Form 8-K Biohaven Pharmaceutical For: Sep 27

NYSE:BHVN Ellie, living with migraine © 2021 Biohaven Pharmaceuticals. All rights reserved. Investor Presentation | September 2021 neuroinnovation

Disclaimer This presentation contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995, including: statements about Biohaven Pharmaceutical Holding Company Ltd. (The ”Company”) and our plans relating to the commercialization and sales of NURTEC® ODT, the potential approval and commercialization of other product candidates, the effect of the ongoing COVID-19 pandemic on the Company, the expected timing, commencement and outcomes of the Company's planned and ongoing clinical trials for our rimegepant, zavegepant (BHV-3500), BHV-3100, troriluzole, BHV-5500, verdiperstat, BHV-1100 and BHV-1200 development programs, the timing of the availability of data from our clinical trials, the timing of our planned regulatory filings, the timing of and our ability to obtain and maintain regulatory approvals for our product candidates, the clinical potential utility of our product candidates, alone and as compared to other existing or potential treatment options, and the potential advancement of our early phase programs including ARMs, MATEs and MoDEs. These statements involve substantial known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements and from the Company's current expectations. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. The forward-looking statements in this presentation represent our views as of the date of this presentation. Subsequent events and developments may cause our views to change. However, the Company does not undertake any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this presentation. Additional important factors to be considered in connection with forward-looking statements are described in the "Risk Factors" section of Biohaven's Annual Report on Form 10-K for the year ended December 31, 2020, filed with the Securities and Exchange Commission on March 1, 2021, and Biohaven's subsequent filings with the Securities and Exchange Commission. This presentation also contains market data and other statistical information that are based on independent industry publications, reports by market research firms or published independent sources. Some market data and statistical information are also based on the Company's good faith estimates, which are derived from management's knowledge of its industry and such independent sources referred to above. While the Company is not aware of any misstatements regarding the market and industry data presented herein, such data involve risks and uncertainties and are subject to change based on various factors. Safety information and the full prescribing information for Nurtec ODT can be found at Nurtec.com. BIOHAVEN INVESTOR PRESENTATION 2SEPTEMBER 2021

Innovative pharmaceutical solutions to address unmet needs in neuroscience PIPELINE INCLUDES 6 products across multiple technology platforms COMMITTED TO IMPROVING THE LIVES OF PATIENTS by advancing first-in-class and best-in-class therapies PROVEN LEADERSHIP in industry & academic settings with unique development approach FULL COMMERCIAL OPERATIONS to support bringing multiple pipeline assets to market 3

Company Achievements BIOHAVEN INVESTOR PRESENTATIONSEPTEMBER 2021 4 >875,000 TRxs of NURTEC to Date 61.5% New to Brand Share $93M Net Sales 2Q2021 $200M Total Net Sales Since Launch


The First and Only Medication Proven to Treat and Prevent Migraine SEPTEMBER 2021 BIOHAVEN INVESTOR PRESENTATION 6 ACUTE Treatment PREVENTIVE Treatment DUAL-THERAPY MIGRAINE TREATMENT Dissolving the line between acute and preventive treatment

SEPTEMBER 2021 BIOHAVEN INVESTOR PRESENTATION 7 Patient

SEPTEMBER 2021 BIOHAVEN INVESTOR PRESENTATION 8

SEPTEMBER 2021 BIOHAVEN INVESTOR PRESENTATION 9

SEPTEMBER 2021 BIOHAVEN INVESTOR PRESENTATION 10

SEPTEMBER 2021 BIOHAVEN INVESTOR PRESENTATION 11

3/ 13 3/ 27 4/ 10 4/ 24 5/ 8 5/ 22 6/ 5 6/ 19 7/ 3 7/ 17 7/ 31 8/ 14 8/ 28 9/ 11 9/ 25 10 /9 10 /2 3 11 /6 11 /2 0 12 /4 12 /1 8 1/ 1 1/ 15 1/ 29 2/ 12 2/ 26 3/ 12 3/ 26 4/ 9 4/ 23 5/ 7 5/ 21 6/ 4 6/ 18 7/ 2 7/ 16 7/ 30 8/ 13 8/ 27 9/ 10 Oral CGRP Class Continues to Show Robust Market Growth, Nurtec leads in TRx at 53.3% share and NBRx at 61.5% share KEY INSIGHTS • Nurtec TRx launch curve shows strong growth, overtaking Ubrelvy in early August, with the brand steadily growing NBRx leadership through the summer months to achieve 61.5% share • Oral CGRP market for migraine on track to reach blockbuster status in U.S. market alone 12 1/ 24 2/ 7 2/ 21 3/ 6 3/ 20 4/ 3 4/ 17 5/ 1 5/ 15 5/ 29 6/ 12 6/ 26 7/ 10 7/ 24 8/ 7 8/ 21 9/ 4 9/ 18 10 /2 10 /1 6 10 /3 0 11 /1 3 11 /2 7 12 /1 1 12 /2 5 1/ 8 1/ 22 2/ 5 2/ 19 3/ 5 3/ 19 4/ 2 4/ 16 4/ 30 5/ 14 5/ 28 6/ 11 6/ 25 7/ 9 7/ 23 8/ 6 8/ 20 9/ 3 9/ 17 Total Rx Volume (9/17)¹ Ubrelvy Nurtec ODT New to Brand Rx Share (9/17)² Ubrelvy Nurtec ODT 23,078 26,344 61.5% 1 2 Week ending Week ending SEPTEMBER 2021 BIOHAVEN INVESTOR PRESENTATION Source: 1. TRX numbers 1/24/20 – 9/17/21, IQVIA SMART, accessed 9/20/21. 2. NBRx numbers 3/13/20 – 9/17/21, IQVIA NPA-MD, accessed 9/27/21 38.5%

40.0% 45.0% 50.0% 55.0% 60.0% 4/ 23 4/ 30 5/ 7 5/ 14 5/ 21 5/ 28 6/ 04 6/ 11 6/ 18 6/ 25 7/ 2 7/ 9 7/ 16 7/ 23 7/ 30 8/ 6 8/ 13 8/ 20 8/ 27 9/ 3 9/ 10 9/ 17 Total Rx Share (9/17)¹ 35% 40% 45% 50% 55% 60% 4/ 23 4/ 30 5/ 7 5/ 14 5/ 21 5/ 28 6/ 04 6/ 11 6/ 18 6/ 25 7/ 2 7/ 9 7/ 16 7/ 23 7/ 30 8/ 6 8/ 13 8/ 20 8/ 27 9/ 3 9/ 10 9/ 17 New to Brand Rx Share (9/17)² SEPTEMBER 2021 13 Prevention Approval Prevention Approval Ubrelvy Nurtec ODT Ubrelvy Nurtec ODT Nurtec ODT Has Gained Significant Market Share in the Weeks Post Prevention Approval BIOHAVEN INVESTOR PRESENTATION Source: 1. TRX numbers 1/24/20 – 9/17/21, IQVIA SMART, accessed 9/20/21. 2. NBRx numbers 3/13/20 – 9/17/21, IQVIA NPA-MD, accessed 9/27/21 Note: Graphs include entire CGRP Oral market 61.5% 38.5% 53.3% 46.7%

476,222 3,924,354 Oral CGRPs Have Significant Growth Opportunity Ahead vs Triptans BIOHAVEN INVESTOR PRESENTATION 14 2Q21 TriptansCGRP orals3 Quarterly TRx volume 2Q21 TRx Volume vs Triptans¹ 11% SEPTEMBER 2021 Source: 1. TRX numbers cover 2Q2021, IQVIA SMART, accessed 8/4/21. 2. NBRx numbers cover 2Q2021, IQVIA XPO, accessed 7/27/21. 3. CGRP orals = Nurtec ODT & Ubrelvy. 121,508 577,863 2Q21 2Q21 TriptansCGRP orals3 Quarterly NBRx volume NBRx Volume vs Triptans² 21%

Orals CGRPs Have Driven CGRP Growth in 2020 and 2021 (vs mAbs) 0 20,000 40,000 60,000 80,000 100,000 120,000 140,000 5/25/1 8 6/25/1 8 7/25/1 8 8/25/1 8 9/25/1 8 10/2 5/18 11/2 5/18 12/2 5/18 1/25/1 9 2/25/1 9 3/25/1 9 4/25/1 9 5/25/1 9 6/25/1 9 7/25/1 9 8/25/1 9 9/25/1 9 10/2 5/19 11/2 5/19 12/2 5/19 1/25/2 0 2/25/2 0 3/25/2 0 4/25/2 0 5/25/2 0 6/25/2 0 7/25/2 0 8/25/2 0 9/25/2 0 10/2 5/20 11/2 5/20 12/2 5/20 1/25/2 1 2/25/2 1 3/25/2 1 4/25/2 1 5/25/2 1 6/25/2 1 7/25/2 1 8/25/2 1 Source: IQVIA SMART: TRx Volume to 9/10/21, accessed 9/20/21. 1. Oral CGRP = Ubrelvy, Nurtec ODT; 2. Injectable mAb = Emgality, Ajovy, Aimovig 15 CGRP Weekly TRx Oral CGRP1 Injectable mAb2 SEPTEMBER 2021 BIOHAVEN INVESTOR PRESENTATION

Significant Unmet Need SEPTEMBER 2021 BIOHAVEN INVESTOR PRESENTATION 16 PREVENTION = TOO FEW, TOO LATE DESPERATION FOR BETTER OPTIONS THE INJECTION BARRIER IS REAL 15% of people who meet clinical criteria for Preventive treatment actually use it 84% of people taking a Preventive treatment wish there was a better option 65% of people do not want to receive an injection as a preventive medication
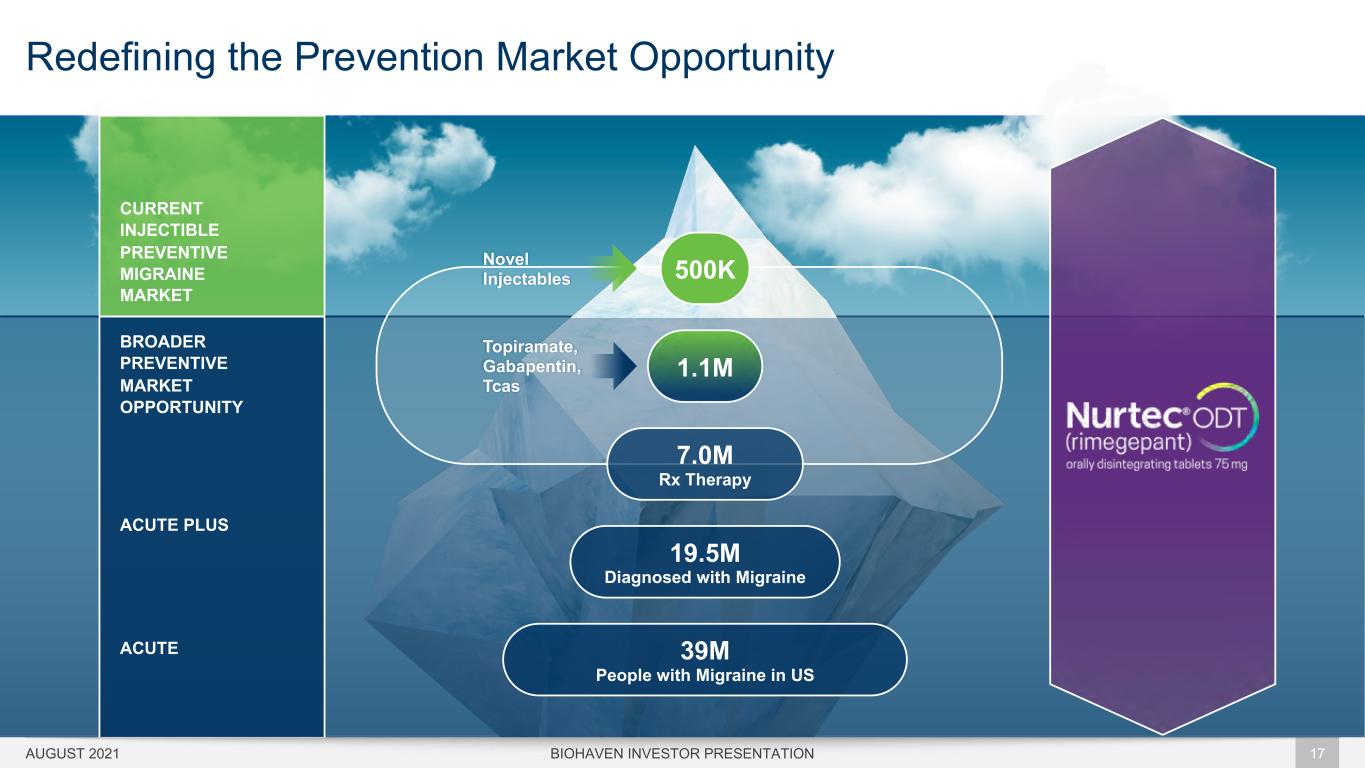
SEPTEMBER 2021 17 39M People with Migraine in US 19.5M Diagnosed with Migraine 500KNovel Injectables 1.1M Topiramate, Gabapentin, Tcas 7.0M Rx Therapy CURRENT INJECTIBLE PREVENTIVE MIGRAINE MARKET BROADER PREVENTIVE MARKET OPPORTUNITY ACUTE PLUS ACUTE AUGUST 2021 Redefining the Prevention Market Opportunity BIOHAVEN INVESTOR PRESENTATION

Perceptions of CGRPs’ Advance Over Triptans Continues to Rise Through 1Q21 BIOHAVEN INVESTOR PRESENTATION 18 13% 12% 16% 15% 12% 33% 32% 42% 38% 51% 0% 41% 51% 53% 58% Q1 2020 (n=98) Q2 2020 (n=101) Q3 2020 (n=101) Q4 2020 (n=101) Q1 2021 (n=106) Percent of Respondents Who Indicate That Recently Launched Brand Represents a Significant Advance Over Triptans Source: Spherix Real Time Dynamix, Specialist fielding Q120-Q121, PCP Q121 Specialist: How much of an advance do you consider [brand] to be over triptans? 1= No advance at all, 10=Significant advance Specialists SEPTEMBER 2021
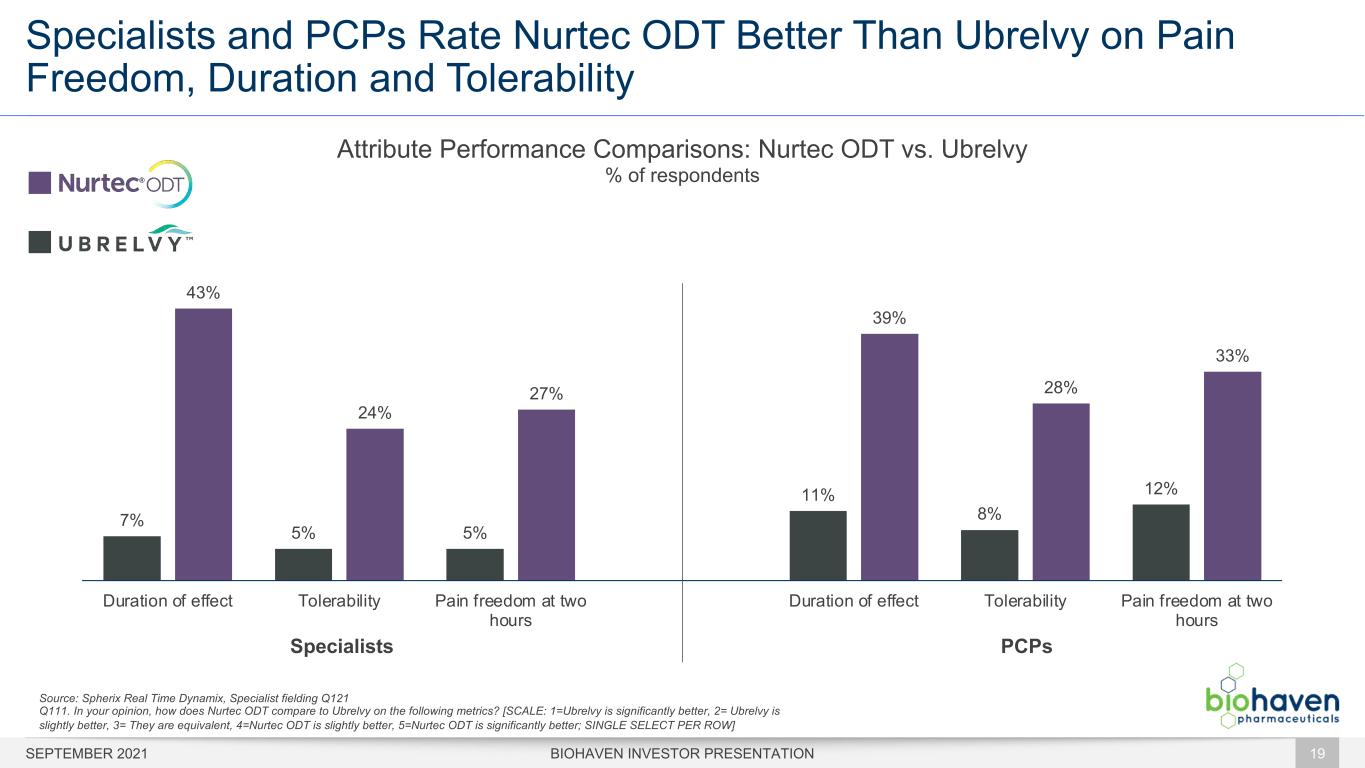
Specialists and PCPs Rate Nurtec ODT Better Than Ubrelvy on Pain Freedom, Duration and Tolerability BIOHAVEN INVESTOR PRESENTATION 19 7% 5% 5% 11% 8% 12% 43% 24% 27% 39% 28% 33% Duration of effect Tolerability Pain freedom at two hours Duration of effect Tolerability Pain freedom at two hours Attribute Performance Comparisons: Nurtec ODT vs. Ubrelvy % of respondents Source: Spherix Real Time Dynamix, Specialist fielding Q121 Q111. In your opinion, how does Nurtec ODT compare to Ubrelvy on the following metrics? [SCALE: 1=Ubrelvy is significantly better, 2= Ubrelvy is slightly better, 3= They are equivalent, 4=Nurtec ODT is slightly better, 5=Nurtec ODT is significantly better; SINGLE SELECT PER ROW] PCPsSpecialists SEPTEMBER 2021

Unparalleled CGRP-Antagonist Franchise Biohaven’s CGRP Platform SEPTEMBER 2021 BIOHAVEN INVESTOR PRESENTATION 20 ORAL Ph 3 prevention Started 1Q21 Zavegepant RAPID DISSOLVING Launched 1Q20 Nurtec® ODT MULTIPLE FORMULATIONS BHV-3100 Ph 1 started 2Q21 Advanced Leads 4 Oral Follow-Ups INTRANASAL 2nd Ph 3 trial underway 4Q20 5 ADVANCED MOLECULES Next-GEN CGRPs

Product Patent Awarded for ODT Formulation, Extends Company's IP for CGRP Platform into 2039 SEPTEMBER 2021 BIOHAVEN INVESTOR PRESENTATION 21 New patent awarded to the Company by the United States Patent and Trademark Office for our drug product, Nurtec ODT (rimegepant), in an ODT form Covers rimegepant as well as other CGRP inhibitors Patent expires in March 2039, not including possible extensions of up to 5 years

CGRP Franchise: Near-Term Value Inflection Milestones BIOHAVEN INVESTOR PRESENTATION SUBMISSION 4Q21 TOP-LINE 4Q22 MIGRAINE ACUTE APPROVED MIGRAINE PREVENTION APPROVED MIGRAINE PEDIATRIC PHASE 3 START MIGRAINE ULTRA- RAPID ACUTE INTRANASAL MIGRAINE CHRONIC ORAL QDZAVEGEPANT ZAVEGEPANT SEPTEMBER 2021 22

United Arab Emirates Acute | APPROVED Israel Acute | APPROVED Kuwait Acute | 4Q20 Europe Dual | 1Q21 Saudi Arabia Acute | 1Q21 Lebanon Acute | 3Q21 Qatar Acute | 3Q21 Bahrain Acute | 4Q20 Japan Ph 2/3 | 4Q21China Ph 3 | 4Q20 Korea Ph 3 | 4Q20 Rimegepant Global Regulatory Expansion in Migraine SEPTEMBER 2021 BIOHAVEN INVESTOR PRESENTATION 23 Acute Migraine2 APPROVALS Acute and Dual By end of 2021 9 SUBMISSIONS Oman Acute | 3Q21
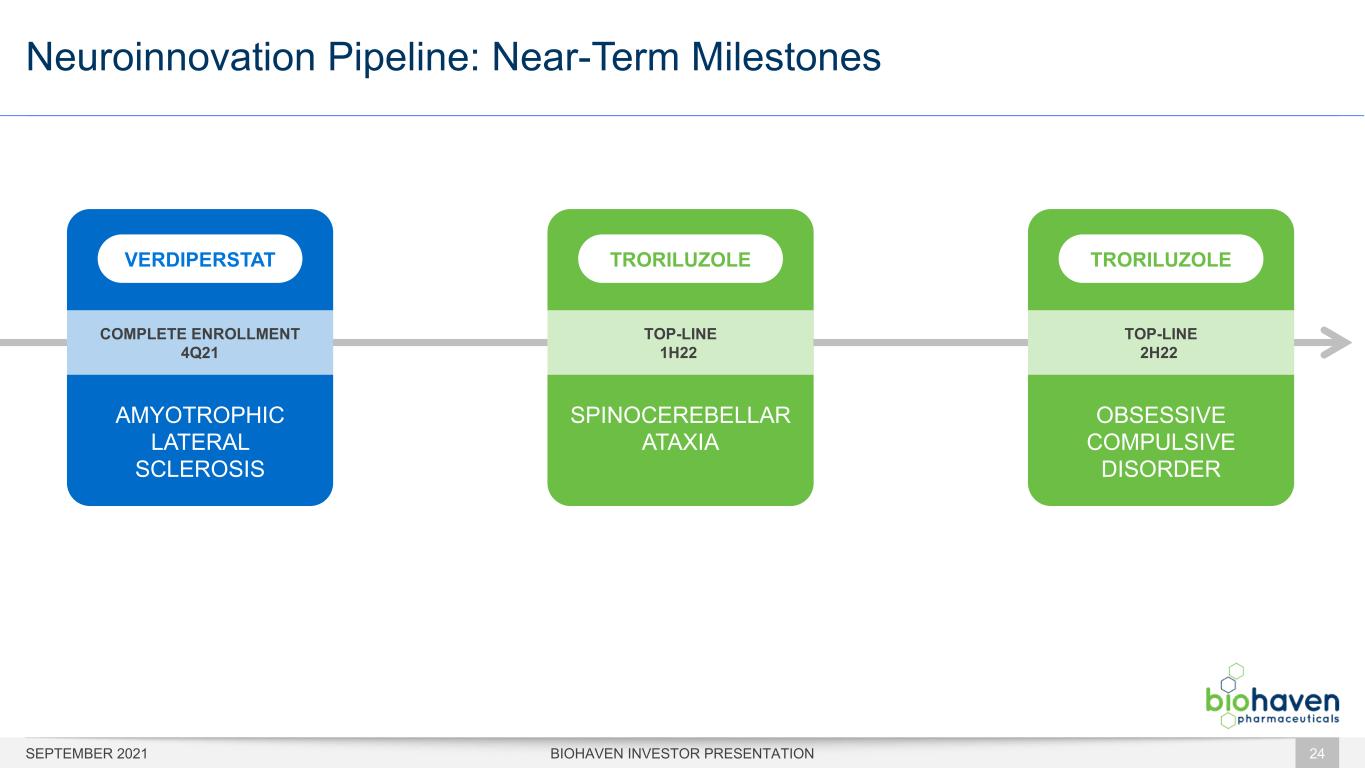
Neuroinnovation Pipeline: Near-Term Milestones BIOHAVEN INVESTOR PRESENTATION OBSESSIVE COMPULSIVE DISORDER TOP-LINE 2H22 TRORILUZOLE AMYOTROPHIC LATERAL SCLEROSIS VERDIPERSTAT COMPLETE ENROLLMENT 4Q21 SPINOCEREBELLAR ATAXIA TRORILUZOLE TOP-LINE 1H22 SEPTEMBER 2021 24

Nurtec® ODT Provides New Hope SEPTEMBER 2021 BIOHAVEN INVESTOR PRESENTATION 25 THE HALO FOR ACUTE IS REAL HCPS ARE LIKELY TO RX FOR MULTIPLE USES DESIRE FOR FLEXIBILITY ACUTE 67% of patients say they want a drug that treats and prevents 85% of HCPs will Rx more for Acute as a result of Preventive indication 77% highly likely to Rx for persistent, every other day 60% also likely to prescribe for ‘intermittent’ or ‘preemptive’ use HORMONAL CHANGES STRESSWEATHER

Broad Commercial Coverage: High Impact Commercial PBM and Health Plan Wins 89% COVERAGE 235M+ TOTAL COVERED LIVES IN ALL CHANNELS SEPTEMBER 2021 BIOHAVEN INVESTOR PRESENTATION 26

Net Sales Progress BIOHAVEN INVESTOR PRESENTATION 27 $10M $18M $35M $44M $93M 2Q20 3Q20 4Q20 1Q21 2Q21 $200M Launch to date approximate net product revenue1 SEPTEMBER 2021 1. 7/7/2021 Press Release - NURTEC® ODT achieved preliminary net product revenue of approximately $93 million for the second quarter of 2021. Launch to date net product revenue for NURTEC ODT is approximately $200 million with over 750,000 prescriptions filled since initial product launch in March 2020. .

20% 2% 17% 20% 1% 14% 27% 14% 1% 17% 16%26% 26% Throughout 2020, Nurtec Maintained a Competitive Share of Voice, With Far Less Budget BIOHAVEN INVESTOR PRESENTATION 28 Source: Nielsen AdIntel January through December Nurtec ODT Ubrelvy 2020 total share of voice 2020 total share of spend Nurtec ODT SEPTEMBER 2021
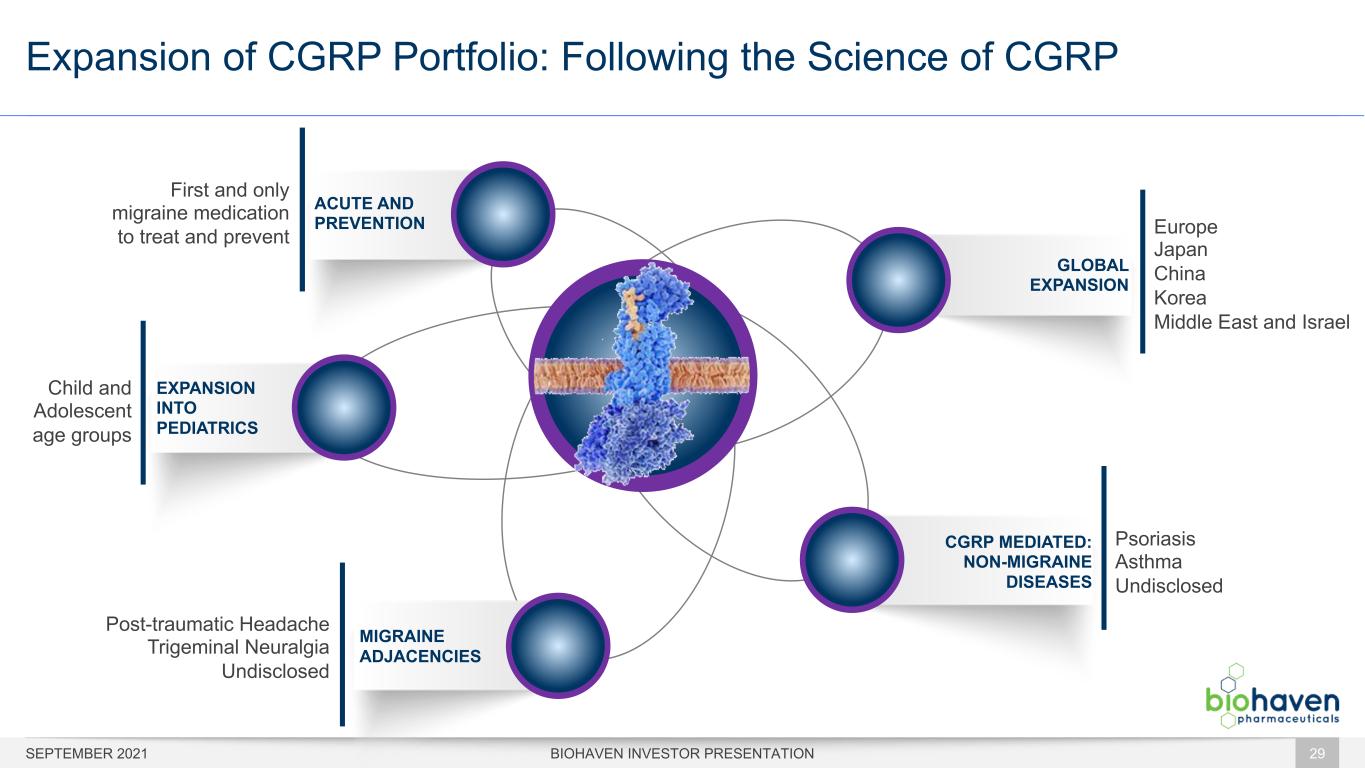
Expansion of CGRP Portfolio: Following the Science of CGRP SEPTEMBER 2021 BIOHAVEN INVESTOR PRESENTATION 29 GLOBAL EXPANSION CGRP MEDIATED: NON-MIGRAINE DISEASES Europe Japan China Korea Middle East and Israel Psoriasis Asthma Undisclosed MIGRAINE ADJACENCIES ACUTE AND PREVENTION EXPANSION INTO PEDIATRICS Child and Adolescent age groups Post-traumatic Headache Trigeminal Neuralgia Undisclosed First and only migraine medication to treat and prevent

Multiple Opportunities Across Multiple Platforms SEPTEMBER 2021 BIOHAVEN INVESTOR PRESENTATION 30 PLATFORM DRUG NAME OPPORTUNITIES CALCITONIN GENE-RELATED PEPTIDE (CGRP) Rimegepant Small molecule/NCE • US NURTEC ODT | Acute Migraine • US NURTEC ODT | Migraine Prevention • EX-US (EU, Japan, China, Korea, Middle East, Israel) • Planned Migraine Adjacencies • Planned Non-Migraine Indications • Pediatrics Zavegepant Small molecule/NCE • US |Intranasal: Acute Migraine • US | Oral: Prevention • COVID19 US | Respiratory Complications • Planned Non-Migraine Indications BHV-3100 Small molecule/NCE Phase 1 initiated GLUTAMATE Troriluzole NCE prodrug of riluzole • Spinocerebellar Ataxia (SCA) • Obsessive-Compulsive Disorder (OCD) BHV-5000/5500 NCE NMDA antagonist Neurologic/Neuropsychiatric Indications MYELOPEROXIDASE (MPO) Verdiperstat NCE oral MPO inhibitor • Amyotrophic Lateral Sclerosis (ALS) BIOHAVEN LABS UC1MT Metallothionein TDP43 MATE MODE ARM • Multiple myeloma • COVID-19 • Undisclosed NCE, new chemical entity; TDP-43: TAR DNA-binding protein 43 UC1MT: monoclonal antibody targeting extracellular metallothionein

Company Achievements BIOHAVEN INVESTOR PRESENTATIONSEPTEMBER 2021 31 >875,000 TRxs of NURTEC to Date 61.5% New to Brand Share $93M Net Sales 2Q2021 $200M Total Net Sales Since Launch

TAKE CONTROL OF MIGRAINE WITH one medication Ellie W Actual Nurtec® ODT Patient

Orphan Disease Opportunities BIOHAVEN INVESTOR PRESENTATION 33 Amyotrophic Lateral Sclerosis Spinocerebellar Ataxia 2 Devastating Diseases 2 Phase 3 Readouts Over Next Year Targeting 2 Global Orphan Drug Approvals 2022/2023 SEPTEMBER 2021
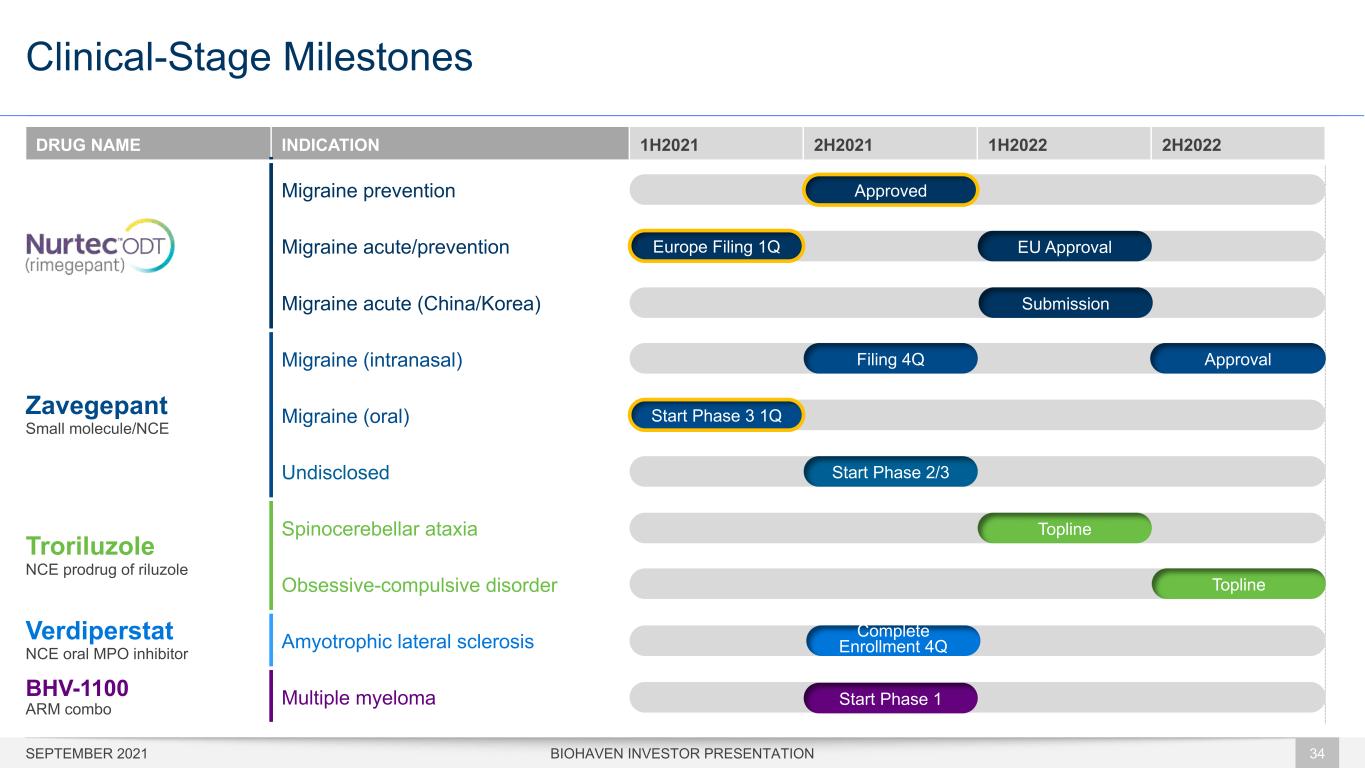
DRUG NAME INDICATION 1H2021 2H2021 1H2022 2H2022 Migraine prevention Migraine acute/prevention Migraine acute (China/Korea) Zavegepant Small molecule/NCE Migraine (intranasal) Migraine (oral) Undisclosed Troriluzole NCE prodrug of riluzole Spinocerebellar ataxia Obsessive-compulsive disorder Verdiperstat NCE oral MPO inhibitor Amyotrophic lateral sclerosis BHV-1100 ARM combo Multiple myeloma Clinical-Stage Milestones BIOHAVEN INVESTOR PRESENTATION 34 Filing 4Q Approval Topline Complete Enrollment 4Q Topline Start Phase 3 1Q Submission Start Phase 2/3 Start Phase 1 EU ApprovalEurope Filing 1Q SEPTEMBER 2021 Approved

Biohaven Labs: Chemistry and Discovery Next-Generation Bispecific Platforms for Pipeline Growth BIOHAVEN INVESTOR PRESENTATION 35 Binds liver (ASGPR) Binds protein target Bifunctional small molecule Target Binder Reactive group Directing group Binding to IgG1/2/4 antibody Conjugation and release of directing group Endogenous IgG Antibody (polyclonal) Macrophages NK cells ARM™ binds to disease target and induces antibody binding ASGPR N-acetyl galactosamine Target protein Target protein ligand ASGPR-binding terminus Target-binding terminus M A T E ™ M U L T I M O D A L A N T I B O D Y T H E R A P Y E N H A N C E R A R M ™ A N T I B O D Y R E C R U I T I N G M O L E C U L E S M o D E s M O L E C U L A R D E G R A D E R S O F E X T R A C E L L U A R P R O T E I N S IgG, Immunoglobulin G; NK, Natural Killer cells ASGPR, Asialoglycoprotein ReceptorSEPTEMBER 2021

Biohaven 2021 Growth Drivers BIOHAVEN INVESTOR PRESENTATION 36 Nurtec (rimegepant) • Treating migraine continuum • Single simple clear dosing with ODT • Significant lifecycle expansion Zavegepant • Intranasal ultrarapid formulation • Oral formulation for chronic migraine • Multiple additional nonmigraine opportunities 3100 CGRP Antagonist • Central role of CGRP across multiple areas • Both rare and common disease opportunities for all CGRP assets Troriluzole • Rare and common disease opportunities • SCA • OCD Verdiperstat • Rare and common disease opportunities • ALS Biohaven Lab Assets • Multiple innovative assets early in development: ARM, MATE, MODE • ARM initial POC in multiple myeloma SEPTEMBER 2021

THANK YOU!

Appendix

Innovative Chemistry & Discovery Initiatives to Advance Next-Generation Bispecific Compounds for Neuroscience, Immunology & Oncology

Binds liver (ASGPR) Binds protein target Bifunctional small molecule Next-Generation Bispecific Compounds – MATEs, ARMs & MoDEs BIOHAVEN INVESTOR PRESENTATION 40 Target Binder Reactive group Directing group Binding to IgG1/2/4 antibody Conjugation and release of directing group Endogenous IgG Antibody (polyclonal) Macrophages NK cells ARM™ binds to disease target and induces antibody binding ASGPR N-acetyl galactosamine Target protein Target protein ligand ASGPR-binding terminus Target-binding terminus M A T E ™ M U L T I M O D A L A N T I B O D Y T H E R A P Y E N H A N C E R A R M ™ A N T I B O D Y R E C R U I T I N G M O L E C U L E S M o D E s M O L E C U L A R D E G R A D E R S o f E X T R A C E L L U A R P R O T E I N S IgG, Immunoglobulin G; NK, Natural Killer cells ASGPR, Asialoglycoprotein ReceptorSEPTEMBER 2021

PRECLINICAL VALIDATION STUDIES: MATE PEPTIDE (HGM) ARM, MATE and MoDE Technologies: Synergies across platforms 41 Synthetic Degraders Immune Cell Engagers Antibody-based Degraders M o D E A R M M AT E Phagocytic Based Degraders Bispecific immune degraders IVIG Conjugates (HGM) MoDe Bind to target protein Bind to receptor that mediate degradation Novel protein degradation technology SyAMs / ARMs Target Binding Terminus (TBT) Binds target protein Immune binding terminus (IBT) Bind to IgG or Immune-cell receptor Linker Novel immune recruiter technology Best-in-class conjugation technology BIOHAVEN INVESTOR PRESENTATIONSEPTEMBER 2021

Chemical Reengineering of Antibodies to Enhance/ Improve their Function Site-directed, chemical conjugation to off-the-shelf therapeutic monoclonal antibodies (mAbs), or therapeutic intravenous immunoglobulin (IVIG) pooled from healthy donors. MATE™ Multimodal Antibody Therapy Enhancer Bioengineering of Antibodies to Optimize Efficacy and/or Safety BIOHAVEN INVESTOR PRESENTATION 42 Target Binder Reactive group Directing group Binding to IgG1/2/4 antibody Conjugation and release of directing group SEPTEMBER 2021

Examples of MATE applications to create Next Generation Antibodies BIOHAVEN INVESTOR PRESENTATION 43 Attachment of anti-CD3 scFV to recombinant antibodies Attachment of targeting peptide to plasma-derived antibodies (IVIG) Attachment of small molecule degrader to recombinant antibodies SEPTEMBER 2021

MATE™ HGM: COVID-19 Three Potential Mechanisms of Action Against SARS-CoV-2 BIOHAVEN INVESTOR PRESENTATION 44 Direct virus neutralization/killing: Blocks virus entry SARS-CoV-2 Spike protein FcγR binding Immune-mediated virus killing HUMAN CELL FcγR binding Long-term vaccination effect NK CELLS Infected cell killing MACROPHAGES Viral particle swallowing FcγRII-III FcγRIII BHV-1200 FcγRII DENDRITIC CELLS Viral antigen presentation to T cells 1 2 3 ACE2 receptor SEPTEMBER 2021

PRECLINICAL VALIDATION STUDIES: MATE PEPTIDE (HGM) MATE Peptide (HGM) Prototype: Convert inert IVIG into potent anti- COVID19 agent (partnered with Bill and Melinda Gates Foundation) BIOHAVEN INVESTOR PRESENTATION 45 Spike Trimer and RBD Binding Antibody Dependent Cellular Phagocytosis Viral Inhibition 0.1 1 10 100 1,000 0 500,000 1,000,000 1,500,000 2,000,000 COVID-19 Spike Proteins Binding to KPMW215 IVIG/S309 MATE COVID-19 Protein Conc. (nM) C he m ilu m in es ce nc e (R LU ) Trimer EC50 = 26.7 nM RBD EC50 = 2.36 nM 0.0001 0.01 1 100 10000 0 10 20 30 40 IC50 KPMW 215 0.07723 % F R EE B EA D S % free beads IvIg rb sera KPMW215 KPMW221 0.1 1 10 100 1000 0 50 100 150 ug/ml % in hi bi tio n KPMW215 ACE 2 KPMW215 ACE2 MATE concentration (nM) SEPTEMBER 2021

Endogenous IgG Antibody (polyclonal) Macrophages NK cells ARM binds to disease target and induces antibody binding Antibody Recruiting Molecules (ARMs) are bispecific molecules that recruit endogenous antibodies to target cancer, virally infected cells, and disease-causing microorganisms for immune destruction. ARMs are comprised of two distinct binding domains connected by a tunable linker domain: • one binding domain attaches to circulating antibodies • the other binding domain attaches to disease cells ARMs are engineered with modular components that are readily interchangeable, giving the platform tremendous flexibility to target a variety of disease cells and tumor types. By creating this “bridge”, ARMs enable endogenous antibodies to coat the cell, leading to the destruction of the disease cell through the body’s innate antibody-mediated immune mechanisms. ARM™ Antibody Recruiting Molecules BIOHAVEN INVESTOR PRESENTATION 46SEPTEMBER 2021

Uptake and degradation of target proteins mediated by MoDE bifunctional molecules MoDEs Molecular Degraders of Extracellular Proteins Utilizes the patients liver to clear unwanted, disease causing proteins BIOHAVEN INVESTOR PRESENTATION 47 ASGPR N-acetyl galactosamine Target protein Target protein ligand ASGPR-binding terminus Target-binding terminus Binds liver (ASGPR) Binds protein target Bifunctional small molecule SEPTEMBER 2021

In vitro evalutiona of cellular updake of cytokine • Cytokine levels are reduced within concentration dependence • Potent activity in nM range Analysis of cell culture supernatants after the addition of MODA-002 • Cytokine disappears from supternatant after MODA-002 treatment SUMMARY • MODA-002 eliminates >95% of cytokine in vivo within minutes • Concentration dependent and no evidence of toxicity PRECLINICAL VALIDATION STUDIES MoDEs PROTOTYPE Constructed cytokine-targeting MoDE rapidly clears targeted cytokine both in vitro and in vivo BIOHAVEN INVESTOR PRESENTATION 48 In vitro validation In vivo validation Pe rc en t C yt ok in e R em ov ed C yt ok in e in c el l c ul tu re s up er na ta nt (n g/ m L) SEPTEMBER 2021

METALLOTHIONEIN BLOCKADE A Novel Anti-Inflammatory Therapeutic
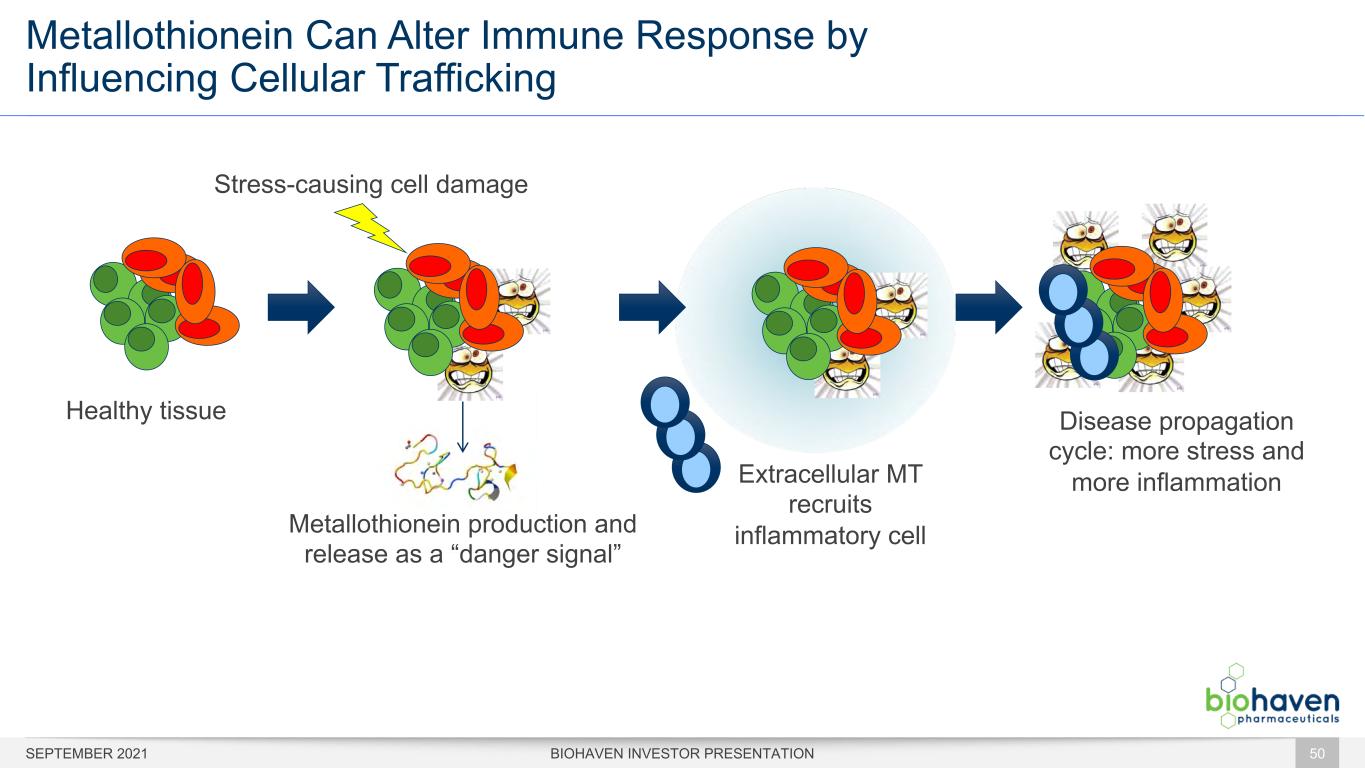
Metallothionein Can Alter Immune Response by Influencing Cellular Trafficking BIOHAVEN INVESTOR PRESENTATION Healthy tissue Stress-causing cell damage Metallothionein production and release as a “danger signal” Extracellular MT recruits inflammatory cell Disease propagation cycle: more stress and more inflammation 50SEPTEMBER 2021
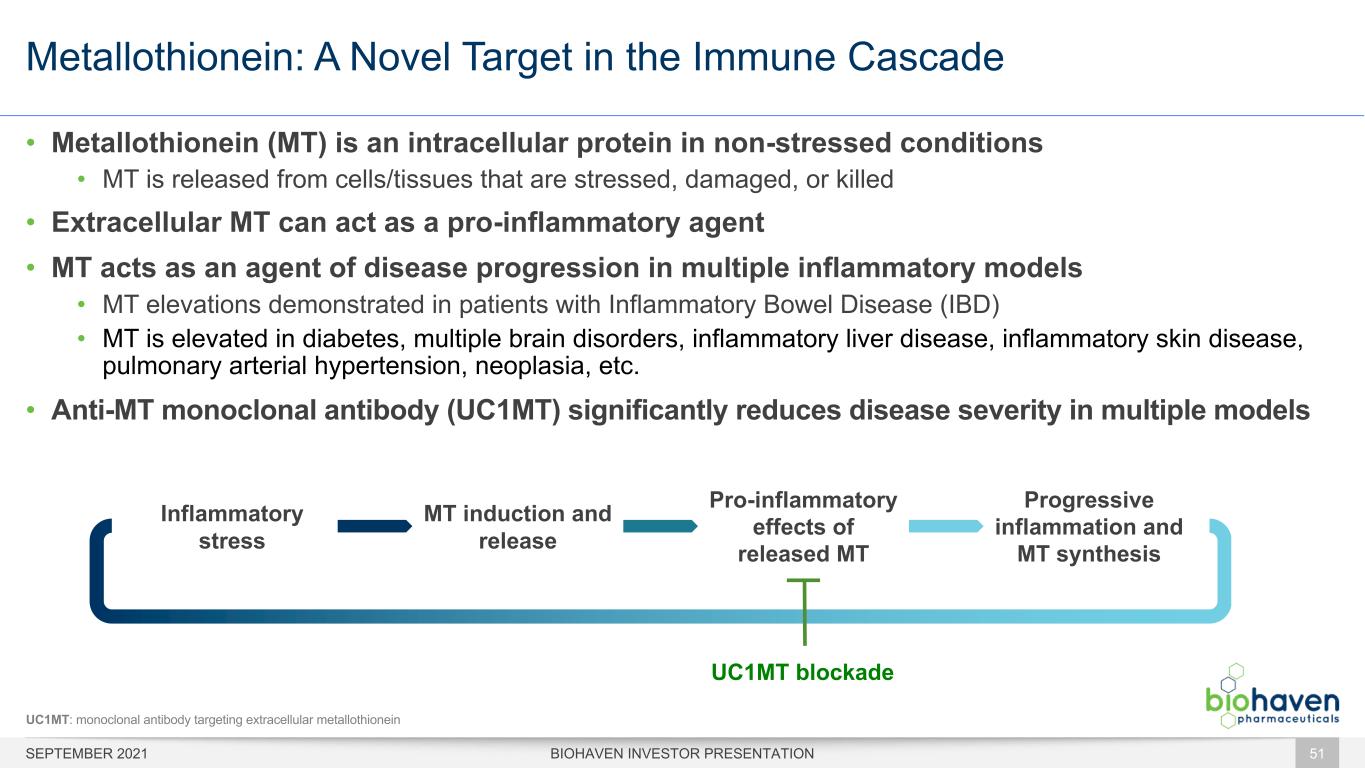
Metallothionein: A Novel Target in the Immune Cascade • Metallothionein (MT) is an intracellular protein in non-stressed conditions • MT is released from cells/tissues that are stressed, damaged, or killed • Extracellular MT can act as a pro-inflammatory agent • MT acts as an agent of disease progression in multiple inflammatory models • MT elevations demonstrated in patients with Inflammatory Bowel Disease (IBD) • MT is elevated in diabetes, multiple brain disorders, inflammatory liver disease, inflammatory skin disease, pulmonary arterial hypertension, neoplasia, etc. • Anti-MT monoclonal antibody (UC1MT) significantly reduces disease severity in multiple models Inflammatory stress MT induction and release Pro-inflammatory effects of released MT Progressive inflammation and MT synthesis UC1MT blockade BIOHAVEN INVESTOR PRESENTATION 51 UC1MT: monoclonal antibody targeting extracellular metallothionein SEPTEMBER 2021

UC1MT Reduces Inflammation in the Adoptive T Cell Transfer Model of IBD BIOHAVEN INVESTOR PRESENTATION Anti-MT performance exceeded conventional anti-TNF therapeutic UC1MT: monoclonal antibody targeting extracellular metallothionein; Anti-MT, anti-metallothionein monoclonal antibody; Anti-TNF, anti-tumor necrosis factor monoclonal antibody; IBD, inflammatory bowel disease 52SEPTEMBER 2021

UC1MT: First in Class Metallothionein Antagonist for a Novel Pro-Inflammatory Target • UC1MT is a high affinity monoclonal antibody that recognizes both mouse and human MT1 and MT2 • Confirmed the role of MT involvement in multiple disease models with better activity than anti-TNF • Evidence of molecular mechanism • Demonstrated the ability reduce markers of inflammation by UC1MT • Strong global patent protection of Anti-MT antibody therapeutic in several disease areas BIOHAVEN INVESTOR PRESENTATION UC1MT: monoclonal antibody targeting extracellular metallothionein; Anti-TNF, anti-tumor necrosis factor monoclonal antibody 53SEPTEMBER 2021

Commercial and Launch Readiness

Doing things differently… 15 sec

Key Characteristics: • Competitive share of voice • Highly targeted • Modern, ‘social first’ mindset Nurtec™ ODT ‘Surround Sound’ Marketing Plan Peer-2-Peer (Engagement & Education) Core HCP Rep Promotion >500 HCP Omni-Channel (Awareness & Engagement) Consumer Omni-Channel (Awareness & Activation) BIOHAVEN INVESTOR PRESENTATION 56SEPTEMBER 2021
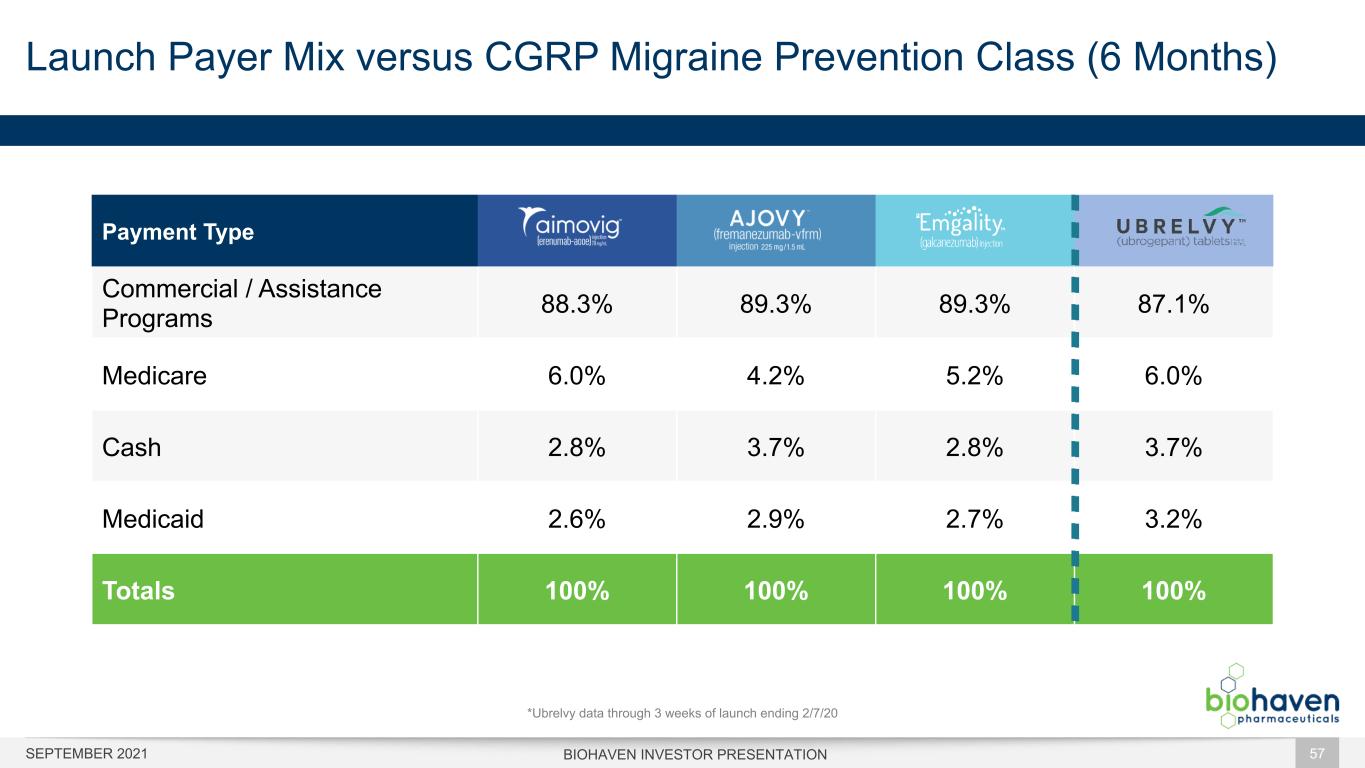
Launch Payer Mix versus CGRP Migraine Prevention Class (6 Months) BIOHAVEN INVESTOR PRESENTATION 57 Payment Type Commercial / Assistance Programs 88.3% 89.3% 89.3% 87.1% Medicare 6.0% 4.2% 5.2% 6.0% Cash 2.8% 3.7% 2.8% 3.7% Medicaid 2.6% 2.9% 2.7% 3.2% Totals 100% 100% 100% 100% *Ubrelvy data through 3 weeks of launch ending 2/7/20 SEPTEMBER 2021

Managed Markets Customer Engagements BIOHAVEN INVESTOR PRESENTATION 58SEPTEMBER 2021
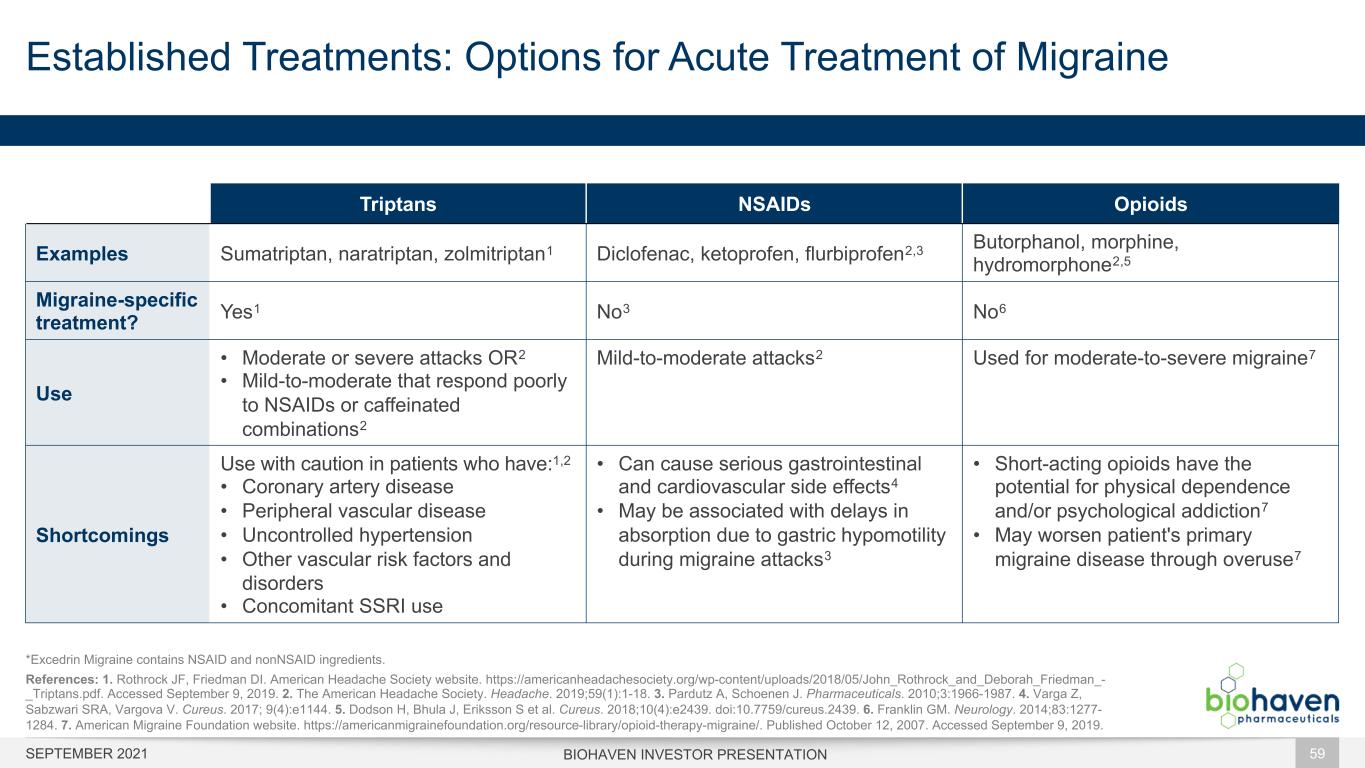
Triptans NSAIDs Opioids Examples Sumatriptan, naratriptan, zolmitriptan1 Diclofenac, ketoprofen, flurbiprofen2,3 Butorphanol, morphine, hydromorphone2,5 Migraine-specific treatment? Yes1 No3 No6 Use • Moderate or severe attacks OR2 • Mild-to-moderate that respond poorly to NSAIDs or caffeinated combinations2 Mild-to-moderate attacks2 Used for moderate-to-severe migraine7 Shortcomings Use with caution in patients who have:1,2 • Coronary artery disease • Peripheral vascular disease • Uncontrolled hypertension • Other vascular risk factors and disorders • Concomitant SSRI use • Can cause serious gastrointestinal and cardiovascular side effects4 • May be associated with delays in absorption due to gastric hypomotility during migraine attacks3 • Short-acting opioids have the potential for physical dependence and/or psychological addiction7 • May worsen patient's primary migraine disease through overuse7 Established Treatments: Options for Acute Treatment of Migraine BIOHAVEN INVESTOR PRESENTATION 59 *Excedrin Migraine contains NSAID and nonNSAID ingredients. References: 1. Rothrock JF, Friedman DI. American Headache Society website. https://americanheadachesociety.org/wp-content/uploads/2018/05/John_Rothrock_and_Deborah_Friedman_- _Triptans.pdf. Accessed September 9, 2019. 2. The American Headache Society. Headache. 2019;59(1):1-18. 3. Pardutz A, Schoenen J. Pharmaceuticals. 2010;3:1966-1987. 4. Varga Z, Sabzwari SRA, Vargova V. Cureus. 2017; 9(4):e1144. 5. Dodson H, Bhula J, Eriksson S et al. Cureus. 2018;10(4):e2439. doi:10.7759/cureus.2439. 6. Franklin GM. Neurology. 2014;83:1277- 1284. 7. American Migraine Foundation website. https://americanmigrainefoundation.org/resource-library/opioid-therapy-migraine/. Published October 12, 2007. Accessed September 9, 2019. SEPTEMBER 2021

Schedule V CNS DEPRESSION, SEROTONIN SYNDROME & MEDICATION OVERUSE HEADACHES (detoxification may be necessary) DRIVING IMPAIRMENT Advise patients not to drive for 8 hours LABEL INDICATION: ACUTE TREATMENT OF MIGRAINE REYVOW™ (Lasmiditan): Serotonin (5-HT) 1F Receptor Agonist BIOHAVEN INVESTOR PRESENTATION 60 Driving Impairment REYVOW may cause significant driving impairment. In a driving study, administration of single 50 mg, 100 mg, or 200 mg doses of REYVOW significantly impaired subjects’ ability to drive. Patient who cannot follow this advice should not take REYVOW. Prescribers and patients should be aware that patients may not be able to assess their own driving competence and the degree of impairment caused by REYVOW. 5.2 Central Nervous System Depression REYVOW may cause central nervous system (CNS) depression, including dizziness and sedation. Because of the potential for REYVOW to cause sedation, other cognitive and/or neuropsychiatric adverse reactions, and driving impairment, REYVOW should be used with caution if used in combination with alcohol or other CNS depressants. Serotonin Syndrome In clinical trials, reactions consistent with serotonin syndrome were reported in patients treated with REYVOW who were not taking any other drugs associated with serotonin syndrome. Serotonin syndrome may also occur with REYVOW during coadministration with serotonergic drugs… Medication Overuse Headache Overuse of acute migraine drugs (e.g., ergotamines, triptans, opioids, or a combination of these drugs for 10 or more days per month) may lead to exacerbation of headache… 9.1 Controlled Substance REYVOW contains lasmiditan, a Schedule V controlled substance. 9.2 Abuse …With all doses of REYVOW, subjects reported statistically significantly higher “drug liking” scores than placebo, indicating that REYVOW has abuse potential…. SEPTEMBER 2021

CGRP Receptor Antagonist Platform: Zavegepant

Zavegepant: First and Only Third-Generation, Intranasal CGRP Receptor Antagonist BIOHAVEN INVESTOR PRESENTATION 62 FIRST INTRANASAL FORMULATION OF A SMALL MOLECULE CGRP RECEPTOR ANTAGONIST • Superior chemical attributes • Potent antagonist at the human CGRP receptor • Highly soluble and high free fraction • US composition of matter protection to March 20311 • Multiple potential routes of delivery • Nasal, inhalation and oral • Zavegepant Achieved Positive Topline Results in Pivotal Phase 2/3 Study • 10 and 20 mg achieved statistical superiority to placebo on regulatory endpoints of pain freedom and freedom from most bothersome symptom at 2 hours • Zavegepant showed evidence of rapid onset with pain relief as early as 15 minutes, return to normal function at 30 min and sustained benefits through 48 hours hCGRP Ki = 23 pM Zavegepant hCGRP Ki = 32 pM Rimegepant 1. Patent expiration, not including patent term adjustment or any potential patent term extensions Zavegepant Structural Diversity vs. Rimegepant SEPTEMBER 2021

ZAVEGEPANT PHASE 2/3 — STUDY BHV3500-201, 10MG & 20MG INTRANASAL Zavegepant 10 mg and 20 mg Demonstrated Early Separation From Placebo at 15, 60 and 120 Minutes, Durable Through 24–48 Hours BIOHAVEN INVESTOR PRESENTATION 63 1. Pain Relief is defined as patients who have either mild-pain or no-pain during the specified interval. 2. Sustained Pain Relief is defined as patients having mild-to-no-pain at 2 hours and continuing to the end of the specified interval. Estimates computed using the mITT population and CMH methods (mean ± Alpha’s Standard Error). Subjects using rescue medications at or before the assessment, and subjects not providing data, are classified as failures. 0 10 20 30 40 50 60 15 min 30 min 60 min 120 min 2-24 hr 2-48 hr Single Dose of Zavegepant, No Rescue Meds // 25% 33% 54% 36% 10% 42% % o f P at ie nt s w ith P ai n R el ie f Pain Relief to 2 Hours1 & Sustained Pain Relief 2-24/48 Hours2 Post-Single Dosing with Zavegepant Intranasal Time Zavegepant 20 mg (n=402) Placebo (n=401) Zavegepant 10 mg (n=391) 27% 39% 61% 45% 15% 40% 61% 43% 18% 30% 46% 50% SEPTEMBER 2021

GLUTAMATE PLATFORM Therapies for Neurologic and Neuropsychiatric Indications
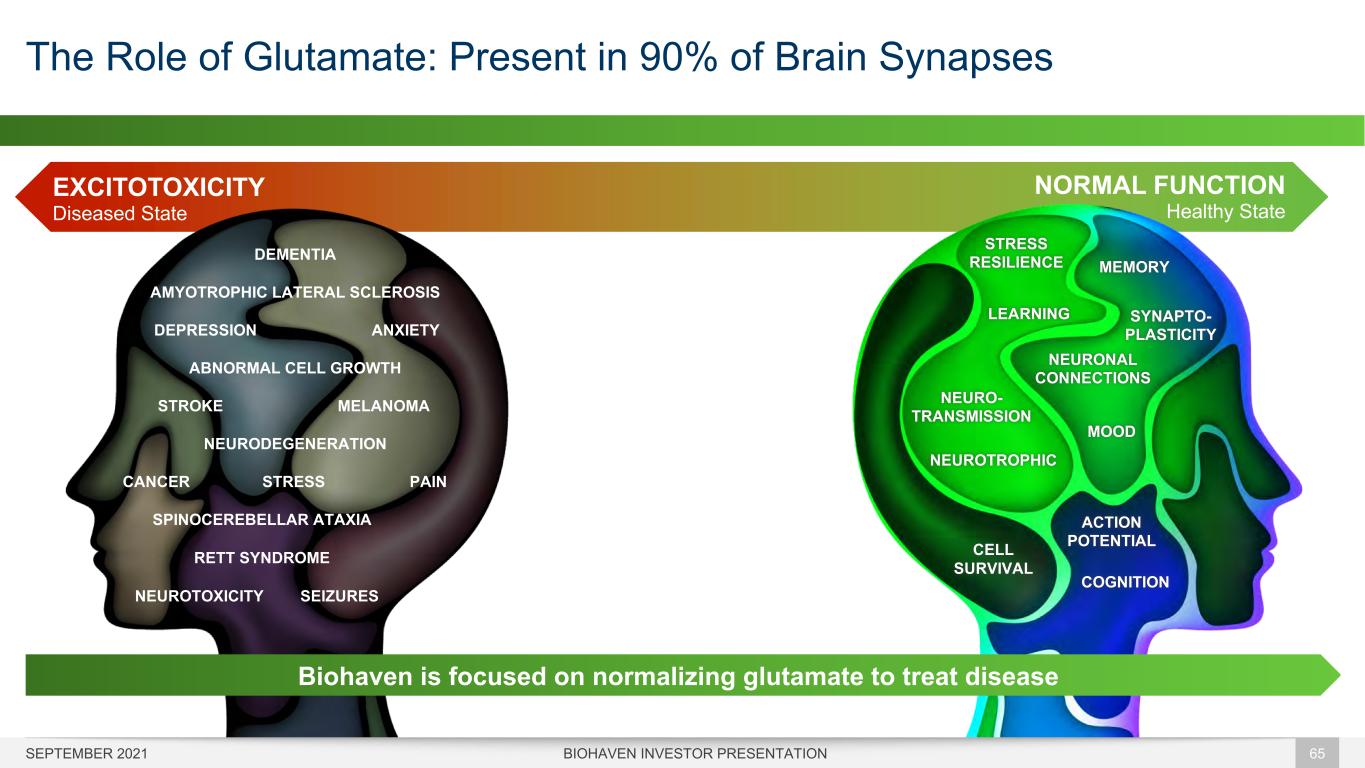
NORMAL FUNCTION Healthy State The Role of Glutamate: Present in 90% of Brain Synapses BIOHAVEN INVESTOR PRESENTATION 65 EXCITOTOXICITY Diseased State AMYOTROPHIC LATERAL SCLEROSIS SPINOCEREBELLAR ATAXIA DEMENTIA NEURODEGENERATION NEUROTOXICITY SEIZURES DEPRESSION ANXIETY STRESSCANCER PAIN STROKE MELANOMA ABNORMAL CELL GROWTH RETT SYNDROME NEURO- TRANSMISSION MEMORY CELL SURVIVAL SYNAPTO- PLASTICITY LEARNING NEUROTROPHIC STRESS RESILIENCE ACTION POTENTIAL COGNITION MOOD NEURONAL CONNECTIONS Biohaven is focused on normalizing glutamate to treat disease SEPTEMBER 2021

Glutamate Mechanisms of Action in CNS BIOHAVEN INVESTOR PRESENTATION 66 Glutamate Transporter Modulation • Troriluzole Glutamate NMDA Receptor Antagonism • BHV-5000 Third-party clinical trials provide basis for exploration of troriluzole and BHV-5000 in multiple neurologic and neuropsychiatric disorders 1 2 1 2 SEPTEMBER 2021

Riluzole (Rilutek®) is approved for the treatment of patients with amyotrophic lateral sclerosis (ALS) and proven to extend survival • Originally marketed by Sanofi, received FDA approval in 1995 • In 2013, the FDA approved the first generic versions of riluzole • Doses above 100 mg for efficacy not approved due to dose-dependent liver effects BENEFITS ü Mechanism of action well understood ü Neuroprotective, survival benefit in ALS ü Well tolerated, safe in clinical settings at approved dose LIMITATIONS ✘ Twice daily dosing, low bioavailability ✘ Fasting required for 6 hours/day, can’t be taken with meals ✘ Dose dependent LFT liability1 ✘ Marked PK variability ✘ High drug burden relative to efficacy2 ✘ Only one approved indication (ALS) Riluzole (Rilutek®): Use and Limitations BIOHAVEN INVESTOR PRESENTATION 67 1. LFT = liver function test; 2. Poor oral bioavailability results in a high liver burden relative to efficacy as ~40% is either not absorbed or is metabolized in the liver SEPTEMBER 2021
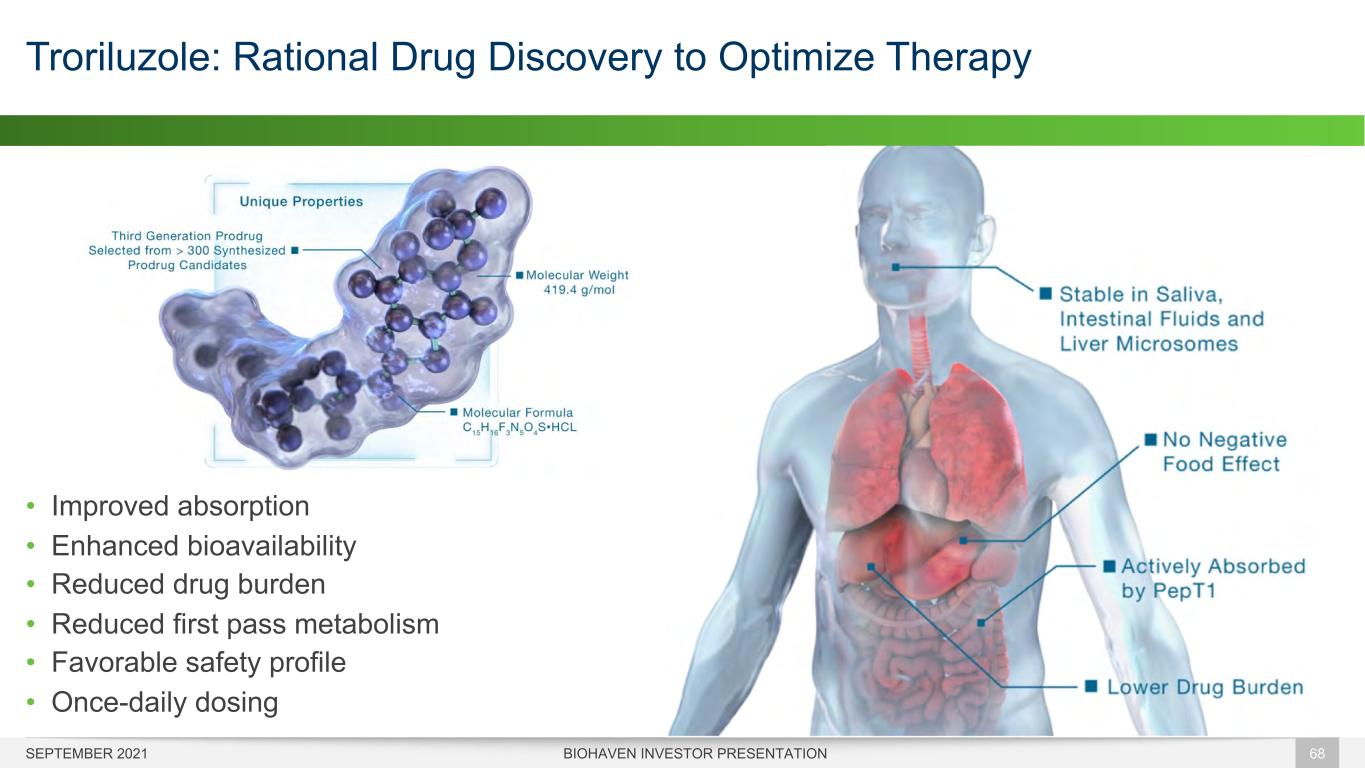
Troriluzole: Rational Drug Discovery to Optimize Therapy BIOHAVEN INVESTOR PRESENTATION 68 • Improved absorption • Enhanced bioavailability • Reduced drug burden • Reduced first pass metabolism • Favorable safety profile • Once-daily dosing SEPTEMBER 2021

Peptide Transporter 1 Enhances Absorption of Troriluzole BIOHAVEN INVESTOR PRESENTATION 69 PepT1 PepT1 = Peptide Transporter 1 ACTIVE ABSORPTION IN INTESTINAL TRACT BY PEPT1 SEPTEMBER 2021

Troriluzole: Targeted Lead-Indication Development Strategy BIOHAVEN INVESTOR PRESENTATION 70 Lead indications across an array of potential neurologic and neuropsychiatric indications Cerebellar Disorders Spinocerebellar Ataxia (SCA) Friedreich’s Ataxia Sporadic Ataxia Other Ataxias Essential Tremor* Obsessive-Compulsive & Related Disorders Obsessive-Compulsive Disorder Trichotillomania Hoarding Disorder SEPTEMBER 2021

Cerebellar Ataxias • Characterized by: • Poor balance with falls • Dysarthria / dysphagia • Incoordination of limbs • Cognitive impairment • Postural or kinetic tremor • Oculomotor dysfunction • Spinocerebellar ataxia (SCA; >40 subtypes) • Affects 1–5.6 per 100,000 (estimated 3,200–18,000 in US) • Neurodegeneration of cerebellum and input/output tracts • Relentlessly progressive, often fatal (aspiration) • Increasing disability over time (requiring wheelchair, assistance with activities of daily living) • The 6 most common genotypes caused by triplet repeat expansion mutations, sharing many phenotypic features • Genetic anticipation in some: subsequent generations affected at earlier ages and with greater severity • Other ataxias have similar core features • Can be recessive, dominant, immune, mitochondrial, post-stroke, e.g., Friedreich’s ataxia, ataxia telangiectasia, multiple system atrophy — cerebellar type BIOHAVEN INVESTOR PRESENTATION 71 Spinocerebellar Ataxia type 2 Cerebellar and brainstem volume loss No FDA-Approved Medications for SCA SEPTEMBER 2021

• Post-hoc analysis of patients enrolled in long-term extension of Phase 2b/3 troriluzole SCA trial • Primary efficacy endpoint: change from baseline in the Total SARA Score after 48 weeks • Patients from BHV4157-201 trial versus eligibility criteria matched Ashizawa Natural History cohort: • SCA Genotype • SCA1, SCA2, SCA3, SCA6 • Age at baseline • 18 to 75 years of age • Gender • SARA Score at baseline • ≥ 8 and ≤ 30, and • Initial SARA gait item score ≥ 2 Troriluzole Treated SCA Patients Treated for 1 Year Compared to Matched Ashizawa Natural History Cohort BIOHAVEN INVESTOR PRESENTATION 72 Troriluzole Study (BHV4157-206) in Spinocerebellar Ataxia (SCA) Achieved Phase 2/3 start in 1Q19 SARA: Scale for the Assessment and Rating of Ataxia SEPTEMBER 2021 SCA Patients on Troriluzole after 48 weeks vs. Natural History Cohort Le as t S qu ar es M ea n2 C ha ng e in T ot al SA R A Sc or e (fr om b as el in e ± SE ) 1. Matched on eligibility criteria 2. ANCOVA model with fixed effects for cohort, sex, & SCA genotype with age and baseline SARA scores as covariates Difference: -1.41 ± 0.411 (95% confidence interval of -2.22 to -0.60) suggesting therapeutic benefits of troriluzole (p=0.0007)

Key Entry Criteria • SCA genotypes (SCA1, SCA2, SCA3, SCA6, SCA7, SCA8, SCA10) Design • Sample size: 230 subjects • Randomization: 1:1 • Stratification: by SCA genotype • Dose: Troriluzole 200 mg QD vs. Placebo QD • Primary Outcome: Modified SARA Scale • FDA aligned outcome measure Status • Phase 3 (initiated 1Q19) • Topline data anticipated 4Q21/1Q22) Troriluzole Study BHV4157-206 Randomized Controlled Trial in SCA BIOHAVEN INVESTOR PRESENTATION 73 Troriluzole 200 mg QD Placebo QD Screening Phase 6 weeks Randomization Phase 48 weeks R Extension Phase 48 weeks Troriluzole 200 mg QD SARA: Scale for the Assessment and Rating of Ataxia SEPTEMBER 2021

• Few FDA-approved treatments • Serotonin reuptake inhibitors (SSRIs and clomipramine) are the only approved monotherapy • No new mechanisms for 30 years • With limited efficacy1,2 • <50% of patients respond well • 25–30% are refractory • Many of those categorized as ‘responders’ continue to suffer • Leading many patients to seek invasive treatments • Deep brain stimulation • Ablative neurosurgery OCD Treatments Are Limited and Need Is Vast BIOHAVEN INVESTOR PRESENTATION 74 1992 19971994 1996 1. Hirschtritt ME et al (2017). JAMA 317(13):1358-1367. 2. Pallanti S et al (2006). Prog Neuropsychopharmacol Biol Psychiatry 30(3):400-412. SEPTEMBER 2021

Accumulating Evidence for Glutamatergic Dysfunction in OCD BIOHAVEN INVESTOR PRESENTATION 75 GENES ANIMAL MODELS NEURAL NETWORKS PATIENTS IN THE CLINIC Genetic association studies: Glutamate transporter gene (SLC1A1 on chromosome 9p24) associated w/OCD1 GWAS studies are focusing attention on DLGAP1, a post-synaptic scaffolding molecule at glutamate synapse ↑Glutamatergic activity worsens OCD behaviors: SAPAP3 knockout mice (- /-) exhibit OCD-like behaviors2 SAPAP3 is an ortholog of DLGAP3, in the same family as a gene implicated by OCD GWAS studies Multiple neuroimaging studies: Increased activity in cortico-striatal-thalamic (CST) pathway3 MRS studies: Glutamate dysfunction in OCD suggested by some studies4,5 Spinal fluid studies: Increased glutamate in OCD patients6 Preliminary efficacy evidence: Glutamate modulating agents show promise in OCD patients 1. Arnold et al 2006; 2. Aida et a; 2015; 3. Baxter et al 1987, 1988, 1992; Nordhal et al 1989; Swedo et al 1989; Sawle et al 1991; Rubin et al 1992, 1995; Adams et al 1993; Perani et al 1995; Adams et al 1993; Perani et al 1995; McGuire et al 1994; Breiter et al 1996; Rausch et al 1996; 4. Rosenberg et al 2000; 5. Bolton et al 2001; 6. Chakrabarty et al 2005 SEPTEMBER 2021
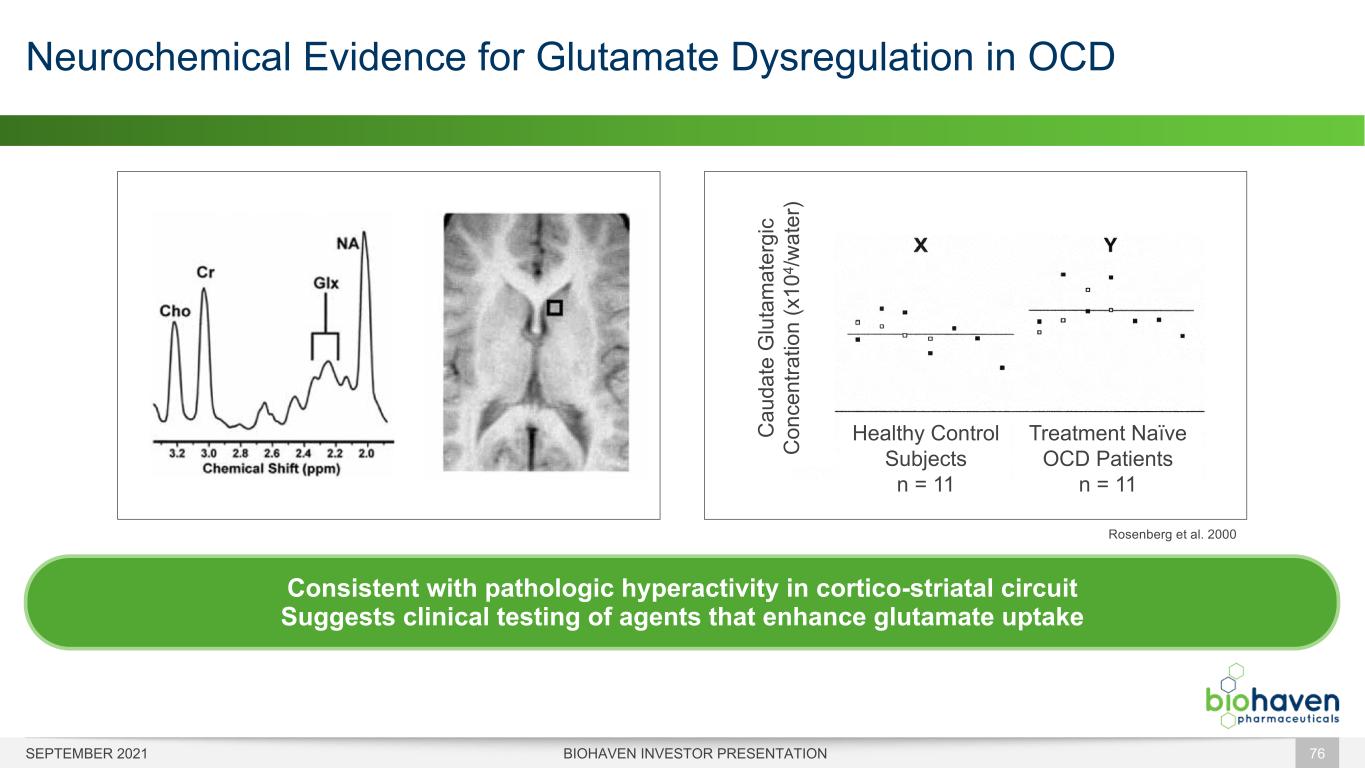
Neurochemical Evidence for Glutamate Dysregulation in OCD BIOHAVEN INVESTOR PRESENTATION 76 Rosenberg et al. 2000 Consistent with pathologic hyperactivity in cortico-striatal circuit Suggests clinical testing of agents that enhance glutamate uptake Healthy Control Subjects n = 11 Treatment Naïve OCD Patients n = 11 C au da te G lu ta m at er gi c C on ce nt ra tio n (x 10 4 /w at er ) SEPTEMBER 2021

Neurochemical Evidence for Glutamate Dysregulation in OCD BIOHAVEN INVESTOR PRESENTATION 77 Consistent with pathologic hyperactivity in cortico-striatal circuit Suggests clinical testing of agents that enhance glutamate uptake CSF Glutamate Concentrations Bhattacharyya et al. 2009 SEPTEMBER 2021
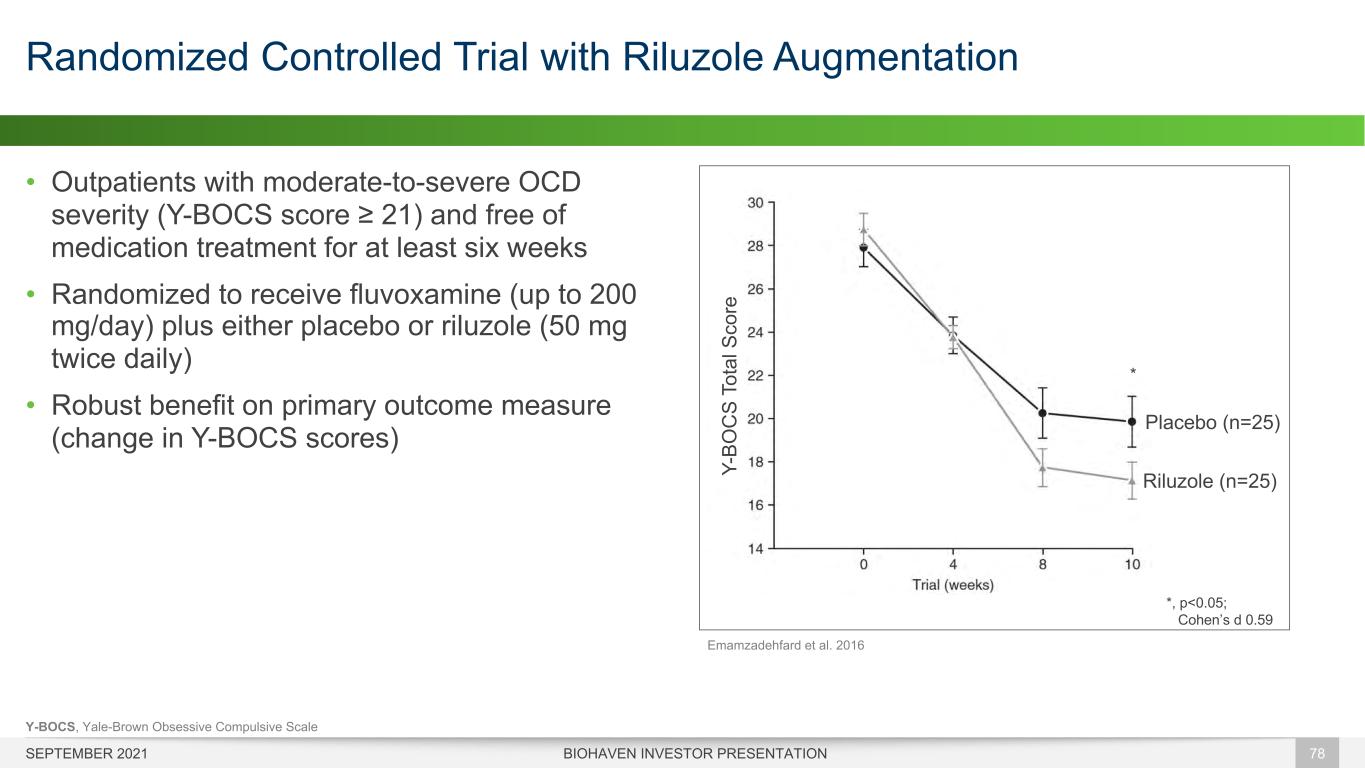
• Outpatients with moderate-to-severe OCD severity (Y-BOCS score ≥ 21) and free of medication treatment for at least six weeks • Randomized to receive fluvoxamine (up to 200 mg/day) plus either placebo or riluzole (50 mg twice daily) • Robust benefit on primary outcome measure (change in Y-BOCS scores) Randomized Controlled Trial with Riluzole Augmentation BIOHAVEN INVESTOR PRESENTATION 78 *, p<0.05; Cohen’s d 0.59 * Placebo (n=25) Riluzole (n=25)Y- BO C S To ta l S co re Y-BOCS, Yale-Brown Obsessive Compulsive Scale Emamzadehfard et al. 2016 SEPTEMBER 2021

KEY ENTRY CRITERIA • Moderate-to-severe OCD and inadequate response to standard of care DESIGN • Sample size: 226 subjects • Randomization: 1:1 • Dose: Troriluzole 200 mg QD vs. Placebo QD (in patients on standard of care) • Primary Outcome: Y-BOCS, a precedented outcome measure accepted by FDA Troriluzole Phase 2/3 Study in OCD (Completed) BIOHAVEN INVESTOR PRESENTATION 79 SOC + Troriluzole 200 mg QD SOC + Placebo QD Screening Phase 42 days Randomization Phase 12 weeks R Extension Phase 48 weeks SOC + Troriluzole 200 mg QD Subjects (n=226) with Moderate to severe OCD and inadequate response to standard of care SOC, standard of care Y-BOCS, Yale-Brown Obsessive Compulsive Scale SEPTEMBER 2021

Troriluzole Phase 2: Strong signal for efficacy and served as basis for powering two ongoing Phase 3 trials needed for approval BIOHAVEN INVESTOR PRESENTATION 80 SOC, standard of care Y-BOCS, Yale-Brown Obsessive Compulsive Scale SEPTEMBER 2021

• TWO PHASE 3 STUDIES • BHV4157-302 (US only) • BHV4157-303 (global) • KEY ENTRY CRITERIA • Moderate-to-severe OCD • Inadequate response to SOC • DESIGN (identical for each Ph 3 study) • Sample size: 600 subjects • Randomization: 1:1 • Treatment: • Troriluzole 280 mg vs. placebo, 1x daily • Adjunctive therapy to SOC • Primary Outcome: Y-BOCS • FDA accepted outcome measure • STATUS • Phase 3 initiated 4Q2020 Troriluzole Phase 3 Program in Obsessive-Compulsive Disorder BIOHAVEN INVESTOR PRESENTATION 81 SOC + Troriluzole 280 mg QD SOC + Placebo QD Screening Phase 42 days Randomization Phase 10 weeks R Extension Study (BHV4157-209) 48 weeks SOC + Troriluzole 280 mg QD Subjects (n=600) with moderate-to-severe OCD and inadequate response to SOC OCD, obsessive-compulsive disorder; SOC, standard of care; Y-BOCS, Yale-Brown Obsessive-Compulsive Scale SEPTEMBER 2021

MPO PLATFORM Therapies for Neuroinflammation
• Rare, rapidly progressive and fatal neurodegenerative disease • Prevalence: 4–8 per 100,000 • Clinical • Characterized on autopsy by degeneration of • Upper motor neurons (brain) • Lower motor neurons (brainstem/spinal cord) • Leads to weakness and paralysis of voluntary muscles • Prognosis • Most die within 3 to 5 years from onset • Treatment • FDA approved therapies • Rilutek (riluzole), Radicava (edaravone) Amyotrophic Lateral Sclerosis (ALS) BIOHAVEN INVESTOR PRESENTATION 83SEPTEMBER 2021

BIOHAVEN INVESTOR PRESENTATION 84 • Targets well accepted ALS disease mechanisms (oxidative stress / nitrosative stress, microglial activation / neuroinflammation) in a physiologically relevant manner • MPO may also play a role in increasingly recognized ALS disease mechanisms mediated by peripheral myeloid cells, including those that migrate into the brain as well as those that remain in the periphery, suggesting relevance of MPO as a therapeutic target at both sites • Human ALS patients exhibit microglial activation / neuroinflammation measured by [11C]-PBR28 TSPO PET • Verdiperstat has demonstrated the ability to decrease TSPO signal in neurodegenerative disease patients Rationale for Studying Verdiperstat in ALS JULY 2021

Neuroinflammation in ALS Indicated by [11C]-PBR28 TSPO Imaging BIOHAVEN INVESTOR PRESENTATION 85 TSPO, translocator protein - positron emission tomography Increased in ALS vs. controls Co-Localizes with Cortical Thinning Correlates with ALS Disease Severity Alshikho MJ, et al. Ann Neurol. 2018 Jun;83(6):1186-1197 SEPTEMBER 2021

SEPTEMBER 2021 BIOHAVEN INVESTOR PRESENTATION 86 Verdiperstat Selected for First-Ever Platform Trial in ALS FDG,

• DESIGN • Sample size: 160 subjects • Randomization: 3:1 • Dose: 600 mg BID vs. Placebo • Primary outcome measure: ALS Functional Rating Scale – Revised (ALS-FRS-R) • Sites: 50 sites (North East ALS Consortium) • STATUS • Study initiated 3Q2020 and recruiting rapidly • Complete Enrollment: expected 4Q21 • COLLABORATOR Verdiperstat Phase 3 Healey ALS Platform Trial Ongoing Verdiperstat 600 mg BID Placebo BID Screening Phase 6 weeks Randomization Phase 24 weeks R BIOHAVEN INVESTOR PRESENTATION 87SEPTEMBER 2021

• Licensed from AstraZeneca • BHV-3241, AZD3241 • Studied in Ph1/Ph2 in ~250 subjects • 4 Phase 1 studies in healthy volunteers • 2 Phase 2a studies in Parkinson’s disease • Generally safe and well tolerated • Achieves target engagement in plasma • ↓ MPO activity • Achieves target engagement in brain • PET shows ↓ TSPO ligand binding • Indicates ↓ microglial activation and neuroinflammation • Pivotal trials in ALS ongoing Verdiperstat Summary BIOHAVEN INVESTOR PRESENTATION 88SEPTEMBER 2021

neuroinnovation WWW.BIOHAVENPHARMA.COM | NYSE: BHVN Zydis® is a registered trademark of Catalent. Rilutek® is a registered trademark of Covis Pharma B.V. Reyvow is a trademark of Eli Lilly and Company
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Biohaven Announces Closing of Public Offering and Full Exercise of the Underwriters' Option to Purchase Additional Shares
- ROSEN, A HIGHLY RECOGNIZED LAW FIRM, Encourages Globe Life Inc. Investors to Inquire About Securities Class Action Investigation – GL
- ROSEN, A LEADING LAW FIRM, Encourages VinFast Auto Ltd. f/k/a Black Spade Acquisition Co. Investors to Secure Counsel Before Important Deadline in Securities Class Action – VFS
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share