Form SC 13E3 SINOVAC BIOTECH LTD Filed by: SINOVAC BIOTECH LTD
UNITED
STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE
13E-3
RULE 13e-3 TRANSACTION STATEMENT UNDER SECTION 13(E)
OF THE SECURITIES EXCHANGE ACT OF 1934
Sinovac
Biotech Ltd.
(Name of the Issuer)
Sinovac
Biotech Ltd.
Mr. Weidong Yin
SAIF Partners IV L.P.
C-Bridge Healthcare Fund II, L.P.
Advantech Capital L.P.
Vivo Capital Fund VIII, L.P.
Vivo Capital Surplus Fund VIII, L.P.
Sinovac (Cayman) Limited
Sinovac Amalgamation Sub Limited
(Names of Persons Filing Statement)
Common Shares, par value $0.001
per share
(Title of Class of Securities)
P8696W104
(CUSIP Number)
No. 39 Shangdi Xi Road
Haidian District, Beijing 100085
People’s Republic of China
Tel: +86.10.8289.0088
|
Sinovac
Biotech Ltd. Tel: +86 10 8289 0088 Haidian District, Beijing 100085 People’s Republic of China
|
Mr. Weidong Yin Sinovac (Cayman) Limited Sinovac Amalgamation Sub Limited Tel: +86 10 8289 0088 Haidian District, Beijing 100085 People’s Republic of China
|
|
SAIF Partners IV L.P. Kenneth Lee Suites 2516-2520, Two Pacific Place 88 Queensway Hong Kong
|
C-Bridge Healthcare Fund II, L.P. Wei Fu Tel: +86 21 8012 7764 Room 7/03 Workingberg Commercial Building, 41-47 Marble Road, North Point, Hong Kong |
|
Advantech Capital L.P. Jianming Yu Tel: +852 2801 6988 Suites 1702-03, 17/F, One Exchange Square 8 Connaught Place, Central Hong Kong
|
Vivo Capital Fund VIII, L.P. Vivo Capital Surplus Fund VIII, L.P. Shan Fu, Lawrence Wang Tel: +1 650 688 0818 505 Hamilton Ave, Suite 207 Palo Alto, CA 94301 United States of America |
(Name, Address and Telephone Number of
Person Authorized to Receive Notices and Communications)
With copies to
|
Allen C. Wang Latham & Watkins 18th Floor, One Exchange Square 8 Connaught Place, Central Hong Kong |
Timothy Gardner William
P. Welty |
|
Henry Yin |
Vincent Ip |
|
Z. Julie Gao Haiping Li Skadden, Arps, Slate, Meagher & Flom LLP c/o 42/F, Edinburgh Tower, The Landmark 15 Queen’s Road, Central Hong Kong |
This statement is filed in connection with (check the appropriate box):
| a. | o The filing of solicitation materials or an information statement subject to Regulation 14A, Regulation 14-C or Rule 13e-3(c) under the Securities Exchange Act of 1934. |
| b. | o The filing of a registration statement under the Securities Act of 1933. |
| c. | ¨ A tender offer |
| d. | x None of the above |
Check the following box if the soliciting materials or information statement referred to in checking box (a) are preliminary copies: o
Check the following box if the filing is a final amendment reporting the results of the transaction: o
Calculation of Filing Fee
|
Transaction Valuation* |
Amount of Filing Fee** |
| $284,716,271 | $35,448 |
| * | Calculated solely for the purpose of determining the filing fee in accordance with Rule 0-11(b) under the Securities Exchange Act. The filing fee is calculated, based on the sum of (a) the proposed cash payment of US$7.00 per common share for 40,119,041 outstanding common shares of the issuer subject to the transaction (which equals the total outstanding common shares less the common shares to be cancelled without consideration (including the common shares held by the Buyer Consortium)) plus (b) the product of options to purchase 1,056,500 common shares and US$2.02 (which is the difference between the amalgamation consideration of US$7.00 per common share and the weighted average exercise price of US$4.98 per common share) plus (c) the product of options to purchase 5,800 common shares and US$4.63 (which is the difference between the amalgamation consideration of US$7.00 per common share and the weighted average exercise price of US2.37 per common share) plus (d) the product of 246,000 restricted shares and US$7.00 ((a), (b), (c) and (d) together, the “Transaction Valuation”). |
| ** | The amount of the filing fee, calculated in accordance with Exchange Act Rule 0-11(b)(1) and the Securities and Exchange Commission Fee Rate Advisory #1 for Fiscal Year 2018, was calculated by multiplying the Transaction Valuation by 0.0001245. |
o Check box if any part of the fee is offset as provided by Rule 0-11(a)(2) and identify the filing with which the offsetting of the fee was previously paid. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing.
TABLE OF CONTENTS
Page
| Item 1 Summary Term Sheet | 2 |
| Item 2 Subject Company Information | 2 |
| Item 3 Identity and Background of Filing Person | 3 |
| Item 4 Terms of the Transaction | 3 |
| Item 5 Past Contracts, Transactions, Negotiations and Agreements | 4 |
| Item 6 Purposes of the Transaction and Plans or Proposals | 5 |
| Item 7 Purposes, Alternatives, Reasons and Effects | 6 |
| Item 8 Fairness of the Transaction | 7 |
| Item 9 Reports, Opinions, Appraisals and Negotiations | 8 |
| Item 10 Source and Amount of Funds or Other Consideration | 9 |
| Item 11 Interest in Securities of the Subject Company | 9 |
| Item 12 The Solicitation or Recommendation | 10 |
| Item 13 Financial Statements | 10 |
| Item 14 Persons/Assets, Retained, Employed, Compensated or Used | 11 |
| Item 15 Additional Information | 11 |
| Item 16 Exhibits | 11 |
INTRODUCTION
This Rule 13e-3 transaction statement on Schedule 13E-3, together with the exhibits hereto (this “Transaction Statement”), is being filed with the SEC pursuant to Section 13(e) of the Exchange Act jointly by the following persons (each, a “Filing Person,” and collectively, the “Filing Persons”): (a) Sinovac Biotech Ltd., a company limited by shares incorporated under the laws of Antigua and Barbuda (the “Company”), the issuer of common shares, par value $0.001 per share (the “Shares”), (b) Mr. Weidong Yin, a citizen of the People’s Republic of China (“Mr. Yin”), (c) SAIF Partners IV L.P. (“SAIF”), an exempted limited partnership formed under the laws of the Cayman Islands, (d) C-Bridge Healthcare Fund II, L.P. (“C-Bridge Capital”), an exempted limited partnership formed under the laws of the Cayman Islands, (e) Advantech Capital L.P. (“Advantech Capital”), an exempted limited partnership formed under the laws of the Cayman Islands, (f) Vivo Capital Fund VIII, L.P., a limited partnership organized and existing under the laws of the State of Delaware (“Vivo Capital Fund”), (g) Vivo Capital Surplus Fund VIII, L.P., a limited partnership organized and existing under the laws of the State of Delaware (“Vivo Capital Surplus Fund,” and together with Mr. Yin, SAIF, C-Bridge, Advantech and Vivo Capital Fund, the “Buyer Consortium”); (h) Sinovac (Cayman) Limited, an exempted company incorporated with limited liability under the laws of the Cayman Islands (“Parent”), and (i) Sinovac Amalgamation Sub Limited, an international business corporation incorporated under the laws of Antigua and Barbuda and a wholly-owned Subsidiary of Parent (“Amalgamation Sub”).
On June 26, 2017, the Company, Parent and Amalgamation Sub entered into an amalgamation agreement (the “Amalgamation Agreement”). If the Amalgamation Agreement is approved and authorized by the Company’s shareholders and the other conditions to the closing of the amalgamation are satisfied or waived, Amalgamation Sub will amalgamate with and into the Company (the “Amalgamation”) in accordance with the International Business Corporations Act, CAP. 222 of the Revised Laws of Antigua and Barbuda (as consolidated and revised) (the “IBCA”), with the Company continuing as the surviving company resulting from the Amalgamation.
Under the Amalgamation Agreement, at the effective time of the Amalgamation, each of the Shares issued and outstanding immediately prior to the effective time of the Amalgamation will be canceled in exchange for the right to receive US$7.00 per Share in cash, without interest (the “Amalgamation Consideration”) and net of any applicable withholding taxes, except for (i) 6,049,500 Shares held by Mr. Yin and 10,780,820 Shares held by SAIF (such Shares, collectively, the “Rollover Shares” and such shareholders, collectively, the “Rollover Shareholders”), (ii) Shares held by Parent, Parent’s affiliates, the Company or any of the Company’s subsidiaries, which will be canceled without payment of any consideration or distribution therefor and (iii) Shares owned by holders who have validly exercised and not effectively withdrawn or lost their rights to dissent from the Amalgamation in accordance with Section 191 of the IBCA, which will be canceled at the effective time of the Amalgamation for the right to receive the fair value of such Shares determined in accordance with the provisions of Section 191(4) or Section 195(2) of the IBCA, as applicable.
| 1 |
In addition to the foregoing, at the effective time of the Amalgamation, all vested and unvested awards granted under the Company’s 2003 Stock Option Plan and 2012 Share Incentive Plan (the “Share Incentive Plans”), including options to purchase Shares and shares of restricted stock, will be converted into a right to receive a cash payment equal to the product of the number of Shares subject to the award and the Amalgamation Consideration (net of the exercise price for each option to purchase Shares).
The Amalgamation remains subject to the satisfaction or waiver of the conditions set forth in the Amalgamation Agreement, including that in order for the Amalgamation to be completed, the Amalgamation Agreement and the transactions contemplated by the Amalgamation Agreement, including the Amalgamation, must be authorized and approved by a special resolution of the shareholders of the Company passed by at least two-thirds of the Shares present and voting in person or by proxy. As of the date of this proxy statement, the Buyer Consortium beneficially owns approximately 29.5% of the issued and outstanding Shares entitled to vote. Pursuant to a support agreement, dated June 26, 2017, among the Rollover Shareholders, Parent and Sinovac Holding (Cayman) Limited, an exempted company incorporated with limited liability under the laws of the Cayman Islands (“Holdco”), these Shares will be voted in favor of the authorization and approval of the Amalgamation Agreement and the transactions contemplated by the Amalgamation Agreement, including the Amalgamation.
The Company will make available to its shareholders a proxy statement (a preliminary copy of which is attached as Exhibit (a)-(1) to this Transaction Statement), relating to the special meeting of shareholders of the Company, at which the shareholders of the Company will consider and vote upon, among other proposals, a proposal to authorize and approve the Amalgamation Agreement and the transactions contemplated by the Amalgamation Agreement, including the Amalgamation. A copy of the Amalgamation Agreement is attached to the proxy statement as Annex A and is incorporated herein by reference. As of the date hereof, the proxy statement is in preliminary form and is subject to completion.
Pursuant to General Instruction F to Schedule 13E-3, the information contained in the proxy statement, including all annexes thereto, is incorporated in its entirety herein by this reference, and the responses to each item in this Schedule 13E-3 are qualified in their entirety by the information contained in the proxy statement and the annexes thereto.
All information contained in this Transaction Statement concerning each Filing Person has been supplied by such Filing Person.
| Item 1 | Summary Term Sheet |
The information set forth in the proxy statement under the following captions is incorporated herein by reference:
| · | “Summary Term Sheet” |
| · | “Questions and Answers about the Special Meeting and the Amalgamation” |
| Item 2 | Subject Company Information |
| (a) | Name and Address. The information set forth in the proxy statement under the following caption is incorporated herein by reference: |
| · | “Summary Term Sheet—The Parties Involved in the Amalgamation” |
| (b) | Securities. The information set forth in the proxy statement under the following captions is incorporated herein by reference: |
| · | “The Special Meeting—Record Date; Shares Entitled to Vote” |
| 2 |
| · | “The Special Meeting—Shareholders Entitled to Vote; Voting Materials” |
| · | “Security Ownership of Certain Beneficial Owners and Management of the Company” |
| (c) | Trading Market and Price. The information set forth in the proxy statement under the following caption is incorporated herein by reference: |
| · | “Market Price of the Company’s Shares, Dividends and Other Matters—Market Price of the Shares” |
| (d) | Dividends. The information set forth in the proxy statement under the following caption is incorporated herein by reference: |
| · | “Market Price of the Company’s Shares, Dividends and Other Matters—Dividend Policy” |
| (e) | Prior Public Offering. The information set forth in the proxy statement under the following caption is incorporated herein by reference: |
| · | “Transactions in Shares—Prior Public Offerings” |
| (f) | Prior Stock Purchases. The information set forth in the proxy statement under the following caption is incorporated herein by reference: |
| · | “Transactions in Shares” |
| · | “Special Factors—Related Party Transactions” |
| Item 3 | Identity and Background of Filing Person |
| (a) | Name and Address. Sinovac Biotech Ltd. is the subject company. The information set forth in the proxy statement under the following captions is incorporated herein by reference: |
| · | “Summary Term Sheet—The Parties Involved in the Amalgamation” |
| · | “Annex E—Directors and Executive Officers of Each Filing Person” |
| (b) | Business and Background of Entities. The information set forth in the proxy statement under the following captions is incorporated herein by reference: |
| · | “Summary Term Sheet—The Parties Involved in the Amalgamation” |
| · | “Annex E—Directors and Executive Officers of Each Filing Person” |
| (c) | Business and Background of Natural Persons. The information set forth in the proxy statement under the following captions is incorporated herein by reference: |
| · | “Summary Term Sheet—The Parties Involved in the Amalgamation” |
| · | “Annex E—Directors and Executive Officers of Each Filing Person” |
| Item 4 | Terms of the Transaction |
| (a) | (1) Material Terms. Not applicable. |
| (b) | (2) Material Terms. The information set forth in the proxy statement under the following captions is incorporated herein by reference: |
| · | “Summary Term Sheet” |
| 3 |
| · | “Questions and Answers about the Special Meeting and the Amalgamation” |
| · | “Special Factors” |
| · | “The Special Meeting” |
| · | “The Amalgamation Agreement” |
| · | “Annex A—Amalgamation Agreement” |
| (c) | Different Terms. The information set forth in the proxy statement under the following captions is incorporated herein by reference: |
| · | “Summary Term Sheet—Interests of Certain Persons in the Amalgamation” |
| · | “Special Factors—Interests of Certain Persons in the Amalgamation” |
| · | “The Special Meeting—Proposals to be Considered at the Special Meeting” |
| · | “The Amalgamation Agreement” |
| · | “Annex A—Amalgamation Agreement” |
| (d) | Dissenters’ Rights. The information set forth in the proxy statement under the following captions is incorporated herein by reference: |
| · | “Questions and Answers about the Special Meeting and the Amalgamation” |
| · | “Dissenters’ Rights” |
| · | “Annex B— Sections 191 to 199 of the International Business Corporations Act, CAP. 222 of the Revised Laws of Antigua and Barbuda” |
| (e) | Provisions for Unaffiliated Security Holders. The information set forth in the proxy statement under the following caption is incorporated herein by reference: |
| · | “Provisions for Unaffiliated Security Holders” |
| (f) | Eligibility of Listing or Trading. Not applicable. |
| Item 5 | Past Contracts, Transactions, Negotiations and Agreements |
| (a) | Transactions. The information set forth in the proxy statement under the following captions is incorporated herein by reference: |
| · | “Special Factors—Related Party Transactions” |
| · | “Transactions in Shares” |
| (b) | Significant Corporate Events. The information set forth in the proxy statement under the following captions is incorporated herein by reference: |
| · | “Special Factors—Background of the Amalgamation” |
| · | “Special Factors—Reasons for the Amalgamation and Recommendation of the Special Committee and the Board” |
| · | “Special Factors—Buyer Consortium’s Purpose of and Reasons for the Amalgamation” |
| 4 |
| · | “Special Factors—Interests of Certain Persons in the Amalgamation” |
| · | “The Amalgamation Agreement” |
| · | “Annex A—Amalgamation Agreement” |
| (c) | Negotiations or Contacts. The information set forth in the proxy statement under the following captions is incorporated herein by reference: |
| · | “Special Factors—Background of the Amalgamation” |
| · | “Special Factors—Plans for the Company after the Amalgamation” |
| · | “Special Factors—Interests of Certain Persons in the Amalgamation” |
| · | “The Amalgamation Agreement” |
| · | “Annex A—Amalgamation Agreement” |
| (d) | Agreements Involving the Subject Company’s Securities. The information set forth in the proxy statement under the following captions is incorporated herein by reference: |
| · | “Summary Term Sheet—Support Agreement” |
| · | “Summary Term Sheet—Financing of the Amalgamation” |
| · | “Special Factors—Background of the Amalgamation” |
| · | “Special Factors—Plans for the Company after the Amalgamation” |
| · | “Special Factors—Financing of the Amalgamation” |
| · | “Special Factors—Interests of Certain Persons in the Amalgamation” |
| · | “Special Factors—Voting by Buyer Consortium at the Special Meeting” |
| · | “The Amalgamation Agreement” |
| · | “Transactions in Shares” |
| · | “Annex A—Amalgamation Agreement” |
| Item 6 | Purposes of the Transaction and Plans or Proposals |
| (a) | Use of Securities Acquired. The information set forth in the proxy statement under the following captions is incorporated herein by reference: |
| · | “Summary Term Sheet” |
| · | “Questions and Answers about the Special Meeting and the Amalgamation” |
| · | “Special Factors—Buyer Consortium’s Purpose of and Reasons for the Amalgamation” |
| · | “Special Factors—Effect of the Amalgamation on the Company” |
| · | “The Amalgamation Agreement” |
| · | “Annex A—Amalgamation Agreement” |
| 5 |
| (b) | (1)-(8) Plans. The information set forth in the proxy statement under the following captions is incorporated herein by reference: |
| · | “Summary Term Sheet—The Amalgamation” |
| · | “Summary Term Sheet—Purposes and Effects of the Amalgamation” |
| · | “Summary Term Sheet—Plans for the Company after the Amalgamation” |
| · | “Summary Term Sheet—Financing of the Amalgamation” |
| · | “Summary Term Sheet—Interests of Certain Persons in the Amalgamation” |
| · | “Special Factors—Background of the Amalgamation” |
| · | “Special Factors—Reasons for the Amalgamation and Recommendation of the Special Committee and the Board” |
| · | “Special Factors—Buyer Consortium’s Purpose of and Reasons for the Amalgamation” |
| · | “Special Factors—Effect of the Amalgamation on the Company” |
| · | “Special Factors—Financing of the Amalgamation” |
| · | “Special Factors—Plans for the Company after the Amalgamation” |
| · | “Special Factors—Interests of Certain Persons in the Amalgamation” |
| · | “The Amalgamation Agreement” |
| · | “Annex A—Amalgamation Agreement” |
| Item 7 | Purposes, Alternatives, Reasons and Effects |
| (a) | Purposes. The information set forth in the proxy statement under the following captions is incorporated herein by reference: |
| · | “Summary Term Sheet—Purposes and Effects of the Amalgamation” |
| · | “Summary Term Sheet—Plans for the Company after the Amalgamation” |
| · | “Special Factors—Reasons for the Amalgamation and Recommendation of the Special Committee and the Board” |
| · | “Special Factors—Buyer Consortium’s Purpose of and Reasons for the Amalgamation” |
| (b) | Alternatives. The information set forth in the proxy statement under the following captions is incorporated herein by reference: |
| · | “Special Factors—Background of the Amalgamation” |
| · | “Special Factors—Reasons for the Amalgamation and Recommendation of the Special Committee and the Board” |
| · | “Special Factors—Position of the Buyer Consortium as to the Fairness of the Amalgamation” |
| · | “Special Factors—Buyer Consortium’s Purpose of and Reasons for the Amalgamation” |
| 6 |
| · | “Special Factors—Alternatives to the Amalgamation” |
| · | “Special Factors—Effect on the Company if the Amalgamation is not Completed” |
| (c) | Reasons. The information set forth in the proxy statement under the following captions is incorporated herein by reference: |
| · | “Summary Term Sheet—Purposes and Effects of the Amalgamation” |
| · | “Special Factors—Background of the Amalgamation” |
| · | “Special Factors—Reasons for the Amalgamation and Recommendation of the Special Committee and the Board” |
| · | “Special Factors—Position of the Buyer Consortium as to the Fairness of the Amalgamation” |
| · | “Special Factors—Buyer Consortium’s Purpose of and Reasons for the Amalgamation” |
| · | “Special Factors—Effect of the Amalgamation on the Company” |
| · | “Special Factors—Alternatives to the Amalgamation” |
| (d) | Effects. The information set forth in the proxy statement under the following captions is incorporated herein by reference: |
| · | “Summary Term Sheet—Purposes and Effects of the Amalgamation” |
| · | “Special Factors—Background of the Amalgamation” |
| · | “Special Factors—Reasons for the Amalgamation and Recommendation of the Special Committee and the Board” |
| · | “Special Factors—Effect of the Amalgamation on the Company” |
| · | “Special Factors—Plans for the Company after the Amalgamation” |
| · | “Special Factors—Effect on the Company if the Amalgamation is not Completed” |
| · | “Special Factors—Effect of the Amalgamation on the Company’s Net Book Value and Net Loss” |
| · | “Special Factors—Interests of Certain Persons in the Amalgamation” |
| · | “Special Factors—Material U.S. Federal Income Tax Consequences” |
| · | “Special Factors—Material PRC Income Tax Consequences” |
| · | “Special Factors—Material Antigua and Barbuda Tax Consequences” |
| · | “The Amalgamation Agreement” |
| · | “Annex A—Amalgamation Agreement” |
| Item 8 | Fairness of the Transaction |
| (a)-(b) | Fairness; Factors Considered in Determining Fairness. The information set forth in the proxy statement under the following captions is incorporated herein by reference: |
| · | “Summary Term Sheet—Position of the Buyer Consortium as to Fairness” |
| · | “Summary Term Sheet—Interests of Certain Persons in the Amalgamation” |
| 7 |
| · | “Special Factors—Background of the Amalgamation” |
| · | “Special Factors—Reasons for the Amalgamation and Recommendation of the Special Committee and the Board” |
| · | “Special Factors—Position of the Buyer Consortium as to the Fairness of the Amalgamation” |
| · | “Special Factors—Opinion of the Special Committee’s Financial Advisor” |
| · | “Special Factors—Interests of Certain Persons in the Amalgamation” |
| · | “Annex D—Opinion of Duff & Phelps, as Financial Advisor” |
| (c) | Approval of Security Holders. The information set forth in the proxy statement under the following captions is incorporated herein by reference: |
| · | “Questions and Answers about the Special Meeting and the Amalgamation” |
| · | “The Special Meeting—Vote Required” |
| (d) | Unaffiliated Representative. The information set forth in the proxy statement under the following captions is incorporated herein by reference: |
| · | “Special Factors—Background of the Amalgamation” |
| · | “Special Factors—Reasons for the Amalgamation and Recommendation of the Special Committee and the Board” |
| (e) | Approval of Directors. The information set forth in the proxy statement under the following captions is incorporated herein by reference: |
| · | “Questions and Answers about the Special Meeting and the Amalgamation” |
| · | “Special Factors—Background of the Amalgamation” |
| · | “Special Factors—Reasons for the Amalgamation and Recommendation of the Special Committee and the Board” |
| (f) | Other Offers. The information set forth in the proxy statement under the following captions is incorporated herein by reference: |
| · | “Special Factors—Background of the Amalgamation” |
| · | “Special Factors—Reasons for the Amalgamation and Recommendation of the Special Committee and the Board” |
| Item 9 | Reports, Opinions, Appraisals and Negotiations |
| (a) | Report, Opinion or Appraisal. The information set forth in the proxy statement under the following captions is incorporated herein by reference: |
| · | “Summary Term Sheet—Opinion of the Special Committee’s Financial Advisor” |
| · | “Special Factors—Background of the Amalgamation” |
| · | “Special Factors—Opinion of the Special Committee’s Financial Advisor” |
| · | “Annex D—Opinion of Duff & Phelps, as Financial Advisor” |
| 8 |
| (b) | Preparer and Summary of the Report, Opinion or Appraisal. The information set forth in the proxy statement under the following captions is incorporated herein by reference: |
| · | “Special Factors—Opinion of the Special Committee’s Financial Advisor” |
| · | “Annex D—Opinion of Duff & Phelps, as Financial Advisor” |
| (c) | Availability of Documents. The information set forth in the proxy statement under the following caption is incorporated herein by reference: |
| · | “Where You Can Find More Information” |
The reports, opinions or appraisals referenced in this Item 9 will be made available for inspection and copying at the principal executive offices of the Company during its regular business hours by any interested holder of the Shares or his, her or its representative who has been so designated in writing.
| Item 10 | Source and Amount of Funds or Other Consideration |
| (a) | Source of Funds. The information set forth in the proxy statement under the following captions is incorporated herein by reference: |
| · | “Summary Term Sheet—Financing of the Amalgamation” |
| · | “Special Factors—Financing of the Amalgamation” |
| · | “The Amalgamation Agreement” |
| · | “Annex A—Amalgamation Agreement” |
| (b) | Conditions. The information set forth in the proxy statement under the following captions is incorporated herein by reference: |
| · | “Summary Term Sheet—Financing of the Amalgamation” |
| · | “Special Factors—Financing of the Amalgamation” |
| (c) | Expenses. The information set forth in the proxy statement under the following caption is incorporated herein by reference: |
| · | “Summary Term Sheet—Fees and Expenses” |
| · | “Special Factors—Fees and Expenses” |
| (d) | Borrowed Funds. Not applicable. |
| Item 11 | Interest in Securities of the Subject Company |
| (a) | Securities Ownership. The information set forth in the proxy statement under the following captions is incorporated herein by reference: |
| · | “Summary Term Sheet—Share Ownership of the Company’s Directors and Executive Officers and Voting Commitments” |
| · | “Special Factors—Interests of Certain Persons in the Amalgamation” |
| · | “Security Ownership of Certain Beneficial Owners and Management of the Company” |
| 9 |
| (b) | Securities Transaction. The information set forth in the proxy statement under the following caption is incorporated herein by reference: |
| · | “Transactions in Shares” |
| Item 12 | The Solicitation or Recommendation |
| (a) | Intent to Tender or Vote in a Going-Private Transaction. The information set forth in the proxy statement under the following captions is incorporated herein by reference: |
| · | “Summary Term Sheet—Share Ownership of the Company’s Directors and Executive Officers and Voting Commitments” |
| · | “Summary Term Sheet—Support Agreement” |
| · | “Questions and Answers about the Special Meeting and the Amalgamation” |
| · | “Special Factors—Voting by Buyer Consortium at the Special Meeting” |
| · | “The Special Meeting—Vote Required” |
| · | “Security Ownership of Certain Beneficial Owners and Management of the Company” |
| (b) | Recommendations of Others. The information set forth in the proxy statement under the following captions is incorporated herein by reference: |
| · | “Summary Term Sheet—Position of the Buyer Consortium as to Fairness” |
| · | “Summary Term Sheet—Share Ownership of the Company’s Directors and Executive Officers and Voting Commitments” |
| · | “Special Factors—Reasons for the Amalgamation and Recommendation of the Special Committee and the Board” |
| · | “Special Factors—Position of the Buyer Consortium as to the Fairness of the Amalgamation” |
| · | “The Special Meeting—Our Board of Directors’ Recommendation” |
| Item 13 | Financial Statements |
| (a) | Financial Information. The audited financial statements of the Company for the two years ended December 31, 2015 and 2016 are incorporated herein by reference to the Company’s Form 20-F for the year ended December 31, 2016, filed on November 22, 2017. The unaudited consolidated financial statements of the Company for the six-month periods ended June 30, 2016 and June 30, 2017 are incorporated herein by reference to the Company’s report on Form 6-K furnished to the SEC on December 1, 2017. |
The information set forth in the proxy statement under the following captions is incorporated herein by reference:
| · | “Financial Information” |
| · | “Where You Can Find More Information” |
| (b) | Pro Forma Information. Not applicable. |
| 10 |
| Item 14 | Persons/Assets, Retained, Employed, Compensated or Used |
| (a) | Solicitation or Recommendations. The information set forth in the proxy statement under the following caption is incorporated herein by reference: |
| · | “The Special Meeting—Solicitation of Proxies” |
| (b) | Employees and Corporate Assets. The information set forth in the proxy statement under the following captions is incorporated herein by reference: |
| · | “Summary Term Sheet—The Parties Involved in the Amalgamation” |
| · | “Special Factors—Interests of Certain Persons in the Amalgamation” |
| · | “Annex E—Directors and Executive Officers of Each Filing Person” |
| Item 15 | Additional Information |
| (a) | Other Material Information. The information contained in the proxy statement, including all annexes thereto, is incorporated herein by reference |
| Item 16 | Exhibits |
| (a)-(1) | Preliminary Proxy Statement of the Company dated , 2018 |
| (a)-(2) | Notice of Special Meeting of Shareholders of the Company, incorporated herein by reference to the proxy statement |
| (a)-(3)* | Form of Proxy Card |
| (a)-(4) | Press Release issued by the Company, dated June 26, 2017, incorporated herein by reference to Exhibit 99.1 to the Report on Form 6-K furnished by the Company to the SEC on June 26, 2017 |
| (b)-(1) | Equity Commitment Letter, dated as of June 26, 2017, by and between C-Bridge, Holdco and Parent, incorporated herein by reference to Exhibit 7.05 to the Schedule 13D/A filed by Mr. Yin with the SEC on June 27, 2017 |
| (b)-(2) | Equity Commitment Letter, dated as of June 26, 2017, by and between Advantech, Holdco and Parent, incorporated herein by reference to Exhibit 7.06 to the Schedule 13D/A filed by Mr. Yin with the SEC on June 27, 2017 |
| (b)-(3) | Equity Commitment Letter, dated as of June 26, 2017, by and between Vivo Capital Fund, Holdco and Parent, incorporated herein by reference to Exhibit 7.07 to the Schedule 13D/A filed by Mr. Yin with the SEC on June 27, 2017 |
| (b)-(4) | Equity Commitment Letter, dated as of June 26, 2017, by and between Vivo Capital Surplus Fund, Holdco and Parent, incorporated herein by reference to Exhibit 7.08 to the Schedule 13D/A filed by Mr. Yin with the SEC on June 27, 2017 |
| (c)-(1) | Opinion of Duff & Phelps, LLC dated June 26, 2017, incorporated herein by reference to Annex D to the proxy statement |
| (c)-(2) | Discussion Materials prepared by Duff & Phelps, LLC for discussion with the special committee of the board of directors of the Company, dated June 26, 2017 |
| (d)-(1) | Amalgamation Agreement, dated as of June 26, 2017, among the Company, Parent and Amalgamation Sub, incorporated herein by reference to Annex A to the proxy statement |
| (d)-(2) | Limited Guarantee dated as of June 26, 2017, by C-Bridge in favor of the Company, incorporated herein by reference to Exhibit 7.09 to the Schedule 13D/A filed by Mr. Yin with the SEC on June 27, 2017 |
| (d)-(3) | Limited Guarantee dated as of June 26, 2017, by Advantech in favor of the Company, incorporated herein by reference to Exhibit 7.10 to the Schedule 13D/A filed by Mr. Yin with the SEC on June 27, 2017 |
| 11 |
| (d)-(4) | Limited Guarantee dated as of June 26, 2017, by Vivo Capital Fund in favor of the Company, incorporated herein by reference to Exhibit 7.11 to the Schedule 13D/A filed by Mr. Yin with the SEC on June 27, 2017 |
| (d)-(5) | Limited Guarantee dated as of June 26, 2017, by Vivo Capital Surplus Fund in favor of the Company, incorporated herein by reference to Exhibit 7.12 to the Schedule 13D/A filed by Mr. Yin with the SEC on June 27, 2017 |
| (d)-(6) | Limited Guarantee, dated as of June 26, 2017, by SAIF in favor of the Company, incorporated herein by reference to Exhibit 7.13 to the Schedule 13D/A filed by Mr. Yin with the SEC on June 27, 2017 |
| (d)-(7) | Support Agreement, dated as of June 26, 2017 by and among Parent, the Rollover Shareholders and Holdco, incorporated herein by reference to Annex C to the proxy statement |
| (f)-(1) | Dissenters’ Rights, incorporated herein by reference to the section entitled “Dissenters’ Rights” in the proxy statement |
| (f)-(2) | Sections 191 to 199 of the International Business Corporations Act, CAP. 222 of the Revised Laws of Antigua and Barbuda, incorporated herein by reference to Annex B to the proxy statement |
| (g) | Not applicable |
________________
* To be filed by amendment
| 12 |
SIGNATURES
After due inquiry and to the best of my knowledge and belief, I certify that the information set forth in this statement is true, complete and correct.
Date: January 5, 2018
| Sinovac Biotech Ltd. | ||
| By: | /s/ Simon Anderson | |
| Name: Simon Anderson | ||
| Title: Chairman of the Special Committee | ||
| 13 |
| Mr. Weidong Yin | ||
| By: | /s/ Weidong Yin | |
| Name: Weidong Yin | ||
| 14 |
|
SAIF Partners IV L.P.
By SAIF IV GP, L.P. its general partner
By SAIF IV GP Capital Ltd., its general partner
| ||
| By: | /s/ Andrew Y. Yan | |
| Name: Andrew Y. Yan | ||
| Title: Director of SAIF IV GP Capital Ltd. | ||
| 15 |
|
C-Bridge Healthcare Fund II, L.P.
(acting by its general partner, C-Bridge Healthcare Fund GP II, L.P.
acting by its general partner, C-Bridge Capital GP, Ltd.)
| ||
| By: | /s/ Wei Fu | |
| Name: Wei Fu | ||
| Title: Authorized Representative | ||
| 16 |
|
Advantech Capital L.P.
By its General Partner, ADVANTECH CAPITAL PARTNERS LTD.
| ||
| By: | /s/ Wong Kok Wai | |
| Name: Wong Kok Wai | ||
| Title: Director | ||
| 17 |
|
Vivo Capital Fund VIII, L.P.
By: Vivo Capital VIII, LLC
Its: General Partner
| ||
| By: | /s/ Frank Kung | |
| Name: Frank Kung | ||
| Title: Managing Member | ||
| 18 |
|
Vivo Capital Surplus Fund VIII, L.P.
By: Vivo Capital VIII, LLC
Its: General Partner
| ||
| By: | /s/ Frank Kung | |
| Name: Frank Kung | ||
| Title: Managing Member | ||
| 19 |
|
Sinovac (Cayman) Limited
| ||
| By: | /s/ Weidong Yin | |
| Name: Weidong Yin | ||
| Title: Director | ||
| 20 |
| Sinovac Amalgamation Sub Limited | ||
| By: | /s/ Weidong Yin | |
| Name: Weidong Yin | ||
| Title: Director | ||
| 21 |
Exhibit Index
| (a)-(1) | Preliminary Proxy Statement of the Company dated , 2018 |
| (a)-(2) | Notice of Special Meeting of Shareholders of the Company, incorporated herein by reference to the proxy statement |
| (a)-(3)* | Form of Proxy Card |
| (a)-(4) | Press Release issued by the Company, dated June 26, 2017, incorporated herein by reference to Exhibit 99.1 to the Report on Form 6-K furnished by the Company to the SEC on June 26, 2017 |
| (b)-(1) | Equity Commitment Letter, dated as of June 26, 2017, by and between C-Bridge, Holdco and Parent, incorporated herein by reference to Exhibit 7.05 to the Schedule 13D/A filed by Mr. Yin with the SEC on June 27, 2017 |
| (b)-(2) | Equity Commitment Letter, dated as of June 26, 2017, by and between Advantech, Holdco and Parent, incorporated herein by reference to Exhibit 7.06 to the Schedule 13D/A filed by Mr. Yin with the SEC on June 27, 2017 |
| (b)-(3) | Equity Commitment Letter, dated as of June 26, 2017, by and between Vivo Capital Fund, Holdco and Parent, incorporated herein by reference to Exhibit 7.07 to the Schedule 13D/A filed by Mr. Yin with the SEC on June 27, 2017 |
| (b)-(4) | Equity Commitment Letter, dated as of June 26, 2017, by and between Vivo Capital Surplus Fund, Holdco and Parent, incorporated herein by reference to Exhibit 7.08 to the Schedule 13D/A filed by Mr. Yin with the SEC on June 27, 2017 |
| (c)-(1) | Opinion of Duff & Phelps, LLC dated June 26, 2017, incorporated herein by reference to Annex D to the proxy statement |
| (c)-(2) | Discussion Materials prepared by Duff & Phelps, LLC for discussion with the special committee of the board of directors of the Company, dated June 26, 2017 |
| (d)-(1) | Amalgamation Agreement, dated as of June 26, 2017, among the Company, Parent and Amalgamation Sub, incorporated herein by reference to Annex A to the proxy statement |
| (d)-(2) | Limited Guarantee dated as of June 26, 2017, by C-Bridge in favor of the Company, incorporated herein by reference to Exhibit 7.09 to the Schedule 13D/A filed by Mr. Yin with the SEC on June 27, 2017 |
| (d)-(3) | Limited Guarantee dated as of June 26, 2017, by Advantech in favor of the Company, incorporated herein by reference to Exhibit 7.10 to the Schedule 13D/A filed by Mr. Yin with the SEC on June 27, 2017 |
| (d)-(4) | Limited Guarantee dated as of June 26, 2017, by Vivo Capital Fund in favor of the Company, incorporated herein by reference to Exhibit 7.11 to the Schedule 13D/A filed by Mr. Yin with the SEC on June 27, 2017 |
| (d)-(5) | Limited Guarantee dated as of June 26, 2017, by Vivo Capital Surplus Fund in favor of the Company, incorporated herein by reference to Exhibit 7.12 to the Schedule 13D/A filed by Mr. Yin with the SEC on June 27, 2017 |
| (d)-(6) | Limited Guarantee, dated as of June 26, 2017, by SAIF in favor of the Company, incorporated herein by reference to Exhibit 7.13 to the Schedule 13D/A filed by Mr. Yin with the SEC on June 27, 2017 |
| (d)-(7) | Support Agreement, dated as of June 26, 2017 by and among Parent, the Rollover Shareholders and Holdco, incorporated herein by reference to Annex C to the proxy statement |
| (f)-(1) | Dissenters’ Rights, incorporated herein by reference to the section entitled “Dissenters’ Rights” in the proxy statement |
| (f)-(2) | Sections 191 to 199 of the International Business Corporations Act, CAP. 222 of the Revised Laws of Antigua and Barbuda, incorporated herein by reference to Annex B to the proxy statement |
| (g) | Not applicable |
________________
* To be filed by amendment
| 22 |
Exhibit (a)-(1)
Sinovac Biotech Ltd.
, 2018
Shareholders of Sinovac Biotech Ltd.
Re: Notice of Special Meeting of Shareholders
Dear Shareholder:
You are cordially invited to attend a special meeting of shareholders of Sinovac Biotech Ltd. (the “Company”) to be held on , 2018 at [a.m./p.m.] ( time). The meeting will be held at . The accompanying notice of the special meeting and proxy statement provide information regarding the matters to be acted on at the special meeting, including at any adjournment thereof.
On June 26, 2017, the Company entered into an amalgamation agreement (the “Amalgamation Agreement”) with Sinovac (Cayman) Limited, an exempted company incorporated with limited liability under the laws of the Cayman Islands (“Parent”), and Sinovac Amalgamation Sub Limited, an international business corporation incorporated under the laws of Antigua and Barbuda and a wholly-owned subsidiary of Parent (“Amalgamation Sub”). Pursuant to the Amalgamation Agreement, Amalgamation Sub will be amalgamated with and into the Company (the “Amalgamation”), with the Company surviving the Amalgamation and becoming a wholly-owned subsidiary of Parent (the “Surviving Corporation”) in accordance with the International Business Corporations Act, CAP. 222 of the Revised Laws of Antigua and Barbuda (as consolidated and revised) (the “IBCA”). At the special meeting, you will be asked to consider and vote upon a proposal to authorize and approve the Amalgamation Agreement and the transactions contemplated by the Amalgamation Agreement, including the Amalgamation (collectively, the “Transactions”). A copy of the Amalgamation Agreement is attached as Annex A to the accompanying proxy statement.
Amalgamation Sub was formed solely for the purpose of effecting the Amalgamation. Parent was formed solely for the purpose of holding equity interests in Amalgamation Sub and the Surviving Corporation following the closing of the Amalgamation, arranging the investment and financing transactions related to the Transactions and completing the Transactions, including the Amalgamation. At the effective time of the Amalgamation (the “Effective Time”), Amalgamation Sub and Parent will be beneficially owned by a consortium (the “Buyer Consortium”) that includes Mr. Weidong Yin, the chairman, president and chief executive officer of the Company (“Mr. Yin”), SAIF Partners IV L.P. (“SAIF”), C-Bridge Healthcare Fund II, L.P. (“C-Bridge Capital”), Advantech Capital L.P. (“Advantech Capital”), Vivo Capital Fund VIII, L.P. and Vivo Capital Surplus Fund VIII, L.P. (together with Vivo Capital Fund VIII, L.P., “Vivo Capital”). If the Amalgamation is completed, the Company will continue its operations as a privately held company and will be beneficially owned by the Buyer Consortium, and, as a result of the Amalgamation, the Company will no longer list its common shares, par value US$0.001 per share (the “Shares”), on the NASDAQ Global Select Market.
At the Effective Time, each of the Shares issued and outstanding immediately prior to the effective time of the Amalgamation will be canceled in exchange for the right to receive US$7.00 per Share in cash, without interest (the “Per Share Amalgamation Consideration”) and net of any applicable withholding taxes, except for (i) 6,049,500 Shares held by Mr. Yin and 10,780,820 Shares held by SAIF (such Shares, collectively, the “Rollover Shares” and such shareholders, collectively, the “Rollover Shareholders”), (ii) Shares held by Parent, Parent’s affiliates, the Company or any of the Company’s subsidiaries, which will be canceled without payment of any consideration or distribution therefor (collectively, the “Affiliate Shares” and, together with the Rollover Shares, the “Excluded Shares”) and (iii) Shares owned by holders who have validly exercised and not effectively withdrawn or lost their rights to dissent from the Amalgamation in accordance with the provisions of Section 191 of the IBCA (“Dissenting Shares”). Dissenting Shares will be canceled at the effective time of the Amalgamation for the right to receive the fair value of such Shares determined in accordance with the provisions of Section 191(4) or Section 195(2) of the IBCA, as applicable.
In addition to the foregoing, at the Effective Time, all vested and unvested awards granted under the Company’s 2003 Stock Option Plan and 2012 Share Incentive Plan (the “Share Incentive Plans”), including options to purchase Shares and shares of restricted stock, will be converted into a right to receive a cash payment equal to the product of the number of Shares subject to the award and the Per Share Amalgamation Consideration (net of the exercise price for each option to purchase Shares and net of any applicable withholding taxes). The Share Incentive Plans will terminate at the Effective Time.
At a meeting held on June 26, 2017, a special committee (the “Special Committee”) of the board of directors of the Company (the “Board”), composed solely of independent directors unaffiliated with the Buyer Consortium or any member of the management of the Company, reviewed and considered the terms and conditions of the Amalgamation Agreement and the Transactions, including the Amalgamation. The Special Committee, after due consideration, unanimously (a) determined that the Amalgamation, on the terms and conditions set forth in the Amalgamation Agreement and the other transaction documents (including the Per Share Amalgamation Consideration), is fair to and in the best interests of the Company and its shareholders other than the holders of Excluded Shares, (b) determined it was advisable that the execution, delivery and performance by the Company of the Amalgamation Agreement and the consummation of the Transactions, including the Amalgamation, be approved, authorized and adopted on the terms and conditions set forth in the Amalgamation Agreement, (c) recommended that the Board authorize and approve the execution, delivery and performance of the Amalgamation Agreement and the other transaction documents and the consummation of the Transactions, including the Amalgamation, and (d) determined it was advisable to recommend that the shareholders of the Company authorize and approve the matters submitted to them in connection with the Amalgamation, including the Amalgamation Agreement and the Transactions, including the Amalgamation.
ii
At a meeting held on June 26, 2017, the Board, acting upon the unanimous recommendation of the Special Committee, unanimously (other than Mr. Yin and Kenneth Lee, who recused themselves from the meeting): (a) determined that the Amalgamation, on the terms and conditions set forth in the Amalgamation Agreement and the other transaction documents (including the Per Share Amalgamation Consideration), is fair to and in the best interests of the Company and the Company’s shareholders (other than holders of the Excluded Shares); (b) approved, authorized and adopted the Amalgamation Agreement and the transactions contemplated thereby, including the Amalgamation, on the terms and conditions set forth in the Amalgamation Agreement; (c) approved and authorized the execution, delivery and performance of the Amalgamation Agreement and the other transaction documents and the consummation of the Transactions, including the Amalgamation; and (d) directed that the Amalgamation Agreement and the transactions contemplated thereby, including, without limitation, the Amalgamation, shall be submitted to the shareholders of the Company for approval with the recommendation of the Board that the Company’s shareholders vote in favor of the matters submitted to them in connection with the Amalgamation.
After careful consideration and upon the unanimous recommendation of the Special Committee, the Board authorized and approved the Amalgamation Agreement and unanimously (other than Mr. Yin or Kenneth Lee) recommends that you vote:
FOR the proposal to authorize and approve the Amalgamation Agreement and the consummation of the Transactions, including the Amalgamation;
FOR the proposal to authorize each of the directors of the Company to do all things necessary to give effect to the Amalgamation Agreement and the consummation of the Transactions, including the Amalgamation; and
FOR the proposal that the chairman of the special meeting be instructed to adjourn the special meeting in order to allow the Company to solicit additional proxies in the event that there are insufficient proxies received to pass the special resolutions during the special meeting.
In considering the recommendation of the Board, you should be aware that some of the Company’s directors and executive officers have interests in the Amalgamation that are different from, or in addition to, the interests of the shareholders generally.
The accompanying proxy statement provides detailed information about the Amalgamation and the special meeting. We encourage you to read the entire document and all of the attachments and other documents referred to or incorporated by reference therein carefully. You may also obtain more information about the Company from documents the Company has filed with or furnished to the United States Securities and Exchange Commission (the “SEC”), which are available for free at the SEC’s website www.sec.gov.
iii
Regardless of the number of Shares that you own, your vote is very important. The Amalgamation cannot be completed unless the Amalgamation Agreement and the Transactions, including the Amalgamation, are authorized and approved by a special resolution passed by at least two-thirds of the Shares present and voting in person or by proxy as a single class. As of the date of this proxy statement, the Buyer Consortium beneficially owns approximately 29.5% of the issued and outstanding Shares entitled to vote. Pursuant to a support agreement, dated June 26, 2017, among the Rollover Shareholders, Parent and Sinovac Holding (Cayman) Limited, an exempted company incorporated with limited liability under the Laws of the Cayman Islands, these Shares will be voted in favor of the authorization and approval of the Amalgamation Agreement and the Transactions, including the Amalgamation.
Whether or not you plan to attend the special meeting, please complete the accompanying proxy card, in accordance with the instructions set forth on the proxy card, as promptly as possible. The deadline to lodge your proxy card is , 2018 at [a.m./p.m.] ( time). Each shareholder has one vote for each Share held as of the close of business in New York on , 2018, being the record date for determining the shareholders entitled to vote at the special meeting (the “Record Date”).
Completing the proxy card in accordance with the instructions set forth on the proxy card will not deprive you of your right to attend the special meeting and vote your Shares in person. Please note, however, that if your Shares are held by a broker, bank or other nominee and you wish to vote at the special meeting in person, you must obtain from the registered holder a proxy issued in your name. If you submit a signed proxy card without indicating how you wish to vote, the Shares represented by your proxy card will be voted:
FOR the proposal to authorize and approve the Amalgamation Agreement and the consummation of the Transactions, including the Amalgamation;
FOR the proposal to authorize each of the directors of the Company to do all things necessary to give effect to the Amalgamation Agreement and the consummation of the Transactions, including the Amalgamation; and
FOR the proposal that the chairman of the special meeting be instructed to adjourn the special meeting in order to allow the Company to solicit additional proxies in the event that there are insufficient proxies received to pass the special resolutions during the special meeting.
Shareholders who elect to dissent from the Amalgamation will have the right to receive payment of the fair value of their Shares if the Amalgamation is completed, but only if they deliver to the Company, before the vote is taken at the special meeting, a written objection to the Amalgamation and subsequently comply with all procedures and requirements of Section 191 of the IBCA for the exercise of dissenters’ rights, which is attached as Annex B to the accompanying proxy statement. The fair value of your Shares as determined under the IBCA could be more than, the same as, or less than the amalgamation consideration you would receive pursuant to the Amalgamation Agreement if you do not exercise dissenters’ rights with respect to your Shares.
Neither the SEC nor any state securities regulatory agency has approved or disapproved the Amalgamation, passed upon the merits or fairness of the Amalgamation or passed upon the adequacy or accuracy of the disclosure in this letter or in the accompanying notice of the special meeting or proxy statement. Any representation to the contrary is a criminal offense.
iv
If you have any questions or need assistance in voting your Shares, you can contact at (+ outside the United States) or at .
Thank you for your cooperation and continued support.
| Sincerely, | Sincerely, | |
| On behalf of the Special Committee | Chairman of the Board |
The proxy statement is dated , 2018, and is first being mailed to the shareholders on or about , 2018.
v
Sinovac Biotech Ltd.
NOTICE OF SPECIAL MEETING OF SHAREHOLDERS
TO BE HELD ON
, 2018
Dear Shareholder:
Notice is hereby given that a special meeting of the shareholders of Sinovac Biotech Ltd. (the “Company”) will be held on , 2018 at [a.m./p.m.] ( time).
Only registered holders of common shares of the Company, par value US$0.001 per share (the “Shares”), as at the close of business in New York on , 2018 or their proxy holders are entitled to vote at this special meeting or any adjournment thereof. At the meeting, you will be asked to consider and vote upon the following resolutions:
As special resolutions:
THAT (i) the amalgamation agreement dated as of June 26, 2017 (the “Amalgamation Agreement”) among the Company, Sinovac (Cayman) Limited, an exempted company incorporated with limited liability under the laws of the Cayman Islands (“Parent”), and Sinovac Amalgamation Sub Limited, an international business corporation incorporated under the laws of Antigua and Barbuda and a wholly-owned subsidiary of Parent (“Amalgamation Sub”) (such Amalgamation Agreement being in the form attached as Annex A to the proxy statement accompanying this notice of special meeting and which will be produced and made available for inspection at the special meeting), in order to give effect to the amalgamation of Amalgamation Sub with and into the Company, with the Company surviving as a subsidiary of Parent (the “Amalgamation”), and any and all transactions contemplated thereby, including the Amalgamation (the “Transactions”), be authorized and approved;
THAT each of the directors of the Company be authorized to do all things necessary to give effect to the Amalgamation Agreement and the Transactions, including the Amalgamation; and
If necessary, as an ordinary resolution:
THAT the chairman of the special meeting be instructed to adjourn the special meeting in order to allow the Company to solicit additional proxies in the event that there are insufficient proxies received to pass the special resolutions during the special meeting.
A list of the shareholders of the Company will be available at its principal executive offices at No. 39 Shangdi Xi Road, Haidian District, Beijing 100085, People’s Republic of China during ordinary business hours for the two business days immediately prior to the special meeting.
vi
After careful consideration and upon the unanimous recommendation of the special committee (the “Special Committee”) of the board of directors of the Company (the “Board”), composed solely of independent directors unaffiliated with Parent, Amalgamation Sub, the consortium (the “Buyer Consortium”) that includes Mr. Weidong Yin, the chairman, president and chief executive officer of the Company (“Mr. Yin”), SAIF Partners IV L.P. (“SAIF”), C-Bridge Healthcare Fund II, L.P. (“C-Bridge Capital”), Advantech Capital L.P. (“Advantech Capital”), Vivo Capital Fund VIII, L.P. and Vivo Capital Surplus Fund VIII, L.P. (together with Vivo Capital Fund VIII, L.P., “Vivo Capital”) or any member of the management of the Company, the Board authorized and approved the Amalgamation Agreement and recommends that you vote:
FOR the proposal to authorize and approve the Amalgamation Agreement and the consummation of the Transactions, including the Amalgamation;
FOR the proposal to authorize each of the directors of the Company to do all things necessary to give effect to the Amalgamation Agreement and the consummation of the Transactions, including the Amalgamation; and
FOR the proposal that the chairman of the special meeting be instructed to adjourn the special meeting in order to allow the Company to solicit additional proxies in the event that there are insufficient proxies received to pass the special resolutions during the special meeting.
In order for the Amalgamation to be completed, the Amalgamation Agreement and the Transactions, including the Amalgamation, must be authorized and approved by a special resolution of the shareholders of the Company passed by at least two-thirds of the Shares present and voting in person or by proxy as a single class.
As of the date of this proxy statement, the Buyer Consortium beneficially owns approximately 29.5% of the issued and outstanding Shares entitled to vote. Pursuant to a support agreement, dated June 26, 2017, among Mr. Yin, SAIF, Parent and Sinovac Holding (Cayman) Limited, an exempted company incorporated with limited liability under the Laws of the Cayman Islands (such support agreement being in the form attached to the proxy statement accompanying this notice of special meeting as Annex C and which will be produced and made available for inspection at the special meeting), these Shares will be voted in favor of each of the foregoing resolutions.
Regardless of the number of Shares that you own, your vote is very important. Even if you plan to attend the special meeting in person, we request that you submit your proxy in accordance with the instructions set forth on the proxy card as promptly as possible. You should simply indicate on your proxy card how you want to vote, sign and date the proxy card, and mail the proxy card in the enclosed return envelope as soon as possible to ensure that it will be received by the Company no later than [a.m./p.m.] on , 2018 ( time), which is the deadline to lodge your proxy card. The proxy card is the “instrument appointing a proxy” referred to in the Company’s by-laws. Each shareholder has one vote for each Share owned by such shareholder as of the close of business in New York on , 2018.
vii
Completing the proxy card in accordance with the instructions set forth on the proxy card will not deprive you of your right to attend the special meeting and vote your Shares in person. Please note, however, that if your Shares are registered in the name of a broker, bank or other nominee and you wish to vote at the special meeting in person, you must obtain from the registered holder a proxy issued in your name.
If you abstain from voting, fail to cast your vote in person, fail to complete and return your proxy card in accordance with the instructions set forth on the proxy card, or fail to give voting instructions to your broker, dealer, commercial bank, trust company or other nominee, your vote will not be counted.
If you receive more than one proxy card because you own Shares that are registered in different names, please vote all of your Shares shown on each of your proxy cards in accordance with the instructions set forth on each such proxy card.
Shareholders who dissent from the Amalgamation will have the right to receive payment of the fair value of their Shares if the Amalgamation is completed, but only if they deliver to the Company, before the vote is taken, a written objection to the Amalgamation and subsequently comply with all procedures and requirements of Section 191 of the International Business Corporations Act, CAP. 222 of the Revised Laws of Antigua and Barbuda (as consolidated and revised) for the exercise of dissenters’ rights, which is attached as Annex B to the accompanying proxy statement. The fair value of your Shares as determined under that statute could be more than, the same as, or less than the amalgamation consideration that you would receive pursuant to the Amalgamation Agreement if you do not exercise dissenters’ rights with respect to your Shares.
PLEASE DO NOT SEND YOUR SHARE CERTIFICATES AT THIS TIME. IF THE AMALGAMATION IS COMPLETED, YOU WILL BE SENT INSTRUCTIONS REGARDING THE SURRENDER OF YOUR SHARE CERTIFICATES.
If you have any questions or need assistance in voting your Shares, you can contact at (+ outside the United States) or at .
The Amalgamation Agreement and the Amalgamation are described in the accompanying proxy statement. A copy of the Amalgamation Agreement is included as Annex A to the accompanying proxy statement. We urge you to read the entire proxy statement carefully.
Notes:
| 1. | In the case of joint holders the vote of the senior holder who tenders a vote, whether in person or by proxy (or, in the case of a corporation or other non-natural person, by its duly authorized representative or proxy), shall be accepted to the exclusion of the votes of the other joint holders, and seniority shall be determined by the order in which the names of the holders stand in the register of members of the Company. |
viii
| 2. | The instrument appointing a proxy must be in writing under the hand of the appointer or his attorney duly authorized in writing, or if the appointer is a corporation, either under seal or under the hand of an officer or attorney duly authorized. |
| 3. | A proxy need not be a shareholder of the Company. |
| BY ORDER OF THE BOARD OF DIRECTORS, | |
| Director | |
| , 2018 | |
|
Registered Office: 36 Long Street City of Saint John Antigua and Barbuda
Executive Office: No. 39 Shangdi Xi Road Haidian District, Beijing 100085 People’s Republic of China |
ix
PROXY STATEMENT
Dated , 2018
SUMMARY VOTING INSTRUCTIONS
Ensure that your shares of Sinovac Biotech Ltd. can be voted at the special meeting by submitting your proxy or contacting your broker, bank or other nominee.
If your shares are registered in the name of a broker, bank or other nominee: check the voting instruction card forwarded by your broker, bank or other nominee to see which voting options are available or contact your broker, bank or other nominee in order to obtain directions as to how to ensure that your shares are voted at the special meeting.
If your shares are registered in your name: submit your proxy as soon as possible by signing, dating and returning the accompanying proxy card in the enclosed postage-paid envelope so that your shares can be voted at the special meeting in accordance with your instruction.
If you submit your signed proxy card without indicating how you wish to vote, the shares represented by your proxy will be voted in favor of the resolutions to be proposed at the special meeting, unless you appoint a person other than the chairman of the meeting as proxy, in which case the shares represented by your proxy will be voted (or not submitted for voting) as your proxy determines.
If you have any questions, require assistance with voting your proxy card, or need additional copies of proxy material, please contact at (+ outside the United States) or at .
x
TABLE OF CONTENTS
| Page | |
| summary term sheet | 5 |
| The Parties Involved in the Amalgamation | 5 |
| The Amalgamation | 6 |
| Amalgamation Consideration | 7 |
| Treatment of Company Options and Company Restricted Shares | 8 |
| Support Agreement | 9 |
| Purposes and Effects of the Amalgamation | 9 |
| Reasons for the Amalgamation and Recommendation of the Board | 9 |
| Plans for the Company after the Amalgamation | 9 |
| Position of the Buyer Consortium as to Fairness | 10 |
| Financing of the Amalgamation | 10 |
| Limited Guarantees | 10 |
| Share Ownership of the Company’s Directors and Executive Officers and Voting Commitments | 10 |
| Opinion of the Special Committee’s Financial Advisor | 11 |
| Interests of Certain Persons in the Amalgamation | 11 |
| Conditions to the Amalgamation | 12 |
| No Solicitation of Competing Transactions | 13 |
| Termination of the Amalgamation Agreement | 13 |
| Termination Fee | 14 |
| Material U.S. Federal Income Tax Consequences | 15 |
| Material PRC Income Tax Consequences | 15 |
| Material Antigua and Barbuda Tax Consequences | 16 |
| Regulatory Matters | 16 |
| Litigation Related to the Amalgamation | 16 |
| Accounting Treatment of the Amalgamation | 17 |
| Market Price of the Shares | 17 |
| Fees and Expenses | 17 |
| Remedies and Limitations on Liabilities | 17 |
| SPECIAL FACTORS | 18 |
| Background of the Amalgamation | 18 |
| Reasons for the Amalgamation and Recommendation of the Special Committee and the Board | 54 |
| Position of the Buyer Consortium as to the Fairness of the Amalgamation | 65 |
| Certain Financial Projections | 68 |
| Opinion of the Special Committee’s Financial Advisor | 70 |
| Buyer Consortium’s Purpose of and Reasons for the Amalgamation | 82 |
| Effect of the Amalgamation on the Company | 83 |
| Effect of the Amalgamation on the Company’s Net Book Value and Net Loss | 86 |
| Net Book Value per Share of Our Shares | 87 |
| 1 |
| Plans for the Company after the Amalgamation | 87 |
| Alternatives to the Amalgamation | 87 |
| Effect on the Company if the Amalgamation is not Completed | 89 |
| Financing of the Amalgamation | 90 |
| Limited Guarantees | 90 |
| Support Agreement | 90 |
| Interim Investors Agreement | 91 |
| Remedies and Limitations on Liability | 91 |
| Interests of Certain Persons in the Amalgamation | 92 |
| Related Party Transactions | 94 |
| Fees and Expenses | 95 |
| Voting by Buyer Consortium at the Special Meeting | 95 |
| Litigation Related to the Amalgamation | 96 |
| Accounting Treatment of the Amalgamation | 96 |
| Regulatory Matters | 96 |
| Dissenters’ Rights | 96 |
| Material U.S. Federal Income Tax Consequences | 97 |
| Material PRC Income Tax Consequences | 101 |
| Material Antigua and Barbuda Tax Consequences | 101 |
| QUESTIONS AND ANSWERS ABOUT THE SPECIAL MEETING AND THE AMALGAMATION | 104 |
| MARKET PRICE OF THE COMPANY’S SHARES, DIVIDENDS AND OTHER MATTERS | 109 |
| Market Price of the Shares | 109 |
| Dividend Policy | 109 |
| THE SPECIAL MEETING | 110 |
| Date, Time and Place of the Special Meeting | 110 |
| Proposals to be Considered at the Special Meeting | 110 |
| Our Board of Directors’ Recommendation | 110 |
| Quorum | 111 |
| Record Date; Shares Entitled to Vote | 111 |
| Vote Required | 111 |
| Shareholders Entitled to Vote; Voting Materials | 112 |
| Proxy Holders for Registered Shareholders | 112 |
| Voting of Proxies and Failure to Vote | 112 |
| Revocability of Proxies | 112 |
| Rights of Shareholders Who Object to the Amalgamation | 113 |
| Whom to Call for Assistance | 113 |
| Solicitation of Proxies | 113 |
| Other Business | 114 |
| 2 |
| THE AMALGAMATION AGREEMENT | 115 |
| Structure and Completion of the Amalgamation | 115 |
| Articles of Incorporation and By-laws; Directors and Officers of the Surviving Corporation | 115 |
| Amalgamation Consideration | 116 |
| Treatment of Company Options and Company Restricted Shares | 117 |
| Exchange Procedures | 117 |
| Representations and Warranties | 118 |
| Conduct of Business Prior to Closing | 124 |
| No Solicitation of Competing Transactions | 127 |
| No Change of Recommendation | 130 |
| Company Shareholders’ Meeting | 132 |
| Directors’ and Officers’ Indemnification and Insurance | 133 |
| Financing | 134 |
| Actions Taken at the Direction of Certain Persons; Knowledge | 135 |
| Agreement to Use Reasonable Best Efforts | 136 |
| Certain Additional Covenants | 136 |
| Conditions to the Amalgamation | 137 |
| Termination of the Amalgamation Agreement | 139 |
| Termination Fees | 141 |
| Fees and Expenses | 142 |
| Modification or Amendment; Waiver of Conditions | 142 |
| Remedies and Limitations on Liabilities | 142 |
| PROVISIONS FOR UNAFFILIATED SECURITY HOLDERS | 144 |
| DISSENTERS’ RIGHTS | 145 |
| FINANCIAL INFORMATION | 147 |
| Ratio of Earnings to Fixed Charges | 148 |
| Net Book Value per Share of Our Shares | 148 |
| TRANSACTIONS IN SHARES | 149 |
| Purchases by the Company | 149 |
| Purchases by the Buyer Consortium | 149 |
| Prior Public Offerings | 149 |
| Transactions in Prior 60 Days | 149 |
| SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT OF THE COMPANY | 150 |
| FUTURE SHAREHOLDER PROPOSALS | 151 |
| CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS | 152 |
| WHERE YOU CAN FIND MORE INFORMATION | 154 |
| 3 |
| ANNEX A: AMALGAMATION AGREEMENT | A-1 |
| ANNEX B: Sections 191 to 199 of the International Business Corporations Act, CAP. 222 of the Revised Laws of Antigua and Barbuda | B-1 |
| ANNEX C: SUPPORT AGREEMENT | C-1 |
| ANNEX D: Opinion of DUFF & PHELPS, as Financial Advisor | D-1 |
| ANNEX E: Directors and Executive Officers of Each Filing Person | E-1 |
| 4 |
summary term sheet
This “Summary Term Sheet,” together with the “Questions and Answers About the Special Meeting and the Amalgamation,” highlight selected information contained in this proxy statement regarding the Amalgamation (as defined below) and may not contain all of the information that may be important to your consideration of the Amalgamation and other transactions contemplated by the Amalgamation Agreement (as defined below). You should carefully read this entire proxy statement and the other documents to which this proxy statement refers for a more complete understanding of the matters being considered at the special meeting. In addition, this proxy statement incorporates by reference important business and financial information about the Company. You are encouraged to read all of the documents incorporated by reference into this proxy statement and you may obtain such information without charge by following the instructions in “Where You Can Find More Information” beginning on page 154. In this proxy statement, the terms “the Company,” “us,” “we” or other terms correlative thereto refer to Sinovac Biotech Ltd, the term “offshore” refers to areas outside of mainland China and the term “onshore” refers to within mainland China. All references to “dollars” and “US$” in this proxy statement are to U.S. dollars, and all references to “RMB” in this proxy statement are to Renminbi, the lawful currency of the People’s Republic of China (the “PRC”).
The Parties Involved in the Amalgamation
The Company
Sinovac Biotech Ltd. (the “Company”) is a company limited by shares incorporated under the laws of Antigua and Barbuda and the issuer of common shares, par value US$0.001 per share (the “Shares”). The Company’s principal executive offices are located at No. 39, Shangdi Xi Road, Haidian District, Beijing 100085, People’s Republic of China. The Company’s telephone number at this address is +86-10-8289-0088.
For a description of the Company’s history, development, business and organizational structure, see the Company’s Annual Report on Form 20-F for the fiscal year ended December 31, 2016, filed with the United States Securities and Exchange Commission (the “SEC”) on November 22, 2017, which is incorporated herein by reference. See “Where You Can Find More Information” beginning on page 154 for a description of how to obtain a copy of the Company’s Annual Report.
The Acquisition Entities
Sinovac Holding (Cayman) Limited (“Holdco”) is an exempted company with limited liability incorporated under the laws of the Cayman Islands. The business address of Holdco is located at c/o No. 39 Shangdi Xi Road, Haidian District, Beijing 100085, People’s Republic of China and its telephone number is +86-10-8289-0088.
Sinovac (Cayman) Limited (“Parent”) is an exempted company with limited liability incorporated under the laws of the Cayman Islands. The business address of Parent is located at c/o No. 39 Shangdi Xi Road, Haidian District, Beijing 100085, People’s Republic of China and its telephone number is +86-10-8289-0088.
| 5 |
Sinovac Amalgamation Sub Limited (“Amalgamation Sub”) is an international business corporation incorporated under the laws of Antigua and Barbuda. The business address of Amalgamation Sub is located at c/o No. 39 Shangdi Xi Road, Haidian District, Beijing 100085, People’s Republic of China and its telephone number is +86-10-8289-0088.
Mr. Weidong Yin (“Mr. Yin”) is the chairman, president and chief executive officer of the Company. The business address of Mr. Yin is located at c/o No. 39 Shangdi Xi Road, Haidian District, Beijing 100085, People’s Republic of China and his telephone number is +86-10-8289-0088.
SAIF Partners IV L.P. (“SAIF”) is an exempted limited partnership registered and existing under the laws of the Cayman Islands. The business address of SAIF is located at c/o SAIF Advisors Limited, Suite 2516-2520, Two Pacific Place, 88 Queensway, Hong Kong and its telephone number is +852-2918-2200.
C-Bridge Healthcare Fund II, L.P. (“C-Bridge Capital”) is an exempted limited partnership registered and existing under the laws of the Cayman Islands. The business address of C-Bridge Capital is located at Room 7/03 Workingberg Commercial Building, 41-47 Marble Road, North Point, Hong Kong and its telephone number is +86-21-8012-7764.
Advantech Capital L.P. (“Advantech Capital”) is an exempted limited partnership registered and existing under the laws of the Cayman Islands. The business address of Advantech Capital is located at 190 Elgin Avenue, George Town, Grand Cayman KY1-9005, Cayman Islands and its telephone number is +852-2801-6988.
Vivo Capital Fund VIII, L.P. (“Vivo Capital Fund”) is a limited partnership organized and existing under the laws of the State of Delaware. The business address of Vivo Capital Fund is located at 505 Hamilton Avenue, Suite 207, Palo Alto, CA 94301 and its telephone number is +1 650-688-0818.
Vivo Capital Surplus Fund VIII, L.P. (“Vivo Capital Surplus Fund,” together with Vivo Capital Fund, collectively, “Vivo Capital”) is a limited partnership organized and existing under the laws of the State of Delaware. The business address of Vivo Capital Surplus Fund is located at 505 Hamilton Avenue, Suite 207, Palo Alto, CA 94301 and its telephone number is +1 650-688-0818.
Throughout this proxy statement, (i) Mr. Yin and SAIF are collectively referred to as the “Rollover Shareholders”; (ii) C-Bridge Capital, Advantech Capital and Vivo Capital are collectively referred to as “Sponsors”; and (iii) Holdco, Parent, Amalgamation Sub, the Rollover Shareholders and the Sponsors are collectively referred to herein as the “Buyer Consortium.” Additional information regarding the Company and members of the Buyer Consortium is set forth in Annex E, which is attached hereto and incorporated herein by reference.
The Amalgamation (Page 115)
The Company, Parent and Amalgamation Sub have entered into an amalgamation agreement, dated as of June 26, 2017 (such agreement as may be amended from time to time, the “Amalgamation Agreement”), pursuant to which Amalgamation Sub will amalgamate with and into the Company (the “Amalgamation”), with the Company continuing as the surviving corporation (the “Surviving Corporation”).
| 6 |
You are being asked to vote to authorize and approve the Amalgamation Agreement, the filing of the Amalgamation Agreement with the Financial Services Regulatory Commission of Antiqua and Barbuda and the transactions contemplated thereby, including the Amalgamation (the “Transactions”). Once the Amalgamation Agreement and the Transactions are authorized and approved by the requisite vote of the shareholders of the Company and the other conditions to the consummation of the Transactions are satisfied or waived in accordance with the terms of the Amalgamation Agreement, the Company shall execute the articles of amalgamation (the “Articles of Amalgamation”) as prescribed in form 8 in the schedule to the International Business Corporations Act, CAP .222 of the Revised Laws of Antigua and Barbuda (as consolidated and revised) (the “ICBA”), the Company will file the Articles of Amalgamation and other documents, including the Amalgamation Agreement, required under the IBCA to effect the Amalgamation with the Financial Services Regulatory Commission of Antiqua and Barbuda as provided by Section 172 of the IBCA and Amalgamation Sub will amalgamate with and into the Company and cease to exist, with the Company continuing as the Surviving Corporation in accordance with Section 191 of the IBCA and becoming a wholly-owned subsidiary of Parent. The Company, as the Surviving Corporation, will continue to do business under the name “Sinovac Biotech Ltd.” following the Amalgamation.
At the effective time of the Amalgamation (the “Effective Time”), the articles of incorporation and by-laws of Amalgamation Sub, as in effect immediately prior to the Effective Time, will be the articles of incorporation and by-laws of the Surviving Corporation, except that (i) Article I of the articles of incorporation of the Surviving Corporation will be amended to read “The name of the Corporation is Sinovac Biotech Ltd.” and the articles of incorporation and by-laws of the Surviving Corporation will be amended to refer to the name of the Surviving Corporation as “Sinovac Biotech Ltd.” and (ii) references therein to the authorized share capital of the Surviving Corporation will be amended to refer to the correct authorized capital of the Surviving Corporation as approved in the Articles of Amalgamation, if necessary.
If the Amalgamation is completed, the Company will cease to be a publicly traded company and all of the Shares will be delisted from the NASDAQ Global Select Market (“NASDAQ”). Upon the completion of the Amalgamation, the Company will become a private company beneficially owned by the Buyer Consortium. A copy of the Amalgamation Agreement is attached as Annex A to this proxy statement. You should read the Amalgamation Agreement in its entirety because it, and not this proxy statement, is the legal document that governs the Amalgamation.
Amalgamation Consideration (Page 116)
Under the Amalgamation Agreement, at the Effective Time, each Share of the Company issued and outstanding immediately prior to the Effective Time will be cancelled in exchange for the right to receive US$7.00 (the “Per Share Amalgamation Consideration”), in cash, without interest and net of any applicable withholding taxes. Notwithstanding the foregoing, if the Amalgamation is completed, the following Shares will be cancelled at the Effective Time but will not be exchanged into the right to receive the Per Share Amalgamation Consideration:
| 7 |
| · | 6,049,500 Shares held by Mr. Yin and 10,780,820 Shares held by SAIF (collectively, the “Rollover Shares”), and Shares held by Parent or any of its affiliates and Shares held by the Company or any of its subsidiaries issued and outstanding immediately prior to the Effective Time (such Shares, together with the Rollover Shares, the “Excluded Shares”), which will be cancelled without payment of any consideration or distribution therefor; |
| · | Shares held by shareholders who have validly exercised and not effectively withdrawn or lost their dissenters’ rights in accordance with Section 191 of the International Business Corporations Act, CAP. 222 of the IBCA (the “Dissenting Shares”), which will be cancelled and will entitle the former holders thereof to receive the payment of the fair value thereof in accordance with the ICBA; and |
| · | all Shares issued and outstanding immediately prior to the Effective Time, together with the associated Series A Junior Participating Preferred Share Purchase Rights (the “Rights”), issued under the Rights Agreement, dated as of March 28, 2016, between the Company and Pacific Stock Transfer Company, as rights agent (as amended from time to time, the “Rights Agreement”), which will cease to be outstanding, will be cancelled and will cease to exist, and the register of shareholders of the Company will be amended accordingly. |
At the Effective Time, each common share, par value US$1.00 per share, of Amalgamation Sub issued and outstanding immediately prior to the Effective Time will be converted into and become one validly issued, fully paid and non-assessable ordinary share, par value US$0.001 per share, of the Surviving Corporation.
Treatment of Company Options and Company Restricted Shares (Page 117)
At the Effective Time, each option to purchase Shares granted under the Company’s Stock Option Plan, as amended (the “Stock Option Plan”), and the Company’s 2012 Share Incentive Plan, as amended (the “2012 Share Incentive Plan” and, together with the Stock Option Plan, the “Share Incentive Plans”), issued, outstanding and unexercised immediately prior to the Effective Time, whether vested or unvested (the “Company Options”), will be cancelled and converted into the right to receive, as soon as practicable after the Effective Time (and in any event no more than five business days after the Effective Time), an amount equal to the product of (a) the total number of Shares issuable under such Company Option immediately prior to the Effective Time, multiplied by (b) the excess of US$7.00 over the exercise price payable per Share under such Company Option, in cash, without interest and net of any applicable withholding taxes. If the exercise price per Share issuable under any such Company Option is equal to or greater than US$7.00, such Company Option will be cancelled without any payment therefor.
At the Effective Time, each restricted Share granted under the 2012 Share Incentive Plan that is issued and outstanding (and with respect to which the restrictions have not lapsed) immediately prior to the Effective Time (the “Company Restricted Shares”) will be cancelled and converted into the right to receive, as soon as practicable after the Effective Time (and in any event no more than five business days after the Effective Time), an amount equal to US$7.00, in cash, without interest and net of any applicable withholding taxes.
| 8 |
The Share Incentive Plans will be terminated at the Effective Time.
Support Agreement (Page 90)
Concurrently with the execution and delivery of the Amalgamation Agreement, the Rollover Shareholders entered into a support agreement dated June 26, 2017 (the “Support Agreement”) in favor of Parent and Holdco, pursuant to which, the Rollover Shareholders have agreed, subject to the terms and conditions set forth therein and among other obligations, to (a) cancel the Rollover Shares held by such Rollover Shareholders for no consideration in connection with the Amalgamation, (b) subscribe for newly issued ordinary shares of Holdco (the “Holdco Shares”) immediately prior to the closing of the Amalgamation (the “Closing”) and (c) vote all of the Shares owned directly or indirectly by them in favor of the Amalgamation. A copy of the Support Agreement is attached as Annex C to this proxy statement and is incorporated herein by reference.
Purposes and Effects of the Amalgamation (Page 82)
The purpose of the Amalgamation is to enable Parent to acquire 100% of the Company in a transaction in which the holders of the Shares (other than holders of Excluded Shares and Dissenting Shares) will be cashed out in exchange for the aggregate Per Share Amalgamation Consideration (in aggregate, the “Amalgamation Consideration”).
The Shares are currently listed on NASDAQ under the symbol “SVA.” Following the consummation of the Amalgamation, the Company will cease to be a publicly traded company and will become a private company beneficially owned by the Buyer Consortium.
Reasons for the Amalgamation and Recommendation of the Board (Page 54)
The board of directors of the Company (the “Board”), upon the unanimous recommendation of a special committee (the “Special Committee”) composed solely of independent directors unaffiliated with the Buyer Consortium or any member of the management of the Company, determined that entering into the Amalgamation Agreement and consummating the Transactions, including the Amalgamation, are fair to and in the best interests of the Company and its shareholders (other than the holders of Excluded Shares) (the “Unaffiliated Holders”). For a detailed discussion of the material factors the Special Committee and the Board considered, see “Special Factors—Reasons for the Amalgamation and Recommendation of the Special Committee and the Board” beginning on page 54 and “Special Factors—Effect of the Amalgamation on the Company—Primary Benefits and Detriments of the Amalgamation” beginning on page 84.
Plans for the Company after the Amalgamation (Page 87)
After the Effective Time, the Buyer Consortium anticipates that the Company will conduct its operations substantially as it currently conducts its operations, except that the Company will cease to be a publicly traded company and will instead be a wholly-owned subsidiary of Parent. See “Special Factors—Plans for the Company after the Amalgamation” beginning on page 87 for additional information.
| 9 |
Position of the Buyer Consortium as to Fairness (Page 65)
The Buyer Consortium believes that the Amalgamation is both procedurally and substantively fair to the Unaffiliated Holders. Their belief is based upon the factors discussed under the caption “Special Factors—Position of the Buyer Consortium as to the Fairness of the Amalgamation” beginning on page 65.
The Buyer Consortium is making the statements included in this section solely for the purpose of complying with the requirements of Rule 13e-3 and related rules under the Securities Exchange Act of 1934, as amended (the “Exchange Act”). The views of the Buyer Consortium as to the fairness of the Amalgamation are not intended to be and should not be construed as a recommendation to any shareholder of the Company as to how to vote on the proposal to authorize and approve the Amalgamation Agreement and the Transactions, including the Amalgamation.
Financing of the Amalgamation (Page 90)
The Company and the Buyer Consortium estimate that the total amount of funds necessary to complete the Transactions, including the Amalgamation, is approximately US$ (assuming no exercise of dissenters’ rights by shareholders of the Company). In calculating this amount, the Company and the Buyer Consortium did not consider the value of the Rollover Shares, which will be canceled for no consideration pursuant to the Amalgamation Agreement. This amount includes (a) the cash to be paid to the Unaffiliated Holders, and (b) the related costs and expenses, in connection with the Transactions, including the Amalgamation. The Transactions, including the Amalgamation, are expected to be funded by equity contributions contemplated by the equity commitment letters (each an “Equity Commitment Letter”), by and between Holdco, Parent, the Company and each of the Sponsors, dated as of June 26, 2017. See “Special Factors—Financing of the Amalgamation” beginning on page 90 for additional information.
Limited Guarantees (Page 90)
Each of SAIF, C-Bridge Capital, Advantech Capital and Vivo Capital (each a “Guarantor” and, collectively, the “Guarantors”) executed and delivered a limited guarantee in favor of the Company on June 26, 2017 (each a “Limited Guarantee” and, collectively, the “Limited Guarantees”), pursuant to which each Guarantor has guaranteed in favor of the Company a portion of the payment obligations of Parent under the Amalgamation Agreement with respect to the termination fee and certain costs and expenses that Parent may be required to pay the Company under the Amalgamation Agreement.
Share Ownership of the Company’s Directors and Executive Officers and Voting Commitments (Page 150)
As of the date of this proxy statement, the directors and executive officers of the Company in the aggregate own 6,688,500 Shares, and no Shares are subject to options and restricted shares that will vest within 60 days after the date of this proxy statement. Each of our directors and executive officers who beneficially owns Shares has informed us that, as of the date of this proxy statement, he or she intends to vote all of his or her Shares in favor of approving and authorizing the Amalgamation Agreement and the consummation of the Transactions, including the Amalgamation, for the reasons described under “Special Factors—Reasons for the Amalgamation and Recommendation of the Special Committee and the Board” beginning on page 54.
| 10 |
As of the date of this proxy statement, the Buyer Consortium beneficially owns approximately 29.5% of the issued and outstanding Shares entitled to vote. Pursuant to the Support Agreement, these Shares will be voted in favor of the authorization and approval of the Amalgamation Agreement and the Transactions, including the Amalgamation.
Opinion of the Special Committee’s Financial Advisor (Page 70)
The Special Committee retained Duff & Phelps, LLC (“Duff & Phelps”) to act as its financial advisor to provide an opinion to the Special Committee as to the fairness, from a financial point of view, to the holders of Shares (other than the Excluded Shares, the Dissenting Shares and Company Restricted Shares) of the Per Share Amalgamation Consideration to be received by such holders in the Amalgamation pursuant to the Amalgamation Agreement (without giving effect to any impact of the Amalgamation on any particular holder of Shares, other than in its capacity as a holder of Shares).
On June 26, 2017, at a meeting of the Special Committee, Duff & Phelps rendered its oral opinion (which was confirmed in writing by delivery of its written opinion later that same day) to the Special Committee that, subject to the factors, assumptions, and limitations set forth therein, as of such date, the Per Share Amalgamation Consideration to be received by the holders of Shares (other than the Excluded Shares, the Dissenting Shares and Company Restricted Shares) in the Amalgamation is fair, from a financial point of view, to such holders (without giving effect to any impact of the Amalgamation on any particular holder of the Shares other than in its capacity as a holder of Shares). The full text of the written opinion of Duff & Phelps is attached as Annex D to this proxy statement and is incorporated herein by reference.
See “Special Factors—Opinion of the Special Committee’s Financial Advisor” beginning on page 70 for additional information.
Interests of Certain Persons in the Amalgamation (Page 92)
In considering the recommendations of the Board, the Company’s shareholders should be aware that, aside from their interests in Shares of the Company, some of the Company’s directors and executive officers have interests in the Amalgamation that are different from, or in addition to, the interests of the Company’s shareholders generally. These interests may include, among others:
| · | the treatment of vested and unvested awards granted to them under the Share Incentive Plans; |
| 11 |
| · | indemnification rights and directors’ and officers’ liability insurance to be provided by the Surviving Corporation to former directors and officers of the Company; and |
| · | the continued service of the executive officers of the Company with the Surviving Corporation in positions that are substantially similar to their current positions. |
The Special Committee and the Board were aware that some of the Company’s directors and executive officers have interests in the Amalgamation that are different from, or in addition to, the interests of the Company’s shareholders generally and considered this, among other matters, in reaching their decisions and recommendations with respect to the Amalgamation Agreement and related matters. Please see “Special Factors—Interests of Certain Persons in the Amalgamation” beginning on page 92 for additional information.
Conditions to the Amalgamation (Page 137)
The consummation of the Amalgamation is subject to the satisfaction of the following conditions:
| · | the authorization and approval, by an affirmative vote of shareholders representing at least two-thirds of the Shares present and voting in person or by proxy as a single class at the special meeting, of the Amalgamation Agreement and the Transactions, including the Amalgamation, at the special meeting; and |
| · | no governmental authority of competent jurisdiction having enacted, issued, promulgated, enforced or entered any law, award, writ, injunction, determination, rule, regulation, judgment, decree or executive order, whether temporary, preliminary or permanent, that is in effect or has or would have the effect of enjoining, restraining, prohibiting or otherwise making illegal the consummation of the Transactions. |
The obligations of Parent and Amalgamation Sub to complete the Amalgamation are also subject to the following conditions:
| · | the accuracy of the representations and warranties of the Company in the Amalgamation Agreement as of the closing date of the Amalgamation; |
| · | the Company’s compliance with its agreements and covenants under the Amalgamation Agreement; |
| · | the delivery by the Company to Parent of a certificate dated the closing date of the Amalgamation as to the satisfaction of the two immediately preceding conditions; |
| · | shareholders of the Company holding no more than 7.5% of the Shares, other than 1Globe Capital LLC and its affiliates (and any investment fund advised or managed by 1Globe Capital LLC or any of its affiliates) and the persons and entities comprising the reporting person identified as “Chiang Li Family” in the Schedule 13G relating to the Company filed by such reporting person with the SEC on April 4, 2016 (and their respective affiliates), having validly served a notice of dissent under Section 191 of the IBCA; and |
| 12 |
| · | there not having been a Company Material Adverse Effect (as defined under “The Amalgamation Agreement—Representations and Warranties” beginning on page 118) since the date of the Amalgamation Agreement that remains continuing. |
The obligations of the Company to complete the Amalgamation are also subject to the following conditions:
| · | the accuracy of the representations and warranties of Parent and Amalgamation Sub in the Amalgamation Agreement as of the closing date of the Amalgamation; |
| · | Parent and Amalgamation Sub’s compliance with their agreements and covenants under the Amalgamation Agreement; and |
| · | the delivery by Parent to the Company of a certificate dated the closing date of the Amalgamation as to the satisfaction of the two immediately preceding conditions. |
No Solicitation of Competing Transactions (Page 127)
The Amalgamation Agreement restricts the Company’s ability, until the Effective Time or, if earlier, the termination of the Amalgamation Agreement, to solicit or engage in discussions or negotiations with third parties regarding any Competing Transaction (as defined under “The Amalgamation Agreement—No Solicitation of Competing Transactions” beginning on page 127). However, subject to specified conditions and prior to obtaining the required shareholder authorization and approval of the Amalgamation Agreement and the Transactions, including the Amalgamation, we may (a) in response to an unsolicited proposal or offer regarding a Competing Transaction from a third party, contact such third party to clarify and understand the terms and conditions of such proposal or offer to the extent the Special Committee determines in good faith that such contact is necessary to determine whether such proposal or offer constitutes a Superior Proposal (as defined under “The Amalgamation Agreement—No Solicitation of Competing Transactions” beginning on page 127) or could reasonably be expected to result in a Superior Proposal and (b) provide information to a third party in response to an unsolicited proposal or offer regarding a Competing Transaction from a third party if the Special Committee determines in its good faith judgement (after consultation with its financial advisor and outside legal counsel) that such proposal or offer constitutes, or could be reasonably expected to result in, a Superior Proposal, and, in light of such Superior Proposal, failure to do so would be inconsistent with the fiduciary duties of the Board under applicable law. Please see and read carefully “The Amalgamation Agreement—No Solicitation of Competing Transactions” and “The Amalgamation Agreement—No Change of Recommendation” beginning on page 127 and page 130, respectively.
Termination of the Amalgamation Agreement (Page 139)
The Amalgamation Agreement may be terminated at any time prior to the Effective Time:
| 13 |
| · | by mutual written consent of the Company (acting only upon the recommendation of the Special Committee) and Parent; |
| · | by either the Company (acting only upon the recommendation of the Special Committee) or Parent if: |
| o | the Amalgamation is not completed on or before March 26, 2018; |
| o | any governmental authority of competent jurisdiction has enacted, issued, promulgated, enforced or entered any final and non-appealable law or award, writ, injunction, determination, rule, regulation, judgment, decree or executive order that has the effect of making illegal, or otherwise preventing or prohibiting, the consummation of the Transactions; or |
| o | the Company’s shareholders do not approve the Amalgamation Agreement and the Transactions, including the Amalgamation, at the special meeting; |
| · | by the Company if: |
| o | Parent or Amalgamation Sub breaches the Amalgamation Agreement in specified ways; |
| o | Parent and Amalgamation Sub fail to complete the Amalgamation; |
| o | the Company accepts a Superior Proposal (as defined under “The Amalgamation Agreement—No Solicitation of Competing Transactions” beginning on page 127) under specified circumstances; or |
| o | the Special Committee authorizes the Company to terminate the Amalgamation Agreement as a result of certain specified intervening events where the Special Committee determines in its good faith judgment that the failure to terminate the Amalgamation Agreement would be inconsistent with the fiduciary duties of the Board under applicable law; or |
| · | by Parent if: |
| o | the Company breaches the Amalgamation Agreement in specified ways; or |
| o | the Board changes its recommendation that the Company’s shareholders approve the Amalgamation Agreement and the Transactions, including the Amalgamation, at the special meeting. |
Termination Fee (Page 141)
If the Amalgamation Agreement is terminated under certain circumstances, the Company may be required to pay Parent a termination fee of US$15.0 million, or if the Amalgamation Agreement is terminated by the Company under certain circumstances, Parent may be required to pay the Company a termination fee of US$15.0 million, in each case, as described under “The Amalgamation Agreement—Termination Fees” beginning on page 141.
| 14 |
Material U.S. Federal Income Tax Consequences (Page 97)
For a U.S. Holder (as defined under “Special Factors—Material U.S. Federal Income Tax Consequences” beginning on page 97), the receipt of cash pursuant to the Amalgamation will be a taxable transaction for U.S. federal income tax purposes and may also be a taxable transaction under applicable state, local and other tax laws. Please see “Special Factors—Material U.S. Federal Income Tax Consequences” beginning on page 97 for additional information. The U.S. federal income tax consequences of the Amalgamation to you will depend upon your personal circumstances. You should consult your tax advisors for a full understanding of the U.S. federal, state, local, non-U.S. and other tax consequences of the Amalgamation to you.
Material PRC Income Tax Consequences (Page 101)
The Company does not believe that it should be considered a PRC tax resident enterprise under the PRC Enterprise Income Tax Law (the “EIT Law”) or that the gain recognized on the receipt of cash for Shares by holders who are not PRC tax residents should be subject to PRC tax. However, there is uncertainty regarding whether PRC tax authorities would deem the Company to be a PRC tax resident enterprise.
If PRC tax authorities determine that the Company is a PRC tax resident enterprise, then gains recognized on the receipt of cash for Shares pursuant to the Amalgamation by shareholders who are not PRC tax residents could be treated as PRC-source income. This income would be subject to PRC income tax at a rate of 10% in the case of enterprises or 20% in the case of individuals (subject to applicable tax treaty relief, if any). Regardless of whether the Company is considered a PRC tax resident enterprise, gain recognized on the receipt of cash for Shares is subject to PRC tax if the holders of such Shares are PRC tax residents.
If the Company were not considered a PRC tax resident enterprise, gains derived through buying and selling such shares in a public stock exchange market by holders of Shares who are not PRC tax residents would not be subject to PRC income tax. If the Company were considered a PRC tax resident enterprise, the PRC tax authorities may determine whether the Amalgamation has bona-fide commercial purposes based on the facts and circumstances as well as the applicable tax circulars.
Announcement [2015] No. 7 issued by the State Administration of Taxation states that an indirect transfer of equity in a Chinese resident enterprise may be re-characterized as a “direct transfer” following the “substance over form” principle if it is assessed as lacking bona-fide commercial purposes. If a “direct transfer” occurs, gains recognized on the receipt of cash for Shares will be subject to PRC income tax.
The bona-fide business purpose test is complicated and depends on the judgment of PRC tax authorities. You should consult your own tax advisors for a full understanding of the tax consequences of the Amalgamation to you, including the above-mentioned potential PRC tax consequences.
| 15 |
Please see “Special Factors—Material PRC Income Tax Consequences” beginning on page 101 for additional information.
Material Antigua and Barbuda Tax Consequences (Page 101)
Under the Antigua and Barbuda International Business Corporations Act, Cap.222 of the Revised Laws of Antigua and Barbuda, an international business corporation (an “IBC”) is granted a fifty-year tax exemption from the date of incorporation that applies to most forms of income, dividends, interest and royalties paid by an IBC to non-residents. An IBC also benefits from the absence of withholding tax, capital gains, estate, inheritance or succession taxes in Antigua and Barbuda.
In addition an IBC is exempt from exchange control under the Exchange Control Act, Cap.157 and the payment of stamp duties under the Stamp Act Cap.410 in respect of all transactions entered into in relation to the IBC’s international trade or business. Antigua and Barbuda has bilateral investment treaties with Germany and the United Kingdom and double taxation agreements with Denmark, Norway, Sweden, the United Kingdom and member states of the Caribbean Community (CARICOM).
The Antigua and Barbuda tax consequences of the Amalgamation will depend upon your personal circumstances. You should consult your tax advisors for a full understanding of the Antigua and Barbuda tax consequences of the Amalgamation to you. Please see “Special Factors—Material Antigua and Barbuda Tax Consequences” beginning on page 101 for additional information.
Regulatory Matters (Page 96)
The Company does not believe that any material federal or state regulatory approvals, filings or notices are required in connection with effecting the Amalgamation other than (a) the approvals, filings or notices required under the federal securities laws and (b) the filing of the Amalgamation Agreement (and supporting documentation as specified in Section 172 of the IBCA) with the Director of the Financial Services Regulatory Commission of Antigua and Barbuda. Adequate notice of the Amalgamation must also be provided to certain creditors of the Company and Amalgamation Sub. Pursuant to Section 172 of the IBCA, adequate notice is given if (i) a notice in writing is sent to each known creditor having a claim against Amalgamation Sub or the Company that exceeds one thousand dollars, (ii) a notice of the Amalgamation is published in a newspaper published or distributed in Antigua and Barbuda and (iii) each notice states that Amalgamation Sub intends to amalgamate with the Company and that a creditor can object to the Amalgamation within thirty days from the date of the notice.
Litigation Related to the Amalgamation (Page 96)
The Board, Parent and Amalgamation Sub have been named as defendants in a shareholder class action brought by purported shareholders of the Company seeking, among other things, to enjoin the Transactions. Please see “Special Factors—Litigation Related to the Amalgamation” beginning on page 96 for additional information.
| 16 |
Accounting Treatment of the Amalgamation (Page 96)
The Amalgamation is expected to be accounted for, at historical cost, as a merger of entities under common control in accordance with Accounting Standards Codification 805-50, “Business Combinations—Related Issues.”
Market Price of the Shares (Page 109)
The Per Share Amalgamation Consideration of US$7.00 per Share represents a premium of 22.8% to the closing price of the Shares as quoted by NASDAQ on June 23, 2017, the last trading day immediately prior to the date that the Company announced that it had entered into the Amalgamation Agreement, and a premium of 32.1% and 30% to the volume-weighted average trading price of the Shares as quoted by NASDAQ during the 30 and 60 trading days, respectively, immediately prior to the Company’s announcement on February 1, 2016 that it had received a preliminary non-binding proposal letter from Mr. Yin and SAIF and/or its affiliates to acquire all of the outstanding Shares not already owned by them.
Fees and Expenses (Page 142)
Whether or not the Amalgamation is completed, all costs and expenses incurred in connection with the Amalgamation Agreement and the transactions contemplated thereby will be paid by the party incurring such costs and expenses, except as otherwise provided in the Amalgamation Agreement.
Remedies and Limitations on Liabilities (Page 142)
The parties to the Amalgamation Agreement may be entitled to specific performance of the terms of the Amalgamation Agreement, including an injunction or injunctions to prevent breaches of the Amalgamation Agreement, in addition to any other remedy at law or equity.
The maximum monetary damages available to the parties to the Amalgamation Agreement are the termination fees described above and under “The Amalgamation Agreement—Termination Fees” beginning on page 141.
| 17 |
SPECIAL FACTORS
Background of the Amalgamation
Events leading to the execution of the Amalgamation Agreement described in this Background of the Amalgamation primarily occurred in the PRC. As a result, PRC Standard Time is used for all dates and times given.
The Board and senior management of the Company have periodically reviewed the Company’s long-term strategic plan with the goal of maximizing shareholder value. As part of this ongoing process, the Board and senior management also have periodically reviewed strategic alternatives that may be available to the Company.
In December 2015, representatives of the Buyer Consortium began discussing with certain potential equity investors and considering the possibility of making a proposal to acquire the Company, in light of the market trends, recent activities of other U.S. public companies with operations primarily in the PRC and the Company’s long term strategic plans.
On January 30, 2016, the Buyer Consortium (which at the time consisted of Mr. Yin and SAIF) submitted to the Board a preliminary non-binding proposal letter, dated January 30, 2016 ( the “Buyer Consortium’s Proposal Letter”), to acquire all of the Company’s outstanding Shares not already owned by the Buyer Consortium and its affiliates in a going-private transaction for US$6.18 in cash per Share (the “Proposed Transaction”). In the Buyer Consortium’s Proposal Letter, the Buyer Consortium also noted that the Proposed Transaction would be financed with a combination of equity capital and debt. Equity financing would be provided by the Buyer Consortium in the form of cash and rollover equity in the Company, and debt financing was expected to be provided by loans from third party financial institutions.
On January 31, 2016, following receipt of the Buyer Consortium’s Proposal Letter, the Board held a telephonic meeting to discuss, among other things, the Proposed Transaction. Mr. Yin and Kenneth Lee, a director nominated by SAIF (the “SAIF Director”), recused themselves from the meeting due to conflicts of interest arising from their roles as members of the Board and Mr. Yin’s and SAIF’s interests in the Proposed Transaction as members of the Buyer Consortium. During the meeting, the Board determined that it was in the best interests of the Company and the unaffiliated shareholders of the Company to form a special committee to consider the Proposed Transaction. After discussing the various qualifications of the members of the Board to serve on the special committee, the Board adopted resolutions to form the Special Committee, comprised of the following three directors, whom the Board determined were sufficiently independent for purposes of serving on the Special Committee: Mr. Simon Anderson (“Mr. Anderson”) (to serve as chairman of the Special Committee), Dr. Yuk Lam Lo (“Dr. Lo”) and Mr. Meng Mei (“Mr. Mei”).
| 18 |
At the same meeting, the Board also adopted resolutions delegating to the Special Committee the power and authority of the Board to, among other things, (a) make such investigation of the Proposed Transaction and any matters relating thereto as the Special Committee, in its sole discretion, deemed appropriate, (b) evaluate the terms of the Proposed Transaction, (c) discuss and negotiate with the Buyer Consortium and its representatives any terms of the Proposed Transaction and implement the Proposed Transaction, in each case, as the Special Committee deemed appropriate, (d) explore any alternatives to the Proposed Transaction (an “Alternative Transaction”) as the Special Committee, in its sole discretion, deemed appropriate, including maintaining the Company’s current status as a public company, (e) if and when appropriate, negotiate and execute ancillary agreements with respect to the Proposed Transaction or any Alternative Transaction, (f) if and when appropriate, negotiate definitive agreements with respect to the Proposed Transaction or any Alternative Transaction, the execution and delivery of any such agreement being subject, however, to the approval of the Board, (g) report to the Board on the Special Committee’s (1) recommendations and conclusions with respect to the Proposed Transaction and/or any Alternative Transaction and any recommendation as to whether the final terms of the Proposed Transaction or any Alternative Transaction are fair to and in the best interests of the minority shareholders of the Company and should be approved by the Board and, if applicable, by the Company’s shareholders, and (2) determinations and recommendations with respect to any other matters requested by the Board, (h) if any when appropriate, at its sole discretion, adopt defensive measures, such as a rights issuance, with respect to unsolicited proposals for an Alternative Transaction and (i) retain, on terms and conditions acceptable to the Special Committee, such advisors, including legal counsel, financial advisors and outside consultants, as the Special Committee in its sole discretion deemed appropriate to assist the Special Committee in discharging its responsibilities.
On February 1, 2016, the Company issued a press release announcing its receipt of the Buyer Consortium’s Proposal Letter and the formation of the Special Committee.
On February 2, 2016, SAIF filed with the SEC an amendment to its Schedule 13D with respect to the submission of the Buyer Consortium’s Proposal Letter.
On February 4, 2016, the Board received a preliminary non-binding proposal letter, dated February 3, 2016 (the “Sinobioway Consortium’s Proposal Letter”), from a consortium comprised of PKU V-Ming (Shanghai) Investment Holdings Co., Ltd. (“PKU Shanghai”), Shandong Sinobioway Biomedicine Co., Ltd. (“Shandong Sinobioway”), CICC Qianhai Development (Shenzhen) Fund Management Co., Ltd. (“CICC Qianhai”), Beijing Sinobioway Group Co., Ltd. (“Beijing Sinobioway”), Heng Feng Investments (International) Limited (“Heng Feng”) and Fuerde Global Investment Limited (“Fuerde”) (such consortium, as the members thereof have changed over time as further described below, the “Sinobioway Consortium”). In the Sinobioway Consortium’s Proposal Letter, the Sinobioway Consortium indicated its interest in acquiring all of the Company’s outstanding Shares for US$7.00 in cash per Share (the “Sinobioway Proposed Transaction”). The Sinobioway Consortium also noted in the Sinobioway Consortium’s Proposal Letter that the Sinobioway Proposed Transaction would be financed with equity from the members of the Sinobioway Consortium and that debt financing might be provided by loans from third party financial institutions. Later on the same day, the Company issued a press release announcing its receipt of the Sinobioway Consortium’s Proposal Letter.
Over the course of the next 17 months, the Special Committee participated in 39 telephonic and in-person meetings in connection with its evaluation of the Proposed Transaction and the Sinobioway Proposed Transaction.
On February 8, 2016, Mr. Yin filed with the SEC an amendment to his Schedule 13D with respect to the submission of the Buyer Consortium’s Proposal Letter.
| 19 |
On February 17, 2016, the Special Committee held a telephonic meeting to discuss, among other things, the engagement of its own independent legal and financial advisors. After the discussion, the Special Committee decided that it should retain independent legal and financial advisors.
During the next few weeks, the Special Committee considered proposals from, and conducted interviews with, multiple law firms and investment banks that had expressed interest in being considered for the role of U.S. legal counsel and financial advisor to the Special Committee, respectively.
On February 25, 2016, after considering proposals from multiple U.S. law firms and evaluating the presentations made by each law firm, the Special Committee engaged Weil, Gotshal & Manges LLP (“Weil”) to serve as its U.S. legal counsel.
On February 26, 2016, management of the Company reported to the Board that they had discussed with the Company’s U.S. legal counsel, Latham & Watkins LLP (“Latham”), and the Company’s Antigua and Barbuda counsel, Delany Law (“Delaney”), the adoption of a shareholder rights plan. Management of the Company proposed that the Board adopt a shareholder rights plan to encourage a potential buyer to negotiate with the Special Committee and provide the Special Committee with adequate time to evaluate different proposals and maximize shareholder value. Management of the Company suggested holding a conference call for the Board to listen to a presentation by Latham and Delaney and further discuss the matter.
On March 2, 2016, the Board held a conference call to discuss the shareholder rights plan, during which Latham and Delany Law gave a presentation regarding the potential adoption of a shareholder rights plan by the Company. Latham and Delany explained that a shareholder rights plan, among other things, is intended to (a) enhance the Board’s ability to protect shareholders against unsolicited attempts to acquire control of the Company that do not offer an adequate price to all shareholders or that are otherwise not in the best interest of the Company and its shareholders, (b) encourage bidders to negotiate with the Board as the elected representatives of the shareholders, (c) provide the Board with the opportunity to preserve existing, more advantageous strategies or to develop and implement superior alternatives and (d) reduce the adverse effects of increased concentration in common stock ownership. They also explained that a shareholder rights plan, among other things, is not intended to (i) lessen the Board’s duties, (ii) prevent all acquisitions, (iii) deter fully-priced and fairly structured offers or (iv) have any impact on the Company’s operation and performance. After a lengthy discussion, the Board determined that Latham and Delany Law should prepare a form of shareholder rights plan with the assistance of the Company’s management to be proposed for adoption and approval by the Board at a future meeting of the Board.
On March 8, 2016, the Special Committee held a conference call with Weil to discuss, among other things, the current status of retaining a financial advisor and Antigua and Barbuda counsel.
On March 17, 2016, the Buyer Consortium retained Lazard Asia (Hong Kong) Limited (“Lazard”) as its financial advisor in connection with the Proposed Transaction, primarily because of Lazard’s demonstrated knowledge of and expertise in the biotechnology sector in the PRC, as well as its reputation and experience in providing financial advice in mergers and acquisitions transactions.
| 20 |
Between mid-March 2016 and early June 2016, representatives of the Buyer Consortium and Lazard contacted, and had preliminary discussions with, various potential equity investors who they thought might be interested in joining the Buyer Consortium and third-party banks who they thought might be willing to provide debt financing to the Buyer Consortium in connection with the Proposed Transaction.
On March 26, 2016, the Special Committee held a conference call with Weil to further discuss the retention of a financial advisor and Antigua and Barbuda counsel. After considering proposals from multiple potential financial advisors and each financial advisor candidate’s respective experience in similar transactions, qualifications, reputation and independence and after evaluating the presentations made by each potential financial advisor, the Special Committee decided to retain Duff & Phelps to act as financial advisor to the Special Committee to provide financial and market related advice and services. In connection with the engagement of Duff & Phelps, the Special Committee also retained Duff & Phelps Securities, LLC (“DPS”), an affiliate of Duff & Phelps, to act as financial advisor to the Special Committee to, at the request of the Special Committee, provide financial and market related advice and services in connection with initiating, soliciting and encouraging any alternative transaction proposals from third parties. In addition, after considering proposals from two Antigua and Barbuda law firms, the Special Committee decided to retain Leslie-Ann Brissett Legal Services (“LABLS”) as its Antigua and Barbuda counsel.
On March 28, 2016, the Company announced that the Board had adopted a Rights Agreement between the Company and Pacific Stock Transfer Company, as Rights Agent (as amended from time to time, the “Rights Agreement”). In connection with the Rights Agreement, the Board declared a dividend distribution of one preferred share purchase right (each, a “Right”) on each Share outstanding at the close of business on April 8, 2016. Pursuant to the Rights Agreement, subject to limited exceptions, the Rights would become exercisable if a person or group acquires 15% or more of the Shares or announces a tender offer for 15% or more of the Shares. Under certain circumstances, each Right would entitle shareholders to purchase from the Company one one-thousandth of a Series A Junior Participating Preferred Share, par value $0.001 per share (the “Series A Preferred Shares”), of the Company at an exercise price of US$30.00. If a person acquires 15% or more of the Shares (an “Acquiring Person”), each Right would entitle the holder of such Right to purchase, at such Right’s then-current exercise price, a number of Shares having a market value at that time of twice the Right’s exercise price. Rights held by the Acquiring Person would become void and would not be exercisable to purchase Shares. Pursuant to the Rights Agreement, if the Company is acquired in a merger or other business combination transaction which has not been approved by the Board, each Right would entitle the holder thereof to purchase, at such Right’s then-current exercise price, a number of shares of the acquiring company’s common stock having a market value at the time of twice the right’s exercise price. Each Series A Preferred Share would have 1,000 votes and would vote together with the holders of common shares as one class. On April 8, 2017, the Company sent a letter to its shareholders to provide a summary of the Rights Agreement. The dividend of the Rights was distributed on April 14, 2016.
| 21 |
On April 12, 2016, the members of the Special Committee convened a meeting by telephone. During the meeting, the Special Committee discussed the current status of the Proposed Transaction and the Sinobioway Proposed Transaction, and Mr. Anderson reported that the engagement of the legal and financial advisors by the Special Committee would soon be announced by the Company.
On April 14, 2016, the Special Committee held a conference call with Duff & Phelps and Weil. During the call, Duff & Phelps and Weil gave an overview of the function, process and timeline of a typical going-private transaction, as well as a typical market check process in similar transactions, and Weil gave a presentation to the Special Committee members regarding, among other things, their responsibilities and fiduciary duties in connection with the Proposed Transaction and the Sinobioway Proposed Transaction. Following the discussion, the Special Committee (a) directed Duff & Phelps and Weil to prepare a list of questions for each of the Buyer Consortium and the Sinobioway Consortium to clarify certain aspects of their respective proposals, (b) instructed Weil to prepare a confidentiality agreement to be executed by the members of the Buyer Consortium and the Sinobioway Consortium prior to the commencement of due diligence and (iii) decided that the Special Committee would have a meeting with LABLS to discuss fiduciary duties under Antigua and Barbuda law and further discuss the market check process with Weil and Duff & Phelps at subsequent Special Committee meetings.
Later on the same day, the Company issued a press release announcing that the Special Committee had retained Weil as its U.S. legal counsel, Duff & Phelps as its financial advisor and LABLS as its Antigua and Barbuda counsel.
On April 19, 2016, the Special Committee held a conference call with LABLS and Weil. During the call, LABLS led a discussion of the fiduciary duties of the Special Committee under Antigua and Barbuda law, as well as other considerations under Antigua and Barbuda law (including the rights of dissenting shareholders) in connection with the Proposed Transaction and the Sinobioway Proposed Transaction.
On the same day, Duff & Phelps held a conference call with Lazard. During the call, Duff & Phelps discussed the Buyer Consortium’s financing plan with Lazard and the preliminary timetable for the Buyer Consortium’s due diligence. Lazard stated that the Buyer Consortium was negotiating equity financing with several potential investors.
On April 20, 2016, the Special Committee held a conference call with Duff & Phelps and Weil. During the call, Duff & Phelps updated the Special Committee on its discussion with Lazard regarding the Buyer Consortium’s proposed financing plan. The Special Committee, Duff & Phelps and Weil further discussed conducting a market check. After the discussion, the Special Committee (a) decided to wait to commence a market check until after the Special Committee had received more information regarding the proposed financing plans of the Buyer Consortium and the Sinobioway Consortium and (b) instructed Duff & Phelps to further clarify with Lazard the Buyer Consortium’s shareholding percentage in the Company and to contact the Sinobioway Consortium to discuss its proposed financing plan.
Later on the same day, Duff & Phelps held a conference call with representatives of the Sinobioway Consortium regarding the Sinobioway Consortium’s financing plan. The Sinobioway Consortium stated that its financing would consist of equity funds and bank loans and that it was negotiating debt financing with several banks.
| 22 |
On April 22, 2016, the Sinobioway Consortium’s U.S. legal counsel, Sidley Austin LLP (“SA”), contacted Weil by telephone. Following the telephone call, Weil provided SA with a list of clarifying questions regarding the Sinobioway Consortium’s proposal, including with respect to the Sinobioway Consortium’s members, its proposed transaction structure and financing plan, any required governmental approvals in connection with its proposed transaction and whether the Sinobioway Consortium would be conducting due diligence on the Company.
On April 27, 2016, Weil distributed a draft confidentiality agreement to SA and Kirkland & Ellis LLP, the Buyer Consortium’s U.S. legal counsel (“K&E”), respectively.
On the same day, the Special Committee held a conference call with Duff & Phelps and Weil, during which Duff & Phelps and Weil updated the Special Committee on their communications with the advisors to the Buyer Consortium and the Sinobioway Consortium.
On April 30, 2016, the Special Committee received responses from the Sinobioway Consortium to the questions that Weil circulated to SA on April 22, 2016. In its responses, the Sinobioway Consortium identified and described each of its consortium members (including a new member, CITIC M&A Fund Management Co., Ltd. (“CITIC M&A”)), and described its proposed transaction structure (a tender offer followed by a second step amalgamation) and the governmental approvals that would be required to consummate its proposed transaction (which included outbound direct investment approvals (the “ODI Approvals”) from PRC governmental authorities). In addition, with respect to financing its proposed transaction, the Sinobioway Consortium stated that (a) its financing would consist of equity funds and bank loans, (b) it had raised RMB3 billion from its members, (c) it was negotiating debt financing with several banks and (d) its debt financing sources would follow the market practice of delivering customary debt financing commitments. The Sinobioway Consortium also indicated that, in addition to SA, it had engaged O’Melveny & Myers LLP (“OMM”) as U.S. legal counsel.
On May 3, 2016, Weil received comments to the draft confidentiality agreement from K&E and SA, respectively.
On May 4, 2016, Duff & Phelps received from Lazard a list of the Buyer Consortium’s potential debt financing sources and equity investors with whom the Buyer Consortium was considering entering into confidentiality agreements.
On May 11, 2016, Weil circulated revised drafts of the confidentiality agreements to SA and K&E, respectively. From May 12, 2016 to July 11, 2016, Weil negotiated the confidentiality agreements with SA and K&E, respectively.
On May 13, 2016, the Special Committee held a conference call with Duff & Phelps, Weil and LABLS. Duff & Phelps described the process and timeline for a typical market check process. Weil also compared and contrasted a market check with a customary “go-shop” process. After the discussion, the Special Committee instructed (a) Duff & Phelps to prepare a summary of the market practice with respect to market checks in recent going-private transactions (and the Special Committee determined that it would further discuss a market check at the next Special Committee meeting) and (b) Weil to prepare a draft amalgamation agreement for distribution to the Buyer Consortium and the Sinobioway Consortium.
| 23 |
Later on the same day, Duff & Phelps circulated to the Special Committee a summary of the market practice with respect to market checks and go-shops in recent going-private transactions.
On May 17, 2016, each of Advantech Advisors (HK) Ltd. and C-Bridge Capital Investment Management, Ltd. entered into a letter agreement with Mr. Yin, on behalf of the Buyer Consortium, with respect to their respective confidentiality obligations in connection with the Proposed Transaction.
On May 18, 2016, the Special Committee held a conference call with Duff & Phelps and Weil. During the call, the Special Committee, Duff & Phelps and Weil further discussed the possibility of a market check in connection with the competing transaction proposals for the Company. Duff & Phelps also reported to the Special Committee that, on May 16, 2016, it had received an email from a representative of 1Globe Capital LLC, a shareholder of the Company holding approximately 16.4% of the Shares at such time (“1Globe”), indicating that 1Globe intended to participate in the Buyer Consortium, subject to the execution of a final consortium agreement.
On May 20, 2016, the Special Committee unanimously resolved in writing to instruct DPS to conduct a market check to solicit interest from qualified third parties regarding a possible transaction with the Company and to evaluate any proposals that it received.
On May 21, 2016, Vivo Capital, LLC entered into a letter agreement with Mr. Yin, on behalf of the Buyer Consortium, with respect to its confidentiality obligations in connection with the Proposed Transaction.
On May 25, 2016, the Special Committee held a conference call with Duff & Phelps and Weil to discuss the market check. Prior to the call, Duff & Phelps had circulated to the Special Committee a list of potential strategic and financial investors that DPS planned to contact during the market check process, based on a review of, among other things, key competitors of the Company, previous investments by financial investors in similar industries, strategic investors with operations in the Company’s industry (both in the PRC and worldwide) and the size of the potential transaction. During the meeting, the Special Committee discussed the list with Duff & Phelps and asked Duff & Phelps to make certain revisions to the list and re-circulate it to the Special Committee after the meeting (which was circulated to, and approved by, the Special Committee later on the same day). Duff & Phelps also reported to the Special Committee that it had been informed that JP Morgan was advising the Sinobioway Consortium as its financial advisor.
On May 30, 2016, Duff & Phelps circulated to the Special Committee an indicative timetable from Lazard regarding the Buyer Consortium’s due diligence, financing arrangements and the negotiation of transaction documents, and another indicative timetable for the Proposed Transaction prepared by Duff & Phelps. The Buyer Consortium’s timetable indicated that the Buyer Consortium would select its equity investors and debt financing sources for the Proposed Transaction by June 24, 2016.
| 24 |
On May 31, 2016, DPS initiated the market check process and contacted 11 potential financial investors and 6 potential strategic investors.
On June 1, 2016, the Special Committee held a conference call with Duff & Phelps and Weil to discuss, among other things, the indicative timetable from Lazard and the indicative timetable prepared by Duff & Phelps. Duff & Phelps also reported to the Special Committee that the Company was in the process of preparing a virtual data room for the purpose of facilitating due diligence on the Company by the two consortiums or other third parties.
On the same day, the Company entered into a confidentiality agreement (which included customary standstill provisions with a “don’t ask, don’t waive” provision) with each member of the Buyer Consortium (which, at the time, consisted of Mr. Yin and SAIF), respectively. The confidentiality agreements also provided that any new member of the Buyer Consortium would require the approval of the Special Committee.
On June 2, 2016, Duff & Phelps, Weil, Lazard and K&E held a conference call to discuss the timetable for the Proposed Transaction and the Buyer Consortium’s due diligence process. Between early June 2016 and late September 2016, following the establishment of the Company’s virtual data room, the Buyer Consortium and its financial, legal and accounting advisors received access to the virtual data room and conducted preliminary financial, legal, business and other due diligence on the Company.
On June 3, 2016, the Company provided Duff & Phelps with certain financial projections for the Company for the fiscal years ending December 31, 2016 through 2025 prepared by the Company’s management. Later on the same day, Duff & Phelps held a conference call with the Company’s senior management and finance team to discuss the Company’s historical financial performance and key assumptions used in the financial projections, as well as management’s views on the Company’s future strategy, industry outlook and market completion.
Between June 3, 2016 and June 8, 2016, Duff & Phelps held various discussions and due diligence meetings with the senior management and finance team of the Company regarding the business, operations and financial performance of the Company. These discussions and meetings focused on, among other things, understanding the nature of the Company’s business and prospects, its historical performance, management’s view on industry outlook, market competition and the risks and challenges facing the Company.
On June 8, 2016, the Special Committee held a conference call with Duff & Phelps and Weil, during which (a) the Special Committee, Duff & Phelps and Weil discussed the financial projections prepared by the Company’s management and Duff & Phelps summarized management’s views on the Company’s future strategy, industry outlook and market competition, as well as the key assumptions used in the financial projections, and (b) Weil updated the Special Committee on its negotiations with SA and OMM regarding the confidentiality agreement for the Sinobioway Consortium. After the discussion, the Special Committee instructed Duff & Phelps to further clarify certain assumptions used in the financial projections with the Company’s management and arrange meetings with the Buyer Consortium and the Sinobioway Consortium to discuss their respective transaction proposals.
| 25 |
On June 15, 2016, the Special Committee held a conference call with Duff & Phelps and Weil to discuss developments since the previous meeting and updates on the progress of negotiations with the Buyer Consortium and the Sinobioway Consortium. During the call, Weil informed the Special Committee that it was in the process of preparing a draft amalgamation agreement for distribution to the Buyer Consortium and the Sinobioway Consortium. In addition, Duff & Phelps informed the Special Committee that (a) the Buyer Consortium had commenced its due diligence on the Company, (b) DPS had not received any indication of interest from any third party during the market check process and (c) Duff & Phelps would arrange meetings with the Buyer Consortium and the Sinobioway Consortium around the middle of July.
On June 20, 2016, Weil, SA and OMM discussed, by telephone, the key remaining issues in the draft confidentiality agreement with the Sinobioway Consortium. OMM informed Weil that the Sinobioway Consortium would not conduct due diligence on the Company and was prepared to sign a simplified confidentiality agreement only covering negotiations and transaction terms, which would not include a standstill provision, with the understanding that the Sinobioway Consortium would not receive confidential information regarding the Company or its business. OMM advised that the Sinobioway Consortium would sign a customary confidentiality agreement with a standstill provision if it subsequently determined to conduct due diligence on the Company.
Later on the same day, Weil reported the discussion with SA and OMM to the Special Committee, and the Special Committee confirmed that a simplified confidentiality agreement with the Sinobioway Consortium would be sufficient as long as the Company did not provide material non-public information to the Sinobioway Consortium.
On June 22, 2016, the Special Committee held a conference call with Duff & Phelps and Weil. During the call, Duff & Phelps reported to the Special Committee that (a) the Buyer Consortium proposed adding new members to its consortium (subject to the consent of the Special Committee), (b) the Buyer Consortium had informed Duff & Phelps that a complete proposal from the Buyer Consortium with respect to its consortium members, financing and final proposed purchase price was unlikely to be delivered until the end of July or early August, (c) the Sinobioway Consortium had yet to make progress in the financing arrangements for its proposal and (d) DPS had not received any indication of interest from any third party during its market check process, except that one third party had told DPS that it would consider participating as an investor in the Buyer Consortium rather than pursuing a separate bid. In addition, the Special Committee, in consultation with Duff & Phelps and DPS, determined to extend the deadline for the market check to June 24, 2016. Following the discussion, the Special Committee decided to schedule meetings with the Buyer Consortium and the Sinobioway Consortium on July 19, 2016 in Beijing.
On June 23, 2016, OMM circulated to Weil a draft of a simplified confidentiality agreement without a standstill provision. From June 23, 2016 to July 9, 2016, Weil held multiple discussions with OMM regarding the simplified confidentiality agreement with the Sinobioway Consortium.
| 26 |
On June 29, 2016, the Special Committee held a conference call with Duff & Phelps and Weil. During the call, Weil updated the Special Committee on the negotiation of the confidentiality agreement with the Sinobioway Consortium and the drafting of the amalgamation agreement. In addition, Duff & Phelps informed the Special Committee that, among other things, (a) the market check process had concluded on June 24, 2016 and that, during the market check process, DPS had contacted 17 potential buyers, comprised of 6 potential strategic buyers and 11 potential financial buyers, but had not received any indication of interest, (b) it had not received any material updates from the Sinobioway Consortium regarding its proposed financing arrangements during the previous week and (c) Lazard had informed Duff & Phelps that (i) the Buyer Consortium had made progress in its financing arrangements and would likely fund the Proposed Transaction entirely with committed equity (and only use debt financing in the unlikely event it was unable to obtain fully committed equity from its consortium members) and (ii) the Buyer Consortium was in discussions with 1Globe regarding the possibility of 1Globe joining the Buyer Consortium and rolling over its Shares in connection with the Proposed Transaction.
On July 5, 2016, Weil provided an initial draft of the amalgamation agreement, together with drafts of a form equity commitment letter and form limited guarantee, to the Special Committee, along with a key issues list regarding the draft documents.
On July 7, 2016, Latham provided to Weil its comments on the initial draft of the amalgamation agreement. From time to time thereafter, Weil discussed the amalgamation agreement with Latham.
On July 11, 2016, Weil circulated to K&E the initial draft of the amalgamation agreement, together with drafts of a form equity commitment letter and form limited guarantee (collectively, the “Transaction Documents”), which reflected, among other things: (a) a one-step amalgamation transaction structure; (b) a closing condition with respect to the approval of a majority of the Shares held by unaffiliated shareholders (the “Majority of the Minority Vote Condition”); (c) the ability of the Special Committee to change its recommendation and/or terminate the amalgamation agreement in certain circumstances, including the right to terminate the amalgamation agreement for fiduciary duty purposes in connection with a superior proposal and for other reasons; (d) the absence of a closing condition with respect to a maximum number of dissenting Shares; and (e) a “no-shop” provision in light of the fact that, since (i) DPS had already conducted a market check, but had not received any indication of interest, and (ii) the Sinobioway Proposed Transaction had been proposed, the Special Committee determined that a post-signing “go-shop” was unlikely to result in any meaningful benefit to the Company or its shareholders. Weil asked K&E to provide comments, on behalf of the Buyer Consortium, to the Transaction Documents by August 1, 2016.
Later on the same day, the Company entered into a simplified confidentiality agreement (without a standstill provision) with all of the members of the Sinobioway Consortium (which, at the time, included PKU Shanghai, Shandong Sinobioway, CICC Qianhai, Beijing Sinobioway, Heng Feng, Fuerde and CITIC M&A).
On the same day, 1Globe entered into a letter agreement with Mr. Yin, on behalf of the Buyer Consortium, with respect to its confidentiality obligations in connection with the Proposed Transaction.
On July 14, 2016, the Special Committee held a conference call with Duff & Phelps and Weil to discuss, among other things, the timing of providing drafts of the transaction documents to the Sinobioway Consortium.
| 27 |
On July 19, 2016, the Special Committee conducted separate in person meetings in Beijing with the Buyer Consortium and the Sinobioway Consortium to discuss each consortium’s proposed transaction, including its proposed financing arrangements. Representatives of Duff & Phelps, Weil and the applicable consortium’s legal and financial advisors were also present at each meeting. During the meetings, (a) the Buyer Consortium reiterated that it would be able to fund the Proposed Transaction with committed equity financing and (b) the Sinobioway Consortium indicated that it would obtain equity commitments for onshore funding in RMB and would need to explore a financing arrangement to exchange such RMB for offshore U.S. dollars to fund the Sinobioway Proposed Transaction.
On July 20, 2016, Lazard circulated to Duff & Phelps an illustrative timetable with respect to the Buyer Consortium’s financing of the Proposed Transaction, which indicated that the Buyer Consortium targeted finalizing the structure of the Proposed Transaction and the allocation of Buyer Consortium’s equity investors’ commitments by the end of August 2016.
On the same day, Weil circulated the initial draft of the amalgamation agreement, together with drafts of a form equity commitment letter and form limited guarantee (collectively, the “Sinobioway Consortium Transaction Documents”) to OMM and SA. Weil asked OMM and SA to provide comments, on behalf of the Sinobioway Consortium, to the Sinobioway Consortium Transaction Documents by August 10, 2016.
On July 21, 2016, the Special Committee engaged Haiwen & Partners (“Haiwen”) to serve as its PRC legal counsel.
On July 24, 2016, SA sent Weil a draft steps plan outlining the Sinobioway Consortium’s transaction structure and the steps that it intended to take in order to consummate the Sinobioway Proposed Transaction (which included, among other things, the PRC regulatory approvals that the Sinobioway Consortium would need to obtain to consummate the Sinobioway Proposed Transaction and the timing thereof).
On August 1, 2016, K&E circulated revised drafts of the Transaction Documents and an initial draft of a support agreement (with respect to Mr. Yin and SAIF voting in favor of, and rolling over their respective Shares in connection with, the Proposed Transaction) to Weil, proposing, among other things: (a) an expansion of the scope of the Company’s representations, warranties and covenants; (b) the deletion of the Majority of the Minority Vote Condition; (c) the deletion of the Company’s right to terminate the amalgamation agreement for fiduciary duty purposes, other than in connection with a superior proposal (the “Fiduciary Out Termination Right”); (d) the addition of a closing condition that would allow the Buyer Consortium not to close if shareholders holding more than 5% of the total outstanding Shares exercised dissenters' rights (the “Dissenting Shareholders Condition”); and (e) a Company termination fee equal to 1% of the Company’s equity value and a termination fee payable by the Buyer Consortium in the event the amalgamation agreement is terminated under certain circumstances equal to 2% of the Company’s equity value. In its cover email to Weil, K&E also confirmed that: (i) subject to the Special Committee’s approval, C-Bridge and Advantech intended to join the Buyer Consortium; (ii) the transaction would be structured as a one-step amalgamation; (iii) the Buyer Consortium had secured sufficient equity financing to consummate the Proposed Transaction (and its members would provide equity commitment letters to the Company for the full amount of the amalgamation consideration to be paid by the Buyer Consortium in connection with the consummation of the Proposed Transaction) and did not expect to require any debt financing; (iv) the Buyer Consortium’s equity financing would be comprised solely of offshore U.S. dollar funds; and (v) the Buyer Consortium did not believe any PRC regulatory approvals would be required to consummate the Proposed Transaction.
| 28 |
The Special Committee and its advisors had previously discussed the potential implications on the Proposed Transaction and the Sinobioway Proposed Transaction of the fact that: (a) the Company’s principal PRC operating subsidiary, Beijing Sinovac Co., Ltd. (“Beijing Sinovac”), was 26.91% owned by Shandong Sinobioway, a member of the Sinobioway Consortium; (b) Mr. Aihua Pan (“Mr. Pan”), a controlling shareholder of Shandong Sinobioway (and who was leading the Sinobioway Consortium), was also the chairman of the board and legal representative of Beijing Sinovac; (c) Beijing Sinovac’s articles of association gave Shandong Sinobioway veto rights over certain matters with respect to Beijing Sinovac, including the nomination or removal of the general manager of Beijing Sinovac; and (d) Beijing Sinovac operated on land leased from an affiliate of Shandong Sinobioway ((a) through (d), the “Beijing Sinovac Matters”).
On August 3, 2016, the Special Committee held a conference call with Duff & Phelps, Weil and Haiwen to discuss, among other things, the status of the Buyer Consortium’s financing and the Beijing Sinovac Matters. During the call, Weil also reported to the Special Committee that (a) it had received a draft steps plan from SA outlining the Sinobioway Consortium’s proposed transaction structure and (b) it was preparing a list of outstanding issues in connection with the Buyer Consortium’s comments on the draft Transaction Documents.
Later on the same day, Weil sent OMM and SA a list of follow-up questions regarding the draft steps plan and regulatory approval timeline of the Sinobioway Consortium to clarify the timing and certainty of closing the Sinobioway Proposed Transaction, including questions relating to the ability of the Sinobioway Consortium to obtain financing commitments in offshore U.S. dollars to fund the Sinobioway Proposed Transaction.
Also on the same day, Lazard sent to Weil preliminary introduction materials for each of Advantech and C-Bridge describing their respective creditworthiness and funding capability.
On August 10, 2016, SA circulated revised drafts of the Sinobioway Consortium Transaction Documents to Weil, proposing, among other things, (a) a two-step transaction structure, consisting of a first-step tender offer followed by a second-step amalgamation, (b) that the tender offer be conditioned on at least a majority of the outstanding Shares having been tendered before the expiration date of the tender offer (the “Tender Offer Condition”), (c) that the Company grant the Sinobioway Consortium a “top-up” option to purchase newly-issued Shares such that (subject to satisfaction of the Tender Offer Condition) after exercise of the option the Sinobioway Consortium would own more than two-thirds of the outstanding Shares (the “Top-Up Option”), (d) an expansion of the scope of the Company’s representations, warranties and covenants, (e) the deletion of the Majority of the Minority Vote Condition, (f) the addition of a “hell or high water” antitrust covenant that would require the Company to agree to any sale or divesture of its assets or business necessary to obtain antitrust clearance (the “Antitrust Covenant”), (g) the addition of a closing condition that would allow the Sinobioway Consortium not to close if the cash and cash equivalents of the Company at closing were less than a minimum target amount to be agreed (the “Minimum Cash Condition”) and (h) that each party reimburse the other party’s expenses in connection with the transaction, up to a maximum amount of US$5 million, if the amalgamation agreement were terminated in certain circumstances (the “Expense Reimbursement Provisions”). The Sinobioway Consortium reserved comments on the financing provisions of the amalgamation agreement pending its determination of whether it would obtain debt financing to finance its proposed transaction. SA also provided an executed consortium agreement, dated July 11, 2016, among all the members of the Sinobioway Consortium.
| 29 |
On August 12, 2016, SA sent Weil its responses to Weil’s follow-up questions regarding the draft steps plan of the Sinobioway Consortium. In response to Weil’s questions relating to the Sinobioway Consortium’s proposed financing arrangements, the Sinobioway Consortium stated that it planned to finance the Sinobioway Proposed Transaction, including the tender offer, the exercise of the top-up option (if any) and the second-step amalgamation, with cash held by the members of the Sinobioway Consortium, which it stated was sufficient to acquire all of the Company’s outstanding Shares.
On August 16, 2016, the Special Committee received a letter (the “Heng Ren Letter”) from Heng Ren Investments LP stating that it believed that the price of US$6.18 per Share proposed by the Buyer Consortium significantly undervalued the Company and requesting that the Special Committee, among other things, seek an increase in the price proposed by the Buyer Consortium.
On August 16, 2016 and August 17, 2016, respectively, Weil and Haiwen had separate conference calls with two representatives of Beijing Sinovac to discuss the Beijing Sinovac Matters, including the potential influence that Mr. Pan might have over Beijing Sinovac.
On August 17, 2016, at the direction of the Special Committee, Weil and Haiwen held a conference call with K&E and King & Wood Mallesons (“KWM”), the Buyer Consortium’s PRC counsel, to clarify certain aspects of the Buyer Consortium’s proposal, including whether it would require a PRC antitrust filing. K&E indicated that (a) the Buyer Consortium was expecting to complete its due diligence on the Company shortly, (b) the Buyer Consortium would finance the Proposed Transaction with offshore U.S. dollar equity contributions from the Buyer Consortium’s members (assuming that US$5 million of the Company’s cash would be used to pay certain transaction expenses of the Buyer Consortium) and that it would not require any debt financing to consummate the Proposed Transaction and (c) two of the Company’s existing shareholders, 1Globe and the persons comprising the reporting person identified as “Chiang Li Family” in the Schedule 13G relating to the Company filed by such reporting person with the SEC on April 11, 2016 (the “Chiang Li Family”), which such shareholders collectively held approximately 22.5% of the Shares at such time, would be willing to join the Buyer Consortium and participate in the Proposed Transaction as rollover shareholders.
On August 18, 2016, at the direction of the Special Committee, Weil and Haiwen held a conference call with SA, OMM and Tianyuan Law Firm (“Tianyuan”), the Sinobioway Consortium’s PRC counsel, to discuss the Sinobioway Consortium’s comments to the Sinobioway Consortium’s Transaction Documents and certain aspects of the Sinobioway Consortium’s proposal, including the Sinobioway Consortium’s steps plan and proposed financing structure and the PRC regulatory approvals that would be required to consummate the Sinobioway Proposed Transaction. SA, OMM and Tianyuan stated that the Sinobioway Consortium planned to finance the Sinobioway Proposed Transaction with cash held by its members, which was in RMB and held within the PRC.
| 30 |
On the same day, Duff & Phelps held a conference call with Lazard. Lazard indicated to Duff & Phelps that the Buyer Consortium expected to complete its due diligence process the following week, except for certain outstanding confirmatory due diligence by Advantech.
On August 22, 2016, Haiwen held a conference call with KWM to further discuss KWM’s analysis of whether the Buyer Consortium’s Proposed Transaction would require PRC antitrust approval. KWM explained that it believed PRC antitrust approval would not be required because the structure of the Buyer Consortium (including the proposed post-closing arrangements among the members of the Buyer Consortium) would ensure that no member of the Buyer Consortium would have affirmative or negative control of the Company following the transaction and the transaction would not result in a change of control of the Company.
On August 25, 2016, SA provided Weil with a backup commitment letter issued by CICC Qianhai, dated July 11, 2016 (the “CICC Qianhai Letter”), pursuant to which CICC Qianhai agreed to provide offshore backup financing for the Sinobioway Proposed Transaction. The backup commitment letter was one page long, was not in a customary form and did not specify the amount, currency and conditions of the funding of the equity commitment. In addition, SA, on behalf of the Sinobioway Consortium, requested the opportunity to conduct simple legal and financial due diligence on the Company and stated that the Sinobioway Consortium had been in contact with certain unaffiliated shareholders of the Company, whose identities were not disclosed by SA, and that, although there was no formal commitment, these shareholders were supportive of the Sinobioway Proposed Transaction.
On August 25, 2016, Tianyuan provided its PRC antitrust analysis with respect to the Sinobioway Proposed Transaction to Haiwen. In its analysis, Tianyuan stated that the Sinobioway Proposed Transaction would require PRC antitrust approval, but simplified filing procedures under applicable PRC law would apply.
On August 26, 2016, the Special Committee held a conference call with Duff & Phelps, Weil, LABLS and Haiwen. During the call, (a) Duff & Phelps reported to the Special Committee that it had a telephone call with Lazard and was still in the process of reviewing the Heng Ren Letter, (b) Weil reviewed with the Special Committee a summary prepared by Weil comparing the key elements of the two consortiums’ proposals, (c) LABLS reviewed directors’ fiduciary duties under Antigua and Barbuda law with the Special Committee and commented on certain other matters from an Antigua and Barbuda law perspective, (d) Haiwen reported to the Special Committee that KWM had reconfirmed that an acquisition of the Company by the Buyer Consortium would not trigger a PRC antitrust filing in light of the structure of the Buyer Consortium and (e) Haiwen further summarized for the Special Committee the PRC approvals, filings and registrations (including a PRC antitrust filing) that would be required in connection with an acquisition of the Company by the Sinobioway Consortium.
| 31 |
On August 30, 2016, at the direction of the Special Committee, Weil held a conference call with K&E to discuss next steps with respect to the Proposed Transaction and provide initial feedback from the Special Committee on the Buyer Consortium’s transaction proposal (including with respect to the positions of the Buyer Consortium reflected in its comments to the Transaction Documents and its proposed transaction and financing structures). In its feedback to the Buyer Consortium, Weil emphasized the disparity in price between the Buyer Consortium and the Sinobioway Consortium and advised the Buyer Consortium to consider a material increase in price. Weil also emphasized the importance of certainty of closing to the Special Committee and advised the Buyer Consortium to consider obtaining written evidence of support from other Company shareholders (such as written evidence of their willingness to sign a support agreement in favor of the Buyer Consortium’s proposal), including 1Globe and the Chiang Li Family. K&E indicated that the Buyer Consortium was expecting to complete its due diligence process within a few days.
Later on the same day, at the direction of the Special Committee, Weil held a conference call with SA and OMM to discuss next steps with respect to the Sinobioway Proposed Transaction and provide initial feedback from the Special Committee on the Sinobioway Consortium’s transaction proposal (including with respect to the positions of the Sinobioway Consortium reflected in its comments to the Sinobioway Consortium Transaction Documents and its proposed transaction and financing structures). In its feedback to the Sinobioway Consortium, Weil emphasized a number of points, including that certainty of closing was very important to the Special Committee and that it was unclear how the Sinobioway Consortium would timely fund, and whether the Sinobioway Consortium would be able to obtain committed financing to fund, the necessary amalgamation consideration in offshore U.S. dollars to consummate the Sinobioway Proposed Transaction, particularly in the context of tightened foreign exchange and investment control by the PRC regulatory authorities. Weil advised the Sinobioway Consortium to consider a simplified financing structure, comprised solely of offshore U.S. dollars instead of onshore RMB financing, and stated that the Special Committee expected customary documentation to be provided with respect to the Sinobioway Consortium’s financing commitments. In addition, Weil advised the Sinobioway Consortium to consider obtaining written evidence of support from other Company shareholders for the Sinobioway Proposed Transaction (such as evidence of their willingness to tender shares to the Sinobioway Consortium) and Weil informed the Sinobioway Consortium that the Special Committee was not willing to accept either the proposed Top-Up Option or the Minimum Cash Condition.
On August 31, 2016, the Special Committee held a conference call with Weil and Duff & Phelps to discuss the latest communications with the two consortiums. After the discussion, the Special Committee instructed (a) Weil to prepare revised drafts of the Transaction Documents and the Sinobioway Consortium Transaction Documents to be circulated to the respective consortiums and (b) Duff & Phelps to reach out to the two consortiums separately to discuss price.
On September 6, 2016, Duff & Phelps held a conference call with Lazard. During the conference call, Lazard stated that (a) the Buyer Consortium expected to complete its due diligence process shortly and that the amount of rollover Shares from the members of the Buyer Consortium that were existing shareholders of the Company had not been finalized yet (including whether 1Globe and the Chiang Li Family would join the Buyer Consortium) and (b) the Buyer Consortium’s offer price of US$6.18 per share would provide superior value to the Company’s shareholders.
| 32 |
Later on the same day, Duff & Phelps contacted the Sinobioway Consortium by telephone and the Sinobioway Consortium indicated that it would not consider an increase in its offer price at such time.
On September 9, 2016, the Special Committee held a conference call with Weil and Duff & Phelps. During the call, Duff & Phelps reported to the Special Committee on its conference call with Lazard.
On September 14, 2016, Weil, on behalf of the Special Committee, provided revised drafts of the Transaction Documents to K&E, which reflected, among other things: (a) the reinstatement of the Company’s Fiduciary Out Termination Right; (b) the reinstatement of the Majority of the Minority Vote Condition; (c) the deletion of the Dissenting Shareholders Condition; and (d) the addition of a provision pursuant to which the Company would not be deemed to be in breach of, and the Buyer Consortium would not have the right to terminate, the amalgamation agreement in certain circumstances relating to the Beijing Sinovac Matters. Weil also asked K&E to provide, by September 29, 2016, a final bid package that would include: (i) the Buyer Consortium’s final comments to each of the Transaction Documents; (ii) a final list of the proposed members of the Buyer Consortium; (iii) a detailed description of the Buyer Consortium’s financing sources; and (iv) a confirmation that the Buyer Consortium had completed all commercial, legal and other due diligence.
Later on the same day, Weil, on behalf of the Special Committee, provided revised drafts of the Sinobioway Consortium Transaction Documents to SA and OMM, which reflected, among other things: (a) the deletion of the Top-Up Option; (b) the reinstatement of the Majority of the Minority Vote Condition; (c) the deletion of the Antitrust Covenant; (d) the addition of a provision pursuant to which the Company would not be deemed to be in breach of, and the Sinobioway Consortium would not have the right to terminate, the amalgamation agreement in certain circumstances relating to the Beijing Sinovac Matters; (e) the deletion of the Minimum Cash Condition; and (f) the deletion of the Expense Reimbursement Provisions. Weil also asked SA and OMM to provide, by September 29, 2016, a final bid package that would include: (i) the Sinobioway Consortium’s final comments to each of the Sinobioway Consortium Transaction Documents; (ii) a final list of the proposed members of the Sinobioway Consortium; (iii) a detailed description of the Sinobioway Consortium’s transaction structure; (iv) a detailed description of the Sinobioway Consortium’s financing sources; and (v) a list of regulatory approvals, filings and registrations required to be obtained or made in connection with the Sinobioway Proposed Transaction (and a timeline for obtaining or making such approvals, filings and registrations).
On September 23, 2016, OMM, on behalf of the Sinobioway Consortium, sent Weil a due diligence request list and asked to conduct due diligence on the Company.
On September 29, 2016, Weil circulated an initial draft of the Company disclosure schedule to K&E and SA and OMM, respectively.
| 33 |
Later on the same day, SA, on behalf of the Sinobioway Consortium, circulated revised drafts of the Sinobioway Consortium Transaction Documents to Weil, proposing, among other things, (a) the deletion of the Majority of the Minority Vote Condition and (b) the deletion of the provision pursuant to which the Company would not be deemed to be in breach of, and the Sinobioway Consortium would not have the right to terminate, the amalgamation agreement in certain circumstances relating to the Beijing Sinovac Matters. In addition, SA provided an updated steps plan and a list of required regulatory approvals, filings and registrations in the PRC in connection with the Sinobioway Proposed Transaction, and further confirmed that (i) there had been no change to the members of the Sinobioway Consortium since the execution of the consortium agreement dated as of July 11, 2016, (ii) the Sinobioway Consortium was unwilling to change its proposed transaction structure (i.e., a first-step tender offer followed by a second-step amalgamation), except that the Sinobioway Consortium would no longer require the Top-Up Option, (iii) the Sinobioway Consortium’s final proposal was not subject to any due diligence of the Company and (iv) the Sinobioway Consortium would not condition closing of the Sinobioway Proposed Transaction on the receipt of any PRC regulatory approvals. With respect to financing the Sinobioway Proposed Transaction, the Sinobioway Consortium provided a one-page unaddressed letter (the “Minsheng Bank Letter”) from Minsheng Bank Suzhou Branch (“Minsheng Bank”), dated September 28, 2016, and stated that in addition to the CICC Qianhai Letter, Minsheng Bank had agreed to provide credit facilities for the transaction. The Minsheng Bank Letter was not in the form of a customary debt commitment letter. In the Minsheng Bank Letter, Minsheng Bank indicated that, subject to the satisfaction of certain conditions, it would fund an unspecified amount of RMB to the offshore bank of the Sinobioway Consortium’s offshore acquisition vehicle and provide assistance in exchanging the RMB into U.S. dollars within two business days thereafter (but did not provide any details on the process by which such RMB would be exchanged into U.S. dollars).
Later on the same day, K&E, on behalf of the Buyer Consortium, submitted its final bid package to Weil, which included (a) its comments to each of the Transaction Documents, (b) a list of the proposed members of the Buyer Consortium (including, subject to the Special Committee’s approval, the addition of Vivo Capital as a new equity investor in the Buyer Consortium and 1Globe and the Chiang Li Family as two new rollover shareholders), (c) a detailed description of the Buyer Consortium’s financing sources and (d) confirmation that the Buyer Consortium had completed all commercial, financial, legal and other due diligence. The Buyer Consortium’s revised Transaction Documents reflected, among other things, (i) the deletion of the Company’s Fiduciary Out Termination Right, (ii) the deletion of the Majority of the Minority Vote Condition, (iii) the reinstatement of the Dissenting Shareholders Condition, but with a 10% threshold, and (iv) the deletion of the provision pursuant to which the Company would not be deemed to be in breach of, and the Buyer Consortium would not have the right to terminate, the amalgamation agreement in certain circumstances relating to the Beijing Sinovac Matters.
On the same day, Duff & Phelps received an email from a representative of 1Globe indicating that the Chiang Li Family intended to join the Buyer Consortium and participate in the Proposed Transaction as rollover shareholders.
| 34 |
On September 30, 2016, SA informed Weil by email that (a) the Sinobioway Consortium had received a confirmation letter for its outbound investment from the National Development and Reform Commission of the PRC (“NDRC”) and (b) the Sinobioway Consortium intended to finance the transaction with a combination of equity and debt in U.S. dollars as follows (no additional financing documentation was provided at the time): (i) Heng Feng and Fuerde would directly make capital contributions in U.S. dollars (in an aggregate amount equal to approximately US$30 million) to the Sinobioway Consortium’s offshore acquisition vehicle, an entity incorporated in the British Virgin Islands; (ii) the PRC consortium members of the Sinobioway Consortium would open a free trade account (the “FTN Account”) in the Shanghai Free Trade Zone through a PRC special purpose vehicle (according to SA, the PRC consortium members had raised approximately RMB 3 billion); (iii) such PRC special purpose vehicle would deposit an amount of RMB equal to the remaining amount of U.S. dollars needed to pay the amalgamation consideration into the FTN Account; and (iv) Minsheng Bank would, subject to the satisfaction of certain closing conditions which were not specified at the time, loan an equivalent amount of U.S. dollars equal to the RMB deposited in the FTN Account to the Sinobioway Consortium’s offshore acquisition vehicle, which such loan of U.S. dollars by the bank would not, according to SA, be subject to any filing or registration with the State Administration of Foreign Exchange of the PRC (“SAFE”) or any other PRC regulatory approvals.
On October 6, 2016, the Special Committee held a conference call with Weil and Duff & Phelps. During the call, Weil reviewed with the Special Committee the revised Transaction Documents and Sinobioway Consortium Transaction Documents received from the respective consortiums and an updated summary chart comparing the key terms of their respective final proposals, including the Sinobioway Consortium’s new financing arrangement with Minsheng Bank. Given that (a) the proposed rollover shareholder members of the Buyer Consortium (including 1Globe and the Chiang Li Family) set forth in the list provided by K&E to Weil collectively owned more than 50% of the outstanding Shares at the time, which would make the Sinobioway Consortium’s Tender Offer Condition impossible to satisfy, (b) all of the Buyer Consortium’s financing would comprise equity financing in offshore U.S. dollars, whereas most of the Sinobioway Consortium’s financing would comprise onshore RMB (which would need to be converted via a loan from Minsheng Bank into offshore U.S. dollars), (c) the debt financing from Minsheng Bank, or any other bank in the Shanghai Free Trade Zone, would be subject to certain unspecified conditions and no bank had provided a customary debt commitment letter and (d) the Proposed Transaction with the Buyer Consortium would not be subject to any PRC approvals whereas the Sinobioway Proposed Transaction would require certain PRC ODI Approvals and MOFCOM antitrust clearance, the Special Committee decided to further negotiate with the Buyer Consortium with respect to the Proposed Transaction, subject to the following conditions: (i) the Buyer Consortium confirming, and providing supporting documentation, that 1Globe and the Chiang Li Family had agreed to participate in the Proposed Transaction as rollover shareholders; (ii) the Buyer Consortium agreeing to bear all risks relating to the Beijing Sinovac Matters; and (iii) the Buyer Consortium increasing its offer price (which at the time was US$6.18 per Share). After the discussion, the Special Committee instructed Weil to provide initial feedback to K&E and Duff & Phelps to contact Lazard to discuss the price increase.
On October 7, 2016 and October 11, 2016, Weil held conference calls with K&E, during which K&E indicated that (a) Mr. Yin and SAIF generally accepted the proposition that the Buyer Consortium would bear all risks related to the Beijing Sinovac Matters, but K&E would discuss with the other members of the Buyer Consortium, and (b) a conference call between Weil and representatives of 1Globe and the Chiang Li Family would be arranged to confirm their participation in the Proposed Transaction as rollover shareholders.
On October 12, 2016, Duff & Phelps held a conference call with Lazard to discuss an increase in the Buyer Consortium’s offer price. Lazard indicated that it would have an internal discussion with the Buyer Consortium but that it would be difficult for the Buyer Consortium to increase its offer price.
| 35 |
On October 19, 2016, Mr. Anderson spoke by telephone with a representative of SAIF to discuss an increase in the Buyer Consortium’s offer price. SAIF indicated that (a) the Sinobioway Consortium had approached the Buyer Consortium to discuss the possibility of joining their respective consortiums and (b) it would be difficult for the Buyer Consortium to increase its offer price at this stage.
On October 20, 2016, Weil and Mr. Anderson held a conference call with K&E and representatives of 1Globe and the Chiang Li Family to discuss their participation in the Proposed Transaction with the Buyer Consortium. On the call, K&E conveyed the verbal confirmation of representatives of 1Globe and the Chiang Li Family regarding their participation in the Proposed Transaction by rolling over their respective Shares. Following the call, Weil circulated a list of questions and requests, through K&E, to 1Globe and the Chiang Li Family seeking, among other things, written confirmation of their equity ownership in the Company and their participation in the Proposed Transaction with the Buyer Consortium. No written responses were received from 1Globe or the Chiang Li Family following such request.
On October 25, 2016, the Special Committee held a conference call with Weil and Duff & Phelps to discuss developments with the Buyer Consortium since the previous Special Committee meeting. After the discussion, the Special Committee decided to (a) request that the Buyer Consortium provide responses regarding (i) the request for written confirmation from 1Globe and the Chiang Li Family, (ii) an increase in the Buyer Consortium’s offer price and (iii) the nature and status of discussions between the Sinobioway Consortium and the Buyer Consortium and (b) circulate revised drafts of the Transaction Documents to K&E upon the receipt of such responses.
Later on the same day, Weil, at the direction of the Special Committee, communicated the Special Committee’s requests to K&E.
On November 1, 2016, the Buyer Consortium and its representatives held a conference call to discuss, among other things, (a) the status of communications with 1Globe and the Chiang Li Family regarding their written confirmation of joining the Buyer Consortium and (b) potential cooperation with the Sinobioway Consortium on a joint transaction.
On November 12, 2016, the Sinobioway Consortium submitted to the Special Committee (a) a letter updating the Special Committee on the Sinobioway Consortium’s progress with respect to financing the Sinobioway Proposed Transaction and informing the Special Committee that its financing structure had changed, (b) a confirmation letter issued by NDRC on September 29, 2016, stating that the Sinobioway Consortium would be permitted to enter into a binding acquisition agreement with respect to the Sinobioway Proposed Transaction, (c) a one-page certification letter (the “SPD Financing Letter”) issued by the Waigaoqiao Free Trade Zone Sub-Branch of Shanghai Pudong Development Bank (“SPD”) to PKU & CC Holdings Limited, the Sinobioway Consortium’s offshore acquisition vehicle (“PKU”), pursuant to which SPD stated that it approved a loan to PKU in offshore U.S. dollars in an amount equal to the RMB 3 billion that would be funded by the members of the Sinobioway Consortium, subject to certain unspecified conditions, and (d) certificates of incorporation for various special purpose vehicles that the Sinobioway Consortium had incorporated in connection with the Sinobioway Proposed Transaction. No explanation was given for the replacement of Minsheng Bank.
| 36 |
On November 17, 2016, the Special Committee held a conference call with Weil, Duff & Phelps and Haiwen. During the call, Weil updated the Special Committee on its discussions with K&E and Haiwen provided an analysis of certain PRC law aspects of the Sinobioway Consortium’s latest proposal submitted on September 30, 2017 and its latest proposed financing structure, including required PRC regulatory approvals. After the discussion, the Special Committee instructed Haiwen and Weil to discuss these matters with the Sinobioway Consortium’s legal advisors with a view to further clarifying the Sinobioway Consortium’s contemplated financing structure, including its proposed financing arrangement with SPD.
Later on the same day, Weil contacted OMM to arrange a conference call to discuss the Sinobioway Consortium’s latest transaction proposal and financing structure, including the Sinobioway Consortium’s financing sources, the Beijing Sinovac Matters and the status of the Sinobioway Consortium’s discussions with key shareholders of the Company.
From November 17, 2016 to November 24, 2016, both Duff & Phelps and Weil held multiple conference calls with Lazard. During these calls, Lazard indicated that (a) the Buyer Consortium would be willing to consider the possibility of accepting the Sinobioway Consortium joining the Buyer Consortium if the Sinobioway Consortium was able to obtain committed financing for the transaction and (b) the Buyer Consortium would consider a small increase in its offer price, but it would be difficult for the Buyer Consortium to increase its price to US$7.00 per Share.
On November 21, 2016, Weil followed up with OMM on its request for a call to discuss the Sinobioway Consortium’s latest proposals. OMM responded that it would discuss the request with the Sinobioway Consortium.
On November 25, 2016, the Special Committee held a conference call with Weil and Duff & Phelps. During the call, Weil and Duff & Phelps updated the Special Committee on their discussions with Lazard. After the discussion, and taking into account, among other factors, the disparity between the Buyer Consortium’s offer price and the Sinobioway Consortium’s offer price and the uncertainties associated with the Sinobioway Consortium’s proposed transaction, including financing and regulatory approval uncertainty, the Special Committee instructed (a) Weil and Duff & Phelps to request, on behalf of the Special Committee, that the Buyer Consortium increase its offer price to US$6.59 per Share and (b) Weil to continue to clarify certain aspects of the SPD Financing Letter with, and to request supporting documents with respect to its proposed financing structure from, the Sinobioway Consortium’s legal advisors to assess the feasibility and certainty of consummating the Sinobioway Consortium’s proposed financing.
Later on the same day Weil, at the direction of the Special Committee, requested, through K&E, that the Buyer Consortium increase its offer price to US$6.59 in cash per Share.
On December 2, 2016, K&E, on behalf of the Buyer Consortium, updated Weil on the status of the internal approval process of the members of the Buyer Consortium with respect to, and requested an extension to December 6, 2016 to respond to, the price increase requested by the Special Committee. The Special Committee granted the extension.
| 37 |
On December 5, 2016, OMM (a) informed the Special Committee and Weil that the Sinobioway Consortium was still working on a number of the matters referred to in its latest proposal submitted to the Special Committee on September 30, 2017 and its latest financing proposal and would respond to the Special Committee’s request for a conference call at a later date and (b) on behalf of the Sinobioway Consortium, requested the opportunity to conduct due diligence on the Company.
On December 6, 2016, K&E informed Weil that the Buyer Consortium had agreed to increase its price to US$6.33 per Share.
On December 7, 2016, the Special Committee held a conference call with Weil and Duff & Phelps. During the call, Duff & Phelps updated the Special Committee on its discussions with Lazard and Weil updated the Special Committee with respect to the Buyer Consortium’s revised offer price. Weil also summarized for the Special Committee the key differences between the proposals of the two consortiums, including with respect to financing and closing certainty. After the discussion, the Special Committee decided to continue its review and analysis of the two proposals at a subsequent meeting later in the same week.
Later on the same day, Weil informed OMM, at the direction of the Special Committee, that the Special Committee did not believe a due diligence investigation of the Company by the Sinobioway Consortium would be appropriate at such an advanced stage in the process.
On December 8, 2016, Duff & Phelps provided the Special Committee with a summary of market practice with respect to premiums paid and offer price improvement in recent going-private transactions.
On December 9, 2016, the Special Committee held a conference call with Weil to continue the discussion of the proposals of the two consortiums. During the call, the Special Committee discussed (a) the increased price proposed by the Buyer Consortium, (b) the key differences between the two consortiums’ proposals and (c) the Special Committee’s duty to act in the best interests of the Company’s unaffiliated shareholders. After the discussion, the Special Committee decided to request that the Buyer Consortium (i) obtain written responses from 1Globe and the Chiang Li Family to the questions and requests submitted on October 20, 2016 and (ii) confirm that the Buyer Consortium would bear the risks associated with the Beijing Sinovac Matters. The Special Committee determined to continue its review and analysis of the two proposals after it received responses from the Buyer Consortium.
Later on the same day, Weil, at the direction of the Special Committee, delivered the Special Committee’s requests to K&E, together with a revised draft of the amalgamation agreement provisions dealing with the Beijing Sinovac Matters.
On December 13, 2016, K&E circulated to Weil a revised draft of the amalgamation agreement provisions dealing with the Beijing Sinovac Matters.
| 38 |
The Company first became aware of certain allegations relating to Mr. Yin as a result of a research report by Geoinvesting, dated December 21, 2016 (the “Geoinvesting Report”). The Geoinvesting Report alleged that Mr. Yin bribed an official of the China Center for Drug Evaluation, an agency of the China Food and Drug Administration, in order to facilitate the Company’s vaccine registration and approval process. On December 23, 2016, the Company issued a press release to respond to the Geoinvesting Report and furnished to the SEC a report on Form 6-K. On December 23, 2016, the Company’s audit committee passed a written resolution to authorize and approve a comprehensive internal investigation into any potential violation of anti-bribery laws raised in the Geoinvesting Report. Following the Company’s press release with respect to the Geoivesting Report, negotiations between the Special Committee and the two consortiums did not progress for several months.
On January 13, 2017, Weil followed up with K&E regarding the status of written responses from 1Globe and the Chiang Li Family to the questions and requests submitted on October 20, 2016. Between mid-January 2017 and mid-February 2017, the Buyer Consortium continued to communicate with 1Globe and the Chiang Li Family regarding the Special Committee’s requests, but 1Globe and the Chiang Li Family did not provide such written responses.
On March 2, 2017, K&E informed Weil that the Buyer Consortium remained committed to pursuing the Proposed Transaction with the Company at an acquisition price of US$6.33 per Share.
On March 3, 2017, 1Globe sent the Special Committee a letter clarifying its position and intention with respect to the proposed transactions by the two consortiums, stating, among other things, that (a) the Company’s daily operations should not be affected by the proposed going-private transaction, (b) the interests of the Company’s shareholders should be respected and protected, (c) 1Globe would support Mr. Yin’s work as general manager of Beijing Sinovac and the CEO of the Company and (d) the Buyer Consortium should negotiate with the Sinobioway Consortium to avoid potential risks relating to the Beijing Sinovac Matters. The letter did not state whether 1Globe would support the Buyer Consortium or the Sinobioway Consortium.
On March 4, 2017, Weil, at the direction of the Special Committee, requested SA to confirm, by March 10, 2017, that the Sinobioway Consortium remained committed to pursuing the Sinobioway Proposed Transaction with the Company at an acquisition price of US$7.00 per Share.
On March 7, 2017, 1Globe sent the Special Committee another letter summarizing various communications and discussions that it had had with the two consortiums between March 9, 2016 and February 13, 2017.
On March 8, 2017, the Special Committee held a conference call with Weil to discuss the letters received from 1Globe and Weil’s latest discussions with the advisors to the Buyer Consortium and the Sinobioway Consortium. After the discussion, the Special Committee authorized Mr. Mei, on behalf of the Special Committee, to meet with 1Globe in Beijing the following week to clarify 1Globe’s position and whether 1Globe intended to join the Buyer Consortium, as discussed on the conference call on October 20, 2016 and as contemplated by the Buyer Consortium’s then-current proposal.
| 39 |
On March 9, 2017, OMM informed Weil that the Sinobioway Consortium remained committed to pursuing the Sinobioway Proposed Transaction with the Company at an acquisition price of US$7.00 per Share. Later on the same day, the Sinobioway Consortium also delivered a letter to the Special Committee stating, in its opinion, that (a) any proposed going-private transaction should be beneficial to both the shareholders of the Company and the shareholders of Beijing Sinovac, (b) the proposed going-private transaction should move forward in an expeditious manner and (c) regarding the Beijing Sinovac Matters, Shandong Sinobioway (i) would not sell any equity interest in Beijing Sinovac, (ii) would not forfeit its veto rights in Beijing Sinovac or agree to any amendment to the articles of Beijing Sinovac and (iii) would exercise its rights in a fair, reasonable and lawful manner.
On March 14, 2017, Mr. Mei and a representative of Weil met in person in Beijing with representatives of 1Globe to discuss 1Globe’s views on the transactions proposed by the Buyer Consortium and the Sinobioway Consortium. During the meeting, 1Globe indicated that it had not yet decided whether to join the Buyer Consortium or the Sinobioway Consortium or pursue other options.
On March 21, 2017, Mr. Mei and Dr. Lo met in person in Beijing with representatives of the Buyer Consortium to discuss the resumption of negotiations with the Buyer Consortium.
Later on the same day, Mr. Mei also met in person in Beijing with representatives of the Sinobioway Consortium to discuss the resumption of negotiations with the Sinobioway Consortium.
On March 27, 2017, the Special Committee held a conference call with Weil. During the call, Mr. Mei reported to the Special Committee on his meetings with the two consortiums and explained that during the meeting with the Buyer Consortium, Mr. Yin emphasized his commitment to the Proposed Transaction and expressed his hope that each consortium’s offshore funding capacity be examined and verified by the Special Committee and its advisors. During the meeting with the Sinobioway Consortium, Mr. Pan emphasized his commitment to the Sinobioway Proposed Transaction and indicated that (a) if the Special Committee chose the Buyer Consortium as the winning bidder or terminated the going-private transaction, he would cause Shandong Sinobioway to exercise its veto powers at Beijing Sinovac to remove Mr. Yin as the general manager of Beijing Sinovac and appoint Mr. Pan as the general manager and (b) the Sinobioway Consortium would be willing to join with the Buyer Consortium, on the condition that the purchase price was no less than US$7.00 per Share. After the discussion, in light of 1Globe’s indication that it had not committed to support the Buyer Consortium and that the Sinobioway Consortium’s prior financing proposals relied on RMB equity commitments (which would be converted into U.S. dollars through an uncommitted loan from SPD), the Special Committee decided to reach out to the legal advisors of the two consortiums and request further information regarding their respective financing plans to assess the feasibility and certainty of closing with respect to each proposal.
Later on the same day, at the direction of the Special Committee, Weil contacted K&E and requested that the Buyer Consortium provide additional evidence of the creditworthiness of its financing sources and documentary support for its financing of the Proposed Transaction, including a detailed description of its sources and uses of financing in the event 1Globe and the Chiang Li Family decided not to join the Buyer Consortium.
| 40 |
On March 29, 2017, at the direction of the Special Committee, Weil sent OMM and SA a list of questions and information requests regarding the Sinobioway Consortium’s financing of the Sinobioway Proposed Transaction, and asked the Sinobioway Consortium to provide evidence of the creditworthiness of its sources of financing and documentary support for its financing of the Sinobioway Proposed Transaction, including a detailed description of its sources and uses of financing, an updated steps plan and timeline for the consummation of the Sinobioway Proposed Transaction and any loan agreement, commitment letter or other agreement between the Sinobioway Consortium and SPD in connection with its proposed financing. Weil also asked the Sinobioway Consortium to provide the name and contact details for a contact person at SPD so that Weil and Haiwen could directly discuss the Sinobioway Consortium’s financing arrangement, and the feasibility of the consummation thereof, with SPD.
On April 7, 2017, K&E sent Weil the Buyer Consortium’s latest financing plan and a description of its proposed sources of financing in the event 1Globe and the Chiang Li Family decided not join the Buyer Consortium. On April 8, 2017, K&E provided Weil with copies of emails from each of Advantech, C-Bridge and Vivo Capital confirming that each such equity investor would have sufficient funds to satisfy their respective equity commitments.
On April 14, 2017, at the direction of the Special Committee, Weil notified OMM and SA that the Special Committee expected to receive the Sinobioway Consortium’s responses to Weil’s questions and information requests by April 21, 2017.
On April 21, 2017, OMM sent to Weil the Sinobioway Consortium’s responses to the Special Committee’s questions and information requests submitted on March 29, 2017, together with a letter issued by SPD to PKU dated April 13, 2017 stating, among other things, that the SPD Financing Letter remained effective and that no regulatory pre-approvals or filings would be required for funding thereunder, except two-week’s prior notice to the People’s Bank of China’s head office in Shanghai. The Sinobioway Consortium also provided an unaddressed, one page capital contribution commitment letter from each member of the Sinobioway Consortium, pursuant to which each member agreed to contribute RMB in a specified amount to the Sinobioway Consortium’s acquisition vehicle. The capital contribution commitment letters were not in a customary form, were not addressed to or signed by the Sinobioway Consortium’s acquisition vehicle and did not include third party beneficiary rights in favor of the Company. No additional debt financing documentation was provided. The Sinobioway Consortium stated that the funds from SPD would be released upon the satisfaction of customary conditions required by SPD. In addition, the Sinobioway Consortium denied Weil’s request to have a direct call with SPD because of, according to the Sinobioway Consortium, SPD’s internal policy and certain unspecified PRC banking regulations and policies. OMM also informed Weil that the Sinobioway Consortium would not be updating its steps plan or its proposed timeline.
From April 25, 2017 to May 10, 2017, after discussions with Haiwen and Duff & Phelps, Weil sent OMM and SA, and OMM and SA responded to, multiple rounds of follow-up questions regarding the Sinobioway Consortium’s proposed financing structure.
| 41 |
On April 27, 2017, Weil and Haiwen held a conference call with OMM and SA to discuss and clarify the Sinobioway Consortium’s financing structure. OMM and SA clarified that the Sinobioway Consortium would finance the Sinobioway Proposed Transaction with a combination of debt and equity as follows: (a) Heng Feng and Fuerde would directly make capital contributions of approximately US$30 million, in the aggregate, in U.S dollars to PKU and (b) the PRC consortium members of the Sinobioway Consortium would utilize an offshore loan arrangement with SPD to fund the remaining portion of the amalgamation consideration in U.S. dollars (the “SPD Offshore Loan Financing”), pursuant to which (i) approximately RMB 2.8 billion would be deposited into an account of the Sinobioway Consortium’s onshore special purpose vehicle with SPD, which would serve as security for an equivalent U.S. dollar denominated loan issued by SPD, (ii) SPD would fund such loan into an account of PKU with SPD in the Shanghai Free Trade Zone (which account would be considered an offshore account for PRC foreign exchange control purposes) and (iii) PKU would use the proceeds of such loan to finance the Sinobioway Proposed Transaction. Due to the complexity of, and regulatory uncertainty associated with, the Sinobioway Consortium’s proposed financing structure, including the SPD Offshore Loan Financing, Weil reiterated its request on two separate occasions that the Sinobioway Consortium arrange a direct call between SPD and the advisors of the Special Committee to understand, and assess the feasibility and certainty of closing of, and SPD’s commitment with respect to, the SPD Offshore Loan Financing arrangement. However, the Sinobioway Consortium denied Weil’s requests for the same reasons previously provided.
On May 3, 2017, in response to Weil’s request for the Sinobioway Consortium to provide draft financing agreements with respect to the contemplated SPD Offshore Loan Financing, the Sinobioway Consortium explained that it would only be able to provide drafts of the financing agreements after the Company agreed to enter into an amalgamation agreement with the Sinobioway Consortium.
On May 12, 2017, the Special Committee held a conference call with Duff & Phelps, Weil and Haiwen to discuss the latest proposals from the Buyer Consortium and the Sinobioway Consortium. During the call, Weil reported to the Special Committee that the Buyer Consortium had confirmed that (a) it would finance the Proposed Transaction solely with rollover Shares from its members and committed equity financing in the form of offshore U.S. dollars from Advantech, C-Bridge and Vivo Capital and (b) Advantech, C-Bridge and Vivo Capital would, collectively, fund any shortfall resulting from a decision by 1Globe and the Chiang Li Family not to join the Buyer Consortium as rollover shareholders. Weil also summarized for the Special Committee the key terms of the proposals of the respective consortiums, and the Special Committee and its advisors discussed various aspects of the proposals, including price, transaction structure, financing, regulatory approvals and certainty of closing. Weil, Haiwen and Duff & Phelps further described in detail for the Special Committee the Sinobioway Consortium’s contemplated SPD Offshore Loan Financing arrangement and the Special Committee and its advisors discussed the feasibility, uncertainties and regulatory risks relating to the SPD Offshore Loan Financing. After the discussion, and taking into account, among other factors, the disparity between the Buyer Consortium’s offer price and the Sinobioway Consortium’s offer price, on the one hand, and the continuing material uncertainties associated with the Sinobioway Consortium’s financing structure, including financing and regulatory approval uncertainty, on the other hand, the Special Committee decided to request a price increase from the Buyer Consortium to US$7.00 per Share.
Later on the same day, Mr. Anderson communicated the requested price increase to a representative of SAIF.
On May 22, 2017, the Sinobioway Consortium informed the Special Committee that according to an announcement issued by Shandong Sinobioway on May 15, 2017, Mr. Pan had proposed to the board of directors of Beijing Sinovac not to reappoint Mr. Yin as the general manager of Beijing Sinovac after the expiration of Mr. Yin’s employment agreement with Beijing Sinovac on April 24, 2017.
| 42 |
On May 25, 2017, K&E informed Weil that the Buyer Consortium had agreed to increase its price to US$7.00 per Share, subject to the Special Committee’s acceptance in principle and confirmation by June 2, 2017 that it would work with the Buyer Consortium to enter into the Transaction Documents on a timeline proposed by the Buyer Consortium.
On May 29, 2017, the Special Committee held a conference call with Duff & Phelps, Weil and Haiwen. During the call, (a) Duff & Phelps indicated that, based on its preliminary analysis, the valuation range for the Shares appeared to be lower than the price of US$7.00 per Share proposed by the Buyer Consortium and (b) Weil summarized for the Special Committee the key terms and key outstanding issues with respect to the Transaction Documents and the Sinobioway Consortium Transaction Documents and discussed with the Special Committee the key differences between each consortium’s proposed transaction. The matters discussed by the Special Committee included the following:
| · | Transaction Structure. The transaction proposed by the Buyer Consortium would result in an acquisition of 100% of the Company if approved by the Company’s shareholders. The transaction proposed by the Sinobioway Consortium would only result in an acquisition of 100% of the Company if the Sinobioway Consortium acquired sufficient Shares in its first-step tender offer to approve a second-step amalgamation (which was not certain because the Buyer Consortium held, at the time, approximately 29.5% of the issued and outstanding Shares entitled to vote). Public shareholders who did not tender their shares to the Sinobioway Consortium could therefore find themselves shareholders in a company with limited trading volume and liquidity. |
| · | Price. The Sinobioway Consortium proposed a price of US$7.00 per Share. The Buyer Consortium, having increased its price on two occasions from an initial offer of US$6.18 per Share, also proposed a price of US$7.00 per Share. |
| · | Financing Plan. The Sinobioway Consortium’s financing plan presented a higher execution risk than that of the Buyer Consortium for a number of reasons, including the following: |
| o | the legality and viability of the SPD Offshore Loan Financing arrangement would be subject to uncertainties under PRC law and would be utilized in the context of tightened foreign exchange/investment control by PRC regulatory authorities; |
| o | Weil had requested access to SPD on three separate occasions in order to discuss the SPD Offshore Loan Financing directly with SPD. This request had been denied by SPD each time, according to the Sinobioway Consortium, because of PRC banking laws and regulations and SPD’s internal policy; |
| 43 |
| o | Weil had requested, but not received, drafts of the proposed financing documentation between the Sinobioway Consortium and SPD; |
| o | the Sinobioway Consortium’s legal advisors had indicated that release of the U.S. dollar funds by SPD would be subject to certain conditions, but the Special Committee had not been provided with a comprehensive list of these conditions; |
| o | given that there would be a significant period of time between the signing and closing of the Sinobioway Proposed Transaction, there would be a risk that either (i) SPD could decide not to approve the transaction for any reason or otherwise fail to fund the U.S. dollar loan at closing or (ii) the relevant PRC regulatory authorities would issue regulations or directives that adversely affect, or render impossible, the SPD Offshore Loan Financing; |
| o | although the Sinobioway Consortium had provided the CICC Qianhai Letter as a back-up U.S. dollar commitment from CICC Qianhai to address some of the risks associated with the proposed SPD Offshore Loan Financing, the Qianhai Letter was not a U.S. dollar equity commitment letter in customary form; and |
| o | in the course of negotiations over many months, the Sinobioway Consortium’s financing structure had changed on multiple occasions and the Sinobioway Consortium had not been able to obtain committed equity or debt financing in offshore U.S. dollars necessary to consummate the Sinobioway Proposed Transaction. |
| · | PRC Regulatory Approvals. The Sinobioway Proposed Transaction proposed by the Sinobioway Consortium would be subject to a number of PRC regulatory filings and approvals, including ODI Approvals and PRC antitrust clearance. |
| · | Beijing Sinovac Matters. The Buyer Consortium had agreed to bear the risks associated with the Beijing Sinovac Matters in certain circumstances. |
After the discussion, the Special Committee directed Weil to finalize the Transaction Documents with K&E.
Immediately following the meeting of the Special Committee, Weil informed K&E that, subject to receipt from the Buyer Consortium of an updated description of its sources and uses of funds with respect to the Proposed Transaction, the Special Committee was willing to finalize the Transaction Documents with the Buyer Consortium.
Later on the same day, K&E provided Weil with an updated description of its sources and uses of funds with respect to the Proposed Transaction, setting forth the Buyer Consortium’s financing sources in the event 1Globe and the Chiang Li Family elected not to join the Buyer Consortium as rollover shareholders.
| 44 |
On June 8, 2017, the Company provided Duff & Phelps with certain updated financial projections, prepared by management, for the Company for the fiscal years ending 2017 through 2025 (which financial projections are summarized under “—Certain Financial Projections”). Later on the same day, Duff & Phelps held a conference call with the Company’s senior management and finance team to discuss the changes made to the assumptions used in the updated financial projections.
On June 9, 2017, the Special Committee held a conference call with Duff & Phelps and Weil. During the call, (a) Duff & Phelps summarized for the Special Committee the key differences between the new projections and the previous set of projections received from the Company and updated the Special Committee on the timing of its valuation analysis and its internal fairness approval process and (b) Weil summarized for the Special Committee the key outstanding issues in the Transaction Documents.
Later on the same day, Weil, at the direction of the Special Committee, provided revised drafts of the Transaction Documents to K&E, which reflected, among other things: (a) the reinstatement of the Company’s Fiduciary Out Termination Right; (b) the reinstatement of the Majority of the Minority Vote Condition; (c) the reinstatement of the provision pursuant to which the Company would not be deemed to be in breach of, and the Buyer Consortium would not have the right to terminate, the amalgamation agreement in certain circumstances relating to the Beijing Sinovac Matters; and (d) reservation on the percentage threshold of the Dissenting Shareholders Condition pending confirmation of whether 1Globe and the Chiang Li Family would join the Buyer Consortium.
On June 14, 2017, K&E, on behalf of the Buyer Consortium, sent Weil its comments to the Transaction Documents, which reflected, among other things: (a) Mr. Yin as one of the guarantors under the limited guarantees; (b) the deletion of the Company’s Fiduciary Out Termination Right; (c) the deletion of the Majority of the Minority Vote Condition; (d) an amount of US$20,000,000 for both the Buyer Consortium’s termination fee and the Company’s termination fee; and (e) the Buyer Consortium’s agreement to the Special Committee’s proposed language relating to the Beijing Sinovac Matters.
On June 15, 2017, Weil held a conference call with K&E to discuss the key outstanding issues in the revised Transaction Documents. K&E informed Weil that 1Globe and the Chiang Li Family were not expected to join the Buyer Consortium at the time.
On June 16, 2017, the Special Committee held a conference call with Weil to discuss the revised Transaction Documents. During the call, Weil summarized for the Special Committee each of the key outstanding issues with respect to the Transaction Documents. The Special Committee then agreed on an approach for each issue.
Later on the same day, Weil, at the direction of the Special Committee, delivered a package proposal to K&E, which included that (a) limited guarantees would be provided by all the investors in the Buyer Consortium other than Mr. Yin (with the other investors covering Mr. Yin’s portion of the guaranteed obligations), (b) the closing would not be subject to the Majority of the Minority Vote Condition, provided that 1Globe and the Chiang Li Family would not join the Buyer Consortium, (c) the Dissenting Shareholders Condition would be subject to a 10% maximum, excluding the Shares held by 1Globe and the Chiang Li Family, (d) the Company would have the Fiduciary Out Termination Right and (e) the termination fee for both the Buyer Consortium and the Company would be US$15,000,000.
| 45 |
On June 17, 2017, K&E responded and informed Weil that the Buyer Consortium was not willing to accept items (a), (c) and (d) of the package proposal.
On June 19, 2017, the Special Committee held a conference call with Weil. During the call, Weil summarized for the Special Committee each of the remaining outstanding issues with respect to the Transaction Documents and K&E’s responses. The Special Committee then agreed on an approach for each issue. The Special Committee decided to continue to insist on items (a) and (c). With respect to item (d), the Special Committee decided to limit the Company’s Fiduciary Out Termination Right to circumstances in which a material intervening event occurs.
From June 19, 2017 to June 25, 2017, Weil had multiple discussions with the Special Committee and K&E about the outstanding issues in the Transaction Documents. Weil and K&E finally reached agreement on the Transaction Documents and the Support Agreement on June 25, 2017, consistent with the Special Committee’s instructions (including, among others, that (a) the limited guarantees would be provided by all investors in the Buyer Consortium other than Mr. Yin, (b) the Dissenting Shareholders Condition would be subject to a 7.5% maximum, excluding the Shares held by 1Globe and the Chiang Li Family, and (c) the Company would have the Fiduciary Out Termination Right in circumstances in which a material intervening event occurs).
On June 23, 2017, Latham sent Weil an initial draft of the Second Amendment to the Rights Agreement (the “Rights Agreement Amendment”) between the Company and the Pacific Stock Transfer Company, which excluded the Proposed Transaction as a triggering event thereunder. Later on the same day, Weil sent Latham its comments to the Rights Agreement Amendment.
On June 26, 2017, the Special Committee held a telephonic meeting with Duff & Phelps, Weil and LABLS to discuss the Proposed Transaction. Prior to the meeting, Duff & Phelps had circulated to the Special Committee discussion materials relating to its financial analysis and Weil had circulated to the Special Committee the final drafts of the Transaction Documents. During the meeting, (a) Weil summarized for the Special Committee the status of negotiations with K&E and reviewed with the Special Committee the Proposed Transaction and the key terms of the Transaction Documents, (b) Duff & Phelps reviewed with the Special Committee its financial analysis and rendered its oral opinion, which was subsequently confirmed in writing by delivery of a written opinion dated the same day, to the Special Committee to the effect that, as of that date and based upon, and subject to, the procedures followed, assumptions made, matters considered and qualifications and limitations on the review undertaken, the amalgamation consideration of US$7.00 per Share payable under the amalgamation agreement was fair, from a financial point of view, to the Company and its shareholders (other than holders of Excluded Shares, Dissenting Shares and Company Restricted Shares) and (c) LABLS summarized the fiduciary duties of the Special Committee members in connection with the Proposed Transaction under Antigua and Barbuda law. Please see “—Opinion of the Special Committee’s Financial Advisor” beginning on page 70 for additional information regarding the financial analyses performed by Duff & Phelps and the opinion rendered by Duff & Phelps to the Special Committee.
| 46 |
Following the discussion, the Special Committee unanimously (a) determined that the Amalgamation, on the terms and conditions set forth in the Transaction Documents (including the Per Share Amalgamation Consideration), is fair to and in the best interests of the Company and the Company’s shareholders (excluding holders of Excluded Shares), (b) determined that it was advisable that the execution, delivery and performance by the Company of the Amalgamation Agreement and the consummation of the Transactions, including the Amalgamation, be approved, authorized and adopted on the terms and conditions set forth in the Amalgamation Agreement, (c) recommended that the Board authorize and approve the execution, delivery and performance of the Amalgamation Agreement and the other Transaction Documents and the consummation of the Transactions, including the Amalgamation, and (d) determined that it is advisable to recommend that the Company’s shareholders vote in favor of the matters submitted to them in connection with the Amalgamation.
Later on the same day, the Board convened a special meeting to consider the Proposed Transaction. At the beginning of the meeting, each director disclosed his interests in the Proposed Transaction and Mr. Yin and the SAIF Director recused themselves from the meeting due to conflicts of interest. After consideration of the Proposed Transaction, the terms of the Amalgamation Agreement, the recommendations of the Special Committee, the fairness opinion issued by Duff & Phelps and the advice from the other advisors, the Board unanimously (a) determined that the Amalgamation, on the terms and conditions set forth in the Transaction Documents, is fair to and in the best interests of the Company and the Company’s shareholders (excluding holders of Excluded Shares), (b) approved and adopted the Amalgamation Agreement and approved the execution, delivery and performance by the Company of the Amalgamation Agreement and the consummation of the Transactions, including the Amalgamation and (c) recommended the adoption of the Amalgamation Agreement by the Company’s shareholders. Please see “—Reasons for the Amalgamation and Recommendation of the Special Committee and the Board” beginning on page 54 for a full description of the resolutions of the Board at this meeting.
On June 26, 2017, the Company, Parent, Amalgamation Sub, Holdco and certain members of the Buyer Consortium, as applicable, executed the Transaction Documents and the Support Agreement and the Company issued a press release announcing the execution of the Transaction Documents. Later on the same day, the Company and the Pacific Stock Transfer Company executed the Rights Agreement Amendment.
On June 27, 2017, each of Mr. Yin and SAIF filed an amendment to its Schedule 13D with the SEC reporting the execution of the Transaction Documents.
On June 28, 2017, the Sinobioway Consortium sent the Special Committee a proposal letter setting forth its revised proposal to acquire the Company’s outstanding Shares by increasing its offer price to US$8.00 per Share and stating that (a) it was prepared to immediately execute an amalgamation agreement on terms substantially similar to those in the Amalgamation Agreement the Company had entered into with the Buyer Consortium, (b) its proposal was intended to be fully funded through equity financing from the Sinobioway Consortium’s offshore cash resources and it would deliver fully committed financing upon the signing of an amalgamation agreement and (c) the closing of its proposed transaction would not be subject to PRC regulatory approvals for outbound investment or remittance of funds offshore (collectively, the “Revised Sinobioway Proposal”).
| 47 |
On June 29, 2017, the Special Committee held a conference call with Weil, Haiwen and Duff & Phelps to discuss the Revised Sinobioway Proposal. During the call, Weil reviewed with the Special Committee its obligations under the Amalgamation Agreement in responding to the Revised Sinobioway Proposal. Following the discussion, the Special Committee decided to submit a list of questions and information requests to OMM and SA to clarify the terms of the Revised Sinobioway Proposal, including with respect to the Sinobioway Consortium’s financing sources and whether it had abandoned the SPD Offshore Loan Financing arrangement with SPD.
Later that day, Weil sent OMM and SA a list of questions and information requests with respect to the Revised Sinobioway Proposal, including clarification of the Sinobioway Consortium’s statement that the Revised Sinobioway Proposal was intended to be fully funded through equity financing from its offshore cash resources (including clarification on which consortium members held offshore funds and what regulatory processes they had gone through to move funds offshore) and clarification on whether its members were prepared to deliver customary financing commitment letters prior to entering into an amalgamation agreement.
On July 7, 2017, 1Globe filed a Schedule 13D with the SEC disclosing that it beneficially owned at such time 16.4% of the Shares, it supported the Revised Sinobioway Proposal and it had expressed its willingness to vote its Shares in favor of, and roll over its Shares in connection with, such proposal.
On July 11, 2017, the Sinobioway Consortium sent a letter to the Special Committee, along with a draft waiver agreement, requesting that the Company waive the provisions of the Rights Agreement as they may apply to the Sinobioway Consortium in connection with its members (and any shareholders of the Company that wanted to join the Sinobioway Consortium) entering into a support agreement. At the instruction of the Special Committee, Weil responded to the request stating that the Special Committee would need to evaluate the totality of the Revised Sinobioway Proposal with complete information and that until the Sinobioway Consortium responded to Weil’s questions and information requests that it submitted on June 29, 2017, the Special Committee would be unable to consider the waiver request. Weil further explained that after the Special Committee received such responses, it would evaluate the Revised Sinobioway Proposal in its entirety (including the waiver request) in accordance with the procedures in the Amalgamation Agreement.
On July 24, 2017, OMM sent to Weil responses from the Sinobioway Consortium to Weil’s questions and information requests submitted on June 29, 2017. In its responses, the Sinobioway Consortium stated that its financing of the Revised Sinobioway Proposal had changed as follows: (a) it believed that shareholders of the Company (including 1Globe) collectively representing 32% of the Shares had expressed a willingness to support, and roll over their Shares in connection with, the Revised Sinobioway Proposal and that it would need to engage in further discussions with shareholders of the Company; (b) if such shareholders agreed to roll over 32% of the Shares, the amalgamation consideration payable by the Sinobioway Consortium with respect to the Revised Sinobioway Proposal would be reduced to approximately US$310 million; and (c) CITIC International Co., Ltd. (“CITIC International”), an affiliate of CITIC M&A, had reserved at least US$400 million in immediately available cash, which had been drawn down from a medium term note issued by CITIC Securities Finance MTN Co., Ltd (“MTN”), which would be used by CITIC International to fund the remaining amount of the amalgamation consideration in offshore U.S. dollars. The Sinobioway Consortium provided a draft equity commitment letter from MTN, which was in substantially the same form as the equity commitment letters executed by the members of the Buyer Consortium, and stated that the timing of, and the conditions to, the equity financing would be subject to CITIC International’s internal approval process and approval of its investment committee. The Sinobioway Consortium further stated that no PRC antitrust regulatory approval or filing would be required in connection with the Revised Sinobioway Proposal because the members of the Sinobioway Consortium would allocate shares in the acquisition vehicle among themselves to achieve a result that no member would obtain control over the surviving company after the amalgamation.
| 48 |
On July 26, 2017, at the direction of the Special Committee, Weil sent a list of follow-up questions and information requests to the Sinobioway Consortium to clarify certain matters raised by the Sinobioway Consortium’s July 24, 2017 responses, including, among others, clarification of (a) the relationship between CITIC International and MTN and CITIC International’s role in the transaction (since the draft equity commitment letter was provided by MTN), (b) the identity of the shareholders that had expressed their willingness to join the Sinobioway Consortium, (c) the details of CITIC International’s approval process (and the timing for obtaining such approval), (d) how the Sinobioway Consortium intended to address the fact that if shareholders of the Company collectively holding less than approximately 90% of the Shares attended the shareholder meeting to approve a transaction with the Sinobioway Consortium, the Buyer Consortium (whose members that were shareholders in the Company indicated that they would oppose any transaction other than the Proposed Transaction) would be able to block shareholder approval of such transaction and (e) how the Sinobioway Consortium intended to structure its consortium to achieve the result that no PRC regulatory antitrust filing or approval would be required in connection with the Revised Sinobioway Proposal.
On August 2, 2017, OMM informed Weil that the Buyer Consortium, the Sinobioway Consortium and 1Globe had scheduled a meeting to take place on August 20, 2017 in Beijing and requested an extension for the Sinobioway Consortium to respond to Weil’s follow-up questions and information requests until after the meeting, which such extension was granted by the Special Committee.
On August 19, 2017, representatives of the Buyer Consortium, the Sinobioway Consortium and 1Globe met in Beijing to discuss the possibility of the Buyer Consortium and the Sinobioway Consortium forming a combined consortium. The Buyer Consortium determined that it would be willing to consider combining with the Sinobioway Consortium if the Sinobioway Consortium could (a) demonstrate that it had sufficient committed offshore U.S. dollars to consummate its proposed transaction and (b) propose an executable cooperation plan to the benefit of both consortiums. The parties agreed to further meet periodically to discuss the possibility of a combined consortium. No consensus was reached regarding the combined consortium between the Buyer Consortium and the Sinobioway Consortium.
| 49 |
On September 2, 2017, the Sinobioway Consortium sent to Weil responses to Weil’s follow-up questions and information requests submitted on July 26, 2017. In its responses, the Sinobioway Consortium declined to identify the shareholders of the Company holding at least 32% of the Shares that expressed their willingness to support its proposal and explained that its proposed financing of the amalgamation consideration (excluding the consideration in respect of the shareholders that it believed would roll over their Shares in connection with its proposed transaction) had changed from equity to debt as follows: (a) Goldstone Investment Co., Ltd. (“Goldstone”), a wholly-owned subsidiary of CITIC Securities Co., Ltd. (“CITIC Securities”), had replaced CITIC M&A as a member of the Sinobioway Consortium; (b) MTN, which is a special purpose vehicle that issues notes and draws down proceeds from a medium term note program guaranteed by CITIC Securities, would provide a debt commitment letter, and each member of the Sinobioway Consortium would provide equity commitment letters, to the Company, and each member of the Sinobioway Consortium would enter into a facility agreement with MTN, the proceeds of which would be used by such members to fund their respective equity commitments; and (c) US$400 million had been reserved for the proposed transaction under the medium term note program. The Sinobioway Consortium also provided the offering documents and a draw-down notice with respect to the medium term note program, an undated amended and restated consortium agreement for the Sinobioway Consortium and a letter from Goldstone confirming that it would cause its parent company, CITIC Securities, to provide the financing for the Revised Sinobioway Proposal and materials describing the creditworthiness of CITIC Securities. An explanation for the replacement of CITIC M&A was not provided and the Sinobioway Consortium did not explain how it intended to address the fact that if shareholders of the Company collectively holding less than approximately 90% of the Shares attended the shareholder meeting to approve a transaction with the Sinobioway Consortium, the Buyer Consortium would be able to block shareholder approval of the transaction. The Sinobioway Consortium stated that its proposal was subject to (i) obtaining both the internal approval of CITIC Securities and the approval of the CITIC investment committee and (ii) confirmatory financial due diligence.
On September 14, 2017, the Special Committee held a conference call with Weil to discuss the latest responses from the Sinobioway Consortium with respect to the Revised Sinobioway Proposal. During the call, Weil reviewed the terms of the Revised Sinobioway Proposal as modified by the Sinobioway Consortium’s responses with the Special Committee. Weil also summarized the obligations of the Special Committee under the Amalgamation Agreement with respect to responding to the Revised Sinobioway Proposal.
On September 18, 2017, Weil sent OMM and SA a list of follow-up questions and information requests with respect to the Revised Sinobioway Proposal, including clarification of (a) the timing of entering into a facility agreement with MTN, (b) the discrepancy between the offering documents and draw-down notice with respect to the medium term note program, which appeared to prevent MTN from lending the proceeds thereof to any member of the Sinobioway Consortium that is not affiliated with the CITIC group, and the Sinobioway Consortium’s proposed financing structure, (c) the identity of the Company’s shareholders that had expressed a willingness to roll over their shares in connection with the Revised Sinobioway Proposal and (d) the discrepancy between the Sinobioway Consortium’s prior PRC antitrust analysis and the Sinobioway Consortium’s amended and restated consortium agreement, under which PKU Shanghai would be permitted to hold a controlling interest in the surviving company following an amalgamation.
On September 29, 2017, SA sent to Weil the Sinobioway Consortium’s responses to Weil’s follow-up questions and information requests submitted on September 18, 2017, which were substantively the same as the Sinobioway Consortium’s responses sent to Weil on September 2, 2017.
| 50 |
On October 9, 2017, the Special Committee received a letter from 1Globe stating that its representatives had held four formal meetings in September with representatives of the Buyer Consortium and the Sinobioway Consortium regarding, and that discussions had advanced with respect to, the Buyer Consortium and the Sinobioway Consortium forming a combined consortium.
On October 11, 2017, the Special Committee held a conference call with Weil to discuss the latest responses from the Sinobioway Consortium with respect to the Revised Sinobioway Proposal and the letter received from 1Globe. After the discussion, based on the information and responses to date provided by the Sinobioway Consortium to the Special Committee and given the time that had elapsed since the Sinobioway Consortium had made the Revised Sinobioway Proposal, the Special Committee determined that the Revised Sinobioway Proposal did not constitute, and could not reasonably be expected to result in, a Superior Proposal (as defined under “The Amalgamation Agreement—No Solicitation of Competing Transactions” beginning on page 127). Factors considered by the Special Committee included the following:
| · | during the nearly four months that had elapsed since the Sinobioway Consortium had made the Revised Sinobioway Proposal, Weil had submitted various questions and information requests with respect to clarifying the Revised Sinobioway Proposal in accordance with the terms of the Amalgamation Agreement to, among other things, enable the Special Committee to assess the likelihood of the consummation thereof, and the Sinobioway Consortium had been afforded ample opportunity to respond to such requests; |
| · | the Sinobioway Consortium’s financing structure with respect to the Revised Sinobioway Proposal had changed on two occasions (and Goldstone had replaced CITIC M&A as a member of the Sinobioway Consortium without an explanation for such replacement), and the Sinobioway Consortium had not been able to obtain committed equity or debt financing in offshore U.S. dollars necessary to consummate the Revised Sinobioway Proposal or provide the Special Committee with draft documentation with respect to the proposed debt financing arrangement with MTN; |
| · | the Sinobioway Consortium had not clarified the discrepancy between the offering documents and draw-down notice with respect to the medium term note program guaranteed by CITIC Securities, which appeared to prevent MTN from lending the proceeds thereof to any member of the Sinobioway Consortium that is not affiliated with the CITIC group, and the Sinobioway Consortium’s proposed financing structure; |
| · | the Sinobioway Consortium had not clarified (i) how the Sinobioway Consortium intended to structure its consortium to achieve the result that no PRC regulatory antitrust filing or approval would be required in connection with the Revised Sinobioway Proposal and (ii) the discrepancy between its claim that shares in the Sinobioway Consortium’s acquisition vehicle would be allocated among the consortium members to achieve the result that no member would obtain control over the surviving company following the amalgamation, and the provisions of the Sinobioway Consortium’s amended and restated consortium agreement that would permit PKU Shanghai to hold a controlling interest in the surviving company; |
| 51 |
| · | the Revised Sinobioway Proposal was subject to obtaining both the internal approval of CITIC Securities and the approval of the CITIC investment committee; |
| · | the Sinobioway Consortium did not explain how it intended to address the fact that if shareholders of the Company collectively holding less than approximately 90% of the issued and outstanding Shares attended a special meeting, in person or by proxy, to approve a transaction with the Sinobioway Consortium, the Buyer Consortium, which holds approximately 29.5% of the issued and outstanding Shares entitled to vote, would be able to block shareholder approval of the transaction; and |
| · | in light of the foregoing, the Special Committee was not able to determine in its good faith judgment that the Revised Sinobioway Proposal was, or could be, reasonably likely to be consummated. |
Later on the same day, Weil, at the direction of the Special Committee, contacted K&E and requested, in accordance with the terms of the Amalgamation Agreement, the Buyer Consortium’s consent to a meeting between the Special Committee and 1Globe to clarify 1Globe’s position with respect to the Transactions, including the Amalgamation, and the status of its discussions with the Buyer Consortium and the Sinobioway Consortium.
On October 12, 2017, K&E sent Weil an email explaining that (a) no consensus had been reached regarding a combined consortium between the Buyer Consortium and the Sinobioway Consortium, (b) the Buyer Consortium had not consented to the letter sent by 1Globe on October 9, 2017 and the letter did not reflect the views of the Buyer Consortium and (c) given that the Sinobioway Consortium was unable to demonstrate that it had sufficient committed financing, the Buyer Consortium did not intend to work with the Sinobioway Consortium on a combined transaction, although it had previously considered that possibility if the Sinobioway Consortium was able to demonstrate that it had sufficient committed financing, and, therefore, the Buyer Consortium did not believe a meeting with 1Globe would be productive at such time.
On October 16, 2017, the Special Committee received an email from 1Globe and its affiliates stating, among other things, that they held more than 30% of the Shares and that they would vote their Shares against any proposed transaction requiring shareholder approval that was not recognized by 1Globe.
On October 17, 2017, the Special Committee held a conference call with Weil to discuss the email from 1Globe and the Buyer Consortium’s response to the Special Committee’s request for its consent to contact 1Globe. Following the discussion, the Special Committee decided to contact Mr. Yin regarding a potential meeting with 1Globe. Mr. Yin confirmed that representatives of the Buyer Consortium and 1Globe would meet in the near future and the Buyer Consortium would consent to the Special Committee contacting and meeting with 1Globe after such meeting.
| 52 |
On the same day, the Special Committee received a letter from the Sinobioway Consortium in which the Sinobioway Consortium, among other things, (a) reiterated the financing structure described in its responses provided to the Special Committee on September 2, 2017, (b) stated that it was willing to provide an executed debt commitment letter and executed equity commitment letters prior to the execution of an amalgamation agreement and that its proposed financing would not delay the execution of an amalgamation agreement, (c) stated that shareholders of the Company collectively owning more than 32% of the Shares, whose identities were not disclosed, had expressed their desire to support the Revised Sinobioway Proposal, (d) explained that Mr. Yin had been removed from his position as the general manager of Beijing Sinovac, (e) confirmed that it was willing to execute an amalgamation agreement as soon as possible and (f) reiterated its request for a waiver under the Rights Agreement.
On October 24, 2017, representatives of the Buyer Consortium met with representatives of 1Globe and the Chiang Li Family to discuss their participation in the Proposed Transaction as members the Buyer Consortium. Mr. Yin later informed Mr. Mei that representatives of the Buyer Consortium and 1Globe had a meeting and that the Buyer Consortium consented to the Special Committee contacting and meeting with 1Globe.
On the same day, Weil, at the direction of the Special Committee, called OMM and explained that, based on the information and responses to date provided by the Sinobioway Consortium to the Special Committee and given the time that had elapsed since the Sinobioway Consortium had made the Revised Sinobioway Proposal, the Special Committee determined that the Revised Sinobioway Proposal did not constitute, and could not reasonably be expected to result in, a Superior Proposal.
Since the end of October 2017, the Buyer Consortium had requested, on various occasions, written confirmation from 1Globe and the Chiang Li Family regarding their intention to participate in, and the amount of Shares they would roll over in connection with, the Proposed Transaction. No written confirmation was received from 1Globe or the Chiang Li Family.
On December 8, 2017, the Special Committee held a conference call with Weil to discuss, among other things, the position of 1Globe and the Chiang Li Family with respect to the Transactions, including the Amalgamation, in light of the fact that the Special Committee had received various reports regarding the intention of 1Globe and the Chiang Li Family to join the Buyer Consortium, but neither 1Globe nor the Chiang Li Family had committed in writing to do so or entered into any written agreement with the Buyer Consortium with respect to its participation in the Proposed Transaction. After the discussion, the Special Committee decided to contact 1Globe to clarify the position of 1Globe and the Chiang Li Family with respect to the Transactions, including the Amalgamation. Following the call, Mr. Anderson contacted a representative of 1Globe by email.
On December 14, 2017, Mr. Anderson received a response by email from a senior representative of 1Globe, in which that representative, among other things, (a) indicated that (i) 1Globe had not agreed to join the Buyer Consortium or participate in the Proposed Transaction and (ii) from 1Globe’s perspective, the optimal consortium with respect to any privatization of the Company would consist of both the Buyer Consortium and the Sinobioway Consortium and (b) referred Mr. Anderson to the Schedule 13D filed by 1Globe with the SEC on July 7, 2017, which had not been amended.
| 53 |
Reasons for the Amalgamation and Recommendation of the Special Committee and the Board
The Special Committee and the Board believe that, as a privately held entity, the Company’s management may have greater flexibility to focus on improving the Company’s long-term financial performance without the pressures created by the public equity market’s emphasis on short-term period-to-period financial performance.
In addition, as an SEC-reporting company, the Company’s management and accounting staff, which comprises a relatively small number of individuals, must devote significant time to SEC reporting and compliance. The Company is also required to disclose a considerable amount of business information to the public, some of which would otherwise be considered competitively sensitive and would not be disclosed by a non-reporting company. As a result, our actual or potential competitors, customers, lenders and vendors all have ready access to this information, which potentially may help them compete against us or make it more difficult for us to negotiate favorable terms with them, as the case may be.
At a meeting held on June 26, 2017, the Special Committee reviewed and considered the terms and conditions of the Amalgamation Agreement and the Transactions, including the Amalgamation. The Special Committee, after due consideration, unanimously (a) determined that the Amalgamation, on the terms and conditions set forth in the Amalgamation Agreement and the other Transaction Documents (including the Per Share Amalgamation Consideration), is fair to and in the best interests of the Company and its Unaffiliated Holders, (b) determined it was advisable that the execution, delivery and performance by the Company of the Amalgamation Agreement and the consummation of the Transactions, including the Amalgamation, be approved, authorized and adopted on the terms and conditions set forth in the Amalgamation Agreement, (c) recommended that the Board authorize and approve the execution, delivery and performance of the Amalgamation Agreement and the other Transaction Documents and the consummation of the Transactions, including the Amalgamation and (d) determined it was advisable to recommend that the shareholders of the Company authorize and approve the matters submitted to them in connection with the Amalgamation, including the Amalgamation Agreement and the Transactions, including the Amalgamation.
At a meeting held on June 26, 2017, the Board, acting upon the unanimous recommendation of the Special Committee, unanimously (other than Mr. Yin and Kenneth Lee, who recused themselves from the meeting) (a) determined that the Amalgamation, on the terms and conditions set forth in the Amalgamation Agreement and the other Transaction Documents (including the Per Share Amalgamation Consideration), is fair to and in the best interests of the Company and the Unaffiliated Holders, (b) approved and adopted the Amalgamation Agreement and approved the execution, delivery and performance by the Company of the Amalgamation Agreement and the other Transaction Documents and the consummation of the Transactions, including the Amalgamation, and (c) directed that the authorization and approval of the Amalgamation Agreement and the Transactions, including the Amalgamation, be submitted to a vote at a special meeting of the shareholders of the Company with the recommendation of the Board that the shareholders of the Company authorize and approve by way of a special resolution the Amalgamation Agreement and the Transactions, including the Amalgamation. The directors who approved the foregoing matters included a majority of the directors not employed by the Company. The Board has produced the fairness determination on behalf of the Company as described in this proxy statement.
| 54 |
In reaching their respective determinations, the Special Committee and the Board considered various substantive factors and potential benefits of the Amalgamation, including the following, which are not listed in any relative order of importance:
| · | the Special Committee’s and the Board’s knowledge of the Company’s business, financial condition, results of operations, prospects and competitive position and their belief that the Amalgamation would, as a whole and considering factors that could impact deal certainty, be financially more favorable to the Unaffiliated Holders than any other alternative reasonably available to the Company and the Unaffiliated Holders; |
| · | estimated forecasts of the Company’s future financial performance prepared by the Company’s management, together with the Company’s management’s view of the Company’s financial condition, results of operations, business, prospects and competitive position; |
| · | the fact that the Per Share Amalgamation Consideration is entirely in cash, which will allow the Unaffiliated Holders to immediately realize liquidity for their investment and provide them with certainty of the value of their Shares; |
| · | the current and historical market prices of the Company’s Shares, and the fact that the Per Share Amalgamation Consideration of US$7.00 per Share represents a premium of 39.4% to the closing price of the Shares on January 29, 2016, the last trading day immediately prior to the date on which the Company announced that it had received the Buyer Consortium’s Proposal Letter, a premium of 32.1% and 30.0% to the volume-weighted average trading price of the Shares as quoted by NASDAQ during the 30 and 60 trading days immediately prior to February 1, 2016 (the date on which the Company announced that it had received the Buyer Consortium’s Proposal Letter), respectively, and a premium of 55.9% to the lowest trading price of the Shares in the 52-week period immediately prior to February 1, 2016; |
| · | the likelihood that the trading price of the Shares would at any time in the foreseeable future reach and sustain a level equal to or greater than the Per Share Amalgamation Consideration of US$7.00, as adjusted for present value; |
| · | the fact that the Per Share Amalgamation Consideration represents an increase of approximately 13.3% from the original US$6.18 per Share offer price in the Buyer Consortium’s Proposal Letter; |
| · | the negotiations with respect to the Per Share Amalgamation Consideration and the Special Committee’s determination that, following extensive negotiations with the Buyer Consortium, US$7.00 was the highest price per Share that the Buyer Consortium would agree to pay, with the Special Committee basing its belief on a number of factors, including the duration and tenor of negotiations and the experience of the Special Committee and its advisors; |
| 55 |
| · | the low probability that any transaction with a third party could be completed given that the members of the Buyer Consortium together hold approximately 29.5% of the issued and outstanding Shares entitled to vote, which is nearly the one-third of the voting power sufficient to prevent the consummation of any transaction with a third party that requires shareholder approval, and agreed not to sell their respective Shares to, and to vote against any transaction requiring shareholder approval with, any third party; |
| · | the likelihood that the Amalgamation would be completed based on, among other things (not in any relative order of importance): |
| o | the fact that the structure of the transaction, and the fact that Parent and Amalgamation Sub have obtained committed equity financing in U.S. dollars necessary to complete the Amalgamation, the conditions to the equity financing, the reputation of the equity financing sources and the location of the equity financing sources outside of the PRC, eliminate the need for certain regulatory approvals that would otherwise be required in connection with an alternative transaction; |
| o | the absence of any financing condition in the Amalgamation Agreement; |
| o | the fact that Parent and Amalgamation Sub do not require any debt financing to complete the Amalgamation; and |
| o | the likelihood and anticipated timing of completing the Amalgamation in light of the scope of the conditions to Closing; |
| · | the fact that the Amalgamation Agreement provides that, in the event of a failure of the Amalgamation to be completed under certain circumstances, Parent will pay the Company a termination fee of US$15.0 million (see “The Amalgamation Agreement—Termination Fees” beginning on page 141); |
| · | following its formation, the Special Committee’s independent control of the sale process with the advice and assistance of Duff & Phelps as its financial advisor and Weil and LABLS as its legal advisors, reporting solely to the Special Committee; and |
| · | the financial analysis reviewed and discussed with the Special Committee by representatives of Duff & Phelps, as well as the oral opinion of Duff & Phelps rendered to the Special Committee on June 26, 2017 (which was subsequently confirmed in writing by delivery of Duff & Phelps’s written opinion addressed to the Special Committee dated June 26, 2017) as to, as of such date, the fairness, from a financial point of view, to the holders of Shares (other than Excluded Shares, Dissenting Shares and Company Restricted Shares), of the Per Share Amalgamation Consideration to be received by such holders in the Amalgamation pursuant to the Amalgamation Agreement (without giving effect to any impact of the Amalgamation on any particular holder of Shares, other than in its capacity as a holder of Shares). |
| 56 |
In addition, the Special Committee and the Board believed that sufficient procedural safeguards were and are present to ensure that the Amalgamation is procedurally fair to the Unaffiliated Holders and to permit the Special Committee and the Board to represent effectively the interests of the Unaffiliated Holders. The procedural safeguards include the following, which are not listed in any relative order of importance:
| · | the consideration and negotiation of the Amalgamation Agreement was conducted entirely under the control and supervision of the Special Committee, which consists of three independent directors, as defined under applicable NASDAQ rules, each of whom is an outside, non-employee director, and that no limitations were placed on the Special Committee’s authority; |
| · | in considering the transaction with the Buyer Consortium, the Special Committee acted solely to represent the interests of the Unaffiliated Holders, and the Special Committee had independent control of the extensive negotiations with the Buyer Consortium and their legal and financial advisors on behalf of the Unaffiliated Holders; |
| · | all of the members of the Special Committee during the entire process were and are independent directors and free from any affiliation with the Buyer Consortium; in addition, none of the members of the Special Committee is or ever was an employee of the Company or any of its subsidiaries or affiliates and none of such members has any financial interest in the Amalgamation that is different from that of the Unaffiliated Holders, other than the members’ receipt of Board and Special Committee compensation (which is not contingent upon completion of the Amalgamation or the Special Committee’s or the Board’s recommendation of the Amalgamation) and their indemnification and liability insurance rights under the Amalgamation Agreement; |
| · | the Special Committee was assisted in negotiations with the Buyer Consortium and in its evaluation of the Amalgamation Agreement by Duff & Phelps as its financial advisor and Weil, Haiwen and LABLS as its legal advisors; |
| · | the Special Committee was empowered to consider, attend to and take any and all actions in connection with the proposal from the Buyer Consortium with respect to the proposed transaction or any alternative transaction with a third party from the date the Special Committee was established, and no evaluation, negotiation or response regarding such proposed transaction or any alternative transaction in connection therewith from that date forward was considered by the Board for approval until the Special Committee had recommended such action to the Board; |
| 57 |
| · | the terms and conditions of the Amalgamation Agreement were the product of extensive negotiations between the Special Committee with the assistance of its advisors and the Buyer Consortium with the assistance of its advisors; |
| · | the Special Committee met on numerous occasions to consider and review the terms of the Amalgamation Agreement and the proposed transaction; |
| · | the recognition by the Special Committee and the Board that the Special Committee had no obligation to recommend the Amalgamation or any other transaction; |
| · | the recognition by the Special Committee and the Board that, under the Amalgamation Agreement, the Special Committee has the ability to consider any acquisition proposal that constitutes, or could reasonably be expected to result in, a Superior Proposal (as defined under “The Amalgamation Agreement—No Solicitation of Competing Transactions” beginning on page 127) until the Company’s shareholders vote upon and authorize and approve the Amalgamation Agreement and the Transactions, including the Amalgamation; |
| · | the Company’s ability, subject to compliance with the terms and conditions of the Amalgamation Agreement, to terminate the Amalgamation Agreement prior to the receipt of shareholder approval in order to accept an alternative transaction proposed by a third party that is a Superior Proposal (as defined under “The Amalgamation Agreement—No Solicitation of Competing Transactions” beginning on page 127); |
| · | the ability of the Board and the Special Committee, under certain circumstances, to change, withhold, withdraw, qualify or modify the Company Recommendation (as defined under “The Amalgamation Agreement—No Change of Recommendation” beginning on page 130) that the Company’s shareholders vote to authorize and approve the Amalgamation Agreement and the Transactions, including the Amalgamation, and/or terminate the Amalgamation Agreement; and |
| · | the availability of dissenters’ rights to the Unaffiliated Holders who comply with all of the required procedures under the IBCA for exercising dissenters’ rights, which allow such shareholders to receive payment from the Company of the fair value of their Shares in the manner set out in the IBCA. |
The Special Committee and the Board also considered a variety of potentially negative factors concerning the Amalgamation Agreement and the Amalgamation, including the following, which are not listed in any relative order of importance:
| · | approval of the Amalgamation Agreement and the Transactions, including the Amalgamation, is not subject to any additional approval by the majority of Unaffiliated Holders and, given that the Buyer Consortium owns approximately 29.5% of the issued and outstanding Shares entitled to vote, the Buyer Consortium may have the ability to authorize and approve the Amalgamation Agreement and the Transactions, including the Amalgamation, at the special meeting unless a substantial majority of the Unaffiliated Holders vote against the proposal to authorize and approve the Amalgamation Agreement and the Transactions, including the Amalgamation; |
| 58 |
| · | the lack of ongoing equity participation in the Company by the Unaffiliated Holders following the Amalgamation, and the inability of the Unaffiliated Holders to participate in the Company’s future earnings or growth, if any, or to benefit from increases, if any, in the value of Shares, and the inability of the Unaffiliated Holders to participate in any potential future sale of the Company to a third party or any potential recapitalization of the Company, which could include a dividend to shareholders; |
| · | the restrictions on the conduct of the Company’s business prior to the completion of the Amalgamation, which may delay or prevent the Company from undertaking business opportunities that may arise or any other action it would otherwise take with respect to the operations of the Company pending completion of the Amalgamation; |
| · | the risks and costs to the Company if the Amalgamation does not close, including the diversion of management and employee attention, potential employee attrition and the potential disruptive effect on the Company’s business and customer relationships; |
| · | the Company’s obligation, under certain circumstances, to pay Parent a termination fee of US$15.0 million in connection with the termination of the Amalgamation Agreement; |
| · | the Company’s remedy in the event of a breach of the Amalgamation Agreement by Parent or Amalgamation Sub is limited, under the Amalgamation Agreement, to receipt of a termination fee of US$15.0 million, and under certain circumstances the Company may not be entitled to a termination fee at all; |
| · | the terms of the Buyer Consortium’s participation in the Amalgamation and the fact that the Buyer Consortium may have interests in the Amalgamation that are different from, or in addition to, those of the Unaffiliated Holders (see “—Interests of Certain Persons in the Amalgamation” beginning on page 92 for additional information); |
| · | the possibility that the Amalgamation might not be completed and the negative impact of public announcement thereof on the Company’s operating results and the Company’s ability to attract and retain key management, and experienced personnel; and |
| · | the taxability of an all-cash transaction to the Unaffiliated Holders. |
| 59 |
In addition, in reaching its determination on June 26, 2017 that the Amalgamation Agreement and the Transactions, including the Amalgamation, are fair to, and in the best interests of, the Company and the Unaffiliated Holders and to recommend the authorization and approval of the Amalgamation Agreement and the Transactions, including the Amalgamation, by the Company’s shareholders, the Special Committee considered a variety of factors concerning the Sinobioway Proposed Transaction, including the following, which are not listed in any relative order of importance:
| · | from February 4, 2016 to May 10, 2017, the Special Committee and its financial and legal advisors held extensive discussions and negotiations with the Sinobioway Consortium with respect to the Sinobioway Proposed Transaction, as further discussed in “—Background of the Amalgamation” beginning on page 18; |
| · | the Transactions, including the Amalgamation, if approved and authorized by the Company’s shareholders, will result in an acquisition by the Buyer Consortium of 100% of the Company, whereas the Sinobioway Proposed Transaction would only result in an acquisition of 100% of the Company if the Sinobioway Consortium acquired sufficient Shares in its first-step tender offer to approve a second-step amalgamation (which would be uncertain because the Buyer Consortium owns approximately 29.5% of the issued and outstanding Shares entitled to vote) and, therefore, shareholders of the Company who did not tender their Shares to the Sinobioway Consortium could remain shareholders in a company with limited trading volume and liquidity; |
| · | the completion of the Sinobioway Proposed Transaction would be less certain, and presented a higher execution risk, than the completion of the Transactions, including the Amalgamation, by the Buyer Consortium for a number of reasons, including the following: |
| o | the Sinobioway Consortium had not sufficiently demonstrated the availability of committed financing: (i) the Special Committee’s legal advisors had requested, but not received, drafts of the proposed financing documentation between the Sinobioway Consortium and SPD with respect to the SPD Offshore Loan Financing, (ii) the Sinobioway Consortium indicated that it would only be able to provide drafts of the financing documentation after an amalgamation agreement was entered into, (iii) the Sinobioway Consortium’s legal advisers indicated that the release of U.S. dollar funds by SPD under the SPD Offshore Loan Financing would be subject to certain conditions, but the Special Committee had not been provided with a comprehensive list of these conditions, and (iv) SPD had denied the requests from the Special Committee’s legal advisors to discuss the SPD Offshore Loan Financing so that the Special Committee could assess the viability and feasibility of the consummation thereof; |
| o | the Sinobioway Proposed Transaction would be mostly financed through the SPD Offshore Loan Financing, and the legality and viability of the SPD Offshore Loan Financing arrangement would be subject to uncertainties under PRC law and utilized in the context of tightened foreign exchange and investment control by PRC regulatory authorities; |
| 60 |
| o | given that there would be a significant period of time between the signing and closing of the Sinobioway Proposed Transaction, there would be a risk that either (i) SPD could decide not to approve the SPD Offshore Loan Financing for any reason or otherwise fail to fund the U.S. dollar loan thereunder at the Closing or (ii) the relevant PRC regulatory authorities would issue regulations or directives that adversely affect, or render impossible, the SPD Offshore Loan Financing; |
| o | in the course of negotiations over many months, the Sinobioway Consortium’s financing structure had changed on multiple occasions and the Sinobioway Consortium had not been able to obtain committed equity or debt financing in offshore U.S. dollars necessary to consummate the Sinobioway Proposed Transaction, whereas Parent and Amalgamation Sub had obtained committed equity financing in offshore U.S. dollars necessary to complete the Transactions, including the Amalgamation; and |
| o | the Sinobioway Proposed Transaction would be subject to a number of PRC regulatory filings and approvals, including ODI Approvals and PRC antitrust clearance, that would not be required in connection with the Transactions, including the Amalgamation. |
| · | if the Company entered into an amalgamation agreement with the Sinobioway Consortium with respect to the Sinobioway Proposed Transaction and the Sinobioway Proposed Transaction were not completed, the public announcement thereof could have a negative impact on the Company’s operating results and the Company’s ability to attract and retain key management, and experienced personnel; |
| · | the Sinobioway Consortium had been unwilling to agree to bear the risks associated with the Beijing Sinovac Matters; and |
| · | the Buyer Consortium, having increased its price on two occasions from an initial offer of US$6.18 per Share, agreed to the Per Share Amalgamation Consideration of US$7.00 per Share, which was the same per Share consideration offered by the Sinobioway Consortium at the time of the Special Committee’s determination. |
At a meeting held on October 11, 2017, the Special Committee reviewed and considered the terms and conditions of the Revised Sinobioway Proposal, as modified by the Sinobioway Consortium’s responses to various clarification questions and information requests submitted to the Sinobioway Consortium by the Special Committee’s legal advisors. The Special Committee determined that, based on such responses provided as of such date and the time that had elapsed since the Sinobioway Consortium had made the Revised Sinobioway Proposal, the Revised Sinobioway Proposal did not constitute, and could not reasonably be expected to result in, a Superior Proposal. In reaching its determination, the Special Committee considered various substantive factors, including the following, which are not listed in any relative order of importance:
| 61 |
| · | during the nearly four months that had elapsed since the Sinobioway Consortium had made the Revised Sinobioway Proposal, the Special Committee’s legal advisors had submitted various questions and information requests with respect to clarifying the Revised Sinobioway Proposal in accordance with the terms of the Amalgamation Agreement to, among other things, enable the Special Committee to assess the likelihood of the consummation thereof, and the Sinobioway Consortium had been afforded ample opportunity to respond to such requests; |
| · | the fact that the Sinobioway Consortium’s financing structure with respect to the Revised Sinobioway Proposal had changed on two occasions (and Goldstone had replaced CITIC M&A as a member of the Sinobioway Consortium without an explanation for such replacement), and the Sinobioway Consortium had not been able to obtain committed equity or debt financing in offshore U.S. dollars necessary to consummate the Revised Sinobioway Proposal or provide the Special Committee with draft documentation with respect to the proposed debt financing arrangement with MTN; |
| · | the ability of the members of the Sinobioway Consortium to fund their respective equity comments would be primarily dependent on entering into, and the funding under, a facility agreement with MTN, the timing of which was uncertain; |
| · | the Sinobioway Consortium had not clarified the discrepancy between the offering documents and draw-down notice with respect to the medium term note program guaranteed by CITIC Securities, which appeared to prevent MTN from lending the proceeds thereof to any member of the Sinobioway Consortium that was not affiliated with the CITIC group, and the Sinobioway Consortium’s proposed financing structure; |
| · | the Sinobioway Consortium had not clarified (i) how the Sinobioway Consortium intended to structure its consortium to achieve the result that no PRC regulatory antitrust filing or approval would be required in connection with the Revised Sinobioway Proposal and (ii) the discrepancy between its claim that shares in the Sinobioway Consortium’s acquisition vehicle would be allocated among the consortium members to achieve the result that no member would obtain control over the surviving company following the amalgamation, and the provisions of the Sinobioway Consortium’s amended and restated consortium agreement that would permit PKU Shanghai to hold a controlling interest in the surviving company; |
| · | the Revised Sinobioway Proposal was subject to obtaining both the internal approval of CITIC Securities and the approval of the CITIC investment committee; |
| · | the Sinobioway Consortium declined to identify the shareholders of the Company holding at least 32% of the outstanding Shares that the Sinobioway Consortium indicated had expressed their willingness to support the Revised Sinobioway Proposal; |
| 62 |
| · | the Sinobioway Consortium did not explain how it would address the fact that, since the Buyer Consortium held approximately 29.5% of the issued and outstanding Shares entitled to vote, if shareholders of the Company collectively holding less than approximately 90% of the issued and outstanding Shares attended a special meeting, in person or by proxy, to approve a transaction with the Sinobioway Consortium, the Buyer Consortium would be able to block shareholder approval of the transaction; and |
| · | in light of the foregoing, the Special Committee was not able to determine in its good faith judgment that the Revised Sinobioway Proposal was, or could be, reasonably likely to be consummated. |
The foregoing discussion of information and factors considered by the Special Committee and the Board is not intended to be exhaustive, but includes a number of the factors considered by the Special Committee and the Board. In view of the wide variety of factors considered by the Special Committee and the Board, neither the Special Committee nor the Board found it practicable to quantify or otherwise assign relative weighting to the foregoing factors in reaching its conclusions. In addition, individual members of the Special Committee and the Board may have given different weighting to different factors and may have viewed some factors more positively or negatively than others.
The Special Committee recommended that the Board authorize and approve, and the Board authorized and approved, the Amalgamation Agreement and the Transactions, including the Amalgamation, and the Special Committee determined that the Revised Sinobioway Proposal did not constitute, and could not reasonably be expected to result in, a Superior Proposal, in each case, based upon the totality of the information presented to and considered by it.
In reaching its conclusion regarding the fairness of the Amalgamation to the Unaffiliated Holders and its decision to recommend the authorization and approval of the Amalgamation Agreement and the Transactions, including the Amalgamation, the Special Committee considered, among other factors, the financial analyses reviewed and discussed with the Special Committee by Duff & Phelps summarized below under the section entitled “—Opinion of the Special Committee’s Financial Advisor” beginning on page 70. The Board expressly adopted these financial analyses, among other factors considered, in reaching its determination as to the fairness of the Transactions, including the Amalgamation.
Neither the Special Committee nor the Board calculated a specific going concern value for the Company in reaching its determination as to the fairness of the Transactions, including the Amalgamation. The Special Committee and the Board consider the Company to be a viable going concern business and the historical market prices of our Shares as described under the section entitled “Market Price of the Company’s Shares, Dividends and Other Matters—Market Price of the Shares” beginning on page 109, to generally be an indication of its going concern value. Neither the Special Committee nor the Board considered the liquidation value of Company’s assets because each considers the Company to be a viable going-concern business where value is derived from cash flows generated from its continuing operations. In addition, the Special Committee and the Board believe that the value of the Company’s assets that might be realized in a liquidation would be significantly less than its going-concern value. Each of the Special Committee and the Board believes the analyses and additional factors it reviewed provided an indication of the Company’s going concern value. Each of the Special Committee and the Board considered the purchase prices paid in previous purchases as described under “Transactions in Shares” beginning on page 149.
| 63 |
Neither the Special Committee nor the Board, however, considered the Company’s net book value, which is defined as total assets minus total liabilities, attributable to the Company’s shareholders, as a factor. The Special Committee and the Board believe that net book value is not a material indicator of the value of the Company as a going concern as it does not take into account the future prospects of the Company, market conditions, trends in the industry or the business risks inherent in competing with larger companies in the same industry. The Company’s net book value per Share as of December 31, 2016 was US$2.03 based on the number of issued and outstanding Shares as of December 31, 2016. Except for the Sinobioway Proposed Transaction and the Revised Sinobioway Proposal, the Company is not aware of any firm offers made by any unaffiliated person, other than the filing persons, during the past two years for (i) the merger or consolidation of the Company with or into another company, or vice-versa; (ii) the sale or other transfer of all or any substantial part of the assets of the Company; or (iii) a purchase of the Company’s securities that would enable the holder to exercise control of the Company.
In reaching its determination that the Amalgamation Agreement and the Transactions, including the Amalgamation, are fair to, and in the best interests of, the Company and the Unaffiliated Holders and its decision to authorize and approve the Amalgamation Agreement and the Transactions, including the Amalgamation, and recommend the authorization and approval of the Amalgamation Agreement and the Transactions, including the Amalgamation, by the Company’s shareholders, the Board, on behalf of the Company, considered the analysis and recommendation of the Special Committee and the factors considered by the Special Committee as described above under this section and under “—Background of the Amalgamation,” including, among other factors, the financial analyses reviewed and discussed with the Special Committee by Duff & Phelps summarized below under the section entitled “—Opinion of the Special Committee’s Financial Advisor,” and adopted the Special Committee’s recommendation and these analyses.
During its consideration of the Amalgamation Agreement and the Transactions, including the Amalgamation, the Board was also aware that some of the Company’s directors and shareholders, including Mr. Yin, the chairman, president and chief executive officer of the Company, and SAIF Partners IV L.P., and other employees of the Company, have interests with respect to the Amalgamation that are, or may be, different from, or in addition to those of the Company’s Unaffiliated Holders generally, as described under the section entitled “—Interests of Certain Persons in the Amalgamation” beginning on page 92.
No unaffiliated representative was engaged by the filing persons to act solely on behalf of unaffiliated security holders for purposes of negotiating the terms of the Rule 13e-3 transaction.
For the foregoing reasons, the Company believes that the Amalgamation Agreement and the Transactions, including the Amalgamation, are fair to, and in the best interests of, the Company and the Unaffiliated Holders.
| 64 |
Position of the Buyer Consortium as to the Fairness of the Amalgamation
Under SEC rules governing going-private transactions, each member of the Buyer Consortium is required to express his, her or its belief as to the fairness of the Amalgamation to the Unaffiliated Holders. Each member of the Buyer Consortium is making the statements included in this section solely for the purpose of complying with the requirements of Rule 13e-3 and related rules under the Exchange Act. The views of the Buyer Consortium as to the fairness of the Amalgamation are not intended to be and should not be construed as a recommendation to any shareholder of the Company as to how that shareholder should vote on the proposal to authorize and approve the Amalgamation Agreement and the Transactions, including the Amalgamation. The Buyer Consortium has interests in the Amalgamation that are different from, and/or in addition to, those of the other shareholders of the Company by virtue of their continuing interests in the Surviving Corporation after the completion of the Amalgamation. These interests are described under the section entitled “—Interests of Certain Persons in the Amalgamation” beginning on page 92.
The Buyer Consortium believes the interests of the Unaffiliated Holders were represented by the Special Committee, which negotiated the terms and conditions of the Amalgamation Agreement with the assistance of its independent legal and financial advisors. The Buyer Consortium attempted to negotiate a transaction that would be most favorable to it, and not to the Unaffiliated Holders, and, accordingly, did not negotiate the Amalgamation Agreement with a goal of obtaining terms that were fair to such holders. The Buyer Consortium did not participate in the deliberations of the Special Committee regarding, and did not receive any advice from the Special Committee’s independent legal or financial advisors as to, the fairness of the Amalgamation to the Unaffiliated Holders. Furthermore, the members of the Buyer Consortium did not themselves undertake a formal evaluation of the fairness of the Amalgamation. No financial advisor provided the Buyer Consortium with any analysis or opinion with respect to the fairness of the Per Share Amalgamation Consideration to the Unaffiliated Holders.
Based on their knowledge and analysis of available information regarding the Company, as well as discussions with the Company’s senior management regarding the Company and its business and the factors considered by, and findings of, the Special Committee and the Board discussed under the section entitled “—Reasons for the Amalgamation and Recommendation of the Special Committee and the Board” beginning on page 54, the Buyer Consortium believes that the Amalgamation is fair to the Unaffiliated Holders based on the following factors, which are not listed in any relative order of importance:
| · | the consideration to be paid to the Unaffiliated Holders represents a premium of 32.1% and 30.0%, to the volume-weighted average trading price of the Shares as quoted by NASDAQ during the 30 and 60 trading days, respectively, immediately prior to the Company’s announcement on February 1, 2016 that it had received a non-binding “going-private” proposal; |
| · | the members of the Special Committee are not officers or employees of the Company, are not affiliated with the Buyer Consortium and do not have any interests in the Amalgamation different from, or in addition to, those of the Unaffiliated Holders, other than the members’ receipt of the Board and Special Committee compensation (which are not contingent upon the completion of the Amalgamation or the Special Committee’s or the Board’s recommendation of the Amalgamation) and their indemnification and liability insurance rights under the Amalgamation Agreement; |
| 65 |
| · | in considering the transaction with the Buyer Consortium, the Special Committee acted solely to represent the interests of the Unaffiliated Holders, and the Special Committee had independent control of the extensive negotiations with the Buyer Consortium and their respective legal and financial advisors on behalf of the Unaffiliated Holders; |
| · | the Special Committee and, based in part upon the unanimous recommendation of the Special Committee, the Board (other than Mr. Yin and Mr. Kenneth Lee, who recused themselves from the meeting) unanimously determined that the Amalgamation Agreement and the Transactions, including the Amalgamation, are fair to, advisable and in the best interests of the Company; |
| · | the Special Committee was assisted in negotiations with the Buyer Consortium and in its evaluation of the Amalgamation by Duff & Phelps LLC as its financial advisor and Weil, Gotshal & Manges LLP, Leslie-Ann Brissett Legal Services and Haiwen & Partners as its legal advisors; |
| · | the Buyer Consortium did not participate in or have any influence over the deliberative process of, or the conclusions reached by, the Special Committee or the negotiating positions of the Special Committee; |
| · | the recognition by the Special Committee and the Board that it had no obligation to recommend the Amalgamation or any other transaction; |
| · | the recognition by the Special Committee and the Board that, under the Amalgamation Agreement, it has the ability to consider any acquisition proposal that constitutes, or could reasonably be expected to result in, a Superior Proposal (as defined under “The Amalgamation Agreement—No Solicitation of Competing Transactions” beginning on page 127) until the Company’s shareholders vote upon and authorize and approve the Amalgamation Agreement and the Transactions, including the Amalgamation; |
| · | the Company’s ability, subject to compliance with the Amalgamation Agreement, to terminate the Amalgamation Agreement prior to the receipt of shareholder approval in order to accept an alternative transaction proposed by a third party that is a Superior Proposal; |
| · | the Company has the ability, under certain circumstances, to specifically enforce the terms of the Amalgamation Agreement; |
| · | the Amalgamation is not conditioned on any financing being obtained by Parent or Amalgamation Sub, thus increasing the likelihood that the Amalgamation will be consummated and the Per Share Amalgamation Consideration will be paid to the Unaffiliated Holders; |
| 66 |
| · | the consideration to be paid to the Unaffiliated Holders in the Amalgamation is all cash, allowing the Unaffiliated Holders to immediately realize a certain and fair value for all of their Shares, without incurring brokerage and other costs typically associated with market sales; |
| · | the authorization and approval of the Amalgamation Agreement and the Transactions, including the Amalgamation, is subject to approval by the affirmative vote of holders of Shares representing at least two-thirds of the Shares present and voting in person or by proxy as a single class under the IBCA; and |
| · | the availability of dissenters’ rights to the Unaffiliated Holders who comply with all of the required procedures under the IBCA for exercising dissenters’ rights, which allow such shareholders to receive payment of the fair value of their Shares as determined by the courts of the Antigua and Barbuda. |
The Buyer Consortium’s consideration of the factors described above reflects its assessment of the fairness of the Per Share Amalgamation Consideration payable in the Amalgamation to the Unaffiliated Holders in relation to the going-concern value of the Company on a stand-alone basis. The Buyer Consortium implicitly considered the value of the Company in a sale as a going concern by taking into account the Company’s current and anticipated business, financial condition, results and operations, prospects and other forward-looking matters. The Buyer Consortium did not, however, explicitly calculate a stand-alone pre-Amalgamation going-concern value of the Company as a public company because the Company will have a significantly different capital structure following the Amalgamation. Therefore, the Buyer Consortium does not believe that the going-concern value of the Company is an appropriate indicator to determine the fairness of the Per Share Amalgamation Consideration. To the extent, however, that the pre-Amalgamation going-concern value of the Company was reflected in the public market trading price of Shares immediately prior to the announcement of the Buyer Consortium’s Proposal Letter on February 1, 2016, the Per Share Amalgamation Consideration of US$7.00 represents a significant premium to the going-concern value of the Company.
The Buyer Consortium did not consider the liquidation value of the Company because the Buyer Consortium considered the Company to be a viable going concern and viewed the trading history of the Shares as an indication of the Company’s going-concern value and, accordingly, did not believe liquidation value to be relevant to a determination as to the fairness of the Amalgamation.
The Buyer Consortium did not consider net book value, which is an accounting concept, as a factor because it believed that net book value is not a material indicator of the value of the Company as a going concern but rather is indicative of historical costs and therefore not a relevant measure in the determination as to the fairness of the Amalgamation. The Buyer Consortium notes, however, that the Per Share Amalgamation Consideration was substantially higher than the net book value of the Shares disclosed in the Company’s Annual Report on Form 20-F for the year ended December 31, 2015 filed on April 25, 2016 with the SEC.
The members of the Buyer Consortium did not consider, any offers or proposals made by any unaffiliated person during the past two years for (a) a merger or consolidation of the Company with another company, (b) the sale or transfer of all or substantially all of the Company’s assets or (c) the purchase of all or a substantial portion of the Shares that would enable such person to exercise control of or significant influence over the Company.
| 67 |
Certain Financial Projections
The Company does not, as a matter of course, make public long-term financial projections as to future performance, earnings or other results beyond the current fiscal year given the inherent unpredictability of the underlying assumptions and estimates for extended periods. From time to time in connection with its internal evaluation of potential strategic options, the Company’s management prepares certain non-public, internal financial projections regarding the Company’s future operations and financial results.
On June 8, 2017, based on assumptions that the Company’s management believed to be reasonable at the time, the Company prepared non-public, internal financial projections for the fiscal years ending December 31, 2017 through December 31, 2025 (the “Management Projections”). The Special Committee considered the Management Projections in connection with its analysis of the Amalgamation and Duff & Phelps considered the Management Projections in connection with the delivery of its fairness opinion. You should not regard this information as an indication that the Company, the Board, the Special Committee, the Special Committee’s financial advisor or any of their respective representatives or affiliates, or any other recipient of this information, considered, or now considers, the Management Projections to be predictive of future results.
The following table summarizes the Management Projections:
| Management Projections | ||||||||||||||||||||||||||||||||||||
| Fiscal Year Ending December 31, | ||||||||||||||||||||||||||||||||||||
| 2017E | 2018E | 2019E | 2020E | 2021E | 2022E | 2023E | 2024E | 2025E | ||||||||||||||||||||||||||||
| (in RMB thousands except percentage) | ||||||||||||||||||||||||||||||||||||
| Net Revenues | 800,959 | 905,501 | 1,146,391 | 1,438,645 | 1,531,359 | 1,601,918 | 1,702,764 | 1,789,683 | 1,855,513 | |||||||||||||||||||||||||||
| % Growth | 64.9% | 13.1% | 26.6% | 25.5% | 6.4% | 4.6% | 6.3% | 5.1% | 3.7% | |||||||||||||||||||||||||||
| Gross Profit* | 655,247 | 758,870 | 941,016 | 1,180,755 | 1,265,422 | 1,318,974 | 1,394,141 | 1,474,889 | 1,533,936 | |||||||||||||||||||||||||||
| % Net Revenues | 81.8% | 83.8% | 82.1% | 82.1% | 82.6% | 82.3% | 81.9% | 82.4% | 82.7% | |||||||||||||||||||||||||||
| Operating Expenses* | 624,667 | 583,461 | 717,559 | 879,274 | 965,990 | 1,001,941 | 1,076,222 | 1,124,287 | 1,180,789 | |||||||||||||||||||||||||||
| % Net Revenues | 78.0% | 64.4% | 62.6% | 61.1% | 63.1% | 62.5% | 63.2% | 62.8% | 63.6% | |||||||||||||||||||||||||||
| Income from Continuing Operations | 607 | 135,983 | 174,539 | 251,393 | 250,607 | 272,307 | 273,919 | 307,596 | 309,462 | |||||||||||||||||||||||||||
| % Net Revenues | 0.1% | 15.0% | 15.2% | 17.5% | 16.4% | 17.0% | 16.1% | 17.2% | 16.7% | |||||||||||||||||||||||||||
| Net Income (loss) attributable to shareholders | (6,091 | ) | 93,013 | 118,007 | 175,707 | 171,196 | 191,226 | 188,259 | 216,698 | 214,673 | ||||||||||||||||||||||||||
% Net Revenues | (0.8% | ) | 10.3% | 10.3% | 12.2% | 11.2% | 11.9% | 11.1% | 12.1% | 11.6% | ||||||||||||||||||||||||||
* Management projected gross profit and operating expenses include depreciation and amortization.
| 68 |
The Company did not prepare the Management Projections with a view to public disclosure. The Company did not prepare the Management Projections with a view to compliance with GAAP, the published guidelines of the SEC regarding projections and forward-looking statements or the guidelines established by the American Institute of Certified Public Accountants for preparation and presentation of prospective financial information. In addition, Ernst & Young Hua Ming LLP, our independent registered public accounting firm, has not examined, reviewed or otherwise applied procedures to the Management Projections.
The Management Projections reflect numerous variables, assumptions and estimates as to future events made by our management that our management believed were reasonable at the time management prepared the Management Projections, taking into account the relevant information available at such time. However, this information is not fact and you should not rely on this information as being necessarily indicative of actual future results.
Important factors may affect actual results and prevent the achievement of the Management Projections. These factors include general economic conditions, the Company’s results and financial condition, accuracy of certain accounting assumptions, changes in actual or projected cash flows, competitive pressures, industry performance, accuracy of certain industry forecasts prepared by third parties, costs or adverse effects on our business, reputation or results from governmental regulations and changes in laws. In addition, the Management Projections do not take into account any circumstances or events occurring after the date that they were prepared and do not give effect to the Amalgamation.
As a result, there can be no assurance that the Management Projections will be realized, and actual results may be materially better or worse than those contained in the Management Projections. Since the Management Projections cover multiple years, that information by its nature becomes less predictive with each successive year. The inclusion of this information should not be regarded as an indication that the Company, the Board, the Special Committee, the Special Committee’s financial advisor, the Buyer Consortium, Parent or their respective representatives or affiliates, or any other recipient of this information, considered, or now considers, the Management Projections to be material information of the Company. Actual results may differ from the Management Projections, and you should not rely upon the Management Projections.
The summary of the Management Projections is not included herein to induce any shareholder to vote in favor of the Amalgamation or any of the other proposals to be voted on at the special meeting or to influence any shareholder to make any investment decision with respect to the Amalgamation, including whether or not to exercise dissenters’ rights with respect to the Amalgamation. You should evaluate the Management Projections, if at all, in conjunction with the historical financial statements, risk factors and other information regarding the Company contained in our public filings with the SEC. See “Where You Can Find More Information” on page 154 of this proxy statement.
| 69 |
The Management Projections are forward-looking statements. For information on factors that may cause the Company's future results to materially vary, see “Cautionary Note Regarding Forward-Looking Statements” on page 152 of this proxy statement.
We do not intend, and expressly disclaim any responsibility, to update or otherwise revise the Management Projections to reflect circumstances existing after the date when the Company prepared the Management Projections or to reflect future events or changes in general economic or industry conditions, even if the assumptions underlying the Management Projections are in error. By including a summary of certain financial projections in this proxy statement, neither the Company, the Board, the Special Committee or any of their respective representatives or affiliates, nor the Buyer Consortium, Parent or any of their respective representatives or affiliates, makes any representation to any person regarding the ultimate performance of the Company compared to the information contained in such financial projections.
In light of the foregoing factors and the uncertainties inherent in the Management Projections, shareholders are cautioned not to unduly rely on the Management Projections included herein.
Opinion of the Special Committee’s Financial Advisor
Pursuant to an engagement letter dated April 12, 2016, the Special Committee retained Duff & Phelps as its financial advisor to deliver a fairness opinion in connection with the Amalgamation. Duff & Phelps is an internationally recognized financial services firm that, among other things, is regularly engaged in the investment banking business, including the valuation of businesses and securities in connection with mergers and acquisitions, public offerings and private placements of securities, and other investment banking services.
At the meeting of the Special Committee on June 26, 2017, Duff & Phelps rendered its oral opinion (which was confirmed in writing by delivery of its written opinion later that same day) to the Special Committee that, as of such date and based upon and subject to the factors, assumptions, and limitations set forth in its opinion, the Per Share Amalgamation Consideration to be received by the holders of Shares (other than the Excluded Shares, the Dissenting Shares and Company Restricted Shares) in the Amalgamation is fair, from a financial point of view, to such holders (without giving effect to any impact of the Amalgamation on any particular holder of the Shares other than in its capacity as a holder of Shares). The Special Committee did not place any limitations upon Duff & Phelps with respect to the investigations made or procedures followed by it in rendering its opinion.
The full text of the written opinion of Duff & Phelps dated June 26, 2017, which sets forth the procedures followed, assumptions made, matters considered and qualifications and limitations on the review undertaken, is attached as Annex D to this proxy statement and is incorporated herein by reference. The summary of the opinion of Duff & Phelps set forth in this proxy statement is qualified in its entirety by reference to the full text of such opinion. The shareholders of the Company are urged to read the opinion in its entirety. Duff & Phelps’ written opinion is addressed to the Special Committee (in its capacity as such), is directed only to the Per Share Amalgamation Consideration and does not constitute a recommendation to any shareholder of the Company as to how such shareholder should vote or act with respect to the Amalgamation or any other matter. Duff & Phelps did not recommend any specific amount of consideration for the Amalgamation or that any specific amount of consideration constituted the only appropriate consideration for the Amalgamation. Duff & Phelps has consented to the inclusion of its opinion in its entirety and the description thereof in this proxy statement and any other filing the Company is required to make with the SEC in connection with the Amalgamation if such inclusion is required by applicable law.
| 70 |
In connection with its opinion, Duff & Phelps made such reviews, analyses and inquiries as it deemed necessary and appropriate under the circumstances. Duff & Phelps also took into account its assessment of general economic, market and financial conditions, as well as its experience in securities and business valuation, in general, and with respect to similar transactions, in particular. Duff & Phelps’ procedures, investigations, and financial analyses with respect to the preparation of its opinion included, but were not limited to, the items summarized below:
| · | reviewed the following documents: |
| o | the Company’s annual reports and audited financial statements on Form 20-F filed with the SEC for the years ended December 31, 2014 and 2015; |
| o | the Company’s unaudited financial statements for the year ended December 31, 2016 contained in a draft Form 20-F provided to Duff & Phelps by the Company’s management; |
| o | a detailed financial projection model for the years ending December 31, 2017 through 2025, prepared and provided to Duff & Phelps by the Company’s management, upon which Duff & Phelps relied, with the Special Committee’s consent, in performing its analysis (the “Management Projections”); |
| o | other internal documents relating to the history, past and current operations, financial conditions, and probable future outlook of the Company, provided to Duff & Phelps by the Company’s management; |
| o | a letter dated June 21, 2017 from the Company’s management, which made certain representations as to the Management Projections and the underlying assumptions with respect to the Company (the “Management Representation Letter”); and |
| o | documents related to the Amalgamation, including a draft of the Amalgamation Agreement dated June 25, 2017; |
| · | discussed the information referred to above and the background and other elements of the Amalgamation with the Company’s management; |
| · | discussed with the Company’s management its plans and intentions with respect to the management and operation of the Company’s business; |
| 71 |
| · | reviewed the historical trading price and trading volume of the Shares and the publicly traded securities of certain other companies that Duff & Phelps deemed relevant; |
| · | performed certain valuation and comparative analyses using generally accepted valuation and analytical techniques including a discounted cash flow analysis, an analysis of selected public companies that Duff & Phelps deemed relevant, an analysis of selected transactions that Duff & Phelps deemed relevant, and a review of premiums paid in selected transactions that Duff & Phelps deemed relevant; and |
| · | conducted such other analyses and considered such other factors as Duff & Phelps deemed necessary or appropriate. |
In performing its analyses and rendering its opinion with respect to the Amalgamation, Duff & Phelps, with the Company’s and the Special Committee’s consent and without independent verification:
| · | relied upon the accuracy, completeness, and fair presentation of all information, data, advice, opinions and representations obtained from public sources or provided to it from private sources, including the Company’s management; |
| · | relied upon the fact that the Special Committee, the Board and the Company had been advised by counsel as to all legal matters with respect to the Amalgamation, including whether all procedures required by law to be taken in connection with the Amalgamation had been duly, validly and timely taken; |
| · | assumed that any estimates, evaluations, forecasts and projections including, without limitation, the Management Projections, furnished to Duff & Phelps were reasonably prepared and based upon the best currently available information and good faith judgment of the person furnishing the same, and Duff & Phelps expressed no view or opinion with respect to such estimates, evaluations, forecasts or projections or their underlying assumptions; |
| · | assumed that the information relating to the Company and the Amalgamation provided to Duff & Phelps and the representations made by the Company’s management regarding the Company and the Amalgamation either orally or in writing were complete and accurate in all material respects, had not and did not omit to state a material fact in respect of the Company and the Amalgamation necessary to make the information not misleading in light of the circumstances under which the information was provided; |
| · | assumed that the representations and warranties made by all parties in the Amalgamation Agreement and in the Management Representation Letter were true and correct in all material respects and that each party to the Amalgamation Agreement would fully and duly perform all covenants, undertakings and obligations required to be performed by such party; |
| 72 |
| · | assumed that the final versions of all documents reviewed by Duff & Phelps in draft form, including the Amalgamation Agreement, would conform in all material respects to the drafts reviewed; |
| · | assumed that there had been no material change in the assets, liabilities, financial condition, cash flows, results of operations, business, or prospects of the Company since the date of the most recent financial statements and other information reviewed by Duff & Phelps, and that no information or facts would make the information reviewed by Duff & Phelps incomplete or misleading; |
| · | assumed that all of the conditions required to implement the Amalgamation would be satisfied and that the Amalgamation would be completed in accordance with the Amalgamation Agreement without any amendments thereto or any waivers of any terms or conditions thereof, and in a manner that complied in all material respects with all applicable laws; and |
| · | assumed that all governmental, regulatory or other consents and approvals necessary for the consummation of the Amalgamation would be obtained without any undue delay, limitation, restriction or condition that would have a material effect on the Company or the contemplated benefits expected to be derived in the Amalgamation. |
To the extent that any of the foregoing assumptions or any of the facts on which the opinion was based prove to be untrue in any material respect, the opinion cannot and should not be relied upon for any purpose. Furthermore, in Duff & Phelps’ analysis and in connection with the preparation of the opinion, Duff & Phelps made numerous assumptions with respect to industry performance, general business, market and economic conditions and other matters, many of which were beyond the control of any party involved in the Amalgamation and as to which Duff & Phelps did not express any view or opinion in the opinion, including as to the reasonableness of such assumptions.
Duff & Phelps prepared its opinion effective as of the date thereof. Its opinion was necessarily based upon the information made available to Duff & Phelps as of the date thereof and market, economic, financial and other conditions as they existed and could be evaluated as of the date thereof, and Duff & Phelps disclaimed any undertaking or obligation to (a) advise any person of any change in any fact or matter affecting its opinion which may come or be brought to the attention of Duff & Phelps after the date thereof or (b) update, revise or reaffirm its opinion after the date thereof.
Duff & Phelps did not evaluate the Company’s solvency or conduct an independent appraisal or physical inspection of any specific assets, properties or liabilities (contingent or otherwise) of the Company, and Duff & Phelps was not provided with any such appraisal or evaluation other than the contents of the Management Representation Letter. Duff & Phelps did not estimate, and expresses no opinion regarding, the liquidation value of any entity or business. Duff & Phelps has not been requested to, and did not, (a) initiate any discussions with, or solicit any indications of interest from, third parties with respect to the Amalgamation, the assets, businesses or operations of the Company, or any alternatives to the Amalgamation, or (b) advise the Special Committee or any other party with respect to alternatives to the Amalgamation. Duff & Phelps did not undertake an independent analysis of any potential or actual litigation, regulatory action, possible unasserted claims or other contingent liabilities, to which the Company is or may be a party or is or may be subject, or of any governmental investigation of any possible unasserted claims or other contingent liabilities to which the Company is or may be a party or is or may be subject.
| 73 |
In rendering its opinion, Duff & Phelps was not expressing any opinion as to the market price or value of the Shares (or anything else) after the announcement or the consummation of the Amalgamation (or any other time). Its opinion should not be construed as a valuation opinion, a credit rating, a solvency opinion, an analysis of the Company’s creditworthiness, tax advice or accounting advice. Duff & Phelps has not made, and assumes no responsibility to make, any representation, or render any opinion, as to any legal matter. Duff & Phelps expressly disclaims any responsibility or liability in this regard.
In rendering its opinion, Duff & Phelps was not expressing any opinion with respect to the amount or nature or any other aspect of any compensation payable to or to be received by any of the Company’s officers, directors or employees, or any class of such persons, relative to the Per Share Amalgamation Consideration, or with respect to the fairness of any such compensation. In addition, the opinion does not address the fairness to, or any other consideration of, the holders of any class of securities, creditors or other constituencies of the Company, other than the holders of the Shares (other than the Excluded Shares, the Dissenting Shares and Company Restricted Shares). Duff & Phelps’ opinion is furnished for the use and benefit of the Special Committee in connection with its consideration of the Amalgamation and is not intended to, and does not, confer any rights or remedies upon any other person, and is not intended to be used, and may not be used, by any other person or for any other purpose, without Duff & Phelps’ prior written consent.
Duff & Phelps has consented to the inclusion of its opinion in its entirety and the description thereof in this proxy statement and any other filing the Company is required to make with the SEC in connection with the Amalgamation if such inclusion is required by applicable law. The opinion (a) does not address the merits of the underlying business decision to enter into the Amalgamation versus any alternative strategy or transaction, (b) does not address any transaction related to the Amalgamation, (c) is not a recommendation as to how the Special Committee, the Board, the Company or any other person including security holders of the Company as to how such person should vote or act with respect to any matters relating to the Amalgamation, or whether to proceed with the Amalgamation or any related transaction, and (d) does not indicate that the Per Share Amalgamation Consideration is the best possibly attainable under any circumstances; instead, it merely states whether the Per Share Amalgamation Consideration is within a range suggested by certain financial analyses. The decision as to whether to proceed with the Amalgamation or any related transaction may depend on an assessment of factors unrelated to the financial analysis on which the opinion is based. The opinion should not be construed as creating any fiduciary duty on the part of Duff & Phelps to any party.
| 74 |
Set forth below is a summary of the material analyses performed by Duff & Phelps in connection with the delivery of its opinion to the Special Committee. This summary is qualified in its entirety by reference to the full text of the opinion, attached hereto as Annex D. While this summary describes the analyses and factors that Duff & Phelps deemed material in its presentation to the Special Committee, it is not a comprehensive description of all analyses and factors considered by Duff & Phelps. The preparation of a fairness opinion is a complex process that involves various determinations as to the most appropriate and relevant methods of financial analysis and the application of these methods to the particular circumstances. Therefore, a fairness opinion is not readily susceptible to partial analysis. In arriving at its opinion, Duff & Phelps did not attribute any particular weight to any analysis or factor considered by it, but rather made qualitative judgments as to the significance and relevance of each analysis and factor. Accordingly, Duff & Phelps believes that its analyses must be considered as a whole and that selecting portions of its analyses and of the factors considered by it in rendering the fairness opinion without considering all analyses and factors could create a misleading or incomplete view of the evaluation process underlying its opinion. The conclusion reached by Duff & Phelps was based on all analyses and factors taken as a whole, and also on the application of Duff & Phelps’ own experience and judgment.
The financial analyses summarized below include information presented in tabular format. In order for Duff & Phelps’ financial analyses to be fully understood, the tables must be read together with the text of each summary. The tables alone do not constitute a complete description of the financial analyses. Considering the data below without considering the full narrative description of the financial analyses, including the methodologies and assumptions underlying the analyses, could create a misleading or incomplete view of Duff & Phelps’ financial analyses.
Discounted Cash Flow Analysis
Duff & Phelps performed a discounted cash flow analysis of the estimated future unlevered free cash flows attributable to the Company for the fiscal years ending December 31, 2017 through December 31, 2025, with unlevered “free cash flow” defined as cash that is available either to reinvest or to distribute to security holders. The discounted cash flow analysis was used to determine the net present value of estimated future free cash flows utilizing a weighted average cost of capital as the applicable discount rate. For the purposes of its discounted cash flow analysis, Duff & Phelps utilized and relied upon the Management Projections, which are described in the section entitled “—Certain Financial Projections” beginning on page 68. The costs associated with the Company being a publicly listed company, as provided by the Company’s management, were excluded from the financial projections because such costs would likely be eliminated as a result of the Amalgamation.
Duff & Phelps estimated the net present value of all cash flows attributable to the Company after fiscal year 2025 (the “Terminal Value”) using a perpetuity growth formula assuming a 6.00% terminal growth rate, which took into consideration an estimate of the expected long-term growth rate of the Chinese economy and the Company’s business. Duff & Phelps used discount rates ranging from 11.00% to 13.00%, reflecting Duff & Phelps’ estimate of the Company’s weighted average cost of capital, to discount the projected free cash flows and the Terminal Value. Duff & Phelps estimated the Company’s weighted average cost of capital by estimating the weighted average of the Company’s cost of equity (derived using the capital asset pricing model) and the Company’s after-tax cost of debt. Duff & Phelps believes that this range of discount rates is consistent with the rate of return that security holders could expect to realize on alternative investment opportunities with similar risk profiles.
| 75 |
Based on these assumptions, Duff & Phelps’ discounted cash flow analysis resulted in an estimated enterprise value for the Company of RMB2,000.70 million to RMB2,932.50 million and a range of implied values of the Shares of US$4.10 to US$5.81 per Share.
The results of the discounted cash flow analysis are summarized as follows:
| 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 | 2025 | Terminal Value | |||||||||||||||||||||||||||||||
| (in RMB thousands) | ||||||||||||||||||||||||||||||||||||||||
| Free Cash Flow | -193,981 | 249,194 | -141,814 | 301,967 | 170,285 | 322,776 | 162,248 | 384,850 | 211,130 | 286,445 | ||||||||||||||||||||||||||||||
| Enterprise Value Range | Low | High | ||||||
| (in RMB thousands, except for percentages) | ||||||||
| Terminal Growth Rate | 6.00% | 6.00% | ||||||
| Weighted Average Cost of Capital | 13.00% | 11.00% | ||||||
| Enterprise Value Range | 2,000,700 | 2,932,500 | ||||||
Selected Public Companies and Merger and Acquisition Transactions Analyses
Duff & Phelps analyzed selected public companies and selected merger and acquisition transactions for purposes of estimating valuation multiples with which to calculate a range of implied enterprise values of the Company. This collective analysis was based on publicly available information and is described in more detail in the sections that follow.
None of the companies utilized for comparative purposes in the following analysis are directly comparable to the Company, and none of the transactions utilized for comparative purposes in the following analysis are directly comparable to the Amalgamation. Duff & Phelps does not have access to non-public information of any of the companies used for comparative purposes. Accordingly, a complete valuation analysis of the Company and the Amalgamation cannot rely solely upon a quantitative review of the selected companies and selected transactions, and involves complex considerations and judgments concerning differences in financial and operating characteristics of such companies and targets, as well as other factors that could affect their value relative to that of the Company. Therefore, the selected public companies and merger and acquisition transactions analysis is subject to certain limitations.
Selected Public Companies Analysis
Duff & Phelps compared certain financial information of the Company to corresponding data and ratios from publicly traded companies in the pharmaceutical and biotechnology industry that Duff & Phelps deemed relevant to its analysis. For purposes of its analysis, Duff & Phelps used certain publicly available historical financial data and consensus equity analyst estimates for the selected publicly traded companies. The 15 companies included in the selected public companies analysis in the pharmaceutical and biotechnology industry were:
| Global Vaccine Companies | • | Emergent BioSolutions Inc. |
| • | Panacea Biotec Limited | |
| • | Valneva SE | |
| • | Allergy Therapeutics plc |
| 76 |
| Global Large Pharmaceutical Companies | • | Pfizer Inc. |
| • | Merck & Co., Inc. | |
| • | GlaxoSmithKline plc | |
| • | Sanofi | |
| • | Eli Lilly and Company | |
| Foreign Listed Chinese Pharmaceutical Companies | • | CSPC Pharmaceutical Group Limited |
| • | Sino Biopharmaceutical Limited | |
| • | 3SBio Inc. | |
| • | China Biologic Products, Inc. | |
| • | Luye Pharma Group Ltd. | |
| • | China NT Pharma Group Company Limited |
Duff & Phelps selected these companies for its analysis based on their relative similarity, primarily in terms of business model, to that of the Company.
The tables below summarize certain observed trading multiples and historical and projected financial performance, on an aggregate basis, of the selected public companies. The estimates for 2017, 2018 and 2019 in the tables below with respect to the selected public companies were derived based on information for the 12-month periods ending closest to the Company’s fiscal year ends for which information was available. Data related to the Company’s earnings before interest, taxes, depreciation and amortization (“EBITDA”) were adjusted for purposes of this analysis to eliminate public company costs and non-recurring income (expenses).
| Revenue Growth (%) | EBITDA Growth (%) | EBITDA Margin (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3-YR CAGR(1) | LTM(2) | 2017 | 2018 | 2019 | 3-YR CAGR | LTM | 2017 | 2018 | 2019 | 3-YR CAGR | LTM | 2017 | 2018 | 2019 | ||||||||||||||||||||||||||||||||||||||||||||||
| Global Vaccine Companies | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Emergent BioSolutions Inc. | 16.0 | 1.5 | 3.5 | 8.9 | 13.4 | 30.5 | -40.4 | 2.2 | 15.0 | 7.2 | 34.8 | 28.0 | 29.2 | 30.8 | 29.1 | |||||||||||||||||||||||||||||||||||||||||||||
| Panacea Biotec Limited | 2.2 | -16.6 | NA | NA | NA | NM | -7.4 | NM | NM | NM | 17.3 | 21.1 | NM | NA | NA | |||||||||||||||||||||||||||||||||||||||||||||
| Valneva SE | NM | 15.6 | 12.9 | 13.4 | -2.4 | NM | NM | NA | 122.3 | -7.8 | -20.3 | -1.0 | 8.0 | 15.6 | 14.8 | |||||||||||||||||||||||||||||||||||||||||||||
| Allergy Therapeutics plc | 7.3 | 36.3 | 14.1 | 18.2 | 10.6 | NM | NM | NM | NM | NM | -4.1 | -7.4 | -11.0 | -3.6 | -4.5 | |||||||||||||||||||||||||||||||||||||||||||||
| Group Median | 7.3 | 8.6 | 12.9 | 13.4 | 10.6 | 30.5 | -23.9 | 2.2 | 68.7 | -0.3 | 6.6 | 10.1 | 8.0 | 15.6 | 14.8 | |||||||||||||||||||||||||||||||||||||||||||||
| Global Large Pharma Companies | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pfizer Inc. | 0.8 | 3.1 | -0.2 | 2.7 | -0.2 | -5.1 | -3.0 | 10.5 | 4.9 | -0.6 | 39.4 | 37.1 | 41.5 | 42.4 | 42.2 | |||||||||||||||||||||||||||||||||||||||||||||
| Merck & Co., Inc. | -3.3 | 1.4 | 0.4 | 2.8 | 3.6 | 6.2 | 14.8 | -2.3 | 1.4 | -0.1 | 42.6 | 46.1 | 45.8 | 45.2 | 43.6 | |||||||||||||||||||||||||||||||||||||||||||||
| GlaxoSmithKline plc | 1.7 | 18.4 | 8.2 | 2.9 | 3.1 | 11.0 | -2.2 | 3.7 | 4.4 | 3.8 | 20.5 | 33.1 | 34.2 | 34.7 | 34.9 | |||||||||||||||||||||||||||||||||||||||||||||
| Sanofi | 3.5 | 5.2 | 5.8 | 2.6 | 4.0 | 2.6 | -2.1 | 6.1 | -4.1 | 10.1 | 33.3 | 32.1 | 33.6 | 31.5 | 33.3 | |||||||||||||||||||||||||||||||||||||||||||||
| Eli Lilly and Company | -2.8 | 7.0 | 4.1 | -0.1 | 5.1 | -8.4 | 10.7 | 22.1 | 6.2 | 6.5 | 25.1 | 26.1 | 29.7 | 31.5 | 32.0 | |||||||||||||||||||||||||||||||||||||||||||||
| Group Median | 0.8 | 5.2 | 4.1 | 2.7 | 3.6 | 2.6 | -2.1 | 6.1 | 4.4 | 3.8 | 33.3 | 33.1 | 34.2 | 34.7 | 34.9 | |||||||||||||||||||||||||||||||||||||||||||||
| Foreign Listed Chinese Pharma Companies | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CSPC Pharmaceutical Group Limited | 7.5 | 10.7 | 16.1 | 17.0 | 14.9 | 20.9 | 18.5 | 25.6 | 21.7 | 18.7 | 23.6 | 26.5 | 28.3 | 29.4 | 30.3 | |||||||||||||||||||||||||||||||||||||||||||||
| Sino Biopharmaceutical Limited | 16.9 | 13.0 | 8.2 | 11.8 | 13.3 | 22.7 | 19.0 | 8.7 | 15.4 | 17.3 | 21.4 | 23.0 | 22.6 | 23.3 | 24.1 | |||||||||||||||||||||||||||||||||||||||||||||
| 3SBio Inc. | 47.3 | 67.2 | 26.9 | 23.5 | 22.9 | 49.6 | 56.7 | 25.2 | 23.7 | 22.9 | 37.4 | 38.1 | 37.6 | 37.7 | 37.7 | |||||||||||||||||||||||||||||||||||||||||||||
| China Biologic Products, Inc. | 18.8 | 11.3 | 10.1 | 16.1 | 15.2 | 18.4 | 9.5 | 4.0 | 21.3 | 19.0 | 47.5 | 45.7 | 43.4 | 45.3 | 46.8 | |||||||||||||||||||||||||||||||||||||||||||||
| Luye Pharma Group Ltd. | 5.1 | 13.8 | 23.5 | 13.1 | 13.6 | 26.1 | 7.9 | 38.7 | 13.6 | 13.1 | 32.7 | 32.3 | 36.3 | 36.5 | 36.3 | |||||||||||||||||||||||||||||||||||||||||||||
| China NT Pharma Group Company Limited | 6.7 | 7.9 | NA | NA | NA | NM | 42.5 | NA | NA | NA | 18.0 | 23.8 | NA | NA | NA | |||||||||||||||||||||||||||||||||||||||||||||
| Group Median | 12.2 | 12.2 | 16.1 | 16.1 | 14.9 | 22.7 | 18.7 | 25.2 | 21.3 | 18.7 | 28.2 | 29.4 | 36.3 | 36.5 | 36.3 | |||||||||||||||||||||||||||||||||||||||||||||
| Aggregate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean | 9.1 | 13.1 | 10.3 | 10.2 | 9.0 | 15.9 | 9.6 | 13.1 | 20.5 | 9.2 | 24.6 | 27.0 | 29.2 | 30.8 | 30.8 | |||||||||||||||||||||||||||||||||||||||||||||
| Median | 5.9 | 10.7 | 8.2 | 11.8 | 10.6 | 18.4 | 9.5 | 8.7 | 14.3 | 8.6 | 25.1 | 28.0 | 33.6 | 31.5 | 33.3 | |||||||||||||||||||||||||||||||||||||||||||||
| Sinovac Biotech Ltd. | 3.3 | 14.7 | 64.9 | 13.1 | 26.6 | -17.1 | -16.4 | 6.4 | 237.1 | 20.5 | 17.0 | 13.3 | 8.6 | 25.6 | 24.4 | |||||||||||||||||||||||||||||||||||||||||||||
| (1) | CAGR = Compounded Annual Growth Rate |
| (2) | LTM = Last Twelve Months |
| 77 |
| Enterprise Value as a Multiple of | ||||||||||||||||||||||||||||||||||||||||||||||||
| LTM EBITDA | 2017 EBITDA | 2018 EBITDA | 2019 EBITDA | LTM EBIT | 2017 EBIT | 2018 EBIT | 2019 EBIT | LTM Revenue | 2017 Revenue | 2018 Revenue | 2019 Revenue | |||||||||||||||||||||||||||||||||||||
| Global Vaccine Companies | ||||||||||||||||||||||||||||||||||||||||||||||||
| Emergent BioSolutions Inc. | 10.4 | x | 9.9 | x | 8.6 | x | 8.0 | x | 14.4 | x | 11.4 | x | 9.3 | x | 8.1 | x | 2.90 | x | 2.88 | x | 2.65 | x | 2.33 | x | ||||||||||||||||||||||||
| Panacea Biotec Limited | 16.0 | NA | NA | NA | 38.7 | NA | NA | NA | 3.37 | NA | NA | NA | ||||||||||||||||||||||||||||||||||||
| Valneva SE | NM | 29.9 | 13.4 | 14.6 | NM | NM | 16.2 | 48.2 | 2.57 | 2.38 | 2.10 | 2.15 | ||||||||||||||||||||||||||||||||||||
| Allergy Therapeutics plc | NM | NM | NM | NM | NM | NM | NM | NM | 2.00 | 2.16 | 1.83 | 1.65 | ||||||||||||||||||||||||||||||||||||
| Group Median | 13.2 | x | 19.9 | x | 11.0 | x | 11.3 | x | 26.6 | x | 11.4 | x | 12.8 | x | 28.2 | x | 2.73 | x | 2.38 | x | 2.10 | x | 2.15 | x | ||||||||||||||||||||||||
| Global Large Pharma Companies | ||||||||||||||||||||||||||||||||||||||||||||||||
| Pfizer Inc. | 11.8 | x | 10.5 | x | 10.1 | x | 10.1 | x | 16.7 | x | 11.5 | x | 11.1 | x | 10.9 | x | 4.39 | x | 4.38 | x | 4.26 | x | 4.27 | x | ||||||||||||||||||||||||
| Merck & Co., Inc. | 10.0 | 10.0 | 9.9 | 9.9 | 13.6 | 13.5 | 12.6 | 11.8 | 4.61 | 4.60 | 4.47 | 4.32 | ||||||||||||||||||||||||||||||||||||
| GlaxoSmithKline plc | 10.3 | 9.6 | 9.2 | 8.9 | 12.1 | 11.7 | 11.3 | 10.6 | 3.42 | 3.29 | 3.20 | 3.10 | ||||||||||||||||||||||||||||||||||||
| Sanofi | 7.6 | 7.1 | 7.4 | 6.7 | 10.2 | 8.9 | 8.6 | 8.2 | 2.45 | 2.38 | 2.32 | 2.23 | ||||||||||||||||||||||||||||||||||||
| Eli Lilly and Company | 16.0 | 13.7 | 12.9 | 12.1 | 21.7 | 16.3 | 15.6 | 13.8 | 4.17 | 4.07 | 4.07 | 3.88 | ||||||||||||||||||||||||||||||||||||
| Group Median | 10.3 | x | 10.0 | x | 9.9 | x | 9.9 | x | 13.6 | x | 11.7 | x | 11.3 | x | 10.9 | x | 4.17 | x | 4.07 | x | 4.07 | x | 3.88 | x | ||||||||||||||||||||||||
| Foreign Listed Chinese Pharma Companies | ||||||||||||||||||||||||||||||||||||||||||||||||
| CSPC Pharmaceutical Group Limited | 20.3 | x | 17.0 | x | 14.0 | x | 11.8 | x | 24.3 | x | 20.3 | x | 16.5 | x | 13.7 | x | 5.38 | x | 4.81 | x | 4.11 | x | 3.58 | x | ||||||||||||||||||||||||
| Sino Biopharmaceutical Limited | 11.3 | 10.8 | 9.4 | 8.0 | 12.6 | 11.8 | 10.6 | 9.0 | 2.58 | 2.44 | 2.18 | 1.93 | ||||||||||||||||||||||||||||||||||||
| 3SBio Inc. | 24.0 | 19.2 | 15.5 | 12.6 | 28.3 | 22.5 | 17.7 | 14.7 | 9.17 | 7.22 | 5.85 | 4.76 | ||||||||||||||||||||||||||||||||||||
| China Biologic Products, Inc. | 18.2 | 17.7 | 14.6 | 12.3 | 20.0 | 19.2 | 16.3 | 13.1 | 8.33 | 7.70 | 6.63 | 5.76 | ||||||||||||||||||||||||||||||||||||
| Luye Pharma Group Ltd. | 13.0 | 9.4 | 8.2 | 7.3 | 15.1 | 10.3 | 9.1 | 7.9 | 4.19 | 3.39 | 3.00 | 2.64 | ||||||||||||||||||||||||||||||||||||
| China NT Pharma Group Company Limited | 13.9 | NA | NA | NA | 15.4 | NA | NA | NA | 3.29 | NA | NA | NA | ||||||||||||||||||||||||||||||||||||
| Group Median | 16.0 | x | 17.0 | x | 14.0 | x | 11.8 | x | 17.7 | x | 19.2 | x | 16.3 | x | 13.1 | x | 4.79 | x | 4.81 | x | 4.11 | x | 3.58 | x | ||||||||||||||||||||||||
| Aggregate | ||||||||||||||||||||||||||||||||||||||||||||||||
| Mean | 14.1 | x | 13.7 | x | 11.1 | x | 10.2 | x | 18.7 | x | 14.3 | x | 12.9 | x | 14.2 | x | 4.19 | x | 3.98 | x | 3.59 | x | 3.28 | x | ||||||||||||||||||||||||
| Median | 13.0 | x | 10.7 | x | 10.0 | x | 10.0 | x | 15.4 | x | 11.8 | x | 11.9 | x | 11.3 | x | 3.42 | x | 3.39 | x | 3.20 | x | 3.10 | x | ||||||||||||||||||||||||
| 78 |
Selected Merger and Acquisition Transactions Analysis
Duff & Phelps compared the Company to the target companies involved in the selected merger and acquisition transactions listed in the tables below. The selection of these transactions was based on, among other things, the target company’s industry, the relative size of the transaction compared to the Amalgamation and the availability of public information related to the transaction. The selected pharmaceutical and biotechnology transactions indicated enterprise value to LTM EBITDA multiples ranging from 8.2x to 32.6x with a median of 14.6x, and enterprise value to LTM revenue multiples ranging from 1.10x to 27.58x with a median of 3.34x.
The Company is not directly comparable to the target companies in the selected merger and acquisition transactions analysis given certain characteristics of the transactions and the target companies, including business and industry comparability and lack of recent relevant transactions. Therefore, although reviewed, Duff & Phelps did not select valuation multiples for the Company based on the selected merger and acquisition transactions analysis.
| 79 |
| Date Announced | Acquirer Name | Target Name | ||
| 03/29/2017 | Tiancheng International Investment Limited | Biotest Aktiengesellschaft | ||
| 01/26/2017 | Johnson & Johnson | Actelion Ltd | ||
| 01/08/2017 | Takeda Pharmaceuticals U.S.A., Inc. | ARIAD Pharmaceuticals, Inc. | ||
| 12/07/2016 | Guangdong Hec Technology Holding Co., Ltd | YiChang HEC ChangJiang Pharmaceutical Co., Ltd. | ||
| 09/11/2016 | LG Chem, Ltd. | LG Life Sciences, Ltd. | ||
| 08/18/2016 | Sichuan Goldstone Orient New Material Equipment Co., Ltd. | Asia Pharmaceutical Group (Hainan) Co., Ltd. | ||
| 8/5/2015 | Guangdong VTR Bio-Tech Co., Ltd. | Henan Lihua Pharmaceutical Company Limited | ||
| 5/13/2015 | Dirui Industrial Co., Ltd. | Ningbo Rui Bio-Technology Co., Ltd. | ||
| 04/29/2015 | Buyer group consisting of certain founders and executives and various funds | WuXi PharmaTech (Cayman) Inc. | ||
| 4/28/2015 | Guangdong Wens Foodstuff Group Co., Ltd. | Guangdong Dahuanong Animal Health Products Co., Ltd. | ||
| 03/29/2015 | Yantai Dongcheng Biochemicals Co., Ltd. | Chengdu Yunke Pharmaceutical Co., Ltd | ||
| 03/14/2015 | Anhui Anke Biotechnology (Group) Co., Ltd. | Shanghai Soho-Yiming Pharmaceuticals Co., Ltd. | ||
| 12/11/2013 | Emergent BioSolutions Inc. | Emergent BioSolutions Canada Inc. | ||
| 9/12/2012 | Jing Lou (Chairman and CEO) | 3SBio Inc. | ||
| 7/6/2012 | TPG Capital | ShangPharma Corporation | ||
| 5/9/2012 | Baizhong Xue (Chairman and CEO) | China Nuokang Bio-Pharmaceutical Inc. | ||
| 5/4/2012 | China Meheco Co., Ltd. | Henan Topfond Pharmaceutical Co., Ltd. |
Summary of Selected Public Companies / Merger and Acquisition Transactions Analyses
In order to estimate a range of enterprise values for the Company, Duff & Phelps applied valuation multiples to the Company’s projected EBITDA for the fiscal years ending December 31, 2018 and December 31, 2019. The projected EBITDA amounts were adjusted for purposes of this analysis to eliminate public company costs and non-recurring income (expenses). Duff & Phelps’ selected valuation multiples were as follows: projected fiscal 2018 EBITDA multiples ranged from 10.0x to 12.0x and projected fiscal 2019 EBITDA multiples ranged from 8.0x to 11.0x.
| 80 |
Valuation multiples were selected taking into consideration historical and projected financial performance metrics of the Company relative to such metrics of the selected public companies. Rather than applying the average or median multiple from the public company set, Duff & Phelps selected multiples that in its judgment, reflected the Company’s size, growth outlook, capital requirements, profit margins, revenue mix, and other characteristics relative to the comparable group. Duff & Phelps noted that while it reviewed the selected merger and acquisition transactions, it did not select valuation multiples for the Company based on the selected merger and acquisition transactions for the reasons described in the section titled “Selected Merger and Acquisition Transactions Analysis” above.
Based on these analyses, Duff & Phelps’ selected public companies analysis resulted in an estimated enterprise value for the Company of RMB2,274.80 million to RMB2,925.20 million and a range of implied values of the Shares of US$4.61 to US$5.80 per Share.
Summary of Analyses
The range of estimated enterprise values for the Company that Duff & Phelps derived from its discounted cash flow analysis was RMB2,000.70 million to RMB2,932.50 million while from its public companies analysis was RMB2,274.80 million to RMB2,925.20 million. Duff & Phelps concluded that the Company’s enterprise value was within a range of RMB2,137.80 million to RMB2,928.90 million based on the analyses described above.
Based on the concluded enterprise value, Duff & Phelps estimated the range of common equity value of the Company to be RMB1,708.78 million to RMB2,330.27 million by:
| · | adding the estimated cash proceeds from the exercise of in-the-money options of RMB0.44 million based on an estimated enterprise value of RMB2,137.80 million or of RMB44.43 million based on an estimated enterprise value of RMB2,928.90 million; |
| · | adding non-current assets of RMB6.85 million as of December 31, 2016; |
| · | adding excess cash of RMB454.36 million as of December 31, 2016; |
| · | subtracting short-term loans of RMB233.17 million as of December 31, 2016; |
| · | subtracting long-term debt of RMB65.60 million as of December 31, 2016; |
| · | subtracting non-current liabilities of RMB2.60 million as of December 31, 2016; and |
| · | subtracting non-controlling interest of RMB589.31 million based on an estimated enterprise value of RMB2,137.80 million or of RMB802.91 million based on an estimated enterprise value of RMB2,928.90 million. |
| 81 |
Based on the foregoing analyses, Duff & Phelps estimated the value of each Share to range from US$4.35 to US$5.81 as of the date of its fairness opinion. Duff & Phelps noted that the Per Share Amalgamation Consideration to be received by the holders of Shares (other than the Excluded Shares, the Dissenting Shares and Company Restricted Shares) in the Amalgamation was above the range of the per Share value indicated by its analyses.
Duff & Phelps’ opinion was only one of the many factors considered by the Special Committee in its evaluation of the Amalgamation and should not be viewed as determinative of the views of the Special Committee.
Fees and Expenses
As compensation for Duff & Phelps’ services in connection with the rendering of its opinion to the Special Committee, the Company agreed to pay Duff & Phelps a fee of US$600,000, consisting of a nonrefundable retainer of US$300,000 payable upon engagement, and US$300,000 payable upon Duff & Phelps rendering the opinion.
The Special Committee also retained DPS, an affiliate of Duff & Phelps, to conduct, under the direction of the Special Committee, a market check process. For this engagement, the Company agreed to pay DPS a nonrefundable retainer of US$100,000 upon the Special Committee’s authorization of the market check
No portion of Duff & Phelps’ fee or DPS’ fee is refundable or contingent upon the consummation of a transaction, including the Amalgamation, or the conclusion reached in the opinion. The Company has also agreed to indemnify Duff & Phelps and DPS for certain liabilities arising out of their engagement. In addition, the Company has agreed to reimburse Duff & Phelps and DPS for their reasonable out-of-pocket expenses incurred in connection with their engagement, not to exceed US$60,000 in the aggregate.
The terms of the fee arrangements with Duff & Phelps and DPS, which the Company believes are customary in transactions of this nature, were negotiated at arm’s length, and the Special Committee and the Board are aware of these fee arrangements. Other than the DPS engagement described above and the Duff & Phelps engagement to render its opinion to the Special Committee, during the two years preceding the date of its opinion, Duff & Phelps did not have any material relationship with any party to the Amalgamation or any member of the Buyer Consortium for which compensation had been received or was intended to be received, nor was any such material relationship or related compensation mutually understood to be contemplated.
Buyer Consortium’s Purpose of and Reasons for the Amalgamation
Under the SEC rules governing going-private transactions, each member of the Buyer Consortium is deemed to be engaged in a going-private transaction and, therefore, required to express his, her or its reasons for the Amalgamation to the Company’s Unaffiliated Holders, as defined in Rule 13e-3 of the Exchange Act. Each member of the Buyer Consortium is making the statements included in this section solely for the purpose of complying with the requirements of Rule 13e-3 and related rules under the Exchange Act. For the Buyer Consortium, the purpose of the Amalgamation is to enable Parent to acquire 100% control of the Company, in a transaction in which the holders of the Shares (other than the Excluded Shares, the Dissenting Shares and the Rights) will be cashed out in exchange for US$7.00 per Share, such that Parent will bear the rewards and risks of the sole ownership of the Company after the Shares are cancelled, including any increases in value of the Company as a result of improvements to the Company’s operations or acquisitions of other businesses.
| 82 |
The Buyer Consortium decided to undertake the going-private transaction at this time because its wants to take advantage of the benefits of the Company’s being a privately held company as described above. In considering the going-private transaction, the Buyer Consortium did not consider alternative transaction structures because the Buyer Consortium believed the Amalgamation was the most direct and effective way to enable the Buyer Consortium to acquire ownership and control of the Company.
Effect of the Amalgamation on the Company
Private Ownership
The Shares are currently listed on NASDAQ under the symbol “SVA.” Following the consummation of the Amalgamation, the Company will cease to be a publicly-traded company and will become a private company beneficially owned by the Buyer Consortium. Following the consummation of the Amalgamation, the Shares will no longer be listed on any securities exchange or quotation system, including NASDAQ, and price quotations for the Shares in the public market will no longer be available.
In addition, registration of Shares under the Exchange Act may be terminated upon the Company’s application to the SEC if Shares are not listed on a national securities exchange and there are fewer than 300 record holders of Shares. Ninety days after the filing of Form 15 in connection with the consummation of the Amalgamation or such longer period as may be determined by the SEC, registration of Shares under the Exchange Act will terminate and the Company will no longer be required to file periodic reports with the SEC or otherwise be subject to the U.S. federal securities laws, including the Sarbanes-Oxley Act, applicable to public companies.
After the consummation of the Amalgamation, the Company’s shareholders will no longer enjoy the rights or protections that the U.S. federal securities laws provide, including reporting obligations for directors, officers and principal securities holders of the Company.
When the Amalgamation is completed, the Shares (other than the Excluded Shares and the Dissenting Shares) will be cashed out in exchange for the Amalgamation Consideration. The Rollover Shares will be cancelled in exchange for Holdco Shares in the Amalgamation. The other Excluded Shares will also be cancelled for no consideration in the Amalgamation. The Dissenting Shares will be cancelled for their fair value determined in accordance with the IBCA.
At the Effective Time, all vested and unvested awards granted under the Share Incentive Plans, including options to purchase Shares and shares of restricted stock, will be converted into a right to receive a cash payment equal to the product of the number of Shares subject to the award and the Per Share Amalgamation Consideration (net of the exercise price for each option to purchase Shares and net of any applicable withholding taxes). The Share Incentive Plans will terminate at the Effective Time.
| 83 |
Current shareholders will no longer have any equity interests in, or be shareholders of, the Company upon the consummation of the Amalgamation and will not participate in the earnings and growth of the Company or have the right to vote on corporate matters. Similarly, our current shareholders will not be exposed to the risk of loss in relation to their investment in the Company.
For a discussion of the Amalgamation Consideration to be received by our directors and executive officers and the effect of the Amalgamation on awards under our Share Incentive Plans, see “—Interests of the Certain Persons in the Amalgamation” beginning on page 92.
Articles of Incorporation and By-laws of the Surviving Corporation; Directors and Management of the Surviving Corporation
If the Amalgamation is completed, the articles of incorporation and by-laws of the Company will be replaced by the articles of incorporation and by-laws of Amalgamation Sub as in effect immediately prior to the completion of the Amalgamation (subject to certain differences, including that, at the Effective Time, (a) Article I of the articles of incorporation of the Surviving Corporation will be amended to read as follows: “The name of the Corporation is Sinovac Biotech Ltd.”; (b) the articles of incorporation and by-laws of the Surviving Corporation shall refer to the name of the Surviving Corporation as “Sinovac Biotech Ltd.” and (c) references therein to the authorized share capital of Amalgamation Sub shall be amended to refer to the authorized share capital of the Surviving Corporation).
In addition, the directors of Amalgamation Sub immediately prior to the completion of the Amalgamation will become the initial directors of the Surviving Corporation and the officers of the Company immediately prior to the completion of the Amalgamation will be the initial officers of the Surviving Corporation (in each case, unless otherwise determined by Parent).
Primary Benefits and Detriments of the Amalgamation
The primary benefits of the Amalgamation to the Unaffiliated Holders include the following:
| · | The Per Share Amalgamation Consideration of US$7.00 represents a premium of 22.8% to the closing price of the Shares as quoted by NASDAQ on June 23, 2017, the last trading day immediately prior to the date that the Company announced that it had entered into the Amalgamation Agreement, and a premium of 32.1% and 30% to the volume-weighted average trading price of the Shares as quoted by NASDAQ during the 30 and 60 trading days, respectively, immediately prior to the Company’s announcement on February 1, 2016 that it had received the Buyer Consortium’s Proposal Letter. |
| · | The Unaffiliated Holders will not bear the risk of a decrease in the value of the Company following the Amalgamation. |
| 84 |
The primary detriments of the Amalgamation to the Unaffiliated Holders include the following:
| · | The Unaffiliated Holders will cease to have an interest in the Company and will not benefit from possible increases in the value of the Company following the Amalgamation. |
| · | In general, the receipt of cash pursuant to the Amalgamation or through the exercise of dissenters’ rights will be a taxable transaction for U.S. federal income tax purposes and may also be a taxable transaction under other applicable tax laws. See “—Material U.S. Federal Income Tax Consequences,” “—Material Antigua and Barbuda Tax Consequences” and “—Material PRC Income Tax Consequences” beginning on page 97. |
The primary benefits of the Amalgamation to the Company’s directors and executive officers include the following:
| · | The continuing service of certain executive officers of the Company with the Surviving Corporation in positions that are substantially similar to their current positions. |
| · | Continued indemnification rights, rights to advancement of fees and directors and officers liability insurance to be provided by the Surviving Corporation to former directors and officers of the Company. |
| · | The treatment under the Amalgamation Agreement of vested and unvested awards granted to them under the Share Incentive Plans. |
The primary detriments of the Amalgamation to the Company’s directors and executive officers include the following:
| · | Certain director and officers of the Company who are presently shareholders of the Company will no longer benefit from possible increases in the future growth or value of the Company or payment of dividends on the Shares, if any. |
The primary benefits of the Amalgamation to the Buyer Consortium include the following:
| · | If the Company successfully executes its business strategies, the value of the Buyer Consortium’s equity investment could increase because of possible increases in future revenues, increases in the underlying value of the Company or the payment of dividends, if any, to Parent. |
| · | The Company will have more flexibility to change its capital spending strategies. |
| · | The Company will no longer bear the costs and administrative burden associated with operating the Company as a publicly traded company, including the costs associated with regulatory filings and compliance requirements. The Company estimates that it will save approximately US$2.9 million per year as a result of ceasing to be a public company. The members of the Buyer Consortium, as the beneficial owners of the Company, will become the beneficiaries of such cost savings. |
| 85 |
| · | The Buyer Consortium may consider re-listing the Company’s equity on another stock exchange, which may have higher valuations. |
The primary detriments of the Amalgamation to the Buyer Consortium include the following:
| · | The Buyer Consortium will bear the risk of a decrease in the value of the Company following the Amalgamation. |
| · | The Buyer Consortium’s equity investment in the Company will involve substantial risk resulting from limited liquidity because there will be no trading market for the Surviving Corporation’s equity securities. |
| · | The business risks facing the Company will be borne by the Buyer Consortium. |
Effect of the Amalgamation on the Company’s Net Book Value and Net Loss
The table below shows the indirect interest in the Company’s net book value and the share of the Company’s net loss for the Buyer Consortium, immediately before and after the Amalgamation, based on the net book value of the Company as of December 31, 2016. The Company’s net book value as of December 31, 2016 was approximately US$115.8 million. The Company’s net loss for the year ended December 31, 2016 was US$0.6 million.
Ownership prior to the Amalgamation(1) | Ownership after the Amalgamation(2) | |||||||||||||||||||||||||||||||
| Net Book Value | Net Loss | Net Book Value | Net Loss | |||||||||||||||||||||||||||||
| US$ millions | % | US$ millions | % | US$ millions | % | US$ millions | % | |||||||||||||||||||||||||
| Mr. Weidong Yin | 12.21 | 10.54 | (0.06 | ) | 10.54 | 12.21 | 10.54 | (0.06 | ) | 10.54 | ||||||||||||||||||||||
| SAIF Partners IV L.P. | 21.75 | 18.78 | (0.11 | ) | 18.78 | 21.75 | 18.78 | (0.11 | ) | 18.78 | ||||||||||||||||||||||
| C-Bridge Healthcare Fund II, L.P. | — | — | — | — | 33.48 | 28.91 | (0.17 | ) | 28.91 | |||||||||||||||||||||||
| Advantech Capital L.P. | — | — | — | — | 33.48 | 28.91 | (0.17 | ) | 28.91 | |||||||||||||||||||||||
| Vivo Capital Fund VIII, L.P. | — | — | — | — | 13.09 | 11.30 | (0.07 | ) | 11.30 | |||||||||||||||||||||||
| Vivo Capital Surplus Fund VIII, L.P. | — | — | — | — | 1.81 | 1.56 | (0.01 | ) | 1.56 | |||||||||||||||||||||||
(1) Ownership percentages based on 57,019,261 issued and outstanding Shares and 1,285,900 Shares issuable under the outstanding Company Options granted under the Share Incentive Plans as of June 23, 2017.
(2) Ownership percentages are subject to adjustment based on the results of the Buyer Consortium’s co-investment and syndication process.
| 86 |
Net Book Value per Share of Our Shares
The net book value per Share as of December 31, 2016 was US$2.03 based on the number of issued and outstanding Shares as of December 31, 2016.
Plans for the Company after the Amalgamation
After the Effective Time, Parent anticipates that the Company will conduct its operations substantially as it currently conducts its operations, except that the Company will cease to be a publicly traded company and will instead be a wholly-owned subsidiary of Parent. See “— Financing of the Amalgamation” beginning on page 90 for additional information.
Other than as described in this proxy statement and transactions already under consideration by the Company, no present plans or proposals relate to or would result in an extraordinary corporate transaction involving the Company’s corporate structure, business or management, such as a merger, reorganization, liquidation, relocation of any material operations, or sale or transfer of a material amount of assets. However, the Buyer Consortium will continue to evaluate the Company’s entire business and operations from time to time, and may propose or develop plans and proposals which they consider to be in the best interests of the Company and its equity holders, including the disposition or acquisition of material assets, alliances, joint ventures and other forms of cooperation with third parties or other extraordinary transactions, including the possibility of relisting the Company or a substantial part of its business on another stock exchange. At this time, however, no actual agreement or understanding as to the particulars of the abovementioned plans has been determined or agreed upon.
Subsequent to the completion of the Amalgamation and the termination of the registration of the Shares under the Exchange Act, the Company will no longer be subject to the Exchange Act and NASDAQ compliance and reporting requirements or the related direct and indirect costs and expenses, and may experience positive effects on profitability as a result of the elimination of such costs and expenses.
Alternatives to the Amalgamation
The Special Committee was formed on January 31, 2016 in response to the receipt of the Buyer Consortium’s Proposal Letter from the Buyer Consortium (which at that time comprised Mr. Yin and SAIF and/or its affiliates) on January 30, 2016.
On February 4, 2016, following the Company’s announcement of its receipt of the Buyer Consortium’s Proposal Letter, the Board received the Sinobioway Consortium’s Proposal Letter from the Sinobioway Consortium (which at the time comprised of PKU Shanghai, Shangdong Sinobioway, CICC Qianhai, Beijing Sinobioway, Heng Fend and Fuerde), pursuant to which the Sinobioway Consortium indicated its interest in acquiring all of the Company’s outstanding Shares for US$7.00 in cash per Share. The Sinobioway Proposed Transaction contemplated a transaction, structured, in light of the Buyer Consortium’s ownership at such time of approximately 29.5% of the voting power of the issued and outstanding Shares, as a two-step amalgamation, with a first-step tender offer for the Shares (conditioned on the tender of Shares representing more than 50% of the voting power of the issued and outstanding Shares) followed by a second-step amalgamation in the event the Sinobioway Consortium acquired at least 66.67% of the issued and outstanding Shares in the tender offer. From February 4, 2016 to May 22, 2017, the Special Committee and its financial and legal advisors held extensive discussions and negotiations with the Sinobioway Consortium with respect to the Sinobioway Proposal Transaction, as further discussed in “—Background of the Amalgamation” beginning on page 18.
| 87 |
In light of the following factors and the considerations set forth in “—Reasons for the Amalgamation and Recommendation of the Special Committee and the Board” beginning on page 54, the Special Committee determined that the Sinobioway Proposed Transaction did not constitute a, and there was no other, viable alternative to the proposed sale of the Company to the Buyer Consortium:
| · | the express intention of the members of the Buyer Consortium not to sell their Shares to any third party, and the Buyer Consortium’s aggregate beneficial ownership of approximately 29.5% of the total voting power of the issued and outstanding Shares (as of the date of this proxy statement), which is nearly the one-third of the voting power sufficient to prevent the consummation of any transaction with a third party that requires shareholder approval; |
| · | in the market check conducted by DPS under the direction of the Special Committee, all the parties deemed to be potentially interested in and capable of acquiring the Company and contacted by DPS declined to engage in discussions with the Special Committee regarding any such acquisition; and |
| · | since the announcement of the Proposed Transaction on February 1, 2016, no party other than the members of the Buyer Consortium and the members of the Sinobioway Consortium had made any proposal to the Company or the Special Committee with respect to any alternative transaction with the Company. |
On June 28, 2017, following the Company’s announcement of the execution of the Transaction Documents, the Special Committee received a proposal letter from the Sinobioway Consortium setting forth the Revised Sinobioway Proposal pursuant to which it proposed to acquire the Company’s outstanding Shares for US$8.00 in cash per Share. From June 29, 2017 to September 29, 2017, the Special Committee and its legal advisors submitted to the Sinobioway Consortium various questions and information requests to clarify and understand the terms and conditions of the Revised Sinobioway Proposal in order to determine whether the Revised Sinobioway Proposal constituted, or could reasonably be expected to result in, a Superior Proposal in accordance with the terms of the Amalgamation Agreement, as further discussed in “—Background of the Amalgamation” beginning on page 18.
On October 11, 2017, based on the responses as of such date provided by the Sinobioway Consortium to the Special Committee and given the time that had elapsed since the Sinobioway Consortium had made the Revised Sinobioway Proposal and in light of the considerations set forth in “—Reasons for the Amalgamation and Recommendation of the Special Committee and the Board” beginning on page 54, the Special Committee determined that the Revised Sinobioway Proposal did not constitute, and could not reasonably be expected to result in, a Superior Proposal.
| 88 |
In addition, the Special Committee and the Board considered, as an alternative to the Amalgamation, that the Company remain a public company. However, based on the considerations set forth under “—Reasons for the Amalgamation and Recommendation of the Special Committee and the Board” beginning on page 54, the Special Committee and the Board have concluded that it was more beneficial to the Unaffiliated Holders for the Company to enter into the Amalgamation Agreement and pursue the consummation of the Transactions, including the Amalgamation, and become a private company rather than to remain a public company. The Special Committee also took into account that, prior to the receipt of shareholder approval, the Company, subject to compliance with the terms and conditions of the Amalgamation Agreement, can terminate the Amalgamation Agreement in order to accept an alternative transaction proposed by a third party that is a Superior Proposal, subject to the payment of a termination fee of US$15.0 million, as provided in the Amalgamation Agreement. In this regard, the Special Committee recognized that it has flexibility under the Amalgamation Agreement to respond to an alternative transaction proposed by a third party that constitutes, or could reasonably be expected to result in, a Superior Proposal, including the ability to provide information to, and engage in discussions and negotiations with, such party (and, if such proposal is a Superior Proposal, recommend such proposal to the Company’s shareholders).
Effect on the Company if the Amalgamation is not Completed
If the Amalgamation Agreement is not authorized and approved by the Company’s shareholders or if the Amalgamation is not completed for any other reason, the Company’s shareholders will not receive any payment for their Shares in connection with the Amalgamation. Instead, the Company will remain a publicly traded company and continue to be listed and traded on NASDAQ, provided that the Company continues to meet NASDAQ’s listing requirements, and the Company will remain subject to SEC reporting obligations.
Therefore, the Company’s shareholders will be subject to similar risks and opportunities as they currently are with respect to their ownership of our Shares. Accordingly, if the Amalgamation is not completed, there can be no assurance as to the effect of these risks and opportunities on the future value of your Shares.
Under specified circumstances in which the Amalgamation Agreement is terminated, the Company may be required to pay Parent a termination fee of US$15,000,000, or Parent may be required to pay the Company a termination fee of US$15,000,000, in each case, as described under the caption “The Amalgamation Agreement—Termination Fees” beginning on page 141.
If the Amalgamation is not completed, from time to time, the Board will evaluate and review, among other things, the business, operations, dividend policy and capitalization of the Company and make changes it deems appropriate and seek to identify strategic alternatives to enhance shareholder value. If the Company’s shareholders do not authorize and approve the Amalgamation Agreement or if the Amalgamation is not completed for any other reason, there can be no assurance that any other transaction acceptable to the Company will be available. As a result, the business, prospects or results of operations of the Company may be adversely impacted.
| 89 |
Financing of the Amalgamation
The Company and the Buyer Consortium estimate that the total amount of funds necessary to complete the Transactions, including the Amalgamation, is approximately US$ (assuming no exercise of dissenters’ rights by shareholders of the Company). In calculating this amount, the Company and the Buyer Consortium did not consider the value of the Rollover Shares, which will be canceled for no consideration pursuant to the Amalgamation Agreement. This amount includes (a) the cash to be paid to the Unaffiliated Holders, and (b) the related costs and expenses, in connection with the Transactions, including the Amalgamation.
Concurrently with the execution of the Amalgamation Agreement on June 26, 2017, each of the Sponsors entered into an Equity Commitment Letter with Holdco, Parent and the Company, dated as of June 26, 2017.
Pursuant to these Equity Commitment Letters, the Sponsors have committed to make, or cause to be made, subject to the terms and conditions therein, equity contributions to Holdco in the following amounts, prior to the Closing in connection with the Amalgamation: (i) US$116,145,527 by Advantech Capital, (ii) US$116,145,527 by C-Bridge Capital, (iii) US$45,412,428 by Vivo Capital Fund and (iv) US$6,270,912 by Vivo Capital Surplus Fund (collectively, the “Holdco Contribution”). The total proceeds of the Holdco Contribution to Holdco under the Equity Commitment Letters shall promptly upon receipt be further contributed by Holdco, as an equity contribution to Parent (the “Holdco to Parent Contribution”). Proceeds of the Holdco to Parent Contribution are to be used to pay such portion of the amounts required to be paid pursuant to the Amalgamation Agreement and related fees and expenses. Notwithstanding anything else to the contrary in the Equity Commitment Letters, the aggregate amount of liability of each Sponsor under its respective Equity Commitment Letter shall at no time exceed the aggregate amount of its respective Holdco Contribution less any portion of such Holdco Contribution that has been funded in accordance with the terms thereof.
Limited Guarantees
Concurrently with the execution and delivery of the Amalgamation Agreement, the Guarantors executed and delivered the Limited Guarantees. Under the Limited Guarantees, each Guarantor has guaranteed in favor of the Company a portion of the payment obligations of Parent under the Amalgamation Agreement for the termination fee and certain costs and expenses that may become payable to the Company by Parent under certain circumstances set forth in the Amalgamation Agreement, provided that the maximum aggregate liability of each Guarantor under the respective Limited Guarantee shall not exceed a certain amount as specified in the respective Limited Guarantee.
Support Agreement
Concurrently with the execution and delivery of the Amalgamation Agreement, the Rollover Shareholders entered into the Support Agreement in favor of Parent and Holdco, pursuant to which, the Rollover Shareholders have agreed to, subject to the terms and conditions set forth therein and among other obligations, to (a) cancel the Rollover Shares held by such Rollover Shareholders for no consideration in connection with the Amalgamation, (b) subscribe for newly issued Holdco Shares immediately prior to the Closing and (c) vote all of the Shares owned directly or indirectly by the Rollover Shareholders in favor of the Amalgamation. A copy of the Support Agreement is attached as Annex C to this proxy statement and is incorporated herein by reference.
| 90 |
Interim Investors Agreement
Concurrently with the execution and delivery of the Amalgamation Agreement, Mr. Yin, SAIF, C-Bridge Capital, Advantech Capital, Vivo Capital, Holdco, Parent and Amalgamation Sub entered into an interim investors agreement, which governs the relationship among the parties thereto with respect to the Amalgamation Agreement and matters relating thereto until the termination of the Amalgamation Agreement or the consummation of the Amalgamation (the “Interim Investors Agreement”). The Interim Investors Agreement provides for, among other things and subject to certain limitations or exceptions therein, (a) the mechanism for making decisions of Holdco, Parent and Amalgamation Sub relating to the Amalgamation Agreement pending consummation of the Amalgamation, (b) the mechanism for making decisions relating to financing pending consummation of the Amalgamation, and (c) the mechanism for allocating certain fees and expenses among the Buyer Consortium.
Remedies and Limitations on Liability
The parties to the Amalgamation Agreement may be entitled to specific performance of the terms of the Amalgamation Agreement, including an injunction or injunctions to prevent breaches of the Amalgamation Agreement, in addition to any other remedy at law or equity.
The Company’s right to obtain an injunction or injunctions, or other appropriate form of specific performance or equitable relief, in each case, with respect to causing Parent and/or Amalgamation Sub to cause the equity financing from the Sponsors to be funded at any time is, however, subject to each of the following: (a) all of the conditions to the obligations of Parent and Amalgamation Sub to consummate the Amalgamation (other than those conditions that by their terms are to be satisfied at the Closing) have been satisfied or waived; (b) Parent and Amalgamation Sub fail to complete the Closing by the date the Closing is required to have occurred pursuant to the Amalgamation Agreement; and (c) the Company has confirmed in writing that (i) all of the conditions to the obligations of the Company to consummate the Amalgamation have been satisfied or that it is willing to waive any of such conditions and (ii) if specific performance is granted and the equity financing is funded, then the Company will take such actions that are within its control to cause the Amalgamation to occur.
The maximum aggregate liabilities of Parent and Amalgamation Sub, on one hand, and the Company, on the other hand, for monetary damages in connection with the Amalgamation Agreement are each limited to a termination fee of US$15 million, and reimbursement of certain expenses in the event the Company or Parent fails to pay the applicable termination fee when due and in accordance with the requirements of the Amalgamation Agreement. While a party may pursue both a grant of specific performance and monetary damages until such time as the other party pays a termination fee (as applicable under the Amalgamation Agreement), no party will be permitted or entitled to receive both a grant of specific performance that results in the Closing and monetary damages.
| 91 |
Interests of Certain Persons in the Amalgamation
In considering the recommendation of the Board, the Company’s shareholders should be aware that, aside from their interests in Shares of the Company, some of the Company’s directors and executive officers have interests in the Amalgamation that are different from, or in addition to, the interests of the Company’s shareholders generally. These interests include, among others:
Treatment of Director and Executive Officer Shares and Awards in the Amalgamation
As is the case for any shareholder, the Company’s directors and executive officers will receive the Per Share Amalgamation Consideration in respect of each Share in which they have interests. In addition, at the Effective Time, all vested and unvested awards granted under the Share Incentive Plans, including options to purchase Shares and shares of restricted stock, will be converted into a right to receive a cash payment equal to the product of the number of Shares subject to the award and the Per Share Amalgamation Consideration (net of the exercise price for each option to purchase Shares).
The following table shows, assuming an Effective Time prior to March 15, 2018 and assuming no options are exercised prior to the Effective Time, for each director and executive officer of the Company, (a) the number of Shares owned that will be cashed out at the Effective Time and the cash payment that will be made in respect of such Shares, (b) the number of Shares subject to options granted under the Share Incentive Plans that will be cashed out at the Effective Time and the cash payment that will be made in respect of such options and (c) the number of restricted shares granted under the Share Incentive Plans that will be cashed out at the Effective Time and the cash payment that will be made in respect of such restricted shares (in all cases before applicable withholding taxes).
| Shares | Company Options | Company Restricted Shares | ||||||||||||||||||||||
| Shares Owned | Cash Payment in US$ | Underlying Shares | Cash Payment in US$ | Underlying Shares | Cash Payment in US$ | |||||||||||||||||||
| Name of Directors and Executive Officers | ||||||||||||||||||||||||
| Weidong Yin | — | — | 150,000 | 303,000 | — | — | ||||||||||||||||||
| Simon Anderson | 50,000 | 350,000 | 40,000 | 80,800 | — | — | ||||||||||||||||||
| Yuk Lam Lo | 50,000 | 350,000 | 40,000 | 80,800 | — | — | ||||||||||||||||||
| Kenneth Lee | — | — | 40,000 | 80,800 | — | — | ||||||||||||||||||
| Meng Mei | — | — | 40,000 | 80,800 | — | — | ||||||||||||||||||
| Nan Wang | 75,000 | 525,000 | 90,000 | 181,800 | 30,000 | 210,000 | ||||||||||||||||||
| Ming Xia | 66,500 | 465,500 | 45,000 | 90,900 | 30,000 | 210,000 | ||||||||||||||||||
| Qiang Gao | 45,000 | 315,000 | 40,000 | 80,800 | 25,000 | 175,000 | ||||||||||||||||||
| Jing Li | 2,500 | 17,500 | 80,000 | 161,600 | 25,000 | 175,000 | ||||||||||||||||||
| 92 |
Indemnification and Insurance
Pursuant to the Amalgamation Agreement, Parent and Amalgamation Sub have agreed that:
| · | the indemnification, advancement and exculpation provisions of the indemnification agreements by and among the Company and its directors and certain executive officers as in effect at the Effective Time shall survive the Amalgamation and shall not be amended, repealed or otherwise modified for a period of six years in a manner that would adversely affect the rights thereunder of the current or former directors or officers of the Company or any of its subsidiaries; |
| · | the articles of incorporation and by-laws of the Surviving Corporation (or in such documents of any successor to the business of the Surviving Corporation) shall contain provisions no less favorable to the intended beneficiaries with respect to exculpation and indemnification of liability and advancement of expenses than are set forth in the articles of incorporation and by-laws of the Company (or in such documents of any successor to the business of the Surviving Corporation) as in effect on the date of the Amalgamation Agreement; |
| · | the Surviving Corporation (a) shall maintain in effect for six years from the Effective Time the current directors’ and officers’ fiduciary liability insurance policies maintained by the Company with respect to matters occurring prior to the Effective Time, including acts or omissions occurring in connection with the Amalgamation Agreement and the consummation of the Transactions on terms, conditions, retentions and limits of liability no less favorable to the indemnified parties than those in effect as of the Effective Time, provided that the cost of such policy does not exceed 300% of the current annual premium paid by the Company for such insurance and (b) may, and at Parent’s request shall, purchase a six-year “tail” prepaid policy prior to the Effective Time on terms, conditions, retentions and limits of liability no less advantageous to the indemnified parties than the existing directors’ and officers’ fiduciary liability insurance policies maintained by the Company; and |
| · | the Surviving Corporation will indemnify and hold harmless (a) the directors and executive officers against any and all costs or expenses (including reasonable attorneys’ fees and expenses), judgments, fines, losses, claims, damages, liabilities and amounts paid in settlement in connection with any actual or threatened claim, action, suit, proceeding or investigation, whether civil, criminal, administrative or investigative (“Damages”), arising out of, relating to or in connection with (i) the fact that an indemnified party is or was a director, officer or employee of the Company or any of its subsidiaries, or (ii) any acts or omissions occurring or alleged to have occurred (including acts or omissions with respect to the approval of the Amalgamation Agreement or the Transactions or arising out of or pertaining to the transactions and actions to enforce this provision or any other indemnification or advancement right of any indemnified party) prior to or at the Effective Time, to the extent provided under the Company’s or its subsidiaries’ respective organizational and governing documents or agreements in effect on the date of the Amalgamation Agreement and to the fullest extent permitted by the IBCA or any other applicable law (provided that such indemnification shall be subject to any limitation imposed from time to time under applicable law), and (b) such persons for any and all Damages arising out of acts or omissions in such persons’ official capacity as an officer, director or other fiduciary in the Company or any of its subsidiaries if such service was at the request or for the benefit of the Company or any of its subsidiaries. |
| 93 |
The Special Committee
On January 31, 2016, the Board established the Special Committee, after receiving the Buyer Consortium’s Proposal Letter. The Board formed the Special Committee to consider this proposal and to take any actions it deemed appropriate to assess the fairness and viability of any proposed transaction and other alternatives.
The Special Committee consists of three independent directors—Mr. Simon Anderson, Mr. Yuk Lam Lo and Mr. Meng Mei. The members of the Special Committee are free from any affiliation with the Buyer Consortium, are not members of the management of the Company, and, aside from any interests in the Shares of the Company, none of such directors has any financial interest in the Amalgamation that is different from that of the Unaffiliated Holders other than (i) the directors’ receipt of board compensation in the ordinary course, (ii) the ownership of options and restricted shares and (iii) the directors’ indemnification and liability insurance rights under the Amalgamation Agreement.
The Board did not place any limitations on the authority of the Special Committee regarding its investigation and evaluation of the Amalgamation. The chairman of the Special Committee receives monthly compensation of US$15,000 and each other member of the Special Committee receives monthly compensation of US$10,000 in exchange for their service on the Special Committee (the payment of which is not contingent upon the completion of the Amalgamation or the Special Committee’s or the Board’s recommendation of the Amalgamation).
Position with the Surviving Corporation
After completion of the Amalgamation, it is anticipated that the executive officers of the Company will hold positions with the Surviving Corporation that are substantially similar to their current positions.
Related Party Transactions
For a description of the Company’s related party transactions, please see “Item 7. Major Shareholders and Related Party Transactions” included in our Annual Report on Form 20-F for the fiscal year ended December 31, 2016, incorporated by reference into this proxy statement. Please see “Where You Can Find More Information” beginning on page 154 for a description of how to obtain a copy of our Annual Report.
| 94 |
Except for the transactions discussed above under this caption titled “Related Party Transactions” or the arrangements in connection with the Amalgamation discussed elsewhere in this proxy statement, during the past two years (i) there were no negotiations, transactions or material contacts between the Company and its affiliates, on the one hand, and any member of the Buyer Consortium, on the other hand, concerning any merger, consolidation, acquisition, tender offer for or other acquisition of any class of the Company’s securities; election of the Company’s directors or sale or other transfer of a material amount of assets of the Company; (ii) the Company and its affiliates did not enter into any other transaction with an aggregate value exceeding 1% of its consolidated revenues with any member of the Buyer Consortium, and (iii) none of the Company’s executive officers, directors or affiliates that is a natural person entered into any transaction during the past two years with an aggregate value (in respect of such transaction or series of similar transactions with that person) exceeding US$60,000 with any member of the Buyer Consortium.
Fees and Expenses
Estimated fees and expenses incurred or to be incurred by the Company and the Buyer Consortium in connection with the Amalgamation are set forth in the table below. The fees are subject to change pending completion of the Amalgamation.
| Description | Amount (US$) | |||
| Financing fees and expenses and other professional fees | ||||
| Legal fees and expenses | ||||
| Special Committee fees | ||||
| Miscellaneous (including accounting, filing fees, printer, proxy solicitation and mailing costs) | ||||
| Total | ||||
Voting by Buyer Consortium at the Special Meeting
Pursuant to the Support Agreement, each of the Rollover Shareholders has agreed, until the earlier of the Effective Time and the termination of the Amalgamation Agreement in accordance with its terms, to appear or otherwise cause its Shares to be counted as present for purposes of calculating a quorum and vote or otherwise cause to be voted all of its Shares:
| · | for authorization and approval of the Amalgamation Agreement; |
| · | against any Competing Transaction or other transaction, proposal, agreement or action made in opposition to the Amalgamation Agreement or in competition or inconsistent with the Amalgamation; |
| · | against any other action, agreement or transaction that is intended or could reasonably be expected to materially interfere with or adversely affect the Amalgamation; |
| · | against any action, proposal, transaction or agreement that would reasonably be expected to result in a breach of any covenant, representation or warranty or any other obligation or agreement of the Company in the Amalgamation Agreement, or of the Rollover Shareholders in the Support Agreement; |
| 95 |
| · | in favor of any adjournment of the special meeting, however called; and |
| · | in favor of any other matter necessary to effect the Transactions. |
Litigation Related to the Amalgamation
On July 12, 2017, a putative shareholder class action lawsuit (Haoquan Wu v. Sinovac Biotech Ltd. et. al., Index No. 654745/2017) was filed in the Supreme Court of the State of New York against Mr. Yin, Simon Anderson, Yul Lam Lo, Kenneth Lee, Meng Mei, the Company, Parent and Amalgamation Sub. The complaint alleges that the Company’s directors breached their fiduciary duties by, among other things, entering into the Transactions at an amalgamation consideration which deprives the plaintiff and the putative class of the true value of their investment in the Company.
The complaint also alleges that the Company, Parent and Amalgamation Sub aided and abetted those alleged breaches of fiduciary duties by the Company’s directors. The complaint seeks, among other things, declaration of the action as a proper class action, certification of the plaintiff as a representative of the class, enjoinment from consummating the Transactions, injunctive relief, damages, including rescissory damages, in favor of the plaintiff and the class, and the fees and costs associated with the litigation.
Accounting Treatment of the Amalgamation
The Amalgamation is expected to be accounted for, at historical cost, as a merger of entities under common control in accordance with Accounting Standards Codification 805-50, “Business Combinations—Related Issues.”
Regulatory Matters
The Company does not believe that any material federal or state regulatory approvals, filings or notices are required in connection with effecting the Amalgamation other than (a) the approvals, filings or notices required under the federal securities laws and (b) the filing of the Amalgamation Agreement (and supporting documentation as specified in Section 172 of the IBCA) with the Director of the Financial Services Regulatory Commission of Antigua and Barbuda. Adequate notice of the Amalgamation must also be provided to certain creditors of the Company and Amalgamation Sub. Pursuant to Section 172 of the IBCA, adequate notice is given if (i) a notice in writing is sent to each known creditor having a claim against Amalgamation Sub or the Company that exceeds one thousand dollars, (ii) a notice of the Amalgamation is published in a newspaper published or distributed in Antigua and Barbuda and (iii) each notice states that Amalgamation Sub intends to amalgamate with the Company and that a creditor can object to the Amalgamation within thirty days from the date of the notice.
Dissenters’ Rights
Registered holders of Shares who exercise dissenters’ rights will have the right to receive payment of the fair value of their Shares in accordance with Section 191 of the International Business Corporations Act, CAP. 222 of the Revised Laws of Antigua and Barbuda (“IBCA”) if the Amalgamation is consummated. To exercise their dissenters’ rights, registered holders of Shares must deliver to the Company, at or before any meeting of shareholders of the Company at which a resolution to amalgamate is to be voted on, a written dissent from the resolution (unless the Company did not give notice to the shareholder of the purpose of the meeting and of his right to dissent) and subsequently comply with all procedures and requirements of Sections 191 to 199 of the IBCA for the exercise of dissenters’ rights.
| 96 |
The fair value of a registered holder’s Shares as determined under the IBCA could be more than, the same as, or less than the Amalgamation Consideration they would receive pursuant to the Amalgamation Agreement if they do not exercise dissenters’ rights with respect to their Shares. These procedures are complex and you should consult your Antigua and Barbuda legal counsel if you are considering exercising such rights. If you do not fully and precisely satisfy the procedural requirements of the IBCA, you will lose your Dissenters’ Rights.
Material U.S. Federal Income Tax Consequences
The following is a general discussion of material U.S. federal income tax consequences to U.S. Holders (as defined below) of the exchange of Shares for cash pursuant to the Amalgamation Agreement, but does not purport to be a complete analysis of all potential tax effects. This discussion is based on the U.S. Internal Revenue Code of 1986, as amended (the “Code”), final and temporary U.S. Treasury Regulations promulgated thereunder, published rulings and administrative pronouncements of the U.S. Internal Revenue Service (the “IRS”), and judicial decisions as of the date hereof, all of which are subject to change, possibly on a retroactive basis, and to differing interpretation, which may result in tax consequences different from those described below.
This discussion does not address any U.S. federal estate, gift, or other non-income tax, the Medicare tax on investment income, or any state, local, or non-U.S. tax consequences of the Amalgamation. This discussion also does not take into account or address changes to United States tax law that may result from tax reforms that may be enacted in 2017 or thereafter. This discussion is not binding on the IRS, and the IRS or a court in the event of an IRS dispute may challenge any of the conclusions set forth below.
This discussion is limited to U.S. Holders that hold the Shares as capital assets within the meaning of Section 1221 of Code (generally, property held for investment). This discussion does not address all U.S. federal income tax consequences that may be relevant in light of a U.S. Holder’s particular circumstances, consequences to Rollover Shareholders or Shareholders exercising Dissenters’ Rights (as described under the section entitled “Dissenters’ Rights” beginning on page 145), alternative minimum tax consequences, or tax consequences applicable to U.S. Holders subject to special rules, such as:
| · | certain financial institutions; |
| · | regulated investment companies; |
| · | real estate investment trusts; |
| · | insurance companies; |
| 97 |
| · | dealers or traders in securities who use a mark-to-market method of tax accounting; |
| · | persons holding Shares as part of a hedge, straddle, integrated transaction, or similar transaction; |
| · | persons whose functional currency for U.S. federal income tax purposes is not the U.S. dollar; |
| · | entities classified as partnerships for U.S. federal income tax purposes; |
| · | tax-exempt entities, including “individual retirement accounts” or “Roth IRAs”; |
| · | persons that own, or have owned, directly, indirectly or constructively, 10% or more of the total voting power of the Company; |
| · | persons who received or acquired Shares pursuant to the exercise of any employee stock option or otherwise as compensation; |
| · | U.S. expatriates and certain former citizens or long-term residents of the U.S.; |
| · | persons that continue to own after the amalgamation, indirectly or constructively, Shares of the Company; or |
| · | persons holding Shares in connection with a trade or business conducted outside the United States. |
If an entity that is treated as a partnership for U.S. federal income tax purposes owns Shares, the U.S. federal income tax treatment of a partner in the partnership will generally depend on the status of the partner, the activities of the partnership, and certain determinations made at the partner level. Accordingly, partnerships owning Shares and partners in such partnerships should consult their tax advisors as to the particular U.S. federal income tax consequences of the disposition of the Shares.
As used herein, a “U.S. Holder” is any beneficial owner of Shares that is (i) an individual citizen or resident of the United States for U.S. federal income tax purposes, (ii) a corporation (or other entity treated as a corporation for U.S. federal income tax purposes) created or organized under the laws of the United States, any state thereof, or the District of Columbia, (iii) an estate the income of which is subject to U.S. federal income taxation regardless of its source or (iv) a trust which (a) is subject to the primary jurisdiction of a court within the United States and for which one or more U.S. persons have authority to control all substantial decisions, or (b) has a valid election in effect under applicable U.S. Treasury Regulations to be treated as a U.S. person for U.S. federal income tax purposes.
U.S. HOLDERS OF SHARES SHOULD CONSULT THEIR TAX ADVISORS REGARDING THE SPECIFIC TAX CONSEQUENCES OF THE AMALGAMATION IN LIGHT OF THEIR PARTICULAR SITUATIONS, INCLUDING THE APPLICABILITY AND EFFECT OF U.S. FEDERAL, STATE, LOCAL, NON-U.S. AND OTHER LAWS.
| 98 |
Consequences of Participation in the Amalgamation
The receipt of cash as consideration in the Amalgamation, will be a taxable transaction for U.S. federal income tax purposes, and a U.S. Holder that exchanges Shares for cash will generally recognize gain or loss in an amount equal to the difference between (i) the amount of cash received and (ii) such U.S. Holder’s adjusted tax basis in the Shares exchanged. Subject to the discussion under “Passive Foreign Investment Company” below, such recognized gain or loss will generally be capital gain or loss, and will constitute long-term capital gain or loss if the U.S. Holder’s holding period for the Shares exchanged is greater than one year at the Effective Time.
Long-term capital gains of non-corporate U.S. Holders are currently subject to U.S. federal income tax at a reduced rate. The ability to use any capital loss to offset other income or gain is subject to certain limitations under the Code. If a U.S. Holder acquired different blocks of Shares at different times and different prices, such U.S. Holder must determine the adjusted tax basis and holding period separately with respect to each such block of Shares.
Any gain or loss recognized by U.S. Holders will generally be treated as U.S. source gain or loss for U.S. foreign tax credit purposes. However, in the event we are deemed to be a PRC “resident enterprise” under the PRC tax law and gain from the disposition of the Shares is regarded as gain sourced from the PRC (and subject to tax in the PRC), you may be eligible to elect to treat such gain as PRC source gain under the income tax treaty between the United States and the PRC (the Agreement Between the Government of the United States of America and the Government of the People’s Republic of China for the Avoidance of Double Taxation and the Prevention of Tax Evasion With Respect to Taxes on Income (the “Treaty”)).
If we are not eligible for the benefits of the Treaty or you fail to make the election to treat any gain as PRC source, then you may not be able to use the foreign tax credit arising from any PRC tax imposed on the exchange of Shares pursuant to the Amalgamation Agreement unless such credit can be applied (subject to applicable limitations) against tax due on other income treated as derived from foreign sources. U.S. Holders are urged to consult their tax advisors regarding the tax consequences if PRC tax is imposed on gain on a disposition of our Shares, including the availability of the foreign tax credit under their particular circumstances.
Passive Foreign Investment Company
In general, we will be classified as a passive foreign investment corporation or “PFIC” in any taxable year if either (a) the average quarterly value of our gross assets that produce passive income or are held for the production of passive income is at least 50% of the average quarterly value of our total gross assets (the “asset test”) or (b) at least 75% of our gross income for the taxable year is passive income (such as certain dividends, interest or royalties).
For this purpose, we will be treated as owning our proportionate share of the assets and earning our proportionate share of the income in any other corporation in which we own, directly or indirectly, at least 25% (by value) of the stock. For purposes of the asset test: (a) any cash and cash invested in short-term, interest bearing debt instruments or bank deposits that are readily convertible into cash will generally count as producing passive income or held for the production of passive income, and (b) the total value of our assets is calculated based on our market capitalization.
| 99 |
We believe we were not a PFIC for our taxable year ended December 31, 2016 or any previous taxable year. Based on the historic market price of our Shares and the historic composition of our income and assets, we do not expect we will be a PFIC for our current taxable year, although there can be no assurance in this regard. Our PFIC status for the current taxable year 2017 will not be determinable until the close of the taxable year ending December 31, 2017.
If we are a PFIC for the current taxable year or have been a PFIC during any prior year in which a U.S. Holder held Shares, any gain recognized by a U.S. Holder on the disposition of a Share generally (a) would be allocated ratably to each day over such U.S. Holder’s holding period for the Share, (b) the amount allocated to the current year and any tax year prior to the first taxable year in which we were a PFIC would be taxed as ordinary income in the current year, (c) the amount allocated to each other taxable year would be subject to tax at the highest applicable marginal rate in effect for that year and (d) an interest charge at the rate for underpayments of taxes would be imposed on the resulting tax allocated to such period. If we are a PFIC for the current taxable year or have been a PFIC during any prior year in which a U.S. Holder held Shares, a U.S. Holder generally would be required to file IRS Form 8621 with respect to the disposition of Shares.
Alternatively, if the Company is a PFIC (or has been treated as a PFIC with respect to a U.S. Holder), a U.S. Holder may have been able to make a “mark-to-market” election with respect to the U.S. Holder’s Shares. If a U.S. Holder made this election in a timely fashion, then instead of the tax treatment described in the preceding paragraph, any gain recognized by the U.S. Holder in the Amalgamation would generally be treated as ordinary income or ordinary loss (but only to the extent of the net amount of previously included income as a result of the mark-to-market election, if any). If a U.S. Holder made a mark-to-market election in respect of a corporation classified as a PFIC and such corporation ceases to be classified as a PFIC, the U.S. Holder will not be required to take into account the gain or loss described above during any period that such corporation is not classified as a PFIC.
The PFIC rules are complex, and each U.S. Holder should consult its tax advisor regarding the applicable consequences of the Amalgamation to such U.S. Holder if we are a PFIC or have been a PFIC during any prior year in which such U.S. Holder held Shares.
Information Reporting and Backup Withholding
A U.S. Holder may be subject, under certain circumstances, to information reporting and backup withholding with respect to the amount of cash received in the Amalgamation. Under the backup withholding rules, a U.S. Holder may be subject to backup withholding unless the U.S. Holder is an exempt recipient and, when required, demonstrates this fact or provides a taxpayer identification number, makes certain certifications on IRS Form W-9, and otherwise complies with the applicable requirements. A U.S. Holder that does not provide its correct taxpayer identification number may also be subject to penalties imposed by the IRS.
| 100 |
Backup withholding is not an additional tax and any amounts withheld under the backup withholding rules may be refunded or credited against a U.S. Holder’s U.S. federal income tax liability, if any, provided that the required procedures are followed. U.S. Holders should consult their tax advisors as to their qualification for exemption from backup withholding and the procedure for obtaining such an exemption.
Certain U.S. Holders may be required to report information with respect to their investment in our Shares not held through a custodial account with a U.S. financial institution to the IRS. U.S. Holders should consult their tax advisors regarding their reporting obligation with respect to the disposition of their Shares.
Material PRC Income Tax Consequences
The Company does not believe that it should be considered a PRC tax resident enterprise under the EIT Law or that the gain recognized on the receipt of cash for Shares by holders who are not PRC tax residents should be subject to PRC tax. However, there is uncertainty regarding whether PRC tax authorities would deem the Company to be a PRC tax resident enterprise.
If PRC tax authorities determine that the Company is a PRC tax resident enterprise, then gains recognized on the receipt of cash for Shares pursuant to the Amalgamation by shareholders who are not PRC tax residents could be treated as PRC-source income. This income would be subject to PRC income tax at a rate of 10% in the case of enterprises or 20% in the case of individuals (subject to applicable tax treaty relief, if any). Regardless of whether the Company is considered a PRC tax resident enterprise, gain recognized on the receipt of cash for Shares is subject to PRC tax if the holders of such Shares are PRC tax residents.
If the Company were not considered a PRC tax resident enterprise, gains derived through buying and selling such shares in a public stock exchange market by holders of Shares who are not PRC tax residents would not be subject to PRC income tax. If the Company were considered a PRC tax resident enterprise, the PRC tax authorities may determine whether the Amalgamation has bona-fide commercial purposes based on the facts and circumstances as well as the applicable tax circulars.
Announcement [2015] No. 7 issued by the State Administration of Taxation states that an indirect transfer of equity in a Chinese resident enterprise may be re-characterized as a “direct transfer” following the “substance over form” principle if it is assessed as lacking bona-fide commercial purposes. If a “direct transfer” occurs, gains recognized on the receipt of cash for Shares will be subject to PRC income tax.
The bona-fide business purpose test is complicated and depends on the judgment of PRC tax authorities. You should consult your own tax advisors for a full understanding of the tax consequences of the Amalgamation to you, including the above-mentioned potential PRC tax consequences.
Material Antigua and Barbuda Tax Consequences
The following is a summary of the material Antigua and Barbuda tax consequences and does not consider all aspects of Antigua and Barbuda taxation that may be relevant to particular shareholders in light of their individual investment circumstances or to certain types of shareholders subject to special tax rules.
| 101 |
Under the IBA please note the following special tax provisions in relation to an IBC:
1) No income tax, capital gains tax or other direct tax or impost may be levied in Antigua and Barbuda upon the profits or gains of an IBC in respect of the international trade and business it carries on from within Antigua and Barbuda or in respect of any securities or assets of an IBC that are beneficially owned by an IBC or by a person who is not a resident.
2) No estate, inheritance, succession or similar tax or impost may be levied in Antigua and Barbuda in respect of any securities or assets of an IBC that are beneficially owned by an IBC or a person who is not a resident. No tax, duty or impost may be levied upon the increment in value of the property or other assets in Antigua and Barbuda or elsewhere of an IBC other than upon such of them as are distributed to residents.
3) No tax, duty or other impost may be levied upon an IBC, its security holders or transferees in respect of the transfer of all or any part of its securities or other assets to another IBC or to a person who is not a resident. When an IBC or a person who is not a resident transfers securities or assets of an IBC that are held by that IBC or person to another IBC or to another person who is not a resident, the transfer is exempt from the payment of any tax, duty or other impost thereon.
4) No income tax or capital gains tax, and no other direct tax or impost may be levied or collected in Antigua and Barbuda, in respect of any dividends, interest or other returns from any securities, deposits or borrowings of an IBC or any assets managed by the IBC if the dividends, interest or other returns are in respect of securities, deposits, borrowings or assets beneficially owned by another IBC or a person who is not a resident; but the onus of establishing ownership lies upon the IBC holding or managing the deposits, borrowings or assets.
5) Notwithstanding any provision of the Income Tax Act Cap. 212, but subject to paragraph 6 below, no IBC need withhold any portion of any dividend, interest or other returns payable to any person in respect of any borrowings of the IBC from that person or in respect of securities of the IBC held by that person.
6) All dividends, interest or other returns attributable to the securities of, or the management of, assets by an IBC that are payable to a resident who is known to be a resident by the IBC or who, with the exercise of reasonable care by the IBC, could be known by him to be a resident must be reported to the Commissioner of Inland Revenue by the IBC.
7) The income, profits, gains and other revenues, and the funds and securities of an IBC that are generated, acquired or managed in the course of the international trade or business of the IBC are exempt from the Exchange Control Act, Cap. 157; and, unless the IBC is a resident, the income, profits, gains and other revenues of the IBC are also exempt from that Act.
8) An exempted corporation is exempt from stamp duties under the Stamp Act Cap. 410 in respect of all transactions entered into in relation to the IBC’s international trade or business.
| 102 |
Any tax exemption provided under the IBCA shall continue in effect for a period of fifty years from the date of incorporation of the IBC.
Antigua and Barbuda has bilateral investment treaties with Germany and the United Kingdom and double taxation agreements with Denmark, Norway, Sweden, the United Kingdom and member states of the Caribbean Community (CARICOM).
ALL REGISTERED HOLDERS OF SHARES SHOULD CONSULT THEIR TAX ADVISORS REGARDING THE SPECIFIC TAX CONSEQUENCES OF THE AMALGAMATION BASED ON THEIR PARTICULAR CIRCUMSTANCES OF THE APPLICABILITY AND EFFECT OF THE SPECIAL TAX PROVISIONS UNDER THE IBCA.
| 103 |
QUESTIONS AND ANSWERS ABOUT THE SPECIAL MEETING AND THE AMALGAMATION
The following questions and answers address briefly some questions you may have regarding the special meeting and the Amalgamation. These questions and answers may not address all questions that may be important to you as a shareholder of the Company. Please refer to the more detailed information contained elsewhere in this proxy statement, the annexes to this proxy statement and the documents referred to or incorporated by reference in this proxy statement.
Q: After the Amalgamation is completed, how will I receive the Per Share Amalgamation Consideration for my Shares?
A: If you are a registered holder of Shares, promptly after the Effective Time, a paying agent appointed by Parent will mail you a form of letter of transmittal specifying how the delivery of the Per Share Amalgamation Consideration to you will be effected, together with instructions for surrendering your Shares in exchange for the Per Share Amalgamation Consideration.
You will receive cash for your Shares from the paying agent after you comply with these instructions. Upon surrender of your Shares, you will receive an amount equal to the number of your Shares multiplied by US$7.00 in cash, without interest and net of any applicable withholding tax, in exchange for the cancellation of your Shares.
If your Shares are held in “street name” by your broker, bank or other nominee, you will receive instructions from your broker, bank or other nominee on how to surrender your Shares and receive the Per Share Amalgamation Consideration for those Shares.
Q: When and where will the special meeting be held?
A: The special meeting will take place on , 2018, at [a.m./p.m.] ( time) at ..
Q: What will the Unaffiliated Holders receive in the Amalgamation and when will they receive it?
A: If you are an Unaffiliated Holder and you own Shares, if and when the Amalgamation is completed, you will be entitled to receive US$7.00 in cash, without interest and net of any applicable withholding taxes, for each Share you own as of the Effective Time (unless you validly exercise and have not effectively withdrawn or lost your dissenters’ rights under section 191 of the IBCA with respect to the Amalgamation, in which event you will be entitled to the fair value of each Share pursuant to the IBCA).
Promptly after the Effective Time, you will receive a letter of transmittal specifying how the delivery of the Per Share Amalgamation Consideration will be effected from a paying agent appointed by Parent if you are a registered holder of Shares.
Please see ‘‘Special Factors—Material U.S. Federal Income Tax Consequences’’, “Special Factors—Material Antigua and Barbuda Tax Consequences” and ‘‘Special Factors—Material PRC Income Tax Consequences’’ beginning on page 97 for a more detailed description of the tax consequences of the Amalgamation. You should consult your own tax advisor for a full understanding of how the Amalgamation will affect your U.S. federal, state, local, foreign and other taxes.
| 104 |
Q: How does the Board recommend that I vote on the proposals?
A: After careful consideration and upon the unanimous recommendation of the Special Committee, the Board unanimously (other than Mr. Yin and Kenneth Lee) recommends that you vote:
| · | FOR the proposal to authorize and approve the Amalgamation Agreement and the consummation of the Transactions, including the Amalgamation; |
| · | FOR the proposal to authorize each of the directors of the Company to do all things necessary to give effect to the Amalgamation Agreement and the consummation of the Transactions, including the Amalgamation; and |
| · | FOR the proposal that the chairman of the special meeting be instructed to adjourn the special meeting in order to allow the Company to solicit additional proxies in the event that there are insufficient proxies received to pass the special resolutions during the special meeting |
You should read “Special Factors—Reasons for the Amalgamation and Recommendation of the Special Committee and the Board” beginning on page 54 for a discussion of the factors that the Special Committee and the Board considered in deciding to recommend the approval of the Amalgamation Agreement. In addition, in considering the recommendation of the Board, you should be aware that some of the Company’s directors and executive officers have interests in the Amalgamation that are different from, or in addition to, the interests of the Company’s shareholders generally. See “Special Factors—Interests of Certain Persons in the Amalgamation” beginning on page 92.
Q: When do you expect the Amalgamation to be completed?
A: We are working toward completing the Amalgamation as quickly as possible and expect the Amalgamation to close during the first quarter of 2018, after all conditions to the Amalgamation have been satisfied or waived. In order to complete the Amalgamation, we must obtain shareholder approval of the Amalgamation at the special meeting and the other closing conditions under the Amalgamation Agreement must be satisfied or waived.
Q: How will our directors and executive officers vote on the proposal to authorize and approve the Amalgamation Agreement?
A: Each of our directors and executive officers who beneficially owns Shares has informed us that, as of the date of this proxy statement, he or she intends to vote all of his or her Shares in favor of approval and authorization of the Amalgamation Agreement and the consummation of the Transactions, including the Amalgamation, for the reasons described under “Special Factors— Reasons for the Amalgamation and Recommendation of the Special Committee and the Board” beginning on page 54.
| 105 |
As of the date of this proxy statement, our directors and executive officers in the aggregate beneficially own 6,688,500 Shares, and no Shares are subject to options and restricted share units that will vest within 60 days after the date of this proxy statement, which represent approximately 11.69% of the total issued and outstanding Shares. See “Security Ownership of Certain Beneficial Owners and Management of the Company” beginning on page 150 for additional information.
Q: What do I need to do now?
A: We urge you to read this proxy statement carefully, including its annexes, exhibits, attachments and the other documents referred to or incorporated by reference herein and to consider how the Amalgamation affects you as a shareholder. After you have done so, please complete, sign and return the proxy card as soon as possible.
Q: How do I vote if my Shares are registered in my name?
A: If Shares are registered in your name as of the close of business in New York on , 2018, being the record date for determining the shareholders entitled to vote at the special meeting (the ‘‘Record Date’’), you should simply indicate on your proxy card how you want to vote, and sign and mail your proxy card in the accompanying return envelope as soon as possible. The deadline to lodge your proxy card so that your Shares may be represented and voted at the special meeting is , 2018 at [a.m./p.m.] ( time).
Alternatively, you can attend the special meeting and vote in person. If you decide to sign and send in your proxy card, and do not indicate how you want to vote, Shares represented by your proxy will be voted FOR the proposal to authorize and approve the Amalgamation Agreement and the consummation of the Transactions, including the Amalgamation, FOR the proposal to authorize each of the directors of the Company to do all things necessary to give effect to the Amalgamation Agreement and the consummation of the Transactions, including the Amalgamation, and FOR the proposal that the chairman of the special meeting be instructed to adjourn the special meeting in order to allow the Company to solicit additional proxies in the event that there are insufficient proxies received to pass the special resolutions during the special meeting, unless you appoint a person other than the chairman of the meeting as proxy, in which case Shares represented by your proxy card will be voted (or not submitted for voting) as your proxy determines.
If your Shares are held by your broker, bank or other nominee, check the voting instruction card forwarded by your broker, bank or other nominee to see which voting options are available or contact your broker, bank or other nominee in order to obtain directions as to how to ensure that your shares are voted at the special meeting.
| 106 |
Q: What will happen if I abstain from voting or fail to vote on the proposal to authorize and approve the Amalgamation Agreement?
A: If you abstain from voting, fail to cast your vote in person, fail to complete and return your proxy card in accordance with the instructions set forth on the proxy card, or fail to give voting instructions to your broker, bank or other nominee, your vote will not be counted.
Q: May I change my vote?
A: Yes. If you are a registered holder of Shares, you may change your vote in one of the following three ways:
| · | First, you may revoke a proxy by written notice of revocation given to the chairman of the special meeting at least two hours before the special meeting commences. Any written notice revoking a proxy should be sent to the Company. |
| · | Second, you may complete, date and submit a new proxy card bearing a later date than the proxy card sought to be revoked to the Company no later than [a.m./p.m.] ( time) on , 2018, which is the deadline to lodge your proxy card. |
| · | Third, you may attend the special meeting and vote in person. Attendance, by itself, will not revoke a proxy. It will only be revoked if the shareholder actually votes at the special meeting. |
If you hold Shares through a broker, bank or other nominee and have instructed the broker, bank or other nominee to vote your Shares, you must follow directions received from the broker, bank or other nominee to change your instructions.
Q: What should I do if I receive more than one set of voting materials?
A: You may receive more than one set of voting materials, including multiple copies of this proxy statement or multiple proxy cards or voting instruction cards. For example, if you hold your Shares in more than one brokerage, bank or other nominee account, you will receive a separate voting instruction card for each brokerage, bank or other nominee account in which you hold Shares. If you are a holder of record and your Shares are registered in more than one name, you will receive more than one proxy or voting instruction card. Please submit each proxy card and voting instruction card that you receive.
Q: What happens if I sell my Shares before the special meeting?
A: The Record Date for determining shareholders entitled to vote at the special meeting is earlier than the date of the special meeting and the date that the Amalgamation is expected to be completed. If you transfer your Shares after the Record Date but before the special meeting, you will retain your right to vote at the special meeting unless you have given, and not revoked, a valid proxy appointing the person to whom you transfer your Shares, but will transfer the right to receive the Per Share Amalgamation Consideration in cash without interest to such person, so long as such person is registered as the holder of such Shares when the Amalgamation is completed. In such case, your vote is still very important and you are encouraged to vote.
| 107 |
Q: Am I entitled to dissenters’ rights?
A: Shareholders who dissent from the Amalgamation will have the right to receive payment of the fair value of their Shares if the Amalgamation is completed, but only if they deliver to the Company, before the vote to authorize and approve the Amalgamation is taken, a written objection to the Amalgamation and subsequently comply with all procedures and requirements of sections 191 to 199 of the IBCA for the exercise of dissenters’ rights, which is attached as Annex B to this proxy statement. The fair value of the Shares as determined under that statute could be more than, the same as, or less than the Per Share Amalgamation Consideration that would be received pursuant to the Amalgamation Agreement if dissenters’ rights were not exercised with respect to the Shares.
We encourage you to read the section of this proxy statement entitled “Dissenters’ Rights” beginning on page 145 as well as ‘‘Annex B—Sections 191 to 199 of the International Business Corporations Act, CAP. 222 of the Revised Laws of Antigua and Barbuda” to this proxy statement carefully and to consult your own Antigua and Barbuda legal counsel if you desire to exercise dissenters’ rights.
Q: Will any proxy solicitors be used in connection with the special meeting?
A: Yes. To assist in the solicitation of proxies, the Company has engaged as its proxy solicitor.
Q: Who can help answer my questions?
A: If you have any questions about the Amalgamation or if you need additional copies of this proxy statement or the accompanying proxy card, you should contact , the proxy solicitor, at (+ outside the United States) or at .
In order for you to receive timely delivery of any additional copy of this proxy statement or the accompanying proxy card in advance of the special meeting, you must make your request no later than ten business days prior to the date of the special meeting.
| 108 |
MARKET PRICE OF THE COMPANY’S SHARES, DIVIDENDS AND OTHER MATTERS
Market Price of the Shares
The following table provides the high and low sales prices for Shares on NASDAQ under the symbol “SVA” for the periods indicated during the past two years:
| Sales Price (in US$) | ||||||||
| Quarterly | High | Low | ||||||
| Fiscal quarter ended March 31, 2016 | 7.16 | 4.38 | ||||||
| Fiscal quarter ended June 30, 2016 | 6.45 | 5.61 | ||||||
| Fiscal quarter ended September 30, 2016 | 6.01 | 5.50 | ||||||
| Fiscal quarter ended December 31, 2016 | 6.45 | 5.25 | ||||||
| Fiscal quarter ended March 31, 2017 | 6.05 | 5.50 | ||||||
| Fiscal quarter ended June 30, 2017 | 6.92 | 4.60 | ||||||
| Fiscal quarter ended September 30, 2017 | 7.16 | 6.50 | ||||||
| Fiscal quarter ended December 31, 2017 | 8.11 | 6.81 | ||||||
| Current fiscal quarter (through [ · ], 2018) | [ · ] | [ · ] | ||||||
The Per Share Amalgamation Consideration of US$7.00 represents a premium of 22.8% to the closing price of the Shares as quoted by NASDAQ on June 23, 2017, the last trading day immediately prior to the date that the Company announced that it had entered into the Amalgamation Agreement, and a premium of 32.1% and 30% to the volume-weighted average trading price of the Shares as quoted by NASDAQ during the 30 and 60 trading days, respectively, immediately prior to the Company’s announcement on February 1, 2016 that it had received the Buyer Consortium’s Proposal Letter.
On , 2018, the most recent practicable date before the printing of this proxy statement, the high and low reported sales prices of the Shares were US$ and US$ , respectively. You are urged to obtain a current market price quotation for the Shares in connection with voting your Shares.
Dividend Policy
The Company has never declared or paid any dividends, nor does it plan to pay any cash dividends on the Shares in the foreseeable future. The Company currently intends to retain most, if not all, of its available funds and any future earnings to operate and expand its business.
The Board has complete discretion on whether to pay dividends. Even if the Board decides to pay dividends, the form, frequency and amount will depend upon the Company’s future operations and earnings, capital requirements and surplus, general financial condition, contractual restrictions and other factors that the Board may deem relevant.
Cash dividends on the Shares, if any, will be paid in U.S. dollars. Under the Amalgamation Agreement, prior to the Effective Time, the Company is not permitted to declare, set aside, make or pay any dividend or other distribution, payable in cash, shares, property or otherwise, with respect to any of its Shares (other than dividends or other distributions from any subsidiary of the Company to the Company or any of its other subsidiaries) without prior written consent of Parent.
| 109 |
THE SPECIAL MEETING
We are furnishing this proxy statement to you, as a holder of our Shares, as part of the solicitation of proxies by the Board for use at the special meeting described below.
Date, Time and Place of the Special Meeting
The special meeting will be held on , 2018 at [a.m./p.m.] ( time) at ..
Proposals to be Considered at the Special Meeting
At the meeting, you will be asked to consider and vote upon:
As special resolutions:
THAT the Amalgamation Agreement and the consummation of the Transactions, including the Amalgamation, be authorized and approved; and
THAT each of the directors of the Company be authorized to do all things necessary to give effect to the Amalgamation Agreement and the consummation of the Transactions, including the Amalgamation.
If necessary, as an ordinary resolution:
THAT the chairman of the special meeting be instructed to adjourn the special meeting in order to allow the Company to solicit additional proxies in the event that there are insufficient proxies received to pass the special resolutions during the special meeting.
If the Amalgamation is completed, at the Effective Time, each outstanding Share, other than the Excluded Shares and the Dissenting Shares, will be cancelled in exchange for the right to receive the Per Share Amalgamation Consideration of US$7.00 in cash without interest. The Excluded Shares will be cancelled and cease to exist for no consideration. The Dissenting Shares will be cancelled and each holder thereof will be entitled to receive only payment of the fair value of such Dissenting Shares as determined in accordance with the IBCA.
Our Board of Directors’ Recommendation
Our board of directors, acting upon the unanimous recommendation of the Special Committee:
| · | determined that the Amalgamation, on the terms and conditions set forth in the Amalgamation Agreement and the other Transactions Documents (including the Per Share Amalgamation Consideration), is fair to and in the best interests of the Company and the Company’s shareholders (other than holders of the Excluded Shares); |
| 110 |
| · | approved, authorized and adopted the Amalgamation Agreement and the transactions contemplated thereby, including the Amalgamation, on the terms and conditions set forth in the Amalgamation Agreement; |
| · | approved and authorized the execution, delivery and performance of the Amalgamation Agreement and the other Transaction Documents and the consummation of the Transactions, including the Amalgamation Agreement; and |
| · | directed that the Amalgamation Agreement and the transactions contemplated thereby, including, without limitation, the Amalgamation, shall be submitted to the shareholders of the Company for approval with the recommendation of the Board that the Company’s shareholders vote in favor of the matters submitted to them in connection with the Amalgamation. |
Quorum
Shareholders present in person or by proxy representing a majority of the Company’s shares shall constitute a quorum.
Record Date; Shares Entitled to Vote
You are entitled to attend and vote at the special meeting if you have Shares registered in your name at the close of business in New York on , 2018, the Record Date for voting at the special meeting. Each outstanding Share on the Record Date entitles the holder to one vote on each matter submitted to the shareholders for authorization and approval at the special meeting and any adjournment thereof. We expect that, as of the Record Date, there will be Shares entitled to be voted at the special meeting.
If you have Shares registered in your name on the Record Date, the deadline for you to lodge your proxy card and vote is , 2018 at [a.m./p.m.] ( time). Please see “Shareholders Entitled to Vote; Voting Materials” below for additional information. If the Amalgamation is not completed, the Company will continue to be a public company in the U.S. and listed on NASDAQ. The Company’s Shares are not listed and cannot be traded on any stock exchange other than NASDAQ.
Vote Required
Under the Amalgamation Agreement, we cannot complete the Amalgamation unless the Amalgamation Agreement and the Transactions, including the Amalgamation, are authorized and approved by a special resolution passed by not less at least two-thirds of the Shares present and voting in person or by proxy as a single class.
Please see “Security Ownership of Certain Beneficial Owners and Management of the Company” beginning on page 150 for additional information. As of the date of this proxy statement, the Buyer Consortium beneficially owns approximately 29.5% of the issued and outstanding Shares entitled to vote. Pursuant to the Support Agreement, these Shares will be voted in favor of the authorization and approval of the Amalgamation Agreement and the Transactions, including the Amalgamation.
| 111 |
Shareholders Entitled to Vote; Voting Materials
Only holders of Shares entered in the register of members of the Company at the close of business in New York on , 2018, the Record Date, will receive the final proxy statement and proxy card directly from the Company. Shareholders registered in the register of members of the Company as of the Record Date or their proxy holders are entitled to vote and may participate in the special meeting or any adjournment thereof.
Shareholders wanting to vote by proxy should simply indicate on their proxy card how they want to vote, sign and date the proxy card, and mail the proxy card in the return envelope as soon as possible but in any event so that it is received no later than [a.m./p.m.] on , 2018 ( time).
Shareholders who have acquired Shares after the close of business in New York on , 2018 may not attend the special meeting unless they receive a proxy from the person or entity who had sold them the Shares.
Proxy Holders for Registered Shareholders
Shareholders registered in the register of members of the Company as of the Record Date who are unable to attend the special meeting may appoint another shareholder, a third party or the chairman of the meeting as their proxy to attend the meeting and vote their Shares on their behalf by completing and returning the form of proxy in accordance with the instructions printed thereon.
Voting of Proxies and Failure to Vote
All Shares represented by valid proxies will be voted at the special meeting in the manner specified by the shareholder. If a shareholder returns a properly signed proxy card but does not indicate how the shareholder wants its Shares voted, the Shares represented by that proxy card will be voted FOR the proposal to authorize and approve the Amalgamation Agreement and the consummation of the Transactions, including the Amalgamation, FOR the proposal to authorize each of the directors of the Company to do all things necessary to give effect to the Amalgamation Agreement and the consummation of the Transactions, including the Amalgamation, and FOR the proposal that the chairman of the special meeting be instructed to adjourn the special meeting in order to allow the Company to solicit additional proxies in the event that there are insufficient proxies received to pass the special resolutions during the special meeting.
Revocability of Proxies
Registered holders of our Shares may revoke their proxies in one of three ways:
| · | first, a registered shareholder may revoke a proxy by written notice of revocation to the Company at its principal executive offices located at No. 39 Shangdi Xi Road, Haidian District, Beijing 100085, People’s Republic of China before the special meeting commences; |
| 112 |
| · | second, a registered shareholder may complete, date and submit a new proxy card bearing a later date than the proxy card sought to be revoked to the Company prior to deadline for submission of proxies; or |
| · | third, a registered shareholder may attend the special meeting and vote in person. Attendance, by itself, will not revoke a proxy. It will only be revoked if the shareholder actually votes at the special meeting. |
If a shareholder holds Shares through a broker, bank or other nominee and has instructed the broker, bank or other nominee to vote the shareholder’s Shares, the shareholder must follow directions received from the broker, bank or other nominee to change those instructions.
Rights of Shareholders Who Object to the Amalgamation
Shareholders who dissent from the Amalgamation will have the right to receive payment of the fair value of their Shares if the Amalgamation is completed, but only if they deliver to the Company, before the vote is taken at the special meeting, a written objection to the Amalgamation and subsequently comply with all procedures and requirements of sections 191 to 199 of the IBCA, which is attached as Annex B to this proxy statement, for the exercise of dissenters’ rights. The fair value of your Shares as determined under that statute could be more than, the same as, or less than the Amalgamation Consideration you would receive pursuant to the Amalgamation Agreement if you do not exercise dissenters’ rights with respect to your Shares.
Whom to Call for Assistance
If you have any questions or need assistance in voting your Shares, please contact , which is acting as the proxy solicitor in connection with the Amalgamation, as follows:
Call toll Free: +
International Shareholders Please Call: +
Solicitation of Proxies
We have engaged to assist in the solicitation of proxies from brokerage, banks or other nominees and individual investors for the special meeting. We expect that ’s fees for its services will be approximately US$ plus certain costs associated with telephone solicitations, if required, and reimbursement of out-of-pocket expenses. In addition, proxies may be solicited by mail, in person, by telephone, by internet or by facsimile by certain of our officers, directors and employees.
These persons will receive no additional compensation for solicitation of proxies but may be reimbursed for reasonable out-of-pocket expenses. We will reimburse banks, brokers, nominees, custodians and fiduciaries for their reasonable expenses in forwarding copies of this proxy statement to the beneficial owners of Shares and in obtaining voting instructions from those owners. We will pay all expenses of filing, printing and mailing this proxy statement.
| 113 |
Other Business
We are not currently aware of any business to be acted upon at the special meeting other than the matters discussed in this proxy statement.
| 114 |
THE AMALGAMATION AGREEMENT
The following summary describes material provisions of the Amalgamation Agreement. This summary may not include all of the information about the Amalgamation Agreement that is important to you. This summary is subject to, and qualified in its entirety by reference to, the Amalgamation Agreement, which is attached as Annex A and incorporated by reference into this section of this proxy statement. You are urged to read the Amalgamation Agreement carefully and in its entirety, as it is the legal document governing the Amalgamation.
The summary of the Amalgamation Agreement below is included in this proxy statement only to provide you with information regarding the terms and conditions of the Amalgamation Agreement, and not to provide any other factual information regarding the Company, Parent, Amalgamation Sub or their respective businesses. Accordingly, the representations and warranties and other provisions of the Amalgamation Agreement should not be read alone, but instead should be read only in conjunction with the information provided elsewhere in this proxy statement and in the documents incorporated by reference into this proxy statement. See “Where You Can Find More Information” beginning on page 154.
Structure and Completion of the Amalgamation
The Amalgamation Agreement provides for the Amalgamation of Amalgamation Sub with and into the Company upon the terms, and subject to the conditions, of the Amalgamation Agreement, with the Company continuing as the Surviving Corporation after the Amalgamation. If the Amalgamation is completed, the Company will cease to be a publicly traded company and will become a subsidiary of Parent. The Closing will occur as soon as practicable, but in any event no later than the tenth business day following the day on which the last of the closing conditions have been satisfied or, if permissible, waived (other than those conditions that by their nature are to be satisfied at the Closing, but subject to the satisfaction or, if permissible, waiver of those conditions at such time), or such other date as the Company and Parent may mutually determine.
At the Effective Time, Amalgamation Sub and the Company will execute the Articles of Amalgamation as prescribed in Form 8 in the schedule to the IBCA and such parties will file the Articles of Amalgamation and other documents required under the IBCA with the Financial Services Regulatory Commission of Antigua and Barbuda. The Amalgamation will become effective upon the date specified in the Articles of Amalgamation in accordance with the IBCA.
We expect that the Amalgamation will be completed during the first quarter of 2018, after all conditions to the Amalgamation have been satisfied or waived. We cannot specify when, or assure you that, all conditions to the Amalgamation will be satisfied or waived. We intend, however, to complete the Amalgamation as promptly as practicable.
Articles of Incorporation and By-laws; Directors and Officers of the Surviving Corporation
At the Effective Time, the articles of incorporation and by-laws of Amalgamation Sub, as in effect immediately prior to the Effective Time, will be the articles of incorporation and by-laws of the Surviving Corporation, except that (a) Article I of the articles of incorporation of the Surviving Corporation will be amended to read “The name of the Corporation is Sinovac Biotech Ltd.” and the articles of incorporation and by-laws of the Surviving Corporation will be amended to refer to the name of the Surviving Corporation as “Sinovac Biotech Ltd.” and (b) references to the authorized share capital of Amalgamation Sub will be amended to refer to the correct authorized capital of the Surviving Corporation as approved in the Articles of Amalgamation, if necessary.
| 115 |
The directors of Amalgamation Sub immediately prior to the Effective Time will be the initial directors of the Surviving Corporation, and the officers of the Company immediately prior to the Effective Time will be the initial officers of the Surviving Corporation, unless otherwise determined by Parent prior to the Effective Time.
Amalgamation Consideration
Each Share (except as noted below) issued and outstanding immediately prior to the Effective Time will be cancelled and cease to exist in exchange for the right to receive US$7.00, in cash, per Share without interest and net of any applicable withholding taxes.
Notwithstanding the foregoing, the following Shares will be cancelled and cease to exist at the Effective Time but will not be converted into the right to receive the consideration described in the immediately preceding sentence:
| (a) | Each of the Excluded Shares issued and outstanding immediately prior to the Effective Time will be cancelled without payment of any consideration or distribution therefor. |
| (b) | Each of the Dissenting Shares will be cancelled and holders of such Shares immediately before such cancellation will only be entitled to receive the payments resulting from the procedures set forth in Section 191(4) of the IBCA or Section 195(2) of the IBCA, as applicable. See “—Dissenters’ Rights” beginning on page 145 for additional information. |
| (c) | Each of the Shares issued and outstanding immediately prior to the Effective Time, together with the associated Series A Junior Participating Preferred Share Purchase Rights, issued under the Rights Agreement will be cancelled and will cease to exist without payment of any consideration or distribution therefor. |
At the Effective Time, each common share, par value US$1.00 per share, of Amalgamation Sub issued and outstanding immediately prior to the Effective Time will be converted into one validly issued, fully paid and non-assessable ordinary share, par value US$0.001 per share, of the Surviving Corporation.
The Per Share Amalgamation Consideration will not be paid to shareholders who are untraceable unless and until they notify the paying agent appointed by Parent of their current contact details. A holder of Shares will be deemed to be untraceable and presumed to have received notice in accordance with Section 321 of the IBCA if (a) such person has no registered address in the register of shareholders maintained by the Company, (b) on the last two consecutive occasions on which a dividend has been paid by the Company a check payable to such person either (i) has been sent to such person and has been returned undelivered or has not been cashed or (ii) has not been sent to such person because on an earlier occasion a check for a dividend so payable has been returned undelivered, and in any such case no valid claim in respect thereof has been communicated in writing to the Company or (c) notice of the special meeting convened to vote to authorize and approve the Amalgamation Agreement and the Transactions, including the Amalgamation, has been sent to such person and has been returned undelivered. Monies due to dissenting shareholders and shareholders of the Company who are untraceable will be returned to the Surviving Corporation on demand and held in a non-interest-bearing bank account for the benefit of dissenting shareholders and shareholders of the Company who are untraceable. Monies unclaimed after a period of ten years from the closing date of the Amalgamation will be classified as “abandoned property” and transferred to the appropriate governmental authority in accordance with Section 256 of the IBCA. Once such transfer has been made, the Surviving Corporation will be relieved of any liability to the beneficial owners of the monies unclaimed.
| 116 |
Treatment of Company Options and Company Restricted Shares
At the Effective Time, each Company Option issued under the Share Incentive Plans that is outstanding as of the Effective Time, whether vested or unvested, will be cancelled and converted into the right to receive, and each former holder of such Company Option will be paid by the Surviving Corporation or one of its subsidiaries, as soon as practicable after the Effective Time (and in any event no more than five business days after the Effective Time), an amount equal to the product of (a) the total number of Shares issuable under such Company Option immediately prior to the Effective Time, multiplied by (b) the excess of US$7.00 over the exercise price payable per Share under such Company Option, in cash, without interest and net of any applicable withholding taxes. If the exercise price per Share of any such Company Option is equal to or greater than US$7.00, such Company Options will be canceled without any payment therefor.
At the Effective Time, each Company Restricted Share issued under the 2012 Share Incentive Plan that is outstanding immediately prior to the Effective Time will be cancelled, and each former holder of such Company Restricted Share will be paid by the Surviving Corporation or one of its subsidiaries, as soon as practicable after the Effective Time (and in any event no more than five business days after the Effective Time), an amount equal to US$7.00, in cash, without interest and net of any applicable withholding taxes.
Notwithstanding the foregoing, each former holder of Company Options or Company Restricted Shares will be personally responsible for the payment of all taxes in connection with the receipt of the payments referenced above in accordance with applicable tax laws, to the extent not deducted or withheld by the Surviving Corporation or one of its subsidiaries, as applicable.
The Share Incentive Plans will be terminated at the Effective Time.
Exchange Procedures
Prior to the Effective Time, Parent will enter into an agreement with a bank or trust company to serve as paying agent with respect to the Amalgamation. At or prior to the Effective Time, or in the case of payments related to the Dissenting Shares, after being ascertained, Parent will deposit, or cause to be deposited, with the paying agent for the benefit of the holders of Shares, sufficient cash for the paying agent to make the payments under the Amalgamation Agreement.
| 117 |
As promptly as practicable after the Effective Time (and in any event within three business days), the paying agent will mail to each registered holder of Shares (other than the Excluded Shares, the Company Restricted Shares and the Dissenting Shares, as the case may be) (a) a letter of transmittal specifying the manner in which the delivery of the Per Share Amalgamation Consideration to registered holders of the Shares will be effected and (b) if applicable, instructions for effecting the surrender of any issued share certificates (or affidavits and indemnities of loss in lieu of the share certificates) or non-certificated Shares represented by book entry (“Uncertificated Shares”) and/or such other documents as may be required in exchange for the Per Share Amalgamation Consideration. For Shares represented by share certificates, if any share certificate has been lost, stolen or destroyed, then the person claiming such share certificate to be lost, stolen or destroyed will have to make an affidavit of loss, theft or destruction, and, if required by the Surviving Corporation or the paying agent, post a bond in a reasonable amount as the Surviving Corporation or the paying agent may direct, as indemnity against any claim that may be made against it with respect to such share certificate before such person will be entitled to receive the Per Share Amalgamation Consideration.
Upon the surrender of, if applicable, any share certificates (or affidavits and indemnities of loss in lieu of the share certificates) or Uncertificated Shares, together with a duly completed and validly executed letter of transmittal, each registered holder of Shares (other than the Excluded Shares, the Company Restricted Shares and the Dissenting Shares, as the case may be) represented by such share certificates and each registered holder of Uncertificated Shares (other than the Excluded Shares, the Company Restricted Shares and the Dissenting Shares, as the case may be) will be entitled to receive a payment in an amount equal to (a) the number of Shares held, multiplied by (b) the Per Share Amalgamation Consideration, without interest and net of any applicable withholding taxes. The share certificate so surrendered will be marked as cancelled.
In the event of a transfer of ownership of Shares that is not registered in the register of shareholders of the Company, the Per Share Amalgamation Consideration may be paid to a person other than the person in whose name the relevant Share is registered in the register of shareholders of the Company if the relevant share certificates are presented to the paying agent, accompanied by all documents reasonably required to evidence and effect such transfer and to evidence that any applicable share transfer taxes have been paid or are not applicable.
Representations and Warranties
The Amalgamation Agreement contains representations and warranties made by the Company to Parent and Amalgamation Sub and representations and warranties made by Parent and Amalgamation Sub to the Company. The statements embodied in those representations and warranties were made for purposes of the Amalgamation Agreement and are subject to important qualifications and limitations agreed by the parties in connection with negotiating the terms of the Amalgamation Agreement (including a disclosure schedule delivered by the Company in connection therewith but not reflected in the Amalgamation Agreement).
| 118 |
In addition, some of those representations and warranties may be subject to a contractual standard of materiality different from that generally applicable to shareholders, may have been made for the principal purposes of establishing the circumstances in which a party to the Amalgamation Agreement may have the right not to close the Amalgamation if the representations and warranties of the other party prove to be untrue due to a change in circumstance or otherwise, and allocating risk between the parties to the Amalgamation Agreement rather than establishing matters as facts. The representations and warranties made by the Company were qualified by its public disclosure with the SEC prior to the date of the Amalgamation Agreement and a disclosure schedule delivered by the Company to Parent and Amalgamation Sub contemporaneously with the execution of the Amalgamation Agreement.
The representations and warranties made by the Company to Parent and Amalgamation Sub include representations and warranties relating to, among other things:
| · | due organization, valid existence, good standing and qualification, license or authority to carry on the Company’s business; |
| · | the articles of incorporation and by-laws of the Company being in full force and effect; |
| · | the Company’s capitalization, the absence of any outstanding subscriptions, options, warrants, conversion rights, call rights or other similar rights or any debt securities that give its holders the right to vote with the Company’s shareholders, and the absence of encumbrances on the Company’s ownership of the equity interests of its subsidiaries; |
| · | the Company’s corporate power and authority to execute, deliver and perform its obligations and to consummate the transactions under the Amalgamation Agreement, and the enforceability of the Amalgamation Agreement against the Company; |
| · | the required vote of the Company’s shareholders to authorize and approve the Amalgamation Agreement and the Transactions, including the Amalgamation; |
| · | the recommendation of the Special Committee, the determination of the fairness of the Amalgamation by the Board, the authorization and approval of the Amalgamation Agreement and the Transactions by the Board and the direction by the Board that the Amalgamation Agreement and the Transactions, including the Amalgamation, be submitted to the shareholders for authorization and approval; |
| · | the receipt by the Special Committee of an opinion from the Special Committee’s financial advisor; |
| 119 |
| · | the absence of (a) any conflict with or violation of the organizational documents of the Company and its subsidiaries, (b) any conflict with or violation of law applicable to the Company or any of its subsidiaries or by which any property or assets of the Company or any of its subsidiaries is bound or affected or (c) any violation, conflict with, requirement of consent under, breach of, or default under, or any right of termination, amendment, acceleration or cancellation given to others of, or creation of any lien or other encumbrance (other than certain permitted encumbrances) on any property or asset of the Company or any of its subsidiaries pursuant to, any contract to which the Company or any of its subsidiaries is a party or by which any of their respective properties or assets are bound, which would have a Company Material Adverse Effect (as defined below) or prevent or materially impair or delay or reasonably be expected to prevent or materially impair or delay, the consummation of Amalgamation or other Transactions, in each case, as a result of the Company’s execution, delivery and performance of the Amalgamation Agreement and the Transactions; |
| · | governmental consents and approvals in connection with the Company’s execution, delivery and performance of the Amalgamation Agreement and the Transactions, including the Amalgamation; |
| · | compliance with applicable laws (including anti-corruption laws), licenses and permits; |
| · | the Company’s SEC filings since January 1, 2014 and the financial statements included therein; |
| · | the status of the Company’s outstanding indebtedness; |
| · | compliance with the Sarbanes-Oxley Act of 2002, the rules and regulations of NASDAQ, the U.S. Securities Act of 1933 (as amended, the “Securities Act”) and the U.S. Exchange Act of 1934 (as amended, the “Exchange Act”); |
| · | the Company’s disclosure controls and procedures and internal controls over financial reporting; |
| · | the absence of a Company Material Adverse Effect and the absence of certain other changes or events from September 30, 2016 to the date of the Amalgamation Agreement; |
| · | the absence of legal proceedings and governmental investigations and orders against the Company or its subsidiaries; |
| · | employee benefits plans and labor and employment matters; |
| · | real property and title to assets; |
| · | intellectual property; |
| · | tax matters; |
| · | material contracts and the absence of any default under, breach or violation of, or termination of, any material contract; |
| 120 |
| · | environmental matters; |
| · | insurance; |
| · | the inapplicability of the Rights Agreement to Parent and Amalgamation Sub in connection with the Transactions, including the Amalgamation; |
| · | the absence of any brokerage, finder’s or other fees or commission, other than those fees payable to Duff & Phelps; and |
| · | acknowledgement by Parent and Amalgamation Sub as to the absence of any other representations and warranties by the Company. |
Many of the representations and warranties made by the Company in the Amalgamation Agreement are qualified as to “materiality” or “Company Material Adverse Effect.” As used herein and for purposes of the Amalgamation Agreement, a “Company Material Adverse Effect” means any fact, event, circumstance, change, condition, occurrence or effect that, individually or in the aggregate with all other facts, events, circumstances, changes, conditions, occurrences and effects, is or would reasonably be expected to be materially adverse to the business, financial condition or results of operations of the Company and its subsidiaries taken as a whole; provided that the determination of whether a Company Material Adverse Effect has occurred will not take into account any fact, event, circumstance, change, condition, occurrence or effect to the extent resulting from:
| (a) | geopolitical conditions, any outbreak of war, any act of sabotage or terrorism or natural or man-made disasters or certain other force majeure events; |
| (b) | changes in laws, GAAP or enforcement or interpretation thereof; |
| (c) | changes or conditions that generally affect the industry and market in which the Company and its subsidiaries operate; |
| (d) | changes in the financial, credit or other securities or capital markets, or in general economic, business, regulatory, legislative or political conditions; |
| (e) | any failure, in and of itself, of the Company and its subsidiaries to meet any internal or published projections, estimates, budgets, plans or forecasts of revenues, earnings or other financial performance measures or operating statistics or predictions or changes in the market price or trading volume of the securities of such person or the credit rating of such person (although the underlying facts giving rise or contributing to such failure or change may be taken into account in determining whether there has been a Company Material Adverse Effect); |
| (f) | the announcement, pendency or consummation of the Transactions, including any loss in respect of or change in relationship with any customer, supplier, employee, vendor, or other business partner of the Company due to such announcement, pendency or consummation or the identity of Parent or its affiliates; |
| 121 |
| (g) | any action taken (or omitted to be taken) by the Company or any of its subsidiaries at the written request or with the written consent of Parent or expressly required by the Amalgamation Agreement; |
| (h) | any suit, claim, request for indemnification or proceeding brought by any current or former shareholder of the Company or any of its subsidiaries (on their own behalf or on behalf of the Company) for breaches of fiduciary duties, violations of securities laws or otherwise in connection with the Amalgamation Agreement or the Transactions; |
| (i) | any action or inaction taken by or at the direction of a Sinobioway Representative (as such term is defined in the Amalgamation Agreement) without the authorization of the board of directors of Beijing Sinovac (including the directors thereof that are not Sinobioway Representatives), |
| (j) | any action or inaction taken by or at the direction of Sinobioway Bio-medicine Co., Ltd. or any of its affiliates, pursuant to or in connection with any lease between such person and Beijing Sinovac; |
| (k) | any action or inaction taken by or at the direction of Sinobioway Bio-medicine Co., Ltd., in its capacity as a shareholder of Beijing Sinovac; or |
| (l) | the matters, allegations or investigations disclosed in the Company’s Form 6-K (including the exhibit thereto) furnished to the SEC on May 16, 2017 or the Company’s Form 6-K (including the exhibit thereto) furnished to the SEC on December 23, 2016; |
provided that any fact, event, circumstance, change, condition, occurrence or effect referred to in clause (b), (c) or (d) may be taken into account in determining whether there has been a Company Material Adverse Effect to the extent having a materially disproportionate effect on the Company and any its subsidiaries, taken as a whole, relative to other participants in the industry in which the Company and its subsidiaries operate (in which case the incremental materially disproportionate impact or impacts may be taken into account in determining whether there has been a Company Material Adverse Effect).
| 122 |
Parent has also agreed that it will not have the right to (a) terminate the Amalgamation Agreement or (b) claim any damage or seek any other remedy at law or in equity, in each case, for any breach or inaccuracy in the representations and warranties made by the Company under the Amalgamation Agreement to the extent such breach or inaccuracy arises out of or relates to matters or circumstances of which Mr. Yin, any other Rollover Shareholder, Parent or any of their respective directors or officers has knowledge as of the date of the Amalgamation Agreement.
The representations and warranties made by Parent and Amalgamation Sub to the Company include representations and warranties relating to, among other things:
| · | their due organization, valid existence and good standing and power and authority to carry on their business; |
| · | their articles of incorporation and by-laws being in full force and effect; |
| · | their corporate power and authority to execute, deliver and perform their obligations under and to consummate the Transactions, including the Amalgamation, and the enforceability of the Amalgamation Agreement against them; |
| · | the absence of (a) any conflict with or violation of the articles of incorporation and by-laws of either Parent or Amalgamation Sub, (b) any conflict with or violation of any law applicable to Parent or Amalgamation Sub or by which any property or asset of either of them is bound or affected or (c) any breach of or default under, or any rights of termination, amendment, acceleration or cancellation given to others of, or the creation of any lien on any property or asset of Parent or Amalgamation Sub pursuant to, any contract or obligation to which Parent or Amalgamation Sub is a party or by which Parent or Amalgamation Sub or any property or asset of either of them is bound or affected, which would, individually or in the aggregate, prevent or materially delay the consummation of any of the Transactions, including the Amalgamation, by Parent or Amalgamation Sub or otherwise be materially adverse to the ability of either of them to perform their material obligations under the Amalgamation Agreement, in each case, as a result of the execution, delivery and performance of the Amalgamation Agreement and consummation of the Transactions; |
| · | governmental consents and approvals in connection with the execution, delivery and performance of the Amalgamation Agreement and consummation of the Transactions, including the Amalgamation, by Parent and Amalgamation Sub; |
| · | their capitalization, ownership structure and operations; |
| · | the sufficiency of funds available to complete the Amalgamation and the other Transactions subject to certain assumptions; |
| · | the absence of any brokerage, finders’ or other fees or commission other than those fees payable to Lazard Asia (Hong Kong) Limited; |
| 123 |
| · | each Limited Guarantee being in full force and effect and the lack of any default thereunder; |
| · | the absence of legal proceedings and governmental investigation or orders against Parent and Amalgamation Sub or any of their respective affiliates; |
| · | the absence of undisclosed Shares or other securities of, any other rights to acquire the Shares or other securities of, or any other economic interest in, the Company, beneficially owned by Parent, Amalgamation Sub, the Rollover Shareholders, the Sponsors or any of their respective affiliates; |
| · | the solvency of the Surviving Corporation at and immediately after the Effective Time, subject to certain assumptions; |
| · | the absence of any undisclosed agreements (a) relating to the Transactions, including the Amalgamation, (i) between or among Parent, Amalgamation Sub, any Rollover Shareholder, any Sponsor or any of their respective affiliates (excluding certain agreements relating to the Surviving Corporation following the Effective Time) or (ii) between or among Parent, Amalgamation Sub, any Rollover Shareholder, any Sponsor or any of their respective affiliates, on the one hand, and any member of the Company’s management, any members of the Board or any of the Company’s shareholders in their capacities as such, on the other hand, or (b) pursuant to which any shareholder of the Company would be entitled to receive consideration of a different amount or nature than the Per Share Amalgamation Consideration or pursuant to which any shareholder of the Company has agreed to vote to approve the Amalgamation Agreement or the Amalgamation or has agreed to vote against any Superior Proposal; |
| · | independent investigation conducted by Parent and Amalgamation Sub; and |
| · | acknowledgement by the Company as to the absence of any other representations and warranties by Parent or Amalgamation Sub. |
Conduct of Business Prior to Closing
Under the Amalgamation Agreement, the Company has agreed that, subject to certain exceptions, from the date of the Amalgamation Agreement until the earlier of the Effective Time or the termination of the Amalgamation Agreement, the business of the Company and its subsidiaries will be conducted in the ordinary course of business and in a manner consistent with past practice in all material respects, and the Company will use its commercially reasonable efforts to preserve intact the assets and business organization of the Company and its subsidiaries in all material respects, to keep available the services of the current officers and key employees of the Company and its subsidiaries and to maintain in all material respects the current relationships of the Company and its subsidiaries with existing customers, suppliers and other persons with which the Company and its subsidiaries have material business relations as of the date of the Amalgamation Agreement, except for any termination of services or relationships as a result of the expiration of any agreements or contracts.
| 124 |
Subject to certain exceptions set forth in the Amalgamation Agreement and the disclosure schedule of the Company delivered in connection with the Amalgamation Agreement or as required by applicable law, unless Parent consents in writing (which consent may not be unreasonably withheld, conditioned or delayed), the Company will not and will not permit its subsidiaries to, among other things:
| · | amend the Company’s articles of incorporation and by-laws or the equivalent organizational documents of any of its subsidiaries; |
| · | issue, sell, transfer, lease, sublease, license, pledge, dispose of, grant or encumber, or authorize the issuance, sale, transfer, lease, sublease, license, pledge, disposition, grant or encumbrance of, with limited exceptions, any shares of any class of the Company or any of its subsidiaries; |
| · | with limited exceptions, sell any property or assets with a value or purchase price (including the value of assumed liabilities) in excess of US$4.5 million, except in the ordinary course of business; |
| · | declare, set aside, make or pay any dividend or other distribution with respect to any shares, except for dividends or other distributions from any subsidiary of the Company to the Company or any other subsidiary of the Company; |
| · | reclassify, combine, split, subdivide or redeem, or purchase or otherwise acquire, directly or indirectly, any of its share capital or securities or other rights exchangeable into or convertible or exercisable for any of its share capital, except for the purchase of Shares to satisfy obligations under the Share Incentive Plans; |
| · | subject to limited exceptions, adopt a plan or agreement of, or commence any proceeding for, complete or partial liquidation, winding up or dissolution, scheme of arrangement, merger, consolidation, amalgamation, restructuring, reorganization, or similar transaction involving the Company or any of its subsidiaries; |
| · | other than pursuant to existing contracts or commitments, acquire, whether by purchase, merger, consolidation, scheme of arrangement, amalgamation or acquisition of stock or assets or otherwise, any assets, securities or properties, in aggregate, with a value or purchase price (including the value of assumed liabilities) in excess of US$3.5 million in any transaction or related series of transactions; |
| · | incur or guarantee any indebtedness for borrowed money, except for the incurrence or guarantee of indebtedness (a) under existing credit facilities of the Company or any of its subsidiaries as in effect on the date of Amalgamation Agreement or (b) not in an aggregate amount in excess of US$5.0 million; |
| · | make capital expenditures in excess of US$4.0 million in the aggregate for the Company and its subsidiaries, taken as a whole, during any twelve month period, except as consistent with past practice and in the ordinary course of business; |
| 125 |
| · | except as required under applicable law or pursuant to any employee benefit plan or the Amalgamation Agreement, (a) enter into any new employment or compensatory agreements, or terminate any such agreements, with any director, officer, employee or consultant of the Company or any of its subsidiaries, other than the hiring or termination of employees below the general manager level or its equivalent or with an annual compensation of less than US$150,000, (b) grant or provide any severance or termination payments or benefits to any director or officer of the Company or any of its subsidiaries, except as required by any contract or agreement in effect as of the date of the Amalgamation Agreement, (c) subject to certain exemptions, increase the compensation, bonus, severance or other benefits of, or pay any bonus to, any director, officer, employee or consultant of the Company or any of its subsidiaries whose annual compensation as of the date of the Amalgamation Agreement is in excess of US$150,000, (d) make any new equity awards to any director, officer or employee of the Company or any of its subsidiaries, (e) establish, adopt, amend or terminate any employee benefit plan of the Company or materially amend the terms of any outstanding Company Options or Company Restricted Shares, (f) subject to certain exceptions, take any action to voluntarily accelerate the vesting of Company Options or Company Restricted Shares or (g) forgive any loans to directors, officers or employees of the Company or any of its subsidiaries; |
| · | make any material changes with respect to financial accounting policies or procedures, except as required by changes in statutory or regulatory accounting rules or GAAP or regulatory requirements with respect to financial accounting policies or procedures or where such changes would better reflect the business and operations of the Company and its subsidiaries due to changes in circumstances; |
| · | other than in the ordinary course of business, enter into or amend, modify, consent to the termination of, or waive any material rights under any material contract that calls for annual aggregate payments of US$4.5 million or more that cannot be terminated without material surviving obligations or material penalty upon notice of 90 days or less; |
| · | enter into any contract between the Company or any of its subsidiaries, on the one hand, with any of their directors or executive officers, on the other hand, required to be disclosed pursuant to Item 7B or Item 19 of Form 20-F under the Exchange Act (except as contemplated or permitted under the Amalgamation Agreement); |
| · | other than renewals in the ordinary course of business, terminate or cancel, let lapse, or amend or modify in any material respect any material insurance policies maintained by it that is not promptly replaced by a comparable amount of insurance coverage; |
| · | commence any action for a claim of more than US$1.0 million (excluding any action seeking injunctive relief or other similar equitable remedies) or settle any action other than any settlement involving the payment of monetary damages not in excess of US$1.0 million; |
| 126 |
| · | permit any material intellectual property owned by the Company or any of its subsidiaries to lapse, be abandoned, dedicated or disclaimed, or fail to perform or make any applicable filings, recordings or other similar actions or filings or fail to pay material fees and taxes required to maintain and protect its interest such material intellectual property; |
| · | engage in the conduct of any new line of business material to the Company and its subsidiaries, taken as a whole; |
| · | make or change any material tax election, materially amend any tax return (except as required by applicable law), enter into any material closing agreement with respect to taxes, surrender any right to claim a material refund of taxes, settle or finally resolve any material controversy with respect to taxes or materially change any method of tax accounting; or |
| · | enter into any agreement or otherwise make a commitment to do any of the foregoing. |
No Solicitation of Competing Transactions
The Company has agreed that, until the earlier of the Effective Time and the termination of the Amalgamation Agreement, neither it nor any of its subsidiaries will, and that the Company will cause its and its subsidiaries’ representatives (including any investment banker, attorney or accountant retained by the Company or any of its subsidiaries) not to, directly or indirectly:
| · | solicit, initiate or knowingly encourage (including by providing nonpublic information concerning the Company or any of its subsidiaries) any inquiries or the making of any proposal or offer (including any proposal or offer to its shareholders) that constitutes, or that in the Company’s good faith judgment could reasonably be expected to lead to, any Competing Transaction (as defined below); |
| · | enter into, maintain or continue discussions or negotiations with, or provide any nonpublic information concerning the Company or any of its subsidiaries to, any third party in furtherance of such inquiries or to obtain such proposal or offer for a Competing Transaction; |
| · | agree to, approve, endorse, recommend or consummate any Competing Transaction or enter into any letter of intent or contract (other than a customary confidentiality agreement) or commitment contemplating or otherwise relating to any Competing Transaction; |
| · | grant any waiver, amendment or release under any standstill, confidentiality or similar agreement or anti-takeover law (and the Company shall promptly take all action necessary to terminate or cause to be terminated any such waiver previously granted with respect to any provision of any such confidentiality, standstill or similar agreement and to enforce each such confidentiality, standstill or similar agreement); or |
| 127 |
| · | authorize or permit any of the representatives of the Company or any of the subsidiaries of the Company to do any of the foregoing. |
As used herein and for purposes of the Amalgamation Agreement, a “Competing Transaction” means any of the following (other than the Transactions): (a) any amalgamation, consolidation, share exchange, business combination, scheme of arrangement, recapitalization, liquidation, dissolution or other similar transaction involving the Company or any of its subsidiaries whose assets, individually or in the aggregate, constitute 20% or more of the consolidated assets of the Company or to which 20% or more of the total revenue or net income of the Company are attributable, (b) any sale, lease, exchange, transfer or other disposition of assets or businesses that constitute or represent 20% or more of the total revenue, net income or assets of the Company and its subsidiaries, taken as a whole, (c) any sale, exchange, transfer or other disposition of 20% or more of any class of equity securities of the Company or securities convertible into or exchangeable for 20% or more of any class of equity securities of the Company, (d) any tender offer or exchange offer that, if consummated, would result in any person beneficially owning 20% or more of any class of equity securities of the Company or (e) any combination of the foregoing.
The Company has agreed to notify Parent as promptly as reasonably practicable (and in any event within 48 hours after the Company has knowledge thereof) of any proposal or offer, or any inquiry or contact with any person, that constitutes a Competing Transaction, or that in the Company’s good faith judgment could reasonably be expected to lead to a Competing Transaction, specifying the material terms and conditions thereof (including material amendments or proposed material amendments) and the identity of the party making such proposal, offer, inquiry or contact. The Company must also keep Parent informed, on a reasonably current basis (and in any event within 48 hours of the occurrence of any material change, development, discussion or negotiation) of the status and terms of any such proposal, offer or request and of any material changes in the status and terms of any such proposal, offer or request (including the material terms and conditions thereof). The Company is required to provide Parent with 48 hours’ prior notice (or such lesser prior notice as is provided to the directors of the Company or the members of the Special Committee, as applicable) of any meeting of the Board or the Special Committee at which the Board or the Special Committee, as applicable, is reasonably expected to consider any Competing Transaction.
The Company has agreed to (a) immediately cease and terminate all existing discussions or negotiations with any parties conducted as of the date of the Amalgamation Agreement with respect to a Competing Transaction and (b) not to, and to cause is subsidiaries not to, enter into any confidentiality agreement with any third party subsequent to the date of the Amalgamation Agreement that prohibits the Company from complying with its obligations in the immediately preceding paragraph.
| 128 |
At any time prior to obtaining the required shareholder authorization and approval of the Amalgamation Agreement and the Transactions, including the Amalgamation, following the receipt of a written bona fide proposal or offer regarding a Competing Transaction that was not obtained in violation of the Company’s “no-shop” obligations under the Amalgamation Agreement described above (other than any immaterial non-compliance that does not adversely affect Parent or Amalgamation Sub), the Company and its representatives may, acting under the recommendation of the Special Committee, (a) contact the person who has made such proposal or offer to clarify and understand the terms and conditions thereof to the extent the Special Committee has determined in good faith that such contact is necessary to determine whether such proposal or offer constitutes a Superior Proposal (as defined below) or could reasonably be expected to result in a Superior Proposal, (b) provide information (including non-public information) in response to the request of the person who has made such proposal or offer if such person has executed a customary confidentiality agreement (provided that the Company will concurrently make available to Parent any material nonpublic information concerning the Company and its subsidiaries that is provided to any such person and that was not previously made available to Parent or its representatives) and (c) engage or participate in any discussions or negotiations with such person. In each case referred to in clauses (b) and (c) above, the Board (acting only upon recommendation of the Special Committee) or the Special Committee shall have (i) determined in good faith (after consultation with its financial advisor and outside legal counsel) that such proposal or offer constitutes or could reasonably be expected to result in a Superior Proposal, (ii) determined in good faith (after consultation with its outside legal counsel) that, in light of such proposal or offer, failure to take such action would be inconsistent with the fiduciary duties of the Board under applicable law and (iii) provided written notice to Parent at least 48 hours prior to taking any such action.
As used herein and for purposes of the Amalgamation Agreement, a “Superior Proposal” means a written proposal or offer with respect to a Competing Transaction that would result in any person (or its shareholders, members or other equity owners) becoming the beneficial owner, directly or indirectly, of more than 50% of the assets (on a consolidated basis), or more than 50% of the total voting power of the equity securities, of the Company that (a) provides for the payment of (i) cash consideration per Share to holders thereof that is in excess of the Per Share Amalgamation Consideration or (ii) consideration in the form of publicly traded securities of a company listed on an internationally recognized securities exchange or automated quotation system with a fair market value that in the good faith judgment of the Special Committee after consultation with its financial advisor is in excess of the Per Share Amalgamation Consideration and (b) the Board (acting only upon the recommendation of the Special Committee) or the Special Committee has determined in its good faith judgment after consultation with its financial advisor and outside legal counsel, (i) is reasonably likely to be consummated in accordance with its terms, taking into account all legal, financial, and regulatory aspects of the proposal (including financing, regulatory approvals, shareholder litigation, the identity of the person or group making such proposal, breakup or termination fee and expense reimbursement provisions, expected timing and risk and likelihood of consummation and other relevant events and circumstances) and (ii) would, if consummated, result in a transaction more favorable to the Company’s shareholders (other than the holders of the Excluded Shares) from a financial point of view than the Transactions, including the Amalgamation; provided that no offer or proposal will be deemed to be a “Superior Proposal” if (x) the receipt of any financing required to consummate the transaction contemplated by such offer or proposal is a condition to the consummation of such transaction, or (y) the consummation of the transactions contemplated by such offer or proposal is expressly conditioned upon obtaining any consent or approval of a governmental authority or other third party that is not expressly set forth as a condition to the consummation of the Amalgamation contained in the Amalgamation Agreement.
| 129 |
No Change of Recommendation
The Board and the Special Committee have each resolved to recommend that the Company’s shareholders authorize and approve the Amalgamation Agreement and the Transactions, including the Amalgamation. Under the Amalgamation Agreement, neither the Board nor any committee of the Board may:
| · | change, withhold, withdraw, qualify or modify (or publicly propose to do so), in a manner adverse to Parent or Amalgamation Sub, the Board’s recommendation in favor of the proposal to authorize and approve the Amalgamation Agreement and the Transactions (the “Company Recommendation”); |
| · | fail to include the Company Recommendation in this proxy statement; |
| · | adopt, approve or recommend (or publicly propose to do so) to the Company’s shareholders a Competing Transaction; |
| · | if a tender offer or exchange offer that constitutes a Competing Transaction is commenced, fail to publicly recommend against acceptance of such tender offer or exchange offer by the Company shareholders (including, for these purposes, by disclosing that it is taking no position with respect to the acceptance of such tender offer or exchange offer by its shareholders, provided that a customary “stop, look and listen” communication by the Board or a statement that the Board has received and is currently evaluating such Competing Transaction will not be prohibited or be deemed to be a Change in the Company Recommendation (as defined below)) within ten business days after commencement thereof (any of the foregoing, a “Change in the Company Recommendation”); or |
| · | subject to certain exceptions, cause or permit the Company or any of its subsidiaries to enter into any letter of intent, memorandum of understanding, agreement in principle, merger agreement, acquisition agreement or other similar document or contract with respect to any Competing Transaction. |
If, however, following the date of the Amalgamation Agreement and prior to obtaining the required shareholder authorization and approval of the Amalgamation Agreement and the Transactions, including the Amalgamation, the Company receives a written bona fide proposal or offer with respect to a Competing Transaction that did not result from a breach of the “no-shop” obligations pursuant to the Amalgamation Agreement described above (other than any immaterial non-compliance that does not adversely affect Parent or Amalgamation Sub) and the Board (acting only upon the recommendation of the Special Committee) or the Special Committee determines in its good faith judgment (after consultation with its financial advisor and outside legal counsel) that such proposal or offer constitutes a Superior Proposal and failure to make a Change in the Company Recommendation with respect to such Superior Proposal would be inconsistent with the fiduciary duties of the Board under applicable law, the Board (acting only upon the recommendation of the Special Committee) or the Special Committee may make a Change in the Company Recommendation with respect to such Superior Proposal and/or authorize the Company to terminate the Amalgamation Agreement, but only:
| 130 |
| · | after providing at least five business days’ written notice to Parent advising Parent that the Board has received a Superior Proposal, specifying the material terms and conditions of such Superior Proposal, identifying the person making such Superior Proposal and indicating that the Board (acting only upon the recommendation of the Special Committee) or the Special Committee, as applicable, intends to effect a Change in the Company Recommendation and/or authorize the Company to terminate the Amalgamation Agreement; |
| · | after negotiating with and causing its financial and legal advisors to negotiate with Parent, Amalgamation Sub and their respective representatives in good faith (to the extent Parent desires to negotiate) to make such adjustments in the terms and conditions of the Amalgamation Agreement and the financing, so that such third party proposal or offer would cease to constitute a Superior Proposal; provided that any material modifications to such third party proposal or offer that the Board or the Special Committee has determined to be a Superior Proposal will be deemed a new Superior Proposal for which the process outlined above (including the issuance of a new notice to Parent) must again be undertaken and complied with; provided further, that with respect to such new Superior Proposal, the notice period will be deemed to be a three-business-day period; and |
| · | following the end of such five-business-day period or three-business-day period (as applicable), the Board (acting only upon the recommendation of the Special Committee) or the Special Committee has determined, in its good faith judgment after consultation with its financial advisor and outside legal counsel, taking into account any changes to the Amalgamation Agreement and the financing proposed by Parent and Amalgamation Sub in response to the notice of Superior Proposal or otherwise, that the proposal or offer with respect to the Competing Transaction giving rise to the notice of Superior Proposal continues to constitute a Superior Proposal. |
In addition, the Company has agreed not to enter into any agreement with respect to a Superior Proposal unless the Company has concurrently paid to Parent the termination fee as described in further detail below in “—Termination Fees” beginning on page 141.
At any time following the date of the Amalgamation Agreement and prior to obtaining the required shareholder authorization and approval of the Amalgamation Agreement and the Transactions, including the Amalgamation, the Board (acting only upon the recommendation of the Special Committee) or the Special Committee may also make a Change in the Company Recommendation if (a) the Board (acting only upon the recommendation of the Special Committee) or the Special Committee determines in its good faith judgment (after consultation with outside legal counsel) that the failure to take such action would be inconsistent with its fiduciary duties under applicable and (b) the Company has (i) provided to Parent at least five business days’ prior written notice that it intends to take such action and specifying in reasonable detail the facts underlying the decision by the Board (acting only upon the recommendation of the Special Committee) or the Special Committee to take such action and (ii) during such five business day period, if requested in writing by Parent, engaged in good faith negotiations with Parent to amend the Amalgamation Agreement in such a manner that would obviate (as determined by the Board (acting only upon the recommendation of the Special Committee) or the Special Committee after consultation with outside legal counsel) the need for such Change in the Company Recommendation.
| 131 |
At any time following the date of the Amalgamation Agreement and prior to obtaining the required shareholder authorization and approval of the Amalgamation Agreement and the Transactions, including the Amalgamation, the Board (acting only upon the recommendation of the Special Committee) or the Special Committee may authorize the Company to terminate the Amalgamation Agreement if (a) an Intervening Event (as defined below) has occurred, (b) the Board (acting only upon the recommendation of the Special Committee) or the Special Committee determines in its good faith judgment (after consultation with outside legal counsel) that the failure to take such action would be inconsistent with its fiduciary duties under applicable law and (c) the Company has (i) provided to Parent at least five business days’ prior written notice that it intends to take such action and specifying in reasonable detail the facts underlying the decision by the Board (acting only upon the recommendation of the Special Committee) or the Special Committee to take such action and (ii) during such five business day period, if requested in writing by Parent, engaged in good faith negotiations with Parent to amend the Amalgamation Agreement in such a manner that would obviate (as determined by the Board (acting only upon the recommendation of the Special Committee) or the Special Committee after consultation with outside legal counsel) the need for such action.
As used herein and for purposes of the Amalgamation Agreement, an “Intervening Event” means an event, occurrence, change or development that materially affects the Company or any of its subsidiaries or their business, assets or operations that (a) was unknown by the Board and the Special Committee as of or prior to the date of the Amalgamation Agreement (or if known, the material consequences of which were not known to or reasonably foreseeable by the Board and the Special Committee as of date of the Amalgamation Agreement) and (b) occurs, arises or becomes known (or any material consequences thereof become known) to the Board or the Special Committee after the date of the Amalgamation Agreement and on or prior to obtaining the required shareholder authorization and approval of the Amalgamation Agreement and the Transactions, including the Amalgamation; provided that the receipt by the Company of a Competing Transaction or Superior Proposal will not be deemed to constitute, or be taken into account to determine whether such event, occurrence, change or development will constitute, an Intervening Event.
Company Shareholders’ Meeting
The Company has agreed that unless the Amalgamation Agreement is terminated, the Company will, as promptly as reasonably practicable after the SEC confirms that it has no further comments on Schedule 13E-3 or this proxy statement, duly convene and cause to occur a special meeting for the purpose of obtaining shareholder authorization and approval of the Amalgamation Agreement and the Transactions, including the Amalgamation. Unless required to do so by the applicable requirements of the Securities Act, the Exchange Act or any other applicable law, the Company may only change the record date or establish a different record date for the special meeting with the prior written consent of Parent.
| 132 |
The Company may adjourn the special meeting of its shareholders (a) to allow reasonable time for the filing and mailing of any supplemental or amended disclosure and for such supplemental or amended disclosure to be disseminated and reviewed by the Company’s shareholders prior to the special meeting or (b) as otherwise required by applicable law or any court of competent jurisdiction; provided that the special meeting of the Company’s shareholders will be adjourned in accordance with Section 113 of the IBCA to the same day two weeks from the originally scheduled special meeting of the Company’s shareholders if at the time of the ordinarily scheduled extraordinary general meeting proceeds to business there are insufficient Shares represented (either in person or by proxy) to constitute a quorum necessary to conduct business at the special meeting of the Company’s shareholders.
The Company has agreed that unless the Board (acting only upon the recommendation of the Special Committee) or the Special Committee, as applicable, effects a Change in the Company Recommendation in the manner described above, the Board is required to recommend that the Company’s shareholders vote to authorize and approve the Amalgamation Agreement and the Transactions, including the Amalgamation, and include such recommendation in this proxy statement, and the Company is required to use its reasonable best efforts to solicit from its shareholders proxies in favor of the proposal to authorize and approve the Amalgamation Agreement and the Transactions, including the Amalgamation, and take all other actions necessary or advisable to secure the required shareholder authorization and approval. The Company has also agreed that, unless the Amalgamation Agreement is validly terminated in accordance with its terms, its obligations described above with respect to the special meeting will not be limited or otherwise affected by the commencement, public proposal, public disclosure or communication to the Company or any other person of any Competing Transaction or by any Change in the Company Recommendation.
Directors’ and Officers’ Indemnification and Insurance
Pursuant to the Amalgamation Agreement, Parent and Amalgamation Sub have agreed that:
| · | The indemnification, advancement and exculpation provisions of certain indemnification agreements by and among the Company and its directors and certain executive officers, as in effect at the Effective Time, will survive the Amalgamation and may not be amended, repealed or otherwise modified for a period of six years from the Effective Time in any manner that would adversely affect the rights thereunder of the current or former directors or officers of the Company or any of its subsidiaries. |
| · | The articles of incorporation and by-laws of the Surviving Corporation will contain provisions no less favorable to the intended beneficiaries with respect to exculpation and indemnification of liability and advancement of expenses than are set forth in the articles of incorporation and by-laws of the Company (or in such documents of any successor to the business of the Surviving Corporation) as in effect on the date of the Amalgamation Agreement, which provisions will not be amended, repealed or otherwise modified for a period of six years from the Effective Time in any manner that would affect adversely the rights thereunder of individuals who, at or prior to the Effective Time, were directors, officers, employees, fiduciaries or agents of the Company, unless such modification is required by applicable law. |
| 133 |
| · | The Surviving Corporation will, and Parent will cause the Surviving Corporation to, maintain in effect for six years from the Effective Time the current directors’ and officers’ fiduciary liability insurance policies maintained by the Company with respect to matters occurring prior to the Effective Time on terms, conditions, retentions and limits of liability no less favorable than the existing insurance; provided that the Surviving Corporation may substitute therefor policies of at least the same coverage containing terms, conditions, retentions and limits of liability that are no less favorable than those provided under the Company’s current policies from an insurance carrier with the same or better credit rating as the Company’s current insurance carrier, and the Surviving Corporation will not be required to expend in any one year an amount in excess of 300% of the current annual premium paid by the Company for such insurance. If the annual premium for such insurance exceeds 300% of the current annual premium paid by the Company for such insurance, the Surviving Corporation must obtain a policy with the greatest coverage for a cost not exceeding such amount. In addition, the Company may and, at Parent’s request, the Company will, purchase a six-year “tail” prepaid policy prior to the Effective Time on terms, conditions, retentions and limits of liability no less advantageous to the indemnified parties than the existing directors’ and officers’ fiduciary liability insurance policies maintained by the Company. If a “tail” prepaid policy has been obtained by the Company prior to the Closing, the Surviving Corporation will (and Parent will cause the Surviving Corporation to) maintain the policy in full force and effect, and continue to honor the obligations thereunder. |
| · | From and after the Effective Time, the Surviving Corporation will (and Parent will cause the Surviving Corporation to) indemnify and hold harmless (a) the indemnified parties against any and all Damages arising out of, relating to or in connection with (i) the fact that such person is or was a director, officer or employee of the Company or any of its subsidiaries, or (ii) any acts or omissions occurring or alleged to have occurred (including acts or omissions with respect to the approval of the Amalgamation Agreement or the Transactions or arising out of or pertaining to the Transaction) prior to or at the Effective Time, to the extent provided under the Company’s or its subsidiaries’ organizational and governing documents or agreements in effect on the date of the Amalgamation Agreement (provided that such indemnification shall be subject to any limitation imposed from time to time under applicable law) and (b) such persons against any and all Damages arising out of acts or omissions in such persons’ official capacity as an officer, director or other fiduciary in the Company or any of its subsidiaries if such service was at the request or for the benefit of the Company or any of its subsidiaries. |
Financing
As of the date of the Amalgamation Agreement, Parent has delivered to the Company executed Equity Commitment Letters from each of the Sponsors pursuant to which each of the Sponsors has committed to purchase, or cause the purchase of, for cash, subject to the terms and conditions therein, equity securities of Holdco equal to the amount set forth therein for the purpose of financing the consummation of the Transactions.
| 134 |
Each of Parent and Amalgamation Sub has agreed that it will use, and will cause its subsidiaries to use, reasonable best efforts to obtain the financing for the Amalgamation on the terms and conditions described in the Equity Commitment Letters, including (a) maintaining in effect the Equity Commitment Letters, (b) satisfying on a timely basis all conditions to the closing of and funding under the Equity Commitment Letters, and not taking or failing to take any action that would reasonably be expected to prevent, impede or delay the availability and consummation of the financing, (c) consummating the financing under the Equity Commitment Letters at or prior to the Effective Time, (d) enforcing the parties’ funding obligations (and the rights of Parent and Amalgamation Sub) under the Equity Commitment Letters, including by seeking specific performance of the parties thereunder, and (e) not amending, altering or waiving, or agreeing to amend, alter or waive, any term of the Equity Commitment Letters without the prior written consent of the Company (acting only upon the recommendation of the Special Committee).
Parent has agreed to give the Company prompt notice (a) upon becoming aware of any breach of any material provision of the Equity Commitment Letters, or termination of any Equity Commitment Letter, or (b) upon the receipt of any written notice from any party to an Equity Commitment Letter with respect to any threatened breach of any material provision or threatened termination of any Equity Commitment Letter.
Actions Taken at the Direction of Certain Persons; Knowledge
Parent and Amalgamation Sub have agreed that the Company will not be deemed to be in breach of any representation, warranty, covenant or agreement in the Amalgamation Agreement if the alleged breach is primarily the result of (a) action or inaction taken by or at the direction of a Sinobioway Representative (as such term is defined in the Amalgamation Agreement) without the authorization of the board of directors of Beijing Sinovac (including the directors thereof that are not Sinobioway Representatives), (b) action or inaction taken by or at the direction of Sinobioway Bio-medicine Co., Ltd., in its capacity as a shareholder of Beijing Sinovac, (c) action or inaction taken by or at the direction of Sinobioway Bio-medicine Co., Ltd. or any of its affiliates pursuant to or in connection with any lease between such person and Beijing Sinovac or (d) action or inaction taken by the Company or any of its subsidiaries at the direction of Mr. Yin, any other Rollover Shareholder, Parent, any of their respective affiliates or any of their respective directors, officers, employees, agents or representatives, in each case, without the authorization of the Board (acting with the concurrence of the Special Committee) or the Special Committee.
In addition, Parent will not have the right to (a) terminate the Amalgamation Agreement or (b) claim any damage or seek any other remedy at law or in equity, in each case, for any breach or inaccuracy in the representations and warranties made by the Company in the Amalgamation Agreement to the extent such breach or inaccuracy arises out of or relates to any matters or circumstance of which Mr. Yin, any other Rollover Shareholder, Parent or any of their respective directors or officers has knowledge, as of the date of the Amalgamation Agreement.
| 135 |
Agreement to Use Reasonable Best Efforts
The parties to the Amalgamation Agreement have agreed to coordinate with one another and use reasonable best efforts to take all appropriate actions and do all things necessary, proper or advisable to consummate and make effective the Transactions; provided that no party will be required to take any action if such action would have a Company Material Adverse Effect.
Certain Additional Covenants
Pursuant to the terms of the Amalgamation Agreement, the Company, Parent and Amalgamation Sub have agreed to certain additional covenants related to the following:
| · | the filing of this proxy statement and the Schedule 13E-3 with the SEC (and cooperation in response to any comments from the SEC); |
| · | access by Parent and its representatives to the offices, properties, books and records of the Company or any of its subsidiaries and other information between the date of the Amalgamation Agreement and the earlier of the Effective Time and termination of the Amalgamation Agreement (subject to all applicable legal or contractual obligations and restrictions); |
| · | notification of certain events; |
| · | cooperation, assistance and exchange of information in connection with making filings and any other required submissions with a governmental authority with jurisdiction over the enforcement of any applicable antitrust laws with respect to the Transactions; |
| · | for Amalgamation Sub to perform its obligations under the Amalgamation Agreement and to consummate the Transactions; |
| · | participation in the defense and settlement of any shareholder litigation relating to the Amalgamation Agreement or the Transactions; |
| · | the prompt delivery to Parent of the resignation of the directors of the Company or any of its subsidiaries designated by Parent; |
| · | consultation with respect to press releases and other public announcements relating to the Amalgamation Agreement and the Transactions; |
| · | delisting by the Surviving Corporation from NASDAQ and the deregistration of the Shares under the Exchange Act as promptly as practicable after the Effective Time; |
| · | restrictions on Parent, Amalgamation Sub and the Parent Group (as defined in the Amalgamation Agreement) entering into or amending, modifying, withdrawing, terminating or waiving any rights under contracts with the Parent Group or any contract pursuant to which any management members, directors or shareholders of the Company, or any of their respective affiliates, receives any consideration or other economic value from any person in connection with the transactions contemplated by the Amalgamation Agreement that is not provided or expressly contemplated in existing contracts as of the date of the Amalgamation Agreement; |
| 136 |
| · | restrictions on Parent, Surviving Corporation and any of their subsidiaries from entering into or modifying any contract pursuant to which any employee of the Company receives any consideration or other economic value from any person in connection with the Transactions that is not provided or expressly contemplated in the contracts with the Buyer Consortium as of the date of the Amalgamation Agreement; and |
| · | certain employee matters. |
Conditions to the Amalgamation
The consummation of the Amalgamation is subject to the satisfaction of the following conditions:
| · | the Amalgamation Agreement and the Transactions, including the Amalgamation, being authorized and approved at the special meeting by the affirmative vote of holders of Shares representing at least two-thirds of the Shares present and voting in person or by proxy as a single class; |
| · | no governmental authority of competent jurisdiction having enacted, issued, promulgated, enforced or entered any law, award, writ, injunction, determination, rule, regulation, judgment, decree or executive order, whether temporary, preliminary or permanent, that is in effect and has or would have the effect of enjoining, restraining, prohibiting or otherwise making illegal the consummation of the Transactions. |
The obligations of Parent and Amalgamation Sub to complete the Amalgamation are also subject to the satisfaction, or waiver by Parent or Amalgamation Sub, of the following conditions:
| · | the representations and warranties of the Company in the Amalgamation Agreement (without giving effect to any qualifications as to “materiality” or “Company Material Adverse Effect”) being true and correct in all respects as of the date of the Amalgamation Agreement and as of the closing date of the Amalgamation, as though made on and as of such date and time (other than representations and warranties that by their terms address matters only as of a specified time, which must be true and correct only as of such time), except where the failure to be true and correct does not constitute a Company Material Adverse Effect; provided that (a) for certain representations and warranties of the Company, such representations and warranties must be true and correct in all respects except for de minimis inaccuracies as of the date of the Amalgamation Agreement and as of the closing date of the Amalgamation, as though made on and as of such date and time (other than representations and warranties that by their terms address matters only as of a specified time, which must be true and correct only as of such time), and (b) for certain representations and warranties of the Company, such representations and warranties must be true and correct in all respects as of the date of the Amalgamation Agreement and as of the closing date of the Amalgamation, as though made on and as of such date and time (other than representations and warranties that by their terms address matters only as of a specified time, which must be true and correct only as of such time); |
| 137 |
| · | the Company having performed or complied in all material respects with all agreements and covenants required by the Amalgamation Agreement to be performed or complied with by it on or prior to the closing date of the Amalgamation; |
| · | the Company having delivered to Parent a certificate dated the closing date of the Amalgamation, signed by a senior executive officer of the Company, certifying as to the satisfaction of the two immediately preceding conditions; |
| · | there not having been any Company Material Adverse Effect since the date of the Amalgamation Agreement that remains continuing; and |
| · | shareholders of the Company holding no more than 7.5% of the total issued and outstanding Shares, other than 1Globe Capital LLC and its affiliates (and any investment fund advised or managed by 1Globe Capital LLC or any of its affiliates) and the persons and entities comprising the reporting person identified as “Chiang Li Family” in the Schedule 13G relating to the Company filed by such reporting person with the SEC on April 11, 2016 (and their respective affiliates), having validly served a notice of dissent under Section 191 of the IBCA. |
The obligations of the Company to complete the Amalgamation are subject to the satisfaction, or waiver by the Company, of the following conditions:
| · | the representations and warranties of Parent and Amalgamation Sub in the Amalgamation Agreement (without giving effect to any qualifications as to “materiality”) being true and correct in all respects as of the date of the Amalgamation Agreement and as of the closing date of the Amalgamation, as though made on and as of such date and time (other than representations and warranties that by their terms address matters only as of a specified time, which must be true and correct only as of such time), except where such failure, individually or in the aggregate, has not, and would not reasonably be expected to, prevent, materially delay or materially impede or impair the ability of Parent and Amalgamation Sub to consummate the Transactions, including the Amalgamation; |
| · | each of Parent and Amalgamation Sub having performed or complied in all material respects with all agreements and covenants required by the Amalgamation Agreement to be performed or complied with by it on or prior to the closing date of the Amalgamation; and |
| 138 |
| · | Parent having delivered to the Company a certificate dated the closing date of the Amalgamation, signed by a director of Parent, certifying as to the satisfaction of the two immediately preceding conditions. |
Termination of the Amalgamation Agreement
The Amalgamation Agreement may be terminated at any time prior to the Effective Time:
| (a) | by mutual written consent of the Company (acting only upon the recommendation of the Special Committee) and Parent; |
| (b) | by either the Company (acting only upon the recommendation of the Special Committee) or Parent (provided that this termination right is not available to either the Company or Parent to the extent such party’s failure to fulfill any of its obligations under the Amalgamation Agreement (or other breach thereof) has been a material cause of, or resulted in, the failure of any applicable condition to the Amalgamation being satisfied), if: |
| · | the Amalgamation is not completed on or before March 26, 2018 (the “Termination Date”) (a “Termination Date Termination Event”); |
| · | any governmental authority of competent jurisdiction having enacted, issued, promulgated, enforced or entered any final and non-appealable law or award, writ, injunction, determination, rule, regulation, judgment, decree or executive order, or taken any other final and non-appealable action, that has the effect of making consummation of the Transactions illegal or otherwise prohibiting consummation of the Transactions; or |
| · | shareholders representing at least two-thirds of the Shares present and voting in person or by proxy as a single class at the special meeting do not authorize and approve the Amalgamation Agreement and the Transactions, including the Amalgamation, at the special meeting or any adjournment thereof (a “No-Vote Termination Event”); |
| (c) | by the Company (acting only upon the recommendation of the Special Committee), if: |
| · | Parent or Amalgamation Sub has breached any of its representations, warranties, covenants or agreements under the Amalgamation Agreement, (i) such that the conditions to the obligations of the Company to complete the Amalgamation would not be satisfied and (ii) which breach is incapable of being cured or, if capable of being cured, is not cured by Parent or Amalgamation Sub, as applicable, within 30 days following receipt of written notice of such breach from the Company (or, if the Termination Date is less than 30 days from the date of receipt of such notice, by the Termination Date); provided that the Company is not in material breach of any representations, warranties, covenants or agreements that would give rise to the failure of a condition to the obligations of Parent and Amalgamation Sub to complete the Amalgamation (a “Parent and Amalgamation Sub Breach Termination Event”); |
| 139 |
| · | (i) all of the conditions to the obligations of Parent and Amalgamation Sub to complete the Amalgamation have been satisfied (other than those conditions that by their nature are to be satisfied by actions taken at the Closing), (ii) the Company has delivered to Parent an irrevocable written notice confirming that all of the conditions to the obligations of the Company to complete the Amalgamation have been satisfied (or that the Company is willing to waive any unsatisfied conditions) and that it is ready, willing and able to consummate the Closing and (iii) Parent and Amalgamation Sub fail to complete the Closing within three business days following the date on which the Closing should have occurred pursuant to the Amalgamation Agreement (a “Parent and Amalgamation Sub Failure to Close Termination Event”); |
| · | prior to the receipt of shareholder authorization and approval of the Amalgamation Agreement and the Transactions, including the Amalgamation, (i) the Board (acting only upon the recommendation of the Special Committee) or the Special Committee has authorized the Company to terminate the Amalgamation Agreement and enter into an alternative acquisition agreement with respect to a Superior Proposal and (ii) the Company concurrently with the termination of the Amalgamation Agreement enters into an alternative acquisition agreement with respect to such Superior Proposal; provided that the Company will not be entitled to terminate the Amalgamation Agreement unless the Company has (a) complied with the “no-shop” obligations under the Amalgamation Agreement with respect to such Superior Proposal and/or alternative acquisition agreement (other than any immaterial non-compliance that does not adversely affect Parent or Amalgamation Sub) and (b) pays in full the Company termination fee prior to or concurrently with taking any such action (a “Superior Proposal Termination Event”); or |
| · | prior to the receipt of shareholder authorization and approval of the Amalgamation Agreement and the Transactions, including the Amalgamation, the Company Board (acting only upon the recommendation of the Special Committee) or the Special Committee has authorized the Company to terminate this Agreement in connection with an Intervening Event (an “Intervening Event Termination Event”); or |
| (d) | by Parent, if: |
| · | the Company has breached any of its representations, warranties, covenants or agreements under the Amalgamation Agreement, (i) such that the conditions to the obligations of Parent and Amalgamation Sub to complete the Amalgamation would not be satisfied and (ii) which breach is incapable of being cured or, if capable of being cured, is not cured by the Company within 30 days following receipt of written notice of such breach from Parent or Amalgamation Sub (or, if the Termination Date is less than 30 days from the date of receipt of such notice, by the Termination Date); provided that Parent and Amalgamation Sub are not in material breach of any representations, warranties, agreements, covenants or agreements that would give rise to the failure of a condition to the obligations of the Company to complete the Amalgamation (a “Company Breach Termination Event”); or |
| 140 |
| · | the Board or any committee thereof has effected a Change in the Company Recommendation (a “Change in the Company Recommendation Termination Event”). |
Termination Fees
The Company is required to pay Parent a termination fee of US$15.0 million in the event the Amalgamation Agreement is terminated:
| · | by either the Company or Parent if (a) a bona fide proposal or offer with respect to a Competing Transaction has been publicly made, proposed or communicated (and not withdrawn or abandoned) after the date of the Amalgamation Agreement and prior to the special meeting (or prior to the termination of the Amalgamation Agreement if there has been no special meeting), (b) following the occurrence of an event described in the preceding clause (a), the Company or Parent terminates the Amalgamation Agreement due to a Termination Date Termination Event or a No-Vote Termination Event, (c) neither Parent nor Amalgamation Sub has materially breached any of its representations, warranties or covenants under the Amalgamation Agreement and (d) within 12 months after the termination of the Amalgamation Agreement, the Company enters into a definitive agreement with respect to the Competing Transaction contemplated by such proposal or offer by a third party (provided that all references to “20%” in the definition of “Competing Transaction” under the Amalgamation Agreement will be deemed to be references to “50%” in this section); |
| · | by Parent pursuant to (a) a Company Breach Termination Event or (b) a Change in the Company Recommendation Termination Event; or |
| · | by the Company pursuant to (a) a Superior Proposal Termination Event or (b) an Intervening Event Termination Event. |
Parent is required to pay the Company a termination fee of US$15.0 million in the event the Amalgamation Agreement is terminated by the Company pursuant to (a) a Parent and Amalgamation Sub Breach Termination Event or (b) a Parent and Amalgamation Sub Failure to Close Termination Event.
In the event that the Company or Parent fails to pay the applicable termination fee when due and in accordance with the requirements of the Amalgamation Agreement, the Company or Parent, as the case may be, is required to reimburse the other party for reasonable costs and expenses actually incurred or accrued by the other party (including fees and expenses of counsel) in connection with collection of such unpaid termination fee, together with accrued interest on such unpaid termination fee.
| 141 |
Fees and Expenses
Whether or not the Amalgamation is completed, all costs and expenses incurred in connection with the Amalgamation Agreement and the transactions contemplated by the Amalgamation Agreement will be paid by the party incurring such costs and expenses, except as otherwise provided in the Amalgamation Agreement and the Interim Investors Agreement.
Modification or Amendment; Waiver of Conditions
The Amalgamation Agreement may be amended by an instrument in writing signed by the parties thereto at any time prior to the Effective Time with the approval of their respective boards of directors (or in the case of the Company, by action taken by or on behalf of the Special Committee); provided that after the authorization and approval of the Amalgamation Agreement and the Transactions, including the Amalgamation, by the Company’s shareholders, no amendment may be made that would reduce the amount or change the type of consideration into which each Share will be converted upon consummation of the Amalgamation.
At any time prior to the Effective Time, each of the parties to the Amalgamation Agreement (by action taken (a) with respect to Parent and Amalgamation Sub, by or on behalf of their respective boards of directors and (b) with respect to the Company, by action taken by or on behalf of the Special Committee) may, by an instrument in writing signed by the party or parties to be bound thereby, (i) extend the time for the performance of any obligation or other act of any other party to the Amalgamation Agreement, (ii) waive any inaccuracy in the representations and warranties of any other party contained in the Amalgamation Agreement or in any document delivered pursuant to the Amalgamation Agreement and (iii) waive compliance with any agreement of any other party or any condition to its obligations contained in the Amalgamation Agreement.
Remedies and Limitations on Liabilities
The parties to the Amalgamation Agreement may be entitled to specific performance of the terms of the Amalgamation Agreement, including an injunction or injunctions to prevent breaches of the Amalgamation Agreement, in addition to any other remedy at law or equity. The Company’s right to obtain an injunction or injunctions, or other appropriate form of specific performance or equitable relief, in each case, with respect to causing the financing from the Sponsors to be funded at any time is, however, subject to (a) all of the conditions to Parent’s and Amalgamation Sub’s obligations to complete the Amalgamation (other than those conditions that by their terms are to be satisfied at the Closing) having been satisfied or waived, (b) Parent and Amalgamation Sub failing to complete the Closing by the date on which the closing should have occurred pursuant to the Amalgamation Agreement, (c) the Company having irrevocably confirmed in writing that (i) all of the conditions to the Company’s obligations to complete the Amalgamation have been satisfied or that it is willing to waive any of such conditions and (ii) if specific performance is granted and the financing is funded, then it would take such actions that are within its control to cause the Closing to occur.
While a party may pursue both a grant of specific performance and monetary damages until such time as the other party pays the applicable termination fee (as applicable under the Amalgamation Agreement), no party will be permitted or entitled to receive both a grant of specific performance that results in the Closing and monetary damages.
| 142 |
The maximum aggregate liabilities of Parent and Amalgamation Sub, on the one hand, and the Company, on the other hand, for monetary damages in connection with the Amalgamation Agreement are limited to a termination fee of US$15.0 million, respectively, and reimbursement of certain expenses in the event the Company or Parent fails to pay the applicable termination fee when due and in accordance with the requirements of the Amalgamation Agreement.
| 143 |
PROVISIONS FOR UNAFFILIATED SECURITY HOLDERS
No provision has been made to (a) grant the Company’s shareholders access to corporate files of the Company and other parties to the Amalgamation or any of their respective affiliates or (b) to obtain counsel or appraisal services at the expense of the Company or any other such party or affiliate.
| 144 |
DISSENTERS’ RIGHTS
The following is a summary of the rights of Shareholders to dissent from the Amalgamation and receive payment for the fair value of their Shares (referred to in this proxy statement as “Dissenters’ Rights”). You must be a registered holder of Shares in order to exercise these rights.
Section 191 of the IBCA provides for the right of dissenting shareholders to be paid the fair value of his Shares if the corporation resolves to amalgamate with another corporation, otherwise than under section 174 (Re-organization) or 175 (Arrangements) as such terms are defined under the IBCA, provided they comply with the following procedures in accordance with sections 191 to 199 of the IBCA:
| a) | The dissenting shareholder must send to the Company, at or before any meeting of shareholders of the corporation at which a resolution to amalgamate (the “Resolution”) is to be voted on, a written dissent from the Resolution, unless the corporation did not give notice to the shareholder of the purpose of the meeting and of his right to dissent. |
| b) | Within 10 days after the shareholders of the corporation adopt the Resolution, the corporation must send notice to the dissenting shareholder that the Resolution has been adopted. The notice need not be sent to the dissenting shareholder if he has voted for the resolution or has withdrawn his dissent. |
| c) | A dissenting shareholder must, within 20 days after he receives notice from the corporation that the Resolution has been adopted, or if he does not receive that notice, within 20 days after he learns that a Resolution has been adopted, send to the Company a written notice of his intention to dissent containing: |
| (i) | His name and address; |
| (ii) | The number and class or series of shares in respect of which he dissents (this must be all the Shares of a class or series that he holds in the corporation); and |
| (iii) | A demand for payment of the fair value of the shares (“Notice”). |
After sending the Notice, the dissenting shareholder ceases to have any rights as a shareholder, other than the right to be paid the fair value of his shares unless:
| (i) | the dissenting shareholder withdraws his notice before the corporation makes an Offer (defined below); |
| (ii) | The corporation fails to make an Offer and the dissenting shareholder withdraws his notice; or |
| (iii) | The directors, as the circumstances require terminate the Amalgamation Agreement; in which case his rights as a shareholder are reinstated as of the date on which the Notice was sent to the Company. |
| 145 |
| d) | A dissenting shareholder must, within 30 days after sending a Notice, send the certificates representing the shares in respect of which he dissents to the Company or its transfer agent. A dissenting shareholder who fails to comply with this requirement has no right to make a claim for a demand for payment of the fair value of the shares. |
| e) | A corporation must, not later than 7 days after the day on which the action approved by the Resolution is effective or the day the corporation received the Notice, whichever is the later date, send to each dissenting shareholder who has sent such a Notice: |
| (i) | A written offer to pay for his shares in an amount considered by the directors of the corporation to be the fair value of those shares (the “Offer”), which must be accompanied with a statement showing how the fair value was determined; or |
| (ii) | If the corporation fails to satisfy the applicable solvency tests, a notification that it is unable lawfully to pay dissenting shareholders for their shares. |
| f) | A corporation must pay for the shares of a dissenting shareholder within 10 days after the Offer has been accepted; but the Offer lapses if the corporation does not receive an acceptance of the Offer within 30 days after it has been made. |
| g) | If a corporation fails to make an Offer, or if a dissenting shareholder fails to accept the Offer made by the corporation, the corporation may, within 50 days after the action approved by the Resolution is effective, apply to the court to fix a fair value for the shares of any dissenting shareholders. If a corporation fails to apply to the court, a dissenting shareholder may, within a further period of 20 days, apply to the court to fix a fair value for the shares of any dissenting shareholders. |
| h) | All dissenting shareholders whose shares have not been purchased by the corporation are to be joined as parties and are bound by the decision of the court. The corporation must notify each affected dissenting shareholder of the date, place and consequences of the application and of his right to appear and be heard in person or by counsel. |
| i) | Upon an application to the court, the court may determine whether any other person is a dissenting shareholder who should be joined as a party and the court must then fix a fair value for the shares of all dissenting shareholders. The court may appoint one or more appraisers to assist the court to fix a fair value for the shares of the dissenting shareholders. The final order of the court must be made against the corporation in favor of each dissenting shareholder of the corporation and for the amount of the shares of the dissenting shareholder as fixed by the court. The court may allow a reasonable rate of interest on the amount payable to each dissenting shareholder from the date the action approved by the resolution is effective until the date of payment by the corporation. |
The above summary is not a complete statement of the law, and is qualified in its entirety by the complete text of Sections 191 to 199 of the IBCA, a copy of which is attached as Annex B to this proxy statement. If you are contemplating the possibility of exercising your Dissenters’ Rights, you should carefully review the text of Annex B and the procedural steps required to exercise Dissenters’ Rights above pursuant to Sections 191 to 199 of the IBCA. These procedures are complex and you should consult your Antigua and Barbuda legal counsel. If you do not fully and precisely satisfy the requirements of the IBCA, you may lose your Dissenters’ Rights.
| 146 |
FINANCIAL INFORMATION
The following sets forth certain selected historical consolidated financial information of the Company. The financial data for the years ended December 31, 2015 and 2016 has been derived from the audited financial statements filed as part of the Company’s Annual Report on Form 20-F for the year ended December 31, 2016. The information set forth below is not necessarily indicative of future results and should be read in conjunction with “Item 5. Operating and Financial Review and Prospects” and the consolidated financial statements, related notes and other financial information included in the Company’s annual report on Form 20-F for the year ended December 31, 2016, which are incorporated into this proxy statement by reference. Please see “Where You Can Find More Information” for a description of how to obtain a copy of such reports.
| Year ended December 31, | ||||||||
| Consolidated statements of comprehensive income (loss) data | 2016 | 2015 | ||||||
| (Restated) | ||||||||
| (U.S. dollars in thousands except share and per share data) | ||||||||
| Sales | 72,431 | 67,414 | ||||||
| Cost of sales(1) | 22,393 | 18,408 | ||||||
| Gross profit | 50,038 | 49,006 | ||||||
| Operating expenses: | ||||||||
| Selling, general and administrative expenses(1) | 41,980 | 37,481 | ||||||
| Provision (recovery) for doubtful accounts | 1,412 | (49 | ) | |||||
| Research and development expenses(1) | 12,648 | 9,490 | ||||||
| Loss on disposal and impairment of property, plant and equipment | 478 | 26 | ||||||
| Government grants recognized in income | (6,984 | ) | (1,637 | ) | ||||
| Total operating expenses | 49,534 | 45,311 | ||||||
| Operating income | 504 | 3,695 | ||||||
| Interest and financing expenses | (1,729 | ) | (1,920 | ) | ||||
| Interest income | 731 | 1,155 | ||||||
| Other income | 100 | (174 | ) | |||||
| Income (loss) from continuing operations before income taxes | (394 | ) | 2,756 | |||||
| Income tax expenses | (2,664 | ) | (2,985 | ) | ||||
| Income (loss) from continuing operations | (3,058 | ) | (229 | ) | ||||
| Income (loss) from discontinued operations, net of tax nil | 2,338 | (728 | ) | |||||
| Net loss | (720 | ) | (957 | ) | ||||
| Less: Loss (income) attributable to non-controlling interests | 124 | (459 | ) | |||||
| Net loss attributable to shareholders of Sinovac | (596 | ) | (1,416 | ) | ||||
| Comprehensive loss | (9,563 | ) | (5,342 | ) | ||||
| Less: comprehensive (income) loss attributable to non-controlling interests | 953 | 82 | ||||||
| Comprehensive loss attributable to shareholders of Sinovac | (8,610 | ) | (5,260 | ) | ||||
| Weighted average number of common shares outstanding | ||||||||
| - basic | 56,949,083 | 56,313,927 | ||||||
| - diluted | 56,949,083 | 56,313,927 | ||||||
| Earnings (loss) per share | ||||||||
| Basic net income (loss) per share: | ||||||||
| Continuing operations | (0.05 | ) | (0.02 | ) | ||||
| Discontinued operations | 0.04 | (0.01 | ) | |||||
| Basic net income (loss) per share | (0.01 | ) | (0.03 | ) | ||||
| Diluted net income (loss) per share: | ||||||||
| Continuing operations | (0.05 | ) | (0.02 | ) | ||||
| Discontinued operations | 0.04 | (0.01 | ) | |||||
| Diluted net income (loss) per share | (0.01 | ) | (0.03 | ) | ||||
| (1) | Includes stock-based compensation of US$2.4 million and US$1.0 million in 2016 and 2015, respectively. |
| 147 |
| As of December 31, | ||||||||
| Balance sheet data | 2016 | 2015 | ||||||
| (Restated) | ||||||||
| (U.S. dollars in thousands) | ||||||||
| Cash and cash equivalents | 62,434 | 63,834 | ||||||
| Total assets | 211,355 | 202,927 | ||||||
| Short-term bank loans and current portion of long-term debt | 31,279 | 21,775 | ||||||
| Total current liabilities | 66,264 | 58,138 | ||||||
| Long term debt (include due to related party) | 9,448 | 756 | ||||||
| Net assets | 129,666 | 136,505 | ||||||
| Non-controlling interests | 13,899 | 14,852 | ||||||
| Common stock | 57 | 57 | ||||||
| Total shareholders’ equity | 115,767 | 121,653 | ||||||
Ratio of Earnings to Fixed Charges
| Year ended December 31, | ||||||||
| 2016 | 2015 | |||||||
| (Unaudited) | (Unaudited) | |||||||
Ratio of earnings to fixed charges(1) | (0.23 | ) | 1.44 | |||||
(1) For purposes of calculating the ratio of earnings to fixed charges, earnings consist of income (loss) from continuing operations before income taxes. Fixed charges consist of (a) interest expensed and capitalized, (b) amortized premiums, discounts and capitalized expenses related to indebtedness, (c) an estimate of the interest within rental expense, and (d) preference security dividend requirements of consolidated subsidiaries. The ratio of earnings to fixed charges should be read in conjunction with “Item 5. Operating and Financial Review and Prospects” and the consolidated financial statements, related notes and other financial information included in the Company’s Annual Report on Form 20-F for the year ended December 31, 2016.
Net Book Value per Share of Our Shares
The Company’s net book value per Share as of December 31, 2016 was US$2.03 based on the number of issued and outstanding Shares as of December 31, 2016.
| 148 |
TRANSACTIONS IN SHARES
Purchases by the Company
The Company has not purchased any Shares at any time within the past two years.
Purchases by the Buyer Consortium
There have been no purchases in the Company’s Shares by any member of the Buyer Consortium at any time during the past two years.
Prior Public Offerings
The Company’s Shares have been listed on the NASDAQ Global Select Market since January 3, 2011 under the symbol “SVA.” On February 2, 2010, the Company completed a follow-on public offering of 11,500,000 Shares at $5.75 per Share.
Transactions in Prior 60 Days
Other than the transactions discussed above, the Amalgamation Agreement and agreements entered into in connection therewith (including the Support Agreement), there have been no transactions in the Company’s Shares during the past 60 days by the Company, any of the Company’s officers or directors, the Buyer Consortium, or any other person with respect to which disclosure is provided in Annex D or any associate or majority-owned subsidiary of the foregoing.
| 149 |
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT OF THE COMPANY
The following table sets forth information with respect to the beneficial ownership of our Shares as of the date of this proxy statement, by:
| · | each of our directors and executive officers; and |
| · | each person known to us to own beneficially more than 5% of our Shares. |
Beneficial ownership is determined in accordance with the rules and regulations of the SEC. In computing the number of Shares beneficially owned by a person and the percentage ownership of that person, we have included Shares that the person has the right to acquire within 60 days of the date of this proxy statement including through the exercise of any option, warrant or other right or the conversion of any other security. These Shares, however, are not included in the computation of the percentage ownership of any other person.
| Shares Beneficially Owned | ||||||||
| Number | % | |||||||
| Directors and Executive Officers: | ||||||||
| Weidong Yin | 6,124,500 | 10.66 | ||||||
| Simon Anderson | 70,000 | * | ||||||
| Yuk Lam Lo | 70,000 | * | ||||||
| Kenneth Lee | 20,000 | * | ||||||
| Meng Mei | 20,000 | * | ||||||
| Nan Wang | 150,000 | * | ||||||
| Ming Xia | 96,500 | * | ||||||
| Qiang Gao | 70,000 | * | ||||||
| Jing Li | 67,500 | * | ||||||
| Principal Shareholders: | ||||||||
| SAIF Partners IV(1) | 10,780,820 | 18.77 | ||||||
| 1Globe Capital LLC(2) | 9,353,092 | 16.28 | ||||||
| Wellington Management Company LLP(3) | 5,521,262 | 9.61 | ||||||
| Chiang Li Family(4) | 3,459,763 | 6.02 | ||||||
| Samuel D. Isaly(5) | 2,667,500 | 4.64 | ||||||
| * | Less than 1% of our Shares. |
(1) According to the Amendment No. 6 to Schedule 13D filed with the SEC on June 27, 2017 by SAIF Partners IV L.P., SAIF IV GP, L.P. and SAIF IV GP Capital Ltd.
(2) According to the Schedule 13D filed with the SEC on July 7, 2017.
(3) According to the Amendment No. 3 to Schedule 13G filed with the SEC on February 9, 2017 by Wellington Management Group LLP, Wellington Group Holdings LLP, Wellington Investment Advisors Holdings LLP and Wellington Management Company LLP. Wellington Management Group LLP is a parent holding company of certain holding companies and certain investment advisors, including Wellington Management Company LLP. Wellington Investment Advisors Holdings LLP is owned by Wellington Group Holdings LLP. Wellington Group Holdings LLP is owned by Wellington Management Group LLP.
(4) According to the Schedule 13G filed with the SEC on April 11, 2016.
(5) According to the Amendment No. 1 to 13G filed with the SEC on February 11, 2016, consists of (i) 1,219,500 Shares beneficially owned by OrbiMed Advisors LLC and (ii) 1,448,000 Shares beneficially owned by OrbiMed Capital LLC. OrbiMed Advisors LLC and OrbiMed Capital LLC are investment advisors, and Samuel D. Isaly is the control person of OrbiMed Advisors LLC and OrbiMed Capital LLC.
| 150 |
FUTURE SHAREHOLDER PROPOSALS
If the Amalgamation is completed, we will not have public shareholders and there will be no public participants in any future shareholders’ meeting.
| 151 |
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Certain statements in this proxy statement, the documents attached hereto and the documents incorporated by reference in this proxy statement are forward-looking statements based on estimates and assumptions. These include statements as to such things as our financial condition, results of operations, plans, objectives, future performance and business, as well as forward-looking statements relating to the Amalgamation. Such forward-looking statements are based on facts and conditions as they exist at the time such statements are made.
Forward-looking statements are also based on current expectations, estimates and projections about our business and the Amalgamation, the accurate prediction of which may be difficult and involve the assessment of events beyond our control. The forward-looking statements are further based on assumptions made by management. Forward-looking statements can be identified by forward-looking language, including words such as “believes,” “anticipates,” “expects,” “estimates,” “intends,” “may,” “plans,” “predicts,” “projects,” “will,” “would” and similar expressions, or the negative of these words.
These statements are not guarantees of the underlying expectations or future performance and involve risks and uncertainties that are difficult to predict. Readers of this proxy statement are cautioned to consider these risks and uncertainties and not to place undue reliance on any forward-looking statements.
The following factors, among others, could cause actual results or matters related to the Amalgamation to differ materially from what is expressed or forecasted in the forward-looking statements:
| · | the satisfaction of the conditions to completion of the Amalgamation, including the authorization and approval of the Amalgamation Agreement by our shareholders; |
| · | the occurrence of any event, change or other circumstance that could give rise to the termination of the Amalgamation Agreement; |
| · | the inability to complete the Amalgamation due to the failure to obtain shareholder approval for the Amalgamation or the failure to satisfy other conditions to completion of the Amalgamation; |
| · | the failure to obtain any necessary financing arrangements set forth in in the equity commitment letters delivered pursuant to the Amalgamation Agreement; |
| · | the effect of the announcement or pendency of the Amalgamation on our business relationships, operating results and business generally; |
| · | the risk that the Amalgamation may not be completed in a timely manner or at all, which may adversely affect our business and the prices of our Shares; |
| · | the potential adverse effect on our business, properties and operations because of certain covenants we agreed to in the Amalgamation Agreement; |
| 152 |
| · | diversion of our management’s attention during the pendency of the Amalgamation from our ongoing operations; |
| · | the amount of the costs, fees, expenses and charges related to the Amalgamation and the actual terms of the financings that will be obtained for the Amalgamation; |
| · | the outcome of any legal proceedings, regulatory proceedings or enforcement matters that may be instituted against us and others relating to the Amalgamation or any other matters; and |
| · | other risks detailed in our filings with the SEC, including the information set forth under the caption “Item 3. Key Information—D. Risk Factors” in our Annual Report on Form 20-F for the year ended December 31, 2016 filed with the SEC on November 22, 2017. Please see “Where You Can Find More Information” beginning on page 154 for additional information. |
Furthermore, the forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures, collaborations, dividends or investments made by the parties. We believe that the assumptions on which our forward-looking statements are based are reasonable. However, many of the factors that will determine our future results are beyond our ability to control or predict and we cannot guarantee any future results, levels of activity, performance or achievements.
We cannot assure you that the actual results or developments we anticipate will be realized or, if realized, that they will have the expected effects on our business or operations. In light of the significant uncertainties inherent in the forward-looking statements, readers should not place undue reliance on forward-looking statements, which speak only as of the date on which the statements were made and it should not be assumed that the statements remain accurate as of any future date.
All subsequent written and oral forward-looking statements concerning the Amalgamation or other matters addressed in this proxy statement and attributable to us or any person acting on our behalf are expressly qualified in their entirety by the cautionary statements contained or referred to in this section. Forward-looking statements speak only as of the date they are made and, except as required by applicable law or regulation, we undertake no obligation to update these forward-looking statements to reflect future events or circumstances.
| 153 |
WHERE YOU CAN FIND MORE INFORMATION
We are subject to the reporting requirements of the Securities Exchange Act of 1934, as amended, applicable to foreign private issuers and we file or furnish our annual and current reports and other information with the SEC. You may read and copy these reports and other information at the SEC’s Public Reference Room at 100 F Street NE, Washington, D.C. 20549 at prescribed rates. Information on the operation of the Public Reference Room may be obtained by calling the SEC at 1-800-SEC-0330. The information we file or furnish is also available free of charge on the SEC’s website at http://www.sec.gov.
You also may obtain free copies of the documents the Company files with the SEC by going to the “Investor Relations” section of our website at www.sinovac.com. Our website address is provided as an inactive textual reference only. The information provided on our website is not part of this proxy statement, and therefore is not incorporated by reference.
Because the Amalgamation is a going private transaction, the Company and the Buyer Consortium have filed with the SEC a transaction statement on Schedule 13E-3 with respect to the Amalgamation. The Schedule 13E-3, including any amendments and exhibits filed or incorporated by reference therein, is available for inspection as set forth above. The Schedule 13E-3 will be amended to report promptly any material changes in the information set forth in the most recent Schedule 13E-3 filed with the SEC.
Statements contained in this proxy statement regarding the contents of any contract or other document are not necessarily complete and each such statement is qualified in its entirety by reference to that contract or other document attached as an exhibit hereto. The SEC allows us to “incorporate by reference” information into this proxy statement. This means that we can disclose important information by referring to another document filed separately with the SEC.
The information incorporated by reference is considered to be part of this proxy statement. This proxy statement and the information that we later file with the SEC may update and supersede the information incorporated by reference. Similarly, the information that we later file with the SEC may update and supersede the information in this proxy statement. The Company’s annual report on Form 20-F for the fiscal year ended December 31, 2016 filed with the SEC on November 22, 2017 is incorporated herein by reference.
To the extent that any of the periodic reports incorporated by reference in this proxy statement contain references to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 with respect to forward-looking statements, we note that these safe harbor provisions do not apply to any forward-looking statements we make in connection with the going private transaction described in this proxy statement.
We undertake to provide you without charge to each person to whom a copy of this proxy statement has been delivered, upon request, by first class mail or other equally prompt means, within one business day of receipt of the request, a copy of any or all of the documents incorporated by reference into this proxy statement, other than the exhibits to these documents, unless the exhibits are specifically incorporated by reference into the information that this proxy statement incorporates.
| 154 |
Requests for copies of our filings should be directed to the Company at the address and phone numbers provided in this proxy statement. Because the Amalgamation is a “going private” transaction, the Company and the Buyer Consortium have filed with the SEC a Transaction Statement on Schedule 13E-3 with respect to the Amalgamation.
The Schedule 13E-3, including any amendments and exhibits filed or incorporated by reference as a part of it, will be made available for inspection and copying at the principal executive offices of the Company during its regular business hours by any interested holder of the Shares or his, her or its representative who has been so designated in writing. Duff & Phelps has consented to the filing of the Discussion Materials, dated June 26, 2017, prepared by Duff & Phelps for discussion with the Special Committee as an exhibit to the Schedule 13E-3.
If you have any questions or need assistance in voting your Shares, you can contact , the proxy solicitor, at (+ outside the United States) or at .
THIS PROXY STATEMENT DOES NOT CONSTITUTE THE SOLICITATION OF A PROXY IN ANY JURISDICTION TO OR FROM ANY PERSON TO WHOM OR FROM WHOM IT IS UNLAWFUL TO MAKE SUCH PROXY SOLICITATION IN THAT JURISDICTION. YOU SHOULD RELY ONLY ON THE INFORMATION CONTAINED OR INCORPORATED BY REFERENCE IN THIS PROXY STATEMENT TO VOTE YOUR SHARES AT THE SPECIAL MEETING. WE HAVE NOT AUTHORIZED ANYONE TO PROVIDE YOU WITH INFORMATION THAT IS DIFFERENT FROM WHAT IS CONTAINED OR INCORPORATED BY REFERENCE IN THIS PROXY STATEMENT.
THIS PROXY STATEMENT IS DATED , 2018. YOU SHOULD NOT ASSUME THAT THE INFORMATION CONTAINED IN THIS PROXY STATEMENT IS ACCURATE AS OF ANY DATE OTHER THAN THAT DATE, AND THE MAILING OF THIS PROXY STATEMENT TO SHAREHOLDERS DOES NOT CREATE ANY IMPLICATION TO THE CONTRARY.
| 155 |
ANNEXES
| 156 |
Annex A
Amalgamation Agreement
EXECUTION VERSION
AMALGAMATION AGREEMENT
among
Sinovac (Cayman) Limited
SINOVAC AMALGAMATION SUB lIMITED
and
Sinovac Biotech LTD.
Dated as of June 26, 2017
| A-1 |
TABLE OF CONTENTS
| Page | ||
| Article I THE AMALGAMATION | A-2 | |
| Section 1.01 | The Amalgamation. | A-2 |
| Section 1.02 | Closing; Closing Date. | A-2 |
| Section 1.03 | Effective Time. | A-2 |
| Section 1.04 | Effects of the Amalgamation. | A-3 |
| Section 1.05 | Articles of Incorporation and By-laws of the Surviving Corporation. | A-3 |
| Section 1.06 | Directors and Officers. | A-3 |
| Article II CONVERSION OF SECURITIES; AMALGAMATION CONSIDERATION | A-3 | |
| Section 2.01 | Conversion of Securities. | A-3 |
| Section 2.02 | Share Incentive Plans, Outstanding Company Options and Company RSs. | A-4 |
| Section 2.03 | Dissenting Shares. | A-5 |
| Section 2.04 | Exchange of Share Certificates, etc. | A-6 |
| Section 2.05 | No Transfers. | A-9 |
| Section 2.06 | Agreement of Fair Value. | A-9 |
| Article III REPRESENTATIONS AND WARRANTIES OF THE COMPANY | A-9 | |
| Section 3.01 | Organization, Good Standing and Qualification. | A-9 |
| Section 3.02 | Articles of Incorporation and By-laws. | A-10 |
| Section 3.03 | Capitalization. | A-10 |
| Section 3.04 | Authority Relative to this Agreement; Fairness. | A-11 |
| Section 3.05 | No Conflict; Required Filings and Consents. | A-12 |
| Section 3.06 | Permits; Compliance with Laws. | A-13 |
| Section 3.07 | SEC Filings; Financial Statements. | A-14 |
| Section 3.08 | Absence of Certain Changes or Events. | A-16 |
| Section 3.09 | Absence of Litigation. | A-16 |
| Section 3.10 | Labor and Employment Matters. | A-17 |
| Section 3.11 | Real Property; Title to Assets. | A-18 |
| Section 3.12 | Intellectual Property. | A-19 |
| Section 3.13 | Taxes. | A-20 |
| Section 3.14 | Material Contracts. | A-20 |
| Section 3.15 | Environmental Matters. | A-22 |
| Section 3.16 | Insurance. | A-22 |
| Section 3.17 | Company Rights Plan. | A-22 |
| Section 3.18 | Brokers. | A-22 |
| Section 3.19 | No Other Representations or Warranties. | A-23 |
-i-
TABLE OF CONTENTS
| Page | ||
| Article IV REPRESENTATIONS AND WARRANTIES OF PARENT AND AMALGAMATION SUB | A-23 | |
| Section 4.01 | Corporate Organization. | A-23 |
| Section 4.02 | Authority Relative to This Agreement. | A-23 |
| Section 4.03 | No Conflict; Required Filings and Consents. | A-24 |
| Section 4.04 | Capitalization. | A-24 |
| Section 4.05 | Available Funds and Financing. | A-25 |
| Section 4.06 | Brokers. | A-26 |
| Section 4.07 | Guarantees. | A-26 |
| Section 4.08 | Absence of Litigation. | A-26 |
| Section 4.09 | Ownership of Company Shares. | A-26 |
| Section 4.10 | Solvency. | A-26 |
| Section 4.11 | Parent Group Contracts. | A-27 |
| Section 4.12 | Independent Investigation. | A-27 |
| Section 4.13 | No Additional Representations. | A-27 |
| Article V CONDUCT OF BUSINESS PENDING THE AMALGAMATION | A-28 | |
| Section 5.01 | Conduct of Business by the Company Pending the Amalgamation. | A-28 |
| Section 5.02 | Operation of Parent’s and Amalgamation Sub’s Business. | A-31 |
| Section 5.03 | No Control of Other Party’s Business. | A-31 |
| Article VI ADDITIONAL AGREEMENTS | A-31 | |
| Section 6.01 | Proxy Statement and Schedule 13E-3. | A-31 |
| Section 6.02 | Company Shareholders’ Meeting. | A-33 |
| Section 6.03 | Access to Information; Confidentiality. | A-34 |
| Section 6.04 | No Solicitation of Transactions. | A-35 |
| Section 6.05 | Directors’ and Officers’ Indemnification and Insurance. | A-39 |
| Section 6.06 | Notification of Certain Matters. | A-41 |
| Section 6.07 | Financing. | A-42 |
| Section 6.08 | Further Action; Reasonable Best Efforts. | A-43 |
| Section 6.09 | Obligations of Amalgamation Sub. | A-43 |
| Section 6.10 | Participation in Litigation. | A-44 |
| Section 6.11 | Resignations. | A-44 |
| Section 6.12 | Public Announcements. | A-44 |
| Section 6.13 | Stock Exchange Delisting; Deregistration. | A-44 |
| Section 6.14 | No Amendment to Parent Group Contracts. | A-45 |
| Section 6.15 | Employee Matters. | A-45 |
| Section 6.16 | Actions Taken at the Direction of Certain Persons; Knowledge. | A-46 |
-ii-
TABLE OF CONTENTS
| Page | ||
| Article VII CONDITIONS TO THE AMALGAMATION | A-46 | |
| Section 7.01 | Conditions to the Obligations of Each Party. | A-46 |
| Section 7.02 | Conditions to the Obligations of Parent and Amalgamation Sub. | A-47 |
| Section 7.03 | Conditions to the Obligations of the Company. | A-47 |
| Section 7.04 | Frustration of Closing Conditions. | A-48 |
| Article VIII TERMINATION | A-48 | |
| Section 8.01 | Termination by Mutual Consent. | A-48 |
| Section 8.02 | Termination by Either the Company or Parent. | A-48 |
| Section 8.03 | Termination by the Company. | A-49 |
| Section 8.04 | Termination by Parent. | A-50 |
| Section 8.05 | Effect of Termination. | A-50 |
| Section 8.06 | Termination Fee and Expenses. | A-50 |
| Article IX GENERAL PROVISIONS | A-53 | |
| Section 9.01 | Non-Survival of Representations, Warranties and Agreements. | A-53 |
| Section 9.02 | Notices. | A-53 |
| Section 9.03 | Certain Definitions. | A-54 |
| Section 9.04 | Severability. | A-62 |
| Section 9.05 | Interpretation. | A-62 |
| Section 9.06 | Entire Agreement; Assignment. | A-63 |
| Section 9.07 | Parties in Interest. | A-63 |
| Section 9.08 | Specific Performance. | A-63 |
| Section 9.09 | Governing Law; Dispute Resolution. | A-64 |
| Section 9.10 | Amendment. | A-65 |
| Section 9.11 | Waiver. | A-65 |
| Section 9.12 | Counterparts. | A-65 |
-iii-
AMALGAMATION AGREEMENT, dated as of June 26, 2017 (this “Agreement”), among Sinovac (Cayman) Limited, an exempted company incorporated with limited liability under the Laws of the Cayman Islands (“Parent”), Sinovac Amalgamation Sub Limited, an international business corporation incorporated under the Laws of Antigua and Barbuda and a wholly-owned Subsidiary of Parent (“Amalgamation Sub”), and Sinovac Biotech Ltd., a company limited by shares incorporated under the Laws of Antigua and Barbuda (the “Company”).
WHEREAS, Parent and the Company intend to enter into a transaction pursuant to which Amalgamation Sub will be amalgamated with and into the Company (the “Amalgamation”), with the Company surviving the Amalgamation and becoming a wholly-owned Subsidiary of Parent as a result of the Amalgamation;
WHEREAS, the board of directors of the Company (the “Company Board”), acting upon the unanimous recommendation of the Special Committee, has acted honestly and with good faith, reasonable care, skill, diligence and a view to the best interests of the Company, and has (a) determined that it is fair to, and in the best interests of, the Company and its shareholders (other than the holders of Excluded Shares), and declared it advisable, to enter into this Agreement and consummate the transactions contemplated by this Agreement, including the Amalgamation (collectively, the “Transactions”), (b) authorized and approved the execution, delivery and performance of this Agreement and the consummation of the Transactions, including the Amalgamation, and (c) resolved to recommend in favor of the authorization and approval of this Agreement and the consummation of the Transactions, including the Amalgamation, to the holders of Shares and direct that this Agreement and the consummation of the Transactions, including the Amalgamation, be submitted to a vote of the holders of Shares for authorization and approval by special resolution passed at the Shareholders’ Meeting;
WHEREAS, the board of directors of each of Parent and Amalgamation Sub has (a) approved the execution, delivery and performance by Parent and Amalgamation Sub, respectively, of this Agreement and the consummation of the Transactions and (b) declared it advisable for Parent and Amalgamation Sub, respectively, to enter into this Agreement;
WHEREAS, as an inducement to Parent’s and Amalgamation Sub’s willingness to enter into this Agreement, concurrently with the execution and delivery of this Agreement, the Rollover Shareholders (as defined below) have executed and delivered a support agreement among such Rollover Shareholders, Sinovac Holding (Cayman) Limited, the sole shareholder of Parent (“Holdco”), and Parent, dated as of the date hereof (the “Support Agreement”, as amended and supplemented from time to time), providing that, among other things, (a) the Rollover Shareholders will vote all Shares held directly or indirectly by them in favor of the authorization and approval of this Agreement and the Transactions, including the Amalgamation, and (b) the Rollover Shareholders agree, upon the terms and subject to the conditions in the Support Agreement, to receive no consideration for the cancellation of the Rollover Shares in accordance with the terms thereof; and
WHEREAS, as a condition and inducement to the Company’s willingness to enter into this Agreement, concurrently with the execution of this Agreement, the entities set out in Schedule A (each, a “Guarantor,” and collectively the “Guarantors”) have executed and delivered limited guarantees in favor of the Company with respect to certain obligations of Parent under this Agreement (each, a “Limited Guarantee” and collectively, the “Limited Guarantees,” as amended and supplemented from time to time).
NOW, THEREFORE, in consideration of the foregoing and the mutual covenants and agreements herein contained, and intending to be legally bound hereby, Parent, Amalgamation Sub and the Company hereby agree as follows:
Article
I
THE AMALGAMATION
Section 1.01 The Amalgamation.
Upon the terms and subject to the conditions set forth in this Agreement, and in accordance with the International Business Corporations Act, CAP. 222 of the Revised Laws of Antigua and Barbuda (as consolidated and revised) (the “IBCA”), at the Effective Time, Amalgamation Sub shall be amalgamated with and into the Company. As a result of the Amalgamation, the separate corporate existence of Amalgamation Sub shall cease and the Company shall continue as the surviving corporation in the Amalgamation (the “Surviving Corporation”) under the Laws of Antigua and Barbuda and become a wholly-owned Subsidiary of Parent.
Section 1.02 Closing; Closing Date.
Unless otherwise agreed in writing between the Company and Parent, the closing for the Amalgamation (the “Closing”) shall take place at 10:00 a.m. (Hong Kong time) at the offices of Weil, Gotshal & Manges, 29/F, Alexandra House, 18 Chater Road, Central, Hong Kong as soon as practicable, but in any event no later than the tenth (10th) Business Day following the day on which the last of the conditions set forth in Article VII to be satisfied or, if permissible, waived (other than those conditions that by their nature are to be satisfied at the Closing, but subject to the satisfaction or, if permissible, waiver of those conditions at such time) shall be satisfied or, if permissible, waived in accordance with this Agreement (such date being the “Closing Date”). By mutual agreement of Parent and the Company, the Closing may take place by conference call and exchange of faxes and/or e-mails of documents in .pdf format.
Section 1.03 Effective Time.
Subject to the provisions of this Agreement, on the Closing Date, Amalgamation Sub and the Company shall execute the articles of amalgamation (the “Articles of Amalgamation”) as prescribed in form 8 in the schedule to the IBCA. Amalgamation Sub and the Company shall file the Articles of Amalgamation and other documents required under the IBCA to effect the Amalgamation with the Financial Services Regulatory Commission as provided by Section 172 of the IBCA. The Amalgamation shall become effective at the time specified in the Articles of Amalgamation in accordance with the IBCA (the “Effective Time”).
| A-2 |
Section 1.04 Effects of the Amalgamation.
At the Effective Time, the Amalgamation shall have the effects specified in the IBCA. Without limiting the generality of the foregoing, and subject thereto, at the Effective Time, the Surviving Corporation shall succeed to and assume all the rights, property of every description, including choses in action, and the business, undertaking, goodwill, benefits, immunities and privileges, mortgages, charges or security interests and all contracts, obligations, claims, debts and liabilities of the Company and Amalgamation Sub in accordance with the IBCA.
Section 1.05 Articles of Incorporation and By-laws of the Surviving Corporation.
At the Effective Time, the articles of incorporation and by-laws of Amalgamation Sub, as in effect immediately prior to the Effective Time, shall be the articles of incorporation and by-laws of the Surviving Corporation until thereafter amended as provided by Law and such articles of incorporation and by-laws; provided that at the Effective Time, (a) Article I of the articles of incorporation of the Surviving Corporation shall be amended to read as follows: “The name of the Corporation is Sinovac Biotech Ltd.” and the articles of incorporation and by-laws of the Surviving Corporation shall be amended to refer to the name of the Surviving Corporation as “Sinovac Biotech Ltd.” and (b) references therein to the authorized share capital of the Surviving Corporation shall be amended to refer to the correct authorized capital of the Surviving Corporation as approved in the Articles of Amalgamation, if necessary.
Section 1.06 Directors and Officers.
The parties hereto shall take all actions necessary so that (a) the directors of Amalgamation Sub immediately prior to the Effective Time shall be the initial directors of the Surviving Corporation and (b) the officers of the Company immediately prior to the Effective Time shall be the initial officers of the Surviving Corporation, in each case, unless otherwise determined by Parent prior to the Effective Time, and until their respective successors are duly elected or appointed and qualified or until the earlier of their death, resignation or removal in accordance with the articles of incorporation and by-laws of the Surviving Corporation.
Article
II
CONVERSION OF SECURITIES; AMALGAMATION CONSIDERATION
Section 2.01 Conversion of Securities.
At the Effective Time, by virtue of the Amalgamation and without any action on the part of Parent, Amalgamation Sub, the Company or the holders of any securities of the Company:
(a) each common share, par value $0.001 per share, of the Company (each, a “Share”) issued and outstanding immediately prior to the Effective Time (other than the Excluded Shares, the Dissenting Shares and Company RSs) shall be cancelled in exchange for the right to receive $7.00 in cash per Share without interest (the “Per Share Amalgamation Consideration”) payable in the manner provided in Section 2.04;
(b) each of the Excluded Shares issued and outstanding immediately prior to the Effective Time shall be cancelled without payment of any consideration or distribution therefor;
(c) each of the Dissenting Shares shall be cancelled in accordance with Section 2.03 and thereafter represent only the right to receive the applicable payments set forth in Section 2.03;
| A-3 |
(d) each of the Company Options shall be cancelled in accordance with Section 2.02(b) and thereafter represent only the right to receive the applicable payments set forth in Section 2.02(c);
(e) each of the Company RSs shall be cancelled in accordance with Section 2.02(b) and thereafter represent only the right to receive the applicable payments set forth in Section 2.02(d);
(f) all Shares issued and outstanding immediately prior to the Effective Time, together with the associated Series A Junior Participating Preferred Share Purchase Rights (the “Rights”) issued under the Rights Agreement, dated as of March 28, 2016, between the Company and Pacific Stock Transfer Company, as rights agent (as amended from time to time, the “Rights Agreement”), shall cease to be outstanding, shall be cancelled and shall cease to exist, and the register of shareholders of the Company shall be amended accordingly; and
(g) each common share, par value $1.00 per share, of Amalgamation Sub issued and outstanding immediately prior to the Effective Time shall be converted into and become one validly issued, fully paid and non-assessable ordinary share, par value $0.001 per share, of the Surviving Corporation. Such common shares shall constitute the only issued and outstanding share capital of the Surviving Corporation, which shall be reflected in the register of shareholders of the Surviving Corporation.
Section 2.02 Share Incentive Plans, Outstanding Company Options and Company RSs.
(a) In accordance with Section 8 of each of the outstanding award agreements entered into with participants under the Stock Option Plan, the Company shall (i) take all actions reasonably necessary to make exercisable each Company Option issued under the Stock Option Plan, whether or not vested, that is then outstanding and unexercised, and (ii) deliver written notice to each holder of a Company Option informing such holder that (x) such Company Option shall be exercisable during the period from receipt of such notice until immediately prior to the Effective Time, (y) in accordance with Section 2.02(b), such Company Option shall be cancelled at the Effective Time to the extent not previously exercised, and (z) to the extent such Company Option is cancelled in accordance with clause (y), such holder shall have no further rights in respect of such Company Option other than the right to receive a payment in respect thereof in accordance with Section 2.02(c).
(b) At the Effective Time, the Company shall (i) terminate the Company’s Share Incentive Plans and any relevant award agreements entered into under the Share Incentive Plans, (ii) cancel each Company Option issued under each Share Incentive Plan that is outstanding and unexercised, whether or not vested or exercisable, and (iii) cancel each Company RS that is outstanding.
(c) Each former holder (or his or her designee) of a Company Option that is cancelled at the Effective Time shall, in exchange thereof, be paid by the Surviving Corporation or one of its Subsidiaries, as soon as practicable (and in any event no more than five Business Days) after the Effective Time (without interest), a cash amount equal to the product of (i) the excess, if any, of the Per Share Amalgamation Consideration over the Exercise Price of such Company Option and (ii) the number of Shares underlying such Company Option; provided that if the Exercise Price of any such Company Option is equal to or greater than the Per Share Amalgamation Consideration, such Company Option shall be cancelled without any payment therefor. Except as set forth in this Section 2.02(c), each such holder shall have no further rights in respect of such a Company Option.
| A-4 |
(d) Each former holder (or his or her designee) of a Company RS that is cancelled at the Effective Time shall, in exchange thereof, be paid by the Surviving Corporation or one of its Subsidiaries, as soon as practicable (and in any event no more than five Business Days) after the Effective Time (without interest), a cash amount equal to the Per Share Amalgamation Consideration. Except as set forth in this Section 2.02(d), each such holder shall have no further rights in respect of such a Company RS.
(e) Any payment under this Section 2.02 shall be made at or as soon as practicable (and in any event no more than five Business Days) after the Effective Time, pursuant to the Company’s ordinary payroll practices and subject to all applicable Taxes and Tax withholding requirements. Notwithstanding the foregoing, each former holder of Company Options and Company RSs shall be personally responsible for the proper reporting and payment of all Taxes related to any distribution contemplated by this Section 2.02.
(f) At or prior to the Effective Time, the Company, the Company Board or the compensation committee of the Company Board, as applicable, shall pass any resolutions and take any actions that are reasonably necessary to effectuate the provisions of this Section 2.02. The Company shall take all reasonable actions necessary to ensure that from and after the Effective Time neither Parent nor the Surviving Corporation will be required to issue Shares or other share capital of the Company or the Surviving Corporation to any person pursuant to the Share Incentive Plans or in settlement of any Company Option or Company RS (as applicable). Without limiting Section 2.02(a), promptly following the date hereof, the Company shall deliver written notice to each holder of Company Options and/or Company RSs informing such holder of the effect of the Amalgamation on his or her Company Options and/or Company RSs (as applicable).
Section 2.03 Dissenting Shares.
(a) Notwithstanding any provision of this Agreement to the contrary and to the extent available under the IBCA, Shares that are issued and outstanding immediately prior to the Effective Time and that are held by shareholders who shall have validly exercised and not effectively withdrawn or lost their rights to dissent from the Amalgamation, or dissenter rights, in accordance with Section 191 of the IBCA (collectively, the “Dissenting Shares” and holders of Dissenting Shares collectively being referred to as “Dissenting Shareholders”) shall be cancelled and the Dissenting Shareholders shall not be entitled to receive the Per Share Amalgamation Consideration and shall instead be entitled to receive only the payment of the fair value of such Dissenting Shares held by them determined in accordance with the provisions of Section 191(4) of the IBCA or Section 195(2) of the IBCA, as applicable.
(b) For the avoidance of doubt, all Shares held by Dissenting Shareholders who shall have failed to exercise or who effectively shall have withdrawn or lost their dissenter rights under Section 192(3) of the IBCA shall thereupon (i) not be deemed to be Dissenting Shares and (ii) be deemed to have been converted into, and to have become exchanged for, as of the Effective Time, the right to receive the Per Share Amalgamation Consideration, without any interest thereon, in the manner provided in Section 2.04. Parent shall promptly deposit or cause to be deposited with the Paying Agent any additional funds necessary to pay in full the aggregate Per Share Amalgamation Consideration so due and payable to such shareholders who have failed to exercise or who shall have effectively withdrawn or lost such dissenter rights under Section 192(3) of the IBCA.
| A-5 |
(c) The Company shall give Parent (i) prompt notice of any notices of objection, notices of dissent or demands for appraisal received by the Company, attempted withdrawals of such notices or demands, and any other instruments served pursuant to applicable Laws of Antigua and Barbuda and received by the Company relating to its shareholders’ rights to dissent from the Amalgamation or appraisal rights and (ii) the opportunity to direct all negotiations and proceedings with respect to any such notice or demand for appraisal under the IBCA. The Company shall not, except with the prior written consent of Parent, make any payment with respect to any exercise by a shareholder of its rights to dissent from the Amalgamation or any demands for appraisal or offer to settle or settle any such demands or approve any withdrawal of any such demands.
(d) In the event that any written notices of objection to the Amalgamation are served by any shareholders of the Company pursuant to Section 191(4) of the IBCA, the Company shall serve written notice of the authorization and approval of this Agreement and the Transactions on such shareholders pursuant to Section 191(7) of the IBCA within ten days of obtaining the Requisite Company Vote at the Shareholders’ Meeting.
Section 2.04 Exchange of Share Certificates, etc.
(a) Paying Agent. Prior to the Effective Time, Parent shall appoint a bank or trust company selected by Parent with the Company’s prior written consent (such consent not to be unreasonably withheld, conditioned or delayed) to act as paying agent (the “Paying Agent”) for all payments required to be made pursuant to Section 2.01(a) and Section 2.03(b) (in the case of Section 2.03(b), when ascertained) (collectively, the “Amalgamation Consideration”), and Parent shall enter into a paying agent agreement with the Paying Agent in form and substance reasonably acceptable to the Company. At or prior to the Effective Time, or in the case of payments pursuant to Section 2.03(b), when ascertained, Parent shall deposit, or cause to be deposited, with the Paying Agent, for the benefit of the holders of Shares, cash in an amount sufficient to pay the Amalgamation Consideration (such cash being hereinafter referred to as the “Exchange Fund”).
| A-6 |
(b) Exchange Procedures. As promptly as practicable after the Effective Time (and in any event within three Business Days), the Surviving Corporation shall cause the Paying Agent to mail to each person who was, at the Effective Time, a registered holder of Shares entitled to receive the Per Share Amalgamation Consideration pursuant to Section 2.01(a): (i) a letter of transmittal (which shall be in customary form for a company incorporated in Antigua and Barbuda reasonably acceptable to Parent and the Company, and shall specify the manner in which the delivery of the Exchange Fund to registered holders of Shares shall be effected and contain such other provisions as Parent and the Company may mutually agree prior to the Effective Time) and (ii) instructions for use in effecting the surrender of any issued share certificates representing Shares (the “Share Certificates”) (or affidavits and indemnities of loss in lieu of the Share Certificates as provided in Section 2.04(c)) or non-certificated Shares represented by book entry (“Uncertificated Shares”) and/or such other documents as may be required in exchange for the Per Share Amalgamation Consideration. Upon surrender of, if applicable, a Share Certificate (or affidavit and indemnity of loss in lieu of the Share Certificate as provided in Section 2.04(c)) or Uncertificated Shares and/or such other documents as may be required pursuant to such instructions to the Paying Agent in accordance with the terms of such letter of transmittal, duly executed in accordance with the instructions thereto, each registered holder of Shares represented by such Share Certificate and each registered holder of Uncertificated Shares shall be entitled to receive in exchange therefor a check, in the amount equal to (x) the number of Shares represented by such Share Certificate (or affidavit and indemnity of loss in lieu of the Share Certificate as provided in Section 2.04(c)) or the number of Uncertificated Shares multiplied by (y) the Per Share Amalgamation Consideration, and any Share Certificate so surrendered shall forthwith be marked as cancelled. No interest shall be paid or will accrue on any amount payable in respect of the Shares pursuant to the provisions of this Article II. In the event of a transfer of ownership of Shares that is not registered in the register of shareholders of the Company, a check for any cash to be exchanged upon due surrender of the Share Certificate may be issued to such transferee if the Share Certificates, if any, that immediately prior to the Effective Time represented such Shares are presented to the Paying Agent, accompanied by all documents reasonably required to evidence and effect such transfer and to evidence that any applicable share transfer Taxes have been paid or are not applicable.
(c) Lost Certificates. If any Share Certificate shall have been lost, stolen or destroyed, upon the making of an affidavit of that fact by the person claiming such Share Certificate to be lost, stolen or destroyed and, if required by the Surviving Corporation or the Paying Agent, the posting by such person of a bond, in such reasonable amount as the Surviving Corporation or the Paying Agent may direct, as indemnity against any claim that may be made against it with respect to such Share Certificate, the Paying Agent will pay in respect of such lost, stolen or destroyed Share Certificate an amount equal to the Per Share Amalgamation Consideration multiplied by the number of Shares represented by such Share Certificate to which the holder thereof is entitled pursuant to Section 2.01(a).
(d) Untraceable and Dissenting Shareholders. Remittances for the Per Share Amalgamation Consideration shall not be sent to holders of Shares who are untraceable unless and until, except as provided below, they notify the Paying Agent of their current contact details. A holder of Shares will be deemed to be untraceable and presumed to have received notice in accordance with Section 321 of the IBCA if (i) such person has no registered address in the register of shareholders maintained by the Company, (ii) on the last two consecutive occasions on which a dividend has been paid by the Company a check payable to such person either (A) has been sent to such person and has been returned undelivered or has not been cashed or (B) has not been sent to such person because on an earlier occasion a check for a dividend so payable has been returned undelivered, and in any such case no valid claim in respect thereof has been communicated in writing to the Company or (iii) notice of the Shareholders’ Meeting convened to vote on the Amalgamation has been sent to such person and has been returned undelivered. Monies due to Dissenting Shareholders and shareholders of the Company who are untraceable shall be returned to the Surviving Corporation on demand and held in a non-interest bearing bank account for the benefit of Dissenting Shareholders and shareholders of the Company who are untraceable. Monies unclaimed after a period of ten years from the Closing Date shall be classified as “abandoned property” and transferred to the appropriate Governmental Authority in accordance with Section 256 of the IBCA. Once such transfer has been made, the Surviving Corporation shall be relieved of any liability to the beneficial owners thereof.
| A-7 |
(e) Adjustments to Amalgamation Consideration. The Per Share Amalgamation Consideration shall be equitably adjusted to reflect appropriately the effect of any share split, reverse share split, share dividend (including any dividend or distribution of securities convertible into Shares), extraordinary cash dividends, reorganization, recapitalization, reclassification, combination, exchange of shares or other like change with respect to Shares occurring on or after the date hereof and prior to the Effective Time and to provide to the holders of Shares, Company Options and Company RSs the same economic effect as contemplated by this Agreement prior to such action.
(f) Investment of Exchange Fund. The Exchange Fund, pending its disbursement to the holders of Shares, shall be invested by the Paying Agent as directed by Parent; provided that (i) Parent shall not direct the Paying Agent to make any such investments that are speculative in nature and (ii) no such investment or losses shall affect the amounts payable to such holders and Parent shall promptly replace or cause to be replaced any funds deposited with the Paying Agent that are lost through any investment so as to ensure that the Exchange Fund is at all times maintained at a level sufficient for the Paying Agent to pay the Amalgamation Consideration. Earnings from investments shall be the sole and exclusive property of Parent and the Surviving Corporation. Except as contemplated by Section 2.04(a) and this Section 2.04(f), the Exchange Fund shall not be used for any other purpose.
(g) Termination of Exchange Fund. Any portion of the Exchange Fund that remains unclaimed by the holders of Shares for six months after the Effective Time shall be delivered to the Surviving Corporation upon demand, and any holders of Shares who have not theretofore complied with this Article II shall thereafter look only to the Surviving Corporation for the cash to which they are entitled pursuant to Section 2.01(a).
(h) No Liability. None of the Paying Agent, the Rollover Shareholders, the Sponsors, Holdco, Parent or the Surviving Corporation shall be liable to any former holder of Shares for any such Shares (or dividends or distributions with respect thereto) or cash properly delivered to a public official pursuant to any applicable abandoned property, bona vacantia, escheat or similar Law. Any amounts remaining unclaimed by such holders at such time at which such amounts would otherwise escheat to or become property of any Governmental Authority shall become, to the extent permitted by applicable Laws, the property of the Surviving Corporation or its designee, free and clear of all claims or interest of any person previously entitled thereto.
(i) Withholding Rights. The Surviving Corporation and the Paying Agent shall be entitled to deduct and withhold from the consideration otherwise payable pursuant to this Agreement to any holder of Shares, Company Options or Company RSs such amounts as it is required to deduct and withhold with respect to the making of such payment under any provision of applicable Tax Law. To the extent that amounts are so withheld by Parent, the Surviving Corporation or the Paying Agent, as the case may be, such withheld amounts shall be (i) remitted by Parent, the Surviving Corporation or the Paying Agent to the applicable Governmental Authority and (ii) to the extent so remitted, treated for all purposes of this Agreement as having been paid to the holder of the Shares, Company Options or Company RSs in respect of which such deduction and withholding was made by Parent, the Surviving Corporation or the Paying Agent, as the case may be. For the avoidance of doubt, each holder of Shares, Company Options and Company RSs shall be personally responsible for the payment of all taxes in connection with receipt of the applicable Amalgamation Consideration in accordance with applicable Tax Laws, to the extent not deducted or withheld pursuant to this Section 2.04(i).
| A-8 |
Section 2.05 No Transfers.
From and after the Effective Time, (a) no transfers of Shares shall be effected in the register of shareholders of the Company and (b) the holders of Shares issued and outstanding immediately prior to the Effective Time shall cease to have any rights with respect to such Shares, except as otherwise provided in this Agreement or by Law. On or after the Effective Time, any Share Certificates presented to the Paying Agent, Parent or Surviving Corporation for transfer or any other reason shall be cancelled, in exchange for the right to receive the cash consideration to which the holders thereof are entitled under this Article II in the case of Shares other than the Excluded Shares, and for no consideration in the case of Excluded Shares.
Section 2.06 Agreement of Fair Value.
Parent, Amalgamation Sub and the Company respectively agree that the Per Share Amalgamation Consideration represents the fair value of the Shares for the purposes of Section 191(4) of the IBCA.
Article
III
REPRESENTATIONS AND WARRANTIES OF THE COMPANY
Except as set forth in (a) the Company Disclosure Schedule delivered to Parent and Amalgamation Sub prior to or contemporaneously with the execution of this Agreement (it being understood that any information set forth in one section or subsection of the Company Disclosure Schedule shall be deemed to apply and qualify the section or subsection of this Agreement to which it corresponds in number and to any other section or subsection of this Agreement the relevance of which is reasonably apparent on its face from the disclosed information) or (b) the Company SEC Reports filed with, or furnished to, the SEC prior to the date hereof, the Company hereby represents and warrants to Parent and Amalgamation Sub that:
Section 3.01 Organization, Good Standing and Qualification.
(a) The Company is an international business company duly organized, validly existing and in good standing under the Laws of Antigua and Barbuda. Each of the Company’s Subsidiaries is a legal entity duly organized or formed, validly existing and in good standing (to the extent the relevant jurisdiction recognizes such concept of good standing) under the Laws of the jurisdiction of its organization or formation, and each Group Company has the requisite corporate or similar power and authority and all necessary governmental approvals to own, lease, operate and use its properties and assets and to carry on its business as it is now being conducted, except where the failure of any Group Company to be so organized, existing or in good standing or of any Group Company to have such power, authority or approvals has not had and would not have a Company Material Adverse Effect. Each Group Company is duly qualified or licensed to do business, and is in good standing (to the extent the relevant jurisdiction recognizes such concept of good standing), in each jurisdiction where the character of the properties and assets owned, leased, operated or used by it or the nature of its business makes such qualification or licensing necessary, except where any such failure to be so qualified or licensed or in good standing would not have a Company Material Adverse Effect.
| A-9 |
Section 3.02 Articles of Incorporation and By-laws.
The Company has heretofore furnished or otherwise made available to Parent a complete and correct copy of the articles of incorporation and by-laws of the Company, as amended to date. Such articles of incorporation and by-laws are in full force and effect as of the date hereof. No Group Company is in violation of any of the provisions of its memorandum and articles of association or equivalent organizational documents in any material respect.
Section 3.03 Capitalization.
(a) The Company is authorized to issue 100,000,000 Shares of a par value of $0.001 per share and 50,000,000 preferred shares of a par value of $0.001 per share. As of the close of business on June 23, 2017, (i) 57,019,261 Shares are issued and outstanding (which number includes 699,000 Company RSs as of the date hereof), all of which have been duly authorized and are validly issued, fully paid and non-assessable, (ii) 1,285,900 Shares are issuable pursuant to outstanding Company Options granted pursuant to the Share Incentive Plans (and for the avoidance of doubt are not included in the number of issued and outstanding Shares set forth in clause (i)) and (iii) no preferred shares are issued and outstanding. Except as set forth in this Section 3.03 and the Rights issued under the Rights Agreement, as of the date hereof there are no outstanding subscriptions, options, warrants, conversion rights, call rights or other agreements, arrangements or commitments issued by the Company or any of its Subsidiaries relating to the issued or unissued share capital of the Company or any of its Subsidiaries or obligating the Company or any of its Subsidiaries to issue, transfer or sell or cause to be issued, transferred or sold any shares of capital stock or other securities of the Company or any of its Subsidiaries or any securities or obligations convertible or exchangeable into or exercisable for, or giving any person a right to subscribe for or acquire, any securities of the Company or any of its Subsidiaries and no securities or obligations evidencing such rights are authorized, issued or outstanding. There are no outstanding contractual obligations of the Company or any of its Subsidiaries to repurchase, redeem or otherwise acquire any Shares or any share capital or other securities of the Company or any of its Subsidiaries. The Company has not issued and does not have outstanding any bonds, debentures, notes or other obligations that entitle the holders thereof to vote (or are convertible into or exchangeable or exercisable for securities having the right to vote) on any matters on which shareholders of the Company may vote.
(b) Section 3.03(b) of the Company Disclosure Schedule sets forth the following information with respect to the Company Options and Company RSs outstanding as of the date hereof: (i) the number of Shares subject to such Company Option, (ii) the number of Company RSs, (iii) the exercise or purchase price of such Company Option or Company RS and (iv) the date on which such Company Option or Company RS expires. The grant of each such outstanding Company Option and Company RS was properly approved in compliance with the terms of the Share Incentive Plans and all applicable Laws. Except as set forth in Section 3.03(b) of the Company Disclosure Schedule or otherwise provided in this Agreement, there are no commitments or agreements of any character to which any Group Company is bound obligating such Group Company to accelerate or otherwise alter the vesting of any Company Option or Company RS as a result of the Transactions. All Shares subject to issuance upon due exercise of a Company Option, upon issuance on the terms and conditions specified in the instruments pursuant to which they are issuable, and all Company RSs, upon vesting on the terms and conditions specified in the instruments pursuant to which they vest, will be duly authorized, validly issued, fully paid and non-assessable. The Company has made available to Parent accurate and complete copies of (i) any Share Incentive Plan pursuant to which the Company has granted Company Options and Company RSs that are currently outstanding, (ii) the form of award agreement evidencing such Company Options and Company RSs and (iii) award agreements evidencing such Company Options and Company RSs with terms that are materially different from those set forth in the form of award agreement.
| A-10 |
(c) Section 3.03(c) of the Company Disclosure Schedule sets forth a true and complete list of each of the Company’s Subsidiaries, together with (i) its respective jurisdiction of organization or formation, (ii) the percentage of the outstanding issued share capital or registered capital, as the case may be, of each such Subsidiary that is owned or otherwise held by a Group Company as of the date hereof and (iii) the other shareholder(s) of such Subsidiary. Other than the Group Companies, no Group Company owns any equity interest in any other corporations, associations or other persons that are legal entities and conduct businesses that are material to the business of the Group Companies, taken as a whole, and neither the Company nor any of its Subsidiaries is a participant in (nor is any part of their businesses conducted through) any joint venture, partnership or similar arrangement that is material to the business of the Group Companies, taken as a whole. The outstanding share capital or registered capital, as the case may be, of each of the Company’s Subsidiaries is duly authorized, validly issued, fully paid and non-assessable, and the portion of the outstanding share capital or registered capital, as the case may be, of each of the Company’s Subsidiaries that is owned by any Group Company is owned by such Group Company free and clear of all Liens (other than Permitted Encumbrances). Except as set forth in Section 3.03(c) of the Company Disclosure Schedule, each such Group Company has the unrestricted right to vote, and (subject to limitations imposed by applicable Law and the applicable constitutional documents) to receive dividends and distributions on, all share capital or registered capital of the Company’s Subsidiaries owned by it. Except as set forth in Section 3.03(c) of the Company Disclosure Schedule or otherwise provided in this Agreement, there are no outstanding contractual obligations of any Group Company to provide funds to, or make any investment (in the form of a loan, capital contribution or otherwise) in any person other than the Company’s wholly-owned Subsidiaries in excess of $3,000,000.
Section 3.04 Authority Relative to this Agreement; Fairness.
(a) The Company has the requisite corporate power and authority to execute and deliver this Agreement, to perform its obligations hereunder and, subject to receipt of the Requisite Company Vote, to consummate the Transactions. The execution, delivery and performance by the Company of this Agreement and the consummation by the Company of the Transactions have been duly authorized by the Company Board and no other corporate action on the part of the Company is necessary to authorize the execution and delivery by the Company of this Agreement and the consummation by it of the Transactions, in each case, subject only to the authorization and approval by way of a shareholders’ special resolution of this Agreement and the Transactions by the affirmative vote of holders of Shares representing at least two-thirds of the Shares present and voting in person or by proxy as a single class at the Shareholders’ Meeting in accordance with Section 169(5) of the IBCA (the “Requisite Company Vote”). This Agreement has been duly and validly executed and delivered by the Company and, assuming the due authorization, execution and delivery by Parent and Amalgamation Sub, constitutes a legal, valid and binding obligation of the Company, enforceable against the Company in accordance with its terms, subject to bankruptcy, insolvency, fraudulent transfer, reorganization, moratorium and similar Laws of general applicability relating to or affecting creditors’ rights and to general principles of equity (the “Bankruptcy and Equity Exception”).
| A-11 |
(b) As of the date hereof, the Special Committee comprises three members of the Company Board, each of whom is not affiliated with Parent or Amalgamation Sub. The Company Board, acting upon the unanimous recommendation of the Special Committee, has (i) determined that it is fair to, and in the best interests of, the Company and its shareholders (other than the holders of Excluded Shares), and declared it advisable, to enter into this Agreement and consummate the Transactions, including the Amalgamation, (ii) authorized and approved the execution, delivery and performance of this Agreement and the consummation of the Transactions, including the Amalgamation, and (iii) resolved to recommend in favor of the authorization and approval of this Agreement and the consummation of the Transactions, including the Amalgamation, to the holders of Shares (the “Company Recommendation”) and direct that this Agreement and the consummation of the Transactions, including the Amalgamation, be submitted to a vote of the holders of Shares for authorization and approval.
(c) The Special Committee has received from Duff & Phelps, LLC or Duff & Phelps Securities, LLC (the “Financial Advisor”) its written opinion, dated the date hereof, subject to the limitations, qualifications and assumptions set forth therein, that the Per Share Amalgamation Consideration to be received by the holders of Shares (other than the Excluded Shares, the Dissenting Shares and Company RSs) is fair, from a financial point of view, to such holders, a copy of which opinion will be delivered to Parent promptly after the execution of this Agreement solely for informational purposes. It is agreed and understood that such opinion may not be relied on by Parent, Amalgamation Sub or any of their respective Affiliates.
Section 3.05 No Conflict; Required Filings and Consents.
(a) The execution and delivery of this Agreement by the Company do not, and the performance of this Agreement by the Company and the consummation of the Transactions will not, (i) assuming that the Requisite Company Vote is obtained, conflict with or violate the articles of incorporation and by-laws of the Company or any equivalent organizational documents of any other Group Company, (ii) assuming (solely with respect to performance of this Agreement and consummation of the Transactions) that the matters referred to in Section 3.05(b) are complied with and the Requisite Company Vote is obtained, conflict with or violate any statute, law, ordinance, regulation, rule, code, executive order, injunction, judgment, decree or other order (“Law”) applicable to any Group Company or by which any property or asset of any Group Company is bound or affected or (iii) violate, conflict with, require consent under, result in any breach of, or constitute a default (or an event that, with notice or lapse of time or both, would become a default) under, or give to others any right of termination, amendment, acceleration or cancellation of, or result in the creation of a Lien (other than Permitted Encumbrances) on any property or asset of any Group Company pursuant to, any Contract to which any Group Company is a party or by which any of their respective properties or assets are bound, except, with respect to clauses (ii) and (iii), for any such conflict, violation, breach, default, right or other occurrence that would not have a Company Material Adverse Effect or prevent or materially impair or delay, or reasonably be expected to prevent or materially impair or delay, the consummation of the Amalgamation or other Transactions.
| A-12 |
(b) The execution and delivery of this Agreement by the Company do not, and the performance of this Agreement by the Company and the consummation by the Company of the Transactions will not, require any consent, approval, authorization or permit of, or filing with or notification to, any nation or government, any agency, public or regulatory authority, instrumentality, department, commission, court, arbitrator, ministry, tribunal or board of any nation or government or political subdivision thereof, in each case, whether foreign or domestic and whether national, supranational, federal, provincial, state, regional, local or municipal (each, a “Governmental Authority”), except (i) for compliance with the applicable requirements of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and the rules and regulations promulgated thereunder (including the joining of the Company in the filing of a Schedule 13E-3, the furnishing of a Form 6-K with documents distributed or required to be distributed to the Company’s shareholders and the filing of one or more amendments to the Schedule 13E-3 to respond to comments of the Securities and Exchange Commission (the “SEC”), if any, on such documents), (ii) for compliance with the rules and regulations of the NASDAQ Stock Market LLC (“NASDAQ”), (iii) for the filing of the Articles of Amalgamation and related documentation with the Financial Services Regulatory Commission pursuant to the IBCA and (iv) any such consent, approval, authorization, permit, action, filing or notification the failure of which to make or obtain would not have a Company Material Adverse Effect or prevent or materially impair or delay, or reasonably be expected to prevent or materially impair or delay, the consummation of the Amalgamation or other Transactions.
Section 3.06 Permits; Compliance with Laws.
(a) Each Group Company is in possession of all material grants, authorizations, licenses, permits, easements, variances, exceptions, consents, certificates, approvals and orders of any Governmental Authority necessary for it to own, lease, operate and use its properties and assets or to carry on its business as it is now being conducted except as would not have a Company Material Adverse Effect (the “Material Company Permits”). As of the date hereof, no suspension or cancellation of any of the Material Company Permits is pending or, to the knowledge of the Company, threatened. All such Material Company Permits are valid and in full force and effect, except where the failure to be valid or in full force and effect would not have a Company Material Adverse Effect. Each Group Company is in compliance with the terms of the Material Company Permits, except where non-compliance would not have a Company Material Adverse Effect. Without limiting the generality of the foregoing, all approvals, filings and registrations with Governmental Authorities in the PRC that are material to the operations of the Group Companies as they are being conducted as of the date hereof, taken as a whole, and are required to be obtained or made in respect of each Group Company incorporated in the PRC, including registrations with the State Administration for Industry and Commerce, the State Administration of Foreign Exchange (“SAFE”) and the State Administration of Taxation, and their respective local counterparts, have been duly completed in all material respects in compliance with applicable PRC Laws. Each Group Company that is organized in the PRC has complied in all material respects with all applicable PRC Laws regarding the contribution and payment of its registered capital. Since January 1, 2014, except as has not had and would not have a Company Material Adverse Effect, no Group Company is in default, breach or violation of any Law applicable to it or by which any of its properties or assets are bound. To the knowledge of the Company, since January 1, 2014, no Group Company has received any written notice or communication from any Governmental Authority or stock exchange of any non-compliance with any applicable Laws that has not been cured except for (i) such non-compliance the outcome of which would not have a Company Material Adverse Effect and/or (ii) any notice or communication relating to investigations or reviews in the trading in the securities of the Company with respect to the Amalgamation.
| A-13 |
(b) Except as would not have a Company Material Adverse Effect, no Group Company, nor, to the knowledge of the Company, the respective directors, officers, employees, or agents of each Group Company, in each case acting on behalf of a Group Company, in the course of his or her actions for, or on behalf of, a Group Company has made or given any bribe, rebate, payoff, influence payment, kickback or any other type of payment that would be unlawful under any Anticorruption Law or (ii) made an offer to pay, a promise to pay or a payment or transfer of money or anything else of value or an authorization of such offer, promise, payment or transfer, directly or indirectly, to any Government Official for the purpose of (A) unlawfully influencing any act or decision of such Government Official in his official capacity, (B) unlawfully securing any improper advantage or (C) unlawfully inducing such Government Official to improperly influence or affect any act or decision of any Governmental Authority, in each case, in order to assist the Company or any of its Subsidiaries in obtaining or retaining business for or with, or in directing business to, any person. No Group Company has conducted or initiated any formal internal investigation or made a voluntary or other disclosure to any Governmental Authority, or, to the knowledge of the Company, received any written notice, citation, report or alleged violations of any applicable Anticorruption Law.
(c) The Company has complied in all material respects with the reporting and/or registration requirements under the applicable SAFE rules and regulations (collectively, the “SAFE Rules and Regulations”) with respect to the registration of its Share Incentive Plans with the Governmental Authorities in the PRC. As of the date hereof, the Company has not received any written inquiries, notifications, orders or any other forms of official written correspondence from SAFE or any of its local branches with respect to any actual or alleged material non-compliance with the SAFE Rules and Regulations.
Section 3.07 SEC Filings; Financial Statements.
(a) The Company has filed or furnished, as the case may be, all forms, reports and documents required to be filed with or furnished to the SEC by the Company since January 1, 2014 (the “Applicable Date”) pursuant to the Securities Act and the Exchange Act (the forms, reports and other documents filed or furnished since the Applicable Date and those filed or furnished subsequent to the date hereof as have been supplemented, modified or amended since the time of filing or furnishing, collectively, the “Company SEC Reports”), except for the Annual Report on Form 20-F for the year ended December 31, 2016. As of the date of filing, in the case of Company SEC Reports filed pursuant to the Exchange Act (and to the extent such Company SEC Reports were amended, then as of the date of filing of such amendment), and as of the date of effectiveness in the case of Company SEC Reports filed pursuant to the Securities Act (and to the extent such Company SEC Reports were amended, then as of the date of effectiveness of such amendment), the Company SEC Reports (i) complied as to form in all material respects with either the requirements of the Securities Act, the Exchange Act or the Sarbanes-Oxley Act of 2002, as the case may be, and the rules and regulations promulgated thereunder, each as in effect on the date so filed or effective and (ii) did not contain any untrue statement of a material fact or omit to state a material fact required to be stated or incorporated by reference therein or necessary in order to make the statements made therein, in light of the circumstances under which they were made, not misleading as of its filing date or effective date (as applicable).
| A-14 |
(b) Each of the consolidated financial statements (including, in each case, any notes thereto) contained in or incorporated by reference into the Company SEC Reports was prepared in accordance with United States generally accepted accounting principles (“GAAP”) (except, in the case of the unaudited statements, as permitted by the SEC) applied on a consistent basis throughout the periods indicated (except as may be indicated in the notes thereto) and each fairly presents, in all material respects, the consolidated financial position of the Group Companies as at the respective dates thereof and the consolidated results of their operations and cash flows for the respective periods indicated therein (subject, in the case of unaudited statements, to normal year-end adjustments and to any other adjustments described therein, the effect of which, individually or in the aggregate, is not material, and to the exclusion of certain notes in accordance with the rules of the SEC relating to unaudited financial statements), in each case in accordance with GAAP except to the extent that such information has been amended or superseded by later Company SEC Reports filed prior to the date hereof.
(c) Except as and to the extent set forth in the unaudited quarterly financial results of the Group Companies as of September 30, 2016, including the notes thereto, no Group Company has outstanding (i) any Indebtedness or any commitments therefor or (ii) any other liability or obligation of any nature (whether accrued, absolute, contingent or otherwise) that are required in accordance with GAAP to be disclosed or reflected or reserved against in the consolidated financial statements of the Group Companies, except for Indebtedness or any commitments therefor or other liabilities or obligations (A) reflected or reserved against on the consolidated balance sheet of the Company as of September 30, 2016, (B) incurred in the ordinary course of business consistent with past practice since September 30, 2016, (C) incurred pursuant to this Agreement or in connection with the Transactions or (D) that do not have a Company Material Adverse Effect.
(d) The Company has made available to Parent complete and correct copies of all material amendments and modifications that have not been filed by the Company with the SEC as of the date of this Agreement to all Material Contracts that previously had been filed by the Company with the SEC and are in effect as of the date of this Agreement.
| A-15 |
(e) The Company has timely filed all certifications and statements required by (i) Rule 13a-14 or Rule 15d-14 under the Exchange Act or (ii) 18 U.S.C. Section 1350 (Section 906 of the Sarbanes-Oxley Act of 2002) with respect to any Company SEC Report. The “disclosure controls and procedures” (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) established and maintained by the Company are reasonably designed to ensure that all material information concerning the Group Companies required to be disclosed by the Company in the reports it files under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and related forms, and that such information is accumulated and communicated to the Company’s chief executive officer and chief financial officer (or persons performing similar functions), as appropriate, to allow timely decisions regarding required disclosure. Since January 1, 2015, neither the Company nor, to the Company’s knowledge, its independent registered public accounting firm has identified or been made aware of any “significant deficiencies” or “material weaknesses” (as defined by the Public Company Accounting Oversight Board) in the design or operation of the internal controls and procedures of the Company that are reasonably likely to adversely affect the ability of the Company to record, process, summarize and report financial data, in each case, which has not been subsequently remediated. Except as set forth in Section 3.07 of the Company Disclosure Schedule, to the Company’s knowledge, there is, and since January 1, 2015, there has been, no fraud, whether or not material, that involves (or involved) the management of the Company or other employees who have (or had) a significant role in the internal controls over financial reporting utilized by the Company. Since the date of the Company’s most recently filed annual report under the Exchange Act, there have been no changes in the Company’s internal control over financial reporting (as such term is defined in the Exchange Act) that have materially affected or are reasonably likely to materially affect, the Company’s internal control over financial reporting. As used in this Section 3.07, the term “file” shall be broadly construed to include any manner in which a document or information is furnished, supplied or otherwise made available to the SEC.
(f) The Group Companies maintain a system of “internal control over financial reporting” (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) sufficient to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with GAAP.
(g) The Company is in compliance, in all material respects, with the applicable listing and corporate governance rules and regulations of the NASDAQ, subject to availing itself of any “home country” exemption from such rules and regulations available to a “foreign private issuer” (as defined under the Exchange Act and under the relevant rules and regulations of the NASDAQ).
Section 3.08 Absence of Certain Changes or Events.
Between September 30, 2016 and the date hereof, except as set forth in Section 3.08 of the Company Disclosure Schedule or as expressly contemplated by this Agreement, (a) each Group Company has conducted business in all material respects in the ordinary course and (b) there has not been any event, occurrence, development or state of circumstances or facts that has had a Company Material Adverse Effect.
Section 3.09 Absence of Litigation.
Except as set forth in Section 3.09 of the Company Disclosure Schedule, as of the date hereof there is no litigation, suit, claim, action, proceeding or investigation (an “Action”) pending or, to the knowledge of the Company, threatened against any Group Company, or any property or asset of any Group Company, before any Governmental Authority that (a) would reasonably be expected to result in a Company Material Adverse Effect or (b) has enjoined, restrained, prevented or materially delayed, or would reasonably be expected to enjoin, restrain, prevent or materially delay the consummation of the Amalgamation. As of the date hereof, no Group Company, nor any property or asset of any Group Company is subject to any continuing order of, consent decree, settlement agreement or other similar written agreement with, or, to the knowledge of the Company, continuing investigation by, any Governmental Authority, or any order, writ, judgment, injunction, decree, determination or award of any Governmental Authority, except those that would not have a Company Material Adverse Effect.
| A-16 |
Section 3.10 Labor and Employment Matters.
(a) Except as set forth in Section 3.10 of the Company Disclosure Schedule, no Group Company is a party to or bound by any collective bargaining agreement or other labor union Contract applicable to persons employed by it, nor is any such agreement being negotiated by any Group Company as of the date hereof. Except for those that would not have a Company Material Adverse Effect, there are no unfair labor practice complaints pending or, to the knowledge of the Company, threatened against any Group Company before any Governmental Authority and there is no organized strike, slowdown, work stoppage or lockout, or similar activity or, to the knowledge of the Company, threatened against or involving any Group Company.
(b) Except as would not have a Company Material Adverse Effect, each Group Company (i) is in compliance with all applicable Laws relating to employment and employment practices, including those related to wages, work hours, shifts, overtime, holidays and leave, collective bargaining terms and conditions of employment and the payment and withholding of Taxes and other sums as required by the appropriate Governmental Authority and (ii) is not liable for any arrears of wages, Taxes, penalties or other sums for failure to comply with any of the foregoing. Except as would not have a Company Material Adverse Effect, (A) there is no claim with respect to payment of wages, salary or overtime pay that is now pending or, to the knowledge of the Company, threatened before any Governmental Authority with respect to any persons currently or formerly employed by any Group Company and (B) there is no charge or proceeding with respect to a violation of any occupational safety or health standards that is now pending or, to the knowledge of the Company, threatened with respect to any Group Company.
(c) The Company has made available to Parent true and complete copies of each material Company Employee Plan including all material amendments thereto.
(d) Each Company Employee Plan is and has at all times been operated and administered in material compliance with the provisions thereof and all applicable legal requirements. There are no material claims (other than for benefits incurred in the ordinary course) or legal proceedings pending, or, to the knowledge of the Company, threatened against any Company Employee Plan or against the assets of any Company Employee Plan.
(e) Except pursuant to or as contemplated under this Agreement, no Company Employee Plan exists that, as a result of the execution of this Agreement, shareholder approval of this Agreement or the consummation of the Transactions alone (and without the occurrence of any additional or subsequent events such as a termination of employment), will entitle any current or former director, employee or consultant of any Group Company to (i) material compensation or benefits (including any severance payment or benefit), or (ii) accelerate the time of payment or vesting or result in any payment or funding of material compensation or benefits under, materially increase the amount payable or result in any other material obligation pursuant to, any of the Company Employee Plans.
| A-17 |
(f) This Section 3.10 constitutes the sole and exclusive representation or warranty of the Group Companies relating to labor and employment matters.
Section 3.11 Real Property; Title to Assets.
(a) Section 3.11(a) of the Company Disclosure Schedule sets forth a true and complete list of each Owned Real Property, including with respect to Owned Real Property in the PRC, the particulars and the issue date of the State-owned Land Use Certificate and Building Ownership Certificate for such Owned Real Property. With respect to each Owned Real Property: (i) the relevant Group Company has good and marketable title, validly granted land use rights or building ownership rights, as applicable, to such Owned Real Property, free and clear of all Liens, except for Liens that do not materially interfere with such Group Company’s use and enjoyment of such Owned Real Property or Permitted Encumbrances or as disclosed in Section 3.11(a)(i) of the Company Disclosure Schedule, (ii) no Group Company has leased or otherwise granted to any person the right to use or occupy any material portion of such Owned Real Property, and (iii) there are no outstanding options, rights of first offer or rights of first refusal to purchase any material portion of or material interest in such Owned Real Property. Except as would not have a Company Material Adverse Effect, the relevant Group Company has duly complied in all respects with all the terms and conditions of, and all of its obligations under, the relevant land use rights contract or real property purchase contract in relation to any Owned Real Property owned by it.
(b) Section 3.11(b) of the Company Disclosure Schedule sets forth, as of the date hereof, the address of each Leased Real Property and a true and complete list of all Leases for each such Leased Real Property (including the date and name of the parties to such Lease). The Company has delivered or otherwise made available to Parent a true and complete copy of each such Lease. Except as would not have a Company Material Adverse Effect, with respect to each Lease: (i) such Lease is legal, valid, binding, enforceable and in full force and effect, subject to the Bankruptcy and Equity Exception, (ii) the Group Companies’ possession and quiet enjoyment of the Leased Real Property under such Lease has not been disturbed since January 1, 2014 and (iii) neither any Group Company nor, to the knowledge of the Company, any other party to such Lease is in breach or default under such Lease, and, to the knowledge of the Company, no event has occurred or circumstance exists that, with the delivery of notice, the passage of time or both, would constitute such a breach or default, or permit the termination, modification or acceleration of rent under such Lease.
(c) The Owned Real Property identified in Section 3.11(a) of the Company Disclosure Schedule and the Leased Real Property identified in Section 3.11(b) of the Company Disclosure Schedule (collectively, the “Company Real Property”) comprise all of the material real property used in the business of the Group Companies as of the date hereof.
(d) To the knowledge of the Company, (i) all buildings, structures, improvements, fixtures, building systems and equipment, and all components thereof, included in the Company Real Property (the “Improvements”) are in good condition and repair and sufficient for the operation of the business of the Group Companies and (ii) there are no structural deficiencies or latent defects materially affecting the intended use or function of the Improvements, in each case, except as would not have a Company Material Adverse Effect.
| A-18 |
(e) Except as would not have a Company Material Adverse Effect, the Group Companies have good and marketable title to, or a valid and binding leasehold interest in, all other properties and assets (excluding Owned Real Property, Leased Real Property and Intellectual Property) that are material to the business of the Group Companies taken as a whole, as currently conducted.
Section 3.12 Intellectual Property.
Except as would not have a Company Material Adverse Effect:
(a) The Group Companies have valid and enforceable rights to use all Intellectual Property used in, or necessary to conduct, the business of the Company or its Subsidiaries as it is currently conducted (the “Company Intellectual Property”), free and clear of all Liens (other than Permitted Encumbrances).
(b) Since January 1, 2014, neither the Company nor any of its Subsidiaries has received written notice of any claim that it, or the business conducted by it, is infringing, diluting or misappropriating or has infringed, diluted or misappropriated any Intellectual Property right of any person, including any demands or unsolicited offers to license any Intellectual Property. To the knowledge of the Company, the right to use the Company Intellectual Property has been obtained through all necessary legal procedures and any applications therefor have been duly filed or registered (as applicable) with the applicable Governmental Authority or other applicable person, have been maintained to the extent necessary, including the submission of all necessary filings and fees in accordance with the legal, administrative and other requirements, and have not lapsed, expired or been abandoned. To the knowledge of the Company, no person is currently infringing, diluting or misappropriating Intellectual Property owned by the Company or any Subsidiary of the Company.
(c) Except as set forth in Section 3.12(c) of the Company Disclosure Schedule, there are no pending or, to the knowledge of the Company, threatened Actions by any person challenging the validity or enforceability of, or the use or ownership by the Company or any of its Subsidiaries of, any of the Company Intellectual Property.
(d) Since January 1, 2010, all employees, consultants or contractors of the Group Companies who have materially contributed to the development of Company Intellectual Property in the course of their employment, engagement or contract with the Company or any of its Subsidiaries have executed and delivered to the Company or such Subsidiary agreements or undertakings (i) providing for the non-disclosure by such person of confidential information and (ii) providing for the assignment by such person to the Company or such Subsidiary of any Intellectual Property developed or arising out of such person’s employment by, engagement by or contract with the Company or such Subsidiary.
(e) The Group Companies have taken all actions reasonably necessary to maintain and protect each item of Intellectual Property that they own in all respects.
| A-19 |
Section 3.13 Taxes.
(a) Except as would not have a Company Material Adverse Effect, each Group Company has duly filed all Tax returns and reports required to be filed by it and has paid and discharged all Taxes required to be paid or discharged, other than such payments as are being contested in good faith by appropriate proceedings, and all such Tax returns are true, accurate and complete in all material respects. As of the date hereof, no taxing authority is asserting in writing or, to the knowledge of the Company, threatening to assert against any Group Company any material deficiency or claim for any Taxes. Except as would not have a Company Material Adverse Effect, each Group Company has properly and timely withheld, collected and deposited all Taxes that are in the Company’s reasonable judgment required to be withheld, collected and deposited under applicable Law. Except as would not have a Company Material Adverse Effect, no Group Company has granted any waiver of any statute of limitations with respect to, or any extension of a period for the assessment of, any material Tax (other than pursuant to extensions of time to file Tax returns in the ordinary course of business).
(b) Each of the Company’s Subsidiaries incorporated in the PRC has, in accordance with applicable PRC Law, duly registered with the relevant PRC Governmental Authority, obtained and maintained the validity of all national and local Tax registration certificates and complied in all respects with all requirements imposed by such Governmental Authorities, in each case, except as would not have a Company Material Adverse Effect. Any submissions made by or on behalf of the Company or any of its Subsidiaries to any Governmental Authority in connection with obtaining Tax exemptions, Tax holidays, Tax deferrals, Tax incentives or other preferential Tax treatments or Tax rebates were accurate and complete in all material respects. As of the date hereof, no suspension, revocation or cancellation of any Tax exemptions, preferential treatments or rebates is pending or, to the knowledge of the Company, threatened.
(c) This Section 3.13 constitutes the sole and exclusive representation or warranty of the Group Companies relating to Tax matters.
Section 3.14 Material Contracts.
(a) Except for this Agreement and except for Contracts filed as exhibits to the Company SEC Reports, as of the date hereof, none of the Group Companies is a party to or bound by:
(i) any Contract that would be required to be filed by the Company pursuant to Item 4 of the Instructions to Exhibits of Form 20-F under the Exchange Act;
(ii) any Contract relating to the formation, creation, operation, management or control of any partnership, joint venture, limited liability company or similar arrangement, in each case, that is material to the business of the Group Companies, taken as a whole;
(iii) any Contract involving Indebtedness of the Company or any of its Subsidiaries for borrowed money having an outstanding principal amount of more than $8,500,000, other than any Indebtedness solely between or among the Group Companies;
(iv) any Contract under which the Company or any of its Subsidiaries has any material obligations that have not been satisfied or performed (other than indemnification and confidentiality obligations) relating to the acquisition, disposition, sale, transfer or lease (including leases in connection with financing transactions) of properties or assets of the Company or any of its Subsidiaries that have a fair market value or purchase price of more than $3,500,000 (other than acquisitions or dispositions of inventory, properties and other assets in the ordinary course of business);
| A-20 |
(v) any Contract relating to any acquisition of equity interest by the Company or any of its Subsidiaries under which the Company or any of its Subsidiaries has continuing “earn-out” or similar contingent payment obligations that would reasonably be expected to result in payments by any Group Company of more than $3,500,000;
(vi) any Contract that contains a call or similar right pursuant to which the Company or any of its Subsidiaries could be required to purchase any equity interest in any person or assets that have a fair market value or purchase price of $3,500,000 or more;
(vii) any non-competition Contract or other Contract that is material to the Group Companies, taken as a whole, and that purports to limit, curtail or restrict in any material respect the ability of the Company or any of its Subsidiaries to compete in any geographic area, industry or line of business;
(viii) any Contract (other than Contracts granting Company Options or Company RSs) giving the other party the right to terminate such Contract as a result of this Agreement or the consummation of the Amalgamation where (A) such Contract requires any payment in excess of $500,000 to be made by the Company or any of its Subsidiaries in any calendar year or (B) the value of the outstanding receivables due to the Group Companies under such Contract is in excess of $500,000 in any calendar year;
(ix) any Contract that prohibits (A) payment of dividends or any distribution with respect to equity interests of the Company or any of its Subsidiaries, (B) pledging of share capital of the Company or any of its Subsidiaries or (C) issuance of guaranty by the Company or any of its Subsidiaries; or
(x) any Contract providing for (A) a license of Intellectual Property of the Company or any of its Subsidiaries to a Third Party, (B) an indemnity of any person by the Company or any of its Subsidiaries against any charge of infringement, misappropriation, unauthorized use or violation of any Intellectual Property right or (C) any royalty, fee or other amount payable by the Company or any of its Subsidiaries to any person by reason of the ownership, use, sale or disposition of Intellectual Property; in each case of (A) through (C), other than agreements for off-the-shelf Software and such Contracts that are not material to the business of the Group Companies, taken as a whole, and in each case of (A) and (B), other than Contracts entered into by the Group Companies in the ordinary course of business;
Each such Contract described in clauses (i) to (ix) and each such Contract that would be a Material Contract but for the exception of being filed as an exhibit to the Company SEC Reports is referred to herein as a “Material Contract.”
(b) Except as would not have a Company Material Adverse Effect, (i) each Material Contract is a legal, valid and binding obligation of the Company or any of its Subsidiaries party thereto and, to the Company’s knowledge, each other party thereto, in each case subject to the Bankruptcy and Equity Exception, (ii) neither the Company nor any of its Subsidiaries nor, to the Company’s knowledge and as of the date hereof, any other party thereto, is in breach or violation of, or default under, any Material Contract and no event has occurred that with notice or lapse of time or both would constitute a breach or violation of, or default under, any Material Contract and (iii) the Group Companies have not received any written claim or notice of default, termination or cancellation under any such Material Contract.
| A-21 |
Section 3.15 Environmental Matters.
Except as would not have a Company Material Adverse Effect, (a) each Group Company is in compliance with all applicable Environmental Laws and has obtained and possesses all material permits, licenses and other authorizations currently required for its establishment and operation under any Environmental Law (the “Environmental Permits”), and all such Environmental Permits are in full force and effect, (b) no Group Company has received any notice, demand, letter, claim or request for information alleging that any Group Company is in violation of or liable under any Environmental Law that remains unresolved and (c) no Group Company is subject to any order, decree or injunction with any Governmental Authority or agreement with any Third Party concerning liability under any Environmental Law or relating to Hazardous Substance. This Section 3.15 constitutes the sole and exclusive representation or warranty of the Group Companies relating to environmental matters.
Section 3.16 Insurance.
Except as would not have a Company Material Adverse Effect, (a) all insurance policies and all self-insurance programs and arrangements relating to the business, assets, liabilities and operations of the Group Companies are in full force and effect, (b) the Company has no reason to believe that it or any of its Subsidiaries will not be able to (i) renew its existing insurance policies as and when such policies expire or (ii) obtain comparable coverage from comparable insurers as may be necessary to continue its business without a significant increase in cost and (c) neither the Company nor any of its Subsidiaries has received any written notice of any threatened termination of, premium increase with respect to, or alteration of coverage under, any of its respective insurance policies.
Section 3.17 Company Rights Plan.
The Company has taken all actions necessary to (a) render the Rights Agreement inapplicable to this Agreement and the transactions contemplated hereby and (b) ensure that (i) none of Parent, Amalgamation Sub or any other Subsidiary of Parent is an Acquiring Person (as defined in the Rights Agreement) pursuant to the Rights Agreement as a result of this Agreement or the Transactions contemplated hereby and (ii) a Distribution Date, a Trigger Event or a Share Acquisition Date (as such terms are defined in the Company Rights Agreement) does not occur, in the case of clauses (i) and (ii), solely by reason of the execution of this Agreement or the Support Agreement or the consummation of the Transactions.
Section 3.18 Brokers.
Except for the Financial Advisor, no broker, finder or investment banker is entitled to any brokerage, finder’s or other fee or commission in connection with the Transactions based upon arrangements made by or on behalf of the Company.
| A-22 |
Section 3.19 No Other Representations or Warranties.
Except for the representations and warranties contained in this Article III, neither the Company nor any other person on behalf of the Company makes any other express or implied representation or warranty with respect to any Group Company or their respective business, operations, assets, liabilities, condition (financial or otherwise) or prospects or any information provided to Parent or Amalgamation Sub or any of their Affiliates or Representatives, notwithstanding the delivery or disclosure to Parent, Amalgamation Sub or any of their Affiliates or Representatives of any documentation, forecasts or other information in connection with the Transactions, and each of Parent and Amalgamation Sub acknowledges the foregoing. Neither the Company nor any other person will have or be subject to any liability or indemnity obligations to Parent, Amalgamation Sub or any other person resulting from the distribution or disclosure or failure to distribute or disclose to Parent, Amalgamation Sub or any of their Affiliates or Representatives, or their use of, any information, unless and to the extent such information is expressly included in the representations and warranties contained in Article III.
Article
IV
REPRESENTATIONS AND WARRANTIES OF PARENT AND AMALGAMATION SUB
Parent and Amalgamation Sub hereby, jointly and severally, represent and warrant to the Company that:
Section 4.01 Corporate Organization.
Parent is an exempted company duly incorporated, validly existing and in good standing under the Laws of the Cayman Islands. Amalgamation Sub is an international business company duly incorporated, validly existing and in good standing under the Laws of Antigua and Barbuda. Each of Parent and Amalgamation Sub has the requisite corporate power and authority and all necessary governmental approvals to own, lease and operate its properties and to carry on its business as it is now being conducted, except where the failure to be so organized, existing or in good standing or to have such power, authority and governmental approvals would not, individually or in the aggregate, prevent or materially delay consummation of any of the Transactions by Parent or Amalgamation Sub or otherwise be materially adverse to the ability of Parent or Amalgamation Sub to perform their material obligations under this Agreement. Parent has heretofore made available to the Company complete and correct copies of the articles of incorporation and by-laws of Parent and Amalgamation Sub, each as amended to date, and each as so delivered is in full force and effect.
Section 4.02 Authority Relative to This Agreement.
Each of Parent and Amalgamation Sub has all necessary corporate power and authority to execute and deliver this Agreement, to perform its obligations hereunder and to consummate the Transactions. The execution and delivery of this Agreement by Parent and Amalgamation Sub and the consummation by Parent and Amalgamation Sub of the Transactions have been duly and validly authorized by all necessary corporate action, and no other corporate proceedings on the part of Parent or Amalgamation Sub are necessary to authorize this Agreement or to consummate the Transactions (other than the filings, notifications and other obligations and actions described in Section 4.03(b)). This Agreement has been duly and validly executed and delivered by Parent and Amalgamation Sub and, assuming due authorization, execution and delivery by the Company, constitutes a legal, valid and binding obligation of each of Parent and Amalgamation Sub, enforceable against each of Parent and Amalgamation Sub in accordance with its terms, subject to the Bankruptcy and Equity Exception.
| A-23 |
Section 4.03 No Conflict; Required Filings and Consents.
(a) The execution and delivery of this Agreement by Parent and Amalgamation Sub do not, and the performance of this Agreement by Parent and Amalgamation Sub will not, (i) conflict with or violate the articles of incorporation and by-laws of either Parent or Amalgamation Sub, (ii) assuming that all consents, approvals, authorizations and other actions described in Section 4.03(b) have been obtained and all filings and obligations described in Section 4.03(b) have been made, conflict with or violate any Law applicable to Parent or Amalgamation Sub or by which any property or asset of either of them is bound or affected or (iii) result in any breach of, or constitute a default (or an event that, with notice or lapse of time or both, would become a default) under, or give to others any rights of termination, amendment, acceleration or cancellation of, or result in the creation of a Lien on any property or asset of Parent or Amalgamation Sub pursuant to, any Contract or obligation to which Parent or Amalgamation Sub is a party or by which Parent or Amalgamation Sub or any property or asset of either of them is bound or affected, except, with respect to clauses (ii) and (iii), for any such conflicts, violations, breaches, defaults or other occurrences that would not, individually or in the aggregate, prevent or materially delay consummation of any of the Transactions by Parent or Amalgamation Sub or otherwise be materially adverse to the ability of Parent and Amalgamation Sub to perform their material obligations under this Agreement.
(b) The execution and delivery of this Agreement by Parent and Amalgamation Sub do not, and the performance of this Agreement by Parent and Amalgamation Sub and the consummation by Parent and Amalgamation Sub of the Transactions will not, require any consent, approval, authorization or permit of, or filing with or notification to, any Governmental Authority, except (i) for the filings and/or notices pursuant to Section 13 of the Exchange Act and the rules and regulations thereunder, (ii) for compliance with the rules and regulations of NASDAQ and (iii) for the filing of the Articles of Amalgamation and related documentation with the Financial Services Regulatory Commission pursuant to the IBCA.
Section 4.04 Capitalization.
(a) The authorized share capital of Parent consists solely of 50,000 shares, par value of $1.00 per share. As of the date hereof, one share of Parent was issued and outstanding, which is duly authorized, validly issued, fully paid and non-assessable and is owned by Holdco. All of the issued and outstanding share capital of Parent is, and at the Effective Time will be, owned by Holdco. Parent was formed solely for the purpose of engaging in the transactions contemplated by this Agreement, and it has not conducted any business prior to the date hereof and has no, and prior to the Effective Time, will have no, assets, liabilities or obligations of any nature other than the Equity Commitment Letters and those incident to its formation and capitalization pursuant to this Agreement and the Transactions.
| A-24 |
(b) The authorized share capital of Amalgamation Sub consists solely of 10,000 shares, par value of $1.00 per share. As of the date hereof, one share of Amalgamation Sub was issued and outstanding, which is duly authorized, validly issued, fully paid and non-assessable and is owned by Parent. All of the issued and outstanding share capital of Amalgamation Sub is, and at the Effective Time will be, owned by Parent. Amalgamation Sub was formed solely for the purpose of engaging in the Transactions, and it has not conducted any business prior to the date hereof and has no, and prior to the Effective Time will have no, assets, liabilities or obligations of any nature other than those incident to its formation and capitalization and pursuant to this Agreement and the Transactions.
Section 4.05 Available Funds and Financing.
(a) Parent has delivered to the Company true and complete copies of executed equity commitment letters from the Sponsors (the “Equity Commitment Letters”) pursuant to which each of the Sponsors has committed to purchase, or cause the purchase of, for cash, subject to the terms and conditions thereof, equity securities of Holdco, up to the aggregate amount set forth therein (the “Financing”). The proceeds of the Financing shall be used to finance the consummation of the Transactions.
(b) (i) Each of the Equity Commitment Letters is in full force and effect and is a legal, valid and binding obligation of Parent and Holdco (as applicable and subject to the Bankruptcy and Equity Exception) and, to the knowledge of Parent, the other parties thereto (subject to the Bankruptcy and Equity Exception) and (ii) none of the Equity Commitment Letters has been amended or modified and no such amendment or modification is contemplated, and the respective commitments contained in the Equity Commitment Letters have not been withdrawn or rescinded in any material respect. Assuming (A) the Financing is funded in accordance with the Equity Commitment Letters and (B) the satisfaction of the conditions to the obligation of Parent and Amalgamation Sub to consummate the Amalgamation as set forth in Section 7.01 and Section 7.02 or the waiver of such conditions, the net proceeds of the Financing contemplated by the Equity Commitment Letters will be sufficient for Parent, Amalgamation Sub and the Surviving Corporation to pay (1) the Amalgamation Consideration and (2) all other amounts required to be paid in connection with the consummation of the Transactions upon the terms and conditions contemplated hereby and all related fees and expenses associated therewith. The Equity Commitment Letters contain all of the conditions precedent to the obligations of the parties thereunder to make the Financing available to Holdco, Parent or Amalgamation Sub on the terms and conditions contained therein. There are no side letters or other agreements, Contracts or arrangements (whether written or oral) to which Parent or any of its Affiliates is a party related to the funding or investing, as applicable, of the full amount of the Financing other than (x) as expressly set forth in the Equity Commitment Letters and (y) any customary non-disclosure agreements that do not impact the conditionality, availability or amount of the Financing. No event has occurred that, with or without notice, lapse of time or both, would either (I) constitute a default or breach under any of the Equity Commitment Letters on the part of Parent or Holdco or, to the knowledge of Parent, any other parties thereto or (II) prevent or materially delay the other parties thereto from providing or funding, as applicable, any portion of the Financing. As of the date hereof, subject to the accuracy of the representations and warranties of the Company set forth in Article III hereof, and the satisfaction of the conditions set forth in Section 7.01 and Section 7.02 hereof, Parent has no reason to believe that it will be unable to satisfy on a timely basis any term or condition of closing to be satisfied by it contained in the Equity Commitment Letters or that any of the conditions to the Financing that are required to be satisfied by Parent or Amalgamation Sub will not be satisfied or that the Financing will not be available to Holdco, Parent or Amalgamation Sub at the Effective Time. For the avoidance of doubt, Parent is not making any representation or warranty regarding the effect of the inaccuracy of the representations and warranties in Article III or non-compliance by the Company with its obligations hereunder.
| A-25 |
Section 4.06 Brokers.
Except for Lazard Asia (Hong Kong) Limited, no broker, finder or investment banker is entitled to any brokerage, finder’s or other fee or commission in connection with the Transactions based upon arrangements made by or on behalf of Parent or Amalgamation Sub.
Section 4.07 Guarantees.
Assuming the due authorization, execution and delivery by the Company, each Limited Guarantee is in full force and effect and is a legal, valid and binding obligation of the Guarantor that executed it, subject to the Bankruptcy and Equity Exception, and no event has occurred that, with or without notice, lapse of time or both, would constitute a default on the part of such Guarantor under such Limited Guarantee.
Section 4.08 Absence of Litigation.
To the knowledge of Parent and Amalgamation Sub, as of the date hereof, (a) there is no Action pending or threatened against Parent or Amalgamation Sub or any of their respective Affiliates before any Governmental Authority and (b) neither Parent nor Amalgamation Sub nor any of their Affiliates is subject to any continuing order of, consent decree, settlement agreement or other similar written agreement with, or continuing investigation by, any Governmental Authority, or any order, writ, judgment, injunction, decree, determination or award of any Governmental Authority, in each case that seeks to, or would reasonably be expected to prevent or materially impair or delay the consummation of the Amalgamation or other Transactions.
Section 4.09 Ownership of Company Shares.
As of the date hereof, other than the Rollover Shares, none of Parent, Amalgamation Sub, the Rollover Shareholders, the Sponsors nor any of their respective Affiliates beneficially own (as such term is used in Rule 13d-3 promulgated under the Exchange Act) any Shares or other securities or any other economic interest (through derivative securities or otherwise) of the Company or any options, warrants or other rights to acquire Shares or other securities of, or any other economic interest (through derivative securities or otherwise) in the Company.
Section 4.10 Solvency.
Neither Parent nor Amalgamation Sub is entering into the Transactions contemplated hereby with the intent to hinder, delay or defraud either present or future creditors. Immediately after giving effect to all of the Transactions contemplated hereby, including the Financing and the payment of the Amalgamation Consideration and all other amounts required to be paid in connection with the consummation of the Transactions, assuming (a) satisfaction of the conditions to the obligation of Parent and Amalgamation Sub to consummate the Amalgamation as set forth herein, or the waiver of such conditions and (b) the accuracy of the representations and warranties of the Company set forth in Article III (for such purposes, the representations and warranties that are qualified as to materiality or “Company Material Adverse Effect” shall be true and correct in all respects and those not so qualified shall be true and correct in all material respects), the Surviving Corporation will be solvent (as such term is used under the Laws of Antigua and Barbuda) at and immediately after the Effective Time.
| A-26 |
Section 4.11 Parent Group Contracts.
Parent has delivered to the Company and the Special Committee a true and complete copy of each of: (a) the Equity Commitment Letters, (b) the Support Agreement, (c) the Limited Guarantees, and (d) the Interim Investors Agreement (collectively, the “Parent Group Contracts”), including all amendments thereto or modifications thereof. As of the date hereof, other than the Parent Group Contracts, there are no side letters or other oral or written Contracts, agreements, arrangements or understandings (whether or not legally enforceable) (i) relating to the Transactions between or among Parent, Amalgamation Sub, any Rollover Shareholder, any Sponsor or any of their respective Affiliates (excluding any agreements among any one or more of the foregoing solely relating to the Surviving Corporation following the Effective Time), (ii) relating to the Transactions between or among Parent, Amalgamation Sub, any Rollover Shareholder, any Sponsor or any of their respective Affiliates, on the one hand, and any member of the Company’s management, any members of the Company Board or any of the Company’s shareholders in their capacities as such, on the other hand or (iii) pursuant to which any shareholder of the Company would be entitled to receive consideration of a different amount or nature than the Per Share Amalgamation Consideration or pursuant to which any shareholder of the Company has agreed to vote to approve this Agreement or the Amalgamation or has agreed to vote against any Superior Proposal.
Section 4.12 Independent Investigation.
Parent and Amalgamation Sub have conducted their own independent investigation, review and analysis of the business, operations, assets, liabilities, results of operations, financial condition and prospects of the Group Companies, which investigation, review and analysis were performed by Parent, Amalgamation Sub, their respective Affiliates and Representatives. Each of Parent and Amalgamation Sub acknowledges that as of the date hereof, it, its Affiliates and their respective Representatives have been provided adequate access to the personnel, properties, facilities and records of the Group Companies for such purpose. In entering into this Agreement, each of Parent and Amalgamation Sub acknowledges that it has relied solely upon the aforementioned investigation, review and analysis and not on any statements, representations or opinions of any of the Company, its Affiliates or their respective Representatives (except the representations and warranties of the Company set forth in this Agreement and in any certificate delivered pursuant to this Agreement).
Section 4.13 No Additional Representations.
Except for the representations and warranties set forth in this Article IV, neither Parent nor Amalgamation Sub nor any other person on behalf of either of them makes any other express or implied representation or warranty with respect to Parent or Amalgamation Sub, or their respective business, operations, assets, liabilities, condition (financial or otherwise) or prospects, notwithstanding the delivery or disclosure to the Company or any of its Affiliates or Representatives of any documentation, forecasts or other information with respect to any one or more of the foregoing, and the Company acknowledges the foregoing.
| A-27 |
Article
V
CONDUCT OF BUSINESS PENDING THE AMALGAMATION
Section 5.01 Conduct of Business by the Company Pending the Amalgamation.
The Company agrees that, from the date hereof until the earlier of the Effective Time and termination of this Agreement pursuant to Article VIII, except as (x) required by applicable Law, (y) set forth in Section 5.01 of the Company Disclosure Schedule or (z) expressly contemplated, permitted or required by this Agreement, unless Parent shall otherwise consent in writing (which consent shall not be unreasonably withheld, conditioned or delayed), (i) the businesses of the Group Companies shall be conducted in the ordinary course of business and in a manner consistent with past practice in all material respects, and (ii) the Company shall use its commercially reasonable efforts to preserve intact the assets and business organization of the Group Companies in all material respects, to keep available the services of the current officers and key employees of the Group Companies and to maintain in all material respects the current relationships of the Group Companies with existing customers, suppliers and other persons with which the Group Companies have material business relations as of the date hereof, except for any termination of services or relationships as a result of the expiration of any agreements or Contracts.
Without limiting the generality of the foregoing paragraph, from the date hereof until the earlier of the Effective Time and termination of this Agreement pursuant to Article VIII, except as (x) required by applicable Law, (y) set forth in Section 5.01 of the Company Disclosure Schedule or (z) expressly contemplated, permitted or required by this Agreement, the Company shall not and shall not permit any other Group Company to, directly or indirectly, do any of the following without the prior written consent of Parent (which consent shall not be unreasonably withheld, conditioned or delayed):
(a) amend or otherwise change its articles of incorporation and by-laws or the equivalent organizational documents of its Subsidiaries;
(b) issue, sell, transfer, lease, sublease, license, pledge, dispose of, grant or encumber, or authorize the issuance, sale, transfer, lease, sublease, license, pledge, disposition, grant or encumbrance of any shares of any class of any Group Company (other than in connection with (A) the exercise of any Company Options or vesting of Company RSs in accordance with the Share Incentive Plans, (B) the withholding of Company securities to satisfy Tax obligations with respect to Company Options or Company RSs, (C) the acquisition by the Company of its securities in connection with the forfeiture of Company Options or Company RSs (D) the acquisition by the Company of its securities in connection with the net exercise of Company Options in accordance with the terms thereof, (E) the issuance of Company securities as required to comply with any Share Incentive Plan or Company Employee Plan in effect as of the date hereof, or (F) pursuant to Contracts in effect as of the date hereof or any options, warrants, securities convertible into any share capital or other rights of any kind to acquire any shares, or any other ownership interest (including any phantom interest), of any Group Company)
(c) sell any property or assets (whether real, personal or mixed, and including leasehold interests and intangible property) of any Group Company with a value or purchase price (including the value of assumed liabilities) in excess of $4,500,000, except (A) in the ordinary course of business, (B) pursuant to Contracts in force on the date hereof, (C) dispositions of obsolete or worthless assets that are no longer useful in the conduct of the business of the Group Companies or (D) transfers among the Group Companies;
| A-28 |
(d) declare, set aside, make or pay any dividend or other distribution, payable in cash, shares, property or otherwise, with respect to any of its shares (other than dividends or other distributions from any Subsidiary of the Company to the Company or any of its other Subsidiaries);
(e) reclassify, combine, split, subdivide or redeem, or purchase or otherwise acquire, directly or indirectly, any of its share capital or securities or other rights exchangeable into or convertible or exercisable for any of its share capital (other than the purchase of Shares to satisfy obligations under the Share Incentive Plans, including the withholding of Shares in connection with the exercise of Company Options or the vesting of Company RSs in accordance with the terms and conditions of such Company Options or Company RSs (as applicable));
(f) adopt a plan or agreement of, or commence any proceeding for, complete or partial liquidation, winding up or dissolution, scheme of arrangement, merger, consolidation, amalgamation, restructuring, reorganization or similar transaction involving any Group Company, other than with respect to any Tax restructuring commenced prior to the date hereof, or as contemplated by this Agreement;
(g) acquire, whether by purchase, merger, consolidation, scheme of arrangement, amalgamation or acquisition of stock or assets or otherwise, any assets, securities or properties, in aggregate, with a value or purchase price (including the value of assumed liabilities) in excess of $3,500,000 in any transaction or related series of transactions, other than pursuant to existing Contracts or commitments;
(h) incur or guarantee any Indebtedness for borrowed money of any Third Party, except for the incurrence or guarantee of Indebtedness (i) under any Group Company’s existing credit facilities as in effect on the date hereof (including any renewal, extension, refinancing or replacement of such Contracts on substantially the same or similar terms) in an aggregate amount not to exceed the maximum amount authorized under the Contracts evidencing such Indebtedness or (ii) in an aggregate amount not in excess of $5,000,000 (in addition to any Indebtedness incurred in accordance with clause (i));
(i) make capital expenditures in excess of $4,000,000 in the aggregate for Group Companies taken as a whole during any twelve month period, except as consistent with past practice and in the ordinary course of business;
(j) except as required under applicable Law or pursuant to any Company Employee Plan or this Agreement, (i) enter into any new employment or compensatory agreements, or terminate any such agreements, with any director, officer, employee or consultant of any Group Company other than the hiring or termination of employees below the general manager level or its equivalent (e.g. the head of business unit) or with an annual compensation of less than $150,000, (ii) grant or provide any severance or termination payments or benefits to any director or officer of any Group Company except as required by applicable Law or any Contract or agreement in effect as of the date hereof, (iii) increase the compensation, bonus, severance or other benefits of, or pay any bonus to, any director, officer, employee or consultant of any Group Company whose annual compensation as of the date hereof is in excess of $150,000 (except such increases or payments that, in the aggregate. do not cause an increase in labor costs to the Group Companies, taken as a whole, by more than five percent (5%)), (iv) make any new equity awards to any director, officer or employee of any Group Company, (v) establish, adopt, amend or terminate any Company Employee Plan or materially amend the terms of any outstanding Company Options and Company RSs, (vi) take any action to voluntarily accelerate the vesting of Company Options or Company RSs to the extent not already required in the Share Incentive Plans or contemplated by this Agreement or otherwise pursuant to any Contract disclosed to Parent in writing as of the date hereof or (vii) forgive any loans to directors, officers or employees of any Group Company;
| A-29 |
(k) make any material changes with respect to financial accounting policies or procedures, including changes affecting the reported consolidated assets, liabilities or results of operations of the Group Companies, except as required by changes in statutory or regulatory accounting rules or GAAP or regulatory requirements with respect thereto or such changes as would better reflect the business and operations of the Group Companies due to changes in circumstances after the date hereof;
(l) enter into or amend, modify, consent to the termination of, or waive any material rights under, any Material Contract (or any Contract that would be a Material Contract if such Contract had been entered into prior to the date hereof) that calls for annual aggregate payments of $4,500,000 or more that cannot be terminated without material surviving obligations or material penalty upon notice of 90 days or less, other than in the ordinary course of business;
(m) enter into any Contract between the Company or any of its Subsidiaries, on the one hand, and any of their directors or executive officers, on the other hand, in each case required to be disclosed pursuant to Item 7B or Item 19 of Form 20-F under the Exchange Act (except as contemplated under this Agreement or permitted under Section 5.01(j));
(n) terminate or cancel, let lapse, or amend or modify in any material respect, other than renewals in the ordinary course of business, any material insurance policies maintained by it that is not promptly replaced by a comparable amount of insurance coverage;
(o) commence any Action for a claim of more than $1,000,000 (excluding any Action seeking injunctive relief or other similar equitable remedies) or settle any Action other than any settlement involving the payment of monetary damages not in excess of $1,000,000;
(p) permit any material Intellectual Property owned by any Group Company to lapse, be abandoned, dedicated or disclaimed, or fail to perform or make any applicable filings, recordings or other similar actions or filings or fail to pay material fees and Taxes required to maintain and protect its interest in the material Intellectual Property owned by any Group Company;
(q) engage in the conduct of any new line of business material to the Group Companies, taken as a whole;
(r) make or change any material Tax election, materially amend any Tax return (except as required by applicable Law), enter into any material closing agreement with respect to Taxes, surrender any right to claim a material refund of Taxes, settle or finally resolve any material controversy with respect to Taxes or materially change any method of Tax accounting; or
| A-30 |
(s) announce an intention, enter into any agreement or otherwise make a commitment, to do any of the foregoing.
Section 5.02 Operation of Parent’s and Amalgamation Sub’s Business.
Each of Parent and Amalgamation Sub agrees that, from the date hereof until the earlier of the Effective Time and termination of this Agreement pursuant to Article VIII, it shall not: (a) take any action that is intended to or would reasonably be likely to result in any of the conditions to effecting the Amalgamation becoming incapable of being satisfied or (b) take any action or fail to take any action that would, or would be reasonably likely to, individually or in the aggregate, prevent, materially delay or materially impede the ability of Parent or Amalgamation Sub to consummate the Amalgamation or the other Transactions in accordance with the terms of the Agreement.
Section 5.03 No Control of Other Party’s Business.
Except as otherwise expressly provided herein, nothing contained in this Agreement is intended to give Parent or Amalgamation Sub, directly or indirectly, the right to control or direct the Company’s or the Company’s Subsidiaries’ operations prior to the Effective Time, and nothing contained in this Agreement is intended to give the Company, directly or indirectly, the right to control or direct Parent’s or Amalgamation Sub’s operations. Prior to the Effective Time, each of Parent, Amalgamation Sub and the Company shall exercise, consistent with the terms and conditions of this Agreement, complete control and supervision over its and its Subsidiaries’ respective operations.
Article
VI
ADDITIONAL AGREEMENTS
Section 6.01 Proxy Statement and Schedule 13E-3.
(a) As soon as practicable following the date hereof, the Company with the assistance of Parent and Amalgamation Sub, shall prepare a proxy statement relating to the authorization and approval of this Agreement and the Transactions by the shareholders of the Company (such proxy statement, as amended or supplemented, being referred to herein as the “Proxy Statement”). Concurrently with the preparation of the Proxy Statement, the Company, Parent and Amalgamation Sub shall jointly prepare and cause to be filed with the SEC a Rule 13e-3 transaction statement on Schedule 13E-3 relating to the authorization and approval of this Agreement and the Transactions by the shareholders of the Company (such Schedule 13E-3, as amended or supplemented, being referred to herein as the “Schedule 13E-3”). Each of the Company, Parent and Amalgamation Sub shall use its reasonable best efforts so that the Proxy Statement and the Schedule 13E-3 complies in all material respects with the requirements of the Exchange Act and the rules and regulations promulgated thereunder. Each of the Company, Parent and Amalgamation Sub shall use its reasonable best efforts to respond promptly to any comments of the SEC with respect to the Proxy Statement and the Schedule 13E-3. Each of Parent and Amalgamation Sub shall provide reasonable assistance and cooperation to the Company in the preparation, filing and distribution of the Proxy Statement, the Schedule 13E-3 and the resolution of comments from the SEC. Upon its receipt of any comments from the SEC or its staff or any request from the SEC or its staff for amendments or supplements to the Proxy Statement and the Schedule 13E-3, the Company shall promptly notify Parent and Amalgamation Sub and shall provide Parent with copies of all correspondence between the Company and its representatives, on the one hand, and the SEC and its staff, on the other hand. No filing of the Schedule 13E-3, the Proxy Statement, any amendments or supplements thereto, or any response to the SEC will be made by the Company, Parent or Amalgamation Sub unless each other party and its counsel has had a reasonable opportunity to review and propose comments which such party shall consider in good faith. If at any time prior to the Shareholders’ Meeting, any information relating to the Company, Parent, Amalgamation Sub or any of their respective Affiliates, officers or directors, is discovered by the Company, Parent or Amalgamation Sub that should be set forth in an amendment or supplement to the Proxy Statement and/or the Schedule 13E-3 so that the Proxy Statement and/or Schedule 13E-3 shall not contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary in order to make the statements therein, in light of the circumstances under which they are made, not misleading, the party that discovers such information shall promptly notify the other parties hereto and the Company shall file an appropriate amendment or supplement describing such information with the SEC and, to the extent required by applicable Law, disseminate to the shareholders of the Company.
| A-31 |
(b) Each of Parent, Amalgamation Sub and the Company agrees, as to itself and its respective Affiliates or Representatives, that none of the information supplied or to be supplied by Parent, Amalgamation Sub or the Company, as applicable, expressly for inclusion or incorporation by reference in the Proxy Statement, the Schedule 13E-3 or any other documents filed or to be filed with the SEC in connection with the Transactions, will, as of the time such documents (or any amendment thereof or supplement thereto) are mailed to the holders of Shares and at the time of the Shareholders’ Meeting, contain any untrue statement of a material fact, or omit to state any material fact required to be stated therein in order to make the statements therein, in light of the circumstances under which they were made, not misleading. Each of Parent, Amalgamation Sub and the Company further agrees that all documents that such party is responsible for filing with the SEC in connection with the Amalgamation will comply as to form and substance in all material respects with the applicable requirements of the Securities Act, the Exchange Act and any other applicable Laws and that all information supplied by such party for inclusion or incorporation by reference in such document will not contain any untrue statement of a material fact, or omit to state any material fact required to be stated therein in order to make the statements therein, in light of the circumstances under which they were made, not misleading. If at any time prior to the Effective Time, any event or circumstance relating to Parent, Amalgamation Sub or the Company, or their respective officers or directors, should be discovered that should be set forth in an amendment or a supplement to the Proxy Statement or the Schedule 13E-3 so that such document would not include any misstatement of a material fact or omit to state any material fact necessary to make the statements therein, in light of the circumstances under which they are made, not misleading, the party discovering such event or circumstance shall promptly inform the other parties and an appropriate amendment or supplement describing such event or circumstance shall be promptly filed with the SEC and disseminated to the shareholders of the Company to the extent required by Law; provided that prior to such filing, the Company and Parent, as the case may be, shall consult with each other with respect to such amendment or supplement and shall afford the other party and their Representatives a reasonable opportunity to comment thereon.
| A-32 |
(c) At the Shareholders’ Meeting, Parent shall vote, or cause to be voted, all of the Shares then beneficially owned by Parent or Amalgamation Sub or with respect to which Parent or Amalgamation Sub otherwise has, directly or indirectly, voting power in favor of the authorization and approval of this Agreement and the Transactions.
Section 6.02 Company Shareholders’ Meeting.
(a) As promptly as reasonably practicable after the SEC confirms it has no further comments on the Schedule 13E-3 (including the Proxy Statement filed therewith as an exhibit), the Company shall (i) take, in accordance with applicable Law, its articles of incorporation and by-laws and the rules of the NASDAQ, all action necessary to call, give notice of, set a record date in accordance with Section 107 of the IBCA (the “Record Date”) for determining shareholders of the Company entitled to vote at, and convene the Shareholders’ Meeting for the purpose of obtaining the Requisite Company Vote and shall not change such Record Date or establish a different record date for the Shareholders’ Meeting without the prior written consent of Parent, unless required to do so by the applicable requirements of the Securities Act, the Exchange Act or any other applicable Law; provided that, in the event that the date of the Shareholders’ Meeting as originally called is adjourned or otherwise delayed in accordance with this Agreement, the Company may establish a new Record Date, as adjourned or delayed, without the prior written consent of Parent and (ii) mail or cause to be mailed to the holders of Shares the Proxy Statement (including notice of the Shareholders’ Meeting) and a form of proxy for use at the Shareholders’ Meeting; provided that the Company may adjourn the Shareholders’ Meeting (i) with the written consent of Parent, (ii) to allow reasonable time for the filing and mailing of any supplemental or amended disclosure and for such supplemental or amended disclosure to be disseminated and reviewed by the Company’s shareholders prior to the Shareholders’ Meeting or (iii) as otherwise required by applicable Law or any court of competent jurisdiction; provided further that the Shareholders’ Meeting shall be adjourned in accordance with Section 113 of the IBCA to the same day two weeks from the originally scheduled Shareholders’ Meeting if at the time the originally scheduled Shareholders’ Meeting proceeds to business there are insufficient Shares represented (either in person or by proxy) to constitute a quorum necessary to conduct business at the Shareholders’ Meeting.
(b) Subject to Section 6.04, (i) the Company Board shall recommend to holders of the Shares that they authorize and approve this Agreement and the Transactions, and shall include such recommendation in the Proxy Statement (which authorization and approval shall be the only matters (other than procedural matters) that are proposed to be voted upon by the holders of Shares at the Shareholders’ Meeting) and (ii) the Company shall use its reasonable best efforts to solicit from its shareholders proxies in favor of the authorization and approval of this Agreement and the Transactions and shall take all other action necessary or advisable to secure the Requisite Company Vote. For the avoidance of doubt, unless this Agreement is validly terminated in accordance with Section 8.03(c) or Section 8.03(d), the Company’s obligations pursuant to this Section 6.02 shall not be limited or otherwise affected by the commencement, public proposal, public disclosure or communication to the Company or any other person of any Competing Transaction or by any Change in the Company Recommendation.
| A-33 |
Section 6.03 Access to Information; Confidentiality.
(a) From the date hereof until the earlier of the Effective Time and termination of this Agreement pursuant to Article VIII and subject to applicable Law and the Confidentiality Agreements, upon reasonable prior notice from Parent, the Company shall (i) provide to Parent and its Representatives reasonable access during normal business hours and upon reasonable prior notice, to the offices, properties, books and records of any Group Company, (ii) to the extent not publicly available, furnish to Parent and its Representatives such existing financial and operating data and other existing information as such persons may reasonably request in writing and (iii) instruct the auditors and other Representatives of the Group Companies to reasonably cooperate with Parent and its Representatives in their investigation. Notwithstanding the foregoing, any such investigation shall be conducted in such a manner as not to interfere unreasonably with the business or operations of the Company or its Subsidiaries or otherwise result in any significant interference with the timely discharge by the employees of the Company or its Subsidiaries of their duties.
(b) Notwithstanding anything to the contrary in Section 6.03(a), nothing in this Agreement shall require the Company or any of its Subsidiaries to provide Parent or any of its Representatives with access to or furnish any books, records, documents or other information to the extent that (i) such books, records, documents or other information are subject to any confidentiality agreement with a Third Party; provided that at the written request of Parent, the Company shall use its commercially reasonable best efforts to obtain a waiver of such confidentiality agreement from such Third Party, (ii) the disclosure of such books, records, documents or other information would reasonably be expected to result in the loss of attorney-client or other legal privilege or (iii) the disclosure of such books, records, documents or other information is prohibited by applicable Law.
(c) All information provided or made available pursuant to this Section 6.03 to Parent or its Representatives shall be subject to the Confidentiality Agreements and, without limiting the generality of the foregoing, Parent shall not, and shall cause its Representatives not to, use such information for any purpose unrelated to the consummation of the Transactions.
| A-34 |
Section 6.04 No Solicitation of Transactions.
(a) Until the earlier of the Effective Time and termination of this Agreement pursuant to Article VIII, except as set forth in this Section 6.04, the Company agrees that neither it nor any of its Subsidiaries, and that it will cause its and its Subsidiaries’ Representatives (including any investment banker, attorney or accountant retained by any Group Company), not to, in each case, directly or indirectly, (i) solicit, initiate or knowingly encourage (including by providing nonpublic information concerning any Group Company) any inquiries or the making of any proposal or offer (including any proposal or offer to its shareholders) that constitutes, or that in the Company’s good faith judgment could reasonably be expected to lead to, any Competing Transaction, (ii) enter into, maintain or continue discussions or negotiations with, or provide any nonpublic information concerning any Group Company to, any Third Party in furtherance of such inquiries or to obtain such proposal or offer for a Competing Transaction, (iii) agree to, approve, endorse, recommend or consummate any Competing Transaction or enter into any letter of intent or Contract (other than a customary confidentiality agreement) or commitment contemplating or otherwise relating to any Competing Transaction, (iv) grant any waiver, amendment or release under any standstill, confidentiality or similar agreement (and the Company shall promptly take all action necessary to terminate or cause to be terminated any such waiver previously granted with respect to any provision of any such confidentiality, standstill or similar agreement and to enforce each such confidentiality, standstill or similar agreement) or any anti-takeover Law or (v) authorize or permit any of the Representatives of the Company or any of its Subsidiaries to take any action set forth in clauses (i) – (iv) of this Section 6.04(a). The Company shall notify Parent as promptly as practicable (and in any event within forty-eight (48) hours after the Company has knowledge thereof), of any proposal or offer, or any inquiry or contact with any person, that constitutes, or that in the Company’s good faith judgment could reasonably be expected to lead to, a Competing Transaction, (x) specifying the material terms and conditions thereof (including material amendments or proposed material amendments) and (y) specifying the identity of the party making such proposal or offer or inquiry or contact. The Company shall keep Parent informed, on a reasonably current basis (and in any event within forty-eight (48) hours of the occurrence of any material changes, developments, discussions or negotiations) of the status and terms of any such proposal, offer, inquiry, contact or request and of any material changes in the status and terms of any such proposal, offer, inquiry, contact or request (including the material terms and conditions thereof). Without limiting the foregoing, the Company shall provide Parent with forty-eight (48) hours prior notice (or such lesser prior notice as is provided to the members of the Company Board or the members of the Special Committee, as applicable) of any meeting of the Company Board or the Special Committee at which the Company Board or the Special Committee, as applicable, is reasonably expected to consider any Competing Transaction. The Company shall, and shall cause its Subsidiaries and their respective Representatives to, immediately following the execution of this Agreement, cease and terminate all existing discussions or negotiations with any parties conducted heretofore with respect to a Competing Transaction. The Company shall not, and shall cause its Subsidiaries not to, enter into any confidentiality agreement with any Third Party subsequent to the date hereof that prohibits the Company from complying with its obligations under this Section 6.04(a).
(b) Notwithstanding anything to the contrary in Section 6.04(a), at any time prior to the receipt of the Requisite Company Vote, following the receipt of a written bona fide proposal or offer regarding a Competing Transaction that did not result from a breach of Section 6.04(a) (other than any immaterial non-compliance that does not adversely affect Parent or Amalgamation Sub), the Company and its Representatives may, with respect to such proposal or offer and acting only upon the recommendation of the Special Committee:
(i) contact the person who has made such proposal or offer to clarify and understand the terms and conditions thereof to the extent the Special Committee shall have determined in good faith that such contact is necessary to determine whether such proposal or offer constitutes a Superior Proposal or could reasonably be expected to result in a Superior Proposal;
(ii) provide information (including non-public information) in response to the request of the person who has made such proposal or offer, if and only if, prior to providing such information, the Company has received from the person so requesting such information an executed customary confidentiality agreement; provided that the Company shall concurrently make available to Parent any material nonpublic information concerning the Company and the Subsidiaries that is provided to any such person and that was not previously made available to Parent or its Representatives; and
| A-35 |
(iii) engage or participate in any discussions or negotiations with the person who has made such proposal or offer;
provided that prior to taking any actions described in clause (ii) or (iii), the Company Board (acting only upon the recommendation of the Special Committee) or the Special Committee has (A) determined, in its good faith judgment, after consultation with its financial advisor and outside legal counsel, that such proposal or offer constitutes or could reasonably be expected to result in a Superior Proposal, (B) determined, in its good faith judgement, after consultation with its outside legal counsel, that in light of such proposal or offer, failure to take such action would be inconsistent with the fiduciary duties of the Company Board under applicable Law and (C) provided written notice to Parent at least forty-eight (48) hours prior to taking any such action.
(c) Except as set forth in Section 6.04(d) or Section 6.04(e), neither the Company Board nor any committee thereof shall (i) (A) change, withhold, withdraw, qualify or modify (or publicly propose to change, withhold, withdraw, qualify or modify), in a manner adverse to Parent or Amalgamation Sub, the Company Recommendation, (B) fail to include the Company Recommendation in the Proxy Statement, (C) adopt, approve or recommend, or publicly propose to adopt, approve or recommend to the shareholders of the Company, a Competing Transaction, or (D) if a tender offer or exchange offer that constitutes a Competing Transaction is commenced, fail to publicly recommend against acceptance of such tender offer or exchange offer by the Company shareholders (including, for these purposes, by disclosing that it is taking no position with respect to the acceptance of such tender offer or exchange offer by its shareholders, which shall constitute a failure to recommend against acceptance of such tender offer or exchange offer; provided that a customary “stop, look and listen” communication by the Company Board pursuant to Rule 14d-9(f) of the Exchange Act or a statement that the Company Board has received and is currently evaluating such Competing Transaction shall not be prohibited or be deemed to be a Change in the Company Recommendation) within ten (10) Business Days after the commencement thereof (any of the foregoing, a “Change in the Company Recommendation”) or (ii) cause or permit the Company or any of its Subsidiaries to enter into any letter of intent, memorandum of understanding, agreement in principle, merger agreement, acquisition agreement or other or similar document or Contract with respect to any Competing Transaction other than a customary confidentiality agreement entered into in compliance with Section 6.04(b) (an “Alternative Acquisition Agreement”).
| A-36 |
(d) Notwithstanding anything to the contrary set forth in this Agreement, from the date hereof and at any time prior to the receipt of the Requisite Company Vote, if the Company has received a written bona fide proposal or offer with respect to a Competing Transaction that did not result from a breach of Section 6.04 (other than any immaterial non-compliance that does not adversely affect Parent or Amalgamation Sub) and the Company Board (acting only upon the recommendation of the Special Committee) or the Special Committee determines in its good faith judgment (after consultation with its financial advisor and outside legal counsel), that such proposal or offer constitutes a Superior Proposal and failure to make a Change in the Company Recommendation with respect to such Superior Proposal would be inconsistent with the fiduciary duties of the Company Board under applicable Law, the Company Board (acting only upon the recommendation of the Special Committee) or the Special Committee may make a Change in the Company Recommendation with respect to such Superior Proposal and/or authorize the Company to terminate this Agreement in accordance with Section 8.03(c), but only (i) after (A) providing at least five (5) Business Days’ (the “Superior Proposal Notice Period”) written notice to Parent (a “Notice of Superior Proposal”) advising Parent that the Company Board has received a Superior Proposal, specifying the material terms and conditions of such Superior Proposal, identifying the person making such Superior Proposal and indicating that the Company Board (acting only upon the recommendation of the Special Committee) or the Special Committee, as applicable, intends to effect a Change in the Company Recommendation and/or authorize the Company to terminate this Agreement in accordance with Section 8.03(c); it being understood that the Notice of Superior Proposal or any amendment or update thereto or the determination to so deliver such notice shall not constitute a Change in the Company Recommendation, and (B) negotiating with and causing its financial and legal advisors to negotiate with Parent, Amalgamation Sub and their respective Representatives in good faith (to the extent Parent desires to negotiate) to make such adjustments in the terms and conditions of this Agreement and the Financing, so that such Third-Party proposal or offer would cease to constitute a Superior Proposal; provided that any material modifications to such Third-Party proposal or offer that the Company Board (acting only upon the recommendation of the Special Committee) or the Special Committee has determined to be a Superior Proposal shall be deemed a new Superior Proposal to which the requirements of this Section 6.04(d) shall apply; provided, further, that with respect to such new Superior Proposal, the Superior Proposal Notice Period shall be deemed to be a three (3) Business Day period rather than the five (5) Business Day period first described above and (ii) following the end of such five (5) Business Day or three (3) Business Day period (as applicable), the Company Board (acting only upon the recommendation of the Special Committee) or the Special Committee shall have determined in its good faith judgment after consultation with its financial advisor and outside legal counsel that taking into account any changes to this Agreement and the Financing proposed by Parent and Amalgamation Sub in response to the Notice of Superior Proposal or otherwise, that the proposal or offer with respect to the Competing Transaction giving rise to the Notice of Superior Proposal continues to constitute a Superior Proposal.
(e) Notwithstanding anything to the contrary set forth in this Agreement, from the date hereof and at any time prior to the receipt of the Requisite Company Vote, the Company Board (acting only upon the recommendation of the Special Committee) or the Special Committee may (i) make a Change in the Company Recommendation or (ii) if an Intervening Event has occurred, authorize the Company to terminate this Agreement pursuant to Section 8.03(d), in each case, if the Company Board (acting only upon the recommendation of the Special Committee) or the Special Committee, as applicable, determines in its good faith judgment (after consultation with outside legal counsel), that the failure to take such action would be inconsistent with its fiduciary duties under applicable Law; provided that the Company Board or the Special Committee, as applicable, shall not take any such action unless the Company has (A) provided to Parent at least five (5) Business Days’ prior written notice that it intends to take such action and specifying in reasonable detail the facts underlying the decision by the Company Board (acting only upon the recommendation of the Special Committee) or the Special Committee, as applicable, to take such action and (B) during such five (5) Business Day period, if requested in writing by Parent, engaged in good faith negotiations with Parent to amend this Agreement in such a manner that would obviate (as determined by the Company Board (acting only upon the recommendation of the Special Committee) or the Special Committee, as applicable, after consultation with outside legal counsel) the need for such action.
| A-37 |
(f) A “Competing Transaction” means any of the following (other than the Transactions): (i) any amalgamation, consolidation, share exchange, business combination, scheme of arrangement, amalgamation, recapitalization, liquidation, dissolution or other similar transaction involving the Company or any of its Subsidiaries whose assets, individually or in the aggregate, constitute twenty percent (20%) or more of the consolidated assets of the Company or to which twenty percent (20%) or more of the total revenue or net income of the Company are attributable, (ii) any sale, lease, exchange, transfer or other disposition of assets or businesses that constitute or represent twenty percent (20%) or more of the total revenue, net income or assets of the Group Companies, taken as a whole, (iii) any sale, exchange, transfer or other disposition of twenty percent (20%) or more of any class of equity securities of the Company, or securities convertible into or exchangeable for twenty percent (20%) or more of any class of equity securities of the Company, (iv) any tender offer or exchange offer that, if consummated, would result in any person beneficially owning twenty percent (20%) or more of any class of equity securities of the Company or (v) any combination of the foregoing.
(g) An “Intervening Event” means an event, occurrence, change or development that materially affects any Group Company or its business, assets or operations that (i) was unknown by the Company Board and the Special Committee as of or prior to the date hereof (or if known, the material consequences of which are not known to or reasonably foreseeable by the Company Board and the Special Committee as of the date hereof) and (ii) occurs, arises or becomes known (or any material consequences thereof become known) to the Company Board or the Special Committee after the date hereof and on or prior to the receipt of the Requisite Company Vote; provided that the receipt by the Company of a Competing Transaction or Superior Proposal will not be deemed to constitute, or be taken into account to determine whether such event, occurrence, change or development will constitute, an Intervening Event.
(h) A “Superior Proposal” means a written proposal or offer with respect to a Competing Transaction that would result in any person (or its shareholders, members or other equity owners) becoming the beneficial owner, directly or indirectly, of more than fifty percent (50%) of the assets (on a consolidated basis), or more than fifty percent (50%) of the total voting power of the equity securities, of the Company that (i) provides for the payment of (A) cash consideration per Share to holders thereof that is in excess of the Per Share Amalgamation Consideration and/or (B) consideration in the form of publicly traded securities of a company listed on an internationally recognized securities exchange or automated quotation system with a fair market value that in the good faith judgment of the Company Board (acting only upon the recommendation of the Special Committee) or the Special Committee after consultation with its financial advisor is in excess of the Per Share Amalgamation Consideration and (ii) the Company Board (acting only upon the recommendation of the Special Committee) or the Special Committee has determined in its good faith judgment after consultation with its financial advisor and outside legal counsel, (A) is reasonably likely to be consummated in accordance with its terms, taking into account all legal, financial, and regulatory aspects of the proposal (including financing, regulatory approvals, shareholder litigation, the identity of the Person or group making such proposal, breakup or termination fee and expense reimbursement provisions, expected timing and risk and likelihood of consummation and other relevant events and circumstances) and (B) would, if consummated, result in a transaction more favorable to the Company’s shareholders (other than the holders of the Excluded Shares) from a financial point of view than the Transactions; provided that no offer or proposal shall be deemed to be a “Superior Proposal” if (x) the receipt of any financing required to consummate the transaction contemplated by such offer or proposal is a condition to the consummation of such transaction, or (y) the consummation of the transactions contemplated by such offer or proposal is expressly conditioned upon obtaining any consent or approval of a Governmental Authority or other third party that is not expressly set forth as a condition to the consummation of the Amalgamation in Article VII.
| A-38 |
(i) Prior to the termination of this Agreement pursuant to Article VIII, the Company shall not submit to the vote of its shareholders any Competing Transaction or enter into any Alternative Acquisition Agreement or propose to do so.
(j) Nothing contained in this Section 6.04 shall be deemed to prohibit the Company, the Company Board or the Special Committee from (i) complying with its disclosure obligations under U.S. federal or state or non-U.S. Law, including (A) disclosure of factual information regarding the business, financial condition or results of operations of the Company or (B) taking and disclosing to its shareholders a position contemplated by Rule 14e-2(a), Rule 14d-9 or Item 1012(a) of Regulation M-A promulgated under the Exchange Act (or any similar communication to shareholders in connection with the making or amendment of a tender offer or exchange offer) or (ii) making any “stop-look-and-listen” communication of the type contemplated by Rule 14d-9(f) under the Exchange Act.
Section 6.05 Directors’ and Officers’ Indemnification and Insurance.
(a) The indemnification, advancement and exculpation provisions of the indemnification agreements by and among the Company and its directors and certain executive officers as in effect at the Effective Time shall survive the Amalgamation and shall not be amended, repealed or otherwise modified for a period of six (6) years from the Effective Time in any manner that would adversely affect the rights thereunder of the current or former directors or officers of the Company or any of its Subsidiaries. The articles of incorporation and by-laws of the Surviving Corporation (or in such documents of any successor to the business of the Surviving Corporation) shall contain provisions no less favorable to the intended beneficiaries with respect to exculpation and indemnification of liability and advancement of expenses than are set forth in the articles of incorporation and by-laws of the Company (or in such documents of any successor to the business of the Surviving Corporation) as in effect on the date hereof, and Parent shall cause such provisions to not be amended, repealed or otherwise modified for a period of six years from the Effective Time in any manner that would affect adversely the rights thereunder of individuals who, at or prior to the Effective Time, were directors, officers, employees, fiduciaries or agents of the Company, unless such modification shall be required by Law. From and after the Effective Time, any agreement of any Indemnified Party with the Company or any of its Subsidiaries regarding exculpation or indemnification of liability or advancement of expenses shall be assumed by the Surviving Corporation, shall survive the Amalgamation and shall continue in full force and effect in accordance with its terms.
| A-39 |
(b) The Surviving Corporation shall, and Parent shall cause the Surviving Corporation to, maintain in effect for six (6) years from the Effective Time the current directors’ and officers’ fiduciary liability insurance policies maintained by the Company with respect to matters occurring prior to the Effective Time, including acts or omissions occurring in connection with this Agreement and the consummation of the Transactions (the parties covered thereby, the “Indemnified Parties”) on terms, conditions, retentions and limits of liability no less favorable to the Indemnified Parties than those in effect as of the Effective Time; provided that the Surviving Corporation may substitute therefor policies of at least the same coverage containing terms, conditions, retentions and limits of liability that are no less favorable than those provided under the Company’s current policies from an insurance carrier with the same or better credit rating as the Company’s current insurance carrier; provided, further, that in no event shall the Surviving Corporation be required to expend pursuant to this Section 6.05(b) more than an amount per year equal to three hundred percent (300%) of current annual premiums paid by the Company for such insurance, and if the cost of such insurance policy exceeds such amount, then the Surviving Corporation shall obtain a policy with the greatest coverage for a cost not exceeding such amount. In addition, the Company may and, at Parent’s request, the Company shall, purchase a six-year “tail” prepaid policy prior to the Effective Time on terms, conditions, retentions and limits of liability no less advantageous to the Indemnified Parties than the existing directors’ and officers’ fiduciary liability insurance policies maintained by the Company. If such “tail” prepaid policy has been obtained by the Company prior to the Effective Time, the Surviving Corporation shall, and Parent shall cause the Surviving Corporation to, maintain such policy in full force and effect, and continue to honor the respective obligations thereunder, and all other obligations of Parent or Surviving Corporation under this Section 6.05(b) shall terminate.
(c) Subject to the terms and conditions of this Section 6.05, from and after the Effective Time, the Surviving Corporation shall comply (and Parent shall cause the Surviving Corporation to comply) with all of the Company’s obligations, and each of the Surviving Corporation and Parent shall cause its Subsidiaries to comply with their respective obligations to indemnify and hold harmless (including any obligations to advance funds for expenses) (i) the Indemnified Parties against any and all costs or expenses (including reasonable attorneys’ fees and expenses), judgments, fines, losses, claims, damages, liabilities and amounts paid in settlement in connection with any actual or threatened claim, action, suit, proceeding or investigation, whether civil, criminal, administrative or investigative (“Damages”), arising out of, relating to or in connection with (A) the fact that an Indemnified Party is or was a director, officer or employee of the Company or any of its Subsidiaries or (B) any acts or omissions occurring or alleged to have occurred (including acts or omissions with respect to the approval of this Agreement or the Transactions or arising out of or pertaining to the Transactions and actions to enforce this provision or any other indemnification or advancement right of any Indemnified Party) prior to or at the Effective Time, to the extent provided under the Company’s or such Subsidiaries’ respective organizational and governing documents or agreements in effect on the date hereof (true and complete copies of which shall have been delivered to Parent prior to the date hereof) and to the fullest extent permitted by the IBCA or any other applicable Law; provided that such indemnification shall be subject to any limitation imposed from time to time under applicable Law and (ii) such persons against any and all Damages arising out of acts or omissions in such persons’ official capacity as an officer, director or other fiduciary in the Company or any of its Subsidiaries if such service was at the request or for the benefit of the Company or any of its Subsidiaries.
(d) In the event the Company or the Surviving Corporation or any of their respective successors or assigns (i) consolidates with or amalgamates into any other person and shall not be the continuing or surviving corporation or entity of such consolidation or amalgamation or (ii) transfers all or substantially all of its properties and assets to any person, then, and in each such case, proper provision shall be made so that the successors and assigns of the Company or the Surviving Corporation, as the case may be, or at Parent’s option, Parent, shall assume the obligations set forth in this Section 6.05.
| A-40 |
(e) The agreements and covenants contained in this Section 6.05 shall be in addition to any other rights an Indemnified Party may have under the articles of association and by-laws of the Company or any of its Subsidiaries (or equivalent constitutional documents), or any agreement between an Indemnified Party and the Company or any of its Subsidiaries, under the IBCA or other applicable Law, or otherwise. The provisions of this Section 6.05 shall survive the consummation of the Amalgamation and are intended to be for the benefit of, and shall be enforceable by, each of the Indemnified Parties and their heirs and legal representatives, each of which shall be a third-party beneficiary of the provisions of this Section 6.05. The obligations of Parent and the Surviving Corporation under this Section 6.05 shall not be terminated or modified in such a manner as to adversely affect the rights of any Indemnified Party without the consent of such Indemnified Party.
(f) Nothing in this Agreement is intended to, shall be construed to or shall release, waive or impair any rights to directors’ and officers’ insurance claims under any policy that is or has been in existence with respect to the Company or any of its Subsidiaries or their respective officers, directors and employees; it being understood and agreed that the indemnification provided for in this Section 6.05 is not prior to or in substitution for any such claims under any such policies.
Section 6.06 Notification of Certain Matters.
Each of the Company and Parent shall promptly notify the other in writing of:
(a) any notice or other communication from any person alleging that the consent of such person is or may be required in connection with the Transactions;
(b) any notice or other communication from any Governmental Authority in connection with the Transactions;
(c) any Actions commenced or, to the knowledge of the Company or the knowledge of Parent, threatened against the Company or any of its Subsidiaries or Parent or any of its Subsidiaries, as the case may be, that, if pending on the date hereof, would have been required to have been disclosed by such party pursuant to any of such party’s representations and warranties contained herein, or that relate to this Agreement or the Transactions, including such party’s ability to consummate the Transactions;
(d) if a breach of any representation or warranty or failure to perform any covenant or agreement on the part of such party set forth in this Agreement shall have occurred that would cause the conditions set forth in Section 7.01, Section 7.02 or Section 7.03 not to be satisfied; and
(e) any person notifying the Company or any of its Subsidiaries in writing that such person is seeking indemnification from the Company or any of its Subsidiaries under any indemnification, advancement or exculpation provisions of the indemnification agreements by and among the Company or any of its Subsidiaries and their respective directors and executive officers or the memorandum and articles of association of the Company or any of its Subsidiaries;
| A-41 |
together, in each case, with a copy of any such notice, communication or Action; provided that the delivery of any notice pursuant to this Section 6.06 shall not limit or otherwise affect the remedies available hereunder to the party receiving such notice; provided, further, that failure to give prompt notice pursuant to this Section 6.06 shall not constitute a failure of a condition to the Amalgamation set forth in Article VII except to the extent that the underlying breach of a representation or warranty or failure to perform any covenant or agreement not so notified would, standing alone, constitute such a failure; provided, further, that the Company’s unintentional failure to give notice under this Section 6.06 shall not be deemed to be a breach of covenant under this Section 6.06 but instead shall constitute only a breach of the underlying representation or warranty or covenant or condition, as the case may be.
Section 6.07 Financing.
(a) Subject to the terms and conditions of this Agreement, each of Parent and Amalgamation Sub shall use, and shall cause their respective Subsidiaries to use, their respective reasonable best efforts to take, or cause to be taken, all actions and to do, or cause to be done, all things necessary, proper or advisable to obtain the Financing at or prior to the Effective Time on the terms and conditions described in the Equity Commitment Letters, including (i) maintaining in effect the Equity Commitment Letters, (ii) satisfying, or causing to be satisfied, on a timely basis (or, if applicable, obtaining waivers of) all conditions to closing of and funding under the Equity Commitment Letters, and not taking or failing to take any action that would reasonably be expected to prevent, impede or delay the availability and consummation of the Financing, (iii) consummating the Financing under the Equity Commitment Letters at or prior to the Effective Time and (iv) enforcing the parties’ funding obligations (and the rights of Parent and Amalgamation Sub) under the Equity Commitment Letters, including by seeking specific performance of the parties thereunder.
(b) Parent shall give the Company prompt notice (i) upon becoming aware of any breach of any material provision of the Equity Commitment Letters or any other definitive documents relating to the Financing, or termination of any such documents relating to the Financing or (ii) upon the receipt of any written notice from any party to the Equity Commitment Letters or any other definitive documents relating to the Financing with respect to any threatened breach of any material provision or threatened termination of any such documents relating to the Financing. In the event any portion of the Financing becomes unavailable on the terms and conditions contemplated in the Equity Commitment Letters or any other definitive documents relating to the Financing, Parent shall promptly notify the Company. If Parent commences an enforcement action to enforce its rights under an Equity Commitment Letter and/or cause the financing sources thereunder to fund the Financing, Parent shall keep the Company reasonably informed of the status thereof.
(c) Subject to Section 6.14 of this Agreement, Parent and/or Amalgamation Sub shall not amend, alter or waive, or agree to amend, alter or waive (in any case whether by action or inaction), any term of the Equity Commitment Letters without the prior written consent of the Company (acting only upon the recommendation of the Special Committee).
| A-42 |
(d) Notwithstanding anything to the contrary, Parent and Amalgamation Sub acknowledge and agree that the obtaining of the Financing is not a condition to the Closing.
Section 6.08 Further Action; Reasonable Best Efforts.
(a) Upon the terms and subject to the conditions of this Agreement, each of the parties hereto and their respective Representatives shall (i) make promptly its respective filings, and thereafter make any other required submissions, with each relevant Governmental Authority with jurisdiction over enforcement of any applicable antitrust or competition Laws with respect to the Transactions, and coordinate and cooperate fully with the other parties in exchanging such information and providing such assistance as the other parties may reasonably request in connection therewith (including (A) obtaining consent (such consent not be unreasonably withheld, conditioned or delayed) from the other parties promptly before making any substantive communication (whether verbal or written) with any Governmental Authority in connection with such filings or submissions, (B) permitting the other parties to review in advance, and consulting with the other parties on, any proposed filing, submission or communication (whether verbal or written) by such party to any Governmental Authority and (C) giving the other parties the opportunity to attend and participate at any meeting with any Governmental Authority in respect of any filing, investigation or other inquiry) and (ii) cooperate with the other parties hereto and use its reasonable best efforts, and cause its Subsidiaries to use their respective reasonable best efforts, to take or cause to be taken, all appropriate action, and to do, or cause to be done, all things necessary, proper or advisable under applicable Laws or otherwise to consummate and make effective the Transactions, including taking any and all steps necessary to avoid or eliminate each and every impediment under any antitrust or competition Law that may be asserted by any Governmental Authority so as to enable the parties hereto to expeditiously consummate the Transactions, including committing to and effecting, by consent decree, hold separate orders, or otherwise, the restructuring, reorganization, sale, divestiture or disposition of such of its assets, properties or businesses; provided that no party hereto shall be required to take any such action if such action would have a Company Material Adverse Effect.
(b) Each party hereto shall, upon request by any other party, furnish such other party with all information concerning itself, its Subsidiaries, directors, officers and shareholders and such other matters as may be reasonably necessary or advisable in connection with the Proxy Statement, the Schedule 13E-3, or any other statement, filing, notice or application made by or on behalf of Parent, Amalgamation Sub, the Company or any of their respective Subsidiaries to any Third Party and/or any Governmental Authority in connection with the Transactions.
Section 6.09 Obligations of Amalgamation Sub.
Parent shall take all action necessary to cause Amalgamation Sub to perform its obligations under this Agreement and to consummate the Transactions on the terms and subject to the conditions set forth in this Agreement.
| A-43 |
Section 6.10 Participation in Litigation.
Prior to the Effective Time or the termination of this Agreement in accordance with its terms, Parent shall give notice as promptly as practicable to the Company, and the Company shall give notice as promptly as practicable to Parent, of any Actions commenced or, to the Company’s knowledge on the one hand and Parent’s knowledge on the other hand, threatened against such party or its directors that relate to this Agreement and the Transactions. The Company shall give Parent the opportunity to participate in the defense or settlement of any shareholder Action against the Company and/or its directors relating to this Agreement or the Transactions, at Parent’s sole expense, and no such Action shall be settled by the Company without Parent’s prior written consent (such consent not be unreasonably withheld, conditioned or delayed); provided that the Company may, without Parent’s prior written consent, settle Actions that involve only the payment of money damages not in excess of $1,000,000.
Section 6.11 Resignations.
To the extent requested by Parent in writing at least five (5) Business Days prior to Closing, on the Closing Date, the Company shall use reasonable best efforts to cause to be delivered to Parent duly signed resignations, effective as of the Effective Time, of the directors of any Group Company designated by Parent.
Section 6.12 Public Announcements.
Except as may be required by applicable Law, the press release announcing the execution of this Agreement shall be issued only in such form as shall be mutually agreed upon by the Company and Parent. Thereafter, at any time prior to termination of this Agreement pursuant to Article VIII, Parent and the Company shall consult with each other before issuing any press release, having any communication with the press (whether or not for attribution), making any other public statement or scheduling any press conference or conference call with investors or analysts with respect to this Agreement or the Transactions and, except in respect of any such press release, communication, other public statement, press conference or conference call as may be required by applicable Law or rules and policies of the NASDAQ, shall not issue any such press release, have any such communication, make any such other public statement or schedule any such press conference or conference call prior to such consultation. Notwithstanding the foregoing, the restrictions set forth in this Section 6.12 shall not apply to any release or announcement made or proposed to be made by the Company in connection with a Change in the Company Recommendation made in compliance with this Agreement.
Section 6.13 Stock Exchange Delisting; Deregistration.
Prior to the Effective Time, the Company shall cooperate with Parent and use reasonable best efforts to take, or cause to be taken, all actions, and do or cause to be done all things, reasonably necessary, proper or advisable on its part under applicable Laws and rules and policies of NASDAQ to enable the delisting of the Shares from the NASDAQ and the deregistration of the Shares under the Exchange Act as promptly as practicable after the Effective Time.
| A-44 |
Section 6.14 No Amendment to Parent Group Contracts.
Without the Company’s prior written consent, (a) Parent and Amalgamation Sub shall not, and shall cause the members of the Parent Group not to, enter into any Contract or amend, modify, withdraw or terminate any Parent Group Contract or waive any rights thereunder in a manner that would (i) result, directly or indirectly, in any of the Rollover Shares ceasing to be treated as Excluded Shares or change the number of Rollover Shares, in each case, other than as provided in the Support Agreement, (ii) individually or in the aggregate, prevent or materially delay the ability of Parent or Amalgamation Sub to consummate the Amalgamation and the other Transactions or (iii) prevent or materially impair the ability of any management member or director of the Company, with respect to any Superior Proposal, taking any of the actions described in Section 6.04 to the extent such actions are permitted to be taken by the Company thereunder and (b) Parent and the members of the Parent Group shall not enter into or modify any Contract pursuant to which any management members, directors or shareholders of the Company, or any of their respective Affiliates receives any consideration or other economic value from any person in connection with the Transactions that is not provided or expressly contemplated in the Parent Group Contracts as of the date hereof, including any carried interest, share option, share appreciation right or other forms of equity or quasi-equity right. Within two (2) Business Days after the execution thereof, Parent and Amalgamation Sub shall provide the Company with a copy of any Contract relating to the Transactions that is entered into after the date hereof and to which a member of the Parent Group is a party. Parent and Amalgamation Sub agree that any action by any member of the Parent Group who is not a party to this Agreement that would constitute a breach of this Section 6.14 if such member of the Parent Group were a party to this Agreement for the purposes of this Section 6.14 shall be deemed to be a breach of this Section 6.14.
Section 6.15 Employee Matters.
Parent shall, for a period of twelve (12) months immediately following the Closing Date, cause the Surviving Corporation and its Subsidiaries to provide employees of the Group Companies who have not been terminated by the Company or who have not given resignation notices to the Company (whether such termination or resignation occurs prior to or following the Closing) as of immediately prior to the Effective Time (the “Company Employees”) with (x) the level of cash compensation at least equal to such employee’s level of cash compensation as of immediately prior to the Effective Time and (y) employee benefits and arrangements (other than equity benefits) that are no less favorable than the employee benefits and arrangements provided by the Group Companies to the Company Employees in effect as of immediately prior to the Effective Time, subject to market adjustments in the ordinary course of business; provided, however, that the requirements of this sentence shall not apply to Company Employees who are covered by a collective bargaining agreement. From and after the Effective Time, Parent shall cause the Surviving Corporation and its Subsidiaries to honor in accordance with their terms, all Contracts, employee benefit plans and arrangements, policies, and commitments of the Group Companies as in effect immediately prior to the Effective Time that are applicable to any current or former employees or directors. Nothing contained in this Section 6.15, expressed or implied, is intended to confer upon any Service Provider any benefits under any employee benefit plans or right to employment or continued employment with Parent or the Surviving Corporation or any of its Subsidiaries for any period by reason of this Agreement. The provisions of this Section 6.15 are solely for the benefit of the parties to this Agreement and no current or former Service Provider or any other individual associated therewith shall be regarded for any purpose as a third-party beneficiary of this Section 6.15, and nothing contained herein shall be construed as an amendment to any employee benefit plan for any purpose.
| A-45 |
Section 6.16 Actions Taken at the Direction of Certain Persons; Knowledge.
Notwithstanding any other provision of this Agreement to the contrary, the Company shall not be deemed to be in breach of any representation, warranty, covenant or agreement hereunder, including Article III, Article V and Article VI hereof, if the alleged breach is primarily the result of (i) action or inaction taken by or at the direction of a Sinobioway Representative without the authorization of the board of directors of Beijing Sinovac (including the directors thereof that are not Sinobioway Representatives), (ii) action or inaction taken by or at the direction of Sinobioway Bio-medicine Co., Ltd., in its capacity as a shareholder of Beijing Sinovac, (iii) action or inaction taken by or at the direction of Sinobioway Bio-medicine Co., Ltd. or any of its Affiliates pursuant to or in connection with any lease between such person and Beijing Sinovac (each of (i), (ii) and (iii), a “Sinobioway Trigger”), or (iv) action or inaction taken by the Company or any of its Subsidiaries at the direction of the Founder, any other Rollover Shareholder, Parent, any of their respective Affiliates or any of their respective directors, officers, employees, agents or representatives, in each case of the foregoing (i), (ii), (iii) and (iv), without the authorization of the Company Board (acting with the concurrence of the Special Committee) or the Special Committee. Parent shall not have the right to (i) terminate this Agreement under Section 8.04 or (ii) claim any damage or seek any other remedy at law or in equity, in each case for any breach or inaccuracy in the representations and warranties made by the Company in Article III to the extent such breach or inaccuracy arises out of or relates to matters or circumstances of which the Founder, any other Rollover Shareholder, Parent or any of their respective directors or officers has knowledge, as of the date of this Agreement. For the avoidance of doubt, any alleged breach by the Company of any representation, warranty, covenant or agreement hereunder (including Article III, Article V and Article VI hereof) shall not be deemed to be primarily the result of a Sinobioway Trigger, if such alleged breach would have occurred or arisen without the occurrence of a Sinobioway Trigger prior to the earlier of the Effective Time and termination of this Agreement pursuant to Article VIII.
Article
VII
CONDITIONS TO THE AMALGAMATION
Section 7.01 Conditions to the Obligations of Each Party.
The obligations of the Company, Parent and Amalgamation Sub to consummate the Amalgamation are subject to the satisfaction or waiver (where permissible under applicable Law) of the following conditions at or prior to the Closing Date:
(a) Shareholder Approval. This Agreement and the Transactions shall have been authorized and approved by holders of Shares constituting the Requisite Company Vote at the Shareholders’ Meeting in accordance with the IBCA and the Company’s articles of incorporation and by-laws.
(b) No Orders. No Governmental Authority of competent jurisdiction shall have enacted, issued, promulgated, enforced or entered any Law or award, writ, injunction, determination, rule, regulation, judgment, decree or executive order (an “Order”), whether temporary, preliminary or permanent that is then in effect and has or would have the effect of enjoining, restraining, prohibiting or otherwise making illegal the consummation of the Transactions.
| A-46 |
Section 7.02 Conditions to the Obligations of Parent and Amalgamation Sub.
The obligations of Parent and Amalgamation Sub to consummate the Amalgamation are subject to the satisfaction or waiver (where permissible under applicable Law) of the following additional conditions at or prior to the Closing Date:
(a) Representations and Warranties. (i) Other than the representations and warranties of the Company contained in Section 3.03(a), the first sentence of Section 3.03(b), Section 3.04(a) and Section 3.18, the representations and warranties of the Company contained in this Agreement (without giving effect to any qualification as to “materiality” or “Company Material Adverse Effect” set forth therein) shall be true and correct in all respects as of the date hereof and as of the Closing Date, as though made on and as of such date and time (other than representations and warranties that by their terms address matters only as of a specified time, which shall be true and correct only as of such time), except where the failure of such representations and warranties of the Company to be so true and correct does not constitute a Company Material Adverse Effect, (ii) the representations and warranties set forth in Section 3.03(a) and the first sentence of Section 3.03(b) shall be true and correct in all respects, except for de minimis inaccuracies, as of the date hereof and as of the Closing Date, as though made on and as of such date and time (other than representations and warranties that by their terms address matters only as of a specified time, which shall be true and correct only as of such time) and (iii) the representations and warranties set forth in Section 3.04(a) and Section 3.18 shall be true and correct in all respects, as of the date hereof and as of the Closing Date, as though made on and as of such date and time (other than representations and warranties that by their terms address matters only as of a specified time, which shall be true and correct only as of such time).
(b) Agreements and Covenants. The Company shall have performed or complied in all material respects with all agreements and covenants required by this Agreement to be performed or complied with by it on or prior to the Closing Date.
(c) No Material Adverse Effect. No Company Material Adverse Effect shall have occurred since the date hereof and be continuing.
(d) Dissenting Shares. Shareholders of the Company, other than 1Globe and the Chiang Li Family, holding no more than seven and one-half percent (7.5%) of the total issued and outstanding Shares shall have validly served a notice of dissent under Section 191 of the IBCA.
(e) Company Certificate. The Company shall have delivered to Parent a certificate, dated the Closing Date, signed by a senior executive officer of the Company, certifying as to the satisfaction of the conditions specified in Section 7.02(a) and Section 7.02(b).
Section 7.03 Conditions to the Obligations of the Company.
The obligations of the Company to consummate the Amalgamation are subject to the satisfaction or waiver (where permissible under applicable Law) of the following additional conditions at or prior to the Closing Date:
| A-47 |
(a) Representations and Warranties. The representations and warranties of Parent and Amalgamation Sub contained in this Agreement (without giving effect to any qualification as to “materiality” set forth therein) shall be true and correct in all respects as of the date hereof and as of the Closing Date, as though made on and as of such date and time (other than representations and warranties that by their terms address matters only as of a specified time, which shall be true and correct only as of such time), except where the failure of such representations and warranties of Parent and Amalgamation Sub to be so true and correct, individually or in the aggregate, has not, and would not reasonably be expected to, prevent, materially delay or materially impede or impair the ability of Parent and Amalgamation Sub to consummate the Transactions.
(b) Agreements and Covenants. Each of Parent and Amalgamation Sub shall have performed or complied in all material respects with all agreements and covenants required by this Agreement to be performed or complied with by it on or prior to the Closing Date.
(c) Parent Certificate. Parent shall have delivered to the Company a certificate, dated the date of the Closing, signed by a director of Parent, certifying as to the satisfaction of the conditions specified in Section 7.03(a) and Section 7.03(b).
Section 7.04 Frustration of Closing Conditions.
Prior to the Termination Date, none of the Company, Parent or Amalgamation Sub may rely on the failure of any condition set forth in Article VII to be satisfied if such failure was caused by such party’s failure to use the standard of efforts required from such party to comply with this Agreement and consummate the Transactions.
Article
VIII
TERMINATION
Section 8.01 Termination by Mutual Consent.
This Agreement may be terminated and the Transactions may be abandoned at any time prior to the Effective Time by mutual written consent of Parent and the Company with the approval of their respective boards of directors (in the case of the Company, acting only upon the recommendation of the Special Committee).
Section 8.02 Termination by Either the Company or Parent.
This Agreement may be terminated by either the Company (acting only upon the recommendation of the Special Committee) or Parent at any time prior to the Effective Time, if:
(a) the Effective Time shall not have occurred on or before March 26, 2018 (the “Termination Date”);
(b) any Governmental Authority of competent jurisdiction shall have enacted, issued, promulgated, enforced or entered any final and non-appealable Order that, or taken any other final and non-appealable action that, has the effect of making consummation of the Transactions illegal or otherwise preventing or prohibiting consummation of the Transactions; or
(c) the Requisite Company Vote shall not have been obtained at the Shareholders’ Meeting duly convened therefor or at any adjournment thereof;
| A-48 |
provided that the right to terminate this Agreement pursuant to this Section 8.02 shall not be available to any party whose failure to fulfill any of its obligations under this Agreement has been a material cause of, or resulted in, the failure of the applicable condition(s) being satisfied.
Section 8.03 Termination by the Company.
This Agreement may be terminated by the Company (acting only upon the recommendation of the Special Committee) at any time prior to the Effective Time, if:
(a) a breach of any representation, warranty, agreement or covenant of Parent or Amalgamation Sub set forth in this Agreement shall have occurred, which breach (i) would give rise to the failure of a condition set forth in Section 7.03(a) or Section 7.03(b) and (ii) is incapable of being cured or, if capable of being cured, is not cured by Parent or Amalgamation Sub, as applicable, within thirty (30) days following receipt of written notice of such breach from the Company (or, if the Termination Date is less than thirty (30) days from the date of receipt of such notice, by the Termination Date); provided that the Company shall not have the right to terminate this Agreement pursuant to this Section 8.03(a) if the Company is then in material breach of any representations, warranties, agreements or covenants of the Company hereunder that would give rise to the failure of a condition set forth in Section 7.02;
(b) (i) all of the conditions set forth in Section 7.01 and Section 7.02 (other than those conditions that by their nature are to be satisfied by actions taken at the Closing) have been satisfied, (ii) the Company has delivered to Parent an irrevocable written notice confirming that all of the conditions set forth in Section 7.03 have been satisfied (or that the Company is willing to waive any unsatisfied conditions in Section 7.03) and that it is ready, willing and able to consummate the Closing and (iii) Parent and Amalgamation Sub fail to complete the Closing within three (3) Business Days following the date on which the Closing should have occurred pursuant to Section 1.02;
(c) prior to the receipt of the Requisite Company Vote, (i) the Company Board (acting only upon the recommendation of the Special Committee) or the Special Committee has authorized the Company to terminate this Agreement and enter into an Alternative Acquisition Agreement with respect to a Superior Proposal and (ii) the Company concurrently with the termination of this Agreement enters into an Alternative Acquisition Agreement with respect to the Superior Proposal referred to in the foregoing clause (i); provided that the Company shall not be entitled to terminate this Agreement pursuant to this Section 8.03(c) unless the Company has (A) complied with the requirements of Section 6.04 with respect to such Superior Proposal and/or Alternative Acquisition Agreement (other than any immaterial non-compliance that does not adversely affect Parent or Amalgamation Sub) and (B) complied with Section 8.06(a) and pays the Company Termination Fee prior to or concurrently with taking any action pursuant to this Section 8.03(c); or
(d) prior to the receipt of the Requisite Company Vote, the Company Board (acting only upon the recommendation of the Special Committee) or the Special Committee has authorized the Company to terminate this Agreement pursuant to Section 6.04(e).
| A-49 |
Section 8.04 Termination by Parent.
This Agreement may be terminated by Parent at any time prior to the Effective Time, if:
(a) a breach of any representation, warranty, agreement or covenant of the Company set forth in this Agreement shall have occurred, which breach (i) would give rise to the failure of a condition set forth in Section 7.02(a) or Section 7.02(b) and (ii) is incapable of being cured or, if capable of being cured, is not cured by the Company within thirty (30) days following receipt of written notice of such breach from Parent or Amalgamation Sub (or, if the Termination Date is less than thirty (30) days from the date of receipt of such notice, by the Termination Date); provided that Parent shall not have the right to terminate this Agreement pursuant to this Section 8.04(a) if either Parent or Amalgamation Sub is then in material breach of any representations, warranties, agreements or covenants of Parent or Amalgamation Sub hereunder that would give rise to the failure of a condition set forth in Section 7.03; or
(b) the Company Board or any committee thereof shall have effected a Change in the Company Recommendation.
Section 8.05 Effect of Termination.
In the event of the termination of this Agreement pursuant to Article VIII, this Agreement shall forthwith become void, and there shall be no liability under this Agreement on the part of any party hereto (or any Representative of such party); provided that the terms of Section 6.03(c), Section 6.12, Article VIII and Article IX shall survive any termination of this Agreement.
Section 8.06 Termination Fee and Expenses.
(a) In the event that:
(i) (A) a bona fide proposal or offer with respect to a Competing Transaction shall have been publicly made, proposed or communicated (and not withdrawn or abandoned), after the date hereof and prior to the Shareholders’ Meeting (or prior to the termination of this Agreement if there has been no Shareholders’ Meeting), (B) following the occurrence of an event described in the preceding clause (A), this Agreement is terminated by the Company or Parent pursuant to Section 8.02(a) or Section 8.02(c), (C) neither Parent nor Amalgamation Sub shall have materially breached any of its representations, warranties or covenants under this Agreement and (D) within twelve (12) months after the termination of this Agreement, the Company enters into a definitive agreement with respect to the Competing Transaction contemplated by such proposal or offer; provided that for purposes of this Section 8.06(a), all references to “20%” in the definition of “Competing Transaction” shall be deemed to be references to “50%”;
(ii) this Agreement is terminated by Parent pursuant to Section 8.04; or
(iii) this Agreement is terminated by the Company pursuant to Section 8.03(c) or Section 8.03(d),
then the Company shall pay to Parent or its designees an amount equal to $15,000,000 (the “Company Termination Fee”) by wire transfer of same day funds as promptly as possible (but in any event (A) within five (5) Business Days after such termination in the case of a termination referred to in clause (ii), (B) within two (2) Business Days following the consummation by the Company of the Competing Transaction in the case of a termination referred to in clause (i) or (C) prior to or concurrently with the termination of this Agreement in the case of a termination pursuant to clause (iii)); it being understood that in no event shall the Company be required to pay the Company Termination Fee on more than one occasion.
| A-50 |
(b) In the event that this Agreement is terminated by the Company pursuant to Section 8.03(a) or Section 8.03(b), then Parent will pay, or cause to be paid, to the Company an amount equal to $15,000,000 (the “Parent Termination Fee”) by wire transfer of same day funds as promptly as possible (but in any event within five (5) Business Days) following such termination; it being understood that in no event shall Parent be required to pay the Parent Termination Fee on more than one occasion.
(c) All Expenses incurred in connection with this Agreement and the Transactions shall be paid by the party incurring such Expenses, whether or not the Amalgamation or any other Transaction is consummated.
(d) In the event that the Company fails to pay the Company Termination Fee, or Parent fails to pay the Parent Termination Fee, when due and in accordance with the requirements of this Agreement, the Company or Parent, as the case may be, shall reimburse the other party for reasonable costs and expenses actually incurred or accrued by the other party (including fees and expenses of counsel) in connection with the collection under and enforcement of this Section 8.06, together with interest on such unpaid Company Termination Fee or Parent Termination Fee, as the case may be, commencing on the date that the Company Termination Fee or Parent Termination Fee, as the case may be, became due, at the prime rate as published in the Wall Street Journal Table of Money Rates on such date plus 3.00%. Such collection expenses shall not otherwise diminish in any way the payment obligations hereunder.
(e) Each of the Company, Parent and Amalgamation Sub acknowledges that (i) the agreements contained in this Section 8.06 are an integral part of the Transactions, (ii) the damages resulting from termination of this Agreement under circumstances where a Company Termination Fee or Parent Termination Fee is payable are uncertain and incapable of accurate calculation and therefore, the amounts payable pursuant to Section 8.06(a) or Section 8.06(b) are not a penalty but rather constitute amounts akin to liquidated damages in a reasonable amount that will compensate Parent or the Company, as the case may be, for the efforts and resources expended and opportunities foregone while negotiating this Agreement and in reliance on this Agreement and on the expectation of the consummation of the Transactions and (iii) without the agreements contained in this Section 8.06, the parties hereto would not have entered into this Agreement.
| A-51 |
(f) (i) Subject to Section 9.08, the Equity Commitment Letters and the Limited Guarantees, in the event that Parent or Amalgamation Sub fails to effect the Closing for any reason or no reason or they otherwise breach this Agreement (whether willfully, intentionally, unintentionally or otherwise) or otherwise fail to perform hereunder (whether willfully, intentionally, unintentionally or otherwise), then the Company’s right to terminate this Agreement and receive the Parent Termination Fee pursuant to Section 8.06(b), and the expenses pursuant to Section 8.06(d) and the guarantee of such obligations pursuant to the Limited Guarantees (subject to their terms, conditions and limitations), shall be the sole and exclusive remedy (whether at law, in equity, in contract, in tort or otherwise) of any Group Company and all members of the Company Group against (A) Parent, Amalgamation Sub, the Rollover Shareholders, the Guarantors and the Sponsors, (B) the former, current and future holders of any equity, partnership or limited liability company interest, controlling persons, directors, officers, employees, agents, attorneys, Affiliates, members, managers, general or limited partners, shareholders, or assignees of Parent, Amalgamation Sub, any Rollover Shareholder, any Guarantor or Sponsor, (C) any lender or prospective lender, lead arranger, arranger, agent or representative of or to Parent, Amalgamation Sub, any Rollover Shareholder or any Guarantor or Sponsor or (D) any holders or future holders of any equity, stock, partnership or limited liability company interest, controlling persons, directors, officers, employees, agents, attorneys, Affiliates, members, managers, general or limited partners, stockholders, assignees of any of the foregoing (clauses (A) – (D), collectively, the “Parent Group”), for any loss or damage suffered as a result of any breach of any representation, warranty, covenant or agreement (whether willfully, intentionally, unintentionally or otherwise) or failure to perform hereunder (whether willfully, intentionally, unintentionally or otherwise) or other failure of the Amalgamation or the other Transactions to be consummated (whether willfully, intentionally, unintentionally or otherwise). For the avoidance of doubt, neither Parent nor any other member of the Parent Group shall have any liability for monetary damages of any kind or nature or arising in any circumstance in connection with this Agreement or any of the Transactions (including the Equity Commitment Letters and the Limited Guarantees) other than the payment of the Parent Termination Fee pursuant to Section 8.06(b) and expenses under Section 8.06(d), and in no event shall any Group Company, the direct or indirect shareholders of the Company or any other Group Company, or any of their respective Affiliates, directors, officers, employees, members, managers, partners, representatives, advisors or agents of the foregoing, (collectively, the “Company Group”) seek, or permit to be sought, on behalf of any member of the Company Group, any monetary damages from any member of the Parent Group in connection with this Agreement or any of the Transactions (including the Equity Commitment Letters and the Limited Guarantees), other than (without duplication) from Parent or Amalgamation Sub to the extent provided in Section 8.06(b) and Section 8.06(d), or the Guarantors to the extent provided in the relevant Limited Guarantees.
(ii) Subject to Section 9.08, Parent’s right to terminate this Agreement and receive the Company Termination Fee pursuant to Section 8.06(a) and expenses under Section 8.06(d), shall be the sole and exclusive remedy (whether at law, in equity, in contract, in tort or otherwise) of any member of the Parent Group against any member of the Company Group for any loss or damage suffered as a result of any breach of any representation, warranty, covenant or agreement (whether willfully, intentionally, unintentionally or otherwise) or failure to perform hereunder (whether willfully, intentionally, unintentionally or otherwise) or other failure of the Amalgamation to be consummated (whether willfully, intentionally, unintentionally or otherwise). Neither the Company nor any other member of the Company Group shall have any liability for monetary damages of any kind or nature or arising in any circumstance in connection with this Agreement or any of the Transactions other than the payment by the Company of the Company Termination Fee pursuant to Section 8.06(a) and expenses under Section 8.06(d), and in no event shall any of Parent, Amalgamation Sub or any other member of the Parent Group seek, or permit to be sought, on behalf of any member of the Parent Group, any monetary damages from any member of the Company Group in connection with this Agreement or any of the Transactions, other than (without duplication) from the Company to the extent provided in Section 8.06(a) and Section 8.06(d).
| A-52 |
Article
IX
GENERAL PROVISIONS
Section 9.01 Non-Survival of Representations, Warranties and Agreements.
The representations, warranties and agreements in this Agreement and in any certificate delivered pursuant hereto shall terminate at the earlier of the Effective Time and termination of this Agreement pursuant to Article VIII, except that this Section 9.01 shall not limit any covenant or agreement of the parties hereto that by its terms contemplates performance after the Effective Time or termination of this Agreement, including the agreements set forth in Article I and Article II, Section 6.05 and this Article IX.
Section 9.02 Notices.
All notices, requests, claims, demands and other communications hereunder shall be in writing and shall be given (and shall be deemed to have been duly given upon receipt) by delivery in person, by email, by facsimile or by international overnight courier to the respective parties at the following addresses (or at such other address for a party as shall be specified in a notice given in accordance with this Section 9.02):
if to Parent or Amalgamation Sub:
c/o No. 39 Shangdi Xi Road
Haidian District, Beijing 100085, China
Attention: Weidong Yin
Email: [email protected]
Facsimile: +86 10 6296 6910
with a copy to:
Kirkland & Ellis
26th Floor, Gloucester Tower, The Landmark
15 Queen’s Road Central
Hong Kong
Attention: David T. Zhang, Esq.
Email: [email protected]
Facsimile: +852 3761 3301
| A-53 |
if to the Company:
No. 39 Shangdi Xi Road
Haidian District, Beijing 100085, China
Attention: Lilian Zhang
Email: [email protected]
Facsimile: +86 10 6296 6910
with a copy to:
Weil, Gotshal & Manges
29/F, Alexandra House
18 Chater Road, Central
Hong Kong
Attention: Tim Gardner, Esq.
Email: [email protected]
Facsimile: +852 3015 9354
Section 9.03 Certain Definitions.
(a) For purposes of this Agreement:
“1Globe” means, collectively, 1Globe Capital LLC and its Affiliates, and any investment fund advised or managed by 1Globe Capital LLC or any of its Affiliates.
“2012 Share Incentive Plan” means the Sinovac Biotech Ltd. 2012 Share Incentive Plan, as amended.
“Affiliate” of a specified person means a person who, directly or indirectly through one or more intermediaries, controls, is controlled by, or is under common control with, such specified person.
“Anticorruption Laws” means Laws relating to anti-bribery or anticorruption (governmental or commercial) that apply to the business and dealings of any Group Company, including the PRC Law on Anti-Unfair Competition adopted on September 2, 1993, the Interim Rules on Prevention of Commercial Bribery issued by the PRC State Administration of Industry and Commerce on November 15, 1996 and the U.S. Foreign Corrupt Practices Act of 1977, as amended from time to time.
“Beijing Sinovac” means Sinovac Biotech Co., Ltd., an equity joint venture incorporated in Beijing.
“Business Day” means any day on which the principal offices of the SEC in Washington, D.C. are open to accept filings, or, in the case of determining a date when any payment is due, any day on which banks are not required or authorized to close in New York, Hong Kong, Antigua and Barbuda or Beijing, PRC.
“Chiang Li Family” means, collectively, the persons comprising the reporting person identified as “Chiang Li Family” in the Schedule 13G relating to the Company filed by such reporting person with the SEC on April 4, 2016 (SEC Accession No. 0001214659-16-010806) and their respective Affiliates.
| A-54 |
“Company Disclosure Schedule” means the disclosure schedule delivered by the Company to and accepted by Parent and Amalgamation Sub on the date hereof.
“Company Employee Plan” means any material plan, program, Contract or other arrangement providing for compensation, severance, termination pay, deferred compensation, performance awards, share or share-related awards, fringe benefits or other employee benefits or remuneration, that is or has been maintained, contributed to or required to be contributed to by any Group Company for the benefit of any current or former employee, director or officer of such Group Company, or with respect to which such Group Company has or may have any liability or obligation.
“Company Material Adverse Effect” means any fact, event, circumstance, change, condition, occurrence or effect that, individually or in the aggregate with all other facts, events, circumstances, changes, conditions, occurrences and effects, is or would reasonably be expected to be materially adverse to the business, financial condition or results of operations of the Group Companies taken as a whole; provided that the determination of whether a Company Material Adverse Effect shall have occurred shall not take into account any fact, event, circumstance, change, condition, occurrence or effect to the extent resulting from: (i) geopolitical conditions, any outbreak or escalation of war or major hostilities or any act of sabotage or terrorism or natural or man-made disasters or other force majeure events, (ii) changes in Laws, GAAP or enforcement or interpretation thereof, (iii) changes or conditions that generally affect the industry and market in which the Group Companies operate, including changes in interest rates or foreign exchange rates, (iv) changes in the financial, credit or other securities or capital markets, or in general economic, business, regulatory, legislative or political conditions, (v) any failure, in and of itself, of the Group Companies to meet any internal or published projections, estimates, budgets, plans or forecasts of revenues, earnings or other financial performance measures or operating statistics or predictions or changes in the market price or trading volume of the securities of such person or the credit rating of such person (it being understood that the underlying facts giving rise or contributing to such failure or change may be taken into account in determining whether there has been a Company Material Adverse Effect if such facts are not otherwise excluded under this definition), (vi) the announcement, pendency or consummation of the Transactions, including any loss in respect of or change in relationship with any customer, supplier, employee, vendor or other business partner of the Company due to such announcement, pendency or consummation or the identity of Parent or its Affiliates, (vii) any action taken (or omitted to be taken) by the Company or any of its Subsidiaries at the written request or with the written consent of Parent or expressly required by this Agreement, (viii) any suit, claim, request for indemnification or proceeding brought by any current or former shareholder of the Company or any of its Subsidiaries (on their own behalf or on behalf of the Company) for breaches of fiduciary duties, violations of securities Laws or otherwise in connection with this Agreement or the Transactions, (ix) any action or inaction taken by or at the direction of a Sinobioway Representative without the authorization of the board of directors of Beijing Sinovac (including the directors thereof that are not Sinobioway Representatives), (x) any action or inaction taken by or at the direction of Sinobioway Bio-medicine Co., Ltd. or any of its Affiliates, pursuant to or in connection with any lease between such person and Beijing Sinovac, (xi) any action or inaction taken by or at the direction of Sinobioway Bio-medicine Co., Ltd., in its capacity as a shareholder of Beijing Sinovac, or (xii) the matters, allegations or investigations disclosed in the Company’s Form 6-K (including the exhibit thereto) furnished to the SEC on May 16, 2017 or the Company’s Form 6-K (including the exhibit thereto) furnished to the SEC on December 23, 2016; except, in the case of clause (ii), (iii) or (iv), to the extent having a materially disproportionate effect on the Group Companies, taken as a whole, relative to other participants in the industry in which the Group Companies operate (in which case the incremental materially disproportionate impact or impacts may be taken into account in determining whether there has been a Company Material Adverse Effect).
| A-55 |
“Company Option” means each option to purchase Shares granted under the Share Incentive Plans on or prior to the Closing Date whether or not such option has become vested on or prior to the Closing Date in accordance with the terms thereof.
“Company RS” means each Share of restricted stock granted under the 2012 Share Incentive Plan on or prior to the Closing Date, the restrictions over which have not lapsed on or prior to the Closing Date in accordance with the terms thereof.
“Confidentiality Agreements” means the confidentiality agreements between the Company and Mr. Weidong Yin and between the Company and SAIF Partners IV L.P., each dated as of June 1, 2016.
“Contract” means any legally enforceable note, bond, mortgage, indenture, or other written contract, agreement, or instrument.
“control” (including the terms “controlled by” and “under common control with”) means the possession, directly or indirectly, or as trustee or executor, of the power to direct or cause the direction of the management and policies of a person, whether through the ownership of voting securities or the possession of voting power, as trustee or executor, by contract or credit arrangement or otherwise.
“Environmental Law” means any applicable PRC local, provincial or national Law relating to (i) the protection of health, safety or the environment or (ii) the handling, use, transportation, disposal, release or threatened release of any Hazardous Substance.
“Excluded Shares” means, collectively, (i) Rollover Shares, (ii) Shares held by Parent or any of its Affiliates and (iii) Shares held by the Company or any of its Subsidiaries.
“Exercise Price” means, with respect to any Company Option, the applicable exercise price per Share underlying such Company Option.
“Expenses” means, with respect to any party hereto, all out-of-pocket fees and expenses (including all fees and expenses of counsel, accountants, investment banking firms and other financial institutions, experts and consultants to such party and its Affiliates) actually incurred or accrued by such party or its Affiliates or on its or their behalf or for which it or they are liable in connection with or related to the authorization, preparation, negotiation, execution and performance of the Transactions, the preparation, printing, filing and mailing of the Schedule 13E-3 and the Proxy Statement, the solicitation of shareholder approvals, the filing of any required notices under applicable Laws and all other matters related to the closing of the Amalgamation and the other Transactions.
| A-56 |
“Founder” means Mr. Weidong Yin, a PRC citizen with the passport number of G44420340.
“Government Official” means any officer, employee or other individual acting in an official capacity for a Governmental Authority or agency or instrumentality thereof (including any state-owned or controlled enterprise).
“Group Company” means any of the Company and its Subsidiaries.
“Hazardous Substance” means any chemical, pollutant, waste or substance that is (i) listed, classified or regulated under any Environmental Law as hazardous substance, toxic substance, pollutant, contaminant or oil or (ii) any petroleum product or by product, asbestos containing material, polychlorinated biphenyls or radioactive material.
“Indebtedness” means, with respect to any person, (i) all indebtedness of such person, whether or not contingent, for borrowed money, (ii) all obligations of such person for the deferred purchase price of property or services, (iii) all obligations of such person evidenced by notes, bonds, debentures or other similar instruments, (iv) all obligations of such person under currency, interest rate or other swaps, and all hedging and other obligations of such person under other derivative instruments, (v) all indebtedness created or arising under any conditional sale or other title retention agreement with respect to property acquired by such person (even though the rights and remedies of the seller or lender under such agreement in the event of default are limited to repossession or sale of such property), (vi) all obligations of such person as lessee under leases that have been or should be, in accordance with GAAP, recorded as capital leases, (vii) all obligations, contingent or otherwise, of such person under acceptance, letter of credit or similar facilities, (viii) all obligations of such person to purchase, redeem, retire, defease or otherwise acquire for value any share capital of such person or any warrants, rights or options to acquire such share capital, valued, in the case of redeemable preferred shares, at the greater of its voluntary or involuntary liquidation preference plus accrued and unpaid dividends, (ix) all Indebtedness of others referred to in clauses (i) through (viii) guaranteed directly or indirectly in any manner by such person and (x) all Indebtedness referred to in clauses (i) through (viii) secured by (or for which the holder of such Indebtedness has an existing right, contingent or otherwise, to be secured by) any Liens on property (including accounts and contract rights) owned by such person, even though such person has not assumed or become liable for the payment of such Indebtedness.
“Intellectual Property” means all (i) patents, utility models, inventions and discoveries, statutory invention registrations, mask works, invention disclosures, and industrial designs, community designs and other designs, (ii) Trademarks, (iii) works of authorship (including Software) and copyrights, and moral rights, design rights and database rights therein and thereto, (iv) confidential and proprietary information, including trade secrets, know-how and invention rights, (v) rights of privacy and publicity, (vi) registrations, applications, renewals and extensions for any of the foregoing in clauses (i)-(v) and (vii) any and all other proprietary rights.
“Interim Investors Agreement” means the interim investors agreement among Holdco, the Rollover Shareholders and the Sponsors, dated as of June 26, 2017, as amended and restated from time to time.
| A-57 |
“knowledge” means, with respect to the Company, the actual knowledge of the individuals listed in Section 9.03(a) of the Company Disclosure Schedule in each case after reasonable inquiry, and with respect to any other party hereto, the actual knowledge of any director or executive officer of such party, in each case, after reasonable inquiry.
“Leased Real Property” shall mean all leasehold or subleasehold estates and other rights to use or occupy any land, buildings, structures, improvements, fixtures or other interest in real property held by any Group Company.
“Leases” shall mean all leases, subleases, licenses, concessions and other agreements (written or oral), including all amendments, extensions, renewals, guarantees and other agreements with respect thereto, pursuant to which any Group Company holds any Leased Real Property, including the right to all security deposits and other amounts and instruments deposited by or on behalf of any Group Company.
“Liens” means any security interest, pledge, hypothecation, mortgage, lien (including environmental and Tax liens), violation, charge, lease, license, encumbrance, servient easement, adverse claim, reversion, reverter, preferential arrangement, restrictive covenant, condition or restriction of any kind, including any restriction on the use, voting, transfer, receipt of income or other exercise of any attributes of ownership.
“Owned Real Property” shall mean all real property and interests in real property, together with all buildings, structures, improvements and fixtures located thereon, and all easements and other rights and interests appurtenant thereto, owned by any Group Company.
“Permitted Encumbrances” shall mean, (i) Liens for Taxes, assessments and charges or levies by Governmental Authorities not yet due and payable or that are being contested in good faith and by appropriate proceedings, (ii) mechanics’, carriers’, workmen’s, repairmen’s, materialmen’s or other Liens or security interests arising or incurred in the ordinary course of business (A) relating to obligations as to which there is no default on the part of the Company or any of its Subsidiaries or (B) that secure a liquidated amount, that are being contested in good faith and by appropriate proceedings, (iii) leases, subleases and licenses (other than capital leases and leases underlying sale and leaseback transactions), (iv) pledges or deposits to secure obligations under workers’ compensation Laws or similar legislation or to secure public or statutory obligations, (v) pledges and deposits to secure the performance of bids, trade contracts, leases, surety and appeal bonds, performance bonds and other obligations of a similar nature, in each case in the ordinary course of business, (vi) easements, covenants and rights of way (unrecorded and of record) and other similar restrictions of record, and zoning, building and other similar restrictions, in each case that do not adversely affect in any material respect the current use of the applicable property owned, leased, used or held for use by the Company or any of its Subsidiaries, (vii) Liens that have otherwise been disclosed to Parent in writing as of the date hereof, or (viii) outbound license agreements and non-disclosure agreements entered into in the ordinary course of business.
“person” means an individual, corporation, partnership, limited partnership, limited liability company, syndicate, person (including a “person” as defined in Section 13(d)(3) of the Exchange Act), trust, association or entity or government, political subdivision, agency or instrumentality of a government.
| A-58 |
“PRC” means the People’s Republic of China.
“Representatives” means, with respect to any party, such party’s officers, directors, employees, accountants, consultants, financial and legal advisors and agents.
“Rollover Shareholders” means each person holding Rollover Shares who is a party to the Support Agreement.
“Rollover Shares” has the meaning ascribed to it in the Support Agreement.
“Securities Act” means the Securities Act of 1933, as amended.
“Service Provider” means each of the officers, employees, directors and independent contractors of the Group Companies.
“Share Incentive Plans” means the Stock Option Plan and the 2012 Share Incentive Plan.
“Shareholders’ Meeting” means the meeting of the Company’s shareholders (including any adjournments thereof) to be held to consider the authorization and approval of this Agreement and the Transactions, including the Amalgamation.
“Sinobioway Representative” means any current or future director or legal representative of Beijing Sinovac who is appointed by or is affiliated with Sinobioway Bio-medicine Co., Ltd., including Mr. Aihua Pan.
“Software” means all (i) computer programs, applications, systems and code, including software implementations of algorithms, models and methodologies, program interfaces, and source code and object code, (ii) Internet and intranet websites, databases and compilations, including data and collections of data, whether machine-readable or otherwise, (iii) development and design tools, library functions and compilers, (iv) technology supporting websites, and the contents and audiovisual displays of websites and (v) media, documentation and other works of authorship, including user manuals and training materials, relating to or embodying any of the foregoing or on which any of the foregoing is recorded.
“Special Committee” means a committee of the Company Board consisting of three members of the Company Board that are not affiliated with Parent or Amalgamation Sub and are not members of the management of the Company.
“Sponsors” means C-Bridge Healthcare Fund II, L.P., Advantech Capital L.P., Vivo Capital Fund VIII, L.P. and Vivo Capital Surplus Fund VIII, L.P.
“Stock Option Plan” means the Sinovac Biotech Ltd. Stock Option Plan, as amended.
“Subsidiary” means, with respect to any party, any person (i) of which such party or any other Subsidiary of such party is a general or managing partner or (ii) of which at least a majority of the securities (or other interests having by their terms ordinary voting power to elect a majority of the board of directors or other performing similar functions with respect to such corporation or other organization) is, directly or indirectly, owned or controlled by such party or by any one or more of its Subsidiaries, or by such party and one or more of its Subsidiaries.
| A-59 |
“Taxes” means any and all taxes, fees, levies, duties, tariffs, imposts and other charges of any kind (together with any and all interest, penalties, additions to tax and additional amounts imposed with respect thereto) imposed by any Governmental Authority or taxing authority, including, taxes or other charges on or with respect to income, franchise, windfall or other profits, gross receipts, occupation, property, real estate, deed, land use, sales, use, capital stock, payroll, severance, employment (including withholding obligations imposed on employer/payer), social security, workers’ compensation, unemployment compensation or net worth; taxes or other charges in the nature of excise, withholding (as payor or payee), ad valorem, stamp, transfer, value-added or gains taxes; license, registration and documentation fees; and customers’ duties, tariffs and similar charges.
“Third Party” means any person or “group” (as defined under Section 13(d) of the Exchange Act) of persons, other than Parent or any of its Affiliates or Representatives.
“Trademarks” means trademarks, service marks, domain names, uniform resource locators, trade dress, trade names, geographical indications and other identifiers of source or goodwill, including the goodwill symbolized thereby or associated therewith.
(b) The following terms have the meaning set forth in the Sections set forth below:
| Defined Term | Location of Definition | |
| Action | Section 3.09 | |
| Agreement | Preamble | |
| Alternative Acquisition Agreement | Section 6.04(c) | |
| Amalgamation | Recitals | |
| Amalgamation Consideration | Section 2.04(a) | |
| Amalgamation Sub | Preamble | |
| Applicable Date | Section 3.07(a) | |
| Arbitrator | Section 9.09(a) | |
| Articles of Amalgamation | Section 1.03 | |
| Bankruptcy and Equity Exception | Section 3.04(a) | |
| Change in the Company Recommendation | Section 6.04(c) | |
| Closing | Section 1.02 | |
| Closing Date | Section 1.02 | |
| Company | Preamble | |
| Company Board | Recitals | |
| Company Employees | Section 6.15 | |
| Company Group | Section 8.06(f)(i) | |
| Company Intellectual Property | Section 3.12(a) | |
| Company Real Property | Section 3.11(c) | |
| Company Recommendation | Section 3.04(b) | |
| Company SEC Reports | Section 3.07(a) | |
| Company Termination Fee | Section 8.06(a) | |
| Competing Transaction | Section 6.04(f) | |
| Damages | Section 6.05(c) | |
| Dissenting Shareholders | Section 2.03(a) |
| A-60 |
| Defined Term | Location of Definition | |
| Dissenting Shares | Section 2.03(a) | |
| Effective Time | Section 1.03 | |
| Environmental Permits | Section 3.15 | |
| Equity Commitment Letters | Section 4.05(a) | |
| Exchange Act | Section 3.05(b) | |
| Exchange Fund | Section 2.04(a) | |
| Financial Advisor | Section 3.04(c) | |
| Financing | Section 4.05(a) | |
| GAAP | Section 3.07(b) | |
| Governmental Authority | Section 3.05(b) | |
| Guarantor or Guarantors | Recitals | |
| HKIAC | Section 9.09(b) | |
| Holdco | Recitals | |
| IBCA | Section 1.01 | |
| Improvements | Section 3.11(d) | |
| Indemnified Parties | Section 6.05(b) | |
| Intervening Event | Section 6.04(g) | |
| Law | Section 3.05(a) | |
| Limited Guarantees | Recitals | |
| Material Contract | Section 3.14(a) | |
| Material Company Permits | Section 3.06(a) | |
| NASDAQ | Section 3.05(b) | |
| Notice of Superior Proposal | Section 6.04(d) | |
| Order | Section 7.01(b) | |
| Parent | Preamble | |
| Parent Group | Section 8.06(f)(i) | |
| Parent Group Contracts | Section 4.11 | |
| Parent Termination Fee | Section 8.06(b) | |
| Paying Agent | Section 2.04(a) | |
| Per Share Amalgamation Consideration | Section 2.01(a) | |
| Proxy Statement | Section 6.01(a) | |
| Record Date | Section 6.02(a) | |
| Requisite Company Vote | Section 3.04(a) | |
| Rights | Section 2.01(f) | |
| Rights Agreement | Section 2.01(f) | |
| Rules | Section 9.09(b) | |
| SAFE | Section 3.06(a) | |
| SAFE Rules and Regulations | Section 3.06(c) | |
| Schedule 13E-3 | Section 6.01(a) | |
| SEC | Section 3.05(b) | |
| Share | Section 2.01(a) | |
| Share Certificates | Section 2.04(b) | |
| Sinobioway Trigger | Section 6.16 | |
| Superior Proposal | Section 6.04(h) | |
| Superior Proposal Notice Period | Section 6.04(d) | |
| Support Agreement | Recitals | |
| Surviving Corporation | Section 1.01 | |
| Termination Date | Section 8.02(a) | |
| Transactions | Recitals | |
| Uncertificated Shares | Section 2.04(b) |
| A-61 |
Section 9.04 Severability.
If any term or other provision of this Agreement is invalid, illegal or incapable of being enforced by any rule of Law or public policy, all other conditions and provisions of this Agreement shall nevertheless remain in full force and effect so long as the economic or legal substance of the Transactions is not affected in any manner adverse to any party. Upon such determination that any term or other provision is invalid, illegal or incapable of being enforced, the parties hereto shall negotiate in good faith to modify this Agreement so as to effect the original intent of the parties as closely as possible in a mutually acceptable manner in order that the Transactions be consummated as originally contemplated to the fullest extent possible.
Section 9.05 Interpretation.
When a reference is made in this Agreement to a Section, Article or Exhibit such reference shall be to a Section, Article or Exhibit of this Agreement unless otherwise indicated. The table of contents and headings contained in this Agreement or in any Exhibit are for convenience of reference purposes only and shall not affect in any way the meaning or interpretation of this Agreement. All words used in this Agreement will be construed to be of such gender or number as the circumstances require. Any capitalized terms used in any Exhibit but not otherwise defined therein shall have the meaning set forth in this Agreement. All Exhibits annexed hereto or referred to herein are hereby incorporated in and made a part of this Agreement as if set forth herein. The word “including” and words of similar import when used in this Agreement will mean “including, without limitation,” unless otherwise specified. The words “hereof,” “herein” and “hereunder” and words of similar import when used in this Agreement shall refer to this Agreement as a whole and not to any particular provision of this Agreement. The definitions contained in this Agreement are applicable to the singular as well as the plural forms of such terms and to the masculine as well as to the feminine and neuter genders of such term. Any agreement, instrument or statute defined or referred to herein or in any agreement or instrument that is referred to herein means such agreement, instrument or statute as from time to time amended, modified or supplemented, including (in the case of agreements or instruments) by waiver or consent and (in the case of statutes) by succession of comparable successor statutes and references to all attachments thereto and instruments incorporated therein. References to a person are also to its permitted successors and assigns. References to clauses without a cross-reference to a Section or subsection are references to clauses within the same Section or, if more specific, subsection. References from or through any date shall mean, unless otherwise specified, from and including or through and including, respectively. The symbol “$” refers to United States Dollars. All $ amounts used in Article III, Article IV and Article V include the equivalent amount denominated in other currencies. The word “extent” in the phrase “to the extent” means the degree to which a subject or other thing extends and such phrase shall not mean simply “if.” References to “day” mean a calendar day unless otherwise indicated as a “Business Day.”
| A-62 |
Section 9.06 Entire Agreement; Assignment.
This Agreement (including the Exhibits and Schedules hereto), the Company Disclosure Schedule and the Confidentiality Agreements constitute the entire agreement among the parties with respect to the subject matter hereof and supersede all prior agreements and undertakings, both written and oral, among the parties, or any of them, with respect to the subject matter hereof. This Agreement shall not be assigned (whether pursuant to an amalgamation, by operation of Law or otherwise), except that Parent and Amalgamation Sub may assign all or any of their rights and obligations hereunder to any Affiliate of Parent; provided that no such assignment shall relieve the assigning party of its obligations hereunder if such assignee does not perform such obligations.
Section 9.07 Parties in Interest.
This Agreement shall be binding upon and inure solely to the benefit of each party hereto, and nothing in this Agreement, express or implied, is intended to or shall confer upon any other person any right, benefit or remedy of any nature whatsoever under or by reason of this Agreement, other than Section 6.05 and Section 8.06(f) (which are intended to be for the benefit of the persons covered thereby and may be enforced by such persons).
Section 9.08 Specific Performance.
(a) Subject to Section 9.08(b) and Section 9.08(d), the parties hereto agree that irreparable damage would occur in the event any provision of this Agreement were not performed in accordance with the terms hereof by the parties, and that money damages or other legal remedies would not be an adequate remedy for such damages. Accordingly, subject to Section 9.08(b) and Section 9.08(d), the parties hereto acknowledge and hereby agree that in the event of any breach by the Company, on the one hand, or Parent or Amalgamation Sub, on the other hand, of any of their respective covenants or obligations set forth in this Agreement, the Company, on the one hand, or Parent or Amalgamation Sub, on the other hand, shall each be entitled to specific performance of the terms hereof (including the obligation of the parties to consummate the Amalgamation, subject in each case to the terms and conditions of this Agreement), including an injunction or injunctions to prevent breaches of this Agreement by any party, in addition to any other remedy at law or equity (including that Parent and Amalgamation Sub use reasonable best efforts to obtain the Financing in accordance with Section 6.07).
(b) Notwithstanding the foregoing, the Company’s right to obtain an injunction or injunctions, or other appropriate form of specific performance or equitable relief, in each case, with respect to causing Parent and/or Amalgamation Sub to cause the Financing to be funded at any time (but not the Company’s right to obtain an injunction or injunctions, or other appropriate form of specific performance or equitable relief with respect to any other matter) shall be subject to the satisfaction of each of the following conditions: (i) all conditions in Section 7.01 and Section 7.02 (other than those conditions that by their terms are to be satisfied at the Closing) have been satisfied or waived, (ii) Parent and Amalgamation Sub fail to complete the Closing by the date the Closing is required to have occurred pursuant to Section 1.02, and (iii) the Company has irrevocably confirmed in writing that (A) all conditions set forth in Section 7.03 have been satisfied or that it is willing to waive any of the conditions to the extent not so satisfied in Section 7.03 and (B) if specific performance is granted and the Financing is funded, then it would take such actions that are within its control to cause the Closing to occur.
| A-63 |
(c) Each party (i) waives any defenses in any action for an injunction or other appropriate form of specific performance or equitable relief, including the defense that a remedy at law would be adequate and (ii) waives any requirement under any Law to post a bond or other security as a prerequisite to obtaining an injunction or other appropriate form of specific performance or equitable relief.
(d) Notwithstanding anything herein to the contrary, (i) Parent and Amalgamation Sub on the one hand and the Company on the other hand, agree that the election to pursue an injunction or other appropriate form of specific performance or equitable relief shall not restrict, impair or otherwise limit Parent and Amalgamation Sub or the Company from, in the alternative, seeking to terminate the Agreement and collect the Company Termination Fee pursuant to Section 8.06(a) and expenses under Section 8.06(d), by Parent on the one hand, or the Parent Termination Fee pursuant to Section 8.06(b) and expenses under Section 8.06(d), by the Company on the other hand and (ii) upon the payment of such amounts, the remedy of specific performance shall not be available against the party making such payment and, if such party is Parent or Amalgamation Sub, any other member of the Parent Group or, if such party is the Company, any other member of the Company Group.
(e) This Section 9.08 shall not be deemed to alter, amend, supplement or otherwise modify the terms of any of the Equity Commitment Letters (including the expiration or termination provisions thereof).
Section 9.09 Governing Law; Dispute Resolution.
(a) This Agreement shall be interpreted, construed and governed by and in accordance with the Laws of the State of New York without regard to the conflicts of Law principles thereof that would subject such matter to the Laws of another jurisdiction, except that the following matters arising out of or relating to this Agreement shall be interpreted, construed and governed by and in accordance with the Laws of Antigua and Barbuda in respect of which the parties hereto hereby irrevocably submit to the nonexclusive jurisdiction of the courts of Antigua and Barbuda: the Amalgamation, the vesting of the undertaking, property and liabilities of Amalgamation Sub in the Surviving Corporation, the cancellation of the Shares, the rights provided for in the IBCA with respect to any Dissenting Shares, the fiduciary or other duties of the Company Board and the directors of Amalgamation Sub and the internal corporate affairs of the Company and Amalgamation Sub.
(b) Subject to Section 9.08 and the last sentence of this Section 9.09(b), any disputes, actions and proceedings against any party or arising out of or in any way relating to this Agreement shall be submitted to the Hong Kong International Arbitration Centre (“HKIAC”) and resolved in accordance with the Arbitration Rules of HKIAC (the “Rules”) in force at the relevant time and as may be amended by this Section 9.09. The place of arbitration shall be Hong Kong. The official language of the arbitration shall be English and the arbitration tribunal shall consist of three (3) arbitrators (each, an “Arbitrator”). The claimant(s), irrespective of number, shall nominate jointly one (1) Arbitrator; the respondent(s), irrespective of number, shall nominate jointly one (1) Arbitrator; and a third (3rd) Arbitrator will be nominated jointly by the first two (2) Arbitrators and shall serve as chairman of the arbitration tribunal. In the event the claimant(s) or respondent(s) or the first two (2) Arbitrators shall fail to nominate or agree the joint nomination of an Arbitrator or the third (3rd) Arbitrator within the time limits specified by the Rules, such Arbitrator shall be appointed promptly by the HKIAC. The arbitration tribunal shall have no authority to award punitive or other punitive-type damages. The award of the arbitration tribunal shall be final and binding upon the disputing parties. Any party to an award may apply to any court of competent jurisdiction for enforcement of such award and, for purposes of the enforcement of such award, the parties irrevocably and unconditionally submit to the jurisdiction of any court of competent jurisdiction and waive any defenses to such enforcement based on lack of personal jurisdiction or inconvenient forum.
| A-64 |
Section 9.10 Amendment.
This Agreement may be amended by the parties hereto by action taken by or on behalf of their respective boards of directors (or in the case of the Company, by action taken by or on behalf of the Special Committee) at any time prior to the Effective Time; provided that after the approval of this Agreement and the Transactions by the shareholders of the Company, no amendment may be made that would reduce the amount or change the type of consideration into which each Share shall be converted upon consummation of the Amalgamation. This Agreement may not be amended except by an instrument in writing signed by each of the parties hereto.
Section 9.11 Waiver.
At any time prior to the Effective Time, any party hereto may by action taken (a) with respect to Parent and Amalgamation Sub, by or on behalf of their respective boards of directors and (b) with respect to the Company, by action taken by or on behalf of the Special Committee, (i) extend the time for the performance of any obligation or other act of any other party hereto, (ii) waive any inaccuracy in the representations and warranties of any other party contained herein or in any document delivered pursuant hereto and (iii) waive compliance with any agreement of any other party or any condition to its own obligations contained herein. Any such extension or waiver shall be valid if set forth in an instrument in writing signed by the party or parties to be bound thereby. No failure or delay by any party in exercising any right, power or privilege hereunder shall operate as a waiver thereof, nor shall any single or partial exercise thereof preclude any other or further exercise thereof or the exercise of any other right, power or privilege.
Section 9.12 Counterparts.
This Agreement may be executed and delivered (including by email or facsimile transmission) in one or more counterparts, and by the different parties hereto in separate counterparts, each of which when executed shall be deemed to be an original but all of which taken together shall constitute one and the same agreement.
[Remainder of Page Left Blank Intentionally]
| A-65 |
IN WITNESS WHEREOF, Parent, Amalgamation Sub and the Company have caused this Agreement to be executed as of the date first written above by their respective directors or officers thereunto duly authorized.
| SINOVAC BIOTECH LTD. | ||
| By: | /s/ Simon Anderson | |
| Name: | Simon Anderson | |
| Title: | Director, Chairman of the Special Committee | |
[Signature Page to Amalgamation Agreement]
IN WITNESS WHEREOF, Parent, Amalgamation Sub and the Company have caused this Agreement to be executed as of the date first written above by their respective directors or officers thereunto duly authorized.
| Sinovac (Cayman) Limited | ||
| By: | /s/ Weidong Yin | |
| Name: | Weidong Yin | |
| Title: | Director | |
[Signature Page to Amalgamation Agreement]
IN WITNESS WHEREOF, Parent, Amalgamation Sub and the Company have caused this Agreement to be executed as of the date first written above by their respective directors or officers thereunto duly authorized.
| sINOVAC Amalgamation sub lIMITED | ||
| By: | /s/ Weidong Yin | |
| Name: | Weidong Yin | |
| Title: | Director | |
[Signature Page to Amalgamation Agreement]
SCHEDULE A
List of Guarantors
C-Bridge Healthcare Fund II, L.P.
Advantech Capital L.P.
Vivo Capital Fund VIII, L.P.
Vivo Capital Surplus Fund VIII, L.P.
SAIF Partners IV L.P.
Annex B
Sections 191 to 199 of the International Business Corporations Act, CAP. 222 of the Revised Laws of Antigua and Barbuda
191. (1) Subject to sections 174 (re-organization) and 204 (restraining oppression), a shareholder of any class of shares of a corporation may dissent if the corporation resolves:
(a) to amend its articles under section 168 to add, change or remove any restriction upon the international trades or businesses that the corporation can carry on;
(b) to amalgamate with another corporation, otherwise than under section 174 or 175; or
(c) to sell, lease or exchange all or substantially all its property under section 125.
(2) Subject to sections 174 and 204, a shareholder of any class of shares of a corporation may dissent if the corporation is subject to an order of the court under section 177 permitting the shareholders to dissent.
(3) The articles of a corporation may provide that a shareholder of any class or series of shares who is entitled to vote under section 163 may dissent if the corporation resolves to amend its articles in a manner described in that section.
(4) In addition to any other right he has, but subject to section 175, a shareholder who complies with this section is entitled, when the action approved by the resolution from which he dissents or an order made under section 175 becomes effective, to be paid by the corporation the fair value of the shares held by him in respect of which he dissents; and the fair value is to be determined as of the close of business on the day before the resolution was adopted or the order made.
(5) A dissenting shareholder may not claim under this section except only with respect to all the shares of a class or series.
(6) A dissenting shareholder must send to the corporation, at or before any meeting of shareholders of the corporation at which a resolution referred to in subsection (1) or (3) is to be voted on, a written dissent from the resolution, unless the corporation did not give notice to the shareholder of the purpose of the meeting and of his right to dissent.
(7) When a shareholder of a corporation has dissented pursuant to subsection (6) to a resolution referred to in subsection (1) or (3), the corporation must, within ten days after the shareholders of the corporation adopt the resolution, send to the shareholder notice that the resolution has been adopted; but the notice need not be sent to the shareholder if he has voted for the resolution or has withdrawn his dissent.
192. (1) A dissenting shareholder must, within twenty days after he receives a notice under subsection (7) of section 191 or, if he does not receive that notice, within twenty days after he learns that a resolution under that section has been adopted, send to the corporation a written notice containing:
| B-1 |
(a) his name and address;
(b) the number and class or series of shares in respect of which he dissents; and
(c) a demand for payment of the fair value of the shares.
(2) A dissenting shareholder must, within thirty days after sending a notice under subsection (1), send the certificates representing the shares in respect of which he dissents to the corporation or its transfer agent.
(3) A dissenting shareholder who fails to comply with subsection (2) has no right to make a claim under this section.
(4) A corporation or its transfer agent must endorse on any share certificate received by it under subsection (2) a notice that the holder of the shares is a dissenting shareholder under this section and forthwith return the share certificate to the dissenting shareholder.
193. After sending a notice under section 192, a dissenting shareholder ceases to have any rights as a shareholder, other than the right to be paid the fair value of his shares as determined under this section unless
(a) the dissenting shareholder withdraws his notice before the corporation makes an offer under section 194;
(b) the corporation fails to make an offer in accordance with section 194 and the dissenting shareholder withdraws his notice; or
(c) the directors, as the circumstances require,
(i) revoke the resolution to amend the articles of the corporation;
(ii) under subsection (6) of section 169, terminate the amalgamation agreement; or
(iii) under subsection (7) of section 125, abandon the sale, lease or exchange of property;
in which case his rights as a shareholder are reinstated as of the date the notice mentioned in section 192 was sent.
194. (1) A corporation must, not later than seven days after the day on which the action approved by the resolution is effective or the day the corporation received the notice referred to in section 192, whichever is the later date, send to each dissenting shareholder who has sent such a notice
(a) a written offer to pay for his shares in an amount considered by the directors of the corporation to be the fair value of those shares, which must be accompanied with a statement showing how the fair value was determined; or
(b) if subsection (3) of section 199 applies, a notification that it is unable lawfully to pay dissenting shareholders for their shares.
(2) Every offer made under subsection (1) for shares of the same class or series must be on the same terms.
| B-2 |
(3) Subject to section 196, a corporation must pay for the shares of a dissenting shareholder within ten days after an offer made under subsection (1) has been accepted; but the offer lapses if the corporation does not receive an acceptance of the offer within thirty days after it has been made.
195. (1) If a corporation fails to make an offer under subsection (1) of section 194, or if a dissenting shareholder fails to accept the offer made by the corporation, the corporation may, within fifty days after the action approved by the resolution is effective, apply to the court to fix a fair value for the shares of any dissenting shareholders.
(2) If a corporation fails to apply to the court in the circumstances described in subsection (1), a dissenting shareholder may, within a further period of twenty days, apply to the court to fix a fair value for the shares of any dissenting shareholders.
196. Upon an application to the court under section 195,
(a) all dissenting shareholders whose shares have not been purchased by the corporation are to be joined as parties and are bound by the decision of the court; and
(b) the corporation must notify each affected dissenting shareholder of the date, place and consequences of the application and of his right to appear and be heard in person or by counsel.
197. (1) Upon an application to the court under section 191, the court may determine whether any other person is a dissenting shareholder who should be joined as a party; and the court must then fix a fair value for the shares of all dissenting shareholders.
(2) The court may appoint one or more appraisers to assist the court to fix a fair value for the shares of the dissenting shareholders.
(3) The final order of the court must be made against the corporation in favour of each dissenting shareholder of the corporation and for the amount of the shares of the dissenting shareholder as fixed by the court.
198. The court may allow a reasonable rate of interest on the amount payable to each dissenting shareholder from the date the action approved by the resolution is effective until the date of payment by the corporation.
199. (1) If subsection (3) applies, the corporation must, within ten days after the making of an order under subsection (3) of section 197, notify each dissenting shareholder that it is unable lawfully to pay dissenting shareholders for their shares.
(2) If subsection (3) applies, a dissenting shareholder, by written notice delivered to the corporation within thirty days after receiving a notice under subsection (1),
(a) may withdraw his notice of dissent, in which case the corporation consents to the withdrawal and the shareholder is reinstated to his full rights as a shareholder; or
| B-3 |
(b) may retain a status as a claimant against the corporation entitled to be paid as soon as the corporation is lawfully able to do so or, in a liquidation, to be ranked subordinate to the rights of creditors of the corporation but in priority to the corporation’s shareholders.
(3) A corporation shall not make a payment to a dissenting shareholder under section 194 if there are reasonable grounds for believing that (a) the corporation is or would, after the payment, be unable to pay its liabilities as they become due; or (b) the realisable value of the corporation’s assets would thereby be less than the aggregate of its liabilities.
| B-4 |
Annex C
Support Agreement
Execution Version
SUPPORT AGREEMENT
This SUPPORT AGREEMENT (this “Agreement”) is entered into as of June 26, 2017 by and among Sinovac (Cayman) Limited, an exempted company incorporated with limited liability under the Laws of the Cayman Islands (“Parent”), Sinovac Holding (Cayman) Limited, an exempted company incorporated with limited liability under the Laws of the Cayman Islands (“Holdco”), Mr. Weidong Yin (the “Chairman”) and SAIF Partners IV L.P., an exempted limited partnership registered under the Laws of the Cayman Islands (“SAIF” and, together with the Chairman, the “Rollover Shareholders” and each, a “Rollover Shareholder”). Capitalized terms used but not defined herein shall have the meanings ascribed to such terms in the Amalgamation Agreement (as defined below).
WHEREAS, Parent, Sinovac Amalgamation Sub Limited, an international business corporation incorporated under the Laws of Antigua and Barbuda and a wholly-owned subsidiary of Parent (“Amalgamation Sub”) and Sinovac Biotech Ltd., a company limited by shares incorporated under the Laws of Antigua and Barbuda and listed on the NASDAQ Global Select Market (the “Company”) have, concurrently with the execution of this Agreement, entered into an amalgamation agreement, dated as of the date hereof (as may be amended, supplemented or otherwise modified from time to time, the “Amalgamation Agreement”), which provides, among other things, for the amalgamation of Amalgamation Sub with and into the Company, with the Company continuing as the surviving corporation and a wholly-owned subsidiary of Parent (the “Amalgamation”), upon the terms and subject to the conditions set forth in the Amalgamation Agreement;
WHEREAS, as of the date hereof, each Rollover Shareholder is the “beneficial owner” (within the meaning of Rule 13d-3 under the Exchange Act) of the issued and outstanding Shares, Company Options and/or Company RSs (as applicable) as set forth opposite such Rollover Shareholder’s name under column “Owned Securities” on Schedule A hereto (with respect to each Rollover Shareholder, the “Owned Securities”) (the Owned Securities, together with any other Shares, Company Options and/or Company RSs acquired (whether beneficially or of record) by such Rollover Shareholder after the date hereof and prior to the earlier of the Effective Time and the termination of all such Rollover Shareholder’s obligations under this Agreement, including any Shares, Company Options and/or Company RSs acquired by means of purchase, dividend or distribution, or issued upon the exercise of any Company Options or warrants or the conversion of any convertible securities or otherwise, being collectively referred to herein as the “Securities”);
WHEREAS, in connection with the consummation of the Amalgamation, each Rollover Shareholder agrees to (a) have his or its respective Shares as set forth opposite such Rollover Shareholder’s name under the column “Rollover Shares” on Schedule A hereto (the “Rollover Shares”) cancelled for no consideration in connection with the Amalgamation, (b) subscribe for newly issued ordinary shares of Holdco (the “Holdco Shares”) immediately prior to the Closing and (c) vote the Securities at the Shareholders’ Meeting in favor of the Amalgamation, in each case, in accordance with and subject to the terms and conditions of this Agreement;
WHEREAS, in order to induce Parent and Amalgamation Sub to enter into the Amalgamation Agreement and consummate the Transactions, the Rollover Shareholders are entering into this Agreement;
| C-1 |
WHEREAS, each Rollover Shareholder acknowledges that Parent and Amalgamation Sub are entering into the Amalgamation Agreement in reliance on the representations, warranties, covenants and other agreements of the Rollover Shareholders set forth in this Agreement; and
NOW, THEREFORE, in consideration of the foregoing, the mutual covenants and agreements set forth herein, and other good and valuable consideration, the receipt and sufficiency of which is hereby acknowledged, the parties hereto agree as follows:
ARTICLE I
VOTING; GRANT AND APPOINTMENT OF PROXY
Section 1.1 Voting. From and after the date hereof until the earlier of (x) the Effective Time and (y) the termination of the Amalgamation Agreement pursuant to and in compliance with the terms therein (such earlier time, the “Expiration Time”), each Rollover Shareholder hereby irrevocably and unconditionally agrees that at the Shareholders’ Meeting or any other meeting (whether annual or special) of the shareholders of the Company, however called, at which any of the matters described in paragraphs (a) – (f) hereof is to be considered (and any adjournment or postponement thereof), or in connection with any written resolution of the Company’s shareholders proposed in accordance with the articles of incorporation and by-laws of the Company, such Rollover Shareholder shall (i) in the case of a meeting, appear or cause his or its Representative(s) to appear at such meeting or otherwise cause his or its Securities to be counted as present thereat for purposes of determining whether a quorum is present and (ii) vote or cause to be voted (including by proxy or written resolution proposed in accordance with the articles of incorporation and by-laws of the Company, if applicable) all of such Rollover Shareholder’s Securities:
(a) for authorization and approval of the Amalgamation Agreement, the Plan of Amalgamation and the Transactions, including the Amalgamation, and any action required in furtherance thereof,
(b) against any Competing Transaction or any other transaction, proposal, agreement or action made in opposition to authorization and approval of the Amalgamation Agreement and the Transactions, including the Amalgamation, or in competition or inconsistent with the Transactions, including the Amalgamation,
(c) against any other action, agreement or transaction that is intended, that could reasonably be expected, or the effect of which could reasonably be expected, to materially impede, interfere with, delay, postpone, discourage or adversely affect the Transactions, including the Amalgamation, or this Agreement or the performance by such Rollover Shareholder of his or its obligations under this Agreement, including, without limitation: (i) any extraordinary corporate transaction, such as a scheme of arrangement, amalgamation, consolidation or other business combination involving any Group Company (other than the Amalgamation); (ii) a sale, lease or transfer of a material amount of assets (whether real, personal or mixed, and including leasehold interests and intangible property) of any Group Company or a reorganization, recapitalization or liquidation of any Group Company; (iii) an election of new members to the Company Board, other than nominees to the Company Board who are serving as directors of the Company on the date of this Agreement or as otherwise provided in the Amalgamation Agreement; (iv) any material change in the present capitalization or dividend policy of the Company or any amendment or other change to the Company’s articles of incorporation and by-laws, except if approved in writing by Parent; or (v) any other action that would require the consent of Parent pursuant to the Amalgamation Agreement, except if approved in writing by Parent,
| C-2 |
(d) against any action, proposal, transaction or agreement that would reasonably be expected to result in a breach in any respect of any covenant, representation or warranty or any other obligation or agreement of the Company contained in the Amalgamation Agreement, or of such Rollover Shareholder contained in this Agreement or otherwise reasonably requested by Parent in order to consummate the Transactions, including the Amalgamation,
(e) in favor of any adjournment or postponement of the Shareholders’ Meeting or other annual or special meeting of the shareholders of the Company, however called, at which any of the matters described in paragraphs (a) through (f) hereof is to be considered (and any adjournment or postponement thereof), and
(f) in favor of any other matter necessary to effect the Transactions, including the Amalgamation.
Any such vote (including by proxy or written resolution proposed in accordance with the articles of incorporation and by-laws of the Company, if applicable) on the matters described in paragraphs (a) – (f) hereof by such Rollover Shareholder shall be cast or submitted in accordance with such procedures relating thereto so as to ensure that it is duly counted, including for purposes of determining that a quorum is present and for purposes of recording the results of such vote (including by proxy or written resolution proposed in accordance with the articles of incorporation and by-laws of the Company, if applicable).
Section 1.2 Grant of Irrevocable Proxy; Appointment of Proxy.
(a) Effective immediately upon the execution of the Amalgamation Agreement, without any further action by any person, and only in the event and to the extent that such Rollover Shareholder fails to perform his or its obligations under Section 1.1 above, each Rollover Shareholder hereby irrevocably appoints Parent and any designee thereof as his or its proxy and attorney-in-fact (with full power of substitution), to vote or cause to be voted (including by proxy or written resolution proposed in accordance with the articles of incorporation and by-laws of the Company, if applicable) such Rollover Shareholder’s Shares in accordance with Section 1.1 above at the Shareholders’ Meeting or other annual or special meeting of the shareholders of the Company, however called, including any adjournment or postponement thereof, at which any of the matters described in Section 1.1 above is to be considered. Each Rollover Shareholder represents that all proxies, powers of attorney, instructions or other requests given by such Rollover Shareholder prior to the execution of this Agreement in respect of the voting of such Rollover Shareholder’s Shares, if any, are not irrevocable and each Rollover Shareholder hereby revokes (and shall cause to be revoked if necessary) any and all previous proxies, powers of attorney, instructions or other requests with respect to such Rollover Shareholder’s Shares. Each Rollover Shareholder shall take such further action or execute such other instruments as may be necessary to effectuate the intent of this proxy.
| C-3 |
(b) Each Rollover Shareholder affirms that the irrevocable proxy set forth in this Section 1.2 is given in connection with the execution of the Amalgamation Agreement, and that such irrevocable proxy is given to secure the performance of the duties of such Rollover Shareholder under this Agreement. Each Rollover Shareholder further affirms that the irrevocable proxy is coupled with an interest and, except as set forth in this Section 1.2, is intended to be irrevocable prior to the Expiration Time. If for any reason the proxy granted herein is not irrevocable, then each Rollover Shareholder agrees to vote such Rollover Shareholder’s Shares in accordance with Section 1.1 above prior to the Expiration Time. The parties hereto agree that the foregoing is a voting agreement.
Section 1.3 Restrictions on Transfers. Except as provided for in Article III below or pursuant to the Amalgamation Agreement, each Rollover Shareholder hereby agrees that, from the date hereof until the Expiration Time, such Rollover Shareholder shall not, and shall cause its Affiliates not to, directly or indirectly, (a) offer for sale, sell (constructively or otherwise), transfer, assign, tender in any tender or exchange offer, pledge, grant, encumber, hypothecate or similarly dispose of (by amalgamation, testamentary disposition, operation of Law or otherwise) (collectively, “Transfer”), either voluntarily or involuntarily, or enter into any Contract, option or other arrangement or understanding with respect to the Transfer of any Securities or any interest therein, including, without limitation, any swap transaction, option, warrant, forward purchase or sale transaction, futures transaction, cap transaction, floor transaction, collar transaction or any other similar transaction (including any option with respect to any such transaction) or combination of any such transactions, in each case involving any Securities (any such transaction, a “Derivative Transaction”), (b) deposit any Securities into a voting trust or enter into a voting agreement or arrangement or grant any proxy or power of attorney with respect thereto that is inconsistent with this Agreement, (c) convert or exchange, or take any action which would result in the conversion or exchange, of any Securities, (d) take any action that would make any representation or warranty of such Rollover Shareholder set forth in this Agreement untrue or incorrect or have the effect of preventing, disabling, or delaying such Rollover Shareholder from performing any of his or its obligations under this Agreement or that is intended, or would reasonably be expected, to impede, frustrate, interfere with, delay, postpone, adversely affect or prevent the consummation of the Transactions or this Agreement or the performance by the Company of its obligations under the Amalgamation Agreement or by any Rollover Shareholder from performing any of his or its obligations under this Agreement, or (e) agree (whether or not in writing) to take any of the actions referred to in the foregoing clauses (a), (b), (c) or (d); provided that the foregoing shall not prevent the conversion of Company Options into the right to receive payment in accordance with the terms of, and to the extent provided in, the Amalgamation Agreement. Any purported Transfer in violation of this Section 1.3 shall be null and void.
ARTICLE II
ROLLOVER SHARES
Section 2.1 Cancellation of Rollover Shares. Subject to the terms and conditions set forth herein, (a) each Rollover Shareholder agrees that his or its Rollover Shares shall be cancelled at the Closing for no consideration, and (b) other than his or its Rollover Shares, all equity securities of the Company held by such Rollover Shareholder, if any, shall be treated as set forth in the Amalgamation Agreement and not be affected by the provisions of this Agreement. Each Rollover Shareholder shall take all actions necessary to cause the number of Rollover Shares opposite such Rollover Shareholder’s name on Schedule A hereto to be treated as set forth herein.
| C-4 |
Section 2.2 Subscription of Holdco Shares. Immediately prior to the Closing, in consideration for the cancellation of the Rollover Shares held by each Rollover Shareholder in accordance with Section 2.1, Holdco shall issue to such Rollover Shareholder (or, if designated by such Rollover Shareholder in writing, an Affiliate of such Rollover Shareholder), and such Rollover Shareholder or his or its Affiliate (as applicable) shall subscribe for, the number of Holdco Shares, at par value per share, equal to the number of Rollover Shares held by such Rollover Shareholder and cancelled pursuant to Section 2.1 above. Each Rollover Shareholder hereby acknowledges and agrees that (a) delivery of such Holdco Shares shall constitute complete satisfaction of all obligations towards or sums due to such Rollover Shareholder by Holdco, Parent and Amalgamation Sub in respect of the Rollover Shares held by such Rollover Shareholder and cancelled pursuant to Section 2.1 above, and (b) such Rollover Shareholder shall have no right to the Amalgamation Consideration in respect of the Rollover Shares held by such Rollover Shareholder.
Section 2.3 Rollover Closing. Subject to the satisfaction in full (or waiver, if permissible) of all of the conditions set forth in Sections 7.01 and 7.02 of the Amalgamation Agreement (other than conditions that by their nature are to be satisfied or waived, as applicable, at the Closing), the closing of the subscription and issuance of Holdco Shares contemplated hereby (the “Rollover Closing”) shall take place immediately prior to the Closing.
Section 2.4 Deposit of Rollover Shares. No later than five (5) Business Days prior to the Closing, each Rollover Shareholder and any agent of such Rollover Shareholder holding certificates evidencing any of the Rollover Shares shall deliver or cause to be delivered to Parent all certificates representing such Rollover Shares in such Person’s possession, for disposition in accordance with the terms of this Agreement; such certificates and documents shall be held by Parent or any agent authorized by Parent until the Closing.
ARTICLE III
REPRESENTATIONS, WARRANTIES AND COVENANTS
OF THE ROLLOVER SHAREHOLDERS
Section 3.1 Representations and Warranties. Each Rollover Shareholder, severally and not jointly, represents and warrants to Parent and Holdco as of the date hereof and as of the Closing:
(a) such Rollover Shareholder has the requisite full legal right, power, capacity and authority to execute and deliver this Agreement, to perform such Rollover Shareholder’s obligations hereunder and to consummate the transactions contemplated hereby;
(b) this Agreement has been duly executed and delivered by such Rollover Shareholder and the execution, delivery and performance of this Agreement by such Rollover Shareholder and the consummation of the transactions contemplated hereby have been duly authorized by all necessary action on the part of such Rollover Shareholder and no other actions or proceedings on the part of such Rollover Shareholder are necessary to authorize this Agreement or to consummate the transactions contemplated hereby;
| C-5 |
(c) assuming due authorization, execution and delivery by Parent, Holdco and the other Rollover Shareholders, this Agreement constitutes a legal, valid and binding agreement of such Rollover Shareholder, enforceable against such Rollover Shareholder in accordance with its terms, except as enforcement may be limited by applicable bankruptcy, insolvency, reorganization, moratorium or similar laws affecting creditors’ rights generally and by general principles of equity (regardless of whether considered in a proceeding in equity or at law);
(d) (i) such Rollover Shareholder (A) is and, immediately prior to the Closing, will be the beneficial owner of, and has and will have good and valid title to, his or its Securities, free and clear of Liens other than as created by this Agreement, and (B) has and will have sole or shared (together with Affiliates controlled by such Rollover Shareholder) voting power, power of disposition, and power to demand dissenter’s rights, in each case with respect to all of his or its Securities, with no limitations, qualifications, or restrictions on such rights, subject to applicable United States federal securities Laws, the Laws of Antigua and Barbuda, the Laws of Cayman Islands, PRC Laws and the terms of this Agreement; (ii) his or its Securities are not subject to any voting trust agreement or other Contract to which such Rollover Shareholder is a party restricting or otherwise relating to the voting or Transfer of such Securities other than this Agreement; (iii) such Rollover Shareholder has not Transferred any interest in any of his or its Securities pursuant to any Derivative Transaction; (iv) as of the date hereof, other than his or its Owned Securities, such Rollover Shareholder does not own, beneficially or of record, any Shares or other securities of the Company, or any direct or indirect interest in any such securities (including by way of derivative securities); and (v) such Rollover Shareholder has not appointed or granted any proxy or power of attorney that is still in effect with respect to any of his or its Rollover Shares, except as contemplated by this Agreement;
(e) except for the applicable requirements of the Exchange Act, the Securities Act, any other U.S. federal or state securities Laws, and the rules and regulations of NASDAQ, (i) no filing with, and no permit, authorization, consent or approval of, any Governmental Authority is necessary on the part of such Rollover Shareholder for the execution, delivery and performance of this Agreement by such Rollover Shareholder or the consummation by such Rollover Shareholder of the transactions contemplated hereby and (ii) neither the execution, delivery or performance of this Agreement by such Rollover Shareholder nor the consummation by such Rollover Shareholder of the transactions contemplated hereby, nor compliance by such Rollover Shareholder with any of the provisions hereof shall (A) conflict with or violate any provision of the organizational documents of any such Rollover Shareholder which is an entity, (B) result in any breach or violation of, or constitute a default (or an event which, with notice or lapse of time or both, would become a default) under, or give to others any rights of termination, amendment, acceleration or cancellation of, or result in the creation of a Lien on property or assets of such Rollover Shareholder pursuant to any Contract to which such Rollover Shareholder is a party or by which such Rollover Shareholder or any property or asset of such Rollover Shareholder is bound or affected, or (C) violate any order, writ, injunction, decree, statute, rule or regulation applicable to such Rollover Shareholder or any of such Rollover Shareholder’s properties or assets;
(f) there is no Action pending against such Rollover Shareholder or, to the knowledge of such Rollover Shareholder, any other person, or, to the knowledge of such Rollover Shareholder, threatened against any such Rollover Shareholder or any other person that restricts or prohibits (or, if successful, would restrict or prohibit) the performance by such Rollover Shareholder of his or its obligations under this Agreement;
| C-6 |
(g) such Rollover Shareholder has been afforded the opportunity to ask such questions as he or it has deemed necessary of, and to receive answers from, representatives of Parent and Holdco concerning the terms and conditions of the transactions contemplated hereby and the merits and risks of owning the Holdco Shares and such Rollover Shareholder acknowledges that he or it has been advised to discuss with his or its own counsel the meaning and legal consequences of such Rollover Shareholder’s representations and warranties in this Agreement and the transactions contemplated hereby; and
(h) such Rollover Shareholder understands and acknowledges that Parent, Amalgamation Sub and the Company are entering into the Amalgamation Agreement in reliance upon such Rollover Shareholder’s execution, delivery and performance of this Agreement.
Section 3.2 Covenants. Each Rollover Shareholder hereby:
(a) agrees, prior to the Expiration Time, not to knowingly take any action that would make any representation or warranty of such Rollover Shareholder contained herein untrue or incorrect or have or could have the effect of preventing, impeding or interfering with or adversely affecting the performance by such Rollover Shareholder of his or its obligations under this Agreement;
(b) irrevocably waives, and agrees not to exercise, any rights of appraisal or rights of dissent from the Amalgamation that such Rollover Shareholder may have with respect to such Rollover Shareholder’s Securities prior to the Expiration Time;
(c) agrees to permit the Company and Parent to publish and disclose in any press release, the Proxy Statement (including all documents filed with the SEC in accordance therewith) and any other disclosure documents in connection with the Amalgamation Agreement and any filings with or notices to any Governmental Authority in connection with the Transactions, such Rollover Shareholder’s identity and beneficial ownership of Shares, Securities or other equity securities of the Company and the nature of such Rollover Shareholder’s commitments, arrangements and understandings under this Agreement (including, for the avoidance of doubt, the disclosure of this Agreement) and any other information, in each case, that the Company or Parent reasonably determines in its good faith judgement is required to be disclosed by Law;
(d) agrees and covenants, severally and not jointly, that such Rollover Shareholder shall promptly (and in any event within forty-eight (48) hours) notify Parent and the Company of any new Shares, Securities and/or other securities of the Company with respect to which beneficial ownership (within the meaning of Rule 13d-3 under the Exchange Act) is acquired by such Rollover Shareholder, including, without limitation, by purchase, as a result of a stock dividend, stock split, recapitalization, combination, reclassification, exchange or change of such Shares, or upon exercise or conversion of any securities of the Company after the date hereof. Any such Shares, Securities and/or other securities of the Company shall automatically be deemed as “Owned Securities” held by such Rollover Shareholder pursuant to the terms of this Agreement, and Schedule A hereto shall be deemed amended accordingly; and
| C-7 |
(e) agrees further that, upon request of Parent, such Rollover Shareholder shall execute and deliver any additional documents, consents or instruments and take such further actions as may reasonably be deemed by Parent to be necessary or desirable to carry out the provisions of this Agreement.
ARTICLE IV
REPRESENTATIONS AND WARRANTIES OF PARENT AND HOLDCO
Each of Parent and Holdco represents and warrants to each Rollover Shareholder that as of the date hereof and as of the Closing:
(a) each of Parent and Holdco is duly incorporated, validly existing and in good standing under the Laws of the Cayman Islands and has all requisite power and authority to execute and deliver this Agreement and to perform its obligations hereunder and to consummate the transactions contemplated hereby. This Agreement has been duly and validly executed and delivered by Parent and Holdco and the execution, delivery and performance of this Agreement by Parent and Holdco and the consummation of the transactions contemplated hereby have been duly authorized by all necessary corporate action on the part of Parent and Holdco and no other corporate actions or proceedings on the part of Parent and Holdco are necessary to authorize this Agreement or to consummate the transactions contemplated hereby. Assuming due authorization, execution and delivery by the Rollover Shareholders, this Agreement constitutes a legal, valid and binding obligation of Parent and Holdco, enforceable against Parent and Holdco in accordance with its terms, except as enforcement may be limited by applicable bankruptcy, insolvency, reorganization, moratorium or similar Laws affecting creditors’ rights generally and by general principles of equity (regardless of whether considered in a proceeding in equity or at law);
(b) except for the applicable requirements of the Exchange Act and Laws of the Cayman Islands, (i) no filing with, and no permit, authorization, consent or approval of, any Governmental Authority is necessary on the part of Parent or Holdco for the execution, delivery and performance of this Agreement by Parent and Holdco or the consummation by Parent and Holdco of the transactions contemplated hereby, and (ii) neither the execution, delivery or performance of this Agreement by Parent and Holdco, nor the consummation by Parent and Holdco of the transactions contemplated hereby, nor compliance by Parent and Holdco with any of the provisions hereof shall (A) conflict with or violate any provision of the organizational documents of Parent or Holdco, (B) result in any breach or violation of, or constitute a default (or an event which, with notice or lapse of time or both, would become a default) under, or give to others any rights of termination, amendment, acceleration or cancellation of, or result in the creation of a Lien on such property or asset of Parent or Holdco pursuant to, any Contract to which Parent or Holdco is a party or by which Parent or Holdco, or any of their property or asset is bound or affected, or (C) violate any order, writ, injunction, decree, statute, rule or regulation applicable to Parent or Holdco any of their properties or assets;
(c) except as contemplated by the Amalgamation Agreement or otherwise agreed to by the parties hereto, at and immediately after the Closing, there shall be (i) no options, warrants, or other rights to acquire share capital of Holdco or Parent, (ii) no outstanding securities exchangeable for or convertible into share capital of Holdco or Parent, and (iii) no outstanding rights to acquire or obligations to issue any such options, warrants, rights or securities; and
| C-8 |
(d) at the Rollover Closing, the Holdco Shares to be issued under this Agreement shall have been duly and validly authorized and when issued and delivered in accordance with the terms hereof, will be validly issued, fully paid and nonassessable, free and clear of all claims, liens and encumbrances, other than restrictions arising under applicable securities Laws.
ARTICLE V
TERMINATION
This Agreement, and the obligations of the Rollover Shareholders hereunder (including, without limitation, Section 1.2 hereof), shall terminate and be of no further force or effect immediately upon the earlier to occur of (a) the Effective Time and (b) the date of termination of the Amalgamation Agreement in accordance with its terms (unless the Company shall have made a claim under this Agreement, in which case this Agreement shall terminate upon the resolution of such action and, to the extent applicable, the satisfaction by the parties hereto of any obligation finally determined or otherwise agreed to be owed by the parties hereto pursuant to this Agreement). Notwithstanding the preceding sentence, this Article V and Article VI shall survive any termination of this Agreement. Nothing in this Article V shall relieve or otherwise limit any party’s liability for any breach of this Agreement prior to the termination of this Agreement. If for any reason the Amalgamation fails to occur but the Rollover Closing contemplated by Article II has already taken place, then Holdco and Parent shall promptly take all such actions as are necessary to restore each such Rollover Shareholder to the position it was in with respect to ownership of the Rollover Shares prior to the Rollover Closing.
ARTICLE VI
MISCELLANEOUS
Section 6.1 Notices. All notices and other communications hereunder shall be in writing in the English language and shall be deemed duly given (a) on the date of delivery if delivered personally, or if by facsimile or e-mail, upon written confirmation of receipt by facsimile or e-mail, or (b) on the first Business Day following the date of dispatch if delivered utilizing a next-day service by a recognized next-day courier. All notices hereunder shall be delivered to the addresses set forth below (or at such other address for a party as shall be specified in a notice given in accordance with this Section 6.1):
(i) If to a Rollover Shareholder, to the address set forth next to such Rollover Shareholder’s name on Schedule A hereto.
| C-9 |
(ii) If to Parent and/or Holdco:
| c/o No. 39 Shangdi Xi Road, | |
| Haidian District, Beijing 100085 | |
| People’s Republic of China | |
| Attention: | Weidong Yin |
| Facsimile: | +86 10 6296 6910 |
| With a copy (which shall not constitute notice) to: | |
| Kirkland & Ellis | |
| 26th Floor, Gloucester Tower | |
| The Landmark | |
| 15 Queen’s Road, Central | |
| Hong Kong | |
| Attention: | David T. Zhang, Esq. |
| Facsimile: | +852-3761-3301 |
| E-mail: | [email protected] |
Section 6.2 Capacity. Notwithstanding anything to the contrary in this Agreement, (i) each Rollover Shareholder is entering into this Agreement, and agreeing to become bound hereby, solely in his or its capacity as a beneficial owner of Securities and not in any other capacity (including without limitation any capacity as a director of the Company) and (ii) nothing in this Agreement shall obligate such Rollover Shareholder or his or its Representatives to take, or forbear from taking, as a director or officer of the Company, any action which is inconsistent with his or its fiduciary duties under applicable Law.
Section 6.3 Severability. Any term or provision of this Agreement which is invalid or unenforceable in any jurisdiction shall, as to that jurisdiction, be ineffective to the extent of such invalidity or unenforceability without rendering invalid or unenforceable the remaining terms and provisions of this Agreement in any other jurisdiction. If any provision of this Agreement is so broad as to be unenforceable, such provision shall be interpreted to be only as broad as is enforceable.
Section 6.4 Entire Agreement. This Agreement and the Amalgamation Agreement embody the complete agreement and understanding among the parties hereto with respect to the subject matter hereof and thereof and supersede and preempt any prior understandings, agreements or representations by or among the parties hereto, written or oral, which may have related to the subject matter hereof in any way.
Section 6.5 Specific Performance. Each Rollover Shareholder acknowledges and agrees that monetary damages would not be an adequate remedy in the event that any covenant or agreement of such Rollover Shareholder in this Agreement is not performed in accordance with its terms, and therefore agrees that, in addition to and without limiting any other remedy or right available to Parent or Holdco and the Company, Parent, Holdco and the Company shall each have the right to an injunction, temporary restraining order or other equitable relief in any court of competent jurisdiction enjoining any such breach and enforcing specifically the terms and provisions hereof. Each Rollover Shareholder agrees not to oppose the granting of such relief in the event a court determines that such a breach has occurred, and to waive any requirement for the securing or posting of any bond in connection with such remedy. All rights, powers, and remedies provided under this Agreement or otherwise available in respect hereof at law or in equity shall be cumulative and not alternative, and the exercise or beginning of the exercise of any thereof by Parent and/or Holdco and/or the Company shall not preclude the simultaneous or later exercise of any other such right, power or remedy by Holdco and/or Parent and/or the Company.
| C-10 |
Section 6.6 Amendments; Waivers. At any time prior to the Expiration Time, any provision of this Agreement may be amended or waived if, and only if, such amendment or waiver is in writing and signed, in the case of an amendment, by the Rollover Shareholders, Holdco, Parent and the Company (acting only upon the recommendation of the Special Committee), or in the case of a waiver, by the party against whom the waiver is to be effective and the Company (acting only upon the recommendation of the Special Committee). Notwithstanding the foregoing, no failure or delay by a party hereto or the Company in exercising any right hereunder shall operate as a waiver thereof nor shall any single or partial exercise thereof preclude any other or further exercise of any other right hereunder.
Section 6.7 Governing Law; Dispute Resolution; Jurisdiction. This Agreement shall be interpreted, construed, performed and enforced in accordance with the Laws of the State of New York without giving effect to its principles or rules of conflict of laws to the extent such principles or rules would require or permit the application of the Laws of another jurisdiction. Subject to the last sentence of this Section 6.7, any disputes, actions and proceedings against any party hereto or arising out of or in any way relating to this Agreement shall be submitted to the Hong Kong International Arbitration Centre (“HKIAC”) and resolved in accordance with the Arbitration Rules of HKIAC (the “Rules”) in force at the relevant time and as may be amended by this Section 6.7. The place of arbitration shall be Hong Kong. The official language of the arbitration shall be English and the arbitration tribunal shall consist of three (3) arbitrators (each, an “Arbitrator”). The claimant(s), irrespective of number, shall nominate jointly one (1) Arbitrator; the respondent(s), irrespective of number, shall nominate jointly one (1) Arbitrator; and a third (3rd) Arbitrator will be nominated jointly by the first two (2) Arbitrators and shall serve as chairman of the arbitration tribunal. In the event the claimant(s) or respondent(s) or the first two (2) Arbitrators shall fail to nominate or agree the joint nomination of an Arbitrator or the third (3rd) Arbitrator within the time limits specified by the Rules, such Arbitrator shall be appointed promptly by the HKIAC. The arbitration tribunal shall have no authority to award punitive or other punitive-type damages. The award of the arbitration tribunal shall be final and binding upon the disputing parties. Any party to an award may apply to any court of competent jurisdiction for enforcement of such award and, for purposes of the enforcement of such award, the parties irrevocably and unconditionally submit to the jurisdiction of any court of competent jurisdiction and waive any defenses to such enforcement based on lack of personal jurisdiction or inconvenient forum.
Section 6.9 No Third Party Beneficiaries. There are no third party beneficiaries of this Agreement and nothing in this Agreement, express or implied, is intended to confer on any person other than the parties hereto (and their respective successors, heirs and permitted assigns), any rights, remedies, obligations or liabilities, except as specifically set forth in this Agreement; provided, however, that, notwithstanding anything to the contrary contained herein, the Company shall be an express third party beneficiary of this Agreement and shall be entitled to specific performance of the terms hereof, including the rights provided under Section 6.5.
| C-11 |
Section 6.10 Assignment; Binding Effect. Neither this Agreement nor any of the rights, interests or obligations hereunder shall be assigned by any of the parties hereto (whether by operation of Law or otherwise) without the prior written consent of the other parties and the Company (acting only upon the recommendation of the Special Committee), except that Parent may assign this Agreement (in whole but not in part) in connection with a permitted assignment of the Amalgamation Agreement by Parent, as applicable. Subject to the preceding sentence, this Agreement shall be binding upon and shall inure to the benefit of the parties hereto and their respective successors and permitted assigns and, in the case of each Rollover Shareholder, his, her or its estate, heirs, beneficiaries, personal representatives and executors.
Section 6.11 No Presumption Against Drafting Party. Each of the parties to this Agreement acknowledges that it has been represented by independent counsel in connection with this Agreement and the transactions contemplated by this Agreement. Accordingly, any rule of Law or any legal decision that would require interpretation of any claimed ambiguities in this Agreement against the drafting party has no application and is expressly waived.
Section 6.12 Counterparts. This Agreement may be executed in two or more consecutive counterparts (including by facsimile or email .pdf format), each of which shall be an original, with the same effect as if the signatures thereto and hereto were upon the same instrument, and shall become effective when one or more counterparts have been signed by each of the parties hereto and delivered (by telecopy, email .pdf format or otherwise) to the other parties; provided, however, that if any of the Rollover Shareholders fails for any reason to execute, or perform his or its obligations under, this Agreement, this Agreement shall remain effective as to all parties executing this Agreement.
[Signature Pages to follow]
| C-12 |
IN WITNESS WHEREOF, the parties hereto have duly executed and delivered this Agreement as of the date and year first written above.
| PARENT | ||
| Sinovac (Cayman) Limited | ||
| By: | /s/ Weidong Yin | |
| Name: Weidong Yin | ||
| Title: Director | ||
| HOLDCO | ||
| SINOVAC HOLDING (CAYMAN) LIMITED | ||
| By: | /s/ Weidong Yin | |
| Name: Weidong Yin | ||
| Title: Director | ||
[Signature Page to Support Agreement]
IN WITNESS WHEREOF, the parties hereto have duly executed and delivered this Agreement as of the date and year first written above.
| ROLLOVER SHAREHOLDERS | |
| WEIDONG YIN | |
| /s/ Weidong Yin |
[Signature Page to Support Agreement]
IN WITNESS WHEREOF, the parties hereto have duly executed and delivered this Agreement as of the date and year first written above.
| ROLLOVER SHAREHOLDERS | ||
| SAIF PARTNERS IV L.P. | ||
| By SAIF IV GP, L.P. its general partner | ||
| By SAIF IV GP Capital Ltd., its general partner | ||
| By: | /s/ Andrew Y. Yan | |
| Name: Andrew Y. Yan | ||
| Title: Authorized Signatory | ||
[Signature Page to Support Agreement]
SCHEDULE A
| Owned Securities | ||||||||||||||||||||||
| Name | Notice Address | Shares | Company Options | Company RSs | Rollover Shares | Holdco Shares | ||||||||||||||||
| Chairman | No. 39 Shangdi West Road, Haidian District, Beijing, 100085, China Facsimile: +86 10 6296 6910 E-mail: [email protected] | 6,049,500 | 150,000 | 0 | 6,049,500 | 6,049,500 | ||||||||||||||||
| SAIF | c/o SAIF Advisors Limited Suites 2516-2520, Two Pacific Place, 88 Queensway, Hong Kong Attention: Kenneth Lee Facsimile: +852 2234 9116 E-mail: [email protected] | 10,780,820 | 0 | 0 | 10,780,820 | 10,780,820 | ||||||||||||||||
Annex D
Opinion of Duff & Phelps, as Financial Advisor

|
Confidential |
June 26, 2017 |
Special Committee of Board of Directors
Sinovac Biotech Ltd.
No. 39 Shangdi Xi Road
Haidian District, Beijing, China, 100085
Dear Members of the Special Committee:
Sinovac
Biotech Ltd., a company limited by shares incorporated under the laws of Antigua and Barbuda (the
“Company”), has engaged Duff & Phelps, LLC (“Duff & Phelps”) to serve as an independent
financial advisor to the special committee comprised of independent directors (the “Special Committee”) of the
board of directors (the “Board of Directors”) of the Company (solely in its capacity as the Special
Committee) to provide this opinion (the “Opinion”) as of the date hereof
as to the fairness, from a financial point of view, to the holders of common shares, each with a par value of US$0.001 per share,
of the Company (each, a “Share” and collectively, the “Shares”), other than the Excluded
Shares, the Dissenting Shares and Company RSs (each as defined in the Amalgamation Agreement), of the Per Share Amalgamation Consideration
(as defined below) to be received by such holders in the Proposed Transaction (as defined below) (without giving effect to any
impact of the Proposed Transaction on any particular holder of the Shares other than in their capacity as holders of Shares).
Description of the Proposed Transaction
It is Duff & Phelps’ understanding that the Company, a company limited by shares incorporated under the laws of Antigua and Barbuda, Sinovac (Cayman) Limited, an exempted company incorporated with limited liability under the laws of the Cayman Islands (“Parent”), and Sinovac Amalgamation Sub Limited, an international business corporation incorporated under the laws of Antigua and Barbuda and a wholly-owned Subsidiary of Parent (“Amalgamation Sub”), propose to enter into an amalgamation agreement (the “Amalgamation Agreement”), dated as of the date hereof, the latest draft of which Duff & Phelps has reviewed is dated June 25, 2017. Pursuant to the Amalgamation Agreement, among other things, Amalgamation Sub will be amalgamated with and into the Company (the “Amalgamation”), with the Company surviving the Amalgamation and becoming a wholly-owned subsidiary of Parent as a result of the Amalgamation. In connection with the Amalgamation, each issued and outstanding Share (other than the Excluded Shares, the Dissenting Shares and Company RSs) shall be cancelled in consideration for the right to receive US$7.00 in cash per Share without interest (such amount, the “Per Share Amalgamation Consideration”). The above is collectively referred to as the “Proposed Transaction”. The terms and conditions of the Proposed Transaction are more fully set forth in the Amalgamation Agreement.
|
Duff & Phelps, LLC 311 South Wacker Drive Suite 4200 Chicago, IL 60606 |
T F
|
+1 312 697 4600 +1 312 697 0112 |
www.duffandphelps.com |
| D-1 |
Special Committee of Independent Directors
Sinovac Biotech Ltd.
June 26, 2017
Scope of Analysis
In connection with this Opinion, Duff & Phelps has made such reviews, analyses and inquiries as it has deemed necessary and appropriate under the circumstances. Duff & Phelps also took into account its assessment of general economic, market and financial conditions, as well as its experience in securities and business valuation, in general, and with respect to similar transactions, in particular. Duff & Phelps’ procedures, investigations, and financial analysis with respect to the preparation of this Opinion included, but were not limited to, the items summarized below:
| 1. | Reviewed the following documents: |
| a. | The Company’s annual reports and audited financial statements on Form 20-F filed with the Securities and Exchange Commission (“SEC”) for the years ended December 31, 2014 and 2015; |
| b. | The Company’s unaudited financial statements for the year ended December 31, 2016 contained in draft Form 20-F provided to Duff & Phelps by the management of the Company; |
| c. | A detailed financial projection model for the years ending December 31, 2017 through 2025, prepared and provided to Duff & Phelps by the management of the Company, upon which Duff & Phelps has relied, with your consent, in performing its analysis (the “Management Projections”); |
| d. | Other internal documents relating to the history, past and current operations, financial conditions, and probable future outlook of the Company, provided to Duff & Phelps by the management of the Company; |
| e. | A letter dated June 21, 2017 from the management of the Company, which made certain representations as to the Management Projections and the underlying assumptions with respect to the Company (the “Management Representation Letter”); and |
| f. | Documents related to the Proposed Transaction, including a draft of the Amalgamation Agreement dated June 25, 2017; |
| 2. | Discussed the information referred to above and the background and other elements of the Proposed Transaction with the management of the Company; |
| 3. | Discussed with the management of the Company its plans and intentions with respect to the management and operation of the Company’s business; |
| D-2 |
Special Committee of Independent Directors
Sinovac Biotech Ltd.
June 26, 2017
| 4. | Reviewed the historical trading price and trading volume of the Shares and the publicly traded securities of certain other companies that Duff & Phelps deemed relevant; |
| 5. | Performed certain valuation and comparative analyses using generally accepted valuation and analytical techniques including a discounted cash flow analysis, an analysis of selected public companies that Duff & Phelps deemed relevant, an analysis of selected transactions that Duff & Phelps deemed relevant, and a review of premiums paid in selected transactions that Duff & Phelps deemed relevant; and |
| 6. | Conducted such other analyses and considered such other factors as Duff & Phelps deemed necessary or appropriate. |
Assumptions, Qualifications and Limiting Conditions
In performing its analyses and rendering this Opinion with respect to the Proposed Transaction, Duff & Phelps, with the Company’s and the Special Committee’s consent and without independent verification:
| 1. | Relied upon the accuracy, completeness, and fair presentation of all information, data, advice, opinions and representations obtained from public sources or provided to it from private sources, including the management of the Company; |
| 2. | Relied upon the fact that the Special Committee, the Board of Directors and the Company have been advised by counsel as to all legal matters with respect to the Proposed Transaction, including whether all procedures required by law to be taken in connection with the Proposed Transaction have been duly, validly and timely taken; |
| 3. | Assumed that any estimates, evaluations, forecasts and projections including, without limitation, the Management Projections, furnished to Duff & Phelps were reasonably prepared and based upon the best currently available information and good faith judgment of the person furnishing the same, and Duff & Phelps expresses no view or opinion with respect to such estimates, evaluations, forecasts or projections or their underlying assumptions; |
| 4. | Assumed that the information relating to the Company and the Proposed Transaction provided to Duff & Phelps and representations made by the management of the Company regarding the Company and the Proposed Transaction either orally or in writing are complete and accurate in all material respects, did not and does not omit to state a material fact in respect of the Company and the Proposed Transaction necessary to make the information not misleading in light of the circumstances under which the information was provided; |
| 5. | Assumed that the representations and warranties made by all parties in the Amalgamation Agreement and in the Management Representation Letter are true and correct in all material respects and that each party to the Amalgamation Agreement will fully and duly perform all covenants, undertakings and obligations required to be performed by such party; |
| D-3 |
Special Committee of Independent Directors
Sinovac Biotech Ltd.
June 26, 2017
| 6. | Assumed that the final versions of all documents reviewed by Duff & Phelps in draft form, including the Amalgamation Agreement, will conform in all material respects to the drafts reviewed; |
| 7. | Assumed that there has been no material change in the assets, liabilities, financial condition, cash flows, results of operations, business, or prospects of the Company since the date of the most recent financial statements and other information reviewed by Duff & Phelps, and that there is no information or facts that would make the information reviewed by Duff & Phelps incomplete or misleading; |
| 8. | Assumed that all of the conditions required to implement the Proposed Transaction will be satisfied and that the Proposed Transaction will be completed in accordance with the Amalgamation Agreement without any amendments thereto or any waivers of any terms or conditions thereof, and in a manner that complies in all material respects with all applicable laws; and |
| 9. | Assumed that all governmental, regulatory or other consents and approvals necessary for the consummation of the Proposed Transaction will be obtained without any undue delay, limitation, restriction or condition that would have a material effect on the Company or the contemplated benefits expected to be derived in the Proposed Transaction. |
To the extent that any of the foregoing assumptions or any of the facts on which this Opinion is based prove to be untrue in any material respect, this Opinion cannot and should not be relied upon for any purpose. Furthermore, in Duff & Phelps’ analysis and in connection with the preparation of this Opinion, Duff & Phelps has made numerous assumptions with respect to industry performance, general business, market and economic conditions and other matters, many of which are beyond the control of any party involved in the Proposed Transaction and as to which Duff & Phelps does not express any view or opinion in this Opinion, including as to the reasonableness of such assumptions.
Duff & Phelps has prepared this Opinion effective as of the date hereof. This Opinion is necessarily based upon the information made available to Duff & Phelps as of the date hereof and market, economic, financial and other conditions as they exist and can be evaluated as of the date hereof, and Duff & Phelps disclaims any undertaking or obligation to (i) advise any person of any change in any fact or matter affecting this Opinion which may come or be brought to the attention of Duff & Phelps after the date hereof or (ii) update, revise or reaffirm this Opinion after the date hereof.
Duff & Phelps did not evaluate the Company’s solvency or conduct an independent appraisal or physical inspection of any specific assets, properties or liabilities (contingent or otherwise) of the Company, nor was Duff & Phelps provided with any such appraisal or evaluation other than the contents of the Management Representation Letter. Duff & Phelps did not estimate, and expresses no opinion regarding, the liquidation value of any entity or business. Duff & Phelps has not been requested to, and did not, (i) initiate any discussions with, or solicit any indications of interest from, third parties with respect to the Proposed Transaction, the assets, businesses or operations of the Company, or any alternatives to the Proposed Transaction, or (ii) advise the Special Committee or any other party with respect to alternatives to the Proposed Transaction. Duff & Phelps did not undertake an independent analysis of any potential or actual litigation, regulatory action, possible unasserted claims or other contingent liabilities, to which the Company is or may be a party or is or may be subject, or of any governmental investigation of any possible unasserted claims or other contingent liabilities to which the Company is or may be a party or is or may be subject.
| D-4 |
Special Committee of Independent Directors
Sinovac Biotech Ltd.
June 26, 2017
Duff & Phelps is not expressing any opinion as to the market price or value of the Shares (or anything else) after the announcement or the consummation of the Proposed Transaction (or any other time). This Opinion should not be construed as a valuation opinion, a credit rating, a solvency opinion, an analysis of the Company’s creditworthiness, tax advice, or accounting advice. Duff & Phelps has not made, and assumes no responsibility to make, any representation, or render any opinion, as to any legal matter. Duff & Phelps expressly disclaims any responsibility or liability in this regard.
In rendering this Opinion, Duff & Phelps is not expressing any opinion with respect to the amount or nature or any other aspect of any compensation payable to or to be received by any of the Company’s officers, directors, or employees, or any class of such persons, relative to the Per Share Amalgamation Consideration, or with respect to the fairness of any such compensation. In addition, this Opinion does not address the fairness to, or any other consideration of, the holders of any class of securities, creditors or other constituencies of the Company, other than the holders of the Shares (other than the Excluded Shares, the Dissenting Shares and Company RSs). This Opinion is furnished solely for the use and benefit of the Special Committee in connection with its consideration of the Proposed Transaction and is not intended to, and does not, confer any rights or remedies upon any other person, and is not intended to be used, and may not be used, by any other person or for any other purpose, without Duff & Phelps’ prior written consent. This Opinion (i) does not address the merits of the underlying business decision to enter into the Proposed Transaction versus any alternative strategy or transaction; (ii) does not address any transaction related to the Proposed Transaction; (iii) is not a recommendation as to how the Special Committee, the Board of Directors, the Company or any other person including security holders of the Company as to how such person should vote or act with respect to any matters relating to the Proposed Transaction, or whether to proceed with the Proposed Transaction or any related transaction, and (iv) does not indicate that the Per Share Amalgamation Consideration is the best possibly attainable under any circumstances; instead, it merely states whether the Per Share Amalgamation Consideration is within a range suggested by certain financial analyses. The decision as to whether to proceed with the Proposed Transaction or any related transaction may depend on an assessment of factors unrelated to the financial analysis on which this Opinion is based. This Opinion should not be construed as creating any fiduciary duty on the part of Duff & Phelps to any party.
This Opinion is solely that of Duff & Phelps, and Duff & Phelps’ liability in connection with this Opinion shall be limited in accordance with the terms set forth in the engagement letter among Duff & Phelps, Duff & Phelps Securities, LLC (“DPS”), the Company, and the Special Committee dated April 12, 2016 (the “Engagement Letter”). This Opinion is confidential, and its use and disclosure is strictly limited in accordance with the terms set forth in the Engagement Letter.
| D-5 |
Special Committee of Independent Directors
Sinovac Biotech Ltd.
June 26, 2017
Disclosure of Prior Relationships
DPS has acted as a financial advisor to the Special Committee (solely in its capacity as the Special Committee) providing such financial and market related advice and assistance as requested by the Special Committee in connection with the Proposed Transaction, and will receive a fee for such services. In addition, pursuant to the Engagement Letter, the Company has agreed to reimburse certain expenses of Duff & Phelps and DPS (subject to a cap) and to indemnify Duff & Phelps and DPS for certain liabilities. Duff & Phelps has acted as a financial advisor to the Special Committee (solely in its capacity as the Special Committee) and will receive a fee for its services. No portion of Duff & Phelps’ fee is contingent upon either the conclusion expressed in this Opinion or whether or not the Proposed Transaction is successfully consummated. Pursuant to the terms of the Engagement Letter, a portion of Duff & Phelps’ fee is payable upon execution of the Engagement Letter, and a portion of Duff & Phelps’ fee is payable upon Duff & Phelps’ rendering of its Opinion. Other than this engagement, during the two years preceding the date of this Opinion, Duff & Phelps has not had any material relationship with any party to the Proposed Transaction for which compensation has been received or is intended to be received, nor is any such material relationship or related compensation mutually understood to be contemplated.
Conclusion
Based upon and subject to the foregoing, Duff & Phelps is of the opinion that as of the date hereof, the Per Share Amalgamation Consideration to be received by the holders of Shares (other than the Excluded Shares, the Dissenting Shares and Company RSs) in the Proposed Transaction is fair, from a financial point of view, to such holders (without giving effect to any impact of the Proposed Transaction on any particular holder of the Shares other than in its capacity as a holder of Shares).
This Opinion has been approved by the Opinion Review Committee of Duff & Phelps.
Respectfully submitted,
| /s/ Duff & Phelps, LLC | |
| Duff & Phelps, LLC |
| D-6 |
Annex E
Directors and Executive Officers of Each Filing Person
| 1. | Directors and Executive Officers of the Company |
Set forth below are the name, telephone number, address, citizenship and respective present principal occupation or employment of each director and executive officer of the Company, the name of the corporation or other organization in which such occupation or employment is conducted and the employment history of each such director and executive officer:
| Name | Telephone | Business Address |
Present Principal Employment |
Citizenship | ||||
| Weidong Yin | * | * | Chairman, President, Chief Executive Officer | People’s Republic of China | ||||
| Simon Anderson | * | * | Independent Director | Canada | ||||
| Yuk Lam Lo | * | * | Independent Director | Hong Kong | ||||
| Kenneth Lee | * | * | Independent Director | Hong Kong | ||||
| Meng Mei | * | * | Independent Director | People’s Republic of China | ||||
| Nan Wang | * | * | Chief Financial Officer, Vice President | People’s Republic of China | ||||
| Ming Xia | * | * | Vice President, Sales and Marketing | People’s Republic of China | ||||
| Qiang Gao | * | * | Vice President, Research and Development | People’s Republic of China | ||||
| Jing Li | * | * | Vice President, Quality and Production | People’s Republic of China |
* The business address and telephone number of each of our directors and executive officers is No. 39 Shangdi Xi Road, Haidian District, Beijing 100085, People’s Republic of China, Tel: +86.10.8289.0088
Mr. Weidong Yin has served as the Company’s chairman, president, chief executive officer and secretary since September 2003. He previously worked as a medical doctor in infectious disease at the China Center for Disease Control and Prevention, Tangshan City, Hebei province. Mr. Yin has been dedicated to hepatitis research for over 20 years and was instrumental in the development of Healive. In addition, Mr. Yin has been appointed as the principal investigator by the Chinese Ministry of Science and Technology for many key governmental R&D programs such as Inactivated Hepatitis A Vaccine R&D, Inactivated SARS Vaccine R&D and New Human Influenza Vaccine (H5N1) R&D. He is also the president of Zhongguancun Listed Companies Association. He obtained his MBA from the National University of Singapore.
| E-1 |
Mr. Simon Anderson has served as an independent director of the Company since July 2004. Mr. Anderson is a member of the audit, compensation and corporate governance and nominating committees. Mr. Anderson advises companies listed on North American stock exchanges and private businesses in the areas of regulatory compliance, exchange listings and financial operations. He is a member of the Chartered Professional Accountants of British Columbia, having qualified as a Chartered Accountant in 1986. Mr. Anderson serves as a director of IBC Advanced Alloys Corp., which manufactures and processes alloys at its U.S. plants.
Mr. Yuk Lam Lo has served as an independent director of the Company since March 2006. Mr. Lo is a member of the audit, compensation and corporate governance and nominating committees. Currently Mr. Lo is serving as the Chairman of the Advisory Council for Food Safety of the Food and Health Bureau HKSAR, an Executive Committee Member of the Chinese Manufacturers’ Association of Hong Kong (CMA) and Chairman of the Education Committee of CMA. Mr. Lo is also the Honorary Founding Chairman of Hong Kong Bio-Organization. In the educational area, Mr. Lo has been elected an Honorary Fellow of the Hong Kong University of Science and Technology. He is an Honorary Chairman of Hong Kong Food Safety Association, Adjunct Professor of the Chinese University of Hong Kong and Honorary Professor of several universities in China. Mr. Lo was heavily involved in several committees of the HKSAR Government. He had been appointed as Director of the Hong Kong Applied R&D Fund Co. Ltd., Chairman of the Biotechnology Committee of the Hong Kong Industry & Technology Development Council, and Chairman of Biotechnology Projects Vetting Committee of the Innovation and Technology Fund, HKSAR. In China, Mr. Lo is a Member of Chinese People’s Political Consultative Conference in Jilin province, and a Consultant of the Centre for Disease Control and Prevention of China. In the business sector, he is an Independent Director of Luye Pharma Group Limited (2186.HK) and CSPC Pharmaceutical Group Limited (1093.HK).
Mr. Kenneth Lee is an independent director of the Company. He has served on the board of directors of the Company since May 2011. In July 2012, the board of directors appointed him as a member of the compensation committee and corporate governance and nominating committee. Mr. Lee is a partner at SAIF Partners. SAIF Partners IV L.P. is currently the largest shareholder of Sinovac. Mr. Lee has more than 20 years of experience across private equity investments, corporate finance and business development in China. He is a non-executive director on the boards of three Chinese portfolio companies publicly listed on the stock exchanges in the United States and Hong Kong and a board director for four other private Chinese companies backed by SAIF Partners. Mr. Lee is a graduate of Amherst College.
| E-2 |
Mr. Meng Mei has served as an independent director of the Company since March 2012. Mr. Mei is the chairman of compensation committee, and member of the audit and corporate governance and nominating committees. Mr. Mei founded TusPark, a science park established by Tsinghua University in 1994, to incubate high growth companies. He has been the director of TusPark’s development center since its inception. Mr. Mei is also the Chairman of TusHoldings Co., Ltd., which is engaged in the development, construction and management of TusPark and is providing services to enterprises based in TusPark. TusHoldings Co., Ltd. is also involved in venture capital investments in China. Mr. Mei sits on the judging expert panel of China’s National Science & Technology Award. He has developed courses on entrepreneurship and new venture formation as a Tsinghua University professor and an entrepreneur. Mr. Mei holds a bachelor’s degree in automation from Tsinghua University, PRC.
Ms. Nan Wang has served as the Company’s chief financial officer since June 2013. Ms. Wang served as the vice president of Beijing Sinovac from 2001 to 2013 where she oversaw business development, investment and clinical research. From 1988 to 1993, Ms. Wang was a researcher in biology at the Life Science College of Peking University, PRC. From 1993 to 2001, she worked as a manager at SinoBioway. Ms. Wang received her bachelor’s degree in biology from Peking University and her master’s degree from University of International Business and Economics, PRC. Ms. Wang also received a diploma in financial management from Beijing College for Entrepreneurs, PRC in 2003.
Mr. Ming Xia has served as the Company’s vice president since April 2016. Mr. Xia has served as the vice president of Beijing Sinovac since 2011 where he oversees sales and marketing departments. Mr. Xia has over 15 years’ experience in vaccine sales and marketing in China. He joined Sinovac in 2002 and has served as Regional Sales Manager, National Sales Manager and Sales Director at Sinovac. Mr. Xia obtained his bachelor degrees in Biochemistry at Anhui University and in International Trade at Shanghai Institute of Foreign Trade. Mr. Xia has made significant contributions to our sales revenue growth in previous years with outstanding leadership and performance results. He kept his top record of generating sales revenue for many years after joining Sinovac. He is a leader with creativity and developed the sales strategy for the Company’s existing products. He is also involved in sales and marketing strategy.
Mr. Qiang Gao has served as the Company’s vice president since April 2016. Mr. Gao joined Beijing Sinovac in 2002 and has served as quality control manager, quality assurance manager, R&D manager and R&D director at Beijing Sinovac in the past years, and the general manager of Sinovac R&D since 2010. He is responsible for developing our new vaccine products, including EV71 vaccine. Mr. Gao received a master’s degree and a bachelor’s degree in microbiology from the University of Agriculture, PRC.
Ms. Jing Li has served as our vice president since April 2016. Ms. Li was named as quality person of Beijing Sinovac in March 2015. Since she joined Beijing Sinovac in 2003, she has worked in different roles in production and quality function, including quality assurance vice manager, department manager of hepatitis A vaccine production and director of vaccine production at Beijing Sinovac. Ms. Li is also in charge of the production of our EV71 vaccine. Ms. Li received a master’s degree in physiology from the University of Agriculture, PRC.
| E-3 |
| 2. | Directors and Executive Officers of Holdco, Parent and Amalgamation Sub |
Holdco, which is wholly owned by Mr. Yin, is an exempted company incorporated with limited liability under the laws of the Cayman Islands with its registered office located at the offices of Maples Corporate Services Limited, PO Box 309, Ugland House, Grand Cayman, KY1-1104, Cayman Islands. The business address of Holdco is located at c/o No. 39 Shangdi Xi Road, Haidian District, Beijing 100085, People’s Republic of China and its telephone number is +86-10-8289-0088. Holdco was formed solely for the purpose of holding equity interests in Parent and arranging the investment and financing transactions related to the Transactions and completing the Transactions, including the Amalgamation.
Parent, which is wholly owned by Holdco, is an exempted company incorporated with limited liability under the laws of the Cayman Islands with its registered office located at the offices of Maples Corporate Services Limited, PO Box 309, Ugland House, Grand Cayman, KY1-1104, Cayman Islands. The business address of Parent is located at c/o No. 39 Shangdi Xi Road, Haidian District, Beijing 100085, People’s Republic of China and its telephone number is +86-10-8289-0088. Parent was formed solely for the purposes of holding equity interests in Amalgamation Sub.
Amalgamation Sub, which is wholly owned by Parent, is an international business corporation incorporated under the laws of Antigua and Barbuda with its registered office located at the offices of CMT Corporate Services Limited, 60 Nevis Street, St Johns, Antigua. The business address of Amalgamation Sub is located at c/o No. 39 Shangdi Xi Road, Haidian District, Beijing 100085, People’s Republic of China and its telephone number is +86-10-8289-0088. Amalgamation Sub was formed solely for the purpose of effecting the Amalgamation.
The following table sets forth information regarding the sole director of each of Holdco, Parent and Amalgamation Sub as of the date of this proxy statement:
| Name | Business Address | Present Principal Employment |
Material Occupation for Past Five Years |
Citizenship | ||||
| Weidong Yin | c/o No. 39 Shangdi Xi Road, Haidian District, Beijing 100085, People’s Republic of China | Chairman, president and chief executive officer of the Company | Chairman, president and chief executive officer of the Company | People’s Republic of China |
None of Holdco, Parent and Amalgamation Sub has any executive officer as of the date of this proxy statement.
During the last five years, none of the persons above has been (i) convicted in a criminal proceeding (excluding traffic violations or similar misdemeanors) or (ii) a party to any judicial or administrative proceeding (except for matters that were dismissed without sanction or settlement) that resulted in a judgment or decree or final order enjoining the person from future violations of, or prohibiting activities subject to, federal or state securities laws, or a finding of any violation of federal or state securities laws.
| E-4 |
Directors and Executive Officers of SAIF
SAIF is an exempted limited partnership registered and existing under the laws of the Cayman Islands with its registered office located at c/o Maples Corporate Services Limited, P.O. Box 309, Ugland House, Grand Cayman, KY1-1104, Cayman Islands. The business address of SAIF is located at c/o SAIF Advisors Limited, Suites 2516-2520, Two Pacific Place, 88 Queensway, Hong Kong and its telephone number is +852-2918-2200. SAIF was formed for the purpose of making investment in companies based in or having a principal place of business in the Asia-Pacific region.
The general partner of SAIF is SAIF IV GP, L.P., an exempted limited partnership registered and existing under the laws of the Cayman Islands, and the general partner of SAIF IV GP, L.P. is SAIF IV GP Capital Ltd., an exempted company incorporated and existing under the laws of the Cayman Islands. Andrew Y. Yan is the sole director and shareholder of SAIF IV GP Capital Ltd.
The following table sets forth information regarding the sole director of SAIF IV GP Capital Ltd. as of the date of this proxy statement:
| Name | Business Address | Present Principal Employment |
Material Occupations for Past Five Years |
Citizenship | ||||
| Andrew Y. Yan | c/o SAIF Advisors Limited, Suites 2516-2520, Two Pacific Place, 88 Queensway, Hong Kong | Managing Partner, SAIF Partners | Managing Partner, SAIF Partners | Hong Kong |
SAIF does not have any executive officers as of the date of this proxy statement.
During the last five years, none of SAIF, or any person listed above has been (i) convicted in a criminal proceeding (excluding traffic violations or similar misdemeanors) or (ii) a party to any judicial or administrative proceeding (except for matters that were dismissed without sanction or settlement) that resulted in a judgment, decree or final order enjoining the person from future violations of, or prohibiting activities subject to, federal or state securities laws, or a finding of any violation of federal or state securities laws.
Directors and Executive Officers of C-Bridge Capital
C-Bridge Capital is an exempted limited partnership registered and existing under the laws of the Cayman Islands with its registered office located at c/o Maples Corporate Services Limited, P.O. Box 309, Ugland House, Grand Cayman, KY1-1104, Cayman Islands. The business address of C-Bridge Capital is located at Room 7/03 Workingberg Commercial Building, 41-47 Marble Road, North Point, Hong Kong and its telephone number is +86-21-8012-7764. C-Bridge Capital was formed for the purpose of making equity investment in companies in the healthcare industry.
The general partner of C-Bridge Capital is C-Bridge Healthcare Fund GP II, L.P., an exempted limited partnership registered and existing under the laws of the Cayman Islands, and the general partner of C-Bridge Healthcare Fund GP II, L.P. is C-Bridge Capital GP. Ltd., an exempted company incorporated and existing under the laws of the Cayman Islands. The business address of C-Bridge Capital GP. Ltd. is located at Room 7/03 Workingberg Commercial Building, 41-47 Marble Road, North Point, Hong Kong and its telephone number is +86-21-8012-7764.
| E-5 |
The following table sets forth information regarding the sole director of C-Bridge Healthcare Fund GP. Ltd. as of the date of this proxy statement:
| Name | Business Address | Present Principal Employment |
Material Occupations for Past Five Years |
Citizenship | ||||
| Fu Wei | Room 7/03 Workingberg Commercial Building, 41-47 Marble Road, North Point, Hong Kong | Director | General Manager of Far East Horizon International | Singapore | ||||
During the last five years, none of C-Bridge Capital, or any person listed above has been (i) convicted in a criminal proceeding (excluding traffic violations or similar misdemeanors) or (ii) a party to any judicial or administrative proceeding (except for matters that were dismissed without sanction or settlement) that resulted in a judgment, decree or final order enjoining the person from future violations of, or prohibiting activities subject to, federal or state securities laws, or a finding of any violation of federal or state securities laws.
Directors and Executive Officers of Advantech Capital
Advantech Capital is an exempted limited partnership registered and existing under the laws of the Cayman Islands with its registered office located at 190 Elgin Avenue, George Town, Grand Cayman KY1-9005, Cayman Islands. The business address of Advantech Capital is located at 190 Elgin Avenue, George Town, Grand Cayman KY1-9005, Cayman Islands and its telephone number is +852-2801-6988. Advantech Capital was formed for the purpose of making equity investment in companies.
The general partner of Advantech Capital is Advantech Capital Partner Ltd., an exempted company incorporated and existing under the laws of the Cayman Islands. The business address of Advantech Capital Partner Ltd. is located at 190 Elgin Avenue, George Town, Grand Cayman KY1-9005, Cayman Islands and its telephone number is +852-2801-6988.
The following table sets forth information regarding the directors of Advantech Capital Partner Ltd. as of the date of this proxy statement:
| Name | Business Address | Present Principal Employment |
Material Occupations for Past Five Years |
Citizenship | ||||
| Hebert Kee Chan Pang | 1702-03, 17/F, One Exchange Square, 8 Connaught Place, Central, Hong Kong | Director |
Partner, Chief Financial Officer, New Horizon Capital |
Malaysia | ||||
| Kok Wai Wong | 1702-03, 17/F, One Exchange Square, 8 Connaught Place, Central, Hong Kong | Director | Partner, Investor Relations, New Horizon Capital | Singapore | ||||
| Jianming Yu | 1702-03, 17/F, One Exchange Square, 8 Connaught Place, Central, Hong Kong | Director |
Managing Partner, New Horizon Capital |
Hong Kong | ||||
| Don Wayne Ebanks | DMS House, 20 Genesis Close, George Town, Grand Cayman KY1-1103, Cayman Islands | Director | Executive Director, DMS Offshore Investment Services | Cayman Islands | ||||
| Roger Howard Hanson | DMS House, 20 Genesis Close, George Town, Grand Cayman KY1-1103, Cayman Islands | Director |
Director, DMS Offshore Investment Services |
Cayman Islands |
| E-6 |
Advantech Capital does not have any executive officers as of the date of this proxy statement.
During the last five years, none of Advantech Capital, or any person listed above has been (i) convicted in a criminal proceeding (excluding traffic violations or similar misdemeanors) or (ii) a party to any judicial or administrative proceeding (except for matters that were dismissed without sanction or settlement) that resulted in a judgment, decree or final order enjoining the person from future violations of, or prohibiting activities subject to, federal or state securities laws, or a finding of any violation of federal or state securities laws.
Directors and Executive Officers of Vivo Capital Fund
Vivo Capital Fund is a limited partnership organized and existing under the laws of the State of Delaware with its registered office located at 1209 Orange Street, Wilmington, County of New Castle, Delaware 19801. The business address of Vivo Capital Fund is located at 505 Hamilton Avenue, Suite 207, Palo Alto, CA 94301 and its telephone number is +1 650-688-0818. Vivo Capital Fund was formed for the purpose of making investment.
The general partner of Vivo Capital Fund is Vivo Capital VIII, LLC, a limited liability company registered and existing under the laws of the State of Delaware. The voting members of Vivo Capital VIII, LLC are Frank Kung, Albert Cha, Shan Fu, Chen Yu and Edgar Engleman.
The following table sets forth information regarding the voting members of Vivo Capital VIII, LLC as of the date of this proxy statement:
| Name | Business Address | Present Principal Employment |
Material Occupations for Past Five Years |
Citizenship | ||||
| Frank Kung | c/o Vivo Capital VIII, LLC, 505 Hamilton Avenue, Suite 207, Palo Alto, CA 94301 | Managing Partner, Vivo Capital VIII, LLC | Managing Partner, Vivo Capital, LLC | United States | ||||
| Albert Cha | c/o Vivo Capital VIII, LLC, 505 Hamilton Avenue, Suite 207, Palo Alto, CA 94301 | Managing Partner, Vivo Capital VIII, LLC | Managing Partner, Vivo Capital, LLC | United States | ||||
| Shan Fu | c/o Vivo Capital VIII, LLC, 505 Hamilton Avenue, Suite 207, Palo Alto, CA 94301 | Managing Partner, Vivo Capital VIII, LLC | Managing Partner, Vivo Capital, LLC | United States | ||||
| Chen Yu | c/o Vivo Capital VIII, LLC, 505 Hamilton Avenue, Suite 207, Palo Alto, CA 94301 | Managing Partner, Vivo Capital VIII, LLC | Managing Partner, Vivo Capital, LLC | United States | ||||
| Edgar Engleman | c/o Vivo Capital VIII, LLC, 505 Hamilton Avenue, Suite 207, Palo Alto, CA 94301 | Managing Partner, Vivo Capital VIII, LLC | Managing Partner, Vivo Capital, LLC | United States |
Vivo Capital Fund does not have any executive officers as of the date of this proxy statement.
| E-7 |
During the last five years, none of Vivo Capital Fund, or any person listed above has been (i) convicted in a criminal proceeding (excluding traffic violations or similar misdemeanors) or (ii) a party to any judicial or administrative proceeding (except for matters that were dismissed without sanction or settlement) that resulted in a judgment, decree or final order enjoining the person from future violations of, or prohibiting activities subject to, federal or state securities laws, or a finding of any violation of federal or state securities laws.
Directors and Executive Officers of Vivo Capital Surplus Fund
Vivo Capital Surplus Fund is a limited partnership organized and existing under the laws of the State of Delaware with its registered office located at 1209 Orange Street, Wilmington, County of New Castle, Delaware 19801. The business address of Vivo Capital Surplus Fund is located at 505 Hamilton Avenue, Suite 207, Palo Alto, CA 94301 and its telephone number is +1 650-688-0818. Vivo Capital Surplus Fund was formed for the purpose of making investment.
The general partner of Vivo Capital Surplus Fund is Vivo Capital VIII, LLC, a limited liability company registered and existing under the laws of the State of Delaware. The voting members of Vivo Capital VIII, LLC are Frank Kung, Albert Cha, Shan Fu, Chen Yu and Edgar Engleman.
The following table sets forth information regarding the voting members of Vivo Capital VIII, LLC as of the date of this proxy statement:
| Name | Business Address | Present Principal Employment |
Material Occupations for Past Five Years |
Citizenship | ||||
| Frank Kung | c/o Vivo Capital VIII, LLC, 505 Hamilton Avenue, Suite 207, Palo Alto, CA 94301 | Managing Partner, Vivo Capital VIII, LLC | Managing Partner, Vivo Capital, LLC | United States | ||||
| Albert Cha | c/o Vivo Capital VIII, LLC, 505 Hamilton Avenue, Suite 207, Palo Alto, CA 94301 | Managing Partner, Vivo Capital VIII, LLC | Managing Partner, Vivo Capital, LLC | United States | ||||
| Shan Fu | c/o Vivo Capital VIII, LLC, 505 Hamilton Avenue, Suite 207, Palo Alto, CA 94301 | Managing Partner, Vivo Capital VIII, LLC | Managing Partner, Vivo Capital, LLC | United States | ||||
| Chen Yu | c/o Vivo Capital VIII, LLC, 505 Hamilton Avenue, Suite 207, Palo Alto, CA 94301 | Managing Partner, Vivo Capital VIII, LLC | Managing Partner, Vivo Capital, LLC | United States | ||||
| Edgar Engleman | c/o Vivo Capital VIII, LLC, 505 Hamilton Avenue, Suite 207, Palo Alto, CA 94301 | Managing Partner, Vivo Capital VIII, LLC | Managing Partner, Vivo Capital, LLC | United States |
Vivo Capital Surplus Fund does not have any executive officers as of the date of this proxy statement.
During the last five years, none of Vivo Capital Surplus Fund, or any person listed above has been (i) convicted in a criminal proceeding (excluding traffic violations or similar misdemeanors) or (ii) a party to any judicial or administrative proceeding (except for matters that were dismissed without sanction or settlement) that resulted in a judgment, decree or final order enjoining the person from future violations of, or prohibiting activities subject to, federal or state securities laws, or a finding of any violation of federal or state securities laws.
| E-8 |
Exhibit (c)-(2)

Sinovac Biotech Ltd. Fairness Analysis Presented to the Special Committee of Independent Directors June 26 , 2017 The information contained herein is of a confidential nature and is intended for the use of the persons or firm to whom it is fu rnished by us. Reproduction, publication, or dissemination of any portion hereof may not be made without prior approval of Duff & Phelps, LL C a nd its affiliates.

2 CONFIDENTIAL Duff & Phelps Disclaimer • The following pages contain materials that are being provided by Duff & Phelps, LLC (“ Duff & Phelps ”) to the special committee of independent directors (the “ Special Committee ”) of the board of directors (the “ Board of Directors ”) (solely in its capacity as the Special Committee) of Sinovac Biotech Ltd . (“ S inovac ” or the “ Company ”) in the context of a meeting of the Special Committee held to consider the Proposed Transaction (as defined herein) . • The accompanying materials are, and any Opinion (as defined herein) will be, compiled and presented on a confidential basis, solely for the use and benefit of the Special Committee in connection with its evaluation of the Proposed Transaction and may not be, in whole or in part, distributed to any other party, publicly disclosed, or relied upon for any other purpose without the prior written consent of Duff & Phelps . • Because these materials were prepared for use in the context of an oral presentation to the Special Committee, whose members are familiar with the business and affairs of the Company, neither Duff & Phelps nor any of its respective legal or financial advisors or accountants, take any responsibility for the accuracy or completeness of any of the accompanying materials if used by persons other than the Special Committee . • These materials are not intended to represent an Opinion and shall not be treated, construed, used or relied upon in any way as an opinion, report, appraisal relating to the fairness of the Proposed Transaction . These materials are intended to serve as discussion materials for the Special Committee and as a summary of the basis upon which Duff & Phelps may render an Opinion, and are incomplete without reference to, and should be viewed solely in conjunction with, the discussion between Duff & Phelps and the Special Committee . • The accompanying material does not, and any Opinion provided by Duff & Phelps would not : (i) address the merits of the underlying business decision of the Special Committee, the Board of Directors or the Company or any other party to the Proposed Transaction to enter into the Proposed Transaction versus any alternative strategy or transaction ; (ii) constitute a recommendation to the Special Committee, the Board of Directors, the Company or any other person including security holders of the Company as to how such person should vote or as to any other specific action that should be taken in connection with the Proposed Transaction ; or (iii) create any fiduciary duty on Duff & Phelps’ part to any party . • The information utilized in preparing this presentation was obtained from the Company and from public sources under the assumption that they are complete and accurate as of the date of provision . Duff & Phelps did not independently verify such information . Any estimates and forecasts contained herein have been prepared by or are based on discussions with the senior management of the Company and involve numerous and significant subjective determinations as of the date hereof, which may or may not prove to be correct . No representation or warranty, expressed or implied, is made as to the accuracy or completeness of such information and nothing contained herein is, or shall be relied upon as, a representation or warranty, whether as to the past or the future . • No selected company or selected transaction used in our analysis is directly comparable to the Company or the Proposed Transaction . • Duff & Phelps’ affiliate, Duff & Phelps Securities, LLC (“ DPS ”), has acted as financial advisor to the Special Committee providing such financial and market related advice and assistance as requested by the Special Committee in connection with the Proposed Transaction, and will receive a fee for certain investment banking services if requested by the Special Committee .

3 CONFIDENTIAL Table of Contents 1. Introduction and Transaction Overview 2. Valuation Analysis – Discounted Cash Flow Analysis – Selected Public Companies / M&A Transactions Analysis Appendix 1. Assumptions, Qualifications, and Limiting Conditions 2. Summary of Premiums Paid – Supplemental

Introduction and Transaction Overview Section 01

5 CONFIDENTIAL Introduction and Transaction Overview The Engagement • Duff & Phelps was retained by the Special Committee to serve as independent financial advisor to the Special Committee (solely in its capacity as such) . • Specifically, Duff & Phelps has been asked to provide an opinion (the “ Opinion ”) to the Special Committee as to the fairness, from a financial point of view, to the holders of common shares, each with a par value of US $ 0 . 001 per share, of the Company (each, a “ Share ” and collectively, the “ Shares ”), other than the Excluded Shares, the Dissenting Shares and Company RSs (each as defined in the Amalgamation Agreement), of the Per Share Amalgamation Consideration (as defined below) to be received by such holders in the Proposed Transaction (as defined below) (without giving effect to any impact of the Proposed Transaction on any particular holder of the Shares other than in their capacity as holders of Shares) . The Proposed Transaction • It is Duff & Phelps’ understanding that the Company, a company limited by shares incorporated under the laws of Antigua and Barbuda, Sinovac (Cayman) Limited, an exempted company incorporated with limited liability under the laws of the Cayman Islands (“ Parent ”), and Sinovac Amalgamation Sub Limited, an international business corporation incorporated under the laws of Antigua and Barbuda and a wholly - owned Subsidiary of Parent (“ Amalgamation Sub ”), propose to enter into an amalgamation agreement (the “ Amalgamation Agreement ”), dated as of the date hereof, the latest draft of which Duff & Phelps has reviewed is dated June 25 , 2017 . • Pursuant to the Amalgamation Agreement, among other things, Amalgamation Sub will be amalgamated with and into the Company (the “ Amalgamation ”), with the Company surviving the Amalgamation and becoming a wholly - owned subsidiary of Parent as a result of the Amalgamation . • In connection with the Amalgamation, each issued and outstanding Share (other than the Excluded Shares, the Dissenting Shares and Company RSs) shall be cancelled in consideration for the right to receive US $ 7 . 00 in cash per Share without interest (such amount, the “ Per Share Amalgamation Consideration ”) . The above is collectively referred to as the “ Proposed Transaction ” . The terms and conditions of the Proposed Transaction are more fully set forth in the Amalgamation Agreement .

6 CONFIDENTIAL Introduction and Transaction Overview Scope of Analysis Duff & Phelps has made such reviews, analyses and inquiries as it has deemed necessary and appropriate under the circumstances . Duff & Phelps also took into account its assessment of general economic, market and financial conditions, as well as its experience in securities and business valuation, in general, and with respect to similar transactions, in particular . Duff & Phelps’ procedures, investigations, and financial analysis with respect to the preparation of its analysis included, but were not limited to, the items summarized below : 1. Reviewed the following documents : - The Company’s annual reports and audited financial statements on Form 20 - F filed with the Securities and Exchange Commission (“ SEC ”) for the years ended December 31 , 2014 and 2015 ; - The Company’s unaudited financial statements for the year ended December 31 , 2016 contained in draft Form 20 - F provided to Duff & Phelps by the management of the Company ; - A detailed financial projection model for the years ending December 31 , 2017 through 2025 , prepared and provided to Duff & Phelps by the management of the Company, upon which Duff & Phelps has relied, with your consent, in performing its analysis (the “ Management Projections ”) ; - Other internal documents relating to the history, past and current operations, financial conditions, and probable future outlook of the Company, provided to Duff & Phelps by the management of the Company ; - A letter dated June 21 , 2017 from the management of the Company, which made certain representations as to the Management Projections and the underlying assumptions with respect to the Company (the “ Management Representation Letter ”) ; and - Documents related to the Proposed Transaction, including a draft of the Amalgamation Agreement dated June 25 , 2017 ; 2. Discussed the information referred to above and the background and other elements of the Proposed Transaction with the management of the Company ; 3. Discussed with the management of the Company its plans and intentions with respect to the management and operation of the Company’s business ; 4. Reviewed the historical trading price and trading volume of the Shares and the publicly traded securities of certain other companies that Duff & Phelps deemed relevant ; 5. Performed certain valuation and comparative analyses using generally accepted valuation and analytical techniques including a discounted cash flow analysis, an analysis of selected public companies that Duff & Phelps deemed relevant, an analysis of selected transactions that Duff & Phelps deemed relevant, and a review of premiums paid in selected transactions that Duff & Phelps deemed relevant ; and 6. Conducted such other analyses and considered such other factors as Duff & Phelps deemed necessary or appropriate .

7 CONFIDENTIAL Introduction and Transaction Overview Ownership Summary Fully Diluted Ownership Source: Company filings, Capital IQ, Company Management Buyer Consortium 28.7% Individual Investor 0.6% Total Institutional Investors 36.9% Public & Other Shareholders 31.0% RSUs & Options 2.9% Sinovac Biotech Ltd. - Ownership % of Fully Diluted Current Shareholders Common Shares Ownership Buyer Consortium SAIF Partners 10,780,820 18.4% Yin, Weidong (Chairman and CEO) 6,049,500 10.3% Buyer Consortium 16,830,320 28.7% Individual Investor Xia, Ming (Vice President of Sales & Marketing) 141,000 0.2% Wang, Nan (CFO) 105,000 0.2% Lo, Yuk Lam (Independent Director) 50,000 0.1% Anderson Simon J. (Independent Director) 50,000 0.1% Individual Investor 346,000 0.6% Top 15 Institutional Investors 1Globe Capital LLC 9,353,092 15.9% Chiang Li Family 3,459,763 5.9% Morgan Stanley, Investment Banking and Brokerage Investments2,721,912 4.6% OrbiMed Advisors, L.L.C. 2,667,500 4.5% Renaissance Technologies Corp. 1,679,490 2.9% CM-CIC Asset Management 231,700 0.4% Two Sigma Investments, LP 191,538 0.3% Massachusetts Financial Services Company 184,855 0.3% Eqis Capital Management Inc. 155,796 0.3% UOB Asset Management Ltd 117,720 0.2% GLG Partners, Inc. 115,446 0.2% Invesco PowerShares Capital Management LLC 99,086 0.2% Sectoral Asset Management Inc 84,410 0.1% California Public Employees' Retirement System 62,000 0.1% British Columbia Investment Management Corporation 59,210 0.1% Top 15 Institutional Investors 21,183,518 36.1% Total Institutional Investors 21,641,120 36.9% Public & Other Shareholders 18,201,821 31.0% Total Shares Outstanding 57,019,261 97.1% RSUs & Options In-the-Money at Offer Price 1,675,400 2.9% Fully Diluted Shares Outstanding at Offer Price 58,694,661 100.0%

8 CONFIDENTIAL Introduction and Transaction Overview Trading Analysis Source: Capital IQ Sinovac Biotech Ltd. - Trading History J anuary 22 , 201 6 to June 23 , 2017 Sinovac Biotech Ltd. Historical Trading Metrics (in thousands, except per share) During one year prior to Offer Post offer Average Closing Price $5.25 Average Closing Price $5.92 High $6.18 High $7.12 Low $4.49 Low $4.64 Average Volume 104.9 Average Volume 96.7 % of Shares Issued and Outstanding 0.2% % of Shares Issued and Outstanding 0.2% % of Float 0.5% % of Float 0.4% 0.0 500.0 1,000.0 1,500.0 2,000.0 2,500.0 3,000.0 3,500.0 $2.00 $3.00 $4.00 $5.00 $6.00 $7.00 $8.00 $9.00 Volume (thousands) Share Price Volume Price 30-day Trailing VWAP 60-day Trailing VWAP 90-day Trailing VWAP Offer (1) Trailing Volume Weighted Average Price (VWAP) at Offer Current (6/23/17) Offer Price Premium Relative to: 22.8% 30 - Day VWAP (1) 32.1% 30.0% 31.6% 60 - Day VWAP (1) 90 - Day VWAP (1) One Day Prior 39.4% $5.70 $5.30 $5.39 $5.32 $5.02 February 1, 2016 Sinovac Biotech Ltd. received a non - binding proposal from Mr. Weidong Yin, chairman, president and CEO of the Company, and SAIF Partners IV L.P. and/or its affiliates to acquire all outstanding common shares of the Company not beneficially owned by the consortium members for $6.18 in cash per common share . August 24, 2016 Sinovac Biotech Ltd. reported Q2'16 earning results. The Company reported revenue of USD 1.4 million and net loss of USD 0.17 per share . February 8 , 2017 Sinovac Biotech Ltd. reported Q3'16 earning results. The Company reported revenue of USD 28.7 million and net income of USD 0.06 per share . May 16 , 2017 Sinovac Biotech Ltd. received Non - Compliance Notice from NASDAQ Decemer 23 , 2016 Sinovac Biotech Ltd.’s Audit Committee authorized the commencement of an internal investigation into the allegations made in the research report by Geoinvesting February 4 , 2016 Sinovac Biotech Ltd. received a non - binding proposal from a consortium comprised of PKU V - Ming, Shandong Sinobioway CICC Qianhai, Beijing Sinobioway, Heng Feng Investments and Fuerde Global Investment Limited to acquire all outstanding common shares of the Company for $7.00 in cash per common share . April 24, 2016 Sinovac Biotech Ltd. reported FY15 earning results. The Company reported revenue of USD 67.4 million and net loss of USD 0.02 per share . June 2, 2016 Sinovac Biotech Ltd. reported Q1'16 earning results. The Company reported revenue of USD 11.0 million and net income of USD 0.02 per share .

9 CONFIDENTIAL (1) Includes noncontrolling interest in Sinovac Beijing and Sinovac Dalian Note: Balance sheet data and LTM as of D ecember 31, 2016. Financial performance metrics presented are adjusted to exclude public company costs and non - recurring income (expens es). Introduction and Transaction Overview Proposed Transaction Sinovac Biotech Ltd. - Offer Premium Share Implied Price Premium Share price of 6/23/17 $5.70 22.8% One-day prior to Offer (1/29/16) $5.02 39.4% 30-days trailing VWAP at Offer $5.30 32.1% 60-days trailing VWAP at Offer $5.39 30.0% 90-days trailing VWAP at Offer $5.32 31.6% Offer Price $7.00 Sinovac Biotech Ltd. - Implied Multiples (USD in millions, except per share data) Offer $7.00 Fully Diluted Shares Issued and Outstanding (millions) 58.7 1.00 Implied Fully Diluted Equity Value $410.9 Plus: Short-term Loans $34.1 34.1 Plus: Long-term Debt $9.6 9.6 Plus: Other Non-current Liabilities $0.4 0.4 Plus: Noncontrolling Interest (1) $143.3 143.3 Less: Cash -66.459477 (66.5) Less: Proceeds from Exercise of Options -6.499359 (6.5) Less: Other Non-current Assets -1.002361 (1.0) Implied Enterprise Value $524.3 Implied Offer Multiples: EV / LTM EBITDA $9.4 55.5x EV / 2017 EBITDA $10.0 52.2x EV / 2018 EBITDA $33.2 15.5x EV / 2019 EBITDA $39.0 12.8x EV / LTM EBIT $4.3 121.1x EV / 2017 EBIT $5.9 88.3x EV / 2018 EBIT $26.6 19.3x EV / 2019 EBIT $32.6 15.4x EV / LTM Revenue $71.1 7.38x EV / 2017 Revenue $117.0 4.48x EV / 2018 Revenue $129.7 3.96x EV / 2019 Revenue $160.0 3.13x

10 CONFIDENTIAL Introduction and Transaction Overview Valuation Summary (1) Includes noncontrolling interest in Sinovac Beijing and Sinovac Dalian Note: Balance sheet data and LTM as of December 31, 2016. Financial performance metrics presented are adjusted to exclude pub lic company costs and non - recurring income (expenses). Valuation Range Conclusions (RMB in thousands, except per share values or otherwise noted) Low High Enterprise Value Discounted Cash Flow Analysis 2,000,700 - 2,932,500 Selected Public Companies / M&A Transactions Analysis 2,274,800 - 2,925,200 Enterprise Value Range 2,137,800 - 2,928,900 Plus: Proceeds from Exercise of Options 442 - 44,434 Plus: Other Non-current Assets 6,853 - 6,853 Less: Short-term Loans (233,167) - (233,167) Less: Long-term Debt (65,597) - (65,597) Less: Other Non-current Liabilities (2,604) - (2,604) Less: Noncontrolling Interest (1) (589,309) - (802,906) Value Attributable to Fully Diluted Shares (Excluding Cash) 1,254,418 - 1,875,913 Fully Diluted Shares Issued and Outstanding 57,402,561 - 58,694,661 Value Per Share (RMB) 21.85 - 31.96 RMB to USD FX rate (as of 6/23/2017) 6.84 - 6.84 Value Per Share Range (Excluding Excess Cash) $3.20 - $4.67 Excess Cash 454,357 - 454,357 Cash Value Per Fully Diluted Shares Issued and Outstanding (RMB) 7.92 - 7.74 Cash Value Per Fully Diluted Shares Issued and Outstanding (USD) $1.16 - $1.13 Offer Price Value Per Share Range $4.35 - $5.81 $7.00 Implied Valuation Multiples EV / LTM EBITDA 64,601 33.1x - 45.3x 55.5x EV / 2017 EBITDA 68,709 31.1x - 42.6x 52.2x EV / 2018 EBITDA 231,649 9.2x - 12.6x 15.5x EV / 2019 EBITDA 279,149 7.7x - 10.5x 12.8x EV / LTM EBIT 29,596 72.2x - 99.0x 121.1x EV / 2017 EBIT 40,580 52.7x - 72.2x 88.3x EV / 2018 EBIT 185,409 11.5x - 15.8x 19.3x EV / 2019 EBIT 233,456 9.2x - 12.5x 15.4x EV / LTM Revenue 485,758 4.40x - 6.03x 7.38x EV / 2017 Revenue 800,959 2.67x - 3.66x 4.48x EV / 2018 Revenue 905,501 2.36x - 3.23x 3.96x EV / 2019 Revenue 1,146,391 1.86x - 2.55x 3.13x

11 CONFIDENTIAL Introduction and Transaction Overview Per Share Valuation Range $4.35 $4.61 $4.10 $5.81 $5.80 $5.81 $3.00 $3.50 $4.00 $4.50 $5.00 $5.50 $6.00 $6.50 $7.00 $7.50 $8.00 Concluded Range Selected Public Companies / M&A Transactions Analysis Discounted Cash Flow Analysis Value Per Share (USD) $7.00 Offer Price

Valuation Analysis Section 02

13 CONFIDENTIAL Valuation Analysis Financial Performance Historical and Projected Financial Performance (RMB in thousands) Management Projections 2012A 2013A 2014A 2015A 2016A 2017P 2018P 2019P 2020P 2021P 2022P 2023P 2024P 2025P Net Revenue 310,184 441,253 387,786 423,542 485,758 800,959 905,501 1,146,391 1,438,645 1,531,359 1,601,918 1,702,764 1,789,683 1,855,513 Growth NA 42.3% (12.1%) 9.2% 14.7% 64.9% 13.1% 26.6% 25.5% 6.4% 4.6% 6.3% 5.1% 3.7% Gross Profit 215,370 337,574 326,753 333,917 356,938 674,190 790,009 971,786 1,214,341 1,300,501 1,358,448 1,432,511 1,515,158 1,576,464 Margin % 69.4% 76.5% 84.3% 78.8% 73.5% 84.2% 87.2% 84.8% 84.4% 84.9% 84.8% 84.1% 84.7% 85.0% EBITDA - 62,640 113,539 75,563 77,295 64,601 68,709 231,649 279,149 361,356 361,523 385,650 384,898 420,400 426,300 Margin % (20.2%) 25.7% 19.5% 18.2% 13.3% 8.6% 25.6% 24.4% 25.1% 23.6% 24.1% 22.6% 23.5% 23.0% Growth NA NM (33.4%) 2.3% (16.4%) 6.4% 237.1% 20.5% 29.4% 0.0% 6.7% (0.2%) 9.2% 1.4% EBIT - 90,954 74,144 25,676 36,395 34,062 40,580 185,409 233,456 311,481 309,431 327,033 327,918 360,602 363,147 Margin % (29.3%) 16.8% 6.6% 8.6% 7.0% 5.1% 20.5% 20.4% 21.7% 20.2% 20.4% 19.3% 20.1% 19.6% Growth NA NM (65.4%) 41.7% (6.4%) 19.1% 356.9% 25.9% 33.4% (0.7%) 5.7% 0.3% 10.0% 0.7% Capital Expenditures 101,929 31,557 67,246 33,292 91,229 83,685 37,599 28,024 39,820 39,000 39,400 40,000 44,000 50,000 % of Net Revenue 32.9% 7.2% 17.3% 7.9% 18.8% 10.4% 4.2% 2.4% 2.8% 2.5% 2.5% 2.3% 2.5% 2.7% % of EBITDA NM 27.79% 89.0% 43.1% 141.2% 121.8% 16.2% 10.0% 11.0% 10.8% 10.2% 10.4% 10.5% 11.7% Note: Financial performance metrics presented are adjusted to exclude public company costs and non-recurring income (expenses). Source: Company filings, Company management

14 CONFIDENTIAL Valuation Analysis Discounted Cash Flow Analysis – Methodology and Key Assumptions Discounted Cash Flow Methodology • Duff & Phelps performed a discounted cash flow analysis of the projected unlevered free cash flows . • Unlevered free cash flow is defined as cash generated by the business that is available to either reinvest or to distribute to security holders . • Projected free cash flows are discounted to the present using a discount rate which reflects their relative risk . • The discount rate is equivalent to the rate of return that security holders could expect to realize on alternative investment opportunities with similar risk profiles . Discounted Cash Flow Key Assumptions • Duff & Phelps utilized and relied upon the Management Projections for the fiscal years ending D ecember 31 , 2017 through 202 5 (excluding public company expenses, as provided by the management of the Company) as well as discussions with the management of the Company, a review of the Company’s historical performance and other factors t o develop the DCF analysis . • Beyond the projection period, Duff & Phelps estimated the “terminal value” using a perpetuity formula and a terminal growth rate based on discussions with the management regarding long - term expected growth for the Company consistent with long - term expected R&D spending levels . • Duff & Phelps discounted the resulting free cash flows and terminal value using a weighted average cost of capital range of 1 1 . 0 % to 1 3 . 0 % , derived from the Capital Asset Pricing Model . • The following is a summary of the Management Projections utilized in the discounted cash flow analysis : - The Company’s net revenue increases at a compound annual growth rate (“ CAGR ”) of 1 6 . 1 % over the nine - year period ending 202 5 . - The Company’s EBITDA increases at a CAGR of 23 . 3 % over the nine - year period ending 202 5 . - The Company’s EBITDA margin averages 2 2 . 3 % from 2017 to 202 5 . - The Company’s net working capital averages 47 . 3 % of revenue from 2017 to 202 5 . - Capital expenditures average 3 . 6 % of revenue from 2017 to 202 5 .
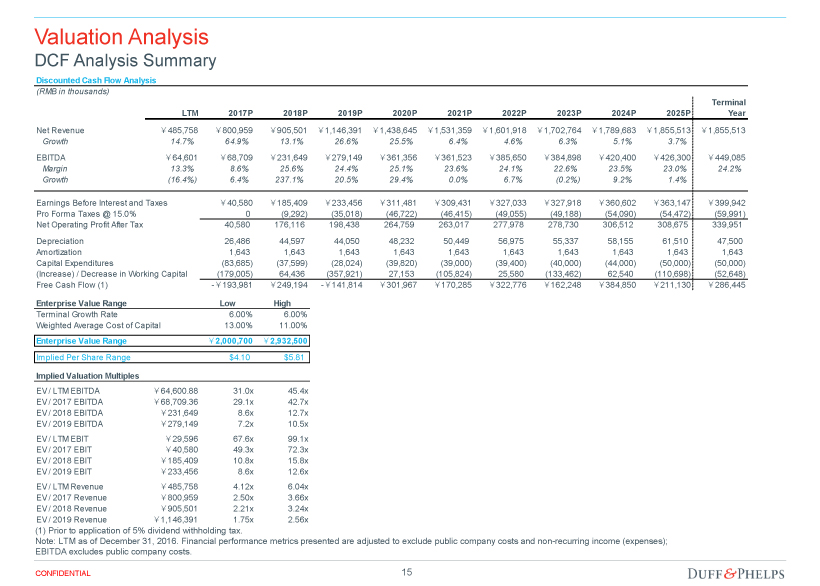
15 CONFIDENTIAL Valuation Analysis DCF Analysis Summary ( 1 ) Prior to application of 5 % dividend withholding tax . Note : LTM as of December 31 , 2016 . Financial performance metrics presented are adjusted to exclude public company costs and non - recurring income (expenses) ; EBITDA excludes public company costs . Discounted Cash Flow Analysis (RMB in thousands) Terminal LTM 2017P 2018P 2019P 2020P 2021P 2022P 2023P 2024P 2025P Year Net Revenue 485,758 800,959 905,501 1,146,391 1,438,645 1,531,359 1,601,918 1,702,764 1,789,683 1,855,513 1,855,513 Growth 14.7% 64.9% 13.1% 26.6% 25.5% 6.4% 4.6% 6.3% 5.1% 3.7% EBITDA 64,601 68,709 231,649 279,149 361,356 361,523 385,650 384,898 420,400 426,300 449,085 Margin 13.3% 8.6% 25.6% 24.4% 25.1% 23.6% 24.1% 22.6% 23.5% 23.0% 24.2% Growth (16.4%) 6.4% 237.1% 20.5% 29.4% 0.0% 6.7% (0.2%) 9.2% 1.4% Earnings Before Interest and Taxes 40,580 185,409 233,456 311,481 309,431 327,033 327,918 360,602 363,147 399,942 Pro Forma Taxes @ 15.0% 0 (9,292) (35,018) (46,722) (46,415) (49,055) (49,188) (54,090) (54,472) (59,991) Net Operating Profit After Tax 40,580 176,116 198,438 264,759 263,017 277,978 278,730 306,512 308,675 339,951 Depreciation 26,486 44,597 44,050 48,232 50,449 56,975 55,337 58,155 61,510 47,500 Amortization 1,643 1,643 1,643 1,643 1,643 1,643 1,643 1,643 1,643 1,643 Capital Expenditures (83,685) (37,599) (28,024) (39,820) (39,000) (39,400) (40,000) (44,000) (50,000) (50,000) (Increase) / Decrease in Working Capital (179,005) 64,436 (357,921) 27,153 (105,824) 25,580 (133,462) 62,540 (110,698) (52,648) Free Cash Flow (1) -193,981 249,194 -141,814 301,967 170,285 322,776 162,248 384,850 211,130 286,445 Enterprise Value Range Low High Terminal Growth Rate 6.00% 6.00% Weighted Average Cost of Capital 13.00% 11.00% Enterprise Value Range 2,000,700 2,932,500 Implied Per Share Range $4.10 $5.81 Implied Valuation Multiples EV / LTM EBITDA 64,600.88 31.0x 45.4x EV / 2017 EBITDA 68,709.36 29.1x 42.7x EV / 2018 EBITDA 231,649 8.6x 12.7x EV / 2019 EBITDA 279,149 7.2x 10.5x EV / LTM EBIT 29,596 67.6x 99.1x EV / 2017 EBIT 40,580 49.3x 72.3x EV / 2018 EBIT 185,409 10.8x 15.8x EV / 2019 EBIT 233,456 8.6x 12.6x EV / LTM Revenue 485,758 4.12x 6.04x EV / 2017 Revenue 800,959 2.50x 3.66x EV / 2018 Revenue 905,501 2.21x 3.24x EV / 2019 Revenue 1,146,391 1.75x 2.56x

16 CONFIDENTIAL Valuation Analysis Selected Public Companies / M&A Transactions Analysis Methodology Selected Public Companies Analysis • Duff & Phelps selected fifteen publicly traded companies in the pharmaceutical and biotechnology industry that were deemed relevant to its analysis . • Duff & Phelps analyzed the financial performance of each of the publicly traded companies . Duff & Phelps then analyzed the selected public companies’ trading multiples, including enterprise value to LTM and projected revenue, EBITDA, and EBIT . Selected M&A Transactions Analysis • Duff & Phelps selected precedent transactions within the pharmaceutical and biotechnology industry that it determined to be relevant to its analysis . Duff & Phelps computed the LTM revenue and EBITDA for each of the target companies (where publicly disclosed) . Duff & Phelps then calculated the implied enterprise value to LTM revenue and enterprise value to LTM EBITDA multiples for each transaction . Duff & Phelps analyzed a number of factors in comparing the Company to the selected public companies and the targets in the selected M&A transactions, including historical and forecasted growth in revenue and profits, profit margins and other characteristics that we deemed relevant . Rather than applying the average or median multiple from the public company set, Duff & Phelps selected multiples that reflect the Company’s size, growth outlook, capital requirements, profit margins, revenue mix, and other characteristics relative to the group . None of the companies utilized for comparative purposes in the following analysis are directly comparable to the Company, and none of the transactions utilized for comparative purposes in the following analysis are directly comparable to the Proposed Transaction . Duff & Phelps does not have access to non - public information of any of the companies used for comparative purposes . Accordingly, a complete valuation analysis of the Company and the Proposed Transaction cannot rely solely upon a quantitative review of the selected companies and selected transactions, and involves complex considerations and judgments concerning differences in financial and operating characteristics of such companies and targets, as well as other factors that could affect their value relative to that of the Company . Therefore, the Selected Public Companies / Selected M&A Transactions Analysis is subject to certain limitations .
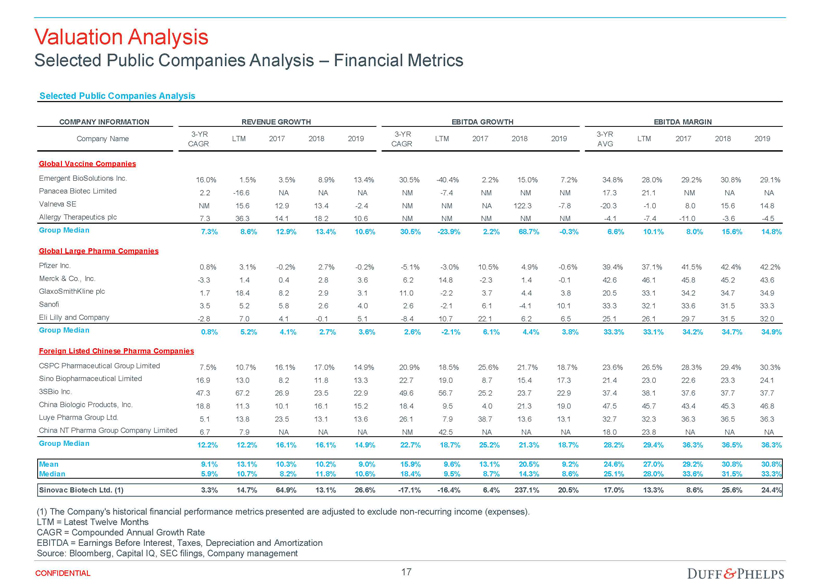
17 CONFIDENTIAL Valuation Analysis Selected Public Companies Analysis – Financial Metrics (1) The Company's historical financial performance metrics presented are adjusted to exclude non - recurring income (expenses). LTM = Latest Twelve Months CAGR = Compounded Annual Growth Rate EBITDA = Earnings Before Interest, Taxes, Depreciation and Amortization Source: Bloomberg, Capital IQ, SEC filings, Company management Selected Public Companies Analysis ($ in millions, except per share data) COMPANY INFORMATION REVENUE GROWTH EBITDA GROWTH EBITDA MARGIN Company Name 3-YR CAGR LTM 2017 2018 2019 3-YR CAGR LTM 2017 2018 2019 3-YR AVG LTM 2017 2018 2019 Global Vaccine Companies Emergent BioSolutions Inc. 16.0% 1.5% 3.5% 8.9% 13.4% 30.5% -40.4% 2.2% 15.0% 7.2% 34.8% 28.0% 29.2% 30.8% 29.1% Panacea Biotec Limited 2.2 -16.6 NA NA NA NM -7.4 NM NM NM 17.3 21.1 NM NA NA Valneva SE NM 15.6 12.9 13.4 -2.4 NM NM NA 122.3 -7.8 -20.3 -1.0 8.0 15.6 14.8 Allergy Therapeutics plc 7.3 36.3 14.1 18.2 10.6 NM NM NM NM NM -4.1 -7.4 -11.0 -3.6 -4.5 Group Median 7.3% 8.6% 12.9% 13.4% 10.6% 30.5% -23.9% 2.2% 68.7% -0.3% 6.6% 10.1% 8.0% 15.6% 14.8% Global Large Pharma Companies Pfizer Inc. 0.8% 3.1% -0.2% 2.7% -0.2% -5.1% -3.0% 10.5% 4.9% -0.6% 39.4% 37.1% 41.5% 42.4% 42.2% Merck & Co., Inc. -3.3 1.4 0.4 2.8 3.6 6.2 14.8 -2.3 1.4 -0.1 42.6 46.1 45.8 45.2 43.6 GlaxoSmithKline plc 1.7 18.4 8.2 2.9 3.1 11.0 -2.2 3.7 4.4 3.8 20.5 33.1 34.2 34.7 34.9 Sanofi 3.5 5.2 5.8 2.6 4.0 2.6 -2.1 6.1 -4.1 10.1 33.3 32.1 33.6 31.5 33.3 Eli Lilly and Company -2.8 7.0 4.1 -0.1 5.1 -8.4 10.7 22.1 6.2 6.5 25.1 26.1 29.7 31.5 32.0 Group Median 0.8% 5.2% 4.1% 2.7% 3.6% 2.6% -2.1% 6.1% 4.4% 3.8% 33.3% 33.1% 34.2% 34.7% 34.9% Foreign Listed Chinese Pharma Companies CSPC Pharmaceutical Group Limited 7.5% 10.7% 16.1% 17.0% 14.9% 20.9% 18.5% 25.6% 21.7% 18.7% 23.6% 26.5% 28.3% 29.4% 30.3% Sino Biopharmaceutical Limited 16.9 13.0 8.2 11.8 13.3 22.7 19.0 8.7 15.4 17.3 21.4 23.0 22.6 23.3 24.1 3SBio Inc. 47.3 67.2 26.9 23.5 22.9 49.6 56.7 25.2 23.7 22.9 37.4 38.1 37.6 37.7 37.7 China Biologic Products, Inc. 18.8 11.3 10.1 16.1 15.2 18.4 9.5 4.0 21.3 19.0 47.5 45.7 43.4 45.3 46.8 Luye Pharma Group Ltd. 5.1 13.8 23.5 13.1 13.6 26.1 7.9 38.7 13.6 13.1 32.7 32.3 36.3 36.5 36.3 China NT Pharma Group Company Limited 6.7 7.9 NA NA NA NM 42.5 NA NA NA 18.0 23.8 NA NA NA Group Median 12.2% 12.2% 16.1% 16.1% 14.9% 22.7% 18.7% 25.2% 21.3% 18.7% 28.2% 29.4% 36.3% 36.5% 36.3% Mean 9.1% 13.1% 10.3% 10.2% 9.0% 15.9% 9.6% 13.1% 20.5% 9.2% 24.6% 27.0% 29.2% 30.8% 30.8% Median 5.9% 10.7% 8.2% 11.8% 10.6% 18.4% 9.5% 8.7% 14.3% 8.6% 25.1% 28.0% 33.6% 31.5% 33.3% Sinovac Biotech Ltd. (1) 3.3% 14.7% 64.9% 13.1% 26.6% -17.1% -16.4% 6.4% 237.1% 20.5% 17.0% 13.3% 8.6% 25.6% 24.4%

18 CONFIDENTIAL Valuation Analysis Selected Public Companies Analysis – Valuation Multiples LTM = Latest Twelve Months Enterprise Value = (Market Capitalization) + (Debt + Preferred Stock + Non - Controlling Interest) – (Cash & Cash Equivalents) – ( Net Non - Operating Assets) EBITDA = Earnings Before Interest, Taxes, Depreciation and Amortization Source: Bloomberg, Capital IQ, SEC filings Selected Public Companies Analysis ($ in millions, except per share data) COMPANY INFORMATION ENTERPRISE VALUE AS A MULTIPLE OF Company Name Stock Price on 6/23/17 % of 52- Wk High Enterprise Value LTM EBITDA 2017 EBITDA 2018 EBITDA 2019 EBITDA LTM EBIT 2017 EBIT 2018 EBIT 2019 EBIT LTM Revenue 2017 Revenue 2018 Revenue 2019 Revenue Global Vaccine Companies Emergent BioSolutions Inc. $34.65 96.6% $1,458 10.4x 9.9x 8.6x 8.0x 14.4x 11.4x 9.3x 8.1x 2.90x 2.88x 2.65x 2.33x Panacea Biotec Limited 2.07 78.2 285 16.0 NA NA NA 38.7 NA NA NA 3.37 NA NA NA Valneva SE 3.36 94.6 294 NM 29.9 13.4 14.6 NM NM 16.2 48.2 2.57 2.38 2.10 2.15 Allergy Therapeutics plc 0.32 82.6 153 NM NM NM NM NM NM NM NM 2.00 2.16 1.83 1.65 Group Median 13.2x 19.9x 11.0x 11.3x 26.6x 11.4x 12.8x 28.2x 2.73x 2.38x 2.10x 2.15x Global Large Pharma Companies Pfizer Inc. $34.17 91.6% $230,823 11.8x 10.5x 10.1x 10.1x 16.7x 11.5x 11.1x 10.9x 4.39x 4.38x 4.26x 4.27x Merck & Co., Inc. 66.16 99.4 183,916 10.0 10.0 9.9 9.9 13.6 13.5 12.6 11.8 4.61 4.60 4.47 4.32 GlaxoSmithKline plc 21.76 99.2 126,639 10.3 9.6 9.2 8.9 12.1 11.7 11.3 10.6 3.42 3.29 3.20 3.10 Sanofi 98.41 94.5 97,978 7.6 7.1 7.4 6.7 10.2 8.9 8.6 8.2 2.45 2.38 2.32 2.23 Eli Lilly and Company 83.89 97.3 89,965 16.0 13.7 12.9 12.1 21.7 16.3 15.6 13.8 4.17 4.07 4.07 3.88 Group Median 10.3x 10.0x 9.9x 9.9x 13.6x 11.7x 11.3x 10.9x 4.17x 4.07x 4.07x 3.88x Foreign Listed Chinese Pharma Companies CSPC Pharmaceutical Group Limited $1.53 100.0% $8,858 20.3x 17.0x 14.0x 11.8x 24.3x 20.3x 16.5x 13.7x 5.38x 4.81x 4.11x 3.58x Sino Biopharmaceutical Limited 0.89 95.7 6,115 11.3 10.8 9.4 8.0 12.6 11.8 10.6 9.0 2.58 2.44 2.18 1.93 3SBio Inc. 1.36 95.8 3,750 24.0 19.2 15.5 12.6 28.3 22.5 17.7 14.7 9.17 7.22 5.85 4.76 China Biologic Products, Inc. 111.84 83.4 2,891 18.2 17.7 14.6 12.3 20.0 19.2 16.3 13.1 8.33 7.70 6.63 5.76 Luye Pharma Group Ltd. 0.56 77.8 1,789 13.0 9.4 8.2 7.3 15.1 10.3 9.1 7.9 4.19 3.39 3.00 2.64 China NT Pharma Group Company Limited 0.22 79.8 441 13.9 NA NA NA 15.4 NA NA NA 3.29 NA NA NA Group Median 16.0x 17.0x 14.0x 11.8x 17.7x 19.2x 16.3x 13.1x 4.79x 4.81x 4.11x 3.58x Mean 14.1x 13.7x 11.1x 10.2x 18.7x 14.3x 12.9x 14.2x 4.19x 3.98x 3.59x 3.28x Median 13.0x 10.7x 10.0x 10.0x 15.4x 11.8x 11.9x 11.3x 3.42x 3.39x 3.20x 3.10x MARKET DATA
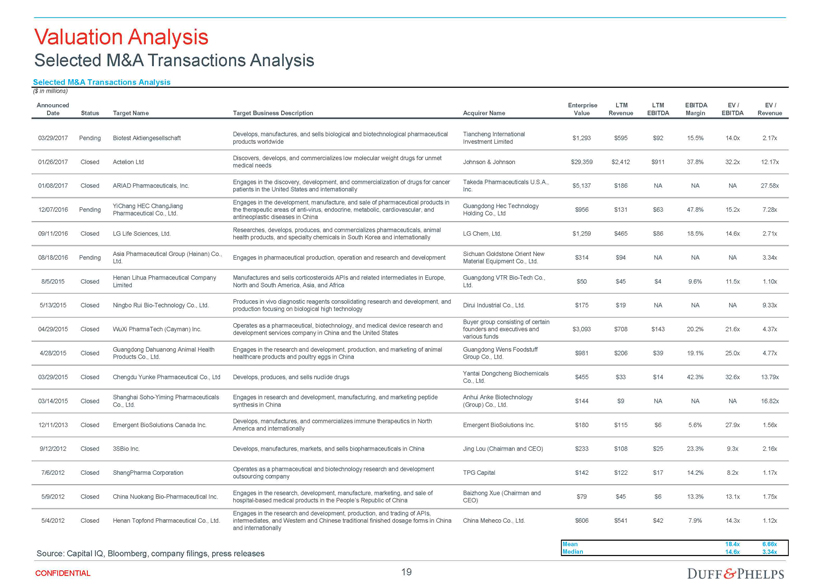
19 CONFIDENTIAL Source: Capital IQ, Bloomberg, company filings, press releases Valuation Analysis Selected M&A Transactions Analysis Selected M&A Transactions Analysis ($ in millions) Announced Date Status Target Name Target Business Description Acquirer Name Enterprise Value LTM Revenue LTM EBITDA EBITDA Margin EV / EBITDA EV / Revenue 03/29/2017 Pending Biotest Aktiengesellschaft Develops, manufactures, and sells biological and biotechnological pharmaceutical products worldwide Tiancheng International Investment Limited $1,293 $595 $92 15.5% 14.0x 2.17x 01/26/2017 Closed Actelion Ltd Discovers, develops, and commercializes low molecular weight drugs for unmet medical needs Johnson & Johnson $29,359 $2,412 $911 37.8% 32.2x 12.17x 01/08/2017 Closed ARIAD Pharmaceuticals, Inc. Engages in the discovery, development, and commercialization of drugs for cancer patients in the United States and internationally Takeda Pharmaceuticals U.S.A., Inc. $5,137 $186 NA NA NA 27.58x 12/07/2016 Pending YiChang HEC ChangJiang Pharmaceutical Co., Ltd. Engages in the development, manufacture, and sale of pharmaceutical products in the therapeutic areas of anti-virus, endocrine, metabolic, cardiovascular, and antineoplastic diseases in China Guangdong Hec Technology Holding Co., Ltd $956 $131 $63 47.8% 15.2x 7.28x 09/11/2016 Closed LG Life Sciences, Ltd. Researches, develops, produces, and commercializes pharmaceuticals, animal health products, and specialty chemicals in South Korea and internationally LG Chem, Ltd. $1,259 $465 $86 18.5% 14.6x 2.71x 08/18/2016 Pending Asia Pharmaceutical Group (Hainan) Co., Ltd. Engages in pharmaceutical production, operation and research and development Sichuan Goldstone Orient New Material Equipment Co., Ltd. $314 $94 NA NA NA 3.34x 8/5/2015 Closed Henan Lihua Pharmaceutical Company Limited Manufactures and sells corticosteroids APIs and related intermediates in Europe, North and South America, Asia, and Africa Guangdong VTR Bio-Tech Co., Ltd. $50 $45 $4 9.6% 11.5x 1.10x 5/13/2015 Closed Ningbo Rui Bio-Technology Co., Ltd. Produces in vivo diagnostic reagents consolidating research and development, and production focusing on biological high technology Dirui Industrial Co., Ltd. $175 $19 NA NA NA 9.33x 04/29/2015 Closed WuXi PharmaTech (Cayman) Inc. Operates as a pharmaceutical, biotechnology, and medical device research and development services company in China and the United States Buyer group consisting of certain founders and executives and various funds $3,093 $708 $143 20.2% 21.6x 4.37x 4/28/2015 Closed Guangdong Dahuanong Animal Health Products Co., Ltd. Engages in the research and development, production, and marketing of animal healthcare products and poultry eggs in China Guangdong Wens Foodstuff Group Co., Ltd. $981 $206 $39 19.1% 25.0x 4.77x 03/29/2015 Closed Chengdu Yunke Pharmaceutical Co., Ltd Develops, produces, and sells nuclide drugs Yantai Dongcheng Biochemicals Co., Ltd. $455 $33 $14 42.3% 32.6x 13.79x 03/14/2015 Closed Shanghai Soho-Yiming Pharmaceuticals Co., Ltd. Engages in research and development, manufacturing, and marketing peptide synthesis in China Anhui Anke Biotechnology (Group) Co., Ltd. $144 $9 NA NA NA 16.82x 12/11/2013 Closed Emergent BioSolutions Canada Inc. Develops, manufactures, and commercializes immune therapeutics in North America and internationally Emergent BioSolutions Inc. $180 $115 $6 5.6% 27.9x 1.56x 9/12/2012 Closed 3SBio Inc. Develops, manufactures, markets, and sells biopharmaceuticals in China Jing Lou (Chairman and CEO) $233 $108 $25 23.3% 9.3x 2.16x 7/6/2012 Closed ShangPharma Corporation Operates as a pharmaceutical and biotechnology research and development outsourcing company TPG Capital $142 $122 $17 14.2% 8.2x 1.17x 5/9/2012 Closed China Nuokang Bio-Pharmaceutical Inc. Engages in the research, development, manufacture, marketing, and sale of hospital-based medical products in the People’s Republic of China Baizhong Xue (Chairman and CEO) $79 $45 $6 13.3% 13.1x 1.75x 5/4/2012 Closed Henan Topfond Pharmaceutical Co., Ltd. Engages in the research and development, production, and trading of APIs, intermediates, and Western and Chinese traditional finished dosage forms in China and internationally China Meheco Co., Ltd. $606 $541 $42 7.9% 14.3x 1.12x Mean 18.4x 6.66x Median 14.6x 3.34x

20 CONFIDENTIAL Valuation Analysis Selected Public Companies / M&A Transactions Analysis Summary Note: LTM data as of December 31, 2016. Financial performance metrics presented are adjusted to exclude public company costs an d non - recurring income (expenses); EBITDA excludes public company costs. Selected Public Companies / M&A Transactions Analysis Summary (RMB in thousands) Enterprise Valuation Multiples Valuation Summary Metric Public Company Median Transaction Median Selected Multiple Range Company Performance Enterprise Value Range EV / 2018 EBITDA 7.4x - 15.5x 10.0x NA 10.0x - 12.0x 231,649 2,316,492 - 2,779,790 EV / 2019 EBITDA 6.7x - 14.6x 10.0x NA 8.0x - 11.0x 279,149 2,233,196 - 3,070,644 Enterprise Value Range 2,274,800 - 2,925,200 Implied Per Share Range (USD) $4.61 - $5.80 Implied Multiples EV / LTM EBITDA 7.6x 24.0x 13.0x 14.6x 64,601 35.2x - 45.3x EV / 2017 EBITDA 7.1x 29.9x 10.7x NA 68,709 33.1x - 42.6x EV / LTM EBIT 10.2x 38.7x 15.4x NA 29,596 76.9x - 98.8x EV / 2017 EBIT 8.9x 22.5x 11.8x NA 40,580 56.1x - 72.1x EV / 2018 EBIT 8.6x 17.7x 11.9x NA 185,409 12.3x - 15.8x EV / 2019 EBIT 7.9x 48.2x 11.3x NA 233,456 9.7x - 12.5x EV / LTM Revenue 2.00x 9.17x 3.42x 3.34x 485,758 4.68x - 6.02x EV / 2017 Revenue 2.16x 7.70x 3.39x NA 800,959 2.84x - 3.65x EV / 2018 Revenue 1.83x 6.63x 3.20x NA 905,501 2.51x - 3.23x EV / 2019 Revenue 1.65x 5.76x 3.10x NA 1,146,391 1.98x - 2.55x Public Company Range

Assumptions, Qualifications, and Limiting Conditions Appendix 01

22 CONFIDENTIAL Assumptions, Qualifications, and Limiting Conditions If issued, our Opinion letter will include assumptions, qualifications and limiting conditions similar to the following . This is not meant to be a complete list of the assumptions, qualifications and limiting conditions which will be included in our Opinion letter, if rendered . Assumptions and Reliance – In performing its analyses and rendering the Opinion with respect to the Proposed Transaction, Duff & Phelps, with the Company’s and the Special Committee’s consent and without independent verification : • Relied upon the accuracy, completeness, and fair presentation of all information, data, advice, opinions and representations obtained from public sources or provided to it from private sources, including the management of the Company ; • Relied upon the fact that the Special Committee, the Board of Directors and the Company have been advised by counsel as to all legal matters with respect to the Proposed Transaction, including whether all procedures required by law to be taken in connection with the Proposed Transaction have been duly, validly and timely taken ; • Assumed that any estimates, evaluations, forecasts and projections including, without limitation, the Management Projections, furnished to Duff & Phelps were reasonably prepared and based upon the best currently available information and good faith judgment of the person furnishing the same, and Duff & Phelps expresses no view or opinion with respect to such estimates, evaluations, forecasts or projections or their underlying assumptions ; • Assumed that the information relating to the Company and the Proposed Transaction provided to Duff & Phelps and representations made by the management of the Company regarding the Company and the Proposed Transaction are accurate in all material respects, did not and does not omit to state a material fact in respect of the Company and the Proposed Transaction necessary to make the information not misleading in light of the circumstances under which the information was provided ; • Assumed that the representations and warranties made by all parties in the Amalgamation Agreement and in the Management Representation Letter are true and correct in all material respects and that each party to the Amalgamation Agreement will fully and duly perform all covenants, undertakings and obligations required to be performed by such party ; • Assumed that the final versions of all documents reviewed by Duff & Phelps in draft form, including the Amalgamation Agreement, conform in all material respects to the drafts reviewed ; • Assumed that there has been no material change in the assets, liabilities, financial condition, results of operations, business, or prospects of the Company since the date of the most recent financial statements and other information made available to Duff & Phelps ; • Assumed that all of the conditions required to implement the Proposed Transaction will be satisfied and that the Proposed Transaction will be completed in accordance with the Amalgamation Agreement without any amendments thereto or any waivers of any terms or conditions thereof, and in a manner that complies in all material respects with all applicable laws ; and • Assumed that all governmental, regulatory or other consents and approvals necessary for the consummation of the Proposed Transaction will be obtained without any undue delay, limitation, restriction or condition that would have a material effect on the Company or the contemplated benefits expected to be derived in the Proposed Transaction . To the extent that any of the foregoing assumptions or any of the facts on which the Opinion is based prove to be untrue in any material respect, the Opinion cannot and should not be relied upon for any purpose . Furthermore, in Duff & Phelps’ analysis and in connection with the preparation of the Opinion, Duff & Phelps has made numerous assumptions with respect to industry performance, general business, market and economic conditions and other matters, many of which are beyond the control of any party involved in the Proposed Transaction and as to which Duff & Phelps does not express any view or opinion in the Opinion, including as to the reasonableness of such assumptions .

23 CONFIDENTIAL Assumptions, Qualifications, and Limiting Conditions Qualifications – If issued, our Opinion will be qualified by the following: • Duff & Phelps has prepared the Opinion effective as of the date thereof . The Opinion is necessarily based upon the information made available to Duff & Phelps as of the date thereof and market, economic, financial and other conditions as they exist and can be evaluated as of the date thereof, and Duff & Phelps disclaims any undertaking or obligation to (i) advise any person of any change in any fact or matter affecting the Opinion which may come or be brought to the attention of Duff & Phelps after the date thereof or (ii) update, revise or reaffirm the Opinion after the date thereof . • Duff & Phelps did not evaluate the Company’s solvency or conduct an independent appraisal or physical inspection of any specific assets or liabilities (contingent or otherwise) of the Company, nor was Duff & Phelps provided with any such appraisal or evaluation other than the contents of the Management Representation Letter . Duff & Phelps did not estimate, and expresses no opinion regarding, the liquidation value of any entity or business . • Duff & Phelps has not been requested to, and did not, (i) initiate any discussions with, or solicit any indications of interest from, third parties with respect to the Proposed Transaction, the assets, businesses or operations of the Company, or any alternatives to the Proposed Transaction, or (ii) advise the Special Committee or any other party with respect to alternatives to the Proposed Transaction . Duff & Phelps did not undertake an independent analysis of any potential or actual litigation, regulatory action, possible unasserted claims or other contingent liabilities, to which the Company is or may be a party or is or may be subject, or of any governmental investigation of any possible unasserted claims or other contingent liabilities to which the Company is or may be a party or is or may be subject . • Duff & Phelps is not expressing any opinion as to the market price or value of the Shares (or anything else) after the announcement or the consummation of the Proposed Transaction (or any other time) . The Opinion should not be construed as a valuation opinion, a credit rating, a solvency opinion, an analysis of the Company’s creditworthiness, tax advice, or accounting advice . Duff & Phelps has not made, and assumes no responsibility to make, any representation, or render any opinion, as to any legal matter . Duff & Phelps expressly disclaims any responsibility or liability in this regard . • In rendering the Opinion, Duff & Phelps is not expressing any opinion with respect to the amount or nature or any other aspect of any compensation payable to or to be received by any of the Company’s officers, directors, or employees, or any class of such persons, relative to the Per Share Amalgamation Consideration, or with respect to the fairness of any such compensation . In addition, the Opinion does not address the fairness to, or any other consideration of, the holders of any class of securities, creditors or other constituencies of the Company, other than the holders of the Shares (other than the Excluded Shares, the Dissenting Shares and Company RSs) .

24 CONFIDENTIAL Assumptions, Qualifications, and Limiting Conditions Limiting Conditions – If issued, the use of our Opinion will be strictly limited and will state: • The Opinion is furnished solely for the use and benefit of the Special Committee in connection with its consideration of the Proposed Transaction and is not intended to, and does not, confer any rights or remedies upon any other person, and is not intended to be used, and may not be used, by any other person or for any other purpose, without Duff & Phelps’ prior written consent . • The Opinion (i) does not address the merits of the underlying business decision to enter into the Proposed Transaction versus any alternative strategy or transaction ; (ii) does not address any transaction related to the Proposed Transaction ; (iii) is not a recommendation as to how the Special Committee, the Board of Directors, the Company or any other person including security holders of the Company as to how such person should vote or act with respect to any matters relating to the Proposed Transaction, or whether to proceed with the Proposed Transaction or any related transaction, and (iv) does not indicate that the Per Share Amalgamation Consideration is the best possibly attainable under any circumstances ; instead, it merely states whether the Per Share Amalgamation Consideration is within a range suggested by certain financial analyses . The decision as to whether to proceed with the Proposed Transaction or any related transaction may depend on an assessment of factors unrelated to the financial analysis on which the Opinion is based . • The Opinion should not be construed as creating any fiduciary duty on the part of Duff & Phelps to any party . • The Opinion is solely that of Duff & Phelps, and Duff & Phelps’ liability in connection with the Opinion shall be limited in accordance with the terms set forth in the engagement letter among Duff & Phelps, DPS, the Company, and the Special Committee dated April 12 , 2016 (the “ Engagement Letter ”) . • The Opinion is confidential, and its use and disclosure is strictly limited in accordance with the terms set forth in the Engagement Letter .

Summary of Premiums Paid Appendix 02

26 CONFIDENTIAL Summary of Premiums Paid Note: Excludes negative premiums. Source: Capital IQ Premiums Paid Analysis - Going Private Transactions Transactions announced, closed, or effective from January 2012 to June 2017 Premium as a % of Number of Deals One-Day Prior to Announcement Date One-Week Prior to Announcement Date One-Month Prior to Announcement Date One-Day Prior as a % of 52-Week High Overall Mean 527 38.8 41.7 43.4 71.8 Overall Median 24.1 27.6 27.7 80.6 Chinese Companies Mean 96 36.2 37.9 40.2 64.8 Chinese Companies Median 24.2 29.8 28.3 68.1 US-Listed Chinese Companies Mean 80 37.4 38.0 39.5 63.3 US-Listed Chinese Companies Median 25.2 29.8 28.1 64.6 Sinovac Biotech Ltd. 39.4 48.0 32.1 81.2 Premiums Paid Analysis - Biotechnology Change of Control Transactions Transactions announced, closed, or effective from January 2012 to June 2017 Premium as a % of Number of Deals One-Day Prior to Announcement Date One-Week Prior to Announcement Date One-Month Prior to Announcement Date One-Day Prior as a % of 52-Week High Overall Industry Mean 63 40.1 40.4 59.2 44.7 Overall Industry Median 37.8 32.2 50.1 38.0 Chinese Companies Mean 2 44.1 49.1 48.7 83.3 Chinese Companies Median 44.1 49.1 48.7 83.3 Sinovac Biotech Ltd. 39.4 48.0 32.1 81.2
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- NOVAGOLD Announces Date of its 2024 Virtual Annual General Meeting of Shareholders
- Guanajuato Silver Announces Brokered LIFE Offering of Units
- RUM Reports Annual Financial Results with Increased Net Comprehensive Income for the Year Ended December 31, 2023
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share