corporate Officer of the Company | |||||
|
January 2012 |
Head of CMSO Office, Takeda Pharmaceuticals International, Inc. | |||||
|
Born on November 6, 1958 (60 years old)
To be Reelected as Internal Director
Tenure as Director: 7 years
Attended 12 of the 12 meetings (100%) of the Board of Directors |
April 2012 |
Senior Vice President, Pharmaceutical Marketing Division of the Company | ||||
|
June 2012 |
Director of the Company (to present) | |||||
|
April 2015 |
President, Japan Pharma Business Unit of the Company (to present) | |||||
[Reason for Election as Director]
Supervises Takeda’s drug business in Japan.
Showed strong leadership in transforming the Japan Pharma Business Unit’s business model by divesting long-listed products to a joint-venture company and taking advantage of the changing market environment where generic products are rapidly penetrating. The Company believes his competency and experience are necessary for its drug business in Japan to be a best-in-class organization that keeps its leadership position in the market and be trusted by society considering the environmental change in Japan, including in the progress of the Community-based Integrated Care System Model.
9
Table of Contents
| Candidate No.3 |
Andrew Plump |
Number of Company Shares Owned (of which, number of Company Shares to be provided |
52,831 shares (52,831 shares) | |||
| (Photo)
|
Profile, Position and Responsibilities at the Company, and Important Duties Concurrently Held | |||||
|
January 2007 |
Executive Director, Cardiovascular Disease Franchise Integrator and Head, Cardiovascular Translational Medicine, Merck & Co. | |||||
|
January 2008 |
Vice President, Cardiovascular Disease Franchise Integrator and Head, Cardiovascular Early Development & Cardiovascular Translational Medicine, Merck & Co. | |||||
|
January 2008 |
Vice President, Cardiovascular Disease Franchise, Worldwide Discovery Head, Merck & Co. | |||||
|
July 2012 |
Vice President & Deputy to the President, Research & Translational Medicine, Sanofi | |||||
|
Born on October 13, 1965 (53 years old)
To be Reelected as Internal Director
Tenure as Director: 4 years
Attended 12 of the 12 meetings (100%) of the Board of Directors |
March 2014 |
Senior Vice President & Deputy to the President for Research & Translational Medicine, Sanofi | ||||
|
February 2015 |
Chief Medical & Scientific Officer Designate of the Company | |||||
|
February 2015 |
Corporate Officer of the Company | |||||
|
June 2015 |
Director of the Company (to present) | |||||
|
June 2015 |
Chief Medical & Scientific Officer of the Company | |||||
|
June 2015 |
Executive Vice President, Takeda Pharmaceuticals International, Inc. (to present) | |||||
|
January 2019 |
President, Research and Development (to present) | |||||
[Reason for Election as Director]
Showed strong leadership in rebuilding the R&D pipeline by implementing key priorities: leveraging therapeutic area expertise to progress innovative assets, enhancing capabilities internally through external collaborations, and strengthening the R&D performance culture.
The Company believes his competency and experience as President, Research and Development are necessary for its success.
10
Table of Contents
| Candidate No.4 |
Constantine Saroukos |
Number of Company Shares Owned (of which, number of Company Shares to be provided |
12,613 shares (12,613 shares) | |||
| (Photo)
|
Profile, Position and Responsibilities at the Company, and Important Duties Concurrently Held | |||||
|
April 2011 |
Regional Finance Director – Africa of MERCK SHARP & DHOME | |||||
|
July 2012 |
Executive Finance Director – Eastern Europe, Middle East & Africa of MERCK SHARP & DHOME | |||||
|
October 2013 |
Executive Finance Director – Greater China & Japan of Allergan | |||||
|
September 2014 |
Head of Finance and Business Development for the Asia-Pacific region of Allergan | |||||
|
Born on April 15, 1971 (48 years old)
To be newly elected as Internal Director |
May 2015 |
Chief Financial Officer of the Europe and Canada Business Unit of the Company | ||||
|
April 2018 |
Chief Financial Officer of the Company (to present) | |||||
|
April 2018 |
Corporate Officer of the Company (to present) | |||||
[Reason for Election as Director]
Over 20 years’ experience in both private and public sectors, having held a number of finance leadership positions with financial responsibility for businesses in over 100 countries across Asia-Pacific, Europe, Africa and the Middle East.
Has a long track record of improving operational business profitability and driving performance by combining effective financial stewardship with business partnership. Throughout his career, he has promoted the use of best-practice sharing and talent development to build strong finance business and strategic partners.
The Company believes that his experience and competencies will contribute to the further acceleration of our transformation to create a global, values-based, R&D-driven biopharmaceutical leader.
11
Table of Contents
| Candidate No.5 |
Masahiro Sakane |
Number of Company Shares Owned (of which, number of Company Shares to be provided |
5,418 shares (4,518 shares) | |||
| (Photo)
|
Profile, Position and Responsibilities at the Company, and Important Duties Concurrently Held | |||||
|
April 1963 |
Joined Komatsu Ltd. | |||||
|
June 2001 |
President and Representative Director, Komatsu Ltd. | |||||
|
June 2007 |
Chairman of the Board and Representative Director, Komatsu Ltd. | |||||
|
June 2008 |
External Director, Nomura Holdings, Inc. | |||||
|
Born on January 7, 1941 (78 years old)
To be Reelected as External Director Independent Director
Tenure as Director: 5 years
Attended 12 of the 12 meetings
(100%) of the |
June 2008 |
External Director, Nomura Securities Co., Ltd. | ||||
|
June 2008 |
External Director, Tokyo Electron Limited | |||||
|
June 2010 |
Chairman of the Board, Komatsu Ltd. | |||||
|
March 2011 |
External Director, Asahi Glass Co., Ltd. | |||||
|
April 2013 |
Director and Councilor, Komatsu Ltd. | |||||
|
June 2013 |
Councilor, Komatsu Ltd. (to present) | |||||
|
June 2014 |
External Director of the Company (to present) | |||||
|
June 2015 |
External Director, Kajima Corporation (to present) | |||||
|
June 2017 |
Chair of the Board of Directors meeting of the Company (to present) | |||||
[Reason for Election as Director]
Proactively expresses his opinions at the Board of Directors meetings by leveraging his ample experience as company top management.
Facilitates Board of Directors meetings as well as leads meetings by External Directors, which contribute to the making of fair and appropriate decisions and securing sound management within the Company.
Has also contributed as chairperson of the Nomination Committee of the Company to provide objectivity and transparency in the Director candidate selection process.
12
Table of Contents
| Candidate No.6 |
Olivier Bohuon |
Number of Company Shares Owned (of which, number of Company Shares to be provided |
0 shares (0 shares) | |||
| (Photo)
|
Profile, Position and Responsibilities at the Company, and Important Duties Concurrently Held | |||||
|
January 1998 |
Chief Executive Officer and President, SmithKline Beecham Pharmaceuticals France | |||||
|
January 2001 |
Senior Vice President & Director European Commercial Operations, GlaxoSmithKline Pharmaceuticals Europe | |||||
|
April 2003 |
President Europe & Corporate Officer, Abbott Laboratories | |||||
|
February 2006 |
Corporate Senior Vice President, Abbott Laboratories | |||||
|
Born on January 3, 1959 (60 years old)
To be Reelected as External Director Independent Director
Tenure as Director: 6 months
Attended 1 of the 2 meetings (-) of the Board of Directors |
July 2009 |
Executive Vice President, Abbott Laboratories | ||||
|
September 2010 |
Chief Executive Officer, Pierre Fabre SA | |||||
|
April 2011 |
Chief Executive Officer, Smith & Nephew plc | |||||
|
June 2011 |
External Director, Virbac SA | |||||
|
July 2015 |
External Director, Shire plc | |||||
|
July 2018 |
External Director, Smiths Group plc (to present) | |||||
|
August 2018 |
External Director and Vice Chairman, LEO Pharma A/S | |||||
|
January 2019 |
External Director of the Company (to present) | |||||
|
February 2019 |
External Director and Chairman of the Board, LEO Pharma A/S (to present) | |||||
[Reason for Election as Director]
He has necessary and sufficient expertise in Legacy Shire’s portfolio and its related therapeutic areas through his experience as an external director of Shire. In addition to his experience at Shire, he has each held key positions, including as CEO, in healthcare companies in Europe and the U.S. and has a deep insight into the management of global healthcare businesses based on their ample experience therein. Among other areas, he has remarkable expertise in the area of marketing in overall healthcare businesses.
13
Table of Contents
| Candidate No.7 |
Ian Clark |
Number of Company Shares Owned (of which, number of Company Shares to be provided |
0 shares (0 shares) | |||
| (Photo)
|
Profile, Position and Responsibilities at the Company, and Important Duties Concurrently Held | |||||
|
December 2005 |
Executive Vice President of Commercial Operations, Genentech, Inc. | |||||
|
April 2009 |
Executive Vice President of Global Marketing, Head of Global Product Strategy and Chief Marketing Officer, Genentech, Inc. | |||||
|
January 2010 |
Director, Chief Executive Officer and Head of North American Commercial Operations, Genentech, Inc. | |||||
|
December 2016 |
External Director, Agios Pharmaceuticals, Inc. (to present) | |||||
|
Born on August 27, 1960 (58 years old)
To be Reelected as External Director Independent Director
Tenure as Director: 6 months
Attended 1 of the 2 meetings (-) of the Board of Directors |
January 2017 |
External Director, Shire plc | ||||
|
January 2017 |
External Director, Corvus Pharmaceuticals, Inc. (to present) | |||||
|
January 2017 |
External Director, Guardant Health, Inc. (to present) | |||||
|
November 2017 |
External Director, AVROBIO Inc. (to present) | |||||
|
April 2018 |
External Director, Forty Seven Inc. (to present) | |||||
|
January 2019 |
External Director of the Company (to present) | |||||
[Reason for Election as Director]
He has necessary and sufficient expertise in Legacy Shire’s portfolio and its related therapeutic areas through his experience as an external director of Shire. In addition to his experience at Shire, he has each held key positions, including as CEO, in healthcare companies in Europe and the U.S. and has a deep insight into the management of global healthcare businesses based on their ample experience therein. Among other areas, he has remarkable expertise in marketing in the area of oncology and the operation of the science and technology division of a healthcare company.
14
Table of Contents
| Candidate No.8 |
Yoshiaki Fujimori |
Number of Company Shares Owned (of which, number of Company Shares to be provided |
6,718 shares (4,518 shares) | |||
| (Photo)
|
Profile, Position and Responsibilities at the Company, and Important Duties Concurrently Held | |||||
|
May 2001 |
Senior Vice President, General Electric Company | |||||
|
October 2008 |
Representative Director, Chairman, President and CEO, General Electric Japan Ltd. | |||||
|
March 2011 |
Representative Director and Chairman, GE Japan Corporation | |||||
|
June 2011 |
Director, LIXIL Corporation | |||||
|
Born on July 3, 1951 (67 years old)
To be Reelected as External Director Independent Director
Tenure as Director: 3 years
Attended 12 of the 12 meetings (100%) of the Board of Directors |
June 2011 |
Director, LIXIL Group Corporation | ||||
|
August 2011 |
Representative Director, President and CEO, LIXIL Corporation | |||||
|
August 2011 |
Director, Representative Executive Officer, President and CEO, LIXIL Group Corporation | |||||
|
June 2012 |
External Director, Tokyo Electric Power Company, Incorporated (currently Tokyo Electric Power Company Holdings, Inc.) (to present) | |||||
|
January 2016 |
Representative Director, Chairman and CEO, LIXIL Corporation | |||||
|
June 2016 |
Senior Advisor, LIXIL Group Corporation (to present) | |||||
|
June 2016 |
External Director of the Company (to present) | |||||
[Reason for Election as Director]
Proactively expresses his opinions at the Board of Directors meetings by leveraging his ample experience as company top management, which contributes to the making of fair and appropriate decisions and securing sound management within the Company. Actively participates in the discussions at the Compensation Committee based on his experience as top management of a global operating company, providing objectivity and transparency in the Company’s compensation plan for Directors.
15
Table of Contents
| Candidate No.9 |
Steven Gillis |
Number of Company Shares Owned (of which, number of Company Shares to be provided |
0 shares (0 shares) | |||
| (Photo)
|
Profile, Position and Responsibilities at the Company, and Important Duties Concurrently Held | |||||
|
August 1981 |
Founder, Director and Executive Vice President, Research and Development, Immunex Corporation (currently, Amgen, Inc.) | |||||
|
June 1988 |
President and Chief Operating Officer, Immunex Research and Development Corporation | |||||
|
July 1990 |
President and Chief Executive Officer, Immunex Research and Development Corporation | |||||
|
May 1993 |
Chief Executive Officer, Immunex Corporation | |||||
|
Born on April 25, 1953 (66 years old)
To be Reelected as External Director Independent Director
Tenure as Director: 6 months
Attended 2 of the 2 meetings (-) of the Board of Directors |
October 1994 |
Founder, Director and Chief Executive Officer, Corixa Corporation (currently, GlaxoSmithKline) | ||||
|
January 1999 |
Director and Chairman, Corixa Corporation | |||||
|
August 2005 |
Managing Director, ARCH Venture Partners (to present) | |||||
|
October 2009 |
External Director, Pulmatrix, Inc. (to present) | |||||
|
October 2012 |
External Director, Shire plc | |||||
|
May 2016 |
External Director and Chairman, VBI Vaccines, Inc. (to present) | |||||
|
January 2019 |
External Director of the Company (to present) | |||||
[Reason for Election as Director]
He has necessary and sufficient expertise in Legacy Shire’s portfolio and its related therapeutic areas through his experience as an external director of Shire. In addition to his experience at Shire, he has each held key positions, including as CEO, in healthcare companies in Europe and the U.S. and has a deep insight into the management of global healthcare businesses based on their ample experience therein. Among other areas, he has remarkable expertise with a Ph.D. in Biological Sciences, in the area of immune-related healthcare businesses.
16
Table of Contents
| Candidate No.10 |
Toshiyuki Shiga |
Number of Company Shares Owned (of which, number of Company Shares to be provided |
6,318 shares (4,518 shares) | |||
| (Photo)
|
Profile, Position and Responsibilities at the Company, and Important Duties Concurrently Held | |||||
|
April 1976 |
Joined Nissan Motor Co., Ltd. | |||||
|
April 2000 |
Senior Vice President (Officer), Nissan Motor Co., Ltd. | |||||
|
April 2005 |
Chief Operating Officer, Nissan Motor Co., Ltd. | |||||
|
June 2005 |
Director, Nissan Motor Co., Ltd. | |||||
|
Born on September 16, 1953 (65 years old)
To be Reelected as External Director Independent Director
Tenure as Director: 3 years
Attended 12 of the 12 meetings (100%) of the Board of Directors |
May 2010 |
Chairman, Japanese Automobile Manufacturers Association, Inc. | ||||
|
November 2013 |
Vice Chairman, Nissan Motor Co., Ltd. | |||||
|
April 2014 |
Vice Chairman, KEIZAI DOYUKAI (Japan Association of Corporate Executives) | |||||
|
June 2015 |
Chairman and CEO, Innovation Network Corporation of Japan | |||||
|
June 2016 |
External Director of the Company (to present) | |||||
|
June 2017 |
Director, Nissan Motor Co., Ltd. (to present) | |||||
|
September 2018 |
Chairman and CEO, INCJ, Ltd. (to present) | |||||
[Reason for Election as Director]
Proactively expresses his opinions at the Board of Directors meetings by leveraging his ample experience as company top management as well as his expertise in general industries in Japan, which contributes to the making of fair and appropriate decisions and securing sound management within the Company.
As chairperson, he actively led discussions at the Compensation Committee by expressing opinions based on his experience as a top executive of a global operating company, providing objectivity and transparency in the Company’s compensation plan for Directors.
17
Table of Contents
| Candidate No. 11 |
Jean-Luc Butel |
Number of Company Shares Owned (of which, number of Company Shares to be provided |
6,532 shares (6,532 shares) | |||
| (Photo)
|
Profile and Important Duties Concurrently Held | |||||
|
January 1994 |
President, Nippon Becton Dickinson Company, Ltd. | |||||
|
January 1998 |
Corporate Officer, President, Worldwide Consumer Healthcare, Becton, Dickinson and Company | |||||
|
November 1999 |
President, Independence Technology, Johnson & Johnson | |||||
|
August 2003 |
Corporate Officer, Executive Committee Member, Senior Vice President and President, Asia Pacific, Medtronic, Inc. | |||||
|
Born on November 8, 1956 (62 years old)
To be newly elected as External Director Independent Director
Tenure as Director: 3 years
Attended 9 of the 10 (90%) meetings of the Board of Directors |
May 2008 |
Corporate Officer, Executive Committee Member, Executive Vice President and Group President, International, Medtronic, Inc. | ||||
|
February 2012 |
Corporate Officer, Operating Committee Member and Corporate Vice President, Baxter International Inc. | |||||
|
January 2015 |
President, International, Baxter International Inc. | |||||
|
July 2015 |
Global Healthcare Advisor, President, K8 Global Pte. Ltd. (to present) | |||||
|
June 2016 |
External Director of the Company who is an ASC Member (to present) | |||||
|
September 2017 |
External Director, Novo Holdings A/S (to present) | |||||
[Reason for Election as Director]
He has ample experience as top management of major western healthcare companies, which contributes to the making of fair and appropriate decisions and securing sound management within the Company.
He has served as External Director who is an ASC Member of the company since 2016.
18
Table of Contents
| Candidate No.12 |
Shiro Kuniya |
Number of Company Shares Owned (of which, number of Company Shares to be provided |
6,318 shares (4,518 shares) | |||
| (Photo)
|
Profile and Important Duties Concurrently Held | |||||
|
April 1982 |
Registered as an attorney-at-law (Osaka Bar Association) | |||||
|
April 1982 |
Joined Oh-Ebashi Law Offices | |||||
|
May 1987 |
Registered as an attorney-at-law at New York Bar Association | |||||
|
June 1997 |
External Corporate Auditor, Sunstar Inc. | |||||
|
Born on February 22, 1957 (62 years old)
To be newly elected as External Director Independent Director
Tenure as Director: 3 years
Attended 12 of the 12 meetings (100%) of the Board of Directors |
April 2002 |
Managing Partner, Oh-Ebashi LPC & Partners (to present) | ||||
|
June 2006 |
External Corporate Auditor, NIDEC CORPORATION | |||||
|
April 2011 |
Chairman, Inter-Pacific Bar Association | |||||
|
March 2012 |
External Director, NEXON Co., Ltd. (to present) | |||||
|
June 2012 |
External Director, EBARA CORPORATION (to present) | |||||
|
June 2013 |
External Corporate Auditor of the Company | |||||
|
June 2013 |
External Director, Sony Financial Holdings Inc. (to present) | |||||
|
June 2016 |
External Director of the Company who is the Head of the ASC (to present) | |||||
[Reason for Election as Director]
As a lawyer, he has wide-ranging experience and expertise in the area of corporate and international legal affairs although he has never been directly involved in company management. He has also contributed as a member of the Nomination Committee of the Company to provide objectivity and transparency in the Director candidate selection process. He has served as External Corporate Auditor since 2013, and External Director who is the Head of ASC since 2016.
19
Table of Contents
(Notes)
| 1. | No special interests exist between the above candidates and the Company. |
| 2. | For the above candidates, the “Number of Company Shares Owned” includes the number of Company shares to be provided (as of March 31, 2019) under the stock compensation plan (for Mr. Andrew Plump and Mr. Constantine Saroukos, under the stock grant plan). Such Company shares are to be provided to each of the directors during his/her term of office or at the time of his/her retirement. |
[Description of the number of Company Shares to be provided under the Stock Compensation Plan, etc.]
The Company introduced a stock compensation plan for Directors (excluding Directors residing overseas who are not External Directors) and a stock grant plan for executives of the Takeda Group in Japan and overseas (collectively, the “Plan”).
The Company shares to be provided under the stock compensation plan for Directors who are not External Directors (excluding Directors who are Audit and Supervisory Committee Members and Directors residing overseas) (“Directors who are eligible for performance-linked compensation”) and the stock grant plan for executives of the Takeda Group in Japan and overseas include the following:
| (i) | a fixed portion which is not linked to the Company’s performance (“Fixed Portion”); and |
| (ii) | a variable portion which is linked to the Company’s performance (“Performance-based Portion”). |
The number of Company shares to be provided to the above candidates in accordance with the Plan includes only the Fixed Portion under (i) above, since such number of Company shares to be provided is already fixed. The number of Company shares relating to the Performance-based Portion under (ii) above is not yet included, since it will vary in the range of 0-200% and is therefore not fixed at this moment. The provision of Company shares under (i) Fixed Portion and (ii) Performance-based Portion to the Directors who are eligible for performance-linked compensation will be made at a certain period during their term of office.
The Company shares to be provided under the stock compensation plan for Directors who are Audit and Supervisory Committee Members and External Directors (“Directors who are not eligible for performance-linked compensation”) are included in the “Number of Company Shares to be provided under the Stock Compensation Plan,” since it is to be provided under (i) Fixed Portion, the number of Company shares to be provided to the above candidates is fixed. The provision of Company shares to the Directors who are not eligible for performance-linked compensation will be made at the end of their term of office.
In addition, with regard to Company shares to be provided under the Plan, (a) the voting rights thereof may not be exercised before such shares are provided to each candidate; and (b) 50% of such shares will be sold in the stock market to secure the necessary funds for tax payments and, thereafter, the proceeds thereof will be provided to each candidate.
| 3. | Mr. Masahiro Sakane, Mr. Olivier Bohuon, Mr. Ian Clark, Mr. Yoshiaki Fujimori, Mr. Steven Gillis, Mr. Toshiyuki Shiga, Mr. Jean-Luc Butel and Mr. Shiro Kuniya are candidates to become External Directors who are not Audit and Supervisory Committee Members of the Company. The Company has set the “Internal criteria for independence of external directors” (the contents of such criteria are as set forth on page 25.) and elected the External Directors based on such criteria. All of these 8 persons have met the requirements for Independent Directors based on the regulations of the financial instruments exchanges that the Company is listed on (e.g.: Tokyo Stock Exchange, Inc.). The Company has appointed these 8 persons as Independent Directors and submitted a notification to each exchange. |
| 4. | The Company receives advice, etc., on legal matters on an as needed basis from other lawyers working at Oh-Ebashi LPC & Partners, the law firm where Mr. Shiro Kuniya works concurrently, but the proportion of the annual value of those transactions to the sales of the Company and of Oh-Ebashi LPC & Partners is less than 1% in both cases. In addition, there is no advisory contract between the Company and Oh-Ebashi LPC & Partners. |
20
Table of Contents
| 5. | Kajima Corporation (“Kajima”), where Mr. Masahiro Sakane serves as an External Director, and an employee of Kajima were prosecuted for a suspected violation of the Antimonopoly Act over the Chuo Shinkansen Projects led by Central Japan Railway Company in March 2018. Mr. Masahiro Sakane didn’t recognize the above fact in advance, however, he has consistently expressed his opinion on the importance of compliance, including in thoroughly complying with applicable laws and regulations, at the Board of Directors meetings and on other occasions at Kajima. After recognizing the fact of the suspected violation mentioned above, Mr. Masahiro Sakane requested Kajima to investigate the matter and performed his duties, including by expressing his opinion on the improvement of the compliance system within the Kajima group and promotion of activities related thereto. |
| 6. | Nissan Motor Co., Ltd. (“Nissan”), where Mr. Toshiyuki Shiga serves as a Director, accepted the Japanese Ministry of Land, Infrastructure, Transport and Tourism’s process improvement orders in March 2018 relating to Nissan’s non-conformity with the final vehicle inspection processes at its plants in Japan during the period of September to November 2017. Moreover, Mr. Carlos Ghosn, Nissan’s former Representative Director and Chairman, and Mr. Greg Kelly, Nissan’s former Representative Director, were indicted for violating the Financial Instruments and Exchange Act, namely making false disclosures in annual securities reports, and Nissan, as a legal entity, was also indicted for the same violation on December 10, 2018 and January 11, 2019. In addition, Mr. Carlos Ghosn was indicted for aggravated breach of trust under the Companies Act on January 11 and April 22, 2019. Both of them are currently under judicial proceedings. |
| 7. | The Company has entered into contracts with Mr. Masahiro Sakane, Mr. Olivier Bohuon, Mr. Ian Clark, Mr. Yoshiaki Fujimori, Mr. Steven Gillis, Mr. Toshiyuki Shiga, Mr. Jean-Luc Butel and Mr. Shiro Kuniya limiting the maximum amount of their liability for the damages set forth in Article 423, Paragraph 1 of the Companies Act to the legally stipulated value. If the re-election of Mr. Masahiro Sakane, Mr. Olivier Bohuon, Mr. Ian Clark, Mr. Yoshiaki Fujimori, Mr. Steven Gillis and Mr. Toshiyuki Shiga is approved, the Company plans to continue the same contracts to limit their liability. Also, if the election of Mr. Jean-Luc Butel and Mr. Shiro Kuniya as the Directors who are not Audit and Supervisory Committee Members is approved, the Company plans to conclude the contracts for limitation of liability with them anew. |
21
Table of Contents
Third Proposal: Election of Two (2) Directors who are Audit and Supervisory Committee Members
The two (2) Directors who are Audit and Supervisory Committee (“ASC”) Members, Shiro Kuniya and Jean-Luc Butel will resign at the close of this General Meeting of Shareholders. Therefore, the Company proposes the election of two (2) Directors who are ASC Members. Please note that the Company proposes that the candidates for the Directors who are ASC Members, Emiko Higashi and Michel Orsinger, will be elected as substitutes for the Directors who are ASC Members, Shiro Kuniya and Jean-Luc Butel. Therefore, their term of office will remain until the expiration of the term of office of the Directors who are ASC Members, Shiro Kuniya and Jean-Luc Butel, who will resign in accordance with the provision of the Articles of Incorporation. This proposal was approved by the ASC.
The candidates for Directors who are ASC Members are as follows (The photographs of the candidates are omitted in this translation.):
| Candidate No. |
Name |
Current position and responsibilities |
Tenure as |
Number of Board of Directors meetings | ||||||
| 1 |
Emiko Higashi | To be newly elected as External Director Independent Director ASC Member |
Director | 3 years | 12/12 (100%) | |||||
| 2 |
Michel Orsinger | To be newly elected as External Director Independent Director ASC Member |
Director | 3 years | 12/12 (100%) | |||||
22
Table of Contents
| Candidate No.1 |
Emiko Higashi |
Number of Company Shares Owned (of which, number of Company Shares to be provided |
6,532 shares (6,532 shares) | |||
| (Photo)
|
Profile and Important Duties Concurrently Held | |||||
|
February 1988 |
Director, Wasserstein Perella & Co., Inc. | |||||
|
May 1994 |
Managing Director, Investment Banking, Merrill Lynch & Co. | |||||
|
April 2000 |
CEO, Gilo Ventures, LLC | |||||
|
January 2003 |
Managing Director, Tomon Partners, LLC (to present) | |||||
|
Born on November 6, 1958 (60 years old)
To be newly elected as External Director Independent Director
Tenure as Director: 3 years
Attended 12 of the 12 meetings (100%) of the Board of Directors |
November 2010 |
External Director, KLA-Tencor Corporation (to present) | ||||
|
October 2014 |
External Director, InvenSense Inc. | |||||
|
June 2016 |
External Director, MetLife Insurance K.K. (to present) | |||||
|
June 2016 |
External Director of the Company (to present) | |||||
|
May 2017 |
External Director, Rambus Inc. (to present) | |||||
[Reason for Election as Director (ASC Member)]
Proactively expresses her opinions at the Board of Directors meetings by leveraging her ample experience and wide expertise on healthcare, technology and financial industries, which contributes to the making of fair and appropriate decisions and securing sound management within the Company. Has also contributed as a member of the Nomination Committee of the Company to provide objectivity and transparency in the Director candidate selection process.
The Company believes she would contribute in the realization of the mission of ASC: to ensure the sound and continuous growth of the Company, realize the creation of mid- and long-term corporate value, and establish a good corporate governance system that will accommodate society’s trust.
23
Table of Contents
| Candidate No.2 |
Michel Orsinger |
Number of Company Shares Owned (of which, number of Company Shares to be provided |
6,532 shares (6,532 shares) | |||
| (Photo)
|
Profile and Important Duties Concurrently Held | |||||
|
January 1996 |
Head of Eastern Europe, Sandoz Nutrition, Consumer Health, Novartis AG | |||||
|
July 1997 |
President, Global Medical Nutrition, Consumer Health, Novartis AG | |||||
|
September 1999 |
Regional President, Europe, Middle East and Africa, Consumer Health, Novartis AG | |||||
|
March 2001 |
Chief Executive Officer and President, OTC Division Worldwide, Consumer Health, Novartis AG | |||||
|
Born on September 15, 1957 (61 years old)
To be Reelected as External Director Independent Director
Tenure as Director: 3 years
Attended 12 of the 12 meetings (100%) of the Board of Directors |
October 2004 |
Chief Operating Officer, Synthes, Inc. (currently Johnson & Johnson) | ||||
|
April 2007 |
President and Chief Executive Officer, Synthes, Inc. | |||||
|
June 2012 |
Worldwide Chairman, Global Orthopedics Group, DePuy Synthes Companies, Johnson & Johnson | |||||
|
June 2012 |
Member of Global Management Team, Johnson & Johnson | |||||
|
June 2016 |
External Director of the Company (to present) | |||||
[Reason for Election as Director (ASC Member)]
Proactively expresses his opinions at the Board of Directors meetings by leveraging his ample experience as top management of major western healthcare companies, which contributes to the making of fair and appropriate decisions and securing sound management within the Company.
The Company believes he would contribute in the realization of the mission of ASC: to ensure the sound and continuous growth of the Company, realize the creation of mid- and long-term corporate value, and establish a good corporate governance system that will accommodate society’s trust.
24
Table of Contents
(Notes)
| 1. | No special interests exist between the above candidates and the Company. |
| 2. | For the above candidates, the “Number of Company Shares Owned” includes the number of Company shares to be provided (as of March 31, 2019) under the stock compensation plan. Such Company shares are to be provided to each of the directors at the time of his/her retirement. Please refer to the [Description of the number of Company Shares to be provided under the Stock Compensation Plan, etc.] in Note No.2 of the “Second Proposal: Election of Twelve (12) Directors who are not Audit and Supervisory Committee Members” with regard to the number of shares to be provided. |
| 3. | Ms. Emiko Higashi and Mr. Michel Orsinger are candidates to become External Directors of the Company who are ASC Members. The Company has set the “Internal criteria for independence of External Directors of the Company” (The contents of such criteria are as set forth below) and elected the External Directors based on such criteria. All of these 2 persons have met the requirement for Independent Directors based on the regulations of the financial instruments exchanges that the Company is listed on (e.g., Tokyo Stock Exchange, Inc.). The Company has appointed these 2 persons as Independent Directors and submitted a notification to each exchange. |
| 4. | The Company has entered into contracts with Ms. Emiko Higashi and Mr. Michel Orsinger limiting the maximum amount of their liability for the damages set forth in Article 423, Paragraph 1 of the Companies Act to the legally stipulated value. If their election as Directors who are Audit and Supervisory Committee Members is approved, the Company plans to continue the same contracts to limit their liability. |
<Reference> Internal criteria for the independence of External Directors of the Company
The Company will judge whether an External Director has sufficient independence against the Company with emphasis on his/her meeting the following quality requirements, on the premise that he/she meets the criteria for independence established by the financial instruments exchanges.
The Company believes that such persons will truly meet the shareholders’ expectations as External Directors of the Company, i.e., persons who can exert a strong presence in a diverse group of people that comprise the directors of the Company by proactively continuing to inquire on the nature of, encourage improvement in, and make suggestions regarding the important matters of the Company doing a pharmaceutical business globally, for the purpose of facilitating an impartial and fair judgment of the Company’s business and securing the sound management of the Company.
The Company requires that persons who will be external directors to meet two (2) or more items out of the following four (4) items of quality requirements:
| (1) | He/She has advanced insight derived from experience in corporate management; |
| (2) | He/She has a high level of knowledge in areas requiring high expertise such as accounting and law; |
| (3) | He/She is well versed in the pharmaceutical and/or global business; and |
| (4) | He/She has advanced linguistic skills and/or broad experience, which enables him/her to understand diverse values and to actively participate in discussions with others. |
25
Table of Contents
<Explanation regarding the compensation system for Directors in relation to the Fourth through Sixth Proposals>
Fourth and Fifth Proposals are intended to make the compensation level and compensation structure for Directors the most advanced as a global bio-pharmaceutical company, in order to maximize the Company’s performance and to ensure the successful completion of the integration with Shire.
Among the Directors, the following “(1) the compensation structure for Directors who are eligible for performance-linked compensation” will be carried on in essence in accordance with the compensation structure for Directors (excluding Directors resident overseas and External Directors) which was approved at the 140th Ordinary General Meeting of Shareholders on June 29, 2016. The following “(2) the compensation structure for Directors who are not eligible for performance-linked compensation” consists of Basic Compensation and stock compensation in the appropriate range (non- performance based) based on their roles.
The Sixth Proposal is regarding payment of Directors’ bonuses for fiscal year 2018 (excluding Directors resident overseas and External Directors).
| (1) | The compensation structure for Directors who are eligible for performance-linked compensation |
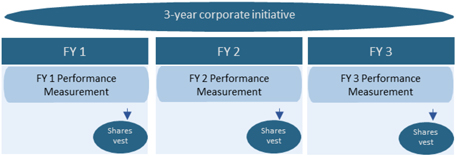
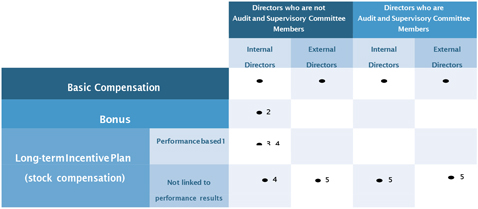
The compensation for Directors who are not Audit and Supervisory Committee Members (excluding External Directors; hereinafter “Directors who are eligible for performance-linked compensation”) consists of “Basic Compensation” which is paid in a fixed amount and “Performance-based Compensation” which is paid in a variable amount based on the achievement, etc. of key performance indicators (KPIs), etc. “Performance-based Compensation” further consists of bonus (Note 1) to be paid based on the consolidated financial results, etc. for each fiscal year, and compensation based on the long-term incentive plan (stock compensation) (Note 2) linked with mid/long-term performance results over 3 years and the Company’s share price. By the Fourth Proposal, the Company proposes to increase the ratio of performance-linked compensation to Basic Compensation to further strengthen the linkage between the Company’s performance and Directors’ compensation and to make other revisions.
| (2) | The compensation structure for Directors who are not eligible for performance-linked compensation |
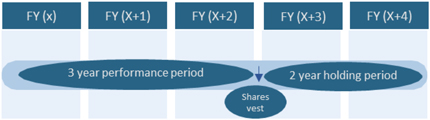
The compensation for Directors who are Audit and Supervisory Committee Members and External Directors (hereinafter “Directors who are not eligible for performance-linked compensation”) consists of “Basic Compensation” which is paid in a fixed amount and “non-performance based compensation” whose payout amount is not related to performance results. Taking into consideration their roles, “non-performance based compensation” is a long-term incentive plan (stock compensation) (Note 3) which is not related to performance results but only to the Company’s share price. By the Fourth Proposal and the Fifth Proposal, the Company proposes changing the timing of payment of non-performance based compensation from the resignation/retirement to the elapse of three (3) years after the award date (meaning the date when base points on which the calculation of such compensation is based will be granted; hereinafter the same for the purposes of this Explanation) to align and maintain consistency of the vesting schedule with Internal Directors and to make other revisions.
26
Table of Contents
(Notes)
| 1. | The payment of the Bonus for the Directors who are eligible for performance-linked compensation ranges from 0% to 200% against the target bonus amount calculated based on the roles and responsibilities of each Director who is eligible for performance-linked compensation, depending on the achievement, etc. of KPIs of business objectives of each fiscal year (consolidated revenue, Core Earnings, Earnings Per Share (EPS) etc.). Regarding Directors who also work as employees, the performance achievement, etc. of the function in charge is also reflected in the variation of bonus payments. |
| 2. | The stock compensation plan for Directors who are eligible for performance-linked compensation is a plan based on the Performance Share system and Restricted Stock system. To enhance commitment to the increase of the corporate value in the mid/long term, Performance Share portion will be linked with the achievement of mid/long-term performance objectives (consolidated revenue, free cash flow, indicators on earnings, R&D target, integration success factor, etc., which are transparent and objective indicators), and payout amount will range in a certain period of time from 0% to 200% against the target amount calculated based on the roles and responsibilities of each Director. |
| 3. | The Stock Compensation for Directors who are not eligible for performance-linked compensation will be up to a ceiling of about 100% of the Basic Compensation for consistency in sharing responsibilities for the Company’s interests as well as corporate value among shareholders and such Directors, and the stock compensation equivalent to the predefined amount regardless of the Company’s performance results, etc. will be paid after the elapse of three (3) years after the award date in order to ensure the adequate supervisory functions which judge the validity of the execution of duties from an objective standpoint and to avoid excessive risk-taking activities to achieve performance results in the short-term. |
|
Directors who are not Audit and Supervisory Committee Members |
Directors who are Audit and Supervisory Committee Members | |||||
|
Internal Directors |
External Directors | Internal Directors | External Directors | |||
|
Directors who are eligible for performance-linked compensation |
Directors who are not eligible for performance-linked compensation | |||||
|
Plan I (*) |
Plan II (*) | Plan III (*) | ||||
|
The Fourth Proposal |
The Fifth Proposal | |||||
| (*) | Please refer to the Fourth Proposal with regard to Plans I and II, and to the Fifth Proposal with regard to Plan III. |
The Company has established the Compensation Committee with an External Director as its Chairperson and a majority of external members, to serve as an advisory organization for the Board of Directors to ensure the appropriateness of Director’s compensation, etc. and transparency in the decision-making process thereof. The revision thereof has been reviewed at the Compensation Committee before resolution by the Board of Directors.
Based on the above, the Company proposes to this General Meeting of Shareholders the Fourth and Fifth Proposals.
27
Table of Contents
As stated above, the compensation plans for Directors who are eligible for performance-linked compensation (excluding Directors who are Audit and Supervisory Committee Members and External Directors) and Directors who are not eligible for performance-linked compensation (Directors who are Audit and Supervisory Committee Members and External Directors) are designed separately but, in the following Fourth Proposal and Fifth Proposal, the Company proposes separately the compensation for Directors who are Audit and Supervisory Committee Members and that for Directors who are not Audit and Supervisory Committee Members.
In the event that the Fourth and Fifth Proposals are approved in the form of the original proposals, the “Director’s Compensation Policy” of the Company will be revised as follows:
| Directors’ Compensation Policy
| ||||
|
1. |
Guiding Principles
|
The Company’s compensation system for Directors has the following guiding principles under the corporate governance code to achieve management objectives: | ||
|
• To attract, retain and motivate managerial talent to realize “Vision 2025” | ||||
| • To increase corporate value through optimizing the Company’s mid- and long-term performance, while reinforcing our patient-focused values
• To be closely linked with company performance, highly transparent and objective
• To support a shared sense of profit with shareholders and improve the managerial mindset focusing on shareholders
• To encourage Directors to challenge and persevere, and to be aligned with the values of Takeda-ism
• To establish transparent and appropriate governance of directors’ compensation to establish the credibility and support of our stakeholders
| ||||
|
2. |
Level of Compensation
|
We aim to be competitive in the global marketplace to attract and retain talent who will continue to transform Takeda into a Global, Values-based, R&D-driven Biopharmaceutical Leader. | ||
| Directors’ compensation should be competitive in the global market consisting of major global companies. Specifically, the global market refers to a “global executive compensation database” developed on the basis of professional survey data with the addition of compensation data from the US, UK and Switzerland, where we need to be competitive with other major pharmaceutical companies.
| ||||
28
Table of Contents
|
3. |
Compensation Mix
|
3-1. Directors who are not Audit & Supervisory Committee Members (excluding External Directors)
| ||
| The compensation of Directors who are not Audit & Supervisory Committee Members (excluding External Directors) consists of “Basic Compensation”, which is paid at a fixed amount and “Performance-based Compensation”, which is paid as a variable amount based on company performance, etc.
| ||||
| “Performance-based Compensation” further consists of a “Bonus” to be paid based on the consolidated financial results, etc. for each fiscal year, and a “Long-term Incentive Plan (stock compensation)” linked with long-term financial results over a 3-year period and with Takeda’s share price.
| ||||
|
⬛ Standard Directors who are not Audit & Supervisory Committee Members (excluding External Directors) Compensation Mix Model
|
The ratio of Long-term Incentives has been increased from prior years (as of fiscal 2018) to better align with the incentives of Takeda’s Directors with Takeda’s shareholders. Moreover, it matches with the peer group and primary industry level. Both Bonus and Long-term incentives as a ratio of Total Direct Compensation is higher putting the directors pay at risk in alignment with the company’s performance. The targets range from 100%-250% of Basic Compensation for “Bonus” and range from 200% to 600% of Basic Compensation for “Long-term Incentive”, reflecting the common practice of global companies.
| |||
|
| ||||
| * Ratio of Bonus and Long-term Incentives to Basic Compensation is determined according to Director’s role.
| ||||
| 3-2. External Directors who are not Audit & Supervisory Committee Members
| ||||
| The compensation of External Directors who are not Audit & Supervisory Committee Members consists of Basic Compensation, which is paid as a fixed amount, and Long-term Incentive (stock compensation). The stock compensation is linked only to share price and not to financial performance results. Newly awarded stock compensation in 2019 and going forward will vest three years after the award date of base points used for the calculation and Directors will be required to hold 75% of their vested share portion until they leave the Company.
| ||||
| Bonus is not available for this category of Director. Committee retainers are paid with Basic Compensation for the chair of board meeting, chair of the compensation committee, and chair of Nomination Committee.
| ||||
29
Table of Contents
|
⬛ Standard External Directors who are not Audit & Supervisory Committee Members Compensation Mix Model |
The current compensation mix is “Basic Compensation” and “Long-term Incentive”, which is a maximum of 100% of the Basic Compensation.
| |||
|
| ||||
| 3-3. Directors who are Audit & Supervisory Committee Members
| ||||
| The compensation of Directors who are Audit & Supervisory Committee Members consists of Basic Compensation, which is paid as a fixed amount, and Long-term Incentive (stock compensation). The stock compensation is linked only to share price and not to financial performance results. Newly awarded stock compensation in 2019 and going forward will vest three years after the award date of base points used for the calculation and Directors will be required to hold 75% of their vested share portion until they leave the Company.
| ||||
| Bonus is not available for this category of Director. Committee retainer is paid with Basic Compensation for external directors who are Audit & Supervisory Committee Members.
| ||||
|
⬛ Standard Directors who are Audit & Supervisory Committee Members Compensation Mix Model
|
The current compensation mix is “Basic Compensation” and “Long-term Incentive”, which is a maximum of 100% of the Basic Compensation.
| |||
|
| ||||
|
4. |
Performance- based Compensation
|
4-1. Directors who are not Audit & Supervisory Committee Members (excluding External Directors) | ||
| For Directors who are not Audit & Supervisory Committee Members (excluding External Directors) a Long-term Incentive Plan that is allocated as 60% Performance Shares and 40% Restricted Stock is in place to strengthen the link between compensation and company performance and share price, and to reinforce the commitment to increasing corporate value in the mid and long term.
| ||||
| Key Performance Indicators (KPI) used for the Long-term Incentive will be linked with the latest mid- to long-term performance objectives over a three-year period such as but not limited to consolidated revenue, operating free cash flow, indicators on earnings, R&D targets and integration success factors, etc., as transparent and objective indicators. The variable range is from 0% to 200% (100% at target), based on performance achievement. For newly awarded Long-term Incentive awards, a two year holding period will be mandated, this includes Performance Share if and when shares become vested.
| ||||
30
Table of Contents
| Annual Performance-based Long-term Incentive Plan (stock compensation) Image
| ||||
|
| ||||
| The company may, from time to time, award special Performance Share awards to Directors who are not Audit & Supervisory Committee Members (excluding External Directors) which are directly linked to point-in-time corporate initiatives and which are aligned with shareholder expectations. Performance against established KPIs for special Performance Share awards are determined independently each year over a three-year period, with shares becoming vested after performance has been determined for the applicable period. There is no post-vesting holding period established for special Performance Share awards.
| ||||
| Special Performance-based Share Awards (stock compensation) Image
| ||||
|
| ||||
| Annual Bonus
| ||||
| Bonuses will be paid based on performance achievement of annual goals. Bonuses will be paid in the range of 0% to 200% (100% at target) in accordance with the achievement of performance indicators such as consolidated revenue, core earnings and core EPS, etc., established for a single fiscal year. For President and CEO, the annual bonus is weighted as 100% to the Corporate KPI.
| ||||
| For other Directors that have divisional responsibilities, 75% of their annual bonus opportunity is linked to the Corporate KPI to drive their commitment to group-wide goals.
|
31
Table of Contents
|
4-2. Directors who are Audit & Supervisory Committee Members and External Directors
| ||||
| The Long-term Incentive (stock compensation) for Directors who are Audit & Supervisory Committee
| ||||
| Members and External Directors is linked only to share price and not linked to financial performance results. Newly awarded stock compensation will vest three years after the award date of base points used for the calculation and Directors will be required to hold 75% of their vested share portion until they leave the Company.
| ||||
| Whole Picture of Directors’ Compensation
| ||||
|
1 Includes Special Performance-based Share Awards | ||||
| 2 Varies from 0% to 200%, depending upon the degree of achievement, etc. of the performance indicators such as consolidated revenue, core earnings, core EPS, etc., established for a single fiscal year. | ||||
| 3 Varies from 0% to 200%, depending upon the degree of achievement, etc. in relation to consolidated revenue, free cash flow, indicators on earnings, R&D targets, integration success factors, etc. over 3 years | ||||
| 4 During term of office | ||||
| 5 Vest three years after the base points used for the calculation is granted.
| ||||
|
5. |
Compensation Governance |
The Compensation Committee has been established with an External Director as its Chairperson and with the majority of members being External Directors, to serve as an advisory organization for the Board of Directors to ensure the appropriateness of Directors’ compensation, etc. and the transparency in its decision-making process. | ||
|
The level of compensation, compensation mix and performance-based compensation (Long-term Incentives and Bonus programs) for Directors are reviewed by the Compensation Committee before resolution by the Board of Directors. The company expanded the authority of the Committee by the board resolution to directly make decisions on Directors who are not Audit & Supervisory Committee Members (excluding External Directors) individual compensations in order to realize the transparency in the process.
| ||||
| The guiding principles for Director Compensation will be revised to develop compensation programs based on Directors’ accountabilities and responsibilities, as well as to develop compensation programs that create shareholder value in alignment with Takeda-ism.
|
32
Table of Contents
Fourth Proposal: Revisions Pertaining to the Amount and the Contents of Stock Compensation, etc. for Directors who are not Audit and Supervisory Committee Members
| 1. | Reason for the proposal and reason for considering such compensation, etc. as appropriate |
The Company received approval at the 140th Ordinary General Meeting of Shareholders on June 29, 2016 regarding the establishment of the stock compensation plan (hereinafter for the purposes of this Proposal, the “Previous Plan”) as compensation, etc. for each fiscal year for the Directors (excluding the Directors who are Audit and Supervisory Committee Members and Directors residing overseas who are not External Directors) as the Company had shifted to Company with Audit and Supervisory Committee. This Proposal requests your approval of the revision to the current stock compensation plan as compensation, etc. for such Directors (hereinafter for the purposes of this Proposal, the “Plan”).
From among this Proposal, the contents of the Proposal for Directors who are not External Directors (excluding Directors who are Audit and Supervisory Committee Members and Directors resident overseas, hereinafter for the purposes of this Proposal, “Internal Directors”) is for the purpose of further enhancing the awareness of contributing to the enhancement of the Company’s mid/long-term achievements as well as the increase of the corporate value of the Company. In addition, with respect to the contents of the Proposal for External Directors (excluding Directors who are Audit and Supervisory Committee Members; the same applies hereinafter for the purposes of this Proposal), in light of the roles of the External Directors and in order to ensure the adequate supervisory functions which judge the validity of the execution of duties from an objective standpoint and to avoid excessive risk-taking activities to achieve performance objectives in the short-term, such Proposal is not linked with the performance achievements, but is for the purpose of further enhancing the awareness of contributing to the increase of our corporate value of the Company. Furthermore, increasing the number of holding shares during their term of office is intended to create unity in sharing the Company’s interests as well as corporate value among shareholders and External Directors. Therefore, the Company considers that such Proposal is appropriate.
In the event that the Second Proposal is approved in the form of the original proposal, the number of Directors (excluding Directors who are Audit and Supervisory Committee Members and Directors resident overseas who are not External Directors) subject to the Plan will be eleven (11) (from among such number of Directors, the number of External Directors will be eight (8)).
| 2. | Details of changes to the Plan |
With respect to the continuation of the Plan, the Company proposes the following changes to the Plan. To clarify, the changes will only be effective for newly established trust in Fiscal Year 2019 given the condition that this proposal is approved in the form of the original proposal and no changes will be made for that already established under the Previous Plan. (Under the Plan, the plan for Internal Directors is hereinafter for the purposes of this Proposal, referred to as “Plan I”, and the plan for External Directors is hereinafter for the purposes of this Proposal, referred to as “Plan II”. The details are as described in and after 3(2). The Company may also implement an incentive plan under the Plan by creating a Trust with a three (3) year trust period, similarly as the Trust created in Fiscal Year 2019, even for each fiscal year in and after Fiscal Year 2020 (“create” includes continuous use of the preexisting Trust by extending the trust period of the Trust; the same applies hereinafter for the purposes of this Proposal).
33
Table of Contents
Notwithstanding the above, with respect to Plan II, the Company will provide the External Directors who were elected at the Extraordinary General Meeting of Shareholders (December 5, 2018) and took office as of January 8, 2019 with base points targeting their term of office from the date of taking office to the date before this General Meeting of Shareholders (hereinafter for the purposes of this Proposal, the “Ex-Post Evaluation Base Points”) as well as base points targeting their term of office starting on the date of this General Meeting of Shareholders (meaning base points for annual grant as described in 3(3) below), which are granted on the condition that such External Directors hold office on July 1 of the year of creation of the Trust, under a newly established trust in Fiscal Year 2019, and the Previous Plan is to be applied to the Ex-Post Evaluation Base Points of such External Directors, the share conversion points calculated by the Ex-Post Evaluation Base Points and the delivery of the shares of the Company corresponding to such share conversion points.
1: Change in Maximum Amount to be contributed by the Company
With respect to Plan I (as described in 3(1) below), the Company would like to increase the compensation amount for Internal Directors from an amount within 2,700 million JPY targeting three (3) fiscal years to 4,500 million JPY targeting three (3) fiscal years in order to increase the Performance Share Unit portion as well as introducing a Special one-time Performance Share Unit. This affects the number of shares that Internal Directors can hold accordingly. In the event that this Proposal is approved in the form of the original proposal, the Company will contribute, as compensation, etc. for Directors, monies up to a total of 4,800 million JPY (4,500 million JPY for Plan I and 300 million JPY (including the compensation amount calculated by the Ex-Post Evaluation Base Points; the same applies hereinafter for the purposes of this Proposal) for Plan II) per three (3) consecutive fiscal years. This compensation, etc. does not include the salaries paid as the employee portion for Directors who also work as employees.
2: Introduce Special one-time Performance Share Unit for Internal Directors
With respect to Plan I, the Company would like to introduce a Special one-time Performance Share Unit deeply embedded in the success of the Shire Integration and delivering on the commitments made for the next three (3) years. The established KPI is related specifically to the Integration milestone (KPI is separately determined by review of Compensation Committee and Board of Directors’ resolution). This way, the Company would like to ensure the success of the Integration with fully committed Internal Directors during this important period. Performance against established KPIs for Special one-time Performance Share Unit is determined independently each year over a three-year period, and the shares to be provided will become vested after performance has been determined for the applicable period.
34
Table of Contents
3: Change in the Calculation Formula of Share Conversion Points for Annual Grant
With respect to Plan I, the Company would like to increase Performance Share awards calculated under the calculation formula of share conversion points using base points for annual grant (as described in 3(3) below and which is substantially the same as the base points calculated under the Previous Plan) among awards granted with a percentage of 60% from 50% so as to be able to strengthen the link between compensation and the Company performance and The Company’s share price, and to reinforce the commitment to increasing corporate value in the mid and long term. Plan II is not subject to such change.
4: Change in Vesting Schedule for External Directors
With respect to Plan II (as described in 3(1) below), the Company would like to change the vesting schedule from resignation/termination of the term of office to the elapse of three (3) years after the award date (meaning the date when base points will be granted, hereinafter the same for the purposes of this Proposal). By implementing this change, the Company seeks to enhance the awareness among External Directors of their contributions to the balance and enhancement of the Company’s mid/long-term achievements as well as the increase of the corporate value. Also, along with the change in the vesting schedule, even in the event of the resignation/retirement of an External Director (excluding resignation due to his/her own convenience or resignation due to dismissal), the share conversion points will be granted similarly as the case that he/she remains in office till the expiry of the Plan Period (as described in 3(2) below) after his/her resignation/retirement, and he/she may receive Deliveries (as described in 3(1) below) from the Trust (as described in 3(2) below) of Company Shares, etc. (as described in 3(1) below) of a number in accordance with the share conversion points.
| 3. | Amount and contents, etc. of the compensation, etc. under the Plan upon obtaining approval |
| (1) | Overview of the Plan |
The Plan is a stock compensation plan in which the shares of the Company are acquired through trust, sourced from the money amount contributed by the Company, and the shares of the Company and the monies equivalent to the cash conversion amount of the shares of the Company (hereinafter for the purposes of this Proposal, collectively, “Company Shares, etc.”) are delivered and provided (hereinafter for the purposes of this Proposal, “Deliveries”) to the Directors (excluding Directors who are Audit and Supervisory Committee Members and Directors resident overseas who are not External Directors; the same hereinafter for the purposes of this Proposal) through such trust.
| (i) | Eligible persons of Deliveries of Company Shares, etc. subject to this Proposal |
<Plan I> Internal Directors
<Plan II> External Directors
| (ii) | Effect of the shares of the Company subject to this Proposal upon the total number of issued shares |
| (a) | Maximum amount to be contributed by the Company (as described in (2) below) |
<Plan I> Total of 4,500 million JPY targeting three (3) fiscal years
<Plan II> Total of 300 million JPY targeting three (3) fiscal years
35
Table of Contents
| (b) | Maximum number of Company Shares, etc. to be acquired by the eligible persons of this Proposal (as described in (3) below) |
<Plan I>
The maximum number of shares is the number obtained by dividing 4,500 million JPY, the maximum monetary amount to be contributed to the Trust as Plan I by the Company, by the closing price of the shares of the Company on the Tokyo Stock Exchange on July 1 of the year in which such Trust was established targeting three (3) fiscal years.
<Plan II>
The maximum number of shares is the number obtained by dividing 300 million JPY, the maximum monetary amount to be contributed to the Trust as Plan II by the Company, by the closing price of the shares of the Company on the Tokyo Stock Exchange on July 1 of the year in which such Trust was established targeting the first of three (3) fiscal years.
| (c) | Method of acquisition of the shares of the Company to be acquired by the eligible persons of this Proposal (as described in (3) below) |
Under both Plans I and II, the shares of the Company are scheduled to be acquired from the stock market.
Therefore, there will be no dilution of the share value of the issued shares.
| (iii) | Contents of the conditions for performance achievement (as described in (3) below) |
<Plan I> Fluctuate depending upon the degree of achievement, etc. in relation to consolidated revenue, free cash flow, indicators on earnings, R&D target, integration success factors, etc. of every fiscal year or over the three (3) fiscal years ending (in either case, as of the end of March) during the Plan Period.
<Plan II> Not linked to performance results (fixed)
| (iv) | Timing of Deliveries of Company Shares, etc. towards the eligible persons of this Proposal (as described in (4) below) |
<Plan I>
With respect to annual grant;
| - | For the portion equivalent to 40% of the base points for annual grant, deliveries will be made every year. |
| - | For the remaining portion equivalent to 60% of the base points for the annual grant, deliveries will be made depending upon the achievement, etc. of the performance objectives after the elapse of three (3) fiscal years. |
| - | Two (2) year stock holding requirement after the vesting date has been introduced for the annual grant. This applies only to Internal Directors. |
With respect to Special one-time Performance Share Unit;
| - | Deliveries of all portions of the base points for Special one-time Performance Share Unit will be made depending upon the achievement of integration success factors, etc. of the performance objectives every year. |
<Plan II> (only for annual grant)
To be delivered three (3) years after the award date and External Directors are required to hold 75% of their vested share portion until they leave the Company.
| * | Both Plans I and II include the shares converted into cash (in which case, the monetary equivalent to the cash conversion amount will be provided to the eligible persons). |
36
Table of Contents
| (2) | Maximum monetary amount to be contributed by the Company |
The Company will contribute, as compensation, etc. for Directors, monies up to a total of 4,800 million JPY (4,500 million JPY for Plan I and 300 million JPY for Plan II) per three (3) consecutive fiscal years (initially the three (3) fiscal years from the fiscal year ending on the last day of March 2020 to the fiscal year ending on the last day of March 2022; hereinafter, the “Plan Period” for the purposes of this Proposal), and create a trust (hereinafter for the purposes of this Proposal, the “Trust” which is created respectively for Plan I and Plan II.) for a three (3) year trust period with the beneficiaries being the Directors meeting the beneficiary requirements. The Trust will acquire the shares of the Company from the stock market, sourced by monies subject to trust, in accordance with the instructions of the trust administrator. During the trust period, the Company will grant share conversion points and make Deliveries of Company Shares, etc. towards the Internal Directors every year during the trust period, and grant share conversion points towards the External Directors and make Deliveries of Company Shares, etc. towards External Directors three (3) years after the award date.
The Company creates a Trust initially in Fiscal Year 2019, but the Company may also implement an incentive plan under the Plan by creating a Trust with a three (3) year trust period, similarly as the Trust created in Fiscal Year 2019, even for each fiscal year in and after Fiscal Year 2020.
For Plan I and Plan II, respectively, the number of the Trust capable of being created in one (1) fiscal year will be one (1), and in the event a Trust is created in every fiscal year, a maximum of three (3) Trusts will coexist. (Notwithstanding the above, in the case that the Plan is to be applied to Directors who are not Audit and Supervisory Committee Members appointed after July 1 of each year, etc. the Company may create more than one Trust in one (1) fiscal year. Even in this case, the total amount of contribution made by the Company to the Plan does not exceed 4,800 million JPY (4,500 million JPY for Plan I and 300 million JPY for Plan II).)
The Trust may be continued by changing the trust agreement and adding trust in place of creating a new Trust at the time of the expiry of the trust period of the Trust. In that case, the trust period will be extended for a three (3) fiscal year period, and the Company will grant base points and share conversion points, and make Deliveries of Company Shares, etc., in the same manner as indicated above, within such extended three (3) fiscal year period of the Plan Period. In the case that additional contribution is made upon the extension of the trust period, the maximum monetary amount which may be additionally contributed when the shares of the Company (excluding, from among Company Shares, etc. equivalent to share conversion points granted to the Directors, those for which the Deliveries are not yet made) and monies (hereinafter for the purposes of this Proposal, “Remaining Shares”) remain within the trust property on the last day of the trust period prior to the extension, will be 4,800 million JPY (4,500 million JPY for Plan I and 300 million JPY for Plan II) minus the amount of the Remaining Shares.
If, at the time of the expiry of the trust period and the extension above is not made, and in the case that an External Director possibly meeting the beneficiary requirements holds office, points will not be granted thereafter to such External Director, but the trust period of the Trust may be extended until the completion of the Deliveries of Company Shares, etc. towards such External Director.
37
Table of Contents
| (3) | Calculation method and maximum of the number of Company Shares, etc. to be acquired by the Directors |
The Company will grant, for the Internal Directors, “share conversion points” calculated in accordance with the degree of achievement, etc. of the performance objectives of the Company, and for the External Directors, “share conversion points” of a fixed number irrespective of the degree of achievement, etc. of the performance objectives of the Company, respectively, in accordance with the base points to be granted in accordance with the nature of the duties and responsibilities, etc. of each Director. The number of shares of the Company to be delivered to each Director through the Plan is determined to be one (1) share per share conversion point granted to each Director. In the event that the shares of the Company subject to the Trust increase or decrease due to share split, share allotment without contribution, share consolidation, etc., the Company will adjust, in accordance with a reasonable method, the number of shares of the Company to be delivered for each share conversion point.
Concretely, first of all, base points will be granted to a Director holding office on July 1 of the year of creation of the Trust in accordance with the following formula; however, base points for Special one-time Performance Share Unit are to be provided only to Internal Directors. Ex-Post Evaluation Base Points will be granted to the External Directors who were elected at the Extraordinary General Meeting of Shareholders (December 5, 2018) and took office as of January 8, 2019.
(Calculation formula of base points)
| - | Base points for annual grant |
Basic annual compensation amount X target ratio of annual grant / closing price of the shares of the Company on the Tokyo Stock Exchange on July 1 of the year of creation of the Trust (if there is no closing price on such date, then the closing price on the date on which a transaction was performed immediately prior to such date)
| - | Base points for Special one-time Performance Share Unit |
Basic annual compensation amount X target ratio of Special one-time Performance Share Unit / closing price of the shares of the Company on the Tokyo Stock Exchange on July 1 of the year of creation of the Trust (if there is no closing price on such date, then the closing price on the date on which a transaction was performed immediately prior to such date)
| - | Ex-Post Evaluation Base Points |
The Ex-Post Evaluation Base Points shall be calculated under the Previous Plan.
| * | Fractions after the decimal point will be disregarded. |
| * | The basic annual compensation amount and the target ratio will be determined taking into account such factors as the nature of the duties and responsibilities of each Director, and the respective percentage shares of the monetary compensation and the stock compensation among the total compensation of Directors. |
38
Table of Contents
<Calculation formula of share conversion points in Plan I>
Share conversion points will be granted to an Internal Director, who are granted the base points based on the above, holding office on June 1 of each year from the year following the creation of the Trust until three (3) fiscal years thereafter, in accordance with the following formula.
(Calculation formula of share conversion points to be granted to Internal Directors)
| (i) | Year following the creation of the Trust and the year after such year [Initial base points for annual grant × 40% × 1/3]+[ Initial base points for Special one-time Performance Share Unit × 1/3 × Performance linked coefficient (a)] |
| (ii) | After three (3) fiscal years from the creation of the Trust [Initial base points for annual grant × 40% × 1/3]+[Initial base points for annual grant × 60% × Performance linked coefficient (b)]+[Initial base points for Special one-time Performance Share Unit × 1/3 × Performance linked coefficient (a)] |
| * | A different performance linked coefficient (performance linked coefficient (a) or (b)) will be applied to initial base points for annual grant and initial base points for Special one-time Performance Share Unit. |
| * | If there occurs any fractions after the decimal point, for the share conversion points for the year following the creation of the Trust and the year following such year, such fractions will be disregarded, and the total number of such fractions (any fractions after the decimal point of such total number will be disregarded) will be added to the share conversion points after three (3) years after the creation of the Trust. |
| * | The performance linked coefficient will be determined within a scope of 0%-200% in accordance with the achievement, etc. of the consolidated revenue, free cash flow, indicators on earnings, R&D target, integration success factor, etc. of every fiscal year or over the three (3) fiscal years ending (in either case, as of the end of March) during the Plan Period set at the time of creation of the Trust. However, in the case that an Internal Director assumes, after his/her resignation/retirement, the position of Director who is an Audit and Supervisory Committee Member, the performance linked coefficient will be calculated as 100% irrespective of the achievement, etc. of the performance objectives during the Plan Period. |
| * | The evaluation of the achievement of the performance objectives will be reported to, and determined at, the Board of Directors upon discussion by the Compensation Committee. |
| * | The number of base points granted to the Internal Directors will be decreased, at the time of the calculation of the share conversion points of each fiscal year, by the portion equivalent to the number of base points used in such calculation (calculated by [ ] within the abovementioned formula). |
In the event of the resignation/retirement of an Internal Director (excluding resignation due to his/her own convenience or resignation due to dismissal), the share conversion points will be granted similarly as the case that he/she remains in office till the expiry of the Plan Period after his/her resignation/retirement, and he/she may receive Deliveries from the Trust of Company Shares, etc. of a number in accordance with the share conversion points.
39
Table of Contents
<Calculation of share conversion points in Plan II>
For an External Director, who are granted the base points based on the above, holding office on June 1 of the year following the creation of the Trust, share conversion points will be granted in accordance with the following formula. In addition to such share conversion points below, share conversion points which will be calculated by Ex-Post Evaluation Base Points under the Previous Plan will be granted to the External Directors who were elected at the Extraordinary General Meeting of Shareholders (December 5, 2018) and took office as of January 8, 2019.
(Calculation formula of share conversion points to be granted to External Directors) Initial base points for annual grant × 100%
| * | The number of base points granted to External Directors will decrease in its entirety at the time of calculation of the share conversion points. |
In the event of the resignation/retirement of an External Director (excluding resignation due to his/her own convenience or resignation due to dismissal), the share conversion points will be granted similarly as the case that he/she remains in office till the expiry of the Plan Period after his/her resignation/retirement, and he/she may receive Deliveries from the Trust of Company Shares, etc. of a number in accordance with the share conversion points.
The maximum of the total number of Company Shares, etc. for which Deliveries are to be made to the Directors from the Trust during the trust period of the Trust under Plans I and II will be the number (fractions after the decimal point are to be disregarded) obtained by dividing (i) 4,800 million JPY (4,500 million JPY for Plan I and 300 million JPY for Plan II), the maximum monetary amount to be contributed to such Trust by the Company, by (ii) the closing price of the shares of the Company on the Tokyo Stock Exchange on July 1 of the year of creation of the Trust (if there is no closing price on such date, then the closing price on the date on which the transaction was performed immediately prior to such date), for each Plan Period.
| (4) | Timing of the Deliveries of Company Shares, etc. towards the Directors |
An Internal Director meeting the beneficiary requirements may, by performing the prescribed beneficiary determination procedures every year during the trust period, receive, after a certain period after being granted share conversion points, the delivery of 50% (the number of shares less than one (1) share unit will be disregarded) of the shares of the Company corresponding to such share conversion points, and in addition, may convert the remainder (including those equivalent to the number of shares less than one (1) share unit described above) within the Trust and receive monies equivalent to such cash conversion amount.
An External Director meeting the beneficiary requirements may, by performing the prescribed beneficiary determination procedures, three (3) years after the award date, receive the delivery of 50% (the number of shares less than one (1) share unit will be disregarded) of the shares of the Company corresponding to the share conversion points, and in addition, may convert the remainder (including those equivalent to the number of shares less than one (1) share unit described above) within the Trust and receive monies equivalent to such cash conversion amount. However, the External Directors who were elected at the Extraordinary General Meeting of Shareholders (December 5, 2018) and took office as of January 8, 2019 may, at the time of their resignation/retirement, receive delivery of the shares of the Company and monies corresponding to the share conversion points calculated by the Ex-Post Evaluation Points under the Previous Plan.
40
Table of Contents
In the event that a Director dies during the trust period, the inheritor of such Director will convert into cash within the Trust any and all of the shares of the Company corresponding to the share conversion points granted to such director, and receive such monies.
| (5) | Voting rights regarding the shares of the Company within the Trust |
With respect to the shares of the Company within the Trust, voting rights will not be exercised during the trust period in order to ensure neutrality towards management.
| (6) | Other contents of the Plan |
Other contents regarding the Plan will be determined by the Board of Directors each time the Trust is created.
Fifth Proposal: Revisions Pertaining to the Contents of Stock Compensation, etc. for Directors who are Audit and Supervisory Committee Members
| 1. | Reason for the proposal and reason for considering such compensation, etc. as appropriate |
The Company received approval at the 140th Ordinary General Meeting of Shareholders on June 29, 2016 regarding the establishment of the stock compensation plan as compensation, etc. for each fiscal year for the Directors who are Audit and Supervisory Committee Members. This Proposal requests for your approval of the revision in current stock compensation plan as compensation, etc. for the Directors who are Audit and Supervisory Committee Members (hereinafter for the purposes of this Proposal, the “Plan”).
This Plan, targeting the Directors who are Audit and Supervisory Committee Members (hereinafter for the purposes of this Proposal, “Audit and Supervisory Committee Members”), is for the purpose of further enhancing the awareness of contribution to the increase of the corporate value of the Company, without linking it to the performance results in light of the roles of the Audit and Supervisory Committee Members, in order to ensure the adequate supervisory functions which judge the validity of the execution of duties from an objective standpoint and to avoid excessive risk taking activities to achieve performance results in the short-term. Therefore, the Company considers that such Proposal is appropriate.
In the event that the Third Proposal is approved in the form of the original proposal, the number of Directors who are Audit and Supervisory Committee Members and who are subject to the Plan will be four (4).
41
Table of Contents
| 2. | Details of changes to the Plan |
With respect to the continuation of the Plan, the Company proposes the following changes to the Plan. To clarify, the changes will only be effective for newly established trust in 2019 given the condition that this proposal is approved in the form of the original proposal and no changes will be made for that already established under the Plan. The Company may also implement an incentive plan under the Plan by creating a Trust with a three (3) year trust period, similarly as the Trust created in Fiscal Year 2019, even for each fiscal year in and after Fiscal Year 2020 (“create” includes continuous use of the preexisting trust by extending the trust period of the trust; the same applies hereinafter for the purposes of this Proposal).
1: Change in Plan Period for Directors who are Audit and Supervisory Committee Members
With respect to Plan III (as described in 3(1) below), the Company would like to change the Plan Period (as described in 3(2) below) from two (2) fiscal years to three (3) fiscal years. In the event that this Proposal is approved in the form of the original proposal, the Company will contribute, as compensation, etc. for Audit and Supervisory Committee Members, monies up to a total of 200 million JPY per three (3) consecutive fiscal years.
2: Change in Vesting Schedule
With respect to Plan III, the Company would like to change the vesting schedule from resignation or termination of the term of office to the elapse of three (3) years after the award date (meaning the date when base points will be granted, hereinafter the same for the purposes of this Proposal). By implementing this change, the Company seeks to enhance the awareness among Audit and Supervisory Committee Members of their contributions to the enhancement of the Company’s mid/long-term achievements as well as the increase of the corporate value. Also, along with the change in the vesting schedule, even in the event of the resignation/retirement of an Audit and Supervisory Committee Member (excluding resignation due to his/her own convenience or resignation due to dismissal), the share conversion points will be granted similarly as the case that he/she remains in office till the expiry of the Plan Period after his/her resignation/retirement, and he/she may receive Deliveries (as described in 3(1) below) from the Trust (as described in 3(2) below) of Company Shares, etc. (as described in 3(1) below) of a number in accordance with the share conversion points.
3: Abolition of Additional Base Points
With respect to Plan III, the Company would like to abolish additional base points to be granted to Audit and Supervisory Committee Members.
| 3. | Amount and contents, etc. of the compensation, etc. under the Plan upon obtaining approval |
| (1) | Overview of the Plan |
The Plan is a stock compensation plan in which the shares of the Company are acquired through trust, sourced from the money amount contributed by the Company, and the shares of the Company and the monies equivalent to the cash conversion amount of the shares of the Company (hereinafter for the purposes of this Proposal, collectively, “Company Shares, etc.”) are delivered and provided (hereinafter for the purposes of this Proposal, “Deliveries”) to the Directors who are Audit and Supervisory Committee Members through such trust. (The plan for the Directors who are Audit and Supervisory Committee Members is hereinafter for the purposes of this Proposal, referred to as “Plan III”.)
42
Table of Contents
| (i) | Eligible persons of Deliveries of Company Shares, etc. subject to this Proposal |
<Plan III> Audit and Supervisory Committee Members
| (ii) | Effect of the shares of the Company subject to this Proposal upon the total number of issued shares |
| (a) | Maximum amount to be contributed by the Company (as described in (2) below) |
<Plan III> Total of 200 million JPY targeting three (3) fiscal years
| (b) | Maximum number of Company Shares, etc. to be acquired by the eligible persons of this Proposal (as described in (3) below) |
<Plan III>
The maximum number of shares is the number obtained by dividing 200 million JPY the maximum monetary amount to be contributed by every targeting fiscal year to the Trust as Plan III by the Company, by the closing price of the shares of the Company on the Tokyo Stock Exchange on July 1 of the year in which such Trust was established targeting three (3) fiscal years.
| (c) | Method of acquisition of the shares of the Company to be acquired by the eligible persons of this Proposal (as described in (3) below) |
Under Plan III, the shares of the Company are scheduled to be acquired from the stock market.
Therefore, there will be no dilution of the share value of the issued shares.
| (iii) | Contents of the conditions for performance achievement (as described in (3) below) |
<Plan III> Not linked to the performance results (fixed)
| (iv) | Timing of Deliveries of Company Shares, etc. towards the eligible persons of this Proposal (as described in (4) below) |
<Plan III> To be delivered three (3) years after the award date and Audit and Supervisory Committee Members are required to hold 75% of their vested share portion until they leave the Company.
| * | Plan III includes the shares converted into cash (in which case, the monetary equivalent to the cash conversion amount will be provided to the eligible persons). |
| (2) | Maximum monetary amount to be contributed by the Company |
The Company will contribute, as compensation, etc. for the Audit and Supervisory Committee Members, monies up to a total of 200 million JPY per three (3) consecutive fiscal years (initially the three (3) fiscal years from the fiscal year ending on the last day of March 2020 to the fiscal year ending on the last day of March 2022; hereinafter, the “Plan Period” for the purposes of this Proposal), and create a trust (hereinafter for the purposes of this Proposal, the “Trust”) for a three (3) year trust period with the beneficiaries being the Audit and Supervisory Committee Members meeting the beneficiary requirements. The Trust will acquire the shares of the Company from the stock market, sourced by monies subject to trust, in accordance with the instructions of the trust administrator. During the trust period, the Company will grant share conversion points and make Deliveries of Company Shares, etc. towards the Audit and Supervisory Committee Members and make Deliveries of Company Shares, etc. towards Audit and Supervisory Committee Members three (3) years after the award date.
43
Table of Contents
The Company creates a Trust initially in Fiscal Year 2019, but the Company may also implement an incentive plan under the Plan by creating a Trust with a three (3) year trust period, similarly as the Trust created in Fiscal Year 2019, even for each fiscal year in and after Fiscal Year 2020.
For Plan III, the number of the Trust capable of being created in one (1) fiscal year will be one (1), and in the event a Trust is created in every fiscal year, a maximum of three (3) Trusts will coexist. (Notwithstanding the above, in the case that the Plan is to be applied to Directors who are Audit and Supervisory Committee Members appointed after July 1 of each year, etc. the Company may create more than one Trust in one (1) fiscal year. Even in this case, the total amount of contribution made by the Company to the Plan does not exceed 200 million JPY.)
The Trust may be continued by changing the trust agreement and adding trust in place of creating a new Trust at the time of the expiry of the trust period of the Trust. In that case, the trust period will be extended for a three (3) fiscal year period, and the Company will grant base points and share conversion points, and make Deliveries of Company Shares, etc., in the same manner as indicated above, within such extended three (3) fiscal year period of the Plan Period. In the case that additional contribution is made upon the extension of the trust period, the maximum monetary amount which may be additionally contributed when the shares of the Company (excluding, from among Company Shares, etc. equivalent to share conversion points granted to the Audit and Supervisory Committee Members, those for which the Deliveries are not yet made) and monies (hereinafter for the purposes of this Proposal, “Remaining Shares”) remain within the trust property on the last day of the trust period prior to the extension, will be 200 million JPY minus the amount of the Remaining Shares.
If, at the time of the expiry of the trust period and the extension above is not made, and in the case that an Audit and Supervisory Committee Member possibly meeting the beneficiary requirements holds office, points will not be granted thereafter to such Audit and Supervisory Committee Member, but the trust period of the Trust may be extended until the completion of the Deliveries of Company Shares, etc. towards such Audit and Supervisory Committee Member.
| (3) | Calculation method and maximum of the number of Company Shares, etc. to be acquired by the Audit and Supervisory Committee Members |
The Company will grant, for the Audit and Supervisory Committee Members, “share conversion points” of a fixed number irrespective of the degree of achievement, etc. of the performance objectives of the Company, in accordance with the base points to be granted in accordance with the nature of the duties and responsibilities, etc. of each Audit and Supervisory Committee Member. The number of shares of the Company to be delivered to each Audit and Supervisory Committee Member through the Plan is determined to be one (1) share per share conversion point granted to each Audit and Supervisory Committee Member. In the event that the shares of the Company subject to the Trust increase or decrease due to share split, share allotment without contribution, share consolidation, etc., the Company will adjust, in accordance with a reasonable method, the number of shares of the Company to be delivered for each share conversion point.
44
Table of Contents
Concretely, base points will be granted to a Director holding office on July 1 of the year of creation of the Trust in accordance with the following formula.
(Calculation formula of base points)
Basic annual compensation amount × target ratio / closing price of the shares of the Company on the Tokyo Stock Exchange on July 1 of the year of creation of the Trust (if there is no closing price on such date, then the closing price on the date on which a transaction was performed immediately prior to such date)
| * | Fractions after the decimal point will be disregarded. |
| * | The basic annual compensation amount and the target ratio will be determined taking into account such factors as the nature of the duties and responsibilities of each Audit and Supervisory Committee Member, and the respective percentage shares of the monetary compensation and the stock compensation among the total compensation of Directors. |
<Calculation of share conversion points in Plan III>
For an Audit and Supervisory Committee Member who is granted base points in accordance with the calculation above and who is holding office on June 1 of the year following the creation of the Trust, share conversion points will be granted in accordance with the following formula.
(Calculation formula of share conversion points to be granted to Audit and Supervisory Committee Members)
Initial base points × 100%
| * | The number of base points granted to Audit and Supervisory Committee Members will decrease in its entirety at the time of calculation of the share conversion points. |
In the event of the resignation/retirement of an Audit and Supervisory Committee Member (excluding resignation due to his/her own convenience or resignation due to dismissal), the share conversion points will be granted similarly as the case that he/she remains in office till the expiry of the Plan Period after his/her resignation/retirement, and he/she may receive Deliveries from the Trust of Company Shares, etc. of a number in accordance with the share conversion points.
The maximum of the total number of Company Shares, etc. for which Deliveries are to be made to the Audit and Supervisory Committee Members from the Trust during the trust period of the Trust under Plan III will be the number (fractions after the decimal point are to be disregarded) obtained by dividing (i) 200 million JPY, the maximum monetary amount to be contributed to such Trust by the Company, by (ii) the closing price of the shares of the Company on the Tokyo Stock Exchange on July 1 of the year of creation of the Trust (if there is no closing price on such date, then the closing price on the date on which a transaction was performed immediately prior to such date), for the Plan Period.
45
Table of Contents
| (4) | Timing of the Deliveries of Company Shares, etc. towards the Audit and Supervisory Committee Members |
An Audit and Supervisory Committee Member meeting the beneficiary requirements may, by performing the prescribed beneficiary determination procedures, three (3) years after the award date, receive the delivery of 50% (the number of shares less than one (1) share unit will be disregarded) of the shares of the Company corresponding to the share conversion points, and in addition, may convert the remainder (including those equivalent to the number of shares less than one (1) share unit described above) within the Trust and receive monies equivalent to such cash conversion amount.
In the event that an Audit and Supervisory Committee Member dies during the trust period, the inheritor of such Audit and Supervisory Committee Member will convert into cash within the Trust any and all of the shares of the Company corresponding to the share conversion points granted to such Audit and Supervisory Committee Member, and receive such monies.
| (5) | Voting rights regarding the shares of the Company within the Trust |
With respect to the shares of the Company within the Trust, voting rights will not be exercised during the trust period in order to ensure neutrality towards management.
| (6) | Other contents of the Plan |
Other contents regarding the Plan will be determined by the Board of Directors each time the Trust is created.
Sixth Proposal: Payment of Bonuses to Directors who are not Audit and Supervisory Committee Members
The Company proposes to pay bonuses up to the total amount of 730 million JPY (excluding bonuses paid to the relevant Directors for their work as employees) to the two (2) Directors (excluding Directors residing overseas and External Directors) in office as of the end of this fiscal year, in keeping with the achievement of the key performance indicators such as the consolidated revenue, Core Earnings and Core EPS set forth for this fiscal year.
<Shareholders’ proposal (Seventh Proposal and Eighth Proposal)>
The Seventh Proposal and Eighth Proposal are proposed by 13 shareholders, the total number of whose voting rights is 20,937.
Note that the “Summary of the Proposal” and the “Reasons for the Proposal,” both of which are proposed by such shareholders, are described as the originals (in Japanese) which we received as of April 24, 2019.
Seventh Proposal: Partial Amendment to the Articles of Incorporation (Individual disclosure of the directors’ compensation)
| (1) | Summary of the Proposal |
Addition of the following clause as Article 20, paragraph 2 to the current Articles of Incorporation (revised on June 28, 2018):
(2) The amount and substance of the directors’ compensation shall be individually disclosed irrespective of the amount, the method to determine the compensation shall be specifically indicated, and all compensation shall be individually evaluated and disclosed in Japanese yen in the business report and the annual securities report each year.
46
Table of Contents
| (2) | Reasons for the proposal |
It is highly necessary to clearly indicate the amount of individual directors’ compensation in order for shareholders to determine, for each director, whether the director’s compensation is suitable for the duties delegated thereto, as the shareholders, being the beneficial owners of a company, elect or dismiss directors through general shareholders meetings. Further, it stands to reason that shareholders encourage the directors to exhibit their abilities and ask them to fully perform their functions. It is also in the interest of society at large to request that the respective directors perform greater functions by the shareholders being aware of the amount of their compensation, having specified by whom and how their compensation is determined, and retaining their right to voice opinions on the propriety of the amount. Accordingly, adopting the individual disclosure of officers’ compensation should be considered also in light of revitalizing the corporate activities. In fact, individual disclosure of officers’ compensation by public companies has already been widely implemented in the U.S., the U.K., and other developed countries. Also, given the globalization of investment activities, Takeda, in its undertaking to become a more internationalized company, must demonstrate to the Japanese and global investors its strict compliance with the internal rules that are on a par with those of first-class companies in developed countries.
| ○ | Opinion of the Board of Directors on the Seventh Proposal |
The Board of Directors objects to this Proposal.
With respect to the disclosure of the compensation of each Director, the Company discloses the amount of the compensations of the Directors whose total compensation paid by the Company and its consolidated subsidiaries is 100 million yen or more in the Securities Report in accordance with applicable laws. Moreover, the Company discloses the total amount of the compensation paid to Directors and the number of Directors who received the compensation, specifying the numbers of Internal Directors who are not Audit and Supervisory Committee members, Directors who are Audit and Supervisory Committee members, and External Directors.
With regard to the disclosure of the Directors’ compensation, the Board of Directors puts weight on showing how the Directors’ compensation effectively works as an incentive for the Directors to increase corporate value over the mid-to-long term. From this perspective, the Company specifies the levels of the compensation, the compensation mix, the contents of performance-based compensation, clear establishment of mid-to-long term KPIs, the procedures for ensuring the appropriateness of the compensation and the existence of transparency in the process for determining the compensation, etc., in the “Directors’ Compensation Policy,” and discloses these matters to shareholders and investors. We believe that the Company has provided shareholders and investors with sufficient and appropriate information to enable them to review the appropriateness of the compensation levels and the relationship between performance and compensation levels through the disclosures described above. In addition, the Company considers that the compensation system for the Directors is one of the important matters with regard to corporate governance. The Company has established the Compensation Committee as an advisory body to the Board of Directors, which serves to ensure the appropriateness of the Directors’ compensation and the existence of transparency in the decision-making process related thereto. The majority of the Compensation Committee members are external members and the Compensation Committee is chaired by an External Director. The Compensation Committee deliberates the Directors’ compensation levels, compensation mix, and the targets of performance-based compensation (i.e., the Long-Term Incentive Plan and bonuses) and gives advice to the Board of Directors; thereafter the Board of Directors determines these matters. In addition, the authority to determine the compensation of the Internal Directors who are not Audit and Supervisory Members is delegated to the Compensation Committee by resolution of the Board of Directors, which makes the process of determining the compensation of each Director more transparent.
47
Table of Contents
Based on the Company’s situation as described above, we believe that the amendment to the Articles of Incorporation related to disclosure of the Directors’ compensation, as proposed by the shareholders, is not necessary and not reasonable; thus, we object to this proposal.
Eighth Proposal: Partial Amendment to the Articles of Incorporation (Adoption of a clawback clause)
| (1) | Summary of the Proposal |
Addition of the following clause as Article 20, Paragraph 3 of the current Articles of Incorporation (revised on June 28, 2018), and addition of the following descriptions at the end of Article 27, Paragraphs 1 and 2 respectively:
Article 20 (Compensation, Etc. for Directors)
(3) If the performance indicators and other figures that serve as the basis for calculating the compensation amount under the performance-based compensation plan are erroneous, or if the compensation amount under the long-term incentive plan (stock compensation) is inflated in proportion to the share price that is unduly inflated by reflecting erroneous information (for example, if impairment loss has arisen on excessive investment in the past or if the correction of previous years’ financial results has been made), the compensation amount shall be recalculated based on correct indicators and other figures, and the difference in the compensation amount arising from the recalculation shall be either returned to the Company or shall be reduced (or unpaid). Details of this arrangement shall be prescribed in the Company’s internal rules, and shall be described in the mandate agreement between each of the directors and the Company.
Article 27 (Exemption of Directors’ Liability)
At the end of Paragraphs 1 and 2 respectively
However, the above shall not apply to the return or reduction (or non-payment) of compensation pursuant to Paragraph 3, Article 20.
| (2) | Reasons for the proposal |
In Europe and the United States, there is a strong prevailing logic that if the fixed compensation proportion is high, the management tends to be conservative; accordingly, the performance-based compensation proportion is set high. Meanwhile, adoption of the clawback clause has already become common practice, from the perspective of deterring executives’ coercive management styles that involve excessive risks or reckless management. Specifically, incorporation of clauses is increasingly prevailing which provide that compensation paid in previous years shall be returned or reduced upon the occurrence of a significant profit correction, unjustifiable conduct, or a huge amount of loss. In Japan, too, Nomura Holdings, Inc. and the Sumitomo Mitsui Financial Group, Inc. have already adopted these types of clause. Takeda, aiming to be a Japan-based global company, deems it necessary to establish this clause in order to deter excessive risk-taking by the Company’s executives, including foreign executive officers. For your information, recently, adoption of the clawback clause is also recommended in the “Guidelines on Executive Compensation (4th edition)” issued by the Japan Association of Corporate Directors.
| ○ | Opinion of the Board of Directors on the Eighth Proposal |
48
Table of Contents
The Board of Directors objects to this Proposal.
We understand that the background of this proposal is the argument that, in cases where amendments to the accounting are required due to inappropriate accounting or financial losses are caused by excessively risky investments, the Company should request the return of the Directors’ compensation regardless of whether or not the Directors breach their duty of due care as prudent managers. In this regard, the Company’s compensation system for Directors has been established, based on the role and responsibility of each Director, so as to enhance the awareness of contributing to the enhancement of the Company’s mid/long-term achievements as well as the increase of the corporate value of the Company and to align with the incentives of Directors with the interest of the Company’s shareholders. Also, the stock compensation system for Internal Directors is reasonably appropriate to deter the executives’ coercive management styles that involve excessive risks or reckless management, which are pointed out in the shareholders’ proposal, since (i) it occupies the majority of the total compensation for Internal Directors, (ii) vesting thereof is divided and made for 3 years, and (iii) two (2) year stock holding requirement after the vesting date has been introduced for the annual grant, which further promotes management from long-term perspective. In addition, in order to ensure the objectivity and transparency of the content and process of deliberations in the Board of Directors, the majority of the Company’s Board of Directors consists of highly independent External Directors (11 of 15 members as of March 31, 2019). These External Directors proactively engage in deliberations, have exhaustive discussions at the time of rendering judgments, and make business decisions which they consider the best possible, based on the shareholders’ interests. The Compensation Committee, the majority of the members of which are external members and is chaired by an External Director also review the company’s performance against approved KPIs, review and approve Cash Bonus (STI) and Equity (LTI) awards for each Internal Director relative to their performance against their approved KPIs. Moreover, the amendment to the Articles of Incorporation proposed by the shareholders, will, without due consideration, place absolute liability on the Directors in cases where misconduct, including inappropriate accounting, has occurred and where the Directors did not breach their duty of due care as prudent managers with respect to the misconduct. As a result, the Directors will be unnecessarily cautious or reluctant to make business decisions which will be detrimental to shareholders.
However, because the Board of Directors must fulfill its duty of due care as a prudent manager, as well as its duty of loyalty, in determining the amount of the compensation of each Director, in a case where a Director actually received an inappropriately high performance-based compensation as a result of misconduct, including an inappropriate accounting, the Company would appropriately exercise its rights in a timely manner, based on the internal rules, mandate agreements, and/or applicable laws, as the case may be, and to request the return of such inappropriate performance-based compensation by considering individual and specific situations such as the content of the actual misconduct. Regardless of whether or not the provision proposed by the shareholders is added to the Articles of Incorporation, the Company believes that it must request the return of the Director’s compensation in a case as described above.
Furthermore, as mentioned above, with regard to a request for return of a Director’s compensation, we believe that this is a matter which must be discreetly examined by the appropriate bodies, such as the Board of Directors, Compensation Committee, and Audit and Supervisory Committee, by considering individual and specific situations. On the other hand, the addition of the unnecessary provision to the Articles of Incorporation relating to the return of Directors’ compensation in certain cases has a possibility of excessively decreasing and limiting the authority and agility of the decision-making of the Board of Directors, the Compensation Committee, and the Audit and Supervisory Committee, thereby interfering with the decisions made by such bodies, and could also distort the Directors’ compensation system.
49
Table of Contents
Based on the Company’s situation and policy as described above, we believe that the amendment to the Articles of Incorporation proposed by the shareholders relating to the return of Directors’ performance-based compensation is not necessary and not reasonable; thus, we object to this proposal.
END OF DOCUMENT
50
Table of Contents
Business Report
(From April 1, 2018 to March 31, 2019)
| 1. | Current State of the Takeda Group |
(1) Business Overview
We are a global, values-based, R&D-driven, biopharmaceutical company with an innovative portfolio, engaged primarily in the research, development, production and marketing of pharmaceutical products. We have a geographically diversified global business base operating in approximately 80 countries, and our prescription drugs are marketed in approximately 100 countries, which are recognized brands in major countries worldwide.
We have grown both organically and through acquisition, completing multiple transactions that have brought growth to our areas of therapeutic, geographic, and pipeline focus; especially through the acquisition of Shire plc (“Shire”) in January 2019 for 6.2 trillion JPY. The acquisition of Shire strengthened our presence in GI and neuroscience, while providing us with a leading position in rare diseases and plasma derived therapies. It also enhanced our R&D engine creating a highly complementary, robust, modality-diverse pipeline. Commercially, the acquisition significantly strengthened our presence in the United States.
We are focused on four core therapeutic areas of oncology, gastroenterology, rare diseases, neuroscience along with targeted R&D investments in plasma-derived therapies and vaccines.
Our business and results of operations are impacted by many factors, including acquisitions and divestitures, changes in the global pharmaceutical industry, and other economic conditions. Examples of these factors include, but are not limited to:
| • | Acquisition of Shire and, to a lesser extent, other acquisitions. In addition to the added business and our ability to integrate successfully, our results are impacted by the amortization expense of the intangible assets acquired, the expense related to the unwinding of inventory fair value adjustment, and the increase in interest expense associated with the debt financing to fund these acquisitions. During the fourth quarter ended March 31, 2019, we recorded amortization expense of 99.2 billion JPY associated with acquired intangible assets, 82.2 billion JPY related to the charge to unwind the fair value step up on inventory, and 41.3 billion JPY of financial expense including incremental interest expense associated with these acquisitions. |
| • | Increase in the global demand for healthcare across markets, driven by increased access to healthcare, particularly in low-income and middle-income countries. |
51
Table of Contents
| • | Government policy to promote innovation and addressing unmet needs. On the other hand increased pressure on pricing mostly due to efforts towards health care cost containment. |
| • | Economic growth continues to be stagnant in many major developed countries, while the pace of growth in many emerging economies has declined |
(2) Business Performance for Fiscal 2018
(i) Consolidated Financial Results (April 1 to March 31, 2019)
| Billion JPY | ||||||||
| For the fiscal year ended March 31, | ||||||||
| 2018 | 2019 | |||||||
| Revenue |
1,770.5 | 2,097.2 | ||||||
| Cost of Sales |
(495.9 | ) | (659.7 | ) | ||||
| Selling, General and Administrative expenses |
(628.1 | ) | (717.6 | ) | ||||
| Research and Development expenses |
(325.4 | ) | (368.3 | ) | ||||
| Amortization and Impairment Losses on Intangible Assets Associated with Products |
(122.1 | ) | (203.4 | ) | ||||
| Other Operating Income |
169.4 | 159.9 | ||||||
| Other Operating Expense |
(126.6 | ) | (103.2 | ) | ||||
|
|
|
|
|
|||||
| Operating Profit |
241.8 | 205.0 | ||||||
| Financial Income |
39.5 | 16.8 | ||||||
| Financial Expense |
(31.9 | ) | (83.3 | ) | ||||
| Shares of Loss of Associates Accounted for Using the Equity Method |
(32.2 | ) | (43.6 | ) | ||||
|
|
|
|
|
|||||
| Profit Before Income Tax |
217.2 | 94.9 | ||||||
| Income Tax expenses |
(30.5 | ) | 14.1 | |||||
|
|
|
|
|
|||||
| Net Profit for the Year |
186.7 | 109.0 | ||||||
|
|
|
|
|
|||||
52
Table of Contents
Fiscal Year Ended March 31, 2019 compared with the Fiscal Year Ended March 31, 2018
The consolidated financial results for the fiscal year ended March 31, 2019 include the impact of acquisition of Shire and Shire operations for the period from January 8 to March 31, 2019, which significantly impacted on our results of operation. The following summarizes the impact on our results of operations in the year end March 31, 2019 and on the change in our results between years.
| Billion JPY except for percentages | ||||||||||||||||||||||||||||||||||||
| For the fiscal year ended March 31, | ||||||||||||||||||||||||||||||||||||
| Consolidated Financial Results | Impact from the Shire acquisition | Remaining Change | ||||||||||||||||||||||||||||||||||
| 2019 | Change vs. the previous year |
Shire operations |
Impact of purchase accounting |
Acquisition/ integration costs |
2019 | Change vs. the previous year |
||||||||||||||||||||||||||||||
| Revenue |
2,097.2 | 326.7 | 18.5 | % | 309.2 | — | — | 1,788.0 | 17.5 | 1.0 | % | |||||||||||||||||||||||||
| Cost of Sales |
(659.7 | ) | (163.8 | ) | 33.0 | % | (101.6 | ) | (81.7 | ) | — | (476.4 | ) | 19.6 | (3.9 | )% | ||||||||||||||||||||
| Selling, general, and administrative expenses |
(717.6 | ) | (89.5 | ) | 14.2 | % | (98.5 | ) | (0.6 | ) | (23.8 | ) | (594.7 | ) | 33.4 | (5.3 | )% | |||||||||||||||||||
| Research and development expenses |
(368.3 | ) | (42.9 | ) | 13.2 | % | (43.0 | ) | — | (1.6 | ) | (323.7 | ) | 1.7 | (0.5 | )% | ||||||||||||||||||||
| Amortization and impairment losses on intangibles assets associated with products |
(203.4 | ) | (81.2 | ) | 66.5 | % | — | (99.2 | ) | — | (104.1 | ) | 18.0 | (14.7 | )% | |||||||||||||||||||||
| Other operating income |
159.9 | (9.5 | ) | (5.6 | )% | (1.4 | ) | — | — | 161.2 | (8.2 | ) | (4.8 | )% | ||||||||||||||||||||||
| Other operating expenses |
(103.2 | ) | 23.4 | (18.5 | )% | (4.9 | ) | — | (59.6 | ) | (38.6 | ) | 88.0 | (69.5 | )% | |||||||||||||||||||||
| Operating profit |
205.0 | (36.8 | ) | (15.2 | )% | 59.8 | (181.6 | ) | (85.0 | ) | 411.8 | 170.0 | 70.3 | % | ||||||||||||||||||||||
| Finance income |
16.8 | (22.7 | ) | (57.4 | )% | — | 0.2 | — | 16.6 | (22.9 | ) | (57.9 | )% | |||||||||||||||||||||||
| Finance expense |
(83.3 | ) | (51.4 | ) | 160.9 | % | (10.6 | ) | (4.2 | ) | (41.3 | ) | (27.1 | ) | 4.8 | (15.1 | )% | |||||||||||||||||||
| Share of profit (loss) of investments accounted for using the equity method |
(43.6 | ) | (11.4 | ) | 35.5 | % | 0.3 | — | (43.9 | ) | (11.7 | ) | 36.4 | % | ||||||||||||||||||||||
| Profit Before Income Tax |
94.9 | (122.3 | ) | (56.3 | )% | 49.4 | (185.6 | ) | (126.3 | ) | 357.4 | 140.2 | 64.5 | % | ||||||||||||||||||||||
| Income Tax expenses |
14.1 | 44.6 | (146.3 | )% | (11.3 | ) | 44.0 | 26.1 | (44.6 | ) | (14.1 | ) | 46.3 | % | ||||||||||||||||||||||
| Net Profit for the Year |
109.0 | (77.7 | ) | (41.6 | )% | 38.1 | (141.7 | ) | (100.2 | ) | 312.8 | 126.1 | 67.5 | % | ||||||||||||||||||||||
Revenue. Revenue increased 326.7 billion JPY, or 18.5%, to 2,097.2 billion JPY for the fiscal year ended March 31, 2019, including 309.2 billion JPY resulting from the Shire Acquisition.
The remaining increase of 17.5 billion JPY, or 1.0%, results from the continued expansion from three business areas (Gastroenterology, Oncology, and Neuroscience), which was partially offset by the divestitures and the unfavorable impact of foreign currency movements. Change in revenue in the three core business areas was primarily attributable to the following products:
53
Table of Contents
| • | GI. In Gastroenterology, revenue was driven by Takeda’s top-selling product ENTYVIO (for ulcerative colitis and Crohn’s disease) with sales of 269.2 billion JPY in the fiscal year ended March 31, 2019, an increase of 67.8 billion JPY, or 33.7%. This increase was mainly attributable to ENTYVIO’s steady expansion of patient share in the bio-naïve segment. Takeda obtained a New Drug Application (“NDA”) approval in July 2018 in Japan for the treatment of patients with moderately to severely active ulcerative colitis and launched the product in November 2018. |
Sales of TAKECAB (for acid-related diseases) were 58.2 billion JPY in the fiscal year ended March 31, 2019, an increase of 9.8 billion JPY, or 20.1%, versus the previous year. The increase was driven by the expansion of new prescriptions in the Japanese market due to TAKECAB’s efficacy in reflux esophagitis and the prevention of recurrence of gastric ulcers during low-dose aspirin administration.
| • | Oncology. In Oncology, sales of NINLARO (for multiple myeloma) were 62.2 billion JPY, an increase of 15.7 billion JPY, or 33.9%, versus the previous year. Strong performance in several regions, particularly in the U.S. continued to contribute to the growth. NINLARO is a once-weekly oral proteasome inhibitor with a profile of efficacy, safety, and convenience. Additionally, sales of ADCETRIS (for malignant lymphomas) increased by 4.4 billion JPY, or 11.4%, reflecting strong performance particularly in Japan and Brazil. Sales of ICLUSIG (for leukemia) and ALUNBRIG (for lung cancer), obtained through the acquisition of ARIAD in February 2017, grew by 5.6 billion JPY, or 24.1% and 2.4 billion JPY, or 84.0%, respectively. Sales of VELCADE (for multiple myeloma), which lost market exclusivity in the U.S. in previous year, decreased by 9.4 billion JPY, or 6.9%. |
| • | Neuroscience. In Neuroscience, sales of TRINTELLIX (for major depressive disorder (MDD)) were 57.6 billion JPY in the fiscal year ended March 31, 2019, an increase of 9.2 billion JPY, or +19.0%, versus the previous year. Prescribers and patients increasingly made TRINTELLIX part of their comprehensive approach to treat MDD. |
The decrease in revenue resulting from divestitures was primarily due to a 18.7 billion JPY decrease resulting from sale of seven long-listed products in Japan to Teva Takeda Yakuhin Ltd. in May 2017, 11.6 billion JPY decrease from the disposition of Guangdong Techpool Bio-Pharma Co in May 2018, and a decrease of 11.0 billion JPY from terminating Takeda’s co-promotion and distribution of Xeljanz in Japan in March 2018.
Shire contributed 309.2 billion JPY to our revenue from the date of acquisition. As part of the integration, this included the application of Takeda’s distribution channel policies to Shire’s portfolio, and a one-time effect resulted in significantly lower days-on-hand of commercial product at wholesalers. Days-on-hand in commercial stock was decreased significantly across various products. The sales were primarily from the following products:
| • | GI. In Gastroenterology, revenue was 21.5 billion JPY primarily from the sales of GATTEX (for the treatment of short bowel syndrome) that were 12.8 billion JPY. |
54
Table of Contents
| • | Rare Diseases. In Rare Diseases, revenue was 111.2 billion JPY including sales of ADYNOVATE (for the treatment of hemophilia A), TAKHZYRO (for the preventive treatment of hereditary angioedema), and NATPARA (for the treatment of hypoparathyroidism) that were 10.7 billion JPY, 9.7 billion JPY, and 7.1 billion JPY, respectively. |
| • | Plasma Derived Therapies. In Plasma Derived Therapies, revenue was 96.3 billion including sales of IMMUNOGLOBULIN (mainly for the treatment of primary immunodeficiency and multifocal motor neuropathy) and ALBUMIN (primarily used for the hypovolemia and hypoalbuminemia 62.2 billion JPY and 10.7 billion JPY, respectively. |
| • | Neuroscience. In Neuroscience, revenue was 60.1 billion JPY including sales of VYVANSE (for the treatment of ADHD and moderate to severe binge eating disorder) of 49.4 billion JPY |
We generated revenue from sales in the following geographies:
| Billion JPY except for percentages | ||||||||||||||||
| For the fiscal year ended March 31, | ||||||||||||||||
| Revenue: |
2018 | 2019 | ||||||||||||||
| Japan |
580.3 | 32.8 | % | 571.0 | 27.2 | % | ||||||||||
| United States |
598.3 | 33.8 | 829.0 | 39.5 | ||||||||||||
| Europe and Canada |
313.7 | 17.7 | 405.6 | 19.3 | ||||||||||||
| Russia/CIS |
68.2 | 3.9 | 59.7 | 2.8 | ||||||||||||
| Latin America |
75.7 | 4.3 | 88.1 | 4.2 | ||||||||||||
| Asia (excluding Japan) |
104.0 | 5.9 | 105.4 | 5.0 | ||||||||||||
| Other |
30.2 | 1.7 | 38.3 | 1.8 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
1,770.5 | 100.0 | % | 2,097.2 | 100.0 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
Cost of Sales. Cost of Sales increased 163.8 billion JPY, or 33.0%, to 659.7 billion JPY for the fiscal year ended March 31, 2019. This includes 101.6 billion JPY related to the sale of Shire products and the impact of purchase price allocation of a 81.7 billion JPY mainly due to non-cash charge from the unwinding of the fair value step up on the inventory from the Shire Acquisition. This increase was offset by a decrease in remaining Cost of Sales 19.6 billion JPY, or 3.9%, primarily due to a more favorable product mix.
Selling, General and Administrative (SG&A) expenses. SG&A expenses increased 89.5 billion JPY, or 14.2%, to 717.6 billion JPY for the fiscal year ended March 31, 2019, primarily due to acquisition of Shire’s operations in our results of 98.5 billion JPY and related acquisition costs of 23.8 billion JPY. This increase was partially offset by a decrease of remaining SG&A expenses of 33.4 billion JPY due to a favorable impact of the Global Opex Initiative as well as lower long-term share-based incentive payments to management.
Research and Development (R&D) expenses. R&D expenses increased 42.9 billion JPY, or 13.2%, to 368.3 billion JPY for the fiscal year ended March 31, 2019, primarily resulting from the acquisition of Shire. The remainder of our R&D expenses remained steady compared to the previous year.
55
Table of Contents
Amortization and Impairment Losses on Intangible Assets Associated with Products. Amortization and Impairment Losses on Intangible Assets Associated with Products increased by 81.2 billion JPY, or 66.5%, to 203.4 billion JPY for the fiscal year ended March 31, 2019. This represents an increase of 99.2 billion JPY related to amortization of intangible assets recorded in the Shire Acquisition and a 22.6 billion reversal of the COLCRYS impairment recorded in the previous year. This increase was offset by lower amortization expense of 36.7 billion JPY, which related to the VELCADE intangible asset fully amortized within the previous year.
Other Operating Income. Other Operating Income decreased 9.5 billion JPY, or 5.6%, to 159.9 billion JPY for the fiscal year ended March 31, 2019. The decrease was primarily due to the net impact of a 106.3 billion JPY gain on the sale of Wako Pure Chemical Industries, Ltd. recorded in the previous year, whereas we recorded a 50.3 billion gain on sale of Property, Plant & Equipment and Investment Property including Takeda’s old headquarter building in Tokyo as well as a 38.2 billion JPY gain on sale of shares of the subsidiary, to which respective real estate businesses were transferred in the current year.
Other Operating Expenses. Other Operating Expenses decreased 23.4 billion JPY, or 18.5%, to 103.2 billion JPY for the fiscal year ended March 31, 2019, which was a decrease of 88.0 billion JPY partially offset by 59.6 billion JPY of Shire integration costs. The decrease was primarily due to a decrease of 22.8 billion JPY in restructuring expense such as R&D transformation costs, and other costs incurred in the prior year that did not reoccur in the current fiscal year such as a 41.5 billion JPY loss on the liquidation of a foreign subsidiary.
Operating Profit. As a result of the above factors, Operating Profit decreased by 36.8 billion JPY, or 15.2%, to 205.0 billion JPY for the fiscal year ended March 31, 2019.
Net Financial Income / (Expense). Net Financial Expense was a 66.4 billion JPY in the current year, an increase of 74.1 billion JPY compared to the previous year, which includes 41.3 billion JPY mainly related to interest on borrowings used to partially fund the Shire Acquisition. The remaining increase is primarily due to the exclusion of a 30.4 billion JPY gain from the adoption of a new accounting standard related to accounting for equity securities that was recorded in the prior year.
Shares of Loss of Associates Accounted for Using the Equity Method. Shares of Loss of Associates Accounted for Using the Equity Method were 43.6 billion JPY, an increase of 11.4 billion JPY from the previous year. This primarily relates to Takeda’s share of an impairment charge recognized by Teva Takeda Pharma Ltd. Teva Takeda Pharma Ltd. operates a business of long-listed products and generics, and conducted a revaluation of its assets in response to changes in the business environment.
Income Tax expenses. Income Tax Expenses decreased 44.6 billion JPY, or -146.3% from 30.5 billion JPY for the fiscal year ended March 31, 2018 to tax benefit of 14.1 billion JPY for the fiscal year ended March 31, 2019. This decrease was mainly due to tax benefit of 58.7 billion JPY from the Shire acquisition. Excluding the Shire acquisition impact, the remaining Income Tax Expenses increased 14.1 billion JPY mainly due to an increase in Profit Before Tax, as well as the impacts from the enactment of the Tax Cuts and Jobs Act (Tax Reform) in the U.S. in the previous year. These factors were partially offset by capital loss related to restructuring of subsidiaries in the current year.
Net Profit for the Year. Net Profit for the Year decreased 86.5 billion JPY, or 46.3%, to 100.2 billion JPY for the fiscal year ended March 31, 2019.
56
Table of Contents
(ii) Underlying Growth (April 1, 2018 to March 31, 2019)
Takeda uses the concept of “Underlying Growth” for internal planning and performance evaluation purposes. For the year ended March 31, 2019, the impact of Shire acquisition has been excluded from our Underlying measures to allow comparison to prior year Underlying measures.
Underlying Growth compares two periods (quarters or years) of financial results under a common basis and is used by management to assess the business. These financial results are calculated on a constant currency basis and excludes the impacts of divestitures and other amounts that are unusual, non-recurring items or unrelated to our ongoing operations. Although these are not measures defined by IFRS, Takeda believes Underlying Growth is useful to investors as it provides a consistent measure of our performance.
Takeda uses “Underlying Revenue(1) Growth”, “Underlying Core Earnings(2) Growth”, and “Underlying Core EPS(3) Growth” as key financial metrics.
| Legacy Takeda Underlying Growth | ||||||||
| Change versus the same period of the previous year | ||||||||
| % | Billion JPY | |||||||
| Underlying Revenue |
+5.3% | +89.1 | ||||||
| Underlying Core Earnings |
+38.7% | +109.5 | ||||||
|
Underlying EPS |
+29.0% | +77.88 | JPY | |||||
| (1) | Underlying Revenue represents revenue on a constant currency basis and excluding non-recurring items and the impact of divestitures occurred during the reporting periods presented and excludes the impact of the Shire Acquisition. |
| In this period, the underlying revenue excludes the impact of the sale of 7 long-listed products in Japan to Teva Takeda Yakuhin Ltd. which is a subsidiary of Teva Takeda Pharma Ltd. and the impact of the divestitures of Multilab Indústria e Comércio de Produtos Farmacêuticos Ltda. and Guangdong Techpool Bio-Pharma Co., Ltd. |
| (2) | Underlying Core Earnings represents Core Earnings based on a constant currency basis and further adjusted to exclude the impacts of divestitures that occurred during the reported periods and the impact of the Shire acquisition. |
| Core Earnings represents net profit adjusted to exclude income tax expenses, our share of profit or loss of investments accounted for using the equity method, finance expenses and income, other operating expenses and income, amortization and impairment losses on acquired intangible assets and other items that management believes are unrelated to our core operations, such as purchase accounting effects and transaction related costs. In this period, divestitures include the impact of the sale of 7 long-listed products in Japan to Teva Takeda Yakuhin Ltd., and the impact of the divestitures of Multilab Indústria e Comércio de Produtos Farmacêuticos Ltda. and Guangdong Techpool Bio-Pharma Co., Ltd. |
| (3) | Underlying Core EPS represents net income based on a constant currency basis, adjusted to exclude the impact of divestitures, the impact of the Shire acquisition, items excluded in the calculation of Core Earnings, and other non-operating items (e.g. amongst other items, fair value adjustments and the imputed financial charge related to contingent consideration) that are unusual, non-recurring in nature or unrelated to its ongoing operations and the tax effect of each of the adjustments, divided by the outstanding shares (excluding treasury shares) as of the end of the comparative period. |
| In this period, the other non-operating significant items that are excluded in calculating Underlying Core EPS include the financial costs such as incremental interest costs from loans payable related to the Shire acquisition in addition to fair value adjustments and the imputed financial charge related to contingent consideration. |
57
Table of Contents
Underlying Revenue. Legacy Takeda Underlying Revenue growth was +5.3% compared to the same period of the previous year, driven by the strong performance of Takeda’s Growth Drivers and more specifically products such as ENTYVIO (for ulcerative colitis and Crohn’s disease) +34.8%, NINLARO (for multiple myeloma) +36.1%, ICLUSIG (for leukemia) +24.6%, TAKECAB (for acid-related diseases) +20.1% and TRINTELLIX (for major depressive disorder) +19.4%. The Underlying Revenue of Takeda’s Growth Drivers grew by +11.1%, which is 63.3% of total revenue.
Underlying Core Earnings. Legacy Takeda Underlying Core Earnings growth was +38.7%, reflecting strong Underlying Revenue growth and the positive impact of the Global Opex Initiative (4). Underlying Cost of Sales as a percentage of sales improved by +1.4pp driven by a more favorable sales mix. Underlying Operating Expenses as a percentage of sales improved by +3.9pp driven by the impact of the Global Opex Initiative. The combination of the above factors led to an improvement in the Core Earnings Margin by 5.4pp to 22.3%.
| (4) | Takeda’s global operating expense reduction initiative with the aim of delivering annual margin improvements driven by reduced consumption, procurement initiatives and organizational optimization. |
Underlying Core EPS. Legacy Takeda Underlying Core EPS growth was +29.0% compared to the same period of the previous year reflecting strong Underlying Core Earnings growth of +38.7%.
58
Table of Contents
(iii) Activities and Results of Research & Development
Research and development expenses for the period ending March 31, 2019 were 368.3 billion JPY.
Takeda initiated a five year R&D Transformation program in July 2016, to re-invigorate the pipeline and build an agile, global R&D organization driven by innovative science. A significant component of the change has been an intensive focus in the following three key areas:
| 1. | Therapeutic Area Focus: Leveraging therapeutic area expertise to progress innovative assets |
| 2. | Partnerships & Capabilities: Enhancing capabilities internally and through external collaborations |
| 3. | Innovative Research Engine: Developing new technologies and new modalities to treat disease |
Upon completion of the Shire Acquisition, Takeda now has focused R&D efforts in four therapeutic areas (Oncology, Gastroenterology, Rare Diseases, and Neuroscience) and two targeted R&D Business Units (Plasma Derived Therapies and Vaccines).
Major progress on R&D events and business development contracts occurring within the period ending March 31 2019, including events and development activities related to Legacy Shire’s products and pipeline for periods after the Shire Acquisition, are listed as follows:
R&D pipeline
Oncology
In oncology, Takeda endeavors to deliver novel medicines to patients with cancer worldwide through the commitment to breakthrough innovation, and a passion for improving the lives of patients. Takeda focuses in 3 key areas in oncology; (1) building on the foundational expertise in hematologic malignancies through continued investment in lifecycle management programs for marketed products NINLARO, ADCETRIS, and ICLUSIG, as well as in pipeline assets in Multiple Myeloma, Acute Myeloid Leukemia, Myelodysplastic Syndromes, and other blood cancers; (2) further developing its portfolio in lung cancer; and (3) pursuing novel immuno-oncology targets and next-generation platforms with external partners as well as exploring innovative cell therapies.
[NINLARO/Generic name: ixazomib]
| - | In July 2018, Takeda announced that the randomized, Phase 3 TOURMALINE-MM3 study met its primary endpoint, demonstrating that single-agent oral NINLARO as a maintenance therapy resulted in a statistically significant improvement in progression-free survival (PFS) versus placebo. The trial evaluated the effect of NINLARO as a maintenance therapy in adult patients diagnosed with multiple myeloma who responded to high-dose therapy (HDT) and autologous stem cell transplant (ASCT). |
| - | In December 2018, the data from the Phase 3 TOURMALINE-MM3 study was presented at the 60th American Society of Hematology (ASH) annual meeting. |
59
Table of Contents
| - | In January 2019, Takeda announced that the data from the TOURMALINE-MM3 study had been submitted to the U.S. Food and Drug Administration (FDA) in November 2018, and after further discussion with the authorities, the decision has been made to withdraw the filing and to resubmit when more mature survival data are available. |
| - | In April 2019, Takeda announced that it has submitted for a partial change to its manufacturing and marketing approval to Japanese Ministry of Health, Labour and Welfare (MHLW) for NINLARO regarding the additional indication as a maintenance therapy for multiple myeloma following ASCT. |
[ALUNBRIG/Generic name: brigatinib]
| - | In July 2018, Takeda announced that the global randomized, Phase 3 ALTA-1L trial met its primary endpoint at the first pre-specified interim analysis, with ALUNBRIG demonstrating a statistically significant improvement in progression-free survival (PFS) compared to crizotinib in adults with anaplastic lymphoma kinase-positive (ALK+) locally advanced or metastatic non-small cell lung cancer (NSCLC) who had not received a prior ALK inhibitor. |
| - | In September 2018, the findings from the first interim analysis of the ALTA-1L trial were presented during the Presidential Symposium at the International Association for the Study of Lung Cancer (IASLC) 19th World Conference on Lung Cancer (WCLC). |
| - | In September 2018, Takeda announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) had adopted a positive opinion, recommending the full approval of ALUNBRIG as a monotherapy for the treatment of adult patients with ALK+ advanced NSCLC previously treated with crizotinib. |
| - | In October 2018, Takeda announced that intracranial efficacy data from the Phase 3 ALTA-1L trial showed improved intracranial progression-free survival (PFS) and intracranial objective response rate (ORR) with ALUNBRIG compared to crizotinib among ALK+ NSCLC patients. Data for these secondary endpoints were presented in a poster discussion at the European Society for Medical Oncology (ESMO) 2018 Congress. |
| - | In December 2018, Takeda announced that the European Commission (EC) had granted marketing authorization for ALUNBRIG as a monotherapy for the treatment of adult patients with ALK+ advanced NSCLC previously treated with crizotinib. |
| [ADCETRIS/Generic | name: brentuximab vedotin] |
| - | In September 2018, Takeda announced that it has obtained an additional indication and dosage & administration from the Japanese Ministry of Health, Labour and Welfare (MHLW) for the use of ADCETRIS in combination with doxorubicin, vinblastine and dacarbazine as a frontline treatment option for CD30-positive Hodgkin lymphoma patients. |
| - | In October 2018, Takeda announced that the Phase 3 ECHELON-2 clinical trial met its primary endpoint, demonstrating a statistically significant improvement in progression-free survival (PFS) of ADCETRIS in combination with CHP (cyclophosphamide, doxorubicin, prednisone) versus the control arm, CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) in previously untreated CD30-expressing peripheral T-cell lymphoma. The ADCETRIS plus CHP arm also demonstrated superior overall survival (OS), a key secondary endpoint, compared to CHOP). |
60
Table of Contents
| - | In December 2018, the data from the Phase 3 ECHELON-2 clinical trial were presented in an oral session at the 60th American Society of Hematology (ASH) Annual Meeting. |
| - | In December 2018, Takeda announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) had adopted a positive opinion for the extension of the marketing authorization of ADCETRIS and recommended its approval in combination with AVD (adriamycin, vinblastine, dacarbazine) in adult patients with previously untreated CD30+ Stage IV Hodgkin lymphoma. |
| - | In February 2019, Takeda announced that the European Commission (EC) extended the current marketing authorization of ADCETRIS to include the treatment of adult patients with previously untreated CD30+ Stage IV Hodgkin lymphoma in combination with AVD. |
| - | In March 2019, Takeda announced that it has submitted an application in Japan for the additional indication and dosage & administration of ADCETRIS in the treatment of patients with CD30-positive peripheral T-cell lymphoma, in pediatric patients with relapsed or refractory CD30-positive Hodgkin lymphoma, and in pediatric patients with anaplastic large cell lymphoma. |
[Generic name: cabozantinib]
| - | In April 2019, Takeda announced that it has submitted to the Ministry of Health, Labor and Welfare of Japan for manufacturing and marketing approval for Cabozantinib for the treatment of unresectable and metastatic renal cell carcinoma. The application is based on the results of an international phase-3 METEOR pivotal trial, an overseas phase-2 CABOSUN trial, and a Japanese phase-2 Cabozantinib-2001 trial that studied the efficacy and safety of Cabozantinib on 35 Japanese patients suffering from advanced renal cell carcinoma, which had progressed after prior vascular endothelial growth factor receptor tyrosine kinase inhibitor (VEGFR-TKI) therapy. |
Gastroenterology
In gastroenterology (GI), Takeda is focused on delivering innovative, life-changing therapeutics for patients with GI and liver diseases. Takeda is expanding its position in specialty GI with ENTYVIO and progressing a pipeline built through partnerships that are exploring opportunities in motility disorders, celiac disease, liver disease, and the microbiome.
[ENTYVIO/Generic name: vedolizumab]
| - | In June 2018, Takeda announced a new analysis of real-world data comparing the safety data of the gut-selective biologic ENTYVIO and tumor necrosis factor-alpha (TNFα)-antagonist therapy. The results showed numerically lower rates of serious infections (SIs) and significantly lower rates of serious adverse events (SAEs) in patients treated with ENTYVIO compared to TNFα-antagonist therapy. This analysis of the VICTORY consortium was presented as an oral presentation at the 2018 Digestive Disease Week (DDW). |
| - | In July 2018, Takeda announced that it has obtained a New Drug Application approval from the Japanese Ministry of Health, Labour and Welfare (MHLW) for ENTYVIO for the treatment of patients with moderately to severely active ulcerative colitis in Japan. |
| - | In July 2018, Takeda announced that it has submitted a New Drug Application to the Japanese MHLW for ENTYVIO for the treatment of adult patients with moderately to severely active Crohn’s disease in Japan. |
61
Table of Contents
| - | In July 2018, Takeda announced top-line results from the Phase 3 VISIBLE 1 clinical trial evaluating the efficacy and safety of an investigational subcutaneous (SC) formulation of vedolizumab for maintenance therapy in adult patients with moderately to severely active ulcerative colitis who achieved clinical response* at week 6 following two doses of open-label vedolizumab intravenous (IV) induction therapy. In the primary endpoint of the trial, a statistically significant proportion of patients receiving vedolizumab SC beginning at week 6 and every two weeks following achieved clinical remission** at week 52 compared to placebo. |
| * | Clinical response is defined as a reduction in Mayo score of ³3 points and ³30% from baseline (week 0) with an accompanying decrease in rectal bleeding subscore of ³1 point or absolute rectal bleeding subscore of £1 point. |
| ** | Clinical remission is defined as a complete Mayo score of £2 points and no individual subscore greater than >1 point. |
| - | In October 2018, the results of the Phase 3 VISIBLE 1 clinical trial were presented at the 2018 United European Gastroenterology (UEG) Week congress. |
| - | In March 2019, Takeda announced results from the Phase 3b head-to-head VARSITY study which demonstrated that ENTYVIO was superior to the anti-tumor necrosis factor-alpha (anti-TNFα) biologic adalimumab (Brand name: HUMIRA®) in achieving clinical remission* in patients with moderately to severely active ulcerative colitis at week 52. Data showed that 31.3% (n=120/383) of patients receiving ENTYVIO intravenous achieved the primary endpoint of clinical remission compared to 22.5% (n=87/386) of patients treated with adalimumab subcutaneous at week 52, with the difference being statistically significant. These results were announced as an oral presentation at the 14th Congress of the European Crohn’s and Colitis Organisation (ECCO). |
| * | Primary endpoint: Clinical remission is defined as a complete Mayo score of £2 points and no individual subscore >1 point. |
| - | In April 2019, Takeda announced that the European Medicines Agency (EMA) has accepted a Marketing Authorization Line Extension Application for a SC formulation of vedolizumab for maintenance therapy in adults with moderately to severely active ulcerative colitis or Crohn’s disease. Takeda proposes to make vedolizumab SC available in both pre-filled syringe and pen options. |
| - | In May 2019, Takeda announced that the U.S. Food & Drug Administration (FDA) has accepted for review a Biologics License Application (BLA) for a SC formulation of vedolizumab for maintenance therapy in adults with moderately to severely active ulcerative colitis (UC). Takeda proposes to make vedolizumab SC available in both pre-filled syringe and pen options. |
Rare Diseases
Takeda added Rare Diseases to its therapeutic area focus after the acquisition of Shire on January 8, 2019, focusing on 3 key areas of deep Shire expertise: (1) Rare Immunology (e.g. Hereditary Angioedema (HAE)), through the recent launch of TAKHZYRO to transform the treatment paradigm; (2) Rare Hematology; with the broadest portfolio across its competitors in Hematology and (3) Rare Metabolic diseases.
| - | In February 2019, Takeda featured 12 presentations, including 11 posters and one oral presentation, at the 15th annual WORLDSymposium™2019. Presentations and other company activities focused on research and development efforts in lysosomal storage disorders including Hunter syndrome (also known as Mucopolysaccharidosis type II or MPS II), type 1 Gaucher disease, Fabry disease, and metachromatic leukodystrophy (MLD). |
[NATPARA/Generic name: Parathyroid hormone]
| - | In March 2019, Takeda presented new data at the Endocrine Society’s 2019 Annual Meeting (ENDO) revealing the burden of chronic hypoparathyroidism on patients and caregivers, as well as potential long-term risks of renal and cardiovascular complications that patients treated with conventional therapy may experience. Takeda also presented six-year results from the open-label long-term safety and efficacy RACE study, showing that treatment with rhPTH (1-84) in patients with chronic hypoparathyroidism had a safety profile consistent with previous clinical studies, and impacted key measurements of mineral homeostasis, notably of urinary calcium. |
62
Table of Contents
Neuroscience
In neuroscience, Takeda aims to bring innovative medicines to patients suffering from neurologic and psychiatric diseases for whom there are no treatments available. Takeda is expanding its presence in psychiatric diseases through continued investment in Trintellix for Major Depressive Disorder, and the Attention Deficit Hyperactivity Disorder portfolio acquired from Shire. Takeda is also building its pipeline in neurology (e.g. Alzheimer’s disease, Parkinson’s disease) and selected rare CNS diseases through a combination of in-house expertise and collaboration with partners.
[TRINTELLIX/Generic name: vortioxetine]
| - | In May 2018, Takeda announced the U.S. Food and Drug Administration (FDA) approved a supplemental new drug application for TRINTELLIX, making it the first FDA-approved treatment for Major Depressive Disorder (MDD) where the U.S. labelling now includes data on an important aspect of cognitive function in acute MDD. The FOCUS and CONNECT studies showed that TRINTELLIX had a positive effect on processing speed, an important aspect of cognitive function that may be impaired in adult patients with acute MDD. |
| - | In June 2018, Takeda announced positive results from a pivotal study in Japan evaluating vortioxetine in adults with Major Depressive Disorder (MDD). |
| - | In September 2018, Takeda announced the submission of a New Drug Application (NDA) to the Japanese Ministry of Health, Labour and Welfare (MHLW) for vortioxetine for the treatment of MDD in adults. |
| - | In October 2018, Takeda announced that the U.S. FDA had approved a supplemental NDA for TRINTELLIX to add new data to its labeling demonstrating superiority over escitalopram in improving selective serotonin reuptake inhibitor (SSRI)-induced sexual dysfunction in adult patients with MDD. TRINTELLIX is the first antidepressant to include head-to-head data in its labeling that shows improvement in treatment-emergent sexual dysfunction (TESD) in MDD patients who switched from certain SSRI treatments. |
[VYVANSE/Generic name: lisdexamfetamine]
| - | In March 2019, Takeda announced that Shionogi obtained approval from the Japanese Ministry of Health, Labour and Welfare (MHLW) for manufacturing and distributing VYVANSE as a treatment for childhood attention deficit/hyperactivity disorder (AD/HD). VYVANSE was collaboratively developed in Japan by Shionogi and Shire, based on a license agreement for joint development and commercialization in Japan that the two companies entered in November 2011. On account of Takeda’s acquisition of Shire, Takeda will be engaging in co-promotion activities. |
Plasma Derived Therapies
Takeda added a new global business unit to focus on Plasma-Derived Therapies (PDT) after the acquisition of Shire on January 8, 2019. PDT will focus on meeting the growing demand for plasma-derived products, which are essential for effectively treating patients with a variety of rare, life-threatening, chronic and genetic diseases across the world.
63
Table of Contents
[FLEXBUMIN /Generic name: Albumin (Human)]
| - | In March 2019, Takeda announced that the U.S. Food and Drug Administration (FDA) has approved the company’s second submission for its new plasma manufacturing facility near Covington, Georgia, for the production of FLEXBUMIN 25% [Albumin (Human)], USP, 25% Solution, indicated for hypovolemia, hypoalbuminemia, (burns, Adult Respiratory Distress Syndrome (ARDS), and nephrosis), cardiopulmonary bypass surgery, and hemolytic disease of the newborn (HDN). |
Vaccine
In vaccines, Takeda is applying innovation to tackle some of the world’s most challenging infectious diseases such as dengue, zika, norovirus, and polio. To support the expansion of our pipeline and the development of our programs, we have entered partnerships with government organizations (in Japan, the U.S., and Singapore) and leading global institutions, such as the Bill & Melinda Gates Foundation. Such partnerships have been essential towards building the critical capabilities necessary to deliver on our programs and realize their full potential.
| - | In January 2019, Takeda announced that the pivotal Phase 3 trial of its dengue vaccine candidate met the primary efficacy endpoint. This first analysis of the TIDES study showed that the company’s investigational live-attenuated tetravalent dengue vaccine (TAK-003) was efficacious in preventing dengue fever caused by any of the four serotypes of the virus. |
Building a sustainable research platform / Enhancing R&D collaboration
Takeda has successfully built a distinct research and development strategy based on therapeutic area focus, a robust research engine, and a comprehensive, differentiated partnership model of collaborations with academia, biotech firms and startups. The research and development program aims to leverage a combination of internal and external expertise to deliver a sustainable pipeline.
| - | In April 2018, Takeda and the Drugs for Neglected Diseases initiative announced that they have signed an agreement to collaborate in conducting preclinical and Phase 1 clinical studies on drug candidate compounds that had been discovered among the aminopyrazole compound class, aimed at developing an innovative drug for the treatment of visceral leishmaniasis (VL). The project has been selected for funding by the Global Health Innovative Technology Fund (GHIT). GHIT is an international public private partnership fund that facilitates global R&D partnerships for the discovery and development of new health technologies needed in developing countries. |
| - | In May 2018, Takeda announced that it has entered into a licensing agreement with ASKA Pharmaceutical Co., Ltd. (ASKA) of Japan to maximize the product value of Takeda-owned relugolix. Takeda grants ASKA exclusive commercialization rights for uterine fibroids and exclusive development and commercialization rights for endometriosis for Japan. Under this agreement, no right of relugolix for prostate cancer treatment in Japan has been granted to ASKA. |
| - | In July 2018, Takeda and Ovid Therapeutics Inc. of the U.S. provided an overview of their TAK-935/OV935 broad clinical development program. The companies plan to initiate three clinical trials: in pediatric patients with Dravet syndrome and Lennox-Gastaut syndrome, in pediatric patients with CDKL5 deficiency disorder (CDD) and Duplication 15q (Dup15q) syndrome, and an extension trial for patients with developmental and epileptic encephalopathies (DEEs) who participated in a previous TAK-935/OV935 clinical study. |
64
Table of Contents
| - | In August 2018, Takeda and Ambys Medicines (Ambys) announced that they have entered into a partnership to support the advancement of the Ambys platform and pipeline. Ambys is pioneering the application of novel modalities, including cell and gene therapy and gain-of-function drug therapy, to meet the urgent need for treatments that restore liver function and prevent the progression to liver failure across multiple liver diseases that are untreatable or poorly treated today. |
| - | In August 2018, Takeda and Japan-based alternative asset management firm, Whiz Partners, Inc. (Whiz) announced they have entered into an agreement to create a joint investment fund, aimed at promoting a drug discovery ecosystem in Japan. Under the terms of the agreement, Whiz established “Drug Discovery Gateway Investment Limited Partnership” (DDG Fund) and assumes the responsibilities of the general partner. Takeda invests Axcelead Drug Discovery Partners, a wholly owned subsidiary of Takeda and a drug discovery platform company, in kind into the DDG Fund, in return for Limited Partner Shares. |
| - | In December 2018, Takeda, the Global Antibiotic Research and Development Partnership (GARDP) and Eisai Co., Ltd. (Eisai) signed an agreement for GARDP to access and screen components of Eisai and Takeda’s chemical libraries. Both libraries will be tested by the Institut Pasteur Korea in the hope of discovering novel compounds with antibacterial activity. This multi-partner agreement supports GARDP’s efforts to tackle serious bacterial infections by developing antibiotics while endeavouring to ensure their sustainable access. |
| - | In January 2019, Takeda announced new research collaborations in immuno-oncology (I-O), an area of key strategic focus for the company. Through these collaborations, Takeda seeks to accelerate the discovery of next-generation cancer immunotherapies, including novel cell therapy approaches that may provide important opportunities for addressing the needs of patients with hard-to-treat cancers. |
| - | Takeda will collaborate with Memorial Sloan Kettering Cancer Center (MSK) to discover and develop novel chimeric antigen receptor T-cell (CAR-T) products for the treatment of multiple myeloma, acute myeloid leukemia and additional solid tumor indications. |
| - | Takeda exercised an option under its existing research collaboration with Noile-Immune Biotech Inc. (Noile), which originated in September 2017. Due to the success of the collaboration, Takeda exclusively licensed NIB-102 and NIB-103 for the treatment of various solid tumor indications, and will co-develop these CAR-T cell therapies with Noile utilizing the company’s proprietary “Prime” (proliferation inducing and migration enhancing) CAR-T platform. |
| - | Takeda exercised an option for an exclusive oncology-targeted Humabody® license from Crescendo Biologics that will allow Takeda to additionally evaluate Humabody® VHs for the development of novel CAR-T therapeutics. |
(3) Facility Investment (Tangible assets) / Fund Procurement
The total amount of investment in tangible assets during the year was 188.4 billion JPY mainly for expansion and renewal of manufacturing facilities as well as enhancement of the headquarters function and R&D facilities.
65
Table of Contents
As regards fund procurement, in order to finance funds necessary for the Shire acquisition, Takeda issued unsecured US dollar dominated senior notes and unsecured Euro dominated senior notes and financed 1,580.4 billion JPY in November, 2018. In addition, on January, 2019, Takeda drew down 1,715.5 billion JPY by exercising the Term Loan Credit Agreement executed on June, 2018, Senior Short Term Loan Facility Agreement executed on October, 2018, and Loan Agreement with the Japan Bank for International Cooperation executed on December, 2018. As a result, the consolidated outstanding balances of bonds and loans as of the end of March 2019 were 3,196.4 billion JPY and 2,554.6 billion JPY respectively.
(4) Issues for the Company to Address
This discussion and analysis contains forward-looking statements based on the current assumptions as of the fiscal year ended March 31, 2019.
Takeda’s stated mission is to “strive towards Better Health and Brighter Future for people worldwide through leading innovation in medicine.” Our culture is based on the achievement of this mission by acting with Integrity, Fairness, Honesty, and Perseverance and prioritizing the Patient (putting the patient at the center), Trust (building trust with society), Reputation (reinforcing our reputation), and Business (developing the business).
Over the past four years, Takeda has been on a transformation journey, focused on becoming an agile, research and development (R&D) driven, global biopharmaceutical company that is well positioned to deliver innovative medicines and transformative care to patients around the world. Takeda has continued to strengthen its reputation through its products and innovation, while remaining true to its values.
Takeda has a strong track record of cross-border merger and acquisition activities and post-acquisition integration, including the acquisition of TiGenix NV in 2018, ARIAD Pharmaceuticals in 2017, Nycomed in 2011 and Millennium Pharmaceuticals in 2008.
Most recently, in January 2019, we completed our acquisition of Shire plc. With the acquisition of Shire, we have taken the next major step in our transformation into a global, values-based, R&D driven biopharmaceutical company, with an attractive geographic footprint. The Shire acquisition strengthens Takeda’s presence in gastroenterology (GI) and neuroscience, which are two of its three core therapeutic areas, and provides leading positions in rare diseases and plasma-derived therapies. It also creates a highly complementary, robust, modality-diverse pipeline and a strengthened R&D engine focused on innovation. The Shire acquisition delivers compelling financial benefits for the combined group, enhancing Takeda’s cash flow profile, with management committed to delivering substantial synergies and generating returns for shareholders.
Takeda’s management team is experienced and diverse and has a proven track record of executing complex business integrations and large-scale transformations. Takeda is dedicated to carrying out integration efforts in a manner consistent with Takeda’s core values.
66
Table of Contents
The following three clear strategic priorities are set to drive sustainable mid- to long-term growth of Takeda.
| 1) | Business Area Focus |
Focus on five key business areas: GI, rare diseases, plasma-derived therapies, oncology, and neuroscience.
| 2) | R&D Engine |
Strengthen the R&D engine based on the therapeutic area focus, a leading partnership model, and patient-centric, science-driven culture of innovation. In particular, we focus our research and development efforts on our four key therapeutic areas: Oncology, GI, rare diseases, neuroscience, plus plasma derived therapies and vaccines, and we continue to transform our research and development structure. In order to deliver values in areas of high unmet medical needs, we strive to progress our pipelines with the focus on innovative medicines.
| 3) | Financial Strength |
Takeda’s financial strength involves a focus on driving margin expansion in mid-to long-term and generating cash flow to invest in the business, de-leverage and return cash to shareholders. We also continue selected disposal of non-core assets to generate cash in order to accelerate the pace of deleveraging.
The acquisition of Shire has expanded our geographic footprint, especially in the United States, an important and innovation-driven market. We have organized our operations into four regions: United States, Japan, Europe & Canada, and a Growth and Emerging Market region comprising China, Latin America, Middle East, Asia Pacific, Russia and the Commonwealth of Independent States in order to manage the execution of our strategy in each region. The execution of our integration plan is underway and we expect the integration will have relatively minimal disruption on the business and our pipeline due to the strong strategic and geographic fit of the two companies. Throughout our integration we will continue to follow our three guiding principles: (i) Patient-Centric (developing more innovative medicines supported by services and support capabilities), (ii) Agile & Simple (minimizing complexity and empowering local leaders to make local decisions), and (iii) Lean & Focused (concentrating our efforts on the five key business areas).
Basic Policy for Profit Distribution
Takeda’s priorities for capital allocation are as follows:
De-leverage rapidly
| - | Target 2x net debt/adjusted EBITDA ratio in 3 to 5 years |
| - | Committed to invest grade credit ratings |
Invest in growth drivers
| - | Strategic internal investment in R&D and product launches |
| - | Disciplined and focused R&D partnerships |
Shareholder returns
| - | Maintain well established dividend policy of 180 yen per share/year |
Dividend
Takeda is strongly committed to shareholder returns with the dividend as a key component.
67
Table of Contents
Financial Forecast for Fiscal 2019
FY2019 Reported Forecast
| Billion JPY | ||||||||||||||||
| Fiscal 2018 | Fiscal 2019 | Change over the previous year | ||||||||||||||
| Revenue |
2,097.2 | 3,300.0 | 1,202.8 | 57.4 | % | |||||||||||
| Operating profit |
205.0 | (193.0 | ) | (398.0 | ) | — | % | |||||||||
| Profit before tax |
94.9 | (369.0 | ) | (463.9 | ) | — | % | |||||||||
| Net profit for the period (attributable to owners of the Company) |
109.1 | (383.0 | ) | (492.1 | ) | — | % | |||||||||
| EPS (JPY) |
113.50 | (246.34 | ) | (359.84 | ) | — | % | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Core Earnings |
459.3 | 883.0 | 423.7 | 92.2 | % | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) | Impact from Shire acquisition related costs |
| Billion JPY | ||||||||||||||||
| Fiscal 2018 | Fiscal 2019 | Change over the previous year | ||||||||||||||
| Revenue |
— | — | — | — | % | |||||||||||
| Operating profit |
(85.0 | ) | (154.0 | ) | (69.0 | ) | (81.3 | )% | ||||||||
| Profit before tax |
(126.3 | ) | (241.0 | ) | (114.7 | ) | (90.8 | )% | ||||||||
| (2) | Impact from Shire’s purchase accounting |
| Billion JPY | ||||||||||||||||
| Fiscal 2018 | Fiscal 2019 | Change over the previous year | ||||||||||||||
| Revenue |
— | — | — | — | % | |||||||||||
| Operating profit |
(181.6 | ) | (693.0 | ) | (511.4 | ) | — | % | ||||||||
| Profit before tax |
(185.6 | ) | (709.0 | ) | (523.4 | ) | — | % | ||||||||
68
Table of Contents
Reported Forecast excluding impact of (1) and (2)
| Billion JPY | ||||||||||||||||
| Fiscal 2018 | Fiscal 2019 | Change over the previous year | ||||||||||||||
| Revenue |
2,097.2 | 3,300.0 | 1,202.8 | 57.4 | % | |||||||||||
| Operating profit |
471.5 | 654.0 | 182.5 | 38.7 | % | |||||||||||
| Profit before tax |
406.8 | 581.0 | 174.2 | 42.8 | % | |||||||||||
| Net profit for the period (attributable to owners of the Company) |
351.0 | 413.0 | 62.0 | 17.7 | % | |||||||||||
| EPS (JPY) |
365.05 | 265.63 | (99.42 | ) | (27.2 | )% | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Core Earnings |
459.3 | 883.0 | 423.7 | 92.2 | % | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
This FY2019 Reported Forecast does not take into consideration the divestitures of XIIDRA (lifitegrast ophthalmic solution) 5% product and TACHOSIL Fibrin Sealant Patch, which were announced on May 9, 2019. At present, Takeda does not expect these divestitures to have a material impact on its FY2019 Reported Forecast. The FY2019 Reported Forecast will be updated at a later date to reflect these divestitures once a reliable estimate of their impact can be made, which will depend upon the exact timing of transaction close.
[Revenue]
Takeda expects revenue to be 3,300.0 billion JPY, an increase of 1,202.8 billion JPY or +57.4% from the prior year, because post-close of the Shire acquisition FY2019 will be the first year its business will be consolidated for the whole year. Of the five key business areas, we expect continued growth from the key legacy Takeda growth products such as ENTYVIO and TAKECAB in Gastroenterology, NINLARO and ADCETRIS in Oncology, TRINTELLIX in Neuroscience. In the Rare Disease business area, expanded by the Shire acquisition, TAKHZYRO, ADYNOVATE, NATPARA, as well as GAMMAGARD and FLEXBUMIN from Plasma Derived Therapies will contribute to increased revenue. However, multiple products including VELCADE are expected to be affected by competitive entrants into the market as well as loss of exclusivity. Also, as part of the integration process of Shire, a temporary decline in revenue is expected from legacy Shire products due to the continued effort of improving days-on-hand of commercial product at wholesalers.
[Operating Profit]
Operating Profit is expected to be a loss of 193.0 billion JPY, a decline of 398.0 billion JPY versus the prior year. Integration costs of 154.0 billion JPY, an increase of 69.0 billion JPY versus the prior year, are expected for FY2019 as part of the Shire acquisition related costs. Also, the effect of purchase accounting due to the Shire acquisition will negatively impact Operating Profit by 693.0 billion JPY, an increase of 511.4 billion JPY versus the prior year, which will include cost of sales related to the unwind of inventory fair value adjustment of 253.0 billion JPY and 439.0 billion JPY amortization of intangible assets for FY2019.
69
Table of Contents
When excluding the effects of these one-time costs and non-cash costs, Operating Profit is expected to be 654.0 billion JPY, an increase of 182.5 billion JPY or +38.7%. Moreover, no gains from the sale of real estate / real estate operations are expected in FY2019 (legacy Takeda had 88.6 billion JPY in the prior year). Also, for impairment losses of intangible assets, a 121.0 billion JPY estimate based on the intangible asset balance increased by the Shire acquisition calculated with the probability from past impairment losses is included, but currently no evidence of impairment is recognized for a specific product or pipeline.
[Profit Before Tax]
Profit Before Tax is expected to be a loss of 369.0 billion JPY, a decrease of 463.9 billion JPY versus the prior year. Shire acquisition related costs will reduce Profit Before Tax by 114.7 billion JPY from FY2019. In addition to the 69.0 billion JPY decline in Operating Profit, an 87.0 billion JPY interest expense is expected from the new debt incurred in relation to the financing for the Shire acquisition, and so finance expenses increase 45.7 billion JPY versus the prior year. Also, the effect of purchase accounting for the Shire acquisition will increase 523.4 billion JPY in FY2019 including finance expenses forecasted to increase 10.8 billion JPY to 15.0 billion JPY.
When excluding these effects, Profit Before Taxes increase 174.2 billion JPY or +42.8% versus prior year to 581.0 billion JPY.
[Net profit for the year (attributable to owners of the Company)]
Net profit for the year (attributable to owners of the Company) is expected to be a loss of 383.0 billion JPY, a decrease of 492.1 billion JPY.
When excluding the effects of the Shire acquisition related costs and the purchase accounting for the Shire acquisition, Net profit for the year (attributable to owners of the Company) is 413.0 billion JPY, an increase of 62.0 billion JPY or +17.7%.
The number of shares used to calculate EPS is the average number of shares throughout the period (excluding treasury shares) of 961,476,993 shares for FY2018, and the 2018 fiscal year end outstanding shares (excluding treasury shares) of 1,554,780,063 for FY2019. On January 8, 2019, Takeda issued 770,303,013 ordinary shares as part of the consideration for the Shire acquisition.
[Core Earnings]
Core Earnings (before adjusting for the impact of foreign exchange rates and divestitures) is expected to be 883.0 billion JPY, an increase of 423.7 billion JPY or +92.2% versus prior year. This significant increase is due to the full year Shire contribution. Also, Core Earnings margin is forecasted to improve 4.9pp to be 26.8% due to the integration of Shire with its high margins, realization of further cost synergies, as well as further improvement of operating expense efficiency with the progress of the Global Opex Initiative.
70
Table of Contents
[Major assumptions used in preparing the FY2019 forecast]
| Billion JPY | ||||||||
| Fiscal 2018 | Fiscal 2019 | |||||||
| FX rates |
|
1 USD = 111 JPY 1 Euro = 129 JPY 1 RUB = 1.7 JPY 1 BRL = 29.5 JPY 1 CNY = 16.5 JPY |
|
|
1 USD = 111 JPY 1 Euro = 124 JPY 1 RUB = 1.7 JPY 1 BRL = 28.4 JPY 1 CNY = 16.4 JPY |
| ||
| R&D expenses |
(368.3 | ) | (491.0 | ) | ||||
| Shire acquisition related costs |
||||||||
| Operating expenses (acquisition costs, etc.) |
(25.3 | ) | — | |||||
| Other operating expenses (integration costs) |
(59.6 | ) | (154.0 | ) | ||||
| Financial expenses (interest costs, etc.) |
(41.3 | ) | (87.0 | ) | ||||
| Impact from Shire’s purchase accounting (major items) |
||||||||
| Cost of sales (unwind of inventory fair value adjustment) |
(82.2 | ) | (253.0 | ) | ||||
| Amortization of intangibles assets (Shire acquisition) |
(99.2 | ) | (439.0 | ) | ||||
| Other non-cash items |
||||||||
| Amortization of intangible assets (Legacy Takeda) |
(95.4 | ) | (99.0 | ) | ||||
| Impairment losses on intangible assets |
(8.7 | ) | (121.0 | ) | ||||
Management Guidance (Excluding any impact of divestitures)
| FY2019 Management Guidance | ||
| Underlying Revenue Growth*1 (pro-forma)*2 |
Flat to slightly declining | |
| Underlying Core Earnings Margin | Mid-twenties | |
| Underlying Core EPS | 350 – 370 yen | |
| Annual dividend per share | 180 yen |
| *1 | Constant Exchange Rate (applying FY2018 full year average foreign exchange rate) |
| *2 | Pro-forma baseline of 3,300.0 billion JPY (12-month April 2018-March 2019 combined revenue of Takeda and Shire) |
This Management Guidance does not take in consideration the divestitures of XIIDRA (lifitegrast ophthalmic solution) 5% product and TACHOSIL Fibrin Sealant Patch, which were announced on May 9, 2019. At present, Takeda does not expect these divestitures to have a meaningful impact on its FY2019 Management Guidance.
Momentum of key growth products in our five key business areas will largely offset loss of exclusivity of VELCADE* and other products. Also, with Shire contributing for the whole year, realization of cost synergies, as well as further improvement of operating expense efficiency, Underlying Core EPS is forecasted to be 350 - 370 yen.
71
Table of Contents
| * | U.S. VELCADE financial assumption is one additional therapeutically non-equivalent competitor with IV (intravenous) and SC (subcutaneous) administration launching in July 2019. If no additional competitor launches, pro-forma Underlying Revenue growth would be “flat to slightly increasing”. |
[Forward looking statement]
All forecasts in this document are based on information currently available to management, and do not represent a promise or guarantee to achieve these forecasts. Various uncertain factors could cause actual results to differ, such as changes in the business environment and fluctuations in foreign exchange rates. Should any significant event occur which requires the forecast to be revised, the Company will disclose it in a timely manner.
72
Table of Contents
(5) Financial Position and Income Summary
(i) Financial Position and Income Summary of the Takeda Group
| (Billion JPY, unless otherwise indicated) | ||||||||||||||||
| 139th fiscal year | 140th fiscal year | 141st fiscal year | 142nd fiscal year | |||||||||||||
| April 1, 2015 to March 31, 2016 |
April 1, 2016 to March 31, 2017 |
April 1, 2017 to March 31, 2018 |
April 1, 2018 to March 31, 2019 |
|||||||||||||
| Revenue |
1,807.4 | 1,732.1 | 1,770.5 | 2,097.2 | ||||||||||||
| Operating profit |
130.8 | 155.9 | 241.8 | 205.0 | ||||||||||||
| Profit before income taxes |
120.5 | 143.3 | 217.2 | 94.9 | ||||||||||||
| Net profit for the year |
83.5 | 115.5 | 186.7 | 109.0 | ||||||||||||
| Net profit for the year attributable to the owners of the Company |
80.2 | 114.9 | 186.9 | 109.1 | ||||||||||||
| Basic earnings per share (JPY) |
102.26 | 147.15 | 239.35 | 113.50 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total assets |
3,824.1 | 4,346.8 | 4,106.5 | 13,872.3 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total equity |
2,011.2 | 1,949.0 | 2,017.4 | 5,163.6 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (Notes). | Consolidated financial statements of the Takeda Group are prepared under the International Financial Reporting Standards (IFRS). |
(ii) Overseas Revenue of the Takeda Group
| (Billions JPY, unless otherwise indicated) | ||||||||||||||||
| 139th fiscal year | 140th fiscal year | 141st fiscal year | 142nd fiscal year | |||||||||||||
| April 1, 2015 to March 31, 2016 |
April 1, 2016 to March 31, 2017 |
April 1, 2017 to March 31, 2018 |
April 1, 2018 to March 31, 2019 |
|||||||||||||
| Overseas revenue |
1,119.3 | 1,076.7 | 1,190.2 | 1,526.2 | ||||||||||||
| Proportion of overseas revenue to the Takeda Group Revenue (%) |
61.9 | 62.2 | 67.2 | 72.8 | ||||||||||||
(iii) R&D Expenses of the Takeda Group
| (Billions JPY, unless otherwise indicated) | ||||||||||||||||
| 139th fiscal year | 140th fiscal year | 141st fiscal year | 142nd fiscal year | |||||||||||||
| April 1, 2015 to March 31, 2016 |
April 1, 2016 to March 31, 2017 |
April 1, 2017 to March 31, 2018 |
April 1, 2018 to March 31, 2019 |
|||||||||||||
| R&D expenses |
335.8 | 312.3 | 325.4 | 368.3 | ||||||||||||
| Ratio of R&D expenses to the Takeda Group Revenue (%) |
18.6 | 18.0 | 18.4 | 17.6 | ||||||||||||
73
Table of Contents
For your reference, the “Financial Position and Income Summary of the Company” is as follows:
| (Billions JPY, unless otherwise indicated) | ||||||||||||||||
| 139th fiscal year | 140th fiscal year | 141st fiscal year | 142nd fiscal year | |||||||||||||
| April 1, 2015 to March 31, 2016 |
April 1, 2016 to March 31, 2017 |
April 1, 2017 to March 31, 2018 |
April 1, 2018 to March 31, 2019 |
|||||||||||||
| Net sales |
777.0 | 737.8 | 659.5 | 651.3 | ||||||||||||
| Operating income |
94.2 | 70.3 | 67.7 | 73.9 | ||||||||||||
| Ordinary income |
292.9 | 81.9 | 125.9 | 17.5 | ||||||||||||
| Net income |
263.0 | 108.4 | 187.0 | 88.2 | ||||||||||||
| Net income per share (JPY) |
335.48 | 138.73 | 239.47 | 91.76 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total assets |
2,699.5 | 3,093.1 | 2,948.6 | 9,534.6 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net assets |
1,572.2 | 1,530.4 | 1,565.9 | 4,647.2 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (Note) | The amount of total assets as of the 141st fiscal year has been retrospectively revised due to the adoption of “Application Guidelines of Accounting Standards for Tax Effect Accounting (ABSJ Statement No.28 February 16, 2018) in the 142nd fiscal year. |
(6) Main Businesses of Takeda Group (as of March 31, 2019)
The main businesses of Takeda Group are research, development, manufacturing and sale of pharmaceuticals.
74
Table of Contents
(7) Material Business Affiliations (as of March 31, 2019)
Principal Subsidiaries and Affiliates
| Name of company (major offices) |
Capital stock |
Percentage of total shares (%) |
Principal business | |||||||
| United States |
Takeda Pharmaceuticals International, Inc. (Head office: Deerfield, Illinois, U.S.) |
US$1 | 100.0 | Supervision of sales of pharmaceuticals | ||||||
| Takeda Pharmaceuticals U.S.A., Inc. (Head office: Deerfield, Illinois, U.S.) |
US$1 thousand (¥111 thousand) | 100.0 | Sales of pharmaceuticals, holding intellectual properties and internal group finance | |||||||
| Millennium Pharmaceuticals, Inc. (Head office: Cambridge, Massachusetts, U.S.) |
US$0.1 | 100.0 | R&D, sales of pharmaceuticals and holding intellectual properties | |||||||
| ARIAD Pharmaceutical, Inc. (Head office: Cambridge, Massachusetts, U.S.) |
US$5,550 (¥614 thousand) | 100.0 | R&D of pharmaceuticals and holding intellectual properties | |||||||
| Takeda California, Inc. (Head office: San Diego, California, U.S.) |
US$1 | 100.0 | R&D of pharmaceuticals | |||||||
| Takeda Vaccines, Inc. (Head office: Deerfield, Illinois, U.S.) |
US$1 | 100.0 | R&D of pharmaceuticals | |||||||
| Takeda Development Center Americas, Inc. (Head office: Deerfield, Illinois, U.S.) |
US$1 | 100.0 | R&D of pharmaceuticals | |||||||
| Cerevance, LLC (Head office: Boston, Massachusetts, U.S.) |
US$916 (¥101 thousand) |
27.8 | R&D of pharmaceuticals | |||||||
| Baxalta Incorporated (Head office: Bannockburn, Illinois, U.S.) |
US$10 (¥1 thousand) |
100.0 | Holding intellectual properties | |||||||
| Baxalta US Inc. (Head office: Bannockburn, Illinois, U.S.) |
US$1 | 100.0 | Holding intellectual properties | |||||||
75
Table of Contents
| Name of company (major offices) |
Capital stock |
Percentage of total shares (%) |
Principal business | |||||||
| United States |
Shire Human Genetic Therapies Inc. (Head office: Lexington, Massachusetts, U.S.) |
US$1 | 100.0 | R&D, production and sales of pharmaceuticals | ||||||
| Shire ViroPharma Incorporated (Head office: Lexington, Massachusetts, U.S.) |
US$1 | 100.0 | Sales of pharmaceuticals | |||||||
| Shire-NPS Pharmaceuticals, Inc. (Head office: Lexington, Massachusetts, U.S.) |
US$1 | 100.0 | Sales of pharmaceuticals | |||||||
| Dyax Corp. (Head office: Lexington, Massachusetts, U.S.) |
US$215 (¥24 thousand) | 100.0 | R&D, sales of pharmaceuticals and holding intellectual properties | |||||||
| Meritage Pharma, Inc. (Head office: Lexington, Massachusetts, U.S.) |
US$1 | 100.0 | R&D of pharmaceuticals and holding intellectual properties | |||||||
| Shire Development LLC (Head office: Lexington, Massachusetts, U.S.) |
US$100 (¥11 thousand) | 100.0 | R&D of pharmaceuticals | |||||||
| Shire North American Group Inc. (Head office: Florence, Kentucky, U.S.) |
US$1 thousand (¥111 thousand) |
100.0 | Holding Company in the U.S. | |||||||
76
Table of Contents
| Name of company (major offices) |
Capital stock |
Percentage of total shares (%) |
Principal business | |||||||
| Europe and Canada |
Takeda Pharmaceuticals International AG (Head office: Zurich, Switzerland) |
3.82 million Swiss francs (¥424 million) | 100.0 | R&D, supervision of sales of pharmaceuticals for the areas other than Japan, holding intellectual properties, supervision of global manufacturing and product supply for all regions | ||||||
| Takeda GmbH (Head office: Konstanz, Germany) (Factory: Singen and Oranienburg, Germany) |
€10.90 million (¥1,353 million) |
100.0 | Holding intellectual properties, production and sales of pharmaceuticals | |||||||
| Takeda Italia S.p.A. (Head office: Rome, Italy) |
€11.25 million (¥1,397 million) |
100.0 | Sales of pharmaceuticals | |||||||
| Takeda Austria GmbH (Head office, Factory: Linz, Austria) |
€14.86 million (¥1.845 million) |
100.0 | Holding intellectual properties, production and sales of pharmaceuticals | |||||||
| Takeda France S.A.S. (Head office: Paris, France) |
€3.24 million (¥402 million) |
100.0 | Sales of pharmaceuticals | |||||||
| Takeda Pharma A/S (Head office: Taastrup, Denmark) (Factory: Hobro, Denmark) |
948.70 million Danish kroner (¥15,777 million) |
100.0 | Holding intellectual properties, production and sales of pharmaceuticals | |||||||
| Takeda AS (Head office, Factory: Asker, Norway) |
272.70 million Norwegian kroner (¥3,492 million) |
100.0 | Holding intellectual properties, production and sales of pharmaceuticals | |||||||
| Takeda UK Limited (Head office: Buckinghamshire, U.K.) |
£50 million (¥7,217 million) |
100.0 | Sales of pharmaceuticals | |||||||
| Takeda Ireland Limited (Head office: Kilruddery, Ireland) (Factory: Bray and Grange Castle, Ireland) |
€396.02 million (¥49,169 million) |
100.0 | Holding intellectual properties, production of pharmaceuticals | |||||||
| Takeda Development Centre Europe Ltd. (Head office: London, U.K.) |
£800 thousand (¥115 million) |
100.0 | R&D of pharmaceuticals | |||||||
77
Table of Contents
| Name of company (major offices) |
Capital stock |
Percentage of total shares (%) |
Principal business | |||||||
| Europe and Canada |
Shire plc (Head office: Jersey) |
£46.27 million (¥6,679 million) |
100.0 | Holding Company | ||||||
| Shire Pharmaceutical Holdings Ireland Limited (Head office: Dublin, Ireland) | US$2,516.00 million (¥278,355 million) | 100.0 | Holding Company | |||||||
| Shire Pharmaceuticals International Unlimited Company (Head office: Dublin, Ireland) | US$4,974.53 million (¥550,573 million) | 100.0 | Holding Company | |||||||
| Shire Pharmaceuticals Ireland Limited (Head office: Dublin, Ireland) |
€100 thousand (¥111 million) |
100.0 | R&D, production and sales of pharmaceuticals | |||||||
| Shire Acquisitions Investments Ireland Designated Activity Company (Head office: Dublin, Ireland) | US$20 (¥2 thousand) |
100.0 | Group finance and treasury | |||||||
| Shire Ireland Finance Trading Limited (Head office: Dublin, Ireland) |
US$3,662.37 million (¥405,183 million) | 100.0 | Group finance and treasury | |||||||
| Shire Pharma Canada ULC (Head office: Vancouver, Canada) |
1.89 million CAD (¥156 million) | 100.0 | Sales of pharmaceuticals | |||||||
| Shire France S.A.S (Head office: Paris, France) |
€5.40 million (¥671 million) |
100.0 | Sales of pharmaceuticals | |||||||
| Shire Deutschland GmbH (Head office: Berlin, Germany) |
€25 thousand (¥3 million) |
100.0 | Sales of pharmaceuticals | |||||||
| Baxalta GmbH (Head office: Zug, Switzerland) |
€20 thousand (¥2 million) |
100.0 | Holding intellectual properties and sales of pharmaceuticals | |||||||
78
Table of Contents
| Name of company (major offices) |
Capital stock |
Percentage of total shares (%) |
Principal business | |||||||
| Europe and Canada |
Shire Pharmaceuticals Iberica S.L.U. (Head office: Madrid, Spain) |
€5.50 million (¥683 million) |
100.0 | Sales of pharmaceuticals | ||||||
| Shire Pharmaceuticals Limited (Head office: London, U.K.) |
£727 thousand (¥105 million) |
100.0 | Sales of pharmaceuticals | |||||||
| Shire Italia S.p.A. (Head office: Milano, Italy) |
€796 thousand (¥99 million) |
100.0 | Sales of pharmaceuticals | |||||||
| Baxter AG (Head office: Vienna, Austria) |
€100 thousand (¥12 million) |
100.0 | Production of pharmaceuticals | |||||||
| Baxalta Manufacturing S.à r.l. (Head office: Neuchatel, Switzerland) |
€2.00 million (¥248 million) |
100.0 | Production of pharmaceuticals | |||||||
| Baxalta Innovations GmbH (Head office: Vienna, Austria) |
€36.34 million (¥4,511 million) |
100.0 | R&D of pharmaceuticals | |||||||
| Shire Pharmaceutical Development Limited (Head office: London, U.K.) |
£230.61 million (¥33,284 million) |
100.0 | R&D of pharmaceuticals | |||||||
| Baxalta Recombinant S.à r.l. (Head office: Neuchatel, Switzerland) |
€20 thousand (¥2 million) |
100.0 | Holding Intellectual properties | |||||||
| Shire International GmbH (Head office: Zug, Switzerland) |
£100 thousand (¥11 million) |
100.0 | Holding Intellectual properties | |||||||
| Russia | Takeda Pharmaceuticals Limited Liability Company (Head office and Factory: Moscow, Russia) |
26 thousand Russian ruble (¥45 thousand) | 100.0 | Production and sales of pharmaceuticals | ||||||
| Latin America |
Takeda Distribuidora Ltda. (Head office: São Paulo, Brazil) |
11.33 million Brazilian reals (¥321 million) | 100.0 | Sales of pharmaceuticals | ||||||
79
Table of Contents
| Name of company (major offices) |
Capital stock |
Percentage of total shares (%) |
Principal business | |||||||
| Asia | Takeda (China) Holdings Co., Ltd. (Head office: Shanghai, China) |
US$75 million (¥8,298 million) |
100.0 | Holding company in China and R&D of pharmaceuticals | ||||||
| Takeda Pharmaceutical (China) Company Limited (Head office: Taizhou, China) | US$61.60 million (¥6,815 million) |
100.0 | Sales of pharmaceuticals | |||||||
| Takeda Pharmaceuticals Korea Co., Ltd. (Head office: Seoul, Korea) |
2,000 million Korean won (¥195 million) |
100.0 | Sales of pharmaceuticals | |||||||
| Takeda Development Center Asia, Pte. Ltd. (Head office: Singapore) |
S $5 million (¥408 million) |
100.0 | R&D of pharmaceuticals | |||||||
| Takeda Vaccines Pte. Ltd. (Head office: Singapore) |
S$32.07 million (¥2,615 million) |
100.0 | R&D of pharmaceuticals | |||||||
| Shire BioScience (Shanghai) Co. Ltd. (Head office: Singapore) |
CNY 0 | 100.0 | Sales of pharmaceuticals | |||||||
| Japan | Takeda Consumer Healthcare Company Limited (Head office: Chiyoda-ku, Tokyo) | ¥490 million | 100.0 | Sales of pharmaceuticals | ||||||
| Nihon Pharmaceutical Co., Ltd. (Head office:
Chuo-ku, Tokyo) |
¥760 million | 87.3 | Production and sales of pharmaceuticals | |||||||
| Shire Japan KK (Head office: Chiyoda-ku, Tokyo) |
¥2,000 million | 100.0 | Sales of pharmaceuticals | |||||||
| Amato Pharmaceutical Products, Ltd. (Head office: Toyonaka City) (Factory: Fukuchiyama City) |
¥96 million | 30.0 | R&D, production and sales of pharmaceuticals | |||||||
| Teva Takeda Pharma Ltd. (Factory: Takayama City) |
¥100 million | 49.0 | R&D, production and sales of pharmaceuticals | |||||||
| (Notes) | 1. | The figures in parentheses under the column “Capital stock” show the Japanese yen equivalents, calculated using the exchange rates as of March 31, 2019. | ||
| 2. | The figures for “Percentage of total shares” include shares that are held indirectly through subsidiaries. | |||
| 3. | As of March 31, 2019, the number of consolidated subsidiaries (including partnership) was 357 and the number of equity method affiliates was 19. | |||
| 4. | No subsidiaries and affiliates fall under “Specific Wholly Owned Subsidiary” as described in the Ordinance for Enforcement of the Companies Act. |
80
Table of Contents
(8) Major Offices of the Company (as of March 31, 2019)
| Head Office | 1-1, Doshomachi 4-chome, Chuo-ku, Osaka | |
| Global Headquarters | 1-1, Nihonbashi-Honcho 2-chome, Chuo-ku, Tokyo | |
| Branches | Sapporo Region, Tohoku Region (located in Sendai), Tokyo Region, Yokohama Region, Chiba/Saitama Region (located in Tokyo), Kita-Kanto/Koshinetsu Region (located in Tokyo), Nagoya Region, Osaka Region, Kobe Region, Kyoto Region, Shikoku Region (located in Takamatsu, Kagawa), Chugoku Region (located in Hiroshima) and Fukuoka Region | |
| Plants | Osaka Plant and Hikari Plant (located in Hikari, Yamaguchi) | |
| Research Centers | Neuroscience Drug Discovery Unit, Gastroenterology Drug Discovery Unit, Immunology Unit, Drug Safety Research and Evaluation, Drug Metabolism & Pharmacokinetics Research, Translational Research and Early Clinical, Regenerative Medicine Unit (the above are located in Fujisawa, Kanagawa) Process Chemistry, Cell Therapies, Drug Product Development, Analytical Development (the above are located in Osaka) Japan CMC, Hikari Biologics Manufacturing (the above are located in Hikari, Yamaguchi) | |
| (Note) Branches were changed as of April 1, 2019 as below.
| ||
| Branches |
Sapporo Region, Tohoku Region (located in Sendai), Tokyo Region, Yokohama Region, Chiba/Saitama Region (located in Tokyo), Kita-Kanto Region (located in Tokyo), Koshinetsu Region (located in Tokyo), Nagoya Region, Osaka Region, Kobe Region, Kyoto Region, Shikoku Region (located in Takamatsu, Kagawa), Chugoku Region (located in Hiroshima) Kyushu Kita Region (located in Fukuoka) and Kyushu Minami Region (located in Fukuoka) | |
(9) Employees (as of March 31, 2019)
| (i) | Number of employees of the Takeda Group |
| Number of employees |
Increase (decrease) from the previous fiscal year end | |
| 49,578 |
22,348 |
| (Note) The | number of employees represents the number of working employees. |
| (ii) | Status of employees of the Company |
| Number of employees |
Increase (decrease) from the previous fiscal |
Average age |
Average length of employment (years) | |||
| 5,291 |
(170) | 41.5 | 15.1 |
(Note) The number of employees represents the number of working employees.
81
Table of Contents
(10) Principal lenders and loan amounts (as of March 31, 2019)
| Lender |
Loan balance | |
| Syndicated loans |
1,918,581 million JPY | |
| Japan Bank for International Cooperation |
409,346 million JPY | |
| The Norinchukin Bank |
80,000 million JPY | |
| Sumitomo Mitsui Trust Bank, Limited |
50,000 million JPY | |
| Shinkin Central Bank |
50,000 million JPY | |
| Mizuho Trust & Banking Co., Ltd. |
30,000 million JPY | |
| Nippon Life Insurance Company |
10,000 million JPY |
| (Note) |
The syndicated loans are joint financing by several lenders arranged by JPMorgan Chase Bank, N.A., Sumitomo Mitsui Banking Corporation and others. |
2. Common Stock of the Company (as of March 31, 2019)
| (1) | Total number of shares authorized to be issued by the Company | 3,500,000,000 shares | ||
| (2) | Total number of issued shares | 1,565,005,908 shares (including 165,150 shares of treasury stock) | ||
| (3) | Number of shareholders | 338,008 | ||
| (4) | Principal Shareholders | |||
| Name of Shareholder |
Number of shares held (thousands) |
Percentage of total shares (%) |
||||||
| THE BANK OF NEW YORK MELLON AS DEPOSITARY BANK FOR DEPOSITARY RECEIPT HOLDERS |
118,250 | 7.56 | ||||||
| The Master Trust Bank of Japan, Ltd. (Trust account) |
109,549 | 7.00 | ||||||
| Japan Trustee Services Bank, Ltd. (Trust account) |
85,405 | 5.46 | ||||||
| Nippon Life Insurance Company |
35,360 | 2.26 | ||||||
| Japan Trustee Services Bank, Ltd. (Trust account 5) |
34,260 | 2.19 | ||||||
| JP Morgan Chase Bank 380055 |
30,324 | 1.94 | ||||||
| SSBTC CLIENT OMNIBUS ACCOUNT |
26,787 | 1.71 | ||||||
| State Street Bank West Client-Treaty 505234 |
24,673 | 1.58 | ||||||
| STATE STREET BANK AND TRUST COMPANY 505001 |
23,775 | 1.52 | ||||||
| Japan Trustee Services Bank, Ltd. (Trust account 1) |
22,798 | 1.46 | ||||||
| (Note) |
The percentage of total shares is based on the number of shares (1,564,840,758 shares) calculated by subtracting the number of treasury stock from the total number of issued shares. |
| (5) | Material items on the Common Stock of the Company other than the items mentioned above |
| (i) | The Company issued 770,303,013 shares as of January 8, 2019 as the part of the compensation for acquisition of Shire based on the delegation of the authority resolved at the Extraordinary General Meeting of Shareholders held on December 5, 2018. |
82
Table of Contents
| (ii) | The Company has introduced the BIP (Board Incentive Plan) trust compensation system for Directors (excluding Directors residing overseas who are not External Directors), based on the resolution of the 140th Ordinary General Meeting of Shareholders held on June 29, 2016 and the resolution of the Board of Directors made in accordance with such shareholders’ resolution. |
The number of stocks of the Company that the trust account for the BIP trust owns is 1,025,109 shares as of March 31, 2019.
| (iii) | From the 138th fiscal year, the Company introduced a stock grant ESOP (Employee Stock Ownership Plan) trust for the senior management of the Takeda Group, based on the resolution of the Board of Directors. |
The number of stocks of the Company that the trust account for the stock grant ESOP trust owns is 8,950,460 shares as of March 31, 2019.
3. Matters Concerning the Stock Acquisition Rights of the Company
Overview of the Stock Acquisition Rights distributed as a consideration for the execution of duties owned by Directors (excluding External Directors) of the Company (as of March 31, 2019)
| Name |
Recipients of time of issuance |
Payment value of Stock Acquisition Rights |
Financial value to be invested upon execution of the Stock Acquisition Rights |
Period during which the Stock Acquisition Rights may be exercised |
Main conditions for execution of the Stock Acquisition Rights |
Type and number of shares subject to Stock Acquisition Rights (and the number of Stock Acquisition Rights) |
Number of Directors (excluding External Directors) possessing the Stock Acquisition Rights and the number of such Stock Acquisition Rights (Note 1) | |||||||
| Stock Acquisition Rights FY2010-issued (June 25, 2010) |
5 Directors (excluding External Directors) |
3,028 JPY per share |
1 JPY per share | July 11, 2013 to July 10, 2020 (Note 2) | (Note 3) | Ordinary shares in the Company; 7,000 shares (70) |
1 Director who is an Audit and Supervisory Committee (ASC) Member: 70 Stock Acquisition Rights | |||||||
| 1st Series of Stock Acquisition Rights FY2011-issued (June 24, 2011) |
4 Directors (excluding External Directors) | 2,726 JPY per share |
1 JPY per share | July 16, 2014 to July 15, 2021 (Note 2) | (Note 3) | Ordinary shares in the Company; 10,100 shares (101) |
1 Director who is an ASC Member: 101 Stock Acquisition Rights | |||||||
| 2nd Series of Stock Acquisition Rights FY2011-issued (June 24, 2011) |
113 members of Corporate Officers and other senior management | 427 JPY per share |
3,705 JPY per share |
July 16, 2014 to July 15, 2031 (Note 4) | (Note 5) | Ordinary shares in the Company; 888,700 shares (8,887) |
1 Director who is not an ASC Member: 429 Stock Acquisition Rights | |||||||
| 1st Series of Stock Acquisition Rights FY2012-issued (June 26, 2012) |
4 Directors (excluding External Directors) | 2,678 JPY per share |
1 JPY per share | July 18, 2015 to July 17, 2022 (Note 2) | (Note 3) | Ordinary shares in the Company; 18,600 shares (186) |
1 Director who is not an ASC Member: 79 Stock Acquisition Rights; 1 Director who is an ASC Member: 107 Stock Acquisition Rights | |||||||
| 1st Series of Stock Acquisition Rights FY2013-issued (June 26, 2013) |
4 Directors (excluding External Directors) | 3,709 JPY per share |
1 JPY per share | July 20, 2016 to July 19, 2023 (Note 2) | (Note 3) | Ordinary shares in the Company; 14,300 shares (143) |
1 Director who is not an ASC Member: 61 Stock Acquisition Rights; 1 Director who is an ASC Member: 82 Stock Acquisition Rights |
83
Table of Contents
| (Notes) |
1. | No Stock Acquisition Rights are possessed by the External Directors. | ||
| 2. | A Director who received an allocation of these Stock Acquisition Rights may exercise said Stock Acquisition Rights from the day following the day of resignation/retirement in cases of resignation/retirement due to the expiration of the Director’s term of office, or, in the case of any other valid reason, even prior to the initial date of the period stated above during which the Stock Acquisition Rights may be exercised. | |||
| 3. | [1] A person who exercises a Stock Acquisition Right must be a Director of the Company at the time the right is exercised. However, this shall not apply if the Director has resigned/retired due to the expiration of the term of office or if there is any other valid reason. | |||
| [2] A single Stock Acquisition Right may not be exercised in part. | ||||
| 4. | A person who received an allocation of these Stock Acquisition Rights may exercise said Stock Acquisition Rights from the day following the day of resignation/retirement in cases of resignation/retirement due to the expiration of the term of office or mandatory retirement, or, in the case of any other valid reason, even prior to the initial date of the period stated above during which the Stock Acquisition Rights may be exercised. | |||
| 5. | [1] A person who exercises a Stock Acquisition Right must be a Director, employee or any other person equivalent thereto of the Company or of subsidiaries of the Company at the time the right is exercised. However, this shall not apply if the person has resigned/retired due to the expiration of the term of office or mandatory retirement or if there is any other valid reason. | |||
| [2] A single Stock Acquisition Right may not be exercised in part. |
84
Table of Contents
4. Executives of the Company
(1) Status of Directors (as of March 31, 2019)
The Company has been appointing persons from inside and outside of the Company as Directors, regardless of nationality and gender, in order to secure a balance of knowledge, experience and capabilities necessary for the management of the Company which conducts business globally. The Company has also been appointing a defined number of Directors to pursue both effective and swift decision making and appropriate monitoring of the management of the Company through sufficient discussions at the Board of Directors meetings. For the purposes of formulating optimal rules for the appointment of Directors and appointing appropriate persons as Directors, the Company has established a Nomination Committee as the advisory body to the Board of Directors, in which an External Director serves as the chairperson.
The status of Directors as of the end of this fiscal year is as follows:
| Name |
Position |
Duty |
Important Positions Held Concurrently, etc. | |||
| Christophe Weber |
President (Representative Director) |
Chief Executive Officer | ||||
| Masato Iwasaki |
Director | President, Japan Pharma Business Unit | ||||
| Andrew Plump |
Director | President, Research & Development | Executive Vice President, Takeda Pharmaceuticals International, Inc. | |||
| Masahiro Sakane |
Director | Chair of the Board of Directors meeting | Councilor, Komatsu Ltd. | |||
| *Olivier Bohuon |
Director | |||||
| *Ian Clark |
Director | |||||
| Yoshiaki Fujimori |
Director | Senior Advisor, LIXIL Group Corporation | ||||
| *Steven Gillis |
Director | Managing Director, ARCH Venture Partners | ||||
| Emiko Higashi |
Director | Managing Director, Tomon Partners, LLC | ||||
| Michel Orsinger |
Director | |||||
| Toshiyuki Shiga |
Director | Director, Nissan Motor Co., Ltd. Chairman and CEO, INCJ, Ltd. | ||||
| Yasuhiko Yamanaka |
Director who is a Full-time Audit and Supervisory Committee Member | |||||
| Shiro Kuniya |
Director who is the Head of the Audit and Supervisory Committee | Managing Partner, Oh-Ebashi LPC & Partners | ||||
| Jean-Luc Butel |
Director who is an Audit and Supervisory Committee Member | |||||
| Koji Hatsukawa |
Director who is an Audit and Supervisory Committee Member | Certified Public Accountant | ||||
85
Table of Contents
| (Notes) |
1. | The Directors marked with an * were newly elected at the Extraordinary General Meeting of Shareholders held on December 5, 2018 and took office as of January 8, 2019. | ||||
| 2. | The following revision was made as of January 8, 2019: |
| Name |
New |
Old | ||
| Andrew Plump | Director, President, Research & Development | Director, Chief Medical & Scientific Officer |
| 3. | Directors Masahiro Sakane, Olivier Bohuon, Ian Clark, Yoshiaki Fujimori, Steven Gillis, Emiko Higashi, Michel Orsinger and Toshiyuki Shiga and Directors who are ASC Members, Shiro Kuniya, Jean-Luc Butel and Koji Hatsukawa, are External Directors as prescribed under Article 2, Item 15 of the Companies Act. | |||||
| 4. | Director who is an ASC Member Koji Hatsukawa is a Certified Public Accountant and has expert knowledge in finance and accounting. | |||||
| 5. | Director who is an ASC Member Yasuhiko Yamanaka is a Full-time ASC Member. The reason for selecting a Full-time ASC Member is to ensure the effective activity of the ASC through (i) acquisition of information by an ASC Member familiar with the Company’s internal situation through his/her attendance in important meetings, daily collection of information, periodically listening to business reports from the business operating division and cooperating with the internal audit division and internal control promoting division, etc., and (ii) sharing such information with all other ASC Members. | |||||
| 6. | The Company receives advice, etc., on legal matters on an as needed basis from other lawyers working at Oh-Ebashi LPC & Partners, the law firm where Director who is an ASC Member Shiro Kuniya works concurrently, but the proportion of the annual value of those transactions to the sales of the Company and of Oh-Ebashi LPC & Partners is less than 1% in both cases. In addition, there is no advisory contract between the Company and Oh-Ebashi LPC & Partners. | |||||
| 7. | There are no relationships between the Company and the organizations in which the External Directors concurrently serve that should be noted other than that described in Note 6 above. | |||||
| 8. | The Company has set the “Internal criteria for independence of external directors of the Company” and has elected the External Directors based on those criteria. Since all the External Directors (i.e., the External Directors Masahiro Sakane, Olivier Bohuon, Ian Clark, Yoshiaki Fujimori, Steven Gillis, Emiko Higashi, Michel Orsinger and Toshiyuki Shiga and the External Directors who are ASC Members Shiro Kuniya, Jean-Luc Butel and Koji Hatsukawa) have met the requirements for Independent Directors based on the regulations of the financial instruments exchanges that the Company is listed on (e.g., Tokyo Stock Exchange, Inc.), the Company has appointed all of them as Independent Directors and submitted notifications to each exchange. | |||||
| 9. | This fiscal year, the Nomination Committee is composed of External Director Masahiro Sakane (Chairperson), External Director Emiko Higashi, External Director who is an ASC Member Shiro Kuniya and President and Representative Director Christophe Weber, and the Compensation Committee is composed of External Director Toshiyuki Shiga (Chairperson), External Director Yoshiaki Fujimori and Director who is an ASC Member Yasuhiko Yamanaka. | |||||
| 10. | The Director who retired from office during this fiscal year is as follows: Director James Kehoe (resigned on May 31, 2018) |
86
Table of Contents
(2) Outline of the terms of the liability limitation agreement
The Company has executed agreements with Non-Executive Directors Masahiro Sakane, Olivier Bohuon, Ian Clark, Yoshiaki Fujimori, Steven Gillis, Emiko Higashi, Michel Orsinger, Toshiyuki Shiga and Non-Executive Directors who are Audit and Supervisory Committee Members Yasuhiko Yamanaka, Shiro Kuniya, Jean-Luc Butel, Koji Hatsukawa stating that the maximum amount of their liabilities for damages as set forth in Article 423, Paragraph 1 of the Companies Act shall be the amount provided by law.
(3) Compensation, etc. for Directors
The Company has formulated the “Director’s Compensation Policy” and determines the composition and level of compensation of the Directors in accordance with the concept and procedure of this Policy. The Policy is designed to attract, retain, and motivate highly qualified and talented executives, as well as closely link compensation to company’s mid-long term performance to align the economic interests of shareholders. Additional modifications to the Director’s Compensation Policy will be proposed at this General Meeting for shareholders to consider that will further reinforce the alignment of executive pay to long-term shareholder value.
The compensation, etc. of Directors who are not Audit and Supervisory Committee Members (excluding External Directors) consists of “Basic Compensation”, which is paid at a fixed amount, and “Performance-based Compensation”, which is paid as a variable amount based on company’s annual and mid-long term performance. “Performance-based Compensation further consists of a “Bonus” to be paid based on the consolidated financial results, etc. for each fiscal year, and a “Long-term Incentive Plan (stock compensation)” linked with mid and long-term financial results over a 3-year period and with Takeda’s share price.
The compensation of External Directors who are not Audit and Supervisory Committee Members consists of Basic Compensation, which is paid as a fixed amount, and Long-term Incentive (stock compensation), which is linked only to share price. Bonus is not available for this category of Director.
The compensation of Directors who are Audit and Supervisory Committee Members consists of Basic Compensation, which is paid as a fixed amount, and Long-term Incentive (stock compensation), which is linked only to share price. Bonus is not available for this category of Director.
The Compensation Committee was established as an advisory body to the Board of Directors. The majority of the Compensation Committee members are External Directors, and it is chaired by an External Director. The committee serves to ensure the appropriateness of and transparency and objectivity in decision-making processes relating to the Directors’ compensation, etc. The criteria and structure of the compensation and performance-based compensation (the Long-term Incentive Plan and Bonus), etc. for Directors shall be determined by the Board of Directors based on the report of the Compensation Committee.
87
Table of Contents
The total amounts of compensation, etc., for Directors for this fiscal year (not including the bonuses and the salaries and bonuses paid to the relevant Directors for their work as employees) are as follows.
| Category |
Number of people | Total amounts of compensation, etc.* * Basic compensation and cost postings relating to the stock | ||
| Directors who are not Audit and Supervisory Committee Members (External Directors) |
12 (8) |
1,262 million JPY (178 million JPY) | ||
| Directors who are Audit and Supervisory Committee Members (External Directors) |
4 (3) |
137 million JPY (87 million JPY) |
| (Notes) | 1. | The aforementioned includes 1 Director who is not an Audit and Supervisory Committee (“ASC”) Member and retired from the office as of May 31, 2018. | ||||||
| 2. | The total amounts of compensation, etc. for Directors who are not ASC Members above include the following basic compensation and cost postings relating to the stock compensation. | |||||||
| [1] | The basic compensation is a fixed amount depending on each position, and its total amount per month is no more than 150 million JPY (within this amount, no more than 30 million JPY per month is for External Directors) (based on a resolution of the 140th Ordinary General Meeting of Shareholders held on June 29, 2016). | |||||||
| [2] | The cost posting relating to stock compensation is the value posted during this fiscal year (814 million JPY, which includes the 61 million JPY for External Directors). This stock compensation is based on the resolution of the 140th Ordinary General Meeting of Shareholders held on June 29, 2016. The upper limit of the amount contributed for that stock compensation and the number of the stocks to be granted are as follows: | |||||||
| (a) | Stock compensation granted to Directors who are neither External Directors nor ASC Members (excluding Directors residing overseas) Upper limit of 2.7 billion JPY per year for three consecutive fiscal years (the upper limit of the number of the stocks to be granted is calculated by dividing the amount of the above-mentioned upper limit by the closing price of the stocks of the Company at the Tokyo Stock Exchange on a predetermined day of each fiscal year) | |||||||
| (b) | Stock compensation granted to External Directors who are not ASC Members Upper limit of 0.3 billion JPY (the upper limit of the number of the stocks to be granted is calculated by dividing the amount of the above-mentioned upper limit by the closing price of the stocks of the Company at the Tokyo Stock Exchange on a predetermined day of each fiscal year) | |||||||
| 3. | If the proposal for the “Payment of Bonuses to Directors who are not Audit and Supervisory Committee Members” is proposed at this General Meeting of Shareholders and approved as proposed, the Directors’ bonuses, included among the compensation, etc., for Directors who are not ASC Members for this fiscal year, will be paid within the amount set forth in the said proposal. Directors’ bonuses are calculated depending on each position based on the Company’s financial results (achievement of key performance indicators such as the consolidated revenue, Core Earnings and EPS). Based on the report of the Compensation Committee, the actual payment amount of bonuses is to be resolved at the meeting of the Board of Directors to be held after this General Meeting of Shareholders. | |||||||
88
Table of Contents
| 4. | The total amounts of compensation, etc. for Directors who are ASC Members include the following basic compensation and cost postings relating to the stock compensation. | |||||||
| [1] | The basic compensation is a fixed amount depending on each portion, and its total amount per month is no more than 15 million JPY (based on a resolution of the 140th Ordinary General Meeting of Shareholders held on June 29, 2016). | |||||||
| [2] | The cost posting relating to stock compensation is the value posted during this fiscal year (40 million JPY). This stock compensation is based on a resolution of the 140th Ordinary General Meeting of Shareholders held on June 29, 2016, for which no more than 200 million JPY will be contributed in this fiscal year for two consecutive fiscal years. The upper limit of the number of the stocks to be granted is calculated by dividing the amount of the above-mentioned upper limit by the closing price of the stocks of the Company at the Tokyo Stock Exchange on a predetermined day of each fiscal year. | |||||||
89
Table of Contents
(4) External Directors
Major activities during this fiscal year
| Category |
Name |
Number of attending the meeting | ||||
| Board of Directors | Audit and Supervisory Committee | |||||
| Directors |
Masahiro Sakane | 12/12 | — | |||
| Olivier Bohuon | 1/2 | — | ||||
| Ian Clark | 1/2 | — | ||||
| Yoshiaki Fujimori | 12/12 | — | ||||
| Steven Gillis | 2/2 | — | ||||
| Emiko Higashi | 12/12 | — | ||||
| Michel Orsinger | 12/12 | — | ||||
| Toshiyuki Shiga |
12/12 | — | ||||
| Directors who are Audit and Supervisory Committee Members |
Shiro Kuniya | 12/12 | 19/20 | |||
| Jean-Luc Butel | 9/10 | 18/19 | ||||
| Koji Hatsukawa | 12/12 | 20/20 | ||||
| (Notes) | 1 | Directors Olivier Bohuon, Ian Clark and Steven Gillis were elected at the Extraordinary General Meeting of Shareholders held on December 5, 2018 and took office as of January 8, 2019. Accordingly, the Board of Directors meetings to be attended by them are the meetings held thereafter. | ||
| 2 | With regard to the Director (Audit and Supervisory Committee Member) Jean-Luc Butel’s “Number of attending the meeting” in the table above, 2 Extraordinary meetings of Board of Directors and 1 meeting of Extraordinary Audit and Supervisory Committee are excluded therefrom because they were held only for discussing the acquisition of Shire and he didn’t join them in order to avoid a conflict of interest as he was a shareholder of Shire. |
External Directors appropriately made statements necessary for the deliberation of the agenda at the Board of Directors meetings based on (i) their advanced insight derived from experience in corporate management, or (ii) their high level of knowledge in areas requiring high expertise such as accounting and law. Also, Shiro Kuniya, Jean-Luc Butel and Koji Hatsukawa, at the Audit and Supervisory Committee, made statements necessary for the deliberation of the respective agenda thereof, based on their specialist perspectives, and vigorously conducted information exchange, etc.
90
Table of Contents
5. Accounting Auditor
| (1) Name of Accounting Auditor |
KPMG AZSA LLC | |
|
(2) Amount of Remuneration, etc. of Accounting Auditor for this Fiscal Year | ||
| (i) | Amount of remuneration, etc. for this fiscal year | 926 million JPY | ||||
| (ii) | Total amount of money and other financial benefits to be paid by the Company and the subsidiaries | 2,886 million JPY |
| (Notes) | 1. As the audit agreement between the Company and its Accounting Auditor does not differentiate the amount of remuneration, etc. for audit under the Companies Act from the one for audit under the Financial Instruments and Exchange Act and such differentiation is impossible in practice, the above amounts show total remuneration, etc. for both audits. | |||
| 2. Audit and Supervisory Committee confirms and examines the auditing plan of the Accounting Auditor, the implementation status of auditing by Accounting Auditor and the rationale for calculating the estimated remuneration thereof based on the Guideline of Practice for Cooperation with Accounting Auditor published by Japan Audit & Supervisory Members Association. As a result of such confirmation ad examination, Board of Corporate Auditors agreed on the remuneration, etc. of the Accounting Auditor pursuant to Section 399, Paragraph 1 of the Companies Act. | ||||
| 3. Among the subsidiaries set forth in “1. Current State of the Takeda Group, (7) Material Business Affiliations (as of March 31, 2019)” herein, audit firms other than KPMG AZSA LLC audit the financial statements of the subsidiaries of the Company located overseas. | ||||
| 4. Total amount of money and other financial benefits to be paid by the Company and the subsidiaries includes the acquired Shire group audit fee as well as the PCAOB audit fee for the past three fiscal years of 2015 to 2017 for the listing of American Depositary Shares. | ||||
| (3) | Services other than Audit |
The Company delegates to the Accounting Auditor the services which fall under services other than the services set forth in Article 2, Paragraph 1 of the Certified Public Accountants Act in respect of services for “Issuance of comfort letter for the bond issue”, etc.
| (4) | Decision-Making Policy on Dismissal or Rejection of the Reappointment of Accounting Auditor |
If the Accounting Auditor is determined to fall under any of the events prescribed in each item of Article 340, Paragraph 1 of the Companies Act, or if an event which has a material adverse effect on the audit procedures of the Company occurs, including, but not limited to, the case in which such Accounting Auditor’s auditing license is suspended, the Accounting Auditor shall be dismissed by the Audit and Supervisory Committee based on the approval of all members thereof.
In addition, the Audit and Supervisory Committee, taking into consideration the audit quality, the quality control and independence of the Accounting Auditor and other factors, shall determine whether or not the Accounting Auditor will be reappointed.
91
Table of Contents
6. Overview of the Systems that Ensure the Appropriateness of Operations of the Company and the Status of Implementation of such Systems
| (1) | Overview of the Systems that ensure the appropriateness of operations |
The Company shares its “Corporate Philosophy,” which comprises its “Mission,” “Vision,” “Values” and “Strategic Roadmap” within the entire Takeda Group and puts an effort to promote the creation of a disciplined and sound corporate culture. Based on the abovementioned principle, the Company undertakes to establish the following measures for its internal control system, treating it as an important component of corporate governance that functions alongside risk management. Also, in order to further enhance corporate governance, necessary changes are conducted, including changes to the decision-making system.
| (i) | Systems that ensure the appropriateness of operations in the Takeda Group |
| • | As a “Company with Audit and Supervisory Committee (“ASC”),” a system that enables ASC to effectively perform its duties relating to audit and supervision shall be established and the composition and diversity of the External Directors in the Board of Directors shall be enhanced. Under the appropriate audit and supervision thereof, the Board of Directors shall make highly transparent and objective decisions and, by resolution, delegate authority to the Directors and expedite the management of business. |
| • | The objectivity and fairness of the appointment of Directors and the compensation paid to them shall be ensured by voluntarily establishing a Nomination Committee and Compensation Committee, as advisory bodies for the Board of Directors, wherein an External Director will serve as the chairperson and external committee members will constitute a majority, respectively. By appointing one or more Directors who are ASC Members as members of such committees, the effectiveness of the ASC’s function of supervising the appointment, etc. of Directors who are not ASC Members and the compensation, etc. paid to them shall be enhanced. By resolution of the Board of Directors, the authority to decide the amount of individual remuneration of Internal Directors who are not ASC Members shall be delegated to the Compensation Committee, through which we have realized a more transparent process in determining individual remuneration. |
| • | Under the system above, the Board of Directors will (i) decide on the most important matters for the business operation of the Takeda Group, including matters relating to Basic Management Policy and matters relating to internal control, including compliance and risk management, and (ii) discuss business strategy, and monitor and supervise the execution of operations. |
92
Table of Contents
| • | To strengthen its global business management system, the Company shall establish the Takeda Executive Team (“TET”), which will consist of the President & CEO and the members who manage and supervise each function of the Takeda Group, and also establish a Business Review Committee (which will be responsible for general management matters), a Portfolio Review Committee (which will be responsible for R&D and product related matters), and an Audit, Risk and Compliance Committee (which will be responsible for internal audit, risk management and compliance matters). These committees will review important matters that will ensure systems through which faster and more flexible work execution and deeper cooperation among the various functions can take place. |
| • | By resolution of the Board of Directors, decision making authority on matters of important business execution shall be partially delegated to the Directors through decision-making bodies such as the Business Review Committee, Portfolio Review Committee, and Audit, Risk and Compliance Committee; the Company shall make flexible and efficient decisions. |
| • | The Company shall clarify the roles and responsibilities of each function based on the “Takeda Group’s Management Policy (T-MAP),” which summarizes the business management systems, decision-making systems and operational rules and other important management rules of the Takeda Group. With regard to certain material items, the Company shall oblige each function to propose or report them to the decision making bodies, including the Board of Directors, depending on the materiality of those items. Concurrently, the Company shall delegate a certain level of decision making authority to the President & CEO or to other TET members, and such decision making authority shall be exercised under proper governance. TET members develop and implement policy manuals (divisional T-MAP) consistent with the T-MAP and establish an adequate internal control structure in the divisions which they oversee. |
| • | In order to manage and supervise the entire Takeda Group in a cross-sectoral and unified manner, the Company shall maintain Global Policies, etc. (Global Policies mean the rules applied to employees of three or more TET organizations) for the respective operations of specialized functions. |
| • | With regard to risk management and management of a crisis that has occurred in the Takeda Group, the “Global Risk Management Policy,” and the “Global Crisis Management Policy” respectively lay out the structure of the risk management system including BCP(Business Continuity Plan)s and the crisis management systems of the Takeda Group. |
93
Table of Contents
| • | The Global Ethics & Compliance division and other divisions in charge of compliance shall disseminate the “Takeda Global Code of Conduct” to all group companies and develop and disseminate compliance programs for all group companies based on that code under the Global Compliance Promotion System. The Global Ethics & Compliance division shall establish a mechanism with monitoring capabilities to ensure that the Takeda Group’s business activities are in compliance with laws and internal rules. In addition, the Global Ethics & Compliance division and other divisions in charge of compliance shall periodically report to the Audit, Risk and Compliance Committee and ASC, and report to the Board of Directors as necessary, on the compliance related affairs of the Takeda Group, including those reported through the internal reporting system for whistleblowers. |
| • | The Group Internal Audit (“GIA”) shall conduct a regular internal audit of each function of the Company and each group company based on the “Group Internal Audit Charter” and report the results thereof to the President & CEO, Board of Directors, and ASC. The GIA shall also conduct an evaluation of the status of the development and implementation of the internal control systems for securing the reliability of financial reporting based on the Financial Instruments and Exchange Act. |
| • | The Global Finance division shall manage the processes of (i) self-inspection based on questionnaires on internal controls over the financial reporting completed by the head of each key subsidiary, and (ii) implementation of the improvement plan in response to warnings or recommendations. |
| • | The Global Quality division shall formulate global quality assurance policies, etc., relating to research, development, manufacturing, and post-marketing safety measures and then audit, monitor, and supervise compliance therewith regularly or as necessary. |
| • | The Corporate EHS (environment, health and safety) department in the Global Manufacturing & Supply division establishes the “Global Policy and Guideline on EHS”, etc. and conducts audits regularly or as necessary. Also, it provides support and advice to reduce risks regarding the environment, occupational health and safety. |
| (ii) | System for retention and management of information in connection with the execution of the duties of Directors |
| • | The minutes of the meetings of the Board of Directors, requests for and approvals of managerial decisions, and other information concerning the execution of the duties of Directors shall be appropriately retained and controlled in keeping with the term, method and place of retention designated for each category of information, as determined in accordance with the “Policy on Document Control,” in either hard copy or electromagnetic record, and to facilitate ease of inspection. |
94
Table of Contents
| (iii) | Risk management rules and other systems |
| • | Based on the “Global Risk Management Policy,” Enterprise Risk Management (ERM) shall be conducted through a five step approach, which is the identification, assessment, mitigation, reporting, and monitoring and control of the risk, and the systems through which the major potential risks and the mitigation plans thereof, etc. will be reported to the Audit, Risk and Compliance Committee and the Board of Directors shall be established. Based on the policy with respect to all risk factors, including major potential risks for the Company (research and development, intellectual property rights, decline of sales due to the expiration of patents, etc., side-effects, drop in prices caused by measures to constrain the cost of medicine, fluctuation of foreign exchange rates, corporate acquisitions, country risks, stable supply, and litigation and other legal matters, IT-security and information management, etc.), the person(s) in charge of each function shall control and manage such risk factors in each area under his/her charge using qualitative and quantitative criteria in designing and implementing mid-range and annual plans, and shall take all necessary measures or remedies available to avoid and minimize such risk factors, depending on the degree and content of the risk the Company is exposed to, in compliance with the countermeasures to cope therewith and any contingency plans. In addition, the Company shall design a BCP for each function under the “Global Risk Management Policy” in order to minimize the negative impact on business when risks are realized. |
| • | In order to prevent and respond to emergency situations, the Company shall establish crisis management systems through the appointment of persons who will be in charge of crisis management, site heads who will lead the incident site and those who will be in charge of site incident management, and shall establish a crisis management committee under the “Policy on Crisis Management.” |
| (iv) | System that ensures the duties of Directors are executed efficiently |
| • | A system that ensures the duties of Directors are executed appropriately and efficiently shall be safeguarded through the “Bylaws of Board of Directors” and other internal company regulations relating to authorities and rules for decision-making. |
| (v) | Systems that ensure Directors and employees comply with laws and regulations and the Company’s Articles of Incorporation in executing their duties |
| • | In accordance with the “Compliance Promotion Rule” that provides for the basic policies and procedures in relation to the implementation of the compliance program for the ethical and legal requirements of the Company, an Ethics & Compliance Officer position, Compliance Promotion Committee and Compliance Secretariat shall be established to promote the compliance policy of the Company. |
| • | The Company has established procedures for the receipt, retention, investigation and treatment of concerns and complaints notified through the internal reporting system related to any violations of laws and regulations, Takeda’s Global Code of Conduct, policies or SOPs, including concerns and complaints related to the Company’s accounting, internal accounting controls, or auditing matters. The Company has also established procedures for the confidential, anonymous submission by Takeda employees of all concerns and complaints. |
95
Table of Contents
| (vi) | System that ensures the audits by the Audit and Supervisory Committee are conducted effectively |
Each of the items stated below shall be carried out in accordance with the “Rules of Audit and Supervisory Committee’s Audit, etc.”
| • | Full-time ASC Members shall be appointed, and an ASC Office, which will be composed of full-time staff, shall be established to provide secretariat assistance to the ASC Members in the performance of their duties and functions. |
| • | The ASC shall make efforts to secure the independence of the ASC Office from the person in charge of executing the business, and the effectiveness of instructions from the ASC and personnel matters with respect to the members of the ASC Office shall be handled by agreement between the Directors and ASC. |
| • | A Director shall inform the ASC of those matters concerning the Company’s basic management policy and plans, and of material matters including the ones involving subsidiaries and affiliated companies (provided, however, that this shall not apply if the ASC Members attend the meeting of the Board of Directors or any other meeting at which such matter is discussed). |
| • | If a Director becomes aware of a fact that might cause material damage to the Takeda Group, such Director shall, without delay, give notice of such fact to the ASC. |
| • | The ASC shall appoint ASC Members who will have the authority to request Directors and employees to report on matters relating to the performance of their duties and investigate the status of the operations and assets of the Company. |
| • | Based on the status of development and operation of the internal control system, the ASC shall have close communications with the internal audit division, internal control promotion division and Accounting Auditor, to which the ASC shall have the authority to give instructions, and it shall enhance the effectiveness and efficiency of the audit by conducting a systematic audit utilizing the information derived therefrom. |
| • | The ASC Members shall request the Company to reimburse their costs for performing their duties, and submit a budget to the Company every year. |
| • | The ASC shall make proposals or state its opinions to the Board of Directors, as necessary, with respect to systems that ensure that any person who makes a report to the ASC and the internal audit divisions, etc., including a report made through the internal reporting system for whistleblowers, would not be subject to any discriminatory treatment due to such reporting. |
96
Table of Contents
| (2) | Overview of the Status of the Implementation of Systems that ensure the appropriateness of operations |
This fiscal year, we made efforts to appropriately implement the systems described in (1) above. Our major efforts this fiscal year considered important points for internal control, including the following:
[Dissemination of Corporate Philosophy and Vision 2025]
| • | The Company further disseminated throughout the Company the “Corporate Philosophy” consisting of the “Mission,” “Vision,” “Values” and “Strategic Roadmap,” as well as “Vision 2025,” which shows what the Company aims to become. Furthermore, TET members, including the President & CEO, disseminated such Corporate Philosophy by posting messages on the intranet, holding town hall meetings etc. |
[Strengthening of the Corporate Governance Structure]
| • | Along with the Company’s conversion into a “Company with Audit and Supervisory Committee,” the Company enhanced the composition ratio of its external directors and diversity so that the Board of Directors and ASC could conduct each of their responsibilities more appropriately. As a result, of the 15 members of the Board of Directors (including one woman director) as of the end of this fiscal year, 11 are External Directors; furthermore, 8 Directors are Japanese and 7 are foreign nationals. Additionally, 4 Directors make up the ASC, and 3 of them are External Directors. |
| • | The decision making process of the former Shire was integrated into the governance system of the Company at the time of the completion of the acquisition of Shire. Also, matters to be complied with cross-functionally are specified, and the Global Policies common in the Company’s group, which shall be the basis of the standard of procedures (SOP), are integrated. |
[Status of the Board of Directors]
| • | 12 Board of Directors meetings were held this fiscal year. At the Board of Directors meetings, the Chairman of the Board, who is an Independent and External Director, lead the discussions, while various Directors, including the External Directors who are highly independent from the Company, delivered statements as were appropriate from their perspectives. |
| • | As mentioned above, by delegating to the Directors the authority to decide on important matters on business execution, the Board of Directors acquired more time both to deliberate issues that can have a significant impact on the Takeda Group and its management strategies and oversee the Directors’ performance on business execution. |
| • | To fulfill the role of a Director of the Company more appropriately, before every Board of Directors’ meeting, External Directors are given a detailed explanation of the agenda of the meeting by the Directors who are not External Directors. In addition, when the External Directors are newly appointed, they are thoroughly educated on their legal obligations as well as provided with information relating to the business environment, strategy, etc., of the Company; and requested to participate in sessions intended to further deepen their understanding thereof. |
97
Table of Contents
| • | After the Shire acquisition, all Directors received training regarding the Corporate Integrity Agreement concluded by Shire and the Office of the Inspector General in the US and the U.S. Compliance program, which are required for listing on the New York Stock Exchange. |
| • | At the Board of Directors meetings, each External Director made appropriate statements during the deliberations on the agenda of the Board of Directors meetings based on (i) their advanced insight derived from experience in corporate management, or (ii) their high level of knowledge in areas requiring high expertise such as accounting and law. In addition, meetings consisting only of the External Directors (“External Directors Meeting”) were held to allow them to share their knowledge or understanding and exchange views and opinions on the management of the Board of Directors and how to engage in management. |
[Efforts to promote the internal control system in the Takeda Group]
| • | With regard to matters other than those that need to be resolved by decision-making bodies, including the Board of Directors, Business Review Committee, Portfolio Review Committee, and Audit, Risk and Compliance Committee, the authority is delegated to the members of the TET which consists of the President & CEO and the representatives of each function. In order to clarify how the TET members delegate their authority, the “Global Policy - Delegation of Authority” was formulated this fiscal year as a global standard. |
| • | The GIA conducted an internal audit of each function of the Company and each company under the Takeda Group, as well as an evaluation of the status of development and implementation of the internal control systems, to secure the reliability of financial reporting based on the Financial Instruments and Exchange Act. |
| • | With regard to the status of internal controls on financial reporting at the key subsidiaries, the Global Finance division confirmed the effectiveness of the internal controls of such subsidiaries based on the answers received from the head of each key subsidiary, which were obtained by self-inspection through questionnaires. |
| • | The Global Quality division clarified the Company’s commitment to, and vision for, quality, and conducts global quality control for the Takeda Group based on the “Global Quality Policy.” |
| • | The Corporate EHS department clarified the roles and responsibilities in order to promote activities for management of the environment, occupational health and safety, and conducted an internal audit from the perspective of management of the environment, occupational health and safety, compliance and crisis management by setting specific targets based on the “Global Policy and the guideline on the EHS”, etc. |
98
Table of Contents
[Efforts to promote compliance]
| • | The monitoring of fields with potentially high compliance-related risks was conducted at each division, and voluntary and continuous improvements are being made. |
| • | The Takeda Group’s compliance-related issues are being regularly reported to the Audit, Risk and Compliance Committee, and to the Board of Directors and TET in a timely manner. |
[Efforts relating to risk management]
| • | This fiscal year, important risks for each region and division were discussed and validated at the Risk Management Committee, and the risks confirmed thereat were again validated at the Audit, Risk and Compliance Committee. Thereafter, such risks were registered as corporate risks and a risk map was developed. |
| • | The risk map was reported to the Board of Directors. Also, a risk mitigation plan for important risks was developed and the effectiveness thereof was monitored. |
| • | Other concrete efforts relating to risk management for this fiscal year are as follows: |
| • | Social Media Playbook, which provides operational rules that include the internal governance structure for social media, was established and an operational administrative tool was adopted. |
| • | The Information Security Working Group, consisting of Legal, Compliance, HR, Risk Management, Crisis Management, R&D, Intellectual Property and IS/IT (Information System/Information Technology) etc,, was held. |
| • | Effective measures and programs on technical matters were implemented in order to secure important data and strengthen capabilities in dealing with a cyber crisis. |
| • | Education and drills for TET and employees, the purpose of which was to enhance consciousness on the appropriate use of social media and of cybersecurity, and consciousness on responding to crises, including earthquakes and pandemic situations, were conducted. |
[Efforts by the Audit and Supervisory Committee]
| • | The ASC is managed based on the “Rules of Audit and Supervisory Committee’s Audit, etc.,” and an External Director serves as its chairman. 20 ASC meetings were held this fiscal year, and information or opinions relating to the agenda at the Board of Directors meetings, status of the execution of the business and the internal control system, etc. were exchanged thereat. All ASC members shared information obtained from a full-time ASC Member activity (attendance in important meetings, periodically listening to reports relating to the business performance of the division in charge of executing the business operation, and through cooperation or collaboration with the internal audit division or internal control promoting division). The audit opinions were formed in ASC through the activities mentioned above. |
| • | The ASC reported on the result of the activities of the previous year and its action policy and activity plan for this fiscal year, and exchanged opinions at the Board of Directors meeting. Also, as necessary, the ASC gave its opinion on the execution of the business by the Directors. |
99
Table of Contents
| • | The ASC exchanged opinions with the GIA regularly or as necessary and made efforts to conduct a systematic audit by providing instructions or requests, in addition to receiving a report relating to the plan and result of the internal audit. |
| • | The ASC exchanged opinions with the GIA regularly or as necessary and made efforts to conduct a systematic audit by providing instructions or requests, in addition to receiving a report relating to the plan and result of the internal audit. |
| • | The ASC oversees the structure and the operation of the employee hotline including the handling of reported concerns and complaints. The Audit & Supervisory Committee also maintains oversight over all reported concerns and complaints through periodic reports from the Global Ethics & Compliance Office. |
****************************************************************
[Note to Business Report]
All monetary amounts indicated in the Business Report are rounded to the nearest unit.
100
Table of Contents
CONSOLIDATED FINANCIAL STATEMENTS [IFRS]
CONSOLIDATED STATEMENT OF OPERATIONS
(April 1, 2018 to March 31, 2019)
| (Million JPY) | ||||||||
| Item |
Amount | [Reference] Amount of previous period |
||||||
| Revenue |
2,097,224 | 1,770,531 | ||||||
| Cost of sales |
(659,690 | ) | (495,921 | ) | ||||
| Selling, general and administrative expenses |
(717,599 | ) | (628,106 | ) | ||||
| Research and development expenses |
(368,298 | ) | (325,441 | ) | ||||
| Amortization and impairment losses on intangible assets associated with products |
(203,372 | ) | (122,131 | ) | ||||
| Other operating income |
159,863 | 169,412 | ||||||
| Other operating expenses |
(103,159 | ) | (126,555 | ) | ||||
|
|
|
|
|
|||||
| Operating profit |
204,969 | 241,789 | ||||||
|
|
|
|
|
|||||
| Financial income |
16,843 | 39,543 | ||||||
| Financial expenses |
(83,289 | ) | (31,928 | ) | ||||
| Share of profit (loss) of associates accounted for using the equity method |
(43,627 | ) | (32,199 | ) | ||||
|
|
|
|
|
|||||
| Profit before tax |
94,896 | 217,205 | ||||||
|
|
|
|
|
|||||
| Income tax expenses |
14,118 | (30,497 | ) | |||||
|
|
|
|
|
|||||
| Net profit for the year |
109,014 | 186,708 | ||||||
|
|
|
|
|
|||||
| Attributable to: |
||||||||
| Owners of the Company |
109,126 | 186,886 | ||||||
| Non-controlling interests |
(112 | ) | (178 | ) | ||||
|
|
|
|
|
|||||
| Net profit for the year |
109,014 | 186,708 | ||||||
|
|
|
|
|
|||||
101
Table of Contents
[Reference] CONSOLIDATED STATEMENT OF
OPERATIONS AND OTHER COMPREHENSIVE INCOME
(April 1, 2018 to March 31, 2019)
| (Million JPY) | ||||||||
| Item |
Amount | [Reference] Amount of previous period |
||||||
| Net profit for the year |
109,014 | 186,708 | ||||||
| Other comprehensive income (loss) |
||||||||
| Items that will not be reclassified to profit or loss |
||||||||
| Changes in fair value of financial assets measured at fair value through other comprehensive income |
6,000 | — | ||||||
| Remeasurement of defined benefit plans |
(11,665 | ) | 724 | |||||
|
|
|
|
|
|||||
| Items that may be reclassified subsequently to profit or loss |
(5,665 | ) | 724 | |||||
| Exchange differences on translating foreign operations |
34,639 | 46,611 | ||||||
| Net changes on revaluation of available-for-sale financial assets |
— | 4,714 | ||||||
| Cash flow hedges |
(33,793 | ) | 1,919 | |||||
| Hedging cost |
(4,909 | ) | 1,606 | |||||
| Share of other comprehensive income of investments accounted for using the equity method |
(94 | ) | 382 | |||||
|
|
|
|
|
|||||
| (4,157 | ) | 55,232 | ||||||
|
|
|
|
|
|||||
| Other comprehensive income (loss) for the year, net of tax |
(9,822 | ) | 55,956 | |||||
|
|
|
|
|
|||||
| Total comprehensive income (loss) for the year |
99,192 | 242,664 | ||||||
|
|
|
|
|
|||||
| Attributable to: |
||||||||
| Owners of the Company |
99,456 | 242,444 | ||||||
| Non-controlling interests |
(264 | ) | 220 | |||||
|
|
|
|
|
|||||
| Total comprehensive income (loss) for the year |
99,192 | 242,664 | ||||||
|
|
|
|
|
|||||
| (Note) |
“CONSOLIDATED STATEMENT OF OPERATIONS AND OTHER COMPREHENSIVE INCOME” is not included in the consolidated financial statements of the Companies Act, but it is displayed for the reference. |
102
Table of Contents
CONSOLIDATED STATEMENT OF FINANCIAL POSITION
(As of March 31, 2019)
| (Million JPY)
|
||||||||
| Item |
Amount | [Reference] Amount of previous period |
||||||
| ASSETS |
||||||||
| Non-current assets |
||||||||
| Property, plant and equipment |
1,316,531 | 536,801 | ||||||
| Goodwill |
4,161,403 | 1,029,248 | ||||||
| Intangible assets |
4,860,368 | 1,014,264 | ||||||
| Investments accounted for using the equity method |
114,658 | 107,949 | ||||||
| Other financial assets |
192,241 | 196,436 | ||||||
| Other non-current assets |
87,472 | 77,977 | ||||||
| Deferred tax assets |
88,991 | 64,980 | ||||||
|
|
|
|
|
|||||
| Total non-current assets |
10,821,664 | 3,027,655 | ||||||
| Current assets |
||||||||
| Inventories |
986,744 | 212,944 | ||||||
| Trade and other receivables |
741,907 | 420,247 | ||||||
| Other financial assets |
23,276 | 80,646 | ||||||
| Income taxes recoverable |
7,212 | 8,545 | ||||||
| Other current assets |
109,666 | 57,912 | ||||||
| Cash and cash equivalents |
702,093 | 294,522 | ||||||
| Assets held for sale |
479,760 | 3,992 | ||||||
|
|
|
|
|
|||||
| Total current assets |
3,050,658 | 1,078,808 | ||||||
|
|
|
|
|
|||||
| TOTAL ASSETS |
13,872,322 | 4,106,463 | ||||||
|
|
|
|
|
|||||
| Item |
Amount | [Reference] Amount of previous period |
||||||
| LIABILITIES |
||||||||
| Non-current liabilities |
||||||||
| Bonds and loans |
4,766,005 | 985,644 | ||||||
| Other financial liabilities |
235,786 | 91,223 | ||||||
| Net defined benefit liabilities |
156,513 | 87,611 | ||||||
| Income taxes payable |
61,900 | — | ||||||
| Provisions |
35,364 | 28,042 | ||||||
| Other non-current liabilities |
75,174 | 68,300 | ||||||
| Deferred tax liabilities |
867,061 | 90,725 | ||||||
|
|
|
|
|
|||||
| Total non-current liabilities |
6,197,803 | 1,351,545 | ||||||
| Current liabilities |
||||||||
| Bonds and loans |
984,946 | 18 | ||||||
| Trade and other payables |
327,394 | 240,259 | ||||||
| Other financial liabilities |
47,340 | 29,613 | ||||||
| Income taxes payable |
119,485 | 67,694 | ||||||
| Provisions |
392,733 | 132,781 | ||||||
| Other current liabilities |
437,888 | 263,930 | ||||||
| Liabilities held for sale |
201,145 | 3,214 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
2,510,931 | 737,509 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
8,708,734 | 2,089,054 | ||||||
| EQUITY |
||||||||
| Share capital |
1,643,585 | 77,914 | ||||||
| Share premium |
1,650,232 | 90,740 | ||||||
| Treasury shares |
(57,142 | ) | (74,373 | ) | ||||
| Retained earnings |
1,569,365 | 1,557,307 | ||||||
| Other components of equity |
353,542 | 350,631 | ||||||
| Other comprehensive income related to assets held for sale |
— | (4,795 | ) | |||||
| Equity attributable to owners of the Company |
5,159,582 | 1,997,424 | ||||||
| Non-controlling interests |
4,006 | 19,985 | ||||||
|
|
|
|
|
|||||
| Total equity |
5,163,588 | 2,017,409 | ||||||
|
|
|
|
|
|||||
| TOTAL LIABILITIES AND EQUITY |
13,872,322 | 4,106,463 | ||||||
|
|
|
|
|
|||||
103
Table of Contents
UNCONSOLIDATED FINANCIAL STATEMENTS
UNCONSOLIDATED BALANCE SHEET
| (As of March 31, 2019) | (Million JPY) | |||||||
| Item |
Amount | [Reference] Amount of previous period |
||||||
| Current assets |
815,299 | 531,728 | ||||||
| Cash and deposits |
303,808 | 174,395 | ||||||
| Notes receivable |
1,830 | 1,804 | ||||||
| Accounts receivable |
141,762 | 129,866 | ||||||
| Securities |
64,982 | — | ||||||
| Merchandise and products |
36,814 | 37,666 | ||||||
| Work in process |
29,476 | 31,564 | ||||||
| Raw materials and supplies |
23,365 | 20,055 | ||||||
| Income taxes recoverable |
4,389 | — | ||||||
| Short-term loans receivable from subsidiaries and associates |
110,634 | 47,128 | ||||||
| Other |
98,264 | 93,015 | ||||||
| Allowance for doubtful receivables |
(-) | 25 | (-) | 3,765 | ||||
| Noncurrent assets |
8,719,346 | 2,412,900 | ||||||
| Tangible noncurrent assets |
202,775 | 215,213 | ||||||
| Buildings and structures |
124,143 | 125,791 | ||||||
| Machinery and equipment |
29,974 | 38,061 | ||||||
| Vehicles |
31 | 45 | ||||||
| Tools and fixtures |
7,841 | 5,052 | ||||||
| Land |
33,477 | 34,364 | ||||||
| Lease assets |
1,643 | 2,110 | ||||||
| Construction in progress |
5,666 | 9,790 | ||||||
| Intangible noncurrent assets |
18,540 | 20,358 | ||||||
| Investments and other assets |
8,498,031 | 2,181,263 | ||||||
| Investment securities |
70,272 | 96,417 | ||||||
| Investment in subsidiaries and affiliates |
8,277,521 | 1,415,005 | ||||||
| Contributions to subsidiaries and affiliates |
30,896 | 560,216 | ||||||
| Long-term deposits |
5,148 | 6,003 | ||||||
| Prepaid pension costs |
38,434 | 36,637 | ||||||
| Defferred tax assets |
64,835 | 57,532 | ||||||
| Other |
10,926 | 9,457 | ||||||
| Allowance for doubtful accounts |
(-) | 1 | (-) | 4 | ||||
|
|
|
|
|
|||||
| TOTAL ASSETS |
9,534,645 | 2,948,562 | ||||||
|
|
|
|
|
|||||
| Item |
Amount | [Reference] Amount of previous period |
||||||
| Current liabilities |
1,208,765 | 354,039 | ||||||
| Accounts payable |
44,112 | 46,156 | ||||||
| Other payable |
161,571 | 88,016 | ||||||
| Accrued expenses |
58,208 | 38,485 | ||||||
| Short-term loans |
646,287 | 78,549 | ||||||
| Deposits received |
137,637 | 52,111 | ||||||
| Current portion of bonds |
60,000 | — | ||||||
| Current portion of long-term loans |
60,000 | — | ||||||
| Reserve for employees’ bonuses |
19,826 | 19,937 | ||||||
| Reserve for share-based payments |
1,833 | 1,391 | ||||||
| Reserve for bonuses for directors and corporate auditors |
633 | 377 | ||||||
| Reserve for restructuring costs |
3,436 | 2,369 | ||||||
| Other reserve |
614 | 2,116 | ||||||
| Other |
14,608 | 20,050 | ||||||
| Noncurrent liabilities |
3,678,709 | 1,028,611 | ||||||
| Bonds |
1,652,027 | 173,179 | ||||||
| Long-term loans |
1,990,874 | 813,151 | ||||||
| Reserve for employees’ retirement benefits |
5,028 | 4,294 | ||||||
| Reserve for SMON compensation |
1,066 | 1,146 | ||||||
| Reserve for share-based payments |
2,031 | 2,155 | ||||||
| Reserve for restructuring costs |
6,732 | 5,440 | ||||||
| Asset retirement obligations |
2,748 | 4,047 | ||||||
| Long-term deferred income |
12,522 | 17,753 | ||||||
| Other |
5,681 | 7,446 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
4,887,474 | 1,382,650 | ||||||
|
|
|
|
|
|||||
| Shareholders’ equity |
4,614,423 | 1,520,637 | ||||||
| Common stock |
1,643,585 | 77,914 | ||||||
| Capital surplus |
1,629,680 | 64,009 | ||||||
| Additional paid-in capital |
1,629,679 | 64,008 | ||||||
| Other capital surplus |
1 | 1 | ||||||
| Retained earnings |
1,398,272 | 1,449,122 | ||||||
| Legal reserve |
15,885 | 15,885 | ||||||
| Other retained earnings |
1,382,387 | 1,437,171 | ||||||
| Reserve for retirement benefits |
5,000 | 5,000 | ||||||
| Reserve for dividends |
11,000 | 11,000 | ||||||
| Reserve for research and development |
2,400 | 2,400 | ||||||
| Reserve for capital improvements |
1,054 | 1,054 | ||||||
| Reserve for promotion of exports |
434 | 434 | ||||||
| Reserve for special depreciation |
— | 24 | ||||||
| Reserve for reduction of noncurrent assets |
29,120 | 32,661 | ||||||
| General reserve |
814,500 | 814,500 | ||||||
| Unappropriated retained earnings |
518,879 | 570,098 | ||||||
| Treasury stock |
(-) | 57,114 | (-) | 74,343 | ||||
| Valuation and translation adjustments |
31,421 | 43,944 | ||||||
| Unrealized gains on available-for-sale securities |
26,814 | 44,056 | ||||||
| Deferred gains on derivatives under hedge accounting |
4,607 | (-) | 112 | |||||
| Stock acquisition rights |
1,327 | 1,332 | ||||||
|
|
|
|
|
|||||
| Total net assets |
4,647,171 | 1,565,913 | ||||||
|
|
|
|
|
|||||
| TOTAL LIABILITIES AND EQUITY |
9,534,645 | 2,948,562 | ||||||
|
|
|
|
|
|||||
| (Note) | The amount as of the 141st fiscal year has been retrospectively revised due to the adoption of “Application Guidelines of Accounting Standards for Tax Effect Accounting (ABSJ Statement No.28 February 16, 2018) in the 142nd fiscal year. |
104
Table of Contents
UNCONSOLIDATED STATEMENT OF OPERATIONS
| (April 1, 2018 to March 31, 2019) | (Million JPY) | |||||||
| Item |
Amount | [Reference] Amount of previous period |
||||||
| Net sales |
651,347 | 659,462 | ||||||
| Cost of sales |
285,681 | 290,952 | ||||||
| Gross profit |
365,666 | 368,510 | ||||||
| Selling, general and administrative expenses |
291,801 | 300,774 | ||||||
| Operating income |
73,865 | 67,736 | ||||||
| Non-operating income |
28,518 | 77,630 | ||||||
| Interest and dividend income |
17,486 | 60,733 | ||||||
| Other |
11,032 | 16,897 | ||||||
| Non-operating expenses |
84,869 | 19,422 | ||||||
| Interest expenses |
28,550 | 6,580 | ||||||
| Expenses associated with acquisition |
38,667 | — | ||||||
| Other |
17,652 | 12,842 | ||||||
| Ordinary income |
17,514 | 125,944 | ||||||
| Extraordinary income |
53,322 | 140,904 | ||||||
| Gain on sales of investment securities |
34,591 | 32,709 | ||||||
| Gain on sales of investment in subsidiaries |
2,926 | 104,923 | ||||||
| Gain on sales of tangible assets |
8,030 | — | ||||||
| Subsidy income |
7,775 | — | ||||||
| Insurance income |
— | 3,272 | ||||||
| Extraordinary loss |
12,541 | 18,911 | ||||||
| Restructuring costs |
12,541 | 9,916 | ||||||
| Impairment loss |
— | 5,202 | ||||||
| Loss on valuation of investment securities |
— | 3,793 | ||||||
| Income before income taxes |
58,295 | 247,937 | ||||||
| Income taxes - current |
(25,179 | ) | (4,641 | ) | ||||
| Income taxes - deferred |
(4,757 | ) | 65,574 | |||||
| Net income |
88,231 | 187,004 | ||||||
105
Table of Contents
[English Translation of the Accounting Auditors’ Report Originally Issued in the Japanese Language]
[Certified Copy of the Accounting Auditors’ Report related to the Consolidated Financial Statements]
Independent Auditor’s Report
May 13, 2019
| The Board of Directors | ||||
| Takeda Pharmaceutical Company Limited | ||||
| KPMG AZSA LLC | ||||
| Koichi Kohori (Seal) | ||||
| Designated Limited Liability Partner | ||||
| Engagement Partner | ||||
| Certified Public Accountant | ||||
| Naohiro Nishida (Seal) | ||||
| Designated Limited Liability Partner | ||||
| Engagement Partner | ||||
| Certified Public Accountant | ||||
We have audited the consolidated financial statements, comprising the consolidated statement of operations, the consolidated statement of financial position, the consolidated statement of changes in equity and the related notes on the consolidated financial statements of Takeda Pharmaceutical Company Limited (the “Company”) as of March 31, 2019 and for the year from April 1, 2018 to March 31, 2019 in accordance with Article 444-4 of the Companies Act.
Management’s Responsibility for the Consolidated Financial Statements
Management is responsible for the preparation and fair presentation of the consolidated financial statements in accordance with the latter part of Article 120-1 of the Ordinance of Companies Accounting that prescribes some omissions of disclosure items required by International Financial Reporting Standards, and for such internal control as management determines is necessary to enable the preparation of consolidated financial statements that are free from material misstatements, whether due to fraud or error.
Auditor’s Responsibility
Our responsibility is to express an opinion on the consolidated financial statements based on our audit as independent auditor. We conducted our audit in accordance with auditing standards generally accepted in Japan. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free from material misstatement.
An audit involves performing procedures to obtain audit evidence about the amounts and disclosures in the consolidated financial statements. The procedures selected depend on our judgement, including the assessment of the risks of material misstatement of the consolidated financial statements, whether due to fraud or error. In making those risk assessments, we consider internal control relevant to the entity’s preparation and fair presentation of the consolidated financial statements in order to design audit procedures that are appropriate in the circumstances, while the objective of the financial statement audit is not for the purpose of expressing an opinion on the effectiveness of the entity’s internal control. An audit also includes evaluating the appropriateness of accounting policies used and the reasonableness of accounting estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements.
106
Table of Contents
We believe that the audit evidence we have obtained is sufficient and appropriate to provide a basis for our audit opinion.
Opinion
In our opinion, the consolidated financial statements referred to above, which were prepared in accordance with the latter part of Article 120-1 of the Ordinance of Companies Accounting that prescribes some omissions of disclosure items required by International Financial Reporting Standards, present fairly, in all material respects, the financial position and the results of operations of the Company and its consolidated subsidiaries for the period, for which the consolidated financial statements were prepared.
Other Matter
Our firm and engagement partners have no interest in the Company which should be disclosed pursuant to the provisions of the Certified Public Accountants Law of Japan.
Notes to the Reader of Independent Auditor’s Report:
The Independent Auditor’s Report herein is the English translation of the Independent Auditor’s Report as required by the Companies Act.
End of Document
107
Table of Contents
[English Translation of the Accounting Auditors’ Report Originally Issued in the Japanese Language]
[Certified Copy of the Accounting Auditors’ Report]
Independent Auditor’s Report
May 13, 2019
| The Board of Directors | ||||
| Takeda Pharmaceutical Company Limited | ||||
| KPMG AZSA LLC | ||||
| Koichi Kohori (Seal) | ||||
| Designated Limited Liability Partner | ||||
| Engagement Partner | ||||
| Certified Public Accountant | ||||
| Naohiro Nishida (Seal) | ||||
| Designated Limited Liability Partner | ||||
| Engagement Partner | ||||
| Certified Public Accountant | ||||
We have audited the financial statements, comprising the balance sheet, the statement of operations, the statement of changes in net assets and the related notes on the accounts, and the supplementary schedules of Takeda Pharmaceutical Company Limited (the “Company”) as of March 31, 2019 and for the 142nd fiscal year from April 1, 2018 to March 31, 2019 in accordance with Article 436-2-1 of the Companies Act.
Management’s Responsibility for the Financial Statements and Others
Management is responsible for the preparation and fair presentation of the financial statements and the supplementary schedules in accordance with accounting principles generally accepted in Japan, and for such internal control as management determines is necessary to enable the preparation of financial statements and the supplementary schedules that are free from material misstatements, whether due to fraud or error.
Auditor’s Responsibility
Our responsibility is to express an opinion on the financial statements and the supplementary schedules based on our audit as independent auditor. We conducted our audit in accordance with auditing standards generally accepted in Japan. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements and the supplementary schedules are free from material misstatement.
An audit involves performing procedures to obtain audit evidence about the amounts and disclosures in the financial statements and the supplementary schedules. The procedures selected depend on our judgement, including the assessment of the risks of material misstatement of the financial statements and the supplementary schedules, whether due to fraud or error. In making those risk assessments, we consider internal control relevant to the entity’s preparation and fair presentation of the financial statements and the supplementary schedules in order to design audit procedures that are appropriate in the circumstances, while the objective of the financial statement audit is not for the purpose of expressing an opinion on the effectiveness of the entity’s internal control. An audit also includes evaluating the appropriateness of accounting policies used and the reasonableness of accounting estimates made by management, as well as evaluating the overall presentation of the financial statements and the supplementary schedules.
108
Table of Contents
We believe that the audit evidence we have obtained is sufficient and appropriate to provide a basis for our audit opinion.
Opinion
In our opinion, the financial statements and the supplementary schedules referred to above present fairly, in all material respects, the financial position and the results of operations of the Company for the period, for which the financial statements and the supplementary schedules were prepared, in accordance with accounting principles generally accepted in Japan.
Other Matter
Our firm and engagement partners have no interest in the Company which should be disclosed pursuant to the provisions of the Certified Public Accountants Law of Japan.
Notes to the Reader of Independent Auditor’s Report:
The Independent Auditor’s Report herein is the English translation of the Independent Auditor’s Report as required by the Companies Act.
End of Document
109
Table of Contents
[English Translation of the Report of the Audit and Supervisory Committee Originally Issued in the Japanese Language]
[Certified Copy of the Audit Report of the Audit and Supervisory Committee]
Audit Report
The Audit and Supervisory Committee has audited the performance of duties of the Directors of the Company during the 142nd fiscal year from April 1, 2018 to March 31, 2019. The Committee hereby reports the methods and results as follows:
| 1. | Auditing Methods and Details Thereof |
| (1) | The Audit and Supervisory Committee received reports regularly from Directors, employees, etc. on the resolutions of the Board of Directors concerning the matters listed in Article 399-13, Paragraph 1, Items (i)(b) and (i)(c) of the Companies Act as well as the status of establishment and implementation of such system that has been put in place based on said resolutions (internal control system), requested explanation as necessary and expressed its opinion. The Committee also received reports from Directors, etc. and KPMG AZSA LLC on the status of the evaluation and audit of internal controls related to financial reporting under the Financial Instruments and Exchange Act and requested explanation as necessary. |
| (2) | The Audit and Supervisory Committee performed its duties based on the Rules of Audit and Supervisory Committee’s Audit, etc. established by the Audit and Supervisory Committee. In accordance with the audit policy, audit plan and duties assigned to each Audit and Supervisory Committee Member, etc., the Committee, in coordination with the internal auditing department, internal control promoting department and other departments concerned, endeavored to gather information and create an improved environment for auditing, attended important meetings, received reports from Directors, employees and other related persons on the status of their performance of duties, and, requested explanations as necessary, inspected the important materials used for the deliberation and reporting, and examined the status of operations and properties. As for the subsidiaries of the Company, the Committee received reports on the businesses of the subsidiaries by having communication with the directors and corporate auditors of the subsidiaries and sharing information among them as necessary. |
| (3) | The Audit and Supervisory Committee monitored and examined whether the Accounting Auditors maintained their independence and conducted their audits in an appropriate manner, received reports from the Accounting Auditors on the performance of their duties and, when necessary, requested their explanations. The Audit and Supervisory Committee received a notification from the Accounting Auditors that they have taken steps to improve the “system for ensuring appropriate execution of the duties of the accounting auditors” (as set forth in Items of Article 131 of the Corporate Accounting Rules) in accordance with the “Quality Control Standard for Auditing” (adopted by the Business Accounting Council on October 28, 2005) and other standards, and requested explanations as necessary. |
Based on the method described above, the Audit and Supervisory Committee reviewed the Business Report and the accompanying supplementary schedule as well as the unconsolidated financial statements (the unconsolidated balance sheet, the unconsolidated statement of operations, the unconsolidated statement of changes in net assets and the notes on the unconsolidated accounts) and their supplementary schedules and the consolidated financial statements (the consolidated statement of financial position, the consolidated statement of operations, the consolidated statement of changes in equity and the notes on the consolidated financial statements, which were prepared omitting a part of items required to disclose by the International Financial Reporting Standards in accordance with the latter clause of Paragraph 1, Article 120 of the Corporate Accounting Rules) for this fiscal year.
| 2. | Results of Audit |
| (1) | Results of Audit of the Business Report, etc. |
| A. | We confirm that the business report and the accompanying supplementary schedules present fairly the status of the Company in conformity with the applicable laws and regulations as well as the Articles of Incorporation of the Company. |
110
Table of Contents
| B. | With regard to the performance of the duties of the Directors, we confirm that there are no fraudulent acts or material facts that violated the applicable laws and regulations or the Articles of Incorporation of the Company in the course of the performance of the duties of the Directors. |
| C. | We confirm that the substance of the resolutions made by the Board of Directors regarding the internal control system is appropriate. We do not recognize any matters that should be pointed out in regard to the content of business report and the performance of the duties of the Directors regarding the internal control system, including the internal control system related to financial reporting. |
| (2) | Results of Audit of the Unconsolidated Financial Statements and the Accompanying Supplementary Schedules |
We confirm that the methods and the results of the audit conducted by the Accounting Auditors, KPMG AZSA LLC are appropriate.
| (3) | Results of Audit of the Consolidated Financial Statements |
We confirm that the methods and the results of the audit conducted by the Accounting Auditors, KPMG AZSA LLC are appropriate.
May 13, 2019
| The Audit and Supervisory Committee of Takeda Pharmaceutical Company Limited | ||||
| Audit and Supervisory Committee Member: Shiro Kuniya | ||||
| Audit and Supervisory Committee Member: Yasuhiko Yamanaka | ||||
| Audit and Supervisory Committee Member: Jean-Luc Butel | ||||
| Audit and Supervisory Committee Member: Koji Hatsukawa | ||||
| Note : | Audit and Supervisory Committee Members Shiro Kuniya, Jean-Luc Butel and Koji Hatsukawa are External Directors as provided in Article 2, Item15 and Article 331, Paragraph 6 of the Companies Act of Japan. |
END
111
Table of Contents
Guidance Notes on the Exercising of Voting Rights via Electronic Means (e.g., the Internet, etc.)
If you wish to exercise your voting rights via electronic means (e.g., the Internet, etc.), please ensure that you do so until 5:30 p.m. on Wednesday, June 26, 2019 after confirming the following items.
If you attend the Meeting in person, exercising your voting rights by mailing (using the Voting Right Exercise Form) or via electronic means (e.g., the Internet, etc.) is not necessary.
Details
| 1. | Website for Exercising Voting Rights |
| (1) | You may exercise your voting rights via the Internet only by accessing the website for exercising voting rights specified by the Company (https://evote.tr.mufg.jp/) using a personal computer, a smartphone or a cellular phone. Please note that you will not be able to access the above URL from 2:00 a.m. to 5:00 a.m. each day. |
| (2) | In some cases, you may not be able to use the website for exercising voting rights, depending upon the network environment, the service and the equipment you are using. |
| 2. | Method for Exercising Voting Rights via the Internet |
| (1) | On the website for exercising voting rights (https://evote.tr.mufg.jp/), please enter your approval or disapproval of the proposals, using the “Code” and “Tentative Password” provided in the Voting Right Exercise Form and following the instructions on the screen. It is possible for you to access the website of exercising voting rights by scanning QR Code(*) with using a kind of gadgets including the cellular phone. With regard to how to use, please see the instructions of the gadgets you use. (QR Code is omitted in this translation.) |
* QR Code is the registered trademark of DENSO WAVE INCORPORATED.
| (2) | Exercising voting rights by using smartphone, neither “Code” nor “Tentative Password” is required only for the first vote. |
| (3) | Please note that if you wish to exercise your voting rights via the Internet, you will be asked to change your “Tentative Password” on the website for exercising voting rights to prevent unauthorized access and falsification of voting by non-shareholders. |
| 3. | Costs Arising from Access to the Website for Exercising Voting Rights |
Any Internet access fees or communication charges, etc., arising from access to the website for exercising voting rights shall be borne by the user.
| For inquiries with respect to systems, please contact:
Mitsubishi UFJ Trust and Banking Corporation Corporate Agency Division (help desk) Telephone: 0120-173-027 (toll-free number) Operating Hours: 9:00 to 21:00 |
To Institutional Investors:
It is possible to use the “Electronic Voting Platform” as a method for exercising voting rights.
END OF DOCUMENT
112
Table of Contents
<TOPICS: General management>
Acquisition of Shire
Takeda Pharmaceutical Company Limited (“Takeda”) has completed the acquisition of Shire plc in January this year, and now has a leading position in the Japanese and US markets, bringing its highly-innovative medicines to approximately 80 countries and regions. Currently, Takeda is executing a smooth integration under the strong leadership of the experienced and diverse Takeda Executive Team. Also, in December last year, Takeda was listed on the New York Stock Exchange (“NYSE”) in addition to the Tokyo Stock Exchange, which is our major listed market. For NYSE listing, 16 members of the Takeda Executive Team participated in the opening bell ceremony held in January this year. The listing means that Takeda is the only pharmaceutical company listed both in Japan and in the United States, making it possible to access to the two largest capital markets in the world. Through this acquisition, Takeda will be a global, values-based, R&D-driven biopharmaceutical leader headquartered in Japan, putting the patients at the center following our corporate philosophy. Takeda’s R&D efforts are focused on its four therapeutic areas of Oncology, Gastroenterology (GI), Neuroscience and Rare Diseases, with targeted R&D investment also committed to Plasma-Derived Therapies (PDT) and Vaccines.
<TOPICS: Ethical drug business>
Approval and release of new drugs and favorable clinical study results
In February this year, the European Commission (EC) extended the current marketing authorization of ADCETRIS, a therapeutic agent for Hodgkin lymphoma, to include treatment of previously untreated CD30+ Stage IV Hodgkin lymphoma in combination with AVD (adriamycin, vinblastine, and dacarbazine). The AVD combination therapy offers a new treatment option to patients with previously untreated CD30+ Stage IV Hodgkin lymphoma for the first time in decades. This decision by EC means that ADCETRIS in combination with AVD is now approved for marketing of this indication in the 28 member states of the European Union (EU) and applicable in Norway, Liechtenstein, and Iceland. In Japan, Entyvio, a therapeutic agent for ulcerative colitis, was launched last November. Entyvio is authorized for the treatment and maintenance of moderately to severely active ulcerative colitis. It is currently approved in more than 60 countries worldwide. Also, in January this year, TAK-003, a tetravalent live-attenuated dengue vaccine, met the primary endpoint in a Phase 3 trial, showing that it is efficacious in preventing dengue fever caused by any of the four serotypes of the virus. We will analyze the results of this ongoing trial and other Phase 3 studies as soon as they are available to apply for marketing authorization of this vaccine.
113
Table of Contents
<TOPICS: CSR>
Commitment to sustainability
Takeda aims to not only create highly innovative medicines but also provide sustainable value. As a patient-centric, global, R&D-driven biopharmaceutical company and responsible global corporate citizen, we contribute to enhancing the sustainability of society. Takeda is highly evaluated for sustainability initiatives by multiple Environment, Social and Governance (ESG) assessment bodies. We obtained high evaluation for our efforts and active information disclosure, such as that our Sustainable Value Report 2018 received the Merit Award in the Environmental Communication Awards. Also, in January this year, Takeda was named as one of the “2019 Global 100 Most Sustainable Corporations in the World Index” for the fourth consecutive year and as “Global Top Employer® 2019,” a certification granted to companies with outstanding personnel systems, for the second consecutive year.
<TOPICS: Consumer Healthcare>
ALINAMIN and Bio-Three brands - Launch of new products
In April this year, Takeda Consumer Healthcare Company Limited (hereinafter “TCHC”), one of affiliate companies mainly responsible for the consumer healthcare business in Japan, launched ALINAMIN MEDICAL Balance (designated quasi-drug), a drink which has the indication for recovery from and prevention of fatigue and is contained in an aluminum pouch with a tap, which is unique in Japan. ALINAMIN MEDICAL Balance is a gel-type product easy to drink with fresh grapefruit flavor, containing vitamin B2 and B6, taurine, and royal jelly in addition to fursultiamine (vitamin B1 derivative) developed by Takeda.
Since the launch of ALINAMIN SUGAR-COATED TABLETS in 1954, Takeda has been proposing various products that match symptoms and scenes of fatigue for 65 years, such as the launch of drink-type ALINAMIN V in 1987, etc. With the launch of ALINAMIN MEDICAL Balance, we will try to meet a wider range of customer needs than ever before.
Also in April this year, we have started an exclusive sale of an intestinal regulation brand product Bio-Three (designated quasi-drug) in Japan. Bio-Three is an intestinal regulation drug containing three types of symbiotic active bacteria (amylolytic bacteria, lactic acid bacteria, and butyric acid bacteria), improving the composition of the intestinal microflora to regulate the intestinal environment. Bio-Three launched by Toa Pharmaceutical Co., Ltd. as an ethical drug is a long-seller product which has also been used as an OTC drug with abundant results by a large number of consumers for more than 30 years.
This time, when we launch small-tablet type Bio-Three Hi Tablets and powder-type Bio-Three H under the Takeda brand, we propose a new idea for improving the activities of the intestines with products containing butyric acid bacteria through TV commercials and in-store promotions with a following key message: Let’s become “Chou-jin (intestinal people),” by starting new method for improving the activities of the intestines with butyric acid bacteria.
114
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- GA-ASI Selected to Build CCA for AFLCMC
- Helo Corp Announces Annual 2023 Results
- International Truck Integrates Allison Fully Automatic Transmissions with S13 Engine
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share