Form 10-K NanoString Technologies For: Dec 31
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
ý | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2017
OR
¨ | TRANSITION REPORT PURSUANT TO SECTION 13 Or 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ______ to______
Commission file number: 001-35980
NANOSTRING TECHNOLOGIES, INC.
(Exact name of registrant as specified in its charter)
Delaware | 20-0094687 | |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification Number) | |
530 Fairview Avenue North
Seattle, Washington 98109
(Address of principal executive offices)
(206) 378-6266
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
Title of Each Class | Name of Exchange on Which Registered | |
Common Stock, $0.0001 par value per share | The NASDAQ Stock Market LLC (The NASDAQ Global Market) | |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No ý
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act Yes ¨ No ý
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ý No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ý
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer | ¨ | Accelerated filer | ý |
Non-accelerated filer | ¨ (Do not check if a smaller reporting company) | Smaller reporting company | ¨ |
Emerging growth company | ý | ||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). (Check one): Yes ¨ No ý
The aggregate market value of the voting and non-voting stock held by non-affiliates of the Registrant, based on the closing sale price of the Registrant’s common stock on the last business day of its most recently completed second fiscal quarter, as reported on The NASDAQ Global Market, was approximately $347.3 million. Shares of common stock held by each executive officer and director and by each other person who may be deemed to be an affiliate of the Registrant, have been excluded from this computation. The determination of affiliate status for this purpose is not necessarily a conclusive determination for other purposes.
There were 25,440,469 shares of the Registrant’s common stock, $0.0001 par value per share, outstanding on February 28, 2018.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive proxy statement to be filed with the Securities and Exchange Commission in connection with the registrant’s 2018 Annual Meeting of Stockholders, which will be filed subsequent to the date hereof, are incorporated by reference into Part III of this Form 10-K. Such proxy statement will be filed with the Securities and Exchange Commission not later than 120 days following the end of the registrant’s fiscal year ended December 31, 2017.
NANOSTRING TECHNOLOGIES, INC.
ANNUAL REPORT ON FORM 10-K
FOR THE FISCAL YEAR ENDED DECEMBER 31, 2017
TABLE OF CONTENTS
Page | |||
-1-
Special Note Regarding Forward-Looking Information
This Annual Report on Form 10-K, including the “Management’s Discussion and Analysis of Financial Condition and Results of Operation” section in Item 7, and other materials accompanying this Annual Report on Form 10-K contain forward-looking statements that are based on our management’s beliefs and assumptions and on information currently available. The statements contained in this Annual Report on Form 10-K that are not purely historical are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended.
Forward-looking statements can be identified by words such as “believe,” “anticipate,” “could,” “continue,” “depends,” “expect,” “expand,” “forecast,” “intend,” “predict,” “plan,” “rely,” “should,” “will,” “may,” “seek,” or the negative of these terms or and other similar expressions, although not all forward-looking statements contain these words. You should read these statements carefully because they discuss future expectations, contain projections of future results of operations or financial condition, or state other “forward-looking” information. These statements relate to our future plans, objectives, expectations, intentions and financial performance and the assumptions that underlie these statements. These forward-looking statements include, but are not limited to:
• | our expectations regarding our future operating results and capital needs, including our expectations regarding instrument, consumable and total revenue, operating expenses, sufficiency of cash on hand and operating and net loss; |
• | the success, costs and timing of implementation of our business model, strategic plans for our business and future product development plans; |
• | the regulatory regime and our ability to secure regulatory clearance or approval or reimbursement for the clinical use of our products, domestically and internationally; |
• | our ability to successfully commercialize Prosigna, our first in vitro diagnostic product; |
• | our ability to realize the potential payments set forth in our collaboration agreements; |
• | our strategic relationships, including with patent holders of our technologies, manufacturers and distributors of our products, collaboration partners and third parties who conduct our clinical studies; |
• | our intellectual property position; |
• | our ability to attract and retain key scientific or management personnel; |
• | our expectations regarding the market size and growth potential for our business; and |
• | our ability to sustain and manage growth, including our ability to expand our customer base, develop new products, enter new markets and hire and retain key personnel. |
All forward-looking statements are based on information available to us on the date of this Annual Report on Form 10-K and we will not update any of the forward-looking statements after the date of this Annual Report on Form 10-K, except as required by law. Our actual results could differ materially from those discussed in this Annual Report on Form 10-K. The forward-looking statements contained in this Annual Report on Form 10-K, and other written and oral forward-looking statements made by us from time to time, are subject to certain risks and uncertainties that could cause actual results to differ materially from those anticipated in the forward-looking statements, and you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all. Factors that might cause such a difference include, but are not limited to, those discussed in the following discussion and within Part I, Item 1A “Risk Factors” of this Annual Report on Form 10-K. In this report, “we,” “our,” “us,” “NanoString,” and “the Company” refer to NanoString Technologies, Inc. and its subsidiaries.
-2-
PART I
Item 1. Business
Overview
We develop, manufacture and sell robust, intuitive products that unlock scientifically valuable and clinically actionable biologic information from minute amounts of tissue. Our nCounter Analysis System directly profiles hundreds of molecules simultaneously using a novel barcoding technology that is powerful enough for use in research, yet simple enough for use in clinical laboratories worldwide. We market our systems and related consumables to researchers in academic, government, and biopharmaceutical laboratories for use in understanding fundamental biology and the molecular basis of disease and to clinical laboratories and medical centers for diagnostic use. As of December 31, 2017, we had an installed base of approximately 605 nCounter systems, which our customers have used to publish more than 1,800 peer-reviewed papers. As researchers using our systems discover new biologic insights to improve clinical decision-making, these discoveries may be translated and validated as diagnostic tests. For example, our first molecular diagnostic product is the Prosigna Breast Cancer Assay, which provides an assessment of a patient’s risk of recurrence for breast cancer. In addition, we collaborate with biopharmaceutical companies to develop companion diagnostics, that may be used to identify which patients are most likely to respond to a particular therapeutic treatment.
Our nCounter Analysis System enables biologic analysis on a scale appropriate for pathway-based biology, the examination of networks of tens or hundreds of genes and proteins that act in concert to produce biologic functions or trigger certain diseases, by digitally quantifying the activity of up to 800 genes or proteins simultaneously in a single minute tissue sample. Our technology platform is enabled by a unique, proprietary optical barcoding chemistry only available to us. We offer a range of instruments to appeal to an array of potential customer types. Our nCounter SPRINT Profiler is designed to appeal to individual researchers running relatively smaller experiments. Our nCounter MAX is a higher throughput instrument with features appealing to larger core laboratories serving multiple researchers. Our nCounter Analysis System instrument has been FDA 510(k) cleared together with Prosigna and is targeted toward clinical laboratories. All three instruments are capable of running our research consumable products and provide comparable, high-quality data. Our revolutionary new 3D Biology products enable researchers to measure combinations of gene expression, protein expression and gene mutations simultaneously from a single minute tissue sample.
Our technology and products address a fundamental challenge in cancer research. With more cancers being detected earlier, tumor samples are becoming smaller and smaller, while researchers and clinicians have a much greater appetite for information regarding the activity of genes and proteins. The sensitivity and precision of our novel barcoding chemistry allows the measurement of subtle changes in genomic and proteomic activity efficiently from minute samples of tissue. Furthermore, tumor samples are often stored in a format known as formalin-fixed paraffin embedded, or FFPE, which complicates subsequent analysis of genetic material. Our chemistry is particularly compatible with FFPE, increasing its popularity among cancer researchers. The nCounter Analysis System is an easy-to-use and flexible solution that allows researchers to efficiently test hypotheses in a high throughput manner across thousands of different samples. As a result, the nCounter Analysis System is particularly useful for validating networks of genes and proteins that characterize and help predict disease states, such as cancer. Using the FLEX configuration of our nCounter Dx Analysis System, researchers also have the potential to translate their discoveries to the clinic as diagnostics on a single instrument system after receiving any necessary regulatory authorizations.
In addition to the nCounter Analysis System, we are currently developing two new systems enabled by our proprietary optical barcoding chemistry. Following completion of product development, each of these new systems is expected to be commercialized as a new instrument along with associated consumables.
The first new platform in development, Digital Spatial Profiling, or DSP, is designed to allow researchers to address important questions regarding how protein and gene expression vary spatially for regions of interest across the landscape of a heterogeneous tissue biopsy. Our DSP instruments are expected to image slide-mounted tissue biopsies, allow selection of regions of interest, and automate the preparation of samples from selected regions for molecular profiling using either an nCounter system or next generation sequencer. DSP technology is expected to offer a number of advantages compared to traditional technologies, including the ability to profile a larger number of different genes or proteins in each region, more flexibility on the selection of regions, and processing of a larger number of samples per day. Early access sales of DSP instruments are expected to start in late 2018 and the commercial launch of DSP instruments is expected during the first half of 2019.
The second new platform in development, Hyb & Seq, is a next generation sequencing platform. Hyb & Seq is designed to have a workflow that is simpler and faster than current sequencing methods, due to the absence of library preparation, enzymes and amplification. Hyb & Seq's simple workflow and compatibility with a variety of sample types offers
-3-
the potential for a sample-to-answer solution for clinical sequencing. The commercial launch of a research version of Hyb & Seq is expected during 2020.
We generated revenue of $114.9 million, $86.5 million and $62.7 million in 2017, 2016 and 2015, respectively, while incurring net losses of $43.6 million, $47.1 million and $45.6 million in 2017, 2016 and 2015, respectively.
We are organized as, and operate in, one reportable segment. For additional information, see Note 2 of the Notes to Consolidated Financial Statements under Item 8 of this report. For financial information regarding our business, see Part II, Item 7 “Management’s Discussion and Analysis of Financial Condition and Results of Operations” of this report and our audited consolidated financial statements and related notes included elsewhere in this report.
We were incorporated in Delaware in June 2003. Our principal executive offices are located at 530 Fairview Avenue, North, Seattle, Washington 98109 and our telephone number is (206) 378-6266. Our common stock trades on The NASDAQ Global Market under the symbol “NSTG.”
This Annual Report on Form 10-K includes our trademarks and registered trademarks, including “NanoString,” “NanoString Technologies,” “nCounter,” “Prosigna,” “nCounter Elements,” “nCounter SPRINT,” “Vantage 3D,” “3D Biology,” and “Hyb & Seq.” Each other trademark, trade name or service mark appearing in this Annual Report on Form 10-K belongs to its holder.
Our Market Opportunity
Every living organism has a genome that contains the full set of biological instructions required to build and maintain life. By analyzing the variations in genomes, genes, gene activity, and proteins in and between organisms, researchers can determine their functions and roles in health and disease. An improved understanding of the genome and its functions allows researchers to drive advancements in scientific discovery. As they make scientific discoveries, researchers have been able to translate some of these findings into clinical applications that improve patient care.
A gene is a specific set of instructions embedded in the DNA of a cell. For a gene to be “turned on,” or “expressed,” the cell must first transcribe a copy of its DNA sequence into molecules of messenger RNA. Then, the cell translates the expressed information contained in the RNA into proteins that control most biological processes. In addition to the translated RNAs, there are many types of non-coding RNAs that are involved in many cellular processes and the control of gene expression, including microRNA, or miRNA.
Biological pathways are the networks of tens or hundreds of genes that work in concert to produce a biological function. Understanding the activation state of pathways and disruptions in individual elements of these pathways provides significant insight into the fundamental basis of disease and facilitates data driven treatment decisions. Therapeutic interventions, such as drugs, can be used to treat disease by activating or inactivating biological pathways that are relevant to disease. As a result, pathway-based biology has become a widely adopted paradigm that researchers use to understand biological processes and has assisted them in the development of diagnostics and drugs to treat disease. This is particularly important in cancer research and treatment.
Over the last decade, methods of measuring genomic information have advanced substantially. However, pathway-based research and the development of diagnostic tests require analysis of multiple genes and sensitivity to small changes in expression, which can be challenging for traditional genomic tools. In both life sciences research and clinical medicine, there is a growing need for improved technologies that can precisely and rapidly measure the activation state of hundreds of genes simultaneously across a large number of precious samples. Furthermore, there is an untapped opportunity for technologies capable of simultaneously profiling the activity of genes and related proteins, which ultimately dictate biological activity.
Life Sciences Research
Academic, government, and biopharmaceutical researchers engaged in gene expression or protein analysis typically focus on making biological discoveries that may lead to the development of relevant medical products and better informed treatment decisions for physicians and patients. They have traditionally performed gene expression experiments using microarrays or quantitative polymerase chain reaction, or PCR, and protein expression experiments using flow cytometry, mass spectrometry, immunohistochemistry or enzyme-linked immunosorbent assay, or ELISA, assays. More recently, RNA sequencing, or RNA-Seq, has dramatically enhanced researchers’ ability to discover patterns of gene expression that have biological meaning. However, related workflows and data analysis can be cumbersome and time consuming, and simultaneous analysis of proteins is not possible. Researchers are increasingly performing analyses on a larger number of genes and samples and are seeking new methods of interrogation that would allow them to:
• | increase the number of molecular targets that can be analyzed simultaneously in order to understand the complete biological pathway involving multiple genes; |
-4-
• | improve the overall efficiency of their laboratories by simplifying workflow and accelerating the rate of successfully completing their research; |
• | provide more reliable, precise and reproducible data about targeted genes and biological pathways; |
• | maximize the amount of biologic information extracted from precious tissue samples; |
• | minimize the computational intensity of complex genomic and proteomic analysis; |
• | process difficult-to-work-with specimens, such as tumor biopsies stored in FFPE format; and |
• | create more systematic and reliable ways to help transition their research discoveries into future clinical products. |
We believe that the above items create an opportunity for technologies like ours that are optimized for pathway-based biology, providing the potential for seamless transition to clinical diagnostic testing laboratories on a distributed basis, with the capability to analyze both genomic and proteomic information.
Molecular Diagnostics
Growth in the molecular diagnostics market is being driven by technological innovations that have enabled unprecedented biological insights that may be used to inform treatment decisions. New and improved technologies have also led to increased test sensitivity, decreased turnaround times, simplified workflow, and lowered costs when compared to previous techniques. In addition, the medical community has seen a trend in favor of decentralized diagnostic testing as a result of the convenience of local testing, hospitals and medical centers increasingly viewing their laboratories as profit centers and a need to increase access to tests for patients outside of the United States. We believe that there is an opportunity to improve the quality of diagnosis and treatment of diseases by developing and commercializing comprehensive, simple and widely available diagnostic products based initially on gene expression analysis, and ultimately based on our 3D Biology capability.
Cancer is a disease generally caused by genetic mutations in cells. The behavior of cancer cells is extremely complex, depending on the activity of many different genes and proteins. It is often impossible for researchers to identify a single gene or protein that adequately predicts a more aggressive or less aggressive type of cancer. In some cases, researchers have been able to identify more aggressive or less aggressive types of cancer through gene expression analysis of biological pathways, enabling oncologists to determine which specific treatments are most likely to be effective for an individual patient, monitor a patient’s response to those treatments, and determine the likelihood of recurrence.
Recently, researchers in the field of oncology have begun to demonstrate the potential of harnessing a patient’s immune system to fight cancer. A new class of therapeutics, referred to generally as immuno-oncology drugs, have begun to come to market with the promise of long-term remissions, or even cures, in certain types of cancer. Unlike cancer therapeutics of the past, these therapeutics do not target genetic abnormalities and there are typically no reliable genetic biomarkers for determining which patients are likely to respond to treatment. The development of diagnostics to inform decisions regarding treatment with immuno-oncology drugs is likely to require analysis of both RNA and proteins.
In addition, the medical community has favored a trend toward decentralized diagnostic testing. Tests for HIV, Hepatitis C, Influenza and MRSA, which were once centralized, are now often conducted in hospital laboratories or at the point of care. We believe that this trend of decentralized testing will continue as a result of many factors, including:
• | Convenience. We believe that physicians would prefer that molecular diagnostic tests be performed at a local level and in the same laboratory that performs other tests that the physicians may order. Local molecular diagnostic testing could provide physicians the same rapid turnaround of test results that they have learned to expect for other types of tests. |
• | Economic Advantages. We believe that hospitals and medical centers desire to make their clinical laboratories profit centers by performing tests and billing third-party payors. As diagnostic technologies become less complicated to administer, hospitals and medical centers tend to favor in-sourcing tests. |
• | International Availability. There is a critical need to increase access to molecular diagnostic tests for patients that live outside the United States. Currently, patients living outside the United States may be challenged to gain access to tests that are provided only by specialized laboratories located within the United States. We believe advanced molecular diagnostic testing will become more available to patients throughout the world when it can be provided by their local clinical laboratories. |
We believe that these factors create an opportunity for technologies like ours that can facilitate the development and use of complex molecular diagnostics, potentially targeting gene mutations, gene expression and protein expression, with a high level of precision on a decentralized basis.
-5-
Our Solution
Our nCounter Analysis System is an automated, multi-application, digital detection and counting system which directly profiles hundreds of molecules simultaneously using a novel barcoding technology that is powerful enough for use in research, yet simple enough for use in clinical laboratories worldwide. Our nCounter Analysis System is based on automated instruments that prepare and analyze tissue samples using proprietary reagents, which can only be obtained from us. Our research customers purchase instruments from us and then purchase our reagents and related consumables for the specific experiment or assay they wish to conduct. Our clinical laboratory customers either purchase or lease instruments from us and also purchase our reagents and related consumables for tests that they intend to run.
Our nCounter Analysis System offers a number of compelling advantages, including:
• | Optimized for Pathway-Based Biology. The nCounter Analysis System can profile up to 800 molecules in a single test tube, which allows customers to analyze interactions among hundreds of genes or proteins that mediate biological pathways. |
• | Digital Precision. Our molecular barcodes hybridize directly to the target molecules in a sample allowing them to be counted. This generates digital data (1 molecule = 1 count) of excellent quality over a wide dynamic range of measurements and provides excellent reproducibility. |
• | Simple Workflow. The nCounter Analysis System’s minimal sample preparation and automated workflow enable the simultaneous analysis of hundreds of genes and proteins in approximately 24 hours between the time a sample is loaded into the system and results are obtained. Our nCounter Analysis System generates data that customers can evaluate without the use of complex bioinformatics. |
• | Flexible Sample Requirements. The nCounter Analysis System is able to unlock biologic information from minute amounts of a variety of challenging tissue samples, including FFPE samples, cell lysates and single cells. |
• | Versatility. The FLEX configuration of the nCounter Analysis System provides clinical laboratories a single platform with the flexibility to support both clinical testing, by running Prosigna or Laboratory Developed Tests using nCounter-based reagents, and research, by processing translational research experiments and multiplexed assays using our research reagents. |
Life Sciences Research
Our nCounter Analysis System is capable of supporting a number of research applications based upon the measurement of the concentration or amount of a target molecule. Additionally, starting in September 2015, we have launched a series of 3D Biology applications, which enable the simultaneous analysis of DNA, RNA and proteins in a single sample. Key applications currently supported include:
• | Gene Expression. Researchers can use the nCounter Analysis System to measure the degree to which individual genes in pathways are turned “on” or “off” by simultaneously quantifying the amount of messenger RNA, or mRNA, associated with each of up to 800 genes. |
• | Protein Expression. Today, researchers can use the nCounter Analysis System to measure simultaneously up to 30 proteins. Ultimately, we intend to expand this capability to an increased number of protein targets, limited only by the 800 target capacity of an assay and the number of antibodies that can be sourced and combined without cross-reaction. |
• | Gene Mutations. In late 2016, we launched our first assay to detect a particular type of gene mutation, known as single nucleotide variations. Our initial panel, targeting solid tumors, gives researchers the power to measure 104 different gene mutations simultaneously, at the same time as measuring the expression of other genes and proteins. |
• | miRNA Expression. Researchers can use the nCounter Analysis System to measure the simultaneous expression levels of up to 800 different miRNAs. The nCounter Analysis System is capable of highly multiplexed, direct digital detection and counting of miRNAs in a single reaction without amplification, thereby delivering high levels of sensitivity, specificity, precision, and linearity. |
• | Copy Number Variation. Researchers can use the nCounter Analysis System to probe for structural variations that result in cells having an abnormal number of copies of one or more sections of the DNA. Researchers are able to conduct large-scale, statistically-powered studies of these copy number variations, or CNVs, by leveraging the nCounter Analysis System’s multiplexing capacity to assay up to 800 DNA regions in a single tube, with as little as 300 ng of DNA. |
• | Gene Fusions. Researchers can use the nCounter Analysis System to detect gene fusion events that occur when one gene fuses to another gene. A number of design options are available for developing assays for |
-6-
these complex structural variants which have been shown to be important in a number of cancers. In 2016, we launched two off-the-shelf panels for analysis of fusion genes relevant to lung cancer and leukemia.
Molecular Diagnostics
We believe that the attributes that make the nCounter Analysis System attractive to researchers also make the system attractive to hospitals and clinical laboratories that desire to conduct molecular diagnostic tests. The precision, ease of use and flexibility of the nCounter Analysis System will allow medical technicians in pathology labs to conduct complex molecular diagnostic tests with minimal training. We expect these tests to encompass both Laboratory Developed Tests based on our nCounter-based reagents and in vitro diagnostic kits.
Our Products and Technology
Instruments and Software
The nCounter Analysis System is an automated, multi-application, digital detection and counting system. In 2008, we began marketing a research use only version of the system, and since that time we have expanded our product line to include three instruments, each targeted at a distinct user segment of our target market.
nCounter SPRINT | nCounter MAX | nCounter FLEX | |||
Target customer | Individual researchers | Core research labs | Clinical labs | ||
Throughput (samples per day) | 24 | 48 | 48 | ||
Expandable with additional prep station (1) | No | Yes | Yes | ||
Diagnostic menu | No | No | Yes | ||
U.S. list price | $149,000 | $235,000 | $265,000 | ||
(1)nCounter MAX and FLEX throughput may be increased to up to 96 samples per day by adding a second prep station.
The nCounter MAX and FLEX systems comprise a Prep Station and a Digital Analyzer. The Prep Station is the automated liquid handling component of the nCounter Analysis System that processes samples after they are hybridized and prepares the samples for data collection on the nCounter Digital Analyzer. The nCounter Digital Analyzer collects data from samples by taking images of the immobilized fluorescent reporters in the sample cartridge and processing the data into output files, which include the target identifier and related count numbers along with a broad set of internal controls that validate the precision of each assay. The nCounter SPRINT Profiler is a single instrument targeted to individual researchers that provides both the liquid handling steps and the digital analysis through use of a microfluidic cartridge. The nCounter FLEX system was designed and is manufactured under ISO 13485:2003, the current quality standard for in vitro diagnostic platforms and medical devices. We also provide our research customers with the nSolver Analysis Software, a data analysis program that offers researchers the ability to quickly and easily quality check, normalize, and analyze their data without having to use any additional software for data analysis. The diagnostic version of our instrument, the nCounter Analysis Dx FLEX System, was FDA 510(k) cleared and CE-marked together with Prosigna. The FLEX System can be enabled with the software that runs Prosigna to generate individualized patient reports, in addition to running any of our research applications.
The nCounter MAX and FLEX Systems employ a simple three-step workflow that takes approximately 24 hours and requires approximately 15 minutes of hands-on time by the user. When run in research mode, a user can process up to 48 samples per day by installing one Prep Station with a single Digital Analyzer. One can increase the number of samples analyzed to 96 samples per day on a single Digital Analyzer if it is coupled with two Prep Stations. This throughput can be quadrupled using sample multiplexing for experiments targeting 200 genes or fewer. For Prosigna, a clinical laboratory can process up to 30 samples per day on an nCounter Dx Analysis System. The nCounter SPRINT Profiler employs an even more streamlined two-step workflow that requires only 10 minutes of hands-on time by the user and can process up to 24 samples per day.
Life Sciences Research Consumables
Following purchase of an nCounter Analysis System, research customers purchase consumables from us to enable their research experiments. These include custom CodeSets targeted to a specific experiment, panels and nCounter-based reagents.
Custom CodeSets
We work with our customers to design and develop custom gene expression CodeSets to enable them to evaluate specific genes that are the subject of their study. Our customers provide us a list of targets for which we subsequently build a unique CodeSet to their specifications. Our design process leverages full length sequences for the DNA or RNA molecules that our customers are interested in detecting and prevents cross hybridization to non-target molecules in the sample. The custom CodeSet design process occurs in four distinct steps: (1) the customer selects the genes of interest, (2) we design probes and
-7-
provide a design report to the customer, (3) the customer reviews and approves the design report, and (4) we manufacture, test and ship the CodeSet to the customer. The manufacturing process typically takes from three to five weeks, depending on the number of genes targeted and samples to be processed by the customer.
Panels
We offer more than 30 gene expression and analysis panels for use with a broad range of sample types and species, including human, mouse, rat, non-human primate and other. These pre-manufactured CodeSets include highly-curated content relevant to a particular research area. Our most significant current panel offering include:
• | Pan Cancer Gene Expression Panels. A portfolio of panels designed to comprehensively analyze genes driving the growth of cancer cells, the immune system’s response, and the progression of the cancer, including: |
◦ | Pathways. A novel set of 770 essential genes representing the signaling pathways implicated in cancer, including key driver genes, selected using a data-driven approach to identifying the genes most relevant to cancer biology. |
◦ | Immune Profiling. A novel set of 770 genes designed in collaboration with cancer immunologists around the globe, combining markers for 24 different immune cell types and populations, 30 common cancer antigens and genes that represent all known categories of immune response including key checkpoint blockade genes. |
◦ | Progression. A novel set of 770 genes addressing the key questions of what happens when cancer metastasizes, including genes for the study of angiogenesis, epithelial mesenchymal transition, extracellular matrix formation, and metastasis. |
• | PanCancer RNA: Protein Immune Profiling Panels. Two panels that combine gene expression analysis of the 770 genes contained in the PanCancer Immune Profiling Gene Expression Panel with the analysis of up to 30 proteins of interest in measuring the immune system’s response to cancer or intracellular signaling. |
• | 360 Gene Expression Panels. These panels include our IO 360 and Breast Cancer 360 and represent a series of next generation panels that combine clinically actionable content for evaluating the tumor microenvironment and immune response along with validated signatures such as the Company's Tumor Inflammation Signature (TIS) and PAM 50 (Breast cancer subtyping) along with up to 30 additional signatures encompassing all aspects of the cancer. These panels may be combined with our 360 Data Analysis Service to provide access to propriety signature algorithms. |
• | Neuropathology and Neuroinflammation Gene Expression Panels. Two new panels built in collaboration with leading drug developers, have been designed to address the growing biomarker needs in the field of neuroscience. These panels, which analyze approximately 770 genes profile mechanisms for neurodegenerative diseases as well as neuropsychiatric disorders. |
• | Autoimmune Disease Gene Expression Panels. Two new panels created to address the specific challenges of autoimmune disease research, and assist with the understanding of the underlying mechanisms of autoimmune disease and for identification of potential responders and non-responders to drug treatments. |
• | nCounter Vantage 3D Panels. A portfolio of panels that enable simultaneous analysis of DNA, RNA, and protein, eliminating the need to divide small samples among different experiments, including: |
◦ | RNA:Protein Solid Tumor Assay — Panels for lysates and FFPE that profile key cancer pathway targets at the RNA, total protein, and phospho-protein level, including targets associated with PI3K, MAPK, EGFR, and more. |
◦ | DNA SNV Solid Tumor Panel — A panel targeting 104 single nucleotide variants in 25 genes that are relevant to a variety of solid tumor types, currently available for MAX and FLEX systems. |
◦ | RNA Panels — A series of seven 192-gene expression panels each designed to interrogate a focused area of cancer biology: Adaptive Immunity, Cancer Metabolism, Intracellular Signaling, Cellular Profiling, Wnt Pathway, Innate Immunity, and DNA Damage & Repair. |
◦ | Lung Fusions — A panel targeting gene fusions that involve the following genes which are important in lung cancer: ALK, ROS, RET, and NTRK1. |
◦ | Leukemia Fusions — A panel targeting 27 gene fusions that are important in leukemia. |
• | Other Gene Expression Panels. A series of panels that allow researchers to conduct a wide variety of gene expression analysis, including analysis of both human and mouse immunology-related genes and inflammation-related genes. |
• | miRNA Expression Panels. A family of panels that provide a cost-effective profiling solution capable of highly multiplexed, direct digital detection and counting of up to 800 miRNAs in a single reaction without amplification. Separate panels are available for use with samples from humans, mice, rats, and fruit flies. |
-8-
• | Cancer Copy Number Variation Panel. Enables copy number quantification for 87 genes commonly amplified or deleted in cancer. |
nCounter-Based Reagents
Our nCounter-based digital molecular barcoding reagents allow users to design their own customized assays using standard sets of barcodes provided by us with the laboratory's choice of oligonucleotide probes that can be purchased independently from an oligonucleotide manufacturer. The highly flexible architecture of these reagents enables a broad range of basic research studies where iterative design and refinement of assays are important.
Master Kits, Cartridges and Reagents
For our nCounter MAX or FLEX systems, the Master Kit includes all of the ancillary reagents and plasticware required for our customers to be able to setup and process samples in the nCounter Prep Station and nCounter Digital Analyzer. The components of the Master Kit include the sample cartridge, strip tubes, tips, buffers, and reagent plates. For our nCounter SPRINT Profiler, customers purchase microfluidic cartridges and separate bottles of reagents which together provide the ancillary components for processing samples with CodeSets, Panels or nCounter-based reagents.
Molecular Diagnostics
Our nCounter Dx Analysis System has the ability to simultaneously quantify gene expression on tens or hundreds of genes from minimal amounts of FFPE tissue, which makes it well suited for profiling pathway activation in tumor samples. In addition, it has the precision, reproducibility, and simple workflow required of technologies used in clinical laboratories. Our clinical laboratory customers use the nCounter Dx Analysis System, nCounter-based reagents and in vitro diagnostic kits to provide clinical diagnostic services. Currently, Prosigna is the only in vitro diagnostic kit available for use on our nCounter Dx Analysis System. Over time, we intend to develop, obtain regulatory authorization for, and sell additional in vitro diagnostic kits, each of which will enable a unique diagnostic test.
Laboratory Developed Tests
Clinical laboratories can use our custom manufacturing services to supply reagents to create Laboratory Developed Tests, or LDTs, which are diagnostic tests that are developed, validated and performed by a single laboratory. These reagents enable assays for gene expression, copy number variation and gene fusions. Clinical laboratories can use these reagents to develop assays to replace tests currently performed using fluorescence-based in situ hybridization, or FISH.
Prosigna
Prosigna, our first molecular diagnostic test, is based on a collection of 50 genes known as the PAM50 gene signature, which was discovered by several of our research customers. Prosigna can provide a breast cancer patient and her physician with a subtype classification based on the fundamental biology of the patient’s tumor, as well as a prognostic score that indicates the probability of cancer recurrence over 10 years. Physicians use Prosigna to help guide therapeutic decisions so that patients receive a therapeutic intervention, such as chemotherapy, only if clinically warranted. Prosigna is regulated as an in vitro diagnostic test and we distribute it as a kit for use on our nCounter Dx Analysis System in clinical laboratories.
Prosigna in the United States. In September 2013, we received 510(k) clearance from the FDA to market in the United States a version of Prosigna providing a prognostic indicator for distant recurrence-free survival at 10 years, which is indicated for postmenopausal women with Stage I/II lymph node-negative or Stage II lymph node-positive (one to three positive nodes) hormone receptor-positive breast cancer who have undergone surgery in conjunction with locoregional treatment consistent with standard of care. For each patient, the Prosigna report includes the Prosigna Score, which is referred to as the ROR Score in the scientific literature and outside the United States, and a risk category based on both the Prosigna Score and nodal status. Node-negative patients are classified as low, intermediate or high risk, while node-positive patients are classified as low or high risk. Prosigna competes with other tests that are currently available as services from specialized central laboratories.
We sell Prosigna kits to our lab customers on a fixed dollars-per-kit basis. These customers are responsible for providing the testing service and contracting and billing payors. Accordingly, we are not directly exposed to third-party payor reimbursement risk.
Prosigna in the European Union and Other Countries that Recognize the CE Mark. In September 2012, we obtained CE mark designation for Prosigna for use as a semi-quantitative in vitro diagnostic assay using the gene expression profile of cells found in FFPE breast tumor tissue to assess the 10-year risk of distant recurrence in postmenopausal women with HR+ early stage breast cancer treated with endocrine therapy alone. This CE-marked product is indicated for use in patients with either node-negative or node-positive disease, and provides physicians and their patients with the intrinsic subtype of a patient’s breast cancer tumor, ROR score, and risk category (high/intermediate/low). In early 2013, we began marketing this test in Europe and Israel.
-9-
Collaborations
Hyb & Seq Technology Development
Lam Research Corporation
In August 2017, we entered into a collaboration agreement with Lam Research Corporation, or Lam, to develop and commercialize our Hyb & Seq sequencing platform and related assays. Under the terms of the agreement, Lam will contribute up to an aggregate of $50.0 million towards the project. The development funding is non-refundable, unless the parties determine that completion of development of the product will not occur and development activities are discontinued, in which case any funds advanced to us by Lam that have not been committed or spent will be refunded to Lam. We will reimburse Lam for the cost of up to 10 full-time Lam employees each year in accordance with the product development plan. Lam is eligible to receive certain single-digit percentage royalty payments from us on net sales of certain products and technologies developed under the agreement. The maximum amount of royalties we may pay to Lam will be capped at an amount up to three times the amount of development funding actually provided by Lam. We will retain exclusive rights to obtain regulatory approval, manufacture and commercialize any Hyb & Seq products.
All intellectual property made or conceived solely by us pursuant to the collaboration will be owned by us and licensed to Lam solely for the purposes of the collaboration. All intellectual property made or conceived solely by Lam pursuant to the collaboration will be owned by Lam and, subject to certain restrictions on use with Lam competitors, licensed to us for the purposes of the collaboration and further development and commercialization of our Hyb & Seq platform, as well as certain other products and technologies resulting from the collaboration in the field of molecular profiling. Jointly created intellectual property will be jointly owned, provided that neither we nor Lam use such jointly owned intellectual property in the other party’s competitive field.
The collaboration agreement establishes a joint steering committee to oversee, review and coordinate our and Lam’s activities under the collaboration agreement and monitor progress and expenditures against the associated development plan. The joint steering committee is comprised of three employees from each of us and Lam, and will be chaired by one of our employees. We will have final decision-making authority on the joint steering committee, subject to certain exceptions for decisions regarding development failure, material changes to the development plan, budget, and the Hyb & Seq product being developed under the agreement, and intellectual property ownership, which require consensus of the parties. The collaboration agreement also contains customary representations, warranties, covenants, indemnities and other obligations of the parties.
The term of the collaboration agreement is 15 years. Either we or Lam may terminate the collaboration agreement in the case of a material breach by the other party after providing notice and an opportunity to cure or in the case of bankruptcy or insolvency of the other party. The joint steering committee may also terminate the collaboration agreement if development is discontinued in the case of a development failure. Lam may also terminate the collaboration agreement on or after the first anniversary in the event we undergo a change of control.
In connection with the execution of the collaboration agreement, we issued Lam a warrant to purchase up to 1.0 million shares of our common stock with the number of underlying shares exercisable at any time proportionate to the amount of the $50.0 million commitment that has been provided by Lam. The exercise price of the warrant is $16.75 per share, and it will expire on the seventh anniversary of the issuance date. The warrant was determined to have a fair value of $6.7 million upon issuance, which will be recorded as additional paid in capital proportionately from the quarterly collaboration payments made by Lam.
In connection with the entry into the collaboration agreement and issuance of the warrant, we and Lam have agreed, subject to certain exceptions applicable to Lam, to be bound by certain “standstill” provisions. Pursuant to the “standstill” provisions, until the third anniversary of the entry into the collaboration agreement, we, Lam and our respective officers, directors, employees or contractors acting on their behalf will not (1) acquire, offer to acquire, agree to acquire or publicly propose or offer to acquire, securities, indebtedness, businesses, properties or assets of the other party or any subsidiary or division thereof; (2) initiate, induce or attempt to induce any other person or group to initiate any transaction referred to in clause (1), any stockholder proposal regarding the other party or call hold or convene a stockholders’ meeting of the other party; (3) make or participate in any solicitation of proxies to vote or seek to advise or influence any person with respect to the voting of any voting securities of the other party; (4) make any public announcement with respect to, or submit a proposal or offer for any extraordinary transaction involving the other party or any of its securities or assets; (5) form, join or in any way participate in a group as defined in Section 13(d)(3) of the Securities Exchange Act of 1934, as amended, in connection with any of the foregoing prohibited activities; (6) act or seek to control or influence the management, board of directors or policies of the other party; (7) take any action that could reasonably be expected to require the other party to make a public announcement regarding the possibility of any of the prohibited activities described in clauses (1) through (6) or (8) advise, assist or encourage any other person in connection with any of the foregoing prohibited activities.
-10-
In addition, Lam has agreed, subject to certain exceptions, not to offer, sell or transfer any of our common stock or securities convertible into or exchangeable or exercisable for our common stock, for three years after the entry into the collaboration agreement without first obtaining our consent, which we may withhold in our sole discretion, unless the collaboration agreement has been terminated, in which case our consent may not be unreasonably withheld.
Companion Diagnostic Development
Celgene Corporation
In March 2014, we entered into a collaboration agreement with Celgene to develop, seek regulatory approval for, and commercialize a companion diagnostic assay using the nCounter Analysis System to identify a subset of patients with DLBCL, who are believed to be the most likely to benefit from treatment with Celgene’s drug REVLIMID. Under the terms of the collaboration agreement, we will develop, seek regulatory approval for, and commercialize the diagnostic test, and we retain the flexibility to independently develop and commercialize additional indications for the test. Pursuant to our agreement as amended in February 2018, we are eligible to receive payments from Celgene totaling up to $27.3 million, of which $5.8 million was received as an upfront payment and $21.5 million is for development funding and potential success-based developmental and regulatory milestones. There have been several amendments to the collaboration agreement and in return we have received additional payments totaling $2.1 million. In the most recent amendment in February 2018, Celgene agreed to provide us with additional funding for work intended to enable a subtype and prognostic indication for the test being developed under the agreement. In addition, the amendment provides an additional milestone payment to us payable upon achievement of certain regulatory activities and timelines. In connection with this amendment, we agreed to remove the right to receive payments from Celgene in the event commercial sales of the companion diagnostic test do not exceed certain pre-specified minimum annual revenues during the first three years following regulatory approval. In addition, the amendment allows Celgene, at its election, to use trial samples with additional technologies for companion diagnostics.
DLBCL is a heterogeneous group of cancers that represents the most common form of Non-Hodgkin Lymphoma. According to the National Cancer Institute, there were approximately 70,000 new cases of Non-Hodgkin Lymphoma in the United States in 2015. DLBCL is the most common type of Non-Hodgkin Lymphoma, representing approximately 1 out of every 3 cases. The subtypes of DLBCL have long been known to have varying prognoses. In January 2014, certain of our research customers published a paper in the journal Blood describing the development and validation of a biomarker assay based on a 20-gene expression DLBCL subtype classifier using our nCounter Analysis System. We have secured a license to the relevant intellectual property to enable the collaboration.
Under the collaboration agreement with Celgene, we have delivered an in vitro companion diagnostic test that will be used to subtype and screen patients who are being enrolled in a pivotal study of REVLIMID for the treatment of DLBCL. The upfront payment, a portion of the success-based milestone payments and the payments related to the subsequent amendments, totaling $13.9 million, have been received from Celgene to date, and we are using these funds in part to cover our costs for clinical development of the test.
Merck & Co., Inc.
In May 2015, we entered into a clinical research collaboration agreement with Merck, to develop an assay intended to optimize immune-related gene expression signatures and evaluate the potential to predict benefit from Merck’s anti-PD-1 therapy, KEYTRUDA, in multiple tumor types. In February 2016, we expanded our collaboration with Merck by entering into a new development collaboration agreement to clinically develop, seek regulatory approval for, and commercialize a companion diagnostic test to predict response to KEYTRUDA in multiple tumor types. In connection with the execution of the development collaboration agreement, we and Merck terminated our May 2015 clinical research collaboration and moved all remaining activities under such clinical research collaboration work plan to the new development collaboration agreement. Under the terms of the new development collaboration agreement, we have received to date $12.0 million as an upfront technology access payment, $8.5 million of preclinical milestone payments, and $19.4 million of development funding. In October 2017, we were notified by Merck of the decision not to pursue regulatory approval of the companion diagnostic test for KEYTRUDA. As a result, the scope of the collaboration was significantly reduced, and activities respecting this collaboration are expected to be materially concluded in 2018.
Medivation, Inc. and Astellas Pharma, Inc.
In January 2016, we entered into a collaboration with Medivation, Inc., or Medivation, and Astellas Pharma Inc., or Astellas, to pursue the translation of a novel gene expression signature algorithm discovered by Medivation into a companion diagnostic assay using the nCounter Analysis System. In September 2016, Medivation was acquired by Pfizer, Inc., or Pfizer, and became a wholly owned subsidiary of Pfizer. In May 2017, we received notification from Pfizer and Astellas terminating the collaboration agreement as a result of a decision to discontinue the related clinical trial. We received a $6.0 million upfront
-11-
payment for technology access, $6.0 million in preclinical milestone payments, $3.3 million in development funding payments, and a $1.0 million termination payment related to this collaboration agreement.
Intellectual Property
We must develop and maintain protection on the proprietary aspects of our technologies in order to remain competitive. We rely on a combination of patents, copyrights, trademarks, trade secret and other intellectual property laws and confidentiality, material transfer agreements, licenses, invention assignment agreements and other contracts to protect our intellectual property rights.
As of December 31, 2017, we owned or exclusively licensed 20 issued U.S. patents and approximately 40 pending U.S. patent applications, including provisional and non-provisional filings. We also owned or licensed approximately 255 pending and granted counterpart applications worldwide, including 111 country-specific validations of 12 European patents. The issued U.S. patents that we own or exclusively license are expected to expire between July 3, 2021 and February 6, 2033. We have either sole or joint ownership positions in all of our pending U.S. patent applications. Where we jointly own cases, we have negotiated license or assignment provisions to obtain exclusive rights. For our material nCounter Analysis System and Prosigna product rights, we are the exclusive licensee. We also generally protect our newly developed intellectual property by entering into confidentiality agreements that include intellectual property assignment clauses with our employees, consultants and collaborators.
Our patent applications relate to the following main areas:
• | our nCounter Analysis System biology, chemistry, methods and hardware; |
• | specific applications for our nCounter Analysis System technology; |
• | our gene expression markers, methods and gene signatures for recurrence and drug response in certain forms of cancer; |
• | biological and chemical compositions, methods and hardware for enzyme and amplification free sequencing; and |
• | biological and chemical compositions, methods and hardware for multiplexed detection and quantification of protein and/or nucleic acid expression in a defined region of a tissue or cell. |
We intend to file additional patent applications in the United States and abroad to strengthen our intellectual property rights; however, our patent applications may not result in issued patents, and we cannot assure investors that any patents that have issued or might issue will protect our technology. We have received notices of claims of potential infringement from third parties and may receive additional notices in the future. When appropriate, we have taken a license to the intellectual property rights from such third parties. For additional information, see the section of this report captioned “Risk Factors — Risks Related to Intellectual Property.”
We own a number of trademarks and develop names for our new products and as appropriate secure trademark protection for them, including domain name registration, in relevant jurisdictions.
License Agreements
We have relied, and expect to continue to rely, on strategic collaborations and licensing agreements with third parties. For example, our base molecular barcoding technology is in-licensed from the Institute for Systems Biology and the intellectual property that forms the basis of Prosigna is in-licensed from Bioclassifier, LLC. In addition to the licenses with the Institute for Systems Biology and Bioclassifier, we have licensed technology related to the DLBCL assay from the National Institutes of Health, and we rely on other license and supply arrangements for proprietary components which require us to pay royalties on the sale of our products. Other research customers are using our nCounter Analysis System to discover gene expression signatures that we believe could form the basis of future diagnostic products. In the future, we may consider these gene signatures for in-licensing. Our licensing arrangements with the Institute for Systems Biology and Bioclassifier are discussed below in greater detail.
Institute for Systems Biology
In 2004, we entered into an agreement with the Institute for Systems Biology pursuant to which the Institute granted to us an exclusive, subject to certain government rights, worldwide license, including the right to sublicense, to the digital molecular barcoding technology on which our nCounter Analysis System is based, including 13 patents and patent applications. Pursuant to the terms of the amended license agreement, we are required to pay the Institute for Systems Biology royalties on net sales of products sold by us, or our sublicensees, at a low single digit percentage rate, which was reduced by 50% in the third quarter of 2016 for the remainder of the license term due to the achievement of a cumulative sales threshold. Through December 31, 2017, we have paid aggregate royalties of $4.9 million under the license agreement. Unless terminated earlier in
-12-
accordance with the terms of the amended license agreement, the agreement will terminate upon the expiration of the last to expire patent licensed to us. The Institute for Systems Biology has the right to terminate the agreement under certain situations, including our failure to meet certain diligence requirements or our uncured material breach of the agreement.
Bioclassifier, LLC
In July 2010, we entered into an exclusive license agreement with Bioclassifier, LLC, pursuant to which Bioclassifier granted to us an exclusive, subject to certain government rights, worldwide license, with the right to sublicense, to certain intellectual property rights and technology, including eight non-provisional patent applications, related to the PAM50 gene signature in the field of research products and prognostic and/or diagnostic tests for cancer, including Prosigna. Bioclassifier has licensed these rights from the academic institutions that employed the cancer researchers that discovered or were involved in the initial development of PAM50. Pursuant to the agreement, we are required to pay Bioclassifier the greater of certain minimum royalty amounts and mid-single digit to low double digit percentage royalties on net sales of products and/or methods sold by us that are covered by patent rights or include, use or are technology licensed to us. Our obligation to pay royalties to Bioclassifier expires on a country-by-country basis upon the expiration of the last patent licensed or, if a product or method includes, uses or is technology licensed to us but is not covered by a patent licensed to us, ten years after the first commercial sale of the product or method in such country. We are also required to pay Bioclassifier a low to mid double digit percentage of any income received by us from the grant of a sublicense to the patents or technology licensed to us under the agreement. The agreement specifies that we will control and be responsible for the costs of prosecuting and enforcing the intellectual property licensed in certain major market countries. The agreement also includes customary rights of termination for Bioclassifier, including for our uncured material breach or our bankruptcy. Through December 31, 2017, we have paid Bioclassifier $1.5 million.
Research and Development
We have committed, and expect to continue to commit, significant resources to developing new technologies and products, improving product performance and reliability and reducing costs. We have assembled experienced research and development teams at our Seattle, Washington location with the scientific, engineering, software and process talent that we believe is required to successfully grow our business. As of December 31, 2017, including clinical, medical and regulatory affairs, we had 161 employees in research and development, of which 64 hold a Ph.D. degree and one holds an M.D. degree. Our research and development expenses for the years ended December 31, 2017, 2016 and 2015 were $46.9 million, $34.7 million and $24.6 million, respectively.
Platform Technology
We are continuously seeking to improve the nCounter Analysis System, including improvements to the technology and accessibility, or to extend its capabilities. As we make improvements or add new capabilities, we anticipate that we will make available new and improved generations of the nCounter Analysis System. In addition to the nCounter Analysis System, we are currently developing two new systems enabled by our proprietary optical barcoding chemistry. Following completion of product development, each of these new systems is expected to be commercialized as a new instrument along with associated consumables.
The first new platform in development, DSP, will allow researchers to address important questions regarding how protein and gene expression vary spatially for regions of interest across the landscape of a heterogeneous tissue biopsy. Our DSP instruments are expected to image slide-mounted tissue biopsies, allow selection of regions of interest, and automate the preparation of samples from selected regions for molecular profiling using either an nCounter system or next generation sequencer. DSP technology is expected to offer a number of advantages compared to traditional technologies, including the ability to profile a larger number of different genes or proteins in each region, more flexibility on the selection of regions, and processing of a larger number of samples per day. Early access sales of DSP instruments are expected to start in late 2018 and the commercial launch of DSP instruments is expected during the first half of 2019.
The second new platform in development, Hyb & Seq, is a next generation sequencing platform. Hyb & Seq is designed to have a workflow that is simpler and faster than current sequencing methods, due to the absence of library preparation, enzymes and amplification. Hyb & Seq's simple workflow and compatibility with a variety of sample types offers the potential for a sample-to-answer solution for clinical sequencing. The commercial launch of a research version of Hyb & Seq is expected during 2020.
Prosigna Breast Cancer Assay
Our Prosigna clinical studies to date have employed a retrospective/prospective design, which means that we use samples that were previously collected from patients and for which the treatment regimen and ultimate outcome of each patient are known. Such studies are capital efficient as they do not require recruiting new patients and running prospective trials and
-13-
they can be completed much more quickly than typical prospective clinical trials. We intend to use a similar approach whenever possible for any additional clinical studies we may conduct for Prosigna.
In the future, we may participate in prospective clinical studies that require recruiting new patients. For example, we are participating in the OPTIMA trial, which is being organized and sponsored by a cooperative group in the United Kingdom. We are not financially responsible for conducting the trial; however, we are providing in-kind support through the sale of Prosigna in vitro diagnostic kits at a discounted price.
Future Molecular Diagnostics
We are continuously monitoring molecular signatures which have the potential to become additional diagnostic products or enable Laboratory Developed Tests based on nCounter-based reagents. We may in-license rights to molecular diagnostic intellectual property as part of our strategy to develop additional diagnostic products and enable Laboratory Developed Tests, with a particular focus on licensing rights from our research customers who are seeking to translate their research into clinical products or services after the necessary regulatory authorizations are secured.
Sales and Marketing
We began selling nCounter Analysis Systems to researchers in 2008 and began sales efforts in the clinical laboratory market in 2013. We sell our instruments and related products primarily through our own sales force in North America and through a combination of direct and distributor channels in Europe, the Middle East, Asia Pacific and South America. We have agreements with 26 distributors, each of which is exclusive within a certain territory. In the event the distributor does not meet minimum performance requirements, we may terminate the distribution agreement or convert from an exclusive to non-exclusive arrangement within the territory, allowing us to enter into arrangements with other distributors for the territory. For additional information regarding geographic revenue, see Note 15 of the Notes to Consolidated Financial Statements under Item 8 of this report. For the year ended December 31, 2017, our collaborators, (1) Merck and (2) Medivation and Astellas, represented 25% and 10%, respectively, of our total revenue. For the year ended December 31, 2016, Merck represented 13% of our total revenue. No customers represented more than 10% of total revenue during the year ended December 31, 2015.
Instrumentation and Research
Our sales and marketing efforts for instrumentation and in the life sciences research market are targeted at department heads, research or clinical laboratory directors, principal investigators, core facility directors, and research scientists and pathologists at leading academic institutions, biopharmaceutical companies, publicly and privately-funded research institutions and contract research organizations. We seek to increase awareness of our products among our target customers through direct sales calls, trade shows, seminars, academic conferences, web presence and other forms of internet marketing.
Our instruments require a significant capital investment or commitment to a lease or reagent rental agreement. Accordingly, our sales process involves numerous interactions with multiple people within an organization, and often includes in-depth analysis by potential customers of our products, proof-of-principle studies, preparation of extensive documentation and a lengthy review process. As a result of these factors, the large capital investment required in purchasing our instruments and the budget cycles of our customers, the time from initial contact with a customer to our receipt of a purchase order can vary significantly and be up to 12 months or longer. Given the length and uncertainty of our sales cycle, we have in the past experienced, and likely will in the future experience, fluctuations in our instrument sales on a period-to-period basis.
We continue to invest in our commercial channel to increase our reach and productivity. During 2017, we added staff focused on sales of consumables to our existing instrument base. We believe these investments enabled our existing sales representatives to focus on instrument sales and help drive the growth of our installed instrument base.
Molecular Diagnostics
The commercialization of Prosigna kits involves a three-pronged effort. First, we seek to establish third-party reimbursement and patient access for clinical testing services that our clinical laboratory customers will provide based upon our products by gaining inclusion in influential treatment guidelines and educating third-party payors regarding the clinical utility and health economic value of the clinical tests enabled by our technology. Second, we seek to establish an installed base of nCounter Analysis Systems by selling or leasing instruments to select clinical laboratories, with initial sales efforts directed at laboratories, hospitals, networks or practices that test or treat a high volume of breast cancer patients. As of December 31, 2017, there were approximately 78 laboratories worldwide that had purchased or rented nCounter Analysis Systems with the intent to market and sell Prosigna testing services. Third, we intend to drive physician demand for clinical testing services enabled by our diagnostic products, and direct test orders toward those laboratories which have adopted our technology. Where appropriate, we intend to coordinate commercial efforts with the sales and marketing personnel of the clinical laboratories offering clinical testing services based on our diagnostic products.
-14-
Manufacturing and Suppliers
We use third-party contract manufacturers to produce our instruments and raw materials for our consumables, and we build the CodeSets and reagent packages at our Seattle, Washington facility.
Instruments
We outsource manufacturing of our nCounter Analysis System instruments. Precision System Science, Co., Ltd. of Chiba, Japan, or PSS, is our sole source supplier for the nCounter Prep Station. Korvis Automation Inc., or Korvis, is our sole source supplier for our nCounter Digital Analyzers at its facility in Corvallis, Oregon. Paramit Corporation, or Paramit, is our sole source supplier for our nCounter SPRINT Profiler at its facility in Morgan Hill, California.
The facilities at which our instruments are built have been certified to ISO 13485:2003 standards. Our contracts with these instrument suppliers do not commit them to carry inventory or make available any particular quantities. Under the terms of the three instrument supply agreements, we are required to place binding purchase orders for instruments that will be delivered to us by the supplier three to six months from the date of placement of the purchase order. Although qualifying alternative third-party manufacturers could be time consuming and expensive, our instruments’ design is similar to other instruments and we believe that alternatives would be available if necessary. However, if our instrument suppliers terminate our relationship with them or if they give other customers’ needs higher priority than ours, then we may not be able to obtain adequate supplies in a timely manner or on commercially reasonable terms.
Consumables
We manufacture our consumables in our Seattle, Washington facility which has been certified to ISO 13485:2003 standards. We expanded our manufacturing capacity in 2015 by relocating certain research and development functions and converting the space to incremental manufacturing labs and offices. In the future, should additional space become necessary, we believe that there will be space available near our existing facility that we believe we can secure; however, we cannot predict that this space will be available if and when it is needed.
We rely on a limited number of suppliers for certain components and materials used in the manufacture of our consumables. Some of these components are sourced from a single supplier. For example, Cidra Precision Services, LLC, of Wallingford, Connecticut, part of IDEX Health & Science, is the sole supplier of the microfluidic cartridge for our nCounter SPRINT Profiler. For some components, we have qualified second sources for several of our critical reagents, including oligonucleotides, adhesives and dyes. We believe that having dual sources for our components helps reduce the risk of a production delay caused by a disruption in the supply of a critical component. We continue to pursue qualifying additional suppliers, but cannot predict how expensive, time-consuming or successful these efforts will be. If we were to lose one or more of our suppliers, it may take significant time and effort to qualify alternative suppliers.
Competition
In the life sciences research market, we compete with companies such as Agilent Technologies, Becton-Dickinson, Bio-Rad, Bio-Techne, Fluidigm, HTG Molecular Diagnostics, Illumina, Luminex, Merck Millipore, O-Link, Perkin Elmer, Qiagen, Roche Applied Science, Thermo Fisher Scientific, and WaferGen Biosystems, some of which also offer diagnostic applications of their technologies. These competitors and others have products for gene and protein expression analysis that compete in certain segments of the market in which we sell our products. In addition, there are a number of new market entrants in the process of developing novel technologies for the life sciences market, such as next generation sequencing, including RNA-sequencing.
In the breast cancer diagnostics market, we compete with Genomic Health’s Oncotype Dx, a service for gene expression analysis performed in its central laboratory in Redwood City, California. We also face competition from companies such as Agendia and bioTheranostics, which also offer centralized laboratories that profile gene or protein expression in breast cancer. Outside the United States, we also face regional competition from Myriad Genetics, and its product EndoPredict, a distributed test for breast cancer recurrence.
We believe that we have multiple competitive advantages in the research market, including the automated nature of our nCounter Analysis System with its simple, rapid and efficient workflow that requires very limited human intervention or labor; the multiplexing capability of our technology to analyze significantly more target molecules in a single tube without amplification, representing multiple biological pathways; the ability to analyze combinations of DNA, RNA and proteins simultaneously in a single experiment; compatibility with many sample types, including difficult samples such as FFPE; and the ability to analyze small sample inputs, in some cases down to a single cell, from a wide variety of sample types. In the diagnostics market, we believe our competitive advantages include the compelling evidence of Prosigna’s ability to inform major medical treatment decisions, including results from our studies; the quality of our nCounter Analysis System, which
-15-
enables consistent and reproducible results in decentralized laboratories; and the improved convenience for physicians and patients, including more rapid test result turnaround time.
While we believe that we compete favorably based on the factors described above, many of our competitors enjoy other competitive advantages over us, including:
• | greater name and brand recognition, financial and human resources; |
• | broader product lines; |
• | larger sales forces and more established distributor networks; |
• | substantial intellectual property portfolios; |
• | larger and more established customer bases and relationships; and |
• | better established, larger scale and lower cost manufacturing capabilities. |
For additional information, see the section of this report captioned “Risk Factors — The life sciences research and diagnostics markets are highly competitive. If we fail to compete effectively, our business and operating results will suffer.”
Government Regulation
Medical Device Regulation
United States
In the United States, medical devices, including in vitro diagnostics, are subject to extensive regulation by the U.S. Food and Drug Administration, or FDA, under the Federal Food, Drug, and Cosmetic Act, or FDC Act, and its implementing regulations, and other federal and state statutes and regulations. The laws and regulations govern, among other things, medical device development, testing, labeling, storage, premarket clearance or approval, advertising and promotion and product sales and distribution.
A medical device is an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including any component part or accessory, which is (1) intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals, or (2) intended to affect the structure or any function of the body of man or other animals, and which does not achieve any of its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of any of its primary intended purposes. In vitro diagnostics are a type of medical device and are tests that can be used in the diagnosis and/or detection of diseases, conditions or infections, including, without limitation, the presence of certain chemicals, genetic or other biomarkers. Some tests are used in laboratories or other health professional settings and other tests are for consumers to use at home.
Medical devices to be commercially distributed in the United States must receive from the FDA either clearance of a premarket notification, or 510(k), or premarket approval of a premarket approval application, or PMA, pursuant to the FDC Act prior to marketing, unless subject to an exemption. Devices deemed to pose relatively low risk are placed in either Class I or II. Placement of a device into Class II generally requires the manufacturer to submit to the FDA a 510(k) seeking clearance for commercial distribution; this is known as the 510(k) clearance process. Preamendment Class III devices for which FDA has not yet required submission of PMAs are also required to submit a 510(k) to FDA. Most Class I devices are exempted from this premarket requirement. Devices deemed by the FDA to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices and some diagnostic tests, are placed into Class III requiring PMA approval. Devices deemed not substantially equivalent to a previously 510(k)-cleared device or novel devices for which no predicate device exists are placed into Class III, but may be reclassified by FDA into Class I or Class II upon the submission by the manufacturer of a de novo reclassification application. A clinical trial is almost always required to support a PMA application or de novo application, and in many cases is required for a 510(k) application. All clinical studies of investigational devices must be conducted in compliance with applicable FDA or Institutional Review Board, or IRB, regulations.
510(k) Clearance Pathway. To obtain 510(k) clearance, a manufacturer must submit a premarket notification demonstrating to the FDA’s satisfaction that the proposed device is substantially equivalent in intended use and in technological characteristics to a previously 510(k) cleared device or a device that was in commercial distribution before May 28, 1976, for which the FDA has not yet called for submission of PMA applications. The previously cleared device is known as a predicate. The FDA’s 510(k) clearance pathway usually takes from four to 12 months, but it can last significantly longer, particularly for a novel type of product.
After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, requires a new 510(k) clearance or could require a PMA approval. The FDA requires each manufacturer to make this determination in the first instance, but the FDA can review any such decision. If
-16-
the FDA disagrees with a manufacturer’s decision not to seek a new 510(k) clearance, the agency may require the manufacturer to seek 510(k) clearance or PMA approval. The FDA also can require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance or PMA approval is obtained.
PMA Approval Pathway. The PMA approval pathway requires a demonstration of reasonable assurance of safety and effectiveness of the device to the FDA’s satisfaction. The PMA approval pathway is costly, lengthy and uncertain.
A PMA application must provide extensive preclinical and clinical trial data and also information about the device and its components regarding, among other things, device design, manufacturing and labeling. As part of the PMA review, the FDA will typically inspect the manufacturer’s facilities for compliance with Quality System Regulation, or QSR, requirements, which impose elaborate testing, control, documentation and other quality assurance procedures.
Upon submission, the FDA determines if the PMA application is sufficiently complete to permit a substantive review, and, if so, the application is accepted for filing. The FDA then commences an in-depth review of the PMA application. The PMA approval process typically takes one to three years, but may last longer. The review time is often significantly extended as a result of the FDA asking for more information or clarification of information already provided. The FDA also may respond with a “not approvable” determination based on deficiencies in the application and require additional clinical studies that are often expensive and time consuming and can delay approval for months or even years. During the review period for a new type of device, an FDA advisory committee, a panel of external experts, likely will be convened to review the application and recommend to the FDA whether, or upon what conditions, the device should be approved. Although the FDA is not bound by the advisory panel decision, the panel’s recommendation is important to the FDA’s overall decision making process.
If the FDA’s evaluation of the PMA application is favorable, the FDA typically issues an “approvable letter” requiring the applicant’s agreement to specific conditions, such as changes in labeling, or specific additional information such as submission of final labeling, in order to secure final approval of the PMA application. Once the approvable letter is satisfied, the FDA will issue a PMA for the approved indications, which can be more limited than those originally sought by the manufacturer. The PMA can include post-approval conditions that the FDA believes necessary to ensure the safety and effectiveness of the device including, among other things, post-approval studies and restrictions on labeling, promotion, sale and distribution. Failure to comply with the conditions of approval can result in material adverse enforcement action, including the loss or withdrawal of the approval or placement of restrictions on the sale of the device until the conditions are satisfied.
Even after approval of a PMA, a new PMA or PMA supplement may be required in the event of a modification to the device, its labeling or its manufacturing process. Supplements to a PMA may require the submission of the same type of information required for an original PMA, except that the supplement is generally limited to that information needed to support the proposed change from the product covered by the original PMA.
De Novo Pathway. If no predicate can be identified, the product is automatically classified as Class III, requiring a PMA. However, the FDA can reclassify, or use “de novo classification” for, a device for which there was no predicate device if the device is low or moderate risk. A device company can ask the FDA at the outset if the de novo route is available for its particular product. When granting a de novo application the FDA will establish special controls that other applicants for the same device type must implement, which often includes labeling restrictions and data requirements. Subsequent applicants can rely upon the de novo product as a predicate for a 510(k) clearance. The de novo route is less burdensome than the PMA process; it is similar in many respects to a 510(k), but generally takes much longer for clearance than the 510(k) process, and almost always requires clinical data. The de novo route has been used for many in vitro diagnostic products.
Postmarket. After a device is placed on the market, numerous regulatory requirements apply. These include: the QSR, labeling regulations, the FDA’s general prohibition against promoting products for unapproved or “off label” uses, registration and listing, the Medical Device Reporting, or MDR, regulation (which requires that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction were to recur), and the Reports of Corrections and Removals regulation (which requires manufacturers to report recalls and field actions to the FDA if initiated to reduce a risk to health posed by the device or to remedy a violation of the FDC Act).
The FDA enforces these requirements by inspection, market surveillance, and other means. If the FDA finds a violation, it can institute a wide variety of enforcement actions, ranging from an untitled letter or a public warning letter to more severe sanctions such as fines, injunctions, and civil penalties; recall or seizure of products; operating restrictions, partial suspension or total shutdown of production; refusing requests for 510(k) clearance or PMA approval of new products; withdrawing 510(k) clearance or PMA approvals already granted; and criminal prosecution. For additional information, see the section of this report captioned “Risk Factors — Risks Related to Government Regulation and Diagnostic Product Reimbursement.”
Research Use Only. Research Use Only, or RUO, products belong to a separate regulatory classification under a long-standing FDA regulation. In essence, RUO products are not regulated as medical devices and are therefore not subject to the
-17-
regulatory requirements discussed above. The products must bear the statement: “For Research Use Only. Not for Use in Diagnostic Procedures.” RUO products cannot make any claims related to safety, effectiveness or diagnostic utility, and they cannot be intended for human clinical diagnostic use. In November 2013, the FDA issued a final guidance on RUO products, which, among other things, reaffirmed that a company may not make any clinical or diagnostic claims about an RUO product. The FDA will also evaluate the totality of the evidence to determine if the product is intended for diagnostic purposes. If FDA were to determine, based on the totality of circumstances, that our products marketed for RUO are intended for diagnostic purposes, they would be considered medical devices that will require clearance or approval.
Dual-Use Instruments. Dual-use instruments are subject to FDA regulation since they are intended, at least in part, for use by customers performing clinical diagnostic testing. In November 2014, FDA issued a guidance that described FDA’s approach to regulating molecular diagnostic instruments that combine in a single molecular instrument both approved/cleared device functions and device functions for which approval/clearance is not required.
Laboratory Developed Tests. Laboratory Developed Tests, or LDTs, are developed, validated and used within a single lab. In the past, the FDA generally exercised its enforcement discretion for LDTs and did not require clearance or approval prior to marketing. On October 3, 2014, FDA issued two draft guidances that proposed to actively regulate LDTs using a risk-based approach, and would have required 510(k)s or PMAs for certain “moderate” or “high” risk devices. However, in late November 2016, FDA announced that it would not be finalizing the 2014 draft LDT Guidances. On January 13, 2017, FDA issued a discussion paper laying out key elements of a possible revised future LDT regulatory framework. We do not expect any guidance document regulating LDTs will go into effect in the near future.
Companion Diagnostics. In August 2014, FDA issued a companion diagnostics final guidance stating that if the device is essential to the safety or efficacy of the drug, FDA will generally require approval or clearance for the device at the time when FDA approves the drug. Most companion diagnostics will require PMA approval.
International
International sales of medical devices are subject to foreign government regulations, which vary substantially from country to country. The European Commission is the legislative body responsible for directives under which manufacturers selling medical products in the European Union, or EU, and the European Economic Area, or EEA, must comply. The EU includes most of the major countries in Europe, while other countries, such as Switzerland, are part of the EEA and have voluntarily adopted laws and regulations that mirror those of the EU with respect to medical devices. The EU has adopted directives that address regulation of the design, manufacture, labeling, clinical studies and post-market vigilance for medical devices. Devices that comply with the requirements of a relevant directive will be entitled to bear the CE conformity marking, indicating that the device conforms to the essential requirements of the applicable directives and, accordingly, can be marketed throughout the EU and EEA.
In September 2012, Prosigna was CE-marked for compliance with IVDD 98/79/EC for use in conjunction with a diagnostic version of our nCounter Analysis System in the EU to assess a breast cancer patient's risk of distant recurrence.
Outside of the EU, regulatory approval needs to be sought on a country-by-country basis in order to market medical devices. Although there is a trend towards harmonization of quality system standards, regulations in each country may vary substantially, which can affect timelines of introduction.
Reimbursement
Our nCounter Dx Analysis Systems are purchased or leased by clinical laboratories, which use our diagnostic products as the basis for testing patients’ samples. These customers can use our products to enable commercial testing services, and generate revenue for their laboratories for this service. In order to collect payment for testing services based upon our diagnostic products, our clinical laboratory customers may bill third parties, including public and private payors. The demand for our diagnostic products will depend indirectly upon the ability for our customers to successfully bill for and receive reimbursement from third-party payors for the clinical testing services based on our products. Therefore, we intend to work with third-party payors in markets where we intend to sell our diagnostic products to ensure that testing services based on our products are covered and paid.
The decision of payors to cover and pay for a specific testing service is driven by many factors, including:
• | strong clinical and analytical validation data; |
• | acceptance into major clinical guidelines, including the National Comprehensive Cancer Network, or NCCN, the American Society of Clinical Oncologists, or ASCO, and the St. Gallen Consensus guidelines; |
• | health economic studies that may indicate that the test improves quality-adjusted survival and leads to reduced costs; and |
• | decision impact studies that show the test leads to better treatment decisions. |
-18-
We have generated dossiers for submission to payors in support of reimbursement for testing services based upon our initial diagnostic product, Prosigna. The dossiers typically contain data from studies supporting the analytical and clinical validity of Prosigna, as well as health economic analyses that examine whether the clinical information supplied by Prosigna changes medical practice in a way that leads to benefit for both the patients and the payors. In some cases, these health economic analyses may be supported by the results of clinical studies of Prosigna’s impact on adjuvant treatment decisions in early stage breast cancer called decision impact studies. We developed a clinical protocol for Prosigna decision impact studies in collaboration with two European cooperative groups, and based on this protocol we have completed three studies to date.
United States
In the United States, clinical laboratory revenue is derived from various third-party payors, including insurance companies, health maintenance organizations, or HMOs, and government healthcare programs, such as Medicare and Medicaid. Clinical laboratory testing services are paid through various methodologies when covered by third-party payors, such as prospective payment systems and fee schedules. For any new clinical test, payment for the clinical laboratory service requires a decision by the third-party payor to cover the particular test, the establishment of a reimbursement rate for the test and the identification of one or more Current Procedural Terminology, or CPT, codes that accurately describe the test.
The American Medical Association, or AMA, has issued a set of CPT codes for billing and reimbursement of complex genomic tests that are based on information from multiple analytes or genes. These new MAAA, or Multianalyte Assays with Algorithmic Analyses, codes are intended to capture tests such as Prosigna and are divided into two categories of unique codes. Category 1 MAAA codes are intended for tests that AMA’s CPT Editorial Panel has vetted and found to meet a certain set of criteria, such as demonstrated clinical validity and utility, as well as current national utilization thresholds. MAAA codes issued to complex genomic tests that have not met all Category 1 coding criteria are referred to as administrative MAAA codes. Assignment of either unique reimbursement code to a particular test may facilitate claims processing by payors; however, assignment of a unique reimbursement code alone does not guarantee favorable reimbursement decisions by payors. A genomic test with an assigned MAAA code must still be vetted and approved by individual payors for coverage and payment before reimbursement is achieved. Given the more stringent requirements for receipt of a Category 1 MAAA, including demonstrated clinical validity and utility and satisfaction of national utilization thresholds, we believe that certain payors may more readily render favorable reimbursement decisions for genomic tests with a Category 1 MAAA rather than an administrative MAAA.
In April 2014, we received an administrative MAAA code (0008M) for use in reimbursement of testing services based on Prosigna. Given the recent commercial launch of Prosigna in the United States, and the lack of utilization data, we expected the issuance of an administrative MAAA initially. In October 2016, we applied for and received a Category 1 MAAA code for Prosigna. The code will be published in the CPT code book in late August 2017, with an effective date of January 1, 2018.
The Centers for Medicare & Medicaid Services, or CMS, administers the Medicare and Medicaid programs, which provide health care to almost one in every three Americans. For any particular geographic region, Medicare claims are processed at the local level by Medicare Administrative Contractors, or MACs. New diagnostic tests typically follow one of three routes to coverage via CMS: National Coverage Determinations, or NCDs, Local Coverage Determinations, or LCDs, or simply payment of claims by a MAC. The NCD applies to Medicare beneficiaries living throughout the United States. Due to cost and CMS bandwidth limitations there are generally few NCDs. The LCD process applies to only beneficiaries in the coverage area of a single MAC, requiring multiple LCDs to cover the testing throughout the United States. Due to the cost of developing an LCD, contractors tend to develop a relatively small number and prefer to tacitly cover services by paying claims. There is also a subset of NCDs known as Coverage with Evidence Development, or CED, that allow a technology (service or procedure) to be covered while evidence of clinical utility is collected through a registry or a study to answer outstanding questions on outcomes. Some MACs have developed Coverage with Data Development, or CDD, policies for the same purpose, which are administered at the local level.
Over the past three years, we have pursued Medicare coverage for Prosigna by working with MACs to obtain favorable LCDs. In 2016, Prosigna achieved Medicare coverage in all 50 states through this process.
For Medicare, the reimbursement rates for individual tests are established under the Clinical Laboratory Fee Schedule (local fee schedules for outpatient clinical laboratory services) or the Physician Fee Schedule, depending on the amount of physician work involved in the test. Molecular diagnostic tests, such as Prosigna, are paid under the Clinical Laboratory Fee Schedule. For additional information, see the section of this report captioned “Risk Factors — Risks Related to Government Regulation and Diagnostic Product Reimbursement.”
With respect to private insurance coverage, we have made significant progress in obtaining third-party reimbursement for the use of tests that incorporate new technology, such as Prosigna. Over the past three years, we have pursued coverage with all of the large private payers to facilitate reimbursement of Prosigna testing. In 2016, coverage policies were adopted by Cigna and Aetna and, in early 2017, Humana adopted a positive coverage policy. Additionally, the Blue Cross and Blue Shield, or
-19-
BCBS, Association Evidence Street recently published a positive assessment of Prosigna. Most individual BCBS entities have updated their coverage policies to include Prosigna based on this evaluation.
Outside the United States
In Europe, governments are primarily responsible for reimbursing diagnostic testing services. A relatively small portion of the market is made up of private payors and cash-pay patients. The primary barrier of adoption of a new in vitro diagnostic test is often reimbursement, and public reimbursement can take several years to achieve, depending on the country. Public reimbursement for genomic testing for breast cancer is available in Canada, Ireland, France, Greece, Switzerland, Denmark and the United Kingdom. Selected private coverage for testing is available in the United Kingdom, Germany, Spain, France, the UAE and Hungary. Reimbursement approval in some countries, such as Spain and Italy, is managed at the regional level. Israel is a market in which genomic testing for breast cancer is widely reimbursed by all four major Sick Funds, the third-party payors that cover a substantial majority of the population.
Our market access approach in Europe is similar to that in the United States and involves data driving clinical and economic publications to support guideline inclusion. Initially, we have targeted the private and cash pay market in Europe. In parallel, we are seeking to establish public reimbursement of Prosigna by national and regional governments in Europe.
Other Regulations
Our operations in the United States and abroad are subject to various fraud and abuse laws, including, without limitation, the federal anti-kickback statute and state and federal marketing compliance laws in the United States. These laws may impact our operations directly, or indirectly through our customers, and may impact, among other things, our proposed sales, marketing and education programs. In addition, we may be subject to patient privacy regulation by both the federal government and the states in which we conduct our business. The laws that may affect our ability to operate include the following federal laws and their counterparts at the state level:
• | the Federal Anti-kickback Law and state anti-kickback prohibitions; |
• | the Federal physician self-referral prohibition, commonly known as the Stark Law, and state equivalents; |
• | the Federal Health Insurance Portability and Accountability Act of 1996, as amended; |
• | the Medicare civil money penalty and exclusion requirements; |
• | the Federal False Claims Act civil and criminal penalties and state equivalents; |
• | the Foreign Corrupt Practices Act, which applies to our international activities; |
• | the Physician Payment Sunshine Act; and |
• | the European Union's General Data Privacy Regulations, or GDPR. |
Employees
As of December 31, 2017, we had 467 employees, of which 119 work in manufacturing, 141 in sales, marketing and business development, 144 in research and development, 17 in clinical, medical and regulatory affairs, and 46 in general and administrative. None of our U.S. employees are represented by a labor union or are the subject of a collective bargaining agreement. As of December 31, 2017, of our 467 employees, 423 were employed in the United States and 44 were employed outside the United States.
Environmental Matters
Our operations require the use of hazardous materials (including biological materials) which subject us to a variety of federal, state and local environmental and safety laws and regulations. Some of the regulations under the current regulatory structure provide for strict liability, holding a party potentially liable without regard to fault or negligence. We could be held liable for damages and fines as a result of our, or others’, business operations should contamination of the environment or individual exposure to hazardous substances occur. We cannot predict how changes in laws or development of new regulations will affect our business operations or the cost of compliance.
Where You Can Find Additional Information
We make available free of charge through our investor relations website, www.nanostring.com, our annual reports, quarterly reports, current reports, proxy statements and all amendments to those reports as soon as reasonably practicable after such material is electronically filed or furnished with the SEC. These reports may also be obtained without charge by contacting Investor Relations, NanoString Technologies, Inc., 530 Fairview Avenue, North, Seattle, Washington 98109, e-mail: [email protected]. Our Internet website and the information contained therein or incorporated therein are not intended to be incorporated into this Annual Report on Form 10-K. In addition, the public may read and copy any materials we
-20-
file or furnish with the SEC at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549 or may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. Moreover, the SEC maintains an Internet site that contains reports, proxy and information statements, and other information regarding reports that we file or furnish electronically with them at www.sec.gov.
Item 1A. Risk Factors
You should carefully consider the following risk factors, in addition to the other information contained in this report, including the section of this report captioned “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes. If any of the events described in the following risk factors and the risks described elsewhere in this report occurs, our business, operating results and financial condition could be seriously harmed. This report on Form 10-K also contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those anticipated in the forward-looking statements as a result of factors that are described below and elsewhere in this report.
Risks Related to Our Business and Strategy
We have incurred losses since we were formed and expect to incur losses in the future. We cannot be certain that we will achieve or sustain profitability.
We have incurred losses since we were formed and expect to incur losses in the future. We incurred net losses of $43.6 million, $47.1 million and $45.6 million for the years ended December 31, 2017, 2016 and 2015, respectively. As of December 31, 2017, we had an accumulated deficit of $313.1 million. We expect that our losses will continue for at least the next several years as we will be required to invest significant additional funds toward ongoing development and commercialization of our technology. We also expect that our operating expenses will continue to increase as we grow our business, but there can be no assurance that our revenue and gross profit will increase sufficiently such that our net losses decline, or we attain profitability, in the future. Our ability to achieve or sustain profitability is based on numerous factors, many of which are beyond our control, including the market acceptance of our products, future product development and our market penetration and margins. We may never be able to generate sufficient revenue to achieve or sustain profitability.
Our financial results may vary significantly from quarter to quarter which may adversely affect our stock price.
Investors should consider our business and prospects in light of the risks and difficulties we expect to encounter in the new, uncertain and rapidly evolving markets in which we compete. Because these markets are new and evolving, predicting their future growth and size is difficult. We expect that our visibility into future sales of our products, including volumes, prices and product mix between instruments and consumables, and the amount and timing of payments pursuant to collaboration agreements will continue to be limited and could result in unexpected fluctuations in our quarterly and annual operating results.
Numerous other factors, many of which are outside our control, may cause or contribute to significant fluctuations in our quarterly and annual operating results. These fluctuations may make financial planning and forecasting difficult. For example, in the third quarter of 2017, product and service revenue did not meet expectations which adversely affected our stock price. In addition, these fluctuations may result in unanticipated changes in our available cash, which could negatively affect our business and prospects. Factors that may contribute to fluctuations in our operating results include many of the risks described in this section. Also, one or more of such factors may cause our revenue or operating expenses in one period to be disproportionately higher or lower relative to the others. For example, in May 2017, our collaboration with Medivation, Inc. and Astellas Pharma Inc., or Astellas Pharma, was terminated, resulting in the recognition of $11.3 million of collaboration revenue for the three months ended June 30, 2017, including the impact of a $1.0 million termination penalty. Also, in October 2017, Merck & Co. Inc., or Merck, notified us of the decision to not continue to pursue regulatory approval of the companion diagnostic for their product, KEYTRUDA, under our collaboration, resulting in the recognition of $11.6 million of collaboration revenue during the fourth quarter of 2017. Furthermore, our instruments involve a significant capital commitment by our customers and accordingly involve a lengthy sales cycle. We may expend significant effort in attempting to make a particular sale, which may be deferred by the customer or never occur. Accordingly, comparing our operating results on a period-to-period basis may not be meaningful, and investors should not rely on our past results as an indication of our future performance. If such fluctuations occur or if our operating results deviate from our expectations or the expectations of securities analysts, our stock price may be adversely affected.
If we do not achieve, sustain or successfully manage our anticipated growth, our business and growth prospects will be harmed.
We have experienced significant revenue growth in recent periods and we may not achieve similar growth rates in the
-21-
future. Investors should not rely on our operating results for any prior periods as an indication of our future operating performance. If we are unable to maintain adequate revenue growth, our financial results could suffer and our stock price could decline. Furthermore, growth will place significant strains on our management and our operational and financial systems and processes. For example, development and commercialization of the Prosigna Breast Cancer Assay, or Prosigna, and other future diagnostic products worldwide are key elements of our growth strategy and have required us to hire and retain additional sales and marketing, medical, regulatory, manufacturing and quality assurance personnel. If we do not successfully generate demand for our products or manage our anticipated expenses accordingly, our operating results will be harmed.
Our future success is dependent upon our ability to expand our customer base and introduce new applications and products.
Our current customer base is primarily composed of academic and government research laboratories, biopharmaceutical companies and clinical laboratories (including physician-owned laboratories) that perform analyses using our nCounter Analysis Systems. Our success will depend, in part, upon our ability to increase our market penetration among all of these customers and to expand our market by developing and marketing new research applications, new instruments, and new diagnostic products. During 2017, in an effort to enhance future results, we have added sales staff focused on consumable sales to existing customers, thus enabling existing sales representatives to increase focus on instrument sales. We expect that increasing the installed base of our nCounter Analysis Systems will drive demand for our relatively high margin consumable products. If we are not able to successfully increase our installed base of nCounter Analysis Systems, sales of our consumable products and our margins may not meet expectations. Moreover, we must convince physicians and third-party payors that our diagnostic products, such as Prosigna, are cost effective in obtaining information that can help inform treatment decisions and that our nCounter Analysis Systems could enable an equivalent or superior approach that lessens reliance on centralized laboratories. Palmetto GBA, a Medicare Administrative Contractor, or MAC, that assesses molecular diagnostic technologies through its Molecular Diagnostics Services Program, or MolDx, issued a positive coverage determination for Prosigna in 2015. Several other Medicare jurisdictions that participate in the MolDx program have adopted the same coverage policy. In the fall of 2017, Palmetto declined to process Medicare claims for Prosigna tests performed at physician-owned laboratories. However, after receiving additional information demonstrating that such labs have the same qualifications required to perform Prosigna as independent labs, Palmetto has agreed to process such claims.
We also plan to develop and introduce new products which would be sold primarily to new customer types, such as our Digital Spatial Profiling, or DSP, instrument for use in pathology labs and a sequencer based on our Hyb & Seq chemistry targeted for use by hospitals and oncology clinics. Attracting new customers and introducing new applications and products requires substantial time and expense. Any failure to expand our existing customer base, or launch new applications and products, would adversely affect our ability to improve our operating results.
Our research business depends on levels of research and development spending by academic and governmental research institutions and biopharmaceutical companies, a reduction in which could limit demand for our products and adversely affect our business and operating results.
In the near term, we expect that a large portion of our revenue will be derived from sales of our nCounter Analysis Systems to academic and government research laboratories and biopharmaceutical companies worldwide for research and development applications. The demand for our products will depend in part upon the research and development budgets of these customers, which are impacted by factors beyond our control, such as:
• | changes in government programs (such as the National Institutes of Health) that provide funding to research institutions and companies; |
• | macroeconomic conditions and the political climate; |
• | changes in the regulatory environment; |
• | differences in budgetary cycles; |
• | competitor product offerings or pricing; |
• | market-driven pressures to consolidate operations and reduce costs; and |
• | market acceptance of relatively new technologies, such as ours. |
In addition, academic, governmental and other research institutions that fund research and development activities may be subject to stringent budgetary constraints that could result in spending reductions, reduced allocations or budget cutbacks, which could jeopardize the ability of these customers to purchase our products. Our operating results may fluctuate substantially due to reductions and delays in research and development expenditures by these customers. Any decrease in our customers’ budgets or expenditures, or in the size, scope or frequency of capital or operating expenditures, could materially and adversely affect our business, operating results and financial condition.
-22-
Our sales cycle is lengthy and variable, which makes it difficult for us to forecast revenue and other operating results.
Our sales process involves numerous interactions with multiple individuals within an organization, and often includes in-depth analysis by potential customers of our products, performance of proof-of-principle studies, preparation of extensive documentation and a lengthy review process. As a result of these factors, the large capital investment required in purchasing our instruments and the budget cycles of our customers, the time from initial contact with a customer to our receipt of a purchase order can vary significantly and be up to 12 months or longer. With the introduction of our nCounter SPRINT system in July 2015, which is targeted at individual researchers that often have less certain funding than other potential customers, our visibility regarding timing of sales has decreased. Given the length and uncertainty of our sales cycle, we have in the past experienced, and likely will in the future experience, fluctuations in our instrument sales on a period-to-period basis. These factors also make it difficult to forecast revenue on a quarterly basis. For example, in the third quarter of 2017, our actual revenues were lower than our forecasts for many reasons that we did not predict, including extended timelines for finalizing purchase decisions by potential customers. Furthermore, from time-to-time, we may lease instruments or place instruments under reagent rental agreements, wherein a customer does not purchase an instrument upfront but instead pays a rental fee associated with each purchase of reagents. An increase in instruments placed under these lease or reagent rental agreements may reduce the number of instruments we would otherwise sell in any period. In addition, any failure to meet customer expectations could result in customers choosing to continue to use their existing systems or to purchase systems other than ours.
Our reliance on distributors for sales of our products outside of the United States, and on clinical laboratories for delivery of Prosigna testing services, could limit or prevent us from selling our products and impact our revenue.
We have established exclusive distribution agreements for our nCounter Analysis Systems and related consumable products in many countries where we do not sell directly. We intend to continue to grow our business internationally, and to do so we must attract additional distributors and retain existing distributors to maximize the commercial opportunity for our products. There is no guarantee that we will be successful in attracting or retaining desirable sales and distribution partners or that we will be able to enter into such arrangements on favorable terms. Distributors may not commit the necessary resources to market and sell our products to the level of our expectations or may choose to favor marketing the products of our competitors. If current or future distributors do not perform adequately, or we are unable to enter into effective arrangements with distributors in particular geographic areas, we may not realize long-term international revenue growth.
Similarly, we or our distributors have entered into agreements with clinical laboratories globally to provide Prosigna testing services. We do not provide testing services directly and, thus, we are reliant on these clinical laboratories to actively promote and sell Prosigna testing services. These clinical laboratories may take longer than anticipated to begin offering Prosigna testing services and may not commit the necessary resources to market and sell Prosigna testing services to the level of our expectations. Furthermore, we intend to contract with additional clinical laboratories to offer Prosigna testing services, including physician-owned laboratories, and we may be unsuccessful in attracting and contracting with new clinical laboratory providers. If current or future Prosigna testing service providers do not perform adequately, or we are unable to enter into contracts with additional clinical laboratories to provide Prosigna testing services, we may not be successful selling Prosigna and our future revenue prospects may be adversely affected.
Our strategy to seek to enter into strategic collaborations and licensing arrangements with third parties to develop diagnostic tests and other products may not be successful.
We have relied, and expect to continue to rely, on strategic collaborations and licensing agreements with third parties for discoveries based on which we develop diagnostic tests. For example, we licensed the rights to intellectual property that forms the basis of Prosigna from Bioclassifier, LLC, which was founded by several of our research customers engaged in translational research. Similarly, in connection with our collaboration with Celgene Corporation, we licensed the rights to intellectual property relating to a gene signature for lymphoma subtyping, which was discovered by a consortium of researchers including several of our research customers, from the National Institutes of Health. In connection with our collaboration with Merck to develop a companion diagnostic test, our partner has licensed the technology for such test to us. We intend to enter into more such arrangements with our research customers and other researchers, including biopharmaceutical companies, for development of future diagnostic products. However, there is no assurance that we will be successful in doing so. Establishing collaborations and licensing arrangements is difficult and time-consuming. Discussions may not lead to collaborations or licenses on favorable terms, if at all. To the extent we agree to work exclusively with a party in a given area, our opportunities to collaborate with others could be limited. Certain parties may seek to partner with companies in addition to us in connection with a project. This, in turn, may limit the commercial potential of any products that are the subject of such collaborations. Potential collaborators or licensors may elect not to work with us based upon their assessment of our financial, regulatory, commercial or intellectual property position. In particular, our customers are not obligated to collaborate with us or license technology to us, and they may choose to develop diagnostic products themselves or collaborate with our competitors.
-23-
In August 2017, we entered into a collaboration agreement with Lam Research Corporation, or Lam, with respect to the development and commercialization of our Hyb & Seq sequencing platform and related assays. Pursuant to the terms of the collaboration agreement, Lam will contribute up to $50.0 million, payable quarterly, for allowable development costs. In exchange, Lam is eligible to receive certain single-digit percentage royalty payments on net sales by us of certain products and technologies developed under the collaboration agreement. In addition, we issued Lam a warrant to purchase up to 1.0 million shares of our common stock. The outcome of this collaboration is uncertain and development costs may exceed $50.0 million, in which case we would need to obtain additional funding to complete development of our Hyb & Seq sequencing platform and related assays. Ultimately the development may not be successful, which would negatively impact our prospects for future revenue growth.
New diagnostic product development involves a lengthy and complex process, and we may be unable to commercialize on a timely basis, or at all, any of the tests or products we develop individually or with our collaborators.
Few research and development projects result in successful commercial products, and success in early clinical studies often is not replicated in later studies. At any point, we may abandon development of a product candidate or we may be required to expend considerable resources repeating clinical studies, which would adversely impact potential revenue and our expenses. In addition, any delay in product development would provide others with additional time to commercialize competing products before we do, which in turn may adversely affect our growth prospects and operating results.
In addition, the success of the development programs for any product candidates or assays developed in collaboration with others will be dependent on the continued pursuit and success of the related drug trials by our collaborators. For example, in October 2017, Merck notified us of their decision not to continue to pursue regulatory approval of the companion diagnostic we were developing for their product, KEYTRUDA. There is no guarantee that our collaborators will continue to pursue clinical trials for product candidates or assays that are the subject of our collaborations or that such clinical trials will be successful and, as a result, we may expend considerable time and resources developing in vitro diagnostic assays that will not gain regulatory approval. Furthermore, significant consolidation in the life sciences industry has occurred during the last several years and in connection with such consolidation, the combined company often reassesses its development priorities which may impact our existing collaborations or future opportunities. For example, in May 2017, Astellas Pharma announced a joint decision with Pfizer Inc., or Pfizer, to discontinue the planned ENDEAR trial which was the subject of our collaboration. We were informed that the decision resulted from an oncology portfolio review by Astellas Pharma and Pfizer. Even if we establish new relationships, we or our collaborators may terminate those relationships or they may never result in the successful development or commercialization of future tests or other products. From time to time we have agreed to modify the terms of our agreements with collaborators, including financial terms, and in the future it is possible that we will agree to modify the terms of existing and future agreements with collaborators.
Although we expect such collaborations to provide funding to cover our costs of development, the failure, discontinuation or modification of these clinical trials could negatively impact our ability to attract new collaboration partners, and would reduce our prospects for introducing new diagnostic products, revenue growth, and future operating results.
Our future capital needs are uncertain and we may need to raise additional funds in the future.
We believe that our existing cash and cash equivalents, together with funds available under our term loan agreement and revolving credit facility, will be sufficient to meet our anticipated cash requirements for at least the next 12 months. However, we may need to raise substantial additional capital to:
• | expand the commercialization of our products; |
• | fund our operations; and |
• | further our research and development. |
Our future funding requirements will depend on many factors, including:
• | market acceptance of our products; |
• | the cost and timing of establishing additional sales, marketing and distribution capabilities; |
• | revenue and cash flow derived from existing or future collaborations; |
• | the cost of our research and development activities; |
• | the cost and timing of regulatory clearances or approvals; |
• | the effect of competing technological and market developments; and |
• | the extent to which we acquire or invest in businesses, products and technologies, including new licensing arrangements for new products. |
We cannot assure you that we will be able to obtain additional funds on acceptable terms, or at all. If we raise additional funds by issuing equity or equity-linked securities, our stockholders may experience dilution. For example, in
-24-
January 2018, we entered into a sales agreement with Cowen and Company, LLC, or Cowen, to sell up to $40.0 million worth of shares of our common stock, from time to time, through an “at the market” equity offering program under which Cowen will act as sales agent. Additional debt financing, if available, may involve additional covenants restricting our operations or our ability to incur additional debt. Any debt or additional equity financing that we raise may contain terms that are not favorable to us or our stockholders. If we raise additional funds through collaboration and licensing arrangements with third parties, it may be necessary to relinquish some rights to our technologies or our products, or grant licenses on terms that are not favorable to us. We have in the past pursued these types of transactions, and may in the future pursue similar transactions or other strategic transactions, on our own or with other advisors, that may impact our business and prospects and the value of our common stock. If we do not have, or are not able to obtain, sufficient funds, we may have to delay development or commercialization of our products or license to third parties the rights to commercialize products or technologies that we would otherwise seek to commercialize. We also may have to reduce marketing, customer support or other resources devoted to our products or cease operations. Any of these factors could harm our operating results.
Our research and development efforts will be hindered if we are not able to contract with third parties for access to archival tissue samples.
Under standard clinical practice, tumor biopsies removed from patients are preserved and stored in formalin-fixed paraffin embedded, or FFPE, format. We rely on our ability to secure access to these archived FFPE tumor biopsy samples, as well as information pertaining to the clinical outcomes of the patients from which they were derived for our clinical development activities. Others compete with us for access to these samples. Additionally, the process of negotiating access to archived samples is lengthy because it typically involves numerous parties and approval levels to resolve complex issues such as usage rights, institutional review board approval, privacy rights, publication rights, intellectual property ownership and research parameters. In January 2017, the Department of Health and Human Services finalized new rules, which became effective as of January 19, 2018, expanding the language to be included in informed consent forms related to the collection of identifiable private information or identifiable biospecimens. If this new requirement, or other factors arising in the future, impact our ability to negotiate access to archived tumor tissue samples with hospitals, clinical partners, pharmaceutical companies, or companies developing therapeutics on a timely basis or on commercially reasonable terms, or at all, or if other laboratories or our competitors secure access to these samples before us, our ability to research, develop and commercialize future products will be limited or delayed.
The life sciences research and diagnostic markets are highly competitive. If we fail to compete effectively, our business and operating results will suffer.
We face significant competition in the life sciences research and diagnostic markets. We currently compete with both established and early stage life sciences research companies that design, manufacture and market instruments and consumables for gene expression analysis, single-cell analysis, polymerase chain reaction, or PCR, digital PCR, other nucleic acid detection and additional applications. These companies use well-established laboratory techniques such as microarrays or quantitative PCR as well as newer technologies such as next generation sequencing such as RNA-sequencing. We believe our principal competitors in the life sciences research and diagnostic markets are Agilent Technologies, Becton-Dickinson, Bio-Rad, Bio-Techne, Fluidigm, HTG Molecular Diagnostics, Illumina, Luminex, Merck Millipore, O-Link, Perkin Elmer, Qiagen, Roche Applied Science, Thermo Fisher Scientific, and WaferGen Biosystems. In addition, there are a number of new market entrants in the process of developing novel technologies for the life sciences market.
We also compete with commercial diagnostic laboratory companies. We believe our principal competitor in the breast cancer diagnostics market is Genomic Health, which provides gene expression analysis at its central laboratory in Redwood City, California and currently commands a substantial majority of the market. We also face competition from companies such as Agendia, bioTheranostics, and Myriad Genetics.
Many of our current competitors are large publicly-traded companies, or are divisions of large publicly-traded companies, and may enjoy a number of competitive advantages over us, including:
• | greater name and brand recognition, financial and human resources; |
• | broader product lines; |
• | larger sales forces and more established distributor networks; |
• | substantial intellectual property portfolios; |
• | larger and more established customer bases and relationships; and |
• | better established, larger scale, and lower cost manufacturing capabilities. |
We believe that the principal competitive factors in all of our target markets include:
• | cost of capital equipment; |
-25-
• | cost of consumables and supplies; |
• | reputation among customers; |
• | innovation in product offerings; |
• | flexibility and ease-of-use; |
• | accuracy and reproducibility of results; and |
• | compatibility with existing laboratory processes, tools and methods. |
We believe that additional competitive factors specific to the diagnostics market include:
• | availability of reimbursement for testing services; |
• | breadth of clinical decisions that can be influenced by information generated by tests; |
• | volume, quality, and strength of clinical and analytical validation data; |
• | inclusion in treatment guidelines; and |
• | economic benefit accrued to customers based on testing services enabled by products. |
We cannot assure investors that our products will compete favorably or that we will be successful in the face of increasing competition from new products and technologies introduced by our existing competitors or new companies entering our markets. In addition, we cannot assure investors that our competitors do not have or will not develop products or technologies that currently or in the future will enable them to produce competitive products with greater capabilities or at lower costs than ours. For example, we recently concluded that certain of our customers have shifted certain types of experiments that previously had been performed on our nCounter system to RNA-sequencing technology. Although we are pursuing several strategies to mitigate this trend, there can be no assurance we will be successful in doing so. Any failure to compete effectively could materially and adversely affect our business, financial condition and operating results.
If Prosigna fails to achieve and sustain sufficient market acceptance, we will not generate expected revenue, and our prospects may be harmed.
Commercialization of Prosigna in Europe, the United States and the other jurisdictions in which we intend to pursue regulatory approval or clearance is a key element of our strategy. Currently, most oncologists seeking sophisticated gene expression analysis for diagnosing and profiling breast cancer in their patients ship tissue samples to a limited number of centralized laboratories typically located in the United States. We may experience reluctance, or refusal, on the part of physicians to order, and third-party payors to pay for, Prosigna if the results of our research and clinical studies, and our sales and marketing activities relating to communication of these results, do not convey to physicians and patients that Prosigna provides equivalent or better prognostic information than those centralized laboratories. In addition, our diagnostic tests are performed by pathologists in local laboratories, rather than by a vendor in a remote centralized laboratory, which requires us to educate pathologists regarding the benefits of this business model and oncologists regarding the reliability and consistency of results generated locally. Also, we offer Prosigna in other countries outside of the United States, where genomic testing for breast cancer is not widely available and the market for such tests is new. The future growth of the market for genomic breast cancer testing will depend on physicians’ acceptance of such testing and the availability of reimbursement for such tests.
These hurdles may make it difficult to convince healthcare providers that tests using our technologies are appropriate options for cancer diagnostics, may be equivalent or superior to available tests, and may be at least as cost effective as alternative technologies. If we fail to successfully commercialize Prosigna, we may never receive a return on the significant investments in sales and marketing, medical, regulatory, manufacturing and quality assurance personnel we have made, and further investments we intend to make, which would adversely affect our growth prospects, operating results and financial condition.
We may not be able to develop new products, enhance the capabilities of our systems to keep pace with rapidly changing technology and customer requirements or successfully manage the transition to new product offerings, any of which could have a material adverse effect on our business and operating results.
Our success depends on our ability to develop new products and applications for our technology in existing and new markets, while improving the performance and cost-effectiveness of our systems. New technologies, techniques or products could emerge that might offer better combinations of price and performance than our current or future products and systems. Existing markets for our products, including gene expression analysis, gene fusions and copy number variation, as well as new markets, such as protein expression and gene mutations, and potential markets for our research and diagnostic product candidates, are characterized by rapid technological change and innovation. Competitors may be able to respond more quickly and effectively than we can to new or changing opportunities, technologies, standards or customer requirements. We anticipate that we will face increased competition in the future as existing companies and competitors develop new or improved products and as new companies enter the market with new technologies. It is critical to our success that we anticipate changes in
-26-
technology and customer requirements and successfully introduce new, enhanced and competitive technologies to meet our customers’ and prospective customers’ needs on a timely and cost-effective basis. For example, we recently announced that we intend to make DSP, which enables the precise quantification of protein and gene expression spatially for regions of interest in a tissue sample, available on an early access basis in late 2018. In addition, we have been developing a unique amplification-free Hyb & Seq chemistry that provides both short and long read capability simultaneously, as well as the ability to sequence both DNA and RNA in parallel. If we do not successfully innovate and introduce new technology into our product lines, our business and operating results will be adversely impacted.
The development of new products typically requires new scientific discoveries or advancements and complex technology and engineering. Such developments may involve external suppliers and service providers, making the management of development projects complex and subject to risks and uncertainties regarding timing, timely delivery of required components or services and satisfactory technical performance of such components or assembled products. For example, in 2017, we continued to work with our supplier of cartridges used in our nCounter SPRINT systems to improve the design which resolved the previous leakage issues in the microfluidic device produced for us. If we do not achieve the required technical specifications or successfully manage new product development processes, or if development work is not performed according to schedule, then such new technologies or products may be adversely impacted and our business and operating results may be harmed.
Additionally, we must carefully manage the introduction of new products. If customers believe that such products will offer enhanced features or be sold for a more attractive price, they may delay purchases until such products are available. In July 2015, we commercially launched a new version of our nCounter Analysis System, the nCounter SPRINT Profiler, that is smaller and less expensive than the previous version. If customers conclude that such new products offer better value as compared to our existing products, we may suffer from reduced sales of our existing products and our overall revenue may decline. We may also have excess or obsolete inventory of older products as we transition to new products and our experience in managing product transitions is limited. If we do not effectively manage the transitions to new product offerings, our revenue, results of operations and business will be adversely affected.
New market opportunities may not develop as quickly as we expect, limiting our ability to successfully market and sell our products.
The market for our products is new and evolving. Accordingly, we expect the application of our technologies to emerging opportunities will take several years to develop and mature and we cannot be certain that these market opportunities will develop as we expect. For example, in September 2015, we launched our first 3D Biology application, a new product that allows users to simultaneously measure gene and protein expression from a single sample. In 2016 and 2017, we launched additional 3D Biology panels, including our first for the measurement of DNA mutations and in 2017 we launched our 360 panels for use in breast cancer, immuno-oncology and hematology. This year, we intend to expand beyond oncology and launch panels in neuroscience and immune-related diseases. At the end of 2018, we intend to launch our DSP product on an early access basis. This product will target the pathology market, which we have not previously targeted.
The future growth of the market for these new products depends on many factors beyond our control, including recognition and acceptance of our applications by the scientific community and the growth, prevalence and costs of competing methods of genomic analysis. If the markets for our new products do not develop as we expect, our business may be adversely affected. If we are not able to successfully market and sell our products or to achieve the revenue or margins we expect, our operating results may be harmed.
We are dependent on single source suppliers for some of the components and materials used in our products, and the loss of any of these suppliers could harm our business.
We rely on Precision System Science, Co., Ltd of Chiba, Japan, to build our nCounter Prep Station, Korvis LLC of Corvallis, Oregon, to build our nCounter Digital Analyzer, Paramit Corporation of Morgan Hill, California, to build our new nCounter SPRINT Profiler and IDEX Corporation of Lake Forest, Illinois to build the fluidics cartridge, a key component of our nCounter SPRINT Profiler. Each of these contract manufacturers are sole suppliers. Since our contracts with these instrument suppliers do not commit them to carry inventory or make available any particular quantities, they may give other customers’ needs higher priority than ours, and we may not be able to obtain adequate supplies in a timely manner or on commercially reasonable terms. We also rely on sole suppliers for various components we use to manufacture our consumable products. We periodically forecast our needs for such components and enter into standard purchase orders with them. If we were to lose such suppliers, there can be no assurance that we will be able to identify or enter into agreements with alternative suppliers on a timely basis on acceptable terms, if at all. If we should encounter delays or difficulties in securing the quality and quantity of materials we require for our products, our supply chain would be interrupted which would adversely affect sales. If any of these events occur, our business and operating results could be harmed.
-27-
We may experience manufacturing problems or delays that could limit our growth or adversely affect our operating results.
Our consumable products are manufactured at our Seattle, Washington facility using complex processes, sophisticated equipment and strict adherence to specifications and quality systems procedures. Any unforeseen manufacturing problems, such as contamination of our facility, equipment malfunction, quality issues with components and materials sourced from third-party suppliers or failure to strictly follow procedures or meet specifications, could result in delays or shortfalls in production or require us to voluntarily recall our consumable products. Identifying and resolving the cause of any such manufacturing or supplier issues could require substantial time and resources. If we are unable to keep up with demand for our products by successfully manufacturing and shipping our products in a timely manner, our revenue could be impaired, market acceptance for our products could be adversely affected and our customers might instead purchase our competitors’ products.
In addition, the introduction of new products may require the development of new manufacturing processes and procedures. For example, our 3D Biology applications for the simultaneous measurement of gene and protein expression and DNA mutations involve new processes for manufacturing our molecular barcodes. While all of our CodeSets are produced using the same basic processes, significant variations may be required to meet new product specifications. Developing new processes can be very time consuming, and any unexpected difficulty in doing so could delay the introduction of a product.
If our Seattle facilities become unavailable or inoperable, we will be unable to continue our research and development, manufacturing our consumables or processing sales orders, and our business will be harmed.
We manufacture our consumable products in our headquarters facilities in Seattle, Washington. In addition, Seattle is the center for research and development, order processing, receipt of our instruments manufactured by third-party contract manufacturers and shipping products to customers. Our facilities and the equipment we use to manufacture our consumable products would be costly, and would require substantial lead time, to repair or replace. Seattle is situated near active earthquake fault lines. These facilities may be harmed or rendered inoperable by natural or man-made disasters, including earthquakes and power outages, which may render it difficult or impossible for us to produce our products for some period of time. The inability to manufacture consumables or to ship products to customers for even a short period of time may result in the loss of customers or harm our reputation, and we may be unable to regain those customers in the future. Although we possess insurance for damage to our property and the disruption of our business, this insurance, and in particular earthquake insurance, which is limited, may not be sufficient to cover all of our potential losses and may not continue to be available to us on acceptable terms, if at all.
We expect to generate a substantial portion of our product and service revenue internationally and are subject to various risks relating to our international activities, which could adversely affect our operating results.
For 2017, 2016 and 2015 approximately 40%, 38% and 39% respectively, of our product and service revenue was generated from sales to customers located outside of North America. We believe that a significant percentage of our future revenue will come from international sources as we expand our overseas operations and develop opportunities in additional areas. Engaging in international business involves a number of difficulties and risks, including:
• | required compliance with existing and changing foreign regulatory requirements and laws; |
• | required compliance with anti-bribery laws, such as the U.S. Foreign Corrupt Practices Act and U.K. Bribery Act, data privacy requirements, labor laws and anti-competition regulations; |
• | export or import restrictions; |
• | various reimbursement and insurance regimes; |
• | laws and business practices favoring local companies; |
• | longer payment cycles and difficulties in enforcing agreements and collecting receivables through certain foreign legal systems; |
• | political and economic instability, such as the anticipated exit of Great Britain from the European Economic Community; |
• | potentially adverse tax consequences, tariffs, customs charges, bureaucratic requirements and other trade barriers; |
• | difficulties and costs of staffing and managing foreign operations; and |
• | difficulties protecting or procuring intellectual property rights. |
As we expand internationally, our results of operations and cash flows will become increasingly subject to fluctuations due to changes in foreign currency exchange rates. Historically, most of our revenue has been denominated in U.S. dollars, although we have sold our products and services in local currency outside of the United States, principally the Euro. Our expenses are generally denominated in the currencies in which our operations are located, which is primarily in the United States. As our operations in countries outside of the United States grow, our results of operations and cash flows will
-28-
increasingly be subject to fluctuations due to changes in foreign currency exchange rates, which could harm our business in the future. For example, if the value of the U.S. dollar increases relative to foreign currencies, our product and service revenue could be adversely affected as we convert revenue from local currencies to U.S. dollars. Similarly, a strong U.S. dollar relative to the local currencies of our international customers can potentially reduce demand for our products, which may compound the adverse effect of foreign exchange translation on our revenue. If we dedicate significant resources to our international operations and are unable to manage these risks effectively, our business, operating results and prospects will suffer.
Significant U.K. or European developments stemming from the U.K.’s referendum on membership in the European Union could have a material adverse effect on us.
In June 2016, the United Kingdom held a referendum and voted in favor of leaving the European Union, and in March 2017, the government of the United Kingdom formally initiated the withdrawal process. This has created political and economic uncertainty, particularly in the United Kingdom and the European Union, and this uncertainty may last for years. Our business in the United Kingdom, the European Union, and worldwide could be affected during this period of uncertainty, and perhaps longer, by the impact of the United Kingdom’s referendum. There are many ways in which our business could be affected, only some of which we can identify as of the date of this report.
The decision of the United Kingdom to withdraw from the European Union has caused and, along with events that could occur in the future as a consequence of the United Kingdom’s withdrawal, including the possible breakup of the United Kingdom, may continue to cause significant volatility in global financial markets, including in global currency and debt markets. This volatility could cause a slowdown in economic activity in the United Kingdom, Europe or globally, which could adversely affect our operating results and growth prospects. In addition, our business could be negatively affected by new trade agreements or data transfer agreements between the United Kingdom and other countries, including the United States, and by the possible imposition of trade or other regulatory and immigration barriers in the United Kingdom. In addition, the Europe-wide market authorization framework for our products (and for the drugs sold by our collaboration partners in the pharmaceutical industry) may also change. Furthermore, we currently operate in Europe through a subsidiary based in the United Kingdom, which provides us with certain operational, tax and other benefits, as well as through other subsidiaries in Europe. The United Kingdom’s withdrawal from the European Union could adversely affect our ability to realize those benefits and we may incur costs and suffer disruptions in our European operations as a result. These possible negative impacts, and others resulting from the United Kingdom’s actual or threatened withdrawal from the European Union, may adversely affect our operating results and growth prospects.
Changes in tax laws or regulations that are applied adversely to us or our customers may have a material adverse effect on our business, cash flow, financial condition or results of operations.
New income, sales, use or other tax laws, statutes, rules, regulations or ordinances could be enacted at any time, which could affect the tax treatment of our domestic and foreign earnings. Any new taxes could adversely affect our domestic and international business operations, and our business and financial performance. Further, existing tax laws, statutes, rules, regulations or ordinances could be interpreted, changed, modified or applied adversely to us. For example, President Trump recently signed tax legislation into law, the Tax Cuts and Jobs Act, that contains many significant changes to the U.S. tax laws, the consequences to us of which have not yet been determined. Changes in corporate tax rates, the availability of the net deferred tax assets relating to our U.S. operations, the taxation of foreign earnings, and the deductibility of expenses contained in the Tax Cuts and Jobs Act or other tax reform legislation could have a material impact on the value of our deferred tax assets, could result in significant one-time charges in the current or future taxable years, and could increase our future U.S. tax expense. Furthermore, changes to the taxation of undistributed foreign earnings could change our future intentions regarding reinvestment of such earnings. The foregoing items could have a material adverse effect on our business, cash flow, financial condition or results of operations.
Our ability to use net operating losses to offset future taxable income may be subject to certain limitations.
As of December 31, 2017, we had federal net operating loss carryforwards, or NOLs, to offset future taxable income of approximately $232.8 million, which expire in various years beginning in 2025, if not utilized. A lack of future taxable income would adversely affect our ability to utilize these NOLs. In addition, under Section 382 of the Internal Revenue Code, a corporation that undergoes an “ownership change” is subject to limitations on its ability to utilize its NOLs to offset future taxable income. We may have already experienced one or more ownership changes. Depending on the timing of any future utilization of our carryforwards, we may be limited as to the amount that can be utilized each year as a result of such previous ownership changes. However, we do not believe such limitations will cause our NOL and credit carryforwards to expire unutilized. In addition, future changes in our stock ownership as well as other changes that may be outside of our control, could result in additional ownership changes under Section 382 of the Internal Revenue Code. Our NOLs may also be impaired under similar provisions of state law. We have recorded a full valuation allowance related to our NOLs and other deferred tax assets due to the uncertainty of the ultimate realization of the future benefits of those assets.
-29-
Provisions of our debt instruments may restrict our ability to pursue our business strategies.
Our term loan agreement and revolving credit facility require us, and any debt instruments we may enter into in the future may require us, to comply with various covenants that limit our ability to, among other things:
• | dispose of assets; |
• | complete mergers or acquisitions; |
• | incur indebtedness; |
• | encumber assets; |
• | pay dividends or make other distributions to holders of our capital stock; |
• | make specified investments; |
• | engage in any new line of business; and |
• | engage in certain transactions with our affiliates. |
These restrictions could inhibit our ability to pursue our business strategies. In addition, we are subject to financial covenants based on total revenue and minimum cash balances. If we default under our term loan agreement or revolving credit facility, and such event of default is not cured or waived, the lenders could terminate commitments to lend and cause all amounts outstanding with respect to the debt to be due and payable immediately, which in turn could result in cross defaults under other debt instruments. Our assets and cash flow may not be sufficient to fully repay borrowings under all of our outstanding debt instruments if some or all of these instruments are accelerated upon a default. We may incur additional indebtedness in the future. The debt instruments governing such indebtedness could contain provisions that are as, or more, restrictive than our existing debt instruments. If we are unable to repay, refinance or restructure our indebtedness when payment is due, the lenders could proceed against the collateral granted to them to secure such indebtedness or force us into bankruptcy or liquidation.
Acquisitions or joint ventures could disrupt our business, cause dilution to our stockholders and otherwise harm our business.
We may acquire other businesses, products or technologies as well as pursue strategic alliances, joint ventures, technology licenses or investments in complementary businesses. We have not made any acquisitions to date, and our ability to do so successfully is unproven. Any of these transactions could be material to our financial condition and operating results and expose us to many risks, including:
• | disruption in our relationships with customers, distributors or suppliers as a result of such a transaction; |
• | unanticipated liabilities related to acquired companies; |
• | difficulties integrating acquired personnel, technologies and operations into our existing business; |
• | diversion of management time and focus from operating our business; |
• | increases in our expenses and reductions in our cash available for operations and other uses; and |
• | possible write-offs or impairment charges relating to acquired businesses. |
Foreign acquisitions involve unique risks in addition to those mentioned above, including those related to integration of operations across different cultures and languages, currency risks and the particular economic, political and regulatory risks associated with specific countries.
Also, the anticipated benefit of any strategic transaction may not materialize. Future acquisitions or dispositions could result in potentially dilutive issuances of our equity securities, the incurrence of debt, contingent liabilities or amortization expenses or write-offs of goodwill, any of which could harm our financial condition. We cannot predict the number, timing or size of future joint ventures or acquisitions, or the effect that any such transactions might have on our operating results.
If we are unable to recruit, train and retain key personnel, we may not achieve our goals.
Our future success depends on our ability to recruit, train, retain and motivate key personnel, including our senior management, research and development, manufacturing and sales and marketing personnel. Competition for qualified personnel is intense, particularly in the Seattle, Washington area. Our growth depends, in particular, on attracting, retaining and motivating highly-trained sales personnel with the necessary scientific background and ability to understand our systems at a technical level to effectively identify and sell to potential new customers. We do not maintain fixed term employment contracts or key man life insurance with any of our employees. Because of the complex and technical nature of our products and the dynamic market in which we compete, any failure to attract, train, retain and motivate qualified personnel could materially harm our operating results and growth prospects.
-30-
Undetected errors or defects in our products could harm our reputation, decrease market acceptance of our products or expose us to product liability claims.
Our products may contain undetected errors or defects when first introduced or as new versions are released. Disruptions or other performance problems with our products may damage our customers’ businesses, harm our reputation and result in reduced revenues. If that occurs, we may also incur significant costs, the attention of our key personnel could be diverted, or other significant customer relations problems may arise. We may also be subject to warranty and liability claims for damages related to errors or defects in our products. A material liability claim or other occurrence that harms our reputation or decreases market acceptance of our products could adversely impact our business and operating results.
The sale and use of products or services based on our technologies, or activities related to our research and clinical studies, could lead to the filing of product liability claims if someone were to allege that one of our products contained a design or manufacturing defect which resulted in the failure to adequately perform the analysis for which it was designed. A product liability claim could result in substantial damages and be costly and time consuming to defend, either of which could materially harm our business or financial condition. We cannot assure investors that our product liability insurance would adequately protect our assets from the financial impact of defending a product liability claim. Any product liability claim brought against us, with or without merit, could increase our product liability insurance rates or prevent us from securing insurance coverage in the future.
We face risks related to handling of hazardous materials and other regulations governing environmental safety.
Our operations are subject to complex and stringent environmental, health, safety and other governmental laws and regulations that both public officials and private individuals may seek to enforce. Our activities that are subject to these regulations include, among other things, our use of hazardous materials and the generation, transportation and storage of waste. We could discover that we or an acquired business are not in material compliance with these regulations. Existing laws and regulations may also be revised or reinterpreted, or new laws and regulations may become applicable to us, whether retroactively or prospectively, that may have a negative effect on our business and results of operations. It is also impossible to eliminate completely the risk of accidental environmental contamination or injury to individuals. In such an event, we could be liable for any damages that result, which could adversely affect our business.
If we experience a significant disruption in our information technology systems or breaches of data security, our business could be adversely affected.
We rely on information technology systems to keep financial records, manage our manufacturing operations, fulfill customer orders, capture laboratory data, maintain corporate records, communicate with staff and external parties and operate other critical functions. Our information technology systems are potentially vulnerable to disruption due to breakdown, malicious intrusion and computer viruses or other disruptive events including but not limited to natural disaster. If we were to experience a prolonged system disruption in our information technology systems or those of certain of our vendors, it could negatively impact our ability to serve our customers, which could adversely impact our business. Although we maintain offsite back-ups of our data, if operations at our facilities were disrupted, it may cause a material disruption in our business if we are not capable of restoring function on an acceptable timeframe. In addition, our information technology systems are potentially vulnerable to data security breaches – whether by employees or others – which may expose sensitive data to unauthorized persons. Such data security breaches could lead to the loss of trade secrets or other intellectual property, or could lead to the public exposure of personal information (including sensitive personal information) of our employees, customers and others, any of which could have a material adverse effect on our business, financial condition and results of operations. If we are unable to prevent such security breaches or privacy violations or implement satisfactory remedial measures, our operations could be disrupted, and we may suffer loss of reputation, financial loss and other negative consequences because of lost or misappropriated information. In addition, these breaches and other inappropriate access can be difficult to detect, and any delay in identifying them may lead to increased harm of the type described above.
We intend to seek strategic collaborations and partnerships and other transactions, which may result in the use of a significant amount of our management resources or significant costs, and we may not be able to fully realize the potential benefit of such transactions.
We intend to seek strategic collaborations and partnerships to support the continued growth of the company. Accordingly, we may be engaged in evaluating potential transactions including, without limitation, strategic partnerships, divestitures of existing businesses or assets, a merger or consolidation with a third party that results in a change in control, a sale or transfer of all or a significant portion of our assets or a purchase by a third party of our securities that may result in a minority or control investment by such third party. From time to time, we may engage in discussions that may result in one or more transactions. Although there would be uncertainty that any of these discussions would result in definitive agreements or the completion of any transaction, we may devote a significant amount of our management resources to such a transaction,
-31-
which could negatively impact our operations. In addition, we may incur significant costs in connection with seeking strategic transactions regardless of whether the transaction is completed. In the event that we consummate a strategic collaboration or partnership or other transaction in the future, we cannot assure you that we would fully realize the potential benefit of such a transaction which could adversely affect our future financial results or that such transaction would positively impact the value of stockholders' investment in us.
Risks Related to Government Regulation and Diagnostic Product Reimbursement
Our “research use only” products for the research market could become subject to regulation as medical devices by the FDA or other regulatory agencies in the future which could increase our costs and delay our commercialization efforts, thereby materially and adversely affecting our business and results of operations.
In the United States, most of our products are currently labeled and sold for research use only, or RUO, and not for the diagnosis or treatment of disease, and are sold to pharmaceutical and biotechnology companies, academic and government institutions and research laboratories. Because such products are not intended for diagnostic use, and the products do not include clinical or diagnostic claims, or directions to use as diagnostic products, they are not subject to regulation by the Food and Drug Administration, or FDA, as medical devices. In particular, while the FDA regulations require that RUO products be labeled, “For Research Use Only. Not for use in diagnostic procedures,” the regulations do not subject such products to the FDA’s pre- and post-market controls for medical devices. Pursuant to FDA guidance on RUO products, a company may not make clinical or diagnostic claims about an RUO product or provide clinical directions or clinical support services to customers for RUO products. If the FDA were to modify its approach to regulating products labeled for research use only, it could reduce our revenue or increase our costs and adversely affect our business, prospects, results of operations or financial condition. In the event that the FDA requires marketing authorization of our RUO products in the future, there can be no assurance that the FDA will ultimately grant any clearance or approval requested by us in a timely manner, or at all.
In addition, we sell dual-use instruments with software that has both FDA-cleared functions and research functions, for which FDA approval or clearance is not required. Dual-use instruments are subject to FDA regulation since they are intended, at least in part, for use by customers performing clinical diagnostic testing. In November 2014, FDA issued a guidance document that described FDA’s approach to regulating molecular diagnostic instruments that combine both approved/cleared device functions and device functions for which approval/clearance is not required. There is a risk that the FDA could take enforcement action against a manufacturer for distributing dual-use instruments if the company does not follow the restrictions discussed in the guidance document. For example, there could be enforcement action if the FDA determines that approval or clearance was required for those functions for which FDA approval or clearance has not been obtained, or the instruments are being promoted off-label. There is also a risk that the FDA could broaden its current regulatory enforcement of dual-use instruments through additional FDA oversight of such products or impose additional requirements upon such products. Earlier this past year, FDA proposed that the clinical diagnostic portions of clinical multiplex test systems, like the ones used with our Prosigna assay, be exempt from the requirement for FDA clearance. FDA adopted the proposal on July 11, 2017 and issued new regulations exempting certain clinical multiplex test systems from premarket notification requirements. However, these new regulations will not impact the FDA clearance requirements for our nCounter Dx Analysis System which will still require 510(k) clearance for use with specific assays like Prosigna.
If Medicare and other third-party payors in the United States and foreign countries do not approve reimbursement for diagnostic tests enabled by our technology, the commercial success of our diagnostic products would be compromised.
Successful commercialization of our diagnostic products depends, in large part, on the availability of adequate reimbursement for testing services that our diagnostic products enable from government insurance plans, managed care organizations and private insurance plans. There is significant uncertainty surrounding third-party reimbursement for the use of tests that incorporate new technology. For example, after the FDA clearance of Prosigna in September 2013, it took over two years to achieve broad Medicare reimbursement of Prosigna testing.
If we are unable to obtain positive policy decisions from third-party payors approving reimbursement for our tests at adequate levels, the commercial success of our diagnostic products would be compromised and our revenue would be significantly limited. Even if we do obtain reimbursement for our tests, Medicare, Medicaid and private and other payors may withdraw their coverage policies, cancel their contracts at any time, review and adjust the rate of reimbursement, require co-payments from patients or stop paying for our tests, which would reduce revenue for testing services based on our technology, and indirectly, demand for diagnostic products. In addition, insurers, including managed care organizations as well as government payors such as Medicare and Medicaid, have increased their efforts to control the cost, utilization and delivery of healthcare services, which may include decreased coverage or reduced reimbursement. From time to time, Congress has considered and implemented changes to the Medicare fee schedules in conjunction with budgetary legislation, and pricing and payment terms, including the possible requirement of a patient co-payment for Medicare beneficiaries for tests covered by
-32-
Medicare, and are subject to change at any time. Most recently the Protecting Access to Medicare Act, or PAMA, of 2014 revises the Medicare Clinical Laboratory Fee Schedule, or CLFS, to base prices on commercial payer rates that are reported to the Centers for Medicare and Medicaid Services, or CMS. In June 2016, CMS released the final Clinical Diagnostic Tests Laboratory Payment System regulations, in response to PAMA. The statute applies different reporting and payment requirements to Advanced Diagnostic Laboratory Tests and to Clinical Diagnostic Laboratory Tests, or CDLTs. Under the definitions in the rules, Prosigna is defined as a CDLT and therefore will be repriced every three years based on a weighted median of commercial payments submitted by labs. As a result, if commercial payment amounts decline, there is a risk that Medicare prices will fall as well, though PAMA limits these reductions to no more than 10% less than the prior year during calendar years 2018-2020 and no more than 15% less during years 2021-2023.
Reductions in the reimbursement rate of third-party payors have also occurred and may occur in the future. For example, in September 2017, CMS published its preliminary determinations of pricing for CDLTs to take effect on January 1, 2018. CMS issued a proposed payment determination that would reduce Medicare reimbursement of Prosigna to our customers from the current rate of $3,443 per test to $508 per test. CMS used a pricing methodology called "crosswalking" pursuant to which a new test such as Prosigna is determined to be similar to an existing test and is assigned the same fee schedule amount as the existing test. CMS recommended crosswalking Prosigna to a colorectal screening test, which has the lowest priced code for advanced diagnostic tests on the fee schedule, despite a recommendation from an advisory panel that it be crosswalked to a different code (0008M). We successfully advocated to CMS to crosswalk Prosigna to the 0008M code category and restore its current reimbursement level. However, as part of its market-based pricing determinations for 2018 required by PAMA, CMS calculated the weighted median of commercial payments for laboratory tests. For Prosigna, only one commercial payment rate from a single commercial laboratory was reported which was lower than the current reimbursement price. CMS used that single payment amount as the weighted median which triggered an automatic reduction in Prosigna's Medicare reimbursement rate of $3,443 to $3,419, effective January 1, 2018. Reductions in the prices at which testing services based on our technology are reimbursed could have a negative impact on our revenue.
In many countries outside of the United States, various coverage, pricing and reimbursement approvals are required. Positive reimbursement decisions for Prosigna have occurred in France, certain regions of Spain, Canada, Israel, Switzerland and Denmark but despite these positive developments, we continue to expect that it will take several years to establish broad coverage and reimbursement for testing services based on our products with most payors in countries outside of the United States, and our efforts may not be successful.
We continue to pursue positive reimbursement and coverage decisions from government insurance plans, managed care organizations and private insurance plans. From time to time, if positive coverage decisions are obtained, we may publicly announce such decisions. In most cases where coverage is denied by a third-party payor, there will be subsequent opportunities to submit additional information or clinical evidence and have such decision reconsidered. We intend to evaluate the benefit of continued pursuit of a positive reimbursement determination on a case by case basis and in most cases expect to continue to pursue a positive coverage decision with those payors based on additional information or subsequent clinical developments; as a result, we do not intend to publicly announce any denials of coverage or the absence of a coverage determination on a regular basis.
Our nCounter-based reagents may be used by clinical laboratories to create Laboratory-Developed Tests, which could in the future be the subject of additional FDA regulation as medical devices, which could materially and adversely affect our business and results of operations.
In February 2014, we launched nCounter Elements reagents, a digital molecular barcoding chemistry that allows users to design their own customized assays using standard sets of barcodes provided by us with the laboratories’ choice of oligonucleotide probes. These reagents, which will now be offered to customers in the United States through a custom manufacturing service, may be used by laboratories in conjunction with appropriate analyte-specific reagents and general purpose reagents to create diagnostic tests or test systems.
A clinical laboratory can use our custom-manufactured reagents to create what is called a Laboratory Developed Test, or LDT. LDTs, according to the FDA, are diagnostic tests that are developed, validated and performed by a single laboratory and include genetic tests. Historically, LDTs generally have not been subject to FDA regulations. In October 2014, the FDA issued draft guidance documents proposing the use of a risk-based approach to regulating LDTs. Any restrictions on LDTs by the FDA could decrease demand for our reagents. Additionally, compliance with additional regulatory burdens could be time consuming and costly for our customers. While FDA announced in November 2016 that it did not intend to seek finalization of the draft LDT guidance in the near term, FDA could alter its position or Congress could enact legislation that could result in FDA regulation of some LDTs. If FDA changed its policy or legislation were enacted, it could adversely affect demand for these specialized reagents.
-33-
If we are unable to obtain additional regulatory clearances or approvals to market Prosigna in additional countries or if regulatory limitations are placed on our diagnostic products, our business and growth will be harmed. In addition, if we do not obtain additional regulatory clearances or approvals necessary to market products other than Prosigna for diagnostic purposes, we will be limited to marketing such products for research use only.
We have received regulatory clearance in the United States under a 510(k) for a version of our first diagnostic product, Prosigna, providing an assessment of a patient’s risk of recurrence for breast cancer, and we have obtained a CE mark for Prosigna which permits us to market that assay for diagnostic purposes in the European Union. We do not have regulatory clearance or approval to market in any additional markets, other than Switzerland, Israel, Canada, Turkey, New Zealand, Hong Kong, Australia, Thailand, Argentina and Singapore, or to promote Prosigna in the United States for additional indications. Other than with respect to Prosigna in such jurisdictions, we are limited to marketing our products for research use only, which means that we cannot make diagnostic or clinical claims. We intend to seek regulatory authorizations to market Prosigna in other jurisdictions and, potentially, for other indications. In addition, pursuant to our collaborations with pharmaceutical companies for the development of companion diagnostic tests for use with their drugs, we are responsible for obtaining any regulatory authorizations needed to use the companion diagnostic tests in clinical trials as well as the regulatory approvals to sell the companion diagnostic tests following completion of such trials. Some of the compensation we expect to receive pursuant to these collaborations is based on the receipt of such approvals.
We cannot assure investors that we will be successful in obtaining these regulatory clearances or approvals. If we do not obtain additional regulatory clearances or approvals to market future products or expand future indications for diagnostic purposes, if additional regulatory limitations are placed on our products or if we fail to successfully commercialize such products, the market potential for our diagnostic products would be constrained, and our business and growth prospects would be adversely affected.
Approval and/or clearance by the FDA and foreign regulatory authorities for our diagnostic tests will take significant time and require significant research, development and clinical study expenditures and ultimately may not succeed.
Before we begin to label and market our products for use as clinical diagnostics in the United States, thereby subjecting them to FDA regulation as medical devices, unless an exemption applies we are required to obtain prior 510(k) clearance, de novo authorization or pre-market application approval, or PMA approval, from the FDA. In September 2013, we received FDA 510(k) clearance for Prosigna as a prognostic indicator for distant recurrence-free survival at 10 years in post-menopausal women with Stage I/II lymph node-negative or Stage II lymph node-positive (1-3 positive nodes) hormone receptor-positive breast cancer who have undergone surgery in conjunction with locoregional treatment and consistent with the standard of care. We may pursue additional intended uses for Prosigna that require a PMA approval, which is a more burdensome regulatory process than the 510(k) clearance process. In addition, we are currently collaborating with Celgene on a companion diagnostic test for their drug REVLIMID. In August 2014, the FDA issued a companion diagnostics final guidance stating that if the device is essential to the safety or efficacy of the drug, the FDA generally will require approval or clearance for the device at the time when the FDA approves the drug. The FDA stated in the companion diagnostics final guidance that while in some instances a companion diagnostic could come to market through a 510(k), FDA expects that companion diagnostics usually will require a PMA. In July 2016, the FDA issued a draft co-development companion diagnostic and therapeutic guidance document which similarly reflected this information. The draft guidance appears to also relate, at least in part, to what may be considered complementary diagnostics, i.e., diagnostics that are beneficial for therapeutic product development or clinical decision making but that do not meet the definition of an IVD companion diagnostic. If we developed a diagnostic device to be used in conjunction with a pharmaceutical product that was then cleared or approved but not as a companion diagnostic for the therapeutic product, this may result in potentially reduced revenue for the test as the labeling of the drug would not reference the need for the diagnostic test.
Any 510(k) clearance, de novo authorization or PMA approval we obtain for any future product would place substantial restrictions on how our device is marketed or sold. The FDA will continue to place considerable restrictions on our products, including, but not limited to, the obligation to comply with the Quality System Regulation, or QSR, registering manufacturing facilities, listing the products with the FDA, and complying with labeling, marketing, complaint handling, medical device reporting requirements, and reporting certain corrections and removals. Obtaining FDA clearance or approval for diagnostics can be expensive and uncertain, and generally takes from several months to several years from submission, and generally requires detailed and comprehensive scientific and clinical data, as well as compliance with FDA regulations. In addition, we have limited experience in obtaining PMA approval from the FDA and are therefore supplementing our operational capabilities to manage the more complex processes needed to obtain and maintain PMAs. Notwithstanding the expense, these efforts may never result in FDA approval or clearance. Even if we were to obtain regulatory approval, authorization or clearance, it may not be for the uses we believe are important or commercially attractive, in which case we would not market our product for those uses.
Sales of our diagnostic products outside the United States are subject to foreign regulatory requirements governing
-34-
clinical studies, vigilance reporting, marketing approval, manufacturing, regulatory inspections, product licensing, pricing and reimbursement. These regulatory requirements vary greatly from country to country. As a result, the time required to obtain approvals outside the United States may differ from that required to obtain FDA approval or clearance, and we may not be able to obtain foreign regulatory approvals on a timely basis or at all. Approval or clearance by the FDA does not ensure approval by regulatory authorities in other countries, and approval by one foreign regulatory authority does not ensure approval or clearance by regulatory authorities in other countries or by the FDA, and foreign regulatory authorities could require additional testing beyond what the FDA requires. In addition, FDA regulates exports of medical devices. Failure to comply with these regulatory requirements or to obtain required approvals or clearances could impair our ability to commercialize our diagnostic products outside of the United States.
We expect to rely on third parties in conducting any future studies of our diagnostic products that may be required by the FDA or other regulatory authorities, and to fulfill product registration requirements in foreign countries, and those third parties may not perform satisfactorily.
We do not have the ability to independently conduct the clinical studies or other studies that may be required to obtain FDA and other regulatory clearance or approval for our diagnostic products, including additional indications for Prosigna. Accordingly, we expect to rely on third parties, such as medical institutions, clinical investigators, consultants, and our pharmaceutical collaborators to conduct such studies. For example, we contract with clinical laboratories to perform the companion diagnostic tests we are developing that are used in the clinical trials run by pharmaceutical companies pursuant to our companion diagnostic collaborations. Our reliance on these third parties for clinical development activities will reduce our control over these activities. These third-party contractors may not complete activities on schedule or conduct studies in accordance with regulatory requirements or the study design. Our reliance on third parties that we do not control will not relieve us of any applicable requirement to ensure compliance with various procedures required under good clinical practices and regulatory requirements. If these third parties do not successfully carry out their contractual duties or regulatory obligations or meet expected deadlines, if the third parties need to be replaced or if the quality or accuracy of the data they obtain is compromised due to their failure to adhere to our clinical protocols or regulatory requirements or for other reasons, the studies may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory approval for our diagnostic products. In addition, under our contracts with our pharmaceutical collaborators, we potentially could be held liable for the failure of our third party subcontractors to perform their contractual obligations.
In many countries, we are not permitted to directly apply for product registrations, and therefore must rely on third-party contractors or product distributors resident in those countries to fulfill the product registration requirements. Our reliance on these third parties reduces our control over the registration activities, and those parties may not appropriately register the products. Our reliance on third parties does not relieve us of the obligation to comply with applicable requirements, and therefore any failure on the part of the third parties could subject us to enforcement action in the country in which the registration was not properly fulfilled.
We are subject to ongoing and extensive regulatory requirements, and our failure to comply with these requirements could substantially harm our business.
Certain of our products are regulated as medical devices, including Prosigna and the nCounter Dx Analysis System. Accordingly, we and certain of our contract manufacturers are subject to ongoing International Organization for Standardization, or ISO, and FDA obligations and continued regulatory oversight and review. These include routine inspections by European Union, or EU, Notified Bodies and by the FDA of our manufacturing facilities and our records for compliance with requirements such as ISO 13485 and the QSR, which establish extensive requirements for quality assurance and control as well as manufacturing and change control procedures. We are also subject to other regulatory obligations, such as requirements pertaining to the registration of our manufacturing facilities and the listing of our devices with the FDA; continued adverse event and malfunction reporting; corrections and removals reporting; and labeling and promotional requirements. Other agencies may also issue guidelines and regulations that could impact the development of our products, including companion diagnostic tests. For example, the European Medicines Agency, a European Union agency which is responsible for the scientific evaluation of medicines used in the EU, recently launched an initiative to determine guidelines for the use of genomic biomarkers in the development and life-cycle of drugs. On May 25, 2017 the European Union adopted the IVD Directive Regulation, which increases the regulatory requirements applicable to some in vitro diagnostics in the EU and would require that we re-classify and obtain pre-approval for our existing CE-marked IVD products within a five-year grace period (by May 25, 2022).
We may also be subject to additional FDA or global regulatory authority post-marketing obligations or requirements by the FDA or global regulatory authority to change our current product classifications which would impose additional regulatory obligations on us. For example, following discussions with the FDA regarding the appropriate classification for our nCounter Elements TagSets as General Purpose Reagents, we submitted a de novo application to the FDA. The FDA requested additional information in support of our application. We subsequently withdrew the application, and plan to offer a custom code
-35-
set manufacturing service to customers developing assays. The promotional claims we can make for Prosigna are limited to the cleared (or equivalent) indication. If we are not able to maintain regulatory compliance, we may not be permitted to market our medical device products and/or may be subject to enforcement by EU Competent Authorities and the FDA and other global regulatory authority such as the issuance of warning or untitled letters, fines, injunctions, and civil penalties; recall or seizure of products; operating restrictions; and criminal prosecution. In addition, we may be subject to similar regulatory regimes of foreign jurisdictions as we continue to commercialize our products in new markets outside of the U.S. and Europe. Adverse Notified Body, EU Competent Authority or FDA or global regulatory authority action in any of these areas could significantly increase our expenses and limit our revenue and profitability.
We may be subject, directly or indirectly, to healthcare fraud and abuse laws and other laws applicable to our marketing practices. If we are unable to comply, or have not complied, with such laws, we could face substantial penalties.
Our operations are directly, or indirectly through our customers, subject to various fraud and abuse laws, including, without limitation, the federal and state anti-kickback statutes and state, federal and foreign marketing compliance laws and gift bans. These laws may impact, among other things, our proposed sales and marketing and education programs and require us to implement additional internal systems for tracking certain marketing expenditures and reporting them to government authorities. In addition, we may be subject to privacy regulations by both the federal government and the states in which we conduct our business as well as by foreign governments and entities. The laws that may affect our ability to operate include:
• | the federal Anti-kickback Law and state anti-kickback prohibitions; |
• | the federal physician self-referral prohibition, commonly known as the Stark Law, and the state equivalents; |
• | the federal Health Insurance Portability and Accountability Act of 1996, as amended; |
• | the Medicare civil money penalty and exclusion requirements; |
• | the federal False Claims Act civil and criminal penalties and state equivalents; |
• | state physician gift bans and state, federal and foreign marketing expenditure disclosure laws; and |
• | the Foreign Corrupt Practices Act, which applies to our international activities; |
• | the European Union's General Data Protection Regulation. |
If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
Healthcare policy changes, including legislation reforming the United States healthcare system, may have a material adverse effect on our financial condition and results of operations.
The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, collectively, the ACA, enacted in March 2010, made changes that significantly impact the pharmaceutical and medical device industries and clinical laboratories. For example, beginning in 2013, each medical device manufacturer must pay a sales tax in an amount equal to 2.3% of the price for which such manufacturer sells its medical devices. In December 2015, Congress passed a two-year suspension of the medical device tax from January 1, 2016 to December 31, 2017. In January 2018, Congress suspended the tax again for a two-year period. The tax applies to our listed medical device products, which include the nCounter Dx Analysis System and Prosigna. The Budget Control Act of 2011, contained automatic spending cuts to the federal budget known as sequestration. As a result of sequestration, Medicare payments are reduced by 2% per year. For Prosigna, pricing changes can occur through the biannual adjustment to the CLFS; this resulted in a 10% reduction in the Medicare reimbursement price for Prosigna starting on January 1, 2018. These or any future proposed or mandated reductions in payments may apply to some or all of the clinical laboratory tests that our customers use our technology to deliver to Medicare beneficiaries, and may indirectly reduce demand for our products.
Other significant measures contained in the ACA include coordination and promotion of research on comparative clinical effectiveness of different technologies and procedures, initiatives to revise Medicare payment methodologies, such as bundling of payments across the continuum of care by providers and physicians, and initiatives to promote quality indicators in payment methodologies. The ACA also includes significant new fraud and abuse measures, including required disclosures of financial arrangements with physician customers, lower thresholds for violations and increasing potential penalties for such violations. In addition, the ACA establishes an Independent Payment Advisory Board, or IPAB, to reduce the per capita rate of growth in Medicare spending. The IPAB has broad discretion to propose policies to reduce healthcare expenditures, which may have a negative impact on payment rates for services, including our tests. Although created in 2010, to date no members have been appointed to IPAB, and it remains non-operational.
In addition to the ACA, the effect of which cannot presently be quantified, various healthcare reform proposals have
-36-
also emerged from federal and state governments. Changes in healthcare policy, such as the creation of broad test utilization limits for diagnostic products in general or requirements that Medicare patients pay for portions of clinical laboratory tests or services received, could substantially impact the sales of our tests, increase costs and divert management’s attention from our business. In addition, sales of our tests outside of the United States will subject us to foreign regulatory requirements, which may also change over time.
We cannot predict whether future healthcare initiatives, including potential repeal of the ACA in whole or in part by Congress following the election of President Trump, will be implemented at the federal or state level or in countries outside of the United States in which we may do business, or the effect any future legislation or regulation will have on us. Changes in the United States healthcare industry may result in decreased profits to us, lower reimbursements by payors for our products or reduced medical procedure volumes, all of which may adversely affect our business, financial condition and results of operations.
Risks Related to Intellectual Property
If we are unable to protect our intellectual property effectively, our business would be harmed.
We rely on patent protection as well as trademark, copyright, trade secret and other intellectual property rights protection and contractual restrictions to protect our proprietary technologies, all of which provide limited protection and may not adequately protect our rights or permit us to gain or keep any competitive advantage. As of December 31, 2017, we owned or licensed 20 issued U.S. patents and approximately 40 pending U.S. patent applications, including provisional and non-provisional filings. We also owned or licensed approximately 255 pending and granted counterpart applications worldwide, including 111 country-specific validations of 12 European patents. We continue to file new patent applications to protect the full range of our technologies. If we fail to protect our intellectual property, third parties may be able to compete more effectively against us and we may incur substantial litigation costs in our attempts to recover or restrict use of our intellectual property.
Our success depends in part on obtaining patent protection for our products and processes, preserving trade secrets, patents, copyrights and trademarks, operating without infringing the proprietary rights of third parties, and acquiring licenses for technology or products. We cannot assure investors that any of our currently pending or future patent applications will result in issued patents, and we cannot predict how long it will take for such patents to be issued. As the patent and prior art landscape for translational research and molecular diagnostic life science products grows more crowded and becomes more complex we may find it more difficult to obtain patent protection for our products including those related to digital spatial profiling and sequencing, for example. Our existing patents and any future patents we obtain may not be sufficiently broad to prevent others from practicing our technologies or from developing competing products and may therefore fail to provide us with any competitive advantage. Additionally, we cannot assure investors that our currently pending or future patent applications have or will be filed in all of our potential markets. Further, we cannot assure investors that other parties will not challenge any patents issued to us or that courts or regulatory agencies will hold our patents to be valid or enforceable. We cannot guarantee investors that we will be successful in defending challenges made against our patents and patent applications. Any successful third-party challenge to our patents could result in the third party or the unenforceability or invalidity of such patents and could deprive us of the ability to prevent others from using the technologies claimed in such issued patents.
The patent positions of life sciences companies can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. No consistent policy regarding the breadth of claims allowed in such companies’ patents has emerged to date in the United States. Furthermore, in the biotechnology field, courts frequently render opinions that may affect the patentability of certain inventions or discoveries, including opinions that may affect the patentability of methods for analyzing or comparing DNA.
In particular, the patent positions of companies engaged in development and commercialization of genomic diagnostic tests, like Prosigna, are particularly uncertain. Various courts, including the U.S. Supreme Court, have rendered decisions that impact the scope of patentability of certain inventions or discoveries relating to genomic diagnostics. Specifically, these decisions stand for the proposition that patent claims that recite laws of nature (for example, the relationships between gene expression levels and the likelihood of risk of recurrence of cancer) are not themselves patentable unless those patent claims have sufficient additional features that provide practical assurance that the processes are genuine inventive applications of those laws rather than patent drafting efforts designed to monopolize the law of nature itself. What constitutes a “sufficient” additional feature is uncertain. Furthermore, in view of these decisions, in December 2014 the U.S. Patent and Trademark Office, or USPTO, published revised guidelines for patent examiners to apply when examining process claims for patent eligibility. This guidance was updated by the USPTO in July 2015 and additional illustrative examples provided in May 2016. The guidance indicates that claims directed to a law of nature, a natural phenomenon, or an abstract idea that do not meet the eligibility requirements should be rejected as non‑statutory, patent ineligible subject matter. We cannot assure you that our patent portfolio will not be negatively impacted by the current uncertain state of the law, new court rulings or changes in
-37-
guidance or procedures issued by the USPTO. From time to time, the U.S. Supreme Court, other federal courts, the U.S. Congress or the USPTO may change the standards of patentability and validity of patents within the genomic diagnostic space, and any such changes could have a negative impact on our business.
The laws of some non-U.S. countries do not protect intellectual property rights to the same extent as the laws of the United States, and many companies have encountered significant problems in protecting and defending such rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to biotechnology, which could make it difficult for us to stop the infringement of our patents. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business.
Changes in either the patent laws or in interpretations of patent laws in the United States or other countries may diminish the value of our intellectual property. We cannot predict the breadth of claims that may be allowed or enforced in our patents or in third-party patents. For example:
• | We might not have been the first to make the inventions covered by each of our pending patent applications. |
• | We might not have been the first to file patent applications for these inventions. |
• | Others may independently develop similar or alternative products and technologies or duplicate any of our products and technologies. |
• | It is possible that our pending patent applications will not result in issued patents, and even if they issue as patents, they may not provide a basis for commercially viable products, may not provide us with any competitive advantages, or may be challenged and invalidated by third parties. |
• | We may not develop additional proprietary products and technologies that are patentable. |
• | The patents of others may have an adverse effect on our business. |
• | We apply for patents covering our products and technologies and uses thereof, as we deem appropriate. However, we may fail to apply for patents on important products and technologies in a timely fashion or at all. |
In addition to pursuing patents on our technology, we take steps to protect our intellectual property and proprietary technology by entering into confidentiality agreements and intellectual property assignment agreements with our employees, consultants, corporate partners and, when needed, our advisors. Such agreements may not be enforceable or may not provide meaningful protection for our trade secrets or other proprietary information in the event of unauthorized use or disclosure or other breaches of the agreements, and we may not be able to prevent such unauthorized disclosure. Monitoring unauthorized disclosure is difficult, and we do not know whether the steps we have taken to prevent such disclosure are, or will be, adequate. If we were to enforce a claim that a third party had illegally obtained and was using our trade secrets, it would be expensive and time consuming, and the outcome would be unpredictable. In addition, courts outside the United States may be less willing to protect trade secrets.
In addition, competitors could purchase our products and attempt to replicate some or all of the competitive advantages we derive from our development efforts, willfully infringe our intellectual property rights, design around our protected technology or develop their own competitive technologies that fall outside of our intellectual property rights. In addition, competitors may develop their own versions of our tests in countries where we did not apply for patents, where our patents have not issued or where our intellectual property rights are not recognized and compete with us in those countries and markets. If our intellectual property is not adequately protected so as to protect our market against competitors’ products and methods, our competitive position could be adversely affected, as could our business.
We have not yet registered certain of our trademarks in all of our potential markets. If we apply to register these trademarks, our applications may not be allowed for registration, and our registered trademarks may not be maintained or enforced. In addition, opposition or cancellation proceedings may be filed against our trademark applications and registrations, and our trademarks may not survive such proceedings. If we do not secure registrations for our trademarks, we may encounter more difficulty in enforcing them against third parties than we otherwise would.
To the extent our intellectual property, including licensed intellectual property, offers inadequate protection, or is found to be invalid or unenforceable, we would be exposed to a greater risk of direct competition. If our intellectual property does not provide adequate protection against our competitors’ products, our competitive position could be adversely affected, as could our business. Both the patent application process and the process of managing patent disputes can be time consuming and expensive.
-38-
We depend on certain technologies that are licensed to us. We do not control these technologies and any loss of our rights to them could prevent us from selling our products.
We rely on licenses in order to be able to use various proprietary technologies that are material to our business, including our core digital molecular barcoding technology licensed from the Institute for Systems Biology, technology relating to Prosigna licensed from Bioclassifier, LLC, intellectual property relating to a gene signature of tumor inflammation from Merck and the intellectual property relating to a gene signature for lymphoma subtyping from the National Institutes of Health for use in our collaboration with Celgene Corporation. We do not own the patents that underlie these licenses. Our rights to use these technologies and employ the inventions claimed in the licensed patents are subject to the continuation of and compliance with the terms of those licenses.
We may need to license other technologies to commercialize future products. We may also need to negotiate licenses to patents and patent applications after launching any of our commercial products. Our business may suffer if the patents or patent applications are unavailable for license or if we are unable to enter into necessary licenses on acceptable terms.
In some cases, we do not control the prosecution, maintenance, or filing of the patents to which we hold licenses, or the enforcement of these patents against third parties. Some of our patents and patent applications were either acquired from another company who acquired those patents and patent applications from yet another company, or are licensed from a third party. Thus, these patents and patent applications are not written by us or our attorneys, and we did not have control over the drafting and prosecution. The former patent owners and our licensors might not have given the same attention to the drafting and prosecution of these patents and applications as we would have if we had been the owners of the patents and applications and had control over the drafting and prosecution. We cannot be certain that drafting or prosecution of the licensed patents and patent applications by the licensors have been or will be conducted in compliance with applicable laws and regulations or will result in valid and enforceable patents and other intellectual property rights.
Enforcement of our licensed patents or defense of any claims asserting the invalidity of these patents is often subject to the control or cooperation of our licensors. Certain of our licenses contain provisions that allow the licensor to terminate the license upon specific conditions. Therefore, our business may suffer if these licenses terminate, if the licensors fail to abide by the terms of the license or fail to prevent infringement by third parties or if the licensed patents or other rights are found to be invalid. Our rights under the licenses are subject to our continued compliance with the terms of the license, including the payment of royalties due under the license. Because of the complexity of our products and the patents we have licensed, determining the scope of the license and related royalty obligation can be difficult and can lead to disputes between us and the licensor. An unfavorable resolution of such a dispute could lead to an increase in the royalties payable pursuant to the license or termination of the license. If a licensor believed we were not paying the royalties due under the license or were otherwise not in compliance with the terms of the license, the licensor might attempt to revoke the license. If such an attempt were successful, we might be barred from producing and selling some or all of our products.
In addition, certain of the patents we have licensed relate to technology that was developed with U.S. government grants. Federal regulations impose certain domestic manufacturing requirements with respect to some of our products embodying these patents.
We may be involved in lawsuits to protect or enforce our patents and proprietary rights, to determine the scope, coverage and validity of others’ proprietary rights, or to defend against third-party claims of intellectual property infringement, any of which could be time-intensive and costly and may adversely impact our business or stock price.
We have received notices of claims of infringement and misappropriation or misuse of other parties’ proprietary rights in the past and may from time to time receive additional notices. Some of these claims may lead to litigation. We cannot assure investors that we will prevail in such actions, or that other actions alleging misappropriation or misuse by us of third-party trade secrets, infringement by us of third-party patents and trademarks or other rights, or the validity of our patents, trademarks or other rights, will not be asserted or prosecuted against us.
Litigation may be necessary for us to protect or enforce our patent and proprietary rights, defend against third-party claims or to determine the scope, coverage and validity of the proprietary rights of others. Litigation could result in substantial legal fees and could adversely affect the scope of our patent protection and reduce our ability to compete in the marketplace. The outcome of any litigation or other proceeding is inherently uncertain and might not be favorable to us. If we resort to legal proceedings to enforce our intellectual property rights or to determine the validity, scope and coverage of the intellectual property or other proprietary rights of others, the proceedings could be burdensome and expensive, even if we were to prevail. Any litigation that may be necessary in the future could result in substantial costs and diversion of resources and could have a material adverse effect on our business, operating results or financial condition.
Numerous significant intellectual property issues have been litigated, and will likely continue to be litigated, between existing and new participants in our existing and targeted markets. Our success depends in part on our non-infringement of the patents or proprietary rights of third parties. We develop complex products that integrate a wide range of technologies which
-39-
may impact our ability to do so clear of third party rights and therefore may need to license other technologies or challenge the scope, coverage and validity of the proprietary rights of others to commercialize future products. As we develop new technologies such as those related to genomic diagnostic tests, digital spatial profiling and sequencing, for example, and move into new markets and applications for our products, we expect incumbent participants in such markets may assert their patents and other proprietary rights against us as part of a business strategy to slow our entry into such markets, impede our successful competition and/or extract substantial license and royalty payments from us. In addition, we may be unaware of pending third-party patent applications that relate to our technology and our competitors and others may have patents or may in the future obtain patents and claim that use of our products infringes these patents. Our competitors and others may now, and in the future, have significantly larger and more mature patent portfolios than we currently have. In addition, future litigation may involve patent holding companies or other adverse patent owners who have no relevant product revenue and against whom our own patents may provide little or no deterrence or protection. We could incur substantial costs and divert the attention of our management and technical personnel in defending against any of these claims. Any adverse ruling or perception of an adverse ruling in defending ourselves against these claims could have an adverse impact on our stock price, which may be disproportionate to the actual impact of the ruling itself. Parties making claims against us may be able to obtain injunctive or other relief, which could block our ability to develop, commercialize and sell products, and could result in the award of substantial damages against us. In the event of a successful claim of infringement against us, we may be required to pay damages and obtain one or more licenses from third parties, or be prohibited from selling certain products. We may not be able to obtain these licenses at a reasonable cost, if at all. We could therefore incur substantial costs related to royalty payments for licenses obtained from third parties, which could negatively affect our gross margins. In addition, we could encounter delays in product introductions while we attempt to develop alternative methods or products to avoid infringing third-party patents or proprietary rights. Defense of any lawsuit or failure to obtain any of these licenses on favorable terms could prevent us from commercializing products, and the prohibition of sale of any of our products could materially affect our ability to grow and gain market acceptance for our products.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, during the course of this kind of litigation, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock.
In addition, our agreements with some of our suppliers, distributors, customers, collaborators and other entities with whom we do business require us to defend or indemnify these parties to the extent they become involved in infringement claims against us, including the claims described above. We could also voluntarily agree to defend or indemnify third parties in instances where we are not obligated to do so if we determine it would be important to our business relationships. If we are required or agree to defend or indemnify any of these third parties in connection with any infringement claims, we could incur significant costs and expenses that could adversely affect our business, operating results, or financial condition.
We may be subject to damages resulting from claims that we or our employees have wrongfully used or disclosed alleged trade secrets of our employees’ former employers.
Many of our employees were previously employed at universities or other life sciences companies, including our competitors or potential competitors. Although no claims against us are currently pending, we or our employees may be subject to claims that these employees or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. If we fail in defending such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights. A loss of key research personnel work product could hamper or prevent our ability to commercialize certain potential products, which could severely harm our business. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
Our products contain third-party open source software components, and failure to comply with the terms of the underlying open source software licenses could restrict our ability to sell our products.
Our products contain software tools licensed by third-party authors under “open source” licenses. Use and distribution of open source software may entail greater risks than use of third-party commercial software, as open source licensors generally do not provide warranties or other contractual protections regarding infringement claims or the quality of the code. Some open source licenses contain requirements that we make available source code for modifications or derivative works we create based upon the type of open source software we use. If we combine our proprietary software with open source software in a certain manner, we could, under certain open source licenses, be required to release the source code of our proprietary software to the public. This would allow our competitors to create similar products with less development effort and time and ultimately could result in a loss of product sales.
-40-
Although we monitor our use of open source software to avoid subjecting our products to conditions we do not intend, the terms of many open source licenses have not been interpreted by U.S. courts, and there is a risk that these licenses could be construed in a way that could impose unanticipated conditions or restrictions on our ability to commercialize our products. Moreover, we cannot assure investors that our processes for controlling our use of open source software in our products will be effective. If we are held to have breached the terms of an open source software license, we could be required to seek licenses from third parties to continue offering our products on terms that are not economically feasible, to re-engineer our products, to discontinue the sale of our products if re-engineering could not be accomplished on a timely basis, or to make generally available, in source code form, our proprietary code, any of which could adversely affect our business, operating results, and financial condition.
We use third-party software that may be difficult to replace or cause errors or failures of our products that could lead to lost customers or harm to our reputation.
We use software licensed from third parties in our products. In the future, this software may not be available to us on commercially reasonable terms, or at all. Any loss of the right to use any of this software could result in delays in the production of our products until equivalent technology is either developed by us, or, if available, is identified, obtained and integrated, which could harm our business. In addition, any errors or defects in third-party software, or other third-party software failures could result in errors, defects or cause our products to fail, which could harm our business and be costly to correct. Many of these providers attempt to impose limitations on their liability for such errors, defects or failures, and if enforceable, we may have additional liability to our customers or third-party providers that could harm our reputation and increase our operating costs.
We will need to maintain our relationships with third-party software providers and to obtain software from such providers that does not contain any errors or defects. Any failure to do so could adversely impact our ability to deliver reliable products to our customers and could harm our results of operations.
Risks Related to Our Common Stock
The price of our common stock may be volatile, and you could lose all or part of your investment.
The trading price of our common stock has fluctuated and may continue to fluctuate substantially. The trading price of our common stock depends on a number of factors, including those described in this “Risk Factors” section, many of which are beyond our control and may not be related to our operating performance. These fluctuations could cause stockholders to lose all or part of their investment in our common stock. Factors that could cause fluctuations in the trading price of our common stock include the following:
• | actual or anticipated quarterly variation in our results of operations or the results of our competitors; |
• | announcements by us or our competitors of new products, significant contracts, commercial relationships or capital commitments; |
• | failure to obtain or delays in obtaining product approvals or clearances from the FDA or foreign regulators; |
• | adverse regulatory or reimbursement announcements; |
• | issuance of new or changed securities analysts’ reports or recommendations for our stock; |
• | developments or disputes concerning our intellectual property or other proprietary rights; |
• | commencement of, or our involvement in, litigation; |
• | market conditions in the research and diagnostics markets; |
• | manufacturing disruptions; |
• | any future sales of our common stock or other securities; |
• | any change to the composition of the board of directors or key personnel; |
• | announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures or capital commitments; |
• | general economic conditions and slow or negative growth of our markets; and |
• | the other factors described in this “Risk Factors” section. |
The stock market in general, and market prices for the securities of life sciences and diagnostic companies like ours in particular, have from time to time experienced volatility that often has been unrelated to the operating performance of the underlying companies. These broad market and industry fluctuations may adversely affect the market price of our common stock, regardless of our operating performance. In several recent situations where the market price of a stock has been volatile, holders of that stock have instituted securities class action litigation against the company that issued the stock. If any of our
-41-
stockholders were to bring a lawsuit against us, the defense and disposition of the lawsuit could be costly and divert the time and attention of our management and harm our operating results.
An active trading market for our common stock may not be sustained.
Although our common stock is listed on The NASDAQ Global Market, the market for our shares has demonstrated varying levels of trading activity and the current level of trading may not be sustained in the future. Purchases or sales of large blocks of our shares relative to the trading volume on a given day can have a disproportionate effect on the price of our common stock. The lack of an active market for our common stock or significant and rapid changes in the price of our common stock may impair investors’ ability to sell their shares at the time they wish to sell them or at a price that they consider reasonable, may reduce the fair market value of their shares and may impair our ability to raise capital.
If securities or industry analysts do not publish research reports about our business, or if they issue an adverse opinion about our business, our stock price and trading volume could decline.
The trading market for our common stock will be influenced by the research and reports that industry or securities analysts publish about us or our business. If one or more of the analysts who cover us issues an adverse opinion about our company, our stock price would likely decline. If one or more of these analysts ceases coverage of us or fails to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
Future sales of our common stock in the public market could cause our stock price to fall.
Our stock price could decline as a result of sales of a large number of shares of our common stock or the perception that these sales could occur. These sales, or the possibility that these sales may occur, also might make it more difficult for us to sell equity securities in the future at a time and at a price that we deem appropriate.
Holders of approximately 3.4 million shares (including shares underlying outstanding warrants), or approximately 13%, of our outstanding shares as of December 31, 2017, have rights, subject to some conditions, to require us to file registration statements covering the sale of their shares or to include their shares in registration statements that we may file for ourselves or other stockholders. We also register the offer and sale of all shares of common stock that we may issue under our equity compensation plans.
In addition, in the future, we may issue additional shares of common stock or other equity or debt securities convertible into common stock in connection with a financing, acquisition, litigation settlement, employee arrangements or otherwise. Any such future issuance, including any issuances pursuant to our “at the market” equity offering program under our sales agreement with Cowen, could result in substantial dilution to our existing stockholders and could cause our stock price to decline.
We will have broad discretion over the use of the proceeds to us from our “at the market” equity offering program and may apply the proceeds to uses that do not improve our operating results or the value of your securities.
We will have broad discretion to use the net proceeds to us from our “at the market” equity offering program put into place in January 2018, and investors will be relying solely on the judgment of our board of directors and management regarding the application of these proceeds. Although we expect to use the net proceeds from our “at the market” equity offering program for general corporate purposes, we have not allocated these net proceeds for specific purposes. Investors will not have the opportunity, as part of their investment decision, to assess whether the proceeds are being used appropriately. Our use of the proceeds may not improve our operating results or increase the value of the securities offered pursuant to the “at the market” equity offering program.
Our officers and directors, and their respective affiliates, own a significant percentage of our stock and will be able to exercise significant influence over matters subject to stockholder approval.
Our executive officers and directors together with their respective affiliates, own approximately 17% of our outstanding common stock as of December 31, 2017. Accordingly, our executive officers and directors together with their respective affiliates, will be able to exert significant influence over matters submitted to our stockholders for approval, as well as our management and affairs. This concentration of ownership could have the effect of delaying or preventing a change in our control or otherwise discouraging a potential acquirer from attempting to obtain control of us, which in turn could have a material adverse effect on our stock price and may prevent attempts by our stockholders to replace or remove the board of directors or management.
-42-
Anti-takeover provisions in our charter documents and under Delaware or Washington law could make an acquisition of us difficult, limit attempts by our stockholders to replace or remove our current management and limit our stock price.
Provisions of our certificate of incorporation and bylaws may delay or discourage transactions involving an actual or potential change in our control or change in our management, including transactions in which stockholders might otherwise receive a premium for their shares, or transactions that our stockholders might otherwise deem to be in their best interests. Therefore, these provisions could adversely affect the price of our stock. Among other things, the certificate of incorporation and bylaws:
• | permit the board of directors to issue up to 15,000,000 shares of preferred stock, with any rights, preferences and privileges as they may designate; |
• | provide that the authorized number of directors may be changed only by resolution of the board of directors; |
• | provide that all vacancies, including newly-created directorships, may, except as otherwise required by law, be filled by the affirmative vote of a majority of directors then in office, even if less than a quorum; |
• | divide the board of directors into three classes; |
• | provide that a director may only be removed from the board of directors by the stockholders for cause; |
• | require that any action to be taken by our stockholders must be effected at a duly called annual or special meeting of stockholders and may not be taken by written consent; |
• | provide that stockholders seeking to present proposals before a meeting of stockholders or to nominate candidates for election as directors at a meeting of stockholders must provide notice in writing in a timely manner, and meet specific requirements as to the form and content of a stockholder’s notice; |
• | prevent cumulative voting rights (therefore allowing the holders of a plurality of the shares of common stock entitled to vote in any election of directors to elect all of the directors standing for election, if they should so choose); |
• | provide that special meetings of our stockholders may be called only by the chairman of the board, our chief executive officer or by the board of directors; and |
• | provide that stockholders are permitted to amend the bylaws only upon receiving at least two-thirds of the total votes entitled to be cast by holders of all outstanding shares then entitled to vote generally in the election of directors, voting together as a single class. |
In addition, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which generally prohibits a Delaware corporation from engaging in any of a broad range of business combinations with any “interested” stockholder for a period of three years following the date on which the stockholder became an “interested” stockholder. Likewise, because our principal executive offices are located in Washington, the anti-takeover provisions of the Washington Business Corporation Act may apply to us under certain circumstances now or in the future. These provisions prohibit a “target corporation” from engaging in any of a broad range of business combinations with any stockholder constituting an “acquiring person” for a period of five years following the date on which the stockholder became an “acquiring person.”
We are an “emerging growth company,” and any decision on our part to comply only with certain reduced reporting and disclosure requirements applicable to emerging growth companies could make our common stock less attractive to investors.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act, or the JOBS Act, enacted in April 2012. We will cease to be an “emerging growth company” on December 31, 2018. As an “emerging growth company,” we have chosen to take advantage of exemptions from various reporting requirements applicable to other public companies but not to “emerging growth companies,” including, but not limited to, not being required to have our independent registered public accounting firm audit our internal control over financial reporting under Section 404, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. If some investors find our common stock less attractive as a result of these exemptions, there may be a less active trading market for our common stock and our stock price may be lower and be more volatile. As an “emerging growth company” the JOBS Act allows us to delay adoption of new or revised accounting pronouncements applicable to public companies until such pronouncements are made applicable to private companies. We have elected to use this extended transition period under the JOBS Act. As a result, our financial statements may not be comparable to the financial statements of issuers who are required to comply with the effective dates for new or revised accounting standards that are applicable to public companies, which may make our common stock less attractive to investors.
-43-
Complying with the laws and regulations affecting public companies increases our costs and the demands on management and could harm our operating results.
As a public company, and particularly after we cease to be an “emerging growth company,” we incur and will continue to incur significant legal, accounting and other expenses that we did not incur as a private company. In addition, the Sarbanes-Oxley Act and rules subsequently implemented by the SEC and The NASDAQ Global Market impose numerous requirements on public companies, including requiring changes in corporate governance practices. Also, the Exchange Act requires, among other things, that we file annual, quarterly and current reports with respect to our business and operating results. Our management and other personnel must devote a substantial amount of time to compliance with these laws and regulations. These burdens may increase as new legislation is passed and implemented, including any new requirements that the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010 may impose on public companies. These requirements have increased and will likely continue to increase our legal, accounting, and financial compliance costs and have made and will continue to make some activities more time consuming and costly. For example, as a public company it is more difficult and more expensive for us to obtain director and officer liability insurance, and in the future we may be required to accept reduced policy limits and coverage or to incur substantial costs to maintain the same or similar coverage. These rules and regulations could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors or our board committees or as executive officers.
The Sarbanes-Oxley Act requires, among other things, that we assess the effectiveness of our internal control over financial reporting annually and the effectiveness of our disclosure controls and procedures quarterly. In particular, Section 404 of the Sarbanes-Oxley Act, or Section 404, requires us to perform system and process evaluation and testing of our internal control over financial reporting to allow management to report on, and our independent registered public accounting firm potentially to attest to, the effectiveness of our internal control over financial reporting. As an “emerging growth company,” we avail ourselves of the exemption from the requirement that our independent registered public accounting firm attest to the effectiveness of our internal control over financial reporting under Section 404. However, we may no longer avail ourselves of this exemption when we cease to be an “emerging growth company” on December 31, 2018. When our independent registered public accounting firm is required to undertake an assessment of our internal control over financial reporting, the cost of our compliance with Section 404 will correspondingly increase. Our compliance with applicable provisions of Section 404 will require that we incur substantial accounting expense and expend significant management time on compliance-related issues as we implement additional corporate governance practices and comply with reporting requirements. Moreover, if we are not able to comply with the requirements of Section 404 applicable to us in a timely manner, or if we or our independent registered public accounting firm identifies deficiencies in our internal control over financial reporting that are deemed to be material weaknesses, the market price of our stock could decline and we could be subject to sanctions or investigations by the SEC or other regulatory authorities, which would require additional financial and management resources.
Furthermore, investor perceptions of our company may suffer if deficiencies are found, and this could cause a decline in the market price of our stock. Irrespective of compliance with Section 404, any failure of our internal control over financial reporting could have a material adverse effect on our stated operating results and harm our reputation. If we are unable to implement these requirements effectively or efficiently, it could harm our operations, financial reporting, or financial results and could result in an adverse opinion on our internal control over financial reporting from our independent registered public accounting firm.
Item 1B. Unresolved Staff Comments
None.
Item 2. Properties
We currently have three long-term operating lease agreements for 104,538 square feet of space used for general office, laboratory, manufacturing, operations, and research and development purposes in Seattle, Washington. The long-term operating leases expire in 2026 and include options to renew at the then fair market rental for each of the facilities. The lease agreements contain rent abatement periods, scheduled rent increases and provide for tenant improvement allowances. In addition, we have two office leases outside of Seattle, Washington, totaling 2,202 square footage, with terms of two years or less.
Our landlords hold security deposits of approximately $317,000. We believe that our existing facilities are adequate to meet our business requirements for the near-term and that additional space will be available on commercially reasonable terms, if required.
Item 3. Legal Proceedings
-44-
We are not engaged in any material legal proceedings. From time to time, we may become involved in litigation relating to claims arising from the ordinary course of business. We believe that there are no claims or actions pending against us currently, the ultimate disposition of which would have a material adverse effect on our consolidated results of operation, financial condition or cash flows.
Item 4. Mine Safety Disclosures
Not applicable.
-45-
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information
Our common stock is traded on The NASDAQ Global Market under the symbol “NSTG.” Trading of our common stock commenced on June 26, 2013 in connection with our initial public offering. The following table sets forth, for the periods indicated, the high and low sales prices for our common stock as reported on The NASDAQ Global Market.
Year ended December 31, 2017 | High | Low | |||||
First quarter | $ | 23.25 | $ | 16.50 | |||
Second quarter | $ | 20.70 | $ | 14.72 | |||
Third quarter | $ | 16.78 | $ | 13.17 | |||
Fourth quarter | $ | 16.60 | $ | 7.03 | |||
Year ended December 31, 2016 | |||||||
First quarter | $ | 17.66 | $ | 11.30 | |||
Second quarter | $ | 16.50 | $ | 11.89 | |||
Third quarter | $ | 20.28 | $ | 12.50 | |||
Fourth quarter | $ | 23.45 | $ | 18.19 | |||
Holders
As of February 28, 2018, there were approximately 25 holders of record of our common stock. The actual number of stockholders is greater than this number of record holders and includes stockholders who are beneficial owners but whose shares are held in street name by brokers and other nominees.
Dividends
We have never declared or paid any cash dividends on our common stock or any other securities. We anticipate that we will retain all available funds and any future earnings, if any, for use in the operation of our business and do not anticipate paying cash dividends in the foreseeable future. In addition, our term loan agreement materially restricts, and future debt instruments we issue may materially restrict, our ability to pay dividends on our common stock. Payment of future cash dividends, if any, will be at the discretion of the board of directors after taking into account various factors, including our financial condition, operating results, current and anticipated cash needs, the requirements of current or then-existing debt instruments and other factors the board of directors deems relevant.
-46-
Performance Graph
This performance graph shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or incorporated by reference into any filing of NanoString Technologies, Inc. under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in such filing.
The following graph compares the performance of our common stock for the periods indicated with the performance of the NASDAQ Composite Index and the NASDAQ Medical Equipment Index. This graph assumes an investment of $100 on June 26, 2013 in each of our common stock, the NASDAQ Composite Index and the NASDAQ Medical Equipment Index, and assumes reinvestment of dividends, if any. The stock price performance shown on the graph below is not necessarily indicative of future stock price performance.
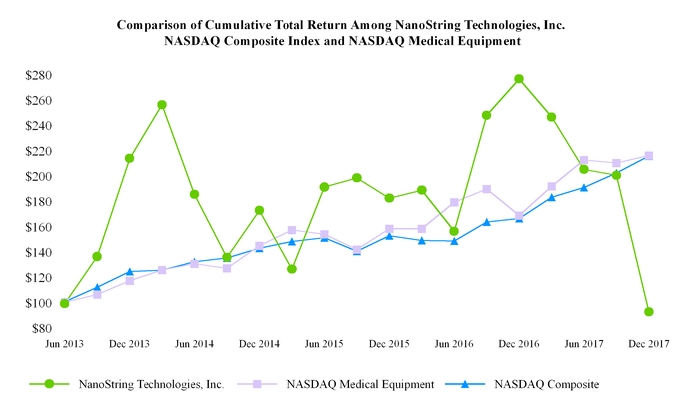
Recent Sales of Unregistered Securities
On July 21, 2017, we issued an aggregate of 28,534 shares of our common stock to a warrant holder upon the exercise of outstanding warrants to purchase an aggregate of 77,677 shares of our common stock pursuant to a net exercise mechanism under the warrants. Each warrant had an exercise price of either $8.448 or $14.397 per share. These issuances were exempt from registration under the Securities Act of 1933, as amended, pursuant to Section 3(a)(9) thereof as an exchange with an existing security holder where no commission or other remuneration is paid or given for soliciting such exchange.
On August 4, 2017, we issued Lam a warrant to purchase up to 1.0 million shares of our common stock, with the exact number of issuable shares equal to (1) 1.0 million shares multiplied by (2)(a) the amount of funding provided by Lam divided by (b) $50.0 million. The exercise price of the warrant is $16.75 per share. The issuance of the warrant was exempt from registration under the Securities Act of 1933, as amended, pursuant to Section 4(a)(2) thereof as a transaction by an issuer not involving a public offering. During 2017, Lam did not exercise any warrants.
-47-
Securities Authorized for Issuance under Equity Compensation Plans
The following table summarizes information about our equity compensation plans as of December 31, 2017. All outstanding awards relate to our common stock.
Plan Category | (a) Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights | (b) Weighted Average Exercise Price of Outstanding Options, Warrants and Rights | (c) Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column (a)) (1) | ||||||
Equity compensation plans approved by security holders: | |||||||||
2004 Stock Option Plan | 932,175 | $ | 3.34 | — | |||||
2013 Equity Incentive Plan | 4,925,785 | 13.42 | 180,497 | ||||||
2013 Employee Stock Purchase Plan | — | N.A. | 269,811 | ||||||
Equity compensation plans not approved by security holders(2): | — | N.A. | — | ||||||
Total | 5,857,960 | N.A. | 450,308 | ||||||
(1) Our 2013 Equity Incentive Plan includes provisions providing for an annual increase in the number of securities available for future issuance on the first day of each fiscal year, equal to the least of: (a) 1,406,250 shares; (b) 5% of the outstanding shares of common stock as of the last day of the immediately preceding fiscal year; and (c) such other amount as the board of directors may determine. Our 2013 Employee Stock Purchase Plan includes provisions providing for an annual increase in the number of securities available for future issuance on the first day of each fiscal year, equal to the least of: (a) 1% of the outstanding shares of common stock on the first day of such fiscal year; (b) 281,250 shares; and (c) such other amount as the board of directors, or a committee appointed by the board of directors, may determine.
(2) On January 15, 2018, our board of directors adopted the NanoString Technologies, Inc. 2018 Inducement Equity Incentive Plan, or the Inducement Plan, and, subject to the adjustment provisions of the Inducement Plan, reserved 250,000 shares of our common stock for issuance pursuant to equity awards granted under the Inducement Plan. The Inducement Plan was adopted without stockholder approval pursuant to Rule 5635(c)(4) and Rule 5635(c)(3) of the Nasdaq Listing Rules. The Inducement Plan provides for the grant of equity-based awards, including nonstatutory stock options, restricted stock units, restricted stock, stock appreciation rights, performance shares and performance units, and its terms are substantially similar to our 2013 Equity Incentive Plan, including with respect to treatment of equity awards in the event of a “merger” or “change in control” as defined under the Inducement Plan, but with such other terms and conditions intended to comply with the NASDAQ inducement award exception or to comply with the NASDAQ acquisition and merger exception. However, our 2013 Equity Incentive Plan permits certain exchange programs (including repricings) without stockholder approval, while the Inducement Plan requires stockholder approval for such exchange programs.
-48-
Item 6. Selected Financial Data
The following selected financial data is derived from our audited financial statements and should be read in conjunction with, and is qualified in its entirety by, Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and Item 8, “Financial Statements and Supplementary Data” contained elsewhere in this Annual Report on Form 10-K. The selected Consolidated Statements of Operations data for the years ended December 31, 2017, 2016 and 2015 and Consolidated Balance Sheet data as of December 31, 2017 and 2016 have been derived from our audited consolidated financial statements appearing elsewhere in this Annual Report on Form 10-K. The selected Consolidated Statements of Operations data for the years ended December 31, 2014 and 2013 and Consolidated Balance Sheet data as of December 31, 2015, 2014 and 2013 have been derived from our audited consolidated financial statements that are not included in this Annual Report on Form 10-K. Historical results are not necessarily indicative of future results.
Year Ended December 31, | |||||||||||||||||||
2017 | 2016 | 2015 | 2014 | 2013 | |||||||||||||||
(In thousands, except per share amounts) | |||||||||||||||||||
Consolidated Statements of Operations: | |||||||||||||||||||
Revenue | $ | 114,905 | $ | 86,489 | $ | 62,667 | $ | 47,593 | $ | 31,403 | |||||||||
Costs and expenses: | |||||||||||||||||||
Cost of product and service revenue | 31,880 | 30,245 | 26,126 | 21,149 | 15,009 | ||||||||||||||
Research and development | 46,888 | 34,720 | 24,597 | 21,404 | 14,979 | ||||||||||||||
Selling, general and administrative | 74,334 | 62,700 | 53,186 | 51,063 | 29,912 | ||||||||||||||
Total costs and expenses | 153,102 | 127,665 | 103,909 | 93,616 | 59,900 | ||||||||||||||
Loss from operations | (38,197 | ) | (41,176 | ) | (41,242 | ) | (46,023 | ) | (28,497 | ) | |||||||||
Other income (expense): | |||||||||||||||||||
Interest income | 809 | 390 | 233 | 272 | 68 | ||||||||||||||
Interest expense | (6,153 | ) | (5,672 | ) | (4,017 | ) | (4,140 | ) | (1,942 | ) | |||||||||
Other income (expense) | 183 | (515 | ) | (389 | ) | (147 | ) | (66 | ) | ||||||||||
Revaluation of preferred stock warrant liability | — | — | — | — | 1,156 | ||||||||||||||
Total other income (expense) | (5,161 | ) | (5,797 | ) | (4,173 | ) | (4,015 | ) | (784 | ) | |||||||||
Net loss before provision for income taxes | (43,358 | ) | (46,973 | ) | (45,415 | ) | (50,038 | ) | (29,281 | ) | |||||||||
Provision for income taxes | (204 | ) | (116 | ) | (166 | ) | — | — | |||||||||||
Net loss | $ | (43,562 | ) | $ | (47,089 | ) | $ | (45,581 | ) | $ | (50,038 | ) | $ | (29,281 | ) | ||||
Accretion of mandatorily redeemable convertible preferred stock | — | — | — | — | (4,653 | ) | |||||||||||||
Net loss attributable to common stockholders | $ | (43,562 | ) | $ | (47,089 | ) | $ | (45,581 | ) | $ | (50,038 | ) | $ | (33,934 | ) | ||||
Net loss per share—basic and diluted | $ | (1.84 | ) | $ | (2.34 | ) | $ | (2.40 | ) | $ | (2.80 | ) | $ | (4.44 | ) | ||||
Weighted-average shares used in computing basic and diluted net loss per share | 23,731 | 20,116 | 19,027 | 17,839 | 7,643 | ||||||||||||||
As of December 31, | |||||||||||||||||||
2017 | 2016 | 2015 | 2014 | 2013 | |||||||||||||||
Consolidated Balance Sheet Data: | |||||||||||||||||||
Cash, cash equivalents and short-term investments | $ | 77,555 | $ | 74,036 | $ | 49,044 | $ | 72,225 | $ | 42,656 | |||||||||
Working capital | 86,002 | 77,402 | 61,882 | 76,411 | 42,106(1) | ||||||||||||||
Total assets | 136,762 | 126,373 | 92,869 | 102,068 | 64,372(1) | ||||||||||||||
Total long-term debt and lease financing obligations, net of unamortized debt issue costs (includes current portion) | 48,931 | 47,424 | 41,226 | 30,246 | 18,293(1) | ||||||||||||||
Total stockholders’ equity | $ | 40,109 | $ | 12,305 | $ | 20,215 | $ | 44,813 | $ | 31,469 | |||||||||
(1)Amounts have not been retrospectively modified to reflect the adoption of Accounting Standard Update No. 2015-03. Interest-Imputation of Interest: Simplifying the Presentation of Debt Issuance Costs.
-49-
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
You should read the following discussion and analysis together with the financial statements and the related notes to those statements included elsewhere in this report. This discussion contains forward-looking statements that involve risks and uncertainties. As a result of many factors, such as those set forth in the section of this report captioned “Risk Factors” and elsewhere in this report, our actual results may differ materially from those anticipated in these forward-looking statements. Throughout this discussion, unless the context specifies or implies otherwise, the terms “NanoString”, “we”, “us” and “our” refer to NanoString Technologies, Inc. and its subsidiaries.
Overview
We develop, manufacture and sell robust, intuitive products that unlock scientifically valuable and clinically actionable biologic information from minute amounts of tissue. Our nCounter Analysis System directly profiles hundreds of molecules simultaneously using a novel barcoding technology that is powerful enough for use in research, yet simple enough for use in clinical laboratories worldwide.
We market our systems and related consumables to researchers in academic, government, and biopharmaceutical laboratories for use in understanding fundamental biology and the molecular basis of disease and to clinical laboratories and medical centers for diagnostic use. As of December 31, 2017, we had an installed base of approximately 605 nCounter systems, which our customers have used to publish more than 1,800 peer-reviewed papers. As researchers using our systems discover new biologic insights to improve clinical decision-making, these discoveries may be translated and validated as diagnostic tests. For example, our first molecular diagnostic product is the Prosigna Breast Cancer Assay, which provides an assessment of a patient’s risk of recurrence for breast cancer. In addition, we collaborate with biopharmaceutical companies to develop companion diagnostics, that may be used to identify which patients are most likely to respond to a particular drug therapy.
We derive a substantial majority of our revenue from the sale of our products to life science researchers, which consist of our nCounter instruments and related proprietary consumables, which we call CodeSets, nCounter-based reagents and Master Kits. After buying an nCounter Analysis System, research customers purchase consumables from us for use in their experiments. Our instruments are designed to work only with our consumable products. Accordingly, as the installed base of our instruments grows, we expect recurring revenue from consumable sales to be an important driver of our operating results. We also derive revenue from processing fees related to proof-of-principle studies we conduct for potential customers and service contracts for our nCounter Analysis Systems.
In 2013, we began offering instruments and consumables for use in diagnostic testing. In September 2013, we received 510(k) clearance from the FDA to market in the United States a version of Prosigna providing an assessment of a patient’s risk of recurrence for breast cancer. In November 2013, we began offering a version of the nCounter Analysis System to high-complexity, CLIA-certified laboratories for research and diagnostics purposes. This configuration of the nCounter Analysis System provides clinical laboratories a single platform with the flexibility to support both clinical testing, by running Prosigna, and research, by processing translational research experiments using our research consumables. The nCounter-based reagents provide further flexibility by allowing laboratories to develop their own Laboratory Developed Tests for gene expression, copy number variation and gene fusion signatures, which can be performed by a laboratory and may include genetic tests and other tests for rare conditions.
We use third-party contract manufacturers to produce the instruments comprising the nCounter Analysis System. We manufacture consumables at our Seattle, Washington facility. This operating model is designed to be capital efficient and to scale efficiently as our product volumes grow. We focus a substantial portion of our resources on developing new technologies, products and solutions. We invested $46.9 million, $34.7 million and $24.6 million in 2017, 2016 and 2015, respectively, in research and development and intend to continue to make significant investments in research and development.
In March 2014, we entered into a collaboration agreement with Celgene Corporation, or Celgene, pursuant to which we are working collaboratively with Celgene to develop, seek regulatory approval for, and commercialize a companion diagnostic assay for use in screening patients with Diffuse Large B-Cell Lymphoma. Under the terms of the collaboration agreement, we will develop, seek regulatory approval for, and commercialize the diagnostic test, and we retain the flexibility to independently develop and commercialize additional indications for the test. We are eligible to receive payments from Celgene totaling up to $27.3 million, of which $5.8 million was received as an upfront payment upon delivery of certain information to Celgene and $21.5 million is for development funding and potential success-based development and regulatory milestones. In February 2018, we entered into an amendment to the collaboration agreement in which Celgene agreed to provide us with additional funding for work intended to enable a subtype and prognostic indication for the test being developed under the agreement for Celgene’s drug REVLIMID. In addition, the amendment provides an additional milestone payment to us payable upon achievement of certain regulatory activities and timelines. In connection with this amendment, we agreed to remove the right to receive payments from Celgene in the event commercial sales of the companion diagnostic test do not exceed certain pre-specified minimum annual revenues during the first three years following regulatory approval. In addition, the amendment
-50-
allows Celgene, at its election, to use trial samples with additional technologies for companion diagnostics. For additional information regarding the development collaboration agreement, see the section of this report captioned “Business—Collaborations—Celgene Corporation” and Note 17 - Subsequent Events of the consolidated financial statements included in this report.
In May 2015, we entered into a clinical research collaboration agreement with Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., or Merck, to develop an assay intended to optimize immune-related gene expression signatures and evaluate the potential to predict benefit from Merck’s anti-PD-1 therapy, KEYTRUDA, in multiple tumor types. In February 2016, we expanded our collaboration with Merck by entering into a new development collaboration agreement to clinically develop and commercialize a novel diagnostic test, based on an optimized gene expression signature, to predict response to KEYTRUDA in multiple tumor types. In October 2017, we were notified by Merck of the decision not to pursue regulatory approval of the companion diagnostic test for KEYTRUDA. As a result, the scope of the collaboration was significantly reduced. For additional information regarding the development collaboration agreement, see the section of this report captioned “Business—Collaborations—Merck & Co., Inc.”.
In January 2016, we entered into a collaboration with Medivation, Inc. and Astellas Pharma Inc. to pursue the translation of a novel gene expression signature algorithm discovered by Medivation into a companion diagnostic assay using the nCounter Analysis System. In September 2016, Medivation was acquired by Pfizer, Inc., or Pfizer, and became a wholly owned subsidiary of Pfizer. In May 2017, we received notification from Pfizer and Astellas terminating the collaboration agreement as a result of a decision to discontinue the related clinical trial. For additional information regarding the development collaboration agreement, see the section of this report captioned “Business—Collaborations—Medivation, Inc. and Astellas Pharma, Inc.”.
In August 2017, we entered into a collaboration agreement with Lam Research Corporation, or Lam, to support the development of our Hyb & Seq product candidate and related assays. For additional information regarding the development collaboration agreement, see the section of this report captioned “Business—Collaborations—Lam Research Corporation”.
Our total revenue increased to $114.9 million in 2017 from $86.5 million in 2016 and $62.7 million in 2015. The increase was driven primarily by increased revenue of $25.5 million recorded from our various collaborations which are discussed in more detail in the above referenced section of this report. Absent the increase in collaboration revenue, our core product and service revenues increased moderately driven in part by growth in our Prosigna revenue, as well as increased revenue from consumables and service contracts associated with our growing install base of nCounter Analysis Systems. Historically, we have generated a substantial majority of our revenue from sales to customers in North America; however, as we have expanded our European direct sales force and entered into agreements with distributors of our products in Europe, the Middle East, Asia Pacific and South America, the amount of revenue generated outside of North America has generally increased, although there have been significant quarter-to-quarter fluctuations. We have never been profitable and had net losses of $43.6 million, $47.1 million, and $45.6 million in 2017, 2016 and 2015, respectively. As of December 31, 2017, our accumulated deficit was $313.1 million.
Key Financial Metrics
We are organized as, and operate in, one reportable segment, which is the development, manufacture and commercialization of instruments, consumables and services for efficiently profiling the activity of hundreds of genes and proteins simultaneously from a single tissue sample. Our chief operating decision maker is the chief executive officer, who manages our operations and evaluates our financial performance on a total company basis. Our principal operations and decision-making functions are located at our corporate headquarters in the United States.
Revenue
We generate revenue from the sale of our products and related services. For a description of our revenue recognition policies, see the section of this report captioned “—Critical Accounting Policies and Significant Estimates—Revenue Recognition.”
Product Revenue
Our products consist of our nCounter Analysis System and related consumables, including Prosigna in vitro diagnostic kits. Our nCounter MAX Analysis System typically consists of one nCounter Digital Analyzer and one nCounter Prep Station, having a U.S. list price of $235,000. The U.S. list price of the similarly configured nCounter Dx Analysis System is $265,000, or $285,000 if fully enabled to run Prosigna. Our newly developed nCounter SPRINT Profiler has a reduced footprint and combines the function of the prep station with the digital analyzer in a single instrument. It has a U.S. list price of $149,000. Outside the United States, depending on the country, list prices are generally higher. In certain cases, customers may pay less than the list price for our various nCounter instruments. For example, some of our systems are sold to customers through
-51-
independent distributors, and these distributors may purchase systems from us at a discount to list price. Our customer base is primarily composed of academic institutions, government laboratories, biopharmaceutical companies and clinical laboratories that perform analyses or testing using our nCounter Analysis System and purchase related consumables, potentially including Prosigna kits.
For our research customers, related consumables include (1) gene and protein expression analysis panels, which are standardized and pre-manufactured, (2) custom CodeSets, which we manufacture to the specific requirements of an individual researcher, and (3) Master Kits, cartridges and reagents, which are ancillary reagents, cartridges, tips and reagent plates required to setup and process samples in our instruments.
For our clinical laboratory customers, related consumables include Prosigna in vitro diagnostic kits and selected nCounter reagents. We sell our nCounter Dx Analysis Systems to clinical laboratory customers or offer to lease them under “reagent rental” arrangements where an instrument is placed at a customer location at minimal direct cost and the customer commits to purchase a minimum volume of consumable products over a period of time. To date, the majority of our clinical laboratory customers have elected to purchase instruments. Our average consumables revenue per installed system was approximately $90,000 for the year ended December 31, 2017.
The list price of a Prosigna test in the United States and Europe is $2,080 and €1,550 per patient, respectively. Although the price of Prosigna and our additional future diagnostic products will depend on many factors, including whether and how much third-party payors will reimburse laboratories for conducting such tests, we expect that the gross margin for our diagnostic kits may be higher than for our research consumables. We sell Prosigna kits to our lab customers, who will be responsible for providing the testing service and contracting and billing payors. Prosigna kits are sold to clinical laboratories on a fixed dollars-per-kit basis, which does not expose us to direct third-party payor reimbursement risk. However, we provide customary volume discounts, and in some cases, introductory pricing during the period in which third-party payor reimbursement is being established. As a result, the average selling price per Prosigna test is lower than list price.
Service Revenue
Service revenue consists of fees associated with service contracts and conducting proof-of-principle studies. We include a one-year warranty with the sale of our instruments and offer service contracts, which are purchased by a majority of our customers. We selectively provide proof-of-principle studies to prospective customers in order to help them better understand the benefits of the nCounter Analysis System, and in some cases allowing customers early access to technologies under development for which we generate data and perform analysis services on their behalf.
Collaboration Revenue
Collaboration revenue has been derived primarily from our collaborations with Celgene, Merck, Lam and, historically, our terminated collaboration with Medivation and Astellas. As of December 31, 2017, we have received a total of $83.4 million from these collaboration agreements, of which $42.3 million, $16.7 million, and $5.9 million has been recorded as collaboration revenue in 2017, 2016, and 2015, respectively, with the remainder recorded as deferred revenue and customer deposits, which will be recognized as collaboration revenue over our remaining development performance period for each of the agreements. Collaboration revenue also includes revenue recognized under several smaller collaborations.
Revenue by Geography
We sell our products through our own sales forces in the United States, Canada, Singapore, Israel and certain European countries. We sell through distributors in other parts of the world. As we have expanded our European direct sales force and entered into agreements with distributors of our products in Europe, the Middle East, Asia Pacific and South America, the amount of revenue generated outside of North America has generally increased, although there have been significant quarter-to-quarter fluctuations. In the future, we intend to continue to expand our sales force and establish additional distributor relationships outside the United States to better access international markets.
The following table reflects total revenue by geography based on the geographic location of our customers, distributors and collaborators. For sales to distributors, their geographic location may be different from the geographic locations of the ultimate end customer. Americas consists of the United States, Canada, Mexico and South America; and Asia Pacific includes Japan, China, South Korea, Singapore, Malaysia, India and Australia.
-52-
Year Ended December 31, | ||||||||||||||||||||
2017 | 2016 | 2015 | ||||||||||||||||||
(Dollars in thousands) | ||||||||||||||||||||
Americas | $ | 86,099 | 75 | % | $ | 60,330 | 70 | % | $ | 41,265 | 66 | % | ||||||||
Europe & Middle East | 21,791 | 19 | % | 18,497 | 21 | % | 14,807 | 24 | % | |||||||||||
Asia Pacific | 7,015 | 6 | % | 7,662 | 9 | % | 6,595 | 10 | % | |||||||||||
Total revenue | $ | 114,905 | 100 | % | $ | 86,489 | 100 | % | $ | 62,667 | 100 | % | ||||||||
Most of our revenue is denominated in U.S. dollars. Our expenses are generally denominated in the currencies in which our operations are located, which is primarily in the United States. Changes in foreign currency exchange rates have not materially affected us to date; however, they may become material to us in the future as our operations outside of the United States expand.
Cost of Product and Service Revenue
Cost of product and service revenue consists primarily of costs incurred in the production process, including costs of purchasing instruments from third-party contract manufacturers, consumable component materials and assembly labor and overhead, installation, warranty, service and packaging and delivery costs. In addition, cost of product and service revenue includes royalty costs for licensed technologies included in our products, provisions for slow-moving and obsolete inventory and stock-based compensation expense. We provide a one-year warranty on each nCounter Analysis System sold and establish a reserve for warranty repairs based on historical warranty repair costs incurred.
The average unit cost of our instruments has declined in the current year as compared to prior years primarily as a result of introducing our lower-cost nCounter SPRINT Profiler in July 2015. We expect the average unit costs of our instruments to continue to decline as we expand our market opportunity among smaller research laboratories and sell a higher proportion of SPRINT systems. We expect the unit costs of consumable products to decline as a result of our ongoing efforts to improve our manufacturing processes and expected increases in production volume and yields. Although the unit costs of our custom CodeSets vary, they are generally higher as a percentage of the related revenue than our standard panel products, in vitro Prosigna diagnostic kits and nCounter-based reagents.
Operating Expenses
Research and Development
Research and development expenses consist primarily of salaries and benefits, occupancy, laboratory supplies, engineering services, consulting fees, costs associated with licensing molecular diagnostics rights and clinical study expenses to support the regulatory approval or clearance of diagnostic products. We have made substantial investments in research and development since our inception. Our research and development efforts have focused primarily on the tasks required to enhance our technologies and to support development and commercialization of new and existing products and applications. We believe that our continued investment in research and development is essential to our long-term competitive position and expect these expenses to increase in future periods. In particular, following the entry into the Lam collaboration in August 2017, which provides up to $50 million of funding for our Hyb & Seq program, we expect to experience a substantial increase in related research and development expenses.
Given the size of our research and development staff and the limited number of active projects at any given time, we have found that, to date, it has been effective for us to manage our research and development activities on a departmental basis. Accordingly, other than for collaborations and certain major technology development projects, we have not required employees to report their time by project, nor have we allocated our research and development costs to individual projects, other than collaborations. Research and development expense by functional area was as follows:
Year Ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
(In thousands) | |||||||||||
Platform technology | $ | 16,645 | $ | 10,312 | $ | 6,749 | |||||
Manufacturing process development | 3,025 | 2,582 | 1,802 | ||||||||
Life sciences research products and applications | 7,933 | 6,298 | 4,982 | ||||||||
Diagnostic product development | 7,161 | 6,648 | 3,727 | ||||||||
Clinical, regulatory and medical affairs | 7,036 | 5,111 | 4,939 | ||||||||
Facility allocation | 5,088 | 3,769 | 2,398 | ||||||||
Total research and development expense | $ | 46,888 | $ | 34,720 | $ | 24,597 | |||||
-53-
Selling, General and Administrative
Selling, general and administrative expenses consist primarily of costs for our sales and marketing, finance, human resources, information technology, business development, legal and general management functions, as well as professional services, such as legal, consulting and accounting services. We expect selling, general and administrative expenses to increase in future periods as the number of sales, technical support and marketing and administrative personnel grows to support the expected introduction of new products and product platforms, including our Digital Spatial Profiling, or DSP, and Hyb and Seq product candidates, as well as the general broadening of our customer base and growth of our existing nCounter business. In 2017, we made additions to our commercial leadership and also expanded our sales force significantly, including the addition new roles which are focused on sales of consumables to our existing instrument base. We believe these changes may enable our existing sales representatives to focus on instrument sales and support the growth of our installed instrument base. Legal, accounting and compliance costs have also increased as a result of our being a public company, and we expect them to continue to increase as our business grows.
Factors Affecting Our Performance
Instrument Installed Base
Our future financial performance will be driven in large part by the rate of sales of our nCounter Analysis Systems. In July 2015, we introduced our new generation of the nCounter Analysis System, the nCounter SPRINT Profiler, which has increased our addressable market by making the technology more appealing to individual researchers. The new system is a single instrument with a reduced footprint that combines a prep station and a digital analyzer and is offered at a more affordable price.
We plan to grow our system sales in the coming years through other strategies, including expanding our sales channel in both direct and distributor territories and by continuing to enhance the underlying technology and applications for both research and clinical diagnostics use. As part of this strategy, we added incremental sales territories and augmented our field sales team with greater inside sales support, and continued to grow our base of distributors in 2017. As our installed base of instruments grows, we solicit feedback from our customers and focus our research and development efforts on enabling the nCounter Analysis System for additional applications, which in turn helps to drive additional sales of our instruments and consumables.
Our sales process involves numerous interactions with multiple individuals within an organization, and often includes in-depth analysis by potential customers of our products, performance of proof-of-principle studies, preparation of extensive documentation and a lengthy review process. As a result of these factors, the large capital investment required in purchasing our instruments and the budget cycles of our customers, the time from initial contact with a customer to our receipt of a purchase order can vary significantly, and may be up to 12 months or longer. Given the length and uncertainty of our sales cycle, we will likely experience fluctuations in our future instrument sales on a period-to-period basis.
As of December 31, 2017 we had an installed base of approximately 605 nCounter Analysis Systems, which we count based on the number of nCounter SPRINT Profilers and nCounter Digital Analyzers sold, given that a system may couple one analyzer with multiple nCounter Prep Stations. Management focuses on instrument unit sales as a primary indicator of current business success and a leading indicator of likely future sales of consumables.
Recurring Consumables Revenue
Our instruments are designed to be used only with our consumables. This closed system model generates recurring revenue from each instrument we sell. Management focuses on recurring consumable revenue per system as an indicator of the continuing value generated by each system. We calculate recurring consumables revenue per system (also known as pull-through) quarterly by dividing consumables and in vitro diagnostic kits revenue recognized in a particular quarter (other than consumables revenue related to proof-of-principle studies) by the total number of nCounter Analysis Systems installed as of the last day in the immediately preceding quarter. Historically, a large majority of our systems and related consumables have been sold to research customers. Our average consumables revenue per installed system was approximately $90,000 for the year ended December 31, 2017.
As the installed base of the nCounter Analysis Systems expands, consumables revenue is expected to increase and over time should continue to be an increasingly important contributor to our total revenue. Our consumables revenue per system installed may fluctuate in the future, reflecting the mix of our installed instruments, and potential shifts in the mix, or type, of consumables sold to our installed customer base. Additionally, we expect Prosigna in vitro diagnostic kit revenue to contribute an increasing amount of recurring revenue as we install more diagnostic systems, Prosigna is included in important breast cancer treatment guidelines and reimbursement by third-party payors becomes more broadly available. In 2016 and 2017, we launched additional 3D Biology panels, including our first for the measurement of DNA mutations and in 2017 we
-54-
launched our “360” panels for use in breast cancer, immuno-oncology and hematology. In 2018, we intend to expand beyond oncology and launch nCounter panels focused on neuroscience and immune-related diseases. The introduction of new applications has the potential to further increase our consumables revenue stream. Over time, we believe that consumables revenue may be subject to less period-to-period fluctuation than our instrument sales revenue.
Revenue Mix and Gross Margin
Our product revenue is derived from sales of nCounter Analysis System instruments and related consumables, including Prosigna in vitro diagnostic kits. Generally, our consumables have higher gross margins than our instruments. There may be fluctuations in sales mix between instruments and consumables from period to period. For example, during 2017 our total product and service revenues increased by 4%, however we experienced a 11% decline in instrument sales compared to 2016. This decline in instrument sales was offset by revenue growth from our consumables, including Prosigna in vitro diagnostic kits, and increased revenue from service contracts resulting from our growing installed base of nCounter Analysis Systems. For 2015 and 2016, we experienced an increase in gross margin on product and service revenues, primarily due to an increasing proportion of our sales being derived from our consumable products and other factors, including improved margins on consumable revenues and service revenue. Although future results may vary period to period, over time, as our installed base of systems grows, consumables may continue to constitute a larger percentage of total product revenue, which would tend to increase our gross margins. Such gross margin increases may be offset by the mix of consumable products sold, or in the event we introduce new instrument product platforms that become increasing components of our product sales, such as DSP or Hyb and Seq. In addition, both the average selling price and manufacturing cost of our instruments has decreased with the introduction of the nCounter SPRINT Profiler and this trend may continue with future generations of our nCounter Analysis System. For example, although we sold approximately 40% more systems in 2016 compared to 2015, our instrument revenue only increased 16%. This was largely due to substantially increased sales of lower priced SPRINT systems in 2016. Future instrument selling prices and gross margins may fluctuate as we grow our volume of distribution partners in geographies outside of the United States, as we introduce new products and reduce our product costs, and from variability in the timing of new product introductions.
We derive service revenue from service contracts, which are purchased by a majority of our customers. Additionally, we selectively provide proof-of-principle studies in connection with prospective sales to customers to demonstrate the performance of our nCounter Analysis System. Collaboration revenue is primarily derived from our diagnostic and other collaborations with Celgene, Merck, Lam, and historically, our collaboration with Medivation and Astellas.
The following table reflects the breakdown of revenue in absolute dollars and as percentage of total revenue.
Year Ended December 31, | ||||||||||||||||||||
2017 | 2016 | 2015 | ||||||||||||||||||
(Dollars in thousands) | ||||||||||||||||||||
Product revenue: | ||||||||||||||||||||
Instruments | $ | 20,839 | 18 | % | $ | 24,229 | 28 | % | $ | 20,974 | 33 | % | ||||||||
Consumables | 38,311 | 33 | % | 37,545 | 43 | % | 30,597 | 49 | % | |||||||||||
In vitro diagnostic kits | 6,745 | 6 | % | 4,168 | 5 | % | 2,457 | 4 | % | |||||||||||
Total product revenue | 65,895 | 57 | % | 65,942 | 76 | % | 54,028 | 86 | % | |||||||||||
Service revenue | 6,115 | 5 | % | 3,192 | 4 | % | 2,611 | 4 | % | |||||||||||
Total product and service revenue | 72,010 | 62 | % | 69,134 | 80 | % | 56,639 | 90 | % | |||||||||||
Collaboration revenue | 42,895 | 38 | % | 17,355 | 20 | % | 6,028 | 10 | % | |||||||||||
Total revenue | $ | 114,905 | 100 | % | $ | 86,489 | 100 | % | $ | 62,667 | 100 | % | ||||||||
Results of Operations
Comparison of Years Ended December 31, 2017 and 2016
-55-
Revenue
Year Ended December 31, | Change | ||||||||||||
2017 | 2016 | Dollars | Percentage | ||||||||||
(Dollars in thousands) | |||||||||||||
Product revenue: | |||||||||||||
Instruments | $ | 20,839 | $ | 24,229 | $ | (3,390 | ) | (14)% | |||||
Consumables | 38,311 | 37,545 | 766 | 2% | |||||||||
In vitro diagnostic kits | 6,745 | 4,168 | 2,577 | 62% | |||||||||
Total product revenue | 65,895 | 65,942 | (47 | ) | —% | ||||||||
Service revenue | 6,115 | 3,192 | 2,923 | 92% | |||||||||
Total product and service revenue | 72,010 | 69,134 | 2,876 | 4% | |||||||||
Collaboration revenue | 42,895 | 17,355 | 25,540 | 147% | |||||||||
Total revenue | $ | 114,905 | $ | 86,489 | $ | 28,416 | 33% | ||||||
Instruments revenue decreased for the year ended December 31, 2017, due primarily to fewer instruments sold during the year, and to a lesser extent, the realization of a lower average price per instrument sold. We sold approximately 125 instruments in 2017, down from approximately 140 instruments in 2016. While our mix of instruments sold remained relatively consistent year over year, the average price per instrument sold in 2017 was impacted by a greater proportion of instruments being sold through distributors during 2017, for which we typically record lower selling prices, as well as lower prices recorded related to sales of our nCounter SPRINT Profilers as compared to 2016. Consumables revenue increased during 2017, primarily as a result of our growing installed base of nCounter Analysis Systems, as well as growth in various European markets. In vitro diagnostic kit revenue represents sales of Prosigna assays, which increased as more testing providers came online, and testing volumes increased. The increase in service revenue was primarily related to an increase in the number of instruments covered by service contracts, and also increases in revenue generated from technology access fees, data analysis, and other services related to new potential customers and technologies which are under development. Our product and service revenue may continue to increase in future periods, as a result of our increased investments in sales and marketing activities, the introduction of new nCounter consumable products, and the potential commercial launch of our DSP and Hyb & Seq product candidates.
Collaboration revenue increased by $25.5 million in 2017, due largely to changes in estimates related to future costs associated with our collaborations with Merck and Medivation and Astellas. Our collaboration with Medivation and Astellas was terminated during the second quarter of 2017, and during the fourth quarter of 2017, we were notified by Merck of a change in scope associated with planned future regulatory activities. Both of these events resulted in a decrease of the total expected costs associated with the collaborations, and as a result, the completion percentage used in the proportional performance model used for revenue recognition increased substantially. These changes in estimates resulted in an acceleration of revenue recognized during 2017, relative to the original planned project time lines and estimated costs. The addition of our new collaboration agreement with Lam, which was entered into during the third quarter of 2017, also contributed to our increased collaboration revenue in 2017 as compared to the prior year.
Cost of Product and Service Revenue; Gross Profit; and Gross Margin
Year Ended December 31, | Change | ||||||||||||
2017 | 2016 | Dollars | Percentage | ||||||||||
(Dollars in thousands) | |||||||||||||
Cost of product and service revenue | $ | 31,880 | $ | 30,245 | $ | 1,635 | 5% | ||||||
Product and service gross profit | $ | 40,130 | $ | 38,889 | $ | 1,241 | 3% | ||||||
Product and service gross margin | 56 | % | 56 | % | |||||||||
For the year ended December 31, 2017, cost of product and service revenue increased due to higher volumes of consumables sold, including our Prosigna in vitro diagnostic kits, as well as increased revenue from service contracts and data analysis services as compared to 2016. These increases were partially offset by the lower volume of instruments sold during the year. Our gross margin on product and service revenue in 2017 benefited from a shift in revenue mix from instruments to consumables, driven in large part by continued growth in Prosigna in vitro diagnostic kit revenue, the addition of new higher margin service revenue from our DSP technology access program, and a lower royalty rate on our license of the foundational nCounter patents due to the achievement of a cumulative revenue milestone that took effect in the third quarter of 2016. These increases were offset by lower average selling prices realized on certain sales of our nCounter Analysis Systems, as a result of selected promotion and sales related activities during the period, the reduction in certain higher gross margin custom consumable orders, and increased reserves for slow-moving inventory. We expect our cost of product and service revenue to increase in future periods, primarily due to our expected continued growth in product and service revenue. We expect our gross
-56-
margin on product and service revenue to remain stable or potentially increase in future periods, as we increase our sales of consumables through a larger instrument installed base, as we introduce new nCounter consumable products that may have lower gross margins during their initial launch period, and as a greater proportion of nCounter SPRINT Profilers are sold in future periods as a percentage of our total instrument sales. Costs related to collaboration revenue are included in research and development expense.
Research and Development Expense
Year Ended December 31, | Change | ||||||||||||
2017 | 2016 | Dollars | Percentage | ||||||||||
(Dollars in thousands) | |||||||||||||
Research and development expense | $ | 46,888 | $ | 34,720 | $ | 12,168 | 35% | ||||||
The increase in research and development expense in 2017 was primarily attributable to an increase in staffing and personnel-related costs of $6.2 million, as well as increased supply costs of $2.2 million and professional fees of $1.8 million. These increased costs were incurred primarily to support the advancement of our collaborations and technology and product development activities, including DSP and Hyb & Seq, product candidates. In addition, facility costs increased by $1.4 million in 2017, due to expansion of our leased space for research and development activities. We expect that research and development costs will continue to increase in future periods in support of remaining development activities relating to our DSP product candidate, and as a result of our new collaboration agreement with Lam and the resulting expansion of our development of the Hyb & Seq product candidate. As an offset to this expected increase in expense relating to Hyb & Seq, Lam has committed to provide up to $50.0 million in funding. We expect the majority of the Hyb and Seq program development efforts and related costs to be incurred during 2018 and the first half of 2019.
Selling, General and Administrative Expense
Year Ended December 31, | Change | ||||||||||||
2017 | 2016 | Dollars | Percentage | ||||||||||
(Dollars in thousands) | |||||||||||||
Selling, general and administrative expense | $ | 74,334 | $ | 62,700 | $ | 11,634 | 19% | ||||||
The increase in selling, general and administrative expense in 2017 was primarily attributable to an increase in staffing and personnel-related costs of $8.8 million to support our sales, marketing and administrative functions, increased sales and marketing costs of $1.6 million related to promotional events and other external activities, and increased professional fees of $0.6 million. These increases were partially offset by $0.6 million of lower state and local gross receipt-based taxes primarily as a result of lower amounts received under our collaboration agreements. We expect selling, general and administrative expenses may increase in future periods, in the event we make additional investments to support the sales of our existing products, or launch activities relating to new product candidates, such as our DSP product candidate, for which material commercial launch activities are expected to commence in 2019.
Other Income (Expense)
Year Ended December 31, | Change | ||||||||||||
2017 | 2016 | Dollars | Percentage | ||||||||||
(Dollars in thousands) | |||||||||||||
Interest income | $ | 809 | $ | 390 | $ | 419 | 107% | ||||||
Interest expense | (6,153 | ) | (5,672 | ) | (481 | ) | 8% | ||||||
Other income (expense), net | 183 | (515 | ) | 698 | (136)% | ||||||||
Total other income (expense), net | $ | (5,161 | ) | $ | (5,797 | ) | $ | 636 | (11)% | ||||
Interest expense increased in 2017, due primarily to increases in our long-term debt borrowings during these periods. The average balance of long-term debt outstanding during 2017 and 2016 was $48.6 million and $44.9 million, respectively. Interest income increased in 2017, due to higher interest rates as well as an increased average investment balance in 2017. Other income (expense), net is primarily related to realized and unrealized gains or losses associated with foreign currency transactions during 2017, in which we benefited from the weakening of the U.S. dollar versus foreign currencies, primarily the Euro and the British pound.
Comparison of Years Ended December 31, 2016 and 2015
-57-
Revenue
Year Ended December 31, | Change | ||||||||||||
2016 | 2015 | Dollars | Percentage | ||||||||||
(Dollars in thousands) | |||||||||||||
Product revenue: | |||||||||||||
Instruments | $ | 24,229 | $ | 20,974 | $ | 3,255 | 16% | ||||||
Consumables | 37,545 | 30,597 | 6,948 | 23% | |||||||||
In vitro diagnostic kits | 4,168 | 2,457 | 1,711 | 70% | |||||||||
Total product revenue | 65,942 | 54,028 | 11,914 | 22% | |||||||||
Service revenue | 3,192 | 2,611 | 581 | 22% | |||||||||
Total product and service revenue | 69,134 | 56,639 | 12,495 | 22% | |||||||||
Collaboration revenue | 17,355 | 6,028 | 11,327 | 188% | |||||||||
Total revenue | $ | 86,489 | $ | 62,667 | $ | 23,822 | 38% | ||||||
Instruments revenue increased for the year ended December 31, 2016 due to the increased volume of instruments sold. We sold approximately 140 instrument systems in 2016, of which approximately 60 were nCounter SPRINT Profilers. Although we sold approximately 40% more systems in 2016 compared to 2015, our instrument revenue only increased 16%. This was largely due to substantially increased sales of the lower priced SPRINT systems in 2016. The increase in consumables revenue was primarily driven by growth in our installed base of instrument systems as the average amount of consumables revenue was over $100,000 per installed system in 2016 and 2015. In vitro diagnostic kit revenue represents sales of Prosigna assays, which increased as more testing providers came online, and testing volumes increased. The increase in service revenue was primarily related to an increase in the number of instruments covered by service contracts. Collaboration revenue increased $9.8 million in 2016 due to our new collaboration agreements with Merck and Medivation and Astellas.
Cost of Product and Service Revenue; Gross Profit; and Gross Margin
Year Ended December 31, | Change | ||||||||||||
2016 | 2015 | Dollars | Percentage | ||||||||||
(Dollars in thousands) | |||||||||||||
Cost of product and service revenue | $ | 30,245 | $ | 26,126 | $ | 4,119 | 16% | ||||||
Product and service gross profit | $ | 38,889 | $ | 30,513 | $ | 8,376 | 27% | ||||||
Product and service gross margin | 56 | % | 54 | % | |||||||||
The increase in cost of product and service revenue for the year ended December 31, 2016 was related to the increased volume of instruments, consumables, in vitro diagnostic kits and services sold. The increase in gross margin on product and service revenues was primarily due to improved gross margin on consumable revenue resulting from efficiencies of scale and a favorable mix of consumable products sold during the year. In addition, gross margin benefited from a reduced technology royalty rate across all products for a portion of the year due to the achievement of a cumulative revenue milestone under the license of our foundational nCounter patents. Costs related to collaboration revenue are included in research and development expense.
Research and Development Expense
Year Ended December 31, | Change | ||||||||||||
2016 | 2015 | Dollars | Percentage | ||||||||||
(Dollars in thousands) | |||||||||||||
Research and development expense | $ | 34,720 | $ | 24,597 | $ | 10,123 | 41% | ||||||
The increase in research and development expense in 2016 reflected a $5.5 million increase in personnel-related expenses and a $3.3 million increase in supply costs to primarily support the advancement of our diagnostic and product development activities, including activities to support our collaboration agreements. In addition, facility costs increased $1.4 million due to expansion of our leased space for research and development activities. These increases were partially offset by decreases of $0.6 million in costs related to clinical trials.
-58-
Selling, General and Administrative Expense
Year Ended December 31, | Change | ||||||||||||
2016 | 2015 | Dollars | Percentage | ||||||||||
(Dollars in thousands) | |||||||||||||
Selling, general and administrative expense | $ | 62,700 | $ | 53,186 | $ | 9,514 | 18% | ||||||
The increase in selling, general and administration expense in 2016 were primarily attributable to a $5.4 million increase in staffing and personnel-related costs to support sales and marketing and administration; increased legal and other professional fees of $1.8 million; increased sales and marketing costs of $1.3 million related to promotional events and other external activities; and increased state and local gross receipts-based taxes of $0.7 million, primarily related to amounts received under our collaboration agreements.
Other Income (Expense)
Year Ended December 31, | Change | ||||||||||||
2016 | 2015 | Dollars | Percentage | ||||||||||
(Dollars in thousands) | |||||||||||||
Interest income | $ | 390 | $ | 233 | $ | 157 | 67% | ||||||
Interest expense | (5,672 | ) | (4,017 | ) | (1,655 | ) | 41% | ||||||
Other income (expense), net | (515 | ) | (389 | ) | (126 | ) | 32% | ||||||
Total other income (expense), net | $ | (5,797 | ) | $ | (4,173 | ) | $ | (1,624 | ) | 39% | |||
The increased interest expense for the year ended December 31, 2016 was due to an increase in borrowing in 2016. Long-term debt and lease financing obligations outstanding increased to $47.4 million as of December 31, 2016 as compared to $41.2 million as of December 31, 2015. The average balance of long-term debt and lease financing obligations outstanding was $44.9 million in 2016 compared to $31.4 million in 2015.
Liquidity and Capital Resources
As of December 31, 2017, we had cash, cash equivalents and short-term investments of $77.6 million, compared to $74.0 million as of December 31, 2016. We believe our existing cash, cash equivalents and short-term investments will be sufficient to meet our working capital and capital expenditure needs for at least the next 12 months. However, we may need to raise additional capital to expand the commercialization of our products, fund our operations and further our research and development activities. Our future funding requirements will depend on many factors, including: the nature and timing of any additional companion diagnostic development collaborations we may establish; market acceptance and the level of sales of our existing products and new product candidates; the nature and timing of any additional research, product development or other partnerships or collaborations we may establish; the cost and timing of establishing additional sales, marketing and distribution capabilities; the cost of our research and development activities; the cost and timing of regulatory clearances or approvals; the effect of competing technological and market developments; and the extent to which we acquire or invest in businesses, products and technologies, although we currently have no commitments or agreements relating to any of these types of transactions.
We will require additional funds in the future and we may not be able to obtain such funds on acceptable terms, or at all. If we raise additional funds by issuing equity or equity-linked securities, our stockholders may experience dilution. Debt financing, if available, may involve covenants restricting our operations or our ability to incur additional debt. Any debt or additional equity financing that we raise may contain terms that are not favorable to us or our stockholders. If we raise additional funds through partnership, collaboration or licensing arrangements with third parties, it may be necessary to relinquish some rights to our technologies or our products, or grant licenses on terms that are not favorable to us. If we are unable to raise adequate funds, we may have to liquidate some or all of our assets, delay or reduce the scope of or eliminate some or all of our research and development programs, delay development, launch activities or commercialization of our products or license to third parties the rights to commercialize products or technologies that we would otherwise seek to commercialize, or reduce marketing, customer support or other resources devoted to our products or cease operations.
Sources of Funds
Since inception, we have financed our operations primarily through the sale of equity securities and, to a lesser extent, from borrowings. Our cash used in operations for the year ended December 31, 2017 was $51.7 million, including $22.7 million in cash receipts from our collaboration agreements. The timing and amount of such receipts in the future are uncertain, and therefore we may be required to secure larger amounts of cash to fund our planned operations.
-59-
In January 2018, we entered into a sales agreement with a sales agent to sell shares of our common stock through an "at the market" equity offering program for up to $40.0 million in gross cash proceeds. The sales agreement allows us to set the parameters for the sale of shares, including the number of shares to be issued, the time period during which sales are requested to be made, limits on the number of shares that may be sold in any one trading day and a minimum price below which sales may not be made. Under the terms of the Sales Agreement, commission expenses to the sales agent will be 3% of the gross sales price per share sold through the sales agent. The Sales Agreement shall automatically terminate upon the issuance and sale of placement shares equaling sales proceeds of $40.0 million and may be terminated earlier by either us, or the sales agent upon five days’ notice. As of the date of this report, there had been no shares of common stock sold under this agreement.
In June 2017, we completed an underwritten public offering of 3,450,000 shares of common stock, including the exercise by the underwriter of an over-allotment option for 450,000 shares of common stock, for total gross proceeds of $57.8 million. After underwriters’ fees and commissions and other expenses of the offering, our aggregate net proceeds were approximately $56.5 million.
In May 2015, we entered into a sales agreement with a sales agent to sell shares of our common stock through an “at the market” equity offering program for up to $40.0 million in total sales proceeds. Pursuant to the sales agreement, we sold 1,331,539 and 960,400 shares during 2016 and 2015, respectively, for net proceeds of $26.1 million and $12.5 million, respectively. The sales agreement automatically terminated when we sold the maximum number of shares allowed under the agreement.
In April 2014, we entered into a term loan agreement under which up to $45.0 million could be borrowed, including an option to defer payment of a portion of the interest that would accrue on the borrowing under the term loan agreement. Upon initial closing, we borrowed $20.0 million and in October 2014, we borrowed an additional $10.0 million under the term loan agreement.
In October 2015, we amended our term loan agreement to, among other provisions, increase the maximum borrowing capacity to $60 million (excluding accrued interest), reduce the applicable interest rate from 12.5% to 12.0%, extend the interest-only period through March 2021, and extend the final maturity to March 2022. Under the amended agreement, borrowings accrue interest at 12.0% annually, payable quarterly, of which 3.0% can be deferred during the first six years of the term at our option and paid together with the principal at maturity. We have elected to exercise the option to defer a portion of the interest and we have recorded $4.3 million of deferred interest through December 31, 2017. In December 2015, we borrowed an additional $10.0 million under the terms of the amended agreement and in June 2016 we borrowed an additional $5.0 million. At December 31, 2016, the option to borrow $15.0 million more under the amended term loan agreement expired. Total borrowings and deferred interest under the amended term loan agreement were $49.3 million as of December 31, 2017.
Under the amended term loan agreement, we may pay interest-only for the first seven years of the term and principal payments are due in four equal installments during the eighth year of the term. We have the option to prepay the term loan, in whole or part, at any time subject to payment of a redemption fee of up to 4%, which declines 1% annually, with no redemption fee payable if prepayment occurs after the fourth year of the loan. In addition, a facility fee equal to 2.0% of the amount borrowed plus any deferred interest is payable at the end of the term or when the loan is repaid in full. A long-term liability of $1.1 million is being accreted using the effective interest method for the facility fee over the term of the loan agreement. Obligations under the term loan agreement are collateralized by substantially all of our assets.
The term loan agreement contains customary conditions to borrowings, events of default and negative covenants, including covenants that could limit our ability to, among other things, incur additional indebtedness, liens or other encumbrances, make dividends or other distributions; buy, sell or transfer assets; engage in any new line of business; and enter into certain transactions with affiliates. The term loan agreement also includes a $2.0 million minimum liquidity covenant and revenue-based financial covenants, which was $85.0 million for 2017 with annual increases of $15.0 million for each subsequent fiscal year thereafter. If our actual revenues are below the minimum annual revenue requirement for any given year, we may avoid a related default by generating proceeds from an equity or subordinated debt issuance equal to the shortfall between our actual revenues and the minimum revenue requirement. We were in compliance with our covenants as of December 31, 2017. In January 2018, we further amended the term loan agreement to provide that the lender’s interest in the collateral under the term loan agreement shall be subordinate to the lender’s interest in such collateral under our secured revolving loan facility, as described below.
In January 2018, we, as borrower, entered into a $15.0 million secured revolving loan facility, with availability subject to a borrowing base consisting of our eligible accounts receivable. The agreement matures in January 2021, at which time any outstanding principal will become due and payable. Interest on borrowings is payable monthly and accrues interest at a yearly rate equal to the greater of the prime rate, as reported in the Wall Street Journal, plus 0.50% or 4.75%. During an event of default, amounts drawn accrue interest at a yearly rate equal to 8.75%. Our obligations under the agreement are secured by our cash and cash equivalents, accounts receivable and proceeds thereof, and inventory and proceeds from the sale thereof. The lender’s interest in the collateral under the loan facility is senior to the lender’s interest in such collateral under the term loan
-60-
agreement. The loan facility contains various customary representations and warranties, conditions to borrowing, events of default, including cross default provisions with respect to the loan facility, and covenants, including financial covenants requiring the maintenance of minimum annual revenue and liquidity. There were no borrowings under this facility as of the date of this report.
Use of Funds
Our principal uses of cash are funding our operations, capital expenditures, satisfaction of our obligations under our debt instruments, and other working capital requirements. Over the past several years, our revenue has increased significantly from year to year and, as a result, our cash flows from customer collections have increased. However, our operating expenses have also increased as we have invested in growing our existing product sales, developing and commercializing Prosigna, supporting our companion diagnostic and other collaborations, and investing in technologies that we believe have the potential to drive the long-term growth of our business.
Our operating cash requirements may increase in the future as we (1) increase sales and marketing activities to expand the installed base of our nCounter Analysis Systems among research customers and clinical laboratories and continue to promote consumable usage, including Prosigna, (2) commercialize, and conduct studies to expand the clinical utility of Prosigna and develop new diagnostic tests, (3) develop new applications, chemistry and instruments for our nCounter platform, and (4) invest in the research and development of new product candidates including DSP and Hyb & Seq, as we cannot be certain our revenues will grow sufficiently to offset our operating expense increases, nor can we be certain that we will be successful in continuing to generate cash from new partnerships or collaborations to help fund our operations. As a result, we will need to raise additional funds to support our operations, and such funding may not be available to us on acceptable terms, or at all. If we are unable to raise additional funds when needed, our operations and ability to execute our business strategy could be adversely affected.
Historical Cash Flow Trends
The following table shows a summary of our cash flows for the periods indicated:
Year Ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
(In thousands) | |||||||||||
Cash used in operating activities | $ | (51,657 | ) | $ | (6,079 | ) | $ | (43,362 | ) | ||
Cash provided by (used in) investing activities | (2,490 | ) | (30,261 | ) | 23,769 | ||||||
Cash provided by financing activities | 59,668 | 35,093 | 24,268 | ||||||||
Operating Cash Flows
We derive operating cash flows from cash collected from the sale of our products and services and from collaborations. These cash flows received are currently outweighed by our use of cash for operating expenses to support the growth of our business. As a result, we have historically experienced negative cash flows from operating activities and this will likely continue for the foreseeable future.
Net cash used in operating activities for 2017 consisted of our net loss of $43.6 million, plus the negative impact of decreases in our deferred revenue related to collaboration agreements of $29.2 million. The decrease in deferred revenue related to collaborations was due primarily to the termination of our Medivation and Astellas collaboration agreement and the change in scope of the Merck collaboration agreement, both of which resulted in the completion percentage used in the proportional performance model used for revenue recognition to increase substantially. As a result, we accelerated the recognition of revenue recognized during 2017, relative to the original planned project time lines and estimated costs. These unfavorable “uses” of funds were partially offset by $3.3 million of changes in our operating assets and liabilities and $17.8 million of net non-cash income and expense items, such as stock-based compensation, depreciation and amortization, deferred interest converted to principal pursuant to our term loan agreement, and provisions for bad debt and inventory obsolescence.
Net cash used in operating activities for 2016 consisted of our net loss of $47.1 million partially offset by $27.5 million of changes in our operating assets and liabilities, including $29.9 million related to our collaboration agreements, and by $13.5 million of net non-cash income and expense items, such as stock-based compensation, depreciation and amortization, deferred interest converted to principal for the term loan, and accretion of discount on short-term investments.
Net cash used in operating activities for 2015 consisted of our net loss of $45.6 million and $7.8 million of changes in our operating assets and liabilities. These uses were partially offset by $10.0 million of net non-cash income and expense items, such as stock-based compensation, depreciation and amortization, deferred interest converted to principal for the term loan, and amortization of premium on short-term investments.
-61-
Investing Cash Flows
Our most significant investing activities for the years ended December 31, 2017, 2016 and 2015 were related to the purchase and sale of short-term investments. Because we manage our cash usage with respect to our total cash, cash equivalents and short-term investments, we do not consider these cash flows to be important to an understanding of our liquidity and capital resources.
In the years ended December 31, 2017, 2016 and 2015, we purchased $4.3 million, $4.0 million, and $3.8 million respectively, of property and equipment required to support the growth and expansion of our operations.
Financing Cash Flows
Historically, we have funded our operations through the issuance of equity securities and debt borrowings.
Net cash provided by financing activities for 2017 consisted of net proceeds of $56.5 million from an underwritten public offering, $1.8 million from proceeds associated with our Employee Stock Purchase Plan, and $1.1 million of proceeds from the exercise of stock options.
Net cash provided by financing activities for 2016 consisted of net proceeds of $26.2 million from the sale of shares through an “at the market” equity offering program, proceeds of $5.0 million from our amended term loan agreement, $1.5 million from proceeds associated with our Employee Stock Purchase Plan, and $2.6 million of proceeds from the exercise of stock options. These proceeds were partially offset by payment of lease financing obligations of $0.2 million.
Net cash provided by financing activities for 2015 consisted of net proceeds of $12.5 million from the sale of shares through an “at the market” equity offering program, proceeds of $10.0 million from our amended term loan agreement, $1.3 million from proceeds associated with our Employee Stock Purchase Plan, and $0.9 million of proceeds from the exercise of stock options. These proceeds were partially offset by payment of lease financing obligations of $0.3 million and payment of deferred offering costs related to the equity offering program of $0.2 million.
Contractual Obligations
The following table reflects a summary of our contractual obligations as of December 31, 2017.
Payments due by period | |||||||||||||||||||
Contractual Obligations(1) | Total | Less than 1 Year | 1-3 Years | 3-5 Years | More than 5 Years | ||||||||||||||
(In thousands) | |||||||||||||||||||
Lease obligations(2) | $ | 46,627 | $ | 5,316 | $ | 10,725 | $ | 11,259 | $ | 19,327 | |||||||||
Long-term debt obligations(3) | 49,315 | — | — | 36,987 | 12,328 | ||||||||||||||
Purchase obligations(4) | 4,407 | 4,407 | — | — | — | ||||||||||||||
Total | $ | 100,349 | $ | 9,723 | $ | 10,725 | $ | 48,246 | $ | 31,655 | |||||||||
(1)Excludes royalty obligations based on net sales of products, including royalties payable to the Institute for Systems Biology, as any such amounts are not currently determinable.
(2)Lease costs are primarily for office, laboratory and manufacturing space.
(3)Includes principal and deferred interest on long-term debt obligations.
(4)Purchase obligations consist of contractual and legally binding commitments under outstanding purchase orders to purchase long lead time inventory and other research and development items.
Critical Accounting Policies and Significant Estimates
Our discussion and analysis of our financial condition and results of operations are based upon our financial statements which have been prepared in accordance with U.S. generally accepted accounting principles, or GAAP. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets and liabilities and related disclosure of contingent assets and liabilities, revenue and expenses at the date of the financial statements. Generally, we base our estimates on historical experience and on various other assumptions in accordance with GAAP that we believe to be reasonable under the circumstances. Actual results may differ from these estimates.
Critical accounting policies and estimates are those that we consider the most important to the portrayal of our financial condition and results of operations because they require our most difficult, subjective or complex judgments, often as a result of the need to make estimates about the effect of matters that are inherently uncertain. Our critical accounting policies and estimates include those related to:
-62-
• | revenue recognition; |
• | stock-based compensation; |
• | inventory valuation; |
• | fair value measurements; and |
• | income taxes. |
Revenue Recognition
We generate the majority of our revenue from sales of products and services. Our products consist of our proprietary nCounter Analysis Systems and related consumables. Services consist of service contracts and service fees for assay processing.
Revenue is recognized when all of the following criteria are met: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred or services have been rendered; (3) the price to the customer is fixed or determinable; and (4) collectability is reasonably assured. The evaluation of these revenue recognition criteria requires significant management judgment. For instance, we use judgment to assess collectability based on factors such as the customer’s creditworthiness and past collection history, if applicable. If we determine that collection of a payment is not reasonably assured, revenue recognition is deferred until receipt of payment. We also use judgment to assess whether a price is fixed or determinable including but not limited to, reviewing contractual terms and conditions related to payment terms.
Instruments, consumables and in vitro diagnostic kits are considered to be separate units of accounting as they are sold separately and revenue is recognized upon transfer of ownership, which is generally upon shipment. Instrument revenue related to installation and calibration services is recognized when services are rendered. For instruments sold for use primarily to run Prosigna assays, training must be provided prior to instrument revenue recognition. Instrument revenue from leased instruments is recognized ratably over the lease term.
Some of our sales arrangements involve the delivery or performance of multiple products or services. Significant interpretation is sometimes required to determine the appropriate accounting, including whether the deliverables specified in a multiple element arrangement should be treated as separate units of accounting for revenue recognition purposes, and, if so, how the related sales price should be allocated among the elements, when to recognize revenue for each element, and the period over which revenue should be recognized. Revenue recognition for arrangements with multiple deliverables is based on the individual units of accounting determined to exist in the arrangement. A delivered element is considered a separate unit of accounting when the delivered element has value to the customer on a stand-alone basis. Elements are considered to have stand-alone value when they are sold separately or when the customer could resell the element on a stand-alone basis.
For multiple-element arrangements, we allocate arrangement consideration at the inception of the arrangement to the deliverables based on the relative selling price method. The selling price used for each deliverable is based on vendor-specific objective evidence, or VSOE, if available, third-party evidence, or TPE, if VSOE is not available, or best estimated selling price, or BESP, if neither VSOE nor TPE is available. BESP is determined in a manner consistent with that used to establish the price to sell the deliverable on a stand-alone basis. To date, selling prices have been established by reference to VSOE based on stand-alone sales transactions for each deliverable. VSOE is considered to have been established when a substantial majority of individual sales transactions within the previous 12-month period fall within a reasonably narrow range, which we have defined to be plus or minus 15% of the median sales price of actual stand-alone sales transactions. Allocated revenue is only recognized for each deliverable when the revenue recognition criteria have been met.
Revenue from the sales of our products that are not part of multiple element arrangements is recognized when no significant obligations remain undelivered and collection of the receivables is reasonably assured, which is generally when delivery has occurred.
Accruals for estimated warranty expenses are made at the time that the associated revenue is recognized. We use judgment to estimate these accruals and, if we were to experience an increase in warranty claims or if costs of servicing our products under warranty were greater than our estimates, our cost of revenue could be adversely affected in future periods.
Revenue from the sales of our services is recognized when no significant obligations remain undelivered and collection of the receivables is reasonably assured, which is generally when delivery has occurred. We offer service contracts on our nCounter Analysis Systems for periods ranging from 12 to 36 months after the end of the standard 12-month warranty period. Revenue from service contracts is deferred and recognized in income on a straight-line basis over the contract period.
We enter into collaborative agreements that may generate upfront fees with subsequent milestone payments that may be earned upon the completion of development-related milestones. We are able to estimate the total cost of services to be provided under the arrangement and recognize collaboration revenue using a proportional performance model. Costs incurred to date compared to total expected costs are used to determine proportional performance, as this is considered to be
-63-
representative of the delivery of outputs under the arrangements. Revenue recognized at any point in time is limited to cash received and amounts contractually due. Changes in estimates of total expected costs are accounted for prospectively as a change in estimate. From period to period, collaboration revenue can fluctuate substantially based on the achievement of development-related milestones. We recognize revenue from collaborative agreements that do not include upfront and/or milestone-based payments when earned. Amounts due to collaboration partners are recognized when the related activities have occurred and are classified in the statement of operations, generally as research and development expense, based on the nature of the related activities.
Stock-based Compensation
We account for stock-based compensation at fair value. Stock-based compensation costs are recognized based on their grant date fair value estimated using the Black-Scholes option pricing model. Stock-based compensation expense recognized in the consolidated statements of operations is based on options ultimately expected to vest and has been reduced by an estimated forfeiture rate based on our historical and expected forfeiture patterns. We use the straight-line method of allocating compensation cost over the requisite service period of the related award.
Determining the fair value of stock-based awards at the grant date under the Black-Scholes option pricing model requires judgment, including estimating the value per share of our common stock, risk-free interest rate, expected term and dividend yield and volatility. The assumptions used in calculating the fair value of stock-based awards represent our best estimates based on management judgment and subjective future expectations. These estimates involve inherent uncertainties. If any of the assumptions used in the Black-Scholes option pricing model significantly change, stock-based compensation for future awards may differ materially from the awards granted previously.
The expected term of options granted is based on historical experience of similar awards and expectations of future employee behavior. The risk-free interest rate for the expected term of the option is based on the U.S. Treasury yield curve in effect at the time of grant. We have not paid and do not anticipate paying cash dividends on our common stock; therefore, the expected dividend yield is assumed to be zero. Volatility through 2016 was based on the estimated volatility of similar companies whose share prices are publicly available. In 2017, we calculated volatility based on our share price activity throughout the year.
Inventory Valuation
Inventory consists of raw materials, certain component parts to be used in manufacturing our products and finished goods. Inventory is stated at the lower of cost or market. Cost is determined using a standard cost system, whereby the standard costs are updated periodically to reflect current costs and market represents the lower of replacement cost or estimated net realizable value. We record adjustments to inventory for potentially excess, obsolete, slow-moving or impaired items. The business environment in which we operate is subject to rapid changes in technology and customer demand. We regularly review inventory for excess and obsolete products and components, taking into account product life cycle and development plans, product expiration and quality issues, historical experience and our current inventory levels. If actual market conditions are less favorable than anticipated, additional inventory adjustments could be required.
Recent Accounting Pronouncements
For information regarding recent accounting pronouncements, see Note 2 of the Notes to the Consolidated Financial Statements under Item 8 of this report.
Off-Balance Sheet Arrangements
We do not have any relationships with unconsolidated entities or financial partnerships, such as entities often referred to as structured finance or special purpose entities, which would have been established for the purpose of facilitating off-balance sheet arrangements or for any other contractually narrow or limited purpose.
Inflation
We do not believe that inflation has had a material effect on our business, financial condition or results of operations. If our costs were to become subject to significant inflationary pressures, we may not be able to fully offset such higher costs through price increases. Our inability or failure to do so could adversely affect our business, financial condition and results of operations.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
-64-
We are exposed to various market risks, including changes in commodity prices and interest rates. Market risk is the potential loss arising from adverse changes in market rates and prices. Prices for our products are largely denominated in U.S. dollars and, as a result, we do not face significant risk with respect to foreign currency exchange rates.
Interest Rate Risk
Generally, our exposure to market risk has been primarily limited to interest income sensitivity, which is affected by changes in the general level of U.S. interest rates, particularly because the majority of our investments are in short-term debt securities. The primary objective of our investment activities is to preserve principal while at the same time maximizing the income we receive without significantly increasing risk. To minimize risk, we maintain our portfolio of cash, cash equivalents and short-term investments in a variety of interest-bearing instruments, which have included U.S. government and agency securities, high-grade U.S. corporate bonds, asset-backed securities, and money market funds. Declines in interest rates, however, would reduce future investment income. A 10% decline in interest rates, occurring on January 1, 2018 and sustained throughout the period ending December 31, 2018, would not be material.
As of December 31, 2017, the principal and deferred interest outstanding under our term borrowings was $49.3 million. The interest rates on our term borrowings under our credit facility are fixed. If overall interest rates had increased by 10% during the periods presented, our interest expense would not have been affected.
Foreign Currency Exchange Risk
As we continue to expand internationally our results of operations and cash flows will become increasingly subject to fluctuations due to changes in foreign currency exchange rates. Historically, a majority of our revenue has been denominated in U.S. dollars, although we sell our products and services directly in certain markets outside of the United States denominated in local currency, principally the Euro. Our expenses are generally denominated in the currencies in which our operations are located, which is primarily in the United States. The effect of a 10% adverse change in exchange rates on foreign denominated cash, receivables and payables would not have been material for the periods presented. As our operations in countries outside of the United States grow, our results of operations and cash flows will be subject to potentially greater fluctuations due to changes in foreign currency exchange rates, which could harm our business in the future. To date, we have not entered into any material foreign currency hedging contracts although we may do so in the future.
-65-
Item 8. Financial Statements and Supplementary Data
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
NANOSTRING TECHNOLOGIES, INC.
Page(s) | |
Financial Statements: | |
-66-
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of NanoString Technologies, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of NanoString Technologies, Inc. and its subsidiaries as of December 31, 2017 and 2016, and the related consolidated statements of operations, of comprehensive loss, of changes in stockholders’ equity and of cash flows for each of the three years in the period ended December 31, 2017, including the related notes (“collectively referred to as the consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2017 and 2016, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2017 in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits of these consolidated financial statements in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ PricewaterhouseCoopers LLP
Seattle, Washington
March 7, 2018
We have served as the Company's auditor since 2008.
-67-
NanoString Technologies, Inc.
Consolidated Balance Sheets
December 31, | |||||||
2017 | 2016 | ||||||
(In thousands, except par value amounts) | |||||||
Assets | |||||||
Current assets: | |||||||
Cash and cash equivalents | $ | 26,136 | $ | 20,583 | |||
Short-term investments | 51,419 | 53,453 | |||||
Accounts receivable, net | 19,564 | 22,193 | |||||
Inventory, net | 20,057 | 13,812 | |||||
Prepaid expenses and other current assets | 4,745 | 3,744 | |||||
Total current assets | 121,921 | 113,785 | |||||
Restricted cash | 143 | 143 | |||||
Property and equipment, net | 14,057 | 12,158 | |||||
Other assets | 641 | 287 | |||||
Total assets | $ | 136,762 | $ | 126,373 | |||
Liabilities and Stockholders’ Equity | |||||||
Current liabilities: | |||||||
Accounts payable | $ | 4,092 | $ | 4,935 | |||
Accrued liabilities | 4,507 | 3,494 | |||||
Accrued compensation and other employee benefits | 8,634 | 8,240 | |||||
Customer deposits | 8,945 | 610 | |||||
Deferred revenue, current portion | 9,229 | 19,033 | |||||
Deferred rent, current portion | 512 | 13 | |||||
Lease financing obligations, current portion | — | 58 | |||||
Total current liabilities | 35,919 | 36,383 | |||||
Deferred revenue, net of current portion | 3,304 | 22,664 | |||||
Deferred rent and other liabilities, net of current portion | 8,499 | 7,655 | |||||
Long-term debt and lease financing obligations, net of current portion and debt issuance costs | 48,931 | 47,366 | |||||
Total liabilities | 96,653 | 114,068 | |||||
Commitments and contingencies (Note 13) | |||||||
Stockholders’ equity | |||||||
Preferred stock, $0.0001 par value, 15,000 shares authorized; none issued | — | — | |||||
Common stock, $0.0001 par value, 150,000 shares authorized; 25,421 and 21,528 shares issued and outstanding at December 31, 2017 and 2016, respectively | 2 | 2 | |||||
Additional paid-in-capital | 353,308 | 281,900 | |||||
Other comprehensive loss | (99 | ) | (57 | ) | |||
Accumulated deficit | (313,102 | ) | (269,540 | ) | |||
Total stockholders’ equity | 40,109 | 12,305 | |||||
Total liabilities and stockholders’ equity | $ | 136,762 | $ | 126,373 | |||
The accompanying notes are an integral part of these consolidated financial statements.
-68-
NanoString Technologies, Inc.
Consolidated Statements of Operations
Years Ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
(In thousands, except per share amounts) | |||||||||||
Revenue: | |||||||||||
Product and service | $ | 72,010 | $ | 69,134 | $ | 56,639 | |||||
Collaboration | 42,895 | 17,355 | 6,028 | ||||||||
Total revenue | 114,905 | 86,489 | 62,667 | ||||||||
Costs and expenses: | |||||||||||
Cost of product and service revenue | 31,880 | 30,245 | 26,126 | ||||||||
Research and development | 46,888 | 34,720 | 24,597 | ||||||||
Selling, general and administrative | 74,334 | 62,700 | 53,186 | ||||||||
Total costs and expenses | 153,102 | 127,665 | 103,909 | ||||||||
Loss from operations | (38,197 | ) | (41,176 | ) | (41,242 | ) | |||||
Other income (expense): | |||||||||||
Interest income | 809 | 390 | 233 | ||||||||
Interest expense | (6,153 | ) | (5,672 | ) | (4,017 | ) | |||||
Other income (expense) | 183 | (515 | ) | (389 | ) | ||||||
Total other income (expense) | (5,161 | ) | (5,797 | ) | (4,173 | ) | |||||
Net loss before provision for income taxes | (43,358 | ) | (46,973 | ) | (45,415 | ) | |||||
Provision for income taxes | (204 | ) | (116 | ) | (166 | ) | |||||
Net loss | $ | (43,562 | ) | $ | (47,089 | ) | $ | (45,581 | ) | ||
Net loss per share—basic and diluted | $ | (1.84 | ) | $ | (2.34 | ) | $ | (2.40 | ) | ||
Weighted average shares used in computing basic and diluted net loss per share | 23,731 | 20,116 | 19,027 | ||||||||
The accompanying notes are an integral part of these consolidated financial statements.
-69-
NanoString Technologies, Inc.
Consolidated Statements of Comprehensive Loss
Years Ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
(In thousands) | |||||||||||
Net loss | $ | (43,562 | ) | $ | (47,089 | ) | $ | (45,581 | ) | ||
Other comprehensive income (loss): | |||||||||||
Change in unrealized gain (loss) on short-term investments | (42 | ) | (28 | ) | 14 | ||||||
Comprehensive loss | $ | (43,604 | ) | $ | (47,117 | ) | $ | (45,567 | ) | ||
The accompanying notes are an integral part of these consolidated financial statements.
-70-
NanoString Technologies, Inc.
Consolidated Statements of Changes in Stockholders’ Equity
Common Stock | Additional Paid-in Capital | Other Comprehensive Loss | Accumulated Deficit | Total Stockholders’ Equity | ||||||||||||||||||
Shares | Amount | |||||||||||||||||||||
(In thousands) | ||||||||||||||||||||||
Balances at January 1, 2015 | 18,272 | $ | 2 | $ | 221,724 | $ | (43 | ) | $ | (176,870 | ) | $ | 44,813 | |||||||||
Issuance of common stock net of issuance costs of $0.5 million | 960 | — | 12,518 | — | — | 12,518 | ||||||||||||||||
Issuance of common stock for employee stock purchase plan | 136 | — | 1,295 | — | — | 1,295 | ||||||||||||||||
Exercise of stock options | 202 | — | 876 | — | — | 876 | ||||||||||||||||
Exercise of common stock warrants, net | — | — | 2 | — | — | 2 | ||||||||||||||||
Stock-based compensation | — | — | 6,278 | — | — | 6,278 | ||||||||||||||||
Net loss | — | — | — | — | (45,581 | ) | (45,581 | ) | ||||||||||||||
Other comprehensive loss | — | — | — | 14 | — | 14 | ||||||||||||||||
Balances at December 31, 2015 | 19,570 | 2 | 242,693 | (29 | ) | (222,451 | ) | 20,215 | ||||||||||||||
Issuance of common stock net of issuance costs of $1.0 million | 1,333 | — | 26,073 | — | — | 26,073 | ||||||||||||||||
Issuance of common stock for employee stock purchase plan | 139 | — | 1,489 | — | — | 1,489 | ||||||||||||||||
Exercise of stock options | 349 | — | 2,607 | — | — | 2,607 | ||||||||||||||||
Exercise of common stock warrants, net | 133 | — | — | — | — | — | ||||||||||||||||
Vesting of restricted stock units | 5 | — | — | — | — | — | ||||||||||||||||
Stock-based compensation | — | — | 9,038 | — | — | 9,038 | ||||||||||||||||
Net loss | — | — | — | — | (47,089 | ) | (47,089 | ) | ||||||||||||||
Other comprehensive income | — | — | — | (28 | ) | — | (28 | ) | ||||||||||||||
Balances at December 31, 2016 | 21,529 | 2 | 281,900 | (57 | ) | (269,540 | ) | 12,305 | ||||||||||||||
Issuance of common stock net of issuance costs of $1.3 million | 3,450 | — | 56,486 | — | — | 56,486 | ||||||||||||||||
Issuance of common stock warrants | — | — | 674 | — | — | 674 | ||||||||||||||||
Issuance of common stock for employee stock purchase plan | 139 | — | 1,793 | — | — | 1,793 | ||||||||||||||||
Exercise of stock options | 228 | — | 1,086 | — | — | 1,086 | ||||||||||||||||
Exercise of common stock warrants, net | 29 | — | — | — | — | — | ||||||||||||||||
Vesting of restricted stock units | 46 | — | — | — | — | — | ||||||||||||||||
Stock-based compensation | — | — | 11,369 | — | — | 11,369 | ||||||||||||||||
Net loss | — | — | — | — | (43,562 | ) | (43,562 | ) | ||||||||||||||
Other comprehensive income | — | — | — | (42 | ) | — | (42 | ) | ||||||||||||||
Balances at December 31, 2017 | 25,421 | $ | 2 | $ | 353,308 | $ | (99 | ) | $ | (313,102 | ) | $ | 40,109 | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
-71-
NanoString Technologies, Inc.
Consolidated Statements of Cash Flows
Years Ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
(In thousands) | |||||||||||
Operating activities | |||||||||||
Net loss | $ | (43,562 | ) | $ | (47,089 | ) | $ | (45,581 | ) | ||
Adjustments to reconcile net loss to net cash used in operating activities | |||||||||||
Depreciation and amortization | 3,354 | 2,977 | 2,377 | ||||||||
Stock-based compensation expense | 11,369 | 9,038 | 6,278 | ||||||||
Amortization (accretion) of discount or premium on short-term investments | 198 | (20 | ) | 270 | |||||||
Interest accrued on long-term debt | 171 | 158 | 18 | ||||||||
Conversion of accrued interest to long-term debt | 1,472 | 1,357 | 1,067 | ||||||||
(Gain) loss on disposal of property and equipment | 15 | (2 | ) | 3 | |||||||
Provision for bad debt | 361 | — | 34 | ||||||||
Provision for inventory obsolescence | 866 | 822 | 248 | ||||||||
Changes in operating assets and liabilities | |||||||||||
Accounts receivable | 2,277 | (2,476 | ) | (7,328 | ) | ||||||
Inventory | (8,742 | ) | (5,857 | ) | (5,602 | ) | |||||
Prepaid expenses and other | (911 | ) | 72 | 1,199 | |||||||
Other assets | (367 | ) | 37 | (7 | ) | ||||||
Accounts payable | (110 | ) | 869 | (166 | ) | ||||||
Accrued liabilities | 1,312 | (40 | ) | (80 | ) | ||||||
Accrued compensation and other employee benefits | 295 | 281 | 1,242 | ||||||||
Customer deposits | 8,335 | 610 | — | ||||||||
Deferred revenue | (29,161 | ) | 29,948 | (127 | ) | ||||||
Deferred rent and other liabilities | 1,171 | 3,236 | 2,793 | ||||||||
Net cash used in operating activities | (51,657 | ) | (6,079 | ) | (43,362 | ) | |||||
Investing activities | |||||||||||
Purchases of property and equipment | (4,284 | ) | (3,991 | ) | (3,796 | ) | |||||
Proceeds from sale of property and equipment | — | 4 | 6 | ||||||||
Proceeds from sale of short-term investments | 3,600 | 4,700 | 3,000 | ||||||||
Proceeds from maturity of short-term investments | 79,599 | 34,800 | 57,309 | ||||||||
Purchases of short-term investments | (81,405 | ) | (65,774 | ) | (32,750 | ) | |||||
Net cash (used in) provided by investing activities | (2,490 | ) | (30,261 | ) | 23,769 | ||||||
Financing activities | |||||||||||
Proceeds from long-term debt | — | 5,000 | 10,000 | ||||||||
Repayment of long-term debt and lease financing obligations | (58 | ) | (226 | ) | (271 | ) | |||||
Proceeds from sale of common stock, net | 56,486 | 26,223 | 12,518 | ||||||||
Proceeds from issuance of common stock warrants | 674 | — | — | ||||||||
Proceeds from exercise of common stock warrants | — | — | 2 | ||||||||
Proceeds from issuance of common stock for employee stock purchase plan | 1,793 | 1,489 | 1,295 | ||||||||
Deferred offering costs | — | — | (152 | ) | |||||||
Tax withholdings related to net share settlements of restricted stock units | (313 | ) | — | — | |||||||
Proceeds from exercise of stock options | 1,086 | 2,607 | 876 | ||||||||
Net cash provided by financing activities | 59,668 | 35,093 | 24,268 | ||||||||
Net increase (decrease) in cash and cash equivalents | 5,521 | (1,247 | ) | 4,675 | |||||||
Effect of exchange rate changes on cash and cash equivalents | 32 | (26 | ) | (42 | ) | ||||||
Cash and cash equivalents | |||||||||||
Beginning of year | 20,583 | 21,856 | 17,223 | ||||||||
End of year | $ | 26,136 | $ | 20,583 | $ | 21,856 | |||||
-72-
NanoString Technologies, Inc.
Consolidated Statements of Cash Flows (continued)
Years Ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
(In thousands) | |||||||||||
Supplemental disclosures | |||||||||||
Cash paid for interest | $ | 4,416 | $ | 4,071 | $ | 2,844 | |||||
Cash paid for taxes | 154 | 217 | 69 | ||||||||
Purchases of property and equipment, accrued but not paid | — | 275 | 640 | ||||||||
Rental instruments reclassified from inventory | 1,023 | 801 | 772 | ||||||||
Non-cash inventory exchange for services | — | 28 | 112 | ||||||||
Non-cash capital lease | — | — | 48 | ||||||||
Accrual of offering costs | — | — | 29 | ||||||||
The accompanying notes are an integral part of these consolidated financial statements.
-73-
NanoString Technologies, Inc.
Notes to Consolidated Financial Statements
1. Description of the Business
NanoString Technologies, Inc. (the “Company”) was incorporated in the state of Delaware on June 20, 2003. The Company’s headquarters is located in Seattle, Washington. The Company’s technology enables direct detection, identification and quantification of individual target molecules in a biological sample by attaching a unique color coded fluorescent reporter to each target molecule of interest. The Company markets its proprietary nCounter Analysis System, consisting of instruments and consumables, including its Prosigna Breast Cancer Assay, to academic, government and biopharmaceutical and clinical laboratory customers. In addition, the Company collaborates with biopharma companies to develop companion diagnostic tests for various cancer therapies.
The Company has incurred losses to date and expects to incur additional losses in the foreseeable future. The Company continues to devote the majority of its resources to the growth of its business in accordance with its business plan. The Company’s activities have been financed primarily through the sale of equity securities and incurrence of indebtedness, and to a lesser extent, capital leases and other borrowings.
Public Offerings
In May 2015, the Company entered into a sales agreement with a sales agent to sell shares of the Company’s common stock through an “at the market” equity offering program for up to $40.0 million in total sales proceeds. Pursuant to the sales agreement, the Company sold 1,331,539 and 960,400 shares during 2016 and 2015, respectively, for net proceeds of $26.1 million and $12.5 million, respectively. The Sales Agreement automatically terminated when the Company sold the maximum number of shares allowed under the agreement.
In June 2017, the Company completed an underwritten public offering of 3,450,000 shares of common stock, including the exercise by the underwriter of an over-allotment option for 450,000 shares of common stock, for total gross proceeds of $57.8 million. After underwriter’s fees and commissions and other expenses of the offering, the Company’s aggregate net proceeds were approximately $56.5 million.
2. Significant Accounting Policies
Accounting Principles and Principles of Consolidation
The consolidated financial statements and accompanying notes were prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). The accompanying consolidated financial statements reflect the accounts of the Company and its wholly-owned subsidiaries. Each of the subsidiaries operates as a sales and support office. The functional currency of each subsidiary is the U.S. dollar. All significant intercompany balances and transactions have been eliminated.
Use of Estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and that affect the reported amounts of revenue and expenditures during the reporting period. Actual results could differ from those estimates. Significant estimates inherent in the preparation of the accompanying consolidated financial statements include the estimation of the valuation of inventory, the fair value of the Company’s equity securities, the calculation of stock-based compensation and the estimated future cost of ongoing collaboration agreements, for which revenues are recognized on a proportional performance basis.
Cash and Cash Equivalents
The Company considers all highly-liquid investments with purchased maturities of three months or less to be cash equivalents. The Company’s cash equivalents consist principally of funds maintained in depository accounts. The Company invests its cash and cash equivalents with major financial institutions; at times these investments exceed federally insured limits.
Investments
The Company classifies its securities as available-for-sale, which are reported at estimated fair value with unrealized gains and losses included in accumulated other comprehensive loss in stockholders’ equity. Realized gains, realized losses and declines in the value of securities judged to be other-than-temporary, are included in other income (expense). The cost of
-74-
investments for purposes of computing realized and unrealized gains and losses is based on the specific identification method. Amortization of premiums and accretion of discounts are included in other income (expense). Interest and dividends earned on all securities are included in other income (expense). Investments in securities with maturities of less than one year, or where management’s intent is to use the investments to fund current operations, or to make them available for current operations, are classified as short-term investments.
If the estimated fair value of a security is below its carrying value, the Company evaluates whether it is more likely than not that it will sell the security before its anticipated recovery in market value and whether evidence indicating that the cost of the investment is recoverable within a reasonable period of time outweighs evidence to the contrary. The Company also evaluates whether or not it intends to sell the investment. If the impairment is considered to be other-than-temporary, the security is written down to its estimated fair value. In addition, the Company considers whether credit losses exist for any securities. A credit loss exists if the present value of cash flows expected to be collected is less than the amortized cost basis of the security. Other-than-temporary declines in estimated fair value and credit losses are charged against other income (expense).
Accounts Receivable and Allowance for Doubtful Accounts
Accounts receivable are stated at the amount management expects to collect from customers based on their outstanding invoices. Management reviews accounts receivable regularly to determine if any receivable will potentially be uncollectible and to estimate the amount of allowance for doubtful accounts necessary to reduce accounts receivable to its estimated net realizable value by analyzing the status of significant past due receivables. The allowance for doubtful accounts was $0.5 million as of December 31, 2017, and $0.1 million as of December 31, 2016, 2015, and 2014. Additions to the allowance were $0.4 million, $0 and $33,600 for the years ended December 31, 2017, 2016 and 2015, respectively. There were write-offs of uncollectible accounts of approximately $1,200 and $5,000 during the years ended December 31, 2017 and 2016, respectively, and there were no write-offs during the year ended December 31, 2015.
Concentration of Credit Risks
Financial instruments that potentially expose the Company to concentrations of credit risk consist principally of cash and cash equivalents, short-term investments and accounts receivable. Cash is invested in accordance with the Company’s investment policy, which includes guidelines intended to minimize and diversify credit risk. Most of the Company’s investments are not federally insured. The Company has credit risk related to the collectability of its accounts receivable. The Company performs initial and ongoing evaluations of its customers’ credit history or financial position and generally extends credit on account without collateral. The Company has not experienced any significant credit losses to date.
The Company had two customers/collaborators, (1) Merck Sharp & Dohme Corp., a subsidiary of Merck& Co., Inc. (“Merck”), and (2) Medivation, Inc. and Astellas Pharma Inc., that represented 25% and 10%, respectively, of total revenue for the year ended December 31, 2017 and one customer/collaborator, Merck, that represented 13% of total revenue for the year ended December 31, 2016. The Company had no customers or collaborators that represented more than 10% of total revenue for the year 2015. The Company had no customers or collaborators that represented more than 10% of total accounts receivable as of December 31, 2017 and 2016.
The Company is also subject to supply chain risks related to the outsourcing of the manufacturing of its instruments to sole suppliers. Although there are a limited number of manufacturers for instruments of this type, the Company believes that other suppliers could provide similar products on comparable terms. A change in suppliers, however, could cause a delay in manufacturing and a possible loss of sales, which would adversely affect operating results.
Fair value of financial instruments
The recorded amounts of certain financial instruments, including cash and cash equivalents, accounts receivable, prepaid expenses and other assets, accounts payable and accrued liabilities approximate fair value due to their relatively short maturities. Investments that are classified as available-for-sale are recorded at fair value. The fair value for securities held is determined using quoted market prices, broker or dealer quotations, or alternative pricing sources with reasonable levels of price transparency. The recorded amount of the Company’s long-term debt approximates fair value because the related interest rates approximate rates currently available to the Company.
-75-
Inventory
Inventory consists of finished goods, work in process, raw materials and certain component parts to be used in manufacturing or servicing the Company’s products. Inventory is stated at the lower of cost or net realizable value. Cost is determined using a standard cost system, whereby the standard costs are updated periodically to reflect current costs and market represents the lower of cost or market (replacement cost or estimated net realizable value). The Company’s policy is to establish inventory reserves when conditions exist that suggest that inventory may be in excess of anticipated demand, obsolete, slow moving or impaired. In the event that the Company identifies these conditions exist in its inventory, its carrying value is reduced to its net realizable value. Inventory reserves were $2.7 million as of December 31, 2017 and $2.2 million as of December 31, 2016, 2015, and 2014. Additions to the reserves were $0.9 million, $0.8 million and $0.2 million for the years ended December 31, 2017, 2016 and 2015, respectively. Write-offs of inventory reserves for the years ended December 31, 2017, 2016 and 2015 were $0.4 million, $0.7 million and $0.3 million, respectively.
The Company outsources the manufacturing of its instruments to third-party contract manufacturers who manufacture them to certain specifications and source certain raw materials from sole source providers. Major delays in shipments, inferior quality, insufficient quantity or any combination of these or other factors may harm the Company’s business and results of operations. In addition, the inability of one or more of these suppliers to provide the Company with an adequate supply of its products or raw materials or the loss of one or more of these suppliers may cause a delay in the Company’s ability to fulfill orders while it obtains a replacement supplier and may harm the Company’s business and results of operations.
Property and Equipment
Property and equipment are recorded at cost, net of accumulated depreciation and amortization. Depreciation and amortization are computed using the straight-line method over the estimated useful lives of the assets. Manufacturing equipment is depreciated over five years, lease and loaner instruments are depreciated over one to five years, prototype systems are depreciated over two years, computer equipment is generally depreciated over three years, furniture and fixtures are depreciated over five years and leasehold improvements are amortized over the life of the related assets or the term of the lease, whichever is shorter. Expenditures for additions are capitalized and expenditures for maintenance and repairs are expensed as incurred. Gains and losses from the disposal of property and equipment are reflected in the consolidated statements of operations in the period of disposition.
Leases and Leasehold Improvements
Rent expense for leases that provide for scheduled rent increases during the lease term is recognized on a straight-line basis over the term of the related lease. Leasehold improvements that are funded by landlord incentives or allowances are recorded in property and equipment and as a component of deferred rent and are amortized as a reduction of rent expense over the term of the related lease.
Impairment of Long-Lived Assets
The Company recognizes impairment losses on long-lived assets when indicators of impairment are present and the anticipated undiscounted cash flows to be generated by those assets are less than the asset’s carrying values. The Company has not experienced any impairment losses on its long-lived assets during the periods presented.
Segments
Operating segments are defined as components of an entity for which separate financial information is available and evaluated regularly by the chief operating decision maker in deciding how to allocate resources and in assessing performance. The Company’s chief operating decision maker is the chief executive officer, who manages the operations and evaluates the financial performance on a total Company basis. The Company’s principal operations and decision-making functions are located at its corporate headquarters in the United States and the Company operates as a single reportable segment.
Revenue Recognition
The Company recognizes revenue when (1) persuasive evidence of an arrangement exists, (2) delivery has occurred or services have been rendered, (3) the price to the customer is fixed or determinable and (4) collectability is reasonably assured. The Company generates revenue from the sale of products and services. The Company’s products consist of its proprietary nCounter Analysis System and related consumables. Services consist of extended warranties and service fees for access to new technologies still under development or for assay processing. A delivered product or service is considered to be a separate unit of accounting when it has value to the customer on a stand-alone basis. Products or services have value on a stand-alone basis if they are sold separately by any vendor or the customer could resell the delivered product.
Instruments, consumables and in vitro diagnostic kits are considered to be separate units of accounting as they are sold separately and revenue is recognized upon transfer of ownership, which is generally upon shipment. Instrument revenue related
-76-
to installation and calibration services is recognized when services are rendered by the Company. Such services can also be provided by the Company’s distribution partners. For instruments sold for use primarily to run Prosigna assays for diagnostic purposes, training must be provided prior to instrument revenue recognition. Instrument revenue from leased instruments is recognized ratably over the lease term.
Service revenue is recognized when earned, which is generally upon the rendering of the related services. Service agreements and service fees for assay processing are each considered separate units of accounting as they are sold separately. The Company offers service agreements on its nCounter Analysis System for periods ranging from 12 to 36 months after the end of the standard 12-month warranty period. Service agreements are generally separately priced. Revenue from service agreements is deferred and recognized on a straight-line basis over the service period.
For arrangements with multiple deliverables, the Company allocates the agreement consideration at the inception of the agreement to the deliverables based upon their relative selling prices. To date, selling prices have been established by reference to vendor specific objective evidence based on stand-alone sales transactions for each deliverable. Vendor specific objective evidence is considered to have been established when a substantial majority of individual sales transactions within the previous 12-month period fall within a reasonably narrow range, which the Company has defined to be plus or minus 15% of the median sales price of actual stand-alone sales transactions. The Company uses its best estimate of selling price for individual deliverables when vendor specific objective evidence or third-party evidence is unavailable. Allocated revenue is only recognized for each deliverable when the revenue recognition criteria have been met.
The Company enters into collaborative agreements that may generate upfront fees with subsequent milestone payments that may be earned upon completion of development-related milestones. The Company is able to estimate the total cost of services under the arrangements and recognizes collaboration revenue using a proportional performance model. Costs incurred to date compared to total expected costs are used to determine proportional performance, as this is considered to be representative of the delivery of outputs under the arrangements. Revenue recognized at any point in time is limited to cash received and amounts contractually due. Changes in estimates of total expected costs are accounted for prospectively as a change in estimate. From period to period, collaboration revenue can fluctuate substantially based on the achievement of development-related milestones.
Cost of Revenue
Cost of revenue consists primarily of costs incurred in the production process, including costs of purchasing instruments from third-party contract manufacturers, consumable component materials and assembly labor and overhead, installation, warranty, service and packaging and delivery costs. In addition, cost of revenue includes royalty costs for licensed technologies included in the Company’s products, provisions for slow-moving and obsolete inventory and stock-based compensation expense. Cost of revenue for instruments and consumables is recognized in the period the related revenue is recognized. Shipping and handling costs incurred for product shipments are included in cost of revenue in the consolidated statements of operations.
Reserve for Product Warranties
The Company generally provides a one-year warranty on its nCounter Analysis Systems and establishes a reserve for future warranty costs based on historical product failure rates and actual warranty costs incurred. Warranty expense is recorded as a component of cost of revenue in the consolidated statements of operations.
Research and Development
Research and development expenses, consisting primarily of salaries and benefits, occupancy costs, laboratory supplies, clinical study costs, contracted services, consulting fees and related costs, are expensed as incurred.
Selling, General and Administrative
Selling expenses consist primarily of personnel related costs for sales and marketing, contracted services and service fees and are expensed as the related costs are incurred. Advertising costs are expensed as incurred and are included in sales and marketing expenses. Advertising costs totaled approximately $5.9 million, $5.3 million and $2.6 million during the years ended December 31, 2017, 2016 and 2015, respectively.
General and administrative expenses consist primarily of personnel related costs for the Company’s finance, human resources, business development, legal, information technology and general management, as well as professional fees for legal, accounting, and other consulting services. General and administrative expenses are expensed as they are incurred.
-77-
Income Taxes
The Company accounts for income taxes under the liability method. Under the liability method, deferred tax assets and liabilities are determined based on the differences between the financial reporting and income tax bases of assets and liabilities and are measured using the tax rates that will be in effect when the differences are expected to reverse. A valuation allowance is recorded when it is more likely than not that some of the deferred tax assets will not be realized.
The Company determines whether a tax position is more likely than not to be sustained upon examination based on the technical merits of the position. For tax positions meeting the more-likely-than-not threshold, the tax amount recognized in the financial statements is reduced by the largest benefit that has a greater than 50% likelihood of being realized upon ultimate settlement with the relevant tax authority.
Stock-Based Compensation
The Company accounts for stock-based compensation under the fair value method. Stock-based compensation costs are based on option awards granted and vested based on their grant-date fair value, estimated using the Black-Scholes option pricing model. The Company uses the straight-line attribution method for recognizing compensation expense.
Guarantees and Indemnifications
In the normal course of business, the Company guarantees and/or indemnifies other parties, including vendors, lessors and parties to transactions with the Company, with respect to certain matters. The Company has agreed to hold the other parties harmless against losses arising from breach of representations or covenants, or out of intellectual property infringement or other claims made against certain parties. It is not possible to determine the maximum potential amount the Company could be required to pay under these indemnification agreements, since the Company has not had any prior indemnification claims, and each claim would be based upon the unique facts and circumstances of the claim and the particular provisions of each agreement. In the opinion of management, any such claims would not be expected to have a material adverse effect on the Company’s consolidated results of operations, financial condition or cash flows. The Company did not have any related liabilities recorded at December 31, 2017 and 2016.
Comprehensive Loss
Comprehensive loss includes certain changes in equity that are excluded from net loss. Specifically, unrealized gains and losses on short-term investments are included in comprehensive loss.
Recently Adopted Accounting Pronouncement
In July 2015, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) entitled “ASU 2015-11, Inventory – Simplifying the Measurement of Inventory.” The standard requires entities to measure inventory at the lower of cost and net realizable value. The Company adopted ASU 2015-11 in the first quarter of 2017 and adoption did not have a material impact on its consolidated results of operations, financial condition, cash flows, and financial statement disclosures.
In March 2016, FASB issued “ASU 2016-09, Improvements to Employee Share-Based Payment Accounting” which amends Accounting Standard Codification Topic 718, “Compensation – Stock Compensation”. The standard includes provisions intended to simplify various aspects related to the accounting and presentation for stock-based payments in the financial statements, including the income tax effects of stock-based payments, minimum withholding requirements upon award settlement, and the method of calculating forfeitures in the recognition of stock compensation expense.
The Company adopted ASU 2016-09 in the first quarter of 2017 and has elected to account for forfeitures as they occur to determine the amount of compensation cost to be recognized. The accounting policy election was adopted applying a modified retrospective approach, and did not have a material impact on the consolidated results of operations, financial condition, cash flows, or financial statement disclosures. Employee taxes paid for withheld shares are presented as a financing activity in the consolidated statements of cash flows, as required by the new standard, and was adopted retrospectively. Other provisions of ASU 2016-09 related to the accounting for the tax effects of stock-based payments have no impact on its consolidated results of operations, as the Company records a valuation allowance for deferred tax assets related to excess tax benefits from stock-based payment transactions.
Recent Accounting Pronouncements
As an “emerging growth company,” the Jumpstart Our Business Startups Act allows the Company to delay adoption of new or revised accounting pronouncements applicable to public companies until such pronouncements are made applicable to private companies. As a result, the Company's financial statements may not be comparable to the financial statements of issuers
-78-
who are required to comply with the effective dates for new or revised accounting standards that are applicable to public companies.
In May 2014, FASB issued “ASU 2014-09, Revenue from Contracts with Customers.” The standard requires an entity to recognize the amount of revenue to which it expects to be entitled for the transfer of promised goods or services to a customer. In March 2016, the FASB issued “ASU 2016-08, Principal vs Agent Considerations (Reporting Revenue Gross versus Net)” which clarifies the implementation guidance on principal versus agent considerations. In April 2016, the FASB issued “ASU 2016-10, Identifying Performance Obligations and Licensing” which clarifies the implementation guidance on identifying performance obligations and the licensing implementation guidance. In May 2016, the FASB issued “ASU 2016-12, Narrow-Scope Improvements and Practical Expedients” which provides practical expedients for contract modifications and clarification on assessing the collectability criterion, presentation of sales taxes, measurement date for noncash consideration and completed contracts at transition.
The Company has formed an implementation team and completed its preliminary assessment of the potential impact of implementing this new standard. The assessment included an analysis of the Company’s current portfolio of customer contracts and related costs, as well as a review of its historical accounting policies and practices to identify potential differences in applying the new standard. The Company has determined that its collaborative agreements fall within the scope of ASC 808, Collaborative Arrangements and intends to apply the principles of ASC 606 in the measurement and recognition of revenue. In addition, the Company has concluded that service contracts will no longer be accounted for under separate accounting guidance, but rather included as separate performance obligations within a contract subject to the new standard, which includes their inclusion in the determination and allocation of the aggregate transaction price based on their relative values, and recognition of revenue upon the delivery of the performance obligation. We have determined that such service contracts comprise two performance obligations, one related to an included right to an annual preventative maintenance service included within the agreements which will be recognized when performed or the right expires, and another related to the Company's stand-ready obligations under the service contracts will be recognized over the service period. The new standard also requires more extensive disclosures related to revenue recognition, particularly in quarterly financial statements. The Company adopted the new guidance on January 1, 2018 using the modified retrospective method of adoption, has evaluated the impact of the standard on all of its revenues, including those mentioned above, and concluded the adoption of the standard will not have a material impact on its consolidated results of operations, financial condition, or cash flows.
In January 2016, FASB issued “ASU 2016-01, Financial Instruments: Overall.” The standard addresses certain aspects of recognition, measurement, presentation and disclosure of financial instruments. The standard will become effective for the Company beginning January 1, 2018. The Company does not anticipate adoption of this standard will have a material impact on its consolidated results of operations, financial condition, cash flows, and financial statement disclosures.
In February 2016, FASB issued “ASU 2016-02, Leases – Recognition and Measurement of Financial Assets and Financial Liabilities.” The standard requires the recognition of lease assets and lease liabilities by lessees for those leases classified as operating leases. Leases will be classified as either finance or operating, with classification affecting the pattern of expense recognition. The standard requires lessors to classify leases as either sales-type, finance or operating. A sales-type lease occurs if the lessor transfers all of the risks and rewards, as well as control of the underlying asset, to the lessee. If risks and rewards are conveyed without the transfer of control, the lease is treated as a financing lease. If the lessor does not convey risks and rewards or control, an operating lease results. The standard will become effective for the Company beginning January 1, 2019. The Company is currently assessing the impact adoption of this standard will have on its consolidated results of operations, financial condition, cash flows, and financial statement disclosures.
In June 2016, FASB issued “ASU 2016-13, Financial Instruments: Credit Losses”. The standard provides information about expected credit losses on financial instruments at each reporting date, and to change how other than temporary impairments on investments securities are recorded. The standard will become effective for the Company beginning on January 1, 2020 with early adoption permitted. The Company is currently assessing the impact adoption of this standard will have on its consolidated results of operations, financial condition, cash flows, and financial statement disclosures.
In August 2016, FASB issued “ASU No. 2016-15, Statement of Cash Flows: Classification of Certain Cash Receipts and Cash Payments”. The standard provides guidance on how certain cash receipts and cash payments are presented and classified in the statement of cash flows and is intended to reduce diversity in practice with respect to these items. The standard is applied using a retrospective transition method and will become effective for the Company on January 1, 2018. The Company does not anticipate adoption of this standard will have a material impact on its consolidated results of operations, financial condition, cash flows, and financial statement disclosures.
In November 2016, FASB issued “ASU 2016-18, Statement of Cash Flows: Restricted Cash”. The standard requires companies to include amounts generally described as restricted cash and restricted cash equivalents, along with cash and cash equivalents, when reconciling the beginning-of-period and end-of-period amounts shown on the statement of cash flows. The standard was adopted by the Company on January 1, 2018. The Company does not anticipate adoption of this standard will
-79-
have a material impact on its consolidated results of operations, financial condition, cash flows, and financial statement disclosures.
In May 2017, FASB issued “ASU 2017-09, Compensation - Stock Compensation: Scope of Modification Accounting”. The standard clarifies which changes to the terms or conditions of a share-based payment award are required to be accounted for as modifications. The standard was adopted by the Company on January 1, 2018. The adoption of this standard did not have a material impact on its consolidated results of operations, financial condition, cash flows, and financial statement disclosures.
3. Short-term Investments
Short-term investments consisted of available-for-sale securities as follows (in thousands):
Type of securities as of December 31, 2017 | Amortized cost | Gross unrealized gains | Gross unrealized losses | Fair value | |||||||||||
Corporate debt securities | $ | 35,567 | $ | — | $ | (53 | ) | $ | 35,514 | ||||||
U.S. government-related debt securities | 15,951 | — | (46 | ) | 15,905 | ||||||||||
Total available-for-sale securities | $ | 51,518 | $ | — | $ | (99 | ) | $ | 51,419 | ||||||
Type of securities as of December 31, 2016 | Amortized cost | Gross unrealized gains | Gross unrealized losses | Fair value | |||||||||||
Corporate debt securities | $ | 36,198 | $ | 4 | $ | (42 | ) | $ | 36,160 | ||||||
U.S. government-related debt securities | 17,312 | 1 | (20 | ) | 17,293 | ||||||||||
Total available-for-sale securities | $ | 53,510 | $ | 5 | $ | (62 | ) | $ | 53,453 | ||||||
The fair values of available-for-sale securities by contractual maturity at December 31 were as follows (in thousands):
2017 | 2016 | ||||||
Maturing in one year or less | $ | 39,985 | $ | 46,310 | |||
Maturing in one to three years | 11,434 | 7,143 | |||||
Total available-for-sale securities | $ | 51,419 | $ | 53,453 | |||
The Company has both the intent and ability to sell its available-for-sale investments maturing greater than one year within 12 months from the balance sheet date and, accordingly, has classified these securities as current in the consolidated balance sheet.
The following table summarizes investments that have been in a continuous unrealized loss position as of December 31, 2017 (in thousands).
Less Than 12 Months | 12 Months or Greater | Total | |||||||||||||||||||||
Fair value | Gross unrealized losses | Fair value | Gross unrealized losses | Fair value | Gross unrealized losses | ||||||||||||||||||
Corporate debt securities | $ | 26,857 | $ | (53 | ) | $ | — | $ | — | $ | 26,857 | $ | (53 | ) | |||||||||
U.S. government-related debt securities | 8,911 | (40 | ) | 3,994 | (6 | ) | 12,905 | (46 | ) | ||||||||||||||
Total | $ | 35,768 | $ | (93 | ) | $ | 3,994 | $ | (6 | ) | $ | 39,762 | $ | (99 | ) | ||||||||
The Company invests in securities that are rated investment grade or better. The unrealized losses on investments as of December 31, 2017 and December 31, 2016 were primarily caused by interest rate increases.
The Company reviews the individual securities in its portfolio to determine whether a decline in a security’s fair value below the amortized cost basis is other-than-temporary. The Company determined that as of December 31, 2017, there were no investments in its portfolio that were other-than-temporarily impaired.
4. Fair Value Measurements
-80-
The Company establishes the fair value of its assets and liabilities using the price that would be received to sell an asset or paid to transfer a financial liability in an orderly transaction between market participants at the measurement date. A fair value hierarchy is used to measure fair value. The three levels of the fair value hierarchy are as follows:
• | Level 1 — Quoted prices in active markets for identical assets and liabilities. |
• | Level 2 — Quoted prices for similar instruments in active markets; quoted prices for identical or similar instruments in markets that are not active; and model-derived valuations in which all significant inputs and significant value drivers are observable in active markets. |
• | Level 3 — Valuations derived from valuation techniques in which one or more significant inputs or significant value drivers are unobservable. |
The Company’s available-for-sale securities by level within the fair value hierarchy were as follows (in thousands):
Type of securities as of December 31, 2017 | Fair value measurement using: | ||||||||||||||
Level 1 | Level 2 | Level 3 | Total | ||||||||||||
Cash equivalents: | |||||||||||||||
Money market fund | $ | 22,398 | $ | — | $ | — | $ | 22,398 | |||||||
Short-term investments: | |||||||||||||||
Corporate debt securities | — | 35,514 | — | 35,514 | |||||||||||
U.S. government-related debt securities | — | 15,905 | — | 15,905 | |||||||||||
Total | $ | 22,398 | $ | 51,419 | $ | — | $ | 73,817 | |||||||
Type of securities as of December 31, 2016 | Fair value measurement using: | ||||||||||||||
Level 1 | Level 2 | Level 3 | Total | ||||||||||||
Cash equivalents: | |||||||||||||||
Money market fund | $ | 16,715 | $ | — | $ | — | $ | 16,715 | |||||||
Short-term investments: | |||||||||||||||
Corporate debt securities | — | 36,160 | — | 36,160 | |||||||||||
U.S. government-related debt securities | — | 17,293 | — | 17,293 | |||||||||||
Total | $ | 16,715 | $ | 53,453 | $ | — | $ | 70,168 | |||||||
5. Inventory, Net
Inventory consisted of the following at December 31 (in thousands):
2017 | 2016 | ||||||
Raw materials | $ | 5,743 | $ | 4,277 | |||
Work in process | 4,845 | 4,046 | |||||
Finished goods | 9,469 | 5,489 | |||||
Total inventory | $ | 20,057 | $ | 13,812 | |||
In 2017 and 2016, the Company transferred into property, plant and equipment net amounts totaling $1.0 million and $0.8 million, respectively, of inventory that was leased, loaned, or assigned for internal use in the Company’s facilities.
6. Property and Equipment
Property and equipment consisted of the following at December 31 (in thousands):
-81-
Useful Life (Years) | 2017 | 2016 | |||||||
Manufacturing equipment | 5 | $ | 8,395 | $ | 6,445 | ||||
Lease and loaner instruments | 1 - 5 | 4,106 | 3,581 | ||||||
Prototype instruments | 2 | 2,938 | 2,975 | ||||||
Computer equipment | 3 | 2,067 | 1,592 | ||||||
Furniture and fixtures | 5 | 1,670 | 1,642 | ||||||
Leasehold improvements | Various | 11,971 | 8,878 | ||||||
Construction in progress | 158 | 1,861 | |||||||
Total property and equipment, gross | 31,305 | 26,974 | |||||||
Less: Accumulated depreciation and amortization | (17,248 | ) | (14,816 | ) | |||||
Total property and equipment, net | $ | 14,057 | $ | 12,158 | |||||
Prototype instruments consist of nCounter instruments used in internal testing and other development activities. Accumulated depreciation on lease and loaner instruments was $1.9 million and $1.5 million at December 31, 2017 and 2016, respectively.
Depreciation and amortization expense related to property and equipment for the years ended December 31, 2017, 2016 and 2015 totaled approximately $3.3 million, $2.9 million and $2.3 million, respectively.
7. Long-Term Debt
2014 Term Loan Agreement
In April 2014, the Company entered into a term loan agreement under which it may borrow up to $45.0 million, including an option to defer payment of a portion of the interest that would accrue on the borrowing under the term loan agreement. Upon initial closing, the Company borrowed $20.0 million, the proceeds of which were primarily used to repay the outstanding balance under the Company’s former credit facility plus a related $1.0 million end of term payment, a $0.3 million make-whole premium, and deferred interest. The Company incurred and recorded a total charge to interest expense of $1.4 million related to the repayment of the former credit facility, including a loss on extinguishment of debt of $0.6 million. In October 2014, the Company borrowed an additional $10.0 million under the term loan agreement.
In October 2015, the Company amended the term loan agreement to, among other provisions, increase the maximum borrowing capacity to $60.0 million (excluding deferred interest), reduce the applicable interest rate from 12.5% to 12.0%, extend the interest-only period through March 2021, and extend the final maturity to March 2022. Under the amended agreement, borrowings accrue interest at 12.0% annually, payable quarterly, of which 3.0% can be deferred during the first six years of the term at the Company’s option and paid together with the principal at maturity. The Company has elected to exercise the option to defer a portion of the interest and has recorded $4.3 million of deferred interest through December 31, 2017. In December 2015, the Company borrowed an additional $10.0 million under the terms of the amended agreement. In June 2016, the Company borrowed an additional $5.0 million. At December 31, 2016, the Company's option to borrow $15.0 million more under the amended term loan agreement expired. Total borrowings and deferred interest under the amended term loan agreement were $49.3 million and $47.8 million as of December 31, 2017 and 2016, respectively.
Under the amended term loan agreement, the Company may pay interest-only for the first seven years of the term and principal payments are due in four equal installments during the eighth year of the term. The Company has the option to prepay the term loan, in whole or part, at any time subject to payment of a redemption fee of up to 4%, which declines 1% annually, with no redemption fee payable if prepayment occurs after the fourth year of the loan. In addition, a facility fee equal to 2.0% of the amount borrowed plus any accrued interest is payable at the end of the term or when the loan is repaid in full. A long-term liability of $1.1 million is being accreted using the effective interest method for the facility fee over the term of the loan agreement. Obligations under the term loan agreement are collateralized by substantially all of the Company’s assets.
The term loan agreement contains customary conditions to borrowings, events of default and negative covenants, including covenants that could limit the Company’s ability to, among other things, incur additional indebtedness, liens or other encumbrances, make dividends or other distributions; buy, sell or transfer assets; engage in any new line of business; and enter into certain transactions with affiliates. The term loan agreement also includes a $2.0 million minimum liquidity covenant and revenue-based financial covenants, which was $85.0 million for 2017 with annual increases of $15.0 million for each subsequent fiscal year thereafter. If the Company’s actual revenues are below the minimum annual revenue requirement for any given year, it may avoid a related default by generating proceeds from an equity or subordinated debt issuance equal to the shortfall between its actual revenues and the minimum revenue requirement. The Company was in compliance with its financial covenants as of December 31, 2017.
-82-
The Company incurred $6.2 million, $5.7 million and $4.0 million of interest expense under the term loan agreement for the years ended December 31, 2017, 2016 and 2015, respectively.
Lease Financing Obligations
The Company entered into agreements to lease certain hardware, software and capitalized installation costs, the longest of which expired in June 2017. Ownership of the leased property transferred to the Company at the end of the lease term. The fair value at lease inception was recorded in property, plant and equipment and depreciated over the shorter of the useful life of the assets or the lease term. A total cost of $0.7 million for leased property is included in property and equipment at December 31, 2017 and 2016, with accumulated depreciation of $0.7 million and $0.5 million at December 31, 2017 and 2016, respectively.
Long-term debt and lease financing obligations consisted of the following at December 31 (in thousands):
2017 | 2016 | ||||||
Term loans payable | $ | 49,315 | $ | 47,844 | |||
Lease financing obligations | — | 58 | |||||
Total long-term debt and lease financing obligations | 49,315 | 47,902 | |||||
Unamortized debt issuance costs | (384 | ) | (478 | ) | |||
Current portion of lease financing obligations | — | (58 | ) | ||||
Long-term debt and lease financing obligations, net of debt issuance costs and current portion | $ | 48,931 | $ | 47,366 | |||
Scheduled future payments of principal for outstanding debt and lease financing obligations were as follows at December 31:
2018 | $ | — | |
2019 | — | ||
2020 | — | ||
2021 | 36,987 | ||
2022 | 12,328 | ||
$ | 49,315 | ||
8. Collaboration Agreements
The Company evaluates the statement of operations classification of payments between the participants in each of its collaboration agreements at inception based on the nature of the arrangement, the nature of its business operations and the contractual terms of the arrangement. The Company has determined that amounts to be received from collaborators in connection with the collaboration agreements entered into through December 31, 2017 are related to revenue generating activities.
The Company uses a contingency-adjusted proportional performance model to recognize revenue over the Company’s performance period for each collaboration agreement that includes upfront and/or milestone-based payments. Costs incurred to date compared to total expected costs are used to determine proportional performance, as this is considered to be representative of the delivery of outputs under the arrangement. Revenue recognized at any point in time is a factor of and limited to cash received and amounts contractually due. Changes in estimates of total expected costs are accounted for prospectively.
The Company recognizes revenue from collaboration agreements that do not include upfront and/or milestone-based payments when earned, which is generally in the same period related costs are incurred. Amounts due to collaboration partners are recognized when the related activities have occurred and are classified in the statement of operations, generally as research and development expense, based on the nature of the related activities.
Lam Research Corporation
In August 2017, the Company entered into a collaboration agreement with Lam Research Corporation (“Lam”) with respect to the development and commercialization of the Hyb & Seq sequencing platform and related assays. Pursuant to the terms of the collaboration agreement, Lam will contribute up to an aggregate of $50.0 million, payable quarterly, based on allowable development costs. Lam is eligible to receive certain single-digit percentage royalty payments from the Company on net sales of certain products and technologies developed under the collaboration agreement. The maximum amount of royalties payable to Lam will be capped at an amount up to three times the amount of development funding actually provided by Lam. The Company will retain exclusive rights to obtain regulatory approval, manufacture and commercialize the Hyb & Seq
-83-
products. Lam will participate in development through a joint steering committee. The Company will reimburse Lam for the cost of up to 10 full-time Lam employees each year in accordance with the product development plan.
In connection with the execution of the collaboration agreement, the Company issued Lam a warrant to purchase up to 1.0 million shares of the Company’s common stock with the number of underlying shares exercisable at any time proportionate to the amount of the $50.0 million commitment that has been provided by Lam. The exercise price of the warrant is $16.75 per share, and it will expire on the seventh anniversary of the issuance date. The warrant was determined to have a fair value of $6.7 million upon issuance, and such amount will be recorded as additional paid in capital proportionately from the quarterly collaboration payments made by Lam.
The Company recognized collaboration revenue of $3.7 million for the year ended December 31, 2017. The Company received development funding of $13.4 million related to the Lam collaboration for the year ended December 31, 2017, of which $8.3 million is included in customer deposits in the consolidated balance sheet as of December 31, 2017 representing amounts received in advance. Through December 31, 2017, no amounts are due or have been paid by the Company to Lam for services provided by Lam employees under the terms of the agreement. During 2017, Lam did not exercise any warrants.
Celgene Corporation
In March 2014, the Company entered into a collaboration agreement with Celgene Corporation (“Celgene”) to develop, seek regulatory approval for, and commercialize a companion diagnostic using the nCounter Analysis System to identify a subset of patients with Diffuse Large B-Cell Lymphoma. Pursuant to the Company's agreement as amended in February 2018, the Company is eligible to receive payments from Celgene totaling up to $27.3 million, of which $5.8 million was received as an upfront payment upon delivery of certain information to Celgene and $21.5 million is for development funding and potential success-based development and regulatory milestones. There have been several amendments to the collaboration agreement and in return the Company has received additional payments totaling $2.1 million. The Company will retain all commercial rights to the diagnostic test developed under this collaboration, subject to certain backup rights granted to Celgene to commercialize the diagnostic test in a particular country if the Company elects to cease distribution or elects not to distribute the diagnostic in such country. Assuming success in the clinical trial process, and subject to regulatory approval, the Company will market and sell the diagnostic assay.
The Company achieved and was paid for milestones totaling $6.0 million during 2014. The process of successfully developing a product candidate, obtaining regulatory approval and ultimately commercializing a product candidate is highly uncertain and the attainment of any additional milestones is therefore uncertain and difficult to predict. In addition, certain milestones are outside the Company’s control and are dependent on the performance of Celgene and the outcome of a clinical trial and related regulatory processes. Accordingly, the Company is not able to reasonably estimate when, if at all, any additional milestone payments may be payable to the Company by Celgene. See Note 17. Subsequent Events for an update to this collaboration agreement.
The Company recognized collaboration revenue related to the Celgene agreement of $0.2 million, $3.2 million and $2.2 million for the years ended December 31, 2017, 2016 and 2015, respectively. At December 31, 2017, the Company had recorded $5.4 million of deferred revenue related to the Celgene collaboration, of which $3.7 million is estimated to be recognizable as revenue within one year.
Merck & Co., Inc.
In May 2015, the Company entered into a clinical research collaboration agreement with Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. (“Merck”), to develop an assay intended to optimize immune-related gene expression signatures and evaluate the potential to predict benefit from Merck’s anti-PD-1 therapy, KEYTRUDA. Under the terms of the collaboration agreement, the Company received $3.9 million in payments during 2015. In connection with the execution of the development collaboration agreement, the Company and Merck terminated the May 2015 clinical research collaboration and moved all remaining activities under the related work plan to the new development collaboration agreement. In February 2016, the Company expanded its collaboration with Merck by entering into a new development collaboration agreement to clinically develop, seek regulatory approval for, and commercialize a companion diagnostic test to predict response to KEYTRUDA in multiple tumor types. During 2016, the Company received $12.0 million upfront as a technology access fee and $8.5 million of preclinical milestone payments. In October 2017, Merck notified the Company of its decision not to pursue regulatory approval of the companion diagnostic test for KEYTRUDA. As a result, the scope of the collaboration was significantly reduced, and activities respecting this collaboration are expected to be materially concluded in 2018.
The Company recognized collaboration revenue related to the Merck agreement of $27.0 million, $8.6 million, and $3.7 million for the years ended December 31, 2017, 2016, and 2015, respectively. As of December 31, 2017, the Company had recorded $1.1 million of deferred revenue related to the Merck collaboration, $1.0 million of which is estimated to be recognized as revenue within one year. The Company received development funding of $6.8 million, $8.7 million, and $3.9 million for the years ended December 31, 2017, 2016, and 2015, respectively.
-84-
Medivation, Inc. and Astellas Pharma, Inc.
In January 2016, the Company entered into a collaboration agreement with Medivation, Inc. ("Medivation") and Astellas Pharma Inc. (“Astellas”) to pursue the translation of a novel gene expression signature algorithm discovered by Medivation into a companion diagnostic assay using the nCounter Analysis System. In September 2016, Medivation was acquired by Pfizer, Inc. (“Pfizer”) and became a wholly owned subsidiary of Pfizer. In May 2017, the Company received notification from Pfizer and Astellas terminating the collaboration agreement as a result of a decision to discontinue the related clinical trial.
The Company recognized collaboration revenue of $11.5 million and $4.8 million related to the Medivation/Astellas agreement for the years ended December 31, 2017 and 2016, respectively, including the favorable impact of a $1.0 million termination penalty during 2017. The Company achieved and was paid for milestones totaling $6.0 million during 2016. The Company received development funding of $0.9 million and $2.4 million for the years ended December 31, 2017 and 2016, respectively.
9. Common Stock and Preferred Stock
Common Stock
Each share of common stock is entitled to one vote. The holders of common stock are also entitled to receive dividends whenever funds are legally available and when declared by the board of directors, subject to the prior rights of holders of other classes of stock outstanding.
Preferred Stock
Pursuant to the amended and restated certificate of incorporation filed by the Company immediately prior to the completion of its initial public offering, the Company’s board of directors is authorized to issue up to 15,000,000 shares of preferred stock in one or more series and to fix the rights, preferences, privileges and restrictions thereof. These rights, preferences and privileges could include dividend rights, conversion rights, voting rights, redemption rights, liquidation preferences, sinking fund terms and the number of shares constituting any series or the designation of such series, any or all of which may be greater than the rights of common stock. The issuance of preferred stock could adversely affect the voting power of holders of common stock and the likelihood that such holders will receive dividend payments and payments upon liquidation. In addition, the issuance of preferred stock could have the effect of delaying, deferring or preventing change in the Company’s control or other corporate action. As of December 31, 2017, no shares of preferred stock were issued or outstanding, and the board of directors has not authorized or designated any rights, preferences, privileges and restrictions for any class of preferred stock.
Warrants
Prior to the Company’s initial public offering, warrants to purchase preferred stock were issued related to certain financing transactions. All preferred stock warrants were converted into warrants to purchase common stock upon the effectiveness of the initial public offering. As of December 31, 2017 there were 355,585 common stock warrants outstanding with a weighted average exercise price of $10.96 per share and expiration dates ranging from 2018 to 2024.
10. Stock-based Compensation
2004 Stock Option Plan and 2013 Equity Incentive Plan
The Company’s 2004 Stock Option Plan and 2013 Equity Incentive Plan (the “Plans”) authorize the grant of options, restricted stock units (“RSUs”) and other equity awards to employees, directors and consultants. As of December 31, 2017, there were 7,501,801 shares authorized under the Plans. All options granted have a ten-year term and generally vest and become exercisable over four years of continued employment or service as defined in each option agreement. The Board of Directors determines the option exercise price and may designate stock options granted as either incentive or nonstatutory stock options. The Company generally grants stock options to employees with exercise prices equal to the estimated fair value of the Company’s common stock on the date of grant.
-85-
Stock Option Activity
A summary of the Company’s stock option activity under the Plans is as follows:
Shares | Weighted- average exercise price per share | Weighted- average remaining contractual term (in years) | Aggregate intrinsic value (in thousands) | |||||||||
Outstanding at January 1,2017 | 4,721,941 | $ | 12.00 | 7.41 | $ | 48,670 | ||||||
Granted | 1,180,086 | 17.90 | ||||||||||
Canceled and forfeited | (478,078 | ) | 15.90 | |||||||||
Exercised | (227,696 | ) | 4.78 | |||||||||
Outstanding at December 31, 2017 | 5,196,253 | $ | 13.32 | 6.98 | $ | 3,861 | ||||||
December 31, 2017: | ||||||||||||
Options vested and expected to vest | 4,792,694 | $ | 13.07 | 6.84 | $ | 3,861 | ||||||
Options exercisable | 3,437,421 | $ | 11.90 | 6.19 | $ | 3,861 | ||||||
The weighted-average grant-date fair value per share of options granted with exercise prices equal to the market price on the date of the grant were $9.08, $6.79, and $7.07 for the years ended December 31, 2017, 2016, and 2015, respectively. The aggregate intrinsic value in the table above is calculated as the difference between the exercise price of the underlying options and the quoted price of the Company’s common stock for all options that were in-the-money at December 31, 2017. The aggregate intrinsic value of options exercised was $2.4 million during 2017, $5.0 million during 2016, and $2.1 million during 2015, determined as of the option exercise date. The fair value of options vested was $8.9 million, $6.8 million, and $6.5 million for the years ended December 31, 2017, 2016, and 2015, respectively.
The following table summarizes information about the Company’s stock options outstanding at December 31, 2017:
Outstanding | Exercisable | ||||||||
Exercise Price | Number of Shares | Weighted- Average Remaining Contractual Life in Years | Number of Shares | Weighted- Average Remaining Contractual Life in Years | |||||
$1.92 | 431,345 | 4.21 | 431,345 | 4.21 | |||||
$2.24 – $6.72 | 494,155 | 3.87 | 494,155 | 3.87 | |||||
$7.47 – $12.56 | 480,694 | 6.76 | 384,717 | 6.28 | |||||
$12.77 | 674,356 | 7.06 | 488,124 | 7.04 | |||||
$12.94 | 577,212 | 8.05 | 276,718 | 7.99 | |||||
$13.01 – $14.95 | 360,230 | 7.62 | 241,553 | 7.42 | |||||
$14.99 – $17.50 | 531,282 | 8.27 | 218,634 | 7.36 | |||||
$17.89 – $18.90 | 1,346,556 | 7.63 | 806,525 | 6.66 | |||||
$19.09 – $22.71 | 300,423 | 8.22 | 95,650 | 7.33 | |||||
5,196,253 | 3,437,421 | ||||||||
In January 2014, the compensation committee of the Company's board of directors delegated authority to a committee of the Company's officers to make new-hire option grants, and in October 2015 further delegated the authority for the committee to make merit option grants, to non-officer employees. The authority of this committee is limited by a charter approved by the compensation committee of the Company's board of directors, the 2013 equity incentive plan and Delaware law. During 2017, the Company became aware of certain new higher option grants that had not been done in accordance with the committee’s charter. The Company took appropriate steps to fix these issues, which included ratification of a number of option grants, adjustments to the grant dates and/or grant prices, and in some cases, cancellation of certain grants. In the aggregate, the Company modified approximately 265,000 stock option awards to correct the exercise price and grant date for these awards which affected 109 employees of the Company, and as a result, during the year ended December 31, 2017, the Company recorded approximately $50,000 of incremental stock based compensation cost resulting from the modifications to these stock awards.
-86-
Restricted Stock Unit (RSU) Activity
A summary of RSU activity under the Plans is as follows:
Non-vested RSUs | Share Equivalent | Weighted-Average Grant Date Fair Value | |||||
Non-vested at January 1, 2017 | 163,602 | $ | 15.70 | ||||
Changes during the year: | |||||||
Granted | 603,493 | 10.73 | |||||
Vested | (66,956 | ) | 15.77 | ||||
Forfeited | (38,432 | ) | 17.20 | ||||
Non-vested at December 31, 2017 | 661,707 | $ | 11.07 | ||||
The fair value of the RSUs is determined based on the closing price of the Company’s common stock on the date of grant. The fair value of vested RSUs was $1.1 million and $64,000 for the years ended December 31, 2017 and 2016. There were no vested RSUs for the year ended December 31, 2015.
Stock-based compensation
The following table sets forth stock-based compensation expense related to stock-based arrangements under the Plans for the years ended December 31 as follows (in thousands):
2017 | 2016 | 2015 | |||||||||
Cost of revenue | $ | 719 | $ | 548 | $ | 471 | |||||
Research and development | 2,853 | 2,046 | 1,453 | ||||||||
Selling, general and administrative | 7,047 | 5,602 | 3,919 | ||||||||
Total stock-based compensation expense | $ | 10,619 | $ | 8,196 | $ | 5,843 | |||||
As of December 31, 2017, total unrecognized stock-based compensation cost related to non-vested options was $19.2 million. This cost will be recognized on a straight-line basis over the weighted-average remaining service period of 2.35 years. The Company utilizes newly issued shares to satisfy option exercises. No tax benefit was recognized related to stock-based compensation cost since the Company has not reported taxable income to date and has established a full valuation allowance to offset all of the potential tax benefits associated with its deferred tax assets.
Valuation assumptions
The fair value of each employee option grant as of December 31 was estimated on the date of grant using the Black-Scholes option pricing model with the following assumptions:
2017 | 2016 | 2015 | |||
Risk-free interest rates | 1.40% - 2.26% | 1.18% - 2.12% | 1.37% - 1.97% | ||
Expected term (years) | 5.50 - 6.25 | 5.50 - 6.50 | 6.25 | ||
Expected dividend yield | — | — | — | ||
Expected volatility | 53.9% - 58.0% | 47.0% | 57.0% | ||
The risk-free interest rates are based on the implied yield currently available in U.S. Treasury securities at maturity with an equivalent term. For purposes of determining the expected term of the awards in the absence of sufficient historical data relating to stock-option exercises, the Company applies a simplified approach in which the expected term of an award is presumed to be the mid-point between the vesting date and the expiration date of the award. The Company has not declared or paid any dividends and does not currently expect to do so in the foreseeable future. The Company based its expected volatility on the historical volatility of similar companies whose share prices are publicly available, as management does not believe that the limited history of the Company’s measureable stock price volatility is representative of future expectations.
Employee Stock Purchase Plan
The Company’s 2013 Employee Stock Purchase Plan (“ESPP”) provides eligible employees with an opportunity to purchase common stock from the Company and to pay for their purchases through payroll deductions. The ESPP has overlapping offering periods of approximately 12 months in length. The offering periods generally start with the first trading day on or after March 1 and September 1 of each year and end on the first trading day on or after March 1 and September 1 of
-87-
the following year, approximately 12 months later. Within each offering period, shares are purchased each six months on an exercise date.
An employee electing to participate in the ESPP (a “participant”) will be granted an option at the start of the offering period to purchase shares with contributions in any whole percentage ranging from 0% to 10% (or greater or lesser percentages or dollar amounts that the administrator determines) of the participant’s eligible compensation. The participant’s contributions will be accumulated and then used to purchase the Company’s shares on each exercise date. The purchase price on the exercise date will be 85% of the fair market value of the lesser of the Company’s share price on either the first trading day of the offering period or on the exercise date.
During 2017, 2016, and 2015, shares issued under the ESPP were 138,972, 139,195, and 136,078, respectively. The Company recorded share-based compensation expense for shares issued from the ESPP of $0.8 million, $0.8 million, and $0.4 million for the years ended December 31, 2017, 2016, and 2015, respectively. A total of 825,442 shares of common stock have been reserved for issuance under the ESPP, of which 269,811 shares were available for issuance as of December 31, 2017.
11. Defined Contribution Retirement Plan
The Company maintains a 401(k) defined contribution retirement plan covering substantially all of its employees. The plan provides for matching and discretionary contributions by the Company. Contributions were $1.2 million, $0.9 million, and $0.5 million for the years ended December 31, 2017, 2016, and 2015, respectively.
12. Income Taxes
Loss before income taxes for the years ended December 31 consisted of the following (in thousands):
2017 | 2016 | 2015 | |||||||||
Domestic | $ | (44,324 | ) | $ | (47,562 | ) | $ | (46,065 | ) | ||
Foreign | 966 | 589 | 650 | ||||||||
Loss before income taxes | $ | (43,358 | ) | $ | (46,973 | ) | $ | (45,415 | ) | ||
Significant components of our provision for income taxes for the years ended December 31 are as follows (in thousands):
2017 | 2016 | 2015 | |||||||||
Current: | |||||||||||
Domestic | $ | — | $ | — | $ | — | |||||
Foreign | 204 | 116 | 166 | ||||||||
Total provision for income taxes | $ | 204 | $ | 116 | $ | 166 | |||||
The Tax Cuts and Jobs Act, or the Act, was enacted on December 22, 2017, which reduced the U.S. federal corporate tax rate from 35% to 21%, among other changes. The Company’s accounting for the elements of the Act is complete and resulted in a $37.7 million reduction in its net deferred tax assets as of December 31, 2017 to reflect the new statutory rate. The rate adjustment to the deferred tax assets was fully offset by a decrease in the valuation allowance, resulting in no rate impact to the Company.
A reconciliation of the federal statutory income tax rate to the effective income tax rate for the years ended December 31 are as follows (in thousands):
2017 | 2016 | 2015 | |||||||||
Income tax provision at statutory rate | $ | (15,076 | ) | $ | (16,010 | ) | $ | (15,662 | ) | ||
Tax on repatriated foreign earnings and other nondeductible items | 179 | 135 | 401 | ||||||||
Change in tax credits | (2,361 | ) | (1,449 | ) | (792 | ) | |||||
Change in valuation allowance | (19,792 | ) | 17,824 | 16,706 | |||||||
Change in tax rate | 37,690 | — | — | ||||||||
Foreign tax and other | (436 | ) | (384 | ) | (487 | ) | |||||
Total provision for income taxes | $ | 204 | $ | 116 | $ | 166 | |||||
Net operating loss (“NOL”) carryforwards created by excess tax benefits from the exercise of non-qualified stock options are not recorded as deferred income tax assets. To the extent such NOL carryforwards are utilized, the benefit realized will increase stockholders’ equity. At December 31, 2017, for income tax return purposes the Company has gross federal and
-88-
state NOL carryforwards totaling $232.8 million and tax credit carryforwards of $7.0 million. These carryforwards may be subject to limitations under the Internal Revenue Code and applicable state tax law. If not utilized, a portion of the carryforwards will begin to expire in 2025 through 2038.
The Company does not expect to utilize any of its net operating loss and tax credit carryforwards in the near term. The Company may have already experienced one or more ownership changes. Depending on the timing of any future utilization of its carryforwards, the Company may be limited as to the amount that can be utilized each year as a result of such previous ownership changes. However, the Company does not believe such limitations will cause its carryforwards to expire unutilized.
Future changes in the Company’s stock ownership as well as other changes that may be outside the Company’s control could potentially result in further limitations on the Company’s ability to utilize its net operating loss and tax credit carryforwards.
The effect of temporary differences and carryforwards that give rise to deferred tax assets for the years ended December 31 were as follows (in thousands):
2017 | 2016 | ||||||
Net operating loss carryforwards | $ | 49,662 | $ | 70,694 | |||
Research and development tax credit carryforwards | 6,505 | 4,572 | |||||
Foreign tax credit carryforwards | 448 | — | |||||
Stock-based compensation | 5,664 | 5,360 | |||||
Other | 6,382 | 7,827 | |||||
Total deferred tax assets | 68,661 | 88,453 | |||||
Less: Valuation allowance | (68,661 | ) | (88,453 | ) | |||
Net deferred tax assets | $ | — | $ | — | |||
The Company has recorded a full valuation allowance related to its deferred tax assets due to the uncertainty of the ultimate realization of the future benefits from those assets.
The table below summarizes changes in the deferred tax asset valuation allowance for the years ended December 31 (in thousands):
2017 | 2016 | 2015 | |||||||||
Balance at beginning of year | $ | 88,453 | $ | 70,629 | $ | 53,923 | |||||
Charged to costs and expenses | 17,898 | 17,824 | 16,706 | ||||||||
Impact of change in tax rate | (37,690 | ) | — | — | |||||||
Balance at end of year | $ | 68,661 | $ | 88,453 | $ | 70,629 | |||||
The total balance of unrecognized gross tax benefits for the years ended December 31, resulting from research and development tax credits claimed on the Company’s annual tax return was as follows (in thousands):
2017 | 2016 | 2015 | |||||||||
Unrecognized tax benefits at beginning of year | $ | 1,524 | $ | 1,041 | $ | 777 | |||||
Additions based on current year tax positions | 644 | 483 | 264 | ||||||||
Unrecognized tax benefits at end of year | $ | 2,168 | $ | 1,524 | $ | 1,041 | |||||
The Company classifies applicable interest and penalties on amounts due to tax authorities as a component of the provision for income taxes. The amount of accrued interest and penalties recorded in 2017, 2016 or 2015 was not significant. The Company does not anticipate that the amount of its existing unrecognized tax benefits will significantly increase or decrease within the next 12 months. Due to the presence of net operating loss carryforwards in most jurisdictions, the Company’s tax years remain open for examination by U.S. taxing authorities back to 2004.
13. Commitments and Contingencies
Operating Leases
The Company is obligated to make future minimum payments under three operating leases for 106,740 square feet of space used for general office, laboratory, manufacturing, operations, and research and development purposes primarily in Seattle. The leases expire beginning in 2018 to 2026 and include options to renew at the then current fair market rental for each of the facilities. The lease agreements contain rent abatement periods, scheduled rent increases and provide for tenant
-89-
improvement allowances. Accordingly, the Company has recorded a deferred rent liability of $8.7 million and $7.5 million as of December 31, 2017 and 2016, respectively. This deferred rent liability is amortized over the term of the related lease.
Rent expense totaled approximately $4.8 million, $3.8 million and $3.2 million for the years ended December 31, 2017, 2016, and 2015, respectively.
Future minimum lease payments under noncancelable operating leases as of December 31, 2017 were as follows (in thousands):
2018 | $ | 5,316 | |
2019 | 5,307 | ||
2020 | 5,418 | ||
2021 | 5,551 | ||
2022 | 5,708 | ||
Thereafter | 19,327 | ||
$ | 46,627 | ||
Purchase Commitments
The Company has non-cancellable purchase obligations totaling $4.4 million at December 31, 2017 related to binding commitments to purchase inventory and other research and development items.
Contingencies
From time to time, the Company may become involved in litigation relating to claims arising from the ordinary course of business. Management believes that there are no claims or actions pending against the Company currently, the ultimate disposition of which would have a material adverse effect on the Company’s consolidated results of operation, financial condition or cash flows.
14. Net Loss Per Share
Net loss per share is computed by dividing the net loss by the weighted average number of shares of common stock outstanding. Outstanding stock options, warrants and preferred stock have not been included in the calculation of diluted net loss per share because to do so would be anti-dilutive. Accordingly, the numerator and the denominator used in computing both basic and diluted net loss per share for each period are the same.
The following outstanding options, restricted stock units and warrants as of December 31 were excluded from the computation of diluted net loss per share for the periods presented because their effect would have been anti-dilutive (in thousands):
2017 | 2016 | 2015 | ||||||
Options to purchase common stock | 5,335 | 4,711 | 4,069 | |||||
Restricted stock units | 313 | 115 | — | |||||
Common stock warrants | 317 | 491 | 572 | |||||
15. Information about Geographic Areas
The following table is based on the geographic location of distributors or end users who purchased products and services and collaborators. For sales to distributors, their geographic location may be different from the geographic locations of the ultimate end user. Revenue by geography as of December 31 was as follows (in thousands):
2017 | 2016 | 2015 | |||||||||
Americas | $ | 86,099 | $ | 60,330 | $ | 41,265 | |||||
Europe & Middle East | 21,791 | 18,497 | 14,807 | ||||||||
Asia Pacific | 7,015 | 7,662 | 6,595 | ||||||||
Total revenue | $ | 114,905 | $ | 86,489 | $ | 62,667 | |||||
Total revenue in the United States was $84.0 million, $58.0 million and $37.9 million for the years ended December 31, 2017, 2016 and 2015, respectively. The Company’s assets are primarily located in the United States and not
-90-
allocated to any specific geographic region. Substantially all of the Company’s long-lived assets are located in the United States.
16. Condensed Quarterly Financial Data (unaudited)
The following table contains selected unaudited financial data for each quarter of 2017 and 2016. The unaudited information should be read in conjunction with the Company’s financial statements and related notes included elsewhere in this report. The Company believes that the following unaudited information reflects all normal recurring adjustments necessary for a fair statement of the information for the periods presented. The operating results for any quarter are not necessarily indicative of results for any future period.
Three months ended | |||||||||||||||
March 31, | June 30, | September 30, | December 31, | ||||||||||||
(in thousands, except per share data) | |||||||||||||||
2017 | |||||||||||||||
Total revenue | $ | 18,063 | $ | 34,592 | $ | 27,016 | $ | 35,234 | |||||||
Net loss | $ | (18,852 | ) | $ | (4,555 | ) | $ | (11,404 | ) | $ | (8,751 | ) | |||
Net loss per share – basic and diluted | $ | (0.87 | ) | $ | (0.20 | ) | $ | (0.45 | ) | $ | (0.34 | ) | |||
2016 | |||||||||||||||
Total revenue | $ | 14,697 | $ | 22,627 | $ | 23,933 | $ | 25,232 | |||||||
Net loss | $ | (14,603 | ) | $ | (10,805 | ) | $ | (10,088 | ) | $ | (11,593 | ) | |||
Net loss per share – basic and diluted | $ | (0.74 | ) | $ | (0.55 | ) | $ | (0.51 | ) | $ | (0.55 | ) | |||
17. Subsequent Events
In January 2018, the Company entered into a sales agreement with a sales agent to sell shares of the Company's common stock through an "at the market" equity offering program for up to $40.0 million in gross cash proceeds. The sales agreement allows the Company to set the parameters for the sale of shares, including the number of shares to be issued, the time period during which sales are requested to be made, limits on the number of shares that may be sold in any one trading day and a minimum price below which sales may not be made. Under the terms of the Sales Agreement, commission expenses to the sales agent will be 3% of the gross sales price per share sold through the sales agent. The Sales Agreement shall automatically terminate upon the issuance and sale of placement shares equaling sales proceeds of $40.0 million and may be terminated earlier by either the Company or the sales agent upon five days’ notice.
In January 2018, the Company, as borrower, entered into a $15.0 million secured revolving loan facility, with availability subject to a borrowing base consisting of eligible accounts receivable. The agreement matures in January 2021, at which time the outstanding principal will become due and payable. Interest on borrowings is payable monthly and accrues at a yearly rate equal to the greater of the prime rate, as reported in the Wall Street Journal, plus 0.50% or 4.75%. During an event of default amounts drawn accrue interest at a yearly rate equal to 8.75%. Obligations under the agreement are collateralized by substantially all of the Company’s assets.
In February 2018, the Company and Celgene entered into an amendment to their collaboration agreement in which Celgene agreed to provide the Company additional funding for work intended to enable a subtype and prognostic indication for the test being developed under the agreement for Celgene’s drug REVLIMID. In addition, the amendment provides an additional milestone payment to the Company payable upon achievement of certain regulatory activities and timelines. In connection with this amendment, the Company agreed to remove the right to receive payments from Celgene in the event commercial sales of the companion diagnostic test do not exceed certain pre-specified minimum annual revenues during the first three years following regulatory approval. In addition, the amendment allows Celgene, at its election, to use trial samples with additional technologies for companion diagnostics.
-91-
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer and our Chief Financial Officer, evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2017. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the U.S.
Securities and Exchange Commission’s (“SEC”) rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the company’s management, including its principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on the evaluation of our disclosure controls and procedures as of December 31, 2017, our Chief Executive Officer and Chief Financial Officer concluded that, as of such date, our disclosure controls and procedures were effective at the reasonable assurance level.
Management’s Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rule 13a-15(f) and Rule 15d-15(f) of the Exchange Act. Our internal control over financial reporting is a process to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Our internal control over financial reporting includes those policies and procedures that:
(i) | pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets; |
(ii) | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and |
(iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
The effectiveness of any system of internal control over financial reporting, including ours, is subject to inherent limitations, including the exercise of judgment in designing, implementing, operating, and evaluating the controls and procedures, and the inability to eliminate misconduct completely. Accordingly, any system of internal control over financial reporting, including ours, no matter how well designed and operated, can only provide reasonable, not absolute, assurances. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions or that the degree of compliance with the policies or procedures may deteriorate. Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2017. In making this assessment, our management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) Internal Control—Integrated Framework (2013). Based on our assessment using those criteria, our management has concluded that, as of December 31, 2017, our internal control over financial reporting was effective.
Changes in Internal Control over Financial Reporting
There was no change in our internal control over financial reporting identified in connection with the evaluation required by Rule 13a-15(d) and 15d-15(d) of the Exchange Act that occurred during the three months ended December 31, 2017 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information
None.
-92-
PART III
Item 10. Directors, Executive Officers and Corporate Governance
The information required by Item 10 of Form 10-K is incorporated by reference to our Proxy Statement for the 2018 Annual Meeting of Stockholders to be filed with the SEC within 120 days after the end of the fiscal year ended December 31, 2017.
Item 11. Executive Compensation
The information required by Item 11 of Form 10-K is incorporated by reference to our Proxy Statement for the 2018 Annual Meeting of Stockholders to be filed with the SEC within 120 days after the end of the fiscal year ended December 31, 2017.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information required by Item 12 of Form 10-K is incorporated by reference to our Proxy Statement for the 2018 Annual Meeting of Stockholders to be filed with the SEC within 120 days after the end of the fiscal year ended December 31, 2017.
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information required by Item 13 of Form 10-K is incorporated by reference to our Proxy Statement for the 2018 Annual Meeting of Stockholders to be filed with the SEC within 120 days after the end of the fiscal year ended December 31, 2017.
Item 14. Principal Accountant Fees and Services
The information required by Item 14 of Form 10-K is incorporated by reference to our Proxy Statement for the 2018 Annual Meeting of Stockholders to be filed with the SEC within 120 days after the end of the fiscal year ended December 31, 2017.
PART IV
Item 15. Exhibits, Financial Statement Schedules
(a) The following documents are filed as part of this report:
(1) Financial Statements — The financial statements filed as part of this Annual Report on Form 10-K are listed on the Index to Consolidated Financial Statements in Item 8.
(2) Financial Statement Schedules — The financial statement schedules have been omitted because the information required to be set forth therein is not applicable or is shown in the financial statements or the notes thereto.
(3) Exhibits — The exhibits required by Item 601 of Regulation S-K are listed in paragraph (b) below.
(b) Exhibits
The exhibits listed on the Exhibit Index (following the Signatures section of this report) are filed herewith or are incorporated by reference to exhibits previously filed with the SEC.
-93-
Exhibit | Incorporated by Reference | |||||||||
Number | Description | Form | Filing Date | Number | Filed Herewith | |||||
3.1 | 10-Q | August 8, 2013 | 3.1 | |||||||
3.2 | 10-Q | August 8, 2013 | 3.2 | |||||||
4.1 | S-1/A | June 13, 2013 | 4.1 | |||||||
4.2 | S-1 | May 20, 2013 | 4.2 | |||||||
4.3 | S-1 | May 20, 2013 | 4.3 | |||||||
4.4 | S-1 | May 20, 2013 | 4.6 | |||||||
4.5 | S-1 | May 20, 2013 | 4.7 | |||||||
4.6 | S-1 | May 20, 2013 | 4.9 | |||||||
4.7 | 8-K | August 8, 2017 | 4.1 | |||||||
10.1 | S-1/A | June 13, 2013 | 10.1 | |||||||
10.2 | S-1 | May 20, 2013 | 10.2 | |||||||
10.3 | S-1 | May 20, 2013 | 10.3 | |||||||
10.4 | S-1 | May 20, 2013 | 10.4 | |||||||
10.5 | S-1/A | June 13, 2013 | 10.5 | |||||||
-94-
Exhibit | Incorporated by Reference | |||||||||
Number | Description | Form | Filing Date | Exhibit | Filed Herewith | |||||
10.6 | S-1/A | June 13, 2013 | 10.6 | |||||||
10.7 | S-1/A | June 13, 2013 | 10.7 | |||||||
10.8 | S-1/A | June 13, 2013 | 10.8 | |||||||
10.9 | S-1/A | June 13, 2013 | 10.9 | |||||||
10.10+ | S-1 | May 20, 2013 | 10.8 | |||||||
10.11+ | 10-Q | August 9, 2017 | 10.1 | |||||||
10.12+ | S-1 | January 13, 2014 | 10.11 | |||||||
10.13+ | 10-Q | August 9, 2017 | 10.2 | |||||||
10.14+ | 10-K | March 11, 2016 | 10.12 | |||||||
10.15+ | 10-Q | August 9, 2017 | 10.3 | |||||||
10.16+ | S-1 | January 13, 2014 | 10.12 | |||||||
10.17+ | X | |||||||||
10.18+ | X | |||||||||
10.19+ | X | |||||||||
10.20+ | X | |||||||||
10.21 | 10-K | March 13, 2015 | 10.14 | |||||||
10.22 | 10-K | March 11, 2016 | 10.13 | |||||||
10.23 | 10-K | March 13, 2015 | 10.15 | |||||||
10.24 | 10-Q | August 4, 2016 | 10.1 | |||||||
10.25 | 10-K | March 13, 2015 | 10.16 | |||||||
10.26 | 10-Q | May 6, 2016 | 10.1 | |||||||
-95-
Exhibit | Incorporated by Reference | |||||||||
Number | Description | Form | Filing Date | Exhibit | Filed Herewith | |||||
10.27 | 10-Q | August 8, 2014 | 10.1 | |||||||
10.28 | 10-Q | August 5, 2015 | 10.2 | |||||||
10.29 | 10-K | March 11, 2016 | 10.19 | |||||||
10.30 | 10-Q | May 5, 2017 | 10.1 | |||||||
10.31 | X | |||||||||
10.32 | 10-Q | August 8, 2014 | 10.2 | |||||||
10.33† | S-1 | May 20, 2013 | 10.19 | |||||||
10.34† | S-1 | May 20, 2013 | 10.20 | |||||||
10.35 | S-1 | May 20, 2013 | 10.21 | |||||||
10.36† | S-1 | May 20, 2013 | 10.22 | |||||||
10.37 | 10-Q | May 11, 2015 | 10.1 | |||||||
10.38 | 10-Q | August 4, 2016 | 10.2 | |||||||
10.39† | 10-Q | November 8, 2017 | 10.1 | |||||||
-96-
Exhibit | Incorporated by Reference | |||||||||
Number | Description | Form | Filing Date | Exhibit | Filed Herewith | |||||
10.40 | 8-K | January 8, 2018 | 1.1 | |||||||
10.41 | 8-K | January 8, 2018 | 1.2 | |||||||
21.1* | X | |||||||||
23.1* | X | |||||||||
24.1* | X | |||||||||
31.1* | X | |||||||||
31.2* | X | |||||||||
32.1* | X | |||||||||
32.2* | X | |||||||||
101.INS* | XBRL Instance Document. | X | ||||||||
101.SCH* | XBRL Taxonomy Extension Schema Document. | X | ||||||||
101.CAL* | XBRL Taxonomy Extension Calculation Linkbase Document. | X | ||||||||
101.DEF* | XBRL Taxonomy Extension Definition Linkbase Document. | X | ||||||||
101.LAB* | XBRL Taxonomy Extension Label Linkbase Document. | X | ||||||||
101.PRE* | XBRL Taxonomy Extension Presentation Linkbase Document. | X | ||||||||
* | Filed herewith. |
+ | Indicates a management contract or compensatory plan. |
† | Confidential treatment has been requested with respect to certain portions of this exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission. |
Item 16. Form 10-K Summary
Not applicable.
-97-
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Dated: March 7, 2018
NANOSTRING TECHNOLOGIES, INC. | |
By: | /s/ R. Bradley Gray |
R. Bradley Gray | |
President and Chief Executive Officer | |
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints R. Bradley Gray and K. Thomas Bailey, and each of them, with full power of substitution and resubstitution and full power to act without the other, as his or her true and lawful attorney-in-fact and agent to act in his or her name, place and stead and to execute in the name and on behalf of each person, individually and in each capacity stated below, and to file, any and all documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing, ratifying and confirming all that said attorneys-in-fact and agents or any of them or their and his or her substitute or substitutes, may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1934, this Annual Report on Form 10-K has been signed by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ R. Bradley Gray | President, Chief Executive Officer and Director (Principal Executive Officer) | March 7, 2018 | ||
R. Bradley Gray | ||||
/s/ K. Thomas Bailey | Chief Financial Officer (Principal Accounting and Financial Officer) | March 7, 2018 | ||
K. Thomas Bailey | ||||
/s/ William D. Young | Chairman of the Board of Directors | March 7, 2018 | ||
William D. Young | ||||
/s/ Elisha W. Finney | Director | March 7, 2018 | ||
Elisha W. Finney | ||||
/s/ Nicholas Galakatos | Director | March 7, 2018 | ||
Nicholas Galakatos | ||||
/s/ Robert M. Hershberg | Director | March 7, 2018 | ||
Robert M. Hershberg | ||||
/s/ Kirk D. Malloy | Director | March 7, 2018 | ||
Kirk D. Malloy | ||||
/s/ Gregory Norden | Director | March 7, 2018 | ||
Gregory Norden | ||||
/s/ Charles P. Waite | Director | March 7, 2018 | ||
Charles P. Waite | ||||
-98-
Exhibit 10.17
NANOSTRING TECHNOLOGIES, INC.
AMENDMENT TO EMPLOYMENT AGREEMENT
This amendment (the “Amendment”) is made by and between Joseph Beechem (“Executive”) and NanoString Technologies, Inc. (the “Company,” and together with Executive, the “Parties”) on the dates set forth below.
WHEREAS, the Parties entered into an employment agreement effective March 31, 2012 (the “Employment Agreement”);
WHEREAS, the Company and Executive desire to amend certain provisions of the Employment Agreement in order to clarify the timing of the severance payment, as set forth below, in accordance with Section VI.B.3 of Internal Revenue Service Notice 2010-6, as amended by Internal Revenue Service Notice 2010-80.
NOW, THEREFORE, for good and valuable consideration, Executive and the Company agree that the Employment Agreement is hereby amended as follows.
1.Section 409A. Section 13 of the Employment Agreement is hereby amended to insert the following paragraph immediately following the first paragraph thereof:
“Any severance payments or benefits under this Agreement that would be considered “deferred compensation” under Section 409A will be paid on, or, in the case of installments, will not commence until, the sixtieth (60th) day following Executive’s Separation From Service, of, if later, such time is required by the final paragraph of this Section 13. Except as required by the final paragraph of this Section 13, any installment payments that would have been made to Executive during the 60 day period immediately following Executive’s Separation From Service but for the preceding sentence will be paid to Executive on the sixtieth (60th) day following Executive’s Separation from Service and the remaining payments shall be made as provided in this Agreement.”
2.Full Force and Effect. To the extent not expressly amended hereby, the Agreement shall remain in full force and effect.
3.Entire Agreement. This Amendment and the Agreement constitute the full and entire understanding and agreement between the Parties with regard to the subjects hereof and thereof. This Amendment may be amended at any time only by mutual written agreement of the Parties.
4.Counterparts. This Amendment may be executed in counterparts, all of which together shall constitute one instrument, and each of which may be executed by less than all of the parties to this Amendment.
5.Governing Law. This Amendment will be governed by the laws of the State of Washington (with the exception of its conflict of laws provisions).
- 1 -
IN WITNESS WHEREOF, each of the Parties has executed this Amendment, in the case of the Company by its duly authorized officer, on the dates set forth below.
NANOSTRING TECHNOLOGIES, INC | Joseph Beechem | |
/s/ Wayne D. Burns | /s/ Joseph Beechem | |
By: Sr. VP, Operations & Administration | SVP of Research & Development | |
Date: December 26, 2012 | Date December 27, 2012 | |
- 2 -
Exhibit 10.18
NANOSTRING TECHNOLOGIES, INC.
AMENDMENT TO EXECUTIVE EMPLOYMENT AGREEMENT
This Amendment to Executive Employment Agreement (this “Amendment”) is made by and between Joseph Beechem (“Executive”) and NanoString Technologies, Inc., a Delaware corporation (the “Company” and together with Executive, the “Parties”) on the dates set forth below.
WHEREAS, the Parties previously entered into an employment agreement effective March 31, 2012, as amended December 27, 2012 (the “Employment Agreement”);
WHEREAS, the Company and Executive desire to amend certain provisions of the Employment Agreement related to severance benefits, as set forth below.
NOW, THEREFORE, for good and valuable consideration, the Parties agree that the Agreement is hereby amended as follows:
1.The Employment Agreement is hereby amended as follows:
A.The period at the end of the first sentence of Section 5(a) is replaced by the following:
“; provided, however, that if such Involuntary Termination occurs within twelve (12) months following a Change in Control (as defined in the Company’s 2013 Equity Incentive Plan), (i) Executive shall be entitled to a lump sum payment equal to Executive’s then-effective Base Salary and target bonus (less applicable withholding taxes) and (ii) if Executive elects continuation coverage pursuant to the Consolidated Omnibus Budget Reconciliation Act of 1985, as amended (“COBRA”) within the time period prescribed pursuant to COBRA for Executive and Executive’s eligible dependents, the Company will reimburse Executive for the premiums necessary to continue group health insurance benefits for Executive and Executive’s eligible dependents for a period of twelve (12) months following the date of Involuntary Termination, except that the right to future COBRA payments shall terminate the date upon which Executive ceases to be eligible for coverage under COBRA.”
B.Clause (A)(1) of Section 6(c) is hereby qualified in its entirety as follows: the continuance of Executive’s duties and responsibilities at the subsidiary or divisional level following a Change in Control, rather than at the parent, combined or surviving company level following such Change in Control shall not be deemed Good Reason within the meaning of clause (A)(1).
2.Full Force and Effect. To the extent not expressly amended hereby, the Agreement shall remain in full force and effect.
3.Entire Agreement. This Amendment and the Agreement (and any other documents referenced therein) constitute the full and entire understanding and agreement between the Parties with regard to the subjects hereof and thereof
4.Governing Law. This Amendment will be governed by the laws of the State of Washington (with the exception of its conflict of laws provisions).
(signature page follows)
- 1 -
IN WITNESS WHEREOF, each of the Parties has executed this Amendment as of the date set forth below.
EXECUTIVE | NANOSTRING TECHNOLOGIES, INC | |
By: /s/ Joseph Beechem | By: /s/ R. Bradley Gray | |
Name: Joseph Beechem | Name: R. Bradley Gray | |
Title: President & CEO | ||
Date: October 30, 2017 | Date: November 7, 2017 | |
- 2 -
Exhibit 10.19
NANOSTRING TECHNOLOGIES, INC.
EMPLOYMENT AGREEMENT
This Employment Agreement (the “Agreement”) is entered into, effective as of October 17, 2017 (the “Effective Date”), by and between NanoString Technologies, Inc. (the “Company”) and J. Chad Brown (“Executive”).
1.Duties and Scope of Employment.
(a)Positions and Duties. Executive will serve as the Senior Vice President, Sales and Marketing of the Company. Executive will render such business and professional services in the performance of Executive’s duties, consistent with Executive’s position within the Company, as shall be assigned to Executive by the Company’s Chief Executive Officer (“CEO”). The period of Executive’s employment under this Agreement is referred to herein as the “Employment Term.” The Employment Term commenced on July 5, 2017, Executive’s actual start date of employment (the “Start Date”).
(b)Obligations. During the Employment Term, Executive will devote Executive’s full business efforts and time to the Company and will use good faith efforts to discharge Executive’s obligations under this Agreement to the best of Executive’s ability and in accordance with each of the Company’s corporate guidance and ethics guidelines, conflict of interest policies, code of conduct and Employee Handbook. For the duration of the Employment Term, Executive agrees not to actively engage in any other employment, occupation, or consulting activity for any direct or indirect remuneration without the prior approval of the Board; provided, however, that Executive may, without the approval of the Board, serve in any capacity with any civic, educational, social, or charitable organization, provided such services do not interfere with Executive’s obligations to the Company.
(c)Prior Agreements. Executive hereby represents and warrants to the Company that he is not party to any contract, understanding, agreement, or policy, written or otherwise, which would be breached by his entering into, or performing services under, this Agreement.
(d)Other Entities. Executive agrees to serve and may be appointed as an officer and director for any of the Company’s subsidiaries, partnerships, joint ventures, limited liability companies and other affiliates, including entities in which the Company has a significant investment as determined by the Company. As used in this Agreement, the term “affiliates” will include any entity controlled by, controlling, or under common control of the Company.
2.At-Will Employment. Executive and the Company agree that Executive’s employment with the Company constitutes “at-will” employment. Executive and the Company acknowledge that this employment relationship may be terminated by either Executive or the Company, at any time, upon written notice to the other Party, with or without cause, for any reason or no reason. Executive understands and agrees that neither Executive’s job performance nor promotions, commendations, bonuses, or the like from the Company alter Executive’s at-will status or give rise to or in any way serve as the basis for modification, amendment, or extension, by implication or otherwise, of Executive’s employment with the Company.
3.Compensation.
(a)Base Salary. During the Employment Term, the Company will pay Executive as compensation for Executive’s services a base salary at the annualized rate of Three Hundred and Sixty Five Thousand dollars ($365,000) (the “Base Salary”). The Base Salary will be paid in accordance with the Company’s normal payroll practices and be subject to the usual, required withholdings. The Base Salary may change at the Company’s discretion.
(b)Target Bonus. Executive shall be eligible to be considered for an annual, performance-based, cash bonus of up to 50% of Executive’s Base Salary for each calendar year, which bonus shall be awarded in the sole discretion of the Compensation Committee of the Board (the “Committee”) based on a recommendation from the CEO, which shall be based on Executive’s performance in the prior calendar year against metrics established for such year by the Company. Any bonus awarded shall be paid by no later than March 15 following
- 1 -
the calendar year to which the bonus corresponds. If Executive’s employment terminates for any reason prior to the end of a given calendar year, then the Company shall have no obligation to pay a bonus to Executive for such year. For the year ending December 31, 2017, Executive’s bonus will be prorated based upon Executive’s Start Date.
(c)Review and Adjustments. Executive’s Base Salary, Target Bonus, and other compensatory arrangements will be subject to review and adjustment in accordance with the Company’s applicable policies.
(d)Stock Options. Executive acknowledges and agrees that Executive is not entitled to any stock, stock options, vesting, or any other form of equity ownership in the Company, except the Stock option to purchase Sixty Thousand (60,000) shares of the Company’s common stock (“Stock Option”) as provided in the July 5, 2017 Stock Option Agreement between Executive and the Company (the “Option Agreement”), which is subject to the terms, definitions and conditions, including vesting requirements, of the Company’s 2013 Equity Incentive Plan (the “Equity Plan”). In the event that there is a Change of Control (as such term is defined in the Plan), and (i) Executive terminates his employment with the Company (or any affiliate) for Good Reason or (ii) the Company (or any affiliate) terminates Executive’s employment without Cause, during the period on, or within twelve (12) months following such Change of Control, then, in each case, one hundred percent (100%) of the unvested portion of the Stock Option shall vest and become exercisable at the time of Executive’s termination from employment. The description of the Stock Option in this Section 3(d) is qualified in its entirety to the actual terms as shall be set forth in the Option Agreement.
4.Limitation on Payments. In the event that the benefits provided for in this Agreement or otherwise payable to Executive (x) constitute “parachute payments” within the meaning of Section 280G of the Internal Revenue Code of 1986, as amended (the “Code”) and (y) but for this Section 5 would be subject to the excise tax imposed by Section 4999 of the Code, then Executive’s benefits will be either (i) delivered in full, or (ii) delivered as to such lesser extent which would result in no portion of such benefits being subject to excise tax under Section 4999 of the Code, whichever of the foregoing amounts, taking into account the applicable federal, state and local income taxes and the excise tax imposed by Section 4999, results in the receipt by Executive on an after-tax basis, of the greatest amount of benefits, notwithstanding that all or some portion of such benefits may be taxable under Section 4999 of the Code. If a reduction in amounts to be paid must be made, reduction shall occur in the following order: first, reduction of cash payments, which shall occur in reverse chronological order such that the cash payment owed on the latest date following the occurrence of the event triggering such excise tax will be the first cash payment to be reduced; second, cancellation of accelerated vesting of equity awards, which shall occur in the reverse order of the date of grant for such stock awards (i.e., the vesting of the most recently granted stock awards will be reduced first); and third, reduction of employee benefits, which shall occur in reverse chronological order such that the benefit owed on the latest date following the occurrence of the event triggering such excise tax will be the first benefit to be reduced. If two or more equity awards are granted on the same date, each award will be reduced on a pro-rata basis. In no event shall Executive have any discretion with respect to the ordering of payment reductions. Unless the Company and Executive otherwise agree in writing, any determination required under this Section will be made in writing by a well-recognized independent public accounting firm chosen by the Company (the “Accountants”), whose determination will be conclusive and binding upon Executive and the Company for all purposes. For purposes of making the calculations required by this Section, the Accountants may make reasonable assumptions and approximations concerning applicable taxes and may rely on reasonable, good faith interpretations concerning the application of Sections 280G and 4999 of the Code. The Company and Executive will furnish to the Accountants such information and documents as the Accountants may reasonably request in order to make a determination under this Section 5. The Company will bear all costs the Accountants may reasonably incur in connection with any calculations contemplated by this Section 5.
5.Employee Benefits.
(a)Generally. During the Employment Term, Executive is eligible to participate in the employee benefit plans currently maintained by the Company without limitation, the medical, dental, vision, life, flexible spending account, and disability plans available to similarly situated employees subject to their terms in effect from time to time. The Company may cancel or change the benefit plans and programs it offers to the Company’s employees at any time.
(b)Paid Time Off. During the Employment Term, Executive will be entitled to paid time off (“PTO”) in accordance with the Company’s Executive PTO policy. PTO shall be taken at such time as mutually and
- 2 -
reasonably agreed by Executive and the Company and in accordance with the Company’s policies in effect from time to time for other similarly situated employees. Executive will receive paid holidays in accordance with the Company’s regular holiday practices.
(c)Expenses. The Company will reimburse Executive for reasonable travel, entertainment, and other expenses incurred by Executive in the furtherance of the performance of Executive’s duties hereunder, in accordance with the Company’s expense reimbursement policy as in effect from time to time.
6.Termination of Employment.
(a)Accrued Obligations. In the event Executive’s employment with the Company terminates for any reason, Executive will be entitled to any (a) unpaid Base Salary accrued up to the effective date of termination; (b) benefits or compensation as provided under the terms of any employee benefit and compensation agreements or plans applicable to Executive; and (c) unreimbursed business expenses required to be reimbursed to Executive pursuant to the Company’s expense reimbursement policy and applicable law; and (d) rights to indemnification Executive may have under the Company’s Certificate of Incorporation and By-Laws, this Agreement, or separate indemnification agreement.
(b)Termination of Employment Without Cause or With Good Reason. If, (i) Executive terminates his employment with the Company (or any affiliate) for Good Reason or (ii) the Company (or any affiliate) terminates Executive’s employment without Cause, subject to Section 6(d) and Section 11 (each of (i) and (ii) referred to as an “Involuntary Termination”), Executive will be eligible to receive severance pay (less applicable withholding taxes) at a rate equal to Executive’s Base Salary rate, as then in effect, for a period of six (6) months (such payments shall be paid periodically in accordance with the Company’s normal payroll policies); provided, however, that if such Involuntary Termination occurs within twelve (12) months following a Change in Control (as defined in the Company’s 2013 Equity Incentive Plan), (i) Executive shall be entitled to a lump sum payment equal to Executive’s then-effective Base Salary and target bonus (less applicable withholding taxes) and (ii) if Executive elects continuation coverage pursuant to the Consolidated Omnibus Budget Reconciliation Act of 1985, as amended (“COBRA”) within the time period prescribed pursuant to COBRA for Executive and Executive’s eligible dependents, the Company will reimburse Executive for the premiums necessary to continue group health insurance benefits for Executive and Executive’s eligible dependents for a period of twelve (12) months following the date of Involuntary Termination, except that the right to future COBRA payments shall terminate the date upon which Executive ceases to be eligible for coverage under COBRA. If Executive becomes entitled to receive severance pay pursuant to this Section, Executive will not be entitled to any other severance benefits or similar payments in accordance with the Company’s established policies as then in effect.
(c)Termination by Reason of Death or Disability. If Executive’s employment with the Company terminates as a result of Executive’s death or Disability (as defined in Section 9 below), Executive or Executive’s estate or representative will receive all salary accrued (plus any other amounts payable as determined by the Board in its sole discretion) as of the date of Executive’s death or Disability and any other benefits payable under the Company’s then-existing benefit plans and policies in accordance with such plans and policies in effect on the date of death or Disability and in accordance with applicable law. Such payments shall be made by the Company periodically in accordance with the Company’s normal payroll policies with respect to each element of such payments.
(d)Release. Notwithstanding anything to the contrary, the payments under Section 7(b) are contingent upon Executive signing and not revoking a release of claims agreement with the Company in a form specified by the Company (the “Release”), which Release shall be provided to Executive within five (5) days after the Executive’s termination of employment, and such Release becoming effective no later than sixty (60) days following the date of termination of employment (such deadline, the “Release Deadline”). The payments under Section 7(b) shall be made within 60 days following the Executive’s termination of employment, provided that the Company has received a properly executed Release by such sixty (60) day period and the revocation period during which Executive is entitled to revoke such Release has expired prior to the sixtieth (60th) day following the Executive’s termination of employment. In no event will Executive’s payments be paid or provided until the Release actually becomes effective and irrevocable.
7.Section 409A.
- 3 -
(a)Notwithstanding anything to the contrary in this Agreement, no severance pay or benefits to be paid or provided to Executive, if any, pursuant to this Agreement that, when considered together with any other severance payments or separation benefits, are considered deferred compensation under Section 409A of the Code, and the final regulations and any guidance promulgated thereunder (“Section 409A”) (together, the “Deferred Payments”) will be paid or otherwise provided until Executive has a “separation from service” within the meaning of Section 409A. Similarly, no severance payable to Executive, if any, pursuant to this Agreement that otherwise would be exempt from Section 409A pursuant to Treasury Regulation Section 1.409A-1(b)(9) will be payable until Executive has a “separation from service” within the meaning of Section 409A.
(b)Notwithstanding anything to the contrary in this Agreement, if Executive is a “specified employee” within the meaning of Section 409A at the time of Executive’s termination (other than due to death), then the Deferred Payments that are payable within the first six months following Executive’s separation from service, will become payable on the first payroll date that occurs on or after the date six months and one day following the date of Executive’s separation from service. All subsequent Deferred Payments, if any, will be payable in accordance with the payment schedule applicable to each payment or benefit. Notwithstanding anything herein to the contrary, if Executive dies following Executive’s separation from service, but prior to the six (6) month anniversary of the separation from service, then any payments delayed in accordance with this paragraph will be payable in a lump sum as soon as administratively practicable after the date of Executive’s death and all other Deferred Payments will be payable in accordance with the payment schedule applicable to each payment or benefit. Each payment and benefit payable under this Agreement is intended to constitute a separate payment for purposes of Section 1.409A-2(b)(2) of the Treasury Regulations.
(c)Any amount paid under this Agreement that satisfies the requirements of the “short-term deferral” rule set forth in Section 1.409A-1(b)(4) of the Treasury Regulations will not constitute Deferred Payments for purposes of this Section.
(d)Any amount paid under this Agreement that qualifies as a payment made as a result of an involuntary separation from service pursuant to Section 1.409A-1(b)(9)(iii) of the Treasury Regulations that does not exceed the Section 409A Limit (as defined below) will not constitute Deferred Payments for purposes of this Section.
(e)The foregoing provisions are intended to comply with the requirements of Section 409A so that none of the severance payments and benefits to be provided hereunder will be subject to the additional tax imposed under Section 409A, and any ambiguities herein will be interpreted to so comply. The Company and Executive agree to work together in good faith to consider amendments to this Agreement and to take such reasonable actions which are necessary, appropriate, or desirable to avoid imposition of any additional tax or income recognition prior to actual payment to Executive under Section 409A. Executive agrees and acknowledges that the Company makes no representations or warranties with respect to the application of Section 409A and other tax consequences to any payments hereunder and, by the acceptance of any such payments, Executive agrees to accept the potential application of Section 409A and the other tax consequences of any payments made hereunder.
8.Arbitration.
(a)General. In consideration of Executive’s service to the Company, Executive’s promise to arbitrate all employment related disputes and Executive’s receipt of the compensation, pay raises and other benefits paid to Executive by the Company, at present and in the future, Executive agrees that any and all controversies, claims, or disputes with anyone (including the Company and any employee, officer, director, stockholder or benefit plan of the Company in their capacity as such or otherwise) arising out of, relating to, or resulting from Executive’s service to the Company under this Agreement or otherwise or the termination of Executive’s service with the Company, including any breach of this Agreement, shall be subject to binding arbitration under the Arbitration Rules set forth in the Revised Code of Washington Chapter 7.04 (the “Rules”) and pursuant to Washington law. Disputes which Executive agrees to arbitrate, and thereby agrees to waive any right to a trial by jury, include any statutory claims under state or federal law, including, but not limited to, claims under Title VII of the Civil Rights Act of 1964, the Americans with Disabilities Act of 1990, the Age Discrimination in Employment Act of 1967, the Older Workers Benefit Protection Act, claims of harassment, discrimination or wrongful termination. Executive further understands that this Agreement to arbitrate also applies to any disputes that the Company may have with Executive.
- 4 -
(b)Procedure. Executive agrees that any arbitration will be administered by the American Arbitration Association (“AAA”) and that a neutral arbitrator will be selected in a manner consistent with its National Rules for the Resolution of Employment Disputes. The arbitration proceedings will allow for discovery according to the National Rules for the Resolution of Employment Disputes and the Washington Code of Civil Procedure. Executive agrees that the arbitrator shall have the power to decide any motions brought by any party to the arbitration, including motions for summary judgment and/or adjudication and motions to dismiss and demurrers, prior to any arbitration hearing. Executive agrees that the arbitrator shall issue a written decision on the merits with findings of fact and conclusions of law. Executive also agrees that the arbitrator shall have the power to award any remedies, including attorneys’ fees and costs, available under applicable law. Executive understands the Company will pay for any administrative or hearing fees charged by the arbitrator or AAA except that Executive shall pay the first $200.00 of any filing fees associated with any arbitration Executive initiates. Executive agrees that the arbitrator shall administer and conduct any arbitration in a manner consistent with the Rules and that to the extent that the AAA’s National Rules for the Resolution of Employment Disputes conflict with the Rules, the Rules shall take precedence.
(c)Remedy. Except as provided by the Rules, arbitration shall be the sole, exclusive and final remedy for any dispute between Executive and the Company. Accordingly, except as provided for by the Rules, neither Executive nor the Company will be permitted to pursue court action regarding claims that are subject to arbitration. Notwithstanding, the arbitrator will not have the authority to disregard or refuse to enforce any lawful Company policy, and the arbitrator shall not order or require the Company to adopt a policy not otherwise required by law which the Company has not adopted.
(d)Availability of Injunctive Relief. In addition to the right under the Rules to petition the court for provisional relief, Executive agrees that any party may also petition the court for injunctive relief where either party alleges or claims a violation of this Agreement or the PHA or any other agreement regarding trade secrets, confidential information, non-competition, non-solicitation or non-disparagement. In the event either party seeks injunctive relief, the prevailing party shall be entitled to recover reasonable costs and attorneys’ fees.
(e)Administrative Relief. Executive understands that this Agreement does not prohibit Executive from pursuing an administrative claim with a local, state or federal administrative body such as the Washington State Human Rights Commission, Equal Employment Opportunity Commission or the workers’ compensation board. This Agreement does, however, preclude Executive from pursuing court action regarding any such claim.
9.Definitions.
(a)Cause. For purposes of this Agreement, “Cause” shall mean:
(i)Executive’s failure to perform Executive’s duties and responsibilities to the Company (other than a failure from Executive’s Disability) after receiving written notice of the alleged failure and ten (10) days opportunity to cure;
(ii)Executive’s commission of any act of fraud, embezzlement, dishonesty or misrepresentation;
(iii)Executive’s violation of any federal or state law or regulation applicable to the business of the Company or its affiliates;
(iv)Executive’s breach of any confidentiality agreement or invention assignment agreement between Executive and the Company (or any affiliate of the Company);
(v)Executive’s being convicted of, or entering a plea of nolo contendere to, a felony or committing any act of moral turpitude, dishonesty or fraud against, or the misappropriation of material property belonging to, the Company or its affiliates;
The determination as to whether Executive is being terminated for Cause shall be based on a good faith determination by the Board.
(b)Disability. For purposes of this Agreement, “Disability” shall mean that Executive has been unable to perform Executive’s duties hereunder, with or without reasonable accommodation, as the result of
- 5 -
Executive’s incapacity due to a physical or mental condition, and such inability, which continues for at least 120 consecutive calendar days or 150 calendar days during any consecutive twelve (12) month period, is determined to be total and permanent by a physician selected by the Company and its insurers and acceptable to Executive or to Executive’s legal representative (with such agreement on acceptability not to be unreasonably withheld).
(c)Good Reason. For purposes of this Agreement, “Good Reason” shall mean Executive’s resignation within thirty (30) days following the expiration of any Company cure period (discussed below) following the occurrence of any of the following, without Executive’s express written consent:
(i)the assignment to Executive of any duties or the reduction of Executive’s duties, either of which results in a material diminution in Executive’s position or responsibilities with the Company; provided that, it being understood that the continuance of Executive’s duties and responsibilities at the subsidiary or divisional level following a Change of Control (as defined in the Equity Plan), rather than at the parent, combined, or surviving company level following such Change of Control shall not deemed Good Reason within the meaning of this clause (i);
(ii)a material reduction by the Company in the base salary of Executive;
(iii)a material change in the geographic location at which Executive must perform services (for purposes of the foregoing, the relocation of Executive to a facility or a location less than 25 miles from Executive’s then-present location shall not be considered a material change in geographic location); or
(iv)any material breach by the Company of any material provision of this Agreement.
Executive’s resignation will not be deemed to be for Good Reason unless Executive has first provided the Company with written notice of the acts or omissions constituting the grounds for Good Reason within ninety (90) days of the initial existence of the grounds for Good Reason and a reasonable cure period of not less than thirty (30) days following the date the Company receives such notice, and such condition has not been cured during such period. The determination as to whether Executive resigned for Good Reason shall be based on a good faith determination by the Board.
10.Indemnification. Subject to applicable law, Executive will be provided indemnification to the maximum extent permitted by the Company’s Certificate of Incorporation or Bylaws, including, if applicable, any directors’ and officers’ insurance policies, with such indemnification to be on terms determined by the Board or any of its committees, but on terms no less favorable than provided to any other Company executive officer and subject to the terms of any separate written indemnification agreement.
11.Proprietary Information and Inventions Agreement. Executive agrees that as a condition of employment, Executive is required to abide by the Proprietary Information and Inventions Agreement dated July 3, 2017 (the “Confidentiality Agreement”), a copy of which is appended to this agreement. Executive’s failure to do this will constitute termination for Cause. Executive agrees and acknowledges that Executive’s right to receive the severance benefits set forth in Section 6 shall be conditioned upon Executive’s continued compliance with Executive’s obligations under the Confidentiality Agreement.
12.Protected Activity Not Prohibited. Executive understands that nothing in this Agreement shall in any way limit or prohibit Executive from engaging in any Protected Activity. For purposes of this Agreement, “Protected Activity” shall mean filing a charge, complaint, or report with, or otherwise communicating, cooperating, or participating in any investigation or proceeding that may be conducted by, any federal, state or local government agency or commission, including the Securities and Exchange Commission, the Equal Employment Opportunity Commission, the Occupational Safety and Health Administration, and the National Labor Relations Board (“Government Agencies”). Executive understands that in connection with such Protected Activity, Executive is permitted to disclose documents or other information as permitted by law, and without giving notice to, or receiving authorization from, the Company. Notwithstanding the foregoing, Executive agrees to take all reasonable precautions to prevent any unauthorized use or disclosure of any information that may constitute Company confidential information under the Confidentiality Agreement to any parties other than the Government Agencies. Executive further understands that “Protected Activity” does not include the disclosure of any Company attorney-client privileged communications. In addition, pursuant to the Defend Trade Secrets Act of 2016, Executive is notified that an individual will not be held criminally or civilly liable under any federal or state trade secret law for
- 6 -
the disclosure of a trade secret that (i) is made in confidence to a federal, state, or local government official (directly or indirectly) or to an attorney solely for the purpose of reporting or investigating a suspected violation of law, or (ii) is made in a complaint or other document filed in a lawsuit or other proceeding, if (and only if) such filing is made under seal. In addition, an individual who files a lawsuit for retaliation by an employer for reporting a suspected violation of law may disclose the trade secret to the individual’s attorney and use the trade secret information in the court proceeding, if the individual files any document containing the trade secret under seal and does not disclose the trade secret, except pursuant to court order.
13.Notices. All notices, requests, demands and other communications called for hereunder will be in writing and will be deemed given (i) on the date of delivery if delivered personally; (ii) one (1) day after being sent overnight by a well-established commercial overnight service; or (iii) four (4) days after being mailed by registered or certified mail, return receipt requested, prepaid and addressed to the Parties or their successors at the following addresses, or at such other addresses as the Parties may later designate in writing:
If to the Company:
NanoString Technologies, Inc.
530 Fairview Ave. N., Suite 2000
Seattle, WA 98109
Attn: CEO
Copy to: General Counsel
If to Executive:
to the last residential address known by the Company.
14.Severability. If any provision hereof becomes or is declared by a court of competent jurisdiction to be illegal, unenforceable, or void, this Agreement will continue in full force and effect without said provision.
15.Integration. This Agreement, together with the Confidentiality Agreement, the Option Agreement, the Equity Plan and the Indemnification Agreement, represents the entire agreement and understanding between the Parties as to the subject matter herein and supersedes all prior or contemporaneous agreements whether written or oral. No waiver, alteration, or modification of any of the provisions of this Agreement will be binding unless in a writing and signed by duly authorized representatives of the Parties hereto. In entering into this Agreement, no Party has relied on or made any representation, warranty, inducement, promise, or understanding that is not in this Agreement.
16.Waiver of Breach. The waiver of a breach of any term or provision of this Agreement, which must be in writing, will not operate as or be construed to be a waiver of any other previous or subsequent breach of this Agreement.
17.Headings. All captions and Section headings used in this Agreement are for convenient reference only and do not form a part of this Agreement.
18.Taxation. All payments made pursuant to this Agreement will be subject to withholding of any applicable taxes. Executive acknowledges that Executive has reviewed with Executive’s own tax advisors the federal, state, local, and foreign tax consequences of payments and transactions described in this Agreement, and Executive is relying solely on such advisors and not on any statements or representations of the Company or any of its agents. Executive understands that Executive (and not the Company) shall be responsible for any tax liability (other than employment tax liability owed by the Company) that may arise as a result of the payments and transactions contemplated by this Agreement.
19.Successors and Assigns. This Agreement will be binding upon and inure to the benefit of any successor of the Company. Any such successor of the Company will be deemed substituted for the Company under the terms of this Agreement for all purposes. For this purpose, “successor” means any person, firm, corporation or other business entity which at any time, whether by purchase, merger or otherwise, directly or indirectly acquires all or substantially all of the assets or business of the Company. None of the rights of Executive to receive any form of compensation payable pursuant to this Agreement may be assigned or transferred except by will or the laws of
- 7 -
descent and distribution. Any other attempted assignment, transfer, conveyance or other disposition of Executive’s right to compensation or other benefits will be null and void.
20.Governing Law. This Agreement will be governed by the laws of the state of Washington without regard to its conflict of laws provisions.
21.Acknowledgment. Executive acknowledges that Executive has had the opportunity to discuss this matter with and obtain advice from an attorney of Executive’s choice, has had sufficient time to review this Agreement, has carefully read this Agreement, and fully understands all the provisions of this Agreement, and is knowingly and voluntarily entering into this Agreement.
22.Counterparts. This Agreement may be executed in counterparts by facsimile or email PDF, and each counterpart will have the same force and effect as an original and will constitute an effective, binding agreement on the part of each of the undersigned.
(signature page follows)
- 8 -
IN WITNESS WHEREOF, each of the Parties has executed this Agreement, in the case of the Company by a duly authorized officer, as of the day and year written below.
“COMPANY”
NANOSTRING TECHNOLOGIES, INC.
By: /s/ R. Bradley Gray | Date: October 23, 2017 | |
Name: R. Bradley Gray | ||
Title: President & Chief Executive Officer | ||
“EXECUTIVE”
J. Chad Brown
/s/ K. Thomas Bailey | Date: October 17, 2017 | |
- 9 -
Exhibit 10.20
NanoString Technologies, Inc.
EMPLOYMENT AGREEMENT
This Employment Agreement (the “Agreement”) is entered into, effective as of January 16, 2018 (the “Effective Date”), by and between NanoString Technologies, Inc. (the “Company”) and K. Thomas Bailey (“Executive”).
1.Duties and Scope of Employment.
(a)Positions and Duties. Executive will serve as the Chief Financial Officer of the Company. Executive will render such business and professional services in the performance of Executive’s duties, consistent with Executive’s position within the Company, as shall be assigned to Executive by the Company’s Chief Executive Officer (“CEO”). The period of Executive’s employment under this Agreement is referred to herein as the “Employment Term.” The Employment Term will commence on Executive’s actual start date of employment with the Company (the “Start Date”), which is expected to occur no later than January 16, 2018.
(b)Obligations. During the Employment Term, Executive will devote Executive’s full business efforts and time to the Company and will use good faith efforts to discharge Executive’s obligations under this Agreement to the best of Executive’s ability and in accordance with each of the Company’s corporate guidance and ethics guidelines, conflict of interest policies, code of conduct and Employee Handbook. For the duration of the Employment Term, Executive agrees not to actively engage in any other employment, occupation, or consulting activity for any direct or indirect remuneration without the prior approval of the Board; provided, however, that Executive may, without the approval of the Board, serve in any capacity with any civic, educational, social, or charitable organization, provided such services do not interfere with Executive’s obligations to the Company. Executive may also continue serving as a business advisor to Peloton Equity Partners, provided that such services (i) do not materially interfere with the effective discharge of Executive’s duties, responsibilities, and obligations under this Section 1 and (ii) do not violate the non-competition and non-solicitation covenants in the “Confidentiality Agreement” (as defined below).
(c)Prior Agreements. Executive hereby represents and warrants to the Company that he is not party to any contract, understanding, agreement, or policy, written or otherwise, which would be breached by his entering into, or performing services under, this Agreement.
(d)Other Entities. Executive agrees to serve and may be appointed as an officer and director for any of the Company’s subsidiaries, partnerships, joint ventures, limited liability companies and other affiliates, including entities in which the Company has a significant investment as determined by the Company. As used in this Agreement, the term “affiliates” will include any entity controlled by, controlling, or under common control of the Company.
(e)External Board Participation. Company agrees that Executive may serve on up to two external boards of directors, provided that such service does not materially interfere with Executive’s obligations to the Company or materially conflict with any business conducted by the Company (or any successor thereto) or its subsidiaries, now or in the future. Prior to accepting any such external board position, Executive will seek written approval of the Company to do so, with such approval not to be unreasonably withheld. Pursuant to such, the Company hereby agrees, and this Agreement shall serve as written permission, that Executive may continue serving as a member of the board of managers of AgaMatrix Holdings, LLC (“AgaMatrix”) (including his service as a member of the boards of directors of AgaMatrix’s wholly-owned subsidiaries AgaMatrix Inc., a developer of diagnostic technologies for diabetes care, and WaveForm Technologies Inc., a developer of technologies for diabetes care) and as a member of the board of managers of SCP Interventional Radiology, LLC (“SCP”) (including his service as a member of the board of directors of SCP’s wholly-owned subsidiary IZI Medical Products, a developer of minimally invasive medical technologies for vascular and spine procedures), provided that such service does not materially interfere with Executive’s obligations to the Company or materially conflict with any business conducted by the Company (or any successor thereto) or its subsidiaries, now or in the future.
2.Immigration Compliance. Executive is required to provide the Company with proof of Executive’s eligibility to work in the U.S. within three (3) business days of Executive’s start date or Executive’s employment will be terminated for Cause.
3.At-Will Employment. Executive and the Company agree that Executive’s employment with the Company constitutes “at-will” employment. Executive and the Company acknowledge that this employment relationship may be terminated by either Executive or the Company, at any time, upon written notice to the other Party, with or without cause, for any reason or no reason. Executive understands and agrees that neither Executive’s job performance nor promotions, commendations, bonuses, or the like from the Company alter Executive’s at-will status or give rise to or in any way serve as the basis for modification, amendment, or extension, by implication or otherwise, of Executive’s employment with the Company.
4.Compensation.
(a)Base Salary. During the Employment Term, the Company will pay Executive as compensation for Executive’s services a base salary at the annualized rate of Four Hundred Thousand dollars ($400,000) (as such base salary may be modified from time to time, the “Base Salary”). The Base Salary will be paid in accordance with the Company’s normal payroll practices and be subject to the usual, required withholdings. The Base Salary may change at the Company’s discretion; for the avoidance of doubt, no such change shall override any rights Executive may have pursuant to this Agreement to resign for Good Reason.
(b)Target Bonus. Executive shall be eligible to be considered for an annual, performance-based, cash bonus (the “Target Bonus”) with a target amount of 50% of Executive’s Base Salary for each calendar year, which bonus shall be awarded in the sole discretion of the Compensation Committee of the Board (the “Committee”) based on a recommendation from the CEO, which shall be based on Executive’s performance in the prior calendar year against metrics established for such year by the Company. Any bonus awarded shall be paid by no later than March 15 following the calendar year to which the bonus corresponds. If Executive’s employment terminates for any reason prior to the end of a given calendar year, then the Company shall have no obligation to pay a bonus to Executive for such year. For the year ending December 31, 2018, Executive’s bonus will be prorated based upon Executive’s Start Date.
(c)Review and Adjustments. Executive’s Base Salary, Target Bonus, and other compensatory arrangements will be subject to review and adjustment in accordance with the Company’s applicable policies.
(d)Equity.
(i)Stock Option. Executive acknowledges and agrees that it will be recommended to the Board that it grant Executive a stock option to purchase Ninety Five Thousand (95,000) shares of the Company’s common stock (the “Option”). The exercise price per share for the Option will be the fair market value per share of an underlying share of Company common stock on the date of grant, as determined in accordance with the 2018 Equity Plan (as defined below). The vesting schedule of the Option will be as follows: twenty-five percent (25%) of the shares subject to the Option shall vest on the one (1) year anniversary of the Start Date, subject to Executive’s continued service with the Company through such date, and the remaining shares subject to the Option will vest monthly over the next thirty-six (36) months in approximately equal monthly amounts subject to Executive’s continued service with the Company through each such vesting date. The Option shall be subject to the terms, definitions and conditions, including vesting requirements, of the Company’s 2018 Inducement Equity Incentive Plan (the “2018 Equity Plan”) and a stock option agreement between Executive and the Company (the “Option Agreement”), both of which are incorporated herein by reference.
(ii)Restricted Stock Units. Additionally, it will be recommended to the Board that it grant Executive Thirty Five Thousand (35,000) Restricted Stock Units (the “RSUs”). The RSUs will be scheduled to vest as to 100% of the award on the second anniversary of Executive’s Start Date. The RSUs shall be subject to the terms, definitions and conditions, including vesting requirements of the Company’s 2018 Equity Plan and a restricted stock unit agreement between Executive and the Company (the “RSU Agreement”), both of which are incorporated herein by reference. No right to any stock is earned or accrued until such time that vesting occurs, nor does the grant confer any right to continue vesting or employment.
(iii)Change in Control. The Company will further recommend to the Board that in the
event that there is a “Change in Control” (as such term is defined in the 2018 Equity Plan) and if upon or during the twelve (12) months following such Change in Control, (i) Executive terminates his employment with the Company (and any affiliate) for “Good Reason” (as such term is defined in Section 10 of this Agreement) or (ii) the Company (or any affiliate) terminates Executive’s employment without “Cause” (as such term is defined in Section 10 of this Agreement), then, in each case, one hundred percent (100%) of the unvested portion of the Stock Option and RSUs, as applicable, shall vest and become exercisable at the time of Executive’s termination of employment. The description of the Stock Option and RSU in this Section 4(d) is qualified in its entirety to the actual terms as shall be set forth in the Option Agreement and the RSU Agreement, as applicable.
5.Limitation on Payments. In the event that the benefits provided for in this Agreement or otherwise payable to Executive (x) constitute “parachute payments” within the meaning of Section 280G of the Internal Revenue Code of 1986, as amended (the “Code”) and (y) but for this Section 5 would be subject to the excise tax imposed by Section 4999 of the Code, then Executive’s benefits will be either (i) delivered in full, or (ii) delivered as to such lesser extent which would result in no portion of such benefits being subject to excise tax under Section 4999 of the Code, whichever of the foregoing amounts, taking into account the applicable federal, state and local income taxes and the excise tax imposed by Section 4999, results in the receipt by Executive on an after-tax basis, of the greatest amount of benefits, notwithstanding that all or some portion of such benefits may be taxable under Section 4999 of the Code. If a reduction in amounts to be paid must be made, reduction shall occur in the following order: first, reduction of cash payments, which shall occur in reverse chronological order such that the cash payment owed on the latest date following the occurrence of the event triggering such excise tax will be the first cash payment to be reduced; second, cancellation of accelerated vesting of equity awards, which shall occur in the reverse order of the date of grant for such stock awards (i.e., the vesting of the most recently granted stock awards will be reduced first); and third, reduction of employee benefits, which shall occur in reverse chronological order such that the benefit owed on the latest date following the occurrence of the event triggering such excise tax will be the first benefit to be reduced. If two or more equity awards are granted on the same date, each award will be reduced on a pro-rata basis. In no event shall Executive have any discretion with respect to the ordering of payment reductions. Unless the Company and Executive otherwise agree in writing, any determination required under this Section will be made in writing by a well-recognized independent public accounting firm chosen by the Company (the “Accountants”), whose determination will be conclusive and binding upon Executive and the Company for all purposes. For purposes of making the calculations required by this Section, the Accountants may make reasonable assumptions and approximations concerning applicable taxes and may rely on reasonable, good faith interpretations concerning the application of Sections 280G and 4999 of the Code. The Company and Executive will furnish to the Accountants such information and documents as the Accountants may reasonably request in order to make a determination under this Section 5. The Company will bear all costs the Accountants may reasonably incur in connection with any calculations contemplated by this Section 5.
6.Employee Benefits.
(a)Generally. During the Employment Term, Executive is eligible to participate in the employee benefit plans currently maintained by the Company without limitation, the medical, dental, vision, life, flexible spending account, and disability plans available to similarly situated employees subject to their terms, including eligibility requirements, in effect from time to time. The Company may cancel or change the benefit plans and programs it offers to the Company’s employees at any time.
(b)Paid Time Off. During the Employment Term, Executive will be entitled to paid time off (“PTO”) in accordance with the Company’s executive PTO policy. PTO shall be taken at such time as mutually and reasonably agreed by Executive and the Company and in accordance with the Company’s policies in effect from time to time for other similarly situated employees. Executive will receive paid holidays in accordance with the Company’s regular holiday practices.
(c)Expenses. The Company will reimburse Executive for reasonable travel, entertainment, and other expenses incurred by Executive in the furtherance of the performance of Executive’s duties hereunder, in accordance with the Company’s expense reimbursement policy as in effect from time to time.
7.Termination of Employment.
(a)Accrued Obligations. In the event Executive’s employment with the Company terminates for any reason, Executive will be entitled to any (a) unpaid Base Salary accrued up to the effective date of termination;
(b) benefits or compensation as provided under the terms of any employee benefit and compensation agreements or plans applicable to Executive; and (c) unreimbursed business expenses required to be reimbursed to Executive pursuant to the Company’s expense reimbursement policy and applicable law; and (d) rights to indemnification Executive may have under the Company’s Certificate of Incorporation, and By-Laws of this Agreement or separate indemnification agreement.
(b)Termination of Employment Without Cause or With Good Reason. If, (i) Executive terminates his employment with the Company (or any affiliate) for Good Reason or (ii) the Company (or any affiliate) terminates Executive’s employment without Cause, subject to Section 7(d), Section 8 and Section 11 (each of (i) and (ii) referred to as an “Involuntary Termination”), Executive will be eligible to receive severance pay (less applicable withholding taxes) at a rate equal to Executive’s Base Salary rate, as then in effect, for a period of six (6) months (such payments shall be paid periodically in accordance with the Company’s normal payroll policies); provided, however, that if such Involuntary Termination occurs within twelve (12) months following a Change in Control (as defined in the Company’s 2018 Equity Plan), (i) Executive shall instead be entitled to a lump sum payment equal to twelve (12) months of Executive’s then-effective Base Salary, and an additional lump sum payment equal to Executive’s target bonus, calculated based on the completion of a full calendar year and at the target bonus percentage (as a percentage of then-current Base Salary) then in effect times the then-effective Base Salary, with no reductions or considerations respecting the Executive’s performance (all less applicable withholding taxes) and (ii) if Executive elects continuation coverage pursuant to the Consolidated Omnibus Budget Reconciliation Act of 1985, as amended (“COBRA”) within the time period prescribed pursuant to COBRA for Executive and Executive’s eligible dependents, the Company will reimburse Executive for the premiums necessary to continue group health insurance benefits for Executive and Executive’s eligible dependents for a period of twelve (12) months following the date of Involuntary Termination, except that the right to future COBRA payments shall terminate the date upon which Executive ceases to be eligible for coverage under COBRA. If Executive becomes entitled to receive severance pay pursuant to this Section, Executive will not be entitled to any other severance benefits or similar payments in accordance with the Company’s established policies as then in effect.
(c)Termination by Reason of Death or Disability. If Executive’s employment with the Company terminates as a result of Executive’s death or “Disability” (as defined in Section 9 below), Executive or Executive’s estate or representative will receive all salary accrued (plus any other amounts payable as determined by the Board in its sole discretion) as of the date of Executive’s death or Disability and any other benefits payable under the Company’s then-existing benefit plans and policies in accordance with such plans and policies in effect on the date of death or Disability and in accordance with applicable law. Such payments shall be made by the Company periodically in accordance with the Company’s normal payroll policies with respect to each element of such payments. For the avoidance of doubt, Executive’s termination of employment or service due to Executive’s death or Disability will not be deemed a termination without Cause for purposes of this Section.
(d)Release. Notwithstanding anything to the contrary, the payments and benefits under Section 7(b) are contingent upon Executive signing and not revoking a release of claims agreement with the Company in a form specified by the Company consistent with such releases entered into with other similarly situated executive employees of the Company (which release provided to Executive will include an agreement not to disparage the Company and an agreement that the Company not disparage the Executive, provided that the Company’s non-disparagement obligation will relate only to the Company’s senior leadership team (or similarly composed group) and only for so long as any members of such group are respectively employed by the Company, and which also will include non-solicit provisions and other standard terms and conditions, but in no event will any restrictive covenants included in such release exceed the geographic and temporal scope of the restrictive covenants included in the Confidentiality Agreement) (the “Release”), which Release shall be provided to Executive within five (5) days after the Executive’s termination of employment, and such Release becoming effective and irrevocable no later than sixty (60) days following the date of termination of employment (such deadline, the “Release Deadline”). If the Release does not become effective and irrevocable by the Release Deadline, Executive will forfeit any rights to severance payments or benefits under this Agreement. In no event will Executive’s payments be paid or provided until the Release actually becomes effective and irrevocable. Subject to Section 8 below, the payments and benefits under Section 7(b) that, but for the delay for the Release effectiveness, would have been made prior to the Release’s effectiveness shall be made as soon as practicable after the effectiveness of the Release (and in all cases, within 60 days following the Executive’s termination of employment) and the remaining payments shall be made as provided in this Agreement, provided that
the Release has become effective and irrevocable by the Release Deadline.
8.Section 409A.
(a)Notwithstanding anything to the contrary in this Agreement, no severance pay or benefits to be paid or provided to Executive, if any, pursuant to this Agreement that, when considered together with any other severance payments or separation benefits, are considered deferred compensation under Section 409A of the Code, and the final regulations and any guidance promulgated thereunder (“Section 409A”) (together, the “Deferred Payments”) will be paid or otherwise provided until Executive has a “separation from service” within the meaning of Section 409A. Similarly, no severance payable to Executive, if any, pursuant to this Agreement that otherwise would be exempt from Section 409A pursuant to Treasury Regulation Section 1.409A-1(b)(9) will be payable until Executive has a “separation from service” within the meaning of Section 409A.
(b)Any severance payments or benefits under this Agreement that would be considered Deferred Payments will be paid on, or, in the case of installments, will not commence until the sixtieth (60th) day following Executive’s separation from service, or if later, such time as required by Section 8(c). Any installment payments that would have been made to Executive during the sixty (60) day period immediately following Executive’s separation from service but for the preceding sentence will be paid to Executive on the sixtieth (60th) day following Executive’s separation from service and the remaining payments shall be made as provided in this Agreement.
(c)Notwithstanding anything to the contrary in this Agreement, if Executive is a “specified employee” within the meaning of Section 409A at the time of Executive’s termination (other than due to death), then the Deferred Payments that are payable within the first six months following Executive’s separation from service, will become payable on the first payroll date that occurs on or after the date six (6) months and one (1) day following the date of Executive’s separation from service. All subsequent Deferred Payments, if any, will be payable in accordance with the payment schedule applicable to each payment or benefit. Notwithstanding anything herein to the contrary, if Executive dies following Executive’s separation from service, but prior to the six (6) month anniversary of the separation from service, then any payments delayed in accordance with this paragraph will be payable in a lump sum as soon as administratively practicable after the date of Executive’s death and all other Deferred Payments will be payable in accordance with the payment schedule applicable to each payment or benefit. Each payment and benefit payable under this Agreement is intended to constitute a separate payment for purposes of Section 1.409A-2(b)(2) of the Treasury Regulations.
(d)Any amount paid under this Agreement that satisfies the requirements of the “short-term deferral” rule set forth in Section 1.409A-1(b)(4) of the Treasury Regulations will not constitute Deferred Payments for purposes of this Section.
(e)Any amount paid under this Agreement that qualifies as a payment made as a result of an involuntary separation from service pursuant to Section 1.409A-1(b)(9)(iii) of the Treasury Regulations that does not exceed the “Section 409A Limit” (as defined below) will not constitute Deferred Payments for purposes of this Section. For purposes of this Agreement, “Section 409A Limit” means two (2) times the lesser of: (x) Executive’s annualized compensation based upon the annual rate of pay paid to Executive during Executive’s taxable year preceding Executive’s taxable year of Executive’s termination of employment as determined under, and with such adjustments as are set forth in, Treasury Regulation 1.409A-1(b)(9)(iii)(A)(1) and any Internal Revenue Service guidance issued with respect thereto, or (y) the maximum amount that may be taken into account under a qualified plan pursuant to Section 401(a)(17) of the Code for the year in which Executive’s employment is terminated.
(f)The foregoing provisions are intended to be exempt from or comply with the requirements of Section 409A so that none of the severance payments and benefits to be provided hereunder will be subject to the additional tax imposed under Section 409A, and any ambiguities or ambiguous terms herein will be interpreted to be so exempt or to so comply. The Company and Executive agree to work together in good faith to consider amendments to this Agreement and to take such reasonable actions which are necessary, appropriate, or desirable to avoid imposition of any additional tax or income recognition prior to actual payment to Executive under Section 409A. Executive agrees and acknowledges that the Company makes no representations or warranties with respect to the application of Section 409A and other tax consequences to any payments hereunder and, by the acceptance of any such payments, Executive agrees to accept the potential application of Section 409A and the other tax consequences of any payments made hereunder.
9.Arbitration.
(a) General. In consideration of Executive’s service to the Company, Executive’s promise to arbitrate all employment related disputes and Executive’s receipt of the compensation, pay raises and other benefits paid to Executive by the Company, at present and in the future, Executive agrees that any and all controversies, claims, or disputes with anyone (including the Company and any employee, officer, director, stockholder or benefit plan of the Company in their capacity as such or otherwise) arising out of, relating to, or resulting from Executive’s service to the Company under this Agreement or otherwise or the termination of Executive’s service with the Company, including any breach of this Agreement, shall be subject to binding arbitration under the Arbitration Rules set forth in the Revised Code of Washington Chapter 7.04 (the “Rules”) and pursuant to Washington law. Disputes which Executive agrees to arbitrate, and thereby agrees to waive any right to a trial by jury, include any statutory claims under state or federal law, including, but not limited to, claims under Title VII of the Civil Rights Act of 1964, the Americans with Disabilities Act of 1990, the Age Discrimination in Employment Act of 1967, the Older Workers Benefit Protection Act, claims of harassment, discrimination or wrongful termination. Executive further understands that this Agreement to arbitrate also applies to any disputes that the Company may have with Executive.
(b) Procedure. Executive agrees that any arbitration will be administered by the American Arbitration Association (“AAA”) and that a neutral arbitrator will be selected in a manner consistent with its National Rules for the Resolution of Employment Disputes. The arbitration proceedings will allow for discovery according to the National Rules for the Resolution of Employment Disputes and the Washington Code of Civil Procedure. Executive agrees that the arbitrator shall have the power to decide any motions brought by any party to the arbitration, including motions for summary judgment and/or adjudication and motions to dismiss and demurrers, prior to any arbitration hearing. Executive agrees that the arbitrator shall issue a written decision on the merits with findings of fact and conclusions of law. Executive also agrees that the arbitrator shall have the power to award any remedies, including attorneys’ fees and costs, available under applicable law. Executive understands the Company will pay for any administrative or hearing fees charged by the arbitrator or AAA except that Executive shall pay the first $200.00 of any filing fees associated with any arbitration Executive initiates. Executive agrees that the arbitrator shall administer and conduct any arbitration in a manner consistent with the Rules and that to the extent that the AAA’s National Rules for the Resolution of Employment Disputes conflict with the Rules, the Rules shall take precedence.
(c) Remedy. Except as provided by the Rules, arbitration shall be the sole, exclusive and final remedy for any dispute between Executive and the Company. Accordingly, except as provided for by the Rules, neither Executive nor the Company will be permitted to pursue court action regarding claims that are subject to arbitration. Notwithstanding, the arbitrator will not have the authority to disregard or refuse to enforce any lawful Company policy, and the arbitrator shall not order or require the Company to adopt a policy not otherwise required by law which the Company has not adopted.
(d) Availability of Injunctive Relief. In addition to the right under the Rules to petition the court for provisional relief, Executive agrees that any party may also petition the court for injunctive relief where either party alleges or claims a violation of this Agreement or the Confidentiality Agreement or any other agreement regarding trade secrets, confidential information, non-competition, nonsolicitation or non-disparagement. In the event either party seeks injunctive relief, the prevailing party shall be entitled to recover reasonable costs and attorneys’ fees.
(e) Administrative Relief. Executive understands that this Agreement does not prohibit Executive from pursuing an administrative claim with a local, state or federal administrative body such as the Washington State Human Rights Commission, Equal Employment Opportunity Commission or the workers’ compensation board. This Agreement does, however, preclude Executive from pursuing court action regarding any such claim.
10. Definitions.
(a) Cause. For purposes of this Agreement, “Cause” for a termination of Executive will exist if Executive is terminated for any of the following reasons:
(i)Executive’s failure to substantially perform Executive’s duties and responsibilities to the Company (other than a failure from Executive’s Disability) after receiving written notice of the alleged failure and ten (10) days opportunity to cure;
(ii)Executive’s commission of any act of fraud, embezzlement, dishonesty or misrepresentation;
(iii)Executive’s violation of any federal or state law or regulation applicable to the business of the Company or its affiliates;
(iv)Executive’s breach of any confidentiality agreement or invention assignment agreement between Executive and the Company (or any affiliate of the Company);
(v)Executive’s being convicted of, or entering a plea of nolo contendere to, a felony or committing any act of moral turpitude, dishonesty or fraud against, or the misappropriation of material property belonging to, the Company or its affiliates; or
(vi)Executive’s failure to provide the Company with proof of Executive’s authorization to work in the U.S.
The determination as to whether Executive is being terminated for Cause shall be based on a good faith determination by the Board.
(b)Disability. For purposes of this Agreement, “Disability” shall mean that Executive has been unable to perform Executive’s duties hereunder, with or without reasonable accommodation, as the result of Executive’s incapacity due to a physical or mental condition, and such inability, which continues for at least 120 consecutive calendar days or 150 calendar days during any consecutive twelve (12) month period, is determined to be total and permanent by a physician selected by the Company and its insurers and acceptable to Executive or to Executive’s legal representative (with such agreement on acceptability not to be unreasonably withheld).
(c)Good Reason. For purposes of this Agreement, “Good Reason” shall mean Executive’s resignation within thirty (30) days following the expiration of any Company cure period (discussed below) following the occurrence of any one or more of the following, without Executive’s express written consent:
(i)the assignment to Executive of any duties or the reduction of Executive’s duties, either of which results in a material diminution in Executive’s position or responsibilities with the Company; provided that, it being understood that the continuance of Executive’s duties and responsibilities at the subsidiary or divisional level following a Change in Control (as defined in the Equity Plan), rather than at the parent, combined, or surviving company level following such Change in Control shall not be deemed Good Reason within the meaning of this clause (i);
(ii)a material reduction by the Company in the base salary of Executive;
(iii)a material change in the geographic location at which Executive must perform services (for purposes of the foregoing, the relocation of Executive to a facility or a location less than 25 miles from Executive’s then-present location shall not be considered a material change in geographic location); or
(iv)any material breach by the Company of any material provision of this Agreement.
Executive’s resignation will not be deemed to be for Good Reason unless Executive has first provided the Company with written notice of the acts or omissions constituting the grounds for Good Reason within ninety (90) days of the initial existence of the grounds for Good Reason and a reasonable cure period of not less than thirty (30) days following the date the Company receives such notice, and such condition has not been cured during such period. The determination as to whether Executive resigned for Good Reason shall be based on a good faith determination by the Board.
10.Indemnification. Subject to applicable law, Executive will be provided indemnification to the maximum extent permitted by the Company’s Certificate of Incorporation or Bylaws, including, if applicable, any directors and officers insurance policies, with such indemnification to be on terms determined by the Board or any of its committees, but on terms no less favorable than provided to any other Company executive officer or director and subject to the terms of any separate written indemnification agreement.
11.Confidential Information, Invention Assignment, and Arbitration. Executive agrees that as a condition of employment, Executive is required to abide by the Company’s standard At-Will Employment, Confidential Information, Invention Assignment, and Arbitration Agreement signed by Executive on January 4, 2018 (the “Confidentiality Agreement”). Executive’s failure to do this will constitute termination for Cause. Executive agrees
and acknowledges that Executive’s right to receive the severance benefits set forth in Section 7 shall be conditioned upon Executive’s continued compliance with Executive’s obligations under the Confidentiality Agreement.
12.Protected Activity Not Prohibited. Executive understands that nothing in this Agreement shall in any way limit or prohibit Executive from engaging in any Protected Activity. For purposes of this Agreement, “Protected Activity” shall mean filing a charge, complaint, or report with, or otherwise communicating, cooperating, or participating in any investigation or proceeding that may be conducted by, any federal, state or local government agency or commission, including the Securities and Exchange Commission, the Equal Employment Opportunity Commission, the Occupational Safety and Health Administration, and the National Labor Relations Board (“Government Agencies”). Executive understands that in connection with such Protected Activity, Executive is permitted to disclose documents or other information as permitted by law, and without giving notice to, or receiving authorization from, the Company. Notwithstanding the foregoing, Executive agrees to take all reasonable precautions to prevent any unauthorized use or disclosure of any information that may constitute Company confidential information under the Confidentiality Agreement to any parties other than the Government Agencies. Executive further understands that “Protected Activity” does not include the disclosure of any Company attorney-client privileged communications. In addition, pursuant to the Defend Trade Secrets Act of 2016, Executive is notified that an individual will not be held criminally or civilly liable under any federal or state trade secret law for the disclosure of a trade secret that (i) is made in confidence to a federal, state, or local government official (directly or indirectly) or to an attorney solely for the purpose of reporting or investigating a suspected violation of law, or (ii) is made in a complaint or other document filed in a lawsuit or other proceeding, if (and only if) such filing is made under seal. In addition, an individual who files a lawsuit for retaliation by an employer for reporting a suspected violation of law may disclose the trade secret to the individual’s attorney and use the trade secret information in the court proceeding, if the individual files any document containing the trade secret under seal and does not disclose the trade secret, except pursuant to court order.
13.Notices. All notices, requests, demands and other communications called for hereunder will be in writing and will be deemed given (i) on the date of delivery if delivered personally; (ii) one (1) day after being sent overnight by a well-established commercial overnight service; or (iii) four (4) days after being mailed by registered or certified mail, return receipt requested, prepaid and addressed to the Parties or their successors at the following addresses, or at such other addresses as the Parties may later designate in writing:
If to the Company:
NanoString Technologies, Inc.
530 Fairview Ave. N., Suite 2000
Seattle, WA 98109
Attn: CEO
Copy to: General Counsel
If to Executive:
to the last residential address known by the Company.
14.Severability. If any provision hereof becomes or is declared by a court of competent jurisdiction to be illegal, unenforceable, or void, this Agreement will continue in full force and effect without said provision.
15.Integration, Entire Agreement. This Agreement, together with the Confidentiality Agreement, the Option Agreement, the RSU Agreement, the 2018 Equity Plan and the Indemnification Agreement, represents the entire agreement and understanding between the Parties as to the subject matter herein and supersedes all prior or contemporaneous agreements whether written or oral, including but not limited to the offer letter (excluding the Confidentiality Agreement) dated January 4, 2018 entered into between Executive and the Company. No waiver, alteration, or modification of any of the provisions of this Agreement will be binding unless in a writing and signed by duly authorized representatives of the Parties hereto. In entering into this Agreement, no Party has relied on or made any representation, warranty, inducement, promise, or understanding that is not in this Agreement.
16.Waiver of Breach. The waiver of a breach of any term or provision of this Agreement, which must be in writing, will not operate as or be construed to be a waiver of any other previous or subsequent breach of this Agreement.
17.Headings. All captions and Section headings used in this Agreement are for convenient reference only
and do not form a part of this Agreement.
18.Taxation. All payments made pursuant to this Agreement will be subject to withholding of any applicable taxes. Executive acknowledges that Executive has reviewed with Executive’s own tax advisors the federal, state, local, and foreign tax consequences of payments and transactions described in this Agreement, and Executive is relying solely on such advisors and not on any statements or representations of the Company or any of its agents. Executive understands that Executive (and not the Company) shall be responsible for any tax liability (other than employment tax liability owed by the Company) that may arise as a result of the payments and transactions contemplated by this Agreement.
19.Successors and Assigns. This Amendment and the rights and obligations of the parties hereunder shall inure to the benefit of, and be binding upon, their respective successors, assigns, and legal representatives.
20.Governing Law. This Agreement will be governed by the laws of the state of Washington without regard to its conflict of laws provisions.
21.Acknowledgment. Executive acknowledges that Executive has had the opportunity to discuss this matter with and obtain advice from an attorney of Executive’s choice, has had sufficient time to review this Agreement, has carefully read this Agreement, and fully understands all the provisions of this Agreement, and is knowingly and voluntarily entering into this Agreement.
22.Counterparts. This Agreement may be executed in counterparts by facsimile or email PDF, and each counterpart will have the same force and effect as an original and will constitute an effective, binding agreement on the part of each of the undersigned.
(signature page follows)
IN WITNESS WHEREOF, each of the Parties has executed this Agreement, in the case of the Company by a duly authorized officer, as of the day and year written below.
“COMPANY”
NANOSTRING TECHNOLOGIES, INC.
By: /s/ R. Bradley Gray | Date: January 16, 2018 | |
Name: R. Bradley Gray | ||
Title: President & Chief Executive Officer | ||
“EXECUTIVE”
K. Thomas Bailey
/s/ K. Thomas Bailey | Date: January 16, 2018 | |
Exhibit 10.31
AMENDMENT AGREEMENT NO. 4
THIS AMENDMENT AGREEMENT NO. 4 (this “Amendment”), dated as of January 5, 2018, is made among NanoString Technologies, Inc., a Delaware corporation (the “Borrower”), the Subsidiary Guarantors listed on the signature pages hereof under the heading “SUBSIDIARY GUARANTORS” (each a “Subsidiary Guarantor” and, collectively, the “Subsidiary Guarantors”, and together with the Borrower, each an “Obligor” and, collectively, the “Obligors”) and the Lenders listed on the signature pages hereof under the heading “LENDERS” (each a “Lender” and, collectively, the “Lenders”).
The Obligors and the Lenders are parties to that certain Term Loan Agreement, dated as of April 1, 2014, amended by Amendment Agreement No. 1, dated as of April 16, 2015, Amendment Agreement No. 2, dated as of October 30, 2015, and Amendment Agreement No. 3, dated as of February 17, 2017 (as further amended, amended and restated, modified or supplemented from time to time, the “Loan Agreement”).
The parties hereto desire to amend the Loan Agreement on the terms and subject to the conditions set forth herein.
Accordingly, the parties hereto agree as follows:
SECTION 1 | Definitions; Interpretation. |
(a)Terms Defined in Loan Agreement. All capitalized terms used in this Amendment (including in the recitals hereof) and not otherwise defined herein shall have the meanings assigned to them in the Loan Agreement.
(b)Interpretation. The rules of interpretation set forth in Section 1.03 of the Loan Agreement shall be applicable to this Amendment and are incorporated herein by this reference.
SECTION 2 Amendments.
Subject to Section 3, the Loan Agreement is hereby amended as follows:
(a)The definition of “Liquidity” set forth in Section 1.01 is hereby amended and restated to read in its entirety as follows:
“Liquidity” means the balance of unencumbered (other than by Liens described in Sections 9.02(a), 9.02(c) (provided that there is no default under the documentation governing Permitted Priority Debt) and 9.02(j)) cash and Permitted Cash Equivalent Investments (which for greater certainty shall not include any undrawn credit lines), in each case to the extent held in an account over which the Lenders have a perfected security interest.”
SECTION 3 | Conditions of Effectiveness. |
The effectiveness of Section 2 shall be subject to the following conditions precedent:
(a)The Obligors and all of the Lenders shall have duly executed and delivered this Amendment.
SECTION 4 Representations and Warranties; Reaffirmation.
(a)Each Obligor hereby ratifies, confirms, reaffirms, and acknowledges its obligations under the Loan Documents to which it is a party and agrees that the Loan Documents remain in full force and effect, undiminished by this Amendment, except as expressly provided herein. By executing this Amendment, each Obligor acknowledges that it has read, consulted with its attorneys regarding, and understands, this Amendment.
SECTION 5 Governing Law; Submission To Jurisdiction; Waiver Of Jury Trial.
(a)Governing Law. This Amendment and the rights and obligations of the parties hereunder shall be governed by, and construed in accordance with, the law of the State of New York, without regard to principles of conflicts of laws that would result in the application of the laws of any other jurisdiction; provided that Section 5-1401 of the New York General Obligations Law shall apply.
1
(b)Submission to Jurisdiction. Each Obligor agrees that any suit, action or proceeding with respect to this Amendment or any other Loan Document to which it is a party or any judgment entered by any court in respect thereof may be brought initially in the federal or state courts in Houston, Texas or in the courts of its own corporate domicile and irrevocably submits to the non-exclusive jurisdiction of each such court for the purpose of any such suit, action, proceeding or judgment. This Section 5 is for the benefit of the Lenders only and, as a result, no Lender shall be prevented from taking proceedings in any other courts with jurisdiction. To the extent allowed by applicable Laws, the Lenders may take concurrent proceedings in any number of jurisdictions.
(c)Waiver of Jury Trial. Each Obligor and each Lender hereby irrevocably waives, to the fullest extent permitted by applicable law, any and all right to trial by jury in any suit, action or proceeding arising out of or relating to this Amendment, the other Loan Documents or the transactions contemplated hereby or thereby.
SECTION 6 Miscellaneous.
(a)No Waiver. Nothing contained herein shall be deemed to constitute a waiver of compliance with any term or condition contained in the Loan Agreement or any of the other Loan Documents or constitute a course of conduct or dealing among the parties. Except as expressly stated herein, the Lenders reserve all rights, privileges and remedies under the Loan Documents. Except as amended hereby, the Loan Agreement and other Loan Documents remain unmodified and in full force and effect. All references in the Loan Documents to the Loan Agreement shall be deemed to be references to the Loan Agreement as amended hereby.
(b)Severability. In case any provision of or obligation under this Amendment shall be invalid, illegal or unenforceable in any jurisdiction, the validity, legality and enforceability of the remaining provisions or obligations, or of such provision or obligation in any other jurisdiction, shall not in any way be affected or impaired thereby.
(c)Headings. Headings and captions used in this Amendment (including the Exhibits, Schedules and Annexes hereto, if any) are included for convenience of reference only and shall not be given any substantive effect.
(d)Integration. This Amendment constitutes a Loan Document and, together with the other Loan Documents, incorporates all negotiations of the parties hereto with respect to the subject matter hereof and is the final expression and agreement of the parties hereto with respect to the subject matter hereof.
(e)Counterparts. This Amendment may be executed in any number of counterparts, all of which taken together shall constitute one and the same instrument and any of the parties hereto may execute this Amendment by signing any such counterpart.
(f)Controlling Provisions. In the event of any inconsistencies between the provisions of this Amendment and the provisions of any other Loan Document, the provisions of this Amendment shall govern and prevail. Except as expressly modified by this Amendment, the Loan Documents shall not be modified and shall remain in full force and effect.
(g)Clarifications.
(i)Borrower and Lenders hereby acknowledge and agree that, in the event that any portion of the Loans becomes due and payable prior to the Stated Maturity Date, whether as a result of acceleration or any other required prepayment event, the “Redemption Date” for purposes of calculating the Prepayment Premium (to the extent due) will be the date of such acceleration or the date of occurrence of the event that triggered such obligation to prepay.
(ii)Borrower further acknowledges that the Prepayment Premium (as a component of the Redemption Price) and the back-end facility fee specified in the Fee Letter shall be due and payable whenever so stated in the Loan Documents, or by any applicable operation of law, regardless of the circumstances causing any related acceleration or payment prior to the Stated Maturity Date, including without limitation any Event of Default or other failure to comply with the terms of any Loan Document, whether or not notice thereof has been given, or any acceleration by, through, or on account of any bankruptcy filing.
[Remainder of page intentionally left blank]
2
IN WITNESS WHEREOF, the parties hereto have duly executed this Amendment, as of the date first above written.
BORROWER
NANOSTRING TECHNOLOGIES, INC.
By: /s/ R. Bradley Gray
R. Bradley Gray
President and Chief Executive Officer
Address for Notices:
530 Fairview Avenue, N. Suite 2000
Seattle, WA 98109
Attn: General Counsel
Tel.: 206-378-6266
Fax: 206-378-6288
Email: [email protected]
SUBSIDIARY GUARANTORS
NANOSTRING TECHNOLOGIES INTERNATIONAL, INC.
By: /s/ Mark Daniel
Mark Daniel
Treasurer
Address for Notices:
530 Fairview Avenue, N. Suite 2000
Seattle, WA 98109
Attn: General Counsel
Tel.: 206-378-6266
Fax: 206-378-6288
Email: [email protected]
LENDERS
CAPITAL ROYALTY PARTNERS II L.P.
By: CAPITAL ROYALTY PARTNERS II GP
L.P., its General Partner
By: CAPITAL ROYALTY PARTNERS II
GP LLC, its General Partner
By: /s/ Nate Hukill
Nate Hukill
Authorized Signatory
PARALLEL INVESTMENT OPPORTUNITIES PARTNERS II L.P.
By: PARALLEL INVESTMENT OPPORTUNITIES PARTNERS II GP L.P., its General Partner
By: PARALLEL INVESTMENT
OPPORTUNITIES PARTNERS II GP LLC,
its General Partner
By: /s/ Nate Hukill
Nate Hukill
Authorized Signatory
CAPITAL ROYALTY PARTNERS II (CAYMAN) L.P.
By: CAPITAL ROYALTY PARTNERS II
(CAYMAN) GP L.P., its General Partner
By: CAPITAL ROYALTY PARTNERS II
(CAYMAN) GP LLC, its General Partner
By: /s/ Nate Hukill
Nate Hukill
Authorized Signatory
WITNESS:
Name:
CAPITAL ROYALTY PARTNERS II -
PARALLEL FUND “B” (CAYMAN) L.P.
By: CAPITAL ROYALTY PARTNERS II
(CAYMAN) GP L.P., its General Partner
By: CAPITAL ROYALTY PARTNERS II
GP LLC, its General Partner
By: /s/ Nate Hukill
Nate Hukill
Authorized Signatory
WITNESS:
Name:
Address for Notices for All Lenders:
1000 Main Street, Suite 2500
Houston, TX 77002
Attn: Portfolio Reporting
Tel.: 713.209.7350
Fax: 713.209.7351
Email: [email protected]
Exhibit 21.1
SUBSIDIARIES OF NANOSTRING TECHNOLOGIES, INC.
Name of Subsidiary | State or other Jurisdiction of Incorporation | |
NanoString Technologies Europe Limited | United Kingdom | |
NanoString Technologies SAS | France | |
NanoString Technologies International, Inc. | Delaware | |
NanoString Technologies Germany GmbH | Germany | |
NanoString Technologies Asia Pacific Limited | Hong Kong | |
NanoString Technologies Singapore Pte Limited | Singapore | |
NanoString Technologies (Bejing) Co. Ltd. | China | |
NanoString Technologies Spain, S.L. | Spain | |
Exhibit 23.1
CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We hereby consent to the incorporation by reference in the Registration Statements on Form S-8 (Nos. 333-189883, 333-194844, 333-202768, 333-210210, 333-216584, 333-222567, and 333-222568) and Form S-3 (No. 333-198465, 333-220255, and 333-220255) of NanoString Technologies, Inc. of our report dated March 7, 2018 relating to the financial statements, which appears in this Form 10‑K.
/s/ PricewaterhouseCoopers LLP
Seattle, Washington
March 7, 2018
Exhibit 31.1
CERTIFICATIONS
I, R. Bradley Gray, certify that:
1. | I have reviewed this Annual Report on Form 10-K of NanoString Technologies, Inc.; |
2. | Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
3. | Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report; |
4. | The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d–15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a–15(f) and 15d–15(f)) for the registrant and have: |
a) | Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
b) | Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
c) | Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
d) | Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and |
5. | The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions): |
a) | All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and |
b) | Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting. |
Date: March 7, 2018
/s/ R. Bradley Gray | |
R. Bradley Gray | |
President and Chief Executive Officer | |
(Principal Executive Officer) | |
Exhibit 31.2
CERTIFICATIONS
I, K. Thomas Bailey, certify that:
1. | I have reviewed this Annual Report on Form 10-K of NanoString Technologies, Inc.; |
2. | Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
3. | Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report; |
4. | The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d–15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a–15(f) and 15d–15(f)) for the registrant and have: |
a) | Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
b) | Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
c) | Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
d) | Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and |
5. | The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions): |
a) | All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and |
b) | Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting. |
Date: March 7, 2018
/s/ K. Thomas Bailey | |
K. Thomas Bailey | |
Chief Financial Officer | |
(Principal Financial and Accounting Officer) | |
Exhibit 32.1
NANOSTRING TECHNOLOGIES, INC.
CERTIFICATION PURSUANT TO
18 U.S.C. SECTION 1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Annual Report of NanoString Technologies, Inc. (the “Company”) on Form 10-K for the year ended December 31, 2017, as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, R. Bradley Gray, President and Chief Executive Officer (Principal Executive Officer) of the Company, certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that to my knowledge:
(1) | The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and |
(2) | The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company. |
/s/ R. Bradley Gray | |
R. Bradley Gray | |
President and Chief Executive Officer | |
(Principal Executive Officer) | |
Date: March 7, 2018
A signed original of this written statement required by Section 906 of the Sarbanes-Oxley Act of 2002 has been provided to the Company and will be retained by the Company and furnished to the Securities and Exchange Commission or its staff upon request.
This certification accompanies the Report to which it relates, is not deemed filed with the Securities and Exchange Commission and is not to be incorporated by reference into any filing of NanoString Technologies, Inc. under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended (whether made before or after the date of the Report), irrespective of any general incorporation language contained in such filing.
Exhibit 32.2
NANOSTRING TECHNOLOGIES, INC.
CERTIFICATION PURSUANT TO
18 U.S.C. SECTION 1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Annual Report of NanoString Technologies, Inc. (the “Company”) on Form 10-K for the year ended December 31, 2017, as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, James A. Johnson, Chief Financial Officer (Principal Financial and Accounting Officer) of the Company, certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that to my knowledge:
(1) | The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and |
(2) | The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company. |
/s/ K. Thomas Bailey |
K. Thomas Bailey |
Chief Financial Officer |
(Principal Financial and Accounting Officer) |
Date: March 7, 2018
A signed original of this written statement required by Section 906 of the Sarbanes-Oxley Act of 2002 has been provided to the Company and will be retained by the Company and furnished to the Securities and Exchange Commission or its staff upon request.
This certification accompanies the Report to which it relates, is not deemed filed with the Securities and Exchange Commission and is not to be incorporated by reference into any filing of NanoString Technologies, Inc. under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended (whether made before or after the date of the Report), irrespective of any general incorporation language contained in such filing.
