Form 8-K Nemaura Medical Inc. For: Sep 27
Exhibit 99.1

BENZINGA HEALTHCARE SMALL CAP INVESTMENT CONFERENCE 29 th September 2021 Dr Faz Chowdhury, CEO Nasdaq : NMRD

Forward Looking Statements This presentation includes forward - looking statements that are subject to many risks and uncertainties. These forward - looking statements, such as statements about Nemaura’s short - term and long - term growth strategies can sometimes be identified by use of terms such as ‘intend,’ ‘expect,’ ‘plan,’ ‘estimate,’ ‘future,’ ‘strive,’ and similar words. These statements involve many ri sks and uncertainties that may cause actual results to differ from what may be expressed or implied in these statements. These risks are discussed in Nemaura’s filings with the Securities and Exchange Commission (the “Commission”), including th e r isks identified under the section captioned “Risk Factors” in Nemaura’s Annual Report on Form 10 - K filed with the Commission on June 29, 2021 as the same may be updated from time to time. Nemaura disclaims any obligation to update information contained in these forward - looking statements whether as a result of new information, future events, or otherwise.

Background About the CEO… • Trained as a Drug Formulation Scientist • Masters in Microsystems and Nanotechnology, Cranfield University UK • Doctorate in Nanomedicine and Drug Delivery, University of Oxford • First patent: Super - absorbent tablets during undergraduate internship at 3M Healthcare • Currently >100 patents across >15 patent families • Chairman and CEO of Nemaura Medical since its foundation in 2011

Introduction We developed the world’s first Daily - Wear non - invasive Continuous Glucose Monitor (CGM) – Class 2b CE approved Medical Device. Launched: sugarBEAT ® and BEAT ® diabetes , supporting Diabetes prevention, management and reversal. Today: Launch of Beta for Mass Market consumer metabolic health application – MiBoKo

Introduction Today’s Focus: Sales and Reimbursement strategy for sugarBEAT® Daily - Wear non - invasive Continuous Glucose Monitor (CGM) – Class 2b CE approved Medical Device. Mass Market consumer metabolic health application – MiBoKo

The Problem… There are over 463 million people living with diabetes worldwide, and over $ 760 Billion was spent in the US alone in 2019 for diabetes related healthcare expenditure 1 . Obesity and Diabetes are two of the major drivers of the chronic disease epidemic The total addressable market exceeds $150 Billion 2,3,4 .

Our Objective: Prevent, Manage or Reverse Type 2 Diabetes Use our suite of technologies to improve metabolic health across the broader population

UNIQUE The world’s first daily wear CGM: no other sensor technology is currently available allowing non - invasive daily use. LIFESTYLE Other competing sensors are worn for 10 - 14 days consecutively. Nemaura’s BEAT® sensors are designed for daily use – any day you choose. Our Unique Solution KNOWLEDGE Glucose sensors based on sugarBEAT provide guidance and insights into the extent of control over sugar levels. ENGAGEMENT A world class digital programs and ecosystems keeping the user engaged for the long term. OUTCOME Improvements in HbA1C, blood cholesterol, blood pressure, and sustainable weight loss. Real results . Sustainable . Affordable . PRICING Highly competitive pricing will yield broader adoption to address unmet clinical needs. $

Our Approach 1. sugarBEAT ® CGM – real time glucose monitoring. 2. BEAT ® diabetes – digital program for diabetes management and reversal, with intermittent glucose profiling. 3. Mass market consumer metabolic health program, targeting obesity, pre - diabetes and Type 2 diabetes.

Total Addressable Market: Diabetes Reversal UK 4.8 million people with diabetes 8 One person diagnosed every 2 minutes Germany 9.5 million have diabetes 9 . 4.5 million of these 9.5 million are undiagnosed and, as a result, may be particularly at risk. U.S. 34.2 million have diabetes 6 88 million people have pre - diabetes 28,000 people diagnosed with diabetes EVERY WEEK in the U.S. alone 7 in a market worth nearly $150B

Impact on Healthcare Cost □ Healthcare costs for persons with Type 2 diabetes cost approximately 2.5x as much as a person without diabetes. If they experience complications, that number soars even higher 10 □ Employers and healthcare insurers are therefore resorting to programs that will provide long - term sustainable results in stemming the onset of diabetes and, where possible, reversing Type 2 diabetes □ Current programs are cost prohibitive, but Nemaura has both a cost advantage as well as user friendliness from its intermittent use sensors and will focus its efforts on the U.S. and European markets initially, for diabetes prevention, management and potential reversal healthy 1 diabetes w/ complications $ spent per year $4k diabetes $12k $21k

Product Portfolio sugarBEAT® CGM BEAT®diabetes Program MiBoKo ® Consumer Metabolic Health Program

CE approved Class IIB Medical Device US FDA PMA approval and launch in the US anticipated by end of 2021 The world’s first daily wearable Continuous Glucose Monitor that doesn’t use needles. CE Approved Class IIb Medical Device sugarBEAT CGM

Core Technology


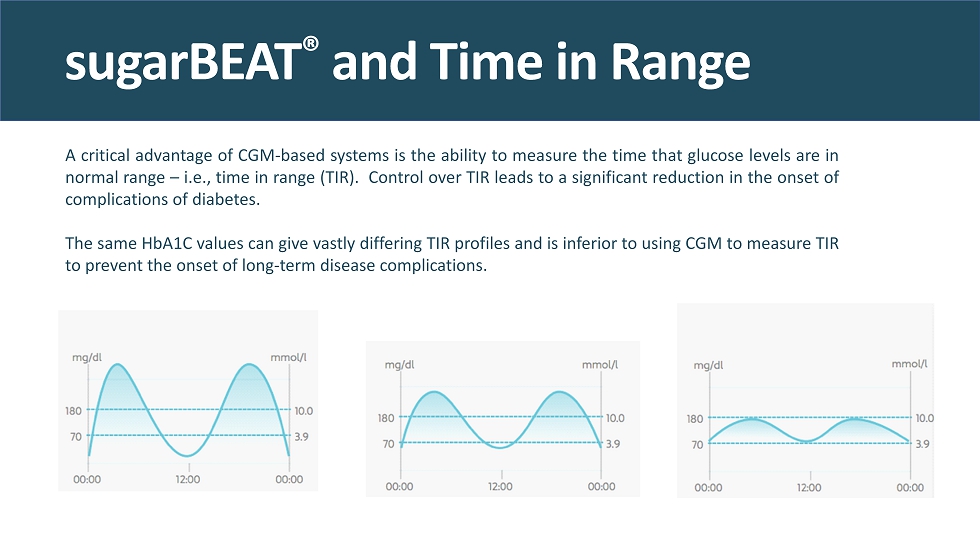
A critical advantage of CGM - based systems is the ability to measure the time that glucose levels are in normal range – i . e . , time in range (TIR) . Control over TIR leads to a significant reduction in the onset of complications of diabetes . The same HbA 1 C values can give vastly differing TIR profiles and is inferior to using CGM to measure TIR to prevent the onset of long - term disease complications . sugarBEAT ® and Time in Range

sugarBEAT ® vs. HbA1C 40% high range 40% in range 20% low range 70% high range 25% in range 5% low range 100% in range The same HbA1C value, yet 3 completely different TIR profiles, demonstrating the power of TIR over HbA1C as the new gold standard 11

sugarBEAT ® Testimonials My Sugar Watch offered me a needle - free blood glucose monitoring solution that’s non - invasive and easy to use. I didn’t even realize I had the My Sugar watch device on my arm as it is so lightweight. It gives me the assurance that my blood sugar reading is accurate, and I have access to my levels on my phone at all times. I was diagnosed with gestational diabetes, and I was informed by my healthcare professional that this may lead to a diagnosis of Type 2 diabetes in the future. Unfortunately, I was diagnosed with Type 2 diabetes after this and I have to manage this diagnosis all by myself and learn to control my blood glucose levels. Using My Sugar Watch has alerted me to changes in my blood glucose levels and helped me understand how these changes make an impact on my body and how I am feeling. To have this information at my fingertips gives me so much control to manage my diabetes. I have been a Type 2 diabetic for 10 years. I sporadically manage my blood sugar with a blood glucose monitoring device. I know that if not controlled or managed effectively I can have real highs and lows and not know when this will happen. I was wearing the My Sugar Watch device and it alerted me to the fact I was about to have a hypo before it happened. This alert enabled me to quickly balance my medication .
sugarBEAT ® Sales Status: UK UK: 200,000 Sensors ordered by licensee following soft launch success. Purchase order forecast for (approximately) a further 100,000 per month for the next 2 years, totaling over 2 million sensors. Licensee selling these based on a diabetes management subscription service.

sugarBEAT ® Sales Status: ROW ◆ Expecting to reveal launch plans for direct to consumer launch of sugarBEAT outside of UK, next quarter.
sugarBEAT ® Reimbursement Strategy ◆ 27 th September 2021 – Announced a deal with MSW Duo - Pack ◆ MSW - DP will purchase devices and sensors from Nemaura ◆ Devices and sensors will be included with widely prescribed diabetes medications – of which there are 2.1 million people on these medications in the 4 key EU territories plus the UK. ◆ Sensors allow patients to check their progress and improvements ◆ Patient does not have to pay for the sensors ◆ Sensors do not need to be separately reimbursed ◆ Nemaura ordered 200,000 devices in anticipation of MSW - DP launch in Q3/4 2022 ◆ Will require 800,000 sensors per month to service 200,000 users ◆ This is IN ADDITION to current orders for 200,000 sensors that are being fulfilled and being offered as part of a private subscription program

Type 2 Diabetes prevention and management program launched in the U.S.

BEAT ® diabetes – 3 Components 1. Weight loss program originally developed at the Joslin Diabetes Centre – over 12 years of clinical evidence (based on an in - clinic program, subsequently replicated using a virtual program). Sustained long term weight loss achieved without loss of muscle mass 2. proBEAT Ρ Intermittent glucose profiling – using world’s first daily - wearable glucose sensor, developed in - house 3. Coaching: digital 24/7 using app, and specialist 1 to 1 coaching

BEAT ® diabetes – Glucose Profiling Intermittent Glucose Profiling: Benefits 7 - point glucose profiles every 4 weeks. Patients received guidance for diet and exercise adjustments based on Self - monitoring of blood glucose (SMBG). Outcome: Significant reductions in HbA1c, weight, BMI, systolic BP, diastolic BP, and LDL Cholesterol Kempf et. al., Diabetes Technol Ther (2010)

BEAT ® diabetes : Potential Outcome for Payers $1,600 $4,700 $6,600 $9,100 Healthy (No conditions) Prediabetes only Hypertension Only Diabetes Only Annual Cost per Employee On average, diabetes costs both employers and insurers over $9,000 per year. Potential savings from prescription medications alone would amount to over $5,000 per year. Healthier Employees = Healthier Business

BEAT ® diabetes : Current Status Pilot study progress to be provided in Q4 2021 together with plans for large scale adoption

MiBoKo.com Metabolism is life. 87 Million people with pre - diabetes in the US Can we help them avoid becoming Diabetic using our low cost wearable glucose sensor and digital ecosystem?

Launching MiBoKo ‘MIND BODY CONNECT’ A metabolic health program comprising an app and integrated glucose sensor

MiBoKo : A Mass - Market Consumer Product The Centers for Disease Control and Prevention currently estimates that more than 42% of U.S. adults are obese. The risks associated with obesity are serious. Obese adults are up to 80 times more likely to get diabetes than individuals in what is considered healthy weight ranges. Adults with obesity also pay an average of $1,429 more per year in out - of - pocket healthcare expenses. 25 Applicable to over 80 million people in the U.S. with prediabetes as well as general health - conscious individuals, and obesity market.

MiBoKo : Market Positioning • A world - first program designed to support weight loss and a better quality of life through continuous feedback on metabolic health • A bespoke app and integrated glucose sensor worn two days per month, at an affordable cost based on subscription • The free app includes functionality to support metabolic health that is normally chargeable content • We believe the integrated glucose sensor places MiBoKo at a competitive and technological advantage compared to all other providers RAIN COMPANY. RETHINK SUSTAINABLE. 4. APPLICATIONS

MiBoKo : Launch Plan and Timings Beta program launched 29 th September 2021 offering free sensors to those who apply and are selected Innovative feature - set, with strong AI capability with continuous improvements MiBoKo will be available globally, although marketing activity will be initially focused on US, UK and key EU territories to leverage higher adoption rates for comparable weight loss programs

CONTINUOUS LACTATE MONITORING Assists in threshold maximization in performance athletes Early identification of tissue hypoperfusion or shock for aggressive early resuscitation of critically ill patients to improve the their chances of survival BODY TEMPERATURE MONITORING Gives a more accurate and large data set. For monitoring viral infections and lower limb blood circulation and tracking the effectiveness of drugs Wearable temperature sensors market is expected to register a CAGR of 8.3% during the forecast period 2021 - 2026 22 Future Product Opportunities Leveraging the BEAT ® Technology A rich portfolio of additional products to complement existing offering and contribute to increased revenues

ALCOHOL MONITORING Support personal health goals and provide warnings prior to driving. Provide physicians with information about individual’s drinking habits. Prevention of progression - to - alcohol - related disease DRUG MONITORING Monitoring the impact of drugs and personalized treatment plan for patients. Global therapeutic drug monitoring device market is expected to reach $3.37B by 2024 23 Future Product Opportunities Leveraging the BEAT ® Technology

The Team We are building a world class team So far includes senior level appointments, with experience from companies including: Dexcom, Lifescan , Roche, Abbvie , & Eli Lilly

Summary 1. UK Order fulfillment for 200,000 sensors and associated devices in progress. 2. Launch in Outside - UK territories (with CE mark) in progress 3. Next steps for BEAT ® diabetes program and adoption/marketing plans for insurers and Employers in the USA to be issued next quarter 4. FDA PMA status update to be provided as the review progresses. 5. MiBoKo metabolic health program – mass market product offering - targeted at the B2C market, backed with the sugarBEAT ® platform – Beta program launched 1. Focus is now on revenues from sales of sugarBEAT ® – and subscriptions for MiBoKo 2. Current balance sheet >$ 27m

References https:// www.nasdaq.com /articles/everything - you - need - to - know - about - noom - 2020 - 07 - 21 25 .
Exhibit 99.2
Nemaura Medical Announces Commercial Agreement with UK Licensee
Nemaura has previously placed order for 200,000 CGMs in anticipation of commercial ramp-up
Loughborough, England – September 27, 2021 (GlobeNewswire) – Nemaura Medical, Inc. (NASDAQ: NMRD)(“Nemaura” or the “Company”), a medical technology company focused on developing and commercializing non-invasive wearable diagnostic devices and supporting personalized lifestyle coaching programs, announces that it has signed a global commercial contract with MySugarWatch DuoPack Limited (“MSW-DP”).
Under terms of the deal, the CGM and sensors will be provided as Duo-Packs with prescription only medicines that are widely prescribed for people with Type 2 diabetes. The initial Duo-Pack presentation will be launched as the first of these medicines loses its patent protection from Q4 2022. MSW-DP has been granted global rights to the sugarBEAT® non-invasive continuous glucose monitor (CGM) devices and sensors, to be provided solely as Duo-Packs with these medicines, under the terms of the agreement, which Nemaura will sell to MSW-DP under the license agreement.
The prescribed medicines are designed to help control and manage Type 2 diabetes, and the CGM and sensor packs will be provided to the patient along with the medicine to help support patient care.
The agreement spans all major global territories, with the first launch planned in the UK in Q4 2022, and all major EU territories thereafter. In 2020 there were approximately 2.1 million people in total in the UK and four key European territories being prescribed this first medicine that will be made available as a Duo-Pack.
“We look forward to this exciting partnership whereupon sugarBEAT® products will support the care of patients with Type 2 diabetes. It is important that patients have a more convenient way to monitor the effectiveness of their therapeutic treatments without the hassle of repeated finger pricks and other invasive techniques,” said Nemaura CEO Dr. Faz Chowdhury. “We look forward to continuing to innovate and develop solutions for patients across the world.”
In anticipation of closing the contract with MSW-DP, and given the lengthy lead times on electronic components, Nemaura placed orders for 200,000 CGM devices in July 2021. Each patient is expected to receive a pack of four sensors each month. Thus, the Company would expect to supply 800,000 sensors each month to service just these initial 200,000 devices, assuming all 200,000 devices are placed in the hands of patients and are used as directed. The company is preparing to be able to fulfill orders to support MSW-DP for a Duo-Pack launch in Q4 of 2022.
Importantly, this contract does not preclude the direct sales of sugarBEAT® sensors in any territories by Nemaura, and the Company expects to have a parallel direct-to-consumer (DTC) offering of the sensors in those markets where the CE Mark is accepted.
About Nemaura Medical, Inc.
Nemaura Medical Inc. is a medical technology company developing and commercializing non-invasive wearable diagnostic devices. The company is currently commercializing sugarBEAT® and proBEAT™. sugarBEAT®, a CE mark approved Class IIb medical device, is a non-invasive and flexible continuous glucose monitor (CGM) providing actionable insights derived from real time glucose measurements and daily glucose trend data, which may help people with diabetes and pre-diabetes to better manage, reverse, and prevent the onset of diabetes. Nemaura has submitted a PMA (Premarket Approval Application) for sugarBEAT® to the U.S. FDA. proBEAT™ combines non-invasive glucose data processed using artificial intelligence and a digital healthcare subscription service and has been launched in the U.S. as a general wellness product as part of its BEAT®diabetes program.
The Company sits at the intersection of the global Type 2 diabetes market that is expected to reach nearly $59 billion by 2025, the $50+ billion pre-diabetic market, and the wearable health-tech sector for weight loss and wellness applications that is estimated to reach $60 billion by 2023.
For more information, please visit www.NemauraMedical.com.
Contact:
Jules Abraham
CORE IR
917-885-7378
[email protected]
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- JERAYGO (aprocitentan) recommended for approval in Europe for the treatment of resistant hypertension
- Lassila & Tikanoja plc: Interim Report 1 January–31 March 2024
- Hexatronic Group AB (publ) Interim report January – March 2024
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share