Form 6-K GALAPAGOS NV For: May 11
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16
UNDER THE SECURITIES EXCHANGE ACT OF 1934
For the month of May 2021
Commission File Number: 001-37384
GALAPAGOS NV
(Translation of registrant’s name into English)
Generaal De Wittelaan L11 A3
2800 Mechelen, Belgium
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F. Form 20-F ☒ Form 40-F ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): ☐
Note: Regulation S-T Rule 101(b)(1) only permits the submission in paper of a Form 6-K if submitted solely to provide an attached annual report to security holders.
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): ☐
Note: Regulation S-T Rule 101(b)(7) only permits the submission in paper of a Form 6-K if submitted to furnish a report or other document that the registrant foreign private issuer must furnish and make public under the laws of the jurisdiction in which the registrant is incorporated, domiciled or legally organized (the registrant’s “home country”), or under the rules of the home country exchange on which the registrant’s securities are traded, as long as the report or other document is not a press release, is not required to be and has not been distributed to the registrant’s security holders, and, if discussing a material event, has already been the subject of a Form 6-K submission or other Commission filing on EDGAR.
First Quarter 2021 Results
On May 6, 2021, the Registrant announced its unaudited first quarter results for 2021, which are further described in the attached report.
| # | Confidential treatment has been requested for portions of this exhibit. These portions have been omitted from this Form 6-K. |
The information contained in this Report on Form 6-K, including the exhibits, except for the quotes of Onno van de Stolpe and the quote of Bart Filius contained in Exhibit 99.1, is hereby incorporated by reference into the Company’s Registration Statements on Forms F-3 (File No. 333-230639) and S-8 (File Nos. 333-204567, 333-208697, 333-211834, 333-215783, 333-218160, 333-225263, 333-231765 and 333-249416).
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| GALAPAGOS NV | ||||||
| (Registrant) | ||||||
| Date: May 11, 2021 | /s/ Xavier Maes | |||||
| Xavier Maes | ||||||
| Company Secretary | ||||||
Exhibit 99.1

Galapagos refocuses pipeline and rightsizes operations
| • | First three months 2021 financial results: |
| • | Group revenues and other income of €124.2 million |
| • | Operating loss of €50.8 million |
| • | Net profit of €9.4 million |
| • | Cash and current financial investments of €5.1 billion on 31 March 2021 |
| • | Refocused clinical pipeline by critically examining risk profile and breadth |
| • | Filgotinib launch in Europe on track |
| • | Initiated €150 million savings program |
Webcast presentation tomorrow, 07 May 2021, at 14.00 CET / 8 AM ET,
www.glpg.com, +32 2 793 38 47, code 5042688
Mechelen, Belgium; 06 May 2021, 22.01 CET; regulated information – Galapagos NV (Euronext & NASDAQ: GLPG) announces its unaudited Q1 results and operational highlights, which are further detailed in the Q1 2021 report available on the Galapagos website, www.glpg.com.
“These last months, we completed a review of our portfolio and development plans with the goal to select a more risk-balanced pipeline. We decided to retain our focus on novel targets to address unmet medical needs in inflammation, fibrosis, and kidney diseases. We also remain fully committed to the launch of Jyseleca in Europe. Moving forward with confidence, we decided to:
| • | Refocus our clinical pipeline by critically examining its risk profile and breadth; |
| • | Cut significant cost in the organization to support this re-sized pipeline development; |
| • | Task our business development team to identify and execute on a transformative opportunity. |
We believe that our strong cash position, expert teams, and solid scientific foundation position us well for future growth,” said Onno van de Stolpe, CEO of Galapagos.
Refocused pipeline
In the revision exercise, Galapagos set goals to focus and adjust the overall risk profile of its clinical pipeline. Consequently, we prioritized those assets with what we believe have enhanced chances of clinical success in our core therapeutic areas. As such, we announce:
| • | We are testing our lead Toledo program ‘3970, a SIK2/3 inhibitor, in five Proof of Concept studies in different indications, and pending the outcome of the studies, we plan to roll out our further development plans in the second half of the year; |
| • | We selected an additional molecule from our Toledo program, SIK2/3 inhibitor ‘4876, as a candidate to accelerate from preclinical phase into clinical development; |
| • | We aim to progress our TYK2 inhibitor ‘3667 into Phase 2b; |
| • | We selected chitinase inhibitor ’4617 to progress to Phase 2 in IPF and decided to stop development of our other IPF molecule ’1205; |
| • | We stopped further work on ‘4059 for metabolic disease, given that this is not a core therapeutic area; |

| • | We discontinued our early research efforts in metabolic diseases and osteoarthritis; and |
| • | We challenged and fine-tuned our stage-gating process to advance compounds. |
Commercial progress
We remain well on track in launching filgotinib in Europe. In the first quarter, we successfully completed the transitions of commercial and medical teams from Gilead in Germany, the UK, Spain, and Italy. We believe everything is in place to complete the final transitions from Gilead to us by year-end. Q1 also saw progress on access and reimbursement for filgotinib in rheumatoid arthritis (RA). Gilead submitted the new drug application in Japan for the treatment of ulcerative colitis (UC). We are encouraged by the primary endpoint outcome with the MANTA/RAy semen parameter studies as we await the Committee for Medicinal Products for Human Use (CHMP) opinion in UC.
Bart Filius, President and COO, added, “In line with our review, we decided to discontinue or cancel certain studies and consequently identified opportunities to reduce operational costs, for a total potential savings of €150M on a full-year basis. Roughly half of these savings will be realized in 2021, resulting in a 2021 cash burni guidance of between €580 million and €620 million. We are working towards a right-sized, refocused version of Galapagos, setting us on a path towards success with our first commercial product, new R&D opportunities, substantial clinical news flow, and a lengthened cash runway for validation of our early pipeline assets.”

Key figures first quarter report 2021 (unaudited)
(€ millions, except basic & diluted gain/loss (-) per share)
| 31 March 2021 group total |
31 March 2020 group total (*) |
|||||||
| Revenues and other income |
124.2 | 103.6 | ||||||
| R&D expenditure |
(130.0 | ) | (115.5 | ) | ||||
| G&Aii and S&Miii expenses |
(45.0 | ) | (34.3 | ) | ||||
| Operating loss |
(50.8 | ) | (46.2 | ) | ||||
| Fair value re-measurement of financial instruments |
2.0 | (20.5 | ) | |||||
| Net other financial result |
36.2 | 14.8 | ||||||
| Income taxes |
(0.2 | ) | (0.3 | ) | ||||
| Net loss from continuing operations |
(12.8 | ) | (52.3 | ) | ||||
| Net profit from discontinued operations |
22.2 | 1.7 | ||||||
| Net profit/loss (-) of the period |
9.4 | (50.6 | ) | |||||
| Basic gain/loss (-) per share (€) |
0.14 | (0.78 | ) | |||||
| Diluted gain/loss (-) per share (€) |
0.14 | (0.78 | ) | |||||
| Current financial investments and cash and cash equivalents |
5,114.7 | 5,722.4 | ||||||
(*) The 2020 comparatives have been restated to consider the impact of classifying the Fidelta business as discontinued operations in 2020.
Details of the financial results
Due to the sale of our fee-for-service business (Fidelta) to Selvita on 4 January 2021 for a total consideration of €37.1 million (including customary adjustments for net cash and working capital), the results of Fidelta are presented as “Net profit from discontinued operations” in our consolidated income statements for the three months ended 31 March 2021 and 31 March 2020.
Revenues and other income from continuing operations
Our revenues and other income from continuing operations for the first three months of 2021 increased to €124.2 million compared to €103.6 million in the first three months of 2020. Our revenues from the Gilead collaboration in the first three months of 2021 (€113.7 million) related to (i) the exclusive access to our drug discovery platform (€57.8 million), (ii) the filgotinib revenue recognition (€55.3 million) and (iii) royalties (€0.7 million).
Our deferred income balance on 31 March 2021 includes €1.9 billion allocated to our drug discovery platform that is recognized linearly over 10 years, and €0.8 billion allocated for the filgotinib development (including considerations for the previous and the renegotiated collaboration combined) that is recognized over time until the end of the development period.

Results from continuing operations
We realized a net loss from continuing operations of €12.8 million for the first three months of 2021, compared to a net loss of €52.3 million for the first three months of 2020.
We reported an operating loss amounting to €50.8 million for the first three months of 2021, compared to an operating loss of €46.2 million for the same period last year.
Our R&D expenditure in the first three months of 2021 amounted to €130.0 million, compared to €115.5 million for the first three months of 2020. This increase was due to an increase in subcontracting costs primarily related to our filgotinib program, our Toledo program and other clinical programs, compensated by a decrease for ziritaxestat, the OA program with GLPG1972 and the program in atopic dermatitis (AtD) with MOR106. Furthermore, the increase in personnel costs is explained by a planned headcount increase following the growth in our activities, and increased cost of the subscription right plans. This factor, and the increased cost of the commercial launch of filgotinib in Europe, contributed to the increase in our S&M and G&A expenses, which were respectively €14.6 million and €30.4 million in the first three months of 2021, compared to respectively €9.8 million and €24.5 million in the first three months of 2020.
We reported a non-cash fair value gain from the re-measurement of initial warrant B issued to Gilead, amounting to €2.0 million, mainly due to the decreased implied volatility of the Galapagos share price and its evolution between 31 December 2020 and 31 March 2021.
Net other financial income in the first three months of 2021 amounted to €36.2 million, compared to net other financial income of €14.8 million for the first three months of 2020, which was primarily attributable to €45.5 million of currency exchange gain on our cash and cash equivalents and current financial investments in U.S. dollars, and to €6.5 million of negative changes in (fair) value of current financial investments and financial assets.
Results from discontinued operations
The net profit from discontinued operations for the three months ended 31March 2021 consisted of the gain on the sale of Fidelta, our fee-for-services business, for €22.2 million.
Group net results
We reported a group net profit for the first three months of 2021 of €9.4 million, compared to a group net loss of €50.6 million for the first three months of 2020.
Cash position
Current financial investments and cash and cash equivalents totaled €5,114.7 million on 31 March 2021, as compared to €5,169.3 million on 31 December 2020.
Total net decrease in cash and cash equivalents and current financial investments amounted to €54.6 million during the first three months of 2021, compared to a net decrease of €58.4 million during the first three months of 2020. This net decrease was composed of (i) €127.7 million of operational cash burn, (ii) offset by €2.3 million of cash proceeds from capital and share premium increase from exercise of subscription rights in the first three months of 2021, (iii) €3.6 million negative changes in (fair) value of current financial investments and €45.7 million of mainly positive exchange rate differences, (iv) €28.7 million cash in from disposal of subsidiaries, net of cash disposed.
Finally, our balance sheet on 31 March 2021 held a receivable from the French government (Crédit d’Impôt Rechercheiv) and a receivable from the Belgian Government for R&D incentives, for a total of both receivables of €142.3 million.

Outlook 2021
We anticipate several regulatory announcements on filgotinib as well as progress in our differentiated pipeline of novel target-based candidates.
We expect reimbursement decisions in most key European markets for filgotinib in RA this year, as we complete the transition to a full European commercial operation by year end. We anticipate a CHMP opinion and a European Commission decision for filgotinib in UC. We expect that our collaboration partner Gilead will complete recruitment for the global DIVERSITY Phase 3 trial in Crohn’s disease this year.
Within our broader inflammation portfolio, we expect to report topline results from several trials this year, including a Phase 1b trial with TYK2 inhibitor ‘3667 in psoriasis, and three Proof of Concept studies with lead Toledo candidate SIK2/3 inhibitor ‘3970 in psoriasis, UC, and RA.
Within our fibrosis portfolio, we expect to progress early clinical compounds with novel mechanisms of action, with the aim to develop novel treatments to help patients suffering from this debilitating condition.
Following the review of our plans for 2021, we give guidance for full year 2021 operational cash burn of €580 to €620 million.
First quarter report 2021
Galapagos’ financial report for the first three months ended 31 March 2021, including details of the unaudited consolidated results, is accessible via www.glpg.com/financial-reports.
Results of annual ordinary shareholders’ meeting
On 28 April 2021, Galapagos held its annual ordinary shareholders’ meeting. All agenda items were approved, including the re-appointments of Ms. Katrine Bosley and Dr. Raj Parekh as members of the supervisory board, and approval of the remuneration report. All documents relating to the shareholders’ meeting are posted on our website at https://www.glpg.com/shareholders-meetings.
Conference call and webcast presentation
Galapagos will conduct a conference call open to the public tomorrow, 07 May 2021, at 14:00 CET / 8 AM ET, which will also be webcasted. To participate in the conference call, please call one of the following numbers ten minutes prior to commencement:
CODE: 5042688
| Standard International: | +44 (0) 2071 928338 | |
| USA: | +1 646 741 3167 | |
| UK: | +44 844 481 9752 | |
| Netherlands: | +31 207 95 66 14 | |
| France: | +33 1 70 70 0781 | |
| Belgium: | +32 2 793 38 47 |

A question and answer session will follow the presentation of the results. Go to www.glpg.com to access the live audio webcast. The archived webcast will also be available for replay shortly after the close of the call.
Financial calendar
| 05 August 2021 |
Half year 2021 results |
(webcast 06 August 2021) | ||
| 04 November 2021 |
Third quarter 2021 results |
(webcast 05 November 2021) | ||
| 24 February 2022 |
Full year 2021 results |
(webcast 25 February 2022) |
About Galapagos
Galapagos NV discovers and develops small molecule medicines with novel modes of action, several of which show promising patient results and are currently in late-stage development in multiple diseases. Our pipeline comprises discovery through Phase 3 programs in inflammation, fibrosis and other indications. Our ambition is to become a leading global biopharmaceutical company focused on the discovery, development, and commercialization of innovative medicines. More information at www.glpg.com.
Except for filgotinib’s approval for the treatment of rheumatoid arthritis by the European Commission and Japanese Ministry of Health, Labour and Welfare, our drug candidates are investigational; their efficacy and safety have not been fully evaluated by any regulatory authority.
Jyseleca® is a trademark of Galapagos NV and Gilead Sciences, Inc. or its related companies.
Contact
Investors:
Elizabeth Goodwin
VP Investor Relations
+1 781 460 1784
Sofie Van Gijsel
Senior Director Investor Relations
+32 485 19 14 15
Media:
Carmen Vroonen
Global Head of Communications & Public Affairs
+32 473 824 874
Kyra Obolensky
Senior Director Corporate Communications
+32 491 92 64 35
Forward-looking statements
This release may contain forward-looking statements, including, among other things, statements regarding the global R&D collaboration with Gilead, the amount and timing of potential future milestones, opt-in and/or royalty payments by Gilead, Galapagos’ strategic R&D ambitions, including progress on our fibrosis portfolio, and potential changes of such ambitions,

the guidance from management (including guidance regarding the expected operational use of cash during financial year 2021), financial results, statements regarding the expected timing, design and readouts of ongoing and planned clinical trials, including recruitment for trials and topline results for our trials and studies in our inflammation portfolio, statements regarding the strategic re-evaluation, statements relating to interactions with regulatory authorities, the timing or likelihood of additional regulatory authorities’ approval of marketing authorization for filgotinib for RA, UC or any other indication, including UC and IBD indication for filgotinib in Europe, the UK, Japan, and the U.S., such additional regulatory authorities requiring additional studies, the timing or likelihood of pricing and reimbursement interactions for filgotinib, statements relating to the build-up of our commercial organization and commercial sales for filgotinib, including in Europe, the expected impact of COVID-19, and our strategy, business plans and focus. Galapagos cautions the reader that forward-looking statements are not guarantees of future performance. Forward-looking statements involve known and unknown risks, uncertainties and other factors which might cause the actual results, financial condition and liquidity, performance or achievements of Galapagos, or industry results, to be materially different from any historic or future results, financial conditions and liquidity, performance or achievements expressed or implied by such forward-looking statements. In addition, even if Galapagos’ results, performance, financial condition and liquidity, and the development of the industry in which it operates are consistent with such forward-looking statements, they may not be predictive of results or developments in future periods. Among the factors that may result in differences are that our expectations regarding our 2021 revenues and financial results and our 2021 operating expenses may be incorrect (including because one or more of its assumptions underlying its expense expectations may not be realized), Galapagos’ expectations regarding its development programs may be incorrect, the inherent uncertainties associated with competitive developments, clinical trial and product development activities and regulatory approval requirements (including the risk that data from Galapagos’ ongoing and planned clinical research programs in rheumatoid arthritis, Crohn’s disease, ulcerative colitis, idiopathic pulmonary fibrosis, osteoarthritis, and other inflammatory indications may not support registration or further development of its product candidates due to safety, efficacy or other reasons), Galapagos’ reliance on collaborations with third parties (including our collaboration partner Gilead), the timing of and the risks related to implementing the amendment of our arrangement with Gilead for the commercialization and development of filgotinib, estimating the commercial potential of our product candidates and Galapagos’ expectations regarding the costs and revenues associated with the transfer of European commercialization rights to filgotinib may be incorrect, and the uncertainties relating to the impact of the COVID-19 pandemic. A further list and description of these risks, uncertainties and other risks can be found in Galapagos’ Securities and Exchange Commission (SEC) filings and reports, including in Galapagos’ most recent annual report on Form 20-F filed with the SEC and other filings and reports filed by Galapagos with the SEC. Given these uncertainties, the reader is advised not to place any undue reliance on such forward-looking statements. These forward-looking statements speak only as of the date of publication of this document. Galapagos expressly disclaims any obligation to update any such forward-looking statements in this document to reflect any change in its expectations with regard thereto or any change in events, conditions or circumstances on which any such statement is based or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements, unless specifically required by law or regulation.
| i | The operational cash burn (or operational cash flow if this performance measure is positive) is equal to the increase or decrease in our cash and cash equivalents (excluding the effect of exchange rate differences on cash and cash equivalents), minus: |
| i. | the net proceeds, if any, from share capital and share premium increases included in the net cash flows generated from/used in (-) financing activities |
| ii. | the net proceeds or cash used, if any, in acquisitions or disposals of businesses; the movement in restricted cash and movement in current financial investments, if any, included in the net cash flows generated from/used in (-) investing activities. |
This alternative performance measure is in our view an important metric for a biotech company in the development stage.
The operational cash burn for the three months ended 31 March 2021 amounted to €127.7 million and can be reconciled to our cash flow statement by considering the increase in cash and cash equivalents of €379.1 million, adjusted by (i) the cash proceeds from capital and share premium increase from the exercise of subscription rights by employees for €2.3 million, (ii) the net sale of current financial investments amounting to €475.8 million, and (iii) the cash in from sale of subsidiaries, net of cash disposed of, of €28.7 million.
| ii | General and administrative |
| iii | Sales and marketing |
| iv | Crédit d’Impôt Recherche refers to an innovation incentive system underwritten by the French government |
Exhibit 99.2

Exhibit 99.2 Forward with confidence Q1 Report 2021

Contents The Galapagos group Letter from the management 4 COVID-19 impact 9 At a glance. 11 Risk factors 13 The Galapagos share 13 Disclaimer and other information 14 Financial statements Unaudited financial statements condensed consolidated interim 17 Notes 24 Other information Glossary of terms 34 Financial calendar 49 Colophon 49 Contact 50 2 Galapagos NV • Q1 Report 2021

The Galapagos group An overview of Galapagos, its strategy and portfolio in the first three months of 2021 Forward with confidence

THE GALAPAGOS GROUP Letter from the management Dear shareholders, We present you with our report on the first quarter of 2021, one in which we both delivered progress with and faced adversity in our pipeline, while driving our commercial business in Europe through the launch of Jyseleca (filgotinib) in rheumatoid arthritis (RA). We move forward with confidence at Galapagos, applying lessons learned to our pipeline decisions, and re-fitting our organization to the new situation. In February, we announced the end of the development program for ziritaxestat, a molecule in Phase 3 trials in idiopathic pulmonary fibrosis (IPF), due to the unfavorable risk/benefit profile observed by an Independent Data Monitoring Committee (IDMC). As disappointed as we were, and for patients in need of a new treatment option in this very complex, debilitating condition, we continue to work hard on our differentiated pipeline. These last months, we embarked on a review of our plans. We listened to our shareholders and had constructive discussions with our R&D teams and management board members, as well as with our supervisory board. We all agree that our scientific foundations are solid, we have outstanding teams, and we have the financial resources to execute our own programs as well as to enrich our pipeline. Onno van de Stolpe, CEO Bart Filius, President & COO We do not intend to deviate from our mission to build on novel targets to address unmet medical needs in inflammation, fibrosis, and kidney disease. Also, we remain fully committed to the launch of filgotinib in Europe. Our teams are working very hard to ensure our drug reaches the greatest number of patients and we’ve already hit some important milestones in RA. Furthermore, Gilead submitted the new drug application in Japan for filgotinib for the treatment of ulcerative colitis (UC) patients with an inadequate response to conventional therapies, and, in Europe, we anticipate a Committee for Medicinal Products for Human Use (CHMP) opinion on the submission for potential approval in UC later this year. The key objective of our exercise was to refocus development plans for 2021, taking lessons learned on board and right-sizing our organization with the goal to select a more risk-balanced pipeline. 4 Galapagos NV • Q1 Report 2021

THE GALAPAGOS GROUP In the end we made three key decisions: 1. Refocus our clinical pipeline by critically examining its risk profile and breadth; 2. Cut significant cost in the organization to support this re-sized pipeline development; and 3. Task our business development team to identify and execute on a transformative opportunity. Starting with our pipeline, we carefully evaluated the risk-balance and prioritized assets with what we believe have enhanced chances of clinical success in our core therapeutic areas: â–ª We are testing our lead Toledo program ‘3970, a SIK2/3 inhibitor, in five Proof of Concept studies in different indications, and pending on the outcome of the studies, we plan to roll out our further development plans in the second half of the year; â–ª We selected an additional molecule from our Toledo program, SIK2/3 inhibitor ‘4876, as a candidate to accelerate from preclinical phase into clinical development; â–ª We aim to progress our TYK2 inhibitor ‘3667 into Phase 2b; â–ª We selected chitinase inhibitor ’4716 to progress to Phase 2 in IPF and decided to stop development of our other IPF molecule ’1205; â–ª We stopped further work on ‘4059 for metabolic disease, given that this is not a core therapeutic area; â–ª We discontinued our early research efforts in metabolic diseases and osteoarthritis (OA); and â–ª We challenged and fine-tuned our stage-gating process to advance compounds. Our reviewed pipeline offers significant newsflow and we look forward to sharing results this year and next. Class Asset Preclinical Phase 1 Phase 2 Phase 3 Filing Approval In line with our review, we decided to discontinue or cancel certain studies, and we identified opportunities to reduce operational costs, for a total potential savings of €150 million on a full-year basis. Roughly half of these savings will be realized in 2021, resulting in a 2021 cash burn guidance of between €580 million and €620 million. Meanwhile, we remain well on track in launching filgotinib in Europe. In the first quarter, we successfully completed the transitions of commercial and medical teams from Gilead in Germany, the UK, Spain, and Italy. Everything is in place to complete the final transitions from Gilead to us by year-end. Q1 also saw progress on access and reimbursement for filgotinib in RA. We are delighted with the recommendation by the National Institute for Health and Care Excellence (NICE) for filgotinib in the UK for the treatment of eligible adult patients 5 Galapagos NV • Q1 Report 2021

THE GALAPAGOS GROUP with moderate to severe active RA. Furthermore, we received an additional benefit in RA by the Gemeinsamer Bundesausschuss (G-BA) in Germany. We are encouraged by the primary endpoint outcome with the MANTA/RAy semen parameter studies as we await the CHMP opinion in UC. We are pleased that our partner Gilead recently announced an extension of the lockup period for their current holding in Galapagos (16,707,477 shares) until 2024, which is testament to their continued support. Altogether, these adjustments make for a right-sized, refocused version of Galapagos, on a path towards success with our first commercial product as well as with new R&D opportunities, substantial clinical news flow, and a lengthened cash runway for clinical validation of our early assets. Operational overview Q1 2021 In inflammation â–ª We and Gilead announced interim data on the MANTA/RAy studies. 8.3% of patients on placebo and 6.7% of patients on filgotinib had a 50% or more decline in sperm concentration at week 13; these results are being shared with regulatory authorities â–ª We initiated two additional Proof of Concept trials with Toledo compound ’3970, a SIK2/3 inhibitor. The first patients were dosed in both the TAPINOMA study in systemic lupus erythematosus and in the GLIDER study in Sjögren’s syndrome â–ª Gilead announced that NICE recommended filgotinib for reimbursement for moderate to severe RA patients in the UK â–ª We published the FINCH 1 Phase 3 data (Combe et al. 2021) and FINCH 3 Phase 3 data (Westhovens et al. 2021) in the Annals of the Rheumatic Diseases In fibrosis â–ª We discontinued development of ziritaxestat in the ISABELA Phase 3 program in IPF Corporate & other â–ª Bart Filius was promoted to President and Chief Operating Officer â–ª We raised €2.3 million from subscription right exercises Recent events â–ª All annual general meeting (AGM) proposals were approved by shareholders at the AGM on 28 April 2021 â–ª We received a transparency notification from the Capital Group indicating they hold 4.65% of the current outstanding Galapagos shares â–ª Gilead submitted the new drug application in Japan for filgotinib for the treatment of UC with an inadequate response to conventional therapies â–ª We and Gilead ended the DIVERGENCE 2 trial with filgotinib in fistulizing CD â–ª We completed enrollment of the SEA TURTLE Phase 2 study with ‘3970 in UC â–ª We announced an extension of the lockup period for Gilead’s current shares in Galapagos to 2024 â–ª We received the second installment of €75 million from Gilead, following payment of an earlier installment of €35 million in January 2021, included under the revised filgotinib agreement as announced in December 2020 Q1 2021 financial result Details of financial results Due to the sale of our fee-for-service business (Fidelta) to Selvita on 4 January 2021 for a total consideration of €37.1 million (including customary adjustments for net cash and working capital), the results of Fidelta are presented as “Net profit from discontinued operations” in our consolidated income statements. 6 Galapagos NV • Q1 Report 2021

THE GALAPAGOS GROUP Revenues and other income from continuing operations Our revenues and other income from continuing operations for the first three months of 2021 amounted to €124.2 million, compared to €103.6 million for the first three months of 2020. Revenues amounted to €113.9 million for the first three months of 2021 compared to €94.8 million for the first three months of 2020, and were higher mainly driven by the increase in revenue recognition of upfront consideration and milestone payments received in the scope of the collaboration with Gilead for filgotinib amounting to €55.3 million for the first three months of 2021 (€35.4 million for the same period last year). Other income (€10.3 million vs €8.7 million for the same period last year) increased, mainly driven by higher incentives income from the government for our R&D activities. Results from continuing operations We realized a net loss from continuing operations of €12.8 million for the first three months of 2021, compared to a net loss of €52.3 million for the first three months of 2020. We reported an operating loss amounting to €50.8 million for the first three months of 2021, compared to an operating loss of €46.2 million for the same period last year. Our R&D expenditure in the first three months of 2021 amounted to €130.0 million, compared to €115.5 million for the first three months of 2020. This increase was due to an increase in subcontracting costs primarily related to our filgotinib program, our Toledo program and other clinical programs, compensated by a decrease for ziritaxestat, the OA program with GLPG1972 and the program in atopic dermatitis (AtD) with MOR106. Furthermore, personnel costs increased explained by a planned headcount increase following the growth of our activities and increased cost of our subscription right plans. This last factor, together with increased costs of the commercial launch of filgotinib in Europe, contributed to the increase in our G&A and S&M expenses which were €45.0 million in the first three months of 2021, compared to €34.3 million in the first three months of 2020. We reported a non-cash fair value gain from the re-measurement of initial warrant B issued to Gilead, amounting to €2.0 million, mainly due to the decreased implied volatility of the Galapagos share price as well as its evolution between 31 December 2020 and 31 March 2021. Net other financial income in the first three months of 2021 amounted to €36.2 million, compared to net other financial income of €14.8 million for the first three months of 2020, which was primarily attributable to €45.5 million of currency exchange gain on our cash and cash equivalents and current financial investments in U.S. dollars and to €6.5 million of negative changes in (fair) value of current financial investments and financial assets. Results from discontinued operations The net profit from discontinued operations for the three months ended 31 March 2021 consisted of the gain on the sale of Fidelta, our fee-for-services business, for €22.2 million. Group net results The group realized a net profit for the first three months of 2021 amounting to €9.4 million, compared to a net loss of €50.6 million for the same period in 2020. Cash position Current financial investments and cash and cash equivalents totaled €5,114.7 million on 31 March 2021 (€5,169.3 million on 31 December 2020, including the cash and cash equivalents included in the assets classified as held for sale). 7 Galapagos NV • Q1 Report 2021

THE GALAPAGOS GROUP Total net decrease in current financial investments and cash and cash equivalents amounted to €54.6 million in the first three months of 2021, compared to a net decrease of €58.4 million during the first three months of 2020. This net decrease was composed of (i) €127.7 million of operational cash burn,1 (ii) offset by €2.3 million of cash proceeds from capital and share premium increase from exercise of subscription rights in the first three months of 2021, (iii) €3.6 million of negative changes in (fair) value of current financial investments and €45.7 million of mainly positive exchange rate differences, (iv) €28.7 million cash in from the disposal of Fidelta, net of cash disposed. Finally, our balance sheet on 31 March 2021 held a receivable from the French government (Crédit d’Impôt Recherche2) and a receivable from the Belgian Government for R&D incentives, for a total of €142.3 million. Outlook 2021 We anticipate regulatory announcements with filgotinib as well as news on progress in our differentiated pipeline of novel target-based candidates this year. We expect reimbursement decisions in most key European markets for filgotinib in RA this year, as we complete the transition to a full European commercial operation by year end. We anticipate a CHMP opinion and a European Commission decision for filgotinib in UC. We expect that our collaboration partner Gilead will complete recruitment for the global DIVERSITY Phase 3 trial in Crohn’s disease this year. Within our broader inflammation portfolio, we expect to report topline results from several trials this year, including a Phase 1b trial with TYK2 inhibitor ‘3667 in psoriasis and three Proof of Concept studies with lead Toledo candidate SIK2/3 inhibitor ‘3970 in psoriasis, UC, and RA. Within our fibrosis portfolio, we expect to progress early clinical compounds with novel mechanisms of action, with the aim to develop novel treatments to help patients suffering from this debilitating condition. Following the review of our plans for 2021, we give guidance for full year 2021 operational cash burn of €580 to €620 million, and potential savings of €150 million on a full-year basis. We thank you for your continued support, as we move forward on our strategy to develop novel mechanism of action drugs aimed at addressing unmet need in inflammation, fibrosis, and kidney disease. We are confident that we have a strong cash position, expert teams, and excellent science to achieve this. Onno van de Stolpe Bart Filius CEO President & COO 1 We refer to the note on the cash position of our condensed consolidated interim financial statements for an explanation and reconciliation of this alternative performance measure. 2 Crédit d’Impôt Recherche refers to an innovation incentive system underwritten by the French government 8 Galapagos NV • Q1 Report 2021

THE GALAPAGOS GROUP COVID-19 impact During the COVID-19 pandemic, we continue to innovate to accommodate for the current situation and minimize the impact to operations. We closely follow local governmental measures and apply these as appropriate within our organization, guided and supported by our dedicated COVID-19 task force teams. All local and global task force teams meet regularly and make recommendations directly to the COO. We report the following impacts: â–ª Staff We implemented strict measures to help prevent the spread of the virus and protect the health of our staff. We rolled out our global and site-specific business continuity plans and took appropriate recommended precautions, including suspending almost all business travel. We continue to believe that during the pandemic most of the international travel can be replaced by virtual meetings, resulting in improved cost efficiency, a better work-life balance, and a reduced carbon footprint. The positive impact of this forced way- of-working will therefore be retained in our future habits and updated work place strategy, called “To the Next Normal.” During lock-down periods, we arranged for essential tasks to be carried out within our facilities. Employees working on site need an authorization letter signed by the line leader and site head. Consequently, the majority of our Research staff continue working from the offices/labs, with periodic exceptions for local lockdowns during which no staff is allowed to come into the facilities. For those employees coming to the office, we have stringent cleaning and sanitation protocols in place, and we strictly respect social distancing policies at all times in order to minimize risk of exposure. Except for employees with laboratory operations and safety roles which require an on-site presence, over 95% of our staff systematically work from home. As the global pandemic continues into 2021, we anticipate that we will maintain our measures and protocols to ensure the health of our employees. â–ª Research portfolio By prioritizing the most advanced projects very early on, increasing the flexibility of our staff in the labs within projects, and maintaining our hiring efforts and outscourcing as planned, we sustain our research delivery, keep the compound management facility running at all times, and continue our early drug research and the implementation of new modalities for target or drug discovery. So far, the scorecard of the research department objectives shows a similar productivity compared to previous years, indicating that we continue to minimize the impact of the pandemic. 9 Galapagos NV • Q1 Report 2021

THE GALAPAGOS GROUP â–ª Development portfolio We have a business continuity plan for our clinical development programs. We closely monitor each program in the context of the current global and local situation of the pandemic and the associated specific regulatory, institutional, and government guidance and policies related to COVID-19. Within the boundaries of these guidances and policies, and in consultation with our CROs and clinical trial sites, we applied various measures to minimize the impact of the COVID-19 pandemic on our clinical development programs, with the primary aim to ensure the safety of our trial participants and to preserve the data integrity and scientific validity of the trials. These measures continue to be implemented on a case-by-case basis, tailored to the specific study and country needs at any given time, with specific attention paid to vulnerable populations and the use of investigational medicines with immunosuppressive properties. The measures include, among others, increased, transparent communication to all stakeholders and the direct supply of investigational medicines to patients. For each clinical trial, we actively monitor and document the impact of COVID-19 on the study where necessary and to facilitate the interpretation and reporting of results. Following the global increase of COVID-19 testing and vaccinations, we issued an internal guidance on the impact of testing and vaccinations on clinical trials. â–ª Filgotinib filing process UC As of publication of this Q1 report, our collaboration partner Gilead has not been informed by the regulatory agencies in Europe of approval timeline delays related to the pandemic. â–ª Manufacturing and supply chain To date, there has been no COVID-19 impact to the commercial supply of filgotinib. Gilead also confirmed that all sites involved in the manufacturing of filgotinib are established sites that currently manufacture other Gilead marketed products and are in good standing with the FDA and are GMP certified. Under the revised agreement with Gilead for filgotinib in Europe, Galapagos plans to become the marketing authorization holder of filgotinib in Europe by year-end 2021, and then become responsible for manufacturing. We intend to work with the same manufacturing sites to ensure continuity. â–ª Commercial organization The form of outreach of our commercial teams to physicians and hospitals was impacted by the COVID-19 pandemic and consequent travel restrictions, becoming virtual instead. The teams invested in virtual channels as part of the overall commercial build strategy, and these channels are being utilized during our commercial launch today. We note as of now no material impact on our commercial operations due to travel restrictions, nor has there been an impact of COVID-19 on our ability to engage in market access discussions thus far. Nevertheless, healthcare systems are under pressure across Europe, increasing the risk of future volatility in reimbursement procedures and potentially reducing the number of new therapy initiations. 10 Galapagos NV • Q1 Report 2021
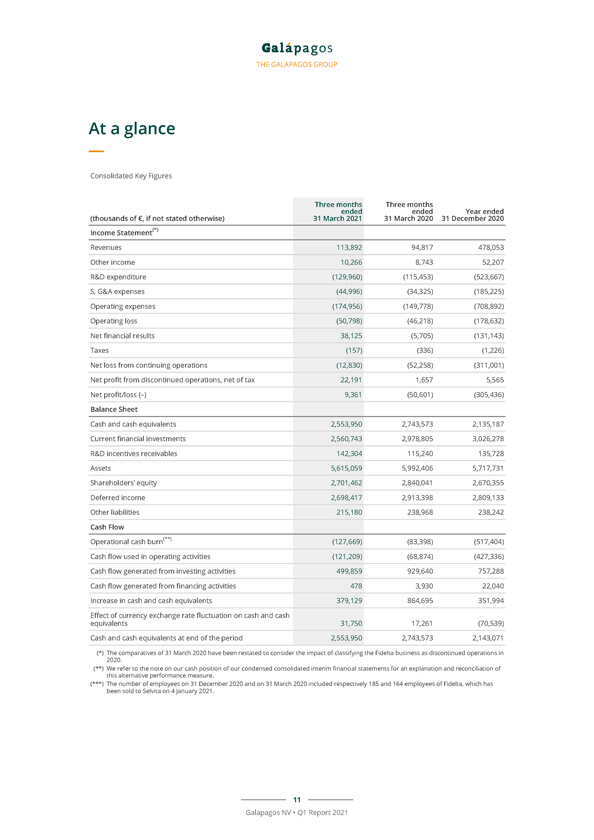
THE GALAPAGOS GROUP At a glance Consolidated Key Figures Three months Three months ended ended Year ended (thousands of €, if not stated otherwise) 31 March 2021 31 March 2020 31 December 2020 Income Statement(*) Revenues 113,892 94,817 478,053 Other income 10,266 8,743 52,207 R&D expenditure (129,960) (115,453) (523,667) S, G&A expenses (44,996) (34,325) (185,225) Operating expenses (174,956) (149,778) (708,892) Operating loss (50,798) (46,218) (178,632) Net financial results 38,125 (5,705) (131,143) Taxes (157) (336) (1,226) Net loss from continuing operations (12,830) (52,258) (311,001) Net profit from discontinued operations, net of tax 22,191 1,657 5,565 Net profit/loss (–) 9,361 (50,601) (305,436) Balance Sheet Cash and cash equivalents 2,553,950 2,743,573 2,135,187 Current financial investments 2,560,743 2,978,805 3,026,278 R&D incentives receivables 142,304 115,240 135,728 Assets 5,615,059 5,992,406 5,717,731 Shareholders’ equity 2,701,462 2,840,041 2,670,355 Deferred income 2,698,417 2,913,398 2,809,133 Other liabilities 215,180 238,968 238,242 Cash Flow Operational cash burn(**) (127,669) (83,398) (517,404) Cash flow used in operating activities (121,209) (68,874) (427,336) Cash flow generated from investing activities 499,859 929,640 757,288 Cash flow generated from financing activities 478 3,930 22,040 Increase in cash and cash equivalents 379,129 864,695 351,994 Effect of currency exchange rate fluctuation on cash and cash equivalents 31,750 17,261 (70,539) Cash and cash equivalents at end of the period 2,553,950 2,743,573 2,143,071 (*) The comparatives of 31 March 2020 have been restated to consider the impact of classifying the Fidelta business as discontinued operations in (**) We 2020 refer . to the note on our cash position of our condensed consolidated interim financial statements for an explanation and reconciliation of (***) The this alternative number of performance employees on measure 31 December . 2020 and on 31 March 2020 included respectively 185 and 164 employees of Fidelta, which has been sold to Selvita on 4 January 2021. 11 Galapagos NV • Q1 Report 2021
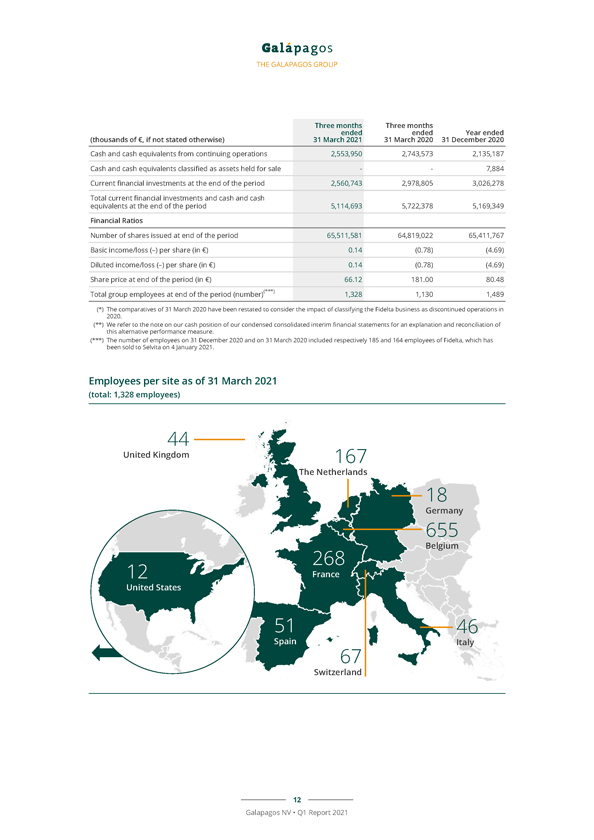
THE GALAPAGOS GROUP Three months Three months ended ended Year ended (thousands of €, if not stated otherwise) 31 March 2021 31 March 2020 31 December 2020 Cash and cash equivalents from continuing operations 2,553,950 2,743,573 2,135,187 Cash and cash equivalents classified as assets held for sale — 7,884 Current financial investments at the end of the period 2,560,743 2,978,805 3,026,278 Total current financial investments and cash and cash equivalents at the end of the period 5,114,693 5,722,378 5,169,349 Financial Ratios Number of shares issued at end of the period 65,511,581 64,819,022 65,411,767 Basic income/loss (–) per share (in €) 0.14 (0.78) (4.69) Diluted income/loss (–) per share (in €) 0.14 (0.78) (4.69) Share price at end of the period (in €) 66.12 181.00 80.48 Total group employees at end of the period (number)(***) 1,328 1,130 1,489 (*) The comparatives of 31 March 2020 have been restated to consider the impact of classifying the Fidelta business as discontinued operations in (**) 2020 We refer . to the note on our cash position of our condensed consolidated interim financial statements for an explanation and reconciliation of (***) The this alternative number of performance employees on measure 31 December . 2020 and on 31 March 2020 included respectively 185 and 164 employees of Fidelta, which has been sold to Selvita on 4 January 2021. Employees per site as of 31 March 2021 (total: 1,328 employees) 12 Galapagos NV • Q1 Report 2021
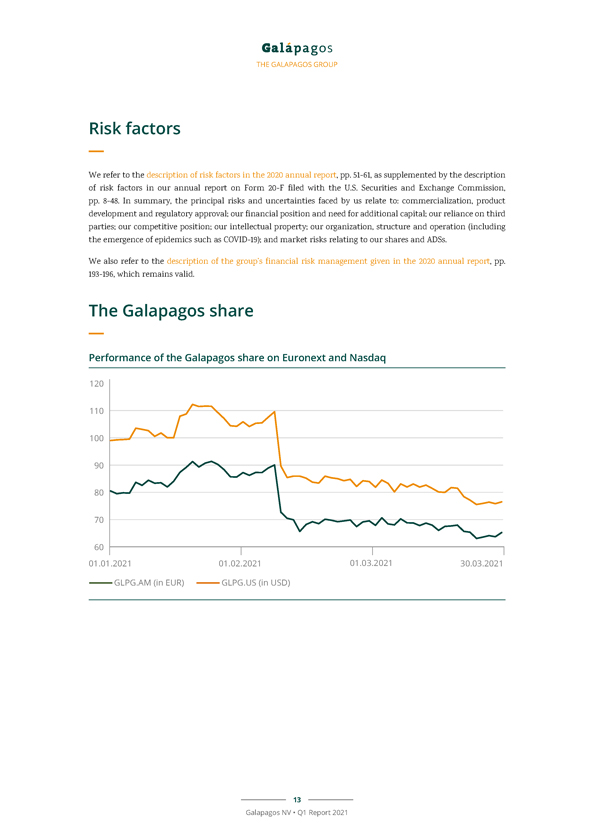
THE GALAPAGOS GROUP Risk factors We refer to the description of risk factors in the 2020 annual report, pp. 51-61, as supplemented by the description of risk factors in our annual report on Form 20-F filed with the U.S. Securities and Exchange Commission, pp. 8-48. In summary, the principal risks and uncertainties faced by us relate to: commercialization, product development and regulatory approval; our financial position and need for additional capital; our reliance on third parties; our competitive position; our intellectual property; our organization, structure and operation (including the emergence of epidemics such as COVID-19); and market risks relating to our shares and ADSs. We also refer to the description of the group’s financial risk management given in the 2020 annual report, pp. 193-196, which remains valid. The Galapagos share Performance of the Galapagos share on Euronext and Nasdaq 13 Galapagos NV • Q1 Report 2021

THE GALAPAGOS GROUP Disclaimer and other information Galapagos NV is a limited liability company organized under the laws of Belgium, having its registered office at Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium. Throughout this report, the term “Galapagos NV” refers solely to the non-consolidated Belgian company and references to “we,” “our,” “the group” or “Galapagos” include Galapagos NV together with its subsidiaries. With the exception of filgotinib’s approval for the treatment of rheumatoid arthritis by the European Commission and Japanese Ministry of Health, Labour and Welfare, our drug candidates mentioned in this report are investigational; their efficacy and safety have not been fully evaluated by any regulatory authority. This report is published in Dutch and in English. In case of inconsistency between the Dutch and the English versions, the Dutch version shall prevail. Galapagos is responsible for the translation and conformity between the Dutch and English version. This report is available free of charge and upon request addressed to: Galapagos NV Investor Relations Generaal De Wittelaan L11 A3 2800 Mechelen, Belgium Tel: +32 15 34 29 00 Email: [email protected] A digital version of this report is available on our website, www.glpg.com. We will use reasonable efforts to ensure the accuracy of the digital version, but we do not assume responsibility if inaccuracies or inconsistencies with the printed document arise as a result of any electronic transmission. Therefore, we consider only the printed version of this report to be legally valid. Other information on our website or on other websites does not form a part of this report. Jyseleca® is a trademark of Galapagos NV and Gilead Sciences, Inc. or its related companies. Listings Euronext Amsterdam and Brussels: GLPG Nasdaq: GLPG Forward-looking statements This report contains forward-looking statements, all of which involve certain risks and uncertainties. These statements are often, but are not always, made through the use of words or phrases such as “believe,” “anticipate,” “expect,” “intend,” “plan,” “seek,” “estimate,” “may,” “will,” “could,” “stand to,” “continue,” as well as similar expressions. Forward-looking statements contained in this report include, but are not limited to, statements made in the “Letter from the management”, the information provided in the section captioned “Outlook 2021”, guidance from management regarding the expected operational use of cash during financial year 2021, statements regarding the amount and timing of potential future milestones, opt-in and/or royalty payments by Gilead, Galapagos’ strategic R&D ambitions, including progress on our fibrosis portfolio, and potential changes of such ambitions, statements regarding the strategic re-evaluation, our statements and expectations regarding commercial sales of filgotinib, statements regarding the global R&D collaboration with Gilead and regarding the amendment of our arrangement with Gilead for the commercialization and development of filgotinib, 14 Galapagos NV • Q1 Report 2021

THE GALAPAGOS GROUP statements regarding the expected timing, design and readouts of ongoing and planned clinical trials (i) with filgotinib in ulcerative colitis and Crohn’s disease, (ii) with GLPG4716 in IPF, (iii) with GLPG3970 in inflammation, ulcerative colitis, rheumatoid arthritis and psoriatic arthritis, (iv) with GLPG0555, GLPG4399 and GLPG3121 in inflammation, (v) with GLPG3667 in psoriasis, (vi) with GLPG2737 in PCKD, (vii) with GLPG4876 in inflammation, and (viii) with GLPG4586 in fibrosis, statements relating to interactions with regulatory authorities, the timing or likelihood of additional regulatory authorities’ approval of marketing authorization for filgotinib for RA, UC or any other indication, including the UC and IBD indications for filgotinib in Europe, the UK, Japan, and the U.S., such additional regulatory authorities requiring additional studies, the timing or likelihood of pricing and reimbursement interactions for filgotinib, statements relating to the build-up of our commercial organization and commercial sales for filgotinib, including in Europe, the expected impact of COVID-19, and our strategy, business plans and focus. We caution the reader that forward-looking statements are not guarantees of future performance. Forward-looking statements may involve known and unknown risks, uncertainties and other factors which might cause our actual results, financial condition and liquidity, performance or achievements, or the development of the industry in which we operate, to be materially different from any historic or future results, financial conditions, performance or achievements expressed or implied by such forward-looking statements. In addition, even if our results of operations, financial condition and liquidity, and the development of the industry in which we operate are consistent with such forward-looking statements, they may not be predictive of results or developments in future periods. Among the factors that may result in differences are that our expectations regarding our 2021 revenues and financial results and our 2021 operating expenses may be incorrect (including because one or more of our assumptions underlying our revenue or expense expectations may not be realized), the inherent uncertainties associated with competitive developments, clinical trial and product development activities and regulatory approval requirements (including the risk that data from our ongoing and planned clinical research programs in rheumatoid arthritis, Crohn’s disease, ulcerative colitis, idiopathic pulmonary fibrosis, osteoarthritis, and other inflammatory indications may not support registration or further development of our product candidates due to safety, efficacy, or other reasons), our reliance on collaborations with third parties (including our collaboration partner, Gilead), the timing of and the risks related to implementing the amendment of our arrangement with Gilead for the commercialization and development of filgotinib, estimating the commercial potential of our product candidates and Galapagos’ expectations regarding the costs and revenues associated with the transfer of European commercialization rights to filgotinib may be incorrect, and the uncertainties relating to the impact of the COVID-19 pandemic. A further list and description of these risks, uncertainties and other risks can be found in our Securities and Exchange Commission filing and reports, including in our most recent annual report on Form 20-F filed with the SEC and our subsequent filings and reports filed with the SEC. We also refer to the “Risk Factors” section of this report. Given these uncertainties, the reader is advised not to place any undue reliance on such forward-looking statements. These forward-looking statements speak only as of the date of publication of this document. We expressly disclaim any obligation to update any such forward-looking statements in this document to reflect any change in our expectations with regard thereto or any change in events, conditions or circumstances on which any such statement is based or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements, unless specifically required by law or regulation. 15 Galapagos NV • Q1 Report 2021

Financial statements Unaudited condensed consolidated interim financial statements for the first three months of 2021 Forward with confidence
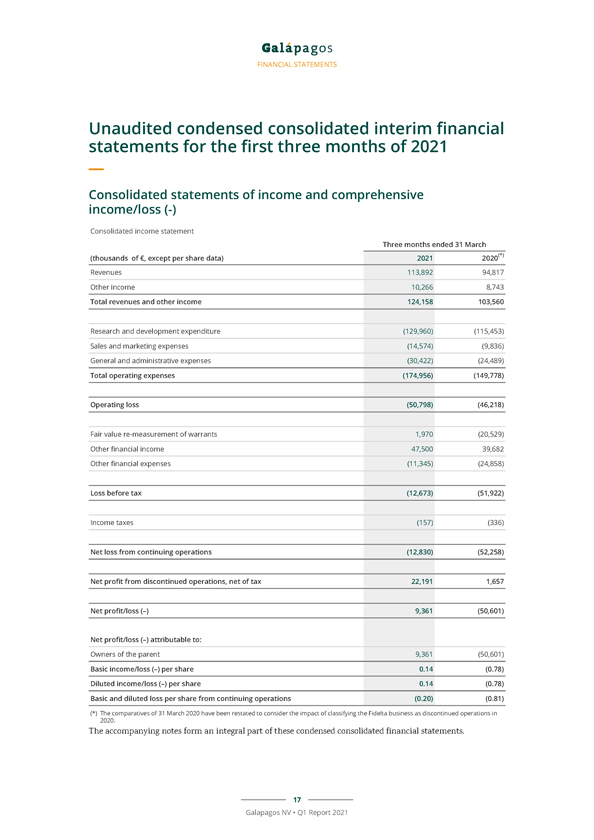
FINANCIAL STATEMENTS Unaudited condensed consolidated interim financial statements for the first three months of 2021 Consolidated statements of income and comprehensive income/loss (-) Consolidated income statement Three months ended 31 March (thousands of €, except per share data) 2021 2020(*) Revenues 113,892 94,817 Other income 10,266 8,743 Total revenues and other income 124,158 103,560 Research and development expenditure (129,960) (115,453) Sales and marketing expenses (14,574) (9,836) General and administrative expenses (30,422) (24,489) Total operating expenses (174,956) (149,778) Operating loss (50,798) (46,218) Fair value re-measurement of warrants 1,970 (20,529) Other financial income 47,500 39,682 Other financial expenses (11,345) (24,858) Loss before tax (12,673) (51,922) Income taxes (157) (336) Net loss from continuing operations (12,830) (52,258) Net profit from discontinued operations, net of tax 22,191 1,657 Net profit/loss (–) 9,361 (50,601) Net profit/loss (–) attributable to: Owners of the parent 9,361 (50,601) Basic income/loss (–) per share 0.14 (0.78) Diluted income/loss (–) per share 0.14 (0.78) Basic and diluted loss per share from continuing operations (0.20) (0.81) (*) The comparatives of 31 March 2020 have been restated to consider the impact of classifying the Fidelta business as discontinued operations in 2020. The accompanying notes form an integral part of these condensed consolidated financial statements. 17 Galapagos NV • Q1 Report 2021
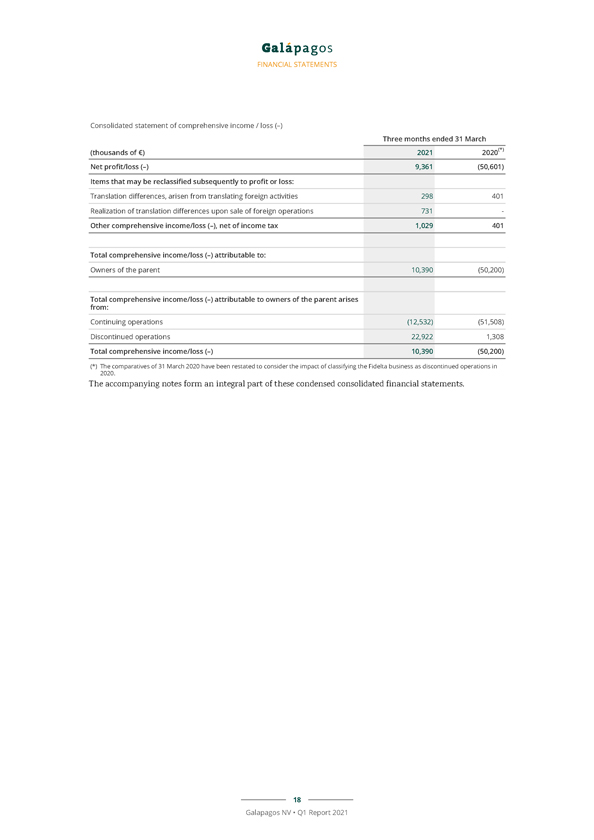
FINANCIAL STATEMENTS Consolidated statement of comprehensive income / loss (–) Three months ended 31 March (thousands of €) 2021 2020(*) Net profit/loss (–) 9,361 (50,601) Items that may be reclassified subsequently to profit or loss: Translation differences, arisen from translating foreign activities 298 401 Realization of translation differences upon sale of foreign operations 731—Other comprehensive income/loss (–), net of income tax 1,029 401 Total comprehensive income/loss (–) attributable to: Owners of the parent 10,390 (50,200) Total comprehensive income/loss (–) attributable to owners of the parent arises from: Continuing operations (12,532) (51,508) Discontinued operations 22,922 1,308 Total comprehensive income/loss (–) 10,390 (50,200) (*) The comparatives of 31 March 2020 have been restated to consider the impact of classifying the Fidelta business as discontinued operations in 2020. The accompanying notes form an integral part of these condensed consolidated financial statements. 18 Galapagos NV • Q1 Report 2021
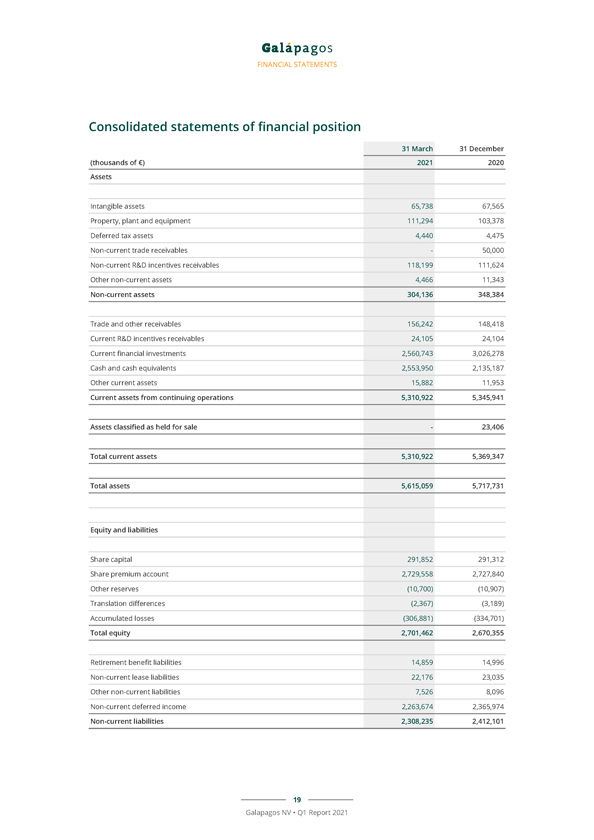
FINANCIAL STATEMENTS Consolidated statements of financial position 31 March 31 December (thousands of €) 2021 2020 Assets Intangible assets 65,738 67,565 Property, plant and equipment 111,294 103,378 Deferred tax assets 4,440 4,475 Non-current trade receivables—50,000 Non-current R&D incentives receivables 118,199 111,624 Other non-current assets 4,466 11,343 Non-current assets 304,136 348,384 Trade and other receivables 156,242 148,418 Current R&D incentives receivables 24,105 24,104 Current financial investments 2,560,743 3,026,278 Cash and cash equivalents 2,553,950 2,135,187 Other current assets 15,882 11,953 Current assets from continuing operations 5,310,922 5,345,941 Assets classified as held for sale—23,406 Total current assets 5,310,922 5,369,347 Total assets 5,615,059 5,717,731 Equity and liabilities Share capital 291,852 291,312 Share premium account 2,729,558 2,727,840 Other reserves (10,700) (10,907) Translation differences (2,367) (3,189) Accumulated losses (306,881) (334,701) Total equity 2,701,462 2,670,355 Retirement benefit liabilities 14,859 14,996 Non-current lease liabilities 22,176 23,035 Other non-current liabilities 7,526 8,096 Non-current deferred income 2,263,674 2,365,974 Non-current liabilities 2,308,235 2,412,101 19 Galapagos NV • Q1 Report 2021
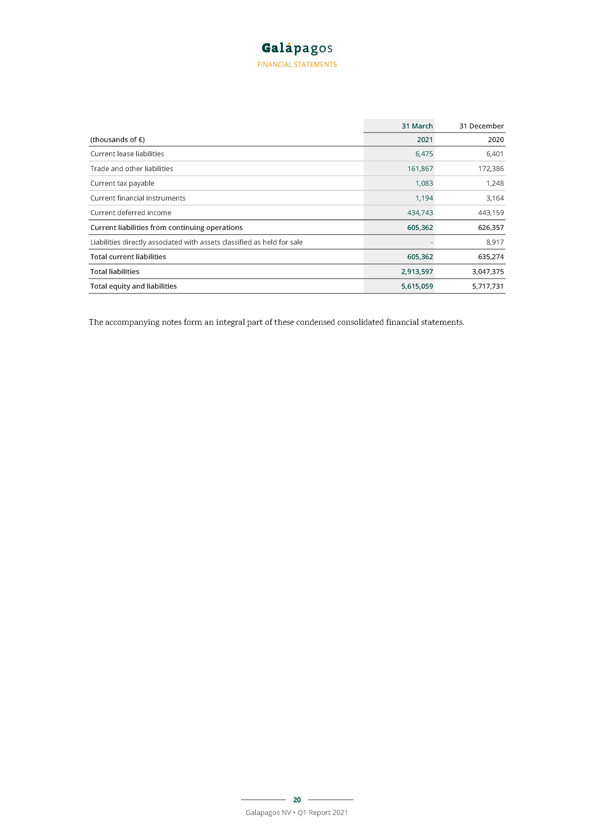
FINANCIAL STATEMENTS 31 March 31 December (thousands of €) 2021 2020 Current lease liabilities 6,475 6,401 Trade and other liabilities 161,867 172,386 Current tax payable 1,083 1,248 Current financial instruments 1,194 3,164 Current deferred income 434,743 443,159 Current liabilities from continuing operations 605,362 626,357 Liabilities directly associated with assets classified as held for sale—8,917 Total current liabilities 605,362 635,274 Total liabilities 2,913,597 3,047,375 Total equity and liabilities 5,615,059 5,717,731 The accompanying notes form an integral part of these condensed consolidated financial statements. 20 Galapagos NV • Q1 Report 2021
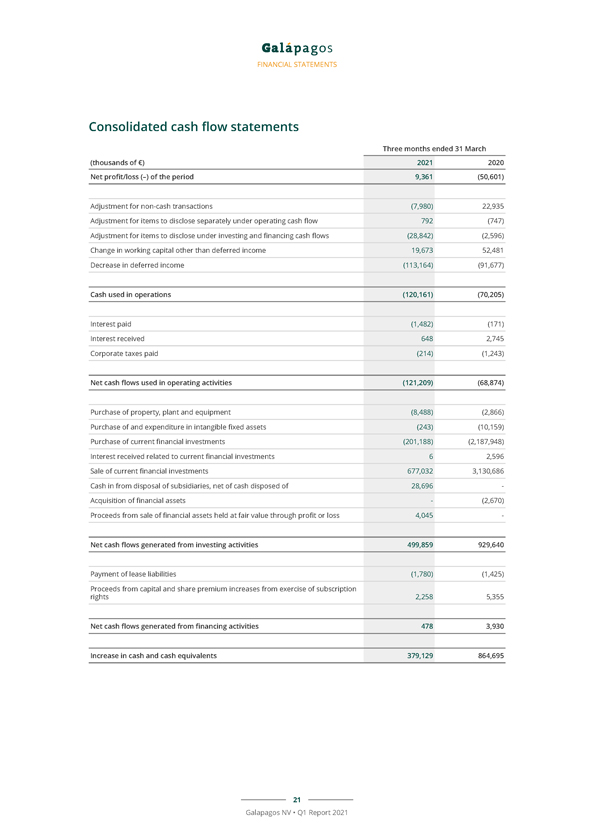
FINANCIAL STATEMENTS Consolidated cash flow statements Three months ended 31 March (thousands of €) 2021 2020 Net profit/loss (–) of the period 9,361 (50,601) Adjustment for non-cash transactions (7,980) 22,935 Adjustment for items to disclose separately under operating cash flow 792 (747) Adjustment for items to disclose under investing and financing cash flows (28,842) (2,596) Change in working capital other than deferred income 19,673 52,481 Decrease in deferred income (113,164) (91,677) Cash used in operations (120,161) (70,205) Interest paid (1,482) (171) Interest received 648 2,745 Corporate taxes paid (214) (1,243) Net cash flows used in operating activities (121,209) (68,874) Purchase of property, plant and equipment (8,488) (2,866) Purchase of and expenditure in intangible fixed assets (243) (10,159) Purchase of current financial investments (201,188) (2,187,948) Interest received related to current financial investments 6 2,596 Sale of current financial investments 677,032 3,130,686 Cash in from disposal of subsidiaries, net of cash disposed of 28,696—Acquisition of financial assets—(2,670) Proceeds from sale of financial assets held at fair value through profit or loss 4,045—Net cash flows generated from investing activities 499,859 929,640 Payment of lease liabilities (1,780) (1,425) Proceeds from capital and share premium increases from exercise of subscription rights 2,258 5,355 Net cash flows generated from financing activities 478 3,930 Increase in cash and cash equivalents 379,129 864,695 21 Galapagos NV • Q1 Report 2021
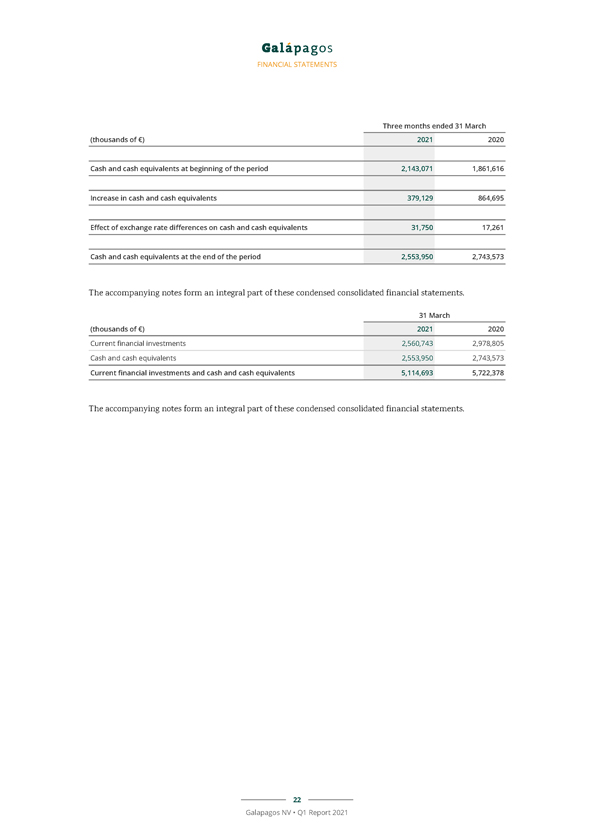
FINANCIAL STATEMENTS Three months ended 31 March (thousands of €) 2021 2020 Cash and cash equivalents at beginning of the period 2,143,071 1,861,616 Increase in cash and cash equivalents 379,129 864,695 Effect of exchange rate differences on cash and cash equivalents 31,750 17,261 Cash and cash equivalents at the end of the period 2,553,950 2,743,573 The accompanying notes form an integral part of these condensed consolidated financial statements. 31 March (thousands of €) 2021 2020 Current financial investments 2,560,743 2,978,805 Cash and cash equivalents 2,553,950 2,743,573 Current financial investments and cash and cash equivalents 5,114,693 5,722,378 The accompanying notes form an integral part of these condensed consolidated financial statements. 22 Galapagos NV • Q1 Report 2021

FINANCIAL STATEMENTS Consolidated statements of changes in equity Share premium Translation Other Accumul. (thousands of €) Share capital account differences reserves Losses Total On 1 January 2020 287,282 2,703,583 (1,142) (4,842) (109,223) 2,875,658 Net loss (50,601) (50,601) Other comprehensive income/loss (–) 478 (77) 401 Total comprehensive income/loss (–) 478 (77) (50,601) (50,200) Share-based compensation 9,227 9,227 Exercise of subscription rights 824 4,531 5,355 On 31 March 2020 288,106 2,708,114 (663) (4,919) (150,597) 2,840,041 On 1 January 2021 291,312 2,727,840 (3,189) (10,907) (334,701) 2,670,355 Net profit 9,361 9,361 Other comprehensive income 822 207 1,029 Total comprehensive income 822 207 9,361 10,390 Share-based compensation 18,459 18,459 Exercise of subscription rights 540 1,718 2,258 On 31 March 2021 291,852 2,729,558 (2,367) (10,700) (306,881) 2,701,462 The accompanying notes form an integral part of these condensed consolidated financial statements. 23 Galapagos NV • Q1 Report 2021

FINANCIAL STATEMENTS Notes to the unaudited condensed consolidated interim financial statements for the first three months of 2021 Basis of preparation These condensed consolidated interim financial statements have been prepared in accordance with IAS 34 ‘Interim Financial Reporting’ as adopted by the European Union and as issued by the IASB. The condensed consolidated interim financial statements do not contain all information required for an annual report and should therefore be read in conjunction with our Annual Report 2020. Impact of COVID-19 on the financial statements To date, we have experienced limited impact on our financial performance, financial position, cash flows and significant judgements and estimates, although we continue to face additional risks and challenges associated with the impact of the outbreak. We refer to the section Covid-19 impact in this Q1 report for a comprehensive overview of the impact of Covid-19 on the business evolution of Galapagos. Significant accounting policies There were no significant changes in accounting policies applied by us in these condensed consolidated interim financial statements compared to those used in the most recent annual consolidated financial statements of 31 December 2020. New standards and interpretations applicable for the annual period beginning on 1 January 2021 did not have any impact on our condensed consolidated interim financial statements. We have not early adopted any other standard, interpretation, or amendment that has been issued but is not yet effective. 24 Galapagos NV • Q1 Report 2021
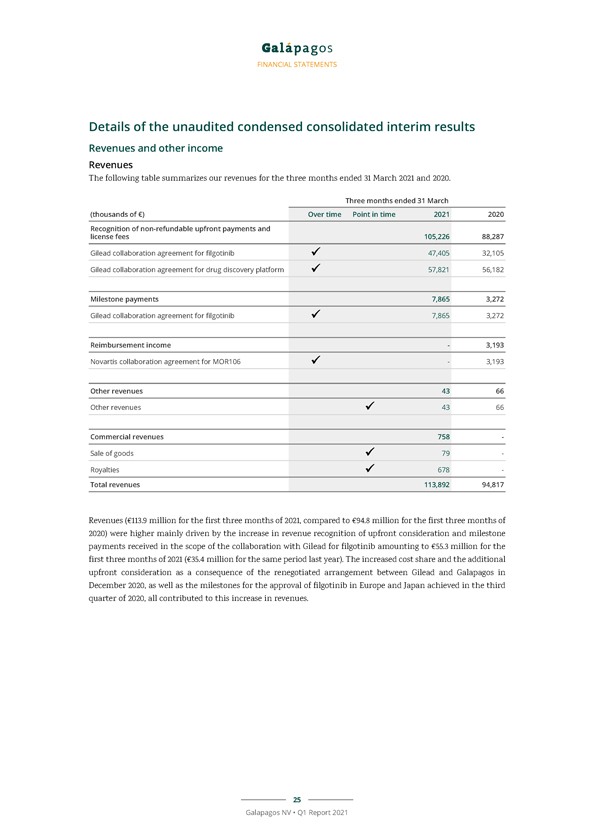
FINANCIAL STATEMENTS Details of the unaudited condensed consolidated interim results Revenues and other income Revenues The following table summarizes our revenues for the three months ended 31 March 2021 and 2020. Three months ended 31 March (thousands of €) Over time Point in time 2021 2020 Recognition of non-refundable upfront payments and license fees 105,226 88,287 Gilead collaboration agreement for filgotinib 47,405 32,105 Gilead collaboration agreement for drug discovery platform 57,821 56,182 Milestone payments 7,865 3,272 Gilead collaboration agreement for filgotinib 7,865 3,272 Reimbursement income—3,193 Novartis collaboration agreement for MOR106—3,193 Other revenues 43 66 Other revenues 43 66 Commercial revenues 758—Sale of goods 79—Royalties 678—Total revenues 113,892 94,817 Revenues (€113.9 million for the first three months of 2021, compared to €94.8 million for the first three months of 2020) were higher mainly driven by the increase in revenue recognition of upfront consideration and milestone payments received in the scope of the collaboration with Gilead for filgotinib amounting to €55.3 million for the first three months of 2021 (€35.4 million for the same period last year). The increased cost share and the additional upfront consideration as a consequence of the renegotiated arrangement between Gilead and Galapagos in December 2020, as well as the milestones for the approval of filgotinib in Europe and Japan achieved in the third quarter of 2020, all contributed to this increase in revenues. 25 Galapagos NV • Q1 Report 2021
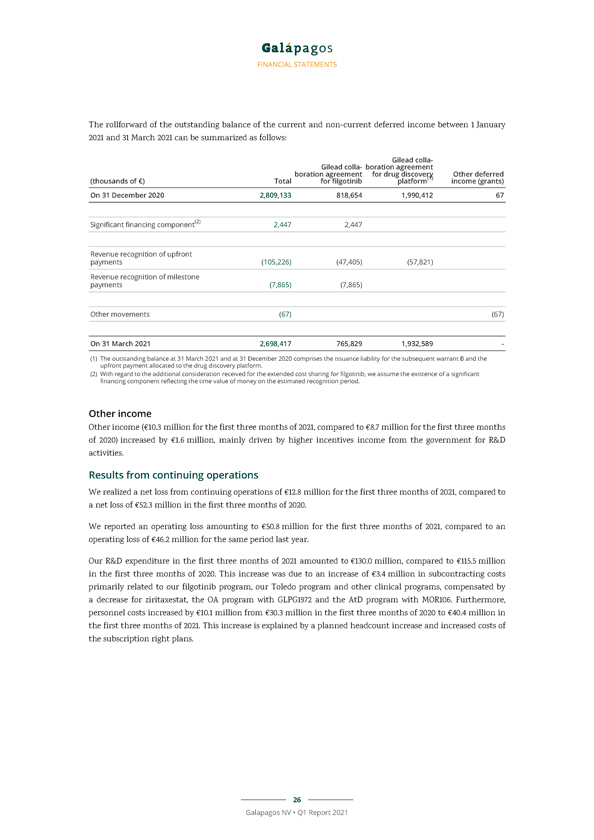
FINANCIAL STATEMENTS The rollforward of the outstanding balance of the current and non-current deferred income between 1 January 2021 and 31 March 2021 can be summarized as follows: Gilead colla- Gilead colla- boration agreement boration agreement for drug discovery (1) Other deferred (thousands of €) Total for filgotinib platform income (grants) On 31 December 2020 2,809,133 818,654 1,990,412 67 Significant financing component(2) 2,447 2,447 Revenue recognition of upfront payments (105,226) (47,405) (57,821) Revenue recognition of milestone payments (7,865) (7,865) Other movements (67) (67) On 31 March 2021 2,698,417 765,829 1,932,589—(1) The outstanding balance at 31 March 2021 and at 31 December 2020 comprises the issuance liability for the subsequent warrant B and the (2) With upfront regard payment to the allocated additional to consideration the drug discovery received platform. for the extended cost sharing for filgotinib, we assume the existence of a significant financing component reflecting the time value of money on the estimated recognition period. Other income Other income (€10.3 million for the first three months of 2021, compared to €8.7 million for the first three months of 2020) increased by €1.6 million, mainly driven by higher incentives income from the government for R&D activities. Results from continuing operations We realized a net loss from continuing operations of €12.8 million for the first three months of 2021, compared to a net loss of €52.3 million in the first three months of 2020. We reported an operating loss amounting to €50.8 million for the first three months of 2021, compared to an operating loss of €46.2 million for the same period last year. Our R&D expenditure in the first three months of 2021 amounted to €130.0 million, compared to €115.5 million in the first three months of 2020. This increase was due to an increase of €3.4 million in subcontracting costs primarily related to our filgotinib program, our Toledo program and other clinical programs, compensated by a decrease for ziritaxestat, the OA program with GLPG1972 and the AtD program with MOR106. Furthermore, personnel costs increased by €10.1 million from €30.3 million in the first three months of 2020 to €40.4 million in the first three months of 2021. This increase is explained by a planned headcount increase and increased costs of the subscription right plans. 26 Galapagos NV • Q1 Report 2021
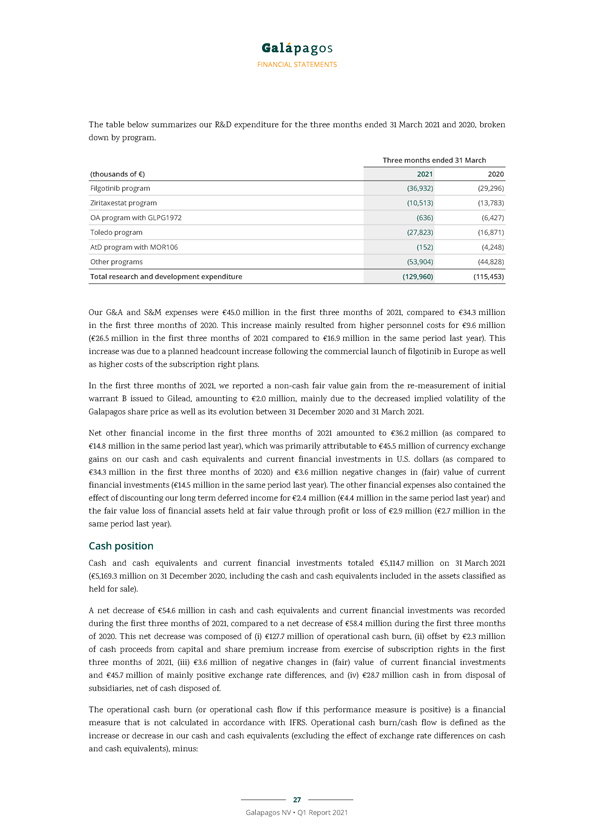
FINANCIAL STATEMENTS The table below summarizes our R&D expenditure for the three months ended 31 March 2021 and 2020, broken down by program. Three months ended 31 March (thousands of €) 2021 2020 Filgotinib program (36,932) (29,296) Ziritaxestat program (10,513) (13,783) OA program with GLPG1972 (636) (6,427) Toledo program (27,823) (16,871) AtD program with MOR106 (152) (4,248) Other programs (53,904) (44,828) Total research and development expenditure (129,960) (115,453) Our G&A and S&M expenses were €45.0 million in the first three months of 2021, compared to €34.3 million in the first three months of 2020. This increase mainly resulted from higher personnel costs for €9.6 million (€26.5 million in the first three months of 2021 compared to €16.9 million in the same period last year). This increase was due to a planned headcount increase following the commercial launch of filgotinib in Europe as well as higher costs of the subscription right plans. In the first three months of 2021, we reported a non-cash fair value gain from the re-measurement of initial warrant B issued to Gilead, amounting to €2.0 million, mainly due to the decreased implied volatility of the Galapagos share price as well as its evolution between 31 December 2020 and 31 March 2021. Net other financial income in the first three months of 2021 amounted to €36.2 million (as compared to €14.8 million in the same period last year), which was primarily attributable to €45.5 million of currency exchange gains on our cash and cash equivalents and current financial investments in U.S. dollars (as compared to €34.3 million in the first three months of 2020) and €3.6 million negative changes in (fair) value of current financial investments (€14.5 million in the same period last year). The other financial expenses also contained the effect of discounting our long term deferred income for €2.4 million (€4.4 million in the same period last year) and the fair value loss of financial assets held at fair value through profit or loss of €2.9 million (€2.7 million in the same period last year). Cash position Cash and cash equivalents and current financial investments totaled €5,114.7 million on 31 March 2021 (€5,169.3 million on 31 December 2020, including the cash and cash equivalents included in the assets classified as held for sale). A net decrease of €54.6 million in cash and cash equivalents and current financial investments was recorded during the first three months of 2021, compared to a net decrease of €58.4 million during the first three months of 2020. This net decrease was composed of (i) €127.7 million of operational cash burn, (ii) offset by €2.3 million of cash proceeds from capital and share premium increase from exercise of subscription rights in the first three months of 2021, (iii) €3.6 million of negative changes in (fair) value of current financial investments and €45.7 million of mainly positive exchange rate differences, and (iv) €28.7 million cash in from disposal of subsidiaries, net of cash disposed of. The operational cash burn (or operational cash flow if this performance measure is positive) is a financial measure that is not calculated in accordance with IFRS. Operational cash burn/cash flow is defined as the increase or decrease in our cash and cash equivalents (excluding the effect of exchange rate differences on cash and cash equivalents), minus: 27 Galapagos NV • Q1 Report 2021
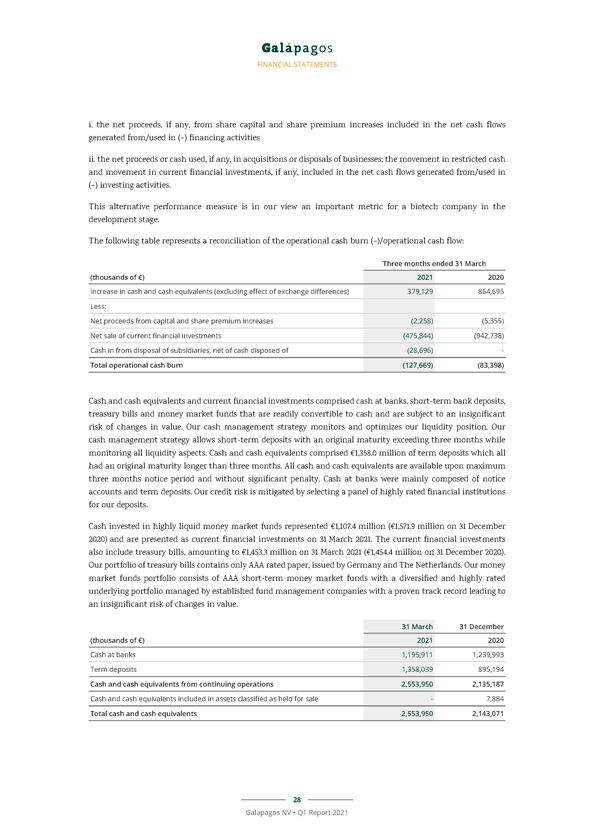
FINANCIAL STATEMENTS i. the net proceeds, if any, from share capital and share premium increases included in the net cash flows generated from/used in (–) financing activities ii. the net proceeds or cash used, if any, in acquisitions or disposals of businesses; the movement in restricted cash and movement in current financial investments, if any, included in the net cash flows generated from/used in (–) investing activities. This alternative performance measure is in our view an important metric for a biotech company in the development stage. The following table represents a reconciliation of the operational cash burn (–)/operational cash flow: Three months ended 31 March (thousands of €) 2021 2020 Increase in cash and cash equivalents (excluding effect of exchange differences) 379,129 864,695 Less: Net proceeds from capital and share premium increases (2,258) (5,355) Net sale of current financial investments (475,844) (942,738) Cash in from disposal of subsidiaries, net of cash disposed of (28,696)—Total operational cash burn (127,669) (83,398) Cash and cash equivalents and current financial investments comprised cash at banks, short-term bank deposits, treasury bills and money market funds that are readily convertible to cash and are subject to an insignificant risk of changes in value. Our cash management strategy monitors and optimizes our liquidity position. Our cash management strategy allows short-term deposits with an original maturity exceeding three months while monitoring all liquidity aspects. Cash and cash equivalents comprised €1,358.0 million of term deposits which all had an original maturity longer than three months. All cash and cash equivalents are available upon maximum three months notice period and without significant penalty. Cash at banks were mainly composed of notice accounts and term deposits. Our credit risk is mitigated by selecting a panel of highly rated financial institutions for our deposits. Cash invested in highly liquid money market funds represented €1,107.4 million (€1,571.9 million on 31 December 2020) and are presented as current financial investments on 31 March 2021. The current financial investments also include treasury bills, amounting to €1,453.3 million on 31 March 2021 (€1,454.4 million on 31 December 2020). Our portfolio of treasury bills contains only AAA rated paper, issued by Germany and The Netherlands. Our money market funds portfolio consists of AAA short-term money market funds with a diversified and highly rated underlying portfolio managed by established fund management companies with a proven track record leading to an insignificant risk of changes in value. 31 March 31 December (thousands of €) 2021 2020 Cash at banks 1,195,911 1,239,993 Term deposits 1,358,039 895,194 Cash and cash equivalents from continuing operations 2,553,950 2,135,187 Cash and cash equivalents included in assets classified as held for sale—7,884 Total cash and cash equivalents 2,553,950 2,143,071 28 Galapagos NV • Q1 Report 2021
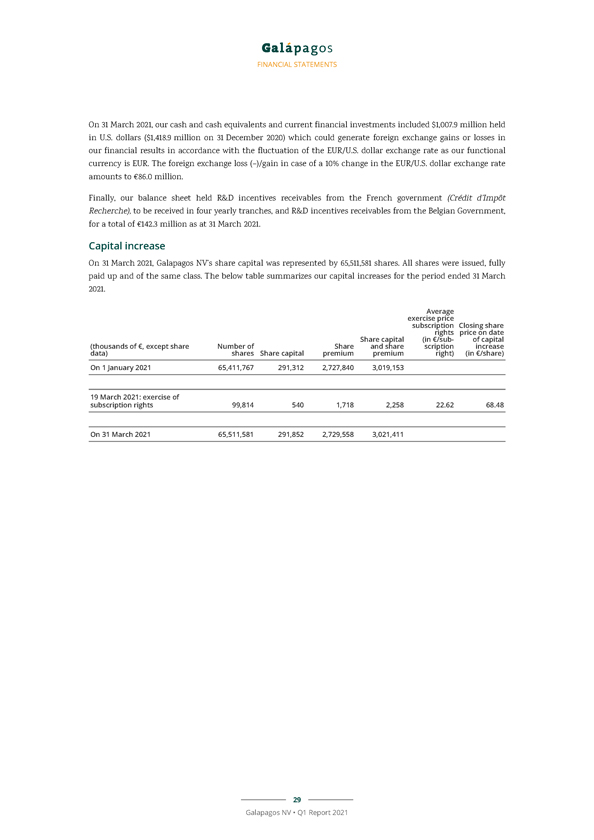
FINANCIAL STATEMENTS On 31 March 2021, our cash and cash equivalents and current financial investments included $1,007.9 million held in U.S. dollars ($1,418.9 million on 31 December 2020) which could generate foreign exchange gains or losses in our financial results in accordance with the fluctuation of the EUR/U.S. dollar exchange rate as our functional currency is EUR. The foreign exchange loss (–)/gain in case of a 10% change in the EUR/U.S. dollar exchange rate amounts to €86.0 million. Finally, our balance sheet held R&D incentives receivables from the French government (Crédit d’Impôt Recherche), to be received in four yearly tranches, and R&D incentives receivables from the Belgian Government, for a total of €142.3 million as at 31 March 2021. Capital increase On 31 March 2021, Galapagos NV’s share capital was represented by 65,511,581 shares. All shares were issued, fully paid up and of the same class. The below table summarizes our capital increases for the period ended 31 March 2021. Average exercise price subscription Closing share rights price on date Share capital (in €/sub- of capital (thousands of €, except share Number of Share and share scription increase data) shares Share capital premium premium right) (in €/share) On 1 January 2021 65,411,767 291,312 2,727,840 3,019,153 19 March 2021: exercise of subscription rights 99,814 540 1,718 2,258 22.62 68.48 On 31 March 2021 65,511,581 291,852 2,729,558 3,021,411 29 Galapagos NV • Q1 Report 2021
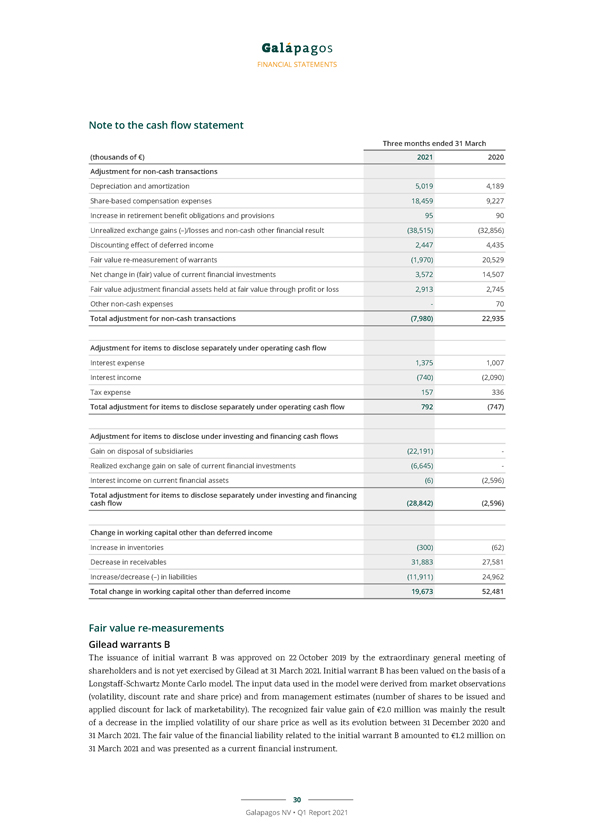
FINANCIAL STATEMENTS Note to the cash flow statement Three months ended 31 March (thousands of €) 2021 2020 Adjustment for non-cash transactions Depreciation and amortization 5,019 4,189 Share-based compensation expenses 18,459 9,227 Increase in retirement benefit obligations and provisions 95 90 Unrealized exchange gains (–)/losses and non-cash other financial result (38,515) (32,856) Discounting effect of deferred income 2,447 4,435 Fair value re-measurement of warrants (1,970) 20,529 Net change in (fair) value of current financial investments 3,572 14,507 Fair value adjustment financial assets held at fair value through profit or loss 2,913 2,745 Other non-cash expenses—70 Total adjustment for non-cash transactions (7,980) 22,935 Adjustment for items to disclose separately under operating cash flow Interest expense 1,375 1,007 Interest income (740) (2,090) Tax expense 157 336 Total adjustment for items to disclose separately under operating cash flow 792 (747) Adjustment for items to disclose under investing and financing cash flows Gain on disposal of subsidiaries (22,191)—Realized exchange gain on sale of current financial investments (6,645)—Interest income on current financial assets (6) (2,596) Total adjustment for items to disclose separately under investing and financing cash flow (28,842) (2,596) Change in working capital other than deferred income Increase in inventories (300) (62) Decrease in receivables 31,883 27,581 Increase/decrease (–) in liabilities (11,911) 24,962 Total change in working capital other than deferred income 19,673 52,481 Fair value re-measurements Gilead warrants B The issuance of initial warrant B was approved on 22 October 2019 by the extraordinary general meeting of shareholders and is not yet exercised by Gilead at 31 March 2021. Initial warrant B has been valued on the basis of a Longstaff-Schwartz Monte Carlo model. The input data used in the model were derived from market observations (volatility, discount rate and share price) and from management estimates (number of shares to be issued and applied discount for lack of marketability). The recognized fair value gain of €2.0 million was mainly the result of a decrease in the implied volatility of our share price as well as its evolution between 31 December 2020 and 31 March 2021. The fair value of the financial liability related to the initial warrant B amounted to €1.2 million on 31 March 2021 and was presented as a current financial instrument. 30 Galapagos NV • Q1 Report 2021
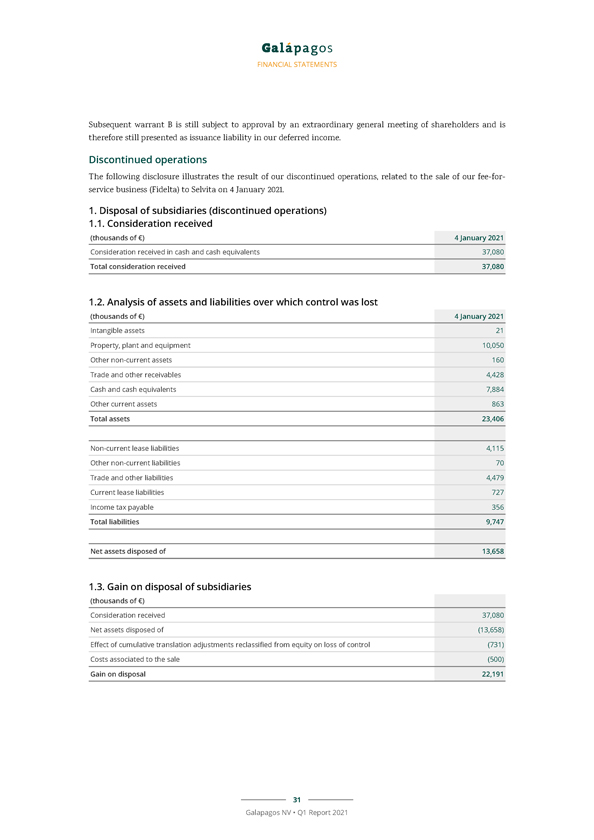
FINANCIAL STATEMENTS Subsequent warrant B is still subject to approval by an extraordinary general meeting of shareholders and is therefore still presented as issuance liability in our deferred income. Discontinued operations The following disclosure illustrates the result of our discontinued operations, related to the sale of our fee-for- service business (Fidelta) to Selvita on 4 January 2021. 1. Disposal of subsidiaries (discontinued operations) 1.1. Consideration received (thousands of €) 4 January 2021 Consideration received in cash and cash equivalents 37,080 Total consideration received 37,080 1.2. Analysis of assets and liabilities over which control was lost (thousands of €) 4 January 2021 Intangible assets 21 Property, plant and equipment 10,050 Other non-current assets 160 Trade and other receivables 4,428 Cash and cash equivalents 7,884 Other current assets 863 Total assets 23,406 Non-current lease liabilities 4,115 Other non-current liabilities 70 Trade and other liabilities 4,479 Current lease liabilities 727 Income tax payable 356 Total liabilities 9,747 Net assets disposed of 13,658 1.3. Gain on disposal of subsidiaries (thousands of €) Consideration received 37,080 Net assets disposed of (13,658) Effect of cumulative translation adjustments reclassified from equity on loss of control (731) Costs associated to the sale (500) Gain on disposal 22,191 31 Galapagos NV • Q1 Report 2021
FINANCIAL STATEMENTS 1.4. Net cash inflow on disposal of subsidiaries (thousands of €) Consideration received in cash and cash equivalents 37,080 Less: cash and cash equivalents balances disposed of (7,884) Total consideration received, net of cash disposed of 29,196 Costs associated to the sale (500) Cash in from disposal of subsidiaries, net of cash disposed of 28,696 2. Result from discontinued operations Three months ended 31 March (thousands of €, except share and per share data) 2021 2020 Revenues—3,356 Other income — Total revenues and other income—3,356 Gain on disposal of subsidiaries 22,191 Research and development expenditure—(1,310) Sales and marketing expenses—General and administrative expenses—(413) Total operating expenses—(1,723) Operating profit 22,191 1,633 Other financial income—40 Other financial expenses—(16) Profit before tax 22,191 1,657 Income taxes — Net profit 22,191 1,657 Basic income per share from discontinued operations 0.34 0.03 Diluted income per share from discontinued operations 0.34 0.02 Weighted average number of shares—Basic (in thousands of shares) 65,425 64,690 Weighted average number of shares—Diluted (in thousands of shares) 65,944 68,123 32 Galapagos NV • Q1 Report 2021
FINANCIAL STATEMENTS 3. Cash flows from discontinued operations Three months ended 31 March (thousands of €) 2021 2020 Net cash flows generated from operating activities—1,998 Net cash flows generated from/used in (-) investing activities 28,696 (447) Net cash flows used in financing activities—(183) Net cash flows from discontinued operations 28,696 1,368 Contingencies and commitments Contractual obligations and commitments We have certain purchase commitments principally with CRO subcontractors and certain collaboration partners. On 31 March 2021, we had outstanding obligations for purchase commitments, which become due as follows: Less than More than (thousands of €) Total 1 year 1 – 3 years 3 – 5 years 5 years Purchase commitments 319,267 252,913 52,599 13,093 662 In addition to the table above, we have a contractual cost sharing obligation related to our collaboration agreement with Gilead for filgotinib. The contractual cost sharing commitment amounted to €482.8 million at 31 March 2021 for which we have direct purchase commitments of €23.5 million at 31 March 2021 reflected in the table above. Contingent liabilities and assets We refer to our Annual Report 2020 for a description of our contingent liabilities and assets. Events after the end of the reporting period On 30 April 2021, the supervisory board of Galapagos approved “Subscription Right Plan 2021 RMV”, a subscription right plan intended for the employees of its French subsidiary, Galapagos SASU, “Subscription Right Plan 2021 ROW”, a subscription right plan intended for the employees of its other non-Belgian subsidiairies and “Subscription Right Plan 2021 BE”, a subscription right plan intended for the employees of the company and its Belgian subsidiaries, within the framework of the authorized capital. Under these subscription right plans, 2,736,250 subscription rights were created, subject to acceptances, and offered to the beneficiaries of the plans. The subscription rights have an exercise term of eight years as of the date of the offer and have an exercise price of €64.76 (the closing price of the share on Euronext Amsterdam and Brussels on the day preceding the date of the offer). The subscription rights are not transferable. Subscription rights granted under Subscription Right Plan 2021 BE can in principle not be exercised prior to 1 January 2025 and subscription rights granted under Subscription Right Plan 2021 RMV and Subscription Right Plan 2021 ROW vest in instalments: with 25% of each grant being exercisable as of 1 January 2023, 25% as of 1 January 2024 and 50% (the remainder) as of 1 January 2025. Each subscription right gives the right to subscribe to one new Galapagos share. We note that Dr. Rajesh Parekh and Ms. Katrine Bosley were re-appointed as members of the supervisory board by the shareholders’ meeting of 28 April 2021 for a period of four years and for a period of one year respectively. Ms. Katrine Bosley is an independent supervisory board member within the meaning of article 7:87 of the Belgian Companies Code and article 3.5 of the Belgian Corporate Governance Code 2020. Approval of interim financial statements The interim financial statements were approved by the management board on 5 May 2021. 33 Galapagos NV • Q1 Report 2021

OTHER INFORMATION Glossary of terms 100 points clinical response Percentage of patients achieving a 100-point decrease in CDAI score during a clinical trial in CD patients ACR American College of Rheumatology ACR20 (ACR 20/50/70) American College of Rheumatology 20% response rate signifies a 20% or greater improvement in the number of swollen and tender joints as well as a 20% or greater improvement in three out of five other disease-activity measures. ACR50 and ACR70 reflect the same, for 50% and 70% response rates, respectively Adenovirus A common virus that causes cold-like symptoms and is used as a research tool for the lab in the discovery of new drugs ADPKD Autosomal dominant polycystic kidney disease, a disease where typically both kidneys become enlarged with fluid-filled cysts, leading to kidney failure. Other organs may be affected as well ADS American Depositary Share; Galapagos has a Level 3 ADS listed on Nasdaq with ticker symbol GLPG and CUSIP number 36315X101. One ADS is equivalent to one ordinary share in Galapagos NV AFM Dutch Authority for the Financial Markets Anemia Condition in which the patient has an inadequate number of red blood cells to carry oxygen to the body’s tissues Ankylosing spondylitis (AS) AS is a systemic, chronic, and progressive spondyoloarthropathy primarily affecting the spine and sacroiliac joints, and progressing into severe inflammation that fuses the spine, leading to permanent painful stiffness of the back Anti-TNF Tumor necrosis factor. An anti-TNF drug acts by modulation of TNF Assays Laboratory tests to determine characteristics 34 Galapagos NV • Q1 Report 2021

OTHER INFORMATION ATS ATS, the American Thoracic Society improves global health by advancing research, patient care, and public health in pulmonary disease, critical illness, and sleep disorders Attrition rate The historical success rate for drug discovery and development, based on publicly known development paths. Statistically seen, investment in at least 12 target-based programs is required to ensure that at least one of these will reach a Phase 3 study. Most new drug R&D programs are discontinued before reaching Phase 3 because they are not successful enough to be approved Autotaxin (ATX) An enzyme important for generating the signaling molecule lypophosphatidic acid (LPA). Ziritaxestat targets autotaxin for IPF and SSc BID dosing Twice-daily dosing (bis in die) Bioavailability Assessment of the amount of product candidate that reaches a body’s systemic circulation after (oral) administration Biomarker Substance used as an indicator of a biological process, particularly to determine whether a product candidate has a biological effect Black & Scholes model A mathematical description of financial markets and derivative investment instruments that is widely used in the pricing of European options and subscription rights Bleomycin model A preclinical model involving use of bleomycin (a cancer medication) to induce IPF symptoms Bridging trial Clinical trial performed to “bridge” or extrapolate one dataset to that for another situation, i.e. to extrapolate data from one population to another for the same drug candidate, or to move from IV to subcutaneous dosing CALOSOMA Phase 1 program with GLPG3970 in psoriasis Cash position Current financial investments and cash and cash equivalents CDAI Crohn’s Disease Activity Index, evaluating patients on eight different factors, each of which has a pre-defined weight as a way to quantify the impact of CD 35 Galapagos NV • Q1 Report 2021

OTHER INFORMATION CDAI remission In the FITZROY trial, the percentage of patients with CD who showed a reduction of CDAI score to <150 CFTR Cystic fibrosis transmembrane conductance regulator (CFTR) is a membrane protein and chloride channel in vertebrates that is encoded by the CFTR gene. It is hypothesized that inhibition of the CFTR channel might reduce cyst growth and enlargement for patients with ADPKD. GLPG2737 is a CFTR inhibitor CHIT1/AMCase Chitotriosidase (CHIT1) is a protein coding gene, and AMCase is an inactive acidic mamalian chitinase. CHIT1 is predominantly involved in macrophage activation. Inhibition of chitinase activity translates into a potential therapeutic benefit in lung diseases like IPF, as shown in preclinical models. GLPG4716 is a CHIT1/AMCase inhibitor targeting a key pathway in tissue remodeling CHMP Committee for Medicinal Products for Human Use is the European Medicines Agency’s (EMA) committee responsible for human medicines and plays a vital role in the authorization of medicines in the European Union (EU) CIR Crédit d’Impôt Recherche, or research credit. Under the CIR, the French government refunds up to 30% of the annual investment in French R&D operations, over a period of three years. Galapagos benefits from the CIR through its operations in Romainville, just outside Paris Clinical Proof of Concept (PoC) Point in the drug development process where the product candidate first shows efficacy in a therapeutic setting Complete Response Letter (CRL) A letter send by the FDA to indicate that the review cycle for an application is complete and the application is not ready for approval in its present form Compound A chemical substance, often a small molecule with drug-like properties Contract research organization (CRO) Organization which provides drug discovery and development services to the pharmaceutical, biotechnology and medical devices industry Corticosteroids Any of a group of steroid hormones produced in the adrenal cortex or made synthetically. They have various metabolic functions and some are used to treat inflammation Crohn’s disease (CD) An IBD involving inflammation of the small and large intestines, leading to pain, bleeding, and ultimately in some cases surgical removal of parts of the bowel 36 Galapagos NV • Q1 Report 2021

OTHER INFORMATION CRP C-reactive protein is a protein found in the blood, the levels of which rise in response to inflammation Cytokine A category of small proteins which play important roles in signaling in processes in the body DARWIN Phase 2 program for filgotinib in RA. DARWIN 1 explored three doses, in twice-daily and once-daily administration, for up to 24 weeks in RA patients with insufficient response to methotrexate (MTX) and who remained on their stable background treatment with MTX. DARWIN 2 explored three once-daily doses for up to 24 weeks in RA patients with insufficient response to methotrexate (MTX) and who washed out of their treatment with MTX. DARWIN 1 and 2 were double-blind, placebo-controlled trials which recruited approximately 900 patients globally and for which results were reported in 2015. DARWIN 3 is a long term extension trial in which all patients are on 200 mg filgotinib, except for U.S. males who are on 100 mg. The week 156 results from DARWIN 3 were reported in 2019 DDI study Drug-drug interaction study. This type of study will assess if there is a change in the action or side effects of a drug caused by concomitant administration with another drug Deep venous thrombosis (DVT) The formation of one or more blood clots in one of the body’s large veins, most commonly in the lower limbs. The blood clots can travel to the lung and cause a pulmonary embolism Degradation The process by which proteins are lost through the use of drugs such as PROTACs or small molecules Development All activities required to bring a new drug to the market. This includes preclinical and clinical development research, chemical and pharmaceutical development and regulatory filings of product candidates Discovery Process by which new medicines are discovered and/or designed. At Galapagos, this is the department that oversees target and drug discovery research through to nomination of preclinical candidates Disease-modifying Addresses the disease itself, modifying the disease progression, not just the symptoms of the disease DIVERGENCE Phase 2 programs with filgotinib in Crohn’s disease. DIVERGENCE 1 was an exploratory study in small bowel CD and DIVERGENCE 2 in fistulizing CD DIVERSITY Phase 3 program evaluating filgotinib in CD 37 Galapagos NV • Q1 Report 2021
OTHER INFORMATION DLCO DLCO (diffusion capacity of the lung for carbon monoxide) is the extent to which oxygen passes from the air sacs of the lungs into the blood. This is measured in IPF patients DMARDs Disease modifying anti rheumatic drugs; these drugs address the disease itself rather than just the symptoms Dose-range finding study Phase 2 clinical study exploring the balance between efficacy and safety among various doses of treatment in patients. Results are used to determine doses for later studies Double-blind Term to characterize a clinical trial in which neither the physician nor the patient knows if the patient is taking placebo or the treatment being evaluated Efficacy Effectiveness for intended use EMA European Medicines Agency, in charge of European market authorization of new medications Endoscopy A non-surgical procedure involving use of an endoscope to examine a person’s digestive tract Esbriet An approved drug (pirfenidone) for IPF, marketed by Roche Fast Track A designation by the FDA of an investigational drug for expedited review to facilitate development of drugs which treat a serious or life-threatening condition and fill an unmet medical need FDA The U.S. Food and Drug Administration is an agency responsible for protecting and promoting public health and in charge of American market approval of new medications Fee-for-service Payment system where the service provider is paid a specific amount for each procedure or service performed Fibrotic score The Ashcroft fibrotic score involves measuring pulmonary fibrosis through examination of histopathology tissue FIH First-in-human clinical trial, usually conducted in healthy volunteers with the aim to assess the safety, tolerability and pharmacokinetics of the product candidate 38 Galapagos NV • Q1 Report 2021

OTHER INFORMATION Filgotinib Formerly known as GLPG0634, commercial name is Jyseleca. Small molecule preferential JAK1 inhibitor, approved in RA in Europa and Japan. Application for approval for ulcerative colitis was filed in Europe and Japan. Filgotinib is partnered with Gilead. Filgotinib currently is in Phase 3 trials in CD FINCH Phase 3 program evaluating filgotinib in RA Fistulizing CD Fistulae are inflammatory tracts that most often occur between the distal colon and the perianal region. Fistulae are one of the most severe sequelae of luminal CD and the lifetime risk of occurrence is close to 50% of those with active CD FITZROY A double-blind, placebo controlled Phase 2 trial with filgotinib in 177 CD patients for up to 20 weeks. Full results were published in The Lancet in 2016 FORM 20-F Form 20-F is an SEC filing submitted to the US Securities and Exchange Commission FRI Functional respiratory imaging is a technology which enhances 3D visualization and quantification of a patient’s airway and lung geometry FSMA The Belgian market authority: Financial Services and Markets Authority, or Autoriteit voor Financiële Diensten en Markten FTE Full-time equivalent; a way to measure an employee’s involvement in a project. For example, an FTE of 1.0 means that the equivalent work of one full-time worker was used on the project Futility analysis Analysis of the likelihood of a trial to meet its primary endpoint, based on a subset of the total information to be gathered. The term ‘futility’ is used to refer to the low likelihood of a clinical trial to achieve its objectives. In particular, stopping a clinical trial when the interim results suggest that it is unlikely to achieve statistical significance can save resources that could be used on more promising research FVC Forced vital capacity is the amount of air which can be forcibly exhaled from the lungs after taking the deepest breath possible. FVC is used to help determine both the presence and severity of lung diseases such as IPF G&A expenses General & administrative expenses 39 Galapagos NV • Q1 Report 2021

OTHER INFORMATION Genome An organism’s complete set of genetic information needed to build that organism and allow it to grow and develop GLIDER Phase 2 Proof of Concept trial with SIK2/SIK3 inhibitor GLPG3970 in Sjögren’s syndrome GLPG0555 A JAK1 inhibitor currently in Phase 1b in osteoarthritis GLPG0634 Molecule number currently known as filgotinib and Jyseleca GLPG1205 A GPR84 inhibitor discovered by us. Development of GLPG1205 was deprioritized in 2021 GLPG1690 Autotaxin inhibitor discovered by us and currently known as ziritaxestat. All development with ziritaxestat was discontinued in February 2021 GLPG2737 A compound currently in Phase 2 in PKCD. This compound is part of the CF collaboration with AbbVie but Galapagos retained rights outside of CF GLPG3121 A compound currently in Phase 1 targeting JAK1/TYK2 directed toward inflammation GLPG3667 A TYK2 kinase inhibitor discovered by us, currently in Phase 1b in psoriasis GLPG3970 A SIK2/SIK3 inhibitor currently in multiple Phase 2 Proof of Concept studies. Currently the lead molecule in the Toledo program GLPG4059 A compound currently in Phase 1 with undisclosed mode of action directed toward metabolic diseases. Development of GLPG4059 was deprioritized in 2021 GLPG4399 A SIK3 inhibitor currently in the preclinical phase directed toward inflammation GLPG4586 A compound with undisclosed mode of action currently in the preclinical phase directed toward fibrosis. This is the first preclinical candidate to emerge from the collaboration with Fibrocor 40 Galapagos NV • Q1 Report 2021
OTHER INFORMATION GLPG4605 A SIK2/SIK3 inhibitor in the preclinical phase, currently directed toward fibrosis GLPG4716 A chitinase inhibitor inlicensed from OncoArendi in preparation for Phase 2 in IPF GLPG4876 A SIK2/SIK3 inhibitor in the preclinical phase, currently directed toward inflammation. This molecule was selected for acceleration in 2021 GPR84 inhibitor Drug candidate aimed at inhibiting or blocking G-protein coupled receptor 84. GLPG1205 is a GPR84 inhibitor aimed at IPF HDL High-density lipoprotein. HDL scavenges and reduces low-density lipoprotein (LDL) which contributes to heart disease at high levels. High levels of HDL reduce the risk for heart disease, while low levels of HDL increase the risk of heart disease Hemoglobin A protein inside red blood cells that carries oxygen from the lungs to tissues and organs in the body and carries carbon dioxide back to the lungs Histology Study of the microscopic structures of tissues Histopathology Microscopic examination of tissues for manifestations of a disease IBD Inflammatory Bowel Disease. This is a general term for an autoimmune disease affecting the bowel, including CD and UC. CD affects the small and large intestine, while UC affects the large intestine. Both diseases involve inflammation of the intestinal wall, leading to pain, bleeding, and ultimately, in some cases, surgical removal of part of the bowel In-/out-licensing Receiving/granting permission from/to another company or institution to use a brand name, patent, or other proprietary right, in exchange for a fee and/or royalty In vitro Studies performed with cells outside their natural context, for example in a laboratory In vivo Studies performed with animals in a laboratory setting Inflammatory diseases A large, unrelated group of disorders associated with abnormalities in inflammation 41 Galapagos NV • Q1 Report 2021

OTHER INFORMATION Inspiratory capacity Total lung capacity or the amount of gas contained in the lung at the end of a maximal inhalation Intellectual property Creations of the mind that have commercial value and are protected or protectable, including by patents, trademarks or copyrights Intersegment Occurring between the different operations of a company Investigational New Drug (IND) Application United States Federal law requires a pharmaceutical company to obtain an exemption to ship an experimental drug across state lines, usually to clinical investigators, before a marketing application for the drug has been approved. The IND is the means by which the sponsor obtains this exemption, allowing them to perform clinical studies IPF Idiopathic pulmonary fibrosis. A chronic and ultimately fatal disease characterized by a progressive decline in lung function. Pulmonary fibrosis involves scarring of lung tissue and is the cause of shortness of breath. Fibrosis is usually associated with a poor prognosis. The term “idiopathic” is used because the cause of pulmonary fibrosis is still unknown ISABELA Phase 3 clinical program investigating ziritaxestat in IPF patients. All development with ziritaxestat was discontinued in February 2021 JAK Janus kinases (JAK) are critical components of signaling mechanisms utilized by a number of cytokines and growth factors, including those that are elevated in RA. Filgotinib is a preferential JAK1 inhibitor Jyseleca® Jyseleca® is the brand name for filgotinib LADYBUG Phase 2 program with GLPG3970 in rheumatoid arthritis LDL Low-density lipoprotein. LDL contributes to heart disease at high levels Lipoprotein Lipoproteins are substances made of protein and fat that carry cholesterol through your bloodstream. There are two main types of cholesterol: High-density lipoprotein (HDL), or “good” cholesterol and Low-density lipoprotein (LDL), or “bad” cholesterol Liver enzymes Inflamed or injured liver cells secrete higher than normal amounts of certain chemicals, including liver enzymes, into the bloodstream 42 Galapagos NV • Q1 Report 2021
OTHER INFORMATION Lymphocyte Type of white blood cell that is part of the immune system MACE Major adverse cardiovascular events; a composite endpoint frequently used in cardiovascular research MANTA A Phase 2 semen parameter trial with filgotinib in male patients with CD or UC MANTA-RAy Phase 2 semen parameter trial with filgotinib in male patients with RA, PsA, or AS MHLW Japanese Ministry of Health, Labor and Welfare (MHLW), in charge of Japanese market authorization of new medications Milestone Major achievement in a project or program; in our alliances, this is usually associated with a payment Modulation The process by which the function of proteins is changed through the use of drugs such as small molecules, peptides, antibodies or cell therapy Molecule collections Chemical libraries, usually consisting of drug-like small molecules that are designed to interact with specific target classes. These collections can be screened against a target to generate initial “hits” in a drug discovery program MTX Methotrexate; a first-line therapy for inflammatory diseases NDA New Drug Application Neutrophil Type of immune system cell which is one of the first cell types to travel to the site of an infection in the body. Neutrophils are another type of white blood cell which fight infection by ingesting and killing microorganisms NICE The National Institute for Health and Care Excellence; an independent public body that provides national guidance and advice to improve health and social care in the UK NK cells Natural killer cells, type of white blood cell with granules of enzymes which can attack tumors or viruses 43 Galapagos NV • Q1 Report 2021

OTHER INFORMATION Ofev An approved drug (nintedanib) for IPF, marketed by Boehringer Ingelheim Oligonucleotide Short DNA or RNA molecule that can be used as research tools or therapeutic drug to change protein expression Oral dosing Administration of medicine by the mouth, either as a solution or solid (capsule, pill) form Osteoarthritis (OA) The most common form of arthritis, usually occurring after middle age, marked by chronic breakdown of cartilage in the joints leading to pain, stiffness, and swelling Outsourcing Contracting work to a third party PCKD Polycystic kidney disease is a genetic disorder in which the renal tubules become structurally abnormal, resulting in the development and growth of multiple cysts within the kidney Pharmacokinetics (PK) Study of what a body does to a drug; the fate of a substance delivered to a body. This includes absorption, distribution to the tissues, metabolism and excretion. These processes determine the blood concentration of the drug and its metabolite(s) as a function of time from dosing Phase 1 First stage of clinical testing of an investigational drug designed to assess the safety and tolerability, pharmacokinetics of a drug, usually performed in a small number of healthy human volunteers Phase 2 Second stage of clinical testing, usually performed in no more than several hundred patients, in order to determine efficacy, tolerability and the dose to use Phase 3 Large clinical trials, usually conducted in several hundred to several thousand patients to gain a definitive understanding of the efficacy and tolerability of the candidate treatment; serves as the principal basis for regulatory approval Phenotypic screening Phenotypic screening is a strategy used in drug discovery to identify molecules with the ability to alter a cell’s disease characteristics. Animal models and cell-based assays are both strategies used to identify these molecules. In contrast to target-based drug discovery, phenotypic screening does not rely on knowing the identity of the specific drug target or its hypothetical role in the disease. A key benefit this approach has over target-based screening, is its capacity to capture complex biological mechanisms that are not otherwise achievable Pivotal trials Registrational clinical trials 44 Galapagos NV • Q1 Report 2021

OTHER INFORMATION Placebo-controlled A substance having no pharmacological effect but administered as a control in testing a biologically active preparation Preclinical Stage of drug research development, undertaken prior to the administration of the drug to humans. Consists of in vitro and in vivo screening, pharmacokinetics, toxicology, and chemical upscaling Preclinical candidate (PCC) A new molecule and potential drug that meets chemical and biological criteria to begin the development process Product candidate Substance that has satisfied the requirements of early preclinical testing and has been selected for development, starting with formal preclinical safety evaluation followed by clinical testing for the treatment of a certain disorder in humans Proof of Concept (POC) A clinical trial in which first evidence for efficacy of a candidate drug is gathered. A Proof of Concept trial is usually with a small number of patients and for short duration to get a first impression of drug activity Proof of Concept study Phase 2 patient study in which activity as well as safety in patients is evaluated, usually for a new mechanism of action PROTAC Proteolysis targeting chimera, a special small molecule capable of removing unwanted proteins that play a role in disease processes Psoriasis A chronic skin disease which results in scaly, often itchy areas in patches. Psoriatic arthritis (PsA) Psoriatic arthritis or PsA is an inflammatory form of arthritis, affecting up to 30% of psoriasis patients. Psoriatic arthritis can cause swelling, stiffness and pain in and around the joints, and cause nail changes and overall fatigue Pulmonary embolism A blockage in one of the pulmonary arteries in the lungs QD dosing Once-daily dosing (qd from the Latin quaque die) R&D operations Research and development operations; unit responsible for discovery and developing new product candidates for internal pipeline or as part of risk/reward sharing alliances with partners 45 Galapagos NV • Q1 Report 2021

OTHER INFORMATION Replication The process by which DNA is copied to produce two identical DNA molecules during the process of cell division Rheumatoid arthritis (RA) A chronic, systemic inflammatory disease that causes joint inflammation, and usually leads to cartilage destruction, bone erosion and disability Screening Method usually applied at the beginning of a drug discovery campaign, where a target is tested in a biochemical assay against a series of small molecules or antibodies to obtain an initial set of “hits” that show activity against the target. These hits are then further tested or optimized SEA TURTLE Phase 2 program with GLPG3970 in ulcerative colitis SEC Securities and Exchange Commission in the US SELECTION Phase 3 program evaluating filgotinib in UC patients SES-CD scores Simple endoscopic score for CD, involving review of five pre-defined bowel segments, assigning values from 0 (unaffected) to 3 (highly affected) Short interfering RNA A research tool that is used to silence the activity of specific genes SIK Salt-inducible kinase. This is the target family for the portfolio of molecules in the Toledo program Sjögrens syndrome Sjögren’s Syndrome is a systemic inflammatory disease which can be felt throughout the body, often resulting in chronic dryness of the eyes and mouth S&M expenses Sales and marketing expenses Small bowel CD (SBCD) CD causes chronic inflammation and erosion of the intestines. It can affect different regions of gastrointestinal tract including the stomach and small and large intestines. While isolated SBCD is an uncommon presentation of CD, involvement of some portion of the small bowel, particularly the ileum, is common 46 Galapagos NV • Q1 Report 2021
OTHER INFORMATION Statin Statins are a class of lipid-lowering medications that reduce illness and mortality in those who are at high risk of cardiovascular disease. They are the most common cholesterol-lowering drugs. Low-density lipoprotein (LDL) carriers of cholesterol play a key role in the development of atherosclerosis and coronary heart disease via the mechanisms described by the lipid hypothesis Systemic lupus erythematosus An autoimmune disease, with systemic manifestations including skin rash, erosion of joints or even kidney failure. TAPINOMA Phase 2 Proof of Concept trial with SIK2/SIK3 inhibitor GLPG3970 in SLE Target Proteïn that has been shown to play a role in a disease process and that forms the basis of a therapeutic intervention or discovery of a medicine Target discovery Identification and validation of proteins that have been shown to play a role in a disease process TEAE Treatment Emergent Adverse Event, is any event not present prior to the initiation of the treatments or any event already present that worsens in either intensity or frequency following exposure to the treatments Technology access fee License payment made in return for access to specific technology (e.g. compound or virus collections) Toledo Toledo is the program name for the target family of SIK inhibitors Topical corticosteroids Corticosteroids which are administered through the skin using an ointment Transcription The process of making an RNA copy of a DNA gene sequence Translation The process by which a protein is synthetized from mRNA TYK Tyrosine kinase is an enzyme that can transfer a phosphate group from ATP to the tyrosine residues of specific proteins inside a cell. It functions as an “on” or “off” switch in many cellular functions. Tyrosine kinases belong to a larger class of enzymes known as protein kinases which also attach phosphates to other amino acids such as serine and threonine. GLPG3667 is a reversible and selective TYK2 kinase domain inhibitor 47 Galapagos NV • Q1 Report 2021

OTHER INFORMATION Ulcerative colitis (UC) UC is an IBD causing chronic inflammation of the lining of the colon and rectum (unlike CD with inflammation throughout the gastrointestinal tract) Venous thrombotic events When a blood clot breaks loose and travels in the blood, this is called a venous thromboembolism (VTE). The abbreviation DVT/PE refers to a VTE where a deep vein thrombosis (DVT) has moved to the lungs (PE or pulmonary embolism) Ziritaxestat Formerly known as GLPG1690. Ziritaxestat is a novel drug candidate targeting autotaxin; all development with ziritaxestat was discontinued in February 2021 48 Galapagos NV • Q1 Report 2021

OTHER INFORMATION Financial calendar Colophon 05 August 2021 Concept, design and online programming nexxar GmbH, Vienna – Online annual reports Half year 2021 results and online sustainability reports 04 November 2021 www.nexxar.com Photography Third quarter 2021 results 24 February 2022 Frank van Delft Copy deadline: 6 May 2021 Full year 2021 results This report is also available in Dutch and available for download in the Downloads section of this report or at www.glpg.com 49 Galapagos NV • Q1 Report 2021

OTHER INFORMATION Contact Elizabeth Goodwin Sofie Van Gijsel Sandra Cauwenberghs Vice President Investor Relations Senior Director Investor Relations Director of Investor Relations Galapagos NV Galapagos NV Galapagos NV 230 Third Ave Generaal De Wittelaan L11 A3 Generaal De Wittelaan L11 A3 Waltham, MA 02451, United 2800 Mechelen, Belgium 2800 Mechelen, Belgium States Tel. +32 485 19 14 15 Tel. +32 15 34 29 00 Tel. +1 781 460 1784 Email: [email protected] Carmen Vroonen Kyra Obolensky Global Head of Communications Senior Director Corporate & Public Affairs Communications Galapagos NV Galapagos NV Generaal De Wittelaan L11 A3 Generaal De Wittelaan L11 A3 2800 Mechelen, Belgium 2800 Mechelen, Belgium Tel. +32 473 82 48 74 Tel. +32 491 92 64 35 Email: [email protected] 50 Galapagos NV • Q1 Report 2021
Exhibit 99.3
CERTAIN CONFIDENTIAL INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND REPLACED
WITH “[…***…]” BECAUSE IT IS NOT MATERIAL AND WOULD BE COMPETITIVELY HARMFUL IF
PUBLICLY DISCLOSED.
TRANSITION & AMENDMENT AGREEMENT
by and between
GALAPAGOS NV
and
GILEAD SCIENCES, INC.
Effective as of April 3, 2021
Table of Contents
| ARTICLE 1 DEFINITIONS |
1 | |||||
| ARTICLE 2 INTERACTION OF AGREEMENTS |
10 | |||||
| 2.1 |
Interaction of Agreements Generally | 10 | ||||
| ARTICLE 3 TIMING OF TRANSITION |
12 | |||||
| 3.1 |
Timing of Transition Generally | 12 | ||||
| 3.2 |
Business Transfer Agreements | 12 | ||||
| ARTICLE 4 GOVERNANCE AND TRANSITION MANAGEMENT |
12 | |||||
| 4.1 |
Existing Committees and Working Groups | 12 | ||||
| 4.2 |
Transition Steering Committee | 13 | ||||
| 4.3 |
Joint Transition Team | 14 | ||||
| 4.4 |
Transition Compliance Working Group. | 16 | ||||
| 4.5 |
Country Transition Teams. | 17 | ||||
| 4.6 |
Functional Teams | 17 | ||||
| 4.7 |
Good Faith | 17 | ||||
| 4.8 |
General Committee Authority | 18 | ||||
| 4.9 |
Disbandment | 18 | ||||
| 4.10 |
General Direction of Galapagos | 18 | ||||
| ARTICLE 5 PERSONNEL |
18 | |||||
| 5.1 |
TUPE Employees | 18 | ||||
| 5.2 |
Non-TUPE Employees | 21 | ||||
| 5.3 |
General rules in relation to the New Galapagos Employees | 22 | ||||
| 5.4 |
Other provisions | 23 | ||||
| ARTICLE 6 REGULATORY MATTERS |
24 | |||||
| 6.1 |
Transfer of Regulatory Documentation | 24 | ||||
| 6.2 |
MAH Related Activities | 26 | ||||
| 6.3 |
MA Transition | 26 | ||||
| 6.4 |
Unallocated MAH Activities | 29 | ||||
| ARTICLE 7 DEVELOPMENT |
29 | |||||
| 7.1 |
Transition Development Activities and Data-Related Activities | 29 | ||||
| 7.2 |
Clinical Trials | 31 | ||||
| 7.3 |
New Joint Development Activities | 32 | ||||
| 7.4 |
Independent Development Activities | 34 | ||||
| 7.5 |
Combination of the Licensed Compound with Other Compounds | 36 | ||||
| 7.6 |
Amendments to A&R Collaboration Agreement; Prior Development Plans | 36 | ||||
| ARTICLE 8 MANUFACTURE AND SUPPLY |
41 | |||||
| 8.1 |
Supply Arrangements | 41 | ||||
| 8.2 |
Manufacturing Process Decisions | 43 | ||||
| 8.3 |
Transferred Inventory | 43 | ||||
| 8.4 |
Manufacturing and Importation Authorization | 44 | ||||
| ARTICLE 9 COMMERCIALIZATION |
44 | |||||
| 9.1 |
Commercialization Arrangements | 44 | ||||
| 9.2 |
Market Access Activities; Medical Affairs Activities | 47 | ||||
| 9.3 |
Pricing Strategies and Decisions | 48 | ||||
| 9.4 |
Transferred Information | 48 | ||||
| 9.5 |
Amendments to A&R Collaboration Agreement | 48 | ||||
| ARTICLE 10 TRANSFERRED TRADEMARKS AND INFORMATION TECHNOLOGY, LICENSES AND EXCLUSIVITY | 49 | |||||
| 10.1 |
Assignment of Transferred Trademarks, Certain Domain Names and Certain Information Technology | 49 | ||||
| 10.2 |
Licenses | 50 | ||||
| 10.3 |
Sublicensing | 54 | ||||
| 10.4 |
Amendment to Exclusivity; Non-Compete | 55 | ||||
| ARTICLE 11 ADDITIONAL COVENANTS |
55 | |||||
| 11.1 |
Further Assurances | 55 | ||||
| 11.2 |
Contracts | 56 | ||||
| 11.3 |
Public Communications | 57 | ||||
| 11.4 |
Competition Law Guidelines | 59 | ||||
| 11.5 |
Social, Social Security and Tax Obligations | 59 | ||||
| 11.6 |
Regulatory Inspections. | 59 | ||||
| ARTICLE 12 FINANCIALS |
59 | |||||
| 12.1 |
Upfront, Milestone Payments, Royalties and Profit Split | 59 | ||||
| 12.2 |
Development Costs | 61 | ||||
| 12.3 |
Regulatory Affairs Costs | 63 | ||||
| 12.4 |
Commercialization Costs Through Initial Transition Date | 63 | ||||
| 12.5 |
Other Transition Costs | 64 | ||||
| 12.6 |
Taxes | 64 | ||||
| 12.7 |
Financial Records; Audits | 65 | ||||
| 12.8 |
Foreign Exchange. | 65 | ||||
| 12.9 |
Interest on Late Payments | 65 | ||||
| 12.10 |
Manner and Place of Payment | 65 | ||||
| ARTICLE 13 REPRESENTATIONS AND WARRANTIES |
66 | |||||
| 13.1 |
Mutual Representations and Warranties | 66 | ||||
| 13.2 |
Representations and Warranties by Gilead | 66 | ||||
| ARTICLE 14 INDEMNIFICATION |
67 | |||||
| 14.1 |
Indemnification by Galapagos | 67 | ||||
| 14.2 |
Indemnification by Gilead | 67 | ||||
| 14.3 |
Indemnification Procedures | 68 | ||||
| 14.4 |
Shared Development Damages. | 68 | ||||
| 14.5 |
Limitations of Liability | 69 | ||||
| 14.6 |
Insurance | 69 | ||||
| ARTICLE 15 TERM OF AGREEMENT |
69 | |||||
| 15.1 |
Term of Agreement | 69 | ||||
| 15.2 |
Impact of Material Breach | 69 | ||||
| 15.3 |
Amendment to A&R Collaboration Agreement | 70 | ||||
| 15.4 |
Survival | 70 | ||||
| ARTICLE 16 MISCELLANEOUS |
70 | |||||
| 16.1 |
Certain Miscellaneous Provisions | 70 | ||||
| 16.2 |
Force Majeure | 70 | ||||
| 16.3 |
Entire Agreement | 71 | ||||
| 16.4 |
Subcontracting | 71 | ||||
| 16.5 |
Assignment | 72 | ||||
| 16.6 |
Dispute Resolution | 72 | ||||
| 16.7 |
Governing Law | 74 | ||||
| 16.8 |
Notices | 74 | ||||
SCHEDULES
SCHEDULE 1 TARGET MA SUBMISSION DATES
SCHEDULE 2 TRANSFERRED TRADEMARKS
SCHEDULE 3 TUPE EMPLOYEES
SCHEDULE 4 DOMAIN NAMES
SCHEDULE 5 EQUITY AWARDS GRANTED TO NEW GALAPAGOS EMPLOYEES
EXHIBITS
EXHIBIT A DEVELOPMENT PLAN AND BUDGET
EXHIBIT B SHARED TERRITORY COMMERCIALIZATION PLAN AND BUDGET
EXHIBIT C TRADEMARK ASSIGNMENT AGREEMENT
EXHIBIT D TRANSITION PLAN
EXHIBIT E INTENTIONALLY OMITTED
EXHIBIT F GILEAD PLANS FOR […***…] TUPE EMPLOYEES
EXHIBIT G […***…] BUSINESS TRANSFER AGREEMENT
EXHIBIT H COMMERCIAL HANDOVER
TRANSITION & AMENDMENT AGREEMENT
This Transition & Amendment Agreement (this “Agreement”) is made and entered into effective as of April 3, 2021 (the “Effective Date”) by and between GALAPAGOS NV, a corporation organized under the laws of Belgium and having its principal place of business at Generaal de Wittelaan L11 A3, 2800 Mechelen, Belgium (“Galapagos”), and GILEAD SCIENCES, INC., a Delaware corporation with offices at 333 Lakeside Drive, Foster City, CA 94404, USA (“Gilead”). Galapagos and Gilead are sometimes referred to herein individually as a “Party” and collectively as the “Parties.”
BACKGROUND
WHEREAS, Galapagos and Gilead Biopharmaceuticals Ireland UC (“GBIUC”) entered into that certain Amended and Restated License and Collaboration Agreement, dated as of August 23, 2019, which was then assigned by GBIUC to Gilead effective September 20, 2019 and was amended by a First Amendment dated as of February 4, 2020 (as so assigned and amended, the “A&R Collaboration Agreement”), pursuant to which, among other things, Galapagos is responsible for Commercializing the Licensed Product in the Benelux Countries, the Parties are jointly Commercializing the Licensed Product in the EU5 Countries and Gilead is responsible for Commercializing the Licensed Product in all other countries in the Galapagos Territory and in the Gilead Territory; and
WHEREAS, the Parties entered into a binding term sheet (the “Term Sheet”) on December 15, 2020 (the “Announcement Date”) agreeing to modify such arrangement with respect to the Galapagos Territory and wish to set forth in greater detail the process for transitioning, and the terms and conditions pursuant to which they will transition, the Development, Manufacture, and Commercialization of the Licensed Compound and the Licensed Products with respect to the Galapagos Territory, along with certain other rights and obligations, from Gilead to Galapagos (the “Transition”), as set forth herein.
NOW, THEREFORE, in consideration of the foregoing premises and the mutual promises, covenants and conditions contained in this Agreement, the Parties agree as follows:
ARTICLE 1
DEFINITIONS
As used in this Agreement, the following initially capitalized terms, whether used in the singular or plural form, shall have the meanings set forth in this ARTICLE 1. Capitalized terms used, but not defined herein, shall have the meanings given to them in the Applicable Collaboration Agreement unless otherwise specifically provided herein (provided, however, that for the period after the Initial Transition Date, if any such capitalized term is not defined in the Second A&R Collaboration Agreement, then such capitalized term shall have the meaning given to it in the A&R Collaboration Agreement). Unless specifically incorporated by reference into the Applicable Collaboration Agreement, the Transition Ancillary Agreements or any other Ancillary Agreement, the meanings given to such capitalized terms shall only apply to this Agreement. In addition, the terms “includes,” “including,” “include” and derivative forms of them shall be deemed followed by the phrase “without limitation” (regardless of whether it is actually written there (and drawing no implication from the actual inclusion of such phrase in some instances after such terms but not others)) and the term “or” has the inclusive meaning represented by the phrase “and/or” (regardless of whether it is actually written (and drawing no implication from the actual use of the phrase “and/or” in some instances but not in others)). Unless otherwise stated, euro (€) amounts are in European euros and Dollars ($) amounts are in the lawful currency of the United States. Unless specified to the contrary, references to Articles, Sections, Schedules or Exhibits shall refer to the particular Articles, Sections, Schedules or Exhibits of or to this Agreement and references to this Agreement include all Schedules and Exhibits hereto. To the extent a Party’s obligations under this Agreement are performed by,
are the responsibility of, or must be completed by an Affiliate of such Party, such Party will procure that the applicable Affiliate perform such obligations in accordance with, and to the standard required under, this Agreement. The words “day,” “quarter” or “year” (and derivative or similar words, e.g., “quarterly”) shall mean a calendar day, calendar quarter or calendar year unless otherwise specified. The word “notice” shall mean notice in writing (whether or not specifically stated) and shall include notices, consents, approvals and other written communications contemplated under this Agreement. The words “hereof,” “herein,” “hereby” and derivative or similar words refer to this Agreement (including any Schedules and Exhibits). The words “will” and “shall” shall have the same obligatory meaning. Provisions that require that a Party, the Parties, the JTT, the TSC, the Transition Compliance Working Group, or a Country Transition Team “agree,” “consent” or “approve” or the like shall require that such agreement, consent or approval be specific and in writing, whether by written agreement, letter, or with respect to the JTT, the TSC, the Transitional Compliance Working Group, or a Country Transition Team only, by approved minutes. Words of any gender include the other gender. Words using the singular or plural number also include the plural or singular number, respectively. References to any specific law or Article, Section or other division thereof, shall be deemed to include the then-current amendments or any replacement law thereto.
[…***…] has the meaning set forth in Section 5.1(b).
“A&R Collaboration Agreement” has the meaning set forth in the recitals.
“Accrued Benefits Amount” has the meaning set forth in Section 5.1(i).
“Acquired Rights Directive” means Council Directive 2001/23/EC of March 12, 2001 on the approximation of the laws of the Member States relating to the safeguarding of employees’ rights in the event of transfers of undertakings, businesses or parts of undertakings or businesses, as amended from time to time.
“Agreement” has the meaning set forth in the preamble hereof.
“Announcement Date” has the meaning set forth in the recitals.
“Applicable Collaboration Agreement” means (a) for the period beginning on the Effective Date and ending on the Initial Transition Date, the A&R Collaboration Agreement and (b) for the period after the Initial Transition Date, the Second A&R Collaboration Agreement.
“Applicable Guidelines” means applicable national and international pharmaceutical industry codes of practice and guidelines, which on and after January 1, 2020, include the International Federation of Pharmaceutical Manufacturers Code of Conduct, the European Federation of Pharmaceutical Industries Associate Code on the Promotion of Prescription-Only Medicines to, and Interactions with, Healthcare Professionals, where applicable, and applicable local country codes and guidelines.
“Applicable Law” means applicable laws, rules and regulations, including any rules, regulations, guidelines or other requirements of Regulatory Authorities that may be in effect from time to time.
“Benelux Co-Commercialization Agreement” means that certain Co-Commercialization Agreement, effective February 4, 2020, between the Parties, pursuant to which the Co-Commercialization Product(s) are being Commercialized by Galapagos in the Benelux Countries.
“Benelux Supply Agreement” means that certain Supply Agreement, effective February 4, 2020, between Gilead Sciences Ireland UC and Galapagos, pursuant to which Gilead Sciences Ireland UC supplies Galapagos with the Co-Commercialization Product(s) in the Benelux Countries.
“Business Day” means a day other than (a) a Saturday or a Sunday, (b) a bank or other public holiday in California, United States, or (c) a bank or other public holiday in Brussels, Belgium.
“cGMP” means the applicable current Good Manufacturing Practices as specified for (a) the United States in the Federal Food, Drug, and Cosmetic Act (21 U.S.C. §§ 301 et seq.) and the relevant U.S. regulations found in Title 21 of the United States Code of Federal Regulations (Chapter 11, 210, 211, 600 and 610), together with the relevant or applicable current International Conference on Harmonization guidance documents, including International Conference on Harmonization Guidance Q7 Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients, as ratified in Annex 19 of the EU Guidelines to Good Manufacturing Practice; (b) the European Union in Directive 2001/83/EC, Directive 2003/94/EC, Eudralex—Volume 4—Good Manufacturing Practice (GMP) guidelines or any other relevant European Union or national regulations; and (c) any other countries, the equivalent legal requirements set forth in the Applicable Law, each as may be amended from time to time.
“Clinical Supply Agreement” means that certain Clinical Materials Supply Agreement, effective February 6, 2017, between the Parties, as may be amended from time to time.
“Commercial Handover” has the meaning set forth on Exhibit H.
“Competition Law Guidelines” has the meaning set forth in Section 11.4(a).
“Contract” means any written contract, agreement, lease, sublease, license, sublicense, instrument, note, guaranty, deed, assignment, purchase order or other legally binding commitment or arrangement.
“Control” means, with respect to any material, Information, Patent, Regulatory Materials or Regulatory Approvals, the possession (whether by ownership or license) by a Party or its Affiliates of the ability to assign, grant to the other Party a license or provide to the other Party access to such item as provided herein, without violating the terms of any agreement or other arrangement with any Third Party, or being obligated to pay any royalties or other consideration therefor (unless such Party has expressly agreed to pay or the other Party has expressly agreed to reimburse such amounts), in existence as of the time such Party or its Affiliates would first be required hereunder to assign or grant to the other Party a license or provide to the other Party access to such item.
“Country Non-TUPE Employees” has the meaning set forth in Section 5.2(c).
“Country Transition Team” has the meaning set forth in Section 4.5(a).
“Cure Period” has the meaning set forth in Section 15.2(a)
“Delegated Galapagos Activities” means all activities expressly reserved for Galapagos or any of its Affiliates from the Effective Date to the MA Transfer Completion Date in a Registered Country pursuant to Section 6.3.
“Delegation of Authority” has the meaning set forth in Section 6.3(a)(iv).
“Development Plan and Budget” means the Development Plan and Budget attached hereto as Exhibit A.
“Effective Date” has the meaning set forth in the preamble hereof.
“EMA” means the European Medicines Agency or its successor.
“Employment Taxes” means withholding, payroll, social security, workers’ compensation, unemployment, disability, and any similar tax imposed by any Tax Authority or social security authority, and any interest, penalties, additions to tax, or additional amounts with respect to the foregoing imposed on any taxpayer or consolidated, combined, or unitary group of taxpayers.
“Encumbrance” means any mortgage, lien, license, pledge, security interest or encumbrance.
“EU5 Co-Commercialization Agreement” means that certain Co-Commercialization Agreement, effective February 4, 2020, between the Parties, pursuant to which the Co-Commercialization Product(s) are being Commercialized by both Parties in the EU5 Countries.
“European Supply Agreement” means (a) the Benelux Supply Agreement, as amended by this Agreement, until its amendment and restatement pursuant to Section 8.1(a) and (b) thereafter such agreement as amended and restated.
“FD&C Act” means the United States Federal Food, Drug and Cosmetic Act, as amended.
“FDA” means the United States Food and Drug Administration or its successor.
“First Overage Share Amount” has the meaning set forth in Section 12.2(c)(iv)(A).
“FIS Supply Agreement” means that certain Supply Agreement, effective February 4, 2020, between Gilead Sciences Ireland UC and Galapagos, pursuant to which Gilead Sciences Ireland UC supplies Galapagos with the Co-Commercialization Product(s) in France, Italy and Spain.
“Functional Team” has the meaning set forth in Section 4.6.
“Galapagos” has the meaning set forth in the preamble hereof.
“Galapagos House Marks” means the […***…].
“Galapagos MAH” means, with respect to a Marketing Authorization or Marketing Authorization application in a Registered Country, Galapagos or, as designated by Galapagos, its agent to whom the Marketing Authorization or Marketing Authorization application is to be transferred pursuant to ARTICLE 6.
“Galapagos Territory” means (a) the member states of the European Union as constituted as of July 14, 2019, including Austria, Belgium, Bulgaria, Croatia, Cyprus, Czechia, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, and the United Kingdom, and all other territories and possessions of the foregoing, (b) […***…], (c) […***…], (d) […***…] and (e) […***…].
“GBIUC” has the meaning set forth in the recitals.
“General Direction” has the meaning set forth in Section 4.10.
“[…***…] Gilead Pension Plans” has the meaning set forth in Section 5.1(l).
“[…***…] Pension Assets” has the meaning set forth in Section 5.1(l).
“[…***…] TUPE Employees” has the meaning set forth in Section 5.1(b).
“[…***…] TUPE Transfer Date” has the meaning set forth in Section 5.1(l).
“[…***…] Business Transfer Agreement” means that certain Business Transfer Agreement, dated on or about the date hereof, between […***…] and […***…], in the form attached as Exhibit G.
“Gilead” has the meaning set forth in the preamble hereof.
“Gilead Copyrights” means all copyrights Controlled as of the Effective Date or during the period prior to the Initial Transition Date by Gilead or its Affiliate(s) and used by Gilead or its Affiliate(s) in the Exploitation of Licensed Compound or Licensed Products in the Field in the Galapagos Territory.
“Gilead House Marks” means […***…].
“Gilead MAH” means, with respect to a Marketing Authorization or Marketing Authorization application in a Registered Country, Gilead or its Affiliate that is the MAH for such Marketing Authorization or Marketing Authorization application as of the Effective Date or thereafter until the MA Transfer Completion Date.
“Gilead Plan” means any Contract, obligation, plan, program, fund, arrangement, policy, or customary practice (whether written or unwritten) maintained, sponsored, owed, adopted, contributed to, or followed by Gilead or its Affiliates, providing compensation (other than salary), benefits, pension, retirement, superannuation, profit sharing, stock bonus, stock option, stock purchase, phantom or stock equivalent, bonus, thirteenth month, incentive, deferred compensation, hospitalization, medical, dental, vision, vacation, life insurance, death benefit, sick pay, disability, severance, termination indemnity, redundancy pay, educational assistance, holiday pay, housing assistance, moving expense reimbursement, fringe benefit or similar employee benefits to current or former directors, officers, employees or agents appointed employed or retained by Gilead or its Affiliates.
“Gilead Territory” means all countries in the world other than the countries included in the Galapagos Territory.
“Gilead’s Knowledge” means […***…].
[…***…]
[…***…]
[…***…]
[…***…]
“Hired Employee” has the meaning set forth in Section 5.2(c)(iii).
“IND” means, with respect to the Licensed Products, (a) an Investigational New Drug Application as defined in the FD&C Act and applicable regulations promulgated thereunder by the FDA, or (b) the equivalent application to the equivalent Regulatory Authority in any other regulatory jurisdiction, the filing of which is necessary to initiate or conduct clinical testing of a pharmaceutical product in humans in such jurisdiction.
“Independent Activities” has the meaning set forth in Section 7.4(a).
“Independent Activities Cost” has the meaning set forth in Section 7.4(g).
“Independent Activities Data” has the meaning set forth in Section 7.4(d).
“Independent Activities Party” has the meaning set forth in Section 7.4(b)(i).
“Independent Activities Plan” has the meaning set forth in Section 7.4(b)(i).
“Independent Activities Regulatory Documentation” has the meaning set forth in Section 7.4(e).
“Initial Transition Date” means 11:59:59 pm Central European Time on December 31, 2021.
“Joint Development Plan and Budget” has the meaning set forth in Section 7.3(b).
“JTT” has the meaning set forth in Section 4.3(a).
“Latest Transition Date” means 11:59:59 pm Central European Time on December 31, 2022.
“MA Maintenance” means (a) the preparation and submission to the applicable Regulatory Authority of (i) all annual and other periodic updates to the Marketing Authorizations in the applicable country or regulatory jurisdiction and (ii) all supplements and amendments to the Marketing Authorizations in the applicable country or regulatory jurisdiction, and (b) all other actions that are necessary to maintain the Marketing Authorization and Marketing Authorization applications in the applicable country or regulatory jurisdiction.
“MA Transfer Completion Date” means with respect to the Licensed Products in the Registered Countries, the date on which the transfer of the Marketing Authorization or Marketing Authorization application in such Registered Country to the Galapagos MAH or the date on which withdrawal of the Marketing Authorization or Marketing Authorization application of the Gilead MAH pursuant to Section 6.1 takes effect.
“MAH” means, with respect to a Marketing Authorization or Marketing Authorization application in a Registered Country, the Person holding such Marketing Authorization or Marketing Authorization application.
“MAH Legal Responsibilities” means, with respect to the Licensed Product, a Party’s or any of its Affiliate’s ongoing legal responsibility as MAH under Regulation (EU) No. 726/2004, Directive 2001/83/EC and other applicable EU legislation and guidance or any equivalent regulation in the applicable Registered Country. […***…].
“MAH Related Activities” has the meaning set forth in Section 6.2(a).
“Marketing Authorization” means, with respect to a Registered Country, the Regulatory Approvals necessary for placing the Licensed Products on the market in such Registered Country.
“Material Breach Notice” has the meaning set forth in Section 15.2(a).
“MIA” has the meaning set forth in Section 8.4.
“NDA” means a New Drug Application, as defined in the FD&C Act and applicable regulations promulgated thereunder by the FDA.
“New Galapagos Employees” has the meaning set forth in Section 5.3.
“New Joint Development Activities” has the meaning set forth in Section 7.3(a).
“Non-TUPE Countries” has the meaning set forth in Section 5.2(c).
“Non-TUPE Employees” has the meaning set forth in Section 5.2(a).
“Offer Date” has the meaning set forth in Section 5.2(c)(iii).
“Original Agreement” means that certain License and Collaboration Agreement, dated December 16, 2015, entered into by the Parties.
“Other Contracts” means all Contracts to which Gilead or any of its Affiliates is a party, other than Transferred Contracts, related to Licensed Compound or Licensed Products and used in the Exploitation of Licensed Compound or Licensed Products in the Field in the Galapagos Territory.
“Participating Employee” has the meeting set forth in Section 5.4(a).
“Participating Entity” has the meeting set forth in Section 5.4(a).
“Party” and “Parties” have the meanings set forth in the preamble hereof.
“Person” means an individual, sole proprietorship, partnership, limited partnership, limited liability partnership, corporation, limited liability company, business trust, joint stock company, trust, unincorporated association, joint venture or other similar entity or organization, including a government or political subdivision, department or agency of a government.
“Power of Attorney” has the meaning set forth in Section 6.3(a)(iv).
“Pre-Transfer Gilead MAH Activities” means all activities expressly reserved for the Gilead MAH or any of its Affiliates from the Effective Date to the MA Transfer Completion Date pursuant to Section 6.3.
“Promotional Review” means the review and oversight of the compliance with Applicable Law and Applicable Guidelines of (a) Promotional Materials and (b) promotional and non-promotional activities (including, for clarity, Commercialization activities, Medical Affairs Activities, public affairs activities and government affairs activities) carried out by business functions in connection with the Commercialization of the Licensed Product(s) in its Respective Territory.
“Prospective Employees” has the meaning set forth in Section 5.2(c)(ii).
“PVA” means the Pharmacovigilance Agreement, dated March 3, 2021, by and between the Parties, as amended from time to time.
“Quality Agreement” means the Quality Agreement, dated February 17, 2020, as amended on November 30, 2020 and as may be amended from time to time, between the Parties (or their Affiliates).
“Regional Non-TUPE Employees” has the meaning set forth in Section 5.2(d).
“Registered Country” means, at any time, each of the countries in the Galapagos Territory with respect to which (a) a Marketing Authorization exists (whether granted by the EMA or locally) or a Marketing Authorization application is pending as of such time and (b) Gilead or its Affiliate is as of such time, or previously was, the MAH.
“Regulatory Approval” means all approvals (including licenses, registrations or authorizations) from any applicable Regulatory Authority in a given country or regulatory jurisdiction necessary for the Development, Manufacture, marketing, commercial distribution, importation and sale of a Licensed Product in such country or jurisdiction, which may include satisfaction of all applicable regulatory and notification requirements and, where applicable, labeling approval, but which, shall exclude any pricing and reimbursement approvals. Regulatory Approvals include approvals by Regulatory Authorities of Marketing Authorization applications.
“Regulatory Authority” means, in a particular country or regulatory jurisdiction, any applicable Governmental Authority involved in granting Regulatory Approval, including (a) the FDA, (b) the EMA, (c) the European Commission, (d) Swissmedic and (e) the MHRA, or the successor of any such Governmental Authority.
“Regulatory Communications” has the meaning set forth in Section 6.3(a)(ii).
“Regulatory Documentation” means the (a) Regulatory Materials, (b) correspondence and information submitted to, received from or retained in order to be made available to a Regulatory Authority (including minutes and official contact reports relating to any communication with any Regulatory Authority) and all supporting documents with respect thereto, (c) Regulatory Approvals and (d) safety or clinical databases, in each case (a) through (d), that are necessary or reasonably desirable in order to Develop, manufacture, market, sell or otherwise Commercialize a Licensed Product in a particular country or regulatory jurisdiction.
“Remedial Measures” has the meaning set forth in Section 15.2(a).
“Respective Territory” means, with respect to Gilead, the Gilead Territory and with respect to Galapagos, the Galapagos Territory.
“RTA” has the meaning set forth in Section 10.2(a)(iii).
“Second A&R Collaboration Agreement” has the meaning set forth in Section 2.1(b).
“Severance Costs” means any amount as legally due to any New Galapagos Employee […***…].
“Shared Territory Commercialization Plan and Budget” means the Shared Territory Commercialization Plan and Budget as defined in the A&R Collaboration Agreement, which is hereby amended as attached hereto as Exhibit B.
“Subcontractor” means any Third Party subcontractor engaged by Gilead or Galapagos to perform its obligations hereunder.
“Sublicensee” means any Third Party other than a Distributor that is granted a sublicense by a Party under the rights licensed to such Party pursuant to ARTICLE 10 of this Agreement.
“Supply Chain Completion Date” has the meaning set forth in Section 8.1(d).
“Target MA Submission Date” means the target date by which the submission to the Regulatory Authority for the transfer of the Marketing Authorization or Marketing Authorization application in a Registered Country to the Galapagos MAH is expected to occur as set forth on Schedule 1, or as otherwise agreed by the JTT, or if applicable, the TSC from time to time.
“Tax” means any income, net income, gross income, gross receipts, profits, capital stock, franchise, property, ad valorem, stamp, excise, severance, occupation, service, sales, use, license, lease, transfer, import, export, customs duties, value added, alternative minimum, estimated or other similar tax (including any fee, assessment, or other charge in the nature of or in lieu of any tax) imposed by any Tax Authority, and any interest, penalties, additions to tax or additional amounts with respect to the foregoing imposed on any taxpayer or consolidated, combined or unitary group of taxpayers.
“Tax Authority” means, with respect to any Tax, the Governmental Authority or political subdivision thereof that imposes such Tax, and the agency (if any) charged with the collection of such Tax for such Governmental Authority or subdivision.
“Term Sheet” has the meaning set forth in the recitals.
“Territory Employee” means any employee who is employed by Gilead in the Galapagos Territory and provides services pursuant to the A&R Collaboration Agreement, as amended by this Agreement.
“Third Party” means any Person other than any Party or any of its respective Affiliates.
“Third Party Assignment Approval” has the meaning set forth in Section 11.2(c).
“Trademark” means any trademark, trade name, service mark, service name, brand, design, trade dress, logo, slogan, or other indicia of origin or ownership, including registrations and applications therefor and the goodwill and activities associated with each of the foregoing.
“Trademark Assignment Agreement” means the Trademark Assignment Agreements in the forms attached hereto as Exhibit C.
“Transferred Assets” means the Transferred Regulatory Documentation, the Marketing Authorizations, the Marketing Authorization applications, the Transferred Trademarks, the Transferred Inventory, the Transferred Contracts, the Transferred Clinical Trials and the Regulatory Documentation applicable thereto and such other assets as are transferred by Gilead to Galapagos pursuant to this Agreement.
“Transferred Clinical Trials” has the meaning set forth in Section 7.2(b).
“Transferred Contracts” has the meaning set forth in Section 11.2.
“Transferred Information” means all Information solely related to the Licensed Compound or Licensed Products that is Controlled by Gilead or its Affiliate(s) and (a) necessary for or otherwise reasonably requested by Galapagos in order to Exploit the Licensed Compound or Licensed Products in the Galapagos Territory, including, as applicable, Registered Country-specific pricing and reimbursement dossiers or official communications, current forecasts and Licensed Products analysis for any Registered Country, and books and records related to research, Development, regulatory activities, Manufacture and commercial data and plans for the Licensed Products in the Galapagos Territory or (b) related to the Transferred Assets. For clarity, […***…].
“Transferred Inventory” has the meaning set forth in Section 8.3(a).
“Transferred Regulatory Documentation” means all Regulatory Documentation that is used or held for use in connection with the Licensed Products in the Galapagos Territory or related to the Transferred Clinical Trials to the extent Controlled by Gilead or any of its Affiliates.
“Transferred Trademarks” means the Trademarks listed on Schedule 2 excluding, for the avoidance of doubt, any Gilead House Marks.
“Transition” has the meaning set forth in the recitals.
“Transition Activity Commercially Reasonable Efforts” means, with respect to each Party, (a) in connection with the transfer or withdrawal of the Marketing Authorizations or Marketing Authorization applications, the level of efforts, expertise and resources reasonably necessary to complete such transfer or withdrawal; and (b) in connection with any other activity under this Agreement, that level of efforts and use of resources commonly dedicated in the research-based pharmaceutical industry by a company to the performance of similar activities intending to achieve the applicable result with respect to such activities; provided, however, […***…].
“Transition Ancillary Agreement” means the Trademark Assignment Agreement, the European Supply Agreement, the Clinical Supply Agreement, the Quality Agreement, the PVA, and the […***…] Business Transfer Agreement.
“Transition Compliance Working Group” has the meaning set forth in Section 4.4(a).
“Transition Development Activities” means the […***…], the […***…] and any New Joint Development Activities for the Licensed Compound or Licensed Products that the Parties agree to conduct under this Agreement.
“Transition Key Messages” has the meaning set forth in Section 9.1(e).
“Transition Plan” means the Transition Plan attached hereto as Exhibit D as the same may be amended from time to time.
“TSC” has the meaning set forth in Section 4.2(a).
“TUPE Employees” has the meaning set forth in Section 5.1(a).
“TUPE Regulations” means (a) when used in relation to employees in a Galapagos Territory other than the United Kingdom, the Acquired Rights Directive and the Applicable Law pursuant to which the Acquired Rights Directive has been transposed into the laws of such Galapagos Territory, and (b) when used in relation to employees in the United Kingdom, the Transfer of Undertakings (Protection of Employment) Regulations 2006 as amended from time to time.
“TUPE Transfer Date” has the meaning set forth in Section 5.1(a).
“VAT” has the meaning set forth in Section 12.6(a).
ARTICLE 2
INTERACTION OF AGREEMENTS
| 2.1 | Interaction of Agreements Generally |
This Section 2.1 shall govern the interaction among the terms of this Agreement and the terms of each Applicable Collaboration Agreement, Ancillary Agreement and Transition Ancillary Agreement. In the event of any inconsistency between the terms of the Applicable Collaboration Agreement and the terms of this Agreement, (x) prior to the Initial Transition Date, the terms of this Agreement shall govern, and (y) after the Initial Transition Date, the terms of the Second A&R Collaboration Agreement shall govern.
| (a) | A&R Collaboration Agreement. The A&R Collaboration Agreement will remain in effect (as amended by this Agreement) until the Initial Transition Date. |
| (b) | Second A&R Collaboration Agreement. As soon as reasonably practicable and, in any event, within […***…], the Parties shall amend and restate the A&R Collaboration Agreement (such agreement as amended and restated, the “Second A&R Collaboration Agreement”). The Second A&R Collaboration Agreement will become effective […***…] and will amend and restate the A&R Collaboration Agreement in its entirety at such time. Upon the effectiveness of the Second A&R Collaboration Agreement, the Second A&R Collaboration Agreement will govern the relationship between the Parties with respect to the […***…]; provided that any Transition activities remaining to be completed under this Agreement shall be performed pursuant to this Agreement but under the purview of the Committees under the Second A&R Collaboration Agreement and subject to the applicable terms of the Second A&R Collaboration Agreement. |
| (c) | Development and Associated Regulatory Activities. Prior to the Initial Transition Date, Transition Development Activities (including any […***…]) and associated regulatory matters will be governed by this Agreement and the A&R Collaboration Agreement as amended hereby. After the Initial Transition Date, all Development Activities […***…]) will be conducted under the Second A&R Collaboration Agreement. |
| (d) | Regulatory Matters. Prior to the Initial Transition Date, regulatory matters related to the Licensed Products in the Galapagos Territory and the Galapagos Combination Products will be governed by this Agreement. Regulatory matters related to the Licensed Products and Gilead Combination Products in the Gilead Territory will continue to be governed by A&R Collaboration Agreement except as provided in this Agreement. After the Initial Transition Date, regulatory matters related to the Licensed Products, Galapagos Combination Product and Gilead Combination Products will be governed by the Second A&R Collaboration Agreement; provided, however, that any on-going activities related to […***…] shall be performed pursuant to this Agreement but under the purview of the Committees under the Second A&R Collaboration Agreement and subject to the applicable terms of the Second A&R Collaboration Agreement. |
| (e) | Supply Arrangements. The commercial supply arrangements for the Licensed Products in […***…] and, once Galapagos assumes the distribution obligations for the Licensed Products in the other countries, in accordance with this Agreement, such other countries in the Galapagos Territory, will be governed by the European Supply Agreement. The supply arrangement for Clinical Trial materials will be governed by the Clinical Supply Agreement. The Parties (on behalf of themselves and their respective Affiliates) hereby terminate the FIS Supply Agreement effective as of the Effective Date. The Parties hereby acknowledge and agree that except as provided in Section 8.1(b), neither Party nor their respective Affiliates shall have any further rights or obligations under the FIS Supply Agreement and that any post-termination obligations that may result from the termination of the FIS Supply Agreement (including Section 13.4 of the FIS Supply Agreement) will not be applicable as a result of such termination pursuant to this Agreement. |
| (f) | Quality Activities. The quality responsibilities relating to manufacture and distribution for the Licensed Products in […***…] and, once Galapagos assumes the distribution obligations for the Licensed Products in other countries in the Galapagos Territory, such other countries in the Galapagos Territory will be governed by the Quality Agreement, as amended pursuant to clause (i) of Section 6.3(e). The Parties hereby acknowledge and agree that, following the last-to-occur MA Transfer Completion Date, the Quality Agreement will be superseded by a new quality agreement as provided in clause (ii) of Section 6.3(e). |
| (g) | Commercialization Activities and Medical Affairs Activities. The Parties hereby terminate the Benelux Co-Commercialization Agreement and the EU5 Co-Commercialization Agreement effective as of the Effective Date. The Parties hereby acknowledge and agree that neither Party shall have any further rights or obligations under the Benelux Co-Commercialization Agreement or the EU5 Co-Commercialization Agreement and that any post-termination obligations that may result from the termination of either the Benelux Co-Commercialization Agreement or the EU5 Co-Commercialization Agreement (including, (i) ARTICLE 8 and Section 11.8 of the Benelux Co-Commercialization Agreement, and (ii) ARTICLE 8 and Section 11.8 of the EU5 Co-Commercialization Agreement) will not be applicable as a result of such terminations pursuant to this Agreement; provided that the termination of the Benelux Co-Commercialization Agreement or the EU5 Co-Commercialization Agreement will not affect any rights or obligations of the Parties that have accrued prior to the date of such termination. The on-going Commercialization activities and Medical Affairs Activities in the Shared Territory and the remainder of the Galapagos Territory will be governed by this Agreement until the Initial Transition Date. |
| After the Initial Transition Date, the Commercialization activities and the Medical Affairs Activities for the Galapagos Territory will be governed by the Second A&R Collaboration Agreement; provided, however, that any on-going Commercialization activities being conducted by Gilead for the Galapagos Territory that have not yet been transitioned to Galapagos shall be performed pursuant to this Agreement but under the purview of the Committees under the Second A&R Collaboration Agreement. |
ARTICLE 3
TIMING OF TRANSITION
| 3.1 | Timing of Transition Generally |
The Parties will use, and will procure that their respective Affiliates use, Transition Activity Commercially Reasonable Efforts to complete the Transition of all activities related to the Development, Manufacture and Commercialization of the Licensed Products in the Galapagos Territory in accordance with and at the times specified in this Agreement and the Transition Plan on or before the Initial Transition Date. If, as of the Effective Date, the transition plan for any given function, or any date or timeline expressly referenced herein as part of the Transition Plan, is not included in the initial Transition Plan attached hereto, the Parties shall discuss in good faith the transition activities for such function, or such date or timeline, as applicable, and amend the Transition Plan to include the mutually agreed plan for such transition activities or the mutually agreed date or timeline. If there are any activities related to the Transition that are not completed on or before the Initial Transition Date due to obstacles outside of the control of Galapagos, the Parties will use Transition Activity Commercially Reasonable Efforts to complete the Transition of such remaining activities as soon as reasonably practicable on or before the Latest Transition Date. Gilead shall not have any obligation to continue any such remaining Transition activities beyond the Latest Transition Date, except that (a) certain agreed upon activities will continue to be obligations of Gilead beyond the Initial Transition Date as described in this Agreement and the Transition Plan and (b) the Transition of certain activities may be subject to Regulatory Approval and such Regulatory Approval processes may continue beyond the Latest Transition Date for reasons outside of Galapagos’ control in which case Gilead shall use, and will procure that its Affiliates use, Transition Activity Commercially Reasonable Efforts to continue such activities until the necessary Regulatory Approvals have been obtained. Galapagos will be responsible for all out-of-pocket costs and expenses reasonably incurred by Gilead pursuant to clause (b) (excluding […***…]). Gilead will invoice Galapagos for any […***…], and Galapagos will pay them not later than […***…] following receipt of such invoice.
| 3.2 | Business Transfer Agreements |
The Parties will execute on the Effective Date (or such other date as mutually agreed) the […***…] Business Transfer Agreement to effect the transfer of assets held at the […***…] country level by Gilead to Galapagos, or its Affiliate(s), as set forth therein, including in the schedules attached thereto. To the extent necessary to effectively consummate the transfer of assets in other jurisdictions in accordance with the terms of this Agreement, the Parties will negotiate in good-faith and enter into such additional business transfer agreements, as may be required by such other jurisdictions.
ARTICLE 4
GOVERNANCE AND TRANSITION MANAGEMENT
| 4.1 | Existing Committees and Working Groups |
| (a) | The JSC and JDC established under the A&R Collaboration Agreement shall remain in effect provided that (i) the JDC shall have no oversight or decision-making authority with respect to any activities conducted under this Agreement other than (A) […***…], which shall remain under the JDC’s purview as set forth in the Applicable Collaboration Agreement, or (B) such other Development activities as may be set forth in the Second A&R Collaboration Agreement, (ii) the JSC shall have no oversight or decision-making authority with respect to any activities conducted under this Agreement other than […***…], and (iii) the last sentence of Section 7.1(a) shall apply with respect to Gilead’s final decision-making authority. |
| (b) | The Compliance Working Groups established under the Benelux Co-Commercialization Agreement and EU5 Co-Commercialization Agreement shall be converted into and continue as the Transition Compliance Working Group as set forth in Section 4.4. |
| (c) | The Joint Teams established for the EU5 Countries under the A&R Collaboration Agreement shall be converted into and continue as Country Transition Teams as set forth in Section 4.5(a). |
| (d) | The following committees and working groups are hereby disbanded: (i) the JCC and the Shared Territory JCC established pursuant to the A&R Collaboration Agreement, and (ii) the EU5 Distribution Working Group, and Country Pricing Committee established pursuant to the EU5 Co-Commercialization Agreement. |
| 4.2 | Transition Steering Committee |
| (a) | Purpose; Formation. Promptly following the execution of the Term Sheet, the Parties established the transition steering committee (the “TSC”). The TSC shall continue under this Agreement; provided that its responsibilities shall be to (i) oversee the JTT and (ii) attempt to resolve any disputes referred to it by the JTT pursuant to Section 4.3(e). |
| (b) | Composition. Each Party shall have the right to appoint […***…] representatives to the TSC, all of whom are executives of the applicable Party and have sufficient seniority within the applicable Party to make decisions arising within the scope of the TSC’s responsibilities. The Alliance Managers will attend TSC meetings as observers. The TSC may change its size from time to time if agreed by consensus among its members. Each Party may replace its TSC representatives at any time upon written notice to the other Party; provided, however, that neither Party may replace a representative on the TSC (except for the Party’s Alliance Manager) with an individual with lower seniority without the approval of the other Party, which approval shall not be unreasonably withheld, conditioned or delayed. The TSC may invite non-members to participate in the discussions and meetings of the TSC; provided that such participants shall have no voting authority at the TSC. The TSC shall have a chairperson who shall be selected by […***…]. The role of the chairperson shall be to convene and preside at meetings of the TSC. The Alliance Managers shall work with the chairperson to prepare and circulate agendas and ensure the preparation and execution of meeting minutes. The chairperson shall have no additional powers or rights beyond those held by the other TSC representatives. |
| (c) | Meetings. The TSC shall meet promptly (and in all cases within […***…] Business Days) to address any disputes referred by the JTT pursuant to Section 4.3(e). Either Party may also call a special meeting of the TSC (by videoconference, teleconference, or in person) by providing at least […***…] Business Days prior written notice to the other Party. To the extent practicable, no later than […***…] Business Days prior to any meeting of the TSC, the chairperson of the TSC shall prepare and circulate an agenda for such meeting; provided, however, that either Party may propose additional topics to be included on such agenda, either prior to or in the course of such meeting. The Parties may mutually agree to |
| additional meetings on shorter or longer notice in the event that matters arise requiring TSC consideration. The TSC may meet in person, by videoconference or by teleconference. Each Party will bear the expense of its respective TSC members’ participation in TSC meetings. Meetings of the TSC shall be effective only if at least one (1) representative of each Party is present or participating in such meeting. The Alliance Manager of […***…] shall be responsible for preparing reasonably detailed written minutes of all TSC meetings that reflect material decisions made and action items identified at such meetings. Such Alliance Manager shall send draft meeting minutes to each member of the TSC for review and approval within […***…] Business Days after each TSC meeting. Such minutes will be deemed approved unless one or more members of the TSC objects (whereby an objection by e-mail shall suffice) to the accuracy of such minutes within […***…] Business Days of receipt, in which case such Alliance Manager shall amend the draft meeting minutes accordingly and send the revised draft meeting minutes to each member of the TSC for review and approval within […***…] Business Days of receipt of the objection(s). The same review procedures and timelines as set out in the immediately preceding sentence shall apply to such revised draft meeting minutes. Minutes will be officially endorsed by the TSC at the next TSC meeting, and will be signed by the Alliance Managers. |
| (d) | Decision-Making. Subject to the remainder of this Section 4.2(d), the TSC shall act by consensus. The representatives from each Party will have, collectively, one (1) vote on behalf of that Party. The TSC shall use good faith efforts to promptly resolve any matters. If the TSC cannot reach consensus on an issue that comes before the TSC within […***…] Business Days of the meeting at which such issue was raised and over which the TSC has oversight, then either Party may elect, by written notice to the other Party, to submit such issue to the Parties’ Executive Officers. The TSC shall submit, in writing and within […***…] Business Days of such notice, the respective positions of the Parties to their respective Executive Officers. Such Executive Officers shall use good faith efforts to resolve promptly such matter, which good faith efforts shall include at least one meeting between such Executive Officers within […***…] Business Days after the TSC’s submission of such matter to them. If the Executive Officers are unable to reach consensus on any such matter, then such matter shall be decided by the Executive Officer of […***…]; provided, however, […***…]. |
| 4.3 | Joint Transition Team |
| (a) | Purpose; Formation. Promptly following the execution of the Term Sheet, the Parties established the joint transition team (the “JTT”). The JTT shall continue under this Agreement; provided that its responsibilities shall be as set forth in this Agreement. |
| (b) | Composition. Each Party shall use reasonable efforts to appoint representatives to the JTT with expertise in the following functional areas: […***…], as well as a representative who shall be responsible for leading the Transition activities for such Party. Each Party’s representatives on the JTT shall have sufficient seniority within the applicable Party to make decisions arising within the scope of the JTT’s responsibilities. The Alliance Managers will attend JTT meetings as observers. The JTT may change its size from time to time; provided that there must be at least […***…] representatives from each Party. Subject to the foregoing expertise and size requirement, each Party may add, remove or replace its JTT representatives at any time upon written notice to the other Party. The JTT representatives may invite non-members to participate in the discussions and meetings of the JTT. The JTT shall have a chairperson who shall be selected by […***…]. The role of the chairperson shall be to convene and preside at meetings of the JTT. |
| (c) | Specific Responsibilities. In addition to its overall responsibilities set forth in Section 4.3(a), the JTT shall in particular: |
| (i) | oversee the execution of the Transition Plan and discuss, monitor, coordinate and provide the necessary support for the Transition activities of the Parties under this Agreement, the Transition Plan, the Development Plan and Budget and the Shared Territory Commercialization Plan and Budget (other than […***…], which remain under the purview of the JDC); |
| (ii) | review, at least […***…], the Shared Territory Commercialization Plan and Budget and suggest any applicable amendments thereto for review and approval by the JTT; |
| (iii) | review and approve any amendments, modifications or changes to the Transition Plan, the Development activities contemplated by the Development Plan and Budget (other than […***…]), the Shared Territory Commercialization Plan and Budget or any other Commercialization plan applicable to […***…]; |
| (iv) | discuss and analyze a material breach by any Party (if referred to it by the non-breaching Party), determine and approve any Remedial Measures to be imposed on such breaching Party and oversee timely implementation of such Remedial Measures by the breaching Party; |
| (v) | approve or consent to any matters under this Agreement that require the approval or consent of the JTT; |
| (vi) | prior to the Initial Transition Date, prepare, review and approve a Joint Development Plan and Budget, if requested by a Party; |
| (vii) | attempt to resolve any disputes referred to it by any Country Transition Teams pursuant to Section 4.5(a) or by the Functional Team, or any disputes that may arise out of Galapagos’ exercise of its General Direction pursuant to Section 4.10; |
| (viii) | establish such additional working groups as it deems necessary to achieve the foregoing objectives; |
| (ix) | discuss compliance issues arising under this Agreement or the Transition Ancillary Agreements that would conflict with […***…] on an ad hoc basis as required; provided that, day-to-day discussions will be had by the Transition Compliance Working Group in accordance with Section 4.4(b). When dealing with such compliance issues, the JTT will invite representatives with relevant compliance knowledge and expertise from both Parties to participate in the discussions; and |
| (x) | prior to the last MA Transfer Completion Date to occur, review and approve Galapagos Public Communications, as need be; provided that, in case of any Galapagos Public Communication that is required by Applicable Law or the rules of a stock exchange on which the securities of Galapagos are listed, (1) Galapagos shall not be required to delay such Galapagos Public Communication for such JTT review, (2) such Galapagos Public Communication shall not require any approval of the JTT, and (3) Galapagos shall not be required to make any disclosure to the JTT that is not permitted by Applicable Law or the rules of a stock exchange on which the securities of Galapagos are listed. |
For clarity, the JTT shall have no oversight or decision-making authority with respect to the Commercialization of any Licensed Products or Gilead Combination Products in the Gilead Territory, as set forth in Section 9.1(g), except to the extent such activities materially adversely affect the Exploitation of the Licensed Product or Galapagos Combination Products by Galapagos in the Galapagos Territory, with respect to which activities neither Party shall have unilateral final decision-making authority notwithstanding anything to the contrary herein (any issues related to such activities not agreed to by the JTT will be resolved in accordance with Section 16.6). Additionally, the JTT shall have no oversight or decision making authority with respect to […***…].
| (d) | Meetings. The JTT shall meet (by videoconference, teleconference, or in person) with such meeting frequency as agreed by the JTT. Either Party’s representatives on the JTT may also call a meeting of the JTT (by videoconference, teleconference, or in person) by providing at least […***…] Business Days prior written notice to the other Party’s representatives. Prior to any meeting of the JTT, the chairperson of the JTT shall prepare and circulate an agenda for such meeting; provided, however, that either Party may propose additional topics to be included on such agenda, either prior to or in the course of such meeting. The Parties may mutually agree to additional meetings on shorter or longer notice in the event that matters arise requiring JTT consideration. The JTT may meet in person, by videoconference or by teleconference. Each Party will bear the expense of its respective JTT members’ participation in JTT meetings. Meetings of the JTT shall be effective only if at least one (1) representative of each Party (which representative is not such Party’s Alliance Manager) is present or participating in such meeting. The Alliance Manager of […***…] shall be responsible for preparing reasonably detailed written minutes of all JTT meetings that reflect material decisions made and action items identified at such meetings. Such Alliance Manager shall send draft meeting minutes to each member of the JTT for review and approval within […***…] Business Days after each JTT meeting. Such minutes will be deemed approved unless one or more members of the JTT objects (whereby an objection by e-mail shall suffice) to the accuracy of such minutes within […***…] Business Days of receipt, in which case such Alliance Manager shall amend the draft meeting minutes accordingly and send the revised draft meeting minutes to each member of the JTT for review and approval within […***…] Business Days of receipt of the objection(s). The same review procedures and timelines as set out in the immediately preceding sentence shall apply to such revised draft meeting minutes. Minutes will be officially endorsed by the JTT at the next JTT meeting, and will be signed by the Alliance Managers. |
| (e) | Decision-Making. Subject to the remainder of this Section 4.3(e) and Section 4.2, the JTT shall act by consensus. The representatives from each Party will have, collectively, one (1) vote on behalf of that Party. If the JTT cannot reach consensus on an issue that comes before the JTT within […***…] Business Days of the meeting at which such issue was raised and over which the JTT has oversight, then either Party may refer such matter to the TSC for resolution in accordance with Section 4.2; provided, however, that […***…]. |
| 4.4 | Transition Compliance Working Group. |
| (a) | Purpose; Formation. Upon the Effective Date, the Compliance Working Groups established under the Benelux Co-Commercialization Agreement and EU5 Co-Commercialization Agreement shall be automatically converted into and continue as a compliance working group of the JTT (the “Transition Compliance Working Group”). |
| (b) | Specific Responsibilities of the Transition Compliance Working Group. The Transition Compliance Working Group will (i) identify and discuss compliance issues arising under this Agreement or the Transition Ancillary Agreements that would conflict with […***…] (and identify such compliance issues that require consideration by the JTT in accordance with Section 4.3(c)(ix)), (ii) address such matters in foregoing clause (i) to ensure that […***…] and (iii) subject to Sections 9.1(e) and 11.3, determine ways of working together to enable appropriate review of Promotional Materials. |
| (c) | Decision-Making. Subject to the remainder of this Section 4.4(c) and Section 4.3, the Transition Compliance Working Group shall act by consensus. The representatives from each Party will have, collectively, one (1) vote on behalf of that Party. If the Transition Compliance Working Group cannot reach consensus on an issue within its responsibilities within […***…] Business Days of the meeting at which such issue was raised and over which the Transition Compliance Working Group has oversight, then either Party may refer such matter to the JTT for resolution. |
| 4.5 | Country Transition Teams. |
| (a) | Purpose; Formation; Decision-Making. For […***…] separately and jointly for all other countries in the Galapagos Territory (other than […***…]), the Parties shall promptly establish (in any event no later than May 3, 2021) a transition team (each, a “Country Transition Team”) to coordinate the Parties’ respective Transition activities in such country/ies and to facilitate the communication and exchange of information between the Parties with respect to the Transition in such country/ies under Galapagos’ General Direction. Each of the Joint Teams established for each […***…] under the A&R Collaboration Agreement are hereby converted into the corresponding Country Transition Team. If a Country Transition Team cannot reach consensus on an issue related to a Party’s Transition activities then either Party may refer such matter to the JTT. |
| (b) | Composition. Each Country Transition Team shall have at least one representative appointed from each Party. |
| 4.6 | Functional Teams |
The day-to-day Transition activities under this Agreement shall be conducted by the functional teams of both Parties (each, a “Functional Team”), it being understood that any issues that arise between the Functional Teams can be brought to the JTT for discussion and resolution if need be.
| 4.7 | Good Faith |
In conducting themselves on the TSC, JTT, Transition Compliance Working Group, the Country Transition Teams and the Functional Teams, and in exercising their rights under this ARTICLE 4, all representatives of each Party shall consider reasonably and in good faith all input received from the other Party. Without limiting or expanding rights or obligations with respect to application of Transition Activity Commercially Reasonable Efforts standards in its performance under this Agreement or the Transition Ancillary Agreements, in making decisions in accordance with its duties in serving on the TSC, JTT, Transition Compliance Working Group, Country Transition Teams, or Functional Teams each Party shall act in accordance with Applicable Law and Applicable Guidelines and based on its good faith judgment taking into consideration the best interests of the Licensed Compound and Licensed Products in the Galapagos Territory and the Gilead Territory and the Transition, provided that, […***…].
| 4.8 | General Committee Authority |
Each of the TSC, JTT, Transition Compliance Working Group, Country Transition Teams and Functional Teams shall have solely the powers expressly assigned to it in this ARTICLE 4 and elsewhere in this Agreement. Except as expressly provided in this Agreement, the TSC, JTT, Country Transition Teams and Functional Teams shall not have any power to amend, modify, or waive compliance with this Agreement, any Transition Ancillary Agreement, the Applicable Collaboration Agreement or any Ancillary Agreement. It is expressly understood and agreed that the control of decision-making authority by a Party, pursuant to Section 4.2(d), so as to resolve a disagreement or deadlock on the TSC for any matter will not authorize either Party to perform any function or exercise any decision-making right not delegated to the TSC, JTT, Country Transition Teams, Functional Teams or such Party, and that neither Galapagos nor Gilead shall have any right to unilaterally modify or amend, or waive its own compliance with, the terms of this Agreement or the Applicable Collaboration Agreement (including any diligence obligation contained herein or therein).
| 4.9 | Disbandment |
The TSC and JTT shall dissolve on the Initial Transition Date and the governance of any ongoing Transition activities under their purview shall be transitioned to and thereafter governed by the Committees under the Second A&R Collaboration Agreement. The Transition Compliance Working Group shall dissolve on the last-to-occur MA Transfer Completion Date. Each Country Transition Team shall dissolve on the date of Commercial Handover for the applicable Registered Country (unless otherwise agreed by the Parties). Each Functional Team shall cease to be considered a “Functional Team” under this Agreement upon completion of the Transition activities hereunder with respect to such function (or upon any earlier mutual written agreement of the Parties). Any escalations from the Transition Compliance Working Group and any Functional Team after the Initial Transition Date shall be made to the JSC.
| 4.10 | General Direction of Galapagos |
As of the Effective Date and until the applicable MA Transfer Completion Date for a given Registered Country, Galapagos, through the TSC, JTT, Country Transition Teams and Functional Teams, shall provide general direction to Gilead on the commercial, medical affairs, and regulatory activities performed by Gilead under this Agreement (“General Direction”).
Gilead shall use Transition Activity Commercially Reasonable Efforts to take any action proposed in good faith by Galapagos in Galapagos’ exercise of its General Direction. Notwithstanding anything to the contrary in this Agreement (including, for clarity, the Transition Plan), […***…]. General Direction of Galapagos does not include the direct supervision or management by Galapagos of Gilead’s personnel and will not impose any requirement on Gilead to […***…].
ARTICLE 5
PERSONNEL
| 5.1 | TUPE Employees |
| (a) | The Parties agree that the employment contracts of the Territory Employees based in […***…], if then still employed by Gilead (or the relevant Affiliate of Gilead) unless the listed Territory Employee has validly objected as per the applicable local legal provisions, shall automatically and in a definitive manner transfer to Galapagos (or the relevant Affiliate of Galapagos) in accordance with the TUPE Regulations (the “TUPE Employees”) upon local completion of the Transition in those countries, respectively (the date of such local completion in relation to such country, the relevant “TUPE Transfer |
| Date”). As a result, as of the relevant TUPE Transfer Date, Galapagos (or the relevant Affiliate of Galapagos) will employ the relevant TUPE Employees on substantially similar contractual terms and conditions, or identical contractual terms and conditions if required under the TUPE Regulations, but not including rights pursuant to any Gilead Plan as in effect for each such TUPE Employee prior to the transfer, except as otherwise required by Applicable Law. |
| (b) | With respect to the Territory Employees of […***…] on Schedule 3, Gilead and Galapagos (or their relevant Affiliates) have jointly informed in writing these employees in accordance with […***…]. Gilead and Galapagos (or their relevant Affiliates) have provided the required information they are responsible for in this respect, i.e. Gilead (or its relevant Affiliate) has provided information on the current employment situation and Galapagos (or its relevant Affiliate) has provided information on any envisaged changes that need to be included in this […***…]. The […***…] contains reference to the statutory deadline of one month after receipt of the […***…] to object to the transfer of employment in writing. The Parties shall promptly inform each other of any notice of objection and consent received from any of these employees and will as soon as practically possible determine which of these employees have transferred in a definitive manner from […***…] to the respective Galapagos Affiliate […***…]. |
| (c) | The Parties will coordinate and use Transition Activity Commercially Reasonable Efforts to effect the transfer of (i) the […***…] as soon as possible following the Effective Date and at the latest on or before […***…] and (ii) the […***…] as soon as possible following the Effective Date and at the latest on or around […***…]. Galapagos will use Transition Activity Commercially Reasonable Efforts to establish legal entities in […***…] as soon as possible following the Effective Date, and the Parties will coordinate and use Transition Activity Commercially Reasonable Efforts to effect the transfer of the TUPE Employees in such countries promptly after the establishment of the applicable legal entity (and in no event later than the Initial Transition Date). |
| (d) | Until the relevant TUPE Transfer Date for the respective TUPE Employees, Gilead will provide to Galapagos, within […***…] after the end of each calendar month, an updated Schedule 3, in each case updated as of the last day of a month, to reflect any TUPE Employees initially included on such Schedule that would no longer be employed by Gilead prior to the relevant TUPE Transfer Date. |
| (e) | From the Effective Date until the relevant TUPE Transfer Date for the respective TUPE Employees, Gilead commits not to implement (and not to agree to, or to announce) […***…]), unless if required by Applicable Law (subject to prior notice to Galapagos), […***…], or if approved in advance by Galapagos; provided that Gilead may […***…] if, […***…]. For clarity, […***…]. |
| (f) | Each Party shall provide the other Party in a timely manner with such information and support as is reasonably required in order to comply with any information and consultation obligation owed to the TUPE Employees under Applicable Law. |
| (g) | The Parties shall coordinate in advance on the substance and timing of any substantive communications to the TUPE Employees and/or their representatives in relation to the transfer of the TUPE Employees. |
| (h) | In accordance with the provisions of Section 5.3(a) and Section 5.3(c), Gilead shall remain exclusively liable for, and solely responsible for the payment to the TUPE Employees of, […***…] compensation these employees would be entitled to under the Gilead Plans for fiscal year ending on […***…], and which are usually paid after […***…]. Galapagos shall be exclusively liable for, and solely responsible for the payment to the TUPE Employees of, any […***…] compensation these employees would be entitled to under the Gilead Plans for fiscal year ending on […***…], and which are usually paid after […***…], as if such TUPE Employees had been employed by Gilead through the end of such fiscal year. |
| (i) | Unless Applicable Law requires Gilead to make such prorated payment directly to the TUPE Employees upon their transfer to Galapagos, or its relevant Affiliate(s), or if determined otherwise by Applicable Law in any other manner, Gilead shall pay to Galapagos within […***…] days of the relevant TUPE Transfer Date an amount in cash in euro equal to the accrued but not yet payable pro rata part of the thirteenth month, accrued vacation rights and variable pay (incentives or other) for the reference period as ongoing at the TUPE Transfer Date for each of the relevant TUPE Employees concerned (together with the applicable Employment Taxes thereon) that relates to the period of employment of the TUPE Employees with Gilead (or the relevant Affiliates of Gilead) prior to the relevant TUPE Transfer Date (the “Accrued Benefits Amount”), subject to the following provisions: |
| (i) | Gilead shall calculate the exact Accrued Benefits Amount as per the relevant TUPE Transfer Date, and communicate the same in writing to Galapagos within […***…] following the relevant TUPE Transfer Date. If Galapagos objects to such calculation, it shall notify Gilead of such objection within […***…] after its receipt of such calculation and specify in reasonable detail the reasons for its objections. The Parties will use Transition Activity Commercially Reasonable Efforts to resolve such disagreement within […***…]. If the Parties are unable to resolve such disagreement within such time period, then either Party may submit it to the JTT for resolution, and the JTT (if applicable, the TSC) will resolve it in accordance with ARTICLE 4. The Accrued Benefits Amount determined following the final resolution of such calculation shall be paid by Gilead or Galapagos, as applicable, within […***…] following the date on which the calculation is agreed upon or the dispute is resolved. |
| (ii) | If the transfer of the TUPE Employees to Galapagos, or its relevant Affiliate(s), occurs in the course of a sales quarter for which target linked incentives are due, the Parties agree that […***…]. |
| (j) | Until […***…], employment of any […***…] may not be terminated by Galapagos or such Affiliate on the grounds of operational reasons |
| (k) | Subject to Applicable Law, Galapagos and its Affiliates will have reasonable access to the personnel records (including performance appraisals, disciplinary actions, grievances and medical records) of Gilead and its Affiliates for the purpose of preparing for the continued employment of the TUPE Employees as from the TUPE Transfer Date. In coordination with the relevant Gilead Human Resources personnel, Galapagos and its Affiliates will also be allowed to conduct meetings with the TUPE Employees prior to the TUPE Transfer Date. |
| (l) | Exhibit F includes (i) a complete and exhaustive list of all of those Gilead Plans, applicable to the […***…]. |
| 5.2 | Non-TUPE Employees |
| (a) | In general, in relation to all Territory Employees other than the TUPE Employees (subject to the provisions of Section 5.1) (the “Non-TUPE Employees”), the Parties agree, […***…]. |
| (b) | Galapagos will not be obligated to hire any of the Non-TUPE Employees, but may make offers of employment as provided for in this Section 5.2 to any or all of such Non-TUPE Employees. |
| (c) | Without prejudice to the general principle set out in Section 5.2(a), in relation to the Non-TUPE Employees that are employed in the Galapagos Territory in […***…] (such countries the “Non-TUPE Countries”, and such Non-TUPE Employees, collectively, the “Country Non-TUPE Employees”), the following rules shall apply: |
| (i) | Until the Initial Transition Date or, if earlier, until Galapagos has adequately staffed the Non-TUPE Countries on a country-by-country basis, Gilead will provide such services as are necessary or reasonably requested by Galapagos to satisfy Gilead’s obligations under this Agreement, the Transition Plan, the Development Plan and Budget and the Shared Territory Commercialization Plan and Budget with respect to the Non-TUPE Countries. |
| (ii) | Galapagos will deliver to Gilead on or before […***…] with respect to […***…], and […***…] with respect to […***…] the list of Country Non-TUPE Employees to whom Galapagos intends to make employment offers (each such employee a “Prospective Employee”), and the Parties will coordinate on communications to the Prospective Employees as soon as possible thereafter in accordance with Applicable Law. |
| (iii) | As soon as Galapagos (or a relevant Affiliate of Galapagos) is legally and practically able to make such informed employment offers on a country-by-country basis in the Non-TUPE Countries, Galapagos (or the relevant Affiliate of Galapagos) will make an employment offer to the respective Prospective Employees (such date, the “Offer Date”). The employment contract of each such Prospective Employee with Gilead (or the relevant Affiliate of Gilead) will be terminated by mutual consent if the Prospective Employee accepts the offer and becomes an employee of Galapagos or a relevant Affiliate of Galapagos (in which event such Prospective Employee will become a “Hired Employee”). Absent such offer or in the event of a definitive or final refusal thereof, Gilead will be allowed to redeploy any such individual Prospective Employees to other roles within Gilead. |
| (iv) | After the Effective Date and prior to the applicable Offer Date, Gilead commits not to implement (and not to agree to, or to announce) […***…] of the relevant Non-TUPE Employees (including […***…]), unless if required by Applicable Law (subject to prior notice to Galapagos), […***…], or if approved in advance by Galapagos; provided that Gilead may […***…] if, […***…]. For clarity, […***…]. |
| (d) | Without prejudice to the general principle set out in Section 5.2(a), in relation to Non-TUPE Employees that are employed by Gilead in […***…] and that provide Galapagos Territory-wide functions that support […***…] (the “Regional Non-TUPE Employees”), until […***…], Gilead will provide services to support all applicable key deliverables and milestones set forth in this Agreement and the Transition Plan applicable to the Regional Non-TUPE Employees that are to be completed by Gilead prior to such date; provided, that Gilead shall continue to provide such services beyond such date if […***…]. |
| (e) | With regard to Non-TUPE Employees in parts of the Galapagos Territory other than those referred to in Section 5.2(c) and Section 5.2(d), the Parties confirm that the discontinuation of local activities pursuant to the Transition will not […***…], unless agreed otherwise between the Parties. |
| (f) | Subject to Applicable Law, Galapagos and its Affiliates will have reasonable access to the personnel records (including performance appraisals, disciplinary actions, grievances and medical records) of Gilead and its Affiliates for the purpose of preparing for and conducting employment interviews with and or making any employment offer to any or all of the Country Non-TUPE Employees. |
| (g) | Galapagos will have the right to set its own initial terms and conditions of employment for the Hired Employees, including compensation and benefits structure, all as permitted by Applicable Law. Unless required by Applicable Law, Galapagos will not be obligated to assume any […***…] as in place at Gilead. |
| (h) | […***…] will be solely liable for any payment required to be made to the Hired Employees in relation to the termination (by mutual consent) of the Hired Employees’ employment contract with […***…], including to any (advance) vacation pay, prorated 13th month and prorated incentive (or other type of variable pay, as applicable). |
| (i) | Neither Galapagos’ nor its Affiliates’ expressed intention to offer employment as set forth in this Section 5.2 will constitute a Contract (express or implied) on the part of Galapagos or such Affiliate of Galapagos to an employment relationship of any term or duration or upon any terms or conditions other than those that Galapagos or such Affiliate of Galapagos may establish pursuant to individual offers of employment. Employment offered by Galapagos or its relevant Affiliate may be terminated by Galapagos or such Affiliate at any time for any reason, subject to Applicable Law. |
| (j) | Nothing in this Agreement will be deemed to prevent or restrict in any way the right of Galapagos or its Affiliates to terminate, reassign, promote or demote any Hired Employees that have accepted an employment offer of Galapagos or its Affiliates, or to change adversely or favorably the title, powers, duties, responsibilities, functions, locations, salaries, other compensation or terms or conditions of employment of such Hired Employees. |
| 5.3 | General rules in relation to the New Galapagos Employees |
Except as otherwise provided in Section 5.1(l) and without prejudice to the detailed provisions of Sections 5.1 and 5.2 above, the following rules shall apply to the TUPE Employees and Hired Employees (the “New Galapagos Employees”):
| (a) | Gilead and its Affiliates will remain solely responsible, and will indemnify Galapagos and its Affiliates, for any and all liabilities to or in respect of the New Galapagos Employees and their beneficiaries and dependents, relating to or arising in connection with the period of employment of each such New Galapagos Employee with Gilead or any of its Affiliates or the termination of their employment by Gilead, whether or not any claims are asserted before, on or after such New Galapagos Employee commences employment with Galapagos (or its Affiliate), including: |
| (i) | […***…], |
| (ii) | any liabilities relating to the participation in or accrual of benefits or compensation under a Gilead Plan, or the failure to participate in or to accrue compensation or benefits under, any Gilead Plan or other employee or retiree benefit or compensation plan, program, practice, policy or other Contract of Gilead or any of its Affiliates, including: |
| (A) | any liabilities in relation to any defined benefit or defined contribution pension plan based […***…], |
| (B) | any liabilities in relation to […***…], other health or other welfare or fringe benefits or expense reimbursements, |
| (C) | any liabilities in relation to any agreements and other commitments, whether of an individual or collective nature regulating […***…]. |
| (b) | Galapagos and its Affiliates will be solely responsible for, and will indemnify Gilead and its Affiliates, for any and all liabilities to or in respect of the New Galapagos Employees and their beneficiaries and dependents, relating to or arising in connection with the period of employment of the New Galapagos Employee with Galapagos or its Affiliates from and including the relevant TUPE Transfer Date for TUPE Employees or start date of employment for Hired Employees, as applicable. |
| (c) | Gilead and its Affiliates will perform and discharge all of their obligations in respect of the New Galapagos Employees for their own account up to the relevant TUPE Transfer Date for TUPE Employees or the date the Hired Employees commence employment with Galapagos or its Affiliates, as applicable (including discharging all remuneration and other costs), unless determined otherwise in this Agreement. |
| (d) | Gilead and Galapagos (or their relevant Affiliate, as applicable) will be jointly responsible for any and all liabilities resulting from incorrect or incomplete information they provided for the […***…] and will indemnify the other Party (or its relevant Affiliate, as applicable) in this respect. |
| 5.4 | Other provisions |
| (a) | Subject to the remainder of this ARTICLE 5 (including Schedule 3), during the Transition, except as otherwise agreed by the Parties in writing and except for the transfer of the New Galapagos Employees as set forth herein, each Party agrees that neither it nor its Affiliates that are engaged in activities under this Agreement (each such Person in such event, a “Participating Entity”), shall actively recruit, solicit or induce, directly or indirectly, any employee or individual consultant of the other Party or its Affiliates who participates in activities relating to the Licensed Product(s) within the Galapagos Territory (the “Participating Employee”) to terminate his or her employment or consulting or similar agreement with such other Participating Entity and become employed by or consult for the first Participating Entity, whether or not such Participating Employee is a full-time employee of such other Participating Entity, and whether or not such employment is pursuant to a written agreement or is at-will; provided that, the Parties may mutually agree |
| in writing for Participating Employees to become employed by or consult for the other Participating Entity. For purposes of this Section 5.4(a), “recruit,” “solicit” and “induce” shall not include (i) general solicitations by Third Party placement specialists or firms (e.g., head-hunters) or (ii) other general solicitations of employment (including responses to general advertisements), in each case (in respect of the foregoing (i) and (ii)) not specifically targeted at Participating Employees of a Participating Entity. |
| (b) | Gilead and its Affiliates will remain solely responsible, and will indemnify Galapagos and its Affiliates, for any and all liabilities to or in respect of any Territory Employee other than the New Galapagos Employees or any employee of Gilead or any of its Affiliates other than a Territory Employee. Galapagos shall promptly notify Gilead if Galapagos becomes aware of any such Territory Employee or other employee of Gilead or any of its Affiliates that claims to have transferred to Galapagos pursuant to TUPE Regulations. |
| (c) | With respect to the services that will be provided by Gilead and its Affiliates to Galapagos and its Affiliates pursuant to the terms of this Agreement, Gilead commits (i) to […***…] in order to be able to meet its transitional obligations under this Agreement in line with the applicable key deliverables and milestones set forth in this Agreement and the Transition Plan (without being limited by any redeployment of Territory Employees as permitted in this ARTICLE 5), and (ii) to […***…] staff performing such services, […***…]. The obligation of Gilead and its Affiliates to provide any specific transition services will cease as soon as Galapagos or the relevant Galapagos Affiliate is adequately staffed and knowledge transfer has been completed in line with the applicable deliverables and milestones set forth in this Agreement and the Transition Plan. |
| (d) | Galapagos will use Transition Activity Commercially Reasonable Efforts to hire personnel throughout Europe and its headquarters to adequately staff itself as soon as possible to meet its transition obligations hereunder and in line with the applicable deliverables and milestones set forth in this Agreement and the Transition Plan. |
| (e) | The Parties and their respective Affiliates will cooperate with any required notification with respect to, or any required consultation with, the employees, employee representatives, work councils, unions, labor boards and relevant Governmental Authorities concerning the transactions contemplated by this Agreement and take whatever other actions as may be necessary to carry out the arrangements described in this ARTICLE 5, including providing each other with such plan documents and summary plan descriptions, employee data or other information as may be reasonably requested. |
| (f) | Nothing contained in this Agreement, expressed or implied, is intended to confer upon any employee of Gilead or its Affiliates any right to employment or continued employment with Galapagos, or any benefits, including severance benefits, by reason of this Agreement. |
ARTICLE 6
REGULATORY MATTERS
| 6.1 | Transfer of Regulatory Documentation |
| (a) | Gilead shall provide Galapagos or its designated Affiliate with a copy of the Transferred Regulatory Documentation for each Registered Country promptly following the Effective Date. On or prior to the date determined by the JTT or, if applicable, the TSC, Gilead will, and will cause its Affiliate(s) to, assign and transfer to Galapagos, or its Affiliate(s), all of the Transferred Regulatory Documentation as specified in the Transition Plan to the extent |
| that such Transferred Regulatory Documentation is not otherwise and would no longer be required to be used or held for use in connection with the Licensed Products in the Gilead Territory or for Clinical Trials being conducted by Gilead or its Affiliate(s) that are not Transferred Clinical Trials. Each Party, or its Affiliate(s), as applicable, will submit all filings, letters and other documentation necessary to effect such assignments and transfers to the applicable Regulatory Authority. |
| (b) | Subject to Section 6.1(c), the Parties will coordinate and plan for filing, and Gilead or Galapagos will file, for the transfer of the Marketing Authorization or Marketing Authorization application in each Registered Country to the Galapagos MAH by […***…] or on such earlier date as mutually agreed by the Parties. Prior to any transfer or withdrawal of the Marketing Authorization or Marketing Authorization application in a Registered Country pursuant to Section 6.1(d)(i), Galapagos shall continue to perform any and all Delegated Galapagos Activities with respect to such Marketing Authorization or Marketing Authorization application, and Gilead shall continue to perform any and all Pre-Transfer Gilead MAH Activities with respect to such Marketing Authorization or Marketing Authorization application. Notwithstanding the foregoing or anything to the contrary in this Agreement, Gilead’s obligations with respect to the transfer or maintenance of Marketing Authorization or Marketing Authorization application in a Registered Country shall terminate upon the earlier of (i) the applicable MA Transfer Completion Date and (ii) the Initial Transition Date except that Gilead’s obligations shall continue to the extent that the regulatory approval process for such Marketing Authorization transfer is not completed for reasons outside of the control of Galapagos or to the extent that the implementation period for the transfer agreed upon by the Gilead MAH and Galapagos MAH with the applicable Regulatory Authority with respect to any such agreed activities has not expired. |
| (c) | The Target MA Submission Date for each applicable Marketing Authorization and Marketing Authorization application is set forth on Schedule 1. At the applicable time in light of the Target MA Submission Date, the Parties shall prepare and, in accordance with Applicable Law and on or prior to the applicable Target MA Submission Date, Gilead MAH or Galapagos MAH shall submit to the applicable Regulatory Authority all documentation necessary to apply for or register the transfer of the Marketing Authorization or Marketing Authorization application in each Registered Country to the applicable Galapagos MAH. If such submission cannot be made in a Registered Country by the applicable Target MA Submission Date, the JTT shall, within […***…] of becoming aware of the situation, meet and discuss in good faith and agree to the next steps to ensure that such documentation is submitted in such Registered Country as promptly as possible thereafter; provided that the applicable MAH shall make the submission no later than […***…]. |
| (d) | The Parties desire to transfer the Marketing Authorization or Marketing Authorization application in each Registered Country to the Galapagos MAH in a manner designed to ensure continuity of regulatory compliance by both Parties, Commercialization of Licensed Products and regulatory progress of Marketing Authorization applications in each Registered Country. |
| (i) | If the Marketing Authorization or Marketing Authorization application in a Registered Country does not transfer to the Galapagos MAH by the anticipated completion date, then the JTT shall agree (i) to next steps to achieve the transfer of the Marketing Authorization or Marketing Authorization application or (ii) […***…]. |
| (ii) | If any such failure to transfer a Marketing Authorization or Marketing Authorization application in accordance with this Section 6.1 is due to any act or omission by either Party or the applicable MAH, then such Party or MAH, as applicable, will use Transition Activity Commercially Reasonable Efforts to cure any such act or omission within […***…] of being notified of such failure. |
| (e) | On the applicable MA Transfer Completion Date, Gilead shall, and shall cause its Affiliates to, deliver to Galapagos (or Galapagos’ designated Affiliate) all of Gilead’s right, title and interest in and to the applicable Marketing Authorization or Marketing Authorization application to the extent Controlled by Gilead or its Affiliate(s). |
| (f) | Gilead will continue to be responsible for the global safety, Clinical Trial and non-clinical studies databases until the transfer thereof pursuant to the Transition Plan. Gilead shall be responsible for any and all costs and expenses related to such global safety database prior to its transfer to Galapagos. |
| (g) | Gilead will continue to provide the pharmacovigilance-activities for Galapagos Territory assigned to it under the PVA and the Applicable Collaboration Agreement in accordance therewith, including the services of Gilead’s EU Qualified Person for Pharmacovigilance, and Galapagos will maintain the appropriate processes to report into Gilead’s pharmacovigilance-system. Both Parties shall use Transition Activity Commercially Reasonable Efforts to effect the transfer of such pharmacovigilance-activities to Galapagos, or its Affiliate(s), on or before the Initial Transition Date in accordance with the Transition Plan. |
| 6.2 | MAH Related Activities |
| (a) | The Parties acknowledge that the MAH for the Licensed Products in a Registered Country has certain rights and obligations under Applicable Law as holder of such Marketing Authorization or Marketing Authorization application, which include performing certain activities for the Licensed Products in such Registered Country (the “MAH Related Activities”). Unless otherwise mutually agreed by the Parties, the performance of MAH Related Activities shall be allocated between Gilead and Galapagos as set forth in this ARTICLE 6. |
| (b) | Without limiting Section 6.1(b), each Party shall use Transition Activity Commercially Reasonable Efforts (i) to perform or cause to be performed the applicable MAH Related Activities as allocated to such Party in this ARTICLE 6 for the Licensed Products in the applicable Registered Country in accordance with the terms of this Agreement and in accordance with all Applicable Law using appropriately qualified and trained personnel and (ii) to cooperate and assist the other Party as reasonably necessary for such other Party to perform the applicable MAH Related Activities as allocated to such other Party in this ARTICLE 6 for the Licensed Products in the applicable Registered Country in accordance with the terms of this Agreement and in accordance with all Applicable Law. |
| 6.3 | MA Transition |
The Parties agree to the following allocation of responsibilities with respect to the MAH Related Activities for each Marketing Authorization or Marketing Authorization application in a Registered Country:
| (a) | Pre-MA Transfer Completion Date. |
| (i) | For the period beginning on the Effective Date and ending on the MA Transfer Completion Date for such Marketing Authorization or Marketing Authorization application, the Gilead MAH shall, under the General Direction of Galapagos, handle all matters related to any Licensed Products involving Regulatory Authorities in a Registered Country, to the extent the Marketing Authorization or Marketing Authorization application has not yet been assigned and transferred to Galapagos. |
| (ii) | For the period beginning on the Effective Date and ending on the MA Transfer Completion Date for such Marketing Authorization or Marketing Authorization application, the Gilead MAH shall be responsible for interfacing, corresponding and meeting with the applicable Regulatory Authority with respect to the Licensed Products in the applicable Registered Country (collectively, “Regulatory Communications”) and shall keep Galapagos reasonably informed with respect to all regulatory matters relating to any Licensed Products in a Registered Country. Gilead shall use Transition Activity Commercially Reasonable Efforts to provide a full copy of any material Regulatory Communications to Galapagos within […***…] of receipt by Gilead. The Parties shall coordinate on all material Regulatory Communication submissions to the applicable Regulatory Authorities. Gilead shall provide a full copy of any proposed material Regulatory Communication submission together with the relevant underlying data to Galapagos in a timely manner. Galapagos shall have the right to review and comment on any such Regulatory Communication prior to the submission of such Regulatory Communication to the applicable Regulatory Authority by the Gilead MAH. Gilead shall promptly provide full copies of any other non-material Regulatory Communications and submissions to Galapagos for information purposes. Transfer of documentation under this Section 6.3(a)(ii) shall be conducted through electronic mail to the attention of the Parties’ respective employee(s) identified to each other or a data sharing site mutually agreed by and accessible to the Parties. |
| (iii) | For the period beginning on the Effective Date and ending on the MA Transfer Completion Date for such Marketing Authorization or Marketing Authorization application, Gilead shall provide Galapagos with reasonable advance notice of all formal meetings and teleconferences with Regulatory Authorities in a Registered Country pertaining to any Licensed Products. To the extent not prohibited by Applicable Law, Gilead shall permit Galapagos to have, at Galapagos’ expense, representatives of Galapagos be present and address Regulatory Authorities, to the extent agreed pursuant to the following sentence, at such formal meetings and teleconferences with Regulatory Authorities in the Galapagos Territory to the extent pertaining to such Licensed Products. Prior to any such meetings or teleconferences, the Parties will meet and agree, on […***…]. |
| (iv) | Gilead will execute and deliver to Galapagos, or its relevant Affiliate(s), (A) no later than the Effective Date, amendments to the existing powers of attorney for the […***…] in the forms mutually agreed, (B) no later than the Effective Date, new powers of attorney for […***…] in the forms mutually agreed and (C) powers of attorney for any other Registered Country or any amendments to existing powers of attorney as may be reasonably requested by Galapagos on or after the Effective Date (each, a “Power of Attorney”) in order to grant, or cause to be granted, to |
| Galapagos or an Affiliate of Galapagos, a delegation of authority with respect to the applicable Marketing Authorization or Marketing Authorization application (the “Delegation of Authority”) pursuant to which Galapagos or its Affiliates shall be responsible for the following, in each case to the extent doing so would not conflict with Gilead’s MAH Legal Responsibilities: |
| (A) | Commercializing the Licensed Products in such Registered Country in accordance with this Agreement, the Transition Plan and the Shared Territory Commercialization Plan and Budget; |
| (B) | any and all pricing strategy and other pricing decisions and determinations for the Licensed Products in such Registered Country in accordance with this Agreement, the Transition Plan and the Shared Territory Commercialization Plan and Budget; and |
| (C) | Medical Affairs Activities and Market Access Activities for the Licensed Products in such Registered Country in accordance with this Agreement, the Transition Plan and the Shared Territory Commercialization Plan and Budget. |
| (b) | Post-MA Transfer Completion Date. On and after the MA Transfer Completion Date for a Registered Country, Galapagos will be solely responsible for all regulatory matters (including for clarity, promotional copy review, local representative responsibilities and quality assurance responsibilities for Affiliates) relating to any Licensed Products in such Registered Country. On and after the MA Transfer Completion Date for a Registered Country, the applicable Galapagos MAH shall have the sole right to conduct all MAH Related Activities in such Registered Country, including the MA Maintenance activities. |
| (c) | Sharing of Regulatory Information; Cooperation. For as long as one Party (or its Affiliate) is the MAH for the Licensed Products in its Respective Territory, the other Party shall provide, or cause to be provided, to the MAH Party in a timely manner full copies of any documents, correspondence or any other regulatory information Controlled by such other Party and relating specifically to any Marketing Authorization or Marketing Authorization application in the MAH Party’s Respective Territory and provide support reasonably necessary for the MAH Party’s MA Maintenance activities upon such MAH Party’s reasonable request. |
| (d) | Withdrawal and Recalls. Prior to the Initial Transition Date, if (i) any Regulatory Authority threatens, initiates or advises any action to remove any Licensed Product from the market in the Galapagos Territory or requires or advises Galapagos, Gilead, or any of their respective Affiliates or Sublicensees to distribute a “Direct Healthcare Professional Communication” letter or its equivalent regarding use of such Licensed Product in the Galapagos Territory, or (ii) either Party determines that an event, incident, or circumstance has occurred that may result in the need for a recall or market withdrawal in the Galapagos Territory, then in each case ((i) or (ii)) Galapagos or Gilead, as applicable, shall, to the extent practicable, notify the other Party of such event or determination immediately, and in any event within […***…] (or sooner if required by Applicable Law) after such Party becomes aware of the event or makes such determination. Such Party shall, to the extent practicable, endeavor to discuss and agree with the other Party upon whether to recall or withdraw the Licensed Product in the Galapagos Territory. The Party responsible for distribution of the Licensed Products for the Development activities or Commercialization |
| activities, as applicable, in the Galapagos Territory shall be responsible for conducting any recalls or taking such other necessary remedial action with respect to Licensed Products in the Galapagos Territory; provided that, […***…]. Each Party shall be responsible for […***…] of the out-of-pocket costs and expenses incurred in conducting such recall or taking such necessary remedial action; provided, however that (x) Gilead will be solely responsible for the costs and expenses incurred in conducting such recall or taking such necessary remedial action, to the extent caused by actions or inactions by or on behalf of Gilead or its Affiliates (for clarity, excluding actions or inactions by or on behalf of Galapagos or its Affiliates) and (y) Galapagos will be solely responsible for the costs and expenses incurred in conducting such recall or taking such necessary remedial action, to the extent caused by actions or inactions by or on behalf of Galapagos or its Affiliates (for clarity, excluding actions or inactions by or on behalf of Gilead or its Affiliates). If not prohibited under Applicable Law, at Galapagos’ request, the Parties shall use Transition Activity Commercially Reasonable Efforts to complete reinstatement of any Licensed Product withdrawn from the Galapagos Territory as a result of such recall or market withdrawal. |
| (e) | Amendments to Quality Agreement and PVA. The Parties shall execute (i) an amendment to the Quality Agreement as needed in light of the Transition, including the manufacture, supply and distribution of Licensed Products for the entire Galapagos Territory, as soon as practicable following the Effective Date, (ii) a separate quality agreement that would supersede the Quality Agreement and take effect on the last-to-occur MA Transfer Completion Date to effectuate the transfer of applicable legal responsibilities of the MAH for a Registered Country or Registered Countries upon the applicable MA Transfer Completion Date, and (iii) an amendment to the PVA in connection with the transfer of global safety database in accordance with this Agreement no later than the Initial Transition Date. |
| 6.4 | Unallocated MAH Activities |
To the extent additional MAH Related Activities are subsequently identified and not otherwise allocated between the Parties pursuant to this ARTICLE 6, the JTT shall discuss the appropriate allocation of such activity between each Party and their MAHs, taking into account the principles set forth in this ARTICLE 6, and the JTT shall coordinate the delegation of any MAH Related Activities that are permitted to be delegated under Applicable Law; provided that […***…]. Notwithstanding the foregoing, for any MAH Related Activity that is required as a new post-approval commitment for the Galapagos Territory, the Party that is responsible for performing the associated underlying Development activity (either […***…]) shall be responsible for performing such MAH Related Activity and any costs incurred in performing such MAH Related Activity shall be allocated between the Parties as if such costs were incurred in performing the associated underlying Development activity (either […***…]) in accordance with Exhibit A.
ARTICLE 7
DEVELOPMENT
| 7.1 | Transition Development Activities and Data-Related Activities |
| (a) | Each Party shall use Commercially Reasonable Efforts to conduct the Transition Development Activities assigned to it under the Development Plan and Budget or the Joint Development Plan and Budget and to achieve the timelines set forth in this Agreement, the Development Plan and Budget, and the Joint Development Plan and Budget, as applicable. Either Party may propose amendments to the Transition Development Activities from time- |
| to-time (i) during the period from the Effective Date until the Initial Transition Date, through its members on the JTT (other than amendments to the […***…], which shall be referred to the JDC) and (ii) from and after the Initial Transition Date, as set forth in the Second A&R Collaboration Agreement. The Parties acknowledge and confirm that […***…] are the only Development activities for Licensed Compound or Licensed Products occurring as of the Effective Date by or on behalf of either Party or their Affiliates. The column entitled “Operational Responsibility” in the Development Plan and Budget sets forth the responsible Party for the operation of each of the […***…]. The decision-making process with respect to the […***…] will continue in accordance with the terms and conditions of the Applicable Collaboration Agreement. […***…] shall not have the right to exercise its final decision making authority (A) to stop the application of any cost-sharing arrangement with respect to any […***…] that are, and for so long as, required by Regulatory Authorities to continue, (B) in a manner that will materially adversely impact the Exploitation of the Licensed Compound or Licensed Product in Galapagos’ Territory provided that […***…], (C) to increase the budget for any […***…], or (D) to increase […***…] level of activities or materially alter the nature of such […***…]. |
| (b) | From and after the Effective Date, Gilead shall deliver or provide to Galapagos, or its Affiliate(s), in accordance with the Transition Plan access to all Information and data Controlled by Gilead or its Affiliate(s) that are related to the Development of the Licensed Product in the Galapagos Territory (including from the global development program, if applicable) and the Transferred Clinical Trials. Notwithstanding the foregoing or anything to the contrary in this Agreement, Gilead shall not have any obligation to provide or transfer to Galapagos any Information related to […***…]. |
| (c) | Each Party shall keep the JTT reasonably informed regarding (i) the progress and results of Transition Development Activities, including by providing a quarterly report in reasonable detail of results versus goals (as such goals are set forth in the Development Plan and Budget, Joint Development Plan and Budget and the Transition Plan) as, in the case of each Party, is typically generated by such Party with respect to its product research and development efforts and (ii) any information with respect to Independent Activities required to be provided pursuant to Section 7.4(g). |
| (d) | Each Party shall maintain complete and accurate records (in the form of technical notebooks or electronic files where appropriate) of all work conducted by it in performance of the Transition Development Activities or any Independent Activities Plan and all Information resulting from such work. Such records shall fully and properly reflect all work done and results achieved in the performance of such plans in sufficient detail and in good scientific manner appropriate for patent and regulatory purposes. Each Party shall have the right to access such records maintained by the other Party to the extent reasonably necessary to perform obligations or to exercise rights under this Agreement. The JTT shall determine the means by which such access will be provided. |
| (e) | Within the timelines set forth in the Transition Plan, Gilead shall (A) transfer to Galapagos (i) the data analysis activities related to the clinical studies set forth in the Transition Plan, together with relevant underlying data and specifications Controlled by Gilead or its Affiliate(s) and (ii) other data analysis activities Controlled by Gilead or its Affiliate(s) […***…], and (B) as reasonably requested by Galapagos, provide the requested internal Gilead support to Galapagos, or its Affiliates, with respect to the activities mentioned in clause (A). At any time prior to […***…], Gilead shall perform data analysis activities in support of the […***…] as reasonably requested by Galapagos. Furthermore, within the |
| timelines set forth in the Transition Plan, upon Galapagos’ reasonable request, Gilead shall perform such value and market access analyses as set forth in the Transition Plan or as are otherwise critical for reimbursement applications in […***…]. Following the transfer of such data analysis activities as described under this Section 7.1(e), upon Gilead’s reasonable request and at Gilead’s reasonable costs (which, for clarity, excludes FTE Costs), Galapagos shall provide Gilead with data relating to the Licensed Products that are Controlled by Galapagos or any applicable contract research organization or qualified vendor as is necessary to support Gilead’s activities with respect to […***…] in the Gilead Territory. |
| 7.2 | Clinical Trials |
| (a) | The Parties acknowledge that the Clinical Trials for the Licensed Product set forth in the Development Plan and Budget are being performed as of Effective Date and that certain of such Clinical Trials are sponsored by Gilead. |
| (b) | The Parties shall coordinate to transfer to Galapagos the sponsorship of each of the following Clinical Trials (the “Transferred Clinical Trials”) together with applicable Regulatory Documentation and Transferred Contracts in accordance with the Transition Plan. The Parties shall mutually establish, by the date indicated in the Transition Plan for the applicable Clinical Trial below for the applicable Transferred Clinical Trial, a plan to transition each of the following Clinical Trials from Gilead to Galapagos, or its relevant Affiliate(s). The start date for the performance of the relevant transfer activities set forth in the Transition Plan shall be no later than the corresponding date specified below: |
| (i) | […***…]; |
| (ii) | […***…]; and |
| (iii) | […***…]. |
Prior to the Effective Date, the sponsorship of […***…] was transferred to Galapagos and shall be deemed to be a Transferred Clinical Trial for purposes of this Agreement.
The Parties shall use Transition Activity Commercially Reasonable Efforts to complete the transfer of the sponsorship of each Transferred Clinical Trial within […***…] of the applicable date set forth above for such Transferred Clinical Trial and, if the Regulatory Approval process is continuing beyond such […***…] period for reasons outside of the Parties’ control, shall continue to use Transition Activity Commercially Reasonable Efforts thereafter until the transfer of the sponsorship of such Transferred Clinical Trial is completed. For clarity, in accordance with Section 16.4, Gilead shall have the option at any time to transfer the management responsibilities with respect to the Transferred Clinical Trials to a contract research organization or qualified vendor approved by Galapagos (such approval not to be unreasonably withheld, conditioned or delayed). To the extent the sponsorship of any additional Clinical Trial needs to be transferred by Gilead or one of its Affiliates to Galapagos, or its Affiliate(s), to result in Galapagos having operational responsibility for all of the Clinical Trials assigned to it in the column entitled “Operational Responsibility” in the Development Plan and Budget, the Parties shall use Transition Activity Commercially Reasonable Efforts to agree upon the timeline and process for transferring the sponsorship of such additional Clinical Trial and related activities; provided that, from the Effective Date until the Initial Transition Date, any disagreements between the Parties with respect to the Clinical Trials relating to […***…]
| shall be resolved by the JTT and any disagreements with respect to Clinical Trials relating to […***…] shall be resolved by the JDC; and following the Initial Transition Date, any disagreements between the Parties with respect to Clinical Trials shall be resolved by the JDC as set forth in the Second A&R Collaboration Agreement. Upon the completion of each such transfer, the applicable additional Clinical Trial shall be deemed to be a Transferred Clinical Trial. Gilead and its Affiliates shall provide copies of relevant documentation and materials Controlled by Gilead or its Affiliate(s) that are necessary for or reasonably requested by Galapagos to perform such Transferred Clinical Trials and any related activities that are transferred to Galapagos, or its Affiliate(s), promptly upon the reasonable request by Galapagos. |
For so long as Gilead is the MAH, notwithstanding the foregoing or anything else to the contrary in this Agreement, (i) following the completion of transfer of the applicable sponsorship, Galapagos shall not conduct or discontinue (or permit to be conducted or discontinued) any of the Transferred Clinical Trials in a manner that would cause Gilead, in its capacity as MAH, to violate Applicable Law.
| (c) | Gilead will retain sponsorship of each of the following Clinical Trials until their termination or completion: |
| (i) | […***…]; |
| (ii) | […***…]; and |
| (iii) | […***…]. |
| (d) | Each Party shall promptly (i) notify the other Party of any discussions, correspondence or meetings that such Party engages in with any Regulatory Authority related to the Transferred Clinical Trials or the Clinical Trials specified in Section 7.2(c) and (ii) provide to the other Party copies of any such correspondence and detailed summaries of any such discussions or meetings, in each case (clauses (i) and (ii)), to the extent they reasonably relate to or affect any activities being conducted in the other Party’s Respective Territory. |
| (e) | Each Party agrees that (a) each Clinical Trial set forth in the Development Plan and Budget that is required to be posted pursuant to Applicable Law or applicable industry codes, including the PhRMA Code, on clinicaltrials.gov or any other similar registry shall be so posted, and (b) all results of such Clinical Trials that are necessary for obtaining a Regulatory Approval for a Licensed Product, Galapagos Combination Product or Gilead Combination Product in the Territory shall be posted on clinictrial.gov, clinicalstudyresults.org or on any other registry with requirements consistent with the registration and publication guidelines of the International Committee of Medical Journal Editors, to the extent required. Each Party shall submit any data and Information generated under such Clinical Trial posted on clinicaltrial.gov, clinicalstudyresults.org or any other registry pursuant to this Section 7.2(e) to the other Party for its review and approval (not to be unreasonably conditioned, delayed or withheld) at least […***…] prior to its intended publication. |
| 7.3 | New Joint Development Activities |
| (a) | From the Effective Date to the Initial Transition Date, the initiation, sponsorship and conduct of any new Development activities (the “New Joint Development Activities”) for the Licensed Products outside of the […***…] that are not covered by the Development Plan and Budget or the Transition Plan will be subject to mutual agreement by the Parties and governed by this Agreement. From and after such Initial Transition Date, the initiation, sponsorship and conduct of any New Joint Development Activities (for clarity, including those initiated prior to the Initial Transition Date) will be governed by the Second A&R Collaboration Agreement. |
| (b) | From the Effective Date to the Initial Transition Date, if either Party desires to conduct any New Joint Development Activities, then it shall present such plans to the JTT for review and discussion. If the other Party desires to have such New Joint Development Activities conducted pursuant to a joint development plan (the “Joint Development Plan and Budget”), then the JTT will prepare, review and approve a Joint Development Plan and Budget for such activities; provided that, in preparing such Joint Development Plan and Budget, the Parties agree to take into account the needs of both Parties in their Respective Territories for the Licensed Product, including with respect to Regulatory Authority requirements and the New Joint Development Activities (e.g., required patient populations and study costs). Such Joint Development Plan and Budget shall include any New Joint Development Activities for the Licensed Products proposed by one Party which the other Party agrees to include and may include any other New Joint Development Activities that the Parties mutually agree to include (including New Joint Development Activities to be conducted on a global basis or in both Parties’ Respective Territories). Unless otherwise mutually agreed by the Parties in writing, each Joint Development Plan and Budget shall provide that each Party shall be responsible for the portion of included New Joint Development Activities in its Respective Territory. Each Party shall conduct the activities assigned to it under the Joint Development Plan and Budget and use Transition Activity Commercially Reasonable Efforts to achieve the timelines set forth therein. Either Party may propose amendments to a Joint Development Plan and Budget from time-to-time through its members on the JTT; provided that neither Party shall have the final decision-making authority over any Joint Development Plan and Budget, including any amendments thereto (either through JTT, TSC, or otherwise); provided further, that such Joint Development Plan and Budget shall include the plan and budget for only those Development activities mutually agreed to by the Parties and each Party shall have the right to propose and conduct (to the extent allowed under Section 7.4) Independent Activities in its Respective Territory in accordance with Section 7.4 and the other terms and conditions of this Agreement if the other Party does not agree to include such activity in the applicable Joint Development Plan and Budget. |
| (c) | The Parties shall share […***…] the Development Costs (as defined in the A&R Collaboration Agreement, which, for clarity, excludes […***…]) incurred by or on behalf of either or both Parties or their Affiliates solely to the extent related to the New Joint Development Activities performed in accordance with the Joint Development Plan and Budget. Within […***…] after the end of each calendar quarter, each Party shall provide to the other Party a report in reasonable detail of any such Development Costs incurred by such Party for the New Joint Development Activities in such calendar quarter (which amount may be based on such Party’s good faith estimate). Such Development Costs so reported shall be used for the calculation of the applicable allocation of the Development Costs for the New Joint Development Activities. Any adjustments made to such reported amounts shall be taken in the calendar quarter in which such adjustments are recorded. Within […***…] following receipt of such report(s), the Party that incurred the higher aggregate Development Costs for the applicable calendar quarter than the amount for which it is responsible pursuant to this Section 7.3(c), shall invoice the other Party for […***…] of |
| such amount. The Party receiving such invoice shall pay it not later than […***…] following receipt thereof, if and to the extent that any spend by the invoicing Party (i) does not exceed […***…] of the budgeted costs and expenses set forth in the Joint Development Plan and Budget for such activities for such calendar year for the Party or (ii) is otherwise approved by the Parties, such approval not to be unreasonably conditioned, withheld or delayed. To the extent that such spend exceeds […***…] of the budgeted costs and expenses set forth in the Joint Development Plan and Budget for such activities for such calendar year for such Party and was not otherwise approved by the Parties in writing, then such additional spend (greater than […***…] of the budgeted costs and expenses) shall not constitute Development Costs in such calendar year and shall be borne by the Party incurring the same. The Parties will further work together and reasonably take into account the internal and external reporting requirements and timelines of the other Party when preparing and delivering reports to the other Party pursuant to this Section 7.3(c). |
| 7.4 | Independent Development Activities |
| (a) | If, prior to the Initial Transition Date, either Party desires to conduct any new Development activities in its Respective Territory that (i) the other Party does not agree to include in a Joint Development Plan and Budget in accordance with Section 7.3(b) and (ii) would not be reasonably expected to have a material adverse effect on the Development or Commercialization of the Licensed Products in the Respective Territory of the other Party (for clarity, in no event shall any Clinical Trial that is required for Regulatory Approval in the Independent Activities Party’s Respective Territory be reasonably expected to have a material adverse effect on the Development or Commercialization of Licensed Products in the Respective Territory of the other Party) (any such activities, “Independent Activities”), then Section 7.4(b) shall apply. From and after the Initial Transition Date, all Independent Activities (for clarity, including those initiated prior to the Initial Transition Date) will be conducted pursuant to the Second A&R Collaboration Agreement and subject to the terms and conditions thereof. |
| (b) | For any Independent Activities: |
| (i) | the Party that desires to conduct such Independent Activities (the “Independent Activities Party”) shall provide the JTT with a written plan for conducting such Independent Activities (“Independent Activities Plan”), which Independent Activities Plan shall describe the applicable Independent Activities in reasonable detail, including for the new Development activities, a description of the applicable Clinical Trial and expected data to be generated therefrom; |
| (ii) | within […***…] after receipt of such Independent Activities Plan, the JTT shall meet to discuss and resolve any comments of the other Party with respect to such Independent Activities Plan; and |
| (iii) | following such meeting of the JTT, such Independent Activities Party shall have the right to conduct Independent Activities (including Clinical Trials) set forth in such Independent Activities Plan, in each case, in accordance with Section 7.4(c). |
| (c) | Any Independent Activities shall be conducted in accordance with this Section 7.4(c). |
| (i) | Any Independent Activities shall be carried out in accordance with the applicable Independent Activities Plan that was last reviewed by the JTT in accordance with Section 7.4(b)(ii); provided that non-substantive changes do not have to be reviewed by the JTT. For clarity, if the Independent Activities Party desires to carry out additional substantive Independent Activities that are not set forth in an Independent Activities Plan that was reviewed by the JTT in accordance with Section 7.4(b)(ii), then such Independent Activities Party shall follow the procedures set forth in Section 7.4(b) with respect to such additional Independent Activities. |
| (ii) | Subject to Section 7.4(g), the Independent Activities Party shall be solely responsible for all costs and expenses for Independent Activities carried out by or on behalf of such Independent Activities Party. |
| (iii) | The applicable Independent Activities Party shall keep the JTT reasonably informed regarding the progress of Independent Activities, including by providing a quarterly report in reasonable detail of results as, in the case of each Party, is typically generated by such Party with respect to its product research and development efforts. Upon completion of any Independent Activities, the Independent Activities Party shall provide the other Party with a report in reasonable detail of the Independent Activities Data generated in connection with such Independent Activities. |
| (d) | As between the Parties, the Independent Activities Party shall solely own all right, title and interest in and to all Information consisting of data generated by or on behalf of such Independent Activities Party or its Affiliates solely from performing the applicable Independent Activities (“Independent Activities Data”). Any Independent Activities Data generated by or on behalf of Gilead shall be deemed to be Gilead Foreground Know-How and, notwithstanding anything to the contrary herein or in the A&R Collaboration Agreement, Galapagos shall not have the right to use such Independent Activities Data (other than safety data) for any purpose, except as set forth in Sections 7.4(d), (e), (f) and (g) or as otherwise agreed in writing by Gilead. Any Independent Activities Data generated by or on behalf of Galapagos shall be deemed to be Galapagos Foreground Know-How and, notwithstanding anything to the contrary herein or in the A&R Collaboration Agreement, Gilead shall not have the right to use such Independent Activities Data (other than safety data), except as set forth in Sections 7.4(d), (e), (f) and (g)or as otherwise agreed in writing by Galapagos. |
| (e) | As between the Parties, the Independent Activities Party shall solely own all right, title and interest in and to all Regulatory Materials and Regulatory Approvals to the extent resulting solely from the applicable Independent Activities (“Independent Activities Regulatory Documentation”). Any Independent Activities Regulatory Documentation resulting solely from Independent Activities for which Gilead is the Independent Activities Party shall be owned solely by Gilead and, notwithstanding anything to the contrary herein or in the A&R Collaboration Agreement, Galapagos shall not have the right to use or reference or access such Independent Activities Regulatory Documentation for any purpose, except as set forth in Sections 7.4(d), (e), (f) and (g) or as otherwise agreed in writing by Gilead. Any Independent Activities Regulatory Documentation resulting solely from Independent Activities for which Galapagos is the Independent Activities Party shall be owned solely by Galapagos and, notwithstanding anything to the contrary herein or in the A&R Collaboration Agreement, Gilead shall not have the right to use or reference or access such Independent Activities Regulatory Documentation for any purpose, except as set forth in Sections 7.4(d), (e), (f) and (g) or as otherwise agreed in writing by Galapagos. |
| (f) | Notwithstanding anything to the contrary in Sections 7.4(d) and (e), any safety-related information (i) shall be covered by the PVA, and (ii) may be used by the non-Independent Activities Party on the Licensed Product label(s) and disclosed by the non-Independent Activities Party to any Regulatory Authority as required by Applicable Law or required by such Regulatory Authority in connection with the Licensed Product as being Developed and Commercialized by the non-Independent Activities Party, without payment of any fee or consideration to the Independent Activities Party and without any such additional agreement as contemplated in Section 7.4(g). |
| (g) | The non-Independent Activities Party with respect to any Independent Activities Data or Independent Activities Regulatory Documentation may provide written notice to the Independent Activities Party that it desires to terminate the restrictions on use of such Independent Activities Data or Independent Activities Regulatory Documentation set forth in Sections 7.4(d) and (e). Upon receipt of such notice, the Independent Activities Party shall provide the other Party with the total Development Costs expended for such Independent Activities (calculated as if such activities were included in Joint Development Plan and Budget) (the “Independent Activities Costs”). The non-Independent Activities Party may, in its discretion, elect to buy back the right to use such Independent Activities Data or Independent Activities Regulatory Documentation by paying to the Independent Activities Party […***…] of the total Independent Activities Costs. Following such payment by the non-Independent Activities Party to the Independent Activities Party, (a) the restrictions on use of such Independent Activities Data or Independent Activities Regulatory Documentation set forth in Sections 7.4(d) and (e) shall terminate and the non-Independent Activities Party shall have a license and right of reference for such Independent Activities Data or Independent Activities Regulatory Documentation as set forth in Section 7.1 of the A&R Collaboration Agreement (as amended by Section 10.2(c) of this Agreement) and Section 10.2(e) of this Agreement, as applicable, and (b) the Independent Activities Party shall provide to the other Party a report covering the records for such Independent Activities described in Section 7.1(d). |
| 7.5 | Combination of the Licensed Compound with Other Compounds |
Notwithstanding anything to the contrary in this Agreement or in the Option, License and Collaboration Agreement, from the Effective Date until the Initial Transition Date, neither Party will conduct hereunder or under the Option, License and Collaboration Agreement, or provide product, compound or financial support for, Development of any […***…], unless the Parties mutually agree in writing; provided, however, that, Galapagos shall have the right to conduct Nonclinical Studies for Galapagos Combination Products at its sole discretion.
| 7.6 | Amendments to A&R Collaboration Agreement; Prior Development Plans |
| (a) | All Development activities shall be conducted in accordance with this Agreement commencing on the Effective Date, and Sections 3.1 through 3.9 of the A&R Collaboration Agreement shall each be deleted in its entirety as of the Effective Date and replaced with the following: |
“3.1 Overview of Development. Gilead shall be primarily responsible for Development and seeking Regulatory Approval of the Licensed Product and Gilead Combination Products in the Gilead Territory and shall use Commercially Reasonable Efforts with respect thereto for the first Licensed Product in […***…] in both […***…]. Notwithstanding anything to the contrary in this Agreement (but subject to the Transition
& Amendment Agreement), the immediately foregoing obligation with respect to Commercially Reasonable Efforts in […***…] and the […***…] indications shall be Gilead’s sole diligence obligation with respect to Development of Licensed Products and Gilead Combination Products, and Gilead shall have the right, but not any obligation, to Develop other Licensed Products and Gilead Combination Products in other countries in the Gilead Territory or for other indications and no diligence obligation of Gilead shall arise under this Agreement with respect to Development of Gilead Combination Products. Galapagos shall use Commercially Reasonable Efforts as reasonably requested by Gilead to assist Gilead with Development activities with respect to the P […***…]. Upon initiation of a […***…], Gilead hereby commits, absent Safety Issues, to complete such Clinical Trial (or such portion thereof), subject to Section 13.3(a)(ii). For the avoidance of doubt, the foregoing obligation does not apply to any long term extension of such […***…]. Following each data read-out of such […***…], at the request of Gilead, the Parties shall discuss the efficacy and safety implications of such data and, in light of such implications, consider whether to continue, amend or wind-down such […***…].”
The Parties agree that the Development Plan and Budget supersedes and replaces all existing Development Plans as of the Effective Date, and the Parties shall have no further obligations under any such Development Plan.
| (b) | Section 1.3 of the A&R Collaboration Agreement shall be deleted in its entirety as of the Effective Date and replaced with the following: |
“1.3 “Affiliate” means, with respect to a particular Person, a person, corporation, partnership, or other entity that controls, is controlled by or is under common control with such Person. For the purposes of this definition, the word “control” (including, with correlative meaning, the terms “controlled by” or “under the common control with”) means the actual power, either directly or indirectly through one or more intermediaries, to direct or cause the direction of the management and policies of such entity, whether by the ownership of more than fifty percent (50%) of the voting stock of such entity, by contract or otherwise; provided, however, that neither Party will be considered an Affiliate of the other Party.”
| (c) | Section 1.27 of the A&R Collaboration Agreement shall be deleted in its entirety as of the Effective Date and replaced with the following: |
“1.27 “Co-Commercialization Term” means, with respect to a country in the Shared Territory, the period from the Amendment Effective Date through the Initial Transition Date (as defined in the Transition & Amendment Agreement).”
| (d) | Section 1.29 of the A&R Collaboration Agreement shall be deleted in its entirety as of the Effective Date and replaced with the following: |
“1.29 “Collaboration Know-How” means, to the extent not Gilead Combination Know-How, all Information related exclusively to a Licensed Product or the Licensed Compound and that is conceived, discovered, developed or otherwise made jointly by the Parties, in each case optionally with their Affiliates or any Third Parties or any employees, consultants or agents of any of the foregoing, in performing the activities under the Original Agreement, this Agreement, the Second A&R Collaboration Agreement, any Ancillary Agreement or the Transition and Amendment Agreement (including any Transition Ancillary Agreement and the performance of the Transition Development Activities, Joint Development Plan and Budget, or Shared Territory Commercialization Plan and Budget (each, as defined in the Transition & Amendment Agreement)).”
| (e) | Section 1.31 of the A&R Collaboration Agreement shall be deleted in its entirety as of the Effective Date and replaced with the following: |
“1.31 “Commercialization” means any and all activities directed to marketing, promoting, distributing, importing, exporting, using, offering to sell, selling or having sold a product, including activities related to the commercial manufacture, marketing, promotion, sale or distribution of a product in the Territory, and, for purposes of setting forth the rights and obligations of the Parties under this Agreement, shall be deemed to include conducting Medical Affairs Activities. Commercialization shall include commercial activities conducted in preparation for a product launch. Commercialization expressly excludes […***…]. “Commercialize” has a correlative meaning.”
| (f) | Section 1.44 of the A&R Collaboration Agreement shall be deleted in its entirety as of the Effective Date and replaced with the following: |
“1.44 “Development” means all activities, including Transition Development Activities, that relate to (a) obtaining or maintaining Regulatory Approval of a Licensed Product, a Galapagos Combination Product or a Gilead Combination Product for one or more indications, (b) developing the process for the Manufacture of clinical and commercial quantities of a Licensed Product, a Galapagos Combination Product or a Gilead Combination Product, or (c) the conduct of Nonclinical Studies and Clinical Trials (including Phase 4 Clinical Trials for a Licensed Product, a Galapagos Combination Product or a Gilead Combination Product), including the preparation, submission, review and development of data or information in support of a submission to a Regulatory Authority to obtain or maintain Regulatory Approval of a Licensed Compound, a Licensed Product, a Galapagos Combination Product or a Gilead Combination Product, as applicable, including the services of outside advisors in connection therewith, including its legal counsel and regulatory consultants, but excluding (A) Commercialization and (B) the Manufacture and accumulation of commercial inventory of a Licensed Product, a Galapagos Combination Product or a Gilead Combination Product, as applicable. “Develop” has a correlative meaning.”
| (g) | Section 1.80 of the A&R Collaboration Agreement shall be deleted in its entirety as of the Effective Date and replaced with the following: |
“1.80 “Galapagos Combination Product” means a pharmaceutical product containing the Licensed Compound in combination with […***…]. The term “in combination,” covers instances where the Licensed Compound and at least […***…].”
| (h) | Section 1.82 of the A&R Collaboration Agreement shall be deleted in its entirety as of the Effective Date and replaced with the following: |
“1.82 “Galapagos Foreground Know-How” means all Information conceived, discovered, developed or otherwise made solely by Galapagos, optionally with its Affiliates or any Third Parties or any employees, consultants or agents of any of the foregoing, in performing the activities under the Original Agreement, this Agreement, the Second A&R Collaboration Agreement, any Ancillary Agreement or the Transition & Amendment Agreement (including any Transition Ancillary Agreement and the performance of the Transition Development Activities, Joint Development Plan and Budget or Shared Territory Commercialization Plan and Budget (each, as defined in the Transition & Amendment Agreement)). For clarity, Galapagos Foreground Know-How shall exclude rights under any Galapagos Patents.”
| (i) | Section 1.94 of the A&R Collaboration Agreement shall be deleted in its entirety as of the Effective Date and replaced with the following: |
“1.94 “Gilead Combination Product” means a pharmaceutical product containing the Licensed Compound in combination with […***…]. The term “in combination,” covers instances where the Licensed Compound and at least […***…].”
| (j) | Section 1.97 of the A&R Collaboration Agreement shall be deleted in its entirety as of the Effective Date and replaced with the following: |
“1.97 “Gilead Foreground Know-How” means all Information conceived, discovered, developed or otherwise made solely by Gilead, optionally with its Affiliates or any Third Parties or any employees, consultants or agents of any of the foregoing, in performing the activities under the Original Agreement, this Agreement, the Second A&R Collaboration Agreement, any Ancillary Agreement or the Transition & Amendment Agreement (including any Transition Ancillary Agreement and the performance of the Transition Development Activities, Joint Development Plan and Budget or Shared Territory Commercialization Plan and Budget (each, as defined in the Transition & Amendment Agreement)). For clarity, Gilead Foreground Know-How shall exclude rights under any Gilead Foreground Patents and Gilead Combination Know-How.”
| (k) | Section 1.120 of the A&R Collaboration Agreement shall be deleted in its entirety as of the Effective Date and replaced with the following: |
“1.120. “Joint Foreground Know-How” means, to the extent not Collaboration Know-How or Gilead Combination Know-How, all Information conceived, discovered, developed or otherwise made jointly by the Parties, optionally with their respective Affiliates, in performing the activities under the Original Agreement, this Agreement, the Second A&R Collaboration Agreement, any Ancillary Agreement or the Transition & Amendment Agreement (including any Transition Ancillary Agreement and the performance of the Transition Development Activities, Joint Development Plan and Budget or Shared Territory Commercialization Plan and Budget (each, as defined in the Transition & Amendment Agreement)). Joint Foreground Know-How shall exclude Collaboration Know-How, rights under any Joint Patents and Gilead Combination Know-How.”
| (l) | Section 1.126 of the A&R Collaboration Agreement shall be deleted in its entirety as of the Effective Date and replaced with the following: |
“1.126. “Licensed Product(s)” means any product, other than a Gilead Combination Product or a Galapagos Combination Product, which product contains a Licensed Compound. Licensed Product includes all such products containing the same Licensed Compound, alone or in combination with one or more active pharmaceutical ingredients, in any and all finished forms, presentations, delivery systems, strength, dosages, and formulations. By the term in combination, it is intended to include where the Licensed Compound and the one or more active pharmaceutical ingredients are sold either as a fixed dose combination or with separate doses in a single package.”
| (m) | The following definition shall be inserted as a new definition between the defined terms for “Option, License and Collaboration Agreement” and “Other Co-Commercialization Indications” in the A&R Collaboration Agreement as of the Effective Date. |
“Original Agreement” means that certain License and Collaboration Agreement, dated December 16, 2015, entered into by the Parties.”
| (n) | The following definition shall be inserted as a new definition between the defined terms for “SEC” and “Shared Program Activities” in the A&R Collaboration Agreement as of the Effective Date. |
“Second A&R Collaboration Agreement” means that certain Second Amended and Restated License and Collaboration Agreement to be effective as of December 31, 2021, as the same may be amended or otherwise supplemented from time to time.”
| (o) | The following definition shall be inserted as a new definition between the defined terms for “Transition Agreement” and “Ulcerative Colitis” in the A&R Collaboration Agreement as of the Effective Date: |
“Transition & Amendment Agreement” means that certain Transition & Amendment Agreement, dated April 3, 2021, by and between the Parties.”
| (p) | Clause (i) of Section 9.1(b) of the A&R Collaboration Agreement shall be deleted in its entirety as of the Effective Date and replaced with the following: |
“(i) all Information conceived, discovered, developed or otherwise made in performing the activities under the Original Agreement, this Agreement, the Second A&R Collaboration Agreement, any Ancillary Agreement or the Transition & Amendment Agreement (including any Transition Ancillary Agreement and the performance of the Transition Development Activities, Joint Development Plan and Budget or Shared Territory Commercialization Plan and Budget (each, as defined in the Transition & Amendment Agreement)), whether solely by one Party or jointly by the Parties, in each case optionally with their Affiliates or any Third Parties or any employees, consultants or agents of any of the foregoing to the extent relating to a Gilead Combination Product (the “Gilead Combination Know-How”) and all Patents arising from such Gilead Combination Know-How (the “Gilead Combination Patents”) (collectively, “Gilead Combination Technology”).”
| (q) | Section 9.1(b) of the A&R Collaboration Agreement shall be amended by adding the following at the end of the existing section: |
“Galapagos shall also solely own all right, title and interest in and to all Information conceived, discovered, developed or otherwise made in performing the activities under the Original Agreement, this Agreement, the Second A&R Collaboration Agreement, any Ancillary Agreement or the Transition & Amendment Agreement (including any Transition Ancillary Agreement and the performance of the Transition Development Activities, Joint Development Plan and Budget or Shared Territory Commercialization Plan and Budget (each, as defined in the Transition & Amendment Agreement)), whether solely by one Party or jointly by the Parties, in each case optionally with their Affiliates or any Third Parties or any employees, consultants or agents of any of the foregoing to the extent relating to a Galapagos Combination Product (the “Galapagos Combination Know-How”) and all Patents arising from such Galapagos Combination Know-How (the “Galapagos Combination Patents”) (collectively, “Galapagos Combination Technology”).”
ARTICLE 8
MANUFACTURE AND SUPPLY
| 8.1 | Supply Arrangements |
| (a) | Notwithstanding Section 13.1 of the Benelux Supply Agreement or anything else to the contrary in this Agreement or any other written agreement between the Parties, the Benelux Supply Agreement shall not terminate upon the termination of the Benelux Co-Commercialization Agreement and shall remain in effect after the Effective Date (subject to the amendments contemplated hereby) until otherwise terminated or expired pursuant to the terms thereof. As soon as reasonably practicable and, in any event, within […***…] following the Effective Date, the Benelux Supply Agreement shall be amended and restated to (i) provide for the supply of the existing Licensed Products by Gilead to Galapagos for the entire Galapagos Territory on a temporary basis (as described in Section 8.1(c)), (ii) address timing of implementation of such supply (e.g., to align with timing of Galapagos’ assumption of distribution responsibilities), (iii) address such supply as portions of the supply chain transition to Galapagos, or its Affiliate(s), (iv) remove references to the terminated Benelux Co-Commercialization Agreement, the EU5 Co-Commercialization Agreement and the FIS Supply Agreement, (v) address process and mechanics for transferring the Transferred Inventory to Galapagos, or its Affiliate(s), (vi) address Galapagos’ rights and obligations to engage in distribution activities with respect to the Licensed Products in the Galapagos Territory, (vii) […***…] and (viii) include such other changes as may be mutually agreed upon by the Parties. The transfer pricing for Licensed Product sold by Gilead to Galapagos under the Benelux Supply Agreement as so amended and restated shall be the same as the pricing at which Galapagos may purchase Co-Commercialization Product from Gilead (i.e., the Transfer Price per Unit) as set forth in the Benelux Supply Agreement as of the Effective Date, and such transfer pricing shall apply with respect to all countries in the Galapagos Territory. |
| (b) | Commencing on the Effective Date and until the Benelux Supply Agreement has been amended and restated pursuant to Section 8.1(a) (and thereafter pursuant to such amended agreement), the Benelux Supply Agreement shall be deemed to include supply for […***…] and, should Galapagos require such supply, all other countries in Europe, in the same manner as the Benelux Countries. Any forecasts or orders provided by Galapagos for […***…] under the FIS Supply Agreement prior to the Effective Date will be deemed made under the Benelux Supply Agreement. Gilead shall provide forecasts for the Licensed Product(s) in the countries in the Galapagos Territory where it is the selling Party, and shall provide monthly updates to such forecasts, in accordance with the applicable terms of the European Supply Agreement. |
| (c) | Until the Initial Transition Date, Gilead will continue to supply the existing Licensed Compound and Licensed Products, including secondary packaging and labeling services and qualified person release, for Commercialization in the Galapagos Territory in accordance with the terms and the prices set forth in the European Supply Agreement. In addition to providing such supply, prior to the Initial Transition Date, Gilead shall build up inventory of finished Licensed Product equal to Galapagos’ forecasted demand for […***…], and such inventory shall be included in the Transferred Inventory. From and after the Initial Transition Date, except as otherwise expressly provided herein, Gilead shall have no obligation to supply (or have supplied) Licensed Products to Galapagos (other than to provide the Transferred Inventory). |
| (d) | The Parties will use Transition Activity Commercially Reasonable Efforts to […***…] to establish its own supply chain for the Licensed Product in the Galapagos Territory, including serialization, artwork management, […***…], secondary packaging, labeling services and batch release. The Parties will use Transition Activity Commercially Reasonable Efforts to ensure that […***…] contemplated to be implemented by Gilead for the Licensed Product is physically implemented before the Initial Transition Date and Gilead (or its applicable Affiliate) shall make any regulatory filings necessary for approval of such design prior to […***…]. Each Party shall be responsible for […***…] of the costs and expenses incurred by or on behalf of either Party in connection with […***…] and the regulatory filing, validation and implementation thereof. Galapagos will use Transition Activity Commercially Reasonable Efforts to ensure that the Supply Chain Completion Date for all portions of the supply chain (other than qualified person release and secondary packaging and labeling) occurs by the Initial Transition Date. Gilead will share with Galapagos the […***…] that are Controlled by Gilead or its Affiliate(s) […***…] for Licensed Products; provided that (i) […***…] and (ii) […***…]; provided that (x) […***…], and (y) […***…]. Notwithstanding the foregoing, Galapagos and its Affiliates shall not use any […***…] that are proprietary to Gilead or its Affiliates (or any associated […***…]) from and after the date that is […***…] or such other date set forth in the Transition Plan, and without limiting any other obligations of Galapagos under this Section 8.1(d), Galapagos shall establish its own source of supply not using any such […***…], including completing any necessary […***…], prior to the date that is […***…] or such other date set forth in the Transition Plan. Subject to the remainder of this Section 8.1(d), when Galapagos determines that, with respect to a given portion of the Manufacturing supply chain for Licensed Product, it has established its own source of supply for such portion of the supply chain and such portion is fully operational (including completing any necessary stability testing), it shall provide written notice thereof to Gilead (the date of each such notice, “Supply Chain Completion Date” with respect to such portion of the supply chain). The Parties shall coordinate to allow for a smooth transition of portions of the supply chain to Galapagos, or its Affiliate(s). Any Licensed Product manufactured by Galapagos or its Affiliates may utilize and bear the Gilead House Marks to the extent Galapagos’ use of such Licensed Product is within the scope of the license granted to Galapagos, or its Affiliate(s), pursuant to Section 10.2(b)(i). |
| (e) | Notwithstanding Section 8.1(c), 8.1(d) or 8.1(f), if required by Galapagos, Gilead will continue (i) to provide qualified person release and secondary packaging and labeling services for the Licensed Products in the Galapagos Territory until […***…] or, solely with respect to secondary packaging and labeling, such later date as may be mutually agreed by the Parties to the extent necessary for Galapagos to resolve any matters that prevent it from taking over the secondary packaging and labeling services for the Galapagos Territory by […***…], and (ii) to provide the safety data sheets and instructions to Galapagos, or its Affiliate(s), for the use, care, handling, storage, or shipment of the Licensed Products in the Galapagos Territory until the applicable MA Transfer Completion Date, in each case (clauses (i) and (ii)), subject to the terms and conditions of this Agreement and pursuant to and in accordance with the applicable payment, delivery and other terms of the European Supply Agreement. Gilead will provide stability test data to Galapagos, or its Affiliate(s), for any batches of the Licensed Products in any country in the Galapagos Territory placed in the market by or on behalf of Gilead prior to the MA Transfer Completion Date for such country. |
| (f) | Unless otherwise determined by the JTT or, if applicable, the TSC and subject to the terms and conditions of this Agreement, Gilead will continue to supply the required Clinical Trial materials for the Transferred Clinical Trials through its manufacturers, pursuant to and in accordance with the terms of the Clinical Supply Agreement, until Galapagos has set up its own supply chain for such materials (other than qualified person release and secondary packaging and labeling services), in no event later than the Initial Transition Date, unless otherwise mutually agreed upon by the Parties. Thereafter, Gilead shall have no obligation to supply (or have supplied) such Clinical Trial materials to Galapagos. The Parties shall amend the Clinical Supply Agreement as needed to reflect the arrangements contemplated by this Agreement as soon as practicable but in no event later than […***…] following the Effective Date. Such Clinical Trial materials will include supplying the Licensed Compound, the Licensed Products and any placebos, ensuring proper release thereof, and providing any other manufacturing, packaging and labeling services required to conduct such Transferred Clinical Trials. |
| (g) | Gilead will remain responsible for supplying the Licensed Products in the Gilead Territory pursuant to and in accordance with the terms of the Applicable Collaboration Agreement and any applicable agreements contemplated thereby. At Galapagos’ request, Gilead shall provide design and artwork services for the Licensed Products at a commercially reasonable rate to be mutually agreed and up to a maximum amount of hours mutually agreed until […***…], or such later date as may be mutually agreed by the Parties. |
| 8.2 | Manufacturing Process Decisions |
| (a) | Until the applicable Supply Chain Completion Date, Gilead shall have decision-making authority with respect to implementing any Manufacture process updates and related filings with Regulatory Authorities with respect to the corresponding portion of the supply chain for Licensed Product in the Galapagos Territory, provided that (i) such decision-making authority shall be subject to the obligations of Gilead and the rights of Galapagos (or their respective Affiliates), solely with respect to implementing such updates or related filings, under the European Supply Agreement, the Quality Agreement and this Agreement and (ii) Galapagos’ prior written consent (not to be unreasonably withheld, conditioned or delayed) shall be required for any update to the Manufacture process that impacts the Galapagos Territory (unless such changes are required by Applicable Law or any Regulatory Authority, in which case Galapagos’ consent shall not be required). |
| (b) | From and after the applicable Supply Chain Completion Date, Galapagos shall have decision-making authority with respect to implementing any Manufacture process updates and related filings with Regulatory Authorities with respect to the corresponding portion of the supply chain for Licensed Product in the Galapagos Territory, provided that Gilead will retain final decision-making authority to the extent such update or filings would conflict with Gilead’s MAH Legal Responsibilities. |
| 8.3 | Transferred Inventory |
| (a) | Promptly (but in any event within […***…]) after the Initial Transition Date or such other date as mutually agreed by the Parties, Galapagos shall purchase, and Gilead shall deliver or cause to be delivered to Galapagos, or its Affiliate(s), all of its and its Affiliates’ right, title and interest in and to the existing Licensed Products and Licensed Compound in […***…] or any other form, whether packaged or labelled or not, held by or on behalf of Gilead for the Galapagos Territory as a result of the Parties’ forecasts and pending orders (including, for clarity, Gilead’s forecasts set forth in Section 8.1(b)) (if applicable, for such country or countries) made prior to the last-to-occur MA Transfer Completion Date or such other date, as applicable (such Licensed Products and Licensed Compound, collectively, the “Transferred Inventory”). The Transferred Inventory will also include […***…] to be agreed by the Parties to be delivered to allow Galapagos to commence its own Manufacture of the Licensed Products. For clarity, the price for […***…] will be set forth in the European Supply Agreement. |
| (b) | The Transferred Inventory sold to Galapagos pursuant to this Section 8.3 shall be sold to Galapagos at the price set forth in the European Supply Agreement, in accordance with the applicable payment, delivery, warranty and other terms set forth therein. |
| (c) | Galapagos shall use Transition Activity Commercially Reasonable Efforts to use the Transferred Inventory before inventory of Licensed Product manufactured using Galapagos’ own source of supply. |
| 8.4 | Manufacturing and Importation Authorization |
The Parties will coordinate and plan for filing, and Galapagos will file, for any Manufacturing and Importation Authorization required for Galapagos to Manufacture the Licensed Products as contemplated hereby (the “MIA”) at such time as Galapagos reasonably determines is appropriate but in no event later than […***…]. Gilead will provide all records, documents and Information Controlled by Gilead or its Affiliate(s), and required or reasonably requested by Galapagos in order for Galapagos to prepare and submit such filing.
ARTICLE 9
COMMERCIALIZATION
| 9.1 | Commercialization Arrangements |
| (a) | For any Registered Country, (i) from the Effective Date until the Commercial Handover for such Registered Country, Galapagos shall have the right to provide General Direction pursuant to Section 4.10 to Gilead with respect to Gilead’s Commercialization activities, including distribution activities, for the Licensed Product in such Registered Country, subject to the oversight authority of the JTT and TSC as provided in ARTICLE 4, and (ii) after the Commercial Handover for such Registered Country, Galapagos shall have full and final decision-making authority with respect to, and shall have the sole right to perform all (and, except as set forth in the Transition Plan or this Agreement, Gilead shall have no responsibility to perform any), Commercialization activities, including distribution activities, for the Licensed Products in such Registered Country, subject to the provisions of this Section 9.1 and the oversight authority of the JTT and TSC as provided in ARTICLE 4; provided, however, that Gilead shall have the responsibility to fulfill, and the right to collect payment (subject to ARTICLE 12) with respect to, any orders for Licensed Product sold in such Registered Country by or on behalf of Gilead or its Affiliate prior to such Commercial Handover and (except as otherwise provided in this Agreement or any other agreement between the Parties or their Affiliates, including in Section 11.1 or 11.4 of the A&R Collaboration Agreement) will also bear any costs and expenses related to any returns, rebates or product liability claims related to such Licensed Products. Notwithstanding anything to the contrary in the A&R Collaboration Agreement, except as expressly provided in this Agreement (including the Transition Plan and Shared Territory Commercialization Plan and Budget), Gilead shall have no obligation to perform (or use any efforts to perform) any Commercialization activities in the Galapagos Territory. |
| (b) | Subject to Section 9.1(a), the Parties agree that the Commercialization activities in the Galapagos Territory that are performed by Gilead as of the Effective Date will continue to be conducted by Gilead until transitioned from Gilead to Galapagos in accordance with the Transition Plan and the Shared Territory Commercialization Plan and Budget, such transition to occur for the […***…] as soon as possible after the Effective Date and for […***…] no later than the MA Transfer Completion Date in such country. As between the Parties, (i) Galapagos shall remain the selling Party (and, for purposes of the A&R Collaboration Agreement, Co-Commercialization Selling Party) for […***…], and (ii) in accordance with the Transition Plan, Galapagos will become the selling Party (and, for purposes of the A&R Collaboration Agreement, Co-Commercialization Selling Party) for the […***…] and shall be or become the selling Party for the remaining countries in the Galapagos Territory, in each case (under this clause (ii)), upon the Commercial Handover for the applicable country. Notwithstanding anything to the contrary in this Agreement or the A&R Collaboration Agreement, at any time after the Effective Date, […***…]. |
| (c) | The JTT shall review, at least […***…], the Shared Territory Commercialization Plan and Budget and suggest any applicable amendments thereto for review and approval by the JTT. Subject to Section 9.1(a) and (b) above, each Party shall conduct the activities assigned to it under the Shared Territory Commercialization Plan and Budget and shall conduct its Commercialization of the Licensed Products in its Respective Territory in a manner consistent with the Shared Territory Commercialization Plan and Budget. Galapagos will prepare, and shall have the right to propose amendments, modifications or changes to the Shared Territory Commercialization Plan and Budget to the JTT. The JTT will analyze any such proposed amendment, modification or change to the Shared Territory Commercialization Plan and Budget and determine whether to approve it in accordance with ARTICLE 4. The Parties agree to implement any such proposed amendment, modification or change to the Shared Territory Commercialization Plan and Budget approved by the JTT or, if applicable, the TSC in accordance with ARTICLE 4. |
| (d) | Subject to Section 16.4, from and after the Effective Date, Gilead shall deliver or provide access to Galapagos to all data that are (i) related to the Commercialization of the Licensed Products in the Galapagos Territory and reasonably requested by Galapagos in order to exercise its rights or perform its obligations under this Agreement or the Applicable Collaboration Agreement (or any Ancillary Agreement or Transition Ancillary Agreement) and (ii) Controlled by Gilead or its Affiliate(s), in each case, on such timelines as may be mutually agreed by the Parties or otherwise set forth in the Transition Plan. |
| (e) | Gilead shall maintain adequate resources with respect to the services performed by […***…] supporting Commercialization of the Licensed Products in the Galapagos Territory (excluding, for clarity, any country-specific staffing or resources or any staffing or resources that are transferred to Galapagos pursuant to this Agreement) to enable it to perform its obligations under this Agreement and the Transition Plan and in line with the applicable key deliverables and milestones set forth in this Agreement and the Transition Plan to be completed on or before […***…]; provided, that Gilead shall continue to perform such obligations beyond such date if and until Gilead shall have completed all such applicable key deliverables and milestones set forth in this Agreement and the Transition Plan that are to be completed by […***…], provided that Galapagos has completed all of its key deliverables and milestones set forth in this Agreement and the Transition Plan that are a prerequisite for Gilead’s ability to complete such key deliverables and milestones of Gilead. |
| (f) | With respect to any Registered Country: |
| (i) | Prior to the earlier of (x) Commercial Handover or (y) the start date for Hired Employees or the TUPE Transfer Date, as applicable, for such Registered Country: |
| (A) | Gilead shall prepare and approve all Promotional Materials for such Registered Country. Such Promotional Materials shall be: (x) consistent with an agreed set of written core messages regarding […***…] in use in each Registered Country, as approved by the Compliance Working Group (“Transition Key Messages”), (y) in compliance with Applicable Law and Gilead policies, and (z) consistent with the applicable Marketing Authorization; |
| (B) | As part of Gilead’s approval process for such Promotional Materials, Gilead will also address any external approvals required prior to the use of the Promotional Materials; and |
| (C) | The Promotional Materials may deviate from the Transition Key Messages (x) for translation purposes, (y) to the extent required by Applicable Law or Applicable Guidelines, and (z) to the extent necessary for successful Commercialization in the Registered Countries. Gilead will inform the Transition Compliance Working Group in case of any other deviations from the Transition Key Messages; and |
| (ii) | From and after the earlier of (x) Commercial Handover or (y) the start date for Hired Employees or the TUPE Transfer Date, as applicable, for such Registered Country: |
| (A) | Galapagos shall prepare and approve all Promotional Materials for such Registered Country. Such Promotional Materials shall be: (x) consistent with the Transition Key Messages, (y) in compliance with Applicable Law and Galapagos policies, and (z) consistent with the applicable Marketing Authorization; |
| (B) | As part of Galapagos’ approval process for such Promotional Materials, Galapagos will also address any external approvals required prior to the use of the Promotional Materials; |
| (C) | The Promotional Materials may deviate from the Transition Key Messages (x) for translation purposes, (y) to the extent required by Applicable Law or Applicable Guidelines, and (z) to the extent necessary for successful Commercialization in the Registered Countries. Galapagos will inform the Transition Compliance Working Group in case of any other deviations from the Transition Key Messages; and |
| (D) | The Parties agree to review the process described in this Section 9.1(f)(ii) if there is a change to either Party’s primary representative responsible for Promotional Review in a Registered Country or in the event of a compliance breach. |
| (g) | Notwithstanding anything to the contrary in the A&R Collaboration Agreement, but subject to Section 7.5, Gilead shall have the sole right, but not any obligation, to Commercialize Licensed Products and Gilead Combination Products in the Gilead Territory and shall have full and final decision-making authority with respect to any related Commercialization activities except to the extent […***…]. |
| (h) | The Parties shall coordinate, on behalf of themselves and their Affiliates, regarding their activities at scientific or medical conferences, including regarding which Party’s or its Affiliate’s or Sublicensee’s employees shall staff any promotional and medical information booths that include any Licensed Product or the Licensed Compound, for global events through the JTT or JTT sub-teams and for local events in the Galapagos Territory through the relevant Country Transition Team. If both Parties participate in any such global conference, then each Party shall maintain its own promotional and medical information booths at such conference unless otherwise agreed between the Parties. |
| (i) | Neither Party shall, unless otherwise agreed by the Parties in writing, market, sell or otherwise distribute Licensed Products to customers outside of their Respective Territory or to customers intending to export Licensed Products outside of their Respective Territory. For clarity, nothing in this Section 9.1(i) is intended or shall be construed to restrict Gilead from performing (i) any of its obligations under this Agreement or (ii) as to any Registered Country, any Commercialization activities with respect to Licensed Products in such Registered Country prior to the Commercial Handover for such Registered Country. |
| 9.2 | Market Access Activities; Medical Affairs Activities |
| (a) | Gilead will use Transition Activity Commercially Reasonable Efforts to continue the Market Access Activities, including dossier preparation and submission, under General Direction of Galapagos in a Registered Country until the execution of a Power of Attorney for such Registered Country covering the applicable Market Access Activity. From and after the execution of such a Power of Attorney for such Registered Country, Galapagos will be solely responsible for such Market Access Activities in such Registered Country, subject to any applicable oversight authority of the JTT and TSC as provided in ARTICLE 4, and Gilead shall have no obligation to perform (or use any efforts to perform) any Market Access Activities that Galapagos or one of its Affiliates is able to conduct under a Power of Attorney that has been issued to Galapagos or one of its Affiliates in such Registered Country. For any Registered Country, from and after the MA Transfer Completion Date for such Registered Country, Gilead shall have no obligation to perform (or use any efforts to perform) any Market Access Activities with respect to such Registered Country. |
| (b) | Gilead will use Transition Activity Commercially Reasonable Efforts to continue the Medical Affairs Activities under General Direction of Galapagos in a Registered Country until the Commercial Handover for such Registered Country. From and after the Commercial Handover for such Registered Country, Galapagos will be solely responsible for all such Medical Affairs Activities in such Registered Country, subject to any applicable oversight authority of the JTT and TSC as provided in ARTICLE 4, and Gilead shall have no obligation to perform (or use any efforts to perform) any Medical Affairs Activities in such Registered Country. |
| (c) | […***…]. |
| 9.3 | Pricing Strategies and Decisions |
For any Registered Country, from the Effective Date until the Commercial Handover for such Registered Country, any and all pricing strategy and other pricing decisions and determinations for the Licensed Product in such Registered Country shall be determined by Galapagos in its sole discretion, and after Galapagos has notified Gilead in writing thereof, Gilead shall implement such decisions and determinations, in each case, unless such decisions or determinations would conflict with Gilead’s MAH Legal Responsibilities. For clarity, Galapagos will be the party to enter into all Licensed Product sales contracts with customers in any Registered Country after the Commercial Handover for any Registered Country, and will continue to have sole discretion over pricing strategy and other pricing decisions and determinations for the Licensed Product in such Registered Country.
| 9.4 | Transferred Information |
As promptly as reasonably practicable after the Effective Date (or, with respect to any Transferred Information created after the Effective Date, after such Transferred Information is created), Gilead shall deliver or provide access to the Transferred Information to Galapagos, or its Affiliate(s). For clarity, Gilead may retain copies of the Transferred Information. All Transferred Information that is solely related to the Galapagos Territory shall be deemed to be the Confidential Information of Galapagos as of the Effective Date and Galapagos shall be deemed to be the disclosing Party and Gilead the receiving Party with respect thereto. All Transferred Information that is not solely related to the Galapagos Territory shall be deemed to be the Confidential Information of both Parties as of the Effective Date and each Party shall be deemed to be both the disclosing Party and the receiving Party with respect thereto.
| 9.5 | Amendments to A&R Collaboration Agreement |
| (a) | Section 5.1(b), Section 5.2(a), Section 5.2(b)(i)-(iv) and (vii), Section 5.4, Section 5.5(b) and (c) and Section 5.6 of the A&R Collaboration Agreement shall each be deleted in its entirety as of the Effective Date and replaced with the following: |
“[omitted.]”
| (b) | Section 5.1(a) of the A&R Collaboration Agreement shall be deleted in its entirety as of the Effective Date and replaced with the following: |
“(a) Licensed Territory. Except as otherwise provided in the Transition & Amendment Agreement, Gilead shall be solely responsible for all Commercialization activities relating to the Licensed Products and Gilead Combination Products in the Licensed Territory. Gilead shall use Commercially Reasonable Efforts to Commercialize the first Licensed Product in each of the Major Markets in the Licensed Territory for each of the Target Indications. […***…].”
| (c) | Section 5.2(b)(xii) of the A&R Collaboration Agreement shall be deleted in its entirety as of the Effective Date and replaced with the following: |
“(xii) (Sub)contracting. Notwithstanding anything in this Agreement to the contrary, except as otherwise provided in (and subject to the terms and conditions of) the Transition & Amendment Agreement, each Party may use (sub)contractors without the consent of the other Party for its Commercialization activities. Each Party may delegate its Commercialization obligations to an Affiliate.”
| (d) | The following shall be added as Section 5.7 of the A&R Collaboration Agreement: |
“5.7 Information and Materials to be Provided to Galapagos and Gilead.
(a) Upon Galapagos’ reasonable request from time to time, Gilead shall promptly deliver or provide access to Galapagos, or its Affiliate(s), to any Information or materials Controlled by Gilead that are related to the Commercialization, Market Access Activities or Medical Affairs activities of the Licensed Compound or Licensed Products and are necessary for or otherwise reasonably requested by Galapagos in order to exercise its rights or perform its obligations under this Agreement or the Transition & Amendment Agreement.
(b) Upon Gilead’s reasonable request from time to time, Galapagos shall promptly deliver or provide access to Gilead to any Information or materials Controlled by Galapagos that are related to the Commercialization, Market Access Activities or Medical Affairs activities of the Licensed Compound or Licensed Products and are necessary for or otherwise reasonably requested by Gilead in order to exercise its rights or perform its obligations under this Agreement or the Transition & Amendment Agreement.”
| (e) | […***…] |
ARTICLE 10
TRANSFERRED TRADEMARKS AND INFORMATION TECHNOLOGY, LICENSES AND EXCLUSIVITY
| 10.1 | Assignment of Transferred Trademarks, Certain Domain Names and Certain Information Technology |
| (a) | Transferred Trademarks. |
| (i) | Within a reasonable period of time after the Effective Date (but in no event more than […***…] after the Effective Date), Gilead shall provide to Galapagos copies of trademark applications and registrations for the Transferred Trademarks (including copies of any filings or communications to or from any trademark office or other Governmental Authority with respect to the Transferred Trademarks), solely to the extent such information is not publicly available. |
| (ii) | Gilead (on behalf of itself and its Affiliates) hereby transfers and assigns to Galapagos exclusively, irrevocably, and unconditionally, free and clear of any Encumbrances and at no cost or expense to Galapagos all of Gilead’s and its Affiliates’ right, title and interest in and to the Transferred Trademarks, including the priority rights related to the Transferred Trademarks, the goodwill associated with the Transferred Trademarks, and any and all rights to bring an action, whether at law or in equity, for infringement, dilution, misappropriation, misuse or other violation of the foregoing Transferred Trademark by a Third Party (“Product Trademark Infringements”), and all rights to secure and recover damages, profits and injunctive relief for all past, present or future infringement, dilution, misappropriation, misuse, or other violation, whether occurring before or after this assignment. On or promptly after the Effective Date, Gilead Sciences Ireland UC and Gilead shall execute the Trademark Assignment Agreement. Gilead and its Affiliates shall also execute and deliver such documents, make reasonably available such information and materials Controlled by and in the possession of |
| Gilead, if any, and take such other actions requested by Galapagos, as are reasonably necessary to enable Gilead to transfer such interest in the Product Trademark Infringements to Galapagos, or its Affiliates. Galapagos shall be solely responsible for recording the assignment of the Transferred Trademarks, including payment of all associated fees and costs and updating chain-of-title for such registrations and applications. Gilead and its Affiliates shall execute and deliver such documents and take such other actions requested by Galapagos as are reasonably necessary to enable Galapagos, or its Affiliate(s), to record the change and effectuate the transfer in title of the Transferred Trademarks. |
| (b) | Domain Names. Gilead (on behalf of itself and its Affiliates) hereby transfers and assigns to Galapagos, exclusively, irrevocably and unconditionally, free and clear of any Encumbrances and at no cost or expense to Galapagos, all of Gilead’s and its Affiliates’ right, title and interest in any of the domain names Controlled by Gilead or its Affiliate(s) and set forth on Schedule 4. Gilead and its Affiliates shall execute and deliver such documents and take such other actions requested by Galapagos as are reasonably necessary to enable Galapagos, or its Affiliate(s), to become the registrant of record for such domain names. Galapagos shall be solely responsible, including payment of all associated fees and costs, for ensuring that domain name registrant information is updated. |
| (c) | Information Technology. Gilead (on behalf of itself and its Affiliates) hereby transfers and assigns to Galapagos, exclusively, irrevocably and, unconditionally, free and clear of any Encumbrances and at no cost or expense to Galapagos, all of Gilead’s and its Affiliates’ right, title and interest in any of the information technology Controlled by Gilead or its Affiliate(s) and set forth in the Transition Plan, if any, in accordance with the requirements set forth in the Transition Plan. Gilead and its Affiliates shall execute and deliver such documents and take such other actions requested by Galapagos as are reasonably necessary to enable Gilead to transfer such information technology to Galapagos, or its Affiliate(s), in accordance with the requirements set forth in the Transition Plan. |
| 10.2 | Licenses |
| (a) | Certain Works Subject to Copyright. Subject to the terms and conditions of this Agreement, Gilead hereby grants, on behalf of itself and its Affiliates, to Galapagos a non-exclusive, sublicensable (solely as permitted in accordance with Section 10.3) license to the Gilead Copyrights, including the following works subject to copyright: |
| (i) | […***…]; |
| (ii) | websites, marketing materials, educational materials, promotional materials and healthcare practitioner reference materials created for the Licensed Products, materials on […***…], in each case, that are Controlled by Gilead or its Affiliate(s); and |
| (iii) | […***…]; |
in each case (clauses (i) through (iii)), to (1) Develop the Licensed Compound and Licensed Product worldwide for purposes of Exploitation in the Galapagos Territory, (2) Manufacture the Licensed Compound and Licensed Products worldwide for purposes of (x) Development under foregoing clause (1) and (y) Exploitation in the Galapagos Territory and (3) Exploit the Licensed Compound and Licensed Product in the Galapagos Territory; provided, however, that, without limiting any other disclaimers or expanding
any other representations or warranties made by Gilead under this Agreement or any other agreement, any […***…] are licensed and provided to Galapagos on an as-is basis, and Gilead makes no, and hereby expressly disclaims any, representation or warranty of any kind with respect thereto, whether express, implied, statutory or otherwise, including without limitation any warranty of title, merchantability, fitness for a particular purpose or non-infringement of Third Party intellectual property rights. Gilead will provide Galapagos with digital copies of the […***…] for Galapagos’ use.
| (b) | Gilead House Marks. |
| (i) | Subject to the terms and conditions of this Agreement and on the timelines set forth in Section 10.2(b)(i)(A) and (B), Gilead hereby grants, on behalf of itself and its Affiliates, to Galapagos: |
| (A) | a non-exclusive, royalty-free, fully paid up license, with the right to sublicense to Affiliates and Distributors (solely as permitted in accordance with Section 10.3, provided that Gilead’s prior written consent (not to be unreasonably withheld, conditioned or delayed) shall be required for any sublicense to Distributors under clause (2) of this Section 10.2(b)(i)(A)), under the Gilead House Marks as used in the labeling and packaging for the Licensed Products for release to end-users (whether in a commercial or clinical packaging presentation), including (1) […***…] and (2) any other written, printed or graphic materials accompanying such Licensed Products (but excluding, in each case, any use of the Gilead House Marks described in clauses (1) through (4) of Section 10.2(b)(i)(B)), in each case, as necessary to Commercialize Licensed Product in the Galapagos Territory until […***…] after the last MA Transfer Completion Date to occur; and |
| (B) | a non-exclusive, royalty-free, fully paid up license, with the right to sublicense to Affiliates and Third Parties, including Distributors (solely as permitted in accordance with Section 10.3), under the Gilead House Marks, to use (1) […***…], as necessary to Commercialize the Licensed Product in the Galapagos Territory until […***…] after the last MA Transfer Completion Date to occur, (2) Commercialize the Transferred Inventory, (3) […***…], as necessary to Commercialize the Licensed Product in the Galapagos Territory until the later of (I) […***…] after the last MA Transfer Completion Date to occur or (II) […***…], and in any case to Commercialize any then-existing inventory of such tablets, and (4) […***…], as necessary to Commercialize the Licensed Product in the Galapagos Territory until […***…]. |
| (ii) | Galapagos shall use the Gilead House Marks as set forth in such reasonable standards as may be provided in writing by Gilead from time to time, and any deviation from such standards shall require Gilead’s prior written approval. If Galapagos without Gilead’s prior written approval uses the Gilead House Marks in any manner that deviates from such standards or otherwise exceeds the scope of the license granted to Galapagos in Section 10.2(b)(i), then, without limiting any other rights or remedies that may be available to Gilead, Gilead shall have the right, upon written notice to Galapagos, to terminate the license granted to Galapagos in Section 10.2(b)(i), and upon any such termination Galapagos shall immediately cease all use of the Gilead House Marks. Notwithstanding anything to the contrary herein, Gilead retains all rights with respect to any domain names that incorporate any Gilead House Marks. |
| (c) | Galapagos Technology. Section 7.1 of the A&R Collaboration Agreement is hereby deleted in its entirety and replaced with the following: |
“7.1 Licenses to Gilead. Subject to the terms and conditions of this Agreement, Galapagos hereby grants Gilead:
| (a) | an exclusive (including as to Galapagos), royalty-bearing, sublicensable (solely as permitted in accordance with Section 7.2) license under the Galapagos Technology (subject to Section 7.4(g) of the Transition & Amendment Agreement) to […***…] in the Gilead Territory; and |
| (b) | an exclusive (including as to Galapagos), royalty-bearing, sublicensable (solely as permitted in accordance with Section 7.2) right of reference under (or right of access to, if such right of reference is unavailable or insufficient) any Regulatory Materials or Regulatory Approvals Controlled by Galapagos with respect to the Licensed Compound or Licensed Product (subject to Section 7.4(g) of the Transition & Amendment Agreement), in each case, to […***…] in the Gilead Territory. |
| (c) | Notwithstanding the exclusive license and rights granted by Galapagos to Gilead pursuant to Sections 7.1(a) and 7.1(b), Galapagos and its Affiliates shall retain, and have the right to grant licenses, sublicenses or rights of reference or access to Third Parties in accordance with Section 7.2 under, the Galapagos Technology described in Section 7.1(a) and the Regulatory Materials and Regulatory Approvals described in 7.1(b), in each case, to perform the activities assigned to Galapagos or its Affiliates under this Agreement or any other agreement between the Parties or their Affiliates, including Developing, Manufacturing and Commercializing the Licensed Compound and Licensed Product as provided herein or therein.” |
| (d) | Other Enabling Grants by Galapagos. Subject to the terms and conditions of this Agreement, Galapagos hereby grants to Gilead a non-exclusive, royalty-free, fully paid-up, sublicensable (solely as permitted in accordance with Section 10.3) (i) license under the Galapagos Technology, Galapagos House Marks, Transferred Trademarks and any domain names and information technology assigned to Galapagos under Section 10.1, and (ii) right of reference under (or right of access to, if such right of reference is unavailable or insufficient) any Regulatory Materials or Regulatory Approvals Controlled by Galapagos with respect to the Licensed Compound or Licensed Product, in each case ((i) and (ii)), solely to perform the activities assigned to Gilead or its Affiliates under this Agreement or any other written agreement between Galapagos or its Affiliate, on the one hand, and Gilead or its Affiliate, on the other hand, including Developing, Manufacturing and Commercializing the Licensed Compound and Licensed Product as provided herein or therein. Gilead shall use the Galapagos House Marks as set forth in such reasonable standards as may be provided in writing by Galapagos from time to time, and any deviation from such standards shall require Galapagos’ prior written approval. If Gilead without Galapagos’ prior written approval uses the Galapagos House Marks in any manner that deviates from such standards or otherwise exceeds the scope of the license granted to Gilead in this Section 10.2(d), then, without limiting any other rights or remedies that may be available to Galapagos, Galapagos shall have the right, upon written notice to Gilead, to terminate the license granted to Gilead in this Section 10.2(d), and upon any such termination Gilead shall immediately cease all use of the Galapagos House Marks. Notwithstanding anything to the contrary herein, Galapagos retains all rights with respect to any domain names that incorporate any Galapagos House Marks. |
| (e) | Gilead Technology. Subject to the terms and conditions of this Agreement, Gilead hereby grants Galapagos: |
| (i) | an exclusive (including as to Gilead), royalty-bearing, sublicensable (solely as permitted in accordance with Section 10.3) license under the Gilead Technology other than Gilead Combination Technology (subject to Section 7.4(g)) to (A) Develop the Licensed Compound and Licensed Product worldwide for purposes of Exploitation in the Galapagos Territory, (B) Manufacture the Licensed Compound and Licensed Products worldwide for purposes of (x) Development under foregoing clause (i) and (y) Exploitation in the Galapagos Territory and (C) Exploit the Licensed Compound and Licensed Product in the Galapagos Territory; |
| (ii) | an exclusive (including as to Gilead), royalty-bearing, sublicensable (solely as permitted in accordance with Section 10.3) right of reference under (or right of access to, if such right of reference is unavailable or insufficient) any Regulatory Materials or Regulatory Approvals for the Galapagos Territory Controlled by Gilead or its Affiliate(s) with respect to the Licensed Compound and Licensed Product (subject to Section 7.4(g)), in each case, to (A) Develop the Licensed Compound and Licensed Product worldwide for purposes of Exploitation in the Galapagos Territory, (B) Manufacture the Licensed Compound and Licensed Products worldwide for purposes of (x) Development under foregoing clause (i) and (y) Exploitation in the Galapagos Territory and (C) Exploit the Licensed Compound and Licensed Product in the Galapagos Territory. For clarity, with respect to any Transferred Regulatory Documentation and Regulatory Approvals to be assigned and transferred to Galapagos, or its Affiliates, under this Agreement, this right of reference under (or right of access to) such Transferred Regulatory Documentation and Regulatory Approvals applies until such time as they are effectively assigned and transferred to Galapagos, or its Affiliates; and |
| (iii) | a non-exclusive, sublicensable (solely as permitted in accordance with Section 10.3) license under the Gilead Technology solely to conduct the activities assigned to Galapagos under this Agreement and the A&R Collaboration Agreement (excluding any activities covered by the license grant set forth in Section 10.2(e)(i)). |
| (f) | Retained Rights of Gilead. Notwithstanding the exclusive license and rights granted by Gilead to Galapagos pursuant to Sections 10.2(e)(i) and 10.2(e)(ii), Gilead and its Affiliates shall retain, and have the right to grant licenses, sublicenses or rights of reference or access to Third Parties in accordance with Section 10.3 (mutatis mutandis) under, the Gilead Technology described in Section 10.2(e)(i) and the Regulatory Materials and Regulatory Approvals described in Section 10.2(e)(ii), in each case, to perform the activities assigned to Gilead or its Affiliates under this Agreement or any other agreement between the Parties or their Affiliates, including Manufacturing and Commercializing the Licensed Compound and Licensed Product as provided herein or therein. |
| 10.3 | Sublicensing. |
| (a) | Section 7.2(a) of the A&R Collaboration Agreement is hereby deleted in its entirety and replaced with the following. |
“7.2(a) Scope of Permissible Sublicensing
(i) Subject to Section 7.2(b), the licenses and rights of reference (and access) granted by Galapagos to Gilead under this Agreement or the Transition & Amendment Agreement may be sublicensed by Gilead through multiple tiers without any requirement of consent, provided that Gilead shall be liable for any act or omission of any such Sublicensee that is a breach of any of Gilead’s obligations under this Agreement as though the same were a breach by Gilead, and Galapagos shall have the right to proceed directly against Gilead with respect to such breach without any obligation to first proceed against such Sublicensee.
(ii) Subject to Section 7.2(b), the licenses and rights of reference (and access) granted by Gilead to Galapagos, or its Affiliates, under this Agreement or the Transition & Amendment Agreement (other than under Section 10.2(b)(i)(A) of the Transition & Amendment Agreement) may be sublicensed by Galapagos through multiple tiers without any requirement of consent, provided that Galapagos shall be liable for any act or omission of any such Sublicensee that is a breach of any of Galapagos’ obligations under this Agreement as though the same were a breach by Galapagos, and Gilead shall have the right to proceed directly against Galapagos with respect to such breach without any obligation to first proceed against such Sublicensee.”
| (b) | Section 7.2(b) of the A&R Collaboration Agreement is hereby deleted in its entirety and replaced with the following. |
“Any sublicenses granted under the licenses set forth in Section 7.1 of this Agreement or Section 10.2 of the Transition & Amendment Agreement shall be under a written sublicense agreement (each, a “Sublicense Agreement”). Gilead shall use reasonable efforts to provide that each Sublicense Agreement entered by Gilead requires the Sublicensee to provide the following to Galapagos if this Agreement terminates, and to Gilead if only such Sublicense Agreement terminates: (i) the assignment and transfer of ownership and possession of, or a right of reference to, all Regulatory Materials and Regulatory Approvals controlled by such Sublicensee with respect to any Licensed Product (which assignment or right of reference may also be provided directly to Gilead prior to any such termination), but solely to the extent such assignment and transfer, or right of reference, would be required of Gilead under Section 15.6, and (ii) the assignment of, or a freely sublicensable exclusive license to, all intellectual property (including Patents) controlled by such Sublicensee that covers or embodies a Licensed Product or its respective use, manufacture, sale, or importation and was conceived, discovered, developed or otherwise made by or on behalf of such Sublicensee during the exercise of its rights or fulfillment of its obligations pursuant to such Sublicense Agreement, but solely to the extent such assignment or exclusive license would be required of Gilead under Section 15.6(a). Galapagos shall use reasonable efforts to provide that each Sublicense Agreement entered by Galapagos requires the Sublicensee to provide the following to Galapagos if such Sublicense Agreement terminates: (x) the assignment and transfer of ownership and possession of, or a right of reference to, all Regulatory Materials and Regulatory Approvals controlled by such Sublicensee with respect to any Licensed Product (which assignment or right of
| reference may also be provided directly to Galapagos prior to any such termination), but solely to the extent such assignment and transfer, or right of reference, would be required of Galapagos under Section 15.6, and (y) the assignment of, or a freely sublicensable exclusive license to, all intellectual property (including Patents) controlled by such Sublicensee that covers or embodies a Licensed Product or its respective use, manufacture, sale, or importation and was conceived, discovered, developed or otherwise made by or on behalf of such Sublicensee during the exercise of its rights or fulfillment of its obligations pursuant to such Sublicense Agreement, but solely to the extent such assignment or exclusive license would be required of Galapagos under Section 15.6(a). Each Sublicense Agreement shall be subject to the applicable terms and conditions of this Agreement. For clarity, in the case of any subcontractor, this Section 7.2(b) shall not apply but the applicable Party shall comply with Section 3.8.” |
| 10.4 | Amendment to Exclusivity; Non-Compete |
Clause (i) of Section 7.6(a) of the A&R Collaboration Agreement is hereby deleted in its entirety and replaced with the following:
“(i) (x) Gilead or its Affiliates may itself or with a Third Party conduct […***…], in all cases that are intended to support the Exploitation of Licensed Products or Gilead Combination Products, and (y) Galapagos or its Affiliates may itself or with a Third Party conduct […***…], in all cases that are intended to support the Exploitation of Licensed Products or Galapagos Combination Products;”.
ARTICLE 11
ADDITIONAL COVENANTS
| 11.1 | Further Assurances |
| (a) | For the period beginning on the Effective Date and ending on the Latest Transition Date, each Party shall, at any time at the request of the other Party, execute and deliver (or cause the execution and delivery) to the other Party of all such instruments of assignment, transfer and conveyance and documents or further assurances as such other Party may reasonably request to effect the Transition and other transactions contemplated herein. |
| (b) | At any time after the Latest Transition Date, if either Gilead or Galapagos becomes aware that any material Transferred Asset has not been transferred to Galapagos, it shall promptly notify the other Party in writing and the Parties shall, as soon as reasonably practicable, ensure that such property is transferred, with any necessary prior Third Party consent or approval, to Galapagos, or its Affiliate(s); provided that, Gilead shall not be required to pay any monetary amounts or provide any other consideration to obtain such consent, approval or waiver (unless Galapagos agrees to reimburse Gilead for such amount). |
| (c) | If, at any time, either Gilead or Galapagos becomes aware that any material regulatory documentation, marketing authorizations, contracts, inventory, clinical trials or any documentation applicable thereto, owned or controlled by Gilead or its Affiliate(s) that is necessary for Galapagos to Develop, Manufacture, or Commercialize the Licensed Compound or the Licensed Products for the Galapagos Territory is neither licensed to Galapagos nor included in the Transferred Assets that have been transferred to Galapagos (e.g., because it was not included in the applicable schedule of assets or cannot be legally transferred) it shall promptly notify the other Party in writing and, at Galapagos’ request, the Parties shall, as soon as reasonably practicable, ensure that such property is transferred, licensed, or the necessary rights are otherwise made available, with any necessary prior Third Party consent or approval, to Galapagos, or its Affiliate(s); provided that, Gilead shall not be required to pay any monetary amounts or provide any other consideration to obtain such consent, approval or waiver (unless Galapagos agrees to reimburse Gilead for such amounts). |
| 11.2 | Contracts |
| (a) | Gilead and its Affiliates shall assign and transfer the Contracts that (i) are solely related to the Transferred Clinical Trials, the Manufacture of Licensed Compound or Licensed Product for the Galapagos Territory, the Exploitation of Licensed Compound or Licensed Product in or for the Galapagos Territory or any of the Transferred Assets, and (ii) Galapagos agrees to assume in accordance with this Section 11.2 (the “Transferred Contracts”) to Galapagos (or its applicable Affiliate, as designated by Galapagos) in accordance with the timeline for the associated activities set forth in the Transition Plan or other timeline agreed by the Parties. Within […***…] after the Effective Date, Gilead shall provide to Galapagos for review a list of Contracts meeting foregoing clause (i) and thereafter shall promptly provide such information related thereto as Galapagos may reasonably request. Galapagos shall identify in writing those Contracts on such list that it wishes to assume and such Contracts shall constitute Transferred Contracts. If there are any additional Contracts that Galapagos wishes to assume that it believes meet foregoing clause (i) it shall provide a list of such additional Contracts to Gilead for review and, if Gilead agrees with Galapagos’ assessment, such Contracts shall also constitute Transferred Contracts. If Gilead disagrees, Galapagos may have such dispute resolved pursuant to Section 16.6. |
| (b) | With respect to each Transferred Contract, Galapagos or one of its Affiliates shall assume the liabilities under such Transferred Contract but only to the extent such liabilities (i) do not result from any failure to perform under such Transferred Contract, or other breach of, default under or violation of such Transferred Contract, by Gilead or any of its Affiliates on or before the date such Transferred Contract is assigned to Galapagos or one of its Affiliates and (ii) arise after the date such Transferred Contract is assigned to Galapagos or one of its Affiliates. Notwithstanding any provision of this Agreement or any Transferred Contract to the contrary, except as expressly set forth in this Section 11.2(b), all other liabilities arising under or related to any such Transferred Contract shall remain the sole obligation and responsibility of Gilead and its Affiliates. |
| (c) | Notwithstanding the foregoing or anything to the contrary in this Agreement, to the extent that Gilead’s rights under any Transferred Contract may not be assigned to Galapagos or its applicable Affiliate without the approval, consent or waiver of another Person (a “Third Party Assignment Approval”), this Agreement shall not constitute an agreement to assign the same if an attempted assignment would constitute a breach thereof or be unlawful. Until such time as the Third Party Assignment Approval is obtained Gilead shall, and shall cause its Affiliates to, at Galapagos’ request, use their respective Transition Activity Commercially Reasonable Efforts to cause the applicable counterparty to (i) provide such Third Party Assignment Approval, or (ii) enter into a replacement or alternative contract with Galapagos, or its Affiliates, to pass along the applicable benefits of such Transferred Contract to Galapagos, or its Affiliates, and Galapagos shall use its Transition Activity Commercially Reasonable Efforts to assist and cooperate in connection therewith. Pending such Third Party Assignment Approval or entry into a replacement or alternative contract, or, if earlier, the Latest Transition Date, Gilead shall, and shall cause its Affiliates to, use |
| their respective Transition Activity Commercially Reasonable Efforts to (i) pass on to Galapagos, or its Affiliate(s), the benefits of such Transferred Contract and (ii) enforce, at the request of and for the account of Galapagos, any rights of Gilead or its Affiliates arising under any such Transferred Contract against any Person. To the extent that Galapagos is provided with substantially comparable benefits of any such non-assignable Transferred Contract pursuant to this Section 11.2(c), Galapagos shall perform on behalf of Gilead or its Affiliates all of the obligations of Gilead or its Affiliates thereunder or in connection therewith. Gilead and its Affiliates shall not be liable to Galapagos for a failure to obtain any Third Party Assignment Approval. During the term of this Agreement, Galapagos shall cooperate with Gilead, upon the request of Gilead, in any reasonable manner in connection with Gilead obtaining any necessary approval, consent or waiver pursuant to this Section 11.2(c). |
| (d) | For each of the Other Contracts, the Parties, at Galapagos’ request, shall, and shall cause their Affiliates to, use Transition Activity Commercially Reasonable Efforts to cause the applicable counterparty to (i) assign the portion of such Other Contract that is solely related to the Transferred Clinical Trials, the Manufacture of Licensed Product for the Galapagos Territory, the Commercialization of Licensed Product in the Galapagos Territory or any of the Transferred Assets to Galapagos or its Affiliates, or (ii) enter into a replacement or alternative contract with Galapagos, or its Affiliate(s), to pass along the applicable benefits of such Other Contract to Galapagos, or its Affiliate(s). Prior to the execution of such assignment, or entry into a replacement or alternative contract or if earlier, the Latest Transition Date, Gilead shall, and shall cause its Affiliates to, use their respective Transition Activity Commercially Reasonable Efforts to (i) pass on to Galapagos, or its Affiliates, the benefits of such Other Contract and (ii) enforce, at the request of and for the account of Galapagos, any rights of Gilead or its Affiliates arising under any such Other Contract against any Person. |
| 11.3 | Public Communications |
| (a) | From the Effective Date until the Initial Transition Date, the Parties agree as follows: |
| (i) | Galapagos shall be solely responsible for all press releases and other public announcements and communications related to the Licensed Products other than any press releases and other public announcements and communications specifically related to the Gilead Territory (each, a “Galapagos Public Communication”). Except to the extent required by Applicable Law or the rules of a stock exchange on which the securities of Galapagos are listed, (x) prior to the last MA Transfer Completion Date to occur, Galapagos shall not issue or make any Galapagos Public Communication unless included in the Transition Plan, a joint communication plan mutually agreed by the Parties, or otherwise approved by Gilead or the JTT (or if applicable, the TSC), and (y) after the last MA Transfer Completion Date to occur, Galapagos shall not issue or make any Galapagos Public Communication without providing Gilead at least […***…] (or such shorter period as necessary to comply with Applicable Law or applicable stock exchange listing rules) written notice prior to issuing or making such Galapagos Public Communication. Galapagos shall consider in good faith any comments provided by Gilead on such Galapagos Public Communications. For clarity, Gilead shall have no right to make any Galapagos Public Communications. |
| (ii) | Gilead shall be solely responsible for all press releases and other public announcements and communications related to the Licensed Products that are specifically related to the Gilead Territory (each, a “Gilead Public Communication”). Except to the extent required by Applicable Law or the rules of a stock exchange on which the securities of Gilead are listed, Gilead shall not issue or make any Gilead Public Communication without providing Galapagos at least […***…] (or such shorter period as necessary to comply with Applicable Law or applicable listing rules) written notice prior to issuing or making such Gilead Public Communication. Gilead shall consider in good faith any comments provided by Galapagos on such Gilead Public Communications. |
| (iii) | The Parties agree that the material terms of this Agreement and the Transition Ancillary Agreements are the Confidential Information of both Parties. Notwithstanding Section 11.3(a)(i) or Section 11.3(a)(ii), neither Party shall issue or make any press release or other public announcement or communication related to the terms of this Agreement or the Transition Ancillary Agreements or the transactions contemplated herein or therein, without the prior written consent of the other Party or otherwise pursuant to and consistent with any joint communication plan mutually agreed upon by the Parties (including any such plan included in the Transition Plan) or permitted under the special authorized disclosure provisions set forth in Sections 12.2 and 12.3 of the Applicable Collaboration Agreement. |
| (b) | Neither Party, except as required by Applicable Law, shall publicly disclose data or results of Clinical Trials or Nonclinical Studies that have not already been publicly disclosed with respect to any Licensed Product except as set forth in this Section 11.3(b). All Publications should be discussed and endorsed by the joint publication team constituted pursuant to Section 4.6 and included in the mutually agreed publication plan (including any such plan included in the Transition Plan) as per good publication practice (GPP). Each Party shall submit any proposed Publications to the other Party for review and mutual approval (not to be unreasonably conditioned, delayed or withheld) (i) at least […***…] prior to its intended submission to a peer-reviewed journal and (ii) any abstract, poster or oral presentation at least […***…] prior to its intended submission to or presentation at scientific congresses. Each Party shall have the right to make Publications containing data or results related solely to its Respective Territory and that does not have relevance or impact outside of its Respective Territory (subject to mutual alignment on the publication plan by the Parties, intellectual property approval by the Parties and courtesy review by the other Party for reasonable comments to such Publication). |
| (c) | This Section 11.3 shall supersede the Parties’ obligations under Section 12.4 of the A&R Collaboration Agreement during the period beginning on the Effective Date and ending on the Initial Transition Date. After the Initial Transition Date, the Parties’ obligations with respect to such matters will be governed by the Second A&R Collaboration Agreement. Except as otherwise provided in this Section 11.3, Sections 12.1, 12.2 and Section 12.3 of the A&R Collaboration Agreement shall govern the respective rights and obligations of the Parties and their respective Affiliates and representatives with respect to Confidential Information (as defined in the A&R Collaboration Agreement and amended by this Agreement). |
| 11.4 | Competition Law Guidelines |
| (a) | To ensure compliance with applicable competition/antitrust laws, the Parties shall finalize and adopt within […***…] after the Effective Date, and after such adoption each Party shall comply with, Commercialization competition law / antitrust guidelines with respect to the Licensed Product (as the same may be modified by mutual agreement of the Parties in writing from time to time) (“Competition Law Guidelines”). |
| (b) | Each Party shall be responsible for and shall ensure that any and all of its employees involved in the Transition hereunder comply with the Competition Law Guidelines (after their adoption) and all applicable competition laws. Each Party shall have in place and shall maintain a competition law compliance program, including a compliance manual, policies and employee trainings. |
| 11.5 | Social, Social Security and Tax Obligations |
| (a) | The Parties will reasonably cooperate and coordinate with each other, as necessary to effect the transactions contemplated herein, with respect to the obtainment and delivery of any necessary tax certifications, including: |
| (i) | from the relevant Spanish tax administrations the certificates as referred to in Article 175.2 of the Spanish General Tax Law (Law 58/2003). |
| (ii) | a Tax certificate (“Certificato carichi pendenti”) from the competent Italian tax administration listing all pending Tax liabilities of Gilead and such relevant Affiliates. |
| 11.6 | Regulatory Inspections. If any Regulatory Authority conducts or provides notice of its intent to conduct any audit or inspection that relates to any activities conducted by Gilead, its Affiliates or Subcontractors with respect to the Galapagos Territory under this Agreement or the A&R Collaboration Agreement, then, upon Galapagos’ reasonable request, Gilead shall coordinate with Galapagos and provide or make available, or procure that its Affiliates or Subcontractor provide or make available, to Galapagos any information Controlled by Gilead or its Affiliates and in the possession of Gilead, its Affiliates or Subcontractors that relates to such activities conducted by Gilead, its Affiliates or Subcontractors with respect to the Galapagos Territory and, in each case, is reasonably necessary for Galapagos to respond to a request or inquiry from the applicable Regulatory Authority in connection with such audit or inspection, including any such information relating to any quality oversight activities (or audits) of Gilead or its Affiliates or Subcontractors related to Licensed Compound or Licensed Products with respect to the Galapagos Territory under this Agreement or the A&R Collaboration Agreement. |
ARTICLE 12
FINANCIALS
| 12.1 | Upfront, Milestone Payments, Royalties and Profit Split |
| (a) | Upfront Payment. The Parties acknowledge that a one-time, non-refundable, non-creditable payment of €35,000,000 was made by Gilead to Galapagos […***…]. |
| (b) | Milestone Payments. Effective on the Announcement Date, Gilead’s obligation to pay milestone payments for the milestone events in the row “Regulatory Approval of a Licensed Product in EMA” in the table included in Section 8.2(a) of the A&R Collaboration Agreement is terminated and of no further force or effect. For clarity, the other milestone payment obligations of Gilead set forth in the A&R Collaboration Agreement and not previously fulfilled remain unchanged and in full force and effect. |
| (c) | Royalties. |
| (i) | Gilead’s royalty payment obligations to Galapagos under Section 8.3 of the A&R Collaboration Agreement for Net Sales of Licensed Products sold by Gilead or its Affiliate or Sublicensee (for clarity, other than by Galapagos or its Affiliate or Sublicensee) in the Licensed Territory shall remain in full force and effect, on a country-by-country basis, until the earlier of (1) the transfer of TUPE Employees or all Hired Employees to Galapagos in such country or (2) the Initial Transition Date, after which time Gilead shall pay royalties to Galapagos on Net Sales of Licensed Products in the Gilead Territory pursuant to the Second A&R Collaboration Agreement. Gilead shall have no royalty payment obligations to Galapagos, under Section 8.3 of the A&R Collaboration Agreement or otherwise, (x) for Net Sales of Licensed Products (if any) sold by Gilead or its Affiliate or Sublicensee in the Galapagos Territory, on behalf of Galapagos if all proceeds of such sale are remitted to Galapagos or (y) based on sales of Licensed Products sold by Galapagos or its Affiliate or Sublicensee in the Galapagos Territory. |
| (ii) | As will be further described in the Second A&R Collaboration Agreement, the Second A&R Collaboration Agreement shall provide that, commencing on […***…], 2024, Galapagos shall pay to Gilead non-refundable royalties on the amount of aggregate Net Sales of Licensed Products in the Galapagos Territory in each calendar year at the rates set forth below. |
| Net Sales of Licensed Products in the Galapagos Territory |
Royalty Rate | |||
| [ *** ] |
8 | % | ||
| [ *** ] |
[ | ***] | ||
| [ *** ] |
[ | ***] | ||
| [ *** ] |
15 | % | ||
Such royalties shall be payable on a country-by-country basis until the end of the Royalty Term for such country. Such royalties shall be subject to step downs and adjustments for compulsory licenses equivalent to those in Sections 8.3(d) and 8.3(e) of the A&R Collaboration Agreement, mutatis mutandis.
| (d) | Operating Profits and Operating Losses Sharing. |
| (i) | Operating Profits and Operating Losses for each Licensed Product in the Shared Territory incurred from the Announcement Date until the Initial Transition Date will continue to be shared equally by each Party as described in, and subject to, Section 8.9 of the A&R Collaboration Agreement; provided, however, that (x) effective as of the Effective Date, that certain letter from Gilead to Galapagos dated October 5, 2020 regarding allocation of Gilead costs regarding the Shared Territory is hereby terminated and of no further force or effect, and (y) until the Initial Transition Date, […***…] of any costs incurred by or on behalf of Gilead or its Affiliates that are incurred in connection with Commercialization activities relating to the Licensed Products and reasonably and directly attributable to the Galapagos Territory shall be deemed Joint Commercialization Costs for purposes of the calculation of Operating Profit (or Loss) under Section 8.9(a)(ii) of the A&R Collaboration Agreement. |
| (ii) | Notwithstanding the A&R Collaboration Agreement, […***…]. |
| (iii) | Beginning on the Initial Transition Date and continuing for all periods thereafter, such profit-sharing arrangement, including any sharing of any costs, with respect to the Shared Territory will terminate and be of no further force or effect (and, for clarity, Gilead shall have no right to share Operating Profits, and no obligation to bear any Operating Losses, with respect to any Licensed Product in the Shared Territory for such periods). |
| 12.2 | Development Costs |
| (a) | Generally. All Development Costs incurred on or after […***…] shall be governed by this Agreement or the Second A&R Collaboration Agreement as set forth below. The provisions in the A&R Collaboration Agreement shall not govern any Development Costs incurred on or after […***…]. |
| (b) | One-Time Development Cost Payments. In consideration for Galapagos assuming responsibility for the Development activities for the Licensed Product as set forth in this Agreement, Gilead irrevocably agrees to make the following one-time payments to Galapagos: |
| (i) | On or before […***…] 2021, Gilead will make a one-time payment of €75,000,000 to Galapagos. |
| (ii) | On or before […***…] 2022, Gilead will make a one-time payment of €50,000,000 to Galapagos. |
The foregoing payments shall be non-refundable, non-creditable, and non-cancelable. Galapagos shall provide Gilead with an applicable invoice (x) for the payment described in clause (i) above, promptly after the Effective Date and (y) for the payment described in clause (ii) above, on or around […***…], and notwithstanding anything to the contrary in this Section 12.2(b) neither such payment shall be due until […***…] after Gilead’s receipt of the applicable invoice.
| (c) | Development Costs Sharing for […***…] Activities. |
| (i) | Section 3.4 of the A&R Collaboration Agreement, which provides that each Party shall be responsible for fifty percent (50%) of the Development Costs incurred by or on behalf of either Party or its Affiliates solely to the extent related to Development of the Licensed Compound or Licensed Products in the Territory, continued to apply to the Development Costs reasonably and directly attributable to […***…] from the Announcement Date until […***…]. |
| (ii) | Subject to the remainder of this Section 12.2(c), Galapagos shall be solely responsible for all Development Costs incurred by or on behalf of either Party or its Affiliates on or after […***…] that are reasonably and directly attributable to […***…]. Each Party shall remain responsible for its own internal costs for the […***…] Activities. Within […***…] after the end of each calendar quarter, Gilead shall provide to Galapagos a report in reasonable detail of any […***…] incurred by Gilead in such calendar quarter together with an invoice for such amount. Galapagos shall pay any such invoice not later than […***…] following receipt thereof. |
| (iii) | For clarity, if Galapagos decides, or is mandated by a Regulatory Authority, to wind down or materially amend any […***…] instead of completing the studies as currently planned, Gilead will not be responsible for any such wind-down or amendment costs incurred by Galapagos or Gilead (and, to the extent any such wind-down or amendment costs are out-of-pocket costs incurred by or on behalf of Gilead, they shall be deemed […***…] and subject to reimbursement by Galapagos in accordance with this Section 12.2(c)). |
| (iv) | In consideration for Galapagos assuming responsibility for the Development activities for the Licensed Product as set forth in this Agreement: |
| (A) | If the aggregate […***…] incurred by or on behalf of both Parties or their Affiliates between […***…] exceed the budgeted amounts for such time period set forth in the Development Plan and Budget, then Gilead shall make a true-up payment to Galapagos in an amount equal to (1) 50% of the amount of any such budget overage for such period, with Gilead’s share of such overage not to exceed […***…] (such share, the “First Overage Share Amount”), plus (2) 50% of the amount of any such budget overage not already captured by clause (1) that is reasonably incurred by the Parties to comply with regulatory requirements imposed by Regulatory Authorities with respect to the […***…] Activities. On or before […***…], Galapagos shall provide to Gilead a report describing in reasonable detail the […***…] for such time period and showing the calculation of such true-up payment amount, together with an invoice for such true-up payment amount. Gilead shall pay to Galapagos the First Overage Share Amount (as invoiced) on or before […***…] (or, if later, the date that is […***…] after receiving such invoice); and |
| (B) | If the aggregate […***…] incurred by or on behalf of both Parties or their Affiliates between […***…] exceed the budgeted amounts for such time period set forth in the Development Plan and Budget, then Gilead shall make a true-up payment to Galapagos in an amount equal to (1) 50% of the amount of any such budget overage for such period, with Gilead’s share of such overage not to exceed […***…] less the First Overage Share Amount, plus (2) 50% of the amount of any such budget overage not already captured by clause (1) that is reasonably incurred by the Parties to comply with regulatory requirements imposed by Regulatory Authorities with respect to the […***…]. On or before […***…], Galapagos shall provide to Gilead a report describing in reasonable detail the […***…] for such time period and showing the calculation of such true-up payment amount, together with an invoice for such true-up payment amount. Gilead shall pay to Galapagos the amount of such invoice on or before […***…] (or, if later, the date that is […***…] after receiving such invoice). |
| (C) | The Parties agree that any extension or other requirement for an existing […***…] that is unilaterally and definitively raised and suggested by the EMA or any other Regulatory Authority in the Galapagos Territory (i.e., not first raised or suggested by Galapagos) as materially important to maintain a Marketing Authorization, and is added as a condition to a Marketing Authorization or otherwise imposed by a decision from the EMA or any other Regulatory Authority, as evidenced by documentation provided by the EMA or such other Regulatory Authority, shall be treated as “imposed” for purposes of clause (2) of Sections 12.2(c)(iv)(A) and (B). |
| (D) | For clarity, if for either period described in Sections 12.2(c)(iv)(A) and (B), the aggregate […***…] incurred by both Parties do not exceed the budgeted amounts for such period set forth in the Development Plan and Budget, then no true-up payment will be owed by Gilead for such period. |
| (d) | Development Costs Sharing for […***…]. From the Effective Date until the applicable […***…] are completed, the Parties agree to continue to share the Development Costs reasonably and directly attributable to […***…] equally. Each Party shall remain responsible for its own internal costs, including […***…], for the […***…]. Within […***…] after the end of each calendar quarter, each Party shall provide to the other Party a report in reasonable detail of any […***…] incurred by such Party in such calendar quarter for each Licensed Product. The […***…] so reported will be used for the calculation of the 50%/50% split for the […***…]. Within […***…] after the end of each calendar quarter, Galapagos shall send Gilead a consolidated report in reasonable detail regarding such […***…] incurred by each Party for such calendar quarter. Within […***…] following receipt of such report, the Party whose […***…] expenditures exceed the portion of the total such expenditures by both Parties for such calendar quarter allocated to such Party in this Section 12.2(d) shall invoice the other Party for the amount of funds necessary to account for such excess. The Party receiving such invoice shall pay it not later than […***…] following receipt thereof. |
| (e) | Development Costs for New Joint Development Activities. The costs for New Joint Development Activities shall be borne by the Parties in accordance with Section 7.3(c). |
| 12.3 | Regulatory Affairs Costs |
With respect to out-of-pocket costs and expenses incurred prior to the MA Transfer Completion Date for any Registered Country in connection with applying for Regulatory Approval with respect to Licensed Products in such Registered Country or transferring to Galapagos or the applicable Galapagos MAH the Marketing Authorization or Marketing Authorization application for such Registered Country, and, in each case, any related regulatory affairs activities (excluding any costs expressly set forth in this Agreement as being at one Party’s sole cost and expense), such costs and expenses shall be shared […***…] and Gilead shall be responsible for […***…] thereof and Galapagos shall be responsible for […***…] thereof. The foregoing costs, expenses and fees will be reported in the same manner as Development Costs and, for those shared […***…], will be included in the reconciliation, invoicing and payment made.
| 12.4 | Commercialization Costs Through Initial Transition Date |
| (a) | Shared Territory. For clarity, […***…] incurred from the Announcement Date until the Initial Transition Date shall be included in the calculation of Operating Profit (or Loss) under Section 8.9(a)(ii) of the A&R Collaboration Agreement. |
| (b) | [Intentionally Omitted] |
| (c) | Other Costs in the Licensed Territory. Except as provided in Section 12.1(d)(i) or 12.4(b), each Party shall be solely responsible for all Commercialization costs incurred by or on behalf of such Party in the Commercialization of Licensed Products in the Licensed Territory. With respect to any country in the Galapagos Territory other than the Shared Territories, if the TUPE Employees or Hired Employees, as applicable, transfer to Galapagos prior to the assumption by Galapagos of the distribution activities in such country, Gilead will pay Galapagos an amount equal to the Net Sales of Licensed Products in such country received by Gilead or its Affiliates during such interim period. To the extent any amounts are owed by Gilead pursuant to the preceding sentence, Galapagos shall provide to Gilead an invoice for such amounts on or before […***…], and Gilead shall pay the amount of such invoice no later than […***…] after its receipt thereof. |
| (d) | Reporting. By the […***…] of each month, each Party shall provide to the other Party a report showing in reasonable detail the costs and expenses incurred by such Party pursuant to the Shared Territory Commercialization Plan and Budget during the preceding month. If requested by either Party, the Parties will have a call or meeting to discuss any costs and expenses included in any such report or related to the Shared Territory Commercialization Plan and Budget. |
| 12.5 | Other Transition Costs |
Except as expressly provided for in this Agreement, each Party shall be responsible for all costs and expenses it incurs in connection with the Transition.
| 12.6 | Taxes |
| (a) | Taxes on Income. Each Party shall be solely responsible for the payment of all taxes imposed on its share of income arising directly or indirectly from the collaborative efforts of the Parties under this Agreement. |
| (b) | Tax Cooperation. The Parties agree to cooperate with one another and use reasonable efforts to avoid or reduce tax withholding or similar obligations in respect of payments made between the Parties under this Agreement. Without limiting the generality of the foregoing, each Party shall provide to the other Party any tax forms and other information that may be reasonably necessary in order to support its claim of no-withholding or reduced withholding based on an applicable treaty. Each Party shall provide the other with reasonable assistance to enable the recovery, as permitted by Applicable Law, of withholding taxes, value added taxes, or similar obligations resulting from payments made under this Agreement, such recovery to be for the benefit of the Party bearing such withholding tax or value added tax. |
| (c) | Payment of Tax. It is understood and agreed between the Parties that any payments made by a Party under this Agreement are exclusive of any value added tax (“VAT”) or similar tax imposed upon such payments. Where VAT is properly added to a payment made under this Agreement, a paying Party will pay the amount of VAT only on receipt of a valid tax invoice issued in accordance with Applicable Law. In addition, to the extent a Party is required by Applicable Law to deduct and withhold taxes on any payment to the other Party, such Party shall pay the amounts of such taxes to the proper Governmental Authority in a timely manner and promptly (within […***…] days of payment) transmit to the other Party an official tax certificate or other evidence of such withholding sufficient to enable the other Party to claim credit for such payment of taxes. |
| 12.7 | Financial Records; Audits |
Each Party and its Affiliates shall use all reasonable efforts to maintain complete and accurate records in sufficient detail to permit the other Party to confirm the accuracy of any amount to be paid, reimbursed, credited, offset or shared in accordance with the terms of this Agreement. Upon reasonable prior notice, such records shall be open during regular business hours for a period of three (3) years from the creation of individual records for examination at the auditing Party’s expense, and not more often than once each calendar year, by an independent certified public accountant selected by the auditing Party and reasonably acceptable to the audited Party or Affiliate for the sole purpose of verifying for the auditing Party the accuracy of the financial statements, reports or notices furnished by the audited Party or Affiliate pursuant to this Agreement or of any payments made, or required to be made, by or to the audited Party or Affiliate to the other pursuant to this Agreement. Any such auditor shall not disclose the audited Party’s or Affiliate’s Confidential Information to the auditing Party, but shall, instead, report that there was or was not a discrepancy uncovered by the audit and if such a discrepancy was uncovered, the amount and direction of it. Any amounts shown to be owed but unpaid, or overpaid and in need of reimbursement, shall be paid or refunded (as the case may be) within […***…] after the accountant’s report, plus interest (as set forth in Section 12.9) from the original due date (unless challenged in good faith by the audited Party, in which case any undisputed portion shall be paid in accordance with the foregoing timetable, any dispute with respect to such challenge shall be resolved in accordance with Section 16.6, any remaining disputed portion shall be paid within […***…] after resolution of the dispute, and interest shall not accrue with respect to the disputed portion during the period of time the dispute is being resolved). The auditing Party shall bear the full cost of such audit unless such audit reveals an overpayment to, or an underpayment by, the audited Party or Affiliate that resulted from a discrepancy in a report that the audited Party or Affiliate provided to the other Party during the applicable audit period, which underpayment or overpayment was more than […***…] of the amount set forth in such report, in which case the audited Party or Affiliate shall bear the full cost of such audit. Each Party, at the request of the other Party, shall make available to the other Party the results of any audit performed by the non-requesting Party on such non-requesting Party’s Sublicensees hereunder.
| 12.8 | Foreign Exchange. |
Notwithstanding Section 8.12 of the A&R Collaboration Agreement, where amounts in this Agreement are expressed in euros, payment should be made in euros.
| 12.9 | Interest on Late Payments |
If a Party does not receive payment of any sum due to it on or before the due date therefor, simple interest shall thereafter accrue on the sum due to such Party from the due date until the date of payment at a per-annum rate of […***…] above the prime rate as reported in The Wall Street Journal, Eastern Edition, or the maximum rate allowable by Applicable Law, whichever is less. Such accrued interest and the payment and acceptance thereof shall not negate or waive the right of the non-paying Party to any other remedy, legal or equitable, to which the non-paying Party may be entitled because of the delinquency of the payment.
| 12.10 | Manner and Place of Payment |
All payments owed under this Agreement shall be made by wire transfer in immediately available funds to a bank and account designated in writing by Galapagos or Gilead (as applicable), unless otherwise specified in writing by such Party.
ARTICLE 13
REPRESENTATIONS AND WARRANTIES
| 13.1 | Mutual Representations and Warranties |
Each Party hereby represents, warrants, and covenants (as applicable) to the other Party, as of the Effective Date, as follows:
| (a) | It is a corporation duly organized, validly existing, and in good standing under the laws of the jurisdiction in which it is incorporated, and has full corporate power and authority and the legal right to own and operate its property and assets and to carry on its business as it is now being conducted and as contemplated in this Agreement, including the right to grant the licenses granted by it hereunder. |
| (b) | Except as set forth in this Section 13.1, (i) it has the corporate power and authority and the legal right to enter into this Agreement and perform its obligations hereunder; (ii) it has taken all necessary corporate action on its part required to authorize the execution and delivery of this Agreement and the performance of its obligations hereunder; and (iii) this Agreement has been duly executed and delivered on behalf of such Party, and constitutes a legal, valid, and binding obligation of such Party that is enforceable against it in accordance with its terms, except to the extent that enforcement of the rights and remedies created hereby is subject to (A) bankruptcy, insolvency, reorganization, moratorium and other similar laws of general application affecting the rights and remedies of creditors or (B) laws governing specific performance, injunctive relief and other equitable remedies. |
| (c) | It is not a party to and will not enter into any agreement that would prevent it from granting the rights or exclusivity granted or intended to be granted to the other Party under this Agreement or performing its obligations under this Agreement. |
| 13.2 | Representations and Warranties by Gilead |
As of the Effective Date, Gilead hereby makes the representations and warranties, and agrees to perform the covenants set forth below in this Section 13.2.
| (a) | To Gilead’s Knowledge, Gilead and its Affiliates own all right, title and interest in and to all of the Transferred Trademarks, free and clear of all Encumbrances. |
| (b) | To Gilead’s Knowledge, neither Gilead nor any of its Affiliates has received written notice in the last 12 months from any person or entity asserting a claim of ownership to any of the Transferred Trademarks. |
| (c) | To Gilead’s Knowledge, no Person is infringing or threatening to infringe or misappropriating or threatening to misappropriate the Transferred Trademarks. |
| (d) | To Gilead’s Knowledge, Gilead has not received any written notice, in the last 12 months, that any person alleges the use of the Transferred Trademarks would be an infringement of their rights. |
| (e) | Neither Gilead nor any of its Affiliates has entered into any sublicense agreement or distribution agreement or granted any sublicense or distribution rights to any Third Party, in each case, with respect to the Licensed Products in the Galapagos Territory, other than sublicenses granted to contract researchers or contract manufacturers in connection with contract research agreements or contract manufacturing agreements pursuant to which such contract researchers or contract manufacturers provide services solely to Gilead. |
| (f) | To Gilead’s Knowledge, neither Gilead nor any of its Affiliates has received, in the last 12 months, any written claim or demand of any person or entity alleging that the Development, Manufacture, use, or Commercialization of the Licensed Products in the Galapagos Territory by Gilead or its Affiliates infringes a Third Party patent. |
| (g) | None of the terms of this Agreement or the transactions contemplated under this Agreement conflict with the terms of the […***…]. |
| (h) | Schedule 5 sets forth the equity awards granted to the New Galapagos Employees under the Gilead Plan, including the quantity of the unvested portions of such awards that will forfeit pursuant to the departure of the New Galapagos Employee from Gilead in the framework of the transaction. This information is, as of the date such information was provided or updated, true and accurate in all material respects. |
| (i) | To Gilead’s Knowledge, neither Gilead nor any of its Affiliates has received, in the last 12 months, any written complaint or is currently the subject of a governmental or regulatory investigation regarding its collection, storage, transfer or use of any information relating to identified or identifiable natural persons that was collected in a Clinical Trial involving the Licensed Compound or Licensed Products or in connection with any Hired Employee or TUPE Employee. |
ARTICLE 14
INDEMNIFICATION
| 14.1 | Indemnification by Galapagos |
Section 11.1 (Indemnification by Galapagos) of the A&R Collaboration Agreement is hereby incorporated by reference into this Agreement, mutatis mutandis, as if references to “this Agreement and any Co-Commercialization Agreement” were to this Agreement or any Transition Ancillary Agreement; provided, however, that as incorporated by reference into this Agreement, Section 11.1(b) of the A&R Collaboration Agreement shall be deemed to read as follows:
“(b) (i) the Exploitation by or on behalf of Galapagos or its Affiliates, subcontractors, licensees or Sublicensees (excluding such conduct by or on behalf of Gilead, its Affiliates and Sublicensees as licensees or Sublicensees of Galapagos hereunder) of any Licensed Product or Licensed Compound excluding any Shared Development Damages (which shall be allocated as set forth in Section 11.7); or (ii) the exercise or use by or on behalf of Galapagos, its Affiliates, subcontractors, licensees, or Sublicensees (excluding such exercise by Gilead, its Affiliates, and Sublicensees as licensees and Sublicensees of Galapagos hereunder) of rights under any license or right of reference, or in or to any Regulatory Materials, Regulatory Approvals, Marks or other Information, in each case granted, transferred or made available by or on behalf of Gilead or any of its Affiliates to Galapagos under this Agreement or any Transition Ancillary Agreement, or (iii) any act or omission taken (or not taken) by Gilead or its Affiliates, subcontractors, licensees or Sublicensees at the express request or direction of Galapagos pursuant to Galapagos’ exercise of its General Direction hereunder.”
| 14.2 | Indemnification by Gilead |
Section 11.2 (Indemnification by Gilead) of the A&R Collaboration Agreement is hereby incorporated by reference into this Agreement, mutatis mutandis, as if references to “this Agreement and any Operational Agreement” were to this Agreement or any Transition Ancillary Agreement; provided, however that as incorporated by reference into this Agreement, Section 11.2 is hereby amended by (i) adding, at the end of subsection (a), “and excluding any Shared Development Damages (which shall be allocated as set forth in Section 11.7),” (ii) deleting the word “or” after subsection (c), (iii) adding the word “or” after subsection (d) and (iv) inserting the following new subsection (e) immediately after subsection (d) that shall read as follows:
“(e) any act or omission taken (or not taken) by Galapagos or its Affiliates, subcontractors, licensees or Sublicensees at the express request or direction of Gilead based on Gilead’s MAH Legal Responsibilities.”
Except to the extent the A&R Collaboration Agreement or this Agreement expressly allocates liability to or sets forth an indemnification obligation of Galapagos or its Affiliate(s), Gilead shall defend, indemnify, and hold the Galapagos Indemnitees harmless from and against any and all Galapagos Damages, all to the extent resulting from Galapagos Claims against such Galapagos Indemnitee arising from or based on the ownership or operation of Transferred Assets prior to the effective date of transfer of such Transferred Assets to Galapagos under this Agreement or a Transition Ancillary Agreement.
Gilead shall also indemnify Galapagos as set forth in Sections 5.3, 5.4 and 6.1 in accordance with the procedures set forth in Section 14.3.
| 14.3 | Indemnification Procedures |
Section 11.3 (Indemnification Procedures) of the A&R Collaboration Agreement is hereby incorporated by reference into this Agreement, mutatis mutandis.
| 14.4 | Shared Development Damages. |
A new Section 11.7 (Shared Development Losses) is added to the A&R Collaboration Agreement as shown below:
“11.7 Shared Development Damages. The Parties shall share equally any Gilead Damages and Galapagos Damages incurred, respectively, by the Gilead Indemnitees and Galapagos Indemnitees (collectively, “Party Indemnitees”) to the extent resulting from Third Party Claims against any such Party Indemnitee that arise from or are based on the performance after the effective date of the Transition & Amendment Agreement of […***…] or the performance of activities under a Joint Development Plan and Budget, in each case to the extent not arising from or based on the events described in Sections 11.1(a) or 11.2(b), (c) or (d) (any such damages, “Shared Development Damages” and any such Third Party Claims, “Shared Development Claims”). If either Party receives notice of any Shared Development Claim, such Party shall inform the other Party in writing as soon as reasonably practicable, and the Parties shall discuss a strategy for defending such Shared Development Claim. This Section 11.7 is not intended, and shall not be construed, to alter either Party’s liability under Section 11.1 or Section 11.2 (as applicable) for any Claims that arise from or are based on any commercially sold Licensed Product. At its option, the Party against whose Party Indemnitees a Third Party raises a Shared Development Claim may assume the defense of any Shared Development Claim by giving written notice to the other Party within […***…] days after the notice of the Shared Development Claim. If such does not assume and conduct the defense of a Shared Development Claim as provided in the preceding sentence, then the other Party may defend against such Shared Development Claim. The non-defending Party may participate in and monitor such defense with counsel of its own choosing, subject to the defending Party’s right to assume and conduct the defense of the Shared Development Claim with counsel of its choice. Neither Party shall settle any Shared Development Claim without the prior written consent of the other Party, not to be unreasonably withheld, conditioned or delayed.”
| 14.5 | Limitations of Liability |
Section 11.5 (Limitation of Liability) of the A&R Collaboration Agreement is hereby incorporated by reference into this Agreement, mutatis mutandis.
| 14.6 | Insurance |
Section 11.6 (Insurance) of the A&R Collaboration Agreement is hereby incorporated by reference into this Agreement, mutatis mutandis.
ARTICLE 15
TERM OF AGREEMENT
| 15.1 | Term of Agreement |
The term of this Agreement shall commence on the Effective Date and shall continue in full force and effect until June 30, 2023.
| 15.2 | Impact of Material Breach |
| (a) | Material Breach. In the event of a material breach of this Agreement by a Party, the non-breaching Party may give the breaching Party a written notice identifying such material breach in reasonable detail (the “Material Breach Notice”) and giving the breaching Party an opportunity to cure such material breach within […***…] days from the date of such notice (or within […***…] from the date of such notice in the event such material breach is solely based upon the breaching Party’s failure to pay any amounts owed to the non-breaching Party hereunder) (the “Cure Period”). If the breaching Party fails to cure such material breach within the Cure Period, then, subject to Section 15.2(b), representatives of the non-breaching Party may escalate the material breach to the Executive Officer of each Party for review and determination of a remedial plan for the breaching Party to remediate the material breach as promptly as reasonably possible (“Remedial Measures”). The Executive Officers or their respective designees shall, within […***…] after such escalation, meet and discuss such material breach and determine the Remedial Measures. The breaching Party shall perform the Remedial Measures and report in writing on a weekly basis to the Executive Officers or their respective designees on the actions taken and the progress in executing the Remedial Measures. The breaching Party will be solely responsible for any costs, expenses and liabilities incurred as a result of the material breach or the implementation of such Remedial Measures. In parallel with the review by the Executive Officers or their respective designees and determination of Remedial Measures, the breaching Party shall use Transition Activity Commercially Reasonable Efforts to remediate any on-going material breach of this Agreement. |
| (b) | Disputed Breach. If the alleged breaching Party disputes in good faith the existence or materiality of a breach specified in a Material Breach Notice, and such alleged breaching Party provides the other Party notice of such dispute within […***…] of receipt of such Material Breach Notice, then the non-breaching Party shall not have the right to seek Remedial Measures under Section 15.2(a) unless and until (i) an arbitrator, in accordance with Section 16.6, has determined that the alleged breaching Party has materially breached this Agreement and (ii) such Party fails to cure such breach within […***…] following such arbitrator’s decision (except to the extent such breach involves the failure to make a payment when owed to the non-breaching Party, which breach must be cured within […***…] following such arbitrator’s decision). |
| 15.3 | Amendment to A&R Collaboration Agreement |
| (a) | Section 13.3(a)(i) of the A&R Collaboration Agreement is hereby deleted in its entirety and replaced with the following: |
“(i) At Will. Subject to Section 13.3(a)(ii), at any time after […***…].”
| (b) | Section 13.3(a)(ii) of the A&R Collaboration Agreement is hereby deleted in its entirety and replaced with the following: |
“(ii) For Safety-Related, Regulatory-Related Reasons. Gilead shall have the right to terminate this Agreement with respect to the Gilead Territory on a Licensed Product-by-Licensed Product, indication-by-indication, or country-by-country basis upon […***…] prior written notice to Galapagos if Gilead concludes, reasonably and in good faith, that […***…].”
| 15.4 | Survival |
The expiration of this Agreement shall not relieve either Party of any of its obligations to the extent such obligations were incurred prior to expiration or that are expressed to survive expiration. Notwithstanding anything to the contrary, the following provisions shall survive and apply after expiration for the period specified therein (or, if no period is specified therein, indefinitely): Sections 2.1 (clause (y) in the first paragraph only), 5.3(a), 5.3(b), 5.3(d), 5.4(b), 8.1(d) (clauses (i) and (ii) of the fifth sentence, and the sixth sentence, only), 10.2(a), 10.2(b)(i)(B) (solely as to clauses (2), (3) and (4) thereof), 10.2(b)(ii), 11.1(b), 11.1(c), 11.2(b), 11.5, 12.2(c)(iv) (provided, however, that if substantially similar terms are set forth in the Second A&R Collaboration Agreement, then Section 12.2(c)(iv) shall not survive and apply after expiration), 12.6 through 12.10 (for payments accruing before the expiration of this Agreement), 14.1, 14.2, 14.3, 14.5, 15.4, ARTICLE 16 (excluding Section 16.4), and any definitions of any applicable capitalized terms.
ARTICLE 16
MISCELLANEOUS
| 16.1 | Certain Miscellaneous Provisions |
Sections 15.5 (No Strict Construction; Headings), 15.7 (Performance by Affiliates), 15.8 (Compliance with Applicable Law), 15.9 (Further Actions), 15.10 (Severability), 15.11 (No Waiver), 15.12 (Independent Contractors) and 15.13 (Counterparts) of the A&R Collaboration Agreement are hereby incorporated by reference into this Agreement, mutatis mutandis.
| 16.2 | Force Majeure |
Both Parties shall be excused from the performance of their obligations under this Agreement to the extent that such performance is prevented or delayed by force majeure and the nonperforming Party promptly provides notice of the prevention to the other Party. Such excuse shall be continued so long as the condition constituting force majeure continues and the nonperforming Party takes reasonable efforts to remove the condition. For purposes of this Agreement, force majeure shall mean conditions beyond the control of the Parties, including an act of God, war, civil commotion, terrorist act, labor strike or lock-out, epidemics or other outbreaks of infectious disease or other public health crises, including COVID-19, failure or default of public utilities or common carriers, destruction of production facilities or materials by fire, earthquake, storm or like catastrophe, and failure of plant or machinery (provided that such failure could not have been prevented by the exercise of skill, diligence, and prudence that would be reasonably and ordinarily expected from a skilled and experienced person engaged in the same type of undertaking under the same or similar circumstances). Notwithstanding the foregoing, a Party shall not be excused from making payments owed hereunder because of a force majeure affecting such Party.
| 16.3 | Entire Agreement |
This Agreement, including the Schedules and Exhibits hereto, the A&R Collaboration Agreement, the Second A&R Collaboration Agreement, the Transition Ancillary Agreements and the Ancillary Agreements set forth the complete, final and exclusive agreement and all the covenants, promises, agreements, warranties, representations, conditions and understandings between the Parties hereto with respect to the subject matter hereof and thereof and supersede all prior agreements and understandings between the Parties existing as of the Effective Date with respect to the subject matter hereof and thereof, including the Term Sheet, which is hereby superseded in its entirety and, from and after the Effective Date, of no further force or effect. There are no, and the Parties expressly disclaim any, covenants, promises, agreements, warranties, representations, conditions or understandings, either oral or written, between the Parties related to the subject matter hereof and thereof other than those, if any, as are expressly set forth herein and therein. No subsequent alteration, amendment, change or addition to this Agreement shall be binding upon the Parties unless reduced to writing and signed by an authorized officer of each Party. In the event of any inconsistency between any Schedules and Exhibits to this Agreement and this Agreement, the terms of this Agreement shall prevail. In the event of any inconsistency between the terms of this Agreement and the terms of any Ancillary Agreement or Transition Ancillary Agreement, the terms of this Agreement shall prevail (unless otherwise provided in the applicable Ancillary Agreement or Transition Ancillary Agreement).
| 16.4 | Subcontracting |
| (a) | Subject to Section 16.4(b), but notwithstanding anything else to the contrary herein, each Party may perform any of its Development, Manufacturing, regulatory, and Commercialization obligations under this Agreement through one or more Subcontractors or consultants, provided that (i) such Party remains responsible for the work allocated to, and payment to, such Subcontractors and consultants to the same extent it would if it had done such work itself; (ii) the Subcontractor or consultant undertakes in writing obligations of confidentiality and non-use regarding Confidential Information that are substantially the same as those undertaken by the Parties with respect to Confidential Information pursuant to ARTICLE 12 of the Applicable Collaboration Agreement; and (iii) such Party undertakes all reasonable efforts to provide that the Subcontractor or consultant undertakes in writing to assign or exclusively license back (with the right to sublicense) all intellectual property with respect to Licensed Compound or Licensed Products developed in the course of performing any such work to such Party. Each Party may also subcontract work on terms other than those set forth in this Section 16.4 with the prior written approval of the other Party. |
| (b) | Subject to Galapagos’ prior written consent as to a specific Subcontractor, which consent will not be unreasonably conditioned, delayed or withheld, Gilead may perform any of its obligations under this Agreement, including Gilead’s pharmacovigilance-activities and Gilead’s data analysis activities, through one or more Subcontractors, which shall include any Third Party contract research organizations, contract manufacturing organizations and other qualified vendors; provided that, subject to Section 11.2, (i) Gilead remains responsible for the work allocated to, and payment to, such Subcontractors to the same extent it would if it had done such work itself; (ii) the Subcontractor undertakes in writing obligations of confidentiality and non-use regarding Confidential Information that are substantially the same as those undertaken by the Parties with respect to Confidential |
| Information pursuant to this Agreement hereof; and (iii) Gilead undertakes all reasonable efforts to provide that the Subcontractor undertakes in writing to assign or exclusively license back (with the right to sublicense) all intellectual property with respect to Licensed Compound or Licensed Products developed in the course of performing any such work to such Party. |
| 16.5 | Assignment |
| (a) | Neither Party may assign or transfer this Agreement or any rights or obligations hereunder without the prior written consent of the other, except that a Party may make such an assignment without the other Party’s consent in connection with the simultaneous assignment of the Applicable Collaboration Agreement pursuant to the terms of such Applicable Collaboration Agreement. Any permitted successor or assignee of rights or obligations hereunder shall, in a writing to the other Party, expressly assume performance of such rights or obligations (and in any event, any Party assigning this Agreement to an Affiliate shall remain bound by the terms and conditions hereof). Any permitted assignment shall be binding on the successors of the assigning Party. Any assignment or attempted assignment by either Party in violation of the terms of this Section 16.5 shall be null, void and of no legal effect. |
| (b) | Sections 15.6(c) of the A&R Collaboration Agreement shall be deleted in its entirety as of the Effective Date and replaced with the following: |
“[omitted.]”
| 16.6 | Dispute Resolution |
| (a) | It is the objective of the Parties to establish procedures to facilitate the resolution of disputes arising under this Agreement in an expedient manner by mutual cooperation and without resort to litigation. In the event of any disputes, controversies or differences which may arise between the Parties out of or in relation to or in connection with this Agreement (excluding any disputes relating to the matters that can be referred to the JTT and the TSC other than determination of Remedial Measures if not agreed by the JTT or TSC), including any alleged failure to perform the provisions, or a breach, of this Agreement, or any issue relating to the interpretation or application of this Agreement, then upon the request of either Party by written notice, the Parties agree to meet and discuss in good faith a possible resolution thereof, which good faith efforts shall include at least one meeting (by videoconference, teleconference, or in person) between the Executive Officers of each Party. If the matter is not resolved within […***…] following the written request for discussions, either Party may then invoke the provisions of Section 16.6(b), as appropriate. For the avoidance of doubt, any disputes, controversies or differences arising from the JTT or the TSC pursuant to ARTICLE 4 shall be resolved solely in accordance with ARTICLE 4. |
| (b) | Any dispute, controversy, difference or claim which may arise between the Parties and not from the JTT or the TSC out of or in relation to or in connection with this Agreement (including arising out of or relating to the validity, construction, interpretation, enforceability, breach, performance, application or termination of this Agreement) that is not resolved pursuant to Section 16.6(a) or as otherwise noted in Section 16.6(a), shall be settled by binding arbitration in accordance with the applicable rules of the International Chamber of Commerce (“ICC Rules”) by three (3) arbitrators, one each chosen by the respective Parties and the third chosen by mutual agreement of the first two, and otherwise |
| in accordance with the ICC Rules. The arbitrators shall have significant experience and shall have expertise in licensing and partnering agreements in the pharmaceutical and biotechnology industries. Either Party, following the end of the […***…] period referenced in Section 16.6(a), may refer such issue to arbitration by submitting a written notice of such request to the other Party. The place of arbitration shall be New York and the language (including all testimony, evidence and written documentation) shall be English. The arbitrators shall establish procedures to facilitate and complete such arbitration as soon and efficiently as practicable. Unless the arbitrators expressly determine otherwise, neither Party shall be required to give general discovery of documents, but may be required only to produce specific, identified documents which are relevant to the dispute. The Parties shall have the right to be represented by counsel. Any judgment or award rendered by the arbitrators shall be final and binding on the Parties, and shall be governed by the terms and conditions hereof, including the limitation on damages set forth in Section 14.5. The Parties agree that such a judgment or award may be enforced in any court of competent jurisdiction. The statute of limitations of the State of New York applicable to the commencement of a lawsuit shall apply to the commencement of arbitration under this Section 16.6. The arbitrators shall determine the allocation of costs and expenses and attorneys’ fees in the arbitration to be borne by each Party. All proceedings and decisions of the arbitrators shall be deemed Confidential Information of each of the Parties, and shall be subject to ARTICLE 12 of the Applicable Collaboration Agreement. |
| (c) | Any award to be paid by one Party to the other Party as determined by the arbitrator as set forth above under Section 16.6(b) shall be promptly paid in U.S. dollars free of any tax, deduction or offset; and any costs, fees or taxes incident to enforcing the award shall, to the maximum extent permitted by law, be charged against the Party resisting enforcement. Each Party agrees to abide by the award rendered in any arbitration conducted pursuant to this Section 16.6, and agrees that, subject to the U.S. Federal Arbitration Act, 9 U.S.C. §§ 1-16, judgment may be entered upon the final award in the U.S. Federal District Court for the Southern District of New York and that other courts may award full faith and credit to such judgment in order to enforce such award. |
| (d) | Nothing in this Section 16.6 will preclude either Party from seeking equitable relief or interim or provisional relief from a court of competent jurisdiction, including a temporary restraining order, preliminary injunction or other interim equitable relief, concerning a dispute either prior to or during any arbitration if necessary to protect the interests of such Party or to preserve the status quo pending the arbitration proceeding. Therefore, in addition to its rights and remedies otherwise available at law, including the recovery of damages for breach of this Agreement, upon an adequate showing of material breach, and without further proof of irreparable harm other than this acknowledgement, such non-breaching Party shall be entitled to seek (a) immediate equitable relief, specifically including, but not limited to, both interim and permanent restraining orders and injunctions, and (b) such other and further equitable relief as the court may deem proper under the circumstances. |
| (e) | Any duty to arbitrate under this Agreement shall remain in effect and be enforceable after termination of this Agreement for any reason. |
| (f) | For the purposes of this Section 16.6, the Parties acknowledge their diversity (Gilead having its principal place of business in the State of California and Galapagos having its principal place of business in Belgium), and agree to accept the jurisdiction of any United States District Court located in New York for the purposes of enforcing or appealing any awards entered pursuant to this Section 16.6 and for enforcing the agreements reflected in this Section 16.6. |
| 16.7 | Governing Law |
This Agreement shall be governed by and construed under the substantive laws of the State of New York, excluding any conflicts or choice of law rule or principle that might otherwise refer construction or interpretation of this Agreement to the substantive law of another jurisdiction.
| 16.8 | Notices |
Any notice required or permitted to be given under this Agreement shall be in writing, shall specifically refer to this Agreement, and shall be addressed to the appropriate Party at the address or electronic mail specified below or such other address or electronic mail as may be specified by such Party in writing in accordance with this Section 16.8 and shall be deemed to have been given for all purposes (a) when received, if hand-delivered, sent by a reputable international expedited delivery service (with receipt confirmed) or facsimile (with transmission confirmed), (b) […***…] after mailing, if mailed by first class certified or registered mail, postage prepaid, return receipt requested or (c) when sent by electronic mail, upon confirmation of receipt by such recipient (which confirmation shall be deemed to include responses to such electronic mail). Any notice delivered by facsimile shall be confirmed by a hard copy delivered by a reputable international expedited delivery service as soon as practicable thereafter. This Section 16.8 is not intended to govern the day-to-day business communications necessary between the Parties in performing their obligations under the terms of this Agreement.
Address for Notice.
| If to Galapagos: | Galapagos NV | |
| Generaal De Wittelaan L11 A3 | ||
| 2800 Mechelen | ||
| Belgium | ||
| Attention: Chief Executive Officer | ||
| Fax: [ *** ] Email: [ *** ] | ||
| With a copy to (which shall not | Galapagos NV | |
| constitute notice): | Generaal De Wittelaan L11 A3 | |
| 2800 Mechelen | ||
| Belgium | ||
| Attention: General Counsel | ||
| Fax: [ *** ] Email: [ *** ] | ||
| With a copy to (which shall not | Baker & McKenzie LLP | |
| constitute notice): | 452 Fifth Avenue | |
| New York, New York 10018 | ||
| United States | ||
| Attention: Olivia Tyrrell | ||
| Oren Livne Fax: [ *** ] Email: [email protected] | ||
| If to Gilead: | Gilead Sciences, Inc. 333 Lakeside Drive Foster City, CA 94404 USA Attention: Alliance Management Email: [ *** ] | |
| With a copy to (which shall not constitute notice): | Gilead Sciences, Inc. 333 Lakeside Drive Foster City, CA 94404 USA Attention: General Counsel Email: [ *** ] | |
| With a copy to (which shall not constitute notice): | Covington & Burling LLP Salesforce Tower 415 Mission Street, Suite 5400 San Francisco, CA 94105-2533 Attention: Amy L. Toro, Esq. Fax: [ *** ] Email: [email protected] | |
[SIGNATURE PAGE FOLLOWS.]
This Agreement is executed by the authorized representatives of the Parties as of the date first written above.
| GALAPAGOS NV | ||
| By: |
| |
| Name: |
| |
| Title: |
| |
| GILEAD SCIENCES, INC. | ||
| By: |
| |
| Name: |
| |
| Title: |
| |
[Signature Page to Transition Agreement]
SCHEDULE 1
TARGET MA SUBMISSION DATES
[…***…] […***…]
SCHEDULE 2
TRANSFERRED TRADEMARKS
[…***…(2 pages omitted)]
SCHEDULE 3
TUPE EMPLOYEES
[…***…(2 pages omitted)]
SCHEDULE 4
DOMAIN NAMES
[…***…]
SCHEDULE 5
EQUITY AWARDS GRANTED TO NEW GALAPAGOS EMPLOYEES
[…***…(2 pages omitted)]
EXHIBIT A
DEVELOPMENT PLAN AND BUDGET
[…***…(2 pages omitted)]
EXHIBIT B
AMENDED SHARED TERRITORY COMMERCIALIZATION PLAN AND BUDGET
[…***…(6 pages omitted)]
EXHIBIT C
TRADEMARK ASSIGNMENT AGREEMENT
[…***…(4 pages omitted)]
EXHIBIT D
TRANSITION PLAN
[…***…(97 pages omitted)]
EXHIBIT E
[INTENTIONALLY OMITTED]
EXHIBIT F
GILEAD PLANS FOR […***…] TUPE EMPLOYEES
[…***…]
EXHIBIT G
[…***…] BUSINESS TRANSFER AGREEMENT
[…***…(55 pages omitted)]
EXHIBIT H
COMMERCIAL HANDOVER
Commercial Handover means the following with respect to the applicable Registered Country:
[…***…]
Exhibit 99.4
Execution copy
Amendment 1 to the
Subscription Agreement of 14 July 2019
Relating to Ordinary Shares in Galapagos NV
This Amendment (hereinafter “Amendment 1”) is made on 7 April 2021 and shall enter into effect as per the same date (the “Amendment 1 Effective Date”)
Between:
| (1) | Galapagos NV, a company organized and existing under the laws of Belgium, with registered office at Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium, registered with the register of legal entities (Antwerp) under number 0466.460.429 (hereinafter referred to as the “Issuer”); |
and
| (2) | Gilead Therapeutics A1 Unlimited Company, an unlimited liability company formed under the laws of Ireland, with registered office at 70 Sir John Rogerson’s Quay, Dublin 2, Ireland (hereinafter referred to as the “Investor”) |
and, for the purposes of Article 3 hereof, in the presence of:
| (3) | Gilead Sciences, Inc., a Delaware corporation having its principal place of business at 333 Lakeside Drive, Foster City, CA, 94404, United States of America (hereinafter referred to as the “Parent Investor”) |
Issuer and Investor are hereinafter individually referred to as “Party” and collectively as “Parties”.
Whereas:
| (A) | On 14 July 2019, Issuer and Investor entered into a Subscription Agreement relating to ordinary shares in Galapagos NV (the “Agreement”); |
| (B) | On 14 July 2019, the Parent Investor agreed to comply with the covenants and obligations set forth in the Agreement, including, without limitation, the lock-up obligations set forth in Article 6.2 of the Agreement; |
| (C) | The Parties wish to enact the outcome of their recent discussions and to amend Article 6.2 of the Agreement. |
Now, therefore, it is agreed as follows:
| 1 | Definitions |
Unless otherwise specifically provided in this Amendment 1, capitalized terms shall have the meaning ascribed thereto in the Agreement.
| 2 | Amendment of the Lock-up provision |
| 2.1 | Article 6.2.1 of the Agreement is hereby deleted in its entirety and replaced by the following: |
| “6.2.1 | During the period running from the Date of this Agreement through 22 August 2024 (the “Lock-up Period”), the Investor and Parent Investor shall not, and shall cause their Affiliates not to, without the prior consent of the Issuer, transfer, sell or otherwise dispose of any Equity Securities held by the Investor, the Parent Investor and their Affiliates, as applicable, (other than transfers, |
Page 1 of 4
Execution copy
| sales or dispositions permitted pursuant to Article 6.2.4); provided, however, that the Lock-up Period shall automatically terminate in the event that (i) the Issuer has received a non-public offer, prior to the end of the Lock-up Period, by a bona fide third party, other than the Investor, the Parent Investor, any of the Affiliates of the Investor or the Parent Investor, or any other party Acting in Concert with the Investor, the Parent Investor or any of the Affiliates of the Investor or the Parent Investor, regarding a bona fide potential takeover bid on the Issuer (including any tender, exchange or other offer or proposal to acquire a majority of the outstanding shares of the Issuer or all or a substantial part of its consolidated assets (except where the disposal of such substantial part of its assets would not adversely affect the Issuer’s ability to comply with its obligations under the Option, License and Collaboration Agreement in any material respect), and such offer is publicly supported or recommended by the Board of Directors, (ii) the Issuer determines to commence, prior to the end of the Lock-Up Period, a process to seek a potential sale of the Issuer or all or a substantial part of its consolidated assets (except where the disposal of such substantial part of its assets would not adversely affect the Issuer’s ability to comply with its obligations under the Option, License and Collaboration Agreement in a material respect), (iii) a bid other than from the Investor, the Parent Investor, any of the Affiliates of the Investor or the Parent Investor, or any other party Acting in Concert with the Investor, the Parent Investor or any Affiliates of the Investor of the Parent Investor, is announced to take over the Issuer pursuant to article 7 or 8, §1, §2 and §3 of the Belgian Royal Decree of 27 April 2007 on public takeover bids, (iv) any bona fide person or entity (other than the Investor, the Parent Investor, any of the Affiliates of the Investor or the Parent Investor, or any other party Acting in Concert with the Investor, the Parent Investor or any of the Affiliates of the Investor or the Parent Investor) publicly discloses any plan, determined as serious by the Board of Directors to further pursue it, to make such a bid, or (v) the Issuer breaches the Option, License and Collaboration Agreement and the Option, License and Collaboration Agreement is terminated by the Parent Investor as a result.” |
| 2.2 | The introductory paragraph of Article 6.2.2 of the Agreement is hereby deleted in its entirety and replaced by the following: |
| “6.2.2 | Upon expiry of the Lock-up Period, the Investor, the Parent Investor and any of their Affiliates may, after notifying the Issuer of their intent to do so, transfer, sell or otherwise dispose of the shares of the Issuer, taking into account that:” |
| 2.3 | Article 6.2.3 of the Agreement is hereby deleted in its entirety and replaced by the following: |
“6.2.3 After the expiry of the Lock-up Period, the Issuer shall, to the extent legally permitted, provide notice to the Investor as promptly as reasonably practicable in the event that the Issuer becomes aware that any person or entity is interested in purchasing or selling shares of the Issuer in a “block trade”.
| 2.4 | The definitions of the terms “Initial Lock-up Period”, “Subsequent Luck-up Period”, and “Subsequent Lock-up Period Threshold” are hereby deleted in their entirety. |
| 3 | Adherence of Parent Investor |
The Parent Investor hereby agrees to comply with Article 6.2 of the Agreement, as amended by this Amendment 1.
| 4 | Unilateral Commitment |
The amendments contemplated by this Amendment 1 result from a unilateral commitment of the Investor, which commitment is enacted by this Amendment 1 and is hereby accepted by the Issuer.
| 5 | Miscellaneous |
| 5.1 | Article 9.2, 10, 11, 12, 13, 14, 15 and 17 of the Agreement are incorporated herein by reference and shall apply mutatis mutandis to this Amendment 1. |
Page 2 of 4
Execution copy
| 5.2 | This Amendment 1 may be executed in counterparts, each of which shall be deemed to be an original, but all of which, taken together, shall constitute one and the same instrument. This Amendment 1may be executed by facsimile, .pdf or other electronically transmitted signatures and such signatures shall be deemed to bind each Party hereto as if they were original signatures. |
| 5.3 | This Amendment 1 shall enter into effect on the Amendment 1 Effective Date. Except as explicitly set forth in this Amendment 1, the Agreement shall remain in full force and effect except that each reference to the “Agreement” or words of like import in the Agreement shall mean and be a reference to the Agreement as amended from time to time, including by this Amendment 1. |
| 5.4 | The validity, construction, performance and interpretation of this Amendment 1 and any non-contractual obligations arising out or in connection with it shall be governed by and construed in accordance with the governing law of the Agreement as set forth in Article 18.1 of the Agreement. Any and all disputes, controversies, differences or claims arising between the Parties out of or in relation to or in connection with this Amendment 1 (including arising out of or relating to the validity, construction, interpretation, enforceability, breach, performance, application or termination of this Amendment 1) shall be submitted for resolution in accordance with Article 18.2 of the Agreement. |
| 5.5 | Articles 15.4 through 15.6 of the Option, License and Collaboration Agreement are incorporated herein by reference and shall apply mutatis mutandis to this Amendment 1. |
Page 3 of 4
Execution copy
In witness whereof, the Parties hereto have caused this Amendment 1 to be executed by their duly authorized representatives. This Amendment 1 shall be binding on the Parties even if it is only executed and exchanged by electronic means.
| Galapagos NV |
|
|
| Name: Bart Filius |
| Title: President & COO |
| Date: |
| Gilead Therapeutics A1 Unlimited Company | ||||||
|
|
|
|||||
| Name: David Cadogan | Name: Padraig Clancy | |||||
| Title: | Title: | |||||
| Date: | Date: | |||||
| Gilead Sciences, Inc. |
|
|
| Name: Andrew Dickinson |
| Title: |
| Date: |
Page 4 of 4
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Galapagos NV (GLPG:NA) (GLPG) PT Lowered to EUR30 at Citi
- Victory Sells Tahlo Lake Property in British Columbia
- Correction: Publication of 2023 Annual Report of Azerion Group N.V.
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share