Form 8-K Seres Therapeutics, Inc. For: Mar 08
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): March 8, 2018
SERES THERAPEUTICS, INC.
(Exact name of Registrant as Specified in Its Charter)
| Delaware | 001-37465 | 27-4326290 | ||
| (State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) | ||
| 200 Sidney Street Cambridge, MA |
02139 | |||
| (Address of Principal Executive Offices) | (Zip Code) | |||
Registrant’s Telephone Number, Including Area Code: (617) 945-9626
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Indicate by check mark whether the registrant is an emerging growth company as defined in as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instructions A.2. below):
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
| Item 2.02. | Results of Operations and Financial Condition. |
On March 8, 2018, Seres Therapeutics, Inc. (the “Company”) announced its financial results for the year and quarter ended December 31, 2017. The full text of the press release issued in connection with the announcement is furnished as Exhibit 99.1 to this Current Report on Form 8-K.
The information in this Item 2.02 of this Form 8-K including Exhibit 99.1 attached hereto, is intended to be furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such filing.
| Item 7.01. | Regulation FD Disclosure. |
On March 8, 2018, the Company posted an updated corporate slide presentation in the “Investors and Media” portion of its website at www.serestherapeutics.com including strategic and operation updates, preliminary results of the Phase 1b study of SER-262, and updated cash guidance. A copy of the slide presentation is attached as Exhibit 99.2 to this Current Report on Form 8-K.
The information in Item 7.01 of this Current Report on Form 8-K, including Exhibit 99.2 attached hereto, is intended to be furnished and shall not be deemed “filed” for purposes of Section 18 of the Exchange Act, or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such filing. The Company undertakes no obligation to update, supplement or amend the materials attached hereto as Exhibit 99.2.
| Item 9.01. | Financial Statements and Exhibits. |
(d) Exhibits
The following exhibits relating to Item 2.02 and Item 7.01, respectively, shall be deemed to be furnished, and not filed:
| Exhibit No. |
Description | |
| 99.1 | Press Release issued on March 8, 2018 | |
| 99.2 | Seres Therapeutics, Inc. Corporate Slide Presentation as of March 8, 2018 | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| SERES THERAPEUTICS, INC. | ||||||
| Date: March 8, 2018 | By: | /s/ Thomas J. DesRosier | ||||
| Name: | Thomas J. DesRosier | |||||
| Title: | Chief Legal Officer and Executive Vice President | |||||
Exhibit 99.1

Seres Therapeutics Reports Fourth Quarter and Full Year 2017 Financial Results and
Provides Business Updates
- Positive SER-287 Phase 1b clinical and microbiome results support further development; Company plans to initiate next clinical trial in mid-2018 -
- Preliminary Phase 1b study data obtained for SER-262, the first ever rationally-designed fermented microbiome therapeutic candidate evaluated in humans–
- Collaboration with MD Anderson and Parker Institute intends to initiate clinical study in 2018 to evaluate microbiome therapeutics enhancing immuno-oncology treatments –
- Conference call at 8:00 a.m. ET today -
CAMBRIDGE, Mass., March 8, 2018 — Seres Therapeutics, Inc. (Nasdaq: MCRB), a leading microbiome therapeutic development company developing a novel class of biological drugs, today reported fourth quarter and full year 2017 financial results and provided operational and strategic updates.
“2017 was a year of significant pipeline progress where Seres obtained promising SER-287 Phase 1b clinical and microbiome results in Ulcerative Colitis, advanced SER-109 into a pivotal Phase 3 study for recurrent C. difficile infection, and entered into a strategic collaboration with MD Anderson and the Parker Institute for Cancer Immunotherapy to initiate a clinical study to evaluate microbiome therapy effects in improving the efficacy of checkpoint inhibitors in cancer patients,” said Roger J. Pomerantz, M.D., President, CEO and Chairman of Seres. “Seres also recently obtained preliminary SER-262 Phase 1b study results in patients with primary C. difficile infection. The SER-262 results, the first ever from a rationally designed microbiome development candidate, provide key mechanistic insights that will inform the progress of Seres microbiome therapeutic candidates, including but not limited to SER-262.”
Dr. Pomerantz continued: “Seres has an array of early and late clinical stage, as well as pre-clinical stage microbiome programs in infectious, metabolic, and immune diseases - each with compelling scientific and clinical rationale. Our near-term focus will be on the highest priority clinical programs to most effectively advance our pipeline: SER-287 for Ulcerative Colitis; SER-109 for Recurrent C. difficile infection; and the SER-401 Immuno-oncology program. We expect 2018 to be an eventful year with continued SER-109 Phase 3 study execution, and the initiation of both a next stage SER-287 Ulcerative Colitis clinical study, as well as a clinical trial evaluating adjunctive microbiome therapy in metastatic melanoma patients being treated with checkpoint inhibitors.”
Recent Highlights
| • | SER-287 Phase 1b study clinical and microbiome results: Seres previously reported positive results from a SER-287 Phase 1b placebo-controlled induction study in 58 patients with mild-to-moderate Ulcerative Colitis (UC) who were failing current therapies. |
SER-287 administration resulted in a dose-dependent improvement of both clinical remission rates and endoscopic scores. Based on an intent to treat ‘missing data counted as a failure’ analysis, 40% (6 of 15) of patients in the vancomycin pre-treatment, daily SER-287 dosing arm achieved clinical remission; whereas in the placebo group 0% (0 of 11) achieved this endpoint (p-value = 0.0237).
High clinical response rates to placebo that were not statistically differentiated from the SER-287 treatment arms were also observed. Clinical response is a subjective endpoint that is prone to high variability and high placebo rates, as previously observed in several other UC trials. In the most recent FDA regulatory guidance in August 2016, clinical remission is the only recommended primary endpoint in UC registrational studies.
The SER-287 safety and tolerability profile was favorable. Study results demonstrated no imbalance in adverse events in patients treated with SER-287, as compared to placebo. There were no drug-related serious adverse events associated with SER-287.
Analyses of study microbiome data demonstrated that SER-287 induced dose-dependent engraftment of SER-287-derived bacterial species. Differences in specific bacterial engraftment signatures were found to be associated with clinical remission. Bacterial engraftment of SER-287-derived bacterial species was durable for at least four weeks after administration of the final SER-287 dose, when final data microbiome samples were collected. In the 11 patients in this trial who achieved clinical remission (all of whom received SER-287), none had flares during the 6 months following SER-287 treatment. Finally, histologic improvement scores were demonstrated to be higher in patients treated with daily SER-287, as compared to placebo.
Seres is in discussion with the FDA regarding the SER-287 study design and plans to initiate the next clinical study of SER-287 in UC patients in mid-2018.
| • | SER-287 Orphan Drug Designation in Pediatric UC: The FDA has granted Orphan Drug Designation to Seres’ microbiome therapeutic candidate SER-287 for the treatment of UC in pediatric patients. The FDA’s designation of SER-287 follows a review of the data that established the potential uses for SER-287. |
| • | Continued execution of the SER-109 ECOSPOR III Phase 3 study: Seres continues to progress its SER-109 Phase 3 clinical study, and plans to enroll approximately 320 patients with multiply recurrent C. difficile infection, at sites in both the U.S. and Canada. Based on previously disclosed interactions with the FDA, ECOSPOR III has been designated a Phase 3 trial and the Company expects that this single pivotal study could support SER-109 registration and approval. SER-109 has been designated by the FDA as a Breakthrough Therapy and has obtained Orphan Drug Designation. |
| • | Preliminary SER-262 Phase 1b study results: Seres obtained preliminary clinical and microbiome results from the SER-262 Phase 1b, first-in-human, dose-escalation clinical study of SER-262 in patients with primary C. difficile infection. SER-262 is the first rationally-designed, fermented microbiome therapeutic candidate ever evaluated in patients. Clinical data have been obtained from seven of the eight planned dose escalation patient cohorts. Each cohort included 10 patients receiving SER-262 and two patients receiving placebo. Based on the first seven patient cohorts, SER-262 had no drug-related serious adverse events reported. No relevant differences were observed in the relative risk of recurrence rate in patients administered SER-262, as compared to placebo; however, this small cohort-based, first-in-human Phase 1b study was not powered to detect a statistically significant difference in recurrence rates. A small group of placebo treated patients were included in this study and, in this group, no recurrences were observed. Of note, a low C. difficile recurrence rate was observed in patients treated with Vancomycin and SER-262, as compared to those treated with Metronidazole and SER-262 (4% versus 31%, respectively). This difference was statistically significant with a p value of 0.0049. The medical literature suggests a recurrence rate of about 25% in patients treated solely with Vancomycin for primary C. difficile infection. Our data suggest that treatment with Vancomycin, followed by SER-262, results in more robust and kinetically more rapid engraftment, and thus may lead to corresponding clinical efficacy. This new finding will be further evaluated to inform future development efforts. |
Preliminary SER-262 microbiome analysis has been conducted on the first five, lowest dose cohorts to assess drug pharmacokinetics. A majority of SER-262-derived strains were detected in patients receiving SER-262; detection of strains was variable across subjects. This is the first-time engraftment of bacteria from a fermented microbiome drug candidate has been demonstrated in the microbiome of humans. Partial engraftment of strains was also a characteristic observed in our SER-109 clinical studies, and has been reported in fecal microbiota transplant treatment of C. difficile infection. In patients where SER-262 engraftment was observed, broader microbiome changes were also observed, indicating that a limited number of engrafting species may cause global restructuring of the human microbiome. Microbiome profile differences, based on the antibiotics used to treat each patient’s C. difficile infection, were also observed. Vancomycin led to more rapid and robust engraftment of SER-262 bacterial strains, as compared to Metronidazole. More detailed microbiome and metabolomic analyses remain ongoing. These unique SER-262 proprietary human data sets will be used to inform future development of SER-262 and other fermented Seres therapeutic candidate, including but not limited to SER-301 for Inflammatory bowel disease (IBD) and SER-155 for hematopoietic stem cell transplantation (HSCT).
| • | Collaboration with MD Anderson Cancer Center and the Parker Institute for Cancer Immunotherapy: Seres, MD Anderson Cancer Center (MD Anderson), and the Parker Institute for Cancer Immunotherapy (Parker Institute), formed a collaboration to evaluate the potential of Seres’ microbiome therapeutic candidates to improve the outcomes of |
| cancer patients treated with immuno-therapy checkpoint inhibitors. The collaborators plan to initiate a randomized, placebo-controlled study at MD Anderson, sponsored by the Parker Institute in patients with metastatic melanoma this year. The clinical trial will evaluate the impact of an anti-PD-1 checkpoint inhibitor with adjunctive microbiome therapy on patient outcomes. Seres also received an exclusive option, with pre-defined financial terms, to license intellectual property rights from MD Anderson related to the use of bacteria in combination with checkpoint inhibitors. |
Financial Results
Seres reported a net loss of $89.4 million for the full year, as compared to a net loss of $91.6 million for the prior year. Seres reported a net loss of $29.0 million for the fourth quarter of 2017, as compared to a net loss of $25.3 million for the same period in 2016. The fourth quarter net loss was driven primarily by clinical and development expenses, personnel expenses, and ongoing development of the Company’s microbiome therapeutics platform. The fourth quarter net loss figure was inclusive of $3.1 million in recognized revenue associated with the Company’s collaboration with Nestlé Health Science.
Research and development expenses for the fourth quarter 2017 were $24.0 million, as compared to $20.3 million for the same period in 2016. The research and development expense was primarily related to Seres’ microbiome therapeutics platform, the clinical development of SER-109, SER-262 and SER-287, as well as the Company’s and immuno-oncology preclinical programs.
General and administrative expenses for the fourth quarter were $8.8 million, as compared to $8.5 million for the same period in the prior year. General and administrative expenses were primarily due to headcount, professional fees, and facility costs.
The decrease in the Company’s cash, cash equivalents and investments balance during the quarter was $21.3 million. Seres ended the fourth quarter with approximately $150.0 million in cash, cash equivalents and investments.
Financial Expectations
Based on the Company’s current operating plan, cash resources are expected to fund operating expenses and capital expenditure requirements, excluding net cash flows from future business development activities or potential incoming milestone payments, through the first quarter 2019.
This projection is a revision to the previous cash funding timing guidance of through 2018.
Seres is eligible to receive a substantial milestone payment, not considered in the financial guidance update, associated with the planned initiation of the next SER-287 clinical study.
Conference Call Information
Seres’ management will host a conference call today, March 8, 2018, at 8:00 a.m. ET. To access the conference call, please dial (844) 277-9450 (domestic) or (336) 525-7139 (international) and reference the conference ID number 5092388. Accompanying slides will be made available on the Seres website prior to the call. To join the live webcast, please visit the “Investors and Media” section of the Seres website at www.serestherapeutics.com.
A webcast replay will be available on the Seres website beginning approximately two hours after the event and will be archived for approximately 21 days.
About Seres Therapeutics
Seres Therapeutics, Inc., (Nasdaq: MCRB) is a leading microbiome therapeutics platform company developing a novel class of biological drugs that are designed to treat disease by restoring the function of a dysbiotic microbiome, where the natural state of bacterial diversity and function is imbalanced. Seres’ lead program, SER-109, has obtained Breakthrough Therapy and Orphan Drug designations from the U.S. Food and Drug Administration and is in Phase 3 development for multiply recurrent C. difficile infection. Seres’ clinical candidate SER-287 has successfully completed a Phase 1b study in patients with mild-to-moderate Ulcerative Colitis. Seres is also evaluating SER-262, a rationally-designed microbiome therapeutic candidate, in a Phase 1b study in patients with primary C. difficile infection. For more information, please visit www.serestherapeutics.com. Follow us on Twitter @SeresTx.
Forward-looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including relating to our prioritization of our assets, our development plans, the ability of ECOSPOR III to support SER-109 approval, ECORSPOR III enrollment, the promise and potential impact of any of our microbiome therapeutics or clinical trial data, our plans to initiate clinical studies of SER-287 and in I-O, the timing and results of any clinical studies, and the sufficiency of cash to fund operations.
These forward-looking statements are based on management’s current expectations. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: we have incurred significant losses, are not currently profitable and may never become profitable; our need for additional funding; our limited operating history; our unproven approach to therapeutic intervention; the lengthy, expensive, and uncertain process of clinical drug development, including potential delays in regulatory approval; our reliance on third parties and collaborators to conduct our clinical trials, manufacture our product candidates, and develop and commercialize our product candidates, if approved; our lack of experience in manufacturing,
selling, marketing, and distributing our product candidates; failure to compete successfully against other drug companies; protection of our proprietary technology and the confidentiality of our trade secrets; potential lawsuits for, or claims of, infringement of third-party intellectual property or challenges to the ownership of our intellectual property; our patents being found invalid or unenforceable; risks associated with international operations; our ability to retain key personnel and to manage our growth; the potential volatility of our common stock; our management and principal stockholders have the ability to control or significantly influence our business; and we are currently subject to securities class action litigation. These and other important factors discussed under the caption “Risk Factors” in our Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission, or SEC, on November 8, 2017 and our other reports filed with the SEC, including the Annual Report we intend to file later today, could cause actual results to differ materially from those indicated by the forward-looking statements made in this press release. Any such forward-looking statements represent management’s estimates as of the date of this press release. While we may elect to update such forward-looking statements at some point in the future, we disclaim any obligation to do so, even if subsequent events cause our views to change. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this press release.
IR or PR Contact:
Carlo Tanzi, Ph.D., Seres Therapeutics, 617-203-3467
Vice President, Investor Relations and Corporate Communications
SERES THERAPEUTICS, INC.
CONSOLIDATED BALANCE SHEETS
(In thousands, except share and per share data)
| December 31, | ||||||||
| 2017 | 2016 | |||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 36,088 | $ | 54,539 | ||||
| Investments |
113,895 | 138,704 | ||||||
| Prepaid expenses and other current assets |
5,095 | 5,126 | ||||||
|
|
|
|
|
|||||
| Total current assets |
155,078 | 198,369 | ||||||
| Property and equipment, net |
32,931 | 36,125 | ||||||
| Long-term investments |
— | 36,752 | ||||||
| Restricted cash |
1,513 | 1,400 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 189,522 | $ | 272,646 | ||||
|
|
|
|
|
|||||
| Liabilities and Stockholder’s Equity |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 7,033 | $ | 7,587 | ||||
| Accrued expenses and other current liabilities |
12,513 | 10,812 | ||||||
| Deferred revenue - related party |
12,079 | 12,058 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
31,625 | 30,457 | ||||||
| Lease incentive obligation, net of current portion |
8,989 | 10,730 | ||||||
| Deferred rent |
2,233 | 2,072 | ||||||
| Deferred revenue, net of current portion - related party |
84,847 | 96,756 | ||||||
| Other long-term liabilities |
1,129 | — | ||||||
|
|
|
|
|
|||||
| Total liabilities |
128,823 | 140,015 | ||||||
|
|
|
|
|
|||||
| Commitments and contingencies |
||||||||
| Stockholders’ equity: |
||||||||
| Preferred stock, $0.001 par value; 10,000,000 shares authorized at December 31, 2017 and 2016; no shares issued and outstanding at December 31, 2017 and 2016 |
— | — | ||||||
| Common stock, $0.001 par value; 200,000,000 shares authorized at December 31, 2017 and 2016; 40,571,015 and 40,355,753 shares issued and outstanding at December 31, 2017 and 2016 |
40 | 40 | ||||||
| Additional paid-in capital |
324,376 | 306,931 | ||||||
| Accumulated other comprehensive income (loss) |
(146 | ) | (149 | ) | ||||
| Accumulated deficit |
(263,571 | ) | (174,191 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders’ equity |
60,699 | 132,631 | ||||||
|
|
|
|
|
|||||
| Total liabilities, convertible preferred stock and stockholders’ equity |
$ | 189,522 | $ | 272,646 | ||||
|
|
|
|
|
|||||
SERES THERAPEUTICS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(In thousands, except share and per share data)
| Year Ended December 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| Revenue: |
||||||||||||
| Collaboration revenue - related party |
$ | 32,100 | $ | 21,766 | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| Total revenue |
32,100 | 21,766 | — | |||||||||
| Operating expenses: |
||||||||||||
| Research and development expenses |
$ | 89,455 | 81,989 | 38,095 | ||||||||
| General and administrative expenses |
34,040 | 32,616 | 16,761 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
123,495 | 114,605 | 54,856 | |||||||||
|
|
|
|
|
|
|
|||||||
| Loss from operations |
(91,395 | ) | (92,839 | ) | (54,856 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Other income (expense): |
||||||||||||
| Interest income (expense), net |
1,590 | 1,260 | 83 | |||||||||
| Other income |
425 | — | — | |||||||||
| Revaluation of preferred stock warrant liability |
— | — | (7 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Total other income (expense), net |
2,015 | 1,260 | 76 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
$ | (89,380 | ) | (91,579 | ) | (54,780 | ) | |||||
| Net loss per share attributable to common stockholders, basic and diluted |
$ | (2.21 | ) | $ | (2.30 | ) | $ | (2.33 | ) | |||
|
|
|
|
|
|
|
|||||||
| Weighted average common shares outstanding, basic and diluted |
40,449,410 | 39,846,928 | 23,532,400 | |||||||||
|
|
|
|
|
|
|
|||||||
| Other comprehensive income (loss): |
||||||||||||
| Unrealized gain (loss) on investments, net of tax of $0 |
3 | (179 | ) | 30 | ||||||||
|
|
|
|
|
|
|
|||||||
| Total other comprehensive income (loss) |
3 | (179 | ) | 30 | ||||||||
|
|
|
|
|
|
|
|||||||
| Comprehensive loss |
$ | (89,377 | ) | $ | (91,758 | ) | $ | (54,750 | ) | |||
|
|
|
|
|
|
|
|||||||
###

Corporate Overview March 2018 Exhibit 99.2

Some of the statements in this presentation constitute “forward looking statements” under the Private Securities Litigation Reform Act of 1995, including, but not limited to, statements on the timing and results of our clinical trials, the sufficiency of our financial resources, and dysbiosis as an underlying cause of disease or failed response to therapy. Such statements are subject to important factors, risks and uncertainties (such as those discussed under the caption "Risk Factors" in the Company's Quarterly Report on Form 10-Q filed on November 8, 2017 and its other filings with the SEC) that may cause actual results to differ materially from those expressed or implied by such forward looking statements. Any forward looking statements included herein represent our views as of today only. We may update these statements, but we disclaim any obligation to do so. Forward looking statements
Seres investor highlights Phase 3 stage company developing microbiome-based therapeutics, a highly promising new area of medicine Leader in microbiome drug development with differentiated capabilities, leading CMC and demonstrated GMP quality, and supportive clinical data Focused R&D efforts in the areas of infectious diseases and inflammation & immunology, including immuno oncology Experienced, highly accomplished leadership team Platform Opportunity Pipeline Team
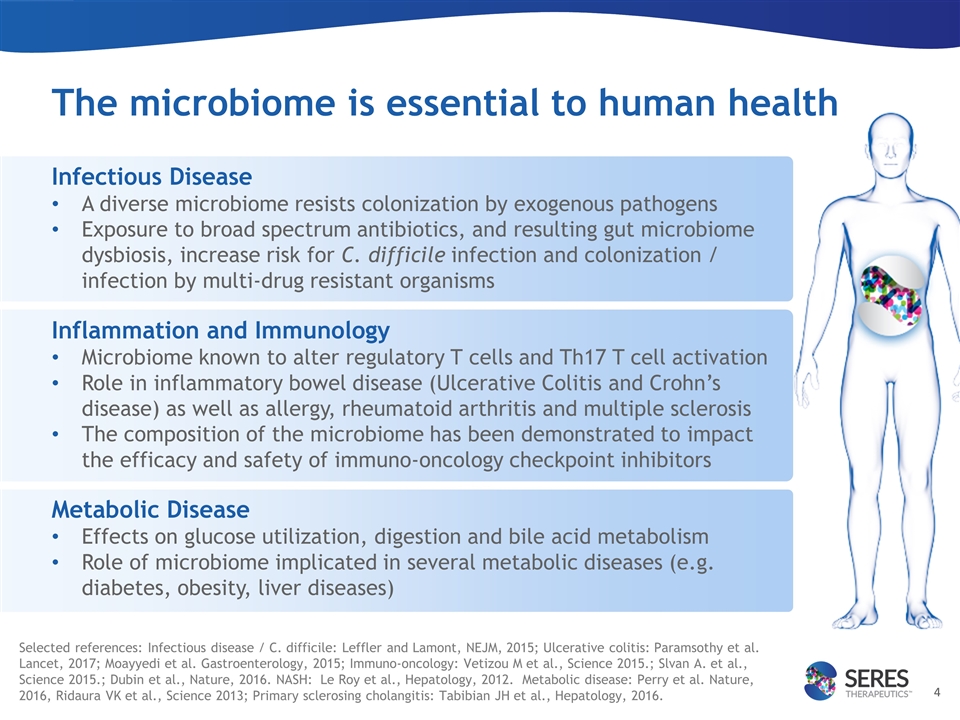
The microbiome is essential to human health Infectious Disease A diverse microbiome resists colonization by exogenous pathogens Exposure to broad spectrum antibiotics, and resulting gut microbiome dysbiosis, increase risk for C. difficile infection and colonization / infection by multi-drug resistant organisms Inflammation and Immunology Microbiome known to alter regulatory T cells and Th17 T cell activation Role in inflammatory bowel disease (Ulcerative Colitis and Crohn’s disease) as well as allergy, rheumatoid arthritis and multiple sclerosis The composition of the microbiome has been demonstrated to impact the efficacy and safety of immuno-oncology checkpoint inhibitors Metabolic Disease Effects on glucose utilization, digestion and bile acid metabolism Role of microbiome implicated in several metabolic diseases (e.g. diabetes, obesity, liver diseases) Selected references: Infectious disease / C. difficile: Leffler and Lamont, NEJM, 2015; Ulcerative colitis: Paramsothy et al. Lancet, 2017; Moayyedi et al. Gastroenterology, 2015; Immuno-oncology: Vetizou M et al., Science 2015.; Slvan A. et al., Science 2015.; Dubin et al., Nature, 2016. NASH: Le Roy et al., Hepatology, 2012. Metabolic disease: Perry et al. Nature, 2016, Ridaura VK et al., Science 2013; Primary sclerosing cholangitis: Tabibian JH et al., Hepatology, 2016.
Business strategy Prioritize serious diseases where dysbiosis of the gut microbiome has a causal role Focused R&D on clinical programs Computational biology Basic microbiome research Microbiology Translational science Clinical development Advanced GMP manufacturing World class, differentiated, microbiome expertise Collaborations with leading academic centers to efficiently advance research in promising new areas Research in new therapeutic areas SER-287 for Ulcerative Colitis SER-109 for recurrent C. difficile infection Adjunctive microbiome therapy with immuno-oncology
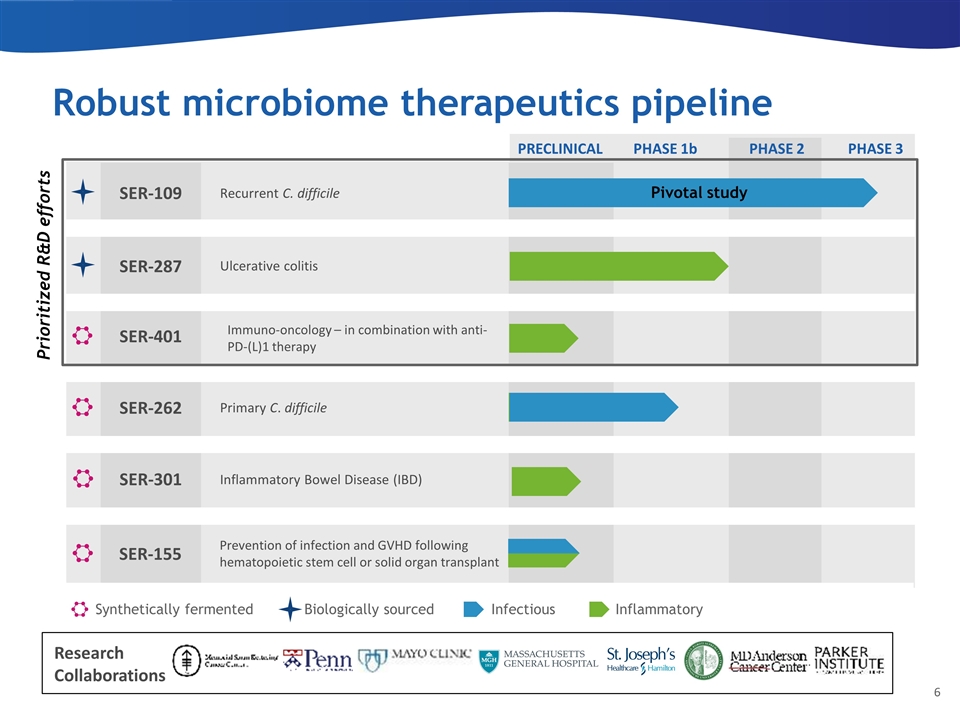
Robust microbiome therapeutics pipeline PRECLINICAL PHASE 1b PHASE 2 Synthetically fermented Infectious Inflammatory SER-262 Primary C. difficile SER-401 Inflammatory Bowel Disease (IBD) SER-301 Biologically sourced SER-287 Ulcerative colitis PHASE 3 SER-109 Recurrent C. difficile SER-155 Prevention of infection and GVHD following hematopoietic stem cell or solid organ transplant Pivotal study Immuno-oncology – in combination with anti-PD-(L)1 therapy Prioritized R&D efforts Research Collaborations

Clostridium difficile Infection Overview and R&D Programs Leading the Microbiome Revolution
C. difficile infection overview Infectious disease caused by toxin-producing anaerobic, spore-forming bacteria, resulting in diarrhea, abdominal pain, fever, and nausea Leading cause of hospital-acquired infection in the US Approximately 29,000 deaths/year ~25% of patients with primary C. diff. recur Risk of relapse increases with each recurrence Multiply recurrent C. difficile infection incidence increased 188% between 2001-2010 Sources: Leffler and Lamont, New England Journal of Medicine, 2015; Ma et al. Annals of Internal Medicine, 2017.
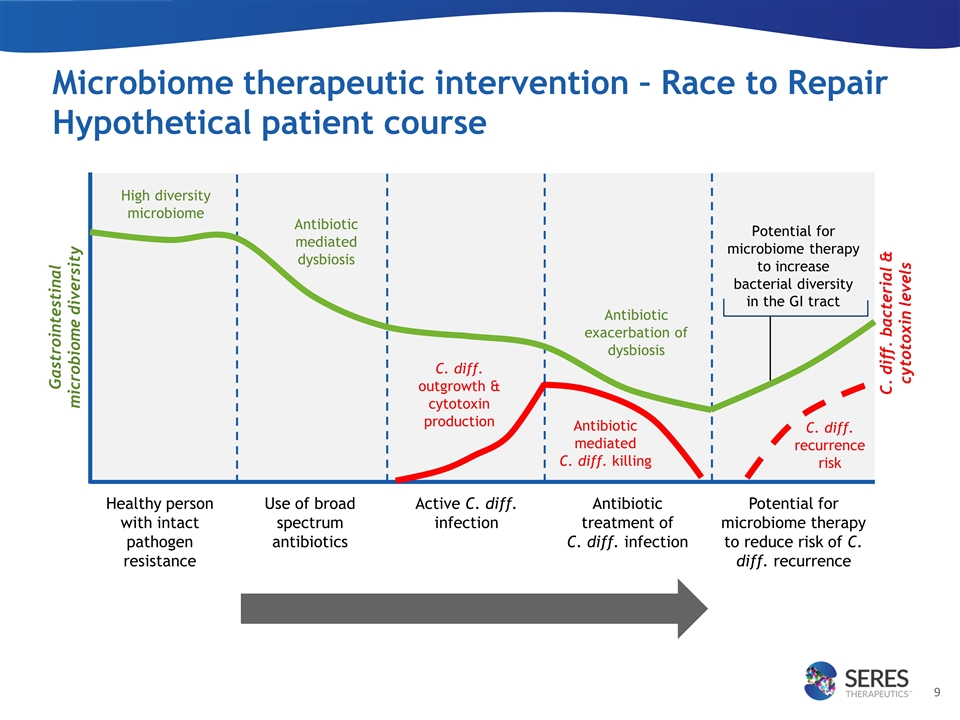
Microbiome therapeutic intervention – Race to Repair Hypothetical patient course Healthy person with intact pathogen resistance High diversity microbiome Use of broad spectrum antibiotics Active C. diff. infection Antibiotic treatment of C. diff. infection Potential for microbiome therapy to reduce risk of C. diff. recurrence Antibiotic mediated C. diff. killing Antibiotic exacerbation of dysbiosis Gastrointestinal microbiome diversity C. diff. bacterial & cytotoxin levels C. diff. recurrence risk C. diff. outgrowth & cytotoxin production Potential for microbiome therapy to increase bacterial diversity in the GI tract Antibiotic mediated dysbiosis
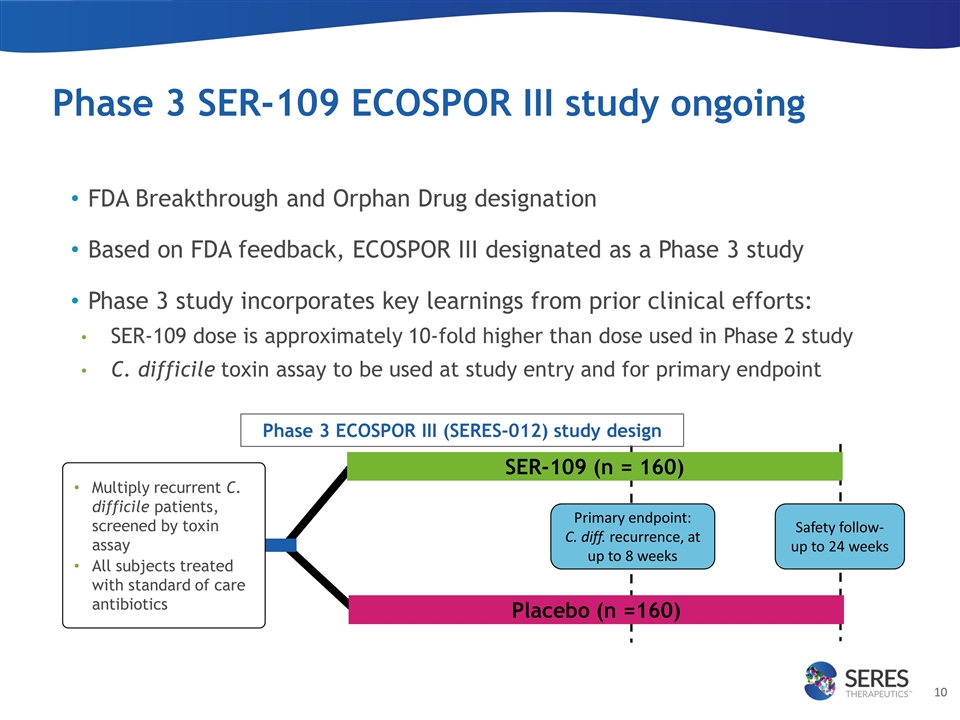
Phase 3 SER-109 ECOSPOR III study ongoing FDA Breakthrough and Orphan Drug designation Based on FDA feedback, ECOSPOR III designated as a Phase 3 study Phase 3 study incorporates key learnings from prior clinical efforts: SER-109 dose is approximately 10-fold higher than dose used in Phase 2 study C. difficile toxin assay to be used at study entry and for primary endpoint Multiply recurrent C. difficile patients, screened by toxin assay All subjects treated with standard of care antibiotics Primary endpoint: C. diff. recurrence, at up to 8 weeks Safety follow-up to 24 weeks Phase 3 ECOSPOR III (SERES-012) study design SER-109 (n = 160) Placebo (n =160)
SER-262: Synthetic, fermented Ecobiotic® therapeutic candidate for primary C. difficile infection Oral, microbiome therapeutic candidate comprising twelve strains of fermented, rationally-selected bacterial spores Bacterial species selected based on analysis of SER-109 Phase 1b microbiome data, biological and phylogenetic heterogeneity, and preclinical efficacy in C. difficile infection mouse model Data support a mechanism of action in which SER-262 strains compete for C. difficile preferred carbon sources SER-262 strains utilize multiple carbon sources In vitro fermentation
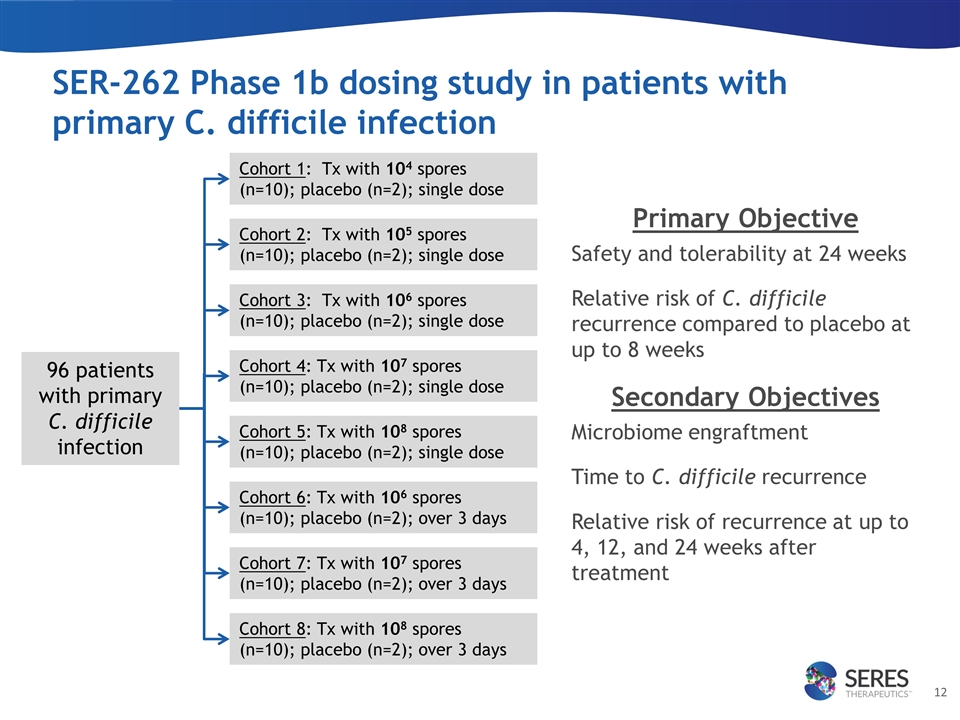
SER-262 Phase 1b dosing study in patients with primary C. difficile infection Primary Objective Safety and tolerability at 24 weeks Relative risk of C. difficile recurrence compared to placebo at up to 8 weeks Secondary Objectives Microbiome engraftment Time to C. difficile recurrence Relative risk of recurrence at up to 4, 12, and 24 weeks after treatment Cohort 1: Tx with 104 spores (n=10); placebo (n=2); single dose Cohort 2: Tx with 105 spores (n=10); placebo (n=2); single dose Cohort 3: Tx with 106 spores (n=10); placebo (n=2); single dose Cohort 4: Tx with 107 spores (n=10); placebo (n=2); single dose 96 patients with primary C. difficile infection Cohort 5: Tx with 108 spores (n=10); placebo (n=2); single dose Cohort 6: Tx with 106 spores (n=10); placebo (n=2); over 3 days Cohort 7: Tx with 107 spores (n=10); placebo (n=2); over 3 days Cohort 8: Tx with 108 spores (n=10); placebo (n=2); over 3 days
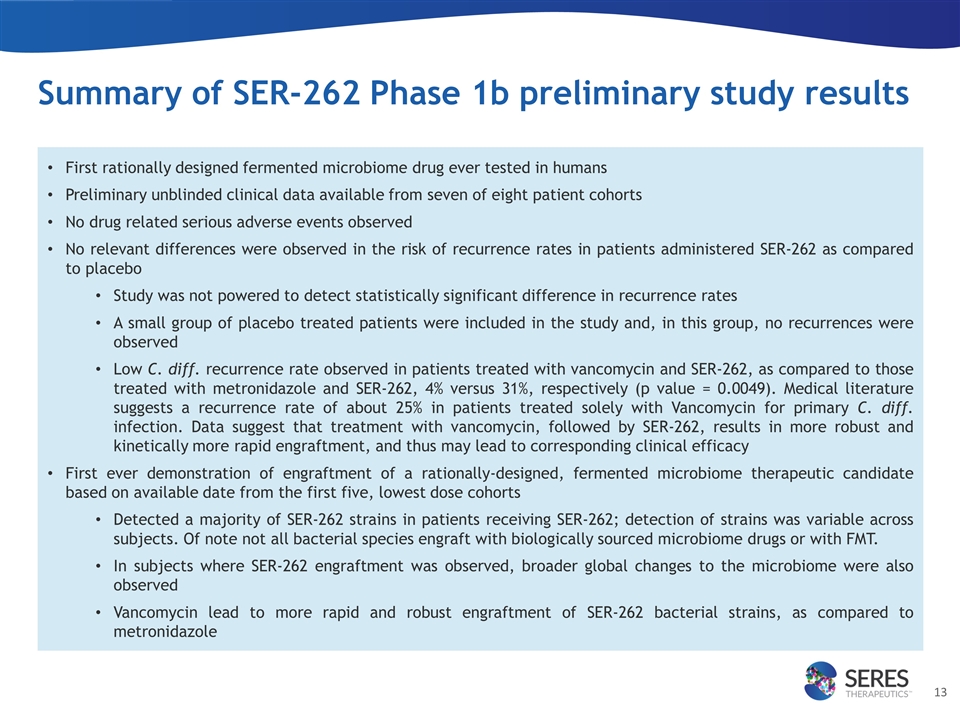
Summary of SER-262 Phase 1b preliminary study results First rationally designed fermented microbiome drug ever tested in humans Preliminary unblinded clinical data available from seven of eight patient cohorts No drug related serious adverse events observed No relevant differences were observed in the risk of recurrence rates in patients administered SER-262 as compared to placebo Study was not powered to detect statistically significant difference in recurrence rates A small group of placebo treated patients were included in the study and, in this group, no recurrences were observed Low C. diff. recurrence rate observed in patients treated with vancomycin and SER-262, as compared to those treated with metronidazole and SER-262, 4% versus 31%, respectively (p value = 0.0049). Medical literature suggests a recurrence rate of about 25% in patients treated solely with Vancomycin for primary C. diff. infection. Data suggest that treatment with vancomycin, followed by SER-262, results in more robust and kinetically more rapid engraftment, and thus may lead to corresponding clinical efficacy First ever demonstration of engraftment of a rationally-designed, fermented microbiome therapeutic candidate based on available date from the first five, lowest dose cohorts Detected a majority of SER-262 strains in patients receiving SER-262; detection of strains was variable across subjects. Of note not all bacterial species engraft with biologically sourced microbiome drugs or with FMT. In subjects where SER-262 engraftment was observed, broader global changes to the microbiome were also observed Vancomycin lead to more rapid and robust engraftment of SER-262 bacterial strains, as compared to metronidazole

SER-287 and Ulcerative Colitis Leading the Microbiome Revolution

Inflammatory Bowel Disease (IBD) opportunity for new mechanistic approaches Significant need for improved therapies Large US population: ~700K ulcerative colitis, ~700K Crohn’s Fewer than ~1/3 of patients achieve remission with current therapies Many therapies are immunosuppressive, limiting widespread use
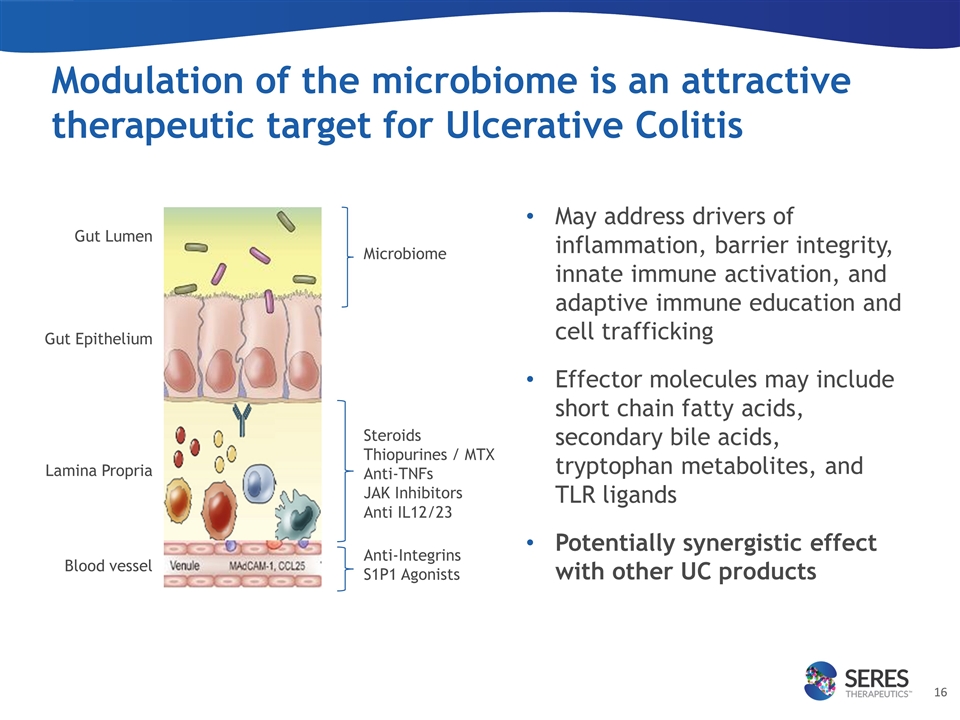
Modulation of the microbiome is an attractive therapeutic target for Ulcerative Colitis May address drivers of inflammation, barrier integrity, innate immune activation, and adaptive immune education and cell trafficking Effector molecules may include short chain fatty acids, secondary bile acids, tryptophan metabolites, and TLR ligands Potentially synergistic effect with other UC products Steroids Thiopurines / MTX Anti-TNFs JAK Inhibitors Anti IL12/23 Microbiome Anti-Integrins S1P1 Agonists Gut Lumen Lamina Propria Blood vessel Gut Epithelium
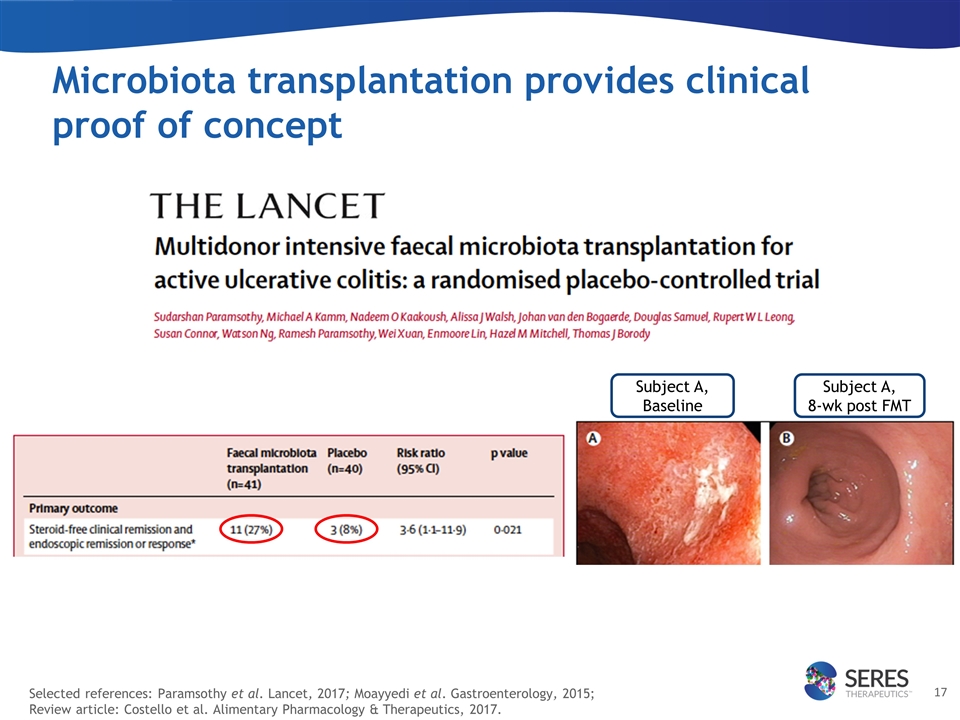
Selected references: Paramsothy et al. Lancet, 2017; Moayyedi et al. Gastroenterology, 2015; Review article: Costello et al. Alimentary Pharmacology & Therapeutics, 2017. Microbiota transplantation provides clinical proof of concept Subject A, Baseline Subject A, 8-wk post FMT
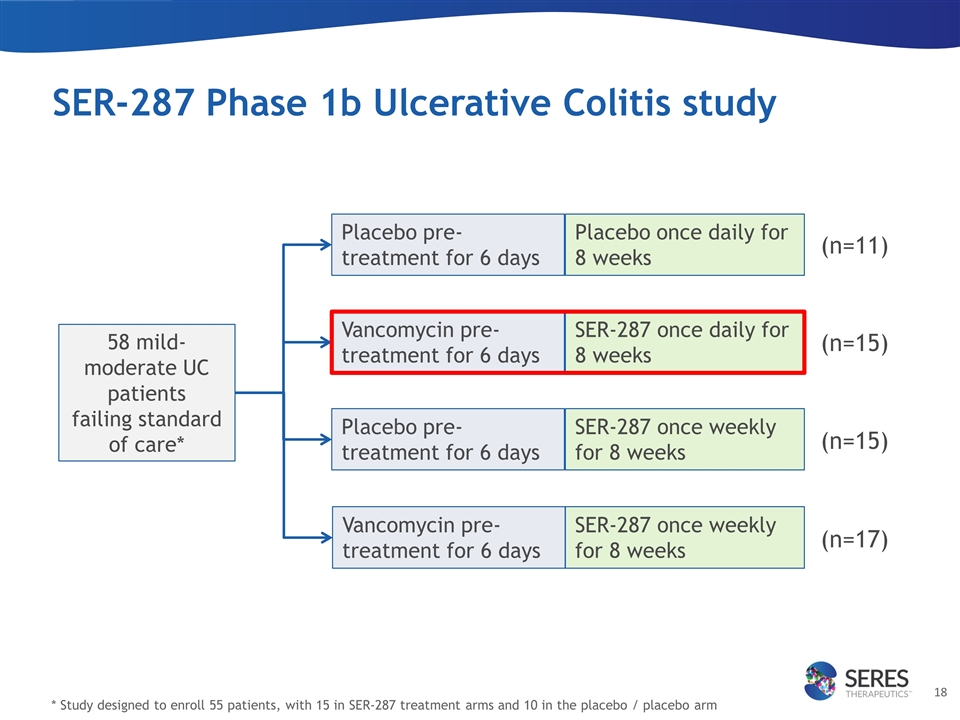
SER-287 Phase 1b Ulcerative Colitis study 58 mild-moderate UC patients failing standard of care* * Study designed to enroll 55 patients, with 15 in SER-287 treatment arms and 10 in the placebo / placebo arm Placebo pre-treatment for 6 days Placebo pre-treatment for 6 days Vancomycin pre-treatment for 6 days Vancomycin pre-treatment for 6 days SER-287 once weekly for 8 weeks Placebo once daily for 8 weeks SER-287 once daily for 8 weeks SER-287 once weekly for 8 weeks (n=11) (n=15) (n=15) (n=17)
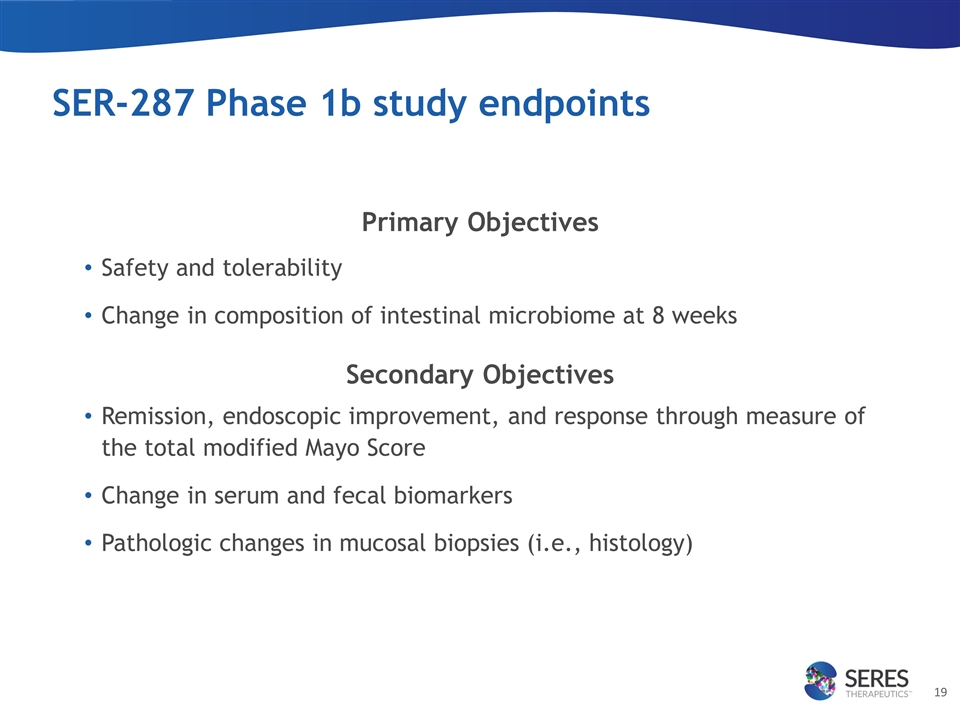
SER-287 Phase 1b study endpoints Primary Objectives Safety and tolerability Change in composition of intestinal microbiome at 8 weeks Secondary Objectives Remission, endoscopic improvement, and response through measure of the total modified Mayo Score Change in serum and fecal biomarkers Pathologic changes in mucosal biopsies (i.e., histology)
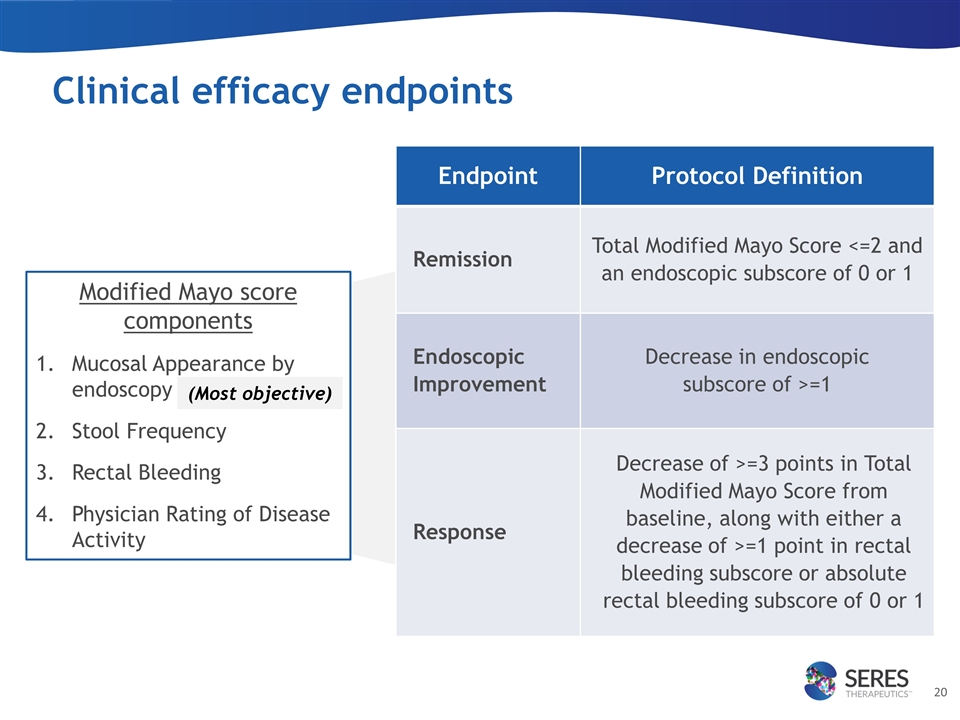
Clinical efficacy endpoints Endpoint Protocol Definition Remission Total Modified Mayo Score <=2 and an endoscopic subscore of 0 or 1 Endoscopic Improvement Decrease in endoscopic subscore of >=1 Response Decrease of >=3 points in Total Modified Mayo Score from baseline, along with either a decrease of >=1 point in rectal bleeding subscore or absolute rectal bleeding subscore of 0 or 1 Modified Mayo score components Mucosal Appearance by endoscopy Stool Frequency Rectal Bleeding Physician Rating of Disease Activity (Most objective)
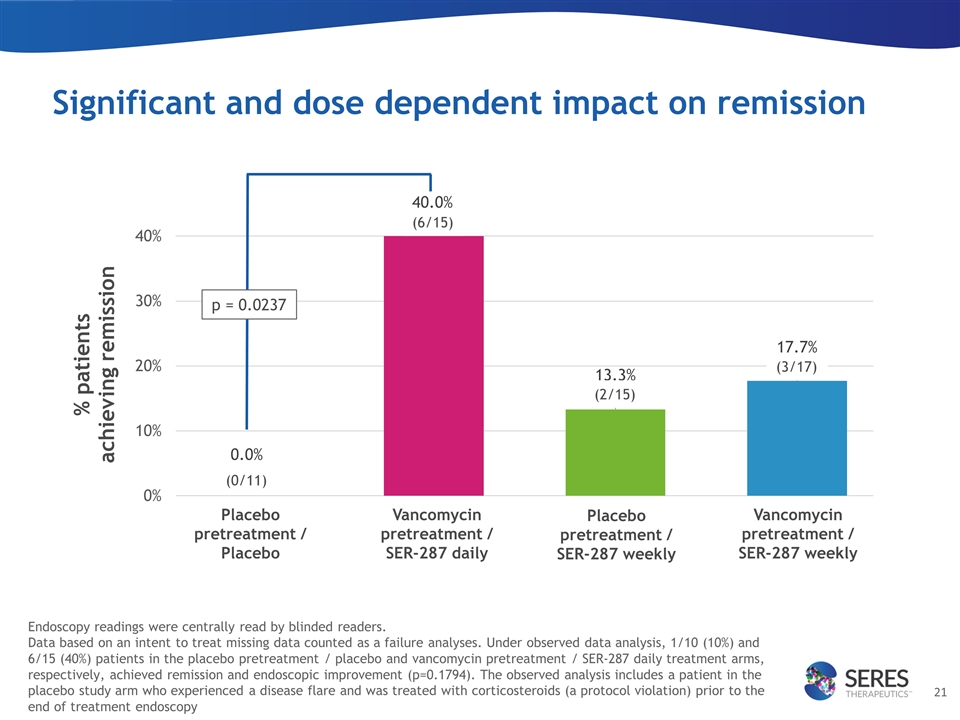
Significant and dose dependent impact on remission Placebo pretreatment / Placebo Vancomycin pretreatment / SER-287 daily Placebo pretreatment / SER-287 weekly Vancomycin pretreatment / SER-287 weekly p = 0.0237 (0/11) (6/15) (2/15) (3/17) Endoscopy readings were centrally read by blinded readers. Data based on an intent to treat missing data counted as a failure analyses. Under observed data analysis, 1/10 (10%) and 6/15 (40%) patients in the placebo pretreatment / placebo and vancomycin pretreatment / SER-287 daily treatment arms, respectively, achieved remission and endoscopic improvement (p=0.1794). The observed analysis includes a patient in the placebo study arm who experienced a disease flare and was treated with corticosteroids (a protocol violation) prior to the end of treatment endoscopy
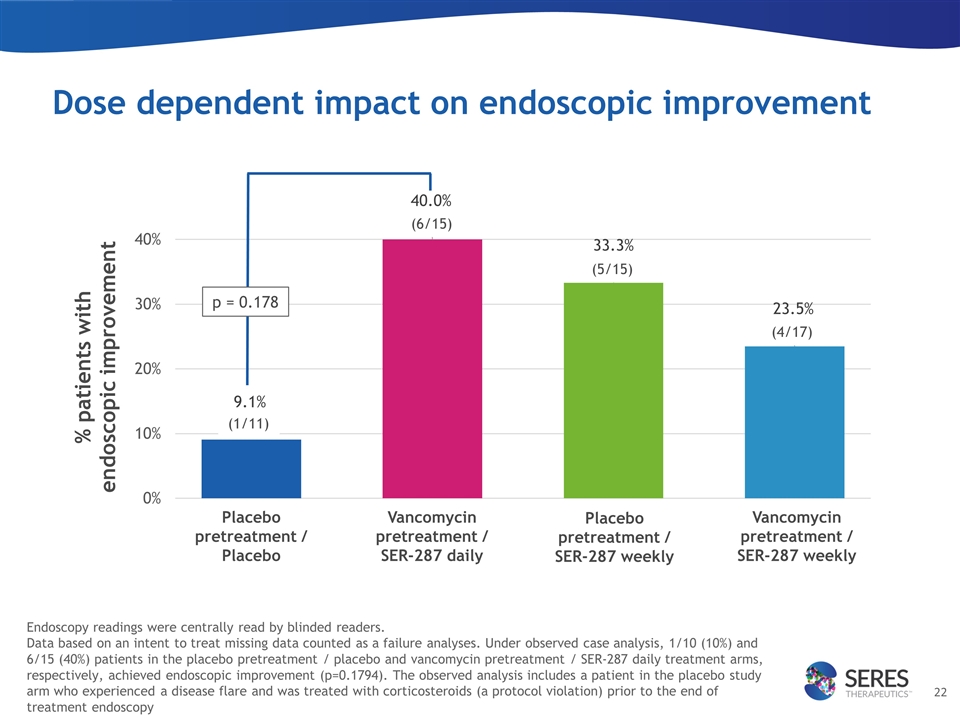
Dose dependent impact on endoscopic improvement Placebo pretreatment / Placebo Vancomycin pretreatment / SER-287 daily Placebo pretreatment / SER-287 weekly Vancomycin pretreatment / SER-287 weekly p = 0.178 (1/11) (6/15) (5/15) (4/17) Endoscopy readings were centrally read by blinded readers. Data based on an intent to treat missing data counted as a failure analyses. Under observed case analysis, 1/10 (10%) and 6/15 (40%) patients in the placebo pretreatment / placebo and vancomycin pretreatment / SER-287 daily treatment arms, respectively, achieved endoscopic improvement (p=0.1794). The observed analysis includes a patient in the placebo study arm who experienced a disease flare and was treated with corticosteroids (a protocol violation) prior to the end of treatment endoscopy
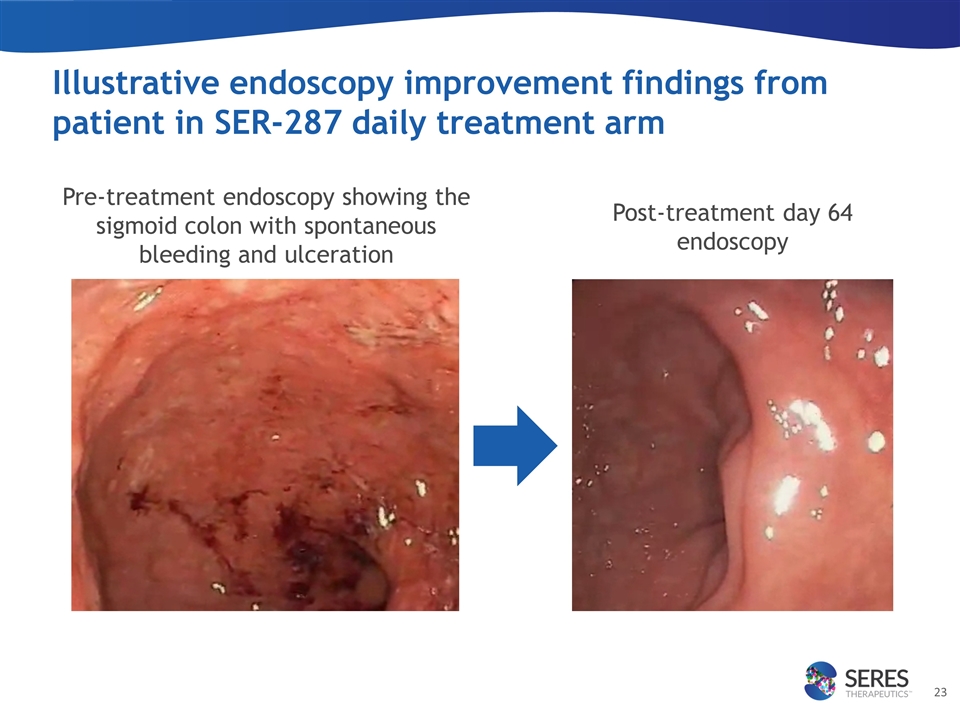
Pre-treatment endoscopy showing the sigmoid colon with spontaneous bleeding and ulceration Post-treatment day 64 endoscopy Illustrative endoscopy improvement findings from patient in SER-287 daily treatment arm
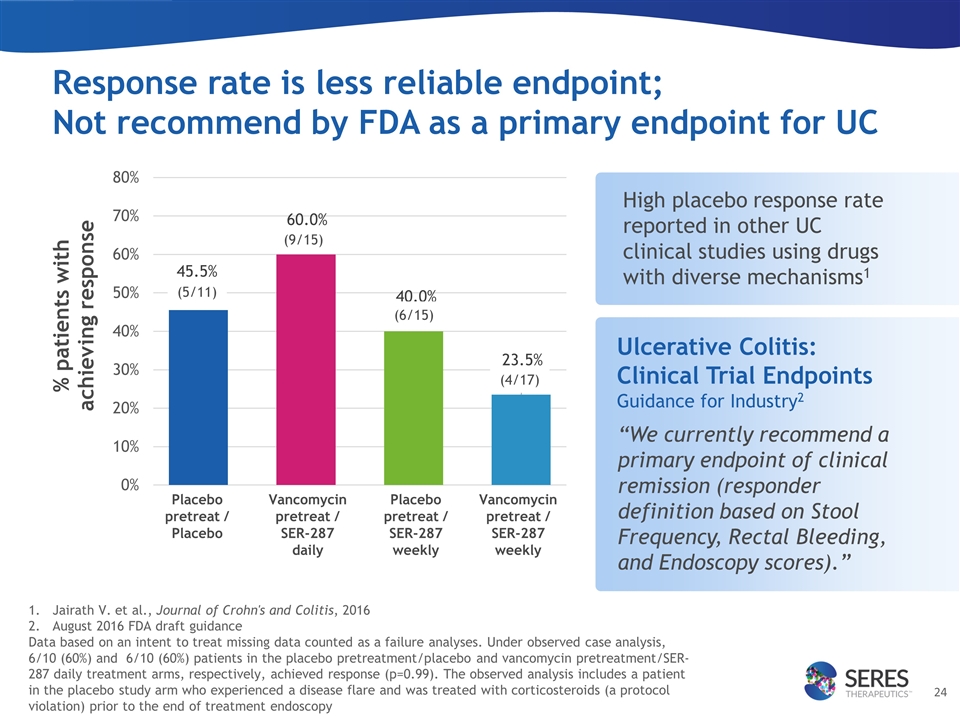
Response rate is less reliable endpoint; Not recommend by FDA as a primary endpoint for UC Placebo pretreat / Placebo Vancomycin pretreat / SER-287 daily Placebo pretreat / SER-287 weekly Vancomycin pretreat / SER-287 weekly Ulcerative Colitis: Clinical Trial Endpoints Guidance for Industry2 (5/11) (9/15) (6/15) (4/17) Jairath V. et al., Journal of Crohn's and Colitis, 2016 August 2016 FDA draft guidance Data based on an intent to treat missing data counted as a failure analyses. Under observed case analysis, 6/10 (60%) and 6/10 (60%) patients in the placebo pretreatment/placebo and vancomycin pretreatment/SER-287 daily treatment arms, respectively, achieved response (p=0.99). The observed analysis includes a patient in the placebo study arm who experienced a disease flare and was treated with corticosteroids (a protocol violation) prior to the end of treatment endoscopy “We currently recommend a primary endpoint of clinical remission (responder definition based on Stool Frequency, Rectal Bleeding, and Endoscopy scores).” High placebo response rate reported in other UC clinical studies using drugs with diverse mechanisms1
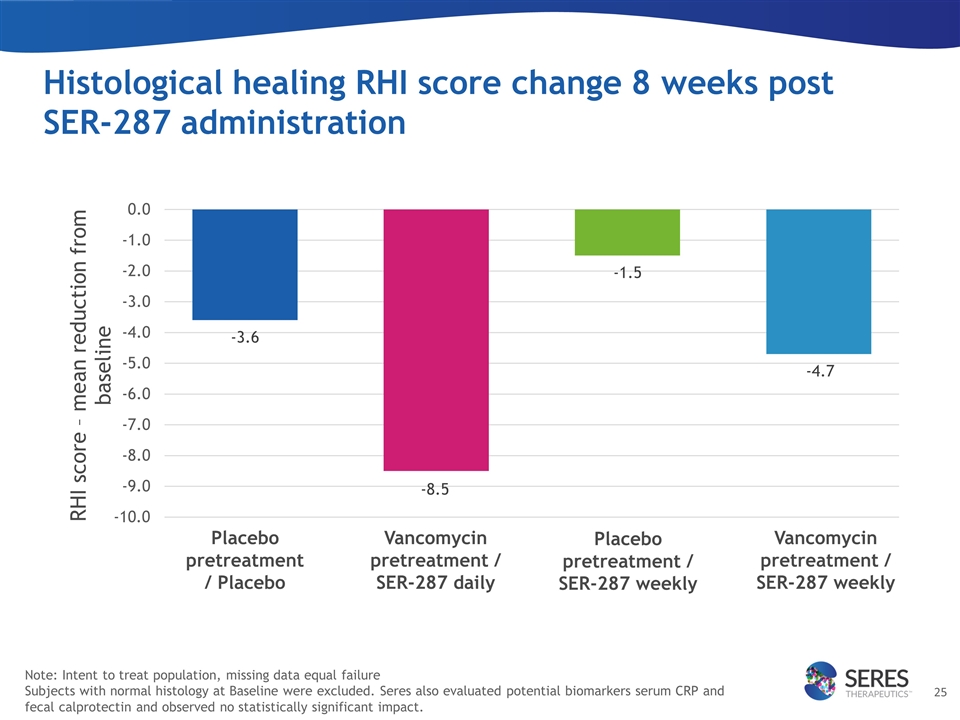
Histological healing RHI score change 8 weeks post SER-287 administration Placebo pretreatment / Placebo Vancomycin pretreatment / SER-287 daily Placebo pretreatment / SER-287 weekly Vancomycin pretreatment / SER-287 weekly Note: Intent to treat population, missing data equal failure Subjects with normal histology at Baseline were excluded. Seres also evaluated potential biomarkers serum CRP and fecal calprotectin and observed no statistically significant impact.

Favorable SER-287 Phase 1b safety profile SER-287 daily arm demonstrated a similar safety profile to placebo No serious drug-related adverse events No subject discontinuations in the SER-287 daily treatment arm Reduced gastrointestinal adverse events provide an independent assessment of efficacy with decreased disease activity SER-287 daily arm GI AEs: 2/15 (13.3%) vs. placebo arm: 5/11 (45.5%)
Analyses of post SER-287 treatment impact on disease activity SER-287 Phase 1b patients were followed for up to 26 weeks post treatment: Of the 11 patients treated with SER-287 who achieved clinical remission, no patients experienced a disease flare in the 26 weeks following the end of treatment (0/11)
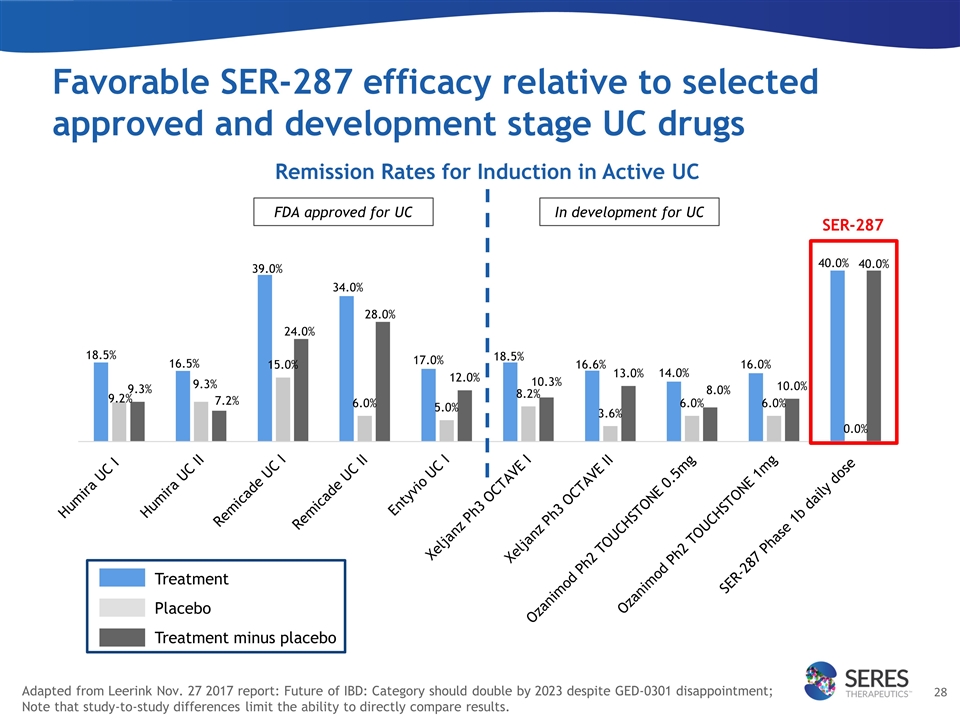
Adapted from Leerink Nov. 27 2017 report: Future of IBD: Category should double by 2023 despite GED-0301 disappointment; Note that study-to-study differences limit the ability to directly compare results. Remission Rates for Induction in Active UC SER-287 FDA approved for UC In development for UC Treatment Placebo Treatment minus placebo Favorable SER-287 efficacy relative to selected approved and development stage UC drugs
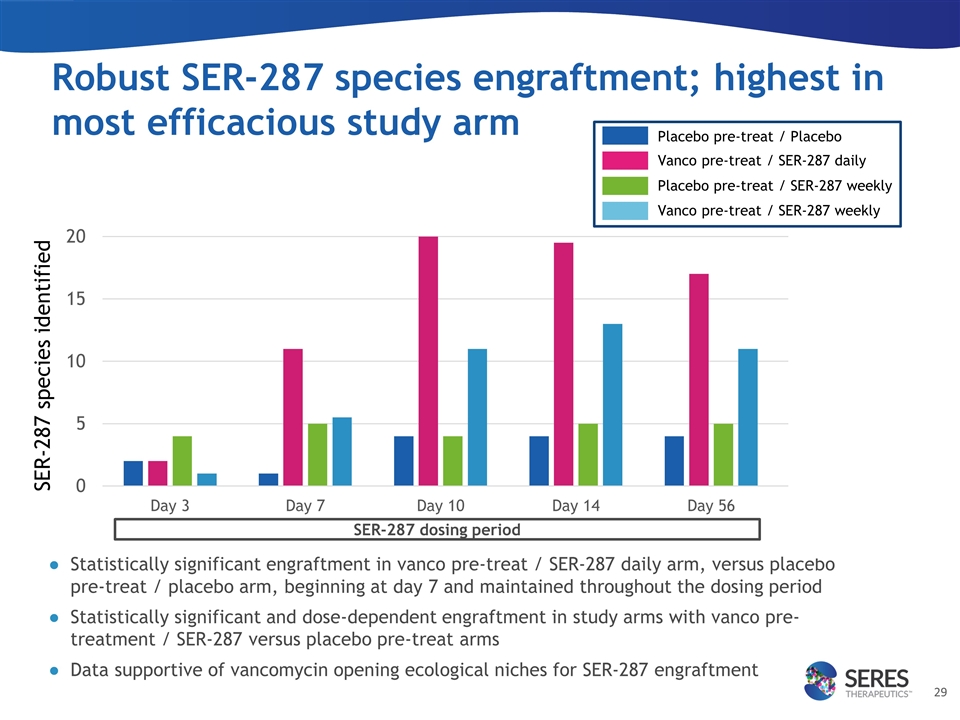
Robust SER-287 species engraftment; highest in most efficacious study arm Statistically significant engraftment in vanco pre-treat / SER-287 daily arm, versus placebo pre-treat / placebo arm, beginning at day 7 and maintained throughout the dosing period Statistically significant and dose-dependent engraftment in study arms with vanco pre-treatment / SER-287 versus placebo pre-treat arms Data supportive of vancomycin opening ecological niches for SER-287 engraftment SER-287 species identified SER-287 dosing period Placebo pre-treat / Placebo Vanco pre-treat / SER-287 daily Placebo pre-treat / SER-287 weekly Vanco pre-treat / SER-287 weekly
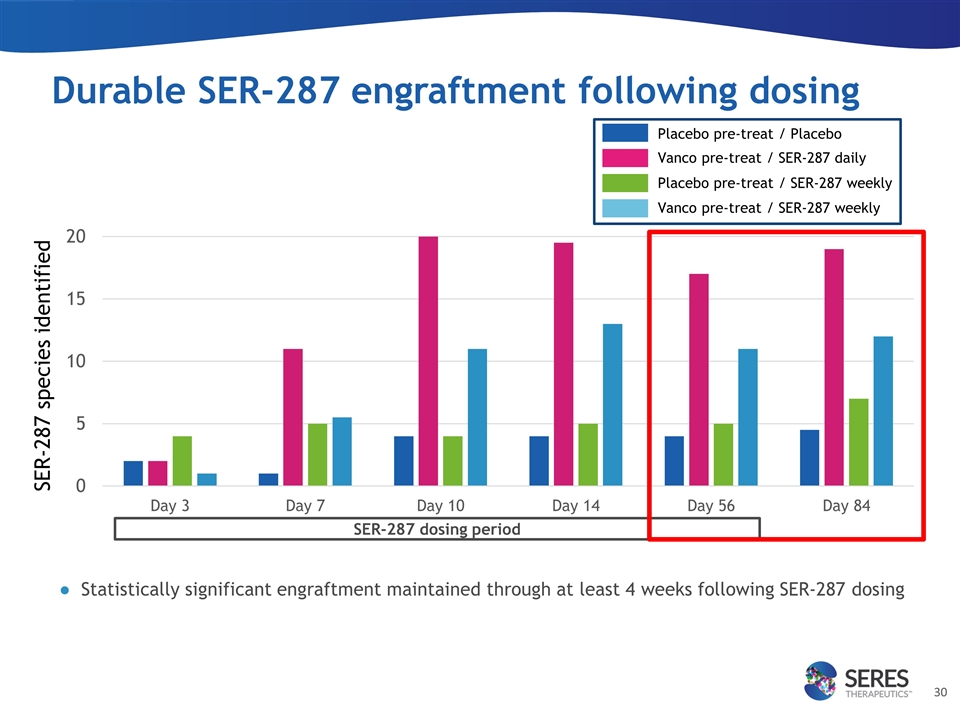
Durable SER-287 engraftment following dosing SER-287 species identified SER-287 dosing period Placebo pre-treat / Placebo Vanco pre-treat / SER-287 daily Placebo pre-treat / SER-287 weekly Vanco pre-treat / SER-287 weekly Statistically significant engraftment maintained through at least 4 weeks following SER-287 dosing
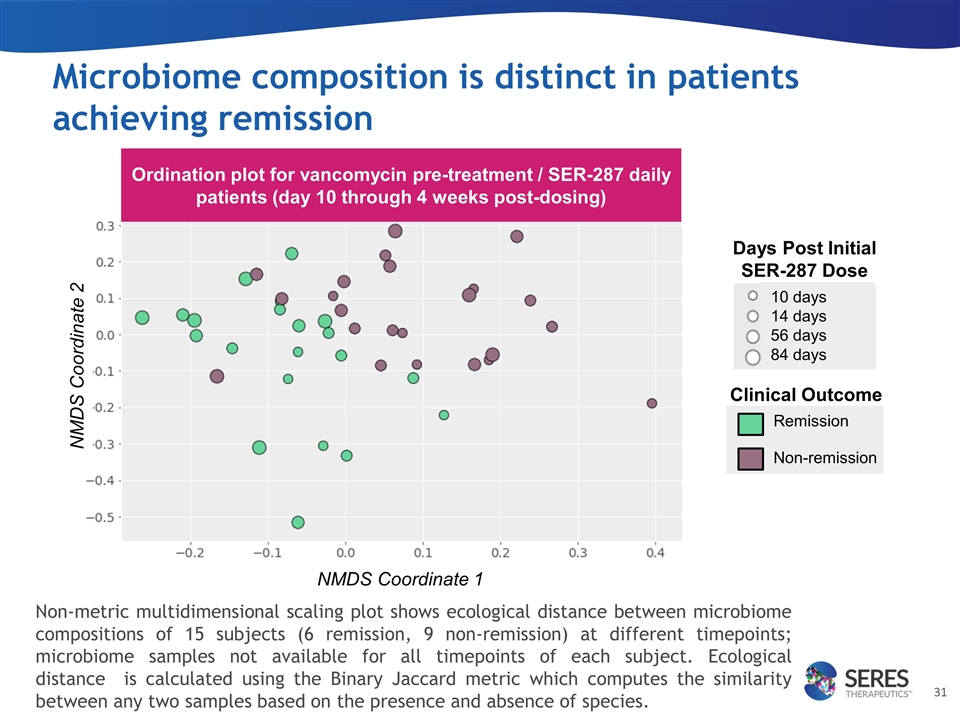
Microbiome composition is distinct in patients achieving remission Days Post Initial SER-287 Dose Clinical Outcome Remission Non-remission 10 days 14 days 56 days 84 days Ordination plot for vancomycin pre-treatment / SER-287 daily patients (day 10 through 4 weeks post-dosing) Non-metric multidimensional scaling plot shows ecological distance between microbiome compositions of 15 subjects (6 remission, 9 non-remission) at different timepoints; microbiome samples not available for all timepoints of each subject. Ecological distance is calculated using the Binary Jaccard metric which computes the similarity between any two samples based on the presence and absence of species. NMDS Coordinate 1 NMDS Coordinate 2
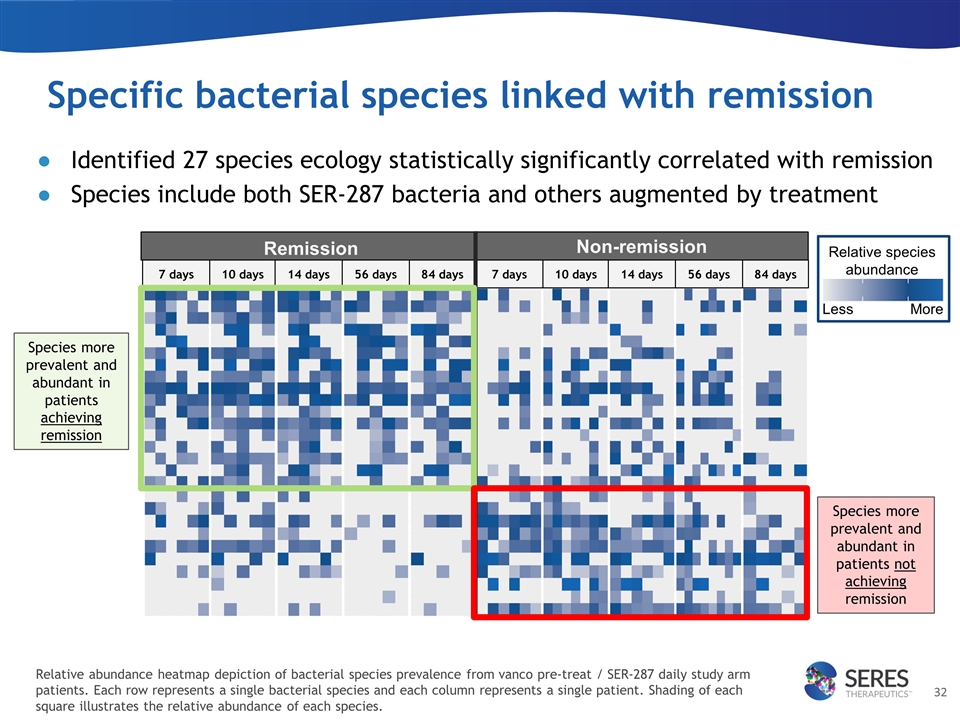
Specific bacterial species linked with remission Remission 7 days 10 days 14 days 56 days 84 days Non-remission 7 days 10 days 14 days 56 days 84 days Identified 27 species ecology statistically significantly correlated with remission Species include both SER-287 bacteria and others augmented by treatment Species more prevalent and abundant in patients achieving remission Species more prevalent and abundant in patients not achieving remission Relative abundance heatmap depiction of bacterial species prevalence from vanco pre-treat / SER-287 daily study arm patients. Each row represents a single bacterial species and each column represents a single patient. Shading of each square illustrates the relative abundance of each species. Less Relative species abundance More

Advancing SER-287 clinical development Compelling Phase 1b results: Beneficial impact on remission and endoscopic improvement Favorable safety and tolerability profile Microbiome data provide mechanistic support for clinical results and demonstrate species-level bacterial signatures associated with efficacy Obtained FDA Orphan Designation for Pediatric Ulcerative Colitis Rapidly advancing SER-287 clinical development: Obtain FDA guidance Expect to start next Ulcerative Colitis clinical study - mid-2018 Evaluate other opportunities (e.g. Crohn’s disease, UC combination therapy) SER-287 Phase 1b study results presented at 13th European Crohn's and Colitis Organisation congress (Feb 14-17, 2018)
SER-301: Synthetic fermented Ecobiotic® therapeutic candidate for inflammatory bowel disease Oral, mechanistically designed follow-on to SER-287 Selection of SER-301 bacterial composition based on: SER-287 study data (clinical and microbiome analysis) Preclinical activity of microbiome compositions Rationally designed composition has shown activity in mouse model

SER-401 and Immuno-oncology Leading the Microbiome Revolution
Gut microbiome composition impacts efficacy of checkpoint inhibitors in oncology patients November 2017 Collaboration
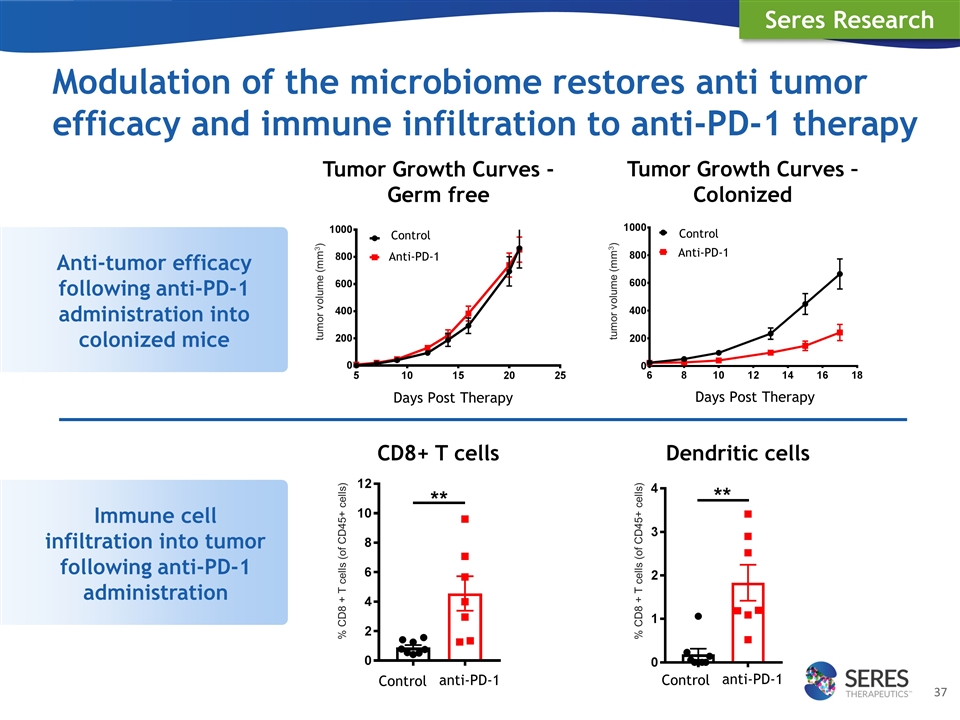
Modulation of the microbiome restores anti tumor efficacy and immune infiltration to anti-PD-1 therapy Immune cell infiltration into tumor following anti-PD-1 administration Dendritic cells CD8+ T cells Seres Research Anti-tumor efficacy following anti-PD-1 administration into colonized mice Tumor Growth Curves – Colonized Days Post Therapy Days Post Therapy Tumor Growth Curves - Germ free anti-PD-1 Control anti-PD-1 Control Control Anti-PD-1 Control Anti-PD-1 tumor volume (mm3) tumor volume (mm3) % CD8 + T cells (of CD45+ cells) % CD8 + T cells (of CD45+ cells)

Collaboration to advance microbiome therapeutic into immuno-oncology Planned placebo-controlled 3 arm clinical study to evaluate impact of checkpoint inhibitors plus adjunctive microbiome therapeutics on clinical outcomes in patients with advanced metastatic melanoma Planned start study in 2018 Seres option to license foundational intellectual property from MD Anderson related to the use of bacteria in combination with checkpoint inhibitors
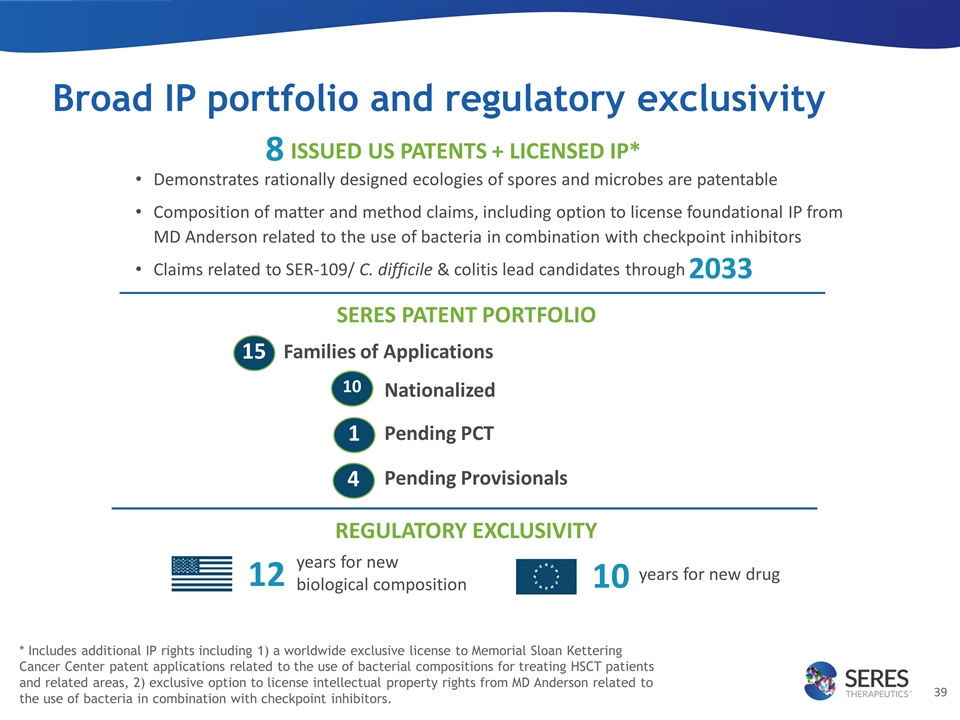
Broad IP portfolio and regulatory exclusivity Families of Applications Nationalized Pending PCT Pending Provisionals SERES PATENT PORTFOLIO 15 10 1 REGULATORY EXCLUSIVITY years for new biological composition 12 years for new drug 10 ISSUED US PATENTS + LICENSED IP* Demonstrates rationally designed ecologies of spores and microbes are patentable Composition of matter and method claims, including option to license foundational IP from MD Anderson related to the use of bacteria in combination with checkpoint inhibitors Claims related to SER-109/ C. difficile & colitis lead candidates through 2033 4 * Includes additional IP rights including 1) a worldwide exclusive license to Memorial Sloan Kettering Cancer Center patent applications related to the use of bacterial compositions for treating HSCT patients and related areas, 2) exclusive option to license intellectual property rights from MD Anderson related to the use of bacteria in combination with checkpoint inhibitors. 8

Resources to operate through Q1 2019 Balance Sheet As of Dec. 31, 2017 Cash, cash equivalents and investments $150 M SER-109: Multiply recurrent C. difficile infection – Phase 3 ongoing SER-287: Ulcerative colitis – Initiate new clinical study (mid-2018) Immuno-oncology clinical study start (2018) Focused R&D efforts to efficiently advance highest priority pipeline programs toward meaningful value inflection points Upcoming Milestones

Appendix Leading the Microbiome Revolution
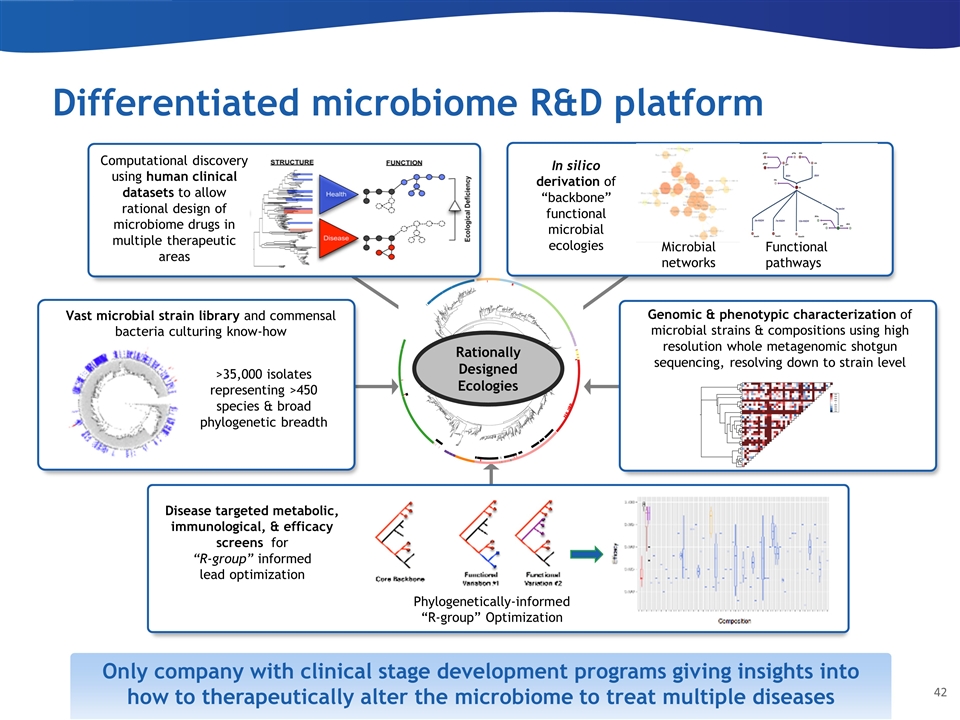
Differentiated microbiome R&D platform Computational discovery using human clinical datasets to allow rational design of microbiome drugs in multiple therapeutic areas Vast microbial strain library and commensal bacteria culturing know-how Disease targeted metabolic, immunological, & efficacy screens for “R-group” informed lead optimization Rationally Designed Ecologies Functional pathways Microbial networks In silico derivation of “backbone” functional microbial ecologies Genomic & phenotypic characterization of microbial strains & compositions using high resolution whole metagenomic shotgun sequencing, resolving down to strain level >35,000 isolates representing >450 species & broad phylogenetic breadth Compositional Variants Efficacy Metric Phylogenetically-informed “R-group” Optimization Only company with clinical stage development programs giving insights into how to therapeutically alter the microbiome to treat multiple diseases
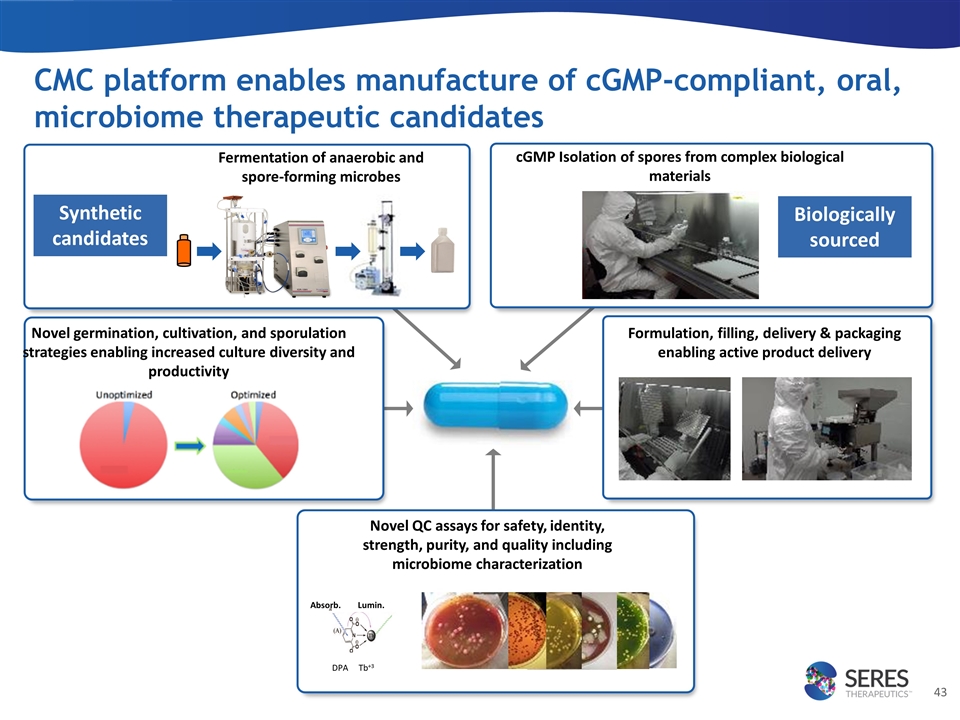
CMC platform enables manufacture of cGMP-compliant, oral, microbiome therapeutic candidates Fermentation of anaerobic and spore-forming microbes Formulation, filling, delivery & packaging enabling active product delivery cGMP Isolation of spores from complex biological materials Synthetic candidates Biologically sourced Novel QC assays for safety, identity, strength, purity, and quality including microbiome characterization Absorb. Lumin. DPA Tb+3 Novel germination, cultivation, and sporulation strategies enabling increased culture diversity and productivity aa
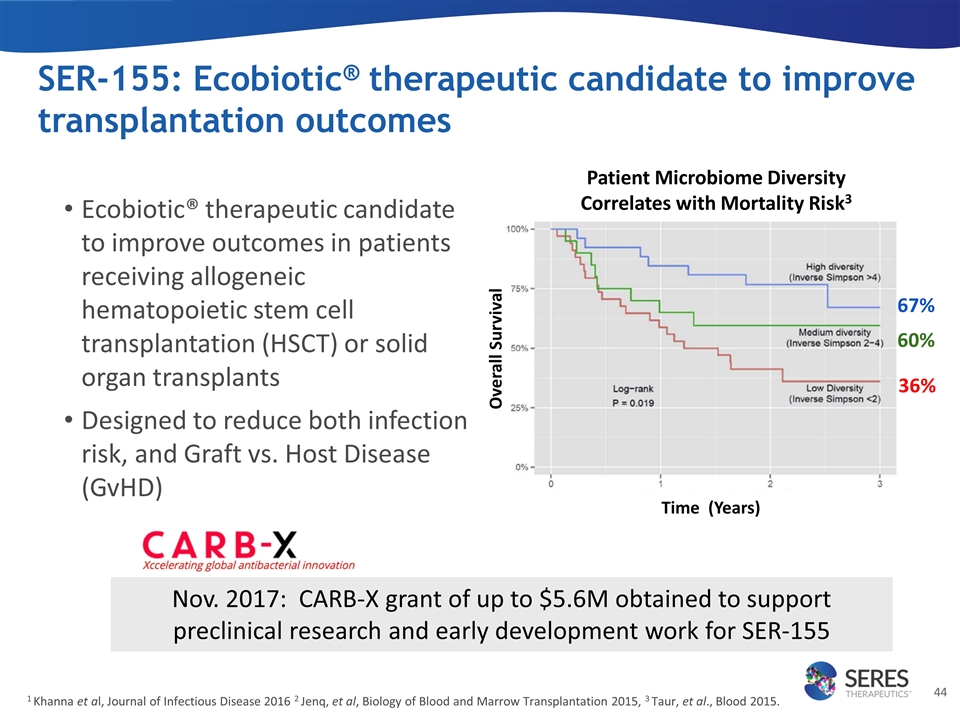
SER-155: Ecobiotic® therapeutic candidate to improve transplantation outcomes 1 Khanna et al, Journal of Infectious Disease 2016 2 Jenq, et al, Biology of Blood and Marrow Transplantation 2015, 3 Taur, et al., Blood 2015. 36% 60% 67% Overall Survival Patient Microbiome Diversity Correlates with Mortality Risk3 Ecobiotic® therapeutic candidate to improve outcomes in patients receiving allogeneic hematopoietic stem cell transplantation (HSCT) or solid organ transplants Designed to reduce both infection risk, and Graft vs. Host Disease (GvHD) Time (Years) Nov. 2017: CARB-X grant of up to $5.6M obtained to support preclinical research and early development work for SER-155
