Form 6-K InflaRx N.V. For: Oct 27
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13A-16 OR 15D-16 UNDER
THE SECURITIES EXCHANGE ACT OF 1934
For the month of October, 2021
Commission File Number: 001-38283
InflaRx N.V.
(Translation of registrant’s name into English)
Winzerlaer Str. 2
07745 Jena, Germany
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): ☐
INFLARX N.V.
On October 27, 2021, InflaRx N.V. (the “Company”) issued a press release titled “InflaRx Announces Positive Data from Third Cohort of Phase IIa Open-Label Study with Vilobelimab in Pyoderma Gangraenosum.” A copy of the press release is attached
hereto as Exhibit 99.1 and is incorporated by reference herein.
The Company will host a conference call and live audio webcast to discuss the clinical data from this study today at 8.30 am EDT / 2.30 pm CEST. A copy of the presentation that will be provided during the conference call and live audio webcast is
attached hereto as Exhibit 99.2 and is incorporated by reference herein. The presentation will also be made available in the Investors section on the Company’s website at www.inflarx.de.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
INFLARX N.V.
|
|
Date: October 27, 2021
|
By:
|
/s/ Niels Riedemann
|
|
Name:
|
Niels Riedemann
|
|
|
Title:
|
Chief Executive Officer
|
EXHIBIT INDEX
|
Exhibit No.
|
Description
|
|
|
Press Release, dated October 27, 2021
|
||
|
InflaRx N.V. “Phase IIa Pyoderma Gangraenosum Top-Line Results Conference Call” presentation
|
Exhibit 99.1

InflaRx Announces Positive Data from Third Cohort of Phase IIa Open-Label Study with Vilobelimab in Pyoderma Gangraenosum
| • |
6 out of 7 patients (85.7%) showed clinical remission (PGA score ≤ 1) and closure of target ulcer in the highest dose cohort
|
| • |
Treatment was well tolerated; no dose-related adverse events observed
|
| • |
Final post treatment observational data will be available in the first half of 2022
|
| • |
InflaRx to host conference call today at 8:30 am EDT / 2:30 pm CEST
|
Jena, Germany, October 27, 2021 – InflaRx N.V. (Nasdaq: IFRX), a clinical-stage
biopharmaceutical company developing anti-inflammatory therapeutics by targeting the complement system, announces positive data from the third cohort of patients in the Phase IIa open-label study with vilobelimab in Pyoderma Gangraenosum (PG).
“We are happy to see more patients responding with the highest dose of vilobelimab in patients with Pyoderma Gangraenosum,” commented Dr. Korinna Pilz, Chief Clinical
Development Officer of InflaRx. “There is a need for better treatment options for this painful and debilitating condition. With the good safety profile and promising efficacy results we have seen in this trial, we will seek FDA guidance on next
steps towards a pivotal program.”
As previously announced, a total of 19 patients were enrolled in the multi-center, proof-of-concept study, with seven patients enrolled in the third cohort. Over a
treatment period of 26 weeks, patients were treated biweekly with vilobelimab 800mg, 1600mg or 2400mg, after an initial run-in phase with three doses of 800mg on days 1, 4 and 8. Following the treatment period, patients continued to be observed for
a period of two months, which is ongoing for the third cohort. Per protocol, a dose increase to the next higher dosing group was possible upon disease assessment on day 57, if at least five patients in the cohort had been treated without safety
concerns and the patient was assessed with a Physician Global Assessment (PGA) score of 4 or higher. The main objectives of the study are the evaluation of the safety and efficacy of vilobelimab in patients with PG. Efficacy is being evaluated by a
responder rate defined as a PGA score of ≤3 of the target ulcer at various timepoints and time to complete closure (remission) of the target ulcer.
In the third dosing cohort at 2400mg biweekly, all seven patients were evaluated at least on the day of last drug administration. Six of the seven patients achieved
clinical remission with a PGA score of ≤ 1, which reflects a closure of the target ulcer. All patients in cohort 3 had elevated C5a levels at baseline that were continuously suppressed after initiation of vilobelimab.
InflaRx previously reported data for ten evaluable patients in the first two dose cohorts at day 99. The patient in the second dosing cohort demonstrating complete target
ulcer closure had been increased from the 1600mg dose group to the highest dose of 2400mg dose on day 57 of the study, and the ulcer closed after the dose escalation. At day 99, this patient had a PGA score of 1, and by the end of the treatment
period at day 189 had a PGA score of 0.
Overall, vilobelimab was well tolerated. From all cohorts, two patients had related severe adverse events (SAEs) that were reported: One patient experienced an erysipelas
leading to hospitalization (judged as non-related by sponsor); another developed a rash due to a delayed hypersensitivity reaction and withdrew from study (which had been previously disclosed from cohort 2). No dose-related adverse events (AEs)
were found. Overall, the observed AE profile was in line with the underlying diseases.

InflaRx will host a conference call and live audio webcast to discuss the clinical data from this study today at 8:30 am EDT / 2:30 pm CEST. To participate in the
conference call, participants may pre-register and will receive dedicated dial-in details to easily and quickly access the call: https://services.choruscall.de/DiamondPassRegistration/register?confirmationNumber=9635740&linkSecurityString=10c5273014
Alternatively, if you have not registered in advance, you can enter the conference assisted by an operator. To reach an operator, please dial one of the following
numbers:
| Germany: |
+49 (0) 69 566 037 000
|
| United Kingdom: |
+44 (0) 203 059 58 69
|
| United States: |
+1 760 294 1674
|
To access the webcast online, please use the following link:
https://services.choruscall.com/mediaframe/webcast.html?webcastid=5Pe5acAk
https://services.choruscall.com/mediaframe/webcast.html?webcastid=5Pe5acAk
After the presentation, a Q&A session will be held. Participants may submit questions via the integrated chat window online or ask questions live by phone. The
archived webcast will be made available in the Investors section of the Company’s website at www.inflarx.com.
About vilobelimab (IFX-1):
Vilobelimab is a first-in-class monoclonal anti-human complement factor C5a antibody, which highly and effectively blocks the biological activity of C5a and demonstrates
high selectivity towards its target in human blood. Thus, vilobelimab leaves the formation of the membrane attack complex (C5b-9) intact as an important defense mechanism, which is not the case for molecules blocking the cleavage of C5. Vilobelimab
has been demonstrated to control the inflammatory response driven tissue and organ damage by specifically blocking C5a as a key “amplifier” of this response in pre-clinical studies. Vilobelimab is believed to be the first monoclonal anti-C5a
antibody introduced into clinical development. Over 300 people have been treated with vilobelimab in completed clinical trials, and the antibody has been shown to be well tolerated. Vilobelimab is currently being developed for various inflammatory
indications, including hidradenitis suppurativa, ANCA-associated vasculitis and pyoderma gangraenosum, as well as severe COVID-19 and cutaneous squamous cell carcinoma (cSCC).

About Pyoderma Gangraenosum (PG):
PG is a rare and debilitating neutrophil-driven, autoinflammatory skin disease, characterized by an acute, destructive ulcerating process of the skin, primarily occurring
on the legs but also other regions of the body. PG can lead to chronic painful and difficult-to-treat wounds with long healing times. Patients frequently suffer from severe pain and frequent relapses. It typically occurs in people in their 40s and
50s. Many PG patients also suffer from other autoimmune disorders, including inflammatory bowel diseases like ulcerative colitis, arthritides like rheumatoid arthritis, and hematological diseases such as multiple myeloma.
The exact prevalence of PG is not yet known, but it is estimated that up to 50,000 patients in the US and Europe are affected by this disease. There are currently no
approved therapies for the treatment of PG in the USA or Europe. Current treatment options include the use of systemic immunosuppression in rapidly progressing cases.
C5a is a key factor for neutrophil tissue infiltration and neutrophil activation, which are believed to play a key amplifying role in PG. Thus, C5a inhibition may be able
to prevent neutrophil infiltration and activation in PG patients. Given the detected activity of C5a inhibition by vilobelimab in another neutrophil-driven skin disorder, hidradenitis suppurativa, InflaRx is currently conducting a Phase IIa
clinical study to investigate a potential benefit of vilobelimab for patients suffering from PG.
About InflaRx N.V.:
InflaRx (Nasdaq: IFRX) is a clinical-stage biopharmaceutical company focused on applying its proprietary anti-C5a technology to discover and develop first-in-class,
potent and specific inhibitors of C5a. Complement C5a is a powerful inflammatory mediator involved in the progression of a wide variety of autoimmune and other inflammatory diseases. InflaRx was founded in 2007, and the group has offices and
subsidiaries in Jena and Munich, Germany, as well as Ann Arbor, MI, USA. For further information please visit www.inflarx.com.

Contacts:
InflaRx N.V.
Jordan Zwick – Chief Strategy Officer
Jason Stewart – Strategy & Investor Relations
Email: [email protected]
Tel: +1 917-338-6523
MC Services AG
Katja Arnold, Laurie Doyle, Andreas Jungfer
Email: [email protected]
Europe: +49 89-210 2280
US: +1-339-832-0752
FORWARD-LOOKING STATEMENTS
This press release contains forward-looking statements. All statements other than statements of historical fact are forward-looking statements, which are often indicated
by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “believe,” “estimate,” “predict,” “potential” or “continue” and similar expressions. Forward-looking statements appear in a number of
places throughout this release and may include statements regarding our intentions, beliefs, projections, outlook, analyses and current expectations concerning, among other things, our ongoing and planned preclinical development and clinical
trials, including when we expect to report final data from our clinical trial of vilobelimab in PG and the safety and efficacy results of the trial; the impact of the COVID-19 pandemic on the Company; the timing of and our ability to commence and
conduct clinical trials; potential results from current or potential future collaborations; our ability to make regulatory filings, obtain positive guidance from regulators, and obtain and maintain regulatory approvals for our product candidates;
our intellectual property position; our ability to develop commercial functions; expectations regarding clinical trial data; our results of operations, cash needs, financial condition, liquidity, prospects, future transactions, growth and
strategies; the industry in which we operate; the trends that may affect the industry or us and the risks, uncertainties and other factors described under the heading “Risk Factors” in InflaRx’s periodic filings with the Securities and Exchange
Commission. These statements speak only as of the date of this press release and involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different
from any future results, performance or achievements expressed or implied by the forward-looking statements. Given these risks, uncertainties and other factors, you should not place undue reliance on these forward-looking statements, and we assume
no obligation to update these forward-looking statements, even if new information becomes available in the future, except as required by law.
Exhibit 99.2

Controlling inflammation Phase IIa Pyoderma Gangraenosum Top-Line Results Conference CallOctober 27,
2021

Important Notice and DisclaimerThis presentation has been prepared by InflaRx N.V. (“InflaRx”), a
US-Nasdaq publicly listed Dutch company having its principal place of business in Germany. This presentation is made for informational purposes only and does not constitute an offer to sell or a solicitation of an offer to buy securities. The
information set forth herein does not purport to be complete or to contain all of the information you may desire. Statements contained herein are made as of the date of this presentation unless stated otherwise, and neither the delivery of this
presentation at any time, nor any sale of securities, shall under any circumstances create an implication that the information contained herein is correct as of any time after such date or that information will be updated or revised to reflect
information that subsequently becomes available or changes occurring after the date hereof.This presentation may contain forward-looking statements. Forward-looking statements are neither historical facts nor assurances of future performance.
Instead, they are based on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, our clinical results and other future conditions. All statements other than statements of historical
facts contained in this presentation, including statements regarding future results of operations and financial position, business strategy, current and prospective product candidates, planned clinical trials and preclinical activities, product
approvals, research and development costs, current and prospective collaborations, timing and likelihood of success, expectations regarding market acceptance and size, plans and objectives of management for future operations, and future results
of anticipated product candidates, are forward-looking statements. These risks and uncertainties include those described under the heading “Risk Factors” in InflaRx’s periodic filings with the Securities and Exchange Commission. New risks and
uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein,
whether as a result of any new information, future events, changed circumstances or otherwise. Although we believe the expectations reflected in such forward-looking statements are reasonable, we can give no assurance that such expectations
will prove to be correct. Accordingly, readers are cautioned not to place undue reliance on these forward-looking statements. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking
statements.Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and InflaRx’s own internal estimates and research. While InflaRx believes
these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party
sources. In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while we believe our
own internal research is reliable, such research has not been verified by any independent source.InflaRx N.V. has an effective shelf registration statement (including a prospectus) on file with the SEC. This presentation does not constitute an
offer to sell, or the solicitation of an offer to buy, any of the Company's securities. Any offering of securities will be made only by means of a prospectus supplement, which will be filed with the SEC. In the event that, the Company conducts
an offering, you may obtain a copy of the prospectus supplement and accompanying prospectus for the offering for free by visiting EDGAR on the SEC website at www.sec.gov. Alternatively, the Company will arrange to send such information if you
request it. InflaRx n.v. | Winzerlaer Str. 2, 07745 Jena, Germany, Email: [email protected], Tel: +49-3641-508180, www.inflarx.com

InflaRx Participants 3 Korinna Pilz, M.D., M.Sc.Chief Clinical Development Officer Thomas Taapken,
Ph.D.Chief Financial Officer Niels Riedemann, M.D., Ph.D.Chief Executive Officer Jordan ZwickChief Strategy Officer Hoda Tawfik, Ph.D.Senior Program Director Dermatology

Overview of Pyoderma Gangraenosum & PGA score Study design and Results Case StudIES

Pyoderma Gangraenosum (PG) 5 An autoimmune condition with high unmet need Clinical featuresPG is a
rare but potentially life-threatening skin disorder that can lead to chronic, highly painful and difficult-to-treat wounds Many PG patients also suffer from other autoimmune disorders, such as ulcerative colitis, rheumatoid arthritis, and
hematological diseases Patients suffer from severe pain, long healing times, and frequent relapsesINCIDENCERare - Estimated that up to 50,000 patients in the US and Europe are affectedCurrent Treatment – Medical NeedNo drugs currently approved
in the US or EUFor less severe cases, topical or intralesional treatments can be used, including topical steroidsUse of systemic immunosuppression in rapidly progressing casesMixed reports about efficacy, long treatment durations, relapses are
frequently seen Strong rationale for treatment with vilobelimab: PG associated with neutrophilic skin infiltration in affected areas and lesions, potentially triggered by C5a. Photo Source: InflaRx study

PGA Score – Physician‘s Global Assessment Score 6 PGA score Description 0 Completely clear except
for possible residual hyperpigmentation 1 Almost clear very significant clearance (about 90%); however, patchy remnants of dusky erythema and/or very small ulceration 2 Marked improvement significant improvement (about 75%); however, a
small amount of disease remaining (i.e., remaining ulcers, although have decreased in size, minimal erythema and/or barely perceptible border elevation) 3 Moderate improvement intermediate between slight and marked; representing about 50%
improvement 4 Slight improvement some improvement (about 25% up to 50%); however, significant disease remaining (i.e., remaining ulcers with only minor decrease in size, erythema or border elevation) 5 No change from
baseline 6 Worse PGA Score in this trial PGA classifies physician-assessed target ulcer improvement compared to photography at Day 1 No PGA score at baseline (Day 1)PGA score is collected from Day 4 until end of studyPGA score of ≤
3 is considered clinical responsePGA score of ≤ 1 is considered clinical remission and closure of target ulcer

Phase IIa Study Design 7 800mg Group 1 N= 6 Initiation Day 1-8, 3 doses
Maintenance Day 15-43, 3 doses PGA ≤ 4 PGA > 4 800mg Q2W Individual titration Day 57-189, 9 doses 1600mg Q2W 800mg Completed Observation Day 219 & 249 800mg Group 2 N= 6 PGA ≤ 4 PGA
> 4 1600mg Q2W 2400mg Q2W 1600mg Completed 800mg Group 3 N= 7 2400mg Q2W 2400mg Ongoing Sequential enrollment of 19 patients reached in April 2021Primary endpoint: Safety Key secondary endpoints:
Responder rate defined as PGA ≤3; Time to complete closure of target ulcer Uptitration to the next dose on day 57 if PGA > 4 and at least 5 patients treated with the current dose showed no safety issues * * *

Key Eligibility Criteria 8 Diagnosis of an ulcerative form of pyoderma gangraenosum confirmed by the
investigatorMust fulfill at least 3 of 6 PG-defining criteria at screening, including but not limited to pathergy, history of papule, pustule or vesicle that rapidly ulcerated, and clinical examination (or photographic evidence) of peripheral
erythema, undermining border, and tenderness at site of ulcerationSubject has a minimum of 1 evaluable ulcer (≥2 cm2) Pyoderma gangraenosum target ulcer for more than 3 years before screeningSurgical wound debridement within the previous 2
weeks before screening Evidence of active tuberculosisInfection requiring suppressive anti-infective therapy (such as latent tuberculosis, pneumocystis, aspergillosis, cytomegalovirus, herpes simplex virus, herpes zoster and atypical
mycobacteria) Use of intravenous antibacterial, antiviral, anti-fungal, or anti-parasitic agents within 30 days before screeningAny drug treatment for pyoderma gangraenosum, including corticosteroids (>10 mg prednisone or prednisone
equivalent), intralesional steroids, cyclosporine A, biologicals and immunosuppressives (with the exception of antibiotics for wound superinfection) used within a time of 5 half-lives of the drug before screening Key Inclusion Criteria Key
Exclusion Criteria

Study Results – Group 1 (Low Dose)PGA-line plot of absolute values over time by patient (Actual days
displayed acc. to visit windows) 9 Two patients (02 and 05) achieved complete remission of target ulcerOne patient (01) with initial response and fluctuating PGAPatients 02 and 05 stopped treatment before Day 189 based on investigator
decision because of complete disease remissionPatient 03 dosed until Day 130 but stopped treatment due to Covid situation. No follow up. PGAscore Group 1 Results Six evaluable subjects: 01 02 03 04 05 06 Uptitration to 1600mg on
day 57 if PGA > 4 and at least 5 patients treated with 800mg show no safety issues. Applied to patient 06 Day 57 * Planned End ofTreatment Visit PlannedMid Visit Assessment * earlier treatment stop Planned Obs. End
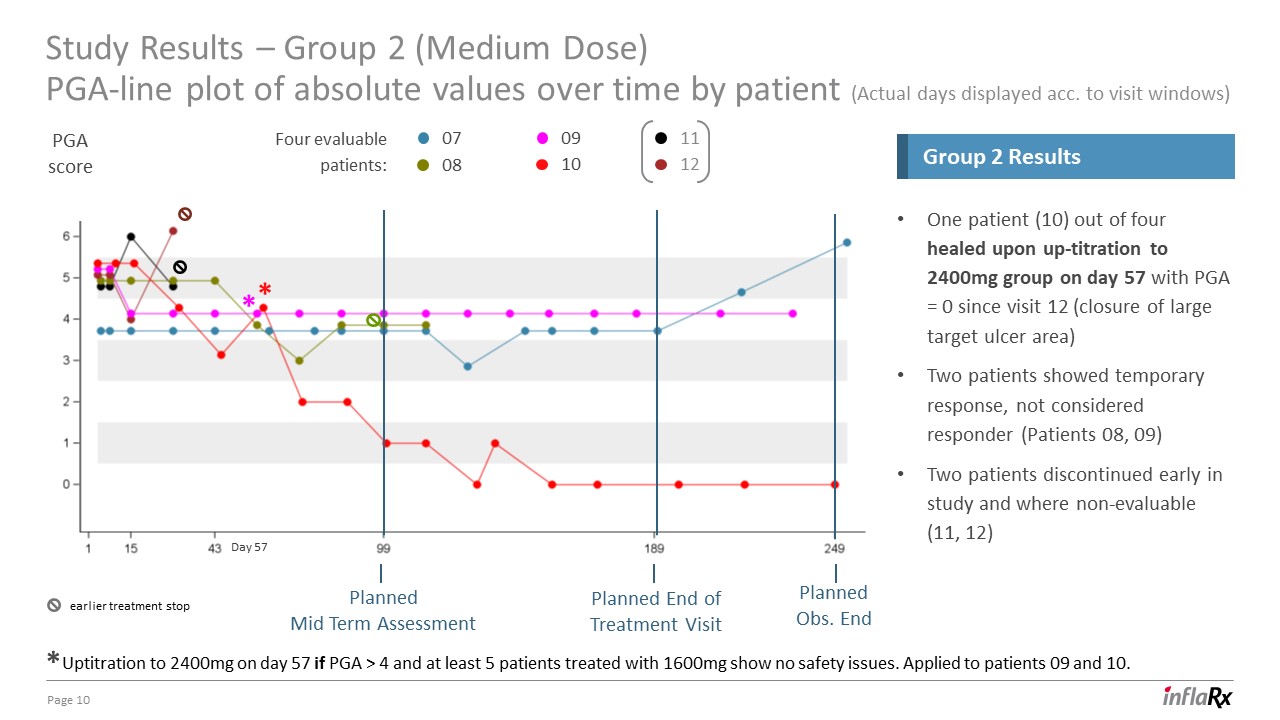
Study Results – Group 2 (Medium Dose)PGA-line plot of absolute values over time by patient (Actual days
displayed acc. to visit windows) 10 One patient (10) out of four healed upon up-titration to 2400mg group on day 57 with PGA = 0 since visit 12 (closure of large target ulcer area)Two patients showed temporary response, not considered
responder (Patients 08, 09)Two patients discontinued early in study and where non-evaluable (11, 12) Group 2 Results 07 08 09 10 11 12 Uptitration to 2400mg on day 57 if PGA > 4 and at least 5 patients treated with 1600mg show
no safety issues. Applied to patients 09 and 10. PGAscore Four evaluable patients: * * * Day 57 Planned End ofTreatment Visit PlannedMid Term Assessment Planned Obs. End earlier treatment stop
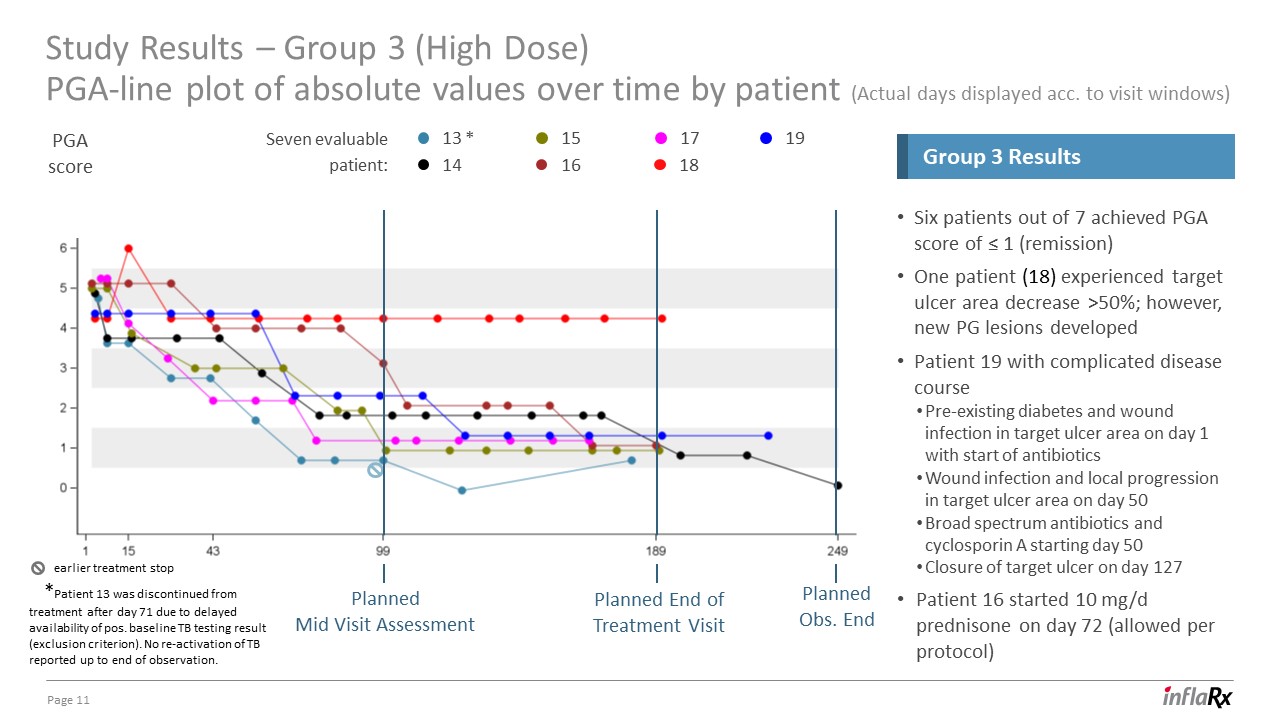
Study Results – Group 3 (High Dose)PGA-line plot of absolute values over time by patient (Actual days
displayed acc. to visit windows) 11 Six patients out of 7 achieved PGA score of ≤ 1 (remission)One patient (18) experienced target ulcer area decrease >50%; however, new PG lesions developedPatient 19 with complicated disease
coursePre-existing diabetes and wound infection in target ulcer area on day 1 with start of antibioticsWound infection and local progression in target ulcer area on day 50Broad spectrum antibiotics and cyclosporin A starting day 50 Closure of
target ulcer on day 127Patient 16 started 10 mg/d prednisone on day 72 (allowed per protocol) Group 3 Results 13 * 19 14 15 16 17 18 *Patient 13 was discontinued from treatment after day 71 due to delayed availability of pos.
baseline TB testing result (exclusion criterion). No re-activation of TB reported up to end of observation. Seven evaluable patient: PGAscore Planned End ofTreatment Visit PlannedMid Visit Assessment Planned Obs. End earlier treatment
stop

Study Results – Group 1 and Group 2C5a levels 12 Patient 10 in Group 2 reached clinical remission at
Day 99 after uptitration to 2400mg at Day 57 Clinical observations Group 1 Group 2 1* 2** 3* 4* 5** 6* 7* 11 8* 12 9 10** Patient Number Patient Number Patients 6, 9, and 10 were uptitrated on day
57 * * * * *Responder (PGA Score ≤ 3)** Responder in remission (PGA ≤1) Values not available

13 Clinical observations Six patients reached PGA≤1Patient 18 only showed minor improvement of target
ulcer but no remission 13 ** 14 ** 15 ** 16 ** 17 ** 18 19 ** Patient Number ** Responder in remission (PGA ≤1) Values not available Study Results – Group 3C5a levels

Summary and Conclusion 14 Vilobelimab Q2W shows good safety and tolerabilityEvidence for dose-dependent
drug activity in PG No infusion-related reactions observedFor 2 patients, related SAEs were reported:Erysipelas leading to hospitalization (judged as non-related by sponsor) Rash due to delayed hypersensitivity reactionObserved AE profile in
line with patients’ underlying diseasesNo dose-related AE detected Safety conclusion Out of 17 evaluable patients at end of treatment visit or day of last drug administration Clinical Remission (PGA ≤ 1): 9 patients (53%)Clinical Response
(PGA ≤ 3): 1 additional patient (6%)Slight Improvement (PGA = 4): 7 patients (41%)High Dose Group shows highest rate of target ulcer closure and clinical remission (85.7%) Clinical response conclusion We will meet with FDA to discuss
next steps

Patient Case Studies

Patient 10 Case Study 16 MH: PG since Jun 2019, Hypertension since 1998; Study Day 1: Feb
2021 Cohort 2: 1600 mg Q2W, individual uptitration to 2400 mg at D57, treatment completedPrevious PG medication: Methylprednisolone only in Jun 2019, Dapsone Jun 2019- Aug 2020, Cyclosporine Oct 2019- Aug 2020 -> ulcer healed and reappeared
soon after discontinuation of immunosuppressants Concomitant Medication: Prednisone 10 mg for PG since October 2020 Day 189, V16 (20 days after last vilo. admin.) PGA = 1 Area: 0.00 mm2 Baseline Area: 3695 mm2 Day 99 PGA = 1 Area:
0.00 mm2 Target ulcer reappeared in August 2020

Patient 14 Case Study 17 MH: PG since October 2018, Obesity since longer time (no exact day
available)Treatment Start: February 2021 Cohort 3: 2400 mg Q2W treatment completedPrevious PG medication: Ciclosporin and methylprednisolone October 2018 – September 2019, failed. Dapsone September 2020 – November 2020. Concomitant
Medication: Prednisone 10 mg since October 2018 Day 189, V16 (20 days after last IFX-1 admin.) PGA = 1 Area: calculation not yet available PG treatment history: ciclosporin, dapsone Baseline Area: 1285 mm2 Day 99 PGA = 2 Area:
0.0 mm2
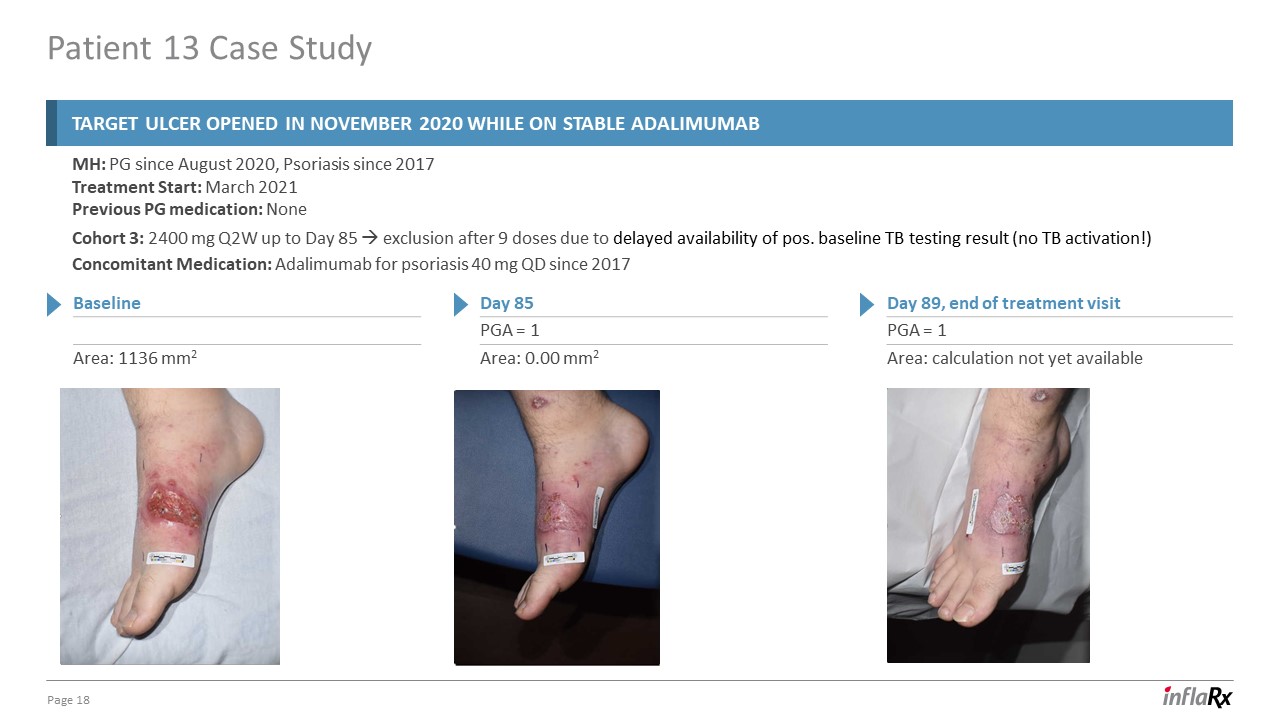
Patient 13 Case Study 18 MH: PG since August 2020, Psoriasis since 2017Treatment Start: March
2021Previous PG medication: None Cohort 3: 2400 mg Q2W up to Day 85 exclusion after 9 doses due to delayed availability of pos. baseline TB testing result (no TB activation!) Concomitant Medication: Adalimumab for psoriasis 40 mg QD since
2017 Day 89, end of treatment visit PGA = 1 Area: calculation not yet available Baseline Area: 1136 mm2 Day 85 PGA = 1 Area: 0.00 mm2 Target ulcer opened in November 2020 while on stable adalimumab

Q&A

Winzerlaer Str. 207745 Jena, GermanyEmail: [email protected]: +49-3641-508180Fax:
+49-3641-508181www.inflarx.com Jordan ZwickChief Strategy OfficerEmail: [email protected] InflaRx N.V. Investor Relations InflaRx N.V.
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- InflaRx to Participate in Capital One Securities 1st Annual Biotech/Biopharma Disrupters Event
- Dimensional Fund Advisors Ltd. : Form 8.3 - Irish Residential Properties REIT PLC
- BitFuFu Files 2023 Annual Report on Form 20-F
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share