Form 10-K SECOND SIGHT MEDICAL For: Dec 31
UNITED
STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 10-K
(Mark One)
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the Fiscal Year Ended December 31, 2017
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from ________ to ________
Commission File Number 333-198073
Second
Sight Medical Products, Inc.
(Exact name of Registrant as specified in its charter)
| California | 02-0692322 |
| (State
or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
12744
San Fernando Road, Suite 400, Sylmar, CA 91342
(Address of principal executive offices, including zip code)
Registrant’s telephone number, including area code: (818) 833-5000
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class | Name of Each Exchange on Which Registered |
| Common Stock, without par value | The Nasdaq Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes ☐ No ☒
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the Registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the Registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 232.405 of this chapter) is not contained herein, and will not be contained, to the best of Registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☒
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definition of “large accelerated filer,” “accelerated filer,” “smaller reporting company”, and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ | Non-accelerated filer ☐ | Smaller reporting company ☒ |
| Emerging growth company ☒ | (Do not check if a smaller reporting company) | ||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☒
The aggregate market value of the shares of the Registrant’s Common Stock held by non-affiliates of the Registrant as of June 30, 2017, computed by reference to the closing sales price on the Nasdaq Capital Market on that date, was approximately $40.6 million.
As of March 13, 2018, the number of shares of the Registrant’s common stock outstanding was 59,858,842.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s Proxy Statement for the 2017 Annual Meeting of Stockholders are incorporated herein by reference in Part III of this Annual Report on Form 10-K to the extent stated herein. Such proxy statement will be filed with the Securities and Exchange Commission within 120 days of the registrant’s fiscal year ended December 31, 2017.
| 1 |
SECOND SIGHT MEDICAL PRODUCTS INC.
FORM 10-K
TABLE OF CONTENTS
| 2 |
Our Company
Overview
Second Sight was founded in 1998 with a mission to develop, manufacture, and market prosthetic devices that are intended to create an artificial form of useful vision for blind individuals. Our principal offices are located in Sylmar, California, approximately 25 miles northwest of downtown Los Angeles. We also have an office in Lausanne, Switzerland, that manages our commercial and clinical operations in Europe, the Middle East and Asia.
Our current product, the Argus® II System, treats outer retinal degenerations, such as retinitis pigmentosa, often referred to as RP. RP is a hereditary disease, affecting an estimated 1.5 million people worldwide including about 100,000 people in the United States. The disease causes a progressive degeneration of the light-sensitive cells of the retina, leading to significant visual impairment and ultimately blindness. The Argus II System is the only retinal prosthesis approved in the United States by the Food and Drug Administration (FDA), and was the first approved retinal prosthesis in the world. By creating an artificial form of useful vision in patients who otherwise have no usable sight, the Argus II System can provide benefits which include:
| ● | restoring independence through a renewed ability to navigate independently in unfamiliar environments, |
| ● | improving patients’ orientation and mobility, such as locating doors and windows, avoiding obstacles, and following the lines of a crosswalk |
| ● | allowing patients to feel more connected with people in their surroundings, such as seeing when someone is approaching or moving away, |
| ● | providing patients with enjoyment from being “visual” again, such as locating the moon, tracking groups of players as they move around a field, and watching the moving streams of lights from fireworks, |
| ● | enabling some patients to re-enter the workforce through multiple vocations that become possible because of Argus II, and |
| ● | improving patients’ well-being and ability to perform activities of daily living. |
The Argus II System provides an artificial form of vision that differs from the vision of people with normal sight. It does not restore normal vision and there is no clear evidence that it can slow or reverse the progression of the disease. The majority of patients receive a significant benefit from the Argus II, however results can vary and some patients report receiving little or no benefit.
Our major corporate, clinical and regulatory milestones include:
| ● | In 1998, Second Sight was founded. |
| ● | In 2002, we commenced clinical trials in the US for our prototype product, the Argus I retinal prosthesis. |
| ● | In 2007, we commenced clinical trials in the US for the Argus II System, which later became our first commercial product. |
| ● | In 2011, we received marketing approval in Europe (CE Mark) for the Argus II System. |
| ● | In 2013, we received marketing approval in the United States (FDA) for the Argus II System. |
| ● | In 2014, we launched the Argus II in the US, completed our initial public offering (“IPO”), and began trading on Nasdaq under the symbol “EYES.” |
| ● | In November 2017, the FDA granted Expedited Access Pathway Designation for the Orion™ Cortical Visual Prosthesis System (Orion). |
| 3 |
| ● | In the first quarter of 2018, the first-in-human Orion was successfully implanted, activated and tested at UCLA. |
We began selling the Argus II System in Europe at the end of 2011, Saudi Arabia in 2012, the United States and Canada in 2014, Turkey in 2015, Iran, Taiwan, South Korea and Russia in 2017, and Singapore in 2018. With the exception of Taiwan, Russia and Singapore, we have full regulatory approval to sell in these regions. In Taiwan, Russia and Singapore we have limited regulatory approval but we are working to obtain full regulatory approval in these countries. We sell primarily through our direct field support teams, but utilize and support distributors in certain countries.
Currently, after more than 18 years of research and development, more than $200 million of investment and over $34 million of grants awarded in support of our technology development, we employ over 110 people in the development (research, engineering and clinical), manufacture, and commercialization of the Argus II System and future products.
Our Technology
The Argus II System employs electrical stimulation to bypass degenerated photoreceptor cells and to stimulate remaining viable retinal cells thereby inducing visual perception in blind individuals. The Argus II System works by converting video images captured by a miniature camera housed in a patient’s glasses into a series of small electrical pulses that are transmitted wirelessly to an array of electrodes that are implanted on the surface of the retina. These pulses are intended to stimulate the retina’s remaining cells, resulting in a corresponding perception of patterns of light in the brain. Following the implant surgery, patients learn to interpret these visual patterns thereby regaining some useful vision, allowing them to detect shapes of people and objects in their surroundings.
We believe the Argus II System (including its implantable components) possesses several unique technological advancements compared to other neurostimulation devices including a hermetic package with the smallest size and largest number of individually programmable electrodes, and a patented electrode material that allows high charge densities and small electrode size. Several other engineering challenges, including device reliability, extended lifetime, and a safe and effective bio-interface, were overcome during the development of the product and these solutions have been protected both by patents and by trade secrets. As of December 31, 2017, we have over 400 issued patents and approximately 80 pending patent applications worldwide. Additionally, from a competitive standpoint, the Argus II System possesses attractive technical and other features that include:
| ● | a unique patented design that allows for a demonstrated lifetime and benefit of over 10.7 years, |
| ● | surgical implantation that can be performed in three to four hours using standard vitreoretinal techniques, |
| ● | a relatively large field of view (20 degrees), |
| ● | implanted patients can undergo MRI procedures, and |
| ● | individually programmable electrodes on the prosthesis which can permit further optimization of the device after implantation. |
We have demonstrated the ability to design products with long-term reliability. The Argus I retinal prosthesis, a proof of concept device that was a predecessor to the Argus II, was implanted in six patients in the United States. Argus I patients were implanted an average of almost six years, with one patient having used the device for over 10 years. The Argus II System has been implanted in over 300 patients. The average implant duration for these patients is nearly three years with several users continuing to use the system 10 years following implantation.
By further developing our visual cortical prosthesis, the Orion visual prosthesis system, we believe we can further expand our market to include nearly all profoundly blind individuals. The only notable exceptions for potential use of the Orion are those who are blind due to otherwise currently treatable diseases (ex. RP or cataracts) or due to direct damage of the visual cortex, which is rare. However, of the estimated 39 million blind people worldwide, there are approximately 5.8 million people who are legally blind due to causes that are not otherwise treatable (including RP) or AMD. If approved for marketing, the FDA and other regulatory agencies will determine the subset of these patients who are eligible for the Orion based on our clinical trial and the associated results.
| 4 |
Our objective in designing and developing the Orion visual prosthesis system is to bypass the optic nerve and directly stimulate the part of the brain responsible for human vision. In January 2018, the first human patient was implanted with an Orion in a feasibility study at the Ronald Reagan UCLA Medical Center (UCLA). In February 2018, this first Orion implant was successfully activated and the patient was able to perceive phosphenes on 56 of 60 electrodes. More recently, the patient has perceived phosphenes when stimulating all 60 electrodes. In the next few months, we plan to expand this feasibility study by implanting four more patients, at UCLA and/or at Baylor College of Medicine (Baylor) in Houston. After preliminary functionality testing is completed, we plan to introduce eyewear with a video camera to the system to produce the first in human test of a fully functional wireless visual cortical stimulator system intended to provide a useful level of vision. This initial study in a small number of subjects, if successful, should also form the basis for an expansion to a pivotal clinical trial in 2019.
In November 2017, the FDA granted Expedited Access Pathway designation for the Orion. This designation is given to a few select medical devices in order to provide more effective treatment of life-threatening or irreversibly debilitating diseases or conditions. This program is intended to help patients have more timely access to these medical devices by expediting their development, assessment, and review. The FDA has also released a draft guidance document for a Breakthrough Devices Program, which, when finalized, will supersede the Expedited Access Pathway. The FDA has indicated that all devices which have Expedited Access Pathway designation will gain Breakthrough Device designation when the guidance document is finalized. With this designation, we believe the Orion will have the following advantages during the FDA review process:
| ● | Greater interactive review both for the Investigational Device Exemption and Premarket Approval application; | |
| ● | Greater reliance on post-market vs. pre-market data collection and greater acceptance of uncertainty in the benefit-risk profile at the time of approval; | |
| ● | Priority review (i.e., review of the submission is placed at the top of the review queue and receives additional review resources); and, | |
| ● | Senior FDA management involvement and assignment of a cross-disciplinary case manager. |
It is our belief that inclusion in the Expedited Access Pathway designation and, in the future, the Breakthrough Devices Program may shorten the timeline required to bring the Orion to market as a commercial product.
Our Markets
Retinitis Pigmentosa (RP)
RP is a group of inherited disorders that affect the retina. The retina is a layer of nerve cells at the back of the eye. RP is a disease that gradually robs relatively young people of their vision over time. Onset of RP is often noted in the teen years or early twenties, typically as night blindness. This is followed by a period of peripheral vision loss, until the patient is left with a tunnel of vision and then no remaining sight. Although there are various genetic causes (over 100) and thus variability in the disease progression, many people with advanced RP have lost all functional vision by their 40s or 50s. The Argus II System works by bypassing rods and cones which are largely defunct in these patients, and sends electrical signals directly to the retina’s remaining healthy cells.
Although there are reported trials for other treatments underway, to our knowledge the Argus II System remains the only approved therapeutic option for end-stage RP in the US, and to our knowledge it is the only treatment option generally available to commercial patients anywhere in the world.
| 5 |
Worldwide, an estimated 1.5 million people suffer from RP1, which includes about 100,000 in the US2. Pan-European data is not readily available, but we believe it is reasonable to estimate that the average prevalence throughout Europe is similar to the average prevalence within the US, and so the ratio of populations could be used to estimate the number of Europeans affected as 167,000 in the 28 EU countries3,4. Approximately 25% of people with RP in the US have vision that is 20/200 or worse (legally blind)5. Since the bare light perception or worse vision criterion (in both eyes) for the US indication is worse than 20/200, we estimate the subset of patients that can be treated by the Argus II System is less than 25,000 in the US. Reliable market data estimating the actual number of patients with bare light perception or worse vision is unavailable. We believe the majority of patients with vision 20/200 or worse have vision that is better than bare light perception and thus, are not currently candidates for Argus II. In Europe, the indicated vision loss for Argus II patients is severe to profound which, while better than bare light perception, remains somewhat worse than 20/200. An estimated 42,000 patients in Europe with RP have vision worse than 20/200 and we estimate the subset of RP patients that can be treated in Europe to be somewhat smaller than this number. As in the US, reliable market data estimating the actual number of patients with severe to profound vision loss or worse is unavailable. We believe that the majority of patients with vision 20/200 or worse in Europe have vision that is too good to be considered a candidate for Argus II with current clinical indications and physician practice. Worldwide, we estimate that 375,000 people are legally blind due to RP, and that a portion of these would be candidates for the Argus II System.
As we improve the quality of vision that the Argus II can produce, we expect to be able to treat a higher percentage of the legally blind population by treating better sighted patients that still suffer from no real usable vision.
Age Related Macular Degeneration (AMD)
AMD is a relatively common eye condition and the leading cause of vision loss among people aged 65 and older6. The macula is a small spot near the center of the retina and its damage results in loss of central vision. AMD can start as a blurred area near the center of vision and over time it can grow larger until loss of central vision occurs. Central vision is extremely important for everyday tasks such as reading, writing, and face recognition.
There are three stages of AMD defined in part by the size of drusen (yellow deposits) under the retina. Early and intermediate stage AMD has few symptoms or vision loss. These earlier stages of the disease are usually left untreated or dealt with using diet supplementation. People with advanced AMD have vision loss from damage to the macula. There are two types of late stage AMD:
| ● | Dry AMD: There is a breakdown of light sensitive cells in the macula that send visual information to the brain, and the supporting tissue beneath the macula. This damage causes vision loss. |
| ● | Wet AMD: Blood vessels grow underneath the retina. These vessels might leak blood which may lead to swelling and damage of the macula. This damage may be severe and can progress quickly. |
1 Weleber, R.G. and Gregory-Evans, K. (2001) ‘Retinitis Pigmentosa and allied disorders.’ In Ryan, S.J. (ed.), Retina. Mosby, St. Louis, pp, 362-470.
2 Foundation Fighting Blindness estimates that about 100,000 Americans are affected by RP or similar diseases. (http://www.ffb.ca/documents/File/rp_guide/Guide_to_RP_and_Other_Related_Diseases.pdf)
3 Eurostat. Retrieved 1 January 2013.
4 Haim M. Epidemiology of Retinitis Pigmentosa in Denmark. Acta Ophthalmol Scand Suppl 2002; 1-34.
5 Grover et al., ‘Visual Acuity Impairment in Patients with Retinitis Pigmentosa at Age 45 Years or Older’, Ophthalmology. 1999 Sept; 106(9):1780-5.
6 The Eye Diseases Prevalence Research Group, 2004a; CDC, 2009.
| 6 |
Worldwide, between 20 and 25 million people are estimated to suffer from vision loss due to AMD7, and of these about two million have vision that is considered legally blind, or worse8. In the US, just over two million people experience vision loss due to AMD according to a 2010 study by the National Eye Institute. Of the 1.3 million legally blind Americans9, we estimate that 42.5% (or 552,500) are due to AMD10. Applying this percent of legally blind due to AMD (42.5%) to the total number of legally blind people in Europe (2.55 million)11, we estimate the population of legally blind individuals from AMD to be about 1.08 million individuals in Europe. We believe the Argus II System may be able to help a subset of these legally blind AMD patients who have severe to profound vision loss. To date, though clinical testing has produced subjective improvements, we have not yet demonstrated objective benefits and no assurance can be given that we will be able to do so. The challenge in demonstrating objective benefits in these patients is that they maintain residual peripheral vision. Thus, we must demonstrate that the quality of the vision we produce is better than their residual vision, which is much more challenging than demonstrating benefit for the RP patients we are currently treating, who have completely lost all vision.
Other diseases resulting in blindness that may be treated by Orion
Many diseases outside of RP and AMD can also cause blindness. Many of the largest causes of visual impairment (i.e. refractive error and cataracts) are avoidable or curable, and their prolonged or untreated impact on vision is largely observed in developing nations and are not part of our target market. Some other causes of blindness, such as brain trauma to the visual cortex, may also not be suitable for treatment by a cortical stimulator. However, the remaining causes of severe vision loss which include glaucoma, diabetic retinopathy, eye trauma, retinopathy of prematurity and many others can result in severe visual impairment that could potentially be treatable by an Orion visual prosthesis system.
According to the World Health Organization (WHO)12, 285 million people suffer from vision loss worldwide. Of these, 39 million people are considered legally blind. The WHO further estimates that 80% of legal blindness is avoidable, leaving 7.8 million legally blind individuals, including those blind due to AMD and RP, or 5.8 million excluding AMD and RP. In the US, 1.3 million people are legally blind13, of whom we estimate 44%, or 575,900, are legally blind due to causes other than preventable/treatable conditions, RP or AMD14. Applying the same logic, we estimate 1.13 million individuals are legally blind in Europe due to causes other than currently treatable conditions (including RP) or AMD. We believe that, if approved by the FDA, the Orion will initially treat a subset of these legally blind individuals, likely starting with the ones who are completely blind. If this is the case, we anticipate that if we are further able to collect additional clinical data demonstrating the efficacy of the Orion for patients with better vision, we will be able to expand the approved indications and addressable market of the Orion to include a larger subset of these 5.8 million individuals for whom no effective treatment currently exists.
Our Strategy
Second Sight’s strategy can be summarized as follows:
| ● | Establish exclusive regional Centers of Excellence (COE), expand reimbursement coverage to cover all levels of care for all eligible patients, and establish highly effective post-surgical vision rehabilitation environment where our artificial sighted patients can thrive, |
| ● | Leverage proven Argus technology to restore useful vision with the Orion cortical stimulator and expand addressable market to include a portion of the almost 6 million patients who are blind from eye trauma, optic nerve disease, and other untreatable causes. |
7 Choptar, A., Chakravarthy, U., and Verma, D. ‘Age Related Macular Degeneration’. BJM 2003;326:485.
8 Global Data on Visual Impairments 2010, World Health Organization.
9 National Eye Institute (http://www.nei.nih.gov/eyedata/blind.asp).
10 Congdon N, O’Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. Apr 2004;122(4):477-485. This percent amount was derived from the rates of different causes of blindness by different races and racial demographic data from 2010 US Census data.
11 Global Data on Visual Impairments 2010, World Health Organization.
12 WHO Fact Sheet number 282, updated October 2013.
13 National Eye Institute (http://www.nei.nih.gov/eyedata/blind.asp).
14 Congdon N, O’Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. Apr 2004;122(4):477-485. This percent amount was derived from the rates of different causes of blindness by different races and racial demographic data from 2010 US Census data.
| 7 |
| ● | Improve Argus II performance and significantly expand use in the RP population by treating better-vision patients. |
Establish Centers of Excellence (COE) and expand reimbursement coverage to reach a larger percentage of eligible patients
We launched the Argus II System in Italy and Germany at the end of 2011; in Saudi Arabia, France, the Netherlands and England in 2013; in Switzerland, Spain, the US and Canada in 2014; and Austria and Turkey in 2015, Taiwan, South Korea and Russia in 2017 and Singapore in 2018. We are employing a refined Centers of Excellence sales strategy, deploying the Argus II at prominent, reputable eye centers which are equipped to handle all aspects of an Argus II program including patient recruitment, surgery, fitting and vision rehabilitation. We believe this strategy represents an efficient use of our capital after giving consideration to the following factors:
| ● | Size of the RP patient population that is currently treatable by Argus II, | |
| ● | Complexity of the technology, surgery, post-surgery programming and rehabilitation, and | |
| ● | Cost of selecting, qualifying, training and building qualified Centers of Excellence. |
When selecting new sites, we focus on high quality health providers considering the following factors:
| ● | Geographic location, | |
| ● | Facility, surgeon skill and reputation, | |
| ● | Site commitment to establish Argus as their core priority, | |
| ● | Desire and capability of institution to perform a significant number of surgeries annually, | |
| ● | Ability of site to perform post-surgery programming of Argus technology, and | |
| ● | Capability of site and/or local resources to direct artificial vision rehabilitation. |
As of December 31, 2017, we have 19 qualified centers in the United States and Canada that are actively implanting the Argus II. Ultimately, we anticipate serving the North American RP market with approximately 35 implanting centers across the US and Canada, with much of that expansion occurring in 2018. In Europe and the Middle East and Asia Pacific, we currently have 21 centers that are actively implanting the Argus II (eight in Germany, three in France, one in Saudi Arabia, four in Turkey, two in Spain, and three in Italy). We believe that we will be able to serve the European and Middle East markets for RP by having approximately 50 centers across Europe, the Middle East and Asia Pacific.
To date, we have employed direct sales and clinical specialists to service our markets in the US and Canada. The majority of our markets in Europe are also serviced by a direct sales and clinical specialist team. As of January 31, 2018, the sales/clinical specialist team for North America numbered nine persons and the sales team for Europe and the Middle East numbered seven persons. In some cases, we believe that we can more efficiently expand our reach by securing distributors in key markets. To date, we have appointed distributors in Turkey, Saudi Arabia, South Korea, Taiwan, Iran, Russia, Singapore and Argentina. We expect that our distributors will commit to providing support services that include marketing, market access, sales, surgical support, post-surgery programming, rehabilitation coordination and service.
The Company is evaluating potential new markets including countries in Latin America or Asia Pacific regions. We will selectively enter markets based on multiple factors including: the presence of RP patients, skilled surgeons, a facility with the necessary support infrastructure, a reliable source of funding or reimbursement along with the assurance that needed clinical, rehabilitation and surgical support can be provided.
| 8 |
Centers of Excellence
Our COE strategy in the US market is designed to help our exclusive regional partners to more effectively manage Argus II patients and achieve inspiring patient outcomes. The COE strategy consists of four major initiatives: (1) financial, (2) patient recruitment, education and screening, (3) post-surgery programming, and (4) patient support and artificial vision rehabilitation.
| ● | First, there are the financial considerations. As reported, the CMS hospital outpatient final rule assigned a payment rate of $122,500 for the Argus II and the associated surgical procedure beginning January 1, 2018. The rate for calendar 2017 was $150,000 and the rate for calendar 2016 was $95,000. Physician fees continue to be reimbursed separately. Our current pricing strategy should generally ensure full reimbursement coverage of hospital surgical procedure costs including the Argus II system. We are also pleased that effective July 1, 2017, CPT codes for post-surgery programming became available. These developments should ensure a favorable economic analysis for any center evaluating an Argus II program. |
| ● | Second, regarding patient recruitment, education and screening, we focus extensive outreach efforts to identify those blind individuals that qualify for Argus II. When identified they are entered into a leading CRM database, supported by interactive marketing automation programs, providing a funnel of patients for each of our strategic sites as patient candidates decide to move forward. We separately clear patient candidates with a team of experienced healthcare professionals, providing our COEs with a streamlined path of higher probability patient candidates. |
| ● | Third, in terms of the post-surgery programming, our recent and future product improvements are aimed at simplifying the programming procedure for the site and for the patient. In fact, we’ve recently reduced the expected time to program our system from two days down to a half day. As with the surgery, repetition will make the programming more routine for the institution. And, as mentioned above, we have secured CPT codes to allow sites to submit for reimbursement when they program an Argus system. |
|
| ● | Fourth, patient support and artificial vision rehabilitation have become our most indispensable tool to managing consistent, positive outcomes across all treated patients. Beginning on day-one of initial activation, the brain works to make sense of the entirely new sensory input from Argus. Each individual patient needs guidance on the activities and tasks he or she can engage in, especially early on, that can best guide the brain to create these important new connections. As we have come to understand the important training curriculum of artificial vision rehabilitation, we are deploying today full-time vision rehabilitation specialists who are experts in all the ways Argus has enabled patients to: regain independence with the world around them; enhance social interactions with the people they associate with; enjoy the near endless aesthetic experiences that derive from visual interactivity; and in a growing number of cases, a form of vision that enables our patients to re-enter the workforce in many special roles. Our objective is to deliver outstanding patient outcomes, while building the know-how and infrastructure of vision rehabilitation for a substantially larger patient population in the near future. |
In summary, the aim of the COE program is to establish implanting centers and physician clinics that are more intimately knowledgeable, self-sufficient, and confident, enabling them to be able to treat a higher volume of patients. We believe the COE program is ultimately the important development work that is preparing the Company to serve expanded patient populations in the near future.
Global Reimbursement
Obtaining reimbursement from governmental and private insurance companies is critical to our commercial success. Due to the cost of the Argus II System, our sales would be limited without the availability of third party reimbursement. In the US, coding, coverage, and payment are necessary for the surgical procedure and Argus II system to be reimbursed by payers. Coding has been established for the device and the surgical procedure. Coverage and payment vary by payer. The majority of Argus II patients are eligible for Medicare, and coverage is primarily provided through traditional Medicare, sometimes referred to as Medicare Fee-for-Service (FFS) or Medicare Advantage. A small percentage of patients are covered by commercial insurers.
| ● | Medicare FFS patients – Coverage is determined by Medicare Administrative Contractors (MACs) that administer various geographic regions of the US. Positive coverage decisions for the Argus II are effective in eight of 12 MAC jurisdictions (comprising 31 states, two territories and the District of Columbia). Effective January 1, 2018, the Centers for Medicare and Medicaid Services (CMS) established a 2018 payment rate of $122,500 for both the procedure and the Argus II Retinal Prosthesis System. |
| 9 |
| ● | Medicare Advantage patients – Medicare Advantage plans are required to cover the same benefits as those covered by the MAC in that jurisdiction. For example, if a MAC in a jurisdiction has favorable coverage for the Argus II, then all Medicare Advantage plans in that MAC jurisdiction are required to offer the same coverage for the Argus II. Individual hospitals and ASCs may negotiate contracts specific to that individual facility, which may include additional separate payment for the Argus II implant system. In addition, procedural payment is variable and can be based on a percentage of billed charges, payment groupings or other individually negotiated payment methodologies. Medicare Advantage plans also allow providers to confirm coverage and payment for the Argus II procedure in advance of implantation. In 2015, 2016 and 2017 combined, a large majority of all Medicare Advantage pre-authorization requests for Argus II procedures were granted. |
| ● | Commercial insurer patients – Commercial insurance plans make coverage and payment rate decisions independent of Medicare, and contracts are individually negotiated with facility and physician providers. |
During the year ended December 31, 2017, 38 individuals in the US and Canada were implanted with the Argus II technology. Of these patients, 31 were in US with most patients having a Medicare FFS coverage and a few having a Medicare Advantage or Veteran’s Administration or another commercial insurance plans and the remaining seven in Canada were treated with private funding.
Second Sight employs dedicated employees and consultants with insurance reimbursement expertise engaged to expand and enhance coverage decisions. Currently, eight of 12 Medicare jurisdictions provide coverage of the Argus II in 31 states, two territories and the District of Columbia when medically necessary, including:
● CGS (J15 -- Ohio and Kentucky),
● Palmetto GBA (JM -- Virginia, (excluding Part B for Arlington and Fairfax counties), West Virginia, North Carolina and South Carolina),
● Palmetto GBA (JJ – Alabama, Georgia and Tennessee),
● NGS (J6 -- Minnesota, Illinois and Wisconsin),
● NGS (JK -- Connecticut, New York, Maine, Massachusetts, New Hampshire, Rhode Island and Vermont),
● FCSO (JN -- Florida, Puerto Rico and the U.S. Virgin Islands),
● Novitas (JH-- Arkansas, Colorado, Louisiana, Mississippi, New Mexico, Oklahoma, and Texas) and
● Novitas (JL -- Delaware, District of Columbia, Maryland, New Jersey and Pennsylvania).
We are actively engaged with the remaining four Medicare jurisdictions covered by two contractors and are committed to supporting their requests for additional information and clinical evidence. We expect that additional positive coverage decisions will be issued over time but cannot predict timing or ultimate success with each MAC.
Within Europe, we have obtained reimbursement approval or funding in Germany, France, one region of Italy, a Commissioning through Evaluation (CtE) in program England. We are seeking additional reimbursement approvals in other countries in Europe and international markets.
In France, the Company was selected to receive the first “Forfait Innovation” (Innovation Bundle) from the Ministry of Health, which is a special funding program for breakthrough procedures to be introduced into clinical practice. As part of this program, the Company is conducting a post-market study in France which has enrolled a total of 18 subjects who are being followed up for two years. The French program also funds implantation of up to 18 additional patients who are not part of the post-market study. After review of the study’s results, we expect Argus II therapy to be covered and funded through the standard payment system in France, however, we can provide no assurance that the French government will continue to fund the Argus II after the first 36 implants.
| 10 |
In December 2016, NHS England announced it would cover 10 Argus implantations as part of a CtE program. The CtE program is especially designed for treatments that show significant promise for the future, while new clinical and patient experience data are collected within a formal evaluation program. This program is similar to the Forfait Innovation program in France. NHS England is known to be under significant financial pressure and also highly selective in adopting innovative technologies – which must demonstrate sufficient value for the cost expended.
To date, our marketing activities have focused on raising awareness of the Argus II System with potential patients, implanting physicians, and referring physicians. Our marketing activities include exhibiting, sponsoring symposia, and securing podium presence at professional and trade shows, securing journalist coverage in popular and trade media, attending patient meetings focused on educating patients about existing and future treatments, and sponsoring information sessions for the Argus II System. In the United States, our efforts will focus on media advertisements dedicated to RP patients and their families. These advertisements are placed in geographic areas where we have Centers of Excellence committed to Argus II.
Improve Argus II performance and significantly expand use in the RP population by treating better-vision patients
The Argus II System is currently approved for RP patients with bare or no light perception in the US, and in Europe for severe to profound vision loss due to outer retinal degeneration, such as from retinitis pigmentosa, choroideremia, and other similar conditions. The number of people who are legally blind due to RP is estimated to be about 25,000 in the US, 42,000 in Europe, and about 375,000 total worldwide. As discussed above, a subset of these patients would be eligible for the Argus II System since the approved baseline vision for the Argus II System is worse than legally blind (20/200). Scarce epidemiological data on visual acuity below legal blindness make it difficult to determine a precise estimate of the potential patient population for this device, but resulting from our commercial efforts thus far we believe most legally blind patients have vision that is too good for Argus II’s current clinical indications.
The Company believes an opportunity exists to expand the use of its technology to better vision individuals with RP who are currently not being treated. In order to achieve this market expansion, the Company plans to start collecting clinical data in 2018 and is undertaking multiple development efforts to improve the technology’s performance. Our clinical and R&D plans for this market segment can be summarized as follows:
| ● | Clinical trials with better-vision individuals – The Company intends to start collecting clinical data at multiple sites in Germany during 2018 to determine if the Argus II provides sufficient clinical benefit to these better-sighted patients. If successful, the Company would proceed with the various required steps to obtain regulatory approval and reimbursement coverage for treatment of this expanded patient group. |
| ● | Retinal stimulation protocols – We believe that we can achieve improved resolution by adjusting retinal stimulation techniques. An example is the use of current steering to cause perception of pixels between electrodes. By producing these ‘virtual’ pixels, we may be able to increase the effective resolution of the Argus II beyond the physical number of electrodes (which today total 60). We expect to continue the patient testing in 2018 with commercial implementation of these revised retinal stimulation protocols in early 2019. |
| ● | External hardware – We plan to submit our new external system to the FDA in mid-2018. The new externals will include redesigns of the head mounted telemetry system (glasses), camera and video processing unit (VPU). The new VPU will possess processing power many times greater than the current Argus II system, which will enable enhanced image processing and support for the commercial implementation of the new retina stimulation protocols discussed above. We anticipate that the new external system will be commercially available in late 2018. |
| ● | Other longer-term R&D efforts – We are developing more advanced software to improve the quality and usefulness of the Argus II vision delivered to patients. If successful, we expect that these software packages will run on the new external system described above. The development of advanced software packages is in the early phases and no assurance can be made that our efforts will be successful nor can we predict commercialization dates. |
| 11 |
Leverage proven Argus technology to restore some vision with cortical stimulation and expand addressable market to include a portion of the almost 6 million patients who are blind from eye trauma, optic nerve disease, and other untreatable causes
We believe we can further expand our market to include nearly all profoundly blind individuals, other than those who are blind due to currently treatable diseases or due to direct brain damage of the visual cortex, by developing a visual cortical prosthesis. We refer to this product as the Orion visual prosthesis system. We estimate that there are approximately 5.8 million people worldwide who are legally blind due to causes other than currently treatable conditions (including RP) or AMD. If approved for marketing, the FDA and other regulatory agencies will determine the subset of these patients who are eligible for the Orion.
Our objective in designing and developing the Orion visual prosthesis system is to bypass the optic nerve and directly stimulate the part of the brain responsible for vision. The Orion visual prosthesis system is based on technology that we utilize in our Argus II system, thereby reducing engineering investment costs and risks, and leveraging the reliability of the Argus II platform.
In November 2017, we received full FDA approval to begin the first human clinical feasibility study of the Orion visual prosthesis system. In January 2018, surgeons at the UCLA implanted the first human with an Orion, and in February 2018, working with UCLA, we activated this first implanted Orion device and began clinical testing. To date, clinical results have been positive, with the first patient being able to perceive phosphenes, or spots of light, when electrodes are turned on. Over the next several months, we plan to implant four more patients at UCLA and Baylor College of Medicine, and to begin cortical stimulation with our external video camera, which we believe may provide these patients with some form of usable vision. If successful, this study should also form the basis for an expansion to a pivotal clinical trial beginning in 2019.
Our Competition
The US life sciences industry is highly competitive and well-positioned for future growth. The treatment of blindness is a significant clinically unmet need and others continue to make progress. There are several approaches to treating blindness including:
| ● | Retinal Prostheses (including the Argus II): aimed at giving more visual ability to a blind patient via implanting a device in the eye to stimulate remaining retina cells. Electrical neurostimulation technology has seen growing use in recent years for numerous applications– such as chronic pain, Parkinson’s disease, essential tremor, epilepsy, and others. |
| ● | Transplants: transplanting retinal tissue to stimulate remaining retina cells. |
| ● | Stem Cells: generally involves implanting immature retinal support cells aimed at slowing retinal degeneration. A single patient with wet AMD was implanted in London in 2015 with an embryonic stem cell line in a study sponsored by Pfizer. This study has been suspended. Patients with dry AMD are also being recruited in Los Angeles for a similar study. No data is yet available as to safety or efficacy of these implantations. A Patient in Japan with AMD was implanted with induced pluripotent stem (ips) cells. These are mature cells reprogramed to be stem cells. |
| ● | Genetics and Gene Therapy: involves identifying a specific gene that is causing retinal problems (there are over 120 for retinitis pigmentosa alone) resulting in visual impairments and blindness; and inserting healthy genes into an individual’s cells using a virus to treat the diseases. A company completed a phase 3 study in 21 patients with a median age of 11 for a gene that affects a very small percentage of retinitis pigmentosa patients, RPE65. That company applied for and received FDA approval in 2018. We believe that there is virtually no overlap with our current market since our patients generally are older (Argus II is indicated for an age minimum of 25 in the US). The other company also injects better sighted patients since its treatment is aimed at improving residual vision. In contrast, Argus II seeks to create artificial vision where vision is completely lost. Pricing for these injections is reported to be approximatly $850,000 for both eyes. |
| 12 |
| ● | Optogenetics Therapy: aimed at slowing down, reversing, and/or eliminating the process by which photoreceptors in the eye are compromised. This therapy also requires infecting the patient’s cells with a virus. However, instead of fixing a gene defect, this approach would cause cells within the eye to become light sensitive. Animal work has shown that these cells are not sensitive enough to respond to ambient light, so this approach currently also requires a light amplifier outside the body to increase light delivered to the retina. The regulatory body in the UK, MHRA, has recently cleared optogenetic clinical trials to begin. |
| ● | Nutritional Therapy: involves diets or supplements that are thought to prevent or slow the progress of vision loss. |
| ● | Implantable Telescope: VisionCare, Ophthalmic Technologies, Inc. offers an FDA approved implantable miniature telescope for AMD, a magnifying device that is implanted in the eye. The VisionCare telescope is approved for use in patients with severe to profound vision impairment (best corrected visual acuity of 20/160 to 20/800) due to dry AMD. |
| ● | Wicab’s The BrainPort® V100 includes a video camera mounted on a pair of sunglasses, a hand-held controller, and tongue array. The tongue array contains 400 electrodes and is connected to the glasses via a flexible cable. White pixels from the camera are felt on the tongue as strong stimulation, black pixels as no stimulation, and gray levels as medium levels of stimulation. This device is indicated for the profoundly blind. |
| ● | There are currently no known treatments for AMD after the disease has caused severe to profound vision loss nor are there any established treatments that delay or reverse the progression of Dry AMD other than supplements. |
| ● | Therapies exist for Wet AMD that delay the progression of visual impairment or slightly improve the vision, rather than completely curing or reversing its course. These therapies are approved in many regions throughout the world, including the US and EU. |
Commercial efforts to develop retinal implants by others include:
| ● | Retina Implant AG: A privately held German company that is developing the Alpha IMS/Alpha AMS, a wireless sub-retinal implant. Although this company obtained a CE Mark in 2013 and was expected to begin commercialization during 2015 in the EU, to our knowledge this product is still not generally available to commercial patients. Publications from the company reported frequent device failures of the Alpha IMS in patients. The company has reportedly improved the design and rebranded its system as the Alpha AMS. Two clinical trial patients are reported to have been implanted in the UK during 2015 and/or 2016. Other reports of implants are unconfirmed. To our knowledge, Retina Implant has not obtained FDA approval to begin a clinical trial in the US but has announced that it plans to advance commercialization efforts that include obtaining reimbursement and opening new implanting centers. |
| ● | Pixium Vision S.A.: A publicly held French company that is developing the IRIS (Intelligent Retinal Implant System) which is surgically placed into the eye and attached to the surface of the retina. Similar to our Argus II technology, its system uses a camera and a wireless transmitter. Pixium received a CE Mark in 2016 for IRIS. In 2017, Pixium halted commercial implants of IRIS II due to shorter than expected implant life. In 2017, Pixium announced approval for two feasibility studies of PRIMA (sub-retinal implant) in Dry-AMD patients. One study is in Paris with five patients. A second study is being conducted in Pittsburgh, Pennsylvania with five patients. To date Pixium has announced the successful implantation and activation of three devices in Paris, but to our knowledge no performance data has yet been reported beyond the perception of light. |
| ● | NanoRetina Inc., a company based in Israel, and several other early stage companies are reported to have developed intellectual property or technology that may improve retinal prostheses in the future, but to our knowledge none of these efforts has resulted in a completed system that has been tested clinically in patients. |
| ● | Academic entities are also working on vision restoring implants. These include Bionic Vision Australia (an early prototype device has been developed and to our knowledge implanted in three human subjects), Boston Retinal Implant project (preclinical phase), Monash Vision Group (preclinical phase), and the Illinois Institute of Technology (preclinical phase). Of these projects, we believe most have not yet demonstrated a working implant, only one has reportedly begun long-term clinical work in humans, and to our knowledge none has received FDA approval to begin clinical trials in the US. |
| 13 |
To our knowledge, no other retinal prosthesis has been successful in long-term human trials, with the Argus II System currently the sole implant generally available to commercial patients for treating RP in the US, Canada, EU, and elsewhere. We anticipate that our competitors are unlikely to obtain significant commercial traction in EU until they have developed in depth clinical data showing the reliability and functionality of their products.
Warranty
We generally provide a standard limited warranty for the Argus II System covering replacement over the following periods after implant:
| ● | three years on implanted epiretinal prosthesis, | |
| ● | two years on external components other than batteries and chargers, and | |
| ● | three months on batteries and chargers. |
Based on our experience to date, the Argus II System has proven to be a reliable device generally performing as intended. We have accrued warranty expense of $1.5 million as of December 31, 2017, which we believe to be adequate.
Our Manufacturing and Quality Assurance
We have a single manufacturing facility, located at our principal office in Sylmar, California. The manufacturing areas at this location are housed in a single building, and include approximately 10,000 square feet of controlled environment rooms (CERs) suitable for implant manufacturing. We currently utilize less than half of this space for Argus II implant production. At the same site we maintain spaces for assembling the external (non-implantable) components of our system and for the labeling, receiving and shipping, and stockroom functions. Finished goods are held at this location and at our contracted logistics partner in Europe.
We rely on many suppliers to provide materials and services necessary to produce and test our products. Many of these materials or services are currently provided by sole source suppliers. In a number of instances we maintain sole source suppliers because our current purchasing volumes do not warrant developing more than one supplier. We expect to secure additional providers as our production volumes increase. If we experience a loss of a sole supplier before confirming an alternative, we risk possible disruptions in our operations. We attempt to mitigate the sole source risk, by among other things, increasing parts inventory as a partial hedge against interruptions in parts supply and by actively seeking to develop alternative suppler sources before experiencing any such disruptions.
Our manufacturing department currently employs 20 persons and the quality assurance department has an additional eight members. We operate a day shift and smaller swing shift, and at this staffing level we can manufacture approximately 10 devices per month. Due to the reduction in sales of the Argus II during 2016, we reduced manufacturing output beginning in the second quarter of 2016. We believe that the space available at the current facility when fully utilized and operating at two full shifts will prove sufficient to build and assemble a combined total of approximately 100 Argus II or Orion devices per month.
Employees
As of December 31, 2017, we had 112 employees, including approximately 28 in operations; 24 in selling, marketing and distribution; 42 in clinical, regulatory and research and development; and 18 in administration. Of these persons, we employed 95 in the United States and 17 in Europe. We believe that the continued success of our business will depend, in part, on our ability to attract and retain qualified personnel, and we are committed to developing our people and providing them with opportunities to contribute to our growth and success. None of these employees is covered by a collective bargaining agreement, and we believe our relationship with our employees is good to excellent.
| 14 |
Properties
Our principal office and facilities are located at 12744 San Fernando Road, Suite 400, Sylmar, California 91342, and consists of approximately 45,351 rentable square feet at a current base rent of about $36,600 per month. Our lease expires in February 2022 and grants us an option to extend the lease term for an additional 60 months. We originally rented these premises from Mann Biomedical Park LLC, an entity affiliated with our former Chairman of the Board, Alfred E. Mann. We believe that the terms of this lease are at least as favorable as those that may have been obtained from a non-affiliated third party. We believe that these premises are adequate for our foreseeable needs. In November 2014, the industrial center in which these premises are located was sold to an independent third party.
Our European office is located on the Innovation Park at Ecole Polytechnique Federal de Lausanne (EPFL), Rue Jean Daniel Colladon, CH 1015 Lausanne, Switzerland. These premises consist of 180 square meters at a base rent of about 8,200 CHF per month, or currently about $8,700 per month. We rent these premises on a month-to-month basis subject to a six month notice required for termination, from the Foundation for the Innovation Park at EPFL.
Legal Proceedings
We are not a party to any pending legal proceedings other than those involving Pixium Vision described in “Risk Factors—Risks Related to Intellectual Property and Other Legal Matters.”
Available Information
Our website address is www.secondsight.com. We make available free of charge through a link provided at such website our Forms 10-K, 10-Q and 8-K as well as any amendments thereto. Such reports are available as soon as reasonably practicable after they are filed with the Securities and Exchange Commission.
The statements that are not historical facts contained in this Form 10-K are forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements reflect the current belief, expectations or intent of our management and are subject to and involve certain risks and uncertainties. Many of these risks and uncertainties are outside of our control and are difficult for us to forecast or mitigate. An investment in our common stock is speculative and involves a high degree of risk. In addition to the risks described elsewhere in this Form 10-K and in certain of our other filings with the US Securities and Exchange Commission, the following important factors, among others, could cause our actual results to differ materially from those expressed or implied by us in any forward-looking statements contained herein or made elsewhere by or on behalf of us. The risks described below are not the only risks we face. If any of the events described in the following risk factors actually occurs, or if additional risks and uncertainties later materialize, that are not presently known to us or that we currently deem immaterial, then our business, prospects, results of operations and financial condition could be materially adversely affected. In that event, the trading price of our common stock or our warrants could decline, and you may lose all or part of your investment in our shares or in our warrants.
Risks Related to Our Dependence on the Argus II System
We depend on the success of our first commercial product, the Argus II System, which received European market clearance (CE Mark) in February 2011 and FDA approval in February 2013, in the United States for RP; and on the regulatory approval of our current product and a new device under development, the Orion visual prosthesis (a modified version of the Argus II System), to treat other diseases causing blindness, in the US and other countries, which may never occur.
Our future success depends upon building a commercial operation in the US and expanding growth in Europe as well as entering additional markets to commercialize our Argus II System for RP. We believe our expanded growth will depend on the further development, regulatory approval and commercialization of the Orion product, which we anticipate can be used by nearly all profoundly blind individuals. If we fail to expand the use of the Argus II System in a timely manner for other forms of retinal degeneration in addition to RP, or to develop the Orion product and penetrate the available markets which those applications are intended to serve, we may not be able to expand our markets or to grow our revenue, our stock values could decline and investors may lose money.
| 15 |
Our revenue from sales of Argus II System is dependent upon the pricing and reimbursement guidelines adopted in each country and if pricing and reimbursement levels are inadequate to achieve profitability our operations will suffer.
Our financial success depends on our ability to price our products in a manner acceptable to government and private payers while still maintaining our profit margins. Numerous factors that may be beyond our control may ultimately impact our pricing of Argus II System and determine whether we are able to obtain reimbursement or reimbursement at adequate levels from governmental programs and private insurance. If we are unable to obtain reimbursement or our product is not adequately reimbursed, we will experience reduced sales, our revenues likely will be adversely affected, and we may not become profitable.
Obtaining reimbursement approvals is time consuming, requires substantial management attention, and is expensive. Our business will be materially adversely affected if we do not receive approval for reimbursement of the Argus II System under government programs and from private insurers on a timely or satisfactory basis. Limitations on coverage could also be imposed at the local Medicare Administrative Contractor level or by fiscal intermediaries in the U.S. and by regional, or national funding agencies in Europe. Our business could be materially adversely affected if the Medicare program, local Medicare Administrative Contractors or fiscal intermediaries were to make such a determination and deny, restrict or limit the reimbursement of Argus II System.
Similarly in Europe these governmental and other agencies could deny, restrict or limit the reimbursement of Argus II System at the hospital, regional or national level. Our business also could be adversely affected if retinal specialists and the facilities within which they operate are not adequately reimbursed by Medicare and other funding agencies for the cost of the procedure in which they implant the Argus II System on a basis satisfactory to the administering retinal specialists and their facilities. If the local contractors that administer the Medicare program and other funding agencies are slow to reimburse retinal specialists or provider facilities for the Argus II System, the retinal specialists may delay their payments to us, which would adversely affect our working capital requirements. If the funding agencies delay reimbursement payments to the hospitals, any increase to their working capital requirements could reduce their willingness to treat blind patients who wish to have our devices implanted. If reimbursement for our products is unavailable, limited in scope or amount, or if pricing is set at unsatisfactory levels, our business will be materially harmed.
Our commercial and financial success depends on the Argus II System being accepted in the market, and if not achieved will result in our not being able to generate revenues to support our operations.
Even if we are able to obtain favorable reimbursement within the markets that we serve, commercial success of our products will depend, among other things, on their acceptance by retinal specialists, ophthalmologists, general practitioners, low vision therapists and mobility experts, hospital purchasing and controlling departments, patients, and other members of the medical community. The degree of market acceptance of any of our product candidates will depend on factors that include:
| ● | cost of treatment, | |
| ● | pricing and availability of future alternative products, | |
| ● | the extent of available third-party coverage or reimbursement, | |
| ● | perceived efficacy of the Argus II System relative to other future products and medical solutions, and | |
| ● | prevalence and severity of adverse side effects associated with treatment. |
The activities of competitive medical device companies, or others, may limit the Argus II System’s revenue.
Our commercial opportunities for the Argus II System may be reduced if our competitors develop or market products that are more effective, are better tolerated, receive better reimbursement terms, are more accepted by physicians, have better distribution channels, or are less costly.
| 16 |
Currently, to our knowledge, no other medical devices comparable to the Argus II System have been approved by regulatory agencies, both in the US and Europe, to restore some functional vision in persons who have become blind due to RP. Other visual prosthesis companies such as Retina Implant AG and Pixium Vision S.A., both based in Europe, are developing retinal implant technologies to partially restore some vision in blind patients. Retina Implant has obtained a CE mark for its Alpha IMS product but has not yet sold it to our knowledge. Pixium Vision has obtained CE Mark for its IRIS II product, but has withdrawn it from the market. Neither Retina Implant nor Pixium has filed for market approval with the FDA. Retina Implant has not to our knowledge obtained an Investigational Device Exemption (IDE) to begin the required clinical trials in the US, and Pixium Vision has not obtained an IDE for its IRIS II product to begin the required clinical trials in the US. Pixium Vision has obtained an IDE for its PRIMA product. This IDE trial is directed toward AMD, not RP. These competitive therapies if or when developed or brought to market may result in pricing and market access pressure even if Argus II System is otherwise viewed as a preferable therapy.
Many privately and publicly funded universities and other organizations are engaged in research and development of potentially competitive products and therapies, such as stem cell and gene therapies, some of which may target RP and other indications as our product candidates. These organizations include pharmaceutical companies, biotechnology companies, public and private universities, hospital centers, government agencies and research organizations. Our competitors include large and small medical device and biotechnology companies that may have significant access to capital resources, competitive product pipelines, substantial research and development staffs and facilities, and substantial experience in medical device development.
We may face substantial competition in the future and may not be able to keep pace with the rapid technological changes which may result from others discovering, developing or commercializing products before or more successfully than we do.
In general the development and commercialization of new medical devices is highly competitive and is characterized by extensive research and development and rapid technological change. Our customers consider many factors including product reliability, clinical outcomes, product availability, inventory consignment, price, and product services provided by the manufacturer. Market share can shift as a result of technological innovation and other business factors. We believe these risk factors are partially mitigated by the Argus II System being the sole product that is currently available for commercial implantation in the US and Europe. Major shifts in industry market share have occurred in connection with product problems, physician advisories and safety alerts, reflecting the importance of product quality in the medical device industry, and any quality problems with our processes, goods and services could harm our reputation for producing high-quality products and would erode our competitive advantage, sales and market share. Our competitors may develop products or other novel technologies that are more effective, safer or less costly than any that we are developing and if those products gain market acceptance our revenue and financial results could be adversely affected.
If we fail to develop new products or enhance existing products, our leadership in the markets we serve could erode, and our business, financial condition and results of operations may be adversely affected.
Risks Related to Our Common Stock
We have not been profitable to date and expect our operating losses to continue for the foreseeable future; we may never be profitable.
We have incurred operating losses and generated negative cash flows since our inception and have financed our operations principally through equity investments and borrowings. Our ability to generate sufficient revenues to fund operations is uncertain. For the fiscal year ended December 31, 2017, we had net revenue of $8.0 million and incurred a net loss of $28.5 million. Our total accumulated deficit through December 31, 2017, was $234.4 million.
As a result of our limited commercial operating history, revenue is difficult to predict with certainty. Current and projected expense levels are based largely on estimates of future revenue. We expect expenses to increase in the future as we expand our activities in connection with the further development of Orion and complete planned enhancements of Argus II. We cannot assure you that we will be profitable in the future. Accordingly, the extent of our future losses and the time required to achieve profitability, if ever, is uncertain. Failure to achieve profitability could materially and adversely affect the value of our Company and our ability to effect additional financings. The success of the business depends on our ability to increase revenues to offset expenses. If our revenues fall short of projections, our business, financial condition and operating results will be materially adversely affected.
| 17 |
Our financial statements have been prepared assuming a going concern qualification by our auditors.
Our independent registered public accounting firm in their report on the Company’s 2017 consolidated financial statements expressed substantial doubt about our ability to continue as a going concern since we have incurred, and are continuing to incur, substantial operating losses and negative cash flows from operations, and we do not have adequate capital to support our operations at current levels through at least the next 12 months from the date the consolidated financial statements are issued. Our ability to continue as a going concern is dependent upon our ability to obtain additional financing, obtain further operating efficiencies, reduce expenditures, attain favorable gross margins and ultimately, generate greater sales and create profitable operations. Such financings may not be available or may not be available on reasonable terms. A “going concern” opinion from our auditors may negatively affect the price of our common stock.
Sales, or the availability for sale, of substantial amounts of our common stock could adversely affect the value of our common stock.
We cannot predict the effect, if any, that future sales of our common stock, or the availability of our common stock for future sales, will have on the market price of our common stock. Sales of substantial amounts of our common stock in the public market and the availability of shares for future sale could adversely affect the prevailing market price of our common stock. This in turn could impair our future ability to raise capital through an offering of our equity securities.
There may be future sales or other dilution of our equity, which may adversely affect the market price of our common stock.
We are not restricted from issuing additional shares of common stock. The market price of our common stock could decline as a result of sales of our common stock and Warrants or the perception that such sales could occur. We may issue and sell additional shares of our common stock in private placements or registered offerings in the future. We also may conduct additional registered rights offerings in the future pursuant to which we may issue shares of our common stock or other securities.
The warrants we issued in our recent rights offering to shareholders may create an overhang on the market and have a negative effect on the market price for our common stock.
We issued 13,652,341 warrants in connection with a completed rights offering of units to our shareholders in 2017. The warrants may be outstanding for up to five years from their issuance date. The warrants may be used in arbitrage transactions and can cause the price of our common stock to remain at the warrant exercise price of $1.47 regardless of our performance.
We have identified and reported on weaknesses in our internal control over financial reporting and if our internal control over financial reporting remains not effective, investor confidence in our Company may be adversely affected.
In response to identified and reported material weaknesses in our internal control over financial reporting, we are continuing to develop and improve our system and process documentation necessary to perform the evaluation needed to comply with Section 404 of the Sarbanes-Oxley Act. For example, in connection with the audit of our consolidated financial statements for fiscal 2015 and 2016, our independent registered public accounting firm identified material weaknesses in our internal control over financial reporting. A “material weakness” is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. Our independent registered public accounting firm identified the following material weaknesses during their audits:
| ● | Control over Financial Reporting. We did not consistently perform timely reconciliation of certain accounts, including revenue, deferred revenue, inventory, prepaid and accrued expenses, and stock-based compensation expense. This resulted in the incorrect recording of certain revenue and expenses that required various adjusting entries which we timely and fully recorded as part of the closing process. |
| 18 |
| ● | Tracking of Back-up Prosthesis Units. For every surgery, we ship a back-up prosthesis unit along with the primary unit in case the primary unit cannot be used for some reason. Following the surgery the unused unit is returned to us. During the year ended December 31, 2015, we did not consistently follow internal procedures regarding the tracking and recordation of returned prosthesis units and the exchange of primary units for back-up units with our customers. When uncorrected this resulted in an understatement of cost of sales and an overstatement of inventory that required various adjusting entries that we timely and fully recorded as part of the closing process. |
| ● | Updating of Standard Costs. It is a customary practice for manufacturing companies to update their standard costs on a regular basis (at least annually) to ensure that inventory costs are accurately and properly stated. During the year ended December 31, 2016, due to the low level of production during the year and the inventory reserves that were established covering most of 2016 production, the Company did not update its standard costs at December 31, 2016. The Company’s failure to update its standard costs at December 31, 2016 represented a material weakness in its internal control over financing reporting. |
While we have taken actions to remediate these specific weaknesses, the Company does not have complete written documentation of its internal control policies, procedures and controls and has not fully completed testing of its key controls. Management evaluated the impact of its failure to have fully tested its internal controls and procedures and has concluded that the control deficiency that resulted represented a material weakness and that our internal control over financial reporting was not effective as of the end of the period covered by this Annual Report on Form 10-K.
If we continue to be unable to conclude that our internal control over financial reporting is effective, or if our independent registered public accounting firm is unable to express an opinion on the effectiveness of our internal controls when it is required to do so by the applicable rules, we could lose investor confidence in the accuracy and completeness of our financial reports, which could cause the price of our common stock to decline, and we may be subject to investigation or sanctions by the regulatory authorities.
As a result, we may need to undertake various actions, such as implementing new internal controls and procedures and hiring additional accounting or internal audit staff. Our remediation efforts may not enable us to avoid a material weakness in the future.
Materials necessary to manufacture Argus II may not be available on commercially reasonable terms, or at all, which may delay development, manufacturing and commercialization of our products.
We rely on numerous suppliers to provide materials, components and services necessary to produce the Argus II System and next generation product candidates. Certain suppliers are currently sole source because of our low manufacturing volumes and our need for specialty technical or other engineering expertise. Our suppliers may be unable or unwilling to deliver these materials and services to us timely as needed or on commercially reasonable terms. Should this occur, we would seek to qualify alternative suppliers or develop in-house manufacturing capability, but may be unable to do so. Substantial design or manufacturing process modifications and regulatory approval might be required to facilitate or qualify an alternate supplier. Even where we could qualify alternative suppliers the substitution of suppliers may be at a higher cost and cause time delays including delays associated with additional possible FDA review, that impede the commercial production of the Argus II System, reduce gross profit margins and impact our abilities to deliver our products as may be timely required to meet demand.
| 19 |
Any failure or delay in completing clinical trials or studies for new product candidates or next generation of the Argus II System and the expense of those trials could adversely affect our business.
Preclinical studies and clinical trials required to demonstrate the safety and efficacy of incremental changes and obtain indication expansion for the next generation of the Argus II System, including new externals and software enhancements and for new product candidates are time consuming and expensive. If we are required to conduct additional clinical trials or other studies with respect to any of our product candidates beyond those that we have contemplated, if we are unable to successfully complete our clinical trials or other studies or if the results of these trials or studies are not positive or are only modestly positive, we may be delayed in obtaining marketing approval for those product candidates, we may not be able to obtain marketing approval or we may obtain approval for indications that are not as broad as intended. Our product development costs also will increase if we experience delays in testing or approvals.
The completion of clinical trials for our product candidates could be delayed because of our inability to manufacture or obtain from third-parties materials sufficient for use in preclinical studies and clinical trials; delays in patient enrollment and variability in the number and types of patients available for clinical trials; difficulty in maintaining contact with patients after treatment, resulting in incomplete data; poor effectiveness of product candidates during clinical trials; unforeseen safety issues or side effects; and governmental or regulatory delays and changes in regulatory requirements and guidelines.
If we incur significant delays in our clinical trials, our competitors may be able to bring their products to market before we do which could result in harming our ability to commercialize our products or potential products. If we experience any of these occurrences our business will be materially harmed.
If we lose key management personnel, or if we fail to recruit additional highly skilled personnel, our ability to identify, develop and commercialize new or next generation product candidates will be impaired, could result in loss of markets or market share and could make us less competitive.
Our executives have significant medical device, regulatory, sales and marketing, operational, and/or corporate finance experience. The loss of any management executive or any other principal member of our management team could impair our ability to identify, develop and market new products or effectively deal with regulatory and reimbursement matters.
We could be adversely affected by violations of the U.S. Foreign Corrupt Practices Act and similar worldwide anti-bribery laws.
The U.S. Foreign Corrupt Practices Act and similar worldwide anti-bribery laws generally prohibit companies and their intermediaries from making improper payments to non-U.S. officials for the purpose of obtaining or retaining business. We intend to adopt policies for compliance with these anti-bribery laws, which often carry substantial penalties. We cannot assure you that our internal control policies and procedures always will protect us from reckless or other inappropriate acts committed by our affiliates, employees or agents. Violations of these laws, or allegations of such violations, could have a material adverse effect on our business, financial position and results of operations and could cause the market value of our common stock to decline.
Risks Related to Intellectual Property and Other Legal Matters
If we or our licensors are unable to protect our/their intellectual property, then our financial condition, results of operations and the value of our technology and products could be adversely affected.
Patents and other proprietary rights are essential to our business and our ability to compete effectively with other companies is dependent upon the proprietary nature of our technologies. We also rely upon trade secrets, know-how, continuing technological innovations and licensing opportunities to develop, maintain and strengthen our competitive position. We seek to protect these, in part, through confidentiality agreements with certain employees, consultants and other parties. Our success will depend in part on the ability of our licensors to obtain, maintain (including making periodic filings and payments) and enforce patent protection for their intellectual property, in particular, those patents to which we have secured exclusive rights. Our licensors may not successfully prosecute or continue to prosecute the patent applications which we have licensed. Even if patents are issued in respect of these patent applications, we or our licensors may fail to maintain these patents, may determine not to pursue litigation against entities that are infringing upon these patents, or may pursue such enforcement less aggressively than we ordinarily would. Without adequate protection for the intellectual property that we own or license, other companies might be able to offer substantially identical products for sale, which could unfavorably affect our competitive business position and harm our business prospects.
| 20 |
Even if issued, patents may be challenged, invalidated, or circumvented, which could limit our ability to stop competitors from marketing similar products or limit the length of term of patent protection that we may have for our products.
Litigation or third-party claims of intellectual property infringement or challenges to the validity of our patents would require us to use resources to protect our technology and may prevent or delay our development, regulatory approval or commercialization of improvements in the Argus II System or new product candidates. Further, the validity of some of our patents has been challenged.
Pixium Vision (Pixium) has filed oppositions in the European Patent Office (EPO) challenging the validity of 21 European patents owned or exclusively licensed by Second Sight. Retina Implant AG has joined Pixium Vision in one Opposition. Two of these patents are owned by Johns Hopkins University (JHU) and exclusively licensed to Second Sight, while 19 of these patents are owned by Second Sight. Although Second Sight was successful in the opposition division in the two JHU cases, at the appeal level one of the JHU patents was upheld and one of JHU patents was invalidated. Second Sight has opposed one Pixium patent. These EPO proceedings involving us and Pixium include:
| ● | EP1061874 Visual Prosthesis – upheld by the opposition and appellate divisions (Second Sight prevailed). No further appeal is available in the EPO. | |
| ● | EP1061996 Apparatus for Preferential Outer Retinal Stimulation – upheld by the opposition division, cancelled in the appellate division. No further appeal is available in the EPO. | |
| ● | EP1171188 Retinal Color Prosthesis for Color Sight Restoration – cancelled in the Opposition Division, we have withdrawn our appeal due to its impending expiration. | |
| ● | EP2219728 Electrode Array for Even Neural Pressure Having Multiple Attachment Points –upheld in the Opposition Division, pending before the Board of Appeal. | |
| ● | EP1937352 Sub-threshold Stimulation to Precondition Neurons for Supra-threshold Stimulation – cancelled in the Opposition Division, pending before the Board of Appeal. | |
| ● | EP2192949 – Return Electrode for a Flexible Circuit Electrode Array – cancelled in the Opposition Division, Pending before the Board of Appeal. | |
| ● | EP1949437 - Implantable Microelectronic Device and Method of Manufacture –Upheld in the Opposition Division, pending before the Board of Appeal. | |
| ● | EP1945835 – Platinum Electrode Surface Coating and Method for Manufacturing the Same – (Pixium joined by Retina Implant) cancelled in the Opposition Division, pending before the Board of Appeal. | |
| ● | EP1986733 (Pixium’s Patent opposed by Second Sight) – Device with Flexible Multilayer System for Contacting or Electro-stimulation of Living Tissue Cells or Nerves –significantly narrowed in the Opposition Division, pending before the Board of Appeal. | |
| ● | EP1562672 – Field Focusing and Mapping in an Electrode Array – cancelled in the Opposition Division, pending before the Board of Appeal. | |
| ● | EP1497483 – Platinum Electrode – Upheld in the Opposition Division, still within the time limit for appeal. | |
| ● | EP2077892 – Automatic Fitting for a Visual Prosthesis – Upheld in the Opposition Division, pending before the Board of Appeal. | |
| ● | EP2061549 – Package for an Implantable Neural Stimulation Device - Cancelled in the Opposition Division, pending before the Board of Appeal. | |
| ● | EP2155327 – System for Providing Stimulation Inputs to a Visual Prosthesis – Upheld in the Opposition Division. Still within the time limit for Appeal. | |
| ● | EP2114514 – Flexible Electrode Array with Film Support - Opposition filed. | |
| ● | EP2089100 – Flexible Circuit Electrode Array - Opposition filed. | |
| ● | EP2185236 – Implantable Device for the Brain – Opposition filed, a hearing is scheduled for May 17, 2018. |
| 21 |
| ● | EP2364179 – Techniques and Functional Electrical Stimulation to Eliminate Discomfort during Electrical Stimulation of the Retina – Cancelled in the Opposition Division. Still within the time limit for Appeal. | |
| ● | EP1874397 – Flexible Circuit Electrode Array – Opposition filed. | |
| ● | EP2136876 – Saliency-Based Apparatus for Visual Prosthesis – Opposition filed. | |
| ● | EP2421602 – Visual Prosthesis Fitting System – Opposition filed. | |
| ● | EP2167186 – Video Processing System for a Visual Prosthetic Apparatus - Opposition filed. |
If we are the target of claims by third parties asserting that our products or intellectual property infringe upon the rights of others we may be forced to incur substantial expenses or divert substantial employee resources from our business and, if successful, those claims could result in our having to pay substantial damages or prevent us from developing one or more product candidates. Further, if a patent infringement suit were brought against us or our collaborators, we or they could be forced to stop or delay research, development, manufacturing or sales of the product or product candidate that is the subject of the suit.
The validity of some of our patents has been challenged. If we experience additional patent infringement claims, or if we elect to avoid current or potential claims others may be able to assert, we or our collaborators may choose to seek, or be required to seek, a license from the third-party and would most likely be required to pay license fees or royalties or both. These licenses may not be available on acceptable terms, or at all. Even if we or our collaborators were able to obtain a license, the rights may be nonexclusive, which would give our competitors access to the same intellectual property. Ultimately, we could be prevented from commercializing a product, or be forced to cease some aspect of our business operations if, as a result of actual or threatened patent infringement claims, we or our collaborators are unable to enter into licenses on acceptable terms. This could harm our business significantly. The cost to us of any litigation or other proceeding, regardless of its merit, even if resolved in our favor, could be substantial. Some of our competitors may be able to bear the costs of such litigation or proceedings more effectively than we can because of their having greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace. Intellectual property litigation and other proceedings may, regardless of their merit, also absorb significant management time and employee resources.
If we fail to comply with our obligations in the agreements under which we license development or commercialization rights to products or technology from third-parties, we could lose license rights that are important to our business.
We hold exclusive licenses from Johns Hopkins University, Duke University, and the Doheny Eye Institute to intellectual property relating to the Argus II visual prosthesis. These licenses impose various commercialization, milestone payment, profit sharing, insurance and other obligations on us. If we fail to comply with any material obligations, the licensor will have the right to terminate the applicable license, which covers part of the system of the eye implant and thus will be a barrier to manufacture the Argus II System and impair our ability to sell the Argus II. The existing or future patents to which we have rights based on our agreements with Johns Hopkins University, Duke University and the Doheny Eye Institute may be too narrow to prevent third-parties from developing or designing around these patents. Additionally, we may lose our rights to the patents and patent applications we license in the event of a breach or termination of the license agreement. Each license expires with the expiration of the last of the licensed patents. In the case of JHU, the license will expire March 13, 2018. While the JHU agreement includes a patent which is a significant obstacle to our competitors, it is one of many other patents which in our view present material obstacles to our competitors. The DEI license includes ongoing research, making the expiration date indeterminate, but in any event the expiration date is no earlier than August 8, 2033. The total aggregate royalty on both agreements does not exceed 3.25% of Argus II System net sales. All of the patents in the DEI agreement are co-owned with the Doheny Eye Institute. We license the Doheny Eye Institute’s interest in the patents to maintain our exclusive use on that intellectual property. Should the license terminate we retain the right to utilize the intellectual property, but may not be able to prevent others from doing so, in which case we may lose a competitive advantage.
| 22 |
If we are unable to protect the confidentiality of our proprietary information and know-how, the value of our technology and products could be adversely affected.
In addition to patented technology, we rely upon, among other things, unpatented proprietary technology, processes, trade secrets and know-how. Any involuntary disclosure to or misappropriation by third-parties of our confidential or proprietary information could enable competitors to duplicate or surpass our technological achievements, potentially eroding our competitive position in our market. We seek to protect confidential or proprietary information in part by confidentiality agreements with our employees, consultants and third-parties. While we require all of our employees, consultants, advisors and any third-parties who have access to our proprietary know-how, information and technology to enter into confidentiality agreements, we cannot be certain that this know-how, information and technology will not be disclosed or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. These agreements may be terminated or breached, and we may not have adequate remedies for any such termination or breach. Furthermore, these agreements may not provide meaningful protection for our trade secrets and know-how in the event of unauthorized use or disclosure. To the extent that any of our staff were previously employed by other pharmaceutical, medical technology or biotechnology companies, those employers may allege violations of trade secrets and other similar claims in relation to their medical device development activities for us.
If we are unable to protect the intellectual property used in our products, others may be able to copy our innovations which may impair our ability to compete effectively in our markets.
The strength of our patents involves complex legal and scientific questions and can be uncertain. We have over 400 issued patents and approximately 80 pending patent applications worldwide as of December 31, 2017. Our patent applications may be challenged or fail to result in issued patents and our existing or future patents may be too narrow to prevent third-parties from developing or designing around our intellectual property and in that event we may lose competitive advantage and our business may suffer.
Further, the patent applications that we license or have filed may fail to result in issued patents. The claims may need to be amended. Even after amendment, a patent may not issue and in that event we may not obtain the exclusive use of the intellectual property that we seek and may lose competitive advantage which could result in harm to our business.
Third-party claims of intellectual property infringement may prevent or delay expanded commercialization efforts for Argus II and our development and commercialization activities for other product candidates.
Although we are not currently aware of any litigation or other proceedings or third-party claims of intellectual property infringement related to the Argus II System, the medical device industry is characterized by many litigation cases regarding patents and other intellectual property rights. Other parties may in the future allege that our activities infringe their patents or that we are employing their proprietary technology without authorization. We may not have identified all the patents, patent applications or published literature that affect our business either by blocking our ability to commercialize our product, by preventing the patentability of one or more aspects of our products or those of our licensors or by covering the same or similar technologies that may affect our ability to market our product.
In addition, even in the absence of litigation, we may need to obtain licenses from third-parties to advance our research or allow commercialization of our product candidates, and we have done so from time to time. We may fail to obtain future licenses at a reasonable cost or on reasonable terms, if at all. In that event, we may be unable to further develop and commercialize one or more of our product candidates, which could harm our business significantly.
We may become involved in future lawsuits to protect or enforce our patents or the patents of our licensors, which could be expensive, time consuming and unsuccessful.
Competitors may infringe our patents or the patents of our licensors. To counter infringement or unauthorized use, we may file infringement claims, which can be expensive and time consuming. In addition, in an infringement proceeding, a court may decide that a patent of ours or of our licensors is not valid or is unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation or defense proceedings could put one or more of our patents at risk of being invalidated or interpreted narrowly and could put our patent applications at risk of not issuing.
| 23 |
The US Patent and Trademark Office may initiate interference proceedings to determine the priority of inventions described in or otherwise affecting our patents and patent applications or those of our collaborators or licensors. An unfavorable outcome could require us to cease using the technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if a prevailing party does not offer us a license on terms that are acceptable to us. Litigation or interference proceedings may fail and, even if successful, may result in substantial costs and distraction of our management and other employees. We may not be able to prevent, alone or with our licensors, misappropriation of our proprietary rights, particularly in countries where the laws may not protect those rights as fully as in the US.
Product liability lawsuits could divert our resources, result in substantial liabilities and reduce the commercial potential of our products.
We face a risk of product liability claims arising from the prosthesis being inserted into the eye, and it is possible that we may be held liable for eye injuries of patients who receive our product. These lawsuits may divert our management from pursuing our business strategy and may be costly to defend. In addition, if we are held liable in any of these lawsuits, we may incur substantial liabilities and may be forced to limit or forego further commercialization of one or more of our products. We maintain product liability insurance relating to our clinical trials and commercial sales, with an aggregate coverage limit under these insurance policies of $10,000,000, and while we believe this amount of insurance currently is sufficient to cover our product liability exposure, these limits may not prove adequate to fully cover potential liabilities. In addition, we may not be able to obtain or maintain sufficient insurance coverage at an acceptable cost or otherwise to protect against potential product liability claims, which could prevent or inhibit the commercial production and sale of our products. If the use of our products harm or are alleged to harm people, we may be subject to costly and damaging product liability claims that exceed our policy limits and cause us significant losses that could seriously harm our financial condition or reputation.
Legislative or regulatory reform of the health care system in the US and foreign jurisdictions may adversely impact our business, operations or financial results.
Our industry is highly regulated and changes in law may adversely impact our business, operations or financial results. In March 2010, the Patient Protection and Affordable Care Act, and a related reconciliation bill were signed into law. This legislation changes the current system of healthcare insurance and benefits intended to broaden coverage and control costs. The law also contains provisions that will affect companies in the medical device industry and other healthcare related industries by imposing additional costs and changes to business practices.
Moreover, in some foreign countries, including countries in Europe and Canada, the pricing of approved medical devices is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take 12 months or longer after the receipt of regulatory approval and product launch. To obtain reimbursement or pricing approval in some countries, we may be required to conduct a clinical trial that compares the cost-effectiveness of our product candidate to other available therapies. Our business could be materially harmed if reimbursement of our products is unavailable or limited in scope or amount or if pricing is set at unsatisfactory levels.
We cannot predict what healthcare reform initiatives may be adopted in the future. Further federal and state legislative and regulatory developments appear likely, and we expect ongoing initiatives in the U.S and Europe. These reforms could have an adverse effect on our ability to obtain timely regulatory approval for new products and on anticipated revenues from the Argus II System and other product candidates, both of which may affect our overall financial condition.
| 24 |
We are an “emerging growth company,” and we cannot be certain if the reduced disclosure requirements applicable to emerging growth companies will make our common stock less attractive to investors.
For so long as we remain an “emerging growth company” as defined in the JOBS Act, we may take advantage of certain exemptions from various requirements that are applicable to public companies that are not “emerging growth companies,” including not being required to comply with the independent auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We may take advantage of these exemptions for so long as we are an “emerging growth company,” which could be as long as five years from November 14, 2014, the date of our initial public offering. Investors may find our common stock less attractive because we rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock, and our stock price may be more volatile or may decline.
In addition, Section 107 of the JOBS Act also provides that an “emerging growth company” can take advantage of an extended transition period for complying with new or revised accounting standards. However, we chose to “opt out” of this extended transition period, and as a result, we intend to comply with new or revised accounting standards on the relevant dates that adoption of those standards may be required for non-emerging growth companies. Our decision to opt out of the extended transition period for complying with new or revised accounting standards is irrevocable.
We are required to evaluate our internal control over financial reporting under Section 404 of the Sarbanes-Oxley Act of 2002, and any adverse results from such evaluation could result in a loss of investor confidence in our financial reports and have an adverse effect on our stock price.
Pursuant to Section 404 of the Sarbanes-Oxley Act of 2002, we are required to furnish a report by our management on our internal control over financial reporting. The report contains, among other matters, an assessment of the effectiveness of our internal control over financial reporting as of the end of our fiscal year, including a statement as to whether or not our internal control over financial reporting is effective. This assessment must include disclosure of any material weaknesses in our internal control over financial reporting identified by management. If we are unable to assert that our internal control over financial reporting is effective, we could lose investor confidence in the accuracy and completeness of our financial reports, which could have an adverse effect on our stock price. See the Risk Factor labeled. “We have identified and reported on weaknesses in our internal control over financial reporting and if our internal control over financial reporting remains not effective, investor confidence in our Company may be adversely affected” as described above.
Risks Relating to Our Financial Results and Need for Financing
Fluctuations in our quarterly operating results and cash flows could adversely affect the price of our common stock.
The revenues we generate and our operating results will be affected by numerous factors such as:
| ● | the general commercial success of the Argus II System, |
| ● | our ability to improve performance and significantly expand the use of Argus II in the larger RP population by treating better-sighted RP patients, |
| ● | our ability to obtain regulatory approval of the Argus II System in additional jurisdictions, |
| ● | the emergence of products that compete with our product candidates, |
| ● | our ability to leverage Argus II technology to restore useful vision with cortical stimulation, |
| ● | the status of our preclinical and clinical development programs, variations in the level of expenses related to our existing product candidates or preclinical and clinical development programs, |
| ● | execution of collaborative, licensing or other arrangements, and the timing of payments received or made under those arrangements, |
| ● | any intellectual property infringement lawsuits to which we may become a party, |
| ● | our ability to obtain reimbursement from government or private payers at levels we deem adequate to sustain our operations. |
| 25 |
If our quarterly operating results fall below the expectations of investors or securities analysts, the price of our common stock could decline substantially. Any quarterly fluctuations in our operating results and cash flows may cause the price of our stock to fluctuate substantially. We believe that, in the near term, quarterly comparisons of our financial results are not necessarily meaningful and should not be relied upon as an indication of our future performance.
We will need additional capital to support our operations and growth. Additional capital, may be difficult to obtain restricting our operations and resulting in additional dilution to our stockholders.
Our business requires additional capital for implementation of our long term business plan. We believe our cash, cash equivalents and other investments, along with the proceeds of approximately $20.1 million from our completed shareholder rights offering, our sale of shares through our ATM together with revenue generated from the sale of Argus II units, may be sufficient to fund our operations through the end of the second quarter of 2018. The actual amount of funds that we will need for our business development will be determined by many factors, some of which are beyond our control, and we may need funds sooner than currently anticipated. These factors include:
| ● | the amount of our future operating losses, |
| ● | third party expenses relating to the ongoing commercialization of Argus II System, |
| ● | the need and cost of conducting additional clinical trials of the Argus II System for other applications, |
| ● | the amount of our research and development, including research and development for Orion visual prosthesis, marketing and general and administrative expenses, and |
| ● | regulatory changes and technological developments in our markets. |
In November 2017, the Company entered into an At-the-Market sales agreement (the “Sales Agreement”) with B. Riley FBR Inc. and H.C. Wainwright & Co., LLC, as agents (“Agents”) pursuant to which the Company may offer and sell, from time to time through either of the Agents, shares of our common stock having an aggregate offering price as set forth in the Sales Agreement and a related prospectus supplement filed with the Securities and Exchange Commission. The Company agreed to pay the Agents a cash commission of 3.0% of the aggregate gross proceeds from each sale of shares under the Sales Agreement. Through December 31, 2017 the Company sold 598,276 shares of common stock and received net proceeds of $1.1 million under the Sales Agreement and thereafter during January and February 2018 the Company sold 2.2 million shares of common stock for additional net proceeds of $4 million.
As we require additional funds, we may seek to fund our operations through the sale of additional equity securities, debt financing and strategic collaboration agreements. We cannot be sure that additional financing from any of these sources will be available when needed or that, if available, the additional financing will be obtained on terms favorable to us or our stockholders. If we raise additional funds by selling shares of our capital stock, the ownership interest of our current stockholders will be diluted. If we are unable to obtain additional funds on a timely basis or on terms favorable to us, we may be required to cease or reduce further commercialization of the Argus II System, to cease or reduce certain research and development projects, to sell some or all of our technology or assets or business units or to merge all or a portion of our business with another entity.
Risks Related to Our Business and Industry
We have incurred operating losses since inception and may continue to incur losses for the foreseeable future.
We have had a history of operating losses and we expect that operating losses will continue into the near term. Although we have had sales of the Argus II product, these limited sales have not been sufficient to cover our operating expenses. Our ability to generate positive cash flow will also hinge on our ability to correctly price our product to our markets, expand the use of the Argus II System, develop the Orion visual prosthesis and obtain government and private insurance reimbursement. As of December 31, 2017 we had total stockholders’ equity of $7.9 million and an accumulated deficit of $234 million. We cannot assure you that we will be profitable even if we successfully commercialize our products. Failure to become and remain profitable may adversely affect the market price of our common stock and our ability to raise capital and continue operations.
| 26 |
Our business is subject to international economic, political and other risks that could negatively affect our results of operations or financial position.
We derive a significant portion of our revenues from Europe, and we anticipate that revenue from Europe and other countries outside the US will increase. Accordingly, our operations are subject to risks associated with doing business internationally, including:
| ● | currency exchange variations, | |
| ● | extended collection timelines for accounts receivable, | |
| ● | greater working capital requirements, | |
| ● | multiple legal frameworks and unexpected changes in legal and regulatory requirements, | |
| ● | the need to ensure compliance with the numerous regulatory and legal requirements applicable to our business in each of these jurisdictions and to maintain an effective compliance program to ensure compliance with these requirements, | |
| ● | political changes in the foreign governments impacting health policy and trade, | |
| ● | tariffs, export restrictions, trade barriers and other regulatory or contractual limitations that could impact our ability to sell or develop our products in certain foreign markets, | |
| ● | trade laws and business practices favoring local competition, and | |
| ● | adverse economic conditions, including the stability and solvency of business financial markets, financial institutions and sovereign nations and the healthcare expenditure of domestic or foreign nations. |
The realization of any of these or other risks associated with operating in Europe or other non-U.S. countries could have a material adverse effect on our business, results of operations or financial condition.
We are subject to stringent domestic and foreign medical device regulation and any unfavorable regulatory action may materially and adversely affect our financial condition and business operations.
Our products, development activities and manufacturing processes are subject to extensive and rigorous regulation by numerous government agencies, including the FDA and comparable foreign agencies. To varying degrees, each of these agencies monitors and enforces our compliance with laws and regulations governing the development, testing, manufacturing, labeling, marketing, distribution, and the safety and effectiveness of our medical devices. The process of obtaining marketing approval or clearance from the FDA and comparable foreign bodies for new products, or for enhancements, expansion of the indications or modifications to existing products, could:
| ● | take a significant, indeterminate amount of time, | |
| ● | result in product shortages due to regulatory delays, | |
| ● | require the expenditure of substantial resources, | |
| ● | involve rigorous pre-clinical and clinical testing, and possibly post-market surveillance, | |
| ● | involve modifications, repairs or replacements of our products, | |
| ● | require design changes of our products, | |
| ● | result in limitations on the indicated uses of our products, and | |
| ● | result in our never being granted the regulatory approval we seek. |
Any of these occurrences that we might experience will cause our operations to suffer, harm our competitive standing and result in further losses that adversely affect our financial condition.
| 27 |
We have ongoing responsibilities under FDA and international regulations, both before and after a product is commercially released. For example, we are required to comply with the FDA’s Quality System Regulation (QSR), which mandates that manufacturers of medical devices adhere to certain quality assurance requirements pertaining among other things to validation of manufacturing processes, controls for purchasing product components, and documentation practices. As another example, the Medical Device Reporting regulation requires us to provide information to the FDA whenever there is evidence that reasonably suggests that a device may have caused or contributed to a death or serious injury or, that a malfunction occurred which would be likely to cause or contribute to a death or serious injury upon recurrence. Compliance with applicable regulatory requirements is subject to continual review and is monitored rigorously through periodic inspections by the FDA. If the FDA were to conclude that we are not in compliance with applicable laws or regulations, or that any of our medical devices are ineffective or pose an unreasonable health risk, the FDA could ban such medical devices, detain or seize such medical devices, order a recall, repair, replacement, or refund of such devices, or require us to notify health professionals and others that the devices present unreasonable risks of substantial harm to the public health. The FDA has been increasing its scrutiny of the medical device industry and the government is expected to continue to scrutinize the industry closely with inspections and possibly enforcement actions by the FDA or other agencies. Additionally, the FDA may restrict manufacturing and impose other operating restrictions, enjoin and restrain certain violations of applicable law pertaining to medical devices and assess civil or criminal penalties against our officers, employees, or us. Any adverse regulatory action, depending on its magnitude, may restrict us from effectively manufacturing, marketing and selling our products. In addition, negative publicity and product liability claims resulting from any adverse regulatory action could have a material adverse effect on our financial condition and results of operations.
The number of preclinical and clinical tests that will be required for regulatory approval varies depending on the disease or condition to be treated, the jurisdiction in which we are seeking approval and the regulations applicable to that particular medical device. Regulatory agencies, including those in the US, Canada, Europe and other countries where medical devices are regulated, can delay, limit or deny approval of a product for many reasons. For example,
| ● | a medical device may not be safe or effective, | |
| ● | regulatory agencies may interpret data from preclinical and clinical testing differently than we do, | |
| ● | regulatory agencies may not approve our manufacturing processes, | |
| ● | regulatory agencies may conclude that our device does not meet quality standards for durability, long-term reliability, biocompatibility, electromagnetic compatibility, electrical safety, and | |
| ● | regulatory agencies may change their approval policies or adopt new regulations. |
The FDA may make requests or suggestions regarding conduct of our clinical trials, resulting in an increased risk of difficulties or delays in obtaining regulatory approval in the US. Any of these occurrences could prove materially harmful to our operations and business.
We are also subject to stringent government regulation in European and other foreign countries, which could delay or prevent our ability to sell our products in those jurisdictions.
We intend to pursue market authorizations for the Argus II System and other product candidates in additional jurisdictions. For us to market our products in Europe and some other international jurisdictions, we and our distributors and agents must obtain required regulatory registrations or approvals. The approval procedure varies among countries and jurisdictions and can involve additional testing, and the time and costs required to obtain approval may differ from that required to obtain an approval by the FDA. Approval by the FDA does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other foreign countries or jurisdictions or by the FDA. Violations of foreign laws governing use of medical devices may lead to actions against us by the FDA as well as by foreign authorities. We must also comply with extensive regulations regarding safety, efficacy and quality in those jurisdictions. We may not be able to obtain all the required regulatory registrations or approvals, or we may be required to incur significant costs in obtaining or maintaining any regulatory registrations or approvals we receive. Delays in obtaining any registrations or approvals required for marketing our products, failure to receive these registrations or approvals, or future loss of previously obtained registrations or approvals would limit our ability to sell our products internationally. For example, international regulatory bodies have adopted various regulations governing product standards, packaging requirements, labeling requirements, import restrictions, tariff regulations, duties and tax requirements. These regulations vary from country to country. In order to sell our products in Europe, we must maintain our ISO 13485:2003 certification and CE mark certification, which is an international symbol of quality and compliance with applicable European medical device directives. Failure to maintain the ISO 13485:2003 certification or CE mark certification or other international regulatory approvals would prevent us from selling in some countries in Europe and elsewhere. The failure to obtain these approvals could harm our business materially.
| 28 |
Even if we obtain clearance or approval to sell our products, we are subject to ongoing requirements and inspections that could lead to the restriction, suspension or revocation of our clearance.
We, as well as any potential collaborative partners such as distributors, will be required to adhere to applicable FDA regulations regarding good manufacturing practice, which include testing, control, and documentation requirements. We are subject to similar regulations in foreign countries. Even if regulatory approval of a product is granted, the approval may be subject to limitations on the indicated uses for which the product may be marketed or to the conditions of approval, or contain requirements for costly post-marketing testing and surveillance to monitor the safety or efficacy of the product. Ongoing compliance with good manufacturing practice and other applicable regulatory requirements is strictly enforced in the United States through periodic inspections by state and federal agencies, including the FDA, and in international jurisdictions by comparable agencies. Failure to comply with these regulatory requirements could result in, among other things, warning letters, fines, injunctions, civil penalties, recall or seizure of products, total or partial suspension of production, failure to obtain premarket clearance or premarket approval for devices, withdrawal of approvals previously obtained, and criminal prosecution. The restriction, suspension or revocation of regulatory approvals or any other failure to comply with regulatory requirements would limit our ability to operate and could increase our costs.
The CE marking regulations are subject to a significant effort to strengthen the regulatory regime for medical devices which, if adopted, will make clearance process more time consuming and costly for us to obtain access to and continue to market within the European markets.
We are subject to an annual audit of compliance with the rules necessary to support our CE Mark. In April 2017 the European Commission adopted a new regulatory scheme that imposes significant additional obligations on medical device companies. As such, devices with a current CE marking have to comply with additional, more challenging regulatory obligations. The changes being made to the regulations include stricter requirements for clinical evidence and pre-market assessment of safety and performance, new classifications to indicate risk levels, requirements for third party testing by government accredited groups for some types of medical devices, and tightened and streamlined quality management system assessment procedures. With the additional provisions adopted by the European Parliament, the European Medicines Agency (EMA) may be involved in regulation of some types of medical devices, in the qualification and monitoring of notified bodies (NBs), and enhancing the roles of other bodies, including a new Medical Devices Coordination Group (MDCG). The European Parliament’s revisions also impose enhanced competence requirements for NBs and “special notified bodies” (SNBs) for specific categories of devices, such as implantable devices. These changes are anticipated to result in stricter conformity assessment procedures. The medical device industry anticipates that there will be significant changes under these initiatives to the regulation of medical devices which will increase the time and costs for obtaining CE marking.
We have no large-scale manufacturing experience, which could limit our growth.
Our limited manufacturing experience may not enable us to make products in the volumes that would be necessary for us to achieve a significant amount of commercial sales. Our product involves new and technologically complex materials and processes and we currently experience low yields on our manufacturing process. As we move from making small quantities of our product for clinical trials to larger quantities for commercial distribution, we must develop new manufacturing techniques and processes that allow us to scale production. We may not be able to establish and maintain reliable, efficient, full scale manufacturing at commercially reasonable costs in a timely fashion. Difficulties we encounter in manufacturing scale-up, or our failure to implement and maintain our manufacturing facilities in accordance with good manufacturing practice regulations, international quality standards or other regulatory requirements, could result in a delay or termination of production. To date, our manufacturing activities have largely been to provide units for clinical testing and initial commercial sales of the Argus II System. We may face substantial difficulties in establishing and maintaining manufacturing for our products at a larger commercial scale and those difficulties may impact the quality of our products and adversely affect our ability to increase sales.
| 29 |
To establish our sales and marketing infrastructure, we will need to grow the size of our organization, and we may experience delays or other difficulties in managing this growth.
As our development and commercialization plans and strategies evolve, we will need to expand the size of our employee base for managerial, operational, sales, marketing, financial and other resources. Future growth would impose significant added responsibilities on members of management, including the need to identify, recruit, maintain, motivate and integrate additional employees. Our management team may have to use a substantial amount of time to manage these growth activities. Our future financial performance and our ability to commercialize the Argus II System and our other product candidates and compete effectively will depend, in part, on our ability timely and effectively to manage any future growth and related costs. We may not be able to effectively manage a rapid pace of growth and timely implement improvements to our management infrastructure and control systems.
We may acquire additional businesses or form strategic alliances in the future, and we may not realize the benefits of such acquisitions or alliances.
We may acquire additional businesses or products, form strategic alliances or create joint ventures with third-parties that we believe will complement or augment our existing business. If we acquire businesses with promising markets or technologies, we may not be able to realize the benefit of acquiring such businesses if we are unable to successfully integrate them with our existing operations and company culture. We may have difficulty in developing, manufacturing and marketing the products of a newly acquired company that enhances the performance of our combined businesses or product lines to realize value from expected synergies. We cannot assure that, following an acquisition, we will achieve the revenues or specific net income that justifies the acquisition.
Our ability to utilize and benefit from our net operating loss carryforwards and certain other tax attributes may be limited.
As of December 31, 2017, we had federal and state of California income tax net operating loss carryforwards, which may be applied to future taxable income, of approximately $44.5 million and $32.9 million, respectively. To the extent that we continue to generate taxable losses, unused losses will carry forward to offset future taxable income, if any, until these unused losses expire. However, we may be unable to use these losses to offset taxable income before our unused losses expire at various dates that range from 2035 through 2037 for federal net operating losses and from 2033 through 2037 for state net operating losses. Under Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, if a corporation undergoes an “ownership change,” generally defined as a greater than 50 percentage point change (by value) in its equity ownership over a three-year period, the corporation’s ability to use its pre-change net operating loss, or NOL, carryforwards to offset its post-change taxable income may be limited. Limitations may also apply to the utilization of other pre-change tax attributes as a result of an ownership change.
The Company experienced an “ownership change” within the meaning of Section 382(g) of the Internal Revenue Code of 1986, as amended, during the second quarter of 2017. The ownership change will subject the Company’s net operating loss carryforwards to an annual limitation, which will significantly restrict its ability to use them to offset taxable income in periods following the ownership change. In general, the annual use limitation equals the aggregate value of the Company’s stock at the time of the ownership change multiplied by a tax-exempt interest rate specified by the Internal Revenue Service. The Company has analyzed the available information to determine the amount of the annual limitation. Based on information available to the Company, the 2017 limitation is estimated to range between be $1.4 million and $3.7 million annually. In total, the Company estimates that the 2017 ownership change will result in approximately $120 million and $56 million of federal and state net operating loss carryforwards expiring unused.
| 30 |
Risks Related to the Securities Market, and Ownership of Our Common Stock
The price of our common stock has been and may continue to be volatile and the value of your investment could decline.
Medical technology stocks have historically experienced high levels of volatility. The trading prices of our common stock have fluctuated and may continue to fluctuate substantially. The market price of our common stock may be higher or lower than the price you pay, depending on many factors, some of which are beyond our control and may not be related to our operating performance. These fluctuations could cause you to lose substantially all or part of your investment in our common stock. Factors that could cause fluctuations in the trading price of our common stock include:
| ● | announcements of new offerings, products, services, therapies, treatments or technologies, commercial relationships, acquisitions or other events by us or our competitors, | |
| ● | challenges to our patents and the patents underlying the patents and intellectual property that we license, | |
| ● | United States and European approvals or denials of our products, | |
| ● | price and volume fluctuations in the overall stock market from time to time, | |
| ● | significant volatility in the market price and trading volume of medical device or technology companies in general, | |
| ● | fluctuations in the trading volume of our shares or the size of our public float, | |
| ● | actual or anticipated changes or fluctuations in our results of operations, | |
| ● | whether our results of operations meet the expectations of securities analysts or investors, | |
| ● | actual or anticipated changes in the expectations of investors or securities analysts, | |
| ● | litigation involving us, our industry, or both, | |
| ● | regulatory developments in the United States, foreign countries, or both, | |
| ● | general economic conditions and trends, | |
| ● | major catastrophic events, | |
| ● | sales of large blocks of our common stock, | |
| ● | departures of key employees, or | |
| ● | an adverse impact on the Company from any of the other risks cited herein. |
In addition, if the market for medical technology stocks or the stock market, in general, experiences a loss of investor confidence, the trading price of our common stock could decline for reasons unrelated to our business, results of operations or financial condition. The trading price of our common stock might also decline in reaction to events that affect other companies in our industry even if these events do not directly affect us. In the past, following periods of volatility in the market price of a company’s securities, securities class action litigation has often been brought against that company. If our stock price is volatile, we may become the target of securities litigation. Securities litigation could result in substantial costs and divert our management’s attention and resources from our business. This could have a material adverse effect on our business, results of operations and financial condition.
Sales of substantial amounts of our common stock in the public or private markets could reduce the price of our common stock and may dilute your voting power and ownership interest in us.
Sales of a substantial number of shares of our common stock in the public or private markets, or the perception that these sales could occur, as well as sales of shares by directors or officers, which have occurred or which may occur from time to time, could adversely affect the market price of our common stock and may make it more difficult for you to sell your common stock at a time and price that you deem appropriate.
Certain of our stockholders have the ability to control the outcome of matters submitted for stockholder approval and may have interests that differ from those of our other stockholders.
As of December 31, 2017 our executive officers, key employees, directors and their affiliates beneficially own in the aggregate approximately 43% of the outstanding shares of our common stock. As a result, these stockholders, if acting together, may be able to exercise significant influence over all matters requiring stockholder approval, including the election of directors and the approval of significant corporate transactions. They may also have interests that differ from yours and may vote in a manner that is adverse to your interests. This concentration of voting power may have the effect of deterring, delaying or impeding actions that could be beneficial to you, including actions that may be supported by our board of directors, and deprive our shareholders of an opportunity to receive a premium for their common stock as part of a sale of our Company and might ultimately affect the market price of our common stock.
| 31 |
We do not intend to pay dividends for the foreseeable future and, consequently, your ability to achieve a return on your investment will depend on appreciation in the price of our common stock.
We have never declared or paid any dividends on our common stock. We intend to retain any earnings to finance the operation and expansion of our business, and we do not anticipate paying any cash dividends in the future. As a result, you may only receive a return on your investment in our common stock if the market price of our common stock increases.
Future sales and issuances of our equity securities or rights to purchase our equity securities, including pursuant to our equity incentive plans, would result in dilution of the percentage ownership of our stockholders and could cause our stock price to fall.
To the extent we raise additional capital by issuing equity securities; our stockholders may experience substantial dilution. We may sell common stock, convertible securities or other equity securities in one or more transactions at prices and in a manner we determine from time to time. If we sell common stock, convertible securities or other equity securities in more than one transaction, investors may be diluted by subsequent sales. Such sales may also result in material dilution to our existing stockholders, and new investors could gain rights superior to existing stockholders.
The public market for our common stock has been volatile since completion of our initial public offering in November 2014. This volatility may affect the ability of our investors to sell their shares as well as the price at which they sell their shares.
We completed our initial public offering in November 2014. Since that time, closing prices of our shares have ranged from $0.96 per share to $23.60 per share and day-to-day trading often has been volatile. This volatility may continue or increase in the future. The market price for the shares may be significantly affected by factors such as progress in the development of our technology, progress in our pre-clinical and clinical trials, agreements with research facilities or co-development partners, commercialization of our technology, coverage by third party payers, variations in quarterly and yearly operating results, general trends in the medical device industry, and changes in FDA and foreign regulations affecting us and our industry. Furthermore, in recent years the stock market has experienced extreme price and volume fluctuations that are unrelated or disproportionate to the operating performance of the affected companies. Those broad market fluctuations may adversely affect the market price of our common stock.
Substantial future sales of shares of our common stock in the public market could cause our stock price to fall.
If our common stockholders (including those persons who may become common stockholders upon exercise of our options or warrants) sell substantial amounts of our common stock, or the public market perceives that stockholders might sell substantial amounts of our common stock, the market price of our common stock could decline significantly. Such sales also might make it more difficult for us to sell equity or equity-related securities in the future at a time and price that our management deems appropriate.
We have the right to issue shares of preferred stock. If we were to issue preferred stock, it is likely to have rights, preferences and privileges that may adversely affect the common stock.
We are authorized to issue 10,000,000 shares of “blank check” preferred stock, with such rights, preferences and privileges as may be determined from time-to-time by our board of directors. Our board of directors is empowered, without stockholder approval, to issue preferred stock in one or more series, and to fix for any series the dividend rights, dissolution or liquidation preferences, redemption prices, conversion rights, voting rights, and other rights, preferences and privileges for the preferred stock. No shares of preferred stock are presently issued and outstanding and we have no immediate plans to issue shares of preferred stock. The issuance of shares of preferred stock, depending on the rights, preferences and privileges attributable to the preferred stock, could adversely reduce the voting rights and powers of the common stock and the portion of our assets allocated for distribution to common stockholders in a liquidation event, and could also result in dilution in the book value per share of our common stock. The preferred stock could also be utilized, under certain circumstances, as a method for raising additional capital or discouraging, delaying or preventing a change in control of our Company, to the detriment of the holders of our common stock. We cannot assure you that we will not, under certain circumstances, issue shares of our preferred stock.
| 32 |
Item 1B. Unresolved Staff Comments
Not applicable.
Our principal office and facilities are located at 12744 San Fernando Road, Suite 400, Sylmar, California 91342, and consists of approximately 45,351 rentable square feet at a base rent of approximately $36,600 per month. Our lease expires in February 2022 and grants us an option to extend the lease term for an additional 60 months period. We believe that these premises are adequate for our foreseeable needs.
Our European office is located on the Innovation Park at EPFL, Rue Jean Daniel Colladon, CH 1015 Lausanne. The lease consists of 180 square meters at a base rent of 8,200 CHF per month, or currently about $8,700 per month. Our lease is currently monthly with a six month notice required for termination, with the Foundation for the Innovation Park at EPFL.
We are not a party to threatened or pending material legal proceedings other than those involving Pixium Vision described in “Risk Factors—Risks Related to Intellectual Property and Other Legal Matters”.
| 33 |
Item 5. Market for Registrant’s Common Equity, Related Shareholder Matters and Issuer Purchases of Equity Securities
(a) Market Price, Dividends and Related Matters
Second Sight’s common stock is traded on the Nasdaq Capital Market under the symbol “EYES.” The following table sets forth the high and low closing sales prices of our common stock as reported on the Nasdaq Capital Market for the following time periods.
| High | Low | |||||||
| Fiscal Year Ended December 31, 2017 | ||||||||
| First quarter | $ | 2.76 | $ | 1.11 | ||||
| Second quarter | $ | 1.31 | $ | 1.10 | ||||
| Third quarter | $ | 1.35 | $ | 0.96 | ||||
| Fourth quarter | $ | 2.35 | $ | 1.06 | ||||
| Fiscal Year Ended December 31, 2016 | ||||||||
| First quarter | $ | 6.79 | $ | 3.78 | ||||
| Second quarter | $ | 5.85 | $ | 3.18 | ||||
| Third quarter | $ | 4.24 | $ | 3.22 | ||||
| Fourth quarter | $ | 3.48 | $ | 1.76 | ||||
On March 13, 2018, the closing sales price reported for our common stock was $1.92 per share, and as of that date there were approximately 111 shareholders of record.
We have never declared or paid cash dividends on our common stock and do not anticipate paying any dividends in the foreseeable future.
The performance graph below compares the cumulative total stockholder return on our common stock with that of the Nasdaq Composite index and the Nasdaq Medical Equipment index. The initial public offering price of our common stock was $9.00 per share and the closing price was $19.97 per share on November 19, 2014 (the date our common stock first commenced trading on Nasdaq). The chart assumes $100 was invested at the close of the market on November 19, 2014 in our common stock, the Nasdaq Composite index and the Nasdaq Medical Equipment index.
34
Second Sight Medical Products, Inc. Comparison of Total Return
Among Second Sight, the Nasdaq Composite Index and the Nasdaq Medical Equipment Index
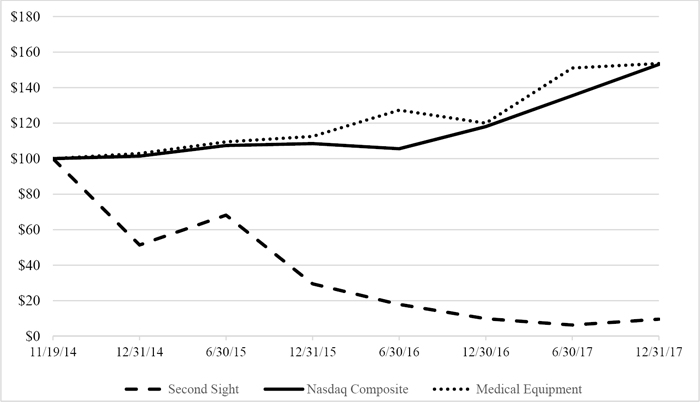
| 11/19/14 | 12/31/14 | 6/30/15 | 12/31/15 | 6/30/16 | 12/30/16 | 6/30/17 | 12/31/17 | |||||||||||||||||||||||||
| Second Sight | $ | 100.00 | $ | 51.38 | $ | 68.15 | $ | 29.49 | $ | 17.93 | $ | 9.86 | $ | 6.31 | $ | 9.56 | ||||||||||||||||
| Nasdaq Composite | $ | 100.00 | $ | 101.39 | $ | 107.37 | $ | 108.45 | $ | 105.57 | $ | 118.07 | $ | 135.44 | $ | 153.06 | ||||||||||||||||
| Medical Equipment | $ | 100.00 | $ | 102.89 | $ | 109.46 | $ | 112.51 | $ | 127.29 | $ | 119.97 | $ | 151.06 | $ | 153.57 | ||||||||||||||||
Use of Proceeds from June 2016 and March 2017 Rights Offerings and our ATM
In June 2016, the Company successfully completed a Rights Offering to existing stockholders (File No. 333-209113), raising proceeds of $19.5 million net of cash offering costs, and selling 5,978,465 shares of common stock at $3.315 per share, representing 85% of the Company’s stock price at the close of the Rights Offering. None of the proceeds was used for construction of plant, building and facilities, the purchase of real estate, or the acquisition of any business
In March 2017 we completed a Rights Offering to existing stockholders (File No. 333-215463), raising proceeds of approximately $19.7 million net of cash offering costs, and selling 13,652,341 units, each consisting of one share of common stock and one warrant, at $1.47 per unit. We have used the proceeds to further develop and enhance our products, support operations and for general corporate purposes.
In November 2017, the Company entered into an At-the-Market issuance sales agreement (the “Sales Agreement”) with B. Riley FBR Inc. and H.C. Wainwright & Co., LLC, as agents (“Agents”) pursuant to which the Company may offer and sell, from time to time through either of the Agents, shares of our common stock having an aggregate offering price as set forth in the Sales Agreement and a related prospectus supplement filed with the Securities and Exchange Commission (File No. 333-221228). The Company agreed to pay the Agents a cash commission of 3.0% of the aggregate gross proceeds from each sale of shares under the Sales Agreement. Through December 31, 2017 the Company sold 598,276 shares of common stock and received net proceeds of $1.1 million under the Sales Agreement and thereafter during January and February 2018 the Company sold 2.2 million shares of common stock for additional net proceeds of $4 million. We are utilizing these proceeds to further develop and enhance our products, support operations and for general corporate purposes.
35
Item 6. Selected Financial Data
The following selected consolidated financial data should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and the notes to those consolidated financial statements. The consolidated statements of operations data set forth below for the years ended December 31, 2017, 2016, and 2015 and the consolidated balance sheet data as of December 31, 2017 and 2016 are derived from, and are qualified in their entirety by reference to, the Company’s audited consolidated financial statements included elsewhere in this Form 10-K. The consolidated balance sheet data as of December 31, 2014 and 2015 and the consolidated statements of operations data for the year ended December 31, 2014 is derived from the audited consolidated financial statements not included herein, but which were previously filed with the Securities and Exchange Commission.
| Fiscal Years Ended December 31, | ||||||||||||||||
| (in thousands, except per share data) | 2017 | 2016 | 2015 | 2014 | ||||||||||||
| Net sales | $ | 7,964 | $ | 3,985 | $ | 8,950 | $ | 3,398 | ||||||||
| Cost of sales | 5,117 | 10,076 | 5,293 | 3,558 | ||||||||||||
| Gross profit (loss) | 2,847 | (6,091 | ) | 3,657 | (160 | ) | ||||||||||
| Operating expenses: | ||||||||||||||||
| Research and development, net of grants | 7,893 | 5,347 | 3,036 | 5,041 | ||||||||||||
| Clinical and regulatory | 3,062 | 2,703 | 3,510 | 2,622 | ||||||||||||
| Selling and marketing | 9,569 | 8,989 | 8,935 | 6,845 | ||||||||||||
| General and administrative | 10,932 | 10,080 | 8,223 | 6,565 | ||||||||||||
| Total operating expenses | 31,456 | 27,119 | 23,704 | 21,073 | ||||||||||||
| Loss from operations | (28,609 | ) | (33,210 | ) | (20,047 | ) | (21,233 | ) | ||||||||
| Interest income | 93 | 31 | 2 | 9 | ||||||||||||
| Other income, net | — | — | 27 | 12 | ||||||||||||
| Interest expense on convertible promissory notes and loan payable | — | — | — | (1,957 | ) | |||||||||||
| Amortization of discount on convertible promissory notes | — | — | — | (5,077 | ) | |||||||||||
| Write-off of unamortized discount on conversion of convertible promissory notes | — | — | — | (6,955 | ) | |||||||||||
| Net loss | $ | (28,516 | ) | $ | (33,179 | ) | $ | (20,018 | ) | $ | (35,201 | ) | ||||
| Net loss per common share – Basic and diluted | $ | (0.53 | ) | $ | (0.84 | ) | $ | (0.56 | ) | $ | (1.41 | ) | ||||
| Weighted average shares outstanding – Basic and diluted | 54,152 | 39,554 | 35,637 | 25,053 | ||||||||||||
As of December 31, | ||||||||||||||||
| (in thousands) | 2017 | 2016 | 2015 | 2014 | ||||||||||||
| Cash | $ | 604 | $ | 539 | $ | 239 | $ | 619 | ||||||||
| Money market funds | $ | 7,235 | $ | 10,336 | $ | 15,721 | $ | 34,000 | ||||||||
| Working capital | $ | 6,550 | $ | 9,620 | $ | 18,782 | $ | 33,525 | ||||||||
| Total assets | $ | 14,497 | $ | 16,810 | $ | 28,245 | $ | 43,069 | ||||||||
| Stockholders’ equity | $ | 7,882 | $ | 11,148 | $ | 20,263 | $ | 34,618 | ||||||||
36
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those anticipated in these forward-looking statements as a result of many factors. The consolidated results of operations for the years ended December 31, 2017, 2016 and 2015 are not necessarily indicative of the results that may be expected for any future period. The following discussion should be read in conjunction with the consolidated financial statements and the notes thereto included in Part IV, Item 15 of this Form 10-K and in conjunction with the “Risk Factors” included in Part I, Item 1A of this Form 10-K.
Business Overview
Second Sight was founded in 1998 with a mission to develop, manufacture, and market prosthetic devices that are intended to create an artificial form of useful vision for blind individuals. Our principal offices are located in Sylmar, California, approximately 25 miles northwest of downtown Los Angeles. We also have an office in Lausanne, Switzerland, that manages our commercial and clinical operations in Europe, the Middle East, and Asia-Pacific.
Our current product, the Argus ® II System, treats outer retinal degenerations, such as retinitis pigmentosa, also referred to as RP. RP is a hereditary disease, affecting an estimated 1.5 million people worldwide including about 100,000 people in the United States, that causes a progressive degeneration of the light-sensitive cells of the retina, leading to significant visual impairment and ultimately blindness. The Argus II System is the only retinal prosthesis approved in the United States by the Food and Drug Administration (FDA), and was the first approved retinal prosthesis in the world. By creating an artificial form of useful vision in patients who otherwise have total sight loss, the Argus II System can provide benefits which include:
| ● | Restoring independence through a renewed ability to navigate independently in unfamiliar environments, |
| ● | improving patients’ orientation and mobility, such as locating doors and windows, avoiding obstacles, and following the lines of a crosswalk |
| ● | allowing patients to feel more connected with people in their surroundings, such as seeing when someone is approaching or moving away, |
| ● | providing patients with enjoyment from being “visual” again, such as locating the moon, tracking groups of players as they move around a field, and watching the moving streams of lights from fireworks, and |
| ● | enabling some patients to re-enter the workforce through multiple vocations that become possible because of Argus II, and |
| ● | improving patients’ well-being and ability to perform activities of daily living. |
The Argus II System provides an artificial form of vision that differs from the vision of people with normal sight. It does not restore normal vision and there is no clear evidence that it can slow or reverse the progression of the disease. The majority of patients receive a significant benefit from the Argus II, however results can vary and some patients report receiving little or no benefit.
Our major corporate, clinical and regulatory milestones include:
| ● | In 1998, Second Sight was founded. |
| ● | In 2002, we commenced clinical trials in the US for our prototype product, the Argus I retinal prosthesis. |
37
| ● | In 2007, we commenced clinical trials in the US for the Argus II System, which later became our first commercial product. |
| ● | In 2011, we received marketing approval in Europe (CE Mark) for the Argus II System. |
| ● | In 2013, we received marketing approval in the United States (FDA) for the Argus II System. |
| ● | In 2014, we launched the Argus II in the US, completed our initial public offering (“IPO”), and began trading on Nasdaq under the symbol “EYES.” |
| ● | In January 2016, we successfully implanted and activated a wireless cortical visual prosthesis in a human. |
| ● | In November 2017, the FDA granted Expedited Access Pathway Designation for the Orion. |
| ● | In the first quarter of 2018, first-in-human Orion was successfully implanted, activated and tested at UCLA. |
Currently, after more than 18 years of research and development, more than $200 million of investment and over $34 million of grants awarded in support of our technology development, we employ over 110 people in the development (research, engineering and clinical), manufacture, and commercialization of the Argus II System and future products.
Going Concern
From inception, our operations have been funded primarily through the sales of our common stock, as well as from the issuance of convertible debt, research and clinical grants, and limited product revenue generated by the sale of our Argus II System. During the years ended December 31, 2017, 2016 and 2015, we funded our business primarily through:
| ● | Issuance of common stock and warrants in our Rights Offering in March 2017, which generated net proceeds of $19.7 million of cash after offering expenses. |
| ● | Issuance of common stock in our Rights Offering in June 2016, which generated net proceeds of $19.5 million of cash after offering expenses. |
| ● | Issuance of common stock through our At-the-Market sales agreement during the fourth quarter of 2017, which generated $1.1 million of cash after offering expenses. |
| ● | Revenue of $8.0 million, $4.0 million, and $8.9 million in 2017, 2016 and 2015, respectively, generated by sales of our Argus II System. |
On March 6, 2017, the Company successfully completed a registered Rights Offering to existing stockholders in which it sold 13,652,341 Units at $1.47 per Unit, which was the closing price of the Company common stock on that date. Each Unit consisted of a share of the Company’s common stock and a warrant to purchase an additional share of the Company’s stock for $1.47. The warrants have a five-year life. At the Company’s discretion, the warrants are redeemable on 30 days’ notice (i) at any time 24 months after the date of issuance, (ii) if the shares of our common stock are trading at 200% of the Subscription Price for 15 consecutive trading days and (iii) if all of the independent directors vote in favor of redeeming the warrants. Holders may be able to sell or exercise warrants prior to any announced redemption date and we will redeem outstanding warrants not exercised by the announced redemption date for a nominal amount of $0.01 per Warrant. We have listed the Warrants for trading on the Nasdaq Stock Market under the symbol “EYESW.”
In November 2017, the Company entered into an At-the-Market sales agreement (the “Sales Agreement”) with B. Riley FBR Inc. and H.C. Wainwright & Co., LLC, as agents (“Agents”) pursuant to which the Company may offer and sell, from time to time through either of the Agents, shares of our common stock having an aggregate offering price as set forth in the Sales Agreement and a related prospectus supplement filed with the Securities and Exchange Commission. The Company agreed to pay the Agents a cash commission of 3.0% of the aggregate gross proceeds from each sale of shares under the Sales Agreement. Through December 31, 2017 the Company sold 598,276 shares of common stock and received net proceeds of $1.1 million under the Sales Agreement and thereafter during January and February 2018 the Company sold 2.2 million shares of common stock for additional net proceeds of $4 million. We are utilizing these proceeds to further develop and enhance our products, support operations and for general corporate purposes.
38
Our financial statements have been presented on the basis that our business is a going concern, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. We are subject to the risks and uncertainties associated with a business with one product line and limited commercial product revenues, including limitations on our operating capital resources and uncertain demand for our products. We have incurred recurring operating losses and negative operating cash flows since inception, and we expect to continue to incur operating losses and negative operating cash flows for at least the next few years. Management has made estimates of future results of operations, using a wide range of assumptions regarding the level of revenue generated, operating expense incurred and future cash flows, which suggest a wide range of possible future outcomes. However, assuming financial results in 2018 similar to the results achieved in 2017, management has concluded that there is substantial doubt about our ability to continue as a going concern, and our independent registered public accounting firm, in its report on our 2017 consolidated financial statements, has raised substantial doubt about our ability to continue as a going concern. No assurances can be given that we will ultimately be able to raise sufficient funds through other means so as to be able to continue operating our business at current levels beyond the end of the end of second quarter of 2018.
Insurance Reimbursement
Obtaining reimbursement from governmental and private insurance companies is critical to our commercial success. Due to the cost of the Argus II System, our sales would be limited without the availability of third party reimbursement. In the US, coding, coverage, and payment are necessary for the surgical procedure and Argus II system to be reimbursed by payers. Coding has been established for the device and the surgical procedure. Coverage and payment vary by payer. The majority of Argus II patients are eligible for Medicare, and coverage is primarily provided through traditional Medicare, sometimes referred to as Medicare Fee-for-Service (FFS) or Medicare Advantage. A small percentage of patients are covered by commercial insurers.
| ● | Medicare FFS patients – Coverage is determined by Medicare Administrative Contractors (MACs) that administer various geographic regions of the US. Positive coverage decisions for the Argus II are effective in eight of 12 MAC jurisdictions (comprising 31 states). Effective January 1, 2018, the Centers for Medicare and Medicaid Services (CMS) established a 2018 payment rate of $122,500 for both the procedure and the Argus II Retinal Prosthesis System. |
| ● | Medicare Advantage patients – Medicare Advantage plans are required to cover the same benefits as those covered by the MAC in that jurisdiction. For example, if a MAC in a jurisdiction has favorable coverage for the Argus II, then all Medicare Advantage plans in that MAC jurisdiction are required to offer the same coverage for the Argus II. Individual hospitals and ASCs may negotiate contracts specific to that individual facility, which may include additional separate payment for the Argus II implant system. In addition, procedural payment is variable and can be based on a percentage of billed charges, payment groupings or other individually negotiated payment methodologies. Medicare Advantage plans also allow providers to confirm coverage and payment for the Argus II procedure in advance of implantation. In 2015, 2016 and 2017 combined, a large majority of all Medicare Advantage pre-authorization requests for Argus II procedures were granted. |
| ● | Commercial insurer patients – Commercial insurance plans make coverage and payment rate decisions independent of Medicare, and contracts are individually negotiated with facility and physician providers. |
During the year ended December 31, 2017, 38 individuals in the US and Canada were implanted with the Argus II technology. Of these patients, 31 were in US with most patients having a Medicare FFS coverage and a few having a Medicare Advantage or Veteran’s Administration or another commercial insurance plans and the remaining seven in Canada were treated with private funding.
Second Sight employs dedicated employees and consultants with insurance reimbursement expertise engaged to expand and enhance coverage decisions. Currently, eight of 12 Medicare jurisdictions provide coverage of the Argus II in 31 states, two territories and the District of Columbia when medically necessary, including:
● CGS (J15 -- Ohio and Kentucky),
39
● Palmetto GBA (JM -- Virginia, (excluding Part B for Arlington and Fairfax counties), West Virginia, North Carolina and South Carolina),
● Palmetto GBA (JJ – Alabama, Georgia and Tennessee),
● NGS (J6 -- Minnesota, Illinois and Wisconsin),
● NGS (JK -- Connecticut, New York, Maine, Massachusetts, New Hampshire, Rhode Island and Vermont),
● FCSO (JN -- Florida, Puerto Rico and the U.S. Virgin Islands),
● Novitas (JH-- Arkansas, Colorado, Louisiana, Mississippi, New Mexico, Oklahoma, and Texas) and
● Novitas (JL -- Delaware, District of Columbia, Maryland, New Jersey and Pennsylvania).
We are actively engaged with the remaining MACs and are committed to supporting their requests for additional information and clinical evidence. We expect that additional positive coverage decisions will be issued over time but cannot predict timing or ultimate success with each MAC.
Within Europe, we have obtained reimbursement approval or funding in Germany, France, one region of Italy, a Commissioning through Evaluation (CtE) in program England.
We are seeking additional reimbursement approvals in other countries in Europe and international markets.
In France, the Company was selected to receive the first “Forfait Innovation” (Innovation Bundle) from the Ministry of Health, which is a special funding program for breakthrough procedures to be introduced into clinical practice. As part of this program, the Company is conducting a post-market study in France which has enrolled a total of 18 subjects who are being followed up for two years. The French program also fund implantation of up to 18 additional patients that are not part of the post-market study. After review of the study’s results, we expect Argus II therapy to be covered and funded through the standard payment system in France, however, we can provide no assurance that the French government will continue to fund the Argus II after the first 36 implants.
In December 2016, NHS England announced it would cover 10 Argus implantations as part of a CtE program. The CtE program is especially designed for treatments that show significant promise for the future, while new clinical and patient experience data are collected within a formal evaluation program. This program is similar to the Forfait Innovation program in France. NHS England is known to be under significant financial pressure and also highly selective in adopting innovative technologies – which must demonstrate sufficient value for the cost expended.
To date, our marketing activities have focused on raising awareness of the Argus II System with potential patients, implanting physicians, and referring physicians. Our marketing activities include exhibiting, sponsoring symposia, and securing podium presence at professional and trade shows, securing journalist coverage in popular and trade media, attending patient meetings focused on educating patients about existing and future treatments, and sponsoring information sessions for the Argus II System. In the United States, our efforts will focus on media advertisements dedicated to RP patients and their families. These advertisements will be placed in geographic areas where we have Centers of Excellence committed to Argus II.
Product and Clinical Development Plans
The Argus II System is currently approved for RP patients with bare or no light perception in the US, and in Europe for severe to profound vision loss due to outer retinal degeneration, such as from retinitis pigmentosa (RP), choroideremia, and other similar conditions. The number of people who are legally blind due to RP is estimated to be about 25,000 in the US, 42,000 in Europe, and about 375,000 total worldwide. A subset of these patients would be eligible for the Argus II System since the approved baseline vision for the Argus II System is worse than legally blind (20/200).
40
The Company believes an opportunity exists to expand the use of its Argus II technology to better sighted individuals with RP who are currently not being treated. In order to achieve this market expansion, the Company plans to start collecting clinical data in 2018 and is undertaking multiple development efforts to improve the technology’s performance, including:
| ● | Clinical trials with better-sighted individuals; |
| ● | Development of retinal stimulation protocols that we believe can achieve improved resolution by adjusting electronic retinal stimulation methods; |
| ● | Redesigns of the externals (glasses, camera, video processing unit) that will possess processing power many times greater than the current Argus II system, which will enable enhanced image processing support for the commercial implementation of the new retina stimulation protocols, possibly by 2018. |
We believe we can further expand our market to include nearly all profoundly blind individuals, other than those who are blind due to preventable diseases or due to brain damage, by developing a visual cortical prosthesis. We refer to this product as the Orion visual prosthesis system. We estimate that there are approximately 5.8 million people worldwide who are legally blind due to causes other than preventable conditions, RP or AMD. If approved for marketing, the FDA and other regulatory agencies will determine the subset of these patients who are eligible for the Orion.
Our objective in designing and developing the Orion visual prosthesis system is to bypass the optic nerve and directly stimulate the part of the brain responsible for vision. We submitted an IDE application to the FDA in 2017 to begin a human feasibility study of the Orion visual prosthesis system. This study will confirm initial findings in our human pilot study we announced in Q4 2016 and provide the first human data of a fully functional wireless visual cortical stimulator system including the external video camera system. This initial study in a small number of subjects, if successful, should also form the basis for an expansion to a pivotal clinical trial in 2018.
We began a five-subject pilot study in the United Kingdom in June 2015, to determine the utility of the Argus II System for use in persons suffering from dry AMD. In Q2 2016 we completed enrollment and continue to track the subjects via the site in Manchester. The subjects have reported the ability to integrate their native peripheral vision with their artificial central vision. Subjects also report that they enjoy using their Argus system. To date, however, the subjects have not demonstrated significant objective benefit over their residual vision when using the Argus II. We’ve opted to finish out the study but not extend or expand at this time. Current performance does not justify enrolling additional patients.
Recently Adopted Accounting Standards
In August 2014, the Financial Accounting Standards Board (the “FASB”) issued Accounting Standards Update No. 2014-15 (ASU 2014-15), Presentation of Financial Statements — Going Concern (Subtopic 205-10). ASU 2014-15 provided guidance as to management’s responsibility to evaluate whether there is substantial doubt about an entity’s ability to continue as a going concern and to provide related footnote disclosures. In connection with our preparing these financial statements we evaluated whether there are conditions or events, considered in the aggregate, that raise substantial doubt about our ability to continue as a going concern within one year after the date that the financial statements are issued. The Company believes that it has sufficient funds to support its operations to sometime late in the second quarter on 2018.
In March 2016, the FASB issued ASU 2016-09, Compensation—Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting. ASU 2016-09 changes how companies account for certain aspects of share-based payment awards to employees, including the accounting for income taxes, forfeitures and statutory tax withholding requirements, as well as classification in the statement of cash flows. ASU 2016-09 is effective for annual periods beginning after December 15, 2016, including interim periods within those annual periods. If an entity early adopts in an interim period, any adjustments should be reflected as of the beginning of the fiscal year that includes that interim period and the entity must adopt all of the amendments from ASU 2016-09 in the same period. Management has determined that the adoption of this standard did not have a material effect to the financial statements and related disclosures.
Recent Accounting Pronouncements
In February 2016, the FASB issued ASU 2016-02, Leases (Topic 842), which supersedes all existing guidance on accounting for leases in ASC Topic 840. ASU 2016-02 is intended to provide enhanced transparency and comparability by requiring lessees to record right-of-use assets and corresponding lease liabilities on the balance sheet. ASU 2016-02 will continue to classify leases as either finance or operating, with classification affecting the pattern of expense recognition in the statement of income. ASU 2016-02 is effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years. Early adoption is permitted. ASU 2016-02 is required to be applied with a modified retrospective approach to each prior reporting period presented with various optional practical expedients. While the Company is continuing to assess all potential impact of this standard, it expects most of its lease commitments will be subject to the updated standard and recognized as lease liabilities and right-of-use assets upon adoption.
In May, 2017, the FASB issued ASU No. 2017-09, “Compensation — Stock Compensation (Topic 718) — Scope of Modification Accounting.” ASU No. 2017-09 provides clarity and reduces complexity when applying the guidance in Topic 718 for changes in terms or conditions of share-based payment awards. It is effective for annual reporting periods beginning after December 15, 2017. The Company is currently evaluating the impact the adoption of this new standard will have on its financial statements. While the Company is continuing to assess all potential impact of this standard, it expects most of its lease commitments will be subject to the updated standard and recognized as lease liabilities and right-of-use assets upon adoption.
41
In May 2014, the FASB issued Accounting Standards Update (“ASU”) No. 2014-09-Revenue from Contracts with Customers (Topic 606) (“ASU 2014-09”), which provides new guidance for revenue recognition. The Financial Accounting Standards Board (“FASB”) subsequently issued ASU No. 2015-14-Revenue from Contracts with Customers (Topic 606), which deferred the effective date of ASU 2014-09, ASU No. 2016-08-Revenue from Contracts with Customers (Topic 606): Principal versus Agent Considerations (Reporting Revenue Gross versus Net), ASU No. 2016-10-Revenue from Contracts with Customers (Topic 606): Identifying Performance Obligations and Licensing, ASU No. 2016-12-Revenue from Contracts with Customers (Topic 606): Narrow-Scope Improvements and Practical Expedients, and ASU No. 2016-20-Technical Corrections and Improvements to Topic 606, Revenue from Contracts with Customers. The above subsequent ASUs did not change the core principle of the guidance in ASU 2014-09. The ASUs referred to above collectively will supersede and replace the revenue recognition requirements in ASC Topic 605-Revenue Recognition, and most of the related industry specific guidance and replace them with ASC Topic 606, Revenue from Contracts with Customers (“ASC 606”). The core principle in ASC 606 is that revenue is recognized when promised goods or services are transferred to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. To achieve that core principle, an entity should apply the following steps:
Step 1: Identify the contract(s) with a customer
Step 2: Identify the performance obligations in the contract.
Step 3: Determine the transaction price.
Step 4: Allocate the transaction price to the performance obligations in the contract.
Step 5: Recognize revenue when (or as) the entity satisfies a performance obligation.
ASU 2014-09 also creates ASC Subtopic 340-40-Other Assets and Deferred Costs-Contracts with Customers (“ASC 340-40”), which requires an entity to recognize an asset certain types of costs related to a contract with a customer within the scope of ASC 606 and amortize the asset over a period consistent with the transfer of the goods and services to which the asset relates. Specifically, the costs required to be capitalized are (a) incremental costs of obtaining a contract with a customer and (b) costs incurred in fulfilling a contract with a customer that are not in the scope of another ASC Topic.
ASC 606 and ASC 340-40 (the “new accounting standards”) require the Company to make significant judgments and estimates. The new accounting standards also require more extensive disclosures regarding the nature, amount, timing and uncertainty of revenue and cash flows arising from contracts with customers. The Company has adopted the new accounting standards as of January 1, 2018 using the modified retrospective transition method, in which the two new accounting standards were applied retrospectively with the cumulative effect of initially applying the new accounting standards as an adjustment to the opening balance of retained earnings at January 1, 2018, the date of initial adoption. In accordance with the modified retrospective transition method, the Company applied the new guidance retrospectively only to contracts that were not completed contracts at January 1, 2018.
42
Also in accordance with the modified retrospective transition method, the Company will provide additional disclosures in its financial statements for each of the quarterly and annual reporting periods in 2018 of (a) the amount by which each financial statement line item is affected in the reporting period by the application of the new accounting standards as compared to the accounting guidance that was in effect before the change, and (b) an explanation of the reasons for significant changes identified.
The Company completed its assessment of adoption of ASC 606 2016, and is currently in the process of updating that assessment to reflect changes in contractual terms and the Company’s customary business practices since completion of the initial assessment.
The Company believes the financial statement impact of the expected changes will be minimal.
Management believes that any other recently issued, but not yet effective, authoritative guidance, if currently adopted, would not have a material impact on the Company’s financial statement presentation or disclosures.
Critical Accounting Policies and Estimates
The following discussion and analysis of financial condition and results of operations is based upon our consolidated financial statements, which have been prepared in conformity with accounting principles generally accepted in the United States of America. Certain accounting policies and estimates are particularly important to the understanding of our financial position and results of operations and require the application of significant judgment by our management or can be materially affected by changes from period to period in economic factors or conditions that are outside of our control. As a result, they are subject to an inherent degree of uncertainty. In applying these policies, our management uses their judgment to determine the appropriate assumptions to be used in the determination of certain estimates. Those estimates are based on our historical operations, our future business plans and projected financial results, the terms of existing contracts, our observance of trends in the industry, information provided by our customers and information available from other outside sources, as appropriate. See Note 2 of notes to our consolidated financial statements for a more complete description of our significant accounting policies.
Revenue Recognition. The Company’s revenue is derived primarily from the sale of its Argus II retinal implant, which is implanted during retinal surgery to restore some functional vision to patients blinded by Retinitis Pigmentosa. The Company sells to a variety of customers including university hospitals, large medical centers and distributors.
43
Revenue is recognized when persuasive evidence of an arrangement exists, the fee is fixed or determinable, collectability is probable, and delivery has occurred.
Revenue is generated under sales agreements with multiple deliverables (multiple-element arrangements), comprising the following deliverables:
| ● | Hospital start up kits (one per site), |
| ● | Surgical support, |
| ● | Training, and |
| ● | The Argus II System. |
The deliverables may vary by transaction.
The Company evaluates each deliverable in a multiple-element arrangement to determine whether it represents a separate unit of accounting. An element constitutes a separate unit of accounting when the delivered item has standalone value and delivery of the undelivered element is probable and within the Company’s control. The Company has determined that the elements listed above do not have standalone value to the customer until delivery of all components has occurred. Accordingly, revenue from multiple-element arrangements is recognized when delivery of all of deliverables has taken place and all other revenue recognition criteria have been met. Generally, revenue recognition occurs at the time of implantation, but revenue recognition can be delayed if certain training has not been delivered to the implanting sites, or if other revenue recognition criteria have not been met.
In the United States, the amount of revenue recognized per unit has been limited in some situations due to the uncertainties of the reimbursement environment and payment terms. In such cases, revenue is not recognized until the consideration becomes fixed, generally when paid to the Company.
In order to determine whether collection is reasonably assured, the Company assesses a number of factors, including creditworthiness of the customer and medical insurance coverage. The Company may periodically grant extended payment terms to customers. In such situations, the Company defers the recognition of revenue until collection becomes probable, which is generally upon receipt of payment.
The Company also sells surgical supplies to customers and recognizes revenue on these products when they are shipped and other revenue recognition criteria have been met.
The Company sells through distributors in certain countries. The Company provides these distributors with clinical start-up kits, surgical supplies and the Argus II System, as well as training them to provide pre- and post-surgical support. The Company monitors the surgery. Other than surgical support which is provided by the Company, the distributor is responsible for delivering products and services to its customers. In the past, the Company has allowed distributors to return or exchange products in certain situations. Due to the Company’s continuing involvement and its returns policy, the Company recognizes revenue from distributors when the implantation procedure has been performed by the distributor’s customer, and all other revenue recognition criteria between the Company and the distributor have been met.
Stock-Based Compensation. Pursuant to Financial Accounting Standards Board ASC 718 Share-Based Payment (“ASC 718”), the Company records stock-based compensation expense for all stock-based awards. Under ASC 718, the Company estimates the fair value of stock options granted using the Black-Scholes option pricing model. The fair value for awards that are expected to vest is then amortized on a straight-line basis over the requisite service period of the award, which is generally the option vesting term.
| ● | The grant price of the issuances is determined based on the fair value of the shares at the date of grant. |
| ● | The risk free interest rate for periods within the contractual life of the option is based on the U.S. treasury yield in effect at the time of grant. |
44
| ● | As permitted by SAB 107, due to the Company’s insufficient history of option activity, management utilizes the simplified approach to estimate the options expected term, which represents the period of time that options granted are expected to be outstanding. |
| ● | Volatility is determined based on average historical volatilities of comparable companies in similar industry. |
| ● | Expected dividend yield is based on current yield at the grant date or the average dividend yield over the historical period. The Company has never declared or paid dividends and has no plans to do so in the foreseeable future. |
Patent Costs. The Company has over 400 domestic and foreign patents. Due to the uncertainty associated with the successful development of one or more commercially viable products based on Company’s research efforts and any related patent applications, all patent costs, including patent-related legal, filing fees and other costs, including internally generated costs, are expensed as incurred. Patent costs are included in general and administrative expenses in the consolidated statements of operations.
Long Term Investor Right. Each beneficial owner (“IPO Shareholder”) of the Company’s common stock, who purchased shares directly in the offering (“IPO Shares”), qualified to receive up to one additional share of common stock from the Company for each share purchased in the offering (“IPO Supplemental Shares”) pursuant to the Long Term Investor Right that was included with each IPO Share. To qualify for receipt of IPO Supplemental Shares, an IPO Shareholder was required to take action to become the direct registered owner of its IPO Shares within 90 days following the closing date of the offering, or by February 22, 2015. Furthermore, IPO Shareholders were required to hold their IPO Shares in their own name and not place them in “street name” or trade them at any time during the 24 month period immediately following the IPO closing date. This Long Term Investors Right was non-detachable and transferable only in limited circumstances.
The Company issued IPO Supplemental Shares to IPO Shareholders who did not otherwise forfeit their Long Term Investor Right since, during the two-year period immediately following the IPO closing date, the Company’s common stock did not trade at or above $18.00 per share (200% of the IPO price per share) for any five consecutive day period.
The formula to determine the number of IPO Supplemental Shares to issue on a trigger of the Long Term Investor Right was: (i) $18.00 minus (ii) the average of the highest consecutive closing prices in any 90 day trading period on the principal exchange during the two years after the Closing Date (the “Measurement Average”) divided by the Measurement Average. Fractional shares issuable to a qualifying IPO Shareholder resulting from the calculation were rounded up to the next whole share of common stock, taking into account the aggregate number of Long Term Investor Rights of a holder. Since the highest average of consecutive closing prices over any 90 calendar day period was $13.96 per share, each Long-Term Investor Right was entitled to 0.2894 additional shares of common stock, which is calculated as: ($18.00 - $13.96)/$13.96.
45
The amount of IPO Supplemental Shares issued was computed by an independent public accountant as soon as practicable following the second anniversary of the Closing Date. The Company in turn delivered 355,095 shares to these shareholders.
The Right was an equity instrument that was accounted for as a component of the actual price per common share paid by the investor in the IPO. For basic earnings per share, the common shares associated with the Right were included in basic earnings per share beginning on their effective issuance date in November 2016.
Results of Operations
Net sales. Our net sales are derived primarily from the sale of our Argus II System. We began selling the Argus II System in Europe at the end of 2011, Saudi Arabia in 2012, the United States and Canada in 2014, Turkey in 2015, Iran, Taiwan, South Korea and Russia in 2017, and Singapore in 2018. Our objective is to increase our product revenue over the next several years as we pursue commercialization of our product, as our product becomes more well-known and accepted in the market, and as insurance coverage becomes more widespread.
Cost of sales. Cost of sales includes the salaries, benefits, material, overhead, third party costs, warranty, charges for excess and obsolete inventory, and other costs required to make our Argus II System at our Sylmar, California facility. Historically, our cost of sales had been greater than our revenues, which resulted in gross losses. During fiscal 2015, due to higher revenues and increased manufacturing output and efficiencies, we generated a positive gross margin. In 2016, in response to growing inventory levels and a decrease in revenue, we reduced our manufacturing output and recorded a reserve for slow-moving inventory, resulting in a gross loss for the year. In 2017, we kept our production low and were able to reduce the reserve for slow-moving inventory, which contributed to generating a positive gross margin in the year. Our product involves technologically complex materials and processes. While we are currently experiencing low yields on our manufacturing process, we expect that over the next few years we will be able to refine our processes and improve our manufacturing yields. We are also producing at less than our capacity which results in unabsorbed overhead costs. In future years, as we produce in greater quantities and improve our manufacturing yields, we expect that we will more consistently generate positive gross margins.
Operating Expenses. We generally recognize our operating expenses as we incur them in four general operational categories: research and development, clinical and regulatory, sales and marketing, and general and administrative. Our operating expenses also include a non-cash component related to the amortization of deferred stock-based compensation allocated to research and development, clinical and regulatory, sales and marketing and general and administrative personnel. From time to time we have received grants from institutions or agencies, such as the National Institutes of Health, to help fund the some of the cost of our development efforts. We have recorded these grants as offsets to the costs as they are incurred to complete the related work.
| ● | Research and development expenses consist primarily of employee compensation and consulting costs related to the design, development, and enhancements of our current and potential future products, offset by grant revenue received in support of specific research projects. We expense our research and development costs as they are incurred. We expect research and development expenses to increase in the future as we pursue further enhancements of our existing product and develop technology for our potential future products, such as the Orion visual cortical prosthesis. We also expect to receive additional grants in the future that will be offset primarily against research and development costs. |
| ● | Clinical and regulatory expenses consist primarily of salaries, travel and related expenses for personnel engaged in clinical and regulatory functions, as well as internal and external costs associated with conducting clinical trials and maintaining relationships with regulatory agencies. We expect clinical and regulatory expenses to increase as we assess the safety and efficacy of enhancements to our current Argus II System, seek to expand the indications for the Argus II System, such as AMD, and prepare to initiate clinical studies of potential future products, such as the Orion visual cortical prosthesis. |
| ● | Sales and marketing expenses consist primarily of salaries, commissions, travel and related expenses for personnel engaged in sales, marketing and business development functions, as well as costs associated with promotional and other marketing activities. We expect sales and marketing expenses to increase as we hire additional sales personnel, initiate additional marketing programs, develop relationships with new distributors, and expand the number of doctors and medical centers that buy and implant our Argus II System and any future products. |
46
| ● | General and administrative expenses consist primarily of salaries and related expenses for executive, legal, finance, human resources, information technology and administrative personnel, as well as recruiting and professional fees, patent filing costs, insurance costs and other general corporate expenses, including rent. We expect general and administrative expenses to increase as we add personnel and incur additional costs related to the growth of our business and operate as a public company. |
Comparison of the Years Ended December 31, 2017 and 2016
Worldwide commercial implant volume for the years ended December 31, 2017, 2016 and 2015 was as follows:
| Years Ended December 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| Europe and the Middle East | 30 | 30 | 43 | |||||||||
| Asia | 7 | — | — | |||||||||
| Canada | 7 | 1 | 4 | |||||||||
| United States | 31 | 11 | 28 | |||||||||
| Total | 75 | 42 | 75 | |||||||||
Net Sales. Our net sales increased from $4.0 million in 2016 to $8.0 million in 2017, an increase of $4.0 million, or 100%. This increase in net sales was due to a higher number of implants in 2017 versus 2016 as well as a higher level of revenue per implant. In 2017 there were 75 implants compared to 42 in 2016, and the amount of revenue recognized per implant increased from $95,000 in 2016 to $106,000 in 2017.
In both 2017 and 2016, there were 30 implants in Europe and the Middle East (EMEA). With the addition of new centers in South Korea and Taiwan, there were seven implants in Asia, compared to none in the prior year. In the North American market, which includes the United States and Canada, implants increased to 38 in 2017 compared to 12 in 2016. The increase in U.S. implants was due, in part, to our emphasis on our Centers of Excellence (COE) strategy, where we support the implanting sites through staff training, screening of patients, surgery support, and after surgery training for patient programming and rehabilitation. Going forward, we expect our COE strategy to drive growth in North America and, when fully deployed, to help revive growth in EMEA. In the Asian markets, we will focus on opening new centers in additional countries and working with our existing centers to foster growth.
The amount of revenue recognized per implant in a period depends on several factors, including reimbursement policies set by private and government payers, the mix of implants between North America and the rest of the world, exchange rates, payment terms that may affect revenue recognition, and sales of ancillary products, such as clinical start-up kits and surgical supplies. Given the higher 2017 CMS rate in the US, as discussed above, and the higher relative growth in North America compared to the rest of the world, where average selling prices are generally lower than in North America, the overall revenue per implant grew from $95,000 in 2016 to $106,000 in 2017. Looking forward to 2018, even with a reduced CMS reimbursement rate of $122,500 in the US, we expect the revenue mix to continue to favor North America and we expect our average revenue per implant to be in the range of $95,000 to $105,000.
In the United States, the amount of sales revenue recognized per unit has been limited in some situations due to the uncertainties of the reimbursement environment and payment terms. Favorable claims outcomes and the development of positive coverage policies in the United States may eventually result in greater and earlier revenue recognition.
47
Cost of sales. Cost of sales decreased to $5.1 million in 2017 from $10.1 million in 2016, a decrease of $5.0 million or 50%, resulting in a positive gross margin of 36% in 2017 compared to a negative gross margin of 153% in 2016. In 2017, cost of sales included a $3.1 million credit related to the reduction of a reserve for slow-moving inventory and a $2.8 million charge related to unabsorbed overhead costs, which combined nets to a $0.3 million credit. In 2016, cost of sales included a $4.7 million charge to increase a reserve for slow-moving inventory and a $2.8 million charge related to unabsorbed overhead costs, which combined totals to a $7.5 million charge. Excluding these amounts for changes in inventory reserves and unabsorbed overhead costs, our cost of goods sold would have been $5.4 million in 2017, yielding an adjusted 32% gross margin, and $1.4 million 2016, yielding an adjusted gross margin of 35%. As we move forward, we hope to keep our production level in line with units sold, reducing the need for additions to our inventory reserves. We also hope to sell and manufacture higher amounts of our product, which would lower, or even eliminate, charges for unabsorbed inventory costs. If we are able to achieve both of these goals, we expect that we will be able to achieve positive gross margins consistently.
Research and development expense. Research and development expense increased from $5.3 million in 2016 to $7.9 million in 2017, an increase of $2.6 million, or 49%. The increase is mainly attributable to a $2.0 million, or 83%, decrease of grant revenues which are used to offset research and development expenses. Grant revenues decreased from $2.4 million in 2016 to $0.4 million in 2017. This decrease in grant revenue relates to a 2014 grant from Johns Hopkins University that had been almost completely utilized in prior years was fully applied by the end of the first quarter of 2017. Excluding the offsetting effect of grant revenue, research and development expense increased by $0.5 million, or 6%, from $7.7 million in 2016 to $8.2 million in 2017. This increase in research and development expenditures relates to $0.9 million of higher spending for people related costs and $0.2 million more spent on consultants and outside services, which was partially offset by $0.6 million in lower expenditures related to supplies and materials used for prototype development. In future years, research and developments expense should continue to increase at a moderate rate, excluding the offsetting benefit of grant revenue, as we continue to move our product development plans ahead.
Clinical and regulatory expense. Clinical and regulatory expense increased from $2.7 million in 2016 to $3.1 million in 2017, an increase of $0.4 million, or 15%. This increase is primarily attributable to higher spending on staff and consultants as we prepared to start new clinical trials for better- vision patients and for the Orion cortical implant. We expect clinical and regulatory expense to increase in 2018 and future years as we expand our clinical trial efforts, especially those related to the Orion cortical implant.
Selling and marketing expense. Selling and marketing expense increased from $9.0 million in 2016 to $9.6 million in 2017, an increase of $0.6 million or 7%. This increase in spending represents an increase of $1.1 million in people related costs, such as salaries, benefits, travel and stock-based compensation, partially offset by a $0.4 million decrease in expenditures on consultants and outside services for items such as legal services, public relations and customer outreach programs. While we expect selling costs to increase in the future as we increase our selling and marketing resources to accelerate the commercialization of our product, we expect selling and marketing expense to decrease over time when expressed as a percentage of product revenue.
General and administrative expense. General and administrative expense increased from $10.1 million in 2016 to $10.9 million in 2017, an increase of $0.8 million, or 8%. This increase is primarily attributable to $0.7 million of higher people related costs, primarily higher salaries, bonuses and stock-based compensation, and $0.5 million of higher expenditures on outside services for items such as legal and recruiting services. Partially offsetting this was a $0.4 million swing in bad debt expense, which dropped from an expense of $0.3 million in 2016 to a net recovery of previously written off receivables of $0.1 million in 2017. In future years, we expect general and administrative costs to increase at rate lower than the future growth of revenue.
Net loss. The net loss was $28.5 million in 2017, as compared to $33.2 million in 2016. The $4.7 million decrease in net loss from 2016 to 2017 was primarily attributable to an $8.9 million increase in gross profit, (from a loss in 2016 to a gross margin in 2017), caused mainly by higher revenues and the reversal of charges for excess inventory, offset by $4.3 million in increased operating expenses.
48
Comparison of the Years Ended December 31, 2016 and 2015
Net Sales. Our net sales decreased from $8.9 million in 2015 to $4.0 million in 2016, a decrease of $4.9 million, or 55%. This decrease in net sales was due to a lower number of implants in 2016 versus 2015 as well as a lower level of revenue per implant. In 2016 there were 42 implants compared to 75 in 2016, and the amount of revenue recognized per implant declined from $119,000 in 2015 to $95,000 in 2016.
In 2016, there were 30 implants in Europe and the Middle East (EMEA) compared to 43 implants in the prior year. The decrease in implants in EMEA is primarily attributable to a decrease of 12 implants in France due, in part, to a potential competitor recruiting RP patients for a clinical trial in France, and a decrease of seven implants in Italy. Germany grew by one implant going from five in 2015 to six in 2016. Elsewhere in EMEA, we saw growth in Saudi Arabia and Turkey with implants increasing by four and one, respectively, in 2016 versus the prior year.
In the United States and Canada (North America), implants decreased to 12 in 2016 compared to 32 in 2015. The decline in U.S. implants was due, in part, to the 2016 Medicare reimbursement level being reduced to $95,000, which in early Q1 2016 was approximately $50,000 below our U.S. list price. We made the decision in late February 2016 to implement temporary discounts in the U.S., lasting through December 2016, to alleviate concerns of our customers that they would lose money on Argus II patient cases due to the difference between the device cost and the reimbursement amount. Additionally, we had hired a new sales leader for North America in the second quarter of 2016 and experienced some turnover in the sales staff. With our new sales team in place, together with a clearly defined Center of Excellence strategy for the U.S., we expect to see a higher level of implants in coming quarters.
The amount of revenue recognized per implant in a period depends on several factors, including reimbursement policies set by private and government payers, the mix of implants between EMEA and North America, exchange rates, payment terms that may affect revenue recognition, and sales of ancillary products, such as clinical start-up kits and surgical supplies. Given the CMS pricing decision made in 2015 to reduce the 2016 reimbursement rate for the Argus II implant and related procedure, we made the determination in late February 2016 to temporarily discount the Argus device in the US to approximately $92,000 compared to the $144,000 we sold the product for in 2015. Accordingly, the average revenue per implant was lower in 2016 than it was in 2015. For 2017, with the CMS reimbursement rate of $150,000 approved for U.S. Medicare patients, we expect our global average revenue per implant to increase to $110,000 to $120,000 depending on the geographic mix of implants. In the United States, the amount of sales revenue recognized per unit has been limited in some situations due to the uncertainties of the reimbursement environment and payment terms. Favorable claims outcomes and the development of positive coverage policies in the United States may eventually result in greater and earlier revenue recognition.
Cost of sales. Cost of sales increased from $5.3 million in 2015 to $10.1 million in 2016, an increase of $4.8 million or 91%. With the reduced level of implants in 2016, the cost of goods sold related to implants declined in 2016, however, we incurred significant charges for excess inventory and unabsorbed overhead costs. Management believed it was prudent to take the reduced level of sales activity during 2016 as compared to 2015 into consideration when estimating excess inventory. Accordingly, we booked a total of $4.7 million of reserves for slow moving inventory in 2016. Also, we made the decision during the second quarter of 2016 to reduce our production levels, lay off certain direct manufacturing personnel, and reassign certain other indirect personnel to where the Company could better utilize their skills. Because of the reduced production output, we are spreading our production costs over a lower number of units, which resulted in approximately $2.8 million of unabsorbed production variances that we recognized as period costs during the year.
Research and development expense. Research and development expense increased from $3.0 million in 2015 to $5.3 million in 2016, an increase of $2.3 million, or 77%. The increase is attributable to increased activity in research and development as work to get new products ready for clinical trials and market release. The increase consists of approximately $962,000 related to charges from manufacturing for work on prototypes, $551,000 for people related costs, $671,000 for outside services and consultants and $468,000 for supplies. These increased expenditures were offset, in part, by $488,000 of higher grant revenue recognized in 2016 versus 2015. Excluding grant revenues, which are used to offset expenses, research and development expense increased from $4.9 million in 2015 to $7.7 million, an increase of $2.8 million, or 57%, in 2016 compared to 2015.
49
Clinical and regulatory expense. Clinical and regulatory expense decreased from $3.5 million in 2015 to $2.7 million in 2016, a decrease of $0.8 million, or 23%. This decrease is primarily attributable to the cost of post-market and other clinical trials to assess the safety and efficacy of our current product, to assess possible enhancements to our existing product, and to assess the efficacy of our technology for treating blindness due to Age-Related Macular Degeneration.
Selling and marketing expense. Selling and marketing expense increased from $8.9 million in 2015 to $9.0 million in 2016, an increase of $0.1 million or 1%. This small increase in costs represent the netting of a decrease of $663,000 in lower people related costs, including lower salaries, stock based compensation, and commissions, against $863,000 in higher costs for consultants related to items such as customer outreach programs and insurance reimbursement for our products in the U.S. and foreign markets.
General and administrative expense. General and administrative expense increased from $8.2 million in 2015 to $10.1 million in 2016, an increase of $1.9 million, or 23%. This increase is primarily attributable to $900,000 of higher stock-based compensation charges than in 2015, and $718,000 in higher cash compensation costs.
Net loss. The net loss was $33.2 million in 2016, as compared to $20.0 million in 2015. The $13.2 million increase in net loss from 2015 to 2016 was primarily attributable to a $9.8 million decrease in gross profit, caused by lower revenues and large charges for excess inventory and unabsorbed manufacturing overhead, and $3.4 million in increased operating expenses.
Liquidity and Capital Resources
Our Company’s consolidated financial statements have been presented on the basis that it is a going concern, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. We have experienced recurring operating losses and negative operating cash flows since inception, and have financed our working capital requirements through the recurring sale of our equity securities in both public and private offerings. As a result, our independent registered public accounting firm, in its current report on our 2017 consolidated financial statements, has raised substantial doubt about our ability to continue as a going concern (see “Going Concern” above”).
In June 2016, the Company successfully completed a Rights Offering to existing shareholders, raising proceeds of $19.5 million net of cash offering costs, and selling 5,978,465 shares of common stock at $3.315 per share.
In March 2017, the Company successfully completed an additional Rights Offering to existing shareholders, raising proceeds of approximately $19.7 million net of cash offering costs, and selling 13,652,341 Units at $1.47 per unit. Each Unit consists of a share of common stock and a five-year warrant with an exercise price of $1.47.
During December 2017, the Company issued 598,276 shares of common stock for gross proceeds of approximately $1.2 million as part of its At-the-Market (“ATM”) sales agreement with two different investment banks. The Company paid expenses of approximately $0.1 million resulting in net proceeds of $1.1 million. In the period from January 1, 2018 to February 28, 2018, the Company sold approximately 2.2 million additional shares through its ATM sales agreement, raising gross proceeds of approximately $4.1 million and net proceeds of approximately $4.0 million after expenses.
Working capital was $6.5 million at December 31, 2017, as compared to $9.6 million at December 31, 2016, a decrease of $3.1 million or 32%. Working capital was $9.6 million at December 31, 2016, as compared to $18.8 million at December 31, 2015, a decrease of $9.2 million or 49%. We use our cash, money market funds and working capital to fund our operating activities.
50
Cash Flows from Operating Activities
During 2017, we used $23.9 million of cash in operating activities, consisting primarily of a net loss of $28.5 million, decreased by non-cash charges of $1.3 million for depreciation and amortization of property and equipment, stock-based compensation, common stock issuable, bad debt recovery and excess inventory reserve, and decreased by a net change in operating assets and liabilities of $3.3 million.
During 2016, we used $25.1 million of cash in operating activities, consisting primarily of a net loss of $33.2 million, offset by non-cash charges of $9.1 million for depreciation and amortization of property and equipment, stock-based compensation, common stock issuable, bad debt expense, excess inventory reserve, and loss on disposal of property and equipment and increased by a net change in operating assets and liabilities of $1.0 million.
During 2015, we used $20.6 million of cash in operating activities, consisting primarily of a net loss of $20.0 million, offset by non-cash charges of $3.3 million for depreciation and amortization of property and equipment, stock-based compensation, and common stock issuable and increased by a net change in operating assets and liabilities of $3.9 million.
Cash Flows from Investing Activities
Investing activities in 2017 provided $2.8 million of cash, reflecting $3.1 million in proceeds from money market investments offset by $0.3 million for the purchase of equipment.
Investing activities in 2016 provided $4.9 million of cash, reflecting $5.4 million in proceeds from money market investments offset by $0.5 million for the purchase of equipment.
Investing activities in 2015 provided $17.5 million of cash, reflecting $18.3 million in proceeds from money market investments offset by $0.8 million for the purchase of equipment.
Cash Flows from Financing Activities
Financing activities provided $21.2 million of cash in 2017, including $19.7 million from the net proceeds from rights offering, $1.1 million from the net proceeds from our ATM share sales and $0.4 million from the issuance of common stock for ESPP purchases.
Financing activities provided $20.5 million of cash in 2016, including $19.5 million from the net proceeds from rights offering, $0.5 million from stock option and warrant exercises and issuance of common stock for ESPP purchases of $0.5 million.
Financing activities provided $2.8 million of cash in 2015, including $2.7 million from stock option and warrant exercises, issuance of common stock for ESPP purchases of $0.2 million offset by $0.1 million for payment of employment taxes related to stock option exercises.
Financial Commitments
Effective August 2012, we entered into a lease agreement (the “Sylmar Lease”) with a company owned by the major stockholder of the Company for office space for a term of five years that was to expire on February 28, 2017. The Sylmar Lease included rental of additional space commencing January 1, 2013 and a five year option to renew. The lease requires us to pay real estate taxes, insurance and common area maintenance each year, and is subject to periodic cost of living adjustments. In April 2014, the Sylmar Lease was renegotiated with the term ending on February 28, 2022, and a five year option to renew. The new lease also requires us to pay real estate taxes, insurance and common area maintenance each year and includes automatic increases in base rent each year. In November 2014, the industrial center in which the Company’s premises are located was sold to an independent third party.
Our Swiss subsidiary rents office space in Switzerland on a month-to-month basis for CHF 8,200 (currently approximately $8,700) per month.
Future minimum rental payments required under the operating leases are as follows for the years ended December 31 (in thousands).
| Years | Amount | ||||
| 2018 | $ | 858 | |||
| 2019 | 884 | ||||
| 2020 | 910 | ||||
| 2021 | 937 | ||||
| 2022 | 158 | ||||
| Total | $ | 3,747 | |||
51
Off-Balance Sheet Arrangements
At December 31, 2017, the Company did not have any transactions, obligations or relationships that could be considered off-balance sheet arrangements.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
Interest Rate Sensitivity
The primary objective of our investment activities is to maintain the safety of principal and preserve liquidity without incurring significant risk. We invest cash in excess of our current needs in money market funds. In general, money market funds are not considered to be subject to interest rate risk because the interest paid on such funds fluctuates with the prevailing interest rate. As of December 31, 2017, our cash equivalents consisted solely of money market funds.
Exchange Rate Sensitivity
During 2017, approximately 58% of our revenue was denominated in U.S. dollars, 35% in Euros, and 7% in Canadian dollars. This compares with 2016 when approximately 53% of our revenue was denominated in U.S. dollars, 44% in Euros, and 3% in Canadian dollars. For 2017, 2016 and 2015, the majority of our operating expenses were denominated in U.S. dollars. We have not entered into foreign currency forward contracts to hedge our operating expense exposure to foreign currencies, but we may do so in the future.
Item 8. Financial Statements and Supplementary Data
Our financial statements and supplementary data required by this Item are provided in the consolidated financial statements of the Company included in this Form 10-K as listed in Item 15(a) of this Form 10-K.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Disclosure controls and procedures are designed to ensure that information required to be disclosed by us in reports filed or submitted under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed in reports filed or submitted under the Exchange Act is accumulated and communicated to management, including our principal executive officer and principal financial officer, or persons performing similar functions, as appropriate to allow for timely decisions regarding required disclosure. Due to inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Further, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that degree of compliance with the policies and procedures may deteriorate. Accordingly, even effective disclosure controls and procedures can only provide reasonable assurance of achieving their control objectives.
52
As of December 31, 2017, management has concluded that our disclosure controls and procedures were not effective because we have not formally documented and tested our key internal controls. Notwithstanding the existence of the material weaknesses discussed below, our management, including our CEO and CFO, has concluded that the consolidated financial statements included in this Annual Report on Form 10-K fairly present, in all material respects, our financial position, results of operations and cash flows for the periods presented in this Annual Report on Form 10-K in conformity with GAAP.
This annual report does not include an attestation report from our independent registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by our independent registered public accounting firm pursuant to the Jumpstart Our Business Startups Act (the “JOBS Act”). Under the JOBS Act, we are not required to comply with Section 404(b) because we are an “emerging growth company.”
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. Our internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Our internal control over financial reporting includes those policies and procedures that:
1. Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets;
2. Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with GAAP, and that our receipts and expenditures are being made only in accordance with the authorization of our management and directors; and
3. Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
As of December 31, 2017, based on the criteria established in “Internal Control — Integrated Framework” (2013 Framework) issued by the Committee of Sponsoring Organizations of the Treadway Commission, management has concluded the Company does not have complete written documentation of its internal control policies, procedures and controls and has not fully completed its testing of its key controls. Management evaluated the impact of its failure to have fully tested its internal controls and procedures and has concluded that the control deficiencies that resulted represented a material weakness and that our internal control over financial reporting was not effective as of the end of the period covered by this Annual Report on Form 10-K.
During its December 31, 2015 and 2016 audits, our independent registered public accounting firm communicated to management and our audit committee that it identified material weaknesses in our internal control over financial reporting due to audit adjustments identified with respect to revenue, accrued expenses, inventory, prepaid and accrued expenses, and stock based compensation. During its December 31, 2017 audit, our independent registered public accounting firm communicated to management and our audit committee that it did not identify any material weaknesses.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. We identified the following material weaknesses:
| ● | Control over Financial Reporting. During the year ended December 31, 2015, we did not consistently perform timely reconciliation of certain accounts, including revenue, deferred revenue, inventory, prepaid and accrued expenses, and stock-based compensation expense. This resulted in the incorrect recording of certain revenue and expenses that required various adjusting entries that we timely and fully recorded as part of the closing process. |
53
| ● | Tracking of Back-up Prosthesis Units. For every surgery, we ship a back-up prosthesis unit along with the primary unit in case the primary unit cannot be used for some reason. Following a surgery, the unused unit is returned to us. During the year ended December 31, 2015, we did not consistently follow internal procedures regarding the tracking and recordation of returned prosthesis units and the exchange of primary units for back-up units with our customers. When uncorrected, this resulted in an understatement of cost of sales and an overstatement of inventory that required various adjusting entries that we timely and fully recorded as part of the closing process. |
| ● | Updating of Standard Costs. It is a customary practice for manufacturing companies to update their standard costs on a regular basis (at least annually) to ensure that inventory costs are accurately and properly stated. During the year ended December 31, 2016, due to the low level of production during the year and the inventory reserves that were established covering most of 2016 production, the Company did not update its standard costs at December 31, 2016. The Company’s failure to update its standard costs at December 31, 2016 represented a material weakness in its internal control over financing reporting. |
While we have taken actions to remediate the specific weaknesses found in fiscal years 2015 and 2016, the Company does not have complete written documentation of its internal control policies, procedures and controls and has not fully completed testing of its key controls. Management evaluated the impact of its failure to have fully tested its internal controls and procedures and has concluded that the control deficiencies that resulted represented a material weakness and that our internal control over financial reporting was not effective as of the end of the period covered by this Annual Report on Form 10-K.
Management’s Remediation Initiatives
In response to the above identified weaknesses in our internal control over financial reporting, we plan to continue our work in documenting in writing our internal control policies and procedures. We do not have a specific timeline within which we expect to conclude this written documentation process but do expect it to be an on-going process for the foreseeable future. We continue to evaluate testing of our internal control policies and procedures, including assessing internal and external resources that may be available to complete these tasks, but do not know when these tasks will be completed.
Our CEO and CFO, along with other key members of management, are and will be active participants in these remediation processes. We believe the steps taken to date have improved the effectiveness of our internal control over financial reporting.
Changes in Internal Control over Financial Reporting
There has been no change in our internal control over financial reporting that occurred during or subsequent to our fourth quarter of the year ended December 31, 2017 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Inherent Limitations on Effectiveness of Controls
The design of any system of control is based upon certain assumptions about the likelihood of future events. There can be no assurance that any design will succeed in achieving its stated objectives under all future events, no matter how remote, or that the degree of compliance with the policies or procedures may not deteriorate. Because of its inherent limitations, disclosure controls and procedures may not prevent or detect all misstatements. Accordingly, even effective disclosure controls and procedures can provide only reasonable assurance of achieving their control objectives. In addition, the design of disclosure controls and procedures must reflect the fact that there are resource constraints and that management is required to apply its judgment in evaluating the benefits of possible controls and procedures relative to their costs. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with policies and procedures may deteriorate.
54
None.
Certain information required by Part III is omitted from this Annual Report on Form 10-K and is incorporated by reference from our definitive proxy statement relating to our 2018 annual meeting of stockholders, pursuant to Regulation 14A of the Securities Exchange Act of 1934, as amended, also referred to in this Annual Report on Form 10-K as our 2018 Proxy Statement, which we will file with the SEC not later than 120 days after the end of the fiscal year covered by this Annual Report on Form 10-K.
Item 10. Directors, Executive Officers and Corporate Governance
Information regarding our directors, including the audit committee and audit committee financial experts, and executive officers and compliance with Section 16(a) of the Exchange Act will be included in an amendment to this Form 10-K or in our 2018 Proxy Statement and is incorporated herein by reference.
Item 11. Executive Compensation
The information required by this item regarding executive compensation will be included in an amendment to this Form 10-K or in our 2018 Proxy Statement and is incorporated herein by reference.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information required by this item regarding security ownership of certain beneficial owners and management will be included in an amendment to this Form 10-K or in our 2018 Proxy Statement and is incorporated herein by reference.
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information required by this item regarding certain relationships and related transactions and director independence will be included in an amendment to this Form 10-K or in our 2018 Proxy Statement and is incorporated herein by reference.
Item 14. Principal Accounting Fees and Services
The information required by this item regarding principal accounting fees and services will be included in an amendment to this Form 10-K or in our 2018 Proxy Statement and is incorporated herein by reference.
Item 15. Exhibits, Financial Statement Schedules
| (a) | The following documents are included in this Annual Report on Form 10-K: |
| 1. | The consolidated financial statements listed in the accompanying Index to Consolidated Financial Statements are filed as part of this report. |
| 2. | All financial schedules have been omitted because the required information is either presented in the consolidated financial statements or the notes thereto or is not applicable or required. |
| 3. | The exhibits required by Item 601 of Regulation S-K and Item 15(b) of this Annual Report on Form 10-K are listed in the Exhibit Index immediately preceding the exhibits and are incorporated herein. We have identified in the Exhibit Index each management contract and compensation plan filed as an exhibit to this Annual Report on Form 10-K in response to Item 15(a)(3) of Form 10-K. |
55
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| Dated: March 20, 2018 | Second Sight Medical Products, Inc. |
| /s/ Jonathan Will McGuire | |
| Jonathan Will McGuire | |
| Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this Report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| Name | Title | Date | ||
| /s/ Jonathan Will McGuire | Chief Executive Officer and Director | March 20, 2018 | ||
| Jonathan Will McGuire | (Principal Executive Officer) | |||
| /s/ Thomas B. Miller | Chief Financial Officer | March 20, 2018 | ||
| Thomas B. Miller | (Principal Financial and Accounting Officer) | |||
| /s/ Gregg Williams | Chairman of the Board | March 20, 2018 | ||
| Gregg Williams | ||||
| /s/ Robert J. Greenberg, M.D., Ph.D. | Director | March 20, 2018 | ||
| Robert J. Greenberg, M.D., Ph.D. | ||||
| /s/ William J. Link | Director | March 20, 2018 | ||
| William J. Link | ||||
| /s/ Aaron Mendelsohn | Director | March 20, 2018 | ||
| Aaron Mendelsohn | ||||
| /s/ Matthew Pfeffer | Director | March 20, 2018 | ||
| Matthew Pfeffer |
56
SECOND SIGHT MEDICAL PRODUCTS, INC.
AND SUBSIDIARY
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
57
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Second Sight Medical Products, Inc. and Subsidiary
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Second Sight Medical Products, Inc. and Subsidiary (the “Company”) as of December 31, 2017 and 2016, and the related consolidated statements of operations, comprehensive loss, stockholders’ equity, and cash flows, for each of the three years in the period ended December 31, 2017, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2017 and 2016, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2017, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
58
Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As more fully discussed in Note 1 to the consolidated financial statements, the Company is subject to the risks and uncertainties associated with a new business and has incurred significant losses from operations since inception. The Company’s operations are dependent upon it raising additional funds through an equity offering or debt financing. The Company has no committed sources of capital and is not certain whether additional financing will be available when needed on terms that are acceptable, if at all. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans regarding these matters are described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ Gumbiner Savett Inc.
We have served as the Company’s auditor since 2014
Santa Monica, California
March 20, 2018
59
SECOND
SIGHT MEDICAL PRODUCTS, INC.
AND SUBSIDIARY
(In thousands)
| December 31, | ||||||||
| 2017 | 2016 | |||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash | $ | 604 | $ | 539 | ||||
| Money market funds | 7,235 | 10,336 | ||||||
| Accounts receivable, net | 1,831 | 274 | ||||||
| Inventories, net | 2,700 | 3,416 | ||||||
| Prepaid expenses and other current assets | 795 | 717 | ||||||
| Total current assets | 13,165 | 15,282 | ||||||
| Property and equipment, net | 1,299 | 1,489 | ||||||
| Deposits and other assets | 33 | 39 | ||||||
| Total assets | $ | 14,497 | $ | 16,810 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 752 | $ | 1,156 | ||||
| Accrued expenses | 2,425 | 2,088 | ||||||
| Accrued compensation expense | 2,611 | 1,600 | ||||||
| Accrued clinical trial expense | 779 | 629 | ||||||
| Deferred revenue | 48 | 85 | ||||||
| Deferred grant revenue | — | 104 | ||||||
| Total current liabilities | 6,615 | 5,662 | ||||||
| Commitments and contingencies | ||||||||
| Stockholders’ equity: | ||||||||
| Preferred stock, no par value, 10,000 shares authorized; none outstanding | — | — | ||||||
| Common stock, no par value; 200,000 shares authorized; shares issued and outstanding: 57,630 and 42,701 at December 31, 2017 and December 31, 2016, respectively | 202,156 | 186,769 | ||||||
| Common stock to be issued | 153 | 153 | ||||||
| Additional paid-in capital | 40,522 | 30,697 | ||||||
| Notes receivable to finance stock option exercises | — | (2 | ) | |||||
| Accumulated other comprehensive loss | (572 | ) | (608 | ) | ||||
| Accumulated deficit | (234,377 | ) | (205,861 | ) | ||||
| Total stockholders’ equity | 7,882 | 11,148 | ||||||
| Total liabilities and stockholders’ equity | $ | 14,497 | $ | 16,810 | ||||
See accompanying notes to consolidated financial statements.
60
SECOND
SIGHT MEDICAL PRODUCTS, INC.
AND SUBSIDIARY
Consolidated Statements of Operations
(In thousands, except per share data)
| Years Ended December 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| Net sales | $ | 7,964 | $ | 3,985 | $ | 8,950 | ||||||
| Cost of sales | 5,117 | 10,076 | 5,293 | |||||||||
| Gross profit (loss) | 2,847 | (6,091 | ) | 3,657 | ||||||||
| Operating expenses: | ||||||||||||
| Research and development, net of grants | 7,893 | 5,347 | 3,036 | |||||||||
| Clinical and regulatory | 3,062 | 2,703 | 3,510 | |||||||||
| Selling and marketing | 9,569 | 8,989 | 8,935 | |||||||||
| General and administrative | 10,932 | 10,080 | 8,223 | |||||||||
| Total operating expenses | 31,456 | 27,119 | 23,704 | |||||||||
| Loss from operations | (28,609 | ) | (33,210 | ) | (20,047 | ) | ||||||
| Interest income | 93 | 31 | 2 | |||||||||
| Other income, net | — | — | 27 | |||||||||
| Net loss | $ | (28,516 | ) | $ | (33,179 | ) | $ | (20,018 | ) | |||
| Net loss per common share – basic and diluted | $ | (0.53 | ) | $ | (0.84 | ) | $ | (0.56 | ) | |||
| Weighted average shares outstanding – basic and diluted | 54,152 | 39,554 | 35,637 | |||||||||
See accompanying notes to consolidated financial statements.
| 61 |
SECOND
SIGHT MEDICAL PRODUCTS, INC.
AND SUBSIDIARY
Consolidated Statements of Comprehensive Loss
(In thousands)
| Years Ended December 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| Net loss | $ | (28,516 | ) | $ | (33,179 | ) | $ | (20,018 | ) | |||
| Other comprehensive income (loss): | ||||||||||||
| Foreign currency translation adjustments | 36 | (27 | ) | (107 | ) | |||||||
| Comprehensive loss | $ | (28,480 | ) | $ | (33,206 | ) | $ | (20,125 | ) | |||
See accompanying notes to consolidated financial statements.
| 62 |
SECOND
SIGHT MEDICAL PRODUCTS, INC.
AND SUBSIDIARY
Consolidated Statements of Stockholders’ Equity
(In thousands)
| Common Stock | Common Stock Issuable | Additional Paid- in | Notes Receivable for Stock Option | Accumulated Other Comprehensive | Accumulated | Total Stockholders’ | ||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Exercises | Loss | Deficit | Equity | ||||||||||||||||||||||||||||
| Balance, December 31, 2014 | 35,241 | $ | 163,171 | 16 | $ | 166 | $ | 24,590 | $ | (171 | ) | $ | (474 | ) | $ | (152,664 | ) | $ | 34,618 | |||||||||||||||||
| Issuance of common stock in connection with warrant exercise | 140 | 702 | — | — | — | — | — | — | 702 | |||||||||||||||||||||||||||
| Issuance of common stock in connection with cashless warrant exercise | 1 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
| Issuance of common stock in connection with Employee Stock Purchase Plan | 53 | 226 | — | — | — | — | — | — | 226 | |||||||||||||||||||||||||||
| Exercise of stock options | 574 | 2,782 | — | — | — | — | — | — | 2,782 | |||||||||||||||||||||||||||
| Stock-based compensation expense | — | — | — | — | 2,687 | — | — | — | 2,687 | |||||||||||||||||||||||||||
| Common stock tendered to exercise stock options | (78 | ) | (993 | ) | — | — | — | — | — | — | (993 | ) | ||||||||||||||||||||||||
| Stock issued or issuable in connection with professional services | 23 | 285 | 17 | 39 | — | — | — | — | 324 | |||||||||||||||||||||||||||
| Common stock tendered to pay taxes on stock option exercise | (12 | ) | (124 | ) | — | — | — | — | — | — | (124 | ) | ||||||||||||||||||||||||
| Repayment of notes receivable for stock option exercises, net | — | — | — | — | — | 166 | — | — | 166 | |||||||||||||||||||||||||||
| Comprehensive loss: | ||||||||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | — | — | — | (20,018 | ) | (20,018 | ) | |||||||||||||||||||||||||
| Foreign currency translation adjustment | — | — | — | — | — | — | (107 | ) | — | (107 | ) | |||||||||||||||||||||||||
| Comprehensive loss | — | — | — | — | — | — | (107 | ) | (20,018 | ) | (20,125 | ) | ||||||||||||||||||||||||
| Balance, December 31, 2015 | 35,942 | $ | 166,049 | 33 | $ | 205 | $ | 27,277 | $ | (5 | ) | $ | (581 | ) | $ | (172,682 | ) | $ | 20,263 | |||||||||||||||||
| 63 |
SECOND
SIGHT MEDICAL PRODUCTS, INC.
AND SUBSIDIARY
Consolidated Statements of Stockholders’ Equity
(In thousands)
(Continued)
| Common Stock | Common Stock Issuable | Additional Paid- in | Notes Receivable for Stock Option | Accumulated Other Comprehensive | Accumulated | Total Stockholders’ | ||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Exercises | Loss | Deficit | Equity | ||||||||||||||||||||||||||||
| Issuance of common stock and options in connection with rights offering net of expenses | 5,978 | 19,430 | — | — | 53 | — | — | — | 19,483 | |||||||||||||||||||||||||||
| Issuance of shares under Long-Term Investor Right | 355 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
| Issuance of common stock in connection with Employee Stock Purchase Plan | 189 | 488 | — | — | — | — | — | — | 488 | |||||||||||||||||||||||||||
| Exercise of stock options | 96 | 478 | — | — | — | 3 | — | — | 481 | |||||||||||||||||||||||||||
| Stock-based compensation expense | — | — | — | — | 3,367 | — | — | — | 3,367 | |||||||||||||||||||||||||||
| Stock issued in connection with professional services | 82 | 324 | 44 | (52 | ) | — | — | — | — | 272 | ||||||||||||||||||||||||||
| Issuance of RSU units | 59 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
| Comprehensive loss: | ||||||||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | — | — | — | (33,179 | ) | (33,179 | ) | |||||||||||||||||||||||||
| Foreign currency translation adjustment | — | — | — | — | — | — | (27 | ) | — | (27 | ) | |||||||||||||||||||||||||
| Comprehensive loss | — | — | — | — | — | — | (27 | ) | (33,179 | ) | (33,206 | ) | ||||||||||||||||||||||||
| Balance, December 31, 2016 | 42,701 | $ | 186,769 | 77 | $ | 153 | $ | 30,697 | $ | (2 | ) | $ | (608 | ) | $ | (205,861 | ) | $ | 11,148 | |||||||||||||||||
| 64 |
SECOND
SIGHT MEDICAL PRODUCTS, INC.
AND SUBSIDIARY
Consolidated Statements of Stockholders’ Equity
(In thousands)
(Continued)
| Common Stock | Common Stock Issuable | Additional Paid- in | Notes Receivable for Stock Option | Accumulated Other Comprehensive | Accumulated | Total Stockholders’ | |||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Exercises | Loss | Deficit | Equity | |||||||||||||||||||||||||||
| Issuance of common stock and warrants in connection with rights offering, net of expenses | 13,653 | 13,647 | — | — | 6,021 | — | — | — | 19,668 | ||||||||||||||||||||||||||
| Issuance of common stock in connection with Employee Stock Purchase Plan | 407 | 394 | — | — | — | — | — | — | 394 | ||||||||||||||||||||||||||
| Repayment of notes receivable for stock option exercises | — | — | — | — | — | 2 | — | — | 2 | ||||||||||||||||||||||||||
| Stock-based compensation expense | — | — | — | — | 3,784 | — | — | — | 3,784 | ||||||||||||||||||||||||||
| Issuance of shares of common stock in connection with ATM, net of expenses | 598 | 1,084 | — | — | — | — | — | — | 1,084 | ||||||||||||||||||||||||||
| Fair value of stock options issued for services in connection with rights offering | — | — | — | — | 20 | — | — | — | 20 | ||||||||||||||||||||||||||
| Stock issued in connection with professional services | 223 | 262 | 5 | — | — | — | — | — | 262 | ||||||||||||||||||||||||||
| Issuance of RSU units | 48 | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||
| Comprehensive loss: | |||||||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | — | — | — | (28,516 | ) | (28,516) | |||||||||||||||||||||||||
| Foreign currency translation adjustment | — | — | — | — | — | — | 36 | — | 36 | ||||||||||||||||||||||||||
| Comprehensive loss | — | — | — | — | — | — | 36 | (28,516 | ) | (28,480) | |||||||||||||||||||||||||
| Balance, December 31, 2017 | 57,630 | $ | 202,156 | 82 | $ | 153 | $ | 40,522 | $ | — | $ | (572 | ) | $ | (234,377 | ) | $ | 7,882 | |||||||||||||||||
See accompanying notes to consolidated financial statements.
| 65 |
SECOND
SIGHT MEDICAL PRODUCTS, INC.
AND SUBSIDIARY
Consolidated Statements of Cash Flows
(In thousands)
| Years Ended December 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| Cash flows from operating activities: | ||||||||||||
| Net loss | $ | (28,516 | ) | $ | (33,179 | ) | $ | (20,018 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||||||
| Depreciation and amortization of property and equipment | 457 | 432 | 335 | |||||||||
| Loss on disposal of property and equipment | — | 2 | — | |||||||||
| Stock-based compensation | 3,784 | 3,367 | 2,687 | |||||||||
| Bad debt (recovery) expense | (142 | ) | 258 | — | ||||||||
| Excess inventory (recovery) reserve | (3,106 | ) | 4,728 | — | ||||||||
| Common stock issued for services | 262 | 272 | 324 | |||||||||
| Changes in operating assets and liabilities: | ||||||||||||
| Accounts receivable | (1,413 | ) | 955 | (793 | ) | |||||||
| Inventories | 3,868 | 10 | (2,488 | ) | ||||||||
| Prepaid expenses and other assets | (71 | ) | 378 | (127 | ) | |||||||
| Accounts payable | (419 | ) | 446 | 197 | ||||||||
| Accrued expenses | 331 | 44 | 656 | |||||||||
| Accrued compensation expenses | 1,013 | (469 | ) | 707 | ||||||||
| Accrued clinical trial expenses | 150 | 13 | 127 | |||||||||
| Deferred revenue | (41 | ) | (234 | ) | (278 | ) | ||||||
| Deferred grant revenue | (104 | ) | (2,093 | ) | (1,878 | ) | ||||||
| Net cash used in operating activities | (23,947 | ) | (25,070 | ) | (20,549 | ) | ||||||
| Cash flows from investing activities: | ||||||||||||
| Purchases of property and equipment | (265 | ) | (490 | ) | (762 | ) | ||||||
| Proceeds from money market funds | 3,106 | 5,378 | 18,279 | |||||||||
| Net cash provided by investing activities | 2,841 | 4,888 | 17,517 | |||||||||
| Cash flows from financing activities: | ||||||||||||
| Net proceeds from sale of common stock in rights offerings and At-the-Market sale of common stock | 20,772 | 19,483 | — | |||||||||
| Proceeds from exercise of options, warrants and employee stock purchase plan options | 396 | 969 | 2,883 | |||||||||
| Payment of employment taxes related to stock option exercises | — | — | (124 | ) | ||||||||
| Net cash provided by financing activities | 21,168 | 20,452 | 2,759 | |||||||||
| Effect of exchange rate changes on cash | 3 | 30 | (107 | ) | ||||||||
| Cash: | ||||||||||||
| Net increase (decrease) | 65 | 300 | (380 | ) | ||||||||
| Balance at beginning of year | 539 | 239 | 619 | |||||||||
| Balance at end of year | $ | 604 | $ | 539 | $ | 239 | ||||||
See accompanying notes to consolidated financial statements.
| 66 |
SECOND
SIGHT MEDICAL PRODUCTS, INC.
AND SUBSIDIARY
Consolidated Statements of Cash Flows
(In thousands)
| Years Ended December 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| Supplemental cash flow information: | ||||||||||||
| Non-cash financing and investing activities: | ||||||||||||
| Fair value of stock options issued for services rendered in connection with rights offering | $ | 20 | $ | 53 | $ | — | ||||||
See accompanying notes to consolidated financial statements.
| 67 |
SECOND
SIGHT MEDICAL PRODUCTS, INC.
AND SUBSIDIARY
Notes to Consolidated Financial Statements
1. Organization and Business Operations
Second Sight Medical Products, Inc. (“Second Sight” or “the Company”), formerly Second Sight LLC, was founded in 1998 as a limited liability company and was subsequently incorporated in the State of California in 2003. Second Sight develops, manufactures and markets implantable prosthetic devices that can restore some functional vision to patients blinded by outer retinal degenerations, such as Retinitis Pigmentosa.
In 2007, Second Sight formed Second Sight (Switzerland) Sarl, initially to manage clinical trials for its products in Europe, and later to manage sales and marketing in Europe and the Middle East. As the laws of Switzerland require at least two corporate stockholders, Second Sight (Switzerland) Sarl is 99.5% owned directly by the Company and 0.5% owned by an executive of Second Sight, who is acting as a nominee of the Company. Accordingly, Second Sight (Switzerland) Sarl is considered 100% owned for financial statement purposes and is consolidated with Second Sight for all periods presented.
The Company’s current product, the Argus II system, entered clinical trials in 2006, received CE Mark approval for marketing and sales in the European Union (“EU”) in 2011, and approval by the United States Food and Drug Administration (“FDA”) for marketing and sales in the United States in 2013. The began selling the Argus II System in Europe at the end of 2011, Saudi Arabia in 2012, the United States and Canada in 2014, Turkey in 2015, Iran, Taiwan, South Korea and Russia in 2017, and Singapore in 2018.
Going Concern
From inception, the Company’s operations have been funded primarily through the sales of its common stock, as well as from the issuance of convertible debt, research and clinical grants, and limited product revenue generated from the sale of its Argus II System. During the years ended December 31, 2017, 2016 and 2015, the Company funded its business primarily through:
| ● | Issuance of common stock and warrants in our Rights Offering in March 2017, which generated net cash proceeds of $19.7 million. |
| ● | Issuance of common stock in our Rights Offering in June 2016, which generated net cash proceeds of $19.5 million. |
| ● | Issuance of common stock through our At-the-Market sales agreement during the fourth quarter of 2017, which generated $1.1 million of net cash proceeds. |
| ● | Revenue of $8.0 million, $4.0 million, and $8.9 million in 2017, 2016 and 2015, respectively, generated by sales of our Argus II System. |
The Company’s financial statements have been presented on the basis that its business is a going concern, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. The Company is subject to the risks and uncertainties associated with a business with one product line and limited commercial product revenues, including limitations on the Company’s operating capital resources and uncertain demand for its product. The Company has incurred recurring operating losses and negative operating cash flows since inception, and it expects to continue to incur operating losses and negative operating cash flows for at least the next few years. As a result, management has concluded that there is substantial doubt about the Company’s ability to continue as a going concern, and the Company’s independent registered public accounting firm, in its report on the Company’s 2017 consolidated financial statements, has raised substantial doubt about the Company’s ability to continue as a going concern.
| 68 |
The Company believes that it does not have sufficient funds to support its operations past the end of the second quarter of 2018. In order to continue business operations past that point, the Company currently anticipates that it will need to raise additional debt and/or equity capital during the next several months. However, there can be no assurances that the Company will be able to secure any such additional financing on acceptable terms and conditions, or at all. If cash resources become insufficient to satisfy the Company’s ongoing cash requirements, the Company would be required to scale back or discontinue its technology and product development programs and/or clinical trials, or obtain funds, if available (although there can be no certainty), through strategic alliances that may require the Company to relinquish rights to its products, or to discontinue its operations entirely.
2. Summary of Significant Accounting Policies
Principles of Consolidation
The accompanying consolidated financial statements of the Company have been prepared in accordance with United States generally accepted accounting principles (“GAAP”) and include the financial statements of Second Sight and Second Sight Switzerland. Intercompany balances and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period. Management bases its estimates on historical experience and on various assumptions that are believed to be reasonable in relation to the financial statements taken as a whole under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Management regularly evaluates the key factors and assumptions used to develop the estimates utilizing currently available information, changes in facts and circumstances, historical experience and reasonable assumptions. After such evaluations, if deemed appropriate, those estimates are adjusted accordingly. Actual results could differ from those estimates. Significant estimates include those related to assumptions used in accruals for potential liabilities, valuing equity instruments issued for services, and the realization of deferred tax assets. Actual results could differ from those estimates
Cash and Cash Equivalents
The Company considers all highly liquid investments with a maturity of three months or less at the date of purchase to be cash equivalents. Cash is carried at cost, which approximates fair value, and cash equivalents are carried at fair value. The Company generally invests funds that are in excess of current needs in high credit quality instruments such as money market funds.
Accounts receivable
Trade accounts receivable are stated net of an allowance for doubtful accounts. The Company performs ongoing credit evaluations of its customers’ financial condition and generally requires no collateral from its customers or interest on past due amounts. Management estimates the allowance for doubtful accounts based on review and analysis of specific customer balances that may not be collectible and how recently payments have been received. Accounts are considered for write-off when they become past due and when it is determined that the probability of collection is remote. Allowance for doubtful accounts amounted to approximately $74,000 and $213,000 at December 31, 2017 and 2016, respectively.
Inventories
Inventories are stated at the lower of cost or market, determined by the first-in, first-out method. Inventories consist primarily of raw materials, work in progress and finished goods, which includes all direct material, labor and other overhead costs. The Company establishes a reserve to mark down its inventory for estimated unmarketable inventory equal to the difference between the cost of inventory and the estimated net realizable value based on assumptions about the usability of the inventory, future demand and market conditions. If actual market conditions are less favorable than those projected by management, additional inventory reserve may be required.
Property and Equipment
Property and equipment are recorded at historical cost less accumulated depreciation and amortization. Improvements are capitalized, while expenditures for maintenance and repairs are charged to expense as incurred. Upon disposal of depreciable property, the appropriate property accounts are reduced by the related costs and accumulated depreciation. The resulting gains and losses are reflected in the consolidated statements of operations.
Depreciation is provided for using the straight-line method in amounts sufficient to relate the cost of assets to operations over their estimated service lives. Leasehold improvements are amortized over the shorter of the life of the asset or the related lease term. Estimated useful lives of the principal classes of assets are as follows:
| Lab equipment | 5 – 7 years |
| Computer hardware and software | 3 – 7 years |
| Leasehold improvements | 2 – 5 years or the term of the lease, if shorter |
| Furniture, fixtures and equipment | 5 – 10 years |
| 69 |
The Company reviews its property and equipment for impairment annually or whenever events or changes in circumstances indicate that the carrying value of such assets may not be recoverable. There were no impairment losses recognized in 2017, 2016, and 2015.
Depreciation and amortization of property and equipment amounted to $457,000, $432,000 and $335,000 for the years ended December 31, 2017, 2016 and 2015, respectively.
Research and Development
Research and development costs are charged to operations in the period incurred and amounted to $7.9 million, $5.3 million and $3.0 million net of grant revenue, for the years ended December 31, 2017, 2016 and 2015, respectively.
Patent Costs
The Company has over 400 domestic and foreign patents at December 31, 2017. Due to the uncertainty associated with the successful development of one or more commercially viable products based on Company’s research efforts and any related patent applications, all patent costs, including patent-related legal, filing fees and other costs, including internally generated costs, are expensed as incurred. Patent costs were $684,000, $652,000 and $679,000 for the years ended December 31, 2017, 2016 and 2015, respectively, and are included in general and administrative expenses in the consolidated statements of operations.
Revenue Recognition
The Company’s revenue is derived primarily from the sale of its Argus II retinal implant, which is implanted during retinal surgery to restore some functional vision to patients blinded by Retinitis Pigmentosa. The Company sells to a variety of customers including university hospitals, large medical centers and distributors.
Revenue is recognized when persuasive evidence of an arrangement exists, the fee is fixed or determinable, collectability is probable, and delivery has occurred.
Revenue is generated under sales agreements with multiple deliverables (multiple-element arrangements), comprising the following deliverables:
| ● | Hospital start up kits (one per site), |
| ● | Surgical support, |
| ● | Training, and |
| ● | The Argus II System |
The deliverables may vary by transaction.
The Company evaluates each deliverable in a multiple-element arrangement to determine whether it represents a separate unit of accounting. An element constitutes a separate unit of accounting when the delivered item has standalone value and delivery of the undelivered element is probable and within the Company’s control. The Company has determined that the elements listed above do not have standalone value to the customer until delivery of all components has occurred. Accordingly, revenue from multiple-element arrangements is recognized when delivery of all of deliverables has taken place and all other revenue recognition criteria have been met. Generally, revenue recognition occurs at the time of implantation, but revenue recognition can be delayed if certain training has not been delivered to the implanting sites, or if other revenue recognition criteria have not been met.
In the United States, the amount of revenue recognized per unit has been limited in some situations due to the uncertainties of the reimbursement environment and payment terms. In such cases, revenue is not recognized until the consideration becomes fixed, generally when paid to the Company.
In order to determine whether collection is reasonably assured, the Company assesses a number of factors, including creditworthiness of the customer and medical insurance coverage. The Company may periodically grant extended payment terms to customers. In such situations, the Company defers the recognition of revenue until collection becomes probable, which is generally upon receipt of payment.
| 70 |
The Company also sells surgical supplies to customers and recognizes revenue on these products when they are shipped and other revenue recognition criteria have been met.
The Company sells through distributors in certain countries. The Company provides these distributors with clinical start-up kits, surgical supplies and the Argus II System, as well as training them to provide pre- and post-surgical support. The Company monitors the surgery. Other than surgical support which is provided by the Company, the distributor is responsible for delivering products and services to its customers. In the past, the Company has allowed distributors to return or exchange products in certain situations. Due to the Company’s continuing involvement and its returns policy, the Company recognizes revenue from distributors when the implantation procedure has been performed by the distributor’s customer, and all other revenue recognition criteria between the Company and the distributor have been met.
Grant Receipts and Liabilities
From time to time, the Company receives grants that help fund specific development programs. Any amounts received pursuant to grants are offset against the related operating expenses as the costs are incurred. During the years ended December 31, 2017, 2016 and 2015 grants offset against operating expenses were $0.4 million, $2.4 million and $1.9 million, respectively.
Concentration of Risk
Credit Risk
Financial instruments that subject the Company to concentrations of credit risk consist primarily of cash, money market funds, and trade accounts receivable. The Company maintains cash and money market funds with financial institutions that management deems reputable, and at times, cash balances may be in excess of FDIC and SIPC insurance limits of $250,000 and $500,000 (including cash of $250,000), respectively. The Company extends differing levels of credit to customers, and typically does not require collateral.
The Company also maintains a cash balance at a bank in Switzerland. Accounts at such bank are insured up to an amount specified by the deposit insurance agency of Switzerland.
Customer Concentration
During the years ended December 31, 2017, 2016 and 2015, one customer represented 10%, 13%, and 14% of revenue, respectively. No other customer represented 10% or more of revenue in any year.
| 71 |
As of December 31, 2017 and 2016, the following customers comprised more than 10% accounts receivable:
| 2017 | 2016 | |||||||
| Customer 1 | 17 | % | 0 | % | ||||
| Customer 2 | 16 | % | 0 | % | ||||
| Customer 3 | 11 | % | 0 | % | ||||
| Customer 4 | 0 | % | 34 | % | ||||
| Customer 5 | 0 | % | 34 | % | ||||
| Customer 6 | 0 | % | 29 | % | ||||
Geographic Concentration
During the years ended December 31, 2017, 2016 and 2015, regional revenue, based on customer locations which comprised more than 10% of revenues, consisted of the following:
| 2017 | 2016 | 2015 | ||||||||||
| United States | 53 | % | 47 | % | 46 | % | ||||||
| Italy | 13 | % | 17 | % | 20 | % | ||||||
| France | 8 | % | 9 | % | 16 | % | ||||||
| Germany | 3 | % | 12 | % | 6 | % | ||||||
Sources of Supply
Several of the components, materials and services used in the Company’s current Argus II product are available from only one supplier, and substitutes for these items cannot be obtained easily or would require substantial design or manufacturing modifications. Any significant problem experienced by one of the Company’s sole source suppliers could result in a delay or interruption in the supply of components to the Company until that supplier cures the problem or an alternative source of the component is located and qualified. Even where the Company could qualify alternative suppliers, the substitution of suppliers may be at a higher cost and cause time delays that impede the commercial production of the Argus II, reduce gross profit margins and impact the Company’s abilities to deliver its products as may be timely required to meet demand.
Foreign Operations
The accompanying consolidated financial statements as of December 31, 2017 and 2016 include assets amounting to approximately $2.7 million and $1.7 million, respectively, relating to operations of the Company in Switzerland. It is always possible unanticipated events in foreign countries could disrupt the Company’s operations.
Fair Value of Financial Instruments
The authoritative guidance with respect to fair value establishes a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value into three levels, and requires that assets and liabilities carried at fair value be classified and disclosed in one of three categories, as presented below. Disclosure as to transfers in and out of Levels 1 and 2, and activity in Level 3 fair value measurements, is also required.
Level 1. Observable inputs such as quoted prices in active markets for an identical asset or liability that the Company has the ability to access as of the measurement date. Financial assets and liabilities utilizing Level 1 inputs include active-exchange traded securities and exchange-based derivatives.
Level 2. Inputs, other than quoted prices included within Level 1, which are directly observable for the asset or liability or indirectly observable through corroboration with observable market data. Financial assets and liabilities utilizing Level 2 inputs include fixed income securities, non-exchange based derivatives, mutual funds, and fair-value hedges.
Level 3. Unobservable inputs in which there is little or no market data for the asset or liability which requires the reporting entity to develop its own assumptions. Financial assets and liabilities utilizing Level 3 inputs include infrequently-traded non-exchange-based derivatives and commingled investment funds, and are measured using present value pricing models.
| 72 |
The Company determines the level in the fair value hierarchy within which each fair value measurement falls in its entirety, based on the lowest level input that is significant to the fair value measurement in its entirety. In determining the appropriate levels, the Company performs an analysis of the assets and liabilities at each reporting period end.
Money market funds are the only financial instrument that is measured and recorded at fair value on the Company’s balance sheet, and they are considered Level 1 valuation securities in both 2017 and 2016.
Stock-Based Compensation
Pursuant to Financial Accounting Standards Board (“FASB”) ASC 718 Share-Based Payment (“ASC 718”), the Company records stock-based compensation expense for all stock-based awards.
Under ASC 718, the Company estimates the fair value of stock options granted using the Black-Scholes option pricing model. The fair value for awards that are expected to vest is then amortized on a straight-line basis over the requisite service period of the award, which is generally the option vesting term.
The fair value of each stock option award is estimated on the date of grant using the Black-Scholes option valuation model. The assumptions used in the Black-Scholes valuation model are as follows:
| ● | The grant price of the issuances, is determined based on the fair value of the shares at the date of grant. |
| ● | The risk free interest rate for periods within the contractual life of the option is based on the U.S. treasury yield in effect at the time of grant. |
| ● | As permitted by SAB 107, due to the Company’s insufficient history of option activity, management utilizes the simplified approach to estimate the options expected term, which represents the period of time that options granted are expected to be outstanding. |
| ● | Volatility is determined based on average historical volatilities of comparable companies in similar industry. |
| ● | Expected dividend yield is based on current yield at the grant date or the average dividend yield over the historical period. The Company has never declared or paid dividends and has no plans to do so in the foreseeable future. |
Long Term Investor Right
Each beneficial owner (“IPO Shareholder”) of the Company’s common stock, who purchased shares directly in the offering (“IPO Shares”), was eligible to receive up to one additional share of common stock from the Company for each share purchased in the offering (“IPO Supplemental Shares”) pursuant to the Long Term Investor Right that was included with each IPO Share. To receive IPO Supplemental Shares, within 90 days following the closing date of the offering, or by February 22, 2015, an IPO Shareholder was required to take action to become the direct registered owner of its IPO Shares. Furthermore, IPO Shareholders were required to hold their IPO Shares in their own name and not place them in “street name” or trade them at any time during the 24 month period immediately following the IPO closing date. This Long Term Investors Right was non-detachable and transferable only in limited circumstances.
The formula to determine the number of IPO Supplemental Shares issued on a trigger of the Long Term Investor Right was: (i) $18.00 minus (ii) the average of the highest consecutive closing prices in any 90 day trading period on the principal exchange during the two years after the IPO closing date (the “Measurement Average”) divided by the Measurement Average. Fractional shares issuable to a qualifying IPO Shareholder resulting from the calculation were rounded up to the next whole share of Common Stock, taking into account the aggregate number of Long Term Investor Rights of a holder. Since the highest average of consecutive closing prices over any 90 calendar day period was $13.96 per share, each Long-Term Investor Right was entitled to 0.2894 additional shares of common stock, which is calculated as: ($18.00 - $13.96)/$13.96.
| 73 |
Shortly after the second anniversary of the IPO closing date, an independent public accountant verified the above formula calculation and determined which IPO Shareholders qualified to receive IPO Supplemental Shares. In total, 355,095 shares were distributed to IPO Shareholders’ accounts.
The Long Term Investor Right was an equity instrument that was accounted for as a component of the actual price per common share paid by the investor in the IPO. For basic earnings per share, the common shares associated with the Long Term Investor Right were treated as contingently issuable shares and were not included in basic earnings per share until the actual number of shares were issued in November 2016.
Comprehensive Income or Loss
The Company complies with provisions of FASB ASC 220, Comprehensive Income, which requires companies to report all changes in equity during a period, except those resulting from investment by owners and distributions to owners, for the period in which they are recognized. Comprehensive income is defined as the change in equity during a period from transactions and other events from non-owner sources.
Comprehensive and other comprehensive income (loss) is reported on the face of the financial statements. For the years ended December 31, 2017, 2016 and 2015 comprehensive income (loss) is the total of net income (loss) and other comprehensive income (loss) which, for the Company, consists entirely of foreign currency translation adjustments and there were no material reclassifications from other comprehensive loss to net loss during the years ended December 31, 2017, 2016 and 2015.
Foreign Currency Translation and Transactions
The financial statements and transactions of the subsidiary’s operations are reported in the local (functional) currency of Swiss francs (CHF) and translated into US dollars in accordance with U.S. GAAP. Assets and liabilities of those operations are translated at exchange rates in effect at the balance sheet date. The resulting gains and losses from translating foreign currency financial statements are recorded as other comprehensive income (loss). Revenues and expenses are translated at the average exchange rate for the reporting period. Foreign currency transaction gains (losses) resulting from exchange rate fluctuations on transactions denominated in a currency other than the foreign operations’ functional currencies are included in expenses in the consolidated statements of operations.
Income Taxes
The Company accounts for income taxes under an asset and liability approach for financial accounting and reporting for income taxes. Accordingly, the Company recognizes deferred tax assets and liabilities for the expected impact of differences between the financial statements and the tax basis of assets and liabilities.
The Company records a valuation allowance to reduce its deferred tax assets to the amount that is more likely than not to be realized. In the event the Company was to determine that it would be able to realize its deferred tax assets in the future in excess of its recorded amount, an adjustment to the deferred tax assets would be credited to operations in the period such determination was made. Likewise, should the Company determine that it would not be able to realize all or part of its deferred tax assets in the future, an adjustment to the deferred tax assets would be charged to operations in the period such determination was made. The Company has incurred losses for tax purposes since inception and has significant tax losses and tax credit carryforwards.
As of December 31, 2017 pursuant to an analysis done under Section 382, Limitations on Net Operating Losses, of the Internal Revenue Code of 1986, as amended, the Company had $44.5 million and $32.9 million of federal and state operating loss carryforwards, respectively, with which to offset any future taxable income. The federal and state net operating loss carryforwards will begin to expire at various dates from 2033 through 2037. If these loss carryforwards are unavailable for use in future periods, the Company’s results of operations and financial position may be adversely affected.
The Company experienced an “ownership change” within the meaning of Section 382(g) of the Internal Revenue Code of 1986, as amended, during the second quarter of 2017. The ownership change will subject the Company’s net operating loss carryforwards to an annual limitation, which will significantly restrict the Company’s ability to use them to offset taxable income in periods following the ownership change. In general, the annual use limitation equals the aggregate value of the Company’s stock at the time of the ownership change multiplied by a tax-exempt interest rate specified by the Internal Revenue Service. The Company has analyzed the available information to determine the amount of the annual limitation. Based on information available to the Company, the limitation arising from this ownership change is estimated to range between $1.4 million and $3.7 million annually. In total, the Company estimates that the 2017 ownership change will result in approximately $120 million and $56 million of federal and state net operating loss carryforwards, respectively, expiring unused.
On December 22, 2017, the President of the United States signed and enacted into law H.R. 1 (the “Tax Reform Law”). The Tax Reform Law, effective for tax years beginning on or after January 1, 2018, except for certain provisions, resulted in significant changes to existing United States tax law, including various provisions that are expected to impact the Company. The Tax Reform Law reduces the federal corporate tax rate from 35% to 21% effective January 1, 2018. The Company will continue to analyze the provisions of the Tax Reform Law to assess the impact on the Company’s consolidated financial statements.
74
Product Warranties
The Company’s policy is to warrant all shipped products against defects in materials and workmanship for up to two years by replacing failed parts. The Company also provides a three-year manufacturer’s warranty covering implant failure by providing a functionally-equivalent replacement implant. Accruals for product warranties are estimated based on historical warranty experience and current product performance trends, and are recorded at the time revenue is recognized as a component of cost of sales. The warranty liabilities are reduced by material and labor costs used to replace parts over the warranty period in the periods in which the costs are incurred. The Company periodically assesses the adequacy of its recorded warranty liabilities and adjusts the amounts as necessary. Although any such adjustments were not material in the years ended December 31, 2017, 2016 and 2015, any such adjustments could be material in the future if estimates differ significantly from actual warranty expense. The warranty liabilities are included in accrued expenses in the consolidated balance sheets.
Presentation of sales and value added taxes
The Company collects value added tax on its sales in Europe and certain states in the United Sates impose a sales tax on the Company’s sales to nonexempt customers. The Company collects that valued added and sales tax from customers and remits the entire amount to the respective authorities. The Company’s accounting policy is to exclude the tax collected and remitted to the authorities from revenues and cost of revenues.
Net Loss per Share
The Company’s computation of earnings per share (“EPS”) includes basic and diluted EPS. Basic EPS is measured as the income (loss) available to common shareholders divided by the weighted average number of common shares outstanding for the period. Diluted EPS is similar to basic EPS but presents the dilutive effect on a per share basis of potential common shares (e.g., convertible notes payable, convertible preferred stock, preferred stock warrants and common stock options) as if they had been converted at the beginning of the periods presented, or issuance date, if later. Potential common shares that have an anti-dilutive effect (i.e., those that increase income per share or decrease loss per share) are excluded from the calculation of diluted EPS.
Loss per common share is computed by dividing net loss by the weighted average number of shares of common stock outstanding during the respective periods. Basic and diluted loss per common share is the same for all periods presented because all common stock warrants and common stock options outstanding were anti-dilutive.
At December 31, 2017, 2016 and 2015, the Company excluded the outstanding securities summarized below, which entitle the holders thereof to ultimately acquire shares of common stock, from its calculation of earnings per share, as their effect would have been anti-dilutive (in thousands).
| 2017 | 2016 | 2015 | ||||||||||
| Long Term Investor Rights | — | — | 400 | |||||||||
| Underwriter’s warrants | 802 | 802 | 802 | |||||||||
| Warrants associated with convertible debt | 676 | 1,039 | 1,039 | |||||||||
| Warrants associated with 2017 Rights Offering | 13,652 | — | — | |||||||||
| Common stock options | 5,675 | 3,667 | 3,472 | |||||||||
| Restricted stock units | 83 | 131 | 190 | |||||||||
| Employee stock purchase plan | 271 | 206 | 93 | |||||||||
| Total | 21,159 | 5,845 | 5,996 | |||||||||
75
Recently Adopted Accounting Standards
In August 2014, the FASB issued Accounting Standards Update No. 2014-15 (ASU 2014-15), Presentation of Financial Statements — Going Concern (Subtopic 205-10). ASU 2014-15 provided guidance as to management’s responsibility to evaluate whether there is substantial doubt about an entity’s ability to continue as a going concern and to provide related footnote disclosures. In connection with preparing these financial statement management evaluated whether there are conditions or events, considered in the aggregate, that raise substantial doubt about the Company’s ability to continue as a going concern within one year after the date that the financial statements are issued. As fully described in Note 1, the Company believes that it does not have sufficient funds to support its operations through the end of second quarter of 2018.
In March 2016, the FASB issued ASU 2016-09, Compensation—Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting. ASU 2016-09 changes how companies account for certain aspects of share-based payment awards to employees, including the accounting for income taxes, forfeitures and statutory tax withholding requirements, as well as classification in the statement of cash flows. ASU 2016-09 is effective for annual periods beginning after December 15, 2016, including interim periods within those annual periods. If an entity early adopts in an interim period, any adjustments should be reflected as of the beginning of the fiscal year that includes that interim period and the entity must adopt all of the amendments from ASU 2016-09 in the same period. Management has determined that adoption of this standard did not have a material effect to the financial statements and related disclosures.
Recent Accounting Pronouncements
In February 2016, the FASB issued ASU 2016-02, Leases (Topic 842), which supersedes all existing guidance on accounting for leases in ASC Topic 840. ASU 2016-02 is intended to provide enhanced transparency and comparability by requiring lessees to record right-of-use assets and corresponding lease liabilities on the balance sheet. ASU 2016-02 will continue to classify leases as either finance or operating, with classification affecting the pattern of expense recognition in the statement of income. ASU 2016-02 is effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years. Early adoption is permitted. ASU 2016-02 is required to be applied with a modified retrospective approach to each prior reporting period presented with various optional practical expedients. The Company generally does not finance purchases of equipment or other capital, but does lease its facilities. While the Company is continuing to assess all potential impact of this standard, it expects most of its lease commitments will be subject to the updated standard and recognized as lease liabilities and right-of-use assets upon adoption.
In May 2017, the FASB issued ASU No. 2017-09, “Compensation – Stock Compensation (Topic 718) – Scope of Modification Accounting.” ASU No. 2017-09 provides clarity and reduces complexity when applying the guidance in Topic 718 for changes in terms or conditions of share-based payment awards. It is effective for annual reporting periods beginning after December 15, 2017. The Company is currently evaluating the impact the adoption of this new standard will have on its financial statements. While the Company is continuing to assess all potential impact of this standard, it expects most of its lease commitments will be subject to the updated standard and recognized as lease liabilities and right-of-use assets upon adoption.
76
In May 2014, the FASB issued Accounting Standards Update (“ASU”) No. 2014-09-Revenue from Contracts with Customers (Topic 606) (“ASU 2014-09”), which provides new guidance for revenue recognition. The Financial Accounting Standards Board (“FASB”) subsequently issued ASU No. 2015-14-Revenue from Contracts with Customers (Topic 606), which deferred the effective date of ASU 2014-09, ASU No. 2016-08-Revenue from Contracts with Customers (Topic 606): Principal versus Agent Considerations (Reporting Revenue Gross versus Net), ASU No. 2016-10-Revenue from Contracts with Customers (Topic 606): Identifying Performance Obligations and Licensing, ASU No. 2016-12-Revenue from Contracts with Customers (Topic 606): Narrow-Scope Improvements and Practical Expedients, and ASU No. 2016-20-Technical Corrections and Improvements to Topic 606, Revenue from Contracts with Customers. The above subsequent ASUs did not change the core principle of the guidance in ASU 2014-09. The ASUs referred to above collectively will supersede and replace the revenue recognition requirements in ASC Topic 605-Revenue Recognition, and most of the related industry specific guidance and replace them with ASC Topic 606, Revenue from Contracts with Customers (“ASC 606”). The core principle in ASC 606 is that revenue is recognized when promised goods or services are transferred to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. To achieve that core principle, an entity should apply the following steps:
Step 1: Identify the contract(s) with a customer
Step 2: Identify the performance obligations in the contract.
Step 3: Determine the transaction price.
Step 4: Allocate the transaction price to the performance obligations in the contract.
Step 5: Recognize revenue when (or as) the entity satisfies a performance obligation.
ASU 2014-09 also creates ASC Subtopic 340-40-Other Assets and Deferred Costs-Contracts with Customers (“ASC 340-40”), which requires an entity to recognize an asset certain types of costs related to a contract with a customer within the scope of ASC 606 and amortize the asset over a period consistent with the transfer of the goods and services to which the asset relates. Specifically, the costs required to be capitalized are (a) incremental costs of obtaining a contract with a customer and (b) costs incurred in fulfilling a contract with a customer that are not in the scope of another ASC Topic.
ASC 606 and ASC 340-40 (the “new accounting standards”) require the Company to make significant judgments and estimates. The new accounting standards also require more extensive disclosures regarding the nature, amount, timing and uncertainty of revenue and cash flows arising from contracts with customers. The Company has adopted the new accounting standards as of January 1, 2018 using the modified retrospective transition method, in which the two new accounting standards were applied retrospectively with the cumulative effect of initially applying the new accounting standards as an adjustment to the opening balance of retained earnings at January 1, 2018, the date of initial adoption. In accordance with the modified retrospective transition method, the Company applied the new guidance retrospectively only to contracts that were not completed contracts at January 1, 2018.
Also in accordance with the modified retrospective transition method, the Company will provide additional disclosures in its financial statements for each of the quarterly and annual reporting periods in 2018 of (a) the amount by which each financial statement line item is affected in the reporting period by the application of the new accounting standards as compared to the accounting guidance that was in effect before the change, and (b) an explanation of the reasons for significant changes identified.
The Company completed its assessment of adoption of ASC 606 2016, and is currently in the process of updating that assessment to reflect changes in contractual terms and the Company’s customary business practices since completion of the initial assessment.
The Company believes the financial statement impact of the expected changes will be minimal.
77
Management believes that any other recently issued, but not yet effective, authoritative guidance, if currently adopted, would not have a material impact on the Company’s financial statement presentation or disclosures.
3. Money Market Funds
Money market funds at December 31, 2017 totaled $7,235,000 and consisted of $698,000 in the City National Rochdale Government Fund Class S, $6,366,000 in the FFI Institutional Fund, and $171,000 held in a deposit account in Switzerland as security for the performance of contracts. Money market funds at December 31, 2016 totaled $10,336,000 and consisted of $218,000 in the City National Rochdale Government Fund Class S, $9,995,000 in the FFI Institutional Fund, and $123,000 held in a deposit account in Switzerland as security for the performance of contracts.
The investment objective of the City National Rochdale Government Money Market Fund is to preserve principal and maintain a high degree of liquidity while providing current income through a portfolio of liquid, high quality, short-term U.S. Government bonds and notes, at least 80% of which is in U.S. Government securities. The City National Rochdale Government Money Market Fund is managed by City National Rochdale, LLC. The investment objective of the FFI Institutional Fund, managed by Merrill Lynch, is to seek maximum current income consistent with liquidity and the maintenance of a portfolio of high-quality, short-term money market securities.
The following table presents money market funds at their level within the fair value hierarchy at December 31, 2017 and 2016 (in thousands).
| Total | Level 1 | Level 2 | Level 3 | |||||||||||||
| December 31, 2017: | ||||||||||||||||
| Money market funds | $ | 7,235 | $ | 7,235 | $ | — | $ | — | ||||||||
| December 31, 2016: | ||||||||||||||||
| Money market funds | $ | 10,336 | $ | 10,336 | $ | — | $ | — | ||||||||
4. Selected Balance Sheet Detail
Inventories, net
Inventories consisted of the following at December 31, 2017 and 2016 (in thousands):
| 2017 | 2016 | |||||||
| Raw materials | $ | 485 | $ | 477 | ||||
| Work in process | 2,620 | 5,032 | ||||||
| Finished goods | 1,660 | 3,284 | ||||||
| 4,765 | 8,793 | |||||||
| Allowance for excess and obsolescence | (2,065 | ) | (5,377 | ) | ||||
| Inventories, net | $ | 2,700 | $ | 3,416 | ||||
During the year-ended December 31, 2017, the Company reversed $ 3.1 million of the 2016 charge for excess inventory based upon increased sales volumes in 2017. During the year-ended December 31, 2016, the Company recorded a charge of $4.7 million for excess inventory determined by management based on projected sales volumes in 2017.
78
Property and equipment, net of accumulated depreciation and amortization
Property and equipment consisted of the following at December 31, 2017 and 2016 (in thousands):
| 2017 | 2016 | |||||||
| Laboratory equipment | $ | 2,450 | $ | 2,300 | ||||
| Computer hardware and software | 1,329 | 1,220 | ||||||
| Leasehold improvements | 298 | 288 | ||||||
| Furniture, fixtures and equipment | 46 | 45 | ||||||
| 4,123 | 3,853 | |||||||
| Accumulated depreciation and amortization | (2,824 | ) | (2,364 | ) | ||||
| Property and equipment, net | $ | 1,299 | $ | 1,489 | ||||
5. Grants
In September 2014, the Company entered into a Joint Research and Development Agreement or JRDA with The Johns Hopkins University Applied Physics Laboratory or APL. The JRDA includes a subcontract to do research under a grant received by APL. Under the JRDA, the Company has agreed to perform research regarding integration of APL research in to a visual prosthesis system. In October, 2014, APL paid the Company $4.1 million in one lump sum to conduct its portion of the research. The JRDA also includes a license from APL to the Company, for the life of any patents resulting from APL’s portion of the research. The APL portion of the research includes image processing enhancements for a visual prosthesis. In exchange for the license, the Company issued 1,000 shares of its common stock to APL, has agreed to pay APL patent prosecution costs, and to pay APL a royalty of 0.25% of net sales of licensed products. The Company recorded funding under the grant as an offset to research and development expenses of $0.1 million in 2017, $2.1 million in 2016, and $1.9 million in 2015.
6. Warrants
Warrants Associated with Convertible Debt
During 2012 and 2013, the Company borrowed money primarily from then existing investors through the issuance of convertible promissory notes (collectively, the “Convertible Notes”) totaling $29.5 million. The Convertible Notes accrued interest at the rate of 7.5% per annum, which was added to the principal amounts. At the time of the Company’s November 2014 IPO, and in accordance with their original terms, the Convertible Notes were converted into 6.6 million shares of the Company’s common stock.
In connection with the Convertible Notes, the Company issued warrants to purchase 1.2 million shares of the Company’s common stock at a price of $5.00 per share. Until their expiration date, the warrants could be exercised at any time, and from time to time, in whole or in part. In accordance with their amended terms, the warrants expire on the earlier of their expiration dates or upon a change in control event. The 361,909 warrants associated with the Convertible Notes issued in 2012 expired on July 31, 2017. The warrants associated with the Convertible Notes issued in 2013 had an expiration date of February 28, 2018. As of December 31, 2017, there were outstanding warrants associated with the Convertible Notes to purchase 676,494 shares of the Company’s common stock, of which warrants for 240,000 shares were held by a related party.
79
Underwriter’s Warrant
As a component of the IPO underwriting fee, the Company granted the underwriter a warrant to purchase 805,000 shares of the Company’s common stock at an exercise price of $11.25 per share, which was 25 percent above the offering price to the investors. The warrant is exercisable, in whole or in part, for a period commencing 180 days after the effective date of the registration statement (November 18, 2014) and ending on the fifth anniversary date of the effective date of the registration statement. Underwriter’s warrants to purchase 802,000 of the Company’s common stock are still outstanding at December 31, 2017.
Warrants from March 2017 Right Offering
On March 6, 2017, the Company completed a registered Rights Offering to existing stockholders in which it sold 13.7 million Units at $1.47 per Unit, which was the closing price of the Company common stock on that date. Each Unit consisted of a share of the Company’s common stock and a warrant to purchase an additional share of the Company’s stock for $1.47. The warrants have a five-year life and have been approved for trading on Nasdaq under the symbol EYESW. None of the warrants associated with the Rights Offering had been converted as of December 31, 2017. (See Rights Offerings in Note 8.)
A summary of warrant activity for the years ended December 31, 2017, 2016 and 2015 is presented below (in thousands, except per share and contractual life data):
| Number of Shares | Weighted Average Exercise Price | Weighted Average Remaining Contractual Life (in Years) | ||||||||||
| Warrants outstanding at December 31, 2014 | 1,984 | $ | 5.00 | |||||||||
| Granted | — | — | ||||||||||
| Exercised | (144 | ) | 5.13 | |||||||||
| Forfeited or expired | — | — | ||||||||||
| Warrants outstanding at December 31, 2015 | 1,840 | $ | 7.72 | |||||||||
| Granted | — | — | ||||||||||
| Exercised | — | — | ||||||||||
| Forfeited or expired | — | — | ||||||||||
| Warrants outstanding at December 31, 2016 | 1,840 | $ | 7.72 | |||||||||
| Granted | 13,652 | 1.47 | ||||||||||
| Exercised | — | — | ||||||||||
| Forfeited or expired | (362 | ) | 5.00 | |||||||||
| Warrants outstanding at December 31, 2017 | 15,130 | $ | 2.15 | 3.90 | ||||||||
| Warrants exercisable at December 31, 2017 | 15,130 | $ | 2.15 | 3.90 | ||||||||
80
The estimated aggregate intrinsic value of warrants exercisable at December 31, 2017 was approximately $6.0 million.
7. Employee Benefit Plans
The Company has a 401(k) Savings Retirement Plan that covers substantially all full-time employees who meet the plan’s eligibility requirements and provides for an employee elective contribution. The Plan provides for employer matching contributions. Employer contributions are discretionary and determined annually by the Board of Directors. For the years ended December 31, 2017, 2016 and 2015, employer contributions to the Plan totaled $142,000, $147,000 and $137,000, respectively.
The Company is required to contribute to a government-sponsored pension plan for the employees of its Switzerland-based subsidiary. For the years ended December 31, 2017, 2016 and 2015, the employer’s portion of the amounts contributed to the subsidiary’s pension plan on behalf of those employees was $139,000, $132,000 and $134,000, respectively.
8. Equity Securities
In June 2014, the Company’s articles of incorporation were amended to increase authorized common shares to 200,000,000, no par value, and to authorize 10,000,000 shares of preferred stock, no par value. The Company’s consolidated financial statements have been retroactively restated to reflect this amendment. The Board of Directors has the authority to establish the rights, preferences, privileges and restrictions granted to and imposed upon the holders of preferred stock and common stock.
Long Term Investor Right
As of November 24, 2016, the Company identified investors who had perfected and maintained Long Term Investor Rights in an aggregate of 1,226,854 shares of common stock that were acquired as part of the Company’s IPO. The highest average closing price for the Company’s common stock on Nasdaq during any consecutive 90 day period ended on or before November 24, 2016 was $13.96. Based on this average closing stock price, an investor who purchased shares as part of the IPO, and who has perfected its Long Term Investor Right, was entitled to 0.2894 shares for each share purchased in the IPO, rounded up to the next whole share, which represents an aggregate of 355,095 shares.
Subsequent to November 24, 2016, the two-year anniversary of the Company’s IPO, the Company distributed 355,095 shares of its common stock to IPO investors who met the qualifying terms of the Long Term Investor Right (LTIR). The shares distributed in connection with the LTIR have been accounted for as an equity transaction in the Company’s Consolidated Statement of Stockholders’ Equity and had no impact on the Consolidated Statements of Operations.
81
Common Stock Issuable
Beginning with services rendered in 2014, and with the first payment in June 2015, non-employee members of the Board of Directors were paid for their services in common stock on June 1 of each year based on the average closing prices for the immediately preceding twenty trading days. For 2017, for these services the Company issued 223,000 shares with a value of $262,000 and accrued $153,000, which equates to 82,000 shares based on the average closing price of $1.86 for the Company’s common stock during last 20 trading days as of December 31, 2017. For 2016, for these services the Company issued 82,000 shares with a value of $324,000 and accrued $153,000, which equates to 77,000 shares based on the average closing price of $1.98 for the Company’s common stock during last 20 trading days as of December 31, 2016. For 2015, for these services the Company issued 23,136 shares with a value of $285,000 and accrued $205,000, which equates to 33,293 shares based on the average closing price of $6.15 for the Company’s common stock during last 20 trading days as of December 31, 2015. The shares, which have not yet been issued, are excluded from the calculation of weighted average common shares outstanding for EPS purposes.
Rights Offerings
In June 2016, the Company completed a Rights Offering to existing stockholders, raising proceeds of $19.5 million net of cash offering costs, and selling 5,978,465 shares of common stock at $3.315 per share, representing 85% of the Company’s stock price at the close of the rights offering. The Company evaluated the financial impact of FASB ASC 260, “Earnings per Share,” which states, among other things, that if a rights issue is offered to all existing stockholders at an exercise price that is less than the fair value of the stock, then the weighted average shares outstanding and basic and diluted earnings per share shall be adjusted retroactively to reflect the bonus element of the rights offering for all periods presented. The Company determined that the application of this specific provision of ASC 260 was immaterial to previously issued financial statements and, therefore, did not retroactively adjust previously reported weighted average shares outstanding and basic and diluted earnings per share.
On March 6, 2017, the Company completed a registered Rights Offering to existing stockholders in which it sold 13.7 million Units at $1.47 per Unit, which was the closing price of the Company common stock on that date. Each Unit consisted of a share of the Company’s common stock and a warrant to purchase an additional share of the Company’s stock for $1.47. The warrants have a five-year life and have been approved for trading on Nasdaq under the symbol EYESW. At the Company’s discretion, the warrants are redeemable on 30 days’ notice (i) at any time 24 months after the date of issuance, (ii) if the shares of the Company’s common stock are trading at $2.94, which is 200% of the Subscription Price, for 15 consecutive trading days and (iii) if all of the independent directors vote in favor of redeeming the warrants. Holders may be able to sell or exercise warrants prior to any announced redemption date and the Company will redeem outstanding warrants not exercised by the announced redemption date for a nominal amount of $0.01 per Warrant.
At-the-Market Sales Agreement
During December 2017, the Company issued 598,276 shares of common stock for gross proceeds of approximately $1.2 million as part of its At-the-Market (“ATM”) sales agreement with two different investment banks. The Company paid expenses of approximately $0.1 million resulting in net proceeds of $1.1 million. In the period from January 1, 2018 to February 28, 2018, the Company sold approximately 2.2 million additional shares through its ATM sales agreement, raising gross proceeds of approximately $4.1 million and net proceeds of approximately $4.0 million after expenses.
9. Stock-Based Compensation
Under the 2003 Plan, as restated in June 2011, the Company was authorized to issue options covering up to 3,500,000 common stock shares. Effective June 1, 2011, the Company adopted the 2011 Equity Incentive Plan (the “2011 Plan”). The maximum number of shares with respect to which options may be granted under the 2011 Plan is 7,500,000 shares, which is offset and reduced by options previously granted under the 2003 Plan. The option price is determined by the Board of Directors but cannot be less than the fair value of the shares at the grant date. Generally, the options vest ratably over either four or five years and expire ten years from the grant date. Both plans provide for accelerated vesting if there is a change of control, as defined in the plans.
82
On May 15, 2015 shareholders approved (1) an increase of 2,000,000 shares in the number of shares available for option awards under the 2011 Equity Incentive Plan, and (2) an Employee Stock Purchase Plan, with an initial 250,000 shares with annual increases of shares available equal to the lesser of (i) 1% of outstanding shares or (ii) 100,000 shares. On May 10, 2016 shareholders approved an increase of 1,500,000 shares in the number of shares available for option awards under the 2011 Equity Incentive Plan.
No option shall be granted under the 2011 Plan after May 31, 2021.
The Company recognized stock-based compensation cost of $3,784,000, $3,367,000 and $2,687,000 during 2017, 2016 and 2015, respectively. The calculated value of each option grant was estimated on the date of grant using the Black-Scholes option-pricing model with the following assumptions:
| 2017 | 2016 | 2015 | ||||||||||
| Risk-free interest rate | 1.92% – 2.25% | 1.40% – 2.03% | 1.93%–2.21% | |||||||||
| Expected dividend yield | 0% | 0% | 0% | |||||||||
| Expected volatility | 48.0% | 47.6% – 48.2% | 47.5%–50.4% | |||||||||
| Expected term | 6.25 years | 6.25 years | 6.25–6.5 years | |||||||||
| Weighted-average grant date calculated fair value | $ | 0.90 | $ | 1.97 | $ | 6.17 | ||||||
As the Company has limited stock trading history, the expected volatility is based on the historical volatility of similar companies that have a trading history. The expected term represents the estimated average period of time that the options are expected to remain outstanding. Since the Company does not have sufficient historical data on the exercise of stock options, the expected term is based on the “simplified” method that measures the expected term as the average of the vesting period and the contractual term. The risk free rate of return reflects the grant date interest rate offered for zero coupon U.S. Treasury bonds over the expected term of the options.
A summary of stock option activity for the years ended December 31, 2017, 2016 and 2015 is presented below (in thousands, except per share and contractual life data):
| Number of Shares | Weighted Average Exercise Price | Weighted Average Remaining Contractual Life (in Years) | ||||||||||
| Options outstanding at December 31, 2014 | 3,252 | $ | 6.07 | |||||||||
| Granted | 998 | 12.29 | ||||||||||
| Exercised | (574 | ) | 4.85 | |||||||||
| Forfeited or expired | (204 | ) | 7.08 | |||||||||
| Options outstanding at December 31, 2015 | 3,472 | $ | 8.01 | |||||||||
| Granted | 745 | 4.18 | ||||||||||
| Exercised | (96 | ) | 5.00 | |||||||||
| Forfeited or expired | (454 | ) | 8.66 | |||||||||
| Options outstanding at December 31, 2016 | 3,667 | $ | 7.23 | |||||||||
| Granted | 2,701 | 1.80 | ||||||||||
| Exercised | — | |||||||||||
| Forfeited or expired | (693 | ) | 5.38 | |||||||||
| Options outstanding at December 31, 2017 | 5,675 | $ | 4.87 | 7.40 | ||||||||
| Options exercisable at December 31, 2017 | 2,161 | $ | 7.19 | 5.25 | ||||||||
83
The exercise prices of common stock options outstanding and exercisable are as follows at December 31, 2017 (in thousands):
| Exercise Price | Options Outstanding (Shares) |
Options
Exercisable (Shares) |
|||||||||
| $ | 1.12 to 1.97 | 2,617 | 40 | ||||||||
| $ | 2.34 to 4.18 | 444 | 169 | ||||||||
| $ | 4.88 to 5.23 | 1,116 | 973 | ||||||||
| $ | 7.00 to 9.01 | 805 | 588 | ||||||||
| $ | 12.43 to 14.06 | 693 | 391 | ||||||||
| 5,675 | 2,161 | ||||||||||
The estimated aggregate intrinsic value of stock options exercisable at December 31, 2017 was approximately $6,000. As of December 31, 2017, there was $4,901,000 of total unrecognized compensation cost related to the outstanding stock options that will be recognized over a weighted average period of 2.69 years.
During the first quarter of 2016, the Company recorded a charge of $55,000 to extend the exercise period of 98,681 vested options for one employee who resigned and became a consultant for the Company. All unvested options for this employee were terminated when this employee ceased full-time employment with the Company.
During the year ended December 31, 2016, the Company granted stock options to purchase 30,000 shares of common stock to an outside attorney in connection with his services relating to the Company’s rights offering to stockholders. The options have fully vested and are exercisable for a period of four years from the date of grant at a price of $5.23 per share, which was 125% of the fair value of the Company’s common stock on the grant date of January 14, 2016. The fair value of these options, as calculated pursuant to the Black-Scholes option-pricing model, was determined to be $53,000 ($1.77 per share). Assumptions used in the model were an expected term of 6.25 years, volatility of 48.2%, a risk-free interest rate of 1.87%, and an expected dividend rate of 0%. The cost of these shares was treated as an issuance cost of the offering and was deducted from the gross proceeds of the offering.
During the year ended December 31, 2017, the Company granted stock options to purchase 2,511,150 shares of common stock to certain employees. The options are exercisable for a period of ten years from the date of grant at prices ranging from $1.12 to $1.97 per share, which was the fair value of the Company’s common stock on the respective grant dates. The options vest over a period of four years. The fair value of these options, as calculated pursuant to the Black-Scholes option-pricing model, was determined to be $2,251,000 ($0.54 to $0.96 per share). Assumptions used in the model were an expected term of 6.25 years, volatility of 48.0%, a risk-free interest rate of 1.92% to 2.25%, and an expected dividend rate of 0%.
In March 2017, the Company granted stock options to purchase 40,000 shares of common stock to an outside attorney in connection with his services relating to the Company’s March, 2017 rights offering to stockholders. The options are exercisable for a period of four years from the date of grant at a price of $1.76 per share, which was 120% of the fair value of the Company’s common stock on the grant date of March 6, 2017. The options vested as of the date of grant. The fair value of these options, as calculated pursuant to the Black-Scholes option-pricing model, was determined to be $19,640 ($0.49 per share). Assumptions used in the model were an expected term of 4.0 years, volatility of 48.0%, a risk-free interest rate of 1.81%, and an expected dividend rate of 0%. The cost of these shares was treated as an issuance cost of the offering and was deducted from the gross proceeds from the offering.
In October 2017, the Company granted stock options to purchase 150,000 shares of common stock to an outside contractor in connection with his services. The options are exercisable for a period of ten years from the date of grant at a price of $1.21 per share, which was the fair value of the Company’s common stock on the grant date. The options vest over a four year period. The unvested portion of these stock options is remeasured by the Company at each reporting period. The fair value of these options, as remeasured pursuant to the Black-Scholes option-pricing model at December 31, 2017, was determined to be $175,067 ($1.17 per share). Assumptions used in the model were an expected term of 6.25 years, volatility of 48.0%, a risk-free interest rate of 2.25%, and an expected dividend rate of 0%. The cost of these shares will be expensed over the life of the grants. As of December 31, 2017 $11,000 has been expensed for this grant.
84
The Company adopted an employee stock purchase plan in June, 2015 for all eligible employees. As of December 31, 2017, the maximum number of shares that may be issued under the plan is 950,000. Under the plan, shares of the Company’s common stock may be purchased at six-month intervals at 85% of the lower of the closing fair market value of the common stock (i) on the first trading day of the offering period or (ii) on the last trading day of the purchase period. An employee may purchase in any one calendar year shares of common stock having an aggregate fair market value of up to $25,000 determined as of the first trading day of the offering period. Additionally, a participating employee may not purchase more than 100,000 shares of common stock in any one offering period. At December 31, 2017, 648,534 shares were issued under the stock purchase plan.
The following table presented below summarizes Restricted Stock Unit (RSU) activity for the years ended December 31, 2017, 2016 and 2015 (in thousands, except per share data):
| Number of Awards | Weighted Average Grant Date Fair Value Per Share | |||||||||
| Outstanding as of December 31, 2014 | — | $ | — | |||||||
| Awarded | 190 | 12.43 | ||||||||
| Vested | — | — | ||||||||
| Forfeited/canceled | — | — | ||||||||
| Outstanding as of December 31, 2015 | 190 | $ | 12.43 | |||||||
| Awarded | — | — | ||||||||
| Vested | 59 | 12.43 | ||||||||
| Forfeited/canceled | — | — | ||||||||
| Outstanding as of December 31, 2016 | 131 | $ | 12.43 | |||||||
| Awarded | — | — | ||||||||
| Vested | 48 | 12.43 | ||||||||
| Forfeited/canceled | — | — | ||||||||
| Outstanding as of December 31, 2017 | 83 | $ | 12.43 | |||||||
As of December 31, 2017, there was $961,000 of total unrecognized compensation cost related to the outstanding RSUs that will be recognized over a weighted average period of 1.63 years.
The total stock-based compensation recognized for stock-based awards granted in the consolidated statements of operations for the years ended December 31, 2017, 2016 and 2015 is as follows (in thousands):
| 2017 | 2016 | 2015 | ||||||||||
| Cost of sales | $ | 235 | $ | 312 | $ | 279 | ||||||
| Research and development | 288 | 303 | 208 | |||||||||
| Clinical and regulatory | 329 | 173 | 235 | |||||||||
| Selling and marketing | 339 | 104 | 442 | |||||||||
| General and administrative | 2,593 | 2,475 | 1,523 | |||||||||
| Total | $ | 3,784 | $ | 3,367 | $ | 2,687 | ||||||
From time to time, the Company has extended full-recourse loans to certain non-officer employees for the purpose of financing stock option exercises. These loans bear interest ranging from 1.27% to 1.91% per annum and are payable over three years in monthly installments of principal and interest. At December 31, 2017, and 2016 the outstanding balance of such loans, including accrued interest, was $0 and $2,000, respectively. These loans receivable are recorded in the Company’s consolidated financial statements as an offset to stockholders’ equity.
85
Employment Agreement
On June 19, 2015 the Company entered into an at will employment agreement with Will McGuire to become the Company’s President and Chief Executive Officer. The Company has agreed to pay Mr. McGuire an initial annual salary of $390,000 and he is also entitled to receive performance bonuses which will be based on performance standards and goals established by the Company’s Board of Directors. Upon termination without cause, Mr. McGuire will be entitled to receive severance consisting of his salary for a period of 12 months following such termination and his pro-rated target bonus through the balance of the calendar year in which such termination occurs. As part of the agreement, the Company agreed to grant Mr. McGuire, effective on his official start date as an employee, options to purchase 420,000 shares of the Company’s common stock, the fair value of which was determined to be $2,574,000, of which $643,000, $645,000 and $240,000 was recognized during the years ended December 31, 2017, 2016 and 2015, respectively, and 190,000 RSUs the fair value of which was determined to be $2,362,000, of which $590,000, $591,000 and $220,000 was recognized during the years ended December 31, 2017, 2016 and 2015, respectively. The fair value of the RSUs and the exercise price of the options were both marked at $12.43 which was the closing price of the Company’s stock on Nasdaq on August 17, 2015. The options and RSUs vest over four years, with 25% vesting on the first anniversary of the grant date, and the remainder vesting thereafter in twelve equal installments of 6.25% on the quarterly anniversaries of the grant date.
10. Income Taxes
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company’s deferred tax assets as of December 31, 2017 and 2016 are summarized below (in thousands):
| 2017 | 2016 | |||||||
| Stock-based compensation | $ | 3,346 | $ | 4,135 | ||||
| Research credits | 5,858 | 5,493 | ||||||
| Depreciation | (41 | ) | (36 | ) | ||||
| Net operating loss carryforwards | 11,646 | 54,509 | ||||||
| Inventory reserve | 480 | 1,958 | ||||||
| Other | 462 | 847 | ||||||
| Total deferred tax assets | 21,751 | 66,906 | ||||||
| Valuation allowance | (21,751 | ) | (66,906 | ) | ||||
| Net deferred tax assets | $ | — | $ | — | ||||
In assessing the potential realization of these deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will be realized. The ultimate realization of deferred tax assets is dependent upon the Company attaining future taxable income during the periods in which those temporary differences become deductible. As of December 31, 2017 and 2016, management was unable to determine if it is more likely than not that the Company’s deferred tax assets will be realized, and has therefore recorded an appropriate valuation allowance against deferred tax assets at such dates.
No federal tax provision has been provided for the years ended December 31, 2017 and 2016 due to the losses incurred during such periods. The Company’s effective tax rate is different from the federal statutory rate of 34% due primarily to operating losses that receive no tax benefit as a result of a valuation allowance recorded for such losses.
86
As of December 31, 2017, after the ownership change under Section 382(g), the Company had federal and state income tax net operating loss carryforwards, which may be applied to future taxable income, of approximately $44.5 million and $32.9 million, respectively. The federal net operating loss carryforwards will expire at various dates from 2035 through 2037. The state net operating loss carryforwards began to expire at various dates from 2033 through 2037. The Company also has a federal and state research and development tax credit carryforwards totaling approximately $3,354,000 and $2,505,000, respectively. The federal research and development tax credit carryforwards will expire at various dates from 2023 through 2037. The state research and development tax credit carryforwards do not expire.
The Company files income tax returns in the U.S. federal jurisdiction and various states and is subject to income tax examinations by federal tax authorities for tax years ended 2014 and later and by state authorities for tax years ended 2013 and later. The Company currently is not under examination by any tax authority. The Company’s policy is to record interest and penalties on uncertain tax positions as income tax expense. As of December 31, 2017 and 2016, the Company has no accrued interest or penalties related to uncertain tax positions. Second Sight Switzerland, the Company’s foreign subsidiary, has not had any taxable income in the prior and current years.
On December 22, 2017, the United States government enacted comprehensive tax legislation commonly referred to as the Tax Cuts and Jobs Act (the "Tax Act"). The Tax Act significantly revises the existing tax law by, among other things, lowering the United States corporate income tax rate from 35% to 21% beginning in 2018. The Company reviewed and incorporated the impact of the Tax Act in its tax calculations and disclosures. The primary impact on the Company stems from the re-measurement of its deferred taxes at the new corporate tax rate of 21%, which reduced the Company's net deferred tax assets, before valuation allowance, by $7.5 million . Due to the full valuation allowance, the change in deferred taxes was fully offset by the change in valuation allowance. The Tax Act did not have a significant impact on the Company's Consolidated Financial Statements for the year ended December 31, 2017.
11. Product Warranties
A summary of activity in the Company’s warranty liabilities, which are included in accrued expenses in the accompanying consolidated balance sheets, for the years ended December 31, 2017, 2016 and 2015 is presented below (in thousands):
| 2017 | 2016 | 2015 | ||||||||||
| Balance, beginning of year | $ | 1,525 | $ | 1,066 | $ | 556 | ||||||
| Additional accruals | 470 | 727 | 991 | |||||||||
| Payments | (236 | ) | (268 | ) | (443 | ) | ||||||
| Adjustments and other | (303 | ) | — | (38 | ) | |||||||
| Total | $ | 1,456 | $ | 1,525 | $ | 1,066 | ||||||
12. Commitments and Contingencies
Lease Commitment
Effective August 2012, the Company entered into a lease agreement (the “Sylmar Lease”) with a company owned by the major stockholder of the Company for office space for a term of five years that was initially set to expire on February 28, 2017. The Sylmar Lease included rental of additional space commencing January 1, 2013 and a five year option to renew. The lease requires the Company to pay real estate taxes, insurance and common area maintenance each year, and is subject to periodic cost of living adjustments. In April 2014, the Sylmar Lease was renegotiated with the term ending on February 28, 2022, and a five year option to renew. The new lease also requires the Company to pay real estate taxes, insurance and common area maintenance each year and includes automatic increases in base rent each year. In November 2014, the property underlying the Sylmar lease was sold to an unrelated party. The current base rent at this facility is $36,600 per month.
Second Sight Switzerland rents office space in Switzerland on a month-to-month basis for CHF 8,200 (approximately $8,400, at December 31, 2017) per month.
87
Total rent expense was approximately $1,017,000, $1,050,000 and $954,000 for the years ended December 31, 2017, 2016 and 2015, respectively, and is allocated based on square footage to general and administrative and manufacturing costs in the accompanying consolidated statement of operations..
Future minimum rental payments required under the operating leases are as follows for the years ended December 31 (in thousands).
| Years | Amount | ||||
| 2018 | $ | 858 | |||
| 2019 | 884 | ||||
| 2020 | 910 | ||||
| 2021 | 937 | ||||
| 2022 | 158 | ||||
| Total | $ | 3,747 | |||
License Agreements
The Company has exclusive licensing agreements to utilize certain patents. These patents are related to the technology for visual prostheses. There are currently two such agreements that the Company has determined there is a reasonable likelihood of future royalty payments. The Company has agreed to pay the licensors’ royalties for licensed products sold or leased by the Company. The royalty rates range from 0.5% to 3.25%, based on related net sales of the patented portion of licensed products, less a credit for royalties paid to others. The 3.25% rate does not reflect a .25% credit for royalties paid to others. Additional discounts may be possible if the Company enters into additional licenses.
One of the licensing agreements requires the Company to pay the licensors a $5,000 annual maintenance fee for the first seven years and a $10,000 annual maintenance fee each year thereafter for as long as the agreement has not been terminated by the Company. The second of these agreements has no stipulated fees. Pursuant to these agreements, the Company has incurred costs of approximately $93,000, $74,000 and $93,000 for the years ended December 31, 2017, 2016 and 2015, respectively.
Clinical Trial Agreements
Based upon FDA approval, which was obtained in February 2013, the Company is required to collect follow-up data from subjects enrolled in its pre-approval trial for a period of up to ten years post-implant, which extends this trial through the year 2019. In addition, the Company is conducting three post-market studies to comply with US FDA, French, and European post-market surveillance regulations and requirements. The Company has contracted with various universities, hospitals, and medical practices to provide these services. Payments are based on procedures performed for each subject and are charged to clinical and regulatory expense as incurred. Total amounts charged to expense for the years ended December 31, 2017, 2016 and 2015 were $814,000, $786,000 and $1,409,000, respectively.
88
Litigation, Claims and Assessments
Twenty-one oppositions have been filed by a third-party in the European Patent Office, each challenging the validity of a European patent owned or exclusively licensed by the Company. The outcome of the challenges is not certain, however, if successful, they may affect the Company’s ability to block competitors from utilizing its patented technology. Management of the Company believes a successful challenge will not have a material effect on its ability to manufacture and sell its products, or otherwise have a material effect on its operations.
The Company is party to litigation arising in the ordinary course of business. It is management’s opinion that the outcome of such matters will have not have a material effect on the Company’s financial statements.
13. Quarterly Financial Summary (unaudited)
| Quarters Ended | ||||||||||||||||
| (in thousands, except per share data) | December
31, 2017 | September
30, 2017 | June
30, 2017 | March
31, 2017 | ||||||||||||
| Product sales | $ | 3,109 | $ | 1,610 | $ | 2,236 | $ | 1,009 | ||||||||
| Gross profit (loss) | $ | 1,247 | $ | 609 | $ | 1,109 | $ | (118 | ) | |||||||
| Operating loss | $ | (7,433 | ) | $ | (6,749 | ) | $ | (6,872 | ) | $ | (7,555 | ) | ||||
| Net loss | $ | (7,409 | ) | $ | (6,716 | ) | $ | (6,843 | ) | $ | (7,548 | ) | ||||
| Net loss per share – basic and diluted | $ | (0.13 | ) | $ | (0.12 | ) | $ | (0.12 | ) | $ | (0.16 | ) | ||||
| Quarters Ended | ||||||||||||||||
| December
31, 2016 | September
30, 2016 | June
30, 2016 | March
31, 2016 | |||||||||||||
| Product sales | $ | 715 | $ | 1,180 | $ | 1,037 | $ | 1,053 | ||||||||
| Gross profit (loss) | $ | (2,593 | ) | $ | (1,435 | ) | $ | (2,204 | ) | $ | 141 | |||||
| Operating loss | $ | (10,383 | ) | $ | (8,499 | ) | $ | (8,507 | ) | $ | (5,821 | ) | ||||
| Net loss | $ | (10,370 | ) | $ | (8,489 | ) | $ | (8,504 | ) | $ | (5,816 | ) | ||||
| Net loss per share – basic and diluted | $ | (0.24 | ) | $ | (0.20 | ) | $ | (0.23 | ) | $ | (0.16 | ) | ||||
14. Subsequent Events
Stock Option Grants
In January 2018, the Company granted stock options to purchase 1,658,872 shares of common stock to employees, including 1,210,000 options that were granted to senior management of the Company. The options are exercisable for a period of ten years from the date of grant with exercise prices ranging from $1.83 to $2.06 per share. The options vest primarily over a four year term, of which one-fourth vests on the one year anniversary of the date of grant and the remaining options vest quarterly over three years thereafter. The fair value of these options, as calculated pursuant to the Black-Scholes option-pricing model, was determined to be $1,625,489 (a weighted average of $0.98 per share).
Severance Agreement
During March 2018, the Company entered into a severance agreement with Gregoire Cosendai, its former Vice President of Clinical Affairs, who was released from work obligations effective January 16, 2018. Under Swiss law, Mr. Cosendai will remain an employee of the Company through April 30, 2018, at which time he will receive five months of voluntary severance pay equal to CHF 103,896, or approximately $110,000.
89
EXHIBIT INDEX
| * | Included herein. |
| + | Indicates management contract or compensatory plan |
| (1) | Incorporated by reference to the registrant’s registration statement on Form S-1, file no. 333-198073, originally filed with the Securities and Exchange Commission on August 12, 2014, as amended. |
| (2) | Incorporated by reference to registrant’s definitive proxy statement on Schedule 14A, filed with the Securities and Exchange Commission on April 16, 2015. |
| (3) | Incorporated by reference to registrant’s current report on Form 8-K filed with the Securities and Exchange Commission on June 25, 2015. |
90
Exhibit 31.1
CERTIFICATION OF THE CHIEF EXECUTIVE OFFICER
Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
I, Jonathan Will McGuire, hereby certify that:
| 1. | I have reviewed this Annual Report on Form 10-K of Second Sight Medical Products, Inc.; |
| 2. | Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
| 3. | Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report; |
| 4. | The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have: |
| (a) | designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
| (b) | designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
| (c) | evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
| (d) | disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of the annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and |
| 5. | The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions): |
| (a) | all significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and | |
| (b) | any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting. |
| Date: March 20, 2018 | /s/ Jonathan Will McGuire |
| Jonathan Will McGuire | |
| Chief Executive Officer | |
| (Principal Executive Officer) |
Exhibit 31.2
CERTIFICATION OF THE CHIEF FINANCIAL OFFICER
Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
I, Thomas B. Miller, certify that:
| 1. | I have reviewed this Annual Report on Form 10-K of Second Sight Medical Products, Inc.; |
| 2. | Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
| 3. | Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report; |
| 4. | The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have: |
| (a) | designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
| (b) | designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
| (c) | evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
| (d) | disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and |
| 5. | The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions): |
| (a) | all significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and |
| (b) | any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting. |
| Date: March 20, 2018 | /s/ Thomas B. Miller |
| Thomas B. Miller | |
| Chief Financial Officer | |
| (Principal Financial and Accounting Officer) |
Exhibit 32.1
Certifications of Principal Executive Officer and Principal Financial Officer
Pursuant to 18 U.S.C. Section 1350, As Adopted Pursuant To
Section 906 of the Sarbanes-Oxley Act of 2002
Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (18 U.S.C. 1350), Jonathan Will McGuire, Chief Executive Officer (Principal Executive Officer) and Thomas B. Miller, Chief Financial Officer (Principal Financial and Accounting Officer) of Second Sight Medical Products, Inc. (the “Company”), each hereby certifies that, to the best of his knowledge:
| 1. | The Annual Report of the Company on Form 10-K (the “Report”) for the fiscal year ended December 31, 2017, to which this Certification is attached as Exhibit 32.1, fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and |
| 2. | The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company at the dates and for periods indicated. |
| Date: March 20, 2018 | /s/ Jonathan Will McGuire |
| Jonathan Will McGuire | |
| Chief Executive Officer | |
| (Principal Executive Officer) | |
| /s/ Thomas B. Miller | |
| Thomas B. Miller | |
| Chief Financial Officer | |
| (Principal Financial and Accounting Officer) |
