Form 20-F AnPac Bio-Medical Scienc For: Dec 31
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 20-F
(Mark One)
|
o |
REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR 12(g) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
|
|
|
OR | |
|
|
|
|
x |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
|
|
|
OR | |
|
|
|
|
o |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
|
|
|
OR | |
|
|
|
|
o |
SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Date of event requiring this shell company report
For the transition period from to
Commission file number: 001-39137
|
AnPac Bio-Medical Science Co., Ltd. |
|
(Exact name of Registrant as specified in its charter) |
|
|
|
N/A |
|
(Translation of Registrant’s name into English) |
|
|
|
British Virgin Islands |
|
(Jurisdiction of incorporation or organization) |
|
|
|
801 Bixing Street, Bihu County Lishui, Zhejiang Province 323006 The People’s Republic of China |
|
(Address of principal executive offices) |
|
|
|
Chris Chang Yu, Chairman and Chief Executive Officer Tel: +86-578-2051-666 801 Bixing Street, Bihu County Lishui, Zhejiang Province 323006 The People’s Republic of China |
|
(Name, Telephone, E-mail and Address of Company Contact Person) |
Securities registered or to be registered pursuant to Section 12(b) of the Act:
|
Title of Each Class |
|
Trading Symbol(s) |
|
Name of Each Exchange on Which |
|
American depositary shares (each representing one Class A ordinary share, par value US$0.01 per share) Class A ordinary share, par value US$0.01 per share * |
|
ANPC |
|
NASDAQ Global Market |
* Not for trading, but only in connection with the listing on the NASDAQ Global Market of the American depositary shares.
Securities registered or to be registered pursuant to Section 12(g) of the Act:
|
None |
|
(Title of Class) |
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act:
|
None |
|
(Title of Class) |
Indicate the number of outstanding shares of each of the issuer’s class the period covered by the annual report:
As of December 31, 2020, there were 12,020,760 ordinary shares, being the sum of (i) 9,192,660 Class A ordinary shares, par value US$0.01 per share (including shares issued and outstanding, as well as (a) 35,000 shares legally issuable as of December 31, 2020 based on the relevant GAAP standard, which were issued subsequently, and (b) 500,000 shares reserved for potential conversion of convertible bonds and convertible debentures), and (ii) 2,863,100 Class B ordinary shares, par value US$0.01 per share.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
o Yes x No
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
o Yes x No
Note — Checking the box above will not relieve any registrant required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 from their obligations under those Sections.
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
x Yes o No
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
x Yes o No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company. See definition of “large accelerated filer,” “accelerated filer,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer o |
|
Accelerated filer o |
|
Non-accelerated filer x |
|
Emerging growth company x |
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards† provided pursuant to Section 13(a) of the Exchange Act. o
† The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012.
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. o
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
|
U.S. GAAP x |
|
International Financial Reporting Standards as issued |
|
Other o |
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow.
o Item 17 o Item 18
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
o Yes x No
(APPLICABLE ONLY TO ISSUERS INVOLVED IN BANKRUPTCY PROCEEDINGS DURING THE PAST FIVE YEARS)
Indicate by check mark whether the registrant has filed all documents and reports required to be filed by Sections 12, 13 or 15(d) of the Securities Exchange Act of 1934 subsequent to the distribution of securities under a plan confirmed by a court.
o Yes o No
|
|
1 | ||
|
|
2 | ||
|
|
|
4 | |
|
|
4 | ||
|
|
4 | ||
|
|
4 | ||
|
|
50 | ||
|
|
87 | ||
|
|
87 | ||
|
|
104 | ||
|
|
114 | ||
|
|
116 | ||
|
|
116 | ||
|
|
117 | ||
|
|
133 | ||
|
|
134 | ||
|
|
|
137 | |
|
|
137 | ||
|
MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS |
|
137 | |
|
|
137 | ||
|
|
139 | ||
|
|
139 | ||
|
|
139 | ||
|
|
139 | ||
|
PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS |
|
139 | |
|
|
139 | ||
|
|
140 | ||
|
|
141 | ||
|
|
|
142 | |
|
|
142 | ||
|
|
142 | ||
|
|
142 | ||
Except where the context otherwise requires:
· “ADME test” refers to our immunology test named AnPac Defense Medical Examination;
· “ADRs” refers to the American depositary receipts that evidence our ADSs;
· “ADSs” refers to our American depositary shares, each of which represents one Class A ordinary share;
· “CDA test” refers to our cancer screening and detection test using the CDA technology;
· “CDA-based tests” refers to either or both of our CDA tests and combination tests;
· “China” or “PRC” refers to the People’s Republic of China, excluding, for the purpose of this annual report only, Hong Kong, Macau and Taiwan;
· “Class A ordinary shares” refers to our Class A ordinary shares of par value US$0.01 per share;
· “Class B ordinary shares” refers to our Class B ordinary shares of par value US$0.01 per share;
· “combination test” refers to a test that combines our CDA test with an auxiliary test based on another cancer screening and detection technology, such as biomarker-based test (which have historically been our primary combination test) and the ct-DNA test (which we refer to as the APCS (AnPac Pan Cancer Screening) test), using our proprietary algorithm;
· “detection” of cancers by our CDA-based device or tests refers to the detection of the risk of whether cancer may occur or has occurred, not to cancer diagnosis, and “detect” has the corresponding meaning;
· “RMB” or “Renminbi” refers to the legal currency of China;
· “shares” or “ordinary shares” refers to our ordinary shares, including Class A and Class B ordinary shares, par value US$0.01 per share;
· “US$,” “U.S. dollars,” “$” or “dollars” refers to the legal currency of the United States; and
· “we,” “us,” “our company,” “our” or “AnPac Bio” refers to AnPac Bio-Medical Science Co., Ltd. and its subsidiaries;
Our reporting currency is the Renminbi. Certain of our financial data in this annual report on Form 20-F are translated into U.S. dollars solely for the reader’s convenience. Unless otherwise noted, all convenience translations from Renminbi to U.S. dollars in this annual report on Form 20-F were made at a rate of RMB6.5250 to US$1.00, the exchange rate set forth in the H.10 statistical release of the Board of Governors of the Federal Reserve System on December 31, 2020. We make no representation that any Renminbi or U.S. dollar amounts could have been, or could be, converted into U.S. dollars or Renminbi, as the case may be, at any particular rate, at the rate stated above, or at all. The PRC government restricts or prohibits the conversion of Renminbi into foreign currency and foreign currency into Renminbi for certain types of transactions.
This annual report on Form 20-F contains forward-looking statements that reflect our current expectations and views of future events. All statements other than statements of historical facts are forward-looking statements. These forward-looking statements are made under the “safe-harbor” provisions of the U.S. Private Securities Litigation Reform Act of 1995. These statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from those expressed or implied by the forward-looking statements.
You can identify some of these forward-looking statements by words or phrases such as “believes,” “estimates,” “anticipates,” “expects,” “plans,” “projects,” “intends,” “potential,” “target,” “aim,” “predict,” “outlook,” “seek,” “goal” “objective,” “assume,” “contemplate,” “continue,” “positioned,” “forecast,” “likely,” “may,” “could,” “might,” “will,” “should,” “approximately” or other similar expressions. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy and financial needs. These forward-looking statements include, but are not limited to, statements about:
· the implementation of our business model and growth strategies;
· trends and competition in the cancer screening and detection market;
· our expectations regarding demand for and market acceptance of our cancer screening and detection tests and our ability to expand our customer base;
· the duration of COVID-19 and its impact on our business and financial performance;
· our ability to obtain and maintain intellectual property protections for our CDA technology and our continued research and development to keep pace with technology developments;
· our ability to obtain and maintain regulatory approvals from the PRC National Medical Products Administration (the “NMPA”), U.S. Food and Drug Administration (the “FDA”) and the relevant U.S. states and to have our laboratories certified or accredited by authorities including under CLIA;
· our future business development, financial condition and results of operations and our ability to obtain financing cost-effectively;
· potential changes of government regulations;
· general economic and business conditions in China and elsewhere;
· our ability to hire and maintain key personnel; and
· our relationship with our major business partners and customers.
This annual report on Form 20-F also contains estimates, projections and statistical data obtained from various government and private publications. This market data speaks as of the date it was published and includes projections that are based on a number of assumptions and are not representations of facts. The cancer screening and detection market may not grow at the rates projected by market data, or at all. The failure of this market to grow at the projected rate may have a material adverse effect on our business and the market price of our ADSs. If any one or more of the assumptions underlying the market data proves to be incorrect, actual results may differ from the projections based on these assumptions. In addition, projections, assumptions and estimates of our future performance and the future performance of the industry in which we operate are necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described in “Risk Factors” and elsewhere in this annual report. You should not place undue reliance on these forward-looking statements.
The forward-looking statements made in this annual report relate only to events or information as of the date on which the statements are made in this annual report. Except as required by U.S. federal securities law, we undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, after the date on which the statements are made or to reflect the occurrence of unanticipated events. You should read this annual report and the documents that we reference in this annual report and have filed as exhibits to this annual report, completely and with the understanding that our actual future results may be materially different from what we expect. Other sections of this annual report include additional factors which could adversely impact our business and financial performance. Moreover, we operate in an evolving environment. New risk factors emerge from time to time and it is not possible for our management to predict all risk factors, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. We qualify all of our forward-looking statements by these cautionary statements.
ITEM 1. IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS
Not applicable.
ITEM 2. OFFER STATISTICS AND EXPECTED TIMETABLE
Not applicable.
A. Selected Financial Data
The following selected consolidated statements of comprehensive income data and selected consolidated cash flows data for the years ended December 31, 2018, 2019 and 2020, and selected consolidated balance sheets data as of December 31, 2019 and 2020 have been derived from our audited consolidated financial statements included elsewhere in this annual report beginning on page F-1. Our selected consolidated balance sheets data as of December 31, 2018 has been derived from our audited consolidated financial statements not included in this annual report. Our consolidated financial statements are prepared and presented in accordance with U.S. GAAP. Our historical results do not necessarily indicate results expected for any future periods. You should read this Selected Financial Data section together with our consolidated financial statements and the related notes and “Item 5. Operating and Financial Review and Prospects” below.
The following table presents our selected consolidated statements of comprehensive loss data for the years ended December 31, 2018, 2019 and 2020.
|
|
|
For the year ended December 31, |
| ||||||
|
|
|
2018 |
|
2019 |
|
2020 |
| ||
|
|
|
RMB |
|
RMB |
|
RMB |
|
US$ |
|
|
|
|
(in thousands) |
| ||||||
|
Selected Consolidated Statements of Comprehensive Loss Data: |
|
|
|
|
|
|
|
|
|
|
Revenues: |
|
|
|
|
|
|
|
|
|
|
Cancer screening and detection tests |
|
9,557 |
|
10,381 |
|
18,445 |
|
2,827 |
|
|
Physical checkup packages, net |
|
693 |
|
464 |
|
2,064 |
|
316 |
|
|
Total revenues |
|
10,250 |
|
10,845 |
|
20,509 |
|
3,143 |
|
|
Cost of revenues, cancer screening(1) |
|
(5,672 |
) |
(6,047 |
) |
(7,628 |
) |
(1,169 |
) |
|
Gross profit |
|
4,578 |
|
4,798 |
|
12,881 |
|
1,974 |
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
Selling and marketing(1) |
|
(9,827 |
) |
(13,633 |
) |
(19,674 |
) |
(3,015 |
) |
|
Research and development(1) |
|
(10,106 |
) |
(9,839 |
) |
(11,576 |
) |
(1,774 |
) |
|
General and administrative(1) |
|
(28,254 |
) |
(69,088 |
) |
(74,757 |
) |
(11,457 |
) |
|
Impairment of long-term investments |
|
— |
|
(1,320 |
) |
(1,430 |
) |
(219 |
) |
|
Loss from operations |
|
(43,609 |
) |
(89,082 |
) |
(94,556 |
) |
(14,491 |
) |
|
Non-operating income and expenses |
|
|
|
|
|
|
|
|
|
|
Interest expense, net |
|
(925 |
) |
(2,609 |
) |
(1,143 |
) |
(175 |
) |
|
Foreign exchange loss, net |
|
(2,776 |
) |
(3,219 |
) |
(667 |
) |
(102 |
) |
|
Share of net (loss) gain in equity method investments |
|
(441 |
) |
190 |
|
(13 |
) |
(2 |
) |
|
Other income (expenses), net |
|
6,040 |
|
(1,823 |
) |
9,096 |
|
1,394 |
|
|
Change in fair value of convertible debt and settlement gain |
|
(784 |
) |
(5,296 |
) |
6,630 |
|
1,016 |
|
|
Loss before income taxes |
|
(42,495 |
) |
(101,839 |
) |
(80,653 |
) |
(12,360 |
) |
|
Income tax benefit |
|
199 |
|
218 |
|
88 |
|
13 |
|
|
Net loss |
|
(42,296 |
) |
(101,621 |
) |
(80,565 |
) |
(12,347 |
) |
|
Net loss attributable to non-controlling interests |
|
(233 |
) |
(561 |
) |
(90 |
) |
(14 |
) |
|
Net loss attributable to ordinary shareholders |
|
(42,063 |
) |
(101,060 |
) |
(80,475 |
) |
(12,333 |
) |
|
Loss per share: |
|
|
|
|
|
|
|
|
|
|
Class A and Class B ordinary shares - basic and diluted |
|
(4.93 |
) |
(11.31 |
) |
(7.19 |
) |
(1.10 |
) |
|
Weighted average shares outstanding used in calculating basic and diluted loss per share: |
|
|
|
|
|
|
|
|
|
|
Class A and Class B ordinary shares - basic and diluted |
|
8,524,100 |
|
8,937,600 |
|
11,190,079 |
|
11,190,079 |
|
Note:
(1) Share-based compensation expenses were allocated as follows:
|
|
|
For the year ended December 31, |
| ||||||
|
|
|
2018 |
|
2019 |
|
2020 |
| ||
|
|
|
RMB |
|
RMB |
|
RMB |
|
US$ |
|
|
|
|
(in thousands) |
| ||||||
|
Cost of revenues |
|
317 |
|
327 |
|
327 |
|
50 |
|
|
Selling and marketing expenses |
|
2,871 |
|
5,393 |
|
1,113 |
|
170 |
|
|
Research and development expenses |
|
1,958 |
|
2,534 |
|
3,534 |
|
542 |
|
|
General and administrative expenses |
|
2,790 |
|
24,601 |
|
12,788 |
|
1,960 |
|
|
Total share-based compensation expenses |
|
7,936 |
|
32,855 |
|
17,762 |
|
2,722 |
|
The following table presents our selected consolidated balance sheet data as of December 31, 2018, 2019 and 2020.
|
|
|
As of December 31, |
| ||||||
|
|
|
2018 |
|
2019 |
|
2020 |
| ||
|
|
|
RMB |
|
RMB |
|
RMB |
|
US$ |
|
|
|
|
(in thousands) |
| ||||||
|
Selected Consolidated Balance Sheet Data: |
|
|
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
12,887 |
|
6,125 |
|
3,016 |
|
462 |
|
|
Total current assets |
|
20,852 |
|
22,171 |
|
21,288 |
|
3,262 |
|
|
Total assets |
|
52,762 |
|
52,982 |
|
49,887 |
|
7,645 |
|
|
Current liabilities |
|
|
|
|
|
|
|
|
|
|
Short-term debt |
|
25,961 |
|
38,568 |
|
8,232 |
|
1,262 |
|
|
Amounts due to related parties |
|
28,687 |
|
4,597 |
|
4,130 |
|
633 |
|
|
Total current liabilities |
|
71,438 |
|
66,197 |
|
43,524 |
|
6,670 |
|
|
Total liabilities |
|
75,155 |
|
68,906 |
|
46,610 |
|
7,143 |
|
|
Total shareholders’ (deficit) equity |
|
(22,393 |
) |
(15,924 |
) |
3,277 |
|
502 |
|
The following table presents our selected consolidated cash flow data for the years ended December 31, 2018, 2019 and 2020.
|
|
|
For the year ended December 31, |
| ||||||
|
|
|
2018 |
|
2019 |
|
2020 |
| ||
|
|
|
RMB |
|
RMB |
|
RMB |
|
US$ |
|
|
|
|
(in thousands) |
| ||||||
|
Selected Consolidated Cash Flow Data: |
|
|
|
|
|
|
|
|
|
|
Net cash used in operating activities |
|
(31,147 |
) |
(48,600 |
) |
(58,967 |
) |
(9,035 |
) |
|
Net cash used in investing activities |
|
(2,680 |
) |
(3,461 |
) |
(2,482 |
) |
(380 |
) |
|
Net cash provided by financing activities |
|
36,271 |
|
46,108 |
|
60,924 |
|
9,339 |
|
|
Effect of foreign exchange rate changes on cash and cash equivalents |
|
(969 |
) |
(809 |
) |
(2,584 |
) |
(401 |
) |
|
Net increase (decrease) in cash and cash equivalents |
|
1,475 |
|
(6,762 |
) |
(3,109 |
) |
(477 |
) |
|
Cash and cash equivalents at the beginning of the year |
|
11,412 |
|
12,887 |
|
6,125 |
|
939 |
|
|
Cash and cash equivalents at the end of the year |
|
12,887 |
|
6,125 |
|
3,016 |
|
462 |
|
Non-GAAP Financial Measure
In evaluating our business, we consider and use adjusted net loss, a non-GAAP measure, as a supplemental measure to review and assess our operating performance. The presentation of this non-GAAP financial measure is not intended to be considered in isolation or as a substitute for financial information prepared and presented in accordance with U.S. GAAP. We define adjusted net loss as net loss adjusted to add back share-based compensation expenses.
We believe that adjusted net loss helps to identify underlying trends in our business that could otherwise be distorted by the effect of the expenses that we add back to net loss. We believe that adjusted net loss provides useful information about our operating results, enhances the overall understanding of our past performance and future prospects, and allows for greater visibility with respect to key metrics used by our management in its financial and operational decision-making.
The non-GAAP financial measure “adjusted net loss” is not defined under U.S. GAAP, is not presented in accordance with U.S. GAAP and has limitations as an analytical tool. One of the key limitations of using adjusted net loss is that it does not reflect all of the items of income and expense that affect our operations. Share-based compensation has been and may continue to be incurred in our business and is not reflected in the presentation of adjusted net loss. Further, the non-GAAP financial measure “adjusted net loss” may differ from the non-GAAP information used by other companies, including peer companies, and therefore their comparability may be limited.
We compensate for these limitations by reconciling the non-GAAP financial measure to the nearest U.S. GAAP performance measure, all of which should be considered when evaluating our performance. This non-GAAP financial measure should be viewed in addition to, and not as a substitute for, our reported results prepared in accordance with U.S. GAAP and should be read only in conjunction with our consolidated financial statements prepared in accordance with U.S. GAAP that are included elsewhere in this annual report.
The table below sets forth a reconciliation of our net loss to adjusted net loss for the years indicated:
|
|
|
Year ended December 31, |
| ||||||
|
|
|
2018 |
|
2019 |
|
2020 |
| ||
|
|
|
RMB |
|
RMB |
|
RMB |
|
US$ |
|
|
|
|
(in thousands) |
| ||||||
|
Net loss |
|
(42,296 |
) |
(101,621 |
) |
(80,565 |
) |
(12,347 |
) |
|
Add: |
|
|
|
|
|
|
|
|
|
|
Share-based compensation expenses |
|
7,936 |
|
32,855 |
|
17,762 |
|
2,722 |
|
|
Adjusted net loss |
|
(34,360 |
) |
(68,766 |
) |
(62,803 |
) |
(9,625 |
) |
B. Capitalization and Indebtedness
Not applicable.
C. Reasons for the Offer and Use of Proceeds
Not applicable.
D. Risk Factors
Risks Related to Our Business
We are a development-stage biotechnology company with a limited operating history, which makes it difficult to evaluate our prospects and may increase the probability that we will not be successful.
We commenced our operations in 2010. We achieved commercialization of our CDA test and started generating revenue in China in 2015; we currently do not have commercial operations in the U.S. We are a development-stage biotechnology company with a limited operating history, and our history may not provide a meaningful basis for you to evaluate our business, financial performance and prospects.
Furthermore, we may not have sufficient experience or resources to address the risks frequently encountered by development-stage biotechnology companies, which include our potential failure to:
· achieve and maintain profitability;
· acquire and retain customers and increase adoption of our cancer screening and detection tests—including primarily our CDA test and combination tests (namely a combination of our CDA test and, on an auxiliary basis, biomarker-based or ct-DNA cancer screening and detection tests)—by physicians, key opinion leaders, or KOLs (including research scientists and doctors in the U.S. who are willing to validate our tests after research), patients, hospitals, medical institutions, healthcare payers and others in the medical community;
· commercialize and/or increase the market adoption for our other products, such as a COVID-19 antibody test and our ADME (AnPac Defense Medical Examination) immunology test, and extend the use of our CDA technology to screen pre-cancer diseases and increase its adoption by the medical community;
· respond to competitive market conditions;
· attract, train, motivate and retain qualified personnel;
· protect our proprietary technologies and intellectual property rights;
· secure a stable supply of blood samples to support our research and clinical studies;
· keep up with evolving industry standards and market developments;
· obtain and maintain the regulatory licenses, certifications, and approvals required for us to further market our cancer screening and detection tests and commercialize our CDA device in China and to commercialize our tests and CDA device in the United States;
· increase the awareness of our tests and protect our reputation;
· maintain adequate control of our operational costs; and
· manage our relationships with our research partners.
If we are unsuccessful in addressing any one or more of these risks, they could adversely affect our business, financial condition and results of operations and increase the probability that we will not be successful.
We have incurred losses each year since our inception, we expect to continue to incur losses for the foreseeable future, and we may not be able to achieve and maintain profitability.
Although our revenue grew rapidly in recent years, we have incurred losses each year since our inception. For the years ended December 31, 2018, 2019 and 2020, we incurred net losses of RMB42.3 million, RMB101.6 million and RMB80.6 million (US$12.3 million), respectively. As of December 31, 2020, we had an accumulated deficit of RMB357.0 million (US$54.7 million). To the date of this annual report, we have financed our operations primarily with proceeds from equity and debt offerings, borrowings, and loans from related parties. We have devoted and expect to continue to devote substantially all of our resources to the research, development and commercialization of our CDA technology, device and test. We expect to continue to incur losses for the foreseeable future. We cannot predict the extent of these future losses, or when we may achieve profitability, if at all. If we are unable to generate sufficient revenue from our business and control our costs and expenses to achieve and maintain profitability, the value of your investment in us could be negatively affected.
Our success depends heavily on the success of our CDA technology and related cancer screening and detection test.
We derive our revenue primarily from our CDA-based tests, which depend on our CDA technology. If we obtain relevant approvals from the NMPA to sell our CDA device, we also anticipate generating revenue from the sales of our CDA device. We believe that our commercial success will depend upon our ability to achieve and maintain market acceptance of our current and future cancer screening and detection tests, which will depend on a number of factors, including:
· our ability to further validate and improve the clinical utility and superiority of our CDA technology by increasing its sensitivity and specificity and through research studies and accompanying publications;
· the timing and scope of additional approvals from the NMPA for our CDA device and test and our ability to maintain these approvals;
· acceptance of our CDA test by physicians, KOLs, patients, hospitals, medical institutions, healthcare payers and others in the medical community;
· our ability to obtain the Class III medical device registration certificate from the NMPA for our CDA device and enter and develop the China hospital market for our CDA device and test;
· sufficient coverage and reimbursement by third-party payers for our services, which may depend on multiple factors such as the enforceability of relevant laws that mandate the coverage of cancer or pre-cancer disease screening;
· our ability to maintain and expand our customer base in China, especially among insurance companies, corporate customers and the hospital market;
· our sales and marketing capabilities, including our success in expanding our sales and marketing team and establishing our own sales network in China;
· the amount and nature of competition from other early cancer screening and detection products and procedures;
· our ability to obtain regulatory approvals for our U.S. laboratories to conduct commercial tests and successfully penetrate the U.S. market; and
· negative publicity regarding our or our competitors’ tests and technologies resulting from defects or errors.
If we are unsuccessful in addressing these or other factors that might affect the market acceptance of our tests, our business and results of operations will suffer.
We face risks related to natural disasters, health epidemics, civil and social disruption and other outbreaks, which could significantly disrupt our operations.
We are vulnerable to social and natural catastrophic events that are beyond our control, such as natural disasters, health epidemics, and other catastrophes, which may materially and adversely affect our business. Since December 2019, there has been an outbreak of a novel strain of coronavirus (COVID-19) in China and around the world. COVID-19 is considered to be highly contagious and poses a serious public health threat. The World Health Organization labeled the coronavirus a pandemic on March 11, 2020, given its threat beyond a public health emergency of international concern that the organization had declared on January 30, 2020. In response to this pandemic, China, the United States and many other countries and jurisdictions have taken, and may continue to adopt, additional restrictive measures to contain the virus’ spread, such as quarantines, travel restrictions and work from home policies. These measures have slowed down the development of the Chinese economy and the U.S. economy and adversely affected the global economic conditions and financial markets. We currently derive all our revenues in China and we have two laboratories in the United States. The outbreak of this virus caused wide-ranging business disruptions and traffic restrictions in China and the United States in 2020, and with its continued spread globally, the virus’ adverse impact on business activities, travels and overall GDP in China, the United States and other parts of the world has been unprecedented and is expected to continue in the foreseeable future. While the Chinese government’s efforts have slowed down the virus’ spread, there has been resurgences in China from time to time, particularly in winter and spring. As the pandemic expands globally, the world economy is suffering a noticeable slowdown. Commercial activities throughout the world have been and could continue to be curtailed with decreased consumer spending, business operation disruptions, interrupted supply chains, difficulties in travel, and reduced workforces.
As a result of the pandemic of COVID-19 in China, the United States and the world, our operations have been, and may continue to be, adversely impacted by disruptions in business activities, commercial transactions and general uncertainties surrounding the duration of the outbreaks and the various governments’ business, travel and other restrictions. These adverse effects could include our ability to market and conduct our tests in China, commercialize our tests in the United States and carry out research studies and activities in China and the United States, temporary closures of our laboratory facilities and offices in China and the United States and our customers’ and suppliers’ facilities, delayed supply of products and services from our suppliers, and delayed or cancelled orders from our customers (such as due to temporary decreased demand for disease screening and detection or physical checkup services or generally due to reduced commercial activities). In addition, our business operations could be disrupted if any of our employees is suspected of contracting the coronavirus or any other epidemic disease, since our employees could be quarantined and/or our offices be shut down for disinfection. In particular, the closing of blood sampling points countrywide in China since the Chinese New Year in 2020, as a measure by the Chinese government to contain the spread of COVID-19, significantly reduced the number of samples that we could collect for our CDA-based tests and adversely affected the sale of our CDA-based tests in the first half of 2020. There were also delays of orders and cancellation of some orders for planned CDA tests and physical checkups from our customers in 2020. In addition, we followed recommendations of local health authorities to minimize exposure risks for our employees, including temporarily closing our laboratories in China from the Chinese New Year to February in 2020 and our laboratories in the United States for a few months in 2020, and having our employees in China and the United States work remotely for some time in 2020. Moreover, our plan to commercialize our CDA test in the United States has been delayed, and will likely continue to be adversely affected, by the COVID-19 outbreak in the United States. Although we have validated a COVID-19 antibody test using Roche’s FDA authorized equipment, we have not begun to commercialize our offering of this test and we cannot guarantee the market acceptance of and demand for this test. We have no control over the development of the COVID-19 situations in China, the United States or around the world and therefore cannot assure you that we will be able to maintain a revenue growth in future periods.
The downturn brought by and the duration of the coronavirus pandemic is difficult to assess or predict and the actual effects will depend on many factors beyond our control, including the increased world-wide spread of COVID-19 and the relevant governments’ actions to contain COVID-19 or treat its impact. While China, the U.S. and many other countries have been administering COVID-19 vaccines, it remains uncertain whether and when the vaccines will be able to effectively contain the pandemic. The extent to which COVID-19 continues to impact our results remains uncertain, and we are closely monitoring its impact on us. Our business, results of operations, financial condition and prospects could be adversely affected directly, as well as to the extent that the coronavirus or any other epidemic harms the Chinese and the United States’ economies in general.
We require substantial funding for our operations. If we cannot raise sufficient capital on acceptable terms, our
business, financial condition and prospects may be materially and adversely affected.
We require substantial capital to expand our business, pursue strategic investments and for other reasons, including to:
· increase our sales and marketing efforts to drive market adoption of our cancer screening and detection tests and address competitive developments;
· expand our technologies into other types of cancer screening and detection products, such as our CDA test’s application in assistance in diagnosis, prognosis and recurrence;
· acquire or invest in technologies or other businesses in our industry;
· seek regulatory and marketing approvals for our cancer screening and detection tests and devices;
· conduct research studies for our CDA test and any additional cancer screening and detection tests;
· maintain, expand and protect our intellectual property portfolio;
· hire and retain additional personnel, such as scientific, quality control and marketing personnel;
· develop, acquire and improve operational, financial and management information systems, including personnel to support our product development and help us comply with our obligations as a public company;
· add equipment and physical infrastructure to support our research and development programs; and
· finance general and administrative expenses.
We will be required to obtain further funding through public or private equity offerings, debt financings or other sources. Further financing may not be available to us on acceptable terms, or at all. If we fail to raise capital as and when needed it would have a negative impact on our financial condition and our ability to pursue our business strategy. In addition, if we raise funds by issuing debt securities or incurring additional borrowings, the terms of the debt securities issued or borrowings could impose significant restrictions on our operations, and we may be unable to repay the indebtedness when due. If we raise funds by issuing equity securities, your investment in our company could be diluted. For example, we issued a US$265,000 10% convertible promissory note at a purchase price of US$250,000 in a private placement to an investor in 2020, and the investor converted the total principal and the accrued interest of this note into 54,642 ADSs of our company in February 2021. In addition, we issued on February 5, 2021 US$2.0 million zero coupon convertible debentures due February 4, 2022 at a purchase price of US$1.7 million in a private placement to several investors, subject to certain condition that may increase the rate to 15% per year. These convertible debentures have placed certain restrictions on us, such as prohibiting us from effecting any issuance of ADSs or the equivalent involving a variable rate transaction. In addition, we have granted registration rights to these investors of our convertible debentures in respect of the underlying ADSs. Our failure to have an effective registration statement covering the resale of the underlying shares prior to April 16, 2021 triggered an event of default under these convertible debentures, which resulted in the outstanding amount under these convertible debentures increasing by 10%.
As of December 31, 2020, we had short-term debt of RMB8.2 million (US$1.3 million). We believe that our cash and cash equivalents on hand, borrowings and financing committed, and our anticipated cash flows generated from our operating activities will be sufficient to meet our current and anticipated needs for general corporate purposes for at least the next 12 months. However, our estimate as to how long we expect these financial resources to be sufficient to fund our operations is based on assumptions that may prove to be wrong. Further, changing circumstances, some of which may be beyond our control, could cause us to consume capital significantly faster than we currently anticipate. Going forward, we expect to need additional fundraising if our cash flows generated from operations do not increase substantially. Our present and future funding requirements will depend on many factors, including:
· the scope, progress, timing, costs and results of the development of our CDA technology and our other products;
· the costs of expanding our laboratory operations and offerings, including our sales and marketing efforts;
· our rate of progress in, and costs of the sales and marketing activities associated with, encouraging adoption of our cancer screening and detection tests;
· our rate of progress in, and cost of research and development activities associated with, our CDA test, any additional cancer screening and detection tests and other tests;
· the impact of competing technological and market developments;
· costs related to entering the U.S. market;
· the costs of preparing, filing and prosecuting patent applications, maintaining and protecting our intellectual property rights and defending against intellectual property related claims;
· the costs, timing and outcome of obtaining regulatory approvals and changes in regulatory policies or laws that may affect our operations; and
· the costs of operating as a public company.
Our ability to grow our China business is substantially dependent on our ability to penetrate the Chinese hospital market.
In China, we currently can only conduct our cancer screening and detection tests on our devices in our own certified laboratories. Given these restrictions, our customer base is primarily direct customers such as corporations and life insurance companies, as well as sales agents such as health management companies and medical device dealers. But China’s largest market for cancer screening and detection tests is the hospital market, in which patients go to Chinese hospitals for cancer screening and other medical tests. Currently we cannot conduct our tests in hospitals. We have applied for an NMPA Class III medical device registration certificate for our CDA devices to assist in multi-cancer diagnosis. If we receive this certificate, together with an updated medical device manufacture license, we would be permitted to place our devices within Chinese hospitals’ laboratories to conduct commercial tests there or sell our devices to the hospitals for the purposes of assisting in physicians’ diagnosis of specified multiple cancers. The timing for us to obtain this certificate or license is uncertain, but we expect it to take at least three years. Even if we obtain the certificate and license, we will need to successfully market our CDA device and test to Chinese hospitals. Our ability to grow our China business depends substantially on our ability successfully to penetrate the Chinese hospital market, and we cannot assure you as to when or whether we will be able to do so.
Our plans to enter the U.S. market may not be successful.
Currently, we conduct commercial operations only in China, and the substantial majority of our business, assets, management and employees are located in China. We have been making efforts to enter the U.S. market. We have obtained a California state laboratory license, accreditation by the College of American Pathologists, or CAP, and a Certificate of Accreditation under the Clinical Laboratory Improvement Amendments of 1988, or CLIA, for our laboratory in San Jose, California. We commenced operations of our new laboratory in Philadelphia, Pennsylvania with the completion of our facility renovation and first phase equipment installation in July 2020. We obtained a CLIA Certificate of Registration for this laboratory in August 2020, have applied for a Pennsylvania state laboratory permit, and will seek accreditation from CAP for this new laboratory. Our U.S. operations currently include collaborating with U.S. health organizations to conduct research tests of our CDA technology, planning to commercialize our CDA tests.
Although our strategy is to expand our U.S. operations and eventually commence commercial sales of our CDA-based tests and other tests (such as COVID-19 antibody tests) in the United States, this strategy is subject to a number of risks and uncertainties, including:
· our ability to secure research agreements with reputable U.S. hospitals, medical institutions and other health organizations to conduct research studies for our test;
· our ability to obtain sufficient blood samples for our planned research tests;
· the substantial costs and time required for U.S. research tests and clinical studies;
· positive outcomes of our U.S. research tests sufficient to support the clinical validity, safety, and effectiveness of our test in the U.S. market;
· U.S. federal and state regulatory risks, including our ability to commence marketing of our CDA test as a laboratory developed test, or LDT, without premarket clearance, market authorization or approval from the United States Food and Drug Administration, or the FDA, our ability to comply with all applicable laws and other regulations, and costs and timing of obtaining relevant approvals;
· development of a U.S. infrastructure, including sales and marketing resources, sufficient to commercialize our test;
· substantial competition in the U.S. cancer screening and detection market, including from companies with substantially greater resources than we have; and
· market acceptance of our test in the U.S.
Our ability to successfully address these factors and penetrate the U.S. market, as well as the costs and timing of these efforts, are highly uncertain. We expect that our commercial activities and revenues will continue to be derived solely from China for the foreseeable future.
Our industry is subject to rapid change, and other companies or institutions may develop and market novel or improved early cancer screening and detection methods, which may make our CDA technology less competitive or obsolete.
Our CDA-based tests depend on the effectiveness of our CDA technology, and we may be unable to maintain the competitiveness of this technology. Our industry is characterized by rapid changes, including technological and scientific breakthroughs, frequent new product introductions and enhancements and evolving industry standards, all of which could make our current CDA-based test obsolete. In recent years, there have been numerous advances in technologies relating to the diagnosis and treatment of cancer. We must continuously enhance our CDA technology and develop new tests to keep abreast of evolving standards of early cancer screening and detection. Other companies and institutions may possess significantly greater financial and other resources and research and development capabilities than we do. These other companies and institutions may devote significant resources to develop new methods of detecting cancers and pre-cancer symptoms, and these methods and related tests could represent significant competition for our CDA technology and cancer screening and detection test, or even render our CDA technology obsolete.
We may be unable to compete effectively against our competitors because their products and services may be superior. They may also have more expertise, experience, financial resources or stronger business relationships in developing and marketing their products and services, more mature technologies and products, greater market adoption and greater brand recognition than we do. Further, even if we do develop new marketable tests or services, our current and future competitors may develop tests and services that are more commercially attractive than ours and they may bring those tests and services to market sooner than we are able to.
We have recorded net working capital deficit and negative cash flows from operating activities and may continue to do so.
We had net working capital deficit of RMB50.6 million, RMB44.0 million and RMB22.2 million (US$3.4 million) as of December 31, 2018, 2019 and 2020, respectively. We cannot assure you that we will not continue to have net working capital deficit in the future, which would expose us to liquidity risk. Our future liquidity and ability to make the additional capital investments necessary for our operations and business expansion will depend primarily on our ability to maintain sufficient cash generated from operating activities and to obtain adequate external financing. There can be no assurance that we will have such cash from operating activities or that we will be able to renew existing loan facilities or obtain other sources of financing.
We have experienced significant cash outflow from operating activities since our inception. We had net cash used in operating activities of RMB31.1 million, RMB48.6 million and RMB59.0 million (US$9.0 million) in 2018, 2019 and 2020, respectively. Our cost of continuing operations could further reduce our cash position, and an increase in our net cash outflow from operating activities could adversely affect our operations by reducing the amount of cash we have available to meet the cash needs for operating our business and to fund our investments in our business expansion.
Our operating results may fluctuate significantly, which makes our future operating results difficult to predict and could cause our operating results to fall below expectations or any guidance we may provide.
Our operating results may fluctuate significantly, which makes it difficult for us to predict our future operating results. These fluctuations may occur due to a variety of factors, many of which are outside of our control, including:
· the level of demand for our cancer screening and detection tests, which may vary significantly;
· the timing and cost of, and level of investment in, research, development, regulatory approval and commercialization activities relating to our CDA technology and our cancer screening and detection tests and device, which may change from time to time;
· the volume, customer mix and product mix for our cancer screening and detection tests;
· the introduction of new cancer screening and detection tests and services by us or others in our industry;
· expenditures that we may incur to acquire, develop or commercialize additional tests, devices and technologies;
· coverage and reimbursement policies with respect to our cancer screening and detection tests and tests that compete with our tests;
· changes in government regulations or in the status of our regulatory approvals or applications;
· future accounting pronouncements or changes in our accounting policies; and
· general market conditions and other factors, including factors unrelated to our operating performance or the operating performance of our competitors.
The cumulative effects of the factors discussed above could result in large fluctuations and unpredictability in our annual operating results. As a result, comparing our operating results on a period-to-period basis may not be meaningful. Investors should not rely on our past results as an indication of our future performance.
If our cancer screening and detection or other tests or our competitors’ comparable tests do not meet customer expectations, our operating results, reputation and business could suffer.
Our success depends on the market’s confidence in our ability to provide reliable, high-quality cancer screening and detection tests and other tests. We believe that our customers are likely to be particularly sensitive to defects or errors in our tests, in particular if our tests fail to accurately detect the risk of pre- and early-stage cancers from blood samples, and we cannot guarantee that our tests will meet their expectations. We may be subject to legal claims arising from any defects or errors in our tests. Furthermore, if comparable tests offered by competing companies fail to perform to expectations, consumers may have lower confidence in cancer screening and detection tests in general. As a result, the failure of our tests or our competitors’ tests to perform as expected could significantly impair our operating results, business prospects and reputation.
We do not carry product liability or professional liability insurance. If we were to be sued for product liability or professional liability, we could face substantial liabilities that exceed our resources.
We could face product liability claims if someone alleges that our cancer screening and detection tests or other tests gave inaccurate or misleading information regarding the patient’s risk of cancer or otherwise failed to perform as designed. A claimant could allege that our test results caused unnecessary treatment or other costs or resulted in the patient missing the best opportunity or timing for treatment. A patient could also allege other mental or physical injury or that our testing provided inaccurate or misleading information concerning the screening and detection, assistance in diagnosis, prognosis or recurrence of, or available therapies for, a cancer or other diseases. We may also be subject to liability for errors in, a misunderstanding of or inappropriate reliance upon, the information we provide in the ordinary course of our business activities. Product liability or professional liability claims could result in substantial damages and be costly and time-consuming for us to defend and could divert our management’s attention.
We do not carry product liability or professional liability insurance. Even if we purchase these kinds of insurance, the insurance may not fully protect us from the financial impact of defending against product liability or professional liability claims. Any product liability or professional liability claim brought against us, with or without merit, could increase our insurance rates or prevent us from securing insurance coverage. Additionally, any product liability or professional liability lawsuit could damage our reputation, or cause our research partners to terminate existing agreements and cause potential research partners to seek other partners, or cause us to lose our current or potential customers. Any of these developments could adversely impact our results of operations, business prospects and financial condition.
We may be subject to liability claims for defective services provided by third-party physical checkup centers, which could harm our reputation and adversely impact our results of operations.
In addition to our cancer screening and detection tests, we also provide annual physical checkup packages to our customers. We typically outsource the physical checkup services in these packages (other than cancer screening and detection tests) to third-party physical checkup centers. As a result, the administration of the physical checkup services by these third parties may subject us to litigation and liability for personal damages to consumers. Potential judgments, settlements or costs relating to these claims, complaints or lawsuits could subject us to significant fees and costs in defending ourselves, adversely affecting our results of operations. In addition, our business, reputation and growth prospects could suffer if we face negative publicity in connection with these liability claims.
We may be unable to support demand for our cancer screening and detection tests and manage our future growth effectively, which could make it difficult to execute our business strategy.
Since our inception, we have experienced rapid growth, and we anticipate further growth in our business operations. Our growth could strain our organizational, administrative and operational infrastructure. As the sales volume of our cancer screening and detection tests grows, we will face increased demands on our capacity and efficiency for sample intake, testing results analysis and other laboratory operations, quality control, customer service, and general workflow management processes. To effectively manage our future growth, we plan to continue to improve our technology, as well as our operational, financial and management controls. We also plan to hire, train and manage additional qualified scientists, laboratory technicians and sales and customer service personnel. We will also need to maintain the quality and expected turnaround time of our tests. The time and resources required for these improvements, and failure to achieve them in a timely and effective manner, could adversely affect our operations, making it difficult for us to execute our business strategy.
We have limited selling and marketing resources and limited sales, marketing, customer support, manufacturing and commercial laboratory experience, which may restrict our success in commercializing our cancer screening and detection tests.
To grow our business as planned, we must expand our sales, marketing, customer support, manufacturing and commercial laboratory management capabilities, which will require developing and administering our commercial infrastructure and/or collaborative commercial arrangements and partnerships. We have limited experience in these respects, and we may encounter difficulties in retaining and managing the specialized workforce that these activities require. For example, our customer base is large and diverse, which requires us to retain a sales team with established industry expertise and experience. We rely on third-party suppliers for the supply of blood samples for our tests and for reagents that we use in the auxiliary biomarker-based tests that form part of our combination tests. We engaged third parties to conduct substantially all of the biomarker-based tests as part of our combination tests in 2018. We later gradually phased out this outsourcing arrangement beginning in 2019 and now perform our combination tests primarily in-house. We also engage third parties to conduct physical checkups. We also rely on contract manufacturers that manufacture key components of our CDA device. While we primarily rely on our own sales and marketing personnel to market our tests, we also engage sales agents, including companies we invested in. However, we may not be able to effectively manage and maintain our relationships with these third parties, including ensuring their compliance with our controls and procedures. Our future growth will also impose significant added responsibilities on our management. If we fail to meet these demands, it would negatively affect our business growth and profitability. We may seek to partner with others to assist us with our sales, marketing and manufacturing functions. However, we may be unable to find appropriate third parties that meet our requirements, in a timely manner or on terms acceptable to us. In addition, our third-party business partners may not perform as we expect or our arrangements with them may otherwise prove to be detrimental to our results. Our third-party arrangements may also be terminated prematurely, including due to factors out of our control. As a result of such developments, our business and prospects may be harmed.
If we are unable to attract and retain qualified key management, scientists, staff and consultants, our ability to implement our business plan may be adversely affected.
We are highly dependent upon certain of our key management, scientists, staff and consultants, particularly Dr. Chris Yu, our founder and chief executive officer, and Dr. He Yu, our co-founder and chief medical officer. Dr. Chris Yu, Dr. He Yu and each of our key management and scientific personnel may terminate his or her employment with us. Our success is also largely attributable to the qualified and experienced key management and scientific personnel that we have been able to train, attract and retain. If we lose any of our key management and scientific personnel, we may be unable to find replacements suitable to us. The loss of their services could significantly delay or prevent our achievement of our technology development, sales and other business objectives. We do not carry any key-man life insurance. In addition, we face intense competition for qualified individuals from numerous biotechnology and pharmaceutical companies, universities, governmental entities and other research institutions. Our limited operating history and the uncertainties attendant to being a development-stage biotechnology company with limited capital resources could limit our ability to attract and retain personnel. We may be unable to attract and retain suitably qualified individuals, and our failure to do so could have an adverse effect on our ability to implement our business plan.
Our future success depends on our ability to promote our brand and protect our reputation.
We believe that enhancing and maintaining awareness of our “AnPac” brand is critical to achieving widespread acceptance of our cancer screening and detection tests, gaining trust for our testing services and attracting new customers. Successful promotion of our brand depends largely on the quality of the services we offer and the effectiveness of our branding and marketing efforts. Currently, we rely primarily on our own sales and marketing team to promote our brand and our cancer screening and detection tests, and we also engage sales agents, including companies we invested in. We expect our branding and marketing efforts will require us to incur significant expenses and devote substantial resources. We cannot guarantee that our marketing efforts will be successful. Brand promotion activities may not yield increased revenue in the near term, and, even if they do, any revenue increases may not offset the expenses we incur to promote our brand. Our failure to establish and promote our brand and any damage to our reputation will hinder our growth.
In addition, some companies that we established in China together with third parties and some of our sales agents for our CDA test—over which we do not have effective control—share with us the “AnPac” trading name and its Chinese characters that we use. Given this shared use, any negative publicity related to these companies as well as their products and services, whether with merit or not and whether or not related to us, could adversely impact our brand and reputation. Furthermore, negative publicity about other market players or isolated incidents such as fraudulent behaviors, whether or not factually correct, may result in negative perception of the early cancer screening and detection industry as a whole and undermine the credibility we have established, which may negatively affect our business and results of operations.
If we are unable to effectively protect our intellectual property, our business would be harmed.
We rely on patent protection as well as trademark, trade secret and other intellectual property rights protection and contractual restrictions to protect our proprietary devices, tests and technologies, all of which provide limited protection and may not adequately protect our rights. If we fail to effectively protect and/or maintain our patented devices, tests and technologies, our competitive position and prospects could be adversely affected. Furthermore, we could incur substantial litigation costs in our attempts to recover or restrict use of our patents and other intellectual property.
We cannot assure investors that any of our currently pending or future patent applications will result in granted patents, and we cannot predict how long it will take for such patents to be issued, if at all. It is possible that, for any of our patents that have been issued or that may be issued in the future, our competitors may design their products around our patented technologies. Further, we cannot assure you that other persons will not challenge any patents granted to us or that courts or regulatory agencies will hold our patents to be valid, enforceable, and/or infringed. We cannot guarantee you that we will be successful in defending challenges made against our patents and patent applications. Any successful third-party challenge or challenges to our patents could result in the unenforceability or invalidity of these patents, or these patents being interpreted narrowly and/or in a manner adverse to our interests. Our ability to establish or maintain a technological or competitive advantage over our competitors and/or market entrants may be diminished because of these uncertainties. For these and other reasons, our intellectual property may not provide us with any competitive advantage. For example:
· we might not have been the first to make the inventions claimed or disclosed by our pending patent applications or issued patents;
· we might not have been the first to file patent applications for these inventions. To determine the priority of these inventions, we may have to participate in interference proceedings or derivation proceedings declared by the United States Patent and Trademark Office, which could result in substantial costs to us, and could possibly result in a loss or narrowing of our patent rights. We cannot assure you that our patent applications or granted patents will have priority over any other patent or patent application involved in such a proceeding, or will be held valid as an outcome of the proceeding;
· other persons may independently develop similar or alternative products and technologies or duplicate any of our products and technologies, which can potentially impact our market share and revenue, regardless of whether our intellectual property rights are successfully enforced against these other persons;
· it is possible that our pending patent applications will not result in granted patents, and even if these pending patent applications are issued as patents, they may not provide intellectual property protection of commercially viable products or product features, may not provide us with any competitive advantages, or may be challenged and invalidated by third parties, patent offices, and/or the courts;
· we may be unaware of or unfamiliar with prior art and/or interpretations of prior art that could potentially impact the validity or scope of our patents or pending patent applications, or patent applications that we intend to file;
· we take efforts and enter into agreements with employees, consultants, collaborators, and advisors to confirm ownership and chain of title in intellectual property rights. However, an inventorship or ownership dispute could arise that may permit one or more third parties to practice or enforce our intellectual property rights, including possible efforts to enforce rights against us;
· we may elect not to maintain or pursue intellectual property rights that, at some point in time, may be considered relevant to or enforceable against a competitor;
· we may not develop additional proprietary products and technologies that are patentable, or we may develop additional proprietary products and technologies that are not patentable;
· the patents or other intellectual property rights of others may have an adverse effect on our business; and
· we apply for patents relating to our devices, tests and technologies, as we deem appropriate. However, we or our representatives or their agents may fail to apply for patents on important devices, tests and technologies in a timely fashion or at all, or we or our representatives or their agents may fail to apply for patents in potentially relevant jurisdictions.
To the extent our intellectual property offers inadequate protection, or is found to be invalid or unenforceable, we would be exposed to a greater risk of direct or indirect competition. If our intellectual property does not provide adequate coverage over our competitors’ products, our competitive position and our business could be adversely affected.
In addition to patent protections, we also try to protect our trade secrets, know-how and other proprietary information through non-disclosure and confidentiality provisions in our agreements with parties who have access to them, such as our employees, consultants and research partners. These agreements may not be enforceable or may not provide meaningful protection for our trade secrets, know-how and/or other proprietary information in the event of unauthorized uses or disclosure or other breaches of the provisions, and we may not be able to prevent such unauthorized uses or disclosure. Moreover, if a party having an agreement with us has an overlapping or conflicting obligation to a third party, our rights in and to certain intellectual property could be undermined. In addition, monitoring unauthorized disclosure and uses of our trade secrets is difficult, and we do not know whether the steps we have taken to prevent such disclosure and uses are, or will be, adequate. If we were to enforce a claim that a third-party had illegally obtained and was using our trade secrets, it would be expensive and time-consuming, and the outcome would be unpredictable, and any remedy may be inadequate. In addition, courts outside the United States may be less willing to protect trade secrets.
In addition, competitors could purchase our devices and tests and attempt to replicate and/or improve some or all of the competitive advantages we derive from our development efforts, willfully infringe our intellectual property rights, and design their devices and tests around our protected technologies or develop their own competitive technologies that fall outside of our intellectual property rights. If our intellectual property does not adequately protect our market share against competitors’ devices and tests, our competitive position could be adversely affected, as could our business.
We may be involved in lawsuits to protect or enforce our patents, which could be expensive, time-consuming and unsuccessful.
Competitors may infringe our patents. In the event of infringement or unauthorized use, we may file one or more infringement lawsuits, which can be expensive and time-consuming. An adverse result in any such litigation proceedings could put one or more of our patents at risk of being invalidated, being found to be unenforceable, and/or being interpreted narrowly. Adverse results of these types could also put our patent applications at risk of not being issued and/or impact the validity or enforceability positions of our other patents. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that part of our confidential information could be compromised by disclosure.
Many of our competitors are larger than we are and have substantially greater resources. They are, therefore, likely to be able to sustain the costs of complex patent litigation longer than we could. In addition, the uncertainties associated with litigation could have a material adverse effect on our ability to raise the funds necessary to continue our operations, continue our internal research programs, pursue, obtain or maintain intellectual property rights, or enter into research and development partnerships that would help to validate and commercialize our tests.
In addition, patent litigation can be very costly and time-consuming. An adverse outcome in such litigation or proceedings may expose us or any of our future development partners to loss of our proprietary position, expose us to significant liabilities, or require us to seek licenses that may not be available on commercially acceptable terms, if at all.
We may be subject to intellectual property infringement or misappropriation claims by third parties, which may force us to incur substantial legal expenses and, if determined adversely against us, could materially disrupt our business.
The validity, enforceability and scope of intellectual property rights protection in biotechnology industries, particularly in China, are uncertain and still evolving. We cannot be certain that our devices, tests and technologies do not or will not infringe patents, copyrights or other intellectual property rights held by third parties. From time to time, we may be subject to legal proceedings and claims alleging infringement of patents, trademarks or copyrights, or misappropriation of creative ideas or formats, or other infringement of proprietary intellectual property rights. Any such proceeding and claims could result in significant costs to us and divert the time and attention of our management and technical personnel from the operation of our business. These types of claims could also potentially adversely impact our reputation and our ability to conduct business and raise capital, even if we are ultimately absolved of all liability. Moreover, third parties making claims against us may be able to obtain injunctive relief against us, which could block our ability to offer one or more devices or tests and could result in a substantial award of damages against us. In addition, since we may indemnify customers or collaboration partners, we may have additional liability in connection with any infringement or alleged infringement of third party intellectual property. Intellectual property litigation can be very expensive, and we may not have the financial means to defend ourselves or our customers or collaboration partners.
Because patent applications can take many years to issue, there may be pending applications, some of which are unknown to us, that may result in issued patents upon which our devices, tests or proprietary technologies may infringe. Moreover, we may fail to identify issued patents of relevance or incorrectly conclude that an issued patent is invalid or not infringed by our technology or any of our devices or tests. There is a substantial amount of litigation involving patents and other intellectual property rights in our industry. If a third-party claims that we or any of our customers or collaboration partners infringe upon a third-party’s intellectual property rights, we may have to:
· seek to obtain licenses that may not be available on commercially reasonable terms, if at all;
· abandon any product alleged or held to infringe, or redesign our products or processes to avoid potential assertion of infringement;
· pay substantial damages including, in exceptional cases, treble damages and attorneys’ fees, if a court decides that the device, test or proprietary technology at issue infringes upon or violates the third-party’s rights;
· pay substantial royalties or fees or grant cross-licenses to our technology; and
· defend litigation or administrative proceedings that may be costly whether we win or lose, and which could result in a substantial diversion of our financial and management resources.
We may be subject to claims that our employees, consultants, or independent contractors have wrongfully used or disclosed confidential information of third parties.
Some of our employees were previously employed at other life science companies, including our potential competitors. Although we try to ensure that our employees, consultants, and independent contractors do not use the proprietary information or know-how of others in their work for us, and we are not currently subject to any claims that our employees, consultants, or independent contractors have wrongfully used or disclosed confidential information of third parties, we may in the future be subject to such claims. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel, which could adversely impact our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
If our laboratories and other facilities become damaged or inoperable, our ability to conduct our laboratory analysis and our research and development efforts may be jeopardized.
We currently derive substantially all of our revenue from cancer screening and detection tests conducted at our laboratory located in Lishui, Zhejiang Province, China. We also intend to sell our CDA device in China after obtaining relevant approvals from the NMPA. We use our own facilities in Lishui to assemble our CDA device, in addition to engaging third-party contract manufacturers to manufacture its key components. In the United States, we intend to perform all our research and commercial tests in our laboratory in San Jose, California as well as in our new laboratory in Philadelphia, Pennsylvania. Our facilities and equipment, or those of our third-party contract manufacturers, could be harmed or rendered inoperable by natural or man-made disasters, including fire, earthquake, power loss, communications failure or terrorism. These types of developments could render it difficult or impossible for us to operate our cancer screening and detection tests and assemble our device for some period of time. If we are unable to perform our tests or to reduce the backlog of analysis that could develop if our facilities are inoperable, for even a short period of time, it could result in a loss of customers or harm to our reputation, and we may be unable to regain those customers or repair our reputation. We have purchased property insurance, but not any business interruption insurance. Damages to, or interruptions in the operations of, our laboratories and other facilities could have a material adverse impact on our results of operations and financial condition. Furthermore, our facilities and the equipment we use to perform our research and development work could be unavailable or costly and time-consuming to repair or replace. It would be difficult, time-consuming and expensive to rebuild our facilities and purchase our equipment, to locate and qualify a new facility or equipment or to license or transfer our proprietary technology to a third-party, particularly in light of licensure and accreditation requirements. Even in the unlikely event that we are able to find a third party with such qualifications to enable us to conduct our test, we may be unable to negotiate commercially reasonable terms.
Security threats to our information technology infrastructure could expose us to liability and damage our reputation and business.
Because our testing services and research and development activities enable us to access customers’ and research partners’ proprietary information, it is essential to our business strategy that our information technology infrastructure remains secure and is perceived by our customers and research partners to be secure. Despite our security measures, we may face cyber-attacks that attempt to penetrate our network security, sabotage or otherwise disable our research, tests and services, misappropriate our or our customers’ and research partners’ proprietary information, which may include personally identifiable information, or cause interruptions of our internal systems and services. We have not purchased any cyber insurance. Any cyber-attacks could negatively affect our reputation, damage our network infrastructure and our ability to deploy our products and services, harm our relationship with customers and research partners that are affected, and expose us to significant financial liabilities.
We depend on third-party suppliers, sales agents, service providers and research partners for different aspects of our business.
We depend on third parties for different aspects of our business, including supplying blood samples for our research studies and reagents required for biomarkers used in our combination tests, performing a portion of auxiliary biomarker-based tests in our combination tests, sales of our cancer screening and detection tests to our customers, and collecting blood samples for our commercial cancer screening and detection tests. Selecting, managing and supervising these third-party suppliers, sales agents and service providers requires significant resources and expertise. Poor performance by these third parties, including their failure to provide services or products according to applicable legal and regulatory requirements, the terms of our contracts or otherwise below standard, could significantly and negatively affect the quality of our cancer screening and detection tests and damage our reputation. Decreases in the level of sales agents’ purchases of tests from us for resale to the end-customers could adversely affect our revenue growth. In addition, the service or cooperative agreements we have with third-party suppliers, sales agents and service providers are subject to a term, and are not on an exclusive basis. If these third parties do not continue to maintain or expand their cooperation with us, we would be required to seek new suppliers and sales agents, which could cause delays in services to us and negatively affect the quality and availability of our cancer screening and detection tests. Any of the above factors could adversely impact our results of operations and financial position.
In addition, certain of our research partners, which are primarily renowned hospitals and medical institutions, collaborate with us and provide blood samples that we use to conduct various research studies. These partners may cease cooperation with us in the future, especially if they enter into similar agreements or arrangements with our competitors. If we are unable to readily access sufficient blood samples to conduct our commercial tests and research studies, we may be unable to compete effectively with other laboratories that have greater access to blood samples, and our business, financial condition and results of operations may be harmed.
We rely on third-party contract manufacturers for the manufacturing of key components of our CDA devices.
We design and configure all of the key components of our CDA device and have outsourced the manufacturing of these components of our CDA devices to third-party contract manufacturers. Our revenue is generated primarily from our CDA tests conducted using our CDA devices. Our contract manufacturers may fail to deliver these key components for reasons beyond our control. For example, they may encounter financial difficulties or experience disruptions in their manufacturing operations due to equipment breakdowns, labor disputes or shortages, raw material shortages, cost increases or other similar reasons. If they fail to timely deliver those key components for us to assemble our CDA device or maintain the quality of their products, our ability to conduct our commercial CDA-based tests could be adversely affected. Currently, we do not have any long-term or exclusive supply contracts with any of our contract manufacturers. Our contract manufacturers may cease to provide us with the key components of our CDA devices. Since qualifying a new contract manufacturer could be costly and time-consuming, the termination of a contract manufacturer could cause disruption to our business and adversely impact our results of operations.
We rely on commercial courier delivery services to transport blood samples to our laboratory facilities in a timely and cost-efficient manner, and if these delivery services are disrupted, our business will be harmed.
Our business depends on our ability to quickly and reliably deliver test results to our customers. We rely on commercial courier delivery services to transport blood samples to our laboratory facilities timely and cost efficiently. Blood samples are typically received within a few days in China for analysis in our laboratories. Disruptions in third-party delivery service, whether due to labor disruptions, bad weather, natural disaster, health epidemics, terrorist acts or threats or for other reasons, could adversely affect specimen integrity and our ability to process blood samples and conduct tests in a timely manner and to service our customers satisfactorily, and ultimately our reputation and our business. In addition, if we are unable to continue to obtain expedited delivery services on commercially reasonable terms, our operating results may be adversely affected.
Material weaknesses in our internal control over financial reporting have been identified, and if we fail to implement and maintain an effective system of internal controls over financial reporting, we may be unable to accurately report our results of operations, meet our reporting obligations or prevent fraud.
As a result of the initial public offering, we have become subject to the reporting requirements of the Exchange Act, the Sarbanes-Oxley Act of 2002 and the rules and regulations of the Nasdaq Stock Market. The Sarbanes-Oxley Act requires, among other things, that we maintain effective disclosure controls and procedures and internal controls over financial reporting. Commencing with our year ended December 31, 2020, we must perform system and process evaluation and testing of our internal controls over financial reporting to allow management to report on the effectiveness of our internal controls over financial reporting in our Form 20-F filing for that year, as required by Section 404 of the Sarbanes-Oxley Act. In addition, when we cease to be an “emerging growth company” as the term is defined in the Jumpstart Our Business Startups Act, our independent registered public accounting firm may be required to attest to and report on the effectiveness of our internal control over financial reporting. Our management may conclude that our internal control over financial reporting is not effective. Moreover, even if our management concludes that our internal control over financial reporting is effective, our independent registered public accounting firm, after conducting its own independent testing, may issue a report that is qualified if it is not satisfied with our internal controls or the level at which our controls are documented, designed, operated or reviewed, or if it interprets the relevant requirements differently from us. This will require that we incur substantial additional professional fees and internal costs to expand our accounting and finance functions and that we expend significant management efforts. We may experience difficulty in meeting these reporting requirements in a timely manner.
In the course of preparing and auditing our consolidated financial statements for the year ended December 31, 2020, we identified three material weaknesses in our internal control over financial reporting as of December 31, 2020, the material weaknesses identified were (i) lack of accounting staff and resources with appropriate knowledge of U.S. GAAP and SEC reporting and compliance requirements; (ii) lack of audit committee and internal audit function to establish formal risk assessment process and internal control framework; and (iii) (a) an IT risk assessment mechanism was not established and conducted; (b) policy and procedures did not address critical area of system development, change management, systems and access monitoring, anti-virus protection management, offsite backup management, and disaster recovery/business resumption planning (DP/BCP); and (c) vulnerability or penetration testing was not performed periodically to identify, assess, and address cybersecurity risk. Following the identification of these material weaknesses, we plan to take measures to remedy our information technology general control. For details, see “Item 15. Controls and Procedures—Internal Control Over Financial Reporting.” However, we cannot assure you that all these measures will be sufficient to remediate our material weakness in time, or at all.
To remedy our identified material weaknesses, we have started to undertake steps to strengthen our internal control over financial reporting, including: (i) hiring additional qualified accounting and financial reporting personnel with U.S. GAAP and SEC reporting experience, (ii) obtaining advisory services from professional consultants with experience in the requirements of the Sarbanes Oxley Act of 2002 and internal audit guidance on SEC reporting, (iii) expanding the capabilities of our existing accounting and financial reporting personnel through continuous training and education in the accounting and reporting requirements under U.S. GAAP, and SEC rules and regulations, (iv) developing, communicating and implementing an accounting policy manual for our accounting and financial reporting personnel for our recurring transactions and period-end closing processes, and (v) establishing effective monitoring and oversight controls for non-recurring and complex transactions to ensure the accuracy and completeness of our company’s consolidated financial statements and related disclosures. However, these measures have not been fully implemented and we concluded that the material weakness in our internal control over financial reporting has not been remediated as of December 31, 2020. We will continue to implement measures to remediate the material weaknesses.
In addition, our internal control over financial reporting will not prevent or detect all errors and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud will be detected.
Our business may suffer if we are unable to collect payments from our corporate customers on a timely basis.
We typically offer credit terms of one to three months to our sales agents and other corporate customers. Any downturn in the businesses of our sales agents and other corporate customers could reduce their willingness or ability to pay us. The failure of any of our sales agents or other corporate customers to make timely payments could require us to recognize an allowance for doubtful accounts. For example, we had allowance for doubtful accounts receivable of RMB198,000, RMB177,000 and RMB304,000 (US$47,000) as of December 31, 2018, 2019 and 2020, respectively. We cannot guarantee that we will be able to collect these doubtful accounts. As a result, our results of operations and financial condition may be adversely affected.
We have granted, and may continue to grant, stock incentive awards, which may result in increased share-based compensation expenses.
We have adopted our 2010 share incentive plan, or 2010 Plan, and 2019 share incentive plan, or 2019 Plan, so that we can grant share-based compensation awards to our directors, officers, employees and consultants to incentivize their performance and align their interests with ours. The maximum number of Class A ordinary shares that may be issued pursuant to all awards under our 2019 Plan is 1,105,300. We have also separately issued options to our directors, officers, employees and consultants under our 2010 Plan. As of the date of this annual report, options to purchase 1,393,400 Class A ordinary shares under the 2010 Plan and the 2019 Plan had been granted and were outstanding.
We believe the granting of stock incentive awards is of significant importance to our ability to attract and retain our management, employees and consultants, and we will continue to grant stock incentive awards to our management, employees and consultants in the future. As a result, our expenses associated with share-based compensation may increase, which may have an adverse effect on our results of operations. In addition, the granting, vesting and exercise of the awards under these stock incentive plans will have a dilutive effect on your shareholding in our company.
We may be subject to litigation and other claims and legal proceedings, and may not always be successful in defending ourselves against these claims or proceedings.
We are subject to lawsuits and other claims in the ordinary course of our business. We have been, and may in the future be, subject to lawsuits and other legal proceedings brought by our customers, competitors, employees, business partners, investors, other shareholders of the companies we invest in, or other entities against us, in matters relating to intellectual property rights, contractual disputes, competition claims and employment disputes, among others. We may also be subject to regulatory proceedings, such as any non-compliance with licensing requirements, advertising practices, and protection of data privacy of the tested individuals. We may not be successful in defending ourselves, and the outcomes of these lawsuits and proceedings may be unfavorable to us. Lawsuits and regulatory proceedings against us may also generate negative publicity that significantly harms our reputation, which may adversely affect our customer base, market position and our relationships with our research partners and other business partners. In addition to the related costs, managing and defending litigation and other legal proceedings and related indemnity obligations can significantly divert our management’s attention from operating our business. We may also need to pay damages or settle lawsuits or other claims with a substantial amount of cash, negatively affecting our liquidity. As a result, our business, financial condition and results of operations could be adversely affected.
We have limited business insurance coverage.
Our business insurance is limited, and we do not carry business interruption insurance to cover our operations. We have determined that the costs of insuring for related risks and the difficulties associated with acquiring such insurance on commercially reasonable terms make it impractical. Any uninsured damage to our facilities or technology infrastructures or disruption of our business operations could require us to incur substantial costs and divert our resources, which could have an adverse effect on our business, financial condition and results of operations.
Risks Relating to Government Regulations
PRC
As a biotechnology company, we are required to comply with extensive regulations and obtain and maintain a number of permits and licenses to carry on our business in China; future government regulation may place additional burdens on our efforts to commercialize our cancer screening and detection tests and device.
As a biotechnology company, we are subject to extensive government regulation and supervision in China. Violation of applicable laws and regulations may materially and adversely affect our business. For example, we are required to obtain a medical institution practice license from the PRC National Health Commission, or the NHC, for our laboratories to conduct cancer screening and detection tests in China. We also need to obtain a medical device manufacture license and a medical device registration certificate from the NMPA for the manufacturing and commercial use and sale of our CDA device.
Each of our current NHC medical institution practice licenses and our NMPA Class II medical device manufacture license and registration certificate has a five-year term. We are applying for a Class III medical device registration certificate from the NMPA. After we obtain this license, we will apply to update our medical device manufacture license to include the manufacture of Class III medical devices. If we are unable to renew our existing licenses and certificates or obtain the Class III medical device license or update our medical device manufacture license, or obtain or renew any other material permits or approvals required for our operations, we may be unable to continue to sell our cancer screening and detection tests or to commercialize our CDA device in China and, as a result, our business may be adversely affected.
In addition, China’s regulatory framework governing biotechnology companies is subject to change and amendment from time to time. Any such change or amendment could materially and adversely impact our business, financial condition and prospects. The PRC government has introduced various reforms to the Chinese healthcare system in recent years and may continue to do so, with an overall objective of expanding basic medical insurance coverage and improve the quality and reliability of healthcare services. The specific regulatory changes under the reforms still remain uncertain. The implementing measures to be issued may not be sufficiently effective to achieve the stated goals, and as a result, we may not be able to benefit from these reforms to the level we expect, if at all. Moreover, the reforms could give rise to regulatory developments, such as more burdensome administrative procedures, which may have an adverse effect on our business and prospects.
If we are unable to maintain our medical device or laboratory related licenses and certificates, our growth strategy may be compromised.
Pursuant to the Regulation on the Supervision and Administration of Medical Devices as amended by the PRC State Council in December 2020, which will come into effect on June 2021, medical devices are classified into three classes according to their risk levels. Class II medical devices are medical devices with moderate risks that must be strictly controlled and regulated to ensure their safety and effectiveness. Class III medical devices are medical devices with relatively high risks that must be strictly controlled and regulated through special measures to ensure their safety and effectiveness. In addition, the Measures for the Supervision and Administration of the Operation of Medical Devices as promulgated by the NMPA’s predecessor, the China Food and Drug Administration, or the CFDA, in November 2017 regulate entities that engage in business activities involving medical devices in the PRC in accordance with the medical devices’ risk levels. The Class II medical device registration certificate and the Class III medical device registration certificate are required for an entity to conduct business activities involving these medical devices.
We have obtained the Class II medical device registration certificate from the NMPA, which allows us to conduct our tests in our licensed laboratories. To perform our CDA test outside of our laboratories and market them to Chinese hospitals, in December 2018, we applied for a Class III medical device registration certificate from the NMPA for our CDA device. We believe it will likely take at least three years for us to obtain this license from the NMPA. After we obtain this license, we will update our medical device manufacture license, which we believe is a relatively straightforward procedure. However, there is no assurance that we will receive this NMPA approvals on a timely basis, or at all. If we fail to maintain and renew our Class II medical device registration certificate or if we are unable to obtain the Class III medical device license and update our medical device manufacture license, our ability to grow our business could be adversely affected.
We believe our NHC medical institution practice license and NMPA Class II medical device registration certificate and manufacture license are effective and cover our current commercialized CDA test, which provides a cancer risk assessment. However, the PRC laws and regulations governing cancer screening and detection devices and tests are subject to uncertainties and regulatory discretion, including changes in interpretation and application, such as in respect of restrictions on foreign investments in clinical laboratories. There is also a risk that the relevant regulatory authorities could disagree with our assessment of the commercial activities permitted by our certificates and licenses. For more information on this, see “Item 4. Information on the Company—B. Business Overview—PRC Regulations—Other Significant PRC Regulations Affecting Our Business Activities in China.” Moreover, if we begin to commercialize our CDA test for other purposes such as assisting in diagnosis, prognosis and recurrence, this regulatory uncertainty and risk would be greater. If the relevant regulatory authorities were to assert that our current or future commercial cancer screening and detection tests were not permitted by our licenses or revoke any of our NMPA or NHC licenses and certificates and require us to take remedial actions to their satisfaction, or if we were unable to obtain amended or additional required licenses or approvals, then our business and financial results would be adversely affected.
We are subject to ongoing obligations and continued regulatory review and to future changes in laws, regulations or enforcement policies in China.
We are subject to ongoing obligations and continued regulatory review in relation to our laboratories and our medical devices. Even if the NMPA grants our application for a Class III medical device registration certificate and allows us to update our medical device manufacture license accordingly, or if we successfully maintain and renew our Class II medical device manufacture license and registration certificate, our CDA device will be subject to extensive and ongoing regulatory requirements.
In addition, there could be a subsequent discovery of previously unknown problems with our device (including problems with third-party manufacturers or manufacturing processes) or failure to comply with existing or future regulatory requirements (including in respect of our conducting of cancer screening and detection tests). For example, if we were found to have conducted any of these tests in premises other than a licensed laboratory, we could be subject to confiscation of revenue from the relevant tests as well as other penalties. For more information on this, see “Item 4. Information on the Company—B. Business Overview—PRC Regulations—Regulation on Medical Devices and Medical Institutions—Medical Institutions Laws and Regulations.” Any government investigation of alleged violations of law could require us to expend significant time and resources and could result in adverse government actions (including penalties on us) and negative publicity on our brand.
Moreover, laws, regulations and enforcement policies in China, including those regulating medical institutions, devices and supplies, are evolving. Changes in these areas could impose more stringent requirements on us, including fines or other penalties, and increase our compliance and other operating costs. Changes in government regulations could also prevent, limit or delay regulatory approvals in relation to our CDA device. If we are unable to maintain regulatory compliance, any regulatory approval that has been obtained may be lost and we may not be able to achieve or sustain profitability. In addition, regulatory changes may relax certain requirements that could benefit our competitors or lower market entry barriers and increase competition. Further, regulatory agencies in China may periodically, and sometimes abruptly, change their enforcement practices. Any litigation or governmental investigation or enforcement proceedings against us in China may be protracted and may result in substantial costs and diversion of resources and management attention, negative publicity, damage to our reputation and decline in the price of our ADSs.
The absence of patent linkage, patent term extension and data and market exclusivity for NMPA-approved medical products could increase the risk of early generic competition against our tests in China.
The life of a patent and the protection it affords are limited under PRC law. Currently, while certain foreign laws regulate patent term extension, patent linkage to products to delay generic entry, or extension of data exclusivity (often referred to as regulatory exclusivity) in certain circumstances, China does not have any effective law or regulation in these aspects. Chinese regulators have set out a framework for delaying generic launches by adding patent linkage and data exclusivity into the Chinese regulatory regime, as well as for establishing a pilot program for patent term extension. However, these measures will require the adoption of specific regulations. The Patent Law of the PRC as amended by the Standing Committee of the National People’s Congress in October 17, 2020, which will come into effect in June 2021, provides for the mechanism of compensation for the patent term in the case of any unreasonable delay. If we are unable to obtain patent term extension or if such extension is shorter in length than requested, our competitors may obtain approval of competing products prior to or following our patent expiration, and our business, financial condition, results of operations and prospects could be materially harmed.
Any change in the regulations governing the use of personal data in China, which are still under development, or any data leakage or unauthorized use of data by third parties could adversely affect our business and reputation.
We provide early cancer screening and detection services to tens of thousands of individuals in China. As a result, we have access to these tested individuals’ personal data, including their age, gender, disease status and medical records. We use this personal data internally to expand our test database and improve the clinical utility of our CDA technology. Any such unauthorized access, loss, or dissemination of information could result in legal claims, proceedings or liability under PRC laws and regulations that protect the privacy of personal data. The Civil Code of the PRC which was promulgated in May 28, 2020 and came into effect on January 2021, provides for the protection of personal data. However, detailed Chinese regulations governing the collection and use of personal data are still under development. Other than the requirements for non-tampering with any personal data collected or retained, we believe that there is no PRC legal restriction on our internal use of such data. Any change in the regulatory regime in this regard could potentially affect our ability with regard to the collection and use of these personal data, which in turn could have a material adverse effect on our business, financial condition and results of operations.
Moreover, we may not be able to prevent third parties from illegally obtaining and misappropriating personal data of the tested individuals that we collect. Concerns about data leakage or unauthorized use of data by third parties, even if unfounded, could damage our reputation and negatively affect our results of operations.
United States
We conduct our business in a heavily regulated industry, and changes in regulations or violations of regulations may, directly or indirectly, reduce our revenue, adversely affect our results of operations and financial condition and harm our business.
The U.S. life sciences industry is highly regulated, and the regulatory environment in which we operate may change significantly and adversely to us in the future. Areas of the regulatory environment that may affect our ability to conduct business in the United States include federal and state laws relating to:
· laboratory testing, including the CLIA and state laboratory licensing laws;
· the development, testing, use, distribution, promotion and advertising of research services, kits and clinical diagnostics, including certain LDTs which are regulated by the FDA under the U.S. Federal Food, Drug, and Cosmetic Act, or the FDCA;
· test ordering, documentation of tests ordered, billing practices and claims payment under the U.S. Centers for Medicare & Medicaid Services, or CMS, and the enforcement of those laws and regulations by the U.S Department of Justice and the U.S. Department of Health and Human Services, or HHS, Office of the Inspector General;
· medical device and in vitro diagnostic, or IVD, clearance, marketing authorization or approval;
· FDA’s policy of enforcement discretion to not regulate the majority of LDTs as IVDs;
· laboratory anti-mark-up laws (which are laws or regulations that can limit the prices of medical tests);
· the handling and disposal of medical and hazardous waste;
· fraud and abuse laws such as the U.S. Federal False Claims Act, or FCA, the Federal Health Care Program Anti-Kickback Statute, or AKS, the Criminal Health Care Fraud Statute and Stark Law (defined below), and state equivalents;
· Occupational Safety and Health Administration rules and regulations;
· the Health Insurance Portability and Accountability Act of 1996, or HIPAA, and other U.S. federal and state medical data privacy and security laws;
· the Genetic Information Non-discrimination Act and similar state laws; and
· coverage and restrictions on coverage and reimbursement for research services, kits, clinical diagnostics and cellular therapies and Medicare, Medicaid, other governmental payers and private insurers reimbursement levels.
In particular, the laws, regulations and policies governing the marketing of an LDT and clinical diagnostic tests and services are extremely complex, and in many instances there are no significant regulatory or judicial interpretations of these laws and regulations. Among other things, pursuant to the FDCA and its implementing regulations, the FDA regulates the research, design, testing, manufacturing, safety, labeling, storage, recordkeeping, premarket clearance, authorization or approval, marketing and promotion and sales and distribution of medical devices in the United States to ensure they are safe and effective. Medical devices are defined by the FDCA to include, among other things, instruments and in vitro reagents or other similar or related articles, which are intended for use in the diagnosis of disease or other conditions. In addition, the FDA regulates the import and export of medical devices. Most LDTs, however, are not currently regulated as medical devices under FDA’s current regulatory framework, although components of LDTs, including, for example, instruments, reagents, and sample collection devices, may be regulated as medical devices. If we are subject to these FDA requirements and do not comply, or later become subject to these requirements and fail to adequately comply, our business operations may be harmed. These requirements may additionally cause delays in our ability to market and sell our products or services, which may, directly or indirectly, reduce our revenue, adversely affect our results of operations and financial condition and harm our business.
We plan to market our CDA test initially as an LDT, and future changes in the FDA’s regulation of LDTs could subject our operations to much more significant regulatory requirements.
We plan to initially market our CDA test in the United States as an LDT. LDTs have generally been considered to be tests that are designed, developed, validated and used within a single laboratory. The FDA has historically exercised a policy of enforcement discretion with respect to LDTs, whereby the FDA does not actively enforce its medical device regulatory requirements for these tests. In October 2014, the FDA issued two draft guidance documents stating that it intended to modify its policy of enforcement discretion with respect to LDTs in a risk-based manner consistent with the existing classification of medical devices. The FDA halted finalization of the draft guidance documents in November 2016 to allow for further public discussion of an appropriate oversight approach to LDTs and to give congressional authorizing committees the opportunity to develop a legislative solution. In January 2017, FDA issued a discussion paper laying out key elements of a possible revised future LDT regulatory framework. On August 19, 2020, HHS rescinded all guidance documents and informal policy statements that FDA had previously issued concerning LDTs, and announced that FDA would no longer require premarket authorization for LDTs unless the FDA engaged in notice-and-comment rulemaking. It is unclear if Congress or the FDA will modify the current approach to the regulation of LDTs in a way that would subject our CDA test to the enforcement of FDA regulatory requirements. The former FDA Commissioner and the Director of the Center for Devices and Radiological Health, or CDRH, have expressed significant concerns regarding disparities between LDTs and IVDs that have been reviewed and cleared, authorized or approved by the FDA. The FDA has also determined that certain LDTs do not qualify for enforcement discretion because these tests pose higher risk to the public health. If we market our test initially as an LDT in the United States and the FDA were to determine that our test is not within the enforcement discretion policy for LDTs for any reason, including as a result of new rules, policies or guidance, or due to changes in law, our laboratory and test may become subject to extensive FDA requirements or otherwise impact our business. These types of changes could reduce our revenue or increase our costs and adversely affect our business, prospects, results of operations or financial condition. If required, the regulatory marketing authorization process required to bring our LDT into compliance may involve, among other things, successfully completing additional clinical validations and submitting to and obtaining from the FDA pre-market clearance (510(k)), authorization for a de novo petition, or approval of a Premarket Approval Application, or PMA. Furthermore, legislative proposals could create new or different regulatory and compliance burdens on us and could have a negative effect on our ability to keep products on the market or develop new products, which could have a material effect on our business. In the event that we market our test initially as an LDT in the United States and then the FDA requires marketing authorization of our LDT in the future, the FDA ultimately may not grant any clearance, authorization or approval requested by us in a timely manner, or at all.
Our proprietary CDA device is an analytical instrument used as part of our CDA test, which may increase our risk that the FDA concludes that our test does not qualify as an LDT.
While the FDA has historically exercised enforcement discretion over the majority of LDTs, there are certain factors that have led to increased regulatory oversight. One such factor is the use of customized equipment and reagents. If the FDA were to conclude that our CDA device requires clearance, market authorization, or approval to be used as part of an LDT, it could prevent us from being able to offer our test. Even if we submit our CDA device for clearance, authorization, or approval, the FDA ultimately may not grant such clearance, authorization or approval requested by us in a timely manner, or at all.
Offering our proprietary cancer screening and detection test from more than one laboratory may increase our risk that the FDA concludes that our test does not qualify as an LDT.
While the FDA has historically exercised enforcement discretion over the majority of LDTs, it has narrowly defined an LDT as a test that is designed, manufactured and used within a single laboratory. However, the FDA has not historically taken enforcement action against laboratories with multiple facilities that offer the same test. If we offer our CDA test from more than one of our laboratories, the FDA could conclude that our test no longer qualifies as an LDT because it is not used within a single laboratory. If the FDA were to conclude that our cancer screening and detection test is not an LDT, that could prevent us from being able to offer our test until we receive appropriate FDA clearance, authorization or approval. Even if we submit for clearance, authorization or approval, the FDA may not ultimately grant such clearance, authorization or approval requested by us in a timely manner, or at all.
Failure to comply with U.S. federal or state laboratory licensing requirements and the applicable requirements of the FDA or any other regulatory authority or accrediting body, could cause us to lose the ability to perform testing in the United States, experience disruptions to our business, or become subject to administrative or judicial sanctions.
We are subject to CLIA, a U.S. federal law that regulates clinical laboratories that perform testing on specimens derived from humans for the purpose of providing information for the diagnosis, prevention or treatment of disease. Any testing subject to CLIA regulation must be performed in a CLIA-certified laboratory. CLIA certification is also required in order for us to be eligible to bill U.S. state and federal healthcare programs, as well as commercial payers, for our tests. We have received accreditation of our laboratory in San Jose, California from CAP, and have obtained a CLIA Certificate of Accreditation for this laboratory. We have commenced operations of our new laboratory in Philadelphia, Pennsylvania with the completion of our facility renovation and first phase equipment installation in July 2020. We obtained a CLIA Certificate of Registration for this laboratory in August 2020. We have applied for a Pennsylvania state laboratory permit and will seek to obtain CAP accreditation for this new laboratory. To maintain our CAP accreditation and CLIA certification, we are subject to survey and unannounced inspection every two years.
We are required to maintain a California clinical laboratory license for our San Jose laboratory and a Pennsylvania clinical laboratory license for our Philadelphia laboratory to conduct testing. In addition, some other states may require our California and Philadelphia laboratories to be licensed there in order to accept blood samples from those states or may have such requirements in the future. To maintain our state licenses, we may be subject to survey and inspection.
Failure to comply with applicable clinical laboratory certification and licensure requirements, including proficiency testing, may result in a range of enforcement actions, including suspension, limitation or revocation of our CAP accreditation, CLIA certificates and/or state licenses, imposition of a directed plan of corrective action, onsite monitoring, civil monetary penalties, criminal sanctions and revocation of the laboratory’s approval to receive Medicare and Medicaid payment for its services. Any of these enforcement actions or our failure to renew our CLIA certificates, a state license or other accreditation could have a material adverse effect on our business, financial condition and results of operations. Even if we were able to bring our laboratory back into compliance, we could incur significant expenses and potentially lose revenue in doing so.
If we are unable to obtain or maintain regulatory clearance or approvals in the United States, or if we experience delays in receiving clearance or approvals, our growth strategy may not be successful.
In the United States, we plan to initially offer our CDA test for clinical use as an LDT in our laboratory in San Jose, California. Because we developed this test and will offer this test solely for use within our laboratory, we believe that we may market the test as an LDT. Under current FDA policies, the FDA does not enforce its premarket clearance or approval requirements for certain LDTs before commercialization. The FDA could disagree with this assessment, however, in which case we would be required to obtain clearance, authorization, or approval for our device and/or test to continue marketing.
A key element of our longer term business strategy is to place our CDA device in other laboratories to broaden access to our technology and increase demand for our tests and any future tests that we may develop. In order to distribute our cancer screening and detection test and device outside of our laboratory, we will need to obtain FDA clearance, authorization, or approval for our test and device.
The FDA regulates medical devices, including IVDs, that are sold and distributed in U.S. interstate commerce. Unless an exemption applies, generally, before a new medical device or a new use for a medical device may be sold or distributed in the United States, the medical device must receive either a 510(k) premarket notification clearance, de novo marketing authorization, or a PMA approval from the FDA. As a result, before we can market or distribute our device and test in the United States for use by other clinical testing laboratories, we must first obtain 510(k) clearance, de novo marketing authorization, or PMA approval from the FDA. We have not yet applied for clearance, marketing authorization, or approval from the FDA for our CDA device, and need to complete additional validations before we are ready to apply. We believe it would likely take two years or more to conduct the clinical studies and trials necessary to obtain clearance, marketing authorization, or approval from the FDA to commercially launch our CDA tests outside of our clinical laboratory. Once we apply, we may not receive the FDA clearance, marketing authorization, or approval for the commercial use of our CDA device and test on a timely basis, or at all.
The FDA can delay, limit or deny clearance, authorization or approval of a device for many reasons, including:
· inability to demonstrate to the satisfaction of the FDA that the products are safe or effective for their intended uses;
· the FDA’s disagreement with the design, conduct or implementation of the clinical studies or the analysis or interpretation of data from preclinical studies, analytical studies or clinical studies;
· serious and unexpected adverse device effects experienced by participants in clinical studies;
· the data from preclinical studies, analytical studies and clinical studies may be insufficient to support clearance, authorization or approval, where required;
· the inability to demonstrate that the clinical and other benefits of the device outweigh the risks;
· an advisory committee, if convened by the FDA, may recommend against approval of a PMA or other application or may recommend that the FDA require, as a condition of approval, additional preclinical studies or clinical studies, limitations on approved labeling or distribution and use restrictions, or even if an advisory committee, if convened, makes a favorable recommendation, the FDA may still not approve the product;
· the FDA may identify deficiencies in our marketing application, and in our or our suppliers’ manufacturing processes, facilities or analytical methods;
· the potential for policies or regulations of the FDA to change significantly in a manner rendering clinical data or regulatory filings insufficient for clearance, authorization or approval; and
· the FDA may audit clinical study data and conclude that the data are not sufficiently reliable to support a PMA application.
There are numerous FDA personnel assigned to review different aspects of marketing submissions, and uncertainties can be presented by their ability to exercise judgment and discretion during the review process. During the course of review, the FDA may request or require additional data and information, and the development and provision of these data and information may be time-consuming and expensive. The process of obtaining regulatory clearances, authorizations or approvals to market a medical device can be costly and time-consuming, and we may not be able to obtain these clearances, authorizations or approvals on a timely basis or at all for our proposed products. If we are unable to achieve clearance or approval or if other laboratories do not accept our device and test, our ability to grow our business could be compromised.
Clinical studies involve a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future trial results.
In order to receive FDA clearance, marketing authorization, or approval for the commercialization of our CDA test and/or device in the United States, we must conduct, at our own expense, extensive analytical testing and clinical studies to demonstrate safety and effectiveness of our device and test for the intended indication of use. Clinical testing is expensive, can take many years to complete, if at all, and its outcome is uncertain. Failure can occur at any time during the clinical study process. Also, our CDA device and test may not prove to be safe and efficacious in the clinical studies, and they may not meet all the applicable regulatory requirements needed to receive FDA clearance, authorization, or approval. The results of our clinical studies may not support the clinical validation needed to offer our cancer screening and detection test in the U.S. In addition, clinical claims for our CDA test that are supported by the clinical studies results may not be commercially viable.
If we receive FDA clearance, marketing authorization, or approval of our CDA device and test, we will continue to be subject to extensive FDA regulatory oversight.
Medical devices are subject to extensive regulation by the FDA in the United States. If our CDA device is cleared, authorized, or approved by the FDA, we will need to comply with the applicable regulatory requirements and our failure to do so could result in enforcement action by the FDA or state agencies. Any of these enforcement actions could also result in higher than anticipated costs or lower than anticipated sales and have a material adverse effect on our reputation, business, results of operations and financial condition.
We also cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative or executive action in the United States. For example, the U.S. has taken several executive actions, including the issuance of a number of executive orders, that could impose significant burdens on, or otherwise materially delay, the FDA’s ability to engage in routine regulatory and oversight activities such as implementing statutes through rulemaking, issuance of guidance and review and approval of marketing applications. It is difficult to predict how these executive actions will be implemented and the extent to which they will affect the FDA’s ability to exercise its regulatory authority. If these executive actions impose constraints on the FDA’s ability to engage in oversight and implementation activities in the normal course, our business may be negatively impacted.
Our plans to offer COVID-19 antibody tests are subject to the FDA’s Emergency Use Authorization authority and the ongoing response to the COVID-19 public health emergency.
Our San Jose laboratory has validated the Roche Elecsys Anti-SARS-CoV-2 assay that FDA granted Emergency Use Authorization, or EUA, to for the qualitative detection of antibodies to SARS-CoV-2 in human serum and plasma. Our Philadelphia laboratory is working to validate the Roche Elecsys Anti-SARS-CoV-2 S assay that was granted an EUA for the qualitative and semi-quantitative determination of antibodies to the SARS-CoV-2 spike protein. The FDA has stated that laboratories that are performing testing using EUA-authorized test kits from commercial manufacturers need not notify FDA of or obtain an EUA from FDA for such testing. We do, however, need to comply with our CLIA licensure and the applicable regulatory requirements set forth in the EUA and failure to do so could result in enforcement action by the FDA or state agencies.
We have not yet offered the COVID-19 antibody tests commercially, and we cannot guarantee the market acceptance of and demand for these tests once we do begin to commercially offer them. We cannot predict how the FDA may act in regards to its EUA policies and COVID-19 testing generally. If the FDA decides to terminate or otherwise cancel or void the EUAs granted to Roche, we may not be able to continue offering the COVID-19 antibody tests. If HHS terminates the emergency declaration for the COVID-19 pandemic, we may not be able to continue to offer the COVID-19 antibody tests.
Our employees may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements, and insider trading.
We are exposed to the risk of fraud or other misconduct by our employees. Misconduct by our employees could include intentional failures to comply with the regulations of the FDA or non-U.S. regulators, to comply with healthcare fraud and abuse laws and regulations in the United States and abroad, or to report financial information or data accurately or to disclose unauthorized activities to us. In particular, sales, marketing, and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Such misconduct could also involve the improper use of information obtained in the course of clinical studies, which could result in regulatory sanctions and cause serious harm to our reputation. We currently have a code of conduct applicable to all of our employees, but it is not always possible to identify and deter employee misconduct, and our code of conduct and the other precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses, or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could result in the imposition of significant civil, criminal and administrative penalties, including, without limitation, damages, monetary fines, individual imprisonment, disgorgement of profits, possible exclusion from participation in Medicare, Medicaid and other U.S. federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings, additional reporting or oversight obligations if we become subject to a corporate integrity agreement or other agreement to resolve allegations of non-compliance with the law and curtailment or restructuring of our operations, which could have a significant impact on our business. Whether or not we are successful in defending against such actions or investigations, we could incur substantial costs, including legal fees, and divert the attention of management in defending ourselves against any of these claims or investigations.
If we fail to comply with healthcare laws and regulations, we could face substantial enforcement actions, including civil and criminal penalties and our business, operations and financial condition could be adversely affected.
We could be subject to healthcare fraud and abuse laws and patient privacy laws of both the U.S. federal government and the states in which we conduct our business. The laws include, but are not limited to:
· the AKS, which prohibits, among other things, persons from soliciting, receiving or providing remuneration, directly or indirectly, to induce either the referral of an individual for an item or service or the purchasing or ordering of a good or service, for which payment may be made under U.S. federal healthcare programs such as the Medicare and Medicaid programs;
· the FCA which prohibits, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other payers that are false or fraudulent, and which may apply to entities like us which provide coding and billing information to customers;
· HIPAA, which prohibits executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters and which also imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information; and
· state law equivalents of each of the above U.S. federal laws, such as anti-kickback and false claims laws which may apply to items or services reimbursed by any third-party payer, including commercial insurers, and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by U.S. federal laws, thus complicating compliance efforts.
If our operations are found to be in violation of any of the laws described above or any governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines and the curtailment or restructuring of our operations. Any penalties, damages, fines, curtailment or restructuring of our operations could adversely affect our ability to operate our business and our financial results. Although compliance programs can mitigate the risk of investigation and prosecution for violations of these laws, the risks cannot be entirely eliminated. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert management’s attention from the operation of our business. Moreover, achieving and sustaining compliance with applicable U.S. federal and state privacy, security and fraud laws may prove costly.
Our collection, use and disclosure of individually identifiable information, including health and/or employee information, is subject to U.S. state, U.S. federal, and foreign privacy and security regulations, and our failure to comply with those regulations or to adequately secure the information we hold could result in significant liability or reputational harm.
The privacy and security of personally identifiable information stored, maintained, received or transmitted, including electronically, is a major issue in the United States and abroad. While we strive to comply with all applicable privacy and security laws and regulations, as well as our own posted privacy policies, legal standards for privacy, including but not limited to “unfairness” and “deception,” as enforced by the U.S. Federal Trade Commission and state attorneys general, continue to evolve, and any failure or perceived failure to comply may result in proceedings or actions against us by government entities or others, or could cause us to lose customers, which could have a material adverse effect on our business. Recently, there has been an increase in public awareness of privacy issues in the wake of revelations about the activities of various government agencies and in the number of private privacy-related lawsuits filed against companies. Concerns about our practices with regard to the collection, use, retention, disclosure or security of personally identifiable information or other privacy-related matters, even if unfounded and even if we are in compliance with applicable laws, could damage our reputation and harm our business.
Numerous U.S. federal and state laws and regulations govern the collection, dissemination, use and confidentiality of personally identifiable health information, or PHI, including state privacy and confidentiality laws (including state laws requiring disclosure of breaches); U.S. federal and state consumer protection and employment laws; HIPAA; and European and other foreign data protection laws. These laws and regulations are increasing in complexity and number, may change frequently and sometimes conflict.
HIPAA establishes a set of national privacy and security standards for the protection of individually identifiable health information, including PHI by health plans, healthcare clearinghouses and healthcare providers that submit certain covered transactions electronically, or covered entities, and their business associates, which are persons or entities that perform certain services for, or on behalf of, a covered entity that involve creating, receiving, maintaining or transmitting PHI.
Penalties for violations of these laws vary. For instance, penalties for failure to comply with a requirement of HIPAA and the Health Information Technology for Economic and Clinical Health Act, or HITECH, vary significantly, and can include civil monetary penalties of up to $59,522 per violation, not to exceed $1.79 million per calendar year for each provision that is violated. A single breach incident can result in findings of violations of multiple provisions, leading to possible civil penalties in excess of $1.78 million in a single year. Violations of HIPAA may also result in criminal penalties. For example, a person who knowingly obtains or discloses individually identifiable health information in violation of HIPAA may face a criminal penalty of up to $50,000 and up to one-year imprisonment. In certain circumstances, criminal fines up to $250,000 per violation and/or up to ten years’ imprisonment may be imposed. The criminal penalties increase if the wrongful conduct involves false pretenses or the intent to sell, transfer, or use identifiable health information for commercial advantage, personal gain, or malicious harm. Responding to government investigations regarding alleged violations of these and other laws and regulations, even if ultimately concluded with no findings of violations or no penalties imposed, can consume company resources and impact our business and, if public, harm our reputation.
Further, various states, such as California and Massachusetts, have implemented similar privacy laws and regulations that impose restrictive requirements regulating the use and disclosure of health information and other personally identifiable information. These laws and regulations are not necessarily preempted by HIPAA, particularly if a state affords greater protection to individuals than HIPAA. Where state laws are more protective, we may have to comply with the stricter provisions. In addition to fines and penalties imposed upon violators, some of these state laws also afford private rights of action to individuals who believe their personal information has been misused. The interplay of U.S. federal and state laws may be subject to varying interpretations by courts and government agencies, creating complex compliance issues for us and our clients and potentially exposing us to additional expense, adverse publicity and liability. Further, as regulatory focus on privacy issues continues to increase and laws and regulations concerning the protection of personal information expand and become more complex, these potential risks to our business could intensify. Changes in laws or regulations associated with the enhanced protection of certain types of sensitive data, such as PHI, or personally identifiable information along with increased customer demands for enhanced data security infrastructure, could greatly increase our cost of providing our services, decrease demand for our services, reduce our revenue and/or subject us to additional liabilities.
We may be exposed to liabilities under the United States Foreign Corrupt Practices Act, or FCPA, and Chinese anti-corruption laws, and any determination that we have violated these laws could have a material adverse effect on our business or our reputation.
We are subject to the FCPA. The FCPA generally prohibits us from making improper payments to non-U.S. officials for the purpose of obtaining or retaining business. We are also subject to the anti-bribery laws of China. Our current customers include state-owned enterprises and, after we obtain the Class III medical device registration certificate, we plan to sell our CDA tests and devices to hospitals in China, many of which are state-owned. As a result, we may engage with Chinese officials or persons of equivalent status during the ordinary course of our business. We do not fully control the interactions that our employees and sales agents have with those officials or persons, and they may try to increase sales volumes of our tests through means that constitute violations of the FCPA, the PRC anti-bribery laws or other related laws. As our business expands, the applicability of the FCPA and other anti-bribery laws to our operations will increase. Our procedures and controls to monitor anti-bribery compliance may fail to protect us from reckless or criminal acts committed by our employees or sales agents. If we, due to either our own deliberate or inadvertent acts or those of others, fail to comply with applicable anti-bribery laws, our reputation could be harmed and we could incur criminal or civil penalties, other sanctions and/or significant expenses, which could have a material adverse effect on our business, including our financial condition, results of operations, cash flows and prospects.
Risks Relating to Doing Business in China
We are subject to many of the economic and political risks associated with emerging markets due to our operations in China. Changes in China’s economic, political or social conditions or government policies and the current tensions in international economic relations could have an adverse effect on our business and operations.
Most of our assets and operations are located in China, the world’s largest emerging market. In light of our operations in an emerging market, we may be subject to risks and uncertainties including fluctuations in GDP, unfavorable or unpredictable treatment in relation to tax matters, expropriation of private assets, exchange controls, restrictions affecting our ability to make cross-border transfer of funds, regulatory proceedings, inflation, currency fluctuations or the absence of, or unexpected changes in, regulations and unforeseeable operational risks. Accordingly, our business, financial condition, results of operations and prospects may be influenced to a significant degree by political, economic and social conditions in China. The Chinese economy differs from the economies of most developed countries in many respects, including the level of government involvement, level of development, growth rate, control of foreign exchange, allocation of resources, evolving regulatory system and lack of sufficient transparency in the regulatory process.
The economies of emerging markets are typically more vulnerable to market downturns and economic slowdowns elsewhere in the world. While the Chinese economy has experienced significant growth over past decades, growth has been uneven, both geographically and among various sectors of the economy, and the rate of growth has been slowing since 2012. Any adverse changes in economic conditions in China, in the policies of the Chinese government or in the laws and regulations in China could have a material adverse effect on China’s overall economic growth. Such developments could adversely affect our business and operating results, lead to a reduction in demand for our cancer screening and detection test and adversely affect our competitive position. The Chinese government has implemented various measures to encourage economic growth and guide the allocation of resources. Some of these measures benefit the overall Chinese economy, but may also have a negative effect on us. For example, our financial condition and results of operations may be adversely affected by government control over capital investments or changes in tax regulations that are applicable to us.
Recently there have been heightened tensions in economic relations between the United States and China. The U.S. government has recently imposed, and proposed to impose additional, new or higher tariffs on products imported from China to penalize China for what it characterizes as unfair trade practices. China has responded by imposing largely commensurate tariffs on products imported from the United States. The lasting impact of these trade conflicts on the PRC economy remains uncertain. As a biotechnology company with operations primarily based in China as well as the United States, our plan to commercialize our CDA test in, and export our CDA device to, the United States after obtaining relevant approvals from the FDA could be adversely affected by these or future trade developments. In addition, political tensions between the United States and China have escalated due to, among other things, the COVID-19 outbreak, sanctions imposed by the U.S. Department of Treasury on certain officials of the Hong Kong Special Administrative Region and the central government of the PRC, and the U.S. sanctions on a number of Chinese entities and relevant individuals. Rising political tensions could reduce levels of trade, investment, technological exchange and other economic activities between the two major economies, which would have a material adverse effect on global economic conditions and the stability of global financial markets. Any of these factors could have a material adverse effect on our business, financial condition and results of operations.
Uncertainties with respect to China’s legal system could have a material adverse effect on our business and operations.
We conduct our businesses in China primarily through our PRC subsidiaries. Our operations in China are governed by PRC laws and regulations. Our PRC subsidiaries are subject to laws and regulations applicable to foreign investment in China. The PRC legal system is a civil law system based on written statutes. Unlike the common law system, prior court decisions may be cited for reference but have limited precedential value. The PRC legal system is evolving rapidly, and the interpretation of many laws, regulations and rules may contain inconsistencies, and the enforcement of these laws, regulations and rules involves uncertainties.
From time to time, we may have to resort to administrative and court proceedings to enforce our legal rights. Any administrative and court proceedings in China may be protracted, resulting in substantial costs and diversion of resources and management attention. Since PRC administrative and court authorities have significant discretion in interpreting and implementing statutory and contractual terms, it may be more difficult to evaluate the outcome of administrative and court proceedings and the level of legal protection we enjoy than in more developed legal systems. These uncertainties may impede our ability to enforce the contracts we have entered into and could materially and adversely affect our business and results of operations. Furthermore, the PRC legal system is based, in part, on government policies and internal rules, some of which are not published in a timely manner, or at all, but which may have retroactive effect. As a result, we may not always be aware of any potential violation of these policies and rules. Such unpredictability towards our contractual, property and procedural rights could adversely affect our business and impede our ability to continue our operations.
Uncertainties exist with respect to the interpretation and implementation of the PRC Foreign Investment Law, which may impose new burdens on us.
The PRC Foreign Investment Law, or the FIL, was enacted by the National People’s Congress of the PRC on March 15, 2019 and became effective on January 1, 2020, which replaces the trio of previous laws regulating foreign investment in China, namely, the Sino-foreign Equity Joint Venture Enterprise Law, the Sino-foreign Cooperative Joint Venture Enterprise Law and the Wholly Foreign-invested Enterprise Law, together with their implementation rules and ancillary regulations. This law has become the legal foundation for foreign investment in the PRC. The FIL embodies an expected PRC regulatory trend to rationalize its foreign investment regulatory regime in line with prevailing international practice and the legislative efforts to unify the corporate legal requirements for both foreign and domestic investments. The Implementation Rules to the Foreign Investment Law were promulgated by the State Council on December 26, 2019 and became effective on January 1, 2020. However, uncertainties exist with respect to interpretation and implementation of the FIL and its Implementation Rules, which may adversely impact our corporate governance practice and increase our compliance costs. For instance, we might be required by governmental interpretations or implementing rules of the FIL to adjust the corporate governance of certain of our PRC subsidiaries in a five-year transition period. In addition, the FIL imposes information reporting requirements on foreign investors or foreign invested enterprises. Failure to take timely and appropriate measures to cope with any of these or other regulatory compliance requirements under the FIL may lead to rectification obligations, penalties, or other regulatory sanctions on us.
PRC regulations of loans and direct investment by offshore holding companies to PRC entities may delay or prevent us from using the proceeds of our offshore equity and debt offerings to make loans or additional capital contributions to our PRC subsidiaries, which could materially and adversely affect our liquidity and our ability to fund and expand our business.
We are an offshore holding company conducting our operations in China through our PRC subsidiaries. We may make loans to our PRC subsidiaries or we may make additional capital contributions to our wholly foreign-owned subsidiaries in China. Any loans by us to our wholly foreign-owned subsidiaries in China to finance their activities cannot exceed statutory limits and must be registered with the local counterpart of the PRC State Administration of Foreign Exchange, or SAFE. In addition, a foreign invested enterprise shall use its capital pursuant to the principle of authenticity and self-use within its business scope.
In March 2015, SAFE promulgated the Circular on Reforming the Administration Measures on Conversion of Foreign Exchange Registered Capital of Foreign-invested Enterprises, or SAFE Circular 19, which took effect and replaced certain previous SAFE regulations from June 1, 2015. SAFE further promulgated the Circular of the SAFE on Reforming and Regulating Policies on the Control over Foreign Exchange Settlement of Capital Accounts, or SAFE Circular 16, which took effective on June 9, 2016 and, among other things, amended certain provisions of SAFE Circular 19. According to SAFE Circular 19 and SAFE Circular 16, the flow and use of Renminbi capital converted from foreign currency-denominated registered capital of a foreign-invested company is regulated such that Renminbi capital may not be used for business beyond its business scope, or to provide loans to persons other than affiliates, unless otherwise permitted under its business scope. SAFE Circular 19 and SAFE Circular 16 may limit our ability to transfer the net proceeds from our offshore equity and debt offerings to our PRC subsidiaries and convert the net proceeds into RMB.
In light of the various requirements imposed by PRC regulations on loans to and direct investment in PRC entities by offshore holding companies, we cannot assure you that we will be able to complete the necessary government registrations or obtain the necessary government approvals on a timely basis, if at all, with respect to future loans to our PRC subsidiaries or future capital contributions by us to our wholly foreign-owned subsidiaries in China. As a result, uncertainties exist as to our ability to provide prompt financial support to our PRC subsidiaries when needed. If we fail to complete such registrations or obtain such approvals, our ability to use the proceeds we expect to receive from our offshore equity and debt offerings and to capitalize or otherwise fund our PRC operations may be negatively affected, which could materially and adversely affect our liquidity and our ability to fund and expand our business.
We rely on dividends paid by our subsidiaries for our cash needs, and any limitation on the ability of our subsidiaries to make payments to us could have a material adverse effect on our ability to conduct our business.
As a holding company, we conduct most of our business through our subsidiaries incorporated in China. We may rely on dividends paid by these PRC subsidiaries for our cash needs, including the funds necessary to pay any dividends and other cash distributions to our shareholders, to service any debt we may incur and to pay our operating expenses. The payment of dividends by entities established in China is subject to limitations. Regulations in China currently permit payment of dividends only out of accumulated profits as determined in accordance with accounting standards and regulations in China. As a result, our PRC subsidiaries are restricted in their ability to transfer a portion of their net assets to us in the form of dividends. In addition, if any of our PRC subsidiaries incurs debt on its own behalf in the future, the instruments governing the debt may restrict its ability to pay dividends or make other distributions to us. Any limitations on the ability of our PRC subsidiaries to transfer funds to us could materially and adversely limit our ability to grow, make investments or acquisitions that could be beneficial to our business, pay dividends and otherwise fund and conduct our business.
Our business benefits from certain financial incentives and discretionary policies granted by local governments. Expiration of, or changes to, these incentives or policies would have an adverse effect on our results of operations.
In the past, local governments in the PRC granted certain financial incentives from time to time to our PRC subsidiaries as part of their efforts to encourage the development of local businesses. The timing, amount and criteria of government financial incentives are determined within the sole discretion of the local government authorities and cannot be predicted with certainty before we actually receive any financial incentive. We generally do not have the ability to influence local governments in making these decisions. Local governments may decide to reduce or eliminate incentives at any time. In addition, some of the government financial incentives are granted on a project basis and subject to the satisfaction of certain conditions, including completion of the specific project therein. We cannot guarantee that we will satisfy all relevant conditions, and if we do not, we may be deprived of the relevant incentives. We cannot assure you of the continued availability of the government incentives currently enjoyed by us. Any reduction or elimination of incentives would have an adverse effect on our results of operations. Government grants and subsidies we recognized for the years ended December 31, 2018, 2019 and 2020 was RMB5.9 million, RMB2.8 million and RMB7.5 million (US$1.1 million), respectively.
Under the PRC Enterprise Income Tax Law, or the EIT Law, we may be classified as a PRC resident enterprise for PRC income tax purposes, which could result in unfavorable tax consequences to us and our non-PRC shareholders or ADS holders, and have a material adverse effect on our results of operations and the value of your investment.
Under the EIT Law and its implementation rules, an enterprise established outside China may be considered as a PRC resident enterprise provided that its “de facto management body” is located within China. According to the implementation rules, “de facto management body” is interpreted as a body that exercises substantial and overall management and control over the business, personnel, accounts and properties of an enterprise. In April 2009, the PRC State Administration of Taxation, or the SAT, issued the Circular of the SAT on Issues Relating to Identification of PRC-Controlled Overseas Registered Enterprises as Resident Enterprises in Accordance With the De Facto Standards of Organizational Management, or SAT Circular 82, which provides certain specific criteria for determining whether the “de facto management body” of a PRC-controlled enterprise incorporated offshore is located in China. Although this circular only applies to offshore enterprises controlled by PRC enterprises or PRC enterprise groups, not those controlled by PRC individuals or foreigners, the criteria set forth in the circular may reflect SAT’s general position on how “de facto management body” rule should be applied in determining the tax resident status of all offshore enterprises. According to SAT Circular 82, an offshore incorporated enterprise controlled by a PRC enterprise or a PRC enterprise group will be regarded as a PRC tax resident by virtue of having its “de facto management body” in China and will be subject to PRC enterprise income tax on its global income only if all of the following conditions are met: (i) the primary location of the day-to-day operational management is in China; (ii) decisions relating to the enterprise’s financial and human resource matters are made or are subject to approval by organizations or personnel in China; (iii) the enterprise’s primary assets, accounting books and records, company seals, and board and shareholder minutes, are located or maintained in China; and (iv) at least 50% of voting board members or senior executives habitually reside in China.
According to these rules and regulations, we may be considered as a PRC resident enterprise by the PRC tax authorities for tax purposes and a number of unfavorable tax consequences could follow. However, the tax resident status of an enterprise is subject to determination by the PRC tax authorities and uncertainties remain with respect to the interpretation of the term “de facto management body.” If the PRC tax authorities determine that we are a PRC resident enterprise for enterprise income tax purposes, we will be subject to PRC tax at a rate of 25% on our worldwide income, which could materially reduce our net income, and we may be required to withhold tax from dividends we pay at a rate of 10% in case to non-PRC enterprise shareholders (including ADS holders) or 20% in case to non-PRC individual shareholders (including ADS holders); in addition, gains realized on the sale or other disposition of our ordinary shares or ADSs may be subject to PRC tax, at a rate of 10% in case of non-PRC enterprise shareholders (including our ADS holders) or 20% in case of non-PRC individual shareholders (including ADS holders), if such dividends or gains are deemed to be from PRC sources. Any such PRC tax liability may be reduced under an applicable tax treaty. However, it is unclear whether non-PRC shareholders (including our ADS holders) of our company would be able to claim the benefits of any tax treaties between their country of tax residence and the PRC in the event that we are treated as a PRC resident enterprise. Any such tax may reduce the returns on your investment in the ADSs.
We face uncertainty with respect to indirect transfers of equity interests in PRC resident enterprises by their non-PRC holding companies.
In February 2015, the SAT issued the Public Notice Regarding Certain Enterprise Income Tax Matters on Indirect Transfer of Properties by Non-Tax Resident Enterprises, or SAT Public Notice 7. SAT Public Notice 7 extends its tax jurisdiction to not only indirect transfers but also transactions involving transfer of other taxable assets, through the offshore transfer of a foreign intermediate holding company. In addition, SAT Public Notice 7 provides certain criteria on how to assess reasonable commercial purposes and has introduced safe harbors for internal group restructurings and the purchase and sale of equity through a public securities market. SAT Public Notice 7 also brings challenges to both foreign transferor and transferee (or other person who is obligated to pay for the transfer) of the taxable assets. Where a non-resident enterprise conducts an “indirect transfer” by transferring the taxable assets indirectly by disposing of the equity interests of an overseas holding company, the non-resident enterprise being the transferor, or the transferee, or the PRC entity that directly owns the taxable assets, may report such indirect transfer to the relevant tax authority. Using a “substance over form” principle, the PRC tax authority may disregard the existence of the overseas holding company if it lacks a reasonable commercial purpose and was established for the purpose of reducing, avoiding or deferring PRC tax. As a result, gains derived from such indirect transfer may be subject to PRC enterprise income tax, and the transferee or other person who is obligated to pay for the transfer is obligated to withhold the applicable taxes, currently at a rate of 10% for the transfer of equity interests in a PRC resident enterprise. Both the transferor and the transferee may be subject to penalties under PRC tax laws if the transferee fails to withhold the taxes and the transferor fails to pay the taxes. However, according to the aforesaid safe harbor rule, the PRC tax would not be applicable to the transfer by any non-resident enterprise of ADSs of our company acquired and sold on public securities markets.
In October 2017, the SAT issued the Public Notice on Issues Concerning the Withholding of Non-resident Enterprise Income Tax at Source, or SAT Public Notice 37, which took effect on December 1, 2017. According to SAT Public Notice 37, where the non-resident enterprise fails to declare the tax payable pursuant to Article 39 of the EIT Law, the tax authority may order it to pay the tax due within required time limits, and the non-resident enterprise shall declare and pay the tax payable within such time limits specified by the tax authority. If the non-resident enterprise voluntarily declares and pays the tax payable before the tax authority orders it to do so, it shall be deemed that such enterprise has paid the tax payable in time.
We face uncertainties on the reporting and consequences of future private equity financing transactions, share exchanges or other transactions involving the transfer of shares in our company by investors that are non-PRC resident enterprises. The PRC tax authorities may pursue such non-resident enterprises with respect to a filing or the transferees with respect to withholding obligation and request our PRC subsidiaries to assist in the filing. As a result, we and non-resident enterprises in such transactions may become at risk of being subject to filing obligations or being taxed under SAT Public Notice 7 and SAT Public Notice 37, and may be required to expend valuable resources to comply with them or to establish that we should not be taxed under these regulations, which may have a material adverse effect on our financial condition and results of operations.
Fluctuations in exchange rates could adversely affect our business and the value of our securities.
Changes in the value of the RMB against the U.S. dollar, Euro and other foreign currencies are affected by, among other things, changes in China’s political and economic conditions. Since July 2005, the RMB is no longer pegged to the U.S. dollar, and the RMB may appreciate or depreciate significantly in value against the U.S. dollar in the medium to long term. Any significant revaluation of the RMB may have a material adverse effect on our revenues and financial condition, and the value of, and any dividends payable on, our shares in U.S. dollar terms. For example, to the extent that we need to convert U.S. dollars we receive from any equity or debt offerings into RMB for our operations, appreciation of the RMB against the U.S. dollar would have an adverse effect on RMB amount we would receive from the conversion. Conversely, if we decide to convert our RMB into U.S. dollars for the purpose of paying dividends on our ordinary shares or for other business purposes, appreciation of the U.S. dollar against the RMB would have a negative effect on the U.S. dollar amount available to us.
Governmental control of currency conversion may limit our ability to utilize our cash balance effectively and affect the value of your investment.
The PRC government imposes controls on the convertibility of the Renminbi into foreign currencies and, in certain cases, the remittance of currency out of China. We receive substantially all of our revenues in Renminbi. Under our current corporate structure, our holding company incorporated in the BVI primarily relies on dividend payments from our PRC subsidiaries to fund our cash and financing requirements. Under existing PRC foreign exchange regulations, payments of current account items, including profit distributions, interest payments and trade and service-related foreign exchange transactions, can be made in foreign currencies without prior approval of SAFE by complying with certain procedural requirements. Specifically, under the existing exchange restrictions, without prior approval of SAFE, cash generated from the operations of our PRC subsidiaries in China may be used to pay dividends to our company. However, approval from or registration with appropriate government authorities is required where Renminbi is to be converted into foreign currency and remitted out of China to pay capital expenses such as the repayment of loans denominated in foreign currencies. As a result, we need to obtain SAFE approval to use cash generated from the operations of our PRC subsidiaries to pay off their respective debt in a currency other than Renminbi owed to entities outside China, or to make other capital expenditure payments outside China in a currency other than Renminbi.
In light of the flood of capital outflows, the PRC government may from time to time impose more restrictive foreign exchange policies and increase scrutiny of major outbound capital movements. More restrictions and substantial vetting processes may be required by SAFE or other government authorities to regulate cross-border transactions falling under the capital account. The PRC government may at its discretion restrict access to foreign currencies for current account transactions in the future. If the foreign exchange control system prevents us from obtaining sufficient foreign currencies to satisfy our foreign currency demands, we may not be able to pay dividends in foreign currencies to our shareholders, including holders of our ADSs.
PRC laws and regulations have more complex procedures for some acquisitions of Chinese companies by foreign investors, which could make it more difficult for us to pursue growth through acquisitions in China.
PRC laws and regulations, such as the Regulations on Mergers and Acquisitions of Domestic Enterprises by Foreign Investors, or the M&A Rules, Anti-Monopoly Law of the PRC and the Rules of the PRC Ministry of Commerce, or the MOFCOM, on Implementation of the Security Review System of Mergers and Acquisitions of Domestic Enterprises by Foreign Investors, or the MOFCOM Security Review Rules, established additional procedures and requirements that are expected to make merger and acquisition activities in China by foreign investors more time-consuming and complex, including requirements in some instances that the MOFCOM be notified in advance of any change of control transaction in which a foreign investor takes control of a PRC domestic enterprise, or that the approval from the MOFCOM be obtained in circumstances where offshore companies established or controlled by PRC enterprises or residents acquire affiliated domestic companies. PRC laws and regulations also require certain merger and acquisition transactions to be subject to merger control review or security review.
According to these laws and regulations, a security review is required for mergers and acquisitions by foreign investors having “national defense and security” concerns, and for mergers and acquisitions by which foreign investors may acquire the “de facto control” of domestic enterprises that have “national security” concerns. In addition, when deciding whether a specific merger or acquisition of a domestic enterprise by foreign investors is subject to the security review, the MOFCOM will look into the substance and actual impact of the transaction. The MOFCOM Security Review Rules further prohibit foreign investors from bypassing the security review requirement by structuring transactions through proxies, trusts, indirect investments, leases, loans, control through contractual arrangements or offshore transactions.
We might grow our business in part by acquiring other companies operating in our industry. Complying with the requirements of the relevant regulations to complete such transactions could be time-consuming, and any required approval processes, including approval from the MOFCOM, may delay or inhibit our ability to complete such transactions, which could affect our ability to expand our business or maintain our market share.
PRC regulations relating to offshore investment activities by PRC residents may limit our PRC subsidiaries’ ability to change their registered capital or distribute profits to us or otherwise expose us or our PRC resident beneficial owners to liability and penalties under PRC law.
SAFE promulgated the Circular on Relevant Issues Concerning Foreign Exchange Administration on Domestic Resident’s Investment and Financing and Roundtrip Investment through Special Purpose Vehicles, or SAFE Circular 37, in July 2014 that requires PRC residents to register with SAFE or its local branch in connection with their establishment or control of an offshore entity established for the purpose of overseas investment or financing, referred to as “offshore special purpose vehicle.” In addition, such PRC residents must update their SAFE registrations when the offshore special purpose vehicle undergoes any change of basic information (including change of such PRC residents, name and operation term), increases or decreases in investment amount, transfers or exchanges of shares, or mergers or divisions. According to the Notice on Further Simplifying and Improving the Foreign Exchange Administration Policies on Direct Investment, or SAFE Notice 13, released on February 2015 by the SAFE, local banks will examine and handle foreign exchange registration for overseas direct investment, including the foreign exchange registration under SAFE Circular 37 from June 2015.
Due to the inherent uncertainty in the implementation of regulatory requirements by the PRC governmental authorities, SAFE Circular 37 registration might not be always practically available under all circumstances as prescribed in those regulations. In addition, we may not at all times be fully aware or informed of the identities of all the PRC residents holding direct or indirect interest in our company. We cannot assure you that all of our PRC resident registered or beneficial owners are in compliance and will comply with SAFE regulations, including those requiring them to make necessary applications, filings and amendments. To our knowledge, certain of our PRC resident individual shareholders who hold an insignificant number of our shares have not completed their SAFE Circular 37 registration yet. The failure or inability of our PRC resident shareholders to comply with the SAFE registrations, or failure by us to update the foreign exchange registrations of our PRC subsidiaries, may subject us to fines and legal sanctions, such as restrictions on our cross-border investment activities, the ability of our wholly foreign-owned subsidiaries in China to distribute dividends and proceeds from any reduction in capital, share transfer or liquidation to us. Moreover, failure to comply with the various foreign exchange registration requirements described above could result in liability under PRC laws for circumventing applicable foreign exchange restrictions. As a result, our business operations and our ability to distribute profits to you could be materially and adversely affected.
Failure to comply with PRC regulations regarding the registration requirements for stock ownership plans or share option plans may subject the PRC plan participants or us to fines and other legal or administrative sanctions.
In February 2012, SAFE promulgated the Notices on Issues Concerning the Foreign Exchange Administration for Domestic Individuals Participating in Stock Incentive Plans of Overseas Publicly-Listed Companies, or the Stock Option Rules. Under the Stock Option Rules and other relevant rules and regulations, PRC residents who participate in stock incentive plan in an overseas publicly-listed company are required to register with SAFE or its local branches and complete certain other procedures. Participants of a stock incentive plan who are PRC residents must retain a qualified PRC agent, which could be a PRC subsidiary of such overseas publicly listed company or another qualified institution selected by such PRC subsidiary, to conduct the SAFE registration and other procedures with respect to the stock incentive plan on behalf of its participants. Such participants must also retain an overseas entrusted institution to handle matters in connection with their exercise of stock options, the purchase and sale of corresponding stocks or interests and fund transfers. In addition, the PRC agent is required to amend the SAFE registration with respect to the stock incentive plan if there is any material change to the stock incentive plan, the PRC agent or the overseas entrusted institution or other material changes. Certain of our directors, executive officers, employees and consultants who are PRC residents may participate in our 2019 Plan, and therefore are subject to these regulations. Failure of these PRC stock option holders to complete their SAFE registrations may subject these PRC residents to fines and legal sanctions and may also limit our ability to contribute additional capital into our PRC subsidiaries, limit our PRC subsidiaries’ ability to distribute dividends to us, or otherwise materially adversely affect our business.
In addition, the SAT has issued certain circulars concerning employee share incentives. Under these circulars, our employees working in the PRC who exercise share options or are granted restricted shares will be subject to PRC individual income tax. Our PRC subsidiaries have obligations to file documents related to employee share options or restricted shares with relevant tax authorities and to withhold individual income taxes of those employees who exercise their share options. If our employees fail to pay or we fail to withhold their income taxes according to relevant laws and regulations, we may face sanctions imposed by the tax authorities or other PRC government authorities.
Our business and our profitability may be negatively affected by the rising labor costs and potential obligations to make additional contributions of social insurance premium and housing funds.
In recent years, labor costs in China have continued to increase, driven by increased inflation, as well as enactment of new labor laws. As a result, we expect our labor costs, including wages and employee benefits, to continue to increase in the foreseeable future. Unless we are able to pass on these increased labor costs to our customers by increasing the prices of our products and services, our financial condition and results of operations may be adversely affected.
In addition, we are required by PRC laws and regulations to participate in various employee social security plans that are organized by municipal and provincial governments, including housing, pension, medical insurance, work-related injury insurance, employment injury insurance, maternity insurance and unemployment insurance. We are required under PRC law to make contributions to employee benefit plans at specified percentages of the salaries, bonuses and certain allowances of our employees, up to a maximum amount specified by the local government from time to time. The relevant government agencies may examine whether an employer has made adequate payments of these requisite statutory employee benefits, and those employers who fail to make adequate payments may be subject to late payment fees, fines and/or other penalties. We have historically failed to promptly make social insurance and housing fund contributions in full with respect to our employees. If the relevant PRC authorities determine that we shall make supplemental social insurance and housing fund contributions, and that we are subject to fines and legal sanctions, our business, financial condition and results of operations may be adversely affected.
Proceedings instituted by the SEC against China-based accounting firms could result in our financial statements being determined to not be in compliance with the requirements of the Exchange Act.
Starting in 2011 five China-based accounting firms were affected by a conflict between U.S. and Chinese law. Specifically, for certain U.S.-listed companies operating and audited in China, the SEC and the PCAOB sought to obtain from the Chinese firms access to their audit work papers and related documents. The firms were, however, advised and directed that under Chinese law, they could not respond directly to the U.S. regulators on those requests, and that requests by foreign regulators for access to such papers in China had to be channeled through the China Securities Regulatory Commission, or the CSRC.
In December 2012, the SEC instituted proceedings under Rule 102(e)(1)(iii) of its Rules of Practice and also under the Sarbanes-Oxley Act of 2002 against five China-based accounting firms alleging that these firms had violated U.S. securities laws and the SEC’s rules and regulations thereunder by failing to provide to the SEC the firms’ work papers related to their audits of certain China-based companies that are publicly traded in the U.S. Rule 102(e)(1)(iii) grants the SEC the authority to deny to any person, temporarily or permanently, the ability to practice before the SEC who is found by the SEC, after notice and opportunity for a hearing, to have willfully violated any such laws or rules and regulations. On January 22, 2014, an initial administrative law decision was issued, censuring these accounting firms and suspending four of the five firms from practicing before the SEC for a period of six months. Four of these China-based accounting firms appealed to the SEC against this decision and, on February 6, 2015, each of the four China-based accounting firms agreed to a censure and to pay a fine to the SEC to settle the dispute and avoid suspension of their ability to practice before the SEC. The firms’ ability to continue to serve all their respective customers is not affected by the settlement. The settlement requires the firms to follow detailed procedures to seek to provide the SEC with access to Chinese firms’ audit documents via the CSRC. Under the terms of the settlement, the underlying proceeding against the four China-based accounting firms was deemed dismissed with prejudice four years after entry of the settlement. The four-year mark occurred on February 6, 2019.
If the SEC restarts the administrative proceedings against audit firms with operations in China, depending upon the final outcome, listed companies in the United States with major PRC operations may find it difficult or impossible to retain auditors in respect of their operations in the PRC, which could result in financial statements being determined to not be in compliance with the requirements of the Exchange Act, including possible delisting. Moreover, any negative news about any such future proceedings against these audit firms may cause investor uncertainty regarding China-based, U.S.-listed companies, and the market price of our ordinary shares may be adversely affected.
If prior our independent registered public accounting firm, Ernst & Young, were was denied, even temporarily, the ability to practice before the SEC and we were unable to timely find another registered public accounting firm to audit and issue an opinion on our financial statements for the years ended December 31, 2018 and 2019, our financial statements could be determined not to be in compliance with the requirements of the Exchange Act. Such a determination could ultimately lead to the delisting of the ADSs from the Nasdaq Global Market or deregistration from the SEC, or both, which would substantially reduce or effectively terminate the trading of the ADSs in the United States.
The recent joint statements by the SEC and the PCAOB, proposed rule changes submitted by Nasdaq, and the Holding Foreign Companies Accountable Act (the "HFCAA") all call for the application of additional and more stringent requirements to emerging market companies upon assessing the qualification of their auditors. These developments could add uncertainties to our listing status.
On April 21, 2020, the SEC and the PCAOB issued a new joint statement, reminding the investors that in many emerging markets, including China, there is substantially greater risk that disclosures will be incomplete or misleading and, in the event of investor harm, substantially less access to recourse, in comparison to U.S. domestic companies, and stressing again the PCAOB’s inability to inspect audit work papers in China and its potential harm to investors.
On May 18, 2020, Nasdaq filed three proposals with the SEC to (i) apply minimum offering size requirement for companies primarily operating in “Restrictive Market,” (ii) adopt a new requirement relating to the qualification of management or board of director for Restrictive Market companies, and (iii) apply additional and more stringent criteria to an applicant or listed company based on the qualifications of the company’s auditors.
The lack of PCAOB inspections in China prevents the PCAOB from regularly evaluating audits and its quality control procedures of auditors based in China. As a result, investors may be deprived of the benefits of PCAOB inspections. Inspections of other firms that the PCAOB has conducted outside China have identified deficiencies in those firms’ audit procedures and quality control procedures, which may be addressed as part of the inspection process to improve future audit quality. The inability of the PCAOB to conduct inspections of auditors in China makes it more difficult to evaluate the effectiveness of these auditors’ audit procedures or quality control procedures as compared to auditors outside of China that are subject to PCAOB inspections. Investors may lose confidence in our reported financial information and procedures and the quality of our financial statements.
As part of a continued regulatory focus in the United States on access to audit and other information currently protected by national law, in particular PRC law, on May 20, 2020, the U.S. Senate passed the Holding Foreign Companies Accountable Act, or the HFCAA, which includes requirements similar to those in the EQUITABLE Act requiring the SEC to identify issuers whose audit reports are prepared by auditors that the PCAOB is unable to inspect or investigate because of restrictions imposed by non-U.S. authorities. The HFCAA would also require public companies on the SEC’s list to certify that they are not owned or controlled by a foreign government and make certain additional disclosures on foreign ownership and control of such issuers in their SEC filings. The HFCAA was approved by the U.S. House of Representatives on December 2, 2020 and was signed into law by the U.S. President on December 18, 2020. The HFCAA amended the Sarbanes-Oxley Act of 2002 to require the SEC to prohibit securities of any U.S.-listed companies from being listed on any of the U.S. securities exchanges, such as the New York Stock Exchange, or traded “over-the-counter,” if the registrant’s financial statements have been audited by an accounting firm branch or office that is not subject to PCAOB inspection for a period of three consecutive years after the HFCAA became effective. On March 24, 2021, the SEC adopted interim final rules to implement portions of the HFCAA, and it gave the public a 30-day comment period. As an initial matter, the process of identifying an issuer, which is audited by an auditor not subject to the PCAOB’s full inspections or investigation because of restrictions by non-U.S. authorities, will require coordination with the PCAOB. The PCAOB is currently considering how it will implement the requirements of the HFCA Act, and any PCAOB rulemaking in this respect will be subject to SEC review and approval prior to implementation.
Our auditor, Friedman LLP, the independent registered public accounting firm that issues the audit report for the year ended December 31, 2020 included elsewhere in this Form 20-F, as an auditor of companies that are traded publicly in the United States and a firm registered with the PCAOB, is subject to laws in the United States pursuant to which the PCAOB conducts regular inspections to assess our auditor’s compliance with the applicable professional standards. Our auditor is headquartered in Manhattan, New York, and has been inspected by the PCAOB on a regular basis with the last inspection in June 2018. However, the recent developments would add uncertainties to our listing status and we cannot assure you whether Nasdaq or regulatory authorities would apply additional and more stringent criteria to us after considering the effectiveness of our auditor’s audit procedures and quality control procedures, adequacy of personnel and training, or sufficiency of resources, geographic reach or experience as it relates to the audit of our financial statements.
Risks Relating to the ADSs
The trading price of our ADSs may be volatile regardless of our operating performance.
The trading price of our ADSs could fluctuate widely due to factors beyond our control. This may happen because of broad market and industry factors, including the performance and fluctuation of the market prices of other companies with business operations located mainly in China that have listed their securities in the United States. Furthermore, the stock market in general has experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of companies like us. These broad market and industry factors may materially reduce the market price of our ADSs, regardless of our operating performance. In addition to market and industry factors, the price and trading volume for our ADSs may be highly volatile for factors specific to our own operations, including the following:
· variations in our revenues, earnings and cash flow;
· announcements of new investments, acquisitions, business partnerships or joint ventures by us or our competitors;
· announcements of new test and service offerings, solutions and expansions by us or our competitors;
· failure on our part to realize monetization opportunities as expected;
· changes in financial estimates by securities analysts;
· detrimental adverse publicity about us, our technology, our tests or our industry;
· additions or departures of key personnel;
· release of lock-up or other transfer restrictions on our outstanding equity securities or sales of additional equity securities;
· regulatory developments affecting us or our industry; and
· potential litigation or regulatory investigations.
Any of these factors may result in large and sudden changes in the volume and price at which our ADSs will trade, and you may not be able to sell your shares at prices you deem acceptable. In the past, shareholders of public companies have often brought securities class action suits against those companies following periods of instability in the market price of their securities. If we were involved in a class action suit, it could divert a significant amount of our management’s attention and other resources from our business and operations and require us to incur significant expenses to defend the suit, which could harm our results of operations. Any such class action suit, whether or not successful, could harm our reputation and restrict our ability to raise capital in the future. In addition, if a claim is successfully made against us, we may be required to pay significant damages, which could have a material adverse effect on our financial condition and results of operations.
Our dual-class share structure with different voting rights will limit your ability to influence our corporate matters and could discourage others from pursuing any change of control transactions that holders of our Class A ordinary shares and ADSs may view as beneficial.
As of the date of this annual report, our issued ordinary shares consisted of 9,793,206 Class A ordinary shares (excluding treasury shares and shares reserved for potential conversion of convertible dentures) and 2,863,100 Class B ordinary shares. In respect of matters requiring the votes of shareholders, holders of Class A ordinary shares will be entitled to one (1) vote per share, while holders of Class B ordinary shares will be entitled to ten (10) votes per share. Each Class B ordinary share is convertible into one Class A ordinary share at any time by its holder, while Class A ordinary shares are not convertible into Class B ordinary shares under any circumstances. Upon any sale, transfer, assignment or disposition of Class B ordinary shares by a holder to any person or entity who is not an affiliate of the holder, or upon a change of ultimate beneficial ownership of the holder of any Class B ordinary share to any person or entity who is not an affiliate of the holder, such Class B ordinary shares will be automatically and immediately converted into the same number of Class A ordinary shares. We sold Class A ordinary shares represented by our ADSs in our initial public offering.
All of the issued and outstanding ordinary shares held by Dr. Chris Chang Yu through CRS Holdings Inc. and a portion of our ordinary shares held by Zhangjiang GU KE Company Limited and Zhijun Sihang Holdings Limited, respectively, have been re-designated as Class B ordinary shares. Dr. Chris Chang Yu, Zhangjiang GU KE Company Limited and Zhijun Sihang Holding Limited beneficially owned approximately 60.0%, 11.4% and 8.0%, respectively, of the aggregate voting power of our company as of the date of this annual report, due to the disparate voting powers associated with our dual-class share structure. As a result of the dual-class share structure and the concentration of ownership, these holders of Class B ordinary shares will have considerable influence over matters such as decisions regarding change of directors, mergers, change of control transactions and other significant corporate actions. They may take actions that are not in the best interest of us or our other shareholders. This concentration of ownership may discourage, delay or prevent a change in control of our company, which could have the effect of depriving our other shareholders of the opportunity to receive a premium for their shares as part of a sale of our company and may reduce the price of our ADSs. This concentrated control will limit your ability to influence corporate matters and could discourage others from pursuing any potential merger, takeover or other change of control transactions that holders of Class A ordinary shares and ADSs may view as beneficial.
Share ownership has remained as of the date of this annual report, and will remain, concentrated in the hands of our principal shareholders and management, who are and will continue to be able to exercise a direct or indirect controlling influence on us.
Our directors, officers and current five percent or greater shareholders and affiliated entities together beneficially owned approximately 84.8% of the voting power of our ordinary shares issued and outstanding as of the date of this annual report, and this concentration of share ownership will remain in the foreseeable future. As a result, these shareholders, acting together, have significant influence over all matters that require approval by our shareholders, including the election of directors and approval of significant corporate transactions. Corporate action might be taken even if other shareholders, including those who own our ADSs, oppose them. This concentration of ownership might also have the effect of delaying or preventing a change of control of our company that other shareholders may view as beneficial.
If securities or industry analysts do not publish research about our business, or if they adversely change their recommendations regarding our ADSs, the market price for our ADSs and trading volume could decline.
The trading market for our ADSs will be influenced by research or reports that industry or securities analysts publish about our business. If one or more analysts who cover us downgrade our ADSs, the market price for our ADSs would likely decline. If one or more of these analysts cease to cover us, or fail to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause the market price or trading volume for our ADSs to decline.
Substantial future sales or perceived potential sales of ADSs or ordinary shares, including upon the exercise of vested options and conversion of convertible securities, in the public market could cause the price of ADSs to decline.
Sales of substantial amounts of our ADSs or ordinary shares, including upon the exercise of vested options and conversion of convertible debentures that we have issued, in the public market or at a discount to the market price, or the perception that these sales could occur, could adversely affect the market price of our ADSs and could materially impair our ability to raise capital through offerings of equity securities or securities convertible into or exchangeable for equity securities in the future. The ADSs sold in our initial public offering are freely tradable without restriction or further registration under the Securities Act, and shares held by our existing shareholders may be sold in the public market in the future subject to the restrictions in Rule 144 and Rule 701 under the Securities Act and the applicable lock-up agreements. There were 12,656,306 ordinary shares (including 9,793,206 Class A ordinary shares represented by ADSs and excluding treasury shares and shares reserved for potential conversion of convertible debentures) outstanding as of the date of this annual report. We issued a US$265,500 10% convertible promissory note at a purchase price of US$250,000 in a private placement to an investor in 2020, and the investor converted the total principal and the accrued interest of this note into 54,642 ADSs of our company in February 2021. In addition, we issued on February 5, 2021 US$2.0 million zero coupon convertible debentures due February 4, 2022 at a purchase price of US$1.7 million in a private placement to several investors, subject to certain condition that may increase the rate to 15% per year. The debenture holders may convert the debentures into our ADSs at any time on or prior to maturity at the lower of (i) $15.00, or (ii) the lower of (x) 82% of the closing bid price in the last reported trade of the ADSs or (y) 80% of the VWAPs (daily dollar volume-weighted average price) during the 10 consecutive trading days, immediately preceding the date of conversion or other date of determination (the “Variable Conversion Price”), but not lower than the floor price of $1.00. Subject to the floor price, the Variable Conversion Price will be 75% of the VWAPs during the 10 consecutive trading days, immediately preceding the conversion date or other date of determination if we trigger certain event of default as set forth in the debentures. Our failure to have an effective registration statement covering the resale of the underlying shares prior to April 16, 2021 triggered an event of default under these convertible debentures, which resulted in the outstanding amount under these convertible debentures increasing by 10%. In addition, the conversion rate of the debentures is subject to adjustments under the terms of the debentures. Sales of substantial amounts of ADSs in the public market or the conversion of these convertible debentures, or the perception that these sales or conversions could occur, could adversely affect the market price of our ADSs.
Our memorandum and articles of association contain anti-takeover provisions that could have a material adverse effect on the rights of holders of our ordinary shares and ADSs.
Our memorandum and articles of association (the “M&A”) contain provisions which may have the effect of limiting the ability of others to acquire control of our company or cause us to engage in change-of-control transactions. These provisions could have the effect of depriving our shareholders of an opportunity to sell their shares at a premium over prevailing market prices by discouraging third parties from seeking to obtain control of our company in a tender offer or similar transaction. Our dual-class voting structure gives disproportionate voting power to the holders of our Class A and Class B ordinary shares. Our board of directors has the authority, without further action by our shareholders, to issue preferred shares in one or more series and to fix their designations, powers, preferences, privileges, and relative participating, optional or special rights and the qualifications, limitations or restrictions, including dividend rights, voting rights, terms of redemption and liquidation preferences, any or all of which may be greater than the rights associated with our ordinary shares, in the form of ADS or otherwise. Preferred shares could be issued quickly with terms calculated to delay or prevent a change in control of our company or make removal of management more difficult. If our board of directors decides to issue preferred shares, the price of our ADSs may fall and the voting and other rights of the holders of our ordinary shares and ADSs may be materially and adversely affected.
As we do not expect to pay dividends in the foreseeable future, you must rely on price appreciation of our ADSs for return on your investment.
We currently intend to retain most, if not all, of our available funds and any future earnings to fund the development and growth of our business. As a result, we do not expect to pay any cash dividends in the foreseeable future. Therefore, you should not rely on an investment in our ADSs as a source for any future dividend income. Accordingly, the return on your investment in our ADSs will likely depend entirely upon any future price appreciation of our ADSs. There is no guarantee that our ADSs will appreciate in value or even maintain the price at which you purchased the ADSs. You may not realize a return on your investment in our ADSs and you may even lose your entire investment in our ADSs.
You may not receive dividends or other distributions on our Class A ordinary shares and you may not receive any value for them, if it is illegal or impractical to make them available to you.
The depositary of the ADSs has agreed that if it or the custodian receives any cash dividends or other distributions on Class A ordinary shares or other deposited securities underlying the ADSs, it will pay them to you after deducting its fees and expenses pursuant to the deposit agreement. You will receive these distributions in proportion to the number of Class A ordinary shares your ADSs represent. However, the depositary or the custodian is not responsible if it decides that it is unlawful or impractical to make a distribution available to any holders of ADSs. For example, it would be unlawful to make a distribution to a holder of ADSs if it consists of securities that require registration under the Securities Act of 1933 but that are not properly registered or distributed under an applicable exemption from registration. The depositary may also determine that it is not feasible to distribute certain property through the mail. Additionally, the value of certain distributions may be less than the cost of mailing them. In these cases, the depositary may determine not to distribute such property. We have no obligation to register under U.S. securities laws any ADSs, Class A ordinary shares, rights or other securities received through such distributions. We also have no obligation to take any other action to permit the distribution of ADSs, Class A ordinary shares, rights or anything else to holders of ADSs. This means that you may not receive distributions we make on our Class A ordinary shares or any value for them if it is illegal or impractical for us to make them available to you. These restrictions may cause a material decline in the value of the ADSs.
The voting rights of holders of ADSs are limited by the terms of the deposit agreement, and you may not be able to exercise your right to direct the voting of the underlying Class A ordinary shares which are represented by your ADSs.
Holders of ADSs do not have the same rights as our registered shareholders. As a holder of our ADSs, you do not have any direct right to attend general meetings of our shareholders or to cast any votes at such meetings. You are only able to exercise the voting rights which are carried by the underlying Class A ordinary shares represented by your ADSs indirectly by giving voting instructions to the depositary in accordance with the provisions of the deposit agreement. Under the deposit agreement, you may vote by giving voting instructions to the depositary. If we instruct the depositary to ask for your instructions, then upon receipt of your voting instructions, the depositary will try, as far as is practicable, to vote the underlying Class A ordinary shares represented by your ADSs in accordance with your instructions. If we do not instruct the depositary to ask for your instructions, the depositary may still vote in accordance with instructions you give, but it is not required to do so. You will not be able to directly exercise any right to vote with respect to the underlying Class A ordinary shares represented by your ADSs unless you withdraw the shares, and become the registered holder of such shares prior to the record date for the general meeting. Under our memorandum and articles of association, the minimum notice period required to be given by our company to our registered shareholders to convene a general meeting is seven days. When a general meeting is convened, you may not receive sufficient advance notice of the meeting to withdraw the shares underlying your ADSs and become the registered holder of such shares to allow you to attend the general meeting and to vote directly with respect to any specific matter or resolution to be considered and voted upon at the general meeting. In addition, under our amended and restated articles of association, for the purposes of determining those shareholders who are entitled to attend and vote at any general meeting, our directors may close our register of members and/or fix in advance a record date for such meeting, and such closure of our register of members or the setting of such a record date may prevent you from withdrawing the Class A ordinary shares underlying your ADSs and becoming the registered holder of such shares prior to the record date, so that you would not be able to attend the general meeting or to vote directly. If we instruct the depositary to ask for your instructions, the depositary will notify you of the upcoming vote and will arrange to deliver our voting materials to you. We cannot assure you that you will receive the voting materials in time to ensure that you can instruct the depositary how to vote the underlying Class A ordinary shares represented by your ADSs. In addition, the depositary and its agents are not responsible for failing to carry out voting instructions or for their manner of carrying out your voting instructions. This means that you may not be able to exercise your right to direct how the shares underlying your ADSs are voted and you may have no legal remedy if the shares underlying your ADSs are not voted as you requested.
You may experience dilution of your holdings due to the inability to participate in rights offerings.
We may, from time to time, distribute rights to our shareholders, including rights to acquire securities. Under the deposit agreement, the depositary will not distribute rights to holders of ADSs unless the distribution and sale of rights and the securities to which these rights relate are either exempt from registration under the Securities Act with respect to all holders of ADSs, or are registered under the provisions of the Securities Act. The depositary may, but is not required to, attempt to sell these undistributed rights to third parties, and may allow the rights to lapse. We may be unable to establish an exemption from registration under the Securities Act, and we are under no obligation to file a registration statement with respect to these rights or underlying securities or to endeavor to have a registration statement declared effective. Accordingly, holders of ADSs may be unable to participate in our rights offerings and may experience dilution of their holdings as a result.
You may be subject to limitations on transfer of your ADSs.
Your ADSs are transferable on the books of the depositary. However, the depositary may close its books at any time or from time to time when it deems expedient in connection with the performance of its duties. The depositary may close its books from time to time for a number of reasons, including in connection with corporate events such as a rights offering, during which time the depositary needs to maintain an exact number of ADS holders on its books for a specified period. The depositary may also close its books in emergencies, and on weekends and public holidays. The depositary may refuse to deliver, transfer or register transfers of our ADSs generally when our share register or the books of the depositary are closed, or at any time if we or the depositary thinks that it is advisable to do so because of any requirement of law or of any government or governmental body, or under any provision of the deposit agreement, or for any other reason in accordance with the terms of the deposit agreement. As a result, you may be unable to transfer your ADSs when you wish to.
Your rights to pursue claims against the depositary as a holder of ADSs are limited by the terms of the deposit agreement.
The deposit agreement governing the ADSs representing our Class A ordinary shares provides that, subject to the depositary’s right to require a claim to be submitted to the U.S. federal or state courts in the City of New York have non-exclusive jurisdiction to hear and determine claims arising under the deposit agreement and in that regard, to the fullest extent permitted by law, ADS holders waive the right to a jury trial of any claim they may have against us or the depositary arising out of or relating to our shares, the ADSs or the deposit agreement, including any claim under the U.S. federal securities laws.
If we or the depositary opposed a jury trial demand based on the waiver, the court would determine whether the waiver was enforceable based on the facts and circumstances of that case in accordance with the applicable U.S. state and federal law. To our knowledge, the enforceability of a contractual pre-dispute jury trial waiver in connection with claims arising under the U.S. federal securities laws has not been finally adjudicated by the United States Supreme Court. However, we believe that a contractual pre-dispute jury trial waiver provision is generally enforceable, including under the laws of the State of New York, which govern the deposit agreement. In determining whether to enforce a contractual pre-dispute jury trial waiver provision, courts will generally consider whether a party knowingly, intelligently and voluntarily waived the right to a jury trial. We believe that this is the case with respect to the deposit agreement and the ADSs. It is advisable that you consult legal counsel regarding the jury waiver provision before investing in the ADSs.
If you or any other holders or beneficial owners of ADSs bring a claim against us or the depositary in connection with matters arising under the deposit agreement or the ADSs, including claims under U.S. federal securities laws, you or such other holder or beneficial owner may not be entitled to a jury trial with respect to such claims, which may have the effect of limiting and discouraging lawsuits against us and/or the depositary. If a lawsuit is brought against us and/or the depositary under the deposit agreement, it may be heard only by a judge or justice of the applicable trial court, which would be conducted according to different civil procedures and may result indifferent outcomes than a trial by jury would have had, including results that could be less favorable to the plaintiff(s) in any such action.
Nevertheless, if this jury trial waiver provision is not enforced, to the extent a court action proceeds, it would proceed under the terms of the deposit agreement with a jury trial. No condition, stipulation or provision of the deposit agreement or ADSs serves as a waiver by any holder or beneficial owner of ADSs or by us or the depositary of compliance with any substantive provision of the U.S. federal securities laws and the rules and regulations promulgated thereunder.
We are subject to liability risks stemming from our foreign status, which could make it more difficult for investors to sue or enforce judgments against our company, and the ability of U.S. authorities to bring actions against us or our management may also be limited.
We are a company incorporated under the laws of the British Virgin Islands. We conduct substantially all of our operations in China and substantially all of our assets are located in China, the world’s largest emerging market. In addition, certain of our directors and executive officers reside within China for a significant portion of a year or are PRC nationals and a substantial portion of their assets are within China. As a result, it could be difficult or impossible for you to bring an action against us or against these individuals in the United States in the event that you believe that your rights have been infringed under the United States federal securities laws or otherwise. Even if you are successful in bringing an action of this kind, the laws of the British Virgin Islands and of China may render you unable to enforce a judgment against our assets or the assets of our directors and officers. In addition, due to jurisdictional limitations, matters of comity and various other factors, the SEC, Department of Justice and other U.S. authorities may be limited in their ability to take enforcement actions, including in instances of fraud, against us or our directors and officers in China. In addition, shareholder claims that are common in the United States, including class action securities law and fraud claims, generally uncommon in China. For example, in China, there are significant legal and other obstacles to obtaining information needed for shareholder investigations or litigation outside China or otherwise with respect to foreign entities. Although the local authorities in China may establish a regulatory cooperation mechanism with the securities regulatory authorities of another country or region to implement cross-border supervision and administration, such regulatory cooperation with the securities regulatory authorities in the U.S. have not been efficient in the absence of mutual and practical cooperation mechanism. According to Article 177 of the PRC Securities Law which became effective in March 2020, no overseas securities regulator is allowed to directly conduct investigation or evidence collection activities within the territory of the PRC. Accordingly, without the consent of the competent PRC securities regulators and relevant authorities, no organization or individual may provide the documents and materials relating to securities business activities to overseas parties.
In addition, BVI companies may not have standing to initiate a shareholder derivative action in a federal court of the United States. The circumstances in which any such action may be brought, and the procedures and defenses that may be available in respect to any such action, may result in the rights of shareholders of a BVI company being more limited than those of shareholders of a company organized in the United States. Accordingly, shareholders may have fewer alternatives available to them if they believe that corporate wrongdoing has occurred. For more information, see “Item 10—B. Memorandum and Articles of Association—Differences in Corporate Law—Shareholders’ Suits”. The BVI courts are also unlikely to recognize or enforce against us judgments of courts in the United States based on certain liability provisions of U.S. securities law, and to impose liabilities against us, in original actions brought in the BVI, based on certain liability provisions of U.S. securities laws that are penal in nature. There is no statutory enforcement in the BVI of judgments obtained in the United States, although the courts of the BVI will in certain circumstances recognize such a foreign judgment and treat it as a cause of action in itself which may be sued upon as a debt at common law so that no retrial of the issues would be necessary. This means that even if shareholders were to sue us successfully, they may not be able to recover anything to make up for the losses suffered.
Lastly, under the provisions of the BVI Act, the memorandum and articles of association of a company are binding as between the company and its members and between the members. In general, members are bound by the decision of the majority or special majorities as set out in the articles of association or in the Act. As for voting, the usual rule is that with respect to normal commercial matters members may act from self-interest when exercising the right to vote attached to their shares.
If the majority members have infringed a minority member’s rights, the minority may seek to enforce its rights either by derivative action or by personal action. The BVI Act provides that any member of a company is entitled to payment of the fair value of his shares upon dissenting from certain matters. For more information, see “Item 10—B. Memorandum and Articles of Association—Differences in Corporate Law—Shareholders’ Suits.”
Generally any other claims against a company by its members must be based on the general laws of contract or tort applicable in the BVI or their individual rights as members as established by the company’s memorandum and articles of association, which are more limited than the rights afforded investors under the laws of many states in the United States.
You may have difficulty enforcing judgment against us or our directors and officers.
We are a BVI holding company and most of our assets are located outside of the United States. In addition, certain of our directors and executive officers are residents of the PRC, and substantially all of their assets and our assets are located in the PRC. As a result, you may not be able to effect service of process upon us or these directors and executive officers, or to enforce against them judgments obtained in courts in the United States in the event that you believe that your rights have been infringed under the U.S. federal securities laws or otherwise. Even if you are successful in bringing an action of this kind, the laws of the BVI and of China may render you unable to enforce a judgment against our assets or the assets of our directors and officers.
We will incur increased costs as a result of being a public company, particularly after we cease to qualify as an “emerging growth company.”
We are a public company and expect to incur significant legal, accounting and other expenses that we did not incur as a private company. The Sarbanes-Oxley Act of 2002, as well as rules subsequently implemented by the SEC and the Nasdaq, impose various requirements on the corporate governance practices of public companies. As a company with less than US$1.07 billion in revenues for our last fiscal year, we qualify as an “emerging growth company” pursuant to the JOBS Act. An emerging growth company may take advantage of specified reduced reporting and other requirements that are otherwise applicable generally to public companies. These provisions include exemption from the auditor attestation requirement under Section 404 in the assessment of the emerging growth company’s ICFR. The JOBS Act also permits an emerging growth company to delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have elected to take advantage of such extended transition period for complying with new or revised accounting standards as required when they are adopted for public companies.
We may take advantage of the aforesaid exemptions for so long as we remain an emerging growth company until the fifth anniversary from the date of our initial listing. After we are no longer an “emerging growth company,” we expect to incur significant expenses and devote substantial management effort toward ensuring compliance with the requirements of Section 404 and the other rules and regulations of the SEC. For example, as a result of becoming a public company, we may need to increase the number of independent directors and will need to adopt policies regarding internal controls and disclosure controls and procedures. In addition, operating as a public company makes it more difficult and more expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. In addition, we may incur additional costs associated with our public company reporting requirements. It may also be more difficult for us to find qualified persons to serve on our board of directors or as executive officers. We are currently evaluating and monitoring developments with respect to these rules and regulations, and we cannot predict or estimate with any degree of certainty the amount of additional costs we may incur or the timing of such costs.
As we are a foreign private issuer and are exempt from certain Nasdaq corporate governance standards applicable to U.S. issuers, you have less protection than you would have if we were a domestic issuer.
The Nasdaq listing rules require listed companies to have, among other things, a majority of their board members be independent. As a foreign private issuer, however, we are permitted to, and we will, follow home country practice in lieu of the above requirements. The corporate governance practice in our home country, the BVI, does not require a majority of our board to consist of independent directors. Since a majority of our board of directors will not consist of independent directors, fewer board members may be exercising independent judgment and the level of board oversight on the management of our company may decrease as a result. In addition, the Nasdaq listing rules also require U.S. domestic issuers to have a compensation committee, a nominating/corporate governance committee composed entirely of independent directors, and an audit committee with a minimum of three members each of whom must be an independent director (unless any exception under the Nasdaq listing rules applies). We, as a foreign private issuer, are not subject to these requirements, except for the aforesaid independence requirement for audit committee members (unless any exception under the Nasdaq listing rules applies). The Nasdaq listing rules may require shareholder approval for certain corporate matters, such as requiring that shareholders be given the opportunity to vote on all equity compensation plans and material revisions to those plans, and certain issuances of ordinary shares and securities convertible into or exchangeable for ordinary shares. We are not required to and may not comply with the requirements of the Nasdaq listing rules in determining whether shareholder approval is required on such matters. While we have appointed a compensation committee and a nominating and corporate governance committee, we have followed home country practice to not have all members of our compensation committee and nomination and corporate governance committee composed entirely of independent directors. In addition, we may consider following home country practice in lieu of the requirements under the Nasdaq listing rules with respect to certain other corporate governance standards which may afford less protection to investors.
We are a foreign private issuer within the meaning of the rules under the Exchange Act, and as such we are exempt from certain provisions applicable to United States domestic public companies.
Because we are a foreign private issuer under the Exchange Act, we are exempt from certain provisions of the securities rules and regulations in the United States that are applicable to U.S. domestic issuers, including: (i) the rules under the Exchange Act requiring the filing of quarterly reports on Form 10-Q or current reports on Form 8-K with the SEC; (ii) the sections of the Exchange Act regulating the solicitation of proxies, consents, or authorizations in respect of a security registered under the Exchange Act; (iii) the sections of the Exchange Act requiring insiders to file public reports of their stock ownership and trading activities and liability for insiders who profit from trades made in a short period of time; and (iv) the selective disclosure rules by issuers of material nonpublic information under Regulation FD.
We are required to file an annual report on Form 20-F within four months of the end of each fiscal year. In addition, we intend to publish our results on a quarterly basis through press releases, distributed pursuant to the rules and regulations of Nasdaq Stock Market LLC. Press releases relating to financial results and material events will also be furnished to the SEC on Form 6-K. However, the information we are required to file with or furnish to the SEC will be less extensive and less timely compared to that required to be filed with the SEC by U.S. domestic issuers. As a result, you may not be afforded the same protections or information, which would be made available to you, were you investing in a U.S. domestic issuer.
There can be no assurance that we will not be a passive foreign investment company, or PFIC, for U.S. federal income tax purposes for any taxable year, which could subject U.S. Holders of our Class A ordinary shares or ADSs to adverse U.S. federal income tax consequences.
A non-U.S. corporation will be a PFIC, if, in any particular year, either (i) 75% or more of its gross income for such year consists of certain types of “passive” income or (ii) the average percentage of the value of its assets that produce or are held for the production of passive income, based on the average of four quarterly testing dates, is at least 50% (the “asset test”). Because the PFIC tests must be applied each year, and the composition of our income and assets and the value of our assets may change, it is possible that we may be a PFIC in the current or a future year. In particular, because the value of our assets for purposes of the asset test may be determined by reference to the market price of our ADSs, fluctuations in the market price of our ADSs may cause us to become a PFIC.
If we are a PFIC in any taxable year, a U.S. Holder (as defined in “Taxation—United States Federal Income Tax Considerations”) may incur significantly increased U.S. federal income tax on gain recognized on the sale or other disposition of our Class A ordinary shares or ADSs and on the receipt of distributions on our Class A ordinary shares or ADSs to the extent such gain or distribution is treated as an “excess distribution” under the U.S. federal income tax rules, and such U.S. Holder may be subject to burdensome reporting requirements. Further, if we are a PFIC for any year during which a U.S. Holder holds our Class A ordinary shares or ADSs, we will generally continue to be treated as a PFIC for all subsequent years during which such U.S. Holder holds our Class A ordinary shares or ADSs, unless we cease to be a PFIC and the U.S. Holder makes a special “purging” election on IRS Form 8621. See “Item 10. Additional Information—E. Taxation—United States Federal Income Tax Considerations—Passive Foreign Investment Company Status” for more details regarding the foregoing.
ITEM 4. INFORMATION ON THE COMPANY
A. History and Development of the Company
We began our operations by incorporating AnPac Bio in January 2010 as a BVI business company limited by shares under the BVI Act. AnPac Bio was established primarily as a holding company and has established our operating subsidiaries in China and the United States.
In March 2010, we established Changhe Bio-Medical Technology (Yangzhou) Co., Ltd., or AnPac Yangzhou, as our wholly foreign owned subsidiary in the PRC to market and sell our cancer screening and detection tests and conduct biology related research and development activities.
In March 2011, we established Changwei System Technology (Shanghai) Co., Ltd., or AnPac Changwei, as our wholly foreign owned subsidiary in the PRC as our global research and development center.
In October 2012, we established AnPac Bio-Medical Technology (Lishui) Co., Ltd. or AnPac Lishui, as our wholly foreign owned subsidiary in the PRC as our headquarters and to manufacture our CDA devices.
In October 2013, we established Shanghai Xinshenpai Technology Co., Ltd., or Shanghai Xinshenpai, as our wholly owned subsidiary in the PRC to market and sell our cancer screening and detection tests. In December 2020, we wound up Shanghai Xinshenpai.
In April 2014, we established AnPac Bio-Medical Technology (Shanghai) Co., Ltd., or AnPac Shanghai, as our wholly owned subsidiary in the PRC to market and sell our cancer screening and detection tests.
In September 2015,we established AnPac Technology USA Co., Ltd., or AnPac US, as our wholly owned subsidiary in the United States to conduct research studies and clinical studies for our research on cancer screening and detection tests.
In July 2016, we established Lishui AnPac Medical Laboratory Co., Ltd., or Lishui Laboratory, as our wholly owned subsidiary in the PRC to conduct cancer screening and detection tests.
In November 2017, we established Shiji (Hainan) Medical Technology Limited, or Shiji Hainan, as our wholly owned subsidiary in the PRC, which we acquired from third parties to conduct cancer screening and detection tests.
In May 2018, we established Penghui Health Management (Shanghai) Co., Ltd., or Penghui Health Management, as our wholly owned subsidiary in the PRC to market and sell our cancer screening and detection tests. In December 2020, we wound up Penghui Health Management.
In March 2019, we established Shanghai Muqing AnPac Health Technology Co., Ltd., or Shanghai Muqing, as our 51% owned subsidiary in the PRC to conduct cancer screening and detection tests.
Our principal executive offices are located at 801 Bixing Street, Bihu County, Lishui, Zhejiang Province 323006, People’s Republic of China. Our telephone number at this address is +86-578-2051-666. Our registered office in the BVI is located at the office of Maples Corporate Services (BVI) Limited at Kingston Chambers, P.O. Box 173, Road Town, Tortola, BVI. Our agent for service of process in the United States is AnPac US, located at 2260 Clove Drive, Suite 127, San Jose, CA 95128.
Investors should submit any inquiries to the address and telephone number of our principal executive offices. Our main website is www.anpacbio.com. The information contained on our website is not a part of this annual report.
SEC maintains an Internet site (http://www.sec.gov) that contains reports, proxy and information statements, and other information regarding us that file electronically with the SEC. See “Item 5. Operating and Financial Review and Prospects—B. Liquidity and Capital Resources—Capital Expenditures” for a discussion of our capital expenditures.
B. Business Overview
We are a biotechnology company focusing on early cancer screening and detection. We market and sell a multi-cancer screening and detection test that uses our innovative, patented CDA technology and our proprietary CDA device. In addition to early cancer screening and detection, our CDA technology has demonstrated potential to assist physicians in cancer diagnosis, prognosis and recurrence.
Our CDA technology provides a comprehensive platform, on which we have developed our CDA test and our proprietary CDA device. Our CDA test can detect and assess an individual’s overall cancer risk with high accuracy, including early stage cancer. We also offer combination tests that combine our CDA test with auxiliary tests based on other cancer screening and detection technologies to detect the risk of specific cancer types. We have historically primarily combined our CDA test with the biomarker-based test in our combination tests. We began offering a new combination test product named APCS in the second quarter of 2020, which combines our CDA test with the ct-DNA test. When we refer to our technology or tests as a “cancer screening and detection” technology or test in this annual report, we refer to the detection and assessment of the risk of cancer occurrence, not to cancer diagnosis.
Our CDA technology focuses on biophysical properties in human blood. Recent studies have shown that there is a correlation between certain biophysical properties, including acoustical, electrical, magnetic, nano-mechanical and optical properties, and cancer occurrence. These studies have revealed that biophysical properties could be important non-genetic aspects of the micro-environment regulating the balance between normal cell growth and carcinogenesis (cancerous growth), which may lead to cancer occurrence. Biophysical properties’ physical expressions of information in the blood can indicate risks of pre-cancerous states and cancers. These biophysical signals change over time as cancer occurs, progresses or regresses. Our proprietary CDA device uses an integrated sensor system to detect certain biophysical signals in blood samples. After collecting data on these signals, we use our CDA technology and proprietary algorithm to measure and analyze these signals at multiple biological levels (including the protein, cellular and molecular levels) and with multiple parameters (including the overall CDA value, the PTF value and the CTF value). According to Frost & Sullivan, we are one of the first biotechnology companies worldwide to focus on the detection and measurement of cancers’ biophysical properties. In our industry and related research fields, our CDA technology, as well as CTCs, ct-DNA, exosome, mRNAs and other emerging technologies, are known as “next-generation” cancer screening and detection technologies.
Our CDA technology provides a highly accurate, early-stage risk assessment of the occurrence of cancer. As of December 31, 2020, our CDA technology had been shown in numerous retrospective validation studies to be able to detect the risk of 26 cancer types with high sensitivity and specificity rates. These 26 cancers accounted for over 80% of the cancer incidences in China from 2013 to 2018, according to Frost & Sullivan. Our CDA technology requires only a standard blood sample from a tested individual, which minimizes the inconvenience and invasive procedures and avoids the harmful side effects that are inherent to many other technologies.
We have established a test database that as of March 31, 2021, consisted of over 222,200 blood samples of various age, sex and disease groups. Our database included approximately 178,300 samples from our commercial CDA-based tests and approximately 43,900 samples from our research studies. According to Frost & Sullivan, we ranked third worldwide among companies offering next-generation early cancer screening and detection technologies in terms of the number of clinical samples for cancer screening and detection as of May 2020. For purposes of these rankings, we had approximately 41,700 clinical samples as of May 2020, which represented the historical aggregate number of participants enrolled in our research studies that were developed in clinical sites qualified by competent authorities, such as the NMPA. In addition, among companies offering next-generation early cancer screening and detection technologies in China, in 2019 we ranked first in terms of volume of commercial cancer screening and detection tests conducted, according to Frost & Sullivan.
We have established two clinical laboratories in China and two clinical laboratories in the United States. Our principal laboratory is a licensed biomedical clinical laboratory located in Lishui, Zhejiang Province, China, where we perform our commercial CDA-based tests (including our CDA tests and combination tests), as well as a variety of other tests (including immunological and biochemical tests). Our laboratory in Haikou, Hainan Province, China is a licensed genomics clinical laboratory where we perform gene sequencing tests. In addition to these two clinical laboratories, we also have a research and development center located in Shanghai, China, where we develop our next-generation cancer screening and detection technology and tests. In the United States, we have a California-licensed clinical laboratory located in San Jose, California for which we obtained CAP accreditation and a CLIA Certificate of Accreditation in March 2020. In addition, we obtained a CLIA Certificate of Registration for our new laboratory in Philadelphia, Pennsylvania in August 2020. We have applied for a Pennsylvania state laboratory permit and plan to seek accreditation from CAP for this new laboratory. Both of our laboratories in the U.S. are equipped to perform our CDA tests and biochemical tests. We plan to also conduct COVID-19 antibody tests in these two laboratories. We have entered into research agreements with U.S. universities and academic medical centers, and we are in discussions with other U.S. hospitals, medical institutions, CROs, managed care companies and other health organizations, to conduct research studies on our CDA technology at our San Jose and Philadelphia laboratories. Our Philadelphia laboratory is currently conducting research using the CDA technology and plans to conduct a correlation study with another qualified laboratory to validate a COVID-19 antibody test using Roche’s FDA authorized equipment.
As of March 31, 2021, we had filed 237 patent applications globally; among these, 142 patents had been granted, including 65 in greater China (including eight in Taiwan) and 20 in the United States, and 95 patent applications were pending in China, the United States and other countries and regions. Our patent applications broadly cover apparatus and methods for early stage disease detection, and they strategically encompass important specific embodiments of these apparatus and methods.
We performed our first commercial CDA-based test in China in 2015 and have generated revenue since then. The number of commercial CDA-based tests (inclusive of CDA tests and combination tests) we sold increased significantly from 41,607 in 2018 to 52,428 in 2019, and it decreased to 41,354 in 2020 primarily due to the impact of COVID-19. In mid-2020, we launched two new products, including our ADME immunology test and APCS cancer screening and detection test (which is included in our combination tests). Our revenue from sales of cancer screening and detection tests increased by 8.6% from RMB9.6 million in 2018 to RMB10.4 million in 2019 and further increased by 77.7% from 2019 to RMB18.5 million (US$2.8 million) in 2020. Our total revenues increased by 5.8% from RMB10.3 million in 2018 to RMB10.8 million in 2019 and further increased by 89.1% from RMB10.8 million in 2019 to RMB20.5 million (US$3.1 million) in 2020. In the United States, we plan to commence marketing our CDA test as an LDT in the future.
Our CDA Technology
Our CDA technology provides an innovative and comprehensive platform for us to develop multi-cancer screening and detection tests with high sensitivity, specificity and cost-efficiency.
Principal Mechanism
Focus on Biophysical Properties
Our CDA technology is a liquid-based technology. The critical difference between our CDA technology and other liquid-based cancer screening and detection technologies is that our technology focuses on biophysical properties rather than conventional biochemical or genomic properties. Specifically, our CDA technology is based on the correlations between biophysical properties and cancer occurrence. Recent studies have shown that there is a correlation between certain biophysical properties and cancer occurrence. These studies have revealed that certain biophysical properties could be important non-genetic aspects of the micro-environment regulating the balance between normal cell growth and carcinogenesis (cancerous growth), which may lead to cancer occurrence. Biophysical properties exist in all human beings, including healthy individuals, and the signals they express can be detected before a tumor has formed. Biophysical properties increase or decrease progressively in a statistically significant way from healthy state to non-cancerous disease, pre-cancer disease, early- and late-stage cancer states. The change in biophysical properties is a potential cause for the loss of immunity and increased occurrence of cancer. On the other hand, the strength of biophysical signals expressed by these biophysical properties—which our CDA technology is designed to detect—increase progressively from healthy through late-stage cancer states.
We have collected testing data on 26 types of cancer, including data on biophysical properties measured in multiple serial samples collected from the same person over time and corresponding pathological data. Our proprietary algorithm is based on this database, and it uses the testing data collected by our CDA device to determine the PTF value, CTF value and overall CDA value of a blood sample. The overall CDA value determined through our test factors in the PTF and CTF value, as well as other biophysical property characteristics of the blood sample. The overall CDA value, as the principal parameter for our CDA technology, is proportional to the cancer risk.
Based on the progressive changes of biophysical properties and their signals from healthy through late-stage cancer states, we believe that our CDA technology is ideally suited for early cancer screening and detection, as well as assistance in cancer diagnosis, prognosis and reoccurrence. Through tracking CDA values, we can obtain both static and dynamic (progression) of information on cancer risk.
Multi-level and Multi-parameter
Our CDA technology is designed to analyze biophysical properties that potentially influence body functions at multiple biological levels, including cellular, protein and molecular levels. By comparison, some other liquid-based cancer screening and detection technologies are based on detection signals that exist at only one of the cellular, protein and molecular levels—for example, conventional biomarkers at the protein level and CTCs at the cellular level. As a result of this multi-level analysis, we believe that our CDA technology is more comprehensive and that it can provide more dimensions of information, potentially making it more accurate in detecting cancers.
Our CDA technology quantitatively measures biophysical properties that are collectively possessed by a biological specimen. These properties may vary by health status at the cellular, protein and molecular levels. At the cellular level, biophysical properties may not only change with a cell’s surface properties, but they may also alter when interactions occur between cells (for example, intercellular repulsions and attractions) as well as possibly cell-to-cell signaling. At the protein and molecular levels, certain biophysical properties may modify proteins’ surface phases and structures and affect the molecular mechanism that maintains the nuclear and genomic integrity of normal cells. Shifts and aberrations in these biophysical properties may potentially lead to alterations in cell interactions and possibly affect functioning and replication of DNA. These shifts and aberrations could therefore cause increased mistakes in gene replications and even increased frequency of gene mutations that result in various diseases, including cancer. In addition, different cancers may share certain common biophysical properties, and our CDA technology captures and quantifies the biophysical signals of malignant cells that are in general distinct from those in normal cells. As a result of these measurements, our CDA technology can detect the risk of multiple cancers in one test. In contrast, certain other liquid-based cancer signals only exist at one of the above three levels (cellular, protein or molecular) and normally a specific signal corresponds to only one cancer. For instance, AFP tumor marker, a protein biomarker, is typically used to screen exclusively for liver cancer; and PSA, another protein biomarker, is typically only used to detect prostate cancer.
Our CDA technology, together with our CDA device, deploys various measurement parameters, primarily PTF, CTF and CDA values, by detecting certain biophysical properties in blood. After testing a blood sample, our CDA device generates a series of testing data, including the PTF value, the CTF value and the overall CDA value. The PTF value refers to the measured level of protein cancer-related factor in the blood. The CTF value refers to the measured level of cellular cancer-related factors in the blood. Using our proprietary algorithm, we arrive at the overall CDA value based on the PTF and CTF values, as well as other biophysical property characteristics of the blood. This overall CDA value is the principal analysis parameter that we use to assess an individual’s overall cancer risk. Based on the results of these parameters, we assess the risk of cancer to be low (normal), medium or high.
Analytical Validation
We have conducted numerous research studies on our CDA technology’s utility and accuracy. Since 2015, we have completed 25 research studies on our CDA technology with hospitals and medical institutes in China. Among them, the results of 15 research studies on which we collaborated with five Chinese hospitals and medical institutes have been published at ASCO annual meetings and other medical conferences and in medical journal supplements. We have also completed an additional ten unpublished research studies with nine hospitals and medical institutes in China. As of March 31, 2021, we had tested more than 222,200 blood samples collected from various age, sex and disease groups, including over 178,300 samples from our commercial CDA-based tests and over 43,900 samples from our research studies.
Our research studies have demonstrated that our CDA technology can detect the risk of multiple cancers with high sensitivity and specificity rates. We have used meta-analysis to analyze the resulting data of all completed research studies for a specific cancer type up to December 31, 2020 and calculated our CDA technology’s sensitivity and specificity rates for that cancer type. Meta-analysis is a statistical analysis of a large collection of analysis results from individual studies for the purpose of integrating the findings. The following table sets forth the sensitivity and specificity rates of our CDA technology in detecting 26 cancers based on our completed research studies up to December 31, 2020:
|
Cancer Type |
|
Aggregate |
|
Sensitivity |
|
Specificity |
|
Publication Information(1) |
|
|
Lung Cancer |
|
2,277 |
|
82.4 |
% |
83.0 |
% |
2015 ASCO Annual Meeting, J Clin Oncol 33, e12578, 2015 (co-author: Cancer Hospital of Chinese Academy of Medical Sciences); 2015 Nobel Prize Laureate Summit on Biomedical Sciences (co-authors: Shanghai Changhai Hospital and School of Life Science of Fudan University); 2015 Annual Congress of Chinese Thoracic Society; 2017 ASCO Annual Meeting, J Clin Oncol 35, e23131, 2017 (co-authors: Shanghai Changhai Hospital and School of Life Science of Fudan University); 2019 ASCO Annual Meeting, J Clin Oncol 37, e20673, 2019 (co-authors: Shanghai Changhai Hospital and Lishui Central Hospital) |
|
|
Cerebral Cancer |
|
93 |
|
89.2 |
% |
89.9 |
% |
2019 ASCO Annual Meeting, J Clin Oncol 37, 2019 (suppl; abstr 2040) |
|
|
Nasopharyngeal Cancer |
|
188 |
|
86.6 |
% |
89.1 |
% |
N/A |
|
|
Oral Cancer |
|
60 |
|
78.3 |
% |
90.8 |
% |
N/A |
|
|
Laryngeal Cancer |
|
61 |
|
93.4 |
% |
88.0 |
% |
N/A |
|
|
Thyroid Cancer |
|
39 |
|
100.0 |
% |
83.6 |
% |
N/A |
|
|
Esophageal Cancer |
|
2,253 |
|
85.8 |
% |
93.0 |
% |
2015 ASCO Annual Meeting, J Clin Oncol 33, e15059, 2015(co-author: Shanghai Changhai Hospital); 2015 Nobel Prize Laureate Summit on Biomedical Sciences (co-authors: Shanghai Changhai Hospital and Fudan University Shanghai Cancer Center); 2017 Gastrointestinal cancers Symposium (San Francisco), J Clin Oncol 35, 2017 (suppl 4S; abstract 42) |
|
|
Lymphoma |
|
528 |
|
87.1 |
% |
92.4 |
% |
N/A |
|
|
Breast Cancer |
|
493 |
|
74.6 |
% |
92.2 |
% |
2015 San Antonio Breast Cancer Symposium(10.1200/JCO.2015.33.28_Suppl.13) |
|
|
Liver Cancer |
|
804 |
|
92.3 |
% |
93.2 |
% |
2015 ASCO Annual Meeting, J Clin Oncol 33, e12578, 2015 and e22171, 2015 (co-author: Lishui Central Hospital, the Fifth Affiliated Hospital of Wenzhou Medical University) |
|
|
Bile Duct Cancer |
|
26 |
|
87.5 |
% |
94.0 |
% |
N/A |
|
|
Gallbladder Cancer |
|
28 |
|
100.0 |
% |
63.4 |
% |
N/A |
|
|
Pancreatic Cancer |
|
162 |
|
89.3 |
% |
90.6 |
% |
N/A |
|
|
Gastric Cancer |
|
1,438 |
|
88.7 |
% |
93.8 |
% |
N/A |
|
|
Kidney Cancer |
|
55 |
|
88.9 |
% |
77.7 |
% |
N/A |
|
|
Bladder Cancer |
|
29 |
|
72.4 |
% |
88.3 |
% |
N/A |
|
|
Colon Cancer |
|
884 |
|
89.4 |
% |
91.2 |
% |
2015 ASCO Annual Meeting, J Clin Oncol 33, e12578, 2015 (co-author: Lishui Central Hospital, the Fifth Affiliated Hospital of Wenzhou Medical University); 2017 Gastrointestinal cancers Symposium (San Francisco), J Clin Oncol 35, 2017 (suppl 4S; abstract 564) |
|
|
Rectum Cancer |
|
653 |
|
89.2 |
% |
88.0 |
% |
N/A |
|
|
Duodenal Cancer |
|
32 |
|
84.4 |
% |
87.5 |
% |
N/A |
|
|
Prostatic Cancer |
|
46 |
|
90.7 |
% |
93.2 |
% |
N/A |
|
|
Cervical Cancer |
|
401 |
|
87.0 |
% |
90.2 |
% |
2019 Shenzhen New Horizons in Cancer Research |
|
|
Ovarian Cancer |
|
474 |
|
90.5 |
% |
90.1 |
% |
2019 Shenzhen New Horizons in Cancer Research |
|
|
Uterine Cancer |
|
164 |
|
87.2 |
% |
92.3 |
% |
N/A |
|
|
Leukemia |
|
196 |
|
77.6 |
% |
88.0 |
% |
N/A |
|
|
Bone Cancer |
|
12 |
|
91.7 |
% |
91.0 |
% |
N/A |
|
|
Skin Cancer |
|
18 |
|
88.9 |
% |
93.7 |
% |
N/A |
|
Note:
(1) For each specific cancer type shown in the table above, the references in this column “Publication Information” indicate the medical conferences and medical journal supplements where we have published any research results for that cancer type up to December 31, 2020, while “N/A” means that none of our completed research studies of that cancer type had been published up to December 31, 2020.
Early Cancer Screening and Detection
Research studies
A number of our research partners, including hospitals and medical institutions in China, have validated our CDA technology’s ability to detect the risk of multiple cancers. This validation has been done through their un-blinding of our single- or double-blinded testing results for tested individuals in their institutions. Single-blinded test refers to the testing process in which we do not know, but our research partners know, about the pathological or clinical information of the tested samples or the makeup of the patient and control groups during the course of testing. By comparison, in double-blinded tests, neither us nor our research partners have this information until the un-blinding step. Un-blinding refers to the disclosure of the previously withheld information to us by our research partners in single-blinded tests, or the publication of this information by a third-party study administrator or by our research partners after they otherwise acquire the information. Set forth below are several representative examples of validation studies on our CDA technology that we have completed with Chinese hospitals:
· Shanghai Changhai Hospital
Since 2015, we have cooperated with Shanghai Changhai Hospital to research various cancers, including lung cancer. We have published six papers under this project. The latest paper was published at the 2019 ASCO Annual Meeting. In this study, 832 blood samples collected from patients with non-small cell lung cancer, or NSCLC, and 642 blood samples from healthy individuals (as the control group) were tested using our CDA technology. The results indicated that our CDA technology had good sensitivity and specificity rates even for lung cancer at stage I—85.2% and 93.0%, respectively.
· A Cancer Hospital in Beijing
This hospital is one of the first hospitals that has cooperated with us in conducting research studies. At the 2015 ASCO Annual Meeting, we published a paper evaluating our multi-level, multi-parameter CDA detection method for digestive system cancer diagnosis based on one of our joint research studies with this hospital. Although the sample size was limited, this was the earliest paper comparing our CDA technology with conventional biomarkers.
In this study, the hospital collected blood samples from nine HCC patients and six colorectal cancer patients, as well as from a control group of 20 healthy individuals. These blood samples were tested by both our CDA technology and methods based on conventional biomarkers, including AFP and carcinoembryonic antigen, or CEA. The results showed that there was a significant statistical difference in the measured overall CDA value between each of the HCC and colorectal cancer patient groups and the control group. Specifically, in the HCC group, our CDA technology had a sensitivity rate of 77.0% compared to the AFP-based method’s 33.0%, while the specificity rates of both methods were similar. In the colorectal cancer group, our CDA technology had a sensitivity rate of 83.0% compared to the CEA-based method’s 33.0%, while the specificity rates of both methods were similar.
· Lishui Central Hospital, the Fifth Affiliated Hospital of Wenzhou Medical University
We have collaborated with Lishui Central Hospital, the Fifth Affiliated Hospital of Wenzhou Medical University, or Lishui Central Hospital, primarily in liver and lung cancer studies. We published two papers, one on HCC and one on NSCLC, at the 2015 ASCO Annual Meeting.
In the HCC study, blood samples were collected from 485 HCC patients, 64 cirrhosis patients and 44 patients with benign liver diseases, or BLD, as well as from a control group of 75 healthy individuals. All the samples were tested using our CDA technology. The results indicated that there was a significant statistical difference in the measured overall CDA value between the HCC patient group and each of the control, BLD, and cirrhosis groups.
In the NSCLC study, three groups of blood samples were tested using our CDA technology, which included 383 samples collected from NSCLC patients, 103 samples from patients with non-cancerous lung diseases and a control group of 149 healthy individuals. The results indicated that our CDA technology can detect NSCLC with the sensitivity of 87.7% and specificity of 79.9%.
Follow-up phone consultations
We conduct follow-up phone consultations with individuals for whom we have conducted commercial CDA-based tests, to validate our CDA technology’s utility in detecting the risk of cancer. These individuals were generally asymptomatic at the time they took our tests. We began our first follow-up call in 2017 and plan to do these follow-up phone consultations for five years. We have obtained preliminary results from this initiative.
We typically call a tested individual for the first time within 15 days (for individuals with high risk results), three months (for those with medium risk results) or six months (for those with low risk results), after issuing a cancer risk assessment report for a tested individual. We also have subsequent phone consultations with the tested individuals on an annual basis. During these consultations, our customer support and service personnel typically ask the tested individuals with medium or high risks of cancer about, among other things, their health conditions, whether or not they have taken follow-up checkup tests as we suggested in the cancer risk assessment reports, and the relevant follow-up diagnoses or test results, if any. As of March 31, 2021, we had contacted over 23,857 tested individuals, of whom 14,127 individuals gave us substantive feedback regarding their health conditions and disease development, and among them, 836 were previously tested as having high risk of cancer, 11,328 with medium risk of cancer and the rest with low risk of cancer. Based on the feedback from these calls, 1,928 of the tested individuals had been diagnosed with various major diseases or cancers by third-party hospitals and medical institutions within two years of taking our CDA-based tests, including 209 cases with cancers, 962 with pre-cancer diseases or benign tumors, and 757 with major non-cancerous diseases. All of these 1,928 individuals were previously tested as having medium or high risk of cancer. Among those 836 and 11,328 individuals tested with high and medium risk of cancer, respectively, 209 (or 25.0%) and 1,719 (or 15.2%) had been diagnosed with cancers, pre-cancer diseases or major non-cancerous diseases, respectively. As it may take years for diseases to progress into cancers or pre-cancer or major non-cancerous diseases, we expect that the percentage of cancer occurrence among these 14,127 cases will likely increase over time.
In addition, based a preliminary data analysis by a research partner in early December 2020 of the data of our follow-up phone consultations with over 13,000 individuals as of June 30, 2020, the initial results indicated that over 20 types of pre-cancer diseases were diagnosed at hospitals or physical testing centers following the individuals’ initial screening utilizing our CDA technology. Of the over 13,000 individuals included in the preliminary data analysis, we screened out pre-cancer cases at roughly 4.5 times of cancer cases.
Assistance in Diagnosis, Prognosis and Recurrence
Assistance in diagnosis
Oncologists typically use tissue biopsy as the “gold standard” method for cancer diagnosis, and they also utilize multiple technologies to provide multi-dimensional input to a cancer diagnosis. These technologies can be used for “assistance in diagnosis” because they provide input complementary to pathologic information drawn from a tissue biopsy, which helps physicians to ensure that their cancer diagnoses are comprehensive and unbiased. For example, a CT scan, in conjunction with the detection of CEA and other tumor markers, is often used to assist in diagnosing lung cancer.
Since 2015, we have collaborated with third-party oncologists and hospitals in utilizing our CDA technology to assist in the diagnosis of multiple cancer types in a number of research studies. These research studies are designed to evaluate the performance of our CDA technology in predicting cancer occurrence in a population with cancer symptoms or abnormal test results. To date, ten of these studies have been published at ASCO annual meetings and other medical conferences and medical journal supplements. The results of these studies demonstrated our CDA technology’s effectiveness in assisting in the diagnosis of multiple cancers—particularly lung and esophageal cancers. For example, in our joint study on NSCLC with Shanghai Changhai Hospital in 2017 (2017 ASCO Annual Meeting; J Clin Oncol 35, e23131, 2017), our CDA technology successfully detected NSCLC with sensitivity of 68.7%, higher than those of CT scans for all NSCLC stage groups. This indicates that compared to a CT scan, our CDA test provides more accurate and reliable diagnostic information and data for oncologists in diagnosing lung cancer.
In another study with Shanghai Changhai Hospital in 2015 (2015 ASCO Annual Meeting; J Clin Oncol 33, e15059, 2015), our CDA technology detected esophageal cancer with relatively high sensitivity of 70.0% and specificity of 90.0%. These results indicated our CDA technology’s effectiveness in assisting in the diagnosis of esophageal cancer.
Prognosis and recurrence
Prognosis refers to an assessment of whether and how a patient responds to cancer treatment. Effective prognostic tools can help oncologists dynamically monitor cancer treatment progression, make necessary and timely adjustments to cancer treatment, and correctly predict a patient’s treatment outcome, such as the survival rate—the percentage of people in a patient group who will be alive for a period of time, the survival time—life expectancy after diagnosis, and whether or not they will go into remission. In some circumstances, prognosis can be effective even before the cancer treatment starts. Recurrence means return of cancer after the patient has been treated and has gone into remission, and happens more frequently for certain cancer types. Patients who have gone into remission have a substantially higher risk of cancer recurrence than the general population. It is therefore important to have technologies to detect cancer recurrence timely, cost-effectively and without side effects. Because biophysical properties in the blood increase or decrease progressively in a statistically significant way from healthy state to late-stage cancer states, we believe that our CDA technology can be used for prognosis of cancer treatment outcomes and for detecting the risk of cancer recurrence.
In a study published at the 2016 ASCO Annual Meeting (2016 ASCO Annual Meeting, J Clin Oncol 34, 2016 (suppl; abstr e23176)), we investigated our CDA technology’s potential for breast cancer prognosis by testing the blood samples collected from three breast cancer patients. The CDA data for each patient’s blood samples were grouped into three categories, namely before, during and after any post-operative treatment. Two of these patients showed favorable responses to the post-operative treatment and their average overall CDA values declined after the treatment. The third patient did not respond well to the post-operative treatment and their average overall CDA values remained high after the treatment. These results indicated that our CDA technology may be useful for monitoring a breast cancer patient’s response to the post-operative treatment, although this utility of our CDA technology needs more validation studies.
Since 2015, we have been working with multiple hospitals in China, including Shanghai Changhai Hospital, Lishui Central Hospital, a cancer hospital in Beijing and a cancer center in Shanghai, in a number of research studies. These studies are designed to explore our CDA technology’s effectiveness as a prognostic tool for lung cancer treatment.
In one of these studies in 2016, we collaborated with Shanghai Changhai Hospital and tested and collected the overall CDA values from 86 lung cancer patients. These patients were divided into two groups: the “good prognosis” group (with each member having an overall CDA value below 47) and the “bad prognosis” group (those with values above 47). We predicted that the “good prognosis” group would have a higher survival rate than that of the “bad prognosis” group due to their relatively low overall CDA values. After the grouping, both groups went through chemotherapy to treat their lung cancers. Two years after the chemotherapy, the survival rate of the “bad prognosis” group dropped below 50%, while that of the “good prognosis” group stayed at the level of 75%. The differences in those two outcomes are statistically significant and meaningful. The results of this clinical study demonstrate our CDA technology’s strong ability in predicting the outcome of lung cancer treatment and validate that it can predict treatment outcomes even before the treatment starts.
The following graph provides a comparison of the predicted progression-free survival rates (the percentage of the measured population that did not demonstrate worsening in their condition over a specified period), or PFS, for those two lung cancer patient groups in this study.
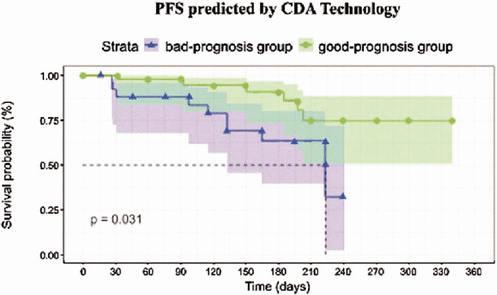
In another study, we tracked a number of patients throughout their approximately three years of cancer treatment. The following graph illustrates the changes of a representative patient’s CDA values throughout the tracking period.
CDA in Long-Term Cancer Monitoring (Stage IIA with Surgery)
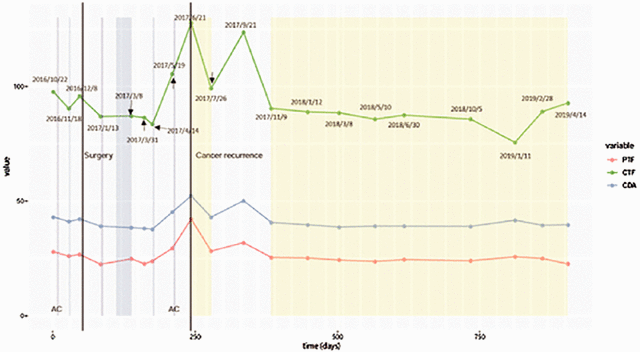
This patient is a middle-aged man diagnosed with a stage IIA lung cancer. As illustrated in the graph above:
· At the beginning of the tracking period, namely Day zero, the patient’s overall CDA value was relatively high, which corroborated the oncologist’s diagnosis that the individual had a cancer;
· From Day 7 to Day 28, as the cancer treatment progressed, the patient’s overall CDA value, as well as PTF and CTF values, continued dropping;
· After his surgery (around Day 52) and during his chemo-therapy treatment, the patient’s overall CDA value dropped below the cut-off value, indicating that by that time, the patient’s stage IIA lung cancer had been effectively controlled and he went into remission;
· However, after a period of remission (around Day 212), the patient’s overall CDA value went up again, which predicted a recurrence of cancer. Shortly after this uptick in the overall CDA value, the oncologists diagnosed that the patient’s cancer had come back and further spread to the liver, corroborating our CDA test’s prediction;
· Subsequently, the patient went through chemotherapy for liver cancer. Following this treatment (around Day 277), the patient showed an overall CDA value below the cut-off value, indicating that the patient responded positively to the chemotherapy and went into remission again; and
· From Day 383 to Day 904, the patient’s overall CDA value, as well as PTF and CTF values, remained relatively low, indicating that he was in remission. This was also confirmed by the oncologists’ clinical observations.
To summarize, this representative example has shown that our CDA test can (i) dynamically monitor a patient’s treatment progression, indicating when the cancer is under control (namely, when the overall CDA value drops below the cut-off value) and when the patient enters the remission phase (namely, after the overall CDA value stays below the cut-off value for a period of time); and (ii) correctly predict cancer recurrence ahead of time (namely, when the overall CDA value resurges and exceeds the cut-off value).
Our CDA Device
Our proprietary CDA device, which we designed in-house and is covered by numerous patents, is used to conduct cancer screening and detection tests based on our proprietary CDA technology. This device uses an integrated, multi-level and multi-parameter sensor system to detect multiple biophysical properties in one single blood test. We believe that we are one of the first biotechnology companies worldwide to use such a sensor system to detect cancers’ biophysical properties.
Working Mechanism
Our CDA device consists of a blood sample input unit, a sample transport unit, a sample mixing chamber, a testing unit and a data storage unit. Because our CDA technology detects biophysical properties, our CDA device’s sensors play a dominant role in biophysical signal detection.
Our CDA device uses a microfluidic device, which is connected to a fluid delivery line inside the testing unit. This microfluidic device contains three primary components: micro-channels, micro-sensors and measurement instruments with automated data recording capabilities. After a blood sample goes into the micro-channels of the microfluidic device, the sensors will probe the blood and measure the relevant data. The measurement instrument that interfaces with the sensors applies a constant input to the blood and records the corresponding biophysical responses as a function of time. The resulting raw data contains both dynamic and static information, which is fed into our proprietary algorithm for further analysis.
Our CDA device is much less costly to manufacture than the equipment used by many of our competitors, especially the complex and expensive gene sequencing machines used in ct-DNA-based tests and micro-electrical mechanical devices used in CTC-based tests. As a result, we can offer our customers cancer screening and detection tests with high accuracy at prices significantly lower than many of our competitors’ tests.
Operation
Our CDA device is a fully-automated system requiring minimal human involvement. After collecting blood samples from the individuals, all our testing personnel needs to do is to properly place these blood samples on the test-tube racks and station the racks inside the sample input unit of our device. Our device will then automatically complete the subsequent test as programmed, including:
· heating the blood samples to prepare them for testing;
· deploying multiple sensors inside the microfluidic device to detect relevant biophysical properties in each blood sample and obtain multi-level information;
· discharging the tested blood samples and cleaning the used test tubes; and
· transferring the testing data collected by the microfluidic device (including PTF and CTF values) to the computer connected to our CDA device, which will process this testing data with our proprietary algorithm and convert it into an overall CDA value. A series of CDA itemized values will also be generated, if we conduct biomarker-based tests in combination with our CDA test while offering our cancer-positioning services.
Based on the resulting CDA values, our professionals can assess a tested individual’s likelihood of having or developing cancers and issue the corresponding cancer risk assessment report.
We design and configure all the key components of our CDA device and outsource production of these components to a number of qualified contract manufacturers. We assemble these components into our CDA devices in-house. We have implemented a strict selection process for our contract manufacturers and evaluate our contract manufacturers’ qualifications on an ongoing basis. We do not disproportionately rely on any particular contract manufacturer and have not entered into any long-term or exclusive supply contract with any of them. For our CDA device, we obtained a Class II medical device manufacture license in June 2013 (renewed in 2018) and a Class II medical device registration certificate April 2015 from the NMPA, Zhejiang Branch. These licenses, along with our clinical laboratory license, allow us to manufacture our device in Lishui, Zhejiang and use the device commercially in our licensed clinical laboratories in China. While conducting the final assembly, testing and packaging of our devices at our plant in Lishui, Zhejiang Province, we thoroughly inspect the key components of our devices sourced from contract manufacturers and closely follow applicable PRC regulations and recognized international quality control standards.
Our CDA-based Tests
Unlike conventional cancer screening and detection approaches such as imaging technology and tissue biopsy, our CDA test uses liquid-based technology to detect the risk of cancer and non-cancerous diseases based on our CDA technology. It is minimally invasive, side effect-free and highly automated. Because it focuses on changes in cancer-related biophysical properties as a disease progresses, we believe that our CDA test can be used for multiple purposes, including early cancer screening and detection, as well as assistance in cancer diagnosis, prognosis and recurrence.
We maintain a comprehensive and flexible test menu to meet different customers’ needs. Our CDA test can detect and assess an individual’s overall risk of having or developing cancer, and we deliver a cancer risk assessment report as the final product of this test. This report presents the analytical parameters that our CDA test uses, including the PTF, CTF and overall CDA values. We set cut-off values for the PTF, CTF and overall CDA values based on the pathological data from our retrospective validation studies and the intended cancer screening and detection objectives. PTF or CTF values in excess of the specified cut-off values indicate a risk of cancer. In addition, we set two cut-offs to divide the overall CDA value into three categories: low risk (healthy), medium risk and high risk. These values, collectively, indicate a tested individual’s overall risk level of having or developing cancer, without identifying the specific types of cancer that the individual may have. For tested individuals with medium or high cancer risks as indicated by the overall CDA value, we normally suggest in our reports that they get follow-up medical examinations on the relevant organs.
In addition to our CDA test, a tested individual can pay a premium for our combination tests, which also include cancer-positioning services to identify the specific type(s) of cancer that he or she has a medium or high risk of having or developing. Our combination tests combine our CDA tests and, on an auxiliary basis, biomarker-based or ct-DNA cancer screening and detection tests performed either by us or by third-party clinical laboratories that we engage. These combination tests typically use two cubic centimeters of blood from the tested individual to perform our CDA test, three cubic centimeters of blood to perform the biomarker-based test and ten cubic centimeters of blood to perform the ct-DNA test. In the combination tests our CDA technology plays a dominant role in identifying the risk of cancer, while biomarkers or ct-DNA provide auxiliary information on the types of cancer that may be involved. We integrate the results of these separate tests using our proprietary algorithm and translate them into a series of itemized CDA values. We then analyze these itemized CDA values to identify the cancer type(s) that a tested individual has a medium or high risk of having or developing. These identified cancer types and the tested individual’s corresponding risk levels of having or developing them will also be included in that individual’s cancer risk assessment report.
We offer standardized CDA-based tests (with or without cancer positioning services). Generally, the more cancer types a standardized test with cancer positioning services can identify, the higher it is priced. In each standardized test with cancer-positioning services, the specific cancer types that can be identified vary between males and females. For instance, our popular CDA six-cancer test with positioning services identifies lung, liver, stomach and colon cancers for both genders, as well as rectal and prostate cancers for males and breast and ovarian cancers for females.
Commercialization
China
In China, we have established clinical laboratories in Lishui, Zhejiang Province and Haikou, Hainan Province. We obtained the medical institutional practice license from the NHC in 2016 and 2015, respectively, for these two laboratories to conduct medical tests, each for a five-year term. Our Lishui laboratory conducts substantially all of our commercial CDA-based tests (including our CDA tests and combination tests), as well as a variety of other tests (including immunology and biochemical tests). In 2020, we launched our ADME immunology test and APCS cancer screening and detection test (which combines our CDA test with the ct-DNA test and is a type of combination test). Both of these new tests are conducted at our Lishui laboratory. We performed our first commercial CDA-based test in 2015 and have generated revenue in China for four consecutive years. The number of our commercial CDA-based tests we sold increased significantly from 41,607 in 2018 to 52,428 in 2019 and decreased to 41,354 in 2020.
In addition to our CDA-based tests, we design annual physical checkup plans for certain of our corporate and life insurance company customers as value-added services and to facilitate these customers to procure physical checkup services from third-party physical checkup service providers. We also sell annual physical checkup packages to our customers, which are designed to include our CDA-based tests as part of the physical checkup services. We outsource a substantial portion of these checkup services in these packages to qualified physical checkup institutions. As of December 31, 2020, we had completed total sales of over 27,006 physical checkup packages.
We have been piloting our genomics tests in our Haikou laboratory operated by our subsidiary Shiji Hainan, which we acquired in November 2017. Our genomics tests primarily consist of genetic testing for the purpose of targeted therapy selection and pharmacogenomics, and ct-DNA mutation testing for multiple purposes, including early cancer screening and detection and prognosis.
Supported by our diverse tests and services, we intend to further expand our customer base in China. To achieve this objective, we plan to market our tests to Chinese hospitals. In December 2018, we applied to the NMPA for a Class III medical device registration certificate for our CDA device to assist in multi-cancer diagnosis. We expect that it would take us at least three years to obtain this registration certificate. After we obtain this license, we will apply to update our medical device manufacture license to include the manufacture of Class III medical devices. With these Class III medical device licenses, we will be permitted to place our devices within Chinese hospitals’ laboratories to conduct commercial tests there or sell our devices to the hospitals for the purposes of assisting in physicians’ diagnosis of specified multiple cancers. We expect our business in China to expand substantially following the commencement of this commercial cooperation with Chinese hospitals.
United States
In the United States, we have established two clinical laboratories in San Jose, California and Philadelphia, Pennsylvania. We have obtained a California state laboratory license, CAP accreditation, and a CLIA Certificate of Accreditation for our laboratory in San Jose, California. We have obtained a CLIA Certificate of Registration for our laboratory in Philadelphia, Pennsylvania. We have applied for a Pennsylvania state laboratory permit and will seek to obtain CAP accreditation for this laboratory. We currently are permitted to conduct our CDA test for research use in the United States. We have entered into research agreements with U.S. universities and academic medical centers, and we are in discussions with U.S. hospitals, medical institutions, CROs, managed care companies and other health organizations, to conduct research studies on our CDA technology in the U.S. To commercialize our CDA test in the United States, we intend to initially market it to U.S. customers as an LDT. As an LDT, we do not expect that our CDA test will require premarket clearance, market authorization, or approval from the FDA prior to marketing. We may begin marketing our test as soon as we complete our validation studies and obtain any state laboratory licenses or other approvals that we are required to hold in order to offer our CDA test in the corresponding states. Under CLIA, CAP, and state licensing requirements, we are required to validate our CDA test with analytical and clinical studies prior to marketing the test as an LDT. These studies are designed to demonstrate the performance of the test. We have entered into research agreements with U.S. universities and academic medical centers, and we are in discussions with other health organizations, to conduct these studies.
After we complete our validation studies for the CDA test, we will be able to market our CDA test following the completion of an administrative process to update our test menu with the CAP, CLIA, and those states in which we are required to hold state laboratory licenses (with the exception of New York State). Assuming that our CDA test falls within one of the disciplines included in the CAP accreditation and CLIA certification that our San Jose laboratory has already received, after we complete our validation studies for the CDA test, we will be able to update our test menu with the CAP electronically, and then immediately offer our CDA test in those states that do not require us to hold state laboratory licenses. In those states where we are required to hold state laboratory licenses, we will need to submit applications to update our test menu. The timeline for these updates is uncertain and will likely depend on the number of applications received by each state at any particular time. Upon completion of this process, we will be able to offer our CDA test throughout the U.S. with the exception of New York State. For more information about the state laboratory license for New York State and its application process, see “Item 4. Information on the Company—B. Business Overview—U.S. Regulations—Federal and State Laboratory Licensing Requirements.”
In addition, we have validated a COVID-19 antibody test using Roche’s FDA authorized equipment but we have not begun to commercialize the test.
Research and Development
The development of our CDA technology and device (together with our proprietary algorithm) is largely attributable to our integrated research and development team that comprises talent from both China and the United States. In our research and development center based in Shanghai, we conduct various ongoing research studies on our CDA technology and continue to improve our CDA device.
We believe that our research and development team possesses industry-leading expertise in the early cancer screening and detection field. As of December 31, 2020, this team had 23 members, including four with M.D. degrees and three with Ph.D. degrees. Our research and development team has a multi-disciplinary background, and most members of this team specialize in areas related or helpful to the development of our CDA technology and device, including mechatronics, physics, biomedical science or computer science. Our founder and chairman, Dr. Chris Chang Yu, our vice president in charge of R&D, Mr. Xuedong Du, and our chief medical officer, Dr. He Yu, have led our research and development team since our inception, leveraging their multi-disciplinary expertise and industry experience. These key members have spearheaded our research and development team in achieving a number of technological breakthroughs, including the design and fabrication of the microfluidic device—the key functioning component of our CDA device—and the testing of multiple cancers in a single blood test. Since 2015, our research and development team had published 15 articles on ASCO and other medical conferences and medical journal supplements to demonstrate our CDA technology’s clinical utility.
We have invested significantly in research and development since our inception. Our research and development expenses were RMB10.1 million, RMB9.8 million and RMB11.6 million (US$1.8 million) in 2018, 2019 and 2020, respectively.
Our Ongoing Research Studies on CDA Technology
In recent years, we have collaborated with a number of Chinese hospitals and medical institutions in conducting clinical studies on our CDA technology. These collaborations have enabled us to validate the effectiveness and utility of our CDA-based test in a clinical setting, explore new applications of our CDA technology, and provide us access to clinically well-characterized patient data. In addition, we have entered into research agreements with U.S. universities and academic medical centers, and we are in discussions with other U.S. hospitals, medical institutions, CROs, managed care companies and other health organizations, to conduct research studies on our CDA technology in the United States. Currently, our ongoing clinical studies on our CDA technology mainly focus on: (i) improving our CDA technology’s utility in detecting early-stage cancers with high incidences in China and the United States, as well as certain cancer types that have been considered difficult for liquid-based technology to detect; (ii) exploring this technology’s potential to dynamically monitor cancer progression and for assistance in cancer diagnosis, prognosis and recurrence; (iii) expanding this technology’s application to different oncological areas, including veterinary cancer screening and detection; and (iv) validating this technology’s ability to detect the risk of major non-cancerous diseases. The following table summarizes our ongoing research studies on CDA technology.
|
Commencement Date |
|
Research Partner |
|
Cancer Type |
|
Estimated |
|
Study Purpose |
|
|
September 2019 |
|
University of Pittsburgh Medical Center |
|
esophageal cancer |
|
100 |
|
for early cancer screening and detection |
|
|
August 2019 |
|
University of Pittsburgh Medical Center |
|
gynecologic cancers |
|
40 |
|
for early cancer screening and detection |
|
|
May 2019 |
|
A university in Shanghai |
|
multiple cancers (with no specification of cancer types) |
|
15,000 |
|
for early cancer screening and detection, as well as assistance in diagnosis, prognosis and recurrence |
|
|
July 2017 |
|
A cancer center in Shanghai |
|
multiple cancers (with no specification of cancer types) |
|
200 |
|
for early cancer screening and detection |
|
|
July 2017 |
|
University of California, Davis |
|
sarcoma and carcinoma cancer |
|
186 |
|
for CDA technology’s application to canine cancer areas |
|
|
May 2017 |
|
Shanghai Changhai Hospital |
|
lung and esophageal cancer |
|
5,000 |
|
for early cancer screening and detection |
|
|
May 2017 |
|
A hospital in Shanghai |
|
lung, colorectal, gastric, breast and pancreatic cancers |
|
1,600 |
|
for assistance in diagnosis, prognosis and recurrence, as well as early cancer screening and detection |
|
These ongoing research studies can be categorized into the following three groups by study purpose:
Studies for Early Cancer Screening and Detection
Our current ongoing research studies in collaboration with Shanghai Changhai Hospital are based on our research agreement dated April 2017. These research studies are designed to validate our CDA technology for the screening and detection of early-stage lung and esophageal cancers. According to Frost & Sullivan, in 2018 there were approximately 867,500 and 271,600 new incidences of lung cancer and esophageal cancer in China, respectively, and lung cancer ranked first among the five most frequent cancers in China. These two cancers are also generally considered difficult for liquid-based technologies to detect with high accuracy, according to Frost & Sullivan. In this project, Shanghai Changhai Hospital is required to provide us with approximately 5,000 blood samples for research studies. Certain preliminary published testing results have shown that our CDA technology can detect the risk of NSCLC with a sensitivity rate of 85.2% and a specificity rate of 93.0% (2019 ASCO Annual Meeting; J Clin Oncol 37, e20673, 2019).
We and a cancer center in Shanghai executed a research project agreement in July 2017. In this ongoing research project, this cancer center is required to provide us with approximately 200 blood samples for the research study to validate our CDA technology’s ability to detect the risk of multiple cancer types. These cancer types include certain cancers that are generally considered difficult for liquid-based technologies to detect, such as esophageal cancer.
We also entered into a research project agreement with a university in Shanghai in May 2019. In this ongoing research project, this university will provide us with approximately 15,000 blood samples for our research studies for multiple purposes, including early cancer screening and detection of multiple cancer types (including lung and esophageal cancers), as well as assistance in diagnosis, prognosis and recurrence.
In addition, in August 2019, we and University of Pittsburgh Medical Center entered into two research agreements. Under the first of these agreements, we retained this university to perform a retrospective, blinded research study to validate our CDA technology for gynecologic cancer screening. This university is required to provide us with at least 20 samples from healthy women and at least 20 samples from ovarian cancer patients for the research study. Under the second agreement, we retained the university to conduct a single-blind research study to validate our CDA technology for esophageal cancer screening. This university is required to provide us with 50 samples for the control group and 50 samples from cancer patients for the research study.
Studies for Assistance in Diagnosis, Prognosis and Recurrence
Since May 2017, we have been working with a hospital in Shanghai on a research study on our CDA technology primarily for assistance in diagnosis, prognosis and recurrence. Under this ongoing study, this hospital is expected to provide us with approximately 1,600 blood samples. These blood samples are collected from patients diagnosed with different subtypes of lung, colorectal, gastric, breast and pancreatic cancers and at different stages of cancer development. By analyzing the pre- and post-treatment CDA values of these patients, we have found correlations between the changes in a patient’s CDA values and the cancer treatment that the patient has received.
Studies for CDA Technology’s Application to Different Oncological Areas
We have been collaborating with the Department of Veterinary Medicine of the University of California, Davis in a study on early cancer screening for canines. Through this study, we plan to expand the application of our CDA technology to veterinary cancer screening and detection.
Studies for Major Non-Cancerous Disease Detection
In addition to the above ongoing studies on our CDA technology’s applications in oncological areas, we are also conducting research on our CDA technology’s ability to detect the risk of pre-cancer diseases and various major non-cancerous diseases, including lung diseases (such as pneumonia and tuberculosis), type II diabetes, heart diseases (such as heart failure and arrhythmia), liver diseases (such as cirrhosis and hepatitis), gastric diseases (such as gastritis and gastric polyp) and biliary diseases (such as calculus of bile duct and cholecystolithiasis). Our preliminary research studies indicate that our CDA technology is able to distinguish individuals with some major non-cancerous diseases from the control group and the cancer group. More studies and further analysis of the study results are needed to validate our findings on our CDA technology’s utility in these major non-cancer areas.
Our Research on Improving our CDA Device
We have conducted substantial research to increase the operational efficiency of our CDA device and, in turn, improve our CDA test’s signal-to-noise ratio to further elevate its accuracy. Our current research in this aspect primarily focuses on enabling our device to improve our CDA technology’s ability to identify cancer types, our CDA technology’s signal-to-noise ratio and its testing throughput.
Sales and Marketing
We currently sell our cancer screening and detection tests only in China. We sell our tests primarily to our customers directly, as well as through our sales agents such as health management companies and medical device dealers. We select our sales agents based on their reputation, market coverage, sales experience and the size of their sales force, and we generally conduct credit assessments of our sales agents.
We set the prices of our tests primarily based on the numbers of cancers that they test. However, we do not set the resale prices for our tests, which our sales agents typically have the sole discretion to determine. We typically give our corporate customers and sales agents a credit term of one to three months for the payments.
Our marketing is focused on expanding the market awareness of our cancer screening and detection test and continuously growing our customer base. We primarily deploy our own sales and marketing personnel to market our tests. As of December 31, 2020, we had 18 sales and marketing personnel. In addition to conducting direct sales to our existing customers, our sales and marketing personnel prepare and deliver our brochures and product presentations to potential customers and attend academic conferences and industrial exhibitions to advertise our CDA technology and tests. Our sales and marketing personnel are generally well trained and educated about the complexities of our tests, and they typically have extensive experience in the cancer early screening and detection field or other medical areas. As our business grows, we plan to build up our sales and marketing team and strengthen our own sales network in China.
We also use sales agents to promote our tests. By referring our tests to their customers and inviting us to deliver product presentations at their promotional events, our sales agents have connected us with their quality customers and enabled us to utilize their network resources for marketing purpose.
Our Customers
We believe that our cancer screening and detection tests have significant market potential in China, as there is strong demand among China’s large, aging population for early cancer screening and detection services. Our existing customer base in China consists primarily of life insurance companies and other large corporations. Generally, they are frequent and high-volume users of our cancer screening and detection tests, because they provide our tests to their individual customers as value-added services or to their employees as benefits. While the majority of our sales has come from our direct sales to our customers, we expect that a significant portion of our sales will continue to be generated through our sales agents.
We believe our customer base provides a meaningful opportunity for our further growth. In addition, we believe an expansion in our customer base will encourage the market acceptance of our CDA technology and raise the public’s awareness of our brand. We plan to acquire additional customers for our CDA-based tests through the annual physical checkup packages we offer. In addition, we plan to further develop our non-CDA cancer screening and detection tests using other technologies, including expanding the genomics tests we currently conduct at our Haikou laboratory. After obtaining the Class III medical device registration certificate and updating our medical device manufacture license, we expect to provide our tests to more individual customers through Chinese hospitals.
Customer Support and Service
We maintain a dedicated team to provide customer support and service for our CDA-based tests. This Shanghai-based team is primarily responsible for operating our service hotline to answer customers’ questions regarding their test results and our cancer risk assessments. In addition, this team periodically conducts follow-up phone consultations with the tested individuals to check their current health conditions, diagnosis results and disease development. These consultations provide us valuable feedback to validate our CDA technology utility in detecting the risk of cancer.
Supply Chain and Quality Control
We devote significant attention to ensuring the accuracy and reliability of our cancer screening and detection tests. We have established a comprehensive quality control system for our tests in accordance with applicable PRC regulations and recognized international quality control standards.
Blood samples for our commercial CDA-based tests are typically delivered to us by a third-party commercial courier. We have also engaged third-party nursing service providers to collect blood samples on our behalf for our commercial cancer screening and detection tests. These service providers are generally responsible for any physical harm caused by the nurses to the tested individuals during the blood collection process. In addition, our research partners are responsible for collecting and delivering blood samples for our research studies. As the quality of blood samples directly affects the accuracy of our tests, we have designed a set of standardized blood sample collection and delivery procedures, including those for sample labeling, preservation and transportation. We require the commercial courier company, nurses and our research partners to follow these standardized procedures to minimize the risks of human errors and sample contamination. During the testing process, we strictly control the temperature and humidity in our laboratories. We carefully preserve the blood samples in a temperature-controlled environment. We also use control samples to ensure that our tests are properly performed and the test results are reliable. After the testing process, our designated personnel will verify the testing results before issuing the cancer risk assessment reports to our customers. In addition, because our CDA technology focuses on biophysical signals, our blood samples can remain stable for testing purpose for up to seven days.
We use a relatively small amount of reagents in our biomarker-based cancer screening and detection tests, which are part of our combination tests. We source these reagents from two third-party suppliers. We do not have an exclusive supply agreement with the supplier. The supplier typically engages commercial courier services to deliver the reagents. In addition, we outsourced substantially all the biomarker-based tests in 2017 and 2018 to two third-party clinical laboratories on a non-exclusive basis. These two laboratories are responsible for conducting the biomarker-based tests and delivering the test results to us for our data consolidation using our algorithm. These two laboratories are obligated to keep confidential all documents relating to the tested samples and the test results. We phased out this outsourcing arrangement in 2019 and are performing our combination tests entirely in-house.
Competition
As early detection of cancer may lead to decreased morbidity with improved survival, more and more biotechnology companies have focused on the immense market opportunities it represents and are attempting to enter the space.
Biotechnology companies worldwide currently use various technologies for early cancer screening and detection. We believe that none of these technologies has yet acquired a dominant market position. As a novel cancer screening and detection technology that focuses on biophysical properties in blood, our CDA technology faces competition primarily from conventional biomarker-based technologies and other next-generation cancer screening and detection technologies, including those based on CTCs and ct-DNA. Recent major advances in CTC- and ct-DNA-based technologies have introduced the possibility of using either or both as tests to screen for cancer, and they have made the possibility for simultaneous screening for multiple primary cancers particularly attractive.
Our major competitors include biotechnology companies that conduct cancer screening and detection using next-generating technologies, such as BGI in China and GRAIL, Guardant Health, and Exact Sciences worldwide. All of these competitors’ cancer screening and detection technologies target CTCs and/or genomics such as ct-DNA, cf-DNA and cf-RNA, as opposed to the biophysical properties that our CDA technology focuses on.
We believe that our competitive advantages include the cost-efficiency, high testing accuracy, and broad test coverage of our CDA-based tests, our expansive patent portfolio and our large proprietary test database. However, many of our competitors have more expertise, experience and financial resources, stronger business relationships in developing and marketing their products, more mature technologies and products, greater market adoption among physicians and patients and others in the medical community, broader test menus, larger test databases, or greater brand recognition than we do. We also cannot assure you that our CDA technology will not become obsolete if we cannot keep pace with constantly changing technologies in the cancer screening and detection market.
Intellectual Property
Intellectual property rights are fundamental to our business, and we devote significant time and resources to their development and protection. We rely on a combination of patent, trade secret and trademark laws, as well as confidentiality agreements, to establish and protect our proprietary rights. We do not rely on third-party licenses of intellectual property when developing our CDA technology and CDA device.
We have developed an early and strong patent position related to our CDA technology, and we continuously seek patent coverage over its new applications. As of March 31, 2021, we had filed 237 patent applications globally; among them, 142 patents had been granted, including 20 patents granted in the United States, 65 in greater China (including eight in Taiwan), and 57 in other countries and regions. Our granted patents are expected to expire between 2031 and 2037. As of the same date, we also had 95 pending patent applications, consisting of 25 in the United States, 34 in greater China (including one in Taiwan), 32 in other countries and regions, and four patent cooperation treaty, or PCT, applications.
Our patents and patent applications broadly cover apparatus and methods for detecting diseases at early stages, and they strategically encompass the important specific embodiments of these apparatus and methods. They generally fall into the following categories:
· those relating to our CDA technology, including claims directed to methods for identifying and measuring various biophysical properties in blood samples and methods for detecting major cancer types and/or non-cancerous diseases, such as methods for detecting multiple cancers in a single blood test;
· those relating to our CDA device, including claims directed to its key components, such as the microfluidic device; and
· those relating to the multi-level, multi-parameter concept underlying our CDA technology, as well as our non-CDA early cancer screening and detection technologies, apparatus and methods.
According to our public searches, some of our patents, including our newly issued U.S. patents, have been cited by patent examiners and third parties (including a number of well-known global corporations and Fortune 50 companies).
Our agreements with our employees generally include assignment provisions, providing that all patents, copyrights and other intellectual property rights arising from the course of their employment with us or their using our facilities belong to us, and the employee-inventors are required assign to us all and any of their rights and title to the relevant granted patents or patent applications. In addition, we also try to protect our trade secrets and know-how through confidentiality agreements and non-disclosure provisions in our other agreements with persons who have access to them, such as our employees, consultants and research partners.
As of March 31, 2021, we also had 29 granted trademarks and no pending trademark applications in greater China, and nine granted trademarks and three pending trademark applications in the U.S.
Legal Proceedings
We may be subject to legal proceedings and claims in the ordinary course of business. We cannot predict the results of any such disputes, and despite the potential outcomes, their existence alone may have an adverse material impact on us because of diversion of management time and attention as well as the financial costs related to resolving such disputes. Neither we nor any of our directors or executive officers are currently a party to, nor is any of our properties the subject of, any material legal or arbitration proceedings.
See “Item 5. Operating and Financial Review and Prospects—A. Operating Results—Key Components of Results of Operations—Revenues” for a breakdown of our net revenues by category of activity.
Seasonality
We do not expect our operating results and operating cash flows to be subject to seasonal variations. This pattern may change, however, as a result of growth, new market opportunities or new product introductions.
PRC Regulations
In China, we are subject to a variety of PRC laws, rules and regulations affecting many aspects of our business. This section summarizes the principal PRC laws, rules and regulations that we believe are relevant to our business and operations.
Regulation on Medical Devices and Medical Institutions
Regulatory Authorities
In the PRC, the newly formed NMPA is the government authority under the State Administration for Market Regulation that monitors and supervises the administration of pharmaceutical products, medical devices, and cosmetics. The NMPA’s predecessor, the CFDA, was established in March 2013 and separated from the Ministry of Health of the PRC, or the MOH, as part of an institutional reform of the State Council. Predecessors of the NMPA also include the former State Food and Drug Administration, or the SFDA, that was established in March 2003 and the State Drug Administration, or the SDA, that was established in August 1998. The primary responsibilities of the NMPA include:
· monitoring and supervising the administration of pharmaceutical products, medical devices, and cosmetics in the PRC;
· formulating administrative rules and policies concerning the supervision and administration of the pharmaceutical, medical device, and cosmetics industry;
· evaluating, registering and approving of new drugs, generic drugs, imported drugs and traditional Chinese medicine;
· approving and issuing permits for the manufacture and export/import of pharmaceutical products, as well as medical devices, and approving the establishment of enterprises to be engaged in the manufacture and distribution of pharmaceutical products; and
· examining and evaluating the safety of pharmaceutical products, medical devices, and cosmetics and handling significant accidents involving these products.
The National Health and Family Planning Commission, or the NHFPC, has been renamed as the NHC. The NHC is an authority at the ministerial level under the State Council and is primarily responsible for national public health. The NHC combines the responsibilities of the former NHFPC, the Leading Group Overseeing Medical and Healthcare Reform under the State Council, the China National Working Commission on Aging, partial responsibilities of the Ministry of Industry and Information Technology in relation to tobacco control, and partial responsibilities from the State Administration of Work Safety in relation to occupational safety. The predecessor of NHFPC is the MOH. Following the establishment of the SFDA in 2003, the MOH was put in charge of the overall administration of the national health in the PRC excluding the pharmaceutical industry.
Medical Institutions Laws and Regulations
The Regulation on the Administration of Medical Institutions as promulgated by the State Council of the PRC on February 1994 and revised in 2016 provides the requirements for the establishment and administration of medical institutions. The establishment of medical institutions must comply with local governments’ plans for the establishment of medical institutions and the basic standards for medical institutions. To establish a medical institution, an entity or individual shall be subject to the examination and approval of the health administrative department of the local government at or above the county level and obtain the written approval for the establishment of medical institutions. A medical institution providing relevant services must register and obtain a medical institution practice license. An entity or individual that has not obtained a medical institution practice license may not carry out diagnosis or treatment activities. The revised Rules for Implementation of the Administrative Regulation on Medical Institutions as promulgated by the NHFPC in February 2017 further regulates the approval on establishment, registration, validation, naming and practice of medical institutions.
Our PRC subsidiaries, AnPac Lishui and Shiji Hainan, obtained their medical institution practice licenses in 2016 and 2015, respectively. We historically conducted a number of our CDA tests in premises other than our Lishui and Haikou laboratories, which could result in the relevant authorities confiscating the revenue we generated from these tests as well as other penalties on us. While we have rectified this practice and have not received any notice of relevant disciplinary governmental action, we cannot assure you that we will not be subject to this penalty.
The Measures for the Administration of Clinical Gene Amplification Testing Laboratories in Medical Institutions as promulgated by Ministry of Health in December 2010 provides the requirements for medical institutions to carry out clinical gene amplification test technique. Clinical gene amplification testing laboratory refers to a laboratory that detects specific DNA or RNA by amplification and to perform disease diagnosis, treatment monitoring and prognosis determination. The PRC Ministry of Health is responsible for supervising and administering clinical gene amplification testing laboratories in medical institutions nationwide. The health administrative authorities at the provincial level is responsible for supervising and administering clinical gene amplification testing laboratories in medical institutions within their respective administrative regions. This regulation also provides the examination and establishment of clinical gene amplification testing laboratories, laboratory quality management and laboratory supervision and management.
Our PRC subsidiary, Shiji Hainan, obtained its Certificate of Clinical Gene Amplification Testing Laboratory in 2016.
Medical Devices Administration Laws and Regulations
The Regulation on the Supervision and Administration of Medical Devices as amended by the State Council in December 2020, which will come into effect in June 2021, regulates entities that engage in the research and development, production, operation, use as well as supervision and administration of medical devices in the PRC. Medical devices are classified according to their risk levels. Class I medical devices are medical devices with low risks, the safety and effectiveness of which can be ensured through routine administration. Class II medical devices are medical devices with moderate risks, which are strictly controlled and administered to ensure their safety and effectiveness. Class III medical devices are medical devices with relatively high risks, which are strictly controlled and administered through special measures to ensure their safety and effectiveness. The evaluation of the risk levels of medical devices take into consideration the expected objectives, structural features, methods of use and other factors of medical devices.
The Measures for the Supervision and Administration of the Manufacture of Medical Device, as promulgated by CFDA in November 2017, regulates entities that engage in the manufacturing of medical devices in the PRC. The food and drug administration at or above the county level regulates medical device manufacturing within its administrative region, including manufacturing related licensing and registration, contract manufacturing and manufacturing quality controls.
The Measures for the Supervision and Administration of the Operation of Medical Devices, as promulgated by CFDA in November 2017, regulates entities that engage in business activities involving medical devices in the PRC. Business activities involving medical devices are regulated in accordance with the medical devices’ risk levels. No registration or license is required for business activities involving Class I medical devices. Registration is required for business activities involving Class II medical devices. A license is required for business activities involving Class III medical devices.
Our PRC subsidiary, AnPac Lishui, obtained its Class II medical device manufacture license and registration certificate for our CDA device in 2013 (renewed in 2018) and 2015.
Packaging of Medical Devices
The Administrative Rules on Instruction Manuals and Labels of Medical Devices, as promulgated by the CFDA in 2014, provides the requirements for instruction manuals and labeling of any medical device to be sold and used in the PRC. The information contained in the instruction manual and label of a medical device must be scientific, authentic, complete, accurate and consistent with product characteristics. The information contained in the instruction manual and label of a medical device must be consistent with the relevant information registered or filed for record. The information contained in the label of a medical device must be consistent with the relevant information in its instructions.
We believe that we are in compliance with these regulations in all material respects.
Clinical Practice Reform
In October 2017, the Chinese government announced an administrative reform of clinical trial institutions. Certification of clinical trial institutions by the former CFDA and the former NHFPC is no longer required. Under this reform, a clinical trial institution can be engaged by a drug and medical device registration applicant (i.e., a sponsor) to conduct a clinical study after it has been duly recorded with the online platform designated by the NMPA. In November 2017, the CFDA and the NHFPC jointly released the Rules for Administration of the Requirements for and Filing of Medical Devices Clinical Trial Institutions. These rules specify requirements for medical devices clinical-trial institutions and filing procedures. Pursuant to these rules, medical devices clinical-trial institutions shall meet the requirements of the Quality Management Standards for Medical Devices Clinical Trials including corresponding professional technical level, organization and management capabilities and ethics review capability.
Other Significant PRC Regulations Affecting Our Business Activities in China
Regulation on Foreign Investment
Investment activities in the PRC by foreign investors are regulated by the Catalog for the Guidance of Foreign Investment Industry, or the Catalog, which was promulgated and is amended from time to time by the MOFCOM, and the National Development and Reform Commission, or NDRC. The Catalog lays out the basic framework for foreign investment in China, classifying businesses into three categories with regard to foreign investment: “encouraged,” “restricted,” and “prohibited.” Industries not listed in the Catalog are generally deemed as falling into a fourth category “permitted” unless specifically restricted by other PRC laws. In addition, on June 23, 2020 the MOFCOM and the NDRC jointly promulgated the Special Management Measures (Negative List) for the Access of Foreign Investment, or the 2020 Negative List, which became effective on July 23, 2020 to amend the Catalog and the previous negative list thereunder. Investment in medical institutions (such as clinical laboratories) belongs to the “restricted” category. In particular, according to relevant PRC foreign investment regulations, only domestic companies and foreign-invested joint ventures are allowed to hold an NHC medical institution practice license. However, it is unclear under PRC law whether a subsidiary of a wholly foreign owned enterprise is eligible to hold this license. We believe that the risks for the NHC medical institution practice license of each of our Lishui and Haikou laboratories—subsidiaries of AnPac Lishui, a wholly foreign owned enterprise—being held invalid or revoked by the NHC is remote, based on our confirmation with relevant regulatory authorities. However, we cannot assure you that the relevant regulatory authorities would not change their interpretation or position regarding the relevant laws and regulations.
On March 15, 2019, the National People’s Congress promulgated the PRC Foreign Investment Law, or the FIL, which came into effect on January 1, 2020 and replaces the trio of previous laws regulating foreign investment in China, namely, the Sino-foreign Equity Joint Venture Enterprise Law, the Sino-foreign Cooperative Joint Venture Enterprise Law and the Wholly Foreign-invested Enterprise Law, together with their implementation rules and ancillary regulations. The FIL embodies an expected regulatory trend in PRC to rationalize its foreign investment regulatory regime in line with prevailing international practice and the legislative efforts to unify the corporate legal requirements for both foreign and domestic investments. The Implementation Rules to the Foreign Investment Law were promulgated by the State Council on December 26, 2019 and became effective on January 1, 2020. The FIL and its Implementation Rules, by means of legislation, have established the basic framework for the access, promotion, protection and administration of foreign investment in view of investment protection and fair competition.
On December 30, 2019, MOFCOM and the State Administration for Market Regulation jointly promulgated the Measures for Information Reporting on Foreign Investment, which became effective on January 1, 2020. Pursuant to these measures, where a foreign investor carries out investment activities in China directly or indirectly, the foreign investor or the foreign-invested enterprise shall submit the investment information to the competent commerce department.
PRC Regulation of Commercial Bribery
Medical device companies involved in a criminal investigation or administrative proceedings related to bribery are listed in the Adverse Records of Commercial Briberies by its provincial health and family planning administrative department. Pursuant to the Provisions on the Establishment of Adverse Records of Commercial Briberies in the Medicine Purchase and Sales Industry, which became effective on March 1, 2014, provincial health and family planning administrative departments formulate the implementing measures for establishment of Adverse Records of Commercial Briberies. If a company is listed in the Adverse Records of Commercial Briberies for the first time, their products may not be purchased by public medical institutions. A company will not be penalized by the relevant PRC government authorities merely by virtue of having contractual relationships with sales agents or third party promoters who are engaged in bribery activities, so long as such company and its employees are not utilizing the sales agents or third party promoters for the implementation of, or acting in conjunction with them in, the prohibited bribery activities. In addition, a company is under no legal obligation to monitor the operating activities of its sales agents and third party promoters, and will not be subject to penalties or sanctions by relevant PRC government authorities as a result of failure to monitor their operating activities.
We believe that we are in compliance with these regulations in all material respects.
PRC Regulation of Product Liability
In addition to the strict new drug approval process, certain PRC laws have been promulgated to protect the rights of consumers and to strengthen the control of medical products in the PRC. Under current PRC law, manufacturers and vendors of defective products in the PRC may incur liability for loss and injury caused by such products. Pursuant to the General Principles of the Civil Law of the PRC promulgated on April 12, 1986 and amended on August 27, 2009, a defective product which causes property damage or physical injury to any person may subject the manufacturer or vendor of such product to civil liability for such damage or injury.
Pursuant to the Civil Code of the PRC promulgated on May 28, 2020, which came into effect on January 1, 2021, the manufacturer shall bear tort liability where a defect of a product causes damage to another person. The infringed person may claim compensation from the manufacturer or the seller of the product where a defect of a product causes damage to another person.
On February 22, 1993, the Product Quality Law of the PRC, or the Product Quality Law, was promulgated to supplement the Civil Law of the PRC aiming to protect the legitimate rights and interests of the end-users and consumers and to strengthen the supervision and control of the quality of products. The Product Quality Law was revised by the Ninth National People’s Congress on July 8, 2000, by the Eleventh National People’s Congress on August 27, 2009 and by the Thirteenth National People’s Congress on December 29, 2018. Pursuant to the revised Product Quality Law, manufacturers who produce defective products may be subject to civil or criminal liability and have their business licenses revoked.
The Law of the PRC on the Protection of the Rights and Interests of Consumers was promulgated on October 31, 1993 and was amended on August 27, 2009 and October 25, 2013 to protect consumers’ rights when they purchase or use goods and accept services. All business operators must comply with this law when they manufacture or sell goods and/or provide services to customers. Under the amendments made on October 25, 2013, all business operators must pay high attention to protecting customers’ privacy and strictly keeping confidential any consumer information they obtain during their business operations. In addition, in extreme situations, pharmaceutical product manufacturers and operators may be subject to criminal liability if their goods or services lead to the death or injuries of customers or other third parties.
We are not aware of any material product liability related litigation or other legal proceedings against us arising from the cancer screening and detection tests that we provide to our customers.
PRC Tort Law
Under the Tort Law of the PRC, which became effective on July 1, 2010, if damages to persons are caused by defective products due to the fault of a third party, such as the parties providing transportation or warehousing, the producers and the sellers of the products have the right to recover their respective losses from such third parties. If defective products are identified after they have been put into circulation, the producers or the sellers must take remedial measures such as issuance of a warning or recall of products. in a timely manner. The producers or the sellers will be liable under tort if they fail to take remedial measures in a timely manner or have not made efforts to take remedial measures, thus causing damages. If the products are produced or sold with known defects, causing deaths or severe adverse health issues, the infringed party has the right to claim punitive damages in addition to compensatory damages.
Under the Civil Code of the PRC promulgated on May 28, 2020, which came into effect on January 1, 2021, where a defect of a product is caused due to the fault of a transporter, a warehouse or any other third party, the manufacturer or the seller shall, after paying compensation, have the right to claim the same from the third party. Where a product is found to be defective after it is put into circulation, the manufacturer or the seller shall timely take such remedial measures as ceasing the sale, giving warning or recall the defective product. If any damage is aggravated due to the manufacturer or the seller’s failure to take timely or effective remedial measures, the manufacturer or the seller shall assume tort liability for the aggravated part of the damage. Where any manufacturer or seller produces or sells the products despite knowing that they are defective or fails to take effective remedial measures as prescribed in the preceding paragraph, thus causing death or serious damage to the health of another person, the infringed person shall have the right to claim appropriate punitive damages.
We are not aware of any material torts related litigation or other legal proceedings against us arising from the cancer screening and detection tests that we provide to our customers.
Regulation on Intellectual Property Rights
China has made substantial efforts to adopt comprehensive legislation governing intellectual property rights, including patents, trademarks, copyrights and domain names.
Patents
Pursuant to the PRC Patent Law, most recently amended in October 2020, which will come into effect on June 1, 2021, and its implementation rules, most recently amended in January 2010, patents in China fall into three categories: invention, utility model and design. An invention patent is granted to a new technical solution proposed in respect of a product or method or an improvement of a product or method. A utility model is granted to a new technical solution that is practicable for application and proposed in respect of the shape, structure or a combination of both of a product. A design patent is granted to the new design of a certain product in shape, pattern or a combination of both and in color, shape and pattern combinations aesthetically suitable for industrial application. Under the PRC Patent Law, the term of patent protection starts from the date of application. Patents relating to invention are effective for twenty years, utility models are effective for ten years, and designs are effective for fifteen years from the date of application. The PRC Patent Law adopts the principle of “first-to-file” system, which provides that where more than one person files a patent application for the same invention, a patent will be granted to the person who first files the application.
Existing patents can become narrowed, invalidated or unenforceable due to a variety of grounds, including lack of novelty, creativity, and deficiencies in patent application. In China, a patent must have novelty, creativity and practical applicability. Under the PRC Patent Law, novelty means that before a patent application is filed, no identical invention or utility model has been publicly disclosed in any publication in China or overseas or has been publicly used or made known to the public by any other means, whether in or outside of China, nor has any other person filed with the patent authority an application that describes an identical invention or utility model and is recorded in patent application documents or patent documents published after the filing date. Creativity means that, compared with existing technology, an invention has prominent substantial features and represents notable progress, and a utility model has substantial features and represents any progress. Practical applicability means an invention or utility model can be manufactured or used and may produce positive results. Patents in China are filed with the State Intellectual Property Office, or SIPO. Normally, the SIPO publishes an application for an invention patent within 18 months after the filing date, which may be shortened at the request of applicant. The applicant must apply to the SIPO for a substantive examination within three years from the date of application.
Article 19 of the PRC Patent Law provides that, for an invention or utility model completed in China, any applicant (not just Chinese companies and individuals), before filing a patent application outside of China, must first submit it to the SIPO for a confidential examination. Failure to comply with this requirement will result in the denial of any Chinese patent for the relevant invention. This added requirement of confidential examination by the SIPO has raised concerns by foreign companies who conduct research and development activities in China or outsource research and development activities to service providers in China.
Patent Enforcement
Unauthorized use of patents without consent from owners of patents, forgery of the patents belonging to other persons, or engagement in other patent infringement acts, will subject the infringers to infringement liability. Serious offenses such as forgery of patents may be subject to criminal penalties.
When a dispute arises out of infringement of the patent owner’s patent right, Chinese law requires that the parties first attempt to settle the dispute through mutual consultation. However, if the dispute cannot be settled through mutual consultation, the patent owner, or an interested party who believes the patent is being infringed, may either file a civil legal suit or file an administrative complaint with the relevant patent administration authority. A Chinese court may issue a preliminary injunction upon the patent owner’s or an interested party’s request before instituting any legal proceedings or during the proceedings. Damages for infringement are calculated as the loss suffered by the patent holder arising from the infringement, and if the loss suffered by the patent holder arising from the infringement cannot be determined, the damages for infringement are calculated as the benefit gained by the infringer from the infringement. If it is difficult to ascertain damages in this manner, damages may be determined using a reasonable multiple of the license fee under a contractual license. Statutory damages may be awarded in the circumstances where the damages cannot be determined by the calculation standards described above. The damage calculation methods will be applied in the order described above. Generally, the patent owner has the burden of proving that the patent is being infringed. However, if the owner of an invention patent for manufacturing process of a new product alleges infringement of its patent, the alleged infringer has the burden of proof.
Exemptions for Unlicensed Manufacture, Use, Sale or Import of Patented Products
The PRC Patent Law provides five exceptions for unauthorized manufacture, use, sale or import of patented products. None of following circumstances are deemed an infringement of the patent rights, and any person may manufacture, use, sell or import patented products without authorization granted by the patent owner as follows:
· Any person who uses, promises to sell, sells or imports any patented product or product directly obtained in accordance with the patented methods after such product is sold by the patent owner or by its licensed entity or individual;
· Any person who has manufactured an identical product, has used an identical method or has made necessary preparations for manufacture or use prior to the date of patent application and continues to manufacture such product or use such method only within the original scope
· Any foreign transportation facility that temporarily passes through the territory, territorial waters or territorial airspace of China and uses the relevant patents in its devices and installations for its own needs in accordance with any agreement concluded between China and that country to which the foreign transportation facility belongs, or any international treaty to which both countries are party, or on the basis of the principle of reciprocity;
· Any person who uses the relevant patents solely for the purposes of scientific research and experimentation; or
· Any person who manufactures, uses or imports patented drugs or patented medical devices for the purpose of providing information required for administrative approval, or manufactures, uses or imports patented drugs or patented medical devices for the abovementioned person.
However, if patented drugs are utilized on the ground of exemptions for unauthorized manufacture, use, sale or import of patented drugs prescribed in PRC Patent Law, such patented drugs cannot be manufactured, used, sold or imported for any commercial purposes without authorization granted by the patent owner.
As of March 31, 2021, we had 65 granted patents (including eight in Taiwan) and 34 pending patent applications (including one in Taiwan) in greater China, and 77 granted patents and 62 pending patent applications outside greater China.
Trade Secrets
According to the PRC Anti-Unfair Competition Law, the term “trade secrets” refers to technical and business information that is unknown to the public, has utility and may create business interests or profits for its legal owners or holders, and is maintained as a secret by its legal owners or holders.
Under the PRC Anti-Unfair Competition Law, which was promulgated on September 2, 1993 and was amended on March 23, 2019, business persons are prohibited from infringing others’ trade secrets by: (1) obtaining the trade secrets from the legal owners or holders by any unfair methods such as theft, bribery, intimidation, solicitation or coercion; (2) disclosing, using or permitting others to use the trade secrets obtained illegally under item (1) above; or (3) disclosing, using or permitting others to use the trade secrets, in violation of any contractual agreements or any requirements of the legal owners or holders to keep such trade secrets in confidence. If a third party knows or should have known of the fact that an employee or former employee of the right owner of trade secrets or any other entity or individual conducts any of the illegal acts above mentioned, but still accepts, publishes, uses or allows any other to use such secrets, such practice shall be deemed as infringement of trade secrets. The parties whose trade secrets are being misappropriated may petition for administrative corrections, and regulatory authorities may stop any illegal activities and fine infringing parties in the amount of RMB100,000 to RMB500,000, where the circumstance is serious, the fine shall be between RMB500,000 to RMB3,000,000. Alternatively, persons whose trade secrets are being misappropriated may file lawsuits in a Chinese court for loss and damages incurred due to the misappropriation.
The measures to protect trade secrets include oral or written non-disclosure agreements or other reasonable measures to require the employees of, or persons in business contact with, legal owners or holders to keep trade secrets confidential. Once the legal owners or holders have asked others to keep trade secrets confidential and have adopted reasonable protection measures, the requested persons bear the responsibility for keeping the trade secrets confidential.
Trademarks and Domain Names
Trademark. The PRC Trademark Law and its implementation rules protect registered trademarks. The PRC Trademark Office of State Administration of Industry and Commerce is responsible for the registration and administration of trademarks throughout the PRC. The Trademark Law has adopted a “first-to-file” principle with respect to trademark registration.
Domain Name. Domain names are protected under the Administrative Measures on the Internet Domain Names promulgated by the Ministry of Industry and Information Technology. The Ministry of Industry and Information Technology is the main regulatory body responsible for the administration of PRC internet domain names.
As of March 31, 2020, we had 29 granted trademarks and no pending trademark applications in greater China, and nine granted trademarks and three pending trademark applications in the U.S. In addition, as of the same date, we had 19 domain names.
PRC Regulation on Data Protection
The Basic Standards for Clinical Laboratories (for Trial Implementation) as promulgated by the NHFPC in 2016 provides that clinical laboratories must establish information management and patient privacy protection policies. The Measures for the Administration of General Population Health Information (for Trial Implementation) as promulgated by the NHFPC in 2014 sets forth the operational measures for patient privacy protection in medical institutions. The measures regulate the collection, use, management, safety and privacy protection of general population health information by medical institutions. Medical institutions are required to establish information management departments in charge of general population health information and establish quality control procedures and relevant information systems to manage general population health information. Medical institutions must adopt stringent procedures to verify the general population health data collected, timely update and maintain the data, establish policies on the authorized use of general population health information, and establish safety protection systems, policies, practice and technical guidance to avoid divulging confidential or private information.
To comply with these laws and regulations, we have required our customers and research partners to consent to, or obtain consent from the tested individuals to, our collecting and using their personal information for our cancer screening and detection tests. We have also established information security systems to protect the tested individuals’ privacy, including data access restrictions and monitoring, data storage, database encryption and backup.
PRC Regulation on Labor Protection
Under the Labor Law of the PRC, effective on January 1, 1995 and subsequently amended on August 27, 2009 and December 29, 2018, the PRC Employment Contract Law, effective on January 1, 2008 and subsequently amended on December 28, 2012 and the Implementing Regulations of the Employment Contract Law, effective on September 18, 2008, employers must establish a comprehensive management system to protect the rights of their employees, including a system governing occupational health and safety to provide employees with occupational training to prevent occupational injury, and employers are required to truthfully inform prospective employees of the job description, working conditions, location, occupational hazards and status of safe production as well as remuneration and other conditions as requested by the Labor Contract Law of the PRC.
Pursuant to the Law of Manufacturing Safety of the PRC effective on November 1, 2002 and amended on August 27, 2009 and August 31, 2014, manufacturers must establish a comprehensive management system to ensure manufacturing safety in accordance with applicable laws, regulations, national standards, and industrial standards. Manufacturers not meeting relevant legal requirements are not permitted to commence their manufacturing activities.
Pursuant to the Administrative Measures Governing the Production Quality of Pharmaceutical Products effective on March 1, 2011, manufacturers of pharmaceutical products are required to establish production safety and labor protection measures in connection with the operation of their manufacturing equipment and manufacturing process.
Pursuant to applicable PRC laws, rules and regulations, including the Social Insurance Law, which became effective on July 1, 2011 and amended on December 29, 2018, the Interim Regulations on the Collection and Payment of Social Security Funds, which became effective on January 22, 1999 and amended on March 24, 2019, Interim Measures concerning the Maternity Insurance of Employees, which become effective on December 14, 1994, and the Regulations on Work-related Injury Insurance, which became effective on January 1, 2004 and was subsequently amended on December 20, 2010, employers are required to contribute, on behalf of their employees, to a number of social security funds, including funds for basic pension insurance, unemployment insurance, basic medical insurance, work-related injury insurance and maternity insurance. If an employer fails to make social insurance contributions timely and in full, the social insurance collecting authority will order the employer to make up outstanding contributions within the prescribed time period and impose a late payment fee at the rate of 0.05% per day from the date on which the contribution becomes due. If such employer fails to make the overdue contributions within such time limit, the relevant administrative department may impose a fine equivalent to one to three times the overdue amount
Regulations Relating to Foreign Exchange Registration of Offshore Investment by PRC Residents
In July 2014, SAFE issued the SAFE Circular 37, and its implementation guidelines. Pursuant to SAFE Circular 37 and its implementation guidelines, PRC residents (including PRC institutions and individuals) must register with local branches of SAFE in connection with their direct or indirect offshore investment in an overseas special purpose vehicle, or SPV, directly established or indirectly controlled by PRC residents for the purposes of offshore investment and financing with their legally owned assets or interests in domestic enterprises, or their legally owned offshore assets or interests. Such PRC residents are also required to amend their registrations with SAFE when there is a change to the basic information of the SPV, such as changes of a PRC resident individual shareholder, the name or operating period of the SPV, or when there is a significant change to the SPV, such as changes of the PRC individual resident’s increase or decrease of its capital contribution in the SPV, or any share transfer or exchange, merger, division of the SPV. Failure to comply with the registration procedures set forth in the Circular 37 may result in restrictions being imposed on the foreign exchange activities of the relevant onshore company, including the payment of dividends and other distributions to its offshore parent or affiliate, the capital inflow from the offshore entities and settlement of foreign exchange capital, and may also subject relevant onshore company or PRC residents to penalties under PRC foreign exchange administration regulations.
Regulations Relating to Employee Stock Incentive Plan
In February 2012, SAFE promulgated the Stock Option Rules. In accordance with the Stock Option Rules and relevant rules and regulations, PRC citizens or non-PRC citizens residing in China for a continuous period of not less than one year, who participate in any stock incentive plan of an overseas publicly listed company, subject to a few exceptions, are required to register with SAFE through a domestic qualified agent, which could be a PRC subsidiary of such overseas listed company, and complete certain procedures. We and our employees who are PRC citizens or who reside in China for a continuous period of not less than one year and who participate in our stock incentive plan will be subject to such regulation. In addition, the SAT has issued circulars concerning employee share options or restricted shares. Under these circulars, employees working in the PRC who exercise share options, or whose restricted shares vest, will be subject to PRC individual income tax, or the IIT. The PRC subsidiaries of an overseas listed company have obligations to file documents related to employee share options or restricted shares with relevant tax authorities and to withhold IIT of these employees related to their share options or restricted shares. If the employees fail to pay, or the PRC subsidiaries fail to withhold, their IIT according to relevant laws, rules and regulations, the PRC subsidiaries may face sanctions imposed by the tax authorities or other PRC government authorities.
Regulations Relating to Dividend Distribution
The principal regulation governing distribution of dividends paid by a PRC enterprise include Company Law of the PRC (1993), as amended in 1999, 2004, 2005, 2013 and 2018;
Under these laws and regulations, foreign-invested enterprises in China may pay dividends only out of their accumulated profits, if any, determined in accordance with PRC accounting standards and regulations. In addition, a wholly foreign-owned enterprise in China is required to set aside at least 10.0% of its after-tax profit based on PRC accounting standards each year to its general reserves until the accumulative amount of such reserves reach 50.0% of its registered capital. These reserves are not distributable as cash dividends. The foreign-invested enterprise has the discretion to allocate a portion of its after-tax profits to staff welfare and bonus funds. A PRC company is not permitted to distribute any profits until any losses from prior fiscal years have been offset. Profits retained from prior fiscal years may be distributed together with distributable profits from the current fiscal year.
Regulations Relating to Foreign Exchange
The principal regulations governing foreign currency exchange in China are the Foreign Exchange Administration Regulations, most recently amended in August 2008. Under the Foreign Exchange Administration Regulations, payments of current account items, such as profit distributions and trade and service-related foreign exchange transactions, can be made in foreign currencies without prior approval from SAFE by complying with certain procedural requirements. However, approval from or registration with appropriate government authorities is required where RMB is to be converted into foreign currency and remitted out of China to pay capital expenses such as the repayment of foreign currency-denominated loans.
In August 2008, SAFE issued the Circular on the Relevant Operating Issues Concerning the Improvement of the Administration of the Payment and Settlement of Foreign Currency Capital of Foreign-Invested Enterprises, or SAFE Circular No. 142, regulating the conversion by a foreign-invested enterprise of foreign currency-registered capital into RMB by restricting how the converted RMB may be used. SAFE Circular No. 142 provides that the RMB capital converted from foreign currency registered capital of a foreign-invested enterprise may only be used for purposes within the business scope approved by the applicable government authority and may not be used for equity investments within China. SAFE also strengthened its oversight of the flow and use of the RMB capital converted from foreign currency registered capital of foreign-invested enterprises. The use of such RMB capital may not be changed without SAFE’s approval, and such RMB capital may not in any case be used to repay RMB loans if the proceeds of such loans have not been used. In March 2015, SAFE issued SAFE Circular No. 19, which took effective and replaced SAFE Circular No. 142 on June 1, 2015. Although SAFE Circular No. 19 allows for the use of RMB converted from the foreign currency-denominated capital for equity investments in China, the restrictions continue to apply as to foreign-invested enterprises’ use of the converted RMB for purposes beyond the business scope, for entrusted loans or for inter-company RMB loans. SAFE promulgated the Notice of the SAFE on Reforming and Standardizing the Foreign Exchange Settlement Management Policy of Capital Account, or Circular 16, effective on June 9, 2016, which reiterates some of the rules set forth in Circular 19, but changes the prohibition against using RMB capital converted from foreign currency-denominated registered capital of a foreign-invested company to issue RMB entrusted loans to a prohibition against using such capital to issue loans to non-associated enterprises. Violations of SAFE Circular 19 or Circular 16 could result in administrative penalties.
In November 2012, SAFE promulgated the Circular of Further Improving and Adjusting Foreign Exchange Administration Policies on Foreign Direct Investment and amended on May 2015, which substantially amends and simplifies the current foreign exchange procedure. Pursuant to this circular, the opening of various special purpose foreign exchange accounts (e.g., pre-establishment expenses accounts, foreign exchange capital accounts and guarantee accounts), the reinvestment of lawful incomes derived by foreign investors in China (e.g. profit, proceeds of equity transfer, capital reduction, liquidation and early repatriation of investment), and purchase and remittance of foreign exchange as a result of capital reduction, liquidation, early repatriation or share transfer in a foreign-invested enterprise no longer require SAFE approval, and multiple capital accounts for the same entity may be opened in different provinces, which was not possible before. In addition, SAFE promulgated the Circular on Printing and Distributing the Provisions on Foreign Exchange Administration over Domestic Direct Investment by Foreign Investors and the Supporting Documents in May 2013, which specifies that the administration by SAFE or its local branches over direct investment by foreign investors in the PRC shall be conducted by way of registration and banks shall process foreign exchange business relating to the direct investment in China based on the registration information provided by SAFE and its branches.
In February 2015, SAFE promulgated the Circular on Further Simplifying and Improving the Policies Concerning Foreign Exchange Control on Direct Investment which took effect on June 1, 2015. The Circular on Further Simplifying and Improving the Policies Concerning Foreign Exchange Control on Direct Investment delegates the authority to enforce the foreign exchange registration in connection with the inbound and outbound direct investment under relevant SAFE rules to certain banks and therefore further simplifies the foreign exchange registration procedures for inbound and outbound direct investment.
Regulations on Enterprise Income Tax
Pursuant to the EIT Law effective as of January 2008 and as last amended in December 2018, the income tax rate for both domestic and foreign-invested enterprises is 25% with certain exceptions. To clarify certain provisions in the EIT Law, the State Council promulgated the Implementation Rules of the EIT Law in December 2007, which became effective in January 2008 and as amended in April 2019. Under the EIT Law and the Implementation Rules of the EIT Law, enterprises are classified as either “resident enterprises” or “non-resident enterprises.” Besides enterprises established within the PRC, enterprises established outside of China whose “de facto management bodies” are located in China are considered “resident enterprises” and subject to the uniform 25% enterprise income tax rate for their global income. In addition, the EIT Law provides that a non-resident enterprise refers to an entity established under foreign law whose “de facto management bodies” are not within the PRC, but has an establishment or place of business in the PRC, or does not have an establishment or place of business in the PRC but has income sourced within the PRC.
The Implementation Rules of the EIT Law provide that since January 2008, an income tax rate of 10% shall normally be applicable to dividends declared to non-PRC resident enterprise investors that do not have an establishment or place of business in the PRC, or have such establishment or place of business but the relevant income is not effectively connected with the establishment or place of business, to the extent such dividends are derived from sources within the PRC. The income tax on the dividends may be reduced pursuant to a tax treaty between China and the jurisdictions in which the non-PRC shareholders reside.
Other PRC National- and Provincial-Level Laws and Regulations
We are subject to changing regulations under many other laws and regulations administered by governmental authorities at the national, provincial and municipal levels, some of which are or may become applicable to our business. For example, regulations control the confidentiality of patients’ medical information and the circumstances under which patient medical information may be released for inclusion in our databases, or released by us to third parties. These laws and regulations governing both the disclosure and the use of confidential patient medical information may become more restrictive in the future.
We also comply with numerous additional national and provincial laws relating to matters such as safe working conditions, manufacturing practices, environmental protection and fire hazard control in all material aspects. We believe that we are currently in compliance with these laws and regulations; however, we may be required to incur significant costs to comply with these laws and regulations in the future. Unanticipated changes in existing regulatory requirements or adoption of new requirements could therefore have a material adverse effect on our business, results of operations and financial condition.
U.S. Regulations
Federal and State Laboratory Licensing Requirements
Pursuant to the CLIA, a laboratory that performs testing on specimens derived from humans for the purpose of providing information for the diagnosis, prevention or treatment of disease, or the impairment of, or assessment of health must hold a certificate applicable to the complexity of the laboratory examinations it performs, and it must comply with, among other things, standards covering operations, personnel, facilities administration, quality, and proficiency testing, which are intended to ensure, among other things, that its clinical laboratory testing services are accurate, reliable and timely. Laboratories performing high-complexity testing are required to meet more stringent requirements than laboratories performing less complex tests. The CLIA requirements do not apply to research laboratories that test human specimens but do not report patient specific results for the diagnosis, prevention or treatment of any disease or impairment of, or the assessment of, the health of individual patients. In order to offer our test in the United States, our laboratories must have the appropriate CLIA certification and the applicable state licenses. A laboratory that has submitted its application but has not yet received CLIA certification, may be issued a CLIA Certificate of Registration which allows the laboratory to perform testing while the laboratory’s survey and inspection are pending. We obtained CAP accreditation and a CLIA Certificate of Accreditation for our San Jose laboratory in March 2020. We obtained a CLIA Certificate of Registration for our laboratory in Philadelphia, Pennsylvania in August 2020. CMS, the agency that oversees CLIA, has deemed CAP standards to be equally or more stringent than CLIA regulations and has approved CAP as a recognized accrediting organization. Inspection by CAP is performed in lieu of CMS inspections for accredited laboratories. To maintain and renew our CAP accreditation and CLIA certification, we are subject to survey and inspection every two years to assess our laboratory’s compliance with program standards. We also may be subject to additional unannounced inspections.
CLIA provides that a state may adopt laboratory regulations with more stringent requirements than those under U.S. federal law, and a number of states have implemented their own laboratory regulatory requirements. State laws may require that laboratory personnel meet certain qualifications, specify certain quality control procedures, facility requirements or prescribe record maintenance requirements.
We are required to maintain a California state laboratory license for our San Jose laboratory pursuant to the relevant state laws. We will be required to maintain a Pennsylvania state laboratory permit for our new Philadelphia laboratory. Each laboratory may also need to maintain licenses in other states with requirements for non-resident laboratories in order to perform tests on samples from patients who reside in those states. For example, in order to offer our test in New York, we must separately apply for a New York State clinical laboratory permit and approval of our test in New York, which will require submission of validation data as well as information regarding the test methods, among other things. Other states may currently have or adopt similar licensure requirements in the future. We will obtain any such necessary licenses before offering our cancer screening and detection test in a state requiring non-resident laboratory licensure.
Failure to comply with CLIA certification and state clinical laboratory licensure requirements may result in a range of enforcement actions, including certificate or license suspension, limitation, or revocation, directed plan of corrective action, on-site monitoring, civil monetary penalties, criminal sanctions, and revocation of the relevant laboratory’s approval to receive Medicare and Medicaid payment for its services, as well as significant adverse publicity.
Regulation of Laboratory Developed Tests
LDTs have generally been considered by the FDA to be tests that are designed, developed, validated and used within a single laboratory. The FDA has the authority to regulate such tests as medical devices under the FDCA. However, the FDA historically has exercised its enforcement discretion and not enforced applicable provisions of the FDCA and FDA regulations with respect to LDTs. However, in recent years, legislative and administrative proposals addressing oversight of LDTs were introduced. For example, in 2014 the FDA issued two draft guidance documents proposing a risk-based framework with respect to applying the FDA’s oversight over LDTs. The draft guidance documents stated that the FDA intended to modify its policy of enforcement discretion with respect to LDTs in a risk-based manner consistent with the existing classification of medical devices. Thus, the FDA planned to begin to enforce its medical device requirements, including premarket submission requirements, on LDTs marketed without FDA premarket review and authorization. In November 2016, the FDA announced its intention not to finalize the 2014 draft guidance documents to allow for further public discussion of an appropriate oversight approach to LDTs and to give congressional authorizing committees the opportunity to develop a legislative solution. In January 2017, the FDA issued a discussion paper on possible approaches to the regulation of LDTs. On August 19, 2020, HHS announced that the FDA would no longer require premarket authorization for LDTs unless the FDA engaged in notice-and-comment rulemaking. HHS also rescinded all guidance documents and informal policy statements that FDA had previously issued concerning LDTs.
We expect that new legislative and administrative proposals regarding the oversight of LDTs will be introduced from time to time. It is possible that legislation could be enacted into law or regulations or guidance could be issued by the FDA, which may result in new or increased regulatory requirements for us to offer our tests as LDTs or to develop and introduce new tests as LDTs in the foreseeable future.
Although we believe we are within the scope of the FDA’s policy for LDTs, the initial commercialization and continued commercial availability of an LDT is subject to uncertainty given the FDA’s latitude in interpreting and applying its laws and policies. For example, FDA does not consider tests to be subject to its LDT enforcement discretion if they are designed or manufactured completely, or partly, outside of the laboratory that offers and uses them, or if they are offered “direct-to-consumer,” as opposed to being available to patients only when prescribed by a health care provider. Even for tests that appear to fall within FDA’s previously stated enforcement discretion, the FDA may decide to take action against certain LDTs on a case-by-case basis at any time if FDA views them as presenting a risk to patients. The former FDA Commissioner and the Director of FDA’s CDRH have expressed significant concerns regarding potential disparities in accuracy and quality between some LDTs and IVDs that have been reviewed and cleared, authorized or approved by FDA. In addition, the U.S. Congress has been considering various legislative proposals that would reform FDA’s regulation of laboratory tests, and such legislation might lead to heightened FDA scrutiny of LDTs, particularly new LDTs. Whether such legislation will be enacted and, if so, what effect it may have on how FDA regulates laboratory tests, including LDTs, is unknown. If FDA disagrees with a laboratory test’s LDT status, FDA may consider the test to be an unapproved medical device, may subject the company to FDA enforcement action, including, without limitation, requiring the company to seek clearance, authorization or approval for the laboratory test.
Regulation of Medical Devices
A medical device is an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including any component part, or accessory which is: (i) recognized in the official National Formulary, or the United States Pharmacopoeia, or any supplement to them; (ii) intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals; or (iii) intended to affect the structure or any function of the body of man or other animals, and which does not achieve any of its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of any of its primary intended purposes. IVDs, are a type of medical device and include reagents and instruments used in the diagnosis or detection of diseases, conditions or infections, including, without limitation, the presence of certain chemicals, genetic information or other biomarkers. Predictive, prognostic and screening tests can also be IVDs.
In the United States, medical devices, including IVDs, are subject to extensive regulation by the FDA under the FDCA and its implementing regulations, and certain other U.S. federal and state statutes and regulations. The laws and regulations govern, among other things, the design, manufacture, storage, recordkeeping, approval, labeling, promotion, post-approval monitoring and reporting, distribution and import and export of medical devices. Failure to comply with applicable requirements may subject a device and/or its manufacturer to a variety of administrative sanctions, such as FDA refusal to approve pending PMAs, issuance of warning letters, mandatory product recalls, import detentions, civil monetary penalties, and/or judicial sanctions, such as product seizures, injunctions, and criminal prosecution.
Device Classification
Under the FDCA, medical devices are classified into one of three classes based on the risk associated with the device and the level of control necessary to provide a reasonable assurance of safety and effectiveness. Class I devices are deemed to be low risk and are subject to the fewest regulatory controls. Class III devices are generally the highest risk devices and are subject to the highest level of regulatory control to provide reasonable assurance of the device’s safety and effectiveness. Class III devices must typically be approved by FDA before they are marketed.
Most Class I devices and a minority of Class II devices are completely exempt from premarket review by FDA. Most Class II and a minority of Class I devices require 510(k) clearance. Devices that pose the highest risk, including life-sustaining, life-supporting or implantable devices, or devices deemed not substantially equivalent to a previously 510(k)-cleared device or a “pre-amendment” Class III device in commercial distribution before May 28, 1976 for which PMA applications have not been called, are placed in Class III requiring PMA approval. A novel device is placed in Class III by default, but it may be eligible to be placed in Class I or Class II via “de novo” classification if it can be shown to pose only low to moderate risk with appropriate regulatory controls.
The PMA approval pathway requires proof of the safety and effectiveness of the device to the FDA’s satisfaction. The 510(k) clearance pathway is much less burdensome and time-consuming than the PMA approval pathway. The de novo pathway has an enhanced burden compared to the 510(k) clearance pathway but is much less burdensome than a PMA approval process.
The 510(k) Clearance Pathway
Under the 510(k) clearance pathway, a device manufacturer must submit to the FDA a premarket notification, demonstrating that the device is “substantially equivalent” to a legally marketed predicate device. A predicate device may be a previously 510(k) cleared device or a pre-amendment device (unless the FDA has issued a regulation calling for PMA applications for this device type). To be “substantially equivalent,” the proposed device must have the same intended use as the predicate device, and either have the same technological characteristics as the predicate device or have different technological characteristics and be shown to be equally safe and effective and not raise different questions of safety or effectiveness than the predicate device.
After the FDA accepts the 510(k) premarket notification, it begins a substantive review. By statute, the FDA is required to complete its review within 90 days of receiving the 510(k) notification. As a practical matter, clearance often takes longer, typically ranging from three to nine months or longer, and clearance is never assured. The FDA’s 510(k) review generally compares a proposed device to a predicate device with respect to intended use and technology (design, materials, software, energy source, etc.). The information necessary to show substantial equivalence will depend upon the differences between the proposed device and the predicate device, which may include bench, cadaver, animal and/or clinical studies.
If the FDA agrees that the proposed device is substantially equivalent to the predicate device, it will grant clearance to commercially market the device. Otherwise, the device manufacturer must fulfill the much more rigorous premarketing requirements of the PMA approval process, or seek reclassification of the device through the de novo process.
After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, requires a new 510(k) clearance or could require reclassification through the de novo process or a PMA approval. The FDA requires each manufacturer to make this determination in the first instance, but the FDA can review any such decision. If the FDA disagrees with a manufacturer’s decision not to seek a new 510(k) clearance, the agency may retroactively require the manufacturer to seek 510(k) clearance, de novo classification, or PMA approval. The FDA also can require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance, de novo classification, or PMA approval is obtained.
The De Novo Pathway
Devices of a new type that the FDA has not previously classified based on risk are automatically classified into Class III, regardless of the level of risk they pose. To avoid requiring PMA review of low- to moderate-risk devices classified in Class III by operation of law, the U.S. Congress created the de novo pathway that allows the FDA to classify a low- to moderate-risk device not previously classified into Class I or II.
Generally, a de novo petition contains a device description, indications for use statement, proposed labeling, data/performance testing (such as bench testing and/or clinical study data), the proposed classification, and a risk/benefit analysis. The risk/benefit analysis is the key element of a de novo petition and typically includes a summary of the benefits of the device, a summary of the known and potential risks, any risk mitigations, and an explanation of whether the benefits outweigh the risks.
The timing for review of a de novo petition is less certain than a 510(k). FDA has agreed to review 65% of de novo submissions received in fiscal year 2021 in 150 calendar days during which a submission is under review at the FDA. As a practical matter, de novo marketing authorization often takes longer, ranging from a year or more, and marketing authorization is never assured due, in part, to stoppages of FDA’s 150-day timeline while the applicant responds to deficiencies identified by FDA. If the FDA authorizes the de novo petition, the device may be legally marketed and used as a predicate device for future 510(k) submissions. If the de novo petition is denied, the device remains in Class III and a PMA approval may be required before the device may be legally marketed in the United States.
The PMA Approval Process
A device not eligible for 510(k) clearance or de novo classification must follow the PMA approval pathway, which requires proof of the safety and effectiveness of the device to the FDA’s satisfaction. The cost of preparing and submitting a PMA is substantial. Under U.S. federal law, the submission of most PMAs is additionally subject to a substantial annually-adjusted application user fee. For example, for fiscal year 2021, the user fee for an original PMA is $365,657. Satisfaction of FDA pre-market approval requirements typically takes years and the actual time required may vary substantially based upon the type, complexity, and novelty of the device or disease.
A PMA application must provide extensive preclinical and clinical trial data and also detailed information about the device and its components regarding, among other things, device design, manufacturing and labeling. There is typically advisory panel review of the clinical data. The FDA typically conducts a preapproval inspection of the manufacturer’s facilities and may also inspect the clinical trial documentation. FDA will not approve a device unless compliance is shown with Quality System Regulation, or QSR, requirements, which impose elaborate testing, control, documentation and other quality assurance procedures. During the review period, the FDA may also request additional information or clarification of information already provided, and the FDA may issue a major deficiency letter to the applicant, requesting the applicant’s response to deficiencies communicated by the FDA.
By statute, the FDA has 180 days to review a filed PMA application, although the review more often occurs over a significantly longer period of time. If its evaluation of a PMA is favorable, the FDA will issue either an approval letter, or an approvable letter. An approvable letter usually contains a number of conditions that must be met in order to secure a final approval of the PMA application. When and if these conditions have been fulfilled to the satisfaction of the FDA, the FDA will issue a PMA approval letter authorizing commercial marketing of the device, subject to the conditions of approval and the limitations established in this approval letter, if any. If the FDA’s evaluation of a PMA application or the relevant manufacturing facilities is not favorable, the FDA will deny approval of the PMA application or issue a not approvable letter. The FDA also may determine that additional tests or clinical trials are necessary, in which case the PMA approval may be delayed for several months or years while the trials are conducted and data is submitted in an amendment to the PMA application, or the PMA application is withdrawn and resubmitted when the data are available. The PMA process can be expensive, uncertain and lengthy and a number of devices for which the FDA approval has been sought by other companies have never been approved by the FDA for marketing.
In approving a PMA application, as a condition of approval, the FDA may also require some form of post-approval study or post-market surveillance, whereby the applicant conducts a follow-up study or follows certain patient groups for a number of years and makes periodic reports to the FDA on the clinical status of these patients when necessary to protect the public health or to provide additional or longer term safety and effectiveness data for the device. The FDA may also approve a PMA application with other post-approval conditions intended to ensure the safety and effectiveness of the device, such as, among other things, restrictions on labeling, promotion, sale, distribution and use.
Even after approval of a PMA, new PMA applications or PMA supplements may also be required for modifications to any approved device, including modifications to the manufacturing processes, device labeling and device design, based on the findings of post-approval studies. Supplements to a PMA often require the submission of the same type of information required for an original PMA, except that the supplement is generally limited to that information needed to support the proposed change from the product covered by the original PMA.
Post-market FDA Regulation
After a medical device enters commercial distribution, numerous regulatory requirements continue to apply. These include:
· the FDA’s QSR, which require manufacturers, including third-party manufacturers, to follow stringent design, testing, production, control, supplier/contractor selection, complaint handling, documentation and other quality assurance procedures during all aspects of the manufacturing process;
· labeling regulations, unique device identification requirements and FDA prohibitions against the promotion of devices for uncleared, unapproved or off-label uses;
· advertising and promotion requirements;
· restrictions on sale, distribution or use of a device;
· PMA annual reporting requirements;
· PMA approval of product modifications, or the potential for new 510(k) clearances for certain modifications to previously 510(k) cleared devices;
· medical device reporting regulations, which require that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction were to recur;
· medical device correction and removal reporting regulations, which require that manufacturers report to the FDA their field corrections and product recalls or removals if undertaken to reduce a risk to health posed by the device or to remedy a violation of the FDCA;
· recall requirements, including a mandatory recall if there is a reasonable probability that the device would cause serious adverse health consequences or death;
· an order of repair, replacement or refund;
· device tracking requirements; and
· post-market surveillance regulations, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device.
The FDA has broad post-market and regulatory enforcement powers. Medical device manufacturers are subject to unannounced inspections by the FDA and other state, local and foreign regulatory authorities to assess compliance with the QSR and other applicable regulations, and these inspections may include the manufacturing facilities of suppliers. Failure to comply with applicable regulatory requirements can result in enforcement action by the FDA, which may include sanctions such as: warning letters, fines, injunctions, consent decrees and civil penalties; unanticipated expenditures, repair, replacement, refunds, recall or seizure of our devices; operating restrictions, partial suspension or total shutdown of manufacturing; the FDA’s refusal of our requests for 510(k) clearances, de novo classification, or premarket approvals of new devices, new intended uses or modifications to existing devices; the FDA’s refusal to issue certificates to foreign governments needed to export devices for sale in other countries; and withdrawing 510(k) clearances, de novo marketing authorization, or premarket approvals that have already been granted; and criminal prosecution.
Emergency Use Authorization
In extraordinary circumstances, such as the COVID-19 pandemic, the FDA may allow the use of unapproved medical devices, including laboratory tests, on an emergency basis through what is known as an Emergency Use Authorization, or EUA. Throughout the COVID-19 public health emergency, FDA has issued guidance documents for clinical laboratories and commercial manufacturers setting forth the FDA’s current thinking and approach to the offering of tests for COVID-19.
When FDA grants emergency authorization to a product, the EUA may include certain conditions for use of that product. For example, the EUA may include conditions limiting who can distribute, administer, or use the product. Manufacturers may also be required to collect and report information regarding the safety and effectiveness of the product once it is available in the market and being used for the emergency.
We plan to offer COVID antibody tests using the Roche Elecsys Anti-SARS-CoV-2 assay that was granted an EUA for the qualitative detection of antibodies to SARS-CoV-2 in human serum and plasma and the Roche Elecsys Anti-SARS-CoV-2 S assay that was granted an EUA for the qualitative and semi-quantitative determination of antibodies to the SARS-CoV-2 spike protein. We have not applied for or obtained an EUA from the FDA for a COVID-19 assay, but can offer these antibody tests pursuant to the EUAs received by Roche. FDA has stated that laboratories that are performing testing using EUA-authorized test kits from commercial manufacturers need not notify FDA of or obtain an EUA from FDA for such testing. As an authorized laboratory, we must comply with the applicable regulatory requirements set forth in the EUA, including labeling requirements and reporting any significant deviations from the established performance characteristics of the product to FDA.
The EUAs are only in effect for the duration of the public health emergency as declared by the Secretary of the HHS. When the public health emergency is terminated, we will not be able to continue to offer the COVID-19 antibody tests unless we or Roche has sought clearance or approval for the assay and come into compliance with the QSR. We expect that HHS or FDA will institute a grace period or enforcement discretion period following termination of the public health emergency for products on the market subject to an EUA.
Federal and State Fraud and Abuse Laws
We are subject to U.S. federal fraud and abuse laws such as the AKS, the U.S. federal prohibition against physician self-referral, or Stark Law, and the FCA. We are also subject to similar state and foreign fraud and abuse laws.
The AKS prohibits knowingly and willfully offering, paying, soliciting, or receiving remuneration, directly or indirectly, overtly or covertly, in cash or in kind, in return for or to induce the referral of an individual, or to purchase, lease, order, arrange for, or recommend purchasing, leasing or ordering, any good, facility, item or service that is reimbursable, in whole or in part, under a U.S. federal healthcare program. Although there are a number of statutory exceptions and regulatory safe harbors protecting certain common activities from prosecution or other regulatory sanctions, the exceptions and safe harbors are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchases or recommendations may be subject to scrutiny if they do not qualify for an exception or safe harbor.
The Stark Law and similar state laws prohibit physician referral of patients for designated health services payable by Medicare/Medicaid to entities with which the physician or an immediate family member has a financial relationship (ownership/investment interest or compensation arrangement), unless an exception applies.
Other U.S. federal fraud and abuse laws to which we are subject include but are not limited to the U.S. federal civil and criminal false claims laws, including the FCA, which imposes liability on any person or entity that, among other things, knowingly presents, or causes to be presented, a false or fraudulent claim for payment to the U.S. federal government, and the U.S. federal Civil Monetary Penalties Law, which prohibits, among other things, the offering or transfer of remuneration to a Medicare or state healthcare program beneficiary if the person knows or should know that remuneration is likely to influence the beneficiary’s selection of a particular provider, practitioner, or supplier of services reimbursable by Medicare or a state healthcare program, unless an exception applies. Under the FCA, private citizens can bring claims on behalf of the government through qui tam actions. We must also operate within the bounds of the fraud and abuse laws of the states in which we do business which may apply to items or services reimbursed by nongovernmental third-party payers, including private insurers.
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, exclusion from government-funded healthcare programs, such as Medicare and Medicaid, disgorgement, contractual damages, reputational harm, diminished profits and future earnings, additional reporting or oversight obligations if we become subject to a corporate integrity agreement or other agreement to resolve allegations of non-compliance with the law and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations. If any of the physicians or other healthcare providers or entities with whom we do business is found to be not in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government-funded healthcare programs.
HIPAA and HITECH
Under the administrative simplification provisions of the HIPAA, as amended by HITECH, HHS issued regulations that establish uniform standards governing the conduct of certain electronic healthcare transactions and requirements for protecting the privacy and security of protected health information, or PHI, used or disclosed by covered entities. Covered entities and business associates are subject to HIPAA and HITECH.
HIPAA and HITECH include the privacy and security rules, breach notification requirements and electronic transaction standards. The privacy rule covers the use and disclosure of PHI by covered entities and business associates and generally prohibits the use or disclosure of PHI except as permitted under the rule. The privacy rule also sets forth individual patient rights, such as the right to access or amend certain records containing PHI, or to request restrictions on the use or disclosure of PHI. The security rule requires covered entities and business associates to safeguard the confidentiality, integrity, and availability of electronically transmitted or stored PHI by implementing administrative, physical and technical safeguards. Under HITECH’s breach notification rule, a covered entity must notify individuals, the Secretary of the HHS, and in some circumstances, the media of breaches of unsecured PHI.
In addition, we may be subject to state health information privacy and data breach notification laws, which may govern the collection, use, disclosure and protection of health-related and other personal information. California, for example, has enacted the Confidentiality of Medical Information Act, which sets forth standards in addition to HIPAA and HITECH with which all California health care providers must abide. State laws may be more stringent, broader in scope or offer greater individual rights with respect to PHI than HIPAA, and state laws may differ from each other, which may complicate compliance efforts.
Entities that are found to be in violation of HIPAA as the result of a breach of unsecured PHI, a complaint about privacy practices or an audit by HHS, may be subject to significant civil and criminal fines and penalties and/or additional reporting and oversight obligations if such entities are required to enter into a resolution agreement and corrective action plan with HHS to settle allegations of HIPAA non-compliance.
U.S. Healthcare Reform
In the United States, there have been a number of legislative and regulatory changes at the U.S. federal and state levels which seek to reduce healthcare costs and improve the quality of healthcare. For example, in March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or the ACA, became law. This law substantially changed the way health care is financed by both commercial payers and government payers, and significantly impacted our industry. Since 2016 there have been efforts to repeal all or part of the ACA. For example, the Tax Cuts and Jobs Act, among other things, removed penalties for not complying with the ACA’s individual mandate to carry health insurance. All or a portion of the ACA and related subsequent legislation may be modified, repealed or otherwise invalidated through judicial challenge, which could result in lower numbers of insured individuals, reduced coverage for insured individuals and adversely affect our business.
The ACA contained a number of provisions expected to impact our business and operations, some of which in ways we cannot currently predict, including those governing enrollment in state and U.S. federal health care programs, reimbursement changes and fraud and abuse, which will impact existing state and U.S. federal health care programs and will result in the development of new programs.
The expansion in the government’s role in the U.S. healthcare industry may result in decreased profits to us and lower reimbursement by payers for our tests, any of which may have a material adverse impact on our business, financial condition, results of operations or cash flows.
In addition, other legislative changes have been proposed and adopted since the ACA was enacted. On August 2, 2011, the Budget Control Act of 2011 was signed into law, which, among other things, reduced Medicare payments to providers by 2% per fiscal year, effective on April 1, 2013 and, due to subsequent legislative amendments to the statute, will remain in effect through 2029 unless additional legislative action is taken.
We anticipate there will continue to be proposals by legislators at both the federal and state levels, and by regulators and commercial payers to reduce costs while expanding individual healthcare benefits. Certain of these changes could impose additional limitations on the prices we will be able to charge for our tests, and the coverage of or the amounts of reimbursement available for our tests from payers, including commercial payers and government payers.
C. Organizational Structure
The following diagram illustrates our corporate structure, including our principal subsidiaries, as of the date of this annual report.
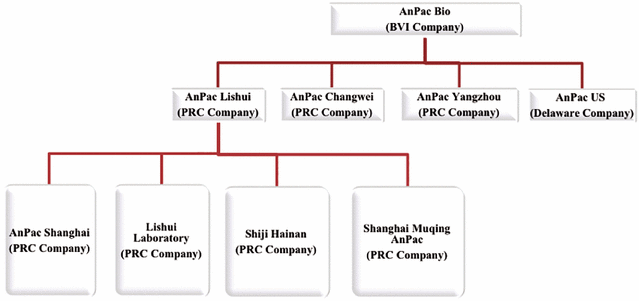
D. Property, Plants and Equipment
Our China headquarters are located in the Bihu Industrial Park in Lishui, Zhejiang Province. Our facilities for manufacturing our CDA device for our performance of commercial CDA-based tests, our principle licensed clinical laboratory to conduct commercial CDA-based tests, as well as our warehouse are all in our headquarters in Lishui. We own the premises of our Lishui headquarters, which have an aggregate floor area of approximately 5,126 square meters. We also own an additional approximately 203 square meters in Lishui and 157 square meters of office space in Yangzhou, Jiangsu Province.
We currently lease several properties with an aggregate floor area of approximately 2,310 square meters in Shanghai, where we operate our primary research and development facilities. We also lease approximately 142 square meters of properties in Haikou, Hainan Province, primarily to operate our government-approved clinical laboratory. Our leases for these properties vary in duration from one to three years.
In the United States, we currently lease approximately 6,700 square feet of office space in Montgomery County, Pennsylvania as the premises for our new CLIA-registered laboratory and U.S. headquarters, which we moved into in the second quarter of 2020. This lease has a term of approximately ten years and we are entitled to early terminate the lease in approximately five years subject to certain conditions. We also currently lease approximately 1,040 square feet of office space in San Jose as the premises for our CAP-accredited laboratory. This lease will expire on April 30, 2021.
ITEM 4A. UNRESOLVED STAFF COMMENTS
None.
ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS
You should read the following discussion and analysis of our financial condition and results of operations in conjunction with the historical consolidated financial statements of our company for the years ended December 31, 2018, 2019 and 2020, and related notes included elsewhere in this annual report on Form 20-F. This discussion contains forward-looking statements that involve risks, uncertainties and assumptions. Our actual results and the timing of selected events could differ materially from those anticipated in these forward-looking statements as a result of various factors, including those set forth under “Risk Factors” and elsewhere in this annual report.
A. Operating Results
Key Factors Affecting Our Results of Operations
Our business and operating results are influenced by certain general factors that affect China’s early cancer screening and detection market, including the increasing prevalence of cancer in China, growth of total healthcare expenditures, and technological trends in cancer diagnosis, treatment and management. Unfavorable changes in these general factors could adversely affect the results of our operations. In addition to these general trends, we believe that our results of operations are more directly affected by certain company-specific factors, including:
Market Adoption of Our CDA-Based Tests
We derive substantially all of our revenues from the sale of our CDA-based tests in China. We expect our business prospects to depend significantly on our ability to increase market adoption of our CDA-based tests in China, as well as our ability to commercialize our CDA-based tests in the U.S.
China’s large, aging population, favorable government policies, and relatively low labor costs represent substantial commercial opportunities for our business and enable us to cost-effectively conduct our cancer screening and detection tests at a large scale. However, compared to conventional, more widely accepted cancer screening and detection technologies, we face additional challenges in raising recognition and adoption of our CDA technology by physicians, patients, hospitals, medical institutions, healthcare payers and others in China’s medical community.
We believe that our CDA technology addresses many limitations of current early cancer screening and detection methods, such as its ability to detect the risk of multiple cancers early, cost-effectively and with high accuracy. We have conducted numerous research studies in cooperation with hospitals and medical institutions in China to validate our CDA technology, and we have published the results of 15 completed research studies at the American Society of Clinical Oncology, or ASCO, annual meetings and other medical conferences and medical journal supplements. To increase market adoption of our CDA-based tests, we intend to continue conducting research studies on our CDA technology on more cancer types and its applications in additional oncological areas, including assistance in diagnosis, prognosis and recurrence, and to present our study results at ASCO annual meetings and other medical conferences and publish them in important medical journals. We are also seeking to cooperate with universities and academic medical centers, hospitals and medical institutions, CROs, managed care companies and other health organizations in the U.S. to conduct research studies on our CDA technology, with a view to commercializing our CDA-based tests in the U.S. market. We plan to initially market our CDA test as an LDT in the U.S. We expect to invest significantly in research studies.
Regulatory Approvals for Our CDA Device by the NMPA
We are currently licensed to manufacture our CDA device and use it to perform our CDA-based tests at our own laboratories in China. To enlarge our total addressable market in China, in December 2018, we applied to the NMPA for a Class III medical device registration certificate for us to use our CDA device to assist in multi-cancer diagnosis. After we obtain this license, we will apply to update our medical device manufacture license to include the manufacture of Class III medical devices. With these licenses, we will be permitted to place our devices within Chinese hospitals’ laboratories to conduct commercial tests there or sell our devices to the hospitals for the purposes of assisting in physicians’ diagnosis of specified multiple cancers. We expect our revenues to grow substantially after our CDA devices are approved to access the Chinese hospital segment. However, it takes at least three years with significant R&D spending and regulatory approvals to obtain a Class III medical device registration certificate and the process is subject to regulatory and other uncertainties.
Our Customer Base and Customer Mix
Our business growth depends significantly on our ability to maintain relationships with our existing customers and attract new customers. Our existing customers in China consist primarily of life insurance companies and other corporations, which offer our CDA-based tests to their insured customers and/or employees. We also attract customers by offering our CDA-based tests as part of annual physical checkup packages and by engaging sales agents to market our tests. We plan to broaden our cancer screening and detection test offerings, including by expanding the range of genomics tests currently conducted at our Haikou laboratory, to attract more customers. If we are able to obtain the Class III medical device registration certificate and update our medical device manufacture license for our CDA device, we will seek to access the Chinese hospital market segment and provide our tests to more individual customers through Chinese hospitals. We expect our marketing expenses to continue to increase as we seek to increase market adoption of our technology and tests and build up our sales channels.
Since our business scale is currently relatively small and our customers are largely corporates, the availability and timing of large CDA-based test orders could cause our revenues to fluctuate significantly from period to period. This makes it difficult to compare our historical operating results or predict our future performance.
Cost Structure
Our results of operations are significantly affected by our cost structure. The largest component of our operating costs and expenses is staff costs, primarily related to our management as well as research and development, sales and marketing personnel. We have also incurred significant share-based compensation expenses to incentivize our directors, officers, employees and consultants, which were RMB7.9 million, RMB32.9 million and RMB17.8 million (US$2.7 million) in 2018, 2019 and 2020, respectively. In addition, we have made substantial investments in customer acquisition, research and development, and patent applications to support our future growth and expansion. As we conduct research studies in the U.S., we expect our research and development expenses to significantly increase. In addition, we expect to incur significant costs in research and development and regulatory approvals to obtain a Class III medical device registration certificate in the PRC. Once we receive this approval, we will incur significant external supplier costs for the manufacture of the devices.
Funding for Our Operations
We have funded our operations primarily through capital contributions from our initial public offering, our shareholders, short-term non-bank borrowings, convertible loans and loans from related parties. With the continuing expansion of our business, we will require further funding, possibly through public or private equity financings, debt financings, or other business arrangements. The availability and costs of funding could significantly impact our results of operations and financial position. Furthermore, debt financings could require us to agree to restrictive financial covenants, which could make it more difficult for us to achieve our goals.
Key Operating Data
We regularly review a number of operating metrics, including those set forth below, to evaluate our business, measure our performance and identify trends affecting our business.
The following table sets forth our key operating data for the periods indicated:
|
|
|
For the year ended December 31, |
| ||||
|
|
|
2018 |
|
2019 |
|
2020 |
|
|
Number of commercial CDA-based tests(1) completed |
|
41,607 |
|
52,428 |
|
41,354 |
|
|
Number of CDA-based tests(1) for research purposes completed |
|
4,873 |
|
6,121 |
|
1,892 |
|
Note:
(1) Including our CDA tests and combination tests.
Key Components of Results of Operation
Revenues
We derive our revenues from two sources: (i) revenue from sales of cancer screening and detection tests (predominantly commercial CDA-based tests) and (ii) net revenue from sales of physical checkup packages.
The table below presents our revenues by type in absolute amount and as a percentage of our total revenues for the periods indicated.
|
|
|
Year ended December 31, |
| ||||||||||||
|
|
|
2018 |
|
2019 |
|
2020 |
| ||||||||
|
|
|
RMB |
|
% |
|
RMB |
|
% |
|
RMB |
|
US$ |
|
% |
|
|
|
|
(in thousands, except %) |
| ||||||||||||
|
Cancer screening and detection tests |
|
9,557 |
|
93.2 |
|
10,381 |
|
95.7 |
|
18,445 |
|
2,827 |
|
89.9 |
|
|
Physical checkup packages |
|
693 |
|
6.8 |
|
464 |
|
4.3 |
|
2,064 |
|
316 |
|
10.1 |
|
|
Total revenues |
|
10,250 |
|
100.0 |
|
10,845 |
|
100.0 |
|
20,509 |
|
3,143 |
|
100.0 |
|
Cancer Screening and Detection Tests
Our revenue from sales of cancer screening and detection tests consists predominantly of revenue from the sales of our commercial CDA-based tests. Our commercial CDA-based tests comprise our CDA tests and our combination tests, which combine our CDA test and, on an auxiliary basis, biomarker-based and ct-DNA-based cancer screening and detection tests performed either by us or by third-party clinical laboratories. We also recognize revenue from sales of commercial CDA-based tests that we provide as part of the physical checkup packages we sell. We expect that our revenue generated from our commercial CDA-based tests will increase as our business grows, including by providing additional tailored CDA-based tests to meet customer demand and exploring other sources of revenue related to our CDA test. We also expect to recognize additional revenue from commercial genomics tests as we devote more resources to marketing and sales of these tests.
Physical Checkup Packages
Our net revenue from physical checkup packages represents our gross billing amount from physical checkup packages that we sell to our customers and have performed during a specified period, less (i) the portion of fees for the commercial CDA-based tests contained in the packages (which are recognized as part of our revenue from sales of CDA-based tests) and (ii) our cost of physical checkup services (other than CDA-based tests) contained in the packages, which are payments we make to third-party physical checkup centers to which we outsource these services. We believe that selling annual physical checkup packages can expand our customer base for commercial CDA-based tests, and we intend to devote more resources to selling physical checkup packages and expect our net revenue from these packages to continue increasing.
Cost of Revenues
Our cost of revenues is related to our sales of cancer screening and detection tests, predominantly our commercial CDA-based tests and, to a lesser extent, our genomics and immunology tests. It mainly consists of staff costs, outsourced testing costs, blood sample taking costs, medical consumable costs, share-based compensation, and depreciation and amortization of our CDA devices. Staff costs mainly include salaries and employee benefit expenses of personnel engaged in laboratory testing functions. Outsourced testing cost represents our cost of engaging third-party clinical laboratories for their performance of auxiliary biomarker-based cancer screening and detection tests, which are included as part of our combination tests. Blood sample taking costs mainly include our cost of engaging third-party nursing service providers who collect blood samples on our behalf for our commercial CDA-based tests. We expect our cost of revenues to continue to grow as we increase the volume of our commercial CDA-based tests.
Gross Profit and Gross Margin
Our gross profit represents our revenue from sales of cancer screening and detection tests minus our cost of revenue, plus our net revenues from sales of physical checkup packages. Our gross profit margin is affected primarily by the mix and relative prices of the cancer screening and detection tests that we sell within a specified period, as well as changes in net revenues from sales of physical checkup packages as a percentage of our total revenues.
Operating Expenses
Our operating expenses include selling and marketing expenses, research and development expenses, and general and administrative expenses. The following table sets forth a breakdown of these expenses for the periods indicated.
|
|
|
For the year ended December 31, |
| ||||||
|
|
|
2018 |
|
2019 |
|
2020 |
| ||
|
|
|
RMB |
|
RMB |
|
RMB |
|
US$ |
|
|
|
|
(in thousands) |
| ||||||
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
Selling and marketing |
|
9,827 |
|
13,633 |
|
19,674 |
|
3,015 |
|
|
Research and development |
|
10,106 |
|
9,839 |
|
11,576 |
|
1,774 |
|
|
General and administrative |
|
28,254 |
|
69,088 |
|
74,757 |
|
11,457 |
|
|
Impairment of long-term investments |
|
— |
|
1,320 |
|
1,430 |
|
219 |
|
|
Total |
|
48,187 |
|
93,880 |
|
107,437 |
|
16,465 |
|
Selling and Marketing Expenses
Our selling and marketing expenses primarily consist of staff costs for personnel engaged in sales, marketing and customer support functions, share-based compensation, marketing expenses, travel expenses and office expenses. We expect that our selling and marketing expenses will increase as we continue to build out our sales and marketing teams and engage more sales agents and other channel partners to increase our market penetration.
Research and Development Expenses
Our research and development expenses primarily consist of staff costs for personnel engaged in research and development functions, share-based compensation, travel expenses, rental costs, costs of consumables and accessories, and depreciation and amortization (mainly related to our clinical laboratory facilities and CDA devices used for research and development purposes). We expect that our research and development expenses will increase significantly in the near future, because we not only have multiple on-going research studies in China, but have also entered into research agreements with U.S. universities and academic medical centers, and we are in discussions with other U.S. hospitals, medical institutions, CROs, managed care companies and other health organizations, to conduct research studies on our CDA technology at our CLIA-registered laboratory in San Jose, California.
General and Administrative Expenses
Our general and administrative expenses primarily include staff costs for personnel engaged in general and administrative functions, share-based compensation, patent service fees, professional service fees, depreciation and amortization (mainly related to our land use rights for the land we acquired in Lishui, Zhejiang Province and the office facilities on that land), rental and property management fees and office expenses. We expect our general and administrative expenses to continue increasing to support our business growth, but we expect that they will eventually decrease as a percentage of our revenues once our business scale increases.
Impairment of Long-term Investments
Our long-term investments include equity method investments and equity investments without readily determinable fair values. An impairment loss is recognized in the consolidated statements of comprehensive loss equal to the amount by which the carrying value exceeds the fair value of the investment.
Non-operating Income and Expenses
Other Income (Expense), Net
Our net other income in 2018 primarily included government grants we received, including for 2018 the price that the government in Lishui, Zhejiang Province of China paid us for repurchase of a portion of a parcel of land that we did not utilize. Our net other expense in 2019 was primarily related to fair value loss as a result of the increase in value of the convertible loans that we borrowed from Jiaxing Zhijun Investment Management Co., Ltd. (“Zhijun”), offset in part by the government grants we received. Our net other income in 2020 primarily included government grants.
Taxation
BVI
Our Company is incorporated in the BVI, and we conduct our business operations primarily through our subsidiaries in China and the U.S.
All dividends, interest, rents, royalties, compensation and other amounts paid by our company to persons who are not resident in the BVI and any capital gains realized with respect to any shares, debt obligations, or other securities of our company by persons who are not resident in the BVI are exempt from all provisions of the Income Tax Ordinance in the BVI.
All instruments relating to transfers of property to or by our company and all instruments relating to transactions in respect of the shares, debt obligations or other securities of our company and all instruments relating to other transactions relating to the business of our company are exempt from payment of stamp duty in the BVI. This assumes that our company does not hold an interest in real estate in the BVI.
There are currently no withholding taxes or exchange control regulations in the BVI applicable to our company or its members.
China
Our subsidiaries in China are subject to the statutory enterprise income tax at a rate of 25%, in accordance with the EIT Law. Some of our PRC subsidiaries enjoy preferential enterprise income tax rates.
Dividends, interest, rent or royalties payable by our PRC subsidiaries to their non-PRC resident enterprise investors, and proceeds from any such non-resident enterprise investor’s disposition of assets (after deducting the net value of such assets) will be subject to withholding tax at a rate of 10%, unless the jurisdiction of incorporation of the respective non-PRC resident enterprise investor has a tax treaty or arrangements with the PRC that provides for a reduced withholding tax rate or an exemption from withholding tax. If our BVI holding company were deemed to be a “resident enterprise” under the PRC Enterprise Income Tax Law, it would also be subject to enterprise income tax on its worldwide income at a rate of 25%. See “Item 3. Key Information—Risk Factors—Risks Relating to Doing Business in China—Under the PRC Enterprise Income Tax Law, we may be classified as a PRC resident enterprise for PRC income tax purposes, which could result in unfavorable tax consequences to us and our non-PRC shareholders or ADS holders, and have a material adverse effect on our results of operations and the value of your investment.” For the foreseeable future, we intend to invest all the undistributed earnings of our subsidiaries incorporated in the PRC and do not plan to have our PRC subsidiaries distribute any dividend. Therefore, no withholding tax is expected to be incurred.
United States
Our U.S. subsidiary, AnPac US, is subject to U.S. federal corporate income tax at a rate of 21% for the years ended December 31, 2018, 2019 and 2020. AnPac US is also subject to state income tax in California for the years ended December 31, 2018, 2019 and 2020.
Critical Accounting Policies and Estimates
An accounting policy is considered critical if it requires an accounting estimate to be made based on assumptions about matters that are highly uncertain at the time such estimate is made, and if different accounting estimates that reasonably could have been used, or changes in the accounting estimates that are reasonably likely to occur periodically, could materially impact the consolidated financial statements.
We prepare our consolidated financial statements in conformity with U.S. GAAP. The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the financial statements and the reported amount of revenue and expenses during the reporting period. Changes in facts and circumstances may result in revised estimates. Actual results could differ from those estimates, and as such, differences could be material to the consolidated financial statements.
The following descriptions of critical accounting policies, judgments and estimates should be read in conjunction with our consolidated financial statements and accompanying notes and other disclosures included in this annual report. When reviewing our financial statements, you should consider (i) our selection of critical accounting policies, (ii) the judgments and other uncertainties affecting the application of these policies and (iii) the sensitivity of reported results to changes in conditions and assumptions.
Revenue Recognition
We derive our revenues principally from customers through our cancer screening and detection tests and physical checkup package services. Revenue is recognized when we satisfy the performance obligations in an amount of consideration to which we expect to be entitled to in exchange for those services. We evaluate the presentation of revenue on a gross or net basis based on whether we control the services provided to customers and are the principal (namely, on a gross basis), or we arrange for other parties to provide the service to the customers and are the agent (namely, on a net basis). We present value-added taxes as a reduction from revenues. Our revenues for the years ended December, 31, 2018, 2019 and 2020 were generated in the PRC.
Revenue from Cancer Screening and Detection Tests
Our revenue from cancer screening and detection tests is primarily generated through administration of the tests to our customer constituents. We offer CDA test and other cancer screening and detection technologies, such as biomarker-based tests, to our customers including corporations and life insurance companies. A contract exists when the master service agreement has been executed and the customer submits a service request, which is a placed order. Our contracts have a single performance obligation which is satisfied upon provision of the CDA-based test(s) and delivery of the CDA-based test result to the customer. We act as the principal as we control the CDA-based test(s) before it is transferred to the customer and record revenue on a gross basis at the point in time when the CDA-based test(s) result is delivered to the customer.
Revenue from Physical Checkup Packages
We facilitate corporations and life insurance companies to procure physical checkup services from third-party physical checkup service providers for their respective employees and policy holders. We enter into contracts with corporations and life insurance companies and physical checkup service providers. We consider both the corporations and life insurance companies and the third-party physical checkup service providers as our customers in this type of transaction. Our performance obligation is to facilitate the corporations and life insurance companies and the third-party physical checkup service providers to complete the purchase of physical checkup services, which is not controlled by us before the services are transferred to the corporations and life insurance companies. Therefore, we fulfill our performance obligation at the point in time when the employees of corporations and policy holders of life insurance companies complete the physical checkups and we record the net amount that we retain from these completed transactions as revenue.
We also enter into arrangements to deliver both CDA-based tests and physical checkup services. We are the principal for the CDA-based tests and the agent for the physical checkup services. Revenues for both services are recognized at the point in time when the performance obligation is satisfied upon delivery of the CDA-based test results to the end customers and completion of the physical checkup services, respectively. As we act as both the principal and agent in the arrangement, we allocate the transaction price to each performance obligation on a relative stand-alone selling price basis.
Contract balances
The payment terms and conditions within our contracts vary by the type of services and the customers.
Contract assets relate to our conditional right to consideration for completed performance obligations under the contract. Accounts receivable are recorded when the right to consideration becomes unconditional. We do not have contract assets for the years presented.
In instances where the timing of revenue recognition differs from the timing of invoicing, we have determined that our contracts generally do not include a significant financing component.
PRC Value-Added Taxes and surcharges
Starting from May 2016, our services are subject to 6% of Value-Added Taxes. We are subject to education surtax and urban maintenance and construction tax, on the services provided in the PRC.
Practical expedients
We have applied the following practical expedients:
(i) The transaction price allocated to the performance obligations that are unsatisfied, or partially unsatisfied has not been disclosed, as substantially all of our contracts have a duration of one year or less.
(ii) We recognize incremental costs to obtain a contract as expenses when incurred because the amortization period would be one year or less. These costs are recorded within sales and marketing expenses.
Costs of revenues
Costs of revenues consists of staff costs, outsourced testing costs, blood sample taking costs, medical consumable costs, share-based compensation and depreciation of CDA equipment.
Research and Development Expenses
Research and development expenses primarily are comprised of costs incurred in performing research and development activities, including related personnel and consultant’s salaries, benefits, share-based compensation and related costs, raw materials and supplies for internally-developed product candidates, and external costs of outside vendors engaged to conduct clinical development activities and trials. We expense our research and development expenses as they are incurred.
Share-Based Compensation
We account for share-based compensation in accordance with ASC 718, Compensation-Stock Compensation (“ASC 718”). In accordance with ASC 718, we determine whether an award should be classified and accounted for as a liability award or an equity award. All of our share-based awards were classified as equity awards and were recognized in the consolidated financial statements based on their grant date fair values.
We have elected to recognize share-based compensation using the straight-line method for all share-based awards granted with graded vesting based on service conditions. We use the accelerated method for all awards granted with graded vesting. We account for forfeitures as they occur in accordance with ASU No. 2016-09, Compensation-Stock Compensation (Topic 718): Improvement to Employee Share-based Payment Accounting. With the assistance of an independent third-party valuation firm, we determined the fair value of the stock options granted to employees. The binomial option pricing model and the Black-Scholes Model were applied in determining the estimated fair value of the options granted to employees and non-employees.
Fair value of options
We use the binomial tree option pricing model to estimate the fair value of share options with the assistance of an independent third-party valuation firm. The assumptions used to value the share options granted to employees and nonemployee were as follows:
|
|
|
For the year ended December 31, |
| ||||
|
|
|
2018 |
|
2019 |
|
2020 |
|
|
Risk-free interest rate |
|
2.46%-3.11% |
|
1.55%-2.50% |
|
0.55%-0.93% |
|
|
Expected volatility range |
|
62.14%-63.61% |
|
60.37%-64.48% |
|
49%-65% |
|
|
Exercise multiple |
|
2.5 |
|
2.5 |
|
2.5 |
|
|
Fair market value per ordinary share as at grant dates |
|
US$9.46-9.61 |
|
US$9.61-9.80 |
|
US$1.74-$4.83 |
|
The estimated fair value of our ordinary shares at their respective grant dates was determined with the assistance of an independent third-party valuation firm. The risk-free interest rate for periods within the contractual life of the options is based on the U.S. Treasury yield curve in effect at the time of grant for a term consistent with the contractual term of the awards. Expected volatility is estimated based on the historical volatility of ordinary shares of several comparable companies in the same industry. The expected exercise multiple is based on management’s estimation, which we believe is representative of the future.
We also entered into a share purchase agreement in 2019 with CRS Holdings Inc., a company controlled by Dr. Chris Chang Yu, who has also served as the Chief Executive Officer since our inception. Pursuant to the share purchase agreement, CRS Holdings Inc. purchased 214,000 ordinary shares at a consideration of $3.27 per share. The offering price in the share purchase agreement with CRS Holdings Inc., which is below fair value, essentially represents compensation to Dr. Chris Chang Yu, for his past services to us.
The following table sets forth the amount of share-based compensation expense included in each of the relevant financial statement line items:
|
|
|
For the year ended December 31, |
| ||||||
|
|
|
2018 |
|
2019 |
|
2020 |
| ||
|
|
|
RMB |
|
RMB |
|
RMB |
|
US$ |
|
|
|
|
(in thousands) |
| ||||||
|
Cost of revenues |
|
317 |
|
327 |
|
327 |
|
50 |
|
|
Selling and marketing expenses |
|
2,871 |
|
5,393 |
|
1,113 |
|
170 |
|
|
Research and development expenses |
|
1,958 |
|
2,534 |
|
3,534 |
|
542 |
|
|
General and administrative expenses |
|
2,790 |
|
24,601 |
|
12,788 |
|
1,960 |
|
|
Total share-based compensation expenses |
|
7,936 |
|
32,855 |
|
17,762 |
|
2,722 |
|
Fair value of financial instruments
We apply ASC 820, Fair Value Measurements and Disclosures, (“ASC 820”). ASC 820 defines fair value, establishes a framework for measuring fair value and expands disclosures about fair value measurements. ASC 820 establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value as follows:
Level 1—Observable inputs that reflect quoted prices (unadjusted) for identical assets or liabilities in active markets.
Level 2—Other inputs that are directly or indirectly observable in the marketplace.
Level 3—Unobservable inputs which are supported by little or no market activity.
ASC 820 describes three main approaches to measuring the fair value of assets and liabilities: (1) market approach; (2) income approach; and (3) cost approach. The market approach uses prices and other relevant information generated from market transactions involving identical or comparable assets or liabilities. The income approach uses valuation techniques to convert future amounts to a single present value amount. The measurement is based on the value indicated by current market expectations about those future amounts. The cost approach is based on the amount that would currently be required to replace an asset.
Our financial instruments include cash and cash equivalents, accounts receivables, accounts payable, other receivables, other payables and short-term debt. The carrying values of these financial instruments approximate their fair values due to their short-term maturities.
We elected the fair value option to account for its convertible loans. We engaged an independent valuation firm to perform the valuation. The fair value of the convertible loans as of December 31, 2019 and 2020 was RMB24.6 million and RMB2.2 million (US$342,000) calculated using the binomial tree model. The convertible loans are classified as level 3 instruments as the valuation was determined based on unobservable inputs which are supported by little or no market activity and reflect our own assumptions in measuring fair value. Significant estimates used in developing the fair value of the convertible loans include time to maturity, risk-free interest rate, straight debt discount rate, probability to convert and expected timing of conversion.
Prior to the adoption of ASU 2016-01 on January 1, 2019, fair value changes relating to our own credit risks of the convertible bonds accounted for under fair value option were recognized together with the total changes in fair value in the consolidated statement of comprehensive loss. After the adoption of ASU 2016-01, such fair value changes related to our own credit risks are recognized separately in accumulated other comprehensive loss.
As the inputs used in developing the fair value for level 3 instruments are unobservable, and require significant management estimate, a change in these inputs could result in a significant change in the fair value measurement.
Results of Operations
The following table summarizes our results of operations for the periods indicated. This information should be read together with our consolidated financial statements and related notes included elsewhere in this annual report. The operating results in any period are not necessarily indicative of the results that may be expected for any future period.
|
|
|
Year ended December 31, |
| ||||||||||||
|
|
|
2018 |
|
2019 |
|
2020 |
| ||||||||
|
|
|
RMB |
|
% of |
|
RMB |
|
% of |
|
RMB |
|
US$ |
|
% of |
|
|
|
|
(in thousands, except %) |
| ||||||||||||
|
Revenues: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cancer screening and detection tests |
|
9,557 |
|
93.2 |
|
10,381 |
|
95.7 |
|
18,445 |
|
2,827 |
|
89.9 |
|
|
Physical checkup packages, net |
|
693 |
|
6.8 |
|
464 |
|
4.3 |
|
2,064 |
|
316 |
|
10.1 |
|
|
Total revenues |
|
10,250 |
|
100.0 |
|
10,845 |
|
100.0 |
|
20,509 |
|
3,143 |
|
100.0 |
|
|
Cost of revenues, cancer screening |
|
(5,672 |
) |
(55.3 |
) |
(6,047 |
) |
(55.8 |
) |
(7,628 |
) |
(1,169 |
) |
(37.2 |
) |
|
Gross profit |
|
4,578 |
|
44.7 |
|
4,798 |
|
44.2 |
|
12,881 |
|
1,974 |
|
62.8 |
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Selling and marketing |
|
(9,827 |
) |
(95.9 |
) |
(13,633 |
) |
(125.7 |
) |
(19,674 |
) |
(3,015 |
) |
(95.9 |
) |
|
Research and development |
|
(10,106 |
) |
(98.6 |
) |
(9,839 |
) |
(90.7 |
) |
(11,576 |
) |
(1,774 |
) |
(56.4 |
) |
|
General and administrative |
|
(28,254 |
) |
(275.6 |
) |
(69,088 |
) |
(637.0 |
) |
(74,757 |
) |
(11,457 |
) |
(364.5 |
) |
|
Impairment of long-term investments |
|
— |
|
— |
|
(1,320 |
) |
(12.2 |
) |
(1,430 |
) |
(219 |
) |
(7.0 |
) |
|
Loss from operations |
|
(43,609 |
) |
(425.5 |
) |
(89,082 |
) |
(821.4 |
) |
(94,556 |
) |
(14,491 |
) |
(461.0 |
) |
|
Non-operating income and expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense, net |
|
(925 |
) |
(9.0 |
) |
(2,609 |
) |
(24.1 |
) |
(1,143 |
) |
(175 |
) |
(5.6 |
) |
|
Foreign exchange loss, net |
|
(2,776 |
) |
(27.1 |
) |
(3,219 |
) |
(29.7 |
) |
(667 |
) |
(102 |
) |
(3.3 |
) |
|
Share of net (loss) gain in equity method investments |
|
(441 |
) |
(4.3 |
) |
190 |
|
1.7 |
|
(13 |
) |
(2 |
) |
(0.1 |
) |
|
Other income (expense), net |
|
6,040 |
|
58.9 |
|
(1,823 |
) |
(16.8 |
) |
9,096 |
|
1,394 |
|
44.4 |
|
|
Change in fair value of convertible debt and settlement gain |
|
(784 |
) |
(7.6 |
) |
(5,296 |
) |
(48.8 |
) |
6,630 |
|
1,016 |
|
32.3 |
|
|
Loss before income taxes |
|
(42,495 |
) |
(414.6 |
) |
(101,839 |
) |
(939.1 |
) |
(80,653 |
) |
(12,360 |
) |
(393.3 |
) |
|
Income tax benefit |
|
199 |
|
1.9 |
|
218 |
|
2.0 |
|
88 |
|
13 |
|
0.4 |
|
|
Net loss |
|
(42,296 |
) |
(412.6 |
) |
(101,621 |
) |
(937.0 |
) |
(80,565 |
) |
(12,347 |
) |
(392.4 |
) |
Year Ended December 31, 2020 Compared to Year Ended December 31, 2019
Revenues
Our total revenues increased by 89.1% to RMB20.5 million (US$3.1 million) for 2020 from RMB10.8 million for 2019, primarily due to a significant increase in our revenue from sales of cancer screening and detection tests.
Our revenue generated from sales of cancer screening and detection tests increased by 77.7% to RMB18.4 million (US$2.8 million) for 2020 from RMB10.4 million for 2019, primarily due to higher test prices charged in 2020. Although we completed approximately 41,354 CDA-based tests in 2020 compared to 52,428 CDA-based tests completed in 2019, our average individual CDA-based test price in 2020 was RMB446 (US$68.4), which increased by 125.3% from RMB198 in 2019. This was primarily because we offered our customers more comprehensive multi-cancer detection tests for which we were able to charge higher test prices.
Our net revenue generated from sales of physical checkup packages increased substantially to RMB2.1 million (US$316,000) for 2020 from RMB464,000 for 2019, primarily due to an increase in the volume of physical checkup packages that we completed in 2020.
Cost of Revenues
Our cost of revenues increased by 26.1% to RMB7.6 million (US$1.2 million) for 2020 from RMB6.0 million for 2019, primarily attributable to more comprehensive multi-cancer detection tests performed, which resulted in increased costs related to testing materials, outsourced biomarker-based tests, blood sample taking and medical consumables. The increase in our cost of revenues was also attributable to an increase in depreciation expense, as we put more CDA devices into use to meet the increased demand for our CDA-based tests.
Gross Profit
Our gross profit more than doubled to RMB12.9 million (US$2.0 million) for 2020 from RMB4.8 million for 2019 , which was in line with the significant increase in our revenue from 2019 to 2020 primarily due to higher test prices we charged for the comprehensive CDA-based tests and physical checkup tests performed. Our gross margin increased to 62.8% for 2020 from 44.2% for 2019.
Operating Expenses
Selling and marketing expenses
Our selling and marketing expenses increased by 44.3% to RMB19.7 million (US$3.0 million) for 2020 from RMB13.6 million for 2019, primarily due to higher marketing expenses as we increased marketing efforts, partially offset by the lower share-based compensation as we granted less options to our marketing and sales personnel.
Research and development expenses
Our research and development expenses increased by 17.7% to RMB11.6 million (US$1.8 million) for 2020 from RMB9.8 million for 2019, primarily because we conducted more research and development activities. This increase was also attributable to an increase in our research and development related depreciation expenses and higher staff costs and share-based compensation for our research and development personnel.
General and administrative expenses
Our general and administrative expenses increased by 8.2% to RMB74.8 million (US$11.5 million) for 2020 from RMB69.1 million for 2019, primarily due to increased listing-related professional fees as well as increased staff compensation incurred in 2020, offset in part by a decrease in share-based compensation.
Impairment of long-term investments
Our impairment of long-term investments increased by 8.3% to RMB1.4 million (US$219,000) for 2020 from RMB1.3 million for 2019, primarily because our investment in Jiangsu Anpac was impaired to zero, we expected the investment of Jiangsu Anpac would not generate any future income.
Non-operating Income and Expenses
Interest expense, net
Our net interest expense decreased significantly to RMB1.1 million (US$175,000) for 2020 from RMB2.6 million for 2019, primarily due to a decrease in our borrowings in 2020.
Other income (expense), net
We recognized net other expense of RMB1.8 million for 2019, which turned into net other income of RMB9.1 million (US$1.4 million) for 2020, primarily due to a government subsidy of RMB7.5 million granted in 2020, which was granted to us as general incentives for our business development.
Change in fair value of convertible debt and settlement gain
We elected to recognize the convertible debt at fair value. For the years ended December 31, 2018 and 2019, we recognized an unrealized loss of RMB784,000 and RMB5.3 million, respectively, due to changes in fair value of our convertible debt borrowed from Zhijun, respectively. For the year ended December 31, 2020, we fully repaid the convertible debt with Zhijun and recognized a settlement gain of RMB7.2 million ($1.1 million). In addition, we recognized an unrealized loss of RMB532,000 (US$82,000) from new convertible debt borrowed from EMA Financial, LLC due to a change in its fair value in 2020.
Net Loss
As a result of the foregoing, our loss for the year was RMB80.6 million (US$12.3 million) for 2020, compared to RMB101.6 million for 2019.
Year Ended December 31, 2019 Compared to Year Ended December 31, 2018
Revenues
Our revenues increased by 5.8% to RMB10.8 million for 2019 from RMB10.3 million for 2018, due to an increase in our revenue from sales of cancer screening and detection tests, partially offset by a decrease in our net revenue from sales of physical checkup packages.
Our revenue generated from sales of cancer screening and detection tests increased by 8.6% to RMB10.4 million for 2019 from RMB9.6 million for 2018, due to an increase in the sales volume of our CDA-based tests, which was partially offset by a decrease in the average selling price of our CDA-based tests as we offered greater discounts to certain customers as a marketing strategy.
Our net revenue generated from sales of physical checkup packages decreased by 33.0% to RMB464,000 for 2019 from RMB693,000 for 2018, primarily due to a substantial increase in the volume of physical checkup packages that we sold to a sales agent at prices lower than our costs as part of our customer acquisition strategy.
Cost of Revenues
Our cost of revenues increased by 6.6% to RMB6.0 million for 2019 from RMB5.7 million for 2018. The increase was primarily attributable to our increased sales volume of CDA-based tests, which resulted in an increase in the testing cost for outsourced biomarker-based tests as well as increases in blood sample taking costs and medical consumables costs. The increase in our cost of revenues was also attributable to an increase in depreciation expense, as we put more CDA devices into use to meet the increased demand for our CDA-based tests.
Gross Profit
Our gross profit was RMB4.6 million and RMB4.8 million for 2018 and 2019, respectively. Our gross margin decreased slightly to 44.2% for 2019 from 44.7% for 2018.
Operating Expenses
Selling and marketing expenses
Our selling and marketing expenses increased by 38.7% to RMB13.6 million for 2019 from RMB9.8 million for 2018, primarily due to (i) higher share-based compensation as we granted more options to our marketing and sales personnel, and (ii) higher marketing expenses as we increased our marketing efforts.
Research and development expenses
Our research and development expenses decreased by 2.6% to RMB9.8 million for 2019 from RMB10.1 million for 2018, primarily because we conducted less research and development activities under one of our research projects, as we came closer to the completion of the project, in 2019 compared to 2018. The decrease in our research and development expenses was also attributable to a decrease in our research and development related depreciation expenses. These factors were partially offset by higher staff costs and share-based compensation for our research and development personnel.
General and administrative expenses
Our general and administrative expenses increased significantly to RMB69.1 million for 2019 from RMB28.3 million for 2018, primarily due to (i) higher share-based compensation to personnel engaged in general and administrative functions, and (ii) higher professional service fees, primarily related to our initial public offering.
Non-operating Income and Expenses
Interest expenses, net
Our net interest expense increased significantly to RMB2.6 million for 2019 from RMB925,000 for 2018, primarily due to an increase in average borrowings.
Other oncome (expense), net
We recognized net other income of RMB6.0 million for 2018, which turned into net other expense of RMB1.8 million for 2019, primarily due to an increase in fair value loss as a result of the increase in value of the convertible loans that we borrowed from Zhijun.
Change in fair value of convertible debt and settlement gain
We recognized an unrealized loss of RMB784,000 in 2018 and RMB5.3 million in 2019 due to changes in fair value of convertible debt with Zhijun.
Net Loss
As a result of the foregoing, our loss for the year was RMB101.6 million for 2019, compared to RMB42.3 million for 2018.
Non-GAAP Financial Measure
In evaluating our business, we consider and use adjusted net loss, a non-GAAP measure, as a supplemental measure to review and assess our operating performance. The presentation of this non-GAAP financial measure is not intended to be considered in isolation or as a substitute for financial information prepared and presented in accordance with U.S. GAAP. We define adjusted net loss as net loss adjusted to add back share-based compensation expenses.
We believe that adjusted net loss helps to identify underlying trends in our business that could otherwise be distorted by the effect of the expenses that we add back to net loss. We believe that adjusted net loss provides useful information about our operating results, enhances the overall understanding of our past performance and future prospects, and allows for greater visibility with respect to key metrics used by our management in its financial and operational decision-making.
The non-GAAP financial measure “adjusted net loss” is not defined under U.S. GAAP, is not presented in accordance with U.S. GAAP and has limitations as an analytical tool. One of the key limitations of using adjusted net loss is that it does not reflect all of the items of income and expense that affect our operations. Share-based compensation has been and may continue to be incurred in our business and is not reflected in the presentation of adjusted net loss. Further, the non-GAAP financial measure “adjusted net loss” may differ from the non-GAAP information used by other companies, including peer companies, and therefore their comparability may be limited.
We compensate for these limitations by reconciling the non-GAAP financial measure to the nearest U.S. GAAP performance measure, all of which should be considered when evaluating our performance. This non-GAAP financial measure should be viewed in addition to, and not as a substitute for, our reported results prepared in accordance with U.S. GAAP and should be read only in conjunction with our consolidated financial statements prepared in accordance with U.S. GAAP that are included elsewhere in this annual report.
|
|
|
Year ended December 31, |
| ||||||
|
|
|
2018 |
|
2019 |
|
2020 |
| ||
|
|
|
RMB |
|
RMB |
|
RMB |
|
US$ |
|
|
|
|
(in thousands) |
| ||||||
|
Net loss |
|
(42,296 |
) |
(101,621 |
) |
(80,565 |
) |
(12,347 |
) |
|
Add: |
|
|
|
|
|
|
|
|
|
|
Share-based compensation expenses |
|
7,936 |
|
32,855 |
|
17,762 |
|
2,722 |
|
|
Adjusted net loss |
|
(34,360 |
) |
(68,766 |
) |
(62,803 |
) |
(9,625 |
) |
Recent Accounting Pronouncements
A list of recent relevant accounting pronouncements is included in Note 2 “Summary of Principal Accounting Policies” of our Consolidated Financial Statements.
Inflation
Since our inception, inflation has not materially affected our results of operations. According to the National Bureau of Statistics of China, the year-over-year percent change in the consumer price index was 1.9% for December 2018, 4.5% for December 2019 and 0.3% for December 2020. Although we have not been materially affected by inflation, we may be affected if China experiences higher rates of inflation in the future.
B. Liquidity and Capital Resources
Our principal sources of liquidity have been cash generated from financing and operating activities. Management expects continuous capital financing through debt or equity issuance to support its working capital. As of December 31, 2020, our short-term debt included (i) convertible loans of RMB2.2 million (US$342,000) we borrowed from EMA Financial, LLC. in 2020, and (ii) short-term borrowings of RMB6.0 million (US$920,000). Subsequently we have completed the following capital raising transactions:
(i) On February 5, 2021, we closed the issuance of convertible debentures in the aggregate principal of US$2,000,000 at a purchase price of US$1,700,000 pursuant to Regulation S under the Securities Act to certain non-U.S. investors. After deducting the original issue discount and offering expenses, the net proceeds will be used for general corporate purposes. The debentures will mature in twelve months on February 4, 2022 and carries an interest rate of 0% per year, which interest rate shall increase to an annual of 15% per year for any such day that the Closing Bid Price is below the Floor Price (means $1.00 per share). See “Item 3.D Risk Factors—Risks Related to Our Business — Substantial future sales or perceived potential sales of ADSs or ordinary shares, including upon the exercise of vested options and conversion of convertible securities, in the public market could cause the price of ADSs to decline” for more information on these debentures.
(ii) On February 5, 2021, we entered into a placement agent agreement with Univest Securities, LLC for potential fundraising around US$15 million. Under the agreement, our engagement of Univest Securities, LLC is exclusive and the engagement is terminable by either party after six months from the date of the agreement. The placement agent’s obligations under this agreement are on a reasonable best efforts only. Any sale of securities by us to any purchaser will be evidenced by a separate securities purchase agreement between us and the purchaser.
(iii) On February 21, 2021, we entered into a share subscription agreement with a Chinese investor (the “Investor”), under which we would issue, and the Investor would acquire, a number of Class A ordinary shares for a consideration of RMB12 million at a purchase price of US$4.8 per share. We issued 387,597 Class A ordinary shares to the Investor on February 24, 2021 according to the subscription agreement at an exchange rate of RMB6.45 to US$1.00. We received the consideration in full, net of expenses, on March 18, 2021. We paid agency finder’s fee in the form of 19,174 Class A ordinary shares to a Chinese consultant on March 22, 2021 in connection with this transaction.
(iv) On April 5, 2021, we entered into a letter of investment intent with Shanghai Shidi Investment Management Center (LLP) (“Shidi”). Pursuant to this letter, Shidi will enter into an investment agreement with us by May 5, 2021, under which it will subscribe to a number of shares in our company for total consideration of US$3 million (or RMB equivalent) at a price that is 80% of the average closing price for the twenty trading days immediately before the effective date of the investment agreement. Shidi is an affiliate of one of our existing shareholders, who is positive on our future business development and willing to support our working capital shortfall.
(v) On April 12, 2021, we entered into a letter of investment intent with Zhijun Sihang Holdings Limited. Pursuant to this letter, Zhijun Sihang Holdings Limited will enter into an investment agreement with us by May 12, 2021, under which it will subscribe to a number of shares in our company for total consideration of US$3 million (or RMB equivalent) at a price that is 80% of the average closing price for the twenty trading days immediately before the effective date of the investment agreement. Zhijun Sihang Holdings Limited is one of our existing shareholders, who is positive on our future business development and willing to support our working capital shortfall.
We believe that the current cash and cash equivalents, together with the financing received subsequent to year end will be sufficient to meet the our anticipated cash needs, including its cash needs for working capital and capital expenditures, for at least the next 12 months from the consolidated financial statement filing date.
We intend to finance our future working capital requirements and capital expenditures from cash generated from funds raised from financing activities until operating activities generate positive cash flows, if ever. We may, however, also require additional cash due to changing business conditions or other future developments, including any investments or acquisitions we may decide to pursue. If our existing cash is insufficient to meet its requirements, we may seek to issue debt or equity securities or obtain additional credit facilities. Financing may be unavailable in the amounts it needs or on terms acceptable to us, if at all. Issuance of additional equity securities or equity-linked securities, including convertible debt securities, would dilute its earnings per share. The incurrence of debt would divert cash for working capital and capital expenditures to service debt obligations and could result in operating and financial covenants that restrict its operations and its ability to pay dividends to our shareholders.
Substantially all of our revenues in the foreseeable future are likely to continue to be in the form of Renminbi. Under existing PRC foreign exchange regulations, payments of current account items, including profit distributions, interest payments and trade and service-related foreign exchange transactions, can be made in foreign currencies without prior SAFE approval as long as certain routine procedural requirements are fulfilled. Therefore, our PRC subsidiaries are allowed to pay dividends in U.S. dollars to us without prior SAFE approval by following these routine procedural requirements. However, approval from or registration with competent government authorities is required where the Renminbi is to be converted into foreign currency and remitted out of China to pay capital expenses such as the repayment of loans denominated in foreign currencies. The PRC government may at its discretion restrict access to foreign currencies for current account transactions in the future.
The following table sets forth selected cash flow statement information for the periods indicated:
|
|
|
Year Ended December 31, |
| ||||||
|
|
|
2018 |
|
2019 |
|
2020 |
| ||
|
|
|
RMB |
|
RMB |
|
RMB |
|
US$ |
|
|
|
|
(in thousands) |
| ||||||
|
Net cash used in operating activities |
|
(31,147 |
) |
(48,600 |
) |
(58,967 |
) |
(9,035 |
) |
|
Net cash used in investing activities |
|
(2,680 |
) |
(3,461 |
) |
(2,482 |
) |
(380 |
) |
|
Net cash provided by financing activities |
|
36,271 |
|
46,108 |
|
60,924 |
|
9,339 |
|
|
Effect of exchange rate changes on cash and cash equivalents |
|
(969 |
) |
(809 |
) |
(2,584 |
) |
(401 |
) |
|
Net increase (decrease) in cash, cash equivalents |
|
1,475 |
|
(6,762 |
) |
(3,109 |
) |
(477 |
) |
|
Cash and cash equivalents at the beginning of the year |
|
11,412 |
|
12,887 |
|
6,125 |
|
939 |
|
|
Cash and cash equivalents at the end of the year |
|
12,887 |
|
6,125 |
|
3,016 |
|
462 |
|
Operating Activities
Net cash used in operating activities for 2020 was RMB59.0 million (US$9.0 million), which was primarily attributable to our net loss of RMB80.1 million (US$12.3 million) for the same year, as adjusted to add back share-based compensation of RMB17.8 million (US$2.7 million) and to deduct gain on settlement of convertible loan of RMB7.2 million (US$1.1 million). Our increase in net operating assets and liabilities of RMB2.0 million (US$0.3 million) was primarily due to (i) an RMB6.8 million (US$1.0 million) increase in accrued expenses and other current liabilities as a result of the increased accrued service costs and consulting fees and (ii) an increase of RMB4.8 million (US$743,000) in other current assets, partially offset by a RMB7.3 million (US$1.1 million) decrease in accounts receivable and an RMB4.8 million (US$732,000) increase in advance to suppliers in line with higher revenue generated in 2020.
Net cash used in operating activities for 2019 was RMB48.6 million, which was primarily attributable to our net loss of RMB101.6 million for the same year, as adjusted to add back share-based compensation of RMB32.9 million, fair value loss on convertible loans of RMB5.3 million, and foreign exchange loss of RMB4.1 million before changes in operating assets and liabilities. Our increase in net operating liabilities of RMB6.4 million was primarily due to an RMB8.2 million increase in accrued expenses and other current liabilities, partially offset by an RMB2.9 million increase in other current assets and an RMB1.9 million decrease in advance from customers.
Net cash used in operating activities for 2018 was RMB31.1 million, which was primarily attributable to our net loss of RMB42.3 million for the same year, as adjusted to deduct gains on disposal of land use right of RMB5.0 million and a foreign exchange loss of RMB2.5 million and to add back share-based compensation of RMB7.9 million and depreciation and amortization of RMB3.1 million before changes in operating assets and liabilities. Our increase in net operating liabilities of RMB874,000 was primarily due to an RMB2.3 million increase in advances from customers, primarily related to our CDA-based tests, and an RMB1.4 million increase in accrued expenses and other current liabilities. These factors were partially offset by an RMB1.6 million increase in advances to suppliers and an RMB1.1 million increase in accounts receivable.
Investing Activities
Net cash used in investing activities for 2020 was RMB2.5 million (US$380,000), which was primarily attributable to our purchase of property and equipment of RMB2.5 million (US$378,000).
Net cash used in investing activities for 2019 was RMB3.5 million, which was primarily attributable to our purchase of property and equipment of RMB2.8 million.
Net cash used in investing activities for 2018 was RMB2.7 million, which was primarily attributable to payments (net of cash received) for our acquisition of our subsidiary, Shiji Hainan, of RMB3.5 million, purchases of property and equipment of RMB2.4 million, and purchases of long-term investments in certain investee companies of RMB1.6 million, partially offset by proceeds from disposal of land use rights of RMB5.3 million.
Financing Activities
Net cash provided by financing activities for 2020 was RMB60.9 million (US$9.3 million), which was primarily attributable to (i) proceeds from issuance of ordinary shares of RMB110.7 million (US$17.0 million), partially offset by our payment for IPO-related expenses of RMB25.6 million (US$3.9 million), and (ii) proceeds from short-term borrowings of RMB13.8 million (US$2.1 million), partially offset by payment for short-term borrowings of RMB20.0 million (US$3.1 million) and repayment of convertible loans of RMB17.3 million (US$2.6 million).
Net cash provided by financing activities for 2019 was RMB46.1 million, which was primarily attributable to (i) proceeds from issuance of ordinary shares of RMB47.6 million, and (ii) proceeds from short-term borrowings of RMB24.3 million, partially offset by our payment for short-term borrowings of RMB18.3 million.
Net cash provided by financing activities for 2018 was RMB36.3 million, which was primarily attributable to (i) advances from Jiaxing Zhijun Sihang Investment Partnership Enterprises (Limited Partnership), or Zhijun Sihang, of RMB25.0 million to one of our PRC subsidiaries in 2018, which constituted a step in the process of Zhijun Sihang making equity contributions of these funds in our company, and (ii) proceeds from short-term borrowings from Zhijun and a non-bank institution of RMB26.6 million, partially offset by payment for short-term borrowings from a non-bank institution of RMB14.7 million.
Capital Expenditures
Our capital expenditures were RMB2.8 million, RMB3.2 million and RMB2.5 million (US$0.4 million) for the years ended December 31, 2018, 2019 and 2020, respectively. In these periods, these capital expenditures included the purchases of property and equipment and intangible assets. We will continue to make capital expenditures to meet the needs of our business’ expected growth.
C. Research and Development, Patents and Licenses, Etc.
See “Item 4. Information on the Company—B. Business Overview—Research and Development.” See “Item 4. Information on the Company—B. Business Overview—Intellectual Property.”
D. Trend Information
Market Trends
Other than as disclosed elsewhere in this annual report, we are not aware of any trends, uncertainties, demands, commitments or events for the fiscal year ended December 31, 2020 that are reasonably likely to have a material and adverse effect on our net revenues, income, profitability, liquidity or capital resources, or that would cause the disclosed financial information to be not necessarily indicative of future results of operations or financial condition.
E. Off Balance Sheet Commitments and Arrangements
We have not entered into any financial guarantees or other commitments to guarantee the payment obligations of any third parties. In addition, we have not entered into any derivative contracts that are indexed to our shares and classified as shareholders’ equity or that are not reflected in our consolidated financial statements. Furthermore, we do not have any retained or contingent interest in assets transferred to an unconsolidated entity that serves as credit, liquidity or market risk support to such entity. We do not have any variable interest in any unconsolidated entity that provides financing, liquidity, market risk or credit support to us or engages in leasing, hedging or product development services with us.
F. Tabular Disclosure of Contractual Obligations
Our contractual obligations include our operating lease commitments related to our business premises. The table below sets forth our contractual obligations for the as of December 31, 2020.
|
|
|
For the year ending December 31, |
| ||||||||||
|
|
|
Total |
|
2021 |
|
2022 |
|
2023 |
|
2024 |
|
2025 and |
|
|
|
|
(RMB in thousands) |
| ||||||||||
|
Operating lease obligations |
|
27,389 |
|
1,838 |
|
1,432 |
|
2,717 |
|
2,739 |
|
18,663 |
|
G. Safe Harbor
See “Forward-Looking Statements” in this annual report.
ITEM 6. DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
A. Directors and Executive Officers
The following table sets forth information regarding our directors and executive as of the date of this annual report.
|
Name |
|
Age |
|
Position/Title |
|
Chris Chang Yu |
|
63 |
|
Founder, chairman of the board of directors and chief executive officer |
|
Feng Guo |
|
41 |
|
Director |
|
Lin Yu |
|
61 |
|
Director |
|
Pu Xing |
|
54 |
|
Independent director |
|
Ren Luo |
|
63 |
|
Independent director |
|
Jianhua Shao |
|
61 |
|
Independent director |
|
Sarah Yu |
|
32 |
|
Director |
|
Edwards Jinqiu Tang |
|
38 |
|
Chief financial officer |
|
He Yu |
|
64 |
|
Chief medical officer |
|
Xuedong Du |
|
41 |
|
Vice president in charge of R&D |
|
Weidong Dai |
|
60 |
|
China president |
Dr. Chris Chang Yu is a co-founder of our company and has served as chairman of our board of directors and chief executive officer since our inception in January 2010. As the first or principal inventor of more than 300 patent applications spanning semiconductor, materials and life science, Dr. Yu has innovated leading technologies and products during his long and successful career since 1990s. Dr. Yu and our team have developed the CDA technology for cancer screening and detection. He is a member of the ASCO. Prior to founding our company, he co-founded Anji Microelectronics (Shanghai) Co., Ltd. (688019.SH) in 2004, and that company recently completed its IPO in China’s science and technology innovation board market in July 2019. Dr. Yu served as a technical director at Semiconductor Manufacturing International Corporation (NYSE: SMI and SEHK: 981) from 2002 to 2004. Dr. Yu served as a vice president of the research and development team of Cabot Microelectronics Corporation, or Cabot, from 1996 to 2002. While working at Cabot, Dr. Yu took a multi-disciplinary approach to developing a new mechanism for a key integrated circuit material. Dr. Yu also worked at three U.S. Fortune 500 companies, including serving as a group leader in the research and development division at Rockwell Co., Ltd. from 1994 to 1995, engineer at Motorola Co., Ltd. from 1992 to 1994, and senior engineer at Micron Technology Co., Ltd. from 1989 to 1992. He has also authored more than 80 papers, some of which are relevant to cancer detection. Dr. Yu received his bachelor and master’s degrees in physics from the University of Missouri Kansas-City Campus in 1983 and 1984, respectively. He received his doctoral degree in physics from the Pennsylvania State University in 1990. His master’s and doctoral dissertations both addressed innovative detection techniques.
Mr. Feng Guo has served as our director since August 2018. He is a co-founder and the president of Jiaxing Zhijun Investment Management Co., Ltd. He is also a sponsor representative and a Chartered Financial Analyst. He has served as an executive director at the Investment Banking Division of Guo Xin Securities Co., Ltd. since 2004 and an executive director at the Investment Banking Division of Huajing Securities since 2017. He also served as a director at China Renaissance Capital from 2015 to 2017. Mr. Guo has approximately 16 years of experience in China’s capital markets and many years of experience in the fields of high-end manufacturing, technology, media and telecom (TMT), medical consumption and energy transportation. He has experience in leading the financial consultation, stock reform, IPO, refinancing, acquisition and capital reduction transactions for many domestic and foreign companies. Mr. Guo received his bachelor’s degree in economics from East China University of Political Science and Law in 2002 and his master’s degree in finance from Shanghai University of Finance and Economics in 2004.
Ms. Lin Yu has served as our director since our inception in January 2010. Ms. Lin Yu served as legal representative at Shanghai Yu Lin Information Science Co., Ltd. from April 2016 to October 2018, a consultant for Yi Mai Fiber Co., Ltd. from 2016 to 2017, a business manager and operation manager from 2000 to 2006 and a technology controller from 2007 to 2015 for Shanghai Fenner Conveyor Belt Co., Ltd., a sales engineer and an operation manager for Trelleborg Sealing Solutions (China) Co., Ltd. (formerly Busak+Shamban Eastern China) from 1994 to 1999, and a sales engineer for Sinopec Shanghai Petrochemical Co., Ltd. from 1986 to 1993. Ms. Lin Yu received her college degree in chemistry from Shanghai University of Science and Technology, Jin Shan Campus, in 1986.
Mr. Pu Xing has served as our independent director since September 2019. Mr. Pu Xing has also served as the head of the compliance and risk management department at Shenzhen Qianhai Kekong Lingdingyang Venture Capital Co., Ltd. since April 2019. Before that, he served as the managing partner at Shanghai Jiyun Investment Management Co., Ltd. from April 2017 to February 2019, an executive vice president and CFO at BesTv New Media Co., Ltd. (a subsidiary of a Shanghai Stock Exchange listed company) from January 2014 to March 2017 and deputy director of the State-owned Assets Supervision and Administration Commission of Shanghai Pudong New Area from March 2013 to February 2014. He also served as vice president and deputy director of the board’s strategic decision-making and investment committee at Shanghai Shengrong Investment Co., Ltd. (now known as Shanghai Guosheng Group) from December 2008 to February 2013, vice general manager at Shanghai Guosheng Group Investment Co., Ltd. from December 2010 to January 2013, executive general manager and executive deputy director at Shanghai Corporate Pavilion from January 2008 to December 2010, special assistant to the president at Shanghai Shengrong Investment Co., Ltd. from July 2008 to November 2008, and vice chairman of SiTV from October 2005 to January 2008. In addition, he served as deputy chief economist from January 2002 to June 2008 and special assistant to the president from October 1999 to January 2002 at Shanghai Automotive Industry (Group) Corporation (a Shanghai Stock Exchange listed company). Previously, he served as an analyst at Lehman Brothers from January 1997 to September 1999 and financial manager at Northeimer Engineering from January 1994 to December 1996. He has been a Special Auditor of Shanghai Audit Bureau since January 2011. Mr. Xing received his bachelor’s degree in economics from Fudan University in 1990, MBA degree in economics and finance from West Chester University in 1993 and master’s degree in accounting from Widener University in 1996.
Mr. Ren Luo has served as our independent director since September 2019. Mr. Luo has served as a senior director and director in charge of industry and government relationships and business development at IQVIA Management Consulting (Shanghai) Co. Ltd. since 2018, and senior manager in charge of industry and government relationships at IMS Health Co. Ltd. since 2013. He also served as supplier services manager at IMS Health Co. Ltd. (a subsidiary of a U.S. listed company) from 2011 to 2013, senior manager and researcher at National Institute for Hospital Administration of the Ministry of Health from 2009 to 2011, senior manager for Greater China and a director at IMS Health Co. Ltd. from 2003 to 2008, general manager and a director of IMS Market Research Consulting (Shanghai) Co. Ltd. from 2002 to 2003, China chief representative of IMS ChinaMetrik Co., Ltd. and manager of China division of IMS Ltd. from 1998 to 2002, chief manager of Chinese projects of ChinaMetrik Ltd. from 1994 to 1998, and consultant of Chinese projects of ChinaMetrik Ltd. from 1991 to 1993. He was also a pharmaceutical chemist at George Washington University Medical Center from 1990 to 1993. He is a consultant to a number of Chinese social associations and a member of American Pharmaceutical Association, American Chemical Society, and Chinese American Pharmacists Association. He is currently a member of the editorial board of Chinese Annual Report of Cardiovascular Disease and was a vice editor-in-chief of China Pharmaceutical Practical Manual for the 2002 and 2003 Edition. Mr. Luo received his bachelor’s degree in medical chemistry from Shanghai Pharmacy College in 1981, and master’s degree in M.S. Medical Chemistry from University of Mary Hardin Baylor in 1990.
Mr. Jianhua Shao was as our independent director and a member of compensation committee since November 2020. Prior to joining us, Mr. Shao has served as a professor of biophysics at Shanghai University of Traditional Chinese Medicine (the “SHUTCM”) since 2012. Before that he successively served as an associate professor from 2003 to 2012 and a lecturer from 1987 to 1992 at SHUTCM. Professor Shao currently serves as the director of the Office of Mathematics and Sciences Teaching and Research at SHUTCM. He is also a member of the council of the Society of Chinese Medical Mathematics and the chief of the Committee of the Society of Chinese Biomedical Engineering (Traditional Chinese Medicine Physics and Engineering). Mr. Shao also serves as the general manager of the project department of SHUTCM Asset Management Company Limited and a director of Shanghai Mingxu Health Management Consulting Co., Ltd. He served as the general manager of Shanghai Traditional Chinese Medicine Technology Co., Ltd. from 2006 to September 2020. Professor Shao has done research and published in the field of biophysics relating to blood vessels, blood fluid dynamics, and the heart. Professor Shao received his bachelor’s degree in physics from Shanghai Normal University in 1982 and his master’s degree in science from the University of the Ryukyus in Japan in 1998.
Ms. Sarah Yu has served as our director since August 2018. Ms. Sarah Yu has served as a senior software engineer at LinkedIn since September 2016. She also served as a software engineer at KQED (California Public Broadcasting) from 2015 to 2016 and a new media producer at KTOO (Alaska Public Broadcasting) from 2013 to 2015. Ms. Sarah Yu received her bachelor’s degrees in biology and English from Dartmouth College in 2011 and her master’s degree in science writing from Massachusetts Institute of Technology in 2012.
Mr. Edwards Jinqiu Tang has served as our chief financial officer since June 2020 and before that he served as our corporate controller beginning from October 2019. Prior to joining our company, Mr. Tang served as a global internal auditor at Natuzzi S.p.A (Italy) from 2016 to 2019. Previously, he worked for Beijing Dongshen CPAs from 2013 to 2016 and Shanghai De’an CPAs from 2011 to 2013, where he provided external audit, finance and tax advisory services across different industries and sectors. He has been a Certified Public Accountant in Australia since 2019 and a Forensic Certified Public Accountant in the U.S. since 2020. Mr. Tang received his bachelor’s degree in accounting from Charles Sturt University in Australia in 2007, MBA from Charles Sturt University in Australia in 2009 and his bachelor’s degree in law from Southwest University of Science and Technology in China in 2017.
Dr. He Yu is a co-founder of our company and has served as our chief medical officer since our inception in 2010. Dr. Yu has served as a professor and program director of cancer epidemiology at the University of Hawaii Cancer Center and an adjunct professor at Yale School of Public Health since 2012. He was a faculty member, from assistant professor to professor, at Yale University, School of Medicine from 2001 to 2011. Being trained in medicine, epidemiology and clinical biochemistry, Dr. Yu conducts various laboratory-based clinical and epidemiologic investigations and has extensive experience in cancer research. He has designed and been involved in many clinical research projects that assess the molecular and genetic features of tumor specimens in relation to cancer characteristics and survival outcomes of patients with various kinds of cancers. As a principal investigator and co-investigator, Dr. Yu has developed and participated in several large population-based epidemiologic studies that investigate gene-environment interactions in breast, endometrial, liver and pancreatic cancers. Biomarkers under his investigation include genetic polymorphisms in DNA repair genes, DNA methylation and methylator phenotype in tumor suppressor genes and detoxification genes, protein markers, peptide growth factors and various non-coding transcripts. Dr. Yu received his bachelor’s degree in medicine from Shanghai First Medical College in 1983. He also received a master of science degree in epidemiology and a PhD in clinical biochemistry from University of Toronto in 1990 and 1996, respectively.
Mr. Xuedong Du has served as our vice president in charge of research and development since April 2011. Prior to joining us, Mr. Du successively served as an engineer, senior engineer, chief engineer and manager at SMIC International IC Manufacturing (Shanghai) Co., Ltd. (a subsidiary of a U.S. listed company) from 2001 to 2010. He has extensive experience in product innovation and research and development. He has participated in more than ten provincial talent projects in relation to municipal science and technology. He is the first or principal inventor of more than 100 Chinese and international patent applications (primarily on medical devices), among which 81 patents have been granted. He has published approximately 20 papers in professional journals and academic conferences. Mr. Du received his bachelor’s degree in physical electronics technology from Fudan University in 2001 and his master’s degree in electronics and communications engineering from Fudan University in 2009.
Mr. Weidong Dai has served as our China president since April 2015. Prior to joining us, Mr. Dai served as a general partner at Stirrfir Investment Management Co. Ltd. from 2008 to 2015, chairman of RTS Management (Shanghai) Co., Ltd. from 2004 to 2013, a managing director of Hong Kong Pro-Health Technology Co., Ltd. and Shanghai Pro-Health Medical Devices Co., Ltd. from 1998 to 2004, and the chief scientific officer at Wex International Inc. ( a subsidiary of a U.S. listed company) from 1994 to 1998. He has served as an adjunct professor at Anhui College of Traditional Chinese Medicine since 2004 and an executive director at the Hainan Branch of China Science Tsing Research Institute of Science and Technology since 2018. He has published a number of medical research papers and scientific articles in professional journals. A medical device that he led in research and development was awarded the Hong Kong Industrial Award in 1999. Mr. Dai received his bachelor’s degree in medicine from Anhui Medical University in 1982, master’s degree in medicine from Sun Yat-San University of Medicine in 1985, and an Advanced Certificate of the EMBA CEO Program from Fudan University, School of Economics in 2002.
None of our directors and executive officers have any family relationships up to the fourth civil degree either by consanguinity or affinity, except that Dr. Chris Chang Yu is Ms. Sarah Yu’s father and Ms. Lin Yu and Dr. He Yu’s first cousin; and Dr. He Yu and Ms. Lin Yu are siblings.
B. Compensation
For the year ended December 31, 2020, we paid an aggregate of approximately RMB2.6 million (US$0.4 million) in cash to our executive officers and directors. We have not set aside or accrued any amount to provide pension, retirement or other similar benefits to our executive officers and directors. Our PRC subsidiaries are required by law to make contributions equal to certain percentages of each employee’s salary for his or her pension insurance, medical insurance, unemployment insurance and other statutory benefits and a housing provident fund.
Employment Agreements and Indemnification Agreements
We have entered into employment agreements with each of our executive officers. Under these agreements, each of our executive officers is employed for a specified time period. We may terminate employment for cause, at any time, without advance notice, for certain acts of the executive officer, such as being investigated for criminal liability, committing serious dereliction of duty, jobbery or malpractice to our detriment, or seriously violating our work discipline, rules and regulations. The executive officer may resign at any time for cause with a one-month advance written notice.
Each executive officer has agreed to hold, both during and after the termination or expiry of his or her employment agreement, in strict confidence and not to disclose, except as required in the performance of his or her duties in connection with the employment or pursuant to applicable law, any of our or our affiliates’ confidential information or trade secrets. The executive officers have also agreed that during the term of employment, all patents, copyrights and other intellectual property rights arising from our work achievement belong to us, and the executive officers have no right to use them for commercial purpose.
In addition, each executive officer has agreed to be bound by non-competition and non-solicitation restrictions during the term of his or her employment and typically for two years following the last date of employment. Specifically, each executive officer has agreed not to (i) approach directly or indirectly our suppliers, clients, customers or contacts or other persons or entities introduced to the executive officer in his or her capacity as a representative of us for the purpose of doing business with such persons or entities that will harm our business relationships with these persons or entities; (ii) assume employment with or provide services directly or indirectly to any of our competitors; (iii) seek directly or indirectly, to solicit the services of any of our employees who is employed by us on or after the date of the executive officer’s termination; or (iv) engage in any business activity, technology development, or sales and marketing related to bio-medical detection devices, targeted therapies or other specified technologies.
We have entered into indemnification agreements with each of our directors and executive officers. Under these agreements, we may agree to indemnify our directors and executive officers against certain liabilities and expenses incurred by such persons in connection with claims made by reason of their being a director or officer of our company.
2019 Share Incentive Plan
On October 31, 2019, our board of directors and shareholders approved our 2019 Plan, to encourage our employees, officers, directors and consultants to continue contributing to our success. The maximum number of ordinary shares that may be issued under the 2019 Plan is 1,105,300 ordinary shares. As of the date of this annual report, options and restricted share units to purchase 649,000 Class A ordinary shares, par value $0.01 each, of our company had been granted and were outstanding under this 2019 Plan, excluding awards that had been exercised, forfeited or cancelled after the relevant grant dates. The following paragraphs describe the principal terms of the 2019 Plan:
Type of Awards. The 2019 Plan permits the awards of options and other awards (such as restricted shares and restricted share units) that the plan administrator decides.
Plan Administration. Our compensation committee or such other committee as appointed by our Board from time to time will administer the 2019 Plan. The committee, as applicable, will determine the participants to receive awards, the time, type and number of awards to be granted to each participant, and the terms and conditions of each award grant.
Award Agreement. Awards granted under the 2019 Plan are evidenced by an award agreement that sets forth terms, conditions and limitations for each award, which may include the term of the award, the provisions applicable in the event of the grantee’s employment or service terminates, and our authority to unilaterally or bilaterally amend, modify, suspend, cancel or rescind the award.
Eligibility. We may grant awards to directors, service provider, advisor, employees and consultants of our company or any of our subsidiaries.
Vesting Conditions. In general, the plan administrator determines the vesting conditions, which is specified in the relevant award agreement.
Exercise of Options. The plan administrator determines the exercise price for each award, which is stated in the award agreement and shall be no less than the fair market value of a share on the date of an award grant. The vested portion of option will expire if not exercised prior to the time as the plan administrator determines at the time of its grant. However, the maximum exercisable term is ten years from the date of a grant.
Transfer Restrictions. Awards may not be transferred in any manner by the recipient other than (i) by trust that was established solely for tax planning purpose; (ii) by gift or pursuant to a qualified domestic relations order to one or more family member; or (iii) by will or the laws of descent and distribution, except as otherwise provided by the plan administrator.
Termination and Amendment. Unless terminated earlier, the 2019 Plan has a term of ten years. The plan administrator has the authority to amend or terminate the 2019 Plan. However, no such action may adversely affect in any material way any awards previously granted without written consent of the recipient, unless the plan administrator expressly reserved the right to make such amendment at the time the relevant awards were granted.
2010 Share Incentive Plan
On February 1, 2010, our shareholders and board of directors authorized the chairman of the board to grant options under a share incentive plan, or the 2010 Plan, to our eligible employees, directors, officers and consultants to purchase not exceeding 1,190,000 ordinary shares of our company by July 1, 2017. On October 19, 2015, our shareholders and board of directors resolved to increase the authorized number of shares underlying the options under the 2010 Plan to 1,866,600 ordinary shares of our company by July 1, 2017. On July 1, 2017, in order to provide additional incentives to attract and retain key employees, directors, officers and consultants of outstanding ability and to motivate them to exert their best efforts, our shareholders and board of directors further resolved to grant additional options under the 2010 Plan, resulting in a total of options to purchase up to 2,726,600 shares of our company by December 31, 2019. As of the date of this annual report, options to purchase 1,393,400 Class A ordinary shares, par value $0.01 each, of our company had been granted and were outstanding under this 2010 Plan, excluding options that had been exercised, forfeited or cancelled after the relevant grant dates. The following paragraphs describe the principal terms of this plan:
Type of Awards. The 2010 Plan only permits the awards of options.
Plan Administration. Our shareholders have authorized the chairman of our board of directors to administer the 2010 Plan. The chairman of the board may determine the grant date, number of options to be granted, participants of the 2010 Plan, vesting conditions, exercise price and other terms and conditions of the options.
Award Agreement. Options granted under the 2010 Plan are evidenced by an award agreement that sets forth terms, conditions and limitations for each option award.
Eligibility. Persons eligible to participate in this plan include our employees, directors, officers and consultants.
Vesting Schedule. The plan administrator determines the vesting schedule. Subject to the terms of the relevant award agreements, the options will vest each year in a four-year schedule for our employees, directors and officers, or based on milestones of performance of the consultants.
Term and termination. The term of each option shall be ten years from the date of grant of the option. Notwithstanding the foregoing, we may forfeit all or part the options granted to a participant under the certain circumstances.
The following table summarizes, as of the date of this annual report, the awards granted to our directors and executive officers and other individuals as authorized by our board of directors under our 2010 Plan and 2019 Plan, excluding awards that were forfeited or canceled after the relevant grant dates and retrospectively reflecting the effectiveness of a share subdivision of each ordinary share of par value of US$1.00 into 100 ordinary shares of par value of US$0.01 each, which became effective on November 12, 2019.
|
Name |
|
Number of Shares* |
|
Exercise Price |
|
Date of Grant |
|
Date of Expiration |
|
Chris Chang Yu |
|
250,000 |
|
US$3.77 |
|
February 1, 2021 |
|
February 1, 2031 |
|
Ren Luo |
|
* |
|
US$0.0005 |
|
October 28, 2010 |
|
October 28, 2020 |
|
Weidong Dai |
|
330,000 |
|
Zero to US$0.0001 |
|
August 1, 2014 and April 1, 2015 |
|
August 1, 2024 and April 1, 2025 |
|
Xuedong Du |
|
488,600 |
|
Zero to US$0.0005 |
|
September 6, 2010 - January 1, 2018 |
|
September 6, 2020 - January 1, 2028 |
|
Edwards Jinqiu Tang |
|
* |
|
US$3.77 to US$7.55 |
|
September 21, 2020 |
|
September 21, 2030 |
|
Other individuals as a group |
|
1,895,033 |
|
Zero to US$12 |
|
August 1, 2010 - April 30, 2021 |
|
August 1, 2020 - April 30, 2031 |
* Less than 1% of our total outstanding ordinary shares.
C. Board Practices
Composition of Board of Directors
Our board of directors consists of seven directors. Unless so fixed by our company in a general meeting, a director is not required to hold any shares in our company to qualify to serve as a director. A director may vote with respect to any contract, proposed contract or transaction in which he is interested, and if he does so, his vote shall be counted and he may be counted in the quorum at any meeting of our directors at which any such contract or proposed contract or arrangement is considered, provided that the nature of the interest of such director shall be disclosed by such director at or prior to its consideration and any vote thereon. None of our non-executive directors has a service contract with us that provides for benefits upon termination of service.
Committees of the Board of Directors
We have established three committees under the board of directors: an audit committee, a compensation committee and a nominating and corporate governance committee. We have adopted a charter for each of the three committees. Each committee’s members and functions are described below.
Audit Committee. Our audit committee consists of Mr. Pu Xing and Mr. Ren Luo. Mr. Pu Xing is the chairman of our audit committee. We have determined that Mr. Pu Xing and Mr. Ren Luo satisfy the “independence” requirements of Rule 5605(c)(2) of the Listing Rules of The NASDAQ Stock Market and Rule 10A-3 under the Exchange Act, as amended. We have determined that Mr. Pu Xing qualifies as an “audit committee financial expert.” The audit committee oversees our accounting and financial reporting processes and the audits of the financial statements of our company. The audit committee is responsible for, among other things:
· appointing the independent auditors and pre-approving all auditing and non-auditing services permitted to be performed by the independent auditors;
· reviewing with the independent auditors any audit problems or difficulties and management’s response;
· discussing the annual audited financial statements with management and the independent auditors;
· reviewing the adequacy and effectiveness of our accounting and internal control policies and procedures and any steps taken to monitor and control major financial risk exposures;
· reviewing and approving all proposed related party transactions;
· meeting separately and periodically with management and the independent auditors; and
· monitoring compliance with our code of business conduct and ethics, including reviewing the adequacy and effectiveness of our procedures to ensure proper compliance.
Compensation Committee. Our compensation committee consists of Mr. Ren Luo, Mr. Jianhua Shao and Dr. Chris Chang Yu. Mr. Ren Luo is the chairman of our compensation committee. We have determined that Mr. Ren Luo and Mr. Jianhua Shao satisfy the “independence” requirements of Rule 5605(a)(2) of the Listing Rules of The NASDAQ Stock Market. The compensation committee assists the board in reviewing and approving the compensation structure, including all forms of compensation, relating to our directors and executive officers. Our chief executive officer may not be present at any committee meeting during which his compensation is deliberated. The compensation committee is responsible for, among other things:
· reviewing and approving, or recommending to the board for its approval, the compensation for our chief executive officer and other executive officers;
· reviewing and recommending to the board for determination with respect to the compensation of our non-employee directors;
· reviewing periodically and approving any incentive compensation or equity plans, programs or similar arrangements; and
· selecting compensation consultant, legal counsel or other adviser only after taking into consideration all factors relevant to that person’s independence from management.
Nominating and Corporate Governance Committee. Our nominating and corporate governance committee consists of Dr. Chris Chang Yu, Mr. Feng Guo and Mr. Pu Xing. Dr. Chris Chang Yu is the chairman of our nominating and corporate governance committee. We have determined that Mr. Pu Xing satisfies the “independence” requirements of Rule 5605(a)(2) of the Listing Rules of The NASDAQ Stock Market. The nominating and corporate governance committee assists the board of directors in selecting individuals qualified to become our directors and in determining the composition of the board and its committees. The nominating and corporate governance committee is responsible for, among other things:
· selecting and recommending to the board nominees for election by the shareholders or appointment by the board;
· reviewing annually with the board the current composition of the board with regards to characteristics such as independence, knowledge, skills, experience and diversity;
· making recommendations on the frequency and structure of board meetings and monitoring the functioning of the committees of the board; and
· advising the board periodically with regards to significant developments in the law and practice of corporate governance as well as our compliance with applicable laws and regulations, and making recommendations to the board on all matters of corporate governance and on any remedial action to be taken.
Duties of Directors
Under BVI law, our directors owe fiduciary duties to our company, including a duty of loyalty, a duty to act honestly and a duty to act in what they consider in good faith to be in our best interests. Our directors also have a duty to exercise the skill they actually possess and such care and diligence that a reasonably prudent person would exercise in comparable circumstances. In fulfilling their duty of care to us, our directors must ensure compliance with our M&A, as amended and restated from time to time, the BVI Act and the class rights vested thereunder in the holders of the shares. Our company has the right to seek damages if a duty owed by our directors is breached. A shareholder may in certain limited exceptional circumstances have the right to seek damages in our name if a duty owed by the directors is breached.
Our Board of Directors has all the powers necessary for managing, and for directing and supervising, our business affairs. The functions and powers of our Board include, among others:
· convening shareholders’ general meetings;
· declaring dividends and distributions;
· appointing officers and determining the term of office of the officers;
· exercising the borrowing powers of our company and mortgaging the property of our company; and
· approving the transfer of shares in our company, including the registration of such shares in our share register.
Terms of Directors and Officers
Our directors may be elected by a resolution of our board of directors, or by a resolution of our shareholders either to appoint any person as a director to fill a casual vacancy on our board or as an addition to the existing board. Our company may by resolution of our shareholders remove any director, notwithstanding any provision in our memorandum and articles of association or in any agreement between such director and us.
D. Employees
As of December 31, 2020, we had 86 full-time employees, including seven in the United States and the remainder in China. The following table sets forth the numbers of our employees categorized by function as of December 31, 2020.
|
Function |
|
Number of Employees |
|
|
Research and development |
|
20 |
|
|
Laboratory technicians and manufacturing personnel |
|
16 |
|
|
Sales and marketing |
|
15 |
|
|
Logistics and customer support and service |
|
5 |
|
|
General and administration |
|
30 |
|
|
Total |
|
86 |
|
We plan to hire additional employees for sales and marketing, customer support and service and manufacturing functions as we grow our business. None of our employees are represented by a labor union with respect to his or her employment with us. We believe that we maintain a good working relationship with our employees, and we have not experienced any material labor disputes.
In accordance with applicable regulations in the PRC, we participate in various employee social security plans that are organized by municipal and provincial governments, including housing, pension, medical insurance, work-related injury insurance, employment injury insurance, maternity insurance and unemployment insurance. We are required under PRC law to make contributions to employee benefit plans at specified percentages of the salaries, bonuses and certain allowances of our employees, up to a maximum amount specified by the local government from time to time.
E. Share Ownership
Except as specifically noted, the following table sets forth information with respect to the beneficial ownership of our ordinary shares as of the date of March 31, 2021 by:
· each of our directors and executive officers; and
· each person known to us to own beneficially more than 5% of our total outstanding ordinary shares.
The calculations in the table below are based on 12,656,306 ordinary shares (including 9,793,206 Class A ordinary shares and 2,863,100 Class B ordinary shares) outstanding as of the date of this annual report, excluding 2,445,358 Class A ordinary shares reserved for potential conversion of convertible debentures.
Beneficial ownership is determined in accordance with the rules and regulations of the SEC. In computing the number of shares beneficially owned by a person and the percentage ownership of that person, we have included shares that the person has the right to acquire within 60 days, including through the exercise of any option, warrant or other right or the conversion of any other security. These shares, however, are not included in the computation of the percentage ownership of any other person.
|
|
|
Class A |
|
Class B |
|
% of total ordinary |
|
% of aggregate |
|
|
Directors and Executive Officers**: |
|
|
|
|
|
|
|
|
|
|
Chris Chang Yu(1) |
|
417,100 |
|
2,263,900 |
|
21.2 |
% |
60.0 |
% |
|
Feng Guo(2) |
|
606,700 |
|
247,900 |
|
6.8 |
% |
8.0 |
% |
|
Lin Yu |
|
— |
|
— |
|
— |
|
— |
|
|
Pu Xing |
|
* |
|
— |
|
* |
|
* |
|
|
Ren Luo |
|
* |
|
— |
|
* |
|
* |
|
|
Jianhua Shao |
|
— |
|
— |
|
— |
|
— |
|
|
Sarah Yu |
|
— |
|
— |
|
— |
|
— |
|
|
Edwards Jinqiu Tang |
|
— |
|
— |
|
— |
|
— |
|
|
He Yu(3) |
|
1,212,700 |
|
— |
|
9.6 |
% |
3.2 |
% |
|
Xuedong Du(4) |
|
465,000 |
|
— |
|
3.6 |
% |
1.2 |
% |
|
Weidong Dai(5) |
|
330,000 |
|
— |
|
2.6 |
% |
0.9 |
% |
|
All Directors and Executive Officers as a Group |
|
3,090,400 |
|
2,511,800 |
|
43.2 |
% |
73.4 |
% |
|
Principal Shareholders: |
|
|
|
|
|
|
|
|
|
|
CRS Holdings Inc.(6) |
|
402,100 |
|
2,263,900 |
|
21.1 |
% |
60.0 |
% |
|
He Yu(3) |
|
1,212,700 |
|
— |
|
9.6 |
% |
3.2 |
% |
|
Zhangjiang GU KE Company Limited(7) |
|
859,200 |
|
351,300 |
|
9.6 |
% |
11.4 |
% |
|
Zhijun Sihang Holdings Limited(2) |
|
606,700 |
|
247,900 |
|
6.8 |
% |
8.0 |
% |
Notes:
* Less than 1% of our total outstanding ordinary shares.
** Except as indicated otherwise below, the business address of our directors and executive officers is 801 Bixing Street, Bihu County, Lishui, Zhejiang Province 323006, People’s Republic of China.
*** For each person or group included in this column, percentage ownership is calculated by dividing the number of shares beneficially owned by such person or group by the sum of the total number of shares outstanding and the number of shares such person or group has the right to acquire upon exercise of an option, restricted share unit or other right within 60 days after March 31, 2021.
† For each person or group included in this column, percentage of total voting power represents voting power based on both Class A and Class B ordinary shares held by such person or group with respect to all outstanding shares of our Class A and Class B ordinary shares as a single class. Each holder of Class A ordinary shares is entitled to one (1) vote per share. Each holder of our Class B ordinary shares is entitled to ten (10) votes per share. Our Class B ordinary shares are convertible at any time by the holder into Class A ordinary shares on a one-for-one basis.
(1) Represents (i) 2,263,900 Class B ordinary shares and 402,100 Class A ordinary shares held by CRS Holdings Inc., a British Virgin Islands company, which is wholly owned by Dr. Chris Chang Yu and (ii) 15,000 Class A ordinary shares issuable upon exercise of options held by the spouse of Dr. Chris Chang Yu. The registered address of CRS Holdings Inc. is Trinity Chambers, P. O. Box 4301, Road Town, Tortola, British Virgin Islands.
(2) Represents (i) 536,000 Class A ordinary shares and 247,900 Class B ordinary shares held by Zhijun Sihang Holding Limited, a British Virgin Islands company, which is wholly owned by Jiaxing Zhijun Sihang Investment Partnership Enterprises (Limited Partnership), and (ii) 70,700 Class A ordinary shares held by Mr. Lei Luo for the benefit of Zhijun Sihang Holdings Limited. The general partner of Jiaxing Zhijun Sihang Investment Partnership Enterprises (Limited Partnership) is Jiaxing Zhijun Investment Management Co., Ltd. Mr. Feng Guo is the controlling shareholder and a member of the three-person investment committee of Jiaxing Zhijun Investment Management Co., Ltd. Mr. Guo disclaims any beneficial ownership with respect to these shares. The registered address of Zhijun Sihang Holding Limited is 113-7, No.100, Zhuyuan Road, Nanhu District, Jiaxing, Zhejiang Province, P.R. China.
(3) Represents 1,212,700 Class A ordinary shares held by Dr. He Yu.
(4) Represents (i) 176,400 Class A ordinary shares held by YuLin Bio-medical Science Co., Ltd. and (ii) 288,600 Class A ordinary shares issuable upon exercise of options held by Mr. Xuedong Du. YuLin Bio-medical Science Co., Ltd. is owned by certain individuals, with none of them holding a controlling interest in YuLin Bio-medical Science Co., Ltd. The registered address of YuLin Bio-medical Science Co., Ltd. is Aequitas International Management Ltd., Grand Pavilion Commercial Centre, Suite 24, 802 West Bay Road, P.O. Box 10281, Grand Cayman KY1-1003, Cayman Islands.
(5) Represents 330,000 Class A ordinary shares held by Mr. Weidong Dai.
(6) Represents 402,100 Class A ordinary shares and 2,263,900 Class B ordinary shares held by CRS Holdings Inc. CRS Holdings Inc., a British Virgin Islands company, is wholly owned by Dr. Chris Chang Yu, and its registered address is Trinity Chambers, P. O. Box 4301, Road Town, Tortola, British Virgin Islands.
(7) Represents 859,200 Class A ordinary shares and 351,300 Class B ordinary shares held by Zhangjiang GU KE Company Limited, a British Virgin Islands company, which is 100% beneficially owned by Shanghai Pudong State-owned Assets Supervision and Administration Commission. The registered address of Zhangjiang GU KE Company Limited is Commence Chambers, P.O. Box 2208, Road Town, Tortola, British Virgin Islands.
As of the date of this annual report, to our knowledge, 7,182,791 Class A ordinary shares were held by one record holder, which is the depositary of our ADS program. In addition, we have seven record holders in the United States of Class A our ordinary shares, who held approximately 18.7% of our total outstanding ordinary shares as of March 31, 2021. As of March 31, 2021, none of our Class B ordinary shares were held by U.S. record holders. The number of beneficial owners of our ADSs in the United States is likely to be much larger than the number of record holders of our ordinary shares in the United States. We are not aware of any arrangement that may, at a subsequent date, result in a change of control of our company.
ITEM 7. MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
A. Major Shareholders
Please refer to “Item 6. Directors, Senior Management and Employees—E. Share Ownership.”
B. Related Party Transactions
Shareholders Agreements
According to shareholders agreements dated June 30, 2017 and August 17, 2017, respectively, that we entered into with certain of our shareholders (the “Investors”), which provide for certain rights, including the right in respect of board composition, right of information and inspection, right of first refusal, co-sale right, anti-dilution protection and registration rights. These rights, except the registration rights, have automatically terminated upon the completion of our initial public offering.
Registration Rights
Upon the demand of any of the Investors, we and certain of our principal shareholders shall procure a company within our group that is conducting a public offering to grant (to the Investors’ satisfaction) the Investors: (i) rights to register their respective shares in the company with the United States Securities and Exchanges Commission, including, but not limited to, three times of demand registration, unlimited times of piggyback registration, and unlimited times of registration under Form F-3/S-3 (or any subsequent registration statements under the U.S. Securities Act of 1933, as amended), or (ii) equivalent or similar registration rights in respect of any issuances of the company’s shares in any other jurisdiction where it commits to a public offering or listing of its shares in a recognized stock exchange.
Employment Agreements and Indemnification Agreements
See “Item 6. Directors, Senior Management and Employees—B. Compensation—Employment Agreements and Indemnification Agreements.”
Share Incentive Plans
See “Item 6. Directors, Senior Management and Employees—B. Compensation.”
Private Placements
Zhijun Sihang provided an advance of RMB25.0 million (US$3.5 million) to one of our PRC subsidiaries in 2018. Zhijun Sihang has made the equity contribution of close to half of these funds in our company.
Convertible Loan Agreements
In 2018, we borrowed from Zhijun a total of US$2.5 million in one-year term loans convertible into our ordinary shares. These loans and the accrued interest expenses amounted to RMB18.0 million and RMB824,000, respectively, as of December 31, 2018. These loans bore interest at 9% per annum. The conversion price would be determined based on the then outstanding principal amount of the loans and an RMB488 million assumed equity value of the Company before granting of the loans in 2018. The convertible loans were convertible into our Class A ordinary shares at the election of Zhijun in whole or in part prior to maturity subject to certain conditions. Zhijun did not convert the loans into our Class A ordinary shares, and we repaid the loans to Zhijun in full in June 2020. We recognized an interest expense of US$35,000 related to these loans in 2020.
Loans From CRS Holdings Inc.
CRS Holdings Inc., wholly owned by our founder and chairman, Dr. Chris Chang Yu, provided loans of RMB2.1 million (US$317,000) to us in 2020. We repaid to CRS Holdings Inc. RMB1.5 million (US$230,000) in 2020. These loans were interest-free, unsecured and repayable on demand.
In February 2021, CRS Holdings Inc. converted RMB4.5 million (US$0.69 million) of loans to us, plus interest at the rate of 8%, into 152,100 Class A ordinary shares of our company.
Sales Agreements and Consultancy Agreement with Investee Companies
We have in the ordinary course of our business engaged certain of our investee companies, including AnPac Beijing Health Management Co., Ltd., Jiangsu Anpac and Anpai (Shanghai) Health Management Consulting Co., Ltd., as sales agents for our CDA-based tests. In 2020, we incurred a consultancy fee of RMB898,000 (US$138,000) to AnPac Beijing Health Management Co., Ltd. for its marketing services to us; and we recognized a rent from Shanghai Muqing Industrial of RMB443,000 (US$68,000).
C. Interests of Experts and Counsel
Not applicable.
A. Consolidated Statements and Other Financial Information
We have appended consolidated financial statements filed as part of this annual report.
Legal Proceedings
We may be subject to legal proceedings and claims in the ordinary course of business. We cannot predict the results of any such disputes, and despite the potential outcomes, their existence alone may have an adverse material impact on us because of diversion of management time and attention as well as the financial costs related to resolving such disputes. Neither we nor any of our directors or executive officers are currently a party to, nor is any of our properties the subject of, any material legal or arbitration proceedings.
Dividend Policy
Our board of directors has discretion on whether to distribute dividends, subject to certain restrictions under British Virgin Islands law, namely that our company may only pay dividends if our directors are satisfied on reasonable grounds that we are solvent immediately after the dividend payment in the sense that we will be able to pay our debts as they become due in the ordinary course of business, and the value of assets of our company will exceed our total liabilities. Even if our board of directors decides to pay dividends, the form, frequency and amount will depend upon our future operations and earnings, capital requirements and surplus, general financial condition, contractual restrictions and other factors that the board of directors may deem relevant.
We have never declared or paid dividends and do not have any plan to pay any cash dividends on our ordinary shares in the foreseeable future. We intend to retain most, if not all, of our available funds and any future earnings to operate and expand our business.
We are a holding company incorporated in the British Virgin Islands. We may rely on dividends from our subsidiaries in China for our cash requirements, including any payment of dividends to our shareholders. PRC regulations may restrict the ability of our PRC subsidiaries to pay dividends to us. See “Item 3. Key Information—D. Risk Factors—Risks Relating to Doing Business in China—We rely on dividends paid by our subsidiaries for our cash needs, and any limitation on the ability of our subsidiaries to make payments to us could have a material adverse effect on our ability to conduct our business.”
If we pay any dividends on our Class A ordinary shares, we will pay those dividends which are payable in respect of the Class A ordinary shares underlying our ADSs to the depositary, as the registered holder of such Class A ordinary shares, and the depositary then will pay such amounts to our ADS holders in proportion to the Class A ordinary shares underlying the ADSs held by such ADS holders, subject to the terms of the deposit agreement, including the fees and expenses payable thereunder. Cash dividends on our Class A ordinary shares, if any, will be paid in U.S. dollars.
B. Significant Changes
Except as disclosed elsewhere in this annual report, we have not experienced any significant changes since the date of our audited consolidated financial statements included in this annual report.
A. Offering and Listing Details
Our ADSs, each representing one Class A ordinary share, have been listed on the NADAQ Global Market since January 30, 2020 and trade under the symbol “ANPC.” No significant trading suspensions have occurred since listing.
B. Plan of Distribution
Not applicable.
C. Markets
See “—A. Offering and Listing Details.”
D. Selling Shareholders
Not applicable.
E. Dilution
Not applicable.
F. Expenses of the Issue
Not applicable.
ITEM 10. ADDITIONAL INFORMATION
A. Share Capital
Not applicable.
B. Memorandum and Articles of Association
We are a BVI business company limited by shares and our affairs are governed by our memorandum and articles of association, as amended and restated from time to time, and the BVI Business Companies Act (the “BVI Act”). The following are summaries of material provisions of our Third Amended and Restated Memorandum and Articles of Association in effect as of the date of this annual report insofar as they relate to the material terms of our ordinary shares.
M&A
The following discussion describes our M&A:
Objects and Purposes, Register, and Shareholders. Subject to the BVI Act, our objects and purposes are unlimited. Our register of members will be maintained by our share registrar, Maples Fund Services (Cayman) Limited. Under the BVI Act, a BVI company may treat the registered holder of a share as the only person entitled to (a) exercise any voting rights attaching to the share, (b) receive notices, (c) receive a distribution in respect of the share and (d) exercise other rights and powers attaching to the share. Consequently, as a matter of BVI law, where a shareholder’s shares are registered in the name of a nominee, the nominee is entitled to receive notices, receive distributions and exercise rights in respect of any such shares registered in its name. The beneficial owners of the shares registered in a nominee’s name will therefore be reliant on their contractual arrangements with the nominee in order to receive notices and dividends and ensure the nominee exercises voting and other rights in respect of the shares in accordance with their directions.
Directors’ Powers. Under the BVI Act, subject to any modifications or limitations in a company’s M&A, a company’s business and affairs are managed by, or under the direction or supervision of, its directors; and directors generally have all powers necessary to manage a company. A director must disclose any interest he has on any proposal, arrangement or contract not entered into in the ordinary course of business and on usual terms and conditions. An interested director may (subject to the M&A) vote on a transaction in which he has an interest. In accordance with, and subject to, our M&A, the directors may by resolution of directors exercise all the powers of the company to incur indebtedness, liabilities or obligations and to secure indebtedness, liabilities or obligations whether of the company or of any third party.
Rights, Preferences and Restrictions of Ordinary Shares. Subject to the restrictions described under the section titled “Dividend Policy” above, our directors may (subject to the M&A) authorize dividends at such time and in such amount as they determine. In the event of a liquidation or dissolution of the company, the holders of ordinary shares are (subject to the M&A) entitled to share ratably in all surplus assets remaining available for distribution to them after payment and discharge of all claims, debts, liabilities and obligations of the company and after provision is made for each class of shares (if any) having preference over the ordinary shares if any at that time. There are no sinking fund provisions applicable to our ordinary shares. Holders of our ordinary shares have no pre-emptive rights. Subject to the provisions of the BVI Act, we may, (subject to the M&A) with board or shareholders’ consent, repurchase our ordinary shares provided always that the company will, immediately after the repurchase, satisfy the solvency test. The company will satisfy the solvency test, if (i) the value of the company’s assets exceeds its liabilities; and (ii) the company is able to pay its debts as they fall due.
In accordance with the BVI Act:
(i) the company may purchase, redeem or otherwise acquire its own shares in accordance with either (a) Sections 60, 61 and 62 of the BVI Act (save to the extent that those Sections are negated, modified or inconsistent with provisions for the purchase, redemption or acquisition of its own shares specified in the company’s M&A); or (b) such other provisions for the purchase, redemption or acquisition of its own shares as may be specified in the company’s M&A. The company’s M&A provide that such Sections 60, 61 and 62 of the BVI Act do not apply to the company; and
(ii) where a company may purchase, redeem or otherwise acquire its own shares otherwise than in accordance with Sections 60, 61 and 62 of the BVI Act, it may not purchase, redeem or otherwise acquire the shares without the consent of the member whose shares are to be purchased, redeemed or otherwise acquired, unless the company is permitted by the M&A to purchase, redeem or otherwise acquire the shares without that consent; and
(iii) unless the shares are held as treasury shares in accordance with Section 64 of the BVI Act, any shares acquired by the company are deemed to be canceled immediately on purchase, redemption or other acquisition.
Variation of the Rights of Shareholders. As permitted by the BVI Act and our M&A, whenever the capital of our company is divided into different classes, the rights attached to any such class may only be materially adversely varied with the consent in writing of the holders of not less than two-thirds (2/3rds) of the issued shares of that class or with the sanction of a resolution of our shareholders passed at a separate meeting of the holders of the shares of that class by the holders of not less than two-thirds (2/3rds) of the issued shares of that class.
Ordinary Shares. Our ordinary shares are divided into Class A ordinary shares and Class B ordinary shares. Holders of our Class A ordinary shares and Class B ordinary shares will have the same rights except for voting and conversion rights. Our ordinary shares are issued in registered form and are issued when registered in our register of members. Our shareholders who are non-residents of the BVI may freely hold and vote their shares.
Conversion. Each Class B ordinary share is convertible into one (1) Class A ordinary share at any time by the holder thereof. Class A ordinary shares are not convertible into Class B ordinary shares under any circumstances. Upon any sale, transfer, assignment or disposition of Class B ordinary shares by a holder thereof to any person other than holders of Class B ordinary shares or their affiliates, or upon a change of ultimate beneficial ownership of the holder of any Class B ordinary share to any person or entity who is not an affiliate of the holder, such Class B ordinary shares shall be automatically and immediately converted into the same number of Class A ordinary shares.
Voting Rights. In respect of all matters subject to a shareholders’ vote, each Class A ordinary share shall entitle the holder thereof to one (1) vote per share and each Class B ordinary share shall entitle the holder to ten (10) votes per share on all matters subject to vote at our general meetings. Our Class A ordinary shares and Class B ordinary shares vote together as a single class on all matters submitted to a vote of our shareholders, except as may otherwise be required by law. Voting at any shareholders’ meeting is by show of hands unless a poll is (before or on the declaration of the result of the show of hands) demanded. A poll may be demanded by the chairman of such meeting or any shareholder present in person or by proxy.
Shareholder Meetings. In accordance with, and subject to, our M&A, (a) the chairman of our board of directors, or a majority of our directors (acting by a resolution of the board), may call general meetings of our shareholders; and (b) upon the written request of shareholders entitled to exercise thirty per cent (30%) or more of the voting rights in respect of the matter for which the meeting is requested, the directors shall convene a meeting of shareholders. Under BVI law, the M&A may be amended to decrease but not increase the required percentage to call a meeting above thirty per cent (30%). In accordance with, and subject to, our M&A, (a) the director convening a meeting shall give not less than ten (10) days’ notice of a meeting of shareholders to those shareholders entitled to vote at the meeting; (b) an annual general meeting of shareholders held in contravention of the requirement to give notice is valid if shareholders holding at least ninety-five per cent (95%) of the total votes attaching to all shares in issue and entitled to attend and vote at such annual general meeting have agreed to waive notice of the meeting; and an extraordinary general meeting of shareholders held in contravention of the requirement to give notice is valid if shareholders holding no less than two-thirds of total votes attaching to all shares in issue and entitled to attend and vote at such extraordinary general meeting have agreed to waive notice of the meeting; (c) a meeting of shareholders is duly constituted if, at the commencement of the meeting, there are present in person or by proxy one or more shareholders holding shares which carry in aggregate not less than a majority of all votes attaching to all shares in issue and entitled to vote at such meeting, and (d) if within half an hour from the time appointed for the meeting a quorum is not present, the meeting shall be dissolved.
Dividends. Subject to the BVI Act and our M&A, our directors may, by resolution, declare dividends at a time and amount as they think fit if they are satisfied, based on reasonable grounds, that, immediately after distribution of the dividend, the value of our assets will exceed our liabilities and we will be able to pay our debts as they fall due. There is no further BVI law restriction on the amount of funds which may be distributed by us by dividend, including all amounts paid by way of the subscription price for ordinary shares regardless of whether such amounts may be wholly or partially treated as share capital or share premium under certain accounting principles. Shareholder approval is not (except as otherwise provided in our M&A) required to pay dividends under BVI law. In accordance with, and subject to, our M&A, no dividend shall bear interest as against the company (except as otherwise provided in our M&A).
Disclosure of the SEC’s Position on Indemnification for Securities Act Liabilities. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling the registrant pursuant to the foregoing provisions, the registrant has been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Transfer of Shares. Subject to any applicable restrictions or limitations arising pursuant to (i) our M&A; or (ii) the BVI Act, any of our shareholders may transfer all or any of his or her shares by an instrument of transfer in the usual or common form or in any other form which our directors may approve (such instrument of transfer being signed by the transferor and containing the name and address of the transferee). Our directors may decline to register any transfer of shares which is not fully paid up or on which our company has a lien. In addition, our directors may also decline to register any transfer of any shares unless (i) the instrument of transfer is lodged with our company, accompanied by the relevant share certificate, (ii) the instrument of transfer is in respect of only one class of shares, (iii) the instrument of transfer is properly stamped, if required, (iv) in the case of a transfer to joint holders, the number of joint holders to whom the share is to be transferred does not exceed four, and (v) a fee of such maximum sum as The NASDAQ Global Market may determine to be payable, or such lesser sum as our board of directors may require, is paid to our company in respect thereof.
Differences in Corporate Law
The BVI Act differs from laws applicable to United States corporations and their shareholders. Set forth below is a summary of the significant differences between the provisions of the BVI Act applicable to us and the laws applicable to companies incorporated in the State of Delaware.
Mergers, Consolidations and Similar Arrangements
The BVI Act provides for mergers as that expression is understood under US corporate law. Common law mergers are also permitted outside of the scope of the BVI Act. Under the BVI Act, two or more companies may either merge into one of such existing companies, or the surviving company, or consolidate with both existing companies ceasing to exist and forming a new company, or the consolidated company. The procedure for a merger or consolidation between our company and another company (which need not be a BVI company) is set out in the BVI Act. The directors of the BVI company or BVI companies which are to merge or consolidate must approve a written plan of merger or consolidation which must also be authorized by a resolution of members (and the outstanding shares of every class of shares that are entitled to vote on the merger or consolidation as a class if the memorandum or articles of association so provide or if the plan of merger or consolidation contains any provisions that, if contained in a proposed amendment to the memorandum or articles, would entitle the class to vote on the proposed amendment as a class) of the shareholders of the BVI company or BVI companies which are to merge. A foreign company which is able under the laws of its foreign jurisdiction to participate in the merger or consolidation is required by the BVI Act to comply with the laws of that foreign jurisdiction in relation to the merger or consolidation. The BVI company must then execute articles of merger or consolidation, containing certain prescribed details. The plan and articles of merger or consolidation are then filed with the Registrar of Corporate Affairs in the BVI, or the Registrar. If the surviving company or the consolidated company is to be incorporated under the laws of a jurisdiction outside BVI, it shall file the additional instruments required under Section 174(2)(b) of the BVI Act. The Registrar then (if he or she is satisfied that the requirements of the BVI Act have been complied with) registers, in the case of a merger, the articles of merger or consolidation and any amendment to the M&A of the surviving company and, in the case of a consolidation, the M&A of the new consolidated company and issues a certificate of merger or consolidation (which is conclusive evidence of compliance with all requirements of the BVI Act in respect of the merger or consolidation). The merger or consolidation is effective on the date that the articles of merger or consolidation are registered by the Registrar or on such subsequent date, not exceeding thirty days, as is stated in the articles of merger or consolidation but if the surviving company or the consolidated company is a company incorporated under the laws of a jurisdiction outside the BVI, the merger or consolidation is effective as provided by the laws of that other jurisdiction.
As soon as a merger or consolidation becomes effective (among other things), (a) the surviving company or consolidated company (so far as is consistent with its amended memorandum and articles of association, as amended or established by the articles of merger or consolidation) has all rights, privileges, immunities, powers, objects and purposes of each of the constituent companies; (b) the memorandum and articles of association of any surviving company are automatically amended to the extent, if any, that changes to its amended memorandum and articles of association are contained in the articles of merger; (c) assets of every description, including choses-in-action and the business of each of the constituent companies, immediately vest in the surviving company or consolidated company; (d) the surviving company or consolidated company is liable for all claims, debts, liabilities and obligations of each of the constituent companies; (e) no conviction, judgment, ruling, order, claim, debt, liability or obligation due or to become due, and no cause existing, against a constituent company or against any shareholder, director, officer or agent thereof, is released or impaired by the merger or consolidation; and (f) no proceedings, whether civil or criminal, pending at the time of a merger or consolidation by or against a constituent company, or against any shareholder, director, officer or agent thereof, are abated or discontinued by the merger or consolidation, but: (i) the proceedings may be enforced, prosecuted, settled or compromised by or against the surviving company or consolidated company or against the shareholder, director, officer or agent thereof, as the case may be or (ii) the surviving company or consolidated company may be substituted in the proceedings for a constituent company but if the surviving company or the consolidated company is incorporated under the laws of a jurisdiction outside the BVI, the effect of the merger or consolidation is the same as noted foregoing except in so far as the laws of the other jurisdiction otherwise provide.
The Registrar shall strike off the register of companies each constituent company that is not the surviving company in the case of a merger and all constituent companies in the case of a consolidation (save that this shall not apply to a foreign company).
If the directors determine it to be in the best interests of us, it is also possible for a merger to be approved as a court approved plan of arrangement or as a scheme of arrangement in accordance with (in each such case) the BVI Act. The convening of any necessary shareholders meetings and subsequently the arrangement must be authorized by the BVI court. A scheme of arrangement requires the approval of 75% of the votes of the shareholders or class of shareholders, as the case may be. If the effect of the scheme is different in relation to different shareholders, it may be necessary for them to vote separately in relation to the scheme, with it being required to secure the requisite approval level of each separate voting group. Under a plan of arrangement, a BVI court may determine what shareholder approvals are required and the manner of obtaining the approval.
Shareholders’ Suits
Under the provisions of the BVI Act, the memorandum and articles of association of a company are binding as between the company and its members and between the members. In general, members are bound by the decision of the majority or special majorities as set out in the articles of association or in the BVI Act. As for voting, the usual rule is that with respect to normal commercial matters members may act from self-interest when exercising the right to vote attached to their shares.
If the majority members have infringed a minority member’s rights, the minority may seek to enforce its rights either by derivative action or by personal action. A derivative action concerns the infringement of the company’s rights where the wrongdoers are in control of the company and are preventing it from taking action, whereas a personal action concerns the infringement of a right that is personal to the particular member concerned.
The BVI Act provides for a series of remedies available to members. Where a company incorporated under the BVI Act conducts some activity which breaches the BVI Act or the company’s memorandum and articles of association, the BVI High Court can issue a restraining or compliance order. Members can now also bring derivative, personal and Representative Actions under certain circumstances.
The traditional English basis for members’ remedies have also been incorporated into the BVI Act: where a member of a company considers that the affairs of the company have been, are being or are likely to be conducted in a manner likely to be oppressive, unfairly discriminating or unfairly prejudicial to him, he may apply to the BVI High Court for an order on such conduct.
Any member of a company may apply to the BVI High Court for the appointment of a liquidator for the company and the Court may appoint a liquidator for the company if it is of the opinion that it is just and equitable to do so.
The BVI Act provides that any member of a company is entitled to payment of the fair value of his shares upon dissenting from any of the following:
(a) a merger;
(b) a consolidation;
(c) any sale, transfer, lease, exchange or other disposition of more than 50 per cent in value of the assets or business of the company if not made in the usual or regular course of the business carried on by the company but not including (i) a disposition pursuant to an order of the court having jurisdiction in the matter; (ii) a disposition for money on terms requiring all or substantially all net proceeds to be distributed to the members in accordance with their respective interest within one year after the date of disposition; or (iii) a transfer pursuant to the power of the directors to transfer assets for the protection thereof;
(d) a redemption of 10 per cent, or fewer, of the issued shares of the company required by the holders of 90 percent, or more, of the shares of the company pursuant to the terms of the BVI Act; and
(e) an arrangement, if permitted by the BVI High Court.
Generally any other claims against a company by its members must be based on the general laws of contract or tort applicable in the BVI or their individual rights as members as established by the company’s memorandum and articles of association.
The BVI Act provides that if a company or a director of a company engages in, proposes to engage in or has engaged in, conduct that contravenes the BVI Act or the memorandum or articles of association of the company, the BVI High Court may, on the application of a member or a director of the company, make an order directing the company or director to comply with, or restraining the company or director from engaging in conduct that contravenes the BVI Act or the memorandum or articles of association.
Indemnification of Directors and Executive Officers and Limitation of Liability
BVI law does not limit the extent to which a company’s articles of association may provide for indemnification of officers and directors, except to the extent any such provision may be held by the BVI High Court to be contrary to public policy (e.g. for purporting to provide indemnification against the consequences of committing a crime). An indemnity will be void and of no effect and will not apply to a person unless the person acted honestly and in good faith and in what he believed to be in the best interests of the company and, in the case of criminal proceedings, the person had no reasonable cause to believe that his conduct was unlawful. Our memorandum and articles of association provide that every director and officer of our company shall be indemnified against all actions, proceedings, costs, charges, expenses, losses, damages or liabilities incurred or sustained by such indemnified person, other than by reason of such indemnified person’s own dishonesty, willful default or fraud, in or about the conduct of our company’s business or affairs (including as a result of any mistake of judgment) or in the execution or discharge of his duties, powers, authorities or discretions, including without prejudice to the generality of the foregoing, any costs, expenses, losses or liabilities incurred by such indemnified person in defending (whether successfully or otherwise) any civil proceedings concerning our company or its affairs in any court whether in the British Virgin Islands or elsewhere. This standard of conduct is generally the same as permitted under the Delaware General Corporation Law for a Delaware corporation. In addition, we have entered into indemnification agreements with our directors and executive officers that provide such persons with additional indemnification beyond that provided in our memorandum and articles of association.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers or persons controlling us under the foregoing provisions, we have been informed that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Directors’ Fiduciary Duties
Under Delaware corporate law, a director of a Delaware corporation has a fiduciary duty to the corporation and its shareholders. This duty has two components: the duty of care and the duty of loyalty. The duty of care requires that a director act in good faith, with the care that an ordinarily prudent person would exercise under similar circumstances. Under this duty, a director must inform himself of, and disclose to shareholders, all material information reasonably available regarding a significant transaction. The duty of loyalty requires that a director acts in a manner he or she reasonably believes to be in the best interests of the corporation. He or she must not use his or her corporate position for personal gain or advantage. This duty prohibits self-dealing by a director and mandates that the best interest of the corporation and its shareholders take precedence over any interest possessed by a director, officer or controlling shareholder and not shared by the shareholders generally. In general, actions of a director are presumed to have been made on an informed basis, in good faith and in the honest belief that the action taken was in the best interests of the corporation. However, this presumption may be rebutted by evidence of a breach of one of the fiduciary duties. Should such evidence be presented concerning a transaction by a director, the director must prove the procedural fairness of the transaction, and that the transaction was of fair value to the corporation.
As a matter of BVI law, directors must not place themselves in a position in which there is a conflict between their duty to the company and their personal interests. This means that, strictly speaking, a director should not participate in a decision in circumstances where he has a potential conflict. That is, he should declare his interest and abstain. The BVI Act provides that a director “shall, forthwith after becoming aware of the fact that he is interested in a transaction entered into or to be entered into by the company, disclose the interest to the board of the company”. The failure of a director to so disclose an interest does not affect the validity of a transaction entered into by the director or the company, provided that the director’s interest was disclosed to the board prior to the company’s entry into the transaction or was not required to be disclosed (for example where the transaction is between the company and the director himself or is otherwise in the ordinary course of business and on usual terms and conditions). Typically a company’s memorandum and articles of association will allow a director interested in a particular transaction to vote on it, attend meetings at which it is considered, and sign documents on behalf of the company which relate to the transaction.
Under the laws of the BVI, a transaction entered into by the company in respect of which a director is interested will not be voidable by the company where the members have approved or ratified the transaction in knowledge of the material facts of the interest of the director in the transaction, or if the company received fair value for the transaction.
Broadly speaking, the duties that a director owes to a company may be divided into two categories. The first category encompasses fiduciary duties, that is, the duties of loyalty, honesty and good faith. The second category encompasses duties of skill and care. Each is considered in turn below.
A director’s fiduciary duties can be summarized as follows:
(a) Bona Fides: The directors must act bona fide in what they consider is in the best interests of the company (or, if permitted as above, that company’s parent company).
(b) Proper Purpose: The directors must exercise the powers that are vested in them for the purpose for which they were conferred and not for a collateral purpose.
(c) Unfettered Discretion: Since the powers of the directors are to be exercised by them in trust for the company, they should not improperly fetter the exercise of future discretion.
(d) Conflict of Duty and Interest: as per the above.
In addition to their fiduciary duties a director has the duties of care, diligence and skill which are owed to the company itself and not, for example, to individual members (subject to the limited exceptions as to enforcement on behalf of the company).
Shareholder Action by Written Consent
Under the Delaware General Corporation Law, a corporation may eliminate the right of shareholders to act by written consent by amendment to its certificate of incorporation. As permitted by BVI law, our articles of association provide that shareholders may approve corporate matters by way of a unanimous written resolution signed by or on behalf of each shareholder who would have been entitled to vote on such matter at a general meeting without a meeting being held.
Shareholder Proposals
Under the Delaware General Corporation Law, a shareholder has the right to put any proposal before the annual meeting of shareholders, provided it complies with the notice provisions in the governing documents. A special meeting may be called by the board of directors or any other person authorized to do so in the governing documents, but shareholders may be precluded from calling special meetings.
BVI law and our M&A provide that upon the written request of shareholders entitled to exercise thirty per cent (30%) or more of the voting rights in respect of the matter for which the meeting is requested, the directors shall convene a meeting of shareholders. As a BVI company, we are not obliged by law to call shareholders’ annual general meetings.
Cumulative Voting
Under the Delaware General Corporation Law, cumulative voting for elections of directors is not permitted unless the corporation’s certificate of incorporation specifically provides for it. Cumulative voting potentially facilitates the representation of investors on a board of directors since it permits the investor to cast all the votes to which the shareholder is entitled on a single director, which increases the shareholder’s voting power with respect to electing such director. There are no prohibitions in relation to cumulative voting under the laws of the British Virgin Islands but our articles of association do not provide for cumulative voting. As a result, our shareholders are not afforded any less protections or rights on this issue than shareholders of a Delaware corporation.
Removal of Directors
Under the Delaware General Corporation Law, a director of a corporation with a classified board may be removed only for cause with the approval of a majority of the outstanding shares entitled to vote, unless the certificate of incorporation provides otherwise. Under our articles of association, a director may be removed from office, by a resolution of shareholders passed at a meeting of shareholders or by a written resolution passed by a least fifty per cent (50%) of the votes of all shareholders of the company entitled to vote, notwithstanding any provision in the memorandum and articles of association or in any agreement between such director and us.
Transactions with Interested Shareholders
The Delaware General Corporation Law contains a business combination statute applicable to Delaware corporations whereby, unless the corporation has specifically elected not to be governed by such statute by amendment to its certificate of incorporation, it is prohibited from engaging in certain business combinations with an “interested shareholder” for three years following the date that such person becomes an interested shareholder. An interested shareholder generally is a person or a group who or which owns or owned 15% or more of the target’s outstanding voting share within the past three years. This has the effect of limiting the ability of a potential acquirer to make a two-tiered bid for the target in which all shareholders would not be treated equally. The statute does not apply if, among other things, prior to the date on which such shareholder becomes an interested shareholder, the board of directors approves either the business combination or the transaction which resulted in the person becoming an interested shareholder. This encourages any potential acquirer of a Delaware corporation to negotiate the terms of any acquisition transaction with the target’s board of directors.
British Virgin Islands law has no comparable statute. As a result, we are not afforded the same statutory protections in the British Virgin Islands as we would be offered by the Delaware business combination statute. However, although British Virgin Islands law does not regulate transactions between a company and its significant shareholders, it does provide that such transactions must be entered into bona fide in the best interests of the company and not with the effect of constituting a fraud on the investors. See also “Shareholders’ Suits” above. We have adopted a code of business conduct and ethics which requires employees to fully disclose any situations that could reasonably be expected to give rise to a conflict of interest, and sets forth relevant restrictions and procedures when a conflict of interest arises to ensure the best interest of the company.
Dissolution; Winding Up
Under the Delaware General Corporation Law, unless the board of directors approves the proposal to dissolve, dissolution must be approved by shareholders holding 100% of the total voting power of the corporation. Only if the dissolution is initiated by the board of directors may it be approved by a simple majority of the corporation’s outstanding shares. Delaware law allows a Delaware corporation to include in its certificate of incorporation a supermajority voting requirement in connection with dissolutions initiated by the board.
The liquidation of a company may be a voluntary solvent liquidation or a liquidation under the Insolvency Act. Where a company has been struck off the Register of Companies under the BVI Act continuously for a period of seven years it is dissolved with effect from the last day of that period.
Voluntary Liquidation
If the liquidation is a solvent liquidation, the provisions of the BVI Act governs the liquidation. A company may only be liquidated under the BVI Act as a solvent liquidation if it has no liabilities or it is able to pay its debts as they fall due and the value of its assets exceeds its liabilities. Subject to the memorandum and articles of association of a company, a liquidator may be appointed by a resolution of directors or resolution of members but if the directors have commenced liquidation by a resolution of directors the members must approve the liquidation plan by a resolution of members save in limited circumstances.
A liquidator is appointed for the purpose of collecting in and realizing the assets of a company and distributing proceeds to creditors.
Liquidation under the Insolvency Act
The Insolvency Act governs an insolvent liquidation. Pursuant to the Insolvency Act, a company is insolvent if it fails to comply with the requirements of a statutory demand that has not be set aside pursuant to the Insolvency Act, execution or other process issued on a judgment, decree or order of court in favor of a creditor of the company is returned wholly or partly unsatisfied or either the value of the company’s liabilities exceeds its assets or the company is unable to pay its debts as they fall due. The liquidator must be either the Official Receiver in BVI or a BVI licensed insolvency practitioner. An individual resident outside the BVI may be appointed to act as liquidator jointly with a BVI licensed insolvency practitioner or the Official Receiver. The members of the company may appoint an insolvency practitioner as liquidator of the company or the court may appoint an Official Receiver or an eligible insolvency practitioner. The application to the court can be made by one or more of the following: (i) the company, (ii) a creditor, (iii) a member, or (iv) the supervisor of a creditors’ arrangement in respect of the company, the Financial Services Commission and the Attorney General in the BVI.
The court may appoint a liquidator if:
(a) the company is insolvent;
(b) the court is of the opinion that it is just and equitable that a liquidator should be appointed; or
(c) the court is of the opinion that it is in the public interest for a liquidator to be appointed.
An application under (a) above by a member may only be made with leave of the court, which shall not be granted unless the court is satisfied that there is prima facie case that the company is insolvent. An application under (c) above may only be made by the Financial Services Commission or the Attorney General and they may only make an application under (c) above if the company concerned is, or at any time has been, a regulated person (i.e. a person that holds a prescribed financial services license) or the company is carrying on, or at any time has carried on, unlicensed financial services business.
Order of Preferential Payments upon Liquidation
Upon the insolvent liquidation of a company, the assets of a company shall be applied in accordance with the following priorities: (a) in paying, in priority to all other claims, the costs and expenses properly incurred in the liquidation in accordance with the prescribed priority; (b) after payment of the costs and expenses of the liquidation, in paying the preferential claims admitted by the liquidator (wages and salary, amounts to the BVI Social Security Board, pension contributions, government taxes) — preferential claims rank equally between themselves and, if the assets of the company are insufficient to meet the claims in full, they shall be paid ratably; (c) after the payment of preferential claims, in paying all other claims admitted by the liquidator, including those of non-secured creditors — the claims of non-secured creditors of the company shall rank equally among themselves and if the assets of the company are insufficient to meet the claims in full, such non-secured creditors shall be paid ratably; (d) after paying all admitted claims, paying any interest payable under the BVI Insolvency Act; and finally (e) any surplus assets remaining after payment of the costs, expenses and claims above shall be distributed to the members in accordance with their rights and interests in the company. Part VIII of the Insolvency Act provides for various applications which may be made by a liquidator to set aside transactions which have unfairly diminished the assets which are available to creditors.
The appointment of a liquidator over the assets of a company does not affect the right of a secured creditor to take possession of and realize or otherwise deal with assets of the company over which that creditor has a security interest. Accordingly, a secured creditor may enforce its security directly without recourse to the liquidator, in priority to the order of payments described in the preceding paragraph. However, so far as the assets of a company in liquidation available for payment of the claims of unsecured creditors are insufficient to pay the costs and expenses of the liquidation and the preferential creditors, those costs, expenses and claims have priority over the claims of charges in respect of assets that are subject to a floating charge created by a company and shall be paid accordingly out of those assets.
The court has authority to order winding up in a number of specified circumstances including where it is, in the opinion of the court, just and equitable to do so. Under the BVI Act and our articles of association, our company may be dissolved, liquidated or wound up by a resolution of our shareholders.
Variation of Rights of Shares
Under the Delaware General Corporation Law, a corporation may vary the rights of a class of shares with the approval of a majority of the outstanding shares of such class, unless the certificate of incorporation provides otherwise. Under British Virgin Islands law and our articles of association, if our share capital is divided into more than one class of shares, the rights attached to any class may only be materially adversely varied with the consent in writing of the holders of not less than two-thirds (2/3rds) of the issued shares of that class or with the sanction of a resolution of our shareholders passed at a separate meeting of the holders of the shares of that class by the holders of not less than two-thirds (2/3rds) of the issued shares of that class. The rights conferred upon the holders of the shares of any class issued with preferred or other rights shall not, subject to any rights or restrictions for the time being attached to the shares of that class, be deemed to be materially adversely varied by, inter alia, the creation, allotment or issue of further shares ranking pari passu with or subsequent to them or the redemption or purchase of any shares of any class by the company. The rights of the holders of shares shall not be deemed to be materially adversely varied by the creation or issue of shares with preferred or other rights including, without limitation, the creation of shares with enhanced or weighted voting rights.
Amendment of Governing Documents
Under the Delaware General Corporation Law, a corporation’s governing documents may be amended with the approval of a majority of the outstanding shares entitled to vote, unless the certificate of incorporation provides otherwise. As permitted by British Virgin Islands law, our memorandum and articles of association may be amended by a resolution of shareholders or by a resolution of directors, save that no amendment may be made by a resolution of directors: (i) to restrict the rights or powers of the shareholders to amend the memorandum or articles; (ii) to change the percentage of shareholders required to pass a resolution of shareholders to amend the memorandum or articles; (iii) in circumstances where the memorandum or articles cannot be amended by the shareholders; or (iv) to certain specified clauses of the articles of association.
Rights of Non-Resident or Foreign Shareholders
There are no limitations imposed by our memorandum and articles of association on the rights of non-resident or foreign shareholders to hold or exercise voting rights on our shares. In addition, there are no provisions in our memorandum and articles of association governing the ownership threshold above which shareholder ownership must be disclosed.
C. Material Contracts
We have not entered into any material contracts other than in the ordinary course of business and other than those described in “Item 4. Information on the Company—B. Business Overview”, “Item 7. Major Shareholders and Related Party Transactions—B. Related Party Transactions,” or elsewhere in this annual report.
D. Exchange Controls
See “Item 4. Information on the Company—B. Business Overview—PRC Regulations—Other Significant PRC Regulations Affecting Our Business Activities in China—Regulations Relating to Foreign Exchange.”
E. Taxation
The following summary of the material BVI, PRC and United States federal income tax consequences of an investment in our Class A ordinary shares or ADSs is based upon laws and relevant interpretations thereof in effect as of the date of this registration statement, all of which are subject to change. This summary does not deal with all possible tax consequences relating to an investment in our Class A ordinary shares or ADSs, such as the tax consequences under U.S. state and local tax laws or under the tax laws of jurisdictions other than the BVI, the People’s Republic of China and the United States.
BVI Taxation
Our company and all dividends, interest, rents, royalties, compensation and other amounts paid by our company to persons who are not resident in the BVI and any capital gains realized with respect to any shares, debt obligations, or other securities of our company by persons who are not resident in the BVI are exempt from all provisions of the Income Tax Ordinance in the BVI.
No estate, inheritance, succession or gift tax, rate, duty, levy or other charge is payable by persons who are not resident in the BVI with respect to any shares, debt obligation or other securities of our company.
All instruments relating to transfers of property to or by our company and all instruments relating to transactions in respect of the shares, debt obligations or other securities of our company and all instruments relating to other transactions relating to the business of our company are exempt from payment of stamp duty in the BVI. This assumes that our company does not hold an interest in real estate in the BVI.
There are currently no withholding taxes or exchange control regulations in the BVI applicable to our company or its members.
People’s Republic of China Taxation
Under the PRC EIT Law and its implementation rules, an enterprise established outside the PRC with a “de facto management body” within the PRC is considered a resident enterprise and will be subject to the enterprise income tax at the rate of 25% on its global income. The implementation rules define the term “de facto management body” as the body that exercises full and substantial control over and overall management of the business, production, personnel, accounts and properties of an enterprise. In April 2009, the SAT issued the Circular of the SAT on Issues Relating to Identification of PRC-Controlled Overseas Registered Enterprises as Resident Enterprises in Accordance With the De Facto Standards of Organizational Management, or SAT Circular 82, which provides certain specific criteria for determining whether the “de facto management body” of a PRC-controlled enterprise that is incorporated offshore is located in China. Although this circular only applies to offshore enterprises controlled by PRC enterprises or PRC enterprise groups, not those controlled by PRC individuals or foreigners, the criteria set forth in the circular may reflect the SAT’s general position on how the “de facto management body” test should be applied in determining the tax resident status of all offshore enterprises. According to SAT Circular 82, an offshore incorporated enterprise controlled by a PRC enterprise or a PRC enterprise group will be regarded as a PRC tax resident by virtue of having its “de facto management body” in the PRC only if all of the following conditions are met: (i) the primary location of the day-to-day operational management is in the PRC; (ii) decisions relating to the enterprise’s financial and human resource matters are made or are subject to approval by organizations or personnel in the PRC; (iii) the enterprise’s primary assets, accounting books and records, company seals, and board and shareholder resolutions, are located or maintained in the PRC; and (iv) at least 50% of voting board members or senior executives habitually reside in the PRC.
We do not believe that AnPac Bio meets all of the conditions above. AnPac Bio is a company incorporated outside the PRC. As a holding company, its key assets are its ownership interests in its subsidiaries, and its key assets are located, and its records (including the resolutions of its board of directors and the resolutions of its shareholders) are maintained, outside the PRC. For the same reasons, we believe our other entities outside of the PRC are not PRC resident enterprises, either. However, the tax resident status of an enterprise is subject to determination by the PRC tax authorities and uncertainties remain with respect to the interpretation of the term “de facto management body.” There can be no assurance that the PRC government will ultimately take a view that is consistent with ours.
If the PRC tax authorities determine that AnPac Bio is a PRC resident enterprise for enterprise income tax purposes, we may be required to withhold a 10% withholding tax from dividends we pay to our shareholders that are non-resident enterprises, including the holders of our ADSs. In addition, non-resident enterprise shareholders (including our ADS holders) may be subject to a 10% PRC tax on gains realized on the sale or other disposition of ADSs or Class A ordinary shares, if such income is treated as sourced from within the PRC. It is unclear whether our non-PRC individual shareholders (including our ADS holders) would be subject to any PRC tax on dividends or gains obtained by such non-PRC individual shareholders in the event we are determined to be a PRC resident enterprise. If any PRC tax were to apply to such dividends or gains, it would generally apply at a rate of 20% unless a reduced rate is available under an applicable tax treaty. However, it is also unclear whether non-PRC shareholders of AnPac Bio would be able to claim the benefits of any tax treaties between their country of tax residence and the PRC in the event that AnPac Bio is treated as a PRC resident enterprise.
Provided that our BVI holding company, AnPac Bio, is not deemed to be a PRC resident enterprise, holders of our ADSs and Class A ordinary shares who are not PRC residents will not be subject to PRC income tax on dividends distributed by us or gains realized from the sale or other disposition of our shares or ADSs. However, under SAT Public Notice 7 and SAT Public Notice 37, where a non-resident enterprise conducts an “indirect transfer” by transferring taxable assets, including, in particular, equity interests in a PRC resident enterprise, indirectly by disposing of the equity interests of an overseas holding company, the non-resident enterprise, being the transferor, or the transferee or the PRC entity which directly owned such taxable assets may report to the relevant tax authority such indirect transfer. Using a “substance over form” principle, the PRC tax authority may disregard the existence of the overseas holding company if it lacks a reasonable commercial purpose and was established for the purpose of reducing, avoiding or deferring PRC tax. As a result, gains derived from such indirect transfer may be subject to PRC enterprise income tax, and the transferee or other person who is obligated to pay for the transfer is obligated to withhold the applicable taxes, currently at a rate of 10% for the transfer of equity interests in a PRC resident enterprise. We and our non-PRC resident investors may be at risk of being required to file a return and being taxed under SAT Public Notice 7 and SAT Public Notice 37, and we may be required to expend valuable resources to comply with SAT Public Notice 7 and SAT Public Notice 37, or to establish that we should not be taxed under these circulars. See “Item 3. Key Information—D. Risk Factors—Risks Relating to Doing Business in China—We face uncertainty with respect to indirect transfers of equity interests in PRC resident enterprises by their non-PRC holding companies.”
United States Federal Income Tax Considerations
The following is a summary of material U.S. federal income tax considerations that are likely to be relevant to the purchase, ownership and disposition of our Class A ordinary shares or ADSs by a U.S. Holder (as defined below).
This summary is based on provisions of the Internal Revenue Code of 1986, as amended (the “Code”), and regulations, rulings and judicial interpretations thereof, in force as of the date hereof. Those authorities may be changed at any time, perhaps retroactively, so as to result in U.S. federal income tax consequences different from those summarized below.
This summary is not a comprehensive discussion of all of the tax considerations that may be relevant to a particular investor’s decision to purchase, hold, or dispose of Class A ordinary shares or ADSs. In particular, this summary is directed only to U.S. Holders that hold Class A ordinary shares or ADSs as capital assets, and does not address particular tax consequences that may be applicable to U.S. Holders who may be subject to special tax rules, such as banks, brokers or dealers in securities or currencies, traders in securities electing to mark to market, financial institutions, life insurance companies, tax-exempt entities, regulated investment companies, entities or arrangements that are treated as partnerships for U.S. federal income tax purposes (or partners therein), holders that own or are treated as owning 10% or more of our stock by vote or value, persons holding Class A ordinary shares or ADSs as part of a hedging or conversion transaction or a straddle, or persons whose functional currency is not the U.S. dollar. Moreover, this summary does not address state, local or non-U.S. taxes, the U.S. federal estate and gift taxes, the Medicare contribution tax applicable to net investment income of certain non-corporate U.S. Holders, or alternative minimum tax consequences of acquiring, holding or disposing of Class A ordinary shares or ADSs.
For purposes of this summary, a “U.S. Holder” is a beneficial owner of Class A ordinary shares or ADSs that is a citizen or resident of the United States or a U.S. domestic corporation or that otherwise is subject to U.S. federal income taxation on a net income basis in respect of such Class A ordinary shares or ADSs.
You should consult your own tax advisors about the consequences of the acquisition, ownership and disposition of the Class A ordinary shares or ADSs, including the relevance to your particular situation of the considerations discussed below and any consequences arising under non-U.S., state, local or other tax laws.
ADSs
In general, if you are a U.S. Holder of ADSs, you will be treated, for U.S. federal income tax purposes, as the beneficial owner of the underlying Class A ordinary shares that are represented by those ADSs. References to “shares” below apply to both Class A ordinary shares and ADSs, unless the context indicates otherwise.
Taxation of Dividends
Subject to the discussion below under “Passive Foreign Investment Company Status,” the gross amount of any distribution of cash or property with respect to our shares (including amounts, if any, withheld in respect of PRC taxes) that is paid out of our current or accumulated earnings and profits (as determined for U.S. federal income tax purposes) will generally be includible in your taxable income as ordinary dividend income on the day on which you receive the dividend, in the case of Class A ordinary shares, or the date the depositary receives the dividends, in the case of ADSs, and will not be eligible for the dividends-received deduction allowed to U.S. corporations under the Code.
We do not expect to maintain calculations of our earnings and profits in accordance with U.S. federal income tax principles. U.S. Holders therefore should expect that distributions generally will be treated as dividends for U.S. federal income tax purposes.
Subject to certain exceptions for short-term and hedged positions, the dividends received by a non-corporate U.S. Holder with respect to the shares will be subject to taxation at a preferential rate if the dividends are “qualified dividends.” Dividends paid on the shares will be treated as qualified dividends if:
· the shares are readily tradable on an established securities market in the United States or we are eligible for the benefits of a comprehensive tax treaty with the United States that the U.S. Treasury determines is satisfactory for purposes of this provision and that includes an exchange of information program; and
· we were not, in the year prior to the year in which the dividend was paid, and are not, in the year in which the dividend is paid, a PFIC.
The ADSs are listed on the NASDAQ Global Market, and will qualify as readily tradable on an established securities market in the United States so long as they are so listed. Based on our audited financial statements, the manner in which we conduct our business, and relevant market and shareholder data, we do not believe we were a PFIC for U.S. federal income tax purposes with respect to our prior taxable year. In addition, based on our audited financial statements, the manner in which we conduct our business, relevant market and shareholder data and our current expectations regarding the value and nature of our assets, and the sources and nature of our income, we do not anticipate becoming a PFIC for our current taxable year or in the foreseeable future. U.S. Holders should consult their own tax advisors regarding the availability of the reduced dividend tax rate in light of their own particular circumstances.
Because the Class A ordinary shares are not themselves listed on a U.S. exchange, dividends received with respect to Class A ordinary shares that are not represented by ADSs may not be treated as qualified dividends. U.S. Holders should consult their own tax advisors regarding the potential availability of the reduced dividend tax rate in respect of the Class A ordinary shares.
In the event that we are deemed to be a PRC resident enterprise under the PRC EIT Law (see “Taxation—People’s Republic of China Taxation”), a U.S. Holder may be subject to PRC withholding taxes on dividends paid on our shares. In that case, we may, however, be eligible for the benefits of the Agreement Between the Government of the United States of America and the Government of the People’s Republic of China for the Avoidance of Double Taxation and the Prevention of Tax Evasion with Respect to Taxes on Income (the “Treaty”). If we are eligible for such benefits, dividends we pay on our shares would be eligible for the reduced rates of taxation described above (assuming we are not a PFIC in the year the dividend is paid or the prior year). Dividend distributions with respect to our shares generally will be treated as “passive category” income from sources outside the United States for purposes of determining a U.S. Holder’s U.S. foreign tax credit limitation. Subject to the limitations and conditions provided in the Code and the applicable U.S. Treasury Regulations, a U.S. Holder may be able to claim a foreign tax credit against its U.S. federal income tax liability in respect of any PRC income taxes withheld at the appropriate rate applicable to the U.S. Holder from a dividend paid to such U.S. Holder. Alternatively, the U.S. Holder may deduct such PRC income taxes from its U.S. federal taxable income, provided that the U.S. Holder elects to deduct rather than credit all foreign income taxes for the relevant taxable year. The rules with respect to foreign tax credits are complex and involve the application of rules that depend on a U.S. Holder’s particular circumstances. Accordingly, U.S. Holders are urged to consult their tax advisors regarding the availability of the foreign tax credit or the deductibility of foreign taxes under their particular circumstances.
U.S. Holders that receive distributions of additional shares or rights to subscribe for shares as part of a pro rata distribution to all our shareholders generally will not be subject to U.S. federal income tax in respect of the distributions, unless the U.S. Holder has the right to receive cash or property, in which case the U.S. Holder will be treated as if it received cash equal to the fair market value of the distribution.
Taxation of Dispositions of Shares
Subject to the discussion below under “Passive Foreign Investment Company Status,” upon a sale, exchange or other taxable disposition of the shares, U.S. Holders will realize gain or loss for U.S. federal income tax purposes in an amount equal to the difference between the amount realized on the disposition and the U.S. Holder’s adjusted tax basis in the shares. Such gain or loss will be capital gain or loss, and will generally be long-term capital gain or loss if the shares have been held for more than one year. Long-term capital gain realized by a U.S. Holder that is an individual generally is subject to taxation at a preferential rate. The deductibility of capital losses is subject to limitations.
Gain, if any, realized by a U.S. Holder on the sale or other disposition of the shares generally will be treated as U.S. source income for U.S. foreign tax credit purposes. Consequently, if a PRC tax is imposed on the sale or other disposition of the shares, a U.S. Holder who does not receive significant foreign source income from other sources may not be able to derive effective U.S. foreign tax credit benefits in respect of such PRC tax. However, in the event that gain from the disposition of the shares is subject to tax in the PRC, and a U.S. Holder is eligible for the benefits of the Treaty, such U.S. Holder may elect to treat such gain as PRC source gain under the Treaty. U.S. Holders should consult their own tax advisors regarding the application of the foreign tax credit rules to their investment in, and disposition of, the shares.
Deposits and withdrawals of Class A ordinary shares by U.S. Holders in exchange for ADSs will not result in the realization of gain or loss for U.S. federal income tax purposes.
Passive Foreign Investment Company Status
Special U.S. tax rules apply to companies that are considered to be PFICs. We will be classified as a PFIC in a particular taxable year if, taking into account our proportionate share of the income and assets of our subsidiaries under applicable “look-through” rules, either
· 75 percent or more of our gross income for the taxable year is passive income; or
· the average percentage of the value of our assets that produce or are held for the production of passive income is, based on the average of four quarterly testing dates, at least 50 percent (the “asset test”).
For this purpose, passive income generally includes dividends, interest, royalties and rents (other than royalties and rents derived in the active conduct of a trade or business and not derived from a related person). If we own at least 25% (by value) of the stock of another corporation, for purposes of determining whether we are a PFIC, we will be treated as owning our proportionate share of the other corporation’s assets and receiving our proportionate share of the other corporation’s income. The asset test is generally applied using the fair market values of a non-U.S. corporation’s assets but is applied using adjusted tax bases of the assets if the non-U.S. corporation is a CFC and is not publicly traded for the year. We have been publicly traded since our initial public offering completed on February 3, 2020 and expect that we will be treated as publicly traded for 2021 and subsequent years. Accordingly, we believe that the PFIC asset test should be applied using the fair market values of our assets. U.S. Holders should consult their own tax advisors regarding the application of these rules and the appropriate valuation of our assets for purposes of the PFIC asset test, as well as the desirability of making a mark-to-market election (discussed below).
Based on our audited financial statements, the manner in which we conduct our business, relevant market and shareholder data and our current expectations regarding the value and nature of our assets and the sources and nature of our income, we do not believe that we were a PFIC in our taxable year ending December 31, 2020, and we do not anticipate becoming a PFIC for our current taxable year or in the foreseeable future. However, because the PFIC tests must be applied each year, and the composition of our income and assets and the value of our assets may change, it is possible that we may become a PFIC in the current or a future year. In particular, because the value of our assets for purposes of the asset test may be determined by reference to the market price of our ADSs, fluctuations in the market price of our ADSs may cause us to become a PFIC for the current or subsequent taxable years.
In the event that we are classified as a PFIC in any year during which a U.S. Holder holds our shares and such U.S. Holder does not make a mark-to-market election, as described in the following paragraph, the U.S. Holder will be subject to a special tax at ordinary income tax rates on “excess distributions,” including certain distributions by us (generally, distributions that are greater than 125% of the average annual distributions received during the shorter of the three preceding taxable years or the U.S. Holder’s holding period for the shares) and gain that the U.S. Holder recognizes on the sale or other disposition of our shares. The amount of income tax on any excess distributions will be increased by an interest charge to compensate for tax deferral, calculated as if the excess distributions were earned ratably over the period that the U.S. Holder holds its shares. Further, if we are a PFIC for any year during which a U.S. Holder holds our shares, we generally will continue to be treated as a PFIC for all subsequent years during which such U.S. Holder holds our shares unless we cease to be a PFIC and the U.S. Holder makes a special “purging” election on IRS Form 8621. Classification as a PFIC may also have other adverse tax consequences, including, in the case of individuals, the denial of a step-up in the basis of his or her shares at death.
A U.S. Holder may be able to avoid the unfavorable rules described in the preceding paragraph by electing to mark its ADSs to market, provided the ADSs are treated as “marketable stock.” The ADSs generally will be treated as marketable stock if the ADSs are “regularly traded” on a “qualified exchange or other market” (which includes the NASDAQ Global Market). It should also be noted that it is not currently intended that the Class A ordinary shares will be listed on any stock exchange. Consequently, a U.S. Holder that holds Class A ordinary shares that are not represented by ADSs may not be eligible to make a mark-to-market election. If the U.S. Holder makes a mark-to-market election, (i) the U.S. Holder will be required in any year in which we are a PFIC to include as ordinary income the excess of the fair market value of its ADSs at year-end over the U.S. Holder’s basis in those ADSs and (ii) the U.S. Holder will be entitled to deduct as an ordinary loss in each such year the excess of the U.S. Holder’s basis in its ADSs over their fair market value at year-end, but only to the extent of the net amount previously included in income as a result of the mark-to-market election. A U.S. Holder’s adjusted tax basis in its ADSs will be increased by the amount of any income inclusion and decreased by the amount of any deductions under the mark-to-market rules. In addition, any gain the U.S. Holder recognizes upon the sale of the U.S. Holder’s ADSs in a year in which we are PFIC will be taxed as ordinary income in the year of sale, and any loss the U.S. Holder recognizes upon the sale will be treated as ordinary loss, but only to the extent of the net amount of previously included income as a result of the mark-to-mark election.
A U.S. Holder that owns an equity interest in a PFIC must annually file IRS Form 8621. A failure to file one or more of these forms as required may toll the running of the statute of limitations in respect of each of the U.S. Holder’s taxable years for which such form is required to be filed. As a result, the taxable years with respect to which the U.S. Holder fails to file the form may remain open to assessment by the IRS indefinitely, until the form is filed.
If we are a PFIC for any taxable year during which a U.S. Holder holds our shares and any of our non-U.S. subsidiaries is also a PFIC, such U.S. Holder will be treated as owning a proportionate amount (by value) of the shares of the lower-tier PFIC for purposes of the application of the PFIC rules. U.S. Holders should consult their own tax advisors about the possible application of the PFIC rules to any of our subsidiaries.
U.S. Holders should consult their own tax advisors regarding the U.S. federal income tax considerations discussed above and the desirability of making a mark-to-market election.
Foreign Financial Asset Reporting
Certain U.S. Holders that own “specified foreign financial assets” with an aggregate value in excess of U.S.$50,000 on the last day of the taxable year or $75,000 at any time during the taxable year are generally required to file an information statement along with their tax returns, currently on IRS Form 8938, with respect to such assets. “Specified foreign financial assets” include any financial accounts held at a non-U.S. financial institution, as well as securities issued by a non-U.S. issuer that are not held in accounts maintained by financial institutions. The understatement of income attributable to “specified foreign financial assets” in excess of U.S.$5,000 extends the statute of limitations with respect to the tax return to six years after the return was filed. U.S. Holders who fail to report the required information could be subject to substantial penalties. Prospective investors are encouraged to consult their own tax advisors regarding the possible application of these rules to their investment, including the application of the rules to their particular circumstances.
Backup Withholding and Information Reporting
Dividends paid on, and proceeds from the sale or other disposition of, the shares that are paid to a U.S. Holder generally may be subject to the information reporting requirements of the Code and may be subject to backup withholding unless the U.S. Holder provides an accurate taxpayer identification number and makes any other required certification or otherwise establishes an exemption. Backup withholding is not an additional tax. The amount of any backup withholding from a payment to a U.S. Holder will be allowed as a refund or credit against the U.S. Holder’s U.S. federal income tax liability, provided the required information is furnished to the IRS in a timely manner.
A holder that is not a U.S. Holder may be required to comply with certification and identification procedures in order to establish its exemption from information reporting and backup withholding.
F. Dividends and Paying Agents
Not applicable.
G. Statement by Experts
Not applicable.
H. Documents on Display
We are subject to periodic reporting and other informational requirements of the Exchange Act as applicable to foreign private issuers. Accordingly, we are required to file reports, including annual reports on Form 20-F, and other information with the SEC. All information filed with the SEC can be obtained over the internet at the SEC’s website at www.sec.gov or inspected and copied at the public reference facilities maintained by the SEC at 100 F Street, N.E., Washington, D.C. 20549. You can request copies of documents, upon payment of a duplicating fee, by writing to the SEC.
As a foreign private issuer, we are exempt under the Exchange Act from, among other things, the rules prescribing the furnishing and content of proxy statements, and our executive officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act. However, we intend to furnish the depositary with our annual reports, which will include a review of operations and annual audited consolidated combined financial statements prepared in conformity with U.S. GAAP, and all notices of shareholders’ meetings and other reports and communications that are made generally available to our shareholders. The depositary will make such notices, reports and communications available to holders of ADSs and, if we so request, will mail to all record holders of ADSs the information contained in any notice of a shareholders’ meeting received by the depositary from us.
I. Subsidiary Information
For a listing of our subsidiaries, see “Item 4. Information on the Company—C. Organizational Structure.”
ITEM 11. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Concentration of credit risk
Financial instruments may subject us to significant concentration of credit risk. These financial instruments consist primarily of cash and cash equivalents and accounts receivables. As of December 31, 2018, 2019 and 2020, the aggregate amount of cash and cash equivalents of RMB7.0 million, RMB5.0 million and RMB2.4 million (US$362,000), respectively, was held at major financial institutions located in the PRC, and RMB5.9 million, RMB1.1 million and RMB650,000 (US$100,000), respectively, was deposited with major financial institutions located outside the PRC. Our management believes that these financial institutions are of high credit quality and continually monitors the credit worthiness of these financial institutions. Historically, deposits in Chinese banks are secure due to the state policy on protecting depositors’ interests. However, China promulgated a new Bankruptcy Law in August 2006 that came into effect on June 1, 2007, which contains a separate article expressly stating that the State Council may promulgate implementation measures for the bankruptcy of Chinese banks based on the Bankruptcy Law. Under the new Bankruptcy Law, a Chinese bank may go into bankruptcy. In addition, since China’s concession to the World Trade Organization, foreign banks have been gradually permitted to operate in China and have been significant competitors against Chinese banks in many aspects, especially since the opening of the Renminbi business to foreign banks in late 2006. Therefore, the risk of bankruptcy of those Chinese banks in which we have deposits has increased. In the event of bankruptcy of one of the banks which holds our deposits, we are unlikely to claim our deposits back in full since the bank is unlikely to be classified as a secured creditor based on PRC laws.
Accounts receivables, unsecured and denominated in Renminbi, derived from sales on our cancer screening and detection tests and physical checkup packages, are exposed to credit risk. As of December 31, 2018, 2019 and 2020, we had one customer, four customers and two customers, respectively, each with a receivable balance exceeding 10% of the total accounts receivable balance. The risk is mitigated by credit evaluations that we perform on our corporate customers.
Equity price risk
We are exposed to equity price risk primarily with respect to convertible loans issued by us accounted for under fair value option. Our investment in Jiangsu Anpac, which is equity securities without readily determinable fair values, is held for strategic purposes. It is accounted for under measurement alternative and not subject to equity price risk.
Currency convertibility risk
A significant portion of our expenses, assets and liabilities are denominated in Renminbi. On January 1, 1994, the PRC government abolished the dual rate system and introduced a single rate of exchange as quoted daily by the People’s Bank of China (the “PBOC”). However, the unification of the exchange rates does not imply that the Renminbi may be readily convertible into U.S. dollar or other foreign currencies. All foreign exchange transactions continue to take place either through the PBOC or other banks authorized to buy and sell foreign currencies at the exchange rates quoted by the PBOC. Approvals of foreign currency payments by the PBOC or other institutions require submitting a payment application form together with relevant documents.
Additionally, the value of the Renminbi is subject to changes in central government policies and international economic and political developments affecting supply and demand in the PRC foreign exchange trading system market.
Foreign currency exchange rate risk
Since July 21, 2005, Renminbi has been permitted to fluctuate within a narrow and managed band against a basket of certain foreign currencies. For U.S. dollar against RMB, there was appreciation of approximately 5.7%, appreciation of approximately 1.3% and depreciation of 6.3% for the years ended December 31, 2018, 2019 and 2020, respectively. It is difficult to predict how market forces or PRC or U.S. government policy may impact the exchange rate between the Renminbi and the U.S. dollar.
The functional currency of our company and AnPac US is the U.S. dollar and the functional currency of our PRC subsidiaries and our reporting currency is Renminbi. Most of our revenues and costs are denominated in RMB, while a portion of cash and cash equivalents and convertible loans are denominated in U.S. dollars. It is difficult to predict how market forces or the PRC or U.S. government policy may impact the exchange rate between the Renminbi and the US$ in the future. Any significant fluctuation of the valuation of RMB may materially affect our cash flows, revenues, earnings and financial position, and the value of any dividends payable on the ADS in US$.
Liquidity risks
As of December 31, 2020, we had RMB3.0 million (US$462,000) of cash and cash equivalents and RMB22.2 million (US$3.4 million) of working capital deficit. For the year ended December 31, 2020, we incurred RMB59.0 million (US$9.0 million) of negative cash flows from operations and RMB2.5 million (US$0.4 million) of capital expenditures. We believe that our cash and cash equivalents on hand, borrowings and future operating cash flows will be adequate to meet our obligations as they come due for the 12 months after the date of this annual report. Going forward, we expect to need additional fundraising if our cash flows generated from operations do not increase substantially.
ITEM 12. DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES
A. Debt Securities
Not applicable.
B. Warrants and Rights
Not applicable.
C. Other Securities
Not applicable.
D. American Depositary Shares
Fees and Charges
As an ADS holder, you will be required to pay the following fees under the terms of the deposit agreement:
|
Services: |
|
Fees: |
|
Issuance of ADSs (e.g., an issuance of ADS upon a deposit of Class A ordinary shares, upon a change in the ADS(s)-to Class A ordinary shares ratio, or for any other reason), excluding ADS issuances as a result of distributions of Class A ordinary shares) |
|
Up to U.S. 5¢ per ADS issued |
|
Cancellation of ADSs (e.g., a cancellation of ADSs for delivery of deposited property, upon a change in the ADS(s)-to-Class A ordinary shares ratio, or for any other reason) |
|
Up to U.S. 5¢ per ADS cancelled |
|
Distribution of cash dividends or other cash distributions (e.g., upon a sale of rights and other entitlements) |
|
Up to U.S. 5¢ per ADS held |
|
Distribution of ADSs pursuant to (i) stock dividends or other free stock distributions, or (ii) exercise of rights to purchase additional ADSs |
|
Up to U.S. 5¢ per ADS held |
|
Distribution of securities other than ADSs or rights to purchase additional ADSs (e.g., upon a spin-off) |
|
Up to U.S. 5¢ per ADS held |
|
ADS Services |
|
Up to U.S. 5¢ per ADS held on the applicable record date(s) established by the depositary |
|
Registration of ADS transfers (e.g., upon a registration of the transfer of registered ownership of ADSs, upon a transfer of ADSs into DTC and vice versa, or for any other reason) |
|
Up to U.S. 5¢ per ADS (or fraction thereof) transferred |
|
Conversion of ADSs of one series for ADSs of another series (e.g., upon conversion of Partial Entitlement ADSs for Full Entitlement ADSs, or upon conversion of Restricted ADSs (each as defined in the Deposit Agreement) into freely transferable ADSs, and vice versa) |
|
Up to U.S. 5¢ per ADS (or fraction thereof) converted |
As an ADS holder you will also be responsible to pay certain charges such as:
· taxes (including applicable interest and penalties) and other governmental charges;
· the registration fees as may from time to time be in effect for the registration of Class A ordinary shares on the share register and applicable to transfers of Class A ordinary shares to or from the name of the custodian, the depositary or any nominees upon the making of deposits and withdrawals, respectively;
· certain cable, telex and facsimile transmission and delivery expenses;
· the fees, expenses, spreads, taxes and other charges of the depositary and/or service providers (which may be a division, branch or affiliate of the depositary) in the conversion of foreign currency;
· the reasonable and customary out-of-pocket expenses incurred by the depositary in connection with compliance with exchange control regulations and other regulatory requirements applicable to Class A ordinary shares, ADSs and ADRs; and
· the fees, charges, costs and expenses incurred by the depositary, the custodian, or any nominee in connection with the ADR program.
ADS fees and charges for (i) the issuance of ADSs, and (ii) the cancellation of ADSs are charged to the person for whom the ADSs are issued (in the case of ADS issuances) and to the person for whom ADSs are cancelled (in the case of ADS cancellations). In the case of ADSs issued by the depositary into DTC, the ADS issuance and cancellation fees and charges may be deducted from distributions made through DTC, and may be charged to the DTC participant(s) receiving the ADSs being issued or the DTC participant(s) holding the ADSs being cancelled, as the case may be, on behalf of the beneficial owner(s) and will be charged by the DTC participant(s) to the account of the applicable beneficial owner(s) in accordance with the procedures and practices of the DTC participants as in effect at the time. ADS fees and charges in respect of distributions and the ADS service fee are charged to the holders as of the applicable ADS record date. In the case of distributions of cash, the amount of the applicable ADS fees and charges is deducted from the funds being distributed. In the case of (i) distributions other than cash and (ii) the ADS service fee, holders as of the ADS record date will be invoiced for the amount of the ADS fees and charges and such ADS fees and charges may be deducted from distributions made to holders of ADSs. For ADSs held through DTC, the ADS fees and charges for distributions other than cash and the ADS service fee may be deducted from distributions made through DTC, and may be charged to the DTC participants in accordance with the procedures and practices prescribed by DTC and the DTC participants in turn charge the amount of such ADS fees and charges to the beneficial owners for whom they hold ADSs. In the case of (i) registration of ADS transfers, the ADS transfer fee will be payable by the ADS Holder whose ADSs are being transferred or by the person to whom the ADSs are transferred, and (ii) conversion of ADSs of one series for ADSs of another series, the ADS conversion fee will be payable by the Holder whose ADSs are converted or by the person to whom the converted ADSs are delivered.
In the event of refusal to pay the depositary fees, the depositary may, under the terms of the deposit agreement, refuse the requested service until payment is received or may set off the amount of the depositary fees from any distribution to be made to the ADS holder. Certain depositary fees and charges (such as the ADS services fee) may become payable shortly after the closing of the ADS offering. Note that the fees and charges you may be required to pay may vary over time and may be changed by us and by the depositary. You will receive prior notice of such changes. The depositary may reimburse us for certain expenses incurred by us in respect of the ADR program, by making available a portion of the ADS fees charged in respect of the ADR program or otherwise, upon such terms and conditions as we and the depositary agree from time to time.
Fees and Other Payments Made by the Depositary to Us
The depositary bank may reimburse us for certain expenses incurred by us in respect of the ADR program, by making available a portion of the ADS fees charged in respect of the ADR program or otherwise, upon such terms and conditions as we and the depositary bank agree from time to time. For the year ended December 31, 2020, the reimbursement we received from the depositary was US$46,042 net of applicable withholding taxes.
ITEM 13. DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES
Not applicable.
ITEM 14. MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS
See “Item 10. Additional Information—B. Memorandum and Articles of Association” for a description of the rights of securities holders, which remain unchanged.
Use of Proceeds
The following “Use of Proceeds” information relates to the registration statement on Form F-1, as amended (File No. 333-234408) in relation to our initial public offering, which was declared effective by the SEC on January 28, 2020. We made our initial public offering on January 29, 2020 and completed the offering on February 3, 2020. In this offering, we issued and sold an aggregate of 1,333,360 ADSs, representing 1,333,360 Class A ordinary shares, at an initial offering price of US$12.00 per ADS.
The total expenses incurred for our company’s account in connection with our initial public offering were approximately US$5.0 million, which included US$1.4 million in underwriting discounts and commissions for the initial public offering and approximately US$3.6 million in other costs and expenses for our initial public offering. None of the transaction expenses included payments to directors or officers of our company or their associates, persons owning more than 10% or more of our equity securities or our affiliates. We have used all of the net proceeds from our initial public offering.
ITEM 15. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
Our management, with the participation of our chief executive officer and chief financial officer, has performed an evaluation of the effectiveness of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Exchange Act) as of the end of the period covered by this report, as required by Rule 13a-15(b) under the Exchange Act.
Based upon that evaluation, our management has concluded that, as of December 31, 2020, our disclosure controls and procedures were ineffective. We have started to undertake steps to remediate the material weakness in our disclosure controls and procedures as set forth below under “Internal Control over Financial Reporting.”
Management’s Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rules 13a-15 (f) under the Exchange Act. Our management, with the participation of our chief executive officer and our chief financial officer, evaluated the effectiveness of our internal control over financial reporting based on criteria established in the framework in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this evaluation, our management has concluded that our internal control over financial reporting was not effective as of December 31, 2020 because of the material weaknesses we identified.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies and procedures may deteriorate.
In connection with the preparation and audit of our consolidated financial statements as of and for the year ended December 31, 2020, our management identified the following three material weaknesses in our internal control over financial reporting: (i) lack of full-time personnel with appropriate levels of accounting knowledge and experience to address complex U.S. GAAP accounting issues and to prepare and review financial statements and related disclosures under the U.S. GAAP, (ii) lack of financial reporting policies and procedures that are commensurate with U.S. GAAP and SEC reporting requirements, and (iii) lack of IT risk management mechanism and policies and procedures to address critical areas of IT system and failure to perform periodic vulnerability or penetration testing to identify, asses and address cybersecurity risk.
After identifying the material weaknesses, we started to implement measures designed to improve our financial control over financial reporting through: (i) hiring additional qualified accounting and financial reporting personnel with U.S. GAAP and SEC reporting experience, (ii) obtaining advisory services from professional consultants with experience in the requirements of the Sarbanes Oxley Act of 2002 and internal audit guidance on SEC reporting, (iii) expanding the capabilities of our existing accounting and financial reporting personnel through continuous training and education in the accounting and reporting requirements under U.S. GAAP, and SEC rules and regulations, (iv) developing, communicating and implementing an accounting policy manual for our accounting and financial reporting personnel for our recurring transactions and period-end closing processes, and (v) establishing effective monitoring and oversight controls for non-recurring and complex transactions to ensure the accuracy and completeness of our company’s consolidated financial statements and related disclosures.
Because such remediation measures were not fully implemented, our management has concluded that the material weaknesses still existed as of December 31, 2020. We expect to complete the measures discussed above and also to take actions to (i) continue to recruit experienced personnel with relevant past experience working on U.S. GAAP and SEC reporting, (ii) improve monitoring and oversight controls for non-recurring and complex transactions to ensure the accuracy and completeness of financial reporting and (iii) engage external experts to assist in non-recurring and complex transactions by the end of 2021 and will continue to implement measures to remediate our internal control deficiencies.
We are fully committed to continue to implement measures to remediate our material weakness, significant deficiency and other control deficiencies in our internal control over financial reporting. However, the implementation of these measures may not fully address the material weaknesses in our internal control over financial reporting. We are not able to estimate with reasonable certainty the costs that we will need to incur to implement these and other measures designed to improve our internal control over financial reporting.
The process of designing and implementing an effective financial reporting system is a continuous effort that requires us to anticipate and react to changes in our business and the economic and regulatory environments and to expend significant resources to maintain a financial reporting system that is adequate to satisfy our reporting obligations. See “Item 3. Key Information—D. Risk Factors—Risks Relating to Our Business and Industry—Material weaknesses in our internal control over financial reporting have been identified, and if we fail to implement and maintain an effective system of internal controls over financial reporting, we may be unable to accurately report our results of operations, meet our reporting obligations or prevent fraud.”
Attestation report of the registered public accounting firm
Since we are an “emerging growth company” as defined under the JOBS Act, we are exempt from the requirement to comply with the auditor attestation requirements that our independent registered public accounting firm attest to and report on the effectiveness of our internal control structure and procedures for financial reporting.
Changes in Internal Control over Financial Reporting
Other than as described above, there were no changes in our internal controls over financial reporting that occurred during the period covered by this annual report on Form 20-F, which have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 16A. AUDIT COMMITTEE FINANCIAL EXPERT
Our Board of Directors has determined that Mr. Pu Xing, an independent director (under the standards set forth in Rule 5605(c)(2) of the NASDAQ and Rule 10A-3 under the Exchange Act), and the chairman of our Audit Committee, is our Audit Committee financial expert.
Our Board of Directors has adopted a code of business conduct and ethics that applies to our all directors, officers and employees in October 2019. We have posted a copy of our code of business conduct and ethics on our website at https://investors.anpacbio.com/static-files/21d2b39d-29e7-4a19-878d-846206d70089, where you can obtain a copy without charge.
ITEM 16C. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The following table sets forth the aggregate fees by categories specified below in connection with certain professional services rendered by Ernst & Young Hua Ming LLP (“Ernst & Young”) and Friedman LLP (“Friedman”), our principal external auditors, for the periods indicated.
|
|
|
Years Ended December 31, |
| ||||||
|
|
|
2019 |
|
2020 |
| ||||
|
|
|
RMB |
|
US$ |
|
RMB |
|
US$ |
|
|
|
|
(in thousands) |
| ||||||
|
Audit fees(1) |
|
|
|
|
|
|
|
|
|
|
- Ernst & Young |
|
4,438 |
|
637 |
|
1,058 |
|
163 |
|
|
- Friedman |
|
— |
|
— |
|
1,240 |
|
190 |
|
(1) Audit fees include the aggregate fees billed in each of the fiscal period listed for professional services rendered by our independent public accountant in relation to the audit of our annual financial statements and services related to our initial public offering.
The policy of our audit committee is to pre-approve all audit services provided by our independent registered public accounting firms, other than those for de minimis services which are approved by the audit committee prior to the completion of the audit.
ITEM 16D. EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES
Not applicable.
ITEM 16E. PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS
Not applicable.
ITEM 16F. CHANGE IN REGISTRANT’S CERTIFYING ACCOUNTANT
On November 3, 2020, Ernst & Young resigned as our independent registered public accounting firm. On December 2, 2020, we engaged Friedman as our independent registered public accounting firm in connection with the audit of our consolidated financial statements for the year ended December 31, 2020. The appointment of Friedman was recommended by our audit committee and approved by our board of directors.
Ernst & Young’s audit report on our consolidated financial statements as of and for the years ended December 31, 2018 and 2019 did not contain an adverse opinion or a disclaimer of opinion and was not qualified or modified as to uncertainty, audit scope or accounting principles. Ernst & Young did not audit any financial statements of our company as of any date or for any period subsequent to December 31, 2019.
During each of the years ended December 31, 2018 and 2019, there were (i) no disagreements, as that term is defined in Item 16F(a)(1)(iv) of Form 20-F and the related instructions to Item 16F of Form 20-F, between us and Ernst & Young on any matter of accounting principles or practices, financial statement disclosure, or audit scope or procedure, any of which, if not resolved to Ernst & Young’s satisfaction, would have caused Ernst & Young to make reference thereto in their reports, and (ii) no “reportable events” requiring disclosure pursuant to Item 16F(a)(1)(v) of the instructions to Form 20-F in connection with our annual report on Form 20-F, other than the material weaknesses in internal control over financial reporting disclosed in this Item 16F.
In the course of auditing our consolidated financial statements for the years ended December 31, 2018 and 2019, two material weaknesses in ICFR were identified, which were (i) lack of sufficient accounting and financial reporting personnel with requisite knowledge and experience in application of U.S. GAAP and SEC rules, and (ii) lack of financial reporting policies and procedures that are commensurate with U.S. GAAP and SEC reporting requirements. The material weaknesses remained as of December 31, 2019.
The matter described above constitutes a reportable event. Our audit committee has discussed such reportable event with Ernst & Young and we authorized Ernst & Young to fully respond to the enquiries from Friedman with respect to this reportable event.
We provided Ernst & Young with a copy of the disclosures under this Item 16F and requested from Ernst & Young a letter addressed to the SEC indicating whether it agrees with such disclosures, and if not, stating the respects in which it does not agree. We have received the requested letter from Ernst & Young, a copy of which is included as Exhibit 16.1 attached herein.
During each of the years ended December 31, 2018 and 2019 and the subsequent interim period through December 2, 2020, neither we nor anyone on behalf of us consulted with Friedman regarding (i) the application of accounting principles to a specific transaction, either completed or proposed, or the type of audit opinion that might be rendered on our financial statements, and neither a written report nor oral advice was provided to us that Friedman concluded was an important factor considered by us in reaching a decision as to any accounting, auditing, or financial reporting issue, or (ii) any matter that was the subject of a disagreement, as that term is defined in Item 16F(a)(1)(iv) of the instructions to Form 20-F (and the related instructions thereto) or a reportable event pursuant to Item 16F(a)(1)(v)(A) through (D) of Form 20-F.
ITEM 16G. CORPORATE GOVERNANCE
We are a “foreign private issuer” (as such term is defined in Rule 3b-4 under the Exchange Act), and our ADSs are listed on the NASDAQ Global Market. The NASDAQ rules provide that foreign private issuers may follow home country practice in lieu of the corporate governance requirements of the NASDAQ Stock Market LLC, subject to certain exceptions and requirements and except to the extent that such exemptions would be contrary to U.S. federal securities laws and regulations. The significant differences between our corporate governance practices and those followed by domestic companies under the NASDAQ rules are summarized as follows:
· shareholder approval for certain events, including the establishment or amendment of equity based compensation plans and arrangements and transactions involving issuances of 20% or more interest in our company;
· a majority of independent directors on our board of directors;
· a compensation committee and a nominating/corporate governance committee composed entirely of independent directors;
· an audit committee with a minimum of three members; and
· regularly scheduled executive sessions of independent directors.
Other than the above, we have followed and intend to continue to follow the applicable corporate governance standards under the NASDAQ rules.
As a result of our reliance on the corporate governance exemptions available to foreign private issuers, holders of our ADSs will not have the same protection afforded to shareholders of companies that are subject to all of NASDAQ Global Market corporate governance requirements.
ITEM 16H. MINE SAFETY DISCLOSURE
Not applicable.
We have elected to provide financial statements pursuant to “Item 18. Financial Statements.”
Our consolidated financial statements are included at the end of this annual report.
* Filed with this annual report on Form 20-F
** Furnished with this annual report on Form 20-F
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
|
|
ANPAC BIO-MEDICAL SCIENCE CO., LTD. | |
|
|
| |
|
|
By: |
/s/ Chris Chang Yu |
|
|
Name: Chris Chang Yu | |
|
|
Title: Chairman of the Board of Directors and Chief Executive Officer | |
|
Date: April 30, 2021 |
| |
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
|
F-2 | |
|
Consolidated balance sheets as of December 31, 2020 and 2019 |
F-4 |
|
F-5 | |
|
F-6 | |
|
Consolidated statements of cash flows for the years ended December 31, 2020, 2019 and 2018 |
F-7 |
|
F-8 – F-44 |

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and the Board of
Directors of AnPac Bio-Medical Science Co., Ltd
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheet of AnPac Bio-Medical Science Co. Ltd. (the “Company”) as of December 31, 2020, and the related consolidated statements of operations and comprehensive income loss, shareholders’ equity (deficit) and cash flows for the year ended December 31, 2020, and the related notes (collectively referred to as the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2020, and the results of its operations and its cash flows for the year ended December 31, 2020, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audit provide a reasonable basis for our opinion.
/s/ Friedman LLP
We have served as the Company’s auditor since 2020
New York, New York
April 30, 2021

REPORTS OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRMS
To the Shareholders and the Board of Directors of AnPac Bio-Medical Science Co., Ltd.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of AnPac Bio-Medical Science Co., Ltd. (the “Company”) as of December 31, 2019, the related consolidated statements of comprehensive loss, shareholders’ deficit and cash flows for each of the two years in the period ended December 31, 2019, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2019, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2019, in conformity with the U.S. generally accepted accounting principles.
Adoption of New Accounting Standards
As discussed in Note 2 to the consolidated financial statements, the Company changed its method for accounting for investments in certain equity securities in the year ended December 31, 2019.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
|
/s/ Ernst & Young Hua Ming LLP |
|
|
We served as the Company’s auditor from 2018 to 2020. |
|
|
Shanghai, the People’s Republic of China |
|
|
May 15, 2020 |
|
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
(Amounts in thousands of Renminbi (“RMB”) and U.S. dollars (“US$”), except for number of shares and per share data)
|
|
|
As of December 31, |
| ||||
|
|
|
2019 |
|
2020 |
|
2020 |
|
|
|
|
RMB |
|
RMB |
|
US$ |
|
|
ASSETS |
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
6,125 |
|
3,016 |
|
462 |
|
|
Advances to suppliers |
|
1,093 |
|
5,588 |
|
856 |
|
|
Accounts receivable, net |
|
1,295 |
|
7,792 |
|
1,194 |
|
|
Amounts due from related parties |
|
555 |
|
1,277 |
|
196 |
|
|
Inventories, net |
|
313 |
|
312 |
|
48 |
|
|
Other current assets, net |
|
12,790 |
|
3,303 |
|
506 |
|
|
Total current assets |
|
22,171 |
|
21,288 |
|
3,262 |
|
|
|
|
|
|
|
|
|
|
|
Property and equipment, net |
|
18,868 |
|
19,267 |
|
2,953 |
|
|
Land use rights, net |
|
1,194 |
|
1,166 |
|
179 |
|
|
Intangible assets, net |
|
5,200 |
|
4,596 |
|
704 |
|
|
Goodwill |
|
2,223 |
|
2,223 |
|
341 |
|
|
Long-term investments, net |
|
2,326 |
|
883 |
|
135 |
|
|
Other assets |
|
1,000 |
|
464 |
|
71 |
|
|
TOTAL ASSETS. |
|
52,982 |
|
49,887 |
|
7,645 |
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND SHAREHOLDERS’ DEFICIT |
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
Short-term debts |
|
38,568 |
|
8,232 |
|
1,262 |
|
|
Accounts payable |
|
1,800 |
|
2,127 |
|
325 |
|
|
Advance from customers |
|
2,450 |
|
3,682 |
|
564 |
|
|
Amounts due to related parties |
|
4,597 |
|
4,130 |
|
633 |
|
|
Accrued expenses and other current liabilities |
|
18,782 |
|
25,353 |
|
3,886 |
|
|
Total current liabilities |
|
66,197 |
|
43,524 |
|
6,670 |
|
|
|
|
|
|
|
|
|
|
|
Deferred tax liabilities |
|
1,134 |
|
1,045 |
|
160 |
|
|
Other long-term liabilities |
|
1,575 |
|
2,041 |
|
313 |
|
|
TOTAL LIABILITIES. |
|
68,906 |
|
46,610 |
|
7,143 |
|
|
|
|
|
|
|
|
|
|
|
Commitments and contingencies |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shareholders’ (deficit) equity: |
|
|
|
|
|
|
|
|
Class A Ordinary shares ((US$0.01 par value per share; 70,000,000 shares authorized, 7,004,900 and 9,192,660 shares issued and outstanding as of December 31, 2019 and 2020, respectively) |
|
466 |
|
618 |
|
95 |
|
|
Class B Ordinary shares ((US$0.01 par value per share; 30,000,000 authorized, 2,863,100 shares issued and outstanding as of December 31, 2019 and 2020) |
|
191 |
|
191 |
|
29 |
|
|
Additional paid-in capital |
|
257,736 |
|
354,295 |
|
54,298 |
|
|
Accumulated deficit |
|
(276,476 |
) |
(356,951 |
) |
(54,705 |
) |
|
Accumulated other comprehensive income |
|
2,110 |
|
4,795 |
|
735 |
|
|
Total AnPac Bio-Medical Science Co., Ltd. shareholders’ (deficit) equity |
|
(15,973 |
) |
2,948 |
|
452 |
|
|
Noncontrolling interests |
|
49 |
|
329 |
|
50 |
|
|
Total shareholders’ (deficit) equity |
|
(15,924 |
) |
3,277 |
|
502 |
|
|
|
|
|
|
|
|
|
|
|
TOTAL LIABILITIES AND EQUITY |
|
52,982 |
|
49,887 |
|
7,645 |
|
The accompanying notes are an integral part of these consolidated financial statements
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
|
|
|
Year Ended December 31, |
| ||||||
|
|
|
2018 |
|
2019 |
|
2020 |
|
2020 |
|
|
|
|
RMB |
|
RMB |
|
RMB |
|
US$ |
|
|
Revenues: |
|
|
|
|
|
|
|
|
|
|
Cancer screening and detection tests |
|
9,557 |
|
10,381 |
|
18,445 |
|
2,827 |
|
|
Physical checkup packages, net |
|
693 |
|
464 |
|
2,064 |
|
316 |
|
|
Total revenues |
|
10,250 |
|
10,845 |
|
20,509 |
|
3,143 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of revenues, cancer screening |
|
(5,672 |
) |
(6,047 |
) |
(7,628 |
) |
(1,169 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
Gross Profit |
|
4,578 |
|
4,798 |
|
12,881 |
|
1,974 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
Selling and marketing |
|
(9,827 |
) |
(13,633 |
) |
(19,674 |
) |
(3,015 |
) |
|
Research and development |
|
(10,106 |
) |
(9,839 |
) |
(11,576 |
) |
(1,774 |
) |
|
General and administrative |
|
(28,254 |
) |
(69,088 |
) |
(74,757 |
) |
(11,457 |
) |
|
Impairment of long-term investments |
|
— |
|
(1,320 |
) |
(1,430 |
) |
(219 |
) |
|
Loss from operations |
|
(43,609 |
) |
(89,082 |
) |
(94,556 |
) |
(14,491 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
Non-operating income and expenses: |
|
|
|
|
|
|
|
|
|
|
Interest expense, net |
|
(925 |
) |
(2,609 |
) |
(1,143 |
) |
(175 |
) |
|
Foreign exchange loss, net |
|
(2,776 |
) |
(3,219 |
) |
(667 |
) |
(102 |
) |
|
Share of net (loss) gain in equity method investments |
|
(441 |
) |
190 |
|
(13 |
) |
(2 |
) |
|
Other income (expense), net |
|
6,040 |
|
(1,823 |
) |
9,096 |
|
1,394 |
|
|
Change in fair value of convertible debt and settlement gain |
|
(784 |
) |
(5,296 |
) |
6,630 |
|
1,016 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss before income taxes |
|
(42,495 |
) |
(101,839 |
) |
(80,653 |
) |
(12,360 |
) |
|
Income tax benefit |
|
199 |
|
218 |
|
88 |
|
13 |
|
|
Net loss |
|
(42,296 |
) |
(101,621 |
) |
(80,565 |
) |
(12,347 |
) |
|
Net loss attributable to noncontrolling interests |
|
(233 |
) |
(561 |
) |
(90 |
) |
(14 |
) |
|
Net loss attributable to ordinary shareholders |
|
(42,063 |
) |
(101,060 |
) |
(80,475 |
) |
(12,333 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
Loss per share |
|
|
|
|
|
|
|
|
|
|
Class A and B ordinary shares - basic and diluted |
|
(4.93 |
) |
(11.31 |
) |
(7.19 |
) |
(1.10 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average shares outstanding used in calculating basic and diluted loss per share |
|
|
|
|
|
|
|
|
|
|
Class A and Class B ordinary shares - basic and diluted |
|
8,524,100 |
|
8,937,600 |
|
11,190,079 |
|
11,190,079 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive (loss) income, net of tax: |
|
|
|
|
|
|
|
|
|
|
Fair value change relating to Company’s own credit risk on convertible loan |
|
— |
|
(955 |
) |
(108 |
) |
(17 |
) |
|
Foreign currency translation adjustment |
|
797 |
|
2,978 |
|
2,793 |
|
428 |
|
|
Total comprehensive loss |
|
(41,499 |
) |
(99,598 |
) |
(77,880 |
) |
(11,936 |
) |
|
Total comprehensive loss attributable to noncontrolling interests |
|
(233 |
) |
(561 |
) |
(90 |
) |
(14 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
Total comprehensive loss attributable to ordinary shareholders |
|
(41,266 |
) |
(99,037 |
) |
(77,790 |
) |
(11,922 |
) |
The accompanying notes are an integral part of these consolidated financial statements.
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ DEFICIT
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
|
|
|
Ordinary Shares |
|
Class A Ordinary |
|
Class B Ordinary |
|
Additional |
|
Accumulated |
|
Accumulated |
|
Total AnPac Bio- |
|
Noncontrolling |
|
Total |
| ||||||
|
|
|
Shares |
|
Amount |
|
Shares |
|
Amount |
|
Shares |
|
Amount |
|
Capital |
|
Deficit |
|
(Note 10) |
|
Equity (Deficit) |
|
interest |
|
(Deficit) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at January 1, 2018 |
|
8,524,000 |
|
564 |
|
— |
|
— |
|
— |
|
— |
|
143,057 |
|
(132,290 |
) |
(1,773 |
) |
9,558 |
|
(61 |
) |
9,497 |
|
|
Net loss |
|
|
|
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
(42,063 |
) |
— |
|
(42,063 |
) |
(233 |
) |
(42,296 |
) |
|
Issuance of ordinary shares |
|
93,700 |
|
6 |
|
— |
|
— |
|
— |
|
— |
|
2,555 |
|
— |
|
— |
|
2,561 |
|
— |
|
2,561 |
|
|
Foreign currency translation differences |
|
|
|
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
797 |
|
797 |
|
— |
|
797 |
|
|
Acquisition of noncontrolling interests |
|
|
|
|
|
— |
|
— |
|
— |
|
— |
|
(454 |
) |
— |
|
— |
|
(454 |
) |
294 |
|
(160 |
) |
|
Repurchase and cancellation of shares |
|
(20,800 |
) |
(1 |
) |
— |
|
— |
|
— |
|
— |
|
(727 |
) |
— |
|
— |
|
(728 |
) |
— |
|
(728 |
) |
|
Share-based compensation |
|
|
|
|
|
— |
|
— |
|
— |
|
— |
|
7,936 |
|
— |
|
— |
|
7,936 |
|
— |
|
7,936 |
|
|
Balance at December 31, 2018 |
|
8,596,900 |
|
569 |
|
— |
|
— |
|
— |
|
— |
|
152,367 |
|
(174,353 |
) |
(976 |
) |
(22,393 |
) |
— |
|
(22,393 |
) |
|
Cumulative effect of the adoption of ASU 2016-01 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(1,063 |
) |
1,063 |
|
— |
|
— |
|
— |
|
|
Balance at January 1, 2019 |
|
8,596,900 |
|
569 |
|
— |
|
— |
|
— |
|
— |
|
152,367 |
|
(175,416 |
) |
87 |
|
(22,393 |
) |
— |
|
(22,393 |
) |
|
Re-designation of authorized ordinary shares |
|
(8,596,900 |
) |
(569 |
) |
5,733,800 |
|
378 |
|
2,863,100 |
|
191 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
|
Net loss |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(101,060 |
) |
— |
|
(101,060 |
) |
(561 |
) |
(101,621 |
) |
|
Issuance of ordinary shares upon private placement |
|
— |
|
— |
|
1,347,200 |
|
93 |
|
— |
|
— |
|
72,509 |
|
— |
|
— |
|
72,602 |
|
— |
|
72,602 |
|
|
Fair value change relating to Company’s own credit risk on convertible loan |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(955 |
) |
(955 |
) |
— |
|
(955 |
) |
|
Foreign currency translation differences |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
2,978 |
|
2,978 |
|
— |
|
2,978 |
|
|
Capital contribution from noncontrolling interest holders |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
610 |
|
610 |
|
|
Repurchase and cancellation of shares |
|
— |
|
— |
|
(76,100 |
) |
(5 |
) |
— |
|
— |
|
5 |
|
— |
|
— |
|
— |
|
— |
|
|
|
|
Share-based compensation |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
32,855 |
|
— |
|
— |
|
32,855 |
|
— |
|
32,855 |
|
|
Balance at December 31, 2019 |
|
— |
|
— |
|
7,004,900 |
|
466 |
|
2,863,100 |
|
191 |
|
257,736 |
|
(276,476 |
) |
2,110 |
|
(15,973 |
) |
49 |
|
(15,924 |
) |
|
Net loss |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(80,475 |
) |
— |
|
(80,475 |
) |
(90 |
) |
(80,565 |
) |
|
Issuance of ordinary shares upon initial public offering, net |
|
— |
|
— |
|
1,333,360 |
|
92 |
|
— |
|
— |
|
75,368 |
|
— |
|
— |
|
75,460 |
|
— |
|
75,460 |
|
|
Issuance shares for exercise of stock option |
|
— |
|
— |
|
284,400 |
|
20 |
|
— |
|
— |
|
763 |
|
— |
|
— |
|
783 |
|
— |
|
783 |
|
|
Issuance shares reserved for convertible loan |
|
— |
|
— |
|
500,000 |
|
35 |
|
— |
|
— |
|
(35 |
) |
— |
|
— |
|
— |
|
— |
|
— |
|
|
Issuance shares for service |
|
— |
|
— |
|
70,000 |
|
5 |
|
— |
|
— |
|
2,701 |
|
— |
|
— |
|
2,706 |
|
— |
|
2,706 |
|
|
Fair value change relating to Company’s own credit risk on convertible loan |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(108 |
) |
(108 |
) |
— |
|
(108 |
) |
|
Foreign currency translation adjustment |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
2,793 |
|
2,793 |
|
— |
|
2,793 |
|
|
Capital contribution from noncontrolling interest holders |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
370 |
|
370 |
|
|
Share-based compensation |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
17,762 |
|
— |
|
— |
|
17,762 |
|
— |
|
17,762 |
|
|
Balance at December 31, 2020 |
|
— |
|
— |
|
9,192,660 |
|
618 |
|
2,863,100 |
|
191 |
|
354,295 |
|
(356,951 |
) |
4,795 |
|
2,948 |
|
329 |
|
3,277 |
|
|
Balance at December 31, 2020 (US$) |
|
|
|
— |
|
|
|
95 |
|
|
|
29 |
|
54,298 |
|
(54,705 |
) |
735 |
|
452 |
|
50 |
|
502 |
|
The accompanying notes are an integral part of these consolidated financial statements.
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
|
|
|
|
|
Year Ended December 31, |
| ||||
|
|
|
2018 |
|
2019 |
|
2020 |
|
2020 |
|
|
|
|
RMB |
|
RMB |
|
RMB |
|
US$ |
|
|
Operating activities: |
|
|
|
|
|
|
|
|
|
|
Net loss |
|
(42,296 |
) |
(101,621 |
) |
(80,565 |
) |
(12,347 |
) |
|
Adjustments to reconcile net loss to net cash provided by operating activities: |
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization |
|
3,144 |
|
2,664 |
|
3096 |
|
474 |
|
|
Share of net loss (gain) in equity method investments |
|
441 |
|
(190 |
) |
13 |
|
2 |
|
|
Bad debt expenses |
|
452 |
|
285 |
|
1,203 |
|
184 |
|
|
Losses on disposal of land use rights and property and equipment |
|
(4,955 |
) |
4 |
|
51 |
|
8 |
|
|
Foreign exchange loss, net |
|
2,473 |
|
4,133 |
|
— |
|
— |
|
|
Issuance shares for service |
|
— |
|
— |
|
2,706 |
|
415 |
|
|
Share-based compensation |
|
7,936 |
|
32,855 |
|
17,762 |
|
2,722 |
|
|
Gain on settlement of convertible loan |
|
— |
|
— |
|
(7,162 |
) |
(1,098 |
) |
|
Loss on change in fair value of convertible loan |
|
784 |
|
5,296 |
|
532 |
|
82 |
|
|
Inventory provision |
|
— |
|
304 |
|
36 |
|
6 |
|
|
Impairment of long-term investment |
|
— |
|
1,320 |
|
1,430 |
|
219 |
|
|
Deferred tax benefit |
|
— |
|
— |
|
(88 |
) |
(13 |
) |
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
|
Advances to suppliers |
|
(1,646 |
) |
1,714 |
|
(4,775 |
) |
(732 |
) |
|
Accounts receivable |
|
(1,095 |
) |
1,286 |
|
(7,256 |
) |
(1,112 |
) |
|
Inventories |
|
178 |
|
(555 |
) |
(44 |
) |
(7 |
) |
|
Amounts due from related parties |
|
13 |
|
(286 |
) |
(219 |
) |
(34 |
) |
|
Other current assets |
|
670 |
|
(2,875 |
) |
4,845 |
|
743 |
|
|
Other assets |
|
(231 |
) |
462 |
|
502 |
|
77 |
|
|
Accounts payable |
|
(843 |
) |
182 |
|
350 |
|
54 |
|
|
Amounts due to related parties |
|
960 |
|
1,060 |
|
128 |
|
20 |
|
|
Advance from customers |
|
2,328 |
|
(1,863 |
) |
1,232 |
|
189 |
|
|
Accrued expenses and other current liabilities |
|
1,412 |
|
8,233 |
|
6,825 |
|
1,046 |
|
|
Other long-term liabilities |
|
(784 |
) |
(920 |
) |
519 |
|
80 |
|
|
Deferred tax liabilities |
|
(88 |
) |
(88 |
) |
(88 |
) |
(13 |
) |
|
Net cash used in operating activities |
|
(31,147 |
) |
(48,600 |
) |
(58,967 |
) |
(9,035 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
Investing activities: |
|
|
|
|
|
|
|
|
|
|
Purchases of property and equipment |
|
(2,417 |
) |
(2,790 |
) |
(2,466 |
) |
(378 |
) |
|
Purchases of intangible assets |
|
(430 |
) |
(371 |
) |
(26 |
) |
(4 |
) |
|
Proceeds from disposal of land use rights |
|
5,257 |
|
— |
|
— |
|
— |
|
|
Proceeds from property and equipment |
|
|
|
|
|
10 |
|
2 |
|
|
Cash paid for business combination, net of cash acquired |
|
(3,540 |
) |
— |
|
— |
|
— |
|
|
Proceeds from short-term investments |
|
12,000 |
|
20,929 |
|
— |
|
— |
|
|
Payments for short-term investments |
|
(12,000 |
) |
(20,929 |
) |
— |
|
— |
|
|
Payments for long-term investments |
|
(1,550 |
) |
(300 |
) |
— |
|
|
|
|
Net cash used in investing activities |
|
(2,680 |
) |
(3,461 |
) |
(2,482 |
) |
(380 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
Financing activities: |
|
|
|
|
|
|
|
|
|
|
Proceeds from short-term borrowings |
|
26,645 |
|
24,300 |
|
13,830 |
|
2,120 |
|
|
Payment for short-term borrowings |
|
(14,700 |
) |
(18,300 |
) |
(20,000 |
) |
(3,065 |
) |
|
Repayment of related party loan |
|
(350 |
) |
(150 |
) |
(1,072 |
) |
(164 |
) |
|
Proceeds from long-term borrowings |
|
— |
|
— |
|
— |
|
— |
|
|
Proceeds from stock options exercised |
|
— |
|
— |
|
8 |
|
1 |
|
|
Capital contribution from noncontrolling interest holders |
|
— |
|
610 |
|
370 |
|
57 |
|
|
Advance from investors |
|
25,000 |
|
— |
|
— |
|
— |
|
|
Payment for convertible loans |
|
(728 |
) |
— |
|
(17,261 |
) |
(2,645 |
) |
|
Proceeds from issuance of ordinary shares in IPO |
|
404 |
|
47,602 |
|
110,668 |
|
16,961 |
|
|
Payment for initial public offering costs |
|
— |
|
(7,954 |
) |
(25,619 |
) |
(3,926 |
) |
|
Net cash provided by financing activities |
|
36,271 |
|
46,108 |
|
60,924 |
|
9,339 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Effect of exchange rate changes on cash and cash equivalents |
|
(969 |
) |
(809 |
) |
(2,584 |
) |
(401 |
) |
|
Net increase (decrease) in cash and cash equivalents |
|
1,475 |
|
(6,762 |
) |
(3,109 |
) |
(477 |
) |
|
Cash and cash equivalents at beginning of year |
|
11,412 |
|
12,887 |
|
6,125 |
|
939 |
|
|
Cash and cash equivalents at end of year |
|
12,887 |
|
6,125 |
|
3,016 |
|
462 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental disclosure of cash flow information: |
|
|
|
|
|
|
|
|
|
|
Interest paid |
|
891 |
|
1,028 |
|
2,413 |
|
370 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental disclosure of non-cash activities: |
|
|
|
|
|
|
|
|
|
|
Purchase of ordinary shares when registered included in advance from investors |
|
2,157 |
|
25,000 |
|
— |
|
— |
|
|
Reclassification of deferred IPO costs |
|
— |
|
— |
|
9,589 |
|
1,470 |
|
|
Purchase of property and equipment included in accrued expenses and other current liabilities |
|
28 |
|
15 |
|
544 |
|
83 |
|
The accompanying notes are an integral part of these consolidated financial statements.
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
1. ORGANIZATION AND PRINCIPAL ACTIVITIES
AnPac Bio-Medical Science Co., Ltd. (the “Company”) was incorporated in the British Virgin Islands in January 2010. The Company and its subsidiaries (collectively, the “Group”) are engaged in marketing and selling a multi-cancer screening and detection test that uses innovative, patented cancer differentiation analysis (the “CDA”) technology and proprietary cancer-detection devices in the People’s Republic of China (the “PRC” or “China”). Dr. Chris Chang Yu is the Founder of the Group (the “Founder”).
Recapitalization
In preparation of its initial public offering in the United States, the Group had undergone a reorganization in 2019. On October 29, 2019, the board of directors approved the re-designation of the authorized share capital of 100,000 ordinary shares to 71,369 Class A ordinary shares and 28,631 Class B ordinary shares. On October 31, 2019, the board of directors approved the increase of authorized share capital of the Class A and Class B ordinary shares to 700,000 and 300,000, respectively. Holders of Class A ordinary shares and Class B ordinary shares have the same rights, except for voting and conversion rights. Each Class A ordinary share is entitled to one vote; and each Class B ordinary share is entitled to ten votes and is convertible into one Class A ordinary share at any time by the holder thereof. Class A ordinary shares are not convertible into Class B ordinary shares under any circumstances. On October 31, 2019, the board of directors approved a share split of 1-for-100, pursuant to which the authorized share capital of the Class A and Class B ordinary shares would increase to 70,000,000 and 30,000,000, respectively, with a par value of US$0.01. The registration of the above changes was completed on November 12, 2019 and the ordinary shares have been retrospectively adjusted accordingly.
Initial public offering (“IPO”)
On January 30, 2020, the Group completed its initial public offering (“IPO”) on the Nasdaq Stock Exchange. The Group offered 1,333,360 American depositary shares (“ADSs”), representing 1,333,360 Class A ordinary shares at offering price of US$12.00 per ADS. The net proceeds to Group from the IPO, after deducting commissions and offering expenses, were RMB 75,460 (or approximately US$ 11.6 million).
COVID-19
As a result of the pandemic of COVID-19 in China, the United States and the world, the Group’s operations have been, and may continue to be, adversely impacted by disruptions in business activities, commercial transactions and general uncertainties surrounding the duration of the outbreaks and the various governments’ business, travel and other restrictions. These adverse effects could include the Group’s ability to market and conduct its tests in China, commercialize its tests in the United States and carry out research studies and activities in China and the United States, temporary closures of its laboratory facilities and offices in China and the United States and its customers’ and suppliers’ facilities, the delay in construction of its new Philadelphia laboratory, delayed supply of products and services from its suppliers, and delayed or cancelled orders from its customers (such as due to temporary decreased demand for disease screening and detection or physical checkup services or generally due to reduced commercial activities). In addition, the Group’s business operations could be disrupted if any of its employees is suspected of contracting the coronavirus or any other epidemic disease, since its employees could be quarantined and/or its offices be shut down for disinfection. In particular, the closing of blood sampling points countrywide in China since the Chinese New Year in 2020, as a measure by the Chinese government to contain the spread of COVID-19, significantly reduced the number of samples that the Group could collect for its CDA tests. Despite partial recovery of the blood sampling points in April 2020, the number of blood samples that the Group can collect was still limited for the year ended December 31, 2020 and there were delays of orders and cancellation of some orders for planned CDA tests and physical checkups from the Group’s customers.
Despite the outbreak of COVID-19, the Group’s total revenue and gross margins for the year ended December 31, 2020 slightly improved comparing to last year, which was mainly attributable to the launch of two new products, including a proprietary immunology test named ADME (AnPac Defense Medical Examination) and a new cancer test package named APCS (AnPac Pan Cancer Screening) combining CDA technology with ct-DNA methods. The Group also continued to work in obtaining the Class III medical device certification in China and laboratory developed test (LDT) designation in the US. While the Group strives to bring in new customers and launch new tests to mitigate the negative impact of COVID-19, it has no control over the development of the COVID-19 situations in China, the United States or around the world and therefore may not be able to achieve a revenue growth or maintain its historical revenue level in future periods. Moreover, the Group’s plan to commercialize its CDA test in the United States has been delayed (as indicated by the delay in construction of their new Philadelphia laboratory) by the COVID-19 outbreak in the United States, which deferred its cooperation with universities, academic medical centers, hospitals and other medical institutions in the U.S. to conduct research studies on its CDA technology and delayed its applications for approval and commercialization plan of its CDA-based tests in the U.S. market.
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
1. ORGANIZATION AND PRINCIPAL ACTIVITIES (Continued)
The downturn brought by and the duration of the coronavirus pandemic is difficult to assess or predict and actual effects will depend on many factors beyond the Group’s control, including the increased world-wide spread of COVID-19 and the relevant governments’ actions to contain COVID-19 or treat its impact. The extent to which COVID-19 may impact the Group’s results continues to remains uncertain. The business, results of operations, financial condition and prospects could be adversely affected directly, as well as to the extent that the coronavirus or any other epidemic harms the Chinese and the United States’ economies in general.
For the year ended December 31, 2020, the details of the Group’s principal subsidiaries are as follows:
|
Major subsidiaries |
|
Percentage of |
|
Date of |
|
Place of |
|
Major Operation |
|
|
Changhe Bio-Medical Technology (Yangzhou) Co., Ltd. |
|
100 |
% |
March 2010 |
|
the PRC |
|
Cancer screening and detection tests |
|
|
Changwei System Technology (Shanghai) Co., Ltd. |
|
100 |
% |
March 2011 |
|
the PRC |
|
Research and development |
|
|
AnPac Bio-Medical Technology (Lishui) Co., Ltd. |
|
100 |
% |
October 2012 |
|
the PRC |
|
Cancer screening detection tests and device manufacturing |
|
|
Shanghai Xinshenpai Technology Co., Ltd.* |
|
100 |
% |
October 2013 |
|
the PRC |
|
Cancer screening and detection tests |
|
|
AnPac Bio-Medical Technology (Shanghai) Co., Ltd. |
|
100 |
% |
April 2014 |
|
the PRC |
|
Cancer screening and detection tests |
|
|
AnPac Technology USA Co., Ltd. (“AnPac US”) |
|
100 |
% |
September 2015 |
|
the U.S. |
|
Clinical trials for research on cancer screening and detection tests |
|
|
Lishui AnPac Medical Laboratory Co., Ltd. |
|
100 |
% |
July 2016 |
|
the PRC |
|
Cancer screening and detection tests |
|
|
Shiji (Hainan) Medical Technology Ltd. |
|
100 |
% |
March 2013 |
|
the PRC |
|
Cancer screening and detection tests |
|
|
Penghui Health Management Co., Ltd.* |
|
100 |
% |
May 2018 |
|
the PRC |
|
Cancer screening and detection tests |
|
|
Shenzhen Anchun Biomedical Technology Co., Ltd. |
|
51 |
% |
December 2017 |
|
the PRC |
|
Cancer screening and detection tests |
|
|
Shanghai Muqing AnPac Health Technology Co., Ltd. |
|
51 |
% |
March 2019 |
|
the PRC |
|
Cancer screening and detection tests |
|
* Shenzhen Anchun Biomedical Technology Co., Ltd., Shanghai Xinshenpai Technology Co., Ltd. and Penghui Health Management Co., Ltd. were deregistered in December 2020. Since these entities were inactive, the deregistration does not have a material impact to the Group’s consolidated financial statements for the years ended December 31 2019 and 2020.
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES
(a) Basis of presentation
The consolidated financial statements have been prepared in accordance with the accounting principles generally accepted in the United States of America (“U.S. GAAP”).
(b) Principles of consolidation
The accompanying consolidated financial statements include the financial statements of the Company and its subsidiaries.
Subsidiaries are those entities in which the Group, directly or indirectly, controls more than one half of the voting power, has the power to appoint or remove the majority of the members of the board of directors, or to cast a majority of votes at the meeting of the board of directors, or has the power to govern the financial and operating policies of the investee under a statute or agreement among the shareholders or equity holders.
All intercompany transactions and balances are eliminated upon consolidation.
(c) Use of estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the financial statements and the reported amount of revenues and expenses during the reporting period. Areas where management uses subjective judgement include, but are not limited to allowance for doubtful accounts, share-based compensation, deferred tax and uncertain tax position, valuation of convertible loans, useful lives of intangible assets and property and equipment, and impairment of long-lived assets, goodwill and long-term investments. Changes in facts and circumstances may result in revised estimates. Actual results could differ from those estimates, and as such, differences could be material to the consolidated financial statements.
(d) Foreign currency
The functional currency of the Group and AnPac US is the United States dollar and its reporting currency is Renminbi (“RMB”). The functional currency of the Group’s PRC subsidiaries is the RMB as determined based on the criteria of Accounting Standards Codification (“ASC”) 830, Foreign Currency Matters.
The financial statements of the Company and AnPac US are translated from the functional currency to the reporting currency, RMB. Transactions denominated in foreign currencies are re-measured into the functional currency at the exchange rates prevailing on the transaction dates. Monetary assets and liabilities denominated in foreign currencies are re-measured at the exchange rates prevailing at the balance sheet date. Non-monetary items that are measured in terms of historical costs in foreign currency are re-measured using the exchange rates at the dates of the initial transactions. Exchange gains and losses are included in the consolidated statements of comprehensive loss.
The Group uses the average exchange rate for the year and the exchange rate at the balance sheet date to translate the operating results and financial position, respectively. Translation differences are recorded in accumulated other comprehensive loss, a component of shareholders’ deficit.
(e) Convenience translation
Amounts in US$ are presented for the convenience of the reader and are translated at the noon buying rate of US$1.00 to RMB6.5250 on December 31, 2020, representing the noon buying rate set forth in the H.10 statistical release of the U.S. Federal Reserve Board. No representation is made that the RMB amounts could have been, or could be converted, realized or settled into US$ at such rate or at any other rate.
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (CONTINUED)
(f) Cash and cash equivalents
Cash and cash equivalents consist of cash on hand and demand deposits placed with banks which are unrestricted as to withdrawal or use and have original maturities less than three months. All highly liquid investments with a stated maturity of 90 days or less from the date of purchase are classified as cash equivalents.
(g) Accounts receivable, net of allowance for doubtful accounts
Accounts receivable are recorded at their invoiced amounts, net of allowances for doubtful accounts. An allowance for doubtful accounts is recorded when the collection of the full amount is no longer probable. In evaluating the collectability of receivable balances, the Group considers specific evidence, including aging of the receivable, the customer’s payment history, its current creditworthiness and current economic trends. Accounts receivable are written off after all collection efforts have ceased. The Group regularly reviews the adequacy and appropriateness of the allowance for doubtful accounts.
Accounts receivable for the years ended December 31, 2019 and 2020 were as follows:
|
|
|
Years ended December 31, |
| ||||
|
|
|
2019 |
|
2020 |
| ||
|
|
|
RMB |
|
RMB |
|
US$ |
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable |
|
1,472 |
|
8,096 |
|
1,241 |
|
|
Allowance for doubtful accounts |
|
(177 |
) |
(304 |
) |
(47 |
) |
|
Balance at end of year |
|
1,295 |
|
7,792 |
|
1,194 |
|
Movement in the allowances for doubtful debts were as follows:
|
|
|
Years ended December 31, |
| ||||||
|
|
|
2018 |
|
2019 |
|
2020 |
| ||
|
|
|
RMB |
|
RMB |
|
RMB |
|
US$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at beginning of year |
|
18 |
|
198 |
|
177 |
|
28 |
|
|
Additional provision |
|
334 |
|
168 |
|
758 |
|
116 |
|
|
Write-offs |
|
(154 |
) |
(189 |
) |
(631 |
) |
(97 |
) |
|
Balance at end of year |
|
198 |
|
177 |
|
304 |
|
47 |
|
(h) Inventories
Inventories are stated at the lower of cost or net realizable value. Cost of inventories are determined using the first in first out method. The Group records inventory reserves for obsolete and slow-moving inventory.
(i) Property and equipment
Property and equipment are stated at cost less accumulated depreciation and any recorded impairment. Property and equipment are depreciated using the straight-line method over the estimated useful lives of the assets, as follows:
|
Category |
|
Estimated useful life |
|
Leasehold improvements |
|
Over the shorter of the lease term or estimated useful lives |
|
Buildings |
|
20 years |
|
Furniture, fixtures and equipment |
|
3-10 years |
|
Motor vehicles |
|
3-5 years |
Repair and maintenance costs are charged to expense as incurred, whereas the costs of betterments that extend the useful life of property and equipment are capitalized as additions to the related assets. Retirements, sale and disposals of assets are recorded by removing the cost and accumulated depreciation with any resulting gain or loss reflected in the consolidated statements of comprehensive loss.
Direct costs that are related to the construction of property and equipment and incurred in connection with bringing the assets to their intended use are capitalized as construction in progress. Construction in progress is transferred to specific property and equipment, and the depreciation of these assets commences when the assets are ready for their intended use.
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (CONTINUED)
(j) Long-term investments
The Group’s long-term investments include equity method investments and equity investments without readily determinable fair values.
Investments in entities in which the Group can exercise significant influence but does not own a majority equity interest or control are accounted for using the equity method of accounting in accordance with ASC 323, Investments-Equity Method and Joint Ventures (“ASC 323”). Under the equity method, the Group initially records its investment at cost and the difference between the cost of the equity investee and the amount of the underlying equity in the net assets of the equity investee is accounted for as if the investee were a consolidated subsidiary. The share of earnings or losses of the investee are recognized in the consolidated statements of comprehensive loss. Equity method adjustments include the Group’s proportionate share of investee income or loss, adjustments to recognize certain differences between the Group’s carrying value and its equity in net assets of the investee at the date of investment, impairments, and other adjustments required by the equity method. The Group assesses its equity investment for other-than-temporary impairment by considering factors as well as all relevant and available information including, but not limited to, current economic and market conditions, the operating performance of the investees including current earnings trends, the general market conditions in the investee’s industry or geographic area, factors related to the investee’s ability to remain in business, such as the investee’s liquidity, debt ratios, and cash burn rate and other company-specific information.
Investments in equity securities without readily determinable fair values are measured at cost minus impairment adjusted by observable price changes in orderly transactions for the identical or a similar investment of the same issuer. These investments are measured at fair value on a nonrecurring basis when there are events or changes in circumstances that may have a significant adverse effect. An impairment loss is recognized in the consolidated statements of comprehensive loss equal to the amount by which the carrying value exceeds the fair value of the investment. Prior to the adoption of ASU 2016-01 on January 1, 2019, these investments were accounted for using the cost method of accounting, measured at cost less other-than-temporary impairment.
For the years ended December 31, 2018, 2019 and 2020, the Group recognized an impairment on its equity investment in Jiangsu Anpac Health Management Co., Ltd. of Nil, RMB1,320 and RMB1,430 (US$219), respectively.
(k) Business combinations
The Group accounts for all business combinations under the purchase method in accordance with ASC 805, Business Combinations. The cost of an acquisition is measured as the aggregate of the fair values at the date of exchange of the assets given, liabilities incurred, and equity instruments issued. The costs directly attributable to the acquisition are expensed as incurred. Identifiable assets, liabilities and contingent liabilities acquired or assumed are measured separately at their fair value as of the acquisition date, irrespective of the extent of any noncontrolling interests. The excess of (i) the total of the cost of the acquisition, fair value of the noncontrolling interests and acquisition date fair value of any previously held equity interest in the acquiree over (ii) the fair value of the identifiable net assets of the acquiree is recorded as goodwill. If the cost of acquisition is less than the fair value of the identifiable net assets of the acquiree, the difference is recognized directly in earnings.
The determination and allocation of fair values to the identifiable net assets acquired, liabilities assumed and noncontrolling interest is based on various assumptions and valuation methodologies requiring considerable judgment. The most significant variables in these valuations are discount rates, terminal values, the number of years on which to base the cash flow projections, as well as the assumptions and estimates used to determine the cash inflows and outflows. The Group determines discount rates to be used based on the risk inherent in the acquiree’s current business model and industry comparisons. Although the Group believes that the assumptions applied in the determination are reasonable based on information available at the date of acquisition, actual results may differ from forecasted amounts and the differences could be material.
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (CONTINUED)
(l) Intangible assets
Intangible assets with finite lives are carried at cost less accumulated amortization. All intangible assets with finite lives are amortized using the straight-line method over the estimated useful lives.
Intangible assets have estimated useful lives from the date of purchase as follows:
|
Category |
|
Estimated useful life |
|
Software |
|
3-10 years |
|
Medical license |
|
15 years |
(m) Land use right, net
All land in the PRC is owned by the PRC government. The PRC government may sell land use rights for a specified period of time. Land use rights represent lease prepayments to the PRC government and are carried at cost less accumulated amortization. Land use rights are amortized on a straight-line basis over the terms of the land use right of 50 years.
(n) Goodwill
Goodwill represents the excess of the cost of an acquisition over the fair value of the identifiable assets acquired less liabilities assumed of an acquired business. The Group’s goodwill at December 31, 2019 and 2020 was related to its business acquisition in November 2017. Goodwill acquired in a business combination are not amortized, but instead tested for impairment at least annually, or more frequently if certain circumstances indicate a possible impairment may exist.
In accordance with ASC 350-20, Intangibles-Goodwill and Other, Goodwill, (“ASC 350-20”) the Group has assigned and assessed goodwill for impairment at the reporting unit level. A reporting unit is an operating segment or one level below the operating segment. The Group has determined that it has one reporting unit, which is also its only reportable segment.
The Group has the option to first assess qualitative factors to determine whether it is necessary to perform the two-step test in accordance with ASC 350-20. If the Group believes, as a result of the qualitative assessment, that it is more-likely-than-not that the fair value of the reporting unit is less than its carrying amount, the two-step quantitative impairment test described below is required. Otherwise, no further testing is required. In the qualitative assessment, the Group considers primary factors such as industry and market considerations, overall financial performance of the reporting unit, and other specific information related to the operations.
In performing the two-step quantitative impairment test, the first step compares the carrying amount of the reporting unit to the fair value of the reporting unit based on either quoted market prices of the ordinary shares or estimated fair value using a combination of the income approach and the market approach. If the fair value of the reporting unit exceeds the carrying value of the reporting unit, goodwill is not impaired, and the Group is not required to perform further testing. If the carrying value of the reporting unit exceeds the fair value of the reporting unit, then the Group must perform the second step of the impairment test in order to determine the implied fair value of the reporting unit’s goodwill. The fair value of the reporting unit is allocated to its assets and liabilities in a manner similar to a purchase price allocation in order to determine the implied fair value of the reporting unit goodwill. If the carrying amount of the goodwill is greater than its implied fair value, the excess is recognized as an impairment loss.
For the year ended December 31, 2018, 2019 and 2020, the Group performed a qualitative assessment for the reporting unit. Based on the requirements of ASC 350-20, the Group evaluated all relevant qualitative and quantitative factors, weighed all factors in their entirety and concluded that it was not more-likely-than-not that the fair value of the reporting unit was less than its carrying amount. Therefore, no goodwill impairment was recognized as of December 31, 2018, 2019 and 2020.
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (CONTINUED)
(o) Impairment of long-lived assets other than goodwill
The Group evaluates its long-lived assets, including property and equipment and intangibles with finite lives, for impairment whenever events or changes in circumstances, such as a significant adverse change to market conditions that will impact the future use of the assets, indicate that the carrying amount of an asset may not be fully recoverable. When these events occur, the Group evaluates the recoverability of long-lived assets by comparing the carrying amount of the assets to the future undiscounted cash flows expected to result from the use of the assets and their eventual disposition. If the sum of the expected undiscounted cash flows is less than the carrying amount of the assets, the Group recognizes an impairment loss based on the excess of the carrying amount of the assets over their fair value. Fair value is generally determined by discounting the cash flows expected to be generated by the assets, when the market prices are not readily available. The adjusted carrying amount of the assets become new cost basis and are depreciated over the assets’ remaining useful lives. Long-lived assets are grouped with other assets and liabilities at the lowest level for which identifiable cash flows are largely independent of the cash flows of other assets and liabilities. Given no events or changes in circumstances indicating the carrying amount of long-lived assets may not be recovered through the related future net cash flows, the Group did not perform such an evaluation for the years ended December 31, 2018, 2019 and 2020.
(p) Fair value of financial instruments
The Group applies ASC 820, Fair Value Measurements and Disclosures, (“ASC 820”). ASC 820 defines fair value, establishes a framework for measuring fair value and expands disclosures about fair value measurements.
ASC 820 establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value as follows:
Level 1—Observable inputs that reflect quoted prices (unadjusted) for identical assets or liabilities in active markets.
Level 2—Other inputs that are directly or indirectly observable in the marketplace.
Level 3—Unobservable inputs which are supported by little or no market activity.
ASC 820 describes three main approaches to measuring the fair value of assets and liabilities: (1) market approach; (2) income approach; and (3) cost approach. The market approach uses prices and other relevant information generated from market transactions involving identical or comparable assets or liabilities. The income approach uses valuation techniques to convert future amounts to a single present value amount. The measurement is based on the value indicated by current market expectations about those future amounts. The cost approach is based on the amount that would currently be required to replace an asset.
The Group’s financial instruments include cash and cash equivalents, accounts receivables, accounts payable, other receivables, other payables and short-term debt. The carrying values of these financial instruments approximate their fair values due to their short-term maturities.
The Group elected the fair value option to account for its convertible loans. The Group engaged an independent valuation firm to perform the valuation. The fair value of the convertible loans as of December 31, 2019 and 2020 was RMB24,568 and RMB2,232 (US$342) calculated using the binomial tree model. The convertible loans are classified as level 3 instruments as the valuation was determined based on unobservable inputs which are supported by little or no market activity and reflect the Group’s own assumptions in measuring fair value. Significant estimates used in developing the fair value of the convertible loans include time to maturity, risk-free interest rate, straight debt discount rate, probability to convert and expected timing of conversion. Refer to Note 8 for additional information.
Prior to the adoption of ASU 2016-01 on January 1, 2019, fair value changes relating to the Group’s own credit risks of the convertible loans accounted for under fair value option were recognized together with the total changes in fair value in the consolidated statement of operations and comprehensive loss. After the adoption of ASU 2016-01, such fair value changes related to the Group’s own credit risks are recognized separately in accumulated other comprehensive loss. There was decrease to beginning retained deficits of RMB1,063 and increase to accumulated other comprehensive income of RMB1,063 in consolidated statements of shareholders’ deficits as a result of applying the new accounting standard.
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (CONTINUED)
(p) Fair value of financial instruments (continued)
As the inputs used in developing the fair value for level 3 instruments are unobservable, and require significant management estimate, a change in these inputs could result in a significant change in the fair value measurement.
The following is a reconciliation of the beginning and ending balances for convertible loans measured at fair value on a recurring basis using significant unobservable inputs (Level 3) as of December 31, 2019 and 2020:
|
|
|
As of December 31, |
| ||||
|
|
|
2019 |
|
2020 |
|
2020 |
|
|
|
|
RMB |
|
RMB |
|
US$ |
|
|
|
|
|
|
|
|
|
|
|
Opening balance |
|
17,961 |
|
24,568 |
|
3,765 |
|
|
Additional convertible loans |
|
— |
|
1,830 |
|
280 |
|
|
Repayments |
|
— |
|
(17,261 |
) |
(2,645 |
) |
|
Loss on change in fair value of convertible loan |
|
5,296 |
|
532 |
|
82 |
|
|
Gain on settlement of convertible loan |
|
— |
|
(7,162 |
) |
(1,098 |
) |
|
Other comprehensive income -foreign exchange translations |
|
1,311 |
|
(275 |
) |
(42 |
) |
|
Total |
|
24,568 |
|
2,232 |
|
342 |
|
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (CONTINUED)
(q) Revenue recognition
Effective January 1, 2017, the Group early adopted ASC 606, Revenue from Contracts with Customers and subsequent amendments to the initial (“ASC 606”).
The Group derives its revenues principally from customers through the Group’s cancer screening and detection test and physical checkup package services. Revenue is recognized when the Group satisfies the performance obligations in an amount of consideration to which the Group expects to be entitled to in exchange for those services. The Group evaluates the presentation of revenue on a gross or net basis based on whether it controls the services provided to customers and is the principal (i.e. “gross”), or the Group arranges for other parties to provide the service to the customers and is an agent (i.e. “net”). The Group presents value-added taxes as a reduction from revenues.
Revenue from cancer screening and detection tests
Revenue from cancer screening and detection test are primarily generated through administration of the tests to the Group’s customer constituents, the Group’s cancer screening and detection tests based on CDA technology and other cancer screening and detection technologies, such as biomarker-based tests, to its customers i.e. corporations and life insurance companies. A contract exists when the master service agreement has been executed and the customer submitting a service request, which is a placed order. The Group’s contracts have a single performance obligation which is satisfied upon rendering of the cancer screening and detection tests and delivery of the cancer screening and detection test results to the customer. The Group acts as the principal as it controls the cancer screening and detection tests before it is transferred to the customer and records revenue on a gross basis at a point in time, when the cancer screening and detection test results are delivered to the customer.
Revenue from physical checkup packages
The Group facilitates corporations and life insurance companies to procure physical checkup package services for their employees and policy holders, respectively, from third-party physical checkup package service providers. The Group enters into contracts with corporations and life insurance companies and physical checkup service providers. The Group considers both the corporations and life insurance companies and the third-party physical checkup package service providers as its customers in this type of transaction. The Group’s performance obligation is to facilitate the corporations and life insurance companies and the third-party physical checkup package service providers to complete the purchase of physical checkup package services, which is not controlled by the Group prior to being transferred to the corporations and life insurance companies. Therefore, the Group fulfills its performance obligation at a point in time when the employees and policy holders of corporations and life insurance companies, respectively, complete the physical checkups and the Group records the net amount that it retains from such completed transaction as revenue.
The Group also enters into arrangements to deliver both cancer screening and detection tests and physical checkup package services. The Group is the principal for the cancer screening and detection tests and the agent for physical checkup package services. Revenues for cancer screening and detection tests and physical checkup are both recognized at a point in time when the performance obligation is satisfied upon delivery of the cancer screening and detection test results to the end customers and completion of physical checkup respectively. As the Group acts as both the principal and agent in the arrangement, the Group allocates the transaction price to each performance obligation on a relative stand-alone selling price basis.
All revenues are generated in the PRC.
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (CONTINUED)
(r) Revenue recognition (continued)
Contract balances
The payment terms and conditions within the Group’s contracts vary by the type of services and the customers.
Contract assets relate to the Group’s conditional right to consideration for completed performance obligations under the contract. Accounts receivable are recorded when the right to consideration becomes unconditional. The Group does not have contract assets for the years presented.
In instances where the timing of revenue recognition differs from the timing of invoicing, the Group has determined that its contracts generally do not include a significant financing component.
Contract liabilities represent considerations received from corporations and life insurance companies in advance of satisfying the Group’s performance obligations under the contract, which are presented in “advance from customers” in the consolidated balance sheets. For the years ended December 31, 2019 and 2020, advance from customers amounted to RMB 2,450 and RMB 3,682 (US$564), respectively.
PRC Value-Added Taxes (“VAT”) and surcharges
The services of the Group are subject to 6% of Value-Added Taxes. The Group is subject to education surtax and urban maintenance and construction tax, on the services provided in the PRC.
Practical expedients
The Group has applied the following practical expedients:
(i) The transaction price allocated to the performance obligations that are unsatisfied, or partially unsatisfied has not been disclosed, as substantially all of the Group’s contracts have a duration of one year or less.
(ii) The Group recognizes incremental costs to obtain a contract as expenses when incurred because the amortization period would be one year or less. These costs are recorded within sales and marketing expenses.
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (CONTINUED)
(s) Costs of revenues
Costs of revenues consists of staff costs, outsourced testing costs, blood sample taking costs, medical consumable costs, share-based compensation and depreciation of CDA equipment.
(t) Research and development expenses
Research and development expenses primarily are comprised of costs incurred in performing research and development activities, including related personnel and consultant’s salaries, benefits, share-based compensation and related costs, raw materials and supplies for internally-developed product candidates and external costs of outside vendors engaged to conduct clinical development activities and trials. The Group expenses research and development expenses as they are incurred.
(u) Government grants
Government grants include financial incentives in the form of cash subsidies that involve no conditions or continuing performance obligations of the Group. Government grants are recognized as other non-operating income upon receipt. For government grants related to assets in the form of land use rights, the government grants are recorded as deferred income when received. The deferred income is then recognized in other income, net in the consolidated statement of comprehensive loss on a systematic basis over the useful life of the related asset. Government grants recognized in other income for the years ended December 31, 2018, 2019 and 2020 were RMB 5.9 million, RMB 2.8 million and RMB 7.5 million (US$1.1 million).
(v) Leases
Leases are classified at the inception date as either a capital lease or an operating lease. The Group assesses a lease to be a capital lease if any of the following conditions exist: (a) ownership is transferred to the lessee by the end of the lease term, (b) there is a bargain purchase option, (c) the lease term is at least 75% of the property’s estimated remaining economic life, or (d) the present value of the minimum lease payments at the beginning of the lease term is 90% or more of the fair value of the leased property to the lessor at the inception date. A capital lease is accounted for as if there was an acquisition of an asset and an occurrence of an obligation at the inception of the lease. The Group has no capital leases for the years presented.
All other leases are accounted for as operating leases wherein rental payments are expensed on a straight-line basis over the periods of their respective lease terms. The Group leases office space, storage unit, research laboratory, employee accommodation and manufacturing space under operating lease agreements. Certain of the lease agreements contain rent holidays. Rent holidays are considered in determining the straight-line rent expense to be recorded over the lease term. The lease term begins on the date of initial possession of the lease property for purposes of recognizing lease expense on straight-line basis over the term of the lease.
(w) Employee benefit expenses
As stipulated by the regulations of the PRC, full-time employees of the Group are entitled to various government statutory employee benefit plans, including medical insurance, maternity insurance, workplace injury insurance, unemployment insurance and pension benefits through a PRC government-mandated multi-employer defined contribution plan. The Group is required to make contributions to the plan and accrues for these benefits based on certain percentages of the qualified employees’ salaries. The total expenses the Group incurred for the plan were RMB 3,250, RMB 3,249 and RMB 1,645 (US$ 252) for the years ended December 31, 2018, 2019 and 2020, respectively.
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (CONTINUED)
(x) Share-based compensation
The Group accounts for share-based compensation in accordance with ASC 718, Compensation — Stock Compensation (“ASC 718”). In accordance with ASC 718, the Group determines whether an award should be classified and accounted for as a liability award or an equity award. All the Group’s share-based awards were classified as equity awards and are recognized in the consolidated financial statements based on their grant date fair values.
The Group has elected to recognize share-based compensation using the straight-line method for all share-based awards granted with graded vesting based on service conditions. The Group uses the accelerated method for all awards granted with graded vesting. The Group accounts for forfeitures as they occur in accordance with ASU No. 2016-09, Compensation — Stock Compensation (Topic 718): Improvement to Employee Share-based Payment Accounting. The Group, with the assistance of an independent third-party valuation firm, determined the fair value of the stock options granted to employees. The binomial option pricing model and Black-Scholes Model were applied in determining the estimated fair value of the options granted to employees and non-employees.
(y) Income taxes
The Group follows the liability method of accounting for income taxes in accordance with ASC 740, Income Taxes (“ASC 740”). Under this method, deferred tax assets and liabilities are determined based on the difference between the financial reporting and tax bases of assets and liabilities using enacted tax rates that will be in effect in the period in which the differences are expected to reverse. The Group records a valuation allowance to offset deferred tax assets if based on the weight of available evidence, it is more-likely-than-not that some portion, or all, of the deferred tax assets will not be realized. The effect on deferred taxes of a change in tax rate is recognized in tax expense in the period that includes the enactment date of the change in tax rate.
The Group accounted for uncertainties in income taxes in accordance with ASC 740. Interest and penalties related to unrecognized tax benefit recognized in accordance with ASC 740 are classified in the consolidated statements of comprehensive loss as income tax expenses.
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (CONTINUED)
(z) Comprehensive loss
Comprehensive loss is defined as the changes in equity of the Group during a period from transactions and other events and circumstances excluding transactions resulting from investments by owners and distributions to owners. Among other disclosures, ASC 220, Comprehensive Income, requires that all items that are required to be recognized under current accounting standards as components of comprehensive loss be reported in a financial statement that is displayed with the same prominence as other financial statements. For each of the periods presented, the Group’s comprehensive loss includes net loss and foreign currency translation differences, and is presented in the consolidated statements of comprehensive loss.
(aa) Segment reporting
The Group’s Chief Executive Officer is the chief operating decision-maker that reviews the consolidated financial results when making decisions about allocating resources and assessing the performance of the Group as a whole and hence, the Group has only one reportable segment in accordance with ASC 280, Segment Reporting. The Group operates and manages its business as a single segment. As the Group’s long-lived assets are substantially all located in the PRC and all the Group revenues are derived from within the PRC, no geographical segments are presented.
(bb) Loss per share
Loss per share is calculated in accordance with ASC 260, Earnings per Share. Basic loss per ordinary share is computed by dividing net loss attributable to ordinary shareholders by the weighted average number of ordinary shares outstanding during the period.
Diluted loss per share is calculated by dividing net loss attributable to ordinary shareholders as adjusted for the effect of dilutive ordinary equivalent shares, if any, by the weighted average number of ordinary and dilutive ordinary equivalent shares outstanding during the period. Ordinary equivalent shares consist of the ordinary shares issuable upon the conversion of the share options, using the treasury stock method. Ordinary share equivalents are excluded from the computation of diluted loss per share if their effects would be anti-dilutive. Basic and diluted loss per ordinary share is presented in the Group’s consolidated statements of comprehensive loss.
The rights, including the liquidation and dividend rights, of the holders of our Class A and Class B ordinary shares are identical, except with respect to voting. Each Class A ordinary share is entitled to one vote; and each Class B ordinary share is entitled to ten votes and is convertible into one Class A ordinary share at any time by the holder thereof. Class A ordinary shares are not convertible into Class B ordinary shares under any circumstances. As the liquidation and dividend rights are identical, the undistributed earnings are allocated on a proportionate basis. For the years ended December 31, 2018, 2019 and 2020, the net loss per share amounts are the same for Class A and Class B common ordinary shares because the holders of each class are entitled to equal per share dividends or distributions in liquidation.
The Group did not include share options in the computation of diluted earnings per share for the years ended December 31, 2018, 2019 and 2020, because those share options were anti-dilutive for loss per share.
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (CONTINUED)
(cc) Concentration of Risks
Concentration of credit risk
Financial instruments that potentially subject the Group to significant concentration of credit risk consist primarily of cash and cash equivalents and accounts receivables. As of December 31,2019 and 2020, the aggregate amounts of cash and cash equivalents of RMB 5,045 and RMB2,366 (US$362), respectively, were held at major financial institutions located in the PRC and RMB 1,080 and RMB 650(US$100), respectively, were deposited with major financial institutions located outside the PRC. Management believes that these financial institutions are of high credit quality and continually monitors the credit worthiness of these financial institutions. Historically, deposits in Chinese banks are secured due to the state policy on protecting depositors’ interests. However, China promulgated a new Bankruptcy Law in August 2006 that came into effect on June 1, 2007 which contains a separate article expressly stating that the State Council may promulgate implementation measures for the bankruptcy of Chinese banks based on the Bankruptcy Law. Under the new Bankruptcy Law, a Chinese bank may go into bankruptcy. In addition, since China’s concession to the World Trade Organization, foreign banks have been gradually permitted to operate in China and have been significant competitors against Chinese banks in many aspects, especially since the opening of the Renminbi business to foreign banks in late 2006. Therefore, the risk of bankruptcy of those Chinese banks in which the Group has deposits has increased. In the event of bankruptcy of one of the banks which holds the Group’s deposits, the Group is unlikely to claim its deposits back in full since the bank is unlikely to be classified as a secured creditor based on PRC laws.
Accounts receivables, unsecured and denominated in RMB, derived from sales of the Group’s cancer screening and detection test and physical checkup package services, are exposed to credit risk. As of December 31, 2019 and 2020, the Group had two and two customers, respectively, each with a receivable balance exceeding 10% of the total accounts receivable balance. The risk is mitigated by credit evaluations the Group performs on its customers.
Business, customer, political, social and economic risks
The Group participates in a dynamic industry and believes that changes in any of the following areas could have a material adverse effect on the Group’s future financial position, results of operations or cash flows: changes in the overall demand for services; competitive pressures due to new entrants; advances and new trends in industry standards; changes in certain strategic relationships or customer relationships; regulatory considerations; intellectual property considerations; and risks associated with the Group’s ability to attract and retain employees necessary to support its growth. The Group’s operations could be also adversely affected by significant political, economic and social uncertainties in the PRC. The Group is also reliant on contract manufacturers that manufacture key components of its CDA device used in its diagnostic testing.
For the years ended December 31, 2018 and 2019, the Group had one and two customers, respectively, that accounted for more than 10% of the total revenues. For the year ended December 31, 2020, the Group had two customers, respectively that accounted for 29% and 15% of total revenues.
For the years ended December 31, 2018, 2019 and 2020, the Group had two, two and four suppliers, respectively, that accounted for more than 10% of cost of revenues. For the year ended December 31, 2020, the Group had four suppliers, respectively that accounted for 17%, 14%, 13% and 11% of total cost of revenues.
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (CONTINUED)
(dd) Concentration of Risks (continued)
Currency convertibility risk
A significant portion of the Group’s expenses, assets and liabilities are denominated in RMB. On January 1, 1994, the PRC government abolished the dual rate system and introduced a single rate of exchange as quoted daily by the People’s Bank of China (the “PBOC”). However, the unification of the exchange rates does not imply that the RMB may be readily convertible into U.S. dollar or other foreign currencies. All foreign exchange transactions continue to take place either through the PBOC or other banks authorized to buy and sell foreign currencies at the exchange rates quoted by the PBOC. Approvals of foreign currency payments by the PBOC or other institutions require submitting a payment application form together with relevant documents.
Additionally, the value of the RMB is subject to changes in central government policies and international economic and political developments affecting supply and demand in the PRC foreign exchange trading system market.
Foreign currency exchange rate risk
From July 21, 2005, the RMB is permitted to fluctuate within a narrow and managed band against a basket of certain foreign currencies. For U.S. dollar against RMB, there was appreciation of approximately 5.7%, appreciation of approximately 1.3% and depreciation of 6.3% in the years ended December 31, 2018, 2019 and 2020, respectively. It is difficult to predict how market forces or PRC or the U.S. government policy may impact the exchange rate between the RMB and the U.S. dollar in the future.
The functional currency and the reporting currency of the Company and AnPac US are the US$ and the RMB, respectively. Most of the revenues and costs of the Group are denominated in RMB, while a portion of cash and cash equivalents and convertible loans (“CL”) are denominated in US$. It is difficult to predict how market forces or PRC or the U.S. government policy may impact the exchange rate between the Renminbi and the US$ in the future. Any significant fluctuation of the valuation of RMB may materially affect the Group’s cash flows, revenues, earnings and financial position, and the value of any dividends payable on the ADS in US$.
(ee) Recent accounting pronouncements
The Group is an emerging growth company (“EGC”) as defined by the Jumpstart Our Business Startups Act (“JOBS Act”). The JOBS Act provides that an EGC can take advantage of extended transition periods for complying with new or revised accounting standards. This allows an EGC to delay adoption of certain accounting standards until those standards would otherwise apply to private companies. The Group elected to take advantage of the extended transition periods. However, this election will not apply should the Group cease to be classified as an EGC.
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (CONTINUED)
(ee) Recent accounting pronouncements (continued)
In February 2016, the FASB issued ASU 2016-02, Leases (Topic 842), which requires lessees to recognize a right-of-use asset and lease liability on the balance sheet for all leases, including operating leases, with a term in excess of 12 months. The guidance also expands the quantitative and qualitative disclosure requirements. In July 2018, the FASB issued updates to the lease standard making transition requirements less burdensome. The update provides an option to apply the transition provisions of the new standard at its adoption date instead of at the earliest comparative period presented in the Group’s financial statements. The new guidance requires the lessee to record operating leases on the balance sheet with a right-of-use asset and corresponding liability for future payment obligations. FASB further issued ASU 2018-11 “Target Improvement” and ASU 2018-20 “Narrow-scope Improvements for Lessors.” In June 2020, the FASB issued ASU No. 2020-05, “Revenue from Contracts with Customers (Topic 606) and Leases (Topic 842) Effective Dates for Certain Entities” (“ASU 2020-05”) in response to the ongoing impacts to businesses in response to the coronavirus (COVID-19) pandemic. ASU 2020-05 provides a limited deferral of the effective dates for implementing previously issued ASU 842 to give some relief to businesses and the difficulties they are facing during the pandemic. ASU 2020-05 affects entities in the “all other” category and public Not-For-Profit entities that have not gone into effect yet regarding ASU 2016-02, Leases (Topic 842). Entities in the “all other” category may defer to fiscal years beginning after December 15, 2021, and interim periods within fiscal years beginning after December 15, 2022. As an emerging growth company, the Group will adopt this guidance effective January 1, 2022. The Group is evaluating the impact on its consolidated financial statements.
In June 2016, the FASB issued ASU No. 2016-13, Financial Instruments — Credit Losses (“ASU 2016-13”). The amendments in ASU 2016-13 update guidance on reporting credit losses for financial assets. These amendments affect loans, debt securities, trade receivables, net investments in leases, off balance sheet credit exposures, reinsurance receivables, and any other financial assets not excluded from the scope that have the contractual right to receive cash. The Group will adopt ASU 2016-13 on January 1, 2022, and is currently evaluating the impact on its consolidated financial statements of adopting this guidance.
In January 2017, the FASB issued ASU 2017-04, “Intangibles — Goodwill and Other (Topic 350): Simplifying the Test for Goodwill Impairment,” which simplifies how an entity is required to test goodwill for impairment by eliminating step two from the goodwill impairment test. Step two of the goodwill impairment test measures a goodwill impairment loss by comparing the implied fair value of a reporting unit’s goodwill with its carrying amount. The new guidance is effective prospectively for us for the year ending December 31, 2021 and interim reporting periods during the year ending December 31, 2021. Early adoption is permitted for interim or annual goodwill impairment tests performed on testing dates after January 1, 2017. The Group is in the process of evaluating the impact of adoption of this guidance on its consolidated financial statements.
In December 2019, the FASB issued ASU 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes. This guidance removes certain exceptions to the general principles in Topic 740 and enhances and simplifies various aspects of the income tax accounting guidance, including requirements such as tax basis step-up in goodwill obtained in a transaction that is not a business combination, ownership changes in investments, and interim-period accounting for enacted changes in tax law. This standard is effective for the Group for the annual reporting periods beginning January 1, 2022 and interim periods beginning January 1, 2023. Early adoption is permitted. The Group does not expect any material impact on the Group’s consolidated financial statements.
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (CONTINUED)
(ee) Recent accounting pronouncements (continued)
In January 2020, the FASB issued ASU 2020-01, Investments-Equity Securities (Topic 321), Investments-Equity Method and Joint Ventures (Topic 323), and Derivatives and Hedging (Topic 815) - Clarifying the Interactions between Topic 321, Topic 323, and Topic 815. This guidance addresses accounting for the transition into and out of the equity method and provides clarification of the interaction of rules for equity securities, the equity method of accounting, and forward contracts and purchase options on certain types of securities. This standard is effective for the Group beginning January 1, 2022 including interim periods within the fiscal year. Early adoption is permitted. The Group does not expect any material impact on its consolidated financial statements.
In August 2020, the FASB issued ASU 2020-06, “Debt — Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging — Contracts in Entity’s Own Equity (Subtopic 815-40)”. This ASU reduces the number of accounting models for convertible debt instruments and convertible preferred stock. As well as amend the guidance for the derivatives scope exception for contracts in an entity’s own equity to reduce form-over-substance-based accounting conclusions. In addition, this ASU improves and amends the related EPS guidance. This standard is effective for the Group on January 1, 2022, including interim periods within those fiscal years. Adoption is either a modified retrospective method or a fully retrospective method of transition. The Group is currently evaluating the impact of the adoption of ASU 2020-06 on its consolidated financial statements.
In October 2020, the FASB issued Accounting Standards Update No. 2020-10, Codification Improvements — Disclosures (“ASU 2020-10”) to align with the SEC’s regulations. This ASU improves consistency by amending the codification to include all disclosure guidance in the appropriate disclosure sections and clarifies application of various provisions in the Codification by amending and adding new headings, cross referencing to other guidance, and refining or correcting terminology. The Group will adopt ASU 2020-10 as of the reporting period beginning January 1, 2021. This ASU will not affect the Group’s results of operations, cash flows or financial position and the Group does not expect the adoption to have a material impact on the disclosures to the consolidated financial statements.
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
3. LIQUDITY
The Group’s principal sources of liquidity have been cash generated from financing and operating activities. As of December 31, 2020, the Group had RMB 3,016 (US$462) of cash and cash equivalents and a working capital deficit of RMB 22,236 (US$3,408). For the years ended December 31, 2018, 2019, and 2020, the Group incurred continuous losses of RMB 42,296, RMB 101,621 and RMB 80,565 (US$12,347), respectively. For the year ended December 31, 2020, the Group incurred RMB 58,967 (US$9,035) of negative cash flows from operations and RMB 2,482 (US$380) of cash flow used in investing activities. Management expects continuous capital financing through debt or equity issuance to supports its working capital. Subsequent to December 31, 2020, the Group closed the issuance of convertible debentures in the aggregate principal of $2,000,000 at a purchase price of $1,700,000 to certain non-U.S. investors and the Group entered into a share subscription agreement with a Chinese investor, under which the Group would issue, and the investor would acquire, a number of Class A ordinary shares in the Group for consideration of RMB12 million at a purchase price of US$4.8 per share (Note 18). The Group believes that the current cash and cash equivalents, together with the financing received subsequent to year end will be sufficient to meet the Group’s anticipated cash needs, including its cash needs for working capital and capital expenditures, for at least the next 12 months from the consolidated financial statement filing date.
The Group intend to finance its future working capital requirements and capital expenditures from cash generated from financing activities until the Group’s operating activities generate positive cash flows, if ever. The Group may, however, require additional cash due to changing business conditions or other future developments, including any investments or acquisitions the Group may decide to pursue. If the Group’s existing cash is insufficient to meet its requirements, the Company may seek to issue debt or equity securities or obtain additional credit facilities. Financing may be unavailable in the amounts it needs or on terms acceptable to the Company, if at all. Issuance of additional equity securities or equity-linked securities, including convertible debt securities, would dilute its earnings per share. The incurrence of debt would divert cash for working capital and capital expenditures to service debt obligations and could result in operating and financial covenants that restrict its operations and its ability to pay dividends to the Group’s shareholders.
4. OTHER CURRENT ASSETS, NET
Other current assets consist of the following:
|
|
|
As of December 31, |
| ||||
|
|
|
2019 |
|
2020 |
| ||
|
|
|
RMB |
|
RMB |
|
US$ |
|
|
|
|
|
|
|
|
|
|
|
Capitalized listing expense |
|
9,764 |
|
— |
|
— |
|
|
Tax recoverable |
|
1,574 |
|
1,649 |
|
253 |
|
|
Others |
|
1,455 |
|
1,753 |
|
268 |
|
|
|
|
12,793 |
|
3,402 |
|
521 |
|
|
Allowance for doubtful accounts |
|
(3 |
) |
(99 |
) |
(15 |
) |
|
Total |
|
12,790 |
|
3,303 |
|
506 |
|
Movement in the allowances for doubtful debts were as follows:
|
|
|
Years ended December 31, |
| ||||
|
|
|
2019 |
|
2020 |
| ||
|
|
|
RMB |
|
RMB |
|
US$ |
|
|
|
|
|
|
|
|
|
|
|
Balance at beginning of year |
|
36 |
|
3 |
|
— |
|
|
Additional provision |
|
117 |
|
96 |
|
15 |
|
|
Write-offs |
|
(150 |
) |
— |
|
— |
|
|
Balance at end of year |
|
3 |
|
99 |
|
15 |
|
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
5. PROPERTY AND EQUIPMENT, NET
Property and equipment, net consists of the following:
|
|
|
As of December 31, |
| ||||
|
|
|
2019 |
|
2020 |
| ||
|
|
|
RMB |
|
RMB |
|
US$ |
|
|
|
|
|
|
|
|
|
|
|
Buildings |
|
16,029 |
|
16,029 |
|
2,457 |
|
|
Leasehold improvements |
|
52 |
|
— |
|
— |
|
|
Furniture, fixtures and equipment |
|
10,352 |
|
11,133 |
|
1,706 |
|
|
Motor vehicles |
|
530 |
|
517 |
|
79 |
|
|
Total |
|
26,963 |
|
27,679 |
|
4,242 |
|
|
Less: |
|
|
|
|
|
|
|
|
Accumulated depreciation |
|
(8,728 |
) |
(10,028 |
) |
(1,537 |
) |
|
|
|
18,235 |
|
17,651 |
|
2,705 |
|
|
Construction in progress |
|
633 |
|
1,616 |
|
248 |
|
|
Property and equipment, net |
|
18,868 |
|
19,267 |
|
2,953 |
|
Construction in progress represents the renovation of an office building and spare parts for medical equipment which the Company will used to assemble new equipment in house.
Depreciation expense was RMB 2,357, RMB 2,059 and RMB 2,441 (US$ 374) for the years ended December 31, 2018, 2019 and 2020, respectively. No impairment charges were recognized on the property and equipment for the years ended December 31, 2018, 2019 and 2020.
6. LAND USE RIGHTS, NET
The land use rights assets as of December 31, 2019 and 2020 are summarized as follows:
|
|
|
As of December 31, |
| ||||
|
|
|
2019 |
|
2020 |
| ||
|
|
|
RMB |
|
RMB |
|
US$ |
|
|
|
|
|
|
|
|
|
|
|
Land use rights, cost |
|
1,388 |
|
1,388 |
|
213 |
|
|
Less: |
|
|
|
|
|
|
|
|
Accumulated depreciation |
|
(194 |
) |
(222 |
) |
(34 |
) |
|
Land use rights, net |
|
1,194 |
|
1,166 |
|
179 |
|
Amortization expense of the land use rights for the years ended December 31, 2018, 2019 and 2020 was RMB 257, RMB 28 and RMB 28 (US$4), respectively. As of December 31, 2020, expected amortization expense for the land use rights is approximately RMB 28 in 2021, RMB28 in 2022, RMB28 in 2023, RMB28 in 2024, RMB28 in 2025 and RMB1,026 in 2026 and thereafter.
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
7. INTANGIBLE ASSETS, NET
Intangible assets, net consist of the following:
|
|
|
As of December 31, |
| ||||
|
|
|
2019 |
|
2020 |
| ||
|
|
|
RMB |
|
RMB |
|
US$ |
|
|
|
|
|
|
|
|
|
|
|
Software |
|
1,305 |
|
1,331 |
|
204 |
|
|
Medical license |
|
5,300 |
|
5,300 |
|
812 |
|
|
Total |
|
6,605 |
|
6,631 |
|
1,016 |
|
|
Less: Accumulated amortization |
|
(1,405 |
) |
(2,035 |
) |
(312 |
) |
|
Land use rights, net |
|
5,200 |
|
4,596 |
|
704 |
|
Amortization expense for the years ended December 31, 2018, 2019 and 2020 amounted to RMB 530, RMB 577 and RMB 630 (US$97), respectively. The estimated aggregate amortization expense for each of the five succeeding years is as follows:
|
Year ending December 31, |
|
RMB |
|
|
2021 |
|
758 |
|
|
2022 |
|
349 |
|
|
2023 |
|
355 |
|
|
2024 |
|
355 |
|
|
2025 |
|
355 |
|
|
Thereafter |
|
2,424 |
|
|
Total |
|
4,596 |
|
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
8. LONG-TERM INVESTMENTS, NET
As at December 31, 2019 and 2020, long-term investments consisted of the following:
|
|
|
As of December 31, |
| ||||
|
|
|
2019 |
|
2020 |
|
2020 |
|
|
|
|
RMB |
|
RMB |
|
US$ |
|
|
|
|
|
|
|
|
|
|
|
Equity method investments |
|
|
|
|
|
|
|
|
Anpac Beijing Health Management Co., Ltd (“Anpac Beijing”). |
|
802 |
|
789 |
|
121 |
|
|
Shanghai Moxu Bio-medical Science Co., Ltd.(“Moxu”) |
|
94 |
|
94 |
|
14 |
|
|
|
|
|
|
|
|
|
|
|
Equity securities without readily determinable fair values |
|
|
|
|
|
|
|
|
Jiangsu Anpac Health Management Co., Ltd. (“Jiangsu Anpac”) |
|
2,750 |
|
2,750 |
|
421 |
|
|
Less: |
|
|
|
|
|
|
|
|
Impairment |
|
(1,320 |
) |
(2,750 |
) |
(421 |
) |
|
Total |
|
2,326 |
|
883 |
|
135 |
|
Equity method investments
On October 19, 2017, the Group and other third parties established Anpac Beijing, of which the Group owned 35% of the investment. In October 2019, the Group’s registered shareholding ratio of Anpac Beijing decreased from 35% to 18% according to the resolution of Anpac Beijing signed in October 2019. The Group’s paid-in shareholding ratio is 35% in both 2018 and 2019, and the shareholding ratio is 18% in 2020. On June 8, 2018, the Group and other third parties established Moxu, of which the Group owned 20% of the investment. The Group assessed the Group had significant influence over these two investees as of December 31, 2019 and 2020.
Equity securities without readily determinable fair values
In January 2016, the Group and other third parties established Jiangsu Anpac, of which the Group owned 10% of the investment. In November 2017, the Group further acquired a 5% equity interest. The Group accounted for the investment under cost method since the Group does not have the ability to exert significant influence over Jiangsu Anpac. With the adoption of ASU 2016-01, the Group accounted for it as equity securities without readily determinable fair values. The Group elected to use the measurement alternative to measure such investments at fair value based on the income approach using the discounted cash flow associated with the underlying assets, which incorporated certain assumptions including the investees’ revenue, growth rates and projected operating costs based on current economic condition, expectation of management and projected trends of current operating results. For the years ended December 31, 2018, 2019 and 2020, the Group recognized impairment loss of Nil, RMB 1,320 and RMB 1,430 (US$219) in Jiangsu Anpac investment, respectively.
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
9. SHORT-TERM DEBTS
|
|
|
As of December 31, |
| ||||
|
|
|
2019 |
|
2020 |
|
2020 |
|
|
|
|
RMB |
|
RMB |
|
US$ |
|
|
|
|
|
|
|
|
|
|
|
Short-term bank and other borrowings (i) |
|
14,000 |
|
6,000 |
|
920 |
|
|
Convertible loan (“CL”) (ii) |
|
24,568 |
|
2,232 |
|
342 |
|
|
Total |
|
38,568 |
|
8,232 |
|
1,262 |
|
(i) The short-term borrowings as of December 31, 2020 consisted of RMB 6,000 borrowings that had a fixed annual interest rate of 4.15% and are due on September 30, 2021. The short-term borrowings as of December 31, 2019 consisted of RMB8,000 and RMB6,000 borrowings that had fixed annual interest rates of 11% and 4.35%, respectively, and were fully repaid upon maturities in fiscal 2020. These borrowings are pledged by certain properties of the Group and the Founder, and guaranteed by the Founder. Interest expense recognized for short-term borrowings for the years ended December 31, 2019 and 2020 were RMB1,037 and RMB728(US$112), respectively.
(ii) During April to August of 2018, the Group issued convertible loans (CL) with an aggregate principal amount of US$2,500 to Jiaxing Zhijun Investment Management Co., Ltd. (“Zhijun”). The CL is originally due in one year and bears interest of 9% per annum if the conversion feature is not triggered. The CL is ultimately guaranteed by the Founder’s personal assets. The convertible loans were fully repaid upon maturity in fiscal 2020.
On July 30, 2020, the Group issued convertible loans with an aggregate principal amount of US$265 to EMA Financial, LLC. (“EMC”). The CL is originally due in nine months and bears interest of 10% per annum if the conversion feature is not triggered. The CL is ultimately guaranteed by the Founder’s personal assets. The loan was converted into 54,642 shares on February 17, 2021.
Interest expense recognized for CL for the years ended December 31, 2019 and 2020 were RMB1,553 and RMB451(US$69), respectively.
Weighted average interest rate for the years ended December 31, 2019 and 2020 were 8.58% and 8.40%, respectively.
Conversion feature
Pursuant to the CL agreement, the conversion price with EMC is lower of the closing price of US$5.9 and 80% of the average trading price of common stock during 5 trading days immediately preceding conversion.
The Group has elected to recognize the CL at fair value and therefore there was no further evaluation of embedded features for bifurcation.
For the years ended December 31, 2018 and 2019, the Group recognized an unrealized loss of RMB 784 and RMB 5,296, respectively, in other expense for the years ended December 31, 2018 and 2019, except for the fair value changes related to the Group’s own credit risks which are recognized in accumulated other comprehensive loss for the year ended December 31, 2019.
For the year ended December 31, 2020, the Group fully repaid the CL with Zhijun of RMB 17,261 ($2,645). Upon the repayment, the Group recognized other income of RMB 7,162 ($1,098) for the year ended December 31, 2020 and reversed in aggregated of RMB 108 ($15) of the fair value changes relating to the Group’s own credit risks previously recognized in the other comprehensive loss. For the year ended December 31, 2020, the Group recognized an unrealized loss of RMB 532 (US$82) from CL with EMC in other expense due to the change in the fair value during the year.
Modifications of CL
On April 26, 2019, the Group and Zhijun agreed to extend the term of the CL to October 31, 2019. No other terms of the CL were modified. On October 30, 2019 the Group and Zhijun agreed to further extend the term of the CL to April 30, 2020, and the conversion feature has also been changed as mentioned above. In accordance with ASC 470-50, Debt, as the present value of cash flows under the term of the new debt instrument did not differ by more than 10% from the present value of the remaining cash flows under the term of the original debt instrument, the modification was accounted for prospectively as yield adjustments based on the revised terms.
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
10. ACCRUED EXPENSES AND OTHER CURRENT LIABILITIES
|
|
|
As of December 31, |
| ||||
|
|
|
2019 |
|
2020 |
|
2020 |
|
|
|
|
RMB |
|
RMB |
|
US$ |
|
|
|
|
|
|
|
|
|
|
|
Salary and welfare payable |
|
9,498 |
|
6,866 |
|
1,053 |
|
|
Payable for acquisition of noncontrolling interests |
|
245 |
|
245 |
|
38 |
|
|
Accrued rental |
|
1,550 |
|
1,768 |
|
271 |
|
|
Accrued expenses |
|
4,430 |
|
14,136 |
|
2,166 |
|
|
Value added tax and other taxes payable |
|
100 |
|
263 |
|
40 |
|
|
Payable for property and equipment |
|
15 |
|
559 |
|
86 |
|
|
Accrued utilities |
|
5 |
|
52 |
|
8 |
|
|
Other payables |
|
2,939 |
|
1,464 |
|
224 |
|
|
Total |
|
18,782 |
|
25,353 |
|
3,886 |
|
11. ACCUMULATED OTHER COMPREHENSIVE (LOSS) INCOME
Other comprehensive (loss) income includes the foreign currency translation differences and fair value change relating to Company’s own credit risk on convertible loan. A rollforward of the amounts included in accumulated other comprehensive (loss) income for the years ended December 31, 2019 and 2020 was as follows:
|
|
|
Foreign currency |
|
Fair value |
|
Total |
|
|
Balance as of January 1, 2019 |
|
(976 |
) |
1,063 |
|
87 |
|
|
Fair value change relating to Company’s own credit risk on convertible loan |
|
— |
|
(955 |
) |
(955 |
) |
|
Foreign currency translation differences |
|
2,978 |
|
— |
|
2,978 |
|
|
Balance as of December 31, 2019 |
|
2,002 |
|
108 |
|
2,110 |
|
|
|
|
|
|
|
|
|
|
|
Fair value change relating to Company’s own credit risk on convertible loan |
|
— |
|
(108 |
) |
(108 |
) |
|
Foreign currency translation differences |
|
2,793 |
|
— |
|
2,793 |
|
|
Balance as of December 31,2020 (RMB) |
|
4,795 |
|
— |
|
4,795 |
|
|
Balance as of December 31, 2020 (US$) |
|
735 |
|
— |
|
735 |
|
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
12. SHAREHOLDERS’ EQUITY
Ordinary Shares
On October 31, 2019, the board of directors approved a share split of 1-for-100, pursuant to which the authorized share capital of the Class A and Class B ordinary shares would further increase to 70,000,000 and 30,000,000 respectively, with a par value of US$0.01. The registration of the above changes was completed on November 12, 2019 and the number of underlying shares and the fair market value per ordinary share as at grant dates have been retrospectively adjusted accordingly.
As of December 31, 2019 and 2020, the Group is authorized to issue 70,000,000 Class A Ordinary shares with US$0.01 par value per share. As of December 31, 2019 and 2020, 7,004,900 and 9,157,660 Class A ordinary shares were issued and outstanding, respectively. As of December 31, 2019 and 2020, the Group is authorized to issue 30,000,000 Class B Ordinary shares with US$0.01 par value per share. As of December 31, 2019 and 2020, 2,863,100 Class B ordinary shares were issued and outstanding. Holders of Class A ordinary shares and Class B ordinary shares have the same rights, except for voting and conversion rights. Each Class A ordinary share is entitled to one vote; and each Class B ordinary share is entitled to ten votes and is convertible into one Class A ordinary share at any time by the holder thereof. Class A ordinary shares are not convertible into Class B ordinary shares under any circumstances.
Completion of IPO
On January 30, 2020, the Group completed its IPO on the Nasdaq Stock Exchange. The Group offered 1,333,360 ADSs, representing 1,333,360 Class A ordinary shares at offering price of US$12.00 per ADS. The net proceeds to the Group from the IPO, after deducting commissions and offering expenses of approximately RMB35,200 (US$5,395), were RMB 75,460 (or approximately US$ 11,565).
Shares issued for service
On July 28, 2020, the Group entered into a service agreement with a public relationship (“PR”) firm. Pursuant to the service agreement, the Group is required to pay 70,000 class A ordinary shares for the PR service by the period ended on September 29, 2020. The fair value of the PR service was RMB 2,706 (US$415) determined based on the Group’s share price on July 28, 2020. The Group issued 35,000 Class A ordinary shares for the year ended December 31, 2020 and the remaining 35,000 Class A ordinary share were issued subsequently to December 31, 2020. Since the remaining 35,000 Class A ordinary shares is legally required to be issued by December 31, 2020, the Group included it in the calculation of the number of shares issued and outstanding as of December 31, 2020.
Shares issued for reserve
For the year ended December 31, 2020, the Group issued 500,000 Class A ordinary shares held in an escrow account as reserve solely for potential convertible note conversion.
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
13. SHARE BASED COMPENSATION
On February 1, 2010, the shareholders and Board of Directors (the “Board”) of the Group approved a resolution which authorized the chairman of the Board to grant share options to its eligible employees, directors, officers and consultants of the Group of a number of shares not exceeding 1,190,000 before July 1, 2017. On October 19, 2015, the shareholders and the Board approved a resolution to increase the authorized number to grant in the future up to 1,866,600. On July 1, 2017, in order to provide additional incentives to attract and retain key employees, directors, officers and consultants of outstanding ability and to motivate them to exert their best efforts, the shareholders and the Board further approved a resolution to grants in the future up to 2,726,600. The options granted are vested either (i) immediately upon grant date; or (ii) over various vesting schedule which no more than four years. After the Group completed its IPO, all the new options were granted under 2019 Share Incentive Plan discussed below.
On October 31, 2019, the shareholders and the Board approved the 2019 Share Incentive Plan (“2019 Plan”) which authorized the compensation committee or such other committee to grant share options to directors, service provider, advisor, employees and consultants of the Group of a number of shares not exceeding 1,105,300. As of December 31, 2019, no share based compensation was granted under 2019 Plan.
For the year ended December 31, 2020, the Board approved to issue share options to its eligible employees, directors, officers and consultants of the Group of 650,000 under 2019 Plan with exercise price ranging from $3.78 per share to $12 per share and contractual life of 10 years. All these options were vested over 1-5 years term based on the related option agreements. As of December 31, 2020, 650,000 options were issued under 2019 Plan.
Employees
The options granted to employees are measured based on the grant date fair value of the equity instrument. They are accounted for as equity awards and contain only service vesting conditions. The following table summarized the Group’s employee share option activities:
|
|
|
Number of |
|
Weighted |
|
Weighted |
|
Weighted |
|
Aggregate |
|
|
|
|
|
|
US$ per |
|
US$ per |
|
Years |
|
US$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Share options outstanding at January 1, 2018 |
|
427,100 |
|
0.0001 |
|
4.93 |
|
7.21 |
|
4,041 |
|
|
Granted |
|
206,000 |
|
0.0005 |
|
9.53 |
|
— |
|
— |
|
|
Forfeited |
|
(1,600 |
) |
0.0010 |
|
4.45 |
|
— |
|
— |
|
|
Share options outstanding at January 1, 2019 |
|
631,500 |
|
0.0002 |
|
6.43 |
|
7.22 |
|
6,090 |
|
|
Granted |
|
327,000 |
|
0.0004 |
|
9.80 |
|
— |
|
— |
|
|
Forfeited |
|
(42,000 |
) |
Nil |
|
9.68 |
|
— |
|
— |
|
|
Share options outstanding at December 31, 2019 |
|
916,500 |
|
0.0003 |
|
7.48 |
|
7.26 |
|
8,985 |
|
|
Granted |
|
379,000 |
|
9.07 |
|
3.10 |
|
— |
|
— |
|
|
Exercised |
|
(213,700 |
) |
— |
|
8.13 |
|
— |
|
— |
|
|
Forfeited |
|
(30,000 |
) |
0.0001 |
|
3.04 |
|
— |
|
— |
|
|
Share options outstanding at December 31, 2020 |
|
1,051,800 |
|
3.27 |
|
5.87 |
|
7.37 |
|
3,616 |
|
|
Vested and exercisable at December 31, 2020 |
|
578,600 |
|
1.91 |
|
6.11 |
|
6.06 |
|
2,579 |
|
The aggregate intrinsic value in the table above represents the difference between the exercise price of the awards and the fair value of the underlying Ordinary Shares at each reporting date, for those awards that had exercise price below the estimated fair value of the relevant Ordinary Shares.
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
For the years ended December 31, 2018, 2019 and 2020, the total fair value of the equity awards vested were RMB 2,312 and RMB 12,376 and RMB 11,725 (US$1,797) respectively. As of December 31, 2020, there was RMB 12,220 (US$1,873) in total unrecognized employee share-based compensation expense related to unvested options, that may be adjusted for actual forfeitures occurring in the future. Total unrecognized compensation cost may be recognized over a weighted-average period of 1.49 years.
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
13. SHARE BASED COMPENSATION (CONTINUED)
Nonemployees
The options granted to nonemployees are accounted for as equity awards with service and/or performance vesting conditions. The following table summarized the Group’s nonemployee share option activity:
|
|
|
Number of |
|
Weighted |
|
Weighted |
|
Weighted |
|
Aggregate |
|
|
|
|
|
|
US$per |
|
US$per |
|
Years |
|
US$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Share options outstanding at January 1, 2018 |
|
219,400 |
|
0.0000 |
|
4.91 |
|
7.22 |
|
2,076 |
|
|
Granted |
|
53,700 |
|
0.0005 |
|
9.55 |
|
|
|
|
|
|
Forfeited |
|
(160,000 |
) |
Nil |
|
4.44 |
|
|
|
|
|
|
Exercised |
|
(19,400 |
) |
Nil |
|
6.50 |
|
|
|
|
|
|
Share options outstanding at January 1, 2019 |
|
93,700 |
|
0.0004 |
|
7.53 |
|
8.11 |
|
904 |
|
|
Granted |
|
153,300 |
|
0.0003 |
|
9.80 |
|
|
|
|
|
|
Share options outstanding at December 31, 2019 |
|
247,000 |
|
0.0003 |
|
8.94 |
|
8.40 |
|
2,422 |
|
|
Granted |
|
271,000 |
|
8.44 |
|
3.07 |
|
— |
|
— |
|
|
Forfeited |
|
— |
|
— |
|
— |
|
— |
|
— |
|
|
Exercised |
|
(70,700 |
) |
1.60 |
|
6.66 |
|
— |
|
— |
|
|
Share options outstanding at December 31, 2020 |
|
447,300 |
|
4.86 |
|
5.85 |
|
8.55 |
|
1,166 |
|
|
Vested and exercisable at December 31, 2020 |
|
268,800 |
|
2.79 |
|
7.73 |
|
7.83 |
|
1,093 |
|
The aggregate intrinsic value in the table above represents the difference between the exercise price of the awards and the fair value of the underlying Ordinary Shares at each reporting date, for those awards that had exercise price below the estimated fair value of the relevant Ordinary Shares.
The total fair value of the equity awards vested during the years ended December 31, 2018, 2019 and 2020 were RMB 3,004, RMB 9,284 and RMB 6,037 (US$925) respectively. As of December 31, 2020, there was RMB 2,546 (US$390) of total unrecognized nonemployee share-based compensation expenses, related to unvested share-based awards. Total unrecognized compensation cost may be recognized over a weighted-average period of 1.06 years.
Fair value of options
The Group estimate the fair value of share options with the assistance of an independent third-party valuation firm. The assumptions used to value the share options granted to employees and nonemployee were as follows:
|
|
|
For the year ended December 31, | ||||
|
|
|
2018 |
|
2019 |
|
2020 |
|
Risk-free interest rate |
|
2.46%-3.11% |
|
1.55%-2.50% |
|
0.55%-0.93% |
|
Expected volatility range |
|
62.14%-63.61% |
|
60.37%-64.48% |
|
49%-65% |
|
Exercise multiple |
|
2.5 |
|
2.5 |
|
2.5 |
|
Fair market value per ordinary share as at grant dates |
|
US$9.46-9.61 |
|
US$9.61-9.80 |
|
US$1.74-$4.83 |
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
13. SHARE BASED COMPENSATION (CONTINUED)
Fair value of options (Continued)
The estimated fair value of the Group’s ordinary shares at their respective grant dates, was determined with the assistance of an independent third-party valuation firm. The risk-free interest rate for periods within the contractual life of the options is based on the U.S. Treasury yield curve in effect at the time of grant for a term consistent with the contractual term of the awards. Expected volatility is estimated based on the historical volatility ordinary shares of several comparable companies in the same industry. The expected exercise multiple is based on management’s estimation, which the Group believes is representative of the future.
The Group also entered into a share purchase agreement with CRS Holdings Inc. (“CRS”, a company controlled by the Founder, who has also been served as the Chief Executive Officer since the inception of the Group) in 2019. Per the share purchase agreement, CRS purchased 214,000 ordinary shares at a consideration of $3.27 per share. The below fair value offering price in the share purchase agreement with CRS essentially represents compensation to the Founder, for past services incurred.
The following table sets forth the amount of share-based compensation expense included in each of the relevant financial statement line items:
|
|
|
For the years ended December 31, |
| ||||||
|
|
|
2018 |
|
2019 |
|
2020 |
|
2020 |
|
|
|
|
RMB |
|
RMB |
|
RMB |
|
US$ |
|
|
Cost of revenues |
|
317 |
|
327 |
|
327 |
|
50 |
|
|
Selling and marketing expenses |
|
2,871 |
|
5,393 |
|
1,113 |
|
170 |
|
|
Research and development expenses |
|
1,958 |
|
2,534 |
|
3,534 |
|
542 |
|
|
General and administrative expenses |
|
2,790 |
|
24,601 |
|
12,788 |
|
1,960 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total share-based compensation expenses |
|
7,936 |
|
32,855 |
|
17,762 |
|
2,722 |
|
14. INCOME TAXES
BVI
The Company is incorporated in the BVI and conducts its primary business operations through the subsidiaries in the PRC and the U.S. Under the current laws of the BVI, the Company is not subject to tax on income or capital gains. Additionally, upon payments of dividends by the Company to its shareholders, no BVI withholding tax will be imposed.
PRC
The Group’s subsidiaries in the PRC are subject to the statutory rate of 25%, in accordance with the Enterprise Income Tax law (the “EIT Law”), which was effective since January 1, 2008. Changhe Bio-Medical Technology (Yangzhou) Co., Ltd., Changwei System Technology (Shanghai) Co., Ltd., Shanghai Xinshenpai Technology Co., Ltd., AnPac Bio-Medical Technology (Shanghai) Co., Ltd., Shiji (Hainan) Medical Technology Ltd. and Penghui Health Management Co., Ltd. are entitled to a preferential income tax rate of 20%, as they qualify as small and micro-sized enterprises.
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
14. INCOME TAXES (CONTINUED)
PRC (Continued)
Dividends, interests, rent and royalties payable by the Group’s PRC subsidiaries, to non-PRC resident enterprises, and proceeds from any such non-resident enterprise investor’s disposition of assets (after deducting the net value of such assets) shall be subject to 10% withholding tax, unless the respective non-PRC resident enterprise’s jurisdiction of incorporation has a tax treaty or arrangements with PRC that provides for a reduced withholding tax rate or an exemption from withholding tax.
United States
AnPac US is subject to the U.S. federal corporate income tax at a rate of 21% for the years ended December 31, 2018, 2019 and 2020, respectively. AnPac US is also subject to state income tax in California for the years ended December 31, 2018, 2019 and 2020.
The Group’s loss before income taxes consisted of:
|
|
|
For the year ended December 31, |
| ||||||
|
|
|
2018 |
|
2019 |
|
2020 |
|
2020 |
|
|
|
|
RMB |
|
RMB |
|
RMB |
|
US$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-PRC |
|
(18,944 |
) |
(56,658 |
) |
(51,328 |
) |
(7,866 |
) |
|
PRC |
|
(23,551 |
) |
(45,181 |
) |
(29,325 |
) |
(4,494 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
Total |
|
(42,495 |
) |
(101,839 |
) |
(80,653 |
) |
(12,360 |
) |
The current and deferred components of income tax benefit appearing in the consolidated statements of comprehensive income are as follows:
|
|
|
For the years ended December 31, |
| ||||||
|
|
|
2018 |
|
2019 |
|
2020 |
|
2020 |
|
|
|
|
RMB |
|
RMB |
|
RMB |
|
US$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
Current tax benefit |
|
111 |
|
130 |
|
— |
|
— |
|
|
Deferred tax benefit |
|
88 |
|
88 |
|
88 |
|
13 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
|
199 |
|
218 |
|
88 |
|
13 |
|
The reconciliation of tax computed by applying the statutory income tax rate of 25% for the year ended December 31, 2018, 2019 and 2020 applicable to the PRC operations to income tax benefit were as follows:
|
|
|
For the years ended December 31, |
| ||||||
|
|
|
2018 |
|
2019 |
|
2020 |
|
2020 |
|
|
|
|
RMB |
|
RMB |
|
RMB |
|
US$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss before income taxes |
|
(42,495 |
) |
(101,839 |
) |
(80,653 |
) |
(12,360 |
) |
|
Income tax benefit computed at the statutory income tax rate at 25% |
|
10,624 |
|
25,460 |
|
20,163 |
|
3,090 |
|
|
Non-deductible expenses |
|
(4,485 |
) |
(5,141 |
) |
2,475 |
|
379 |
|
|
International rate differences |
|
(2,227 |
) |
(11,367 |
) |
(9,606 |
) |
(1,472 |
) |
|
Preferential tax rate differences |
|
(210 |
) |
(710 |
) |
(552 |
) |
(85 |
) |
|
Effect of change in tax rate |
|
(826 |
) |
789 |
|
— |
|
— |
|
|
Change in valuation allowance |
|
(2,677 |
) |
(8,813 |
) |
(12,392 |
) |
(1,899 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
Income tax benefit |
|
199 |
|
218 |
|
88 |
|
13 |
|
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
14. INCOME TAXES (CONTINUED)
Deferred Taxes
The significant components of deferred taxes were as follows:
|
|
|
As of December 31, |
| ||||
|
|
|
2019 |
|
2020 |
|
2020 |
|
|
|
|
RMB |
|
RMB |
|
US$ |
|
|
|
|
|
|
|
|
|
|
|
Deferred tax assets: |
|
|
|
|
|
|
|
|
Net loss carryforward |
|
23,148 |
|
34,417 |
|
5,275 |
|
|
Accrued expenses |
|
1,479 |
|
1,359 |
|
208 |
|
|
Provision for doubtful accounts |
|
70 |
|
857 |
|
131 |
|
|
Others |
|
69 |
|
— |
|
— |
|
|
Valuation allowance |
|
(24,766 |
) |
(36,633 |
) |
(5,614 |
) |
|
Total deferred tax assets. |
|
— |
|
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
Deferred tax liabilities: |
|
|
|
|
|
|
|
|
Long-lived assets arising from acquisition |
|
(1,134 |
) |
(1,045 |
) |
(160 |
) |
|
|
|
|
|
|
|
|
|
|
Total deferred tax liabilities. |
|
(1,134 |
) |
(1,045 |
) |
(160 |
) |
The Group operates through several subsidiaries. Valuation allowance is considered for each of the entities.
Realization of the net deferred tax assets is dependent on factors including future reversals of existing taxable temporary differences and adequate future taxable income, exclusive of reversing deductible temporary differences and tax loss carry forwards. The Group evaluates the potential realization of deferred tax assets on an entity-by-entity basis. As of December 31, 2020 and 2019, the Company and all of its subsidiaries were in cumulative loss position, valuation allowances were provided against deferred tax assets in entities where it was determined it was more likely than not that the benefits of the deferred tax assets will not be realized.
As of December 31, 2020, the Group had net operating losses carryforward of RMB 141,892 (US$ 21,746) derived from entities in the PRC and the U.S., of which can be carried forward per tax regulation to offset future taxable income. The PRC net operating losses of RMB 124,333 (US$ 19,055) will expire from 2021 to 2025 if not utilized. The U.S. net operating losses of RMB 17,559 (US$ 2,691) can be utilized indefinitely.
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
15. RELATED PARTY TRANSACTIONS AND BALANCES
Parties are considered to be related if one party has the ability, directly or indirectly, to control the other party or exercise significant influence over the other party in making financial and operational decisions. The related parties that had transactions or balances with the Group in 2019 and 2020 consisted of:
|
Related Party |
|
Nature of the party |
|
Relationship with the Group |
|
Dr. Chris Chang Yu |
|
Individual |
|
Founder and Chairman with majority voting control |
|
Ms. Lin Yu |
|
Individual |
|
Director of the Group |
|
Anpai (Shanghai) Healthcare Management and Consulting Co., Ltd. (“Anpai”) |
|
Health management |
|
Equity investee of the Group |
|
Anpac Beijing |
|
Health management |
|
Equity investee of the Group |
|
Jiaxing Zhijun Sihang Investment Partnership Enterprises (limited partnership) (“Jiaxing Zhijun”) |
|
Private equity investment |
|
Shareholder |
|
Jiaxing Zhijun Investment Management Co., Ltd. (“Zhijun”) |
|
Investment management |
|
General partner of the shareholder |
|
CRS |
|
Investor |
|
Controlled by Dr. Chris Chang Yu |
|
Jiangsu Anpac |
|
Health management |
|
Equity investee of the Group |
|
Shanghai Yulin Information Technology Co., Ltd. (“Shanghai Yulin”) |
|
Information technology |
|
Controlled by Ms. Lin Yu |
|
Weidong Dai |
|
Individual |
|
Director of the Group |
|
Xuedong Du |
|
Individual |
|
Director of the Group |
|
Rouou Ying |
|
Individual |
|
Supervisor of Apanc lishui |
|
Xing Pu |
|
Individual |
|
Director of Lishui anpac |
|
Shanghai Muqing Industrial Co., Ltd. (“Shanghai Muqing Industrial”) |
|
Investor |
|
Equity investee of Muqing Anpanc |
|
Shanghai Muqing Jiahe Healthcare Management Co., Ltd. (Shanghai Muqing Jiahe) |
|
Health management |
|
Controlled by Shanghai Muqing industrial |
(a) Related party balances
|
|
|
As of December 31, |
| ||||
|
|
|
2019 |
|
2020 |
|
2020 |
|
|
|
|
RMB |
|
RMB |
|
US$ |
|
|
Due from related parties: |
|
|
|
|
|
|
|
|
Anpai |
|
535 |
|
215 |
|
33 |
|
|
Jiangsu Anpac |
|
1 |
|
— |
|
— |
|
|
Zhijun |
|
6 |
|
— |
|
— |
|
|
Shanghai Yulin |
|
13 |
|
13 |
|
2 |
|
|
Shanghai Muqing Jiahe |
|
— |
|
9 |
|
1 |
|
|
Anpac Beijing |
|
— |
|
200 |
|
31 |
|
|
Xuedong Du |
|
— |
|
832 |
|
128 |
|
|
Xing Pu |
|
— |
|
8 |
|
1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
555 |
|
1,277 |
|
196 |
|
Amounts due from Anpai, Jingsu Anpac and Jiaxing Zhijun comprise of accounts receivable. Amounts due from Shanghai Yulin comprise of other current assets.
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
|
|
|
As of December 31, |
| ||||
|
|
|
2019 |
|
2020 |
|
2020 |
|
|
|
|
RMB |
|
RMB |
|
US$ |
|
|
Due to related parties: |
|
|
|
|
|
|
|
|
CRS |
|
1,894 |
|
2,802 |
|
430 |
|
|
Zhijun |
|
— |
|
55 |
|
8 |
|
|
Jiaxing Zhijun |
|
2,403 |
|
877 |
|
135 |
|
|
Jiangsu Anpac |
|
300 |
|
302 |
|
46 |
|
|
Weidong Dai |
|
— |
|
22 |
|
3 |
|
|
Rouou Ying |
|
— |
|
4 |
|
1 |
|
|
Shanghai Muqing Industrial |
|
— |
|
68 |
|
10 |
|
|
|
|
|
|
|
|
|
|
|
|
|
4,597 |
|
4,130 |
|
633 |
|
Amounts due to Jiangsu Anpac comprise of loans which were interest-free, unsecured and repayable on demand, while amounts due to Jiaxing Zhijun and CRS comprise of the accrued interest expense of RMB 876(US$134) and RMB 59 (US$9).
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
15. RELATED PARTY TRANSACTIONS AND BALANCES (CONTINUED)
(b) Related party transactions
During the year ended December 31, 2018, 2019 and 2020, related party transactions consisted of the following:
|
|
|
For the years ended December 31, |
| ||||||
|
|
|
2018 |
|
2019 |
|
2020 |
|
2020 |
|
|
|
|
RMB |
|
RMB |
|
RMB |
|
US$ |
|
|
Revenue rendered to Anpac Beijing |
|
231 |
|
3 |
|
1 |
|
— |
|
|
Revenue rendered to Jiangsu Anpac |
|
110 |
|
64 |
|
39 |
|
6 |
|
|
Revenue rendered to Anpai. |
|
298 |
|
616 |
|
96 |
|
15 |
|
|
Consulting service received from Anpac Beijing |
|
700 |
|
2,199 |
|
898 |
|
138 |
|
|
Consulting service received from Jiangsu Anpac. |
|
— |
|
— |
|
8 |
|
1 |
|
|
Rent from Shanghai muqing industrial |
|
— |
|
— |
|
443 |
|
68 |
|
|
Advance from Jiaxing Zhijun |
|
25,000 |
|
— |
|
— |
|
— |
|
|
Purchase ordinary shares with the advance from Jiaxing Zhijun |
|
— |
|
25,000 |
|
— |
|
— |
|
|
CL from Zhijun |
|
16,445 |
|
— |
|
— |
|
— |
|
|
Repayment to Zhijun |
|
— |
|
— |
|
(17,261 |
) |
(2,645 |
) |
|
Interest expense to Zhijun |
|
824 |
|
1,579 |
|
1,664 |
|
255 |
|
|
Loan from CRS |
|
1,431 |
|
1,202 |
|
(2,071 |
) |
(317 |
) |
|
Repayment of loan to CRS |
|
(1,144 |
) |
(1,262 |
) |
1,498 |
|
230 |
|
|
Loan to Shanghai Yulin |
|
— |
|
(2,885 |
) |
— |
|
— |
|
|
Repayment of loan from Shanghai Yulin |
|
— |
|
2,872 |
|
— |
|
— |
|
|
Repayment to Jiangsu Anpac |
|
(350 |
) |
(150 |
) |
— |
|
— |
|
(c) Guarantor
The Group’s short-term borrowings in 2019 consisting of an RMB 8,000 and RMB 6,000 borrowing are guaranteed by the Founder.
16. RESTRICTED NET ASSETS
In accordance with the PRC Regulations on Enterprises with Foreign Investment, an enterprise established in the PRC with foreign investment is required to make appropriations to certain statutory reserves, namely a general reserve fund, an enterprise expansion fund, a staff welfare fund and a bonus fund, all of which are appropriated from net profit as reported in its PRC statutory accounts. A foreign invested enterprise is required to allocate at least 10% of its annual after-tax profits to a general reserve fund until such fund has reached 50% of its respective registered capital. Appropriations to the enterprise expansion fund and staff welfare and bonus funds are at the discretion of the board of directors for the foreign invested enterprises. For other subsidiaries incorporated in the PRC, the general reserve fund was appropriated based on 10% of net profits as reported in each subsidiary’s PRC statutory accounts. General reserve and statutory surplus funds are restricted to set-off against losses, expansion of production and operation and increasing registered capital of the respective company. Staff welfare and bonus fund and statutory public welfare funds are restricted to capital expenditures for the collective welfare of employees. The reserves are not allowed to be transferred to the Company in terms of cash dividends, loans or advances, nor are they allowed for distribution except under liquidation. As of December 31, 2019 and 2020, the PRC subsidiaries did not have after-tax profit and therefore no statutory reserves were allocated.
In addition, under PRC laws and regulations, the Company’s PRC subsidiaries are restricted in their ability to transfer their net assets to the Company in the form of dividend payments, loans or advances. As of December 31, 2019 and 2020, restricted net assets of the Company’s PRC subsidiaries were RMB 5,406 and RMB 166,729 (US$ 25,552), respectively.
Furthermore, cash transfers from the Group’s PRC subsidiaries to the Group’s subsidiaries outside of the PRC are subject to the PRC government control of currency conversion. Shortages in the availability of foreign currency may restrict the ability of the Group’s PRC subsidiaries to remit sufficient foreign currency to pay dividends or other payments to the Company, or otherwise satisfy their foreign currency denominated obligations.
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
17. COMMITMENTS AND CONTINGENCIES
(a) Operating lease commitments
The Group has entered into lease agreements for its business operations. Such leases are classified as operating leases.
Future minimum lease payments under non-cancellable operating lease agreements at December 31, 2020 were as follows:
|
Twelve months ending December 31, |
|
Minimum lease payment |
| ||
|
|
|
RMB |
|
US$ |
|
|
2021 |
|
1,838 |
|
282 |
|
|
2022 |
|
1,432 |
|
219 |
|
|
2023 |
|
2,717 |
|
416 |
|
|
2024 |
|
2,739 |
|
420 |
|
|
2025 and thereafter |
|
18,663 |
|
2,860 |
|
|
Total |
|
27,389 |
|
4,197 |
|
(b) Litigation
In the ordinary course of the business, the Group is subject to periodic legal or administrative proceedings. As of December 31, 2019 and 2020, the Group is not a party to any legal or administrative proceedings which will have a material adverse effect on the Group’s business, financial position, results of operations and cash flows.
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
18. SUBSEQUENT EVENTS
On February 1, 2021, the Board of directors approved to issue stock options of 250,000 to the Founder, the Chief Executive Officer of the Group to incentivize the future performance under the 2019 Plan with exercise price of $3.78 per share and expected life of 10 years. All these options were vested by February 1, 2021 based on the related option agreements. In addition, between January 2021 and April 2021, the Company issued 101,333 stock options to four consultants and two employees under the 2019 Plan with exercise price between $0 to $7.55 per share and expected life of 10 years.
On February 5, 2021, the Group closed the issuance of convertible debentures (the “Debentures”) in the aggregate principal of $2,000,000 at a purchase price of $1,700,000 pursuant to Regulation S of the Securities Act of 1933, as amended, to certain non-U.S. investors (the “Note Holders”). After deducting the original issue discount and offering expenses, the net proceeds will be used for general corporate purposes. The Debentures will mature in twelve months on February 4, 2022 and carries an interest rate of 0% per year, subject to certain condition that may increase the rate to 15% per year. The Note Holders may convert the Debentures into the Group’s ADSs, each currently representing one Class A ordinary share of the Group, at any time on or prior to maturity at the lower of (i) $15.00, or (ii) the lower of (x) 82% of the closing bid price in the last reported trade of the ADSs or (y) 80% of the VWAPs (daily dollar volume-weighted average price) during the 10 consecutive trading days, immediately preceding the date of conversion or other date of determination (the “Variable Conversion Price”), but not lower than the floor price of $1.00. Subject to the floor price, the Variable Conversion Price shall be 75% of the VWAPs during the 10 consecutive trading days, immediately preceding the conversion date or other date of determination if the Group shall trigger certain event of default as set forth in the Debenture. The conversion rate of the Debentures is subject to adjustments under the terms of the Debentures. The Group has agreed to register for resale the ADSs underlying the Debentures with the U.S. Securities and Exchange Commission pursuant to a registration rights agreement dated February 5, 2021.
On February 17, 2021, EMC converted the CL (Note 8) into 54,642 shares of ADS at the conversion price of $5.12 per share.
On February 20, 2021, the Group entered into a share purchase agreement with CEO, under which the CEO will purchase the Group’s 152,100 ordinary shares at the price of $4.56 per share with net proceeds of approximately $0.7 million
On February 21, 2021, the Group entered into a share subscription agreement with a Chinese investor (the “investor”), under which the Group would issue, and the investor would acquire, a number of Class A ordinary shares in the Group for consideration of RMB12 million (or approximately $1.8 million) at a purchase price of US$4.8 per share. The Group issued 387,597 Class A ordinary Shares to the investor on February 24, 2021 according to the subscription agreement, based on an exchange rate of RMB6.45 to US$1.00, and in return, the Group received the consideration in full on March 18, 2021. The Group paid a finder’s fee in the form of 19,174 Class A ordinary shares to a Chinese consultant on March 22, 2021 in connection with this transaction.
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
19. PARENT COMPANY ONLY CONDENSED FINANCIAL INFORMATION
Condensed balance sheets
|
|
|
As of December 31, |
| ||||
|
|
|
2019 |
|
2020 |
|
2020 |
|
|
|
|
RMB |
|
RMB |
|
US$ |
|
|
ASSETS |
|
|
|
|
|
|
|
|
Current assets |
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
44 |
|
68 |
|
10 |
|
|
Advances to suppliers |
|
— |
|
4,467 |
|
685 |
|
|
Amounts due from affiliates and subsidiaries |
|
99,704 |
|
58,341 |
|
8,941 |
|
|
Other current assets |
|
872 |
|
1,187 |
|
182 |
|
|
Total current assets |
|
100,620 |
|
64,063 |
|
9,818 |
|
|
Non-current assets: |
|
|
|
|
|
|
|
|
Investments in subsidiaries |
|
(72,289 |
) |
(49,424 |
) |
(7,573 |
) |
|
Other assets |
|
760 |
|
— |
|
— |
|
|
TOTAL ASSETS |
|
29,091 |
|
14,639 |
|
2,245 |
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND SHAREHOLDERS’ DEFICIT |
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
Short-term debts |
|
24,568 |
|
2,232 |
|
343 |
|
|
Amounts due to related parties |
|
18,194 |
|
4,206 |
|
645 |
|
|
Accrued expenses and other current liabilities |
|
2,302 |
|
5,253 |
|
805 |
|
|
Total liabilities |
|
45,064 |
|
11,691 |
|
1,793 |
|
|
|
|
|
|
|
|
|
|
|
Shareholders’ (deficit) equity: |
|
|
|
|
|
|
|
|
Class A Ordinary shares (US$0.01 par value per share; 70,000,000 shares authorized, 7,004,900 and 9,192,660 shares issued and outstanding as of December 31, 2019 and 2020, respectively) |
|
466 |
|
618 |
|
95 |
|
|
Class B Ordinary shares (US$0.01 par value per share; 30,000,000 authorized, 2,863,100 shares issued and outstanding as of December 31, 2019 and 2020) |
|
191 |
|
191 |
|
29 |
|
|
Additional paid-in capital |
|
257,736 |
|
354,295 |
|
54,298 |
|
|
Accumulated deficits |
|
(276,476 |
) |
(356,951 |
) |
(54,705 |
) |
|
Accumulated other comprehensive income |
|
2,110 |
|
4,795 |
|
735 |
|
|
Total shareholders’ (deficit) equity |
|
(15,973 |
) |
2,948 |
|
452 |
|
|
TOTAL LIABILITIES AND SHAREHOLDERS’ DEFICIT |
|
29,091 |
|
14,639 |
|
2,245 |
|
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
(Amounts in thousands of RMB and US$, except for number of shares and per share data)
19. PARENT COMPANY ONLY CONDENSED FINANCIAL INFORMATION (Continued)
Condensed statements of comprehensive loss
|
|
|
For the years ended December 31, |
| ||||||
|
|
|
2018 |
|
2019 |
|
2020 |
|
2020 |
|
|
|
|
RMB |
|
RMB |
|
RMB |
|
US$ |
|
|
Operating loss: |
|
|
|
|
|
|
|
|
|
|
Selling and marketing expenses |
|
(2,871 |
) |
(5,393 |
) |
(3,922 |
) |
(601 |
) |
|
Research and development expenses |
|
(1,958 |
) |
(2,534 |
) |
(4,800 |
) |
(736 |
) |
|
General and administrative expenses |
|
(3,537 |
) |
(31,884 |
) |
(33,499 |
) |
(5,134 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations |
|
(8,366 |
) |
(39,811 |
) |
(42,221 |
) |
(6,471 |
) |
|
Interest expense |
|
(828 |
) |
(1,576 |
) |
(393 |
) |
(60 |
) |
|
Other (expense) income, net |
|
— |
|
23 |
) |
(692 |
) |
(106 |
) |
|
Change in fair value of convertible debt |
|
(784 |
) |
(5,296 |
) |
6,630 |
|
1,016 |
|
|
Share of losses of subsidiaries |
|
(32,085 |
) |
(54,400 |
) |
(43,799 |
) |
(6,712 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
Loss before income taxes and net loss |
|
(42,063 |
) |
(101,060 |
) |
(80,475 |
) |
(12,333 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive income, net of tax |
|
|
|
|
|
|
|
|
|
|
—Fair value change relating to Company’s own credit risk on convertible loan |
|
— |
|
(955 |
) |
(108 |
) |
(17 |
) |
|
—Foreign currency translation adjustment |
|
797 |
|
2,978 |
|
2,793 |
|
428 |
|
|
Total comprehensive loss |
|
(41,266 |
) |
(99,037 |
) |
(77,790 |
) |
(11,922 |
) |
Condensed statements of cash flows
|
|
|
As of December 31 |
| ||||||
|
|
|
2018 |
|
2019 |
|
2020 |
|
2020 |
|
|
|
|
RMB |
|
RMB |
|
RMB |
|
US$ |
|
|
Net cash used in operating activities |
|
(1,259 |
) |
(11,922 |
) |
(65,043 |
) |
(9,968 |
) |
|
Net cash used in investing activities |
|
(12,475 |
) |
(31,415 |
) |
(79,461 |
) |
(12,178 |
) |
|
Net cash provided by financing activities |
|
15,150 |
|
39,648 |
|
144,408 |
|
22,132 |
|
|
Effect of exchange rate changes on cash and cash equivalents |
|
125 |
|
30 |
|
120 |
|
18 |
|
|
Net increase (decrease) in cash and cash equivalents |
|
1,541 |
|
(3,659 |
) |
24 |
|
3 |
|
|
Cash and cash equivalents at beginning of year |
|
2,162 |
|
3,703 |
|
44 |
|
7 |
|
|
Cash and cash equivalents at end of year |
|
3,703 |
|
44 |
|
68 |
|
10 |
|
REGISTRATION RIGHTS AGREEMENT
THIS REGISTRATION RIGHTS AGREEMENT (this “Agreement”), dated as of February 5, 2021, by and among AnPac Bio-Medical Science Co., Ltd. a British Virgin Islands corporation (the “Company”), and investors identified on the signatory pages to this Agreement (the “Investors”).
WHEREAS:
A. In connection with the Securities Purchase Agreement by and among the parties hereto of even date herewith (the “Securities Purchase Agreement”), the Company has agreed, upon the terms and subject to the conditions of the Securities Purchase Agreement, to issue and sell to the Investors up to $2,000,000 of secured convertible debentures (the “Convertible Debentures”), which shall be convertible into shares of the Company’s American Depositary Shares (“ADSs”, each an “ADS”) with each representing one Class A ordinary shares, par value $0.01 (the “Common Stock”) (as converted, the “Conversion Shares”). Capitalized terms not defined herein shall have the meaning ascribed to them in the Securities Purchase Agreement.
B. To induce the Investors to execute and deliver the Securities Purchase Agreement, the Company has agreed to provide certain registration rights under the Securities Act of 1933, as amended, and the rules and regulations thereunder, or any similar successor statute (collectively, the “Securities Act”), and applicable state securities laws and other rights as provided for herein.
NOW, THEREFORE, in consideration of the premises and the mutual covenants contained herein and other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Company and the Investors hereby agree as follows:
1. DEFINITIONS.
As used in this Agreement, the following terms shall have the following meanings:
(a) “Effectiveness Deadline” means, with respect to a Registration Statement filed hereunder, the 70th calendar day following the date hereof, provided, however, in the event the Company is notified by the U.S. Securities and Exchange Commission (“SEC”) that one of the Registration Statements, as defined below, will not be reviewed or is no longer subject to further review and comments, the Effectiveness Deadline as to such Registration Statement shall be the fifth calendar day following the date on which the Company is so notified if such date precedes the date required above.
(b) “Person” means a corporation, a limited liability company, an association, a partnership, an organization, a business, an individual, a governmental or political subdivision thereof or a governmental agency.
(c) “Prospectus” means the prospectus included in a Registration Statement (including, without limitation, a prospectus that includes any information previously omitted from a prospectus filed as part of an effective registration statement in reliance upon Rule 430A promulgated under the Securities Act), as amended or supplemented by any prospectus supplement, with respect to the terms of the offering of any portion of the Registrable Securities covered by a Registration Statement, and all other amendments and supplements to the Prospectus, including post-effective amendments, and all material incorporated by reference or deemed to be incorporated by reference in such Prospectus.
(d) “Registrable Securities” means all of (i) the Conversion Shares issuable upon conversion of the Convertible Debentures, and (ii) any additional shares issuable in connection with any anti-dilution provisions of the Convertible Debentures (without giving effect to any limitations on exercise set forth in the Convertible Debentures) and (iii) any ADS issued or issuable with respect to the Conversion Shares as a result of any stock split, dividend or other distribution, recapitalization or similar event or otherwise (in each case without giving effect to any limitations on exercise set forth in the Convertible Debentures).
(e) “Registration Statement” means the registration statements required to be filed hereunder (including any additional registration statements contemplated by Section 2(c)), including (in each case) the Prospectus, amendments and supplements to such registration statement or Prospectus, including pre- and post-effective amendments, all exhibits thereto, and all material incorporated by reference or deemed to be incorporated by reference in such registration statement.
(f) “Required Registration Amount” means at least such number of shares of Common Stock as shall equal up to 100% of the number of shares of ADSs issuable upon conversion of all Convertible Debentures then outstanding (assuming for purposes hereof that (x) such Convertible Debentures are convertible at the Floor Price (as defined therein) in effect as of the date of determination, and (y) any such conversion shall not take into account any limitations on the conversion of the Convertible Debentures set forth in the Convertible Debentures), in each case subject to any cutback set forth in Section 2(d); provided that in no event shall the Required Registration Amount be more than 2,420,950 ordinary shares.
(g) “Rule 415” means Rule 415 promulgated by the SEC pursuant to the Securities Act, as such Rule may be amended from time to time, or any similar rule or regulation hereafter adopted by the SEC having substantially the same purpose and effect as such Rule.
2. REGISTRATION.
(a) The Company’s registration obligations set forth in this Section 2 including its obligations to file Registration Statements, obtain effectiveness of Registration Statements, and maintain the continuous effectiveness of Registration Statement that have been declared effective shall begin on the date hereof and continue until all the Registrable Securities have been sold or may permanently be sold without any restrictions pursuant to Rule 144, as determined by the counsel to the Company pursuant to a written opinion letter to such effect, addressed and acceptable to the Company’s transfer agent and the affected Holders (the “Registration Period”).
(b) Subject to the terms and conditions of this Agreement, the Company shall, on or prior to the Filing Deadline, prepare and file with the SEC a Registration Statement on Form F-1 covering the resale by the Investor of Registrable Securities. Each Registration Statement prepared pursuant hereto shall register for resale at least the number of ADSs equal to the Required Registration Amount as of date the Registration Statement is initially filed with the SEC. Each Registration Statement shall contain the “Selling Stockholders” and “Plan of Distribution” sections. The Company shall use its best efforts to have each Registration Statement declared effective by the SEC as soon as practicable. By 9:30 am on the business day following the date of effectiveness, the Company shall file with the SEC in accordance with Rule 424 under the 1933 Act the final Prospectus to be used in connection with sales pursuant to such Registration Statement. Prior to the filing of the Registration Statement with the SEC, the Company shall furnish a draft of the Registration Statement to the Investor for their review and comment. The Investor shall furnish comments on the Registration Statement to the Company within twenty-four (24) hours of the receipt thereof from the Company.
(c) During the Registration Period, the Company shall (i) promptly prepare and file with the SEC such amendments (including post-effective amendments) and supplements to a Registration Statement and the Prospectus used in connection with a Registration Statement, which Prospectus is to be filed pursuant to Rule 424 promulgated under the Securities Act, as may be necessary to keep such Registration Statement effective at all times during the Registration Period, (ii) prepare and file with the SEC additional Registration Statements in order to register for resale under the Securities Act all of the Registrable Securities; (iii) cause the related Prospectus to be amended or supplemented by any required Prospectus supplement (subject to the terms of this Agreement), and as so supplemented or amended to be filed pursuant to Rule 424; (iv) respond as promptly as reasonably possible to any comments received from the SEC with respect to a Registration Statement or any amendment thereto and as promptly as reasonably possible provide the Investors true and complete copies of all correspondence from and to the SEC relating to a Registration Statement (provided that the Company may excise any information contained therein which would constitute material non-public information as to any Investor which has not executed a confidentiality agreement with the Company); and (v) comply with the provisions of the Securities Act with respect to the disposition of all Registrable Securities of the Company covered by such Registration Statement until such time as all of such Registrable Securities shall have been disposed of in accordance with the intended methods of disposition by the seller or sellers thereof as set forth in such Registration Statement. In the case of amendments and supplements to a Registration Statement which are required to be filed pursuant to this Agreement (including pursuant to this Section 2(c)) by reason of the Company’s filing a report on Form 20-F or Form 6-K or any analogous report under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), the Company shall incorporate such report by reference into the Registration Statement, if applicable, or shall file such amendments or supplements with the SEC on the same day on which the Exchange Act report is filed which created the requirement for the Company to amend or supplement the Registration Statement.
(d) Reduction of Registrable Securities Included in a Registration Statement. Notwithstanding anything contained herein, in the event that the SEC requires the Company to reduce the number of Registrable Securities to be included in a Registration Statement in order to allow the Company to rely on Rule 415 with respect to a Registration Statement, then the Company shall be obligated to include in such Registration Statement (which may be a subsequent Registration Statement if the Company needs to withdraw a Registration Statement and refile a new Registration Statement in order to rely on Rule 415) only such limited portion of the Registrable Securities as the SEC shall permit. Any Registrable Securities that are excluded in accordance with the foregoing terms are hereinafter referred to as “Cut Back Securities.” To the extent Cut Back Securities exist, as soon as may be permitted by the SEC, the Company shall be required to file a Registration Statement covering the resale of the Cut Back Securities (subject also to the terms of this Section) and shall use best efforts to cause such Registration Statement to be declared effective as promptly as practicable thereafter.
(e) Failure to File or Obtain Effectiveness of the Registration Statement or Remain Current. If a Registration Statement is not declared effective on or prior to Effectiveness Deadline or the Company fails to file with the SEC a request for acceleration in accordance with Rule 461 promulgated under the Securities Act, within five (5) Trading Days of the date that the Company is notified (orally or in writing, whichever is earlier) by the SEC that a Registration Statement will not be “reviewed,” or not subject to further review, or (iii) after the effectiveness, a Registration Statement ceases for any reason to remain continuously effective as to all Registrable Securities, except for Cut Back Securities, for which it is required to be effective, or the Holders are otherwise not permitted to utilize the Prospectus therein to resell such Registrable Securities for more than 30 consecutive calendar days or more than an aggregate of 40 calendar days during any 12-month period (which need not be consecutive calendar days), or (iv) if after the six month anniversary of the date hereof, the Company does not have available adequate current public information as set forth in Rule 144(c) (any such failure or breach being referred to as an “Event”), then in addition to any other rights the holders of the Convertible Debentures may have hereunder or under applicable law, the Company shall be in breach of the term and conditions of this Agreement and such Event shall be deemed an event of default under the Convertible Debentures (provided that such event of default shall cease at such time that the Registration Statement is filed if the Event is due to (i) above, the Registration Statement becomes effective if the Event is due to (ii) above, and once all Registrable Securities become eligible for public resale pursuant to an exemption from registration under the Securities Act).
3. REMOVED AND RESERVED.
4. OBLIGATIONS OF THE INVESTORS.
(a) The Investor agrees that, upon receipt of any notice from the Company of the happening of any event of the kind described in Section 3(d) such Investor will immediately discontinue disposition of Registrable Securities pursuant to any Registration Statement covering such Registrable Securities until the Investor’s receipt of the copies of the supplemented or amended prospectus contemplated by Section 3(d) or receipt of notice that no supplement or amendment is required. Notwithstanding anything to the contrary, the Company shall cause its transfer agent to deliver unlegended certificates for shares of Common Stock to a transferee of an Investor in accordance with the terms of the Securities Purchase Agreement in connection with any sale of Registrable Securities with respect to which an Investor has entered into a contract for sale prior to the Investor’s receipt of a notice from the Company of the happening of any event of the kind described in Section 3(d) and for which the Investor has not yet settled.
(b) The Investor covenants and agrees that it will comply with the prospectus delivery requirements of the Securities Act as applicable to it or an exemption therefrom in connection with sales of Registrable Securities pursuant to the Registration Statement.
5. EXPENSES OF REGISTRATION.
All expenses incurred in connection with registrations, filings or qualifications pursuant to Sections 2 and 3, including, without limitation, all registration, listing and qualifications fees, printers, legal and accounting fees, except legal fees of Investor’s counsel associated with the review of the Registration Statement and any comment letters issued by the SEC relating to such Registration Statement, shall be paid by the Company.
6. INDEMNIFICATION.
With respect to Registrable Securities which are included in a Registration Statement under this Agreement:
(a) To the fullest extent permitted by law, the Company will, and hereby does, indemnify, hold harmless and defend the Investor, the directors, officers, partners, employees, agents, representatives of, and each Person, if any, who controls any Investor within the meaning of the Securities Act or the Exchange Act (each, an “Indemnified Person”), against any losses, claims, damages, liabilities, judgments, fines, penalties, charges, costs, reasonable attorneys’ fees, amounts paid in settlement or expenses, joint or several (collectively, “Claims”) incurred in investigating, preparing or defending any action, claim, suit, inquiry, proceeding, investigation or appeal taken from the foregoing by or before any court or governmental, administrative or other regulatory agency, body or the SEC, whether pending or threatened, whether or not an indemnified party is or may be a party thereto (“Indemnified Damages”), to which any of them may become subject insofar as such Claims (or actions or proceedings, whether commenced or threatened, in respect thereof) arise out of or are based upon: (i) any untrue statement or alleged untrue statement of a material fact in a Registration Statement or any post-effective amendment thereto or in any filing made in connection with the qualification of the offering under the securities or other “blue sky” laws of any jurisdiction in which Registrable Securities are offered (“Blue Sky Filing”), or the omission or alleged omission to state a material fact required to be stated therein or necessary to make the statements therein not misleading; (ii) any untrue statement or alleged untrue statement of a material fact contained in any final prospectus (as amended or supplemented, if the Company files any amendment thereof or supplement thereto with the SEC) or the omission or alleged omission to state therein any material fact necessary to make the statements made therein, in light of the circumstances under which the statements therein were made, not misleading; or (iii) any violation or alleged violation by the Company of the Securities Act, the Exchange Act, any other law, including, without limitation, any state securities law, or any rule or regulation there under relating to the offer or sale of the Registrable Securities pursuant to a Registration Statement (the matters in the foregoing clauses (i) through (iii) being, collectively, “Violations”). The Company shall reimburse the Investors and each such controlling person promptly as such expenses are incurred and are due and payable, for any legal fees or disbursements or other reasonable expenses incurred by them in connection with investigating or defending any such Claim. Notwithstanding anything to the contrary contained herein, the indemnification agreement contained in this Section 6(a): (x) shall not apply to a Claim by an Indemnified Person arising out of or based upon a Violation which occurs in reliance upon and in conformity with information furnished in writing to the Company by such Indemnified Person expressly for use in connection with the preparation of the Registration Statement or any such amendment thereof or supplement thereto; (y) shall not be available to the extent such Claim is based on a failure of the Investor to deliver or to cause to be delivered the prospectus made available by the Company, if such prospectus was timely made available by the Company pursuant to Section 3(c); and (z) shall not apply to amounts paid in settlement of any Claim if such settlement is effected without the prior written consent of the Company, which consent shall not be unreasonably withheld. Such indemnity shall remain in full force and effect regardless of any investigation made by or on behalf of the Indemnified Person.
(b) In connection with a Registration Statement, the Investor agrees to severally and not jointly indemnify, hold harmless and defend, to the same extent and in the same manner as is set forth in Section 6(a), the Company, each of its directors, each of its officers, employees, representatives, or agents and each Person, if any, who controls the Company within the meaning of the Securities Act or the Exchange Act (each an “Indemnified Party”), against any Claim or Indemnified Damages to which any of them may become subject, under the Securities Act, the Exchange Act or otherwise, insofar as such Claim or Indemnified Damages arise out of or is based upon any Violation, in each case to the extent, and only to the extent, that such Violation occurs in reliance upon and in conformity with written information furnished to the Company by such Investor expressly for use in connection with such Registration Statement; and, subject to Section 6(d), such Investor will reimburse any legal or other expenses reasonably incurred by them in connection with investigating or defending any such Claim; provided, however, that the indemnity agreement contained in this Section 6(b) and the agreement with respect to contribution contained in Section 7 shall not apply to amounts paid in settlement of any Claim if such settlement is effected without the prior written consent of such Investor, which consent shall not be unreasonably withheld; provided, further, however, that the Investor shall be liable under this Section 6(b) for only that amount of a Claim or Indemnified Damages as does not exceed the net proceeds to such Investor as a result of the sale of Registrable Securities pursuant to such Registration Statement. Such indemnity shall remain in full force and effect regardless of any investigation made by or on behalf of such Indemnified Party. Notwithstanding anything to the contrary contained herein, the indemnification agreement contained in this Section 6(b) with respect to any prospectus shall not inure to the benefit of any Indemnified Party if the untrue statement or omission of material fact contained in the prospectus was corrected and such new prospectus was delivered to each Investor prior to such Investor’s use of the prospectus to which the Claim relates.
(c) Promptly after receipt by an Indemnified Person or Indemnified Party under this Section 6 of notice of the commencement of any action or proceeding (including any governmental action or proceeding) involving a Claim, such Indemnified Person or Indemnified Party shall, if a Claim in respect thereof is to be made against any indemnifying party under this Section 6, deliver to the indemnifying party a written notice of the commencement thereof, and the indemnifying party shall have the right to participate in, and, to the extent the indemnifying party so desires, jointly with any other indemnifying party similarly noticed, to assume control of the defense thereof with counsel mutually satisfactory to the indemnifying party and the Indemnified Person or the Indemnified Party, as the case may be; provided, however, that an Indemnified Person or Indemnified Party shall have the right to retain its own counsel with the fees and expenses of not more than one (1) counsel for such Indemnified Person or Indemnified Party to be paid by the indemnifying party, if, in the reasonable opinion of counsel retained by the indemnifying party, the representation by such counsel of the Indemnified Person or Indemnified Party and the indemnifying party would be inappropriate due to actual or potential differing interests between such Indemnified Person or Indemnified Party and any other party represented by such counsel in such proceeding. The Indemnified Party or Indemnified Person shall cooperate fully with the indemnifying party in connection with any negotiation or defense of any such action or claim by the indemnifying party and shall furnish to the indemnifying party all information reasonably available to the Indemnified Party or Indemnified Person which relates to such action or claim. The indemnifying party shall keep the Indemnified Party or Indemnified Person fully apprised at all times as to the status of the defense or any settlement negotiations with respect thereto. No indemnifying party shall be liable for any settlement of any action, claim or proceeding effected without its prior written consent; provided, however, that the indemnifying party shall not unreasonably withhold, delay or condition its consent. No indemnifying party shall, without the prior written consent of the Indemnified Party or Indemnified Person, consent to entry of any judgment or enter into any settlement or other compromise which does not include as an unconditional term thereof the giving by the claimant or plaintiff to such Indemnified Party or Indemnified Person of a release from all liability in respect to such claim or litigation. Following indemnification as provided for hereunder, the indemnifying party shall be subrogated to all rights of the Indemnified Party or Indemnified Person with respect to all third parties, firms or corporations relating to the matter for which indemnification has been made. The failure to deliver written notice to the indemnifying party within a reasonable time of the commencement of any such action shall not relieve such indemnifying party of any liability to the Indemnified Person or Indemnified Party under this Section 6, except to the extent that the indemnifying party is prejudiced in its ability to defend such action.
(d) The indemnification required by this Section 6 shall be made by periodic payments of the amount thereof during the course of the investigation or defense, as and when bills are received or Indemnified Damages are incurred.
(e) The indemnity agreements contained herein shall be in addition to (i) any cause of action or similar right of the Indemnified Party or Indemnified Person against the indemnifying party or others, and (ii) any liabilities the indemnifying party may be subject to pursuant to the law.
7. REPORTS UNDER THE EXCHANGE ACT.
With a view to making available to the Investors the benefits of Rule 144 promulgated under the Securities Act or any similar rule or regulation of the SEC that may at any time permit the Investors to sell securities of the Company to the public without registration (“Rule 144”), and as a material inducement to the Investor’s purchase of the Convertible Debentures, the Company represents, warrants, and covenants to the following:
(a) The Company is subject to the reporting requirements of section 13 or 15(d) of the Exchange Act and has filed all required reports under section 13 or 15(d) of the Exchange Act during the 12 months prior to the date hereof (or for such shorter period that the issuer was required to file such reports), other than Form 8-K reports
(b) During the Registration Period, the Company shall file with the SEC in a timely manner all required reports under section 13 or 15(d) of the Exchange Act (it being understood that nothing herein shall limit the Company’s obligations under the Securities Purchase Agreement) and such reports shall conform to the requirement of the Exchange Act and the SEC for filing thereunder.
(c) The Company shall furnish to the Investor so long as such Investor owns Registrable Securities, promptly upon request, (i) a written statement by the Company that it has complied with the reporting requirements of Rule 144, (ii) a copy of the most recent annual or quarterly report of the Company and such other reports and documents so filed by the Company, and (iii) such other information as may be reasonably requested to permit the Investors to sell such securities pursuant to Rule 144 without registration.
8. AMENDMENT OF REGISTRATION RIGHTS.
Provisions of this Agreement may be amended and the observance thereof may be waived (either generally or in a particular instance and either retroactively or prospectively), only with the written consent of the Company and Investors who then hold at least two-thirds (2/3) of the Registrable Securities. Any amendment or waiver effected in accordance with this Section 9 shall be binding upon each Investor and the Company. No such amendment shall be effective to the extent that it applies to fewer than all of the holders of the Registrable Securities. No consideration shall be offered or paid to any Person to amend or consent to a waiver or modification of any provision of any of this Agreement unless the same consideration also is offered to all of the parties to this Agreement.
9. MISCELLANEOUS.
(a) A Person is deemed to be a holder of Registrable Securities whenever such Person owns or is deemed to own of record such Registrable Securities or owns the right to receive the Registrable Securities. If the Company receives conflicting instructions, notices or elections from two (2) or more Persons with respect to the same Registrable Securities, the Company shall act upon the basis of instructions, notice or election received from the registered owner of such Registrable Securities.
(b) Subsequent Registrations. The Company shall not file any other registration statements on Form F-3, Form F-1, or otherwise until the 15 Trading Days after such date the initial Registration Statement required hereunder is declared effective by the SEC, provided that this Section 10(b) shall not prohibit the Company from filing amendments to registration statements already filed. The Company shall not include any other securities on a Registration Statement unless otherwise agreed by the Investor.
(c) Piggy-Back Registrations. If at any time there is not an effective Registration Statement covering all of the Registrable Securities and the Company shall determine to prepare and file with the SEC a registration statement relating to an offering for its own account or the account of others under the Securities Act of any of its equity securities, other than on Form S-4 or Form S-8 (each as promulgated under the Securities Act) or their then equivalents relating to equity securities to be issued solely in connection with any acquisition of any entity or business or equity securities issuable in connection with the stock option or other employee benefit plans, then the Company shall send to each Investor a written notice of such determination and, if within fifteen (15) days after the date of such notice, any such Investor shall so request in writing, the Company shall include in such registration statement all or any part of such Registrable Securities such Investor requests to be registered; provided, however, that, the Company shall not be required to register any Registrable Securities pursuant to this Section 10(c) that are eligible for resale pursuant to Rule 144 promulgated under the Securities Act or that are the subject of a then effective Registration Statement.
(d) Any notices, consents, waivers or other communications required or permitted to be given under the terms of this Agreement must be in writing and will be deemed to have been delivered pursuant to the notice provisions of the Securities Purchase Agreement or to such other address and/or electronic mail address and/or to the attention of such other person as the recipient party has specified by written notice given to each other party five (5) days prior to the effectiveness of such change. Written confirmation of receipt (A) given by the recipient of such notice, consent, waiver or other communication, (B) electronically generated by the sender’s email service provider containing the time, date, and recipient email or (C) provided by a courier or overnight courier service shall be rebuttable evidence of personal service, receipt by facsimile or receipt from a nationally recognized overnight delivery service in accordance with this section.
(e) Failure of any party to exercise any right or remedy under this Agreement or otherwise, or delay by a party in exercising such right or remedy, shall not operate as a waiver thereof.
(f) The laws of the State of New York shall govern all issues concerning the relative rights of the Company and the Investors as its stockholders. All other questions concerning the construction, validity, enforcement and interpretation of this Agreement shall be governed by the internal laws of the State of New York, without giving effect to any choice of law or conflict of law provision or rule (whether of the State of New York or any other jurisdiction) that would cause the application of the laws of any jurisdiction other than the State of New York. Each party hereby irrevocably submits to the non-exclusive jurisdiction of the Supreme Court of the State of New York, sitting in New York County, New York and federal courts for the Southern District of New York sitting New York, New York, for the adjudication of any dispute hereunder or in connection herewith or with any transaction contemplated hereby or discussed herein, and hereby irrevocably waives, and agrees not to assert in any suit, action or proceeding, any claim that it is not personally subject to the jurisdiction of any such court, that such suit, action or proceeding is brought in an inconvenient forum or that the venue of such suit, action or proceeding is improper. Each party hereby irrevocably waives personal service of process and consents to process being served in any such suit, action or proceeding by mailing a copy thereof to such party at the address for such notices to it under this Agreement and agrees that such service shall constitute good and sufficient service of process and notice thereof. Nothing contained herein shall be deemed to limit in any way any right to serve process in any manner permitted by law. If any provision of this Agreement shall be invalid or unenforceable in any jurisdiction, such invalidity or unenforceability shall not affect the validity or enforceability of the remainder of this Agreement in that jurisdiction or the validity or enforceability of any provision of this Agreement in any other jurisdiction. EACH PARTY HEREBY IRREVOCABLY WAIVES ANY RIGHT IT MAY HAVE, AND AGREES NOT TO REQUEST, A JURY TRIAL FOR THE ADJUDICATION OF ANY DISPUTE HEREUNDER OR IN CONNECTION HEREWITH OR ARISING OUT OF THIS AGREEMENT OR ANY TRANSACTION CONTEMPLATED HEREBY.
(g) This Agreement shall inure to the benefit of and be binding upon the permitted successors and assigns of each of the parties hereto.
(h) The headings in this Agreement are for convenience of reference only and shall not limit or otherwise affect the meaning hereof.
(i) This Agreement may be executed in identical counterparts, each of which shall be deemed an original but all of which shall constitute one and the same agreement. This Agreement, once executed by a party, may be delivered to the other party hereto as an attachment to an email of a copy of this Agreement bearing the signature of the party so delivering this Agreement.
(j) Each party shall do and perform, or cause to be done and performed, all such further acts and things, and shall execute and deliver all such other agreements, certificates, instruments and documents, as the other party may reasonably request in order to carry out the intent and accomplish the purposes of this Agreement and the consummation of the transactions contemplated hereby.
(k) The language used in this Agreement will be deemed to be the language chosen by the parties to express their mutual intent and no rules of strict construction will be applied against any party.
(l) This Agreement is intended for the benefit of the parties hereto and their respective permitted successors and assigns, and is not for the benefit of, nor may any provision hereof be enforced by, any other Person.
[REMAINDER OF PAGE INTENTIONALLY LEFT BLANK]
IN WITNESS WHEREOF, the Investor and the Company have caused their signature page to this Registration Rights Agreement to be duly executed as of the date first above written.
|
|
COMPANY: | |
|
|
| |
|
|
ANPAC BIO-MEDICAL SCIENCE CO., LTD. | |
|
|
| |
|
|
By: |
/s/Chris Chang Yu |
|
|
Name: |
Chris Chang Yu |
|
|
Title: |
Chief Executive Officer |
IN WITNESS WHEREOF, the Investor and the Company have caused their signature page to this Registration Rights Agreement to be duly executed as of the date first above written.
|
|
INVESTOR: | |
|
|
| |
|
|
By: |
/s/ Heng Zhang |
|
|
Name: |
Heng Zhang |
|
|
Title: |
|
IN WITNESS WHEREOF, the Investor and the Company have caused their signature page to this Registration Rights Agreement to be duly executed as of the date first above written.
|
|
INVESTOR: | |
|
|
| |
|
|
By: |
/s/ Layette Holdings Inc. |
|
|
Name: |
Layette Holdings Inc. |
|
|
Title: |
|
IN WITNESS WHEREOF, the Investor and the Company have caused their signature page to this Registration Rights Agreement to be duly executed as of the date first above written.
|
|
INVESTOR: | |
|
|
| |
|
|
By: |
/s/ Jie Wang |
|
|
Name: |
Jie Wang |
|
|
Title: |
|
IN WITNESS WHEREOF, the Investor and the Company have caused their signature page to this Registration Rights Agreement to be duly executed as of the date first above written.
|
|
INVESTOR: | |
|
|
| |
|
|
By: |
/s/ Hongyu Wang |
|
|
Name: |
Hongyu Wang |
|
|
Title: |
|
SECURITIES PURCHASE AGREEMENT
THIS SECURITIES PURCHASE AGREEMENT (this “Agreement”), dated as of February 5, 2021, is between AnPac Bio-Medical Science Co., Ltd., a company incorporated under the laws of the British Virgin Islands, with headquarter located at 801 Bixing Street, Bihu County, Lishui, Zhejiang Province 323006, People’s Republic of China (the “Company”), and each of the investors listed on the Schedule of Buyers attached hereto (individually, a “Buyer” and collectively the “Buyers”).
WITNESSETH
WHEREAS, the Company and each Buyer desire to enter into this transaction for the Company to sell and the Buyers to purchase the Convertible Debentures (as defined below) pursuant to an exemption from registration pursuant to Regulation S (“Regulation S”) as promulgated by the U.S. Securities and Exchange Commission (the “SEC”) under the Securities Act of 1933, as amended (the “Securities Act”);
WHEREAS, the parties desire that, upon the terms and subject to the conditions contained herein, the Company shall issue and sell to the Buyer(s), as provided herein, and the Buyers shall purchase up to an aggregate of $2,000,000 of convertible debentures in the form attached hereto as “Exhibit A” (the “Convertible Debentures”), which shall be convertible into the Company’s American Depositary Shares (“ADSs”, each an “ADS”) with each representing one Class A ordinary shares, par value $0.01 (the “Common Stock”) (as converted, the “Conversion Shares”), of which the Convertible Debentures shall be purchased upon signing this Agreement (the “ Closing”), for a total purchase price of up to $1,700,000 (the “Purchase Price”) in the respective amounts in any combination of U.S. dollar or Chinese Yuan (based on the exchange rate of RMB 6.5408 to US$1.0000) set forth opposite each Buyer(s) name on Schedule I (the “Subscription Amount”);
WHEREAS, contemporaneously with the execution and delivery of this Agreement, the parties hereto are executing and delivering a Registration Rights Agreement (the “Registration Rights Agreement”) pursuant to which the Company has agreed to provide certain registration rights under the Securities Act and the rules and regulations promulgated there under, and applicable state securities laws;
WHEREAS, contemporaneously with the execution and delivery of this Agreement, the Company is delivering Irrevocable Transfer Agent Instructions (the “Irrevocable Transfer Agent Instructions”) to its transfer agent; and
WHEREAS, the Convertible Debentures and the Conversion Shares are collectively referred to herein as the “Securities.”
AGREEMENT
NOW, THEREFORE, in consideration of the premises and the mutual covenants contained herein and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Company and each Buyer hereby agree as follows:
1. PURCHASE AND SALE OF CONVERTIBLE DEBENTURES.
(a) Purchase of Convertible Debentures. Subject to the satisfaction (or waiver) of the conditions set forth in Sections 6 and 7 below, the Company shall issue and sell to each Buyer, and each Buyer severally, but not jointly, agrees to purchase from the Company at each Closing Convertible Debentures in amounts corresponding with the Subscription Amount set forth opposite each Buyer’s name on Schedule of Buyers attached as Schedule I hereto.
(b) Closing Dates. Each Closing of the purchase of Convertible Debentures by the Buyers shall occur at the offices Ortoli Rosenstadt LLP, 366 Madison Avenue, 3rd Floor, New York, NY 10017. The date and time of the Closing shall be 10:00 a.m., New York time, on the first Business Day on which the conditions to the Closing set forth in Sections 6 and 7 below are satisfied or waived (or such other date as is mutually agreed to by the Company and each Buyer) (the “Closing Date”). As used herein “Business Day” means any day other than a Saturday, Sunday or other day on which commercial banks in New York, New York are authorized or required by law to remain closed.
(c) Form of Payment; Deliveries. Subject to the satisfaction of the terms and conditions of this Agreement, on each Closing Date, (i) the Buyers shall deliver to the Company such aggregate proceeds for the Convertible Debentures to be issued and sold to such Buyer at such Closing, minus the fees to be paid directly from the proceeds of such Closing as set forth herein, and (ii) the Company shall deliver to each Buyer, Convertible Debentures which such Buyer is purchasing at such Closing in amounts indicated opposite such Buyer’s name on Schedule I, duly executed on behalf of the Company.
2. BUYER’S REPRESENTATIONS AND WARRANTIES.
Each Buyer, severally and not jointly, represents and warrants to the Company with respect to only itself that, as of the date hereof and as of each Closing Date:
(a) Investment Purpose. The Buyer is acquiring the Securities for its own account for investment only and not with a view towards, or for resale in connection with, the public sale or distribution thereof, except pursuant to sales registered or exempted under the Securities Act; provided, however, that by making the representations herein, such Buyer reserves the right to dispose of the Securities at any time in accordance with or pursuant to an effective registration statement covering such Securities or an available exemption under the Securities Act. Such Buyer does not presently have any agreement or understanding, directly or indirectly, with any Person to distribute any of the Securities.
(b) Reliance on Exemptions. The Buyer understands that the Securities are being offered and sold to it in reliance on specific exemptions from the registration requirements of United States federal and state securities laws and that the Company is relying in part upon the truth and accuracy of, and such Buyer’s compliance with, the representations, warranties, agreements, acknowledgments and understandings of such Buyer set forth herein in order to determine the availability of such exemptions and the eligibility of such Buyer to acquire the Securities.
(c) Information. The Buyer and its advisors (and his or, its counsel), if any, have been furnished with all materials relating to the business, finances and operations of the Company and information he deemed material to making an informed investment decision regarding his purchase of the Securities, which have been requested by such Buyer. The Buyer and its advisors, if any, have been afforded the opportunity to ask questions of the Company and its management. Neither such inquiries nor any other due diligence investigations conducted by such Buyer or its advisors, if any, or its representatives shall modify, amend or affect such Buyer’s right to rely on the Company’s representations and warranties contained in Section 3 below. The Buyer understands that its investment in the Securities involves a high degree of risk. The Buyer has sought such accounting, legal and tax advice, as it has considered necessary to make an informed investment decision with respect to its acquisition of the Securities.
(d) Transfer or Resale. The Buyer understands that: (i) the Securities have not been registered under the Securities Act or any state securities laws, and may not be offered for sale, sold, assigned or transferred unless (A) subsequently registered thereunder, (B) such Buyer shall have delivered to the Company an opinion of counsel, in a generally acceptable form, to the effect that such Securities to be sold, assigned or transferred may be sold, assigned or transferred pursuant to an exemption from such registration requirements, or (C) such Buyer provides the Company with reasonable assurances (in the form of seller and broker representation letters) that such Securities can be sold, assigned or transferred pursuant to Rule 144 promulgated under the Securities Act, as amended (or a successor rule thereto) (collectively, “Rule 144”), in each case following the applicable holding period set forth therein; and (ii) any sale of the Securities made in reliance on Rule 144 may be made only in accordance with the terms of Rule 144 and further, if Rule 144 is not applicable, any resale of the Securities under circumstances in which the seller (or the person through whom the sale is made) may be deemed to be an underwriter (as that term is defined in the Securities Act) may require compliance with some other exemption under the Securities Act or the rules and regulations of the SEC thereunder.
(e) Legends. The Buyer agrees to the imprinting, so long as its required by this Section 2(f), of a restrictive legend on the Securities in substantially the following form:
THE SECURITIES REPRESENTED BY THIS CERTIFICATE AND THOSE SECURITIES INTO WHICH THEY ARE CONVERTIBLE HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED, OR APPLICABLE STATE SECURITIES LAWS. THE SECURITIES AND THOSE SECURITIES INTO WHICH THEY ARE CONVERTIBLE HAVE BEEN ACQUIRED SOLELY FOR INVESTMENT PURPOSES AND NOT WITH A VIEW TOWARD RESALE AND MAY NOT BE OFFERED FOR SALE, SOLD, TRANSFERRED OR ASSIGNED IN THE ABSENCE OF AN EFFECTIVE REGISTRATION STATEMENT FOR THE SECURITIES UNDER THE SECURITIES ACT OF 1933, AS AMENDED, OR APPLICABLE STATE SECURITIES LAWS, OR AN OPINION OF COUNSEL, IN A GENERALLY ACCEPTABLE FORM, THAT REGISTRATION IS NOT REQUIRED UNDER SAID ACT OR APPLICABLE STATE SECURITIES LAWS
Certificates evidencing the Conversion Shares shall not contain any legend (including the legend set forth above), (i) while a registration statement covering the resale of such security is effective under the Securities Act, (ii) following any sale of such Conversion Shares pursuant to Rule 144, (iii) if such Conversion Shares are eligible for sale under Rule 144, or (iv) if such legend is not required under applicable requirements of the Securities Act (including judicial interpretations and pronouncements issued by the staff of the SEC). The Buyer agrees that the removal of restrictive legend from certificates representing Securities as set forth in this Section 3(f) is predicated upon the Company’s reliance that the Buyer will sell any Securities pursuant to either the registration requirements of the Securities Act, including any applicable prospectus delivery requirements, or an exemption therefrom, and that if Securities are sold pursuant to a registration statement, they will be sold in compliance with the plan of distribution set forth therein.
(f) Organization; Authority. Such Buyer is an entity duly organized, validly existing and in good standing under the laws of the jurisdiction of its organization with the requisite power and authority to enter into and to consummate the transactions contemplated by the Transaction Documents (as defined below) to which it is a party and otherwise to carry out its obligations hereunder and thereunder.
(g) Authorization, Enforcement. This Agreement has been duly and validly authorized, executed and delivered on behalf of such Buyer and shall constitute the legal, valid and binding obligations of such Buyer enforceable against such Buyer in accordance with its terms, except as such enforceability may be limited by general principles of equity or to applicable bankruptcy, insolvency, reorganization, moratorium, liquidation and other similar laws relating to, or affecting generally, the enforcement of applicable creditors’ rights and remedies.
(h) No Conflicts. The execution, delivery and performance by such Buyer of this Agreement and the consummation by such Buyer of the transactions contemplated hereby will not (i) result in a violation of the organizational documents of such Buyer, (ii) conflict with, or constitute a default (or an event which with notice or lapse of time or both would become a default) under, or give to others any rights of termination, amendment, acceleration or cancellation of, any agreement, indenture or instrument to which such Buyer is a party or (iii) result in a violation of any law, rule, regulation, order, judgment or decree (including federal and state securities laws) applicable to such Buyer, except, in the case of clauses (ii) and (iii) above, for such conflicts, defaults, rights or violations which could not, individually or in the aggregate, reasonably be expected to have a material adverse effect on the ability of such Buyer to perform its obligations hereunder.
(i) Certain Trading Activities. The Buyer has not directly or indirectly, nor has any Person acting on behalf of or pursuant to any understanding with the Buyer, engaged in any transactions in the securities of the Company (including, without limitation, any Short Sales (as defined below) involving the Company’s securities) during the period commencing as of the time that the Buyer first contacted the Company or the Company’s agents regarding the specific investment in the Company contemplated by this Agreement and ending immediately prior to the execution of this Agreement by such Buyer. The Buyer hereby agrees that it shall not directly or indirectly, engage in any Short Sales involving the Company’s securities during the period commencing on the date hereof and ending when no Convertible Debentures remain outstanding. “Short Sales” means all “short sales” as defined in Rule 200 promulgated under Regulation SHO under the 1934 Act (as defined below). The Buyer is aware that Short Sales and other hedging activities may be subject to applicable federal and state securities laws, rules and regulations and the Buyer acknowledges that the responsibility of compliance with any such federal or state securities laws, rules and regulations is solely the responsibility of the Buyer.
3. REPRESENTATIONS AND WARRANTIES OF THE COMPANY.
Except as set forth under the corresponding section of the Disclosure Schedules which Disclosure Schedules shall be deemed a part hereof and to qualify any representation or warranty otherwise made herein to the extent of such disclosure, the Company hereby makes the representations and warranties set forth below to The Buyer:
(a) Organization and Qualification. The Company and each of its Subsidiaries are entities duly formed, validly existing and in good standing under the laws of the jurisdiction in which they are formed, and have the requisite power and authority to own their properties and to carry on their business as now being conducted and as presently proposed to be conducted. The Company and each of its Subsidiaries is duly qualified as a foreign entity to do business and is in good standing in every jurisdiction in which its ownership of property or the nature of the business conducted by it makes such qualification necessary, except to the extent that the failure to be so qualified or be in good standing would not reasonably be expected to have a Material Adverse Effect (as defined below). As used in this Agreement, “Material Adverse Effect” means any material adverse effect on (i) the business, properties, assets, liabilities, operations (including results thereof), condition (financial or otherwise) or prospects of the Company and its Subsidiaries, taken as a whole, (ii) the transactions contemplated hereby or in any of the other Transaction Documents or any other agreements or instruments to be entered into by the Company in connection herewith or therewith or (iii) the authority or ability of the Company to perform any of its obligations under any of the Transaction Documents (as defined below). “Subsidiaries” means any Person in which the Company, directly or indirectly, owns a majority of the outstanding capital stock having voting power or holds a majority of the equity or similar interest of such Person, and each of the foregoing, is individually referred to herein as a “Subsidiary”.
(b) Authorization; Enforcement; Validity. The Company has the requisite power and authority to enter into and perform its obligations under this Agreement and the other Transaction Documents and to issue the Securities in accordance with the terms hereof and thereof. The execution and delivery of this Agreement and the other Transaction Documents by the Company and the consummation by the Company of the transactions contemplated hereby and thereby (including, without limitation, the issuance of the Convertible Debentures, the reservation for issuance and issuance of the Conversion Shares issuable upon conversion of the Convertible Debentures), have been duly authorized by the Company’s board of directors and no further filing, consent or authorization is required by the Company, its board of directors or its stockholders or other governmental body. This Agreement has been, and the other Transaction Documents to which the Company is a party will be prior to the Closing, duly executed and delivered by the Company, and each constitutes the legal, valid and binding obligations of the Company, enforceable against the Company in accordance with its respective terms, except as such enforceability may be limited by general principles of equity or applicable bankruptcy, insolvency, reorganization, moratorium, liquidation or similar laws relating to, or affecting generally, the enforcement of applicable creditors’ rights and remedies and except as rights to indemnification and to contribution may be limited by federal or state securities law. “Transaction Documents” means, collectively, this Agreement, Registration Rights Agreement, the Convertible Debentures, the Irrevocable Transfer Agent Instructions, and each of the other agreements and instruments entered into by the Company or delivered by the Company in connection with the transactions contemplated hereby and thereby, as may be amended from time to time.
(c) Issuance of Securities. The issuance of the Securities are duly authorized and, upon issuance and payment in accordance with the terms of the Transaction Documents the Securities shall be validly issued, fully paid and non-assessable and free from all preemptive or similar rights, mortgages, defects, claims, liens, pledges, charges, taxes, rights of first refusal, encumbrances, security interests and other encumbrances (collectively “Liens”) with respect to the issuance thereof. As of each Closing Date, the Company shall have reserved from its duly authorized capital stock not less than 100% of the maximum number of shares of ADSs issuable upon conversion of all Convertible Debentures (assuming for purposes hereof that (x) such Convertible Debentures are convertible at the Conversion Price (as defined therein) as of the date of determination, (y) any such conversion shall not take into account any limitations on the conversion of the Convertible Debentures set forth therein, including the Floor Price). Upon issuance or conversion in accordance with the Convertible Debentures, the Conversion Shares, when issued, will be validly issued, fully paid and nonassessable and free from all preemptive or similar rights or Liens with respect to the issue thereof, with the holders being entitled to all rights accorded to a holder of ADSs.
(d) No Conflicts. The execution, delivery and performance of the Transaction Documents by the Company and the consummation by the Company of the transactions contemplated hereby and thereby (including, without limitation, the issuance of the Convertible Debentures, the Conversion Shares, and the reservation for issuance of the Conversion Shares) will not (i) result in a violation of the Articles of Incorporation (as defined below), Bylaws (as defined below), certificate of formation, memorandum of association, articles of association, bylaws or other organizational documents of the Company or any of its Subsidiaries, or any capital stock or other securities of the Company or any of its Subsidiaries, (ii) conflict with, or constitute a default under, or give to others any rights of termination, amendment, acceleration or cancellation of, any agreement, indenture or instrument to which the Company or any of its Subsidiaries is a party, or (iii) result in a violation of any law, rule, regulation, order, judgment or decree (including, without limitation, U.S. federal and state securities laws and regulations, the securities laws of the jurisdictions of the Company’s incorporation or in which it or its subsidiaries operate and the rules and regulations of the Nasdaq Stock Market (the “Principal Market”) and including all applicable laws, rules and regulations of the British Virgin Islands) applicable to the Company or any of its Subsidiaries or by which any property or asset of the Company or any of its Subsidiaries is bound or affected, except in the case of (ii) and (iii) for any conflict, default, right or violation that would not reasonably be expected to result in a Material Adverse Effect.
(e) Consents. The Company is not required to obtain any material consent from, authorization or order of, or make any filing or registration with (other than any filings as may be required by any federal or state securities agencies and any filings as may be required by the Principal Market), any Governmental Entity (as defined below) or any regulatory or self-regulatory agency or any other Person in order for it to execute, deliver or perform any of its obligations under or contemplated by the Transaction Documents, in each case, in accordance with the terms hereof or thereof. All consents, authorizations, orders, filings and registrations which the Company or any Subsidiary is required to obtain pursuant to the preceding sentence have been or will be obtained or effected on or prior to each Closing Date, and neither the Company nor any of its Subsidiaries are aware of any facts or circumstances which might prevent the Company or any of its Subsidiaries from obtaining or effecting any of the registration, application or filings contemplated by the Transaction Documents. The Company is not in violation of the requirements of the Principal Market and has no knowledge of any facts or circumstances which could reasonably lead to delisting or suspension of the ADSs in the foreseeable future. The Company has notified the Principal Market of the issuance of all of the Securities hereunder, which does not require obtaining the approval of the stockholders of the Company or any other Person or Governmental Entity, and the Principal Market has completed its review of the related Listing of Additional Share form. “Governmental Entity” means any nation, state, county, city, town, village, district, or other political jurisdiction of any nature, federal, state, local, municipal, foreign, or other government, governmental or quasi-governmental authority of any nature (including any governmental agency, branch, department, official, or entity and any court or other tribunal), multi-national organization or body; or body exercising, or entitled to exercise, any administrative, executive, judicial, legislative, police, regulatory, or taxing authority or power of any nature or instrumentality of any of the foregoing, including any entity or enterprise owned or controlled by a government or a public international organization or any of the foregoing.
(f) Acknowledgment Regarding Buyer’s Purchase of Securities. The Company acknowledges and agrees that each Buyer is acting solely in the capacity of an arm’s length purchaser with respect to the Transaction Documents and the transactions contemplated hereby and thereby and that no Buyer is (i) an officer or director of the Company or any of its Subsidiaries, (ii) to its knowledge, an “affiliate” (as defined in Rule 144 promulgated under the 1933 Act (or a successor rule thereto) (collectively, “Rule 144”)) of the Company or any of its Subsidiaries or (iii) to its knowledge, a “beneficial owner” of more than 10% of the ADSs (as defined for purposes of Rule 13d-3 of the 1934 Act). The Company further acknowledges that no Buyer is acting as a financial advisor or fiduciary of the Company or any of its Subsidiaries (or in any similar capacity) with respect to the Transaction Documents and the transactions contemplated hereby and thereby, and any advice given by a Buyer or any of its representatives or agents in connection with the Transaction Documents and the transactions contemplated hereby and thereby is merely incidental to such Buyer’s purchase of the Securities. The Company further represents to each Buyer that the Company’s decision to enter into the Transaction Documents to which it is a party has been based solely on the independent evaluation by the Company and its representatives.
(g) No Integrated Offering. None of the Company, its Subsidiaries or any of their affiliates, nor any Person acting on their behalf has, directly or indirectly, made any offers or sales of any security or solicited any offers to buy any security, under circumstances that would cause this offering of the Securities to require approval of stockholders of the Company under any applicable stockholder approval provisions, including, without limitation, under the rules and regulations of any exchange or automated quotation system on which any of the securities of the Company are listed or designated for quotation. None of the Company, its Subsidiaries, their affiliates nor any Person acting on their behalf will take any action or steps that would cause the offering of any of the Securities to be integrated with other offerings of securities of the Company.
(h) Dilutive Effect. The Company understands and acknowledges that the number of Conversion Shares will increase in certain circumstances. The Company further acknowledges its obligation to issue the Conversion Shares upon conversion of the Convertible Debentures in accordance with this Agreement and the Convertible Debentures is, absolute and unconditional regardless of the dilutive effect that such issuance may have on the ownership interests of other stockholders of the Company.
(i) Application of Takeover Protections; Rights Agreement. The Company and its board of directors have taken all necessary action, if any, in order to render inapplicable any control share acquisition, interested stockholder, business combination, poison pill (including, without limitation, any distribution under a rights agreement), stockholder rights plan or other similar anti-takeover provision under the Articles of Incorporation, Bylaws or other organizational documents or the laws of the jurisdiction of its incorporation or otherwise which is or could become applicable to any Buyer as a result of the transactions contemplated by this Agreement, including, without limitation, the Company’s issuance of the Securities and any Buyer’s ownership of the Securities.
(j) SEC Documents; Financial Statements. The Company has timely filed all reports, schedules, forms, proxy statements, statements and other documents required to be filed by it with the SEC pursuant to the reporting requirements of the Securities Exchange Act of 1934, as amended (the “1934 Act”) (all of the foregoing filed prior to the date hereof and all exhibits and appendices included therein and financial statements, notes and schedules thereto and documents incorporated by reference therein being hereinafter referred to as the “SEC Documents”). The Company has delivered or has made available to the Buyers or their respective representatives true, correct and complete copies of each of the SEC Documents not available on the EDGAR system. As of their respective dates, the SEC Documents complied in all material respects with the requirements of the 1934 Act and the rules and regulations of the SEC promulgated thereunder applicable to the SEC Documents, and none of the SEC Documents, at the time they were filed with the SEC, contained any untrue statement of a material fact or omitted to state a material fact required to be stated therein or necessary in order to make the statements therein, in the light of the circumstances under which they were made, not misleading. As of their respective dates, the financial statements of the Company included in the SEC Documents complied in all material respects with applicable accounting requirements and the published rules and regulations of the SEC with respect thereto as in effect as of the time of filing. Such financial statements have been prepared in accordance with generally accepted accounting principles (“GAAP”), consistently applied, during the periods involved (except (i) as may be otherwise indicated in such financial statements or the notes thereto, or (ii) in the case of unaudited interim statements, to the extent they may exclude footnotes or may be condensed or summary statements) and fairly present in all material respects the financial position of the Company as of the dates thereof and the results of its operations and cash flows for the periods then ended (subject, in the case of unaudited statements, to normal year-end audit adjustments which will not be material, either individually or in the aggregate). The reserves, if any, established by the Company or the lack of reserves, if applicable, are reasonable based upon facts and circumstances known by the Company on the date hereof and there are no loss contingencies that are required to be accrued by the Statement of Financial Accounting Standard No. 5 of the Financial Accounting Standards Board which are not provided for by the Company in its financial statements or otherwise. No other information provided by or on behalf of the Company to any of the Buyers which is not included in the SEC Documents (including, without limitation, information in the disclosure schedules to this Agreement) contains any untrue statement of a material fact or omits to state any material fact necessary in order to make the statements therein not misleading, in the light of the circumstance under which they are or were made. The Company is not currently contemplating to amend or restate any of the financial statements (including, without limitation, any notes or any letter of the independent accountants of the Company with respect thereto) included in the SEC Documents (the “Financial Statements”), nor is the Company currently aware of facts or circumstances which would require the Company to amend or restate any of the Financial Statements, in each case, in order for any of the Financials Statements to be in compliance with GAAP and the rules and regulations of the SEC. The Company has not been informed by its independent accountants that they recommend that the Company amend or restate any of the Financial Statements or that there is any need for the Company to amend or restate any of the Financial Statements.
(k) Absence of Certain Changes. Since the date of the Company’s most recent audited financial statements contained in a Form 20-F, there has been no Material Adverse Effect, nor any event or occurrence specifically affecting the Company or its Subsidiaries that would be reasonably expected to result in a Material Adverse Effect. Since the date of the Company’s most recent audited financial statements contained in a Form 20-F, neither the Company nor any of its Subsidiaries has (i) declared or paid any dividends, (ii) sold any material assets, individually or in the aggregate, outside of the ordinary course of business or (iii) made any material capital expenditures, individually or in the aggregate, outside of the ordinary course of business. Neither the Company nor any of its Subsidiaries has taken any steps to seek protection pursuant to any law or statute relating to bankruptcy, insolvency, reorganization, receivership, liquidation or winding up, nor does the Company or any Subsidiary have any knowledge or reason to believe that any of their respective creditors intend to initiate involuntary bankruptcy proceedings or any actual knowledge of any fact which would reasonably lead a creditor to do so.
(l) No Undisclosed Events, Liabilities, Developments or Circumstances. No event, liability, development or circumstance has occurred or exists, or is reasonably expected to exist or occur specific to the Company, any of its Subsidiaries or any of their respective businesses, properties, liabilities, prospects, operations (including results thereof) or condition (financial or otherwise), that has not been publicly disclosed and would reasonably be expected to have a Material Adverse Effect.
(m) Conduct of Business; Regulatory Permits. Neither the Company nor any of its Subsidiaries is in violation of any term under its Articles of Incorporation, any certificate of designation, preferences or rights of any other outstanding series of preferred stock of the Company or any of its Subsidiaries or Bylaws or their organizational charter, certificate of formation, memorandum of association, articles of association, Articles of Incorporation or certificate of incorporation or bylaws, respectively. Neither the Company nor any of its Subsidiaries is in violation of any judgment, decree or order or any statute, ordinance, rule or regulation applicable to the Company or any of its Subsidiaries, and neither the Company nor any of its Subsidiaries will conduct its business in violation of any of the foregoing, except in all cases for violations which would not reasonably be expected to have a Material Adverse Effect. Without limiting the generality of the foregoing, the Company is not in violation of any of the rules, regulations or requirements of the Principal Market and has no knowledge of any facts or circumstances that could reasonably lead to delisting or suspension of the ADSs by the Principal Market in the foreseeable future. During the one year prior to the date hereof, (i) the ADSs has been listed or designated for quotation on the Principal Market, (ii) trading in the ADSs has not been suspended by the SEC or the Principal Market and (iii) the Company has received no communication, written or oral, from the SEC or the Principal Market regarding the suspension or delisting of the ADSs from the Principal Market, which has not been publicly disclosed. The Company and each of its Subsidiaries possess all certificates, authorizations and permits issued by the appropriate regulatory authorities necessary to conduct their respective businesses, except where the failure to possess such certificates, authorizations or permits would not reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect, and neither the Company nor any of its Subsidiaries has received any notice of proceedings relating to the revocation or modification of any such certificate, authorization or permit. There is no agreement, commitment, judgment, injunction, order or decree binding upon the Company or any of its Subsidiaries or to which the Company or any of its Subsidiaries is a party which has or would reasonably be expected to have the effect of prohibiting or materially impairing any business practice of the Company or any of its Subsidiaries, any acquisition of property by the Company or any of its Subsidiaries or the conduct of business by the Company or any of its Subsidiaries as currently conducted other than such effects, individually or in the aggregate, which have not had and would not reasonably be expected to have a Material Adverse Effect on the Company or any of its Subsidiaries.
(n) Equity Capitalization.
(i) Authorized and Outstanding Capital Stock. As of the date hereof, the authorized capital stock of the Company consists of (A) 70,000,000 Class A ordinary shares with a par value of $0.01 per share, of which, 9,157,660 are issued and outstanding and (B) 30,000,000 Class B ordinary shares with a par value of $0.01 per share, of which, 2,863,100 are issued and outstanding.
(ii) Valid Issuance; Available Shares. All of such outstanding shares are duly authorized and have been validly issued and are fully paid and nonassessable.
(iii) Organizational Documents. The Company has furnished to the Buyers or filed on EDGAR true, correct and complete copies of the Company’s Articles of Incorporation, as amended and as in effect on the date hereof (the “Articles of Incorporation”), and the Company’s bylaws, as amended and as in effect on the date hereof (the “Bylaws”), and the terms of all convertible securities and the material rights of the holders thereof in respect thereto.
(o) Litigation. Except as disclosed in the SEC Documents, there is no action, suit, arbitration, proceeding, inquiry or investigation before or by the Principal Market, any court, public board, other Governmental Entity, self-regulatory organization or body pending or, to the knowledge of the Company, threatened against or affecting the Company or any of its Subsidiaries, the ADSs or any of the Company’s or its Subsidiaries’ officers or directors, whether of a civil or criminal nature or otherwise, in their capacities as such, which would reasonably be expected to result in a Material Adverse Effect. After reasonable inquiry of its employees, the Company is not aware of any event which might result in or form the basis for any such action, suit, arbitration, investigation, inquiry or other proceeding. Without limitation of the foregoing, there has not been, and to the knowledge of the Company, there is not pending or contemplated, any investigation by the SEC involving the Company, any of its Subsidiaries or any current or former director or officer of the Company or any of its Subsidiaries. Neither the Company nor any of its Subsidiaries is the subject of any order, writ, judgment, injunction, decree, determination or award of any Governmental Entity that would reasonably be expected to result in a Material Adverse Effect.
(p) Removed and Reserved.
(q) Manipulation of Price. Neither the Company nor any of its Subsidiaries has, and, to the knowledge of the Company, no Person acting on their behalf has, directly or indirectly, (i) taken any action designed to cause or to result in the stabilization or manipulation of the price of any security of the Company or any of its Subsidiaries to facilitate the sale or resale of any of the Securities, (ii) sold, bid for, purchased, or paid any compensation for soliciting purchases of, any of the Securities, or (iii) paid or agreed to pay to any Person any compensation for soliciting another to purchase any other securities of the Company or any of its Subsidiaries.
(r) Registration Eligibility. The Company is eligible to register the resale of the Conversion Shares by the Buyers using Form F-1 promulgated under the 1933 Act.
(s) Shell Company Status. The Company is not, and has never been, an issuer identified in, or subject to, Rule 144(i).
(t) Money Laundering. The Company and its Subsidiaries are in compliance with, and have not previously violated, the USA Patriot Act of 2001 and all other applicable U.S. and non-U.S. anti-money laundering laws and regulations, including, but not limited to, the laws, regulations and Executive Orders and sanctions programs (“Sanctions Programs”) administered by the U.S. Office of Foreign Assets Control (“OFAC”), including, without limitation, (i) Executive Order 13224 of September 23, 2001 entitled, “Blocking Property and Prohibiting Transactions With Persons Who Commit, Threaten to Commit, or Support Terrorism” (66 Fed. Reg. 49079 (2001)); and any regulations contained in 31 CFR, Subtitle B, Chapter V.
(u) Disclosure. The Company confirms that neither it nor any other Person acting on its behalf has provided any of the Buyers or their agents or counsel with any information that constitutes or could reasonably be expected to constitute material, non-public information concerning the Company or any of its Subsidiaries, other than the existence of the transactions contemplated by this Agreement and the other Transaction Documents. The Company understands and confirms that each of the Buyers will rely on the foregoing representations in effecting transactions in securities of the Company. All disclosure provided to the Buyers regarding the Company and its Subsidiaries, their businesses and the transactions contemplated hereby, including the schedules to this Agreement, furnished by or on behalf of the Company or any of its Subsidiaries, taken as a whole, is true and correct and does not contain any untrue statement of a material fact or omit to state any material fact necessary in order to make the statements made therein, in the light of the circumstances under which they were made, not misleading. All of the written information furnished after the date hereof by or on behalf of the Company or any of its Subsidiaries to each Buyer pursuant to or in connection with this Agreement and the other Transaction Documents, taken as a whole, will be true and correct in all material respects as of the date on which such information is so provided and will not contain any untrue statement of a material fact or omit to state any material fact necessary in order to make the statements made therein, in the light of the circumstances under which they were made, not misleading. No event or circumstance has occurred or information exists with respect to the Company or any of its Subsidiaries or its or their business, properties, liabilities, prospects, operations (including results thereof) or conditions (financial or otherwise), which, under applicable law, rule or regulation, requires public disclosure at or before the date hereof or announcement by the Company but which has not been so publicly disclosed. All financial projections and forecasts that have been prepared by or on behalf of the Company or any of its Subsidiaries and made available to the Buyers have been prepared in good faith based upon reasonable assumptions and represented, at the time each such financial projection or forecast was delivered to each Buyer, the Company’s best estimate of future financial performance (it being recognized that such financial projections or forecasts are not to be viewed as facts and that the actual results during the period or periods covered by any such financial projections or forecasts may differ from the projected or forecasted results). The Company acknowledges and agrees that no Buyer makes or has made any representations or warranties with respect to the transactions contemplated hereby other than those specifically set forth in Section 2.
(v) No General Solicitation. Neither the Company, nor any of its affiliates, nor any Person acting on its or their behalf, has engaged in any form of general solicitation or general advertising (within the meaning of Regulation D under the Securities Act) in connection with the offer or sale of the Securities.
(w) Private Placement. Assuming the accuracy of the Buyers’ representations and warranties set forth in Section 2, no registration under the Securities Act is required for the offer and sale of the Securities by the Company to the Buyers as contemplated hereby. The issuance and sale of the Securities hereunder does not contravene the rules and regulations of the Primary Market.
4. COVENANTS.
(a) Reporting Status. For the period beginning on the date hereof, and ending 6 months after the date on which all the Convertible Debentures are no longer outstanding (the “Reporting Period”), the Company shall use its best efforts to file on a timely basis all reports required to be filed with the SEC pursuant to the 1934 Act, and the Company shall not terminate its status as an issuer required to file reports under the 1934 Act even if the 1934 Act or the rules and regulations thereunder would no longer require or otherwise permit such termination.
(b) Use of Proceeds. Neither the Company nor any Subsidiary will, directly or indirectly, use the proceeds of the transactions contemplated herein to repay any loans to any executives or employees of the Company. Neither the Company nor any Subsidiary will, directly or indirectly, use the proceeds of the transactions contemplated herein, or lend, contribute, facilitate or otherwise make available such proceeds to any Person (i) to fund, either directly or indirectly, any activities or business of or with any Person that is identified on the list of Specially Designated Nationals and Blocker Persons maintained by OFAC, or in any country or territory, that, at the time of such funding, is, or whose government is, the subject of Sanctions Programs, or (ii) in any other manner that will result in a violation of Sanctions Programs.
(c) Listing. To the extent applicable, the Company shall promptly secure the listing or designation for quotation (as the case may be) of all of the Underlying Securities (as defined below) upon each national securities exchange and automated quotation system, if any, upon which the ADS is then listed or designated for quotation (as the case may be, each an “Eligible Market”), subject to official notice of issuance, and shall use reasonable efforts to maintain such listing or designation for quotation (as the case may be) of all Underlying Securities from time to time issuable under the terms of the Transaction Documents on such Eligible Market for the Reporting Period. Neither the Company nor any of its Subsidiaries shall take any action which could be reasonably expected to result in the delisting or suspension of the ADSs on an Eligible Market during the Reporting Period. The Company shall pay all fees and expenses in connection with satisfying its obligations under this Section 4(c). “Underlying Securities” means the (i) the Conversion Shares, and (ii) any ADS of the Company issued or issuable with respect to the Conversion Shares, including, without limitation, (1) as a result of any stock split, stock dividend, recapitalization, exchange or similar event or otherwise and (2) shares of capital stock of the Company into which the shares of ADSs are converted or exchanged without regard to any limitations on conversion of the Convertible Debentures.
(d) Fees. The Company shall reimburse the Buyers a one-time due diligence and structuring fee of $15,000. The Company authorizes each Buyer to deduct any fees due hereunder from the gross process of the purchase of any Convertible Debentures.
(e) Pledge of Securities. Notwithstanding anything to the contrary contained in this Agreement, the Company acknowledges and agrees that, subject to compliance with applicable federal and state securities laws, the Securities may be pledged by an Investor in connection with a bona fide margin agreement or other loan or financing arrangement that is secured by the Securities. The Company hereby agrees to execute and deliver such documentation as a pledgee of the Securities may reasonably request in connection with a pledge of the Securities to such pledgee by a Buyer.
(f) Disclosure of Transactions and Other Material Information. On or before 9:30 a.m., New York time, within four (4) Business Day after the date of this Agreement, the Company shall file a current report of foreign private issuer on Form 6-K describing all the material terms of the transactions contemplated by the Transaction Documents in the form required by the 1934 Act and attaching all the material Transaction Documents (including, without limitation, this Agreement (and all schedules to this Agreement) and the form of Statement of Designations) (including all attachments, the “Current Report”). From and after the filing of the Current Report, the Company shall have disclosed all material, non-public information (if any) provided to any of the Buyers by the Company or any of its Subsidiaries or any of their respective officers, directors, employees or agents in connection with the transactions contemplated by the Transaction Documents. In addition, effective upon the filing of the Current Report, the Company acknowledges and agrees that any and all confidentiality or similar obligations with respect to the transactions contemplated by the Transaction Documents under any agreement, whether written or oral, between the Company, any of its Subsidiaries or any of their respective officers, directors, affiliates, employees or agents, on the one hand, and any of the Buyers or any of their affiliates, on the other hand, shall terminate. The Company shall not, and the Company shall cause each of its Subsidiaries and each of its and their respective officers, directors, employees and agents not to, provide any Buyer with any material, non-public information regarding the Company or any of its Subsidiaries from and after the date hereof without the express prior written consent of such Buyer (which may be granted or withheld in such Buyer’s sole discretion).
(g) Reservation of Shares. So long as any of the Convertible Debentures remain outstanding, the Company shall take all action necessary to at all times have authorized, and reserved for the purpose of issuance, no less than the lesser of 100% of the maximum number of shares of ADSs issuable upon conversion of all the Convertible Debentures then outstanding (assuming for purposes hereof that (x) the Convertible Debentures are convertible at the Conversion Price then in effect, and (y) any such conversion shall not take into account any limitations on the conversion of the Convertible Debentures, including the Floor Price) (the “Required Reserve Amount”); provided that at no time shall the number of ADSs reserved pursuant to this Section 4(g) be reduced other than proportionally in connection with any conversion and/or redemption, or reverse stock split. If at any time the number of shares of ADSs authorized and reserved for issuance is not sufficient to meet the Required Reserved Amount, the Company will promptly take all corporate action necessary to authorize and reserve a sufficient number of shares, including, without limitation, calling a special meeting of stockholders to authorize additional shares to meet the Company’s obligations pursuant to the Transaction Documents, in the case of an insufficient number of authorized shares, recommending that stockholders vote in favor of an increase in such authorized number of shares sufficient to meet the Required Reserved Amount.
(h) Conduct of Business. The business of the Company and its Subsidiaries shall not be conducted in violation of any law, ordinance or regulation of any Governmental Entity, except where such violations would not reasonably be expected to result, either individually or in the aggregate, in a Material Adverse Effect.
(i) Subsequent Equity Sales. So long as any of the Convertible Debentures remain outstanding, the Company shall be prohibited from effecting or entering into an agreement to effect any issuance by the Company or any of its subsidiaries of ADSs or equivalents (or a combination of units thereof) involving a Variable Rate Transaction (as defined below). Notwithstanding the foregoing, Variable Rate Transactions shall be permitted if there is no shelf registration statement on Form F-3 declared effective by the SEC prior to February 28, 2021, provided however that proceeds from such subsequent Variable Rate Financing shall be used to repay no less than 45% of outstanding Convertible Debentures and any accrued interest.
“Variable Rate Transaction” means a transaction in which the Company (i) issues or sells any debt or equity securities that are convertible into, exchangeable or exercisable for, or include the right to receive additional ADSs either (A) at a conversion price, exercise price or exchange rate or other price that is based upon and/or varies with the trading prices of or quotations for the ADSs at any time after the initial issuance of such debt or equity securities, or (B) with a conversion, exercise or exchange price that is subject to being reset at some future date after the initial issuance of such debt or equity security or upon the occurrence of specified or contingent events directly or indirectly related to the business of the Company or the market for the ADSs or (ii) enters into, or effects a transaction under, any agreement, including, but not limited to, an equity line of credit or at-the-market offering facility, whereby the Company may issue securities at a future determined price.
5. REGISTER; TRANSFER AGENT INSTRUCTIONS; LEGEND.
(a) Register. The Company shall maintain at its principal executive offices or with the Transfer Agent (or at such other office or agency of the Company as it may designate by notice to each holder of Securities), a register for the Convertible Debentures in which the Company shall record the name and address of the Person in whose name the Convertible Debentures have been issued (including the name and address of each transferee), the amount of Convertible Debentures held by such Person, and the number of Conversion Shares issuable upon conversion of the Convertible Debentures held by such Person. The Company shall keep the register open and available at all times during business hours for inspection of any Buyer or its legal representatives.
(b) Transfer Restrictions. The Securities may only be disposed of in compliance with state and federal securities laws. In connection with any transfer of Securities other than pursuant to an effective registration statement or Rule 144, to the Company or to an Affiliate of a Buyer or in connection with a pledge as contemplated herein, the Company may require the transferor thereof to provide to the Company an opinion of counsel selected by the transferor and reasonably acceptable to the Company, the form and substance of which opinion shall be reasonably satisfactory to the Company, to the effect that such transfer does not require registration of such transferred Securities under the Securities Act. As a condition of transfer, any such transferee shall agree in writing to be bound by the terms of this Agreement and shall have the rights and obligations of a Buyer under this Agreement.
6. CONDITIONS TO THE COMPANY’S OBLIGATION TO SELL.
The obligation of the Company hereunder to issue and sell the Convertible Debentures to each Buyer at each Closing is subject to the satisfaction, at or before each Closing Date, of each of the following conditions, provided that these conditions are for the Company’s sole benefit and may be waived by the Company at any time in its sole discretion by providing each Buyer with prior written notice thereof:
(a) Such Buyer shall have executed each of the Transaction Documents to which it is a party and delivered the same to the Company.
(b) Such Buyer and each other Buyer shall have delivered to the Company the Purchase Price (less, in the case of any Buyer, the amounts withheld pursuant to Section 4(d) and expenses designated by the Company) for the Convertible Debentures being purchased by such Buyer at the Closing by wire transfer of immediately available funds in accordance with the Closing Statement.
(c) The representations and warranties of such Buyer shall be true and correct in all material respects as of the date when made and as of each Closing Date as though originally made at that time (except for representations and warranties that speak as of a specific date, which shall be true and correct as of such specific date), and such Buyer shall have performed, satisfied and complied in all material respects with the covenants, agreements and conditions required by this Agreement to be performed, satisfied or complied with by such Buyer at or prior to such Closing Date.
7. CONDITIONS TO EACH BUYER’S OBLIGATION TO PURCHASE.
The obligation of each Buyer hereunder to purchase its Convertible Debentures at each Closing is subject to the satisfaction, at or before each Closing Date, of each of the following conditions, provided that these conditions are for each Buyer’s sole benefit and may be waived by such Buyer at any time in its sole discretion by providing the Company with prior written notice thereof:
(a) The Company shall have duly executed and delivered to such Buyer each of the Transaction Documents to which it is a party and the Company shall have duly executed and delivered to such Buyer such aggregate principal amount of Convertible Debentures as is set forth opposite such Buyer’s name in column (b) of the Schedule of Buyers for each Closing.
(b) The Company shall have delivered to such Buyer a certificate evidencing the incorporation and good standing of the Company issued by the Registrar for the British Virgin Islands as of a date within ten (10) days of the Closing Date.
(c) The ADSs (A) shall be designated for quotation or listed (as applicable) on the Principal Market and (B) shall not have been suspended, as of the Closing Date, by the SEC or the Principal Market from trading on the Principal Market nor shall suspension by the SEC or the Principal Market have been threatened, as of each Closing Date, either (I) in writing by the SEC or the Principal Market or (II) by falling below the minimum maintenance requirements of the Principal Market.
(d) The Company shall have obtained all governmental, regulatory or third-party consents and approvals, if any, necessary for the sale of the Securities, including without limitation, those required by the Principal Market, if any.
(e) No statute, rule, regulation, executive order, decree, ruling or injunction shall have been enacted, entered, promulgated or endorsed by any court or Governmental Entity of competent jurisdiction that prohibits the consummation of any of the transactions contemplated by the Transaction Documents.
(f) Since the date of execution of this Agreement, no event or series of events shall have occurred that has resulted in or would reasonably be expected to result in a Material Adverse Effect.
(g) Such Buyer shall have received a letter, duly executed by an officer of the Company, setting forth the wire amounts of each Buyer and the wire transfer instructions of the Company (the “Closing Statement”).
(h) From the date hereof to the applicable Closing Date, (i) trading in the ADSs shall not have been suspended by the SEC or the Principal Market (except for any suspension of trading of limited duration agreed to by the Company, which suspension shall be terminated prior to the Closing), and (ii) at any time prior to the applicable Closing Date, trading in securities generally as reported by Bloomberg L.P. shall not have been suspended or limited, or minimum prices shall not have been established on securities whose trades are reported by such service, or on the Principal Market, nor shall a banking moratorium have been declared either by the United States or New York State authorities nor shall there have occurred any material outbreak or escalation of hostilities or other national or international calamity of such magnitude in its effect on, or any material adverse change in, any financial market which, in each case, in the reasonable judgment of each Buyer, makes it impracticable or inadvisable to purchase the Securities at the Closing.
(i) The Company and its Subsidiaries shall have delivered to such Buyer such other documents, instruments or certificates relating to the transactions contemplated by this Agreement as such Buyer or its counsel may reasonably request.
(j) The Company shall use its best efforts to cause the Registration Statement be declared effective by the SEC within sixty (60) days of the Closing Date.
8. TERMINATION.
In the event that the Closing shall not have occurred with respect to a Buyer within five (5) days of the date hereof due to Company’s failure to deliver the Transaction Documents or satisfy the conditions to the Closing, then such Buyer shall have the right to terminate its obligations under this Agreement with respect to itself at any time on or after the close of business on such date without liability of such Buyer to any other party; provided, however, (i) the right to terminate this Agreement under this Section 8 shall not be available to such Buyer if the failure of the transactions contemplated by this Agreement to have been consummated by such date is the result of such Buyer’s breach of this Agreement and (ii) the abandonment of the sale and purchase of the Convertible Debentures shall be applicable only to such Buyer providing such written notice, provided further that no such termination shall affect any obligation of the Company under this Agreement to reimburse such Buyer for the expenses described herein. Nothing contained in this Section 8 shall be deemed to release any party from any liability for any breach by such party of the terms and provisions of this Agreement or the other Transaction Documents or to impair the right of any party to compel specific performance by any other party of its obligations under this Agreement or the other Transaction Documents.
9. MISCELLANEOUS.
(a) Governing Law; Jurisdiction; Jury Trial. All questions concerning the construction, validity, enforcement and interpretation of this Agreement shall be governed by the internal laws of the State of New York, without giving effect to any choice of law or conflict of law provision or rule (whether of the State of New York or any other jurisdictions) that would cause the application of the laws of any jurisdictions other than the State of New York. The Company hereby irrevocably submits to the exclusive jurisdiction of the state and federal courts sitting in The City of New York, Borough of Manhattan, for the adjudication of any dispute hereunder or in connection herewith or under any of the other Transaction Documents or with any transaction contemplated hereby or thereby, and hereby irrevocably waives, and agrees not to assert in any suit, action or proceeding, any claim that it is not personally subject to the jurisdiction of any such court, that such suit, action or proceeding is brought in an inconvenient forum or that the venue of such suit, action or proceeding is improper. Each party hereby irrevocably waives personal service of process and consents to process being served in any such suit, action or proceeding by mailing a copy thereof to such party at the address for such notices to it under this Agreement and agrees that such service shall constitute good and sufficient service of process and notice thereof. Nothing contained herein shall be deemed to limit in any way any right to serve process in any manner permitted by law. Nothing contained herein shall be deemed or operate to preclude any Buyer from bringing suit or taking other legal action against the Company in any other jurisdiction to collect on the Company’s obligations to such Buyer or to enforce a judgment or other court ruling in favor of such Buyer. EACH PARTY HEREBY IRREVOCABLY WAIVES ANY RIGHT IT MAY HAVE TO, AND AGREES NOT TO REQUEST, A JURY TRIAL FOR THE ADJUDICATION OF ANY DISPUTE HEREUNDER OR UNDER ANY OTHER TRANSACTION DOCUMENT OR IN CONNECTION WITH OR ARISING OUT OF THIS AGREEMENT, ANY OTHER TRANSACTION DOCUMENT OR ANY TRANSACTION CONTEMPLATED HEREBY OR THEREBY.
(b) Counterparts. This Agreement may be executed in two or more identical counterparts, all of which shall be considered one and the same agreement and shall become effective when counterparts have been signed by each party and delivered to the other party. In the event that any signature is delivered by facsimile transmission or by an e-mail which contains a portable document format (.pdf) file of an executed signature page, such signature page shall create a valid and binding obligation of the party executing (or on whose behalf such signature is executed) with the same force and effect as if such signature page were an original thereof.
(c) Headings; Gender. The headings of this Agreement are for convenience of reference and shall not form part of, or affect the interpretation of, this Agreement. Unless the context clearly indicates otherwise, each pronoun herein shall be deemed to include the masculine, feminine, neuter, singular and plural forms thereof. The terms “including,” “includes,” “include” and words of like import shall be construed broadly as if followed by the words “without limitation.” The terms “herein,” “hereunder,” “hereof” and words of like import refer to this entire Agreement instead of just the provision in which they are found.
(d) Entire Agreement, Amendments. This Agreement supersedes all other prior oral or written agreements between the Buyer, the Company, their affiliates and persons acting on their behalf with respect to the matters discussed herein, and this Agreement and the instruments referenced herein contain the entire understanding of the parties with respect to the matters covered herein and therein and, except as specifically set forth herein or therein, neither the Company nor any Buyer makes any representation, warranty, covenant or undertaking with respect to such matters. No provision of this Agreement may be waived or amended other than by an instrument in writing signed by the party to be charged with enforcement.
(e) Notices. Any notices, consents, waivers or other communications required or permitted to be given under the terms of this Agreement must be in writing by letter and email and will be deemed to have been delivered: upon the later of (A) either (i) receipt, when delivered personally or (ii) one (1) Business Day after deposit with an overnight courier service with next-day international delivery specified, in each case, properly addressed to the party to receive the same and (B) receipt, when sent by electronic mail.
(f) Successors and Assigns. This Agreement shall be binding upon and inure to the benefit of the parties and their respective successors and assigns, including any purchasers of any of the Convertible Debentures (but excluding any purchasers of Underlying Securities, unless pursuant to a written assignment by such Buyer). The Company shall not assign this Agreement or any rights or obligations hereunder without the prior written consent of the Buyers. In connection with any transfer of any or all of its Securities, a Buyer may assign all, or a portion, of its rights and obligations hereunder in connection with such Securities without the consent of the Company, in which event such assignee shall be deemed to be a Buyer hereunder with respect to such transferred Securities.
(g) No Strict Construction. The language used in this Agreement will be deemed to be the language chosen by the parties to express their mutual intent, and no rules of strict construction will be applied against any party.
[REMAINDER PAGE INTENTIONALLY LEFT BLANK]
IN WITNESS WHEREOF, each Buyer and the Company have caused their respective signature page to this Securities Purchase Agreement to be duly executed as of the date first written above.
|
|
COMPANY: | |
|
|
| |
|
|
AnPac Bio-Medical Science Co., Ltd. | |
|
|
| |
|
|
By: |
/s/ Chris Chang Yu |
|
|
Name: |
Chris Chang Yu |
|
|
Title: |
CEO |
IN WITNESS WHEREOF, each Buyer and the Company have caused their respective signature page to this Securities Purchase Agreement to be duly executed as of the date first written above.
|
|
BUYER: | |
|
|
| |
|
|
Hongyu Wang | |
|
|
| |
|
|
By: |
/s/ Hongyu Wang |
|
|
Name: |
Hongyu Wang |
|
|
Title: |
|
IN WITNESS WHEREOF, each Buyer and the Company have caused their respective signature page to this Securities Purchase Agreement to be duly executed as of the date first written above.
|
|
BUYER: | |
|
|
| |
|
|
Heng Zhang | |
|
|
| |
|
|
By: |
/s/ Heng Zhang |
|
|
Name: |
Heng Zhang |
|
|
Title: |
|
IN WITNESS WHEREOF, each Buyer and the Company have caused their respective signature page to this Securities Purchase Agreement to be duly executed as of the date first written above.
|
|
BUYER: | |
|
|
| |
|
|
Jie Wang | |
|
|
| |
|
|
By: |
/s/ Jie Wang |
|
|
Name: |
Jie Wang |
|
|
Title: |
|
IN WITNESS WHEREOF, each Buyer and the Company have caused their respective signature page to this Securities Purchase Agreement to be duly executed as of the date first written above.
|
|
BUYER: | |
|
|
| |
|
|
Layette Holdings Inc | |
|
|
| |
|
|
By: |
/s/ Layette Holdings Inc. |
|
|
Name: |
Layette Holdings Inc. |
|
|
Title: |
|
EXHIBIT I
CONVERSION NOTICE
(To be executed by the Holder in order to Convert the Debenture)
TO: AnPac Bio-Medical Science Co., Ltd.
Via Email:
The undersigned hereby irrevocably elects to convert a portion of the outstanding and unpaid Conversion Amount of Debenture No. ANPC-[-] into ADSs of AnPac Bio-Medical Science Co., Ltd., according to the conditions stated therein, as of the Conversion Date written below.
Conversion Date:
Principal Amount to be Converted:
Accrued Interest to be Converted:
Total Conversion Amount to be converted:
Fixed Conversion Price:
Variable Conversion Price:
Applicable Conversion Price:
Number of ADSs to be issued:
Please issue the ADSs in the following name and to the following address: Issue to:
|
Authorized Signature: |
|
|
|
|
|
Name: |
|
|
|
|
|
Title: |
|
|
|
|
|
Broker DTC Participant Code: |
|
|
|
|
|
Account Number: |
|
SCHEDULE OF BUYERS
|
|
|
(b) |
|
(c) |
| ||
|
|
|
Principal Amount |
|
Purchase Price |
| ||
|
(a) |
|
of Convertible |
|
(85% of Face |
| ||
|
Buyer |
|
Debentures |
|
Value) |
| ||
|
Heng Zhang |
|
$ |
550,000 |
|
$ |
467,5000 |
|
|
Layette Holdings Inc. |
|
$ |
300,000 |
|
$ |
255,000 |
|
|
Jie Wang |
|
$ |
150,000 |
|
$ |
127,500 |
|
|
Hongyu Wang |
|
$ |
1,000,000 |
|
$ |
850,000 |
|
|
|
|
|
|
|
| ||
|
|
Aggregate: |
$ |
2,000,000.00 |
|
$ |
1,700,000.00 |
|
Form of Debentures Issued to Certain Investors
NEITHER THIS DEBENTURE NOR THE SECURITIES INTO WHICH THIS DEBENTURE IS CONVERTIBLE HAVE BEEN REGISTERED WITH THE SECURITIES AND EXCHANGE COMMISSION OR THE SECURITIES COMMISSION OF ANY STATE. THESE SECURITIES HAVE BEEN SOLD IN RELIANCE UPON AN EXEMPTION FROM REGISTRATION UNDER THE SECURITIES ACT OF 1933, AS AMENDED (THE “SECURITIES ACT”), AND, ACCORDINGLY, MAY NOT BE OFFERED OR SOLD EXCEPT PURSUANT TO AN EFFECTIVE REGISTRATION STATEMENT UNDER THE SECURITIES ACT OR PURSUANT TO AN AVAILABLE EXEMPTION FROM, OR IN A TRANSACTION NOT SUBJECT TO, THE REGISTRATION REQUIREMENTS OF THE SECURITIES ACT AND IN ACCORDANCE WITH APPLICABLE STATE SECURITIES LAWS.
ANPAC BIO-MEDICAL SCIENCE CO., LTD.
CONVERTIBLE DEBENTURE
Principal Amount: [ ]
Debenture Issuance Date: February 5, 2021
Debenture Number: ANPC-5
FOR VALUE RECEIVED, AnPac Bio-Medical Science Co., Ltd., a British Virgin Islands company (the “Company”), hereby promises to pay to the order of [ ], or its registered assigns (the “Holder”) the amount set out above as the Principal Amount (as reduced pursuant to the terms hereof pursuant to redemption, conversion or otherwise, the “Principal”) when due, whether upon the Maturity Date (as defined below), acceleration, redemption or otherwise (in each case in accordance with the terms hereof) and to pay interest (“Interest”) on any outstanding Principal at the applicable Interest Rate from the date set out above as the Debenture Issuance Date (the “Issuance Date”) until the same becomes due and payable, whether upon an Interest Date (as defined below), the Maturity Date or acceleration, conversion, redemption or otherwise (in each case in accordance with the terms hereof). This Convertible Debenture (including all debentures issued in exchange, transfer or replacement hereof, this “Debenture”) was originally issued pursuant to the Securities Purchase Agreement dated February 5, 2021 (the “Securities Purchase Agreement”) between the Company and the Buyers listed on the Schedule of Buyers attached thereto. Certain capitalized terms used herein are defined in Section (13).
(1) GENERAL TERMS
(a) Maturity Date. On the Maturity Date, the Company shall pay to the Holder an amount in cash representing all outstanding Principal, accrued and unpaid Interest, and any other amounts outstanding pursuant to the terms of this Debenture. The “Maturity Date” shall be February 4, 2022, as may be extended at the option of the Holder. Other than as specifically permitted by this Debenture, the Company may not prepay or redeem any portion of the outstanding Principal and accrued and unpaid Interest
(b) Interest Rate and Payment of Interest. Interest shall accrue on the outstanding Principal balance hereof at an annual rate equal to 0% (“Interest Rate”), which Interest Rate shall increase to an annual rate of 15% for any such day that the Closing Bid Price is below the Floor Price. Interest shall be calculated on the basis of a 365-day year and the actual number of days elapsed, to the extent permitted by applicable law.
(c) Early Redemption. The Company shall have the right, but not the obligation, to redeem (“Optional Redemption”) early a portion or all amounts outstanding under this Debenture as described in this Section; provided that the Company provides the Holder with at least 5 Business Days’ prior written notice (each, a “Redemption Notice”) of its desire to exercise an Optional Redemption. Each Redemption Notice shall be irrevocable and shall specify the outstanding balance of the Convertible Debentures to be redeemed and the applicable Redemption Premium. The “Redemption Amount” shall be equal to the outstanding Principal balance being redeemed by the Company, plus the applicable Redemption Premium, plus all accrued and unpaid interest. After receipt of the Redemption Notice, the Holder shall have 5 Business Days to elect to convert all or any portion of Convertible Debentures. On the 6th Business Day after the Redemption Notice, the Company shall deliver to the Holder the Redemption Amount with respect to the Principal amount redeemed after giving effect to conversions effected during the 5 Business Day period.
(2) EVENTS OF DEFAULT.
(a) The outstanding amount under this Debenture shall increase by 10% in an Event of Default and if the Company fails to cure such default within five (5) Trading Dyas. An “Event of Default”, wherever used herein, means any one of the following events (whatever the reason and whether it shall be voluntary or involuntary or effected by operation of law or pursuant to any judgment, decree or order of any court, or any order, rule or regulation of any administrative or governmental body):
(i) the Company’s failure to pay to the Holder any amount of Principal, Interest, or other amounts when and as due under this Debenture or any other Transaction Document within five (5) Business Days after such payment is due;
(ii) the Company or any subsidiary of the Company shall commence, or there shall be commenced against the Company or any subsidiary of the Company under any applicable bankruptcy or insolvency laws as now or hereafter in effect or any successor thereto, or the Company or any subsidiary of the Company commences any other proceeding under any reorganization, arrangement, adjustment of debt, relief of debtors, dissolution, insolvency or liquidation or similar law of any jurisdiction whether now or hereafter in effect relating to the Company or any subsidiary of the Company or there is commenced against the Company or any subsidiary of the Company any such bankruptcy, insolvency or other proceeding which remains undismissed for a period of 61 days; or the Company or any subsidiary of the Company is adjudicated insolvent or bankrupt; or any order of relief or other order approving any such case or proceeding is entered; or the Company or any subsidiary of the Company suffers any appointment of any custodian, private or court appointed receiver or the like for it or any substantial part of its property which continues undischarged or unstayed for a period of sixty one (61) days; or the Company or any subsidiary of the Company makes a general assignment for the benefit of creditors; or the Company or any subsidiary of the Company shall fail to pay, or shall state that it is unable to pay, or shall be unable to pay, its debts generally as they become due; or the Company or any subsidiary of the Company shall call a meeting of its creditors with a view to arranging a composition, adjustment or restructuring of its debts; or the Company or any subsidiary of the Company shall by any act or failure to act expressly indicate its consent to, approval of or acquiescence in any of the foregoing; or any corporate or other action is taken by the Company or any subsidiary of the Company for the purpose of effecting any of the foregoing;
(iii) the ADS shall cease to be quoted or listed for trading, as applicable, on any Primary Market for a period of 10 consecutive Trading Days;
(iv) the Company or any subsidiary of the Company shall be a party to any Change of Control Transaction (as defined in Section (13) unless in connection with such Change of Control Transaction this Debenture is retired;
(v) the Company’s (A) failure to cure a Conversion Failure by delivery of (I) the required number of ADSs or (II) the Buy-In Price within five (5) Business Days after the applicable Conversion Failure or (B) notice, written or oral, to any holder of the Debentures, including by way of public announcement, at any time, of its intention not to comply with a request for conversion of any Debentures into ADSs that is tendered in accordance with the provisions of the Debentures, other than pursuant to Section (4)(b);
(vi) the Company shall fail for any reason to deliver the payment in cash pursuant to a Buy-In (as defined herein) within five (5) Business Days after such payment is due;
(vii) the Company shall fail to observe or perform any other material covenant, agreement or warranty contained in, or otherwise commit any material breach or default of any provision of this Debenture or any Transaction Document (as defined in Section (13)) which is not cured within the time prescribed; or
(viii) the Company shall fail to have an effective registration statement covering the resale of the Underlying Shares prior to April 16, 2021.
(3) CONVERSION OF DEBENTURE. This Debenture shall be convertible into ADSs, on the terms and conditions set forth in this Section (3).
(a) Conversion Right. Subject to the provisions of Section (3)(c), at any time or times on or after the Issuance Date, the Holder shall be entitled to convert any portion of the outstanding and unpaid Conversion Amount (as defined below) into fully paid and nonassessable ADSs in accordance with Section (3)(b), at the Conversion Rate (as defined below). The number of ADSs issuable upon conversion of any Conversion Amount pursuant to this Section (3)(a) shall be determined by dividing (x) such Conversion Amount by (y) the Conversion Price (the “Conversion Rate”). The Company shall not issue any fraction of ADS upon any conversion. All calculations under this Section (3) shall be rounded to the nearest $0.0001. If the issuance would result in the issuance of a fraction of ADS, the Company shall round such fraction of an ADS up to the nearest whole share.
(i) “Conversion Amount” means the portion of the Principal and accrued Interest to be converted, redeemed or otherwise with respect to which this determination is being made.
(ii) “Conversion Price” means, as of any Conversion Date (as defined below) or other date of determination the lower of (i) $15.00 (the “Fixed Conversion Price”), or (ii) the lower of (x) 82% of the Closing Bid Price or (y) 80% of the VWAPs during the 10 consecutive Trading Days, immediately preceding the Conversion Date or other date of determination (the “Variable Conversion Price”), but not lower than the Floor Price. Subject to the Floor Price, the Variable Conversion Price shall be 75% of the VWAPs during the 10 consecutive Trading Days, immediately preceding the Conversion Date or other date of determination if the Company shall trigger an Event of Default pursuant to Section 2(a)(viii). The Conversion Price shall be adjusted from time to time pursuant to the other terms and conditions of this Debenture.
(b) Mechanics of Conversion.
(i) Optional Conversion. To convert any Conversion Amount into ADSs on any date (a “Conversion Date”), the Holder shall (A) transmit by facsimile (or otherwise deliver), for receipt on or prior to 11:59 p.m., New York Time, on such date, a copy of an executed notice of conversion in the form attached hereto as Exhibit I (the “Conversion Notice”) to the Company and (B) if required by Section (3)(b)(iii), surrender this Debenture to a nationally recognized overnight delivery service for delivery to the Company (or an indemnification undertaking reasonably satisfactory to the Company with respect to this Debenture in the case of its loss, theft or destruction). On or before the third Business Day following the date of receipt of a Conversion Notice (the “Share Delivery Date”), the Company shall (X) if legends are not required to be placed on certificates of ADSs and provided that the Transfer Agent is participating in the Depository Trust Company’s (“DTC”) Fast Automated Securities Transfer Program, credit such aggregate number of ADSs to which the Holder shall be entitled to the Holder’s or its designee’s balance account with DTC through its Deposit Withdrawal Agent Commission system or (Y) if the Transfer Agent is not participating in the DTC Fast Automated Securities Transfer Program, issue and deliver to the address as specified in the Conversion Notice, a certificate, registered in the name of the Holder or its designee, for the number of ADSs to which the Holder shall be entitled which certificates shall not bear any restrictive legends unless required pursuant to rules and regulations of the Commission. If this Debenture is physically surrendered for conversion and the outstanding Principal of this Debenture is greater than the Principal portion of the Conversion Amount being converted, then the Company shall as soon as practicable and in no event later than three (3) Business Days after receipt of this Debenture and at its own expense, issue and deliver to the holder a new Debenture representing the outstanding Principal not converted. The Person or Persons entitled to receive the ADSs issuable upon a conversion of this Debenture shall be treated for all purposes as the record holder or holders of such ADSs upon the transmission of a Conversion Notice.
(ii) Company’s Failure to Timely Convert. If within three (3) Trading Days after the Company’s receipt of the facsimile copy of a Conversion Notice the Company shall fail to issue and deliver a certificate to the Holder or credit the Holder’s balance account with DTC for the number of ADSs to which the Holder is entitled upon such holder’s conversion of any Conversion Amount (a “Conversion Failure”), and if on or after such Trading Day the Holder purchases (in an open market transaction or otherwise) ADSs to deliver in satisfaction of a sale by the Holder of ADSs issuable upon such conversion that the Holder anticipated receiving from the Company (a “Buy-In”), then the Company shall, within three (3) Business Days after the Holder’s request and in the Holder’s discretion, either (i) pay cash to the Holder in an amount equal to the Holder’s total purchase price (including brokerage commissions and other out of pocket expenses, if any) for the ADSs so purchased (the “Buy-In Price”), at which point the Company’s obligation to deliver such certificate (and to issue such ADSs) shall terminate, or (ii) promptly honor its obligation to deliver to the Holder a certificate or certificates representing such ADSs and pay cash to the Holder in an amount equal to the excess (if any) of the Buy-In Price over the product of (A) such number of ADSs, times (B) the Closing Bid Price on the Conversion Date.
(iii) Book-Entry. Notwithstanding anything to the contrary set forth herein, upon conversion of any portion of this Debenture in accordance with the terms hereof, the Holder shall not be required to physically surrender this Debenture to the Company unless (A) the full Conversion Amount represented by this Debenture is being converted or (B) the Holder has provided the Company with prior written notice (which notice may be included in a Conversion Notice) requesting reissuance of this Debenture upon physical surrender of this Debenture. The Holder and the Company shall maintain records showing the Principal and Interest converted and the dates of such conversions or shall use such other method, reasonably satisfactory to the Holder and the Company, so as not to require physical surrender of this Debenture upon conversion.
(c) Limitations on Conversions.
(i) Beneficial Ownership. The Holder shall not have the right to convert any portion of this Debenture or receive ADSs hereunder to the extent that after giving effect to such conversion or receipt of such Shares, the Holder, together with any affiliate thereof, would beneficially own (as determined in accordance with Section 13(d) of the Exchange Act and the rules promulgated thereunder) in excess of 4.99% of the number of ADSs outstanding immediately after giving effect to such conversion or receipt of shares as payment of interest. Since the Holder will not be obligated to report to the Company the number of ADSs it may hold at the time of a conversion hereunder, unless the conversion at issue would result in the issuance of ADSs in excess of 4.99% of the then outstanding ADSs without regard to any other shares which may be beneficially owned by the Holder or an affiliate thereof, the Holder shall have the authority and obligation to determine whether the restriction contained in this Section will limit any particular conversion hereunder and to the extent that the Holder determines that the limitation contained in this Section applies, the determination of which portion of the Principal amount of this Debenture is convertible shall be the responsibility and obligation of the Holder. If the Holder has delivered a Conversion Notice for a Principal amount of this Debenture that, without regard to any other shares that the Holder or its affiliates may beneficially own, would result in the issuance in excess of the permitted amount hereunder, the Company shall notify the Holder of this fact and shall honor the conversion for the maximum Principal amount permitted to be converted on such Conversion Date in accordance with Section (3)(a) and, any Principal amount tendered for conversion in excess of the permitted amount hereunder shall remain outstanding under this Debenture. The provisions of this Section may be waived by a Holder (but only as to itself and not to any other Holder) upon not less than 65 days prior notice to the Company. Other Holders shall be unaffected by any such waiver.
(ii) Nasdaq Rule 5635(d) Limitations. The Company shall not issue any ADS pursuant to the terms of this Debenture if the issuance of such ADSs would exceed the aggregate number of ADSs that the Company may issue upon conversion of the Debenture and the Other Debentures in compliance with the Company’s obligations under the rules or regulations of the Nasdaq Capital Market (the number of shares which may be issued without violating such rules and regulations is 2,402,949 ordinary shares and shall be referred to as the “Exchange Cap”), except that such limitation shall not apply in the event that the Company (A) obtains the approval of its stockholders as required by the applicable rules of the Nasdaq Capital Market for issuances of ADSs in excess of such amount or (B) obtains a written opinion from outside counsel to the Company that such approval is not required, which opinion shall be reasonably satisfactory to the Holder.
(d) Other Provisions.
(i) The Company shall at all times reserve and keep available out of its authorized ADSs the full number of ADSs issuable upon conversion of all outstanding amounts under this Debenture; and within three (3) Business Days following the receipt by the Company of a Holder’s notice that such minimum number of Underlying Shares is not so reserved, the Company shall promptly reserve a sufficient number of ADSs to comply with such requirement.
(ii) All calculations under this Section (3) shall be rounded to the nearest $0.0001 or whole share.
(iii) The Company covenants that it will at all times reserve and keep available out of its authorized and unissued ADSs solely for the purpose of issuance upon conversion of this Debenture and payment of interest on this Debenture, each as herein provided, free from preemptive rights or any other actual contingent purchase rights of persons other than the Holder, not less than such number of shares of the ADSs as shall be issuable (taking into account the adjustments and restrictions set forth herein) upon the conversion of the outstanding Principal amount of this Debenture and payment of interest hereunder. The Company covenants that all ADSs that shall be so issuable shall, upon issue, be duly and validly authorized, issued and fully paid, nonassessable.
(iv) Nothing herein shall limit a Holder’s right to pursue actual damages or declare an Event of Default pursuant to Section (2) herein for the Company’s failure to deliver certificates representing ADSs upon conversion within the period specified herein and such Holder shall have the right to pursue all remedies available to it at law or in equity including, without limitation, a decree of specific performance and/or injunctive relief, in each case without the need to post a bond or provide other security. The exercise of any such rights shall not prohibit the Holder from seeking to enforce damages pursuant to any other Section hereof or under applicable law.
(4) Adjustments to Conversion Price
(a) Adjustment of Conversion Price upon Subdivision of ADSs. If the Company, at any time while this Debenture is outstanding, shall (a) pay a stock dividend or otherwise make a distribution or distributions on ADSs or any other equity or equity equivalent securities payable in ADSs, (b) subdivide outstanding ADSs into a larger number of shares, (c) combine (including by way of reverse stock split) outstanding ADSs into a smaller number of shares, or (d) issue by reclassification of shares of the ADSs any shares of capital stock of the Company, then each of the Fixed Conversion Price and the Floor Price shall be multiplied by a fraction of which the numerator shall be the number of ADSs (excluding treasury shares, if any) outstanding before such event and of which the denominator shall be the number of ADSs outstanding after such event. Any adjustment made pursuant to this Section shall become effective immediately after the record date for the determination of stockholders entitled to receive such dividend or distribution and shall become effective immediately after the effective date in the case of a subdivision, combination or re-classification.
(b) Other Corporate Events. In addition to and not in substitution for any other rights hereunder, prior to the consummation of any Fundamental Transaction pursuant to which holders of ADSs are entitled to receive securities or other assets with respect to or in exchange for ADSs (a “Corporate Event”), the Company shall make appropriate provision to ensure that the Holder will thereafter have the right to receive upon a conversion of this Debenture, at the Holder’s option, (i) in addition to the ADSs receivable upon such conversion, such securities or other assets to which the Holder would have been entitled with respect to such ADSs had such ADSs been held by the Holder upon the consummation of such Corporate Event (without taking into account any limitations or restrictions on the convertibility of this Debenture) or (ii) in lieu of the ADSs otherwise receivable upon such conversion, such securities or other assets received by the holders of ADSs in connection with the consummation of such Corporate Event in such amounts as the Holder would have been entitled to receive had this Debenture initially been issued with conversion rights for the form of such consideration (as opposed to ADSs) at a conversion rate for such consideration commensurate with the Conversion Rate. Provision made pursuant to the preceding sentence shall be in a form and substance satisfactory to the Required Holders. The provisions of this Section shall apply similarly and equally to successive Corporate Events and shall be applied without regard to any limitations on the conversion or redemption of this Debenture.
(c) Whenever the Conversion Price is adjusted pursuant to Section (4) hereof, the Company shall promptly mail to the Holder a notice setting forth the Conversion Price after such adjustment and setting forth a brief statement of the facts requiring such adjustment.
(d) In case of any (1) merger or consolidation of the Company or any subsidiary of the Company with or into another Person, or (2) sale by the Company or any subsidiary of the Company of more than one-half of the assets of the Company in one or a series of related transactions, a Holder shall have the right to (A) exercise any rights, (B) convert the aggregate amount of this Debenture then outstanding into the shares of stock and other securities, cash and property receivable upon or deemed to be held by holders of ADSs following such merger, consolidation or sale, and such Holder shall be entitled upon such event or series of related events to receive such amount of securities, cash and property as the ADSs into which such aggregate Principal amount of this Debenture could have been converted immediately prior to such merger, consolidation or sales would have been entitled, or (C) in the case of a merger or consolidation, require the surviving entity to issue to the Holder a convertible Debenture with a Principal amount equal to the aggregate Principal amount of this Debenture then held by such Holder, plus all accrued and unpaid interest and other amounts owing thereon, which such newly issued convertible Debenture shall have terms identical (including with respect to conversion) to the terms of this Debenture, and shall be entitled to all of the rights and privileges of the Holder of this Debenture set forth herein and the agreements pursuant to which this Debentures were issued. In the case of clause (C), the conversion price applicable for the newly issued shares of convertible preferred stock or convertible Debentures shall be based upon the amount of securities, cash and property that each share of ADSs would receive in such transaction and the Conversion Price in effect immediately prior to the effectiveness or closing date for such transaction. The terms of any such merger, sale or consolidation shall include such terms so as to continue to give the Holder the right to receive the securities, cash and property set forth in this Section upon any conversion or redemption following such event. This provision shall similarly apply to successive such events.
(5) REISSUANCE OF THIS DEBENTURE.
(a) Transfer. If this Debenture is to be transferred, the Holder shall surrender this Debenture to the Company, whereupon the Company will forthwith issue and deliver upon the order of the Holder a new Debenture, registered in the name of the registered transferee or assignee, representing the outstanding Principal being transferred by the Holder (along with any accrued and unpaid interest thereof) and, if less then the entire outstanding Principal is being transferred, a new Debenture to the Holder representing the outstanding Principal not being transferred. The Holder and any assignee, by acceptance of this Debenture, acknowledge and agree that, by reason of the provisions of Section (3)(b)(iii) following conversion or redemption of any portion of this Debenture, the outstanding Principal represented by this Debenture may be less than the Principal stated on the face of this Debenture.
(b) Lost, Stolen or Mutilated Debenture. Upon receipt by the Company of evidence reasonably satisfactory to the Company of the loss, theft, destruction or mutilation of this Debenture, and, in the case of loss, theft or destruction, of any indemnification undertaking by the Holder to the Company in customary form and, in the case of mutilation, upon surrender and cancellation of this Debenture, the Company shall execute and deliver to the Holder a new Debenture representing the outstanding Principal.
(c) Debenture Exchangeable for Different Denominations. This Debenture is exchangeable, upon the surrender hereof by the Holder at the principal office of the Company, for a new Debenture or Debentures representing in the aggregate the outstanding Principal of this Debenture, and each such new Debenture will represent such portion of such outstanding Principal as is designated by the Holder at the time of such surrender.
(6) NOTICES. Any notices, consents, waivers or other communications required or permitted to be given under the terms hereof must be in writing by letter and email and will be deemed to have been delivered: upon the later of (A) either (i) receipt, when delivered personally or (ii) one (1) Business Day after deposit with an overnight courier service with next-day international delivery specified, in each case, properly addressed to the party to receive the same and (B) receipt, when sent by electronic mail.
(7) Except as expressly provided herein, no provision of this Debenture shall alter or impair the obligations of the Company, which are absolute and unconditional, to pay the Principal of, interest and other charges (if any) on, this Debenture at the time, place, and rate, and in the coin or currency, herein prescribed. This Debenture is a direct obligation of the Company. As long as this Debenture is outstanding, the Company shall not and shall cause its subsidiaries not to, without the consent of the Holder, (i) amend its certificate of incorporation, memorandum or articles of association, bylaws or other charter documents so as to adversely affect any rights of the Holder; (ii) repay, repurchase or offer to repay, repurchase or otherwise acquire ADSs or other equity securities; or (iii) enter into any agreement with respect to any of the foregoing.
(8) This Debenture shall not entitle the Holder to any of the rights of a stockholder of the Company, including without limitation, the right to vote, to receive dividends and other distributions, or to receive any notice of, or to attend, meetings of stockholders or any other proceedings of the Company, unless and to the extent converted into ADSs in accordance with the terms hereof.
(9) This Debenture shall be governed by and construed in accordance with the laws of the State of New York, without giving effect to conflicts of laws thereof. Each of the parties consents to the jurisdiction of the Supreme Court of the State of New York located in the City of New York, Borough of Manhattan, and the U.S. District Court for the Southern District of New York in connection with any dispute arising under this Debenture and hereby waives, to the maximum extent permitted by law, any objection, including any objection based on forum non conveniens to the bringing of any such proceeding in such jurisdictions. THE PARTIES HEREBY KNOWINGLY, VOLUNTARILY AND INTENTIONALLY WAIVE THE RIGHT ANY OF THEM MAY HAVE TO A TRIAL BY JURY IN RESPECT OF ANY LITIGATION BASED HEREON OR ARISING OUT OF, UNDER OR IN CONNECTION WITH THIS AGREEMENT OR ANY TRANSACTION DOCUMENT OR ANY COURSE OF CONDUCT, COURSE OF DEALING, STATEMENTS (WHETHER VERBAL OR WRITTEN) OR ACTIONS OF ANY PARTY. THIS PROVISION IS A MATERIAL INDUCEMENT FOR THE PARTIES’ ACCEPTANCE OF THIS AGREEMENT.
(10) If the Company fails to strictly comply with the terms of this Debenture, then the Company shall reimburse the Holder promptly for all fees, costs and expenses, including, without limitation, attorneys’ fees and expenses incurred by the Holder in any action in connection with this Debenture, including, without limitation, those incurred: (i) during any workout, attempted workout, and/or in connection with the rendering of legal advice as to the Holder’s rights, remedies and obligations, (ii) collecting any sums which become due to the Holder, (iii) defending or prosecuting any proceeding or any counterclaim to any proceeding or appeal; or (iv) the protection, preservation or enforcement of any rights or remedies of the Holder.
(11) Any waiver by the Holder of a breach of any provision of this Debenture shall not operate as or be construed to be a waiver of any other breach of such provision or of any breach of any other provision of this Debenture. The failure of the Holder to insist upon strict adherence to any term of this Debenture on one or more occasions shall not be considered a waiver or deprive that party of the right thereafter to insist upon strict adherence to that term or any other term of this Debenture. Any waiver must be in writing.
(12) If any provision of this Debenture is invalid, illegal or unenforceable, the balance of this Debenture shall remain in effect, and if any provision is inapplicable to any person or circumstance, it shall nevertheless remain applicable to all other persons and circumstances. If it shall be found that any interest or other amount deemed interest due hereunder shall violate applicable laws governing usury, the applicable rate of interest due hereunder shall automatically be lowered to equal the maximum permitted rate of interest. The Company covenants (to the extent that it may lawfully do so) that it shall not at any time insist upon, plead, or in any manner whatsoever claim or take the benefit or advantage of, any stay, extension or usury law or other law which would prohibit or forgive the Company from paying all or any portion of the Principal of or interest on this Debenture as contemplated herein, wherever enacted, now or at any time hereafter in force, or which may affect the covenants or the performance of this indenture, and the Company (to the extent it may lawfully do so) hereby expressly waives all benefits or advantage of any such law, and covenants that it will not, by resort to any such law, hinder, delay or impeded the execution of any power herein granted to the Holder, but will suffer and permit the execution of every such as though no such law has been enacted.
(13) CERTAIN DEFINITIONS For purposes of this Debenture, the following terms shall have the following meanings:
(a) “ADS” means American Depository Share, each representing one Class A ordinary share in the capital of the Company with a par value of $0.01 each and shares of any other class into which such shares may hereafter be changed or reclassified.
(b) “Approved Stock Plan” means a stock option plan that has been approved by the Board of Directors of the Company, pursuant to which the Company’s securities may be issued only to any employee, officer, or director for services provided to the Company.
(c) “Bloomberg” means Bloomberg Financial Markets.
(d) “Business Day” means any day except Saturday, Sunday and any day which shall be a federal legal holiday in the United States or a day on which banking institutions are authorized or required by law or other government action to close.
(e) “Change of Control Transaction” means the occurrence of (a) an acquisition after the date hereof by an individual or legal entity or “group” (as described in Rule 13d-5(b)(1) promulgated under the Exchange Act) of effective control (whether through legal or beneficial ownership of capital stock of the Company, by contract or otherwise) of in excess of fifty percent (50%) of the voting securities of the Company (except that the acquisition of voting securities by the Holder or any other current holder of convertible securities of the Company shall not constitute a Change of Control Transaction for purposes hereof), (b) a replacement at one time or over time of more than one-half of the members of the board of directors of the Company (other than as due to the death or disability of a member of the board of directors) which is not approved by a majority of those individuals who are members of the board of directors on the date hereof (or by those individuals who are serving as members of the board of directors on any date whose nomination to the board of directors was approved by a majority of the members of the board of directors who are members on the date hereof), (c) the merger, consolidation or sale of fifty percent (50%) or more of the assets of the Company or any subsidiary of the Company in one or a series of related transactions with or into another entity, or (d) the execution by the Company of an agreement to which the Company is a party or by which it is bound, providing for any of the events set forth above in (a), (b) or (c). No transfer to a wholly-owned subsidiary shall be deemed a Change of Control Transaction under this provision.
(f) “Closing Bid Price” means the price per share in the last reported trade of the ADSs on a Primary Market or on the exchange which the ADSs is then listed as quoted by Bloomberg.
(g) “Convertible Securities” means any stock or securities (other than Options) directly or indirectly convertible into or exercisable or exchangeable for ADSs.
(h) “Commission” means the Securities and Exchange Commission.
(i) “Exchange Act” means the Securities Exchange Act of 1934, as amended.
(j) “Floor Price” means $1.00 per share.
(k) “Fundamental Transaction” means any of the following: (1) the Company effects any merger or consolidation of the Company with or into another Person and the Company is the non-surviving company (other than a merger or consolidation with a wholly owned subsidiary of the Company for the purpose of redomiciling the Company), (2) the Company effects any sale of all or substantially all of its assets in one or a series of related transactions, (3) any tender offer or exchange offer (whether by the Company or another Person) is completed pursuant to which holders of ADSs are permitted to tender or exchange their shares for other securities, cash or property, or (4) the Company effects any reclassification of the ADSs or any compulsory share exchange pursuant to which the ADSs is effectively converted into or exchanged for other securities, cash or property.
(l) “Options” means any rights, warrants or options to subscribe for or purchase ADSs or Convertible Securities
(m) “Original Issue Date” means the date of the first issuance of this Debenture regardless of the number of transfers and regardless of the number of instruments, which may be issued to evidence such Debenture.
(n) “Person” means a corporation, an association, a partnership, organization, a business, an individual, a government or political subdivision thereof or a governmental agency.
(o) “Primary Market” means any of the New York Stock Exchange, the NYSE MKT, the Nasdaq Global Market, the Nasdaq Global Select Market, or the OTC QB, and any successor to any of the foregoing markets or exchanges.
(p) “Redemption Premium” means, 20% of the Principal amount being redeemed.
(q) “Securities Act” means the Securities Act of 1933, as amended, and the rules and regulations promulgated thereunder.
(r) “Trading Day” means a day on which the ADSs are quoted or traded on a Primary Market on which the ADSs are then quoted or listed; provided, that in the event that the ADSs are not listed or quoted, then Trading Day shall mean a Business Day.
(s) “Transaction Document(s)” shall mean this Debenture, along with the Securities Purchase Agreement, the Registration Rights Agreement and any other documents or agreements entered into in connection with the foregoing.
(t) “Underlying Shares” means the ADSs issuable upon conversion of this Debenture or as payment of interest in accordance with the terms hereof.
(u) “VWAP” means, for any security as of any date, the daily dollar volume-weighted average price for such security on the Primary Market as reported by Bloomberg through its “Historical Prices — Px Table with Average Daily Volume” functions, or, if no dollar volume-weighted average price is reported for such security by Bloomberg.
[Signature Page Follows]
IN WITNESS WHEREOF, the Company has caused this Convertible Debenture to be duly executed by a duly authorized officer as of the date set forth above.
|
|
COMPANY: | |
|
|
| |
|
|
AnPac Bio-Medical Science Co., Ltd. | |
|
|
|
|
|
|
|
|
|
|
By: |
|
|
|
Name: |
|
|
|
Title: |
|
|
|
|
|
|
|
|
|
|
|
Accepted by | |
|
|
| |
|
|
INVESTOR: | |
|
|
|
|
|
|
|
|
|
|
By: |
|
|
|
Name: |
|
|
|
Title: |
|
EXHIBIT I
CONVERSION NOTICE
(To be executed by the Holder in order to Convert the Debenture)
TO: AnPac Bio-Medical Science Co., Ltd.
Via Email:
The undersigned hereby irrevocably elects to convert a portion of the outstanding and unpaid Conversion Amount of Debenture No. ANPC-[-] into ADSs of AnPac Bio-Medical Science Co., Ltd., according to the conditions stated therein, as of the Conversion Date written below.
Conversion Date:
Principal Amount to be Converted:
Accrued Interest to be Converted:
Total Conversion Amount to be converted:
Fixed Conversion Price:
Variable Conversion Price:
Applicable Conversion Price:
Number of ADSs to be issued:
Please issue the ADSs in the following name and to the following address: Issue to:
|
Authorized Signature: |
|
|
Name: |
|
|
Title: |
|
|
Broker DTC Participant |
|
|
Code: |
|
|
Account Number: |
|

02/05/2021
Dr. Chris Yu
Chairman
AnPac Bio-Medical Science Co., Ltd..
Dear Dr. Yu,
This letter (the “Agreement”) constitutes the agreement between Univest Securities, LLC (“Univest”), or the (“Placement Agent/(s)”) and AnPac Bio-Medical Science Co., Ltd.., a company incorporated under the laws in the Cayman Island (the “Company”), pursuant to which the Placement Agent shall serve as the exclusive placement agent of public offerings for the Company, on a “reasonable best efforts”, timely and effective basis, in connection with the proposed placement (the “Placement”) of securities and instruments, including Class A ordinary shares being represented by American depositary shares (ADSs), being registered within Company’s shelf registration statement to be on Form F-3(and F1 relating to or required for public offerings) (collectively the “Securities”), with the intention to conduct an raise sizing around $15,000,000. Univest shall use its best efforts to complete the Placement as soon as practical but no later than the Termination Date (as defined below) set forth herein. For clarify, both parties agree that the scope of this engagement excludes any private placement by the Company and related resale offering by such Investors on Form F-1 or Form F-3. The terms of the Placement and the Securities shall be mutually agreed upon by the Company and the purchasers (each, a “Purchaser” and collectively, the “Purchasers”) and nothing herein constitutes that the Placement Agent would have the power or authority to bind the Company or any Purchaser or an obligation for the Company to issue any Securities or complete the Placement. This Agreement and the documents executed and delivered by the Company and the Purchasers in connection with the Placement, including but not limited to the Purchase Agreement (as defined below) shall be collectively referred to herein as the “Transaction Documents.” The date of the closing of the Placement shall be referred to herein as the “Closing Date.” The Company expressly acknowledges and agrees that the Placement Agent’ obligations hereunder are on a reasonable best efforts basis only and that the execution of this Agreement does not constitute a commitment by the Placement Agent to purchase the Securities and does not ensure the successful placement of the Securities or any portion thereof or the success of the Placement Agent’ with respect to securing any other financing on behalf of the Company. The Placement Agent may retain other brokers or dealers to act as sub-agents or selected dealers on its behalf in connection with the Placement. The sale of the Securities to any Purchaser will be evidenced by a securities purchase agreement (the “Purchase Agreement”) between the Company and such Purchaser in a form reasonably acceptable to the Company and the Placement Agent. Capitalized terms that are not otherwise defined herein have the meanings given to such terms in the Purchase Agreement. Prior to the signing of any Purchase Agreement, officers of the Company will be available to answer inquiries from prospective Purchasers.
SECTION 1. REPRESENTATIONS AND WARRANTIES OF THE COMPANY; COVENANTS OF THE COMPANY.
A. Representations of the Company. Each of the representations and warranties (together with any related disclosure schedules thereto) and covenants made by the Company to the Purchasers in the Purchase Agreement in connection with the Placement is hereby incorporated herein by reference into this Agreement (as though fully restated herein) and is, as of the date of this Agreement and as of the Closing Date, hereby made to, and in favor of, the Placement Agent. In addition to the foregoing, the Company represents and warrants that:
|
UNIVEST SECURITIES, LLC |
375 PARK AVE SUITE 1502 |
+1 212.343.8888 |
|
|
NEW YORK, NEW YORK |
|
1. The Company is eligible to use free writing prospectuses in connection with the Placement pursuant to Rules 164 and 433 under the Securities Act. Any free writing prospectus that the Company is required to file pursuant to Rule 433(d) under the Securities Act has been, or will be, filed with the Commission in accordance with the requirements of the Securities Act and the applicable rules and regulations of the Commission thereunder. Each free writing prospectus that the Company has filed, or is required to file, pursuant to Rule 433(d) under the Securities Act or that was prepared by or on behalf of or used by the Company complies or will comply in all material respects with the requirements of the Securities Act and the applicable rules and regulations of the Commission thereunder. The Company will not, without the prior consent of the Placement Agent, prepare, use or refer to, any free writing prospectus.
2. No registration under the Securities Act is required for the offer and sale of the Warrants or the ADSs underlying the Warrants by the Company to the Purchasers as contemplated hereby.
3. There are no affiliations with any FINRA member firm among the Company’s officers, directors or, to the knowledge of the Company, any five percent (5.0%) or greater stockholder of the Company, except as set forth in the Registration Statement and SEC Reports.
B. Covenants of the Company. The Company has delivered, or will as promptly as practicable deliver, to the Placement Agent complete conformed copies of the a registration statement on Form F-1 or F-3 (collectively the “Registration Statement”) and of each consent and certificate of experts, as applicable, filed as a part thereof, and conformed copies of the Registration Statement (without exhibits), the Base Prospectus, the Time of Sale Prospectus and the Prospectus Supplement, as amended or supplemented, in such quantities and at such places as the Placement Agent reasonably requests. Neither the Company nor any of its directors and officers has distributed and none of them will distribute, prior to the Closing Date, any offering material in connection with the offering and sale of the Securities pursuant to the Placement other than the Base Prospectus, the Time of Sale Prospectus, the Prospectus Supplement, the Registration Statement, copies of the documents incorporated by reference therein and any other materials permitted by the Securities Act.
SECTION 2. REPRESENTATIONS OF THE PLACEMENT AGENT. Placement Agent represents and warrants that it (i) is a member in good standing of FINRA, (ii) is registered as a broker/dealer under the Exchange Act, (iii) is licensed as a broker/dealer under the laws of the States applicable to the offers and sales of the Securities by such Placement Agent, (iv) is and will be a body corporate validly existing under the laws of its place of incorporation, and (v) has full power and authority to enter into and perform its obligations under this Agreement. Placement Agent will immediately notify the Company in writing of any change in its status as such. Placement Agent, covenants that it will use its reasonable best efforts to conduct the Placement hereunder in compliance with the provisions of this Agreement and the requirements of applicable law.
SECTION 3. COMPENSATION. In consideration of the services to be provided for hereunder, the Company shall pay to the Placement Agent or their respective designees their pro rata portion (based on the Securities placed) of the following compensation with respect to the Securities which they are placing. During the term of this agreement, if the Company receive any written placement agreement from any other FINRA registered full service investment bank in the US which has more beneficial compensation terms to the Company, the Company shall have the right to ask the Placement Agent to match those terms:
A. A cash fee (the “Cash Fee”) equal to an aggregate of seven percent (7%) of the aggregate gross proceeds raised in the Placement to be paid at the Closing of the Placement. This fee shall not apply to any investors who are in the Company’s direct placement program.
B. Subject to compliance with FINRA Rule 5110(f)(2)(D), the Company also agrees to reimburse the Placement Agent for all reasonable travel and other out-of-pocket expenses, including the reasonable fees, costs and disbursements of its legal counsel, in an amount not to exceed an aggregate of $50,000. The Company will reimburse Placement Agent directly out of the Closing of the Placement. In the event this Agreement shall terminate prior to the consummation of the Placement, the Placement Agent shall be entitled to reimbursement for actual expenses; provided. The Company further agrees that, in addition to the expenses payable pursuant to this Section, on the Closing Date it shall pay to the Placement Agent, by deduction from the net proceeds of the Placement contemplated herein, a non-accountable expense allowance equal to one percent (1%) of the gross proceeds received by the Company from the sale of the Securities.
C. Each Placement Agent reserves the right to reduce any item of its compensation or adjust the terms thereof as specified herein in the event that a determination shall be made by FINRA to the effect that such Placement Agent’s aggregate compensation is in excess of FINRA Rules or that the terms thereof require adjustment.
SECTION 4. INDEMNIFICATION. The Company agrees to the indemnification and other agreements set forth in the Indemnification Provisions (the “Indemnification”) attached hereto as Addendum A, the provisions of which are incorporated herein by reference and shall survive the termination or expiration of this Agreement.
SECTION 5. ENGAGEMENT TERM. The Placement Agent’ engagement for public offering hereunder shall be exclusive until the earlier of (i) the final closing date of the Placement and (ii) the date a party terminates the engagement according to the terms of the next sentence (such date, the “Termination Date” and the period of time during which this Agreement remains in effect is referred to herein as the “Term”). After an initial period of six (6) month(s) from the date hereof, the engagement may be terminated at any time by either party upon 10 days written notice to the other party, effective upon receipt of written notice to that effect by the other party. If the Company elects to terminate this Agreement for any reason even though the Placement Agent were prepared to proceed with the Placement reasonably within the intent of this Agreement, and if within six (6) months following such termination, the Company completes any financing of equity, equity-linked or debt or other capital raising activity of the Company (other than the exercise by any person or entity of any options, warrants or other convertible securities) with any of the investors contacted by Placement Agent during the term of this Agreement, then the Company will pay the Placement Agent upon the closing of such financing the compensation set forth in Section 3 herein. Notwithstanding anything to the contrary contained herein, the provisions concerning the Company’s obligation to pay any fees actually earned pursuant to Section 3 hereof and the provisions concerning confidentiality, indemnification and contribution contained herein and the Company’s obligations contained in the Indemnification Provisions will survive any expiration or termination of this Agreement. If this Agreement is terminated prior to the completion of the Placement, all fees due to the Placement Agent shall be paid by the Company to the Placement Agent on or before the Termination Date (in the event such fees are earned or owed as of the Termination Date). The Placement Agent agree, not to use any confidential information concerning the Company provided to the Placement Agent by the Company for any purposes other than those contemplated under this Agreement.
SECTION 6. FUTURE SERVICE. The Company and Univest agree that for a period of eight months from the date of the Closing Date, whether or not the engagement contemplated under this Agreement is terminated (other than termination for Cause, as defined below), the Company grants Univest the right (provided the initial public offering is completed) to provide investment banking services to the Company on an exclusive basis in all public offerings (F3 and F1 related to and required for public offerings) on a registration statement or prospectus conducted by the Company (such right, the “Right of First Refusal”), which right is exercisable in Univest’s discretion. If Univest elects to exercise its right, Univest agrees to perform and complete public offerings on a “reasonable best efforts”, timely and effective basis. For these purposes, investment banking services shall only include acting as lead manager for any public offerings. Univest shall notify the Company of its intention to exercise the Right of First Refusal within 5 business days following notice in writing by the Company. For clarity, the Right of First Refusal shall not apply to any private placement by the Company and resale offerings on Form F-1 or Form F-3 thereof. Any decision by Univest to act in any such capacity shall be contained in separate agreements, which agreements would contain, among other matters, provisions for customary fees for transactions of similar size and nature, as may be mutually agreed upon, and indemnification of Univest and shall be subject to general market conditions. If Univest declines or fails to notify to exercise the Right of First Refusal, the Company shall have the right to retain any other person or persons to provide such services on terms and conditions which are not more favorable to such other person or persons than the terms declined by Univest. The Right of First Refusal granted hereunder may be terminated by the Company for “Cause,” which shall mean a material breach by Univest of this Agreement or a material failure by Univest to provide the services as contemplated by this Agreement. The services provided by Univest hereunder are solely for the benefit of the Company and are not intended to confer any rights upon any persons or entities not a party hereto (including, without limitation, securityholders, employees or creditors of the Company) as against Univest or its directors, officers, agents and employees.
SECTION 7. PLACEMENT AGENT INFORMATION. The Company agrees that any information or advice rendered by the Placement Agent in connection with this engagement is for the confidential use of the Company only in their evaluation of the Placement and, except as otherwise required by law, the Company will not disclose or otherwise refer to the advice or information in any manner without the Placement Agent’ prior written consent.
SECTION 8. NO FIDUCIARY RELATIONSHIP. This Agreement does not create, and shall not be construed as creating rights enforceable by any person or entity not a party hereto, except those entitled hereto by virtue of the Indemnification Provisions hereof. The Company acknowledges and agrees that each Placement Agent is not and shall not be construed as a fiduciary of the Company and shall have no duties or liabilities to the equity holders or the creditors of the Company or any other person by virtue of this Agreement or the retention of such Placement Agent hereunder, all of which are hereby expressly waived.
SECTION 9. CLOSING. The obligations of the Placement Agent, and the closing of the sale of the Securities hereunder are subject to the accuracy, when made and on the Closing Date, of the representations and warranties on the part of the Company and its Subsidiaries contained herein and in the Purchase Agreement, to the accuracy of the statements of the Company and its Subsidiaries made in any certificates pursuant to the provisions hereof, to the performance by the Company and its Subsidiaries of their obligations hereunder, and to each of the following additional terms and conditions, except as otherwise disclosed to and acknowledged and waived by the Placement Agent by the Company:
A. No stop order suspending the effectiveness of the Registration Statement shall have been issued and no proceedings for that purpose shall have been initiated or threatened by the Commission, and any request for additional information on the part of the Commission (to be included in the Registration Statement, the Base Prospectus, the Prospectus Supplement or otherwise) shall have been complied with to the reasonable satisfaction of the Placement Agent. Any filings required to be made by the Company in connection with the Placement shall have been timely filed with the Commission.
B. The Placement Agent shall not have discovered and disclosed to the Company on or prior to the Closing Date that the Registration Statement, the Base Prospectus, the Prospectus Supplement or any amendment or supplement thereto contains an untrue statement of a fact which, in the opinion of counsel for the Placement Agent, is material or omits to state any fact which, in the opinion of such counsel, is material and is required to be stated therein or is necessary to make the statements therein not misleading.
C. All corporate proceedings and other legal matters incident to the authorization, form, execution, delivery and validity of each of this Agreement, the Securities, the Registration Statement, the Base Prospectus and the Prospectus Supplement and all other legal matters relating to this Agreement and the transactions contemplated hereby shall be reasonably satisfactory in all material respects to counsel for the Placement Agent, and the Company shall have furnished to such counsel all documents and information that they may reasonably request to enable them to pass upon such matters.
D. The Placement Agent shall have received from outside counsels to the Company such counsels’ written opinions, addressed to the Placement Agent and the Purchasers and dated as of the Closing Date, in form and substance reasonably satisfactory to the Placement Agent.
E. On the date of this Agreement and on the Closing Date, the Placement Agent shall have received a “comfort” letter from the Company’s auditor at the time, and a certificate of the chief financial officer of the Company, as of each such date, addressed to each of the Placement Agent and inform and substance satisfactory in all respects to the Placement Agent and Placement Agent’ counsel.
F. On the Closing Date, Placement Agent shall have received a certificate of the chief executive officer of the Company, dated, as applicable, as of the date of such Closing, to the effect that, as of the date of this Agreement and as of the applicable date, the representations and warranties of the Company contained herein and in the Purchase Agreement were and are accurate in all material respects, except for such changes as are contemplated by this Agreement and except as to representations and warranties that were expressly limited to a state of facts existing at a time prior to the applicable Closing Date, and that, as of the applicable date, the obligations to be performed by the Company hereunder on or prior thereto have been fully performed in all material respects.
G. On the Closing Date, Placement Agent shall have received a certificate of the Secretary of the Company, dated, as applicable, as of the date of such Closing, certifying to the organizational documents, good standing in the state of incorporation of the Company and board resolutions relating to the Placement of the Securities from the Company.
H. Neither the Company nor any of its Subsidiaries (i) shall have sustained since the date of the latest audited financial statements included or incorporated by reference in the Registration Statement, the Base Prospectus and the Prospectus Supplement, any loss or interference with its business from fire, explosion, flood, terrorist act or other calamity, whether or not covered by insurance, or from any labor dispute or court or governmental action, order or decree, otherwise than as set forth in or contemplated by the Registration Statement, the Base Prospectus and the Prospectus Supplement, and (ii) since such date there shall not have been any change in the capital stock or long-term debt of the Company or any of its Subsidiaries or any change, or any development involving a prospective change, in or affecting the business, general affairs, management, financial position, stockholders’ equity, results of operations or prospects of the Company and its Subsidiaries, otherwise than as set forth in or contemplated by the Registration Statement, the Base Prospectus and the Prospectus Supplement, the effect of which, in any such case described in clause (i) or (ii), is, in the judgment of the Placement Agent, so material and adverse as to make it impracticable or inadvisable to proceed with the sale or delivery of the Securities on the terms and in the manner contemplated by the Base Prospectus, Time of Sale Prospectus and Prospectus Supplement.
I. The ADSs are registered under the Exchange Act and, as of the Closing Date, the Shares shall be listed and admitted and authorized for trading on the Trading Market or other applicable U.S. national exchange and satisfactory evidence of such action shall have been provided to the Placement Agent. The Company shall have taken no action designed to, or likely to have the effect of terminating the registration of the ADSs under the Exchange Act or delisting or suspending from trading the ADSs from the Trading Market or other applicable U.S. national exchange, nor has the Company received any information suggesting that the Commission or the Trading Market or other U.S. applicable national exchange is contemplating terminating such registration or listing.
J. No action shall have been taken and no statute, rule, regulation or order shall have been enacted, adopted or issued by any governmental agency or body which would, as of the Closing Date, prevent the issuance or sale of the Securities or materially and adversely affect or potentially and adversely affect the business or operations of the Company; and no injunction, restraining order or order of any other nature by any federal or state court of competent jurisdiction shall have been issued as of the Closing Date which would prevent the issuance or sale of the Securities or materially and adversely affect or potentially and adversely affect the business or operations of the Company.
K. The Company shall have prepared and filed with the Commission a Current Report on Form 6-K with respect to the Placement, including as an exhibit thereto this Agreement.
L. The Company shall have entered into a Purchase Agreement with each of the Purchasers and such agreements shall be in full force and effect and shall contain representations, warranties and covenants of the Company as agreed between the Company and the Purchasers.
M. FINRA shall have raised no objection to the fairness and reasonableness of the terms and arrangements of this Agreement. In addition, the Company shall, if requested by the Placement Agent, make or authorize Placement Agent’ counsel to make on the Company’s behalf, any filing with the FINRA Corporate Financing Department pursuant to FINRA Rule 5110 with respect to the Placement and pay all filing fees required in connection therewith.
N. Prior to the Closing Date, the Company shall have furnished to the Placement Agent such further information, certificates and documents as the Placement Agent may reasonably request.
If any of the conditions specified in this Section 8 shall not have been fulfilled when and as required by this Agreement, or if any of the certificates, opinions, written statements or letters furnished to the Placement Agent or to Placement Agent’ counsel pursuant to this Section 8 shall not be reasonably satisfactory in form and substance to the Placement Agent and to Placement Agent’ counsel, all obligations of the Placement Agent hereunder may be cancelled by the Placement Agent at, or at any time prior to, the consummation of the Closing. Notice of such cancellation shall be given to the Company in writing or orally. Any such oral notice shall be confirmed promptly thereafter in writing.
SECTION 10. GOVERNING LAW. This Agreement will be governed by, and construed in accordance with, the laws of the State of New York applicable to agreements made and to be performed entirely in such State. This Agreement may not be assigned by either party without the prior written consent of the other party. This Agreement shall be binding upon and inure to the benefit of the parties hereto, and their respective successors and permitted assigns. Any right to trial by jury with respect to any dispute arising under this Agreement or any transaction or conduct in connection herewith is waived. Any dispute arising under this Agreement may be brought into the courts of the State of New York or into the Federal Court located in New York, New York and, by execution and delivery of this Agreement, the Company hereby accepts for itself and in respect of its property, generally and unconditionally, the jurisdiction of aforesaid courts. Each party hereto hereby irrevocably waives personal service of process and consents to process being served in any such suit, action or proceeding by delivering a copy thereof via overnight delivery (with evidence of delivery) to such party at the address in effect for notices to it under this Agreement and agrees that such service shall constitute good and sufficient service of process and notice thereof. Nothing contained herein shall be deemed to limit in any way any right to serve process in any manner permitted by law. If either party shall commence an action or proceeding to enforce any provisions of a Transaction Document, then the prevailing party in such action or proceeding shall be reimbursed by the other party for its attorney’s fees and other costs and expenses incurred with the investigation, preparation and prosecution of such action or proceeding.
SECTION 11. ENTIRE AGREEMENT/MISC. This Agreement (including the attached Indemnification Provisions) embodies the entire agreement and understanding between the parties hereto, and supersedes all prior agreements and understandings, relating to the subject matter hereof. If any provision of this Agreement is determined to be invalid or unenforceable in any respect, such determination will not affect such provision in any other respect or any other provision of this Agreement, which will remain in full force and effect. This Agreement may not be amended or otherwise modified or waived except by an instrument in writing signed by both Placement Agent and the Company. The representations, warranties, agreements and covenants contained herein shall survive the closing of the Placement and delivery of the Securities. This Agreement may be executed in two or more counterparts, all of which when taken together shall be considered one and the same agreement and shall become effective when counterparts have been signed by each party and delivered to the other party, it being understood that both parties need not sign the same counterpart. In the event that any signature is delivered by facsimile transmission or a .pdf format file, such signature shall create a valid and binding obligation of the party executing (or on whose behalf such signature is executed) with the same force and effect as if such facsimile or .pdf signature page were an original thereof.
SECTION 12. CONFIDENTIALITY. The Placement Agent, (i) will keep the Confidential Information (as such term is defined below) confidential and will not (except as required by applicable law or stock exchange requirement, regulation or legal process (“Legal Requirement”)), without the Company’s prior written consent, disclose to any person any Confidential Information, and (ii) will not use any Confidential Information other than in connection with the Placement. The Placement Agent further agree, severally and not jointly, to disclose the Confidential Information only to its Representatives (as such term is defined below) who need to know the Confidential Information for the purpose of the Placement, and who are informed by the Placement Agent of the confidential nature of the Confidential Information. The term “Confidential Information” shall mean, all confidential, proprietary and non-public information (whether written, oral or electronic communications) furnished by the Company to a Placement Agent or its Representatives in connection with such Placement Agent’s evaluation of the Placement. The term “Confidential Information” will not, however, include information which (i) is or becomes publicly available other than as a result of a disclosure by a Placement Agent or its Representatives in violation of this Agreement, (ii) is or becomes available to a Placement Agent or any of its Representatives on a non-confidential basis from a third- party, (in) is known to a Placement Agent or any of its Representatives prior to disclosure by the Company or any of its Representatives, or (iv) is or has been independently developed by a Placement Agent and/or the Representatives without use of any Confidential Information furnished to it by the Company. The term “Representatives” shall mean each Placement Agent’s directors, board committees, officers, employees, financial advisors, attorneys and accountants. This provision shall be in full force until the earlier of (a) the date that the Confidential Information ceases to be confidential and (b) two years from the date hereof. Notwithstanding any of the foregoing, in the event that the Placement Agent or any of their respective Representatives are required by Legal Requirement to disclose any of the Confidential Information, such Placement Agent and their respective Representatives will furnish only that portion of the Confidential Information which such Placement Agent or their respective Representative, as applicable, is required to disclose by Legal Requirement as advised by counsel, and will use reasonable efforts to obtain reliable assurance that confidential treatment will be accorded the Confidential Information so disclosed.
SECTION 13. NOTICES. Any and all notices or other communications or deliveries required or permitted to be provided hereunder shall be in writing and shall be deemed given and effective on the earliest of (a) the date of transmission, if such notice or communication is sent to the email address specified on the signature pages attached hereto prior to 6:30 p.m. (Eastern Standard Time) on a business day, (b) the next business day after the date of transmission, if such notice or communication is sent to the email address on the signature pages attached hereto on a day that is not a business day or later than 6:30 p.m. (Eastern Standard Time) on any business day, (c) the third business day following the date of mailing, if sent by U.S. internationally recognized air courier service, or (d) upon actual receipt by the party to whom such notice is required to be given. The address for such notices and communications shall be as set forth on the signature pages hereto.
SECTION 14. PRESS ANNOUNCEMENTS. The Company agrees that the Placement Agent shall, from and after any Closing, have the right to reference the Placement and the Placement Agent’ role in connection therewith in the Placement Agent’ marketing materials and on its website and to place advertisements in financial and other newspapers and journals, in each case at its own expense.
SECTION 15. ENGLISH LANGUAGE. This Agreement is expressed in the English language. If this Agreement is translated by either party to another language for any purpose, the English language version shall govern over any translation in the event of any inconsistency, discrepancy or conflict in interpretation. All communications, notices, and other actions relating to this Agreement shall be in the English language
[The remainder of this page has been intentionally left blank.]
Please confirm that the foregoing correctly sets forth our agreement by signing and returning to Univest the enclosed copy of this Agreement.
|
|
UNIVEST SECURITIES, LLC | ||
|
|
|
| |
|
|
|
Name: |
Edric Yi Guo |
|
|
|
Title: |
COO and Head of Investment Banking |
|
|
/s/ Edric Yi Guo |
|
|
|
|
|
Address for notice: |
|
|
375 Park Avenue Unit 1502, |
|
|
New York, NY 10152 |
|
|
Attention: Edric Guo |
|
|
Email: [ ] |
|
Accepted and Agreed to as of the date first written above: |
| |
|
|
| |
|
AnPac Bio-Medical Science Co., Ltd. |
| |
|
|
| |
|
By: |
/s/ Chris Chang Yu |
|
|
|
Chris Chang Yu |
|
|
|
CEO and Chairman |
|
[Signature Page to Placement Agency Agreement Between AnPac Bio-Medical Science Co., Ltd. and Univest Securities, LLC.]
ADDENDUM A
INDEMNIFICATION PROVISIONS
In connection with the engagement of Univest Securities, LLC(the “Lead Manager”) or together with other broker dealers registered with FINRA and caused by Univest to also act as a manager (the “Lead Managers”) by AnPac Bio-Medical Science Co., Ltd.. (the “Company”) pursuant to a placement agency agreement dated as of the date hereof, between the Company and the Lead Manager(s), as it may be amended from time to time in writing (the “Agreement”), the Company hereby agrees as follows:
1. To the extent permitted by law and in the absence of willful misconduct by the Lead Manager, its syndicate or affiliates, the Company will indemnify the Lead Manager(s) and each of their affiliates, directors, officers, employees and controlling persons (within the meaning of Section 15 of the Securities Act of 1933, as amended, or Section 20 of the Securities Exchange Act of 1934) against all losses, claims, damages, expenses and liabilities, as the same are incurred (including the reasonable fees and expenses of counsel), relating to or arising out of its activities hereunder or pursuant to the Agreement, except, with regard to the Lead Managers, to the extent that any losses, claims, damages, expenses or liabilities (or actions in respect thereof) are found in a final judgment (not subject to appeal) by a court of law to have resulted primarily and directly from the Lead Managers’ willful misconduct or gross negligence in performing the services described herein, as the case may be.
2. Promptly after receipt by the Lead Managers of notice of any claim or the commencement of any action or proceeding with respect to which the Lead Managers are entitled to indemnity hereunder, the Lead Managers will notify the Company in writing of such claim or of the commencement of such action or proceeding, and the Company will assume the defense of such action or proceeding and will employ counsel reasonably satisfactory to the Lead Managers and will pay the fees and expenses of such counsel. Notwithstanding the preceding sentence, the Lead Managers will be entitled to employ counsel separate from counsel for the Company and from any other party in such action if counsel for the Lead Managers reasonably determines that it would be inappropriate under the applicable rules of professional responsibility for the same counsel to represent both the Company and the Lead Managers. In such event, the reasonable fees and disbursements of no more than one such separate counsel will be paid by the Company. The Company will have the exclusive right to settle the claim or proceeding provided that the Company will not settle any such claim, action or proceeding without the prior written consent of the Lead Managers, which will not be unreasonably withheld.
3. The Company agrees to notify the Lead Managers promptly of the assertion against it or any other person of any claim or the commencement of any action or proceeding relating to a transaction contemplated by the Agreement.
4. If for any reason the foregoing indemnity is unavailable to the Lead Managers or insufficient to hold the Lead Managers harmless, then the Company shall contribute to the amount paid or payable by the Lead Managers, as the case may be, as a result of such losses, claims, damages or liabilities in such proportion as is appropriate to reflect not only the relative benefits received by the Company on the one hand, and the Lead Managers on the other, but also the relative fault of the Company on the one hand and the Lead Managers on the other that resulted in such losses, claims, damages or liabilities, as well as any relevant equitable considerations. The amounts paid or payable by a party in respect of losses, claims, damages and liabilities referred to above shall be deemed to include any legal or other fees and expenses incurred in defending any litigation, proceeding or other action or claim. Notwithstanding the provisions hereof, the Lead Managers’ share of the liability hereunder shall not be in excess of the amount of fees actually received, or to be received, by the Lead Managers under the Agreement (excluding any amounts received as reimbursement of expenses incurred by the Lead Managers).
5. These Indemnification Provisions shall remain in full force and effect whether or not the transaction contemplated by the Agreement is completed and shall survive the termination of the Agreement, and shall be in addition to any liability that the Company might otherwise have to any indemnified party under the Agreement or otherwise.
[The remainder of this page has been intentionally left blank]
|
|
Name: Edric Yi Guo |
|
|
|
|
|
Title: COO and Head of Investment Banking |
|
|
|
|
|
/s/ Edric Yi Guo |
|
|
|
|
|
Address for notice: |
|
|
375 Park Avenue Unit 1502, |
|
|
New York, NY 10152 |
|
|
Attention: Edric Guo |
|
|
Email: [ ] |
|
Accepted and Agreed to as of the date first written above: |
| ||
|
|
| ||
|
AnPac Bio-Medical Science Co., Ltd. |
| ||
|
|
| ||
|
By: |
/s/ Chris Chang Yu |
|
|
|
|
Chris Chang Yu |
| |
[Signature Page to Placement Agency Agreement Between AnPac Bio Bio-Medical Science Co., Ltd. and Univest Securities, LLC.]
Letter of Investment Intent
Party A: AnPac Bio-Medical Science Co., Ltd.
Party B: Shanghai Shidi Investment Management Center (Limited Partnership)
Party A is Anpac Bio-medical Science Co., Ltd, a NASDAQ listed company (NASDAQ: ANPC) registered at Trinity Chambers, P.O. Box 4301, Road Town, Tortola, British Virgin Islands (“AnPac”). AnPac focuses on the development and industrialization of the world’s leading biomedical technologies. The results of retrospective studies of tens of thousands of samples have demonstrated that AnPac’s original CDA detection technology platform has higher sensitivity and specificity compared to certain traditional technologies in respect of detection and assessment of early-stage cancers. AnPac is actively promoting its CDA technology platform in the health management market and testing market in China and the United States, and accelerate the research and development and marketing of new products in these markets.
Party B is an investment institution with abundant resources in a wide range of fields, and is an investor of AnPac’s series-B financing.
Party B intends to provide investment to Party A, and Party A intends to accept Party B’s investment. Based on good-faith negotiation, the following terms have been agreed between parties:
I. Investment amount: Party B proposes to invest a total of US$3 million (or RMB equivalent) in Party A.
II. Investment price: Party B shall be entitled to obtain Party A’s ordinary shares at 80% of the average closing share price in the 20 trading days prior to the effective date of the formal investment agreement signed by parties. The current total share capital of Party A is approximately 12,586,000 shares.
III. Closing: Within one month from the effective date of this letter, a formal investment agreement shall be entered into by the parties to determine the investment price. Party B shall remit the full investment amount to Party A’s designated account in lump-sum or in installments in accordance with the formal investment agreement.
IV. Party A undertakes to complete the issuance of shares to Party B and the registration of such shares within five business days upon receiving the full investment amount from Party B.
V. Party B hereby certifies that Party B is a non-U.S. entity, and acknowledges that shares purchased under this letter are not registered in accordance with the U.S. Securities Act of 1933, as amended, and under an exemption under relevant rules is available, Party B shall not transfer such shares for a period of 180 days from the date of obtaining such shares. Party B shall bear all taxes with respect of the investment and transaction.
VI. during the term of holding Party A’s shares, Party B shall not directly or indirectly engage in any business competitive with Party A’s business, and shall support Party A’s business development with its resources.
VII. Party B acknowledges that during the financing process, all information in respect of Party A obtained by Party B either orally or in writing shall be confidential and owned by Party A. Party B shall be obligated to maintain the confidentiality of such information until the information is in public domain. Party B undertakes to enter into a confidentiality agreement at the time of signing this letter and strictly fulfill its confidentiality obligations thereunder.
VIII. This letter does not constitute a legally binding agreement between parties. The final terms of this letter are subject to the formal investment agreement as contemplated therein.
IX. This letter shall be effective upon signing by the parties in two original copies with the same effect. Parties shall keep this letter strictly confidential.
|
Party A (Seal) |
|
Party B (Seal) | ||
|
By: |
/s/Chris Chang Yu |
|
By: |
/s/Hong Pan |
|
Date: April 5, 2021 |
|
Date: April 5, 2021 | ||
Letter of Investment Intent
Party A: AnPac Bio-Medical Science Co., Ltd.
Party B: Zhijun Sihang Holdings Limited
Party A is Anpac Bio-medical Science Co., Ltd, a NASDAQ listed company (NASDAQ: ANPC) registered at Trinity Chambers, P.O. Box 4301, Road Town, Tortola, British Virgin Islands (“AnPac”). AnPac focuses on the development and industrialization of the world’s leading biomedical technologies. The results of retrospective studies of tens of thousands of samples have demonstrated that AnPac’s original CDA detection technology platform has higher sensitivity and specificity compared to certain traditional technologies in respect of detection and assessment of early-stage cancers. AnPac is actively promoting its CDA technology platform in the health management market and testing market in China and the United States, and accelerate the research and development and marketing of new products in these markets.
Party B is an investment institution with abundant resources in a wide range of fields, and is an investor of AnPac’s series-B financing.
Party B intends to provide investment to Party A, and Party A intends to accept Party B’s investment. Based on good-faith negotiation, the following terms have been agreed between parties:
I. Investment amount: Party B proposes to invest a total of US$3 million (or RMB equivalent) in Party A.
II. Investment price: Party B shall be entitled to obtain Party A’s ordinary shares at 80% of the average closing share price in the 20 trading days prior to the effective date of the formal investment agreement signed by parties. The current total share capital of Party A is approximately 12,586,000 shares.
III. Closing: Within one month from the effective date of this letter, a formal investment agreement shall be entered into by the parties to determine the investment price. Party B shall remit the full investment amount to Party A’s designated account in lump-sum or in installments in accordance with the formal investment agreement.
IV. Party A undertakes to complete the issuance of shares to Party B and the registration of such shares within five business days upon receiving the full investment amount from Party B.
V. During the term of holding Party A’s shares, Party B shall not directly or indirectly engage in any business competitive with Party A’s business, and shall support Party A’s business development with its resources.
VI. Party B acknowledges that during the financing process, all information in respect of Party A obtained by Party B either orally or in writing shall be confidential and owned by Party A. Party B shall be obligated to maintain the confidentiality of such information until the information is in public domain. Party B undertakes to enter into a confidentiality agreement at the time of signing this letter and strictly fulfill its confidentiality obligations thereunder.
VII. This letter does not constitute a legally binding agreement between parties. The final terms of this letter are subject to the formal investment agreement as contemplated therein.
VIII. This letter shall be effective upon signing by parties in two original copies with the same effect. The parties shall keep this letter strictly confidential.
|
Party A (Seal) |
|
Party B (Seal) | ||
|
By: |
/s/Chris Chang Yu |
|
By: |
/s/Feng Guo |
|
Date: April 12, 2021 |
|
Date: April 12, 2021 | ||
List of Principal Subsidiaries of the Registrant
|
Name of Subsidiaries |
|
Percentage |
|
Place of Incorporation |
|
|
|
|
|
|
|
AnPac Technology USA Co., Ltd. |
|
100% |
|
United States of America |
|
Changwei System Technology (Shanghai) Co., Ltd. |
|
100% |
|
People’s Republic of China |
|
AnPac Bio-Medical Technology (Lishui) Co., Ltd. |
|
100% |
|
People’s Republic of China |
|
Changhe Bio-Medical Technology (Yangzhou) Co., Ltd. |
|
100% |
|
People’s Republic of China |
|
AnPac Bio-Medical Technology (Shanghai) Co., Ltd. |
|
100% |
|
People’s Republic of China |
|
Lishui AnPac Medical Laboratory Co., Ltd. |
|
100% |
|
People’s Republic of China |
|
Shiji (Hainan) Medical Technology Limited |
|
100% |
|
People’s Republic of China |
|
Shanghai Muqing Anpac Health Technology Co., Ltd |
|
51% |
|
People’s Republic of China |
Certification by the Principal Executive Officer
Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
I, Chris Chang Yu, certify that:
1. I have reviewed this annual report on Form 20-F of AnPac Bio-Medical Science Co., Ltd..;
2. Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3. Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the company as of, and for, the periods presented in this report;
4. The company’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) for the company and have:
(a) Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the company, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
(b) [intentionally omitted];
(c) Evaluated the effectiveness of the company’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
(d) Disclosed in this report any change in the company’s internal control over financial reporting that occurred during the period covered by the annual report that has materially affected, or is reasonably likely to materially affect, the company’s internal control over financial reporting; and
5. The company’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the company’s auditors and the Audit Committee of the company’s Board of Directors (or persons performing the equivalent functions):
(a) All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the company’s ability to record, process, summarize and report financial information; and
(b) Any fraud, whether or not material, that involves management or other employees who have a significant role in the company’s internal control over financial reporting.
Date: April 30, 2021
|
By: |
/s/ Chris Chang Yu |
|
|
|
Name: Chris Chang Yu |
|
|
|
Title: Chief Executive Officer |
|
[CEO’s Section 302 Certification]
Certification by the Principal Financial Officer
Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
I, Edwards Jinqiu Tang, certify that:
1. I have reviewed this annual report on Form 20-F of AnPac Bio-Medical Science Co., Ltd.;
2. Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3. Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the company as of, and for, the periods presented in this report;
4. The company’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) for the company and have:
(a) Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the company, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
(b) [intentionally omitted];
(c) Evaluated the effectiveness of the company’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
(d) Disclosed in this report any change in the company’s internal control over financial reporting that occurred during the period covered by the annual report that has materially affected, or is reasonably likely to materially affect, the company’s internal control over financial reporting; and
5. The company’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the company’s auditors and the Audit Committee of the company’s Board of Directors (or persons performing the equivalent functions):
(a) All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the company’s ability to record, process, summarize and report financial information; and
(b) Any fraud, whether or not material, that involves management or other employees who have a significant role in the company’s internal control over financial reporting.
Date: April 30, 2021
|
By: |
/s/ Edwards Jinqiu Tang |
|
|
|
Name: Edwards Jinqiu Tang |
|
|
|
Title: Chief Financial Officer |
|
[CFO’s Section 302 Certification]
Certification by the Principal Executive Officer
Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
In connection with the Annual Report of AnPac Bio-Medical Science Co., Ltd. (the “Company”) on Form 20-F for the year ended December 31, 2020 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, Chris Chang Yu, Chief Executive Officer of the Company, hereby certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that to my knowledge:
(1) The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and
(2) The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
Date: April 30, 2021
|
By: |
/s/ Chris Yu Chang |
| |
|
|
Name: |
Chris Yu Chang |
|
|
|
Title: |
Chief Executive Officer |
|
[CEO’s Section 906 Certification]
Certification by the Principal Financial Officer
Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
In connection with the Annual Report of AnPac Bio-Medical Science Co., Ltd. (the “Company”) on Form 20-F for the year ended December 31, 2020 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, Edwards Jinqiu Tang, Chief Financial Officer of the Company, hereby certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that to my knowledge:
(1) The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and
(2) The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
Date: April 30, 2021
|
By: |
/s/ Edwards Jinqiu Tang |
| |
|
|
Name: |
Edwards Jinqiu Tang |
|
|
|
Title: |
Chief Financial Officer |
|
[CFO’s Section 906 Certification]

CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We consent to the incorporation by reference in the Form S-8 Registration Statement pertaining to the 2010 and 2019 Share Incentive Plans of AnPac Bio-Medical Science Co., Ltd. of our report dated April 30, 2021 relating to the consolidated balance sheet of AnPac Bio-Medical Science Co., Ltd. as of December 31, 2020, and the related consolidated statements of operations and comprehensive loss, statement equity and cash flows for the year ended December 31, 2020 filed with the Securities and Exchange Commission on April 30, 2021 on Form 20-F.
/s/ Friedman LLP
New York, New York
April 30, 2021

Consent of Independent Registered Public Accounting Firm
We consent to the incorporation by reference in the Registration Statement (Form S-8 No. 333-238679) pertaining to the 2010 and 2019 Share Incentive Plans of AnPac Bio-Medical Science Co., Ltd. of our report dated May 15, 2020, with respect to the consolidated financial statements of AnPac Bio-Medical Science Co., Ltd. included in this Annual Report (Form 20-F) for the year ended December 31, 2020.
/s/ Ernst & Young Hua Ming LLP
Shanghai, The People’s Republic of China
April 30, 2021
April 30, 2021
Securities and Exchange Commission
100 F Street, N.E.
Washington, DC 20549
Ladies and Gentlemen:
We have read Item 16F of Form 20-F dated April 30, 2021, of AnPac Bio-Medical Science Co., Ltd. (the “Company”) for the fiscal year ended December 31, 2020 and are in agreement with the statements made in the first sentence of paragraph 1, paragraph 2, paragraph 3, paragraph 4, paragraph 5 and paragraph 6 of that section. We have no basis to agree or disagree with other statements of the registrant contained therein.
/s/ Ernst & Young Hua Ming LLP
Shanghai, the People’s Republic of China
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Resolutions of Harvia Plc's Annual General Meeting on 26 April 2024
- Dimensional Fund Advisors Ltd. : Form 8.3 - BARRATT DEVELOPMENTS PLC - Ordinary Shares
- Entergy Texas’ First Solar Resource Helps Customers Meet Sustainability Goals
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share