Form 8-K TITAN PHARMACEUTICALS For: Mar 07
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of The Securities Exchange Act of 1934
Date of Report (Date of Earliest Event Reported): March 7, 2016
Titan Pharmaceuticals, Inc.
(Exact name of registrant as specified in its charter)
Delaware
(State or other jurisdiction of incorporation)
| 0-27436 | 94-3171940 |
| (Commission File Number) | (IRS Employer Identification No.) |
400 Oyster Point Blvd., Suite 505, South San Francisco, CA 94080
(Address of principal executive offices and zip code)
650-244-4990
(Registrant's telephone number including area code)
(Registrant's former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of registrant under any of the following provisions:
| ¨ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ | Soliciting material pursuant to Rule 14a-12(b) under the Exchange Act (17 CFR 240.14a-12(b)) |
| ¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Item 7.01. Regulation FD Disclosure.
On March 7, 2016, Titan Pharmaceuticals, Inc. (the “Company”) posted an updated corporate presentation on its website, a copy of which is attached hereto as Exhibit 99.1 and incorporated herein by reference.
The foregoing information, including the presentation attached hereto as an Exhibit, is being furnished pursuant to Item 7.01 of this Current Report and shall not be deemed "filed" for the purposes of Section 18 of the Securities and Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that Section. This information shall not be incorporated by reference into any registration statement pursuant to the Securities Act of 1933, as amended.
Item 8.01. Other Events.
On March 7, 2016, the Company issued a press release announcing that it had received written feedback from the FDA on the initial development plan for its proprietary ropinirole hydrochloride (HCL) implant for Parkinson's disease.
A copy of the press release is attached hereto as Exhibit 99.2 and is incorporated herein by reference.
Item 9.01. Financial Statement and Exhibits.
(d) Exhibits.
| Exhibit No. | Description | |
| 99.1 | Corporate Presentation | |
| 99.2 | Press Release dated March 7, 2016 |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| TITAN PHARMACEUTICALS, INC. | ||
| By: | /s/ Sunil Bhonsle | |
| Name: | Sunil Bhonsle | |
| Title: | President | |
Dated: March 7, 2016
Exhibit 99.1
Corporate Presentation Sunil Bhonsle Mar 2016

Forward - Looking Statements The presentation may contain “forward - looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Reference is made in particular to the description of our plans and objectives for future operations, assumptions underlying such plans and objectives and other forward - looking terminology such as “may,” “expects,” “believes,” “anticipates,” “intends,” “projects,” or similar terms, variations of such terms or the negative of such terms. Forward - looking statements are based on management’s current expectations. Actual results could differ materially from those currently anticipated and such statements involve risks and uncertainties, including, but not limited to, those risks and uncertainties relating to availability of financing, difficulties or delays in development, testing, regulatory approval, production and marketing of the Company's drug candidates, adverse side effects or inadequate therapeutic efficacy of the Company's drug candidates that could slow or prevent product development or commercialization and the uncertainty of patent protection for the Company's intellectual property or trade secrets. Titan Pharmaceuticals Company Overview | Proprietary & Confidential | ©2014 Titan Pharmaceutical, Inc. 2

Company Overview Titan Pharmaceuticals specializes in the development of treatments for select chronic diseases utilizing its proprietary ProNeura ™ technology platform • ProNeura: Proprietary Long - term Drug Delivery Platform • Provides continuous drug release and non - fluctuating medication levels over long periods of up to a year • Ideal for use in the treatment of chronic diseases for which maintenance of non - fluctuating medication levels may offer advantages over daily administration • Probuphine® for the Maintenance Treatment of Opioid Addiction • Long - acting formulation of buprenorphine providing six months of steady - state levels • January 12, 2016 FDA Advisory Committee (PDAC) vote 12 - 5 in favor of approval of Probuphine • Probuphine NDA under review by the FDA with action date of May 27, 2016 • ProNeura Product Pipeline • Ropinirole implant for treatment of Parkinson’s disease – file IND and commence proof of concept clinical study: Q4 - 2016 • Triiodothyronine (T3) implant for treatment of hypothyroidism – pre - IND meeting target date: Q4 - 2016 • Feasibility studies with additional compounds in progress Titan Pharmaceuticals Company Overview | Proprietary & Confidential | ©2014 Titan Pharmaceutical, Inc. 3

The Epidemic of Opioid Addiction • Increasingly recognized as a global epidemic by world health authorities • Addiction - a primary, chronic disease of brain reward, motivation, memory and neurobiological circuitry • Cravings, accompanied by lack of impulse control • Abstinence is rarely a successful therapeutic approach • Cycles of relapse and remission • Progressive, and often results in disability or premature death if untreated Source: National Center on Health Statistics, CDC WONDER Titan Pharmaceuticals Company Overview | Proprietary & Confidential | ©2014 Titan Pharmaceutical, Inc. 4 0 5,000 10,000 15,000 20,000 25,000 30,000 2009 2010 2011 2012 2013 2014 Deaths from Opioid Overdose Overdose Deaths
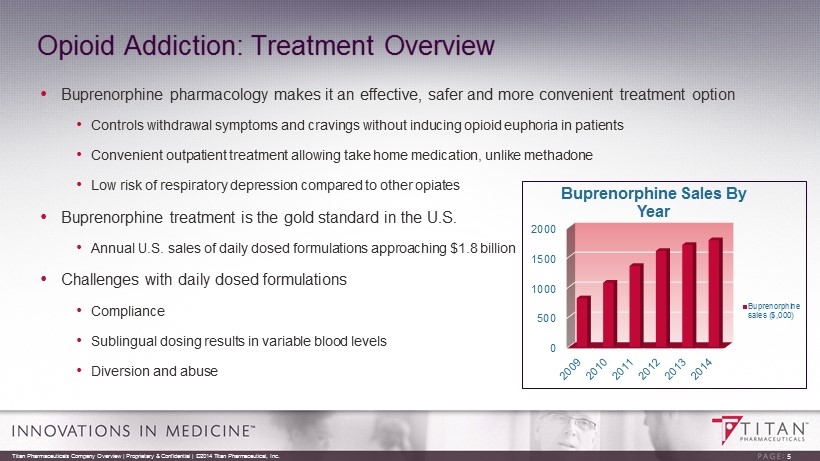
Opioid Addiction: Treatment Overview • Buprenorphine pharmacology makes it an effective, safer and more convenient treatment option • Controls withdrawal symptoms and cravings without inducing opioid euphoria in patients • Convenient outpatient treatment allowing take home medication, unlike methadone • Low risk of respiratory depression compared to other opiates • Buprenorphine treatment is the gold standard in the U.S. • Annual U.S. sales of daily dosed formulations approaching $1.8 billion • Challenges with daily dosed formulations • Compliance • Sublingual dosing results in variable blood levels • Diversion and abuse Titan Pharmaceuticals Company Overview | Proprietary & Confidential | ©2014 Titan Pharmaceutical, Inc. 5 0 500 1000 1500 2000 Buprenorphine Sales By Year Buprenorphine sales ($,000)

Buprenorphine Probuphine EVA polymer Proprietary ProNeura Technology: Probuphine Implant • Implant contains 80 mg of buprenorphine HCl, uniformly distributed throughout the ethylene vinyl acetate co - polymer (EVA) matrix • No reservoir, therefore no risk of drug dumping 6 Titan Pharmaceuticals Company Overview | Proprietary & Confidential | ©2014 Titan Pharmaceutical, Inc. 26 mm long ,2.5mm diameter Blended & Extruded

Probuphine Administration 7 Four probuphine implants are inserted subdermally in the inner, upper arm in a brief office procedure. After six months, the implants are removed and new implants may be inserted in the opposite arm.

Probuphine Value Proposition Probuphine is the first and only potential treatment for opioid dependence that provides non - fluctuating blood levels of buprenorphine around - the - clock for a period of six months 8 Titan Pharmaceuticals Company Overview | Proprietary & Confidential | ©2014 Titan Pharmaceutical, Inc. Efficacy Effective in reducing illicit opioid use Safety Non - fluctuating drug exposure over six months may provide superior safety and tolerability Compliance Treatment with implant expected to enhance compliance Ease of Use Patients dosed once every six months in an outpatient setting Diversion Limited access to implants

• Six clinical studies completed and NDA submitted in October 2012 • NDA accepted for Priority Review in January 2013 • Positive advisory committee vote (10 - 4 for approval) in March 2013 • Receipt of CRL in April 2013 requesting additional clinical testing and a few other items • Additional Phase 3 study, as requested by FDA successfully completed in June 2015 • A randomized, double blind, double dummy study evaluating a dose of four Probuphine implants in stable patients who have been receiving maintenance therapy at a dose of 8mg/day or less of buprenorphine. The primary efficacy analysis: non - inferiority comparison between the two arms • NDA resubmitted at the end of August 2015 with additional clinical data and validation of the training program through human factors testing • FDA reviewed the NDA at a Psychopharmacologic Advisory Committee meeting on January 12, 2016 • PDAC voted 12 - 5 in favor of approval of Probuphine Titan Pharmaceuticals Company Overview | Proprietary & Confidential | ©2014 Titan Pharmaceutical, Inc. 9 Probuphine Clinical and Regulatory Background

Positive Results From PRO 814 Phase 3 Study • Primary efficacy endpoint based on non - inferiority comparison of ‘responders’ following six months of treatment with either four Probuphine implants or 8 mg or less of an approved daily dosed sublingual tablet formulation of buprenorphine 10 Titan Pharmaceuticals Company Overview | Proprietary & Confidential | ©2014 Titan Pharmaceutical, Inc. 24 Weeks (Weeks 1 to 24 ) Monthly Visits Group A : Daily SL BPN ≤ 8 mg 4 placebo implants • Clinically stable patients • Daily ≤ 8 mg SL BPN for at least 90 days • Opioid - negative urine toxicology for last 90 days 2 Weeks ( 25 to 26) 24 Weeks (6 months) on Treatment Urine Toxicology & Other Study Assessments Group B : 4 Probuphine implants Daily SL placebo Randomization takes place on Day 1 (day of implant) SL BPN = sublingual buprenorphine or sublingual buprenorphine/naloxone Screening Maintenance Phase Follow - up R R Results: Probuphine SL BPN Proportion Difference(95% CI ) Superiority P value Responder - ITT population 81/84 (96%) 78/89 (88%) 0.088 (0.009, 0.167) 0.03 Responder - modified ITT 81/87 (93%) 78/89 (88%) 0.055 ( - 0.032, 0.141) 0.22

Advisory Committee Meeting (January 12, 2016) • Focus of the discussion: • Efficacy - What is the most appropriate definition of a treatment responder, given the use of supplemental “dose adjustments,” and missing or incomplete urine toxicology data ? • Safety/HF validation - Given the possibility of procedural complications, is the training program adequate to insure that clinicians will be able to safely perform Probuphine insertion and removals ? • FDA explained that it was seeking guidance from the committee on the best way to analyze the data and presented several sensitivity analyses focused on measuring efficacy • FDA recommended method for analysis included • zero months with illicit opioid use • missing urines considered positive • up to two instances of supplemental buprenorphine use in the Probuphine arm / unlimited supplemental use in the SL BPN arm • N on - inferiority endpoint met with lower bound of CL at - 0.09, once again favoring Probuphine • The safety data and HF validation information were presented and discussed by the committee along with the Risk Evaluation and Mitigation Strategy (REMS) • The committee voted 12 – 5 in favor of approval of Probuphine 11 Titan Pharmaceuticals Company Overview | Proprietary & Confidential | ©2014 Titan Pharmaceutical, Inc.

Probuphine Summary - The First of its Kind • NDA review in process with FDA action date of May 27, 2016 • Partnership with Braeburn Pharmaceuticals for development and commercialization in U.S. and Canada • Upfront: $15.75 mil; Approval: $15 mil; Sales Milestones: $165 mil; Tiered Royalties: mid teens - low 20s (%) • Braeburn has sublicensed Canadian rights to Knight Therapeutics • Analyst projections of peak sales: $300 - $500 million • U.S. patent protection to 2024 • Pursuing ex - U.S. opportunities in opioid addiction treatment • Progressing discussions with interested companies in select regions • Planning regulatory discussions following U.S. approval • Opportunity to develop Probuphine for treatment of chronic pain 12 Titan Pharmaceuticals Company Overview | Proprietary & Confidential | ©2014 Titan Pharmaceutical, Inc.

Titan: Adding Value Beyond Probuphine Proprietary ProNeura Technology Platform • Long - term drug delivery technology validated through the Probuphine program • Potential for the treatment of select chronic diseases for which low dose, long - term delivery and stable drug levels may offer advantages over other forms of administration • Product development programs in progress: • Ropinirole implant for the treatment of Parkinson’s disease (PD) • Triiodothyronine (T3) implant for the treatment of hypothyroidism • Conducting feasibility evaluation of additional compounds in other chronic disease settings to add to the product pipeline • Benign Prostate Hyperplasia, Pre - exposure prophylaxis therapy for HIV - 1 prevention, Type 2 Diabetes, Attention Deficit Hyperactivity Disorder 13 Titan Pharmaceuticals Company Overview | Proprietary & Confidential | ©2014 Titan Pharmaceutical, Inc.

. Titan Pharmaceuticals Company Overview | Proprietary & Confidential | ©2014 Titan Pharmaceutical, Inc. 14 Definition Characterized by the loss of dopamine, which alters activity in the brain region impacting movement and motor function Treatment Treated with drugs designed to replace or mimic dopamine in the brain Following several years of chronic treatment, these drugs lose their benefit and trigger serious side effects in up to 80% of patients Research Pulsatile dopaminergic stimulation from current oral treatment may cause motor side effects Continuous dopaminergic stimulation (CDS) by systemic infusion of dopamine replacement medications has been shown to palliate these motor complications and also delay or prevent the onset of dyskinesias Product Opportunity Titan’s ProNeura drug delivery technology has the potential to deliver continuous non - fluctuating levels of dopamine agonists and provide CDS for three months or longer from a single treatment Parkinson’s Disease Overview

Parkinson’s Disease Population Increasing Worldwide 15 Based on information from Titan and other sources believed to be reliable and prepared exclusively for Titan. Woodside Capita l P artners is not responsible for any use that Titan may make of this material. Titan Pharmaceuticals Company Overview | Proprietary & Confidential | ©2014 Titan Pharmaceutical, Inc.

Parkinson’s Disease - Therapeutics Market • As many as one million people in the US affected by Parkinson’s disease • The number is expected to almost double by 2030 because of the aging population • About 60,000 newly diagnosed for Parkinson’s disease annually • More than 23,000 die from Parkinson’s disease each year 16 Titan Pharmaceuticals Company Overview | Proprietary & Confidential | ©2014 Titan Pharmaceutical, Inc. * GlobalData; **Parkinson’s Action Network, National Center for Health Statistics; “The Current and Projected Economic Burden of Parkinson’s Disease in the United States” Movement Disorders, March 2013 Based on information from Titan and other sources believed to be reliable and prepared exclusively for Titan. Woodside Capita l P artners is not responsible for any use that Titan may make of this material. Sales of Dopamine Agonists, U.S.* Year Total Sales % DA $ DA 2012 $1.1 Billion 26% $286 Million 2022 $2.3 Billion 18% $414 Million Cost to American Society ** $14.4 Billion Annually Treatment Costs $8.1 Billion Indirect Costs $6.3 Billion If costs continue to rise they will double by 2040

ProNeura Parkinson’s Disease Program • Ropinirole (Requip ® ), a generic dopamine agonist marketed by GSK for PD, was evaluated in a Parkinsonian primate model using ProNeura drug delivery platform • Results presented in June 2015 - 19th International Congress of Parkinson's Disease and Movement Disorders • Sustained plasma ropinirole levels for several months following implantation • No local skin irritation at implant site • Controlled PD symptoms without triggering dyskinesias • Ropinirole implant program status • Implant formulation selected for clinical development • Non - clinical development program defined and initial clinical study design established • FDA feedback received on pre - IND meeting briefing material and now initiating the required non - clinical studies • On target to file IND in Q4 2016 followed by the initial pharmacokinetic and proof of concept clinical study 17 Titan Pharmaceuticals Company Overview | Proprietary & Confidential | ©2014 Titan Pharmaceutical, Inc.

. Titan Pharmaceuticals Company Overview | Proprietary & Confidential | ©2014 Titan Pharmaceutical, Inc. 18 Definition Hypothyroidism is a disorder that occurs when the thyroid gland does not make enough thyroid hormone to meet the body’s needs Thyroid hormone regulates metabolism and affects nearly every organ in the body Cause Primary hypothyroidism is caused by a problem with the thyroid gland Secondary hypothyroidism occurs when another problem interferes with the thyroid's ability to produce hormones, such as the inability of the pituitary gland and hypothalamus to produce hormones that trigger the release of thyroid hormone Treatment Estimated number of people affected with hypothyroidism in the U.S. – 14 million Patients diagnosed using standard blood tests and receive treatment typically consisting of synthetic prohormone thyroxine (T4) given orally once a day, which in turn is converted by the body to the active triiodothyronine (T3) Based upon symptoms and blood tests it is estimated that 15 - 20% of patients are not adequately treated with T4 and physicians typically add an oral T3 dose to the treatment regimen Product Opportunity Oral T3 treatment is effective but comes with potential side effects like headache, nervousness, irritability, depression and arrhythmia caused by the peak and trough blood level fluctuations Titan’s ProNeura drug delivery platform has the potential to deliver continuous, non - fluctuating levels of T3 and provide a stable blood level for several months following a single treatment. Hypothyroidism Disease Overview

ProNeura Hypothyroidism Program • Completed initial formulation development of the implant and conducted in - vitro and in - vivo drug release studies to further define implant formulation • In - vivo non - clinical studies in progress evaluating implant formulations for drug release characteristics • Demonstrated non - fluctuating release of T3 over several months in small and large animal models • Testing in a non - clinical model of hypothyroidism in process • Next steps • Complete proof - of - concept in the non - clinical studies • Establish the non - clinical study plan that will provide safety data for the IND • Target meeting with the FDA for a pre - IND meeting in Q4 2016 • Goal is to start a proof of concept clinical study in mid - 2017 19 Titan Pharmaceuticals Company Overview | Proprietary & Confidential | ©2014 Titan Pharmaceutical, Inc.

Titan Pharmaceuticals Summary • Titan Pharmaceuticals specializes in the development of treatments for select chronic diseases, utilizing its innovative ProNeura long term drug delivery platform • Probuphine, a six month buprenorphine implant for the maintenance treatment of opioid addiction; NDA resubmitted at the end of August 2015; Advisory Committee voted 12 - 5 in favor of approval of Probuphine in January 2016; FDA has set action date of May 27, 2016 • U.S. and Canadian partnership with Braeburn Pharmaceuticals - $15 mil milestone at approval • Pursuing ex - U.S. opportunities for approval and commercialization • Potential for treatment of chronic pain • ProNeura for Parkinson’s disease (ropinirole implant) and hypothyroidism(T3 implant) have the potential to significantly enhance Titan’s value • Active evaluation of ProNeura long - term drug delivery platform for other chronic diseases • Proven management team with strong track record of success • Strong news flow expected to provide multiple value inflection opportunities 20 Titan Pharmaceuticals Company Overview | Proprietary & Confidential | ©2014 Titan Pharmaceutical, Inc.

• Marc Rubin, M.D, Executive Chairman and Director • 9 years with Titan Pharmaceuticals • Former Head of Global Research & Development and member of the Board of Management at Bayer Pharma • Executive R&D and commercial responsibilities at GSK for 13 years • 25 years in the pharmaceutical industry following 7 years at NIH • Sunil Bhonsle, M.B.A., President, CEO and Director • 20 years with Titan Pharmaceuticals • 20 years with Bayer Corporation in Biological and Pharmaceutical finance and operations management • Kate Beebe, Ph.D., Executive Vice President, Chief Development Officer • 9 years with Titan Pharmaceuticals • 19 years in industry, with senior positions in clinical development and medical affairs at GSK and Merck • 10 years in academic medicine Titan Executive Management Titan Pharmaceuticals Company Overview | Proprietary & Confidential | ©2014 Titan Pharmaceutical, Inc. 21

Thank You Sunil Bhonsle Mar 2016
Exhibit 99.2
 |
Titan Pharmaceuticals, Inc. |
TITAN PHARMACEUTICALS RECEIVES FEEDBACK FROM FDA ON ROPINIROLE IMPLANT DEVELOPMENT PROGRAM FOR PARKINSON'S DISEASE
SOUTH SAN FRANCISCO, CA – MARCH 7, 2016 – Titan Pharmaceuticals, Inc. (NASDAQ: TTNP) announced today that the U.S. Food and Drug Administration has provided written feedback on the initial development plan for its proprietary ropinirole hydrochloride (HCL) implant for Parkinson's disease. Based on the FDA's feedback on the development plan submitted in December 2015, Titan is proceeding with the required non-clinical studies to support the potential submission of an investigational new drug (IND) application in the fourth quarter of 2016, followed by the initial pharmacokinetic and proof-of-concept clinical study. Titan is pursuing a 505(b)(2) registration pathway for the product candidate.
The ropinirole implant, which employs Titan’s novel ProNeura technology platform, is designed for the long-term, continuous delivery of ropinirole HCL for the treatment of signs and symptoms of Parkinson's disease, including stiffness, tremors, muscle spasms, and poor muscle control. Ropinirole is a dopamine agonist currently available in daily or more frequently dosed oral formulations for the treatment of Parkinson's disease symptoms and restless leg syndrome.
The U.S. patent for Titan's ropinirole implant, which was allowed last year, is scheduled to be issued on March 8, 2016.
"Our early studies of a ropinirole implant utilizing the ProNeura technology have shown promise, and we are encouraged by the FDA's feedback on the product development plan ahead of an IND filing later this year," said Kate Beebe Ph.D., Titan's chief development officer and executive vice president. "Parkinson's disease affects nearly one million people in the U.S., a number that is rapidly increasing with the aging population. Symptoms of the disease can be very debilitating, and more treatments are needed to improve the quality of life for these patients until a cure is found."
In June 2015 Titan presented nonclinical data from a dose-escalating study of a ropinirole implant in a Parkinsonian primate model at the 19th International Congress of Parkinson's Disease and Movement Disorders. The study showed that motor function could be significantly improved with no onset of dyskinesias (involuntary movements), following the continuous, non-fluctuating release of ropinirole with the subdermal implant. There were also no observed signs of irritation, inflammation or fibrotic capsule formation at the implant site. Continuous, non-fluctuating release of ropinirole was observed for a period of several months following implantation.
Symptoms of Parkinson's disease are primarily treated today by dopamine replacement therapy (DRT). However, DRT is often associated with the pulsatile stimulation of dopamine receptors due to peak-trough fluctuations of medication in the blood. Over time the non-physiologic stimulation of dopamine receptors in the brain causes patients to develop serious motor complications and dyskinesias, limiting treatment effectiveness. Current treatments that offer continuous delivery of medication providing non-pulsatile stimulation of dopamine receptors in the brain appear to be more effective in controlling motor complications, but are surgically invasive and with potential risk of serious adverse effects.
Titan's ropinirole implant will be inserted subdermally in a brief office procedure and could potentially provide continuous, non-fluctuating therapeutic drug levels for several months from a single treatment. Probuphine, the company's first product utilizing the ProNeura long-term continuous drug delivery platform, has demonstrated safety and efficacy in a Phase 3 clinical program for the maintenance treatment of opioid addiction, and a new drug application (NDA) is currently under review by the FDA with an action date of May 27, 2016.
About the ProNeura Long-term Drug Delivery Platform
ProNeura is Titan’s proprietary, long-term drug delivery platform utilized in the development of products for the treatment
of select chronic conditions that may benefit from the delivery of continuous, non-fluctuating levels of certain medications over
an extended period of six months to a year. ProNeura consists of a small, solid rod made from a mixture of ethylene-vinyl acetate
(EVA) and a drug substance. The resulting product is a solid matrix that is placed subdermally, normally in the inner part of the
upper arm, during a simple office procedure, and is removed in a similar manner at the end of treatment. The drug substance is
released continuously through the process of dissolution, resulting in a stable, non-fluctuating blood level similar to that seen
with intravenous administration. These long-term, linear-release characteristics are medically desirable to avoid the peak and
trough swings from oral dosing that pose problems in the current treatments for many diseases, especially diseases of the central
nervous system. Titan has issued patents as well as patent applications covering the use of the ProNeura long-term drug delivery
platform for the formulation of specific products for the treatment of certain chronic diseases, such as opioid addiction, Parkinson’s
disease, and others.
About Titan Pharmaceuticals
Titan Pharmaceuticals Inc. (NASDAQ: TTNP), based in South San Francisco, CA, is a specialty pharmaceutical company developing proprietary therapeutics primarily for the treatment of serious medical disorders. The company’s lead product candidate is Probuphine®, a novel and long-acting formulation of buprenorphine for the long-term maintenance treatment of opioid dependence. Probuphine employs Titan’s proprietary drug delivery system ProNeura™, which is capable of delivering sustained, consistent levels of medication for three months or longer. Titan has granted U.S. and Canadian commercial rights for Probuphine to Braeburn Pharmaceuticals. If approved, Probuphine would be the first and only commercialized treatment of opioid dependence to provide continuous, around-the-clock blood levels of buprenorphine for six months following a single procedure. The ProNeura technology has the potential to be used in developing products for treating other chronic conditions, such as Parkinson’s disease, where maintaining consistent blood levels of a therapeutic agent may benefit the patient and improve medical outcomes. For more information about Titan, please visit www.titanpharm.com.
This press release may contain "forward-looking statements" within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Such statements include, but are not limited to, any statements relating to our product development programs and any other statements that are not historical facts. Such statements involve risks and uncertainties that could negatively affect our business, operating results, financial condition and stock price. Factors that could cause actual results to differ materially from management's current expectations include those risks and uncertainties relating to the regulatory approval process, the development, testing, production and marketing of our drug candidates, patent and intellectual property matters and strategic agreements and relationships. We expressly disclaim any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in our expectations or any changes in events, conditions or circumstances on which any such statement is based, except as required by law.
###
CONTACT:
Titan Pharmaceuticals, Inc.:
Sunil Bhonsle, President
(650) 244-4990
Investors:
Stephen Kilmer
(650) 989-2215
Media:
Susan Thomas
(650) 989-2216
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Southern Energy Corp. Announces Fourth Quarter and Year End 2023 Financial and Operating Results
- Burning Rock Announces ADS Ratio Change
- JERAYGO (aprocitentan) recommended for approval in Europe for the treatment of resistant hypertension
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share