Form 8-K HUMANIGEN, INC For: Nov 05
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of
the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): November 5, 2020
Humanigen, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 001-35798 | 77-0557236 | ||
| (State or other Jurisdiction of Incorporation) |
(Commission File No.) |
(IRS Employer Identification No.)
|
533 Airport Boulevard, Suite 400
Burlingame, CA 94010
(Address of principal executive offices, including zip code)
(650) 243-3100
(Registrant’s telephone number, including area code)
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2):
| ¨ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| Common Stock | HGEN | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (17 CFR §230.405) or Rule 12b-2 of the Securities Exchange Act of 1934 (17 CFR §240.12b-2).
Emerging growth company ¨
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
| Item 1.01. | Entry into a Material Definitive Agreement. |
On November 5, 2020, Humanigen, Inc. (the “Company”) and the Department of Defense (DoD) Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense (JPEO-CBRND or JPEO) entered into a Cooperative Research and Development Agreement (“CRADA”) in collaboration with the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response (ASPR) at the U.S. Department of Health and Human Services (HHS), in support of Operation Warp Speed (“OWS”), to assist in the development of Humanigen’s lead product candidate, lenzilumabTM, in advance of a potential Emergency Use Authorization (“EUA”) for COVID-19.
Pursuant to the CRADA, Humanigen will be provided access to a full-scale, integrated team of OWS manufacturing, and regulatory subject matter experts, leading decision makers and statistical support in anticipation of applying for EUA and subsequently a Biologics License Application (“BLA”), for lenzilumab as a potential treatment for COVID-19. The CRADA also provides that OWS regulatory experts will work with the Company on U.S. Food and Drug Administration (FDA) communications, meetings and regulatory filings. The CRADA aims to support the ongoing lenzilumab Phase 3 clinical trials, focusing on efficiently generating an EUA and BLA submission. In addition to providing access under EUA, a goal of the CRADA is to ensure lenzilumab receives the benefits provided by Public Law 115-92.
The foregoing summary of the CRADA does not purport to be complete and is qualified in its entirety by reference to the full text of the CRADA, which will be filed with the Securities and Exchange Commission (the “SEC”) as an exhibit to the Company’s Annual Report on Form 10-K for the year ended December 31, 2020 (the “Form 10-K”) or via an amendment to this Current Report on Form 8-K. Pursuant to Item 601(b)(10) of Regulation S-K, certain terms of the CRADA have been omitted from this Current Report on Form 8-K, and will be omitted from the version of the CRADA to be filed with the SEC, because such terms are both (i) not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
A copy of the Company’s press release relating to the CRADA is filed as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
| Item 7.01. | Regulation FD Disclosure |
On November 6, 2020, the Company issued a press release announcing interim data from its Phase 3 trial of lenzilumab in patients hospitalized with COVID-19. A copy of the press release is attached hereto as Exhibit 99.2 and is incorporated herein by reference.
On November 6, 2020, the Company will host a conference call for investors and other interested stakeholders to discuss the interim data from its Phase 3 trial as well as the CRADA. A copy of the presentation materials to be discussed on the conference call is furnished as Exhibit 99.3 to this report.
The information in this Item 7.01, including Exhibit 99.2 and Exhibit 99.3, is being furnished pursuant to Item 7.01 of Form 8-K and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference into any of the Company’s filings under the Securities Act of 1933, as amended, except to the extent expressly set forth by specific reference in such a filing.
| Item 9.01. | Financial Statements and Exhibits |
| (d) | Exhibits |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Humanigen, Inc. | |||
| By: | /s/ Cameron Durrant | ||
| Name: Cameron Durrant Title: Chairman of the Board and Chief Executive Officer | |||
Dated: November 6, 2020
Exhibit 99.1
Humanigen Announces Cooperative Research and Development Agreement with the Department of Defense to Develop Lenzilumab for COVID-19
| · | The Cooperative Research and Development Agreement (CRADA) with the Department of Defense (DoD) in support of Operation Warp Speed (OWS) aims to improve the availability of lenzilumab for patients with COVID-19 |
| · | Humanigen’s development efforts complemented by full-scale, integrated team of OWS leading experts and U.S. Government decision makers dedicated to advancing lenzilumab ahead of a potential Emergency Use Authorization (EUA) submission |
Burlingame, CA – November 6, 2020 – Humanigen, Inc., (HGEN) (“Humanigen”), a clinical stage biopharmaceutical company focused on preventing and treating an immune hyper-response called ‘cytokine storm’ with its lead drug candidate lenzilumab™, today announced that the company and the Department of Defense (DoD) Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense (JPEO-CBRND or JPEO) have entered into a Cooperative Research and Development Agreement (CRADA) in collaboration with the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response (ASPR) at the U.S. Department of Health and Human Services (HHS), in support of Operation Warp Speed (OWS) to assist in the development of lenzilumab in advance of a potential Emergency Use Authorization (EUA) for COVID-19.
The CRADA complements Humanigen’s development efforts, providing access to a full-scale, integrated team of OWS manufacturing and regulatory subject matter experts, leading decision makers and statistical support in anticipation of applying for EUA and subsequently a Biologics License Application for lenzilumab as a potential treatment for COVID-19. The CRADA also provides that OWS regulatory experts will work hand in hand with the Company on U.S. Food and Drug Administration (FDA) communications, meetings and regulatory filings. The CRADA aims to support the ongoing lenzilumab Phase 3 clinical trials, focusing on efficiently generating EUA and BLA submissions. In addition to providing access under EUA, a goal of the CRADA is to ensure lenzilumab receives the benefits provided by Public Law 115-92.
Humanigen's investigational treatment lenzilumab, a proprietary Humaneered® anti-human granulocyte macrophage-colony stimulating factor (GM-CSF) monoclonal antibody, is designed to prevent and treat an immune hyper-response called ‘cytokine storm’, a complication considered to be a leading cause of COVID-19 death. Data show that up to 89 percent of patients hospitalized with COVID-19 are at risk of this immune hyper-response, which is believed to trigger the acute respiratory distress syndrome in severe cases of COVID-19.
Cameron Durrant, MD, MBA, chief executive officer of Humanigen said, “We are honored to be part of Operation Warp Speed, receive this CRADA, and collaborate with JPEO to advance lenzilumab as a potential response treatment and seek a potential EUA. We have been working tirelessly to advance lenzilumab for COVID-19 and are excited to have the integrated expert team at OWS prioritize lenzilumab research and development during this critical time.”
Lenzilumab was also selected by the National Institutes of Health (NIH) to be evaluated among the promising COVID-19 agents for its ACTIV-5 “Big Effect Trial” (ACTIV-5/BET) which will enroll patients at up to 40 sites in the U.S.
More details on Humanigen’s programs in COVID-19 can be found on the company’s website at www.humanigen.com under the COVID-19 tab. Details on the U.S. Phase 3 lenzilumab clinical trial can be found at clinicaltrials.gov using Identifier NCT04351152. Details on ACTIV-5/BET can be found at clinicaltrials.gov using Identifier NCT04583969.
Humanigen to Host Investor Conference Call
The Company will host an investor call and webcast today, Friday, November 6, 2020 at 9:00 a.m. EST.
To participate in the conference call, please dial toll free 1-800-410-4983 or toll/International number 1-303-223-4366. The conference ID number is 21972012. A simultaneous webcast of the call and presentation can be accessed by visiting: http://public.viavid.com/index.php?id=142380.
In addition, a replay of the webcast will be available on the company website for 30 days following the event.
About Humanigen, Inc.
Humanigen, Inc. is developing its portfolio of clinical and pre-clinical therapies for the treatment of cancers and infectious diseases via its novel, cutting-edge GM-CSF neutralization and gene-knockout platforms. We believe that our GM-CSF neutralization and gene-editing platform technologies have the potential to reduce the inflammatory cascade associated with coronavirus infection. The company’s immediate focus is to prevent or minimize the cytokine release syndrome that precedes severe lung dysfunction and ARDS in serious cases of SARS-CoV-2 infection. The company is also focused on creating next-generation combinatory gene-edited CAR-T therapies using strategies to improve efficacy while employing GM-CSF gene knockout technologies to control toxicity. In addition, the company is developing its own portfolio of proprietary first-in-class EphA3-CAR-T for various solid cancers and EMR1-CAR-T for various eosinophilic disorders. The company is also exploring the effectiveness of its GM-CSF neutralization technologies (either through the use of lenzilumab as a neutralizing antibody or through GM-CSF gene knockout) in combination with other CAR-T, bispecific or natural killer (NK) T cell engaging immunotherapy treatments to break the efficacy/toxicity linkage, including to prevent and/or treat graft-versus-host disease (GvHD) in patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT). Additionally, Humanigen and Kite, a Gilead Company, are evaluating lenzilumab in combination with Yescarta® (axicabtagene ciloleucel) in patients with relapsed or refractory large B-cell lymphoma in a clinical collaboration. For more information, visit www.humanigen.com.
Forward-Looking Statements
This release contains forward-looking statements. Forward-looking statements reflect management's current knowledge, assumptions, judgment and expectations regarding future performance or events. Although management believes that the expectations reflected in such statements are reasonable, they give no assurance that such expectations will prove to be correct and you should be aware that actual events or results may differ materially from those contained in the forward-looking statements. Words such as "will," "expect," "intend," "plan," "potential," "possible," "goals," "accelerate," "continue," and similar expressions identify forward-looking statements, including, without limitation, statements regarding the potential future development of lenzilumab to minimize or reduce the immune hyper-response called cytokine storm, which is believed to trigger the acute respiratory distress syndrome in severe cases of COVID-19, the potential benefits of the CRADA with JPEO and other assistance from the federal government to the Company’s efforts to seek an Emergency Use Authorization and ultimately a BLA from, be approved by, FDA for such use or to help CAR-T reach its full potential or to deliver benefit in preventing GvHD. Forward-looking statements are subject to a number of risks and uncertainties including, but not limited to, the risks inherent in our lack of profitability and need for additional capital to grow our business; our dependence on partners to further the development of our product candidates; the uncertainties inherent in the development, attainment of requisite regulatory approvals and launch of any new pharmaceutical product; the outcome of pending or future litigation; and the various risks and uncertainties described in the "Risk Factors" sections and elsewhere in the Company's periodic and other filings with the Securities and Exchange Commission.
All forward-looking statements are expressly qualified in their entirety by this cautionary notice. You should not place undue reliance on any forward-looking statements, which speak only as of the date of this release. We undertake no obligation to revise or update any forward-looking statements made in this press release to reflect events or circumstances after the date hereof or to reflect new information or the occurrence of unanticipated events, except as required by law.
CONTACT:
Media
Cammy Duong
Westwicke, an ICR company
203-682-8380
Investors
Alan Lada
Solebury Trout
856-313-8206
Exhibit 99.2
Humanigen Announces Positive Interim Phase 3 Data of Lenzilumab™ in Patients Hospitalized with COVID-19
| · | Interim data suggest clinically meaningful impact on patient recovery from COVID-19 with an estimated 37 percent more recoveries observed in lenzilumab arm of Phase 3 trial versus current standard of care |
| · | Data safety monitoring board recommendation demonstrates the Phase 3 trial is in the “promising zone” of the adaptive trial design |
| · | CRADA between Humanigen and U.S. government provides for regulatory and other support to submit an EUA and BLA |
Burlingame, CA – November 6, 2020 – Humanigen, Inc., (Nasdaq: HGEN) (“Humanigen”), a clinical stage biopharmaceutical company focused on preventing and treating an immune hyper-response called ‘cytokine storm’ with its lead investigational treatment lenzilumab, today announced positive interim Phase 3 data of lenzilumab in patients hospitalized with COVID-19. This interim analysis for sizing and powering suggests that lenzilumab had a clinically meaningful impact on patient recovery, with an estimated 37 percent more recoveries observed in the lenzilumab arm of the randomized, placebo-controlled, double-blinded study versus current standard of care (SOC).
“These interim data demonstrate the potential of lenzilumab as a frontline treatment option for patients hospitalized with COVID-19,” said Cameron Durrant, MD, MBA, chief executive officer of Humanigen. “We are encouraged by these data and the clinically meaningful impact that lenzilumab may have for patients with COVID-19 over and above remdesivir and/or steroids.”
The data safety monitoring board (DSMB) composed of independent subject matter experts conducted an interim analysis of the unblinded data for trial sizing and powering and recommended increasing the target number of events (recoveries) from 257 to 402 to maintain the power of the study at 90 percent. The adaptive trial design only allows for the addition of patients if interim data are in the “promising zone” (i.e., achieving or surpassing an average improvement in recoveries of 29 percent (hazard ratio (HR) > 1.29) through day 28).
The company remains blinded to the data and based on the recommended number of events, the HR was calculated to be 1.37, an average of 37 percent more recoveries observed in the lenzilumab arm compared to the control arm. Any observed benefit in the lenzilumab arm would be over and above the use of remdesivir and/or steroids which are among the treatments that have been used as SOC in both the lenzilumab treatment arm and the placebo arm of the study.
At the recommendation of the DSMB, the company plans to increase enrollment to achieve 402 events (approximately 515 patients). This increase in enrollment ensures an even higher probability of success in meeting the primary endpoint and maintains the power of the study at 90 percent. The next interim analysis for efficacy is planned when the study reaches 75 percent events (302 events) which will require approximately 390 patients to be enrolled in the trial.
“Based on this feedback from the DSMB, we believe the Phase 3 trial is significantly de-risked. Targeting 402 events improves the probability of success, maintains the power of the study at 90 percent, and further supports our plans for Emergency Use Authorization (EUA) and Biologics License Application (BLA) submission,” said Dale Chappell, MD, MBA, chief scientific officer of Humanigen. “We are working to quickly activate additional trial sites across the U.S. to support rapid enrollment and increase access to lenzilumab with a continued commitment to inclusion and diversity.”
A Cooperative Research and Development Agreement (CRADA) that Humanigen entered into with the Joint Program Executive Office for Chemical, Biological Radiological and Nuclear Defense (JPEO-CBRND), provides for, among other support, regulatory, statistical and manufacturing subject matter advice to anticipation of an application for an EUA and eventually a BLA submission. The activities under the CRADA are being funded by the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response (ASPR) at the U.S. Department of Health and Human Services (HHS), working in collaboration with JPEO-CBRND. These activities include providing regulatory representatives who may participate in informal or formal meetings with the U.S. Food and Drug Administration (FDA) and providing comments on submission prior to submitting to FDA.
Humanigen intends to file for EUA in the first quarter of 2021 either following interim data at 75 percent or at study completion. The Phase 3 trial evaluating patients hospitalized with COVID-19 is enrolling at sites across the U.S. and Latin America. Current enrollment stands at 300 patients.
Interim Phase 3 lenzilumab in COVID-19 data analysis highlights:
| · | 78 percent of trial participants on either remdesivir or dexamethasone (or other steroids) or both (across both arms of the study) |
| · | 65 percent of trial participants with oxygen saturation £ 94 percent or on low-flow oxygen support |
| · | 35 percent of trial participants on high-flow oxygen or non-invasive positive pressure ventilation at time of enrollment |
| · | 45 percent of trial participants were 65 years of age or older |
| · | 50 percent of trial participants from diverse populations |
| · | No serious adverse events have been attributed to lenzilumab |
| · | These findings apply across both arms of the study |
More details on Humanigen’s programs in COVID-19 can be found on the company’s website at www.humanigen.com under the COVID-19 tab. Details on the U.S. Phase 3 lenzilumab clinical trial can be found at clinicaltrials.gov using Identifier NCT04351152 or by visiting www.StopStorm.com.
Humanigen to Host Investor Conference Call
The Company will host an investor call and webcast today, Friday, November 6, 2020 at 9:00 a.m. EST to discuss these results and the recently announced CRADA issued through a collaboration between the Department of Defense and the U.S. Department of Health and Human Services to meet the U.S. government’s Operation Warp Speed goals.
To participate in the conference call, please dial toll free 1-800-410-4983 or toll/International number 1-303-223-4366. The conference ID number is 21972012. A simultaneous webcast of the call and presentation can be accessed by visiting: http://public.viavid.com/index.php?id=142380.
In addition, a replay of the webcast will be available on the company website for 30 days following the event.
About Humanigen, Inc.
Humanigen, Inc. is developing its portfolio of clinical and pre-clinical therapies for the treatment of cancers and infectious diseases via its novel, cutting-edge GM-CSF neutralization and gene-knockout platforms. We believe that our GM-CSF neutralization and gene-editing platform technologies have the potential to reduce the inflammatory cascade associated with coronavirus infection. The company’s immediate focus is to prevent or minimize the cytokine release syndrome that precedes severe lung dysfunction and ARDS in serious cases of SARS-CoV-2 infection. The company is also focused on creating next-generation combinatory gene-edited CAR-T therapies using strategies to improve efficacy while employing GM-CSF gene knockout technologies to control toxicity. In addition, the company is developing its own portfolio of proprietary first-in-class EphA3-CAR-T for various solid cancers and EMR1-CAR-T for various eosinophilic disorders. The company is also exploring the effectiveness of its GM-CSF neutralization technologies (either through the use of lenzilumab as a neutralizing antibody or through GM-CSF gene knockout) in combination with other CAR-T, bispecific or natural killer (NK) T cell engaging immunotherapy treatments to break the efficacy/toxicity linkage, including to prevent and/or treat graft-versus-host disease (GvHD) in patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT). Additionally, Humanigen and Kite, a Gilead Company, are evaluating lenzilumab in combination with Yescarta® (axicabtagene ciloleucel) in patients with relapsed or refractory large B-cell lymphoma in a clinical collaboration. For more information, visit www.humanigen.com.
Forward-Looking Statements
This release contains forward-looking statements. Forward-looking statements reflect management's current knowledge, assumptions, judgment and expectations regarding future performance or events. Although management believes that the expectations reflected in such statements are reasonable, they give no assurance that such expectations will prove to be correct and you should be aware that actual events or results may differ materially from those contained in the forward-looking statements. Words such as "will," "expect," "intend," "plan," "potential," "possible," "goals," "accelerate," "continue," and similar expressions identify forward-looking statements, including, without limitation, statements regarding our expectations for the Phase 3 study and the potential future development of lenzilumab, our pathway to our intended submission for, and potential receipt of, an Emergency Use Authorization and potential subsequent BLA from FDA, and statements regarding the potential for lenzilumab to be used to prevent or treat GvHD and, as sequenced therapy with Kite’s Yescarta, in CAR-T therapies. Forward-looking statements are subject to a number of risks and uncertainties including, but not limited to, the risks inherent in our lack of profitability; our dependence on partners to further the development of our product candidates; the costs and the uncertainties inherent in the development and launch of any new pharmaceutical product; the outcome of pending or future litigation; and the various risks and uncertainties described in the "Risk Factors" sections and elsewhere in the company's periodic and other filings with the Securities and Exchange Commission.
All forward-looking statements are expressly qualified in their entirety by this cautionary notice. You should not place undue reliance on any forward-looking statements, which speak only as of the date of this release. We undertake no obligation to revise or update any forward-looking statements made in this press release to reflect events or circumstances after the date hereof or to reflect new information or the occurrence of unanticipated events, except as required by law.
CONTACTS:
Media
Cammy Duong
Westwicke, an ICR company
203-682-8380
Investors
Alan Lada
Solebury Trout
617-221-8006
Exhibit 99.3

Humanigen November 2020

2 Cautionary Note Regarding Forward - Looking Statements All statements other than statements of historical facts contained in this presentation, including information concerning the of fering, our possible or assumed future results of operations and expenses, business strategies and plans, competitive position, business and industry environment an d p otential growth opportunities, are forward - looking statements. Forward - looking statements reflect management's current knowledge, assumptions, judgment and expecta tions regarding future performance or events. Although management believes that the expectations reflected in such statements are reasonable, they g ive no assurance that such expectations will prove to be correct and you should be aware that actual events or results may differ materially from those con tained in the forward - looking statements. Words such as "will," "expect," "intend," "plan," "potential," "possible," "goals," "accelerate," "continue," and similar exp res sions identify forward - looking statements, including, without limitation, statements regarding: Our expectations for timing and achievement of the key corporate and COVID - 19 milestones described in the presentation, includin g; The target date for completion of patient enrollment in our Phase 3 trial of lenzilumab in COVID - 19 patients; The anticipated use of lenzilumab in the ACTIV - 5 Trial sponsored by NIAID, and the anticipated scope of that trial and timeline for same; Our potential request for and receipt of an Emergency Use Authorization from FDA for lenzilumab in COVID - 19 and our expectations for filing a BLA; and Our plans to launch and commercialize lenzilumab following receipt of the requisite regulatory authorizations or approvals; a nd Our expectations for timing and achievement of milestones for our pipeline outside of COVID - 19, including in respect of our ZUMA - 19 Phase Ib trial of lenzilumab that is being conducted with Kite, a Gilead company; our ongoing Phase I trial of ifabotuzumab in GBM patients; our plans for a study of lenzilumab in GvHD expected to be conducted with the IMPACT Group in the United Kingdom and a study in the US, and our plans for a study of lenz ilu mab in CMML in Australia; Forward - looking statements are subject to a number of risks and uncertainties including, but not limited to, the risks inherent in our lack o f p rofitability and need for additional capital to grow our business; our dependence on partners to further the development of our product candidates; the un certainties inherent in the development and launch of any new pharmaceutical product; the outcome of pending or future litigation; and the various risks and uncertainties described in the "Risk Factors" sections of our latest annual and quarterly reports and other filings with the SEC. All forward - looking statements are expressly qualified in their entirety by this cautionary notice. You should not rely upon any forward - looking statements, as predictions of future events. We undertake no obligation to revise or update any forward - looking statements made in this presentation to ref lect events or circumstances after the date hereof, to reflect new information or the occurrence of unanticipated events, to update the reasons why actual results c oul d differ materially from those anticipated in the forward - looking statements, in each case, except as required by law.

3 Agenda • Introduction Cameron Durrant, CEO • Phase 3 update Dale Chappell, CSO • CRADA/OWS Tim Morris COO/CFO • Questions All

4 Positive Interim Phase 3 Data of Lenzilumab Ρ in COVID - 19 • Current enrollment is 300 patients • Interim analysis conducted on the first 165 patients • Estimated 37% more recoveries observed with lenzilumab vs current standard of care • Phase 3 study is tracking to a successful outcome with a strong signal of efficacy on the primary endpoint • Lenzilumab is tracking to a result that is significantly above and beyond steroids and/or remdesivir • DSMB recommendation to expand the study • Phase 3 trial is significantly de - risked by this data and current study plan • With the current study plan, the trial is 90% powered and has a high probability of success • Total of 515 patients to be enrolled in phase 3 study • Interim efficacy analysis at 75% event rate to be performed (approximately 390 patients) • EUA filing expected in Q1 2021 • Recent CRADA with DoD in support of OWS provides regulatory and manufacturing support for lenzilumab

5 Lenzilumab Phase 3 - Understanding the Interim DSMB Analysis • Time to event analysis has four components (p - value, power, events, hazard ratio) • G iven three of the four components, the fourth component can be derived from the other three • P - value is fixed at p=0.05 • Power is selected by HGEN/sponsor at 90% • DSMB determines events to maintain power at 90% based on unblinded data and observed conditional power calculation in an adaptive trial design • Hazard ratio (HR) can be derived/estimated from the number of events determined by the DSMB, p - value, and power • Once HR is known, conditional power estimate at the interim can be derived from the known variables, original number of events, p - value, HR
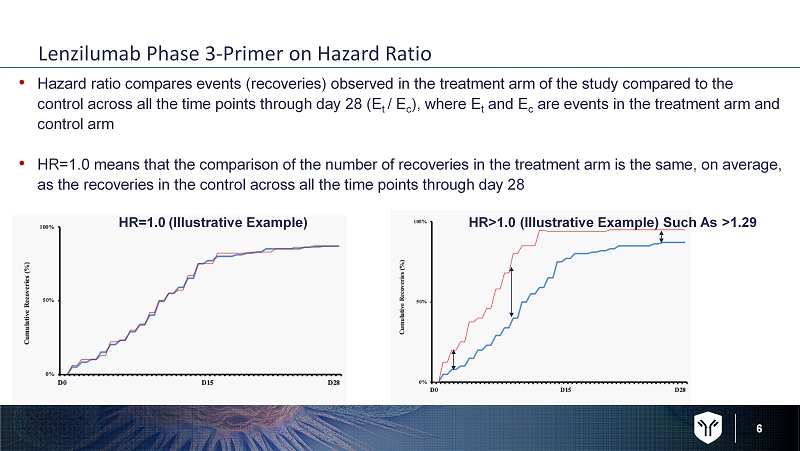
6 Lenzilumab Phase 3 - Primer on Hazard Ratio • Hazard ratio compares events ( recoveries) observed in the treatment arm of the study compared to the control across all the time points through day 28 (E t / E c ), where E t and E c are events in the treatment arm and control arm • HR=1.0 means that the comparison of the number of recoveries in the treatment arm is the same, on average, as the recoveries in the control across all the time points through day 28 HR=1.0 (Illustrative Example) HR>1.0 (Illustrative Example) Such As >1.29

7 Lenzilumab Phase 3 Adaptive Trial Design • Humanigen’s phase 3 study is an adaptive trial design • Initial trial design included n=300 to generate 257 events • Trial designed as “real world”, patients allowed access to remdesivir and steroids as background SOC in both arms. All patients, whether in the ‘placebo’ arm or the lenzilumab arm, received similar background care • Trial designed to add patients only if the observed effect on the time to recovery is HR > 1.29 • HR=1.29 means 29% more recoveries on average observed in the lenzilumab arm compared to the control arm (steroids and/or remdesivir ) through day 28 • Lenzilumab trial tracking at an estimate HR=1.37 and DSMB recommendation is to add events as trial is in the “promising zone” of the adaptive trial design, demonstrating a high probability of success • 37% more recoveries on average observed in the lenzilumab arm compared to the control arm (steroids and/or remdesivir ) through day 28

8 Lenzilumab Phase 3 - Adaptive Trial Design 50% HR=1.50 HR=1.29 HR ≤ 1.0 % Increase in sample size 25% 50% 10% S teroids and/or remdesivir Baricitinib 1 Tocilizumab 2 HR=1.16 Lenzilumab 3 HR=1.37 1 ACTT - 2 2 EMPACTA 3 Lenzilumab HR based on observed interim analysis with the large majority of patients on concomitant remdesivir and/or steroids • Adaptive trial design allows for addition of patients during the study if interim results ar e in the “promising zone” • Addition of patients maintains 90% power • Increases probability of success

9 Lenzilumab Phase 3 - Interim Analysis Interpretation * Benefit that is in addition to any benefit from steroids and/or remdesivir in the control arm • Patient characteristics for first 165 patients show good distribution of • Disease severity • Age • Diversity • Extensive use of concomitant therapies • Estimated HR of 1.37 suggests 37% increase in recoveries with lenzilumab

10 Lenzilumab Phase 3 - Interim Analysis Interpretation • Based on the first 165 patients (randomized, double - blind, placebo - controlled, multicenter), l enzilumab tracking to a result that is significantly better than steroids and/or remdesivir alone (estimated HR=1.37): • 37% more recoveries on average in the lenzilumab arm compared to the control arm (steroids and/or remdesivir ) through day 28 • Lenzilumab interim results even more impressive given large majority of patients on concomitant active therapies ( remdesivir and/or steroids) • The observed benefit of lenzilumab is additional to remdesivir and/or steroids as background SOC therapies • Baracitinib result in ACTT - 2 is more typical of an add - on therapy (HR=1.16) • No SAE’s have been attributable to lenzilumab (no SAE’s related to infections, Pulmonary Alveolar Proteinosis, etc.) • The trial is 90% powered (402 events, approximately 515 patients) to show the observed benefit at the interim analysis, HR=1.37 and has a very high probability of success • Planned interim analysis for efficacy at 75% enrollment (approximately 390 patients) may stop enrollment for overwhelming efficacy (current enrollment 285 patients)

11 Lenzilumab Phase 3 - Interim Analysis Conclusion • Lenzilumab treatment tracking better than standard of care • Phase 3 study being expanded, a dditional study sites being added • 90% powering maintained • Program significantly de - risked • EUA filing expected Q1 2021 • Interim efficacy analysis to be completed at 75% events • CRADA provides regulatory and manufacturing support

12 CRADA and Operation Warp Speed CRADA signed with Department of Defense Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense (JPEO - CBRND) • In collaboration with Biomedical Advanced Research and Development Authority (BARDA) • Part of Assistant Secretary for Preparedness and Response (ASPR) at the US Department of Health and Human Services (HHS) • In support of Operation Warp Speed (OWS) • CRADA provides access to OWS Subject Matter Experts • Chief Advisor, Dr. Moncef Slaoui, former Chairman, Global Research and Development and Chairman, Global Vaccines, GlaxoSmithKline • Regulatory input and assistance (including FDA staffers) • Statistical analysis • Manufacturing and Supply Chain Cooperative Research and Development Agreement with DoD aims to improve availability to lenzilumab

13 Key Members of OWS • Moncef Slaoui • General Gustave Perna • Janet Woodcock • Carlo De Notaristefano • Colonel Deacon Maddox • Lt. General Paul Ostrowski

14 OWS Regulatory Participation and Expertise • Interim results (unblinded) shared with OWS • OWS therapeutic efforts headed by Janet Woodcock, director of the Center for Drug Evaluation and Research (CDER) at FDA • Review of all submissions and correspondence to FDA • OWS FDA SME attendance at company meetings with FDA • OWS will be included as a Named Contact on all FDA filings • OWS concurs with expansion of the Phase 3 study and path forward

15 OWS Manufacturing and Supply Chain Expertise • Manufacturing experts include Carlo De Notaristefano , Exec VP, Global Ops, Teva • OWS efforts lead by General Gustave Perna , US Army four - star general, Chief Operating Officer (previously served as the 19th commanding general of United States Army Material Command) • Logistics expertise provided by Colonel Deacon Maddox, career logistics expert • Contracting and procurement assistance provided by Lieutenant General Paul Ostrowski

16 Benefits of CRADA • Regulatory guidance and assistance provided by FDA experts enhances FDA interaction • Provides to access to BARDA existing relationships with CMOs • Potential for preferential scheduling and capacity allocation • Ability to tap into current stock of critical components • CRADA precursor to potential contract for development of lenzilumab

17 Questions and Discussion
Questions and Discussion
