Form 424B4 MorphoSys AG
Table of Contents
Filed pursuant to Rule 424(b)(4)
Registration Nos. 333-223843 and 333-130614
8,300,000 American Depositary Shares

| Representing | 2,075,000 | Ordinary Shares |
This is the initial public offering of American Depositary Shares, or the ADSs, representing ordinary shares of MorphoSys AG, a German stock corporation. We are offering 8,300,000 ADSs, at a public offering price of $25.04 per ADS.
Each ADS represents 0.25 of an ordinary share. Our ordinary shares are bearer shares with no par value.
The ADSs have been approved for listing on the Nasdaq Global Market, or the Nasdaq, under the symbol “MOR”. Our shares are listed on the Frankfurt Stock Exchange under the symbol “MOR”.
We are an “emerging growth company” as defined under Section 2(a) of the Securities Act of 1933, and, as such, we have elected to comply with certain reduced public company reporting requirements for this prospectus and may elect to comply with reduced public company reporting requirements for future filings.
Investing in our ADSs involves risks. See “Risk Factors” beginning on page 14.
| Per ADS | Total | |||||||
| Price to public |
$ | 25.0400 | $ | 207,832,000.00 | ||||
| Underwriting discounts and commissions(1) |
$ | 1.7528 | $ | 14,548,240.00 | ||||
| Proceeds, before estimated expenses, to us |
$ | 23.2872 | $ | 193,283,760.00 | ||||
| (1) | We have agreed to reimburse the underwriters for certain expenses in connection with this offering. See “Underwriting.” |
We have granted the underwriters the right to purchase up to an additional 1,245,000 ADSs. If the underwriters exercise their option in full, the total underwriting discounts and commissions payable by us will be $16,730,476, and the total proceeds to us, before expenses will be $222,276,324.
The underwriters expect to deliver the ADSs to purchasers on or about April 23, 2018.
Neither the U.S. Securities and Exchange Commission, or SEC, nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| Goldman Sachs & Co. LLC | J.P. Morgan | Leerink Partners | ||
| Berenberg Capital Markets, LLC | JMP Securities | |||
Prospectus dated April 18, 2018
Table of Contents
| Page | ||||
| iii | ||||
| iv | ||||
| 1 | ||||
| 10 | ||||
| 12 | ||||
| 14 | ||||
| 66 | ||||
| 68 | ||||
| 69 | ||||
| 70 | ||||
| 72 | ||||
| 73 | ||||
| 74 | ||||
| 76 | ||||
| MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
78 | |||
| 94 | ||||
| 169 | ||||
| 191 | ||||
| 192 | ||||
| 195 | ||||
| 218 | ||||
| 228 | ||||
| 230 | ||||
| 231 | ||||
| 246 | ||||
| 247 | ||||
| 259 | ||||
| 260 | ||||
| 261 | ||||
| 262 | ||||
| F-1 | ||||
You should rely only on the information contained in this prospectus or contained in any free writing prospectus we file with the SEC. Neither we nor the underwriters have authorized anyone to provide you with additional information or information different from that contained in this prospectus or in any free writing prospectus filed with the SEC. We are offering to sell, and seeking offers to buy, our ADSs only in jurisdictions where offers and sales of these securities are legally permitted. The information contained in this prospectus or in any free writing prospectus we file is accurate only as of its date, regardless of the time of delivery of this prospectus or of any sale of our ADSs. Our business, financial condition, results of operation and prospects may have changed since that date.
i
Table of Contents
Through and including May 13, 2018 (the 25th day after the date of this prospectus), federal securities laws may require all dealers that buy, sell or trade the ADSs, whether or not participating in this offering, to deliver a prospectus. This requirement is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
ii
Table of Contents
PRESENTATION OF FINANCIAL AND OTHER INFORMATION
All references in this prospectus to “U.S. dollars” or “$” are to the legal currency of the United States, all references to “€” or “euro” are to the currency introduced at the start of the third stage of the European economic and monetary union pursuant to the treaty establishing the European Community, as amended.
Unless otherwise indicated, the consolidated financial statements and related notes included in this prospectus have been prepared in accordance with International Accounting Standards and also comply with International Financial Reporting Standards and interpretations issued by the International Accounting Standards Board, or IASB, which differ in certain significant respects from U.S. generally accepted accounting principles, or U.S. GAAP.
Financial information in thousands or millions, and percentage figures in this prospectus have been rounded. Rounded total and sub-total figures in tables in this prospectus may differ marginally from unrounded figures indicated elsewhere in this prospectus or in the consolidated financial statements. Moreover, rounded individual figures and percentages may not produce the exact arithmetic totals and sub-totals indicated elsewhere in this prospectus.
iii
Table of Contents
This prospectus contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. We obtained the industry and market data in this prospectus from our own research as well as from industry and general publications, surveys and studies conducted by third parties some of which may not be publicly available. These data involve a number of assumptions and limitations and contain projections and estimates of the future performance of the industries in which we operate that are subject to a high degree of uncertainty. We caution you not to give undue weight to such projections, assumptions and estimates.
iv
Table of Contents
This summary highlights information contained elsewhere in this prospectus. This summary does not contain all of the information that you should consider in making your investment decision. Before investing in our ADSs, you should read this entire prospectus carefully, including the sections entitled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and MorphoSys AG’s consolidated financial statements and related notes appearing elsewhere in this prospectus, for a more complete understanding of our business and this offering. Except as otherwise required by the context, references to “MorphoSys”, “Company”, “we”, “us” and “our” are to MorphoSys AG and its subsidiaries on a consolidated basis.
Overview
We are a late-stage biopharmaceutical company devoted to the development of innovative and differentiated therapies for patients suffering from serious diseases. Based on our proprietary technology platforms and leadership in the field of therapeutic antibody discovery, generation and engineering, we, together with our partners, have participated in the development of more than 100 therapeutic product candidates. Our broad pipeline spans two business segments: Proprietary Development, in which we invest in and develop product candidates, and Partnered Discovery, in which we generate product candidates for our partners in the pharmaceutical and biotechnology industries against targets identified by our partners. We currently have 28 product candidates in clinical development, including our most advanced proprietary product candidate, MOR208, for the treatment of relapsed or refractory diffuse large B cell lymphoma, or r/r DLBCL. We believe our pipeline of novel and differentiated product candidates has the potential to treat serious diseases and improve the lives of patients.
Our late-stage and most advanced proprietary product candidate, MOR208, received breakthrough therapy designation, or BTD, from the U.S. Food and Drug Administration, or the FDA, in 2017 in combination with lenalidomide for the treatment of patients with r/r DLBCL, who are not eligible for high-dose chemotherapy and autologous stem cell transplantation. We hold worldwide rights to develop and market MOR208. Also in 2017, Tremfya®, developed by our partner Janssen, became the first therapeutic antibody based on our proprietary technology to reach the market. Tremfya®, which is approved to treat moderate-to-severe plaque psoriasis, was launched in the United States in July 2017 and received approval for marketing in the European Union and Canada in November 2017.
Based on our heritage as an antibody discovery and development company, we have a large and diverse pipeline, composed of both proprietary and partnered programs, in multiple therapeutic areas and across all development phases. The combination of our technology platforms and antibody expertise has allowed us to generate promising product candidates and enter into multiple strategic collaborations with leading global pharmaceutical and biotechnology companies. These collaborations provide us with an additional funding source and allow us to leverage our collaborators’ expertise to advance the development of our proprietary product candidates.
1
Table of Contents
The following table summarizes our product candidates and programs:
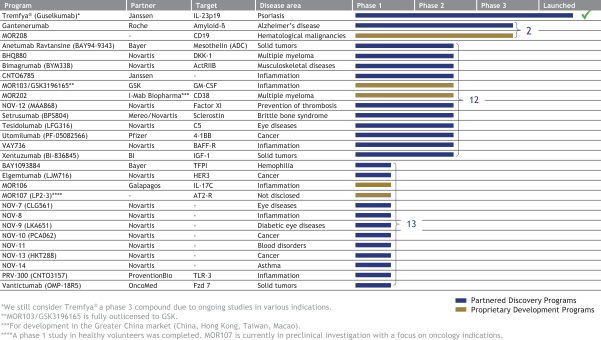
Our most advanced Proprietary Development programs include:
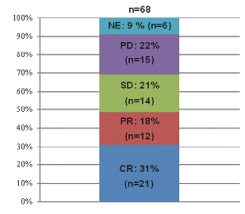
| · | MOR208—an investigational, humanized Fc-engineered monoclonal antibody directed against CD19. CD19 is a target for the treatment of B cell malignancies, including DLBCL, follicular lymphoma, or FL, chronic lymphocytic leukemia, or CLL, and others. On October 23, 2017, the FDA granted BTD for the use of MOR208 in combination with lenalidomide to treat patients with r/r DLBCL who are not eligible for high-dose chemotherapy and autologous stem cell transplantation. The granting of BTD was based on the interim results as of June 13, 2017 from our single arm Phase 2 L-MIND study, which showed a response in 23 out of 44 patients (Overall Response Rate, or ORR, 52%) and progression-free survival estimate based on a Kaplan Meier curve of 11.3 months based on investigator response assessment. Interim data from our most recent cut-off date, December 12, 2017, were consistent with our prior interim results, with a response in 33 out of 68 patients (ORR: 49%) and a progression-free survival, or PFS, rate at 12 months of 50.4% based on investigator response assessment, with the preliminary median progression-free survival, or mPFS, not yet reached. We are also investigating MOR208 in combination with bendamustine, in our pivotal Phase 2/3 B-MIND study targeting a comparable DLBCL patient population as the L-MIND study. B-MIND started as a randomized safety run-in with 10 patients in each treatment arm (the Phase 2 stage of the trial assessing safety of the combination with bendamustine) and continued then comparing MOR208 plus bendamustine with a standard of care rituximab plus bendamustine control arm (the Phase 3 stage of the trial comparing to standard of care). A third clinical trial, known as COSMOS, is an exploratory Phase 2 study in CLL patients who have progressed on or who do not tolerate treatment with Bruton tyrosine kinase inhibitors. Pursuant to our BTD, we are working closely with the FDA regarding further clinical development of MOR208. We |
2
Table of Contents
| hold worldwide rights to develop and market MOR208 and intend to commercialize it either alone or together with a partner. |
| · | MOR202—an investigational, human monoclonal HuCAL antibody directed against CD38. MOR202 is currently under clinical investigation in a Phase 1/2a trial in patients with relapsed or refractory multiple myeloma, or r/r MM. The study is evaluating the safety and preliminary efficacy of MOR202 alone and in combination with the immunomodulatory drugs pomalidomide or lenalidomide, plus dexamethasone, respectively. We believe MOR202 offers a potentially differentiated safety and administration profile relative to infusion site reaction and infusion time of other agents targeting CD38. Scientific research suggests that an anti-CD38 antibody such as MOR202 may have therapeutic activity in solid tumors including non-small cell lung cancer, or NSCLC. We plan to explore this potential activity in NSCLC in combination with nivolumab (an anti-PD-1 antibody) or more oncology indications either alone or together with a partner. |
| · | MOR106—a human monoclonal antibody targeting IL-17C, currently under clinical development for the treatment of atopic dermatitis, or AD. We believe MOR106 is the first antibody directed against IL-17C in clinical development worldwide. MOR106 is also the first antibody from our Ylanthia platform to enter clinical development. Based on findings from pre-clinical models, we believe IL-17C plays an important role in inflammatory skin disorders. We are developing MOR106 in collaboration with our partner, Galapagos, and share equally the research and development costs as well as all future economics. In September 2017, we reported results from a randomized placebo-controlled Phase 1 study, testing MOR106 in single ascending doses in healthy volunteers and in multiple ascending doses in patients suffering from AD. In the trial, MOR106 was generally well-tolerated and showed first signs of clinical activity in five of six patients with moderate-to-severe AD. We plan to initiate a Phase 2 study with our partner Galapagos in the first half of 2018. |
Our most advanced Partnered Discovery products and product candidates include:
| · | Tremfya®—a HuCAL antibody directed against IL-23 marketed by our partner Janssen to treat moderate-to-severe plaque psoriasis was launched in the United States and received marketing approval in the EU and Canada in 2017. We are entitled to royalty payments on net sales of Tremfya®. Janssen is currently investigating Tremfya® in additional Phase 3 trials in psoriasis and in psoriatic arthritis and plans to develop the product in Crohn’s disease. |
| · | Gantenerumab—a HuCAL antibody directed against amyloid beta that is being developed by Roche for the treatment of Alzheimer’s disease. In Phase 1 trials, gantenerumab has been shown to reduce brain amyloid in mild-to-moderate Alzheimer’s disease patients. Roche is currently planning to test a higher dose of gantenerumab in two Phase 3 trials, called GRADUATE 1 & 2, for patients with prodromal and mild Alzheimer’s disease. |
The majority of our Proprietary Development product candidates and all product candidates in our Partnered Discovery programs have been discovered and engineered using our advanced antibody technology platforms. Our core platforms include:
| · | HuCAL (Human Combinatorial Antibody Library)—HuCAL is our original technology platform, which constitutes a collection or “library” of several billion distinct fully human antibodies. |
3
Table of Contents
| This platform enables rapid selection of antibodies having high affinity and specificity as well as systematic optimization of antibodies to precisely-defined specifications to increase the probability of successful clinical development. |
| · | Ylanthia—Ylanthia is our newest antibody library, which comprises over 100 billion fully human antibodies. Ylanthia enables the generation of fully human antibody candidates with optimized biophysical properties, which we believe offer a number of important advantages over competing platforms. This platform builds on our experience in generating more than 100 therapeutic product candidates using our original HuCAL platform. We believe Ylanthia will be the source of the next generation of therapeutic antibody candidates in our and our partners’ future pipelines. |
| · | Lanthipeptides—Our lanthipeptide platform opens up new possibilities for discovering product candidates based on highly specific and stable peptides, which are intended to bind and activate only one target receptor subtype. |
We are committed to investing in our platforms, generating new therapeutics and developing them into products that address significant unmet medical needs.
We have an internationally-trained, multi-cultural team of more than 300 employees and consultants, including a research and development team of approximately 260 scientists, clinicians and support staff. Our management team and senior experts have deep experience and capabilities in biology, chemistry, product discovery and clinical development.
Our Strengths
We believe our core strengths include:
| · | Our lead product candidate MOR208, which has been granted BTD in r/r DLBCL by the FDA and has further potential in additional hematological malignancies. |
| · | Additional differentiated proprietary product candidates, such as MOR202 and MOR106, in clinical trials for the treatment of cancer and inflammatory diseases. |
| · | Tremfya®, the first approved product based on our technology which offers a royalty opportunity. |
| · | Our antibody pipeline, which we believe is one of the broadest and deepest in the biotech industry and which provides us with multiple opportunities for value creation. |
| · | Our long-term technology leadership in antibody discovery, generation and engineering, as demonstrated by multiple collaborations with leading pharmaceutical and biotechnology companies as well as by the breadth and depth of our technology platforms. |
| · | Our diversified business model with both proprietary and partnered development programs, which provides us with a strong financial base and strategic flexibility. |
| · | A broad intellectual property portfolio protecting product candidates as well as our technology platforms. |
| · | Our experienced management team, comprising industry leaders in antibody discovery and development. |
4
Table of Contents
Our Strategy
Our goal is to make differentiated, innovative biopharmaceuticals to improve the lives of patients suffering from serious diseases. Our strategy to achieve this goal is:
| · | Continue to advance the development of our lead product candidate MOR208 towards regulatory approval—The MOR208 program is currently in an advanced clinical development stage following receipt of BTD by the FDA in October 2017 based on interim data from our L-MIND study. We believe that with BTD, we can establish a path to approval of MOR208 in r/r DLBCL. In addition, we are evaluating opportunities to develop MOR208 in other treatment lines and hematological malignancies. |
| · | Build our commercial capabilities in connection with the potential future approval of MOR208—We are finalizing different commercialization strategies for MOR208. If we receive marketing approval for MOR208, our preferred option at this stage is to retain either sole or co-promotion rights in the United States and seek partners for commercialization elsewhere. We are currently commencing our commercialization preparation efforts with the goal of being ready to promote MOR208 as early as the first half of 2020. |
| · | Continue the development of MOR202—In order to maximize the value of MOR202, we are exploring opportunities for its further development, either alone or together with a partner, in one or more oncology indications, including in solid tumors, with an initial focus on NSCLC. |
| · | Advance our earlier stage product candidates—We continue to advance the development of our early-stage product candidates, in particular MOR106, for which we and our partner Galapagos intend to initiate a Phase 2 program in AD in 2018. |
| · | Realize the value of our technology platforms by using them to discover and develop additional early-stage proprietary programs—We continue to capitalize on the advantages of our antibody technologies and our internal expertise and know-how to discover and develop novel product candidates. |
Corporate Information
MorphoSys AG was founded in 1992 as a German limited liability company (Gesellschaft mit beschränkter Haftung). In June 1998, MorphoSys became a German stock corporation (Aktiengesellschaft) and is registered in the commercial register (Handelsregister) of the local court (Amtsgericht) of Munich under number HRB 121023. In March 1999, we listed on the Frankfurt Stock Exchange, and our shares currently trade under the symbol “MOR”.
Our registered address is Semmelweisstrasse 7, 82152 Planegg, Germany. Our telephone number is +49 89-89927-0. Our principal website is www.morphosys.com. The information contained on our website is not a part of this prospectus.
Our agent for service of process in the United States is CT Corporation System, 111 Eighth Avenue, New York, NY 10011.
5
Table of Contents
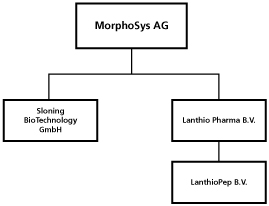
Organizational Chart
The following chart shows our organizational structure. All of the subsidiaries listed below are, directly or indirectly, wholly-owned by MorphoSys AG.
Our Risks and Challenges
Our business is subject to numerous risks, as more fully described in the section entitled “Risk Factors”. You should read these risks before you invest in the ADSs. We may be unable, for many reasons, including those that are beyond our control, to implement our business strategy. In particular, risks associated with our business include, but are not limited to, the following:
| · | we cannot assure you of the adequacy of our capital resources, including the proceeds from this offering, to successfully complete the development and commercialization of our product candidates, and a failure to obtain additional capital, if needed, could force us to delay, limit, reduce or terminate one or more of our product development programs or commercialization efforts; |
| · | we have incurred significant losses since inception and anticipate that we will continue to incur losses in the future; |
| · | all of our proprietary product candidates are still in pre-clinical or clinical development, and only one of our partnered products has been approved for marketing and sale. We cannot give any assurance that any of our product candidates will receive regulatory approval, and if we are unable to obtain regulatory approval and ultimately commercialize our product candidates or experience significant delays in doing so, our business will be materially harmed; |
| · | clinical trials are very expensive, time consuming and difficult to design and implement and involve uncertain outcomes. If clinical trials of our product candidates are prolonged, delayed or terminated, we may be unable to obtain required regulatory approvals, and therefore be unable to commercialize our product candidates on a timely basis or at all, which may materially adversely affect our business, financial condition, results of operations and prospects; |
| · | the FDA may rescind the breakthrough designation for MOR208 in combination with lenalidomide for the treatment of patients with r/r DLBCL who are not eligible for high-dose |
6
Table of Contents
| chemotherapy and autologous stem cell transplantation and we may be unable to obtain breakthrough therapy designation for other indications. In addition, breakthrough therapy designation by the FDA may not lead to a faster development, regulatory review or approval process, and it may not increase the likelihood that MOR208 will receive marketing approval in the United States; |
| · | we currently do not have a sales and marketing organization and we have no history of commercializing our proprietary products; |
| · | we face significant competition in seeking new partnerships, including for MOR202; |
| · | if we are unable to obtain and maintain sufficient intellectual property protection for our products or product candidates, or if the scope of our intellectual property protection is not sufficiently broad, our ability to commercialize our products or product candidates successfully and to compete effectively may be materially adversely affected; |
| · | we currently rely on third-party suppliers and single source third-party CMOs for the manufacturing of our product candidates and our dependence on these third parties may impair the development of our product candidates; |
| · | we are currently, and may in the future, be involved in lawsuits to protect or enforce our patents, which could be expensive, time-consuming and unsuccessful; |
| · | we face substantial competition from companies with considerably more resources and experience than we have, which may result in others discovering, developing, receiving approval for or commercializing products before or more successfully than us; |
| · | we do not currently intend to pay dividends on our securities, and, consequently, your ability to achieve a return on your investment will depend on appreciation in the price of the ADSs; and |
| · | the dual listing of our shares and the ADSs following this offering may adversely affect the liquidity and value of the ADSs. |
Implications of Being an Emerging Growth Company
As a company with less than $1.07 billion in revenue during our last fiscal year, we qualify as an “emerging growth company”, as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. An emerging growth company may take advantage of specified reduced reporting and is exempt from other burdens that are otherwise applicable generally to public companies. These provisions include:
| · | the ability to include only two years of audited financial statements and only two years of related Management’s Discussion and Analysis of Financial Condition and Results of Operations disclosure; |
| · | an exemption from the auditor attestation requirement in the assessment of our internal control over financial reporting pursuant to the Sarbanes-Oxley Act of 2002, as amended, or the Sarbanes-Oxley Act; |
| · | to the extent that we no longer qualify as a foreign private issuer (1) reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, |
7
Table of Contents
| and (2) exemptions from the requirements of holding a non-binding advisory vote on executive compensation, including golden parachute compensation; and |
| · | an exemption from compliance with the requirement that the Public Company Accounting Oversight Board, or the PCAOB, has adopted regarding a supplement to the auditor’s report providing additional information about the audit and the financial statements. |
We may take advantage of these provisions for up to five years following the completion of this offering or such earlier time that we are no longer an emerging growth company. We would cease to be an emerging growth company if we have more than $1.07 billion in annual revenue, have more than $700 million in market value of our shares held by non-affiliates, or issue more than $1.0 billion of non-convertible debt over a three-year period. We may choose to take advantage of some but not all of these reduced burdens. For example, Section 107 of the JOBS Act provides that an emerging growth company that uses U.S. GAAP for financial reporting can use the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended, or the Securities Act, for complying with new or revised accounting standards. Given that we currently report and expect to continue to report under International Financial Reporting Standards as issued by the IASB, or IFRS, we have irrevocably elected not to avail ourselves of this extended transition period and, as a result, we will adopt new or revised accounting standards on the relevant dates on which adoption of such standards is required by the IASB. We have taken advantage of reduced reporting requirements in this prospectus. Accordingly, the information contained herein may be different than the information you receive from other public companies in which you hold equity securities.
Implications of Being a Foreign Private Issuer
Upon consummation of this offering, we will report under the Securities Exchange Act of 1934, as amended, or the Exchange Act, as a non-U.S. company with foreign private issuer status. Even after we no longer qualify as an emerging growth company, as long as we qualify as a foreign private issuer under the Exchange Act, we will be exempt from certain provisions of the Exchange Act that are applicable to U.S. domestic public companies, including:
| · | the rules under the Exchange Act requiring domestic filers to issue financial statements prepared under U.S. GAAP; |
| · | the sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations in respect of a security registered under the Exchange Act; |
| · | the sections of the Exchange Act requiring insiders to file public reports of their stock ownership and trading activities and liability for insiders who profit from trades made in a short period of time; and |
| · | the rules under the Exchange Act requiring the filing with the SEC of quarterly reports on Form 10-Q, containing unaudited financial and other specified information, and current reports on Form 8-K, upon the occurrence of specified significant events. |
Notwithstanding these exemptions, we will file with the SEC, within four months after the end of each fiscal year, or such applicable time as required by the SEC, an annual report on Form 20-F containing financial statements audited by an independent registered public accounting firm.
8
Table of Contents
We may take advantage of these exemptions until such time as we are no longer a foreign private issuer. We would cease to be a foreign private issuer at such time as more than 50% of our outstanding voting securities are held by U.S. residents and any of the following three circumstances applies: the majority of our executive officers or directors are U.S. citizens or residents, more than 50% of our assets are located in the United States or our business is administered principally in the United States.
Both foreign private issuers and emerging growth companies are also exempt from certain more stringent executive compensation disclosure rules. Thus, even if we no longer qualify as an emerging growth company but remain a foreign private issuer, we will continue to be exempt from the more stringent compensation disclosure requirements for companies that are not emerging growth companies and will continue to be permitted to follow our home country practice on such matters.
9
Table of Contents
| ADSs offered by us |
8,300,000 ADSs |
| Option to purchase additional ADSs |
We granted the underwriters an option to purchase up to an additional 1,245,000 ADSs from us. |
| The ADSs |
Each ADS represents 0.25 of an ordinary share. |
| The depositary, the custodian or any of their respective nominees will hold the ordinary shares and any other rights or property underlying your ADSs. As an ADS holder, we will not treat you as one of our shareholders. The depositary, The Bank of New York Mellon, will be the holder of the ordinary shares underlying your ADSs. You will have rights as provided in the deposit agreement. You may surrender your ADSs and withdraw the underlying ordinary shares as provided, and pursuant to, the limitations set forth in, the deposit agreement. The depositary will charge you fees for, among other acts, any such surrender for the purpose of withdrawal. In certain limited instances described in the deposit agreement, we may amend or terminate the deposit agreement without your consent. If you continue to hold your ADSs, you agree to be bound by the terms of the deposit agreement then in effect. |
| To better understand the terms of the ADSs, you should carefully read the “Description of American Depositary Shares” section of this prospectus. You should also read the deposit agreement, which is an exhibit to the Registration Statement of which this prospectus forms a part. |
| Depositary |
The Bank of New York Mellon |
| Custodian |
The Bank of New York Mellon SA/NV |
| Use of proceeds |
We estimate that we will receive total net proceeds of approximately $189.9 million, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. If the underwriters exercise in full their option to purchase additional ADSs, we estimate that the net proceeds of the offering will be approximately $218.9 million, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. |
| We intend to use the net proceeds from this offering, together with a portion of our cash and cash equivalents, available-for-sale financial assets, bonds available-for-sale, and financial assets classified as loans and receivables to continue the development of our proprietary clinical pipeline, initiate pre-commercial and |
10
Table of Contents
| commercial activities and to advance earlier stage product candidates in particular as follows: (i) further development of MOR208, our wholly-owned anti-CD19 antibody currently being evaluated in r/r DLBCL (a) in combination with lenalidomide for patients ineligible for high-dose chemotherapy, or HDCT, and autologous stem cell transplantation, or ASCT, (b) in combination with bendamustine for patients ineligible for HDCT and ASCT, and (c) in additional treatment lines and additional indications; (ii) build our commercial capabilities in connection with the potential future approval of MOR208; (iii) to fund clinical development of MOR202 in MM and NSCLC and MOR106 in atopic dermatitis; and (iv) for general corporate purposes, including the addition of further compounds to our pipeline via own research, in-licensing or acquisition, if promising compounds are available, and potential addition of technologies to expand our existing technology base. See the “Use of Proceeds” section of this prospectus beginning on page 70 for a more complete description of the intended use of proceeds from this offering. |
| Dividend policy |
We have not paid any dividends on our ordinary shares since our inception, and we currently intend to retain any future earnings to finance the growth and development of our business. Therefore, we do not anticipate that we will declare or pay any cash dividends in the foreseeable future. Except as required by law, any future determination to pay cash dividends will be at the discretion of our management board and supervisory board and will be dependent upon our financial condition, results of operations, capital requirements, and other factors our management board and supervisory board deem relevant. See “Dividend Policy”. |
| Lock-up agreements |
We have agreed with the underwriters, subject to certain exceptions, not to offer, sell, or dispose of any of our share capital or securities convertible into or exchangeable or exercisable for any of our share capital during the 90-day period following the date of this prospectus. Members of our management board have agreed to substantially similar 90-day lock-up provisions, subject to certain exceptions. |
| Risk factors |
You should carefully read the information set forth in the “Risk Factors” section of this prospectus beginning on page 14 and the other information set forth in this prospectus before deciding to invest in the ADSs. |
| Nasdaq Global Market Symbol |
“MOR” |
11
Table of Contents
SUMMARY HISTORICAL CONSOLIDATED FINANCIAL DATA
We present below summary historical consolidated financial data of MorphoSys AG. The financial data as of and for the years ended December 31, 2016 and 2017, have been derived from our audited consolidated financial statements and the related notes, which are included elsewhere in this prospectus and which have been prepared in accordance with IFRS.
The summary historical consolidated financial data presented below are not necessarily indicative of the financial results expected for any future periods. The summary historical consolidated financial data below do not contain all the information included in our financial statements. You should read this information in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations”, “Selected Consolidated Financial Data” and our consolidated financial statements and related notes, each included elsewhere in this prospectus.
The following tables also contain translations of euro amounts into U.S. dollars for amounts presented as of and for the year ended December 31, 2017. These translations are solely for convenience of the reader and were calculated at the rate of €1.00 per $1.2022, which equals the noon buying rate of the Federal Reserve Bank of New York on December 29, 2017. You should not assume that, on that or any other date, one could have converted these amounts of euro into U.S. dollars at this exchange rate.
Consolidated Statement of Income Data:
| Year ended December 31, | ||||||||||||
| 2016 | 2017 | |||||||||||
| (in €) | (in €) | (in $) | ||||||||||
| (in thousands except per share data) | ||||||||||||
| Revenues |
49,744 | 66,791 | 80,296 | |||||||||
| Operating Expenses |
||||||||||||
| Research and Development |
(95,723 | ) | (116,809 | ) | (140,428 | ) | ||||||
| General and Administrative |
(14,116 | ) | (17,039 | ) | (20,484 | ) | ||||||
| Total Operating Expenses |
(109,839 | ) | (133,848 | ) | (160,912 | ) | ||||||
| Other Income |
709 | 1,120 | 1,346 | |||||||||
| Other Expenses |
(554 | ) | (1,671 | ) | (2,009 | ) | ||||||
| Earnings before Interest and Taxes (EBIT) |
(59,941 | ) | (67,608 | ) | (81,278 | ) | ||||||
| Finance Income |
1,385 | 712 | 856 | |||||||||
| Finance Expenses |
(1,308 | ) | (1,895 | ) | (2,278 | ) | ||||||
| Income Tax Expenses |
(519 | ) | (1,036 | ) | (1,245 | ) | ||||||
| Consolidated Net Profit/(Loss) |
(60,383 | ) | (69,827 | ) | (83,946 | ) | ||||||
| Earnings per Share, basic and diluted(1) |
(2.28 | ) | (2.41 | ) | (2.90 | ) | ||||||
| Shares Used In Computing Earnings per Share (in units), basic and diluted(1) |
26,443,415 | 28,947,566 | n/a | |||||||||
| Dividends Declared per Share |
— | — | — | |||||||||
| (1) | Basic and diluted Earnings per Share are the same in each of the years ended December 31, 2016 and 2017, because the assumed exercise of outstanding stock options and convertible bonds would be anti-dilutive due to our consolidated net loss in the respective period. |
12
Table of Contents
Consolidated Balance Sheet Data:
| As of December 31, | ||||||||||||
| 2016 | 2017 | |||||||||||
| (in €) | (in €) | (in $) | ||||||||||
| (in thousands) | ||||||||||||
| Cash and Cash Equivalents |
73,929 | 76,589 | 92,075 | |||||||||
| Available-for-sale Financial Assets |
63,362 | 86,538 | 104,036 | |||||||||
| Bonds, Available-for-sale |
6,532 | — | — | |||||||||
| Financial Assets classified as Loans and Receivables (current and non-current) |
215,630 | 149,059 | 179,199 | |||||||||
| Total Current Assets |
308,056 | 340,681 | 409,567 | |||||||||
| Total Assets |
463,600 | 415,398 | 499,391 | |||||||||
| Total Liabilities |
48,140 | 56,727 | 68,197 | |||||||||
| Total Stockholders’ Equity |
415,460 | 358,671 | 431,194 | |||||||||
13
Table of Contents
Investing in our ADSs involves a high degree of risk. You should carefully consider the risks and uncertainties described below, which we believe are the material risks of our business, our industry, our intellectual property, the ADSs and this offering, before making an investment decision. If any of the following risks actually occurs, our business, financial condition and operating results could be harmed. In that case, the trading price of the ADSs could decline and you might lose all or part of your investment. In assessing these risks, you should also refer to the other information contained in this prospectus, including MorphoSys AG’s consolidated financial statements and the related notes thereto appearing elsewhere in this prospectus.
Risks Related to Our Financial Condition
We cannot assure you of the adequacy of our capital resources, including the proceeds from this offering, to successfully complete the development and commercialization of our product candidates, and a failure to obtain additional capital, if needed, could force us to delay, limit, reduce or terminate one or more of our product development programs or commercialization efforts.
As of December 31, 2017, we had cash and cash equivalents, available-for-sale financial assets and current financial assets classified as loans and receivables amounting to €312.2 million. We believe that we will continue to expend substantial resources for the foreseeable future developing our proprietary product candidates and in particular MOR208. These expenditures will include costs associated with research and development, conducting pre-clinical studies and clinical trials, seeking regulatory approvals, as well as launching and commercializing products approved for sale, if any, and potentially acquiring new products. In addition, other unanticipated costs may arise. Because the outcome of our anticipated clinical trials is highly uncertain, we cannot reasonably estimate the actual amounts necessary to successfully complete the development and commercialization of our proprietary product candidates.
Our future funding requirements will depend on many factors, including but not limited to:
| · | the numerous risks and uncertainties associated with developing therapeutic product candidates; |
| · | the number and characteristics of product candidates that we pursue; |
| · | the rate of enrollment, progress, cost and outcomes of our clinical trials, which may or may not meet their primary end-points; |
| · | the timing of, and cost involved in, conducting non-clinical studies that are regulatory prerequisites to conducting clinical trials of sufficient duration for successful product registration; |
| · | the cost of manufacturing clinical supply and establishing commercial supply of our product candidates; |
| · | the timing of, and the costs involved in, obtaining regulatory approvals for our product candidates if clinical trials are successful; |
| · | the timing of, and costs involved in, conducting post-approval studies that may be required by regulatory authorities; |
14
Table of Contents
| · | the cost of commercialization activities for our product candidates, if any of our product candidates are approved for sale; |
| · | the terms and timing of any collaborative, licensing, and other arrangements that we may establish, including any required milestone and royalty payments thereunder and any non-dilutive funding that we may receive; |
| · | the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims, including litigation costs, if any, and the outcome of any such litigation; |
| · | the timing, receipt, and amount of sales of, or royalties or milestones on, our future products, if any; and |
| · | the costs to recruit and build the organization including key executives needed to transform to a commercial organization. |
In addition, our operating plan may change as a result of many factors currently unknown to us. As a result of these factors, we may need additional funds sooner than planned. We expect to finance future cash needs primarily through a combination of public or private equity offerings, strategic collaborations and non-dilutive funding. If sufficient funds on acceptable terms are not available when needed, or at all, we could be forced to significantly reduce operating expenses and delay, limit, reduce or terminate one or more of our product development programs or commercialization efforts.
We have incurred significant losses since inception and anticipate that we will continue to incur losses in the future.
We are a late-stage biopharmaceutical company. We have incurred significant losses since our inception. Our consolidated net loss for the year ended December 31, 2017 was €69.8 million. As of December 31, 2017, our accumulated deficit was approximately €97.4 million. We expect to continue to incur losses in the future as we continue our research and development of, and seek regulatory approvals for, our product candidates, prepare for and begin to commercialize any approved product candidates and add infrastructure and personnel to support our product development efforts and operations as a public company in the United States. The net losses and negative cash flows incurred to date, together with expected future losses, have had, and likely will continue to have, an adverse effect on our shareholders’ deficit and working capital. The amount of future net losses will depend, in part, on the rate of future growth of our expenses and our ability to generate revenue.
Because of the numerous risks and uncertainties associated with biopharmaceutical product development, we are unable to accurately predict the timing or amount of increased expenses or when, or if, we will be able to achieve profitability. For example, our expenses could increase if we are required by the FDA or EMA to perform trials in addition to those that we currently expect to perform, or if there are any delays in completing our currently planned clinical trials, the partnering process for our proprietary product candidates or in the development of any of our proprietary product candidates.
Our revenue to date has been primarily revenue from the license of our proprietary technology platforms and from milestone payments for the development of product candidates against targets provided by our collaborators. Our ability to generate revenue and achieve profitability in the future depends in large part on our ability, alone or with our collaborators, to achieve milestones and to successfully complete the development of, obtain the necessary regulatory
15
Table of Contents
approvals for, and commercialize, product candidates. This will require us to be successful in a range of challenging activities, including developing product candidates, obtaining regulatory approval for such product candidates, and manufacturing, marketing and selling those product candidates for which we may obtain regulatory approval. We may never succeed in these activities and may never generate revenue from product sales that is significant enough to achieve profitability. Even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods. Our failure to become or remain profitable would depress our market value and could impair our ability to raise capital, expand our business, develop other product candidates, or continue our operations. A decline in the value of our company could also cause you to lose all or part of your investment.
Our operating results may fluctuate significantly in the future.
Our results of operations may fluctuate significantly in the future due to a variety of factors, many of which are outside of our control. The revenues we generate, if any, and our operating results will be affected by numerous factors, including, but not limited to:
| · | the development status of our product candidates and, particularly, the timing of any milestone payments to be paid or received by us under our collaboration agreements; |
| · | the incurrence of clinical expenses that could fluctuate significantly from period to period; |
| · | the commercial success of the products marketed by our partners, in particular Tremfya®, and the amount of royalties to us associated therewith; |
| · | the unpredictable effects of collaborations during these periods; |
| · | the timing of our satisfaction of applicable regulatory requirements; |
| · | the rate of expansion of our clinical development and other development efforts; |
| · | the effect of competing technologies and products and market developments; and |
| · | general and industry-specific economic conditions. |
For example, we expect our revenues in 2018 to be significantly less than we generated in 2017 as a result of the conclusion of our collaboration with Novartis and the corresponding reduction in revenues from funded research and license fee payments. If our operating results fall below the expectations of investors or securities analysts, the price of our ordinary shares could decline substantially. Furthermore, any fluctuations in our operating results and cash flows may, in turn, cause the price of our ADSs to fluctuate substantially.
Raising additional capital may cause dilution to our shareholders, restrict our operations or require us to relinquish rights to our technologies or product candidates.
Identifying and acquiring rights to develop potential product candidates and conducting pre-clinical testing and clinical trials is a time-consuming, expensive and uncertain process that may take years to complete. We may never generate the necessary data or results required to obtain regulatory approval and achieve product sales, and even if one or more of our product candidates is approved, they may not achieve commercial success. Accordingly, we will need to continue to rely on additional financing to achieve our business objectives. Adequate additional financing may not be available to us on acceptable terms, or at all.
We may seek additional funding through a combination of equity offerings, debt financings, including convertible bond offerings, collaborations, licensing arrangements, strategic alliances
16
Table of Contents
and marketing or distribution arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms may include liquidation or other preferences that adversely affect your rights as a holder of our ADSs. The incurrence of indebtedness or the issuance of certain equity securities could result in increased fixed payment obligations and could also result in certain additional restrictive covenants, such as limitations on our ability to incur additional debt or issue additional equity, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. In addition, issuance of additional equity securities, or the possibility of such issuance, may cause the market price of our ADSs to decline. In the event that we enter into collaborations or licensing arrangements to raise capital, we may be required to accept unfavorable terms, including relinquishing or licensing to a third party on unfavorable terms our rights to technologies or product candidates that we otherwise would seek to develop or commercialize ourselves or potentially reserve for future potential arrangements when we might be able to achieve more favorable terms.
A substantial portion of our historical revenues are from a limited number of strategic collaborations and partnerships, and the termination of these collaborations could have a material adverse effect on our business, financial condition and results of operations.
Historically, we derived a substantial portion of our revenues from a limited number of collaborations, under which we generated revenues through licensing arrangements such as research and development payments, up-front payments, milestone payments, and, once a product is commercialized, royalty payments based on a portion of the revenue of product sold. For the year ended December 31, 2017, 90.4% of our revenues were derived from our collaboration partners Novartis, I-Mab Biopharma and Janssen. We do not have further research and development collaborations with Janssen and Novartis at this time and, as a result, we expect our revenues from funded research and license fee payments to be significantly lower in 2018. We expect royalties from Janssen on sales of Tremfya® to account for a substantial portion of our revenues for the next several years. The loss of any significant collaborator or any significant reduction in payments by a collaborator may have a material adverse effect on our business, financial condition and results of operations.
Risks Related to the Development, Clinical Testing and Commercialization of Our Product Candidates
All of our proprietary product candidates are still in pre-clinical or clinical development, and only one of our partnered products has been approved for marketing and sale. We cannot give any assurance that any of our product candidates will receive regulatory approval, and if we are unable to obtain regulatory approval and ultimately commercialize our product candidates or experience significant delays in doing so, our business will be materially harmed.
All of our proprietary product candidates are still in pre-clinical or clinical development, and only one of our partnered products, Tremfya®, has received regulatory approval. Although we may receive certain payments from our collaboration partners, including upfront payments, payments for achieving certain development, regulatory or commercial milestones and royalties, our ability to generate revenue from our product candidates’ sales is dependent on receipt of regulatory approval for, and successful commercialization of, such product candidates, which
17
Table of Contents
may never occur. Our business and future success is in particular dependent on our ability to develop, either alone or in partnership, successfully, receive regulatory approval for, and then successfully commercialize our proprietary product candidates, in particular MOR208. Each of our product candidates will require additional pre-clinical and/or clinical development, regulatory approval in multiple jurisdictions, manufacturing supply, substantial investment and significant marketing efforts before we generate any revenue from product sales or royalties. We are not permitted to market or promote any of our product candidates before we receive regulatory approval from applicable regulatory authorities. The success of our product candidates will depend on several factors, including the following:
| · | successful completion of pre-clinical and/or clinical studies; |
| · | successful enrollment of patients in, and completion of, clinical trials; |
| · | strategic commitment to particular product candidates and indications by us and our collaborators; |
| · | receipt of regulatory authorizations from applicable regulatory authorities for future clinical trials; |
| · | receipt of product approvals, including marketing approvals, from applicable regulatory authorities; |
| · | successful completion of all safety studies required to obtain regulatory approval in the United States, the European Union and other jurisdictions for our product candidates; |
| · | obtaining and maintaining patent and trade secret protection or regulatory exclusivity for our product candidates; |
| · | launching commercial sales of our product candidates, if and when approved, whether alone or in collaboration with others; |
| · | acceptance of the product candidates, if and when approved, by patients, the medical community and third-party payors; |
| · | effectively competing with other therapies; |
| · | obtaining and maintaining coverage and adequate reimbursement from third-party payors; |
| · | enforcing and defending intellectual property rights and claims; |
| · | maintaining a continued acceptable safety and quality profile of the product candidates following approval; and |
| · | maintaining a continued, sufficient supply of drug substance in acceptable quality. |
If we do not achieve one or more of these factors in a complete and timely manner or at all, we could experience significant delays or an inability to successfully commercialize our product candidates, which would materially adversely affect our business, financial condition, results of operations and prospects and, in case of product candidates, technologies and licenses we have acquired, may result in a significant impairment of assets.
We have not previously submitted a biologics license application, or BLA, to the FDA, or similar regulatory approval filings to comparable foreign authorities, for any product candidate, and we cannot be certain that any of our product candidates will be successful in clinical trials or receive regulatory approval. Further, our product candidates may not receive regulatory
18
Table of Contents
approval even if they are successful in clinical trials. If we do not receive regulatory approvals for our product candidates, we may not be able to continue our operations. Even if we successfully obtain regulatory approvals to market one or more of our product candidates, our revenues will be dependent, in part, upon the size of the markets in the territories for which we gain regulatory approval and have commercial rights. If the markets for patient subsets that we are targeting are not as significant as we estimate, we may not generate significant revenues from sales of such products, if approved.
We plan to seek regulatory approval to commercialize our product candidates both in the United States and the EU, and potentially in additional foreign countries. While the scope of regulatory approval is similar in other countries, to obtain separate regulatory approval in many other countries, we must comply with numerous and varying regulatory requirements of such countries regarding safety and efficacy and governing, among other things, clinical trials and commercial sales, pricing and distribution of our product candidates, and we cannot predict success in these jurisdictions.
Clinical trials are very expensive, time consuming and difficult to design and implement and involve uncertain outcomes. If clinical trials of our product candidates are prolonged, delayed or terminated, we may be unable to obtain required regulatory approvals, and therefore be unable to commercialize our product candidates on a timely basis or at all, which may materially adversely affect our business, financial condition, results of operations and prospects.
We are currently conducting clinical trials for MOR208, MOR202, and MOR106. Each of our clinical trials requires the investment of substantial expense and time and the timing of the commencement, continuation and completion of these clinical trials may be subject to significant delays or termination relating to various causes, including, among other things:
| · | scheduling conflicts with participating clinicians and clinical institutions; |
| · | difficulties in identifying and enrolling patients who meet trial eligibility criteria; |
| · | failure of patients to complete the clinical trials or return for post-treatment follow-up; |
| · | delays in accumulating the required number of clinical events for data analyses; |
| · | clinical investigators or sites deviating from trial protocol or failing to comply with regulatory requirements or meet their contractual obligations; |
| · | delay or failure to obtain required approvals; |
| · | delays in or failure to reach agreement on acceptable terms with prospective contract research organizations, or CROs, and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
| · | delays in or failure to obtain institutional review board, or IRB, approval at each site; |
| · | failure of third-party contractors used in our clinical trials or contract manufacturing organizations, or CMOs, to comply with regulatory requirements or meet their contractual obligations in a timely manner, or not at all; |
| · | changes in regulatory requirements; |
| · | the development and approval of competitive products, in particular with respect to MOR202 for the treatment of MM; |
19
Table of Contents
| · | results from clinical trials of competing compounds, which may give rise to concerns about the target, the envisioned mode of action, the compound class or the commercial potential of the product candidate we are evaluating — in particular, in respect of MOR208, data being produced from other product candidates in CLL may make our ability to get an approval for MOR208 in this indication more difficult; |
| · | higher-than-expected costs of clinical trials of our product candidates; and |
| · | insufficient, inadequate or prohibitively expensive supply or quality of our product candidates or other materials necessary to conduct clinical trials of our product candidate. |
We do not know whether any of our clinical trials will begin as planned, will need to be redesigned or amended or will be completed on schedule, or at all. Clinical testing is expensive and can take many years to complete, and its outcome is inherently uncertain. Failure can occur at any time during the clinical trial process. We could encounter delays if a clinical trial is suspended or terminated by us, by the IRBs of the institutions in which such trials are being conducted, by a data review committee or data safety monitoring board for such trial or by the FDA or other regulatory authorities. Such authorities may impose such a suspension or termination due to a number of factors, including failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols, inspection of the clinical trial operations or trial site by the FDA or other regulatory authorities resulting in the imposition of a clinical hold, safety issues or adverse side effects, failure to demonstrate a benefit from using a drug, changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial. If we experience delays in the completion of, or termination of, any clinical trial of our product candidates, the commercial prospects of our product candidates will be harmed, and our ability to generate product revenues from any of these product candidates will be delayed. In addition, any delays in completing our clinical trials will increase our costs, slow down our product candidate development and approval process and jeopardize our ability to commence product sales and generate revenues. Significant clinical trial delays could also allow our competitors to bring products to market before we do or shorten any periods during which we have the exclusive right to commercialize our product candidates and impair our ability to commercialize our product candidates, and may harm our business and results of operations. In addition, some of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates.
Clinical trials must be conducted with supplies of our product candidates produced under current good manufacturing practice, or cGMP, requirements and other regulations. Furthermore, we rely on CROs and clinical trial sites to ensure the proper and timely conduct of our clinical trials and while we have agreements governing their committed activities, we have limited influence over their actual performance. We depend on our collaborators and on medical institutions and CROs to conduct our clinical trials in compliance with Good Clinical Practice, or GCP, requirements. To the extent our collaborators or the CROs fail to enroll participants for our clinical trials, fail to conduct the study to GCP standards or are delayed for a significant time or fail in the execution of trials, including achieving full enrollment, we may be affected by increased costs, program delays or both, which may harm our business.
If we are unable to successfully complete clinical trials of our product candidates or other testing, if the results of these trials or tests are unfavorable or are only modestly favorable, if
20
Table of Contents
there are safety concerns associated with our product candidates we may decide to develop in the future, or if we are required to conduct additional clinical trials or other testing of our product candidates that we may develop in future beyond the trials and testing that we contemplate, we may:
| · | be delayed in obtaining marketing approval for our product candidates; |
| · | not obtain marketing approval at all; |
| · | obtain approval for indications or patient populations that are not as broad as intended or desired; |
| · | obtain approval with product labeling that includes significant use or distribution restrictions or significant safety warnings, including boxed warnings; |
| · | be subject to additional post-marketing testing or other requirements; or |
| · | remove the product from the market after obtaining marketing approval. |
The occurrence of any such events may materially adversely affect our business, financial condition, results of operations and prospects.
The incidence and prevalence for target patient populations of our product candidates are based on estimates and third-party sources. If the market opportunities for our product candidates are smaller than we estimate or if any approval that we obtain is based on a narrower definition of the patient population, our business, financial condition, results of operations and prospects may be materially adversely affected.
Periodically, we make estimates regarding the incidence and prevalence of target patient populations for particular diseases based on various third-party sources and internally generated analysis and use such estimates in making decisions regarding our product development strategy, including determining indications on which to focus in pre-clinical or clinical trials.
These estimates may be inaccurate or based on imprecise data. For example, the total addressable market opportunity will depend on, among other things, the acceptance of such data by the medical community and patient access, product pricing and reimbursement. The number of patients in the addressable markets may turn out to be lower than expected, patients may not be otherwise amenable to treatment with our products, or new patients may become increasingly difficult to identify or gain access to, all of which could materially adversely affect our business, financial condition, results of operations and prospects.
The speed at which we complete our pre-clinical studies and clinical trials depend on many factors, including, but not limited to, patient enrollment. If we are unable to enroll patients in our clinical trials, our research and development efforts and business, financial condition, results of operations and prospects could be materially adversely affected.
Patient enrollment, a significant factor in the timing and successful completion of clinical trials, is affected by many factors, including the size and nature of the patient population, the proximity of patients to clinical sites, the eligibility criteria for the trial, the design of the clinical trial, competing clinical trials and clinicians’ and patients’ perceptions as to the potential advantages of the product being studied in relation to other available therapies, including any new products that may be approved for the indications we are investigating. Because there is a relatively limited number of patients worldwide, patient enrollment may be challenging. Trials
21
Table of Contents
may be subject to delays as a result of patient enrollment taking longer than anticipated or patient withdrawal. Delays in the completion of any clinical trial of our product candidates will increase our costs, slow down our product candidate development and delay or potentially jeopardize our ability to receive regulatory approval, commence product sales and generate revenue. Any of these occurrences may harm our clinical trials, which could materially adversely affect our business, financial condition, results of operations and prospects.
Results of previous pre-clinical studies and clinical trials may not be predictive of future results, and the results of our current and planned clinical trials may not satisfy the requirements of the FDA, the EMA or comparable foreign regulatory authorities.
Positive or timely results from pre-clinical or early-stage trials do not ensure positive or timely results in late-stage clinical trials or product approval by the FDA, European Medicines Agency, or the EMA, or comparable foreign regulatory authorities. This includes the interim data presented from the L-MIND study as of June 13, 2017, which will evolve over time and may not be as positive as interim data that were the basis on which the FDA awarded breakthrough therapy designation. We will generally be required to demonstrate with substantial evidence through well-conducted, possibly controlled clinical trials that our product candidates are safe and effective for use in a well-defined patient population before we can seek regulatory approvals for their commercial sale. Our planned clinical trials may produce negative or inconclusive results, and we or any of our current and future collaborators may decide, or regulators may require us, to conduct additional clinical or pre-clinical testing. Success in pre-clinical studies or early-stage clinical trials does not mean that future clinical trials or registration clinical trials will be successful because product candidates in later-stage clinical trials may fail to demonstrate sufficient safety or efficacy to the satisfaction of the FDA, EMA and comparable foreign regulatory authorities, despite having progressed through pre-clinical studies and initial clinical trials. Product candidates that have shown promising results in early clinical trials may still suffer significant setbacks in subsequent clinical trials. For example, a number of companies in the pharmaceutical industry, including those with greater resources and experience than us, have suffered significant setbacks in advanced clinical trials, even after obtaining promising results in earlier clinical trials. Similarly, interim results of a clinical trial do not necessarily predict final results.
The regulatory approval processes of the FDA and comparable foreign authorities are lengthy, time consuming and inherently unpredictable and if we fail to obtain regulatory approval in any jurisdiction, we will not be able to commercialize our products in that jurisdiction and our business, results of operations, financial condition and prospects, may be materially adversely affected.
The time required to obtain approval by the FDA and comparable foreign authorities is unpredictable but typically takes many years following the commencement of clinical trials and depends upon numerous factors, including the substantial discretion of the regulatory authorities. In addition, approval laws, regulations, policies or the type and amount of clinical data or other information necessary to gain approval may change during the course of a product candidate’s clinical development and may vary among jurisdictions. We have not obtained regulatory approval for any product candidate, and it is possible that none of our existing product candidates or any product candidates we may seek to develop in the future will ever obtain regulatory approval.
22
Table of Contents
Our product candidates could fail to receive regulatory approval for many reasons, including the following:
| · | the FDA or comparable foreign regulatory authorities may disagree with the design or implementation of our clinical trials; |
| · | we may be unable to demonstrate to the satisfaction of the FDA or comparable foreign regulatory authorities that a product candidate is safe and effective for its proposed indication; |
| · | the designs of clinical trials might not be considered adequate, or the results of clinical trials may not meet the level of statistical significance required, by the FDA or comparable foreign regulatory authorities for approval; |
| · | we may be unable to demonstrate that a product candidate’s clinical and other benefits outweigh its safety risks; |
| · | the FDA or comparable foreign regulatory authorities may disagree with our interpretation of data from pre-clinical studies or clinical trials; |
| · | the data collected may not be sufficient to support the submission of a BLA or other submission, or to obtain regulatory approval in the United States, the EU or elsewhere; |
| · | the FDA or comparable foreign regulatory authorities may fail to approve the manufacturing processes or facilities of third-party manufacturers with which we contract for clinical and commercial supplies; and |
| · | the laws, regulations or policies of the FDA or comparable foreign regulatory authorities may significantly change in a manner rendering our clinical data or other regulatory submissions insufficient for approval. |
This lengthy approval process as well as the unpredictability of future clinical trial results may result in our failing to obtain regulatory approval to market any of our product candidates, which would significantly harm our business, results of operations and prospects. The FDA, the EMA and other regulatory authorities have substantial discretion in the approval process and determining when or whether regulatory approval will be obtained for any of our product candidates. Even if we believe the data collected from clinical trials of our product candidates are promising, such data may not be sufficient to support approval by the FDA, the EMA or any other regulatory authority.
In addition, even if we were to obtain approval, regulatory authorities may approve any of our product candidates for fewer or more limited indications than we request, may not approve the price we intend to charge for our products, may grant approval contingent on the performance of costly post-marketing clinical trials, or may approve a product candidate with a label that does not include the labeling claims necessary or desirable for the successful commercialization of that product candidate. Any of the foregoing scenarios could materially harm the commercial prospects for our product candidates.
In order to commercialize our products in more than one jurisdiction, we will require separate regulatory approval in each market and compliance with numerous and varying regulatory requirements. The approval procedures vary from country to country and may require additional testing or other steps. Satisfying these and other regulatory requirements is costly, time
23
Table of Contents
consuming, uncertain and subject to unanticipated delays. In addition, in many countries outside the United States and in particular in many of the Member States of the European Union, a product must undergo health economic assessments to agree on pricing and/or be approved for reimbursement before it can be approved for sale in that country, or before it becomes commercially viable. The FDA and the EMA may come to different conclusions regarding approval of a marketing application. Approval by the FDA or EMA does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other foreign countries or by the FDA or EMA. In addition, our failure to obtain regulatory approval in any country may delay or have negative effects on the process for regulatory approval in other countries. Clinical trials conducted in one country may not be accepted by regulatory authorities in other countries. We may not obtain regulatory approvals on a timely basis, if at all. We may not be able to file for regulatory approvals and may not receive necessary approvals to commercialize our products in any market. We may be required to conduct additional pre-clinical studies or clinical trials, which would be costly and time consuming. If we or any future partner are unable to obtain regulatory approval for our product candidates in one or more significant jurisdictions, then the commercial opportunity for our product candidates, and our business, results of operations, financial condition and prospects, may be materially adversely affected.
The FDA may rescind the breakthrough designation for MOR208 in combination with lenalidomide for the treatment of patients with r/r DLBCL who are not eligible for high-dose chemotherapy and autologous stem cell transplantation and we may be unable to obtain breakthrough therapy designation for other indications. In addition, breakthrough therapy designation by the FDA may not lead to a faster development, regulatory review or approval process, and it may not increase the likelihood that MOR208 will receive marketing approval in the United States.
Under the Food and Drug Administration Safety and Innovation Act, or FDASIA, the FDA is authorized to give certain products “breakthrough therapy designation”. Breakthrough therapy product candidate is defined as a product candidate that is intended, alone or in combination with one or more other drugs, to treat a serious or life-threatening disease or condition and preliminary clinical evidence indicates that such product candidate may demonstrate substantial improvement on one or more clinically significant endpoints over existing therapies. The FDA will seek to ensure the sponsor of a breakthrough therapy product candidate receives intensive guidance on an efficient drug development program, intensive involvement of senior managers and experienced staff on a proactive, collaborative and cross-disciplinary review and a rolling review process whereby the FDA may consider reviewing portions of a marketing application before the sponsor submits the complete application. Product candidates designated as breakthrough therapies by the FDA may be eligible for other expedited programs, such as priority review, if supported by clinical data.
The receipt of breakthrough therapy designation for a product candidate, or acceptance for one or more of the FDA’s other expedited programs, may not result in a faster development process, review or approval compared to products considered for approval under conventional FDA procedures and does not guarantee ultimate approval by the FDA. For example, we are evaluating MOR208 in combination with lenalidomide for the treatment of r/r DLBCL; however, lenalidomide (being marketed by Celgene) is currently not approved for the treatment of r/r
24
Table of Contents
DLBCL. There are a number of reasons why the FDA may not grant an approval of a registration package for a product candidate. Among these reasons, a pivotal study of the
combination of two unapproved product candidates in a particular indication may not alone be acceptable to support approval. Additionally, the FDA may later decide that the product candidate no longer meets the conditions for designation and may
withdraw designation at any time or decide that the time period for FDA review or approval will not be shortened.
Fast track designation for one or more of our product candidates may not actually lead to a faster development or regulatory review or approval process.
In 2014, we received fast track designation for MOR208 for the treatment of r/r DLBCL. If a product candidate is intended for the treatment of a serious condition, and pre-clinical or clinical data demonstrate the potential to address unmet medical need for this condition, a product sponsor may apply for FDA fast track designation. Even though we have received fast track designation for MOR208 for the treatment of r/r DLBCL, fast track designation does not ensure that we will receive marketing approval or that approval will be granted within any particular timeframe. We may not experience a faster development or regulatory review or approval process with fast track designation compared to conventional FDA procedures. In addition, the FDA may withdraw fast track designation if it believes that the designation is no longer supported by data from our clinical development program. Fast track designation alone does not guarantee qualification for the FDA’s priority review procedures.
Our product candidates may cause undesirable side effects that could delay or prevent their regulatory approval, limit the commercial profile of an approved label, or result in significant negative consequences following regulatory approval, if any.
Undesirable side effects that may be caused by our product candidates could cause us, our collaboration partners or the regulatory authorities to interrupt, delay or halt clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA, EMA or comparable foreign regulatory authorities. Results of our trials could reveal a high and unacceptable severity and prevalence of side effects. In such an event, our trials could be suspended or terminated and the FDA, EMA or comparable foreign regulatory authorities could order us to cease further development of or deny approval of our product candidates for any or all targeted indications. The product-related side effects could affect patient recruitment or the ability of enrolled patients to complete the trial or result in potential product liability claims.
Clinical trials assess a sample of the potential patient population. With a limited number of patients and duration of exposure, rare and severe side effects of our product candidates may only be uncovered with a significantly larger number of patients exposed to the product candidate. If our product candidates receive regulatory approval and we or others identify undesirable side effects caused by such product candidates (or any other similar products) after such approval, a number of potentially significant negative consequences could result, including:
| · | regulatory authorities may withdraw or limit their approval of such product candidates and require us to take our approved product(s) off the market; |
| · | regulatory authorities may require the addition of labeling statements, such as a “boxed” warning or a contra-indication, or field alerts to physicians and pharmacies; |
25
Table of Contents
| · | we may be required to create a medication guide outlining the risks of such side effects for distribution to patients; |
| · | we may be required to change the way such product candidates are distributed or administered, conduct additional clinical trials or change the labeling of the product candidates; |
| · | regulatory authorities may require a Risk Evaluation and Mitigation Strategy, or REMS, plan to mitigate risks, which could include medication guides, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries and other risk minimization tools; |
| · | we may be subject to regulatory investigations and government enforcement actions; |
| · | we may decide or be required to remove such product candidates from the marketplace; |
| · | we could be sued and potentially held liable for injury caused to individuals exposed to or taking our product candidates; |
| · | sales of the product(s) may decrease substantially; and |
| · | our reputation may suffer. |
Any of these events could prevent us from achieving or maintaining market acceptance of the affected product candidates and could substantially increase the costs of commercializing our product candidates, if approved, and therefore could have a material adverse effect on our business, financial condition, results of operations and prospects.
We and our collaboration partners have conducted and intend to conduct additional clinical trials for selected product candidates at sites outside the United States, and the FDA may not accept data from trials conducted in such locations or may require additional U.S.-based trials.
We and our collaboration partners have conducted, currently are conducting and intend in the future to conduct, clinical trials outside the United States, particularly in the European Union where we are headquartered.
Although the FDA may accept data from clinical trials conducted outside the United States, acceptance of this data is subject to certain conditions imposed by the FDA. For example, the clinical trial must be well designed and conducted by qualified investigators in accordance with GCPs, including review and approval by an independent ethics committee and receipt of informed consent from trial patients. The trial population must also adequately represent the U.S. population, and the data must be applicable to the U.S. population and U.S. medical practice in ways that the FDA deems clinically meaningful. Generally, the patient population for any clinical trial conducted outside of the United States must be representative of the population for which we intend to seek approval in the United States. In addition, while these clinical trials are subject to applicable local laws, FDA acceptance of the data will be dependent upon its determination that the trials also comply with all applicable U.S. laws and regulations. There can be no assurance that the FDA will accept data from trials conducted outside of the United States. If the FDA does not accept the data from any clinical trials that we or our collaboration partners conduct outside the United States, it would likely result in the need for additional clinical trials, which would be costly and time-consuming and delay or permanently halt our ability to develop and market these or other product candidates in the United States. In
26
Table of Contents
other jurisdictions, for instance, in Japan, there is a similar risk regarding the acceptability of clinical trial data conducted outside of that jurisdiction.
In addition, there are risks inherent in conducting clinical trials in multiple jurisdictions, inside and outside of the United States, such as:
| · | regulatory and administrative requirements of the jurisdiction where the trial is conducted that could burden or limit our ability to conduct our clinical trials; |
| · | foreign exchange fluctuations; |
| · | manufacturing, customs, shipment and storage requirements; |
| · | cultural differences in medical practice and clinical research; and |
| · | the risk that the patient populations in such trials are not considered representative as compared to the patient population in the target markets where approval is being sought. |
The design or our execution of clinical trials may not support regulatory approval.
The design or execution of a clinical trial can determine whether its results will support regulatory approval and flaws in the design or execution of a clinical trial may not become apparent until the clinical trial is well advanced. In some instances, there can be significant variability in safety or efficacy results between different trials of the same product candidate due to numerous factors, including changes in trial protocols, differences in size and type of the patient populations, adherence to the dosing regimen and other trial protocols and the rate of dropout among clinical trial participants. We do not know whether any Phase 2, Phase 3 or other clinical trials we or any of our strategic partners may conduct will demonstrate consistent or adequate efficacy and safety to obtain regulatory approval to market our product candidates or whether the regulatory authorities will agree that the design of our or our partners’ studies is adequate to support approval.
Further, the FDA, EMA or other regulatory authorities have substantial discretion in the approval process and in determining when or whether regulatory approval will be obtained for any of our product candidates. Our product candidates may not be approved even if they achieve their primary endpoints in future Phase 3 clinical trials or registration trials. The FDA, EMA or other regulatory authorities may disagree with our trial design and our interpretation of data from pre-clinical studies and clinical trials. In addition, any of these regulatory authorities may change requirements for the approval of a product candidate even after reviewing and providing comments or advice on a protocol for a pivotal Phase 3 clinical trial that has the potential to result in FDA, EMA or other agencies’ approval. In addition, any of these regulatory authorities may also approve a product candidate for fewer or more limited indications than we request or may grant approval contingent on the performance of costly post-marketing clinical trials. The FDA, EMA or other regulatory authorities may not approve the labeling claims that we believe would be necessary or desirable for the successful commercialization of our product candidates.
Even if we receive regulatory approval for any of our product candidates, we will be subject to ongoing obligations and continued regulatory review, which may materially adversely affect our business, prospects, financial condition and results of operations.
If the FDA, EMA or a comparable foreign regulatory authority approves any of our product candidates, the manufacturing processes, labeling, packaging, distribution, adverse event
27
Table of Contents
reporting, storage, advertising, marketing, promotion and recordkeeping for the product will be subject to extensive and ongoing regulatory requirements. These requirements include submissions of safety and other post-marketing information and reports, registration, as well as continued compliance with cGMPs and GCPs for any clinical trials that we conduct post-approval, all of which may result in significant expense and limit our ability to commercialize such products. Any regulatory approvals that we receive for our product candidates may also be subject to limitations on the approved indicated uses for which the product may be marketed or to the conditions of approval, or contain requirements for potentially costly post-marketing testing and surveillance to monitor the safety and efficacy of the product.
In addition, regulatory policies may change or additional government regulations or legislation may be enacted that could prevent, limit or delay regulatory approval of our product candidates. If we fail to comply with existing requirements, are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any regulatory approval that we may have obtained or face regulatory or enforcement actions, which may materially adversely affect our business, prospects, financial condition and results of operations.
We may be subject to penalties if we fail to comply with regulatory requirements or experience unanticipated problems with any of our products that receive regulatory approval, which may materially adversely affect our business, prospects, financial condition and results of operations.
Once a product is approved by the FDA, EMA or a comparable foreign regulatory authority for marketing, it is possible that previously unknown problems may occur with the product, including problems with third-party manufacturers or manufacturing processes, or failure to comply with regulatory requirements. If any of the foregoing occurs with respect to our products, it may result in, among other things:
| · | restrictions on the marketing or manufacturing of the product, withdrawal of the product from the market, or voluntary or mandatory product recalls; |
| · | fines, warning letters or holds on clinical trials; |
| · | refusal by the FDA, EMA or comparable foreign regulatory authority to approve pending applications or supplements to approved applications filed by us, or suspension or revocation of product license approvals; |
| · | requirements to conduct additional clinical trials, change our product labeling or submit additional applications or application supplements; |
| · | product seizure or detention, or refusal to permit the import or export of products; and |
| · | injunctions or the imposition of civil or criminal penalties. |
The occurrence of any of these events, or any government investigation of alleged violations of law could require us to expend significant time and resources and could generate negative publicity, and our ability to sell such product may be impaired. If we or our collaborators are not able to maintain regulatory compliance, regulatory approval that has been obtained may be lost and we may not achieve or sustain profitability, which may materially adversely affect our business, prospects, financial condition and results of operations.
28
Table of Contents
We may allocate our limited resources to pursue a particular product candidate or indication and fail to capitalize on product candidates or indications that may later prove to be more profitable or for which there is a greater likelihood of success, which may materially adversely affect our business, financial condition, and results of operations.
Because we have limited financial and managerial resources, we must limit our licensing, research and development programs to specific product candidates that we identify for specific indications. As a result, we may forego or delay pursuit of opportunities with other product candidates or for other indications that later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial product candidates or profitable market opportunities, and our decisions concerning the allocation of research, collaboration, management and financial resources towards particular product candidates may not lead to the development of viable commercial products. In addition, if we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through collaboration, licensing or other royalty arrangements when it would have been more advantageous for us to retain sole development and commercialization rights to such product candidate. If we make incorrect determinations regarding the market potential of our product candidates or misread trends in the biopharmaceutical industry, in particular for our late stage product candidates, our business, financial condition, and results of operations could be materially adversely affected.
We currently do not have a sales and marketing organization and we have no history of commercializing our proprietary products.
The development of our proprietary product candidates has been limited to developing and screening our technology and undertaking pre-clinical studies and clinical trials thereof, either independently or with strategic partners. We have not yet demonstrated the ability to successfully complete the development of our proprietary product candidates, obtain marketing approvals, manufacture them at a commercial scale with our CMOs, or conduct sales and regulatory activities necessary for successful product commercialization of our proprietary product candidates. Any predictions about our future success or viability may not be as accurate as they could be if we had a history of successfully developing and commercializing our proprietary pharmaceutical products.
Factors that may affect our ability to commercialize our product candidates on our own include recruiting and retaining adequate numbers of effective sales and marketing personnel, obtaining access to or persuading adequate numbers of physicians to prescribe our product candidates and other unforeseen costs associated with creating an independent sales and marketing organization. In addition, we may seek to acquire a commercialization platform. There is no guarantee that any such acquisition targets will be available.
We do not currently have a sales and marketing organization, and developing or acquiring a sales and marketing organization will be expensive and time consuming and could delay the launch of our product candidates. We may not be able to build an effective sales and marketing organization. If we are unable to build our own distribution and marketing capabilities or to find suitable partners for the commercialization of our product candidates, we may not be able to generate revenues from them or to reach or sustain profitability.
29
Table of Contents
If any of our product candidates receive regulatory approval, the approved products may not achieve broad market acceptance among physicians, patients, the medical community and third-party payors, in which case revenue generated from their sales would be limited.
The commercial success of our product candidates will depend upon their acceptance among physicians, patients and the medical community. The degree of market acceptance of our product candidates will depend on a number of factors, including:
| · | limitations or warnings contained in the approved labeling for a product candidate; |
| · | changes in the standard of care for the targeted indications for any of our product candidates; |
| · | limitations in the approved clinical indications for our product candidates; |
| · | demonstrated clinical safety and efficacy compared to other products; |
| · | lack of significant adverse side effects; |
| · | sales, marketing and distribution support; |
| · | availability of coverage and extent of reimbursement from managed care plans and other third-party payors; |
| · | timing of market introduction and perceived effectiveness of competitive products; |
| · | the degree of cost-effectiveness of our product candidates; |
| · | availability of alternative therapies at similar or lower cost, including generic and over-the-counter products; |
| · | whether the product is designated under physician treatment guidelines as a first-line therapy or as a second or third-line therapy for particular diseases; |
| · | whether the product can be used effectively with other therapies to achieve higher response rates; |
| · | adverse publicity about our product candidates or favorable publicity about competitive products; |
| · | convenience and ease of administration of our products; and |
| · | potential product liability claims. |
If any of our product candidates are approved, but do not achieve an adequate level of acceptance by physicians, patients and the medical community, we may not generate sufficient revenue from these products, and we may not become or remain profitable. In addition, efforts to educate the medical community and third-party payors on the benefits of our product candidates may require significant resources and may never be successful.
30
Table of Contents
Risks Related to Our Reliance on Collaborators and Other Third Parties
Collaborations on products and product candidates are important to our business, and future collaborations may also be important to us. If we are unable to maintain any of these collaborations or if these collaborations are not successful, our business could be materially adversely affected.
We have in the past entered into, and intend to continue to enter into, collaborations with other companies that we believe provide us with valuable funding and other benefits. However, we cannot ensure that any such collaboration will continue or be successful. For example, in March 2015, we and Celgene Corporation agreed to end the existing co-development and co-promotion agreement for MOR202, following which we regained the rights to MOR202. Although we have subsequently partnered Chinese regional rights to MOR202, we are currently investigating the possibility of further developing MOR202 outside of China with a partner. We cannot ensure that any such partner will be found or that such collaboration will be successful. Our inability to find a partner for any of our product candidates, may result in our termination of that specific product candidate program or evaluation of a product candidate in a particular indication. Due to the competition in MM and the size and the duration of a potential pivotal study in this indication, we intend to develop MOR202 in MM only with a strong and committed partner. In addition, we have entered into various other collaboration and license arrangements with third parties. We cannot ensure that any such collaboration or license agreement will be successful.
In the future, we may enter into additional collaborations to fund our development programs or to gain access to sales, marketing or distribution capabilities. Under our collaboration agreements, we typically grant our partners an exclusive license to certain therapeutic antibodies for specific targets and receive license fees, research and development funding, milestone payments and/or, if a product is approved for marketing, sales royalties in return. Following the discovery and pre-clinical testing phase, our partners are typically solely responsible for the further development of the product candidate and therefore exercise full control over its further development and potential commercialization. Our existing collaborations, and any future collaborations we enter into, therefore may pose a number of risks, including the following:
| · | collaborators may have significant discretion in determining the efforts and resources that they will apply to these collaborations; |
| · | collaborators may not perform their obligations as expected by us or by health authorities, such as the FDA, the EMA or comparable foreign regulatory authorities; |
| · | collaborators may dissolve, merge, be bought, or may otherwise become unwilling to fulfill the initial terms of the collaboration with us; |
| · | collaborators may not pursue development and commercialization of any product candidates that achieve regulatory approval or may elect not to continue or renew development or commercialization programs based on clinical trial results, changes in the collaborators’ strategic focus or available funding, or external factors, such as an acquisition, that divert resources or create competing priorities or the actual or perceived competitive situation in a specific indication; |
31
Table of Contents
| · | collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial or abandon a product candidate, repeat or conduct new clinical trials or may require a new formulation of a product candidate for clinical testing; |
| · | collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our products or product candidates if the collaborators believe that competitive products are more likely to be successfully developed or can be commercialized under terms that are more economically attractive than ours; |
| · | product candidates discovered in collaboration with us may be viewed by our collaborators as competitive with their own product candidates or products, which may cause collaborators to cease to devote resources to the commercialization of our product candidates; |
| · | a collaborator with marketing and distribution rights to one or more of our product candidates that achieve regulatory approval may not commit sufficient resources to the marketing and distribution of such product or products; |
| · | disagreements with collaborators or licensors, including disagreements over proprietary rights, contract interpretation and breach of contract claims, payment obligations or the preferred course of development, might cause delays or termination of the research, development or commercialization of products or product candidates, might lead to additional responsibilities, including financial obligations for us with respect to products or product candidates, or delays or withholding of any payments due or might result in litigation or arbitration, any of which would be time consuming and expensive; |
| · | collaborators may not properly maintain or defend our intellectual property rights or may use our proprietary information in such a way that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential litigation; |
| · | collaborators may infringe the intellectual property rights of third parties, which may expose us to litigation and potential liability; and |
| · | collaborations may be terminated for the convenience of the collaborator and, if terminated, we could be required to raise additional capital to pursue further development or commercialization of the applicable product candidates. |
If our collaborations on research and development candidates do not result in the successful development and commercialization of products or if one of our collaborators terminates its agreement with us, we may not receive any future research funding or milestone or royalty payments under the collaboration. If we do not receive the funding we expect under these agreements, our development of our product candidates could be delayed and we may need additional resources to develop our proprietary product candidates. All of the risks relating to product development, regulatory approval and commercialization described in this prospectus also apply to the activities of our program collaborators.
Additionally, subject to its contractual obligations to us, if one of our collaborators is involved in a business combination, the collaborator might deemphasize or terminate the development or commercialization of any product candidate licensed to it by us. If one of our collaborators terminates its agreement with us, we may find it more difficult to attract new collaborators in a timely manner.
32
Table of Contents
We face significant competition in seeking new partnerships, including for MOR202.
We are considering entering into a partnership or collaboration agreement for the further development of MOR202 and may in the future determine to collaborate with additional pharmaceutical and biotechnology companies for development and potential commercialization of certain other of our product candidates for various territories in the world. We face significant competition in seeking appropriate collaborators or partners. Our ability to reach a definitive agreement for a partnership will depend, among other things, upon our assessment of the partner’s resources and expertise, the terms and conditions of the proposed partnership and the proposed partner’s evaluation of a number of factors. These factors may include the design or results of clinical trials, the likelihood of approval by the FDA, the EMA or comparable foreign regulatory authorities, the potential market for the subject product candidate, market access and pricing considerations in the respective territory, the costs and complexities of manufacturing and delivering such product candidate to patients, the potential of competing products, incidence and prevalence of the respective disease, and industry and market conditions generally. The partner may also consider alternative product candidates for similar indications that may be available to collaborate on and whether such collaboration could be more attractive than the one with us for our product candidate. For example, MOR202, if approved for multiple myeloma, would compete with Janssen’s daratumumab and potentially isatuximab, and, as a result, it may be difficult to find a suitable partner with adequate resources. Due to the competition in MM and the size and the duration of a potential pivotal study in this indication, we intend to develop MOR202 in MM only with a strong and committed partner.
Collaborations and commercialization partnerships are complex and time consuming to negotiate and document. If we are unable to reach agreements with suitable partners on a timely basis, on acceptable terms, or at all, we may have to curtail or even stop the development of a product candidate, in one or all indications, in one or all territories in the world, reduce or delay one or more of our other discovery and development programs, delay its potential commercialization, or increase our expenditures and undertake development or commercialization activities at our own expense. If we elect to fund and undertake development or commercialization activities on our own — for instance, as we have done so far for MOR208 — we may need to obtain additional expertise and additional capital, which may not be available to us on acceptable terms or at all. If we fail to enter into collaborations and other partnerships and do not have sufficient funds or expertise to undertake the necessary development and commercialization activities, we may not be able to further develop our product candidates in any or all indications or bring them to market in any or all territories in the world and our business may be materially and adversely affected.
We rely, and expect to continue to rely, on third parties, including independent clinical investigators, and CROs, to conduct our pre-clinical studies and clinical trials and perform data collection and analysis, which may result in costs and delays in the development of our product candidates. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may not be able to obtain regulatory approval for or commercialize our product candidates and our business could be materially adversely affected.
We currently, and expect to continue to, selectively rely on public and private research institutions, medical institutions, clinical investigators, CROs, contract laboratories and collaboration partners to conduct some of our early-stage product development activities,
33
Table of Contents
perform data collection and analysis and carry out our clinical trials. Our development activities or clinical trials conducted in reliance on third parties may be delayed, suspended or terminated, including for the following reasons:
| · | the third parties do not devote a sufficient amount of time or effort to our activities or otherwise fail to successfully carry out their contractual duties or to meet regulatory obligations or expected deadlines; |
| · | we replace a third party; or |
| · | the quality or accuracy of the data obtained by third parties is compromised due to their failure to adhere to clinical protocols, regulatory requirements or for other reasons. |
While we have performance monitoring and metrics in place, we do not have the ability to control every action of third parties in their conduct of development activities. Nevertheless, we are responsible for ensuring that each of our studies and trials is conducted in accordance with the applicable protocol, legal, regulatory and scientific standards, and our reliance on these third parties does not relieve us of our regulatory responsibilities. We and our third-party contractors and CROs are required to comply with GCPs, which are regulations and guidelines enforced by the FDA, the competent authorities of the member states of the European Economic Area, or EEA, and comparable foreign regulatory authorities for all of our products in clinical development. Regulatory authorities enforce these GCPs through periodic inspections of trial sponsors, principal investigators and trial sites. If we or any of our CROs fail to comply with applicable GCPs, the clinical data generated in our clinical trials may be deemed unreliable and the FDA, the EMA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot assure you that upon inspection by a given regulatory authority, such regulatory authority will determine that any of our clinical trials comply with GCP regulations. In addition, our clinical trials must be conducted with product produced under cGMP regulations. If CROs do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols, regulatory requirements or for other reasons, our clinical trials may be extended, delayed or terminated and we may not be able to obtain regulatory approval for or successfully commercialize our product candidates.
Third-party performance failures may increase our development costs, delay our ability to obtain regulatory approval and delay or prevent the commercialization of our product candidates. While we believe that there are alternative sources to provide these services, in the event that we seek such alternative sources, we may not be able to enter into replacement arrangements without incurring delays or additional costs.
34
Table of Contents
We currently rely on third-party suppliers and single source third-party CMOs for the manufacturing of our product candidates and our dependence on these third parties may impair the development of our product candidates. Moreover, we intend to rely on third parties to produce commercial supplies of any approved product candidate and our commercialization of any of our product candidates could be stopped, delayed or made less profitable if those third parties fail to provide us with sufficient quantities of product or fail to do so at acceptable quality levels or prices or fail to otherwise complete their duties in compliance with their obligations to us or in compliance with applicable laws. Service or supply failures, or other failures, business interruptions, or other disasters affecting the manufacturing facilities of any party participating in the supply chain, would adversely affect our ability to supply our product candidates and products.
We do not currently have, nor do we plan to acquire, the infrastructure or capability internally to manufacture our pre-clinical (with the exclusion of non-GLP testing) and clinical product supplies and we lack the resources and the capability to manufacture any of our product candidates on a clinical or commercial scale. We therefore rely on and expect to continue to rely on third-party contract manufacturing organizations, or CMOs, for the supply of cGMP-grade, clinical trial materials and commercial quantities of our product candidates and products, if approved. The facilities used by our CMOs or other third-party manufacturers to manufacture our product candidates are subject to the FDA’s, the EMA’s and other comparable regulatory authorities’ preapproval inspections that will be conducted after we submit our BLA to the FDA or the required approval documents to any other relevant regulatory authority. Except for our legally required qualification audit prior to contracting a CMO and subsequent regular audits of such facilities and GMP procedures, we do not control the implementation of the manufacturing process of, and are completely dependent on, our contract manufacturers or other third-party manufacturers for compliance with the regulatory requirements, known as current good manufacturing practices, or cGMPs, for manufacture of both active drug substances and finished drug products. If our contract manufacturers or other third-party manufacturers cannot successfully manufacture material that conforms to applicable specifications and the strict regulatory requirements of the FDA, the EMA or another comparable regulatory authority, we may not be able to secure and/or maintain regulatory approvals for our products manufactured at these facilities. In addition (except for our audit obligations described above), we have no control over the ability of our contract manufacturers or other third-party manufacturers to maintain adequate quality control and quality assurance procedures and qualified personnel. If the FDA, the EMA or another comparable regulatory authority finds deficiencies at these facilities for the manufacture of our product candidates or if it withdraws any approval because of deficiencies at these facilities in the future, we may need to find alternative manufacturing facilities, which would significantly impact our ability to develop, obtain regulatory approval for or market our product candidates, if approved. If, for any reason, we were to experience an unexpected loss of supply of our product candidates, combination drug, or placebo or comparator product used in certain of our clinical trials, whether as a result of manufacturing, supply or storage issues or otherwise, we could experience delays, disruptions, suspensions or terminations of, or be required to restart or repeat, any pending or ongoing clinical trials.
We rely on our manufacturers to purchase from third-party suppliers the materials necessary to produce our product candidates for our clinical trials. For certain items, there are a limited number of suppliers for raw materials that we use to manufacture our products and appropriate lead times for ordering such materials are factored into the manufacturing plans. However,
35
Table of Contents
there may be a need to assess alternate suppliers to prevent a possible disruption of the manufacture of the materials necessary to produce our product candidates for our clinical trials, and if approved, for commercial sale. Moreover, we currently do not have any agreements in place for the commercial production of these raw materials. Although we generally do not begin a clinical trial unless we believe we have access to a sufficient supply of a product candidate to complete the clinical trial, any significant delay in the supply of a product candidate, or the raw material components thereof, for an ongoing clinical trial due to the need to implement corrective actions at the supplier, or to replace a contract manufacturer or other third-party manufacturer could considerably delay completion of our clinical trials, product testing and potential regulatory approval of our product candidates. If our manufacturers or we are unable to purchase these raw materials after regulatory approval has been obtained for our product candidates, the commercial launch of our product candidates would be delayed or there would be a shortage in supply, which would impair our ability to generate revenue from the sale of our product candidates. Additionally, if we receive regulatory approval for our product candidates, we may experience unforeseen difficulties or challenges in the manufacture of our product candidates on a commercial scale compared to the manufacture for clinical purposes. We currently rely on single source CMOs for the manufacturing of each of our proprietary product candidates, including Boehringer Ingelheim, or BI, in respect of MOR208. Thus any regulatory action, service failure, business interruptions, or other disasters affecting BI’s facilities or the facilities of our other CMOs for our other proprietary product candidates could materially and adversely impact the supply of our product candidate.
The manufacture of our product candidates is complex. Our third-party manufacturers may encounter difficulties in production. If we encounter any such difficulties, our ability to supply our product candidates for clinical trials or, if approved, for commercial sale could be delayed or halted entirely.
The manufacture of biopharmaceutical products is complex and requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls. The process of manufacturing biopharmaceuticals, including our product candidates, is susceptible to product loss due to contamination, equipment failure or improper installation or operation of equipment, vendor or operator error, contamination and inconsistency in yields, variability in product characteristics and difficulties in scaling the production process or product loss during fill and finishing. Even minor deviations from normal manufacturing processes could result in reduced production yields, product defects and other supply disruptions. If microbial, viral or other contaminations are discovered in our product candidates or in the manufacturing facilities in which our product candidates are made, such manufacturing facilities may need to be closed for an extended period of time to investigate and remedy the contamination. Any adverse developments affecting manufacturing operations for our product candidates, if any are approved, may result in shipment delays, inventory shortages, lot failures, product withdrawals or recalls, or other interruptions in the supply of our products. We may also have to take inventory write-offs and incur other charges and expenses for products that fail to meet specifications, undertake costly remediation efforts or seek more costly manufacturing alternatives.
36
Table of Contents
Risks Related To Our Intellectual Property Rights
If we are unable to obtain and maintain sufficient intellectual property protection for our products or product candidates, or if the scope of our intellectual property protection is not sufficiently broad, our ability to commercialize our products or product candidates successfully and to compete effectively may be materially adversely affected.
Our success depends in large part on our ability to obtain and maintain protection with respect to our intellectual property and proprietary technology. We rely upon a combination of patents, trade secret protection and confidentiality agreements to protect the intellectual property related to our products and product candidates. The patent position of pharmaceutical companies is generally uncertain because it involves complex legal and factual considerations. The standards applied by the United States Patent and Trademark Office, or USPTO, and foreign patent offices in granting patents are not always applied uniformly or predictably, and can change. The patent applications that we own or in-license may fail to result in issued patents, and if they do, such patents may not cover our products or product candidates in the United States or in other countries. Accordingly, we cannot predict whether additional patents protecting our technology or our product candidates will issue in the United States or in non-U.S. jurisdictions, or whether any patents that do issue will have claims of adequate scope to provide us with a competitive advantage. Additionally, the laws of some foreign countries do not protect intellectual property rights to the same extent as the laws of the United States, and many companies have encountered significant problems in protecting and defending such rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property rights, particularly those relating to biotechnology, which could make it difficult for us to stop the infringement of our licensed and owned patents, the reproduction of our manufacturing or other know-how or marketing of competing products in violation of our proprietary rights generally. Any of these outcomes could impair our ability to prevent competition from third parties, which may have a material adverse effect on our business.
Competitors may use our technologies in jurisdictions where we have not obtained or are unable to adequately enforce patent protection to develop their own products and further, may export otherwise infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States and Europe. These products may compete with our products, and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing. Proceedings to enforce our patent rights, whether or not successful, could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
The patent prosecution process is expensive and time consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we may fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. In addition, we or our
37
Table of Contents
licensors, may only pursue, obtain or maintain patent protection in a limited number of countries. There is no assurance that all potentially relevant prior art relating to our patents and patent applications has been found. We may be unaware of prior art that could be used to invalidate an issued patent or prevent our pending patent applications from issuing as patents. Even if patents do successfully issue and even if such patents cover our products or product candidates, third parties (including our licensees) may challenge their validity, enforceability or scope, which may result in such patents being narrowed or invalidated. Further, the existence of issued patents does not guarantee our right to practice the patented technology or commercialize the patented product. Third parties may have or obtain rights to patents which they may use to prevent or attempt to prevent us from commercializing any of our patented product candidates. If these other parties are successful in obtaining valid and enforceable patents, and establishing our infringement of those patents, we could be prevented from selling our products unless we were able to obtain a license under such third-party patents. In addition, third parties may seek approval to market their own products similar to or otherwise competitive with our products. In these circumstances, we may need to defend and/or assert our patents, including by filing lawsuits alleging patent infringement. In any of these types of proceedings, a court or agency of competent jurisdiction may find our patents invalid and/or unenforceable.
Furthermore, even if they are unchallenged, our patents and patent applications may not adequately protect our intellectual property, provide exclusivity for our products or product candidates, prevent others from designing around our claims or otherwise provide us with a competitive advantage. Additionally, our confidentiality agreements and other contractual protections may not be adequate to protect our intellectual property from unauthorized disclosure, third-party infringement or misappropriation. We may not have adequate remedies in the case of a breach of any such agreements, and our trade secrets and other proprietary information could be disclosed to our competitors or others may independently develop substantially equivalent or superior proprietary information and techniques or otherwise gain access to our trade secrets or disclose such technologies. In addition, the research resulting in certain of our licensed patent rights and technology has been, and may in the future be, funded by the government or other institutional organizations that may have certain rights, including march-in rights, to such patent rights and technology.
If the patent applications we own or have in-licensed with respect to our product candidates fail to issue as patents, if their breadth or strength of protection is narrowed or threatened, or if they fail to provide meaningful exclusivity, it could dissuade companies from collaborating with us and adversely affect our competitive position. We cannot offer any assurances about which, if any, patents will issue, the breadth of any such patents or whether any issued patents will be found invalid or unenforceable or will be threatened by third parties. Any successful challenge to any patents owned by or licensed to us could deprive us of rights necessary for the successful commercialization of any product or product candidate that we may develop and could impair or eliminate our ability to collect future revenues and royalties with respect to such products or product candidates. Since patent applications in the United States and most other countries are confidential for a period of time after filing, and some remain so until issued, we cannot be certain that we were the first to file any patent application related to a product or product candidate. In addition, patents have a limited lifespan. In the United States and most foreign jurisdictions, the natural expiration of a patent is generally 20 years after its effective filing date. Various extensions may be available; however, the life of a patent and the protection it
38
Table of Contents
affords is limited. If we encounter delays in obtaining regulatory approvals, the period of time during which we could market a product under patent protection could be reduced. Even if patents covering our product candidates are obtained, once such patents expire, we may be vulnerable to competition from similar or biosimilar products. The launch of a biosimilar version of one of our products in particular would be likely to result in an immediate and substantial reduction in the demand for our product, which could have a material adverse effect on our business, financial condition, results of operations or prospects.
Obtaining and maintaining our patent protection, including patents licensed from third parties, depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
The USPTO, and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other provisions during the patent process. There are situations in which noncompliance can result in abandonment or lapse of a patent or patent application, resulting in partial loss, complete loss or unenforceability of patent rights in the relevant jurisdiction. In such an event, competitors might be able to enter the market earlier than would otherwise have been the case.
If we or our licensors fail to maintain the patents and patent applications covering or otherwise protecting our product candidates, it could materially harm our business. In addition, to the extent that we have responsibility for taking any action related to the prosecution or maintenance of patents or patent applications in-licensed from a third party, any failure on our part to maintain the in-licensed intellectual property could jeopardize our rights under the relevant license and may expose us to liability.
We currently are, and may in the future, be involved in lawsuits to protect or enforce our patents, which could be expensive, time-consuming and unsuccessful.
Even if the patent applications we own or license are issued, competitors may infringe these patents. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time-consuming. For instance, we are currently involved in a patent litigation lawsuit as a plaintiff against Janssen Biotech Inc., Genmab A/S and Genmab US, Inc. at the District Court of Delaware seeking redress for infringement in connection with the manufacture, use and sale of Janssen’s and Genmab’s daratumumab, an antibody targeting CD38, approved for the treatment of certain patients with MM and the same target against which MOR202 is directed. Defendants have filed counterclaims asserting that our patents are not infringed, invalid and have recently asked the court to add further counterclaims of unenforceability. In addition, Janssen, Genmab, Sanofi and Takeda opposed a European counterpart of the litigated U.S. patents, EP2511297. The patent was revoked in opposition proceedings. We appealed and the proceedings are currently pending.
In an infringement proceeding, a court may decide that a patent of ours or our licensors is not valid, is unenforceable and/or is not infringed, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation or defense proceedings could put our patents or our licensors’ patents at risk of being invalidated or interpreted narrowly and could put our patent applications at risk of not issuing.
39
Table of Contents
Interference proceedings, or other similar enforcement and revocation proceedings, provoked by third parties or brought by us may be necessary to determine the priority of inventions with respect to our patents or patent applications or those of our licensors. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms. Our defense of litigation or interference proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees. We may not be able to prevent, alone or with our licensors, misappropriation of our intellectual property rights, particularly in countries where the laws may not protect those rights as fully as in the United States.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a material adverse effect on the price of our common shares.
Even if resolved in our favor, litigation or other legal proceedings relating to our, our licensor’s or other third parties’ intellectual property claims may cause us to incur significant expenses and could distract our personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions, or other interim proceedings or developments, and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our ordinary shares. If not resolved in our favor, litigation may require us to pay any portion of our opponents’ legal fees. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing, or distribution activities. We may not have sufficient financial or other resources to conduct such litigation or proceedings adequately. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources and more mature and developed intellectual property portfolios. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace.
Developments in patent law could have a negative impact on our business.
From time to time, authorities in the United States, the European Union and other government authorities may change the standards of patentability, and any such changes could have a negative impact on our business.
For example, in the United States, the Leahy-Smith America Invents Act, or the America Invents Act, which was signed into law in 2011, includes a number of significant changes to U.S. patent law. These changes include a transition from a “first-to-invent” system to a “first-to-file” system, changes to the way issued patents are challenged, and changes to the way patent applications are disputed during the examination process. As a result of these changes, patent law in the United States may favor larger and more established companies that have greater resources to devote to patent application filing and prosecution. The USPTO has developed new and untested regulations and procedures to govern the full implementation of the America Invents Act, and many of the substantive changes to patent law associated with the America Invents
40
Table of Contents
Act, and, in particular, the first-to-file provisions became effective on March 16, 2013. Substantive changes to patent law associated with the America Invents Act may affect our ability to obtain patents, and if obtained, to enforce or defend them.
Developments of patent law in other jurisdictions may impact our business. For example, it is currently not clear what impact the planned introduction of the Unified Patent Court in the European Union will have. Patents that are valid and enforceable under the current system may be considered invalid and/or unenforceable under the new system. Also patents may be invalidated not just in one single jurisdiction, but across all countries of the European Union in one single trial.
Our commercial success depends significantly on our ability to operate without infringing the patents and other proprietary rights of third parties.
Our success will depend in part on our ability to operate without infringing the proprietary rights of third parties. Other entities may have or obtain patents or proprietary rights that could limit our ability to make, use, sell, offer for sale or import our products and future approved products or impair our competitive position.
Patents could be issued to third parties that we may ultimately be found to infringe. Third parties may have or may obtain valid and enforceable patents or proprietary rights that could block us from developing product candidates using our technology. Our failure to identify or misinterpret third-party patents, to obtain or maintain a license to any technology that we require may materially harm our business, financial condition, results of operations or prospects. Furthermore, we could be exposed to a threat of litigation.
In the pharmaceutical and biotechnology industry, significant litigation and other proceedings regarding patents, patent applications, trademarks and other intellectual property rights have become commonplace. The types of situations in which we may become a party to such litigation or proceedings include:
| · | we or our collaborators may initiate litigation or other proceedings against third parties seeking to invalidate the patents held by those third parties or to obtain a judgment that our products or processes do not infringe those third parties’ patents; |
| · | if our competitors file patent applications that claim technology also claimed by us or our licensors, we or our licensors may be required to participate in interference, derivation, inter partes review or opposition proceedings to determine the priority of invention, inventorship or validity of the applicable patent rights which could jeopardize our patent rights and potentially provide a third party with a dominant patent position; |
| · | if third parties initiate litigation claiming that our processes, products or uses thereof infringe their patent or other intellectual property rights, we and our collaborators will need to defend against such proceedings; and |
| · | if a license to necessary technology is terminated, the licensor may initiate litigation claiming that our processes or products infringe or misappropriate their patent or other intellectual property rights and/or that we breached our obligations under the license agreement, and we and our collaborators would need to defend against such proceedings. |
Any such lawsuit would be costly and could affect our results of operations and divert the attention of our management and scientific personnel. There is a risk that a court would decide
41
Table of Contents
that we or our collaborators are infringing the third party’s patents and would order us or our collaborators to stop the activities covered by the patents. In that event, we or our collaborators may not have a viable alternative to the technology protected by the patent and may need to halt work on the affected product candidate or cease commercialization of an approved product. In addition, there is a risk that a court may order us or our collaborators to pay the other party damages. An adverse outcome in any litigation or other proceeding could subject us to significant liabilities to third parties and require us to cease using the technology that is at issue or to license the technology from third parties. We may not be able to obtain any required licenses on commercially acceptable terms or at all. Any of these outcomes could have a material adverse effect on our business, financial condition, results of operations or prospects.
The pharmaceutical and biotechnology industries have produced a significant number of patents, and it may not always be clear to industry participants, including us, which patents cover various types of products or methods of use.
The coverage of patents is subject to interpretation by the courts, and the interpretation is not always uniform or predictable. If we are sued for patent infringement, we would need to demonstrate that our products, methods or uses thereof either do not infringe the patent claims of the relevant patent or that the patent claims are invalid or unenforceable, and we may not be able to do this. Proving invalidity is difficult. For example, in the United States, proving invalidity requires a showing of clear and convincing evidence to overcome the presumption of validity enjoyed by issued patents. Even if we are successful in these proceedings, we may incur substantial costs and divert management’s time and attention in pursuing these proceedings, which could have a material adverse effect on our business. If we are unable to avoid infringing the patent rights of others, we may be required to seek a license, defend an infringement action or challenge the validity or enforceability of the patents in court. We may not have sufficient resources to bring these actions to a successful conclusion and there is no assurance that such a license would be available or that a court would find in our favor. In addition, if we do not obtain a license, develop or obtain non-infringing technology, fail to defend an infringement action successfully or have infringed patents declared invalid or unenforceable, we may incur substantial monetary damages, encounter significant delays in bringing our product candidates to market and be precluded from manufacturing or selling our product candidates.
The cost of any patent litigation or other proceeding, even if resolved in our favor, could be substantial. Some of our competitors may be able to sustain the cost of such litigation and proceedings more effectively than we can because of their substantially greater resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our business, financial condition, results of operations or prospects.
We are dependent on third parties for the prosecution, protection, and enforcement of intellectual property rights relating to some of our products and product candidates.
While we normally seek to obtain the right to control the prosecution, maintenance, enforcement and defense of intellectual property rights related to our products and product candidates, there may be times when our licensors or collaborators control, or have a first right to control, the filing, prosecution, enforcement and defense of such rights. For instance, pursuant to the 2nd amended and restated collaboration and license agreement, Novartis has a first right to file, prosecute and enforce all patent rights related to products generated under
42
Table of Contents
this agreement. Also, pursuant to the development and license agreement with GlaxoSmithKline, or GSK, GSK has a first right to file, prosecute and enforce all patent rights related to MOR103 and pursuant to the development and license agreement with Xencor, Xencor has a first right to file, prosecute and enforce patent rights which are in-licensed by us and relate to MOR208. We cannot be certain that our licensors or collaborators will prosecute, maintain, enforce and defend such intellectual property rights in a manner consistent with the best interests of our business, including by taking reasonable measures to protect the confidentiality of know-how and trade secrets, or the payment of all applicable prosecution and maintenance fees related to our technologies or any of our product candidates. We also cannot be certain that the drafting or prosecution of the licensed patents by our licensors have been conducted accurately and in compliance with applicable laws and regulations, and will result in valid and enforceable patents and other intellectual property rights. If they fail to do so, we could lose our rights to the intellectual property, our ability to develop and commercialize those products or product candidates may be adversely affected and we may not be able to prevent competitors from making, using and selling competing products.
If trademarks and trade names related to our products or product candidates are not adequately protected, then we may not be able to build name recognition in our markets of interest and our business may be materially adversely affected.
Our registered or unregistered trademarks or trade names, as well as the registered or unregistered trademarks or trade names used by our licensees or distributors in relation with our products or product candidates, may be challenged, infringed, circumvented or declared generic or determined to be infringing on other trademarks. We may not be able to protect our rights to these trademarks and trade names, which we need to build name recognition by potential partners or customers. Over the long term, if we are unable to establish name recognition based on our trademarks and trade names, then we may not be able to compete effectively and our business may be materially adversely affected.
If we are unable to protect the confidentiality of our proprietary information, the value of our technology and products could be materially adversely affected.
In addition to patent protection, we also rely on other proprietary rights, including protection of trade secrets, and other proprietary information. To maintain the confidentiality of trade secrets and proprietary information, we enter into confidentiality agreements with our employees, consultants, collaborators, CMOs, CROs and others upon the commencement of their relationships with us. These agreements require that all confidential information developed by the individual or made known to the individual by us during the course of the individual’s relationship with us be kept confidential and not disclosed to third parties. Our agreements with employees as well as our personnel policies also generally provide that any inventions conceived by the individual in the course of rendering services to us shall be our exclusive property or that we may obtain full rights to such inventions at our election. However, we may not obtain these agreements in all circumstances, and individuals with whom we have these agreements may not comply with their terms. We also face the risk that present or former employees could continue to hold rights to intellectual property used by us, may demand the registration of intellectual property rights in their name and demand damages pursuant to the German Employee Invention Act. In the event of unauthorized use or disclosure of our trade secrets or proprietary information, these agreements, even if obtained, may not provide
43
Table of Contents
meaningful protection, particularly for our trade secrets or other confidential information. To the extent that our employees, consultants or contractors use technology or know-how owned by third parties in their work for us, disputes may arise between us and those third parties as to the rights in related inventions. To the extent that an individual who is not obligated to assign rights in intellectual property to us is rightfully an inventor of intellectual property, we may need to obtain an assignment or a license to that intellectual property from that individual, or a third party or from that individual’s assignee. Such assignment or license may not be available on commercially reasonable terms or at all.
Adequate remedies may not exist in the event of unauthorized use or disclosure of our proprietary information. The disclosure of our trade secrets would impair our competitive position and may materially harm our business, financial condition and results of operations. Costly and time consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and failure to maintain trade secret protection could adversely affect our competitive business position. In addition, others may independently discover or develop our trade secrets and proprietary information, and the existence of our own trade secrets affords no protection against such independent discovery.
As is common in the biotechnology and pharmaceutical industries, we employ individuals who were previously or concurrently employed at research institutions and/or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. We may be subject to claims that these employees, or we, have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers, or that patents and applications we have filed to protect inventions of these employees, even those related to one or more of our product candidates, are rightfully owned by their former or concurrent employer. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
We may not be successful in obtaining necessary intellectual property rights to product candidates for our development pipeline through acquisitions and in-licenses.
Although we intend to develop product candidates through our own internal research, we may also seek to acquire or in-license product candidates to grow our product candidate pipeline. However, we may be unable to acquire or in-license intellectual property rights relating to, or necessary for, any such product candidates from third parties on commercially reasonable terms or at all. In that event, we may be unable to develop or commercialize such product candidates. We may also be unable to identify product candidates that we believe are an appropriate strategic fit for our company and intellectual property relating to, or necessary for, such product candidates.
The in-licensing and acquisition of third-party intellectual property rights for product candidates is a competitive area, and a number of more established companies are also pursuing strategies to in-license or acquire third-party intellectual property rights for product candidates that we may consider attractive or necessary. These established companies may have a competitive advantage over us due to their size, cash resources and greater clinical development and commercialization capabilities. Furthermore, companies that perceive us to be a competitor may be unwilling to assign or license rights to us. If we are unable to successfully obtain rights to
44
Table of Contents
suitable product candidates, our business, financial condition, results of operations and prospects for growth could suffer.
In addition, we expect that competition for the in-licensing or acquisition of third-party intellectual property rights for product candidates that are attractive to us may increase in the future, which may mean fewer suitable opportunities for us as well as higher acquisition or licensing costs. We may be unable to in-license or acquire the third-party intellectual property rights for product candidates on terms that would allow us to make an appropriate return on our investment.
We may not be able to adequately protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on our products in all countries throughout the world would be prohibitively expensive. The requirements for patentability may differ in certain countries, particularly developing countries, and the breadth of patent claims allowed can be inconsistent. In addition, the laws of some foreign countries may not protect our intellectual property rights to the same extent as laws in the U.S. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the U.S. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and, furthermore, may export otherwise infringing products to territories in which we have patent protection that may not be sufficient to terminate infringing activities.
We do not have patent rights in certain foreign countries in which a market may exist. Moreover, in foreign jurisdictions where we do have patent rights, proceedings to enforce such rights could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly, and our patent applications at risk of not issuing. Additionally, such proceedings could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Thus, we may not be able to stop a competitor from marketing and selling in foreign countries products that are the same as or similar to our products, and our competitive position in the international market would be harmed.
Our intellectual property agreements with third parties may be subject to disagreements over contract interpretation, which could narrow the scope of our rights to the relevant intellectual property or technology or increase our financial or other obligations to our licensors.
Certain provisions in our intellectual property agreements may be susceptible to multiple interpretations. The resolution of any contract interpretation disagreement that may arise could affect the scope of our rights to the relevant intellectual property or technology, or affect financial or other obligations under the relevant agreement, either of which could have a material adverse effect on our business, financial condition, results of operations and prospects.
In addition, while it is our policy to require our employees and contractors who may be involved in the conception or development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who in fact conceives or develops intellectual property that we regard as our own. Our assignment agreements may not be self-executing or may be breached, and we may be forced
45
Table of Contents
to bring claims against third parties, or defend claims they may bring against us, to determine the ownership of what we regard as our intellectual property.
Risks Related to Our Business and Industry
Our relationships with health care professionals, institutional providers, principal investigators, consultants, customers (actual and potential) and third-party payors are, and will continue to be, subject, directly and indirectly, to health care fraud and abuse, false claims, marketing expenditure tracking and disclosure, government price reporting, and health information privacy and security laws. If we are unable to comply, or have not fully complied, with such laws, we could face penalties, including, without limitation, civil, criminal, and administrative penalties, damages, fines, exclusion from government-funded health care programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations.
Our business operations and activities may be directly or indirectly subject to various fraud and abuse laws, including, without limitation, the federal Anti-Kickback Statute and the federal False Claims Act. If we obtain FDA approval for any of our proprietary product candidates and begin commercializing those products in the United States, our potential exposure under such laws will increase significantly, and our costs associated with compliance with such laws are also likely to increase. These laws may impact, among other things, our current activities with principal investigators and research subjects, as well as proposed and future sales, marketing and education programs. In addition, we may be subject to patient privacy regulation by the federal government and state governments in which we conduct our business. The laws that may affect our ability to operate include, but are not limited to:
| · | the federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made, in whole or in part, under a federal health care program such as Medicare and Medicaid. A person or entity need not have actual knowledge of the federal Anti-Kickback Statute or specific intent to violate it in order to have committed a violation; in addition, the government may assert that a claim that includes items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the False Claims Act; |
| · | the federal civil and criminal false claims laws, including the civil False Claims Act, and civil monetary penalties laws, which prohibit individuals or entities from, among other things, knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false, fictitious or fraudulent; knowingly making a false statement or record material to a false or fraudulent claim or obligation to pay or transmit money or property to the federal government; or knowingly concealing or knowingly and improperly avoiding or decreasing an obligation to pay money to the federal government; |
| · | the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created additional federal criminal laws that prohibit, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program or knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for |
46
Table of Contents
| healthcare benefits, items or services; similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation; |
| · | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009 and their respective implementing regulations, which impose requirements on certain covered health care providers, health plans, and health care clearinghouses as well as their respective business associates that perform services for them that involve the use, or disclosure of, individually identifiable health information, relating to the privacy, security and transmission of individually identifiable health information without appropriate authorization; |
| · | the federal transparency requirements under the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, or collectively the ACA will require manufacturers of products, devices, biologics and medical supplies to report to the Department of Health and Human Services information related to payments and other transfers of value to physicians and teaching hospitals and physician ownership and investment interests; |
| · | federal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers; |
| · | federal government price reporting laws, changed by the ACA to, among other things, increase the minimum Medicaid rebates owed by most manufacturers under the Medicaid Drug Rebate Program and offer such rebates to additional populations, that may require us to calculate and report complex pricing metrics to government programs, where such reported prices may be used in the calculation of reimbursement and/or discounts on our marketed products (participation in these programs and compliance with the applicable requirements may subject us to potentially significant discounts on our products, increased infrastructure costs, and potentially limit our ability to offer certain marketplace discounts); |
| · | the Foreign Corrupt Practices Act, a U.S. law which regulates certain financial relationships with foreign government officials (which could include, for example, certain medical professionals); and |
| · | analogous state laws and regulations, such as state anti-kickback and false claims laws, which may apply to healthcare items or services that are reimbursed by non-governmental third-party payors, including private insurers. |
Some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring manufacturers to report information related to payments to physicians and other healthcare providers or marketing expenditures and pricing information. State and foreign laws also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
In addition, the regulatory approval and commercialization of any of our product candidates outside the United States will also likely subject us to foreign equivalents of the health care laws mentioned above, among other foreign laws.
47
Table of Contents
In the European Union, the Data Protection Directive, or DPD, which will be superseded by the General Data Protection Regulation, or GDPR, effective from May 2018, imposes strict regulations and establishes a series of requirements regarding the storage of personally identifiable information on computers or recorded on other electronic media. This has been implemented by all European Union member states through national laws. The DPD and GDPR provide for specific regulations requiring all non-European Union countries doing business with European Union member states to provide adequate data privacy protection when receiving personal data from persons in any of the European Union member states. In addition, the use and disclosure of personal health and other private information is subject to regulation in other jurisdictions in which we do business or expect to do business in the future. Those jurisdictions may attempt to apply such laws extraterritorially or through treaties or other arrangements with European governmental entities. We cannot assure you that our privacy and security policies and practices will be found sufficient to protect us from liability or adverse publicity relating to the privacy and security of personal information.
Efforts to ensure that our business arrangements will comply with applicable health care laws may involve substantial costs. It is possible that governmental and enforcement authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law interpreting applicable fraud and abuse or other health care laws and regulations. If we are unable to comply, or have not fully complied, with such laws, we could face penalties, including, without limitation, civil, criminal, and administrative penalties, damages, fines, individual imprisonment, exclusion from government funded health care programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. Additionally, if our collaborators’ operations or relationships with healthcare providers, customers and third-party payors are found to be non-compliant with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs, which could also have a negative impact on us. Even if successful, defending against any such actions can be costly, time-consuming and may require significant financial and personnel resources. We may become exposed to costly and damaging liability claims, either when testing our product candidates in the clinic or at the commercial stage; and our product liability insurance may not cover all damages from such claims.
We are exposed to potential product liability and professional indemnity risks that are inherent in the research, development, manufacturing, marketing and use of pharmaceutical products.
The use of our investigational medicinal products in clinical trials and the sale of any approved products in the future may expose us to liability claims. These claims might be made by patients who use the product, health care providers, pharmaceutical companies or others selling such products. Any claims against us, regardless of their merit, could be difficult and costly to defend and could materially adversely affect the market for our product candidates or any prospects for commercialization of our product candidates.
Although the clinical trial process is designed to identify and assess potential side effects, it is always possible that a product, even after regulatory approval, may exhibit unforeseen side effects. If any of our product candidates were to cause adverse side effects during clinical trials or after approval of the product candidate, we may be exposed to substantial liabilities. Physicians and patients may not comply with any warnings that identify known potential adverse effects and patients who should not use our product candidates.
48
Table of Contents
To cover such liability claims, we purchase clinical trial insurances in the conduct of each of our clinical trials. It is possible that our liabilities could exceed our insurance coverage or that our insurance will not cover all situations in which a claim against us could be made. We also intend to expand our insurance coverage to include the sale of commercial products if we receive marketing approval for any of our proprietary products. However, we may not be able to maintain insurance coverage at a reasonable cost or obtain insurance coverage that will be adequate to satisfy any liability that may arise. If a successful product liability claim or series of claims is brought against us for uninsured liabilities or in excess of insured liabilities, our assets may not be sufficient to cover such claims and our business operations could be impaired. Should any of the events described above occur, this could have a material adverse effect on our business, financial condition and results of operations, including, but not limited to:
| · | decreased demand for our future product candidates; |
| · | adverse publicity and injury to our reputation; |
| · | withdrawal of clinical trial participants; |
| · | initiation of investigations by regulators; |
| · | costs to defend the related litigation; |
| · | a diversion of management’s time and our resources; |
| · | compensation in response to a liability claim; |
| · | product recalls, withdrawals or labeling, marketing or promotional restrictions; |
| · | loss of revenue; |
| · | exhaustion of any available insurance and our capital resources; and |
| · | the inability to commercialize our products or product candidates. |
We could be adversely affected if we are subject to negative publicity. We could also be adversely affected if any of our products or any similar products distributed by other companies prove to be, or are asserted to be, harmful to patients. Any adverse publicity associated with illness or other adverse effects resulting from patients’ use or misuse of our products or any similar products distributed by other companies could have a material adverse impact on our business, financial condition, results of operations or prospects.
Even if we, or any future collaborators, are able to commercialize any product candidate that we, or they, develop, the product may become subject to unfavorable pricing regulations or third-party payor coverage and reimbursement policies, any of which could materially harm our business.
Patients who are provided medical treatment for their conditions generally rely on third-party payors to reimburse all or part of the costs associated with their treatment. Therefore, our ability, and the ability of any future collaborators to commercialize any of our product candidates will depend in part on the extent to which coverage and reimbursement for these products and related treatments will be available from third-party payors including government health administration authorities and private health coverage insurers. Third-party payors decide which medications they will cover and establish reimbursement levels. We cannot be certain that coverage will be available and reimbursement will be adequate for any of our
49
Table of Contents
product candidates. Also, we cannot be certain that reimbursement policies will not reduce the demand for, or the price paid for, our products.
Obtaining coverage and adequate reimbursement for our products may be particularly difficult because of the higher prices often associated with drugs administered under the supervision of a physician. A decision by a third-party payor not to cover our products could reduce physician utilization of our products once approved. Even if coverage is provided, the approved reimbursement amount may not be high enough to allow us, or any future collaborators, to establish or maintain pricing sufficient to realize a sufficient return on our or their investment. In the United States, no uniform policy of coverage and reimbursement for products exists among third-party payors and coverage and reimbursement for products can differ significantly from payor to payor. As a result, the coverage determination process is often a time-consuming and costly process that will require us to provide scientific and clinical support for the use of our products to each payor separately, with no assurance that coverage and adequate reimbursement will be applied consistently or obtained in the first instance.
In addition, increasingly, third-party payors are requiring higher levels of evidence of the benefits and clinical outcomes of new technologies and are challenging prices. We cannot be sure that coverage will be available for any product candidate that we, or any future collaborator, commercialize and, if available, that the reimbursement rates will be adequate. Further, the net reimbursement for products may be subject to additional reductions if there are changes to laws that presently restrict imports of drugs from one country to another. An inability to promptly obtain coverage and adequate payment rates from both government-funded and private payors for any of our product candidates for which we, or any future collaborator, obtain marketing approval could significantly harm our operating results, our ability to raise capital needed to commercialize products and our overall financial condition.
Price controls may be imposed in certain markets, which may adversely affect our future profitability.
In some countries, particularly member states of the European Union, the pricing of prescription drugs is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after receipt of marketing approval for a product. In addition, there can be considerable pressure by governments and other stakeholders on prices and reimbursement levels, including as part of cost containment measures. Political, economic and regulatory developments may further complicate pricing negotiations, and pricing negotiations may continue after reimbursement has been obtained. Reference pricing used by various countries and parallel distribution, or arbitrage between low-priced and high-priced countries, can further reduce prices. In some countries, in particular in many member states of the European Union, we may be required to conduct a clinical trial or other studies that compare the cost-effectiveness of our product candidates to other available therapies in order to obtain or maintain reimbursement or pricing approval. Publication of discounts by third-party payors or authorities may lead to further pressure on the prices or reimbursement levels within the country of publication and other countries. If reimbursement of our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our business, financial condition, results of operations or prospects could be materially adversely affected.
50
Table of Contents
Current and future legislation may increase the difficulty and cost for us and any collaborators to obtain marketing approval of and commercialize our product candidates and affect the prices we, or they, may obtain.
In the United States and foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities and affect our ability to profitably sell any product candidates for which we obtain marketing approval. We expect that current laws, as well as other healthcare reform measures that may be adopted in the future, may result in additional reductions in Medicare and other healthcare funding, more rigorous coverage criteria, new payment methodologies and in additional downward pressure on the price that we, or any collaborators, may receive for any approved products.
In March 2010, President Obama signed the ACA into law. Among the provisions of the ACA of potential importance to our business and our product candidates are the following:
| · | an annual, non-deductible fee on any entity that manufactures or imports specified branded prescription products and biologic products; |
| · | an increase in the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program; |
| · | a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for products that are inhaled, infused, instilled, implanted or injected; |
| · | extension of manufacturers’ Medicaid rebate liability to individuals enrolled in Medicaid managed care organizations; |
| · | expansion of the entities eligible for discounts under the Public Health Service pharmaceutical pricing program; |
| · | a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research; |
| · | a new Independent Payment Advisory Board, or IPAB, which has authority to recommend certain changes to the Medicare program to reduce expenditures by the program that could result in reduced payments for prescription products; and |
| · | established the Center for Medicare and Medicaid Innovation within CMS to test innovative payment and service delivery models. |
Since its enactment, there have been judicial and Congressional challenges to numerous aspects of the ACA. With the current Presidential administration and U.S. Congress, there will likely be additional administrative or legislative changes, including modification, repeal, or replacement of all, or certain provisions of, the ACA.
In addition, other legislative changes have been proposed and adopted since the ACA was enacted. In August 2011, the Budget Control Act of 2011, among other things, included aggregate reductions to Medicare payments to providers of 2% per fiscal year, which went into effect in April 2013 and will remain in effect through 2025 unless additional Congressional action is taken. The American Taxpayer Relief Act of 2012, among other things, reduced
51
Table of Contents
Medicare payments to several providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. These new laws may result in additional reductions in Medicare and other healthcare funding and otherwise affect the prices we may obtain for any of our product candidates for which we may obtain regulatory approval or the frequency with which any such product candidate is prescribed or used.
The costs of prescription pharmaceuticals in the United States has also been the subject of considerable discussion in the United States, and members of Congress and the Administration have stated that they will address such costs through new legislative and administrative measures. There have been several U.S. Congressional inquiries and proposed bills designed to, among other things, bring more transparency to drug pricing, reduce the cost of prescription drugs under Medicare, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs.
In addition, individual states have also become increasingly active in passing legislation and implementing regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access, and to encourage importation from other countries and bulk purchasing.
The policies of the FDA or similar regulatory authorities may change and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our product candidates. For example, in December 2016, the 21st Century Cures Act, or Cures Act, was signed into law. The Cures Act, among other things, is intended to modernize the regulation of drugs and biologics and spur innovation, but it has not yet been implemented and its ultimate implementation is unclear. If we or our collaborators are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we or our collaborators are not able to maintain regulatory compliance, our product candidates may lose any regulatory approval that may have been obtained and we may not achieve or sustain profitability, which would adversely affect our business.
We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. For example, certain policies of the Trump administration may impact our business and industry. Namely, the Trump administration has taken several executive actions, including the issuance of a number of Executive Orders, that could impose significant burdens on, or otherwise materially delay, FDA’s ability to engage in routine regulatory and oversight activities such as implementing statutes through rulemaking, issuance of guidance, and review and approval of marketing applications. If these executive actions impose constraints on FDA’s ability to engage in oversight and implementation activities in the normal course, our business may be negatively impacted.
We cannot predict whether future healthcare legislative or policy changes will be implemented at the federal or state level or in countries outside of the United States in which we may do business, or the effect any future legislation or regulation will have on us.
52
Table of Contents
We and our contract manufacturers and our suppliers could be subject to liabilities, fines, penalties or other sanctions under environmental, health and safety laws and regulations if we or they fail to comply with such laws or regulations or otherwise incur costs that could have a material adverse effect on our business.
We currently rely on and expect to continue to rely on third parties for the manufacturing and supply of active pharmaceutical ingredients, or API, and drug products of our product candidates. These third parties are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, transportation, use, storage, treatment and disposal of hazardous materials and wastes. Although we have auditing rights and obligations (according to cGMP regulations for sponsors of clinical trials) with all our CMOs for production of API and drug products, we do not have control over a manufacturer’s or supplier’s compliance with environmental, health and safety laws and regulations. Liabilities they incur pursuant to these laws and regulations could result in significant costs or in certain circumstances, an interruption in operations, any of which could adversely affect our business and financial condition if delayed manufacturing activities impact our clinical development activities.
With respect to any hazardous materials or waste which we are currently, or in the future will be, handling, using, storing or disposing of, we cannot eliminate the risk of contamination or injury from these materials or waste, including at third-party disposal sites. In the event of such contamination or injury, we could be held liable for any resulting damages and liability. We also could incur significant costs associated with civil or criminal fines and penalties for failure to comply with applicable environmental, health and safety laws. In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or production efforts. Failure to comply with these laws and regulations may also result in substantial fines, penalties or other sanctions.
We may not be successful in our efforts to use and expand our lanthipeptide technology platform.
We are using our proprietary lanthipeptide technology platform to generate peptide product candidates that exhibit enhanced stability and selectivity. Our lanthipeptide technology platform has led to one clinical stage product candidate MOR107. We are at a very early stage of development and the lanthipeptide technology platform has not yet, and may never lead to, approved or marketable peptide products, including with respect to MOR107. Even if we are successful in continuing to build our lanthipeptide pipeline, the potential product candidates that we identify may not be suitable for clinical development, including as a result of harmful side effects, limited efficacy or other characteristics that indicate that such products are unlikely to receive marketing approval and achieve market acceptance. If we are not able to successfully develop and commercialize peptide product candidates based upon our lanthipeptide platform, our business, prospects, financial condition and results of operations may be materially adversely affected.
53
Table of Contents
Our internal computer systems, or those of our collaborators or other contractors or consultants, may fail or suffer security breaches, which could result in a material disruption of our product development programs.
Our computer systems and those of our current and any future collaborators and other contractors or consultants are vulnerable to damage from cyber-attacks, computer viruses, natural disasters, terrorism, war and telecommunication and electrical failures. While we have not experienced any such computer system failure, accident or security breach to date, if such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our development programs and our business operations, whether due to a loss of our trade secrets or other proprietary information or other disruptions. For example, the loss of clinical trial data from completed or future clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability, our competitive position could be harmed and the further development and commercialization of our product candidates could be delayed.
Our product candidates for which we intend to seek approval as biologic products may face competition sooner than anticipated.
The ACA, includes a subtitle called the Biologics Price Competition and Innovation Act of 2009, or BPCIA, which created an abbreviated approval pathway for biological products that are biosimilar to or interchangeable with an FDA-licensed reference biological product. Under the BPCIA, an application for a biosimilar product may not be submitted to the FDA until four years following the date that the reference product was first licensed by the FDA. In addition, the approval of a biosimilar product may not be made effective by the FDA until 12 years from the date on which the reference product was first licensed. During this 12-year period of exclusivity, another company may still market a competing version of the reference product if the FDA approves a full BLA for the competing product containing the sponsor’s own pre-clinical data and data from adequate and well-controlled clinical trials to demonstrate the safety, purity and potency of their product. The law is complex and is still being interpreted and implemented by the FDA. As a result, its ultimate impact, implementation, and meaning are subject to uncertainty. While it is uncertain when such processes intended to implement BPCIA may be fully adopted by the FDA, any such processes could have a material adverse effect on the future commercial prospects for our biological products.
One or more of our product candidates approved as a biological product under a BLA may qualify for the 12-year period of exclusivity. However, there is a risk that this exclusivity could be shortened due to congressional action or otherwise, or that the FDA will not consider our product candidates to be reference products for competing products, potentially creating the opportunity for generic competition sooner than anticipated. Other aspects of the BPCIA, some of which may impact the BPCIA exclusivity provisions, have also been the subject of recent litigation. Moreover, the extent to which a biosimilar, once approved, will be substituted for any one of our reference products in a way that is similar to traditional generic substitution for non-biological products is not yet clear, and will depend on a number of marketplace and regulatory factors that are still developing.
54
Table of Contents
We face substantial competition from companies with considerably more resources and experience than we have, which may result in others discovering, developing, receiving approval for or commercializing products before or more successfully than us.
The pharmaceutical and biotechnology industries are characterized by intense competition and significant and rapid technological change as researchers learn more about diseases and develop new technologies and treatments. Any product candidates that we successfully develop and commercialize will compete with existing products and new products that may become available in the future. We have competitors in each of the disease fields in which we research and develop our product candidates, many of which have substantially greater name recognition, commercial infrastructure and financial, technical and personnel resources than we have. Smaller or early-stage companies may also prove to be significant competitors, particularly through partnerships with larger and established companies. Significant competitive factors in our industry include product efficacy and safety, quality and breadth of an organization’s technology, skill of an organization’s employees and its ability to recruit and retain key employees, timing and scope of regulatory approvals, government reimbursement rates for, and the average selling price of, products, the availability of raw materials and qualified manufacturing capacity, manufacturing costs, intellectual property and patent rights and their protection and sales and marketing capabilities. While we believe that our product candidate platform, antibody discovery and development expertise and scientific knowledge provide us with competitive advantages, we face potential competition from many different sources, including major pharmaceutical, specialty pharmaceutical and biotechnology companies, academic institutions and governmental agencies and public and private research institutions. In particular for MOR208, we compete with all companies that have products on the market or are developing product candidates for r/r DLBCL. With regard to our other proprietary or partnered product candidates, we are, alone or in partnerships, for example, developing products to combat diseases such as multiple myeloma, other cancers, atopic dermatitis, psoriasis, Alzheimer’s, where our competitors primarily are comprised of large pharmaceutical companies, including Roche, Celgene, Novartis, Janssen, Gilead, Abbvie and many others. This competition includes a number of alternative therapies to combat such diseases that are being researched and are in various stages of development. Should these therapies prove effective, it could reduce the potential size of the market for our products. Given the intense competition in our industry, we cannot assure you that any of the products that we develop will be clinically superior or scientifically or commercially preferable to products developed or introduced by our competitors.
In addition, significant delays in the development of our product candidates could allow our competitors to succeed in obtaining the FDA, the EMA or other regulatory approvals for their product candidates more rapidly than us, which could place us at a significant competitive disadvantage or deny us marketing exclusivity rights.
Competitors may develop novel products or other technologies that could make our product candidates obsolete or uneconomical. Any of our product candidates that competes with an approved product may need to demonstrate compelling advantages, such as increased efficacy, convenience, pricing, tolerability and/or safety in order to be commercially successful. Any of our product candidates that are approved could also face other competitive factors in the future, including biosimilar competition, which could force us to lower prices or could result in reduced sales. If we fail to respond to this environment by improving our products, by licensing new
55
Table of Contents
third-party products or by developing new product candidates in a timely fashion, or if such new or improved products do not achieve adequate market acceptance, our business, financial condition, results of operations and prospects could be materially and adversely effected.
In addition, many of our competitors have significantly greater financial resources and expertise in R&D, including manufacturing, conducting pre-clinical studies and clinical trials, as well as in obtaining regulatory and reimbursement approvals and marketing and selling products. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of competitors, particularly through partnership arrangements with large established companies. These companies also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient recruitment for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
Our future success depends on our ability to retain key executives and to attract, retain and motivate qualified personnel.
We are highly dependent on the expertise of the members of our research and development team, as well as the other principal members of our management, including Simon Moroney, our Chief Executive Officer, Jens Holstein, our Chief Financial Officer, Malte Peters, our Chief Development Officer, and Markus Enzelberger, our Chief Scientific Officer. Our management board members have fixed-term contracts typically of three years.
Recruiting and retaining qualified management, scientific, clinical, manufacturing and sales and marketing personnel is also critical to our success. The loss of the services of our executive officers or other key employees could impede the achievement of our research, development and commercialization objectives and seriously harm our ability to successfully implement our business strategy. Furthermore, replacing executive officers and key employees may be difficult and may take an extended period of time because of the limited number of individuals in our industry with the breadth of skills and experience required to successfully develop, gain regulatory approval of and commercialize drugs. Competition to hire from this limited pool is intense, and we may be unable to hire, train, retain or motivate these key personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. We also experience competition for the hiring of scientific and clinical personnel from universities and research institutions. Failure to succeed in clinical trials may make it more challenging to recruit and retain qualified scientific personnel.
We may acquire businesses or products, or form strategic alliances, in the future, and we may not realize the benefits of such acquisitions.
We may acquire additional businesses or products, form strategic alliances or create joint ventures with third parties that we believe will complement or augment our existing business. If we acquire businesses with promising products or technologies, we may not be able to realize the benefit of acquiring such businesses if we are unable to successfully integrate them with our existing operations and company culture. We may encounter numerous difficulties in developing, manufacturing and marketing any new products resulting from a strategic alliance or acquisition that delay or prevent us from realizing their expected benefits or enhancing our business. We cannot assure you that, following any such acquisition, we will achieve the expected synergies to justify the transaction. If we are unsuccessful in realizing any of the
56
Table of Contents
benefits following an acquisition, we may incur impairment charges in respect of the assets acquired, which could adversely affect our results of operations.
We may be subject to tax audits or disputes or changes in tax laws.
Pending and future tax audits within our group, disputes with tax authorities and changes in tax law or fiscal regulations could lead to additional tax liabilities. We are subject to routine tax audits by the respective local tax authorities. Any additional tax liability could have an adverse effect on our business, financial condition, results of operations or prospects.
We are subject to currency exchange rate fluctuations.
Due to the international scope of our operations, our assets, earnings and cash flows are influenced by movements in exchange rates of several currencies, particularly the U.S. dollar and the euro. Our functional currency is the euro and the majority of our operating expenses are paid in euro, but we also receive payments from our collaboration partners in U.S. dollars and we regularly acquire services, consumables and materials in U.S. dollars. Further, future revenue will be derived from abroad, particularly from the United States. As a result, our business may be affected by fluctuations in foreign exchange rates between the euro and the U.S. dollar, which may also have a significant impact on our reported results of operations and cash flows from period to period.
Risks Related to the American Depositary Shares and this Offering
There has been no prior market for the ADSs on a U.S. national securities exchange and an active and liquid market for our securities may fail to develop, which could harm the market price of the ADSs.
Prior to this offering, while our shares have been traded on Frankfurt Stock Exchange since 1999, there has been no public market on a U.S. national securities exchange for the ADSs or our shares.
Although the ADSs have been approved for listing on The Nasdaq Global Market, or Nasdaq, an active trading market for the ADSs may never develop or be sustained following this offering. The offering price may not be indicative of the market price of the ADSs or shares after this offering. In the absence of an active trading market for the ADSs or shares, investors may not be able to sell their ADSs at or above the offering price or at the time that they would like to sell.
The price of our ADSs may be volatile and fluctuate due to factors beyond our control.
The price of the securities of publicly traded biopharmaceutical and drug discovery and development companies has been highly volatile and is likely to remain highly volatile in the future. The market price of our ADSs may fluctuate significantly due to a variety of factors, including the following:
| · | positive or negative results of testing and clinical trials by us, our partners or competitors; |
| · | delay in entering into partnerships with respect to the development or commercialization of proprietary product candidates or entry into partnerships on terms that are not deemed favorable to us; |
57
Table of Contents
| · | technological innovations or commercial product introductions by us or competitors; |
| · | changes in government regulation; |
| · | developments concerning proprietary rights, including patents and litigation matters; |
| · | public concerns relating to the commercial value or safety of our product candidates; |
| · | financing or other corporate transactions; |
| · | publication of research reports or comments by securities or industry analysts; |
| · | general market conditions in the pharmaceutical industry or in the economy as a whole; |
| · | foreign exchange rate fluctuations; and |
| · | other events and factors, many of which are beyond our control. |
These and other industry and market factors may cause the market price and demand for our securities to fluctuate substantially, regardless of our actual operating performance, which may limit or prevent investors from readily selling their ADSs and may otherwise negatively affect the liquidity of the ADSs. In addition, the stock market in general, and biopharmaceutical companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies.
You will incur immediate and substantial dilution as a result of this offering.
If you purchase ADSs in this offering, you will incur immediate and substantial dilution of €16.66 ($20.63) per ADS, after giving effect to the sale by us of the 8,300,000 ADSs (representing 2,075,000 ordinary shares) offered by us in the offering, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. Dilution for this purpose represents the difference between the price per ADS paid by new investors and net tangible book value per ADS immediately after the completion of the offering. As a result of the dilution to investors purchasing ADSs in this offering, investors may receive significantly less than the purchase price paid in this offering, if anything, in the event of our liquidation.
We do not currently intend to pay dividends on our securities, and, consequently, your ability to achieve a return on your investment will depend on appreciation in the price of the ADSs.
Although we engage from time to time in share repurchases, we have never declared or paid any dividends on our ordinary shares and do not intend to do so in the foreseeable future, and any share repurchases will likely occur on the Frankfurt Stock Exchange. You are not likely to receive any dividends on your ADSs, and the success of an investment in ADSs will depend upon any future appreciation in its value. Investors may need to sell all or part of their holdings of ADSs after price appreciation, which may never occur, to realize any future gains on their investment. There is no guarantee that the ADSs will appreciate in value or even maintain the price at which our shareholders have purchased the ADSs.
You may not receive distributions on the ordinary shares underlying our ADSs or any value for them if it is illegal or impractical to make them available to holders of ADSs.
The depositary of our ADSs has agreed to pay to you the cash dividends or other distributions, if any, it or the custodian receives on our ordinary shares after deducting any withholding taxes or
58
Table of Contents
other governmental expenses that must be paid and its fees and expenses. You will receive these distributions in proportion to the number of ordinary shares your ADSs represent. However, the depositary is not responsible if it decides that it is unlawful or impracticable to make a distribution available to any holders of ADSs. We have no obligation to take any other action to permit the distribution to ADS holders. This means that you may not receive the distributions we make on our ordinary shares or any value from them if it is unlawful or impractical for us to make them available for you. These restrictions may have a material adverse effect on the value of your ADSs.
The dual listing of our shares and the ADSs following this offering may adversely affect the liquidity and value of the ADSs.
Following this offering and after the ADSs are traded on the Nasdaq, our shares will continue to be listed on the Frankfurt Stock Exchange. Trading of the ADSs or shares in these markets will take place in different currencies (U.S. dollars on the Nasdaq and euros on the Frankfurt Stock Exchange), and at different times (resulting from different time zones, different trading days and different public holidays in the United States and Germany). The trading prices of our shares on these two markets may differ due to these and other factors. Any decrease in the price of our shares on the Frankfurt Stock Exchange could cause a decrease in the trading price of the ADSs on the Nasdaq. Investors could seek to sell or buy our shares to take advantage of any price differences between the markets through a practice referred to as arbitrage. Any arbitrage activity could create unexpected volatility in both our share prices on one exchange, and the shares available for trading on the other exchange. In addition, holders of ADSs will not be immediately able to surrender their ADSs and withdraw the underlying shares for trading on the other market without effecting necessary procedures with the depositary. This could result in time delays and additional cost for holders of ADSs. We cannot predict the effect of this dual listing on the value of our ordinary shares and the ADSs. However, the dual listing of our shares and the ADSs may reduce the liquidity of these securities in one or both markets and may adversely affect the development of an active trading market for the ADSs in the United States. Furthermore, in connection with any distributions of payments under the deposit agreement, the depositary may convert foreign currency itself or through any of its affiliates and, in those cases, acts as principal for its own account and not as an agent, fiduciary or broker on behalf of any other person and earns revenue, including, without limitation, fees and spreads that it will retain for its own account. The depositary makes no representation that the exchange rate used or obtained in any currency conversion will be the most favorable rate that could be obtained at the time or as to the method by which that rate will be determined, subject to its obligations under the deposit agreement.
Future sales, or the perception of future sales, of a substantial number of our shares or ADSs could adversely affect the price of the ADSs, and actual sales of our equity will dilute shareholders and ADS holders.
Future sales of a substantial number of our shares or ADSs, or the perception that such sales will occur, could cause a decline in the market price of the ADSs. As of April 18, 2018, we had 31,495,785 ordinary shares issued (assuming no exercise of the underwriters’ option to purchase additional ADSs). This includes the shares underlying the ADSs offered in this offering, which may be resold in the public market immediately without restriction, unless purchased by our affiliates. If, after the period during which such lock-up agreements restrict sales of the ADSs
59
Table of Contents
and shares, these shareholders sell substantial amounts of shares or ADSs in the public market, or the market perceives that such sales may occur, the market price of the ADSs and our ability to raise capital through an issue of equity securities in the future could be adversely affected.
After the completion of the offering, we may be at an increased risk of securities class action litigation.
Historically, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us because biotechnology and biopharmaceutical companies have experienced significant share price volatility in recent years. If we were to be sued, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business.
Holders of the ADSs are not treated as shareholders of our company.
By participating in this offering you will become a holder of ADSs with underlying shares in a German public company. Holders of the ADSs are not treated as our shareholders, unless they withdraw the shares underlying the ADSs from the depositary. The depositary is the holder of the shares underlying the ADSs. Holders of ADSs therefore do not have any rights as shareholders of our company, other than the rights that they have pursuant to the deposit agreement.
You may not be able to exercise your right to vote the shares underlying your ADSs.
Holders of ADSs may exercise voting rights with respect to the shares represented by the ADSs only in accordance with the provisions of the deposit agreement. You may instruct the depositary to vote the number of whole deposited shares your ADSs represent. The depositary will notify you of shareholders’ meetings or other solicitations of consents and arrange to deliver our voting materials to you if we ask it to. Those materials will describe the matters to be voted on and explain how you may instruct the depositary how to vote. For instructions to be valid, they must reach the depositary by a date set by the depositary.
You may instruct the depositary to vote the shares underlying your ADSs. Otherwise, you will not be able to exercise your right to vote, unless you withdraw the shares underlying the ADSs you hold. We cannot assure you that you will receive the voting materials in time to ensure that you can instruct the depositary to vote your shares. In addition, the depositary and its agents are not responsible for failing to carry out voting instructions or for the manner of carrying out voting instructions provided that any such failure is in good faith. This means that you may not be able to exercise your right to vote and there may be no remedies available to you if the shares underlying your ADSs are not voted as you requested. If we do not instruct the depositary to obtain your voting instructions, you can still instruct the depositary how to vote, and the depositary may vote as you instruct, but it is not required to do so.
Your right as a holder of ADSs to participate in any future preemptive subscription rights issues or to elect to receive dividends in shares may be limited, which may cause dilution to your holdings.
Under German law, the existing shareholders have a preemptive right to subscribe for shares offered in proportion to the amount of shares they hold in connection with any offering of shares. However, a shareholders’ meeting may vote, by a majority, which represents at least
60
Table of Contents
three quarters of the share capital represented at the meeting, to waive this preemptive right provided that, from the company’s perspective, there exists good and objective cause for such waiver.
Certain non-German shareholders may not be able to exercise their preemptive subscription rights in our future offerings due to the legislation and regulations of their home country. For example, ADS holders in the United States will not be entitled to exercise or sell such rights unless we register the rights and the securities to which the rights relate under the Securities Act or an exemption from the registration requirements is available. In addition, the deposit agreement provides that the depositary need not make rights available to you unless the distribution to ADS holders of both the rights and any related securities are either registered under the Securities Act or exempted from registration under the Securities Act. We are under no obligation to file a registration statement with respect to any such rights or securities or to endeavor to cause such a registration statement to be declared effective. Moreover, we may not be able to establish an exemption from registration under the Securities Act. Accordingly, ADS holders may be unable to participate in our rights offerings and may experience dilution in their holdings. In addition, if the depositary is unable to sell rights that are not exercised or not distributed or if the sale is not lawful or reasonably practicable, it will allow the rights to lapse, in which case you will receive no value for these rights.
As a foreign private issuer, we are permitted to adopt certain home country practices in relation to corporate governance matters that differ significantly from Nasdaq corporate governance listing standards. These practices may afford less protection to shareholders than they would enjoy if we complied fully with corporate governance listing standards.
We are a “foreign private issuer”, as defined in the SEC’s rules and regulations. The Nasdaq Listing Rules include certain accommodations in the corporate governance requirements that allow foreign private issuers to follow “home country” corporate governance practices in lieu of the otherwise applicable corporate governance standards of Nasdaq. The application of such exceptions requires that we disclose the Nasdaq Listing Rules that we do not follow and describe the German corporate governance practices we do follow in lieu of the relevant Nasdaq corporate governance standard. If and when the ADSs are listed on Nasdaq, we intend to continue to follow German corporate governance practices in lieu of the corporate governance requirements of Nasdaq in certain respects. In particular, we intend to follow German corporate governance practices in connection with the distribution of annual and interim reports to shareholders, the application of our code of conduct to our supervisory board, proxy solicitation in connection with shareholders’ meetings, and obtaining shareholder approval in connection with the issuance of shares in connection with an acquisition, change of control transactions, the establishment or material amendment to any equity-based compensation plans and the issuance of shares in a private placement in excess of 20% of the outstanding share capital at less than the greater of book or market value. To this extent, our practice varies from the requirements of Nasdaq.
U.S. holders of ADSs may suffer adverse tax consequences if we are characterized as a passive foreign investment company.
A non-U.S. corporation will be classified as a passive foreign investment company, or PFIC, for U.S. federal income tax purposes for any taxable year, if either (i) 75% or more of its gross income for such year consists of certain types of “passive” income or (ii) 50% or more of the
61
Table of Contents
value of its assets (determined on the basis of a quarterly average) during such year produce or are held for the production of passive income. Passive income generally includes dividends, interest, royalties, rents, annuities, net gains from the sale or exchange of property producing such income and net foreign currency gains. In addition, a non-U.S. corporation will be treated as owning its proportionate share of the assets and earning its proportionate share of the income of any other corporation in which it owns, directly or indirectly, more than 25% (by value) of the stock.
Based on certain estimates of our gross income and gross assets, the latter determined by reference to the expected value of the ADSs and shares, we believe that we will not be classified as a PFIC for the taxable year ending December 31, 2018 and we do not expect to be treated as a PFIC in any future taxable year. However, because PFIC status is based on our income, assets and activities for the entire taxable year, which we expect may vary substantially over time, it is not possible to determine whether we will be characterized as a PFIC for any taxable year until after the close of the taxable year. Moreover, we must determine our PFIC status annually based on tests that are factual in nature, and our status in future years will depend on our income, assets and activities in each of those years. There can be no assurance that we will not be considered a PFIC for any taxable year.
If we were to be or become a PFIC for any taxable year during which a U.S. holder (defined below in “Taxation—U.S. Taxation”) holds ADSs, certain adverse U.S. federal income tax consequences could apply to such U.S. holder. See “Taxation—U.S. Taxation—PFIC Rules”.
The interpretation of the treatment of ADSs by the German tax authorities is subject to change
The specific treatment of ADSs under German tax law is based on administrative provisions by the fiscal authorities, which are not codified law and are subject to change. Tax authorities may modify their interpretation and the current treatment of ADSs may change, as the circular issued by the German Federal Ministry of Finance (BMF-Schreiben), dated November 8, 2017, reference number IV C 1 – S 1980-1/16/10010 :10, shows. According to this new circular, ADSs are not treated as capital participation (Kapitalbeteiligung) within the meaning of Section 2 Para. 8 of the Investment Tax Code (Investmentsteuergesetz). Such changes in the interpretation by the fiscal authorities may have adverse effects on the taxation of investors.
We may lose our foreign private issuer status in the future, which could result in significant additional cost and expense.
While we currently qualify as a foreign private issuer, the determination of foreign private issuer status is made annually on the last business day of an issuer’s most recently completed second fiscal quarter.
In the future, we would lose our foreign private issuer status if we to fail to meet the requirements necessary to maintain our foreign private issuer status as of the relevant determination date. Our foreign private issuer status will be tested on June 30 of each year. We expect that we will maintain our status on June 30, 2018, but in the future we may lose that status. This could occur if, for instance, a majority of our shareholders of record were U.S. citizens or residents and a majority of the executive officers or directors were U.S. citizens or residents or if a majority of our assets were located in the U.S.
The regulatory and compliance costs to us under U.S. securities laws as a U.S. domestic issuer may be significantly more than the costs we incur as a foreign private issuer. If we are not a
62
Table of Contents
foreign private issuer, we will be required to file periodic reports and registration statements on U.S. domestic issuer forms with the SEC, which are more detailed and extensive in certain respects than the forms available to a foreign private issuer. We would be required under current SEC rules to prepare our financial statements in accordance with U.S. GAAP rather than IFRS. Such conversion of our financial statements to U.S. GAAP would involve significant time and cost, and we would still be required to prepare financial statements in accordance with IFRS under the rules of the Frankfurt Stock Exchange. In addition, we may lose our ability to rely upon exemptions from certain corporate governance requirements on United States stock exchanges that are available to foreign private issuers such as the ones described above and exemptions from procedural requirements related to the solicitation of proxies.
We are eligible to be treated as an “emerging growth company”, as defined in the JOBS Act, and we cannot be certain if the reduced disclosure requirements applicable to emerging growth companies will make the ADSs less attractive to investors.
We are an emerging growth company, as defined in the JOBS Act. For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including (1) the ability to include only two years of audited financial statements and only two years of related Management’s Discussion and Analysis of Financial Condition and Results of Operations disclosure; (2) an exemption from the auditor attestation requirement in the assessment of our internal control over financial reporting pursuant to the Sarbanes-Oxley Act; (3) to the extent that we no longer qualify as a foreign private issuer, (a) reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements; and (b) exemptions from the requirements of holding a nonbinding advisory vote on executive compensation, including golden parachute compensation; and (4) an exemption from compliance with the requirement that the PCAOB has adopted regarding a supplement to the auditor’s report providing additional information about the audit and the financial statements.
We may take advantage of these provisions for up to five years or such earlier time that we are no longer an emerging growth company. We would cease to be an emerging growth company if we have more than $1.07 billion in annual revenue, have more than $700 million in market value of our shares held by non-affiliates, or issue more than $1.0 billion of nonconvertible debt over a three-year period. We may choose to take advantage of some but not all of these reduced burdens. For example, Section 107 of the JOBS Act provides that an emerging growth company that uses U.S. GAAP for financial reporting can use the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. Given that we currently report and expect to continue to report under IFRS we have irrevocably elected not to avail ourselves of this extended transition period and, as a result, we will adopt new or revised accounting standards on the relevant dates on which adoption of such standards is required by the IASB. We have taken advantage of reduced reporting requirements in this prospectus. Accordingly, the information contained herein may be different than the information you receive from other public companies in which you hold equity securities.
U.S. investors may have difficulty enforcing civil liabilities against our company and members of our supervisory board and management board and the experts named in this prospectus.
We are incorporated under the laws of Germany. The majority of our assets are located outside the United States and all of the members of our management board, five out of six supervisory
63
Table of Contents
board members and all members of our management board reside outside of the United States. As a result, it may not be possible for investors to effect service of process within the United States upon such persons or to enforce against them or us in U.S. courts’ judgments predicated upon the civil liability provisions of the federal securities laws of the United States. Foreign courts may refuse to hear a United States securities law claim because foreign courts may not be the most appropriate forums in which to bring such a claim. Even if a foreign court agrees to hear a claim, it may determine that the law of the jurisdiction in which the foreign court resides, and not U.S. law, is applicable to the claim.
Further, if U.S. law is found to be applicable, the content of applicable U.S. law must be proved as a fact, which can be a time-consuming and costly process, and certain matters of procedure would still be governed by the law of the jurisdiction in which the foreign court resides. We have been advised by Skadden, Arps, Slate, Meagher & Flom LLP, our German counsel, that there is currently no treaty between the United States and Germany providing for reciprocal recognition and enforceability of judgments rendered in connection with civil and commercial disputes and, accordingly, a final judgment rendered by a U.S. court based on civil liability would not be enforceable in Germany as such. However, a U.S. court’s judgment may carry evidentiary value in any proceedings for civil liability brought in the German courts.
The rights of shareholders in a stock corporation subject to German law differ in material respects from the rights of shareholders of corporations incorporated in the United States.
We are a German stock corporation with our registered office in Germany. Our corporate affairs are governed by the laws governing stock corporations incorporated in Germany and our articles of association. The rights of shareholders and the responsibilities of members of our management board (Vorstand) and supervisory board (Aufsichtsrat) may be different from the rights and obligations of shareholders in companies governed by the laws of U.S. jurisdictions. In the performance of their duties, our management board and supervisory board may take into account a broad range of considerations, including our interests, the interests of our shareholders, employees, creditors and, to a limited extent, the general public. It is possible that some of these parties will have interests that are different from, or in addition to, your interests as a holder of ADSs. See “Description of Share Capital—Differences in Corporate Law”.
If we fail to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results or prevent fraud. As a result, shareholders could lose confidence in our financial and other public reporting, which would harm our business and the trading price of our shares.
Effective internal controls over financial reporting are necessary for us to provide reliable financial reports and, together with adequate disclosure controls and procedures, are designed to prevent fraud. Any failure to implement required new or improved controls, or difficulties encountered in their implementation could cause us to fail to meet our reporting obligations. In addition, any testing by us conducted in connection with Section 404 of the Sarbanes-Oxley Act of 2002, or any subsequent testing by our independent registered public accounting firm, may reveal deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses or that may require prospective or retroactive changes to our financial statements, or identify other areas for further attention or improvement. Inferior internal controls could also cause investors to lose confidence in our reported financial information, which could have a negative effect on the trading price of our shares.
64
Table of Contents
For as long as we are an “emerging growth company” under the JOBS Act, our independent registered public accounting firm will not be required to attest to the effectiveness of our internal controls over financial reporting pursuant to Section 404. We could be an “emerging growth company” for up to five years. An independent assessment of the effectiveness of our internal controls could detect problems that our management’s assessment might not. Undetected material weaknesses in our internal controls could lead to financial statement restatements and require us to incur the expense of remediation.
You may be subject to limitations on transfers of your ADSs.
Your ADSs are transferable on the books of the depositary. However, the depositary may close its transfer books at any time or from time to time when it deems expedient in connection with the performance of its duties. In addition, the depositary may refuse to deliver, transfer or register transfers of ADSs generally when our books or the books of the depositary are closed, or at any time if we or the depositary deems it advisable to do so because of any requirement of law or of any government or governmental body, or under any provision of the deposit agreement, or for any other reason.
65
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements concerning our business, operations and financial performance and condition as well as our plans, objectives and expectations for our business operations and financial performance and condition. Any statements that are not of historical facts may be deemed to be forward-looking statements. You can identify these forward-looking statements by words such as “believes”, “estimates”, “anticipates”, “expects”, “plans”, “intends”, “may”, “could”, “might”, “will”, “should”, “aims” or other similar expressions that convey uncertainty of future events or outcomes. Forward-looking statements appear in a number of places throughout this prospectus and include statements regarding our intentions, beliefs, assumptions, projections, outlook, analyses or current expectations concerning, among other things, our intellectual property position, results of operations, cash needs, spending of the proceeds from this offering, financial condition, liquidity, prospects, growth and strategies, the industry in which we operate and the trends that may affect the industry or us.
By their nature, forward-looking statements involve risks and uncertainties because they relate to events, competitive dynamics and industry change, and depend on economic circumstances that may or may not occur in the future or may occur on longer or shorter timelines than anticipated. Although we believe that we have a reasonable basis for each forward-looking statement contained in this prospectus, we caution you that forward-looking statements are not guarantees of future performance and involve known and unknown risks, uncertainties and other factors that are in some cases beyond our control. All of our forward-looking statements are subject to risks and uncertainties that may cause our actual results to differ materially from our expectations. These forward-looking statements include, without limitation, statements about the following:
| · | the timing, progress and results of pre-clinical studies and clinical trials for our product candidates, including statements regarding the timing of initiation and completion of studies or trials and related preparatory work, the period during which the results of the trials will become available and our research and development programs; |
| · | the timing of and our ability to obtain and maintain regulatory approval for our product candidates; |
| · | the proposed clinical development pathway for MOR208 and our other product candidates, and the acceptability of the results of such trials for regulatory approval of such product candidates by the FDA or comparable foreign regulatory authorities; |
| · | our expectations regarding the size of the patient populations for our product candidates, if approved for commercial use; |
| · | our expectations regarding timing for meetings with regulatory agencies; |
| · | our intent regarding the commercialization of MOR208; |
| · | our estimates of our expenses, ongoing losses, future revenue, capital requirements and our needs for or ability to obtain additional financing; |
| · | our ability to identify and develop new product candidates; |
66
Table of Contents
| · | our ability to identify new collaboration partners and successfully enter into new collaboration arrangements; |
| · | our ability to identify, recruit and retain key personnel; |
| · | our and our collaborators’ ability to protect and enforce our intellectual property protection for our proprietary and partnered product candidates, and the scope of such protection; |
| · | our expectations with regard to our future revenues; |
| · | our expectations regarding the future development of MOR202 in MM; |
| · | the development of and projections relating to our competitors or our industry; and |
| · | our expectations regarding the time during which we will be an emerging growth company under the JOBS Act and a foreign private issuer. |
Actual results could differ materially from our forward-looking statements due to a number of factors, including, the risks set forth under the section “Risk Factors” of this prospectus and elsewhere in this prospectus.
Any forward-looking statements that we make in this prospectus speak only as of the date of such statement, and we undertake no obligation to update such statements to reflect events or circumstances after the date of this prospectus or to reflect the occurrence of unanticipated events.
67
Table of Contents
The table below shows the period end, average, high and low exchange rates of U.S. dollars per euro for the periods shown. Average rates are computed by using the noon buying rate of the Federal Reserve Bank of New York for the euro on the last business day of each month during the relevant year indicated and for each business day during the relevant month indicated. The rates set forth below are provided solely for your convenience and may differ from the actual rates used in the preparation of our consolidated financial statements and other financial data included in this prospectus. You should not assume that, on that or any other date, one could have converted these amounts of euro into U.S. dollars at this exchange rate.
Fluctuations in the exchange rates between the euro and the U.S. dollar will affect the U.S. dollar amounts received by owners of our ADSs on conversion of dividends, if any, paid in euro on our ordinary shares. The following table presents the information on the exchange rates between the euro and the U.S. dollar for the periods indicated:
| Year Ended December 31, | High | Low | Average | Period end | ||||||||||||
| 2013 |
1.3816 | 1.2774 | 1.3281 | 1.3779 | ||||||||||||
| 2014 |
1.3927 | 1.2101 | 1.3297 | 1.2101 | ||||||||||||
| 2015 |
1.2015 | 1.0524 | 1.1096 | 1.0895 | ||||||||||||
| 2016 |
1.1516 | 1.0375 | 1.1072 | 1.0552 | ||||||||||||
| 2017 |
1.2041 | 1.0416 | 1.1301 | 1.2022 | ||||||||||||
| Month Ended | High | Low | Average | Period end | ||||||||||||
| September 2017 |
1.2041 | 1.1747 | 1.1913 | 1.1813 | ||||||||||||
| October 2017 |
1.1847 | 1.1580 | 1.1755 | 1.1648 | ||||||||||||
| November 2017 |
1.1936 | 1.1577 | 1.1743 | 1.1898 | ||||||||||||
| December 2017 |
1.2022 | 1.1725 | 1.1836 | 1.2022 | ||||||||||||
| January 2018 |
1.2488 | 1.1922 | 1.2197 | 1.2428 | ||||||||||||
| February 2018 |
1.2482 | 1.2211 | 1.2340 | 1.2211 | ||||||||||||
| March 2018 |
1.2440 | 1.2216 | 1.2334 | 1.2320 | ||||||||||||
On April 18, 2018, the exchange rate for the euro was €1.00 = $1.2381.
68
Table of Contents
Our shares have been trading on the Frankfurt Stock Exchange under the symbol “MOR” since 1999.
The following table sets forth for the periods indicated the reported high and low closing sale prices per ordinary share in Xetra trading in euros on the Frankfurt Stock Exchange.
| (in €) | High | Low | ||||||
| Annual |
||||||||
| 2013 |
61.35 | 29.29 | ||||||
| 2014 |
86.72 | 55.45 | ||||||
| 2015 |
78.65 | 52.52 | ||||||
| 2016 |
56.07 | 33.25 | ||||||
| 2017 |
82.95 | 47.60 | ||||||
| Quarterly |
||||||||
| First Quarter 2016 |
56.07 | 35.00 | ||||||
| Second Quarter 2016 |
51.31 | 33.25 | ||||||
| Third Quarter 2016 |
40.50 | 35.90 | ||||||
| Fourth Quarter 2016 |
48.75 | 38.77 | ||||||
| First Quarter 2017 |
60.38 | 47.60 | ||||||
| Second Quarter 2017 |
66.67 | 51.00 | ||||||
| Third Quarter 2017 |
71.71 | 56.14 | ||||||
| Fourth Quarter 2017 |
82.95 | 71.86 | ||||||
| Monthly |
||||||||
| October 2017 |
79.04 | 71.86 | ||||||
| November 2017 |
82.95 | 76.50 | ||||||
| December 2017 |
81.26 | 74.77 | ||||||
| January 2018 |
87.05 | 77.50 | ||||||
| February 2018 |
82.15 | 72.05 | ||||||
| March 2018 |
87.25 | 77.60 | ||||||
69
Table of Contents
We estimate that we will receive total net proceeds of approximately $189.9 million after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. If the underwriters exercise in full their option to purchase additional ADSs, we estimate that the net proceeds of the offering will be approximately $218.9 million, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
We intend to use the net proceeds from this offering, together with a portion of our cash and cash equivalents, available-for-sale financial assets, and current financial assets classified as loans and receivables (in aggregate of €312.2 million as of December 31, 2017) to continue the development of our proprietary clinical pipeline, initiate pre-commercial and commercial activities and to advance earlier stage product candidates in particular, in order of priority, as follows:
| · | Approximately $225 million for the continuation and further development of MOR208, our wholly-owned anti-CD19 antibody currently being evaluated in r/r DLBCL (a) in combination with lenalidomide for patients ineligible for HDCT and ASCT, (b) in combination with bendamustine for patients ineligible for HDCT and ASCT, and (c) in CLL in combination with either idelalisib or venetoclax in patients who have relapsed after prior therapy with a Bruton’s tyrosine kinase inhibitor; |
| · | Approximately $90 million to build our commercial capabilities in connection with the potential future approval of MOR208; |
| · | Approximately $20 million for the financing of clinical development of MOR202 (in MM and in NSCLC in combination with a checkpoint inhibitor) and approximately $30 million for the clinical development of MOR106 (in atopic dermatitis); and |
| · | Approximately $45 million for discovery programs and the development of our technology platforms. |
We expect to use the remainder of any net proceeds from the offering as well as existing cash and cash equivalents for general corporate purposes. We may also use a portion of the net proceeds to in-license, acquire or invest in complementary technologies, products, businesses or assets, either alone or together with a collaboration partner. However, we have no current plans, commitments or obligations to do so.
With the net proceeds from this offering as well as our other available sources of liquidity, we expect (a) in respect of MOR208, to be able to complete BLA submissions for our L-MIND and B-MIND trials and to reach primary completion in our L-MIND study, (b) in respect of MOR202, to conduct a Phase 1/2 trial in NSCLC and to complete the current Phase 1/2a in MM, and (c) in respect of MOR106, to conduct a Phase 2 trial in AD. Our expected use of the net proceeds from this offering represents our current expectations of our funding needs in respect of those uses through 2020, based upon our present plans, business conditions and expectations for further clinical development, each of which could change in the future as our plans and business conditions evolve. After 2020, we expect that there will be continued clinical trial costs associated with MOR202 and our earlier stage product candidates, in particular MOR106, as well as costs associated with the commercialization of MOR208, though we cannot estimate these
70
Table of Contents
costs with certainty. As of the date of this prospectus, we cannot predict with certainty all of the particular uses for the net proceeds to be received upon the completion of this offering or the amounts that we will specifically spend on the uses set forth above. The amounts and timing of our actual expenditures depend on numerous factors, including the ongoing status of and results from our clinical trials and pre-clinical studies, our ability to obtain regulatory approvals in respect of our product candidates, changes in the competitive landscape, management decisions to work together with collaboration partners, the level and timing of milestones and royalty payments received, and any unforeseen cash needs. Please see also “Risk Factors” and “Special Note Regarding Forward-Looking Statements”. As a result, our management will have broad discretion in applying the net proceeds of this offering and investors will be relying on our judgment regarding the application of the net proceeds of this offering. In addition, we might decide to postpone or not pursue other clinical trials or pre-clinical activities if the net proceeds from this offering and our other sources of cash are less than expected.
Pending their use, we intend to invest the net proceeds from this offering in a variety of capital preservation investments, including money market deposits and investment funds that will be designated as financial assets at fair value through profit and loss in our consolidated statement of financial position.
71
Table of Contents
We have not paid any dividends on our ordinary shares since our inception, and we currently intend to retain any future earnings to finance the growth and development of our business. Therefore, we do not anticipate that we will declare or pay any cash dividends in the foreseeable future. Except as required by law, any future determination to pay cash dividends will be at the discretion of our management board and supervisory board and will be dependent upon our financial condition, results of operations, capital requirements, and other factors our management board and supervisory board deem relevant.
All of the shares represented by the ADSs offered by this prospectus will generally have the same dividend rights as all of our other outstanding shares. However, the depositary may limit distributions based on practical considerations and legal limitations. See “Description of American Depositary Shares—Dividends and Other Distributions”. Any distribution of dividends proposed by our management and supervisory boards requires the approval of our shareholders in a shareholders’ meeting. See “Description of Share Capital—Dividend Rights”, which explains in more detail the procedures we must follow and the German law provisions that determine whether we are entitled to declare a dividend.
For information regarding the German withholding tax applicable to dividends and related United States refund procedures, see “Taxation—German Taxation—German Taxation of Holders of ADSs”.
72
Table of Contents
The following table sets forth our cash, cash equivalents and other financial assets and capitalization on a consolidated basis as of December 31, 2017:
| · | on an actual basis, and |
| · | on an as adjusted basis to give effect to the issuance and sale of 8,300,000 ADSs representing 2,075,000 ordinary shares in this offering by us at an initial public offering price of $25.04 per ADS, after deducting underwriting discounts and commissions and estimated offering expenses payable by us, as set forth under “Use of Proceeds” and excluding the underwriters’ option to purchase additional ADSs. |
Investors should read this table together with our consolidated financial statements, including the notes thereto, included in this prospectus, as well as “Use of Proceeds”, “Selected Consolidated Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations”.
| As of December 31, 2017 | ||||||||
| (in € thousands) | Actual | As adjusted(1) | ||||||
| Cash, cash equivalents and other financial assets(2) |
312,187 | 465,565 | ||||||
|
|
|
|||||||
| Borrowings (long and short term) |
— | — | ||||||
| Stockholders’ Equity |
||||||||
| Common Stock |
29,421 | 31,496 | ||||||
| Treasury Stock, at Cost |
(11,827 | ) | (11,827 | ) | ||||
| Additional Paid-in Capital |
438,558 | 589,861 | ||||||
| Revaluation Reserve |
(105 | ) | (105 | ) | ||||
| Accumulated Income/(Deficit) |
(97,375 | ) | (97,375 | ) | ||||
|
|
|
|||||||
| Total Stockholders’ Equity |
358,671 | 512,050 | ||||||
|
|
|
|||||||
| Total Capitalization |
358,671 | 512,050 | ||||||
| (1) | The as adjusted information is presented for informational purposes only and is not necessarily indicative of what our financial position and results would have been had these transactions actually occurred at such date nor is it indicative of our future financial position or performance. |
| (2) | Includes cash and cash equivalents (€76,589 thousand as of December 31, 2017), available-for-sale financial assets (€86,538 thousand as of December 31, 2017), and current financial assets classified as loans and receivables (€149,059 thousand as of December 31, 2017). |
73
Table of Contents
If you invest in the ADSs, your ownership interest will be diluted to the extent of the difference between the initial public offering price per ADS paid by purchasers of the ADSs and the pro forma as adjusted net tangible book value per ADS immediately after the completion of this offering. As of December 31, 2017, we had a net tangible book value of €290.8 million, corresponding to a net tangible book value of €9.99 per ordinary share or €2.50 per ADS based on an ordinary share to ADS ratio of 1 to 4. Historical net tangible book value per ordinary share represents the amount of our total assets less our total liabilities, excluding goodwill and other intangible assets, divided by the total number of our ordinary shares outstanding at December 31, 2017. Historical net tangible book value per ADS represents the amount of our total assets less our total liabilities, excluding goodwill and other intangible assets, divided by the total number of our ordinary shares outstanding at December 31, 2017 converted to ADS at a ratio of 1 to 4.
After giving effect to the issuance and sale of 8,300,000 ADSs (representing an aggregate of 2,075,000 ordinary shares) at an offering price of $25.04 per ADS, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us, our pro forma as adjusted net tangible book value as of December 31, 2017 would have been approximately €444.2 million (or $550.0 million), representing €14.25 (or $17.64) per ordinary share outstanding or €3.56 (or $4.41) per ADS. This represents an immediate increase in pro forma as adjusted net tangible book value of €4.25 (or $5.27) per ordinary share outstanding or €1.06 (or $1.32) per ADS to existing shareholders and an immediate dilution in net tangible book value of €66.65 (or $82.52) per ordinary share outstanding or €16.66 (or $20.63) per ADS to new investors purchasing ADSs in this offering. Dilution for this purpose represents the difference between the price per ADS paid by these purchasers and the pro forma as adjusted net tangible book value per ADS immediately after the completion of this offering.
The following table illustrates this dilution to new investors purchasing ADSs in the offering, assuming either no exercise or full exercise of the underwriters’ option to purchase additional ADSs:
| No exercise | Full exercise | |||||||||||||||
| (in €) | (in $) | (in €) | (in $) | |||||||||||||
| Initial public offering price per ADS |
20.22 | 25.04 | 20.22 | 25.04 | ||||||||||||
| Historical net tangible book value as of December 31, 2017 per ADS |
2.50 | 3.09 | 2.50 | 3.09 | ||||||||||||
| Increase in net tangible book value attributable to investors purchasing ADSs in this offering |
1.06 | 1.32 | 1.21 | 1.50 | ||||||||||||
| Pro forma as adjusted net tangible book value as of December 31, 2017 per ADS |
3.56 | 4.41 | 3.71 | 4.60 | ||||||||||||
| Dilution to new investors per ADS |
16.66 | 20.63 | 16.51 | 20.44 | ||||||||||||
| Dilution to new investors per ordinary share outstanding (on ADS basis) |
66.65 | 82.52 | 66.05 | 81.77 | ||||||||||||
74
Table of Contents
The following table summarizes on a pro forma as adjusted basis, as of December 31, 2017, the differences between the shareholders as of December 31, 2017, and the new investors with respect to the number of ordinary shares and ADSs purchased from us (using an ordinary share to ADS ratio of 1 to 4), the total consideration paid (for existing shareholders, translated into US dollars at $1.2381 per euro) and the average price per ordinary share paid by existing shareholders (translated into US dollars at $1.2381 per euro) and by investors participating in this offering at an initial public offering price of $25.04 per ADS after deducting the underwriting discounts and commissions and estimated offering expenses payable by us and excluding the underwriters’ option to purchase additional ADSs:
| ADS Purchased | Ordinary Shares Purchased |
Total Consideration | ||||||||||||||||||||||||||||||
| Number | Percent | Number | Percent | Amount (in million) |
Percent | Average Price per Share |
Average Price per ADS |
|||||||||||||||||||||||||
| Existing shareholders |
116,404,428 | 93.3 | % | 29,101,107 | 93.3 | % | $ | 538.1 | 73.9 | % | $ | 18.49 | $ | 4.62 | ||||||||||||||||||
| New investors |
8,300,000 | 6.7 | % | 2,075,000 | 6.7 | % | $ | 189.9 | 26.1 | % | $ | 91.52 | $ | 22.88 | ||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||||||
| Total |
124,704,428 | 100.0 | % | 31,176,107 | 100.0 | % | $ | 728.0 | 100.0 | % | ||||||||||||||||||||||
If the underwriters exercise their option to purchase additional ADSs in full, our existing shareholders would own 29,101,107 ordinary shares, or 92.4%, in the aggregate, and our new investors would own 2,386,250 ordinary shares, or 7.6%, in the aggregate.
The number of our ordinary shares to be outstanding after this offering is based on the number of ordinary shares outstanding as of December 31, 2017, and excludes any ordinary shares that may be reserved for future issuance under our convertible bonds and performance share and stock option plans. See “Management—Management Board—Share-Based Incentive Plans”.
To the extent we grant options or other equity awards to our employees or members of our management board in the future, and those options or other equity awards are exercised in the future or other issuances of our ordinary shares are made, there will be further dilution to new investors.
75
Table of Contents
SELECTED CONSOLIDATED FINANCIAL DATA
The financial data as of and for the years ended December 31, 2016 and 2017, have been derived from our audited consolidated financial statements and the related notes, which are included elsewhere in this prospectus and which have been prepared in accordance with IFRS.
The financial data presented below are not necessarily indicative of the financial results to be expected for any future periods. The financial data below do not contain all the information included in our financial statements. You should read this information in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations”, and our consolidated financial statements and related notes, each included elsewhere in this prospectus.
The following tables also contain translations of euro amounts into U.S. dollars for amounts presented as of and for the year ended December 31, 2017. These translations are solely for convenience of the reader and were calculated at the rate of €1.00 per $1.2022, which equals the noon buying rate of the Federal Reserve Bank of New York on December 29, 2017. You should not assume that, on that or any other date, one could have converted these amounts of euro into U.S. dollars at this exchange rate.
Consolidated Statement of Income Data:
| Year ended December 31, | ||||||||||||
| 2016 | 2017 | |||||||||||
| (in €) | (in €) | (in $) | ||||||||||
| (in thousands except per share data) | ||||||||||||
| Revenues |
49,744 | 66,791 | 80,296 | |||||||||
| Operating Expenses |
||||||||||||
| Research and Development |
(95,723 | ) | (116,809 | ) | (140,428 | ) | ||||||
| General and Administrative |
(14,116 | ) | (17,039 | ) | (20,484 | ) | ||||||
| Total Operating Expenses |
(109,839 | ) | (133,848 | ) | (160,912 | ) | ||||||
| Other Income |
709 | 1,120 | 1,346 | |||||||||
| Other Expenses |
(554 | ) | (1,671 | ) | (2,009 | ) | ||||||
| Earnings before Interest and Taxes (EBIT) |
(59,941 | ) | (67,608 | ) | (81,278 | ) | ||||||
| Finance Income |
1,385 | 712 | 856 | |||||||||
| Finance Expenses |
(1,308 | ) | (1,895 | ) | (2,278 | ) | ||||||
| Income Tax Expenses |
(519 | ) | (1,036 | ) | (1,245 | ) | ||||||
| Consolidated Net Profit/(Loss) |
(60,383 | ) | (69,827 | ) | (83,946 | ) | ||||||
| Earnings per Share, basic and diluted(1) |
(2.28 | ) | (2.41 | ) | (2.90 | ) | ||||||
| Shares Used In Computing Earnings per Share (in units), basic and diluted(1) |
26,443,415 | 28,947,566 | n/a | |||||||||
| Dividends Declared per Share |
— | — | — | |||||||||
| (1) | Basic and diluted Earnings per Share are the same in each of the years ended December 31, 2016 and 2017, because the assumed exercise of outstanding stock options and convertible bonds would be anti-dilutive due to our consolidated net loss in the respective period. |
76
Table of Contents
Consolidated Balance Sheet Data:
| As of December 31, | ||||||||||||
| 2016 | 2017 | |||||||||||
| (in €) | (in €) | (in $) | ||||||||||
| (in thousands) | ||||||||||||
| Cash and Cash Equivalents |
73,929 | 76,589 | 92,075 | |||||||||
| Available-for-sale Financial Assets |
63,362 | 86,538 | 104,036 | |||||||||
| Bonds, Available-for-sale |
6,532 | — | — | |||||||||
| Financial Assets classified as Loans and Receivables (current and non-current) |
215,630 | 149,059 | 179,199 | |||||||||
| Total Current Assets |
308,056 | 340,681 | 409,567 | |||||||||
| Total Assets |
463,600 | 415,398 | 499,391 | |||||||||
| Total Liabilities |
48,140 | 56,727 | 68,197 | |||||||||
| Total Stockholders’ Equity |
415,460 | 358,671 | 431,194 | |||||||||
77
Table of Contents
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of the financial condition and results of operations of the Company in conjunction with the section entitled “Selected Consolidated Financial Data” and the consolidated financial statements and the related notes thereto included elsewhere in this prospectus. In addition to historical financial information, the following discussion contains forward-looking statements that reflect our plans, estimates and opinions. Our actual results could differ materially from those discussed in these forward-looking statements. Factors that could cause or contribute to these differences or cause our actual results or the timing of selected events to differ materially from those anticipated in these forward-looking statements include those set forth under “Risk Factors”, “Special Note Regarding Forward-Looking Statements” and elsewhere in this prospectus.
Overview
We are a late-stage biopharmaceutical company devoted to the development of innovative and differentiated therapies for patients suffering from serious diseases. Based on our proprietary technology platforms and leadership in the field of therapeutic antibody discovery, generation and engineering, we, together with our partners, have participated in the development of more than 100 therapeutic product candidates. Our broad pipeline spans two business segments: Proprietary Development, in which we invest in and develop product candidates, and Partnered Discovery, in which we generate product candidates for our partners in the pharmaceutical and biotechnology industries against targets identified by our partners. We currently have 28 product candidates in clinical development, including our most advanced proprietary product candidate, MOR208, for the treatment of relapsed or refractory diffuse large B cell lymphoma, or r/r DLBCL. We believe our pipeline of novel and differentiated product candidates has the potential to treat serious diseases and improve the lives of patients.
Our late-stage and most advanced proprietary product candidate, MOR208, received breakthrough therapy designation, or BTD, from the U.S. Food and Drug Administration, or the FDA, in 2017 in combination with lenalidomide for the treatment of patients with r/r DLBCL, who are not eligible for high-dose chemotherapy and autologous stem cell transplantation. We hold worldwide rights to develop and market MOR208. Also in 2017, Tremfya®, developed by our partner Janssen, became the first therapeutic antibody based on our proprietary technology to reach the market. Tremfya®, which is approved to treat moderate-to-severe plaque psoriasis, was launched in the United States in July 2017 and received approval for marketing in the European Union and Canada in November 2017.
Based on our heritage as an antibody discovery and development company, we have a large and diverse pipeline, composed of both proprietary and partnered programs, in multiple therapeutic areas and across all development phases. The combination of our technology platforms and antibody expertise has allowed us to generate promising product candidates and enter into multiple strategic collaborations with leading global pharmaceutical and biotechnology companies. These collaborations provide us with an additional funding source and allow us to leverage our collaborators’ expertise to advance the development of our proprietary product candidates.
78
Table of Contents
Financially, our goal is to maximize our portfolio’s full value by investing in proprietary product candidates while maintaining financial discipline and strict cost controls to increase enterprise value. Our business performance is influenced by factors such as license and milestone payments, research and development expenses, other operating cash flows, existing liquidity resources, expected cash inflows and working capital. As of December 31, 2017, we had cash and cash equivalents, available-for-sale financial assets, and current financial assets classified as loans and receivables of €312.2 million.
In recent years, we have incurred significant operating losses due to substantial research and development expenses incurred for the development of our proprietary product candidates. None of our proprietary product candidates have been approved. We have only generated revenues from collaboration agreements and have never generated any revenue from proprietary product sales. Our ability to generate revenue sufficient to achieve profitability depends significantly upon the successful development and eventual commercialization of one or more of our proprietary or partnered product candidates, which may never occur. In 2017, we incurred earnings before finance income, finance expenses, and income taxes of negative €67.6 million, and as of December 31, 2017, we had an accumulated deficit of €97.4 million.
We expect our expenses to increase substantially in connection with our continuing development activities related to our pre-clinical and clinical programs. We anticipate that our costs will increase substantially if and as we:
| · | continue to fund the B-MIND, L-MIND and COSMOS studies for our lead product candidate MOR208; |
| · | build our commercial capabilities in connection with the potential future approval of MOR208; |
| · | continue to develop MOR202, potentially in one or more oncology indications either alone or together with a partner; |
| · | develop our earlier stage product candidates, in particular MOR106; |
| · | seek to invest in and enhance our technology platform; |
| · | seek to discover and develop additional product candidates; |
| · | seek regulatory approval for any product candidates that successfully complete clinical trials; |
| · | obtain, maintain, expand and protect our intellectual property portfolio, including litigation costs associated with the assertion of our intellectual property rights and the defense against alleged patent or other intellectual property infringement claims; |
| · | add clinical, scientific, operational, financial and management information systems and personnel, including personnel to support our product development and potential future commercialization efforts; |
| · | experience any delays or encounter any issues with respect to any of the above, including failed studies, ambiguous trial results, safety issues or other regulatory challenges; and |
| · | operate as a publicly listed company in the United States. |
As a result, we will need additional financing to support our continuing operations. Until such time as we can generate significant revenue from own product sales, if ever, we expect to
79
Table of Contents
finance our operations through a combination of public or private equity or debt financings or other sources, which may include collaborations with third parties as well as royalties and milestones from partnered programs. Adequate additional financing may not be available to us on acceptable terms, or at all. Our inability to raise capital as and when needed would have a negative impact on our financial condition and our ability to pursue our business strategy.
Segments
We operate two reportable segments:
| · | Proprietary Development—The Proprietary Development segment focuses on developing therapeutic antibodies and peptides developed using our proprietary technology platforms and candidates in-licensed from other companies. During clinical development, we determine whether and at which point we may pursue a partnership for later development and commercialization. The product candidate can then be either completely out-licensed or developed further in cooperation with a pharmaceutical or biotechnology company (co-development). Projects may be developed on a proprietary basis until they are ready for commercialization. In selected cases, we intend to retain certain geographic rights and commercialize products in these areas on our own while seeking partners for commercialization elsewhere. |
| · | Partnered Discovery—In the Partnered Discovery segment, we generate antibody candidates for partners in the pharmaceutical and biotechnology industries. We receive contractual payments including license fees for technologies and funded research, as well as success-based milestone payments and royalties on product sales. The funds generated from these partnerships support our long-term business model and help fund our proprietary development activities. |
Key Factors Affecting Results of Operations
| · | Research and development expenses—Our ability to successfully develop innovative product candidates will be the primary factor affecting our future growth and development. Developing novel product candidates requires a significant investment of resources over a prolonged period of time, and a core part of our strategy is to continue making sustained investments in this area. We currently have five Proprietary Development product candidates under clinical investigation. For more information on these product candidates, see “Business—Proprietary Development”. |
All of the product candidates in our Proprietary Development segment are still in development, and we have incurred and will continue to incur significant research and development costs for pre-clinical studies and clinical trials. We expect that our research and development expenses will continue to constitute the substantial majority of our expenses in future periods in line with the advance and expansion of the development of our product candidates.
We have historically been able to fund the research and development expenses from a variety of sources, including cash flows generated from our Partnered Discovery segment, milestone payments from our partnerships in our Proprietary Development segment and proceeds received from capital increases.
80
Table of Contents
If completed, the net proceeds to us from this offering will also be an important source of funds for our research and development. For more information on the nature of the intended uses for the proceeds from this offering, see “Use of Proceeds”.
| · | Our and our collaborators’ ability to commercialize our product candidates—Our ability to generate revenue from our product candidates depends on our and our collaborators’ ability to successfully complete clinical trials for our product candidates and receive regulatory approval, particularly in the United States, Europe, and other major markets. |
We believe that our broad portfolio of product candidates with both novel and validated targets enhances the likelihood that our research and development efforts will yield successful product candidates. Nonetheless, we cannot be certain if any of our product candidates will receive regulatory approvals. Even if such approvals are granted, we will thereafter need to establish manufacturing and supply arrangements and engage in extensive marketing prior to generating any revenue from such products, and the ultimate commercial success of our products will depend on their acceptance by patients, the medical community and third-party payors and their ability to compete effectively with other therapies on the market.
The competitive environment is also an important factor with the commercial success of our product candidates, and our ability to successfully commercialize a product candidate will depend on whether there are competing product candidates being developed or already marketed by other companies.
| · | Our collaborations—Our results of operations have been, and we expect them to continue to be, affected by our collaborations with third parties for the development and commercialization of certain of our product candidates. In 2016 and 2017, our results of operations were significantly affected by our collaboration agreements with Janssen, Novartis, I-Mab Biopharma and Pfizer. Our collaboration partners have historically covered all or a significant portion of our research and development costs for product candidates developed in collaboration with them. In many of our collaboration arrangements, our research and development obligations have ceased, including in respect of our former collaboration with Novartis and our obligations under our agreement with Janssen. Generally, we receive upfront payments upon our entry into a collaboration agreement and milestone payments upon the achievement of certain development, regulatory or commercial milestones for the relevant product candidate. Nearly all of our revenue in 2016 and 2017 was derived from licensing fees, service fees and milestone payments. We are also eligible to receive royalty payments (generally a mid single-digit to low-teens percentage rate) upon commercial sale of products. |
Following the conclusion of our partnership with Novartis, we expect our future revenues to depend mainly on product sales and royalty payments to us on such product sales. Revenues from funded research and license fee payments are expected to be significantly negatively affected in 2018 by the conclusion of this collaboration, which will in turn have a significant negative impact on our future revenues.
Key Components of Results of Operations
Revenues
Our revenue currently consists of license fees, including royalties, milestone payments, and service fees.
81
Table of Contents
License fees consist of non-refundable fees for providing access to technologies and fees for the use of technologies as well as upfront payments made to us in connection with collaboration and license agreements and royalties paid on net sales of licensed products.
We receive milestone payments under our collaboration agreements, upon the occurrence of meeting certain milestones as set forth in the respective collaboration agreement. Generally, milestone payments are achieved upon meeting certain clinical trial phases and on obtaining final approval of a specified therapeutic.
We receive service fees for the performance of research and development activities.
Research and Development Expenses
Research and development expenses consist of expenses incurred in performing research and development activities. These expenses include conducting research experiments, pre-clinical and clinical studies, and other indirect expenses in support of advancing our product candidates. The following items are included in research and development expenses:
| · | employee-related expenses such as salaries and benefits (including share-based payment expense); |
| · | amounts paid to vendors and suppliers for laboratory supplies; |
| · | research and development-related maintenance and travel expenses; |
| · | amortization, depreciation and other costs of tangible and intangible assets (such as impairment charges for intangible assets as well as patent and license costs); |
| · | costs to file and protect intellectual property; |
| · | fees paid to consultants, subcontractors, CROs, CMOs and other third-party vendors for work performed under our clinical trials and pre-clinical studies; and |
| · | depreciation and other costs for infrastructure (such as depreciation of office and laboratory equipment and furniture and fixtures as well as rent and utilities). |
Our research and development expenses may vary from period to period based on the timing of our research and development activities, including the timing of initiation of clinical trials and enrolment of patients in clinical trials. Research and development expenses are expected to continue to constitute the substantial majority of our expenses as we advance the clinical development of our current product candidates.
General and Administrative Expenses
General and administrative expenses consist of personnel expenses (including share-based payment expenses), consumable supplies, general and administrative related maintenance and travel expenses, amortization of intangible assets (such as software), expenses for external services, depreciation and other costs for infrastructure.
Income Tax Expense
MorphoSys AG and our German subsidiary Sloning BioTechnology GmbH are subject to German corporate taxes, the solidarity surcharge and trade taxes. In 2017, the overall tax rate of the German entities comprises corporate income tax of 15%, a solidarity surcharge of 5.5% on the corporate tax and an average trade tax of 10.85%.
82
Table of Contents
The Dutch entities Lanthio Pharma B.V. and LanthioPep B.V. are subject to an income tax rate of 25% on annual income exceeding €200,000, and annual income below €200,000 is subject to a tax rate of 20%. Subject to certain conditions, a tax rate of 5% may be applicable under what is known as the “Innovation Box”.
Critical Accounting Policies and Significant Judgments and Estimates
Revenue Recognition
Under IAS 18.9, revenues are measured at the fair value of the consideration received or receivable. In accordance with IAS 18.20b, revenues are recognized only to the extent that it is sufficiently probable that we will receive the economic benefits associated with the transaction.
Discounts that are likely to be granted and whose amount can be reliably determined are recognized as a reduction in revenue at the time of revenue recognition. The timing of the transfer of risks and rewards varies depending on the terms of the sales contract. In accordance with IAS 18.21 and 18.25, revenue from multiple-component contracts is recognized by allocating the total consideration to the separately identifiable components based on their respective fair values and by applying IAS 18.20. The applicable revenue recognition criteria are assessed separately for each component.
License fees and milestone payments
Revenues related to non-refundable fees for providing access to technologies, fees for the use of technologies and license fees are recognized on a straight-line basis over the period of the agreement unless a more appropriate method of revenue recognition is available. The period of the agreement usually corresponds to the contractually agreed term of the research project or, in the case of contracts without an agreed project term, management’s expected term of the collaboration. If all IAS 18.14 criteria are met, revenue is recognized immediately and in full. Revenues from milestone payments are recognized upon achievement of certain contractual criteria.
Service fees
Service fees from research and development collaborations are recognized in the period the services are provided.
Share-Based Payments
We apply the provisions under IFRS 2 “Share-based Payment”, which require us to spread compensation expenses from the estimated fair values of share-based payments on the reporting date over the period in which the beneficiaries provide the services which triggered the granting of the share-based payments.
IFRS 2 “Share-based Payment” requires the consideration of the effects of share-based payments if we acquire goods or services in exchange for shares or stock options (“settlement in equity instruments”) or other assets that represent the value of a specific number of shares or stock options (“cash settlement”). The key impact of IFRS 2 on us is the expense resulting from the use of an option pricing model in relation to share-based incentives for employees and the management board.
83
Table of Contents
Impairments
Non-derivative financial instruments
A financial instrument not carried at fair value through profit or loss is assessed at each reporting date to determine if there is objective evidence for impairment. A financial instrument is impaired if objective evidence indicates that an event has occurred after the initial recognition of the asset that could result in a loss and whether that event could have a negative effect on the asset’s estimated future cash flows, which can be assessed reliably.
Objective evidence that financial instruments are impaired can include the default or delinquency of a debtor, indications that a debtor or issuer will enter insolvency, adverse changes in the payment status of borrowers or issuers in our group as well as economic conditions that correlate with defaults or the disappearance of an active market for a marketable security. A significant or prolonged decline in a financial instrument’s fair value below its acquisition cost is objective evidence of impairment.
Receivables
We consider evidence of the impairment of receivables on an individual level. All individually significant receivables are tested specifically for impairment.
For a financial instrument measured at amortized cost less impairment, impairment is calculated as the difference between its carrying amount and the present value of the estimated future cash flows. Cash flows are discounted at the asset’s initial effective interest rate. Losses are recognized in profit or loss and reflected in an allowance account against receivables. When a subsequent event (e.g., repayment by a debtor) causes the amount of impairment to decrease, the impairment is reversed through profit and loss.
Available-for-sale financial assets
In case of objective indications, impairment of available-for-sale financial assets is recognized by reclassifying the accumulated losses from the revaluation reserve in equity to profit and loss. The amount of the accumulated loss to be reclassified from equity to profit and loss is the difference between the acquisition cost less amortization and any principal repayment and the current fair value less any impairment previously recognized in profit or loss. Impairment losses recognized in profit and loss for an investment in a financial instrument classified as available-for-sale are not reversed through profit and loss. If in a subsequent period the fair value of an impaired available-for-sale debt instrument increases and this increase can be objectively linked to an event occurring after the impairment was recognized in profit or loss, then the impairment loss is reversed, and the amount of the reversal is recognized in profit or loss.
Non-financial assets
The carrying amounts of our non-financial assets and inventories are reviewed at each reporting date for any indication of impairment. The non-financial asset’s recoverable amount and inventories’ net realizable value is estimated if such indication exists. For goodwill and intangible assets that have indefinite useful lives or are not yet available for use, the recoverable amount is estimated at the same time each year, or on an interim basis, if required. Impairment is recognized if the carrying amount of an asset or the cash-generating unit, or CGU, exceeds its estimated recoverable amount.
84
Table of Contents
The recoverable amount of an asset or CGU is the greater of its value-in-use or its fair value less costs of disposal. In assessing value-in-use, the estimated future pre-tax cash flows are discounted to their present value using a pre-tax discount rate that reflects current market assessments of the time value of money and the risks specific to the asset or CGU. For the purposes of impairment testing, assets that cannot be tested individually are grouped into the smallest group of assets that generates cash flows from ongoing use that are largely independent of the cash flows of other assets or CGUs. A ceiling test for the operating segment must be carried out for goodwill impairment testing. CGUs that have been allocated goodwill are aggregated so that the level at which impairment testing is performed reflects the lowest level at which goodwill is monitored for internal reporting purposes. Goodwill acquired in a business combination may be allocated to groups of CGUs that are expected to benefit from the combination’s synergies.
Our corporate assets do not generate separate cash flows and are utilized by more than one CGU. Corporate assets are allocated to CGUs on a reasonable and consistent basis and are tested for impairment as part of the impairment testing of the CGU that was allocated the corporate asset.
Impairment losses are recognized in profit and loss. Goodwill impairment cannot be reversed. For all other assets, impairment recognized in prior periods is assessed on each reporting date for any indications that the losses decreased or no longer exist. Impairment is reversed when there has been a change in the estimates used to determine the recoverable amount. Impairment losses can only be reversed to the extent that the asset’s carrying amount does not exceed the carrying amount net of depreciation or amortization that would have been determined if an impairment had not been recognized.
Fair Value Measurements
Fair value is measured in accordance with IFRS 13 “Fair Value Measurement” by using the same assumptions and taking into account the same characteristics of the asset or liability as would an independent market participant. Fair value is a market-based, not an entity-specific measurement. The fair value of non-financial assets is based on the best use of the asset by a market participant. For financial instruments, the use of bid prices for assets and ask prices for liabilities is permitted but not required if those prices best reflect the fair value in the respective circumstances. For simplification, mean rates are also permitted. Thus, IFRS 13 not only applies to financial assets but all assets and liabilities.
We use the following hierarchy for determining and disclosing the fair value of financial instruments:
| Level 1: | Quoted (unadjusted) prices in active markets for identical assets or liabilities to which the Company has access. |
| Level 2: | Inputs other than quoted prices included within Level 1 that are observable for the assets or liabilities, either directly (i.e., as prices) or indirectly (i.e., derived from prices). |
| Level 3: | Inputs for the asset or liability that are not based on observable market data (that is, unobservable inputs). |
85
Table of Contents
Deferred Taxes
The calculation of deferred taxes is based on the balance sheet liability method that refers to the temporary differences between the carrying amounts of assets and liabilities and the amounts used for taxation purposes. The method of calculating deferred taxes depends on how the asset’s carrying amount is expected to be realized and how the liabilities will be repaid. The calculation is based on the prevailing tax rates or those adopted on the reporting date.
Deferred tax assets are offset against deferred tax liabilities if the taxes are levied by the same taxation authority and the entity has a legally enforceable right to set off current tax assets against current tax liabilities.
Deferred tax assets are recognized only to the extent that it is likely that there will be future taxable income to offset. Deferred tax assets are reduced by the amount that the related tax benefit is no longer expected to be realized.
Changes in accounting policies and disclosures
For a discussion of new accounting standards and interpretations not yet adopted by us, please see note 2.1.2 “Changes in accounting policies and disclosures” in the notes to our consolidated financial statements included in this prospectus.
Results of Operations
Revenues
In 2017, revenues increased by 34.4%, or €17.1 million, from €49.7 million in 2016 to €66.8 million in 2017. The increase in revenues was primarily a result of a $20.0 million (equal to €16.8 million at the then-prevailing exchange rate) upfront payment received and fully recognized in 2017 following the signing of an exclusive regional license agreement with I-Mab Biopharma for the development and commercialization of MOR202 in China, Taiwan, Hong Kong and Macao. In 2016 and 2017, revenues were significantly and positively affected by funded research and license fees from a collaboration agreement with Novartis that concluded at the end of 2017. On a regional basis, revenues with biotechnology and pharmaceutical companies in the United States and Canada increased by 70.6%, or €3.6 million, from €5.1 million in 2016 to €8.7 million in 2017 primarily due to higher success-based payments received mainly from Janssen. Revenues with customers in Europe or Asia increased by 30.0%, or €13.4 million, from €44.7 million in 2016 to €58.1 million in 2017 primarily due to the upfront payment received from I-Mab Biopharma in 2017 and was partially offset by lower revenues received from Novartis in 2017.
In 2017, 90.4% of our revenues were attributable to activities with our partners Novartis, I-Mab Biopharma and Janssen, whereas 94.8% of our revenues in 2016 were attributable to activities with our partners Novartis, Pfizer and Janssen. This change is due to entry into the agreement with I-Mab Biopharma in 2017 and receipt of the related upfront payment.
Proprietary Development
In 2017, revenue in our Proprietary Development segment increased by €17.0 million, from €0.6 million in 2016 to €17.6 million in 2017. This increase was due to the revenue recognized from the upfront payment received under our 2017 agreement with I-Mab Biopharma.
86
Table of Contents
Partnered Discovery
In 2017, revenue in our Partnered Discovery segment increased by €0.1 million, from €49.1 million in 2016 to €49.2 million in 2017. These amounts include €43.6 million in 2016 and €41.9 million in 2017 in funded research and license fees, received primarily in connection with the collaboration with Novartis as well as €5.6 million in 2016 and €7.3 million in 2017 in success-based payments received primarily from Janssen and Novartis. Revenue in our Partnered Discovery segment included €1.9 million of royalties on net sales of Tremfya® in 2017. As a result of the conclusion of our collaboration arrangement with Novartis, we no longer expect to receive significant recurring research and license fees from Novartis, and further revenue received from Novartis, if any, will consist of milestone payments and royalties from sales of approved products.
Operating Expenses
In 2017, operating expenses increased by 21.9%, or €24.0 million, from €109.8 million in 2016 to €133.8 million in 2017. This increase was driven by higher research and development as well as general and administrative expenses. Research and development expenses increased by 22.0%, or €21.1 million, from €95.7 million in 2016 to €116.8 million in 2017 mainly as a result of increased expenses for external services related to development in our Proprietary Development segment. General and administrative expenses increased by 20.6%, or €2.9 million, from €14.1 million in 2016 to €17.0 million in 2017 mainly due to higher personnel expenses and external services.
In 2017, operating expenses in our Proprietary Development segment increased by 26.2%, or €20.6 million, from €78.5 million in 2016 to €99.1 million in 2017, primarily due to an increase in research and development expenses. Research and development expenses in our Proprietary Development segment, including technology development, increased by 26.2%, or €20.6 million, from €78.5 million in 2016 to €99.1 million in 2017 due to increases mainly in research and development expenses for our Proprietary Development segment, in particular MOR208, MOR106 and MOR202.
In 2017, operating expenses in our Partnered Discovery segment increased by 4.4%, or €0.8 million, from €18.1 million in 2016 to €18.9 million in 2017, primarily due to an increase in research and development expenses. Research and development expenses in our Partnered Discovery segment increased by 2.9%, or €0.5 million, from €17.2 million in 2016 to €17.7 million in 2017. Research and development expenses in our Partnered Discovery segment related primarily to the Novartis collaboration, which is now concluded.
Research and Development
In 2017, research and development expenses increased by 22.0%, or €21.1 million, from €95.7 million in 2016 to €116.8 million in 2017, primarily due to higher expenses for external laboratory services and personnel. External laboratory services and other expenses (including legal and scientific consulting services) increased from €44.4 million in 2016 to €63.1 million in 2017, primarily due to increased expenses related to our Proprietary Development segment. Personnel expenses increased from €26.5 million in 2016 to €29.7 million in 2017, primarily due to higher share-based compensation and severance expense (in the aggregate by €2.5 million) in connection with the conclusion of the Novartis collaboration, which were only partially offset by a decrease in the number of employees active in research and development.
87
Table of Contents
Expenses for intangible assets remained almost unchanged and decreased slightly from €13.7 million in 2016 to €13.5 million in 2017. Expenses for intangible assets mainly represent impairment charges of €9.8 million in 2017 related to the termination of the cooperation with Aptevo Therapeutics for the development of MOR209 and €10.1 million in 2016. In 2017, the reason for the impairment was the termination of the cooperation with Aptevo Therapeutics due to the expectation of a delay in the development plan, a delayed market entry and a delay in the occurrence of future cash flows compared to previous assumptions. In 2016, the reason for the partial impairment was the expectation of a lower inflow of benefits and of a delay in the occurrence of future cash flows. Depreciation and other costs for infrastructure expenses decreased from €5.9 million in 2016 to €4.9 million in 2017, primarily due to one-time costs related to our move to a new building in 2016. Other expenses increased from €2.9 million in 2016 to €3.1 million in 2017 primarily due to higher maintenance expenses for laboratory equipment. Expenses for consumable supplies increased from €2.3 million in 2016 to €2.6 million in 2017 in line with the increase in our research and development operations.
General and Administrative
In 2017, general and administrative expenses increased by 20.6%, or €2.9 million, from €14.1 million in 2016 to €17.0 million in 2017, primarily due to higher personnel expenses and external services. Personnel expenses increased from €9.5 million in 2016 to €12.3 million in 2017, primarily due to higher deferred compensation for share-based incentive plans and bonus payments. Expenses for external services increased from €2.5 million in 2016 to €2.9 million in 2017, primarily due to increased project costs for commercialization. Other expenses decreased from €1.0 million in 2016 to €0.8 million in 2017, primarily due to one-time costs related to our move in 2016 to a new building.
Other Income
In 2017, other income increased by 57.1%, or €0.4 million, from €0.7 million in 2016 to €1.1 million in 2017 and mainly consisted of grant income in an amount of €0.2 million in 2017 and €0.3 million in 2016, currency gains in an amount of €0.5 million in 2017 and €0.2 million in 2016 and miscellaneous income of €0.5 million in 2017 and €0.2 million in 2016.
Other Expenses
In 2017, other expenses increased by €1.1 million, from €0.6 million in 2016 to €1.7 million in 2017. Other expenses mainly consisted of currency losses in an amount of €0.8 million in 2017 and €0.4 million in 2016 and miscellaneous expenses of €0.8 million in 2017 and €0.2 million in 2016.
Finance Income
In 2017, finance income decreased by 50.0%, or €0.7 million, from €1.4 million in 2016 to €0.7 million in 2017 reflecting lower returns from investments. Finance income mainly consisted of interest income of €1.0 million in 2016 and €0.2 million in 2017 received from investments in term deposits with fixed or variable interest rates, €0.3 million in 2016 and less than €0.1 million in 2017 in realized gains from the divestment of available-for-sale financial assets and bonds and €0.1 million in 2016 and €0.4 million in 2017 in realized gains from derivatives.
88
Table of Contents
Finance Expenses
In 2017, finance expenses increased by 46.2%, or €0.6 million, from €1.3 million in 2016 to €1.9 million in 2017 and consisted primarily of losses on derivatives of €1.4 million and interest expenses of €0.4 million in 2017. In 2016, finance expenses mainly consisted of €1.2 million in realized losses from the sale of available-for-sale financial assets and bonds.
Income Tax Expenses
In 2017, income tax expenses increased by 100.0%, or €0.5 million, from €0.5 million in 2016 to €1.0 million in 2017, due in large part to an income tax benefit in 2016 related to certain losses that were carried back to offset 2015 taxable income. In 2017, no such tax loss carry back was possible. The effective income tax rate changed from negative 0.9% in 2016 to negative 1.5% in 2017. The difference between the expected tax rate of 26.7% (which would have resulted in an expected income tax benefit of €18.3 million in 2017 and €16.0 million in 2016) is primarily the result of the non-recognition of deferred tax assets on current year tax losses of €22.0 million in 2017 and €13.4 million in 2016 and the non-recognition of deferred tax assets on temporary differences of negative €3.3 million in 2017 and €3.8 million in 2016.
Liquidity and Capital Resources
Sources of Funding
We have funded our operations primarily through the issuance of ordinary shares and through cash received in the ongoing operations of our business, including upfront fees, milestone payments, license fees, and support fees from our strategic partners and government grants.
As of December 31, 2017, we had €76.6 million in cash and cash equivalents, €86.5 million in available-for-sale financial assets, and €149.1 million in current other financial assets categorized as “loans and receivables”. Cash in excess of immediate working capital requirements is invested in accordance with our investment policy, primarily with a view to liquidity and capital preservation. Investments are primarily made in money market funds, corporate bonds, and term deposits with fixed or variable interest.
We do not have any financial indebtedness, and we are not subject to any operating covenants or capital requirements.
Uses of Funding
Our primary use of cash is to fund research and development costs related to the development of our product candidates. Our primary future funding requirements include the development of our proprietary clinical pipeline (primarily, MOR208, MOR202 and MOR106) and the advancement of our earlier stage wholly-owned or co-developed product candidates.
We believe that our existing cash and cash equivalents and other financial instruments (including cash invested in various financial instruments as described above) will be sufficient to fund our anticipated operating expenses for at least the next twelve months.
We have based this estimate on assumptions that may prove to be wrong, and we could use our capital resources sooner than we currently expect. Additionally, the process of testing product candidates in clinical trials is costly, and the timing of progress in these trials is uncertain.
89
Table of Contents
Because our product candidates are in various stages of development and the outcome of these efforts is uncertain, we cannot estimate the actual amounts necessary to successfully complete the development and commercialization of our product candidates or whether, or when, we may achieve profitability.
We will likely require additional capital for the further development of our existing product candidates, regulatory approval processes, the potential buildout of a commercial organization and for our operation as a public company in the U.S. and may also need to raise additional funds sooner to pursue other development activities related to additional product candidates. Until we can generate a sufficient amount of revenue, we expect to finance future cash needs primarily through public or private equity or debt offerings, including convertible bonds. Additional capital may not be available on reasonable terms, if at all. If we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may have to significantly delay, scale back or discontinue the development or commercialization of one or more of our product candidates. If we raise additional funds through the issuance of debt or equity securities, it could result in dilution to our existing shareholders, increased fixed payment obligations or the securities may have rights senior to those of our ordinary shares or the ADSs. If we incur indebtedness, we could become subject to covenants that would restrict our operations and potentially impair our competitiveness, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business.
Cash Flows
The following table sets forth our cash flows for the years ended December 31, 2016 and 2017.
| For the year ended December 31, |
||||||||
| (in € thousands) | 2016 | 2017 | ||||||
| Net Cash Provided by/(Used in) Operating Activities |
(46,616 | ) | (38,446 | ) | ||||
| Net Cash Provided by/(Used in) Investing Activities |
(80,786 | ) | 32,933 | |||||
| Net Cash Provided by/(Used in) Financing Activities |
110,403 | 8,174 | ||||||
|
|
|
|||||||
| Increase/(Decrease) in Cash and Cash Equivalents |
(16,999 | ) | 2,660 | |||||
| Cash and Cash Equivalents at the Beginning of the Period |
90,928 | 73,929 | ||||||
|
|
|
|||||||
| Cash and Cash Equivalents at the End of the Period |
73,929 | 76,589 | ||||||
|
|
|
|||||||
Cash flows provided by / used in operating activities
In 2017, net cash used in operating activities was €38.4 million, primarily driven by the consolidated net loss of €69.8 million, partially offset by non-cash charges of positive €0.7 million, and changes in operating assets and liabilities and taxes paid of €30.6 million. Consolidated net loss of €69.8 million was primarily driven by expenses incurred to fund our ongoing operations, in particular research and development expenses and general and administrative expenses. Changes in operating assets and liabilities for 2017 consisted primarily of €18.4 million in deferred revenue received during the year, a €7.8 million increase in accounts payable and accrued expenses and a €3.1 million increase in other liabilities. The deferred revenue received during the year mainly related to annual license fees. The increase in accounts
90
Table of Contents
payable and accrued expenses was mainly due to an increase in external laboratory services primarily related to the MOR208 program that were outstanding at year end. The increase in other liabilities was mainly due to the deferral of the rent-free period for the rental agreement for our headquarters.
In 2016, net cash used in operating activities was €46.6 million, primarily driven by the consolidated net loss of €60.4 million, after consideration of the net non-cash charges of negative €0.7 million, and changes in operating assets and liabilities as well as taxes paid of €14.4 million. Consolidated net loss of €60.4 million, after consideration of the net non-cash charges of negative €0.7 million, was primarily driven by expenses incurred to fund our ongoing operations, in particular research and development expenses and general and administrative expenses. Net cash provided by changes in operating assets and liabilities for 2016, consisted primarily of €17.4 million in deferred revenue prepayments received during the year and a €13.0 million increase in accounts payable and accrued expenses, partially offset by a €13.9 million increase in prepaid expenses and other assets. The prepayments for deferred revenue received during the year mainly related to annual license fees. The increase in accounts payable and accrued expenses was mainly due to an increase in external laboratory services. The increase in prepaid expenses and other assets was mainly due to an increase in the purchase of combination compounds and prepaid fees for external laboratory services, in each case primarily related to our MOR208 program.
Cash flows provided by / used in investing activities
In 2017, net cash provided by investing activities was €32.9 million, primarily driven by proceeds from financial assets of €210.2 million, partially offset by the purchase of financial assets of €164.4 million, of which €108 million are classified as loans and receivables. Cash provided by investing activities primarily related to a shift in the composition in our investment portfolio as financial assets matured and were sold and new, similar financial assets were purchased.
In 2016, net cash used in investing activities was €80.8 million, primarily driven by purchase of financial assets of €423.4 million, partially offset by sales of financial assets and bonds of €343.5 million. Use of cash in investing activities during the period primarily related to a shift in the composition in our investment portfolio.
Cash flows provided by / used in financing activities
In 2017, net cash provided by financing activities was €8.2 million and mainly related to exercises of convertible bonds by members of the management board and the senior management.
In 2016, net cash provided by financing activities was €110.4 million. Cash provided by financing activities during the period primarily related to our capital increase in November 2016, resulting in gross proceeds of €115.4 million.
91
Table of Contents
Contractual Obligations
The following table summarizes our contractual obligations at December 31, 2017.
| Payments due by period | ||||||||||||||||||||
| (in € thousands) | Total | Less than 1 year |
1 to 3 years |
3 to 5 years |
More than 5 years |
|||||||||||||||
| Operating Lease Obligations |
25,317 | 2,918 | 5,727 | 5,482 | 11,190 | |||||||||||||||
Operating Lease Obligations
We lease facilities and equipment under long-term operating leases. In 2017, leasing expenses amounted to €2.8 million. Leasing expenses also include leasing of company cars and machinery. The majority of these leasing contracts can be renewed on a yearly or quarterly basis, and some agreements may be terminated prematurely.
In 2016, we signed a rental agreement for our headquarters at Semmelweisstrasse 7, 82152 Planegg, Germany. The contract includes a minimum rental period of approximately ten years.
Other Commitments
Other commitments may become due for future payments for outsourced studies. As of December 31, 2017, we expected to incur approximately €122.2 million of fees for outsourced studies, of which approximately €56.1 million will be paid in the next twelve months. Additionally, if certain milestones are achieved in the Proprietary Development segment, for example, filing an application for an investigational new drug, or IND, for specific target molecules, this may trigger regulatory and sales milestone payments to licensors of up to an aggregate of $287 million. The next milestone payment in the amount of $12.5 million could occur in approximately 18 to 24 months. No accrued expenses have been recorded in our consolidated balance sheet for these amounts.
Off-Balance Sheet Arrangements
We did not have, during 2017 and 2016, and we do not currently have, any off-balance sheet arrangements.
Quantitative and Qualitative Disclosure About Market Risk
Market Risk
We are exposed to currency and interest rate risks.
Currency Risk
Our financial statements are prepared in euros. While our expenses are predominately incurred in euros, a portion of our revenue is dependent on the prevailing exchange rate of the U.S. dollar. Throughout the year, we monitor the need to hedge foreign exchange rates to minimize currency risk and address this risk by using a variety of derivative financial instruments.
A 10% increase in the euro versus the U.S. dollar as of December 31, 2017, would have reduced our income by €0.2 million. A 10% decline in the euro versus the U.S. dollar would have increased our income by €0.2 million.
92
Table of Contents
Interest Rate Risk
Our risk exposure to changes in interest rates mainly relates to our fixed term deposits and available-for-sale bonds. Changes in the general level of interest rates may lead to an increase or decrease in the value of these securities. Our investment focus places the safety of an investment ahead of its return. Interest rate risk is limited because all securities can be liquidated within a maximum of two years.
We are not subject to significant interest rate risks from liabilities reported in our balance sheet.
Credit and Liquidity Risk
Financial instruments that could subject us to a concentration of credit and liquidity risk include primarily cash and cash equivalents, available-for-sale financial assets, fixed term deposits and bonds, financial assets classified as loans and receivables, derivative financial instruments and receivables. Our cash and cash equivalents are principally denominated in euros. Available-for-sale financial assets, fixed term deposits, bonds and financial assets classified as loans and receivables represent investments in high quality securities, meaning the majority of securities having a rating of A3/A- (long-term) with any of the three primary rating agencies and/or being guaranteed by a financial institution having a minimum rating of A3/A- (long-term) with any of the three primary rating agencies. In case the investment or the financial institution is downgraded by one or more rating agencies, a review of the investment(s) is initiated and a sell / hold recommendation is made. Cash, cash equivalents, available-for-sale financial assets and bonds, and financial assets classified as loans and receivables are held at several reputable financial institutions in Germany. We limit our exposure as far as possible to any given financial institution through diversification and/or low-risk investments. We continuously monitor our positions with financial institutions that are counterparts to our financial instruments and these institutions’ credit ratings.
One of our policies requires all customers who wish to transact business on credit terms to undergo a credit assessment based on external ratings. Nevertheless, our revenue and accounts receivable are still subject to credit risk from customer concentration. Our most significant single customer accounted for €5.1 million, or 45.5%, of accounts receivable as of December 31, 2017 (as of December 31, 2016: €8.4 million). Three individual customers accounted for 55.2%, 25.1% and 10.0%, respectively, of the total revenue in 2017. In 2016, our top three customers had individually accounted for 85%, 5% and 5% of our revenues. Based on our management board’s assessment, no allowances were required in 2017.
As of December 31, 2017, we were not subject to credit risk from derivative financial instruments. As of December 31, 2017, we had rent deposits of €1.1 million outstanding, which represented the maximum credit risk of financial guarantees.
93
Table of Contents
Overview
We are a late-stage biopharmaceutical company devoted to the development of innovative and differentiated therapies for patients suffering from serious diseases. Based on our proprietary technology platforms and leadership in the field of therapeutic antibody discovery, generation and engineering, we, together with our partners, have participated in the development of more than 100 therapeutic product candidates. Our broad pipeline spans two business segments: Proprietary Development, in which we invest in and develop product candidates, and Partnered Discovery, in which we generate product candidates for our partners in the pharmaceutical and biotechnology industries against targets identified by our partners. We currently have 28 product candidates in clinical development, including our most advanced proprietary product candidate, MOR208, for the treatment of relapsed or refractory diffuse large B cell lymphoma, or r/r DLBCL. We believe our pipeline of novel and differentiated product candidates has the potential to treat serious diseases and improve the lives of patients.
Our late-stage and most advanced proprietary product candidate, MOR208, received breakthrough therapy designation, or BTD, from the U.S. Food and Drug Administration, or the FDA, in 2017 in combination with lenalidomide for the treatment of patients with r/r DLBCL, who are not eligible for high-dose chemotherapy and autologous stem cell transplantation. We hold worldwide rights to develop and market MOR208. Also in 2017, Tremfya®, developed by our partner Janssen, became the first therapeutic antibody based on our proprietary technology to reach the market. Tremfya®, which is approved to treat moderate-to-severe plaque psoriasis, was launched in the United States in July 2017 and received approval for marketing in the European Union and Canada in November 2017.
Based on our heritage as an antibody discovery and development company, we have a large and diverse pipeline, composed of both proprietary and partnered programs, in multiple therapeutic areas and across all development phases. The combination of our technology platforms and antibody expertise has allowed us to generate promising product candidates and enter into multiple strategic collaborations with leading global pharmaceutical and biotechnology companies. These collaborations provide us with an additional funding source and allow us to leverage our collaborators’ expertise to advance the development of our proprietary product candidates.
94
Table of Contents
The following table summarizes our product candidates and programs:

Our most advanced Proprietary Development programs include:
| · | MOR208—an investigational, humanized Fc-engineered monoclonal antibody directed against CD19. CD19 is a target for the treatment of B cell malignancies, including DLBCL, follicular lymphoma, or FL, chronic lymphocytic leukemia, or CLL, and others. On October 23, 2017, the FDA granted BTD for the use of MOR208 in combination with lenalidomide to treat patients with r/r DLBCL who are not eligible for high-dose chemotherapy and autologous stem cell transplantation. The granting of BTD was based on the interim results as of June 13, 2017 from our single arm Phase 2 L-MIND study, which showed a response in 23 out of 44 patients (Overall Response Rate, or ORR, 52%) and progression-free survival estimate based on a Kaplan Meier curve of 11.3 months based on investigator response assessment. Interim data from our most recent cut-off date December 12, 2017 were consistent with our prior interim results, with a response in 33 out of 68 patients (ORR: 49%) and a progression-free survival, or PFS, rate at 12 months of 50.4% based on investigator response assessment, with the preliminary median progression-free survival, or mPFS, not yet reached. We are also investigating MOR208 in combination with bendamustine, in our pivotal Phase 2/3 B-MIND study targeting a comparable DLBCL patient population as the L-MIND study. B-MIND started as a randomized safety run-in with ten patients in each treatment arm (the Phase 2 stage of the trial assessing safety of the combination with bendamustine) and continued then comparing MOR208 plus bendamustine with a standard of care Rituximab plus bendamustine control arm (the Phase 3 stage of the trial comparing to standard of care). A third clinical trial, known as COSMOS, is an exploratory Phase 2 study in CLL patients who have progressed on or who do not tolerate treatment with Bruton tyrosine kinase inhibitors. Pursuant to our BTD, we are working closely with the FDA regarding further clinical development of MOR208. We |
95
Table of Contents
| hold worldwide rights to develop and market MOR208 and intend to commercialize it either alone or together with a partner. |
| · | MOR202—an investigational, human monoclonal HuCAL antibody directed against CD38. MOR202 is currently under clinical investigation in a Phase 1/2a trial in patients with relapsed or refractory multiple myeloma, or r/r MM. The study is evaluating the safety and preliminary efficacy of MOR202 alone and in combination with the immunomodulatory drugs pomalidomide or lenalidomide, plus dexamethasone, respectively. We believe MOR202 offers a potentially differentiated safety and administration profile relative to infusion site reaction and infusion time of other agents targeting CD38. Scientific research suggests that an anti-CD38 antibody such as MOR202 may have therapeutic activity in solid tumors including non-small cell lung cancer, or NSCLC. We plan to explore this potential activity in NSCLC in combination with nivolumab (an anti-PD-1 antibody) or more oncology indications either alone or together with a partner. |
| · | MOR106—a human monoclonal antibody targeting IL-17C, currently under clinical development for the treatment of atopic dermatitis, or AD. We believe MOR106 is the first antibody directed against IL-17C in clinical development worldwide. MOR106 is also the first antibody from our Ylanthia platform to enter clinical development. Based on findings from pre-clinical models, we believe IL-17C plays an important role in inflammatory skin disorders. We are developing MOR106 in collaboration with our partner, Galapagos, and share equally the research and development costs as well as all future economics. In September 2017, we reported results from a randomized placebo-controlled Phase 1 study, testing MOR106 in single ascending doses in healthy volunteers and in multiple ascending doses in patients suffering from AD. In the trial, MOR106 was generally well-tolerated and showed first signs of clinical activity in five of six patients with moderate-to-severe AD. We plan to initiate a Phase 2 study with our partner Galapagos in the first half of 2018. |
Our most advanced Partnered Discovery products and product candidates include:
| · | Tremfya®—a HuCAL antibody directed against IL-23 marketed by our partner Janssen to treat moderate-to-severe plaque psoriasis was launched in the United States and received marketing approval in the EU and Canada in 2017. We are entitled to royalty payments on net sales of Tremfya®. Janssen is currently investigating Tremfya® in additional Phase 3 trials in psoriasis and in psoriatic arthritis and plans to develop the product in Crohn’s disease. |
| · | Gantenerumab—a HuCAL antibody directed against amyloid beta that is being developed by Roche for the treatment of Alzheimer’s disease. In Phase 1 trials, gantenerumab has been shown to reduce brain amyloid in mild-to-moderate Alzheimer’s disease patients. Roche is currently planning to test a higher dose of gantenerumab in two Phase 3 trials, called GRADUATE 1 & 2, for patients with prodromal and mild Alzheimer’s disease. |
The majority of our Proprietary Development product candidates and all product candidates in our Partnered Discovery programs have been discovered and engineered using our advanced antibody technology platforms. Our core platforms include:
| · | HuCAL (Human Combinatorial Antibody Library)—HuCAL is our original technology platform, which constitutes a collection or “library” of several billion distinct fully human antibodies. This platform enables rapid selection of antibodies having high affinity and specificity as well as systematic optimization of antibodies to precisely-defined specifications to increase the probability of successful clinical development. |
96
Table of Contents
| · | Ylanthia—Ylanthia is our newest antibody library, which comprises over 100 billion fully human antibodies. Ylanthia enables the generation of fully human antibody candidates with optimized biophysical properties, which we believe offer a number of important advantages over competing platforms. This platform builds on our experience in generating more than 100 therapeutic product candidates using our original HuCAL platform. We believe Ylanthia will be the source of the next generation of therapeutic antibody candidates in our and our partners’ future pipelines. |
| · | Lanthipeptides—Our lanthipeptide platform opens up new possibilities for discovering product candidates based on highly specific and stable peptides, which are intended to bind and activate only one target receptor subtype. |
We are committed to investing in our platforms, generating new therapeutics and developing them into products that address significant unmet medical needs.
We have an internationally-trained, multi-cultural team of more than 300 employees and consultants, including a research and development team of approximately 260 scientists, clinicians and support staff. Our management team and senior experts have deep experience and capabilities in biology, chemistry, product discovery and clinical development.
Our Strengths
We believe our core strengths include:
| · | Our lead product candidate MOR208, which has been granted BTD in r/r DLBCL by the FDA and has further potential in additional hematological malignancies. |
| · | Additional differentiated proprietary product candidates, such as MOR202 and MOR106, in clinical trials for the treatment of cancer and inflammatory diseases. |
| · | Tremfya®, the first approved product based on our technology which offers a royalty opportunity. |
| · | Our antibody pipeline, which we believe is one of the broadest and deepest in the biotech industry and which provides us with multiple opportunities for value creation. |
| · | Our long-term technology leadership in antibody discovery, generation and engineering, as demonstrated by multiple collaborations with leading pharmaceutical and biotechnology companies as well as by the breadth and depth of our technology platforms. |
| · | Our diversified business model with both proprietary and partnered development programs, which provides us with a strong financial base and strategic flexibility. |
| · | A broad intellectual property portfolio protecting product candidates as well as our technology platforms. |
| · | Our experienced management team, comprising industry leaders in antibody discovery and development. |
Our Strategy
Our goal is to make differentiated, innovative biopharmaceuticals to improve the lives of patients suffering from serious diseases. Our strategy to achieve this goal is:
| · | Continue to advance the development of our lead product candidate MOR208 towards regulatory approval—The MOR208 program is currently in an advanced clinical development |
97
Table of Contents
| stage following receipt of BTD by the FDA in October 2017 based on interim data from our L-MIND study. We believe that with BTD, we can establish a path to approval of MOR208 in r/r DLBCL. In addition, we are evaluating opportunities to develop MOR208 in other treatment lines and hematological malignancies. |
| · | Build our commercial capabilities in connection with the potential future approval of MOR208—We are finalizing different commercialization strategies for MOR208. If we receive marketing approval for MOR208, our preferred option at this stage is to retain either sole or co-promotion rights in the United States and seek partners for commercialization elsewhere. We are currently commencing our commercialization preparation efforts with the goal of being ready to promote MOR208 as early as the first half of 2020. |
| · | Continue the development of MOR202—In order to maximize the value of MOR202, we are exploring opportunities for its further development, either alone or together with a partner, in one or more oncology indications, including in solid tumors, with an initial focus on NSCLC. |
| · | Advance our earlier stage product candidates—We continue to advance the development of our early-stage product candidates, in particular MOR106, for which we and our partner Galapagos intend to initiate a Phase 2 program in AD in 2018. |
| · | Realize the value of our technology platforms by using them to discover and develop additional early-stage proprietary programs—We continue to capitalize on the advantages of our antibody technologies and our internal expertise and know-how to discover and develop novel product candidates. |
Background on Antibodies as Therapeutic Agents
Antibodies, also known as immunoglobulins (Ig) are large, Y-shaped complex proteins that the immune system uses to neutralize pathogens. Antibodies recognize and bind to foreign entities, such as bacteria and viruses, and remove them from the bloodstream. Antibodies are essential to human life and between one and two billion antibodies are continuously flowing throughout our bloodstream, fighting infections and diseases.
98
Table of Contents
The antibody molecule itself has two separable functions: First, antibodies have the ability to recognize and attach themselves to pathogenic, or disease-causing, foreign molecules; and second, in recognizing and attaching themselves to these pathogenic molecules, antibodies act as markers, signaling to other parts of the bodies’ own immune system to attack and eliminate the pathogen.
As illustrated above, an IgG antibody, for example, consists of four polypeptide chains, two identical heavy chains and two identical light chains, joined by chemical linkages known as disulfide bridges. The antigen-binding fragment, or Fab, is a region on an antibody that binds to antigens. It is composed of one constant and one variable region of each of the heavy and the light chain. The variable region acts as the “business” end of the antibody for recognizing pathogens. The specific structure recognized by the variable region of an antibody, whether a portion of a protein, another biological molecule or a unique molecule of a pathogen, is known as an antigen. An antibody and the antigen that it recognizes fit together like a lock and key.
The Fc region, which resides at the other end of the antibody, interacts with the effector cells of the immune system, and provides the signal that activates these cells to attack the pathogen. When an antigen is detected, several types of immune system cells work together to recognize and respond to it. These responses include the stimulation of B cells to produce additional antibodies and the stimulation of effector cells, including T cells and natural killer, or NK, cells that act to eliminate the pathogen or foreign molecule.
The first methods for producing specific single defined antibodies that recognize a single antigen, or monoclonal antibodies, in order to use them as therapeutics were developed approximately 40 years ago. While cancer and inflammatory conditions have been the two largest disease areas for therapeutic antibody discovery, the broad applicability of antibodies has led to a rapid expansion of their use in other indications, including infectious diseases, metabolic conditions and neurodegenerative diseases. As a result, more than 50 antibodies are currently approved for marketing in various clinical applications. Amongst these are therapeutic antibodies which label and/or block the activity of cell surface receptors or signaling molecules,
99
Table of Contents
stimulate the activity of cells or lead to their elimination by effector cells, and bind toxic substances from the bloodstream to accelerate their elimination.
Initially, monoclonal antibodies were derived from mice. However, antibodies derived from mice are of limited use as therapeutic agents since the human immune system recognizes such antibodies as foreign molecules and may trigger a defense reaction against them. Technological advances over the last three decades have allowed the modification of antibody structures to make them more “human-like”, culminating in the creation of fully human antibodies. Currently, it is possible to generate fully human antibodies from transgenic mice. With our Human Combinatorial Antibody Library, we have developed a technology for the in vitro generation of highly specific and fully human antibodies. Ylanthia, which is our most recent antibody technology platform, comprises more than 100 billion distinct, different, fully human antibodies.
With our acquisition of Lanthio Pharma B.V. in 2015, we gained full access to Lanthio Pharma’s proprietary LanthioPep technology, which is focused on the generation and development of lanthipeptides. Our lanthipeptide platform opens up new possibilities for discovering product candidates based on highly specific and stable peptides, which are intended to bind and activate only one target receptor subtype.
Please see “—Our Technology Platforms” for a further description of our antibody technologies.
Proprietary Development
Within our Proprietary Development segment we focus on the development of therapeutic antibody candidates in the area of oncology and inflammatory diseases. Below is an overview of our Proprietary Development product candidates. In addition, we have seven proprietary or co-developed product candidates that are in the discovery phase and one in pre-clinical phase.
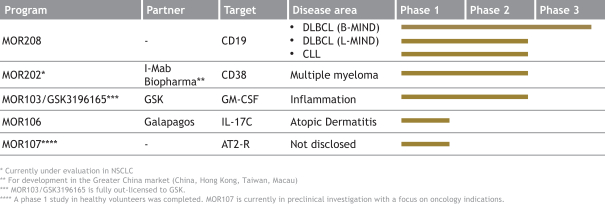
MOR208
Overview
MOR208 (formerly known as XmAb5574) is an investigational, humanized Fc-engineered monoclonal antibody directed against the antigen CD19, which is broadly expressed on the surface of B cells, a type of white blood cell. It is a target for the treatment of B cell malignancies, including DLBCL, FL, CLL and others. Fc-modification of the parental anti-CD19 IgG1 antibody is intended to lead to a potentiation, or increase, of antibody-dependent cell-
100
Table of Contents
mediated cytotoxicity, or ADCC, and antibody-dependent cellular phagocytosis, or ADCP, two key mechanisms involved in tumor cell killing. Furthermore, MOR208 induces direct apoptosis by binding to CD19, which is a crucial component for B cell receptor signaling. We are currently investigating MOR208 as an immunotherapeutic option in B cell malignancies.
We are developing MOR208 pursuant to a collaboration and license agreement that we entered into in June 2010 with Xencor, Inc., or Xencor. Under this agreement, Xencor granted us an exclusive worldwide license to MOR208 for all indications. Pursuant to this agreement, except for the Phase 1 clinical trial of MOR208 in CLL, which was completed in January 2013, we are and have been, responsible for all development and commercialization activities in connection with MOR208.
In 2014, we were granted fast track designation for MOR208 by the FDA for the treatment of r/r DLBCL. The FDA’s fast track program is designed to facilitate the development and expedite the review of product candidates intended, alone or in combination with one or more other drugs, for the treatment of a serious or life-threatening disease or condition, and that demonstrates the potential to meet unmet medical need for such diseases or conditions. Also in 2014 and 2015, respectively, the FDA as well as the European Commission granted orphan drug designation and orphan medicinal product status respectively for MOR208 for the indications of DLBCL and CLL/small lymphocytic lymphoma, or SLL.
The main focus of the current MOR208 development program is on r/r DLBCL. Our L-MIND (Phase 2) and B-MIND (Phase 2/3) trials are being conducted in r/r DLBCL in patients who are not eligible for high-dose chemotherapy and subsequent autologous stem cell transplantation. Interim data from the L-MIND study showed, at the data cut-off of June 13, 2017, when 51 patients had been enrolled in the study, a response in 23 out of the 44 patients who were evaluable for efficacy assessments by the investigators (ORR: 52%), 19 (83%) of whom showed ongoing responses. Complete responses were seen in 14 out of 44 patients (CR: 32%). At the time of data cut-off, recruitment was still ongoing and seven patients in L-MIND were not yet evaluable for efficacy since a radiological post-baseline response assessment had not yet been performed. The median time to response was 1.8 months and the median time to complete response was 2.3 months. The preliminary median progression-free survival based on Kaplan Meier estimate was 11.3 months (95% confidence interval: 5.4 months—not reached).
On October 23, 2017, based on interim data from the ongoing L-MIND study as of June 13, 2017, the FDA granted breakthrough therapy designation for MOR208 in combination with lenalidomide, or len, for the treatment of patients with r/r DLBCL who are not eligible for high-dose chemotherapy and autologous stem cell transplantation. The FDA grants this designation to a product candidate intended alone or in combination with one or more other drugs to treat a serious or life-threatening disease or condition when preliminary data indicate that the product candidate may demonstrate substantial improvement over existing therapies on one or more clinically-significant endpoints, such as substantial treatment effects observed early in clinical development. The FDA’s grant of breakthrough therapy designation is intended to expedite the development and review of product candidates.
Latest interim data with a cut-off date of December 12, 2017 (median follow-up 8.3 months) based on 81 patients enrolled, of whom 68 had reached at least the first post-baseline time point of tumor assessment and therefore were available for efficacy assessment by the investigators, showed an ORR of 49% (n=33) and a CR rate of 31% (n=21). At the time of
101
Table of Contents
data cut-off, the PFS rate at 12 months was 50.4% (95% confidence interval 40 – 67%) and the preliminary median PFS had not been reached (95% confidence interval: 4.3 months—not reached). 29 out of 33 responses (88%) were ongoing at the time of data-cut off; median time to response was 1.8 months, median time to complete response was 3.6 months.
Pursuant to our BTD, we are working closely with the FDA regarding further clinical development of MOR208 in combination with lenalidomide based on the current L-MIND study.
Description of B cell malignancies and DLBCL and Current Treatments
B cell malignancies include lymphomas such as non-Hodgkin lymphomas, or NHL, and leukemias such as CLL, and acute lymphoblastic leukemia, or ALL. Collectively, lymphomas represent approximately five percent of all cancers diagnosed in the United States. NHL is the most prevalent of all lymphoproliferative diseases, with the National Cancer Institute estimating that 72,240 new cases occurred in the United States in 2017. Worldwide, 385,741 new cases per year were estimated in 2012. DLBCL is the most frequent type of malignant lymphoma worldwide and accounts for approximately one third of all NHLs worldwide. As of today first line treatment of B cell malignancies most commonly consists of a combination chemotherapy regimen including Rituxan®, also referred to commonly as R-CHOP (R, rituximab; CHOP, cyclophosphamide, doxorubicin, vincristine and prednisone, each a chemotherapy agent), in DLBCL. The addition of Rituxan®, an anti-CD20 antibody, has led to major therapeutic improvements in response rates and progression-free survival. Yet, in DLBCL, despite the therapeutic success of first line R-CHOP, up to 40% of patients are refractory or relapse after initial treatment with fast progression of disease.
Currently, standard of care treatments across almost all B cell lymphomas consist of a combination of a CD20 monoclonal antibody, or mAb, (rituximab (Rituxan®) or rituximab biosimilars, obinutuzumab (Gazyva®) and ofatumumab (Arzerra®)), with either chemotherapy or targeted agents. There is a scientific rationale for the replacement of the CD20 with a CD19 targeting antibody. First, CD19 has been shown to be expressed earlier and more broadly during B cell development than CD20. Second, in clinical practice an anti-CD20 approach is often applied by physicians in the relapsed or refractory, or r/r, setting, even though patients have already shown a relapse to a prior therapy containing an anti-CD20 antibody. With blinatumomab (Blincyto®), Yescarta® and tisagenlecleucel (CTL-019) there are three CD19-targeting therapeutic approaches approved, with only Yescarta® for the treatment of r/r DLBCL and the other two for treatment of ALL. Other clinical programs directed against the same target molecule use alternative approaches to increase the antibody’s efficacy, for example by coupling with toxic substances or changing the antibody’s glycosylation pattern. Alternative approaches using small molecules are also being developed in the field of B cell malignancies.
DLBCL patients who are refractory or relapse after R-CHOP have a poor prognosis and few therapeutic options. Treatment options for r/r DLBCL are currently curative HDCT followed by ASCT, if the patients are relatively fit. For those DLBCL patients who are not eligible for HDCT and ASCT, current treatments options are limited. In late 2017, a first chimeric antigen receptor-T cell (CAR-T) therapy (Yescarta®) was approved for the treatment of r/r DLBCL. It remains to be seen what proportion of r/r patients will be considered to be eligible for such therapies, and how broadly such therapies will be available for the foreseeable future.
In r/r DLBCL, approximately half of patients are eligible for HDCT followed by ASCT. Of those r/r DLBCL patients who can be transplanted, about 50% suffer a further relapse. Therefore, the vast
102
Table of Contents
majority of DLBCL patients relapsing after first-line treatment will ultimately be treated in a palliative setting, utilizing such regimens as rituximab plus bendamustine. There remains a high unmet need for novel therapies in r/r patients, especially in those not able to undergo HDCT and ASCT.
MOR208 for Treatment of B cell malignancies, including DLBCL
MOR208 binds to the CD19 antigen, which is broadly and homogeneously expressed across different B cell-derived blood cancers including DLBCL, CLL and SLL. According to pre-clinical findings, CD19 is able to enhance B cell receptor signaling, which is important for B cell survival and is considered an important therapeutic target for the treatment of B cell-related lymphomas and leukemias.
The suggested mechanism of action of MOR208 is as follows: The Fc-engineered antibody MOR208 binds to the CD19 antigen on the surface of blood cancer cells. This attracts the immune system’s natural killer cells, and/or macrophages. Natural killer cells and macrophages attach themselves to the cancer cells by way of the MOR208 antibody and kill them through the processes of ADCC and ADCP. MOR208’s engineered Fc region is designed to produce potentiation of the body’s immune reaction to cancer cells. In addition to its immune-mediated functions, the binding of MOR208 to CD19 may also lead to the direct killing of the tumor cells, or direct cytotoxicity. The below figure depicts the suggested mechanisms of action of MOR208:

We believe that, if approved by the authorities, MOR208 may offer a differentiated therapeutic approach in DLBCL. In particular for r/r DLBCL patients who are ineligible for or not willing to undergo HDCT and ASCT, available treatment options are limited. It is our goal to further clinically investigate MOR208 in this patient segment who are in need of more treatment options.
103
Table of Contents
Development of MOR208
MOR208 has been or is being investigated in seven clinical trials in the following indications: NHL, CLL/SLL and B cell acute lymphoblastic leukemia, or B-ALL. A Phase 1 trial in CLL/SLL and a Phase 2 trial in B-ALL were completed in 2013 and 2015, respectively. A Phase 2a trial in NHL (including r/r DLBCL, mantle cell lymphoma, or MCL, FL, and other indolent NHL) and an investigator sponsored Phase 2 trial in CLL/SLL are currently ongoing. Three additional trials (two in DLBCL and one in CLL) are currently ongoing.
The main focus of the current MOR208 development program is on r/r DLBCL. Our L-MIND and B-MIND trials are being conducted in this indication. Both trials are focusing on r/r DLBCL patients who are not eligible for high-dose chemotherapy and subsequent autologous stem cell transplantation. Following its review of data from the L-MIND study, in October 2017 the FDA awarded breakthrough therapy designation for MOR208, in combination with lenalidomide, for the treatment of non-transplant eligible patients with r/r DLBCL.
Active Clinical Combination Trials
Currently three clinical combination studies with MOR208 are ongoing:
| · | L-MIND: The L-MIND study is a Phase 2 trial initiated in April 2016 to evaluate MOR208 in combination with lenalidomide in patients suffering from r/r DLBCL. The trial is designed as an open-label, single-arm study with the primary endpoint being overall response rate, or ORR by independent review committee, with multiple secondary endpoints, including progression-free survival, overall survival and time to progression. We completed the safety run-in phase of the L-MIND trial in August 2016. No unexpected safety signals have been detected and the trial was continued as planned. The trial is enrolling patients with r/r DLBCL after up to three prior lines of therapy, with at least one prior therapy including an anti-CD20 targeting therapy (such as Rituxan®). Patients enrolled cannot be candidates for HDCT and ASCT. |
At the interim data cut-off of June 13, 2017, 51 patients had been enrolled in the study, of whom 44 were evaluated for efficacy assessments. Preliminary data per investigator assessment showed an objective response in 23 out of 44 patients (ORR: 52%), 19 (83%) of whom showed ongoing responses. Complete responses were seen in 14 out of 44 patients (CR: 32%). The median time to response was 1.8 months, the median time to complete response was 2.3 months. The preliminary median progression-free survival based on Kaplan Meier estimate was 11.3 months (95% confidence interval: 5.4 months—not reached).
No unexpected toxicities were observed for the treatment combination and no infusion-related reactions were reported for MOR208. The most frequent adverse events with a grade of 3 or higher were neutropenia in 36% (18/51), thrombocytopenia in 10% (5/51), and pneumonia in 10% (5/51), observed in 35% (18/51), 10% (5/51), and 10% (5/51) of patients, respectively. Leukopenia and hypokalemia of grade 3 or higher were reported for 8% (4/51) of the patients. At the data cut-off, 45% (23/51) of patients required a reduction of their lenalidomide dose, from a starting dose of 25mg daily. These data were presented at the ASH 2017 Annual Meeting on December 11, 2017.
Adverse events in this study were assessed and classified according to the Common Terminology Criteria for Adverse Events (CTCAE). Grade refers to the severity of the adverse event, with grade 1 adverse events generally including mild or asymptomatic conditions or
104
Table of Contents
clinical or diagnostic observations only, grade 2 adverse events generally including moderate events or minimal, local or noninvasive intervention events, grade 3 adverse events generally including severe or medically significant events that are not immediately life-threatening, events requiring hospitalization or prolongation of hospitalization or disabling events and grade 4 adverse events generally including life-threatening consequences or urgent intervention events.
In November 2017, the trial completed patient enrollment with 81 patients. Patients enrolled had a median age of 72 years, 40/81 (49%) were in advanced Ann Arbor stage III/IV, a median of two prior lines, 32/81 (40%) were refractory to the previous therapy line and 30/81 (37%) were rituximab-refractory in any line.
The latest interim data with a cut-off date of December 12, 2017 based on 81 patients enrolled, of whom 68 were available for efficacy assessment as per investigator, show an overall response rate (ORR) of 49% (n=33) and a CR rate of 31% (n=21). At the time of data cut-off, the PFS rate at 12 months was 50.4% (95% confidence interval 40–67%) and the preliminary median PFS had not been reached (95% confidence interval: 4.3 months—not reached). 29 out of 33 responses (88%) were ongoing at the time of data cut-off; median time to response was 1.8 months, median time to complete response was 3.6 months. No unexpected toxicities were observed for the treatment combination and no infusion-related reactions were reported for MOR208. The most frequent adverse events with a toxicity grade 3 or higher were neutropenia, thrombocytopenia, febrile neutropenia and pneumonia, observed in 36% (29/81), 12% (10/81), 7% (6/81) and 7% (6/81) of patients, respectively. 41% (33/81) of patients required a reduction of their lenalidomide dose, from a starting dose of 25mg daily.
The figures below present the ORR and mPFS in the L-MIND study at the cut-off date of December 12, 2017:
| ORR | mPFS | |
|
|
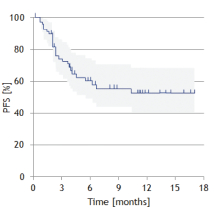
|
The primary analysis for the study is anticipated to be published in the fourth quarter of 2019. For a description of other studies with r/r-DLBCL patients, please see “—Competition—MOR208”.
| · | B-MIND: The B-MIND trial is a Phase 2/3 randomized multicenter study in which patients are randomized in a one-to-one fashion to receive either MOR208 in combination with bendamustine or rituximab in combination with bendamustine. The study was initiated in |
105
Table of Contents
| September 2016 at 180 centers across Europe, the Asia/Pacific region and the United States and will aim to enroll approximately 330 adult patients suffering from r/r DLBCL. Patients must have been treated previously with at least one but not more than three prior lines of therapy, including one anti-CD20 targeted therapy (such as rituximab). Patients must not be candidates for HDCT and ASCT. In June 2017, the Phase 3 part of B-MIND commenced. Prior to that, the Independent Data Monitoring Committee of the trial had supported its continuation as per protocol and the transition of the study into its Phase 3 part based on the available data from the Phase 2 safety evaluation. We expect to obtain the primary analysis of the study in the fourth quarter of 2019. |
| · | COSMOS: In addition to the two trials in r/r DLBCL described above, we are currently evaluating MOR208 in a Phase 2 trial in CLL and SLL. The trial, named COSMOS, is designed to evaluate MOR208 in combination with either idelalisib or venetoclax in patients who have relapsed or have become refractory after or have shown an intolerance to a prior therapy with a Bruton’s tyrosine kinase, or BTK, inhibitor such as ibrutinib. Currently these patients have very limited therapy options. 24 patients are enrolled in the study. The study is primarily investigating the clinical safety of the treatment combinations. We plan on presenting the initial results of the first cohort at an appropriate medical conference in 2018. |
Phase 2a Clinical Trial with MOR208 as a single agent in r/r NHL including DLBCL
We have conducted an open-label, Phase 2a, multicenter study to assess the activity and safety of weekly doses of 12 mg/kg MOR208 as a single agent in 92 pre-treated patients with various subtypes of r/r NHL including DLBCL, mantle cell lymphoma, or MCL, and other indolent NHL, or iNHL, including follicular lymphoma, or FL. 76 of the 92 patients were evaluable for post-baseline response assessment (with 16 patients discontinuing or being withdrawn from the study prior to any post-baseline radiological assessment). The ORR of MOR208 reached 36% in the DLBCL subgroup and 30% in iNHL patients (both based on evaluable patients). Based on all patients with DLBCL and iNHL in the study, the ORR was 26% and 29%, respectively. In addition to patients achieving a partial or complete response (PR, CR), a clinical benefit was also observed in other patients treated with MOR208. The majority of patients (5/6 DLBCL and 12/16 iNHL) with stable disease also had a reduction in the size of the target lesions. This resulted in a disease control rate of 40% in DLBCL and 73% in iNHL patients. Moreover, progression-free survival with MOR208 therapy was observed to be comparable in rituximab refractory and non-refractory NHL patients. The median progression-free survival, or PFS, was 2.7 months (95% CI 2.1–15.4) in patients with DLBCL, 8.8 months (95% CI 5.3–27.1) in patients with FL and not reached (95% CI 2.0–NA) in patients with other iNHL. Nine patients treated with MOR208 were still in remission, the longest responses were ongoing for 26 months at data cut-off. Of the 16 patients who discontinued the study or were withdrawn from the study prior to any post-baseline radiological assessment, three patients discontinued or were withdrawn as a result of adverse events (one for dyspnea and one for thrombocytopenia (both considered treatment-related by investigator); one for cytomegalovirus infection (not considered treatment-related by investigator)), four discontinued or were withdrawn as a result of death (none of these deaths were considered treatment related by investigator), one discontinued or was withdrawn as a result of protocol violation, eight discontinued or were withdrawn as a result of clinical progression or on the investigators’ decision without confirmation by imaging (CT or PET-CT).
According to data reported at the American Society of Hematology (ASH) 2016 conference, the most common adverse events were infusion-related reactions occurring in 12% (11/92) of the
106
Table of Contents
patients and neutropenia occurring in 12% (11/92) of patients. The incidence of MOR208 treatment-related serious adverse events was 6% (2/35) in DLBCL and 4% (2/45) in iNHL patients. No treatment-related deaths were reported.
A total of 28 patients (30%) experienced an aggregate of 35 serious adverse events, or SAEs, during the course of the study as set forth below. SAEs were experienced in System Organ Classes of general disorders and administration site conditions (eleven patients (12%) experienced twelve SAEs), infections and infestations (seven patients (8%) experienced seven SAEs), blood and lymphatic system disorders (three patients (3%) experienced five SAEs), gastrointestinal disorders (three patients (3%) experienced three SAEs), renal disorders (two patients (2%) experienced two SAEs), respiratory disorders (two patients (2%) experienced two SAEs), cardiac disorders (one patient (1%) experienced one SAE), injury and infestations (one patient (1%) experienced one SAE), hepatobiliary disorders (one patient (1%) experienced one SAE), and neoplasms (one patient (1%) experienced one SAE).
A causal relationship to the administration of MOR208 was suspected in four patients (4%). All of these SAEs occurred in patients with an FL subtype (two patients (6%) experienced two SAEs) and a DLBCL subtype (two patients (6%) experienced two SAEs). These four SAEs included myelodysplastic syndrome, genital herpes, dyspnoea, and febrile neutropenia. An SAE of myelodysplastic syndrome was reported for one patient approximately a year after the start of therapy with MOR208. According to the investigator, the event was attributed to previous anti-lymphoma treatment (eight cycles of R-CHOP administered during 2008), in addition to the MOR208 therapy. The sponsor’s assessment of the SAE also revealed a family history of cancer and prior exposure to chemotherapeutic agents which are a commonly reported cause of therapy-induced myelodysplastic syndrome, and the chromosomal abnormalities in this patient were consistent with those reported after exposure to alkylating agents with or without rituximab.
Phase 2 Investigator-Sponsored Trial (IST) with MOR208 in CLL (OSU-13031)
In an investigator-sponsored trial presented in December 2016, investigators evaluated MOR208 in combination with lenalidomide in three cohorts of patients with CLL: previously untreated CLL patients, r/r CLL patients, and patients with Richter’s Transformation. The trial also included a fourth cohort of ibrutinib-treated CLL patients with identified resistance mutations to ibrutinib in the tumors (molecular relapse) but no confirmed clinical relapse, where MOR208 was added to ibrutinib therapy. Historical data had generally shown poor clinical outcomes in patients who relapse after a therapy with the BTK inhibitor ibrutinib and whose leukemia cells carry a mutation in the BTK gene prior to relapse.
According to the data presented by investigators, 34 patients had been enrolled in the study so far, 27 receiving MOR208 in combination with lenalidomide (eleven of whom were in the previously untreated cohort, eleven in the r/r cohort, five in the Richter’s Transformation cohort) and seven receiving MOR208 plus ibrutinib, with patient accrual still ongoing.
A total of ten out of 34 patients (safety cut-off May 31, 2016) (29%) experienced an aggregate of 19 SAEs across all cohorts. SAEs were most frequently reported in the System Organ Classes of metabolism and nutrition disorders (three patients (9%) experienced five SAEs), infections and infestations (three patients (9%) experienced four SAEs), respiratory, thoracic and mediastinal disorders (two patients (6%) experienced two SAEs), general disorders and administration site conditions (two patients (6%) experienced two SAEs) and investigations (two patients (6%) experienced two SAEs). By Preferred Term, the most frequently reported SAEs were dyspnoea
107
Table of Contents
(two patients (6%) experienced two SAEs) and hypercalcaemia (one patient (3%) experienced two SAEs). All other reported SAEs were single occurrences including clostridium difficile colitis, metapneumovirus infection, pneumocystis jirovecii pneumonia, sepsis, dehydration, fluid retention, hyponatraemia, confusional state, cholelithiasis, renal failure, death (related to progression of underlying disease), systemic inflammatory response syndrome, blood lactate dehydrogenase increased, weight decreased and infusion related reaction.
A causal relationship to the administration of MOR208 was suspected in two patients (6%). These two SAEs included an infusion related reaction with symptoms of feeling warm, facial flushing, nausea, vomiting and dizziness, and one dyspnoea.
The most frequent hematological adverse events over all cohorts was thrombocytopenia in 47% of patients (6% grade 3 or higher) and neutropenia in 35% (21% grade 3 or higher). There were no unexpected SAEs reported, and no patient had developed progressive disease at the time of the abstract date cut-off. All enrolled patients were evaluable as per the protocol. In the cohorts of patients with treatment-naive or relapsed disease, six out of ten evaluable patients reached partial response as best response. Importantly, responses generally deepened over time in both cohorts. In addition, preliminary evidence of activity against CLL cells with BTK C481S has been observed in the cohort of patients with molecular progression on ibrutinib. BTK C481S variant allele frequency decreased or at least stabilized in six patients out of seven. The trial has been suspended for enrollment of new patients, while treatment of enrolled patients is still ongoing.
Phase 2 Trial with MOR208 as single agent in ALL
In 2013, we commenced a Phase 2 clinical trial of MOR208 in B cell acute lymphoblastic leukemia, or B-ALL. The U.S.-based open-label, multicenter, single-arm clinical trial was designed to assess the efficacy of MOR208 in patients suffering from r/r B-ALL. Secondary outcome measures included response duration, safety and pharmacokinetics of MOR208. Out of the 22 patients treated in this study, responses to MOR208 therapy were observed in two patients (one complete remission, or CR, and one complete remission with incomplete hematologic recovery), yielding an ORR of 9.1%. Recruitment was stopped after 22 patients had entered the treatment period.
CLL Phase 1 and Pre-clinical Development
In pre-clinical studies conducted by Xencor, MOR208 demonstrated FcgR-dependent anti-tumor activity against multiple human B cell lymphomas in vitro and anti-tumor effects in mouse lymphoma models. Xencor also demonstrated favorable half-life and B cell depletion in monkey models. Xencor submitted the IND for MOR208 to the FDA in February 2010.
In January 2013, Xencor completed a Phase 1 clinical trial of MOR208 in patients with high-risk, heavily-pretreated CLL. 27 patients were enrolled and were evaluable for response. Dose levels from 0.3 to 12 mg/kg were tested. The trial protocol was amended to include a period of extended dosing for a total of eight patients at the 12 mg/kg dose to study the effect of longer duration of exposure on safety and response rate. The primary endpoints for this clinical trial were safety, pharmacokinetics and immunogenicity. The secondary endpoints for this clinical trial included clinical responses assessed according to International Working Group on CLL, or IWCLL, 2008 and 1996 Guidelines. ORR by IWCLL 2008 criteria was 29.6% (eight partial responses in 27 evaluable patients). Using IWCLL 1996 response criteria resulted in a response rate of 66.7% (18 partial responses).
108
Table of Contents
MOR202
Overview
MOR202 is a recombinant human IgG1 HuCAL monoclonal antibody, or mAb, directed against the CD38 cell surface antigen, a therapeutic target for the treatment of multiple myeloma, or MM, and certain leukemias. Multiple myeloma is seldom curable and a majority of patients experience a relapse after initial responses. This has led to a particularly high demand for alternative treatments, such as those that target the CD38 surface antigen. CD38 is one of the most strongly and uniformly expressed antigens on the surface of malignant plasma cells, and is an established diagnostic marker for multiple myeloma. Scientific research suggest that an anti-CD38 antibody may have therapeutic activity in solid tumors including non-small cell lung cancer, or NSCLC, or autoimmune and other diseases driven by autoantibodies such as light chain amyloidosis or systemic lupus erythematosus.
We are currently investigating MOR202 alone and in combination with immunomodulatory drugs in a Phase 1/2a clinical trial program in patients with r/r multiple myeloma. We expect to present results from this trial in 2018. In addition, we plan to initiate a Phase 1/2 clinical trial evaluating MOR202 in NSCLC in 2018.
In November 2017, we and I-Mab entered into an exclusive regional licensing agreement to develop and commercialize MOR202 in Greater China (China, Taiwan, Hong Kong and Macao). Under the terms of the agreement, I-Mab Biopharma, Shanghai/China, will assume exclusive responsibility for all subsequent development and commercialization of MOR202 in the agreed territory. We received an immediate upfront payment of $20.0 million (equal to €16.8 million at the then-prevailing exchange rate). We will be entitled to receive additional success-based clinical and commercial milestone payments from I-Mab of up to approximately $100.0 million, as well as tiered double-digit royalties on net sales of MOR202 in the territory. I-Mab Biopharma intends to start clinical development of MOR202 in patients with multiple myeloma in China by the end of 2018.
We are currently investigating the possibility of further developing MOR202 outside China with a partner. We cannot ensure that any such partner will be found or that such collaboration will be successful. Due to the competition in MM and the size and the duration of a potential study in this indication, we intend to develop MOR202 in MM only with a strong and committed partner.
Description of Multiple Myeloma and Current Treatment
According to the National Cancer Institute SEER database, MM has an estimated annual U.S. incidence of 30,280. Multiple myeloma causes cancer cells to accumulate in the bone marrow, where they displace and suppress healthy blood progenitor cell populations. Multiple myeloma is also characterized by destructive lytic bone lesions (rounded, punched-out areas of bone), diffuse osteoporosis, bone pain, and the production of abnormal proteins, which accumulate in the urine. Anemia is also present in most multiple myeloma patients at the time of diagnosis and during follow-up. Anemia in multiple myeloma is multifactorial, and is secondary to bone marrow replacement by malignant plasma cells, chronic inflammation, relative erythropoietin deficiency, and vitamin deficiency.
There is currently no standard multiple myeloma treatment. A patient’s individual treatment plan is based on such factors as age and general health, results of laboratory and cytogenetic
109
Table of Contents
(genomic) tests, symptoms and disease complications, prior myeloma treatment, patient’s lifestyle, goals, views on quality of life, and personal preferences. In addition, many cancer centers have developed their own guidelines for treating myeloma.
Multiple myeloma drug therapies consist of two categories. The primary treatment regimens are cytoreductive chemotherapies, in combination with stem cell transplantation, aimed at achieving a cure, if possible. In addition, myeloma patients require substantial supportive therapy aimed at managing the complications of the disease (such as bone damage) and ameliorating the side effects of treatment. There are a number of drug classes for the treatment of multiple myeloma: monoclonal antibodies, immunomodulatory drugs (IMiDs®), proteasome inhibitors, chemotherapy, histone deacetylase inhibitor, and steroids.
Among monoclonal antibodies, Empliciti® (elotumumab) is a monoclonal antibody developed by Bristol-Myers Squibb and AbbVie against an antigen called SLAM7 and was the first monoclonal antibody approved for use in multiple myeloma. The anti-CD38 antibody Darzalex® (daratumumab), developed by Janssen and Genmab, was first approved for use in multiple myeloma in 2015. Among IMiDs® are Revlimid® (lenalidomide), an oral medication that is effective across the spectrum of myeloma disease and Pomalyst® (pomalidomide), both developed by Celgene. Proteasome inhibitors include Ninlaro® (ixazomib) or Velcade® (bortezomib) and Kyprolis® (carfilzomib). Steroids (corticosteroids) such as dexamethasone and prednisone have been used for decades to treat myeloma throughout the spectrum of disease. They are currently used in combination with other myeloma drugs.
The introduction of CD38 monoclonal antibodies to the treatment landscape of multiple myeloma, highlighted by the approval of daratumumab, might be transformative. Based on their distinct mechanisms of action, generally favorable toxicity profile, and single agent activity, CD38 antibodies are considered attractive partners in combination regimens. Deep responses and prolonged progression-free survival have been achieved in r/r MM patients when CD38 antibodies are combined with immunomodulatory agents or proteasome inhibitors.
MOR202 for Treatment of MM
MOR202 is our anti-CD38 antibody candidate, which is currently being developed in multiple myeloma. This antibody recognizes a unique epitope on CD38. Its key activities are ADCC and ADCP. It does not involve complement dependent cytotoxicity, or CDC, an additional mechanism involved in tumor cell killing. In addition, pre-clinical data point to a low NK-cell depletion.
The figure below depicts the suggested mechanism of action of MOR202:

110
Table of Contents
One of the key features of MOR202 is a low frequency of infusion-related reactions leading to a short infusion time of two hours or less. We believe the favorable safety and tolerability observed for MOR202 (trial code MOR202C101) may support further clinical development of MOR202 as a treatment option for subjects with MM, though we anticipate only advancing MOR202 with a partner.
We are also exploring on our own the use of MOR202 in the solid tumor setting in combination with an immune checkpoint inhibitor with an initial focus on NSCLC. Pre-clinical experiments in mouse NSCLC models demonstrated highly synergistic anti-tumor activity of immune checkpoint inhibitor (for example anti-PD-L1) and anti-CD38 combination therapy.
Development of MOR202
We are currently investigating MOR202 alone and in combination with immunomodulatory drugs in a Phase 1/2a clinical trial program in patients with r/r MM. We filed an IND-equivalent in Germany and Austria to evaluate MOR202 in 2011.
Active Clinical Trials
MOR202 is undergoing a Phase 1/2a trial in patients with r/r MM. The study is evaluating the safety and preliminary efficacy of MOR202 alone and in combination with the immunomodulatory drugs pomalidomide, or pom, and lenalidomide in patients with r/r MM. These regimens include the addition of dexamethasone, or dex, in each cohort. The primary endpoints of the trial are the safety, tolerability and recommended dose of MOR202 alone and in combination with immunomodulatory drugs. Secondary outcome measures are pharmacokinetics and preliminary efficacy based on ORR, duration of response, time-to-progression, and progression-free survival.
In the trial, MOR202 was administered as a two-hour or shorter infusion up to the highest planned dose of 16 mg/kg. The latest publication of clinical data at the American Society of Clinical Oncology Annual Meeting 2017 (with a data cut-off from April 19, 2017) reported on 48 patients receiving at least one dose of study drug of the clinically relevant cohorts, i.e. patients exposed to doses and combination therapies potentially further explored in clinical development of MOR202. The cohort of patients treated with MOR202 in combination with len/dex had a median of two prior lines of therapy and an ORR of 71%, based on the “intent to treat” population, with the treatment of nine patients still ongoing at the data cut-off. The median PFS of this patient group was not yet reached.
The cohort of patients treated with MOR202 in combination with pom/dex had a median of three prior lines of therapy and have thus far shown an ORR of 46% with treatment of eight patients still ongoing at the data cut-off. It is important to note that the data of this combination treatment were still relatively immature and that responses in this patient group are often detected after a longer treatment time. The current median PFS of this combination is 17.5 months with a median follow-up period of 8.5 months.
The cohort of patients treated with MOR202 plus dex had a median of three prior lines of treatment before study entry. Median PFS of this cohort was 4.7 months, with a median follow-up of 22.1 months. Five out of 18 patients had a response (ORR of 28%, based on the intention-to-treat population).
Infusion-related reactions occurred in only 6% of patients in the clinically relevant dose cohorts of MOR202 (4 mg/kg, 8 mg/kg, 16 mg/kg) plus dex, len/dex or pom/dex and were limited to
111
Table of Contents
grades 1 and 2. Differentiated by the treatment arms with MOR202 in the study, two out of 18 patients (11%) treated with MOR202 plus dex showed an infusion-related reaction, or IRR, one out of 17 patients (6%) treated with MOR202 plus len/dex recorded an IRR and no patient in the MOR202 plus pom/dex cohort experienced an IRR. No unexpected safety signals have been observed: based on published literature, observed safety findings for MOR202 were qualitatively and quantitatively (i.e., regarding nature and incidence) comparable to daratumumab, another antibody directed against CD38. Expected safety events included low cell counts of various types of blood cells (for example, white blood cells, platelets, and thrombocytes). Two patients discontinued due to a MOR202-related adverse event (one grade 4 thrombocytopenia, meaning occurrence of a low platelet count of below 25,000 per microliter (platelets are a type of blood cell involved in the stopping of bleeding); one grade 3 acute kidney failure). 41 (85%) patients experienced any frequent adverse events of grade 3 or higher, defined as an adverse event of grade 3 or higher occurring in at least 10% of patients of any cohort. The most frequent treatment emergent adverse events of grade 3 or higher were lymphopenia, neutropenia and leukopenia. The numbers were 19 (40%), 20 (42%) and 16 (33%) patients, respectively. No MOR202 treatment-related deaths were reported. Adverse events in this study were assessed and classified according to the CTCAE.
In August 2017, enrollment for the study completed.
Pre-clinical Development
In vitro binding studies showed that the binding affinity of MOR202 to CD38 is in the low nanomolar range. The in vivo efficacy of MOR202 was demonstrated in MM and lymphoma xenograft models in severe combined immunodeficient, or SCID, mice. MOR202 reduced tumor growth, increased survival and decreased bone lysis induced by MM cells inoculated into the tibiae of SCID mice. In vitro combination studies of MOR202 with lenalidomide enhanced cytotoxicity on MM cell lines. In vivo, the combination of MOR202 with lenalidomide showed synergistic effects on survival and inhibition of MM cell-induced bone lysis.
MOR106
Overview
MOR106 is an investigational human monoclonal anti-IL-17C antibody derived from our Ylanthia library, currently in development for the treatment of atopic dermatitis, or AD. In our pre-clinical studies, MOR106 has been shown to inhibit the binding of IL-17C to its receptor thus abolishing its biological activity. Results from rodent inflammatory skin models of atopic dermatitis and psoriasis support clinical development of MOR106.
We are developing MOR106 in collaboration with Galapagos. We share research and development costs equally with Galapagos, as well as all future economics.
In September 2017, we announced results of a Phase 1 clinical study with MOR106 in healthy volunteers and patients with atopic dermatitis. In the study, MOR106 was generally well-tolerated and showed first signs of clinical activity in five of six patients with moderate-to-severe AD, supporting progression to Phase 2 clinical studies. We plan to initiate a Phase 2 study with our partner Galapagos in the first half of 2018.
112
Table of Contents
Description of Atopic Dermatitis and Current Treatments
AD, the most severe and common type of eczema, is a chronic relapsing inflammatory skin disease that causes severe itch, dry skin and rashes, predominantly on the face, inner side of the elbows and knees, and on hands and feet. Scratching of the afflicted skin leads to a vicious cycle causing redness, swelling, cracking, scaling of the skin and an increased risk of bacterial infections. Lichenification, thickening of the skin, is characteristic in older children and adults. The National Eczema Association estimates that atopic dermatitis affects over 30 million Americans or up to 25% of children and 2-3% of adults. 60% of AD-patients are diagnosed in the first year of life, and 90% of patients have a disease onset before age five. Symptoms commonly fade during childhood, however, approximately 10-30% of the patients will suffer from atopic dermatitis for life. A smaller percentage first develop symptoms as adults.
An array of treatment options is used to moisturize skin, reduce inflammation, clear eczema, alleviate itch, and maximize restful sleep in AD patients. AD management begins with over-the-counter emollients. Pharmacotherapy or, in some cases, UV phototherapy is prescribed as needed to better control disease manifestations. Topical pharmacotherapies like topical corticosteroids, topical calcineurin-inhibitors or topical PDE4-inhibitors are mainly used to treat mild-to-moderate cases of AD. In moderate-to-severe cases of AD, which cannot be controlled by topical agents alone, oral or parenteral systemic therapies are employed. Current systemic therapies for moderate-to-severe, topical treatment-refractory patients comprise conventional orally administered immunosuppressants such as systemic corticosteroids, cyclosporine A, mycophenolate, methotrexate, azathioprine, phototherapy, and a targeted biologic agent applied as subcutaneous injection—Sanofi/Regeneron’s anti-IL-4 receptor alpha antibody Dupixent®, which was launched in 2017.
MOR106 for Treatment of Atopic Dermatitis
We believe MOR106 is the first antibody in clinical development worldwide targeting the IL-17C antigen. MOR106 binds to the cytokine IL-17C, a novel target, which is up-regulated in inflammatory skin disorders such as psoriasis and atopic dermatitis. Based on findings from pre-clinical models, IL-17C plays an important and pro-inflammatory role in such skin disorders. Importantly, IL-17C has been shown to be distinct from other members of the IL-17 cytokine family in its biological function, as it is produced by different cell types, predominantly epithelial cells, and signals via a different, specific receptor.
113
Table of Contents
In inflammatory skin disorders, IL-17C has been identified as an important pro-inflammatory mediator. IL-17C is a cytokine expressed by epithelial cells, such as skin keratinocytes, and binds to its receptor consisting of the subunits IL-17-RA and IL-17-RE. Binding of IL-17C to its receptor is assumed to trigger an inflammatory cascade that plays a promoting role in skin diseases. By specifically binding to IL-17C, MOR106 is designed to block the binding of the cytokine to its receptor, thus neutralizing the cytokine’s biological activity. The figure below depicts the suggested mechanism of action of MOR106:
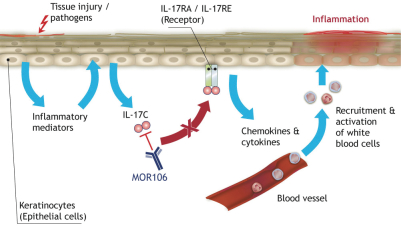
Development of MOR106
We filed clinical trial applications in various European countries in 2016 to evaluate MOR106 in clinical studies. We have investigated MOR106 in a Phase 1 clinical trial in healthy volunteers and patients with AD and are preparing initiation of a Phase 2 trial, with our co-development partner Galapagos, in 2018.
Active Clinical Trials
We have reported results from a Phase 1 study in September 2017 and in February 2018 investigating the safety, tolerability and pharmacokinetic profile of MOR106 when administered intravenously, or IV, in single ascending doses in healthy volunteers as well as in multiple ascending doses in patients suffering from atopic dermatitis. The primary objective of the Phase 1 study was to evaluate the safety and tolerability of MOR106. The study’s secondary objective was to characterize the pharmacokinetic profile of MOR106 in patients. Exploratory objectives included the measurement of early signs of efficacy. In the single ascending dose part of the study, 56 healthy volunteers received an infusion (MOR106 (n=42); placebo (n=14)), followed by a 7-week follow up period. In the multiple ascending dose part of the study, 25 patients diagnosed with moderate-to-severe atopic dermatitis (MOR106 (n=18); placebo (n=7)), received four infusions, once a week for four weeks, followed by a 10-week follow-up period. Patients received either placebo or MOR106 in a one-to-three ratio of placebo and three different dose levels of MOR106, 1, 4, and 10 mg/kg body weight.
In the study, MOR106 was generally well tolerated. Any adverse drug reactions observed in relation to MOR106 were mild-to-moderate and transient in nature. No serious adverse events or infusion-related reactions were recorded. MOR106 reported a favorable pharmacokinetic profile with dose-dependent exposure and a half-life in patients in line with what was observed
114
Table of Contents
in healthy volunteers. Even though the study was not designed to show statistical differences in efficacy between treatment groups, an improvement of at least 50% measured by the eczema area and severity index (or EASI-50) at week four occurred in 83% of patients (five out of six) at the highest dose level of MOR106 compared to 17% of patients (one out of six) with an EASI-50 improvement at week four who were receiving placebo. Pooled data across all dose cohorts showed that patients treated with MOR106 achieved an EASI improvement compared to baseline of 58%, 62%, 72%, and 64% at week 4, 8, 12, and 14, respectively. For patients receiving placebo, the EASI improvement was 32%, 40%, 38%, and 50%, respectively, (as depicted in the figure below). A fast onset of response was observed, occurring within two to four weeks, depending on the dose administered. We consider responses observed as long-lasting given that they were maintained up to over two months after stopping treatment.
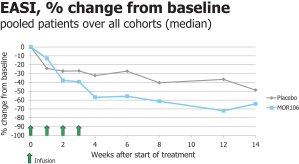
Figure: Effect of MOR106 on the EASI compared to placebo as seen in the Phase 1 study in atopic dermatitis. Changes in the EASI (in %) compared to baseline in patients receiving either MOR106 or placebo. Pooled data over all cohorts are shown for patients who received MOR106 or placebo, respectively.
These first signs of MOR106’s clinical activity, coupled with the observations that the compound was generally well tolerated in the Phase 1 study, support the plan to progress development to a Phase 2 clinical study.
In consideration of the chronic treatment envisaged and the importance of patient convenience, implementation of a subcutaneous application for MOR106 is under preparation.
Pre-clinical Development
In vitro, MOR106 inhibits the binding of human IL-17C to its specific receptor subunit IL-17-RE and inhibits the biological activity of IL-17C as determined in a NF-kB reporter gene assay in mouse NIH3T3 cells and in a more physiological assay using primary human keratinocytes endogenously expressing IL-17-RE/RA. In these functional assays, MOR106 inhibits the activity of cynomolgus monkey and mouse IL-17C with similar potencies as human IL-17C.
MOR106 prevented the occurrence of an atopic dermatitis-like skin inflammation in the MC903 (calcipotriol-induced) mouse model, with a significant impact on epidermal and dermal thickening, inflammation and type 2 T helper cell (Th2)-like gene expression. A therapeutic effect of MOR106 administration was also shown in the flaky tail mutant mouse, which exhibits a defective skin barrier function and spontaneously develops atopy evolving into a progressive overt AD-like dermatitis, reproducing cardinal features of AD in man. Beyond models of AD, MOR106 also displayed a protective effect in the IL-23-induced psoriasis-like skin inflammation
115
Table of Contents
mouse model with a significant impact on ear thickness, epidermal thickening and IL-23-induced gene expression.
For the non-clinical safety assessment of MOR106, mouse and cynomolgus monkey were identified as pharmacologically relevant animal species. The results of the in vivo toxicity studies demonstrated that MOR106 was well-tolerated in mouse and cynomolgus monkey.
MOR103/GSK3196165
Overview
MOR103/GSK3196165 is a fully human HuCAL antibody directed against the granulocyte-macrophage colony-stimulating factor, or GM-CSF. We discovered and advanced MOR103/GSK3196165 into clinical development and our partner GSK is now developing the antibody in the area of inflammatory diseases. Due to its diverse functions in the immune system, GM-CSF can be considered a target for a broad spectrum of anti-inflammatory therapies. GSK acquired the rights to MOR103/GSK3196165 pursuant to an exclusive worldwide development and license agreement that was entered into in June 2013.
We were responsible for completing a Phase 1b/2a clinical trial of MOR103/GSK3196165 in rheumatoid arthritis, or RA, which was completed in September 2012, and in multiple sclerosis, or MS, which was completed in September 2014. GSK is solely responsible, at its own cost, for all other development and commercialization activities. GSK is currently evaluating this antibody in several Phase 2 studies in patients with RA as well as in a Phase 2a trial in patients suffering from inflammatory hand osteoarthritis. GSK has reached primary completion of a Phase 2b study in RA as well as a Phase 2a study in hand OA, and we expect GSK will publish results from these studies in 2018.
Description of GM-CSF and Current Treatments
GM-CSF
GM-CSF stimulates stem cells to produce granulocytes and macrophages and can subsequently activate these differentiated immune cells. GM-CSF is part of the natural immune and inflammatory cascade but has also been identified as an inflammatory mediator in autoimmune disorders like RA leading to an increased production of pro-inflammatory cytokines, chemokines and proteases and thereby ultimately to articular destruction.
GM-CSF can act as a pro-inflammatory cytokine mainly by inducing the activation, maturation and differentiation of macrophages and dendritic cells, which are essential for the initiation and propagation of cell-mediated immune responses.
Rheumatoid arthritis
Rheumatoid arthritis, or RA, is a disabling and painful inflammatory condition, which can lead to substantial loss of mobility. The disease affects approximately four to six million people worldwide. In patients suffering from RA, white blood cells move from the bloodstream into the synovium, where they cause inflammation.
Disease-modifying anti-rheumatic drugs, or DMARDs, are routinely prescribed as first-line therapies for RA and have become the cornerstone of treatment, often prescribed to patients at all levels of disease severity. The conventional DMARDs most commonly used are methotrexate
116
Table of Contents
(Rheumatrex, Otrexup, Rasuvo, generics), leflunomide (Arava, generics), sulfasalazine (Azulfidine/Salazopyrin, generics), and hydroxychloroquine (Plaquenil, generics). For patients not responding to conventional DMARD treatment, TNF-a inhibitors are universally accepted as first-line biologic agents owing to their efficacy and to physician familiarity and comfort with these agents’ long-term post-marketing experience. At least five TNF-a agents are approved to treat moderately to severely active RA, under which are Enbrel (etanercept), a TNF-a receptor fusion protein, Remicade (infliximab), Humira (adalimumab), Simponi/Simponi Aria (golimumab), and Cimzia (certolizumab pegol), which are monoclonal antibodies against TNF-a. Multiple treatment options with different mechanisms of action are available for patients for whom TNF-a antibodies are contraindicated or who are not responding to TNF-a inhibitor treatment. The selective co-stimulation modulator Orencia® (abatacept) and IL-6 inhibitors Actemra®/RoActemra® (tocilizumab) and Kevzara® (sarilumab) have been approved for biologic-naive RA patients, and the B cell modulator Rituxan®/MabThera® (rituximab) is approved for patients who have failed at least one TNF-a inhibitor. Another biologic, Kineret® (anakinra), is marketed but rarely used for RA because of its lower efficacy.
Biosimilar versions of four molecules (infliximab, etanercept, rituximab, adalimumab) have been approved in the United States and Europe, though not all are available due to pending patent and exclusivity litigation. The availability of Jak inhibitors (Xeljanz®, Olumiant®), an oral class of agents with efficacy comparable to that of established biologic agents, is also expanding. A RANKL inhibitor, Prolia® (denosumab), has been approved for RA in Japan in July 2017, though this agent is specifically approved for inhibiting the structural damage (bone erosion) associated with RA and does not alleviate disease progression.
Osteoarthritis
Osteoarthritis, or OA, is a condition that causes damage to the surface of joints in the body leading to joint pain and stiffness. In some patients, it can adversely affect work and normal daily activities.
Currently available OA therapies focus on symptom relief, as there are no disease-modifying drugs approved for OA. Conventional pharmacological treatments include topical and oral nonsteroidal anti-inflammatory drugs, or NSAIDs, opioid analgesics, and intra-articular corticosteroid and hyaluronic acid injections. At present, a stepped-care approach is broadly used in the management of OA. First-line therapy generally involves non-pharmacologic interventions, followed by topical treatments and oral NSAIDs. For patients who do not achieve adequate pain relief with these first-line therapies, opioid analgesics may be prescribed. Both NSAIDs and opioids are associated with multiple adverse events, and it is recommended that they are prescribed in low doses for the shortest time possible. Acetaminophen was once widely used as a first-line therapy for mild OA. However, it has been associated with hepatic toxicity and multi-organ failure, and recent studies have indicated greater risk of adverse events associated with acetaminophen use than previously thought. More invasive modes of therapy involve corticosteroid injections, which provide effective but brief pain relief and are offered to patients who failed conventional oral therapies. A maximum of four steroid injections are recommended per year, making them a short-term solution. Patients with severe OA who do not respond sufficiently to any available pharmacological treatments are referred for joint replacement surgery, which represents the only curative medical intervention for OA.
117
Table of Contents
MOR103/GSK3196165 for Treatment of Anti-Inflammatory Diseases
MOR103/GSK3196165 a fully human HuCAL antibody directed against GM-CSF (granulocyte-macrophage colony-stimulating factor). GM-CSF levels are significantly elevated in several inflammatory disorders and in the joints of rheumatoid arthritis patients. By neutralizing GM-CSF, the HuCAL antibody MOR103 has demonstrated its ability to reduce GM-CSF induced proliferation and activation of inflammatory cells and to intervene in several pathophysiological pathways in pre-clinical models of RA. The figure below depicts the suggested mechanism of action of MOR103/GSK3196165:
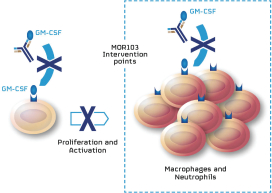
Development of MOR103/GSK3196165
MOR103/GSK3196165 has been investigated in various clinical trials addressing the indications RA, OA and MS. Phase 1/2 trials conducted by MorphoSys in RA and MS were completed in 2012 and 2014, respectively. GSK is currently investigating MOR103/GSK3196165 in several Phase 2 clinical studies in RA and hand OA.
Active Clinical GSK Trials
In the third quarter of 2015, GSK announced the start of Phase 2b clinical study to investigate MOR103/GSK3196165 in RA. The primary objective of the randomized, dose-adaptive, multicenter, double-blind, parallel group, placebo-controlled study is to assess the efficacy of MOR103/GSK3196165, in combination with methotrexate in 210 patients with active moderate-severe RA despite treatment with methotrexate.
In April 2016, GSK initiated a Phase 2a clinical study to investigate the effectiveness and safety of MOR103/GSK3196165 in patients with inflammatory hand OA. The goal of the European multi-center double-blind, placebo-controlled study is to investigate the efficacy and safety of subcutaneous injections of MOR103/GSK3196165 in adult subjects with inflammatory hand OA.
The main objective of the study is to assess the efficacy potential of MOR103/GSK3196165 on pain in inflammatory hand OA. Secondary objectives include the assessment of safety and pharmacokinetics of MOR103/GSK3196165.
GSK is currently investigating MOR103/GSK3196165 in combination with methotrexate in other Phase 2 clinical studies in RA.
Phase 1b Clinical Trial in RA
In a randomized, double-blind, placebo-controlled Phase 1b/2a trial in 96 mild-to-moderate RA patients, MOR103/GSK3196165 was administered in four weekly doses of 0.3 mg/kg, 1.0 mg/kg
118
Table of Contents
or 1.5 mg/kg. The primary aim of the trial was to determine the safety and tolerability of multiple doses of MOR103/GSK3196165 in patients with active RA. Secondary outcome measures were pharmacokinetics, immunogenicity, and the product’s potential to improve clinical signs and symptoms of RA as measured by Disease Activity Score in 28 joints, or DAS28, American College of Rheumatology score measuring 20% / 50% / 70% improvement, or ACR20/50/70, and other criteria.
The best response was achieved in the 1.0 mg/kg dose cohort with an ACR20 score of 68% at week four, which was significantly higher than in the control arm. Of particular importance was the fast onset of action observed: within two weeks, up to 40% of patients achieved an ACR20 score. Improvement of DAS28 scores was rapid and significant over the treatment period of the study. ACR20/50 scores at week four are depicted in the table below.
| Results at week four (Majority of patients were on stable regimen of DMARDS) |
Placebo | MOR103 [0.3 mg/ kg] |
MOR103 [1.0 mg/ kg] |
MOR103 [1.5 mg/ kg] |
||||||||||||
| Number of patients |
27 | 24 | 22 | 23 | ||||||||||||
| Proportion of patients achieving ACR20 |
7% | 25% | 68% | 30% | ||||||||||||
| Proportion of patients achieving ACR50 |
4% | 4% | 23% | 9% | ||||||||||||
A total of 144 treatment-emergent adverse effects were reported in 54 (56.3%) subjects (42 subjects (60.9%) in the MOR103/GSK3196165 groups and 12 (44.4%) in the pooled placebo group). The most common treatment-emergent adverse event by preferred term in the active and placebo groups was nasopharyngitis. The incidences of fatigue, cough and adverse events related to RA (worsening or flares) in the MOR103/GSK3196165 group were more than four percentage points higher than in the placebo group. None of the adverse events were considered to be probably or definitely related to treatment. Adverse events possibly related to treatment were reported in seven placebo (14 adverse events) and ten MOR103/GSK3196165 subjects (19 adverse events). Only three adverse events (fatigue, scalingia and decreased diffusion capacity of the lung for carbon monoxide) were considered possibly related to treatment in more than one subject. All adverse events were judged to be of mild or moderate intensity except for one severe adverse event of hospitalization due to paronychia in the placebo group.
Phase 1b Clinical Trial in MS
In September 2014, we concluded a Phase 1b clinical trial of MOR103/GSK3196165 in patients with relapsing-remitting MS or secondary progressive MS with relapses. In this 20-week, double-blind, placebo-controlled, Phase 1b dose escalation study, patients received an intravenous infusion of placebo or 0.5 mg/kg, 1 mg/kg or 2 mg/kg MOR103/GSK3196165 every two weeks for ten weeks. 31 patients received treatment. The primary endpoint was safety assessed by adverse events, physical examinations, vital signs, clinical laboratory data, electrocardiograms, pulmonary function tests, and MS relapses.
A total of 184 treatment-associated adverse events were reported in 31 (96.8%) patients, with no overall indication of increased adverse event frequencies in the MOR103/GSK3196165 groups compared with placebo. The most common adverse events in all groups were nasopharyngitis, headache, and MS exacerbation. No cases of infusion-related reaction were reported.
119
Table of Contents
Eleven MS exacerbation events occurred in nine patients. MS exacerbation occurred in three (50.0%), five (62.5%), one (12.5%), and zero (0%) patients in the placebo, MOR103/GSK3196165 0.5, 1.0, and 2.0 mg/kg groups, respectively. Events occurred in both the treatment and follow-up periods in the placebo and MOR103/GSK3196165 0.5 mg/kg groups and during follow-up in the MOR 1.0 mg/kg group. All events either resolved/recovered or were resolving/recovering at the end of the follow-up period; recovery with sequelae was reported for three exacerbations.
A total of 71 adverse events considered to be possibly, probably, or definitely related to treatment, were reported in 18 (58.1%) patients. Adverse events were generally of mild or moderate intensity. There were two severe adverse events (urinary tract infection, placebo group; decreased vibratory sense in right lower limb, MOR103/GSK3196165 2.0 mg/kg group) both of which were considered unlikely to be related to treatment. No adverse events led to death or trial discontinuation.
MOR107
Overview
MOR107 is a lanthipeptide based on our proprietary technology platform and a selective agonist of the angiotensin II receptor type 2, or AT2R. Lanthipeptides are a novel class of therapeutics with potential high target molecule selectivity and high in vivo stability. Lanthipeptides can be designed to combine agonistic or antagonistic activity.
Development of MOR107
Clinical Trials
On February 16, 2017, we initiated a first-in-human Phase 1 study of MOR107 in healthy male volunteers. 24 participants were dosed. The study was conducted by Quotient Clinical (now Quotient Sciences) at their phase 1 unit in Nottingham, United Kingdom. The primary endpoint of the single-center randomized, double-blind, placebo-controlled study was to assess safety and tolerability of subcutaneously administered single ascending doses of MOR107 in healthy male volunteers. The secondary endpoint of the study was to assess the pharmacokinetics and pharmacodynamics of subcutaneously administered single ascending doses of MOR107.
MOR107 is formulated as a solution that is administered by subcutaneous injection. In May 2017, the first part of the clinical study in healthy volunteers was completed. The trial included three dose cohorts of MOR107. MOR107 was well tolerated following single doses of 0.001, 0.01 and 0.1 mg. Following single subcutaneous administration MOR107 demonstrated a rapid absorption from the subcutaneous injection site and exposure increased in an apparent dose-proportional manner. There were no deaths or SAEs, and no subject was withdrawn from the safety follow up as a result of an AE. The incidence of AEs was low. For each MOR107 dose group, one AE was reported by one participant (16.7%). All AEs were classed as mild in severity. No laboratory test result, vital sign measurement, ECG measurement, physical examination or injection site assessment was considered clinically significant.
Pre-clinical Development
In pre-clinical in vivo studies, MOR107 has demonstrated AT2R-dependent activity.
Three in vivo and one in vitro safety pharmacology studies that investigated potential physiological effects of MOR107 on the central nervous system, respiratory and cardiovascular
120
Table of Contents
systems did not reveal any detrimental effects over the dose range tested. Dose ranging toxicology studies using both the intravenous and subcutaneous routes in rat and dog were carried out but no maximum tolerated dose has yet been identified. Based on initial anti-tumor data, MOR107 is currently in pre-clinical investigation with a focus on oncology indications.
Partnered Discovery Programs
In our Partnered Discovery programs, we apply technologies for the research, development and optimization of therapeutic antibodies as product candidates in partnership with pharmaceutical and biotechnology companies.
The table below sets forth the clinical pipeline for our Partnered Discovery programs. In addition, we have 54 partnered product candidates that are in the discovery phase and 24 partnered product candidates that are in pre-clinical development.
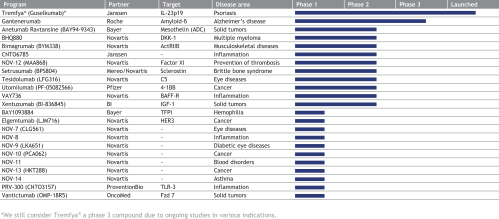
Tremfya®
Overview
Tremfya® is a fully human anti-interleukin (IL)-23 monoclonal antibody developed by Janssen. The HuCAL antibody library technology was used to generate the Tremfya® antibody under a license from us. IL-23 is a pro-inflammatory protein which has been identified as a cytokine in autoimmune diseases. IL-23 is found in the skin of patients with psoriasis and in other inflammatory diseases. It is therefore considered as a potential treatment target for inflammatory diseases. The antibody binds to the so-called p19 subunit unique to IL-23. Antibodies that bind to IL-23’s p40 subunit will also neutralize IL-12 and are therefore less specific. Tremfya® is the first approved antibody binding the p19 subunit of IL-23.
In July 2017, Janssen announced it had received U.S. market approval from the FDA for Tremfya® for the treatment of adult patients suffering from moderate-to-severe plaque psoriasis. We received a milestone payment from Janssen related to the approval. In mid-September 2017, the Committee for Medicinal Products for Human Use, or CHMP, of the EMA recommended approval in Europe of Tremfya® for the treatment of patients with moderate-to-severe plaque psoriasis. The EU Commission granted European approval in November 2017. Also in November 2017, Janssen announced that it had received Health Canada approval in Canada for Tremfya® for the treatment of adult patients suffering from
121
Table of Contents
moderate-to-severe plaque psoriasis. At the beginning of April 2018, Janssen reported that Tremfya® was approved for the treatment of adults suffering from moderate-to-severe plaque psoriasis in Brazil and Australia. Also early April 2018, Janssen reported that Tremfya® was approved in Japan for the treatment of three forms of psoriasis (plaque, pustular, and erythrodermic) and psoriatic arthritis in patients with moderate-to-severe disease for whom other existing treatments have failed.
Tremfya® is also being developed by Janssen for the treatment of patients with psoriatic arthritis, or PsA. Janssen has also announced plans for a Phase 3 program to investigate Tremfya® in patients with Crohn’s disease. It is also pursuing additional marketing-based studies in plaque psoriasis.
Description of Psoriasis and Current Treatments
Psoriasis is an inflammatory autoimmune disease of the skin. The associated inflamed skin patches may vary in severity from small and localized to complete body coverage. There are five main types of psoriasis: plaque, guttate, inverse, pustular, and erythrodermic. Plaque psoriasis, also known as psoriasis vulgaris, makes up about 90% of cases. It typically presents with red, itchy and scaly patches with white scales on top (plaques). Psoriasis is usually chronic and has a high morbidity and negative impact on patients’ quality of life. It is estimated that more than 7.5 million Americans live with the disease. Approximately 80% of those affected with psoriasis have mild-to-moderate disease, while 20% have moderate-to-severe plaque psoriasis.
Psoriatic arthritis is a chronic immune-mediated inflammatory disease characterized by both joint inflammation and the skin lesions associated with psoriasis. It is estimated that one third of the 125 million people living with psoriasis worldwide will also develop psoriatic arthritis. The disease causes pain, stiffness and swelling in and around the joints and commonly appears between the ages of 30 and 50 but can develop at any time. Though the exact cause of psoriatic arthritis is unknown, genes, the immune system and environmental factors are all believed to play a role in the onset of the disease.
122
Table of Contents
Tremfya® mechanism of action
Tremfya® is a fully human monoclonal antibody directed against the p19 subunit of interleukin (IL)-23. IL-23 is a pro-inflammatory protein which has been identified as a cytokine in autoimmune diseases and IL-23 levels are elevated in the skin of patients with psoriasis and in other inflammatory diseases. By binding to IL-23, the antibody prevents the cytokine from binding to its receptor, thereby silencing ongoing autoimmune responses. The figure below illustrates the suggested mechanism of action of Tremfya®:
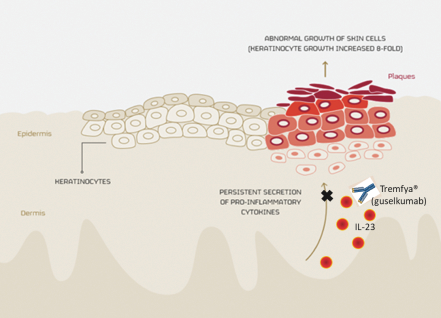
Clinical Development of Tremfya®
To date Tremfya® has been investigated in 16 clinical trials led by Janssen. These addressed the indications moderate-to-severe plaque-type psoriasis, pustular psoriasis, palmoplantar pustulosis and psoriatic arthritis and included two Phase 1 studies conducted and completed in healthy volunteers to assess the safety and pharmacokinetics of the antibody.
The majority of studies (nine in total) were initiated in patients with moderate-to-severe plaque-type psoriasis (plaque psoriasis), of which three have already been completed and the remaining trials are ongoing. In this indication, two Phase 1 trials had been completed analyzing either Tremfya® alone or Tremfya® in combination with P450 enzyme inhibitors. The remaining Phase 3 studies were either studies with Tremfya® versus a placebo and/or an active comparator drug: the Phase 3 VOYAGE-1 and VOYAGE-2 compared Tremfya® to adalimumab (Humira®), while NAVIGATE compared Tremfya® to ustekinumab (Stelara®).
Another Phase 3 study, POLARIS, is still ongoing and compares Tremfya® to fumaric acid esters. The Phase 3 ECLIPSE trial is a head-to-head comparison trial to secukinumab (Cosentyx®).
Two Phase 3 trials are ongoing investigating Tremfya® versus placebo in pustular psoriasis and palmoplantar pustulosis, respectively.
In addition, Janssen has conducted three trials in psoriatic arthritis. One Phase 2 trial has been completed, while two Phase 3 trials are currently ongoing and recruiting patients.
123
Table of Contents
Clinical Development of Tremfya® in psoriasis
In October 2016, Janssen reported results from its Phase 3 clinical VOYAGE-1 trial in 837 patients with moderate-to-severe plaque psoriasis. In the study, the efficacy and safety of Tremfya® were compared with placebo and with Humira®. The data presented by Janssen showed that Tremfya® exhibited significantly better efficacy than placebo and superiority over Humira®. According to Janssen, the study met both the primary endpoints and all major secondary endpoints. For the primary endpoints, it was assessed whether signs and symptoms of psoriasis were improved, while delivering clear or almost clear skin (IGA 0 or 1 and PASI 90) at week 16, in patients receiving Tremfya® compared to those receiving placebo. An investigator global assessment score, or IGA, of 0 or 1 means a patient has either achieved completely clear skin (IGA 0) or almost completely clear skin (IGA 1). PASI 90 means that 90% of the psoriatic lesions have disappeared. For the secondary endpoints it was assessed in what percentage of patients the signs and symptoms of psoriasis were improved under treatment with Tremfya® compared to patients receiving Humira®.
In March 2017, Janssen presented results from two further Phase 3 studies, VOYAGE-2 and NAVIGATE, in patients with moderate-to-severe plaque psoriasis. Both studies met all primary endpoints, according to the abstracts submitted by Janssen to the American Academy of Dermatology 2017 meeting. According to Janssen, data from the VOYAGE-2 study showed that patients treated with Tremfya® experienced significant improvements in skin clearance and other measures of disease activity compared with placebo, and significantly greater improvements compared with Humira® (adalimumab). Data from Janssen’s NAVIGATE study showed that patients who had an inadequate response following treatment with the IL-12/23 monoclonal antibody STELARA® (ustekinumab) and who then switched to Tremfya®, showed significantly greater improvements in skin clearance compared with patients who continued to receive STELARA® (ustekinumab). In February 2018, Janssen reported additional data from the Phase 3 VOYAGE-2 study of Tremfya®, which demonstrated long-term skin clearance in patients with moderate-to-severe plaque psoriasis. According to Janssen, the new data showed that 86% of patients with moderate-to-severe plaque psoriasis receiving Tremfya® who achieved at least 90% improvement of the signs and symptoms of psoriasis measured by the Psoriasis Area and Severity Index (PASI 90) at week 28, maintained a PASI 90 response with continuous treatment through week 72. 11.5% of patients who were withdrawn from treatment maintained PASI 90 response. According to Janssen, 87.6% of patients originally randomized to Tremfya®, but withdrawn from treatment at week 28, regained a PASI 90 response within six months of reinitiating treatment with Tremfya®. Janssen further reported that no new safety signals were observed with continuous treatment or retreatment therapy with Tremfya® through week 100.
In May 2017, Janssen announced plans for new Phase 3 clinical studies with Tremfya®, which include a Phase 3 study to evaluate the comparative efficacy of Tremfya® versus Cosentyx® (secukinumab) for the treatment of moderate-to-severe plaque psoriasis (ECLIPSE study). Janssen initiated the ECLIPSE study in the first half of 2017.
Various other Phase 2 and Phase 3 studies with Tremfya® in patients with plaque psoriasis or other forms of psoriasis have been conducted or are still ongoing.
Clinical Development of Tremfya® in psoriatic arthritis and Crohn’s disease
In November 2016, Janssen had presented positive results from a Phase 2a clinical study evaluating Tremfya® in patients with PsA. The data published by Janssen showed that a
124
Table of Contents
substantially higher percentage of patients receiving Tremfya® achieved at least a 20 % improvement in signs and symptoms of the disease (ACR 20) at week 24, the study’s primary endpoint, compared with patients receiving placebo.
In September 2017, Janssen initiated two Phase 3 studies in psoriatic arthritis evaluating the efficacy and safety of Tremfya® in this inflammatory disease, which affects both the joints and the skin. Janssen made a milestone payment to us in connection with the initiation of these Phase 3 studies.
In May 2017, Janssen announced plans for a Phase 3 program to investigate Tremfya® in Crohn’s disease.
Commercialization of Tremfya®
Following FDA approval on July 13, 2017, Tremfya® has been made available to patients with plaque psoriasis in the U.S. since the end of July 2017, and we received our first royalty payment soon after. Following approvals in Canada and the EU, Tremfya® has also been made available to patients in Canada and in the EU. We received milestone payments from Janssen together with the FDA approval in July 2017 as well with Janssen’s filing for US approval announced in November 2016. We also received a milestone payment from Janssen at the start of Phase 3 development of Tremfya® in psoriatic arthritis in 2017. We are eligible to further milestone payments from Janssen for certain defined clinical and/or regulatory milestones related to the product. In addition, we are entitled to royalties on net sales of Tremfya®.
Gantenerumab
Gantenerumab is a HuCAL antibody directed against amyloid beta that is being developed by Roche for the treatment of Alzheimer’s disease.
Description of Alzheimer’s Disease and Current Treatments
Eight cognitive domains are commonly impaired in Alzheimer’s disease: memory, language, reception, attention, constructive ability, orientation, problem solving, and functional ability. Cognitive impairments in Alzheimer’s disease are progressive, and decline is inexorable. No product currently available can stop, prevent, or modify the progression of Alzheimer’s disease; instead, currently available therapies are prescribed with the goal of improving the quality of life of both patients and their caregivers, who must cope with the significant burden of this disease. These products provide only marginal, transient benefits, highlighting the need for new, more-effective therapies. Symptomatic therapies—AChEIs (donepezil, galantamine, and rivastigmine) and NMDA receptor antagonists (memantine)—are used to ameliorate the cognitive and functional symptoms associated with Alzheimer’s disease, whereas antipsychotics and antidepressants target secondary behavioral symptoms (such as depression and psychosis).
Gantenerumab for Treatment of Alzheimer’s Disease
Gantenerumab is an IgG1 antibody derived from a Partnered Discovery project with Hoffmann La Roche. The HuCAL antibody is directed against amyloid-beta (Ab) and binds the N-terminus and a section in the middle of the amyloid beta peptide. On binding, the antibody seems to neutralize and disrupt the formation of amyloid plaque and amyloid oligomers or might dissolve existing ones. In Phase 1 clinical trials, gantenerumab has been shown to reduce brain amyloid in mild-to-moderate Alzheimer’s disease patients. Load and location of brain Ab was determined
125
Table of Contents
by using positron emission tomography, or PET, imaging. After treatment with infusions of intravenous gantenerumab or placebo PET was performed using a radioactive Carbon-11 labelled tracer. Using this method a dose dependent reduction of brain amyloid level was measured.
The figure below depicts the suggested mechanism of action of gantenerumab:
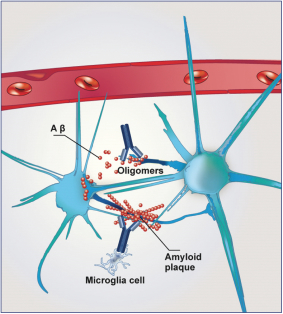
Clinical Development of Gantenerumab
Gantenerumab has been studied by Roche in several clinical trials in patients with Alzheimer’s disease, including two Phase 3 studies.
In 2014, we announced that gantenerumab had failed a futility analysis in the first Phase 3 trial.
In a later analysis, it was established that gantenerumab had been dosed significantly lower when compared to clinical trials conducted with aducanumab, an antibody against amyloid beta developed by Biogen with similar characteristics (e.g. epitope, affinity or IgG subtype) to gantenerumab. Aducanumab is the first antibody to show dose-dependent amyloid plaque reduction in combination with a clinical benefit measured by different readouts in patients. At the same time, aducanumab showed an acceptable side effect profile at the doses tested.
Roche is currently planning to examine a higher dose of gantenerumab in two Phase 3 trials, called GRADUATE 1 & 2, for patients with prodromal and mild Alzheimer’s disease.
Active Clinical Trials
In March 2017, Roche has announced to plan for the initiation of a new pivotal Phase 3 program for gantenerumab in patients with prodromal to mild Alzheimer’s disease. We have been informed that Roche intends to commence preparations for two studies which will test gantenerumab at higher doses compared to the previous Phase 3 trials. The name of the planned studies was disclosed as GRADUATE 1 and GRADUATE 2.
126
Table of Contents
Commercialization of Gantenerumab
We are entitled to further milestone payments from Roche for certain defined clinical and/or regulatory milestones related to the product candidate. In addition, in the event of a future approval and commercialization of the antibody, we are entitled to receive from Roche tiered royalties of between 5.5% and 7% of the net sales generated with gantenerumab.
Key Partnered Development Programs in Phase 2 Clinical Trials
Anetumab Ravtansine
Anetumab ravtansine is an antibody-drug conjugate directed against mesothelin and is based on an antibody developed using our HuCAL technology. The mesothelin antigen targeted by anetumab ravtansine is expressed on various cancer types including malignant pleural mesothelioma, pancreatic and ovarian cancer.
Malignant pleural mesothelioma is a rare cancer disease affecting parts of the lung and the thorax, in particular the membrane that covers the surface of each lung. The disease is typically associated with asbestos exposure. The tumor is characterized as very aggressive leading to a poor prognosis in affected patients. Treatment options are very limited and there is currently no cure available. After initial diagnosis, patients are reported to have a median life expectancy of between 12 and 21 months depending on the stage of disease at diagnosis. About 10% of patients are reported to survive for five years from the time of diagnosis. Approximately 3,000 people in the U.S. are diagnosed with mesothelioma each year. On average, about 2,500 mesothelioma-related deaths occur in the U.S. each year.
In July 2017, our partner Bayer reported that a Phase 2 clinical study examining anetumab ravtansine in patients with malignant pleural mesothelioma did not meet its primary endpoint of progression-free survival in comparison to vinorelbine. The Phase 2 clinical trial was a randomized, open-label, active-controlled, multicenter superiority study evaluating the safety and efficacy of anetumab ravtansine as second-line treatment in 248 patients with advanced or metastatic mesothelin-positive malignant pleural mesothelioma whose disease had progressed after treatment with first-line platinum/pemetrexed-based chemotherapy.
Bayer reported further that anetumab ravtansine is currently being investigated in additional studies, as monotherapy and in combination, including a Phase 1b multi-indication study in six different types of advanced solid tumors, as well as a Phase 1b combination-study in patients with recurrent platinum-resistant ovarian cancer. Bayer has stated that based on the available data, the company remains committed to further evaluating the utility and safety of anetumab ravtansine across multiple tumor types with significant unmet medical need. Bayer further announced that, in the trial reported, the safety and tolerability of anetumab ravtansine were consistent with earlier clinical findings and that detailed study results are expected to be presented at an upcoming medical meeting.
Xentuzumab (BI 836845)
Xentuzumab (BI 836845) is a HuCAL antibody directed against IGF1-R which we identified in collaboration with Boehringer Ingelheim. The antibody is currently in Phase 1b/2 clinical development in solid tumors including metastatic breast cancer and lung cancer.
127
Table of Contents
Clinical studies with xentuzumab (BI 836845) include:
| · | a Phase 1b open-label trial of once daily oral treatment of afatinib plus weekly intravenous infusion of xentuzumab (BI 836845) in patients with epidermal growth factor receptor, or EGFR, mutant non-small cell lung cancer with progression following prior EGFR tyrosine kinase inhibitors; |
| · | a Phase 1b study in patients with different types of solid tumors aiming to find a safe dose of xentuzumab in combination with abemaciclib with or without hormonal therapies. The study also tests how effective these medicines are in patients with lung and breast cancer; and |
| · | a Phase 1b/2 randomized study of xentuzumab (BI 836845) in combination with exemestane and everolimus versus exemestane and everolimus alone in women with locally advanced or metastatic breast cancer. |
Bimagrumab (BYM338)
Bimagrumab is a HuCAL antibody directed against Activin receptor IIB, or ActRIIB.
In April 2016, Novartis confirmed that a Phase 2b/3 study investigating bimagrumab (BYM338) in the rare disease sporadic inclusion body myositis, or sIBM, did not meet its primary endpoint. All three of the Phase 3 studies in this indication were discontinued.
The HuCAL antibody’s active Phase 2 clinical trials in sarcopenia, a form of age-related muscle loss, and muscular atrophy after hip surgery were continued as planned.
In December 2016, Novartis announced on the website clinicaltrials.gov, that a Phase 2 trial with bimagrumab in another indication will be started. This trial is designed to assess the safety, pharmacokinetics and efficacy of the HuCAL antibody versus a placebo in around 60 obese patients with type 2 diabetes.
Setrusumab (BPS804)
Setrusumab (formerly BPS804) is a HuCAL antibody directed against sclerostin, or SOST, which we identified in collaboration with Novartis. The antibody was out-licensed from Novartis to Mereo Biopharma which is developing the antibody in the indication osteogenesis imperfecta (brittle bone disease), or OI.
OI is a rare genetic disorder that is characterized by fragile bones and reduced bone mass resulting in bones that fracture easily, loose joints and weakened teeth. In severe cases patients may experience hundreds of fractures in a lifetime. In addition, OI-patients often suffer muscle weakness, early hearing loss, fatigue, curved bones, scoliosis, respiratory problems and short stature. The majority of cases of OI (estimated at 85-90%) are caused by a dominant mutation in a gene coding for type I collagen, a key component of healthy bone. Current treatment of OI is supportive, focusing on minimizing fractures and maximizing mobility, but to date, there are no EMA or FDA approved treatments.
A Phase 2b, multicenter, multinational, placebo-controlled, double-blind, dose-finding study (ASTEROID study) in 140 adult patients with type I, III or IV OI is currently ongoing.
In November 2017, Mereo announced that the EMA granted PRIME designation to BPS804 for the treatment of OI. The PRIME initiative is a program launched by the EMA to enhance the EMA’s support for the development of treatments in diseases of high unmet medical need which
128
Table of Contents
may offer a significant therapeutic benefit over existing treatment options or address a disease where there are currently no treatment options. PRIME is designed to accelerate availability of these new treatment options to patients by appointing a CHMP rapporteur before submission of the marketing authorization application, offering early dialogue and scientific advice at key development milestones, and the potential to qualify products for accelerated review earlier in the application process.
Utomilumab
Utomilumab is an antibody derived from a partnered discovery project with Pfizer against solid and liquid tumors. The antibody targets CD137 (4-1BB), which is expressed by cell types of the hemato-poietic lineage, including T cells, B cells, T, NK-cells and dendritic cells. By binding to its target, the antibody is supposed to trigger different effects such as induction of proliferation, up-regulation of anti-apoptotic pathways, induction of maturation and upregulation of co-stimulatory molecules and production of pro-inflammatory cytokines, enhancing the immune system and anti-tumoral immune responses.
Utomilumab is currently being tested in Phase 1b/3 studies (Javelin DLBCL) in combination with various agents in DLBCL and in Phase 1b/2 study (JAVELIN Medley) in combination with avelumab in various solid tumors. Although it showed a manageable safety profile, early signs of efficacy were insufficient and did not support the advancing of utomilumab in combination with avelumab alone into Phase 3 trials.
The results of other ongoing studies involving utomilumab, including the triple combination of avelumab, utomilumab and PF-04518600 (OX40 agonist) in solid tumors, will further inform next steps for utomilumab.
In addition, Phase 2 combination studies have been announced (AVIATOR Phase 2 combination study in advanced breast cancer).
Other Partnered Development Programs
BHQ880—BHQ880 is an antibody directed against DKK-1 developed for Novartis. It is currently being tested in Phase 2 trials in multiple myeloma (renal insufficiency) and smoldering multiple myeloma.
CNTO6785—CNTO6785 is an antibody directed against an undisclosed target developed for Janssen /Johnson & Johnson. It is currently being tested in Phase 2 trials in chronic obstructive pulmonary disease and rheumatoid arthritis.
Elgemtumab (LJM716)—Elgemtumab (LJM716) is an antibody directed against HER3 developed for Novartis. It is currently being tested in a Phase 2 trial in esophageal squamous cell carcinomas, or ESCC, (in combination with BYL719) and in Phase 1 in HER2 positive cancer (in combination with BYL719 and trastuzumab and in combination with trastuzumab only).
Tesidolumab (LFG316)—Tesidolumab (LFG316) is an antibody directed against C5 developed for Novartis. It is currently being tested in clinical Phase 2 trials in age-related geographic atrophy (single-agent and in combination with CLG561), in panuveitis and paroxysmal nocturnal hemoglobinuria. Further, tesidolumab (LFG316) is being tested in a Phase 1 trial in patients with renal disease awaiting kidney transplants.
VAY736—VAY736 is an antibody targeting BAFF-R developed for Novartis. It is currently being tested in clinical Phase 2 trials in the indications pemphigus vulgaris, primary Sjögren’s
129
Table of Contents
syndrome, rheumatoid arthritis using ADCC mediated B cell depletion and BAFF-R blockade. Further, a clinical Phase 1 trial is ongoing in the indication primary Sjögren’s syndrome.
BAY1093884—BAY1093884 is an antibody targeting tissue factor pathway inhibitor developed for Bayer. It is currently being tested in a clinical Phase 1 trial in hemophilia.
NOV-7 through NOV 14—we have a number of early-stage product candidates directed against undisclosed targets being developed by Novartis. Each of the product candidates is currently being tested in clinical Phase 1 trials and are being developed in eye disease, diabetic eye disease, inflammation, cancer, blood disorders, thrombosis, and asthma.
PRV-300 (CNTO3157)—PVR-300 (CNTO3157) is an antibody targeting TLR-3 that has been developed for Janssen/Johnson & Johnson and was out-licensed to Provention Bio in 2017. Provention Bio announced its intention to start a Phase 1/2 clinical study with the antibody in moderate-to-severe ulcerative colitis in the first half of 2018.
Vantictumab (OMP-18R5)—Vantictumab (OMP-18R5) is an antibody targeting Fzd7 that has been developed for OncoMed /Bayer. It is currently being tested in clinical Phase 1 trials in breast cancer (combination with paclitaxel) and pancreatic cancer (combination with nap-paclitaxel and gemcitabine).
Additionally, as of December 31, 2017, for both our proprietary and partnered programs, we had 62 programs in discovery and 25 programs in the pre-clinical phase.
Further Development of Partnered Discovery Programs
Below is an overview of Phase 3 trials of partnered programs that are scheduled for primary completion in 2018, or were scheduled for primary completion in 2017 but did not lead to any newsflow so far, according to www.clinicaltrials.gov. as of April 6, 2018. Since our partners are solely responsible for clinical development, actual events might differ.
| NCT Number | Program / Study Name |
Partner | Title | Indication | Estimated Primary Completion Date | |||||
| NCT01224106 |
Gantenerumab | Roche | A Study of Gantenerumab in Participants With Prodromal Alzheimer’s Disease | Alzheimer’s Disease |
April 13, 2018 | |||||
| NCT03090100 |
Guselkumab, Secukinumab (ECLIPSE) |
Janssen /J&J |
A Study to Evaluate the Comparative Efficacy of CNTO 1959 (Guselkumab) and Secukinumab for the Treatment of Moderate to Severe Plaque-type Psoriasis | Psoriasis | September 28, 2018 | |||||
130
Table of Contents
| NCT Number | Program / Study Name |
Partner | Title | Indication | Estimated Primary Completion Date | |||||
| NCT02951533 |
Guselkumab / Fumaric Acid Esters (POLARIS) |
Janssen /J&J |
A Study to Compare the Efficacy of Guselkumab to Fumaric Acid Esters for the Treatment of Participants With Moderate to Severe Plaque Psoriasis | Psoriasis | September 13, 2017 | |||||
| NCT02325219 |
Guselkumab | Janssen /J&J |
An Efficacy and Safety of CNTO 1959 (Guselkumab) in Participants With Moderate to Severe Plaque-type Psoriasis | Psoriasis | September 21, 2018 | |||||
| NCT02343744 |
Guselkumab | Janssen /J&J |
An Efficacy and Safety Study of CNTO1959 (Guselkumab) in the Treatment of Participants With Generalized Pustular Psoriasis or Erythrodermic Psoriasis | Pustular Psoriasis |
August 31, 2018 | |||||
| NCT02641730 |
Guselkumab | Janssen /J&J |
An Efficacy and Safety of Guselkumab in Participants With Palmoplantar Pustulosis | Palmoplantar Pustulosis |
January 31, 2018 | |||||
131
Table of Contents
Below is an overview of Phase 2 trials of partnered programs that are scheduled for primary completion in 2018, or were scheduled for primary completion in 2017 but did not lead to any newsflow so far, according to www.clinicaltrials.gov. as of April 6, 2018. Since our partners are solely responsible for clinical development, actual events might differ.
| NCT Number | Program | Partner | Title | Conditions | Primary Completion Date | |||||
| NCT03118570 |
Setrusumab (BPS804) | Mereo/ Novartis | A Study in Adult Patients With Type I, III or IV Osteogenesis Imperfecta Treated With BPS804 (ASTEROID) | Osteogenesis Imperfecta, Type I, Osteogenesis Imperfecta Type III, Osteogenesis Imperfecta Type IV | June 30, 2018 | |||||
| NCT02152761 |
Bimagrumab (BYM338) | Novartis | Study of Efficacy and Safety of Bimagrumab in Patients After Hip Fracture Surgery | Muscle Wasting (Atrophy) After Hip Fracture Surgery | May 15, 2018 | |||||
| NCT02333331 |
Bimagrumab (BYM338) | Novartis | Dose Range Finding Study of Bimagrumab in Sarcopenia | Sarcopenia | December 16, 2018 | |||||
| NCT02468674 |
Bimagrumab (BYM338) | Novartis | A 24-week Off-drug Extension Study in Sarcopenic Elderly Who Completed Treatment in the 6-month Core Study | Sarcopenia | September 18, 2018 | |||||
| NCT02675803 |
VAY736 | Novartis | Safety, Tolerability, Pharmacokinetics and Pharmacodynamics Study of VAY736 in Rheumatoid Arthritis Patients | Rheumatoid Arthritis | January 22, 2018 | |||||
132
Table of Contents
| NCT Number | Program | Partner | Title | Conditions | Primary Completion Date | |||||
| NCT02149420 |
VAY736 | Novartis | PD of VAY736 in Patients With Primary Sjögren’s Syndrome | Primary Sjögren’s Syndrome | February 7, 2018 | |||||
| NCT02204072 |
Xentuzumab (BI-836845) | Böhringer Ingelheim | BI836845 Plus Enzalutamide in Castrate Resistant Prostate Cancer (CRPC) | Prostatic Neoplasms, Castration-Resistant | September 25, 2017 | |||||
| NCT02123823 |
Xentuzumab (BI-836845) | Böhringer Ingelheim | BI 836845 in Estrogen Receptor Positive Metastatic Breast Cancer | Neoplasms | May 28, 2018 | |||||
Our Technology Platforms
Our core technology platforms are HuCAL, Ylanthia and Lanthipeptides. HuCAL was our first-generation antibody platform. Its successor, Ylanthia, is based on our engineering know-how and experience in development of over 100 therapeutic antibody projects.
Human Combinatorial Antibody Library (HuCAL)
Our HuCAL technology permits the in vitro generation of highly diverse, fully human antibodies. The structural diversity of the human antibody repertoire is approximately 95% composed of seven variable heavy chain, or VH, and seven variable light chain, or VL, region genes. The combination of these genes gives rise to 49 frameworks in the HuCAL master library, which form the scaffolds for several billion distinct fully human antibodies. The seven VH and seven VL HuCAL library is then combined with a highly variable genetic “cassette” using our trinucleotide mutagenesis technology to permit any combination of amino acids at each single position of the CDR region in a ratio reflecting the one found in humans.
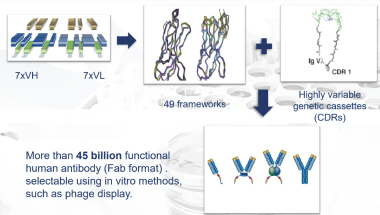
133
Table of Contents
A laboratory technique critical for the identification and eventual production of therapeutic antibodies aimed at specific antigens is known as “phage display”. Phage display enables the selection of specifically binding antibodies out of libraries containing billions of different antibodies.
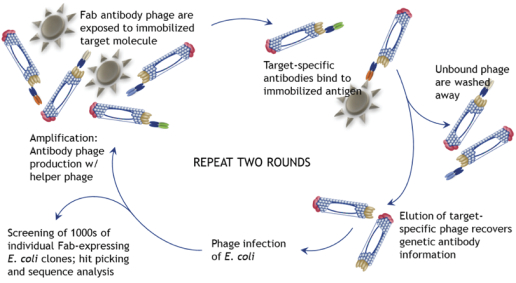
Phage display utilizes bacteriophages (viruses that infect bacteria) to connect proteins with the genetic information that encodes them. In traditional phage display as applied to antibody production, the gene encoding the antibody’s Fab fragment is inserted into a phage coat protein gene, causing the phage to “display” the Fab fragment on its outside while containing the gene for the Fab fragment. Displaying phages can be screened against the epitope(s) of interest which has been immobilized to the surface of a microtiter plate or is presented on the surface of a cell, and those phages displaying the Fab fragment of interest will bind to the surface. Those phages displaying other Fab sequences will be removed by washing. Phages that remain can be removed via the process of elution, and used to produce more phage by re-infection of bacteria, resulting in a phage mixture enriched for the Fab fragment of interest. The repetition of these cycles to create an increasingly purified phage mixture is known as the process of ‘panning’ (comparable to the original method of searching for gold in the beds of rivers). The ease of attachment and detachment of phages from the microtiter surface, and the overall speed of each cycle, can have a profound impact on the efficiency of antibody isolation and production.
Unlike conventional phage display technologies, in HuCAL, the antibody Fab fragment is not genetically fused to the phage coat protein. Instead, the Fab fragment forms a disulfide bond with an engineered gene III protein on the phage surface. This disulfide bond is sensitive to reducing agents, which allows for an efficient elution protocol to be used to recover phage displaying antibody fragments. Through this proprietary process, we are able to identify antibodies with high affinity for the antigen of interest in a highly efficient manner.
Generating antibodies using HuCAL technology involves seven steps: antigen immobilization, phage display selection, subcloning, primary screening, sequencing, expression and purification, and antibody quality control. The HuCAL process of production of monoclonal antibodies takes
134
Table of Contents
approximately eight weeks, in comparison to four to nine months for traditional conventional monoclonal discovery techniques. In addition, the antibodies produced are highly specific, maintain high production yields, exhibit a high degree of product purity and are capable of being produced in a number of formats, including monovalent or human immunoglobulin G.
Another key advantage of HuCAL phage display is the enhanced control of the selection process. The design of the selection process permits rapid identification of antibodies against specific antigens, the elimination or enhancement of cross reactivity against other antigens (as desired) and the generation of mouse cross-reactive antibodies for use in murine models. The modular design of HuCAL allows straightforward enhancement of affinities and switching among different antibody formats (such as ones which activate the immune system or are immunologically silent).
Today, thousands of antibodies have been made using the HuCAL technology, and over 20 HuCAL antibodies are in clinical trials.
Ylanthia
The Ylanthia antibody library is based on a concept that incorporates desirable antibody characteristics in its design through selection of optimal framework pairs and design of the complementary determining regions. Ylanthia is a new platform that provides fully human antibody candidates with optimized biophysical properties. This feature, called “developability”, is crucial for modern biologics development and production. In contrast to small molecules, the production of protein-based therapeutics (biologics) is a highly complex process. Final formulation requirements, including the production of proteins soluble at high concentrations in small volumes for subcutaneous injections, further raise the bar for success. Multiple biologics have failed in their development due to a poor “developability” profile. In Ylanthia, properties such as production yield, solubility, monomeric content, lack of immunogenicity, and absence of post-translational modifications have been optimized by the design of the library using 25 years of our protein engineering know-how. The size, sequence correctness and structural diversity also reflects the lessons we learned in modern biologics development in over 100 therapeutic antibody projects.
Key distinguishing and industry leading features of Ylanthia include:
| · | Size and heavy/light chain pairing: Ylanthia is one of the industry’s largest known antibody Fab library, comprising over 100 billion distinct, fully human antibodies. Ylanthia uses 36 fixed, naturally-occurring heavy and light chain framework combinations, which translates into extensive structural diversity. The library’s diversity delivers antibodies against previously inaccessible target molecules and unique epitope coverage. |
| · | Enhanced biophysical properties: Antibody frameworks were pre-selected for expression levels, stability and aggregation behavior. A shift towards higher stability and stress tolerance will increase shelf life and serum stability of resulting antibody products, making them more cost-effective to produce and administer. A higher solubility in turn opens up the path for more convenient formulations for patients, such as subcutaneous administration. These features obviate the need to engineer Ylanthia antibodies, which is common practice with other technologies. By avoiding engineering steps, development timelines are shortened and the risk that Ylanthia antibodies fail in the manufacturing and formulation process is reduced. |
135
Table of Contents
| · | Ability to address antigens that are difficult to target with antibodies, such as G-protein coupled receptors, or GPCRs, which are a very important product target class. This target class is notoriously difficult to address using other antibody technologies. GPCRs are proteins that are embedded in the cellular membrane with only small protruding portions (domains) are accessible to the antibody. Ylanthia was designed in a way that these small domains can also be targeted. |
| · | Rapid, highly efficient optimization: When needed, antibodies from the Ylanthia library are optimized using our Slonomics technology. Slonomics is a fully automated DNA synthesis platform that utilizes sets of double stranded DNA triplets in the controlled fabrication of highly diverse combinatorial gene libraries. With Slonomics, Ylanthia distinguishes itself from HuCAL, which relies on a modular gene design and pre-formed cassettes for antibody optimization. |
| · | Ylanthia demonstrated its ability to deliver drug-quality antibodies when MOR106 became the first Ylanthia antibody to enter clinical trials. |
| · | Ylanthia is used in all of our ongoing proprietary discovery projects. |
One goal using Ylanthia is to enhance the efficacy of direct tumor targeting using bi-specific antibodies that activate T cells, which are expected to kill the tumor cells. This approach is intended to allow for a binding of a bi-specific antibody to T cells via CD137 (4-1BB) and to the antigen present on the tumor cell, thereby enhancing T cell recruitment to the tumor as well as co-stimulation of tumor-specific effector T cells. Consequently, this is intended to result in enhanced activation of T cells and tumor cell killing.
CD137 is a validated, co-stimulatory checkpoint target expressed on T cells. First signs of efficacy with monospecific antibodies against CD137 (such as Utomilumab) in patients with advanced solid cancer have been shown. Furthermore. A bi-specific approach against CD137 and a tumor target is meant to increase efficacy and the therapeutic window by co-stimulation of tumor-specific effector T cells in the tumor microenvironment. Additionally, a potential advantage of this approach is reduced toxicity compared to a CD3-based bi-specific antibody due to the fact, that no activation of T cells will occur in the periphery. Moreover, the approach might offer a combination potential with further checkpoint modulators such as PD-1, PD-L1, CTLA-4 and others.
136
Table of Contents
The figure below depicts the suggested mode of action of a bi-specific anti-CD137 antibody within the tumor microenvironment in a schematic overview:
A) Tumor microenvironment: An antibody binding to both the tumor cell and CD137 is intended to induce activation of tumor-specific effector T cells and subsequent enhancement of tumor cell killing. B) In the periphery, binding of the bi-specific antibody to the T cell does not lead to T cell stimulation due to the absence of the tumor cell antigen.
Today, proprietary Ylanthia anti-CD137 antibodies have been generated that are equipotent to a competitor reference antibody as analyzed in relevant in vitro assays and a bi-specific, bi-valent effector-format with IgG-like properties has been established.
Lanthipeptides
With our acquisition of Lanthio Pharma B.V. in 2015, we gained full access to Lanthio Pharma’s proprietary LanthioPep technology. We believe this technology may be used to generate novel peptide products with therapeutic potential. We have subsequently developed a proprietary platform which permits phage display of lanthipeptides. This platform synergistically combines our expertise in phage display and library diversification of monoclonal antibodies with Lanthio Pharma’s knowledge of lanthipeptide biosynthesis.
As natural ligands to many targets, peptides may have agonistic or antagonistic activity with low toxicity risk and can be applied to disease targets, where small molecules or antibody-based products cannot be used. Furthermore, they can be chemically produced and alternative routes for drug delivery (such as inhalation) may be possible. However, the number of therapeutic peptide products is limited by properties that are inherent to many natural (wild-type) peptides, for example, that they often bind to multiple receptor subtypes and that the time they remain active in the body is usually very short. Our lanthipeptides offer the potential to overcome these problems and make it possible to discover highly target-selective peptides, which only bind and
137
Table of Contents
activate one specific target subtype. The following sets forth some of the benefits of our lanthipeptide technology:
| · | A peptide can have different structural conformations, which can allow it to bind to several different receptors. One conformation is often optimal for binding to one specific receptor. Our technology can be used to make lanthipeptides that are selective for one specific receptor by constraining them in the optimal structural conformation for binding to that receptor. In this way we have identified several selective and highly active agonistic peptides for various GPCR targets. |
| · | In addition, our technology not only constrains peptides in their optimal target binding conformation, but through the introduction of lanthionines, we have also developed protection against peptidase degradation. We have also established phage display of lanthipeptides, enabling the creation of lanthipeptide libraries from which lead molecules can be selected. We completed a clinical Phase 1 trial in the first synthetic lanthipeptide, MOR107, in 2017. |
Collaboration and License Agreements
A core component of our business model, and key aspect of our heritage as an antibody discovery and development company, is the entry into collaboration and licenses or partnership agreements with leading global pharmaceutical and biotechnology companies. Many of these research and development, collaboration and license agreements are entered into in the ordinary course of our business, and may or may not become significant or material to us, depending primarily on the development of the underlying product candidates.
Generally, our collaboration and license agreement may be for a specific therapeutic program or may be for multiple therapeutic programs across diseases. For programs that we out-license, we may participate in the development and generation of an antibody for a specified target and will have limited pre-clinical and clinical research and development obligations, with the licensee being primarily responsible for clinical development and commercialization. In general, pursuant to the collaboration agreements we enter into for programs that we out-license, most of our partners have the first right to prosecute, maintain and enforce patents for antibodies (and other patentable technology) developed with our technology. In the event that our partners determine to relinquish any such patent right, we generally have a first right to obtain ownership of such patents. We are generally entitled to milestone payments during the course of development of the therapeutic product and to royalty payments (generally a mid single-digit to low-teens percentage rate) upon commercial sale of the products. The royalty term generally will be on a product-by-product and country-by-country starting on the first commercial sale and ending on the later of: (i) the expiration of certain specified patent rights, (ii) a certain defined period of years following the first commercial sale, or (iii) the expiration of regulatory exclusivity. The agreements will generally terminate or expire once the obligation of the licensee to pay royalties has ceased.
Below is a description of our current significant, or material, collaboration and license agreements.
Collaboration and License Agreement with Xencor
In June 2010, we entered into a collaboration and license agreement with Xencor which was subsequently amended in March 2012 (which we refer to, as amended, as the Xencor
138
Table of Contents
Collaboration Agreement). Under the Xencor Collaboration Agreement, Xencor granted us an exclusive, worldwide license, including the right to sublicense under certain conditions.
Under the terms of the agreement, Xencor initiated and sponsored a Phase 1 clinical trial for MOR208 in patients with CLL which was completed in January 2013. Since the completion of such clinical trial, we have been responsible for all additional clinical development of MOR208.
Xencor already received an upfront payment of $13 million and received $15.5 million for development milestones under the Xencor Collaboration Agreement and is entitled to receive up to an additional $286.5 million in aggregated milestone payments upon the achievement of certain development events including $50 million in the aggregate with respect to sales of licensed antibody products. Furthermore, Xencor will also be eligible to receive tiered royalty payments upon commercialization of MOR208 in the mid single-digit to sub-teen double-digit percent range based upon net sales of licensed antibody sold by us or our licensees. Our royalty obligations continue on a product-by-product and country-by-country basis until the later to occur of the expiration of the last valid claim in the licensed patent covering a licensed product in such country, or 11 years after the first sale of a licensed product following marketing authorization in such country.
Under the Xencor Collaboration Agreement, Xencor retained the rights to prosecute, maintain and enforce certain patents licensed to us, including those patents licensed to us that were already filed as of the effective date of the Xencor Collaboration Agreement and whose claims cover MOR208 and certain other antibodies. We retain the rights to prosecute, maintain and enforce patents that cover MOR208 and no other antibody. Furthermore, Xencor retained the rights to prosecute, maintain and enforce certain patents directed to inventions developed under the Xencor Collaboration Agreement that were solely invented by or on behalf of Xencor.
The term of the Xencor Collaboration Agreement will continue until all of our royalty payment obligations have expired, unless terminated earlier. The Xencor Collaboration Agreement may be terminated by either party upon written notice to the other party immediately in the event of the other party’s insolvency or upon 120 days’ written notice for the other party’s uncured material breach (or upon 30 days’ written notice in the case of a breach of a payment obligation). Moreover, we may terminate the Xencor Collaboration Agreement without cause upon 90 days’ advance written notice to Xencor. In the event that (i) we terminate this agreement for convenience or (ii) Xencor terminates due to our material breach, our challenge of Xencor’s licensed patents or our insolvency, worldwide rights to develop, manufacture and commercialize licensed products, including MOR208, revert back to Xencor.
Research and License Agreement with Janssen (formerly Centocor)
In December 2000, we entered into a research and license agreement with Centocor (now Janssen), which was amended and restated in December 2004 (which we refer to as the Janssen Collaboration Agreement). Under the Janssen Collaboration Agreement, we obtained technology license fees and research and development funding and are now eligible to receive milestone payments, including up to €21.5 million in aggregated development and commercial milestone payments for therapeutic products, on a per target basis. In addition, we are eligible to receive tiered royalty payments in the mid single-digit percent range, on a product-by-product and country-by-country basis, until the later of (i) the expiration of the last licensed patent in such country having a valid claim covering such product and (ii) twelve years beginning from the first commercial sale of such product in such country.
139
Table of Contents
Under the Janssen Collaboration Agreement, we shared certain research and development responsibilities with Janssen to generate and develop HuCAL antibodies. Janssen provided funding for our research costs in support of the collaboration at a predetermined fee per full-time equivalent employee involved in research at our facilities. All of our research and development responsibilities have now ceased. Janssen is solely responsible for the further research, development, manufacturing, and commercialization of the products.
Either party may terminate the Janssen Collaboration Agreement for the other party’s uncured material breach or bankruptcy. We may terminate certain of Janssen’s commercial licenses if Janssen fails to diligently pursue the development of at least one therapeutic antibody product under such licenses, and Janssen may terminate its commercial licenses under the agreement at its sole discretion at any time, in each case after a certain notice period to the other party. Unless earlier terminated, the Janssen Collaboration Agreement will expire when all of Janssen’s obligations to pay royalties to us have ceased.
Intellectual property
Our commercial success depends in part on our ability to obtain and maintain proprietary or intellectual property protection for our product candidates and our core technologies and other know-how to operate without infringing, misappropriating or otherwise violating the proprietary rights of others and to prevent others from infringing, misappropriating or otherwise violating our proprietary or intellectual property rights. We protect our proprietary and intellectual property position by, among other methods, licensing or filing of patent applications covering our proprietary technologies and products in our home country and all major markets, with a particular emphasis on the United States. We also rely on trade secrets, know-how and continuing technological innovation to develop and maintain our proprietary and intellectual property position, which we generally seek to protect through contractual obligations with third parties.
Patents
Patents, patent applications and other intellectual property rights are important in the sector in which we operate. We consider on a case-by-case basis filing patent applications with a view to protecting certain innovative technologies, products, processes, and methods of treatment. We may also license or acquire rights to patents, patent applications or other intellectual property rights owned by third parties, academic partners or commercial companies, which are of interest to us.
As with other biotechnology and pharmaceutical companies, our ability to maintain and solidify our proprietary and intellectual property position for our product candidates and technologies will depend on our success in obtaining effective patent claims and enforcing those claims if granted. However, our pending patent applications, and any patent applications that we may in the future file or license from third parties may not result in the issuance of patents. We also cannot predict the breadth of claims that may be allowed or enforced in our patents or whether the claims of any issued patent will provide sufficient proprietary protection from competitors. Any issued patents that we may receive or license in the future may be challenged, invalidated or circumvented. For example, we cannot be certain of the priority of our patents and patent applications over third-party patents and patent applications. In addition, because of the extensive time required for clinical development and regulatory review of a product candidate we may develop, it is possible that, before any of our product candidates can be
140
Table of Contents
commercialized, any related patent may expire or remain in force for only a short period following commercialization, thereby limiting the protection such patent would afford the respective product and any competitive advantage such patent may provide. For more information regarding the risks related to our intellectual property, please see “Risk factors—Risks related to intellectual property”.
HuCAL
Our HuCAL platform patent portfolio is wholly owned and the platform is protected by several patent families. The basic HuCAL patents, based on WO97/08320, expired in August 2016. These patents covered the composition of the library, methods to isolate antibodies from the library and methods to diversify antibodies isolated from the library. At least four additional patent families protecting other technological aspects of the library, such as the specific CDR design (based on WO2008/053275) and certain display methods used with the library (based on WO2001/05950) as well as improvements of this method (based on WO2009/024593) are still in force in all major jurisdictions, including Australia, Canada, China, the European Union (EP2190987), Israel, Japan, New Zealand, South Africa and the United States. The last U.S. patent (US9062097) expires on February 18, 2030. Patents in other jurisdictions expire in August 2028. The HuCAL library is also protected by considerable know-how proprietary to us.
Ylanthia
Our Ylanthia antibody library patent portfolio is wholly owned and the platform is protected by two key patent families covering the composition of the library and nucleic acid collections encoding the library. Patent applications (based on WO2010/136598 and WO2012/066129) are filed in all major jurisdictions, including Australia, Canada, China, the European Union, Hong Kong, India, Israel, Japan, Mexico, New Zealand, Russia, Singapore, South Africa, South Korea and the United States. The patent term is expected to last at least until November 2031 (EP2640742—pending, intention to grant issued; US8367586). One material US patent, US9541559, expires on May 6, 2032. Certain patent protected ancillary technologies are used as well, including the Slonomics technology. Like the HuCAL antibody library, the Ylanthia library encompasses considerable know-how proprietary to us.
Slonomics
Our Slonomics technology patent portfolio is wholly owned by our subsidiary Sloning BioTechnology GmbH and the technology is protected by five patent families. The patent family covering the key technology, being a method used for the generation of diversified libraries, such as antibody libraries, has an expiry date of at least March 2029. The most relevant U.S. patent, US9115352, has an expiry date of December 6, 2030. Counterparts in the European Union (EP2110435) and Japan expire in March 2029.
MOR208
Our MOR208 patent portfolio is fully owned and/or exclusively licensed from Xencor and the program is currently protected by ten different patent families covering various aspects of the molecule, its compositions, methods of use, combination treatments, and formulation, as well as other aspects. The basic composition-of-matter patent was in-licensed from Xencor and was filed in Australia, Canada, the European Union, Hong Kong, India, Japan and the United States. The expiry date for the composition of matter patent is August 2029 for the United States and August 2027 for the other countries, not including any potential patent term extensions.
141
Table of Contents
Other patent families were filed and are prosecuted in Australia, Brazil, Canada, China, the European Union, Israel, India, Japan, Mexico, New Zealand, Qatar, Russia, Singapore, South Africa, South Korea and the United States.
MOR202
Our MOR202 patent portfolio is fully owned and the program is currently protected by eight different patent families covering various aspects of the molecule, its compositions, combination treatments, dosage regimens, radioconjugates, as well as assays utilized in clinical development. The basic composition-of-matter patent expires in October 2026, outside the United States, and in January 2028, in the United States, in both cases not including any potential patent term extensions. Patent were filed and are prosecuted in Argentina, Australia, Brazil, Canada, China, the European Union, Hong Kong, Israel, India, Japan, Mexico, New Zealand, Russia, Singapore, South Africa, South Korea, Taiwan and the United States. Rights to the Greater Chinese territory were exclusively licensed to I-Mab.
MOR103/GSK3196165
Our MOR103 patent portfolio is fully owned and exclusively licensed to GSK. The patent portfolio related to MOR103 consists of at least eight patent families covering various aspects of the program (composition-of-matter, indications, combination therapy, aspects of patient selection, as well as assays utilized in clinical development). Some of the patents were in-licensed from the University of Melbourne. The expiry date of the composition-of-matter patent is May 2026, not including any patent term adjustments or potential patent term extensions. Composition-of-matter patents were filed and are prosecuted in Argentina, Australia, Brazil, Canada, China, the European Union, Hong Kong, India, Israel, Japan, Mexico, New Zealand, Russia, Singapore, South Korea, Taiwan and the United States. Certain additional aspects were filed and are prosecuted in additional jurisdictions.
MOR106
The MOR106 patent portfolio is co-owned by us and Galapagos. The program is currently protected by six different patent families covering various aspects of the molecule, its composition, indications, methods of use, as well as other aspects. The basic composition-of-matter patent was filed as an international PCT patent application and in addition was filed in non-PCT countries Argentina, Taiwan and Venezuela. The Patent Cooperation Treaty, or PCT, is an international patent law treaty providing for a unified procedure for filing patent applications to protect inventions in each country party to the PCT. A patent application filed under the PCT is called an international application, or PCT application. The projected expiry date for the composition of matter patent is February 2037, not including any potential patent term extensions. Other patent families were filed and are being prosecuted in Australia, Canada, China, the European Union, Hong Kong, Israel, India, Japan, Korea, Mexico, New Zealand, Russia, Singapore, United States and South Africa.
Patent Term
The term of an individual patent depends upon the legal term for patents in the countries in which such patent is granted. In most countries, including the United States, the patent term is generally 20 years from the date on which the application for the patent was filed or, if the patent claims priority to an earlier filed application or applications, 20 years from the filing date
142
Table of Contents
of the earliest filed application where priority was claimed. In the United States, a patent’s term may, in certain cases, be lengthened by patent term adjustment, which compensates a patentee for administrative delays by the USPTO in examining and granting a patent, or may be shortened if a patent is terminally disclaimed over a commonly owned patent or a patent naming a common inventor and having an earlier expiration date. The Hatch-Waxman Act permits a patent term extension of up to five years beyond the expiration date of a U.S. patent as partial compensation for the length of time the product is under regulatory review while the patent is in force. The length of the patent term extension is related to the length of time the product is under regulatory review. Patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval and only one patent applicable to an approved product may be extended. Similar provisions are available in other jurisdictions to extend the term of a patent that covers an approved product, or to offer similar protection for an extended period, as is the case in the European Union. In the future, if and when our product candidates receive approval from the FDA or other regulatory authorities, we expect to apply for patent term extensions on patents covering those products where such extensions are available; however there is no guarantee that the applicable authorities, including the FDA, will agree with our assessment of whether such extensions should be granted and, even if granted, the length of such extensions.
Trade Secrets
In addition to patents, we rely upon unpatented trade secrets and know-how and continuing technological innovation to develop and maintain our competitive position. However, trade secrets and know-how can be difficult to protect. We seek to protect our proprietary information, in part, by executing confidentiality agreements with our partners, collaborators, scientific advisors, employees, consultants and other third parties, and invention assignment agreements with our consultants and employees. We have also executed agreements requiring assignment of inventions with selected scientific advisors and collaborators. The confidentiality agreements we enter into are designed to protect our proprietary information and the agreements or clauses requiring assignment of inventions to us are designed to provide mechanisms to grant us ownership of technologies that are developed through our relationship with the respective counterparty. We cannot guarantee that we have entered into such agreements with each party that may have or have had access to our trade secrets or proprietary technology and processes or that these agreements will afford us adequate protection of our intellectual property and proprietary information rights. If any of the partners, collaborators, scientific advisors, employees and consultants who are parties to these agreements breaches or violates the terms of any of these agreements or otherwise discloses our proprietary information, we may not have adequate remedies for any such breach or violation, and we could lose our trade secrets as a result.
Trademarks and domain names
We conduct our business using trademarks with various forms of the “MorphoSys” brand and numerous additional trademarks, as well as domain names incorporating some or all of these trademarks. Key trademarks are protected in all major jurisdictions, including the United States, the European Union, Switzerland, Canada, Australia and Japan.
143
Table of Contents
Manufacturing
We have adopted a manufacturing strategy of contracting with third parties in accordance with cGMP for the manufacture of drug substance and product. Additional contract manufacturers are used to fill, label, package and distribute investigational drug products. This allows us to maintain a more flexible infrastructure while focusing our expertise on developing our products. We will ultimately depend on contract manufacturers, or CMOs, for the manufacture of our products for commercial sale, as well as for process development. CMOs are subject to extensive governmental regulation. We currently rely on single source CMOs for each of MOR208, MOR202, MOR106 and MOR107. Although multiple potential CMOs are available as additional or alternative manufacturing partners for these product candidates, any such change in CMO would likely result in a delay in the development process of such product candidate.
We are able to internally manufacture the quantities of our product candidates required for relatively short non-GLP animal studies. We believe that this allows us to accelerate the product development process by not having to rely on third parties for all of our manufacturing needs. However, we do rely and expect to rely on a number of CMOs to produce sufficient quantities of our product candidates for use in lengthier non-GLP or GLP pre-clinical research.
Competition
We compete in an industry that is characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. Our competitors include pharmaceutical companies, biotechnology companies, academic institutions and other research organizations. We compete with these parties for promising targets for antibody-based therapeutics, new technology for optimizing antibodies and in recruiting highly qualified personnel. Many competitors and potential competitors have substantially greater scientific, research and product development capabilities as well as greater financial, marketing and sales and human resources than we do. In addition, many specialized biotechnology firms have formed collaborations with large, established companies to support the research, development and commercialization of products that may be competitive with ours. Accordingly, our competitors may be more successful than we may be in developing, commercializing and achieving widespread market acceptance. In addition, our competitors’ products may be more effective or more effectively marketed and sold than any treatment we or our development partners may commercialize and may render our product candidates obsolete or noncompetitive before we can recover the expenses related to developing and commercializing any of our product candidates.
Below is a description of competition in certain of our products and product candidates.
MOR208
DLBCL is curable in 60-70% of patients with the current standard treatment, a combination of chemotherapy and Roche’s monoclonal antibody rituximab (Rituxan®). The most widely used treatment is R-CHOP (rituximab plus a chemotherapeutic regimen consisting of cyclophosphamide, doxorubicin, vincristine, and prednisone). High-dose chemotherapy coupled with stem cell transplantation can be used as a potentially curative treatment in patients with DLBCL whose disease is refractory or relapsed following initial therapy. However, fewer than half of the patients relapsing after frontline therapy will eventually receive a stem cell transplant and fewer than half of those actually obtain a lasting remission.
144
Table of Contents
R/r DLBCL patients who are not candidates for stem cell transplantation, or who choose not to have a stem cell transplant, may be treated with various combination chemotherapy regimens with or without rituximab or lenalidomide with or without rituximab, although none of these agents or regimens have been approved in r/r DLBCL. In October 2017, a first CD19-directed CAR-T (Chimeric antigen receptor-T cell) therapy, axicabtagene ciloleucel (Yescarta®), developed by Kite (now Gilead), received accelerated FDA approval for r/r DLBCL.
In addition to MOR208, several novel individual and combination therapies are being tested in clinical trials for the treatment of patients with both newly diagnosed and r/r DLBCL.
In frontline DLBCL, the most promising therapies in pivotal clinical trials are currently considered to be: combination therapies of R-CHOP with targeted agents ibrutinib (a Btk-inhibitor from Janssen/AbbVie) or lenalidomide (an immune modulating agent from Celgene) or treatment with polatuzumab vedotin (an antibody drug conjugate targeting CD79b from Roche). For r/r DLBCL patients ineligible for stem cell transplantation, other important pivotal trials are ongoing with combination regimens: with polatuzumab, bendamustine plus rituximab, with the checkpoint inhibitors avelumab and/or utomilumab (both from Pfizer) plus rituximab plus chemotherapy and with pixantrone (CTI BioPharma/Servier) plus rituximab, and with the TG Therapeutics compounds umbralisib (a PI3K inhibitor) plus ublituximab (a CD20 antibody) in combination with bendamustine.
In almost all combination regimens, the CD20 antibody rituximab (Rituxan®) serves as a backbone therapy, meaning it is a therapeutic that can be combined with many other therapies for the treatment of the indication and has value for the overall efficacy of the treatment regimen. Other CD20 targeting antibodies obinutuzumab (Gazyva®) and ofatumumab (Arzerra®) have not been successful in showing superiority to rituximab in the treatment of DLBCL.
CD19 also serves as a target for: antibody drug conjugates (ADCs), bispecific antibodies and CAR-T cells. ADCs are antibodies that carry a cytotoxic payload that kills the cells upon internalization of the drug and release of the toxin (denintuzumab mafodotin). The bispecific antibody blinatumomab (Blincyto®) induces killing of tumor cells by linking cytotoxic T cells via its CD3 binding moiety to the B cell derived tumor cells via its CD19 binding arm. It is approved in r/r ALL and is in Phase 2 development in DLBCL. Various CAR-T approaches with T cells collected from the patient and engineered to express a CD19 binding antibody fragment on their surface are also in clinical trials. One of these therapies, Yescarta® obtained accelerated approval for treatment of refractory non-transplant-eligible DLBCL patients in October 2017. Despite showing promising efficacies in patients with an extremely dismal prognosis, CAR-T therapies have to date been associated with severe toxicities, as well as significant and costly manufacturing.
Interim L-MIND results in the context of other r/r DLBCL trial data
There is currently no approved treatment for patients with r/r DLBCL who are not eligible for HDCT and ASCT. The assessment of the activity of MOR208 combined with lenalidomide may be drawn from comparisons of the interim L-MIND data as of December 12, 2017 (N=68, 49% ORR; 31% CR; preliminary mPFS not yet reached) with previously published data from studies carried out in related patient populations. However, there are significant limitations in cross trial comparisons due to differences in patient population, different endpoints, different sites and
145
Table of Contents
investigators, different methodologies of laboratory analyses, and other factors. Such comparisons, therefore, must be interpreted with caution.
Monotherapy studies
Single agent lenalidomide was investigated by Czuczman et al. (2017) in a Phase 2/3 study of 51 patients with r/r DLBCL. The study included patients with DLBCL who had relapsed after or who were refractory to one chemotherapy regimen containing rituximab and an anthracycline or equivalent, as well as one or more additional combination chemotherapy regimens. Similar to L-MIND, trial participants were not eligible for stem-cell transplantation. For r/r DLBCL patients the ORR was 28%, with a CR rate of 10%. The median PFS was 3.1 months (13.6 weeks).
Witzig et al. (2011) also reported a Phase 2 study investigating single agent lenalidomide in patients with r/r aggressive NHL. Efficacy was studied in the subgroup of 108 patients with r/r DLBCL. The ORR was 28%, with a CR of 7%. The median progression free survival, or PFS, was 2.7 months.
Dual and triple combination therapy studies
Other studies in r/r DLBCL have examined the outcome for patients treated with two-agent or three-agent combination regimens.
Wang et al. (2013) investigated Rituximab® combined with lenalidomide in a Phase 2 study in patients with r/r DLBCL, transformed large cell lymphoma or follicular lymphoma who had received one to four prior lines of treatment. In the subgroup of 32 patients with DLBCL, ORR was 28%, with 22% of patients having a CR. The median PFS was 2.8 months.
Morschhauser et al. (ASH 2016) presented data of a combination study with obinutuzumab and lenalidomide. The Phase 1/2 trial in mantle cell lymphoma and DLBCL enrolled patients who had relapsed or became refractory after at least one prior anti-CD20 therapy with a median age of 70 years. In the 71 patient cohort with DLBCL, an ORR of 45% and a CR rate of 16% was reported. The median PFS was 4.1 month.
A further combination regimen, Rituximab® plus the cytotoxic chemotherapy agent bendamustine, was investigated by Vacirca et al (2014) in a Phase 2 trial in 59 patients (median age 74 years) with CD20-positive r/r DLBCL who had received at least one prior regimen. The ORR was 46%, with 15% of patients having a CR. The median PFS was 3.6 months.
Also in 2014, Dang et al. (ASH 2014) reported data from a Phase 3 trial in which Rituxan® plus the cytotoxic chemotherapy agent bendamustine was investigated in older patients (137 patients; median age was 69 years) with CD20-positive r/r DLBCL who had received at least one prior regimen. The ORR was 49%, with 18% of patients having a CR. The median PFS was 4.2 months. Median overall survival, or OS, was 9.5 months.
Sehn et al. (ASH, 2017) reported a randomized Phase 2 study comparing a combination of polatuzumab vedotin (an antibody drug conjugate directed against CD79b), Rituxan®, and bendamustine against Rituxan® and bendamustine in r/r DLBCL. The 80 patient trial (N=40 in each treatment arm) showed 33% ORR and 20% CR in the Rituxan® bendamustine arm compared to 70% ORR and 58% CR in the Rituxan® bendamustine plus polatuzumab vedotin arm. The median PFS of the doublet of 2.0 months was lower compared to the triplet with 6.7 months. Median OS was 4.7 months in the doublet compared to 11.8 months in the bendamustine plus polatuzumab vedotin arm.
146
Table of Contents
A recently published multi-cohort study (SCHOLAR-1) by Crump et al. (2017) retrospectively assessed outcomes in 636 patients with chemo-refractory DLBCL in order to provide a benchmark for future clinical trials in this population. Patients were treated with at least CD20 monoclonal antibody and an anthracycline. The ORR was 26%, with 7% of patients having a CR. Median OS was 6.3 months.
Chimeric antigen receptor (CAR) T cell therapy studies
Neelapu et al. (2017) reported the results from a Phase 2 study (ZUMA-1) with Axicabtagene ciloleucel, an autologous CAR-T cell therapy targeting CD19. Overall, 111 patients with refractory DLBCL, transformed follicular lymphoma or primary mediastinal B cell lymphoma were enrolled, of which 101 were treated. In the DLBCL subgroup, 81, mainly young (median age 58) and heavily pretreated patients were included of which 77 patients could be infused. Side effects like cytokine release syndrome, or CRS, and neurologic toxicities were reported. Three patients were reported to have died during treatment. In all groups, the ORR was 82% and the CR 55%; at six months, ORR was 48% and CR was 46%. In the DLBCL group, the ORR was 82% with a 49% CR (N=77). The median PFS (including also the patients with PMBCL and TFL) was 5.8 months.
Another CD19 targeted autologous T cell construct called CTL019 or Tisagenlecleucel is being investigated in r/r DLBCL. In the Phase 2 Juliet trial, 147 patients with r/r DLBCL were enrolled, of which 81 were infused. Side effects like CRS and neurologic toxicities were reported. According to data reported at the ASH 2017 conference, the ORR was 53% and the CR 40%; at six months, ORR was 37% and CR rate was 30%. In December 2017, results of another trial with CTL019 in lymphoma patients (r/r DLBCL, FL) were published in the New England Journal of Medicine (Schuster et al. (NEJM, 2017)): 28 patients, as in the ZUMA-1 trial, mainly young and heavily pretreated (median age 58) were included. Side effects like CRS and neurologic toxicities and one death were reported. For the r/r DLBCL subgroup (N=14) an ORR of 50%, a CR rate of 43% and a median PFS of 3.2 months were reported.
MOR202
There is no curative treatment for r/r MM. Products approved for the treatment of r/r MM include: Darzalex® (daratumumab; Janssen Biotech/Genmab), Empliciti® (elotuzumab, Bristol-Meyers Squibb/AbbVie), Revlimid® (lenalidomide, Celgene), Pomalyst® (pomalidomide, Celgene), Thalomid® (thalidomide, Celgene), Ninlaro® (ixazomib, Takeda), Velcade® (bortezomib, Takeda), Kyprolis® (carfilzomib, Amgen), Farydak® (panobinostat, Novartis). There are multiple treatments and various combinations in clinical development for different lines of multiple myeloma therapy. In addition to MOR202, products in clinical development for the treatment of multiple myeloma include: the CD38 antibody isatuximab, CC-4047, siltuximab, atezolizumab, pembrolizumab, nivolumab, durvalumab, venetoclax, bendamustine, amrubicin, filanesib, various BCMA-CAR T cell constructs, oprozomib, ricolinostat, selinexor, and plitidepsin.
MOR103/GSK3196165
Rheumatoid arthritis, RA, is an autoimmune disease characterized by inflammation of the joints, bone and cartilage erosion, and joint deformity. Additionally, the condition manifests itself in multiple joints in the body. Although the disease primarily affects the joints, it can also affect other organs, including the skin, eyes, lungs, heart, and blood vessels. Despite the many advances in both the understanding and the treatment of RA, it remains largely incurable, and
147
Table of Contents
its causes are still unknown. The disease can lead to premature mortality, disability, and decreased quality of life. Methotrexate and other conventional synthetic disease-modifying anti-rheumatic drugs, DMARDs, such as hydroxychloroquine, leflunomide, minocycline, and sulfasalazine are frequently used prior to the introduction of a biologic. Within most markets, the first-generation anti-TNFs (etanercept, adalimumab and infliximab) are frequently favored as a first line of therapy when the decision to introduce a biologic is made. The second-generation anti-TNFs (certolizumab pegol and golimumab) are popular as a second or third line of therapy. Other approved products are rituximab (CD20), tocilizumab (IL-6 receptor), sarilumab (IL-6), abatacept (CD80), anakinra (IL-1 receptor antagonist) and tofacitinib (JAK3/1). In addition, to several biosimilars also several novel JAK inhibitors and antibodies are currently in Phase 3 development.
MOR103 is also being evaluated in osteoarthritis, where the current treatment focus is on relief of symptoms as no disease modifying agent is approved yet. In late stage development pipelines there are other antibodies next to MOR103 (Phase 2) in development: tanezumab and fasinumab (nerve growth factor) both in Phase 3 and canakinumab (IL-1b) as well as ABT-981 (IL-1a/b) in Phase 2.
Tremfya®
Psoriasis is a chronic disease and currently there are no curative treatments available. Topical therapies are largely used to treat mild psoriasis, and more-potent systemic therapies (such as methotrexate or biologics) are often prescribed concomitantly with topical agents for moderate to severe disease. In general, topical corticosteroids are well tolerated when used for short courses in the appropriate areas and have a fast onset of action. Other topical therapies include vitamin D3 analogues and the combination therapy of a vitamin D3 analogue and topical corticosteroid, calcipotriene/betamethasone dipropionate.
Systemic therapies are divided into conventional systemic therapies—which include oral drugs and phototherapies—and biological agents. Eight biologics are approved for psoriasis: the TNF-a inhibitors etanercept, infliximab, and adalimumab; the IL-12/23 inhibitor ustekinumab; the specific IL-23 inhibitor Tremfya®; the IL-17A inhibitors secukinumab, ixekizumab and the IL-17A receptor antagonist brodalumab. An oral treatment with a novel mechanism of action—apremilast—is also available for the treatment of psoriasis. Phototherapy is a non-pharmaceutical therapy for moderate-to-severe psoriasis that involves exposure of the skin to ultraviolet light.
Currently, there are two on-target competitors to Tremfya® either under review for approval (tildrakizumab) or close to submission for BLA filing (risankizumab). There are also various TNF-a biosimilars currently in development.
Tremfya® is also being investigated for treatment of PsA, which is closely related to psoriasis and also a chronic disease. NSAIDs, selective COX-2 inhibitors, and intra-articular corticosteroids provide symptomatic relief to cutaneous or articular pain and inflammation. The cDMARDs (such as, methotrexate, sulfasalazine, leflunomide), bDMARDs (such as, TNF-a inhibitors: adalimumab, etanercept, infliximab; IL-12/23 inhibitor: ustekinumab; IL-17 inhibitors: secukinumab, ixekizumab, brodalumab; JAK inhibitor: tofacitinib), and tsDMARDs (apremilast) are used to improve the signs and symptoms of PsA. Most agents now used for PsA were extrapolated from rheumatoid arthritis and psoriasis.
148
Table of Contents
Gantenerumab
Alzheimer’s disease is a progressive neurodegenerative disease that is characterized by memory loss, cognitive impairment, and functional decline. During the early stages of the disease, sleep disturbances and forgetfulness are generally the first presenting symptoms. The currently available therapies for Alzheimer’s disease provide only symptomatic relief, and will not cure the disease or prevent it from worsening over time. Since Alzheimer’s disease is multifarious, with various etiologies, it is unlikely that any one treatment or approach will be able to cure it. Most prescribed products are cholinesterase inhibitors (donepezil hydrochloride, rivastigmine and galantamine), N-methyl-D-aspartate receptor antagonists (memantine hydrochloride) and psychotropics (risperidone). Currently there are three on-target competitors to gantenerumab in Phase 3 development: crenezumab, solanezumab and aducanumab. Innovative treatment options are in demand given the very high unmet medical need in this indication.
Government Regulation
Government authorities in the United States, at the federal, state and local level, and in the European Union and other countries and jurisdictions, extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, packaging, storage, recordkeeping, labeling, advertising, promotion, distribution, marketing, post-approval monitoring and reporting, and import and export of pharmaceutical products, including biological products. In addition, some jurisdictions regulate the pricing of pharmaceutical products. The processes for obtaining marketing approvals in the United States and in foreign countries and jurisdictions, along with subsequent compliance with applicable statutes and regulations and other requirements of regulatory authorities, require the expenditure of substantial time and financial resources.
Regulation and Procedures Governing Approval of Biological Products in the United States
In the United States, our product candidates are regulated as biological products, or biologics, under the Public Health Service Act, or PHSA, and the Federal Food, Drug, and Cosmetic Act and their implementing regulations. The failure to comply with the applicable U.S. requirements at any time during the product development process, including during nonclinical testing, clinical testing, the approval process or post-approval process, may subject an applicant to delays in the conduct of a study or regulatory review and approval, and/or to administrative or judicial sanctions and adverse publicity. Sanctions may include, but are not limited to, the FDA’s refusal to allow an applicant to proceed with clinical testing, refusal to approve pending applications, withdrawal of an approval, warning or untitled letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, debarment, disgorgement of profits and civil or criminal investigations and penalties brought by the FDA or the Department of Justice or other governmental entities.
An applicant seeking approval to market and distribute a new biologic in the United States generally must satisfactorily complete each of the following steps:
| · | nonclinical laboratory tests, animal studies and formulation studies all performed in accordance with applicable regulations, including the FDA’s good laboratory practices, or GLP, regulations; |
| · | submission to the FDA of an IND application for human clinical testing, which must become effective before human clinical trials may begin; |
149
Table of Contents
| · | approval by an IRB representing each clinical site before each clinical trial may be initiated; |
| · | performance of adequate and well-controlled human clinical trials to establish the safety, potency and purity of the product candidate for each proposed indication, in accordance applicable regulations, including with Good Clinical Practices, or GCP, regulations; |
| · | preparation and submission to the FDA of a BLA for a biologic product requesting marketing approval for one or more proposed indications, including submission of detailed information on the manufacture and composition of the product in clinical development, evidence of safety, purity and potency from nonclinical testing and clinical trials, and proposed labeling; |
| · | review of the product by an FDA advisory committee, if applicable; |
| · | satisfactory completion of one or more FDA inspections of the manufacturing facility or facilities, including those of third parties, at which the product, or components thereof, are produced to assess compliance with current Good Manufacturing Practices, or cGMP, requirements and to assure that the facilities, methods and controls are adequate to preserve the product’s identity, strength, quality and purity; |
| · | satisfactory completion of any FDA audits of the clinical study sites to assure compliance with GCPs, and the integrity of clinical data in support of the BLA; |
| · | payment of user fees and securing FDA approval of the BLA; and |
| · | compliance with any post-approval requirements, including the potential requirement to implement a REMS and to conduct any post-approval studies required by the FDA. |
Nonclinical Studies and Investigational New Drug Application
Before testing any biologic product candidate in humans, the product candidate must undergo nonclinical testing. Nonclinical tests include laboratory evaluations of product chemistry, formulation and stability, as well as animal studies to evaluate the potential for activity and toxicity. The conduct of the nonclinical tests and formulation of the compounds for testing must comply with federal regulations and requirements. The results of the nonclinical tests, together with manufacturing information, analytical data, any available clinical data or literature and a proposed clinical protocol, are submitted to the FDA as part of an IND application. The IND automatically becomes effective 30 days after receipt by the FDA, unless before that time the FDA raises concerns or questions about the product or conduct of the proposed clinical trial, including concerns that human research subjects will be exposed to unreasonable health risks, and places the trial on a clinical hold. In that case, the IND sponsor and the FDA must resolve any outstanding FDA concerns before the clinical trial can begin.
As a result, submission of the IND may result in the FDA not allowing the trial to commence or not be conducted on the terms originally specified by the sponsor in the IND. If the FDA raises concerns or questions either during this initial 30-day period, or at any time during the IND process, it may choose to impose a partial or complete clinical hold. If the FDA imposes a clinical hold, trials may not recommence without FDA authorization and then only under terms authorized by the FDA. A clinical hold issued by the FDA may therefore delay either a proposed clinical study or cause suspension of an ongoing study, until all outstanding concerns have been adequately addressed and the FDA has notified the company that investigation may proceed. This could cause significant difficulties in completing planned clinical trials in a timely manner.
150
Table of Contents
The FDA may impose clinical holds on a biologic product candidate at any time before or during clinical trials due to safety concerns or non-compliance.
Human Clinical Trials in Support of a BLA
Clinical trials involve the administration of the investigational product candidate to healthy volunteers or patients with the disease to be treated under the supervision of a qualified principal investigator in accordance with GCP requirements. Clinical trials are conducted under study protocols detailing, among other things, the objectives of the study, inclusion and exclusion criteria, the parameters to be used in monitoring safety, dosing procedures and the effectiveness criteria to be evaluated. A protocol for each clinical trial and any subsequent protocol amendments must be submitted to the FDA as part of the IND.
A sponsor who wishes to conduct a clinical trial outside the United States may, but need not, obtain FDA authorization to conduct the clinical trial under an IND. If a foreign clinical trial is not conducted under an IND, the sponsor may submit data from the clinical trial to the FDA in support of the BLA so long as the clinical trial is well-designed and well-conducted in accordance with GCP, including review and approval by an independent ethics committee, and the FDA is able to validate the study data through an onsite inspection, if necessary.
Further, each clinical trial must be reviewed and approved by an IRB either centrally or individually at each institution at which the clinical trial will be conducted. The IRB will consider, among other things, clinical trial design, patient informed consent, ethical factors and the safety of human subjects. An IRB must operate in compliance with FDA regulations. The FDA, IRB, or the clinical trial sponsor may suspend or discontinue a clinical trial at any time for various reasons, including a finding that the clinical trial is not being conducted in accordance with FDA requirements or that the subjects or patients are being exposed to an unacceptable health risk. Clinical testing also must satisfy extensive GCP rules and the requirements for informed consent. The IRB also approves the form and content of the informed consent that must be signed by each clinical trial subject or his or her legal representative and must monitor the clinical trial until completed. Additionally, some clinical trials are overseen by an independent group of qualified experts organized by the clinical trial sponsor, known as a data safety monitoring board or committee. This group may recommend continuation of the study as planned, changes in study conduct, or cessation of the study at designated check points based on access to certain data from the study.
Clinical trials typically are conducted in three sequential phases, but the phases may overlap or be combined. Additional studies may be required after approval.
| · | Phase 1 clinical trials (or Phase 1) are initially conducted in a limited population to test the product candidate for safety, including adverse effects, dose tolerance, absorption, metabolism, distribution, excretion and pharmacodynamics in healthy humans or, on occasion, in patients, such as in the case of some products for severe or life-threatening diseases, especially when the product may be too inherently toxic to ethically administer to healthy volunteers. |
| · | Phase 2 clinical trials (or Phase 2) are generally conducted in a limited patient population to identify possible adverse effects and safety risks, preliminarily evaluate the efficacy of the product candidate for specific targeted indications and determine dose tolerance and optimal dosage. Multiple Phase 2 clinical trials may be conducted by the sponsor to obtain information prior to beginning larger Phase 3 clinical trials. |
151
Table of Contents
| · | Phase 3 clinical trials (or Phase 3) proceed if the Phase 2 clinical trials demonstrate that a certain dose or dose range of the product candidate is potentially effective and has an acceptable safety profile. Phase 3 clinical trials are undertaken within an expanded patient population, often at geographically dispersed clinical trial sites, to gather additional information about safety and effectiveness necessary to evaluate the overall benefit-risk relationship of the product and to provide an adequate basis for physician labeling. |
In some cases, the FDA may approve a BLA for a product candidate but require the sponsor to conduct additional clinical trials to further assess the product candidate’s safety and effectiveness after approval. Such post-approval trials are typically referred to as Phase 4 clinical trials (or Phase 4). These studies may be used to gain additional experience from the treatment of patients in the intended therapeutic indication and to document a clinical benefit in the case of biologics approved under accelerated approval regulations. If the FDA approves a product while a company has ongoing clinical trials that were not necessary for approval, a company may be able to use the data from these clinical trials to meet all or part of any Phase 4 clinical trial requirement or to request a change in the product labeling. Failure to exhibit due diligence with regard to conducting required Phase 4 clinical trials could result in withdrawal of approval for products.
During all phases of clinical development, regulatory agencies require extensive monitoring and auditing of all clinical activities, clinical data, and clinical trial investigators. Annual progress reports detailing the results of the clinical trials must be submitted to the FDA. Written IND safety reports must be promptly submitted to the FDA and the investigators for serious and unexpected adverse events, any findings from other trials, tests in laboratory animals or in vitro testing that suggest a significant risk for human subjects, or any clinically important increase in the rate of a serious suspected adverse reaction over that listed in the protocol or investigator brochure. The sponsor must submit an IND safety report within 15 calendar days after the sponsor determines that the information qualifies for reporting. The sponsor also must notify the FDA of any unexpected fatal or life-threatening suspected adverse reaction within seven calendar days after the sponsor’s initial receipt of the information. The FDA or the sponsor or its data safety monitoring board may suspend a clinical trial at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the biological candidate has been associated with unexpected serious harm to patients.
There are also requirements governing the reporting of ongoing clinical trials and completed clinical trial results to public registries. Sponsors of clinical trials of FDA-regulated products, including biologics, are required to register and disclose certain clinical trial information, which is publicly available at www.clinicaltrials.gov. Information related to the product, patient population, phase of investigation, trial sites and investigators, and other aspects of the clinical trial is then made public as part of the registration. Sponsors are also obligated to discuss the results of their clinical trials after completion. Disclosure of the results of these trials can be delayed until the new product or new indication being studied has been approved.
Compliance with cGMP Requirements
Before approving a BLA, the FDA typically will inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the
152
Table of Contents
manufacturing processes and facilities are in full compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Among other things, the sponsor must develop methods for testing the identity, strength, quality, potency and purity of the final biological product. Additionally, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the biological product does not undergo unacceptable deterioration over its shelf life. The PHSA emphasizes the importance of manufacturing control for products like biologics whose attributes cannot be precisely defined.
Manufacturers and others involved in the manufacture and distribution of biological products must also register their establishments with the FDA and certain state agencies. Both domestic and foreign manufacturing establishments must register and provide additional information to the FDA upon their initial participation in the manufacturing process.
Establishments may be subject to periodic unannounced inspections by government authorities to ensure compliance with cGMPs and other laws. Manufacturers may have to provide, on request, electronic or physical records regarding their establishments. Delaying, denying, limiting, or refusing inspection by the FDA may lead to a product being deemed to be adulterated.
Review and Approval of a BLA
The results of product candidate development, nonclinical testing and clinical trials, including negative or ambiguous results as well as positive findings, are submitted to the FDA as part of a BLA requesting a license to market the product. The BLA must contain extensive manufacturing information and detailed information on the composition of the product and proposed labeling. The FDA adjusts the Prescription Drug User Fee Act, or PDUFA, user fees on an annual basis. Fee waivers or reductions are available in certain circumstances, including a waiver of the application fee for the first application filed by a small business. Additionally, no user fees are assessed on BLAs for products designated as orphan drugs, unless the product also includes a non-orphan indication.
The FDA has 60 days after submission of the application to conduct an initial review to determine whether the BLA is sufficient to accept for filing based on the agency’s threshold determination that it is substantially complete so as to permit substantive review. Once the submission has been accepted for filing, the FDA begins an in-depth review of the application. Under the goals and policies agreed to by the FDA under PDUFA, the FDA aims to complete its initial review of a standard application and respond to the applicant within ten months of the 60-day filing date, and for a priority review application within six months. The FDA does not always meet its PDUFA goal dates for standard and priority BLAs, and its review goals are subject to change from time to time. The review process may often be significantly extended by FDA requests for additional information or clarification. The review process and the PDUFA goal date may also be extended by three months if the FDA requests or if the applicant otherwise provides additional information or clarification regarding information already provided in the submission within the last three months before the PDUFA goal date.
The FDA reviews a BLA to determine, among other things, whether the proposed product is safe and potent, or effective, for its intended use, and has an acceptable purity profile, and whether the product is being manufactured in accordance with cGMP requirements to assure and preserve the product’s identity, safety, strength, quality, potency and purity. On the basis of the
153
Table of Contents
FDA’s evaluation of the application and accompanying information, including the results of the inspection of the manufacturing facilities and any FDA audits of clinical trial sites to assure compliance with GCPs, the FDA may issue an approval letter, denial letter, or a complete response letter. An approval letter authorizes commercial marketing of the product with specific prescribing information for specific indications. Under the PHSA, the FDA may approve a BLA if it determines that the product is safe, pure and potent and the facility where the product will be manufactured meets standards designed to ensure that it continues to be safe, pure and potent. If the application is not approved, the FDA may issue a complete response letter, which will contain the conditions that must be met in order to secure final approval of the application, and when possible will outline recommended actions the sponsor might take to obtain approval of the application. If a complete response letter is issued, the applicant may either resubmit the BLA, addressing all of the deficiencies identified in the letter, or withdraw the application.
Sponsors that receive a complete response letter who elect to address the deficiencies may submit to the FDA information that represents a complete response to the issues identified by the FDA in the response letter. Such resubmissions are classified under PDUFA as either Class 1 or Class 2, based on the information submitted by an applicant in response to an action letter. Under the goals and policies agreed to by the FDA under PDUFA, the FDA aims to review and act on a Class 1 resubmission with two months of receipt and, with respect to a Class 2 resubmission, within six months of receipt. The FDA will not approve an application until issues identified in the complete response letter have been addressed.
The FDA may also refer the application to an advisory committee for review, evaluation and recommendation as to whether the application should be approved and under what conditions. In particular, the FDA may refer applications for novel biologic products or biologic products that present difficult questions of safety or efficacy to an advisory committee. Typically, an advisory committee is a panel of independent experts, including clinicians and other scientific experts. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
If the FDA approves a new product, it may limit the approved indications for use of the product, or limit the approval to specific dosages. It may also require that certain contraindications, warnings or precautions be included in the product labeling. In addition, the FDA may call for post-approval studies, including Phase 4 clinical trials, to further assess the product’s safety after approval. The agency may also require testing and surveillance programs to monitor the product after commercialization, or impose other conditions, including distribution restrictions or other risk management mechanisms, including REMS, to help ensure that the benefits of the product outweigh the potential risks. REMS can include medication guides, communication plans for healthcare professionals, and elements to assure safe use, or ETASU. ETASU can include, but are not limited to, special training or certification for prescribing or dispensing, dispensing only under certain circumstances, special monitoring and the use of patent registries. If the FDA concludes a REMS is needed, the sponsor of the BLA must submit a proposed REMS; the FDA will not approve the BLA without a REMS, if required. The FDA may prevent or limit further marketing of a product based on the results of post-market studies or surveillance programs. After approval, many types of changes to the approved product, such as adding new indications, manufacturing changes and additional labeling claims, are subject to further testing requirements and FDA review and approval.
154
Table of Contents
Fast Track, Breakthrough Therapy and Priority Review Designations
The FDA may designate certain products for expedited review if they are intended to address an unmet medical need in the treatment of a serious or life-threatening disease or condition. These programs include fast track designation, breakthrough therapy designation and priority review designation.
The FDA may designate a product for fast track review if it is intended, whether alone or in combination with one or more other products, for the treatment of a serious or life-threatening disease or condition, and it demonstrates the potential to address unmet medical needs for such a disease or condition. Fast track designation applies to the combination of the product and the specific indication for which it is being studied. The sponsor of a new biologic may request that the FDA designate the biologic as a fast track product at any time during the clinical development of the product. For fast track products, sponsors may have greater interactions with the FDA and the FDA may initiate review of sections of a fast track product’s application before the application is complete. This rolling review may be available if the FDA determines, after preliminary evaluation of clinical data submitted by the sponsor, that a fast track product may be effective. The sponsor must also provide, and the FDA must approve, a schedule for the submission of the remaining information and the sponsor must pay applicable user fees. However, the FDA’s time period goal for reviewing a fast track application does not begin until the last section of the application is submitted. Fast track designation may be withdrawn by the FDA if the FDA believes that the designation is no longer supported by data emerging in the clinical trial process.
In 2012, Congress enacted the FDASIA. This law established a new regulatory scheme allowing for expedited review of products designated as “breakthrough therapies”. A product may be designated as a breakthrough therapy if it is intended, either alone or in combination with one or more other products, to treat a serious or life-threatening disease or condition and preliminary clinical evidence indicates that the product may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. The FDA may take certain actions with respect to breakthrough therapies, including holding meetings with the sponsor throughout the development process; providing timely advice to the product sponsor regarding development and approval; involving more senior staff in the review process; assigning a cross-disciplinary project lead for the review team; and taking other steps to facilitate the design of clinical trials in an efficient manner.
The FDA may designate a product for priority review if it is a product that treats a serious condition and, if approved, would provide a significant improvement in safety or effectiveness. The FDA determines, on a case-by-case basis, whether the proposed product represents a significant improvement when compared with other available therapies. Significant improvement may be illustrated by evidence of increased effectiveness in the treatment of a condition, elimination or substantial reduction of a treatment-limiting product reaction, documented enhancement of patient compliance that may lead to improvement in serious outcomes and evidence of safety and effectiveness in a new subpopulation. A priority designation is intended to direct overall attention and resources to the evaluation of such applications, and to shorten the FDA’s goal for taking action on a marketing application from ten months to six months.
155
Table of Contents
Fast track designation, priority review and breakthrough therapy designation may expedite the development or approval process, but do not change the standards for approval.
Accelerated Approval Pathway
The FDA may grant accelerated approval to a product for a serious or life-threatening condition that provides meaningful therapeutic advantage to patients over existing treatments based upon a determination that the product has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit. The FDA may also grant accelerated approval for such a condition when the product has an effect on an intermediate clinical endpoint that can be measured earlier than an effect on irreversible morbidity or mortality, or IMM, and that is reasonably likely to predict an effect on IMM or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments. Products granted accelerated approval must meet the same statutory standards for safety and effectiveness as those granted traditional approval.
For the purposes of accelerated approval, a surrogate endpoint is a marker, such as a laboratory measurement, radiographic image, physical sign, or other measure that is thought to predict clinical benefit, but is not itself a measure of clinical benefit. Surrogate endpoints can often be measured more easily or more rapidly than clinical endpoints. An intermediate clinical endpoint is a measurement of a therapeutic effect that is considered reasonably likely to predict the clinical benefit of a product, such as an effect on IMM. The FDA has stated that although it has limited experience with accelerated approvals based on intermediate clinical endpoints, such endpoints generally may support accelerated approval where the therapeutic effect measured by the endpoint is not itself a clinical benefit and basis for traditional approval, if there is a basis for concluding that the therapeutic effect is reasonably likely to predict the ultimate clinical benefit of a product.
The accelerated approval pathway is most often used in settings in which the course of a disease is long and an extended period of time is required to measure the intended clinical benefit of a product. Thus, accelerated approval has been used extensively in the development and approval of products for treatment of a variety of cancers in which the goal of therapy is generally to improve survival or decrease morbidity and the duration of the typical disease course requires lengthy and sometimes large trials to demonstrate a clinical or survival benefit.
The accelerated approval pathway is usually contingent on a sponsor’s agreement to conduct, in a diligent manner, additional post-approval confirmatory studies to verify and describe the product’s clinical benefit. As a result, a product candidate approved on this basis is subject to rigorous post-marketing compliance requirements, including the completion of Phase 4 or post-approval clinical trials to confirm the effect on the clinical endpoint. Failure to conduct required post-approval studies, or confirm a clinical benefit during post-marketing studies, may lead the FDA to withdraw the product from the market. All promotional materials for product candidates approved under accelerated regulations are subject to prior review by the FDA.
Post-Approval Regulation
If regulatory approval for marketing of a product or for a new indication for an existing product is obtained, the sponsor will be required to comply with rigorous and extensive post-approval regulatory requirements as well as any post-approval requirements that the FDA has imposed on the particular product as part of the approval process. The sponsor will be required, among
156
Table of Contents
other things, to report certain adverse reactions and production problems to the FDA, provide updated safety and efficacy information and comply with requirements concerning advertising and promotional labeling. Manufacturers and certain of their subcontractors are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with ongoing regulatory requirements, including cGMP regulations, which impose certain procedural and documentation requirements upon manufacturers. Accordingly, the BLA-holder and its third-party manufacturers must continue to expend time, money and effort in the areas of production and quality control to maintain compliance with cGMP regulations and other regulatory requirements. In addition, changes to the manufacturing process or facility generally require prior FDA approval before being implemented, and other types of changes to the approved product, such as adding new indications and additional labeling claims, are also subject to further FDA review and approval.
Once an approval is granted, the FDA may withdraw the approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information; imposition of post-market study requirements or clinical trial requirements to assess new safety risks; or imposition of distribution restrictions or other restrictions under a REMS program. Other potential consequences include, among other things:
| · | restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls; |
| · | fines, untitled letters or warning letters or holds on post-approval clinical trials; |
| · | adverse publicity; |
| · | refusal of the FDA to approve pending applications or supplements to approved applications, or suspension or revocation of product license approvals; |
| · | product seizure or detention, or refusal to permit the import or export of products; or |
| · | injunctions, fines, debarment, disgorgement of profits or the imposition of civil or criminal penalties. |
The FDA strictly regulates marketing, labeling, advertising and promotion of products that are placed on the market. Pharmaceutical products may be promoted only for the approved indications and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant liability.
Orphan Drug Designation
Orphan drug designation in the United States is designed to encourage sponsors to develop products intended for rare diseases or conditions. In the United States, a rare disease or condition is statutorily defined as a disease or condition that affects fewer than 200,000 individuals in the United States or that affects more than 200,000 individuals in the United States but for which there is no reasonable expectation that the cost of developing and making
157
Table of Contents
available the product for the disease or condition will be recovered from sales of the product in the United States.
Orphan drug designation qualifies a company for certain financial incentives, including tax advantages and, if the product receives the first FDA approval for the indication for which it has orphan designation, market exclusivity for seven years following the date of the product’s marketing approval. An application for designation as an orphan product can be made any time prior to the filing of an application for approval to market the product. Once a product receives orphan drug designation from the Office of Orphan Products Development at the FDA, the product must then go through the review and approval process like any other product.
In addition, a sponsor of a product that is otherwise the same product as an already approved orphan drug may seek and obtain orphan drug designation for the subsequent product for the same rare disease or condition if it can present a plausible hypothesis that its product may be clinically superior to the first product. More than one sponsor may receive orphan drug designation for the same product for the same rare disease or condition, but each sponsor seeking orphan drug designation must file a complete request for designation.
The period of exclusivity begins on the date that the marketing application is approved by the FDA and applies only to the indication for which the product has been designated. The FDA may approve a second application for the same product for a different use or a second application for a clinically superior version of the product for the same use. The FDA cannot, however, approve the same product made by another manufacturer for the same indication during the market exclusivity period unless it has the consent of the sponsor, the manufacturer makes a showing of clinical superiority over the product with orphan exclusivity, or the sponsor is unable to provide sufficient quantities.
Orphan product designation does not convey any advantage in or shorten the duration of the regulatory review and approval process.
Pediatric Studies and Exclusivity
Under the Pediatric Research Equity Act of 2003, a BLA or supplement thereto must contain data that are adequate to assess the safety and effectiveness of the product for the claimed indications in all relevant pediatric subpopulations, and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. Sponsors who are planning to submit a marketing application for a biological product that includes a new active ingredient, new indication, new dosage form, new dosing regimen or new route of administration must also submit pediatric study plans prior to the assessment data, and no later than 60 calendar days following an end-of-phase 2 meeting with the FDA. Pediatric study plans must contain an outline of the proposed pediatric study or studies the sponsor plans to conduct, including study objectives and design, any deferral or waiver requests and other information required by regulation. The applicant, the FDA, and the FDA’s internal review committee must then review the information submitted, consult with each other and agree upon a final plan. The FDA or the applicant may request an amendment to the plan at any time.
The FDA may, on its own initiative or at the request of the applicant, grant deferrals for submission of some or all pediatric data until after approval of the product for use in adults, or full or partial waivers from the pediatric data requirements. Additional requirements and procedures relating to deferral requests and requests for extension of deferrals are contained in
158
Table of Contents
FDASIA. Unless otherwise required by regulation, the pediatric data requirements do not apply to products with orphan designation.
Pediatric exclusivity is another type of non-patent marketing exclusivity in the United States and, if granted, provides for the attachment of an additional six months of marketing protection to the term of any existing regulatory exclusivity, including the non-patent and orphan exclusivity. This six-month exclusivity may be granted if a BLA sponsor submits pediatric data that fairly respond to a written request from the FDA for such data. The data do not need to show the product to be effective in the pediatric population studied; rather, if the clinical trial is deemed to fairly respond to the FDA’s request, the additional protection is granted. If reports of requested pediatric studies are submitted to and accepted by the FDA within the statutory time limits, whatever statutory or regulatory periods of exclusivity or patent protection cover the product are extended by six months. This is not a patent term extension, but it effectively extends the regulatory period during which the FDA cannot approve another application.
Regulation of Combination Products in the United States
Certain products may be comprised of components that would normally be regulated under different types of regulatory authorities and frequently by different centers at the FDA. These products are known as combination products. Specifically, under regulations issued by the FDA, a combination product may be:
| · | A product comprised of two or more regulated components that are physically, chemically, or otherwise combined or mixed and produced as a single entity; |
| · | Two or more separate products packaged together in a single package or as a unit and comprised of drug and device products, device and biological products, or biological and drug products; |
| · | A drug, or device, or biological product packaged separately that according to its investigational plan or proposed labeling is intended for use only with an approved individually specified drug, or device, or biological product where both are required to achieve the intended use, indication, or effect and where upon approval of the proposed product the labeling of the approved product would need to be changed, e.g., to reflect a change in intended use, dosage form, strength, route of administration, or significant change in dose; or |
| · | Any investigational drug, device, or biological product packaged separately that according to its proposed labeling is for use only with another individually specified investigational drug, device, or biological product where both are required to achieve the intended use, indication, or effect. |
Under the United States Federal Food, Drug, and Cosmetic Act, the FDA is charged with assigning a center with primary jurisdiction, or a lead center, for review of a combination product. That determination is based on the “primary mode of action” of the combination product. Thus, if the primary mode of action of a device-biologic combination product is attributable to the biologic product, the FDA center responsible for premarket review of the biologic product would have primary jurisdiction for the combination product. The FDA has also established an Office of Combination Products to address issues surrounding combination products and provide more certainty to the regulatory review process. That office serves as a
159
Table of Contents
focal point for combination product issues for agency reviewers and industry. It is also responsible for developing guidance and regulations to clarify the regulation of combination products, and for assignment of the FDA center that has primary jurisdiction for review of combination products where the jurisdiction is unclear or in dispute.
Regulation and Procedures Governing Approval of Medicinal Products in the European Union
In order to market any product outside of the United States, a company also must comply with numerous and varying regulatory requirements of other countries and jurisdictions regarding quality, safety and efficacy and governing, among other things, clinical trials, marketing authorization, commercial sales and distribution of products. Whether or not it obtains FDA approval for a product, an applicant will need to obtain the necessary approvals by the comparable foreign regulatory authorities before it can initiate clinical trials or marketing of the product in those countries or jurisdictions. Specifically, the process governing approval of medicinal products in the European Union generally follows the same lines as in the United States. It entails satisfactory completion of pharmaceutical development, nonclinical studies and adequate and well-controlled clinical trials to establish the safety and efficacy of the medicinal product for each proposed indication. It also requires the submission to relevant competent authorities for clinical trials authorization for a marketing authorization application, or MAA, and granting of a marketing authorization by these authorities before the product can be marketed and sold in the European Union or its Member States.
Clinical Trial Approval
Pursuant to the currently applicable Clinical Trials Directive 2001/20/EC and the Directive 2005/28/EC on GCP, a system for the approval of clinical trials in the European Union has been implemented through national legislation of the Member States. Under this system, an applicant must obtain approval from the competent national authority of a European Union Member State in which the clinical trial is to be conducted or in multiple Member States if the clinical trial is to be conducted in a number of Member States. Furthermore, the applicant may only start a clinical trial at a specific study site after the independent ethics committee has issued a favorable opinion. The clinical trial application must be accompanied by an investigational medicinal product dossier with supporting information prescribed by Directive 2001/20/EC and Directive 2005/28/EC and corresponding national laws of the Member States and further detailed in applicable guidance documents.
In April 2014, the European Union adopted a new Clinical Trials Regulation (EU) No 536/2014, which is set to replace the current Clinical Trials Directive 2001/20/EC. It is expected that the new Clinical Trials Regulation will apply in the second half of 2019. It will overhaul the current system of approvals for clinical trials in the European Union. Specifically, the new regulation, which will be directly applicable in all Member States, aims at simplifying and streamlining the approval of clinical trials in the European Union. For instance, the new Clinical Trials Regulation provides for a streamlined application procedure using a single entry point and strictly defined deadlines for the assessment of clinical trial applications.
Marketing Authorization
To obtain a marketing authorization for a product under the European Union regulatory system, an applicant must submit an MAA, either under a centralized procedure administered by the EMA or one of the procedures administered by competent authorities in European Union
160
Table of Contents
Member States (decentralized procedure, national procedure, or mutual recognition procedure). A marketing authorization may be granted only to an applicant established in the European Union. Regulation (EC) No. 1901/2006 on medicinal products for paediatric use provides that prior to obtaining a marketing authorization in the European Union in the centralized procedure, an applicant must demonstrate compliance with all measures included in an EMA-approved Pediatric Investigation Plan covering all subsets of the pediatric population, unless the EMA has granted a product-specific waiver, class waiver, or a deferral for one or more of the measures included in the Pediatric Investigation Plan.
The centralized procedure provides for the grant of a single marketing authorization by the European Commission that is valid for all European Union Member States. Pursuant to Regulation (EC) No. 726/2004, the centralized procedure is compulsory for specific products, including for medicines produced by certain biotechnological processes, products designated as orphan medicinal products, advanced therapy products and products with a new active substance indicated for the treatment of certain diseases, including products for the treatment of cancer. For products with a new active substance indicated for the treatment of other diseases and products that are highly innovative or for which a centralized process is in the interest of patients, the centralized procedure may be optional.
Under the centralized procedure, the CHMP established at the EMA is responsible for conducting the assessment of a product to define its risk/benefit profile. Under the centralized procedure, the maximum timeframe for the evaluation of an MAA is 210 days, excluding clock stops when additional information or written or oral explanation is to be provided by the applicant in response to questions of the CHMP. Accelerated evaluation may be granted by the CHMP in exceptional cases, when a medicinal product is of major interest from the point of view of public health and, in particular, from the viewpoint of therapeutic innovation.
If the CHMP accepts such a request, the time limit of 210 days will be reduced to 150 days, but it is possible that the CHMP may revert to the standard time limit for the centralized procedure if it determines that it is no longer appropriate to conduct an accelerated assessment.
Periods of Authorization and Renewals
A marketing authorization is valid for five years, in principle, and it may be renewed after five years on the basis of a reevaluation of the risk benefit balance by the EMA or by the competent authority of the authorizing Member State. To that end, the marketing authorization holder must provide the EMA or the competent authority with a consolidated version of the file in respect of quality, safety and efficacy, including all variations introduced since the marketing authorization was granted, at least six months before the marketing authorization ceases to be valid. Once renewed, the marketing authorization is valid for an unlimited period, unless the European Commission or the competent authority decides, on justified grounds relating to pharmacovigilance, to proceed with one additional five-year renewal period. Any authorization that is not followed by the placement of the product on the European Union market (in the case of the centralized procedure) or on the market of the authorizing Member State within three years after authorization ceases to be valid.
Regulatory Requirements after Marketing Authorization
Following approval, the holder of the marketing authorization is required to comply with a range of requirements applicable to the manufacturing, marketing, promotion and sale of the
161
Table of Contents
medicinal product. These include compliance with the European Union’s stringent pharmacovigilance or safety reporting rules, pursuant to which post-authorization studies and additional monitoring obligations can be imposed. In addition, the manufacturing of authorized products, for which a separate manufacturer’s license is mandatory, must also be conducted in strict compliance with the EMA’s cGMP requirements and comparable requirements of other regulatory bodies in the European Union, which mandate the methods, facilities and controls used in manufacturing, processing and packing of products to assure their safety and identity. Finally, the marketing and promotion of authorized products, including industry-sponsored continuing medical education and advertising directed toward the prescribers of products and/or the general public, are strictly regulated in the European Union under Directive 2001/83EC, as amended.
Orphan Drug Designation and Exclusivity
Regulation (EC) No. 141/2000 and Regulation (EC) No. 847/2000 provide that a product can be designated as an orphan drug by the European Commission if its sponsor can establish: that the product is intended for the diagnosis, prevention or treatment of (1) a life-threatening or chronically debilitating condition affecting not more than five in ten thousand persons in the European Union when the application is made, or (2) a life-threatening, seriously debilitating or serious and chronic condition in the European Union and that without incentives it is unlikely that the marketing of the product in the European Union would generate sufficient return to justify the necessary investment. For either of these conditions, the applicant must demonstrate that there exists no satisfactory method of diagnosis, prevention, or treatment of the condition in question that has been authorized in the European Union or, if such method exists, the product has to be of significant benefit compared to products available for the condition.
An orphan drug designation provides a number of benefits, including fee reductions, regulatory assistance and the possibility to apply for a centralized European Union marketing authorization. Marketing authorization for an orphan drug leads to a ten-year period of market exclusivity. During this market exclusivity period, neither the EMA nor the European Commission or the Member States can accept an application or grant a marketing authorization for a “similar medicinal product”. A “similar medicinal product” is defined as a medicinal product containing a similar active substance or substances as contained in an authorized orphan medicinal product, and which is intended for the same therapeutic indication. The market exclusivity period for the authorized therapeutic indication may, however, be reduced to six years if, at the end of the fifth year, it is established that the product no longer meets the criteria for orphan drug designation because, for example, the product is sufficiently profitable not to justify market exclusivity.
Coverage, Pricing and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of any product candidates for which we may obtain regulatory approval. Even if our product candidates are approved for marketing, sales of such product candidates will depend, in part, on the extent to which third-party payors, including government health programs in the United States (such as Medicare and Medicaid), commercial health insurers, and managed care organizations, provide coverage and establish adequate reimbursement levels for such product candidates. In the United States, the Member States of the European Union and markets in other countries, patients who are prescribed treatments for their conditions and providers performing the
162
Table of Contents
prescribed services generally rely on third-party payors to reimburse all or part of the associated healthcare costs. Reimbursement rules and levels are not harmonized in the European Union and therefore differ from Member State to Member State. Patients are unlikely to use any product candidates we may develop unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of such product candidates. The process for determining whether a payor will provide coverage for a product may be separate from the process for setting the price or reimbursement rate that the payor will pay for the product once coverage is approved. Third-party payors are increasingly challenging the price and examining the medical necessity and cost-effectiveness of medical products and services and imposing controls to manage costs.
In order to secure coverage and reimbursement for any product that might be approved for sale, a company may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of the product, and the cost of these studies would be in addition to the costs required to obtain FDA or other comparable marketing approvals. Even after pharmacoeconomic studies are conducted, product candidates may not be considered medically necessary or cost effective. A decision by a third-party payor not to cover any product candidates we may develop could reduce physician utilization of such product candidates once approved and have a material adverse effect on our sales, results of operations and financial condition. Additionally, a payor’s decision to provide coverage for a product does not imply that an adequate reimbursement rate will be approved. For example, the payor may require co-payments that patients find unacceptably high. Further, one payor’s determination to provide coverage for a product does not assure that such coverage will continue or that other payors will also provide coverage and reimbursement for the product, and the level of coverage and reimbursement can differ significantly from payor to payor. Third-party reimbursement and coverage may not be adequate to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development. The insurance coverage and reimbursement status of newly approved products for orphan diseases is particularly uncertain, and failure to obtain or maintain adequate coverage and reimbursement for any such product candidates could limit a company’s ability to generate revenue.
The containment of healthcare costs also has become a priority of federal, state and foreign governments as well as other third-party payors such as statutory health insurance funds and the prices of pharmaceuticals have been a focus in this effort. Governments have shown significant interest in implementing cost-containment programs, including price controls, restrictions on reimbursement and requirements for substitution of generic products. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit a company’s revenue from the sale of any approved products. Coverage policies and third-party reimbursement rates may change at any time. Even if favorable coverage and reimbursement status is attained for one or more products for which a company or its collaborators receive marketing approval, less favorable coverage policies and reimbursement rates may be implemented or coverage may be ended in the future.
Outside the United States, we will face challenges in ensuring obtaining adequate coverage and payment for any product candidates we may develop. Pricing of prescription pharmaceuticals is subject to governmental control in many countries. Pricing negotiations with governmental authorities or other third-party payors such as statutory health insurance funds can extend well
163
Table of Contents
beyond the receipt of regulatory marketing approval for a product and may require us to conduct a clinical trial that compares the cost effectiveness of any product candidates we may develop to other available therapies. The conduct of such a clinical trial could be expensive and result in delays in our commercialization efforts.
In the European Union, pricing and reimbursement schemes vary widely from country to country. Some countries provide that products may be marketed only after a reimbursement price has been agreed. Some countries may require the completion of additional studies that compare the cost effectiveness of a particular product candidate to currently available therapies (so called health technology assessments) in order to obtain reimbursement or pricing approval. For example, the European Union provides options for its Member States to restrict the range of products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. European Union Member States may approve a specific price for a product or may instead adopt a system of direct or indirect controls on the profitability of the company placing the product on the market. Other Member States allow companies to fix their own prices for products, but monitor and control prescription volumes and issue guidance to physicians to limit prescriptions. Recently, many countries in the European Union have increased the amount of discounts required on pharmaceuticals and these efforts could continue as countries attempt to manage healthcare expenditures, especially in light of the severe fiscal and debt crises experienced by many countries in the European Union. The downward pressure on health care costs in general, particularly prescription products, has become intense. As a result, increasingly high barriers are being erected to the entry of new products. Political, economic and regulatory developments may further complicate pricing negotiations, and pricing negotiations may continue after reimbursement has been obtained. Reference pricing used by various European Union Member States and parallel trade (arbitrage between low-priced and high-priced Member States) can further reduce prices. Special pricing and reimbursement rules may apply to orphan drugs. Inclusion of orphan drugs in reimbursement systems tend to focus on the medical usefulness, need, quality and economic benefits to patients and the healthcare system as for any product. Acceptance of any medicinal product for reimbursement may come with cost, use and often volume restrictions, which again can vary by country. In addition, results based rules of reimbursement may apply. There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of our products, if approved in those countries.
Healthcare Law and Regulation
Healthcare providers and third-party payors play a primary role in the recommendation and prescription of pharmaceutical products that are granted marketing approval. Our current and future arrangements with providers, researchers, consultants, third-party payors and customers are subject to broadly applicable federal and state fraud and abuse, anti-kickback, false claims, transparency and patient privacy laws and regulations and other healthcare laws and regulations that may constrain our business and/or financial arrangements. Restrictions under applicable federal and state healthcare laws and regulations include, without limitation, the following:
| · | the U.S. federal Anti-Kickback Statute, which prohibits, among other things, persons and entities from knowingly and willfully soliciting, receiving, offering, or paying remuneration, directly or indirectly, in-cash or in kind, to induce or reward either the referral of an individual |
164
Table of Contents
| for, or the purchase, order or recommendation of, any good or service, for which payment may be made, in whole or in part, under a federal healthcare program such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the statute or a specific intent to violate it in order to have committed a violation. Moreover, the government may assert that a claim that includes items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the civil False Claims Act; |
| · | the federal civil and criminal false claims laws, including the civil False Claims Act, and civil monetary penalties laws, which prohibit individuals or entities from, among other things, knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false, fictitious, or fraudulent or knowingly making, using, or causing to be made or used a false record or statement to avoid, decrease, or conceal an obligation to pay money to the federal government; |
| · | HIPAA, which created additional federal criminal laws that prohibit, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or a specific intent to violate it in order to have committed a violation; |
| · | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, and their respective implementing regulations, including the Final Omnibus Rule published in January 2013, which impose obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information without the appropriate authorization by entities subject to the law, such as healthcare providers, health plans and healthcare clearinghouses and their respective business associates; |
| · | the federal transparency requirements known as the federal Physician Payments Sunshine Act, under the ACA, which requires certain manufacturers of drugs, devices, biologics and medical supplies to report annually to the Centers for Medicare & Medicaid Services, or CMS, within the U.S. Department of Health and Human Services, information related to payments and other transfers of value made by that entity to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members; |
| · | federal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers; |
| · | federal government price reporting laws, which require us to calculate and report complex pricing metrics to government programs and which may be used in the calculation of reimbursement and/or discounts on marketed products; |
| · | the Foreign Corrupt Practices Act, a U.S. law which regulates certain financial relationships with foreign government officials (which could include, for example, certain medical professionals); and |
| · | analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, which may apply to healthcare items or services that are reimbursed by non-governmental third-party payors, including private insurers. |
165
Table of Contents
Some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring pharmaceutical manufacturers to report information related to payments to physicians and other health care providers or marketing expenditures and pricing information. State and foreign laws also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
Violations of these laws can subject us to criminal, civil and administrative sanctions including monetary penalties, damages, fines, disgorgement, individual imprisonment and exclusion from participation in government funded healthcare programs, such as Medicare and Medicaid, additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws, reputational harm, and we may be required to curtail or restructure our operations. Moreover, we expect that there will continue to be federal and state laws and regulations, proposed and implemented, that could impact our future operations and business.
Healthcare Reform
A primary trend in the U.S. healthcare industry and elsewhere is cost containment. There have been a number of federal and state proposals during the last few years to reduce the cost of care through changes in the healthcare system, including limits on the pricing, coverage and reimbursement of pharmaceutical and biopharmaceutical products, especially under government-funded health care programs, and increased governmental control of drug pricing.
By way of example, in March 2010, the U.S. Congress enacted the ACA, which, among other things, includes changes to the coverage and payment for products under governmental and private insurance plans. Among the provisions of the ACA that may be of importance to our potential product candidates are:
| · | an annual, nondeductible fee on any entity that manufactures or imports specified branded prescription drugs and biologic products, apportioned among these entities according to their market share in certain government healthcare programs, although this fee would not apply to sales of certain products approved exclusively for orphan indications; |
| · | expansion of eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to certain individuals with income at or below 133% of the federal poverty level, thereby potentially increasing a manufacturer’s Medicaid rebate liability; |
| · | expansion of manufacturers’ rebate liability under the Medicaid Drug Rebate Program by increasing the minimum rebate for both branded and generic drugs and revising the definition of “average manufacturer price” for calculating and reporting Medicaid drug rebates on outpatient prescription drug prices and extending rebate liability to prescriptions for individuals enrolled in Medicare Advantage plans; |
| · | a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for products that are inhaled, infused, instilled, implanted or injected; |
| · | expanding the types of entities eligible for the 340B drug discount program; |
| · | a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research; |
166
Table of Contents
| · | creation of the IPAB which, if impaneled, would have authority to recommend certain changes to the Medicare program to reduce expenditures by the program that could result in reduced payments for prescription products; and |
| · | establishment of the Center for Medicare and Medicaid Innovation within CMS to test innovative payment and service delivery models to lower Medicare and Medicaid spending, potentially including prescription product spending (funding has been allocated to support the mission of the Center for Medicare and Medicaid Innovation through 2019). |
At this point, healthcare reform and its impacts on us are highly uncertain in many respects. For example, since its enactment, there have been judicial and Congressional challenges to numerous aspects of the ACA. The current U.S. administration and U.S. Congress have focused on additional executive and legislative changes, including in particular repeal and replacement of certain provisions of the ACA. It remains to be seen, however, whether new legislation reforming, repealing or replacing the ACA will be enacted and, if so, precisely what the new legislation will provide and what impact it will have on the availability of healthcare and containing or lowering the cost of healthcare. It is possible that these reform, repeal and replacement initiatives, if enacted into law, could ultimately result in fewer individuals having health insurance coverage or in individuals having insurance coverage with less generous benefits. It is also possible that some ACA provisions that generally are not favorable for the research-based pharmaceutical industry could also be repealed along with ACA coverage expansion provisions.
Other legislative changes have been proposed and adopted in the United States since the ACA was enacted. For example, in August 2011, the Budget Control Act of 2011, among other things, included aggregate reductions of Medicare payments to providers of 2% per fiscal year, which went into effect in April 2013 and, due to subsequent legislative amendments, will remain in effect through 2025 unless additional Congressional action is taken. In January 2013, the American Taxpayer Relief Act of 2012, among other things, further reduced Medicare payments to several providers, including hospitals, imaging centers and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years.
Additionally, there has been increasing legislative and enforcement interest in the United States with respect to specialty drug pricing practices. Specifically, there have been several recent U.S. Congressional inquiries and proposed bills designed to, among other things, bring more transparency to drug pricing, reduce the cost of prescription drugs under Medicare, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs. Individual states have also become increasingly active in passing legislation and implementing regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access, and to encourage importation from other countries and bulk purchasing.
There have been, and likely will continue to be, legislative and regulatory proposals at the foreign, federal and state levels directed at broadening the availability of healthcare and containing or lowering the cost of healthcare. Such reforms could have an adverse effect on anticipated revenues from product candidates that we may successfully develop and for which
167
Table of Contents
we may obtain marketing approval and may affect our overall financial condition and ability to develop product candidates.
Facilities
Our headquarters are in the suburbs of Munich, Germany, where we occupy office and laboratory space under a ten-year fixed term lease that started on January 1, 2017. Our subsidiary, Lanthio Pharma, is based in Groningen, the Netherlands, where it occupies office space under a six-year lease. The lease expires in 2022.
Employees
As of December 31, 2017, we had 326 active employees of whom 263 were involved in research and development activities and 63 were involved in general administration. All of these employees are located in our offices in Munich, Germany and in Groningen, Netherlands. We have no collective bargaining agreements with our employees and we have not experienced any labor strikes. We consider our relationship with our employees as good.
Legal Proceedings
From time to time, we are subject to various legal proceedings and claims that arise in the ordinary course of our business activities. Although the results of litigation and claims cannot be predicted with certainty, as of the date of this prospectus, we do not believe we are party to any claim or litigation, the outcome of which, if determined adversely to us, would individually or in the aggregate be reasonably expected to have a material adverse effect on our business. Regardless of the outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
Currently we are involved in a patent litigation lawsuit as a plaintiff against Janssen Biotech Inc., Genmab A/S and Genmab US, Inc at the District Court of Delaware. The lawsuit was filed on April 4, 2016. We are seeking redress for infringement in connection with the manufacture, use and sale of Janssen’s and Genmab’s daratumumab, an antibody targeting CD38, approved for the treatment of certain patients with MM and the same target against which MOR202 is directed. While we believe we have a strong claim in this proceeding, the outcome of the proceeding is uncertain. Defendants have filed counterclaims asserting that our patents are not infringed, invalid and have recently asked the court to add further counterclaims of unenforceability. The case is currently in pre-trial discovery, and a jury trial is scheduled to start in February 2019. The currently litigated patents are US 8,263,746, US 9,200,061 and US 9,758,590. In addition, Janssen, Genmab, Sanofi and Takeda opposed a European counterpart of the litigated U.S. patents, EP2511297. The patent was revoked in opposition proceedings. We appealed and the proceedings are currently pending.
Within the past twelve months, we have not been party to any litigation, arbitration proceedings or administrative proceedings that may have a material effect on our financial condition or profitability, and we are not aware of any such proceedings being pending or threatened.
168
Table of Contents
Overview
We are a German stock corporation (Aktiengesellschaft or AG) with our registered offices in Germany. We are subject to German legislation on stock corporations, most importantly the German Stock Corporation Act (Aktiengesetz). In accordance with the German Stock Corporation Act, our corporate bodies are the management board (Vorstand), the supervisory board (Aufsichtsrat) and the shareholders’ meeting (Hauptversammlung). Our management and supervisory boards are entirely separate and, as a rule, no individual may simultaneously be a member of both boards.
Our management board is responsible for the day-to-day management of our business in accordance with applicable laws, our articles of association (Satzung) and the management board’s internal rules of procedure (Geschäftsordnung). Our management board represents us in our dealings with third parties.
The principal function of our supervisory board is to supervise our management board. The supervisory board is also responsible for appointing and dismissing the members of our management board, representing us in connection with transactions between a current or former member of the management board and us and granting approvals for certain significant matters.
Under German law, members of both boards owe a duty of loyalty and care to us. In carrying out their duties, these members are required to exercise the standard of care of a prudent and diligent businessperson. If they fail to observe the appropriate standard of care, they may become liable to us.
In carrying out their duties, the members of both boards may take into account a broad range of considerations when making decisions, including our interests and the interests of our shareholders, employees, creditors and, to a limited extent, the general public, while respecting the rights of our shareholders to be treated on equal terms. Additionally, the management board is responsible for implementing an internal monitoring system for risk management purposes.
Our supervisory board has comprehensive monitoring responsibilities. To ensure that our supervisory board can carry out these functions properly, our management board must, among other duties, regularly report to our supervisory board regarding our current business operations and future business planning (including financial, investment and personnel planning). In addition, our supervisory board is entitled to request special reports from the management board at any time.
Under German law, our shareholders have no direct recourse against the members of our management or supervisory boards if they have breached their duty of loyalty and care to us. Apart from insolvency or other special circumstances, only MorphoSys AG has the ability to claim damages against the members of our two boards. We may waive these claims to damages or settle these claims only if at least three years have passed since any violation of a duty occurred and our shareholders approve the waiver or settlement at a shareholders’ meeting with a simple majority of the votes cast; provided that shareholders who in the aggregate hold one-tenth or more of our share capital do not oppose the waiver or settlement and have their opposition formally recorded in the meeting’s minutes by a German civil law notary.
169
Table of Contents
Supervisory Board
German law requires that the supervisory board consists of at least three members, whereby the articles of association may stipulate a certain higher number. Our supervisory board currently consists of six members. German law further requires the number of supervisory board members to be divisible by three if this is necessary for the fulfilment of co-determination requirements. This does not apply to us as we are currently not subject to co-determination. As we grow, this may change and our supervisory board may be required to include employee representatives subject to the provisions of the German One-Third Employee Representation Act (Drittelbeteiligungsgesetz), which applies to companies that have at least 500 employees, and the German Codetermination Act (Mitbestimmungsgesetz), which applies to companies that have at least 2,000 employees. As of January 1, 2016, 30% of the supervisory board members must be women in case the Company is a fully co-determined (voll mitbestimmungspflichtig) company, which requires that the company has at least 2,000 employees. This currently does not apply to us.
The supervisory board has set certain targets for the composition of the supervisory board, including:
| · | at least two non-German members or at least two members having extensive international experience; |
| · | at least four independent members (within the meaning of the German Corporate Governance Code (Deutscher Corporate Governance Kodex)); |
| · | at least a 33.33% ratio of women to men serving on the supervisory board; and |
| · | an age limit of 75 years at the time of appointment. |
All of the members of our current supervisory board were elected by the shareholders’ meeting in accordance with the provisions of the German Stock Corporation Act.
Under German law, a member of a supervisory board may be elected for a term of up to approximately five years, depending on the date of the shareholders’ meeting at which such member is elected. Re-election, including repeated re-election, is permissible. The shareholders’ meeting may specify a term of office for individual members or all of the members of our supervisory board which is shorter than the standard term of office and, subject to statutory limits, may set different start and end dates for the terms of members of our supervisory board. In accordance with the German Corporate Governance Code, our supervisory board has agreed on the following regular limits to supervisory board members’ term of office to be proposed to the shareholders’ meeting when supervisory board members are to be elected: first term of two years, reappointment of supervisory board members twice for a further three-year term of office each and in justified individual cases our supervisory board reserves the right to make exceptions to this rule and to propose reappointment of supervisory board members for a fourth term of office of three years.
Except for our supervisory board member Krisja Vermeylen, who was first appointed by our shareholders’ meeting in 2017 for a two-year term, all other members of our supervisory board were each elected to serve for a three-year term.
The shareholders’ meeting may, at the same time as it elects the members of the supervisory board, elect one or more substitute members. The substitute members replace members who
170
Table of Contents
cease to be members of our supervisory board and take their place for the remainder of their respective terms of office. Currently, no substitute members have been elected or have been proposed to be elected.
Members of our supervisory board may be dismissed at any time during their term of office by a resolution of the shareholders’ meeting adopted by a majority of three quarters of the votes cast. In addition, any member of our supervisory board may resign at any time by giving one month’s written notice of his or her resignation to the chairman of our supervisory board (in case the chairman resigns, such notice is to be given to the deputy chairman) or to the management board. Our supervisory board may agree upon a shorter notice period.
Our supervisory board elects a chairman and a deputy chairman from its members. The deputy chairman exercises the chairman’s rights and obligations whenever the chairman is unable to do so. The members of our supervisory board have elected Dr. Gerald Möller as chairman and Dr. Frank Morich as deputy chairman, each for the term of their respective membership on our supervisory board.
The supervisory board meets at least four times each calendar year. Our articles of association and the supervisory board’s rules of procedure provide that a quorum of the supervisory board members is present if at least two-thirds of its members, but in any case no less than three members, participate in the vote, provided that the chairman or the deputy chairman is among the participating members. Members of our supervisory board are deemed present if they participate via telephone or video conference unless the chairman issues an order deviating therefrom. Any absent member may also participate in the voting by submitting his or her written vote through another member.
Resolutions of our supervisory board are passed by the vote of a simple majority unless otherwise required by law, our articles of association or the rules of procedure of our supervisory board. In the event of a tie, the chairman has the casting vote.
Our supervisory board is not permitted to make management decisions, but, in accordance with German law and in addition to its statutory responsibilities, it has determined that certain matters require its prior consent, including:
| · | annual planning, in particular the annual budget plan for each financial year; |
| · | entering, materially amending or terminating any agreement or other commitment of MorphoSys having a real or potential volume in excess of €5 million, or committing to any payment in excess of €0.3 million, prior to entering into such agreement; |
| · | concluding, materially amending or terminating agreements of the Company with or for the benefit of any of our supervisory board or management board members, their respective relatives (as defined in Section 15 of the German Fiscal Code (Abgabenordnung)) or companies related with them as defined in Section 15 German Stock Corporation Act; |
| · | founding or dissolving companies; |
| · | acquiring or selling participations in companies; |
| · | granting, materially amending or terminating profit participation rights in MorphoSys, including silent interest participations; |
| · | establishing, acquiring, closing or selling plants or branches of MorphoSys; |
171
Table of Contents
| · | acquiring, selling or encumbering real estate or rights equivalent to real estate; |
| · | granting of loans to (i) companies in which MorphoSys does not hold an equity stake or (ii) companies in which MorphoSys holds an equity stake and which loans have a real or potential value in excess of €2 million; |
| · | transferring MorphoSys’s assets in whole or in a substantial part; |
| · | mortgaging or permitting the creation of any mortgage over the whole or a substantial part of the assets of MorphoSys; and |
| · | changing the schedules of responsibilities (Geschäftsverteilungsplan) of the management board. |
Our supervisory board may designate further types of actions requiring its approval.
The following table sets forth the names and functions of the current members of our supervisory board, their ages, their terms (which expire on the date of the relevant year’s general shareholders’ meeting) and their principal occupations outside of our Company:
| Name | Age | Term expires |
Principal business activities performed outside of MorphoSys | |||||||
| Dr. Gerald Möller* |
74 | 2018 | Chief executive officer of Adrenomed AG; chairman of the board of directors of 4sigma, Inc.; chairman of the advisory board of AYOXXA Biosystems GmbH. | |||||||
| Dr. Marc Cluzel |
63 | 2018 | Consultant & business professional; member of the board of directors of Moleac Pte. Ltd. | |||||||
| Krisja Vermeylen |
55 | 2019 | Senior vice president, corporate people & organization, Novo Nordisk A/S. | |||||||
| Wendy Johnson |
66 | 2020 | Chief operating officer, Reneo Pharmaceuticals, Inc.; managing director of Gemini Advisors. | |||||||
| Klaus Kühn** |
66 | 2020 | Chairman of the supervisory board of Flossbach von Storch AG; member of the supervisory board and member of the shareholder committee of Hella KGaA Hueck & Co. | |||||||
| Dr. Frank Morich |
64 | 2020 | Independent consultant of the life sciences and healthcare industries. | |||||||
| * | Dr. Gerald Möller is to retire as a member of our supervisory board at the end of his term at the end of the annual shareholders’ meeting in 2018. |
| ** | Mr. Klaus Kühn has announced to us that he will resign as a member of the supervisory board effective as of the end of the annual shareholders’ meeting in 2018. |
172
Table of Contents
The business address of the members of our supervisory board is the same as our business address: MorphoSys AG, Semmelweisstrasse 7, 82152 Planegg, Germany. Our supervisory board has determined that all members of our supervisory board are independent under German law and the Nasdaq Stock Market Rules.
The following is a brief summary of the prior business experience of the members of our supervisory board:
Dr. Gerald Möller
Dr. Gerald Möller has been a member of and chairman of our supervisory board since 1999. He currently serves as chief operating officer of Adrenomed AG. Dr. Möller was an advisor at HBM Partners from 2002 to 2014. He was chief executive officer and member of the executive committee of 4sigma Ltd. from 1999 to 2000. Dr. Möller was head of development and strategic marketing pharmaceuticals at Roche in 1998. Prior to that, Dr. Möller held several positions at Boehringer Mannheim Group, including chief executive officer from 1995 to 1998. Dr. Möller received his Ph.D. from the Institute for Physical Chemistry in Kiel, Germany.
Dr. Marc Cluzel
Dr. Marc Cluzel has been a member of our supervisory board since 2012. He was executive vice president of product development at HÙYÀ Bioscience International, LLC from 2011 to 2012. Prior to that, between 1993 and 2010, he held several positions at Sanofi-Aventis, including head of research and development. Dr. Cluzel received his Ph.D. in biochemistry and his doctor of medicine from the University of Montpellier, France.
Krisja Vermeylen
Krisja Vermeylen has been a member of our supervisory board since 2017. Since 1997, Mrs. Vermeylen has held several positions at Novo Nordisk, including her current position as senior vice president corporate people & organization. Prior to that, she held several positions at Pharmacia and Upjohn. Mrs. Vermeylen graduated with a masters in pharmaceutical sciences from the University of Antwerp, Belgium.
Wendy Johnson
Wendy Johnson has been a member of our supervisory board since 2015. Mrs. Johnson currently serves as the chief operating officer at Reneo Pharmaceuticals and as managing director of Gemini Advisors. Mrs. Johnson was the founder, president and chief executive officer of Aires Pharmaceuticals, Inc. from 2007 to 2014. Mrs. Johnson was also a venture partner in ProQuest Investments (2005 to 2014), senior vice president corporate development at Salmedix, Inc. (2001 to 2005), vice president business development at Women First HealthCare (1998 to 2000), vice president corporate development & operations at Selective Genetics (1994 to 1998), vice president business development & regulatory affairs at Cytel Corp. (1990 to 1994), manager business development at Synbiotics Corp. (1988 to 1990) and international business development & regulatory affairs manager at Murex Corp. (1986 to 1988). Prior to that, Mrs. Johnson served as assistant director at the Center for Devices & Radiological Health at the U.S. Food and Drug Administration from 1976 to 1986. Mrs. Johnson graduated with a masters in business administration from Loyola Marymount University, USA, a masters in science in clinical microbiology from Hahnemann University Hospital, USA and a Bachelor of science in microbiology from the University of Maryland, USA.
173
Table of Contents
Klaus Kühn
Klaus Kühn has been a member of our supervisory board since 2015. Mr. Kühn served as the chief financial officer of Bayer from 2002 to 2010 and the head of finance at Bayer from 1998 to 2002. Prior to that, Mr. Kühn served as head of corporate staff and head of finance of Schering from 1981 to 1998. Mr. Kühn graduated with a masters of business administration in international business studies from the University of South Carolina, USA, and a diploma in mathematics/physics from the Technical University Berlin, Germany.
Dr. Frank Morich
Dr. Frank Morich has been a member of our supervisory board since 2015. Dr. Morich also serves as a consultant in the life sciences and health care industries. Dr. Morich previously served as chief commercial officer (2011 to 2014) and executive vice president international operations (2010 to 2011) at Takeda Pharmaceutical. Prior to that, Dr. Morich served as chief executive officer of NOXXON Pharma AG (2008 to 2010), chief executive officer and member of the board of directors of Innogenetics N.V. (2005 to 2007), and chief executive officer and chairman of the executive board of AM Pharma B.V. (2004). Prior to that, Dr. Morich held several positions at Bayer, including member of the board of management of Bayer AG, head of global product development and head of research and development. Dr. Morich graduated in medical studies at the University of Marburg, Germany.
The supervisory board has nominated Dr. George Golumbeski and Michael Brosnan as candidates to be elected as new supervisory board members at our annual shareholders’ meeting, which is expected to take place on May 17, 2018. Dr. Gerald Möller is to retire as a member of our supervisory board at the end of his term at the end of that annual shareholders’ meeting, and Mr. Klaus Kühn will resign as a member of the supervisory board effective as of the end of that annual shareholders’ meeting. The biographies of Dr. George Golumbeski and Michael Brosnan are set forth below.
Dr. George Golumbeski
Dr. George Golumbeski currently serves as executive vice president & executive advisor for innovation at Celgene Corporation. He will retire from this position on April 16, 2018. Over the last 27 years, he has held leadership roles in business and corporate development, partnering and M&A with global pharmaceutical and life science companies, including Celgene, Novartis, Elan Corporation (today: Perrigo), and Schwarz Pharma (today: UCB). Dr. Golumbeski obtained his doctorate in genetics from the University of Wisconsin in Madison, USA and holds a degree in biology from the University of Virginia, Charlottesville, USA.
Michael Brosnan
Michael Brosnan has over 40 years of experience in finance, controlling and auditing. Since 2010, he has served as chief financial officer of Fresenius Medical Care Management AG, a company with a dual listing in Germany (Frankfurt) and the United States (NYSE). Over the last 20 years, he has worked in various leadership and executive positions for Fresenius Medical Care in the United States and Germany. Prior to joining Fresenius Medical Care, he held senior financial positions at Polaroid Corporation and was an audit partner at KPMG. Mr. Brosnan holds a degree in business administration and accounting from Northeastern University, Boston, MA, USA.
174
Table of Contents
Supervisory Board Practices
Decisions are generally made by our supervisory board as a whole; however, decisions on certain matters may be delegated to committees of our supervisory board to the extent permitted by law. The chairman, or if he or she is prevented from doing so, the deputy chairman, chairs the meetings of the supervisory board and determines the order in which the agenda items are discussed, the method and order of voting, any adjournment of the discussion and passing of resolutions on individual agenda items after a due assessment of the circumstances.
In addition, under German law, each member of the supervisory board is obliged to carry out his or her duties and responsibilities personally, and such duties and responsibilities cannot be generally and permanently delegated to third parties. However, the supervisory board and its committees have the right to appoint independent experts for the review and analysis of specific circumstances in accordance with its control and supervision duties under German law. We would bear the costs for any such independent experts that are retained by the supervisory board or any of its committees.
Pursuant to Section 107 Para. 3 of the German Stock Corporation Act, the supervisory board may form committees from among its members and charge them with the performance of specific tasks. The committees’ tasks, authorizations and processes are determined by the supervisory board. Where permissible by law, important powers of the supervisory board may also be transferred to committees.
Under Article 10 of its rules of procedure, the supervisory board has established an Audit Committee (Prüfungsausschuss), a Remuneration and Nomination Committee (Vergütungs- und Ernennungsausschuss), and a Science and Technology Committee (Wissenschafts- und Technologieausschuss).
Audit Committee
Our Audit Committee assists the supervisory board in overseeing the accuracy and integrity of our accounting and financial reporting processes and audits of our financial statements, the effectiveness of our internal control system, our risk management system and our compliance with legal and regulatory requirements, the independent auditors’ qualifications and independence and the performance of the independent auditors.
The Audit Committee consists of at least three members and its duties and responsibilities include, among others:
| · | considering the appointment of the external auditor, the audit fee, and any questions of resignation or dismissal; |
| · | reviewing and recommending the annual financial statements for approval before submission to our supervisory board; |
| · | discussing the half-year and quarterly financial reports and the report on the review of the half-year financial statements with the management board and, regarding the half-year financial reports, also with the auditor; |
| · | monitoring the financial reporting process and the effectiveness of the internal control system, the compliance management system, the risk management system and the internal audit system; and |
175
Table of Contents
| · | discussing the investment strategy with our management board and approving the investment proposals by our management board. |
The Audit Committee consists of three members of our supervisory board: Krisja Vermeylen, Wendy Johnson, and Klaus Kühn (chairman). Our supervisory board has determined that each member of the Audit Committee satisfies the independence requirements of Nasdaq and the Exchange Act and that Klaus Kühn qualifies as an “audit committee financial expert” as defined under the Exchange Act.
Remuneration and Nomination Committee
Pursuant to our articles of association and the rules of procedure of our supervisory board, the Remuneration and Nomination Committee proposes suitable supervisory board candidates for recommendation to the shareholders’ meeting and prepares hiring and personnel decisions for approval by the supervisory board. The Remuneration and Nomination Committee consists of at least three members and its duties and responsibilities include, among others:
| · | preparing the personnel decisions of the supervisory board, in particular appointing and removing members of the management board, the conclusion, amendment and termination of service agreements with our management board and fixing of an adequate remuneration of our management board members consistent with a sustainable development of the company; and |
| · | proposing suitable candidates for appointment to our supervisory board for recommendation to the shareholders’ meeting of the Company. |
The Remuneration and Nomination Committee consists of three members of our supervisory board: Dr. Gerald Möller (chairman), Dr. Marc Cluzel, and Krisja Vermeylen. Our supervisory board has determined that each member of the Remuneration and Nomination Committee satisfies the independence requirements of Nasdaq.
Science and Technology Committee
Pursuant to our articles of association and the rules of procedure of our supervisory board, the Science and Technology Committee is responsible for technically reviewing and discussing our technology development efforts, in particular our research, discovery and development activities with respect to the establishment of a proprietary antibody product pipeline (including related in-licensing and mergers and acquisitions activity).
The Science and Technology Committee consists of at least two members and its duties and responsibilities include, among others:
| · | technically reviewing and discussing our research, discovery and development activities directed at building a proprietary antibody product pipeline; |
| · | reviewing and discussing our R&D activities in the context of our overall strategy; and |
| · | reviewing and discussing our R&D activities in the context of the competitive environment. |
The Science and Technology Committee consists of three members of our supervisory board: Dr. Marc Cluzel (chairman), Wendy Johnson, and Dr. Frank Morich.
176
Table of Contents
Compensation of Supervisory Board Members
The following table sets forth the aggregate compensation and benefits provided to our supervisory board in the year ended December 31, 2017:
| (in €) | Fixed Compensation |
Attendance Fees |
Total Compensation |
|||||||||
| Dr. Gerald Möller |
95,156 | 36,800 | 131,956 | |||||||||
| Dr. Frank Morich |
57,240 | 23,200 | 80,440 | |||||||||
| Dr. Marc Cluzel |
52,160 | 26,800 | 78,960 | |||||||||
| Krisja Vermeylen* |
28,961 | 16,000 | 44,961 | |||||||||
| Wendy Johnson |
46,160 | 38,000 | 84,160 | |||||||||
| Klaus Kühn |
46,160 | 22,000 | 68,160 | |||||||||
| Karin Eastham** |
19,578 | 14,800 | 34,378 | |||||||||
| * | Krisja Vermeylen joined our supervisory board on May 17, 2017. |
| ** | Karin Eastham resigned from her office as a member of our supervisory board with effect from the end of the annual general meeting on May 17, 2017. |
The table below provides an overview, as of April 6, 2018, of the shares held by members of our supervisory board.
| Shares | ||||
| Dr. Gerald Möller |
11,000 | |||
| Dr. Frank Morich |
1,000 | |||
| Dr. Marc Cluzel |
500 | |||
| Krisja Vermeylen |
350 | |||
| Wendy Johnson |
500 | |||
| Klaus Kühn |
0 | |||
For information on the shareholdings of our supervisory board, see “Principal Shareholders”.
Management Board
Overview
Under the Company’s articles of association, the management board must consist of at least two members, with the supervisory board determining the exact number. The supervisory board also appoints the chairman and the deputy chairman of the management board, if any.
The members of our management board conduct our daily business in accordance with applicable laws, our articles of association and the rules of procedure for the management board. The management board is in general responsible for our management and for handling our daily business relations with third parties, the internal organization of our business and communications with our shareholders. In addition, the management board has the responsibility for:
| · | the preparation of our annual financial statements; |
| · | jointly with the supervisory board, the making of a proposal to our shareholders’ meeting on how our profits (if any) should be allocated; and |
177
Table of Contents
| · | regular reporting to the supervisory board on our current operating and financial performance, our budgeting and planning processes and our performance under them and on future business planning (including strategic, financial, investment and personnel planning). |
The supervisory board can appoint the members of the management board for a maximum term of five years. Reappointment or extension, including a repeated reappointment or extension, for terms of up to five years is permissible. The supervisory board may revoke the appointment of a management board member prior to the expiration of his or her term only for good cause, such as for gross breach of fiduciary duties or if the shareholders’ meeting passes a vote of no-confidence with respect to such member, unless the supervisory board deems the no-confidence vote to be clearly unreasonable. The supervisory board is also responsible for entering into, amending and terminating service agreements with the management board members and, in general, for representing us in disputes with the management board, both in and out of court.
The supervisory board has set a non-binding target quota for female members of the management board at 25%. This quota is currently not fulfilled.
The rules of procedure for our management board provide for certain matters that require a resolution adopted by the management board, in addition to transactions for which a resolution adopted by the management board is required by law or required by our articles of association. Such resolutions include the following:
| · | preparation of the annual financial statements and the management report of MorphoSys AG as well as the consolidated annual financial statements, and the management report on our group; |
| · | convening of our shareholders’ meetings and proposed resolutions of the management board to be submitted to the shareholders’ meetings for a resolution; |
| · | matters which require the approval of our supervisory board pursuant to the rules of procedure of the management board; |
| · | matters which impact more than one member of the management board’s area of responsibility; |
| · | matters which relate to personnel at or above the level of director; and |
| · | drafting and publication of press releases. |
Members of the Management Board
The following table sets forth the names and functions of the current members of our management board, their ages and their terms:
| Name | Age | Term ends | Position | |||||||
| Dr. Simon Moroney |
59 | June 30, 2020 | Chief Executive Officer | |||||||
| Jens Holstein |
54 | June 30, 2020 | Chief Financial Officer | |||||||
| Dr. Malte Peters |
55 | June 30, 2019 | Chief Development Officer | |||||||
| Dr. Markus Enzelberger |
48 | June 30, 2020 | Chief Scientific Officer | |||||||
The business address of the members of our management board is the same as our business address: MorphoSys AG, Semmelweisstrasse 7, 82152 Planegg, Germany.
178
Table of Contents
The following is a brief summary of the business experience of the members of our management board:
Dr. Simon Moroney
Dr. Moroney is one of our co-founders. Prior to that, Dr. Moroney held positions in the Department of Pharmacology, University of Cambridge, United Kingdom, as assistant professor in the Chemistry Department of the University of British Columbia, Vancouver, Canada, and as associate in the Chemistry Department of the ETH in Zurich, Switzerland, where he also held a position as lecturer. He was an associate in the Harvard Medical School, Boston, USA, and an employee of ImmunoGen Inc., where he worked on the first generation of anti-cancer antibody conjugates. Dr. Moroney studied chemistry in New Zealand, where he completed a masters in science with 1st class honors and was a Commonwealth Scholar to the University of Oxford, where he was awarded a doctor in philosophy in Chemistry.
In 2002, Dr. Moroney received the German Cross of the Order of Merit by Dr. Johannes Rau, President of the Federal Republic of Germany, for his services to the biotechnology industry.
Jens Holstein
Jens Holstein joined MorphoSys in May 2011. Prior to his time at MorphoSys, Mr. Holstein served as regional chief financial officer for the EME (Europe/Middle East) region for Fresenius Kabi AG and as managing director of Fresenius Kabi Deutschland GmbH. Over almost 16 years at Fresenius he had held a variety of financial and general management positions. From 2006 to 2010, he was regional chief financial officer of Fresenius Kabi Asia Pacific Ltd., based in Hong Kong. Prior to this appointment, Mr. Holstein was managing director of Fresenius ProServe GmbH and finance director and labor director of Fresenius’s subsidiary Wittgensteiner Kliniken AG. Earlier positions within Fresenius included general manager of hospitalia care GmbH, commercial manager of the projects & service business unit of Fresenius AG and commercial manager of hospitalia international GmbH. Prior to joining Fresenius, Mr. Holstein spent several years in the consulting industry, with positions in Frankfurt and London. Mr. Holstein holds a diploma in business administration from the University of Münster, Germany.
Dr. Malte Peters
Dr. Peters joined MorphoSys in March 2017. Prior to his time at MorphoSys, Dr. Peters served as the global head of clinical development of the biopharmaceuticals business unit at Sandoz International. Prior to this position, he served as clinical head and site head for Basel and East Hanover in the department of oncology translational medicine at Novartis. Dr. Peters held teaching appointments in internal medicine and biochemistry at the University of Mainz, Germany. Dr. Peters also served as research scientist at the Amgen Research Institute in Toronto, Canada, as director of cancer research at Merck KGaA and as medical director at Micromet AG.
Dr. Peters received his doctor of medicine from the Freie Universität Berlin, Germany, and was trained at the Universities of Padova, Italy, and Bochum and Berlin, Germany. After scientific work at different universities he habilitated in internal medicine at the University of Mainz, Germany.
Dr. Markus Enzelberger
Dr. Enzelberger joined MorphoSys in March 2002 and served in different leadership positions within R&D. Since 2012 he acted as senior vice president discovery, alliances and technologies,
179
Table of Contents
taking responsibility for discovery programs for our partners and our proprietary pipeline as well as for the technology development. He was appointed Interim-Chief Scientific Officer effective April 15, 2017 and Chief Scientific Officer effective November 1, 2017. His new areas of responsibility include discovery, technology development, protein sciences, manufacturing and alliance management. Dr. Enzelberger is co-inventor of the HuCAL Platinum and the Ylanthia libraries and worked on Tremfya® and many other programs within our pipeline.
Before joining MorphoSys, Dr. Enzelberger completed a post-doctorate with Steven Quake at the California Institute of Technology on microfluidic biological assays, where he co-invented the key technologies of Fluidigm. Dr. Enzelberger studied chemistry and was awarded a doctor of natural sciences by the Technical University of Stuttgart, Germany.
Compensation of Management Board Members
The following table sets forth the aggregate compensation and benefits provided to our management board in the year ended December 31, 2017:
| (in €) | Dr. Simon Moroney |
Jens Holstein |
Dr. Malte Peters |
Dr. Markus Enzelberger3 |
||||||||||||
| Fixed Compensation |
500,876 | 372,652 | 281,500 | 204,698 | ||||||||||||
| Fringe Benefits1 |
35,912 | 42,905 | 568,644 | 417,158 | ||||||||||||
| One-Year Variable Compensation |
368,144 | 273,899 | 206,903 | 121,688 | ||||||||||||
| Total Short-Term Employee Benefits |
904,932 | 689,456 | 1,057,047 | 743,544 | ||||||||||||
| Service Cost |
149,567 | 99,949 | 60,967 | 29,186 | ||||||||||||
| Total Benefit Expenses—Post-Employment Benefits |
149,567 | 99,949 | 60,967 | 29,186 | ||||||||||||
| Multi-Year Variable Compensation2 |
||||||||||||||||
| 2013 Convertible Bond Program |
58,224 | 59,641 | — | — | ||||||||||||
| 2013 Long-Term Incentive Program |
202,349 | 138,585 | — | — | ||||||||||||
| 2014 Long-Term Incentive Program |
22,460 | 15,383 | — | — | ||||||||||||
| 2015 Long-Term Incentive Program |
67,635 | 46,324 | — | — | ||||||||||||
| 2016 Long-Term Incentive Program |
171,688 | 112,481 | — | — | ||||||||||||
| 2017 Long-Term Incentive Program |
163,906 | 107,395 | 107,395 | 68,979 | ||||||||||||
| 2017 Stock Option Plan |
127,997 | 83,861 | 83,861 | 53,875 | ||||||||||||
| Total Stock-Based Compensation |
814,259 | 563,670 | 191,256 | 122,854 | ||||||||||||
| Total Compensation |
1,868,758 | 1,353,075 | 1,309,270 | 895,584 | ||||||||||||
180
Table of Contents
| (in €) | Dr. Marlies Sproll4 |
Dr. Arndt Schottelius5 |
||||||||||||||
| Fixed Compensation |
222,450 | 103,253 | ||||||||||||||
| Fringe Benefits |
20,427 | 9,161 | ||||||||||||||
| One-Year Variable Compensation |
67,745 | 23,490 | ||||||||||||||
| Total Short-Term Employee Benefits |
310,622 | 135,904 | ||||||||||||||
| Service Cost |
77,976 | 28,245 | ||||||||||||||
| Total Benefit Expenses—Post-Employment Benefits |
77,976 | 28,245 | ||||||||||||||
| Multi-Year Variable Compensation2 |
||||||||||||||||
| 2013 Convertible Bond Program |
39,879 | 39,879 | ||||||||||||||
| 2013 Long-Term Incentive Program |
138,585 | 138,585 | ||||||||||||||
| 2014 Long-Term Incentive Program |
15,383 | (42,038 | ) | |||||||||||||
| 2015 Long-Term Incentive Program |
46,324 | (79,105 | ) | |||||||||||||
| 2016 Long-Term Incentive Program |
112,481 | (76,828 | ) | |||||||||||||
| 2017 Long-Term Incentive Program |
80,538 | — | ||||||||||||||
| 2017 Stock Option Plan |
62,898 | — | ||||||||||||||
| Total Stock-Based Compensation |
496,088 | (19,507 | ) | |||||||||||||
| Total Compensation |
884,686 | 144,642 | ||||||||||||||
| 1 | In 2017, the fringe benefits of Dr. Malte Peters und Dr. Markus Enzelberger each included a one-time compensation in the form of MorphoSys AG shares as an incentive to join the Management Board of MorphoSys AG. |
| 2 | The fair value was determined pursuant to the regulations of IFRS 2 “Share-based Payments”. This table shows the pro-rata share of personnel expenses resulting from stock-based compensation for the respective financial year. |
| 3 | The figures presented for Dr. Markus Enzelberger do not include any compensation granted for his activities as a member of the Senior Management Group as they do not relate to his appointment as a member of the Management Board. |
| 4 | Dr. Marlies Sproll left the Management Board of MorphoSys AG on October 31, 2017. Since November 1, 2017, Dr. Marlies Sproll has taken on a new part-time role at MorphoSys as Special Adviser to the CEO. Therefore, the figures presented for Dr. Marlies Sproll do not include any remuneration granted for these activities. |
| 5 | Dr. Arndt Schottelius resigned from his office as member of the Management Board with effect as of February 28, 2017 and was replaced by Dr. Malte Peters with effect as of March 1, 2017. |
The table below provides an overview, as of April 6, 2018, of the shares, convertible bonds and performance shares held by members of our management board.
| Shares | Convertible Bonds* |
Performance Shares** |
Options*** | |||||||||||||
| Dr. Simon Moroney |
483,709 | 88,386 | 30,060 | 12,511 | ||||||||||||
| Jens Holstein |
11,000 | 60,537 | 20,086 | 8,197 | ||||||||||||
| Dr. Malte Peters |
9,505 | — | 3,187 | 8,197 | ||||||||||||
| Dr. Markus Enzelberger |
7,262 | — | 5,987 | 5,266 | ||||||||||||
| * | The convertible bonds vested in April 2017. Subject to several conditions, each converted bond can be exchanged for one of MorphoSys AG’s ordinary bearer shares equal to the proportional amount of ordinary bearer shares (currently being €1.00) for a conversion price of €31.88. Please see “—Share-Based Incentive Plans—Convertible Bonds—2013 Program” below. |
181
Table of Contents
| ** | Performance shares are subject a vesting/performance period and certain vesting criteria. Please see “—Share-Based Incentive Plans” below. |
| *** | Options were granted to members of the management board pursuant to the 2017 Stock Option Plan on April 1, 2017. The options are exercisable four years after the grant date, being April 1, 2021, and expire on April 1, 2024. The exercise price of the options are €55.52. |
For information on the shareholdings of our management board, see “Principal Shareholders”.
We do not separately set aside any amounts for pensions, retirement or other benefits for members of our management board, other than pursuant to relevant statutory requirements.
Service Agreements
The service agreements with our management board members generally have a total term of three years. The current service agreements of our management board members Dr. Simon Moroney, Jens Holstein and Dr. Markus Enzelberger run until June 30, 2020. The current service agreement of our management board member Dr. Malte Peters runs until June 30, 2019.
In the event of a change of control, our management board members are entitled to exercise a right to terminate their employment contracts and receive any outstanding fixed salary for the remainder of the fixed contract period.
Share-Based Incentive Plans
Below is a summary of the equity plans that are currently active, which form part of our incentive and commitment program for our key personnel.
Convertible Bonds – 2013 Program
On April 1, 2013, we granted the members of the management board and members of the senior management group convertible bonds with a total nominal value of €225,000, divided into 449,999 bearer bonds with equal rights from the “Conditional Capital 2008-III” (see “Description of Share Capital—Conditional Capital”). The convertible bonds mature on March 31, 2020 and do not carry any interest. The beneficiaries have the right to convert the bonds into ordinary shares of MorphoSys AG. Each convertible bond can be exchanged for one of MorphoSys AG’s ordinary shares equal to the proportional amount of ordinary shares, which currently stands at €1.00. Exercise of the convertible bonds is subject to several conditions, such as the achievement of performance targets, the expiration of vesting periods, the exercisability of the conversion rights, the existence of an employment or service contract that is not under notice and the commencement of the exercise period.
The conversion price amounted to €31.88 and was derived from MorphoSys AG’s share price in the XETRA closing auction of the Frankfurt Stock Exchange on the trading day preceding the issue of the convertible bonds. The exercise of the conversion rights is admissible since on at least one trading day during the lifetime of the convertible bonds, the share price of the Company has risen to more than 120% of the price in the XETRA closing auction of the Frankfurt Stock Exchange on the trading day preceding the issue of the convertible bonds.
The exercise of the conversion rights is only admissible since the expiration of the four-year vesting period from the grant date. For every year without a notice of termination of the employment relationship with the Company or an affiliated company, 25% of the conversion rights become vested. All convertible bonds have vested by April 1, 2017.
182
Table of Contents
2012 Long-Term Incentive Program
On April 1, 2012, we established a long-term incentive plan, which we refer to as the “2012 LTI Plan 1”, for members of the management board and the senior management group. The vesting period of this plan expired on April 1, 2016. The 2012 LTI Plan 1 was a performance-related share plan and was paid out in ordinary shares (performance shares) of MorphoSys AG if predefined key performance criteria were achieved. The key performance criteria are based on the absolute MorphoSys share price performance and the relative MorphoSys share price performance compared to the Nasdaq Biotechnology Index and the TecDAX Index. These criteria were approved annually by the supervisory board. The fulfillment of these criteria was set at 200% for two years, 54% for one year and 0% for one year. The supervisory board set the “company factor” at 0.88, meaning the number of performance shares to be allocated was scaled by a factor of 0.88.
On October 1, 2012, we established a second long-term incentive plan for senior management group members. The vesting period of this plan expired on October 1, 2016. The terms of this plan were identical to the 2012 LTI Plan 1. The supervisory board set the “company factor” at 1.57, meaning the number of performance shares to be allocated was scaled by a factor of 1.57. All performance shares have vested.
2013 Long-Term Incentive Program
On April 1, 2013, we established a long-term incentive plan, which we refer to as the “2013 LTI Plan 1”, for members of the management board and the senior management group. The vesting period of this plan expired on April 1, 2017. The 2013 LTI Plan 1 was a performance-related share plan and was paid out in ordinary shares (performance shares) of MorphoSys AG if predefined key performance criteria were achieved. The key performance criteria were based on the absolute MorphoSys share price performance and the relative MorphoSys share price performance compared to the NASDAQ Biotechnology Index and the TecDAX Index. These criteria were approved annually by the supervisory board. The fulfillment of these criteria was set at 200% for one year, 54% for one year and 0% for two years. The supervisory board set the “company factor” at 1.57, meaning the number of performance shares to be allocated was scaled by a factor of 1.57.
On October 1, 2013, we established a second long-term incentive plan for senior management group members. The vesting period of this plan expired on October 1, 2017. The terms of this plan were identical to the 2013 LTI Plan 1. The supervisory board set the “company factor” at 1.57, meaning the number of performance shares to be allocated was scaled by a factor of 1.57. All performance shares have vested.
2014 Long-Term Incentive Program
On April 1, 2014, we established a long-term incentive plan, which we refer to as the “2014 LTI Plan”, for members of the management board and the senior management group. The 2014 LTI Plan is a performance-related share plan and will be paid out in ordinary shares (performance shares) of MorphoSys AG if predefined key performance criteria are achieved. These criteria are evaluated annually by the supervisory board. The grant date was April 1, 2014 and the vesting/performance period is four years. If the predefined key performance criteria for the respective period are fully met, 25% of the performance shares vest in each year of the four-year vesting period. The number of performance shares vested per year is calculated based on the key
183
Table of Contents
performance criteria of the absolute MorphoSys share price performance and the relative MorphoSys share price performance compared to the Nasdaq Biotechnology Index and the TecDAX Index. The number of performance shares vesting each year will be reduced or increased to the extent that the performance criteria of the respective year have been achieved between only 50% and 99.9% (<100 %) or the achievement of the performance criteria has exceeded 100% (maximum 200%). If in one year the performance criteria are met by less than 50%, no performance shares will vest in that year. In any case, the maximum pay-out at the end of the four-year period is limited by a factor we determine, which generally amounts to 1. However, in justified cases, the supervisory board may set this factor in its discretion between 0 and 2, for example, if the level of payment is regarded as unreasonable in view of our general development. The right to receive a certain allocation of performance shares under the 2014 LTI Plan, however, will only occur at the end of the four-year vesting period.
If the number of treasury shares is not sufficient for servicing the 2014 LTI Plan, we reserve the right to pay a certain amount of the 2014 LTI Plan in cash in the amount of the performance shares at the end of the vesting period, provided the cash amount does not exceed 200% of the fair value of the performance shares on the grant date.
If a member of the management board ceases to hold an office at our group because of termination (or if the management board member terminates the employment contract), resignation, death, injury, disability, by reaching retirement age (receipt of a normal retirement pension, early-retirement pension or disability pension, as long as the requirements for the disability pension entitlement are met) or under other circumstances subject to the supervisory board’s discretion, the management board member (or the member’s heirs) is entitled to performance shares determined on a precise daily pro rata basis.
If a member of the management board ceases to hold an office at our group for good reason as defined by Section 626 Para. 2 of the German Civil Code and/or as defined by Section 84 Para. 3 of the German Stock Corporation Act, the beneficiary will not be entitled to performance shares.
If a change of control occurs during the four-year vesting period, all performance shares will fully vest. In this case, the right to receive a certain allocation of performance shares under the 2014 LTI Plan will only occur at the end of the four-year vesting period.
2015 Long-Term Incentive Program
On April 1, 2015, we established a long-term incentive plan, which we refer to as the “2015 LTI Plan”, for members of the management board and the senior management group. The 2015 LTI Plan is a performance-related share plan and will be paid out in ordinary shares (performance shares) of MorphoSys AG if predefined key performance criteria are achieved. These criteria are evaluated annually by the supervisory board. The grant date was April 1, 2015 and the vesting/performance period is four years. If the predefined key performance criteria for the respective period are fully met, 25% of the performance shares vest in each year of the four-year vesting period. The number of performance shares vested per year is calculated based on the key performance criteria of the absolute MorphoSys share price performance and the relative MorphoSys share price performance compared to the Nasdaq Biotechnology Index and the TecDAX Index. The number of performance shares vesting each year will be reduced or increased to the extent that the performance criteria of the respective year have been achieved
184
Table of Contents
between only 50% and 99.9% (<100%) or the achievement of the performance criteria has exceeded 100% (maximum 200%). If in one year the performance criteria are met by less than 50%, no performance shares will vest in that year. In any case, the maximum pay-out at the end of the four-year period is limited by a factor we determine, which generally amounts to 1. However, in justified cases, the supervisory board may set this factor in its discretion between 0 and 2, for example, if the level of payment is regarded as unreasonable in view of our general development. The right to receive a certain allocation of performance shares under the 2015 LTI Plan will only occur at the end of the four-year vesting period.
If the number of treasury shares is not sufficient for servicing the 2015 LTI Plan, we reserve the right to pay a certain amount of the 2015 LTI Plan in cash in the amount of the performance shares at the end of the vesting period, provided the cash amount does not exceed 200% of the fair value of the performance shares on the grant date.
If a member of the management board ceases to hold an office at our group because of termination (or if the management board member terminates the employment contract), resignation, death, injury, disability, by reaching retirement age (receipt of a normal retirement pension, early-retirement pension or disability pension, as long as the requirements for the disability pension entitlement are met) or under other circumstances subject to the supervisory board’s discretion, the management board member (or the member’s heirs) is entitled to performance shares determined on a precise daily pro rata basis.
If a member of the management board ceases to hold an office at our group for good reason as defined by Section 626 Para. 2 of the German Civil Code and/or as defined by Section 84 Para. 3 of the German Stock Corporation Act, the beneficiary will not be entitled to performance shares.
If a change of control occurs during the four-year vesting period, all performance shares will fully vest. In this case, the right to receive a certain allocation of performance shares under the 2015 LTI Plan will only occur at the end of the four-year vesting period.
2016 Long-Term Incentive Program
On April 1, 2016, we established a long-term incentive plan, which we refer to as the “2016 LTI Plan”, for members of the management board and the senior management group. The 2016 LTI Plan is a performance-related share plan and will be paid out in ordinary shares (performance shares) of MorphoSys AG if predefined key performance criteria are achieved. These criteria are evaluated annually by the supervisory board. The grant date was April 1, 2016 and the vesting/performance period is four years. If the predefined key performance criteria for the respective period are fully met, 25% of the performance shares vest in each year of the four-year vesting period. The number of performance shares vested per year is calculated based on the key performance criteria of the absolute MorphoSys share price performance and the relative MorphoSys share price performance compared to the Nasdaq Biotechnology Index and the TecDAX Index. The number of performance shares vesting each year will be reduced or increased to the extent that the performance criteria of the respective year have been achieved between only 50% and 99.9% (<100%) or the achievement of the performance criteria has exceeded 100% (maximum 200%). If in one year the performance criteria are met by less than 50%, no performance shares will vest in that year. In any case, the maximum pay-out at the end of the four-year period is limited by a factor we determine, which generally amounts to 1.
185
Table of Contents
However, in justified cases, the supervisory board may set this factor in its discretion between 0 and 2, for example, if the level of payment is regarded as unreasonable in view of our general development. The right to receive a certain allocation of performance shares under the 2016 LTI Plan will only occur at the end of the four-year vesting period.
There is a six-month exercise period following the four-year vesting period, during which the performance shares can be transferred to the beneficiaries. Beneficiaries are free to choose the exercise date within this exercise period.
If the number of treasury shares is not sufficient for servicing the 2016 LTI Plan, we reserve the right to pay a certain amount of the 2016 LTI Plan in cash in the amount of the performance shares at the end of the vesting period, provided the cash amount does not exceed 200% of the fair value of the performance shares on the grant date.
If a member of the management board ceases to hold an office at our group because of termination (or if the management board member terminates the employment contract), resignation, death, injury, disability, by reaching retirement age (receipt of a normal retirement pension, early-retirement pension or disability pension, as long as the requirements for the disability pension entitlement are met) or under other circumstances subject to the supervisory board’s discretion, the management board member (or the member’s heirs) is entitled to performance shares determined on a precise daily pro rata basis.
If a member of the management board ceases to hold an office at our group for good reason as defined by Section 626 Para. 2 of the German Civil Code and/or as defined by Section 84 Para. 3 of the German Stock Corporation Act, the beneficiary will not be entitled to performance shares.
If a change of control occurs during the four-year vesting period, all performance shares will fully vest. In this case, the right to receive a certain allocation of shares under the 2016 LTI Plan will only occur at the end of the four-year vesting period.
2017 Long-Term Incentive Program
On April 1, 2017, we established a long-term incentive plan, which we refer to as the “2017 LTI Plan”, for members of the management board and the senior management group. The 2017 LTI Plan is a performance-related share plan and will be paid out in ordinary shares (performance shares) of MorphoSys AG if predefined key performance criteria are achieved. These criteria are evaluated annually by the supervisory board. The grant date was April 1, 2017 and the vesting/performance period is four years. If the predefined key performance criteria for the respective period are fully met, 25% of the performance shares vest in each year of the four-year vesting period. The number of performance shares vested per year is calculated based on the key performance criteria of the absolute MorphoSys share price performance and the relative MorphoSys share price performance compared to the Nasdaq Biotechnology Index and the TecDAX Index. The number of performance shares vesting each year will be reduced or increased to the extent that the performance criteria of the respective year have been achieved between only 50% and 99.9% (<100%) or the achievement of the performance criteria has exceeded 100% (maximum 200%). If in one year the performance criteria are met by less than 50%, no performance shares will vest in that year. In any case, the maximum pay-out at the end of the four-year period is limited by a factor we determine, which generally amounts to 1. However, in justified cases, the supervisory board may set this factor in its discretion between
186
Table of Contents
0 and 2, for example, if the level of payment is regarded as unreasonable in view of our general development. The right to receive a certain allocation of performance shares under the 2017 LTI Plan will only occur at the end of the four-year vesting period.
There is a six-month exercise period following the four-year vesting period, during which the performance shares can be transferred to the beneficiaries. Beneficiaries are free to choose the exercise date within this exercise period.
If the number of treasury shares is not sufficient for servicing the 2017 LTI Plan, we reserve the right to pay a certain amount of the 2017 LTI Plan in cash in the amount of the performance shares at the end of the vesting period, provided the cash amount does not exceed 200% of the fair value of the performance shares on the grant date.
If a member of the management board ceases to hold an office at our group because of termination (or if the management board member terminates the employment contract), resignation, death, injury, disability, by reaching retirement age (receipt of a normal retirement pension, early-retirement pension or disability pension, as long as the requirements for the disability pension entitlement are met) or under other circumstances subject to the supervisory board’s discretion, the management board member (or the member’s heirs) is entitled to performance shares determined on a precise daily pro rata basis.
If a member of the management board ceases to hold an office at our group for good reason as defined by Section 626 Para. 2 of the German Civil Code and/or as defined by Section 84 Para. 3 of the German Stock Corporation Act, the beneficiary will not be entitled to performance shares.
If a change of control occurs during the four-year vesting period, all performance shares will fully vest. In this case, the right to receive a certain allocation of shares under the 2017 LTI Plan will only occur at the end of the four-year vesting period.
2017 Stock Option Plan
With effect as of April 1, 2017, we established a stock option plan, or the 2017 Stock Option Plan, for the management board, the senior management group and our employees who are not part of the senior management group. The program is considered a share-based compensation program with settlement in equity instruments in accordance with IFRS 2 and is accounted for accordingly. The grant date was April 1, 2017, and the vesting/performance period is four years. If the predefined performance criteria for the respective period are fully met, 25% of the stock options will vest in each year of the four-year vesting period. The number of stock options vested per year is calculated based on the key performance criteria of the absolute MorphoSys share price performance and the relative MorphoSys share price performance compared to the Nasdaq Biotechnology Index and the TecDAX Index. The performance criteria can be achieved annually up to a maximum of 200%. If less than 0% of the predefined performance criteria are achieved in one year, “0” shares will become vested (entitlement) for that year. The right to exercise a stock option, however, occurs only at the end of the four-year vesting/performance period.
We reserve the right to settle the exercise of stock options through newly created shares from “Conditional Capital 2016-III”, through the issue of own shares or by payment in cash. The exercise period is three years, following the end of the four-year vesting/performance period.
187
Table of Contents
The exercise price is, subject to certain adjustments, equal to the 30-day average closing price in Xetra trading on the Frankfurt Stock Exchange that ended on March 31, 2017.
If a member of the management board ceases to hold an office with us through termination (or the management board member terminates the employment contract), resignation, death, injury, disability or the attainment of retirement age (receipt of a standard retirement pension, early-retirement pension or disability pension, as long as the requirements for the disability pension entitlement are met) or under other circumstances subject to the supervisory board’s discretion, the management board member (or the member’s heirs) is entitled to a precise daily pro rata number of stock options.
If a member of the management board ceases to hold an office at our group for good reason as defined by Section 626 Para. 2 of the German Civil Code and/or as defined by Section 84 Para. 3 of the German Stock Corporation Act, all unexercised stock options will be forfeited without any entitlement to compensation.
If a change of control occurs during the four-year vesting period, all stock options will be considered fully vested and the performance criteria will be deemed to be achieved at a level of 100%. In this case, the right to exercise the stock options occurs only at the end of the four-year vesting period.
German Corporate Governance Code
The German Corporate Governance Code, or Corporate Governance Code, was originally published by the German Federal Ministry of Justice (Bundesministerium der Justiz) in 2002 and was most recently amended on February 7, 2017 and published in the German Federal Gazette (Bundesanzeiger) on April 24, 2017. The Corporate Governance Code contains recommendations (Empfehlungen) and suggestions (Anregungen) relating to the management and supervision of German companies that are listed on a stock exchange. It follows internationally and nationally recognized standards for good and responsible corporate governance. The purpose of the Corporate Governance Code is to make the German system of corporate governance transparent for investors. The Corporate Governance Code includes corporate governance recommendations and suggestions with respect to shareholders and shareholders’ meetings, the management and supervisory boards, transparency, accounting policies and auditing.
There is no obligation to comply with the recommendations or suggestions of the Corporate Governance Code. The German Stock Corporation Act requires only that the management board and supervisory board of a German listed company issue an annual declaration that either (i) states that the company has complied with the recommendations of the Corporate Governance Code or (ii) lists the recommendations that the company has not complied with and explains its reasons for deviating from the recommendations of the Corporate Governance Code (Entsprechenserklärung). In addition, a listed company is also required to state in this annual declaration whether it intends to comply with the recommendations or list the recommendations it does not plan to comply with in the future. These declarations have to be published permanently on the company’s website. If the company changes its policy on certain recommendations between such annual declarations, it must disclose this fact and explain its reasons for deviating from the recommendations. Non-compliance with suggestions contained in the Corporate Governance Code need not be disclosed.
188
Table of Contents
Following our listing on Nasdaq, the Corporate Governance Code will continue to apply to us and we will be required to issue the annual declarations described above.
Our management board and supervisory board will be obliged to comply with the Corporate Governance Code except for such provisions, which are explicitly listed in the annual declaration and for which they provide an explanation of non-compliance.
In particular, we will adhere to the following significant recommendations of the Corporate Governance Code: (i) the supervisory board will establish a compensation and nomination committee (Vergütungs-und Nominierungsausschuss) and an audit committee (Prüfungsausschuss); (ii) the management board must keep the supervisory board closely informed, in particular with respect to measures which can fundamentally affect our financial situation; and (iii) significant management measures are subject to supervisory board approval.
However, we currently deviate from one recommendation of the Corporate Governance Code and expect to deviate from certain recommendations and suggestions of the Corporate Governance Code in the future. All deviations from the Corporate Governance Code recommendations will be published in the official annual declarations and are available on our website: www.morphosys.com. The information and other content appearing on our website are not part of this prospectus.
Code of Business Conduct and Ethics
We have adopted a written code of business conduct and ethics, or code of conduct, which outlines the principles of legal and ethical business conduct under which we do business. The code of conduct applies to all of our management board members and employees. The full text of the code of conduct is available on our website at www.morphosys.com. The information and other content appearing on our website are not part of this prospectus.
In addition, we have implemented a compliance handbook which describes the compliance management system implemented at MorphoSys, which is designed to ensure compliance with all legal requirements, while at the same time implementing high ethical standards that are mandatory for both management and each employee. The overall responsibility for the compliance management system lies with the management board, which reports regularly to the Audit Committee. In the performance of its compliance responsibilities, the management board has delegated the corresponding tasks to various functions at MorphoSys.
Differences between Our Corporate Governance Practices and Those Set Forth in the Nasdaq Stock Market Rules
In general, Nasdaq Stock Market Rule 5615(a)(3) permits foreign private issuers such as us, to follow home country corporate governance practices instead of certain provisions of the Nasdaq Stock Market Rules without having to seek individual exemptions from Nasdaq. A foreign private issuer making its initial U.S. listing on Nasdaq and following home country corporate governance practices in lieu of the corresponding corporate governance provisions of the Nasdaq Stock Market Rules must disclose in its registration statement or on its website any significant ways in which its corporate governance practices differ from those followed by U.S. companies under the Nasdaq Stock Market Rules. In addition, we also may qualify for certain exemptions under the Nasdaq Stock Market Rules as a foreign private issuer that may affect our corporate governance practices.
189
Table of Contents
The significant differences between the corporate governance practices that we follow and those set forth in the Nasdaq Stock Market Rules are described below:
| · | Distribution of Annual and Interim Reports. Nasdaq Listing Rule 5250(d) requires that our annual and interim reports be distributed or made available to shareholders within a reasonable period of time following filing with the SEC. Consistent with applicable rules and regulations in Germany, we do not distribute annual and interim reports automatically to shareholders. Instead, our annual and interim reports are available to shareholders on our website and delivery of printed versions thereof can be requested online. Furthermore, our annual and semi-annual reports are also filed with the German Company Register (Unternehmensregister). |
| · | Code of Conduct. Nasdaq Listing Rule 5610 requires companies to adopt one or more codes of conduct applicable to all directors, officers and employees. Although there is no requirement under German law for a company to have a code of conduct, we nevertheless have one in place applying to our management board and employees but not to our supervisory board. |
| · | Proxy Solicitation. Nasdaq Listing Rule 5620(b) requires companies that are not a limited partnership to solicit proxies and provide proxy statements for all meetings of shareholders and to provide copies of such proxy solicitation to Nasdaq. Under German law, there is no requirement for companies to solicit proxies in connection with a meeting of shareholders. Shareholders have the right to exercise their voting rights in the shareholders’ meeting through proxies appointed by them in writing. The proxies appointed by us are obligated to vote only in accordance with the instructions of the represented shareholder. |
| · | Shareholder approval requirements. Nasdaq Listing Rule 5635 requires companies to obtain shareholder approval before undertaking any of the following transactions: |
| · | acquiring the stock or assets of another company, where such acquisition results in the issuance of 20% or more of our outstanding share capital or voting power; |
| · | entering into any change of control transaction; |
| · | establishing or materially amending any equity compensation arrangement; and |
| · | entering into any transaction other than a public offering involving the sale, issuance or potential issuance by us of shares (or securities convertible into or exercisable for shares) equal to 20% or more of our outstanding share capital or 20% or more of the voting power outstanding before the issuance for less than the greater of book or market value of the stock. |
Consistent with the German Stock Corporation Act (Aktiengesetz), approval by the shareholders’ meeting is generally required for the issuance of any shares as well as any securities granting the respective holder the right to acquire shares (including options and convertibles).
Share Ownership by Members of Supervisory Board and Management Board
Please see “Principal Shareholders”.
190
Table of Contents
Since January 1, 2015, there has not been, nor is there currently proposed, any material transaction or series of similar material transactions to which we were or are a party in which any of the members of our supervisory or management boards, executive officers, holders of more than 10% of any class of our voting securities, or any member of the immediate family of any of the foregoing persons, had or will have a direct or indirect material interest, other than the compensation and shareholding arrangements we describe in the “Management” and “Principal Shareholders” sections of this prospectus.
191
Table of Contents
The following table sets forth information, as of April 6, 2018, regarding the beneficial ownership of our ordinary shares: (i) prior to the consummation of this offering and (ii) as adjusted to reflect the sale of our ADSs in this offering, for:
| · | members of our supervisory board; |
| · | members of our management board; |
| · | members of our supervisory and management boards as a group; and |
| · | each person who has reported to us that such person beneficially owns 3% or more of our outstanding ordinary shares pursuant to applicable German law. |
Beneficial ownership is determined in accordance with the rules of the SEC, and the information is not necessarily indicative of beneficial ownership for any other purpose. Under such rules, beneficial ownership includes any shares over which the individual has sole or shared voting power or investment power as well as any shares that the individual has the right to acquire within 60 days of April 6, 2018, through the exercise of any option, warrant or other right. Except as otherwise indicated, and subject to applicable community property laws, the persons named in the table have sole voting and investment power with respect to all shares held by that person.
The percentage of shares beneficially owned before this offering is computed on the basis of 29,420,785 issued shares as of April 6, 2018. The percentage of shares beneficially owned on an adjusted basis after this offering is based on 31,495,785 shares to be issued after this offering after giving effect to completion of this offering assuming no exercise of the underwriters’ option to purchase additional ADSs, and 31,807,035 shares to be issued after this offering after giving effect to completion of this offering and assuming full exercise of the underwriters’ option to purchase additional ADSs from us. Shares that a person has the right to acquire within 60 days of April 6, 2018, are deemed issued for purposes of computing the percentage ownership of the person holding such rights, but are not deemed issued for purposes of computing the percentage ownership of any other person, except with respect to the percentage ownership of all members of our supervisory board and management board, as a group.
| Shareholder | Shares beneficially owned before this offering |
Shares beneficially owned after this offering |
||||||||||||||||||||||
| Assuming underwriters’ option to purchase additional ADSs is not exercised |
Assuming underwriters’ option to purchase additional ADSs is exercised in full |
|||||||||||||||||||||||
| Number | Percent | Number | Percent | Number | Percent | |||||||||||||||||||
| 3% Shareholders |
||||||||||||||||||||||||
| Baillie Gifford & Co(1) |
1,267,457 | 4.31 | 1,267,457 | 4.02 | 1,267,457 | 3.98 | ||||||||||||||||||
| Oppenheimer Global Opportunities Fund(2) |
1,484,241 | 5.04 | 1,484,241 | 4.71 | 1,484,241 | 4.67 | ||||||||||||||||||
| Templeton Investment Counsel, LLC(3) |
821,235 | 2.79 | 821,235 | 2.61 | 821,235 | 2.58 | ||||||||||||||||||
| Templeton Global Advisors Limited(4) |
808,190 | 2.75 | 808,190 | 2.57 | 808,190 | 2.54 | ||||||||||||||||||
| Templeton Funds Trust(5) |
906,960 | 3.08 | 906,960 | 2.88 | 906,960 | 2.85 | ||||||||||||||||||
192
Table of Contents
| Shareholder | Shares beneficially owned before this offering |
Shares beneficially owned after this offering |
||||||||||||||||||||||
| Assuming underwriters’ option to purchase additional ADSs is not exercised |
Assuming underwriters’ option to purchase additional ADSs is exercised in full |
|||||||||||||||||||||||
| Number | Percent | Number | Percent | Number | Percent | |||||||||||||||||||
| Schroder International Selection Fund(6) |
984,076 | 3.34 | 984,076 | 3.12 | 984,076 | 3.09 | ||||||||||||||||||
| Invesco Holding Company Limited(7) |
700,937 | 2.38 | 700,937 | 2.22 | 700,937 | 2.20 | ||||||||||||||||||
| Consonance Capital Master Account, L.P.(8) |
965,355 | 3.28 | 965,355 | 3.07 | 965,355 | 3.04 | ||||||||||||||||||
| Members of supervisory board and management board |
||||||||||||||||||||||||
| Dr. Simon Moroney |
572,095 | (9) | 1.94 | (11) | 572,095 | (9) | 1.82 | (11) | 572,095 | (9) | 1.80 | (11) | ||||||||||||
| Jens Holstein |
71,537 | (10) | * | (11) | 71,537 | (10) | * | (11) | 71,537 | (10) | * | (11) | ||||||||||||
| Dr. Malte Peters |
9,505 | * | 9,505 | * | 9,505 | * | ||||||||||||||||||
| Dr. Markus Enzelberger |
7,262 | * | 7,262 | * | 7,262 | * | ||||||||||||||||||
| Dr. Gerald Möller |
11,000 | * | 11,000 | * | 11,000 | * | ||||||||||||||||||
| Dr. Marc Cluzel |
500 | * | 500 | * | 500 | * | ||||||||||||||||||
| Krisja Vermeylen |
350 | * | 350 | * | 350 | * | ||||||||||||||||||
| Wendy Johnson |
500 | * | 500 | * | 500 | * | ||||||||||||||||||
| Klaus Kühn |
0 | — | 0 | — | 0 | — | ||||||||||||||||||
| Dr. Frank Morich |
1,000 | * | 1,000 | * | 1,000 | * | ||||||||||||||||||
| Members of supervisory board and management board (as a group) |
668,249 | 2.28 | (11) | 668,249 | 2.12 | (11) | 668,249 | 2.10 | (11) | |||||||||||||||
| * | Indicates beneficial ownership of less than 1% of the total outstanding shares. |
| (1) | The information is based solely on a notification provided by Baillie Gifford & Co. pursuant to the German Securities Trading Act (Wertpapierhandelsgesetz). MorphoSys issued a respective voting rights announcement on March 6, 2018. |
| (2) | The information is based solely on a notification provided by OppenheimerFunds, Inc. pursuant to the German Securities Trading Act (Wertpapierhandelsgesetz). MorphoSys issued a respective voting rights announcement on December 14, 2017. |
| (3) | The information is based solely on a notification provided by Templeton Investment Counsel, LLC pursuant to the German Securities Trading Act (Wertpapierhandelsgesetz). MorphoSys issued a respective voting rights announcement on May 6, 2015. |
| (4) | The information is based solely on a notification provided by Templeton Global Advisors Limited pursuant to the German Securities Trading Act (Wertpapierhandelsgesetz). MorphoSys issued a respective voting rights announcement on April 21, 2015. |
| (5) | The information is based solely on a notification provided by Templeton Funds Trust pursuant to the German Securities Trading Act (Wertpapierhandelsgesetz). MorphoSys issued a respective voting rights announcement on June 21, 2017. |
| (6) | The information is based solely on a notification provided by Schroders plc pursuant to the German Securities Trading Act (Wertpapierhandelsgesetz). MorphoSys issued a respective voting rights announcement on August 17, 2016. |
| (7) | The information is based solely on a notification provided jointly by Invesco Holding Company Limited, Invesco Management Group Inc., Invesco North American Holdings Inc., Invesco Group Services, Inc., IVZ, Inc., IVZ UK Limited pursuant to the German Securities Trading Act (Wertpapierhandelsgesetz). MorphoSys issued a respective voting rights announcement on May 22, 2015. |
| (8) | The information is based solely on a notification provided by Mr. Mitchell Blutt pursuant to the German Securities Trading Act (Wertpapierhandelsgesetz). MorphoSys issued a respective voting rights announcement on March 21, 2018, |
| (9) | Includes convertible bonds with a principal amount of €88,386 convertible into 88,386 ordinary shares. See “Management—Share-Based Incentive Plans—Convertible Bonds—2013 Program”. |
| (10) | Includes convertible bonds with a principal amount of €60,537 convertible into 60,537 ordinary shares. See “Management—Share-Based Incentive Plans—Convertible Bonds—2013 Program”. |
| (11) | Calculated as percentage of the outstanding share capital assuming exercise of all convertible bonds held by such person. |
193
Table of Contents
Our ordinary shares are issued only in bearer form. Accordingly, we cannot determine the identity of our shareholders or how many shares a particular shareholder owns and the number of ordinary shares directly held by persons with U.S. addresses.
None of our shareholders will have different voting rights from other shareholders after the closing of this offering. We are not aware of any arrangement that may, at a subsequent date, result in a change of control of our company.
194
Table of Contents
The following description is a summary of certain information relating to our share capital as well as certain provisions of our articles of association and the German Stock Corporation Act (Aktiengesetz). Unless stated otherwise, the description insofar as it relates to our articles of association is based on the amended version of our articles of association as of April 18, 2018, which was registered in the commercial register on April 18, 2018. This summary does not purport to be complete and speaks as of the date of this prospectus. Copies of the articles of association will be publicly available from the commercial register (Handelsregister) of the local court (Amtsgericht) in Munich, Germany, electronically at www.unternehmensregister.de and as an exhibit to the registration statement of which this prospectus forms a part.
Share Capital
As of the date of this prospectus, we have share capital registered in the commercial register in the amount of €31,495,785.00 which is divided into 31,495,785 ordinary bearer shares (Inhaberaktien). All shares are shares with no par value (Stückaktien ohne Nennbetrag) with a notional amount attributable to each ordinary share of €1.00.
Incorporation of the Company
MorphoSys AG was formed on March 3, 1998 and registered in the commercial register of the local court of Munich under number HRB121023 on June 30, 1998.
Form, Certification and Transferability of the Shares
The form and contents of our global share certificates, any dividend certificates, renewal certificates and interest coupons are determined by our management board with the approval of our supervisory board. A shareholder’s right to certificated shares is excluded, to the extent permitted by law and to the extent that certification is not required by the stock exchange on which the shares are admitted to trading. We are permitted to issue global share certificates that represent one or more shares.
All of our outstanding shares are no par-value bearer shares (auf den Inhaber lautende Stückaktien ohne Nennbetrag). Any resolution regarding a capital increase may determine the profit participation of the new shares resulting from such capital increase.
Our shares are freely transferable under German law, with the transfer of ownership governed by the rules of the relevant clearing system.
Our articles of association do not include any provisions that would have a direct effect of delaying, deferring or preventing a change of control. However, in the event of a hostile takeover, we could use our authorized capital to increase our share capital to issue new shares to an investor at a premium. See “—Authorized Capital”. An increase in the number of shares outstanding could have a negative effect on a party’s ability to carry out a hostile takeover.
General Information on Capital Measures
Pursuant to our articles of association, an increase of our share capital generally requires a resolution passed at our shareholders’ meeting with both a simple majority of the share capital
195
Table of Contents
represented at the relevant shareholders’ meeting and a simple majority of the votes cast. The shareholders at such meeting may authorize our management board to increase our share capital with the consent of our supervisory board within a period of five years by issuing shares for a certain total amount, which we refer to as authorized capital (genehmigtes Kapital) and is a concept under German law that enables us to issue shares without going through the process of obtaining another shareholders’ resolution. The aggregate nominal amount of the authorized capital created by the shareholders may not exceed one-half of the share capital existing at the time of registration of the authorized capital with the commercial register.
Furthermore, our shareholders may resolve to amend or create conditional capital (bedingtes Kapital). However, they may do so only to issue conversion or subscription rights to holders of convertible bonds, in preparation for a merger with another company or to issue subscription rights to employees and members of the management of our company or of an affiliated company by way of a consent or authorization resolution. According to German law, the aggregate nominal amount of the conditional capital created at the shareholders’ meeting may not exceed one-half of the share capital existing at the time of the shareholders’ meeting adopting such resolution. The aggregate nominal amount of the conditional capital created for the purpose of granting subscription rights to employees and members of the management of our company or of an affiliated company may not exceed 10% of the share capital existing at the time of the shareholders’ meeting adopting such resolution.
According to German law, any resolution pertaining to the creation of authorized or conditional capital requires the vote of at least three-quarters of the share capital represented at the relevant shareholders’ meeting and a simple majority of the votes cast. The shareholders may also resolve to increase the share capital from company resources by converting capital reserves and profit reserves into registered share capital. Pursuant to our articles of association, any resolution pertaining to an increase in share capital from company resources requires the vote of a simple majority of the share capital represented at the relevant shareholders’ meeting and a simple majority of the votes cast.
All shares issued by the Company are fully paid in (meaning that shareholders are not liable to the Company to pay in any further amount in relation to their existing shares). Any resolution relating to a reduction of our share capital requires the vote of at least three-quarters of the share capital represented at the relevant shareholders’ meeting as well as a simple majority of the votes cast according to German law.
Changes in Our Share Capital during the Last Three Fiscal Years
As of April 18, 2018, our share capital as registered with the commercial register amounted to €31,495,785.00. Since January 1, 2015, our share capital has changed as follows:
As of February 12, 2015 our share capital as registered with the commercial register amounted to €26,456,834.00. Our share capital changed to reflect the issuance of new shares based on a share participation program.
As of February 1, 2016 our share capital as registered with the commercial register amounted to €26,537,682.00. Our share capital changed to reflect the issuance of new shares based on a share participation program.
196
Table of Contents
As of November 17, 2016 our share capital as registered with the commercial register amounted to €29,159,770.00. Our share capital changed to reflect the issuance of new shares in a private placement.
As of January 4, 2018 our share capital as registered with the commercial register amounted to €29,420,785.00. Our share capital changed to reflect the issuance of new shares based on the exercise of convertible bonds by the beneficiaries in the financial year 2017. See “Management—Management Board—Share-Based Incentive Plans—Convertible Bond 2013 Program”.
As of April 18, 2018 our share capital as registered with the commercial register amounted to €31,495,785.00. Our share capital was increased to reflect the issuance of shares underlying the 8,300,000 ADSs offered hereby.
Authorized Capital
As of the date of this prospectus we have two authorized capitals, the so-called Authorized Capital 2017-I and Authorized Capital 2017-II.
Our Authorized Capital 2017-I as of the date of this prospectus amounted to €2,915,977.00 and was created by shareholders’ resolution on May 17, 2017. The Authorized Capital 2017-I can be summarized as follows:
The management board, with the consent of the supervisory board, is authorized, until and including April 30, 2022, to increase the registered share capital of MorphoSys AG by up to €2,915,977.00 by issuing up to 2,915,977 new ordinary bearer shares with no par value of MorphoSys AG, against contribution in cash. Our shareholders are principally entitled to subscription rights. The shares may also be subscribed to by one or several credit institutions with the obligation to offer the shares to shareholders for subscription. With the supervisory board’s consent, the management board is, however, until and including April 30, 2022, authorized to exclude the subscription rights of shareholders in the following cases:
| · | to the extent such exclusion is necessary to avoid fractional shares; or |
| · | if the issue price of the new shares is not significantly below the market price of shares of the same class already listed and the total number of shares issued pursuant to or based on the analogous application of Section 186 Para. 3, sentence 4 German Stock Corporation Act in return for cash contributions and for which the shareholders’ subscription right is excluded while the authorization is in effect does not exceed 10% of the share capital, specifically neither on the effective date of this authorization nor at the time this authorization is exercised. |
The total number of shares to be issued via capital increases against contribution in cash, excluding subscription rights and based on the authorizations mentioned above shall not exceed 20% of the share capital when calculated based on the authorizations’ effective date or exercise, whichever amount is lower. This 20% limit mentioned above shall take into account (i) treasury shares sold with the exclusion of subscription rights after the effective date of these authorizations (unless they service the entitlements of members of the management board and/or employees under employee participation programs); (ii) shares to be issued with the exclusion of subscription rights during the effective period of these authorizations from other authorized capital existing on the effective date of these authorizations or to be resolved by the same
197
Table of Contents
shareholders’ meeting resolving these authorizations; and (iii) shares to be issued during the effective period of these authorizations to service bonds with conversion or warrant rights, whose authorization basis exists on the effective date of these authorizations, to the extent the bonds with conversion or warrant rights were issued with the exclusion of shareholders’ subscription rights (unless they service the entitlements of members of the management board and/or employees under employee participation programs).
Our Authorized Capital 2017-II as of the date of this prospectus amounted to €9,588,908.00 and was created by shareholders’ resolution on May 17, 2017. The Authorized Capital 2017-II can be summarized as follows: the management board, with the consent of the supervisory board, is authorized, until and including April 30, 2022, to increase the registered share capital of MorphoSys AG by up to €9,588,908.00 by issuing up to 9,588,908 new ordinary bearer shares with no par value of MorphoSys AG. Our shareholders are principally entitled to subscription rights. The shares may also be subscribed to by one or several credit institutions with the obligation to offer the shares to shareholders for subscription. With the supervisory board’s consent, the management board is, however, until and including the date of April 30, 2022 authorized to exclude the subscription rights of shareholders in the following cases:
| · | in the case of a capital increase against contribution in cash, to the extent such exclusion is necessary to avoid fractional shares; |
| · | in the case of a capital increase against contribution in kind; or |
| · | in the case of a capital increase against contribution in cash to the extent the new shares shall be placed on a foreign stock exchange in the context of a new listing. |
The total number of shares to be issued via capital increases against contribution in cash and/or in kind, excluding subscription rights and based on the authorizations mentioned above, shall not exceed 20% of the share capital when calculated based on the authorizations’ effective date or exercise, whichever amount is lower. This 20% limit mentioned above shall take into account (i) treasury shares sold with the exclusion of subscription rights after the effective date of these authorizations (unless they service the entitlements of members of the management board and/or employees under employee participation programs); (ii) shares that are issued excluding subscription rights during the effective period of these authorizations from other authorized capital existing on the effective date of these authorizations or to be resolved by the same shareholders’ meeting resolving these authorizations; and (iii) shares to be issued during the effective period of these authorizations to service bonds with conversion or warrant rights, whose authorization basis exists on the effective date of these authorizations, to the extend the bonds with conversion or warrant rights were issued with the exclusion of shareholders´ subscription rights (unless they service the entitlements of members of the management board and/or employees under employee participation programs).
Conditional Capital
MorphoSys AG’s share capital is increased conditionally by up to €5,307,536.00 through the issue of up to 5,307,536 new ordinary bearer shares with no par value of MorphoSys AG, or Conditional Capital 2016-I. The Conditional Capital 2016-I may solely be used as a means to grant new shares to the holders of conversion or warrant rights that will be issued by MorphoSys AG or companies in which MorphoSys AG has a direct or indirect majority interest according to the authorizing resolution of the shareholders’ meeting on June 2, 2016 under
198
Table of Contents
agenda item 7 a). The issue of the shares will be carried out at the respective conversion or exercise price to be determined in accordance with the resolution above. The conditional capital increase will only be carried out to the extent that the holders of conversion or warrant rights exercise their rights or fulfill conversion obligations under such bonds. The shares will be entitled to dividends as of the beginning of the previous financial year if they were issued before the start of the shareholders’ meeting or otherwise as of the beginning of the financial year in which they were issued.
MorphoSys AG’s share capital is conditionally increased by up to €188,985.00 through the issuance of up to 188,985 new ordinary bearer shares with no par value shares of MorphoSys AG, or Conditional Capital 2008-III. The Conditional Capital 2008-III may solely be used to the extent the holders of convertible bonds, exercise their conversion rights in exchange for ordinary shares of MorphoSys AG. The new shares participate in earnings from the beginning of the fiscal year in which they are issued by virtue of the exercise of conversion rights. The management board is authorized, with the consent of the supervisory board, to establish additional details regarding the conditional capital increase and its execution.
MorphoSys AG ‘s share capital is increased conditionally by up to €995,162.00 through the issuance of up to 995,162 new ordinary bearer shares with no par value shares of MorphoSys AG, or Conditional Capital 2016-III. The Conditional Capital 2016-III may solely be used to meet the obligations of subscription rights that have been issued and exercised based on the authorization resolved by the shareholders’ meeting of June 2, 2016 under agenda item 9 letter a). Any such conditional capital increase will be executed only to the extent that holders of subscription rights exercise their right to subscribe to shares of MorphoSys AG. The shares will be issued at the exercise price set in each case as the issue price in accordance with agenda item 9 letter a) subparagraph (8) of the resolution of the shareholders’ meeting dated June 2, 2016; Section 9 Para. 1 of the German Stock Corporation Act remains unaffected. The new shares are entitled to a dividend for the financial year for which no resolution of a shareholders’ meeting has yet been made on the appropriation of profits at the time of the shares’ issue. The management board, and the supervisory board where members of the management board are concerned, is authorized to determine the additional details of the conditional capital increase and its execution.
Subscription Rights
According to the German Stock Corporation Act, every shareholder is generally entitled to subscription rights (commonly known as pre-emptive rights) to any new shares issued within the framework of a capital increase, including convertible bonds, bonds with warrants, profit-sharing rights or income bonds in proportion to the number of shares the respective shareholder holds in the corporation’s existing share capital. Under German law, these rights do not apply to shares issued out of conditional capital. A minimum subscription period of two weeks must be provided for the exercise of such subscription rights.
Under German law, the shareholders’ meeting may pass a resolution excluding subscription rights if at least three-quarters of the share capital represented adopts the resolution. To exclude subscription rights, the management board must also make a report available to the shareholders justifying the exclusion and demonstrating that the company’s interest in excluding the subscription rights outweighs the shareholders’ interest in having them. In addition to approval by the general shareholders’ meeting, the exclusion of subscription rights
199
Table of Contents
requires a justification. The justification must be based on the principle that our interest in excluding subscription rights outweighs the shareholders’ interest in their subscription rights and may be subject to judicial review. Accordingly, under German law, the exclusion of subscription rights upon the issuance of new shares is permitted, in particular, if we increase the share capital against cash contributions, if the amount of the capital increase does not exceed 10% of the existing share capital and the issue price of the new shares is not significantly lower than the market price of our shares (for this purpose, the market price may also be considered the market price of an ADS listed on Nasdaq divided by the number of our shares or the fraction of one of our shares represented by an ADS, as the case may be).
The authorization of the management board to issue convertible bonds or other securities convertible into shares must be limited to a period not exceeding five years as of the respective shareholder resolution.
Shareholders’ Meetings, Resolutions and Voting Rights
Pursuant to our articles of association, shareholders’ meetings may be held at our registered offices or at the registered seat of a German stock exchange. In general, shareholders’ meetings are convened by our management board. The supervisory board is additionally required to convene a shareholders’ meeting in cases where this is required under binding statutory law (i.e., if this is in the best interest of our company). In addition, shareholders who, individually or as a group, own at least 5% of our share capital may request that our management board convenes a shareholders’ meeting. If our management board does not convene a shareholders’ meeting upon such a request, the shareholders may petition the competent German court for authorization to convene a shareholders’ meeting.
Pursuant to our articles of association, the convening notice for a shareholders’ meeting must be made public at least 36 days prior to the meeting. Shareholders who, individually or as a group, own at least 5% or €500,000 of our share capital may require that modified or additional items be added to the agenda of the shareholders’ meeting. For each new item, an explanation of the requested change must be provided or a voting proposal (Beschlussvorlage). Any request for an amendment of the agenda of the shareholders’ meeting must be received by the Company within 30 days prior to the meeting. The Company must publish any requests for the amendment of the agenda of the shareholders’ meeting immediately. Under German law, our annual general shareholders’ meeting must take place within the first eight months of each fiscal year. Among other things, the general shareholders’ meeting is required to decide on the following issues:
| · | appropriation and use of annual net income; |
| · | discharge or ratification of the actions taken by the members of our management board and our supervisory board; |
| · | the approval of our statutory auditors; |
| · | increases or decreases in our share capital; |
| · | the election of supervisory board members; and |
| · | to the extent legally required, the approval of our financial statements. |
Each ordinary share grants one vote in a shareholders’ meeting. Voting rights may be exercised by authorized proxies, which may be appointed by the Company (Stimmrechtsvertreter). The
200
Table of Contents
granting of a power of attorney must be made in text form. Generally, the shareholder or an authorized proxy must be present at the shareholders’ meeting to cast a vote. However, under the Company’s articles of association, the management board may determine in the invitation to the shareholders’ meeting that shareholders may submit their votes in writing or by means of electronic communication without attending the shareholders’ meeting in person.
Our articles of association provide in Article 20 that the resolutions of the shareholders’ meeting are adopted by a simple majority of the votes cast. To the extent required by law, certain resolutions may have to be approved by a simple majority of share capital represented at the meeting, in addition to the majority of votes cast.
Neither German law nor our articles of association provide for a minimum participation for a quorum for our shareholders’ meetings.
Under German law, certain resolutions of fundamental importance require the vote of at least three-quarters of the share capital present or represented in the voting at the time of adoption of the resolution. Resolutions of fundamental importance include, in particular, capital increases with exclusion of subscription rights, capital decreases, the creation of authorized or conditional share capital, the dissolution of a company, a merger into or with another company, split-offs and split-ups, the conclusion of inter-company agreements (Unternehmensverträge) as defined in the German Stock Corporation Act (in particular domination agreements (Beherrschungsverträge) and profit and loss transfer agreements (Ergebnisabführungsverträge)), and a change of the legal form of a company.
Dividend Rights
Under German law, distributions of dividends on shares for a given fiscal year are generally determined by a process in which the management board and supervisory board submit a proposal to our annual general shareholders’ meeting held in the subsequent fiscal year and such annual general shareholders’ meeting adopts a resolution.
German law provides that a resolution concerning dividends and distribution thereof may be adopted only if the company’s unconsolidated financial statements prepared in accordance with German law show net retained profits. In determining the profit available for distribution, the result for the relevant year must be adjusted for profits and losses brought forward from the previous year and for withdrawals from or transfers to reserves. Certain reserves are required by law and must be deducted when calculating the profit available for distribution.
Shareholders participate in profit distributions in proportion to the number of shares they hold. Dividends on shares resolved by the general shareholders’ meeting are paid annually, shortly after the general shareholders’ meeting, in compliance with the rules of the respective clearing system. Dividend payment claims are subject to a three-year statute of limitation in the company’s favor.
Liquidation Rights
Apart from liquidation as a result of insolvency proceedings, we may be liquidated only with a vote of the holders of at least three-quarters of the share capital represented at the shareholders’ meeting at which such a vote is taken. If we are liquidated, any assets remaining after all of our liabilities have been paid off would be distributed among our shareholders in
201
Table of Contents
proportion to their holdings in accordance with German statutory law. The German Stock Corporation Act provides certain protections for creditors which must be observed in the event of liquidation.
Authorization to Acquire Our Own Shares
We may not acquire our own shares unless authorized by the shareholders’ meeting or in other very limited circumstances as set out in the German Stock Corporation Act. Shareholders may not grant a share repurchase authorization lasting for more than five years. The German Stock Corporation Act generally limits repurchases to 10% of our share capital and resales must generally be made either on a stock exchange, in a manner that treats all shareholders equally, or in accordance with the rules that apply to subscription rights relating to a capital increase.
The shareholders’ meeting adopted a resolution on May 23, 2014 authorizing the management board, for a period until April 30, 2019, subject to the consent of the supervisory board and provided it complies with the legal requirement of equal treatment, to purchase our shares in an amount up to 10% of our total share capital existing on May 23, 2014 or—if the relevant amount is lower—the total share capital existing at the time the authorization is exercised. At the discretion of the management board, such purchase may be effected on the stock market or by means of a public offer or a public solicitation to submit sales offers.
The management board is authorized to generally use treasury shares for all legally permissible purposes.
Squeeze-Out of Minority Shareholders
Under German law, the shareholders’ meeting of a stock corporation may resolve upon request of a shareholder that holds at least 95% of the share capital that the shares held by any remaining minority shareholders be transferred to this shareholder against payment of “adequate cash compensation” (Ausschluss von Minderheitsaktionären). This amount must take into account the full value of the company at the time of the resolution, which is generally determined using the future earnings value method (Ertragswertmethode).
A squeeze-out in the context of a merger (umwandlungsrechtlicher Squeeze-Out) only requires a majority shareholder to hold at least 90% of the share capital. A squeeze-out following a successful public take-over offer (übernahmerechtlicher Squeeze-Out) requires a majority shareholder to hold at least 95% of the share capital, but has a simplified process.
Disclosure Requirements for Shareholdings and Mandatory Offer
The German Securities Trading Act (Wertpapierhandelsgesetz) requires every shareholder whose equity participation in a company with a registered seat in Germany, and that is listed for trading on an organized market in a member state of the European Union or a country that is a party to the Treaty on the European Economic Area, reaches, exceeds, or falls below thresholds of 3%, 5%, 10%, 25%, 30%, 50%, or 75% of the voting rights of such company to inform the company and the German Federal Financial Supervisory Authority (Bundesanstalt für Finanzdienstleistungsaufsicht, or “BaFin”) without undue delay and, in any case, no later than four trading days after reaching, exceeding or falling below these thresholds, using a standardized form. In the context of this requirement, the German Securities Trading Act and other regulations contains various rules that are meant to ensure that share ownership is
202
Table of Contents
attributed to the person that actually controls the voting rights pertaining to such shares. As long as a shareholder fails to make such notification, such shareholder may generally not exercise any rights pertaining to these shares (including voting rights and dividend rights). Upon receipt of any such shareholder notification, the German company is required to immediately publish the notification by a so-called European media bundle.
In addition, the European Market Abuse Regulation requires, inter alia, the members of the management board and the supervisory board, their spouses and close relatives, who purchase or sell shares, or other types of securities representing the right to acquire shares, including convertible bonds and bonds with warrants attached, issued by a company whose shares have been admitted to trading on a German stock exchange in excess of a de minimis number, to immediately notify the issuer and the BaFin of such purchases or sales. Upon receipt of such notice, the issuer is required to publish this notification by, among other things, posting it on its website.
Pursuant to the German Securities Acquisition and Takeover Act (Wertpapiererwerbs- und Übernahmegesetz), every person gaining control over a listed company, that is whose shares of voting rights reach or exceed 30% of the voting rights in such company, is obliged to publish this fact, including the percentage of its voting rights, immediately but within seven calendar days latest by (i) publication on the internet and (ii) though electronic media for disseminating financial information. Furthermore, this person has to submit a mandatory public tender offer to all shareholders of the company unless an exemption from this obligation has been granted by the BaFin. If the respective shareholder fails to publish the mandatory notice, this shareholder is obliged to pay interests for the consideration owed to the other shareholders for the duration of the delinquency. In addition, the respective shareholder has to submit an offer document for a public takeover bid to the BaFin within four weeks after the publishing of gaining control which is also to be published (i) on the internet and (ii) as an announcement in the German Federal Gazette.
Objective of Our Company
Our business purpose, as described in Article 2 of our articles of association, is to identify, explore, optimize, develop, apply, commercialize, and sell technologies, processes and products in the field of medicine, pharmaceutical compounds and related intermediate products, as well as to provide related services. We may engage in all measures that relate to or appear, directly or indirectly, conducive to achieving the object of our company. In particular, we may establish, acquire or take participating interests in other companies.
Registration of the Company with Commercial Register
We are a German stock corporation that is organized under the laws of Germany. On June 30, 1998, our company was registered in the commercial register (Handelsregister) of the local court (Amtsgericht) in Munich, Germany under the number HRB 121023.
Differences in Corporate Law
The applicable provisions of the German Stock Corporation Act differ from laws applicable to U.S. corporations and their shareholders. Set forth below is a summary of certain differences
203
Table of Contents
between the provisions of the German Stock Corporation Act applicable to us and the Delaware General Corporation Law relating to shareholders’ rights and protections.
| Germany | Delaware | |||
| Board System |
Under German law, a stock corporation has a two-tier board structure composed of the management board (Vorstand) and the supervisory board (Aufsichtsrat).
The management board is responsible for running the company’s affairs and representing the company in dealings with third parties.
The supervisory board of a German stock corporation has a control and supervisory function. The supervisory board does not actively manage the company but certain management board actions require the approval of the supervisory board. |
Under Delaware law, a corporation has a unitary board structure, and it is the responsibility of the board of directors to appoint and oversee the management of the corporation on behalf of and in the best interests of the shareholders of the corporation.
Management is responsible for running the corporation and overseeing its day-to-day operations. | ||
| Number of Board Members / Directors |
Under German law, a stock corporation must have at least one member on its management board and the number of members shall be determined by or in the manner provided in the company’s articles of association. | Under Delaware law, a corporation must have at least one director and the number of directors shall be fixed by or in the manner provided in the bylaws. | ||
| A stock corporation must have at least three but no more than 21 supervisory board members, whereby the number of supervisory board members must be divisible by three if this is necessary for the fulfilment of co-determination requirements. The articles of association of the company | ||||
204
Table of Contents
| Germany | Delaware | |||
| must specify if the supervisory board has more than three members. | ||||
| Supervisory board members are either appointed by the shareholders’ meeting or delegated by one or more individual shareholders if so provided for in the company’s articles of association. | ||||
| Depending on the number of employees of the company, the supervisory board may be required to include employee representatives subject to the provisions of the German One-Third Employee Representation Act (Drittelbeteiligungsgesetz), which applies to companies that have at least 500 employees, or the German Codetermination Act (Mitbestimmungsgesetz), which applies to companies that have at least 2,000 employees. Such rules result in different appointment rules for supervisory board members, i.e. in companies which are subject to the German One-Third Employee Representation Act two-thirds of supervisory board members are representatives of the shareholders, while one-third are representatives of the employees. In companies which are subject to the German Codetermination Act the rule of parity applies, i.e., half of the supervisory | ||||
205
Table of Contents
| Germany | Delaware | |||
| board members are representatives of the shareholders and the other half are representatives of the employees. In cases of dead lock the chairman has a casting vote. The employee representatives in the supervisory board are elected by the employees following certain procedures depending on the number of employees. | ||||
| Additionally, 30% of the supervisory board members must be women in case the company is a fully co-determined (voll mitbestimmungspflichtige) company, which requires that the company has at least 2,000 employees. | ||||
| Appointment and Removal of Board Members / Directors |
Members of the management board of a German stock corporation are appointed by the supervisory board for a maximum period of five years with an opportunity to be reelected. The supervisory board may remove a member of the management board prior to the expiration of his or her term only for good cause, such as gross breach of duties (grobe Pflichtverletzung), the inability to manage the business properly (Unfähigkeit zur ordnungsgemäßen Pflichtausübung) or a vote of no-confidence during the shareholders’ meeting (Vertrauensentzug). The | Under Delaware law, any director or the entire board of directors may be removed, with or without cause, by the holders of a majority of the shares then entitled to vote at an election of directors, except (a) unless the certificate of incorporation provides otherwise, in the case of a corporation whose board of directors is classified, shareholders may effect such removal only for cause, or (b) in the case of a corporation having cumulative voting, if less than the entire board of directors is to be removed, no director may be removed without cause if the votes cast against his or | ||
206
Table of Contents
| Germany | Delaware | |||
| shareholders themselves are not entitled to appoint or dismiss the members of the management board.
Under German law, a member of a supervisory board may be elected for a term of up to approximately five years (except for the first supervisory board of a newly incorporated company which may only be elected for a term of approximately one year), depending on the date of the annual shareholders’ meeting at which such member is elected, which is the standard term of office. Reelection, including repeated reelection, is permissible. Prior to the expiration of his or her term, supervisory board members which have been appointed by the shareholders’ meeting may be removed by a resolution of the shareholders’ meeting requiring a three-quarter majority of the votes cast, unless otherwise provided by the company’s articles of association. Supervisory board members who are delegated by a shareholder or the company’s employees may be revoked and the resulting vacancy filled at the sole discretion of such shareholder or the employees. |
her removal would be sufficient to elect him or her if then cumulatively voted at an election of the entire board of directors, or, if there are classes of directors, at an election of the class of directors of which he or she is a part. |
207
Table of Contents
| Germany | Delaware | |||
| Vacancies on the Boards |
Under German law, vacant positions on the management board are filled by the supervisory board in accordance with the general rules of appointment, which provide that vacancies are filled by the simple majority of supervisory board votes cast, unless otherwise provided by the company’s articles of association. In case of emergencies, a vacant position on the management board may be filled by an individual appointed by the court. | Under Delaware law, vacancies and newly created directorships may be filled by a majority of the directors then in office (even though less than a quorum) or by a sole remaining director unless (a) otherwise provided in the certificate of incorporation or bylaws of the corporation or (b) the certificate of incorporation directs that a particular class of stock is to elect such director, in which case a majority of the other directors elected by such class, or a sole remaining director elected by such class, will fill such vacancy. | ||
| Vacant positions on the supervisory board are filled in accordance with the general rules of appointment. If the number of supervisory board members falls below three, the statutory minimum required, the vacant position on the supervisory board may be filled by an individual appointed by the court. | ||||
| Annual Shareholders’ Meeting |
Under German law, a stock corporation must hold an annual shareholders’ meeting within eight months of the end of its fiscal year. The annual shareholders’ meeting must be held in Germany at a location determined by the articles of association. If the articles of association do not provide for a specific location, the shareholders’ meeting shall be held at the company’s | Under Delaware law, the annual meeting of shareholders shall be held at such place, on such date and at such time as may be designated from time to time by the board of directors or as provided in the certificate of incorporation or by the bylaws. | ||
208
Table of Contents
| Germany | Delaware | |||
| seat or, if applicable, at the venue where its shares are listed. | ||||
| Calling of Shareholders’ Meetings |
Under German law, extraordinary shareholders’ meetings, in addition to the annual shareholders’ meetings, may be called by either the management board, or by the supervisory board if it is in the best interest of the company. Shareholders holding at least 5% of the company’s share capital are entitled to request that the management board convenes an extraordinary shareholders’ meeting and may also address their request to the court, which then may authorize the requesting minority shareholders to convene a special meeting by themselves. | Under Delaware law, special meetings of the shareholders may be called by the board of directors or by such person or persons as may be authorized by the certificate of incorporation or by the bylaws. | ||
| Notice of Shareholders’ Meetings |
Under German law, unless a longer period is otherwise provided for in the articles of association, the shareholders must be given at least 30 days’ advance notice of the shareholders’ meeting. Such notices must at least specify the name of the company, the statutory seat of the company, as well as the location, date and time of the shareholders’ meeting. In addition, the invitation must contain the agenda items as well as the management board’s and the supervisory | Under Delaware law, unless otherwise provided in the certificate of incorporation or bylaws, written notice of any meeting of the shareholders must be given to each shareholder entitled to vote at the meeting not less than ten nor more than 60 days before the date of the meeting and shall specify the place, date, hour and purpose or purposes of the meeting. | ||
209
Table of Contents
| Germany | Delaware | |||
| board’s voting proposal for each agenda item. | ||||
| If all shareholders entitled to attend the shareholders’ meeting are present or represented and provide their consent thereto, the formalities of calling and holding of a shareholders’ meeting can be waived. | ||||
| Proxy Voting | Under German law, a shareholder may designate another person to attend, speak and vote at a shareholders’ meeting of the company on such shareholder’s behalf by proxy. | Under Delaware law, at any meeting of shareholders, a shareholder may designate another person to act for such shareholder by proxy, but no such proxy shall be voted or acted upon after three years from its date, unless the proxy provides for a longer period. A director of a Delaware corporation may not issue a proxy representing the director’s voting rights as a director. | ||
| With respect to management board meetings, a management board member may issue a proxy to another management board member representing the member’s voting rights as a management board member. | ||||
| With respect to supervisory board meetings, a supervisory board member may participate in voting by issuing a written vote to another supervisory board member or third party entitled to attend the supervisory board meeting. | ||||
| Preemptive / Subscription Rights | Under German law, existing shareholders have a statutory subscription right for any additional issue of shares or any security convertible into shares pro rata to the nominal value of their respective holdings in the | Under Delaware law, shareholders have no preemptive rights to subscribe to additional issuances of stock or to any security convertible into such stock unless, and except to the extent that | ||
210
Table of Contents
| Germany | Delaware | |||
| company, unless (i) shareholders representing three-quarters of the registered share capital present at the shareholders’ meeting have resolved upon the whole or partial exclusion of the subscription right and (ii) there exists good and objective cause for such exclusion. No separate resolution on the exclusion of subscription rights is required if all shareholders waive their statutory subscription rights. | such rights are expressly provided for in the certificate of incorporation. | |||
| Authority to Allot | Under German law, the management board may not allot shares, grant rights to subscribe for or to convert any security into shares unless a shareholder resolution to that effect has been passed at the company’s shareholders’ meeting granting the management board with such authority—subject to the approval of the supervisory board—, in each case in accordance with the provisions of the German Stock Corporation Act. | Under Delaware law, if the corporation’s certificate of incorporation so provides, the board of directors has the power to authorize the issuance of stock. It may authorize capital stock to be issued for consideration consisting of cash, any tangible or intangible property or any benefit to the corporation or any combination thereof. It may determine the amount of such consideration by approving a formula. In the absence of actual fraud in the transaction, the judgment of the directors as to the value of such consideration is conclusive. | ||
| Voting Rights | Under German law, each share, except for statutory non-voting preferred shares (nicht stimmberechtigte Vorzugsaktien), entitles its holder to vote at the shareholders’ meeting and to | Delaware law provides that, unless otherwise provided in the certificate of incorporation, each shareholder is entitled to one vote for each share of | ||
211
Table of Contents
| Germany | Delaware | |||
| participate with such number of votes with respect to one share which correspond to the quota of such share in the company’s share capital. While German law does not provide for a minimum attendance quorum for shareholders’ meetings, the company’s articles of association may so provide. In general, resolutions adopted at a shareholders’ meeting may be passed by a simple majority of votes cast, unless a higher majority is required by law or under the company’s articles of association. | capital stock held by such shareholder. | |||
| Shareholder Vote on Certain Transactions |
Under German law, certain shareholders’ resolutions of fundamental importance require the vote of at least three-quarters of the share capital present or represented in the voting at the time of adoption of the resolution. Resolutions of fundamental importance include, in particular, capital increases with exclusion of subscription rights, capital decreases, the creation of authorized or conditional share capital, the dissolution of a company, a merger into or with another company, split-offs and split-ups, the conclusion of inter-company agreements (Unternehmensverträge), in particular domination agreements (Beherrschungsverträge) and profit and loss transfer | Generally, under Delaware law, unless the certificate of incorporation provides for the vote of a larger portion of the stock, completion of a merger, consolidation, sale, lease or exchange of all or substantially all of a corporation’s assets or dissolution requires:
· the approval of the board of directors; and
· approval by the vote of the holders of a majority of the outstanding stock or, if the certificate of incorporation provides for more or less than one vote per share, a majority of the votes of the outstanding stock of a corporation entitled to vote on the matter. | ||
212
Table of Contents
| Germany | Delaware | |||
| agreements (Ergebnisabführungsverträge), and a change of the legal form of a company. | ||||
| Liability of Directors and Officers |
Under German law, any provision, whether contained in the company’s articles of association or any contract or otherwise, that purports to exempt a management or supervisory board member from any liability that would otherwise attach to such board member in connection with any negligence, default, breach of duty or breach of trust in relation to the company is void. | Under Delaware law, a corporation’s certificate of incorporation may include a provision eliminating or limiting the personal liability of a director to the corporation and its shareholders for damages arising from a breach of fiduciary duty as a director. However, no provision can limit the liability of a director for:
| ||
|
Under German law, members of both the management board and members of the supervisory board are liable to the company, and in certain cases to third parties or shareholders, for any damage caused to them due to a breach of such member’s duty of care. Apart from insolvency or special circumstances, only the company has the right to claim damages from members of either board. The company may waive claims for damages against a negligent management or supervisory board member only after the expiry of three years. |
· any breach of the director’s duty of loyalty to the corporation or its shareholders; · acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law; · intentional or negligent payment of unlawful dividends or stock purchases or redemptions; or · any transaction from which the director derives an improper personal benefit. | |||
| Standard of Conduct for Directors and Officers |
Under German law, both management and supervisory board members must conduct their affairs with “the care and diligence of a prudent | Delaware law does not contain specific provisions setting forth the standard of conduct of a director. The scope of the fiduciary | ||
213
Table of Contents
| Germany | Delaware | |||
| business man” and act in the best interest of the company. The scope of the fiduciary duties of management and supervisory board members is generally determined by German legislation and by the German courts.
Statutory and fiduciary duties of members of the management board to the company include, among others:
· to act in accordance with the law, the company’s articles of association and the rules of procedure for the management board, if any;
· to report to the supervisory board on a regular basis as well as on certain important occasions;
· to exercise reasonable care, skill and diligence;
· to maintain a proper accounting system;
· to not compete, directly or indirectly, with the company without permission by the supervisory board; and
· to secure that no further transactions are made in case of insolvency.
Members of the supervisory board owe substantially the same statutory and fiduciary duties to the company as members of the management |
duties of directors is generally determined by the courts of the State of Delaware. In general, directors have a duty to act without self-interest, on a well-informed basis and in a manner they reasonably believe to be in the best interest of the shareholders.
Directors of a Delaware corporation owe fiduciary duties of care and loyalty to the corporation and to its shareholders. The duty of care generally requires that a director act in good faith, with the care that an ordinarily prudent person would exercise under similar circumstances. Under this duty, a director must inform himself or herself of all material information reasonably available regarding a significant transaction. The duty of loyalty requires that a director act in a manner he or she reasonably believes to be in the best interests of the corporation. He or she must not use his corporate position for personal gain or advantage. In general, but subject to certain exceptions, actions of a director are presumed to have been made on an informed basis, in good faith and in the honest belief that the action taken was in the best interest of |
214
Table of Contents
| Germany | Delaware | |||
| board. Additionally, their duties include:
· to effectively supervise the company’s affairs and the management board;
· to evaluate and issue a resolution on certain transactions which can only be conducted by the management board after approval of the supervisory board;
· to approve the company’s financial statements;
· to appoint the management board members and to represent the company in transactions between the company and members of the management board; and
|
the corporation. However, this presumption may be rebutted by evidence of a breach of one of the fiduciary duties. Delaware courts have also imposed a heightened standard of conduct upon directors of a Delaware corporation who take any action designed to defeat a threatened change in control of the corporation.
In addition, under Delaware law, when the board of directors of a Delaware corporation approves the sale or break-up of a corporation, the board of directors may, in certain circumstances, have a duty to obtain the highest value reasonably available to the shareholders. | |||
| · to approve service contracts between individual members of the supervisory board and the company. |
||||
| Shareholder Suits | Under German law, generally, the company, rather than its shareholders, is the proper claimant in an action with respect to a wrong committed against the company, or in cases where there is an irregularity in the company’s internal management or supervision. Therefore, such claims may only be raised by the company represented by its management board, or, in | Under Delaware law, a shareholder may initiate a derivative action to enforce a right of a corporation if the corporation fails to enforce the right itself. The complaint must:
· state that the plaintiff was a shareholder at the time of the transaction of which the plaintiff complains or that the plaintiff’s shares thereafter devolved on | ||
215
Table of Contents
| Germany | Delaware | |||
| the case of a wrong committed by a member of the management board, by the supervisory board.
Additionally, pursuant to German case law the supervisory board is obliged to pursue the company’s claims against the management board, unless the interest of the company keeps them from doing so.
The management board, or, if a claim is against a member of the management board, the supervisory board, is obliged to pursue the company’s claims against the designated individuals if so resolved by a simple majority of votes cast during a shareholders’ meeting. With a simple majority of votes, shareholders can request that a representative pursues the claim on behalf of the company. |
the plaintiff by operation of law; and
· allege with particularity the efforts made by the plaintiff to obtain the action the plaintiff desires from the directors and the reasons for the plaintiff’s failure to obtain the action; or
· state the reasons for not making the effort.
Additionally, the plaintiff must remain a shareholder through the duration of the derivative suit. The action will not be dismissed or compromised without the approval of the Delaware Court of Chancery. | |||
| If the company is unable to fulfill its third-party obligations, the company’s creditors may pursue the company’s damage claims against members of the management board for certain wrongdoings. | ||||
| Under certain circumstances, shareholders can bring forward damage claims of the company against its management on their own behalf. In order to bring forward such a claim one shareholder alone or | ||||
216
Table of Contents
| Germany | Delaware | |||
| together with other shareholders needs to hold at least 1% of the company’s share capital or a participation of €100,000 in the share capital. Additionally, the claimant needs to pass through special claim approval procedures. |
217
Table of Contents
DESCRIPTION OF AMERICAN DEPOSITARY SHARES
American Depositary Shares
The Bank of New York Mellon, as depositary, will register and deliver American Depositary Shares, also referred to as ADSs. Each ADS will represent one-quarter (1/4) of an ordinary share (or a right to receive one-quarter (1/4) of an ordinary share of MorphoSys AG) deposited with The Bank of New York Mellon SA/NV, as custodian for the depositary in Frankfurt. Each ADS will also represent any other securities, cash or other property which may be held by the depositary. The deposited shares together with any other securities, cash or other property held by the depositary are referred to as the deposited securities. The depositary’s office at which the ADSs will be administered is located at 101 Barclay Street, New York, New York 10286. The Bank of New York Mellon’s principal executive office is located at 225 Liberty Street, New York, New York 10286.
You may hold ADSs either (A) directly (i) by having an American Depositary Receipt, also referred to as an ADR, which is a certificate evidencing a specific number of ADSs, registered in your name, or (ii) by having uncertificated ADSs registered in your name, or (B) indirectly by holding a security entitlement in ADSs through your broker or other financial institution that is a direct or indirect participant in The Depository Trust Company, also called DTC. If you hold ADSs directly, you are a registered ADS holder, also referred to as an ADS holder. This description assumes you are an ADS holder. If you hold the ADSs indirectly, you must rely on the procedures of your broker or other financial institution to assert the rights of ADS holders described in this section. You should consult with your broker or financial institution to find out what those procedures are.
Registered holders of uncertificated ADSs will receive statements from the depositary confirming their holdings.
As an ADS holder, we will not treat you as one of our shareholders and you will not have shareholder rights. German law governs shareholder rights. The depositary will be the holder of the ordinary shares underlying your ADSs. As a registered holder of ADSs, you will have ADS holder rights. A deposit agreement among us, the depositary, ADS holders and all other persons indirectly or beneficially holding ADSs sets out ADS holder rights as well as the rights and obligations of the depositary. New York law governs the deposit agreement and the ADSs.
The following is a summary of the material provisions of the deposit agreement. For more complete information, you should read the entire deposit agreement and the form of ADR. Directions on how to obtain copies of those documents are described in “Where You Can Find More Information”.
Dividends and Other Distributions
How will you receive dividends and other distributions on the shares?
The depositary has agreed to pay or distribute to ADS holders the cash dividends or other distributions it or the custodian receives on ordinary shares or other deposited securities, upon payment or deduction of its fees and expenses. You will receive these distributions in proportion to the number of shares your ADSs represent.
Cash. The depositary will convert any cash dividend or other cash distribution we pay on our ordinary shares into U.S. dollars, if it can do so on a reasonable basis and can transfer the U.S.
218
Table of Contents
dollars to the United States and will promptly distribute the amount thus received. If that is not possible or if any government approval is needed and cannot be obtained, the deposit agreement allows the depositary to distribute the foreign currency only to those ADS holders to whom it is possible to do so. It will hold the foreign currency it cannot convert for the account of the ADS holders who have not been paid. It will not invest the foreign currency and it will not be liable for any interest.
Before making a distribution, any withholding taxes, or other governmental charges that must be paid will be deducted. See ”Taxation”. The depositary will distribute only whole U.S. dollars and cents and will round fractional cents to the nearest whole cent. If the exchange rates fluctuate during a time when the depositary cannot convert the foreign currency, you may lose some of the value of the distribution.
Shares. The depositary may distribute additional ADSs representing any ordinary shares we distribute as a dividend or free distribution. The depositary will only distribute whole ADSs. It will sell ordinary shares which would require it to deliver a fraction of an ADS (or ADSs representing those shares) and distribute the net proceeds in the same way as it does with cash. If the depositary does not distribute additional ADSs, the outstanding ADSs will also represent the new shares. The depositary may sell a portion of the distributed ordinary shares (or ADSs representing those shares) sufficient to pay its fees and expenses in connection with that distribution.
Rights to purchase additional shares. If we offer holders of our securities any rights to subscribe for additional ordinary shares or any other rights, the depositary may (i) exercise those rights on behalf of ADS holders if instructed to do so by the relevant ADS holders, (ii) distribute those rights to ADS holders or (iii) sell those rights and distribute the net proceeds to ADS holders, in each case after deduction or upon payment of its fees and expenses. To the extent the depositary does not do any of those things, it will allow the rights to lapse. In that case, you will receive no value for them. The depositary will exercise or distribute rights only if we ask it to and provide satisfactory assurances to the depositary that it is legal to do so. If the depositary will exercise rights, it will purchase the securities to which the rights relate and distribute those securities or, in the case of shares, new ADSs representing the new shares, to subscribing ADS holders, but only if ADS holders have paid the exercise price to the depositary. U.S. securities laws may restrict the ability of the depositary to distribute rights or ADSs or other securities issued on exercise of rights to all or certain ADS holders, and the securities distributed may be subject to restrictions on transfer.
There can be no assurance that you will be given the opportunity to exercise rights on the same terms and conditions as the holders of our ordinary shares or be able to exercise such rights at all.
Other Distributions. The depositary will send to ADS holders anything else we distribute on deposited securities by any means it thinks is legal, equitable and practical. If it cannot make the distribution in that way, the depositary has a choice. It may decide to sell what we distributed and distribute the net proceeds, in the same way as it does with cash. Or, it may decide to hold what we distributed, in which case ADSs will also represent the newly distributed property. However, the depositary is not required to distribute any securities (other than ADSs) to ADS holders unless it receives satisfactory evidence from us that it is legal to make that distribution. The depositary may sell a portion of the distributed securities or property sufficient to pay its
219
Table of Contents
fees and expenses in connection with that distribution. U.S. securities laws may restrict the ability of the depositary to distribute securities to all or certain ADS holders, and the securities distributed may be subject to restrictions on transfer.
The depositary is not responsible if it decides that it is unlawful or impractical to make a distribution available to any ADS holders. We have no obligation to register ADSs, shares, rights or other securities under the Securities Act. We also have no obligation to take any other action to permit the distribution of ADSs, shares, rights or anything else to ADS holders. This means that you may not receive the distributions we make on our shares or any value for them if it is illegal or impractical for us to make them available to you.
Deposit, Withdrawal and Cancellation
How are ADSs issued?
The depositary will deliver ADSs if you or your broker deposits shares or evidence of rights to receive shares with the custodian. Upon payment of its fees and expenses and of any taxes or charges, such as stamp taxes or stock transfer taxes or fees, the depositary will register the appropriate number of ADSs in the names you request and will deliver the ADSs to or upon the order of the person or persons that made the deposit.
How can ADS holders withdraw the deposited securities?
You may surrender your ADSs for the purpose of withdrawal at the depositary’s office. Upon payment of its fees and expenses and of any taxes or charges, such as stamp taxes or stock transfer taxes or fees, the depositary will deliver the ordinary shares and any other deposited securities underlying the ADSs to the ADS holder or a person the ADS holder designates at the office of the custodian. Or, at your request, risk and expense, the depositary will deliver the deposited securities at its office, if feasible. The depositary may charge you a fee and its expenses for instructing the custodian regarding delivery of deposited securities.
How do ADS holders interchange between certificated ADSs and uncertificated ADSs?
You may surrender your ADR to the depositary for the purpose of exchanging your ADR for uncertificated ADSs. The depositary will cancel that ADR and will send to the ADS holder a statement confirming that the ADS holder is the registered holder of uncertificated ADSs. Upon receipt by the depositary of a proper instruction from a registered holder of uncertificated ADSs requesting the exchange of uncertificated ADSs for certificated ADSs, the depositary will execute and deliver to the ADS holder an ADR evidencing those ADSs.
Voting Rights
How do you vote?
ADS holders may instruct the depositary how to vote the number of deposited ordinary shares their ADSs represent at any meeting at which you are entitled to vote pursuant to applicable law and our articles of association. Upon receipt of notice of any shareholders’ meeting, the depositary will notify you of such shareholders’ meeting and send or make voting materials available to you. Those materials will describe the matters to be voted on and explain how ADS holders may instruct the depositary how to vote. For instructions to be valid, they must reach the depositary by a date set by the depositary. The depositary will try, as far as practical, subject
220
Table of Contents
to the laws of Germany and the provisions of our articles of association or similar documents, to vote or to have its agents vote the ordinary shares or other deposited securities as instructed by ADS holders. If we do not request the depositary to solicit your voting instructions, you can still send voting instructions, and, in that case, the depositary may try to vote as you instruct, but it is not required to do so.
Except by instructing the depositary as described above, you will not be able to exercise voting rights unless you surrender your ADSs and withdraw the ordinary shares. However, you may not know about the meeting enough in advance to withdraw the ordinary shares. In any event, except as set out below the depositary will not exercise any discretion in voting deposited securities and it will only vote or attempt to vote as instructed.
We cannot assure you that you will receive the voting materials in time to ensure that you can instruct the depositary to vote your ordinary shares. In addition, the depositary and its agents are not responsible for failing to carry out voting instructions or for the manner of carrying out voting instructions. This means that you may not be able to exercise voting rights and there may be nothing you can do if your ordinary shares are not voted as you requested.
In order to give you a reasonable opportunity to instruct the depositary as to the exercise of voting rights relating to Deposited Securities, if we request the Depositary to act, we agree to give the depositary notice of any such meeting and details concerning the matters to be voted upon at least 30 days in advance of the meeting date.
If we request the Depositary to act and comply with the notice period referred to above and you do not instruct the Depositary to vote, the Depositary shall deem that you have given a discretionary proxy to a person designated by us to vote on that matter, except that no proxy of that kind shall be deemed given as to any matters as to which we inform the Depositary that (i) we do not wish a proxy is given, (ii) substantial opposition exists or (iii) the matter materially and adversely affects the rights of the holders of our shares.
221
Table of Contents
Fees and Expenses
| Persons depositing or withdrawing shares or ADS holders must pay: | For: | |
| $5.00 (or less) per 100 ADSs (or portion of 100 ADSs) | Issuance of ADSs, including issuances resulting from a distribution of shares or rights or other property | |
| Cancellation of ADSs for the purpose of withdrawal, including if the deposit agreement terminates | ||
| $.05 (or less) per ADS | Any cash distribution to ADS holders | |
| A fee equivalent to the fee that would be payable if securities distributed to you had been shares and the shares had been deposited for issuance of ADSs | Distribution of securities distributed to holders of deposited securities (including rights) that are distributed by the depositary to ADS holders | |
| $.05 (or less) per ADS per calendar year | Depositary services | |
| Registration or transfer fees | Transfer and registration of shares on our share register to or from the name of the depositary or its agent when you deposit or withdraw shares | |
| Expenses of the depositary | Cable and facsimile transmissions (when expressly provided in the deposit agreement) | |
| Converting foreign currency to U.S. dollars | ||
| Taxes and other governmental charges the depositary or the custodian has to pay on any ADSs or shares underlying ADSs, such as stock transfer taxes, stamp duty or withholding taxes | As necessary | |
| Any charges incurred by the depositary or its agents for servicing the deposited securities | As necessary | |
The depositary collects its fees for delivery and surrender of ADSs directly from investors depositing ordinary shares or surrendering ADSs for the purpose of withdrawal or from intermediaries acting for them. The depositary collects fees for making distributions to investors by deducting those fees from the amounts distributed or by selling a portion of distributable property to pay the fees. The depositary may collect its annual fee for depositary services by deduction from cash distributions or by directly billing investors or by charging the book-entry system accounts of participants acting for them. The depositary may collect any of its fees by deduction from any cash distribution payable (or by selling a portion of securities or other
222
Table of Contents
property distributable) to ADS holders that are obligated to pay those fees. The depositary may generally refuse to provide fee-attracting services until its fees for those services are paid.
From time to time, the depositary may make payments to us to reimburse us for costs and expenses generally arising out of establishment and maintenance of the ADS program, waive fees and expenses for services provided to us by the depositary or share revenue from the fees collected from ADS holders. In performing its duties under the deposit agreement, the depositary may use brokers, dealers, foreign currency dealers or other service providers that are owned by or affiliated with the depositary and that may earn or share fees, spreads or commissions.
The depositary may convert currency itself or through any of its affiliates and, in those cases, acts as principal for its own account and not as agent, advisor, broker or fiduciary on behalf of any other person and earns revenue, including, without limitation, transaction spreads, that it will retain for its own account. The revenue is based on, among other things, the difference between the exchange rate assigned to the currency conversion made under the deposit agreement and the rate that the depositary or its affiliate receives when buying or selling foreign currency for its own account. The depositary makes no representation that the exchange rate used or obtained in any currency conversion under the deposit agreement will be the most favorable rate that could be obtained at the time or that the method by which that rate will be determined will be the most favorable to ADS holders, subject to the depositary’s obligations under the deposit agreement. The methodology used to determine exchange rates used in currency conversions is available upon request.
Payment of Taxes
You will be responsible for any taxes or other governmental charges payable on your ADSs or on the deposited securities represented by any of your ADSs. The depositary may refuse to register any transfer of your ADSs or allow you to withdraw the deposited securities represented by your ADSs until those taxes or other charges are paid. It may apply payments owed to you or sell deposited securities represented by your American Depositary Shares to pay any taxes owed and you will remain liable for any deficiency. If the depositary sells deposited securities, it will, if appropriate, reduce the number of ADSs to reflect the sale and pay to ADS holders any proceeds, or send to ADS holders any property, remaining after it has paid the taxes.
Tender and Exchange Offers; Redemption, Replacement or Cancellation of Deposited Securities
The depositary will not tender deposited securities in any voluntary tender or exchange offer unless instructed to do by an ADS holder surrendering ADSs and subject to any conditions or procedures the depositary may establish.
If deposited securities are redeemed for cash in a transaction that is mandatory for the depositary as a holder of deposited securities, the depositary will call for surrender of a corresponding number of ADSs and distribute the net redemption money to the holders of called ADSs upon surrender of those ADSs.
If there is any change in the deposited securities such as a subdivision, combination or other reclassification, or any merger, consolidation, recapitalization or reorganization affecting the issuer of deposited securities in which the depositary receives new securities in exchange for or
223
Table of Contents
in lieu of the old deposited securities, the depositary will hold those replacement securities as deposited securities under the deposit agreement. However, if the depositary decides it would not be lawful and to hold the replacement securities because those securities could not be distributed to ADS holders or for any other reason, the depositary may instead sell the replacement securities and distribute the net proceeds upon surrender of the ADSs.
If there is a replacement of the deposited securities and the depositary will continue to hold the replacement securities, the depositary may distribute new ADSs representing the new deposited securities or ask you to surrender your outstanding ADRs in exchange for new ADRs identifying the new deposited securities.
If there are no deposited securities underlying ADSs, including if the deposited securities are cancelled, or if the deposited securities underlying ADSs have become apparently worthless, the depositary may call for surrender or of those ADSs or cancel those ADSs upon notice to the ADS holders.
Amendment and Termination
How may the deposit agreement be amended?
We may agree with the depositary to amend the deposit agreement and the ADRs without your consent for any reason. If an amendment adds or increases fees or charges, except for taxes and other governmental charges or expenses of the depositary for registration fees, facsimile costs, delivery charges or similar items, or prejudices a substantial right of ADS holders, it will not become effective for outstanding ADSs until 30 days after the depositary notifies ADS holders of the amendment. At the time an amendment becomes effective, you are considered, by continuing to hold your ADSs, to agree to the amendment and to be bound by the ADRs and the deposit agreement as amended.
How may the deposit agreement be terminated?
The depositary will initiate termination of the deposit agreement if we instruct it to do so. The depositary may initiate termination of the deposit agreement if
| · | 60 days have passed since the depositary told us it wants to resign but a successor depositary has not been appointed and accepted its appointment; |
| · | we delist our shares from an exchange on which they were listed and do not list the shares on another exchange; |
| · | we appear to be insolvent or enter insolvency proceedings |
| · | all or substantially all the value of the deposited securities has been distributed either in cash or in the form of securities; |
| · | there are no deposited securities underlying the ADSs or the underlying deposited securities have become apparently worthless; or |
| · | there has been a replacement of deposited securities. |
| · | If the deposit agreement will terminate, the depositary will notify ADS holders at least 90 days before the termination date. At any time after the termination date, the depositary may sell the deposited securities. After that, the depositary will hold the money it received on the sale, as well as any other cash it is holding under the deposit agreement, unsegregated and |
224
Table of Contents
| without liability for interest, for the pro rata benefit of the ADS holders that have not surrendered their ADSs. Normally, the depositary will sell as soon as practicable after the termination date. |
After the termination date and before the depositary sells, ADS holders can still surrender their ADSs and receive delivery of deposited securities, except that the depositary may refuse to accept a surrender for the purpose of withdrawing deposited securities if it would interfere with the selling process. The depositary may refuse to accept a surrender for the purpose of withdrawing sale proceeds until all the deposited securities have been sold. The depositary will continue to collect distributions on deposited securities, but, after the termination date, the depositary is not required to register any transfer of ADSs or distribute any dividends or other distributions on deposited securities to the ADSs holder (until they surrender their ADSs) or give any notices or perform any other duties under the deposit agreement except as described in this paragraph.
Limitations on Obligations and Liability
Limits on our Obligations and the Obligations of the Depositary; Limits on Liability to Holders of ADSs
The deposit agreement expressly limits our and our directors’, officers’, employees’, agents’ and affiliates’ obligations and the obligations of the depositary and its directors, officers, employees, agents and affiliates. It also limits our liability and the liability of the depositary. We and the depositary:
| · | are only obligated to take the actions specifically set forth in the deposit agreement without negligence or bad faith; |
| · | are not liable if we are or it is prevented or delayed by law or by events or circumstances beyond our or its ability to prevent or counteract with reasonable care or effort from performing our or its obligations under the deposit agreement; |
| · | are not liable if we or it exercises discretion permitted under the deposit agreement; |
| · | are not liable for the inability of any holder of ADSs to benefit from any distribution on deposited securities that is not made available to holders of ADSs under the terms of the deposit agreement, or for any special, consequential or punitive damages for any breach of the terms of the deposit agreement; |
| · | have no obligation to become involved in a lawsuit or other proceeding related to the ADSs or the deposit agreement on your behalf or on behalf of any other person; |
| · | are not liable for the acts or omissions of any securities depository, clearing agency or settlement system; and |
| · | may rely upon any documents we believe or it believes in good faith to be genuine and to have been signed or presented by the proper person. |
In the deposit agreement, we and the depositary agree to indemnify each other under certain circumstances.
225
Table of Contents
Requirements for Depositary Actions
Before the depositary will deliver or register a transfer of ADSs, make a distribution on ADSs, or permit withdrawal of ordinary shares, the depositary may require:
| · | payment of stock transfer or other taxes or other governmental charges and transfer or registration fees charged by third parties for the transfer of any shares or other deposited securities; |
| · | satisfactory proof of the identity and genuineness of any signature or other information it deems necessary; and |
| · | compliance with regulations it may establish, from time to time, consistent with the deposit agreement, including presentation of transfer documents. |
The depositary may refuse to deliver ADSs or register transfers of ADSs when the transfer books of the depositary or our transfer books are closed or at any time if the depositary or we think it advisable to do so.
Your Right to Receive the Shares Underlying your ADSs
ADS holders have the right to cancel their ADSs and withdraw the underlying ordinary shares at any time except:
| · | when temporary delays arise because: (i) the depositary has closed its transfer books or we have closed our transfer books; (ii) the transfer ordinary of shares is blocked to permit voting at a shareholders’ meeting; or (iii) we are paying a dividend on our ordinary shares; |
| · | when you owe money to pay fees, taxes and similar charges; or |
| · | when it is necessary to prohibit withdrawals in order to comply with any laws or governmental regulations that apply to ADSs or to the withdrawal of shares or other deposited securities. |
This right of withdrawal may not be limited by any other provision of the deposit agreement.
Pre-release of ADSs
The deposit agreement permits the depositary to deliver ADSs before deposit of the underlying ordinary shares. This is called a pre-release of the ADSs. The depositary may also deliver shares upon cancellation of pre-released ADSs (even if the ADSs are canceled before the pre-release transaction has been closed out). A pre-release is closed out as soon as the underlying shares are delivered to the depositary. The depositary may receive ADSs instead of shares to close out a pre-release. The depositary may pre-release ADSs only under the following conditions: (1) before or at the time of the pre-release, the person to whom the pre-release is being made represents to the depositary in writing that it or its customer owns the shares or ADSs to be deposited; (2) the pre-release is fully collateralized with cash, U.S. government securities or other collateral that the depositary determines, in good faith, will provide substantially similar liquidity and security; (3) the depositary must be able to close out the pre-release on not more than five business days’ notice; and (4) subject to all indemnities and credit regulations the depositary deems appropriate. In addition, the depositary will limit the number of ADSs that may be outstanding at any time as a result of pre-release, although the depositary may disregard the limit from time to time if it thinks it is appropriate to do so.
226
Table of Contents
Direct Registration System
In the deposit agreement, all parties to the deposit agreement acknowledge that the Direct Registration System, also referred to as DRS, and Profile Modification System, also referred to as Profile, will apply to the ADSs. DRS is a system administered by DTC that facilitates interchange between registered holding of uncertificated ADSs and holding of security entitlements in ADSs through DTC and a DTC participant. Profile is feature of DRS that allows a DTC participant, claiming to act on behalf of a registered holder of uncertificated ADSs, to direct the depositary to register a transfer of those ADSs to DTC or its nominee and to deliver those ADSs to the DTC account of that DTC participant without receipt by the depositary of prior authorization from the ADS holder to register that transfer.
In connection with and in accordance with the arrangements and procedures relating to DRS/Profile, the parties to the deposit agreement understand that the depositary will not determine whether the DTC participant that is claiming to be acting on behalf of an ADS holder in requesting registration of transfer and delivery as described in the paragraph above has the actual authority to act on behalf of the ADS holder (notwithstanding any requirements under the Uniform Commercial Code). In the deposit agreement, the parties agree that the depositary’s reliance on and compliance with instructions received by the depositary through the DRS/Profile system and in accordance with the deposit agreement will not constitute negligence or bad faith on the part of the depositary.
Shareholder Communications; Inspection of Register of Holders of ADSs
The depositary will make available for your inspection at its office all communications that it receives from us as a holder of deposited securities that we make generally available to holders of deposited securities. The depositary will send you copies of those communications or otherwise make those communications available to you if we ask it to. You have a right to inspect the register of holders of ADSs, but not for the purpose of contacting those holders about a matter unrelated to our business or the ADSs.
227
Table of Contents
SHARES ELIGIBLE FOR FUTURE SALE
Upon completion of this offering, we will have 31,495,785 ordinary shares issued (including ordinary shares represented by ADSs) or, if the underwriters exercise in full their option to buy additional ADSs, 31,807,035 ordinary shares issued (including ordinary shares represented by ADSs). All of the ADSs sold in this offering will be freely transferable by persons other than by our “affiliates” without restriction or further registration under the Securities Act. Sales of substantial amounts of our ordinary shares or ADSs in the public market could adversely affect prevailing market prices of our ADSs. Prior to this offering, there has been no public market for our ordinary shares in the United States or the ADSs, and although the ADSs have been approved for listing on Nasdaq, we cannot assure you that a regular trading market will develop in the ADSs. We do not intend to list our ordinary shares on a trading market in the United States and therefore do not expect that a trading market in the United States will develop for our ordinary shares not represented by the ADSs.
Lock-Up Agreements
For a description of the lock-up arrangements that we and members of our management board have entered into in connection with this offering, see “Underwriting”.
Rule 144
In general, under Rule 144 under the Securities Act as in effect on the date of this prospectus, beginning 90 days after the effective date of the registration statement of which this prospectus forms a part, a person who is not an affiliate of ours at any time during the 90 days preceding a sale, and who has held their ordinary shares for at least six months, as measured by SEC rule, including the holding period of any prior owner other than one of our affiliates, may sell ordinary shares without restriction, provided current public information about us is available. In addition, under Rule 144, any person who is not an affiliate of ours at any time during the three months preceding a sale, and who has held their ordinary shares for at least one year, as measured by SEC rule, including the holding period of any prior owner other than one of our affiliates, would be entitled to sell an unlimited number of ordinary shares immediately upon consummation of this offering without regard to whether current public information about us is available.
Beginning 90 days after the effective date of the registration statement of which this prospectus forms a part, a person who is an affiliate of ours and who has beneficially owned “restricted” ordinary shares for at least six months, as measured by applicable SEC rules, including the holding period of any prior owner other than one of our affiliates, is entitled to sell a number of restricted ordinary shares within any three-month period that does not exceed the greater of:
| · | 1% of the number of ordinary shares then outstanding, in the form of ADSs or otherwise; and |
| · | the average weekly trading volume of our ADSs on Nasdaq during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale. |
Sales of restricted ordinary shares under Rule 144 held by our affiliates are also subject to requirements regarding the manner of sale, notice and the availability of current public information about us. Rule 144 also requires that affiliates relying on Rule 144 to sell ordinary
228
Table of Contents
shares that are not restricted shares must nonetheless comply with the same restrictions applicable to restricted shares, other than the holding period requirement.
In addition, in each case, these ordinary shares would remain subject to lock-up arrangements and would only become eligible for sale when the lock-up period expires.
Regulation S
Regulation S under the Securities Act provides that ordinary shares or ADSs owned by any person may be sold without registration in the United States, provided that the sale is effected in an offshore transaction and no directed selling efforts are made in the United States (as these terms are defined in Regulation S), subject to certain other conditions. In general, this means that our shares or ADSs may be sold outside the United States without registration in the United States being required.
Rule 701
Under Rule 701 under the Securities Act, ordinary shares or ADSs acquired by any of our employees, members of the management board, members of the supervisory board, consultants or advisors upon the exercise of options or pursuant to other rights granted under a written compensatory stock or option plan or other written agreement in compliance with Rule 701 may be resold, by:
| · | persons other than affiliates, beginning 90 days after the effective date of the registration statement of which this prospectus forms a part, subject only to the manner-of-sale provisions of Rule 144; and |
| · | our affiliates, beginning 90 days after the effective date of the registration statement of which this prospectus forms a part, subject to the manner-of-sale and volume limitations, current public information and filing requirements of Rule 144, in each case, without compliance with the six-month holding period requirement of Rule 144. |
Form S-8 Registration Statement
After the completion of this offering, we intend to file one or more registration statements on Form S-8 under the Securities Act covering our shares subject to outstanding options and shares issuable under our share-based incentive schemes. We expect to file the registration statement covering shares offered pursuant to our share-based incentive schemes on or shortly after the date of this prospectus, permitting the resale of such shares by nonaffiliates in the public market without restriction under the Securities Act and the sale by affiliates in the public market, subject to compliance with the resale provisions of Rule 144. Accordingly, our shares registered under any such registration statement will be available for sale in the open market upon exercise by the holders, subject to vesting and holding restrictions, as applicable, Rule 144 limitations applicable to our affiliates and the contractual lock-up provisions described in “Underwriting”.
229
Table of Contents
EXCHANGE CONTROLS AND LIMITATIONS AFFECTING SHAREHOLDERS
There are currently no legal restrictions in Germany on international capital movements and foreign exchange transactions, except in limited embargo circumstances (Teilembargo) relating to certain areas, entities or persons as a result of applicable resolutions adopted by the United Nations and the EU. Restrictions currently exist with respect to, among others, Belarus, Congo, Egypt, Eritrea, Guinea, Guinea-Bissau, Iran, Iraq, Ivory Coast, Lebanon, Liberia, Libya, North Korea, Somalia, South Sudan, Sudan, Syria, Tunisia and Zimbabwe.
For statistical purposes, there are, however, limited notification requirements regarding transactions involving cross-border monetary transfers. With some exceptions, every corporation or individual residing in Germany must report to the German Central Bank (Deutsche Bundesbank) (i) any payment received from, or made to, a non-resident corporation or individual that exceeds €12,500 (or the equivalent in a foreign currency) and (ii) in case the sum of claims against, or liabilities payable to, non-residents or corporations exceeds €5,000,000 (or the equivalent in a foreign currency) at the end of any calendar month. Payments include cash payments made by means of direct debit, checks and bills, remittances denominated in euros and other currencies made through financial institutions, as well as netting and clearing arrangements.
230
Table of Contents
The following discussion is a summary of U.S. and German tax consequences of owning and disposing of the ADSs. It does not purport to be a comprehensive description of all tax considerations that may be relevant to a decision to purchase ADSs by any particular investor. In particular, this discussion does not address tax considerations applicable to a U.S. holder (as defined in “—U.S. Taxation” below) that may be subject to special tax rules, including, without limitation, dealers or traders in securities, notional principal contracts or currencies, financial institutions, insurance companies, U.S. expatriates and inverted companies, certain stapled companies, tax-exempt organizations, tax-deferred or other retirement accounts, regulated investment companies, real estate investment trusts, a person that holds ADSs as part of a hedge, straddle, conversion or other integrated transaction for tax purposes, a person that purchases or sells ADSs as part of a wash sale for tax purposes, a person whose functional currency for tax purposes is not the U.S. dollar, a person subject to the U.S. alternative minimum tax, a person who does not hold the ADSs as capital assets for tax purposes, a person subject to special tax accounting rules as a result of any item of gross income with respect to the ADSs being taken into account in an applicable financial statement; or a person that owns or is deemed to own 10% or more of the company’s shares by vote or value. In addition, the discussion does not address tax consequences to an entity treated as a partnership (or other pass-through entity) for U.S. federal income tax purposes that holds ADSs. Prospective purchasers that are partners in a partnership holding ADSs should consult their own tax advisors.
German Taxation
The following discussion addresses certain German tax consequences of acquiring, owning or disposing of the ADSs. With the exception of the subsection “Taxation of Holders Tax Resident in Germany” below, which provides an overview of dividend taxation to holders that are residents of Germany, this discussion applies only to U.S. treaty beneficiaries (defined below) that acquire ADSs in the offering.
This discussion is based on domestic German tax laws, including, but not limited to, circulars issued by German tax authorities, which are not binding on the German courts, and the Treaty (defined below). It is based upon tax laws in effect at the time of filing of this prospectus. These laws are subject to change, possibly with retroactive effect. For example, certain member states of the European Union are considering introducing a financial transaction tax (Finanztransaktionssteuer) which, if and when introduced, may also be applicable on sales and/or transfer of ADSs. In addition, in Germany, for example, there are currently ongoing discussions on the raise of the top tax rate, which may also have an effect on the German tax consequences of acquiring, owning and disposing of the ADSs. There is no assurance that German tax authorities will not challenge one or more of the tax consequences described in this discussion.
In addition, this discussion is based upon the assumption that each obligation in the deposit agreement and any related agreement will be performed in accordance with its terms. It does not purport to be a comprehensive or exhaustive description of all German tax considerations that may be of relevance in the context of acquiring, owning and disposing of ADSs.
231
Table of Contents
The tax information presented in this Prospectus is not a substitute for tax advice. Prospective holders of ADSs should consult their own tax advisors regarding the German tax consequences of the purchase, ownership, disposition, donation or inheritance of ADSs in light of their particular circumstances, including the effect of any state, local, or other foreign or domestic laws or changes in tax law or interpretation. The same applies with respect to the rules governing the refund of any German dividend withholding tax (Kapitalertragsteuer) withheld. Only an individual tax consultation can appropriately account for the particular tax situation of each investor.
MorphoSys does not assume any responsibility for withholding tax at source.
Taxation of MorphoSys
MorphoSys’s taxable income, whether distributed or retained, is generally subject to corporate income tax (Körperschaftsteuer) at a uniform rate of 15% plus the solidarity surcharge (Solidaritätszuschlag) of 5.5% thereon, resulting in a total corporate income tax liability of 15.825%.
Dividends (Gewinnanteile) and other distributions received by MorphoSys from domestic or foreign corporations are exempt from corporate income tax, inter alia, if MorphoSys held at the beginning of the calendar year at least 10% of the registered share capital (Grundkapital or Stammkapital) of the distributing corporation which did not deduct the distributions from its own tax base; however, 5% of such revenue is treated as a non-deductible business expense and, as such, is subject to corporate income tax plus the solidarity surcharge. The acquisition of a participation of at least 10% in the course of a calendar year is deemed to have occurred at the beginning of such calendar year for the purpose of this rule. Participations in the share capital of other corporations which MorphoSys holds through a partnership, including co- entrepreneurships (Mitunternehmerschaften), are attributable to MorphoSys only on a pro rata basis at its entitlement to the profits of the relevant partnership. Subject to the above-mentioned requirements, 95% of the amount of dividends and other distributions that MorphoSys receives from corporations are exempt from corporate income tax. The same applies, in general and irrespective of the size of the shareholding, to profits earned by MorphoSys from the sale of shares in another domestic or foreign corporation. Losses incurred from the sale of such shares are not deductible for tax purposes.
In addition, MorphoSys is subject to trade tax (Gewerbesteuer) with respect to its taxable trade profit (Gewerbeertrag) from its permanent establishments in Germany (inländische gewerbesteuerliche Betriebsstätten). Trade tax is generally based on the taxable income as determined for corporate income tax purposes taking into account, however, certain add-backs and deductions.
The trade tax rate depends on the local municipalities in which MorphoSys maintains its permanent establishments. Dividends received from other corporations and capital gains from the sale of shares in other corporations are treated in principle in the same manner for trade tax purposes as for corporate income tax purposes. However, dividends received from domestic and foreign corporations are effectively 95% exempt from trade tax only if MorphoSys held at least 15% (10% in the event of companies resident for tax purposes in EU member states other than Germany) of the registered share capital (Grundkapital or Stammkapital) of the distributing corporation at the beginning or—in the event of foreign corporations—since the beginning of
232
Table of Contents
the relevant tax assessment period. Additional limitations apply with respect to dividends received from foreign non-EU corporations.
Expenditures for external financing are subject to the “interest barrier” (Zinsschranke) rules. When MorphoSys calculates its taxable income, the interest barrier rules generally prevent MorphoSys from deducting certain net interest expense, i.e., the excess of interest expense over interest income for a given fiscal year, exceeding 30% of its taxable EBITDA (taxable earnings adjusted for interest expense, interest income and certain depreciation/amortization and other reductions) if its net interest expense is, or exceeds, EUR 3 million (Freigrenze) and no other exceptions apply. Special rules apply in the event of external financing undertaken by shareholders or related parties. Interest expense that is not deductible in a given year may be carried forward to subsequent fiscal years of MorphoSys (interest carry-forward) and will increase the interest expense in those subsequent years. EBITDA amounts that could not be utilized may, under certain conditions, be carried forward into future fiscal years. If such EBITDA carry-forward is not used within five fiscal years it will be forfeited. An EBITDA carry-forward that arose in an earlier year must be used before a carry-forward that arose in a later year is used. By the decision dated October 14, 2015, the German Federal Fiscal Court (Bundesfinanzhof) submitted to the German Federal Constitutional Court (Bundesverfassungsgericht) the question as to whether or not the interest barrier rule is unconstitutional. The final decision on whether the interest barrier rule violates the constitution now lies with the German Federal Constitutional Court. While a decision has not been issued as of the date of the filing of this Prospectus, it may take a few years until this Court will decide. For the time being, the interest barrier remains applicable, and tax assessments may be kept open.
Tax-loss carry-forwards can be used to fully offset taxable income for corporate income tax and trade tax purposes up to an amount of EUR 1 million. If the taxable profit for the year or taxable profit subject to trade taxation exceeds this threshold, only up to 60% of the amount exceeding the threshold may be offset by tax-loss carry-forwards. The remaining 40% is subject to tax (minimum taxation) (Mindestbesteuerung). The rules also provide for a tax carryback to the previous year with regard to corporate income tax. Unused tax-loss carry-forwards may be generally carried forward indefinitely and used in subsequent assessment periods to offset future taxable income in accordance with this rule.
However, unused losses, loss carry forwards and interest carry-forwards are fully forfeited in full if within five years more than 50% of the subscribed capital, membership interests, equity interests or voting rights of MorphoSys are transferred, whether directly or indirectly, to an acquiring party or affiliated individuals/entities, or a similar change of ownership occurs (harmful acquisition) (schädlicher Beteiligungserwerb). A group of acquirers with aligned interests is also considered to be an acquiring party for these purposes. In addition, any current year losses incurred prior to the acquisition will not be deductible. If between 25% and 50% of the subscribed capital, membership interests, equity interests or voting rights of MorphoSys is transferred, a proportional amount of the unused losses and interest carry-forwards is forfeited. A capital increase shall be deemed as equivalent to a transfer of the subscribed capital to the extent that it causes a change of the interest ratio in the capital of the corporation. Unused losses, loss carry-forwards, and interest carry-forwards are not forfeited (i) in the event of certain intra-group transactions, (ii) or to the extent that they are covered at the time of the harmful acquisition by certain built-in gains (stille Reserven) which are subject to tax in
233
Table of Contents
Germany. Alternatively to (i) and (ii), MorphoSys may, under certain requirements, opt for the continuity of business exemption (Fortführungsgebundener Verlustvortrag) to preserve unused losses, loss carry forwards and interest carry-forwards. By the decision dated March 29, 2017, the German Federal Constitutional Court decided that the proportional, i.e., between 25% and 50%, change of ownership rule is unconstitutional. The legislator was requested to change this rule with retroactive effect until December 31, 2018. By the decision dated August 29, 2017, the Lower Tax Court of Hamburg (Finanzgericht Hamburg) submitted to the German Federal Constitutional Court the question as to whether or not the change of ownership rule stipulating a full forfeiture of unused losses, loss carry forwards and interest carry-forwards is also unconstitutional.
German Taxation of Holders of ADSs
General
Based on the circular issued by the German Federal Ministry of Finance (BMF-Schreiben), dated May 24, 2013, reference number IV C 1-S2204/12/10003, in respect of the taxation of American Depositary Receipts (ADRs) on domestic shares or the “ADR Tax Circular,” for German tax purposes, the ADSs represent a beneficial ownership interest in the underlying shares of MorphoSys and qualify as ADRs for the purpose of the ADR Tax Circular. If the ADSs qualify as ADRs under the ADR Tax Circular, dividends would accordingly be attributable to holders of the ADSs for tax purposes, and not to the legal owner of the ordinary shares (i.e., the financial institution on behalf of which the ordinary shares are stored at a domestic depository for the ADS holders). Furthermore, holders of the ADSs should be treated as beneficial owners of the capital of MorphoSys with respect to capital gains (see below in section “German Taxation of Capital Gains of the U.S. Treaty Beneficiaries of the ADSs”). However, investors should note that circulars published by the German tax authorities (including the ADR Tax Circular) are not binding on German courts, including German tax courts, and it is unclear whether a German court would follow the ADR Tax Circular in determining the German tax treatment of the ADSs. For the purpose of this German tax section, it is assumed that the ADSs qualify as ADRs within the meaning of the ADR Tax Circular.
Taxation of Holders Not Tax Resident in Germany
The following discussion describes the material German tax consequences for a holder that is a U.S. treaty beneficiary of acquiring, owning and disposing of the ADSs. For purposes of this discussion, a “U.S. treaty beneficiary” is a resident of the United States for purposes of the Agreement between the Federal Republic of Germany and United States of America for the Avoidance of Double Taxation and the Prevention of Fiscal Evasion with respect to Taxes on Income and on Capital as of June 4, 2008 (Abkommen zwischen der Bundesrepublik Deutschland und den Vereinigten Staaten von Amerika zur Vermeidung der Doppelbesteuerung und zur Verhinderung der Steuerverkürzung auf dem Gebiet der Steuern vom Einkommen und vom Vermögen und einiger anderer Steuern in der Fassung vom 4. Juni 2008), hereinafter referred to as the “Treaty”, who is fully eligible for benefits under the Treaty.
A holder will be a U.S. treaty beneficiary entitled to full Treaty benefits in respect of the ADSs if it is, inter alia:
| · | the beneficial owner of the ADSs (and the dividends paid with respect thereto); |
| · | a U.S. holder; |
234
Table of Contents
| · | not also a resident of Germany for German tax purposes; and |
| · | not subject to the limitation on benefits (i.e., anti-treaty shopping) article of the Treaty that applies in limited circumstances. |
Special rules apply to pension funds and certain other tax-exempt investors.
This discussion does not address the treatment of ADSs that are (i) held in connection with a permanent establishment or fixed base through which a U.S. treaty beneficiary carries on business or performs personal services in Germany or (ii) part of business assets for which a permanent representative in Germany has been appointed.
General Rules for the Taxation of Holders Not Tax Resident in Germany
Non-German resident holders of ADSs are subject to German taxation with respect to German source income (beschränkte Steuerpflicht). According to the ADR Tax Circular, income from the shares should be attributed to the holder of the ADSs for German tax purposes. As a consequence, income from the ADSs should be treated as German source income.
The full amount of a dividend distributed by MorphoSys to a non-German resident holder which does not maintain a permanent establishment or other taxable presence in Germany is subject to (final) German withholding tax at an aggregate rate of 26.375%. German withholding tax is withheld and remitted to the German tax authorities by the disbursing agent (i.e., the German credit institution, financial services institution, securities trading enterprise or securities trading bank (each as defined in the German Banking Act (Kreditwesengesetz) and in each case including a German branch of a foreign enterprise, but excluding a foreign branch of a German enterprise)) that holds or administers the underlying shares in custody and disburses or credits the dividend income from the underlying shares or disburses or credits the dividend income from the underlying shares on delivery of the dividend coupons or disburses such dividend income to a foreign agent or the central securities depository (Wertpapiersammelbank) in terms of the German Depositary Act (Depotgesetz) holding the underlying shares in a collective deposit, if such central securities depository disburses the dividend income from the underlying shares to a foreign agent, regardless of whether a holder must report the dividend for tax purposes and regardless of whether or not a holder is a resident of Germany.
Pursuant to the Treaty, the German withholding tax may not exceed 15% of the gross amount of the dividends received by U.S. treaty beneficiaries. The excess of the total withholding tax, including the solidarity surcharge, over the maximum rate of withholding tax permitted by the Treaty is refunded to U.S. treaty beneficiaries upon application. For example, for a declared dividend of 100, a U.S. treaty beneficiary initially receives 73.625 (100 minus the 26.375% withholding tax including solidarity surcharge). The U.S. treaty beneficiary is entitled to a partial refund from the German tax authorities in the amount of 11.375% of the gross dividend (of 100). As a result, the U.S. treaty beneficiary ultimately receives a total of 85 (85% of the declared dividend) following the refund of the excess withholding. However, investors should note that it is unclear how the German tax authorities will apply the refund process to dividends on the ADSs with respect to non-German resident holders of the ADSs. Further, such refund is subject to the German anti-avoidance treaty shopping rule (as described below in section “—Withholding Tax Refund for U.S. Treaty Beneficiaries”).
235
Table of Contents
German Taxation of Capital Gains of the U.S. Treaty Beneficiaries of the ADSs
The capital gains from the disposition of the ADSs realized by a non-German resident holder which does not maintain a permanent establishment or other taxable presence in Germany would be treated as German source income and be subject to German tax if such holder at any time during the five years preceding the disposition, directly or indirectly, owned 1% or more of MorphoSys’s share capital, irrespective of whether through the ADSs or shares of MorphoSys. If such holder had acquired the ADSs without consideration, the previous owner’s holding period and quota would be taken into account.
Pursuant to the Treaty, U.S. treaty beneficiaries are not subject to German tax even under the circumstances described in the preceding paragraph and therefore should not be taxed on capital gains from the disposition of the ADSs.
German statutory law requires the disbursing agent to levy withholding tax on capital gains from the sale of ADSs or other securities held in a custodial account in Germany. With regard to the German taxation of capital gains, disbursing agent means a German credit institution, a financial services institution, a securities trading enterprise or a securities trading bank (each as defined in the German Banking Act and, in each case including a German branch of a foreign enterprise, but excluding a foreign branch of a German enterprise) that holds the ADSs in custody or administers the ADSs for the investor or conducts sales or other dispositions and disburses or credits the income from the ADSs to the holder of the ADSs. The German statutory law does not explicitly condition the obligation to withhold taxes on capital gains being subject to taxation in Germany under German statutory law or on an applicable income tax treaty permitting Germany to tax such capital gains.
However, a circular issued by the German Federal Ministry of Finance, dated January 18, 2016, reference number IV C 1-S2252/08/10004 :017, provides that taxes need not be withheld when the holder of the custody account is not a resident of Germany for tax purposes and the income is not subject to German taxation. The circular further states that there is no obligation to withhold such tax even if the non-resident holder owns 1% or more of the share capital of a German company. While circulars issued by the German Federal Ministry of Finance are only binding on the German tax authorities but not on the German courts, in practice, the disbursing agents nevertheless typically rely on guidance contained in such circulars. Therefore, a disbursing agent would only withhold tax at 26.375% on capital gains derived by a U.S. treaty beneficiary from the sale of ADSs held in a custodial account in Germany in the event that the disbursing agent did not follow the abovementioned guidance. In this case, the U.S. treaty beneficiary may be entitled to claim a refund of the withholding tax from the German tax authorities under the Treaty, as described below in the section “—Withholding Tax Refund for U.S. Treaty Beneficiaries”.
Withholding Tax Refund for U.S. Treaty Beneficiaries
U.S. treaty beneficiaries are generally eligible for treaty benefits under the Treaty, as described above in Section “—Taxation of Holders Not Tax Resident in Germany”. Accordingly, U.S. treaty beneficiaries are in general entitled to claim a refund of the portion of the otherwise applicable 26.375% German withholding tax (corporate income tax including solidarity surcharge) on dividends that exceeds the applicable Treaty rate. However, such refund is only possible, provided that pursuant to special rules on the restriction of withholding tax credit, the following three cumulative requirements are met: (i) the shareholder must qualify as beneficial
236
Table of Contents
owner of the ADSs for an uninterrupted minimum holding period of 45 days within a period starting 45 days prior to and ending 45 days after the due date of the dividends, (ii) the shareholder has to bear at least 70% of the change in value risk related to the ADSs during the minimum holding period as described under (i) of this paragraph and has not entered into (acting by itself or through a related party) hedging transactions which lower the change in value risk by more than 30%, and (iii) the shareholder must not be obliged to fully or largely compensate directly or indirectly the dividends to third parties. If these requirements are not met, then for a shareholder not being tax-resident in Germany who applied for a full or partial refund of the withholding tax pursuant to a double taxation treaty, no refund is available. This restriction generally does only apply, if (i) the tax underlying the refund application is below a tax rate of 15% based on the gross amount of the dividends or capital gains and (ii) the shareholder does not directly own 10% or more in the shares of the company and is subject to income taxes in its state of residence, without being tax-exempt.
In general, as previously discussed, investors should note that it is unclear how the German tax administration will apply the refund process to dividends on the ADSs. Further, such refund is subject to the German anti-avoidance treaty shopping rule. Generally, this rule requires that the U.S. treaty beneficiary (in case it is a non-German resident company) maintains its own administrative substance and conducts its own business activities. In particular, a foreign company has no right to a full or partial refund to the extent persons holding ownership interests in MorphoSys would not be entitled to the refund if they derived the income directly and the gross income realized by the foreign company is not caused by the business activities of the foreign company, and there are either no economic or other considerable reasons for the interposition of the foreign company, or the foreign company does not participate in general commerce by means of a business organization with resources appropriate to its business purpose. However, this shall not apply if the foreign company’s principal class of stock is regularly traded in substantial volume on a recognized stock exchange, or if the foreign company is subject to the provisions of the German Investment Tax Act (Investmentsteuergesetz). Whether or not and to which extent the anti-avoidance treaty shopping rule applies, has to be analyzed on a case by case basis taking into account all relevant tests. In addition, the interpretation of these tests is disputed and to date no published decisions of the German Federal Finance Court exist in this regard.
Due to the legal structure of the ADSs, only limited guidance of the German tax authorities exists on the practical application of this procedure with respect to the ADSs.
Taxation of Holders Tax Resident in Germany
This subsection provides an overview of dividend taxation with regard to the general principles applicable to MorphoSys’s holders that are tax resident in Germany. A holder is a German tax resident if, in case of an individual, he or she maintains a domicile (Wohnsitz) or a usual residence (gewöhnlicher Aufenthalt) in Germany or if, in case of a corporation, it has its place of management (Geschäftsleitung) or registered office (Sitz) in Germany.
The German dividend and capital gains taxation rules applicable to German tax residents require a distinction between ADSs held as private assets (Privatvermögen) and ADSs held as business assets (Betriebsvermögen).
237
Table of Contents
ADSs as Private Assets (Privatvermögen)
If the ADSs are held as private assets by a German tax resident, dividends and capital gains are taxed as investment income and are principally subject to 25% German flat income tax on capital income (Abgeltungsteuer) (plus a 5.5% solidarity surcharge (Solidaritätszuschlag) thereon, resulting in an aggregate rate of 26.375%), which is levied in the form of withholding tax (Kapitalertragsteuer). In other words, once deducted, the shareholder’s income tax liability on the dividends will be settled.
Shareholders may apply to have their capital investment income assessed in accordance with the general rules and with an individual’s personal income tax rate if this would result in a lower tax burden in which case actually incurred expenses are not deductible. The holder would be taxed on gross personal investment income (including dividends or gains with respect to ADSs), less the saver’s allowance of €801 for an individual or €1,602 for a married couple and a registered civil union (eingetragene Lebenspartnerschaft) filing taxes jointly. The deduction of expenses related to the investment income (including dividends or gains with respect to ADSs) is generally not possible for private investors.
Losses resulting from the disposal of ADSs can only be offset by capital gains from the sale of any shares (Aktien) and other ADSs. If, however, a holder directly or indirectly held at least 1% of the share capital of the company at any time during the five years preceding the sale, 60% of any capital gains resulting from the sale are taxable at the holder’s personal income tax rate (plus 5.5% solidarity surcharge thereon). Conversely, 60% of any capital losses are recognized for tax purposes.
Church tax generally has to be withheld, if applicable, based on an automatic data access procedure, unless the shareholder has filed a blocking notice (Sperrvermerk) with the Federal Central Tax Office. Where church tax is not levied by way of withholding, it is determined by means of income tax assessment.
ADSs as Business Assets (Betriebsvermögen)
In case the ADSs are held as business assets, the taxation depends on the legal form of the holder (i.e., whether the holder is a corporation or an individual). Irrespective of the legal form of the holder, dividends are subject to the aggregate withholding tax rate of 26.375%. The withholding tax is credited against the respective holder’s income tax liability, provided that pursuant to special rules on the restriction of withholding tax credit, the following three cumulative requirements are met: (i) the shareholder must qualify as beneficial owner of the ADSs for an uninterrupted minimum holding period of 45 days occurring within a period starting 45 days prior to and ending 45 days after the due date of the dividends, (ii) the shareholder has to bear at least 70% of the change in value risk related to the ADSs during the minimum holding period as described under (i) of this paragraph and has not entered into (acting by itself or through a related party) hedging transactions which lower the change in value risk for more than 30%, and (iii) the shareholder must not be obliged to fully or largely compensate directly or indirectly the dividends to third parties. If these requirements are not met, three-fifths of the withholding tax imposed on the dividends must not be credited against the shareholder’s (corporate) income tax liability, but may, upon application, be deducted from the shareholder’s tax base for the relevant tax assessment period. Such requirements also apply to ADSs, which lead to domestic income in Germany and which are held by a non-German depositary bank. A shareholder that is generally subject to German income tax or corporate
238
Table of Contents
income tax and that has received gross dividends without any deduction of withholding tax due to a tax exemption without qualifying for a full tax credit under the aforementioned requirements has to notify the competent local tax office accordingly and has to make a payment in the amount of the omitted withholding tax deduction. The special rules on the restriction of withholding tax credit do not apply to a shareholder whose overall dividend earnings within an assessment period do not exceed €20,000 or that has been the beneficial owner of the ADSs in the company for at least one uninterrupted year upon receipt of the dividends.
To the extent the amount withheld exceeds the income tax liability, the withholding tax will be refunded, provided that certain requirements are met (including the aforementioned requirements).
Special rules apply to credit institutions (Kreditinstitute), financial services institutions (Finanzdienstleistungsinstitute), financial enterprises (Finanzunternehmen), life insurance and health insurance companies, and pension funds.
With regard to holders in the legal form of a corporation, dividends and capital gains are in general 95% tax exempt from corporate income tax (including solidarity surcharge), inter alia, if the shareholder held at least 10% of the registered share capital (Grundkapital oder Stammkapital) of MorphoSys at the beginning of the calendar year. The remaining 5% is treated as non-deductible business expense and, as such, is subject to corporate income tax (including solidarity surcharge). The acquisition of a participation of at least 10% in the course of a calendar year is deemed to have occurred at the beginning of such calendar year for the purpose of this rule. Participations in the share capital of other corporations which MorphoSys holds through a partnership, including co-entrepreneurships (Mitunternehmerschaften), are attributable to MorphoSys only on a pro rata basis at the ratio of its entitlement to the profits of the relevant partnership. Moreover, actual business expenses incurred to generate the dividends may be deducted.
However, the amount of any dividends after deducting business expenses related to the dividends is subject to the trade tax, unless the corporation held at least 15% of MorphoSys’s registered share capital at the beginning of the relevant tax assessment period. In the latter case, the aforementioned exemption of 95% of the dividend income also applies for trade tax purposes. Losses from the sale of ADSs are generally not tax deductible for corporate income tax and trade tax purposes.
With regard to individuals holding ADSs as business assets, 60% of dividends and capital gains are taxed at the individual’s personal income tax rate (plus 5.5% solidarity surcharge thereon). Correspondingly, only 60% of business expenses related to the dividends and capital gains as well as losses from the sale of ADSs are principally deductible for income tax purposes.
German Inheritance and Gift Tax (Erbschaft- und Schenkungsteuer)
The transfer of ADSs to another person by inheritance or gift should be generally subject to German inheritance and gift tax only if:
| (1) | the decedent or donor or heir, beneficiary or other transferee maintained his or her domicile or a usual residence in Germany or had its place of management or registered office in Germany at the time of the transfer, or is a German citizen who has spent no more |
239
Table of Contents
| than five consecutive years outside of Germany without maintaining a domicile in Germany or is a German citizen who serves for a German entity established under public law and is remunerated for his or her service from German public funds (including family members who form part of such person’s household, if they are German citizens) and is only subject to estate or inheritance tax in his or her country of domicile or usual residence with respect to assets located in such country (special rules apply to certain former German citizens who neither maintain a domicile nor have their usual residence in Germany); |
| (2) | at the time of the transfer, the ADSs are held by the decedent or donor as business assets forming part of a permanent establishment in Germany or for which a permanent representative in Germany has been appointed; or |
| (3) | the ADSs subject to such transfer form part of a portfolio that represents at the time of the transfer 10% or more of the registered share capital of the company and that has been held directly or indirectly by the decedent or donor, either alone or together with related persons. |
The Agreement between the Federal Republic of Germany and the United States of America for the avoidance of double taxation with respect to taxes on inheritances and gifts as of December 21, 2000 (Abkommen zwischen der Bundesrepublik Deutschland und den Vereinigten Staaten von Amerika zur Vermeidung der Doppelbesteuerung auf dem Gebiet der Nachlass-, Erbschaft- und Schenkungssteuern in der Fassung vom 21. Dezember 2000), hereinafter referred to as the “United States-Germany Inheritance and Gifts Tax Treaty”, provides that the German inheritance tax or gift tax can, with certain restrictions, only be levied in the cases of (1) and (2) above. Special provisions apply to certain German citizens living outside of Germany and former German citizens.
Other Taxes
No German transfer tax, value-added tax, stamp duty or similar taxes are assessed on the purchase, sale or other transfer of ADSs. Provided that certain requirements are met, an entrepreneur may, however, opt for the payment of value-added tax on transactions that are otherwise tax-exempt. Net wealth tax (Vermögensteuer) is currently not imposed in Germany. Certain member states of the European Union are considering introducing a financial transaction tax (Finanztransaktionssteuer) which, if and when introduced, may also be applicable on sales and/or transfer of ADSs.
U.S. Taxation
The following discussion is a summary of U.S. federal income tax considerations to U.S. holders (as defined below), and solely to the extent described below under “—Withholding on Foreign Accounts”, to non-U.S. persons, of owning and disposing of the ADSs who purchase such ADSs in the offering.
The information provided below is based on the Internal Revenue Code of 1986, as amended, or the Code, Treasury Regulations, the Treaty, Internal Revenue Service, or IRS, rulings and pronouncements, and judicial decisions all as now in effect and all of which are subject to change or differing interpretations, possibly with retroactive effect. There can be no assurance that the IRS or a court will not take a contrary position with respect to any U.S. federal income tax considerations described below.
240
Table of Contents
This discussion does not provide a complete analysis of all potential tax considerations. For example, the summary does not address the 3.8% Medicare tax imposed on certain net investment income or any aspect of U.S. federal estate and gift tax laws or any foreign, state or local laws that may be applicable to a holder.
For purposes of this summary, a “U.S. holder” is a beneficial owner of ADSs that for U.S. federal income tax purposes, is (1) an individual who is a citizen or resident of the United States, (2) a corporation, or an entity treated as a corporation for U.S. federal income tax purposes, created or organized in or under the laws of the United States or any state of the United States, including the District of Columbia, (3) an estate, the income of which is subject to U.S. federal income taxation regardless of its source, or (4) a trust (i) the administration of which is subject to the primary supervision of a U.S. court and which has one or more U.S. persons who have the authority to control all substantial decisions of the trust or (ii) that has otherwise elected to be treated as a U.S. person under the applicable regulations. A “non-U.S. holder” is a beneficial owner of ADSs, other than a partnership or an entity or arrangement treated as a partnership, that is not a U.S. holder.
If a partnership (including an entity or arrangement, domestic or foreign, treated as a partnership for U.S. federal income tax purposes) holds ADSs, the tax treatment of a partner in the partnership will depend upon the status of the partner and the activities of the partnership. A holder of ADSs that is a partnership, and partners in such partnership, should consult their own tax advisors about the U.S. federal income tax consequences of owning and disposing of the ADSs.
In general, a holder of ADSs should be treated as the owner of our ordinary shares for U.S. federal income tax purposes. Holders should consult their own tax advisers concerning the tax consequences of converting ADSs to ordinary shares.
Each prospective holder of ADSs should consult its own tax advisors regarding the U.S. federal, state and local or other tax consequences of acquiring, owning and disposing of the company’s ADSs in light of their particular circumstances. U.S. holders should also review the discussion under “—German Taxation” for the German tax consequences to a U.S. holder of the ownership of the ADSs.
U.S. Holders
This subsection describes the tax consequences to a U.S. holder.
Distributions
Under the United States federal income tax laws, and subject to the discussion below under “—PFIC Rules,” the gross amount of any distribution that is actually or constructively received by a U.S. holder with respect to its ADSs without reduction for any German taxes withheld will be a dividend to the extent the amount of such distribution is paid out of our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. To the extent the amount of such distribution exceeds our current or accumulated earnings and profits, such amount will be treated first as a non-taxable return of capital to the extent of such U.S. holder’s adjusted tax basis in its ADSs, and to the extent the amount of such distribution exceeds such adjusted tax basis, will be treated as capital gain from the sale of the ADSs. Because we do not intend to determine our earnings and profits on the basis of U.S. federal income tax principles,
241
Table of Contents
any distribution we pay will generally be reported as dividend income for U.S. federal income tax purposes. If you are a non-corporate U.S. holder, dividends paid to you that constitute qualified dividend income should be taxable to you at a preferential rate (rather than the higher rates of tax generally applicable to items of ordinary income) provided that you are not under any obligation to make related payments with respect to positions in substantially similar or related property, you hold our ADSs for more than 60 days during the 121-day period beginning 60 days before the ex-dividend date and your risk of loss with respect to the ADSs is not otherwise diminished. If we are a PFIC (as discussed below under “—Additional United States Federal Income Tax Consequences—PFIC Rules”) during the year of distribution or the year preceding the distribution, distributions paid by us with respect to our ADSs will not be eligible for the preferential income tax rate. Prospective investors should consult their own tax advisors regarding the taxation of distributions under these rules.
Dividends paid on our ADSs will not be eligible for the dividends-received deduction generally available to corporate U.S. holders.
Subject to applicable limitations, non-refundable German taxes withheld from dividends on the ADSs can be claimed as a credit against the U.S. holder’s U.S. federal income tax liability. For purposes of the U.S. foreign tax credit rules, dividends with respect to our ADSs should constitute income from sources outside of the United States and should generally be passive income for purposes of computing the foreign tax credit allowable to the U.S. holder. The amount of the qualified dividend income, if any, paid to a U.S. holder that is subject to the reduced dividend income tax rate and that is taken into account for purposes of calculating the U.S. holder’s U.S. foreign tax credit limitation must be reduced by the rate differential portion of the dividend. In lieu of claiming a foreign tax credit, U.S. holders may, at their election, deduct foreign taxes, including any German income tax, in computing their taxable income, subject to generally applicable limitations under U.S. law. An election to deduct foreign taxes instead of claiming foreign tax credits applies to all foreign taxes paid or accrued in the taxable year. The rules applicable to foreign tax credits are complex. Prospective investors should consult their tax advisors regarding the implications of the foreign tax credit provisions for them, in light of their particular situation.
The gross amount of any dividend paid in foreign currency will be included in the gross income of a U.S. holder in an amount equal to the U.S. dollar value of the foreign currency calculated by reference to the exchange rate in effect on the date the dividend distribution is includable in the U.S. holder’s income, regardless of whether the payment is in fact converted into U.S. dollars. If the foreign currency is converted into U.S. dollars on the date of receipt by the depositary, a U.S. holder generally should not be required to recognize foreign currency gain or loss in respect of the dividend. If the foreign currency received is not converted into U.S. dollars on the date of receipt, a U.S. holder will have a basis in the foreign currency equal to its U.S. dollar value on the date of receipt. Any gain or loss on a subsequent conversion or other disposition of the foreign currency will be treated as ordinary income or loss, and will generally be income or loss from sources within the United States for foreign tax credit limitation purposes.
Sales or Other Taxable Dispositions
A U.S. holder will generally recognize a gain or loss for U.S. federal income tax purposes upon the sale or other disposition of ADSs in an amount equal to the difference between the U.S.
242
Table of Contents
dollar value of the amount realized from such sale or other disposition and the U.S. holder’s tax basis in such ADSs. Subject to the discussion below under “—PFIC Rules,” such gain or loss generally will be capital gain or loss. Capital gains of individuals and certain other non-corporate U.S. holders recognized on the sale or other disposition of ADSs held for more than one year are generally eligible for a reduced rate of taxation. The gain or loss will generally be income or loss from sources within the United States for foreign tax credit limitation purposes. The deductibility of capital losses is subject to limitations.
A U.S. holder’s adjusted tax basis in the ADSs will generally equal the U.S. dollar value of the purchase price for the ADSs, based on the prevailing exchange rate on the date of such purchase. The amount realized on a disposition of the ADSs in exchange for foreign currency, will generally equal the U.S. dollar value of such currency translated at the spot exchange rate in effect on the date of the disposition. If, however, the ADSs are treated as traded on an “established securities market” for U.S. federal income tax purposes, a cash basis U.S. holder (or, if it elects, an accrual basis U.S. holder) will determine the U.S. dollar value of the purchase price for the ADSs or the amount realized on a disposition of the ADSs in exchange for non-U.S. currency, as the case may be, by translating the amount paid or received at the spot exchange rate in effect on the settlement date of the purchase or disposition, as the case may be. Any such election by an accrual basis U.S. holder must be applied consistently from year to year and cannot be changed without the consent of the IRS. A U.S. holder’s tax basis in any non-U.S. currency received on a disposition of the ADSs will generally equal the U.S. dollar value of such currency on the date of receipt. Any gain or loss realized by a U.S. holder on a subsequent conversion or other disposition of the non-U.S. dollar currency will generally be foreign currency gain or loss and treated as U.S. source ordinary income or loss. U.S. holders should consult their tax advisors regarding the sale or other taxable disposition of the ADSs under their particular circumstances.
PFIC Rules
A non-U.S. corporation will be classified as a passive foreign investment company, or PFIC, for U.S. federal income tax purposes for any taxable year, if either (i) 75% or more of its gross income for such year consists of certain types of “passive” income or (ii) 50% or more of the value of its assets (determined on the basis of a quarterly average) during such year produce or are held for the production of passive income. Passive income generally includes dividends, interest, royalties, rents, annuities, net gains from the sale or exchange of property producing such income and net foreign currency gains. In addition, a non-U.S. corporation will be treated as owning its proportionate share of the assets and earning its proportionate share of the income of any other corporation in which it owns, directly or indirectly, more than 25% (by value) of the stock.
Based on certain estimates of our gross income and gross assets, the latter determined by reference to the expected value of the ADSs and shares, we believe that we will not be classified as a PFIC for the taxable year ending December 31, 2018 and we do not expect to be treated as a PFIC in any future taxable year. However, because PFIC status is based on our income, assets and activities for the entire taxable year, (including goodwill, which is based on the market value of our shares and ADSs and is subject to change) which we expect may vary substantially over time, it is not possible to determine whether we will be characterized as a PFIC for any taxable year until after the close of the taxable year. Moreover, we must determine our PFIC status annually based on tests that are factual in nature, and our status in future years will
243
Table of Contents
depend on our income, assets and activities in each of those years. There can be no assurance that we will not be considered a PFIC for any taxable year.
If we are classified as a PFIC for any taxable year during which a U.S. Holder (as defined below) holds ADSs, unless the U.S. Holder makes a “mark-to-market” election (as described below), the U.S. Holder will generally be subject to special tax rules that have a generally penalizing effect, regardless of whether we remain a PFIC, on (i) any excess distribution that we make to the U.S. Holder (which generally means any distribution paid during a taxable year to a U.S. Holder that is greater than 125% of the average annual distributions paid in the three preceding taxable years or, if shorter, the U.S. Holder’s holding period for its ADSs), and (ii) any gain realized on the sale or other disposition of its ADSs.
If we are a PFIC for any taxable year during which a U.S. Holder holds ADSs and any of our subsidiaries is also a PFIC, such U.S. Holder will be treated as owning a proportionate amount (by value) of the shares of the lower-tier PFIC. U.S. Holders should consult their tax advisors regarding the application of the PFIC rules to any of our subsidiaries.
If we were to be classified as a PFIC, a U.S. Holder may make a mark-to-market election with respect to its ADSs provided the ADSs are treated as regularly traded on a qualified exchange or other market as defined in applicable Regulations. Because, as a technical matter, a mark-to-market election cannot be made for any lower-tier PFICs that we may own, however, a U.S. Holder may continue to be subject to the PFIC rules with respect to such holder’s indirect interest in any investments held by us that are treated as an equity interest in a PFIC for U.S. federal income tax purposes. U.S. Holders should consult their tax advisors regarding the potential availability and consequences of a mark-to-market election in case we are classified as a PFIC in any taxable year.
We do not intend to make available the information necessary for a U.S. Holder to make a “qualified electing fund” election.
If a U.S. Holder holds ADSs in any year in which we are treated as a PFIC with respect to such U.S. Holder, such U.S. Holder will generally be required to file IRS Form 8621 and such other forms as may be required by the U.S. Treasury Department.
U.S. holders should consult their own tax advisors regarding the application of the PFIC rules to their investment in our ADSs and the elections discussed above.
Information with Respect to Foreign Financial Assets
Owners of “specified foreign financial assets” with an aggregate value in excess of $50,000 (and in some circumstances, a higher threshold) may be required to file IRS Form 8938 (Statement of Specified Foreign Financial Assets) with respect to such assets on their tax returns. “Specified foreign financial assets” may include financial accounts maintained by foreign financial institutions, as well as any of the following, if they are held for investment and not held in accounts maintained by financial institutions: (i) stocks and securities issued by non-U.S. persons, (ii) financial instruments and contracts held for investment that have non-U.S. issuers or counterparties, and (iii) interests in foreign entities. U.S. holders are urged to consult their tax advisors regarding the application of these rules to their ownership of the ADSs.
244
Table of Contents
Withholding on Foreign Accounts
Legislation commonly referred to as “FATCA” generally imposes a withholding tax of 30% in certain circumstances on certain “withholdable payments” and, in the future, may impose such withholding on “foreign passthru payments” made by a “foreign financial institution” (each as defined in the Code) that has entered into an agreement with the IRS to perform certain diligence and reporting obligations with respect to the foreign financial institution’s U.S.-owned accounts. An intergovernmental agreement between the United States and the non-U.S. entity’s jurisdiction may modify these requirements. If we are treated as a “foreign financial institution” under a relevant intergovernmental agreement, we would be subject to these diligence, withholding and reporting obligations under FATCA. It is not yet clear how foreign passthru payments will be addressed under FATCA. Non-U.S. holders, and U.S. holders holding ADSs through a non-U.S. intermediary, should consult their tax advisors regarding the potential application of FATCA to the ADSs.
245
Table of Contents
SERVICE OF PROCESS AND ENFORCEMENT OF CIVIL LIABILITIES
We are organized as a German stock corporation (Aktiengesellschaft) and our registered offices and most of our assets are located outside of the United States. In addition, all of the members of our management board, five out of six supervisory board members, and the experts named herein are residents of Germany or jurisdictions other than the United States. As a result, it may not be possible for you to effect service of process within the United States upon these individuals or upon MorphoSys AG or to enforce judgments obtained in U.S. courts based on the civil liability provisions of the U.S. securities laws against MorphoSys AG in the United States. Awards of punitive damages in actions brought in the United States or elsewhere are generally not enforceable in Germany. In addition, actions brought in a German court against MorphoSys AG or the members of its management board and supervisory board, and the experts named herein to enforce liabilities based on U.S. federal securities laws may be subject to certain restrictions; in particular, German courts generally do not award punitive damages. Litigation in Germany is also subject to rules of procedure that differ from the U.S. rules, including with respect to the taking and admissibility of evidence, the conduct of the proceedings and the allocation of costs. Proceedings in Germany would have to be conducted in the German language, and all documents submitted to the court would, in principle, have to be translated into German. For these reasons, it may be difficult for a U.S. investor to bring an original action in a German court predicated upon the civil liability provisions of the U.S. federal securities laws against us, the members of our management board, supervisory board and the experts named in this prospectus. In addition, even if a judgment against our company, members of our management board, supervisory board, or the experts named in this prospectus based on the civil liability provisions of the U.S. federal securities laws is obtained, a U.S. investor may not be able to enforce it in U.S. or German courts.
246
Table of Contents
We are offering the ADSs described in the prospectus through a number of underwriters. Goldman Sachs & Co. LLC, J.P. Morgan Securities LLC and Leerink Partners LLC are acting as representatives of the underwriters. We have entered into an underwriting agreement with each of the underwriters. Subject to the terms and conditions of the underwriting agreement, we have agreed to sell to the underwriters, and each underwriter has severally agreed to purchase, at the public offering price less the underwriting discounts and commissions set forth on the cover page of this prospectus, the number of ADSs listed next to its name in the following table.
| Name | Number of ADSs |
|||
| Goldman Sachs & Co. LLC |
3,050,250 | |||
| J.P. Morgan Securities LLC |
3,050,250 | |||
| Leerink Partners LLC |
1,701,500 | |||
| JMP Securities LLC |
332,000 | |||
| Berenberg Capital Markets, LLC |
166,000 | |||
|
|
|
|||
| Total |
8,300,000 | |||
The underwriters are committed to purchase all the ADSs offered by us if they purchase any ADSs. The underwriting agreement also provides that if an underwriter defaults, the purchase commitments of non-defaulting underwriters may also be increased or the offering may be terminated.
The underwriters propose to offer the ADSs directly to the public at the initial public offering price set forth on the cover page of this prospectus and to certain dealers at that price less a concession not in excess of $1.05 per ADS. Any such dealers may resell shares to certain other brokers or dealers at a discount of up to $1.05 per ADS from the initial public offering price. The offering of the ADSs by the underwriters is subject to receipt and acceptance and subject to the underwriters’ right to reject any order in whole or in part. After the initial offering of the shares to the public, the offering price and other selling terms may be changed by the underwriters. Sales of shares made outside of the United States may be made by affiliates of the underwriters.
The underwriters have an option to buy up to 1,245,000 additional ADSs from us to cover sales of shares by the underwriters that exceed the number of shares specified in the table above. The underwriters have 30 days from the date of this prospectus to exercise this option to purchase additional ADSs. If any ADSs are purchased with this option to purchase additional ADSs, the underwriters will purchase ADSs in approximately the same proportion as shown in the table above. If any additional ADSs are purchased, the underwriters will offer the additional ADSs on the same terms as those on which the ADSs are being offered.
For reasons of German law, Goldman Sachs AG will initially subscribe for all of the new ordinary shares represented by the ADSs on behalf of the underwriters, at an issue price of €1.00 per share. This issue price will be credited against the amount due from the underwriters at closing. If the underwriters exercise their option to buy additional ADSs, Goldman Sachs AG will initially subscribe for the new ordinary shares representing such additional ADS at an issue price equal to the price to the public less underwriting discounts and commissions.
247
Table of Contents
The underwriting fee is equal to the public offering price per ADS less the amount paid by the underwriters to us per ADS. The underwriting fee is $1.75 per ADS. The following table shows the per ADS and total underwriting discounts and commissions to be paid to the underwriters assuming both no exercise and full exercise of the underwriters’ option to purchase additional ADSs.
| Without option to purchase additional ADSs exercised |
With full option to purchase additional ADSs exercised |
|||||||
| Per ADS |
$ | 1.7528 | $ | 1.7528 | ||||
| Total |
$ | 14,548,240 | $ | 16,730,476 | ||||
We estimate that the total expenses of this offering, including registration, filing and listing fees, printing fees and legal and accounting expenses, but excluding the underwriting discounts and commissions, will be approximately $3,346,400. We have also agreed to reimburse the underwriters for certain of their expenses in an amount of up to $35,000.
A prospectus in electronic format may be made available on the websites maintained by one or more underwriters, or selling group members, if any, participating in the offering. The underwriters may agree to allocate a number of shares to underwriters and selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the representatives to underwriters and selling group members that may make Internet distributions on the same basis as other allocations.
We have agreed that, subject to certain exceptions, we will not (i) offer, pledge, announce the intention to sell, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase or otherwise dispose of, directly or indirectly, or file with the Securities and Exchange Commission a registration statement under the Securities Act relating to, any of our ordinary shares or ADSs or securities convertible into or exchangeable or exercisable for any of our ordinary shares or ADSs, or publicly disclose the intention to make any offer, sale, pledge, disposition or filing, or (ii) enter into any swap or other arrangement that transfers all or a portion of the economic consequences associated with the ownership of any ADSs or ordinary shares or any such other securities (regardless of whether any of these transactions are to be settled by the delivery of ADSs, ordinary shares or such other securities, in cash or otherwise), in each case without the prior written consent of Goldman Sachs & Co. LLC, J.P. Morgan Securities LLC and Leerink Partners LLC for a period of 90 days after the date of this prospectus.
The members of our management board have entered into lock-up agreements with the underwriters prior to the commencement of this offering pursuant to which each of these persons or entities, with limited exceptions, for a period of 90 days after the date of this prospectus, may not, without the prior written consent of Goldman Sachs & Co. LLC, J.P. Morgan Securities LLC and Leerink Partners LLC, (1) offer, pledge, announce the intention to sell, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwise transfer or dispose of, directly or
248
Table of Contents
indirectly, any of our ADSs, ordinary shares or any securities convertible into or exercisable or exchangeable for our ADSs or ordinary shares (including, without limitation, ADSs, ordinary shares or such other securities which may be deemed to be beneficially owned by such directors, executive officers, managers and members in accordance with the rules and regulations of the SEC and securities which may be issued upon exercise of a stock option or warrant) or (2) enter into any swap or other agreement that transfers, in whole or in part, any of the economic consequences of ownership of the ADSs, ordinary shares or such other securities, whether any such transaction described in clause (1) or (2) above is to be settled by delivery of our ADSs, ordinary shares or such other securities, in cash or otherwise, or (3) make any demand for or exercise any right with respect to the registration of any of our ADSs, ordinary shares or any security convertible into or exercisable or exchangeable for our ADSs or ordinary shares.
We have agreed to indemnify the several underwriters against certain liabilities, including liabilities under the Securities Act.
Our ADSs have been approved for listing on the Nasdaq Global Market under the symbol “MOR”.
In connection with this offering, the underwriters may engage in stabilizing transactions, which involves making bids for, purchasing and selling ordinary shares in the open market for the purpose of preventing or retarding a decline in the market price of the ordinary shares while this offering is in progress. These stabilizing transactions may include making short sales of the ADSs, which involves the sale by the underwriters of a greater number of ADSs than they are required to purchase in this offering, and purchasing ADSs on the open market to cover positions created by short sales. Short sales may be “covered” shorts, which are short positions in an amount not greater than the underwriters’ option to purchase additional ADSs referred to above, or may be “naked” shorts, which are short positions in excess of that amount. The underwriters may close out any covered short position either by exercising their option to purchase additional ADSs, in whole or in part, or by purchasing ADSs in the open market. In making this determination, the underwriters will consider, among other things, the price of ADSs available for purchase in the open market compared to the price at which the underwriters may purchase ADSs through the option to purchase additional ADSs. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the ADSs in the open market that could adversely affect investors who purchase in this offering. To the extent that the underwriters create a naked short position, they will purchase ADSs in the open market to cover the position.
The underwriters have advised us that, pursuant to Regulation M of the Securities Act, they may also engage in other activities that stabilize, maintain or otherwise affect the price of the ADSs, including the imposition of penalty bids. This means that if the representatives of the underwriters purchase ADSs in the open market in stabilizing transactions or to cover short sales, the representatives can require the underwriters that sold those shares as part of this offering to repay the underwriting discount received by them.
These activities may have the effect of raising or maintaining the market price of the ADSs or preventing or retarding a decline in the market price of the ADSs, and, as a result, the price of the ordinary shares may be higher than the price that otherwise might exist in the open market. If the underwriters commence these activities, they may discontinue them at any time. The underwriters may carry out these transactions on the Nasdaq Global Market, in the over-the-counter market or otherwise.
249
Table of Contents
Prior to this offering, there has been no public market in the United States for our ADSs. Our ordinary shares are listed on the Frankfurt Stock Exchange (Frankfurter Wertpapierbörse) under the symbol “MOR”.
Neither we nor the underwriters can assure investors that an active trading market will develop in the United States for our ADSs, or that the ADSs will trade in such public market at or above the initial public offering price.
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
Notice to Prospective Investors in the European Economic Area
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (each, a “Relevant Member State”), with effect from and including the date on which the Prospectus Directive is implemented in that Relevant Member State, no offer of ADSs may be made to the public in that Relevant Member State other than:
| · | to any legal entity which is a qualified investor as defined in the Prospectus Directive; |
| · | to fewer than 150 natural or legal persons (other than qualified investors as defined in the Prospectus Directive), subject to obtaining the prior consent of representatives; or |
| · | in any other circumstances falling within Article 3(2) of the Prospectus Directive, |
provided that no such offer of ADSs shall require the Company or any underwriter to publish a prospectus pursuant to Article 3 of the Prospectus Directive or supplement a prospectus pursuant to Article 16 of the Prospectus Directive and each person who initially acquires any ADSs or to whom any offer is made will be deemed to have represented, acknowledged and agreed to and with each of the underwriters and the Company that it is a “qualified investor” within the meaning of the law in that Relevant Member State implementing Article 2(1)(e) of the Prospectus Directive.
In the case of any ADSs being offered to a financial intermediary as that term is used in Article 3(2) of the Prospectus Directive, each such financial intermediary will be deemed to have represented, acknowledged and agreed that the ADSs acquired by it in the offer have not been acquired on a non-discretionary basis on behalf of, nor have they been acquired with a view to their offer or resale to, persons in circumstances which may give rise to an offer of any ADSs to the public other than their offer or resale in a Relevant Member State to qualified investors as so defined or in circumstances in which the prior consent of the representatives has been obtained to each such proposed offer or resale.
250
Table of Contents
For the purposes of this provision, the expression an “offer to the public” in relation to any ADSs in any Relevant Member State means the communication in any form and by means of sufficient information on the terms of the offer and the ADSs to be offered so as to enable an investor to decide to purchase ADSs, as the same may be varied in that Member State by any measure implementing the Prospectus Directive in that Member State, the expression “Prospectus Directive” means Directive 2003/71/EC (as amended, including by Directive 2010/73/EU), and includes any relevant implementing measure in the Relevant Member State.
Notice to Prospective Investors in the United Kingdom
In addition, in the United Kingdom, this document is being distributed only to, and is directed only at, and any offer subsequently made may only be directed at persons who are “qualified investors” (as defined in the Prospectus Directive) (i) who have professional experience in matters relating to investments falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, as amended (the “Order”) and/or (ii) who are high net worth companies (or persons to whom it may otherwise be lawfully communicated) falling within Article 49(2)(a) to (d) of the Order (all such persons together being referred to as “relevant persons”) or otherwise in circumstances which have not resulted and will not result in an offer to the public of the ADSs in the United Kingdom within the meaning of the Financial Services and Markets Act 2000.
Any person in the United Kingdom that is not a relevant person should not act or rely on the information included in this document or use it as basis for taking any action. In the United Kingdom, any investment or investment activity that this document relates to may be made or taken exclusively by relevant persons.
Notice to Prospective Investors in Canada
The ADSs may be sold only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the ADSs must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor.
Pursuant to section 3A.3 of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriters are not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
Notice to Prospective Investors in Switzerland
The ADSs may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (“SIX”) or on any other stock exchange or regulated trading facility in Switzerland.
251
Table of Contents
This document does not constitute a prospectus within the meaning of, and has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering or marketing material relating to the ADSs or the offering may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering or marketing material relating to the offering, the Company, the ADSs have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of ADSs will not be supervised by, the Swiss Financial Market Supervisory Authority FINMA (FINMA), and the offer of ADSs has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes (“CISA”). The investor protection afforded to acquirers of interests in collective investment schemes under the CISA does not extend to acquirers of ADSs.
Notice to Prospective Investors in the United Arab Emirates
The ADSs have not been, and are not being, publicly offered, sold, promoted or advertised in the United Arab Emirates (including the Dubai International Financial Centre) other than in compliance with the laws of the United Arab Emirates (and the Dubai International Financial Centre) governing the issue, offering and sale of securities. Further, this prospectus does not constitute a public offer of securities in the United Arab Emirates (including the Dubai International Financial Centre) and is not intended to be a public offer. This prospectus has not been approved by or filed with the Central Bank of the United Arab Emirates, the Securities and Commodities Authority or the Dubai Financial Services Authority.
Notice to Prospective Investors in Australia
This prospectus:
| · | does not constitute a product disclosure document or a prospectus under Chapter 6D.2 of the Corporations Act 2001 (Cth) (the “Corporations Act”); |
| · | has not been, and will not be, lodged with the Australian Securities and Investments Commission (“ASIC”), as a disclosure document for the purposes of the Corporations Act and does not purport to include the information required of a disclosure document under Chapter 6D.2 of the Corporations Act; |
| · | does not constitute or involve a recommendation to acquire, an offer or invitation for issue or sale, an offer or invitation to arrange the issue or sale, or an issue or sale, of interests to a “retail client” (as defined in section 761G of the Corporations Act and applicable regulations) in Australia; and |
| · | may only be provided in Australia to select investors who are able to demonstrate that they fall within one or more of the categories of investors, or Exempt Investors, available under section 708 of the Corporations Act. |
The ADSs may not be directly or indirectly offered for subscription or purchased or sold, and no invitations to subscribe for or buy the ADSs may be issued, and no draft or definitive offering memorandum, advertisement or other offering material relating to any ADSs may be distributed
252
Table of Contents
in Australia, except where disclosure to investors is not required under Chapter 6D of the Corporations Act or is otherwise in compliance with all applicable Australian laws and regulations. By submitting an application for the ADSs, you represent and warrant to us that you are an Exempt Investor.
As any offer of ADSs under this document will be made without disclosure in Australia under Chapter 6D.2 of the Corporations Act, the offer of those securities for resale in Australia within 12 months may, under section 707 of the Corporations Act, require disclosure to investors under Chapter 6D.2 if none of the exemptions in section 708 applies to that resale. By applying for the ADSs you undertake to us that you will not, for a period of 12 months from the date of issue of the ADSs, offer, transfer, assign or otherwise alienate those securities to investors in Australia except in circumstances where disclosure to investors is not required under Chapter 6D.2 of the Corporations Act or where a compliant disclosure document is prepared and lodged with ASIC.
Notice to Prospective Investors in Japan
The ADSs have not been and will not be registered pursuant to Article 4, Paragraph 1 of the Financial Instruments and Exchange Act. Accordingly, none of the ADSs nor any interest therein may be offered or sold, directly or indirectly, in Japan or to, or for the benefit of, any “resident” of Japan (which term as used herein means any person resident in Japan, including any corporation or other entity organized under the laws of Japan), or to others for re-offering or resale, directly or indirectly, in Japan or to or for the benefit of a resident of Japan, except pursuant to an exemption from the registration requirements of, and otherwise in compliance with, the Financial Instruments and Exchange Act and any other applicable laws, regulations and ministerial guidelines of Japan in effect at the relevant time.
Notice to Prospective Investors in Hong Kong
The ADSs have not been offered or sold and will not be offered or sold in Hong Kong, by means of any document, other than (a) to “professional investors” as defined in the Securities and Futures Ordinance (Cap. 571) of Hong Kong and any rules made under that Ordinance; or (b) in other circumstances which do not result in the document being a “prospectus” as defined in the Companies (Winding Up and Miscellaneous Provisions) Ordinance (Cap. 32) of Hong Kong or which do not constitute an offer to the public within the meaning of that Ordinance. No advertisement, invitation or document relating to the ADSs has been or may be issued or has been or may be in the possession of any person for the purposes of issue, whether in Hong Kong or elsewhere, which is directed at, or the contents of which are likely to be accessed or read by, the public of Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than with respect to ADSs which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” as defined in the Securities and Futures Ordinance and any rules made under that Ordinance.
WARNING
The contents of this document have not been reviewed by any regulatory authority in Hong Kong. You are advised to exercise caution in relation to the offer. If you are in any doubt about any of the contents of this document, you should obtain independent professional advice.
253
Table of Contents
Notice to Prospective Investors in Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of ADSs may not be circulated or distributed, nor may the ADSs be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore (the “SFA”), (ii) to a relevant person pursuant to Section 275(1), or any person pursuant to Section 275(1A), and in accordance with the conditions specified in Section 275 of the SFA, or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
Where the ADSs are subscribed or purchased under Section 275 of the SFA by a relevant person which is:
| · | a corporation (which is not an accredited investor (as defined in Section 4A of the SFA)) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or |
| · | a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary of the trust is an individual who is an accredited investor, |
securities (as defined in Section 239(1) of the SFA) of that corporation or the beneficiaries’ rights and interest (howsoever described) in that trust shall not be transferred within six months after that corporation or that trust has acquired the ADS pursuant to an offer made under Section 275 of the SFA except:
| · | to an institutional investor or to a relevant person defined in Section 275(2) of the SFA, or to any person arising from an offer referred to in Section 275(1A) or Section 276(4)(i)(B) of the SFA; |
| · | where no consideration is or will be given for the transfer; |
| · | where the transfer is by operation of law; |
| · | as specified in Section 276(7) of the SFA; or |
| · | as specified in Regulation 32 of the Securities and Futures (Offers of Investments) (Shares and Debentures) Regulations 2005 of Singapore. |
Notice to Prospective Investors in Bermuda
ADSs may be offered or sold in Bermuda only in compliance with the provisions of the Investment Business Act of 2003 of Bermuda which regulates the sale of securities in Bermuda. Additionally, non-Bermudian persons (including companies) may not carry on or engage in any trade or business in Bermuda unless such persons are permitted to do so under applicable Bermuda legislation.
Notice to Prospective Investors in Saudi Arabia
This prospectus may not be distributed in the Kingdom of Saudi Arabia except to such persons as are permitted under the Offers of Securities Regulations as issued by the board of the Saudi
254
Table of Contents
Arabian Capital Market Authority (“CMA”) pursuant to resolution number 2-11-2004 dated 4 October 2004 as amended by resolution number 1-28-2008, as amended (the “CMA Regulations”). The CMA does not make any representation as to the accuracy or completeness of this prospectus and expressly disclaims any liability whatsoever for any loss arising from, or incurred in reliance upon, any part of this prospectus. Prospective purchasers of the securities offered hereby should conduct their own due diligence on the accuracy of the information relating to the securities. If you do not understand the contents of this document, you should consult an authorized financial adviser.
Notice to Prospective Investors in the British Virgin Islands
The ADSs are not being, and may not be offered to the public or to any person in the British Virgin Islands for purchase or subscription by or on behalf of MorphoSys AG. The ADSs may be offered to companies incorporated under the BVI Business Companies Act, 2004 (British Virgin Islands),“BVI Companies”), but only where the offer will be made to, and received by, the relevant BVI Company entirely outside of the British Virgin Islands.
This prospectus has not been, and will not be, registered with the Financial Services Commission of the British Virgin Islands. No registered prospectus has been or will be prepared in respect of the ADSs for the purposes of the Securities and Investment Business Act, 2010 (“SIBA”) or the Public Issuers Code of the British Virgin Islands.
Notice to Prospective Investors in China
This prospectus does not constitute a public offer of ADSs, whether by sale or subscription, in the People’s Republic of China (the “PRC”). The ADSs are not being offered or sold directly or indirectly in the PRC to or for the benefit of, legal or natural persons of the PRC.
Further, no legal or natural persons of the PRC may directly or indirectly purchase any of the ADSs without obtaining all prior PRC’s governmental approvals that are required, whether statutorily or otherwise. Persons who come into possession of this document are required by the issuer and its representatives to observe these restrictions.
Notice to Prospective Investors in Korea
The ADSs have not been and will not be registered under the Financial Investments Services and Capital Markets Act of Korea and the decrees and regulations thereunder (the “FSCMA”), and the ADSs have been and will be offered in Korea as a private placement under the FSCMA. None of the ADSs may be offered, sold or delivered directly or indirectly, or offered or sold to any person for re-offering or resale, directly or indirectly, in Korea or to any resident of Korea except pursuant to the applicable laws and regulations of Korea, including the FSCMA and the Foreign Exchange Transaction Law of Korea and the decrees and regulations thereunder (the “FETL”). Furthermore, the purchaser of the ADSs shall comply with all applicable regulatory requirements (including but not limited to requirements under the FETL) in connection with the purchase of the ADSs. By the purchase of the ADSs, the relevant holder thereof will be deemed to represent and warrant that if it is in Korea or is a resident of Korea, it purchased the ADSs pursuant to the applicable laws and regulations of Korea.
255
Table of Contents
Notice to Prospective Investors in Malaysia
No prospectus or other offering material or document in connection with the offer and sale of the ADSs has been or will be registered with the Securities Commission of Malaysia (“Commission”) for the Commission’s approval pursuant to the Capital Markets and Services Act 2007. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the ADSs may not be circulated or distributed, nor may the ADSs be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Malaysia other than (i) a closed end fund approved by the Commission; (ii) a holder of a Capital Markets Services Licence; (iii) a person who acquires the ADSs, as principal, if the offer is on terms that the ADSs may only be acquired at a consideration of not less than RM 250,000 (or its equivalent in foreign currencies) for each transaction; (iv) an individual whose total net personal assets or total net joint assets with his or her spouse exceeds RM 3 million (or its equivalent in foreign currencies), excluding the value of the primary residence of the individual; (v) an individual who has a gross annual income exceeding RM 300,000 (or its equivalent in foreign currencies) per annum in the preceding twelve months; (vi) an individual who, jointly with his or her spouse, has a gross annual income of RM 400,000 (or its equivalent in foreign currencies), per annum in the preceding twelve months; (vii) a corporation with total net assets exceeding RM 10 million (or its equivalent in a foreign currencies) based on the last audited accounts; (viii) a partnership with total net assets exceeding RM 10 million (or its equivalent in foreign currencies); (ix) a bank licensee or insurance licensee as defined in the Labuan Financial Services and Securities Act 2010; (x) an Islamic bank licensee or takaful licensee as defined in the Labuan Financial Services and Securities Act 2010; and (xi) any other person as may be specified by the Commission; provided that, in the each of the preceding categories (i) to (xi), the distribution of the ADSs is made by a holder of a Capital Markets Services Licence who carries on the business of dealing in securities. The distribution in Malaysia of this prospectus is subject to Malaysian laws. This prospectus does not constitute and may not be used for the purpose of public offering or an issue, offer for subscription or purchase, invitation to subscribe for or purchase any securities requiring the registration of a prospectus with the Commission under the Capital Markets and Services Act 2007.
Notice to Prospective Investors in Taiwan
The ADSs have not been and will not be registered with the Financial Supervisory Commission of Taiwan pursuant to relevant securities laws and regulations and may not be sold, issued or offered within Taiwan through a public offering or in circumstances which constitutes an offer within the meaning of the Securities and Exchange Act of Taiwan that requires a registration or approval of the Financial Supervisory Commission of Taiwan. No person or entity in Taiwan has been authorized to offer, sell, give advice regarding or otherwise intermediate the offering and sale of the ADSs in Taiwan.
256
Table of Contents
Notice to Prospective Investors in South Africa
Due to restrictions under the securities laws of South Africa, the ADSs are not offered, and the offer shall not be transferred, sold, renounced or delivered, in South Africa or to a person with an address in South Africa, unless one or other of the following exemptions applies:
| · | the offer, transfer, sale, renunciation or delivery is to: |
| (a) | persons whose ordinary business is to deal in securities, as principal or agent; |
| (b) | the South African Public Investment Corporation; |
| (c) | persons or entities regulated by the Reserve Bank of South Africa; |
| (d) | authorized financial service providers under South African law; |
| (e) | financial institutions recognized as such under South African law; |
| (f) | a wholly-owned subsidiary of any person or entity contemplated in (c), (d) or (e), acting as agent in the capacity of an authorized portfolio manager for a pension fund or collective investment scheme (in each case duly registered as such under South African law); or |
| (g) | any combination of the person referred to in (a) to (f); or |
| · | the total contemplated acquisition cost of the securities, for any single addressee acting as principal is equal to or greater than ZAR1,000,000. |
No “offer to the public” (as such term is defined in the South African Companies Act, No. 71 of 2008 (as amended or re-enacted) (the “South African Companies Act”)) in South Africa is being made in connection with the issue of the ADSs. Accordingly, this prospectus does not, nor is it intended to, constitute a “registered prospectus” (as that term is defined in the South African Companies Act) prepared and registered under the South African Companies Act and has not been approved by, and/or filed with, the South African Companies and Intellectual Property Commission or any other regulatory authority in South Africa. Any issue or offering of the ADSs in South Africa constitutes an offer of the ADSs in South Africa for subscription or sale in South Africa only to persons who fall within the exemption from “offers to the public” set out in section 96(1)(a) of the South African Companies Act. Accordingly, this document must not be acted on or relied on by persons in South Africa who do not fall within section 96(1)(a) of the South African Companies Act (such persons being referred to as “SA Relevant Persons”). Any investment or investment activity to which this prospectus relates is available in South Africa only to SA Relevant Persons and will be engaged in South Africa only with SA relevant persons.
No South African residents or offshore subsidiary of a South African resident may subscribe for or purchase any of the ADSs or beneficially own or hold any of the ADSs unless specific approval has been obtained from the financial surveillance department of the South African Reserve Bank (the “SARB”) by such persons or such subscription, purchase or beneficial holding or ownership is otherwise permitted under the South African Exchange Control Regulations or the rulings promulgated thereunder (including, without limitation, the rulings issued by the SARB providing for foreign investment allowances applicable to persons who are residents of South Africa under the applicable exchange control laws of South Africa).
257
Table of Contents
Notice to Prospective Investors in Germany
The ADSs may be offered and sold in the Federal Republic of Germany only in compliance with the German Securities Prospectus Act (Wertpapierprospektgesetz), as amended, Commission Regulation No. (EC) 809/2004 of April 29, 2004, as amended, or any other laws applicable in Germany governing the issue, offering and sale of securities. This prospectus has not been approved under the German Securities Prospectus Act or the Prospectus Directive and, accordingly, the ADSs may not be offered publicly in the Federal Republic of Germany. The ADSs will be offered in the Federal Republic of Germany in reliance on an exemption from the requirement to publish an approved securities prospectus under the German Securities Prospectus Act. Any resale of the ADSs in Germany may only be made in accordance with the German Securities Prospectus Act and other applicable laws. The Company has not filed, and does not intend to file, a securities prospectus with the German Federal Financial Supervisory Authority (Bundesanstalt für Finanzdienstleistungsaufsicht) or obtain a notification to BaFin from another competent authority of a member state of the European Economic Area, with which a securities prospectus may have been filed, pursuant to Section 17 Para. 3 of the German Securities Prospectus Act.
Other relationships
The underwriters and their respective affiliates are full service financial institutions engaged in various activities, which may include sales and trading, commercial and investment banking, advisory, investment management, investment research, principal investment, hedging, market making, brokerage and other financial and non-financial activities and services. Certain of the underwriters and their affiliates have provided in the past to us and our affiliates, and may provide from time to time in the future, certain commercial banking, financial advisory, investment banking, lending and other services for us and such affiliates in the ordinary course of their business, for which they have received and may continue to receive customary fees and commissions.
In addition, in the ordinary course of their various business activities, the underwriters and their respective affiliates, officers, directors and employees may purchase, sell or hold a broad array of investments and actively trade securities, derivatives, loans, commodities, currencies, credit default swaps and other financial instruments for their own account and for the accounts of their customers. Therefore certain of the underwriters and their affiliates may from time to time effect transactions for their own account or the account of customers, and hold on behalf of themselves or their customers, long or short positions in our debt or equity securities or loans (directly, as collateral securing other obligations or otherwise) and/or persons and entities with relationships with us. The underwriters and their respective affiliates may also communicate independent investment recommendations, market color or trading ideas and/or publish or express independent research views in respect of such assets, securities or instruments and may at any time hold, or recommend to clients that they should acquire, long and/or short positions in such assets, securities and instruments.
258
Table of Contents
EXPENSES RELATED TO THIS OFFERING
The following table sets forth the main expenses we will be required to pay in connection with this offering, other than underwriting discounts and commissions. All amounts in the table below are estimates, except the SEC registration fee, the FINRA filing fee and the Nasdaq listing fee. We will pay all of the expenses of this offering. Expenses incurred or estimated in euros have been calculated at the rate of €1.00 per $1.2234, which equals the rate published by the European Central Bank for the euro on April 6, 2018.
| Expenses | Amount | |||
| SEC registration fee |
$ | 28,664 | ||
| FINRA filing fee |
35,033 | |||
| Nasdaq listing fee |
125,000 | |||
| Legal fees and expenses |
1,223,400 | |||
| Accounting fees and expenses |
1,052,124 | |||
| Corporate advisory fees and expenses |
0 | |||
| Printing fees |
350,000 | |||
| Depositary expenses |
0 | |||
| Other fees and expenses |
532,179 | |||
| Total |
$ | 3,346,400 | ||
259
Table of Contents
The validity of the shares and the ADSs with respect to German and U.S. federal law and New York state law in connection with this offering will be passed upon for us by Skadden, Arps, Slate, Meagher & Flom LLP, our German and U.S. counsel. Certain legal matters with respect to German and U.S. federal law and New York state law in connection with this offering will be passed upon for the underwriters by Latham & Watkins LLP, German and U.S. counsel for the underwriters.
260
Table of Contents
The consolidated financial statements as of December 31, 2016 and 2017 and for each of the two years in the period ended December 31, 2017 included in this prospectus have been so included in reliance on the report of PricewaterhouseCoopers GmbH Wirtschaftsprüfungsgesellschaft, an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting. PricewaterhouseCoopers GmbH Wirtschaftsprüfungsgesellschaft is a member of the Chamber of Public Accountants (Wirtschaftsprüferkammer), Berlin, Germany.
261
Table of Contents
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form F-1 under the Securities Act, including amendments and relevant exhibits and schedules, covering the underlying ordinary shares represented by the ADSs to be sold in this offering. The ADS depositary has also filed with the SEC a related registration statement on Form F-6 to register the ADSs. This prospectus, which constitutes a part of the registration statement on Form F-1, summarizes material provisions of contracts and other documents included in the Registration Statement. Since this prospectus does not contain all of the information contained in the registration statement on Form F-1, you should read the registration statement on Form F-1 and its exhibits and schedules for further information with respect to us and our ADSs.
Immediately upon the effectiveness of the registration statement on Form F-1, we will become subject to periodic reporting and other informational requirements of the Exchange Act as applicable to foreign private issuers. Our annual reports on Form 20-F for the year ended December 31, 2018 and for all subsequent years will be due within four months after fiscal year-end in accordance with applicable SEC rules. We are not required to disclose certain other information that is required from U.S. domestic issuers. Also, as a foreign private issuer, we are exempt from the rules of the Exchange Act prescribing the furnishing of proxy statements to shareholders and members of our management and supervisory boards and our principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act.
You may review and copy the registration statement on Form F-1, reports and other information we file at the SEC’s public reference room at 100 F Street, N.E., Washington, D.C. 20549. You may also request copies of these documents upon payment of a duplicating fee by writing to the SEC. For further information on the public reference facility, please call the SEC at 1-800-SEC-0330. Our SEC filings, including the registration statement on Form F-1, are also available to you on the SEC’s website at http://www.sec.gov.
As a foreign private issuer, we are also exempt from the requirements of Regulation FD (Fair Disclosure) which, generally, are meant to ensure that select groups of investors are not privy to specific information about an issuer before other investors. We are, however, still subject to the anti-fraud and anti-manipulation rules of the SEC, such as Rule 10b-5 of the Exchange Act. Since many of the disclosure obligations required of us as a foreign private issuer are different than those required by other U.S. domestic reporting companies, our shareholders, potential shareholders and the investing public in general should not expect to receive information about us in the same amount and at the same time as information is received from, or provided by, U.S. domestic reporting companies. We are liable for violations of the rules and regulations of the SEC that apply to us as a foreign private issuer.
262
Table of Contents
Table of Contents
Report of Independent Registered Public Accounting Firm
To the Supervisory Board and Stockholders of MorphoSys AG
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of MorphoSys AG and its subsidiaries as of December 31, 2017 and 2016, and the related consolidated statements of income, comprehensive income, changes in stockholders’ equity and cash flows for each of the two years in the period ended December 31, 2017, including the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2017 and 2016, and the results of their operations and their cash flows for each of the two years in the period ended December 31, 2017 in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits of these consolidated financial statements in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Munich, Germany
March 14, 2018
PricewaterhouseCoopers GmbH
Wirtschaftsprüfungsgesellschaft
| /s/ Dietmar Eglauer | /s/ Holger Lutz | |
| Wirtschaftsprüfer | Wirtschaftsprüfer | |
| (German Public Auditor) | (German Public Auditor) |
We have served as the Company’s auditor since 2011.
F-2
Table of Contents
MorphoSys Group: Consolidated Financial Statements
F-3
Table of Contents
F-4
Table of Contents
F-5
Table of Contents
Consolidated Statement of Income (IFRS)
| in € | Note | 2017 | 2016 | |||||||||
| Revenues |
2.7.1, 4.1 | 66,790,840 | 49,743,515 | |||||||||
| Operating Expenses |
||||||||||||
| Research and Development |
2.7.2, 4.2.1 | (116,808,575 | ) | (95,723,069 | ) | |||||||
| General and Administrative |
2.7.2, 4.2.2 | (17,038,720 | ) | (14,116,085 | ) | |||||||
|
|
|
|||||||||||
| Total Operating Expenses |
(133,847,295 | ) | (109,839,154 | ) | ||||||||
|
|
|
|||||||||||
| Other Income |
2.7.3, 4.3 | 1,119,598 | 708,571 | |||||||||
| Other Expenses |
2.7.4, 4.3 | (1,670,792 | ) | (553,925 | ) | |||||||
|
|
|
|||||||||||
| Earnings before Interest and Taxes (EBIT) |
3 | (67,607,649 | ) | (59,940,993 | ) | |||||||
|
|
|
|||||||||||
| Finance Income |
2.7.5, 4.3 | 712,397 | 1,385,164 | |||||||||
| Finance Expenses |
2.7.6, 4.3 | (1,894,852 | ) | (1,308,322 | ) | |||||||
| Income Tax Expenses |
2.7.7, 4.4 | (1,036,365 | ) | (518,625 | ) | |||||||
|
|
|
|||||||||||
| Consolidated Net Loss |
(69,826,469 | ) | (60,382,776 | ) | ||||||||
|
|
|
|||||||||||
| Earnings per Share, basic and diluted |
2.7.8, 4.5 | (2.41 | ) | (2.28 | ) | |||||||
| Shares Used in Computing Earnings per Share, basic and diluted |
2.7.8, 4.5 | 28,947,566 | 26,443,415 | |||||||||
The notes are an integral part of these consolidated financial statements.
F-6
Table of Contents
Consolidated Statement of Comprehensive Income (IFRS)1
| in € | 2017 | 2016 | ||||||
| Consolidated Net Loss |
(69,826,469 | ) | (60,382,776 | ) | ||||
|
|
|
|||||||
| Change in Unrealized Gains and Losses on Available-for-sale Financial Assets and Bonds |
||||||||
| (Thereof € 86,685 and € 251,455 for 2017 and 2016, respectively, Reclassifications of realized Gains and Losses to Profit and Loss) |
54,170 | 115,396 | ||||||
| Change of Tax Effects presented in Other Comprehensive Income on Available-for-sale Financial Assets and Bonds |
63,659 | (136,550 | ) | |||||
|
|
|
|||||||
| Change in Unrealized Gains and Losses on Available-for-sale Financial Assets and Bonds, Net of Tax Effects |
117,829 | (21,154 | ) | |||||
|
|
|
|||||||
| Change in Unrealized Gains and Losses on Cash Flow Hedges |
||||||||
| (Thereof € 256,085 and € 0 for 2017 and 2016, respectively, Reclassifications of realized Losses to Profit and Loss) |
(490,164 | ) | 490,164 | |||||
| Change of Tax Effects presented in Other Comprehensive Income on Cash Flow Hedges |
130,751 | (130,751 | ) | |||||
|
|
|
|||||||
| Change in Unrealized Gains and Losses on Cash Flow Hedges, Net of Tax Effects |
(359,413 | ) | 359,413 | |||||
|
|
|
|||||||
| Other Comprehensive Income |
(241,584 | ) | 338,259 | |||||
|
|
|
|||||||
| Total Comprehensive Income |
(70,068,053 | ) | (60,044,517 | ) | ||||
| 1 | In financial years 2017 and 2016, the statement of comprehensive income only comprised components which will be reclassified in terms of IAS 1.82A(b) to profit and loss in subsequent periods when specific conditions are met. |
The notes are an integral part of these consolidated financial statements.
F-7
Table of Contents
Consolidated Balance Sheet (IFRS)
| in € | Note | 12/31/2017 | 12/31/2016 | |||||||||
| ASSETS |
||||||||||||
| Current Assets |
||||||||||||
| Cash and Cash Equivalents |
2.8.1, 5.1 | 76,589,129 | 73,928,661 | |||||||||
| Available-for-sale Financial Assets |
2.8.1, 5.2 | 86,538,195 | 63,361,727 | |||||||||
| Bonds, Available-for-sale |
2.8.1, 5.2 | 0 | 6,532,060 | |||||||||
| Financial Assets classified as Loans and Receivables |
2.8.1, 5.2 | 149,059,254 | 136,108,749 | |||||||||
| Accounts Receivable |
2.8.2, 5.3 | 11,234,308 | 12,596,655 | |||||||||
| Income Tax Receivables |
2.8.2, 5.5 | 654,511 | 519,915 | |||||||||
| Other Receivables |
2.8.2, 5.4 | 84,727 | 656,887 | |||||||||
| Inventories, Net |
2.8.3, 5.5 | 300,753 | 310,366 | |||||||||
| Prepaid Expenses and Other Current Assets |
2.8.4, 5.5 | 16,219,761 | 14,041,469 | |||||||||
|
|
|
|||||||||||
| Total Current Assets |
340,680,638 | 308,056,489 | ||||||||||
|
|
|
|||||||||||
| Non-current Assets |
||||||||||||
| Property, Plant and Equipment, Net |
2.8.5, 5.6 | 3,526,351 | 4,189,108 | |||||||||
| Patents, Net |
2.8.6, 5.7.1 | 4,669,128 | 5,323,341 | |||||||||
| Licenses, Net |
2.8.6, 5.7.2 | 2,999,074 | 3,146,937 | |||||||||
| In-process R&D Programs |
2.8.6, 5.7.3 | 52,158,527 | 50,818,700 | |||||||||
| Software, Net |
2.8.6, 5.7.4 | 655,399 | 1,285,474 | |||||||||
| Goodwill |
2.8.6, 5.7.5 | 7,364,802 | 7,364,802 | |||||||||
| Financial Assets classified as Loans and Receivables, Net of Current Portion |
2.8.1, 5.2 | 0 | 79,521,181 | |||||||||
| Prepaid Expenses and Other Assets, Net of Current Portion |
2.8.7, 5.8 | 3,344,292 | 3,894,085 | |||||||||
| Total Non-current Assets |
74,717,573 | 155,543,628 | ||||||||||
|
|
|
|||||||||||
| Total Assets |
415,398,211 | 463,600,117 | ||||||||||
|
|
|
|||||||||||
The notes are an integral part of these consolidated financial statements.
F-8
Table of Contents
| in € | Note | 12/31/2017 | 12/31/2016 | |||||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY |
||||||||||||
| Current Liabilities |
||||||||||||
| Accounts Payable and Accrued Expenses |
2.9.1, 6.1 | 44,811,718 | 32,222,616 | |||||||||
| Tax Provisions |
2.9.2, 6.2 | 314,944 | 1,652,006 | |||||||||
| Provisions |
2.9.1, 6.2 | 1,185,741 | 3,195,252 | |||||||||
| Current Portion of Deferred Revenue |
2.9.3, 6.3 | 1,388,638 | 1,232,072 | |||||||||
|
|
|
|||||||||||
| Total Current Liabilities |
47,701,041 | 38,301,946 | ||||||||||
|
|
|
|||||||||||
| Non-current Liabilities |
||||||||||||
| Provisions, Net of Current Portion |
2.9.1, 6.2 | 23,166 | 23,166 | |||||||||
| Deferred Revenue, Net of Current Portion |
2.9.4, 6.3 | 306,385 | 1,672,872 | |||||||||
| Convertible Bonds due to Related Parties |
2.9.5 | 87,785 | 218,293 | |||||||||
| Deferred Tax Liability |
2.9.6, 4.4 | 7,811,258 | 7,421,835 | |||||||||
| Other Liabilities, Net of Current Portion |
2.9.7, 6.4 | 797,537 | 501,840 | |||||||||
|
|
|
|||||||||||
| Total Non-current Liabilities |
9,026,131 | 9,838,006 | ||||||||||
|
|
|
|||||||||||
| Total Liabilities |
56,727,172 | 48,139,952 | ||||||||||
| Stockholders’ Equity |
||||||||||||
| Common Stock |
2.9.8, 6.5.1 | 29,420,785 | 29,159,770 | |||||||||
| Ordinary Shares Issued (29,420,785 and 29,159,770 for 2017 and 2016, respectively) |
||||||||||||
| Ordinary Shares Outstanding (29,101,107 and 28,763,760 for 2017 and 2016, respectively) |
||||||||||||
| Treasury Stock (319,678 and 396,010 shares for 2017 and 2016, respectively), at Cost |
2.9.8, 6.5.4 | (11,826,981 | ) | (14,648,212 | ) | |||||||
| Additional Paid-in Capital |
2.9.8, 6.5.5 | 438,557,856 | 428,361,175 | |||||||||
| Revaluation Reserve |
2.9.8, 6.5.6 | (105,483 | ) | 136,101 | ||||||||
| Accumulated Deficit |
2.9.8, 6.5.7 | (97,375,138 | ) | (27,548,669 | ) | |||||||
|
|
|
|||||||||||
| Total Stockholders’ Equity |
358,671,039 | 415,460,165 | ||||||||||
|
|
|
|||||||||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY |
415,398,211 | 463,600,117 | ||||||||||
|
|
|
|||||||||||
The notes are an integral part of these consolidated financial statements.
F-9
Table of Contents
Consolidated Statement of Changes in Stockholders’ Equity (IFRS)
| Note | Common Stock | Treasury Stock | Additional Paid-in Capital |
Revaluation Reserve |
Accumulated Income / (Deficit) |
Total Stockholders’ Equity |
||||||||||||||||||||||||||||||
| Shares | € | Shares | € | € | € | € | € | |||||||||||||||||||||||||||||
| Balance as of January 1, 2016 |
26,537,682 | 26,537,682 | 434,670 | (15,827,946 | ) | 319,394,322 | (202,158 | ) | 32,834,107 | 362,736,007 | ||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Capital Increase, Net of Issuance Cost of € 2,778,652 |
2,622,088 | 2,622,088 | 0 | 0 | 109,971,132 | 0 | 0 | 112,593,220 | ||||||||||||||||||||||||||||
| Compensation Related to the Grant of Convertible Bonds and Performance Shares |
0 | 0 | 0 | 0 | 2,357,418 | 0 | 0 | 2,357,418 | ||||||||||||||||||||||||||||
| Repurchase of Treasury Stock, Net of Bank Fees |
6.5.4 | 0 | 0 | 52,295 | (2,181,963 | ) | 0 | 0 | 0 | (2,181,963 | ) | |||||||||||||||||||||||||
| Transfer of Treasury Stock for Long-Term Incentive Program |
0 | 0 | (90,955 | ) | 3,361,697 | (3,361,697 | ) | 0 | 0 | 0 | ||||||||||||||||||||||||||
| Reserves: |
||||||||||||||||||||||||||||||||||||
| Change in Unrealized Gains and Losses on Available-for-sale Financial Assets and Bonds, Net of Tax Effects |
0 | 0 | 0 | 0 | 0 | (21,154 | ) | 0 | (21,154 | ) | ||||||||||||||||||||||||||
| Change in Unrealized Gains on Cash Flow Hedges, Net of Tax Effects |
0 | 0 | 0 | 0 | 0 | 359,413 | 0 | 359,413 | ||||||||||||||||||||||||||||
| Consolidated Net Loss |
0 | 0 | 0 | 0 | 0 | 0 | (60,382,776 | ) | (60,382,776 | ) | ||||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||||||||||
| Total Comprehensive Income |
0 | 0 | 0 | 0 | 0 | 338,259 | (60,382,776 | ) | (60,044,517 | ) | ||||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||||||||||
| Balance as of December 31, 2016 |
29,159,770 | 29,159,770 | 396,010 | (14,648,212 | ) | 428,361,175 | 136,101 | (27,548,669 | ) | 415,460,165 | ||||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||||||||||
F-10
Table of Contents
| Note | Common Stock | Treasury Stock | Additional Paid-in Capital |
Revaluation Reserve |
Accumulated Income / (Deficit) |
Total Stockholders’ Equity |
||||||||||||||||||||||||||||||
| Shares | € | Shares | € | € | € | € | € | |||||||||||||||||||||||||||||
| Balance as of January 1, 2017 |
29,159,770 | 29,159,770 | 396,010 | (14,648,212 | ) | 428,361,175 | 136,101 | (27,548,669 | ) | 415,460,165 | ||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Compensation Related to the Grant of Stock Options, Convertible Bonds and Performance Shares |
7.1, 7.2, 7.3 | 0 | 0 | 0 | 0 | 4,974,599 | 0 | 0 | 4,974,599 | |||||||||||||||||||||||||||
| Exercise of Convertible Bonds Issued to Related Parties |
7.2, 7.4 | 261,015 | 261,015 | 0 | 0 | 8,043,313 | 0 | 0 | 8,304,328 | |||||||||||||||||||||||||||
| Transfer of Treasury Stock for Long-Term Incentive Program |
7.3.1, 7.4 | 0 | 0 | (61,871 | ) | 2,286,752 | (2,286,752 | ) | 0 | 0 | 0 | |||||||||||||||||||||||||
| Transfer of Treasury Stock to Members of the Management Board |
6.5.1, 7.4 | 0 | 0 | (14,461 | ) | 534,479 | (534,479 | ) | 0 | 0 | 0 | |||||||||||||||||||||||||
| Reserves: |
||||||||||||||||||||||||||||||||||||
| Change in Unrealized Gains and Losses on Available-for-sale Financial Assets and Bonds, Net of Tax Effects |
6.5.6 | 0 | 0 | 0 | 0 | 0 | 117,829 | 0 | 117,829 | |||||||||||||||||||||||||||
| Change in Unrealized Gains and Losses on Cash Flow Hedges, Net of Tax Effects |
6.5.6 | 0 | 0 | 0 | 0 | 0 | (359,413 | ) | 0 | (359,413 | ) | |||||||||||||||||||||||||
| Consolidated Net Loss |
6.5.7 | 0 | 0 | 0 | 0 | 0 | 0 | (69,826,469 | ) | (69,826,469 | ) | |||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||||||||||
| Total Comprehensive Income |
0 | 0 | 0 | 0 | 0 | (241,584 | ) | (69,826,469 | ) | (70,068,053 | ) | |||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||||||||||
| Balance as of December 31, 2017 |
29,420,785 | 29,420,785 | 319,678 | (11,826,981 | ) | 438,557,856 | (105,483 | ) | (97,375,138 | ) | 358,671,039 | |||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||||||||||
The notes are an integral part of these consolidated financial statements.
F-11
Table of Contents
Consolidated Statement of Cash Flows (IFRS)
| in € | Note | 2017 | 2016 | |||||||||
| Operating Activities: |
||||||||||||
| Consolidated Net Loss |
(69,826,469 | ) | (60,382,776 | ) | ||||||||
| Adjustments to Reconcile Net Loss to Net Cash |
||||||||||||
| Impairment of Assets |
5.6, 5.7 | 9,863,582 | 10,141,187 | |||||||||
| Depreciation and Amortization of Tangible and Intangible Assets |
5.6, 5.7 | 4,028,948 | 3,763,813 | |||||||||
| Net (Gain) / Loss on Sales of Available-for-sale Financial Assets |
5.2 | 84,841 | 915,201 | |||||||||
| Proceeds from Derivative Financial Instruments |
5.4 | (589,134 | ) | 725,157 | ||||||||
| Net (Gain) / Loss on Derivative Financial Instruments |
5.4 | 919,042 | (29,879 | ) | ||||||||
| Net (Gain) / Loss on Sale of Property, Plant and Equipment |
11,314 | (4,037 | ) | |||||||||
| Recognition of Deferred Revenue |
6.3 | (19,595,746 | ) | (19,042,772 | ) | |||||||
| Stock-based Compensation |
4.2.3, 7 | 4,974,599 | 2,357,418 | |||||||||
| Income Tax Expenses |
4.4 | 1,036,365 | 518,625 | |||||||||
| Changes in Operating Assets and Liabilities: |
||||||||||||
| Accounts Receivable |
5.3 | 1,362,347 | (1,154,597 | ) | ||||||||
| Prepaid Expenses and Other Assets, Tax Receivables and Other Receivables |
5.4, 5.5 | 1,807,670 | (13,912,263 | ) | ||||||||
| Accounts Payable and Accrued Expenses, Tax Provisions and Provisions |
6.1, 6.2 | 7,819,386 | 13,010,160 | |||||||||
| Other Liabilities |
6.1 | 3,133,558 | (421,492 | ) | ||||||||
| Deferred Revenue |
6.3 | 18,385,824 | 17,440,930 | |||||||||
| Income Taxes Paid |
(1,861,982 | ) | (540,383 | ) | ||||||||
|
|
|
|||||||||||
| Net Cash Provided by / (Used in) Operating Activities |
(38,445,855 | ) | (46,615,708 | ) | ||||||||
|
|
|
|||||||||||
The notes are an integral part of these consolidated financial statements.
F-12
Table of Contents
| in € | Note | 2017 | 2016 | |||||||||
| Investing Activities: |
||||||||||||
| Purchase of Available-for-sale Financial Assets |
5.2 | (56,406,580 | ) | (166,923,795 | ) | |||||||
| Proceeds from Sales of Available-for-sale Financial Assets |
5.2 | 33,231,500 | 167,873,152 | |||||||||
| Proceeds from Sales of Bonds, Available-for-sale |
5.2 | 6,500,000 | 25,770,000 | |||||||||
| Purchase of Financial Assets Classified as Loans and Receivables |
5.2 | (108,000,000 | ) | (256,499,997 | ) | |||||||
| Proceeds from Sales of Financial Assets Classified as Loans and Receivables |
5.2 | 170,498,593 | 149,894,769 | |||||||||
| Purchase of Property, Plant and Equipment |
5.6 | (1,317,058 | ) | (2,502,286 | ) | |||||||
| Proceeds from Disposals of Property, Plant and Equipment |
84 | 5,000 | ||||||||||
| Purchase of Intangible Assets |
5.7 | (11,831,789 | ) | (411,204 | ) | |||||||
| Interest Received |
257,752 | 2,008,325 | ||||||||||
|
|
|
|||||||||||
| Net Cash Provided by / (Used in) Investing Activities |
32,932,502 | (80,786,036 | ) | |||||||||
|
|
|
|||||||||||
| Financing Activities: |
||||||||||||
| Repurchase of Treasury Stock, Net of Bank Fees |
6.5.4 | 0 | (2,181,963 | ) | ||||||||
| Proceeds of Share Issuance |
6.5 | 0 | 115,371,872 | |||||||||
| Cost of Share Issuance |
(15,525 | ) | (2,778,652 | ) | ||||||||
| Proceeds in Connection with Convertible Bonds Granted to Related Parties |
8,189,345 | 0 | ||||||||||
| Outflows in Connection with Convertible Bonds Granted to Related Parties |
0 | (6,707 | ) | |||||||||
| Interest Paid |
0 | (1,819 | ) | |||||||||
|
|
|
|||||||||||
| Net Cash Provided by / (Used in) Financing Activities |
8,173,820 | 110,402,731 | ||||||||||
|
|
|
|||||||||||
| Increase / (Decrease) in Cash and Cash Equivalents |
2,660,467 | (16,999,013 | ) | |||||||||
|
|
|
|||||||||||
| Cash and Cash Equivalents at the Beginning of the Period |
73,928,661 | 90,927,673 | ||||||||||
|
|
|
|||||||||||
| Cash and Cash Equivalents at the End of the Period |
76,589,129 | 73,928,661 | ||||||||||
|
|
|
|||||||||||
The notes are an integral part of these consolidated financial statements.
F-13
Table of Contents
BUSINESS ACTIVITIES AND THE COMPANY
MorphoSys AG (“the Company” or “MorphoSys”) develops and applies technologies for generating therapeutic antibodies. The Company has a broad proprietary portfolio of compounds and a broad pipeline of compounds developed with partners from the pharmaceutical and biotechnology industry. The Group was founded as a German limited liability company in July 1992. In June 1998, MorphoSys became a German stock corporation. In March 1999, the Company completed its initial public offering on Germany’s “Neuer Markt”: the previous segment of the Deutsche Börse designated for high-growth companies. On January 15, 2003, MorphoSys AG was admitted to the Prime Standard segment of the Frankfurt Stock Exchange.
2 Summary of Significant Accounting Policies
| 2.1 | BASIS OF AND CHANGES IN ACCOUNTING STANDARDS |
| 2.1.1 | BASIS OF APPLICATION |
These consolidated financial statements were prepared in accordance with the International Financial Reporting Standards as issued by the International Accounting Standards Board (IASB), (“IFRS”). The statements take into account the recommendations of the International Financial Reporting Standards Interpretations Committee (IFRS IC) and also give consideration to the supplementary German commercial law provisions, applicable in accordance with Sec. 315a Para. 1 of the German Commercial Code (HGB).
These consolidated financial statements as of and for the financial years ended December 31, 2017 and 2016, comprise MorphoSys AG and its subsidiaries (collectively referred to as the “MorphoSys Group” or the “Group”).
In preparing the consolidated financial statements in accordance with IFRS, the Management Board is required to make certain estimates and assumptions, which have an effect on the amounts recognized in the consolidated financial statements and the accompanying notes. The actual results may differ from these estimates. The estimates and the underlying assumptions are subject to continuous review. Any changes in estimates are recognized in the period in which the changes are made and in all relevant future periods.
The consolidated financial statements were prepared in Euro – the functional currency of all entities in the MorphoSys Group. Statements are prepared on the basis of historical cost, except for derivative financial instruments and available-for-sale financial assets, which are recognized at their respective fair value. All figures in this report are rounded to the nearest euro, thousand euros or million euros.
Unless stated otherwise, the accounting policies set out below have been applied consistently to all periods presented in these consolidated financial statements.
| 2.1.2 | CHANGES IN ACCOUNTING POLICIES AND DISCLOSURES |
The accounting principles applied generally correspond to the policies used in the prior year.
F-14
Table of Contents
The following new and revised standards and interpretations were applied for the first time in the financial year.
| Standard / Interpretation | Mandatory application for financial years starting on |
Impact on MorphoSys | ||||
| IAS 7 (A) |
Disclosure Initiative | 01/01/2017 | none | |||
| IAS 12 (A) |
Recognition of Deferred Tax Assets for Unrealised Losses | 01/01/2017 | yes | |||
| Annual Improvements to IFRS Standards 2014–2016 Cycle | 01/01/2017 | none | ||||
| (A) Amendments |
||||||
The impact on the consolidated financial statements of the Amendments to IAS 12 is not deemed to be material.
F-15
Table of Contents
The following new and revised standards and interpretations, which were not yet mandatory for the financial year, were not applied. Standards with the remark “yes” are likely to have an impact on the consolidated financial statements, and their impact is currently being assessed by the Group. Only material impacts will be described in more detail. The impact on the consolidated financial statements of the Amendments to IFRS 2and IFRIC 22 is not expected to be material and is therefore not individually described. Standards with the remark “none” are not likely to have a material impact on the consolidated financial statements.
| Standard / Interpretation | Mandatory application for financial years starting on |
Possible Impact on MorphoSys | ||||||
| IFRS 9 |
Financial Instruments | 01/01/2018 | yes | |||||
| IFRS 15 and IFRS 15 (A) |
Revenue from Contracts with Customers | 01/01/2018 | yes | |||||
| IFRS 16 |
Leases | 01/01/2019 | yes | |||||
| IFRS 17 |
Insurance Contracts | 01/01/2021 | none | |||||
| IFRS 2 (A) |
Classification and Measurement of Share-based Payment Transactions | 01/01/2018 | yes | |||||
| IFRS 4 (A) |
Applying IFRS 9 Financial Instruments with IFRS 4 Insurance Contracts | 01/01/2018 | none | |||||
| IFRS 9 (A) |
Prepayment Features with Negative Compensation | 01/01/2019 | none | |||||
| IFRS 15 (C) |
Revenue from Contracts with Customers | 01/01/2018 | yes | |||||
| IAS 19 (A) |
Plan Amendment, Curtailment or Settlement | 01/01/2019 | none | |||||
| IAS 28 (A) |
Long-term Interests in Associates and Joint Ventures | 01/01/2019 | none | |||||
| IAS 40 (A) |
Transfers of Investment Property | 01/01/2018 | none | |||||
| IFRIC (I) 22 |
Foreign Currency Transactions and Advance Consideration | 01/01/2018 | yes | |||||
| IFRIC (I) 23 |
Uncertainty over Income Tax Treatments | 01/01/2019 | none | |||||
| Annual Improvements to IFRS Standards 2014–2016 Cycle | 01/01/2018 | none | ||||||
| Annual Improvements to IFRS Standards 2015–2017 Cycle | 01/01/2019 | none | ||||||
| (A) Amendments |
||||||||
| (C) Clarifications |
||||||||
| (I) Interpretation |
||||||||
IFRS 9, the new standard governing financial instruments, may lead to changes in the classification and measurement of financial assets and financial liabilities. Upon first-time recognition, financial assets are classified as assets to be measured “at fair value” or “at amortized cost”, depending on the business model and the contractually agreed cash flows of
F-16
Table of Contents
the respective financial instruments. Depending on the classification, the subsequent measurement of financial assets is carried out either at amortized cost or at fair value. Changes in the fair value are to be recognized in profit or loss or in other comprehensive income. The requirements for the de-recognition of financial assets and liabilities and the general accounting of financial liabilities have been adopted to a large extent from IAS 39. Changes to the classification result in changes to MorphoSys’s financial assets that are classified as “available-for-sale” or “loans and receivables” in accordance with IAS 39. There are no material conversion effects with regard to the measurement of financial assets and financial liabilities. Hitherto, “available-for-sale” financial instruments are measured already at fair value in accordance with IAS 39 and thus no conversion effects will arise.
The provisions in the new standard for the recognition of impairments are based on the expected credit loss model and replace the model of incurred losses applied under IAS 39. Unlike under IAS 39, financial assets are to be divided into different risk classes according to historical and future expected loss probabilities, and a risk provision must be recognized before the occurrence of loss events. Past experience and the Group’s expectations regarding the performance of existing assets do only suggest minor future losses. Therefore no additional impairment should be recognized at the time of initial application other than the twelve-month expected credit loss in accordance with IFRS 9. For “Accounts receivable” the simplified impairment model will be applied with recognition of a loss allowance based on lifetime expected credit losses.
IFRS 9 is not expected to have an impact on the recognition of hedging relationships. As of December 31, 2017, there is neither a forward rate agreement that is subject to hedge accounting in accordance with IAS 39 nor any other hedging instrument that will be subject to hedge accounting.
Qualitative and quantitative adjustments to the disclosures in accordance with IFRS 7 are expected due to the implementation of IFRS 9, however, only for the fiscal year 2018.
The new IFRS 15 standard on revenue recognition was reviewed for its potential impact on the revenue recognition of existing contracts and future contracts with partners and/or licensees. IFRS 15 establishes principles for reporting information about the nature, amount, timing and uncertainty of revenue and cash flows arising from contracts with customers and replaces IAS 18 “Revenue”. This review revealed that, compared to the regulations currently applied to the existing contractual arrangements, quantitative effects on the consolidated financial statements are to be expected since for some contracts, revenue under IFRS 15 has to be recognized at a point in time rather than over time as under IAS 18. The Group will implement the new standard on January 1, 2018 and will apply the modified retrospective method, which requires the recognition of the cumulative effect of applying IFRS 15 as at January 1, 2018 to accumulated deficit, and not restate prior years. Therefore, the Group estimates that deferred revenue will be reduced by € 1.1 million and accumulated deficit will be reduced by € 1.1 million on January 1, 2018, accordingly. Qualitative adjustments of the required disclosures in the Notes under IFRS 15 are expected, however, not before the standard’s first-time application as of January 1, 2018.
The Group also reviewed the new IFRS 16 standard governing leases for its potential impact on existing lease contracts. Currently, all leases are accounted for as operating leases pursuant to IAS 17. As of January 1, 2019, right-of-use assets under existing lease contracts will be capitalized and lease liabilities will be recognized. Rental costs currently recognized in the
F-17
Table of Contents
statement of income will be replaced by depreciation on the respective assets and interest expenses, i.e. the related costs will be presented in different line items in the statement of income and may differ in their overall amount compared to the application of IAS 17. From today´s perspective, the implementation of IFRS 16 will have material quantitative effects on the consolidated balance sheet due to the rented premises at Semmelweisstraße 7, Planegg. The exact amount of assets and lease liabilities and the transitional provisions to be applied when switching from IAS 17 to IFRS 16 have not yet been determined.
| 2.2 | CONSOLIDATION PRINCIPLES |
Intercompany balances and transactions and any unrealized gains arising from intercompany transactions are eliminated when preparing consolidated financial statements pursuant to IFRS 10.B86. Unrealized losses are eliminated in the same manner as unrealized gains. Accounting policies have been applied consistently for all subsidiaries.
For all contracts and business transactions between group entities, the arm’s length principle was applied.
| 2.2.1 | CONSOLIDATED COMPANIES AND SCOPE OF CONSOLIDATION |
MorphoSys AG as ultimate parent company of the Group is located in Planegg, near Munich. MorphoSys AG has two wholly owned subsidiaries (collectively referred to as the “MorphoSys Group” or the “Group”): Sloning BioTechnology GmbH (Planegg) and Lanthio Pharma B.V. (Groningen, The Netherlands). Additionally, MorphoSys AG’s investment in Lanthio Pharma B.V. indirectly gives it 100% ownership in LanthioPep B.V. (Groningen, The Netherlands).
The consolidated financial statements for the year ended December 31, 2017 were prepared and approved by the Management Board in its meeting on March 8, 2018 by means of a resolution. The Management Board members are Dr. Simon Moroney (Chief Executive Officer), Jens Holstein (Chief Financial Officer), Dr. Markus Enzelberger (Chief Scientific Officer), and Dr. Malte Peters (Chief Development Officer).
Dr. Arndt Schottelius was Chief Development Officer until February 28, 2017. Dr. Malte Peters assumed the position on March 1, 2017. Dr. Markus Enzelberger, who served as Interim CSO from April 15, 2017, was appointed Chief Scientific Officer (CSO) effective November 1, 2017. He succeeded Dr. Marlies Sproll, who resigned from her CSO position effective end of October 31, 2017.
The Supervisory Board is authorized to amend the financial statements after their approval by the Management Board. MorphoSys Group’s registered head office is located in Planegg (district of Munich), and the registered business address is Semmelweisstraße 7, 82152 Planegg, Germany. The company is registered in the Commercial Register, Section B, of the District Court of Munich under the number HRB 121023.
F-18
Table of Contents
| 2.2.2 | CONSOLIDATION METHODS |
The following Group subsidiaries are included in the scope of consolidation as shown in the following table.
| Company | Purchase of Shares | Included in Basis of Consolidation since |
||||||
| Sloning BioTechnology GmbH |
October 2010 | 10/07/2010 | ||||||
| Lanthio Pharma B.V. |
May 2015 | 05/07/2015 | ||||||
| LanthioPep B.V. |
May 2015 | 05/07/2015 | ||||||
These subsidiaries are fully consolidated because they are either directly or indirectly wholly owned. MorphoSys controls these subsidiaries because it possesses full power over the investees. Additionally, MorphoSys is subject to risk exposure or has rights to variable returns from its involvement with the investees. MorphoSys also has unlimited capacity to exert power over the investees to influence their returns.
The Group does not have any entities consolidated as joint ventures by using the equity method as defined by IFRS 11 “Joint Arrangements” nor does it exercise a controlling influence as defined by IAS 28 “Investments in Associates and Joint Ventures”. Interests in such entities would be measured at fair value or historic cost in accordance with IAS 39.
Assets and liabilities of fully consolidated domestic and international entities are recognized using Group-wide uniform accounting and valuation methods. The consolidation methods applied have not changed from the previous year.
Receivables, liabilities, expenses and income among consolidated entities are eliminated in the consolidated financial statements.
| 2.2.3 | BASIS OF FOREIGN CURRENCY TRANSLATION |
IAS 21 “The Effects of Changes in Foreign Exchange Rates” governs the accounting for transactions and balances denominated in foreign currencies. Transactions denominated in foreign currencies are translated at the exchange rates prevailing on the date of the transaction. Any resulting translation differences are recognized in profit and loss. On the reporting date, assets and liabilities are translated at the closing rate for the financial year. Any foreign exchange rate differences derived from these translations are recognized in the consolidated statement of income.
| 2.3 | FINANCIAL INSTRUMENTS AND FINANCIAL RISK MANAGEMENT |
| 2.3.1 | CREDIT RISK AND LIQUIDITY RISK |
Financial instruments that could subject the Group to a concentration of credit and liquidity risk include primarily cash and cash equivalents, marketable securities (consisting of available-for-sale financial assets and bonds), financial assets of the loans and receivables category, derivative financial instruments and receivables. The Group’s cash and cash equivalents are principally denominated in euros. Marketable securities and financial assets of the loans and receivables category represent investments in high-quality securities. Cash, cash equivalents, marketable securities and financial assets of the loans and receivables category are held at several renowned financial institutions in Germany. The Group continuously monitors its
F-19
Table of Contents
positions with financial institutions that are counterparts to its financial instruments and these institutions’ credit ratings and does not expect any risk of non-performance.
One of the Group’s policies requires all customers who wish to transact business on credit terms to undergo a credit assessment based on external ratings. Nevertheless, the Group’s revenues and accounts receivable are still subject to credit risk from customer concentration. The Group’s most significant single customer accounted for € 5.1 million of accounts receivables as of December 31, 2017 (December 31, 2016: € 8.4 million) or 45% of the Group’s accounts receivable at the end of 2017. The top three individual customers of the Group accounted for of 55%, 25% and 10%, respectively, of the total revenues in 2017. On December 31, 2016, one customer had accounted for 66% of the Group’s accounts receivable, and the top three customers had individually accounted for 85%, 5% and 5% of the Group’s revenues in 2016. Based on the Management Board’s assessment, no allowances were required in the financial years 2017 and 2016. The carrying amounts of financial assets represent the maximum credit risk.
The table below shows the accounts receivables by region as of the reporting date.
| in € | 12/31/2017 | 12/31/2016 | ||||||
| Europe and Asia |
8,838,884 | 9,852,273 | ||||||
| USA and Canada |
2,395,424 | 2,744,382 | ||||||
| Other |
0 | 0 | ||||||
|
|
|
|||||||
| Total |
11,234,308 | 12,596,655 | ||||||
|
|
|
|||||||
The following table shows the aging of trade receivables as of the reporting date.
| in €; Accounts Receivable are due since | 12/31/2017 0 - 30 days |
12/31/2017 30 - 60 days |
12/31/2017 60 + days |
12/31/2017 Total |
||||||||||||
| Accounts Receivable |
11,234,308 | 0 | 0 | 11,234,308 | ||||||||||||
| Write-off |
0 | 0 | 0 | 0 | ||||||||||||
|
|
|
|||||||||||||||
| Accounts Receivable, Net of Allowance |
11,234,308 | 0 | 0 | 11,234,308 | ||||||||||||
|
|
|
|||||||||||||||
| in €; Accounts Receivable are due since | 12/31/2016 0 - 30 days |
12/31/2016 30 - 60 days |
12/31/2016 60 + days |
12/31/2016 Total |
||||||||||||
| Accounts Receivable |
12,596,655 | 0 | 0 | 12,596,655 | ||||||||||||
| Write-off |
0 | 0 | 0 | 0 | ||||||||||||
|
|
|
|||||||||||||||
| Accounts Receivable, Net of Allowance |
12,596,655 | 0 | 0 | 12,596,655 | ||||||||||||
|
|
|
|||||||||||||||
On December 31, 2017 and December 31, 2016, the Group was not exposed to a credit risk from derivative financial instruments. The maximum credit risk of financial guarantees (rent deposits) on the reporting date amounted to € 1.1 million (December 31, 2016: € 1.3 million).
The contractually agreed maturities and the corresponding cash outflows of accounts payable are within one year. Convertible bonds issued to related parties mature on March 31, 2020 (maximum cash outflow: € 0.1 million).
F-20
Table of Contents
| 2.3.2 | MARKET RISK |
Market risk is the risk that changes in market prices, such as foreign exchange rates, interest rates and equity prices will affect the Group’s results of operations or the value of the financial instruments held. The Group is exposed to currency and interest rate risks.
CURRENCY RISK
The consolidated financial statements are prepared in euros. Whereas MorphoSys’s expenses are predominantly incurred in euros, a portion of the revenue is dependent on the prevailing exchange rate of the US dollar. Throughout the year, the Group monitors the need to hedge foreign exchange rates to minimize currency risk and addresses this risk by using derivative financial instruments.
Under the Group’s hedging policy, highly probable cash flows and definite foreign currency receivables collectable within a twelve-month period are tested to determine if they should be hedged. MorphoSys began using foreign currency options and forwards to hedge its foreign exchange risk against US dollar receivables in 2003. These derivatives are recorded at their fair values under provisions as of December 31, 2017, since the fair value is negative.
As of December 31, 2017, there were 12 unsettled forward rate agreements with terms of one month to twelve months (December 31, 2016: 10 unsettled forward rate agreements). The unrealized gross loss from these agreements amounted to € 0.3 million as of December 31, 2017 and was reported in the finance result (December 31, 2016: less than € 0.1 million unrealized gross gain).
One forward rate agreement dating back to January 2016 with an original maturity in early April 2017 was subject to hedge accounting as a cash flow hedge and at the original term’s expiry was extended until the beginning of July 2017. In July 2017, a net loss of € 0.3 million was recognized in the income statement for this hedging instrument, which was previously recognized as gross gains and losses in other comprehensive income.
The table below shows the Group’s exposure to foreign currency risk based on the items’ carrying amounts.
| as of December 31, 2017; in € | EUR | USD | Other | Total | ||||||||||||
| Cash and Cash Equivalents |
74,289,250 | 2,299,879 | 0 | 76,589,129 | ||||||||||||
| Available-for-sale Financial Assets |
86,538,195 | 0 | 0 | 86,538,195 | ||||||||||||
| Financial Assets classified as Loans and Receivables |
149,059,254 | 0 | 0 | 149,059,254 | ||||||||||||
| Accounts Receivable |
11,199,652 | 34,656 | 0 | 11,234,308 | ||||||||||||
| Restricted Cash (included in Other Current Assets) |
1,132,782 | 0 | 0 | 1,132,782 | ||||||||||||
| Accounts Payable and Accrued Expenses |
(44,655,328 | ) | (156,390 | ) | 0 | (44,811,718 | ) | |||||||||
|
|
|
|||||||||||||||
| Total |
277,563,805 | 2,178,145 | 0 | 279,741,950 | ||||||||||||
|
|
|
|||||||||||||||
F-21
Table of Contents
| as of December 31, 2016; in € | EUR | USD | Other | Total | ||||||||||||
| Cash and Cash Equivalents |
73,456,907 | 471,754 | 0 | 73,928,661 | ||||||||||||
| Available-for-sale Financial Assets |
63,361,727 | 0 | 0 | 63,361,727 | ||||||||||||
| Bonds, Available-for-sale |
6,532,060 | 0 | 0 | 6,532,060 | ||||||||||||
| Financial Assets classified as Loans and Receivables |
136,108,749 | 0 | 0 | 136,108,749 | ||||||||||||
| Financial Assets classified as Loans and Receivables, Net of Current Portion |
79,521,181 | 0 | 0 | 79,521,181 | ||||||||||||
| Accounts Receivable |
12,215,814 | 380,841 | 0 | 12,596,655 | ||||||||||||
| Restricted Cash (included in Other Current Assets) |
1,252,405 | 0 | 0 | 1,252,458 | ||||||||||||
| Accounts Payable and Accrued Expenses |
(31,794,114 | ) | (428,502 | ) | 0 | (32,222,616 | ) | |||||||||
|
|
|
|||||||||||||||
| Total |
340,654,782 | 424,093 | 0 | 341,078,875 | ||||||||||||
|
|
|
|||||||||||||||
Various foreign exchange rates and their impact on assets and liabilities were simulated in an in-depth sensitivity analysis to determine the effects on income. A 10% increase in the euro versus the US dollar as of December 31, 2017 would have reduced the Group’s income by € 0.2 million. A 10% decline in the euro versus the US dollar would have increased the Group’s income by € 0.2 million.
A 10% increase in the euro versus the US dollar as of December 31, 2016 would have reduced the Group’s income by less than € 0.1 million. A 10% decline in the euro versus the US dollar would have increased the Group’s income by less than € 0.1 million.
INTEREST RATE RISK
The Group’s risk exposure to changes in interest rates mainly relates to fixed term deposits and bonds, available-for-sale. Changes in the general level of interest rates may lead to an increase or decrease in the fair value of these securities. The Group’s investment focus places the safety of an investment ahead of its return. Interest rate risk is limited because all securities can be liquidated within a maximum of two years.
The Group is not subject to significant interest rate risks from the liabilities currently reported in the balance sheet.
| 2.3.3 | FAIR VALUE HIERARCHY AND MEASUREMENT PROCEDURES |
The IFRS 13 “Fair Value Measurement” guidelines must always be applied when measurement at fair value is required or permitted or disclosures regarding measurement at fair value are required based on another IAS/IFRS guideline. The fair value is the price that would be achieved for the sale of an asset in an arm’s length transaction between independent market participants or the price to be paid for the transfer of a liability (disposal or exit price). Accordingly, the fair value of a liability reflects the default risk (i.e., own credit risk). Measurement at fair value requires that the sale of the asset or the transfer of the liability takes place on the principal market or, if no such principal market is available, on the most advantageous market. The principal market is the market a company has access to that has the highest volume and level of activity.
F-22
Table of Contents
Fair value is measured by using the same assumptions and taking into account the same characteristics of the asset or liability as would an independent market participant. Fair value is a market-based, not an entity-specific measurement. The fair value of non-financial assets is based on the best use of the asset by a market participant. For financial instruments, the use of bid prices for assets and ask prices for liabilities is permitted but not required if those prices best reflect the fair value in the respective circumstances. For simplification, mean rates are also permitted. Thus, IFRS 13 not only applies to financial assets, but all assets and liabilities.
MorphoSys uses the following hierarchy for determining and disclosing the fair value of financial instruments:
| Level 1: |
Quoted (unadjusted) prices in active markets for identical assets or liabilities to which the Company has access. |
| Level 2: |
Inputs other than quoted prices included within Level 1 that are observable for the assets or liabilities, either directly (i.e., as prices) or indirectly (i.e., derived from prices). |
| Level 3: |
Inputs for the asset or liability that are not based on observable market data (that is, unobservable inputs). |
The carrying amounts of financial assets and liabilities, such as financial assets of the loans and receivables category and accounts receivable and accounts payable approximate their fair value because of their short-term maturities.
HIERARCHY LEVEL 1
The fair value of financial instruments traded in active markets is based on the quoted market prices on the reporting date. A market is considered active if quoted prices are available from an exchange, dealer, broker, industry group, pricing service or regulatory body that is easily and regularly accessible and prices reflect current and regularly occurring market transactions at arm’s length conditions. For assets held by the Group, the appropriate quoted market price is the buyer’s bid price. These instruments fall under level 1 of the hierarchy (see also Item 5.2 of these Notes).
HIERARCHY LEVEL 2 AND 3
The fair value of financial instruments not traded in active markets can be determined using valuation methods. In this case, fair value is estimated using the results of a valuation method that makes maximum use of market data and relies as little as possible on entity-specific inputs. If all significant inputs required for measuring fair value by using valuation methods are observable, the instrument is allocated to level 2. If significant inputs are not based on observable market data, the instrument is allocated to level 3.
Hierarchy level 2 contains the forward exchange contracts used for currency hedging. Future cash flows for these forward exchange contracts are determined based on forward exchange rate curves. The fair value of these instruments corresponds to their discounted cash flows.
There were no financial assets or liabilities allocated to hierarchy level 3.
There were no transfers from one fair value hierarchy level to another in 2017 or 2016.
F-23
Table of Contents
The table below shows the fair values of financial assets and liabilities and the carrying amounts presented in the consolidated balance sheet.
| December 31, 2017 | Note | Hierarchy Level |
Loans and Receivables |
Available- for-sale |
Other Financial Liabilities |
Total Carrying Amount |
Fair value |
|||||||||||||||||||||
| (in 000’s €) | ||||||||||||||||||||||||||||
| Cash and Cash Equivalents |
5.1 | * | 76,589 | 0 | 0 | 76,589 | * | |||||||||||||||||||||
| Financial Assets classified as Loans and Receivables |
5.2 | * | 149,059 | 0 | 0 | 149,059 | * | |||||||||||||||||||||
| Accounts Receivable |
5.3 | * | 11,234 | 0 | 0 | 11,234 | * | |||||||||||||||||||||
| Restricted Cash (included in Other Current Assets) |
5.4 | * | 1,133 | 0 | 0 | 1,133 | * | |||||||||||||||||||||
| Other Receivables |
5.4 | * | 85 | 0 | 0 | 85 | * | |||||||||||||||||||||
| Available-for-sale Financial Assets |
5.2 | 1 | 0 | 86,538 | 0 | 86,538 | 86,538 | |||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| Total |
238,100 | 86,538 | 0 | 324,638 | ||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| Convertible Bonds - Liability Component |
7.2 | 2 | 0 | 0 | (88 | ) | (88 | ) | (88 | ) | ||||||||||||||||||
| Accounts Payable and Accrued Expenses |
6.1 | * | 0 | 0 | (44,812 | ) | (44,812 | ) | * | |||||||||||||||||||
| Forward Exchange Contracts Used for Hedging (included in Provisions) |
6.2 | 2 | 0 | 0 | (300 | ) | (300 | ) | (300 | ) | ||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| Total |
0 | 0 | (45,200 | ) | (45,200 | ) | ||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| * | Declaration waived in line with IFRS 7.29 (a). For these instruments carrying value is a reasonable approximation of fair value. |
F-24
Table of Contents
| December 31, 2016 | Note | Hierarchy Level |
Loans and Receivables |
Available- for-sale |
Other Financial Liabilities |
Total Carrying Amount |
Fair value |
|||||||||||||||||||||
| (in 000’s €) | ||||||||||||||||||||||||||||
| Cash and Cash Equivalents |
5.1 | * | 73,929 | 0 | 0 | 73,929 | * | |||||||||||||||||||||
| Financial Assets classified as Loans and Receivables |
5.2 | * | 136,109 | 0 | 0 | 136,109 | * | |||||||||||||||||||||
| Accounts Receivable |
5.3 | * | 12,597 | 0 | 0 | 12,597 | * | |||||||||||||||||||||
| Forward Exchange Contracts Used for Hedging (included in Other Receivables) |
5.4 | 2 | 520 | 0 | 0 | 520 | 520 | |||||||||||||||||||||
| Restricted Cash (included in Other Current Assets) |
5.4 | * | 1,252 | 0 | 0 | 1,252 | * | |||||||||||||||||||||
| Other Receivables |
5.4 | * | 137 | 0 | 0 | 137 | * | |||||||||||||||||||||
| Financial Assets classified as Loans and Receivables, Net of Current Portion |
5.2 | * | 79,521 | 0 | 0 | 79,521 | 79,521 | |||||||||||||||||||||
| Available-for-sale Financial Assets |
5.2 | 1 | 0 | 63,362 | 0 | 63,362 | 63,362 | |||||||||||||||||||||
| Bonds, Available-for-sale |
5.2 | 1 | 0 | 6,532 | 0 | 6,532 | 6,532 | |||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| Total |
304,065 | 69,894 | 0 | 373,959 | ||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| Convertible Bonds - Liability Component |
7.2 | 2 | 0 | 0 | (218 | ) | (218 | ) | (218 | ) | ||||||||||||||||||
| Accounts Payable and Accrued Expenses |
6.1 | * | 0 | 0 | (32,223 | ) | (32,223 | ) | * | |||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| Total |
0 | 0 | (32,441 | ) | (32,441 | ) | ||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| * | Declaration waived in line with IFRS 7.29 (a). For these instruments carrying value is a reasonable approximation of fair value. |
| 2.4 | IMPAIRMENTS |
| 2.4.1 | NON-DERIVATIVE FINANCIAL INSTRUMENTS |
A financial instrument not carried at fair value through profit or loss is assessed at each reporting date to determine if there is objective evidence for impairment. A financial instrument is impaired if objective evidence indicates that an event has occurred after the initial recognition of the asset that could result in a loss and whether that event could have a negative effect on the asset’s estimated future cash flows, which can be assessed reliably.
Objective evidence that financial instruments are impaired can include the default or delinquency of a debtor, indications that a debtor or issuer will enter insolvency, adverse changes in the payment status of borrowers or issuers in the Group as well as economic conditions that correlate with defaults or the disappearance of an active market for a marketable security. A significant or prolonged decline in a financial instrument’s fair value below its acquisition cost is objective evidence of impairment.
F-25
Table of Contents
| 2.4.2 | RECEIVABLES |
The Group considers evidence of the impairment of receivables on an individual level. All individually significant receivables are tested specifically for impairment.
For a financial instrument measured at amortized cost less impairment, impairment is calculated as the difference between its carrying amount and the present value of the estimated future cash flows. Cash flows are discounted at the asset’s initial effective interest rate. Losses are recognized in profit or loss and reflected in an allowance account against receivables. When a subsequent event (e.g., repayment by a debtor) causes the amount of impairment to decrease, the impairment is reversed through profit and loss.
| 2.4.3 | AVAILABLE-FOR-SALE FINANCIAL ASSETS |
In case of objective indications, impairment of available-for-sale financial assets is recognized by reclassifying the accumulated losses from the revaluation reserve in equity to profit and loss. The amount of the accumulated loss to be reclassified from equity to profit and loss is the difference between the acquisition cost less amortization and any principal repayment and the current fair value less any impairment previously recognized in profit or loss. Impairment losses recognized in profit and loss for an investment in an equity instrument classified as available-for-sale are not reversed through profit and loss. If in a subsequent period the fair value of an impaired available-for-sale debt instrument increases and this increase can be objectively linked to an event occurring after the impairment was recognized in profit or loss, then the impairment loss is reversed, and the amount of the reversal is recognized in profit or loss.
| 2.4.4 | NON-FINANCIAL ASSETS |
The carrying amounts of the Group’s non-financial assets and inventories are reviewed at each reporting date for any indication of impairment. The non-financial asset’s recoverable amount and inventories’ net realizable value is estimated if such indication exists. For goodwill and intangible assets that have indefinite useful lives or are not yet available for use, the recoverable amount is estimated at the same time each year, or on an interim basis, if required. Impairment is recognized if the carrying amount of an asset or the cash-generating unit (CGU) exceeds its estimated recoverable amount.
The recoverable amount of an asset or CGU is the greater of its value-in-use or its fair value less costs of disposal. In assessing value-in-use, the estimated future pre-tax cash flows are discounted to their present value using a pre-tax discount rate that reflects current market assessments of the time value of money and the risks specific to the asset or CGU. For the purposes of impairment testing, assets that cannot be tested individually are grouped into the smallest group of assets that generates cash flows from ongoing use that are largely independent of the cash flows of other assets or CGUs. A ceiling test for the operating segment must be carried out for goodwill impairment testing. CGUs that have been allocated goodwill are aggregated so that the level at which impairment testing is performed reflects the lowest level at which goodwill is monitored for internal reporting purposes. Goodwill acquired in a business combination may be allocated to groups of CGUs that are expected to benefit from the combination’s synergies.
The Group’s corporate assets do not generate separate cash flows and are utilized by more than one CGU. Corporate assets are allocated to CGUs on a reasonable and consistent basis and are
F-26
Table of Contents
tested for impairment as part of the impairment testing of the CGU that was allocated the corporate asset.
Impairment losses are recognized in profit and loss. Goodwill impairment cannot be reversed. For all other assets, impairment recognized in prior periods is assessed on each reporting date for any indications that the losses decreased or no longer exist. Impairment is reversed when there has been a change in the estimates used to determine the recoverable amount. Impairment losses can only be reversed to the extent that the asset’s carrying amount does not exceed the carrying amount net of depreciation or amortization that would have been determined if an impairment had not been recognized.
| 2.5 | ADDITIONAL INFORMATION |
| 2.5.1 | KEY ESTIMATES AND ASSUMPTIONS |
Estimates and judgments are continually evaluated and based on historical experience and other factors that include expectations of future events that are believed to be realistic under the prevailing circumstances.
The Group makes estimates and assumptions concerning the future. The resulting accounting-related estimates will, by definition, seldom correspond to the actual results. The estimates and assumptions that carry a significant risk of causing material adjustments to the carrying amounts of assets and liabilities in the next financial year are addressed below.
IN-PROCESS R&D PROGRAMS AND GOODWILL
The Group performs a yearly test to determine whether in-process R&D programs or goodwill is subject to impairment in accordance with the accounting policies discussed in Item 2.4.4. The recoverable amounts from in-process R&D programs and cash-generating units have been determined using value-in-use calculations and are subjected to a sensitivity analysis. These calculations require the use of estimates (see also Items 5.7.3 and 5.7.5 in the Notes).
INCOME TAXES
The Group is subject to income taxes in a number of tax jurisdictions. Due to the increasing complexity of tax laws and the corresponding uncertainty regarding the legal interpretation by the fiscal authority, tax calculations are generally subject to an elevated amount of uncertainty. To the extent necessary, possible tax risks were taken into account in the form of provisions.
Deferred tax assets on tax loss carryforwards are recognized based on the expected business performance of the relevant Group entity. For details on tax loss carryforwards and any recognized deferred tax assets, please refer to Item 4.4 in the Notes.
The Management Board’s policy for capital management is to preserve a strong and sustainable capital base in order to maintain the confidence of investors, business partners, and the capital market and to support future business development. As of December 31, 2017, the equity ratio was 86.3% (December 31, 2016: 89.6%; see also the following overview). The Group does not currently have any financial debt.
F-27
Table of Contents
Under the respective incentive plans resolved by the Annual General Meeting, the Management Board and employees may participate in the Group’s performance through long-term performance-related remuneration consisting of convertible bonds issued in 2013 and a stock option plan (SOP) set up in 2017. MorphoSys also established long-term incentive programs (LTI plan) in 2013, 2014, 2015, 2016 and 2017. These programs are based on the performance-related issue of shares, or “performance shares”, which are granted when certain predefined success criteria have been achieved and the vesting period has expired (for more information, please refer to Item 7.3 in the Notes). There were no changes in the Group’s approach to capital management during the year.
| in 000’ € | 12/31/2017 | 12/31/2016 | ||||||
| Stockholders’ Equity |
358,671 | 415,460 | ||||||
| In % of Total Capital |
86.3% | 89.6% | ||||||
| Total Liabilities |
56,727 | 48,140 | ||||||
| In % of Total Capital |
13.7% | 10.4% | ||||||
|
|
|
|||||||
| Total Capital |
415,398 | 463,600 | ||||||
|
|
|
|||||||
| 2.6 | USE OF INTEREST RATES FOR VALUATION |
The Group uses interest rates to measure fair value. When calculating stock-based compensation, MorphoSys uses interest rates of German government bonds with maturities of five or seven years on the date they were granted to determine the fair value of convertible bonds.
| 2.7 | ACCOUNTING POLICIES APPLIED TO LINE ITEMS OF THE INCOME STATEMENT |
| 2.7.1 | REVENUES AND REVENUE RECOGNITION |
The Group’s revenue includes license fees, milestone payments and service fees. Under IAS 18.9, revenues are measured at the fair value of the consideration received or receivable. In accordance with IAS 18.20b, revenues are recognized only to the extent that it is sufficiently probable that the Company will receive the economic benefits associated with the transaction.
LICENSE FEES AND MILESTONE PAYMENTS
Revenues related to non-refundable fees for providing access to technologies, fees for the use of technologies and license fees are recognized on a straight-line basis over the period of the agreement unless a more appropriate method of revenue recognition is available. The period of the agreement usually corresponds to the contractually agreed term of the research project or, in the case of contracts without an agreed project term, the expected term of the collaboration. If all IAS 18.14 criteria are met, revenue is recognized immediately and in full. Revenues from milestone payments are recognized upon achievement of certain contractual criteria.
SERVICE FEES
Service fees from research and development collaborations are recognized in the period the services are provided.
Discounts that are likely to be granted and whose amount can be reliably determined are recognized as a reduction in revenue at the time of revenue recognition. The timing of the
F-28
Table of Contents
transfer of risks and rewards varies depending on the terms of the sales contract. In accordance with IAS 18.21 and 18.25, revenue from multiple-component contracts is recognized by allocating the total consideration to the separately identifiable components based on their respective fair values and by applying IAS 18.20. The applicable revenue recognition criteria are assessed separately for each component.
Deferred revenue consists of customer payments that were not yet recognized as revenue because the related services specified in the contract were not yet rendered.
| 2.7.2 | OPERATING EXPENSES |
PERSONNEL EXPENSES RESULTING FROM STOCK OPTIONS
The Group applies the provisions under IFRS 2 “Share-based Payment”, which require the Group to spread compensation expenses from the estimated fair values of share-based payments on the reporting date over the period in which the beneficiaries provide the services which triggered the granting of the share-based payments.
IFRS 2 “Share-based Payment” requires the consideration of the effects of share-based payments if the Group acquires goods or services in exchange for shares or stock options (“settlement in equity instruments”) or other assets that represent the value of a specific number of shares or stock options (“cash settlement”). The key impact of IFRS 2 on the Group is the expense resulting from the use of an option pricing model in relation to share-based incentives for employees and the Management Board. Additional information can be found under Items 7.1, 7.2, 7.3 and 7.4 in the Notes.
RESEARCH AND DEVELOPMENT
Research costs are expensed in the period they occur. Development costs are generally expensed as incurred in accordance with IAS 38.5 and IAS 38.11 to 38.23. Development costs are recognized as an intangible asset when the criteria of IAS 38.21 (probability of expected future economic benefits, reliability of cost measurement) are met and if the Group can provide proof under IAS 38.57.
This line item contains personnel expenses, consumables supplies, other operating expenses, impairment, amortization and other costs of intangible assets (additional information can be found under Item 5.7 in the Notes), external services and depreciation and other costs for infrastructure.
GENERAL AND ADMINISTRATIVE
This line item contains personnel expenses, consumable supplies, other operating expenses, amortization of intangible assets (software; additional information can be found under Item 5.7 in the Notes), expenses for external services, and depreciation and other costs for infrastructure.
OPERATING LEASE PAYMENTS
Payments made under operating leases are recognized in the income statement on a straight-line basis over the term of the lease. According to SIC-15, all incentive agreements in the context of operating leases are recognized as an integral part of the net consideration agreed for the use of the leased asset. The total amount of income from incentives is recognized as a reduction in lease expenses on a straight-line basis over the term of the lease.
F-29
Table of Contents
All of the Group’s lease agreements are classified exclusively as operating leases. The Group did not engage in any finance lease arrangements.
| 2.7.3 | OTHER INCOME |
GOVERNMENT GRANTS
Grants received from government agencies to fund specific research and development projects are recognized in the income statement in the separate line item “other income” to the extent that the related expenses have already occurred. Under the terms of the grants, government agencies generally have the right to audit the use of the funds granted to the Group.
Basically, government grants are cost subsidies, and their recognition through profit and loss is limited to the corresponding costs.
When the repayment of cost subsidies depends on the success of the development project, these cost subsidies are recognized as other liabilities until success has been achieved. If the condition for repayment is not met, then the grant is recognized under “other income”.
No payments were granted in the 2017 financial year that are required to be classified as investment subsidies.
| 2.7.4 | OTHER EXPENSES |
The line item “other expenses” consists mainly of currency losses from the operating business and the repayment of cost subsidies.
| 2.7.5 | FINANCE INCOME |
Interest income is recognized in the income statement as it occurs and takes into account the asset’s effective interest rate.
| 2.7.6 | FINANCE EXPENSES |
Finance expenses are expensed in the income statement in the period they occur.
| 2.7.7 | INCOME TAX EXPENSES/INCOME |
Income taxes consist of current and deferred taxes and are recognized in the income statement unless they relate to items recognized directly in equity.
Current taxes are the taxes expected to be payable on the year’s taxable income based on prevailing tax rates on the reporting date and any adjustments to taxes payable in previous years.
The calculation of deferred taxes is based on the balance sheet liability method that refers to the temporary differences between the carrying amounts of assets and liabilities and the amounts used for taxation purposes. The method of calculating deferred taxes depends on how the asset’s carrying amount is expected to be realized and how the liabilities will be repaid. The calculation is based on the prevailing tax rates or those adopted on the reporting date.
Deferred tax assets are offset against deferred tax liabilities if the taxes are levied by the same taxation authority and the entity has a legally enforceable right to set off current tax assets against current tax liabilities.
F-30
Table of Contents
Deferred tax assets are recognized only to the extent that it is likely that there will be future taxable income to offset. Deferred tax assets are reduced by the amount that the related tax benefit is no longer expected to be realized.
| 2.7.8 | EARNINGS PER SHARE |
The Group reports basic and diluted earnings per share under consideration of IAS 33.41. Basic earnings per share is computed by dividing the net profit or loss attributable to parent company shareholders by the weighted-average number of ordinary shares outstanding during the reporting period. Diluted earnings per share is calculated in the same manner with the exception that the net profit or loss attributable to parent company shareholders and the weighted-average number of ordinary shares outstanding are adjusted for any dilutive effects resulting from stock options and convertible bonds granted to the Management Board and employees.
In 2017 and 2016, diluted earnings per share equal basic earnings per share. The effect of 87,904 potentially dilutive shares in 2017 (2016: 99,764 dilutive shares) resulting from stock options and convertible bonds granted to the Management Board, the Senior Management Group and employees of the Company who are not members of the Senior Management Group, has been excluded from the diluted earnings per share because it would result in a decrease in the loss per share and is therefore not to be treated as dilutive.
The 62,071 stock options not yet vested as of December 31, 2017, were not included in the calculation of potentially dilutive shares, as they are antidilutive for the 2017 financial year. These shares could potentially have a dilutive effect in the future.
| 2.8 | ACCOUNTING POLICIES APPLIED TO THE ASSETS OF THE BALANCE SHEET |
| 2.8.1 | LIQUIDITY |
CASH AND CASH EQUIVALENTS AND MARKETABLE SECURITIES
The Group regards all cash at banks and on hand and all short-term deposits with a maturity of three months or less as cash and cash equivalents. The Group invests most of its cash and cash equivalents at several major financial institutions: Commerzbank, UniCredit, BayernLB, LBBW, BNP Paribas, Deutsche Bank, Sparkasse and Rabobank.
Cash and cash equivalents are recognized at nominal value. Marketable securities are recognized and measured at fair value. Any fluctuations in the fair value of marketable securities are directly recognized in equity. Permanent impairment is recognized in profit and loss
NON-DERIVATIVE FINANCIAL INSTRUMENTS
Depending on how they are classified, existing financial instruments are either measured at amortized cost (category “loans and receivables”) or fair value (category “available-for-sale financial assets”). The amortized cost of current receivables and current liabilities generally corresponds to either the nominal amount or repayment amount.
All non-derivative financial instruments are initially recognized at fair value, which is defined as the fair value of the consideration provided net of transaction costs.
F-31
Table of Contents
The Group applies IAS 39 for financial instruments in the form of debt and equity instruments. At the time of purchase, the Management Board determines the financial instrument’s classification and reviews this classification at each reporting date. The classification depends on the purpose of acquiring the financial instrument. As of December 31, 2017 and December 31, 2016, some financial instruments held by the Group were classified as “available-for-sale”. These financial instruments are recognized or derecognized as of the date on which the Group commits to the financial instrument’s purchase or sale. Following their initial recognition, available-for-sale financial assets are measured at fair value, and any resulting gain or loss is reported directly in the revaluation reserve within equity until the financial instruments are sold, redeemed, otherwise disposed of or considered impaired, at which time the accumulated loss is reported in profit and loss.
Guarantees granted for rent deposits and obligations from convertible bonds issued to employees are recorded under other assets as restricted cash since they are not available for use in the Group’s operations.
DERIVATIVE FINANCIAL INSTRUMENTS
The Group uses derivative financial instruments to hedge its foreign exchange rate risk and cash flows. In accordance with IAS 39.9, stand-alone derivative financial instruments are predominantly held for trading and are initially recognized at fair value. After their initial recognition, derivative financial instruments are measured at fair value, which is defined as their quoted market price on the reporting date. Any resulting gain or loss from derivatives is recognized in profit and loss unless the derivatives are effective and designated as hedging instruments under a hedging relationship (hedge accounting). According to the Group’s foreign currency hedging policy, the Group only hedges highly probable future cash flows and clearly identifiable receivables that can be collected within a twelve-month period.
The use of derivative financial instruments is subject to a Group policy that is a written guideline approved by the Management Board for dealing with derivative financial instruments. Any changes in the fair value of derivative financial instruments are documented.
HEDGE ACCOUNTING
The Group has designated hedging instruments to hedge cash flows (cash flow hedges) during the fiscal years 2017 and 2016.
At the beginning of the hedge accounting, the hedging relationship between the underlying and the hedge transaction are documented, including the risk management objectives and corporate strategy underlying the hedging relationship. Additionally, when concluding the hedge and also during the term of the hedge, the Group regularly provides documentation if the hedging instrument designated for the hedging relationship is highly effective in terms of the hedged risk to compensate for any changes of the underlying transaction’s cash flows.
For information on the fair value of derivatives used for hedging, please refer to Item 2.3.2 in the Notes.
CASHFLOW HEDGES
The effective portion of the change in fair value of derivatives that are suitable for cash flow hedges and designated as such is recognized within other comprehensive income. The gain/loss
F-32
Table of Contents
attributable to the ineffective portion is immediately recognized in profit and loss with “other operating income/expenses”.
Amounts recognized within other comprehensive income are reclassified to the consolidated statement of income in the period in which the underlying transaction is recognized in profit and loss. The gain/loss is recorded in the same line item of the consolidated statement of income as the underlying transaction.
The hedging relationship is no longer accounted for if the Group dissolves the hedging relationship, the hedging instrument expires, is sold, terminated or exercised or no longer is suitable for hedging purposes. The full gain/loss recognized in other comprehensive income and accrued within equity remains in equity when the hedge accounting ends and is only recognized in profit and loss once the expected transaction is also recognized in profit and loss. If the transaction is no longer expected to materialize, the full gain/loss recognized in equity is immediately reclassified into the consolidated statement of income.
| 2.8.2 | ACCOUNTS RECEIVABLE, INCOME TAX RECEIVABLES AND OTHER RECEIVABLES |
Accounts receivable are measured at amortized cost less any impairment; for example, allowances for doubtful accounts (see Items 2.4.2 and 5.3 in the Notes).
Income tax receivables mainly include receivables due from tax authorities in the context of capital gain taxes withheld.
Other non-derivative financial instruments are measured at amortized cost using the effective interest method less any impairment.
| 2.8.3 | INVENTORIES |
Inventories are measured at the lower value of production or acquisition cost and net realizable value under the first-in first-out method. Acquisition costs comprise all costs of purchase and those incurred in bringing the inventories into operating condition while taking into account purchase price reductions, such as bonuses and discounts. Net realizable value is the estimated selling price less the estimated expenses necessary for completion and sale. Inventories are divided into the categories of raw materials and supplies.
| 2.8.4 | PREPAID EXPENSES AND OTHER CURRENT ASSETS |
Prepaid expenses include expenses resulting from an outflow of liquid assets prior to the reporting date that are only recognized as expenses in the subsequent financial year. Such expenses usually involve maintenance contracts, sublicenses and prepayments for external laboratory services not yet performed. Other current assets primarily consist of receivables from tax authorities resulting from value-added taxes and restricted cash, such as rent deposits. This item is recognized at nominal value.
| 2.8.5 | PROPERTY, PLANT AND EQUIPMENT |
Property, plant and equipment is recorded at historical cost less accumulated depreciation (see also Item 5.6 in the Notes) and any impairment (see Item 2.4.4 in the Notes). Historical cost includes expenditures directly related to the purchase at the time of the acquisition. Replacement purchases, building alterations and improvements are capitalized while repair and
F-33
Table of Contents
maintenance expenses are charged as expenses as they are incurred. Property, plant and equipment is depreciated on a straight-line basis over its useful life (see table below). Leasehold improvements are depreciated on a straight-line basis over the lesser of the asset’s estimated useful life or the remaining term of the lease.
| Asset Class | Useful Life | Depreciation Rates | ||||||
| Computer Hardware |
3 years | 33% | ||||||
| Low-value Laboratory and Office Equipment below € 410 |
Immediately | 100% | ||||||
| Permanent Improvements to Property/Buildings |
10 years | 10% | ||||||
| Office Equipment |
8 years | 13% | ||||||
| Laboratory Equipment |
4 years | 25% | ||||||
Asset’s residual values and useful lives are reviewed at the end of each reporting period and adjusted if appropriate.
Borrowing costs that can be directly attributed to the acquisition, construction or production of a qualifying asset are not included in the acquisition or production costs because the Group finances the entire operating business with equity.
| 2.8.6 | INTANGIBLE ASSETS |
Purchased intangible assets are capitalized at acquisition cost and exclusively amortized on a straight-line basis over their useful lives. Internally generated intangible assets are recognized to the degree the recognition criteria set out in IAS 38 are met.
Development costs are capitalized as intangible assets when the capitalization criteria described in IAS 38 have been met, namely, clear specification of the product or procedure, technical feasibility, intention of completion, use, commercialization, coverage of development costs through future free cash flows, reliable determination of these free cash flows and availability of sufficient resources for completion of development and sale. Amortization is recorded in research and development expenses.
Expenses to be classified as research expenses are allocated to research and development expenses as defined by IAS 38.
Subsequent expenditures for capitalized intangible assets are capitalized only when they substantially increase the future economic benefits of the specific asset to which they relate. All other expenditures are expensed as incurred.
PATENTS
Patents obtained by the Group are recorded at acquisition cost less accumulated amortization (see below) and any impairment (see Item 2.4.4 in the Notes). Patent costs are amortized on a straight-line basis over the lower of the estimated useful life of the patent (ten years) or the remaining patent term. Amortization starts when the patent is issued. Technology identified in the purchase price allocation for the acquisition of Sloning BioTechnology GmbH is recorded at the fair value at the time of acquisition, less accumulated amortization (useful life of ten years).
F-34
Table of Contents
LICENSE RIGHTS
The Group has acquired license rights from third parties by making upfront license payments, paying annual fees to maintain the license and paying fees for sublicenses. The Group amortizes upfront license payments on a straight-line basis over the estimated useful life of the acquired license (eight to ten years). The amortization period and method are reviewed at the end of each financial year under IAS 38.104. Annual fees to maintain a license are amortized over the term of each annual agreement. Sublicense fees are amortized on a straight-line basis over the term of the contract or the estimated useful life of the collaboration for contracts without a set duration.
IN-PROCESS R&D PROGRAMS
This line item contains capitalized upfront payments from the in-licensing of compounds for the Proprietary Development segment, as well as milestone payments for these compounds subsequently paid as milestones are achieved. Additionally, the line item also includes compounds resulting from acquisitions. The assets are recorded at acquisition cost and are not yet available for use and therefore not subject to scheduled amortization. The assets are tested for impairment annually or in case of triggering events, as required by IAS 36.
SOFTWARE
Software is recorded at acquisition cost less accumulated amortization (see below) and any impairment (see Item 2.4.4 in the Notes). Amortization is recognized in profit and loss on a straight-line basis over the estimated useful life of three to five years. Software is amortized from the date the software is operational.
GOODWILL
Goodwill is recognized for expected synergies from business combinations and the skills of the acquired workforce. Goodwill is tested annually for impairment as required by IAS 36 (see also Item 5.7.5 in the Notes).
| Intangible Asset Class | Useful Life | Amortization Rates | ||||||
| Patents |
10 years | 10% | ||||||
| License Rights |
8 - 10 years | 13% - 10% | ||||||
| In-process R&D Programs |
|
Not yet amortized, Impairment Only |
|
– | ||||
| Software |
3 - 5 years | 33% - 20% | ||||||
| Goodwill |
Impairment Only | – | ||||||
| 2.8.7 | PREPAID EXPENSES AND OTHER ASSETS, NET OF CURRENT PORTION |
The non-current portion of expenses that occurred prior to the reporting date but to be recognized in subsequent financial years is also recorded under prepaid expenses. This line item contains maintenance contracts and sublicenses.
This line item also includes other non-current assets, which are recognized at fair value. Other non-current assets consist mainly of restricted cash, such as rent deposits.
F-35
Table of Contents
| 2.9 | ACCOUNTING POLICIES APPLIED TO EQUITY AND LIABILITY ITEMS OF THE BALANCE SHEET |
| 2.9.1 | ACCOUNTS PAYABLE, OTHER LIABILITIES AND PROVISIONS |
Trade payables and other liabilities are recognized at amortized cost. Liabilities with a term of more than one year are discounted to their net present value. Liabilities with uncertain timing or amount are recorded as provisions.
IAS 37 requires the recognition of provisions for obligations to third parties arising from past events. Furthermore, provisions are only recognized for legal or factual obligations to third parties if the event’s occurrence is more likely than not. Provisions are recognized at the amount required to settle the respective obligation and discounted to the reporting date if the interest effect is material. The amount required to meet the obligation also includes expected price and cost increases. The interest portion of the added provisions is recorded in the finance result. The measurement of provisions is based on past experience and considers the circumstances in existence on the reporting date.
The Group has entered into various research and development contracts with research institutions and other companies. These agreements are generally cancelable, and related payments are recorded as research and development expenses as incurred. The Group records accruals for estimated ongoing research costs that have been incurred. When evaluating the adequacy of the accrued expenses, the Group analyzes progress of the studies, including the phase or completion of events, invoices received and contracted costs. Significant judgments and estimates are made in determining the accrued balances at the end of any reporting period. Actual results could differ from the Group’s estimates. The Group’s historical accrual estimates have not been materially different from the actual costs.
| 2.9.2 | TAX PROVISIONS |
Tax liabilities are recognized and measured at their nominal value. Tax liabilities contain obligations from current taxes, excluding deferred taxes. Provisions for trade taxes, corporate taxes and similar taxes on income are determined based on the taxable income of the consolidated entities less any prepayments made.
| 2.9.3 | CURRENT PORTION OF DEFERRED REVENUE |
Upfront payments from customers for services to be rendered by the Group are recognized as deferred revenue in accordance with IAS 18.13 and measured at the lower of fair value or nominal amount of cash received or receivable. The corresponding rendering of services and revenue recognition is expected to occur within a twelve-month period after the reporting date.
| 2.9.4 | DEFERRED REVENUE, NET OF CURRENT PORTION |
This line item includes the non-current portion of deferred upfront payments from customers in accordance with IAS 18.13, which are measured at the lower of fair value or nominal amount of cash received or receivable.
| 2.9.5 | CONVERTIBLE BONDS DUE TO RELATED PARTIES |
The Group issued convertible bonds to the Group’s Management Board and employees. In accordance with IAS 32.28, the equity component of a convertible bond must be recorded separately under additional paid-in capital. The equity component is determined by deducting
F-36
Table of Contents
the separately determined amount of the liability component from the fair value of the convertible bond. The effect of the equity component is recognized in profit and loss in personnel expenses from share-based payments, whereas the effect on profit and loss from the liability component is recognized as interest expense. The Group applies the provisions of IFRS 2 “Share-based Payments” for all convertible bonds granted to the Management Board and the Group’s employees.
| 2.9.6 | DEFERRED TAXES |
The recognition and measurement of deferred taxes are based on the provisions of IAS 12. Deferred tax assets and liabilities are calculated using the liability method, which is common practice internationally. Under this method, taxes expected to be paid or recovered in subsequent financial years are based on the applicable tax rate at the time of recognition.
Deferred tax assets and liabilities are recorded separately in the balance sheet and take into account the future tax effects resulting from temporary differences between values in the balance sheet for assets, liabilities as well as for tax loss carryforwards.
Deferred tax assets are offset against deferred tax liabilities if the taxes are levied by the same taxation authority and the entity has a legally enforceable right to set off current tax assets against current tax liabilities. Pursuant to IAS 12, deferred tax assets and liabilities may not be discounted.
| 2.9.7 | OTHER LIABILITIES |
Other liabilities are made up of rent-free periods. The corresponding release over the minimum rent period are calculated based on the effective interest method. Other liabilities are discounted due to their long-term maturities.
| 2.9.8 | STOCKHOLDERS’ EQUITY |
COMMON STOCK
Ordinary shares are classified as stockholders’ equity. Incremental costs directly attributable to the issue of ordinary shares and stock options are recognized as a deduction from stockholders’ equity.
TREASURY STOCK
Repurchases of the Company’s own shares at prices quoted on an exchange or at market value are recorded in this line item as a deduction from common stock.
When common stock that was recorded as stockholders’ equity is repurchased, the amount of consideration paid, including directly attributable costs, is recognized as a deduction from stockholders’ equity net of taxes and is classified as treasury shares. When treasury shares are subsequently sold or reissued, the proceeds are recognized as an increase in stockholders’ equity, and any difference between the proceeds from the transaction and the initial acquisition costs is recognized in additional paid-in capital.
The allocation of treasury shares to beneficiaries (in this case: performance shares) under long-term incentive programs is reflected in this line item based on the set number of shares to be allocated after the expiration of the four-year vesting period (quantity structure) multiplied by
F-37
Table of Contents
the weighted-average purchase price of the treasury shares (value structure). The adjustment is carried out directly in equity by reducing the treasury stock line item, which is a deduction from common stock, while simultaneously reducing the amount of additional paid-in capital. Further information can be found in Item 7.3.1 in the Notes.
ADDITIONAL PAID- IN CAPITAL
Additional paid-in capital mainly consists of personnel expenses resulting from the grant of convertible bonds and performance shares and the proceeds from newly created shares in excess of their nominal value.
REVALUATION RESERVE
The revaluation reserve mainly consists of unrealized gains and losses on available-for-sale financial assets and bonds that are measured directly in equity until they are sold as well as cash flow hedges.
ACCUMULATED INCOME/DEFICIT
The “accumulated income/deficit” line item consists of the Group’s accumulated consolidated net profits/losses. A separate measurement of this item is not made.
MorphoSys Group applies IFRS 8 “Operating Segments ”. An operating segment is defined as a unit of an entity that engages in business activities from which it can earn revenues and incur expenses and whose operating results are regularly reviewed by the entity’s chief operating decision maker, the Management Board, and for which discrete financial information is available.
Segment information is provided for the Group’s operating segments based on the Group’s management and internal reporting structures. The segment results and segment assets include items that can be either directly attributed to the individual segment or allocated to the segments on a reasonable basis.
The Management Board evaluates a segment’s economic success using selected key figures so that all relevant income and expenses are included. EBIT, which the Company defines as earnings before finance income, finance expenses and income taxes, is the key benchmark for measuring and evaluating the operating results. Refer to the table in Note 3.3 for a reconciliation of EBIT to Net income as well as to the table in Note 4.3 for a breakdown of finance income and expenses. Other key internal reporting figures include revenues, operating expenses, segment results and the liquidity position.
The Group consists of the following operating segments.
| 3.1 | PROPRIETARY DEVELOPMENT |
The segment comprises all activities related to the proprietary development of therapeutic antibodies and peptides. These activities currently comprise a total of 13 antibodies and peptides, including the proprietary clinical programs MOR208, MOR202, and MOR106, which is co-developed with Galapagos. The proprietary program MOR103, also included in this segment,
F-38
Table of Contents
was out-licensed to GlaxoSmithKline (GSK) in 2013 and all activities since that time are conducted by GSK. This program has been allocated to this segment since the beginning of its development and will, therefore, continue to be reported under this segment. MorphoSys is also pursuing other programs that are either at an early stage of proprietary development or fall under co-development agreements. One of these programs is the clinical program MOR107 (formerly LP2) resulting from the acquisition of Lanthio Pharma B.V. A further eight programs are in the discovery phase. The development of proprietary technologies is allocated to the Proprietary Development segment.
| 3.2 | PARTNERED DISCOVERY |
MorphoSys possesses one of the leading technologies for generating therapeutics based on human antibodies. The Group markets this technology commercially through its partnerships with numerous pharmaceutical and biotechnology companies. The Partnered Discovery segment encompasses all operating activities relating to these commercial agreements.
| 3.3 | CROSS-SEGMENT DISCLOSURE |
The information on segment assets is based on the assets’ respective locations.
| For the Twelve-month Period Ended 31 December |
Proprietary Development |
Partnered Discovery |
Unallocated | Group | ||||||||||||||||||||||||||||
| (in 000’s €) | 2017 | 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | 2016 | ||||||||||||||||||||||||
| External Revenues |
17,635 | 621 | 49,156 | 49,123 | 0 | 0 | 66,791 | 49,744 | ||||||||||||||||||||||||
| Other Operating Expenses |
(99,106 | ) | (78,515 | ) | (18,906 | ) | (18,113 | ) | (15,835 | ) | (13,212 | ) | (133,847 | ) | (109,840 | ) | ||||||||||||||||
|
|
|
|||||||||||||||||||||||||||||||
| Segment Result |
(81,471 | ) | (77,894 | ) | 30,250 | 31,010 | (15,835 | ) | (13,212 | ) | (67,056 | ) | (60,096 | ) | ||||||||||||||||||
| Other Income |
157 | 327 | 0 | 0 | 963 | 382 | 1,120 | 709 | ||||||||||||||||||||||||
| Other Expenses |
0 | 0 | 0 | 0 | (1,671 | ) | (554 | ) | (1,671 | ) | (554 | ) | ||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||||||
| Segment EBIT |
(81,314 | ) | (77,567 | ) | 30,250 | 31,010 | (16,543 | ) | (13,384 | ) | (67,607 | ) | (59,941 | ) | ||||||||||||||||||
| Finance Income |
712 | 1,385 | ||||||||||||||||||||||||||||||
| Finance Expenses |
(1,895 | ) | (1,308 | ) | ||||||||||||||||||||||||||||
| Profit before Taxes |
(68,790 | ) | (59,864 | ) | ||||||||||||||||||||||||||||
| Income Tax Expenses |
(1,036 | ) | (519 | ) | ||||||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||||||
| Net Loss |
(69,826 | ) | (60,383 | ) | ||||||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||||||
| Current Assets |
8,802 | 13,157 | 18,054 | 18,415 | 313,825 | 276,484 | 340,681 | 308,056 | ||||||||||||||||||||||||
| Non-current Assets |
60,658 | 59,292 | 8,490 | 10,165 | 5,569 | 86,087 | 74,717 | 155,544 | ||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||||||
| Total Segment Assets |
69,460 | 72,449 | 26,544 | 28,580 | 319,394 | 362,571 | 415,398 | 463,600 | ||||||||||||||||||||||||
| Current Liabilities |
33,008 | 20,948 | 4,083 | 2,512 | 10,610 | 14,842 | 47,701 | 38,302 | ||||||||||||||||||||||||
| Non-current Liabilities |
7,072 | 6,930 | 1,045 | 2,165 | 909 | 743 | 9,026 | 9,838 | ||||||||||||||||||||||||
| Stockholders’ Equity |
0 | 0 | 0 | 0 | 358,671 | 415,460 | 358,671 | 415,460 | ||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||||||
| Total Segment Liabilities and Equity |
40,080 | 27,878 | 5,128 | 4,677 | 370,190 | 431,045 | 415,398 | 463,600 | ||||||||||||||||||||||||
| Capital Expenditure |
12,344 | 1,358 | 602 | 1,181 | 204 | 374 | 13,150 | 2,913 | ||||||||||||||||||||||||
| Depreciation and Amortization |
1,555 | 1,272 | 2,075 | 2,117 | 400 | 375 | 4,030 | 3,764 | ||||||||||||||||||||||||
The segment result is defined as a segment’s revenue less the segment’s operating expenses. The unallocated other operating expenses of € 15.8 million (2016: € 13.2 million) includes primarily
F-39
Table of Contents
expenses for central administrative functions that are not allocated to one of the two segments. Finance income, finance expense and income tax are also not allocated to the segments as they are managed on a group basis. In the 2017 financial year, impairments totaling € 9.9 million were recognized in the Proprietary Development segment (2016: impairments of € 10.1 million in the Proprietary Discovery segment).
The Group’s key customers are allocated to the Partnered Discovery and Proprietary Development segments. As of December 31, 2017, the single most important customer represented accounts receivable with of a carrying amount of € 5.1 million (December 31, 2016: € 8.4 million). The largest customer accounted for revenues in 2017 of € 36.9 million, the second largest for € 16.8 million and the third largest for € 6.7 million. The largest and third largest customers were allocated to the Partnered Discovery segment and the second largest customer to the Proprietary Development segment. The top three of the Group’s customers that were all allocated to the Partnered Discovery segment accounted for € 42.1 million, € 2.5 million and € 2.5 million, respectively, of the total revenues in 2016.
The following overview shows the Group’s regional distribution of revenue.
| in 000’ € | 2017 | 2016 | ||||||
| Germany |
851 | 1,621 | ||||||
| Europe and Asia |
57,229 | 43,046 | ||||||
| USA and Canada |
8,711 | 5,077 | ||||||
|
|
|
|||||||
| Total |
66,791 | 49,744 | ||||||
|
|
|
|||||||
A total of € 42.2 million (December 31, 2016: € 123.7 million) and € 32.6 million (December 31, 2016: € 32.6 million) of the Group’s non-current assets, excluding deferred tax assets, are located in Germany and the Netherlands, respectively. The Group’s total investments of € 13.1 million (December 31, 2016: € 2.8 million) were made in Germany, except for € 0.1 million (December 31, 2016: € 0.1 million), which were made in the Netherlands. In accordance with internal definitions, investments only included additions to property, plant and equipment as well as intangible assets which are not related to business combinations. MorphoSys defines investments as additions to non-current assets that are not related to acquisitions.
4 Notes to the Income Statement
| 4.1 | REVENUES |
In 2017, revenues consisted of license fees and milestone payments totaling € 44.8 million (2016: € 28.4 million). Of this total, € 16.8 million was generated by the Proprietary Development segment and € 28.0 million was generated by the Partnered Discovery segment. In 2016, all such revenues of € 28.4 million were generated by the Partnered Discovery segment.
Of the service fee revenues totaling € 22.0 million (2016: € 21.4 million), € 0.8 million (2016: € 0.6 million) were attributable to the Proprietary Development segment and € 21.2 million (2016: € 20.8 million) to the Partnered Discovery segment.
F-40
Table of Contents
| 4.2 | OPERATING EXPENSES |
| 4.2.1 | RESEARCH AND DEVELOPMENT EXPENSES |
Research and development increased compared to the prior year due to a high level of investment in our proprietary product pipeline (namely, external services) and increased personnel expenses. Research and development expenses consisted of the items below.
| in 000’ € | 2017 | 2016 | ||||||
| Personnel Expenses |
29,735 | 26,493 | ||||||
| Consumable Supplies |
2,588 | 2,321 | ||||||
| Other Operating Expenses |
3,065 | 2,922 | ||||||
| Impairment, Amortization and Other Costs of Intangible Assets |
13,503 | 13,689 | ||||||
| External Services |
63,053 | 44,409 | ||||||
| Depreciation and Other Costs for Infrastructure |
4,865 | 5,889 | ||||||
|
|
|
|||||||
| Total |
116,809 | 95,723 | ||||||
|
|
|
|||||||
| in million € | 2017 | 2016 | ||||||
| R&D Expenses on behalf of Partners |
17.7 | 17.2 | ||||||
| Proprietary Development Expenses |
97.7 | 77.1 | ||||||
| Technology Development Expenses |
1.4 | 1.4 | ||||||
|
|
|
|||||||
| R&D Total |
116.8 | 95.7 | ||||||
|
|
|
|||||||
| 4.2.2 | GENERAL AND ADMINISTRATIVE EXPENSES |
General and administrative expenses included the items below.
| in 000’ € | 2017 | 2016 | ||||||
| Personnel Expenses |
12,315 | 9,521 | ||||||
| Consumable Supplies |
33 | 97 | ||||||
| Other Operating Expenses |
794 | 978 | ||||||
| Amortization of Intangible Assets |
112 | 111 | ||||||
| External Services |
2,947 | 2,484 | ||||||
| Depreciation and Other Costs for Infrastructure |
838 | 925 | ||||||
|
|
|
|||||||
| Total |
17,039 | 14,116 | ||||||
|
|
|
|||||||
| 4.2.3 | PERSONNEL EXPENSES |
Personnel expenses included the items below.
| in 000’ € | 2017 | 2016 | ||||||
| Wages and Salaries |
28,196 | 27,146 | ||||||
| Social Security Contributions |
4,542 | 4,570 | ||||||
| Stock-based Compensation Expense |
4,975 | 2,357 | ||||||
| Temporary Staff (External) |
881 | 1,061 | ||||||
| Other |
3,456 | 880 | ||||||
|
|
|
|||||||
| Total |
42,050 | 36,014 | ||||||
|
|
|
|||||||
F-41
Table of Contents
In 2017, other personnel expenses consisted primarily of severance payments, recruitment and development costs. In 2016, other personnel expenses consisted mainly of recruitment costs.
The average number of employees in the 2017 financial year was 344 (2016: 354). Of the 326 employees on December 31, 2017 (December 31, 2016: 345), 263 were active in research and development (December 31, 2016: 289) and 63 were engaged in general and administrative functions (December 31, 2016: 56 employees). As of December 31, 2017, there were 161 employees in the Proprietary Development segment and 105 employees in the Partnered Discovery segment; 60 employees were not allocated to a segment (December 31, 2016: 135 in the Proprietary Development segment, 156 employees in the Partnered Discovery segment and 54 employees were unallocated). Costs for defined-contribution plans amounted to € 0.6 million in 2017 (2016: € 0.5 million).
| 4.3 | OTHER INCOME AND EXPENSES, FINANCE INCOME AND FINANCE EXPENSES |
The line items “other income and expenses” and “finance income and finance expenses” include the following items.
| in 000’ € | 2017 | 2016 | ||||||
| Grant Income |
157 | 327 | ||||||
| Gain on Foreign Exchange |
485 | 192 | ||||||
| Reversal of Impairment for Accounts Receivable Previously Deemed Impaired |
76 | 15 | ||||||
| Miscellaneous Income |
402 | 175 | ||||||
|
|
|
|||||||
| Other Income |
1,120 | 709 | ||||||
|
|
|
|||||||
| Loss on Foreign Exchange |
(844 | ) | (400 | ) | ||||
| Impairment of Other Receivables |
0 | (7 | ) | |||||
| Miscellaneous Expenses |
(827 | ) | (147 | ) | ||||
|
|
|
|||||||
| Other Expenses |
(1,671 | ) | (554 | ) | ||||
|
|
|
|||||||
| Gain on Available-for-sale Financial Assets and Bonds |
35 | 294 | ||||||
| Interest Income |
236 | 1,017 | ||||||
| Gain on Derivatives |
441 | 74 | ||||||
|
|
|
|||||||
| Finance Income |
712 | 1,385 | ||||||
|
|
|
|||||||
| Interest Expenses |
(374 | ) | (20 | ) | ||||
| Loss on Derivatives |
(1,360 | ) | (44 | ) | ||||
| Bank Fees |
(41 | ) | (35 | ) | ||||
| Loss on Available-for-sale Financial Assets and Bonds |
(120 | ) | (1,209 | ) | ||||
|
|
|
|||||||
| Finance Expenses |
(1,895 | ) | (1,308 | ) | ||||
|
|
|
|||||||
F-42
Table of Contents
| 4.4 | INCOME TAX EXPENSES/INCOME |
MorphoSys AG and its German subsidiary Sloning BioTechnology GmbH are subject to corporate taxes, the solidarity surcharge and trade taxes. The Company’s corporate tax rate is 15.0% and the solidarity surcharge 5.5%. The effective trade tax rate is 10.85% and remained unchanged.
The Dutch entities Lanthio Pharma B.V. and LanthioPep B.V. are subject to an income tax rate of 25% on annual income exceeding € 200,000; annual income below € 200,000 is subject to a tax rate of 20%. Subject to certain conditions, a tax rate of 5% may be applicable under what is known as the “Innovation Box.”
Income taxes consist of the items listed below.
| in 000’ € | 2017 | 2016 | ||||||
| Current Tax Income / (Expense) (Thereof Regarding Prior Years: k€ 171; 2016: k€ (60)) |
(534 | ) | 45 | |||||
| Deferred Tax Expenses |
(502 | ) | (564 | ) | ||||
|
|
|
|||||||
| Total Income Tax Expense |
(1,036 | ) | (519 | ) | ||||
|
|
|
|||||||
| Total Amount of Current Taxes Resulting from Entries Directly Recognized in Other Comprehensive Income |
0 | (82 | ) | |||||
| Total Amount of Deferred Taxes Resulting from Entries Directly Recognized in Other Comprehensive Income |
0 | (112 | ) | |||||
|
|
|
|||||||
| Total Amount of Tax-Effects Resulting from Entries Directly Recognized in Equity or Other Comprehensive Income |
0 | (194 | ) | |||||
|
|
|
|||||||
The following table reconciles the expected income tax expense with the actual income tax expense as presented in the consolidated financial statements. The combined income tax rate of 26.675% in the 2017 financial year (2016: 26.675%) was applied to profit before taxes to calculate the statutory income tax expense. This rate consisted of a corporate income tax of 15.0%, a solidarity surcharge of 5.5% on the corporate tax and an average trade tax of 10.85% applicable to the Group.
| in 000’ € | 2017 | 2016 | ||||||
| Profit Before Income Taxes |
(68,790 | ) | (59,864 | ) | ||||
| Expected Tax Rate |
26.675% | 26,675% | ||||||
| Expected Income Tax |
18,350 | 15,969 | ||||||
| Tax Effects Resulting from: |
||||||||
| Stock-based Compensation |
(290 | ) | 5 | |||||
| Non-Tax-Deductible Items |
(134 | ) | (135 | ) | ||||
| Differences in Profit and Loss Neutral Adjustments |
37 | 812 | ||||||
| Non-Recognition of Deferred Tax Assets on Temporary Differences |
3,256 | (3,766 | ) | |||||
| Non-Recognition of Deferred Tax Assets on Current Year Tax Losses |
(22,007 | ) | (13,354 | ) | ||||
| Tax Rate Differences to Local Tax Rates |
(71 | ) | (46 | ) | ||||
| Prior Year Taxes |
(171 | ) | 0 | |||||
| Other Effects |
(6 | ) | (4 | ) | ||||
|
|
|
|||||||
| Actual Income Tax |
(1,036 | ) | (519 | ) | ||||
|
|
|
|||||||
F-43
Table of Contents
As of December 31, 2017, neither deferred tax assets in the amount of € 33.6 million on tax loss carryforwards (December 31, 2016: € 12.8 million) nor deferred tax assets on temporary differences in the amount of € 0.5 million (December 31, 2016: € 3.8 million) were recognized by MorphoSys AG due to continued substantial investments in proprietary product development and related business development.
As of December 31, 2017, tax loss carryforwards of Sloning BioTechnology GmbH were fully exhausted. As of December 31, 2016, deferred tax assets in the amount of € 0.5 million were recognized on tax loss carryforwards.
As of December 31, 2017, deferred tax assets in the amount of € 3.8 million on tax loss carryforwards (December 31, 2016: € 2.5 million) were not recognized for the Lanthio Group due to continued substantial investments in proprietary product development and related business development.
Deferred tax assets and deferred tax liabilities are composed as follows.
| in 000’s €, as of December 31 | Deferred Tax Asset 2017 |
Deferred Tax Asset 2016 |
Deferred Tax Liability 2017 |
Deferred Tax Liability 2016 |
||||||||||||
| Intangible Assets |
0 | 0 | 8,297 | 8,068 | ||||||||||||
| Receivables and Other Assets |
0 | 0 | 0 | 8 | ||||||||||||
| Prepaid Expenses and Deferred Charges |
0 | 0 | 3 | 3 | ||||||||||||
| Short-term Securities Investments |
0 | 19 | 0 | 131 | ||||||||||||
| Provisions |
253 | 130 | 0 | 0 | ||||||||||||
| Other Liabilities |
236 | 123 | 0 | 0 | ||||||||||||
| Tax Losses |
0 | 516 | 0 | 0 | ||||||||||||
|
|
|
|||||||||||||||
| Total |
489 | 788 | 8,300 | 8,210 | ||||||||||||
|
|
|
|||||||||||||||
| Changes in Deferred Taxes in 2017 | ||||||||
| in 000’s €, as of December 31 | Recognized in Profit and Loss Income / (Expense) |
Recognized in Other Comprehensive Income |
||||||
| Intangible Assets |
(229 | ) | 0 | |||||
| Receivables and Other Assets |
8 | 0 | ||||||
| Short-term Securities Investments and cash flow hedge |
0 | 112 | ||||||
| Provisions |
123 | 0 | ||||||
| Other Liabilities |
113 | 0 | ||||||
| Tax Losses |
(516 | ) | 0 | |||||
|
|
|
|||||||
| Total |
(501 | ) | 112 | |||||
|
|
|
|||||||
As of December 31, 2017, temporary differences existed in connection with investments in subsidiaries (known as outside basis differences) of € 0.2 million (December 31, 2016: € 0.3 million) for which no deferred tax liabilities were recognized.
F-44
Table of Contents
| 4.5 | EARNINGS PER SHARE |
Earnings per share is computed by dividing the 2017 consolidated net loss of € 69,826,469 (2016: consolidated net loss of € 60,382,776) by the weighted-average number of ordinary shares outstanding during the respective year (2017: 28,947,566; 2016: 26,443,415).
The table below shows the calculation of the weighted-average number of ordinary shares.
| 2017 | 2016 | |||||||
| Shares Issued on January 1 |
29,159,770 | 26,537,682 | ||||||
| Effect of Treasury Shares Held on January 1 |
(396,010 | ) | (434,670 | ) | ||||
| Effect of Repurchase of Treasury Stock |
0 | (34,812 | ) | |||||
| Effect of Share Issuance |
0 | 327,761 | ||||||
| Effect of Transfer of Treasury Stock to Members of the Management Board |
7,759 | 0 | ||||||
| Effect of Transfer of Treasury Stock / Shares Issued in January |
0 | 0 | ||||||
| Effect of Transfer of Treasury Stock / Shares Issued in February |
0 | 0 | ||||||
| Effect of Transfer of Treasury Stock / Shares Issued in March |
0 | 0 | ||||||
| Effect of Transfer of Treasury Stock / Shares Issued in April |
154,250 | 12,638 | ||||||
| Effect of Transfer of Treasury Stock / Shares Issued in May |
3,778 | 10,039 | ||||||
| Effect of Transfer of Treasury Stock / Shares Issued in June |
1,094 | 17,749 | ||||||
| Effect of Transfer of Treasury Stock / Shares Issued in July |
2,038 | 0 | ||||||
| Effect of Transfer of Treasury Stock / Shares Issued in August |
2,669 | 6,463 | ||||||
| Effect of Transfer of Treasury Stock / Shares Issued in September |
3,976 | 490 | ||||||
| Effect of Transfer of Treasury Stock / Shares Issued in October |
2,566 | 76 | ||||||
| Effect of Transfer of Treasury Stock / Shares Issued in November |
5,549 | 0 | ||||||
| Effect of Transfer of Treasury Stock / Shares Issued in December |
127 | 0 | ||||||
| Weighted-average Number of Shares of Common Stock |
28,947,566 | 26,443,415 | ||||||
In 2017 and 2016, diluted earnings per share equal basic earnings per share. The effect of 87,904 potentially dilutive shares in 2017 (2016: 99,764 dilutive shares) resulting from stock options and convertible bonds granted to the Management Board, the Senior Management Group and employees of the Company who are not members of the Senior Management Group, has been excluded from the diluted earnings per share because it would result in a decrease in the loss per share and is therefore not to be treated as dilutive.
5 Notes to the Assets of the Balance Sheet
| 5.1 | CASH AND CASH EQUIVALENTS |
| in 000’ € | 12/31/2017 | 12/31/2016 | ||||||
| Bank Balances and Cash in Hand |
76,589 | 73,929 | ||||||
| Term Deposits |
1,133 | 1,252 | ||||||
| Restricted Cash |
(1,133 | ) | (1,252 | ) | ||||
|
|
|
|||||||
| Cash and Cash Equivalents |
76,589 | 73,929 | ||||||
|
|
|
|||||||
Restricted cash of € 1.1 million mainly consisted of rent deposits (2016: € 1.3 million).
F-45
Table of Contents
| 5.2 | FINANCIAL ASSETS AND BONDS, AVAILABLE-FOR-SALE AND FINANCIAL ASSETS CLASSIFIED AS LOANS AND RECEIVABLES |
As of December 31, 2017 and December 31, 2016, available-for-sale financial assets consisted of the items below.
| Gross Unrealized | ||||||||||||||||||||
| in 000’ € | Maturity | Cost | Gains | Losses | Market Value |
|||||||||||||||
| December 31, 2017 |
||||||||||||||||||||
| Money Market Funds |
daily | 86,644 | 0 | 106 | 86,538 | |||||||||||||||
|
|
|
|||||||||||||||||||
| Total |
86,538 | |||||||||||||||||||
|
|
|
|||||||||||||||||||
| December 31, 2016 |
||||||||||||||||||||
| Money Market Funds |
daily | 63,433 | 2 | 73 | 63,362 | |||||||||||||||
|
|
|
|||||||||||||||||||
| Total |
63,362 | |||||||||||||||||||
|
|
|
|||||||||||||||||||
In 2017, the Group recorded a net gain of less than € 0.1 million in the income statement from the disposal of financial assets. This gain was previously recognized in stockholders’ equity (2016: net gain of € 0.3 million).
As of December 31, 2017 and December 31, 2016, bonds, available-for-sale consisted of the items below.
| Gross Unrealized | ||||||||||||||||||||
| in 000’ € | Maturity | Cost | Gains | Losses | Market Value |
|||||||||||||||
| December 31, 2017 |
||||||||||||||||||||
| Bonds |
daily | 0 | 0 | 0 | 0 | |||||||||||||||
|
|
|
|||||||||||||||||||
| Total |
0 | |||||||||||||||||||
|
|
|
|||||||||||||||||||
| December 31, 2016 |
||||||||||||||||||||
| Bonds |
daily | 6,620 | 2 | 90 | 6,532 | |||||||||||||||
|
|
|
|||||||||||||||||||
| Total |
6,532 | |||||||||||||||||||
|
|
|
|||||||||||||||||||
In 2017, the Group recorded a net loss of € 0.1 million from the disposal of financial assets contained in the income statement that were previously recognized in stockholders’ equity (2016: net loss of € 1.2 million). The bonds were purchased at a price above their nominal value. The loss that resulted from the product-specific price development is more than offset by the bond’s interest income.
As of December 31, 2017, the Company held current financial assets of € 149.1 million (December 31, 2016: € 136.1 million) and no non-current financial assets (December 31, 2016: € 79.5 million), which were allocated to the “loans and receivables” category in accordance with IAS 39 “Financial Instruments”. These financial assets consisted mainly of term deposits with fixed or variable interest rates. The decline in financial assets resulted from the expiry of their
F-46
Table of Contents
agreed holding periods and the use of the related cash released for operating activities. The carrying amounts included interest receivables of € 0.1 million (December 31, 2016: € 0.1 million).
Interest income from financial assets under “loans and receivables” amounted to € 0.2 million (2016: € 0.9 million) and was recorded in the finance result. The risk associated with these financial instruments primarily resulted from bank credit risks. There was no indication of impairment in the financial year 2017.
Further information on the accounting for financial assets is provided in Item 2.8.1 in the Notes.
| 5.3 | ACCOUNTS RECEIVABLE |
All accounts receivable are non-interest bearing, and generally have payment terms of between 30 and 45 days. As of December 31, 2017 and December 31, 2016, accounts receivable included unbilled receivables amounting to € 5.3 million and € 3.3 million, respectively.
Based on the Management Board’s estimate, no net loss for allowances for doubtful receivables was recognized in profit and loss in 2017 and 2016.
| 5.4 | OTHER RECEIVABLES |
As of December 31, 2017, there were no impairments recognized for other receivables. An immaterial amount of impairments had been recognized as of December 31, 2016.
| 5.5 | INCOME TAX RECEIVABLES, INVENTORIES, PREPAID EXPENSES AND OTHER CURRENT ASSETS |
As of December 31, 2017 income tax receivables amounted to € 0.7 million (December 31, 2016: € 0.5 million) and consisted of receivables from capital gain taxes withheld and income taxes for prior years.
Inventories amounting to € 0.3 million as of December 31, 2017 (December 31, 2016: € 0.3 million) were stored at the Planegg location and consisted of raw materials and supplies. As in the previous year, no inventories were carried at fair value less selling costs as of the reporting date.
As of December 31, 2017, prepaid expenses and other current assets mainly consisted of combination compounds of € 11.2 million (December 31, 2016: € 7.3 million), receivables due from tax authorities for the remaining surplus from prepayments for value-added taxes of € 2.4 million (December 31, 2016: € 2.8 million), prepaid fees for external laboratory services of € 0.6 million (December 31, 2016: € 2.4 million), prepaid fees for sublicenses of € 0.4 million (December 31, 2016: € 0.3 million), restricted cash for rent deposits of € 0.4 million (December 31, 2016: € 0.4 million) and other prepayments amounting to € 1.1 million (December 31, 2016: € 0.8 million).
F-47
Table of Contents
| 5.6 | PROPERTY, PLANT AND EQUIPMENT |
| in 000’ € | Office and Laboratory Equipment |
Furniture and Fixtures |
Total | |||||||||
| Cost |
||||||||||||
| January 1, 2017 |
16,658 | 2,389 | 19,047 | |||||||||
| Additions |
1,205 | 112 | 1,317 | |||||||||
| Disposals |
(528 | ) | 0 | (528 | ) | |||||||
|
|
|
|||||||||||
| December 31, 2017 |
17,335 | 2,501 | 19,836 | |||||||||
|
|
|
|||||||||||
| Accumulated Depreciation and Impairment |
||||||||||||
| January 1, 2017 |
13,120 | 1,738 | 14,858 | |||||||||
| Depreciation Charge for the Year |
1,887 | 82 | 1,969 | |||||||||
| Impairment |
0 | 0 | 0 | |||||||||
| Disposals |
(517 | ) | 0 | (517 | ) | |||||||
|
|
|
|||||||||||
| December 31, 2017 |
14,490 | 1,820 | 16,310 | |||||||||
|
|
|
|||||||||||
| Carrying Amount |
||||||||||||
| January 1, 2017 |
3,538 | 651 | 4,189 | |||||||||
| December 31, 2017 |
2,845 | 681 | 3,526 | |||||||||
| Cost |
||||||||||||
| January 1, 2016 |
15,040 | 1,780 | 16,820 | |||||||||
| Additions |
1,890 | 612 | 2,502 | |||||||||
| Disposals |
(272 | ) | (3 | ) | (275 | ) | ||||||
|
|
|
|||||||||||
| December 31, 2016 |
16,658 | 2,389 | 19,047 | |||||||||
|
|
|
|||||||||||
| Accumulated Depreciation and Impairment |
||||||||||||
| January 1, 2016 |
11,691 | 1,655 | 13,346 | |||||||||
| Depreciation Charge for the Year |
1,700 | 86 | 1,786 | |||||||||
| Impairment |
0 | 0 | 0 | |||||||||
| Disposals |
(271 | ) | (3 | ) | (274 | ) | ||||||
|
|
|
|||||||||||
| December 31, 2016 |
13,120 | 1,738 | 14,858 | |||||||||
|
|
|
|||||||||||
| Carrying Amount |
||||||||||||
| January 1, 2016 |
3,349 | 125 | 3,474 | |||||||||
| December 31, 2016 |
3,538 | 651 | 4,189 | |||||||||
No impairment of property, plant and equipment was recognized in the 2017 and 2016 financial years.
No borrowing costs were capitalized during the reporting period. There were neither restrictions on retention of title nor property, plant and equipment pledged as security for liabilities. There were no material contractual commitments for the purchase of property, plant and equipment as of the reporting date.
F-48
Table of Contents
Depreciation is included in the following line items of the income statement.
| in 000’ € | 2017 | 2016 | ||||||
| Research and Development |
1,672 | 1,518 | ||||||
| General and Administrative |
297 | 268 | ||||||
|
|
|
|||||||
| Total |
1,969 | 1,786 | ||||||
|
|
|
|||||||
|
|
|
|||||||
| 5.7 | INTANGIBLE ASSETS |
| in 000’ € | Patents | License Rights |
In-process R&D Programs |
Software | Goodwill | Total | ||||||||||||||||||
| Cost |
||||||||||||||||||||||||
| January 1, 2017 |
16,419 | 23,896 | 60,960 | 5,800 | 11,041 | 118,116 | ||||||||||||||||||
| Additions |
640 | 0 | 11,140 | 53 | 0 | 11,833 | ||||||||||||||||||
| Disposals |
(64 | ) | 0 | (19,941 | ) | 0 | 0 | (20,005 | ) | |||||||||||||||
|
|
|
|||||||||||||||||||||||
| December 31, 2017 |
16,995 | 23,896 | 52,159 | 5,853 | 11,041 | 109,944 | ||||||||||||||||||
|
|
|
|||||||||||||||||||||||
| Accumulated Amortization and Impairment |
||||||||||||||||||||||||
| January 1, 2017 |
11,096 | 20,749 | 10,141 | 4,515 | 3,676 | 50,177 | ||||||||||||||||||
| Amortization Charge for the Year |
1,230 | 148 | 0 | 683 | 0 | 2,061 | ||||||||||||||||||
| Impairment |
64 | 0 | 9,800 | 0 | 0 | 9,864 | ||||||||||||||||||
| Disposals |
(64 | ) | 0 | (19,941 | ) | 0 | 0 | (20,005 | ) | |||||||||||||||
|
|
|
|||||||||||||||||||||||
| December 31, 2017 |
12,326 | 20,897 | 0 | 5,198 | 3,676 | 42,097 | ||||||||||||||||||
|
|
|
|||||||||||||||||||||||
| Carrying Amount |
||||||||||||||||||||||||
| January 1, 2017 |
5,323 | 3,147 | 50,819 | 1,285 | 7,365 | 67,939 | ||||||||||||||||||
| December 31, 2017 |
4,669 | 2,999 | 52,159 | 655 | 7,365 | 67,847 | ||||||||||||||||||
| Cost |
||||||||||||||||||||||||
| January 1, 2016 |
16,064 | 23,896 | 60,960 | 5,744 | 11,041 | 117,705 | ||||||||||||||||||
| Additions |
355 | 0 | 0 | 56 | 0 | 411 | ||||||||||||||||||
|
|
|
|||||||||||||||||||||||
| December 31, 2016 |
16,419 | 23,896 | 60,960 | 5,800 | 11,041 | 118,116 | ||||||||||||||||||
|
|
|
|||||||||||||||||||||||
| Accumulated Amortization and Impairment |
||||||||||||||||||||||||
| January 1, 2016 |
9,923 | 20,651 | 0 | 3,808 | 3,676 | 38,058 | ||||||||||||||||||
| Amortization Charge for the Year |
1,173 | 98 | 0 | 707 | 0 | 1,978 | ||||||||||||||||||
| Impairment |
0 | 0 | 10,141 | 0 | 0 | 10,141 | ||||||||||||||||||
|
|
|
|||||||||||||||||||||||
| December 31, 2016 |
11,096 | 20,749 | 10,141 | 4,515 | 3,676 | 50,177 | ||||||||||||||||||
|
|
|
|||||||||||||||||||||||
| Carrying Amount |
||||||||||||||||||||||||
| January 1, 2016 |
6,141 | 3,245 | 60,960 | 1,936 | 7,365 | 79,647 | ||||||||||||||||||
| December 31, 2016 |
5,323 | 3,147 | 50,819 | 1,285 | 7,365 | 67,939 | ||||||||||||||||||
F-49
Table of Contents
In the 2017 financial year, impairment losses of € 0.1 million were recognized on patents and licenses. No impairment of patents and licenses was recognized in the 2016 financial year.
As of December 31, 2017, in-process research and development programs were subject to an impairment test as required by IAS 36. This test indicated no need for impairment. Further details on the impairment of in-process research and development programs can be found in Item 5.7.3 in the Notes.
The carrying amount of intangible assets pledged as security amounts to € 26.5 million and relates to a government grant in the amount of € 1.5 million.
Amortization is included in the following line items of the income statement.
| in 000’ € | 2017 | 2016 | ||||||
| Research and Development |
1,958 | 1,872 | ||||||
| Research and Development (Write-off) |
9,864 | 10,141 | ||||||
| General and Administrative |
103 | 106 | ||||||
|
|
|
|||||||
| Total |
11,925 | 12,119 | ||||||
|
|
|
|||||||
| 5.7.1 | PATENTS |
In the 2017 financial year, the carrying amount of patents declined by € 0.6 million from € 5.3 million to € 4.7 million. This was the result of additions amounting to € 0.6 million for patent applications, particularly for proprietary programs and technologies, which were offset by straight-line amortization of € 1.2 million.
| 5.7.2 | LICENSES |
In the 2017 financial year, the carrying amount of licenses declined by € 0.1 million from € 3.1 million to € 3.0 million.
| 5.7.3 | IN-PROCESS R&D PROGRAMS |
In the 2017 financial year, the carrying amount of in-process R&D programs increased by € 1.3 million to € 52.2 million. The reason for this increase was the capitalization of a milestone payment made in the amount of € 11.1 million, which was offset by an impairment on MOR209/ES414 of € 9.8 million. The reason for the impairment was the termination of the cooperation with Aptevo Therapeutics in 2017 due to the expectation of a delay in the development plan, a delayed market entry and a delay in the occurrence of future cash flows compared to previous assumptions.
As of December 31, 2017, this balance sheet item contained capitalized upfront payments from the in-licensing of one compound for the Proprietary Development segment as well as subsequent milestone payments for this compound which were paid at a later point in time. Additionally, the line item also included two compounds resulting from an acquisition.
The annual impairment test was performed on September 30, 2017.At that time, the compound MOR208, an intangible asset with indefinite useful life and a carrying amount of € 23.9 million, was subject to an impairment test as required by IAS 36. The recoverable amount of the cash-generating unit MOR208, which is part of the Proprietary Development segment, was determined on the basis of value-in-use calculations. The calculation showed that the recoverable amount was higher than the carrying amount of the cash-generating unit. The cash
F-50
Table of Contents
flow forecasts took into account expected cash inflows from the potential commercialization of the compound and cash outflows from the expected research and development as well as commercialization costs. The cash flow forecasts are based on the term with patent protection for MOR208. For this reason, a planning horizon of about 20 years is considered appropriate for the value-in-use calculation. The values of the underlying assumptions were determined using both internal (past experience) and external sources of information (market information). Based on the updated cash flow forecast, the value-in-use was determined as follows: A beta factor of 1.2 (2016: 1.2) and WACC before taxes of 9.4% (2016: 8.6%). A detailed sensitivity analysis was performed for the discount rate. A sensitivity analysis for changes in the cash flows has not been performed since the cash flows from research and development as well as commercialization of the compound have already been probability-adjusted in the value-in-use calculations so as to reflect the probabilities of success of phases in clinical trials. The analysis did not reveal any need for impairment. The values ascribed to the assumptions correspond to the Management Board’s forecasts for future development and are based on internal planning scenarios as well as external sources of information. No indicators for impairments were identified at December 31, 2017.
| 5.7.4 | SOFTWARE |
In the 2017 financial year, additions to this line item totaled € 0.1 million. The carrying amount decreased by € 0.6 million from € 1.3 million in 2016 to € 0.7 million in 2017. Additions were offset by amortization of € 0.7 million.
| 5.7.5 | GOODWILL |
The annual goodwill impairment test was performed on September 30, 2017.
As of September 30, 2017, goodwill of € 3.7 million from the 2010 acquisition of Sloning BioTechnology GmbH was subject to an impairment test as required by IAS 36. The recoverable amount of the cash-generating unit Slonomics technology, which is part of the Partnered Discovery segment, was determined on the basis of value-in-use calculations. The calculation showed that the recoverable amount was higher than the carrying amount of the cash-generating unit. The cash flow forecasts took into account the payments expected under existing contracts as well as the future free cash flows from the contribution of the Slonomics technology to partnered programs and was offset by expected personnel and administrative expenses. Cash flow forecasts are based on a period of ten years because the Management Board believes that commercialization through licensing agreements, upfront payments, milestone payments, funded development services and royalties is only feasible by means of medium- to long-term contracts. For this reason, a planning horizon of ten years is considered appropriate for the value-in-use calculation. The cash flow forecasts are largely based on the assumption that the Slonomics technology is very beneficial for existing customers. The values of the underlying assumptions were determined using both internal (past experience) and external sources of information (market information). Based on the updated ten-year cash flow forecast, the value-in-use was determined as follows: A beta factor of 1.2 (2016: 1.2), WACC before taxes of 10.6% (2016: 12.2%) and a perpetual growth rate of 1% (2016: 1%). A detailed sensitivity analysis was performed for the growth rate and the discount rate for calculating value-in-use. The sensitivity analysis took into account the change in one assumption, with the remaining assumptions remaining unchanged from the original calculation. A sensitivity analysis for changes in the cash flows has not been performed since the cash flows have already been
F-51
Table of Contents
probability-adjusted in the value-in-use calculations so as to reflect the probabilities of success of phases in clinical trials. This analysis did not reveal any additional need for impairment. The values ascribed to the assumptions correspond to the Management Board’s forecasts for future development and are based on internal planning scenarios as well as external sources of information.
As of September 30, 2017, goodwill of € 3.7 million and related intangible assets with indefinite useful life of € 28.2 million from the Lanthio Group acquisition was tested for impairment. The recoverable amount of the cash-generating unit Lanthio Group, which is part of the Proprietary Development segment, was determined on the basis of value-in-use calculations. The value-in-use was higher than the carrying amount of the cash-generating unit. The cash flow forecasts included planned cash inflows from the potential sale of compounds based on lanthipeptides expected to achieve market approval. These cash inflows were offset by expected operating expenses for compound development and clinical trials as well as sales and administrative expenses. The duration and likelihood of individual stages of the study were taken into consideration. Cash flow forecasts are based on a period of 30 years because the Management Board believes that after the successful approval of compounds, the drugs that follow can generate free cash flows within that period of time. The values of the underlying assumptions were determined using both internal (past experience) and external sources of information (market information). On the basis of the updated cash flow forecast, the value-in-use was determined as follows: A beta factor of 1.2 (2016: 1.2) and WACC before taxes of 12.1% (2016: 11.9%). A detailed sensitivity analysis was performed with regard to the discount rate. A sensitivity analysis for changes in the cash flows has not been performed since the cash flows from research and development as well as commercialization of the compounds have already been probability-adjusted in the value-in-use calculations so as to reflect the probabilities of success of phases in clinical trials. This analysis did not reveal any need for impairment. The values ascribed to the assumptions correspond to the Management Board’s forecasts for future development and are based on internal planning scenarios as well as external sources of information.
No indicators for impairments were identified at December 31, 2017.
| 5.8 | PREPAID EXPENSES AND OTHER ASSETS, NET OF CURRENT PORTION |
This line item included the non-current portion of prepaid expenses and other assets and mainly resulted from prepaid rent for the premises in Semmelweisstraße 7 in Planegg. The Group classified certain line items under other assets as “restricted cash” that are not available for use in the Group’s operations (see Items 2.8.1 and 5.1 in the Notes). As of December 31, 2017 and December 31, 2016, the Group held long-term restricted cash in the amount of € 0.7 million and € 0.9 million, respectively, for issued rent guarantees and of € 0.1 million for convertible bonds granted to employees (December 31, 2016: € 0.2 million).
The table below shows the breakdown of this line item.
| in 000’ € | 12/31/2017 | 12/31/2016 | ||||||
| Prepaid Expenses, Net of Current Portion |
2,546 | 2,783 | ||||||
| Other Current Assets |
798 | 1,111 | ||||||
|
|
|
|||||||
| Total |
3,344 | 3,894 | ||||||
|
|
|
|||||||
F-52
Table of Contents
6 Notes to Equity and Liabilities of the Balance Sheet
| 6.1 | ACCOUNTS PAYABLE AND ACCRUED EXPENSES |
Accounts payable were non-interest-bearing and under normal circumstances had payment terms of no more than 30 days.
Accounts payable are listed in the table below.
| in 000’ € | 12/31/2017 | 12/31/2016 | ||||||
| Trade Accounts Payable |
4,622 | 8,457 | ||||||
| Licenses Payable |
196 | 179 | ||||||
| Accrued Expenses |
36,408 | 22,838 | ||||||
| Other Liabilities |
3,586 | 749 | ||||||
|
|
|
|||||||
| Total |
44,812 | 32,223 | ||||||
|
|
|
|||||||
Accrued expenses mainly included accrued personnel expenses for payments to employees and management amounting to € 5.0 million (December 31, 2016: € 2.8 million), provisions for outstanding invoices in the amount of € 2.6 million (December 31, 2016: € 2.6 million), external laboratory services in the amount of € 26.3 million (December 31, 2016: € 16.2 million), license payments in the amount of € 0.2 million (December 31, 2016: € 0.1 million), audit fees and other audit-related costs in the amount of € 0.2 million (December 31, 2016: € 0.1 million) and expenses for legal advice in the amount of € 2.1 million (December 31, 2016: € 1.0 million).
At the Company’s Annual General Meeting in May 2017, the Supervisory Board was authorized to appoint PricewaterhouseCoopers GmbH Wirtschaftsprüfungsgesellschaft (PwC GmbH), Munich, as the auditor.
In the 2017 financial year, PwC GmbH received compensation from MorphoSys in the amount of € 351,044, including audit fees in the amount of € 252,725 as well as fees for other services in the amount of € 98,319. PwC GmbH did neither provide other audit-related and valuation services nor tax consultation services in 2017.
| 6.2 | TAX PROVISIONS AND PROVISIONS |
As of December 31, 2017, the Group recorded tax provisions and provisions of € 1.5 million (2016: € 4.9 million).
Tax provisions mainly consisted of income tax expenses and provisions included provisions for onerous contracts and lease obligations for office premises, which will not be used anymore in the future, as well as for potential losses resulting from unsettled forward rate agreements. Furthermore provisions comprised obligations resulting from an agreement with a contract manufacturing organization. .
As of December 31, 2017, tax provisions and provisions are uncertain in their amount and are expected to be utilized in 2018.
F-53
Table of Contents
The table below shows the development of tax provisions and current and non-current provisions in the 2017 financial year.
| in 000’ € | 01/01/2017 | Additions | Utilized | Released | 12/31/2017 | |||||||||||||||
| Tax Provisions |
1,652 | 147 | 1,484 | 0 | 315 | |||||||||||||||
| Provisions |
3,218 | 1,116 | 1,841 | 1,284 | 1,209 | |||||||||||||||
|
|
|
|||||||||||||||||||
| Total |
4,870 | 1,263 | 3,325 | 1,284 | 1,524 | |||||||||||||||
|
|
|
|||||||||||||||||||
| 6.3 | DEFERRED REVENUES |
Deferred revenues are payments received from customers for which the services have not been rendered. The table below shows the development of this line item.
| in 000’ € | 2017 | 2016 | ||||||
| Opening Balance |
2,905 | 4,507 | ||||||
|
|
|
|||||||
| Prepayments Received in the Fiscal Year |
18,386 | 17,441 | ||||||
| Revenue Recognized through Release of Prepayments in line with Services Performed in the Fiscal Year |
(19,596 | ) | (19,043 | ) | ||||
|
|
|
|||||||
| Closing Balance |
1,695 | 2,905 | ||||||
|
|
|
|||||||
| thereof short-term |
1,389 | 1,232 | ||||||
| thereof long-term |
306 | 1,673 | ||||||
| 6.4 | OTHER LIABILITIES |
Other liabilities exclusively consisted of the deferred amount of the rent-free period for the building located at Semmelweisstraße 7, Planegg, as agreed in the lease contract. This item is released over the contractually agreed minimum rent period.
The current portion amounting to € 0.1 million of this liability was included in the item accounts payable and accrued expenses.
| 6.5 | STOCKHOLDERS’ EQUITY |
| 6.5.1 | COMMON STOCK |
On December 31, 2017, the Company’s common stock, including treasury stock, increased by € 261,015 to € 29,420,785 from its level of € 29,159,770 on December 31, 2016. Each no-par value share is entitled to one vote. Common stock increased by € 261,015 as a result of the exercise of 261,015 convertible bonds granted to the Management Board and the Senior Management Group. The weighted-average exercise price of the exercised convertible bonds was € 31.88.
On December 31, 2017, the Company held 319,678 shares of treasury stock amounting to € 11,826,981 which represents a decrease of € 2,811,104 compared to December 31, 2016 (396,010 shares, € 14,648,212). This decrease was the result of the transfer of 61,871 shares of treasury stock to the Management Board and Senior Management under the performance-based 2013 long-term incentive plan (LTI plan) totaling € 2,286,752. The vesting period for this LTI program expired on April 1, 2017 and October 1, 2017 and provides or provided beneficiaries
F-54
Table of Contents
a six-month option to receive a total of 61,871 shares. In addition, in March 2017, Chief Development Officer Dr. Peters received 9,505 treasury shares worth € 351,305. In November 2017, Chief Scientific Officer Dr. Enzelberger received 4,956 treasury shares worth € 183,174. As a result, the number of MorphoSys shares held by the Company as of December 31, 2017 amounted to 319,678 (December 31 2016: 396,010).
| 6.5.2 | AUTHORIZED CAPITAL |
The number of authorized ordinary shares increased from 10,584,333 on December 31, 2016, to 14,579,885. This increase resulted from the cancellation of Authorized Capital 2015-I amounting to € 10,584,333 and the creation of Authorized Capital 2017-I in the amount of € 2,915,977 and Authorized Capital 2017-II in the amount of € 11,663,908 at the Annual General Meeting on May 17, 2017. Within the scope of Authorized Capital 2017-I and 2017-II, with the Supervisory Board’s approval, the Management Board received authorization to increase the Company’s common stock on one or more occasions until and including April 30, 2022 by up to € 2,915,977 and € 11,663,908, respectively, by issuing up to 2,915,977 and 11,663,908 new, no-par-value bearer shares.
Pursuant to the Company’s articles of association, the shareholders may authorize the Management Board to increase the share capital with the consent of the Supervisory Board within a period of five years by issuing shares for a certain total amount, which are referred to as authorized capital (genehmigtes Kapital) and is a concept under German law that enables the Company to issue shares without going through the process of obtaining another shareholders’ resolution. The aggregate nominal amount of the authorized capital created by the shareholders may not exceed one-half of the share capital existing at the time of registration of the authorized capital with the commercial register.
| 6.5.3 | CONDITIONAL CAPITAL |
The number of ordinary shares of conditional capital compared to December 31, 2016 decreased from 6,752,698 to 6,491,683 shares due to the exercise of 261,015 conversion rights in 2017. The reduction in ordinary shares of conditional capital through the exercise of 261,015 conversion rights was entered in the commercial register in December 2017.
The shareholders may resolve to amend or create conditional capital (bedingtes Kapital). However, they may do so only to issue conversion or subscription rights to holders of convertible bonds, in preparation for a merger with another company or to issue subscription rights to employees and members of the Management Board of the Company or of an affiliated company by way of a consent or authorization resolution. According to German law, the aggregate nominal amount of the conditional capital created at the shareholders’ meeting may not exceed one-half of the share capital existing at the time of the shareholders’ meeting adopting such resolution. The aggregate nominal amount of the conditional capital created for the purpose of granting subscription rights to employees and members of the management of our company or of an affiliated company may not exceed 10% of the share capital existing at the time of the shareholders’ meeting adopting such resolution.
F-55
Table of Contents
| 6.5.4 | TREASURY STOCK |
In contrast to the year 2016, the Group did not repurchase any of its own shares in 2017. The composition and development of this line item is listed in the following table.
| Number of Shares |
Value | |||||||
| As of 12/31/2010 |
79,896 | 9,774 | ||||||
| Purchase in 2011 |
84,019 | 1,747,067 | ||||||
|
|
|
|||||||
| As of 12/31/2011 |
163,915 | 1,756,841 | ||||||
| Purchase in 2012 |
91,500 | 1,837,552 | ||||||
|
|
|
|||||||
| As of 12/31/2012 |
255,415 | 3,594,393 | ||||||
| Purchase in 2013 |
84,475 | 2,823,625 | ||||||
|
|
|
|||||||
| As of 12/31/2013 |
339,890 | 6,418,018 | ||||||
| Purchase in 2014 |
111,000 | 7,833,944 | ||||||
|
|
|
|||||||
| As of 12/31/2014 |
450,890 | 14,251,962 | ||||||
| Purchase in 2015 |
88,670 | 5,392,931 | ||||||
| Transfer in 2015 |
(104,890 | ) | (3,816,947 | ) | ||||
|
|
|
|||||||
| As of 12/31/2015 |
434,670 | 15,827,946 | ||||||
| Purchase in 2016 |
52,295 | 2,181,963 | ||||||
| Transfer in 2016 |
(90,955 | ) | (3,361,697 | ) | ||||
|
|
|
|||||||
| As of 12/31/2016 |
396,010 | 14,648,212 | ||||||
| Transfer in 2017 |
(76,332 | ) | (2,821,231 | ) | ||||
|
|
|
|||||||
| As of 12/31/2017 |
319,678 | 11,826,981 | ||||||
|
|
|
|||||||
| 6.5.5 | ADDITIONAL PAID-IN CAPITAL |
On December 31, 2017, additional paid-in capital amounted to € 438,557,857 (December 31, 2016: € 428,361,175). The total increase of € 10,196,682 resulted mainly from the exercise of convertible bonds in the amount of € 8,043,313 and the allocation of personnel expenses resulting from share-based payments in the amount of € 4,974,599. There was an offsetting effect from the decline in the reclassification of treasury shares in the context of the allocation of shares under the 2013 performance-based share plan in the amount of € 2,286,752 and the allocation of treasury shares to Dr. Peters and Dr. Enzelberger in the amount of € 534,479.
| 6.5.6 | REVALUATION RESERVE |
As of December 31, 2017, the revaluation reserve amounted to € -105,483 (December 31, 2016: € 136,101). The decline of € 241,584 resulted from the change in the unrealized gains and losses from available-for-sale securities and bonds in the amount of € 117,829 and the change in unrealized losses of € -359,413 from cash flow hedges.
| 6.5.7 | ACCUMULATED DEFICIT |
The consolidated net loss of € -69,826,469 is reported in accumulated deficit. The accumulated deficit increased from € -27,548,669 in the year 2016 to € -97,375,138 in 2017.
F-56
Table of Contents
7 Remuneration System for the Management Board and Employees of the Group
| 7.1 | 2017 STOCK OPTION PLAN |
On April 1, 2017, MorphoSys established a stock option plan (SOP) for the Management Board, the Senior Management Group and employees of the Company who are not members of the Senior Management Group. In accordance with IFRS 2, the program is considered an equity-settled share-based payment and is accounted for accordingly. The grant date was April 1, 2017 and the vesting period/performance period is four years. The stock options vest each year by 25% within the four-year vesting period, provided that the performance criteria specified for the respective period have been 100% fulfilled. The number of stock options vested per year is calculated based on the key performance criteria of the absolute MorphoSys share price performance and the relative MorphoSys share price performance compared to the NASDAQ Biotechnology Index and the TecDAX Index. The performance criteria can be met annually up to a maximum of 200%. If the specified performance criteria are met by less than 0% in one year, no shares will be earned for that year (entitlement). The right to exercise a stock option, however, arises only at the end of the four-year vesting period/performance period.
The exercise price, derived from the average market price of the Company’s shares in the XETRA closing auction on the Frankfurt Stock Exchange from the 30 trading days prior to the issue of the stock options, is € 55.52.
MorphoSys reserves the right to settle the exercise of stock options through newly created shares from Conditional Capital 2016-III, through the issuance of treasury shares or in cash. The exercise period is three years after the end of the four-year vesting period/performance period, which is March 31, 2024.
If a member of the Management Board ceases to hold an office at the MorphoSys Group through termination (or the Management Board member terminates the employment contract), resignation, death, injury, disability or the attainment of retirement age (receipt of a standard retirement pension, early-retirement pension or disability pension, as long as the requirements for the disability pension entitlement are met) or under other circumstances subject to the Supervisory Board’s discretion, the Management Board member (or the member’s heirs) is entitled to a precise daily pro rata number of stock options.
If a member of the Management Board ceases to hold an office at the MorphoSys Group for good reason as defined by Sec. 626 Para. 2 of the German Civil Code (BGB), all unexercized stock options will be forfeited without any entitlement to compensation.
If a change of control occurs during the four-year vesting period, the stock options will become fully vested. In this case, however, the right to exercise the stock options arises only at the end of the four-year vesting period.
As of April 1, 2017, a total of 81,157 stock options had been granted to the beneficiaries, of which 40,319 had been granted to the Management Board (further details can be found in the “Stock Options” table in Note 7.4 “Related Parties”), 37,660 to the Senior Management Group and 3,178 to the Company employees who do not belong to the Senior Management Group. The stated number of stock options granted is based on 100% target achievement. The fair value of the stock options on the grant date (April 1, 2017) was € 21.41 per stock option. In the period from the grant date to December 31, 2017, one beneficiary had left MorphoSys, resulting
F-57
Table of Contents
in the forfeiture of 1,402 stock options. For the calculation of personnel expenses resulting from share-based payments under the 2017 Stock Option Plan, the assumption is that two beneficiaries would leave the company during the four-year period.
In 2017, personnel expenses from stock options under the Group’s 2017 SOP amounted to € 801,330.
The fair value of the stock options from the 2017 Stock Option Plan has been determined with a Monte Carlo simulation. The expected volatility is based on the development of the share volatility of the last four years. Furthermore, the calculation of fair value equally considered the performance criteria of the absolute and relative performance of MorphoSys shares compared to the development of the NASDAQ Biotech Index and the TecDAX Index. The parameters of each program are listed in the table below.
| April 2017 Stock Option Plan |
||||
| Share Price on Grant Date in € |
55.07 | |||
| Strike Pricein € |
55.52 | |||
| Expected Volatility of the MorphoSys share in % |
37.49 | |||
| Expected Volatility of the NASDAQ Biotech Index in % |
25.07 | |||
| Expected Volatility of the TecDAX Index in % |
16.94 | |||
| Performance Term of Program in Years |
4.0 | |||
| Dividend Yield in % |
n/a | |||
| Risk-free Interest Rate in % |
between 0.03 and 0.23 | |||
| 7.2 | CONVERTIBLE BONDS – 2013 PROGRAM |
On April 1, 2013, MorphoSys AG granted the Management Board and members of the Senior Management Group convertible bonds with a total nominal value of € 225,000 and divided into 449,999 bearer bonds with equal rights from “Conditional Capital 2008-III”. The beneficiaries have the right to convert the bonds into Company shares. Each convertible bond can be exchanged for one of the Company’s bearer shares equal to the proportional amount of common stock, which currently stands at € 1. Exercise of the convertible bonds is subject to several conditions, such as the achievement of performance targets, the expiration of vesting periods, the exercisability of the conversion rights, the existence of an employment or service contract that is not under notice and the commencement of the exercise period.
The conversion price amounted to € 31.88 and was derived from the Company’s share price in the XETRA closing auction of the Frankfurt Stock Exchange on the trading day preceding the issue of the convertible bonds. The exercise of the conversion rights is admissible since, on at least one trading day during the lifetime of the convertible bonds, the share price of the Company has risen to more than 120% of the price in the XETRA closing auction of the Frankfurt Stock Exchange on the trading day preceding the issue of the convertible bonds.
The exercise of the conversion rights is only admissible since the expiration of the four-year vesting period from the grant date. For every year without a notice of termination of the employment relationship with the Company or an affiliated company, 25% of the conversion rights become vested.
F-58
Table of Contents
The following table shows the development of the convertible bond plans for Group employees in the 2017 and 2016 financial years.
| Convertible Bonds |
Weighted- average Price (€) |
|||||||
| Outstanding on January 1, 2016 |
449,999 | 31.88 | ||||||
|
|
|
|||||||
| Granted |
0 | 0.00 | ||||||
| Exercised |
0 | 0.00 | ||||||
| Forfeited |
(13,414 | ) | 31.88 | |||||
| Expired |
0 | 0.00 | ||||||
|
|
|
|||||||
| Outstanding on December 31, 2016 |
436,585 | 31.88 | ||||||
|
|
|
|||||||
| Outstanding on January 1, 2017 |
436,585 | 31.88 | ||||||
|
|
|
|||||||
| Granted |
0 | 0.00 | ||||||
| Exercised |
(261,015 | ) | 31.88 | |||||
| Forfeited |
0 | 0.00 | ||||||
| Expired |
0 | 0.00 | ||||||
|
|
|
|||||||
| Outstanding on December 31, 2017 |
175,570 | 31.88 | ||||||
|
|
|
|||||||
From the grant date until December 31, 2017, one beneficiary left MorphoSys and, therefore, 13,414 convertible bonds were forfeited. As December 31, 2017, the number of vested convertible bonds totaled 175,570 shares (December 31, 2016: 327,439 shares).
The following overview includes the weighted-average exercise price as well as information on the contract duration of significant groups of convertible bonds as of December 31, 2017.
| Range of Exercise Prices | Number Outstanding |
Remaining Contractual Life (in Years) |
Weighted- average Exercise Price (€) |
Number Exercisable |
Weighted- average Exercise Price (€) |
|||||||||||||||
| €25.00 - €40.00 |
175,570 | 2.25 | 31.88 | 175,570 | 31.88 | |||||||||||||||
| 175,570 | 2.25 | 31.88 | 175,570 | 31.88 | ||||||||||||||||
The Group recognizes personnel expenses resulting from convertible bonds on a straight-line basis in accordance with IFRS 2 and IAS 32.28. The equity component of the convertible bonds is presented separately under additional paid-in capital. The corresponding amount is recognized as personnel expenses from convertible bonds. In 2017 and 2016, compensation expenses related to convertible bonds amounted to € 287,601 and € 40,375, respectively.
| 7.3 | LONG-TERM INCENTIVE PROGRAMS |
| 7.3.1 2013 | LONG-TERM INCENTIVE PROGRAM |
On April 1, 2013, MorphoSys established a long-term incentive plan (LTI plan) for the Management Board and the Senior Management Group. The vesting period of this plan expired on April 1, 2017. According to IFRS 2, this program is considered a share-based payment program with settlement in equity instruments and is accounted for accordingly. The LTI plan is
F-59
Table of Contents
a performance-related share plan and is paid out in ordinary shares (performance shares) of MorphoSys AG if predefined key performance criteria are achieved. The key performance criteria are based on the absolute MorphoSys share price performance and the relative MorphoSys share price performance compared to the NASDAQ Biotechnology Index and the TecDAX Index. These criteria are approved annually by the Supervisory Board. The fulfillment of these criteria was set at 200% for one year, 54% for one year and 0% for two years. The Supervisory Board set the “company factor” at 1.57, meaning the number of performance shares to be allocated was scaled by a factor of 1.57. This factor resulted in an adjustment of previously recognized personnel expenses of € 1.0 million in the 2017 financial year. Previously, personnel expenses resulting from the 2013 LTI program were recognized based on the assumption of a company factor of 1.0. Based on these terms and the company factor, a total of 61,323 performance shares of MorphoSys AG was transferred to beneficiaries on October 2, 2017 after the expiration of the four-year vesting period. The Management Board received 36,729 performance shares (for further information, please see the tables titled “Shares” and “Performance Shares” in Item 7.4 “Related Parties”), the Senior Management Group received 21,248 performance shares and former members of the Senior Management Group who have since left the Company received 3,346 performance shares.
On October 1, 2013, MorphoSys established another long-term incentive plan (LTI plan) for Senior Management Group members. The vesting period of this plan expired on October 1, 2017. The terms of this plan were identical to the April 1, 2013 plan. The fulfillment of the performance criteria was set at 200% for one year, 54.8% for one year and 0% for two years. The Supervisory Board set the “company factor” at 1.57, meaning the number of performance shares to be allocated was scaled by a factor of 1.57. This factor resulted in an adjustment of previously recognized personnel expenses of € 0.02 million in the 2017 financial year. Previously, personnel expenses resulting from the 2013 LTI program were recognized based on the assumption of a company factor of 1.0. Based on these terms and the company factor, a total of 548 performance shares of MorphoSys AG was allocated to beneficiaries after the expiration of the four-year vesting period in December 2017. The Senior Management Group received all of the 548 performance shares.
In 2017, personnel expenses from stock options under the Group’s 2013 LTI plan amounted to € 1,038,639 (2016: € -23,571).
| 7.3.2 2014 | LONG-TERM INCENTIVE PROGRAM |
On April 1, 2014, MorphoSys established a long-term incentive plan (LTI plan) for the Management Board and the Senior Management Group. According to IFRS 2, this program is considered a share-based payment program with settlement in equity instruments and is accounted for accordingly. The LTI plan is a performance-related share plan and will be paid out in ordinary shares (performance shares) of MorphoSys AG if predefined key performance criteria are achieved. These criteria are evaluated annually by the Supervisory Board. The grant date was April 1, 2014 and the vesting/performance period is four years. If the predefined key performance criteria for the respective period are fully met, 25% of the performance shares become vested in each year of the four-year vesting period. The number of performance shares vested per year is calculated based on the key performance criteria of the absolute MorphoSys share price performance and the relative MorphoSys share price performance compared to the NASDAQ Biotechnology Index and the TecDAX Index. The number of performance shares vested
F-60
Table of Contents
each year will be reduced or increased to the extent that the performance criteria of the respective year have been achieved between only 50% and 99.9% (<100%) or the achievement of the performance criteria has exceeded 100% (maximum 200%). If in one year the performance criteria are met by less than 50%, no performance shares will become vested in that year. In any case, the maximum pay-out at the end of the four-year period is limited by a factor determined by the Group, which generally amounts to 1. However, in justified cases, the Supervisory Board may set this factor freely between 0 and 2, for example, if the level of payment is regarded as unreasonable in view of the general development of the Company. The right to receive a certain allocation of performance shares under the LTI plan, however, occurs only at the end of the four-year vesting period.
At the end of the four-year vesting period, there is a six-month exercise period during which the Company can transfer the shares to the beneficiaries. Beneficiaries are free to choose the exercise date within this exercise period.
If the number of repurchased shares is not sufficient for servicing the LTI plan, MorphoSys reserves the right to pay a certain amount of the LTI plan in cash in the amount of the performance shares at the end of the vesting period, provided the cash amount does not exceed 200% of the fair value of the performance shares on the grant date.
If a member of the Management Board ceases to hold an office at the MorphoSys Group because of termination (or if the Management Board member terminates the employment contract), resignation, death, injury, disability, by reaching retirement age (receipt of a normal retirement pension, early-retirement pension or disability pension, as long as the requirements for the disability pension entitlement are met) or under other circumstances subject to the Supervisory Board’s discretion, the Management Board member (or the member’s heirs) is entitled to performance shares determined on a precise daily pro rata basis.
If a member of the Management Board ceases to hold an office at the MorphoSys Group for good reason as defined by Sec. 626 Para. 2 of the German Civil Code (BGB) and/or as defined by Sec. 84 Para. 3 of the German Stock Corporation Act (AktG), the beneficiary will not be entitled to performance shares.
If a change of control occurs during the four-year vesting period, all performance shares will become fully vested. In this case, the right to receive a certain allocation of performance shares under the LTI plan occurs only at the end of the four-year vesting period.
In March 2014, MorphoSys repurchased 111,000 of its own shares on the stock exchange at an average price of € 70.53 per share. The repurchased shares may be used for all purposes named in the authorizations of the Annual General Meetings on May 19, 2011 and May 23, 2014 and particularly for any existing or future employee participation schemes and/or to finance acquisitions. The shares may also be redeemed.
A total of 32,513 of these shares were allocated to beneficiaries on April 1, 2014 with 18,264 performance shares allocated to the Management Board (further details may be found in the table titled “Performance Shares” in Item 7.4 “Related parties”) and 14,249 performance shares to the Senior Management Group. The number of performance shares allocated is based on the full achievement of performance criteria and a company factor of 1. The fair value of the performance shares on the grant date (April 1, 2014) was € 62.17 per share. No dividends were included in the determination of the fair value of the performance shares because the Group
F-61
Table of Contents
does not intend to distribute any dividends in the foreseeable future. From the grant date until December 31, 2017, three beneficiaries left MorphoSys and, therefore, 1,829 performance shares were forfeited. For the calculation of the personnel expenses from share-based payments under the 2014 LTI plan, it was initially assumed that one beneficiary would leave the Company during the four-year period. This assumption was updated in 2017.
In 2017, personnel expenses resulting from performance shares under the Group’s 2014 LTI plan amounted to € 55,759 (2016: € 178,518).
| 7.3.3 2015 | LONG-TERM INCENTIVE PROGRAM |
On April 1, 2015, MorphoSys established a long-term incentive plan (LTI plan) for the Management Board and the Senior Management Group. According to IFRS 2, this program is considered a share-based payment program with settlement in equity instruments and is accounted for accordingly. The LTI plan is a performance-related share plan and will be paid out in ordinary shares (performance shares) of MorphoSys AG if predefined key performance criteria are achieved. These criteria are evaluated annually by the Supervisory Board. The grant date was April 1, 2015 and the vesting/performance period is four years. If the predefined key performance criteria for the respective period are fully met, 25% of the performance shares become vested in each year of the four-year vesting period. The number of performance shares vested per year is calculated based on the key performance criteria of the absolute MorphoSys share price performance and the relative MorphoSys share price performance compared to the NASDAQ Biotechnology Index and the TecDAX Index. The number of performance shares vested each year will be reduced or increased to the extent that the performance criteria of the respective year have been achieved between only 50% and 99.9% (<100%) or the achievement of the performance criteria has exceeded 100% (maximum 200%). If in one year the performance criteria are met by less than 50%, no performance shares will become vested in that year. In any case, the maximum pay-out at the end of the four-year period is limited by a factor determined by the Group, which generally amounts to 1. However, in justified cases, the Supervisory Board may set this factor freely between 0 and 2, for example, if the level of payment is regarded as unreasonable in view of the general development of the Company. The right to receive a certain allocation of performance shares under the LTI plan, however, occurs only at the end of the four-year vesting period.
At the end of the four-year waiting period, there is a six-month exercise period during which the Company can transfer the shares to the beneficiaries. Beneficiaries are free to choose the exercise date within this exercise period.
If the number of repurchased shares is not sufficient for servicing the LTI plan, MorphoSys reserves the right to pay a certain amount of the LTI plan in cash in the amount of the performance shares at the end of the vesting period, provided the cash amount does not exceed 200% of the fair value of the performance shares on the grant date.
If a member of the Management Board ceases to hold an office at the MorphoSys Group because of termination (or if the Management Board member terminates the employment contract), resignation, death, injury, disability, by reaching retirement age (receipt of a normal retirement pension, early-retirement pension or disability pension, as long as the requirements for the disability pension entitlement are met) or under other circumstances subject to the Supervisory Board’s discretion, the Management Board member (or the member’s heirs) is entitled to performance shares determined on a precise daily pro rata basis.
F-62
Table of Contents
If a member of the Management Board ceases to hold an office at the MorphoSys Group for good reason as defined by Sec. 626 Para. 2 of the German Civil Code (BGB) and/or as defined by Sec. 84 Para. 3 of the German Stock Corporation Act (AktG), the beneficiary will not be entitled to performance shares.
If a change of control occurs during the four-year vesting period, all performance shares will become fully vested. In this case, the right to receive a certain allocation of performance shares under the LTI plan occurs only at the end of the four-year vesting period.
In April 2015, MorphoSys repurchased 88,670 of its own shares on the stock exchange at an average price of € 60.79 per share. The repurchased shares may be used for all purposes named in the authorization of the Annual General Meeting on May 23, 2014 and particularly for any existing or future employee participation schemes and/or to finance acquisitions. The shares may also be redeemed.
A total of 40,425 of these shares were allocated to beneficiaries on April 1, 2015 with 21,948 performance shares allocated to the Management Board (further details may be found in the table titled “Performance Shares” in Item 7.4 “Related parties”) and 18,477 performance shares to the Senior Management Group. The number of performance shares allocated is based on the full achievement of the performance criteria and a company factor of 1. The fair value of the performance shares on the grant date (April 1, 2015) was € 61.40 per share. No dividends were included in the determination of the fair value of the performance shares because the Group does not intend to distribute any dividends in the foreseeable future. From the grant date until December 31, 2017, two beneficiaries left MorphoSys, and therefore 3,055 performance shares were forfeited. For the calculation of the personnel expenses from share-based payments under the 2015 LTI plan, it was initially assumed that one beneficiary would leave the Company during the four-year period. This assumption was updated in 2017.
In 2017, personnel expenses resulting from performance shares under the Group’s 2015 LTI plan amounted to € 201,608 (2016: € 837,153).
| 7.3.4 2016 | LONG-TERM INCENTIVE PROGRAM |
On April 1, 2016, MorphoSys established a long-term incentive plan (LTI plan) for the Management Board and the Senior Management Group. According to IFRS 2, this program is considered a share-based payment program with settlement in equity instruments and is accounted for accordingly. The LTI plan is a performance-related share plan and will be paid out in ordinary shares (performance shares) of MorphoSys AG if predefined key performance criteria are achieved. These criteria are evaluated annually by the Supervisory Board. The grant date was April 1, 2016 and the vesting/performance period is four years. If the predefined key performance criteria for the respective period are fully met, 25% of the performance shares become vested in each year of the four-year vesting period. The number of performance shares vested per year is calculated based on the key performance criteria of the absolute MorphoSys share price performance and the relative MorphoSys share price performance compared to the NASDAQ Biotechnology Index and the TecDAX Index. The number of performance shares vested each year will be reduced or increased to the extent that the performance criteria of the respective year have been achieved between only 50% and 99.9% (<100%) or the achievement of the performance criteria has exceeded 100% (maximum 200%). If in one year the performance criteria are met by less than 50%, no performance shares will become vested in
F-63
Table of Contents
that year. In any case, the maximum pay-out at the end of the four-year period is limited by a factor determined by the Group, which generally amounts to 1. However, in justified cases, the Supervisory Board may set this factor freely between 0 and 2, for example, if the level of payment is regarded as unreasonable in view of the general development of the Company. The right to receive a certain allocation of performance shares under the LTI plan, however, occurs only at the end of the four-year vesting/performance period.
At the end of the four-year waiting period, there is a six-month exercise period during which the Company can transfer the shares to the beneficiaries. Beneficiaries are free to choose the exercise date within this exercise period.
If the number of repurchased shares is not sufficient for servicing the LTI plan, MorphoSys reserves the right to pay a certain amount of the LTI plan in cash in the amount of the performance shares at the end of the vesting period, provided the cash amount does not exceed 200% of the fair value of the performance shares on the grant date.
If a member of the Management Board ceases to hold an office at the MorphoSys Group because of termination (or if the Management Board member terminates the employment contract), resignation, death, injury, disability, by reaching retirement age (receipt of a normal retirement pension, early-retirement pension or disability pension, as long as the requirements for the disability pension entitlement are met) or under other circumstances subject to the Supervisory Board’s discretion, the Management Board member (or the member’s heirs) is entitled to performance shares determined on a precise daily pro rata basis.
If a member of the Management Board ceases to hold an office at the MorphoSys Group for good reason as defined by Sec. 626 Para. 2 of the German Civil Code (BGB) and/or as defined by Sec. 84 Para. 3 of the German Stock Corporation Act (AktG), the beneficiary will not be entitled to performance shares.
If a change of control occurs during the four-year vesting period, all performance shares will become fully vested. In this case, the right to receive a certain allocation of performance shares under the LTI plan occurs only at the end of the four-year vesting period.
In March 2016, MorphoSys repurchased 52,295 of its own shares on the stock exchange at an average price of € 41.69 per share. The repurchased shares may be used for all purposes named in the authorization of the Annual General Meeting on May 23, 2014 and particularly for any existing or future employee participation schemes and/or to finance acquisitions. The shares may also be redeemed.
A total of 68,143 of these shares were allocated to beneficiaries on April 1, 2016 with 35,681 performance shares allocated to the Management Board (further details may be found in the table titled “Performance Shares” in Item 7.4 “Related parties”) and 32,462 performance shares to the Senior Management Group. The number of performance shares allocated is based on the full achievement of the performance criteria and a company factor of 1. The fair value of the performance shares on the grant date (April 1, 2016) was € 46.86 per share. No dividends were included in the determination of the fair value of the performance shares because the Group does not intend to distribute any dividends in the foreseeable future. From the grant date until December 31, 2017, four beneficiaries left MorphoSys, and therefore 9,350 performance shares were forfeited. For the calculation of the personnel expenses from share-based payments under the 2016 LTI plan, it was initially assumed that one beneficiary would leave the Company during the four-year period. This assumption was updated in 2017.
F-64
Table of Contents
In 2017, personnel expenses resulting from performance shares under the Group’s 2016 LTI plan amounted to € 663,624 (2016: € 1,483,694).
| 7.3.5 2017 | LONG-TERM INCENTIVE PLAN |
On April 1, 2017, MorphoSys established another long-term incentive plan (LTI plan) for the Management Board, the Senior Management Group and employees of the Company who are not members of the Senior Management Group. According to IFRS 2, this program is considered a share-based payment program with settlement in equity instruments and is accounted for accordingly. The LTI plan is a performance-related share plan and will be paid out in ordinary shares (performance shares) of MorphoSys AG if predefined key performance criteria are achieved. The grant date was April 1, 2017 and the vesting/performance period is four years. If the predefined performance criteria for the respective period are fully met, 25% of the performance shares become vested in each year of the four-year vesting period. The number of performance shares vested per year is calculated based on the key performance criteria of the absolute MorphoSys share price performance and the relative MorphoSys share price performance compared to the NASDAQ Biotechnology Index and the TecDAX Index. The performance criteria can be met annually up to a maximum of 300% and up to 200% for the entire four-year period. If the specified performance criteria are met by less than 0% in one year, no shares will be earned for that year (entitlement). In any case, the maximum pay-out at the end of the four-year period is limited by a factor determined by the Group, which generally amounts to 1. However, in justified cases, the Supervisory Board may set this factor freely between 0 and 2, for example, if the level of payment is regarded as unreasonable in view of the general development of the Company. The right to receive a certain allocation of performance shares under the LTI plan, however, occurs only at the end of the four-year vesting/performance period.
At the end of the four-year waiting period, there is a six-month exercise period during which the Company can transfer the shares to the beneficiaries. Beneficiaries are free to choose the exercise date within this exercise period.
If the number of repurchased shares is not sufficient for servicing the LTI plan, MorphoSys reserves the right to pay a certain amount of the LTI plan in cash in the amount of the performance shares at the end of the vesting period, provided the cash amount does not exceed 200% of the fair value of the performance shares on the grant date.
If a member of the Management Board ceases to hold an office at the MorphoSys Group because of termination (or if the Management Board member terminates the employment contract), resignation, death, injury, disability, by reaching retirement age (receipt of a normal retirement pension, early-retirement pension or disability pension, as long as the requirements for the disability pension entitlement are met) or under other circumstances subject to the Supervisory Board’s discretion, the Management Board member (or the member’s heirs) is entitled to performance shares determined on a precise daily pro rata basis.
If a member of the Management Board ceases to hold an office at the MorphoSys Group for good reason as defined by Sec. 626 Para. 2 of the German Civil Code (BGB) and/or as defined by Sec. 84 Para. 3 of the German Stock Corporation Act (AktG), the beneficiary will not be entitled to performance shares.
F-65
Table of Contents
If a change of control occurs during the four-year vesting period, all performance shares will become fully vested. In this case, the right to receive a certain allocation of performance shares under the LTI plan occurs only at the end of the four-year vesting period.
A total of 31,549 of these shares were allocated to beneficiaries on April 1, 2017 with 15,675 performance shares allocated to the Management Board (further details may be found in the table titled “Performance Shares” in Item 7.4 “Related parties”), 14,640 performance shares allocated to the Senior Management Group and 1,234 performance shares allocated to employees of the Company who are not members of the Senior Management Group. The number of s performance hares allocated is based on 100% achievement of the performance criteria and a company factor of 1. The fair value of the performance shares on the grant date (April 1, 2017) was € 70.52 per share. From the grant date until December 31, 2017, one beneficiary left MorphoSys, and therefore 545 performance shares were forfeited. For the calculation of the personnel expenses from share-based payments under the 2017 LTI plan, the assumption is that two beneficiaries would leave the company during the four-year period.
In 2017, personnel expenses resulting from performance shares under the Group’s 2017 LTI plan amounted to € 1,026,037.
The fair value of the performance shares from the long-term incentive plans 2014 until 2017 has been determined with a Monte Carlo simulation. The expected volatility is based on the development of the share volatility of the last four years. Furthermore, the calculation of fair value equally considered the performance criteria of the absolute and relative performance of MorphoSys shares compared to the development of the NASDAQ Biotech Index and the TecDAX Index. The parameters of each program are listed in the table below.
| April 2014 Long-Term Incentive Program |
April 2015 Long-Term Incentive Program |
April 2016 Long-Term Incentive Program |
April 2017 Long-Term Incentive Program |
|||||||||||||
| Share Price on Grant Date in € |
68.08 | 57.18 | 43.28 | 55.07 | ||||||||||||
| Strike Pricein € |
n/a | n/a | n/a | n/a | ||||||||||||
| Expected Volatility of the MorphoSys share in % |
30.87 | 33.09 | 34.64 | 37.49 | ||||||||||||
| Expected Volatility of the NASDAQ Biotech Index in % |
20.28 | 20.70 | 23.39 | 25.07 | ||||||||||||
| Expected Volatility of the TecDAX Index in % |
20.18 | 20.10 | 17.01 | 16.94 | ||||||||||||
| Performance Term of Program in Years |
4.0 | 4.0 | 4.0 | 4.0 | ||||||||||||
| Dividend Yield in % |
n/a | n/a | n/a | n/a | ||||||||||||
| Risk-free Interest Rate in % |
0.44 | 0.07 | 0.05 | |
between 0.03 and 0.23 |
| ||||||||||
Related parties that can be influenced by the Group or can have a significant influence on the Group can be divided into subsidiaries, members of management in key positions and other related entities.
F-66
Table of Contents
The Group engages in business relationships with members of the Management Board and Supervisory Board as related parties responsible for the planning, management and monitoring of the Group. In addition to cash compensation, the Group has granted the Management Board convertible bonds and performance shares. The tables below show the shares, stock options, convertible bonds and performance shares held by the members of the Management Board and Supervisory Board, as well as the changes in their ownership during the 2017 financial year.
| Shares | ||||||||||||||||
| 01/01/2017 | Additions | Sales | 12/31/2017 | |||||||||||||
| Management Board |
||||||||||||||||
| Dr. Simon Moroney |
514,214 | 12,024 | 42,529 | 483,709 | ||||||||||||
| Jens Holstein |
7,000 | 38,235 | 34,235 | 11,000 | ||||||||||||
| Dr. Malte Peters1 |
– | 9,505 | 0 | 9,505 | ||||||||||||
| Dr. Markus Enzelberger2 |
– | 4,956 | 2,600 | 7,262 | ||||||||||||
| Dr. Arndt Schottelius3 |
10,397 | 68,772 | 0 | – | ||||||||||||
| Dr. Marlies Sproll4 |
57,512 | 68,772 | 0 | – | ||||||||||||
|
|
|
|||||||||||||||
| Total |
589,123 | 202,264 | 79,364 | 511,476 | ||||||||||||
|
|
|
|||||||||||||||
| Supervisory Board |
||||||||||||||||
| Dr. Gerald Möller |
11,000 | 0 | 0 | 11,000 | ||||||||||||
| Dr. Frank Morich |
1,000 | 0 | 0 | 1,000 | ||||||||||||
| Dr. Marc Cluzel |
500 | 0 | 0 | 500 | ||||||||||||
| Krisja Vermeylen5 |
– | 350 | 0 | 350 | ||||||||||||
| Wendy Johnson |
500 | 0 | 0 | 500 | ||||||||||||
| Klaus Kühn |
0 | 0 | 0 | 0 | ||||||||||||
| Karin Eastham6 |
2,000 | 0 | 0 | – | ||||||||||||
|
|
|
|||||||||||||||
| Total |
15,000 | 350 | 0 | 13,350 | ||||||||||||
| Stock Options | ||||||||||||||||||||
| 01/01/2017 | Additions | Forfeitures | Exercises | 12/31/2017 | ||||||||||||||||
| Management Board |
||||||||||||||||||||
| Dr. Simon Moroney |
0 | 12,511 | 0 | 0 | 12,511 | |||||||||||||||
| Jens Holstein |
0 | 8,197 | 0 | 0 | 8,197 | |||||||||||||||
| Dr. Malte Peters 1 |
– | 8,197 | 0 | 0 | 8,197 | |||||||||||||||
| Dr. Markus Enzelberger 2 |
– | 5,266 | 0 | 0 | 5,266 | |||||||||||||||
| Dr. Marlies Sproll 4 |
0 | 6,148 | 0 | 0 | – | |||||||||||||||
|
|
|
|||||||||||||||||||
| Total |
0 | 40,319 | 0 | 0 | 34,171 | |||||||||||||||
F-67
Table of Contents
| Convertible Bonds | ||||||||||||||||||||
| 01/01/2017 | Additions | Forfeitures | Exercises | 12/31/2017 | ||||||||||||||||
| Management Board |
||||||||||||||||||||
| Dr. Simon Moroney |
88,386 | 0 | 0 | 0 | 88,386 | |||||||||||||||
| Jens Holstein |
90,537 | 0 | 0 | 30,000 | 60,537 | |||||||||||||||
| Dr. Malte Peters1 |
– | 0 | 0 | 0 | 0 | |||||||||||||||
| Dr. Markus Enzelberger2 |
– | 0 | 0 | 0 | 0 | |||||||||||||||
| Dr. Arndt Schottelius3 |
60,537 | 0 | 0 | 60,537 | – | |||||||||||||||
| Dr. Marlies Sproll4 |
60,537 | 0 | 0 | 60,537 | – | |||||||||||||||
|
|
|
|||||||||||||||||||
| Total |
299,997 | 0 | 0 | 151,074 | 148,923 | |||||||||||||||
| Performance Shares | ||||||||||||||||||||
| 01/01/2017 | Additions | Forfeitures | Allocations | 12/31/2017 | ||||||||||||||||
| Management Board |
||||||||||||||||||||
| Dr. Simon Moroney |
37,220 | 4,864 | 0 | 12,024 | 30,060 | |||||||||||||||
| Jens Holstein |
25,134 | 3,187 | 0 | 8,235 | 20,086 | |||||||||||||||
| Dr. Malte Peters1 |
– | 3,187 | 0 | 0 | 3,187 | |||||||||||||||
| Dr. Markus Enzelberger2 |
– | 2,047 | 0 | 0 | 5,987 | |||||||||||||||
| Dr. Arndt Schottelius3 |
25,134 | 0 | 0 | 8,235 | – | |||||||||||||||
| Dr. Marlies Sproll4 |
25,134 | 2,390 | 0 | 8,235 | – | |||||||||||||||
|
|
|
|||||||||||||||||||
| Total |
112,622 | 15,675 | 0 | 36,729 | 59,320 | |||||||||||||||
| 1 | Dr. Malte Peters joined the Management Board of MorphoSys AG on March 1, 2017. |
| 2 | Dr. Markus Enzelberger joined the Management Board of MorphoSys AG on November 1, 2017. Prior to his appointment as member of the Management Board 4,906 shares have been held by Dr. Markus Enzelberger. Under the Long-Term Incentive Programs 2014 to 2016, Dr. Markus Enzelberger was granted 3,940 performance shares as a member of the Senior Management prior to his appointment as member of the Management Board. |
| 3 | Dr. Arndt Schottelius left the Management Board of MorphoSys AG on February 28, 2017. The exercises and allocations presented in the tables “Convertible Bonds” and “Performance Shares” were made after resignation from the Management Board. The respective convertible bonds and performance shares were granted in previous years. The table “Shares” shows no further changes in the number of shares after resignation from the Management Board of MorphoSys AG. |
| 4 | Dr. Marlies Sproll left the Management Board of MorphoSys AG on October 31, 2017. The exercises presented in the table “Convertible Bonds” were made after resignation from the Management Board. The respective convertible bonds were granted in a previous year. The table “Shares” shows no further changes in the number of shares after resignation from the Management Board of MorphoSys |
| 5 | Krisja Vermeylen joined the Supervisory Board of MorphoSys AG on May 17, 2017. |
| 6 | Karin Eastham left the Supervisory Board of MorphoSys AG on May 17, 2017. Changes in the number of shares after resignation from the Supervisory Board of MorphoSys AG are not presented in the tables. |
The Supervisory Board of MorphoSys AG does not hold any stock options, convertible bonds or performance shares.
The remuneration system for the Management Board is intended to encourage sustainable, results-oriented corporate governance. The Management Board’s total remuneration consists of several components, including fixed compensation, an annual cash bonus that is dependent upon the achievement of corporate targets (short-term incentives – STI), variable compensation components with long-term incentives (LTI) and other remuneration components. Variable remuneration components with long-term incentive consist of performance share plans from previous years and the current year, a convertible bond program from 2013 and a stock option plan from the current year. The members of the Management Board additionally receive fringe
F-68
Table of Contents
benefits in the form of benefits in kind, essentially consisting of a company car and insurance premiums. All total remuneration packages are reviewed annually by the Remuneration and Nomination Committee and compared to an annual Management Board remuneration analysis to check the scope and appropriateness of the remuneration packages. The amount of remuneration paid to members of the Management Board is based largely on the duties of the respective Management Board member, the financial situation and the performance and business outlook for the Company versus its competition. All resolutions on adjustments to the overall remuneration packages are passed by the plenum of the Supervisory Board. The remuneration of the Management Board and the index-linked pension scheme were last adjusted in July 2017. The remuneration of the new Management Board member, Dr. Markus Enzelberger, was amended as of November 1, 2017.
If a Management Board member’s employment contract terminates due to death, the member’s spouse or life partner is entitled to the fixed monthly salary for the month of death and the 12 months thereafter. In the event of a change of control, Management Board members are entitled to exercise their extraordinary right to terminate their employment contracts and receive any outstanding fixed salary for the remainder of the agreed contract period. Moreover, in such a case, all stock options and performance shares granted will become vested immediately and can be exercised after the expiration of the statutory vesting periods. A change of control has occurred when (i) MorphoSys transfers assets or a substantial portion of its assets to unaffiliated third parties, (ii) MorphoSys merges with an unaffiliated company or (iii) a shareholder or third party holds 30% or more of MorphoSys’s voting rights.
While in the management report the remuneration of the Management Board and Supervisory Boards as members in key management positions is presented in accordance with the provisions of the German Corporate Governance Code, the following tables show the expense-based view in accordance with IAS 24.
F-69
Table of Contents
MANAGEMENT BOARD REMUNERATION FOR THE YEARS 2017 AND 2016 (IAS 24):
| Dr. Simon Moroney Chief Executive Officer |
Jens Holstein Chief Financial Officer |
Dr. Malte Peters Chief Development Officer |
Dr. Markus Enzelberger 3 Chief Scientific Officer |
Dr. Marlies Sproll 4 Chief Scientific Officer |
Dr. Arndt Schottelius Chief Development Officer |
Total | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Appointment: March 1, 2017 |
Appointment (Interim-CSO): April 15, 2017 Appointment: November 1, 2017 |
Temporary Leave: April 15, 2017 - October 31, 2017 Resignation: October 31, 2017 |
Resignation: February 28, 2017 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | |||||||||||||||||||||||||||||||||||||||||||
| Fixed Compensation |
463,457 | 500,876 | 314,405 | 372,652 | – | 281,500 | – | 204,698 | 314,405 | 222,450 | 309,759 | 103,253 | 1,402,026 | 1,685,429 | ||||||||||||||||||||||||||||||||||||||||||
| Fringe Benefits1 |
34,270 | 35,912 | 46,300 | 42,905 | – | 568,644 | – | 417,158 | 24,141 | 20,427 | 28,388 | 9,161 | 133,099 | 1,094,207 | ||||||||||||||||||||||||||||||||||||||||||
| One -Year Variable Compensation |
210,873 | 368,144 | 143,054 | 273,899 | – | 206,903 | – | 121,688 | 143,054 | 67,745 | 140,940 | 23,490 | 637,921 | 1,061,869 | ||||||||||||||||||||||||||||||||||||||||||
| Total Short-Term Employee Benefits (IAS 24.17 (a)) |
708,600 | 904,932 | 503,759 | 689,456 | – | 1,057,047 | – | 743,544 | 481,600 | 310,622 | 479,087 | 135,904 | 2,173,046 | 3,841,505 | ||||||||||||||||||||||||||||||||||||||||||
| Service Cost |
142,096 | 149,567 | 92,875 | 99,949 | – | 60,967 | – | 29,186 | 92,876 | 77,976 | 95,473 | 28,245 | 423,320 | 445,890 | ||||||||||||||||||||||||||||||||||||||||||
| Total Benefit Expenses - Post-Employment Benefits (IAS 24.17 (b)) |
142,096 | 149,567 | 92,875 | 99,949 | – | 60,967 | – | 29,186 | 92,876 | 77,976 | 95,473 | 28,245 | 423,320 | 445,890 | ||||||||||||||||||||||||||||||||||||||||||
| Multi-Year Variable Compensation2: |
0 | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2013 Convertible Bonds Program |
33,964 | 58,224 | 34,791 | 59,641 | – | 0 | – | 0 | 23,263 | 39,879 | 23,263 | 39,879 | 115,281 | 197,623 | ||||||||||||||||||||||||||||||||||||||||||
| 2012 Long-Term Incentive Program |
(42,350 | ) | 0 | (29,007 | ) | 0 | – | 0 | – | 0 | (29,007 | ) | 0 | (29,007 | ) | 0 | (129,371 | ) | 0 | |||||||||||||||||||||||||||||||||||||
| 2013 Long-Term Incentive Program |
(10,303 | ) | 202,349 | (7,075 | ) | 138,585 | – | 0 | – | 0 | (7,075 | ) | 138,585 | (7,075 | ) | 138,585 | (31,528 | ) | 618,104 | |||||||||||||||||||||||||||||||||||||
| 2014 Long-Term Incentive Program |
32,972 | 22,460 | 22,572 | 15,383 | – | 0 | – | 0 | 22,572 | 15,383 | 22,572 | (42,038 | ) | 100,688 | 11,188 | |||||||||||||||||||||||||||||||||||||||||
| 2015 Long-Term Incentive Program |
148,799 | 67,635 | 101,906 | 46,324 | – | 0 | – | 0 | 101,906 | 46,324 | 101,906 | (79,105 | ) | 454,517 | 81,178 | |||||||||||||||||||||||||||||||||||||||||
F-70
Table of Contents
| Dr. Simon Moroney Chief Executive Officer |
Jens Holstein Chief Financial Officer |
Dr. Malte Peters Chief Development Officer |
Dr. Markus Enzelberger 3 Chief Scientific Officer |
Dr. Marlies Sproll 4 Chief Scientific Officer |
Dr. Arndt Schottelius Chief Development Officer |
Total | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Appointment: March 1, 2017 |
Appointment (Interim-CSO): April 15, 2017 Appointment: November 1, 2017 |
Temporary Leave: April 15, 2017 - October 31, 2017 Resignation: October 31, 2017 |
Resignation: February 28, 2017 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | |||||||||||||||||||||||||||||||||||||||||||
| 2016 Long-Term Incentive Program |
269,420 | 171,688 | 176,511 | 112,481 | – | 0 | – | 0 | 176,511 | 112,481 | 176,511 | (76,828 | ) | 798,953 | 319,822 | |||||||||||||||||||||||||||||||||||||||||
| 2017 Long-Term Incentive Program |
0 | 163,906 | 0 | 107,395 | – | 107,395 | – | 68,979 | 0 | 80,538 | 0 | – | 0 | 528,213 | ||||||||||||||||||||||||||||||||||||||||||
| 2017 Stock Option Plan (Vesting Period 4 Years) |
0 | 127,997 | 0 | 83,861 | – | 83,861 | – | 53,875 | 0 | 62,898 | 0 | – | 0 | 412,492 | ||||||||||||||||||||||||||||||||||||||||||
| Total Stock-Based Compensation (IAS 24.17 (e)) |
432,502 | 814,259 | 299,698 | 563,670 | – | 191,256 | – | 122,854 | 288,170 | 496,088 | 288,170 | (19,507 | ) | 1,308,540 | 2,168,620 | |||||||||||||||||||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total Compensation |
1,283,198 | 1,868,758 | 896,332 | 1,353,075 | – | 1,309,270 | – | 895,584 | 862,646 | 884,686 | 862,730 | 144,642 | 3,904,906 | 6,456,015 | ||||||||||||||||||||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 | In 2017, the fringe benefits of Dr. Malte Peters und Dr. Markus Enzelberger each included a one-time compensation in the form of MorphoSys shares as an incentive to join the Management Board of MorphoSys AG. |
| 2 | The fair value was determined pursuant to the regulations of IFRS 2 “Share-based Payments”. This table shows the pro-rata share of personnel expenses resulting from stock-based compensation for the respective financial year. Further details can be found in Sections 7.1, 7.2. and 7.3. |
| 3 | The figures presented for Dr. Markus Enzelberger do not include any compensation granted for his activities as a member of the Senior Management Group as they do not relate to his appointment as a member of the Management Board. |
| 4 | Dr. Marlies Sproll left the Management Board of MorphoSys AG on October 31, 2017. Since November 1, 2017, Dr. Marlies Sproll has taken on a new part-time role at MorphoSys as Special Adviser to the CEO. Therefore, the figures presented for Dr. Marlies Sproll do not include any remuneration granted for these activities. |
F-71
Table of Contents
On January 5, 2017, MorphoSys announced that Dr. Malte Peters would succeed Dr. Arndt Schottelius as the Chief Development Officer and member of the Management Board of MorphoSys AG. Dr. Schottelius resigned from his position as Chief Development Officer effective February 28, 2017 to pursue new challenges. For the period leading up to the end of his employment contract on April 30, 2017, Dr. Schottelius and MorphoSys entered into an exemption agreement. According to the agreement, Dr. Schottelius was entitled to the remuneration agreed in his employment contract until the date of April 30, 2017. The remuneration included a contractually agreed payment of a pro rata amount of his annual gross base salary of € 103,252.96 and a bonus of € 23,490.05. Dr. Schottelius also exercised the convertible bonds granted to him in 2013. In addition, he received shares that had vested after the four-year vesting period under the 2013 Performance Share Plan. Dr. Schottelius still has a pro rata entitlement based on the 2014, 2015 and 2016 Performance Share Plans, which can be exercised after a total of four years at the earliest. Dr. Schottelius did not participate in the 2017 Performance Share Plan. Effective March 1, 2017, Dr. Malte Peters was appointed Chief Development Officer of MorphoSys AG. His employment contract runs until June 30, 2019. As an additional incentive to join MorphoSys, Dr. Peters was granted a one-time compensation payment for the lost compensation from his former employment. This compensation was in the form of treasury shares held by MorphoSys valued at € 500,000. In the 2017 financial year, the granting of these shares was recognized as personnel expenses from performance shares as defined by IFRS 2.
On October 30, 2017, MorphoSys announced that Dr. Markus Enzelberger would succeed Dr. Marlies Sproll as Chief Scientific Officer at MorphoSys AG. Dr. Sproll had been on a temporary leave of absence since April 15, 2017 and eventually resigned from her post as Chief Scientific Officer effective October 31, 2017. She was working as a Special Advisor to the CEO of MorphoSys, Simon Moroney, on a parttime basis since November 1, 2017. She received remuneration until October 31, 2017 in accordance with her employment contract. Dr. Sproll’s long-term compensation granted to her during her time as a member of the Management Board will be settled in accordance with the plans’ terms. Effective November 1, 2017, Dr. Enzelberger was appointed Chief Scientific Officer of MorphoSys AG after having served as the Interim Chief Scientific Officer since April 15, 2017. Dr. Enzelberger has held various management positions in research and development at MorphoSys since 2002. His Management Board employment contract runs until June 30, 2020. Upon joining the Management Board of MorphoSys AG, Dr. Enzelberger was granted a one-time incentive consisting of treasury shares held by MorphoSys valued at € 400,000. In the 2017 financial year, the granting of these shares was recognized as personnel expenses from performance shares as defined by IFRS 2.
In the years 2017 and 2016, there were no other long-term benefits in accordance with IAS 24.17 (c) or benefits upon termination of employment in accordance with IAS 24.17 (d) accruing to the Management Board or Supervisory Board.
In 2017, the total remuneration for the Supervisory Board, excluding reimbursed travel costs, amounted to € 523,015 (2016: € 529,680).
F-72
Table of Contents
SUPERVISORY BOARD REMUNERATION FOR THE YEARS 2017 AND 2016:
| Fixed Compensation |
Attendance Fees1 | Total Compensation |
||||||||||||||||||||||
| in € | 2017 | 2016 | 2017 | 2016 | 2017 | 2016 | ||||||||||||||||||
| Dr. Gerald Möller |
95,156 | 91,400 | 36,800 | 43,400 | 131,956 | 134,800 | ||||||||||||||||||
| Dr. Frank Morich |
57,240 | 57,240 | 23,200 | 26,800 | 80,440 | 84,040 | ||||||||||||||||||
| Dr. Marc Cluzel |
52,160 | 52,160 | 26,800 | 34,600 | 78,960 | 86,760 | ||||||||||||||||||
| Krisja Vermeylen2 |
28,961 | – | 16,000 | – | 44,961 | – | ||||||||||||||||||
| Wendy Johnson |
46,160 | 46,160 | 38,000 | 33,800 | 84,160 | 79,960 | ||||||||||||||||||
| Klaus Kühn |
46,160 | 46,160 | 22,000 | 21,400 | 68,160 | 67,560 | ||||||||||||||||||
| Karin Eastham3 |
19,578 | 52,160 | 14,800 | 24,400 | 34,378 | 76,560 | ||||||||||||||||||
|
|
|
|||||||||||||||||||||||
| Total |
345,415 | 345,280 | 177,600 | 184,400 | 523,015 | 529,680 | ||||||||||||||||||
| 1 | The attendance fee contains expense allowances for the attendance at the Supervisory Board and the Committee meetings. |
| 2 | Krisja Vermeylen joined the Supervisory Board of MorphoSys AG on May 17, 2017. |
| 3 | Karin Eastham has left the Supervisory Board of MorphoSys AG on May 17, 2017. |
No other agreements presently exist with current or former members of the Supervisory Board.
On December 31, 2017, the Senior Management Group held 35,978 stock options (December 31, 2016: 0), 13,233 convertible bonds (December 31, 2016: 136,588) and 67,149 performance shares (December 31, 2016: 82,143) granted by the Company. In 2017, a new stock option program and a new performance share program were granted to the Senior Management Group (see Items 7.1 and 7.3.5). On April 1, 2017, the Senior Management Group was allocated 21,248 shares from the 2013 LTI program and 548 shares on October 1, 2017. In each case there was the option to receive these shares within a six-month period. As of December 2017, the Senior Management Group had exercised options to receive 21,796 shares.
| 8.1 | OBLIGATIONS ARISING FROM OPERATING LEASES, RENTAL AND OTHER CONTRACTS |
The Group leases facilities and equipment under long-term operating leases. In financial years 2017 and 2016, leasing expenses amounted to € 2.6 million and € 3.1 million. The 2016 amount includes the recognition of a provision for onerous contracts from rent obligations for office premises. Leasing expenses for 2017 and 2016 include expenses for company cars and machinery totaling € 0.2 million and € 0.2 million, respectively. The majority of these contracts can be renewed on a yearly or quarterly basis. Some of these agreements may be terminated prematurely.
In 2016 a rental agreement was signed for the premises at Semmelweisstraße 7, Planegg. The contract includes a minimum rental period of ten years.
F-73
Table of Contents
The future minimum payments under non-terminable operating leases, insurance contracts and other services as of December 31, 2017 are shown in the following table.
| in 000’ € | Rent and Leasing |
Other | Total | |||||||||
| Up to One Year |
2,918 | 733 | 3,651 | |||||||||
| Between One and Five Years |
11,209 | 0 | 11,209 | |||||||||
| More than Five Years |
11,190 | 0 | 11,190 | |||||||||
|
|
|
|||||||||||
| Total |
25,317 | 733 | 26,050 | |||||||||
|
|
|
|||||||||||
Additionally, the future payments shown in the table below may become due for outsourced studies after December 31, 2017. These amounts could be shifted or substantially lower due to changes in the study timeline or premature study termination.
| in million € | Total 2017 |
|||
| Up to One Year |
56.1 | |||
| Between One and Five Years |
66.1 | |||
| More than Five Years |
0.0 | |||
|
|
|
|||
| Total |
122.2 | |||
|
|
|
|||
| 8.2 | CONTINGENT ASSETS/CONTINGENT LIABILITIES |
Contingent liabilities are potential obligations from past events that exist only when the occurrence of one or more uncertain future events – beyond the Company’s control – is confirmed. Current obligations can represent a contingent liability if it is not probable enough that an outflow of resources justifies the recognition of a provision. Moreover, it is not possible to make a sufficiently reliable estimate of the amount of the obligations.
The Management Board is unaware of any proceedings that may result in a significant obligation for the Group and may lead to a material adverse effect on the Group’s net assets, financial position or results of operations.
If certain milestones are achieved in the Proprietary Development segment, for example, filing an application for an investigational new drug, or IND, for specific target molecules, this may trigger regulatory and sales milestone payments to licensors of up to an aggregate of $287 million. The next milestone payment in the amount of $12.5 million could occur in approximately 18 to 24 months.
If a partner achieves certain milestones in the Partnered Discovery segment, for example, filing an application for an investigational new drug (IND) for specific target molecules or the transfer of technology, this may trigger milestone payments to MorphoSys. However, no further details can be published since the timing, and the achievement of such milestones are uncertain.
Obligations may arise from enforcing the Company’s patents against third parties. It is also conceivable that competitors may challenge the patents of the MorphoSys Group companies. MorphoSys may also come to the conclusion that MorphoSys’s patents or patent families have been infringed upon by competitors, which may prompt MorphoSys to take legal action against competitors. At present, there are no specific indications that liabilities have occurred as described above.
F-74
Table of Contents
| 8.3 | CORPORATE GOVERNANCE |
The Group has submitted the Declaration of Conformity with the recommendations of the Government Commission on the German Corporate Governance Code for the 2017 financial year under Sec. 161 of the German Stock Corporation Act (AktG). This declaration was published on the Group’s website (www.morphosys.com) on December 1, 2017 and made permanently available to the public.
| 8.4 | RESEARCH AND DEVELOPMENT AGREEMENTS |
The Group has entered numerous research and development agreements as part of its proprietary research and development activities and its partnered research strategy. The following information describes the agreements that have a material effect on the Group and the developments under the research and development agreements in the 2017 financial year.
| 8.4.1 | PROPRIETARY DEVELOPMENT SEGMENT |
In the Proprietary Development segment, partnerships are entered into as part of the Group’s strategy to develop its own drugs in its core areas of oncology and inflammatory diseases. Our partners include (in alphabetical order): G7 Therapeutics, Galapagos, GlaxoSmithKline, I-Mab Biopharma, Immatics Biotechnologies, Merck Serono, MD Anderson Cancer Center and Xencor.
In August 2014, MorphoSys and Aptevo Therapeutics Inc., a spin-off of Emergent BioSolutions, announced a co-development and co-promotion agreement for MOR209/ES414. MOR209/ES414 is a bi-specific anti-PSMA/anti-CD3 antibody based on Aptevo’s (formerly Emergent) proprietary ADAPTIR™ platform (modular protein technology). In the process of prioritizing its development programs, MorphoSys ended the cooperation with Aptevo Therapeutics Inc. at the end of 2017. The rights to the drug’s development and commercialization were returned to Aptevo. As a result of ending the cooperation, impairment for the in-process research and development MOR209/ES414 program in the amount of € 9.8 million was recognized in 2017.
In August 2015, MorphoSys and Swiss-based G7 Therapeutics AG announced a new collaboration to develop novel antibody therapeutics targeting G protein-coupled receptors (GPCRs) and other potentially disease-related transmembrane proteins, such as ion channels. Under this agreement, G7 Therapeutics will give MorphoSys a choice of various receptors that can be linked to the emergence of a variety of diseases. MorphoSys will use its proprietary Ylanthia antibody library to identify and develop antibody compounds directed against these receptors. MorphoSys has the right to sublicense to partners access to these target molecules in conjunction with therapeutic antibody programs.
In November 2008, MorphoSys and Galapagos announced a long-term drug discovery and co-development cooperation aimed at exploring novel mechanisms for the treatment of inflammatory diseases and developing antibody therapies against these diseases. The agreement covers all activities ranging from the probing of target molecules to the completion of clinical trials for novel therapeutic antibodies. After demonstrating clinical efficacy in humans, the programs may be out-licensed to partners for further development, approval, and commercialization. Both companies contributed their core technologies and expertise to the alliance. Along with the use of its adenovirus-based platform for the exploration of new target molecules for the development of antibodies, Galapagos provided access to target molecules already identified that are associated with bone and joint diseases. MorphoSys provided access
F-75
Table of Contents
to its antibody technologies used for generating fully human antibodies directed against these target molecules. Under the terms of the agreement, Galapagos and MorphoSys will share the research and development costs. In July 2014, the collaboration advanced into the preclinical development of MOR106, an antibody from MorphoSys’ next-generation library Ylanthia directed against a novel Galapagos target molecule. The antibody will be co-developed in the area of inflammatory diseases.
In June 2013, MorphoSys announced it had entered into a global agreement with GlaxoSmithKline (GSK) for the development and commercialization of MOR103. MOR103/GSK3196165 is MorphoSys’s proprietary HuCAL antibody against the GM-CSF target molecule. Under the agreement, GSK assumes responsibility for the compound’s entire development and commercialization. MorphoSys received an immediate upfront payment of € 22.5 million as part of this agreement. Depending on the achievement of certain developmental stages and regulatory, commercial and revenue-related milestones, MorphoSys is eligible to receive additional payments from GSK in the amount of up to € 423 million, as well as tiered double-digit royalties on net sales. The drug is currently undergoing development in a phase 2b study in patients with rheumatoid arthritis and a 2a study in patients with osteoarthritis of the hand. GSK also initiated a mechanistic phase 2a study of MOR103/GSK3196165 in rheumatoid arthritis to further investigate the GM-CSF signaling pathway affected by the HuCAL antibody.
In the reporting year, MorphoSys announced it had signed an exclusive regional licensing agreement with I-Mab Biopharma to develop and commercialize MOR202 in China, Taiwan, Hong Kong and Macao. MOR202 is MorphoSys’s proprietary antibody targeting CD38. MOR202 is being evaluated in a phase 1/2a clinical trial in Europe in patients with multiple myeloma. Under the terms of the agreement, I-Mab Biopharma has the exclusive rights for the subsequent development and commercialization of MOR202 in the agreed regions. MorphoSys received an immediate upfront payment of USD 20.0 million. MorphoSys is also entitled to receive additional success-based clinical and commercial milestone payments from I-Mab of up to approximately USD 100 million, as well as tiered double-digit, staggered royalties on net sales of MOR202 in the agreed regions.
In August 2015, MorphoSys announced a strategic alliance in the field of immuno-oncology with the German company Immatics Biotechnologies GmbH. The alliance was formed to develop novel antibody-based therapies against a variety of cancer antigens that are recognized by T cells. The alliance agreement gives MorphoSys access to several of Immatics’s proprietary tumor-associated peptides (TUMAPs). In return, Immatics receives the right to develop MorphoSys’s Ylanthia antibodies against several TUMAPs. The companies will pay each other milestone payments and royalties on commercialized products based on the companies’ development progress.
In June 2014, MorphoSys and Merck KGaA announced an agreement to identify and develop therapeutic antibodies against target molecules of the class of immune checkpoints. Under this agreement, both MorphoSys and Merck Serono, the biopharmaceutical division of Merck, will co-develop therapies intended to trigger the immune system to attack tumors. MorphoSys will use its proprietary Ylanthia antibody library and other technology platforms to generate antibodies directed against the selected target molecules. Merck Serono is contributing its expertise in the field of immuno-oncology and clinical development and will assume full project responsibility starting with phase 1 of clinical development.
F-76
Table of Contents
In May 2016, MorphoSys and the University of Texas MD Anderson Cancer Center announced a long-term strategic alliance. With MorphoSys applying its Ylanthia technology platform, the partners will work together to identify, validate and develop novel anti-cancer antibodies through to clinical proof of concept by researching targets in a variety of oncology indications. MorphoSys and MD Anderson will conduct early clinical studies of therapeutic antibody candidates after which MorphoSys has the option to continue developing selected antibodies in later stages of clinical development for its own proprietary pipeline.
In June 2010, MorphoSys AG and the US-based biopharmaceutical company Xencor signed an exclusive global licensing and cooperation agreement under which MorphoSys receives exclusive global licensing rights to the XmAb5574/MOR208 antibody for the treatment of cancer and other indications. The companies jointly conducted a phase 1/2a trial in the US in patients with chronic lymphocytic leukemia. MorphoSys is solely responsible for further clinical development after the successful completion of the phase 1 clinical trial. Xencor received an upfront payment of USD 13.0 million (approx. € 10.5 million) from MorphoSys, which was capitalized under in-process R&D programs. Xencor is entitled to development, regulatory, and commercially-related milestone payments as well as tiered royalties on product sales.
| 8.4.2 | PARTNERED DISCOVERY SEGMENT |
Commercial partnerships in the Partnered Discovery segment provide MorphoSys with various types of payments that are spread over the duration of the agreements or recognized in full as revenue when reaching a predefined target or milestone. These payments include upfront payments upon signature, annual license fees in exchange for access to MorphoSys’s technologies and payments for funded research to be performed by MorphoSys on behalf of the partner. In addition, MorphoSys is entitled to development-related milestone payments and royalties on product sales for specific antibody programs.
Prior to the 2017 financial year, active collaborations with a number of partners had already ended because the agreements had expired. However, drug development programs initiated in the active phase are designed so that they can be continued by the partner and, therefore, still result in performance-based payments for the achievement of the defined milestones.
Partnerships in the Partnered Discovery segment that ended before the beginning of 2017 but where drug development programs were still being pursued, include (in alphabetical order): Astellas, Bayer AG, Boehringer Ingelheim, Daiichi-Sankyo, Fibron Ltd. (continuation of contract with Prochon Biotech Ltd.), Janssen Biotech, Merck & Co., OncoMed Pharmaceuticals, Pfizer, Roche and Schering-Plough (a subsidiary of Merck & Co.).
Partnerships that were still active in 2017 include (in alphabetical order): GeneFrontier Corporation/Kaneka, Heptares, LEO Pharma and Novartis.
The Group’s alliance with Novartis AG ended in November 2017. The companies started working together in 2004, which has led to the creation of several ongoing therapeutic antibody programs against a number of diseases. In December 2007, MorphoSys and Novartis significantly expanded their existing relationship and forged a strategic alliance in the discovery and development of biopharmaceuticals. The payments for technology access, internalization charges, and R&D services amounted to € 450.5 million over the ten-year contract. Additionally, MorphoSys receives performance-based milestones, contingent upon the successful clinical development and regulatory approval of several products. In addition to these payments,
F-77
Table of Contents
MorphoSys is also entitled to royalties on any future product sales. The partnership with Novartis ended at the end of November 2017 according to the contract. Novartis did not exercise its option to extend the contract.
| 8.5 | SUBSEQUENT EVENTS |
No other events occurred after the balance sheet date of December 31, 2017 that require reporting.
F-78
Table of Contents
8,300,000 American Depositary Shares
Representing 2,075,000 Ordinary Shares
PROSPECTUS
| Goldman Sachs & Co. LLC | J.P. Morgan | Leerink Partners |
| Berenberg Capital Markets, LLC | JMP Securities |
Prospectus dated April 18, 2018
Through and including May 13, 2018 (the 25th day after the date of this prospectus), all dealers effecting transactions in these securities, whether or not participating in this international offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Woodside Energy Group Ltd Annual General Meeting Address by Chair Richard Goyder and CEO Meg O'Neill
- Heineken Holding N.V. reports on 2024 first-quarter trading
- Eurofins Achieves Organic Growth of 6.8% in Q1 2024, Ahead of Its Objectives
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share