Form 6-K GENETIC TECHNOLOGIES For: Apr 28
FORM 6-K
U.S. SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16 UNDER THE
SECURITIES EXCHANGE ACT OF 1934
dated April 28, 2016
Commission File Number 0-51504
GENETIC TECHNOLOGIES LIMITED
(Exact Name as Specified in its Charter)
N/A
(Translation of Registrant’s Name)
60-66 Hanover Street
Fitzroy
Victoria 3065 Australia
(Address of principal executive offices)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form 20-F x Form 40-F o
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): o
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): o
Indicate by check mark whether by furnishing the information contained in this Form, the registrant is also thereby furnishing the information to the Commission pursuant to Rule 12g3-2(b) under the Securities Exchange Act of 1934.
Yes o No x
If “Yes” is marked, indicate below the file number assigned to the registrant in connection with Rule 12g3-2(b): Not applicable.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused the Report to be signed on its behalf by the undersigned, thereunto duly authorized.
Date: April 28, 2016
|
|
GENETIC TECHNOLOGIES LIMITED | ||
|
|
|
| |
|
|
By: |
/s/ Kevin Fischer | |
|
|
|
Name: |
Kevin Fischer |
|
|
|
Title: |
Company Secretary |
EXHIBIT INDEX
|
Exhibit |
|
Description of Exhibit |
|
|
|
|
|
99.1 |
|
Quarterly Activities Report and Appendix 4C of the ASX Listing Rules for the Quarter Ended 31 March 2016 |
Exhibit 99.1
genetic technolog GENETIC TECHNOLOGIES LIMITED A.B.N. 17 009 212 328 Quarterly Activities Report and Appendix 4C of the ASX Listing Rules for the quarter ended 31 March 2016

GENETIC TECHNOLOGIES LIMITED QUARTERLY ACTIVITIES REPORT FOR THE QUARTER ENDED 31 March 2016 Highlights • • Achieved quarter on quarter sales growth Established partnership with international race car driver Pippa Mann to promote BREVAGenp/us® Continued to progress research program to support marketing of BREVAGenp/us Strong cash position with $12.5m in cash • • Melbourne, Australia, 28 April 2016: Genetic Technologies Limited (ASX: GTG; NASDAQ: GENE, "Company"), a molecular diagnostics company specialising in women's health, and provider of BREVAGenp/us®, a first-in-class, clinically validated risk assessment test for sporadic breast cancer, is pleased to provide its Quarterly Activities Report for the period ending 31 March 2016, together with the attached Appendix 4C. In February, Genetic Technologies established a partnership with international race car driver Pippa Mann as part of a marketing program to raise the profile of BREVAGenp/us. This agreement marks the Company's first marketing campaign, with a national reach, to promote BREVAGenp/us in the U.S. Our association with Mann is reflective of her commitment to working with research organisations and corporations who share her passion for reducing the impact that breast cancer has on women. In addition to Genetic Technologies, Mann has an established partnership with Susan G. Kamen®, the world's largest breast cancer organisation, which funds more breast cancer research than any other non-profit while providing screening, education, treatment and psychosocial support programs. This is an exciting time for the Company as we commence this robust partnership with Mann and ramp up commercial activities for BREVAGenp/us. Mann shares our passion for the integral role BREVAGenp/us can play in women's health and is steadfast in leveraging her brand power to promote breast cancer awareness and raise money to help fund cures. Mann is one of only nine female athletes to ever compete in the Indianapolis 500 and the only female driver to start in the race over the past three consecutive years. The BREVAGenp/us logo will feature on Mann's racing and promotional apparel as well as the Dale Coyne Racing car supporting Susan G. Kamen. In addition to Mann's participation in key BREVAGenp/us oriented events, her messaging and images will also appear in the Company's promotional print, video and social media platforms. The 1DOth running of the Indianapolis 500 on May 29, 2016 will serve as the first event under the partnership program. The Indianapolis 500 is the largest single day sporting event in the world, in terms of on-site attendance, and offers significant promotional opportunities throughout the month of May leading up to race day on Sunday May 29th, Memorial Day weekend. More detail regarding Genetic Technologies relationship with Mann can be found in the Company's announcement dated 22 February, 2016. 1

Quarterly Activities Report for the quarter ended 31 March 2016 OPERATIONS Financial summary For the quarter ended 31 March 2016, 287 BREVAGen/BREVAGenplus test samples were received, compared to 284 in the previous quarter. While this represents a modest 1% quarter on quarter gain, it indicates that the slide in tests received has been arrested. Weekly figures, while more subjective and arbitrary, also support this view, with a trend toward improving sales growth. Total cash receipts from customers during the quarter ended 31 March 2016 were $0.3m (PCP: $0.4m), taking the equivalent figure to $0.9m for the nine months ended on that date (PCP: $2.1m). The previous corresponding period YTD revenue number includes revenue generated from the Australian heritage business that was divested on 19 November 2014. Efficiencies gained from ongoing restructuring activities and the timing of payments of a recurring nature saw operational cash spend, for the March quarter, down $0.6m compared to the previous quarter. Based on the nine months to date run rate, the annualised FY16 cash spend equates to $9.4m compared to $13.2m in FY15 and $15.2m in FY14, representing a reduction in annual cash spend of 28.8% and 38.2% respectively. As at 31 March 2016, the Company had $12.5m in cash. BREVAGenp/usbreast cancer risk test Clinical utility studies and peer-review publications: The Company recognises that scientific papers are the ultimate marketing material for medical device companies and that scientific and clinical study data are key drivers to help strengthen the Company's commercial position. Physicians, the major breast health centres and health insurance companies seek multiple points of confirmation that the medical device works as intended and leads to a meaningful improvement in women's health. Therefore, the more papers that are published on BREVAGenplus, profiling its performance characteristics, the more likely physicians will be to use the test. They will also strongly influence how much insurers will be willing to pay for the test. The Company has previously conducted multiple scientific studies to develop and validate the first generation BREVAGen test, in addition to developing two health economic models to demonstrate potential cost savings and health benefits associated with the use of the BREVAGen test. Importantly, due to the nature of the technology and the specific improvements incorporated in BREVAGenplus, the research undertaken and published based on the original version of the test remains applicable to the new and improved BREVAGenplus test. Two further papers were published in the December 2015 quarter. The first paper provided compelling scientific evidence indicating that improved risk assessment has the potential to substantially lower the impact of breast cancer while the second paper supported the use of BREVAGenplus testing for African American and Hispanic women. These two new publications further add to the already existing scientific evidence base for BREVAGenplus. An important next step for the Company is to undertake studies that demonstrate the "clinical utility" of the test. To this end, the Company has recently commenced two such studies designed to measure how the BREVAGenplus test results influence physician decision making. The first such study is expected to be completed in 01, FY17, while the second in 02, FY17. A third longer term prospective study is currently in the late stages of planning, and is being designed to specifically evaluate the impact of BREVAGenplus on patient outcomes. The anticipated start date, for this study, is 01, FY 2017. 2

Quarterly Activities Report for the quarter ended 31 March 2016 Combined, these three studies are designed to inform the medical community of the measureable improvement in health outcomes associated with BREVAGenp/us testing. Whilst we continue to make significant investment into the future success of the BREVAGenp/us breast cancer risk assessment test, I am also pleased to report that Auslndustry has accepted and approved that the costs associated with these overseas research activities are eligible for the R&D Tax Incentive, representing a 45% cash refund from the Australian Tax Office. NON • CODING ASSERTION PROGRAM On 7 December 2015, Genetic Technologies argued before the Federal Circuit Court of Appeals in Washington DC that Claim 1 of the Company's foundation '179 patent is patent eligible under the standards set forth in the Mayo/Alice line of Supreme Court cases, and that Judge Stark's decision to grant motions to dismiss finding Claim 1 patent ineligible should be reversed. On 8 April 2016, the Federal Circuit affirmed the District Court and found that Claim 1 of the Company's '179 patent is patent-ineligible under 35 U.S.C. § 101. As at the date of this report, the Company's U.S. attorney is still in the process of evaluating the decision. CORPORATE MATTERS Settlement and Release Agreement with Hendrix Genetics B.V. On 5 February, the Company reported that it has executed a Settlement and Release Agreement with Hendrix Genetics B.V. (Hendrix), of Boxmeer, The Netherlands. With the execution of this Agreement completed, Genetic Technologies authorised the dismissal of legal action against Hendrix. The precise commercial terms of the Agreement are covered by formal confidentiality provisions and cannot be disclosed. Appendix 4D and 31 December 2015 Half Year Financial Report The Company published its Half Year Financial Report on 24 February 2016. The Half Year Financial Report is available on the Company's website at www.gtglabs.com Signed on behalf of Genetic Technologies Limited Date: 28 April, 2016 Eutillio Buccilli Executive Director & Chief Executive Officer 3

Appendix 4C Quarterly report for entities admitted on the basis of commitments Rule 4.7B Appendix 4C Quarterly report for entities admitted on the basis of commitments Introduced 31/3/2000. Amended 30/9/2001, 24/10/2005. Name of entity ABN Quarter ended (“current quarter”) Consolidated statement of cash flows + See chapter 19 for defined terms. 17/12/2010 Appendix 4C Page 1 Cash flows related to operating activities 1.1Receipts from customers 1.2Payments for(a) staff costs (b) advertising and marketing (c) research and development (d) leased assets (e) other working capital 1.3Dividends received 1.4Interest and items of a similar nature received 1.5Interest and other costs of finance paid 1.6Income taxes paid 1.7Grant and other income Net operating cash flows Current quarter (March 2016) A$ Year to date A$ 310,807 (1,013,903) (159,936) (95,411) - (759,168) - 24,904 - - - 918,967 (3,267,313) (553,839) (211,333) - (3,059,919) - 42,399 (5,870) - - (1,692,707) (6,136,908) 31 March 2016 17 009 212 328 GENETIC TECHNOLOGIES LIMITED
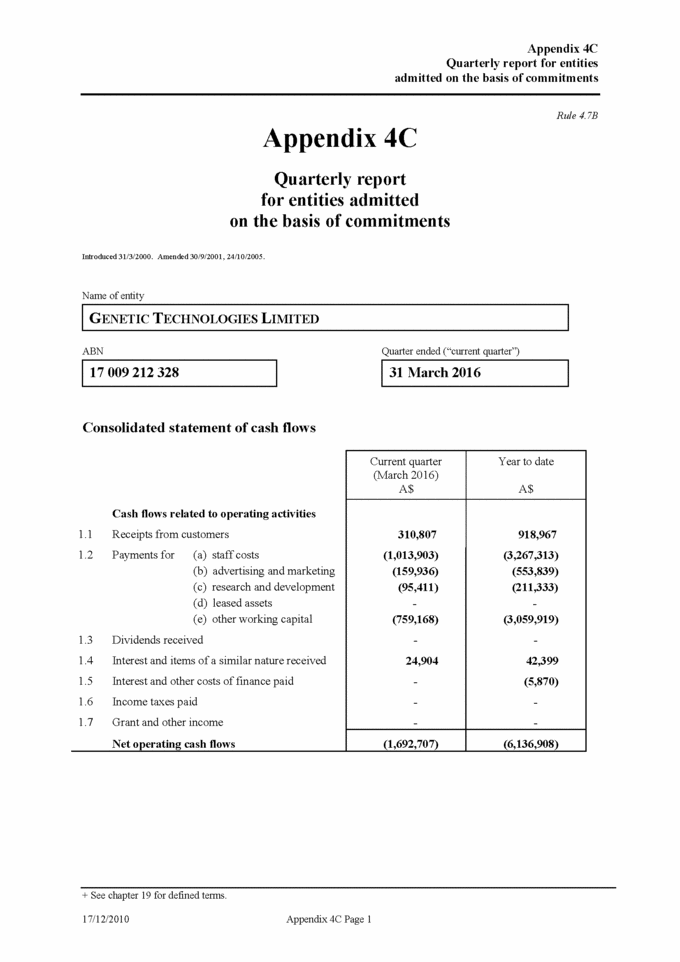
Appendix 4C Quarterly report for entities admitted on the basis of commitments Consolidated statement of cash flows (cont.) + See chapter 19 for defined terms. Appendix 4C Page 2 17/12/2010 Current quarter (March 2016) A$ Year to date A$ 1.8Net operating cash flows (carried forward) (1,692,707) (6,136,908) Cash flows related to investing activities 1.9Payment for the acquisition of: a)businesses (item 5) b)equity investments c)intellectual property d)physical non-current assets e)other non-current assets 1.10Proceeds from the disposal of: a)businesses (item 5) b)equity investments c)intellectual property d)physical non-current assets e)joint venture interest f)other assets 1.11Loans to other entities 1.12Loans repaid by other entities (refer note below) 1.13Other (provide details if material) Net investing cash flows 1.14Total operating and investing cash flows - - - (29,835) - - - - - - - - - - - - - (293,533) - - - - - - - - - - (29,835) (293,533) (1,722,542) (6,430,441) Cash flows related to financing activities 1.15Net proceeds from the issue of shares 1.16Equity transaction costs 1.17Net proceeds from borrowings 1.18Net proceeds from the issue of unlisted secured debt notes 1.19Dividends paid Net financing cash flows - - - - - (1,654) - - - - - (1,654) Net increase / (decrease) in cash held 1.20Cash at beginning of quarter / year to date 1.21Exchange rate adjustments 1.22Cash at end of quarter (1,722,542) 14,519,541 (249,595) (6,432,095) 18,341,357 638,142 12,547,404 12,547,404
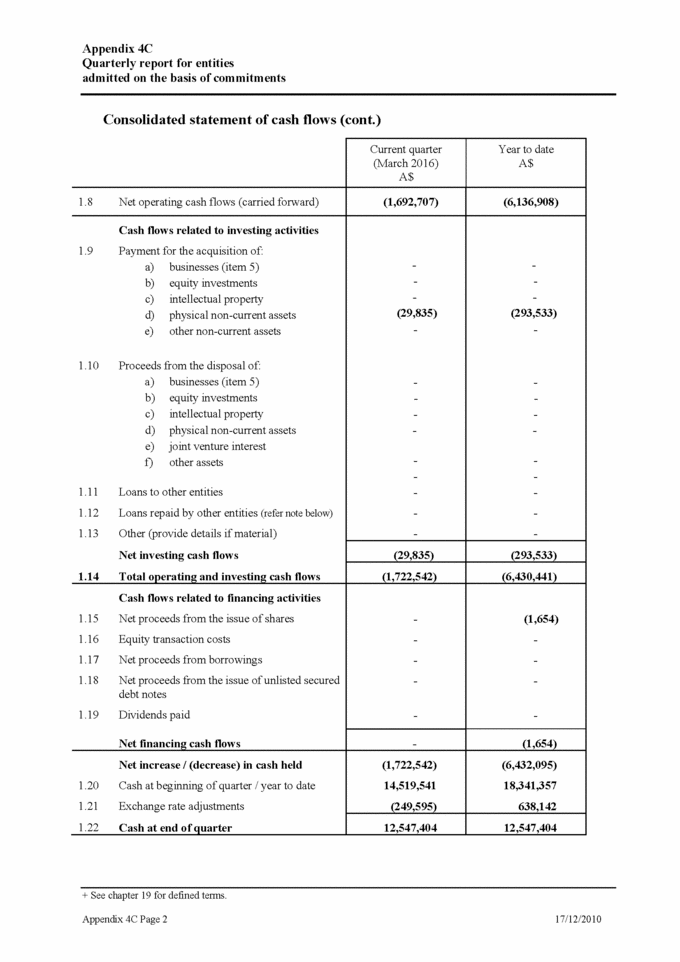
Appendix 4C Quarterly report for entities admitted on the basis of commitments Payments to directors of the entity and associates of the directors Payments to related entities of the entity and associates of the related entities 1.23 Aggregate amount of payments to the parties included in item 1.2 1.24 Aggregate amount of loans to the parties included in item 1.11 1.25 Explanation necessary for an understanding of the transactions Non-cash financing and investing activities 2.1 Details of financing and investing transactions which have had a material effect on consolidated assets and liabilities but did not involve cash flows 2.2 Details of outlays made by other entities to establish or increase their share in businesses in which the reporting entity has an interest Financing facilities available Add notes as necessary for an understanding of the position. (See AASB 1026 paragraph 12.2). 3.1 Loan facilities 3.2 Credit standby arrangements Hire purchase facility + See chapter 19 for defined terms. 17/12/2010 Appendix 4C Page 3 Amount available $A Amount used $A - - - - None during the quarter under review None during the quarter under review The amount included at Item 1.23 includes $153,767 paid to Directors during the quarter in respect of fees and superannuation. Current quarter $A 153,767 -
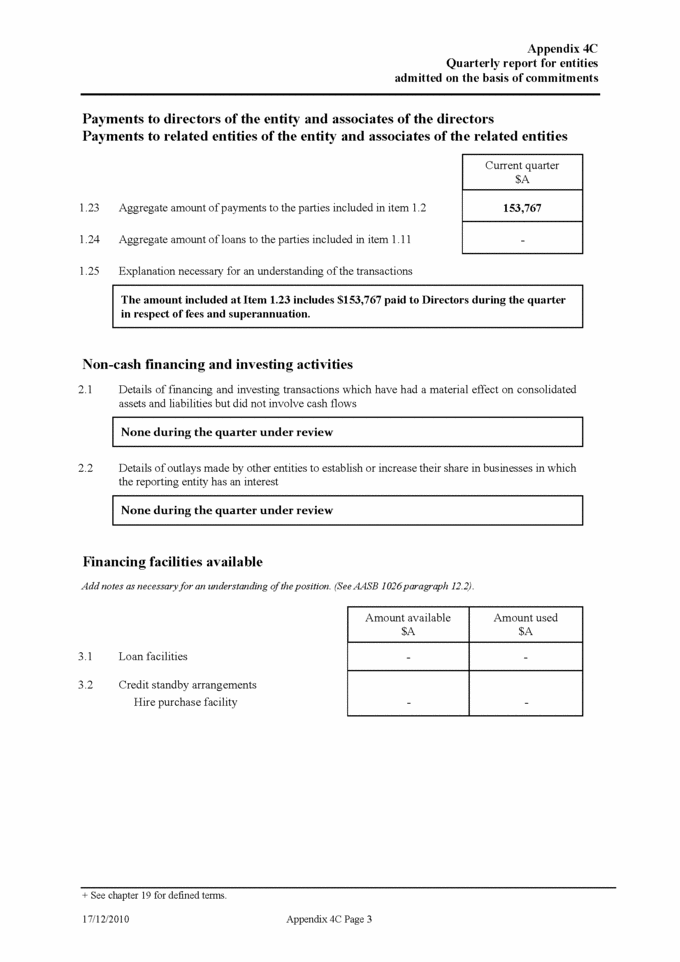
Appendix 4C Quarterly report for entities admitted on the basis of commitments Reconciliation of cash Reconciliation of cash at the end of the quarter (as shown in the consolidated statement of cash flows) to the related items in the accounts is as follows: 4.1 Cash on hand and at bank 4.2 Term deposits 4.3 Bank overdraft 4.4 Commercial Bills of Exchange Total cash at end of quarter (item 1.23) Acquisitions and disposals of business entities 5.1 Name of entity 5.2 Place of incorporation or registration 5.3 Consideration for acquisition or disposal 5.4 Total net liabilities 5.5 Nature of business Compliance statement 1 This statement has been prepared under accounting policies which comply with accounting standards as defined in the Corporations Act (except to the extent that information is not required because of note 2) or other standards acceptable to ASX. 2 This statement does give a true and fair view of the matters disclosed. Sign here: ............................................................ CFO/ Company Secretary Date: 28 April 2016 Print name: Kevin Fischer + See chapter 19 for defined terms. Appendix 4C Page 4 17/12/2010 Acquisitions (Item 1.9(a)) Disposals (Item 1.10(a)) Not applicable Not applicable Current quarter (March 2016) $A Previous quarter (December 2015) $A 7,984,420 12,329,804 4,562,984 2,189,737 - - - - 12,547,404 14,519,541
Appendix 4C Quarterly report for entities admitted on the basis of commitments Notes 1. The quarterly report provides a basis for informing the market how the entity’s activities have been financed for the past quarter and the effect on its cash position. An entity wanting to disclose additional information is encouraged to do so, in a note or notes attached to this report. 2. The definitions in, and provisions of, AASB 107: Statement of Cash Flows apply to this report except for any additional disclosure requested by AASB 107 that are not already itemised in this report. 3. Accounting Standards.ASX will accept, for example, the use of International Financial Reporting Standards for foreign entities. If the standards used do not address a topic, the Australian standard on that topic (if any) must be complied with. + See chapter 19 for defined terms. 17/12/2010 Appendix 4C Page 5

Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Genetic Technologies Announces Closing of US$2 Million Registered Direct Offering
- Perk Labs Announces Closing of Non-Brokered Private Placements
- International Horticultural Exhibition 2024 Chengdu Opens
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share