Form F-10 Cresco Labs Inc.
Table of Contents
As filed with the Securities and Exchange Commission on February 26, 2021.
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM F-10
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Cresco Labs Inc.
(Exact name of Registrant as specified in its charter)
| British Columbia, Canada | 2833 | 98-1505364 | ||
| (Province or other Jurisdiction of Incorporation or Organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number, if applicable) |
400 W Erie St Suite 110
Chicago, IL 60654
United States
(312) 929-0993
(Address and telephone number of Registrant’s principal executive offices)
John Schetz, General Counsel
Cresco Labs Inc.
400 W Erie St Suite 110
Chicago, IL 60654
United States
(312) 929-0993
(Name, address (including zip code) and telephone number (including area code) of agent for service in the United States)
Copies to:
Heidi Steele
McDermott Will & Emery LLP
444 West Lake Street Suite 4000
Chicago, IL 60606
Telephone: (312) 372-2000
Approximate date of commencement of proposed sale of the securities to the public:
As soon as practicable after this registration statement becomes effective
British Columbia, Canada
(Principal jurisdiction regulating this offering)
It is proposed that this filing shall become effective (check appropriate box below):
| A. | ☐ upon filing with the Commission, pursuant to Rule 467(a) (if in connection with an offering being made contemporaneously in the United States and Canada). |
| B. | ☒ at some future date (check the appropriate box below) |
| 1. | ☐ pursuant to Rule 467(b) on ( ) at ( ) (designate a time not sooner than 7 calendar days after filing). |
| 2. | ☐ pursuant to Rule 467(b) on ( ) at ( ) (designate a time 7 calendar days or sooner after filing) because the securities regulatory authority in the review jurisdiction has issued a receipt or notification of clearance on ( ). |
| 3. | ☐ pursuant to Rule 467(b) as soon as practicable after notification of the Commission by the Registrant or the Canadian securities regulatory authority of the review jurisdiction that a receipt or notification of clearance has been issued with respect hereto. |
| 4. | ☒ after the filing of the next amendment to this Form (if preliminary material is being filed). |
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to the home jurisdiction’s shelf prospectus offering procedures, check the following box. ☒
CALCULATION OF REGISTRATION FEE
|
| ||||||
| Title of Each Class of Securities to be Registered |
Amount to be Registered(1) |
Proposed Maximum Aggregate Offering Price(1)(2) |
Amount of Registration Fee(2) | |||
| Subordinate Voting Shares |
||||||
| Debt Securities |
||||||
| Subscription Receipts |
||||||
| Warrants |
||||||
| Units |
||||||
| Total |
US$1,000,000,000 | US$1,000,000,000 | US$109,100 | |||
|
| ||||||
|
| ||||||
| (1) | There are being registered under this registration statement such indeterminate number of Subordinate Voting Shares, Debt Securities, Subscription Receipts, Warrants and Units of the Registrant as shall have an aggregate initial offering price of US$1,000,000,000. Any securities registered by this registration statement may be sold separately or as units with other securities registered under this registration statement. The proposed maximum initial offering price per security will be determined, from time to time, by the Registrant in connection with the sale of the securities under this registration statement. |
| (2) | Estimated solely for the purpose of calculating the amount of the registration fee pursuant to Rule 457 of the Securities Act of 1933, as amended (the “U.S. Securities Act”). |
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registration statement shall become effective as provided in Rule 467 under the Securities Act of 1933 or on such date as the Commission, acting pursuant to Section 8(a) of the Act, may determine.
Table of Contents
PART I
INFORMATION REQUIRED TO BE DELIVERED TO OFFEREES OR PURCHASERS
Table of Contents
A copy of this preliminary short form base shelf prospectus has been filed with securities regulatory authorities in each of the provinces of Canada but has not yet become final for the purpose of the sale of securities. A registration statement relating to these securities has also been filed with the U.S. Securities and Exchange Commission. Information contained in this preliminary short form base shelf prospectus may not be complete and may have to be amended. The securities may not be sold nor may offers to buy be accepted until a receipt for the short form base shelf prospectus is obtained from the applicable securities regulatory authorities. This short form base shelf prospectus does not constitute an offer to sell or a solicitation of an offer to buy, nor shall there be any sales of these securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction.
This preliminary short form base shelf prospectus has been filed under legislation in each of the provinces of Canada that permits certain information about these securities to be determined after this prospectus has become final and that permits the omission from this prospectus of that information. The legislation requires the delivery to purchasers of a prospectus supplement containing the omitted information within a specified period of time after agreeing to purchase any of these securities.
No securities regulatory authority has expressed an opinion about these securities and it is an offence to claim otherwise. This short form base shelf prospectus constitutes a public offering of these securities only in those jurisdictions where they may be lawfully offered for sale and therein only by persons permitted to sell such securities.
Information has been incorporated by reference in this short form base shelf prospectus from documents filed with securities commissions or similar authorities in Canada. Copies of the documents incorporated herein by reference may be obtained on request without charge from Cresco Labs Inc., at 400 W Erie St. #110, Chicago, IL, 60654, telephone 312-929-0993, and are also available electronically at www.sedar.com.
PRELIMINARY SHORT FORM BASE SHELF PROSPECTUS
| New Issue and Secondary Offering | February 26, 2021 |

CRESCO LABS INC.
US$1,000,000,000
Subordinate Voting Shares
Debt Securities
Subscription Receipts
Warrants
Units
Cresco Labs Inc. (“Cresco” or the “Corporation”) may from time to time offer and issue the following securities: (i) subordinate voting shares of the Corporation (“Subordinate Voting Shares”); (ii) debt securities of the Corporation (“Debt Securities”); (iii) subscription receipts (“Subscription Receipts”) exchangeable for Subordinate Voting Shares and/or other securities of the Corporation; (iv) warrants exercisable to acquire Subordinate Voting Shares and/or other securities of the Corporation (“Warrants”); and (v) securities comprised of more than one of Subordinate Voting Shares, Debt Securities, Subscription Receipts and/or Warrants offered together as a unit (“Units”), or any combination thereof having an offer price of up to US$1,000,000,000 in aggregate (or the equivalent thereof, at the date of issue, in any other currency or currencies, as the case may be) at any time during the 25-month period that this short form base shelf prospectus (including any amendments hereto, the “Prospectus”) remains valid. The Subordinate Voting Shares, Debt Securities, Subscription Receipts, Warrants and Units (collectively, the “Securities”) offered hereby may be offered in one or more offerings, separately or together, in separate series, in amounts, at prices and on terms to be set forth in one or more prospectus supplements (collectively or individually, as the case may be, “Prospectus Supplements”). In addition, one or more securityholders (each, a “Selling Securityholder”) of the Corporation may also offer and sell Securities under this Prospectus. See “Selling Securityholders”.
The Securities may be sold, from time to time in one or more transactions at a fixed price or prices which may be changed or at market prices prevailing at the time of sale, at prices related to such prevailing market prices or at negotiated prices, including sales made directly on the Canadian Securities Exchange (the “CSE”) or other existing trading markets for the Securities, and as set forth in an accompanying Prospectus Supplement. See “Plan of Distribution”.
Table of Contents
The specific terms of any offering of Securities will be set forth in the applicable Prospectus Supplement and may include, without limitation, where applicable: (i) in the case of Subordinate Voting Shares, the number of Subordinate Voting Shares being offered, the offering price, whether the Subordinate Voting Shares are being offered for cash, the person offering the Subordinate Voting Shares (the Corporation and/or the Selling Securityholder) and any other terms specific to the Subordinate Voting Shares being offered; (ii) in the case of Debt Securities, the specific designation, aggregate principal amount, the currency or the currency unit for which the Debt Securities may be purchased, maturity, interest provisions, authorized denominations, offering price, whether the Debt Securities are being offered for cash, the covenants, the events of default, any terms for redemption or retraction, any exchange or conversion rights attached to the Debt Securities, the person offering the Debt Securities (the Corporation and/or the Selling Securityholder) and any other terms specific to the Debt Securities being offered; (iii) in the case of Subscription Receipts, the number of Subscription Receipts being offered, the offering price, whether the Subscription Receipts are being offered for cash, the terms, conditions and procedures for the exchange of the Subscription Receipts into or for Subordinate Voting Shares and/or other securities of the Corporation, the person offering the Subscription Receipts (the Corporation and/or the Selling Securityholder) and any other terms specific to the Subscription Receipts being offered; (iv) in the case of Warrants, the number of such Warrants offered, the offering price, whether the Warrants are being offered for cash, the terms, conditions and procedures for the exercise of such Warrants into or for Subordinate Voting Shares and/or other securities of the Corporation, the person offering the Warrants (the Corporation and/or the Selling Securityholder) and any other specific terms; and (v) in the case of Units, the number of Units being offered, the offering price, the terms of the Subordinate Voting Shares, Debt Securities, Subscription Receipts and/or Warrants underlying the Units, the person offering the Units (the Corporation and/or the Selling Securityholder) and any other specific terms.
All shelf information permitted under applicable securities legislation to be omitted from this Prospectus will be contained in one or more Prospectus Supplements that will be delivered to purchasers together with this Prospectus. Each Prospectus Supplement will be incorporated by reference into this Prospectus as of the date of such Prospectus Supplement and only for the purposes of the distribution of the Securities covered by that Prospectus Supplement. The offerings are subject to approval of certain legal matters on behalf of the Corporation by Bennett Jones LLP.
This Prospectus does not qualify for issuance Debt Securities, or Securities convertible or exchangeable into Debt Securities, in respect of which the payment of principal and/or interest may be determined, in whole or in part, by reference to one or more underlying interests including, for example, an equity or debt security, a statistical measure of economic or financial performance including, without limitation, any currency, consumer price or mortgage index, or the price or value of one or more commodities, indices or other items, or any other item or formula, or any combination or basket of the foregoing items. This Prospectus may qualify for issuance Debt Securities, or Securities convertible or exchangeable into Debt Securities, in respect of which the payment of principal and/or interest may be determined, in whole or in part, by reference to published rates of a central banking authority or one or more financial institutions, such as a prime rate or bankers’ acceptance rate, or to recognized market benchmark interest rates such as CDOR (the Canadian Dollar Offered Rate) or LIBOR (the London Interbank Offered Rate), and/or convertible into or exchangeable for Subordinate Voting Shares and/or other securities of the Corporation.
The Corporation and the Selling Securityholders may sell the Securities, separately or together: (i) to one or more underwriters or dealers; (ii) through one or more agents; or (iii) directly to one or more purchasers. The Prospectus Supplement relating to a particular offering of Securities will describe the terms of such offering of Securities, including: (i) the terms of the Securities to which the Prospectus Supplement relates, including the type of Security being offered, and the method of distribution; (ii) the name or names of any underwriters, dealers or agents involved in such offering of Securities; (iii) the purchase price of the Securities offered thereby and the proceeds to, and the expenses borne by, the Corporation or the Selling Securityholder from the sale of such Securities; (iv) any commission, underwriting discounts and other items constituting compensation payable to underwriters, dealers or agents; (v) any discounts or concessions allowed or re-allowed or paid to underwriters, dealers or agents; and (vi) the identity of the Selling Securityholder, if any. See “Plan of Distribution”.
In connection with any offering of the Securities, subject to applicable laws (unless otherwise specified in the relevant Prospectus Supplement), the underwriters or agents may over-allot or effect transactions that stabilize or maintain the market price of the offered Securities at a level above that which might otherwise prevail on the open market. Such transactions, if commenced, may be interrupted or discontinued at any time. See “Plan of Distribution”.
- ii -
Table of Contents
The issued and outstanding Subordinate Voting Shares are listed and posted for trading on the CSE under the symbol “CL”. On February 25, 2021, the last trading day prior to the date of this Prospectus, the closing price per Subordinate Voting Share on the CSE was $18.19. Unless otherwise specified in the applicable Prospectus Supplement, the Debt Securities, Subscription Receipts, Warrants and Units will not be listed on any securities exchange. There is no market through which these Securities may be sold and purchasers may not be able to resell such Securities purchased under this Prospectus. This may affect the pricing of the Securities in the secondary market, the transparency and availability of trading prices, the liquidity of the Securities, and the extent of issuer regulation.
Investing in Securities is speculative and involves a high degree of risk and should only be made by persons who can afford the total loss of their investment. A prospective purchaser should therefore review this Prospectus and the documents incorporated by reference herein in their entirety and carefully consider the risk factors described or referenced under “Risk Factors” prior to investing in such Securities.
No underwriter, dealer or agent has been involved in the preparation of this Prospectus or performed any review of the contents of this Prospectus.
Note to U.S. Holders:
THESE SECURITIES HAVE NOT BEEN APPROVED OR DISAPPROVED BY THE U.S. SECURITIES AND EXCHANGE COMMISSION (THE “SEC”) NOR HAS THE SEC PASSED UPON THE ACCURACY OR ADEQUACY OF THIS PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The Corporation is permitted, under a multijurisdictional disclosure system adopted in the United States and Canada, to prepare this Prospectus in accordance with Canadian disclosure requirements. Prospective investors should be aware that such requirements are different from those of the United States. The Corporation prepares its annual financial statements and its interim financial statements, which have been included or incorporated by reference herein, in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board, and thus may not be comparable to financial statements of United States companies prepared under United States generally accepted accounting principles.
Purchasers of Securities should be aware that the acquisition of Securities may have tax consequences both in the United States and in Canada. This Prospectus does not discuss U.S. or Canadian tax consequences and any such tax consequences may not be described fully in any applicable Prospectus Supplement with respect to a particular offering of Securities. Prospective investors should consult their own tax advisors prior to deciding to purchase any of the Securities.
The enforcement by investors of civil liabilities under U.S. federal securities laws may be affected adversely by the fact that the Corporation is incorporated under the laws of British Columbia, Canada.
The Corporation has four classes of issued and outstanding shares: the Subordinate Voting Shares, the Proportionate Voting Shares of the Corporation (the “Proportionate Voting Shares”), the Super Voting Shares of the Corporation (the “Super Voting Shares”) and the Special Subordinate Voting Shares of the Corporation (the “Special Subordinate Voting Shares”). The Subordinate Voting Shares are “restricted securities” within the meaning of such term under applicable Canadian securities laws. Each Subordinate Voting Share is entitled to one vote per Subordinate Voting Share, each Proportionate Voting Share is entitled to one vote in respect of each Subordinate Voting Share into which such Proportionate Voting Share could ultimately then be converted, which is currently equal to 200 votes per Proportionate Voting Share, each Super Voting Share is currently entitled to 2,000 votes per Super Voting Share, and each Special Subordinate Voting Share is entitled to one vote in respect of each Subordinate Voting Share into which such Special Subordinate Voting Share could ultimately be converted, which is currently equal to 0.00001 of a vote per Special Subordinate Voting Share, in each case, on all matters upon which the holders of shares of the Corporation are entitled to vote, and holders of Subordinate Voting Shares, Proportionate Voting Shares, Super Voting Shares and Special Subordinate Voting Shares will vote together on all matters subject to a vote of holders of those
- iii -
Table of Contents
classes of shares as if they were one class of shares, except to the extent that a separate vote of holders as a separate class is required by law or provided by the articles of the Corporation. Other than the return of the issue price for their Super Voting Shares, the holders of Super Voting Shares are not entitled to receive, directly or indirectly, as holders of Super Voting Shares, any other assets or property of the Corporation. Holders of Subordinate Voting Shares, Proportionate Voting Shares and Special Subordinate Voting Shares are entitled to receive, as and when declared by the board of directors of the Corporation, dividends in cash or property of the Corporation. In the event of the liquidation, dissolution or winding-up of the Corporation, whether voluntary or involuntary, or in the event of any other distribution of assets of the Corporation among its shareholders for the purpose of winding up its affairs, the holders of Subordinate Voting Shares are, subject to the prior rights of the holders of any shares of the Corporation ranking in priority to the Subordinate Voting Shares (including, without restriction, the Super Voting Shares as to the issue price paid in respect thereof), entitled to participate rateably along with all other holders of Subordinate Voting Shares. In the event that a take-over bid is made for the Super Voting Shares, the holders of Subordinate Voting Shares will not be entitled to participate in such offer and may not tender their shares into any such offer, whether under the terms of the Subordinate Voting Shares or under any coattail trust or similar agreement. Notwithstanding this, any take-over bid for solely the Super Voting Shares is unlikely given that by the terms of the investment agreement entered into by the Corporation and the Founders (as defined herein) in connection with the issuance to the Founders of the Super Voting Shares, upon any sale of Super Voting Shares to an unrelated third party purchaser, such Super Voting Shares will be redeemed by the Corporation for their issue price. See “Description of Share Capital of the Corporation” for further details.
The directors, chief executive officer and chief financial officer of the Corporation reside outside of Canada and each has appointed Bennett Jones LLP, 3400 One First Canadian Place, Toronto, Ontario, M5X 1A4, as his or her agent for service of process in Canada. Marcum LLP, the auditor in respect of the audited financial statements of the Corporation, as at and for the years ended December 31, 2020 and 2019, is incorporated, continued or otherwise organized under the laws of a foreign jurisdiction. Purchasers are advised that it may not be possible for investors to enforce judgments obtained in Canada against any person or company that resides outside of Canada or is incorporated, continued or otherwise organized under the laws of a foreign jurisdiction, even if the party has appointed an agent for service of process.
The Corporation’s head office is located at 400 W Erie St. #110, Chicago, IL, 60654 and registered office is located at Suite 2500, 666 Burrard Street, Vancouver, BC, V6C 2X8.
This Prospectus qualifies the distribution of securities of an entity that currently directly derives a substantial portion of its revenues from the cannabis industry in certain U.S. states, which industry is illegal under U.S. federal law. The Corporation is directly involved (through its licensed subsidiaries) in both the adult-use and medical cannabis industry in the States of Illinois, Pennsylvania, Ohio, Nevada, Arizona and California, as permitted within such states under applicable state law which states have regulated such industries, and is in the process of acquiring businesses which would allow the Corporation to directly participate in the adult-use and medical cannabis industry in the States of New York, Massachusetts, Florida and Maryland, as permitted within such states under applicable state law and which states have regulated such industries.
The cultivation, sale and use of cannabis is illegal under United States federal law pursuant to the Controlled Substance Act (21 U.S.C. §811) (the “CSA”). The United States federal government regulates drugs through the CSA, which places controlled substances, including cannabis, in a schedule. Other than industrial hemp, cannabis is classified as a Schedule I drug. Under United States federal law, a Schedule I drug or substance has a high potential for abuse, no accepted medical use in the United States, and a lack of accepted safety for the use of the drug under medical supervision. Under the CSA, the policies and regulations of the United States federal government and its agencies are that cannabis has no medical benefit and a range of activities including cultivation and the personal use of cannabis is prohibited. The United States Food and Drug Administration (“FDA”) has not approved cannabis for the treatment of any disease or condition. The agency has, however, approved one cannabis-derived drug product, Epidiolex, for the treatment of seizures associated with Lennos-Gastaut syndrome or Dravet syndrome.
Despite the current state of the federal law and the CSA, 35 U.S. states, Washington D.C., and the territories of Puerto Rico, the U.S. Virgin Islands, the Northern Mariana Islands and Guam have laws and/or regulations that recognize, in one form or another, legitimate medical uses for cannabis and consumer use of cannabis in
- iv -
Table of Contents
connection with medical treatment for patients with certain qualifying conditions. The States of Alaska, Arizona, California, Colorado, Illinois, Maine, Massachusetts, Michigan, Montana, Nevada, New Jersey, Oregon, South Dakota, Vermont, Washington, and the District of Columbia, have legalized recreational use of cannabis, although the District of Columbia has not legalized commercial sale of cannabis. In early 2018, Vermont became the first state to legalize recreational cannabis by passage in a state legislature, but does not yet allow commercial sales of recreational cannabis.
Over half of the U.S. states have enacted legislation to legalize and regulate the sale and use of medical cannabis, provided that there are strict limits on the levels of THC. However, there is no guarantee that state laws legalizing and regulating the sale and use of cannabis will not be repealed or overturned, or that local governmental authorities will not limit the applicability of state laws within their respective jurisdictions.
Accordingly, in the U.S., cannabis is largely regulated at the state level. State laws that permit and regulate the production, distribution and use of cannabis for adult-use or medical purposes are in direct conflict with the CSA. Although certain states authorize medical or adult-use cannabis production and distribution by licensed or registered entities, under United States federal law, the possession, use, cultivation, and transfer of cannabis and any related drug paraphernalia is illegal and any such acts are criminal acts. The Supremacy Clause of the United States Constitution establishes that the United States Constitution and federal laws made pursuant to it are paramount and in case of conflict between federal and state law, the federal law shall apply.
On January 4, 2018, former U.S. Attorney General Jeff Sessions issued a memorandum to U.S. district attorneys which rescinded previous guidance from the U.S. Department of Justice (“DOJ”) specific to cannabis enforcement in the United States, including the Cole Memorandum (as defined herein). With the Cole Memorandum rescinded, U.S. federal prosecutors have been given discretion in determining whether to prosecute cannabis related violations of U.S. federal law. Mr. Sessions resigned on November 7, 2018. Following the brief tenure of Matthew Whitaker as the acting United States Attorney General, on December 7, 2018, President Donald Trump announced the nomination of William Barr and, on February 14, 2019, Mr. Barr was confirmed as Attorney General. The DOJ under Mr. Barr did not take a formal position on federal enforcement of laws relating to cannabis. On December 14, 2020, President Trump announced that Mr. Barr would be resigning from his post as Attorney General, effective December 23, 2020. President Joseph Biden has nominated Merrick Garland to succeed Mr. Barr as the U.S. Attorney General. It is unclear what impact, if any, the new administration will have on U.S. federal government enforcement policy on cannabis. If the DOJ policy shifts to aggressively pursue financiers or equity owners of cannabis-related business, and United States Attorneys followed such policies through pursuing prosecutions, then the Corporation could face (i) seizure of its cash and other assets used to support or derived from its cannabis subsidiaries, and (ii) the arrest of its employees, directors, officers, managers and investors, who could face charges of ancillary criminal violations of the CSA for aiding and abetting and conspiring to violate the CSA by virtue of providing financial support to state-licensed or permitted cultivators, processors, distributors, and/or retailers of cannabis. Additionally, as has recently been affirmed by the U.S. Customs and Border Protection, employees, directors, officers, managers and investors of the Corporation who are not U.S. citizens face the risk of being barred from entry into the United States for life.
On December 27, 2020, President Donald Trump signed the Consolidated Appropriations Act of 2021, which included the Rohrabacher-Farr Amendment (as defined herein), which prohibits the funding of federal prosecutions with respect to medical cannabis activities that are legal under state law. The Consolidated Appropriations Act of 2021 makes appropriations for the fiscal year ending September 30, 2021. There can be no assurances that the Rohrabacher- Farr Amendment will be included in future appropriations bills or budget resolutions. See “United States Regulatory Environment” for additional information.
The Corporation’s objective is to capitalize on the opportunities presented as a result of the changing regulatory environment governing the cannabis industry in the United States. Accordingly, there are a number of significant risks associated with the business of the Corporation. Unless and until the United States Congress amends the CSA with respect to medical and/or adult-use cannabis (and as to the timing or scope of any such potential amendments there can be no assurance), there is a significant risk that federal authorities may enforce current U.S. federal law, and the business of the Corporation may be deemed to be producing, cultivating, extracting, or dispensing cannabis or aiding or abetting or otherwise engaging in a conspiracy to commit such
- v -
Table of Contents
acts in violation of federal law in the United States. If the U.S. federal government begins to enforce U.S. federal laws relating to cannabis in states where the sale and use of cannabis is currently legal, or if existing applicable state laws are repealed or curtailed, the Corporation’s business, results of operations, financial condition and prospects would be materially adversely affected.
In light of the political and regulatory uncertainty surrounding the treatment of United States cannabis-related activities, on February 8, 2018, the Canadian Securities Administrators published CSA Staff Notice 51-352 – (Revised) Issuers with U.S. Marijuana-Related Activities (“Staff Notice 51-352”) setting out the Canadian Securities Administrator’s disclosure expectations for specific risks facing issuers with cannabis-related activities in the United States. Staff Notice 51-352 includes additional disclosure expectations that apply to all issuers with United States cannabis-related activities, including those with direct and indirect involvement in the cultivation and distribution of cannabis, as well as issuers that provide goods and services to third parties involved in the United States cannabis industry.
For these reasons, the Corporation’s investments in the United States cannabis market may subject the Corporation to heightened scrutiny by regulators, stock exchanges, clearing agencies and other United States and Canadian authorities. There are a number of risks associated with the business of the Corporation. See the section entitled “Risk Factors” herein and within the AIF (as defined herein).
- vi -
Table of Contents
| 10 | ||||
| 10 | ||||
| 10 | ||||
| 11 | ||||
| 11 | ||||
| 11 | ||||
| 13 | ||||
| 15 | ||||
| 18 | ||||
| 24 | ||||
| 25 | ||||
| 26 | ||||
| 27 | ||||
| 27 | ||||
| 27 | ||||
| 28 | ||||
| 28 | ||||
| 29 | ||||
| 30 | ||||
| 30 | ||||
| 30 | ||||
| 38 | ||||
| 38 | ||||
| 38 | ||||
| 43 | ||||
| 43 | ||||
| 43 | ||||
| 43 | ||||
| 43 | ||||
| 44 | ||||
Table of Contents
ABOUT THIS SHORT FORM BASE SHELF PROSPECTUS
An investor should rely only on the information contained in this Prospectus (including the documents incorporated by reference herein) and is not entitled to rely on parts of the information contained in this Prospectus (including the documents incorporated by reference herein) to the exclusion of others. The Corporation has not authorized anyone to provide investors with additional or different information. The Corporation takes no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give readers of this Prospectus. Information contained on, or otherwise accessed through, the Corporation’s website shall not be deemed to be a part of this Prospectus and such information is not incorporated by reference herein.
The Corporation is not offering to sell the Securities in any jurisdictions where the offer or sale of the Securities is not permitted. The information contained in this Prospectus (including the documents incorporated by reference herein) is accurate only as of the date of this Prospectus or as of the date as otherwise set out herein (or as of the date of the document incorporated by reference herein or as of the date as otherwise set out in the document incorporated by reference herein, as applicable), regardless of the time of delivery of this Prospectus or any sale of the Subordinate Voting Shares, Debt Securities, Subscription Receipts, Warrants and/or Units. The business, financial condition, capital, results of operations and prospects of the Corporation may have changed since those dates. The Corporation does not undertake to update the information contained or incorporated by reference herein, except as required by applicable Canadian securities laws.
This Prospectus shall not be used by anyone for any purpose other than in connection with an offering of Securities as described in one or more Prospectus Supplements.
The documents incorporated or deemed to be incorporated by reference herein contain meaningful and material information relating to the Corporation and readers of this Prospectus should review all information contained in this Prospectus, the applicable Prospectus Supplement and the documents incorporated or deemed to be incorporated by reference herein and therein.
ENFORCEMENT OF CIVIL LIABILITIES
Cresco is incorporated under and governed by the Business Corporations Act (British Columbia). The Corporation has appointed an agent for service of process in the United States; however it may nevertheless be difficult for investors who reside in the United States to effect service of process in the United States upon the Corporation or to enforce a U.S. court judgment predicated upon the civil liability provisions of the U.S. federal securities laws against the Corporation. There is substantial doubt whether an action could be brought in Canada in the first instance predicated solely upon U.S. federal securities laws.
The Corporation filed with the SEC, concurrently with the Registration Statement (as defined herein), an appointment of agent for service of process on Form F-X. Under the Form F-X, the Corporation appointed Cresco Labs, LLC (the “LLC”) as its agent for service of process in the United States in connection with any investigation or administrative proceeding conducted by the SEC and any civil suit or action brought against or involving the Corporation in a U.S. court arising out of or related to or concerning the offering of Securities under this Prospectus.
MEANING OF CERTAIN REFERENCES AND CURRENCY PRESENTATION
References to dollars or “$” are to Canadian currency unless otherwise indicated. All references to “US$” refer to United States dollars. On February 25, 2021, the daily exchange rate for the United States dollar in terms of Canadian dollars, as quoted by the Bank of Canada, was US$1.00 = $1.2530.
Unless the context otherwise requires, all references in this Prospectus to the “Corporation” refer to the Corporation and its subsidiary entities on a consolidated basis.
Table of Contents
WHERE YOU CAN FIND MORE INFORMATION
The Corporation files certain reports with, and furnishes other information to, each of the SEC and certain securities regulatory authorities of Canada. Under a multijurisdictional disclosure system adopted by the United States and Canada, such reports and other information may be prepared in accordance with the disclosure requirements of the provincial and territorial securities regulatory authorities of Canada, which requirements are different from those of the United States. As a foreign private issuer, the Corporation is exempt from the rules under the U.S. Securities Exchange Act of 1934, as amended (the “U.S. Exchange Act”), prescribing the furnishing and content of proxy statements, and the Corporation’s officers and directors are exempt from the reporting and short swing profit recovery provisions contained in Section 16 of the U.S. Exchange Act. The Corporation’s reports and other information filed or furnished with or to the SEC are available, from EDGAR at www.sec.gov, as well as from commercial document retrieval services. The Corporation’s Canadian filings are available on SEDAR at www.sedar.com.
The Corporation has filed with the SEC under the U.S. Securities Act of 1933, as amended (the “U.S. Securities Act”) the Registration Statement (as defined herein) relating to the Securities being offered hereunder, of which this Prospectus forms a part. This Prospectus does not contain all of the information set forth in the Registration Statement, certain items of which are contained in the exhibits to the Registration Statement as permitted or required by the rules and regulations of the SEC. Items of information omitted from this Prospectus but contained in the Registration Statement will be available on the SEC’s website at www.sec.gov.
Unless otherwise indicated, the market and industry data contained or incorporated by reference in this Prospectus is based upon information from independent industry publications, market research, analyst reports and surveys and other publicly available sources. Although the Corporation believes these sources to be generally reliable, market and industry data is subject to interpretation and cannot be verified with complete certainty due to limits on the availability and reliability of raw data, the voluntary nature of the data gathering process and other limitations and uncertainties inherent in any survey. The Corporation has not independently verified any of the data from third party sources referred to or incorporated by reference herein, and accordingly the accuracy and completeness of such data is not guaranteed.
CAUTION REGARDING FORWARD-LOOKING STATEMENTS
This Prospectus includes “forward-looking information” and “forward-looking statements” within the meaning of Canadian securities laws and United States securities laws. In addition to the following cautionary statement, with respect to forward-looking information contained in the documents incorporated by reference herein, prospective purchasers should refer to “Cautionary Statement Regarding Forward-Looking Information” in the AIF (as defined herein) or any subsequently filed annual information form of the Corporation, as well as the advisories section of any documents incorporated or deemed to be by reference herein, including those that are filed after the date hereof.
All information, other than statements of historical facts, included in this Prospectus that address activities, events or developments that the Corporation expects or anticipates will or may occur in the future is forward-looking information. Forward-looking information is often identified by the words “may”, “would”, “could”, “should”, “will”, “intend”, “plan”, “anticipate”, “believe”, “estimate”, “expect” or similar expressions and includes, among others, information regarding: the Corporation’s intention regarding cash flows from operating activities in future periods, the expected timing of the closing of the Arrangement (as defined herein), statements relating to the business and future activities of, and developments related to, the Corporation after the date of this Prospectus, including but not limited to, such things as future business strategy, competitive strengths, goals, expansion and growth of the Corporation’s business, operations and plans, including new revenue streams, the completion of contemplated acquisitions by the Corporation, the application for additional licenses and the grant of licenses that have been applied for, the expansion of existing cultivation and production facilities, the completion of cultivation and production facilities that are under construction, the construction of additional cultivation and production facilities, the expansion into additional States within the United States, international markets and Canada, any potential future legalization of adult-use and/or medical marijuana under U.S. federal law; expectations of market size and growth in the United States and the States in which the Corporation operates; expectations for other economic, business, regulatory and/or competitive factors related to the Corporation or the cannabis industry generally; and other events or conditions that may occur in the future.
- 2 -
Table of Contents
Readers are cautioned that forward-looking information and statements are not based on historical facts but instead are based on reasonable assumptions, estimates, analysis and opinions of management of the Corporation at the time they were provided or made, in light of its experience and its perception of trends, current conditions and expected developments, as well as other factors that management believes to be relevant and reasonable in the circumstances, and involve known and unknown risks, uncertainties and other factors which may cause the actual results, performance or achievements of the Corporation, as applicable, to be materially different from any future results, performance or achievements expressed or implied by such forward-looking information and statements.
Forward-looking information and statements are not a guarantee of future performance and are based upon a number of estimates and assumptions of management at the date the statements are made including, among other things, assumptions about: the contemplated acquisitions and dispositions being completed on the current terms and current contemplated timeline, including that all conditions to the completion of the Arrangement will be satisfied in a timely manner; development costs remaining consistent with budgets; ability to manage anticipated and unanticipated costs; favorable equity and debt capital markets; the ability to raise sufficient capital to advance the business of the Corporation; favorable operating and economic conditions; political and regulatory stability; obtaining and maintaining all required licenses and permits; receipt of governmental approvals and permits; sustained labor stability; stability in financial and capital goods markets; favourable production levels and costs from the Corporation’s operations; the pricing of various cannabis products; the level of demand for cannabis products; the availability of third party service providers and other inputs for the Corporation’s operations; and the Corporation’s ability to conduct operations in a safe, efficient and effective manner. While the Corporation considers these assumptions to be reasonable, the assumptions are inherently subject to significant business, social, economic, political, regulatory, competitive and other risks and uncertainties, contingencies and other factors that could cause actual performance, achievements, actions, events, results or conditions to be materially different from those projected in the forward-looking information and statements. Many assumptions are based on factors and events that are not within the control of the Corporation and there is no assurance they will prove to be correct.
Risks, uncertainties and other factors which may cause the actual results, performance or achievements of the Corporation, as applicable, to be materially different from any future results, performance or achievements expressed or implied by such forward-looking information and statements include, among others, risks relating to the availability of future financing; the use of proceeds of future financings; the accuracy of forward-looking information; regulatory uncertainty; money laundering laws and access to banking; heightened scrutiny of cannabis companies in Canada; proceedings against the Corporation; significant ongoing costs and obligations related to the Corporation’s investment in infrastructure; regulatory compliance and operations; availability of favourable locations; unfavorable tax treatment of cannabis businesses; the tax classification of the Corporation; the Corporation being a holding corporation; enforcement of contracts; competition; limitations on owners of licenses; difficulty in forecasting; the concentrated voting control of the Corporation and the unpredictability caused by the existing capital structure; dilution; volatility of market price; negative cash flows; U.S. regulatory landscape and enforcement related to cannabis, including political risks; risks relating to anti-money laundering laws and regulation; other governmental and environmental regulation; public opinion and perception of the cannabis industry; risks related to the ability to consummate the proposed acquisitions and the ability to obtain requisite regulatory approvals and third party consents and the satisfaction of other conditions to the consummation of the Arrangement and other proposed acquisitions on the proposed terms and schedule; the potential impact of the announcement or consummation of the proposed acquisitions on relationships, including with regulatory bodies, employees, suppliers, customers and competitors; the diversion of management time on the proposed acquisitions; risks related to contracts with third party service providers; risks related to the enforceability of contracts; the limited operating history of the Corporation; reliance on the expertise and judgment of senior management of the Corporation; risks inherent in an agricultural business; risks related to co-investment with parties with different interests to the Corporation; risks related to proprietary intellectual property and potential infringement by third parties; risks relating to financing activities including leverage; risks relating to the management of growth; increased costs associated with the Corporation becoming a publicly traded company; increasing competition in the industry; risks relating to energy costs; risks associated to cannabis products manufactured for human consumption including potential product recalls; reliance on key inputs, suppliers and skilled labour (the availability and retention of which is subject to uncertainty); cybersecurity risks; ability and constraints on marketing products; fraudulent activity by employees, contractors and consultants; tax and insurance related risks;
- 3 -
Table of Contents
risks related to the economy generally; risk of litigation; conflicts of interest; risks relating to certain remedies being limited and the difficulty of enforcement of judgments and effecting service outside of Canada; risks related to future acquisitions or dispositions; sales by existing shareholders; the limited market for securities of the Corporation; limited research and data relating to cannabis; the effects of the COVID-19 pandemic; as well as those risk factors discussed elsewhere herein and in the documents incorporated by reference herein, including the AIF.
Readers are cautioned that the foregoing lists are not exhaustive of all factors and assumptions that may have been used. Although the Corporation has attempted to identify important factors that could cause actual results to differ materially, there may be other factors that cause results not to be as anticipated, estimated or intended. There can be no assurance that such forward-looking information and statements will prove to be accurate as actual results and future events could differ materially from those anticipated in such information and statements. Accordingly, readers should not place undue reliance on forward-looking information and statements. The forward-looking information and statements contained herein are presented for the purposes of assisting readers in understanding the Corporation’s expected financial and operating performance and the Corporation’s plans and objectives and may not be appropriate for other purposes.
The forward-looking information and statements contained in this Prospectus represent the Corporation’s views and expectations as of the date of this Prospectus and forward-looking information and statements contained in the documents incorporated by reference herein represent the Corporation’s views and expectations as of the date of such documents, unless otherwise indicated in such documents. The Corporation anticipates that subsequent events and developments may cause its views and expectations to change. However, while the Corporation may elect to update such forward- looking information and statements at a future time, it has no current intention of and assumes no obligation for doing so except to the extent required by applicable law.
DOCUMENTS INCORPORATED BY REFERENCE
Information has been incorporated by reference in this Prospectus from documents filed with the securities commissions or similar regulatory authorities in Canada. Copies of the documents incorporated herein by reference may be obtained on request without charge from the General Counsel of the Corporation, at 400 W Erie St. #110, Chicago, IL, 60654, 312-929-0993, and are also available electronically at www.sedar.com.
As of the date hereof, the following documents (or the sections or sub-sections thereof set out below), filed with the various securities commissions or similar authorities in each of the provinces of Canada, are specifically incorporated by reference into, and form an integral part of, this Prospectus:
| 1. | the annual information form of the Corporation dated April 28, 2020 (the “AIF”); |
| 2. | the unaudited condensed interim financial statements of the Corporation for the three and nine month periods ended September 30, 2020 and 2019, together with the notes thereon filed on November 18, 2020; |
| 3. | the management’s discussion and analysis of financial condition and results of operations of the Corporation for the three months and nine month periods ended September 30, 2020 and 2019, filed on November 18, 2020; |
| 4. | the audited financial statements of the Corporation for the years ended December 31, 2019 and 2018, together with the notes thereto and the auditor’s report for the year ended December 31, 2019 attached thereto, filed on April 28, 2020; |
| 5. | the management’s discussion and analysis of financial condition and results of operations of the Corporation for the three and twelve month periods ended December 31, 2019 and 2018, filed on April 28, 2020; |
| 6. | the sub-section entitled “Summary of the Equity Plan” of Section 9 (Options to Purchase Securities) of the listing statement of the Corporation dated November 30, 2018; |
- 4 -
Table of Contents
| 7. | the management information circular of the Corporation dated June 3, 2020, prepared in connection with an annual general and special meeting of shareholders held on June 29, 2020, filed on June 8, 2020; |
| 8. | the material change report dated January 13, 2020, in connection with the completion of the court approved plan of arrangement pursuant to which the Corporation acquired all of the issued and outstanding shares of CannaRoyalty Corp. d/b/a Origin House (“Origin House”); |
| 9. | the material change report of the Corporation dated February 3, 2020, in connection with the Corporation’s entry into a credit agreement for a senior secured term loan (the “Senior Secured Loan”) in an initial aggregate principal amount of US$100 million; |
| 10. | the material change report of the Corporation dated March 12, 2020, in connection with the resignation of Joseph Caltabiano from his former position as the Corporation’s President; |
| 11. | the material change report of the Corporation dated January 19, 2021, in connection with the Corporation’s entry into an arrangement agreement with Bluma Wellness Inc. (“Bluma”), pursuant to which the Corporation will acquire all of the issued and outstanding shares of Bluma; and |
| 12. | the material change report of the Corporation dated February 1, 2021, in connection with the closing of the Corporation’s overnight marketed offering of Subordinate Voting Shares. |
Any document of the type required by National Instrument 44-101 — Short Form Prospectus Distributions to be incorporated by reference into a short form prospectus, including any annual information forms, material change reports (except confidential material change reports), business acquisition reports, interim financial statements, annual financial statements and the auditor’s report thereon, management’s discussion and analysis and information circulars of the Corporation filed by the Corporation with securities commissions or similar authorities in Canada after the date of this Prospectus and prior to the completion or withdrawal of any offering under this Prospectus shall be deemed to be incorporated by reference into this Prospectus. In addition, all documents filed on Form 6-K or Form 40-F by the Corporation with the SEC on or after the date of this Prospectus shall be deemed to be incorporated by reference into the registration statement on Form F-10 (the “Registration Statement”) of which this Prospectus forms a part, if and to the extent, in the case of any report on Form 6-K, expressly provided in such document.
Upon a new interim financial report and related management’s discussion and analysis of the Corporation being filed with the applicable securities regulatory authorities during the currency of this Prospectus, the previous interim financial report and related management’s discussion and analysis of the Corporation most recently filed shall be deemed no longer to be incorporated by reference into this Prospectus for purposes of future offers and sales of Securities hereunder. Upon new annual financial statements and related management’s discussion and analysis of the Corporation being filed with the applicable securities regulatory authorities during the currency of this Prospectus, the previous annual financial statements and related management’s discussion and analysis and the previous interim financial report and related management’s discussion and analysis of the Corporation most recently filed shall be deemed no longer to be incorporated by reference into this Prospectus for purposes of future offers and sales of Securities hereunder. Upon a new annual information form of the Corporation being filed with the applicable securities regulatory authorities during the currency of this Prospectus, the following documents shall be deemed no longer to be incorporated by reference into this Prospectus for purposes of future offers and sales of Securities hereunder: (i) the previous annual information form, if any; (ii) material change reports filed by the Corporation prior to the end of the financial year in respect of which the new annual information form is filed; (iii) business acquisition reports filed by the Corporation for acquisitions completed prior to the beginning of the financial year in respect of which the new annual information form is filed; and (iv) any information circular of the Corporation filed by the Corporation prior to the beginning of the financial year in respect of which the new annual information form is filed. Upon a new information circular of the Corporation prepared in connection with an annual general meeting of the Corporation being filed with the applicable securities regulatory authorities during the currency of this Prospectus, the previous information circular of the Corporation prepared in connection with an annual general meeting of the Corporation shall be deemed no longer to be incorporated by reference into this Prospectus for purposes of future offers and sales of Securities hereunder.
- 5 -
Table of Contents
A Prospectus Supplement to this Prospectus containing the specific variable terms in respect of an offering of the Securities will be delivered to purchasers of such Securities together with this Prospectus, unless an exemption from the prospectus delivery requirements has been granted or is otherwise available, and will be deemed to be incorporated by reference into this Prospectus as of the date of such Prospectus Supplement only for the purposes of the offering of the Securities covered by such Prospectus Supplement.
Notwithstanding anything herein to the contrary, any statement contained in this Prospectus or in a document incorporated or deemed to be incorporated by reference herein shall be deemed to be modified or superseded, for purposes of this Prospectus, to the extent that a statement contained herein or in any other subsequently filed document incorporated or deemed to be incorporated by reference herein modifies or supersedes such prior statement. The modifying or superseding statement need not state that it has modified or superseded a prior statement or include any other information set forth in the document that it modifies or supersedes. The making of a modifying or superseding statement shall not be deemed an admission for any purpose that the modified or superseded statement, when made, constituted a misrepresentation, an untrue statement of a material fact or an omission to state a material fact that is required to be stated or that is necessary to make a statement not misleading in light of the circumstances in which it was made. Any statement so modified or superseded shall thereafter neither constitute, nor be deemed to constitute, a part of this Prospectus, except as so modified or superseded.
Corporate Structure
The Corporation was incorporated in the Province of British Columbia under the Business Corporations Act (British Columbia) on July 6, 1990. On December 30, 1997, the Corporation changed its name from Randsburg Gold Corporation to Randsburg International Gold Corp. (“Randsburg”), and consolidated its outstanding common shares on a five old for one new basis. On November 30, 2018, in connection with the Business Combination (as defined herein), the Corporation (i) consolidated its outstanding Randsburg common shares on a 812.63 old for one new basis by way of resolution of its board of directors (without any corporate filings being necessary), and (ii) filed an alteration to its Notice of Articles with the British Columbia Registrar of Companies to change its name from Randsburg International Gold Corp. to Cresco Labs Inc. and to amend the rights and restrictions of its existing class of common shares, redesignate such class as the class of Subordinate Voting Shares and create the Proportionate Voting Shares and the Super Voting Shares (collectively, the “Share Terms Amendment”). On June 29, 2020, the Corporation filed an alteration to its Notice of Articles with the British Columbia Registrar of Companies to create a class of Special Subordinate Voting Shares and amend the rights and restrictions of the Subordinate Voting Shares, the Super Voting Shares and the Proportionate Voting Shares.
The Corporation’s head office is located at 400 W Erie St #110, Chicago, IL 60654 and the Corporation’s registered office is located at Suite 2500, 666 Burrard Street, Vancouver, BC V6C 2X8.
On November 30, 2018, a series of transactions were completed among the Corporation (then Randsburg) and Cresco resulting in a reorganization of Cresco and Randsburg and pursuant to which Randsburg became the indirect parent and sole voting unitholder of Cresco (the “Business Combination”). The Business Combination constituted a reverse takeover of Randsburg by Cresco under applicable securities laws.
Cresco Labs LLC was formed as a limited liability company under the laws of the state of Illinois on October 8, 2013 and is governed by the Cresco limited liability company agreement dated October 8, 2013, as amended and restated as of March 28, 2015 and as further amended and restated as of March 17, 2018 and as of July 1, 2018 (the “Pre-Combination LLC Agreement”). The Pre-Combination LLC Agreement was further amended and restated in connection with the completion of the Business Combination.
Set forth below is the condensed organization chart of the Corporation. The material subsidiaries of Cresco did not change in connection with the Business Combination.
- 6 -
Table of Contents
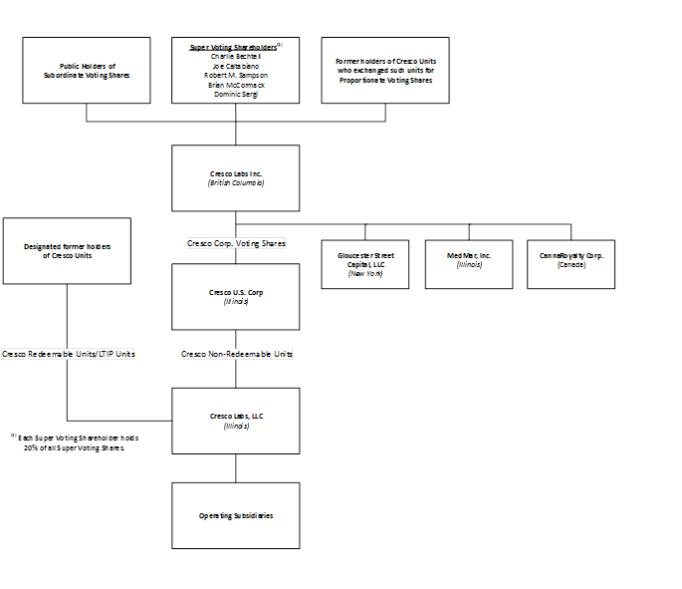
Note: See “Description of Share Capital of the Corporation” herein, “Description of Share Capital of Cresco Corp.” in the AIF and “Description of Unit Capital of Cresco” in the AIF for additional details as to the share and unit capital of the Corporation, Cresco U.S. Corp. (“Cresco Corp.”) and the LLC, respectively.
Summary Description of the Business
Cresco exists to provide high-quality and consistent cannabis-based products to consumers. Cresco blends regulatory compliance expertise with best practices from the agricultural, pharmaceutical and consumer packaged goods industries. Cresco (either directly or indirectly through subsidiaries) has been awarded three licenses to cultivate and manufacture medicinal cannabis in the State of Illinois. Cresco was awarded a cultivation license in Pennsylvania and was one of only five cultivators that was initially also awarded a dispensary license which allows for up to three dispensaries, with a second license granted in December of 2018 for up to three additional dispensaries. Cresco was awarded a cultivation license in Ohio and a dispensary license in Ohio and was the first approved dispensary to begin dispensary operations in Ohio in December 2018. Cresco received prequalification from the State of Michigan, which will allow Cresco to operate growing, processing and provisioning center facilities in Michigan. Cresco also has an interest in a cultivation, processing, and dispensary license in Nevada, an ownership interest in cultivation and processing licenses in California, owns and operates five dispensaries in Illinois and owns and operates two cultivation centers and one dispensary location in Arizona. Cresco acquired one medical cannabis cultivation center license and
- 7 -
Table of Contents
four dispensary locations in New York. Most recently, Cresco was the first cultivator in Illinois to receive approvals to grow adult-use cannabis; all three cultivation facilities were granted approvals in the state. Additionally, Cresco’s five Illinois dispensary locations were approved for dispensing adult-use cannabis in the state upon legalization, effective January 1, 2020. Cresco has completed an agreement to acquire assets in Massachusetts, including state registration and licensing that will allow for cultivation, manufacturing, processing, and the establishment and operation of a medical marijuana dispensary, with the ability to obtain up to three medical marijuana dispensary licenses and three adult-use dispensary licenses. Cresco has also completed its acquisition of operations in California, via the acquisition of Origin House (as defined herein). Additionally, Cresco has entered into an agreement to acquire operations in Florida, via Bluma. See “Recent Developments”.
Cresco plans to leverage the success in these markets to expand into legalized cannabis markets in other states, while focusing on compliance, control, efficiency, and product performance in the medicinal or adult-use cannabis industry.
Cresco owns and operates cultivation, manufacturing and retail dispensary businesses. The manufacturing and retail businesses are operational today and vertically integrated across eight highly regulated and/or limited licenses, and therefore limited legal supply markets: Illinois, Nevada, Ohio, Arizona, Pennsylvania, California, New York and Massachusetts, with processing operations in Maryland, and is expected to commence cultivation, manufacturing and retail dispensary operations in Michigan. These markets, where supply and demand can be reasonably predicted and forecasted, create the foundation upon which Cresco has created the opportunity for sustainable growth. Importantly, Cresco is not yet active in markets popularized by mainstream media like Washington, Oregon and Colorado where loose regulatory frameworks create unpredictable supply-demand market dynamics.
This ownership of wholesale and retail businesses supports Cresco’s strategy of distributing brands at scale by enabling Cresco to capture market share, generate brand awareness, and earn customer loyalty in its operating markets by guaranteeing share-of-shelf in its own retail stores and its ability to foster mutually beneficial relationships with its third-party dispensary customers as a large supplier of a portfolio of distinct and trusted cannabis brands. More detailed information regarding the business of the Corporation as well as its operations, assets, and properties can be found in the AIF and other documents incorporated by reference herein, as supplemented by the disclosure herein. See “Documents Incorporated by Reference” and “Recent Developments”.
Recent Developments
Increase and Extension of Credit Facility
On December 14, 2020, the Corporation announced an increase in its Senior Secured Loan from US$100 million to US$200 million. Also, as part of the agreement with the Corporation’s lenders, the Senior Secured Loan was extended to January 23, 2023 at a reduced interest rate of 12% per annum and the Corporation was provided with greater prepayment optionality.
Bluma Wellness Inc.
On January 14, 2021, the Corporation announced that it had entered into an arrangement agreement with Bluma, a publicly traded company, to acquire all of the issued and outstanding shares of Bluma (the “Arrangement”) on the basis of 0.0859 of a Subordinate Voting Share for each common share of Bluma, subject to certain adjustments as described in the arrangement agreement. On the date of announcement, total consideration for the Arrangement was equal to US$213 million, or US$1.12 per Bluma share. The Arrangement is proposed to be effected by way of a plan of arrangement under the Business Corporations Act (British Columbia). Bluma, under its operating subsidiary, One Plant Florida, has seven strategically located dispensaries with eight more locations under legal control and planned to open.
The Arrangement is subject to, among other things, the approval of Bluma shareholders at a special meeting, and receipt of all required CSE, regulatory and court approvals, including clearance under the U.S. Hart-Scott-Rodino Antitrust Improvements Act.
- 8 -
Table of Contents
Chief Operating Officer
On January 19, 2021, the Corporation announced that it had hired Ty Gent as its new chief operating officer, replacing David Ellis who became regional president of operations for emerging markets in Massachusetts, New York, Pennsylvania, Ohio and Maryland. As chief operating officer, Mr. Gent is responsible for operational consistency and efficiency across markets and implementation of structural enhancements to facilitate scaling.
Equity Offering
On January 21, 2021, the Corporation announced the closing of an overnight marketed offering of Subordinate Voting Shares at a price of $16.00 per share for total gross proceeds of approximately US$125 million.
DESCRIPTION OF SHARE CAPITAL OF THE CORPORATION
The authorized share capital of the Corporation consists of an unlimited number of Subordinate Voting Shares, of which 216,851,695 were issued and outstanding as of February 25, 2021, an unlimited number of Proportionate Voting Shares, of which 138,204 (which are convertible on a 1:200 basis into 27,640,896 Subordinate Voting Shares) were issued and outstanding as of February 25, 2021, an unlimited number of Super Voting Shares, of which 500,000 were issued and outstanding as of February 25, 2021, and an unlimited number of Special Subordinate Voting Shares, of which 63,868,296 (which are convertible on a 100,000:1 basis into 639 Subordinate Voting Shares) were issued and outstanding as of February 25, 2021. All of the issued and outstanding Super Voting Shares are held by the Corporation’s founders, Charlie Bachtell, Joe Caltabiano, Robert Sampson, Dominic Sergi and Brian McCormack (together, the “Founders”). In addition, members of the LLC hold 120,907,764 redeemable units that are convertible into Proportionate Voting Shares on a 200:1 basis.
The Subordinate Voting Shares are “restricted securities” within the meaning of such term under applicable Canadian securities laws. The Corporation has complied with the requirements of Part 12 of National Instrument 41- 101 — General Prospectus Requirements (“NI 41-101”) to be able to file a prospectus under which the Subordinate Voting Shares or securities that are, directly or indirectly, convertible into, or exercisable or exchangeable for, the Subordinate Voting Shares are distributed, as the Corporation received the requisite prior majority approval of shareholders of the Corporation, at the annual and special meeting of shareholders held on November 14, 2018, in accordance with applicable law, including Section 12.3 of NI 41-101, for the Share Terms Amendment. The Share Terms Amendment constituted a “restricted security reorganization” within the meaning of such term under applicable Canadian securities laws.
As of February 25, 2021, the Subordinate Voting Shares represent approximately 17% of the voting rights attached to outstanding securities of the Corporation, the Proportionate Voting Shares represent approximately 2%, the Super Voting Shares represent approximately 80%, and the Special Subordinate Voting Shares represent approximately 0.0001% of the voting rights attached to outstanding securities of the Corporation.
The following is a summary of the rights, privileges, restrictions and conditions attached to the Subordinate Voting Shares, the Proportionate Voting Shares, the Super Voting Shares, and the Special Subordinate Voting Shares but does not purport to be complete. Reference should be made to the articles of the Corporation and the full text of their provisions for a complete description thereof, which are available under the Corporation’s profile on SEDAR at www.sedar.com.
Subordinate Voting Shares
| Right to Notice and Vote |
Holders of Subordinate Voting Shares will be entitled to notice of and to attend at any meeting of the shareholders of the Corporation, except a meeting of which only holders of another particular class or series of shares of the Corporation will have the right to vote. At each such meeting, holders of Subordinate Voting Shares will be entitled to one vote in respect of each Subordinate Voting Share held. |
- 9 -
Table of Contents
| Class Rights & Right of First Refusal |
As long as any Subordinate Voting Shares remain outstanding, the Corporation will not, without the consent of the holders of the Subordinate Voting Shares by separate special resolution, prejudice or interfere with any right attached to the Subordinate Voting Shares. Holders of Subordinate Voting Shares will not be entitled to a right of first refusal to subscribe for, purchase or receive any part of any issue of Subordinate Voting Shares, or bonds, debentures or other securities of the Corporation. | |
| Dividends |
Holders of Subordinate Voting Shares will be entitled to receive as and when declared by the directors of the Corporation, dividends in cash or property of the Corporation. | |
| Participation |
In the event of the liquidation, dissolution or winding-up of the Corporation, whether voluntary or involuntary, or in the event of any other distribution of assets of the Corporation among its shareholders for the purpose of winding up its affairs, the holders of Subordinate Voting Shares shall, subject to the prior rights of the holders of any shares of the Corporation ranking in priority to the Subordinate Voting Shares (including, without restriction, the Super Voting Shares) be entitled to participate ratably along with all other holders of Subordinate Voting Shares, Special Subordinate Voting Shares (on an as converted to Subordinate Voting Shares basis) and the Proportionate Voting Shares (on an as converted to Subordinate Voting Shares basis). | |
| Changes |
No subdivision or consolidation of the Subordinate Voting Shares shall occur unless, simultaneously, the Subordinate Voting Shares, the Special Subordinate Voting Shares, the Proportionate Voting Shares and the Super Voting Shares are subdivided or consolidated in the same manner or such other adjustment is made so as to maintain and preserve the relative rights of the holders of the shares of each of the said classes. | |
| Conversion |
In the event that an offer is made to purchase Proportionate Voting Shares and the offer is one which is required, pursuant to applicable securities legislation or the rules or conditions of listing of a stock exchange on which the Proportionate Voting Shares are then listed, to be made to all or substantially all the holders of Proportionate Voting Shares in a given province or territory of Canada to which these requirements apply, each Subordinate Voting Share shall become convertible at the option of the holder into Proportionate Voting Shares at the inverse of the Conversion Ratio then in effect at any time while the offer is in effect until one day after the time prescribed by applicable securities legislation for the offeror to take up and pay for such shares as are to be acquired pursuant to the offer. The conversion right may only be exercised in respect of Subordinate Voting Shares for the purpose of depositing the resulting Proportionate Voting Shares pursuant to the offer, and for no other reason. In such event, the Corporation’s transfer agent shall deposit the resulting Proportionate Voting Shares on behalf of the holder. Should the Proportionate Voting Shares issued upon conversion and tendered in response to the offer be withdrawn by shareholders or not taken up by the offeror, or should the offer be abandoned or withdrawn, the Proportionate Voting Shares resulting from the conversion shall be automatically reconverted, without further intervention on the part of the Corporation or on the part of the holder, into Subordinate Voting Shares at the Conversion Ratio then in effect. | |
Take-Over Bid Protection
The Super Voting Shares are transferable only among the Founders and their respective affiliates for planning and similar purposes. The Founders have entered into an investment agreement with the Corporation whereby, upon any sale of Super Voting Shares to a third party purchaser not listed above, such Super Voting Shares will immediately be redeemed by the Corporation for their issue price. See “Super Voting Shares – Investment Agreement” below.
Additionally, as noted above, the Corporation’s articles entitle the holders of Subordinate Voting Shares to convert to Proportionate Voting Shares and tender to any take-over bid made solely to the holders of Proportionate Voting Shares.
- 10 -
Table of Contents
Proportionate Voting Shares
| Right to Vote |
Holders of Proportionate Voting Shares will be entitled to notice of and to attend at any meeting of the shareholders of the Corporation, except a meeting of which only holders of another particular class or series of shares of the Corporation will have the right to vote. At each such meeting, holders of Proportionate Voting Shares will be entitled to one vote in respect of each Subordinate Voting Share into which such Proportionate Voting Share could ultimately then be converted, which for greater certainty, shall initially be equal to 200 votes per Proportionate Voting Share (subject to adjustment at the discretion of the board of directors of the Corporation, depending upon the ratios necessary to preserve foreign private issuer status). | |
| Class Rights |
As long as any Proportionate Voting Shares remain outstanding, the Corporation will not, without the consent of the holders of the Proportionate Voting Shares and Super Voting Shares by separate special resolution, prejudice or interfere with any right or special right attached to the Proportionate Voting Shares. Consent of the holders of a majority of the outstanding Proportionate Voting Shares and Super Voting Shares shall be required for any action that authorizes or creates shares of any class having preferences superior to or on a parity with the Proportionate Voting Shares. In connection with the exercise of the voting rights for the foregoing only, each holder of Proportionate Voting Shares will have one vote in respect of each Proportionate Voting Share held. | |
| Dividends |
The holder of Proportionate Voting Shares shall have the right to receive dividends, out of any cash or other assets legally available therefor, pari passu (on an as converted basis, assuming conversion of all Proportionate Voting Shares into Subordinate Voting Shares) as to dividends and any declaration or payment of any dividend on the Subordinate Voting Shares. No dividend will be declared or paid on the Proportionate Voting Shares unless the Corporation simultaneously declares or pays, as applicable, equivalent dividends (on an as-converted to Subordinate Voting Share basis) on the Subordinate Voting Shares and the Special Subordinate Voting Shares. | |
| Participation |
In the event of the liquidation, dissolution or winding-up of the Corporation, whether voluntary or involuntary, or in the event of any other distribution of assets of the Corporation among its shareholders for the purpose of winding up its affairs, the holders of Proportionate Voting Shares will, subject to the prior rights of the holders of any shares of the Corporation ranking in priority to the Proportionate Voting Shares (including, without restriction, the Super Voting Shares), be entitled to participate ratably along with all other holders of Proportionate Voting Shares (on an as-converted to Subordinate Voting Share basis), the Special Subordinate Voting Shares (on an as-converted to Subordinate Voting Share basis) and the Subordinate Voting Shares. | |
| Changes |
No subdivision or consolidation of the Proportionate Voting Shares shall occur unless, simultaneously, the Subordinate Voting Shares, the Special Subordinate Voting Shares, the Proportionate Voting Shares and the Super Voting Shares are subdivided or consolidated in the same manner or such other adjustment is made so as to maintain and preserve the relative rights of the holders of the shares of each of the said classes. | |
| Conversion |
Holders of Proportionate Voting Shares have a restricted right to convert into 200 Subordinate Voting Shares per Proportionate Voting Share (the “Conversion Ratio”), subject to customary adjustments for certain customary corporate changes. The ability to convert the Proportionate Voting Shares is subject to a restriction that the aggregate number of Subordinate Voting Shares, Special Subordinate Voting Shares, Proportionate Voting Shares and Super Voting Shares held of record, directly or indirectly, by residents of the United States (as determined in accordance with Rules 3b-4 and 12g3-2(a) under the U.S. Exchange Act), may not exceed forty percent (40%) (subject to adjustment) of the aggregate number of Subordinate Voting Shares, Special Subordinate Voting Shares, Proportionate Voting Shares and Super Voting Shares issued and outstanding after giving effect to such conversions and to a restriction on beneficial ownership of Subordinate Voting Shares exceeding certain levels. In addition, the Proportionate Voting Shares will be automatically converted into Subordinate Voting Shares in certain circumstances, including upon the registration of the Subordinate Voting Shares under the U.S. Securities Act. | |
- 11 -
Table of Contents
Super Voting Shares
| Right to Vote |
Holders of Super Voting Shares shall be entitled to notice of and to attend at any meeting of the shareholders of the Corporation, except a meeting of which only holders of another particular class or series of shares of the Corporation shall have the right to vote. At each such meeting, holders of Super Voting Shares shall be entitled to 2,000 votes in respect of each Super Voting Share held provided that, if at any time the aggregate number of issued and outstanding (i) redeemable shares in the capital of Cresco Corp (“Cresco Corp Redeemable Shares”) (if applicable) and (ii) redeemable units in the capital of the Corporation (“Cresco Redeemable Units”) (or such securities of any successor to Cresco Corp or the Corporation as may exist from time to time) beneficially owned, directly or indirectly by a holder of the Super Voting Shares (the “Holder”) and the Holder’s predecessor or transferor, permitted transferees and permitted successors, and any prior transferor’s transferor and any prior permitted transferee’s permitted transferee (the “Holder’s Group”), divided by the aggregate number of (i) Cresco Corp Redeemable Shares (if applicable) and (ii) Cresco Redeemable Units beneficially owned, directly or indirectly by the Holders and the Holder’s Group as at the date of completion of the business combination transaction involving, among others, the Corporation, Cresco Corp and Cresco be less than 50% (the “Triggering Event”), the Holder shall from that time forward be entitled to 50 votes in respect of each Super Voting Share held. The holders of Super Voting Shares shall, from time to time upon the request of the Corporation, provide to the Corporation evidence as to such holders’ direct and indirect beneficial ownership (and that of its permitted transferees and permitted successors) of Cresco Corp Redeemable Shares (if applicable) and Cresco Redeemable Units to enable the Corporation to determine the voting entitlement of the Super Voting Shares. For the purposes of these calculations, a Holder shall be deemed to beneficially own Cresco Corp Redeemable Shares (if applicable) held by an intermediate company or fund in proportion to their equity ownership of such company or fund. | |
| Class Rights |
As long as any Super Voting Shares remain outstanding, the Corporation will not, without the consent of the holders of the Super Voting Shares by separate special resolution, prejudice or interfere with any right or special right attached to the Super Voting Shares. Consent of the holders of a majority of the outstanding Super Voting Shares shall be required for any action that authorizes or creates shares of any class having preferences superior to or on a parity with the Super Voting Shares. In connection with the exercise of these voting rights, each holder of Super Voting Shares will have one vote in respect of each Super Voting Share held. | |
| Dividends |
The holders of the Super Voting Shares shall not be entitled to receive dividends. | |
| Participation |
In the event of the liquidation, dissolution or winding-up of the Corporation, whether voluntary or involuntary, or in the event of any other distribution of assets of the Corporation among its shareholders for the purpose of winding up its affairs, the Corporation will distribute its assets firstly and in priority to the rights of holders of any other class of shares of the Corporation (including the holders of Subordinate Voting Shares, Special Subordinate Voting Shares and the Proportionate Voting Shares) to return the issue price of the Super Voting Shares to the holders thereof and if there are insufficient assets to fully return the issue price to the holders of the Super Voting Shares, such holders will receive an amount equal to their pro rata share in proportion to the issue price of their Super Voting Shares along with all other holders of Super Voting Shares. The holders of Super Voting Shares shall not be entitled to receive directly or indirectly as holders of Super Voting Shares any other assets or property of the Corporation and their sole rights will be to the return of the issue price of such Super Voting Shares in accordance with this paragraph. | |
- 12 -
Table of Contents
| Changes |
No subdivision or consolidation of the Super Voting Shares shall occur unless, simultaneously, the Super Voting Shares, the Proportionate Voting Shares, the Special Subordinate Voting Shares and the Subordinate Voting Shares are subdivided or consolidated in the same manner, so as to maintain and preserve the relative rights of the holders of the shares of each of the said classes. | |
| Conversion |
The holders of the Super Voting Shares shall have no right of conversion. | |
| Redemption Rights |
Upon the occurrence of a Triggering Event, the Corporation has the right to redeem all or some of the Super Voting Shares from the Holder and Holder’s Group who caused the Triggering Event to occur, by providing two days prior written notice to the Holder and Holder’s Group of such Super Voting Shares, for an amount equal to the issue price for each Super Voting Share, payable in cash to the holders of the Super Voting Shares so redeemed. The Corporation need not redeem Super Voting Shares on a pro-rata basis among the Holders or Holder’s Group. Holders of Super Voting Shares to be redeemed by the Corporation shall surrender the certificate or certificates representing such Super Voting Shares to the Corporation at its records office duly assigned or endorsed for transfer to the Corporation (or accompanied by duly executed share transfers relating thereto). | |
| Transfer |
No Super Voting Share may be transferred by the holder thereof unless such transfer is to an immediate family member or a transfer for the purposes of estate or tax planning to a company or person that is wholly beneficially owned by such holder or immediate family members of such holder or which such holder or immediate family members of such holder are the sole beneficiaries thereof. In order to be effective, any transfer shall require the prior written consent of the Corporation. | |
| Investment Agreement |
To supplement the rights, privileges, restrictions and conditions attached to the Super Voting Shares, the Corporation and the Founders, being the initial holders of Super Voting Shares, entered into an investment agreement effective as of the completion of the Business Combination which, among other things, provides that (i) each Super Voting Share will be transferable only to the holder’s immediate family members or an affiliated entity or a transfer to the other Founder or an entity affiliated with the other Founder, and (ii) upon any sale of Super Voting Shares to a third party purchaser not listed in clause (i), such Super Voting Shares will immediately be redeemed by the Corporation for their issue price. | |
Special Subordinate Voting Shares
| Right to Vote |
Holders of Special Subordinate Voting Shares shall be entitled to notice of and to attend at any meeting of the shareholders of the Corporation, except a meeting of which only holders of another particular class or series of shares of the Corporation shall have the right to vote. At each such meeting, holders of Special Subordinate Voting Shares will be entitled to one vote in respect of each Subordinate Voting Share into which such Special Subordinate Voting Shares could ultimately then be converted, which for greater certainty, shall initially be equal to 0.00001 of a vote per Special Subordinate Voting Share. | |
| Class Rights |
As long as any Special Subordinate Voting Shares remain outstanding, the Corporation will not, without the consent of the holders of the Special Subordinate Voting Shares by separate special resolution, prejudice or interfere with any right or special right attached to the Special Subordinate Voting Shares. In connection with the exercise of these voting rights, each holder of Special Subordinate Voting Shares will have one vote in respect of each Special Subordinate Voting Share held. | |
- 13 -
Table of Contents
| Dividends |
The holders of Special Subordinate Voting Shares shall have the right to receive dividends, out of any cash or other assets legally available therefor, pari passu (on an as converted basis, assuming conversion of all Special Subordinate Voting Shares into Subordinate Voting Shares at the Special Conversion Ratio) as to dividends and any declaration or payment of any dividend on the Subordinate Voting Shares. No dividend will be declared or paid on the Special Subordinate Voting Shares unless the Corporation simultaneously declares or pays, as applicable, equivalent dividends (on an as-converted to Subordinate Voting Share basis) on the Subordinate Voting Shares and the Proportionate Voting Shares. | |
| Participation |
In the event of the liquidation, dissolution or winding-up of the Corporation, whether voluntary or involuntary, or in the event of any other distribution of assets of the Corporation among its shareholders for the purpose of winding up its affairs, the holders of Special Subordinate Voting Shares will, subject to the prior rights of the holders of any shares of the Corporation ranking in priority to the Special Subordinate Voting Shares (including, without restriction, the Super Voting Shares), be entitled to participate ratably along with all other holders of Special Subordinate Voting Shares (on an as-converted to Subordinate Voting Share basis), the Proportionate Voting Shares (on an as-converted to Subordinate Voting Share basis) and the Subordinate Voting Shares. | |
| Changes |
The Special Subordinate Voting Shares may be subdivided or consolidated by resolution of the directors (or a committee thereof) without the simultaneous subdivision or consolidation of the Subordinate Voting Shares, the Proportionate Voting Shares and the Super Voting Shares in the same manner, provided that the Special Conversion Ratio is correspondingly adjusted and the voting rights of the Special Subordinate Voting Shares are correspondingly adjusted such that the aggregate number of votes held by all holders of Special Subordinate Voting Shares prior to subdivision or consolidation is equal to the aggregate number of votes held by all holders of Special Subordinate Voting Shares following the subdivision or consolidation. | |
| Ownership Restrictions |
The Special Subordinate Voting Shares may only be beneficially owned or controlled, directly or indirectly, by a person or persons who are not specified U.S. Persons. | |
| Transfer Restrictions |
No Special Subordinate Voting Share or any rights or interests therein may be transferred legally, beneficially or in any other manner by the holder thereof without the prior written consent of the board of directors of the Corporation (or a committee thereof), which may be withheld in its sole discretion. | |
| Redemption Rights |
The Corporation has the right to redeem all or some of the Special Subordinate Voting Shares from any holder thereof at any time by providing two days prior written notice (the “Redemption Notice”) to such holder for either: (i) cash, at a price per Special Subordinate Voting Share equal to the Special Conversion Ratio (as may be adjusted in accordance with its terms) multiplied by the average volume weighted average trading price of the Subordinate Voting Shares on the CSE (or such other stock exchange or quotation system the Subordinate Voting Shares are then principally listed or quoted) for the 10 trading days immediately prior to the date of the Redemption Notice; or (ii) Subordinate Voting Shares at the Special Conversion Ratio, as may be adjusted in accordance with its terms. The Corporation need not redeem Special Subordinate Voting Shares on a pro-rata basis among the holders of Special Subordinate Voting Shares. | |
- 14 -
Table of Contents
| Conversion |
Holders of Special Subordinate Voting Shares have a restricted right to convert into 0.00001 Subordinate Voting Shares per Special Subordinate Voting Share (the “Special Conversion Ratio”), subject to customary adjustments for certain corporate changes. The ability to convert the Special Subordinate Voting Shares is subject to the prior written consent of the Corporation’s board of directors or a committee thereof. The Corporation may require each holder of Special Subordinate Voting Shares to convert all, and not less than all, of the Special Subordinate Voting Shares at the applicable Special Conversion Ratio if at any time all the following conditions are satisfied (or otherwise waived by special resolution of holders of Special Subordinate Voting Shares): (A) the Corporation is no longer a “foreign private issuer” (as determined in accordance with Rule 3b-4 of the U.S. Exchange Act); or (B) the board of directors of the Corporation (or a committee thereof) determine that the Special Subordinate Voting Shares are no longer necessary or required. | |
See “Description of Share Capital of Cresco Corp.” and “Description of Unit Capital of Cresco” in the AIF for details as to the share and unit capital respectively of Cresco Corp. and the LLC.
DESCRIPTION OF DEBT SECURITIES
The Corporation may issue Debt Securities, separately or together, with Subordinate Voting Shares, Warrants, Subscription Receipts or Units or any combination thereof, as the case may be. The Debt Securities will be issued in one or more series under an indenture (the “Indenture”) to be entered into between the Corporation and one or more trustees that will be named in a Prospectus Supplement for the applicable series of Debt Securities. To the extent applicable, the Indenture will be subject to and governed by the U.S. Trust Indenture Act of 1939, as amended. Each such Indenture is expected to be governed by and construed either in accordance with the laws of the State of New York or the laws of the Province of British Columbia or such other laws as set forth in the applicable Prospectus Supplement. The description of certain provisions of the Indenture in this section do not purport to be complete and are subject to, and are qualified in their entirety by reference to, the provisions of the Indenture. Terms used in this summary that are not otherwise defined herein have the meaning ascribed to them in the Indenture. The particular terms relating to Debt Securities offered by a Prospectus Supplement will be described in the related Prospectus Supplement. This description may include, but may not be limited to, any of the following, if applicable:
| • | the specific designation of the Debt Securities; any limit on the aggregate principal amount of the Debt Securities; the date or dates, if any, on which the Debt Securities will mature and the portion (if less than all of the principal amount) of the Debt Securities to be payable upon declaration of acceleration of maturity; |
| • | the rate or rates (whether fixed or variable) at which the Debt Securities will bear interest, if any, the date or dates from which any such interest will accrue and on which any such interest will be payable and the record dates for any interest payable on the Debt Securities that are in registered form; |
| • | the terms and conditions under which the Corporation may be obligated to redeem, repay or purchase the Debt Securities pursuant to any sinking fund or analogous provisions or otherwise; |
| • | the terms and conditions upon which the Corporation may redeem the Debt Securities, in whole or in part, at the Corporation’s option; |
| • | the covenants applicable to the Debt Securities; |
| • | the terms and conditions for any conversion or exchange of the Debt Securities for any other securities; |
| • | whether the Debt Securities will be issuable in registered form or bearer form or both, and, if issuable in bearer form, the restrictions as to the offer, sale and delivery of the Debt Securities which are in bearer form and as to exchanges between registered form and bearer form; |
| • | whether the Debt Securities will be issuable in the form of registered global securities (“Global Securities”), and, if so, the identity of the depositary for such registered Global Securities; |
| • | the denominations in which registered Debt Securities will be issuable; |
- 15 -
Table of Contents
| • | each office or agency where payments on the Debt Securities will be made and each office or agency where the Debt Securities may be presented for registration of transfer or exchange; |
| • | the currency in which the Debt Securities are denominated or the currency in which the Corporation will make payments on the Debt Securities; |
| • | material Canadian federal income tax consequences and U.S. federal income tax consequences of owning the Debt Securities; |
| • | any index, formula or other method used to determine the amount of payments of principal of (and premium, if any) or interest, if any, on the Debt Securities; and |
| • | any other terms of the Debt Securities which apply solely to the Debt Securities. |
Each series of Debt Securities may be issued at various times with different maturity dates, may bear interest at different rates and may otherwise vary.
The terms on which a series of Debt Securities may be convertible into or exchangeable for Subordinate Voting Shares or other securities of the Corporation will be described in the applicable Prospectus Supplement. These terms may include provisions as to whether conversion or exchange is mandatory, at the option of the holder or at the option of the Corporation, and may include provisions pursuant to which the number of Subordinate Voting Shares or other securities to be received by the holders of such series of Debt Securities would be subject to adjustment.
To the extent any Debt Securities are convertible into Subordinate Voting Shares or other securities of the Corporation, prior to such conversion the holders of such Debt Securities will not have any of the rights of holders of the securities into which the Debt Securities are convertible, including the right to receive payments of dividends or the right to vote such underlying securities.
DESCRIPTION OF SUBSCRIPTION RECEIPTS
The following sets forth certain general terms and provisions of the Subscription Receipts. The particular terms and provisions of the Subscription Receipts offered pursuant to this Prospectus will be set forth in the applicable Prospectus Supplement, which particular terms and provisions of such Subscription Receipts may differ from the general terms and provisions described below in some or all respects.
The Corporation may issue Subscription Receipts that may be exchanged by the holders thereof for Subordinate Voting Shares and/or other Securities of the Corporation upon the satisfaction of certain conditions. The Corporation may offer Subscription Receipts separately or together with Subordinate Voting Shares, Debt Securities, Warrants or Units, as the case may be. The Corporation will issue Subscription Receipts under one or more subscription receipt agreements. Under each subscription receipt agreement, a purchaser of Subscription Receipts will have a contractual right of rescission following the issuance of the Subordinate Voting Shares and/or other Securities of the Corporation, as the case may be, to such purchaser upon exchange of Subscription Receipts, entitling the purchaser to receive the amount paid for the Subscription Receipts upon surrender of the Subordinate Voting Shares and/or other Securities of the Corporation, as the case may be, if this Prospectus, the relevant Prospectus Supplement, and any amendment thereto, contains a misrepresentation, provided such remedy for rescission is exercised within 180 days of the date the Subscription Receipts are issued.
Any Prospectus Supplement will contain the terms and conditions and other information relating to the Subscription Receipts being offered, including:
| • | the number of Subscription Receipts; |
| • | the price at which the Subscription Receipts will be offered and whether the price is payable in installment; |
- 16 -
Table of Contents
| • | any conditions to the exchange of Subscription Receipts into Subordinate Voting Shares, and/or other Securities of the Corporation, as the case may be, and the consequences of such conditions not being satisfied; |
| • | the procedures for the exchange of the Subscription Receipts into Subordinate Voting Shares and/or other Securities of the Corporation, as the case may be; |
| • | the number of Subordinate Voting Shares and/or other Securities of the Corporation, as the case may be, that may be exchanged upon exercise of each Subscription Receipt; |
| • | the designation and terms of any other Securities with which the Subscription Receipts will be offered, if any, and the number of Subscription Receipts that will be offered with each Security; |
| • | the dates or periods during which the Subscription Receipts may be exchanged into Subordinate Voting Shares and/or other Securities of the Corporation; |
| • | whether such Subscription Receipts will be listed on any securities exchange; |
| • | any other rights, privileges, restrictions and conditions attaching to the Subscription Receipts; and |
| • | any other specific terms. |
Prior to the exchange of their Subscription Receipts, holders of Subscription Receipts will not have any of the rights of holders of the securities issuable on the exchange of the Subscription Receipts.
The following sets forth certain general terms and provisions of the Warrants. The particular terms and provisions of the Warrants offered pursuant to this Prospectus will be set forth in the applicable Prospectus Supplement, which particular terms and provisions of such Warrants may differ from the general terms and provisions described below in some or all respects.
The Corporation may issue Warrants for the purchase of Subordinate Voting Shares and/or other Securities of the Corporation. Warrants may be issued independently or together with Subordinate Voting Shares, Debt Securities and Subscription Receipts offered by any Prospectus Supplement and may be attached to, or separate from, any such offered Securities. Warrants will be issued under one or more warrant agreements entered into between the Corporation and a warrant agent named in the applicable Prospectus Supplement.
Selected provisions of the Warrants and the warrant agreements are summarized below. This summary is not complete. The statements made in this Prospectus relating to any warrant agreement and Warrants to be issued thereunder are summaries of certain anticipated provisions thereof and are subject to, and are qualified in their entirety by reference to, all provisions of the applicable warrant agreement.
Any Prospectus Supplement will contain the terms and other information relating to the Warrants being offered, including:
| • | the exercise price of the Warrants; |
| • | the designation of the Warrants; |
| • | the aggregate number of Warrants offered and the offering price; |
| • | the designation, number and terms of the Subordinate Voting Shares and/or other Securities of the Corporation purchasable upon exercise of the Warrants, and procedures that will result in the adjustment of those numbers; |
- 17 -
Table of Contents
| • | the dates or periods during which the Warrants are exercisable; |
| • | the designation and terms of any securities with which the Warrants are issued; |
| • | if the Warrants are issued as a unit with another security, the date on and after which the Warrants and the other security will be separately transferable; |
| • | the currency or currency unit in which the exercise price is denominated; |
| • | any minimum or maximum amount of Warrants that may be exercised at any one time; |
| • | whether such Warrants will be listed on any securities exchange; |
| • | any terms, procedures and limitations relating to the transferability, exchange or exercise of the Warrants; |
| • | any rights, privileges, restrictions and conditions attaching to the Warrants; and |
| • | any other specific terms. |
Prior to the exercise of their Warrants, holders of Warrants will not have any of the rights of holders of the Securities subject to the Warrants.
Units are a security comprised of more than one of the other Securities described in this Prospectus offered together as a “Unit”. A Unit is typically issued so the holder thereof is also the holder of each Security included in the Unit. As a result, the holder of a Unit will have the rights and obligations of a holder of each Security comprising the Unit. The agreement, if any, under which a Unit is issued may provide that the Securities comprising the Unit may not be held or transferred separately at any time or at any time before a specified date.
The particular terms and provisions of the Units offered pursuant to this Prospectus will be set forth in the applicable Prospectus Supplement, which particular terms and provisions of such Units may differ from the general terms and provisions described below in some or all respects. This description will include, where applicable:
| • | the designation and terms of the Units and of the Securities comprising the Units, including whether and under what circumstances those Securities may be held or transferred separately; |
| • | any provisions for the issuance, payment, settlement, transfer or exchange of the Units or of the Securities comprising the Units; |
| • | whether the Units will be issued in registered or global form; and |
| • | any other material terms and conditions of the Units. |
Prior sales of Securities will be provided, as required, in a Prospectus Supplement with respect to the issuance of Securities pursuant to such Prospectus Supplement.
Trading price and volume of Securities will be provided, as required, in each Prospectus Supplement to this Prospectus.
- 18 -
Table of Contents
This Prospectus may also, from time to time, relate to the offering of the Securities by way of a secondary offering by certain Selling Securityholders.
The terms under which the Securities may be offered by Selling Securityholders will be described in the applicable Prospectus Supplement. The Prospectus Supplement for or including any offering of Securities by Selling Securityholders will include, without limitation, where applicable: (i) the names of the Selling Securityholders; (ii) the number and type of Securities owned, controlled or directed by each Selling Securityholder; (iii) the number of Securities being distributed for the accounts of each Selling Securityholder; (iv) the number of Securities to be owned, controlled or directed by each Selling Securityholder after the distribution and the percentage that number or amount represents out of the total number of outstanding Securities; (v) whether the Securities are owned by the Selling Securityholders, both of record and beneficially, of record only or beneficially only; (vi) if a Selling Securityholder purchased any of the Securities held by him, her or it in the 12 months preceding the date of the Prospectus Supplement, the date or dates the Selling Securityholder acquired the Securities; and (vii) if a Selling Securityholder acquired the Securities held by him, her or it in the 12 months preceding the date of the Prospectus Supplement, the cost thereof to the Selling Securityholder in the aggregate and on a per security basis.
The Corporation may offer and sell Securities directly to one or more purchasers, through agents, or through underwriters or dealers designated by it from time to time. The Corporation may distribute the Securities from time to time in one or more transactions at a fixed price or prices (which may be changed from time to time), at market prices prevailing at the times of sale, at prices related to prevailing market prices or at negotiated prices. A description of such pricing will be disclosed in the applicable Prospectus Supplement. The Corporation may offer Securities in the same offering, or the Corporation may offer Securities in separate offerings.
This Prospectus may also, from time to time, relate to the offering of Securities by certain Selling Securityholders. The Selling Securityholders may sell all or a portion of the Securities beneficially owned by them and offered thereby from time to time directly or through one or more underwriters, broker-dealers or agents. Securities may be sold by the Selling Securityholders in one or more transactions at fixed prices, at prevailing market prices at the time of the sale, at varying prices determined at the time of sale, or at negotiated prices.
A Prospectus Supplement will describe the terms of each specific offering of Securities, including (i) the terms of the Securities to which the Prospectus Supplement relates, including the type of Security being offered; (ii) the name or names of any agents, underwriters or dealers involved in such offering of Securities; (iii) the name or names of any Selling Securityholders; (iv) the purchase price of the Securities offered thereby and the proceeds to, and the portion of expenses borne by, the Corporation from the sale of such Securities; (v) any agents’ commission, underwriting discounts and other items constituting compensation payable to agents, underwriters or dealers; and (vi) any discounts or concessions allowed or re-allowed or paid to agents, underwriters or dealers.
If underwriters are used in an offering, the Securities offered thereby will be acquired by the underwriters for their own account and may be resold from time to time in one or more transactions at a fixed public offering price or at varying prices determined at the time of sale. Securities may be either offered to the public through underwriting syndicates represented by managing underwriters or by underwriters without a syndicate. Only underwriters named in a Prospectus Supplement are deemed to be underwriters in connection with the Securities offered thereby. The obligations of the underwriters to purchase Securities will be subject to the conditions precedent agreed upon by the parties and the underwriters will be obligated to purchase all Securities under that offering if any are purchased. Any public offering price and any discounts or concessions allowed or re-allowed or paid to agents, underwriters or dealers may be changed from time to time.
The Securities may also be sold: (i) directly by the Corporation or the Selling Securityholders at such prices and upon such terms as agreed to; or (ii) through agents designated by the Corporation or the Selling Securityholders from time to time. Any agent involved in the offering and sale of the Securities in respect of which this Prospectus is delivered will be named, and any commissions payable by the Corporation and/or Selling Securityholder to such agent will be set forth, in the Prospectus Supplement. Unless otherwise indicated in the Prospectus Supplement, any agent is acting on a “best efforts” basis for the period of its appointment.
- 19 -
Table of Contents
The Corporation and/or the Selling Securityholders may agree to pay the underwriters a commission for various services relating to the issue and sale of any Securities offered under any Prospectus Supplement. Agents, underwriters or dealers who participate in the distribution of the Securities may be entitled under agreements to be entered into with the Corporation and/or the Selling Securityholders to indemnification by the Corporation and/or the Selling Securityholders against certain liabilities, including liabilities under securities legislation, or to contribution with respect to payments which such underwriters, dealers or agents may be required to make in respect thereof.
Agents, underwriters or dealers may make sales of Securities in privately negotiated transactions and/or any other method permitted by law, including sales deemed to be an ‘‘at-the-market’’ offering as defined in and subject to limitations imposed by applicable Canadian securities laws which includes sales made directly on an existing trading market for the Subordinate Voting Shares, or sales made to or through a market maker other than on an exchange. In connection with any offering of Securities, except with respect to ‘‘at-the-market’’ offerings, underwriters may over-allot or effect transactions which stabilize or maintain the market price of the offered Securities at a level above that which might otherwise prevail in the open market. Such transactions may be commenced, interrupted or discontinued at any time. No underwriter or dealer involved in an ‘‘at-the-market’’ offering, as defined under applicable Canadian securities laws, no affiliate of such an underwriter or dealer and no person or company acting jointly or in concert with such an underwriter or dealer may enter into any transaction that is intended to stabilize or maintain the market price of the Securities or Securities of the same class as the Securities distributed under the applicable Prospectus Supplement, including selling an aggregate number or principal amount of Securities that would result in the underwriter or dealer creating an over-allocation position in the Securities.
The Corporation may authorize agents or underwriters to solicit offers by eligible institutions to purchase Securities from it at the public offering price set forth in the applicable Prospectus Supplement under delayed delivery contracts providing for payment and delivery on a specified date in the future. The conditions to these contracts and the commissions payable for solicitation of these contracts will be set forth in the applicable Prospectus Supplement.
Each class or series of Securities, other than the Subordinate Voting Shares, that is not issued in a secondary offering will be a new issue of Securities with no established trading market. Unless otherwise specified in the applicable Prospectus Supplement, the Debt Securities, Warrants, Subscription Receipts or Units will not be listed on any securities exchange. Unless otherwise specified in the applicable Prospectus Supplement, there is no market through which the Debt Securities, Warrants, Subscription Receipts or Units may be sold and purchasers may not be able to resell Debt Securities, Warrants, Subscription Receipts or Units purchased under this Prospectus or any Prospectus Supplement. This may affect the pricing of the Debt Securities, Warrants, Subscription Receipts or Units in the secondary market, the transparency and availability of trading prices, the liquidity of the securities, and the extent of issuer regulation. Subject to applicable laws, certain dealers may make a market in the Debt Securities, Warrants, Subscription Receipts or Units, as applicable, but will not be obligated to do so and may discontinue any market making at any time without notice. No assurance can be given that any dealer will make a market in the Debt Securities, Warrants, Subscription Receipts or Units or as to the liquidity of the trading market, if any, for the Debt Securities, Warrants, Subscription Receipts or Units.
The net proceeds to the Corporation from any offering of Securities and the proposed use of those proceeds will be set forth in the applicable Prospectus Supplement relating to that offering of Securities. The Corporation will not receive any proceeds of the sale of Securities from a Selling Securityholder.
Pursuant to the audited financial statements of the Corporation for the years ended December 31, 2019 and 2018, the Corporation has negative cash flow from operating activities. Each applicable Prospectus Supplement will contain specific information concerning whether, and if so, to what extent, the Corporation will use the proceeds of the distribution to fund any anticipated negative cash flow from operating activities in future periods.
- 20 -
Table of Contents
The applicable Prospectus Supplement will provide, as required by applicable Canadian securities laws, the earnings coverage ratios with respect to the issuance of Securities pursuant to such Prospectus Supplement.
The applicable Prospectus Supplement will describe any material change in, and the effect of such material change on, the share and loan capitalization of the Corporation since the date of the Corporation’s financial statements for its most recently completed financial period included in such Prospectus Supplement, including any material change that will result from the issuance of Securities pursuant to such Prospectus Supplement.
UNITED STATES REGULATORY ENVIRONMENT
The emergence of the legal cannabis sector in the United States, both for medical and adult-use, has been rapid as more states adopt regulations for its production and sale. Today, 60% of Americans live in a state where cannabis is legal in some form and almost a quarter of the population lives in states where it is fully legalized for adult use.1
The use of cannabis and cannabis derivatives to treat or alleviate the symptoms of a wide variety of chronic conditions has been generally accepted by a majority of citizens with a growing acceptance by the medical community as well. A review of the research, published in 2015 in the Journal of the American Medical Association, found strong evidence that cannabis can treat pain and muscle spasms.2 The pain component is particularly important because other studies have suggested that cannabis can replace pain patients’ use of highly addictive, potentially deadly opiates — meaning marijuana legalization has the potential to save lives.3
Polls throughout the United States consistently show overwhelming support for the legalization of medical cannabis, together with strong majority support for the full legalization of recreational adult-use cannabis. It is estimated that 94% of the U.S. voters support legalizing cannabis for medical use.4 In addition, 64% of the U.S. public supports legalizing cannabis for adult recreational use.5 These represent large increases in public support over the past 40 years in favor of legal cannabis use.
Notwithstanding that more than half of the U.S. states have now legalized adult-use and/or medical marijuana, marijuana remains illegal under U.S. federal law with marijuana listed as a Schedule I drug under the CSA. See “Description of the Business” and “Risk Factors” in the AIF for further information. The DOJ defines Schedule I drugs, substances or chemicals as “drugs with no currently accepted medical use and a high potential for abuse.” The FDA has not approved marijuana as a safe and effective drug for any indication.
Unlike in Canada, which has federal legislation uniformly governing the cultivation, distribution, sale and possession of medical marijuana under the Cannabis Act (Canada), marijuana is largely regulated at the state level in the United States.
State laws regulating cannabis are in direct conflict with the CSA, which makes cannabis use and possession federally illegal in the United States. Although certain states and territories of the United States authorize medical or recreational cannabis production and distribution by licensed or registered entities, under U.S. federal law, the possession, use, cultivation, and transfer of cannabis and any related drug paraphernalia is illegal and any such acts are criminal acts under U.S. federal law under any and all circumstances under the
| 1 | Ripley, Eve. (2016 November 30). Nearly 60 percent of U.S. population now lives in states with marijuana legalization. Retrieved from https://news.medicalmarijuanainc.com/nearly-60-percent-u-s-population-now-lives-states-marijuana-legalization/. |
| 2 | Grant, Igor MD (2015). Medical Use of Cannabinoids. Journal of American Medical Association, 314: 16, 1750-1751. doi: 10.1001/jama.2015.11429. |
| 3 | Bachhuber, MA, Saloner B, Cunningham CO, Barry CL. (2014). Medical Cannabis Laws and Opioid Analgesic Overdose Mortality in the United States, 1999-2010. JAMA Intern Med. 174(10):1668-1673. doi: 10.1001/jamainternmed.2014.4005. |
| 4 | Quinnipiac University. (2017 April 20). U.S. Voter Support For Marijuana Hits New High; Quinnipiac University National Poll Finds; 76 Percent Say Their Finances Are Excellent Or Good. Retrieved from https://poll.qu.edu/national/release-detail?ReleaseID=2453. |
| 5 | Gallup. (2017 October 25). Record-High Support for Legalizing Marijuana Use in U.S. Retrieved from http://news.gallup.com/poll/221018/record-high-support-legalizing-marijuana.aspx. |
- 21 -
Table of Contents
CSA. Although Cresco’s and its subsidiaries activities are compliant with applicable United States state and local law, strict compliance with state and local laws with respect to cannabis may neither absolve Cresco and its subsidiaries of liability under United States federal law, nor provide a defense to any U.S. federal proceeding that may be brought against Cresco or its subsidiaries.
The risk of U.S. federal enforcement and other risks associated with the Corporation’s business are described in the “Risk Factors” section of the AIF.
Current U.S. Cannabis Market
Source: https://thecannabisindustry.org/ncia-news-resources/state-by-state-policies/
Due to the support for legal access to marijuana at the state level, there has been rapid opportunity growth in the U.S. market. Sales of legal cannabis flower and cannabis-infused derivative and edible products totaled US$6.1 billion in 2017, and are expected to reach US$8.8 billion in 2018 with approximately 36% of sales for medical use and 64% for full adult use6. The U.S. market for direct legal cannabis sales alone is projected to grow to US$17 billion by 20217 and the total addressable market for direct cannabis sales in the United States today is estimated at US$45-50 billion if every state legalized full adult recreational consumption.8 By 2030, the size of the U.S. cannabis market is projected to be approximately US$63 billion.9 Going forward, the Corporation expects that the U.S. cannabis industry will continue to be subject to state legislation, with additional states regulating the medical and recreational use of cannabis.
The number of medical cannabis patients in states with existing comprehensive medical cannabis programs was approximately 1.5 million by the end of 2017, served by approximately 1500-2000 medical dispensaries nationwide, a disproportionate number of those in California. It is currently estimated that each patient spends about US$2,000 annually,10 and that the total number of medical cannabis patients nationwide is expected to grow to 2.5 million by 2021.11
| 6 | Marijuana Business Daily. (2017). Marijuana Business Factbook, 2017. Available from https://mjbizdaily.com/factbook/. |
| 7 | Arcview Market Research & New Frontier Data. (2016). The State of Legal Marijuana Markets (4th ed.), pp. 11. Available from https://www.arcviewmarketresearch.com/4th-edition-legal-marijuana-market/. |
| 8 | Marijuana Business Daily. (2017). Marijuana Business Factbook, 2017. Available from https://mjbizdaily.com/factbook/. |
| 9 | Eight Capital. (2018). What’s Going on Down There? A $63 B Market Cannot be Ignored. |
| 10 | Marijuana Business Daily. (2017). Marijuana Business Factbook, 2017. Available from https://mjbizdaily.com/factbook/. |
| 11 | New Frontier Financial. (2015). Modeling of State Patient Counts. Cannabis Weekly. |
- 22 -
Table of Contents
Currently the Corporation operates in the States of Illinois, Pennsylvania, Ohio, California, Nevada, Arizona, and Maryland and with firm plans to expand into New York, Massachusetts, Florida and Michigan. It intends to expand into other states within the United States that have legalized cannabis use either medicinally or recreationally.
On December 20, 2018, the 2018 Farm Bill (the “Farm Bill”) became law in the United States. Under the Farm Bill, industrial and commercial hemp is no longer be classified as a Schedule I controlled substance in the United States. Hemp includes the plant Cannabis sativa L and any part of that plant, including seeds, derivatives, extracts, cannabinoids and isomers. To qualify under the Farm Bill, hemp must contain no more than 0.3 percent of delta-9-tetrahydrocannabinol (“THC”). The Farm Bill explicitly allows interstate commerce of hemp which will enable the transportation and shipment of hemp. In February 2019, the Corporation announced the formation of a new wellness subsidiary, Well Beings, which will offer a full line of high-quality hemp-based CBD wellness products eligible for national distribution. The subsidiary, currently in early development stage, will have its own unique product line and produce CBD versions of Cresco Labs’ house of branded products including Cresco, Remedi and Mindy’s Edibles and provide the potential to expand its footprint to all 50 states and reach a new customer base outside of the licensed dispensary channel.
Illinois
The Compassionate Use of Medical Cannabis Pilot Program Act, which allows individuals diagnosed with a debilitating medical condition access to medical marijuana, became effective January 1, 2014 and is extended through July 1, 2020. There are more than 50 qualifying conditions as part of the medical program, including epilepsy, traumatic brain injury, and post-traumatic stress disorder (“PTSD”). Illinois’ retail market size for 2017 was over $86 million, representing an over 140% year-over-year increase. As of October 3, 2018, total retail sales were over $97 million representing an approximate 12% increase over 2017 retail sales (with two months remaining). On August 28, 2018, the Alternatives to Opioids Act (Public Act 100-1114) was signed into law. The Alternative to Opioids Act significantly expands the Illinois’ medical marijuana market by enabling patients to access medical marijuana in place of pharmaceutical opioid medications. The Illinois Department of Public Health reports that there were more than 5.3 million prescriptions for opioid-based painkillers filled last year. This paves the way for the single-largest expansion of the existing Illinois Medical Cannabis Pilot Program, which has about 42,000 authorized patients. Those patients have brought the state about $200 million in sales tax revenue since the program’s inception in late 2015. On August 9, 2019, the “Pilot” status was removed and the medical program became permanent.
The Opioid Alternative Pilot Program launched January 31, 2018 with registration open through the Illinois Department of Public Health. The pilot program is part of the Alternative to Opioids Act, which former Gov. Bruce Rauner signed into law in August 2018, with the aim of combating the opioid epidemic. The pilot program will allow patients that receive or are qualified to receive opioid prescriptions access to medical marijuana as an alternative to prescription opioid medications such as OxyContin, Percocet and Vicodin. Medical Cannabis Pilot Program patients with one of more than 50 qualifying medical conditions designated by the state of Illinois, and a doctor recommendation can also receive a temporary medical cannabis card online and make immediate cannabis purchases without waiting for their permanent card to be processed. In January 2019, one of Cresco’s Illinois dispensary locations launched its participation in this pilot program and made the first sale of medical cannabis thereunder.
In January 2019, JB Pritzker was sworn into office as Governor of Illinois. Cresco’s CEO and co-founder, Charles Bachtell, has been appointed to the Cannabis Legalization Subcommittee of the governor’s transition team. Cannabis Legalization is one of four subcommittees under the Governor’s Restorative Justice and Safe Communities Transition Committee. The primary goals of the Cannabis Legalization Subcommittee are to evaluate and develop implementation recommendations for the Governor-elects platform on legalizing cannabis. As outlined during the Governor’s campaign, these priorities include safely legalizing and decriminalizing cannabis, reviewing and commuting the sentences of people incarcerated for cannabis offenses in Illinois, as well as a focus on diversity and community outreach.
On June 25, 2019, Governor Pritzker signed into law the Cannabis Regulation and Tax Act, thereby legalizing the recreational use of cannabis. The establishment of a regulatory scheme and the grant of licenses to cultivate, distribute, and sell recreational cannabis in Illinois are in process for new operators. Sales of recreational cannabis began on or about January 1, 2020.
- 23 -
Table of Contents
Pennsylvania
The Pennsylvania medical marijuana program was signed into law on April 17, 2016 under Act 16 and provided access to state residents with one of 17 qualifying conditions, including epilepsy, chronic pain, and PTSD. The state operates as a high-barrier market with very limited market participation. Retail sales opened in February 2018 to a limited number of retail locations across the state. Pennsylvania is the fifth-largest state in the country, home to nearly 13 million people. Pennsylvania’s medical marijuana market is expected to become one of the biggest markets in the United States.12
Ohio
House Bill 523, effective on September 8, 2016, legalized medical marijuana in Ohio. The Ohio Medical Marijuana Control Program allows people with certain medical conditions, upon the recommendation of an Ohio-licensed physician certified by the State Medical Board, to purchase and use medical marijuana. Ohio’s medical cannabis sales are projected to be between US$200 and US$400 million once the system is fully matured.13 According to industry experts, Ohio could become a national “powerhouse” for the medical marijuana industry, largely because of its population — it’s the seventh largest state — and because the broad list of conditions eligible for treatment with medical marijuana includes “pain.”14 House Bill 523 required that the framework for the MMCP will be in place no later than September 2018. This timeframe allowed for a deliberate process to ensure the safety of the public and to promote access to a safe product. The first medical marijuana sales were on January 16, 2019.
The three following state government agencies are responsible for the operation of Ohio’s Medical Marijuana Control Program: (i) the Ohio Department of Commerce is responsible for overseeing medical marijuana cultivators, processors and testing laboratories; (ii) the State of Ohio Board of Pharmacy is responsible for overseeing medical marijuana retail dispensaries, the registration of medical marijuana patients and caregivers, the approval of new forms of medical marijuana and coordinating the Medical Marijuana Advisory Committee; and (iii) the State Medical Board of Ohio is responsible for certifying physicians to recommend medical marijuana and may add to the list of qualifying conditions for which medical marijuana can be recommended. Qualifying medical conditions for medical marijuana include: HIV/AIDS, Lou Gehrig’s disease, Alzheimer’s disease, cancer, chronic traumatic encephalopathy, Crohn’s disease, epilepsy or other seizure disorder, fibromyalgia, glaucoma, hepatitis C, inflammatory bowel disease, multiple sclerosis (MS), pain (either chronic, severe, or intractable), Parkinson’s disease, PTSD, sickle cell anemia, spinal cord disease or injury, tourette’s syndrome, traumatic brain injury and ulcerative colitis. In order for a patient to be eligible to obtain medical marijuana, a physician must make the diagnosis of one of these conditions.
Several forms of medical marijuana are legal in Ohio, these include: inhalation of marijuana through a vaporizer (not direct smoking), oils, tinctures, plant material, edibles, patches and any other forms approved by the State Board of Pharmacy.
California
In 1996, California was the first state to legalize medical marijuana through Proposition 215, the Compassionate Use Act of 1996. This legalized the use, possession and cultivation of medical marijuana by patients with a physician recommendation for treatment of cancer, anorexia, AIDS, chronic pain, spasticity, glaucoma, arthritis, migraine, or any other illness for which marijuana provides relief.
In 2003, Senate Bill 420 was signed into law establishing an optional identification card system for medical marijuana patients.
In September 2015, the California legislature passed three bills collectively known as the Medical Cannabis Regulation and Safety Act (“MCRSA”). The MCRSA established a licensing and regulatory framework for medical marijuana businesses in California. The system created multiple license types for dispensaries, infused products manufacturers, cultivation facilities, testing laboratories, transportation companies, and distributors. Edible infused
| 12 | https://mjbizdaily.com/chart-pennsylvanias-medical-marijuana-market-set-become-one-countrys-biggest/ |
| 13 | https://cannabusinessplans.com/ohios-medical-cannabis-market/ |
| 14 | https://cannabusinessplans.com/ohios-medical-cannabis-market/ |
- 24 -
Table of Contents
product manufacturers would require either volatile solvent or non-volatile solvent manufacturing licenses depending on their specific extraction methodology. Multiple agencies would oversee different aspects of the program and businesses would require a state license and local approval to operate. However, in November 2016, voters in California overwhelmingly passed Proposition 64, the Adult Use of Marijuana Act (“AUMA”) creating an adult-use marijuana program for adult-use 21 years of age or older. AUMA had some conflicting provisions with MCRSA, so in June 2017, the California State Legislature passed Senate Bill No. 94, known as Medicinal and Adult-Use Cannabis Regulation and Safety Act (“MAUCRSA”), which amalgamates MCRSA and AUMA to provide a set of regulations to govern medical and adult-use licensing regime for cannabis businesses in the State of California. The four agencies that regulate marijuana at the state level are the California Bureau of Cannabis Control, California Department of Food and Agriculture, California Department of Public Health, and California Department of Tax and Fee Administration.
In order to legally operate a medical or adult-use cannabis business in California, the operator must have both a local and state license. This requires license holders to operate in cities with marijuana licensing programs. Therefore, cities in California are allowed to determine the number of licenses they will issue to marijuana operators or can choose to ban marijuana outright.
MAUCRSA went into effect on January 1, 2018.
The California marijuana market is expected to be one of the fastest growing industries in California over the next five years. Market analysts forecast a stabilized market to occur after 2025 where the California marijuana market is estimated to be valued at approximately US$10 billion.15 In 2016, California recorded approximately US$850 million in medical marijuana retail sales from operated dispensaries state wide; however, it is estimated approximately 85% of total transactions are unrecorded for revenue and are carried out through illegal transactions. The University of California Agricultural Issues Center predicts the illegal market to shrink to less than 30%, legal adult-use sales to increase to approximately 62%, and legal medical sales to decrease from approximately 15% to less than 10% as patients are provided with an alternative to obtaining medical marijuana physician recommendations for a fee.16
Nevada
Nevada is one of the most dynamic markets anticipated for the full development of the recreational market. By certain estimates, the recreational market in Nevada is projected to have a cumulative average growth rate of 25%.17 With most of the state population and tourism located in Las Vegas, the opportunity in Las Vegas is strengthened by the fact that Las Vegas has a limited number of licenses and the city of Las Vegas has placed a priority for current license holders to be preferred in obtaining other non-operating retail licenses. The City of Las Vegas has historically seen nearly forty million tourists in a year, making it one of the most visited cities in the United States. Industry estimates put the overall cannabis market size in Las Vegas to be over US$800 million per year.18
Medical marijuana use was legalized in Nevada by a ballot initiative in 2000. In November 2016, voters in Nevada passed an adult use marijuana measure to allow for the sale of recreational marijuana in the state. The first dispensaries to sell adult use marijuana began sales in July 2017. The Nevada Department of Taxation is the regulatory agency overseeing the medical and adult use cannabis programs. Similar to California, cities and counties in Nevada are allowed to determine the number of local marijuana licenses they will issue.
| 15 | Sources: Berke, Jeremy. (2017 December 8). The legal marijuana market is exploding – it’ll hit almost $10 billion sales this year. Retrieved from http://www.businessinsider.com/legal-weed-market-to-hit-10-billion-in-sales-report-says-2017-12; Morris, Chris. (2017 December 6). Legal Marijuana Sales Are Expected to Hit $10 Billion This Year. Retrieved from http://fortune.com/2017/12/06/legal-marijuana-sales-10-billion/; The Arcview Group. (2017 December 6). NEW REPORT: Legal Marijuana Sales to Grow 33% to $10 Billion in 2017. Retrieved from https://globenewswire.com/news-release/2017/12/06/1234230/0/en/NEW-REPORT-Legal-Marijuana-Sales-to-Grow-33-to-10-Billion-in-2017.html. |
| 16 | McGreevy, Patrick. (2017 June 11). Legal marijuana could be a $5-billion boon to California’s economy. Retrieved from http://www.latimes.com/politics/la-pol-ca-pot-economic-study-20170611-story.html. |
| 17 | Frontier Financial Group Inc. (2017). Change in Compensation: Working in Cannabis. Retrieved from https://newfrontierdata.com/marijuana-insights/change-in-compensation-working-in-cannabis/. |
| 18 | Retrieved from https://newfrontierdata.com/cannabits/. |
- 25 -
Table of Contents
Arizona
In 2010, Arizona passed Ballot Proposition 203, which amended Title 36 to the Arizona Revised Statutes (“ARS”). This amendment added Chapter 28.1, titled the Arizona Medical Marijuana Act (the “AMMA”). The AMMA also appointed the Arizona Department of Health Services (the “ADHS”) as the regulator for the program and authorized ADHS to promulgate, adopt and enforce regulations for the AMMA. The ADHS has established the Arizona Department of Health Services Medical Marijuana Program (“MMJ Program”), which includes a vertically integrated license, meaning if allocated a Medical Marijuana Dispensary Registration Certificate (“AZ Dispensary License”), entities are authorized to dispense and cultivate medical cannabis. Arizona’s medical marijuana market is one of the largest in the nation as well as one of the hottest. The amount of medical marijuana sold has more than doubled from 5,012 pounds in August 2016 to 10,826 pounds in August 2018, according to the ADHS. The patient count during that period has surged from 105,076 to 178,257.19
The ADHS regulations are embodied in the Arizona Administrative Code Title 9 Chapter 17. ARS § 36-2801(11) defines a ‘‘nonprofit medical cannabis dispensary’’ as a not-for-profit entity that acquires, possesses, cultivates, manufactures, delivers, transfers, transports, supplies, sells or dispenses cannabis or related supplies and educational materials to cardholders.
The ADHS has established the MMJ Program, which includes a vertically integrated license, meaning if allocated an AZ Dispensary License, entities are authorized to dispense and cultivate medical cannabis. Each AZ Dispensary License allows the holding entity to operate one on-site cultivation facility, and one off-site cultivation facility which can be located anywhere within the State of Arizona. An entity holding an AZ Dispensary License is required to file an application to renew with the ADHS on an annual basis, which must also include audited annual financial statements. While an AZ Dispensary License may not be sold, transferred or otherwise conveyed, AZ Dispensary License holders typically contract with third parties to provide various services related to the ongoing operation, maintenance and governance of its dispensary and/or cultivation facility so long as such contracts do not violate the requirements of the AMMA or the MMJ Program.
The ADHS has established a registration application system for patients and nonprofit marijuana dispensaries, as well as a web-based verification platform for use by law enforcement officials and dispensaries to verify a patient’s status as such. The ADHS also specified patients’ rights, qualifying medical conditions, and allowed out-of-state medical marijuana patients to maintain their patient status (though not to purchase cannabis).
On December 6, 2012, Arizona’s first licensed medical marijuana dispensary opened in Glendale.
In order to qualify to use medical marijuana under the AMMA, a patient is required to have a “debilitating medical condition”. Valid medical conditions include: HIV, cancer, glaucoma, immune deficiency syndrome, hepatitis C, Chron’s disease, agitation of Alzheimer’s disease, ALS, cachexia/wasting syndrome, muscle spasms, nausea, seizures, severe and chronic pain or another chronic or debilitating condition.
New York
New York is one of the most promising medical cannabis markets that opened in 2016. The state population numbers near twenty million and New York City is among the most populous and visited cities in the United States.20 The New York program, when initially implemented, allowed for only five fully vertically integrated licenses. The licenses allowed each license holder the opportunity to operate a cultivation facility, extraction and manufacturing, and four retail medical marijuana dispensaries. The State program was adjusted to increase the range of qualifying conditions which, as of the date hereof, includes chronic and severe pain. In August 2017, the State of New York also increased the number of licensed operators in the state to a total of ten. Each of the newly added licenses can carry out the same operations as the original license holders. The State has made progress towards the ability to increase the outreach to qualified patients through the ten licensed operators via the disbursement of retail locations across the state, the
| 19 |
https://mjbizdaily.com/arizonas-sizzling-medical-marijuana-market-entices-investors-despite-legal-uncertainties/ |
| 20 | United States Census Bureau. (2017). QuickFacts United States. Retrieved from https://www.census.gov/quickfacts/NY; see also NYC and Company. NYC Travel & Tourism Visitation Statistics. Retrieved from http://www.nycandcompany.org/research/nyc-statistics-page; see also World Atlas. (2017 November 9). The Most Visited Cities In The US. Retrieved from https://www.worldatlas.com/articles/the-most-visited-cities-in-the-us.html. |
- 26 -
Table of Contents
increase in range of qualifying conditions, and other various methods to support patient access. In July, the New York Department of Health filed emergency regulations to add any condition, for which an opioid could be prescribed, as a qualifying condition for medical marijuana. This legislation was signed into law on September 24, 2018. From July 10 to September 25, 2018, the number of certified patients in the system rose to 18%.21
In July 2014, the New York Legislature and Governor enacted the Compassionate Care Act (A06357E, S07923) to provide a comprehensive, safe and effective medical marijuana program to meet the needs of New Yorkers. The program allows 10 “Registered Organizations” to hold vertically-integrated licenses and service qualified patients and caregivers. Limited product types are allowed in the state and smoking of cannabis flower is prohibited. The NYSDOH is the regulatory agency overseeing the medical marijuana program.
Massachusetts
In November 2015, Massachusetts, a medical cannabis market since January 2013, voted in favour of “Question 4”, approving the legalization of adult use. Research firm Arcview Market Research projects that the Massachusetts market will grow to over US$1 billion by 2020 at a compound annual growth rate of 113%. The Question 4 ballot initiative requiring the state legislature to authorize the adult use of cannabis in the state was approved by the Massachusetts electorate in November 2016. The first adult use dispensaries opened their doors on July 1, 2018. Located in the very populous North-Eastern region of the U.S., tourism is anticipated to be an important factor in driving market growth in a state that itself has a growing population of 6.8 million. The adjoining states represent an additional “tourist” market of 26 million, vastly exceeding the very successful industry in Colorado. The new legislation allows local control policy, allowing local government officials in towns that voted “no” on the 2016 ballot initiative to ban marijuana businesses until December 2019. For towns that voted “yes” in 2016, any bans must be placed on a local ballot for voters to approve. The maximum sales tax rate will increase from 12% to 20%. Under the bill, the state tax will be 17% and the local option will be 3%.
The Medical Use of Marijuana Program (the “MUMP”) registers qualifying patients, personal caregivers, Registered Marijuana Dispensaries (“RMD”), and RMD agents. The MUMP was established by Chapter 369 of the Acts of 2012, ‘‘An Act for the Humanitarian Medical Use of Marijuana,’’ following the passage of Ballot Question 3 in the 2012 general election. RMD certifications are vertically integrated licenses in that each RMD license entitles a license holder to three cultivation facilities, three processing facilities and up to three dispensary locations. There is a limit of three RMD licenses per person/entity.
The Massachusetts Department regulations 105 CMR 725.000 et seq. provide a regulatory framework that requires licensed producers, which are statutorily defined as ‘‘Registered Marijuana Dispensaries’’, to cultivate, process, transport and dispense medical cannabis in a vertically integrated marketplace. Patients with debilitating medical conditions qualify to participate in the program, including conditions such as cancer, glaucoma, positive status for human immunodeficiency virus (HIV), acquired immune deficiency virus (AIDS), hepatitis C, ALS, Crohn’s disease, Parkinson’s disease, and multiple sclerosis (MS) when such diseases are debilitating, and other debilitating conditions as determined in writing by a qualifying patient’s healthcare provider.
Maryland
Maryland adopted a comprehensive law legalizing medical cannabis in 2014. The Maryland program will result in a large medical marijuana market as a result of an expansive list of qualifying conditions, less restrictive provisions for obtaining cannabis certifications from doctors, and patient freedom to choose preferred methods of ingestions. The Maryland Medical Cannabis Commission began to sell through dispensaries on December 1, 2017. 14 growers, 12 processors and nine dispensaries have been licensed by the Maryland Medical Cannabis Commission, and on the day sales began, approximately 15,000 people had signed up to be prospective patients. Almost 550 healthcare providers have registered with Maryland to recommend cannabis to their patients. Maryland has a population of over 6 million people.
| 21 | https://mjbizdaily.com/new-york-formalizes-medical-cannabis-as-alternative-to-opioids-market-boost-seen/ |
- 27 -
Table of Contents
The Maryland Medical Cannabis Commission (the “MMCC”) grants medical cannabis grower, processor, dispensary and transportation licenses. A licensee may hold a license in each category to obtain vertical integration. The applicant must first seek pre-approval from the MMCC in order to be granted a license. As part of the pre-approval application, the applicant must submit information related to its operations; safety and security; medical cannabis professionalism; retail management factors; business and economic factors; and other additional factors that may apply.
Michigan
In November 2008, Michigan residents approved the Michigan Medical Marihuana Act (the “MMMA”) to provide a legal framework for a safe and effective medical marijuana program. In September 2016, the Michigan Senate passed the Medical Marihuana Facilities Licensing Act (the “MMFLA”) and the Marihuana Tracking Act (the “MTA” and together with the MMMA and the MMFLA, the “Michigan Cannabis Regulations”) to provide a comprehensive licensing and tracking scheme, respectively, for the medical marijuana program. Additionally, the Michigan Department of Licensing and Regulatory Affairs and its licensing board (“LARA”) has supplemented the Michigan Cannabis Regulations with “Emergency Rules” to further clarify the regulatory landscape surrounding the medical marijuana program. LARA is the main regulatory authority for the licensing of marijuana businesses.
Under the MMFLA, LARA administrates five types of “state operating licenses” for medical marijuana businesses: (a) a “grower” license, (b) a “processor” license, (c) a “secure transporter” license, (d) a “provisioning center” license and (e) a “safety compliance facility” license. There are no stated limits on the number of licenses that can be made available on a state level; however, LARA has discretion over the approval of applications and municipalities can pass additional restrictions.
On November 6, 2018, Michigan voters approved Proposal 1, to make marijuana legal under state and local law for adults 21 years of age or older and to control the commercial production and distribution of marijuana under a system that licenses, regulates, and taxes the businesses involved. The act will be known as the Michigan Regulation and Taxation of Marihuana Act. According to Proposal 1, LARA is required to start accepting applications for retail (recreational) dispensaries within 12 months of the measure’s effective date.
On November 13, 2019, the state’s Marijuana Regulatory Agency announced that any existing medically licensed businesses would be allowed to sell recreational use cannabis beginning December 1, 2019.
In December 2019, the state’s Marijuana Regulatory Agency adopted rules for adult-use cannabis.
Florida
In 2014, the Florida Legislature passed the Compassionate Use Act (the “CUA”) which was a low-THC (CBD) law, allowing cannabis containing not more than 0.8%THC to be sold to patients diagnosed with severe seizures or muscle spasms and cancer. The CUA created a competitive licensing structure and originally allowed for one vertically integrated license to be awarded in each of five regions. The CUA set forth the criteria for applicants as well as the minimum qualifying criteria which included the requirement to hold a nursery certificate evidencing the capacity to cultivate a minimum of 400,000 plants, to be operated by a nurseryman and to be a registered nursery for at least 30 continuous years. The CUA also created a state registry to track dispensations. In 2016, the Florida Legislature passed the Right to Try Act (the “RTA”), which expanded the State’s medical cannabis program to allow for full potency THC products to be sold as “medical marijuana” to qualified patients.
In November of 2016, the Florida Medical Marijuana Legalization ballot initiative (the “Initiative”) to expand the medical cannabis program under the RTA was approved by 71.3% of voters, thereby amending the Florida constitution. The Initiative is now codified as Article X, Section 29 of the Florida Constitution.
The Initiative expanded the list of qualifying medical conditions include cancer, epilepsy, glaucoma, HIV and AIDS, ALS, Crohn’s disease, Parkinson’s disease, multiple sclerosis, or other debilitating medical conditions of the same kind or class or comparable to those other qualifying conditions and for which a physician believes the benefits outweigh the risks to the patient. The Initiative also provided for the implementation of state-issued medical cannabis identification cards. In 2017, the Florida Legislature passed legislation implementing the constitutional amendment and further codifying the changes set forth in the constitution into law. The 2017 law provides for the issuance of 10 licenses to specific entities and another four licenses to be issued for every 100,000 active qualified patients added to the registry. The 2017 law also initially limited license holders to a maximum of 25 dispensary locations with the ability to purchase additional dispensary locations from one another, and for an additional five locations to be allowed by the State for every 100,000 active qualified patients added to the registry. The 2017 legislation’s cap on dispensing facilities expired in April 2020.
- 28 -
Table of Contents
Please see also “United States Regulatory Environment” and “State Regulatory Environment” in the AIF for a further description of the legal and regulatory landscape in respect of the states in which the Corporation currently operates.
Nonetheless, for the reasons referenced above and the risks further described under “Risk Factors” in the AIF, there are significant risks associated with the businesses of the Corporation. Readers are strongly encouraged to carefully read all of the risk factors contained in the AIF and other documents incorporated or deemed to be incorporated by reference herein, the applicable Prospectus Supplement and the documents incorporated or deemed to be incorporated by reference therein.
CERTAIN CANADIAN FEDERAL INCOME TAX CONSIDERATIONS
The applicable Prospectus Supplement may describe certain Canadian federal income tax considerations generally applicable to investors described therein of purchasing, holding and disposing of the applicable Securities, including, in the case of an investor who is not a resident of Canada, Canadian non-resident withholding tax considerations.
CERTAIN UNITED STATES FEDERAL INCOME TAX CONSIDERATIONS
The applicable Prospectus Supplement may describe certain United States federal income tax considerations generally applicable to investors described therein of purchasing, holding and disposing of the applicable Securities.
Before making an investment decision, prospective purchasers of Securities should carefully consider the information described in this Prospectus and the documents incorporated by reference herein (including subsequently filed documents incorporated by reference herein), including the applicable Prospectus Supplement. Additional risk factors relating to a specific offering of Securities may be described in the applicable Prospectus Supplement. Some of the risk factors described herein and in the documents incorporated by reference herein (including subsequently filed documents incorporated by reference herein), including the applicable Prospectus Supplement are interrelated and, consequently, investors should treat such risk factors as a whole. If any event arising from these risks occurs, the Corporation’s business, prospects, financial condition, results of operations and cash flows, and an investment in the Securities, could be materially adversely affected. Additional risks and uncertainties of which the Corporation is currently unaware or that are unknown or that the Corporation currently deems to be immaterial could have a material adverse effect on the Corporation’s business, prospects, financial condition, results of operations and cash flows. The Corporation cannot provide any assurances that it will successfully address any or all of these risks.
Risks Associated with the Securities of the Corporation
Founder Voting Control
As a result of the Super Voting Shares, the Founders exercise approximately 80.5% of the voting power in respect of the Corporation’s outstanding shares. The Subordinate Voting Shares are entitled to one vote per share, the Proportionate Voting Shares are entitled to 200 votes per share (subject to adjustment in accordance with the terms thereof) and the Super Voting Shares are entitled to 2,000 votes per share. As a result, the Founders (and any three of the Founders for certain actions not requiring a 2/3 majority) potentially have the ability to control the outcome of matters submitted to the Corporation’s shareholders for approval, including the election and removal of directors and any arrangement or sale of all or substantially all of the assets of the Corporation. Mr. Caltabiano left the Corporation in March 2020. If the remaining Founders’ employment with the Corporation is terminated, or they resign from their positions with the Corporation, they will continue to have the ability to exercise the same significant voting power. Additionally, each Super Voting Share, may be so transferred to the holder’s immediate family members, or in connection with estate or tax planning matters.
- 29 -
Table of Contents
In addition, because the number of Super Voting Shares held by a holder thereof from time to time is dependent upon the number of Cresco Redeemable Units (and Cresco Corp Redeemable Shares, if and when issued) beneficially owned, directly or indirectly, or deemed to be so beneficially owned by such holder from time to time, should the Corporation cause Cresco to issue additional Cresco Redeemable Units or Cresco Redeemable Units in the future to a Founder in connection with employee equity incentive programs, it would prolong the Founder’s voting control.
To supplement the rights, privileges, restrictions and conditions attached to the Super Voting Shares, the Corporation and the Founders, being the initial holders of Super Voting Shares, entered into an investment agreement effective as of the completion of the Business Combination which, among other things, provides that (i) each Super Voting Share will be transferable only to the holder’s immediate family members or an affiliated entity or a transfer to the other Founder or an entity affiliated with the other Founder, and (ii) upon any sale of Super Voting Shares to a third party purchaser not listed in clause (i), such Super Voting Shares will immediately be redeemed by the Corporation for their issue price.
The concentrated control through the Super Voting Shares could delay, defer, or prevent a change of control of the Corporation, arrangement involving the Corporation or sale of all or substantially all of the assets of the Corporation that it’s other shareholders support. Conversely, this concentrated control could allow the Founders to consummate such a transaction that the Corporation’s other shareholders do not support. In addition, the Founders may make long-term strategic investment decisions and take risks that may not be successful and may seriously harm the Corporation’s business.
As directors and officers of the Corporation, the remaining Founders have control over the day-to-day management and the implementation of major strategic decisions of the Corporation, subject to authorization and oversight by the Corporation’s board of directors. As board members and officers, the remaining Founders owe a fiduciary duty to the Corporation’s shareholders and are obligated to act honestly and in good faith with a view to the best interests of the Corporation. As shareholders, even controlling shareholders, the Founders are entitled to vote their shares, and shares over which they have voting control, in their own interests, which may not always be in the interests of the Corporation or the other shareholders of the Corporation.
Unpredictability Caused by the Capital Structure and Founder Voting Control
Although other Canadian-based companies have dual class or multiple voting share structures, given the concentration of voting control that is held by the Founders and given the other unique features of the capital structure of the Corporation, including the existence of a significant amount of redeemable equity securities that have been issued by, and are issuable pursuant to the exercise, conversion or exchange of the applicable convertible securities of, Cresco Labs, LLC, which equity securities are redeemable from time to time for Proportionate Voting Shares, in accordance with their terms, the Corporation is not able to predict whether this structure and control will result in a lower trading price for or greater fluctuations in the trading price of the Subordinate Voting Shares or will result in adverse publicity to the Corporation or other adverse consequences.
Additional Issuance of Subordinate Voting Shares or Subsidiary Securities May Result in Dilution
The Corporation may issue additional securities in the future, which may dilute a shareholder’s holdings in the Corporation. The Corporation’s articles permit the issuance of an unlimited number of Subordinate Voting Shares, and existing shareholders will have no pre-emptive rights in connection with such further issuance. The Corporation’s board of directors has discretion to determine the price and the terms of further issuances. Moreover, additional Subordinate Voting Shares will be issued by the Corporation on the conversion of the Proportionate Voting Shares in accordance with their terms. The Corporation may also issue Subordinate Voting Shares to finance future acquisitions. The Corporation cannot predict the size of future issuances of Subordinate Voting Shares or the effect that future issuances and sales of Subordinate Voting Shares will have on the market price of the Subordinate Voting Shares. Issuances of a substantial number of additional Subordinate Voting Shares, or the perception that such issuances could occur, may adversely affect prevailing market prices for the Subordinate Voting Shares. With any additional issuance of Subordinate Voting Shares, investors will suffer dilution to their voting power and the Corporation may experience dilution in its revenue per share.
- 30 -
Table of Contents
Additionally, the subsidiaries of the Corporation, such as Cresco U.S. Corp. and Cresco Labs, LLC, may issue additional securities, including Cresco Corp Redeemable Shares, Cresco Redeemable Units and long-term incentive plan units to new or existing shareholders, members or securityholders, including in exchange for services performed or to be performed on behalf of such entities or to finance future acquisitions. Any such issuances could result in substantial dilution to the indirect equity interest of the holders of Subordinate Voting Shares in Cresco Labs, LLC. Moreover, certain unitholders of the LLC and shareholders of Cresco may sell their securities of the LLC or the Corporation. The sale of a substantial number of such securities, or the perception in the market that holders of a large number of securities intend to sell securities, could reduce the market price of the Subordinate Voting Shares and could impair the Corporation’s ability to raise capital through the sale of additional equity securities. The effect of any such sales on the prevailing market price of the Subordinate Voting Shares is not predictable.
Additional Financing
The Corporation expects to require substantial additional capital in the near future to continue operations at its cultivation and production facilities, dispensaries, expansion of its product lines, development of its intellectual property base, increasing production capabilities and expanding its operations in states where it currently operates and states where it currently does not have operations. The Corporation may not be able to obtain additional financing on terms acceptable to it, or at all. If the Corporation fails to raise additional capital, as needed, its ability to implement its business model and strategy could be compromised.
Even if the Corporation obtains financing for its near-term operations, it expects that it will require additional capital thereafter. The capital needs of the Corporation will depend on numerous factors including: (i) profitability; (ii) the release of competitive products by competitors; (iii) the level of investment in research and development; and (iv) the amount of the Corporation’s capital expenditures, including acquisitions. There can be no assurance that the Corporation will be able to obtain capital in the future to meet its needs.
Although the Corporation has accessed private financing in the past, there is neither a broad nor deep pool of institutional capital that is available to companies in the U.S. cannabis industry. There can be no assurance that additional financing, if raised privately, will be available to the Corporation when needed or on terms which are acceptable.
Volatile Market Price of the Subordinate Voting Shares and Other Listed Securities
The market price of the Subordinate Voting Shares and other listed securities of the Corporation from time to time, cannot be predicted and has been and may be volatile and subject to wide fluctuations in response to numerous factors, many of which are beyond the Corporation’s control. This volatility may affect the ability of holders of Subordinate Voting Shares or such other securities to sell their securities at an advantageous price. Market price fluctuations in the Subordinate Voting Shares or such other securities may be due to the Corporation’s operating results failing to meet expectations of securities analysts or investors in any period, downward revision in securities analysts’ estimates, adverse changes in general market conditions or competitive, regulatory or economic trends, adverse changes in the economic performance or market valuations of companies in the industry in which the Corporation operates, acquisitions, dispositions, strategic partnerships, joint ventures, capital commitments or other material public announcements by the Corporation or its competitors or government and regulatory authorities, operating and share price performance of the companies that investors deem comparable to the Corporation, addition or departure of the Corporation’s executive officers and other key personnel, along with a variety of additional factors. These broad market fluctuations may adversely affect the market price of the Subordinate Voting Shares or such other securities.
Financial markets have at times historically experienced significant price and volume fluctuations that have particularly affected the market prices of equity and convertible securities of companies and that have often been unrelated to the operating performance, underlying asset values or prospects of such companies. Accordingly, the market price of the Subordinate Voting Shares and other listed securities of the Corporation, from time to time, may decline even if the Corporation’s operating results, underlying asset values or prospects have not changed. Additionally, these factors, as well as other related factors, may cause decreases in asset values that are deemed to be other than temporary, which may result in impairment losses. There can be no assurance that continuing fluctuations in price and volume will not occur. If such increased levels of volatility and market turmoil continue or arise, the Corporation’s operations may be adversely impacted and the trading price of the Subordinate Voting Shares and such other securities may be materially adversely affected.
- 31 -
Table of Contents
Negative Cash Flow from Operating Activities
The Corporation has incurred operating losses in recent periods. The Corporation may not be able to achieve or maintain profitability and may continue to incur significant losses in the future. In addition, the Corporation expects to continue to increase operating expenses as it implements initiatives to continue to grow its business. If the Corporation’s revenues do not increase to offset its costs and operating expenses or if the Corporation is unable to raise financing to fund capital or operating expenditures or acquisitions, it could limit its growth and may have a material adverse effect upon the Corporation’s business, financial condition, cash flows, results of operations or prospects.
Risks Associated with the Business of the Corporation
U.S. Federal Regulation
The Corporation could be found to be violating laws related to medical cannabis.
Currently, there are 33 states plus the District of Columbia, Puerto Rico and Guam that have laws and/or regulations that recognize, in one form or another, legitimate medical uses for cannabis and consumer use of cannabis in connection with medical treatment. Other states are considering similar legislation. Conversely, under the CSA, the policies and regulations of the federal government and its agencies are that cannabis has no proven medical benefit and a range of activities including cultivation and the personal use of cannabis is prohibited. Unless and until Congress amends the CSA with respect to medical cannabis, as to the timing or scope of any such amendments there can be no assurance, there is a risk that federal authorities may enforce current U.S. federal law. The risk of strict enforcement of the CSA in light of Congressional activity, judicial holdings, and stated federal policy remains uncertain. This would cause a direct and adverse effect on the Corporation’s subsidiaries’ businesses, or intended businesses, and on its revenue and prospective profits.
Marijuana is a Schedule-I controlled substance and is illegal under U.S. federal law. Even in those States in which the use of marijuana has been legalized, its use remains a violation of U.S. federal law. Since U.S. federal law criminalizing the use of marijuana pre-empts State laws that legalize its use, strict enforcement of U.S. federal law regarding marijuana would likely result in the Corporation’s inability to proceed with its business plan.
Laws and regulations affecting the medical marijuana industry are constantly changing, which could detrimentally affect the proposed operations of the Corporation.
Local, state, and U.S. federal medical marijuana laws and regulations are broad in scope and subject to evolving interpretations, which could require the Corporation to incur substantial costs associated with compliance or alter certain aspects of its business plan. In addition, violations of these laws, or allegations of such violations, could disrupt certain aspects of the Corporation’s business plan and result in a material adverse effect on certain aspects of its planned operations. In addition, it is possible that regulations may be enacted in the future that will be directly applicable to certain aspects of the Corporation’s business. No prediction can be made as to the nature of any future laws, regulations, interpretations or applications, nor can it be determined what effect additional governmental regulations or administrative policies and procedures, when and if promulgated, could have on the Corporation’s business.
Notwithstanding the permissive regulatory environment of medical marijuana at the state level, marijuana continues to be categorized as a controlled substance under the CSA. Under the CSA, the policies and regulations of the U.S. federal government and its agencies are that cannabis has no “proven” medical benefits. Unless and until Congress amends the CSA with respect to medical marijuana, as to the timing or scope of any such potential amendments there can be no assurance, there is a risk that U.S. federal authorities may enforce current U.S. federal law, and the Corporation may be deemed to be producing, cultivating, or dispensing marijuana in violation of U.S. federal law with respect to the Corporation’s current or proposed business operations, or the Corporation may be deemed to be facilitating the sale or distribution of drug paraphernalia in violation of U.S. federal law. A change in the U.S. federal government’s approach to begin more active enforcement of cannabis may adversely affect the Corporation’s revenues and profits. The risk of strict enforcement of the CSA in light of Congressional activity, judicial holdings, and stated U.S. federal policy remains uncertain.
- 32 -
Table of Contents
Risk of U.S. Federal Law Proceedings Against the Corporation
Potential proceedings under U.S. federal law could involve significant restrictions being imposed upon the Corporation or third parties, while diverting the attention of key executives. Such proceedings could have a material adverse effect on the Corporation’s business, revenues, operating results and financial condition as well as the Corporation’s reputation, even if such proceedings were concluded successfully in favour of the Corporation. In the extreme case, such proceedings could ultimately involve the prosecution of key executives of the Corporation or the seizure of corporate assets. However, as of the date hereof, the Corporation has obtained legal advice in respect thereof that proceedings of this nature have historically been sufficiently uncommon to be characterized as remote absent a shift by federal authorities to a more aggressive enforcement approach. The Corporation has also received advice from its legal counsel regarding the potential exposure and implications arising from U.S. federal law generally. As the legal landscape at both the U.S. federal level and the state level is evolving, all such legal advice is historical in nature, and is only effective up to the date such advice was received.
Following the issuance of the Sessions Memo and the Barr Comments, the Corporation continues to look to the guidelines of the Cole Memorandum as an industry best practice and continues to do the following to ensure compliance with the Cole Memorandum:
| • | ensuring the operations of its subsidiaries are compliant with all licensing requirements that are set forth with regards to cannabis operation by the applicable state, county, municipality, town, township, borough, and other political/administrative divisions. To this end, the Corporation retains appropriately experienced legal counsel and other professionals to conduct the necessary due diligence to ensure compliance of such operations with all applicable; |
| • | the activities relating to the cannabis business adhere to the scope of the licensing obtained. Accordingly, in the states where only medical cannabis is permitted, the products are only sold to patients who hold the necessary documentation to permit the possession of the cannabis; and in the states where cannabis is permitted for adult recreational use, the products are only sold to individuals who meet the requisite age requirements; |
| • | the Corporation only works through licensed operators, which must pass a range of requirements, adhere to strict business practice standards and be subjected to strict regulatory oversight whereby sufficient checks and balances ensure that no revenue is distributed to criminal enterprises, gangs and cartels; and |
| • | the Corporation conducts reviews of products and product packaging to ensure that the products comply with applicable regulations and contain necessary disclaimers about the contents of the products to prevent adverse public health consequences from cannabis use and prevent impaired driving. |
The Corporation will continue to monitor compliance on an ongoing basis in accordance with its compliance program and standard operating procedures. While the Corporation’s operations are in full compliance with all applicable state laws, regulations and licensing requirements, such activities remain illegal under U.S. federal law. For the reasons described above and the risks further described below, there are significant risks associated with the business of the Corporation.
Risks Associated with the Corporation’s Acquisition Strategy
The Corporation Could Fail to Complete its Proposed or Contemplated Acquisitions or They May Be Completed On Different Terms
There can be no assurances that any of the Corporation’s proposed or contemplated acquisitions will be completed or that they will be completed on the same or similar terms currently contemplated by the Corporation. In addition, if the proposed or contemplated acquisitions are not completed, the ongoing business of the Corporation may be adversely affected as a results of the costs (including opportunity costs) incurred in respect of pursuing potential acquisitions. Failure to complete the Corporation’s proposed or contemplated acquisitions could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
- 33 -
Table of Contents
Anticipated Benefits of Acquisition Strategy May Not Occur
The Corporation’s acquisition strategy may result in the Corporation failing to realize the growth opportunities and synergies currently anticipated due to, among other things, challenges associated with integration the operations and personnel of the Corporation with potential acquisition targets and the ability of the combined company to attract capital.
Pursuant to a decision of the Autorité des marchés financiers dated February 25, 2021, the Corporation was granted a permanent exemption from the requirement to translate into French this Prospectus as well as the documents incorporated by reference therein and any Prospectus Supplement to be filed in relation to any future “at-the-market” distribution. This exemption is granted on the condition that this Prospectus and any Prospectus Supplement (other than in relation to an “at-the-market” distribution) be translated into French if the Corporation offers Securities to Québec purchasers in connection with an offering other than in relation to an “at-the-market” distribution.
Unless otherwise specified in the Prospectus Supplement relating to an offering of Securities, certain legal matters relating to the offering of Securities will be passed upon on behalf of the Corporation by Bennett Jones LLP with respect to matters of Canadian law and by McDermott Will & Emery LLP with respect to matters of U.S. law. As of the date hereof, Bennett Jones LLP, and its partners, counsel and associates, beneficially own, directly or indirectly, as a group, less than 1% of any class of outstanding securities of the Corporation, Cresco Corp. and the LLC.
AUDITORS, TRANSFER AGENT AND REGISTRAR
Marcum LLP is the auditor of the Corporation and has confirmed that they are independent within the meaning of the relevant rules and related interpretations prescribed by the relevant professional bodies in Canada and any applicable legislation or regulations. Marcum LLP has performed the audit in respect of certain financial statements incorporated by reference herein or attached hereto.
The transfer agent and registrar for the Subordinate Voting Shares is Odyssey Trust Company at its principal offices in Calgary, Alberta.
DOCUMENTS FILED AS PART OF THE REGISTRATION STATEMENT
The following documents have been filed or furnished with the SEC as part of the Registration Statement of which this Prospectus forms a part: (i) the documents listed under the heading “Documents Incorporated by Reference”; (ii) powers of attorney from the Corporation’s directors and officers, as applicable; (iii) the consent of Marcum LLP; and (iv) the form of indenture relating to the Debt Securities. A copy of the form of warrant agreement, subscription receipt agreement or statement of eligibility of trustee on Form T-1, as applicable, will be filed by post-effective amendment or by incorporation by reference to documents filed or furnished with the SEC under the U.S. Exchange Act.
Securities legislation in certain of the provinces and territories of Canada provides purchasers with the right to withdraw from an agreement to purchase securities. This right may be exercised within two business days after receipt or deemed receipt of a prospectus and any amendment. In several of the provinces and territories, the securities
- 34 -
Table of Contents
legislation further provides a purchaser with remedies for rescission or, in some jurisdictions, revisions of the price or damages if the prospectus and any amendment contains a misrepresentation or is not delivered to the purchaser, provided that the remedies for rescission, revision of the price or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for the particulars of these rights or consult with a legal advisor.
Original purchasers of Securities that are convertible, exchangeable or exercisable for other securities of the Corporation will have a contractual right of rescission against the Corporation in respect of the conversion, exchange or exercise of such Securities. The contractual right of rescission will be further described in any applicable Prospectus Supplement, but will, in general, entitle such original purchasers to receive, upon surrender of the underlying securities, the amount paid for the applicable convertible, exchangeable or exercisable Securities in the event that this Prospectus, the relevant Prospectus Supplement or an amendment thereto contains a misrepresentation, provided that: (i) the conversion, exchange or exercise takes place within 180 days of the date of the purchase of such Securities under this Prospectus and the applicable Prospectus Supplement; and (ii) the right of rescission is exercised within 180 days of the date of the purchase of such Securities under this Prospectus and the applicable Prospectus Supplement.
In an offering of Debt Securities, Subscription Receipts, Warrants and Units which are convertible, exchangeable or exercisable for other securities of the Corporation, investors are cautioned that the statutory right of action for damages for a misrepresentation contained in this Prospectus, the relevant Prospectus Supplement or an amendment thereto is limited, in certain provincial and territorial securities legislation, to the price at which the Debt Securities, Subscription Receipts, Warrants and Units which are convertible, exchangeable or exercisable for other securities of the Corporation are offered to the public under the prospectus offering. This means that, under the securities legislation of certain provinces and territories, if the purchaser pays additional amounts upon conversion, exchange or exercise of the Security, those amounts may not be recoverable under the statutory right of action for damages that applies in those provinces and territories. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for the particulars of this right of action for damages, or consult with a legal adviser.
ENFORCEMENT OF JUDGMENTS AGAINST FOREIGN PERSONS
The directors, chief executive officer and chief financial officer of the Corporation, being Charles Bachtell, Dominic A. Sergi, Robert M. Sampson, John R. Walter, Gerald Corcoran, Thomas Manning, Randy Podolsky, Dennis Olis, Marc Lustig, Michele Roberts, and Carol Vallone reside outside of Canada and each has appointed Bennett Jones LLP, Suite 3400, One First Canadian Place, P.O. Box 130, Toronto, Ontario M5X 1A4, as his or her agent for service of process in Canada. Marcum LLP, the auditor in respect of the audited financial statements of the Corporation for the years ended January 31, 2019 and 2018, is incorporated, continued or otherwise organized under the laws of a foreign jurisdiction. Purchasers are advised that it may not be possible for investors to enforce judgments obtained in Canada against any person or company that resides outside of Canada or is incorporated, continued or otherwise organized under the laws of a foreign jurisdiction, even if the party has appointed an agent for service of process.
- 35 -
Table of Contents
PART II
INFORMATION NOT REQUIRED TO BE DELIVERED TO
OFFEREES OR PURCHASERS
Indemnification of Directors and Officers.
Section 160 of the Business Corporations Act (British Columbia) (the “BCBCA”) authorizes a company to indemnify past and present directors and officers of the company and past and present directors and officers of a corporation of which the company is or was a shareholder, against liabilities incurred in connection with the provision of their services as such if the director or officer acted honestly and in good faith with a view to the best interests of the company and, in the case of a criminal or administrative proceeding, if he or she had reasonable grounds for believing that his or her conduct was lawful. Section 165 of the BCBCA provides that a company may purchase and maintain liability insurance for the benefit of such directors and officers.
Under the Company’s articles and subject to the provisions of the BCBCA, the Company shall indemnify a director, former director or alternate director of the Company and his or her heirs and legal personal representatives against all eligible penalties to which such person is or may be liable, and the Company shall, after the final disposition of an eligible proceeding, pay the expenses actually and reasonably incurred by such person in respect of that proceeding. Under the Company’s articles and subject to any restrictions in the BCBCA, the Company may indemnify any other person, including the officers, former officers and alternate officers of the Company.
A policy of directors’ and officers’ liability insurance is maintained by the Company which insures directors and officers against losses incurred as a result of claims against the directors and officers of the Company pursuant to the indemnity provisions under the Company’s articles and the BCBCA.
Insofar as indemnification for liabilities arising under the U.S. Securities Act may be permitted to directors, officers or persons controlling the Company pursuant to the foregoing provisions, the Company has been informed that in the opinion of the Commission such indemnification is against public policy as expressed in the U.S. Securities Act and is therefore unenforceable.
EXHIBIT INDEX
Table of Contents
| * | Filed herewith. |
Table of Contents
PART III
UNDERTAKING AND CONSENT TO SERVICE OF PROCESS
Item 1. Undertaking.
The registrant undertakes to make available, in person or by telephone, representatives to respond to inquiries made by the Commission staff, and to furnish promptly, when requested to do so by the Commission staff, information relating to the securities registered pursuant to Form F-10 or to transactions in said securities.
Item 2. Consent to Service of Process.
| (a) | A written Appointment of Agent for Service of Process and Undertaking on Form F-X for the Registrant and its agent for service of process was filed concurrently with the initial filing of this Registration Statement on Form F-10. |
| (b) | Any change to the name or address of the Registrant’s agent for service shall be communicated promptly to the Commission by amendment to Form F-X referencing the file number of this Registration Statement. |
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form F-10 and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the city of Chicago, Illinois on February 26, 2021
| Cresco Labs Inc. | ||
| By: | /s/ Charles Bachtell | |
| Name: | Charles Bachtell | |
| Title: | Chief Executive Officer | |
Table of Contents
Each person whose signature appears below hereby appoints each of Charles Bachtell and Dennis Olis, severally, acting alone and without the other, his or her true and lawful attorney-in-fact, with full power of substitution, and with the authority to execute in the name of each such person, any and all amendments (including without limitation, post-effective amendments) to this registration statement, which amendments may make such other changes in the registration statement as the aforesaid attorney-in-fact executing the same deems appropriate, and to file such registration statements with the Securities and Exchange Commission, together with any exhibits thereto and other documents therewith, and granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing necessary or advisable to enable the registrant to comply with the Securities Act of 1933, and any rules, regulations and requirements of the Securities and Exchange Commission in respect thereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on February 26, 2021.
| Signature |
Title | |||
| /s/ Charles Bachtell |
Chief Executive Officer and Director | |||
| Charles Bachtell | (Principal Executive Officer) | |||
| /s/ Dennis Olis |
||||
| Dennis Olis | Chief Financial Officer | |||
| (Principal Financial and Accounting Officer) | ||||
| /s/ Tom Manning |
||||
| Tom Manning | Executive Chairman of the Board of Directors | |||
| /s/ Gerry Corcoran |
||||
| Gerry Corcoran | Director | |||
| /s/ Marc Lustig |
||||
| Marc Lustig | Director | |||
| /s/ Randy Podolsky |
||||
| Randy Podolsky | Director | |||
| /s/ Michele Roberts |
||||
| Michele Roberts | Director | |||
Table of Contents
| /s/ Rob Sampson |
||||
| Rob Sampson | Director | |||
| /s/ Dominic Sergi |
||||
| Dominic Sergi | Director | |||
| /s/ Carol Vallone |
||||
| Carol Vallone | Director | |||
| /s/ John Walter |
||||
| John Walter | Director | |||
Table of Contents
AUTHORIZED REPRESENTATIVE
Pursuant to the requirements of Section 6(a) of the Securities Act of 1933, the undersigned has signed this Registration Statement, solely in its capacity as the duly authorized representative of Cresco Labs Inc. in the United States, on February 26, 2021.
| By: | /s/ John Schetz | |||
| Name: | John Schetz | |||
| Title: | General Counsel | |||
Exhibit 4.6
CRESCO LABS INC.

November 30, 2018
LISTING STATEMENT - FORM 2A
IN CONNECTION WITH THE LISTING OF THE SHARES OF CRESCO LABS INC., THE ENTITY FORMERLY KNOWN AS RANDSBURG INTERNATIONAL GOLD CORP., AFTER THE REVERSE TAKEOVER BY CRESCO LABS, LLC
|
MARIJUANA IS ILLEGAL UNDER U.S. FEDERAL LAW AND THE ENFORCEMENT OF RELEVANT LAWS IS A SIGNIFICANT RISK.
Cresco Labs Inc. indirectly holds all the voting interests and an approximate 53% equity interest in Cresco Labs, LLC, the entity through which all of the operating subsidiaries of Cresco Labs, LLC are held. Shareholders are cautioned that at this time, the outstanding Subordinate Voting Shares and Proportionate Voting Shares does not represent all of the equity interest in Cresco Labs, LLC.
Cresco Labs Inc. derives a substantial portion of its revenues from the cannabis industry in certain states of the United States, which industry is illegal under United States federal law. Cresco Labs Inc. is indirectly involved (through its licensed subsidiaries) in the cannabis industry in the United States where local state laws permit such activities. Currently, its subsidiaries are directly engaged in the manufacturing, possession, use, sale or distribution of cannabis in the recreational and/or medicinal cannabis marketplace in the States of Illinois, Pennsylvania, Ohio, Nevada, Arizona and California and will begin such activities in New York, Massachusetts and Maryland in the near term.
The United States federal government regulates drugs through the Controlled Substances Act, 21 U.S.C. § 811 (the “CSA”), which places controlled substances, including cannabis, in a schedule. Cannabis is classified as a Schedule I drug. Under United States federal law, a Schedule I drug or substance has a high potential for abuse, no accepted medical use in the United States, and a lack of accepted safety for the use of the drug under medical supervision. The United States Food and Drug Administration (“FDA”) has not approved marijuana as a safe and effective drug for any indication.
In the United States, marijuana is largely regulated at the state level. State laws regulating cannabis are in direct conflict with the CSA, which makes cannabis use and possession federally illegal in the United States. Although certain states authorize medical or recreational cannabis production and distribution by licensed or registered entities, under U.S. federal law, the possession, use, cultivation, and transfer of cannabis and any related drug paraphernalia is illegal and any such acts are criminal acts under U.S. federal law. The Supremacy Clause of the United States Constitution establishes that the United States Constitution and federal laws made pursuant to it are paramount and in case of conflict between U.S. federal and state law, the U.S. federal law shall apply.
On January 4, 2018, U.S. Attorney General Jeff Sessions issued a memorandum to U.S. district attorneys, which rescinded previous guidance from the U.S. Department of Justice specific to cannabis enforcement in the United States, including the Cole Memorandum (as defined herein). With the Cole Memorandum rescinded, U.S. federal prosecutors have been given discretion in determining whether to prosecute cannabis related violations of U.S. federal law. |
|
There is no guarantee that state laws legalizing and regulating the sale and use of cannabis will not be repealed or overturned, or that local governmental authorities will not limit the applicability of state laws within their respective jurisdictions. Unless and until the United States Congress amends the CSA with respect to medical and/or adult-use cannabis (and there can be no assurance as to the timing or scope of any such potential amendments), there is a risk that U.S. federal authorities may enforce current U.S. federal law. If the U.S. federal government begins to enforce U.S. federal laws relating to cannabis in states where the sale and use of cannabis is currently legal, or if existing applicable state laws are repealed or curtailed, Cresco Labs Inc.’s business, results of operations, financial condition and prospects would be materially adversely affected. See Section 17 of this Listing Statement – Risk Factors for additional information on this risk.
In light of the political and regulatory uncertainty surrounding the treatment of U.S. cannabis-related activities, including the rescission of the Cole Memorandum discussed above, on February 8, 2018 the Canadian Securities Administrators published a staff notice (Staff Notice 51-352) setting out the Canadian Securities Administrator’s disclosure expectations for specific risks facing issuers with cannabis-related activities in the United States. Staff Notice 51-352 confirms that a disclosure-based approach remains appropriate for issuers with U.S. cannabis-related activities. Staff Notice 51-352 includes additional disclosure expectations that apply to all issuers with U.S. cannabis- related activities, including those with direct and indirect involvement in the cultivation and distribution of cannabis, as well as issuers that provide goods and services to third parties involved in the U.S. cannabis industry. |
-2-
CAUTIONARY NOTE REGARDING FORWARD-LOOKING INFORMATION
This Listing Statement includes “forward-looking information” and “forward-looking statements” within the meaning of Canadian securities laws and United States securities laws. All information, other than statements of historical facts, included in this Listing Statement that address activities, events or developments that the Resulting Issuer expects or anticipates will or may occur in the future is forward-looking information. Forward-looking information is often identified by the words “may”, “would”, “could”, “should”, “will”, “intend”, “plan”, “anticipate”, “believe”, “estimate”, “expect” or similar expressions and includes, among others, information regarding: expectations for the effects of the Business Combination, the potential benefits of the Business Combination; statements relating to the business and future activities of, and developments related to, the Resulting Issuer after the date of this Listing Statement, including but not limited to, such things as future business strategy, competitive strengths, goals, expansion and growth of the Resulting Issuer’s business, operations and plans, including new revenue streams, the completion of contemplated acquisitions by the Resulting Issuer, the application for additional licenses and the grant of licenses that have been applied for, the expansion of existing cultivation and production facilities, the completion of cultivation and production facilities that are under construction, the construction of additional cultivation and production facilities, the expansion into additional States within the United States, international markets and Canada, any potential future legalization of adult-use and/or medical marijuana under U.S. federal law; expectations of market size and growth in the United States and the States in which the Resulting Issuer operates; expectations for other economic, business, regulatory and/or competitive factors related to the Resulting Issuer or the cannabis industry generally; and other events or conditions that may occur in the future.
Resulting Issuer Shareholders are cautioned that forward-looking information and statements are not based on historical facts but instead are based on reasonable assumptions and estimates of management of the Resulting Issuer at the time they were provided or made and involve known and unknown risks, uncertainties and other factors which may cause the actual results, performance or achievements of the Resulting Issuer, as applicable, to be materially different from any future results, performance or achievements expressed or implied by such forward-looking information and statements. Such factors include, among others, risks relating to the founder voting control, risks relating to U.S. federal regulation, the variation in state regulation, risks relating to U.S. regulatory landscape and enforcement related to cannabis, including political risks; risks relating to anti-money laundering laws and regulation; risks relating to changes in cannabis laws and regulatory uncertainty; risks relating to legal, regulatory or political change; risks relating to Canadian investors in the U.S. cannabis sector; risks relating to the market price and volatility of the cannabis sector; risks relating to the internal controls of the Resulting Issuer and dilution; risks relating to the global economic condition; risks relating to the value of the Subordinate Voting Shares; tax and insurance related risks; risks relating to the limited operating history of the Resulting Issuer and the reliance on the expertise and judgment of senior management of the Resulting Issuer; risks relating to competition; risks relating to the difficulty in recruiting and retaining management and key personnel and managing growth; risks relating to the unreliability of forecasts; risks relating to the inability to innovate and find efficiencies; website and operational risks; risks relating to the reliance on third-party suppliers, manufacturers and contractors; risks relating to revenue shortfalls; risks relating to the ability to obtain the necessary permits and authorizations; risks relating to potential conflicts of interest; risks related to proprietary intellectual property and potential infringement by third parties; risks relating to the lack of U.S. bankruptcy protection, currency fluctuations and lack of earnings and dividend record; risks relating to anti-money laundering laws and regulation; risks relating to civil asset forfeiture; risks relating to the heightened scrutiny of investments in the U.S.; risks relating to the ability and constraints on marketing products; risks relating to the settlements of trades, access to banks and legality of contracts; risks relating to the unfavourable tax treatment of cannabis businesses in the U.S. and the classification of the Resulting Issuer for U.S. tax purposes; risks relating to the public opinion, consumer acceptance and perception of the cannabis industry; security risks; risks relating to litigation; risks inherent in an agricultural business; risks relating to the Resulting Issuers reliance on licenses; risks relating to product liability and product recall; risks relating to regulatory or agency proceedings, investigations and audits; risks relating to the newly established legal regimes; and general economic risks as well as those risk factors discussed in Section 17 of this Listing Statement below. Although the Resulting Issuer has attempted to identify important factors that could cause actual results to differ materially, there may be other factors that cause results not to be as anticipated, estimated or intended. There can be no assurance that such forward-looking information and statements will prove to be accurate as actual results and future events could differ materially from those anticipated in such information and statements. Accordingly, readers should not place undue reliance on forward-looking information and statements. Forward-looking information and statements are provided and made as of the date of this Listing Statement and the Resulting Issuer does not undertake any obligation to revise or update any forward-looking information or statements other than as required by applicable law.
Market and Industry Data
This Listing Statement includes market and industry data that has been obtained from third- party sources, including industry publications. The Reporting Issuer believes that the industry data is accurate and that its estimates and assumptions are reasonable, but there is no assurance as to the accuracy or completeness of this data. Third party sources generally state that the information contained therein has been obtained from sources believed to be reliable, but there is no assurance as to the accuracy or completeness of included information. Although the data is believed to be reliable, the Reporting Issuer has not independently verified any of the data from third-party sources referred to in this Listing Statement or ascertained the underlying economic assumptions relied upon by such sources.
Currency
Unless otherwise indicated, all references to “$” or “US$” in this Listing Statement refer to United States dollars and all references to “C$” in this Listing Statement refer to Canadian dollars.
-2-
TABLE OF CONTENTS
| 1. | Glossary of Terms |
1 | ||||
| 2. | Corporate Structure |
8 | ||||
| 3. | General Development of the Business |
10 | ||||
| 4. | Narrative Description of the Business |
16 | ||||
| 5. | Selected Consolidated Financial Information |
60 | ||||
| 6. | Management’s Discussion and Analysis |
61 | ||||
| 7. | Market for Securities |
61 | ||||
| 8. | Consolidated Capitalization |
61 | ||||
| 9. | Options to Purchase Securities |
62 | ||||
| 10. | Description of the Securities |
66 | ||||
| 11. | Escrowed Securities |
83 | ||||
| 12. | Principal Shareholders |
84 | ||||
| 13. | Directors and Officers |
84 | ||||
| 14. | Capitalization |
89 | ||||
| 15. | Executive Compensation |
92 | ||||
| 16. | Indebtedness of Directors and Executive Officers |
92 | ||||
| 17. | Risk Factors |
93 | ||||
| 18. | Promoters |
115 | ||||
| 19. | Legal Proceedings |
115 | ||||
| 20. | Interest of Management and Others in Material Transactions |
116 | ||||
| 21. | Auditors, Transfer Agents and Registrars |
116 | ||||
| 22. | Material Contracts |
116 | ||||
| 23. | Interest of Experts |
116 | ||||
| 24. | Other Material Facts |
117 | ||||
| 25. | Financial Statements |
123 | ||||
| Schedule “A” | Audited annual consolidated financial statements for Cresco as of and for the years ended December 31, 2017 and 2016 |
| ||||
| Schedule “B” | Unaudited consolidated financial statements for Cresco as of and for the three and six months ended June 30, 2018 and 2017 |
| ||||
| Schedule “C” | Cresco’s Management Discussion & Analysis |
| ||||
| Schedule “D” | Audited annual financial statements for Randsburg as of and for the years ended January 31, 2018 and 2017 |
| ||||
| Schedule “E” | Condensed interim financial statements (restated) of Randsburg as at July 31, 2018 and for the three and six-month periods ended July 31, 2018 and 2017 |
| ||||
| Schedule “F” | Consolidated Pro Forma Financial Statements of the Resulting Issuer |
| ||||
| 1. | Glossary of Terms |
The following is a glossary of certain general terms used in this Listing Statement including in the summary hereof. Terms and abbreviations used in the financial statements appended to this Listing Statement are defined separately and the terms and abbreviations defined below are not used therein, except where otherwise indicated. Words importing the singular, where the context requires, include the plural and vice versa and words importing any gender include all genders.
“A&M” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“A&R LLC Agreement” means the Amended and Restated Limited Liability Company Agreement of Cresco, entered into by Cresco and each of the members of Cresco on the Closing Date.
“Agents” means Canaccord Genuity Inc. and GMP Securities L.P., as agents in connection with the SR Offering.
“Agency Agreement” has the meaning ascribed thereto in Section 3 of the Listing Statement.
“Agent Fee” has the meaning ascribed thereto in Section 3 of the Listing Statement.
“ADHS” has the meaning ascribed thereto in Section 3 of the Listing Statement.
“AFS” has the meaning ascribed thereto in Section 3 of the Listing Statement.
“AFS Maryland” has the meaning ascribed thereto in Section 3 of the Listing Statement.
“ALS” means Amyotrophic Lateral Sclerosis.
“AMMA” has the meaning ascribed thereto in Section 3 of the Listing Statement.
“AO LTIP Unit” means a unit of Cresco which is designated as an “Appreciation Only LTIP Unit” in the applicable vesting agreement or other documentation pursuant to which such AO LTIP Unit is granted or issued, having the rights, powers, privileges, restrictions, qualifications and limitations set forth in Exhibit A to the A&R LLC Agreement in respect of the holder thereof, as well as any applicable vesting agreement or other documentation pursuant to which such AO LTIP Unit is granted or issued.
“API” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“ARS” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“Assumed tax liability” has the meaning ascribed thereto under the A&R LLC Agreement.
“AUMA” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“AZ Dispensary License” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“Bank Secrecy Act” has the meaning ascribed thereto in Section 3 of the Listing Statement.
“Basis Adjustments” has the meaning ascribed thereto in Section 10 of the Listing Statement.
“BCBCA” means the Business Corporations Act (British Columbia), as amended.
“BCC” means California Bureau of Cannabis Control.
“Broker Warrants” has the meaning ascribed thereto in Section 3 of the Listing Statement.
“Business Combination” means the business combination among Randsburg and Cresco pursuant to which Cresco completed a reverse take-over of Randsburg.
“CalCannabis” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“Cal Subsidiaries” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“Canadian Securities Administrators” refers to an umbrella organization of Canada’s provincial and territorial securities regulators.
“CARERS Act” means the Compassionate Access, Research Expansion, and Respect States Act of 2015, as amended.
“CBP” has the meaning ascribed thereto in Section 17 of the Listing Statement.
“CCA” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“CDS” has the meaning ascribed thereto in Section 17 of the Listing Statement.
“Certificate” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“Certificate of Operation” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“Class 1, Div 1” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“Class F Units” means Class F units in the capital of Cresco existing prior to the recapitalization of Cresco in connection with the completion of the Business Combination.
“CME Group” has the meaning ascribed thereto in Section 13 of the Listing Statement.
“Code” means the United States Internal Revenue Code of 1986, as amended.
“Cole Memo” has the meaning ascribed thereto in Section 3 of the Listing Statement.
“Common Units” means those units designated by Cresco after Cresco effected a recapitalization of its outstanding unit capital in connection with the Business Combination, whereby under such recapitalization all previously issued Cresco Units were combined into a single class of non-voting units of Cresco.
“Compensation Committee” means the Compensation Committee of the Resulting Issuer to be appointed by the Resulting Issuer Board.
“Conversion Ratio” has the meaning ascribed thereto in Section 10 of the Listing Statement.
“Cresco” means Cresco Labs, LLC, a limited liability company existing under the laws of the state of Illinois.
“Cresco Acquisition” means Cresco Labs Finco Ltd., a company existing prior to the completion of the Business Combination under the laws of the Province of British Columbia, which entity was amalgamated with a subsidiary of Randsburg in connection with the completion of the Business Combination.
“Cresco Acquisition Shares” means the common shares in the capital of Cresco Acquisition.
“Cresco Canada” means Cresco SPV Inc., a company existing prior to the completion of the Business Combination under the laws of the Province of British Columbia, which entity was amalgamated with a subsidiary of Randsburg in connection with the completion of the Business Combination.
“Cresco Canada Shares” means the voting common shares in the capital of Cresco Canada.
-2-
“Cresco Corp” means Cresco U.S. Corp., a company existing under the laws of the state of Illinois and an entity that became a direct subsidiary of Randsburg as a result of the Business Combination.
“Cresco Corp Redeemable Shares” means the non-voting common shares in the capital of Cresco Corp.
“Cresco Corp Voting Shares” means the voting common shares in the capital of Cresco Corp.
“Cresco LTIP Unitholder” means the holders of the LTIP Units.
“Cresco Members” means the holders of Cresco Units.
“Cresco Ohio” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“Cresco Redeemable Units” means the Common Units of Cresco following completion of the Business Combination held by Cresco Members other than Cresco Corp.
“Cresco Units” means the Class A units, Class B units Class C units, Class D units, Class E units and Class F units in the capital of Cresco existing prior to the recapitalization of Cresco in connection with the completion of the Business Combination.
“Cresco Yeltrah” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“CSA” has the meaning ascribed thereto on the cover page of the Listing Statement.
“CSE” means the Canadian Securities Exchange.
“CUA” has the meaning ascribed thereto on the cover page of the Listing Statement.
“DCO” has the meaning ascribed thereto in Section 13 of the Listing Statement.
‘‘Dispensary License’’ has the meaning ascribed thereto in Section 3 of the Listing Statement.
“Distributable cash” has the meaning ascribed thereto under the A&R LLC Agreement.
“DOJ” has the meaning ascribed thereto in Section 17 of the Listing Statement.
“DOT” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“Equity Plan” means the new equity incentive plan approved by Randsburg Shareholders at the Meeting and adopted by the Resulting Issuer.
“Escrow Agent” means Odyssey Trust Company.
“Escrowed Funds” has the meaning ascribed thereto in Section 3 of the Listing Statement.
“Expanded Affiliated Group” has the meaning ascribed thereto in Section 17 of the Listing Statement.
“FATCA” means Foreign Account Tax Compliance Act.
“FCEN” has the meaning ascribed thereto in Section 17 of the Listing Statement.
“FCEN Memo” has the meaning ascribed thereto in Section 17 of the Listing Statement.
“FDA” has the meaning ascribed thereto on the cover page of the Listing Statement.
-3-
“FinCEN” has the meaning ascribed thereto in Section 3 of the Listing Statement.
“FIRPTA” has the meaning ascribed thereto in Section 18 of the Listing Statement.
“FloraMedex” has the meaning ascribed thereto in Section 3 of the Listing Statement.
“Founders” has the meaning ascribed thereto in Section 17 of the Listing Statement.
“FV LTIP Unit” means a unit of Cresco which is designated as a “Full Value LTIP Unit” in the applicable vesting agreement or other documentation pursuant to which such FV LTIP Unit is granted or issued, having the rights, powers, privileges, restrictions, qualifications and limitations set forth in Exhibit A to the A&R LLC Agreement in respect of the holder thereof, as well as any applicable vesting agreement or other documentation pursuant to which such FV LTIP Unit is granted or issued.
“HHH” has the meaning ascribed thereto in Section 3 of the Listing Statement.
“Holder” has the meaning ascribed thereto in Section 10 of the Listing Statement.
“Holder’s Group” has the meaning ascribed thereto in Section 10 of the Listing Statement.
“iCORI” means the Criminal Offender Record Information System.
“IFM” has the meaning ascribed thereto in Section 13 of the Listing Statement.
“ILLCA” Illinois Limited Liability Company Act, as amended.
“IRS” means the Internal Revenue Service.
“ISO” has the meaning ascribed thereto in Section 9 of the Listing Statement.
“ITA” has the meaning ascribed thereto in Section 17 of the Listing Statement.
“Leahy Amendment” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“LIBOR” means the London Inter-bank Offered Rate.
“Lighthouse” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“LTIP Units” has the meaning ascribed thereto in Section 10 of the Listing Statement.
“Massachusetts Department” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“Massachusetts Regulation” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“MAUCRSA” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“MCRSA” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“MCSB” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“Meeting” means the special meeting of Randsburg Shareholders held on November 14, 2018.
“METRC” an end-to-end tracking and tracing software for marijuana plants and products provided by Franwell Inc.
“MIPs” has the meaning ascribed thereto in Section 4 of the Listing Statement.
-4-
“MJ Freeway” means MJ Freeway Inc. a corporation providing cloud-based, seed-to-sale, cannabis compliance software for marijuana businesses including retail, delivery, wholesale, cultivation, and manufacturing.
“MMCC” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“MMCP” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“MMJ Program” has the meaning ascribed thereto in Section 3 of the Listing Statement.
“MOU” has the meaning ascribed thereto in Section 17 of the Listing Statement.
“MUMP” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“Names Executive Officers” has the meaning ascribed thereto in Section 15 of the Listing Statement.
“NBSG” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“Nevada Licenses” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“NFA” has the meaning ascribed thereto in Section 13 of the Listing Statement.
“Non U.S. Holder” has the meaning ascribed thereto in Section 18 of the Listing Statement.
“NQSO” has the meaning ascribed thereto in Section 9 of the Listing Statement.
“NY Licenses” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“NY Purchase Price” has the meaning ascribed thereto in Section 3 of the Listing Statement.
“NYSDOH” has the meaning ascribed thereto in Section 3 of the Listing Statement.
“Option” has the meaning ascribed thereto in Section 9 of the Listing Statement.
“Paradise Wellness” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“Participants” has the meaning ascribed thereto in Section 9 of the Listing Statement.
“Phoenix Farms” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“PMP” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“Pre-Combination LLC Agreement” means the Cresco limited liability company agreement dated October 8, 2013, as amended and restated as of March 28, 2015 and as further amended and restated as of March 17, 2018.
“Prior LLC Agreement” has the meaning ascribed thereto in Section 2 of the Listing Statement.
“Promissory Notes” has the meaning ascribed thereto in Section 5 of the Listing Statement.
“Proportionate Voting Shares” means the Proportionate Voting Shares in the capital of the Resulting Issuer, after giving effect to the Business Combination.
“PTSD” has the meaning ascribed thereto in Section 3 of the Listing Statement.
“Public Securityholders” has the meaning ascribed thereto in Section 14 of the Listing Statement.
-5-
“Publicly traded partnership” has the meaning ascribed thereto under the Code.
“Randsburg” means Randsburg International Gold Corp. a corporation existing under the BCBCA, which entity was renamed to Cresco Labs Inc. in connection with the Business Combination and for the purposes herein following the completion of the Business Combination is referred to herein as the Resulting Issuer.
“Randsburg Articles Amendment” means the amendment of the rights and restrictions of the existing class of Randsburg Shares and re-designation of such class as the class of Subordinate Voting Shares and the creation of the class of Super Voting Shares and the class of Proportionate Voting Shares.
“Randsburg Share Consolidation” means the consolidation of the Randsburg Shares being one (1) post-consolidation Randsburg Shares for every existing 812.63 pre-consolidation Randsburg Shares.
“Randsburg Shareholders” means the holders of Randsburg Shares.
“Randsburg Shares” means the common shares in the capital of Randsburg, prior to giving effect to the Business Combination, including the Randsburg Share Consolidation, the Randsburg Articles Amendment and the change of its name from Randsburg to “Cresco Labs Inc.”.
“REFER Act” means the H.R.4779: REFER Act of 2018.
“Regulatory Counsel” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“Registered Organizations has the meaning ascribed thereto in Section 4 of the Listing Statement.
“Resulting Issuer” means Cresco Labs Inc., a corporation existing under the BCBCA and being Randsburg after completion of the Business Combination.
“Resulting Issuer Board” means the board of directors of the Resulting Issuer as the same is constituted from time to time.
“Resulting Issuer Options” has the meaning ascribed thereto in Section 9 of the Listing Statement.
“RFID” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“RJO” has the meaning ascribed thereto in Section 13 of the Listing Statement.
“RMD” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“Rohrabacher Blumenauer Amendment” has the meaning ascribed thereto in Section 17 of the Listing Statement.
“RSU” has the meaning ascribed thereto in Section 9 of the Listing Statement.
“Rules” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“SAR” has the meaning ascribed thereto in Section 9 of the Listing Statement.
“SEC” means the U.S. Securities & Exchange Commission.
“Seed-to-Sale” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“Sessions Memorandum” has the meaning ascribed thereto in Section 17 of the Listing Statement.
“Silver State” has the meaning ascribed thereto in Section 4 of the Listing Statement.
-6-
“SLO” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“SR Offering” has the meaning ascribed thereto in Section 3 of the Listing Statement.
“SR Offering Price” has the meaning ascribed thereto in Section 3 of the Listing Statement.
“States Act” has the meaning ascribed thereto in Section 17 of the Listing Statement.
“Subordinate Voting Shares” means the Subordinate Voting Shares in the capital of the Resulting Issuer, after giving effect to the Business Combination.
“Subscription Receipts” means the subscription receipts offered by Cresco Acquisition in the SR Offering.
“Super Voting Shares” means the non-participating Super Voting Shares in the capital of the Resulting Issuer, after giving effect to the Business Combination.
“Support Agreement” has the meaning ascribed thereto under Section 8 of this Listing Statement.
“T&T” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“Tax Receivable Agreement” has the meaning ascribed thereto in Section 10 of the Listing Statement.
“TINAD” has the meaning ascribed thereto in Section 4 of the Listing Statement.
“Treasury Regulations” refers to the United States Treasury Regulations issued by the United States Internal Revenue Service, a bureau of the United States Department of the Treasury.
“Triggering Event” has the meaning ascribed thereto in Section 10 of the Listing Statement.
“U.S. Holder” has the meaning ascribed thereto in Section 18 of the Listing Statement.
“U.S. Marijuana Issuers” as defined in the CSA Staff Notice 51-352 Issuers with U.S. Marijuana-Related Activities.
“U.S. Tax Code” has the meaning ascribed thereto in Section 17 of the Listing Statement.
“USRPHC” has the meaning ascribed thereto in Section 24 of the Listing Statement.
“USRPI” has the meaning ascribed thereto in Section 24 of the Listing Statement.
“VA” has the meaning ascribed thereto in Section 3 of the Listing Statement.
“Valley Ag” has the meaning ascribed thereto in Section 3 of the Listing Statement.
“VARE” has the meaning ascribed thereto in Section 3 of the Listing Statement.
-7-
| 2. | Corporate Structure |
Randsburg (formerly, Randsburg Gold Corporation) was incorporated in the Province of British Columbia under the Company Act (British Columbia) on July 6, 1990. On December 30, 1997, Randsburg changed its name from Randsburg Gold Corporation to Randsburg International Gold Corp., and consolidated its common shares on a five old for one new basis.
In connection with the Business Combination, Randsburg filed a notice of alteration to effect the change of its name from Randsburg to “Cresco Labs Inc.”, the Randsburg Articles of Amendment and the Randsburg Share Consolidation by way of resolution of the Randsburg Shareholders.
The Resulting Issuer’s head office is located at 520 W Erie St #220, Chicago, IL 60654 and registered office is located at Suite 2200, 1055 West Hastings Street, Vancouver, BC V6E 2E9.
Cresco was formed as a limited liability company under the laws of the state of Illinois on October 8, 2013 and is governed by the Pre-Combination LLC Agreement. The Pre-Combination LLC Agreement was further amended and restated in connection with the completion of the Business Combination. Please see Section 10 below for further details in respect of the Post-Combination LLC Agreement.
Pursuant to the Business Combination, a series of transactions were completed resulting in a reorganization of Cresco and Randsburg as a result of which, the Resulting Issuer became the indirect parent and sole voting unitholder of Cresco. Assuming the redemption in full of all redeemable securities of Cresco and its affiliates (being the effective economic equivalent of the Subordinate Voting Shares) outstanding immediately following the completion of the Business Combination (but otherwise assuming that other convertible securities of the Resulting Issuer, Cresco and its affiliates remain outstanding), securityholders of Cresco and Cresco Corp hold approximately 91.8% of the equity of the Resulting Issuer, while holders of Subordinate Voting Shares (former Randsburg Shareholders, former holders of Cresco Canada Shares and former holders of Subscription Receipts) hold approximately 8.1% of the equity of the Resulting Issuer. See Schedule “F” – Consolidated Pro Forma Financial Statements of the Resulting Issuer.
The Resulting Issuer will carry on the business currently carried on by Cresco. Set forth below is the corporate chart of the Resulting Issuer. The material subsidiaries of Cresco were not changed in connection with the Business Combination.
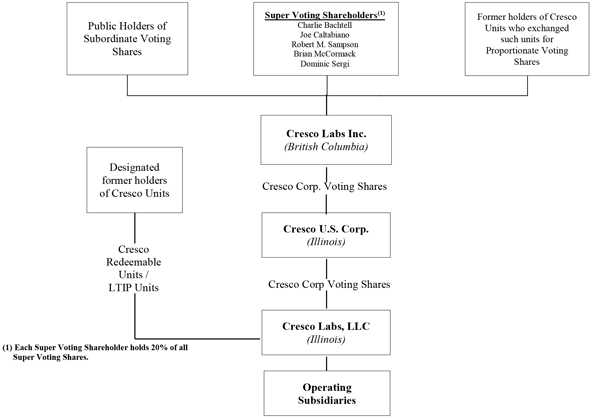
-8-
The current organization chart of Cresco, setting out active subsidiaries of Cresco, is set forth below.
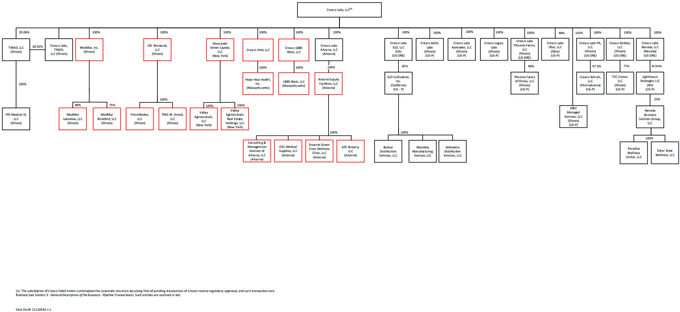
-9-
| 3. | General Development of the Business |
Cresco was formed as a limited liability company under the laws of the State of Illinois on October 8, 2013, primarily to engage in the business of cultivating medical grade cannabis, manufacturing medical products derived from cannabis cultivation and the distribution of such products to medical or adult use consumers in legalized cannabis markets. Cresco exists to provide high-quality and consistent cannabis-based products to consumers. It currently has ownership interests in and/or operates in Illinois, Pennsylvania Nevada, Arizona and Ohio. Cresco’s business activities pertaining to cultivation, manufacturing, logistics and distribution are conducted by wholly owned subsidiaries, which subsidiaries are in turn managed by Cresco. A similar legal structure will be utilized for new opportunities in future states.
In July 2018, Cresco acquired ownership in a cultivation operation in California. In October 2018, Cresco entered into an agreement to acquire a company involved in the cultivation and processing of medical cannabis as well as the establishment of four medical cannabis dispensaries in the State of New York.
Pursuant to the Business Combination, a series of transactions were completed on November 30 2018, resulting in a reorganization of Cresco and Randsburg. The Resulting Issuer became the indirect parent and sole voting unitholder of Cresco. The Resulting Issuer will carry on the business currently carried on by Cresco.
Acquisitions and Dispositions
Cresco has conducted or is planning on conducting the following acquisitions and/or dispositions:
New York
On or about October 24, 2018, Cresco entered into a definitive agreement to merge a subsidiary with and into Gloucester Street Capital, LLC, the parent entity of Valley Agriceuticals, LLC (“Valley Ag”). Valley Ag is one of the ten holders of a vertically integrated license from the New York State Department of Health (“NYSDOH”) allowing for the cultivation and processing of medical cannabis as well as the establishment of four medical cannabis dispensaries in the State of New York for consideration consisting of cash, equity, and contingent consideration based upon the achievement or occurrence of certain milestones or events. To date, the only material asset of Valley Ag is the vertically integrated license from the NYSDOH (for additional details, please see Section 3 – State Level U.S. Cannabis Operations – New York Licenses). Closing of the transaction is subject to customary closing conditions, including receipt of regulatory approval from the NYSDOH. Cresco expects the closing to occur in the fourth quarter of 2018 or the first quarter of 2019.
Completed Transactions
On or about November 21, 2018, Cresco completed its acquisition of 100% of the membership interests of FloraMedex, LLC (“FloraMedex”) and an affiliated real estate entity for cash consideration. FloraMedex operates a medical marijuana dispensary in Elmwood Park, Illinois. On or about November 16, 2018, Cresco acquired 100% of the membership interests of Arizona Facilities Supply, LLC (“AFS”) and its subsidiaries for cash consideration. AFS provides management and advisory services to Encanto Green Cross Dispensary (“Encanto”), a non-profit entity that holds a vertical license to cultivate, process and dispense medical marijuana in the State of Arizona and operates a medical marijuana dispensary in Phoenix, Arizona, and owns real property used for cultivation in Salome, Arizona. In September 2018, Cresco accomplished a series of transactions whereby it increased its ownership interest in its existing businesses as follows: Illinois from 94% to 100%, Pennsylvania from 45% to 98%, Ohio from 55% to 98%, and California from 60% to 80%. Other strategic transactions completed by Cresco in 2018 include the investment in its Nevada business in January and the acquisition of its California operation in June 2018.
-10-
Pipeline Transactions
Cresco is actively pursuing growth opportunities to expand its asset portfolio in the medical and adult-use cannabis industry. Cresco currently has a number of transactions in its pipeline, including the following:
| • | On or about November 19, 2018, Cresco entered into a definitive agreement to acquire 100% of the shares and membership interests, as applicable, of Hope Heal Health, Inc. (“HHH”) and an affiliated real estate entity for consideration consisting of cash and the assumption of certain indebtedness. HHH holds a provisional certificate of registration from the Massachusetts Department of Health that will allow for cultivation, manufacturing and processing and the establishment and operation of a medical cannabis dispensary in Fall River, Massachusetts once a final certificate of registration is granted, and has the ability to apply for up to two additional such licenses. HHH has entered into host community agreements with the municipalities of Rockland, North Attleborough, and Fall River to allow the siting of a medical cannabis dispensary, subject to site approval, and is in the process of applying for adult-use licenses from the Massachusetts Cannabis Control Commission. It is anticipated that closing of the transaction will occur in the fourth quarter of 2018 or the first quarter of 2019, subject to receipt of applicable regulatory approvals. |
| • | On or about November 26, 2018, Cresco entered into a definitive agreement with MedMar, Inc. (“MedMar”) and certain other equityholders to acquire the shares of MedMar and membership interests of MedMar Lakeview, LLC (“MedMar Lakeview”) and MedMar Rockford, LLC (“MedMar Rockford”) for a combination of cash and equity consideration. MedMar Lakeview and MedMar Rockford currently operate medical marijuana dispensaries in Chicago, Illinois and Rockford, Illinois, respectively. It is expected that closing of the acquisition will occur in the fourth quarter of 2018, subject to receipt of applicable regulatory approvals. |
| • | In connection with the AFS transaction described above, Cresco will enter into a financing and consulting arrangement with AFS Maryland, LLC, a former affiliate of AFS (“AFS Maryland”). AFS Maryland holds a processing license for medical cannabis issued by the Maryland Medical Cannabis Commission. Under the arrangement, Cresco will provide funding and consulting services to support the development and operations of AFS Maryland and will receive interest and fee payments and the right to take assignment of the membership interests of AFS Maryland in lieu of repayment of the outstanding balance of debt owed to Cresco by AFS Maryland receiving such payments as and when such transfer of membership interests is permitted by applicable law. |
For additional information in respect of Cresco’s strategy for expansion, please see Section 4 below.
Financing Activities
On October 4, 2018, Cresco completed a brokered private placement of 26,666,667 Class F Units at a price of US$3.75 per Unit, for aggregate gross proceeds of US$100,000,000. The net proceeds of the offering will be used for working capital, including without limitation, to purchase assets. Certain Canadian investors participated in this offering by subscribing for an aggregate of approximately US$18,600,000 Cresco Canada Shares the proceeds of which were then used to subscribe for Class F Units of Cresco. The net proceeds of the offering will be used for working capital, including without limitation, to purchase assets. As part of the Business Combination, Cresco Canada was amalgamated with a subsidiary of Randsburg and upon amalgamation each holder of Cresco Canada Shares received Subordinate Voting Shares in exchange for their Cresco Canada Shares on a 1:1 basis without payment of additional consideration or further action on the part of the holder.
Pursuant to an agency agreement dated as of November 26, 2018 (the “Agency Agreement”) between Cresco Acquisition, Cresco, Cresco Corp, Randsburg and the Agents, Cresco Acquisition completed a private placement of 12,624,054 Subscription Receipts (the “SR Offering”) at a price of C$8.50 per Subscription Receipt (the “SR Offering Price”) for aggregate gross proceeds of approximately C$107,304,459.
Each Subscription Receipt will be automatically converted into one Cresco Acquisition Share immediately prior to and in connection with the completion of the Business Combination, without payment of additional consideration or further action on the part of the holder. Upon completion of the Business Combination each Cresco Acquisition Share will be converted into one Subordinate Voting Share, without payment of additional consideration or further action on the part of the holder.
-11-
The Agents’ fee in connection with the SR Offering was (i) a cash commission equal to 6.0% of the gross proceeds from the SR Offering; (ii) a financial advisory fee for the non-brokered portion of the SR Offering equal to 1.0% of the gross proceeds from the sale of Subscription Receipts, up to a maximum of US$7,500,000; and (iii) a financial advisory fee for the non-brokered portion of the SR Offering equal to 6.0% of the gross proceeds from the sale of the Subscription Receipts, above US$7,500,000, for a total of C$5,931,786.80 (the “Agent Fee”) plus reimbursements for the Agents’ expenses in connection with the SR Offering (including legal fees, disbursements and applicable taxes) in the amount of C$507,047.80. Fifty percent (50%) of the Agent Fee was paid on November 26, 2018 and the remaining in fifty percent (50%) was held in escrow by the Escrow Agent pending the closing of the Business Combination. The funds that were held in escrow by the Escrow Agent, together with all interest and other income earned thereon, are referred to herein as the “Escrowed Funds”.
In connection with the completion of the Business Combination, the Escrowed Funds were released from escrow by the Escrow Agent as follows on November 30, 2018: (a) to the Agents, an amount equal to the 50% of the Agent Fee, in the amount of C$2,965,893.40; and (b) to Cresco Acquisition (or as directed by Cresco Acquisition), an amount equal to the Escrowed Funds, less the foregoing deductions.
In addition the Agents’ received 343,745 broker warrants which is equal to 3.0% of the number of Subscription Receipts sold pursuant to the SR Offering (the “Broker Warrants”), excluding the non-brokered portion. Each Broker Warrant will be exercisable at any time prior to the date that is 24 months following the completion of the Business Combination (which occurred on November 30, 2018) to acquire one Subordinate Voting Share of the Resulting Issuer at the issue price for the Subscription Receipts.
In May 2018, Cresco completed a non-brokered private placement of Class F Units of Cresco at a price of $2.25 per Unit, for aggregate gross proceeds of US$24 million. The net proceeds of the offering were used to expand our operations in Illinois and Pennsylvania, acquire an additional dispensary in Illinois, increase the size of our California operation, and invest in executive level hires to drive future growth and expansion.
In November 2017, Cresco completed a non-brokered private placement of Class F Units of Cresco at a price of $1.14 per Unit, for aggregate gross proceeds of US$16 million. The net proceeds of the offering were used to complete acquisitions in Nevada, California, and two dispensaries in Illinois, and for working capital.
United States Industry Background and Trends
The emergence of the legal cannabis sector in the United States, both for medical and adult-use, has been rapid as more states adopt regulations for its production and sale. Today 60% of Americans live in a state where cannabis is legal in some form and almost a quarter of the population lives in states where it is fully legalized for adult use.1
The use of cannabis and cannabis derivatives to treat or alleviate the symptoms of a wide variety of chronic conditions has been generally accepted by a majority of citizens with a growing acceptance by the medical community as well. A review of the research, published in 2015 in the Journal of the American Medical Association, found strong evidence that cannabis can treat pain and muscle spasms.2 The pain component is particularly important, because other studies have suggested that cannabis can replace pain patients’ use of highly addictive, potentially deadly opiates — meaning marijuana legalization has the potential to save lives.3
Polls throughout the U.S. consistently show overwhelming support for the legalization of medical cannabis, together with strong majority support for the full legalization of recreational adult-use cannabis. It is estimated that 94% of the U.S. voters support legalizing cannabis for medical use.4 In addition, 64% of the U.S. public supports legalizing cannabis for adult recreational use.5 These represent large increases in public support over the past 40 years in favor of legal cannabis use.
| 1 | Ripley, Eve. (2016 November 30). Nearly 60 percent of U.S. population now lives in states with marijuana legalization. Retrieved from https://news.medicalmarijuanainc.com/nearly-60-percent-u-s-population-now-lives-states-marijuana-legalization/. |
| 2 | Grant, Igor MD (2015). Medical Use of Cannabinoids. Journal of American Medical Association, 314: 16, 1750-1751. doi: 10.1001/jama.2015.11429. |
| 3 | Bachhuber, MA, Saloner B, Cunningham CO, Barry CL. (2014). Medical Cannabis Laws and Opioid Analgesic Overdose Mortality in the United States, 1999-2010. JAMA Intern Med. 174(10):1668-1673. doi: 10.1001/jamainternmed.2014.4005. |
| 4 | Quinnipiac University. (2017 April 20). U.S. Voter Support For Marijuana Hits New High; Quinnipiac University National Poll Finds; 76 Percent Say Their Finances Are Excellent Or Good. Retrieved from https://poll.qu.edu/national/release-detail?ReleaseID=2453. |
| 5 | Gallup. (2017 October 25). Record-High Support for Legalizing Marijuana Use in U.S. Retrieved from http://news.gallup.com/poll/221018/record-high-support-legalizing-marijuana.aspx. |
-12-
Notwithstanding that more than half of the U.S. states have now legalized adult-use and/or medical marijuana, marijuana remains illegal under U.S. federal law with marijuana listed as a Schedule I drug under the CSA. See Section 4 (Narrative Description of the Business) and Section 17 (Risk Factors) below. The Department of Justice defines Schedule I drugs, substances or chemicals as “drugs with no currently accepted medical use and a high potential for abuse.” The FDA has not approved marijuana as a safe and effective drug for any indication.
Unlike in Canada, which has U.S. federal legislation uniformly governing the cultivation, distribution, sale and possession of medical marijuana under the Cannabis Act, marijuana is largely regulated at the state level in the United States.
State laws regulating cannabis are in direct conflict with the U.S. federal CSA, which makes cannabis use and possession federally illegal in the United States. Although certain states and territories of the U.S. authorize medical or recreational cannabis production and distribution by licensed or registered entities, under U.S. federal law, the possession, use, cultivation, and transfer of cannabis and any related drug paraphernalia is illegal and any such acts are criminal acts under U.S. federal law under any and all circumstances under the CSA. Although Cresco’s and its subsidiaries activities are compliant with applicable United States state and local law, strict compliance with state and local laws with respect to cannabis may neither absolve Cresco and its subsidiaries of liability under United States federal law, nor provide a defense to any U.S. federal proceeding which may be brought against Cresco or its subsidiaries.
The risk of U.S. federal enforcement and other risks associated with the Resulting Issuer’s business are described in Section 17 – Risk Factors.
Current U.S. Cannabis Market
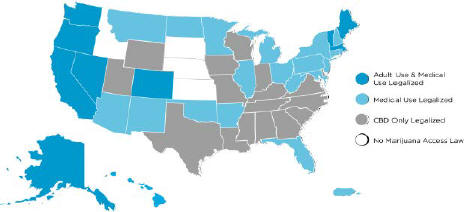
Due to the support for legal access to marijuana at the state level, there has been rapid opportunity growth in the U.S. market. Sales of legal cannabis flowers and cannabis-infused derivative and edible products totaled US$6.1 billion in 2017, and are expected to reach US$8.8 billion in 2018 with approximately 36% of sales for medical use and 64% for full adult use6. The U.S. market for direct legal cannabis sales alone is projected to grow to US$17 billion by 20217 and the total addressable market for direct cannabis sales in the U.S. today is estimated at US$45-50 billion if every state legalized full adult recreational consumption.8
| 6 | Marijuana Business Daily. (2017). Marijuana Business Factbook, 2017. Available from https://mjbizdaily.com/factbook/. |
| 7 | Arcview Market Research & New Frontier Data. (2016). The State of Legal Marijuana Markets (4th ed.), pp. 11. Available from https://www.arcviewmarketresearch.com/4th-edition-legal-marijuana-market/. |
| 8 | Marijuana Business Daily. (2017). Marijuana Business Factbook, 2017. Available from https://mjbizdaily.com/factbook/. |
-13-
The number of medical cannabis patients in states with existing comprehensive medical cannabis programs was approximately 1.5 million by the end of 2017, served by approximately 1500-2000 medical dispensaries nationwide, a disproportionate number of those in California. It is currently estimated that each patient spends about US$2,000 annually,9 and that the total number of medical cannabis patients nationwide is expected to grow to 2.5 million by 2021.10
Currently the Resulting Issuer operates in the States of Illinois, Pennsylvania, Ohio, California, Nevada, and Arizona, and with firm plans to expand into New York, Massachusetts and Maryland. It intends to expand into other states within the U.S. that have legalized cannabis use either medicinally or recreationally.
Illinois
The Compassionate Use of Medical Cannabis Pilot Program Act, which allows individuals diagnosed with a debilitating medical condition access to medical marijuana, became effective January 1, 2014 and is extended through July 1, 2020. There are over 41 qualifying conditions as part of the medical program, including epilepsy, traumatic brain injury, and post-traumatic stress disorder (“PTSD”). Illinois’ retail market size for 2017 was over $86 million, representing an over 140% year-over-year increase. As of October 3, 2018, total retail sales were over $97 million representing an approximate 12% increase over 2017 retail sales (with 2 months remaining).11 On August 28, 2018, the Alternatives to Opioids Act (Public Act 100-1114) was signed into law. The Alternative to Opioids Act significantly expands the Illinois’ medical marijuana market by enabling patients to access medical marijuana in place of pharmaceutical opioid medications. The Illinois Department of Public Health reports that there were more than 5.3 million prescriptions for opioid-based painkillers filled last year. This paves the way for the single-largest expansion of the existing Illinois Medical Cannabis Pilot Program, which has about 42,000 authorized patients. Those patients have brought the state about $200 million in sales tax revenue since the program’s inception in late 2015.12
Pennsylvania
The Pennsylvania medical marijuana program was signed into law on April 17, 2016 under Act 16 and provided access to state residents with one of 17 qualifying conditions, including epilepsy, chronic pain, and PTSD. The state, operates as a high-barrier market with very limited market participation. Retail sales opened in February 2018 to a limited number of retail locations across the state. Pennsylvania is the fifth-largest state in the country, home to nearly 13 million people. Pennsylvania’s medical marijuana market is expected to become one of the biggest markets in the U.S.13
Ohio
House Bill 523, effective on September 8, 2016, legalized medical marijuana in Ohio. The Ohio Medical Marijuana Control Program allows people with certain medical conditions, upon the recommendation of an Ohio-licensed physician certified by the State Medical Board, to purchase and use medical marijuana. Ohio’s medical cannabis sales are projected to be between $200 and $400 million once the system is fully matured.14 According to industry experts, Ohio could become a national “powerhouse” for the medical marijuana industry, largely because of its population — it’s the seventh largest state — and because the broad list of conditions eligible for treatment with medical marijuana includes “pain.“15
| 9 | Marijuana Business Daily. (2017). Marijuana Business Factbook, 2017. Available from https://mjbizdaily.com/factbook/. |
| 10 | New Frontier Financial. (2015). Modeling of State Patient Counts. Cannabis Weekly. |
| 11 | Illinois Medical Cannabis Pilot Program. (2018 October 3). Overall Medical Cannabis Pilot Program Data, as of 24/10/2018. Retrieved from https://www2.illinois.gov/sites/mcpp/Pages/update10032018.aspx |
| 12 | Illinois News Network (2018 August 28). New law expands access to medical marijuana in Illinois to curb opioid use https://www.ilnews.org/news/health/new-law-expands-access-to-medical-marijuana-in-illinois-to/article_4b2a156c-ab05-11e8-95a9-037d97496f1a.html |
| 13 | https://mjbizdaily.com/chart-pennsylvanias-medical-marijuana-market-set-become-one-countrys-biggest/ |
| 14 | https://cannabusinessplans.com/ohios-medical-cannabis-market/ |
| 15 | https://cannabusinessplans.com/ohios-medical-cannabis-market/ |
-14-
California
The California marijuana market is expected to be one of the fastest growing industries in California over the next five years. Market analysts forecast a stabilized market to occur after 2025 where the California marijuana market is estimated to be valued at approximately US$10 billion.16 In 2016, California recorded approximately US$850 million in medical marijuana retail sales from operated dispensaries state wide; however, it is estimated approximately 85% of total transactions are unrecorded for revenue and are carried out through illegal transactions. The University of California Agricultural Issues Center predicts the illegal market to shrink to less than 30%, legal adult-use sales to increase to approximately 62%, and legal medical sales to decrease from approximately 15% to less than 10% as patients are provided with an alternative to obtaining medical marijuana physician recommendations for a fee.17
Nevada
Nevada is one of the most dynamic markets anticipated for the full development of the recreational market. By certain estimates, the recreational market in Nevada is projected to have a cumulative average growth rate of 25%.18 With most of the state population and tourism located in Las Vegas, the opportunity in Las Vegas is strengthened by the fact that Las Vegas has a limited number of licenses and the city of Las Vegas has placed a priority for current license holders to be preferred in obtaining other non-operating retail licenses. The City of Las Vegas has historically seen nearly forty million tourists in a year, making it one of the most visited cities in the United States. Industry estimates put the overall cannabis market size in Las Vegas to be over US$800 million per year.19
Arizona
In 2010, Arizona passed Ballot Proposition 203, which amended Title 36 to the Arizona Revised Statutes. This amendment added Chapter 28.1, titled the Arizona Medical Marijuana Act (the “AMMA”). The AMMA also appointed the Arizona Department of Health Services (the “ADHS”) as the regulator for the program and authorized ADHS to promulgate, adopt and enforce regulations for the AMMA. The ADHS has established the Arizona Department of Health Services Medical Marijuana Program (“MMJ Program”), which includes a vertically integrated license, meaning if allocated a Medical Marijuana Dispensary Registration Certificate, entities are authorized to dispense and cultivate medical cannabis. Arizona’s medical marijuana market is one of the largest in the nation as well as one of the hottest. The amount of medical marijuana sold has more than doubled from 5,012 pounds in August 2016 to 10,826 pounds in August 2018, according to the Arizona Department of Health Services. The patient count during that period has surged from 105,076 to 178,257.20
New York
New York is one of the most promising medical cannabis markets that opened in 2016. The state population numbers near twenty million and New York City is among the most populous and visited cities in the U.S.21 The New York
| 16 | Sources: Berke, Jeremy. (2017 December 8). The legal marijuana market is exploding – it’ll hit almost $10 billion sales this year. Retrieved from http://www.businessinsider.com/legal-weed-market-to-hit-10-billion-in-sales-report-says-2017-12; Morris, Chris. (2017 December 6). Legal Marijuana Sales Are Expected to Hit $10 Billion This Year. Retrieved from http://fortune.com/2017/12/06/legal-marijuana-sales-10-billion/; The Arcview Group. (2017 December 6). NEW REPORT: Legal Marijuana Sales to Grow 33% to $10 Billion in 2017. Retrieved from https://globenewswire.com/news-release/2017/12/06/1234230/0/en/NEW-REPORT-Legal-Marijuana-Sales-to-Grow-33-to-10-Billion-in-2017.html. |
| 17 | McGreevy, Patrick. (2017 June 11). Legal marijuana could be a $5-billion boon to California’s economy. Retrieved from http://www.latimes.com/politics/la-pol-ca-pot-economic-study-20170611-story.html. |
| 18 | Frontier Financial Group Inc. (2017). Change in Compensation: Working in Cannabis. Retrieved from https://newfrontierdata.com/marijuana-insights/change-in-compensation-working-in-cannabis/. |
| 19 | Retrieved from https://newfrontierdata.com/cannabits/. |
| 20 |
https://mjbizdaily.com/arizonas-sizzling-medical-marijuana-market-entices-investors-despite-legal-uncertainties/ |
| 21 | United States Census Bureau. (2017). QuickFacts United States. Retrieved from https://www.census.gov/quickfacts/NY; see also NYC and Company. NYC Travel & Tourism Visitation Statistics. Retrieved from http://www.nycandcompany.org/research/nyc-statistics-page; see also World Atlas. (2017 November 9). The Most Visited Cities In The US. Retrieved from https://www.worldatlas.com/articles/the-most-visited-cities-in-the-us.html. |
-15-
program, when initially implemented, allowed for only five fully vertically integrated licenses. The licenses allowed each license holder the opportunity to operate a cultivation facility, extraction and manufacturing, and four retail medical marijuana dispensaries. The State program was adjusted to increase the range of qualifying conditions which, as of the date hereof, includes chronic and severe pain. In August 2017, the State of New York also increased the number of licensed operators in the state to a total of ten. Each of the newly added licenses can carry out the same operations as the original license holders. The State has made progress towards the ability to increase the outreach to qualified patients through the ten licensed operators via the disbursement of retail locations across the state, the increase in range of qualifying conditions, and other various methods to support patient access. In July, the New York Department of Health filed emergency regulations to add any condition, for which an opioid could be prescribed, as a qualifying condition for medical marijuana. This legislation was signed into law on September 24, 2018. From July 10 to September 25, 2018 the number of certified patients in the system rose to 18%.22
Massachusetts
In November 2015, Massachusetts, a medical cannabis market since January 2013, voted in favour of “Question 4”, approving the legalization of adult use. Research firm Arcview Market Research projects that the Massachusetts market will grow to over $1 billion by 2020 at a compound annual growth rate of 113%. The Question 4 ballot initiative requiring the state legislature to authorize the adult use of cannabis in the state was approved by the Massachusetts electorate in November 2016. The first adult use dispensaries opened their doors on July 1, 2018. Located in the very populous North-Eastern region of the U.S., tourism is anticipated to be an important factor in driving market growth in a state that itself has a growing population of 6.8 million. The adjoining states represent an additional “tourist” market of 26 million, vastly exceeding the very successful industry in Colorado. The new legislation allows local control policy, allowing local government officials in towns that voted “no” on the 2016 ballot initiative to ban marijuana businesses until December 2019. For towns that voted “yes” in 2016, any bans must be placed on a local ballot for voters to approve. The maximum sales tax rate will increase from 12% to 20%. Under the bill, the state tax will be 17% and the local option will be 3%.
Maryland
Maryland adopted a comprehensive law legalizing medical cannabis in 2014. The Maryland program will result in a large medical marijuana market as a result of an expansive list of qualifying conditions, less restrictive provisions for obtaining cannabis certifications from doctors, and patient freedom to choose preferred methods of ingestions. The Maryland Medical Cannabis Commission began to sell through dispensaries on December 1, 2017. 14 growers, 12 processors and nine dispensaries have been licensed by the Maryland Medical Cannabis Commission, and on the day sales began, approximately 15,000 people had signed up to be prospective patients. Almost 550 healthcare providers have registered with Maryland to recommend Cannabis to their patients. Maryland has a population of over 6 million people.
| 4. | Narrative Description of the Business |
| General | |
The below description of Cresco, will become the Resulting Issuer’s business.
Cresco exists to provide high-quality and consistent cannabis-based products to consumers. Cresco blends regulatory compliance expertise with best practices from the agricultural, pharmaceutical and consumer packaged goods industries. Cresco (either directly or indirectly through subsidiaries) has been awarded three licenses to cultivate and manufacture medicinal cannabis in the State of Illinois. Cresco was awarded a cultivation license in Pennsylvania and was one of only five cultivators that was also awarded a dispensary license which allows for up to three dispensaries. Most recently, Cresco was awarded a cultivation license in Ohio and a dispensary license in Ohio. It also has pending applications for cultivation, processing, and dispensary licenses in Michigan. Cresco recently acquired ownership interest in a cultivation, processing, and dispensary license in Nevada, an ownership interest in a cultivation, processing, an ownership interest in cultivation and processing licenses in California, an ownership interest in three dispensaries in Illinois. Additionally, Cresco has entered into an agreement to acquire a company involved in the cultivation and processing of medical cannabis as well as the establishment of four medical cannabis dispensaries in the State of New York (See Section 3 – General Development of the Business – Pipeline Transactions).
| 22 | https://mjbizdaily.com/new-york-formalizes-medical-cannabis-as-alternative-to-opioids-market-boost-seen/ |
-16-
Cresco plans to leverage the success in these markets to expand into legalized cannabis markets in other states, while focusing on compliance, control, efficiency, and product performance in the medicinal or adult-use cannabis industry.
Cresco owns and operates cultivation, manufacturing and retail dispensary businesses. The manufacturing and retail businesses are operational today and vertically integrated across six highly regulated, limited licensed, and therefore limited legal supply markets: Illinois, Nevada, Ohio, Arizona, Pennsylvania and California and is expected to commence cultivation, manufacturing and retail dispensary operations in New York, Michigan, Massachusetts and New Jersey. These markets, where supply and demand can be reasonably predicted and forecasted, create the foundation upon which Cresco has created the opportunity for sustainable growth. Importantly, Cresco is not yet active in markets popularized by mainstream media like Washington, Oregon and Colorado where loose regulatory frameworks create unpredictable supply-demand market dynamics.
This ownership of wholesale and retail businesses supports Cresco’s strategy of distributing brands at scale by enabling Cresco to capture market share, generate brand awareness, and earn customer loyalty in its operating markets. By guaranteeing share-of-shelf in its own retail stores and its ability to foster mutually beneficial relationships with its third-party dispensary customers as a large supplier of a portfolio of distinct and trusted cannabis brands.
Significant Events or Milestones
The principal milestones that must occur during the next 12-month period for the business objectives described herein to be accomplished are as follows: hire key personnel, obtain necessary regulatory approvals, implement marketing plans and commence production and sales in Cresco’s new markets, including retail stores for recreational and medical cannabis where legislation permits.
Cresco has put in place a team of executives, board of advisors and consultants with various areas of expertise and experience in multiple industries including commercial agriculture, pharmaceutical, manufacturing, consumer packaged goods and traditional healthcare. In the interest of progressing a professional medical dialogue and educating as many physicians as possible on the use of medical cannabis as a therapeutic treatment for patients, it has organized a team of physicians to educate, train, and inform medical professionals on all aspects of cannabis as medicine.
Cresco previously engaged Denver Relief Consulting, LLC, to provide advisory services to acquire new licenses in merit-based application states and to review potential acquisitions as it seeks to expand into new legalized cannabis markets. In addition, Cresco has an equity partnership with Mindy Segal, a James Beard awarding winning pastry chef, who is responsible for the product development of its edible line of medical products.
Available Funds/Financing
Cresco has historically relied upon equity and debt financings to satisfy its capital requirements and may require further equity and debt capital to finance its development, expansion and acquisition activities moving forward.
The working capital deficiency of Randsburg as at July 31, 2018 was C$1,189,489. The working capital position of Cresco as at October 31, 2018 was approximately C$100,745,090. The total net proceeds raised from the SR Offering were C$107,304,459. As a result, the Resulting Issuer has an estimated C$207,779,549 of funds available for use (assuming a USD:CAD exchange rate of US$1.00 = C$1.30).
-17-
The Resulting Issuer intends to use the estimated funds available to it as set out in the following table:
| Use of Available Funds |
Amount (Canadian Dollars) | |||
| Acquisition Pipeline(1) |
$ | 107,000,000 | ||
| Capital Expenditures |
$ | 74,000,000 | ||
| Operating Expenditures(2) |
$ | 10,000,000 | ||
| Excess Working Capital |
$ | 16,779,549 | ||
|
|
|
|||
| Total Available Funds: |
$ | 207,779,549 | ||
|
|
|
|||
| (1) | Please see Section 3 – Pipeline Transactions. |
| (2) | Existing operations are also funded by operating revenue. |
Notwithstanding the foregoing, there may be circumstances where, for sound business reasons, a reallocation of funds may be necessary for the Resulting Issuer to achieve its objectives. The Resulting Issuer may require additional funds in order to fulfill all of its expenditure requirements to meet its business objectives and may either issue additional securities or incur debt. There can be no assurance that additional funding required by the Resulting Issuer will be available, if required.
Growth Strategy
The legalization of cannabis throughout the United States continues to expand both recreationally and medically. Analysts at Cowen and Co. have established that the size of the U.S. cannabis market could surpass US$50 billion by 2026.23 On the recreational side, there are currently multiple states in which the recreational sale of cannabis has been approved. These states include Alaska, Oregon, Washington, Nevada, California, Colorado, Massachusetts and Maine. In these markets, recreational sales should continue to grow as cannabis retailers benefit from a shift in consumers from illegal sales to legal sales and from new cannabis consumers. Cresco plans on capitalizing on the significant increase in cannabis consumption in these recreational markets through both an expansion of its retail footprint, as well an entry into other sizable recreational markets. Cresco will also seek opportunities to expand its cultivation and production operations in recreational markets through expansions of its existing facilities or through acquisitions of additional licenses or cultivation operators.
With respect to medical marijuana, as more research centers study the effects of cannabis-based products in treating or addressing therapeutic needs, and assuming that research findings demonstrate that such products are effective in doing so, management believes that the size of the U.S. medical cannabis market will also continue to grow as more states expand their medical marijuana programs and new states legalize medical marijuana. Given Cresco’s existing operations in Illinois and Pennsylvania, Cresco is well-versed in operating within a medical-only market and will continue to seek opportunities to expand into new medical-only markets.
Cresco plans to leverage the success in Illinois, Pennsylvania, Ohio, Nevada, California, and Arizona to expand into legalized cannabis markets in other states, while focusing on compliance, control, efficiency, and product performance in the medicinal or adult use cannabis industry. Its strategy is to create a national brand by executing on the following actions:
| • | Pursue acquisition of license or existing cannabis operations in other legal cannabis markets |
| • | Complete application process for new states beginning or expanding medical cannabis programs such as Florida, Michigan, and New Jersey, etc. |
| • | Create licensing and/or distribution partnerships to expand Mindy Segal edible products into growing markets such as Colorado, Oregon, Washington, etc. |
Cresco has proven its ability to become operational in new markets and plans on continuing this trend. It completed construction of three cultivation facilities, with over 110,000 square feet of cultivation space, in approximately six months. It was one of the first operators in the State of Pennsylvania to be approved and come to market as a cultivator and dispensary. It was also one of the first cultivators to be deemed operational after acquiring a cultivation license in the State of Ohio.
| 23 | https://www.marketwatch.com/story/marijuana-industry-could-be-worth-50-billion-annually-by-2026-2017-04-20 |
-18-
Cultivation
Cresco is building or has built and/or renovated thirteen separate cultivation facilities, totaling approximately 1,200,00024 cultivation square feet, across eight states (being Illinois, Nevada, Arizona, Ohio, California, Pennsylvania currently and Massachusetts and New York pending closing of the acquisitions disclosed in Section 2), which includes other non-cultivation activities. It operates both indoor and hybrid green houses. Cresco’s multiple cultivation, extraction, and processing facilities allow it to produce cannabis products across several product categories.
Cultivation
Cresco currently has (or will have upon closing of the transactions described in Section 2) the following approximate cultivation square footage in each respective state of operation: 39,000 square feet in Illinois, 20,000 square feet in Pennsylvania, 25,000 square feet in Ohio, 132,000 square feet in California, 13,000 square feet in Massachusetts and 128,000 square feet in Arizona.25 Cresco is expanding26 to add approximately the following additional cultivation square footage in each respective state of operation: 100,000 square feet in Illinois, 45,000 square feet in Pennsylvania, 25,000 square feet in Ohio, 460,000 square feet in California, 36,000 square feet in Nevada, 100,000 square feet in New York and 77,000 square feet in Massachusetts. Designed to provide consistency of product, increase yields and minimize the possibility of crop failure, each of Cresco’s facilities is equipped with traditional commercial agriculture components, automated environmental control systems, and watering and feed fertigation systems. Developed over years of research, its proprietary nutritional regimen is utilized to ensure crop quality. Using organic and soluble-based plant nutritional supplements, secondary metabolites (cannabinoids/terpenes) are maximized resulting in superior flower quality, yield and consistency. Cresco has invested more than US$45 million in cultivation and processing facilities and plans on continuing to do so.
Manufacturing
Cresco’s laboratory instrumentation gives it the ability to formulate and develop a variety of products based on traditional pharmaceutical delivery systems – pills, tinctures, topical salves, transdermal patches and edible forms with a variety of cannabinoid profiles. Cresco’s kitchen is outfitted with equipment that allows us to produce shelf-stable quality confections with consistency. Cresco has plans to develop a kitchen and laboratory in every state it operates in with the exception of Pennsylvania and New York due to regulatory restrictions on production and sales of edible products. It is expected that 40-50% of the raw cannabis produced at Cresco’s production facilities (except for raw cannabis produced in Pennsylvania and New York) will be used at Cresco’s kitchens and laboratories to make the vaporizable, oral and topical and edible products sold under the Cresco, ‘Reserve’, ‘Remedi’, and ‘Mindy’s’ brands.
Production
Cresco’s production capacity and expected production capacities in each state where it operates is as follow.
| State |
Current Production (expressed in lbs.) |
Planned Production after Completion of all Planned Expansions of Cultivation Facilities(26) (expressed in lbs.) |
Expected Total Production by Year-End 2019(26)) (expressed in lbs.) |
|||||||||
| Illinois |
14,190 | 53,170 | 67,360 | |||||||||
| Pennsylvania |
8,250 | 21,638 | 29,888 | |||||||||
| Ohio |
9,376 | 9,543 | 18,919 | |||||||||
| California |
42,408 | 140,284 | 182,692 | |||||||||
| 24 | These statements constitute forward-looking information related to possible events, conditions or financial performance based on future economic conditions and courses of action. These statements involve known and unknown risks, assumptions, uncertainties and other factors that may cause actual results or events to differ materially. Cresco believes there is reasonable basis for the expectation reflected in the forward-looking statements, however these expectations may not prove to be correct. |
| 25 | These figures are approximate measurements of square footage. |
| 26 | Cresco’s expansion, production and cultivation plans are subject to a number of risks and uncertainties, including the need for new licenses or amendments to existing licenses, additional regulatory or municipal approvals, including zoning and other risks associated with construction and cultivation generally, See – Section 17 – Risk Factors. No assurances are given as to the precise cost or timing. |
-19-
| Nevada |
N/A | 13,578 | 13,578 | |||||||||
| Arizona |
34,265 | N/A | 34,265 | |||||||||
| New York |
N/A | 44,123 | 44,123 | |||||||||
| Massachusetts |
5,071 | 29,968 | 35,039 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
113,560 | 312,304 | 425,864 | |||||||||
|
|
|
|
|
|
|
Dispensaries
Wholesale
Cresco collaborates with its retail partners on strategic in-store promotions, customer events, and shelf space tactics to ensure maximum sell throughput. It takes a data-driven approach in its efforts to create an optimized sale process.
Retail
Cresco has an ownership interest (assuming the completion of and regulatory approval for the transactions contemplated herein) in the following: five (5) operational dispensaries in the State of Illinois; three (3) dispensaries in the State of Pennsylvania (two of which are currently operational); one (1) dispensary in the State of Nevada; one (1) dispensaries in the State of Arizona; one (1) dispensary in the State of Ohio; and four (4) dispensaries in the State of New York.
Real Estate Strategy
Cresco is focused on entering cannabis markets with demand potential, supply constraints and high barriers to entry. Within its core markets, Cresco spends time and resources in selecting real estate in premium locations with significant foot traffic and proximity to popular attractions (restaurants, malls, sports arenas, hotels, etc.). Cresco targets retail spaces based on the market and available real estate. Cresco utilizes both its internal real estate team and a network of real estate brokers to negotiate leases and purchases on behalf of the company. Cresco typically prefers purchasing the underlying real estate for its retail operations and has been successful in securing debt financing for its real estate acquisitions. When purchasing the real estate is not possible, it attempts to secure long-term leases and purchase options.
Branding and Marketing
Cresco utilizes a multi-brand approach to product development. The brand “Cresco” features THC-focused products available in flower, vape pens, and multiple forms of extracts. Each product falls into one of three proprietary categories: “Rise”, “Refresh”, “Rest”, named and color-coded to help the user intuitively identify the desired effects of the relevant strain’s cannabinoid profile. “Mindy’s Artisanal Edibles” and “Mindy’s Kitchen” are brands created in collaboration with James Beard Award Winning Chef Mindy Segal and are the industry’s first true culinary-backed edible option. Both of Mindy’s lines are lauded for their unique flavor profiles and delectability. “Reserve” products are made from Cresco’s most premium and exclusive plants, and are the reward of years of selective breeding. “Remedi” products are designed for the medically-minded patient, with forms reminiscent of traditional pharmaceuticals.
Banking and Processing
Cresco deposits funds from its dispensary operations into its banking partners in each respective market. These state chartered banks are fully aware of the nature of Cresco’s business and continue to remain supportive of Cresco’s growth plans. Cresco’s dispensaries currently accept only cash and debit card and do not process credit card payments. It is anticipated that over time all forms of payment will be accepted by each of the dispensaries.
-20-
Product Selection and Offerings
Product selection decisions are currently made by leaders from the Product Development, Operations, Finance, Marketing and Sales teams, who negotiate and receive bids from potential brand vendors across all product categories including flower, vape pens, oils, extracts, edibles and pre-rolls. Cresco bases its product selection decisions on market demand and opportunity, product quality, margin potential, consumer feedback and the ability for the respective brands to scale. Cresco also anticipates requiring brands to pay slotting fees for shelf space.
Cresco’s manufactured products are sold through Company-owned and managed dispensaries as well as third-party dispensaries. In the future, as production capacity increases, Cresco expects to sell bulk product, as well as new branded products to other dispensaries through both Company-owned and third party distributors. The full extent of this will depend upon the ultimate extent of Cresco-owned and managed retail footprint, as well as the ultimate expanded production capacity of Cresco’s cultivation and production facilities.
Cresco offers or plans to offer, the following products in the following states:
| State |
Offering | |
| Illinois (Currently manufactures) |
Cannabis dry flower, vaporizer forms of cannabis, cannabis oil in capsule, oral and sublingual solutions, cannabis in topical products, cannabis edible products and other cannabis products. Product lines include: THC focused products available in flower, vape pens, and multiple forms of extracts under the “Cresco” brand; “Reserve” products made from premium and exclusive plants and “Remedi” products which include precisely-dosed, non-combustible products including: tinctures, capsules, salves, sublingual oils and transdermal patches. Cresco also sells cannabis infused edibles through its partnership with James Beard Award Winning Chef Mindy Segal. Under the brands “Mindy’s Artisanal Edibles” and “Mindy’s Kitchen” Cresco sells cannabis infused edibles including: chocolate and toffee confections, fruit- forward gummies, hard sweet and chews. Retail locations in Illinois sell a variety of these brands and their corresponding products. | |
| Pennsylvania (Currently manufactures) |
Cannabis dry flower, vaporizer forms of cannabis, cannabis oil in capsule, oral and sublingual solutions, cannabis in topical products and other cannabis products. The product lines include THC focused products available in flower, vape pens, and multiple forms of extracts under their “Cresco” brand; “Reserve” products made from premium and exclusive plants and “Remedi” products which include precisely-dosed non-combustible products including tinctures, capsules, salves, sublingual oils and transdermal patches. Retail locations in Pennsylvania sell a variety of these brands and their corresponding products. | |
| Ohio (Currently manufactures) |
Cannabis dry flower for vaporizerization. If awarded a processor license, Cresco will also manufacture cannabis oil in capsule, oral and sublingual solutions, cannabis in topical products and other cannabis products. The product lines include (or will include if Cresco receives a processor license) THC focused products available in flower, vape pens, and multiple forms of extracts under their “Cresco” brand; “Reserve” products made from premium and exclusive plants and “Remedi” products which include precisely-dosed non-combustible products including tinctures, capsules, salves, sublingual oils and transdermal patches. Retail locations in Ohio will sell a variety of these brands and their corresponding products. | |
| California (Currently manufactures) |
Cannabis dry flower, vaporizer forms of cannabis, cannabis oil in capsule, oral and sublingual solutions, cannabis in topical products, cannabis edible products and other cannabis products. The product lines include, THC focused products available in flower, vape pens, and multiple forms of extracts under their “Cresco” brand; “Reserve” products made from premium and exclusive plants and “Remedi” products which include precisely-dosed, non-combustible products including: tinctures, capsules, salves, sublingual oils and transdermal patches. Cresco also sells cannabis infused edibles through its partnership with James Beard Award Winning Chef Mindy Segal. Under the brands “Mindy’s Artisanal Edibles” and “Mindy’s Kitchen”. Cresco sells cannabis infused edibles including: chocolate and toffee confections, fruit- forward gummies, hard sweet and chews. Retail locations in California sell a variety of these brands and their corresponding products. | |
| Nevada (Plans to manufacture) |
Cannabis dry flower, vaporizer forms of cannabis, cannabis oil in capsule, oral and sublingual solutions, cannabis in topical products, cannabis edible products and other cannabis products. The product lines will include, THC focused products available in flower, vape pens, and multiple forms of extracts under their “Cresco” brand; “Reserve” products made from premium and exclusive plants and “Remedi” products which include precisely-dosed, non-combustible products including: tinctures, capsules, salves, sublingual oils and transdermal patches. Cresco also sells cannabis infused edibles through its partnership with James Beard Award Winning Chef Mindy Segal. Under the brands “Mindy’s Artisanal Edibles” and “Mindy’s Kitchen”. Mindy’s products are currently available in Nevada. Cresco will also sell cannabis infused edibles including: chocolate and toffee confections, fruit-forward gummies, hard sweet and chews. Retail locations in Nevada will sell a variety of these brands and their corresponding products. | |
| Arizona (Plans to manufacture) |
Cannabis dry flower, vaporizer forms of cannabis, cannabis oil in capsule, oral and sublingual solutions, cannabis in topical products, cannabis edible products and other cannabis products. The product lines will include, THC focused products available in flower, vape pens, and multiple forms of extracts under their “Cresco” brand; “Reserve” products made from premium and exclusive plants and “Remedi” products which include precisely-dosed, non-combustible | |
-21-
| products including: tinctures, capsules, salves, sublingual oils and transdermal patches. Cresco will also sell cannabis infused edibles through its partnership with James Beard Award Winning Chef Mindy Segal. Under the brands “Mindy’s Artisanal Edibles” and “Mindy’s Kitchen”. Cresco will also sell cannabis infused edibles including: chocolate and toffee confections, fruit-forward gummies, hard sweet and chews. Retail locations in Arizona will sell a variety of these brands and their corresponding products. | ||
| Massachusetts (Plans to manufacture) |
Cannabis dry flower, vaporizer forms of cannabis, cannabis oil in capsule, oral and sublingual solutions, cannabis in topical products, cannabis edible products and other cannabis products. The product lines will include, THC focused products available in flower, vape pens, and multiple forms of extracts under their “Cresco” brand; “Reserve” products made from premium and exclusive plants and “Remedi” products which include precisely-dosed, non-combustible products including: tinctures, capsules, salves, sublingual oils and transdermal patches. Cresco will also cannabis infused edibles through its partnership with James Beard Award Winning Chef Mindy Segal. Under the brands “Mindy’s Artisanal Edibles” and “Mindy’s Kitchen”. Cresco will also sell cannabis infused edibles including: chocolate and toffee confections, fruit-forward gummies, hard sweet and chews. Retail locations in Massachusetts will sell a variety of these brands and their corresponding products. | |
| New York (Plans to manufacture) |
Vaporizer forms of cannabis, cannabis oil in capsule, oral and sublingual solutions, cannabis in topical products and other cannabis products. The product lines include THC focused products available in vape carts and pens under their “Cresco” brand; “Reserve” products made from premium and exclusive plants and “Remedi” products which include precisely- dosed non-combustible products including tinctures, capsules, salves, sublingual oils and transdermal patches. Retail locations in New York will sell a variety of these brands and their corresponding products. | |
Product Pricing
Cresco’s prices vary based on the market conditions and product pricing of vendor partners. Generally, Cresco strives to keep pricing consistent across all store locations. Cannabis and cannabis product pricing is based on operating costs, materials costs, growth time, and other applicable variables. Additionally, product pricing reflects existing pricing regulations in Cresco’s markets where applicable. For example, the State of Nevada does not regulate pricing and licensed dispensing organizations within the State of Nevada may set their own prices for cannabis and cannabis products. However, products sold at dispensaries in Nevada are subject to a 10% cannabis excise and sales tax.
Inventory Management
Cresco has comprehensive inventory management procedures, which are compliant with the rules set forth by the applicable state and local laws, regulations, ordinances, and other requirements. These procedures ensure strict control over Cresco’s cannabis and cannabis product inventory from delivery by a licensed distributor to sale or delivery to a consumer, or disposal as cannabis waste. Such inventory management procedures also include measures to prevent contamination and maintain the safety and quality of the products dispensed at Cresco’s retail locations. Cresco understands its responsibility to the greater community and the environment and is committed to providing consumers with a safe, consistent, and high-quality supply of cannabis.
Employees
As of September 30, 2018, Cresco had 275 employees. The employees are distributed among the following departments:
| Department |
Number of Employees | |||
| Administrative |
4 | |||
| Executive |
5 | |||
| Finance |
8 | |||
| Cultivation |
41 | |||
| Human Resources |
4 | |||
| Kitchen |
10 | |||
| Legal |
7 | |||
| Logistics |
13 | |||
-22-
| Department |
Number of Employees | |||
| Maintenance |
4 | |||
| Manufacturing |
17 | |||
| Marketing |
7 | |||
| Operations |
27 | |||
| Packaging/Processing |
72 | |||
| Retail |
42 | |||
| Sales |
9 | |||
| Security |
1 | |||
| Supply Chain |
2 | |||
| Technology |
2 | |||
|
|
|
|||
| Total |
275 | |||
|
|
|
|||
Cresco recruits, hires and promotes individuals that are best qualified for each position, priding itself on using a selection process that recruits people who are trainable, cooperative and share its core values as a company. For 2017, the annualized turnover rate for the businesses that then existed and now form a part of Resulting Issuer was 28%, which is relatively low in view of the rapid growth and change such businesses have experienced. In addition, the safety of employees is a priority and Cresco is committed to the prevention of illness and injury through the provision and maintenance of a healthy workplace. Cresco takes all reasonable steps to ensure staff are appropriately informed and trained to ensure the safety of themselves as well as others around them. In 2017, the businesses that then existed and now form a part of Resulting Issuer had zero workplace injuries.
Competition
With respect to retail operations, Cresco expects to compete with other retail license holders across the markets that it operates in. Many of Cresco’s competitors in those markets are small local operators. In certain markets such as California, there are also a number of illegally operating dispensaries, which serve as competition. However, it is expected that the majority of these dispensaries will be forced to cease operations in the next twelve months. In addition to physical dispensaries, Cresco also expects to compete with third-party delivery services, which provide direct-to-consumer delivery services.
In terms of cultivation and production, Cresco expects to compete with other licensed cultivators and operators in the states in which it operates. Similar to retail, there are a number of illegally operating cultivators in California which will serve as competition in the near-term. However, it is expected that the majority of these cultivators will cease operations over the next twelve months.
Intellectual Property
Cresco has developed numerous proprietary technologies and processes. These proprietary technologies and processes include its cultivation and extraction techniques, and certain cultivation equipment and irrigation systems. While exploring the patentability of these techniques and processes, Cresco relies on non-disclosure and confidentiality arrangements and trade secret protection.
Cresco has invested significant resources towards developing recognizable and unique brands and is in the process of seeking registration of trademarks with the United States Patent and Trademark Office and the states in which it operates. Cresco owns nine website domains, (including www.crescolabs.com, www.cydispensary.com, www.crescoyeltrah.com, www.stateofrelief.com, www.reservebycresco.com, www.crescocannabis.com, www.summerorrelief.com, www.mindysedibles.com and www.smiledispensaries.com) numerous social media accounts across all major platforms and various phone and web application platforms.
-23-
Cresco’s legal counsel monitors and proactively addresses potential intellectual property infringement. Additionally, Cresco maintains strict standards and operating procedures regarding its intellectual property, including the standard use of non-disclosure, confidentiality, and intellectual property assignment agreements.
Trademarks
Cresco is in the process of registering the following brands for trademark protection at the U.S. federal level and/or in the states in which the brands are offered. For additional details on the risks associated with the lack of trademark protection please see Section 17- Risk Factors – Intellectual Property:
| • | The text and stylized logo for “Cresco”, produced here: |

| • | The text and stylized logo for “Cresco Labs”, produced here: |

| • | The text and stylized logo for “remedi”, produced here: |

| • | The text and stylized logo for “reserve”, produced here: |

| • | The stylized logo, produced here: |

-24-
| • | The text and stylized logo for “Mindy’s Kitchen”, produced here: |

| • | The text and stylized logo for “Mindy’s Artisanal Edibles”, produced here: |

Cresco will continue to rely on common law protection for these brands during the trademark registration process. For additional details on the risks associated with the lack of trademark protection please see Section 17- Risk Factors – Intellectual Property.
United States Regulatory Environment
Federal Regulatory Environment
Under U.S. federal law, marijuana is currently a Schedule I drug. The CSA has five different tiers or schedules. A Schedule I drug means the Drug Enforcement Agency considers it to have a high potential for abuse, no accepted medical treatment, and lack of accepted safety for the use of it even under medical supervision. Other Schedule I drugs are heroin, LSD and ecstasy. Cresco believes the CSA categorization as a Schedule I drug is not reflective of the medicinal properties of marijuana or the public perception thereof, and numerous studies show cannabis is not able to be abused in the same way as other Schedule I drugs, has medicinal properties, and can be safely administered. Additionally, while studies show cannabis is less harmful than alcohol,27 alcohol is not classified under the CSA.
Given that more than half of the U.S. states have now legalized adult-use and/or medical marijuana, the federal government sought to provide guidance to enforcement agencies and banking institutions with the introduction of the United States Department of Justice Memorandum drafted by former Deputy Attorney General James Michael Cole in 2013 (the “Cole Memo”)28 and the Department of the Treasury Financial Crimes Enforcement Network (“FinCEN”) guidance in 2014.29
| 27 | See Lachenmeier, DW & Rehm, J. (2015). Comparative risk assessment of alcohol, tobacco, cannabis and other illicit drugs using the margin of exposure approach. Scientific Reports, 5, 8126. doi: 10.1038/srep08126; Thomas, G & Davis, C. (2009). Cannabis, Tobacco and Alcohol Use in Canada: Comparing risks of harm and costs to society. Visions Journal, 5. Retrieved from http://www.heretohelp.bc.ca/sites/default/files/visions_cannabis.pdf; Jacobus et al. (2009). White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicology and Teratology, 31, 349-355. https://doi.org/10.1016/j.ntt.2009.07.006; Could smoking pot cut risk of head, neck cancer? (2009 August 25). Retrieved from https://www.reuters.com/article/us-smoking-pot/could-smoking-pot-cut-risk-of-head-neck-cancer-idUSTRE57O5DC20090825; Watson, SJ, Benson JA Jr. & Joy, JE. (2000). Marijuana and medicine: assessing the science base: a summary of the 1999 Institute of Medicine report. Arch Gen Psychiatry Review, 57, 547-552. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10839332; Hoaken, Peter N.S. & Stewart, Sherry H. (2003). Drugs of abuse and the elicitation of human aggressive behavior. Addictive Behaviours, 28, 1533-1554. Retrieved from http://www.ukcia.org/research/AgressiveBehavior.pdf; and Fals-Steward, W.,Golden, J. & Schumacher, JA. (2003). Intimate partner violence and substance use: a longitudinal day-to-day examination. Addictive Behaviors, 28, 1555-1574. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/14656545. |
| 28 | U.S. Dept. of Justice. (2013). Memorandum for all United States Attorneys re: Guidance Regarding Marijuana Enforcement. Washington, DC: US Government Printing Office. Retrieved from https://www.justice.gov/iso/opa/resources/3052013829132756857467.pdf. |
| 29 | Department of the Treasury Financial Crimes Enforcement Network. (2014). Guidance re: BSA Expectations Regarding Marijuana-Related Businesses (FIN-2014-G001). Retrieved from https://www.fincen.gov/resources/statutes-regulations/guidance/bsa-expectations-regarding-marijuana-related-businesses. |
-25-
The Cole Memo offered guidance to federal enforcement agencies as to how to prioritize civil enforcement, criminal investigations and prosecutions regarding marijuana in all states. The memo put forth eight prosecution priorities:
| 1. | Preventing the distribution of marijuana to minors; |
| 2. | Preventing revenue from the sale of marijuana from going to criminal enterprises, gangs and cartels; |
| 3. | Preventing the diversion of marijuana from states where it is legal under state law in some form to other states; |
| 4. | Preventing the state-authorized marijuana activity from being used as a cover or pretext for the trafficking of other illegal drugs or other illegal activity; |
| 5. | Preventing the violence and the use of firearms in the cultivation and distribution of marijuana; |
| 6. | Preventing the drugged driving and the exacerbation of other adverse public health consequences associated with marijuana use; |
| 7. | Preventing the growing of marijuana on public lands and the attendant public safety and environmental dangers posed by marijuana production on public lands; and |
| 8. | Preventing marijuana possession or use on federal property. |
In January 2018, United States Attorney General, Jeff Sessions, rescinded the Cole Memo and thereby created a vacuum of guidance for enforcement agencies and the Department of Justice. As an industry best practice, despite the recent rescission of the Cole Memo, Cresco continues to do the following to ensure compliance with the guidance provided by the Cole Memo:
| • | ensure the operations of its subsidiaries (or third parties, in the jurisdictions where Cresco conducts its business as an ancillary services provider) are compliant with all licensing requirements that are set forth with regards to cannabis operation by the applicable state, county, municipality, town, township, borough, and other political/administrative divisions. To this end, Cresco retains appropriately experienced legal counsel to conduct the necessary due diligence to ensure compliance of such operations with all applicable regulations; |
| • | the activities relating to cannabis business adhere to the scope of the licensing obtained – for example, in the states where only medical cannabis is permitted, the products are only sold to patients who hold the necessary documentation to permit the possession of the cannabis; and in the states where cannabis is permitted for adult recreational use, the products are only sold to individuals who meet the requisite age requirements; |
| • | in working with licensed operators, such as cultivators and manufacturers in due diligence on the policies and procedures to ensure that the products are not distributed to minors. Additionally, Cresco employs professional consultants to investigate any past license violations and ensure that the business has not been involved in these types of violations; |
| • | Cresco only works through licensed operators, which must pass a range of requirements, adhere to strict business practice standards and be subjected to strict regulatory oversight with sufficient checks and balances to ensure that no revenue is distributed to criminal enterprises, gangs and cartels. Furthermore, as a part of its due diligence, Cresco retains professional consultants to vet the ownership of such cannabis businesses to ensure that no profits or revenues are used for the benefit of criminal enterprises; |
| • | as a part of its compliance audit, Cresco also ensures that the licensed operators have an adequate inventory tracking system and necessary procedures in place to ensure that such compliance system is effective in tracking inventory. This is done to ensure that there is no diversion of cannabis or cannabis products into the states where cannabis is not permitted by state law, or cross the state lines in general; |
-26-
| • | Cresco conducts the necessary review of financial records and where appropriate retains professional third-party consultants to do so, to ensure that the state-authorized cannabis business activity is not used as a cover or pre-text for trafficking of other illegal drugs or engaged in other illegal activity or any activities that are contrary to any applicable anti-money laundering statutes; |
| • | Cresco conducts background checks to ensure that the principals and management of the licensed operators are of good character, and have not been involved with other illegal drugs, engaged in illegal activity or activities involving violence, or use of firearms in cultivation, manufacturing or distribution of cannabis; |
| • | Cresco conducts reviews of activities of the cannabis businesses, the premises on which they operate and the policies and procedures that are related to possession of cannabis or cannabis products outside of licensed premises (including the cases where such possession permitted by regulation – e.g. transfer of products between licensed premises). These activities are done to ensure that no licensed operators possess or use cannabis on federal property or engage in manufacturing or cultivation of cannabis on federal lands; and |
| • | Cresco conducts reviews of products and product packaging to ensure that the products comply with applicable regulations and contain necessary disclaimers about the contents of the products to prevent adverse public health consequences from cannabis use and prevent impaired driving. |
Due to the CSA categorization of marijuana as a Schedule I drug, U.S. federal law makes it illegal for financial institutions that depend on the Federal Reserve’s money transfer system to take any proceeds from marijuana sales as deposits. Banks and other financial institutions could be prosecuted and possibly convicted of money laundering for providing services to cannabis businesses under the United States Currency and Foreign Transactions Reporting Act of 1970 (the “Bank Secrecy Act”). Under U.S. federal law, banks or other financial institutions that provide a cannabis business with a checking account, debit or credit card, small business loan, or any other service could be found guilty of money laundering or conspiracy.
While there has been no change in U.S. federal banking laws to account for the trend towards legalizing medical and recreational marijuana by U.S. states, FinCEN has issued guidance advising prosecutors of money laundering and other financial crimes not to focus their enforcement efforts on banks and other financial institutions that serve marijuana-related businesses, so long as that business is legal in their state and none of the federal enforcement priorities are being violated (such as keeping marijuana away from children and out of the hands of organized crime). The FinCEN guidance also clarifies how financial institutions can provide services to marijuana-related businesses consistent with their Bank Secrecy Act obligations, including thorough customer due diligence, but makes it clear that they are doing so at their own risk. The customer due diligence steps include:
| 1. | verifying with the appropriate state authorities whether the business is duly licensed and registered; |
| 2. | reviewing the license application (and related documentation) submitted by the business for obtaining a state license to operate its marijuana-related business; |
| 3. | requesting from state licensing and enforcement authorities available information about the business and related parties; |
| 4. | developing an understanding of the normal and expected activity for the business, including the types of products to be sold and the type of customers to be served (e.g., medical versus recreational customers); |
| 5. | ongoing monitoring of publicly available sources for adverse information about the business and related parties; |
-27-
| 6. | ongoing monitoring for suspicious activity, including for any of the red flags described in this guidance; and |
| 7. | refreshing information obtained as part of customer due diligence on a periodic basis and commensurate with the risk. With respect to information regarding state licensure obtained in connection with such customer due diligence, a financial institution may reasonably rely on the accuracy of information provided by state licensing authorities, where states make such information available. |
Due to the fear by financial institutions of being implicated in or prosecuted for money laundering, marijuana businesses are often forced into becoming “cash-only” businesses. As banks and other financial institutions in the U.S. are generally unwilling to risk a potential violation of federal law without guaranteed immunity from prosecution, most refuse to provide any kind of services to marijuana businesses. Despite the attempt by FinCEN to legitimize marijuana banking, in practice its guidance has not made banks much more willing to provide services to marijuana businesses. This is because, as described above, the current law does not guarantee banks immunity from prosecution, and it also requires banks and other financial institutions to undertake time-consuming and costly due diligence on each marijuana business they take on as a customer. Recently, some banks that have been servicing marijuana businesses have been closing accounts operated by marijuana businesses and are now refusing to open accounts for new marijuana businesses for the reasons enumerated above.
The few credit unions who have agreed to work with marijuana businesses are limiting those accounts to no more than 5% of their total deposits to avoid creating a liquidity risk. Since the federal government could change the banking laws as it relates to marijuana businesses at any time and without notice, these credit unions must keep sufficient cash on hand to be able to return the full value of all deposits from marijuana businesses in a single day, while also servicing the need of their other customers.
The U.S. Treasury Department, headed by Stephen Mnuchin, has publicly stated they were not informed of the Attorney General Jeff Sessions’ desire to rescind the Cole Memo and do not have a desire to rescind the FinCEN guidance for financial institutions.30 Multiple legislators believe that Sessions’ rescinding of the Cole Memo invites an opportunity for Congress to pass more definitive protections for marijuana businesses in states with legal marijuana programs during this Congress.31
Both Congress and marijuana-related businesses recognize that guidance is not law and thus have worked to continually renew the Rohrabacher Blumenauer Appropriations Amendment (originally the Rohrabacher Farr Amendment) since 2014. This amendment prevents the Department of Justice from using congressional funds to prosecute cannabis businesses in states that have medical marijuana laws and programs. In 2017, Senator Patrick Leahy (D-Vermont) introduced a similar amendment to H.R.1625 – a vehicle for the Consolidated Appropriations Act of 2018), preventing federal prosecutors from using federal funds to impede the implementation of medical cannabis laws enacted at the state level, subject to Congress restoring such funding (“Leahy Amendment”). The Leahy Amendment expired with the 2018 fiscal year on September 30, 2018. It may or may not be included in the 2019 fiscal year omnibus appropriations package or a continuing budget resolution, and its inclusion or non-inclusion, as applicable, is subject to political changes.
| 30 | Angell, Tom. (2018 February 6). Trump Treasury Secretary Wants Marijuana Money In Banks. Retrieved from https://www.forbes.com/sites/tomangell/2018/02/06/trump-treasury-secretary-wants-marijuana-money-in-banks/#2848046a3a53; see also Mnuchin: Treasury is reviewing cannabis policies. (2018 February 7). Retrieved from http://www.scotsmanguide.com/News/2018/02/Mnuchin--Treasury-is-reviewing-cannabis-policies/. |
| 31 | Jackson, Cherese. (2018 January 30). State-by-State Analysis of Sessions Move to Rescind Cole Memo. Retrieved from http://guardianlv.com/2018/01/state-state-analysis-sessions-move-rescind-cole-memo/; see also Velasquez, Josefa. (2018 January 23). NY Lawmarker Asks US Attorneys to Keep Hands Off State’s Med Marijuana Programs. Retrieved from https://www.law.com/newyorklawjournal/sites/newyorklawjournal/2018/01/22/ny-lawmaker-asks-us-attorneys-to-keep-hands-off-states-med-marijuana-programs/?slreturn=20180205182803; see also “This is Outrageous”: Politicians react to news that A.G. Sessions is rescinding Cole Memo. (January 4 2018). Retrieved from https://www.thecannabist.co/2018/01/04/sessions-marijuana-cole-memo-politicians/95890/. |
-28-
For fiscal year 2019, the strategy amongst the Congressional Marijuana Working Group, is to introduce numerous marijuana-related appropriations amendments in the Appropriations Committee in both the House and Senate, similar to the strategy employed in fiscal year 2018.32 The amendments will include protections for marijuana-related businesses in states with medical and adult use marijuana laws, as well as protections for financial institutions that provide banking services to state-legal marijuana businesses.33 However it should be noted that there is no assurance that such amendments will be passed into law.
Since 2014, Congress has made immense strides in marijuana policy. The bipartisan Congressional Cannabis Caucus launched in 2017 and is headed by Representatives Dana Rohrabacher (CA-48), Earl Blumenauer (OR-03), Don Young (AK-At Large), and Jared Polis (CO-02). The group is “dedicated to developing policy reforms that bridge the gap between federal laws banning marijuana and the laws in an ever-growing number of states that have legalized it for medical or recreational purposes.“34 Additionally, each year more Representatives and Senators sign on and co-sponsor marijuana legalization bills including the CARERS Act, REFER Act and others. While there are different perspectives on the most effective route to end U.S. federal marijuana prohibition, Congressman Blumenauer and Senator Wyden introduced the three-bill package, Path to Marijuana Reform which would fix the 280E provision, eliminate civil asset forfeiture and federal criminal penalties for businesses complying with state law, reduce barriers to banking, and would de-schedule, tax and regulate marijuana in 2017.35 Senator Booker has also introduced the Marijuana Justice Act, which would de-schedule marijuana, and in 2018 Congresswoman Barbara Lee introduced the House companion.
Notwithstanding the foregoing, there is no guarantee that the current Presidential administration will not change the stated policy of the previous administration regarding the low-priority enforcement of U.S. federal laws that conflict with state laws. The Trump administration and the Congress could decide to enforce U.S. federal laws vigorously.
An additional challenge to marijuana-related businesses is that the provisions of the Code, Section 280E, are being applied by the IRS to businesses operating in the medical and adult use marijuana industry. Section 280E of the Code prohibits marijuana businesses from deducting their ordinary and necessary business expenses, forcing them to pay higher effective U.S. federal tax rates than similar companies in other industries. The effective tax rate on a marijuana business depends on how large its ratio of non-deductible expenses is to its total revenues. Therefore, businesses in the legal cannabis industry may be less profitable than they would otherwise be.
State Regulatory Environment
The following sections describe the legal and regulatory landscape in the states in which Cresco operates. While Cresco’s operations are in full compliance with all applicable state laws, regulations and licensing requirements, for the reasons described above and the risks further described in Section 17 - Risk Factors, there are significant risks associated with the business of Cresco. Readers are strongly encouraged to carefully read all of the risk factors contained in Section 17 below.
| 32 | Congress of the United States. (2018 January 12). Letter to The Honorable Paul Ryan, The Honorable Nancy Pelosi, Chairman Rodney P. Frelinghuysen and Ranking Member Nita Lowey. Retrieved from https://polis.house.gov/uploadedfiles/marijuana_appropriations_mcclintock-polis_language_1-12-18.pdf. |
| 33 | Congress of the United States. (2018 January 17). Letter to Director Kenneth Blanco of the Financial Crimes Enforcement Network of the Department of the Treasury. Retrieved from https://dennyheck.house.gov/sites/dennyheck.house.gov/files/FINCEN%20MJ%20Guidance%20Letter%20FINAL.pdf; see also United States Senate. (2018 January 11). Letter to Director Kenneth Blanco of the Financial Crimes Enforcement Network of the Department of the Treasury. Retrieved from https://www.documentcloud.org/documents/4347431-368944892-Letter-Urging-FinCEN-to-Maintain.html#document/p1; see also United States Senate. (2018 January 18). Letter to Director Kenneth Blanco of the Financial Crimes Enforcement Network of the Department of the Treasury. Retrieved from https://www.documentcloud.org/documents/4356160- 18-01-18-FinCEN-LTR-Cannabis-Banking.html; see also Congress of the United States. (2018 January 25). Letter to The Honorable Donald Trump. Retrieved from https://www.warren.senate.gov/files/documents/2018_01_25%20Letter%20to%20Trump%20on%20Sessions%20withdrawal%20of% 20the%20Cole%20memo.pdf. |
| 34 | Huddleston, Tom Jr. (2017 February 17). Pro-Pot Lawmakers Launch a Congressional Cannabis Caucus. Retrieved from http://fortune.com/2017/02/16/congress-cannabis-caucus/. |
| 35 | Wyden, Blumenauer. (2017 March 30). Wyden, Blumenauer announce bipartisan path to marijuana reform. Retrieved from https://blumenauer.house.gov/media-center/press-releases/wyden-blumenauer-announce-bipartisan-path-marijuana-reform. |
-29-
STATE LEVEL U.S. CANNABIS OPERATIONS
Illinois
Illinois Regulatory Landscape
The Compassionate Use of Medical Cannabis Pilot Program Act, which allows individuals diagnosed with a debilitating medical condition access to medical marijuana, became effective January 1, 2014 and is extended through July 1, 2020. There are over 41 qualifying conditions as part of the medical program, including epilepsy, traumatic brain injury, and PTSD.
Illinois’ retail market size for 2017 was over $85 million, representing an over 140% year-over-year increase. In the first three calendar months of 2018, recorded state-wide sales are already over 1/3 of the total market size for all of 2017. The first quarter net revenues of 2018 represent an approximate 14% sequential increase over the fourth quarter of 2017.
In March 2018, Cook County voters (which is by far and large the most populous county in the state, encompassing all of Chicagoland metro area) responded positively for state-wide recreational legalization with a 63% majority. Although the vote was non-binding, the voting leverage of Cook County, which encompasses more than 130 municipalities, is anticipated to play a significant role in the November 2018 gubernatorial elections for which numerous candidates have outwardly pledged their support for cannabis legislation.
Illinois Licenses
Cresco currently operates three medical cannabis cultivation and manufacturing centers in Illinois and holds an ownership interest in five dispensary locations in Illinois assuming completion of the MedMar transaction described in Section 3 above. Licenses were awarded based on merit in a competitive application process to applicants who demonstrated operational expertise and financial backing.
Cresco is licensed to operate in the state of Illinois as a medical cultivator and medical product manufacturer. Phoenix Farms of Illinois, LLC (“Phoenix Farms”), PDI Medical III, LLC d/b/a PDI Medical (“PDI”), FloraMedex, MedMar Lakeview, and MedMar Rockford, subsidiaries of Cresco, are licensed to operate retail dispensaries in the State of Illinois. The Table below lists the licenses issued to Cresco, Phoenix Farms, PDI, FloraMedex, MedMar Lakeview, and MedMar Rockford in respect of its operations in Illinois. Under applicable laws, the licenses permit Cresco, Phoenix Farms, PDI, FloraMedex, MedMar Lakeview and MedMar Rockford to cultivate, manufacture, process, package, sell, and purchase (as applicable) marijuana pursuant to the terms of the licenses, which are issued by the Department of Agriculture and the Department of Financial and Professional Regulation under the provisions of the Illinois Revised Statutes 410 ILCS 130. All licenses are, as of the date hereof, active with the State of Illinois. There are two categories of licenses in Illinois: (i) cultivation/processing and (ii) dispensary. The licenses are independently issued for each approved activity.
All cultivation/processing establishments must register with Illinois Department of Agriculture. All dispensaries must register with the Illinois Department of Financial and Professional Regulation. If applications contain all required information and after vetting by officers, establishments are issued a medical marijuana establishment registration certificate. Registration certificates are valid for a period of one year and are subject to annual renewals after required fees are paid and the business remains in good standing. Renewal requests are typically communicated through email from the Department of Agriculture or Illinois Department of Financial and Professional Regulation and include a renewal form.
-30-
Licenses in the State of Illinois
| Holding Entity |
Permit/License |
City |
Expiration/Renewal Date (if applicable) (MM/DD/YY) |
Description | ||||
| Cresco Labs, LLC | Medical Cannabis Cultivation Center Operating Permit Permit: 1503060739 |
Kankakee | 05/18/2019 | Permit to operate medical cannabis cultivation center | ||||
| Medical Cannabis Cultivation Center Operating Permit Permit: 1503060740 |
Lincoln | 03/09/2019 | Permit to operate medical cannabis cultivation center | |||||
| Medical Cannabis Cultivation Center Operating Permit Permit: 1503060741 |
Joliet | 03/09/2019 | Permit to operate medical cannabis cultivation center | |||||
| Phoenix Farms of Illinois, LLC d/b/a Phoenix Botanical | Registered Medical Cannabis Dispensing Registry ID: 10-002 License: DISP.000035 |
Champaign | 04/26/2019 | Permit to operate a medical cannabis dispensary | ||||
| PDI Medical, III, LLC d/b/a PDI Medical | Registered Medical Cannabis Dispensing Registry ID: 27-002 License: DISP.000016 |
Buffalo Grove | 12/7/2018 | Permit to operate a medical cannabis dispensary | ||||
| FloraMedex, LLC | Registered Medical Cannabis Dispensing Registry ID: 35-001 License: DISP.000034 |
Elmwood Park | 04/18/19 | Permit to operate a medical cannabis dispensary | ||||
| MedMar Lakeview, LLC | Registered Medical Cannabis Dispensing Registry ID: 44-002 License: DISP.000050 |
Chicago | 1/13/19 | Permit to operate a medical cannabis dispensary | ||||
| MedMar Rockford, LLC | Registered Medical Cannabis Dispensing Registry ID: 16-001 License: DISP.000013 |
Rockford | 11/24/18 | Permit to operate a medical cannabis dispensary | ||||
Illinois License and Regulations
The retail dispensary license permits Cresco to purchase marijuana and marijuana products from cultivation/processing facilities, and allows the sale of marijuana and marijuana products to registered patients.
The medical cultivation licenses permit Cresco to acquire, possess, cultivate, manufacture/process into edible medical marijuana products and/or medical marijuana-infused products, deliver, transfer, have tested, transport, supply or sell marijuana and related supplies to medical marijuana dispensaries.
Illinois imposes an income surtax, equal to the amount of federal income tax levied, on any direct or indirect transfer of Illinois license permit.
-31-
Illinois Reporting Requirements
The State of Illinois uses BioTrack as the State’s computerized track-and-trace (“T&T”) system for seed-to-sale. Individual licensees whether directly or through third-party integration systems are required to push data to the state to meet all reporting requirements. Cresco uses the commercial version of BioTrack as its in-house computerized seed to sale software, which integrates with the State’s BioTrack program and captures the required data points for cultivation, manufacturing and retail as required in the Illinois Compassionate Use of Medical Cannabis Pilot Program Act.
Illinois Storage and Security Requirements
As to its cultivation facility, the regulations require Cresco to store marijuana and marijuana infused products in a safe, vault or secured room in such a manner to prevent diversion, theft or loss. Any marijuana that is not a finished product must likewise be maintained in a secured area within the facility only accessible to authorized personnel. All locks and security equipment safeguarding the marijuana must be kept in good working order, and the storage areas must be locked and protected from unauthorized access at all times.
The cultivation facility must also have an operational 24-hour, seven day a week, closed circuit television surveillance system on the premises that complies with certain regulatory minimum standards. Access to the surveillance area is restricted to only those people who are essential to surveillance operations, law enforcement agencies, security system service personnel and the regulator. In addition, video surveillance recordings shall be retained for 90 days at the facility and an additional 90 days off site.
Cresco must also maintain an alarm system at its cultivation facility. The cultivation facility must maintain and use a professionally monitored robbery and burglary alarm system that meets certain regulatory minimum standards. A qualified alarm system vendor must test the system annually.
With respect to its Illinois dispensaries, Cresco must store inventory on site in a secured and restricted access area consistent with the security regulations and tracked in accordance with the inventory tracking regulations. Any containers storing medical marijuana that have been tampered with or opened must be stored separately until disposed; such materials can only be stored at the dispensary for one week.
The dispensaries must also implement security measures to deter and prevent entry into and theft from restricted access areas that contain marijuana and/or currency, including having a commercial grade alarm and surveillance system installed by an Illinois licensed private alarm contractor or private alarm contractor agency. The facility must also have security measures to protect the premises, registered qualifying patients, designated caregivers and dispensing organization agents.
Pennsylvania
Pennsylvania Regulatory Landscape
The Pennsylvania medical marijuana program was signed into law on April 17, 2016 under Act 16 and provided access to state residents with one of 17 qualifying conditions, including epilepsy, chronic pain, and PTSD. The state, which consists of over 12 million U.S. citizens and qualifies as the fifth largest population in the US, operates as a high-barrier market with very limited market participation. The state originally awarded only 12 licenses to grow/process and 27 licenses to operate retail dispensaries (which entitled holders to up to three medical dispensary locations). Cresco Yeltrah, LLC (“Cresco Yeltrah”), a subsidiary of Creso, was awarded one medical cannabis grow and processing license and one dispensary license in Pennsylvania (allowing for three (3) dispensary locations in Pennsylvania).
Retail sales opened in February 2018 to a limited number of retail locations across the state. Cresco Yeltrah, on February 15, was the first cultivator/processor to release product into Pennsylvania market (approximately 6 weeks ahead of any other producer) and its dispensary was the first to sell product to patients in the state.
-32-
On March 22, 2018, it was announced that the final phase of the Pennsylvania medical marijuana program would initiate its rollout, which will include 13 additional grow/processing licenses and 23 additional dispensary licenses. The application period ran from April 2018 through May 17, 2018. Cresco Yeltrah submitted additional dispensary applications and anticipates that the Pennsylvania Department of Health will announce the license awards in the fourth quarter of 2018.
It was announced on April 17, 2018 that dry flower would be included in the regulations as an approved product form for sale and consumption (in addition to the already approved forms of concentrates, pills, and tinctures). Simultaneously, it was announced that the list of qualifying conditions would expand from 17 to 21, including additions of cancer remission therapy and opioid-addiction therapy.
Pennsylvania Licenses
Cresco Yeltrah is licensed to operate in the Commonwealth of Pennsylvania as a medical cannabis grower/processor and to operate three (3) medical cannabis dispensaries. The table below lists the licenses issued to Cresco Yeltrah in respect of its operations in Pennsylvania. Under applicable laws, the licenses permit Cresco Yeltrah to cultivate, manufacture, process, package, sell, and purchase medical marijuana pursuant to the terms of the licenses, which are issued by the Pennsylvania Department of Health under the provisions of Medical Marijuana Act (35 P.S. § § 10231.101— 10231.2110) and Chapters 1141, 1151 and 1161 of the Pennsylvania regulations. All licenses are, as of the date hereof, active with the Commonwealth of Pennsylvania. There are two categories of licenses in Pennsylvania: (i) cultivation/processing; and (ii) dispensary. The licenses are independently issued for each approved activity for use at Cresco Yeltrah facilities in Pennsylvania.
All cultivation/processing establishments must register with Pennsylvania Department of Health. All dispensaries must register with the Pennsylvania Department of Health. Registration certificates are valid for a period of one year and are subject to annual renewals after required fees are paid and the business remains in good standing. Specifically, for licenses that Cresco Yeltrah currently holds, each have undergone one renewal period.
| Holding Entity |
Permit/License |
City |
Expiration/Renewal |
Description | ||||
| Cresco Yeltrah, LLC | Medical Marijuana Dispensary Permit Applicant ID: D-5016-17 | Butler | 6/30/2019 | Permit to operate a medical marijuana dispensary | ||||
| Medical Marijuana Dispensary Permit Applicant ID: D-5016-17 | Pittsburgh | 6/30/2019 | Permit to operate a medical marijuana dispensary | |||||
| Medical Marijuana Dispensary Permit Applicant ID: D-5016-17 | TBD | 6/30/2019 | Permit to operate a medical marijuana dispensary | |||||
| Medical Marijuana Grower/Processor Permit Applicant ID: GP-6012-17 |
Brookville | 6/30/2019 | Permit to grow and process medical marijuana |
Pennsylvania License and Regulations
The retail dispensary licenses permit Cresco Yeltrah to purchase marijuana and marijuana products from growing/processing facilities, and allows the sale of marijuana and marijuana products to registered patients.
The medical cultivation licenses permit Cresco Yeltrah to acquire, possess, cultivate, manufacture/process into edible medical marijuana products and/or medical marijuana-infused products, deliver, transfer, have tested, transport, supply or sell marijuana and related supplies to medical marijuana dispensaries.
-33-
Pennsylvania Reporting Requirements
The Commonwealth of Pennsylvania uses MJ Freeway as the state’s computerized T&T system for seed-to-sale. Individual licensees are required to use MJ Freeway to push data to the state to meet all reporting requirements. Cresco Yeltrah uses MJ Freeway as its in-house computerized seed to sale software, which integrates with the state’s MJ Freeway program and captures the required data points for cultivation, manufacturing and retail as required in the Pennsylvania medical marijuana laws and regulations.
Pennsylvania Storage and Security
The regulations require the maintenance of storage areas at Cresco Yeltrah’s grower/processor location in a clean and orderly condition, free from infestation. These separate and locked limited access areas are used for the grower/processor to store seeds, immature plants, mature plants and medical marijuana that is expired, damaged, deteriorated, mislabeled, contaminated, recalled or whose containers or packages have been opened or breached until such product is destroyed or otherwise disposed of.
The regulations also require Cresco Yeltrah’s grower/processor facility to have a commercial grade security system to prevent unauthorized entry and to prevent and detect any attempted diversion. This security must include an alarm system that covers the interior and exterior of the facility, including a silent alarm.
A dispensary must also have a locked limited access area for the storage of medical marijuana that is expired, damaged, deteriorated, mislabeled, contaminated, recalled or whose containers or packages have been opened or breached until such product is returned to the grower/processor.
Cresco Yeltrah’s dispensaries must have a security system with the same features as that for the grower/processor facility. This system must be professionally monitored 24-hours a day and seven days a week with fixed cameras on the interior and exterior of the facilities. The surveillance system must store data for a period of four years in a readily available format for investigative purposes.
Ohio
Ohio Regulatory Landscape
House Bill 523, effective on September 8, 2016, legalized medical marijuana in Ohio. The Ohio Medical Marijuana Control Program (“MMCP”) allows people with certain medical conditions, upon the recommendation of an Ohio-licensed physician certified by the State Medical Board, to purchase and use medical marijuana. House Bill 523 required that the framework for the MMCP will be in place no later than September 2018. This timeframe allowed for a deliberate process to ensure the safety of the public and to promote access to a safe product. Due to construction delays and lawsuits, as of the end of September, 2018 medical marijuana is not yet sold in Ohio. It is speculated that January, 2019 is new target date for medical marijuana sales to begin in Ohio.
The three following state government agencies are responsible for the operation of Ohio’s Medical Marijuana Control Program: (i) the Ohio Department of Commerce is responsible for overseeing medical marijuana cultivators, processors and testing laboratories; (ii) the State of Ohio Board of Pharmacy is responsible for overseeing medical marijuana retail dispensaries, the registration of medical marijuana patients and caregivers, the approval of new forms of medical marijuana and coordinating the Medical Marijuana Advisory Committee; and (iii) the State Medical Board of Ohio is responsible for certifying physicians to recommend medical marijuana and may add to the list of qualifying conditions for which medical marijuana can be recommended. Qualifying medical conditions for medical marijuana include: HIV/AIDS, Lou Gehrig’s disease, Alzheimer’s disease, cancer, chronic traumatic encephalopathy, Crohn’s disease, epilepsy or other seizure disorder, fibromyalgia, glaucoma, hepatitis C, inflammatory bowel disease, multiple sclerosis (MS), pain (either chronic, severe, or intractable), Parkinson’s disease, PTSD, sickle cell anemia, spinal cord disease or injury, tourette’s syndrome, traumatic brain injury, ulcerative colitis. In order for a patient to be eligible to obtain medical marijuana, a physician must make the diagnosis of one of these conditions.
-34-
Several forms of medical marijuana are legal in Ohio, these include: inhalation of marijuana through a vaporizer (not direct smoking), oils, Tinctures, plant material, edibles, patches and any other forms approved by the State Board of Pharmacy.
Ohio Licenses
On June 4, 2018, the State of Ohio Board of Pharmacy awarded 56 medical marijuana provisional dispensary licenses. The licenses were awarded after a review of 376 submitted dispensary applications.
Provisional licensees are authorized to begin the process of establishing a dispensary in accordance with the representations in their applications and the rules adopted by the State of Ohio Board of Pharmacy. Per rule, all provisional license holders have a maximum of six months to demonstrate compliance with the dispensary operational requirements to obtain a certificate of operation (a “Certificate of Operation”). Compliance will be determined through an inspection by a Board of Medical Marijuana Compliance Agent. Once a dispensary is awarded a Certificate of Operation, it can begin selling medical marijuana to Ohio patients and caregivers in accordance with Ohio laws and rules.
By rule, the State of Ohio Board of Pharmacy is limited to issuing up to 60 dispensary licenses across the state, but will have the authority to increase the number of licenses after September 8, 2018. To date, no announcement has been made if the number of licenses will be increased. Per the program rules, the Board will consider, on at least a biennial basis, whether enough medical marijuana dispensaries exist, considering the state population, the number of patients seeking to use medical marijuana, and the geographic distribution of dispensary sites.
Cresco Labs Ohio, LLC (“Cresco Ohio”), a subsidiary of Cresco, was awarded one provisional dispensary license on June 7, 2018. Cresco Ohio will take the steps needed to meet the six-month operational deadline at its dispensary location. A provisional license serves as authorization from the Board for Cresco Ohio to begin the construction or modification of your facility and to secure any other applicable permits you may need from your local jurisdiction in order to receive a certificate of operation. The dispensary will be located in Winterville, Ohio and is expected to commence operations in December 2018.36
Cresco Ohio applied for and on November 30, 2017 received one provisional cultivation license. Cresco Ohio’s cultivation facility is a hybrid greenhouse structure located in Yellow Springs, Ohio and is currently comprised of approximately 25,000 square feet of cultivation space. This facility is currently undergoing an expansion which, when completed, would increase the cultivation space to a total of approximately 50,000 square feet.37 All of the 50,000 square feet will be used for cultivation.
A holder of a provisional cultivation license is prohibited from operating as a licensed cultivator and performing any cultivation or production activities, including the procurement of seeds, seedlings, or other starting plant material until a Certificate of Operation is issued by the Ohio Department of Commerce. This provisional license serves as authorization from the Ohio Department of Commerce for Cresco Ohio to begin the construction or modification of the facility and to secure any other applicable permits needed from local jurisdictions in order to receive a Certificate of Operation. Pursuant to Ohio Administrative Code s. 3796:2-1-06(B), a provisional license holder has nine months to obtain a Certificate of Operation. On September 14, 2018, Cresco Ohio received its Certificate of Operation.
| 36 | These statements constitute forward-looking information related to possible events, conditions or financial performance based on future economic conditions and courses of action. These statements involve known and unknown risks, assumptions, uncertainties and other factors that may cause actual results or events to differ materially. Cresco believes there is a reasonable basis for the expectations reflected in the forward-looking statements, however these expectations may not prove to be correct. |
| 37 | These statements constitute forward-looking information related to possible events, conditions or financial performance based on future economic conditions and courses of action. These statements involve known and unknown risks, assumptions, uncertainties and other factors that may cause actual results or events to differ materially. Cresco believes there is a reasonable basis for the expectations reflected in the forward-looking statements, however these expectations may not prove to be correct. |
-35-
Licenses in the State of Ohio
| Holding Entity |
Permit/License |
City |
Expiration/Renewal |
Description | ||||
| Cresco Labs Ohio, LLC | MMCPC00017 | Yellow Springs | 9/13/2019 | Cultivation License | ||||
| MMD 04051 | Wintersville | Not Issued | Provisional Dispensary License | |||||
| N/A | [●] | N/A | Medical Processor License38 | |||||
Ohio License and Regulations
The dispensary license will permit Cresco Ohio to purchase marijuana and marijuana products from cultivation and/or processing facilities, and allows the sale of marijuana and marijuana products to registered patients.
The medical cultivation licenses permit, will permit Cresco Ohio to acquire, possess, cultivate, manufacture/process into medical marijuana products, deliver, transfer, have tested, transport, supply or sell marijuana and related supplies to medical marijuana dispensaries.
A medical processor license allows for the manufacturing and production of medical marijuana products. Cresco, Ohio has submitted an application for a processor license, which is currently pending.
Ohio Reporting Requirements
Ohio uses METRC as its seed-to-sale tracking system. Licensees are required to use METRC in Ohio to push data to the state to meet all of the reporting requirements. Cresco Ohio integrates its in-house seed-to-sale tracking system (BioTrack) with METRC to capture the required data points as required in the Ohio medical marijuana laws and regulations.
Ohio Storage and Security Requirements
For Cresco Ohio’s dispensaries, a designated representative is responsible for providing supervision and control of medical marijuana and medical marijuana products to ensure that they are dispensed in accordance with the law and regulations. In addition, the dispensary must have physical or electronic security over such items. In particular, Cresco Ohio’s dispensaries must also maintain security (with alarms and surveillance equipment) as required by the regulations to prevent diversion and theft, as well as to protect patients, caregivers and employees. The dispensary department, restricted access areas and stock of medical marijuana must be secured by a physical barrier with suitable locks and an electronic barrier. Medical marijuana must also be stored in a secure area and tracked in the inventory tracking system. No person is permitted in this secure area unless under the personal supervision of a licensed dispensary employee. The storage area must be clean and free of infestation. Containers storing expired, damaged, deteriorated, misbranded, adulterated or opened medical marijuana shall be separated from other medical marijuana until they are properly destroyed; these materials can only be stored for one week.
The regulations permit Cresco Ohio to store medical marijuana inventory at its cultivation facility in a designated, enclosed, locked facility identified in Cresco Ohio’s plans and specifications that it submitted to the Ohio Department of Commerce. This storage area can only be accessible by authorized individuals. On an annual basis and as a condition to renewal of its cultivator license, Cresco Ohio must perform a physical, manual inventory, of the medical marijuana on hand and compare it to the annual report generated by the inventory tracking system. The cultivation facility must install a commercial grade security alarm system to prevent and detect diversion, theft, or loss. The
| 38 | Application is pending. |
-36-
facility also must maintain surveillance equipment to capture the entire facility, and provide direct access to the regulator on a real-time basis. All of this equipment must be kept in good working order and inspected and tested on an annual basis by a third party.
California
California Regulatory Landscape
In 1996, California was the first state to legalize medical marijuana through Proposition 215, the Compassionate Use Act of 1996 (“CUA”). This legalized the use, possession and cultivation of medical marijuana by patients with a physician recommendation for treatment of cancer, anorexia, AIDS, chronic pain, spasticity, glaucoma, arthritis, migraine, or any other illness for which marijuana provides relief.
In 2003, Senate Bill 420 was signed into law establishing an optional identification card system for medical marijuana patients.
In September 2015, the California legislature passed three bills collectively known as the “Medical Cannabis Regulation and Safety Act” (“MCRSA”). The MCRSA established a licensing and regulatory framework for medical marijuana businesses in California. The system created multiple license types for dispensaries, infused products manufacturers, cultivation facilities, testing laboratories, transportation companies, and distributors. Edible infused product manufacturers would require either volatile solvent or non-volatile solvent manufacturing licenses depending on their specific extraction methodology. Multiple agencies would oversee different aspects of the program and businesses would require a state license and local approval to operate. However, in November 2016, voters in California overwhelmingly passed Proposition 64, the “Adult Use of Marijuana Act” (“AUMA”) creating an adult-use marijuana program for adult-use 21 years of age or older. AUMA had some conflicting provisions with MCRSA, so in June 2017, the California State Legislature passed Senate Bill No. 94, known as Medicinal and Adult-Use Cannabis Regulation and Safety Act (“MAUCRSA”), which amalgamates MCRSA and AUMA to provide a set of regulations to govern medical and adult-use licensing regime for cannabis businesses in the State of California. The four agencies that regulate marijuana at the state level are BCC, California Department of Food and Agriculture, California Department of Public Health, and California Department of Tax and Fee Administration.
In order to legally operate a medical or adult-use cannabis business in California, the operator must have both a local and state license. This requires license holders to operate in cities with marijuana licensing programs. Therefore, cities in California are allowed to determine the number of licenses they will issue to marijuana operators or can choose to ban marijuana outright.
MAUCRSA went into effect on January 1, 2018.
On June 7, 2018, Cresco acquired a 60% ownership interest in SLO Cultivation Inc. (“SLO”). On September 27, 2018, Cresco acquired a further 20% ownership interest in SLO bringing its total ownership to 80%. SLO wholly-owns Bolivar Distribution Services, LLC, Mandela Manufacturing Services, LLC and Mahatma Distribution Services, LLC (the “Cal Subsidiaries”). The Cal Subsidiaries operate a marijuana cultivation operation in the cities of Carpinteria (Santa Barbara County) and San Luis Obispo (San Luis Obispo County) California. The cultivation facility has a capacity of up to 600,000 square feet of greenhouse production space. As a result of Cresco’s acquisition, Cresco has essentially complete management and operational control (except for certain farm-specific functions that remain under the supervised management of SLO). The cannabis will initially be cultivated from the Carpinteria facilities. From there, the harvest will ultimately be transported as fresh (or fresh frozen) material to our manufacturing facilities at 1269 Marie Street in Mendota (Fresno County) California. However, as the facilities at Mendota continue to be built out (expected operations beginning by 1Q 2019)39, Cresco will have its harvested product converted into cannabis-related products through certain third-party contractors. Though Cresco contemplates having its own distribution license by the end of this year, distribution into the southern California and northern California markets will be undertaken by certain third-party contractors. These contract distributors will deliver the Cresco cannabis-related products directly to retail dispensaries for sale to the public.
| 39 | These statements constitute forward-looking information related to possible events, conditions or financial performance based on future economic conditions and courses of action. These statements involve known and unknown risks, assumptions, uncertainties and other factors that may cause actual results or events to differ materially. Cresco believes there is a reasonable basis for the expectations reflected in the forward-looking statements, however these expectations may not prove to be correct. |
-37-
Because the County of Santa Barbara has not yet approved recreational cannabis (though such approval is contemplated in the near future), all cultivation, manufacture, distribution and retail sale of Cresco products in Santa Barbara shall be limited to medical cannabis, only. In October, 2018 the County of Santa Barbara ratified its ordinance to allow for “commercial” cannabis, which includes both medical and recreational cannabis under California State law.
Licenses
Pursuant to MAUCRSA, as set out herein, SLO will be applying for and has been granted licenses permitting it to cultivate, manufacture, distribute and retail medical (and in some instances, adult use) cannabis and cannabis-related products:
Mendota (Fresno County)
| • | SLO has been issued a temporary license for Type 7 (Manufacturing 2 – Volatile), Adult Use & Medical (“A&M”). |
| • | SLO has been issued a temporary license for a temporary Type 11 (Distribution), A&M. |
Willow Road (SLO County)
| • | SLO plans to submit an application for a Type 3B, Tier 2 (Cultivation; Mixed-Light; Medium, greater than 6 but less than 25 watts per light), Type M (medical) only. Cresco has also initiated a license application for a non-store front Type 9 Retailer. |
Carpinteria (SB County)
| • | SLO been issued temporary licenses for Type 2B, Tier 1 (Cultivation; Specialty Mixed-Light; Small, less than 6 watts per light (Tier 1)), Medical only. |
| • | Another three (3) license applications are pending o Type 2B, Tier 1, Cultivation, Specialty, Mixed-light, Medical only. |
| • | Nursery, allowing for the planting and cultivation of medical cannabis from seeds, clones, and immature plants. |
| • | Processor Type, allowing for the harvesting, drying, curing, grading or tanning of cannabis as well as the packaging and labelling of certain non-manufactured cannabis. |
Cresco also has submitted applications with the state for both a Cannabis Event Permit (which is pending) and Cannabis Event Organizer Permit (which has been granted), enabling the company to host events.
-38-
Licenses in the State of California
| Holding Entity |
Permit/License |
City |
Expiration/ |
Description |
Notes | |||||
| Bolivar Distribution Services, LLC | Temporary License Number: C11-18-0000160- TEMP | Mendota | 23/02/2019 | Medical and Adult Use Distribution | ||||||
| Mandela Manufacturing Services, LLC | Temp Manufacturing Number: CDPH-T00001273 | Mendota | 20/12/2018 | Medical and Adult Use Manufacturing | ||||||
| Mahatma Distribution Services, LLC | Pending Distribution Applicant ID: 18TMP-040075 | Carpinteria | N/A | Medical distribution | Application submitted; awaiting license issuance | |||||
| SLO Cultivation, Inc. | Pending Processing Applicant ID: 18TMP-022762 | Arroyo Grande & Willow | N/A | Medical cultivation Small Indoor (for processing) | Application not submitted at this time for strategic purposes. The Company plans to submit an application at a future date. | |||||
| SLO Cultivation, Inc. | Pending Cultivation Applicant ID: 18TMP-022962 | Arroyo Grande & Willow | N/A | Medical cultivation Medium Indoor, Mixed Light, Tier 2 | Application not submitted at this time for strategic purposes. The Company plans to submit an application at a future date. | |||||
| SLO Cultivation, Inc. | Pending Retail Applicant ID: 18TMP-045242 | Arroyo Grande & Willow | N/A | Medical and Adult Use non-storefront retail dispensary | Application not submitted at this time for strategic purposes. The Company plans to submit an application at a future date. | |||||
| SLO Cultivation, Inc. | Pending Retail Applicant ID: 18TMP-039937 | San Luis Obispo | N/A | Medical and Adult Use retail dispensary | Application not submitted at this time for strategic purposes. The Company plans to submit an application at a future date. | |||||
| SLO Cultivation, Inc. | Temporary License Number: TML18-0006715 | Carpinteria | 22/02/2019 | Medical cultivation Small Indoor, Mixed Light, Tier 1 | ||||||
| SLO Cultivation, Inc. | Temporary License Number: TML18-0006716 | Carpinteria | 22/02/2019 | Medical cultivation Small Indoor, Mixed Light, Tier 1 | ||||||
| SLO Cultivation, Inc. | Temporary License Number: TML18-0006723 | Carpinteria | 03/03/2019 | Medical cultivation Small Indoor, Mixed Light, Tier 1 | ||||||
| SLO Cultivation, Inc. | Temporary License Number: TML18-0006724 | Carpinteria | 03/03/2019 | Medical cultivation Small Indoor, Mixed Light, Tier 1 | ||||||
| SLO Cultivation, Inc. | Temporary License Number: TML18-0006725 | Carpinteria | 03/03/2019 | Medical cultivation Small Indoor, Mixed Light, Tier 1 | ||||||
| SLO Cultivation, Inc. | Temporary License Number: TML18-0006726 | Carpinteria | 03/03/2019 | Medical cultivation Small Indoor, Mixed Light, Tier 1 | ||||||
-39-
| SLO Cultivation, Inc. | Temporary License Number: TML18-0006727 | Carpinteria | 03/03/2019 | Medical cultivation Small Indoor, Mixed Light, Tier 1 | ||||||
| SLO Cultivation, Inc. | Temporary License Number: TML18-0006728 | Carpinteria | 03/03/2019 | Medical cultivation Small Indoor, Mixed Light, Tier 1 | ||||||
| SLO Cultivation, Inc. | Temporary License Number: TML18-0006729 | Carpinteria | 03/03/2019 | Medical cultivation Small Indoor, Mixed Light, Tier 1 | ||||||
| SLO Cultivation, Inc. | Temporary License Number: TML18-0006730 | Carpinteria | 03/03/2019 | Medical cultivation Small Indoor, Mixed Light, Tier 1 | ||||||
| SLO Cultivation, Inc. | Temporary License Number: TML18-0006731 | Carpinteria | 03/03/2019 | Medical cultivation Small Indoor, Mixed Light, Tier 1 | ||||||
| SLO Cultivation, Inc. | Temporary License Number: TML18-0006732 | Carpinteria | 03/03/2019 | Medical cultivation Small Indoor, Mixed Light, Tier 1 | ||||||
| SLO Cultivation, Inc. | Temporary License Number: TML18-0006734 | Carpinteria | 03/03/2019 | Medical cultivation Small Indoor, Mixed Light, Tier 1 | ||||||
| SLO Cultivation, Inc. | Temporary License Number: TML18-0006735 | Carpinteria | 03/03/2019 | Medical cultivation Small Indoor, Mixed Light, Tier 1 | ||||||
| SLO Cultivation, Inc. | Temporary License Number: TML18-0006736 | Carpinteria | 03/03/2019 | Medical cultivation Small Indoor, Mixed Light, Tier 1 | ||||||
| SLO Cultivation, Inc. | Temporary License Number: TML18-0006737 | Carpinteria | 03/03/2019 | Medical cultivation Small Indoor, Mixed Light, Tier 1 | ||||||
| SLO Cultivation, Inc. | Temporary License Number: TML18-0006738 | Carpinteria | 03/03/2019 | Medical cultivation Small Indoor, Mixed Light, Tier 1 | ||||||
| SLO Cultivation, Inc. | Temporary License Number: TML18-0006739 | Carpinteria | 03/03/2019 | Medical cultivation Small Indoor, Mixed Light, Tier 1 | ||||||
| SLO Cultivation, Inc. | Temporary License Number: TML18-0006740 | Carpinteria | 03/03/2019 | Medical cultivation Small Indoor, Mixed Light, Tier 1 | ||||||
-40-
| SLO Cultivation, Inc. | Temporary License Number: TML18-0006741 | Carpinteria | 03/03/2019 | Medical cultivation Small Indoor, Mixed Light, Tier 1 | ||||||
| SLO Cultivation, Inc. | Temporary License Number: TML18-0006742 | Carpinteria | 03/03/2019 | Medical cultivation Small Indoor, Mixed Light, Tier 1 | ||||||
| SLO Cultivation, Inc. | Temporary License Number: TML18-0006743 | Carpinteria | 03/03/2019 | Medical cultivation Small Indoor, Mixed Light, Tier 1 | ||||||
| SLO Cultivation, Inc. | Temporary License Number: TML18-0006744 | Carpinteria | 03/03/2019 | Medical cultivation Small Indoor, Mixed Light, Tier 1 | ||||||
| SLO Cultivation, Inc. | Temporary License Number: TML18-0006745 | Carpinteria | 03/03/2019 | Medical cultivation Small Indoor, Mixed Light, Tier 1 | ||||||
| SLO Cultivation, Inc. | Temporary License Number: TML18-0006746 | Carpinteria | 03/03/2019 | Medical cultivation Small Indoor, Mixed Light, Tier 1 | ||||||
| SLO Cultivation, Inc. | Temporary License Number: TML18-0006747 | Carpinteria | 03/03/2019 | Medical cultivation Small Indoor, Mixed Light, Tier 1 | ||||||
| SLO Cultivation, Inc. | Temporary License Number: TML18-0006748 | Carpinteria | 03/03/2019 | Medical cultivation Small Indoor, Mixed Light, Tier 1 | ||||||
| SLO Cultivation, Inc. | Pending Processing Applicant ID: 18TMP-029647 | Carpinteria | N/A | Medical and Adult Use (for processing) | Application to be submitted during annual application process. | |||||
| SLO Cultivation, Inc. | Pending Nursery | Carpinteria | N/A | Medical and Adult Use (for nursery) | Application to be submitted during annual application process. | |||||
| SLO Cultivation, Inc. | Pending Cultivation | Carpinteria | N/A | Medical cultivation Specialty, Mixed Light, Tier 1 | Application to be submitted during annual application process. | |||||
California state and local licenses are renewed annually. Each year, licensees are required to submit a renewal application per guidelines published by the corresponding regulatory agency. While renewals are annual, there is no limit to the number of renewals a licensee may obtain. Additionally, in respect of the renewal process, provided that the requisite renewal fees are paid, the renewal application is submitted in a timely manner, and there are no material violations noted against the applicable license, SLO and the Cal Subsidiaries would expect to receive the applicable renewed licenses in the ordinary course of business. While SLO’s and the Cal Subsidiaries’ compliance controls have been developed to mitigate the risk of any material violations of a license arising, there is no assurance that the licenses will be renewed in the future in a timely manner. Any unexpected delays or costs associated with the licensing renewal process could impede the ongoing or planned operations of the Resulting Issuer and could have a material adverse effect on its business, financial condition, results of operations or prospects.
-41-
California License and Regulations
A Medicinal Retailer licenses permits the sale of medicinal cannabis and cannabis products by a medicinal cannabis patient in California who possesses a physician’s recommendation. Only certified physicians may provide medicinal marijuana recommendations.
An Adult-Use Retailer license permits the sale of cannabis and cannabis products to any individual 21 years of age or older. It does not require the individual to possess a physician’s recommendation. Under the terms of such licenses, the holder is permitted to sell adult-use cannabis and cannabis products to any person, provided the local jurisdiction permits the sale of adult use cannabis and the person presents a valid government- issued photo identification.
The Medicinal Cultivation licenses that have been granted to the Cal Subsidiaries permits commercial cannabis cultivation activity involving the planting, growing, harvesting, drying, curing, grading or trimming of cannabis. Such licenses further permit the production, labeling and packaging of a limited number of non-manufactured cannabis products (e.g., pre-rolled joints) and permit the licensee to sell cannabis to certain licensed entities (both Medical and Adult-Use licensees) within the State of California for resale or manufacturing purposes.
Adult-Use and Medicinal Distribution licenses permit cannabis related distribution activity, which means the procurement, sale, and transportation of cannabis and cannabis products between licensed entities. Distribution activity is permissible to and from certain Cresco owned and non-Cresco owned licensees.
Cresco maintains an open and collaborative relationship with the each of the four California regulatory agencies: the Bureau of Cannabis Control (BCC), Manufactured Cannabis Safety Branch of the California Department of Public Heath (MCSB), the CalCannabis Licensing Division of the California Department of Food and Agriculture (CalCannabis)) and the California Department of Tax and Fee Administration, as well as the local jurisdiction’s cannabis regulatory agencies.
In the State of California, only cannabis that is grown in the state can be sold in the state. Although California is not a vertically integrated system, the state also allows SLO and the Cal Subsidiaries to make wholesale purchase of cannabis from, or a distribution of cannabis and cannabis product to, another licensed entity within the state.
California Reporting Requirements
The State of California has selected Franwell Inc.’s METRC solution as the state’s T&T system used to track commercial cannabis activity and movement across the distribution chain (“seed-to-sale”). The METRC system is in the process of being implemented state-wide but has not yet been released. When operational, the system will allow for other third-party system integration via application programming interface (“API”). Cresco will utilize an electronic system independent of METRC that will integrate with METRC via API. T&T currently captures required data points for cultivation, distribution and retail as stipulated in the corresponding regulatory agencies’ regulations. Cresco will implement its own interim track-and-trace solution, leveraging its own internal controls and standard operating procedures, ensuring maximum compliance and transparency, until the statewide T&T solution is ready to be deployed.
California Storage and Security
To ensure the safety and security of cannabis business premises and to maintain adequate controls against the diversion, theft, and loss of cannabis or cannabis products, Cresco is required to do the following:
| • | maintain a fully operational security alarm system; • contract for security guard services; |
| • | maintain a video surveillance system that records continuously 24 hours a day; • ensure that the facility’s outdoor premises have sufficient lighting; • not dispense from its premises outside of permissible hours of operation; |
| • | store cannabis and cannabis product only in areas per the premises diagram submitted to the State of California during the licensing process; |
-42-
| • | store all cannabis and cannabis products in a secured, locked room or a vault; |
| • | report to local law enforcement within 24 hours after being notified or becoming aware of the theft, diversion, or loss of cannabis; and |
| • | to ensure the safe transport of cannabis and cannabis products between licensed facilities, maintain a delivery manifest in any vehicle transporting cannabis and cannabis products. Only vehicles registered with the BCC, that meet BCC distribution requirements, are to be used to transport cannabis and cannabis products. |
Nevada
Nevada Regulatory Landscape
Medical marijuana use was legalized in Nevada by a ballot initiative in 2000. In November 2016, voters in Nevada passed an adult use marijuana measure to allow for the sale of recreational marijuana in the state. The first dispensaries to sell adult use marijuana began sales in July 2017. The Nevada Department of Taxation (“DOT”) is the regulatory agency overseeing the medical and adult use cannabis programs. Similar to California, cities and counties in Nevada are allowed to determine the number of local marijuana licenses they will issue.
Cresco via a Unit Purchase and Sales Agreement with Lighthouse Strategies Inc. (“Lighthouse”), through their subsidiary Cresco Labs Nevada, LLC, acquired a 25% ownership interest in Nevada Business Services Group (“NBSG”) who wholly owns Paradise Wellness Center, LLC (“Paradise Wellness”) d/b/a Las Vegas Releaf and Silver State Wellness, LLC (“Silver State”), entities licensed to operate in the State of Nevada.
Nevada Licenses
NBSG is licensed to operate in the State of Nevada as a medical dispensary and retail dispensary. Silver State is licensed to operate in the State of Nevada as a cultivator. The table below lists the licenses issued to Paradise Wellness and Silver State in respect to their operations in Nevada (the “Nevada Licenses”). Under applicable laws, the licenses permit the applicable entities to cultivate, manufacture process, package, sell or purchase pursuant to the terms of the license, which is issued by the DOT under the provisions of Nevada Revised Statutes section 453A. All Nevada Licenses are, as of the date hereof, active with the State of Nevada. All licenses are independently issued for each approved activity for use at Paradise Wellness and Silver State facilities and retail location in Nevada. Silver State is currently building a 36,000 sq/ft cultivation space.40 Paradise Wellness currently operates an approximately 12,000 sq/ft processing centre and dispensary called “Releaf” located one block from the Las Vegas strip, near the SLS hotel.
Licenses in the State of Nevada
| Holding Entity |
Permit/License |
City |
Expiration/Renewal Date (if applicable) (MM/DD/YY) |
Description | ||||
| Paradise Wellness Vegas Releaf |
M63-00010 | Las Vegas | 01/01/2019 | Medical Dispensary | ||||
| P65-00126 | Las Vegas | 01/01/2019 | Recreational Dispensary | |||||
| Silver State Wellness, LLC |
2000081.MMR-301 | Las Vegas | 12/31/2018 | Clark County Limited Business License: Cultivation | ||||
All marijuana establishments must register with DOT. If applications contain all required information and after vetting by officers, establishments are issued a medical marijuana establishment registration certificate. In a local governmental jurisdiction that issues business licenses, the issuance by DOT of a medical marijuana establishment registration certificate is considered provisional until the local government has issued a business license for operation
| 40 | These statements constitute forward-looking information related to possible events, conditions or financial performance based on future economic conditions and courses of action. These statements involve known and unknown risks, assumptions, uncertainties and other factors that may cause actual results or events to differ materially. Cresco believes there is a reasonable basis for the expectations reflected in the forward-looking statements, however these expectations may not prove to be correct. |
-43-
and the establishment is in compliance with all applicable local governmental ordinances. Final registration certificates are valid for a period of one year and are subject to annual renewals after required fees are paid and the business remains in good standing. Renewal requests are typically communicated through email from DOT and include a renewal form. The renewal periods serve as an update for DOT on the licensee’s status toward active licensure. It is important to note that provisional licenses do not permit the operation of any commercial or medical cannabis activity. Only after a provisional licensee has gone through necessary state and local inspections, if applicable, and has received a final registration certificate from DOT may an entity engage in cannabis business operation.
Nevada License and Regulations
In the State of Nevada, only cannabis that is grown or produced in the state by a licensed establishment may be sold in the state. Although Nevada is not a vertically integrated system, NBSG is vertically integrated and has the capabilities to cultivate, harvest, process and sell, dispense and/or deliver cannabis and cannabis products.
The retail dispensary license and registration certificate permit NBSG to purchase marijuana from cultivation facilities, marijuana and marijuana products from product manufacturing facilities and marijuana from other retail stores, and allows the sale of marijuana and marijuana products to consumers.
The medical cultivation license permit NBSG to acquire, possess, cultivate, deliver, transfer, have tested, transport, supply or sell marijuana and related supplies to medical marijuana dispensaries, facilities for the production of edible medical marijuana products and/or medical marijuana-infused products, or other medical marijuana cultivation facilities.
The medical product manufacturing license permits NBSG to acquire, possess, manufacture, deliver, transfer, transport, supply, or sell edible marijuana products or marijuana infused products to other medical marijuana production facilities or medical marijuana dispensaries.
Nevada Reporting Requirements
The State of Nevada uses METRC as the state’s computerized T&T system used to track commercial cannabis activity and seed-to-sale. Individual licensees whether directly or through third-party integration systems are required to push data to the state to meet all reporting requirements. For the Nevada Licenses, Silver State will designate an in-house computerized seed to sale software that will integrate with METRC via API. The chosen seed-to-sale system captures the required data points for cultivation, manufacturing and retail as required in Nevada Revised Statutes section 453A. For the operating dispensary, Silver State currently uses BioTrackTHC’s seed-to-sale solution and anticipates full integration of processes through METRC.
Storage and Security
To ensure the safety and security of cannabis business premises and to maintain adequate controls against the diversion, theft, and loss of cannabis or cannabis products, Paradise Wellness and Silver State are required to do the following:
| • | be an enclosed, locked facility; |
| • | have a single secure entrance; |
| • | train employees in security measures and controls, emergency response protocol, confidentiality requirements, safe handling of equipment, procedures for handling products, as well as the differences in strains, methods of consumption, methods of cultivation, methods of fertilization and methods for health monitoring; |
| • | install security equipment to deter and prevent unauthorized entrances, which includes: |
| • | devices that detect unauthorized intrusion which may include a signal system; |
| • | exterior lighting to facilitate surveillance; |
| • | electronic monitoring including, without limitation: |
| • | at least one call-up monitor that is 19 inches or more; |
-44-
| • | a video printer capable of immediately producing a clear still photo from any video camera image; |
| • | video cameras with a recording resolution of at least 704 x 480 which provide coverage of all entrances to and exits from limited access areas and all entrances to and exits from the building and which can identify any activity occurring in or adjacent to the building; |
| • | a video camera at each point-of-sale location which allows for the identification of any person who holds a valid registry identification card, including, without limitation, a designated primary caregiver, purchasing medical marijuana; |
| • | a video camera in each grow room that can identify any activity occurring within the grow room in low light conditions; |
| • | a method for storing video recordings from the video cameras for at least 30 calendar days; |
| • | a failure notification system that provides an audible and visual notification of any failure in the electronic monitoring system; |
| • | sufficient battery backup for video cameras and recording equipment to support at least five (5) minutes of recording in the event of a power outage; and |
| • | a security alarm to alert local law enforcement of unauthorized breach of security; and |
| • | implement security procedures that: |
| • | restrict access of the establishment to only those persons/employees authorized to be there; |
| • | deter and prevent theft; |
| • | provide identification (badge) for those persons/employees authorized to be in the establishment; |
| • | prevent loitering; |
| • | require and explain electronic monitoring; and |
| • | require and explain the use of automatic or electronic notification to alert local law enforcement of an unauthorized breach of security. |
Arizona
Arizona Regulatory Landscape
In 2010, Arizona passed Ballot Proposition 203, which amended Title 36 to the Arizona Revised Statutes. This amendment added Chapter 28.1, titled the Arizona Medical Marijuana Act (the “AMMA”). The AMMA is codified in Arizona Revised Statutes (“ARS”) § 36-2801 et. Seq. The AMMA also appointed the ADHS as the regulator for the program and authorized ADHS to promulgate, adopt and enforce regulations for the AMMA. These ADHS Regulations are embodied in the Arizona Administrative Code Title 9 Chapter 17 (the “Rules”). ARS § 36-2801(11) defines a ‘‘nonprofit medical cannabis dispensary’’ as a not-for-profit entity that acquires, possesses, cultivates, manufactures, delivers, transfers, transports, supplies, sells or dispenses cannabis or related supplies and educational materials to cardholders.
The ADHS has established the MMJ Program, which includes a vertically integrated license, meaning if allocated a Medical Marijuana Dispensary Registration Certificate (“AZ Dispensary License”), entities are authorized to dispense and cultivate medical cannabis. Each AZ Dispensary License allows the holding entity to operate one on-site cultivation facility, and one off-site cultivation facility which can be located anywhere within the State of Arizona. An entity holding an AZ Dispensary License is required to file an application to renew with the ADHS on an annual basis, which must also include audited annual financial statements. While an AZ Dispensary License may not be sold, transferred or otherwise conveyed, AZ Dispensary License holders typically contract with third parties to provide various services related to the ongoing operation, maintenance and governance of its dispensary and/or cultivation facility so long as such contracts do not violate the requirements of the AMMA or the MMJ Program.
The ADHS had until April 2012 to establish a registration application system for patients and nonprofit marijuana dispensaries, as well as a web-based verification platform for use by law officials and dispensaries to verify a patient’s status as such. It also specified patients’ rights, qualifying medical conditions, and allowed out-of-state medical marijuana patients to maintain their patient status (though not to purchase cannabis).
On December 6, 2012, Arizona’s first licensed medical marijuana dispensary opened in Glendale.
In order to qualify to use medical marijuana under the AMMA, a patient is required to have a “debilitating medical condition”. Valid medical conditions include: HIV, cancer, glaucoma, immune deficiency syndrome, hepatitis C, Chron’s disease, agitation of Alzheimer’s disease, ALS, cachexia/wasting syndrome, muscle spasms, nausea, seizures, severe and chronic pain or another chronic or debilitating condition.
-45-
Arizona Licensing Requirements
In order for an applicant to receive a Dispensary Registration Certificate (a “Certificate”) they must: (i) fill out an application on the form proscribed by ADHS, (ii) submit the applying entity’s articles of incorporation and by-laws, (iii) submit fingerprints for each principal officer or board member of the applicant for a background check to exclude felonies, (iv) submit a business plan and policies and procedures for inventory control, security, patient education, and patient recordkeeping that are consistent with the AMMA and the Rules to ensure that the Dispensary will operate in compliance and (v) designate an Arizona licensed physician as the Medical Director for the Dispensary. Certificates are renewed annually so long as the Dispensary is in good standing with ADHS and pays the renewal fee and submits an independent third party financial audit.
Once an applicant has been issued a Certificate, they are allowed to establish one physical retail dispensary location, one cultivation location which is co-located at the dispensary’s retail site (if allowed by local zoning) and one additional off-site cultivation location. None of these sites can be operational, however, until the Dispensary receives an approval to operate from ADHS for the applicable site. This approval to operate requires: (i) an application on the ADHS form, (ii) demonstration of compliance with local zoning regulations, (iii) a site plan and floor plan for the applicable property, and (iv) an in-person inspection by ADHS of the applicable location to ensure compliance with the Rules and consistency with the Dispensary’s applicable policies and procedures.
On or about November 16, 2018, Cresco acquired 100% of the membership interests of AFS. AFS provides management and advisory services to Encanto, a non-profit entity that holds a vertical license to cultivate, process and dispense medical marijuana in the State of Arizona and operates a medical marijuana dispensary in Phoenix, Arizona, and owns real property used for cultivation in Salome, Arizona.
Arizona Licenses
| Holding Entity |
Permit/License |
Registration Number |
City |
Expiration/Renewal |
Description | |||||
| Encanto Green Cross Dispensary |
Medical Marijuana Dispensary Registration Certificate; Approval to Operate |
00000080DCQI00709964 | Phoenix | 08/07/19 | Approval to cultivate medical marijuana | |||||
| Encanto Green Cross Dispensary |
Medical Marijuana Dispensary Registration Certificate; Approval to Operate |
00000080DCQI00709964 | Salome | 08/07/19 | Approval to cultivate medical marijuana | |||||
The Arizona license is renewed annually. Before expiry, licensees are required to submit a renewal application. While renewals are granted annually, there is no ultimate expiry after which no renewals are permitted. Additionally, in respect of the renewal process, provided that the requisite renewal fees are paid, the renewal application is submitted in a timely manner, and there are no material violations noted against the applicable license, Cresco would expect to receive the applicable renewed license in the ordinary course of business. While Cresco’s compliance controls have been developed to mitigate the risk of any material violations of a license, there is no assurance that the license will be renewed in the future in a timely manner. Any unexpected delays or costs associated with the licensing renewal process could impede the ongoing or planned operations and could have a material adverse effect on the Resulting Issuer’s business, financial condition, results of operations or prospects.
-46-
Arizona Security Requirements for Dispensary Facilities
Any Dispensary facility (both retail and cultivation) must abide by the following security requirements: (i) ensure that access to the facilities is limited to authorized agents of the dispensary who are in possession of a dispensary agent identification card; and (ii) equip the facility with: (a) intrusion alarms and surveillance equipment, (b) exterior and interior lighting to facilitate surveillance, (c) at least one 19-inch monitor for surveillance and a video capable of printing a high resolution still image, (d) high resolution video cameras at all points of sale, entrances, exits, and limited access areas, both in and around the building, (e) 30 days’ video storage, (f) failure notifications and battery backups for the security system and (g) panic buttons inside each building.
Arizona Storage Requirements
Any Dispensary facility (both retail and cultivation) must abide by the following requirements for the storage of product: (i) product must be stored in an area that is separate from areas used to store toxic and flammable materials; (ii) product must be stored in a manner that is clean and sanitary; (iii) product must be protected from flies, dust, dirt, and any other contamination; and (iv) all surfaces and objects used in the handling and storage of product must be cleaned daily. Additionally, the Rules establish strict inventory protocols for tracking product from “seed to sale,” which requires all product to be traceable to the original plants used to grow the cannabis used in the product.
Arizona Transportation Requirements
Dispensaries may transport medical cannabis between their own sites or between their sites and another Dispensary’s site and must comply with the following Rules: (i) prior to transportation, the dispensary agent must complete a trip plan showing: (a) the name of the dispensary agent in charge of transporting the cannabis, (b) the date and start time of the trip, (c) a description of the cannabis, cannabis plants, or cannabis paraphernalia being transported; and (d) the anticipated route of transportation; (ii) during transport the dispensary agent shall: (a) carry a copy of the trip plan at all times, (b) use a vehicle with no medical cannabis identification, (c) carry a cell phone, and (d) ensure that no cannabis is visible; and (iii) Dispensaries must maintain trip plan records.
ADHS Inspections and Enforcement
ADHS may inspect a facility at any time upon five (5) days’ notice to the Dispensary. However, if someone has alleged that the Dispensary is not in compliance with the AMMA or the Rules, ADHS may conduct an unannounced inspection. ADHS will provide written notice to the Dispensary of any violations found during any inspection and the Dispensary then has 20 working days to take corrective action and notify ADHS.
ADHS must revoke a Certificate if a Dispensary: (i) operates before obtaining approval to operate a dispensary from the ADHS; (ii) dispenses, delivers, or otherwise transfers cannabis to an entity other than another dispensary with a valid dispensary registration certificate issued by the ADHS, a qualifying patient with a valid registry identification card, or a designated caregiver with a valid registry identification card; (iii) acquires usable cannabis or mature cannabis plants from any entity other than another dispensary with a valid dispensary registration certificate issued by the ADHS, a qualifying patient with a valid registry identification card, or a designated caregiver with a valid registry identification card; or (iv) if a principal officer or board member has been convicted of an excluded felony offense.
Furthermore, ADHS may revoke a Certificate if a Dispensary does not: (i) comply with the requirements of the AMMA or the Rules, or (ii) implement the policies and procedures or comply with the statements provided to the ADHS with the dispensary’s application.
New York
New York Regulatory Landscape
In July 2014, the New York Legislature and Governor enacted the Compassionate Care Act (A06357E, S07923) (the “CCA”) to provide a comprehensive, safe and effective medical marijuana program to meet the needs of New Yorkers. The program allows ten (10) “Registered Organizations” to hold vertically-integrated licenses and service qualified patients and caregivers. Limited product types are allowed in the state and smoking of cannabis flower is prohibited. The NYSDOH is the regulatory agency overseeing the medical marijuana program.
-47-
New York Licenses
Valley Ag is licensed to operate as a medical marijuana cultivator, manufacturer and retailer, as a “Registered Organization”, under applicable New York jurisdictional law. Valley Ag holds five licenses, one cultivation/manufacturing license and four dispensary licenses (collectively, the “NY Licenses”), under the CCA and Medical Use of Marihuana Regulations (Title 10, Chapter XIII, Part 1004) by the NYSDOH, permitting Valley Ag to possess, cultivate, process, transport, dispense and sell medical cannabis in the State of New York. Cresco will obtain the rights to the NY Licenses following closing of its acquisition of Valley Ag’s parent entity as described in Section 3 above.
While there are individual licenses issued for each site in New York, at present there are no material assets held by Valley Ag other than the NY Licenses. Following the acquisition of Valley Ag, the Resulting Issuer intends to develop and invest appropriate funding to develop Valley Ag into a vertically-integrated cannabis company whereby through the licenses to operate one cultivation/manufacturing facility in Middletown and four dispensaries geographically dispersed throughout the state per the CCA (in Brooklyn, Huntington, Poughkeepsie and Utica), Valley Ag will become a vertically-integrated cannabis company. Please see the table below for a list of the licenses issued to Valley Ag in New York.
New York Licenses
| Holding Entity |
Permit/License |
City |
Expiration/Renewal Date (if applicable) (MM/DD/YY) |
Description | ||||
| Valley Agriceuticals, LLC | Registration Number: MM0801M | Middletown | 07/31/2019 | Manufacturing License | ||||
| Registration Number: MM0802D | Brooklyn | 07/31/2019 | Dispensary License | |||||
| Registration Number: MM0803D | Huntington | 07/31/2019 | Dispensary License | |||||
| Registration Number: MM0804D | Poughkeepsie | 07/31/2019 | Dispensary License | |||||
| Registration Number: MM0805D | Utica | 07/31/2019 | Dispensary License |
The state licenses in New York are renewed every two years. Before the two year period ends, licensees are required to submit a renewal application per guidelines published by the NYSDOH. While renewals are granted every two years, there is no ultimate expiry after which no renewals are permitted. Additionally, in respect of the renewal process, provided that the requisite renewal fees are paid, the renewal application is submitted in a timely manner, and there are no material violations noted against the applicable license, Valley Ag would expect to receive the applicable renewed license in the ordinary course of business. While Valley Ag’s compliance controls have been developed to mitigate the risk of any material violations of a license arising, there is no assurance that Valley Ag’s licenses will be renewed in the future in a timely manner. Any unexpected delays or costs associated with the licensing renewal process could impede the ongoing or planned operations of Valley Ag and have a material adverse effect on the Resulting Issuer’s business, financial condition, results of operations or prospects.
New York Regulations
The NY Licenses permit the sale of medical cannabis products to any qualified patient who possess a physician’s recommendation. Under the terms of the NY Licenses, Valley Ag is permitted to sell NYSDOH approved medical marijuana manufactured products to any qualified patient, provided that the patient presents a valid government-issued photo identification and NYSDOH-issued Registry Identification Card proving that the patient or designated caregiver meets the statutory conditions to be a qualified patient or designated caregiver. Registry Identification Cards are valid for one year after the date the certification is signed. The card contains the recommendation from the physician and the limitation on form or dosage of medical marijuana.
-48-
In order for a physician to recommend medical marijuana, the physician must pay for and pass a NYSDOH approved physician certification training program which lasts for four hours. The content of the course includes: “pharmacology of marijuana; contraindications; side effects; adverse reactions; overdose prevention; drug interactions; dosing; routes of administration; risks and benefits; warnings and precautions; abuse and dependence; and such other components as determined by the commissioner”.
In order for a patient or registered caregiver to receive dispensed marijuana, they must be logged into the Prescription Monitoring Program (“PMP”) registry. The PMP registry is monitored by the NYSDOH and contains controlled substance prescription dispensing history and medical marijuana dispensing history to ensure that patients only receive a maximum of 30 days’ worth of dispensed product from one Registered Organization. Only registered pharmacists can dispense medical marijuana to approved patients and caregivers.
Allowable forms of medical marijuana in New York State are the following: metered liquid or oil preparations, solid and semisolid preparations (e.g. capsules, chewable and effervescent tablets, lozenges), metered ground plant preparations, topical forms and transdermal patches.
Medical marijuana may not be incorporated into food products by the Registered Organization, unless approved by the Commissioner of Health. Smoking is not an approved route of administration.
Qualifying conditions in the State of New York are the following: cancer, HIV infection or AIDS, amyotrophic lateral sclerosis (ALS), Parkinson’s disease, multiple sclerosis, spinal cord injury with spasticity, epilepsy, inflammatory bowel disease, neuropathy, Huntington’s disease, post-traumatic stress disorder or chronic pain. The severe debilitating or life threatening condition must also be accompanied by one or more of the following associated or complicating conditions: cachexia or wasting syndrome, severe or chronic pain, severe nausea, seizures, or severe or persistent muscle spasms.
In the State of New York, only cannabis that is grown and manufactured in the state can be sold in the State. New York is a vertically integrated system however it does allow Registered Organizations to wholesale manufactured products to one another. As such, Valley Ag has the ability to be vertically integrated and cultivate, harvest, process, transport, sell and dispense cannabis products. Delivery is allowed from dispensaries to patients, however the delivery plan must be pre-approved by the NYSDOH. As of the date hereof, Valley Ag has not submitted a delivery plan to the NYSDOH.
New York Reporting Requirements
The State of New York has selected BioTrackTHC’s solution as the state’s T&T system used to track commercial cannabis activity and seed-to-sale. The BioTrackTHC system is required to serve as all Registered Organizations’ patient verification system, but is optional as the Registered Organizations’ facing tracking system. Valley Ag currently uses BioTrackTHC as its seed-to-sale tracking system, but is also exploring more robust options for the future that more seamlessly integrate with its tracking systems used in other states.
Every month the NYSDOH requests a dispensing report in Excel format, via email, showing all products dispensed for the month. This is the only report Valley Ag is required to submit to the NYSDOH. All other data is pulled by the NYSDOH directly from Valley Ag’s seed-to-sale tracking system.
Storage and Security
To ensure the safety and security of cannabis business premises and to maintain adequate controls against the diversion, theft, and loss of cannabis or cannabis products, Valley Ag is required to:
| • | Maintain a security operations plan that includes the following at a minimum: |
-49-
| • | a perimeter alarm; |
| • | motion detectors; |
| • | video cameras in all areas that may contain marijuana and at all points of entry and exit, which shall be appropriate for the normal lighting conditions of the area under surveillance. The manufacturing facility or dispensing facility shall direct cameras at all approved safes, approved vaults, dispensing areas, marijuana sales areas and any other area where marijuana is being manufactured, stored, handled, dispensed or disposed of. At entry and exit points, the manufacturing facility or dispensing facility shall angle cameras so as to allow for the capture of clear and certain identification of any person entering or exiting the facility; |
| • | twenty-four hour recordings from all video cameras, which the manufacturing facility or dispensing facility shall make available for immediate viewing by the department or the department’s authorized representative upon request and shall be retained for at least 90 days. The registered organization shall provide the department with an unaltered copy of such recording upon request. If a registered organization is aware of a pending criminal, civil or administrative investigation or legal proceeding for which a recording may contain relevant information, the registered organization shall retain an unaltered copy of the recording until the investigation or proceeding is closed or the entity conducting the investigation or proceeding notifies the registered organization that it is not necessary to retain the recording; |
| • | a duress alarm, which for purposes of this section means a silent security alarm system signal generated by the entry of a designated code into an arming station in order to signal that the alarm user is being forced to turn off the system; |
| • | a panic alarm, which for purposes of this section means an audible security alarm system signal generated by the manual activation of a device intended to signal a life threatening or emergency situation requiring a law enforcement response; |
| • | a holdup alarm, which for purposes of this section means a silent alarm signal generated by the manual activation of a device intended to signal a robbery in progress; |
| • | an automatic voice dialer or digital dialer, which for purposes of this section means any electrical, electronic, mechanical, or other device capable of being programmed to send a prerecorded voice message, when activated, over a telephone line, radio or other communication system, to a law enforcement, public safety or emergency services agency requesting dispatch, or other department-approved industry standard equivalent; |
| • | a failure notification system that provides an audible, text or visual notification of any failure in the surveillance system. The failure notification system shall provide an alert to the manufacturing facility or dispensing facility within five minutes of the failure, either by telephone, email, or text message; |
| • | the ability to immediately produce a clear color still photo that is a minimum of 9600 dpi from any camera image (live or recorded); |
| • | a date and time stamp embedded on all recordings. The date and time shall be synchronized and set correctly and shall not significantly obscure the picture; and |
| • | the ability to remain operational during a power outage. |
| • | As a registered organization, ensure that any manufacturing facility and dispensing facility maintains all security system equipment and recordings in a secure location so as to prevent theft, loss, destruction or alterations; |
-50-
| • | In addition to the requirements listed in of the first bullet above, ensure that each manufacturing facility and dispensing facility shall have a back-up alarm system approved by the department that shall detect unauthorized entry during times when no employees are present at the facility and that it shall be provided by a company supplying commercial grade equipment; |
| • | As a registered organization, limit access to any surveillance areas solely to persons that are essential to surveillance operations, law enforcement agencies, security system service employees, the department or the department’s authorized representative, and others when approved by the department. A registered organization shall make available to the department or the department’s authorized representative, upon request, a current list of authorized employees and service employees who have access to any surveillance room. A manufacturing facility and dispensing facility shall keep all on-site surveillance rooms locked and shall not use such rooms for any other function; |
| • | As a registered organization, keep illuminated the outside perimeter of any manufacturing facility and dispensing facility that is operated under the registered organization’s license; |
| • | Ensure that all video recordings shall allow for the exporting of still images in an industry-standard image format (including .jpeg, .bmp, and .gif). Exported video shall have the ability to be archived in a proprietary format that ensures authentication of the video and guarantees that no alteration of the recorded image has taken place. Exported video shall also have the ability to be saved in an industry-standard file format that can be played on a standard computer operating system. A registered organization shall erase all recordings prior to disposal or sale of the facility; |
| • | As a registered organization, keep all security equipment in full operating order and test such equipment no less than semi-annually at each manufacturing facility and dispensing facility that is operated under the registered organization’s registration. Records of security tests must be maintained for five years and made available to the department upon request; |
| • | With respect to the manufacturing facility of the registered organization, it must be securely locked and protected from unauthorized entry at all times. In this regard: |
| • | The registered organization shall be responsible for ensuring the integrity of the security of the manufacturing facility and the maintenance of sanitary operations when permitting access to the facility; and |
| • | The manufacturing facility shall maintain a visitor log of all persons other than registered organization’s employees or emergency personnel responding to an emergency that access any secured areas, which shall include the name of the visitor, date, time and purpose of the visit. The visitor log shall be available to the department at all times during operating hours and upon request. |
| • | Ensure that all marijuana is stored in a secure area or location within the registered organization accessible to the minimum number of employees essential for efficient operation and in such a manner as approved by the department in advance, to prevent diversion, theft or loss; |
| • | Return marijuana to its secure location immediately after completion of manufacture, distribution, transfer or analysis; |
| • | Ensure that all medical marijuana is stored in such a manner as to protect against physical, chemical and microbial contamination and deterioration of the product; |
| • | Ensure that all approved safes, vaults and any other approved equipment or areas used for the manufacturing or storage of marijuana and approved medical marijuana products are securely locked or protected from entry, except for the actual time required to remove or replace marijuana or approved medical marijuana products; |
-51-
| • | Ensure that keys are not left in the locks or stored or placed in a location accessible to individuals who are not authorized access to marijuana or manufactured medical marijuana products; |
| • | Ensure that all security measures, such as combination numbers, passwords or biometric security systems, are not be accessible to individuals other than those specifically authorized to access marijuana or manufactured medical marijuana products; |
| • | Prior to transporting any medical marijuana, a registered organization shall complete a shipping manifest using a form determined by the department; |
| • | A copy of the shipping manifest must be transmitted to the destination that will receive the products and to the department at least two business days prior to transport unless otherwise expressly approved by the department. In this regard: |
| • | The registered organization shall maintain all shipping manifests and make them available to the department for inspection upon request, for a period of 5 years; and |
| • | Approved medical marijuana products must be transported in a locked storage compartment that is part of the vehicle transporting the marijuana and in a storage compartment that is not visible from outside the vehicle. |
| • | Ensure that its employees, when transporting approved medical marijuana products, travel directly to his or her destination(s) and shall not make any unnecessary stops in between; |
| • | As a registered organization, ensure that all approved medical marijuana product delivery times are randomized; |
| • | As a registered organization, staff all transport vehicles with a minimum of two employees. At least one transport team member shall remain with the vehicle at all times that the vehicle contains approved medical marijuana products; |
| • | Ensure that its transport team member shall have access to a secure form of communication with employees at the registered organization’s manufacturing facility at all times that the vehicle contains approved medical marijuana products; |
| • | Ensure its transport team member possesses a copy of the shipping manifest at all times when transporting or delivering approved medical marijuana products and produces it to the commissioner, the commissioner’s authorized representative or law enforcement official upon request. |
Regulation of Cannabis in Massachusetts
The Medical Use of Marijuana Program (the “MUMP”) registers qualifying patients, personal caregivers, Registered Marijuana Dispensaries (“RMD”), and RMD agents. The MUMP was established by Chapter 369 of the Acts of 2012, “An Act for the Humanitarian Medical Use of Marijuana,” following the passage of Ballot Question 3 in the 2012 general election. Registered Marijuana Dispensary certifications are vertically integrated licenses in that each RMD license entitles a license holder to one cultivation facility, one processing facility and up to three (3) dispensary locations. There is a limit of three RMD licenses per person/entity.
Massachusetts Regulatory Framework
The State of Massachusetts Department of Health regulations 105 CMR 725.000 et seq. provide a regulatory framework that requires licensed producers, which are statutorily defined as “Registered Marijuana Dispensaries”, to cultivate, process, transport and dispense medical cannabis in a vertically integrated marketplace. Patients with debilitating medical conditions qualify to participate in the program, including conditions such as cancer, glaucoma, positive status for human immunodeficiency virus (HIV), acquired immune deficiency virus (AIDS), hepatitis C,
-52-
amyotrophic lateral sclerosis (ALS), Crohn’s disease, Parkinson’s disease, and multiple sclerosis (MS) when such diseases are debilitating, and other debilitating conditions as determined in writing by a qualifying patient’s healthcare provider.
Massachusetts Licensing Requirements
The State of Massachusetts Department of Health (the “Massachusetts Department”) regulations 105 CMR 725.100 (the “Massachusetts Regulations”) delineates the licensing requirements for RMD’s in Massachusetts. Licensed entities must demonstrate the following: (i) they are licensed and in good standing with the Secretary of the Commonwealth of Massachusetts; (ii) no executive, member or any entity owned or controlled by such executive or member directly or indirectly controls more than three RMD licenses; (iii) vaporizers must be made available for sale; (iv) a RMD may not cultivate and dispense medical cannabis from more than two locations statewide; (v) all dispensary agents must be registered with the Massachusetts Department; (vi) a RMD must have a program to provide reduced cost or free marijuana to patients with documented verifiable financial hardships; (vii) one executive of a RMD must register with the Massachusetts Department of Criminal Justice Information Services on behalf of the entity as an organization user of the Criminal Offender Record Information (iCORI) system; (viii) the RMD applicant has at least $500,000 in its control as evidenced by bank statements, lines of credit or equivalent; and (ix) payment of the required application fee. In a RMD application, an applicant must also demonstrate or include: (i) the name, address date of birth and resumes of each executive of the applicant and of the members of the entity; (ii) proof of liability insurance coverage in compliance with statutes; (iii) a detailed summary of the business plan for the RMD; (iv) an operational plan for the cultivation of marijuana including a detailed summary of all policies and procedures; and (v) a detailed summary of the operating policies and procedures for the operations of the RMD including security, prevention of diversion, storage of marijuana, transportation of marijuana, inventory procedures, procedures for quality control and testing of product for potential contaminants, procedures for maintaining confidentiality as required by law, personnel policies, dispensing procedures, record keeping procedures, plans for patient education and any plans for patient or personal caregiver home delivery. A RMD applicant must also demonstrate that it has (i) a successful track record of running a business; (ii) a history of providing healthcare services or services providing marijuana for medical purposes in or outside of Massachusetts; (iii) proof of compliance with the laws of the Commonwealth of Massachusetts; (iv) complied with all laws and orders of the Commonwealth of Massachusetts; and (v) a satisfactory criminal and civil background. Upon the determination by the Massachusetts Department that a RMD applicant has responded to the application requirements in a satisfactory fashion, the RMD applicant is required to pay the applicable registration fee and shall be issued a provisional certificate of registration. Thereafter, the Massachusetts Department shall review architectural plans for the building of the RMD’s cultivation facility and/or dispensing facilities, and shall either approve, modify or deny the same. Once approved, the RMD provisional license holder shall construct its facilities in conformance with the requirements of the Massachusetts Regulations. Once the Massachusetts Department completes all inspections and issues approval for a RMD of its facilities, the Massachusetts Department shall issue a final certificate of registration to the RMD applicant. RMD final certificates of registration are valid for one year, and shall be renewed by filing the required renewal application no later than sixty days prior to the expiration of the certificate of registration.
Massachusetts Licenses
As described in Section 3 above, Cresco has entered into an agreement to acquire ownership of HHH. HHH holds a provisional certificate of registration from the Massachusetts Department of Health that will allow for cultivation, manufacturing and processing and the establishment and operation of a medical cannabis dispensary in Fall River, Massachusetts once a final certificate of registration is granted, and has the ability to apply for up to two additional such licenses. HHH has entered into host community agreements with the municipalities of Rockland, North Attleborough, and Fall River to allow for the siting of a medical cannabis dispensary, subject to site approval, and is in the process of applying for adult-use licenses from the Massachusetts Cannabis Control Commission. It is anticipated that closing of the transaction will occur in the fourth quarter of 2018 or the first quarter of 2019, subject to receipt of applicable regulatory approvals.
-53-
| Holding Entity |
Permit/License |
City |
Expiration/Renewal Date (if applicable) (MM/DD/YY) |
Description | ||||
| HHH | Provisional Certificate and Registration |
Fall River | N/A | Cultivation, manufacturing and processing and it establishes and allows operating for dispensary |
Before expiry, licensees are required to submit a renewal application. While renewals are granted annually, there is no ultimate expiry after which no renewals are permitted. Additionally, in respect of the renewal process, provided that the requisite renewal fees are paid, the renewal application is submitted in a timely manner, and there are no material violations noted against the applicable license, Cresco would expect to receive the applicable renewed license in the ordinary course of business. While Cresco’s compliance controls have been developed to mitigate the risk of any material violations of a license arising, there is no assurance that licenses will be renewed in the future in a timely manner. Any unexpected delays or costs associated with the licensing renewal process could impede the ongoing or planned operations and could have a material adverse effect on the Resulting Issuer’s business, financial condition, results of operations or prospects.
Massachusetts Dispensary Requirements
A RMD shall follow its written and approved operation procedures in the operation of its dispensary locations. Operating procedures shall include (i) security measures in compliance with the Massachusetts Regulations; (ii) employee security policies including personal safety and crime prevention techniques; (iii) hours of operation and after-hours contact information; (iv) a price list for marijuana; (v) storage protocols in compliance with state law; (vi) a description of the various strains of marijuana that will be cultivated and dispensed, and the forms that will be dispensed; (vii) procedures to ensure accurate recordkeeping including inventory protocols; (viii) plans for quality control; (ix) a staffing plan and staffing records; (x) diversion identification and reporting protocols; and (xi) policies and procedures for the handling of cash on RMD premises including storage, collection frequency and transport to financial institutions. The siting of dispensary locations is expressly subject to local/municipal approvals pursuant to state law, and municipalities control the permitting application process that a RMD must comply with. More specifically, a RMD shall comply with all local requirements regarding siting, provided, however, that if no local requirements exist, a RMD shall not be sited within a radius of five hundred feet of a school, daycare center, or any facility in which children commonly congregate. The 500-foot distance under this section is measured in a straight line from the nearest point of the facility in question to the nearest point of the proposed RMD. There is no specified numeric maximum amount that a RMD may have on its premises. The Massachusetts Regulations require that RMDs must limit their inventory of seeds, plants, and useable marijuana to reflect the projected needs of registered qualifying patients. A RMD shall only dispense to a registered qualifying patient who has a current valid certification.
Massachusetts Security Requirements
A RMD shall implement sufficient security measures to deter and prevent unauthorized entrance into areas containing marijuana and theft of marijuana at the RMD. These measures must include: (i) allowing only registered qualifying patients, caregivers, dispensary agents, authorized persons, or approved outside contractors access to the RMD facility; (ii) preventing individuals from remaining on the premises of a RMD if they are not engaging in activities that are permitted; (iii) disposing of marijuana or byproducts in compliance with law; (iv) establishing limited access areas accessible only to authorized personnel; (v) storing all finished marijuana in a secure locked safe or vault; (vi) keeping all equipment, safes, vaults or secured areas securely locked at all times; (vii) ensuring that the outside perimeter of the RMD is sufficiently lit to facilitate surveillance; and (viii) ensuring that all landscaping or foliage outside of the RMD does not allow a person to conceal themselves. A RMD shall also utilize a security/alarm system that: (i) monitors all entry and exit points and windows and doors; (ii) includes a panic/duress alarm; (iii) includes system failure notifications; (iv) includes 24 hour video surveillance of all safes, vaults, sales areas, areas where marijuana is cultivated, processed or dispensed; and (v) includes date and time stamping of all records and the ability to produce a clear, color still photo. The video surveillance system shall have the capacity to remain operational during a power outage. The RMD shall also maintain a backup alarm system with all of the capabilities of the primary system, and both systems shall be in good working order at all times and shall be inspected and tested on regular intervals.
-54-
Massachusetts Transportation
Marijuana or marijuana-infused products (“MIPs”) may only be transported by dispensary agents on behalf of a RMD: (i) between separately-owned RMDs in compliance with 725.105(B)(2) of the Massachusetts Regulations; (ii) between RMD sites owned by the same non-profit entity; (iii) between a RMD and a testing laboratory; (iv) from the RMD to the destruction or disposal site; or (v) from a RMD to the primary residences of registered qualifying patients. A RMD shall staff all transport vehicles with a minimum of two dispensary agents. At least one dispensary agent shall remain with the vehicle at all times that the vehicle contains marijuana or MIPs. Prior to leaving the origination location, a RMD must weigh, inventory, and account for, on video, all marijuana to be transported.
Marijuana must be packaged in sealed, labeled, and tamper-proof packaging prior to and during transportation. In the case of an emergency stop, a log must be maintained describing the reason for the stop, the duration, the location, and any activities of personnel exiting the vehicle. A RMD shall ensure that all delivery times and routes are randomized. Each dispensary agent shall carry his or her Massachusetts Department-issued MUMP ID Card at all times when transporting marijuana or MIPs and shall produce it to Massachusetts Department representatives or law enforcement officials upon request. Where videotaping is required when weighing, inventorying, and accounting of marijuana before transportation or after receipt, the video must show each product being weighed, the weight, and the manifest. A RMD must document and report any unusual discrepancy in weight or inventory to the Massachusetts Department and local law enforcement within 24 hours. A RMD shall report to the Massachusetts Department and local law enforcement any vehicle accidents, diversions, losses, or other reportable incidents that occur during transport, within 24 hours. A RMD shall retain all transportation manifests for no less than one year and make them available to the Massachusetts Department upon request. Any cash received from a qualifying patient or personal caregiver must be transported to a RMD immediately upon completion of the scheduled deliveries. Vehicles used in transportation must be owned, leased or rented by the RMD, be properly registered, and contain a GPS system that is monitored by the RMD during transport of marijuana and said vehicle must be inspected and approved by the Massachusetts Department prior to use.
During transit, a RMD shall ensure that: (i) marijuana or MIPs are transported in a secure, locked storage compartment that is part of the vehicle transporting the marijuana or MIPs; (ii) the storage compartment cannot be easily removed (for example, bolts, fittings, straps or other types of fasteners may not be easily accessible and not capable of being manipulated with commonly available tools); (iii) marijuana or MIPs are not visible from outside the vehicle; and (iv) all product is transported in a vehicle that bears no markings indicating that the vehicle is being used to transport marijuana or MIPs and does not indicate the name of the RMD. Each dispensary agent transporting marijuana or MIPs shall have access to a secure form of communication with personnel at the origination location at all times that the vehicle contains marijuana or MIPs.
Massachusetts Department Inspections
The Massachusetts Department or its agents may inspect a RMD and affiliated vehicles at any time without prior notice. A RMD shall immediately upon request make available to the Massachusetts Department all information that may be relevant to a Massachusetts Department inspection, and the Massachusetts Department may direct a RMD to test marijuana for contaminants. Any violations found will be noted in a deficiency statement that will be provided to the RMD, and the RMD shall thereafter submit a Plan of Correction to the Massachusetts Department outlining with particularity each deficiency and the timetable and steps to remediate the same. The Massachusetts Department shall have the authority to suspend or revoke a certificate of registration in accordance with 105 CMR 725.405 of the Regulation of adult-use cannabis in Massachusetts. Adult-use cannabis “Marijuana Establishments” are regulated in Massachusetts by the Cannabis Control Commission pursuant to 935 CMR 500.000 et seq. Pursuant to section 500.101(2), RMDs that have received a provisional or final certificate of registration are authorized to apply for a vertically-integrated Marijuana Establishment license on a priority basis over new applicants without a RMD certification. The same application requirements exist for a Marijuana Establishment license as a RMD application, and each owner, officer or member must undergo background checks and fingerprinting with the Cannabis Control Commission. Applicants must submit the location and identification of each site, and must establish a property interest in the same, and the applicant and the local municipality must have entered into a host agreement authorizing the
-55-
location of the adult-use Marijuana Establishment within the municipality, and said agreement must be included in the application. Applicants must include disclosure of any and all regulatory actions against it by the Commonwealth of Massachusetts, as well as the civil and criminal history of the applicant and all owners, officers, principals or members. The application must include the RMD applicant’s plans for separating medical and adult-use operations, proposed timeline for achieving operations, liability insurance, business plan, and a detailed summary describing and/or updating or modifying the RMD’s existing medical marijuana operating policies and procedures for adult-use including security, prevention of diversion, storage, transportation, inventory procedures, quality control, dispensing procedures, personnel policies, record keeping, maintenance of financial records and employee training protocols.
The adult-use license application process commenced on April 1, 2018 for existing RMD license holders, and will commence for all non-RMD license holders on July 1, 2018. Existing RMD license holders that timely applied for a adult-use license on or before April 1, 2018 are eligible to receive three adult-use licenses per medical RMD license. Namely, one integrated RMD medical license is eligible, if awarded by the Cannabis Control Commission, to receive three adult-use licenses as follows: one for cultivation, one for processing and one for dispensary. Additionally, there is a 100,000 square foot cultivation canopy for adult-use licenses; however, there is no canopy restriction for RMD license holders relative to their cultivation facility.
Regulation of Cannabis in Maryland
The Maryland Medical Cannabis Commission (the “MMCC”) grants medical cannabis grower, processor, dispensary and transportation licenses. A licensee may hold a license in each category to obtain vertical integration. The applicant must first seek pre-approval from the MMCC in order to be granted a license. As part of the pre-approval application, the applicant must submit information related to its operations; safety and security; medical cannabis professionalism; retail management factors; business and economic factors; and other additional factors that may apply.
Maryland Licensing Requirements
In order to become a licensed medical cannabis dispensary, each applicant must submit an application detailing the location of the proposed dispensary, the personal details of each principal officer or director, and operating procedures the dispensary will use. All owners, members, shareholders, officers, and directors of dispensary holding a 5% or greater interest in the company must undergo a criminal and financial background checks. All employee, volunteers and personnel who will be working in the dispensary with access to the non-public areas are required to undergo background checks and register as a dispensary agent with the MMCC.
Maryland Licenses
As described in Section 3 above, in connection with the AFS transaction, Cresco will enter into a financing and consulting arrangement with AFS Maryland. AFS Maryland holds a processing license for medical cannabis issued by the Maryland Medical Cannabis Commission. Under the arrangement, Cresco will provide funding and consulting services to support the development and operations of AFS Maryland and will receive interest and fee payments and the right to take assignment of the membership interests of AFS Maryland in lieu of receiving such payments as and when permitted by applicable law.
| Holding Entity |
Permit/License |
City |
Expiration/Renewal Date (if applicable) (MM/DD/YY) |
Description | ||||
| AFS Maryland | P-17-00010 | Snow Hill | 10/03/19 | Business License for an establishment |
Maryland Reporting Requirements
Once licensed, the medical cannabis dispensary is required to submit to the MMCC quarterly reports including the following information: (i) the number of patients served; (ii) the county of residence of each patient served; (iii) the medical condition for which medical cannabis was recommended; (iv) the type and amount of medical cannabis dispensed; and (v) if available, a summary of clinical outcomes, including adverse events and any cases of suspected diversion. The medical cannabis dispensary must not include any patient personal information in the quarterly report.
-56-
Maryland Inspections
Licensees must be inspected by the MMCC prior to receiving approval from the MMCC to be authorized to begin cultivation, processing, and dispensing. Licensees are eligible to apply to renew their license every two years during which time a full inspection of the facility is performed. Spot-inspections may be performed at the dispensary at any time and without advance notice.
Maryland Safety and Security Requirements
As part of the medical cannabis dispensary application, the applicant must provide information about the dispensary’s operating procedures consistent with the oversight regulations established by the MMCC, including the following: (i) storage of cannabis and products containing cannabis only in enclosed and locked facilities; (ii) security features and procedures; (iii) how the dispensary will prevent diversion; and (iv) safety procedures. As part of the safety and security requirements, the applicant must detail how the premises will be constructed to prevent unauthorized entry, including a designation of a secured room that meets high-security requirements. The applicant must describe how it would train all registered dispensary agents on safety procedures, including responding to: (i) a medical emergency; (ii) a fire; (iii) a chemical spill; and (iv) a threatening event including: (a) an armed robbery, (b) an invasion, (c) a burglary, or (d) any other criminal incident.
The applicant must describe its security and surveillance plan with information including the following: (i) an alarm system that covers all perimeter entry points, windows, and portals at the premises that: (a) will be continuously monitored; (b) detects smoke and fire capabilities; (c) detects power loss capabilities; (d) includes panic alarm devices mounted at convenient, readily-accessible locations through the licensed premises; (e) inclusion of a second, independent alarm system to protect where records are stored on-and off-site and where any secure room holds medical cannabis; (f) equipped with auxiliary power to continue operation for at least 48 hours; (ii) a video surveillance that: (a) records continuously for 24 hours per day for 365 days a year without interruption, (b) has cameras in fixed places that allow for the clear facial identification and of activities in the controlled areas of the premises, including where medical cannabis is packaged, tested, processed, stored, or dispensed, (c) has the capability of recording clear images and displays the time and date of the recording, and (d) demonstrates a plan for retention of recordings for at least 30 days.
Following licensure, no major renovation or modification may be undertaken without notification to the MMCC. Other than while the dispensary is open for business and one hour before and one hour after, the medical cannabis inventory must be stored in the secure room.
Maryland Operating Requirements
As part of the dispensary application, the applicant must provide information about the dispensary’s operations, including the following: (i) communication systems; (ii) facility odour mitigation; and (iii) back-up systems for all cultivation and processing systems. The applicant must establish a standard operating procedure of all aspects of the receipt, storage, packaging, labelling, handling, tracking, and dispensing of products containing medical cannabis and medical cannabis waste.
In addition, the applicant must provide information about the dispensary’s medical cannabis professionalism, including the following information: (i) experience, knowledge, and training in training dispensary agents in the science and use of medical cannabis; and (ii) use of a clinical director (optional).
The applicant must also provide information about the dispensary’s retail management operations, including the following: (i) a detailed plan to preserve the quality of the medical cannabis; (ii) a plan to minimize any negative impact on the surrounding community and businesses; (iii) a detailed inventory control plan; and (iv) a detailed medical cannabis waste disposal plan.
-57-
The business and economic factors of the dispensary business must also detail the following information: (i) a business plan demonstrating a likelihood of success, demonstrating sufficient business ability and experience on the part of the applicant, and providing for appropriate employee working conditions, benefits, and training; (ii) demonstration of adequate capitalization; and (iii) a detailed plan evidencing how the dispensary will enforce the alcohol and drug free workplace policy.
Additional information the applicant must also provide includes the following: (i) demonstration of Maryland residency among the owners and investors; (ii) evidence that the applicant is not in arrears regarding any tax obligation in Maryland or other jurisdictions; and (iii) the medical cannabis extracts and medical cannabis-infused products proposed to be dispensed with proposed cannabinoid profiles, including varieties with high cannabidiol content, and the varieties of routes of administration.
Maryland Record Keeping and Inventory Tracking
Maryland requires use of a seed-to-sale tracking system operated by METRC. Licensees must create and use a perpetual inventory control system that identifies and tracks the stock of medical cannabis from the time it is delivered or produced to the time it is delivered to a patient or qualified caregiver. The applicant must describe how it will assure the integrity of the electronic manifest and inventory control system and that a cannabis transportation agent will continue the chain of custody to a dispensary agent.
The applicant must retain attendance records and ensure dispensary agents are trained on the record retention and standard operating procedure. MMCC regulators have the authority to audit the records of licensees to ensure they comport with the reporting in METRC.
Maryland Transportation
Only licensed medical cannabis growers, processors and authorized secure transportation companies may transport business-to-business packages containing medical cannabis. Dispensaries are not authorized to pick up medical cannabis products from licensed growers or processors. Owners and employees of secure transportation companies must register as transportation agents with the MMCC by undergoing criminal and financial background checks, and they must carry identification cards evidencing that they hold current registration at all times while in possession of medical cannabis. Transportation agents must possess a current, valid driver’s license and may not wear any clothing or symbols that indicate ownership or possession of medical cannabis while on duty. Medical cannabis transport vehicles must be approved by the MMCC and shall display current registration from the state, be insured, and may not display any sign or illustration related to medical cannabis or a licensee.
Electronic manifests must accompany all shipments to record the chain of custody and includes (i) the name and address of the shipping licensee; (ii) the shipping licensee’s shipment identification number; (iii) the weight and description of each individual package that is part of the shipment, and the total number of individual packages; (iv) the name of the licensee agent that prepared the shipment; (v) the name and address of the receiving licensee; (vi) any special handling or storage instructions; (vii) the date and time the shipment was prepared; (viii) the date and time the package was placed in the secure transport vehicle; and (ix) a listing of any other people who had custody or control over the shipment, and the person’s identity, circumstances, duration and disposition.
Dispensary licensees in Maryland are authorized to perform home delivery directly to patients. To do so, the dispensary must (i) independently verify the patients identification and registration status; (ii) enter the transaction in METRC prior to delivery; (iii) perform the delivery through a registered dispensary agent; and (iv) confirm that the transaction otherwise complies with all other requirements regarding the sale of medical cannabis under applicable regulations. The Policy Committee of the MMCC recently recommended several changes to the home delivery rules that would require all home delivery be performed using a secure medical cannabis transport vehicle.
Cresco Compliance Program
Cresco oversees, maintains, and implements a compliance program in conjunction with its operations in each jurisdiction. In addition to Cresco’s robust legal and compliance departments, Cresco also has local
-58-
regulatory/compliance counsel engaged in every jurisdiction (state and local) in which it operates. The Facility Directors and / or Compliance Managers for each jurisdiction serve as the liaison to state and local regulators during both regular business hours and after hours. The compliance department is responsible for ensuring operations and employees strictly comply with applicable laws, regulations and licensing conditions and ensure that operations do not endanger the health, safety or welfare of the community. The Facility Director and / or the Compliance Manager for each location coordinate with each operational unit within each facility to ensure that the operation and all employees are following and complying with Cresco’s written security procedures and all regulatory compliance standards.
In conjunction with Cresco’s human resources and operations departments, the compliance and quality departments help oversee and implement training for all employees, including on the following topics:
| • | Compliance with state and local laws |
| • | Dispensing procedures |
| • | Security & safety policies and procedures |
| • | Inventory control |
| • | Track-and-Trace training session |
| • | Quality control |
| • | Transportation procedures |
Cresco’s compliance program emphasizes security and inventory control to ensure strict monitoring of cannabis and inventory from delivery by a licensed distributor to sale or disposal. Only authorized, properly trained employees are allowed to access Cresco’s computerized seed-to-sale system.
Cresco’s compliance department and legal team, comprised of in-house and local outside counsel, monitors all compliance notifications from the regulators and inspectors in each market, timely resolving any issues identified. The team maintains records of all compliance notifications received from the state regulators or inspectors and how and when the issue was resolved Cresco has created comprehensive standard operating procedures that include detailed descriptions and instructions for receiving shipments of inventory, inventory tracking, recordkeeping and record retention practices related to inventory, as well as procedures for performing inventory reconciliation and ensuring the accuracy of inventory tracking and recordkeeping. Cresco maintains accurate records of its inventory at all licensed facilities. Adherence to Cresco’s standard operating procedures is mandatory and ensures that Cresco’s operations are compliant with the rules set forth by the applicable state and local laws, regulations, ordinances, licenses and other requirements. Training on these standard operating procedures is mandatory by all employees and defined by function and role.
In addition to the above disclosure, please see Section 17 below for further risk factors associated with the operations of Cresco and the Resulting Issuer.
Compliance Software
The Corporation utilizes an enterprise compliance platform, which integrates the inventory management program of the Manager and Licensed Entities with the software’s compliance checklists and auditing features to facilitate continued compliance with state and local requirements. The software features a robust auditing system that allows for both internal as well as third-party compliance auditing, covering all state and municipal, facility and operational requirements. The enterprise compliance platform offers building tools to facilitate the implementation and maintenance of compliant operations and tracks all required licensing maintenance criteria, which include countdown features and automatically generated reminders for initiating renewals and required reporting
-59-
Compliance Program
Cresco has developed and continues to refine a robust compliance program designed to ensure operational and regulatory requirements continue to be satisfied, and has worked closely with outside counsel, (“Regulatory Counsel”) to assist Cresco in the development of compliance procedures which will assist the Resulting Issuer monitor its compliance with U.S. state law on an ongoing basis. Cresco will continue to work closely with Regulatory Counsel and other compliance experts, and is evaluating the engagement of one or more independent third party providers to further develop, enhance and improve its compliance and risk management and mitigation processes and procedures in furtherance of continued compliance with the complex regulatory frameworks of the states to the jurisdiction of which Cresco’s operations are subject. The internal compliance program currently in place includes continued monitoring by managers and executives of Cresco and its subsidiaries to ensure that all operations conform to and comply with required laws, regulations and operating procedures. Cresco further requires its operating subsidiaries to report and disclose all instances of non-compliance, regulatory, administrative, or legal proceedings that may be initiated against them.
| 5. | Selected Consolidated Financial Information |
The following table sets out certain selected financial information of Cresco in summary form for the year ended December 31, 2017 and the six-month period ended June 30, 2018. This selected financial information has been derived from and should be read in conjunction with the Cresco’s financial statements for the year ended December 31, 2017 and the three and six month periods ended June 30, 2018 and June 30, 2017, which are attached to this Listing Statement as Schedules “A” and “B”, respectively:
| As at and for the six months ended June 30, 2018 (US$) |
As at and for the year ended December 31, 2017 (US$) |
|||||||
| Current assets |
45,619,603 | 34,065,117 | ||||||
| Total assets |
74,465,442 | 41,617,307 | ||||||
| Current liabilities |
5,359,875 | 4,093,507 | ||||||
| Total liabilities |
7,253,951 | 5,680,737 | ||||||
| Members’ equity |
67,211,491 | 35,936,570 | ||||||
| Revenue |
13,566,187 | 10,982,313 | ||||||
| Net Income (Loss) Attributable to Controlling Interest |
1,981,487 | (3,175,353 | ) | |||||
Description of Certain Existing and Recent Indebtedness
On December 30, 2016, Cresco entered into promissory note agreements (“Promissory Notes”) with its founders in the aggregate amount of $562,500. The terms of the Promissory Notes include maturity on the first anniversary of issuance with the Resulting Issuer having an election to extend the maturity date for up to two successive 12-month periods, but in no event beyond the third anniversary date of the Promissory Notes. Additionally, the Promissory Notes accrue interest at an annual rate of 10%. Cresco elected to extend the maturity date for two 12-month periods.As of June 30, 2018 and December 31, 2017, the amount outstanding under these agreements was $187,500 and $328,125, respectively.
On November 19, 2018, Cresco entered in a definitive agreement with HHH and a related real estate entity to acquire 100% of the shares and equity interests of those entities in exchange for cash and assumption of an outstanding mortgage loan of approximately $850,000 on certain real property. Cresco’s obligation to assume the mortgage on the real property is contingent on the closing of the transaction, which remains subject to regulatory approval.
-60-
| 6. | Management’s Discussion and Analysis |
| Annual | & Interim MD&A |
Please see attached the Cresco MD&A for the year ended December 31, 2017 and the three and six months ended June 30, 2018, attached hereto as Schedule “C”.
| 7. | Market for Securities |
Cresco’s securities were not listed prior to completion of the Business Combination. The Subordinate Voting Shares are listed for trading on the CSE under the symbol “CL”.
| 8. | Consolidated Capitalization |
The following table summarizes the share capital of Cresco, Randsburg and the Resulting Issuer both prior and after giving effect to the SR Offering and the Business Combination, including the consolidation of the pre-transaction Randsburg Shares:
| RANDSBURG (EXISTING PRIOR TO THE RANDSBURG SHARE CONSOLIDATION PRE-CONSOLIDATION) |
||||
| Security |
Number | |||
| Randsburg Shares |
210,327,446 | |||
| Randsburg Warrants |
43,333,333 | |||
| CRESCO(1) (PRIOR TO THE BUSINESS COMBINATION) |
||||
| Security |
Number | |||
| Founder Units |
33,000,000 | |||
| Class A Units |
94,400,000 | |||
| Class B Units |
14,195,556 | |||
| Class C Units |
17,020,000 | |||
| Class D Units |
5,184,859 | |||
| Class E Units |
14,006,523 | |||
| Class F Units(2) |
75,598,111 | |||
| Cresco Warrants(3) |
4,100,000 | |||
| Cresco Options(4) |
20,000,000 | |||
| (1) | All Cresco Units (Founder Units through Class F Units) convert in the Business Combination into either Cresco Redeemable Units or Proportionate Voting Shares, all of which are convertible to Subordinate Voting Shares on a 1:1 basis. |
| (2) | Includes 16,384,065 Class F Units issuable, in the aggregate, to the vendors in the pending acquisitions of Valley Ag, MedMar Inc. and Cresco Labs TINAD d/b/a PDI Medical. |
| (3) | 4,000,000 warrants exercisable at C$6.10 per Subordinate Voting Share, issuable in connection with Valley Ag acquisition, 2,000,000 million of which are contingent on the achievement of certain performance milestones. 100,000 warrants exercisable at C$1.30 per Subordinate Voting Share. |
| (4) | 17,960,000 options outstanding at a blended average exercise price of C$2.26 per Subordinate Voting Share. 2,040,000 options reserved for future grants. |
-61-
| RESULTING ISSUER (POST BUSINESS COMBINATION, SUBSCRIPTION RECEIPT FINANCING AND RANDSBURG CONSOLIDATION) |
||||
| Security |
Number | |||
| Super-Voting Shares(5) |
500,000 | |||
| Cresco Members converting to Proportionate Voting Shares (presented on an as converted to Subordinate Voting Shares basis at 1:200)(6) |
100,727,910 | (7) | ||
| Cresco Members holding Cresco Redeemable Units(8) |
143,690,687 | |||
| Subordinate Voting Shares issued on the conversion of Cresco Canada Shares in the Business Combination |
8,990,819 | |||
| Subordinate Voting Shares issued to participants in the SR Offering |
12,624,054 | |||
| Proportionate Voting Shares issuable in pending acquisitions |
3,278,510 | |||
| Subordinate Voting Shares held by existing Randsburg Shareholders |
258,824 | |||
| Basic Shares Outstanding (on an as converted to Subordinate Voting Share basis) |
270,070,803 | |||
| Resulting Issuer Replacement Options(9) |
20,000,000 | |||
| Cresco Replacement Warrants(10) |
4,100,000 | |||
| Existing Warrants(11) |
53,325 | |||
| Broker Warrants from Subscription Receipt Financing(12) |
343,745 | |||
| Fully-Diluted Outstanding |
294,567,873 | |||
| (5) | Each carrying 2,000 votes. In the aggregate, the Super Voting Shares represent approximately 77% voting control upon closing of the Business Combination. |
| (6) | As discussed above under the heading “Description of the Securities”, in order to maintain foreign private issuer status, certain US resident members of Cresco will receive Proportionate Voting Shares rather than SVS on a 1:200 basis. Proportionate Voting Shares carry voting and economic rights proportionate to Subordinate Voting Shares. Each Proportionate Voting Share is convertible into 200 Subordinate Voting Shares. This table presents the Proportionate Voting Shares on an as-converted basis. |
| (7) | Inclusive of 13,466,667 issuable in pending acquisitions. |
| (8) | Cresco Redeemable Units are convertible to Proportionate Voting Shares on a 200:1 basis and such Proportionate Voting Shares are convertible into Subordinate Voting Shares on a 1:200 basis. |
| (9) | 17,960,000 options outstanding at a blended average exercise price of C$2.26 per Subordinate Voting Shares. 2,040,000 options reserved for future grants. |
| (10) | 4,000,000 warrants exercisable at C$6.10 per Subordinate Voting Shares, issuable in connection with Valley Ag acquisition, 2,000,000 of which are contingent on the achievement of certain performance milestones. 100,000 warrants exercisable at C$1.30 per Subordinate Voting Share. |
| (11) | Each exercisable into one Subordinate Voting Share at a price of C$7.53. |
| (12) | Each exercisable into one Subordinate Voting Share at C$8.50. |
| 9. | Options to Purchase Securities |
The following table sets forth the aggregate number of Options of the Resulting Issuer (the “Resulting Issuer Options”) that are outstanding as of the date hereof. The Resulting Issuer Options are subject to the Equity Plan, which is described below.
| Subordinate Voting Shares Under Options Granted(1) |
Exercise Price (US$) (Range) |
Date of Grant (Range) | ||||||||
| All executive officers and directors of Cresco |
13,800,000 | $ | 0.50 - $3.75 | May 15, 2015 to September 1, 2018 | ||||||
| All other employees of any subsidiaries of Cresco |
4,160,000 | $ | 1.00 - $3.75 | September 14, 2015 to August 27, 2018 | ||||||
| (1) | The Resulting Issuer Options outstanding have a 3 or 4 year term and generally have, in the case of 3-year options, generally 1/36th of such grant vests monthly and in the case of 4-year options generally 25% of such grants vest annually. |
The Resulting Issuer has implemented the Equity Plan, the principal terms of which are described below. Also, Cresco has separate discretion to grant LTIP Units under the A&R LLC Agreement. See the description of the A&R LLC Agreement under Section 10 below.
-62-
Summary of the Equity Plan
The principal features of the Equity Plan are summarized below.
Purpose
The purpose of the Equity Plan will be to: (i) promote and retain employees, officers, consultants, advisors and directors capable of assuring the future success of the Resulting Issuer after the completion of the Business Combination (the “Resulting Issuer”) and its affiliated companies; (ii) motivate management to achieve long-range goals; and (iii) to provide compensation and opportunities for ownership and alignment of interests with the Resulting Issuer shareholders.
The Equity Plan permits the grant of (i) nonqualified stock options (“NQSOs”) and incentive stock options (“ISOs”) (collectively, “Options”); (ii) restricted stock awards; (iii) restricted stock units (“RSUs”); (iv) stock appreciation rights (“SARs”); and (v) performance compensation awards, which are referred to herein collectively as “Awards,” as more fully described below. The Equity Plan will be administered by the Resulting Issuer’s Compensation Committee, provided that, unless and until a Compensation Committee is appointed, all rights and obligations described below with respect to the Compensation Committee and the Equity Plan shall be those of the full Resulting Issuer Board. When used below, the term “Compensation Committee” will include the Resulting Issuer Board or any other committee thereof tasked with administering the Equity Plan.
Eligibility
Any of the Resulting Issuer’s employees, officers, directors, consultants (who are natural persons) are eligible to participate in the Equity Plan if selected by the Compensation Committee of the Resulting Issuer (the “Participants”). The basis of participation of an individual under the Equity Plan, and the type and amount of any Award that an individual will be entitled to receive under the Equity Plan, will be determined by the Compensation Committee based on its judgment as to the best interests of the Resulting Issuer and its shareholders, and therefore cannot be determined in advance.
The maximum number of Subordinate Voting Shares that may be issued under the Equity Plan shall be determined on a rolling basis and shall not exceed 10% of all issued and outstanding Subordinate Voting Shares of the Resulting Issuer. Any shares subject to an Award under the Equity Plan that are forfeited, cancelled, expire unexercised, are settled in cash, or are used or withheld to satisfy tax withholding obligations of a Participant, shall again be available for Awards under the Equity Plan.
In the event of any dividend, recapitalization, forward or reverse stock split, reorganization, merger, amalgamation, consolidation, split-up, split-off, combination, repurchase or exchange of Subordinate Voting Shares or other securities of the Resulting Issuer, issuance of warrants or other rights to acquire Subordinate Voting Shares or other securities of the Resulting Issuer, or other similar corporate transaction or event, which affects the Subordinate Voting Shares, or unusual or nonrecurring events affecting the Resulting Issuer, or the financial statements of the Resulting Issuer, or changes in applicable rules, rulings, regulations or other requirements of any governmental body or securities exchange or inter-dealer quotation system, accounting principles or law, the Compensation Committee may (and in some cases, shall) adjust, as appropriate in order to prevent dilution or enlargement of, the rights of Participants under the Equity Plan, to (i) the number and kind of shares which may thereafter be issued in connection with Awards, (ii) the number and kind of shares issuable in respect of outstanding Awards, (iii) the purchase price or exercise price relating to any Award or, if deemed appropriate, make provision for a cash payment with respect to any outstanding Award, and (iv) any share limit set forth in the Equity Plan.
Awards
Options
The Compensation Committee is authorized to grant Options under the Equity Plan to purchase Subordinate Voting Shares that are either ISOs meaning they are intended to satisfy the requirements of Section 422 of the Code, or
-63-
NQSOs, not intended to satisfy the requirements of Section 422 of the Code. Unless the Compensation Committee determines otherwise in the case of an Option substituted for another Option in connection with a corporate transaction, the exercise price of an Option will not be less than the fair market value (as determined under the Equity Plan) of the shares at the time of grant. Options will be subject to such terms, including the exercise price and the conditions and timing of exercise, as may be determined by the Compensation Committee and specified in the applicable award agreement. The maximum term of an Option will be ten years from the date of grant (or five years in the case of an ISO granted to a 10% shareholder). Payment in respect of the exercise of an Option may be made in cash or by check, or by such other method as the Compensation Committee may determine to be appropriate, including by surrender of unrestricted shares (at their fair market value on the date of exercise) and other cashless exercise arrangements. The Compensation Committee may, in its discretion, accelerate the vesting and exercisability of Options. Unless otherwise provided in the applicable award agreement or as may be determined by the Compensation Committee, upon a Participant’s termination of service with the Resulting Issuer the unvested portion of an Option will be forfeited.
Restricted Stock
A restricted stock award is a grant of Subordinate Voting Shares, which are subject to forfeiture restrictions during a restriction period. The Compensation Committee will determine the price, if any, to be paid by the Participant for each Subordinate Voting Shares subject to a restricted stock award. The Compensation Committee may condition the expiration of the restriction period, if any, upon: (i) the Participant’s continued service over a period of time with the Resulting Issuer or its affiliates; (ii) the achievement by the Participant, the Resulting Issuer or its affiliates of any other performance goals set by the Compensation Committee; or (iii) any combination of the above conditions as specified in the applicable award agreement. If the specified conditions are not attained, the Participant will forfeit the portion of the restricted stock award with respect to which of those conditions are not attained, and the underlying Subordinate Voting Shares will be forfeited. At the end of the restriction period, if the conditions (if any) have been satisfied, the restrictions imposed will lapse with respect to the applicable number of Subordinate Voting Shares. During the restriction period, unless otherwise provided in the applicable award agreement, a Participant will have the right to vote the shares underlying the restricted stock and dividends will be paid as determined by the Compensation Committee. The Compensation Committee may, in its discretion, accelerate the vesting and delivery of shares of restricted stock. Unless otherwise provided in the applicable award agreement or as may be determined by the Compensation Committee, upon a Participant’s termination of service with the Resulting Issuer, the unvested portion of a restricted stock award will be forfeited.
RSUs
RSUs are granted in reference to a specified number of Subordinate Voting Shares and entitle the holder to receive, on achievement of specific performance goals established by the Compensation Committee, after a period of continued service with the Resulting Issuer or its affiliates or any combination of the above as set forth in the applicable award agreement, one Subordinate Voting Share for each such Subordinate Voting Share covered by the RSU; provided, that the Compensation Committee may elect to pay cash, or part cash and part Subordinate Voting Shares in lieu of delivering only Subordinate Voting Shares. The Compensation Committee may, in its discretion, accelerate the vesting of RSUs. Unless otherwise provided in the applicable award agreement or as may be determined by the Compensation Committee, upon a Participant’s termination of service with the Resulting Issuer, the unvested portion of the RSUs will be forfeited. RSU holders will not have any shareholder rights with respect to their RSUs, including voting or dividend rights, provided that the Compensation Committee may provide for dividend equivalents, subject to applicable terms and conditions. The Compensation Committee may, in its discretion, accelerate the vesting of RSUs. Unless otherwise provided in the applicable award agreement or as may be determined by the Compensation Committee, upon a Participant’s termination of service with the Resulting Issuer, the unvested portion of an RSU award will be forfeited.
Stock Appreciation Rights
An SAR entitles the recipient to receive, upon exercise of the SAR, the increase in the fair market value of a specified number of Subordinate Voting Shares from the date of the grant of the SAR and the date of exercise payable in Subordinate Voting Shares. Any grant may specify a vesting period or periods before the SAR may become exercisable and permissible dates or periods on or during which the SAR shall be exercisable. No SAR may be
-64-
exercised more than ten years from the grant date. Upon a Participant’s termination of service, the same general conditions applicable to Options as described above would be applicable to the SAR. The Compensation Committee may, in its discretion, accelerate the vesting of SARs. Unless otherwise provided in the applicable award agreement or as may be determined by the Compensation Committee, upon a Participant’s termination of service with the Resulting Issuer, the unvested portion of an SAR will be forfeited.
General
The Compensation Committee may impose restrictions on the grant, exercise or payment of an Award as it determines appropriate. Generally, Awards granted under the Equity Plan shall be nontransferable except by will or by the laws of descent and distribution.
In general, no Participant shall have any rights as a shareholder with respect to Subordinate Voting Shares covered by Options, SARs, or RSUs, unless and until such Awards are settled in Subordinate Voting Shares.
No Option (or, if applicable, SARs) shall be exercisable, no Subordinate Voting Shares shall be issued, no certificates for Subordinate Voting Shares shall be delivered and no payment shall be made under the Equity Plan except in compliance with all applicable laws.
The Resulting Issuer Board or the Compensation Committee may amend, alter, suspend, discontinue or terminate the Equity Plan and the Compensation Committee may amend any outstanding Award at any time; provided that (i) such amendment, alteration, suspension, discontinuation, or termination shall be subject to the approval of the Resulting Issuer’s shareholders if such approval is necessary to comply with any tax or regulatory requirement applicable to the Equity Plan (including, without limitation, as necessary to comply with any rules or requirements of applicable securities exchange), and (ii) no such amendment or termination may adversely affect Awards then outstanding without the Award holder’s permission.
In the event of a change in control, as defined in the Equity Plan, the Compensation Committee may, in its sole discretion, provide for any (or a combination) of the following to be effective upon the consummation of the event (or effective immediately prior to the consummation of the event, provided that the consummation of the event subsequently occurs):
| • | termination of the Award, whether or not vested, in exchange for cash and/or other property, if any, equal to the amount that would have been attained upon the exercise of the vested portion of the Award or realization of the Participant’s vested rights (with any applicable performance conditions deemed to be fulfilled as determined by the Compensation Committee); |
| • | the replacement of the Award with other rights or property selected by the Compensation Committee, in its sole discretion; |
| • | assumption of the Award by the successor or survivor corporation, or a parent or subsidiary thereof, or shall be substituted for by similar options, rights or awards covering the stock of the successor or survivor corporation, or a parent or subsidiary thereof, with appropriate adjustments as to the number and kind of shares and prices; |
| • | that the Award shall be exercisable or payable or fully vested with respect to all Subordinate Voting Shares covered thereby, notwithstanding anything to the contrary in the applicable award agreement; or |
| • | that the Award cannot vest, be exercised or become payable after a date certain in the future, which may be the effective date of the event. |
-65-
Tax Withholding
The Resulting Issuer may take such action as it deems appropriate to ensure that all applicable federal, state, local and/or foreign payroll, withholding income or other taxes, which are the sole and absolute responsibility of a Participant, are withheld or collected from such Participant.
| 10. | Description of the Securities |
DESCRIPTION OF SHARE CAPITAL OF THE RESULTING ISSUER
The Resulting Issuer is authorized to issue an unlimited number of Subordinate Voting Shares, an unlimited number of Proportionate Voting Shares and 500,000 Super Voting Shares. Upon completion of the transaction, the outstanding capital of the Resulting Issuer consists of: (i) 21,873,696 Subordinate Voting Shares; (ii) 520,032 Proportionate Voting Shares (which includes securities to be issued in connection with an acquisition and are convertible on a 1:200 basis into 104,006,420 Subordinate Voting Shares); and (iii) 500,000 Super Voting Shares.
Summary of Share Provisions
Subordinate Voting Shares (formerly post-consolidation common shares of Randsburg)
| Consolidation and Reclassification | Each Randsburg Share shall undergo the Randsburg Share Consolidation and each post-consolidation common share held by a shareholder of the Resulting Issuer was be reclassified into one Subordinate Voting Share. | |
| Right to Notice and Vote | Holders of Subordinate Voting Shares will be entitled to notice of and to attend at any meeting of the shareholders of the Resulting Issuer, except a meeting of which only holders of another particular class or series of shares of the Resulting Issuer will have the right to vote. At each such meeting, holders of Subordinate Voting Shares will be entitled to one vote in respect of each Subordinate Voting Share held. | |
| Class Rights & Right of First Refusal | As long as any Subordinate Voting Shares remain outstanding, the Resulting Issuer will not, without the consent of the holders of the Subordinate Voting Shares by separate special resolution, prejudice or interfere with any right attached to the Subordinate Voting Shares. Holders of Subordinate Voting Shares will not be entitled to a right of first refusal to subscribe for, purchase or receive any part of any issue of Subordinate Voting Shares, or bonds, debentures or other securities of the Resulting Issuer. | |
| Dividends | Holders of Subordinate Voting Shares will be entitled to receive as and when declared by the directors of the Resulting Issuer, dividends in cash or property of the Resulting Issuer. | |
| Participation | In the event of the liquidation, dissolution or winding-up of the Resulting Issuer, whether voluntary or involuntary, or in the event of any other distribution of assets of the Resulting Issuer among its shareholders for the purpose of winding up its affairs, the holders of Subordinate Voting Shares shall, subject to the prior rights of the holders of any shares of the Resulting Issuer ranking in priority to the Subordinate Voting Shares (including, without restriction, the Super Voting Shares) be entitled to participate rateably along with all other holders of Subordinate Voting Shares and the Proportionate Voting Shares (on an as converted to Subordinate Voting Shares basis). | |
| Changes | No subdivision or consolidation of the Subordinate Voting Shares shall occur unless, simultaneously, the Subordinate Voting Shares, the Proportionate Voting Shares and the Super Voting Shares are subdivided or consolidated in the same manner or such other adjustment is made so as to maintain and preserve the relative rights of the holders of the shares of each of the said classes. | |
| Conversion | In the event that an offer is made to purchase Proportionate Voting Shares and the offer is one which is required, pursuant to applicable securities legislation or the rules or conditions | |
-66-
| of listing of a stock exchange on which the Proportionate Voting Shares are then listed, to be made to all or substantially all the holders of Proportionate Voting Shares in a given province or territory of Canada to which these requirements apply, each Subordinate Voting Share shall become convertible at the option of the holder into Proportionate Voting Shares at the inverse of the Conversion Ratio then in effect at any time while the offer is in effect until one day after the time prescribed by applicable securities legislation for the offeror to take up and pay for such shares as are to be acquired pursuant to the offer. The conversion right may only be exercised in respect of Subordinate Voting Shares for the purpose of depositing the resulting Proportionate Voting Shares pursuant to the offer, and for no other reason. In such event, the Resulting Issuer’s transfer agent shall deposit the resulting Proportionate Voting Shares on behalf of the holder. Should the Proportionate Voting Shares issued upon conversion and tendered in response to the offer be withdrawn by shareholders or not taken up by the offeror, or should the offer be abandoned or withdrawn, the Proportionate Voting Shares resulting from the conversion shall be automatically reconverted, without further intervention on the part of the Resulting Issuer or on the part of the holder, into Subordinate Voting Shares at the Conversion Ratio then in effect. |
Take-Over Bid Protection
The Super Voting Shares are transferable only among the Founders and their respective affiliates for planning and similar purposes. The Founders have entered into an investment agreement with the Resulting Issuer whereby, upon any sale of Super Voting Shares to a third party purchaser not listed above, such Super Voting Shares will immediately be redeemed by the Resulting Issuer for their issue price. See “Super Voting Shares – Investment Agreement” below.
Additionally, as noted above, the Resulting Issuer’s articles entitle the holders of Subordinate Voting Shares to convert to Proportionate Voting Shares and tender to any take-over bid made solely to the holders of Proportionate Voting Shares.
Proportionate Voting Shares
| Right to Vote | Holders of Proportionate Voting Shares will be entitled to notice of and to attend at any meeting of the shareholders of the Resulting Issuer, except a meeting of which only holders of another particular class or series of shares of the Resulting Issuer will have the right to vote. At each such meeting, holders of Proportionate Voting Shares will be entitled to one vote in respect of each Subordinate Voting Share into which such Proportionate Voting Share could ultimately then be converted, which for greater certainty, shall initially be equal to 200 votes per Proportionate Voting Share (subject to adjustment at the discretion of the Board, depending upon the ratios necessary to preserve foreign private issuer status). | |
| Class Rights | As long as any Proportionate Voting Shares remain outstanding, the Resulting Issuer will not, without the consent of the holders of the Proportionate Voting Shares and Super Voting Shares by separate special resolution, prejudice or interfere with any right or special right attached to the Proportionate Voting Shares. Consent of the holders of a majority of the outstanding Proportionate Voting Shares and Super Voting Shares shall be required for any action that authorizes or creates shares of any class having preferences superior to or on a parity with the Proportionate Voting Shares. In connection with the exercise of the voting rights for the foregoing only, each holder of Proportionate Voting Shares will have one vote in respect of each Proportionate Voting Share held. | |
| Dividends | The holder of Proportionate Voting Shares shall have the right to receive dividends, out of any cash or other assets legally available therefor, pari passu (on an as converted basis, assuming conversion of all Proportionate Voting Shares into Subordinate Voting Shares) as to dividends and any declaration or payment of any dividend on the Subordinate Voting Shares. No dividend will be declared or paid on the Proportionate Voting Shares unless the Resulting Issuer simultaneously declares or pays, as applicable, equivalent dividends (on an as-converted to Subordinate Voting Share basis) on the Subordinate Voting Shares. | |
-67-
| Participation | In the event of the liquidation, dissolution or winding-up of the Resulting Issuer, whether voluntary or involuntary, or in the event of any other distribution of assets of the Resulting Issuer among its shareholders for the purpose of winding up its affairs, the holders of Proportionate Voting Shares will, subject to the prior rights of the holders of any shares of the Resulting Issuer ranking in priority to the Proportionate Voting Shares (including, without restriction, the Super Voting Shares), be entitled to participate rateably along with all other holders of Proportionate Voting Shares (on an as-converted to Subordinate Voting Share basis) and the Subordinate Voting Shares. | |
| Changes | No subdivision or consolidation of the Proportionate Voting Shares shall occur unless, simultaneously, the Subordinate Voting Shares, the Proportionate Voting Shares and the Super Voting Shares are subdivided or consolidated in the same manner or such other adjustment is made so as to maintain and preserve the relative rights of the holders of the shares of each of the said classes. | |
| Conversion | The Proportionate Voting Shares each have a restricted right to convert into 200 Subordinate Voting Shares (the “Conversion Ratio”), subject to adjustments for certain customary corporate changes and foreign private issuer considerations. The ability to convert the Proportionate Voting Shares is subject to a restriction that the aggregate number of Subordinate Voting Shares, Proportionate Voting Shares and Super Voting Shares held of record, directly or indirectly, by residents of the United States (as determined in accordance with Rules 3b-4 and 12g3-2(a) under the Securities Exchange Act of 1934, as amended), may not exceed forty percent (40%) (subject to adjustment) of the aggregate number of Subordinate Voting Shares, Proportionate Voting Shares and Super Voting Shares issued and outstanding after giving effect to such conversions and to a restriction on beneficial ownership of Subordinate Voting Shares exceeding certain levels. In addition, the Proportionate Voting Shares will be automatically converted into Subordinate Voting Shares in certain circumstances, including upon the registration of the Subordinate Voting Shares under the United States Securities Act of 1933, as amended. | |
|
Super Voting Shares
|
||
| Right to Vote | Holders of Super Voting Shares shall be entitled to notice of and to attend at any meeting of the shareholders of the Resulting Issuer, except a meeting of which only holders of another particular class or series of shares of the Resulting Issuer shall have the right to vote. At each such meeting holders of Super Voting Shares shall be entitled to 2,000 votes in respect of each Super Voting Share held provided that, if at any time the aggregate number of issued and outstanding (i) Cresco Corp Redeemable Shares in the capital of Cresco Corp (if applicable) and (ii) Cresco Redeemable Units in the capital of Cresco (or such securities of any successor to Cresco Corp or Cresco as may exist from time to time) beneficially owned, directly or indirectly by a holder of the Super Voting Shares (the “Holder”) and the Holder’s predecessor or transferor, permitted transferees and permitted successors, and any prior tranferor’s transferor and any prior permitted transferee’s permitted transferee (the “Holder’s Group”), divided by the aggregate number of (i) Cresco Corp Redeemable Shares (if applicable) and (ii) Cresco Redeemable Units beneficially owned, directly or indirectly by the Holders and the Holder’s Group as at the date of completion of the business combination transaction involving, among others, the Resulting Issuer, Cresco Corp and Cresco be less than 50% (the “Triggering Event”), the Holder shall from that time forward be entitled to 50 votes in respect of each Super Voting Share held. The holders of Super Voting Shares shall, from time to time upon the request of the Resulting Issuer, provide to the Resulting Issuer evidence as to such holders’ direct and indirect beneficial ownership (and that of its permitted transferees and permitted successors) of Cresco Corp. Redeemable Shares (if applicable) and Cresco Redeemable | |
-68-
| Units to enable the Resulting Issuer to determine the voting entitlement of the Super Voting Shares. For the purposes of these calculations, a Holder shall be deemed to beneficially own Cresco Corp Redeemable Shares (if applicable) held by an intermediate company or fund in proportion to their equity ownership of such company or fund. | ||
| Class Rights | As long as any Super Voting Shares remain outstanding, the Resulting Issuer will not, without the consent of the holders of the Super Voting Shares by separate special resolution, prejudice or interfere with any right or special right attached to the Super Voting Shares. Consent of the holders of a majority of the outstanding Super Voting Shares shall be required for any action that authorizes or creates shares of any class having preferences superior to or on a parity with the Super Voting Shares. In connection with the exercise of the these voting rights, each holder of Super Voting Shares will have one vote in respect of each Super Voting Share held. | |
| Dividends | The holders of the Super Voting Shares shall not be entitled to receive dividends. | |
| Participation | In the event of the liquidation, dissolution or winding-up of the Resulting Issuer, whether voluntary or involuntary, or in the event of any other distribution of assets of the Resulting Issuer among its shareholders for the purpose of winding up its affairs, the Resulting Issuer will distribute its assets firstly and in priority to the rights of holders of any other class of shares of the Resulting Issuer (including the holders of Subordinate Voting Shares and the Proportionate Voting Shares) to return the issue price of the Super Voting Shares to the holders, thereof and if there are insufficient assets to fully return the issue price to the holders of the Super Voting Shares, such holders will receive an amount equal to their pro rata share in proportion to the issue price of their Super Voting Shares along with all other holders of Super Voting Shares. The holders of Super Voting Shares shall not be entitled to receive directly or indirectly as holders of Super Voting Shares any other assets or property of the Resulting Issuer and their sole rights will be to the return of the issue price of such Super Voting Shares in accordance with this paragraph. | |
| Changes | No subdivision or consolidation of the Super Voting Shares shall occur unless, simultaneously, the Super Voting Shares, Proportionate Voting Shares and the Subordinate Voting Shares are subdivided or consolidated in the same manner, so as to maintain and preserve the relative rights of the holders of the shares of each of the said classes. | |
| Conversion | The holders of the Super Voting Shares shall have no right of conversion. | |
| Redemption Rights | Upon the occurrence of a Triggering Event, the Resulting Issuer has the right to redeem all or some of the Super Voting Shares from the Holder and Holder’s Group who caused the Triggering Event to occur, by providing two days prior written notice to the Holder and Holder’s Group of such Super Voting Shares, for an amount equal to the issue price for each Super Voting Share, payable in cash to the holders of the Super Voting Shares so redeemed. The Resulting Issuer need not redeem Super Voting Shares on a pro-rata basis among the Holders or Holder’s Group. Holders of Super Voting Shares to be redeemed by the Resulting Issuer shall surrender the certificate or certificates representing such Super Voting Shares to the Resulting Issuer at its records office duly assigned or endorsed for transfer to the Resulting Issuer (or accompanied by duly executed share transfers relating thereto). | |
| Transfer | No Super Voting Share may be transferred by the holder thereof unless such transfer is to an Immediate Family Member or a transfer for the purposes of estate or tax planning to a company or person that is wholly beneficially owned by such holder or immediate family members of such holder or which such holder or immediate family members of such holder are the sole beneficiaries thereof. In order to be effective, any transfer shall require the prior written consent of the Resulting Issuer. | |
-69-
| Investment Agreement | To supplement the rights, privileges, restrictions and conditions attached to the Super Voting Shares, the Resulting Issuer and the Founders, being the initial holders of Super Voting Shares, entered into an investment agreement effective as of the completion of the Business Combination which, among other things, provides that (i) each Super Voting Share will be transferable only to the holder’s immediate family members or an affiliated entity or a transfer to the other Founder or an entity affiliated with the other Founder, and (ii) upon any sale of Super Voting Shares to a third party purchaser not listed in clause (i), such Super Voting Shares will immediately be redeemed by the Resulting Issuer for their issue price. |
DESCRIPTION OF SHARE CAPITAL OF CRESCO CORP
The share capital of Cresco Corp consists of Cresco Corp Voting Shares and Cresco Corp Redeemable Shares. As of the date hereof no Cresco Corp Redeemable Shares are issued or outstanding.
Holders of Cresco Corp Voting Shares are entitled to receive notice of, attend and vote at meetings of the securityholders of Cresco Corp (other than meetings at which only holders of another class or series of shares are entitled to vote separately as a class or series). Each Cresco Corp Voting Share entitles the holder thereof to one vote on all matters upon which holders of Cresco Corp Voting Shares are entitled to vote.
Holders of Cresco Corp Redeemable Shares (if and when issued) are entitled to exchange or redeem their Cresco Corp Redeemable Shares for Proportionate Voting Shares pursuant to the terms specified in the articles of incorporation of Cresco Corp. Cresco Corp Redeemable Shares do not entitle the holders thereof to receive notice of, attend or vote at meetings of the securityholders.
A holder of Cresco Corp Redeemable Shares (other than the Resulting Issuer), if and when issued, has the right to cause Cresco Corp to redeem its Cresco Corp Redeemable Shares. If a holder of Cresco Corp Redeemable Shares (other than the Resulting Issuer) exercises its redemption or exchange right, Cresco Corp will repurchase for cancellation each such Cresco Corp Redeemable Share submitted for redemption or exchange in consideration for either Proportionate Voting Shares (currently, at a ratio of 1 Proportionate Voting Share for every 200 Cresco Corp Redeemable Shares exchanged) or a cash amount equal to the cash settlement amount applicable to such Cresco Corp Redeemable Share, as determined by Cresco Corp; provided that Cresco Corp may assign to the Resulting Issuer its rights and obligations to effect a redemption or exchange directly with the redeeming holder. For further details on the rights attached to Proportionate Voting Shares, please see – “Proportionate Voting Shares” above. For greater certainty, Cresco Corp or Cresco may elect to deliver Subordinate Voting Shares (currently, on a 1:1 basis) in lieu of the Proportionate Voting Shares for the Cresco Corp Redeemable Shares exchanged.
DESCRIPTION OF UNIT CAPITAL OF CRESCO
Management of Cresco
Following consummation of the Business Combination, Cresco Corp will be the sole manager of Cresco and will have the exclusive right, power and authority to manage, control, administer and operate the business and affairs and to make decisions regarding the undertaking and business of Cresco, subject to the terms of the A&R LLC Agreement and applicable laws.
A&R LLC Agreement
The following is a summary of the material provisions set forth in the A&R LLC Agreement to be entered into between Cresco and each of the Cresco Members in accordance with the provisions of the Prior LLC Agreement, which A&R LLC Agreement will amend and restate the Prior LLC Agreement and come into effect on the Closing Date.
-70-
Duration
Cresco has perpetual existence and will continue as a limited liability company until and unless Cresco is terminated or dissolved in accordance with the A&R LLC Agreement and the ILLCA.
Purpose of Cresco
The principal purpose and business of Cresco shall be to engage in any lawful act or activity for which a limited liability company may be organized under the ILLCA and to conduct such other activities as may be necessary, advisable, convenient or appropriate to promote or conduct the business of Cresco as set forth herein, including, but not limited to, entering into partnership agreements in the capacity of a general or limited partner, becoming a member of a joint venture or a limited liability company, participating in forms of syndication for investment, owning stock in corporations and the incurring of indebtedness and the granting of liens and security interests on the real and personal property of Cresco.
Management: The Manager
Cresco Corp is the sole manager of Cresco and will manage all of Cresco’s operations and activities in accordance with the A&R LLC Agreement. Cresco Corp has the capacity and authority to act as the manager of Cresco.
Subject to the terms of the A&R LLC Agreement and the ILLCA, Cresco Corp has the full and exclusive right, power and authority to manage, control, administer and operate the business and affairs and to make decisions regarding the undertaking and business of Cresco. Among other things, Cresco Corp is empowered to negotiate, execute and perform all agreements, conveyances or other instruments on behalf of Cresco, and to mortgage, charge or otherwise create a security interest over any or all of the property of Cresco or its subsidiaries, and to sell property subject to such a security interest.
The A&R LLC Agreement provides that, where Cresco Corp is permitted or required to take any action or to make a decision in its “sole discretion”, “discretion”, with “complete discretion” or any other grant of similar authority and latitude under the A&R LLC Agreement in managing Cresco’s operations and activities, Cresco Corp shall be entitled to consider only such interests and factors as it desires, including its own interests, and shall, to the fullest extent permitted by the ILLCA, have no duty or obligation (fiduciary or otherwise) to give any consideration to any interest of, or factors affecting, Cresco or the other Cresco Members.
Despite the foregoing, Cresco Corp will only be able to take certain types of actions (as set forth in the A&R LLC Agreement) if the same are approved, consented to or directed by a majority of the Cresco Members.
Capital Structure of Cresco and Cresco Corp
Upon the closing of the Business Combination, the capital of Cresco shall initially consist of three classes of units: the interest of Cresco Corp is to be represented by Common Units with the number of issued Common Units immediately following the Business Combination to be equal to the respective number of Subordinate Voting Shares issued and outstanding, provided that such Common Units held by Cresco Corp shall not entitle Cresco Corp to any exchange or redemption rights with respect to such Common Units. The interests of other Cresco Members will be represented by Common Units, pursuant to which all such other Cresco Members shall be entitled to certain exchange rights and redemption rights, as provided in the A&R LLC Agreement. Such Common Units held by such other Cresco Members are referred to herein as “Cresco Redeemable Units.” The A&R LLC Agreement shall also authorize the issuance of AO LTIP Units, FV LTIP Units, or other classes or series of membership units issued in accordance with Exhibit A of the A&R LLC Agreement (“LTIP Units”) to persons who provide services for or on behalf of Cresco, which such LTIP Units shall entitle the holder to certain rights and privileges, including the right to convert such LTIP Units to Common Units, subject to certain restrictions, qualifications and limitations, each as provided in the A&R LLC Agreement.
When the Resulting Issuer issues Subordinate Voting Shares, it may contribute all or a portion of the net proceeds to Cresco Corp in exchange for additional shares of Cresco Corp stock. Upon receipt of any such net proceeds from the
-71-
Resulting Issuer, Cresco Corp will generally contribute such net proceeds to Cresco as a capital contribution on account of its Common Units. In the event that a new class of shares in the capital of the Resulting Issuer is created, Cresco Corp may create a corresponding new class of Cresco units that has corresponding distribution rights to such new class of Resulting Issuer shares and will cause Cresco to issue new units of such class to Cresco Corp. The Resulting Issuer may contribute all or a portion of the net proceeds from the issuance of any such shares to Cresco Corp and Cresco Corp, upon receipt of such proceeds, will generally contribute such net proceeds to Cresco in exchange for units of Cresco.
If the Resulting Issuer proposes to redeem, repurchase or otherwise acquire any Subordinate Voting Shares for cash, the A&R LLC Agreement requires that Cresco Corp cause Cresco to redeem a corresponding number of Common Units held by Cresco Corp at an aggregate redemption price equal to the aggregate purchase or redemption price of the Subordinate Voting Shares being repurchased or redeemed by the Resulting Issuer (plus any expenses related thereto) and upon such other terms as are the same for the redemption by the Resulting Issuer, and the A&R LLC Agreement further requires that Cresco Corp, immediately prior to such redemption, repurchase or acquisition by the Resulting Issuer, but immediately following the redemption by Cresco, to redeem a corresponding number of shares of Cresco Corp stock held by the Resulting Issuer at an aggregate redemption price equal to the aggregate purchase or redemption price of the Subordinate Voting Shares being repurchased or redeemed by the Resulting Issuer (plus any expenses related thereto) and upon such other terms as are the same for the redemption by the Resulting Issuer.
In the event that any change is effected in the share capital of the Resulting Issuer, Cresco shall undertake all actions requested by Cresco Corp, including a reclassification, distribution, division or recapitalization of the Common Units to maintain at all times the same ratios between the number of Subordinate Voting Shares, the number of Cresco Corp shares and the number of Common Units issued and outstanding immediately prior to any such reclassification, consolidation, split, dividend of securities or other recapitalization including, without limitation, also effecting a reclassification, consolidation, split, dividend of securities or other recapitalization with respect to, as applicable, the Subordinate Voting Shares, Cresco Corp shares and Common Units.
Exchange Mechanism
A holder of Common Units (other than Cresco Corp) will have the right to cause Cresco to redeem its Common Units. If a holder of Common Units (other than Cresco Corp) exercises its exchange right, Cresco will repurchase for cancellation each such Common Unit submitted for exchange in consideration for either Proportionate Voting Shares (at a ratio of 1 Proportionate Voting Share for every 200 Common Units exchanged) or a cash amount equal to the cash settlement amount applicable to such Common Unit, as determined by Cresco Corp, provided that Cresco Corp shall have the right to complete such exchange directly with the redeeming holder or may assign to the Resulting Issuer its rights and obligations to effect an exchange directly with the redeeming holder. For greater certainty, Cresco may elect to deliver Subordinate Voting Shares (currently, on a 1:1 basis) in lieu of the Proportionate Voting Shares for the Common Units exchanged.
Any holder that causes Cresco to redeem its Common Units pursuant to the terms of the A&R LLC Agreement and otherwise fails to comply with the documentation requirements of Code Section 1446, including the requirement that such holder provide to Cresco a properly completed IRS Form W-9 or satisfy another exception as permitted within Code Section 1446, prior to the effective time of any such redemption or exchange, will generally be subject to U.S. withholding tax equal to ten percent (10%) of the fair market value of the Proportionate Voting Shares or the cash, as applicable, to be delivered to such holder pursuant to such redemption or exchange.
Additional Common Units; No Preemptive Rights
Except as described above, the A&R LLC Agreement authorizes Cresco Corp to cause Cresco to issue additional Common Units and securities convertible or exchangeable into Common Units on any terms and conditions of offering and sale as Cresco Corp in its discretion may determine, including with respect to acquisitions by Cresco of additional assets or equity interests in corporations, partnerships, limited liability companies and other entities and with respect to executive compensation. Unless otherwise determined by Cresco Corp, no person or entity shall have preemptive, preferential or any other similar right with respect to the issuances of any interest in Cresco.
-72-
LTIP Units
Cresco may issue LTIP Units to new or existing Cresco Members in exchange for services performed or to be performed on behalf of Cresco. LTIP Units are intended to qualify as “profits interests” for U.S. federal income tax purposes in Cresco. Two initial series of LTIP Units designated as AO LTIP Units and FV LTIP Units, respectively, will be established. The number of LTIP Units, AO LTIP Units and FV LTIP Units that may be issued by Cresco shall not be limited.
LTIP Units may, in the sole discretion of Cresco Corp, be issued subject to vesting, forfeiture and additional restrictions on transfer pursuant to the terms of an award, vesting or other similar agreement. The terms of any such award, vesting or similar agreement may be modified by Cresco Corp from time to time in its sole discretion, subject to any restrictions on amendment imposed by the relevant award, vesting or similar agreement or by the terms of any plan pursuant to which the LTIP Units are issued, if applicable.
Unless otherwise specified in the relevant award, vesting or similar agreement, upon the occurrence of any event specified in such an agreement resulting in either the forfeiture of any LTIP Units or the repurchase thereof by Cresco at a specified purchase price, then, upon the occurrence of the circumstances resulting in such forfeiture or repurchase by Cresco, the relevant LTIP Units shall immediately and without any further action be treated as cancelled and no longer outstanding for any purpose or as transferred to Cresco.
Upon the occurrence of certain events, including (A) Cresco making a distribution on all outstanding Common Units in Units; (B) Cresco subdividing the outstanding Common Units into a greater number of Units or combining the outstanding Common Units into a smaller number of Units; or (C) Cresco issuing any Units in exchange for its outstanding Common Units by way of reclassification or recapitalization, then Cresco Corp shall make a corresponding adjustment to the LTIP Units to maintain the same correspondence between the Common Units and LTIP Units as existed prior to the occurrence of any such actions.
A holder of LTIP Units shall have the right, at his or her option, at any time to convert all or a portion of his or her vested LTIP Units as follows:
| (1) | an AO LTIP Unit that that has become a vested LTIP Unit shall be converted into a number (or fraction thereof) of fully paid and non-assessable Common Units, giving effect to all adjustments (if any) made pursuant to terms of the A&R LLC Agreement equal to the applicable conversion factor as provided in the A&R LLC Agreement; and |
| (2) | A FV LTIP Unit that that has become a vested LTIP Unit shall be converted into a number (or fraction thereof) of fully paid and non-assessable Common Units, giving effect to all adjustments (if any) made pursuant to the terms of the A&R LLC Agreement equal to the applicable conversion factor as provided in the A&R LLC Agreement. |
If Cresco or Cresco Corp is a party to any transaction (including without limitation a merger, consolidation, unit exchange, self-tender offer for all or substantially all Common Units or other business combination or reorganization, or sale of all or substantially all of Cresco’s assets, but excluding any transaction which constitutes an event requiring an adjustment to the LTIP Units to maintain the same correspondence between the Common Units and the LTIP Units, as described above) as a result of which Common Units shall be exchanged for or converted into the right, or the holders of Common Units shall otherwise be entitled to receive cash, securities or other property or any combination thereof, then Cresco Corp shall, immediately prior to such transaction, insure the conversion of the maximum number of LTIP Units then eligible for conversion, taking into account any allocations that occur in connection with such transaction or that would occur in connection with such transaction if the assets of Cresco were sold at the applicable price of such transaction or, if applicable, at a value determined by Cresco Corp in good faith using the value attributed to the Common Units in the context of the such transaction (in which case the date of the forced LTIP Unit conversion shall be the effective date of such transaction and the conversion shall occur immediately prior to the effectiveness of such transaction).
-73-
LTIP Units will not be redeemable at the option of Cresco; provided, however, that the foregoing shall not prohibit Cresco from repurchasing LTIP Units from the holder thereof if and to the extent that such holder agrees to sell such LTIP Units.
Except as otherwise set forth in the relevant award, vesting or similar agreement or other separate agreement entered into between Cresco and an LTIP Unit holder, and subject to the terms and conditions set forth in the A&R LLC Agreement, on or at any time after an applicable LTIP Unit conversion date each LTIP Unit holder will have the right to require Cresco to redeem all or a portion of the Common Units into which such LTIP Unit holder’s LTIP Units were converted in exchange for cash, unless the terms of the A&R LLC Agreement, the relevant award, vesting or similar agreement or other separate agreement entered into between Cresco and the LTIP Unit holder expressly provide that such Common Units are not entitled to such redemption right.
Except as otherwise provided in the A&R LLC Agreement, holders of LTIP Units shall not have the right to vote on any matters submitted to a vote of the Cresco Members.
Subject to the terms of the relevant award, vesting or similar agreement or other documentation pursuant to which LTIP Units are granted, except in connection with the exercise of a redemption, a holder of LTIP Units may not transfer all or any portion of his or her LTIP Units without the prior written consent of Cresco Corp, which consent may be given or withheld in Cresco Corp’s sole and absolute discretion.
Transfer of Common Units
Except as permitted by the A&R LLC Agreement, no holder of Common Units may transfer any interest in such Common Units. The A&R LLC Agreement permits a transfer of Common Units pursuant to (i) the prior written approval of Cresco Corp; (ii) certain transactions that cause a change of control of Cresco; (iii) the exercise of exchange or redemption rights by any holder of Common Units; or (iv) certain other limited circumstances. Prior to transferring any Common Units (other than pursuant to certain transactions that cause a change of control of Cresco) the transferring holder of Common Units will cause the transferee to execute a joinder to the A&R LLC Agreement and any other agreements required pursuant to the terms of the A&R LLC Agreement. Any transfer or attempted transfer of any Common Units in violation of any provision of the A&R LLC Agreement shall be void and Cresco shall not record such transfer on its books or treat any purported transferee as the owner of such Common Units for any purpose.
In no event shall any transfer of Common Units be effective to the extent that such transfer could, in the reasonable determination of Cresco Corp:
| • | result in a violation of the United States Securities Act of 1933, as amended, or any other applicable federal, state or foreign laws; |
| • | cause an assignment under the United States Investment Company Act of 1940, as amended; |
| • | be a violation of or a default (or an event that, with notice or the lapse of time or both, would constitute a default) under, or result in an acceleration of any indebtedness under, any promissory note, mortgage, loan agreement, indenture or similar instrument or agreement to which Cresco or Cresco Corp is a party; provided that the payee or creditor to whom Cresco or Cresco Corp owes such obligation is not an affiliate of Cresco or Cresco Corp; |
| • | be a transfer to a person who is not legally competent or who has not achieved his or her majority under applicable law (excluding trusts for the benefit of minors); |
| • | cause Cresco to lose its status as a partnership for U.S. federal income tax purposes or, without limiting the generality of the foregoing, be effected on or through an “established securities market” or a “secondary market or the substantial equivalent thereof,” as such terms are used in Section 1.7704- 1 of United States Treasury Regulations; |
-74-
| • | cause Cresco or any Cresco Member or Cresco Corp to be treated as a fiduciary under the United States Employee Retirement Income Security Act of 1974, as amended; |
| • | cause Cresco (as determined by Cresco Corp in its sole discretion) to be treated as a “publicly traded partnership” or to be taxed as a corporation pursuant to Section 7704 of the Code or successor provision of the Code; or |
| • | result in Cresco having more than one hundred (100) partners, within the meaning of Treasury Regulations Section 1.7704-1(h)(1) (determined pursuant to the rules of Treasury Regulations Section 1.7704-1(h)(3)) in any taxable year that is not a “restricted taxable year” (as defined in the A&R LLC Agreement). |
Any holder that transfers its Common Units pursuant to the terms of the A&R LLC Agreement and otherwise fails to comply with the documentation requirements of Code Section 1446, including the requirement that such holder provide to Cresco a properly completed IRS Form W-9 or satisfy another exception as permitted within Code Section 1446, prior to the effective time of any such transfer, will generally be subject to U.S. withholding tax equal to ten percent (10%) of the fair market value of the consideration to be delivered to such holder pursuant to such redemption or exchange.
Power of Attorney
Each Cresco Member who is an individual, including those persons who become Cresco Members in connection with receiving any Common Units, automatically and irrevocably will appoint Cresco Corp, with full power of substitution, as that Cresco Member’s agent to execute and file documents or instruments required for, among other things, but subject in each case to the other provisions of the A&R LLC Agreement, the A&R LLC Agreement (or a joinder thereto), all instruments that Cresco Corp deems appropriate or necessary to reflect any amendment, change, modification or restatement of the A&R LLC Agreement, all conveyances and other instruments or documents which Cresco Corp deems appropriate or necessary to reflect the dissolution or liquidation of Cresco pursuant to the terms of the A&R LLC Agreement, all instruments relating to the admission, withdrawal or substitution of a Cresco Member pursuant to the terms of the A&R LLC Agreement, and any other ballots, consents, approvals, waivers, certificates and other instruments appropriate or necessary, in the reasonable judgment of Cresco Corp, to evidence, confirm or ratify any vote, consent, approval, agreement, or other action made or given by the Cresco Members in accordance with the terms of the A&R LLC Agreement.
Capital Contributions
Following the issuance of the Common Units to the Cresco Members pursuant to the adoption of the A&R LLC Agreement, the Cresco Members will not be required to make further contributions to Cresco.
Neither Cresco nor Cresco Corp is liable for the return of any capital contribution made by a Cresco Member to Cresco.
Limited Liability of the Cresco Members
Subject to the provisions of the ILLCA and of similar legislation in other jurisdictions of the United States and the A&R LLC Agreement: (i) the liability of each Cresco Member for the debts, liabilities and obligations of Cresco will be limited to the Cresco Member’s capital contribution, plus the Cresco Member’s share of any undistributed income of Cresco; and (ii) following payment of a Cresco Member’s capital contribution, such Cresco Member may be required to return amounts previously distributed to such Cresco Member in accordance with the ILLCA and the laws of the State of Illinois.
Limitation on Authority of the Cresco Members and Limited Liability
The A&R LLC Agreement states that a Cresco Member (in its capacity as a Cresco Member) does not have the authority or power to do any of the following:
-75-
| • | act for or on behalf of Cresco; |
| • | to do any act that would be binding upon Cresco; |
| • | make any expenditure on behalf of Cresco; |
| • | seek or obtain partition by court decree or operation of law of any Cresco property; or |
| • | own or use particular or individual assets of Cresco. |
The A&R LLC Agreement provides that Cresco will indemnify each Cresco Member for all liabilities incurred by the Cresco Member that arises solely by reason of such Cresco Member being a member of Cresco.
Distributions
Subject to the provisions set forth in the A&R LLC Agreement, Cresco Corp will cause distributions to be made by Cresco as follows: (i) “distributable cash” (as defined in the A&R LLC Agreement) or other funds or property legally available to the extent permitted by the ILLCA and applicable law, to the Cresco Members pro rata in accordance to each Cresco Member’s proportionate ownership interest in Cresco in amounts on terms as Cresco Corp will determine; and (ii) not less than five business days prior to the due date of a U.S. federal income tax return for an individual calendar year taxpayer, cash in an amount equal to the excess of each Cresco Member’s “assumed tax liability” (as defined in the A&R LLC Agreement) over distributions previously made to such Cresco Member with respect to each such taxable period.
In no case will Cresco be required to make a distribution if such distribution would violate the ILLCA or any other applicable law.
Amendment of the A&R LLC Agreement
The A&R LLC Agreement may be amended or modified by Cresco Corp as determined to be necessary or advisable, in the sole discretion of Cresco Corp, in connection with the adoption, implementation, modification or termination of certain equity plans by the Resulting Issuer. Subject to the right of Cresco Corp to amend the A&R LLC Agreement in connection with the adoption, implementation, modification or termination of certain equity plans by the Resulting Issuer, unless otherwise specified in the A&R LLC Agreement that a specific amendment requires the approval or action of certain persons, the A&R LLC Agreement may only be amended with the consent of Cresco Corp and Cresco Members holding a majority of the outstanding Common Units.
Merger, Sale or Other Disposition of Assets
Cresco Corp shall have the power and authority to effectuate the sale, lease, transfer, exchange or other disposition of any, all or substantially all of the assets of Cresco (including the exercise or grant of any conversion, option, privilege or subscription right or any other right available in connection with any assets at any time held by Cresco) or the merger, consolidation, reorganization or other combination of Cresco with or into another entity.
Treatment of Cresco as a Partnership for U.S. Federal Income Tax Purposes
The Cresco Members intend that Cresco be treated as a partnership for U.S. federal and, if applicable, state or local income tax purposes. Each Cresco Member and Cresco will file all tax returns and will otherwise take all tax and financial reporting positions in a manner consistent with such treatment.
Dissolution
Cresco will dissolve, and its affairs will be wound up, upon the occurrence of any of the following:
-76-
| • | the decision of Cresco Corp together with the holders of a majority of the then- outstanding Common Units entitled to vote to dissolve Cresco; |
| • | a dissolution of Cresco under the ILLCA; or |
| • | the entry of a decree of judicial dissolution of Cresco under the ILLCA. |
Except as otherwise provided in the A&R LLC Agreement, Cresco is intended to have perpetual existence. The withdrawal of a Cresco Member shall not cause a dissolution of Cresco and Cresco shall continue in existence subject to the terms and conditions of the A&R LLC Agreement.
Procedure on Dissolution
Upon dissolution of Cresco, the procedure is as follows:
| • | the liquidators shall cause a proper accounting to be made by a recognized firm of certified public accountants of Cresco’s assets, liabilities and operations through the last day of the calendar month in which the dissolution occurs or the final liquidation is completed, as applicable; |
| • | the liquidators shall cause the notice described in the ILLCA to be mailed to each known creditor of and claimant against Cresco in the manner described thereunder; |
| • | the liquidators shall pay, satisfy or discharge from Cresco funds, or otherwise make adequate provision for payment and discharge thereof (including the establishment of a cash fund for contingent liabilities in such amount and for such term as the liquidators may reasonably determine): first, all expenses incurred in liquidation; and second, all of the debts, liabilities and obligations of Cresco; and |
| • | all remaining assets of Cresco shall be distributed to the Cresco Members in accordance with the terms of the A&R LLC Agreement by the end of the taxable year during which the liquidation of Cresco occurs (or, if later, by ninety (90) days after the date of the liquidation), which shall constitute a complete return to the Cresco Members of their capital contributions to Cresco, a complete distribution to the Cresco Members of their interest in Cresco and all of Cresco’s property. To the extent that a Cresco Member returns funds to Cresco, it has no claim against any other Cresco Member for those funds. |
Withdrawal and Removal of the Manager
Cresco Corp may resign as the sole manager of Cresco at any time by giving written notice to the Cresco Members. Unless otherwise specified in the notice, the resignation shall take effect upon receipt thereof by the Cresco Members, and the acceptance of the resignation shall not be necessary to make it effective. The Cresco Members have no right under the A&R LLC Agreement to remove or replace Cresco Corp as the sole manager of Cresco. Vacancies in the position of manager occurring for any reason will be filled by Cresco Corp (or, if Cresco Corp has ceased to exist without any successor or assign, then by the holders of a majority in interest of the voting capital stock of Cresco Corp immediately prior to such cessation).
Indemnification
Under the A&R LLC Agreement, in most circumstances, Cresco will indemnify and hold harmless any person to the fullest extent permitted under the ILLCA, as the same now exists or may hereafter be amended, substituted or replaced (but, in the case of any such amendment, substitution or replacement only to the extent that such amendment, substitution or replacement permits Cresco to provide broader indemnification rights than Cresco is providing immediately prior to such amendment, substitution or replacement), against all expenses, liabilities and losses (including attorneys’ fees, judgments, fines, excise taxes or penalties) reasonably incurred or suffered by such person (or one or more of such person’s affiliates) by reason of the fact that such person is or was a Cresco Member or is or was serving at the request of Cresco as the manager, an officer, an employee or another agent of Cresco or is or was serving at the request of Cresco as a manager, member, employee or agent of another limited-liability company,
-77-
corporation, partnership, joint venture, trust or other enterprise; provided, however, that no such person shall be indemnified for actions against Cresco, the Manager or Managers or any other Cresco Members, or which are not made in good faith and not or in a manner which he or she reasonably believed to be in or not opposed to the best interests of Cresco, or, with respect to any criminal action or proceeding other than by or in the right of Cresco, had reasonable cause to believe the conduct was unlawful, or for any present or future breaches of any representations, warranties or covenants by such person or its affiliates as provided in the A&R LLC Agreement or other agreements to which Cresco is a party.
Expenses, including attorneys’ fees, incurred by any such person in defending a proceeding shall be paid by Cresco as they are incurred and in advance of the final disposition of such action, suit or proceeding, upon receipt of an undertaking by or on behalf of such person to repay such amount if it is ultimately determined by a court of competent jurisdiction that such person is not entitled to be indemnified by Cresco.
Cresco will maintain directors’ and officers’ liability insurance, or make other financial arrangements at its expense, to protect any person indemnified pursuant to the A&R LLC Agreement against certain expenses, liabilities or losses described in the A&R LLC Agreement whether or not Cresco would otherwise have the power to indemnify such person against such expenses, liabilities or losses under the provisions of the A&R LLC Agreement. Cresco shall use its commercially reasonable efforts to purchase directors’ and officers’ liability insurance (including employment practices coverage) with a carrier and in an amount determined necessary or desirable as determined in good faith by Cresco Corp.
Books and Records
Cresco shall keep, or cause to be kept, appropriate books and records with respect to Cresco’s business, including all books and records necessary to provide any information, lists and copies of documents required to be provided to each person who was a Cresco Member during each fiscal year of Cresco as is reasonably necessary for the preparation of such person’s U.S. federal and applicable state income tax returns.
Tax Matters
All decisions to make or refrain from making any tax elections will be determined by Cresco Corp. Cresco Corp is authorized to represent Cresco, at Cresco’s expense, in connection with all examinations of Cresco’s affairs by tax authorities, including resulting administrative and judicial proceedings. Each Cresco Member agrees to cooperate with Cresco Corp and to do or refrain from doing any or all things with regard to all things reasonably required by Cresco Corp to conduct such proceedings. Cresco Corp shall keep all Cresco Members fully advised on a current basis of any contacts by or discussions with the tax authorities, and the Cresco Members shall have the right to observe and participate through representatives of their own choosing (at their sole expense) in any tax proceedings.
TAX RECEIVABLE AGREEMENT
In connection with the Business Combination, Cresco Corp will enter into a tax receivable agreement with Cresco, the Cresco Members and the Cresco LTIP Unitholders (the “Tax Receivable Agreement”). Cresco Corp expects to obtain an increase in its share of the tax basis of the assets of Cresco when a Cresco Member receives cash or Subordinate Voting Shares in connection with a redemption or exchange of such Cresco Member’s Common Units for Subordinate Voting Shares or cash (such basis increase is referred to as the “Basis Adjustments”).
The Tax Receivable Agreement provides for the payment by Cresco Corp to Cresco Members and Cresco LTIP Unitholders of 85% of the amount of tax benefits, if any, that Cresco Corp actually realizes, or in some circumstances is deemed to realize, as a result of the redemption and exchange transactions described above, including increases in the tax basis of the assets of Cresco arising from such transactions, tax basis increases attributable to payments made under the Tax Receivable Agreement and deductions attributable to imputed interest and other payments of interest pursuant to the Tax Receivable Agreement. Cresco Corp expects to benefit from the remaining 15% of tax benefits, if any, that Cresco Corp may actually realize.
-78-
Cresco intends to treat such acquisition of Common Units as a direct purchase by Cresco of Common Units from a Cresco Member for U.S. federal income and other applicable tax purposes, regardless of whether such Common Units are surrendered by a Cresco Member to Cresco, Cresco Corp or the Resulting Issuer upon the exercise by Cresco Corp of its election to acquire such Common Units directly or the exercise by Cresco Corp to assign its rights to acquire such Common Units directly to the Resulting Issuer. Basis Adjustments may have the effect of reducing the amounts that Cresco Corp may otherwise owe in the future to various tax authorities. The Basis Adjustments may also decrease gains (or increase losses) on future dispositions of certain capital assets to the extent tax basis is allocated to those capital assets. The actual Basis Adjustments, as well as any amounts paid to the Cresco Members under the Tax Receivable Agreement, will vary depending on a number of factors, including:
| • | the timing of any subsequent redemptions or exchanges—for instance, the increase in any tax deductions will vary depending on the fair value, which may fluctuate over time, of the depreciable or amortizable assets of Cresco at the time of each redemption or exchange; |
| • | the price of Subordinate Voting Shares at the time of redemptions or exchanges—the Basis Adjustments, as well as any related increase in any tax deductions, is directly related to the price of Subordinate Voting Shares at the time of each redemption or exchange; |
| • | the extent to which such redemptions or exchanges are taxable—if a redemption or exchange is not taxable for any reason, increased tax deductions will not be available; and |
| • | the amount and timing of Cresco Corp’s income—the Tax Receivable Agreement generally will require Cresco Corp to pay 85% of the tax benefits as and when those benefits are treated as realized under the terms of the Tax Receivable Agreement. If Cresco Corp does not have taxable income, it generally will not be required (absent a change of control or other circumstances requiring an early termination payment) to make payments under the Tax Receivable Agreement for that taxable year because no tax benefits will have been actually realized. However, any tax benefits that do not result in realized tax benefits in a given taxable year will likely generate tax attributes that may be utilized to generate tax benefits in previous or future taxable years. The utilization of any such tax attributes will result in payments under the Tax Receivable Agreement. |
Cresco will have in effect an election under Section 754 of the Code effective for each taxable year in which a redemption or exchange of Common Units for Subordinate Voting Shares or cash occurs. These Tax Receivable Agreement payments are not conditioned upon any continued ownership interest in either Cresco or the Resulting Issuer by any Cresco Member. The rights of each Member under the Tax Receivable Agreement are assignable to transferees of its Common Units (other than Cresco Corp as transferee pursuant to subsequent redemptions or exchanges of the transferred Common Units), subject to the satisfaction of certain requirements.
For purposes of the Tax Receivable Agreement, cash savings in income and franchise taxes will be computed by comparing Cresco Corp’s actual income and franchise tax liability to the amount of such taxes that Cresco Corp would have been required to pay had there been no Basis Adjustments and had the Tax Receivable Agreement not been entered into. The Tax Receivable Agreement will generally apply to each taxable year in which the Tax Receivable Agreement remains effective, beginning with the first taxable year ending after the completion of the Business Combination. There is no maximum term for the Tax Receivable Agreement; however, the Tax Receivable Agreement may be terminated by Cresco Corp pursuant to an early termination procedure that requires Cresco Corp to pay the Cresco Members and Cresco LTIP Unitholders an agreed upon amount equal to the estimated present value of the remaining payments to be made under the Tax Receivable Agreement (calculated based on certain assumptions, including regarding tax rates and utilization of the Basis Adjustments).
The payment obligations under the Tax Receivable Agreement are obligations of Cresco Corp and not of the Resulting Issuer or Cresco. The actual timing and amount of any payments that may be made under the Tax Receivable Agreement will vary. Any payments made by Cresco Corp to Cresco Members and Cresco LTIP Unitholders under the Tax Receivable Agreement will generally reduce the amount of overall cash flow that might have otherwise been available to Cresco Corp (or to the Resulting Issuer or Cresco) and, to the extent that Cresco Corp is unable to make payments under the Tax Receivable Agreement for any reason, the unpaid amounts generally will be deferred and will accrue interest until paid by Cresco Corp.
-79-
Decisions made by Cresco Corp in the course of running its business, such as with respect to mergers, asset sales, other forms of business combinations or other changes in control, may influence the timing and amount of payments that are received by a Cresco Member or Cresco LTIP Unitholder under the Tax Receivable Agreement. For example, the earlier disposition of assets following a transaction that results in a Basis Adjustment will generally accelerate payments under the Tax Receivable Agreement and increase the present value of such payments.
The Tax Receivable Agreement provides that if (i) Cresco Corp materially breaches any of its material obligations under the Tax Receivable Agreement; (ii) certain mergers, asset sales, other forms of business combinations, or other changes of control were to occur; or (iii) Cresco Corp elects an early termination of the Tax Receivable Agreement, then Cresco Corp’s (or its successor’s) obligations under the Tax Receivable Agreement would accelerate and become due and payable, based on certain assumptions, including an assumption that Cresco Corp would have sufficient taxable income to fully utilize all potential future tax benefits that are subject to the Tax Receivable Agreement.
As a result, (i) Cresco Corp could be required to make cash payments to the Cresco Members and Cresco LTIP Unitholders that are greater than the specified percentage of the actual benefits it ultimately realizes in respect of the tax benefits that are subject to the Tax Receivable Agreement, and (ii) if Cresco Corp elects to terminate the Tax Receivable Agreement early, Cresco Corp would be required to make an immediate cash payment equal to the present value of the anticipated future tax benefits that are the subject of the Tax Receivable Agreement , which payment may be made significantly in advance of the actual realization, if any, of such future tax benefits. In these situations, Cresco Corp’s obligations under the Tax Receivable Agreement could have a material adverse effect on its or the Resulting Issuer’s liquidity and could have the effect of delaying, deferring or preventing certain mergers, asset sales, other forms of business combinations, or other changes of control. There can be no assurance that Cresco Corp will be able to finance its obligations under the Tax Receivable Agreement.
Payments under the Tax Receivable Agreement will be based on the tax reporting positions that Cresco Corp determines. If any such position is subject to a challenge by a taxing authority the outcome of which would reasonably be expected to materially affect a recipient’s payments under the Tax Receivable Agreement, then Cresco Corp will not be permitted to settle or fail to contest such challenge without the consent (not to be unreasonably withheld or delayed) of each Cresco Member that directly or indirectly owns at least 10% of the outstanding Common Units and LTIP Units. Cresco Corp will not be reimbursed for any cash payments previously made to any Cresco Member pursuant to the Tax Receivable Agreement if any tax benefits initially claimed by Cresco Corp are subsequently challenged by a taxing authority and ultimately disallowed. Instead, in such circumstances, any excess cash payments made by Cresco Corp to a Cresco Member or Cresco LTIP Unitholder will be netted against any future cash payments that Cresco Corp might otherwise be required to make under the terms of the Tax Receivable Agreement. However, Cresco Corp might not determine that it has effectively made an excess cash payment to the Cresco Members or Cresco LTIP Unitholders for a number of years following the initial time of such payment and, if Cresco Corp’s tax reporting positions are challenged by a taxing authority, it will not be permitted to reduce any future cash payments under the Tax Receivable Agreement until any such challenge is finally settled or determined. As a result, it is possible that Cresco Corp could make cash payments under the Tax Receivable Agreement that are substantially greater than its actual cash tax savings.
Payments are generally due under the Tax Receivable Agreement within a specified period of time following the filing of Cresco Corp’s U.S. federal income tax return (or, if Cresco Corp becomes a member of an affiliated or consolidated group of corporations that files a consolidated U.S. federal income tax return pursuant to Section 1501 of the Code or any provision of U.S. state or local law, then such consolidated U.S. federal income tax return) for the taxable year with respect to which the payment obligation arises, although interest on such payments will begin to accrue at a rate of LIBOR plus 100 basis points from the due date (without extensions) of such tax return. Any late payments that may be made under the Tax Receivable Agreement will continue to accrue interest at LIBOR plus 500 basis points until such payments are made, including any late payments that Cresco Corp may subsequently make because Cresco Corp did not have enough available cash to satisfy its payment obligations at the time at which they originally arose.
-80-
SUPPORT AGREEMENT
Pursuant to the support agreement entered into by and among the Resulting Issuer, Cresco Corp and Cresco (the “Support Agreement”), the Resulting Issuer will agree that, so long as any Common Units not owned by Cresco Corp or its affiliates are outstanding or Common Units are issuable pursuant to the exercise, conversion or exchange of any outstanding securities of Cresco, the Resulting Issuer shall:
| (a) | take all such actions and do all such things as are reasonably necessary or desirable to enable and permit Cresco, in accordance with applicable law, to pay and otherwise perform its obligations with respect to the satisfaction of a redemption of Common Units by a holder thereof in respect of each issued and outstanding Common Unit upon a redemption of such Common Units by Cresco and, without limiting the generality of the foregoing, take all such actions and do all such things as are necessary or desirable to enable and permit Cresco to cause to be delivered Proportionate Voting Shares, Subordinate Voting Shares and/or amounts in cash, as applicable, to the holders of Common Units in accordance with the provisions of the A&R LLC Agreement, together with an amount in cash sufficient to pay any amount to be paid in respect of unpaid distributions with respect to such Common Units (if any); |
| (b) | take all such actions and do all such things as are reasonably necessary or desirable to enable and permit Cresco Corp, if it elects to effect an exchange of Common Units directly with the holder thereof, in accordance with applicable law, to pay and otherwise perform its obligations with respect to the satisfaction of the redemption or exchange of Common Units by a holder thereof and, without limiting the generality of the foregoing, take all such actions and do all such things as are necessary or desirable to enable and permit Cresco Corp to cause to be delivered Proportionate Voting Shares, Subordinate Voting Shares and/or amounts in cash, as applicable, to the holders of Common Units in accordance with the provisions of the A&R LLC Agreement, together with an amount in cash sufficient to pay any amount to be paid in respect of unpaid distributions with respect to such Common Units (if any); |
| (c) | if Cresco Corp so elects, take all such actions and do all things as are reasonably necessary or desirable to effect the exchange of Common Units directly with the holder thereof, in accordance with applicable law, take all such actions and do all such things as are necessary or desirable to cause to be delivered directly Proportionate Voting Shares, Subordinate Voting Shares and/or amounts in cash, as applicable, to the holders of Common Units in accordance with the provisions of the A&R LLC Agreement, together with an amount in cash sufficient to pay any amount to be paid in respect of unpaid distributions with respect to such Common Units (if any); and |
| (d) | ensure that Cresco Corp does not exercise its vote as the manager of Cresco to initiate the voluntary liquidation, dissolution or winding up of Cresco nor take any action or omit to take any action that is designed to result in the liquidation, dissolution or winding-up of Cresco. |
The Resulting Issuer will further agree that, so long as any Cresco Corp Redeemable Shares (if and when issued) not owned by the Resulting Issuer or its affiliates which are redeemable or exchangeable for Proportionate Voting Shares (or Subordinate Voting Shares, at the election of Cresco Corp and/or Cresco) are outstanding or any Cresco Corp Redeemable Shares are issuable pursuant to the exercise, conversion or exchange of any outstanding securities of Cresco Corp, the Resulting Issuer shall:
| (e) | take all such actions and do all such things as are reasonably necessary or desirable to enable and permit Cresco Corp, in accordance with applicable law, to pay and otherwise perform its obligations with respect to the satisfaction of a redemption of Cresco Corp Redeemable Shares by a holder thereof in respect of each issued and outstanding Cresco Corp Redeemable Share upon the redemption of such Cresco Corp Redeemable Shares by Cresco Corp and, without limiting the generality of the foregoing, take all such actions and do all such things as are necessary or desirable to enable and permit Cresco Corp to cause to be delivered Proportionate Voting Shares, Subordinate Voting Shares and/or amounts in cash, as applicable, to the holders of Cresco Corp Redeemable Shares in accordance with the articles of incorporation and bylaws of Cresco Corp, together with an amount in cash sufficient to pay any amount to be paid in respect of unpaid distributions with respect to such Cresco Corp Redeemable Shares (if any); |
-81-
| (f) | upon the election of Cresco Corp for the Resulting Issuer to effect an exchange directly with a holder of Cresco Corp Redeemable Shares, take all such actions and do all things as are reasonably necessary or desirable to effect the exchange of Cresco Corp Redeemable Shares directly with the holder thereof, in accordance with applicable law, without limiting the generality of the foregoing, take all such actions and do all such things as are necessary or desirable to cause to be delivered directly Proportionate Voting Shares, Subordinate Voting Shares and/or amounts in cash, as applicable, to the holders of Cresco Corp Redeemable Shares in accordance with the provisions of the articles of incorporation of Cresco Corp, together with an amount in cash sufficient to pay any amount to be paid in respect of unpaid distributions (if any) with respect to such Cresco Corp Redeemable Shares; and |
| (g) | ensure that Cresco Corp is not voluntarily liquidated, dissolved or wound up nor take any action or omit to take any action that is designed to result in the liquidation, dissolution or winding-up of Cresco Corp. |
The Support Agreement provides that in the event that a tender offer, share exchange offer, issuer bid, take-over bid or similar transaction with respect to Proportionate Voting Shares and/or Subordinate Voting Shares is proposed by the Resulting Issuer or is proposed to the Resulting Issuer or its shareholders and is recommended to the Resulting Issuer Board, or is otherwise effected or to be effected with the consent or approval of the Resulting Issuer Board, and the Common Units are not redeemed by Cresco or purchased by Cresco Corp or the Resulting Issuer pursuant to the terms of the A&R LLC Agreement or the Cresco Corp Redeemable Shares (if and when issued) are not redeemed by Cresco Corp or purchased by Cresco Corp or the Resulting Issuer pursuant to the terms of the articles of incorporation of Cresco Corp, the Resulting Issuer will use its reasonable efforts in good faith to take all such actions and do all such things as are necessary or desirable to enable and permit holders of Common Units (other than Cresco Corp and its affiliates) and Cresco Corp Redeemable Shares (other than the Resulting Issuer and its affiliates) to participate in such offer to the same extent and on an economically equivalent basis as the holders of Proportionate Voting Shares and/or Subordinate Voting Shares, without discrimination. Without limiting the generality of the foregoing, the Resulting Issuer will use its reasonable efforts in good faith to ensure that holders of Common Units and Cresco Corp Redeemable Shares (if and when issued) may participate in each such offer without being required to redeem Common Units as against Cresco and Cresco Corp Redeemable Shares against Cresco Corp (or, if so required, to ensure that any such retraction, shall be effective only upon, and shall be conditional upon, the closing of such offer and only to the extent necessary to tender or deposit to the offer). Nothing in the Support Agreement will limit the ability of the Resulting Issuer (or any of its subsidiaries including, without limitation, Cresco Corp or Cresco) to make ordinary market purchases of Subordinate Voting Shares in accordance with applicable laws and regulatory and stock exchange requirements.
The Support Agreement provides that while any Common Units (or other rights pursuant to which Common Units may be acquired upon the exercise thereof) other than Common Units held by Cresco Corp or its affiliates are outstanding, and at all times while any Cresco Corp Redeemable Shares (or other rights pursuant to which Cresco Corp Redeemable Shares may be acquired upon the exercise thereof) other than Cresco Corp Redeemable Shares held by the Resulting Issuer or its affiliates are outstanding, the Resulting Issuer will make available such number of Proportionate Voting Shares and/or Subordinate Voting Shares (or other shares or securities into which Proportionate Voting Shares and/or Subordinate Voting Shares may be reclassified or changed) without duplication equal to the sum of (i) the number of Common Units issued and outstanding from time to time; (ii) the number of Common Units issuable upon the exercise of all rights to acquire Common Units outstanding from time to time; (iii) the number of Cresco Corp Redeemable Shares issued and outstanding from time to time; and (iv) the number of Cresco Corp Redeemable Shares issuable upon the exercise of all rights to acquire Cresco Corp Redeemable Shares outstanding from time to time in addition to any additional Proportionate Voting Shares and/or Subordinate Voting Shares as may be required to enable and permit the Resulting Issuer to meet its obligations under the A&R LLC Agreement, the Tax Receivable Agreement and under any other security or commitment pursuant to which the Resulting Issuer may be required to deliver Proportionate Voting Shares and/or Subordinate Voting Shares to any person, to enable and permit Cresco Corp to meet its obligations under each of the A&R LLC Agreement and the Tax Receivable Agreement with respect to the delivery of Proportionate Voting Shares and/or Subordinate Voting Shares and payment of the tax benefits contemplated under the Tax Receivable Agreement and to enable and permit Cresco to meet its obligations under the Support Agreement and under the A&R LLC Agreement.
-82-
With the exception of changes for the purpose of (i) adding to the covenants of any or all of the parties; (ii) making such amendments or modifications not inconsistent with the Support Agreement as may be necessary or desirable with respect to matters or questions arising thereunder; or (iii) curing or correcting any ambiguities or defect or inconsistent provision or clerical omission or mistake or manifest errors (provided, in the case of (i), (ii) or (iii) that the board of directors of each of the Resulting Issuer and Cresco Corp and the manager of Cresco are of the good faith opinion that such amendments are not prejudicial to the rights or interests of the holders of Common Units or Cresco Corp Redeemable Shares), the Support Agreement may not be amended except by agreement in writing executed by Cresco, Cresco Corp, and the Resulting Issuer and approved by the holders of a majority of the Common Units in accordance with the terms of the A&R LLC Agreement and a majority of the Cresco Corp Redeemable Shares in accordance with the terms of the articles of incorporation and the bylaws of Cresco Corp.
Prior Sales of Cresco Securities
The Corporation
The following tables set forth the issuances of Cresco Units within the last twelve (12) months before the date of this Listing Statement (excluding securities issued upon closing of the Transaction).
| Date Issued |
Number of Cresco Securities |
Issue Price per Unit ($) | Aggregate Issue Price ($) |
Nature of Consideration | ||||
| November 26, 2018 |
12,624,054 Subscription Receipts |
C$8.50 | C$107,304,459 | Cash | ||||
| October 4, 2018 |
26,666,667 Class F Units | $3.75 | $100,000,000 | Cash | ||||
| May 20, 2018 |
717,556 Class F Units | $2.25 | $29,114,500 | Cash | ||||
| January 26, 2018 |
500,000 Class F Units | $1.14 | $570,000 | Acquisition | ||||
| November 27, 2017 |
14,006,523 Class E Units | $1.14 | $15,967,436 | Cash | ||||
| OPTIONS | ||||||||
| Date Issued |
Number of Units | Exercise Price per Unit ($) |
Total Aggregate Proceeds Assuming the Exercise of all Options |
Nature of Consideration | ||||
| October 16, 2017 to September 1, 2018 |
15,050,000 Options to purchase Class F Units |
$1.00 to 3.75 | $29,254,285 (assuming the exercise of all options) |
Cash | ||||
| 11. | Escrowed Securities |
Pursuant to National Policy 46-201, the Resulting Issuer is an “exempt issuer” as defined therein and is thus not subject to escrow. Directors, officers and significant shareholders have entered into lock-up agreements pursuant to which such parties have agreed, subject to customary carve-outs and exceptions, not to sell any Subordinate Voting Shares (or announce any intention to do so), or any securities issuable in exchange therefor, for a period of 180 days from the date of the Business Combination.
-83-
| 12. | Principal Shareholders |
The following table sets forth, to the best of the Resulting Issuer’s knowledge, as of the date hereof, the persons or companies who beneficially own, directly or indirectly, or exercise control or direction over, directly or indirectly, 10% or more of the Subordinate Voting Shares.
| Name of Securityholder Jurisdiction of Residence |
Type of Ownership |
Number and Percentage of Subordinate Voting Shares (on an as converted basis) | ||
| McCormack, LLC(1) Chicago, IL, United States |
Beneficial and of Record | 27,643,297 / 10.26% | ||
| GG&K Holdings LLC(2) Chicago, IL, United States |
Beneficial and of Record | 28,320,000 / 10.51% | ||
| (1) | Controlled by Brian McCormack. |
| (2) | Controlled by John Gorman. |
| 13. | Directors and Officers |
The following table sets out, for each of the Resulting Issuer’s directors and executive officers, the person’s name, age, state and country of residence, position with the Resulting Issuer, principal occupation(s) during the last five (5) years, and, if an existing officer of Cresco prior to the Business Combination, the date on which the person became such an officer. The Resulting Issuer’s directors were elected as such at the Meeting and are expected to hold office until its next annual general meeting of shareholders unless they resign prior thereto or are removed by the shareholders of the Resulting Issuer. The Resulting Issuer’s directors will be elected annually and, unless re-elected, will retire from office at the end of the next annual general meeting of shareholders.
Under NI 52-110, an independent director is one who is free from any direct or indirect relationship which could, in the view of the Resulting Issuer Board, be reasonably expected to interfere with a director’s exercise of independent judgment. Charles Bachtell and Joe Caltabiano, officers of the Resulting Issuer, are not considered independent and Dominic A. Sergi, Brian McCormack, Robert M. Sampson, John R. Walter, Gerald Corcoran, Thomas Manning and Randy Podolsky are considered independent.
Directors and Officers
| Name and State and Country of |
Age | Position(s) with the Resulting Issuer |
Cresco Officer/Director Since |
Principal Occupation(s)(1) |
Number of Securities of Resulting Issuer Directly or Indirectly Held(2) | |||||
| Charles Bachtell Chicago IL, United States |
40 | Chief Executive Officer and Director | 02/2015 | Chief Executive Officer, Cresco | 15,690,478 / 5.81% | |||||
| Joe Caltabiano Chicago IL, United States |
41 | President and Director | 02/2015 | President, Cresco | 17,642,446 / 6.53% | |||||
| Ken Amann Chicago IL, United States |
47 | Chief Financial Officer | 09/2015 | Chief Financial Officer, Cresco | 1,600,157 / 0.59% | |||||
-84-
| Name and State and Country of |
Age | Position(s) with the Resulting Issuer |
Cresco Officer/Director Since |
Principal Occupation(s)(1) |
Number of Securities of Resulting Issuer Directly or Indirectly Held(2) | |||||
| Zach Marburger Chicago IL, United States |
33 | Chief Information Officer | 09/2015 | Chief Information Officer, Cresco | 533 / 0.0002% | |||||
| David Ellis Chicago IL, United States |
36 | Chief Operating Officer | 06/2017 | Chief Operating Officer, Cresco | 139,740 / 0.05% | |||||
| Jason Erkes, Chicago, IL, United States |
48 | Chief Communications Officer | 09/2018 | Chief Communications Officer, Cresco | 66,693 / 0.025% | |||||
| John Schetz, Chicago, IL, United States |
42 | General Counsel | 06/2018 | General Counsel, Cresco | 9,387 / 0.003% | |||||
| Dominic A. Sergi Glen Ellyn, IL, United States |
35 | Director | 02/2015 | Real Estate Investor | 11,274,827 / 4.18% | |||||
| Brian McCormack Chicago, Illinois, United States |
50 | Director | 02/2015 | Founder and Director of InnerWorkings | 27,635,077 / 10.23% | |||||
| Robert M. Sampson Downers Grove, IL United States |
44 | Director | 05/2015 | Chief Executive Officer of Bemortgage | 13,105,710 / 4.85% | |||||
| John R. Walter(3) Naples, FL, United States |
71 | Director | 03/2017 | Chief Executive Officer of Ashlin Management Company | 135,340 / 0.05% | |||||
| Gerald Corcoran(3) Winnetka, Illinois, United States |
63 | Director | 03/2017 | Chairman of the Board of O’Brien & Associates, LLC | 997,395 / 0.37% | |||||
| Thomas Manning(3) Evanston, Illinois, United States |
63 | Director | 10/2016 | Chief Executive Officer of Dun and Bradstreet | 132,000 / 0.05% | |||||
| Randy Podolsky Lincolnshire, IL, United States |
63 | Director | 12/2016 | Principal of Podolsky Circle CORFAC International | 1,014,387 / 0.38% | |||||
Notes:
| (1) | For prior occupations of each director and officer for the last 5 years, if applicable, please see biographies below. |
-85-
| (2) | Excludes all options. |
| (3) | Denotes proposed members of the audit committee for the Resulting Issuer. All three proposed members are independent and financially literate within the meaning of NI52-110. |
Biographies
The following are brief profiles of the Resulting Issuer’s executive officers and directors.
Charles Bachtell, Chief Executive Officer and Director
Charles Bachtell is the CEO of Cresco Labs, LLC. Bachtell is an attorney and brings with him legal expertise in both corporate governance and regulatory compliance. Bachtell is a founding member of the Illinois Cannabis Bar Association and industry trade associations in IL, PA, and OH. Prior to Cresco Labs, Bachtell served for 8 years as the Executive Vice President and General Counsel of Guaranteed Rate. Bachtell also serves as an adjunct Professor at Northwestern University Pritzker School of Law teaching a course on the legal and regulatory issues in the emerging cannabis industry.
Joe Caltabiano, President and Director
Joe Caltabiano is the President of Cresco Labs, LLC. bringing more than a decade of finance experience to his role. He is also a leukemia survivor who is committed to supporting organizations and efforts that help patients in their fight against cancer. Caltabiano has expertise working in the regulated mortgage industry. He has received awards for mortgage production, client experience and community involvement.
Ken Amann, Chief Financial Officer
Ken Amann brings nearly 20 years of financial management and consulting experience as the Chief Financial Officer of the Resulting Issuer. Prior to joining Cresco Labs, Amann spent two years as the Chief Financial Officer for nSource, a BPO and consulting practice located in Chicago. Prior to that, he was the Chief Financial Officer for eight years at Williams Lea, a $2+ billion dollar global BPO business specializing in corporate information solutions that re-engineer end-to-end business processes. He was responsible for over 170 employees located in 7 different countries. He is a Certified Public Accountant.
Zach Marburger, Chief Information Officer
Zach Marburger is the Chief Information Officer for the Resulting Issuer and has over 8 years’ experience in automated technologies and systems. In recent years Marburger created Whaxy.com, the fastest growing medical cannabis resource and compliant online ordering platform in the industry. Previously, Marburger founded an anti-piracy software company with 4,000 copyright holders under management, which was acquired in 2014. Marburger ensures all Cresco Lab’s systems and information storage are compliant, forward-thinking, and enterprise grade.
David Ellis, Chief Operating Officer
David Ellis is the Chief Operating Officer for the Resulting Issuer. Ellis brings a decade of executive level operations experience across several industries to the team. Most recently, Ellis founded and served as the President and CEO of Greens & Gills, a 10,000 square foot, controlled environment, hydroponic and aquaponic farm on the South Side of Chicago. Through operational best practices and proprietary software, Ellis was able to build one of the only profitable urban farming businesses in the country. Ellis will work with the Operations Team to design, implement and ensure that all operational activities at Cresco Labs are optimized to industry leading standards.
Jason Erkes, Chief Communications Officer
Jason Erkes is Cresco Labs’ Chief Communications Officer and has been managing the company’s media relations and external messaging since its inception. He is a former Emmy-Award winning journalist and political advisor who has advised dozens of CEOs, thought-leaders, and politicians on executing effective communication strategies. Jason has penned guest columns for several cannabis-focused publications discussing managing crisis situations and has been quoted as a media expert in Forbes and Entrepreneur.
-86-
John Schetz, General Counsel
John Schetz recently joined as Cresco Labs’ General Counsel. John combines experience as a Partner in a large law firm and as Chief Legal Officer of a multinational public company with broad and deep legal and business experience in multiple industries. John previously served as Executive Vice President and General Counsel of Stericycle, Inc. (NASDAQ: SRCL) where he was responsible for global legal affairs, completed approximately 70 M&A transactions, including leading all aspects of a $2.3B acquisition, engineered the favorable settlement of enterprise-threatening class action litigation, and spearheaded the creation of the company’s legal and compliance functions. Prior to his tenure at Stericycle, John was a Partner in the Corporate Department of McDermott Will & Emery LLP in Chicago where he advised public and private companies and private equity firms on a wide range of acquisitions, dispositions, investments, joint ventures and strategic partnerships, capital markets transactions, corporate governance, SEC reporting and compliance, and general corporate matters. John received his undergraduate and law degrees from the University of Michigan in Ann Arbor.
Dominic A. Sergi, Director
Dominic Sergi is a successful real estate, business and financial expert who devotes much of his free time raising funds to help patients fighting leukemia and lymphoma. As president and chief executive officer of a Chicago-based investment real estate company, Sergi draws on his strategic and business acumen to deliver on the company’s long-term vision and provide asset budgeting forecasts, which have exceeded $120 million in value. Sergi’s many other responsibilities as leader of a growing firm include oversight of all operations and personnel management. He is also very involved in his family’s $100 million dollar Union Electrical Contracting business and serves on the board of the 400-employee company. Sergi is active in a number of community and charitable organizations and is passionate about giving back to the community.
Brian McCormack, Director
Brian McCormack, one of Illinois’ most innovative and successful business entrepreneurs, brings a creative, progressive approach to business, finance and manufacturing at Cresco Labs as he seeks to position the company at the forefront of medical cannabis cultivation in Illinois. McCormack is the founder and director of InnerWorkings (NASDAQ: INWK), a print management company in Chicago that he built into a successful global operation that employs more than 1,800 people and grosses over $1 billion in annual revenue. His innovative business strategies, manufacturing expertise and focus on employee satisfaction led InnerWorkings to financial growth and to being named by Forbes as one of the Best Companies to Work For in 2010. Prior to founding InnerWorkings, McCormack founded and served as CEO of McWitt Graphic Communications. He also provided financial and manufacturing operations insight to uBid.com and EZLinks Golf Corporation as a director on the companies’ advisory boards.
Robert M. Sampson, Director
Prior to forming Cresco Labs, Robert Sampson had more than 20 years of operating experience in large business, including 12 years in the heavily regulated mortgage industry, having served as Chief Operating Officer at Guaranteed Rate, the nation’s seventh largest retail mortgage bank. As the former COO of Cresco Labs, Sampson oversaw the construction of two 40,000 sq/ft cement precast structures and one 30,000 sq/ft hybrid greenhouse structure and was responsible for all facility operations and systems including the design and implementation of fertigation and irrigation systems, inventory control systems, compliance process procedures, audits, security, and IT. Sampson is currently the CEO of Bemortgage based in Chicago.
John R. Walter, Director
John R. Walter is Chairman of Ashlin Management Company, a private investment and management services firm. He is the retired President and COO of AT&T Corporation, a position he held from 1996 to 1997, and retired Chairman and CEO of R.R. Donnelley & Sons Company, a position he held from 1989 through 1996. Mr. Walter joined R.R. Donnelley & Sons Company in 1969 and held various positions during his career. Mr. Walter has served as a Director
-87-
of Manpower Inc. since 1998, and served as Non-Executive Chairman of the company from 1999 to 2001. Mr. Walter also serves as a Director of Cresco Labs, ecoAmerica and The Conservancy of Southwest Florida. He previously served on the boards of other companies, including Innerworkings, Echo Global Logistics, VASCO Data Securities, Media Bank, LLC, Groupon, Deere & Company, Abbott Laboratories, Inc., AT&T Corporation, Target Corporation and Jones Lang LaSalle. Mr. Walter was the founding Chairman of InnerWorkings, Inc. Mr. Walter also served as Chairman of SNP Corp. of Singapore and an Advisory member of the Singapore Economic Development Board. Mr. Walter is on the Board of Trustees for Steppenwolf Theater, Northwestern University and The Naples Children & Education Foundation. Mr. Walter was previously a Director of NorthShore University HealthSystem and Chairman Emeritus of the NorthShore University HealthSystem Foundation Board. In addition, he was a Director of the African Wildlife Foundation, the Metropolitan Pier and Exposition Authority and the Chicago Symphony Orchestra. Mr. Walter earned a Bachelor of Science Degree in Management and holds an Honorary Doctorate Degree from Miami University, Oxford, Ohio.
Gerald Corcoran, Director
Gerry Corcoran has served as Chief Executive Officer of R.J. O’Brien & Associates, LLC (RJO) since 2000 and Chairman of the Board since 2007. Chicago-based RJO, which celebrated its Centennial in 2014, is the nation’s oldest and largest independent futures brokerage firm and the last surviving founding member of the Chicago Mercantile Exchange (now CME Group). In July 2014, Corcoran was elected Chairman of the FIA (formerly Futures Industry Association), and he served in that position until March 2016. At that time, following the January merger of the organization with its European and Asian counterparts, he was elected Treasurer of the Board of Directors of the newly unified FIA, the leading trade organization for the futures, options and cleared swaps markets worldwide. He served in that role until March 2017. Corcoran serves on the FIA’s Executive Committee as well as its Americas Advisory Board. He has been a member of FIA’s Board of Directors since March 2008 and served as Vice Chairman from March 2013 until July 2014. Corcoran also serves on the Board of Directors and Executive Committee of the National Futures Association (NFA), the self-regulatory organization for the futures industry. In addition, he is on the Board of the Clearing Corporation Charitable Foundation and is a member of the Risk Committee of CME Group. He previously served on the Board of the Institute for Financial Markets (IFM). In November 2013, he received the Heart of Mercy Award from Misericordia Home, which supports more than 600 children and adults living with developmental disabilities.
Thomas Manning, Director
Tom Manning is the Chief Executive Officer of Dun & Bradstreet (NYSE), a leading provider of corporate information and analytics. He is a director of CommScope (NASDAQ) and Clear Media (HKSE). He is also an executive-in-residence at the Booth School of Business at the University of Chicago and teaches corporate governance, private equity, innovative problem-solving, and US-China relations at the University of Chicago Law School. While based in Hong Kong for nearly 20 years, he served as CEO of Cerberus Capital Asia, Capgemini Asia, and Ernst & Young Consulting Asia, and also as a senior partner at Bain & Company. During the past decade, he served on the boards of several large, publicly-listed Chinese companies, including Bank of Communications, Gome Electrical Appliances, AsiaInfo-Linkage, and iSoftStone. Earlier in his career, he was extensively involved in the medical field as the founder and CEO of a biomedical device company and the founder of McKinsey’s health care consulting practice. A graduate of Harvard and Stanford, he speaks Mandarin and is a frequent speaker at conferences and contributor to media.
Randy Podolsky, Director
Randy D. Podolsky has served the entrepreneurial, corporate, institutional and Not-For-Profit clients of Podolsky Circle CORFAC International for over 40 years, and served as Managing Principal of the firm from 1986 to 2015. As a Principal of the firm, Randy provided personalized transaction and contract negotiation and advisory services to financial institutions, users, owners and Not-For-Profits for all facets of commercial real estate. Randy leads the firms Development Team, which has completed numerous development and redevelopment projects. Strategizing and executing complex real estate and debt transaction solutions is his passion. In 2005, Randy recognized increased overspending on commercial real estate and, anticipating a near-term bubble burst, advised nearly 100 investors to divest of their commercial real estate holdings. Mr. Podolsky serves as a Board Member and Chair of the Real Estate Committee of the Waukegan Port District. Additionally, he is a volunteer member of the U.S. Coast Guard Auxiliary since 1991 and served as the elected District Commodore (DCO) of the Ninth Western Region in 2009-2010.
-88-
Cease Trade and Bankruptcy
None of the Resulting Issuer’s directors or executive officers has, within the 10 years prior to the date of this Listing Statement, been a director, chief executive officer or chief financial officer of any company (including the Resulting Issuer) that, while such person was acting in that capacity (or after such person ceased to act in that capacity but resulting from an event that occurred while that person was acting in such capacity) was the subject of a cease trade order, an order similar to a cease trade order, or an order that denied the company access to any exemption under securities legislation, in each case for a period of more than 30 consecutive days.
None of the Resulting Issuer’s directors or executive officers has, within the 10 years prior to the date of this Listing Statement, become bankrupt, made a proposal under any legislation relating to bankruptcy or insolvency or was subject to or instituted any proceedings, arrangement or comprise with creditors or had a receiver, receiver manager or trustee appointed to hold the assets of such director or executive officer, been a director or executive officer of any company, that, while that person was acting in that capacity, or within a year of that person ceasing to act in that capacity, became bankrupt, made a proposal under any legislation relating to bankruptcy or insolvency or was subject to or instituted any proceedings, arrangement or comprise with creditors or had a receiver, receiver manager or trustee appointed to hold its assets.
No director or executive officer of the Resulting Issuer has: (i) been subject to any penalties or sanctions imposed by a court relating to securities legislation or by a securities regulatory authority or has entered into a settlement agreement with a securities regulatory authority; or (ii) been subject to any other penalties or sanctions imposed by a court or regulatory body that would likely be considered important to a reasonable investor in making an investment decision.
To the best of the Resulting Issuer’s knowledge, there are no known existing or potential material conflicts of interest among the Resulting Issuer or a subsidiary of the Resulting Issuer and a director or officer of the Resulting Issuer or a subsidiary of the Resulting Issuer as a result of their outside business interests except that certain of the Resulting Issuer’s or its subsidiaries’ directors and officers serve as directors and officers of other companies, and therefore it is possible that a conflict may arise between their duties to the Resulting Issuer and their duties as a director or officer of such other companies.
14. Capitalization
Issued Capital
| Number of Securities (non- diluted) |
Number of Securities (fully- diluted)(1) |
% of Issued (non- diluted) |
% of Issued (fully diluted) |
|||||||||||||
| Public Float |
||||||||||||||||
| Total outstanding (A) |
21,873,696 | 294,567,873 | 100 | % | 100 | % | ||||||||||
| Held by Related Persons or employees of the Issuer or Related Person of the Issuer, or by persons or companies who beneficially own or control, directly or indirectly, more than a 5% voting position in the Issuer (or who would beneficially own or control, directly or indirectly, more than a 5% voting position in the Issuer upon exercise or conversion of other securities held) (B) |
0 | 122,010,816 | 0 | % | 41.4 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Public Float (A-B) |
21,873,696 | 172,557,057 | 100 | % | 58.6 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
-89-
| Number of Securities (non- diluted) |
Number of Securities (fully- diluted)(1) |
% of Issued (non- diluted) |
% of Issued (fully diluted) |
|||||||||||||
| Freely-Tradeable Float |
||||||||||||||||
| Number of outstanding securities subject to resale restrictions, including restrictions imposed by pooling or other arrangements or in a shareholder agreement and securities held by control block holders (C) |
0 | 0 | 0 | % | 0 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Tradeable Float (A-C) |
21,873,696 | 294,567,873 | 100 | % | 100 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) | There will be 520,032 Proportionate Voting Shares (which includes securities to be issued in connection with certain acquisitions) which convert to Subordinate Voting Shares 200:1 and 143,690,687 Cresco Redeemable Units that convert 1:1 into Subordinate Voting Shares. There will also be 500,000 Super Voting Shares issued and outstanding immediately following the completion of the Business Combination. The Super Voting Shares are non-participating and are not convertible into any other class of shares. The fully diluted figure also includes an aggregate of 24,497,070 Resulting Issuer Replacement Options, Cresco Replacement Warrants, Existing Warrants, and Broker Warrants. See Section 8 – Consolidated Capitalization. |
Public Securityholders (Registered)
Class of Shares: Subordinate Voting Shares
| Size of Holding |
Number of holders | Total number of securities | ||||
| 1 – 99 securities |
51 | 1,328 | ||||
| 100 – 499 securities |
7 | 1,609 | ||||
| 500 – 999 securities |
— | — | ||||
| 1,000 – 1,999 securities |
— | — | ||||
| 2,000 – 2,999 securities |
— | — | ||||
| 3,000 – 3,999 securities |
33 | 128,238 | ||||
| 4,000 – 4,999 securities |
5 | 23,159 | ||||
| 5,000 or more securities |
111 | 4,254,002 | ||||
|
|
|
| ||||
| Total |
200 | 4,408,336 | ||||
|
|
|
| ||||
Public Securityholders (Beneficial)
Class of Shares: Subordinate Voting Shares
| Size of Holding |
Number of holders |
Total number of securities | ||
| 1 – 99 securities |
233 | 12,184 | ||
| 100 – 499 securities |
30 | 8,099 | ||
| 500 – 999 securities |
9 | 7,413 | ||
| 1,000 – 1,999 securities |
12 | 20,516 | ||
| 2,000 – 2,999 securities |
16 | 42,320 | ||
| 3,000 – 3,999 securities |
12 | 36,000 | ||
| 4,000 – 4,999 securities |
5 | 21,905 | ||
| 5,000 or more securities |
111 | 17,316,923 | ||
|
|
| |||
| Total |
428 | 17,465,360 | ||
|
|
|
-90-
Non-Public Securityholders (Registered)
Class of Shares: Subordinate Voting Shares
| Size of Holding |
Number of holders |
Total number of securities | ||
| 1 – 99 securities |
— | — | ||
| 100 – 499 securities |
— | — | ||
| 500 – 999 securities |
— | — | ||
| 1,000 – 1,999 securities |
— | — | ||
| 2,000 – 2,999 securities |
— | — | ||
| 3,000 – 3,999 securities |
— | — | ||
| 4,000 – 4,999 securities |
— | — | ||
| 5,000 or more securities |
— | — | ||
|
|
| |||
| Total |
— | — | ||
|
|
|
Convertible Securities
| Description of Security (include conversion / exercise terms, including conversion / exercise price) |
Number of convertible/exchangeable securities outstanding |
Number of listed securities issuable upon conversion/exercise |
||||||
| Warrants to acquire Subordinate Voting Shares at an exercise price equal to C$8.50 per Subordinate Voting Share, subject to vesting and certain other conditions |
343,745 | 343,745 | ||||||
| Warrants to acquire Subordinate Voting Shares at an exercise price of C$6.10 per Subordinate Voting Share(1) |
4,000,000 | 4,000,000 | ||||||
| Warrants of Cresco exercisable at an exercise price of C$2.26 per Subordinate Voting Share |
100,000 | 100,000 | ||||||
| Options to acquire Subordinate Voting Shares at an exercise price of C$1.30 per Subordinate Voting Share |
100,000 | 100,000 | ||||||
| Options reserved for issuance under the Cresco Stock Option Plan at a blended exercise price of C$2.26 per Subordinate Voting Share |
17,960,000 | 17,960,000 | ||||||
| Proportionate Voting Shares |
520,032 | 104,006,420 | ||||||
| Cresco Corp Redeemable Shares |
Nil. | Nil. | ||||||
| Cresco Redeemable Units |
143,690,687 | 143,690,687 | ||||||
| (1) | 2,000,000 of which are contingent on the achievement of certain performance milestones. |
-91-
15. Executive Compensation
The following table sets forth the anticipated compensation to be paid or awarded to the following executive officers of the Resulting Issuer: (i) the Chief Executive Officer; (ii) the Chief Financial Officer; and (iii) the three most highly compensated individuals whose total compensation was more than $150,000 (the “Named Executive Officers”):
| Table of Compensation Excluding Compensation Securities |
||||||||||||||||||||||||||||
| Name & Position |
Year | Salary, Consulting Fee, Retainer or Commission ($) |
Bonus ($) | Committee or meeting fees ($) |
Value of Perquisites ($) |
Value of all other compensation ($) |
Total compensation ($) |
|||||||||||||||||||||
| Charles Bachtell, CEO and Director |
2018 | $ | 350,000 | $ | 262,500 | Nil | Nil | Nil | $ | 612,500 | ||||||||||||||||||
| Ken Amann, CFO |
2018 | $ | 250,000 | $ | 125,000 | Nil | Nil | Nil | $ | 375,000 | ||||||||||||||||||
| Joe Caltabiano, President |
2018 | $ | 350,000 | $ | 262,500 | Nil | Nil | Nil | $ | 612,500 | ||||||||||||||||||
| David Ellis, COO |
2018 | $ | 200,000 | $ | 100,000 | Nil | Nil | Nil | $ | 300,000 | ||||||||||||||||||
| John Schetz, General Counsel |
2018 | $ | 200,000 | $ | 66,667 | Nil | Nil | Nil | $ | 266,667 | ||||||||||||||||||
Termination and Change of Control Benefits
Except as described herein, the Resulting Issuer will not have any contracts, agreements, plans or arrangements that provide for payments to a Named Executive Officer at, following or in connection with any termination (whether voluntary, involuntary or constructive), resignation, retirement, a change in control of the Resulting Issuer or change in a Named Executive Officer’s responsibilities.
Review and Oversight of Executive Officer Compensation
The Board of the Resulting Issuer, or the Compensation Committee thereof, will review the compensation of its executives following completion of the Business Combination and from time to time thereafter and make such changes as it deems appropriate.
16. Indebtedness of Directors and Executive Officers
No person who is or at any time during the most recently completed financial year was a director, executive officer or senior officer of the Company, no proposed nominee for election as a director of the Company, and no associate of any of the foregoing persons has been indebted to the Company at any time since the commencement of the Company’s last completed financial year. No guarantee, support agreement, letter of credit or other similar arrangement or understanding has been provided by the Company at any time since the beginning of the most recently completed financial year with respect to any indebtedness of any such person.
-92-
17. Risk Factors
MARIJUANA IS ILLEGAL UNDER U.S. FEDERAL LAW AND ENFORCEMENT OF RELEVANT LAWS IS A SIGNIFICANT RISK.
READERS ARE STRONGLY ENCOURAGED TO CAREFULLY READ ALL OF THE RISK FACTORS CONTAINED IN THIS SECTION.
The following are certain factors relating to the business of the Resulting Issuer. These risks and uncertainties are not the only ones facing the Resulting Issuer. Additional risks and uncertainties not presently known to the Resulting Issuer or currently deemed immaterial by the Resulting Issuer, may also impair the operations of the Resulting Issuer. If any such risks actually occur, shareholders of the Resulting Issuer could lose all or part of their investment and the business, financial condition, liquidity, results of operations and prospects of the Resulting Issuer could be materially adversely affected and the ability of the Resulting Issuer to implement its growth plans could be adversely affected.
The acquisition of any of the securities of the Resulting Issuer is speculative, involving a high degree of risk and should be undertaken only by persons whose financial resources are sufficient to enable them to assume such risks and who have no need for immediate liquidity in their investment. An investment in the securities of the Resulting Issuer should not constitute a major portion of an individual’s investment portfolio and should only be made by persons who can afford a total loss of their investment. Resulting Issuer Shareholders should evaluate carefully the following risk factors associated with the Resulting Issuer’s securities, along with the risk factors described elsewhere in this Listing Statement.
The following table is intended to assist readers in identifying those parts of this Listing Statement that address the disclosure expectations outlined in Canadian Securities Administrators Staff Notice 51-352 (Revised) – Issuers with U.S. Marijuana-Related Activities (“Staff Notice 51-352”) for issuers that currently have marijuana-related activities in U.S. states where such activity has been authorized within a state regulatory framework.
| Industry Involvement |
Specific Disclosure Necessary to Fairly Present |
Listing Statement Cross Reference | ||
| All Issuers with U.S. Marijuana-Related Activities | Describe the nature of the issuer’s involvement in the U.S. marijuana industry and include the disclosures indicated for at least one of the direct, indirect and ancillary industry involvement types noted in this table. | Section 3 – General Development of the Business - State Level U.S. Cannabis Operations
Section 4 – Narrative Description of the Business | ||
| Prominently state that marijuana is illegal under U.S. federal law and that enforcement of relevant laws is a significant risk. | Cover Page (disclosure in bold and capital typeface) | |||
-93-
| Industry Involvement |
Specific Disclosure Necessary to Fairly Present |
Listing Statement Cross Reference | ||
| Discuss any statements and other available guidance made by federal authorities or prosecutors regarding the risk of enforcement action in any jurisdiction where the issuer conducts U.S. marijuana-related activities.
Outline related risks including, among others, the risk that third party service providers could suspend or withdraw services and the risk that regulatory bodies could impose certain restrictions on the issuer’s ability to operate in the U.S. |
Section 17 – Risk Factors - U.S. Federal Regulation
Section 17 – Risk Factors – Variation in State Regulations
Section 17 – Risk Factors – Anti-money Laundering Laws and Regulations
Section 17 – Risk Factors – Access to Banks
Section 17 – Risk Factors – Investments in the United States May be Subject to Heightened Scrutiny
Section 17 – Risk Factors – Constraints on marketing products
Section 17 – Risk Factors – Heightened scrutiny by Canadian regulatory authorities
Section 17 – Risk Factors – Intellectual Property
Section 17 – Risk Factors – Lack of access to U.S. bankruptcy protections
Section 17 – Risk Factors – Legality of contracts
Section 17 – Risk Factors – Newly established legal regime
Section 17 – Risk Factors – Risk of civil asset forfeiture | |||
| Given the illegality of marijuana under U.S. federal law, discuss the issuer’s ability to access both public and private capital and indicate what financing options are / are not available in order to support continuing operations. | Section 4 – Narrative Description of the Business — Total Funds Available
Section 4 – Narrative Description of the Business – Ability to Access Public and Private Capital
Section 17 – Risk Factors – Newly established legal regime
Section 17 – Risk Factors – Access to Banks
Section 17 – Risk Factors – Access to Capital | |||
| Quantify the issuer’s balance sheet and operating statement exposure to U.S. marijuana-related activities. | Section 5 – Selected Consolidated Financial Information
Schedules “A” and “B” to the Listing Statement.
Note: at the time of the Listing Statement, the major operations of the Resulting Issuer are only in the United States | |||
| Disclose if legal advice has not been obtained, either in the form of a legal opinion or otherwise, regarding (a) compliance with applicable state regulatory frameworks and (b) potential exposure and implications arising from U.S. federal law. | Legal advice has been obtained. | |||
-94-
| Industry Involvement |
Specific Disclosure Necessary to Fairly Present |
Listing Statement Cross Reference | ||
| U.S. Marijuana Issuers with direct involvement in cultivation or distribution | Outline the regulations for U.S. states in which the issuer operates and confirm how the issuer complies with applicable licensing requirements and the regulatory framework enacted by the applicable U.S. state. | Section 3 – General Development of the Business - State Level U.S. Cannabis Operations | ||
| Discuss the issuer’s program for monitoring compliance with U.S. state law on an ongoing basis, outline internal compliance procedures and provide a positive statement indicating that the issuer is in compliance with U.S. state law and the related licensing framework. Promptly disclose any non-compliance, citations or notices of violation which may have an impact on the issuer’s licence, business activities or operations. | Section 3 – General Development of the Business - State Level U.S. Cannabis Operations
Section 3 – General Development of the Business – Compliance with Applicable State Law in the United States
Section 17 – Risk Factors – U.S. state regulatory uncertainty | |||
| U.S. Marijuana Issuers with indirect involvement in cultivation or distribution | Outline the regulations for U.S. states in which the issuer’s investee(s) operate. | Section 4 – Narrative Description of the Business – State Level U.S. Cannabis Operations – Nevada | ||
| Provide reasonable assurance through either positive or negative statements, that the investee’s business is in compliance with applicable licensing requirements and the regulatory framework enacted by the applicable U.S. state. | Cresco is not aware of any non-compliance | |||
| U.S. Marijuana Issuers with material ancillary involvement | Provide reasonable assurance, through either positive or negative statements, that the applicable customer’s or investee’s business is in compliance with applicable licensing requirements and the regulatory framework enacted by the applicable U.S. State. | Not applicable. | ||
In accordance with Staff Notice 51-352, Cresco’s subsidiaries are directly engaged in the manufacture, possession, use, sale or distribution of cannabis in the recreational and/or medicinal cannabis marketplace in the States of Illinois, Pennsylvania, Ohio, Nevada, Arizona and California with firm plans to expand into New York, Maryland and Massachusetts. In accordance with Staff Notice 51-352, Cresco will evaluate, monitor and reassess this disclosure, and any related risks, on an ongoing basis and the same will be supplemented and amended to investors in public filings, including in the event of government policy changes or the introduction of new or amended guidance, laws or regulations regarding marijuana regulation. Any non-compliance, citations or notices of violation which may have an impact on any license, business activities or operations will be promptly disclosed by the Resulting Issuer.
Founder Voting Control
As a result of the Super Voting Shares, Charlie Bachtell, Joe Caltabiano, Robert Sampson, Dominic Sergi and Brian McCormack (the “Founders”) will exercise approximately 77.3% of the voting power in respect of the Resulting Issuer’s outstanding shares. The Subordinate Voting Shares are expected to be entitled to one vote per share, the Proportionate Voting Shares are expected to be entitled to two-hundred vote per share and the Super Voting Shares are expected to be entitled to two-thousand votes per share. As a result, the Founders (and any three of the Founders for certain actions not requiring a 2/3 majority) potentially have the ability to control the outcome of all matters submitted to the Resulting Issuer’s shareholders for approval, including the election and removal of directors and any arrangement or sale of all or substantially all of the assets of the Resulting Issuer. If the Founders’ employment with
-95-
the Resulting Issuer is terminated or they resign from their positions with the Resulting Issuer, they will continue to have the ability to exercise the same significant voting power. Additionally, each Super Voting Share, may be so transferred to the holder’s immediate family members, or in connection with estate or tax planning matters.
In addition, because the number of Super Voting Shares held by a holder thereof from time to time is dependent upon the number of Cresco Redeemable Units (and Cresco Corp Redeemable Shares, if and when issued) beneficially owned, directly or indirectly, or deemed to be so beneficially owned by such holder from time to time, should the Resulting Issuer cause Cresco to issue additional Cresco Redeemable Units or Cresco Redeemable Units in the future to a Founder in connection with employee equity incentive programs, it would prolong the Founder’s voting control.
To supplement the rights, privileges, restrictions and conditions attached to the Super Voting Shares, the Resulting Issuer and the Founders, being the initial holders of Super Voting Shares, entered into an investment agreement effective as of the completion of the Business Combination which, among other things, provides that (i) each Super Voting Share will be transferable only to the holder’s immediate family members or an affiliated entity or a transfer to the other Founder or an entity affiliated with the other Founder, and (ii) upon any sale of Super Voting Shares to a third party purchaser not listed in clause (i), such Super Voting Shares will immediately be redeemed by the Resulting Issuer for their issue price.
The concentrated control through the Super Voting Shares could delay, defer, or prevent a change of control of the Resulting Issuer, arrangement involving the Resulting Issuer or sale of all or substantially all of the assets of the Resulting Issuer that it’s other shareholders support. Conversely, this concentrated control could allow the Founders to consummate such a transaction that the Resulting Issuer’s other shareholders do not support. In addition, the Founders may make long-term strategic investment decisions and take risks that may not be successful and may seriously harm the Resulting Issuer’s business.
As directors and officers of the Resulting Issuer, the Founders are anticipated to have control over the day-to-day management and the implementation of major strategic decisions of the Resulting Issuer, subject to authorization and oversight by the Resulting Issuer Board. As board members and officers, the Founders will owe a fiduciary duty to the Resulting Issuer’s shareholders and will be obligated to act honestly and in good faith with a view to the best interests of the Resulting Issuer. As shareholders, even controlling shareholders, the Founders will be entitled to vote their shares, and shares over which they have voting control, in their own interests, which may not always be in the interests of the Resulting Issuer or the other shareholders of the Resulting Issuer.
U.S. Federal Regulation
U.S. federal regulation and enforcement may adversely affect the implementation of medical and recreational marijuana laws and regulations and may negatively impact our revenues and profits.
Investors are cautioned that in the U.S., marijuana is largely regulated at the state level. Currently, there are 30 States plus the District of Columbia, Puerto Rico and Guam that have laws and/or regulations that recognize, in one form or another, legitimate medical uses for cannabis and/or recreational use of cannabis.
Notwithstanding the permissive regulatory environment of medical marijuana at the state level, marijuana continues to be categorized as a controlled substance under the CSA. Under the CSA, the policies and regulations of the U.S. federal government and its agencies are that cannabis has no “proven” medical benefits. Unless and until Congress amends the CSA with respect to medical marijuana, as to the timing or scope of any such potential amendments there can be no assurance, there is a risk that U.S. federal authorities may enforce current U.S. federal law, and we may be deemed to be producing, cultivating, or dispensing marijuana in violation of U.S. federal law with respect to the Resulting Issuer’s current or proposed business operations, or the Resulting Issuer may be deemed to be facilitating the sale or distribution of drug paraphernalia in violation of U.S. federal law. A change in the U.S. federal government’s approach to begin more active enforcement of cannabis may adversely affect our revenues and profits. The risk of strict enforcement of the CSA in light of Congressional activity, judicial holdings, and stated U.S. federal policy remains uncertain.
-96-
The U.S. Supreme Court declined to hear a case brought by San Diego County, California that sought to establish federal pre-emption over State medical marijuana laws. The pre-emption claim was rejected by every court that reviewed the case. The California Fourth District Court of Appeals wrote in its unanimous ruling, “Congress does not have the authority to compel the States to direct their law enforcement personnel to enforce U.S. federal laws.” However, in another case, the U.S. Supreme Court held that, as long as the CSA contains prohibitions against marijuana, under the Commerce Clause of the U.S. Constitution, the U.S. may criminalize the production and use of homegrown cannabis even where States approve its use for medical purposes.
In an effort to provide guidance to U.S. federal law enforcement, the Department of Justice (“DOJ”) issued Guidance Regarding Marijuana Enforcement to all U.S. Attorneys in a memorandum from: (1) Deputy Attorney General David Ogden on October 19, 2009; (2) Deputy Attorney General James Cole on June 29, 2011; and (3) Mr. Cole on August 29, 2013 (the “Cole Memorandum”). Each memorandum included statements that the DOJ is committed to the enforcement of the CSA, but also included that the DOJ is also committed to using its limited investigative and prosecutorial resources to address the most significant threats in the most effective, consistent, and rational way.
On February 14, 2014, Mr. Cole supplemented the Cole Memorandum to add guidance regarding the impact of the prior memoranda on “financial crimes” for which marijuana-related conduct is a predicate. Among other things he noted that the provisions of the money laundering statutes, the unlicensed money remitter statute and the Bank Secrecy Act remain in effect with respect to marijuana-related conduct. However, he also reiterated the position reflected in the August 29, 2013 guidance, stating that the investigation and prosecution of financial crimes “would be subject to the same consideration and prioritization.”
On December 20, 2014, President Obama signed into law a federal spending bill with a Congressional appropriation rider for the year ending September 30, 2015, providing that “None of the funds made available to the DOJ pursuant to the 2015 Consolidated and Further Continuing Appropriations Act may be used to prevent certain States, including Arizona, Nevada and California, from implementing their own laws that have authorized the use, distribution, possession, or cultivation of medical marijuana” (the “Rohrabacher-Blumenauer Amendment”). This limitation was carried over for the year ending September 30, 2016. The DOJ addressed the impact of the Rohrabacher-Blumenauer Amendment in a memorandum dated February 27, 2015, which was released to the public in August 2015. That memorandum took the position that the Rohrabacher-Blumenauer Amendment does not bar the use of funds for civil and criminal enforcement “consistent with the existing DOJ guidance….” The DOJ’s interpretation appears to have been firmly rejected by the U.S. Court of Appeals for the Ninth Circuit (which includes federal court districts of Arizona and Nevada). In a decision dated August 16, 2016, the Court specifically ruled that the Rohrabacher-Blumenauer Amendment prohibited the use of DOJ funds for “conduct completely authorized by State law” United States v McIntosh, No.15-10117, 2016 WL 4363168, at 32 (9th Cir. Aug. 16, 2016).
Following the inauguration of President Trump, a Task Force on Crime Reduction and Public Safety was established through an executive order by the President of the U.S. in February 2017. The Task Force was to deliver its recommendations by July 27, 2017. To date, its recommendations have not been made public.
In March, 2017, U.S. Attorney General Jeff Sessions acknowledged the validity of the Cole Memorandum and noted limited federal resources due to the appropriations restrictions.
However, Mr. Sessions disagreed that the Cole Memorandum had been implemented effectively and, on January 4, 2018, Attorney General Jeff Sessions issued a memorandum (the “Sessions Memorandum”), which rescinded the Cole Memorandum. The Sessions Memorandum rescinded previous nationwide guidance specific to the prosecutorial authority of U.S. Attorneys relative to marijuana enforcement on the basis that they are unnecessary, given the well-established principles governing federal prosecution that are already in place. Those principals are included in chapter 9.27.000 of the U.S. Attorneys’ Manual and require federal prosecutors deciding which cases to prosecute to weigh all relevant considerations, including federal law enforcement priorities set by the Attorney General, the seriousness of the crime, the deterrent effect of criminal prosecution, and the cumulative impact of particular crimes on the community.
As a result of the Sessions Memorandum, federal prosecutors will now be free to utilize their prosecutorial discretion to decide whether to prosecute marijuana activities despite the existence of state-level laws that may be inconsistent with federal prohibitions. No direction was given to federal prosecutors in the Sessions Memorandum as to the priority
-97-
they should ascribe to such marijuana activities, and it is uncertain how active federal prosecutors will be in relation to such activities. Furthermore, the Sessions Memorandum did not discuss the treatment of medical marijuana by federal prosecutors. Medical marijuana is currently protected against enforcement by enacted legislation from U.S. Congress in the form of the Rohrabacher-Blumenauer Amendment which similarly prevents federal prosecutors from using federal funds to impede the implementation of medical marijuana laws enacted at the state level, subject to Congress restoring such funding. Although the appropriations restriction on the use of DOJ funds imposed by the Rohrabacher-Blumenauer Amendment has been effectively extended through September 30, 2018 by the continuing resolution contained in the Consolidated Appropriations Act, 2018, there can be no assurance that future federal appropriations will continue to restrict the DOJ’s use of funds.
Due to the ambiguity of the Sessions Memorandum in relation to medical marijuana, there can be no assurance that the federal government will not seek to prosecute cases involving marijuana businesses that are otherwise compliant with state law. The DOJ has not historically devoted resources to prosecuting individuals whose conduct is limited to possession of small amounts of marijuana for use on private property, but has relied on State and local law enforcement to address marijuana activity. In the event the DOJ reverses its stated policy and begins strict enforcement of the CSA in States that have laws legalizing medical marijuana and adult-use marijuana in small amounts, there may be a direct and adverse impact to our business and our revenue and profits.
Since the issuance of the Sessions Memorandum, limited public comments have been made by the U.S. Attorneys in the jurisdictions in which the Reporting Issuer operates regarding the enforcement of federal law related to cannabis. The U.S. Attorneys who have made statements where the Reporting Issuer operates are outlined below.
Benjamin C. Glassman U.S. Attorney for the Southern District of Ohio stated that “Congress made marijuana illegal under the Controlled Substances Act. That was true under Deputy Attorney General Cole’s 2013 memorandum on marijuana enforcement, and it’s just as true under the [Sessions Memorandum] today.” Further Mr. Glassman has taken the position that federal law enforcement continues to work with limited resources and is focused on prosecutions that have the biggest impact on public safety.
Justin E. Herdman U.S. Attorney for the Northern District of Ohio stated that he does not plan to change his approach to bringing criminal cases involving marijuana, even in light of guidance from the Sessions Memorandum the has freed the ability of prosecutors to pursue such cases.
A spokesperson for McGregor Scott, U.S. Attorney for the Eastern District of California has stated that that marijuana violations in the Eastern District will be evaluated in accordance with our district’s federal law enforcement priorities and resources.
Adam Braverman, U.S. Attorney for the Southern District of California commented that the DOJ is committed to reducing violent crime and enforcing the laws as enacted by Congress. The cultivation, distribution, and possession of marijuana has long been and remains a violation of federal law and the Southern District of California will utilize long-established prosecutorial priorities to carry out its mission to combat violent crime, disrupt and dismantle transnational criminal organizations, and stem the rising tide of the drug crisis.
Andrew Lelling, U.S. Attorney for the District of Massachusetts commented that Congress has unambiguously made it a federal crime to cultivate, distribute and/or possess marijuana and as a law enforcement officer in the Executive Branch, it is his sworn responsibility to enforce that law, guided by the Principles of Federal Prosecution. Mr. Lelling acknowledged that he will proceed on a case-by-case basis, assessing each matter according to those principles and deciding whether to use limited federal resources to pursue it. Mr. Lelling expressed concern deciding, in advance, to immunize a certain category of actors from federal prosecution would be to effectively amend the laws Congress has already passed. The kind of categorical relief sought by those engaged in state-level marijuana legalization efforts can only come from the legislative process.
John Childress, U.S. Attorney for the Central District of Illinois commented that for citizens of central Illinois, the Sessions Memorandum does not change long-established prosecutorial principles to enforce federal law and that his office will continue to work together with our law enforcement partners, to promote the safety and interests of our local communities.
-98-
Matthew Schneider, U.S. Attorney for the Eastern District of Michigan was reported as saying that his office will review marijuana cases in terms of where those cases fit within our priorities and our limited federal resources.
Scott Brady, U.S. Attorney for the Western District of Pennsylvania released a statement which provided noted that his office will continue to deploy all prosecutorial tools at our disposal to protect the citizens of western Pennsylvania from those individuals and criminal organizations which traffic in all illegal controlled substances, including marijuana. This was in contrast to Pennsylvania Governor Tom Wolf who views the Sessions Memorandum as a backwards move and vowed to protect cancer patients, kids with epilepsy, veterans with PTSD and all Pennsylvanians seeking relief from legal medical marijuana. Governor Wolf was critical of the Trump Administration and affirmed his commitment to do everything in his power to protect Pennsylvania patients.
On January 8, 2018, Mr. Andrew E. Lelling, U.S. Attorney for the District of Massachusetts commented that his office cannot provide assurances that certain categories of participants in the state-level marijuana trade will be immune from federal prosecution. In addition, Mr. Lellin’s office released the following statement:
“This is a straightforward rule of law issue. Congress has unambiguously made it a federal crime to cultivate, distribute and/or possess marijuana. As a law enforcement officer in the Executive Branch, it is [Mr Lellin’s] sworn responsibility to enforce that law, guided by the Principles of Federal Prosecution. To do that, however, [Mr Lellin] must proceed on a case-by-case basis, assessing each matter according to those principles and deciding whether to use limited federal resources to pursue it. Deciding, in advance, to immunize a certain category of actors from federal prosecution would be to effectively amend the laws Congress has already passed, and that [Mr Lellin] will not do. The kind of categorical relief sought by those engaged in state-level marijuana legalization efforts can only come from the legislative process.”
On January 16, 2018, a coalition of 19 attorney generals from various States, including: Ms. Lisa Madigan, Illinois Attorney General, Xavier Becerra, California Attorney General, Eric T. Schneiderman, New York Attorney General (who has since been replaced by Barbara Underwood) and Josh Shapiro, Pennsylvania Attorney General released a joint statement urging Congress to advance legislation that would allow states with legalized medical or recreational marijuana to participate in the banking system. Banks and other depository institutions are currently hindered by federal law from providing financial services to marijuana businesses, even in states where those businesses are regulated.
In July, 2018 U.S. Senators Cory Gardner (R-CO) and Elizabeth Warren (D-MA) introduced the bipartisan Strengthening the Tenth Amendment Through Entrusting States Act (the “States Act”). If passed and signed into law, the States Act would, among other things, (i) allow legal cannabis business to have bank accounts at financial institutions; (ii) make State-legal cannabis retailers exempt from federal prosecution; (iii) set the legal age to purhcase adult-use cannabis to 21 nationally, expect for medical purposes and ban cannabis distribution and consumption at highway rest areas and truck stops; and (iv) remove industrial hemp from the definition of Cannabis. The States Act does not propose to remove Cannabis as a Schedule I drug under the CSA.
Potential proceedings under U.S. federal law could involve significant restrictions being imposed upon the Resulting Issuer or third parties, while diverting the attention of key executives. Such proceedings could have a material adverse effect on the Resulting Issuer’s business, revenues, operating results and financial condition as well as the Resulting Issuer’s reputation, even if such proceedings were concluded successfully in favour of the Resulting Issuer. In the extreme case, such proceedings could ultimately involve the prosecution of key executives of the Resulting Issuer or the seizure of corporate assets. However, as of the date hereof, the Resulting Issuer and has obtained legal advice in respect thereof that proceedings of this nature have historically been sufficiently uncommon to be characterized as remote absent a shift by federal authorities to a more aggressive enforcement approach. The Resulting Issuer has also received advice from its legal counsel regarding the potential exposure and implications arising from U.S. federal law generally. As the legal landscape at both the U.S. federal level and the state level is evolving, all such legal advice is historical in nature, and is only effective up to the date such advice was received.
Following the issuance of the Sessions Memorandum, the Resulting Issuer continues to look to the guidelines of the Cole Memorandum as an industry best practice and continues to do the following to ensure compliance with the Cole Memorandum:
-99-
| • | ensuring the operations of its subsidiaries are compliant with all licensing requirements that are set forth with regards to cannabis operation by the applicable state, county, municipality, town, township, borough, and other political/administrative divisions. To this end, the Resulting Issuer retains appropriately experienced legal counsel and other professionals to conduct the necessary due diligence to ensure compliance of such operations with all applicable; |
| • | the activities relating to the cannabis business adhere to the scope of the licensing obtained. Accordingly, in the states where only medical cannabis is permitted, the products are only sold to patients who hold the necessary documentation to permit the possession of the cannabis; and in the states where cannabis is permitted for adult recreational use, the products are only sold to individuals who meet the requisite age requirements; |
| • | the Resulting Issuer only works through licensed operators, which must pass a range of requirements, adhere to strict business practice standards and be subjected to strict regulatory oversight whereby sufficient checks and balances ensure that no revenue is distributed to criminal enterprises, gangs and cartels; and |
| • | the Resulting Issuer conducts reviews of products and product packaging to ensure that the products comply with applicable regulations and contain necessary disclaimers about the contents of the products to prevent adverse public health consequences from cannabis use and prevent impaired driving. |
The Resulting Issuer will continue to monitor compliance on an ongoing basis in accordance with its compliance program and standard operating procedures. While the Resulting Issuer’s operations are in full compliance with all applicable state laws, regulations and licensing requirements, such activities remain illegal under U.S. federal law. For the reasons described above and the risks further described below, there are significant risks associated with the business of the Resulting Issuer.
Variation in State Regulations
Variations in State and local regulation, and enforcement in States that have legalized medical cannabis, may restrict marijuana-related activities, including activities related to medical cannabis, which may negatively impact our revenues and prospective profits.
The marijuana laws of each state are not necessarily consistent with those of other states. A number of states have decriminalized marijuana to varying degrees, other states have created exemptions specifically for medical cannabis, and several have both decriminalization and medical laws. Four States, Alaska, Colorado, Oregon, Washington and the District of Columbia have previously legalized the adult-use of cannabis. In November 2016, four additional states, California, Massachusetts, Maine and Nevada, voted to legalize adult-use cannabis, although adult-use will not commence in those states until appropriate regulatory frameworks have been put in place. The exception is Nevada and California, which began its adult-use program on July 1, 2017 and January 2018, respectively. Variations exist among states that have legalized, decriminalized, or created medical marijuana exemptions. For example, Alaska, Colorado, and the District of Columbia have limits on the number of marijuana plants that can be homegrown. In most states, the cultivation of marijuana for personal use continues to be prohibited except for those states that allow small-scale cultivation by the individual in possession of medical marijuana needing care or that person’s caregiver. Active enforcement of state laws that prohibit personal cultivation of marijuana may indirectly and adversely affect our business and our revenue and profits.
The Resulting Issuer is in compliance with, and has obtained legal advice in respect of its compliance with, U.S. state laws and the related licensing framework of Illinois, Pennsylvania, Ohio, Nevada, Arizona, California and New York applicable to its respective business operations.
MARIJUANA IS ILLEGAL UNDER U.S. FEDERAL LAW AND ENFORCEMENT OF RELEVANT LAWS IS A SIGNIFICANT RISK.
The Resulting Issuer could be found to be violating laws related to medical cannabis.
-100-
Currently, there are 30 states plus the District of Columbia, Puerto Rico and Guam that have laws and/or regulations that recognize, in one form or another, legitimate medical uses for cannabis and consumer use of cannabis in connection with medical treatment. Other states are considering similar legislation. Conversely, under the CSA, the policies and regulations of the federal government and its agencies are that cannabis has no proven medical benefit and a range of activities including cultivation and the personal use of cannabis is prohibited. Unless and until Congress amends the CSA with respect to medical cannabis, as to the timing or scope of any such amendments there can be no assurance, there is a risk that federal authorities may enforce current U.S. federal law. The risk of strict enforcement of the CSA in light of Congressional activity, judicial holdings, and stated federal policy remains uncertain. This would cause a direct and adverse effect on the Resulting Issuer’s subsidiaries’ businesses, or intended businesses, and on its revenue and prospective profits.
Marijuana is a Schedule-I controlled substance and is illegal under U.S. federal law. Even in those States in which the use of marijuana has been legalized, its use remains a violation of U.S. federal law. Since U.S. federal law criminalizing the use of marijuana pre-empts State laws that legalize its use, strict enforcement of U.S. federal law regarding marijuana would likely result in the Resulting Issuers inability to proceed with its business plan.
Laws and regulations affecting the medical marijuana industry are constantly changing, which could detrimentally affect the proposed operations of the Resulting Issuer.
Local, state, and U.S. federal medical marijuana laws and regulations are broad in scope and subject to evolving interpretations, which could require the Resulting Issuer to incur substantial costs associated with compliance or alter certain aspects of its business plan. In addition, violations of these laws, or allegations of such violations, could disrupt certain aspects of the Resulting Issuers business plan and result in a material adverse effect on certain aspects of its planned operations. In addition, it is possible that regulations may be enacted in the future that will be directly applicable to certain aspects of the Resulting Issuer’s business. No prediction can be made as to the nature of any future laws, regulations, interpretations or applications, nor can it be determined what effect additional governmental regulations or administrative policies and procedures, when and if promulgated, could have on our business.
Change of Cannabis Laws
It is possible that U.S. federal or State legislation could be enacted in the future that would prohibit the Resulting Issuer from selling cannabis and cannabis products, and if such legislation were enacted, the Resulting Issuer’s revenues could decline, leading to a loss of shareholder investment. Additionally, it is possible that regulatory bodies could impose new restrictions on our ability to operate in the U.S. which could lead to a loss of shareholder investment.
U.S. State Regulatory Uncertainty
The rulemaking process for cannabis operators at the state level in any state will be ongoing and result in frequent changes. As a result, a compliance program is essential to manage regulatory risk. All operating policies and procedures implemented in the operation will be compliance-based and derived from the state regulatory structure governing ancillary cannabis businesses and their relationships to state-licensed or permitted cannabis operators, if any. Notwithstanding the Resulting Issuer’s efforts, regulatory compliance and the process of obtaining regulatory approvals can be costly and time-consuming. No assurance can be given that the Resulting Issuer will receive the requisite licenses, permits or cards to operate its businesses.
In addition, local laws and ordinances could restrict the Resulting Issuer’s business activity. Although legal under the laws of the states in which the Resulting Issuer’s business will operate, local governments have the ability to limit, restrict, and ban cannabis businesses from operating within their jurisdiction. Land use, zoning, local ordinances, and similar laws could be adopted or changed, and have a material adverse effect on the Resulting Issuer’s business.
The Resulting Issuer is aware that proportionate states are considering special taxes or fees on businesses in the marijuana industry. Illinois has, for example, imposed a license transfer surtax. It is a potential yet unknown risk at this time that other states are in the process of reviewing such additional fees and taxation. This could have a material adverse effect upon the Resulting Issuer’s business, results of operations, financial condition or prospects.
-101-
Approval of Cannabis Related Drug
While under the CSA, the policies and regulations of the U.S. federal government and its agencies are that cannabis has no proven medical benefit. On June 25, 2018 The U.S. Food and Drug Administration (“FDA”) approved a drug named Epidiolex, which is the first drug comprised of an active ingredient derived from marijuana. Epidiolex is used to treat rare, severe forms of epilepsy. Prior to this the FDA had not approved cannabis as a safe and effective drug for any indication. In September, 2018 Epidiolex cleared the final hurdle to market, as the medicine has been reclassified by the Drug Enforcement Administration from Schedule I to Schedule V, thereby legally allowing its sale.
Risk of Legal, Regulatory or Political Change
Delays in enactment of new state or U.S. federal regulations could restrict the ability of the Resulting Issuer to reach strategic growth targets and lower return on investor capital. The strategic growth strategy of the Resulting Issuer, is reliant upon certain federal and state regulations being enacted to facilitate the legalization of medical and adult-use marijuana. If such regulations are not enacted, or enacted but subsequently repealed or amended, or enacted with prolonged phase-in periods, the growth targets of the Resulting Issuer, and thus, the effect on the return of investor capital, could be detrimental. The Resulting Issuer is unable to predict with certainty when and how the outcome of these complex regulatory and legislative proceedings will affect its business and growth.
Further, there is no guarantee that state laws legalizing and regulating the sale and use of cannabis will not be repealed or overturned, or that local governmental authorities will not limit the applicability of state laws within their respective jurisdictions. If the U.S. federal government begins to enforce federal laws relating to cannabis in states where the sale and use of cannabis is currently legal, or if existing applicable state laws are repealed or curtailed, the Resulting Issuer’s business, results of operations, financial condition and prospects would be materially adversely affected. It is also important to note that local and city ordinances may strictly limit and/or restrict disbursement of marijuana in a manner that will make it extremely difficult or impossible to transact business that is necessary for the continued operation of the marijuana industry. Federal actions against individuals or entities engaged in the marijuana industry or a repeal of applicable marijuana related legislation could adversely affect the Resulting Issuer and its business, results of operations, financial condition and prospects.
The Resulting Issuer is aware that multiple states are considering special taxes or fees on businesses in the marijuana industry. It is a potential yet unknown risk at this time that other states are in the process of reviewing such additional fees and taxation. This could have a material adverse effect upon the Resulting Issuer’s business, results of operations, financial condition or prospects.
The commercial medical and adult-use marijuana industry is in its infancy and the Resulting Issuer anticipates that such regulations will be subject to change as the jurisdictions in which the Resulting Issuer does business matures. The Resulting Issuer has in place a detailed compliance program with dedicated staff who oversee, maintain, and implement the compliance program and personnel. In addition to the Resulting Issuer’s robust legal and compliance departments, the Resulting Issuer also has local regulatory/compliance counsel engaged in every jurisdiction in which it operates. The Resulting Issuer’s compliance program emphasizes security and inventory control to ensure strict monitoring of cannabis and inventory from delivery by a licensed distributor to sale or disposal. Additionally, the Resulting Issuer has created comprehensive standard operating procedures that include detailed descriptions and instructions for monitoring inventory at all stages of development and distribution. The Resulting Issuer will continue to monitor compliance on an ongoing basis in accordance with its compliance program, standard operating procedures, and any changes to regulation in the marijuana industry.
Overall, the medical and adult-use marijuana industry is subject to significant regulatory change at both the state and federal level. The inability of the Resulting Issuer to respond to the changing regulatory landscape may cause it to not be successful in capturing significant market share and could otherwise harm its business, results of operations, financial condition or prospects.
The Resulting Issuer is aware that multiple states are considering special taxes or fees on businesses in the marijuana industry. It is a potential yet unknown risk at this time that other states are in the process of reviewing such additional fees and taxation. This could have a material adverse effect upon the Resulting Issuer’s business, results of operations, financial condition or prospects.
-102-
WARNING TO CANADIAN INVESTORS - Canadian Investors May be Barred from Entering the U.S.
Todd Owen, executive assistant commissioner for the Office of Field Operations of the U.S. Customs and Border Protection Agency (“CBP”) has stated that Canadians who work in the marijuana industry and those who invest in the cannabis sector are risk a lifetime ban on travel to the U.S. The CBP will continue to apply long-standing U.S. federal laws and regulations that treat marijuana as a banned substance and participants in the cannabis industry as drug traffickers who are inadmissible into the U.S. Although some U.S. states have eased marijuana laws, the U.S. continues to maintain a federal prohibition that applies at the border. CBP officials are not planning to go out of their way to interrogate every Canadian traveler about marijuana use. However, other factors may cause them to raise the topic. In July, 2018 a venture-capitalist from Vancouver, British Columbia who had invested more than $100,000 into legal American cannabis companies, was denied entry to the U.S. and barred from future entry as his investments were deemed to be assisting and abetting in the illicit trafficking of drugs.
Market Price and Volatility
Publically traded securities in the cannabis industry have experienced an extreme level of price and volume volatility over the past few of years and the market price of securities of many companies has experienced wide fluctuations which, in many cases, have not necessarily been related to the performance, underlying asset values or prospects of such companies. The trading price of the securities in the Resulting Issuer may be subject to large fluctuations and, therefore, may result in losses to investors. In addition, following periods of volatility in the market price of publically traded securities in the cannabis industry, holders of such securities have instituted class action securities litigation against those companies. Such litigation, if instituted, could result in substantial costs and diversion of management attention and resources, which could significantly harm the Resulting Issuer’s business, condition, prospects and reputation.
Internal Controls
The failure to implement and maintain proper and effective internal controls and disclosure controls could result in material weaknesses in the Resulting Issuer’s financial reporting, such as errors in its financial statements and in the accompanying footnote disclosures that could require restatements. Investors may lose confidence in the Resulting Issuer’s reported financial information and disclosure, which could negatively impact its share price.
The Resulting Issuer does not expect that its internal controls over financial reporting will prevent all errors and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Controls can be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the controls. Over time, controls may become inadequate because changes in conditions or deterioration in the degree of compliance with policies or procedures may occur. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
Access to Capital
The Resulting Issuer has limited capital resources and operations. Prior to the Business Combination, Cresco’s operations have been funded entirely from the proceeds of debt and equity financings including the private placement offerings. The Resulting Issuer expects to require substantial additional capital in the near future to continue operations at its cultivation and production facilities, dispensaries, expansion of its product lines, development of its intellectual property base, increasing production capabilities and expanding its operations in states where it currently operates and states where it currently does not have operations. As a result, the Resulting Issuer may not be able to obtain additional financing on terms acceptable to it, or at all. If the Resulting Issuer fail to raise additional capital, as needed, its ability to implement its business model and strategy could be compromised.
Even if the Resulting Issuer obtains financing for its near-term operations, it expects that it will require additional capital thereafter. The capital needs of the Resulting Issuer will depend on numerous factors including: (i) profitability; (ii) the release of competitive products by competitors; (iii) the level of investment in research and development; and (iv) the amount of our capital expenditures, including acquisitions. There can be no assurance that the Resulting Issuer will be able to obtain capital in the future to meet its needs.
-103-
Although the Resulting Issuer has accessed private financing in the past, there is neither a broad nor deep pool of institutional capital that is available to companies in the U.S. cannabis industry. There can be no assurance that additional financing, if raised privately, will be available to the Resulting Issuer when needed or on terms which are acceptable.
Dilution
Any additional funds raised through the issuance of equity or convertible debt securities will result in the percentage ownership held by our existing shareholders to be reduced and shareholders may experience significant dilution. In addition, new securities may contain rights, preferences, or privileges that are senior to those existing today. If additional capital is raised by incurring debt, this will result in increased interest expenses. If additional funds are raised through the issuance of securities, market fluctuations in the price of the Subordinate Voting Shares could limit the Resulting Issuer’s ability to obtain equity financing.
No assurance can be given that any additional financing will be available to the Resulting Issuer, or if available, will be on favorable terms. If the Resulting Issuer is unable to raise capital when needed, its business, financial condition, and results of operations would be materially adversely affected, and it could be forced to reduce or discontinue our operations.
Global Economic Conditions
The Resulting Issuer’s business, financial condition, results of operations, and cash flow have been, and may in the future be, negatively impacted by challenging global economic conditions.
A global economic slowdown would cause disruptions and extreme volatility in global financial markets, increased rates of default and bankruptcy, and declining consumer and business confidence, which can lead to decreased levels of consumer spending. These macroeconomic developments have and could negatively impact the Resulting Issuer’s business, which depends on the general economic environment and levels of consumer spending. As a result, the Resulting Issuer may not be able to maintain its existing customers or attract new customers, or it may be forced to reduce the price of its products. The Resulting Issuer is unable to predict the likelihood of the occurrence, duration, or severity of such disruptions in the credit and financial markets and adverse global economic conditions. Any general or market-specific economic downturn could have a material adverse effect on the business, financial condition, results of operations, and cash flow of the Resulting Issuer.
No Guaranteed Return
There is no guarantee that an investment in the Subordinate Voting Shares will earn any positive return in the short, medium or long term. There is no assurance that holders of the Subordinate Voting Shares will receive cash distributions or any rate of return on, or repayment of, their investment in the Subordinate Voting Shares. In fact, an investor could lose its entire investment in the Subordinate Voting Shares.
Unknown Value of the Subordinate Voting Shares
The value of the Subordinate Voting Shares is subject to the ability of the Resulting Issuer to build equity in the enterprise. If insufficient proceeds are raised and alternative financing is not available, the completion of the Resulting Issuer’s business plan may not be fulfilled. There can be no assurance that a profitable business will be achieved by the Resulting Issuer.
Tax
Canadian federal and provincial and U.S. federal and state tax issues should be taken into consideration prior to investing in the Subordinate Voting Shares. The return on an investor’s investment is subject to taxes and to changes
-104-
in Canadian and U.S. tax laws. There can be no assurance that tax laws, regulations or judicial or administrative interpretations of these laws and regulations will change in a manner that fundamentally alters the tax consequences to investors holding or disposing of the Subordinate Voting Shares.
If you are purchasing the Subordinate Voting Shares outside of Canada, you should consult your own tax advisor for advice for your local jurisdiction.
Limited Operating History
As a high growth enterprise, Cresco does not have a history of profitability. As such, Cresco has no immediate prospect of generating profit from its intended operations. The Resulting Issuer is therefore subject to many of the risks common to early-stage enterprises, including under-capitalization, cash shortages, limitations with respect to personnel, financial, and other resources and lack of revenues. There is no assurance that the Resulting Issuer will be successful in achieving a return on shareholders’ investment and the likelihood of success must be considered in light of the early stage of operations.
Reliance on Management
The success of the Resulting Issuer is dependent upon the ability, expertise, judgment, discretion and good faith of its senior management. While employment agreements or management agreements are customarily used as a primary method of retaining the services of key employees, these agreements cannot assure the continued services of such employees. Any loss of the services of such individuals could have a material adverse effect on the Resulting Issuer’s business, operating results, financial condition or prospects.
Competition
There is potential that the Resulting Issuer will face intense competition from other companies, some of which can be expected to have longer operating histories and more financial resources and experience than the Resulting Issuer. Increased competition by larger and better financed competitors could materially and adversely affect the business, financial condition, results of operations or prospects of the Resulting Issuer.
Because of the early stage of the industry in which the Resulting Issuer operates, the Resulting Issuer expects to face additional competition from new entrants. To become and remain competitive, the Resulting Issuer will require research and development, marketing, sales and support. The Resulting Issuer may not have sufficient resources to maintain research and development, marketing, sales and support efforts on a competitive basis which could materially and adversely affect the business, financial condition, results of operations or prospects of the Resulting Issuer.
Difficulty in Recruiting and Retaining Management and Key Personnel
The Resulting Issuer’s future success depends on its key executive officers and its ability to attract, retain, and motivate qualified personnel.
Future success largely depends upon the continued services of the Resulting Issuer’s executive officers and management team. If one or more of the executive officers are unable or unwilling to continue in their present positions, replacements may not be readily available, if at all. Additionally, the Resulting Issuer may incur additional expenses to recruit and retain new executive officers. If any of the executive officers joins a competitor or forms a competing corporation, we may lose some or all of our customers. Finally, we do not maintain “key person” life insurance on any of our executive officers. Because of these factors, the loss of the services of any of these key persons could adversely affect our business, financial condition, and results of operations, and thereby an investment in the Subordinate Voting Shares.
The continuing ability to attract and retain highly qualified personnel will also be critical to the Reporting Issuer’s success because it will need to hire and retain additional personnel as the business grows. There can be no assurance that highly qualified personnel will be retained or available. Due to the competition for skilled personnel in the U.S. cannabis industry it is more difficult and expensive to attract, hire, and retain qualified managers and employees.
-105-
Because of these factors, The Resulting Issuer may not be able to effectively manage or grow its business, which could adversely affect its financial condition and the value of any investment in the Resulting Issuer could be significantly reduced or completely lost.
Unreliability of Forecasts
Any forecasts made by the Resulting Issuer about its operations may prove to be inaccurate. The Resulting Issuer must, among other things, determine appropriate risks, rewards, and level of investment in its product lines, respond to economic and market variables outside of its control, respond to competitive developments and continue to attract, retain, and motivate qualified employees. There can be no assurance that the Resulting Issuer will be successful in meeting these challenges and addressing such risks and the failure to do so could have a materially adverse effect on our business, results of operations, and financial condition. The prospects of the Resulting Issuer must be considered in light of the risks, expenses, and difficulties frequently encountered by companies in the early stage of development. As a result of these risks, challenges, and uncertainties, the value of any investment in the Resulting Issuer could be significantly reduced or completely lost.
Managing Growth
The Resulting Issuer may not be able to effectively manage its growth or improve its operational, financial, and management information systems, which would impair its results of operations.
In the near term, the Resulting Issuer intends to expand the scope of its operations and activities significantly. If it is successful in executing its business plan, it will experience growth that could place a significant strain on its business operations, finances, management, and other resources. The factors that may place strain on the Resulting Issuer’s resources include, but are not limited to, the following:
| (3) | the need for continued development of financial and information management systems; |
| (4) | the need to manage strategic relationships and agreements with manufacturers, customers, and partners; and |
| (5) | difficulties in hiring and retaining skilled management, technical, and other personnel necessary to support and manage the business. |
Additionally, the strategy of the Resulting Issuer envisions a period of rapid growth that may impose a significant burden on its administrative and operational resources. The ability to effectively manage growth will require it to substantially expand the capabilities of its administrative and operational resources and to attract, train, manage, and retain qualified management and other personnel. There can be no assurance that the Resulting Issuer will be successful in recruiting and retaining new employees, or retaining existing employees.
The Resulting Issuer cannot provide assurances that its management will be able to manage this growth effectively and the failure to successfully manage growth could result in its sales not increasing commensurately with capital investments or otherwise materially adversely affecting the business, financial condition, or results of operations.
Inability to Innovate and Find Efficiencies
If the Resulting Issuer is unable to continually innovate and increase efficiencies, our ability to attract new customers may be adversely affected. In the area of innovation, the Resulting Issuer must be able to develop new technologies and products that appeal to its customers. This depends, in part, on the technological and creative skills of the Resulting Issuer’s personnel and on our ability to protect our intellectual property rights. The Resulting Issuer may not be successful in the development, introduction, marketing, and sourcing of new technologies or innovations, that satisfy customer needs, achieve market acceptance, or generate satisfactory financial returns.
-106-
Website
Prospective customers may be deterred from doing business with the Resulting Issuer with a significant nationwide online presence because of fears of U.S. federal or state enforcement of laws prohibiting possession and sale of medical or adult-use marijuana.
The Resulting Issuer’s website is visible in jurisdictions where medicinal and/or adult-use of marijuana is not permitted and, as a result, the Resulting Issuer may be found to be violating the laws of those jurisdictions. The Resulting Issuer could lose potential customers as they could fear federal prosecution for buying its marijuana, reducing its revenue.
Operational Risk
The Resulting Issuer will be affected by a number of operational risks and the it may not be adequately insured for certain risks, including: labour disputes; catastrophic accidents; fires; blockades or other acts of social activism; changes in the regulatory environment; impact of non-compliance with laws and regulations; natural phenomena, such as inclement weather conditions, floods, earthquakes and ground movements. There is no assurance that the foregoing risks and hazards will not result in damage to, or destruction of, the Resulting Issuer’s properties, grow facilities and extraction facilities, personal injury or death, environmental damage, adverse impacts on the Resulting Issuer’s operation, costs, monetary losses, potential legal liability and adverse governmental action, any of which could have an adverse impact on the Resulting Issuer’s future cash flows, earnings and financial condition. Also, the Resulting Issuer may be subject to or affected by liability or sustain loss for certain risks and hcorards against which the Resulting Issuer cannot insure or which the Resulting Issuer may elect not to insure because of the cost. This lack of insurance coverage could have an adverse impact on the Resulting Issuer’s future cash flows, earnings, results of operations and financial condition.
Reliance on third-party suppliers, manufacturers and contractors; Reliance on Key Inputs
The Resulting Issuer’s business is dependent on a number of key inputs from third-parties and their related costs including raw materials and supplies related to its cultivation and production operations, as well as electricity, water and other local utilities. Due to the uncertain regulatory landscape for regulating cannabis in the U.S., the Resulting Issuer’s third party suppliers, manufacturers and contractors may elect, at any time, to decline or withdraw services necessary for the Resulting Issuer’s operations. Any significant interruption or negative change in the availability or economics of the supply chain for key inputs from third-parties could materially impact the business, financial condition and operating results of the Resulting Issuer. Some of these inputs may only be available from a single supplier or a limited group of suppliers in the future. If the Resulting Issuer becomes reliant upon a sole source supplier and it was to go out of business or suspend services the Resulting Issuer might be unable to find a replacement for such source in a timely manner or at all. Similarly, if any future sole source supplier were to be acquired by a competitor, that competitor may elect not to sell to the Resulting Issuer in the future. Additionally, any supplier could at any time suspend or withdraw services. Any inability to secure required supplies and services or to do so on appropriate terms could have a materially adverse impact on the Resulting Issuer’s business, financial condition and operating results.
Revenue Shortfalls
Revenue shortfalls from budget may result from lower than expected sales volume, sale price and/or inventory due to inadequate marketing or lower than expected market stimulation. Average sales prices may be less than budgeted due to aggressive competitor pricing below the Resulting Issuer’s prices.
Permits and Authorizations
The Resulting Issuer may be able to obtain or maintain the necessary licenses, permits, authorizations, or accreditations, or may only be able to do so at great cost, to operate its medical marijuana business. In addition, the Resulting Issuer may not be able to comply fully with the wide variety of laws and regulations applicable to the medical marijuana industry. Failure to comply with or to obtain or maintain the necessary licenses, permits, authorizations, or accreditations could result in restrictions on our ability to operate the medical marijuana business, which could have a material adverse effect on our business.
-107-
Potential for Conflict of Interest
All decisions to be made by such directors and officers involving the Resulting Issuer are required to be made in accordance with their duties and obligations to act honestly and in good faith with a view to the best interests of the Resulting Issuer. In addition, such directors and officers are required to declare their interests in, and such directors are required to refrain from voting on any matter in which they may have a material conflict of interest.
Difficulty in Enforcing Judgments and Effecting Service of Process on Directors and Officers
The directors and officers of the Resulting Issuer reside outside of Canada. Some or all of the assets of such persons may be located outside of Canada. Therefore, it may not be possible for Resulting Issuer shareholders to collect or to enforce judgments obtained in Canadian courts predicated upon the civil liability provisions of applicable Canadian securities laws against such persons. Moreover, it may not be possible for Resulting Issuer shareholders to effect service of process within Canada upon such persons.
Intellectual Property
If the Resulting Issuer fail to protect our intellectual property, its business could be adversely affected. Viability will depend, in part, on the Resulting Issuer’s ability to develop and maintain the proprietary aspects of its technology to distinguish its products from its competitors’ products. The Resulting Issuer relies on copyrights, trademarks, trade secrets, and confidentiality provisions to establish and protect our intellectual property.
The Resulting Issuer will not be able to register any United States federal trademarks or patents for its cannabis products due to producing, manufacturing, processing, possessing, distributing, selling, and using cannabis being a crime under the CSA. The United States Patent and Trademark Office will not permit the registration of any patent or trademark that identifies cannabis products. As a result, the Resulting Issuer likely will be unable to protect its cannabis product trademarks beyond the geographic areas in which it conducts business. The use of its trademarks outside the states in which it operates by one or more other persons could have a material adverse effect on the value of such trademarks.
Any infringement or misappropriation of the Resulting Issuer’s intellectual property could damage its value and limit its ability to compete. The Resulting Issuer may have to engage in litigation to protect the rights to its intellectual property, which could result in significant litigation costs and require a significant amount of its time. In addition, the Resulting Issuer’s ability to enforce and protect its intellectual property rights may be limited in certain countries outside the U.S., which could make it easier for competitors to capture market position in such countries by utilizing technologies that are similar to those developed or licensed by the Resulting Issuer.
Competitors may also harm the Resulting Issuer’s sales by designing products that mirror the capabilities of its products or technology without infringing on its intellectual property rights. If the Resulting Issuer does not obtain sufficient protection for its intellectual property, or if it is unable to effectively enforce its intellectual property rights, its competitiveness could be impaired, which would limit its growth and future revenue.
The Resulting Issuer may also find it necessary to bring infringement or other actions against third parties to seek to protect its intellectual property rights. Litigation of this nature, even if successful, is often expensive and time-consuming to prosecute and there can be no assurance that the Resulting Issuer will have the financial or other resources to enforce its rights or be able to enforce its rights or prevent other parties from developing similar technology or designing around its intellectual property.
Although the Resulting Issuer believes that its technology does not and will not infringe upon the patents or violate the proprietary rights of others, it is possible such infringement or violation has occurred or may occur, which could have a material adverse effect on the business.
-108-
The Resulting Issuer is not aware of any infringement by it of any person’s or entity’s intellectual property rights. In the event that products sold by the Resulting Issuer are deemed to infringe upon the patents or proprietary rights of others, the Resulting Issuer could be required to modify its products or obtain a license for the manufacture and/or sale of such products or cease selling such products. In such event, there can be no assurance that the Resulting Issuer would be able to do so in a timely manner, upon acceptable terms and conditions, or at all, and the failure to do any of the foregoing could have a material adverse effect upon the Resulting Issuer’s business.
There can be no assurance that the Resulting Issuer will have the financial or other resources necessary to enforce or defend a patent infringement or proprietary rights violation action. If the Resulting Issuer’s products or proposed products are deemed to infringe or likely to infringe upon the patents or proprietary rights of others, the Resulting Issuer could be subject to injunctive relief and, under certain circumstances, become liable for damages, which could also have a material adverse effect on the Resulting Issuer’s business and its financial condition.
Trade Secrets
The Resulting Issuer’s trade secrets may be difficult to protect as it depends upon the skills, knowledge, and experience of its scientific and technical personnel, consultants and advisors, as well as licensors and contractors. Because of the highly competitive nature of the U.S. cannabis industry, the Resulting Issuer relies in part on trade secrets to protect its proprietary technology and processes. However, trade secrets are difficult to protect. The Resulting Issuer enters into confidentiality or non-disclosure agreements with its corporate partners, employees, consultants, outside scientific collaborators, developers, and other advisors. These agreements generally require that the receiving party keep confidential and not disclose to third parties confidential information developed by the receiving party or made known to the receiving party by the Resulting Issuer during the course of the receiving party’s relationship with the Resulting Issuer. These agreements also generally provide that inventions conceived by the receiving party in the course of rendering services to the Resulting Issuer will be the Resulting Issuer’s exclusive property, and the Resulting Issuer enters into assignment agreements to perfect its rights.
These confidentiality, inventions, and assignment agreements may be breached and may not effectively assign intellectual property rights to the Resulting Issuer. Trade secrets also could be independently discovered by competitors, in which case the Resulting Issuer would not be able to prevent the use of such trade secrets by competitors. The enforcement of a claim alleging that a party illegally obtained and was using the Resulting Issuer’s trade secrets could be difficult, expensive, and time consuming and the outcome would be unpredictable. In addition, courts outside the U.S. may be less willing to protect trade secrets. The failure to obtain or maintain meaningful trade secret protection could adversely affect the Resulting Issuer’s competitive position.
Lack of Access to U.S. Bankruptcy Protections
Because the use of cannabis is illegal under U.S. federal law, many courts have denied cannabis businesses bankruptcy protections, thus making it very difficult for lenders to recoup their investments in the cannabis industry in the event of a bankruptcy. If the Resulting Issuer were to experience a bankruptcy, there is no guarantee that U.S. federal bankruptcy protections would be available to the Resulting Issuer, which would have a material adverse effect.
Fluctuations in Currency Exchange Rates
Fluctuations in currency exchange rates may adversely affect Cresco’s financial position. Fluctuations in currency exchange rates may significantly impact Cresco’s financial position and results. Cresco does not have in place a policy for managing or controlling foreign currency risks since, to date, its primary activities have not resulted in material exposure to foreign currency risk.
Lack of Earnings and Dividend Record
The Resulting Issuer does not anticipate declaring or paying dividends. Payments of any dividends will be at the discretion of the Resulting Issuer Board after taking into account many factors, including the financial condition and current and anticipated cash needs of the Resulting Issuer.
-109-
Insurance Coverage
The Resulting Issuer’s insurance coverage may be inadequate to cover all significant risk exposures as it will be exposed to liabilities that are unique to the products we provide. While the Resulting Issuer intends to maintain insurance for certain risks, the amount of its insurance coverage may not be adequate to cover all claims or liabilities, and it may be forced to bear substantial costs resulting from risks and uncertainties of its business. It is also not possible to obtain insurance to protect against all operational risks and liabilities. The failure to obtain adequate insurance coverage on terms favorable to the Reporting Issuer, or at all, could have a material adverse effect on its business, financial condition, and results of operations. The Reporting Issuer does not have any business interruption insurance. Any business disruption or natural disaster could result in substantial costs and diversion of resources.
Anti-money Laundering Laws and Regulations
The Resulting Issuer is subject to a variety of laws and regulations domestically and in the U.S. that involve money laundering, financial recordkeeping and proceeds of crime, including the Currency and Foreign Transactions Reporting Act of 1970 (commonly known as the Bank Secrecy Act), as amended by Title III of the Uniting and Strengthening America by Providing Appropriate Tools Required to Intercept and Obstruct Terrorism Act of 2001 (USA PATRIOT Act), the Proceeds of Crime (Money Laundering) and Terrorist Financing Act (Canada), as amended and the rules and regulations thereunder, the Criminal Code (Canada) and any related or similar rules, regulations or guidelines, issued, administered or enforced by governmental authorities in the U.S. and Canada.
In February 2014, the Financial Crimes Enforcement Network (“FCEN”) of the Treasury Department issued a memorandum (the “FCEN Memo”) providing instructions to banks seeking to provide services to marijuana-related businesses. The FCEN Memo states that in some circumstances, it is permissible for banks to provide services to marijuana-related businesses without risking prosecution for violation of U.S. federal money laundering laws. It refers to supplementary guidance that Deputy Attorney General Cole issued to U.S. federal prosecutors relating to the prosecution of money laundering offenses predicated on marijuana-related violations of the CSA. It is unclear at this time whether the current administration will follow the guidelines of the FCEN Memo.
In the event that any of the Resulting Issuer’s operations, or any proceeds thereof, any dividends or distributions therefrom, or any profits or revenues accruing from such operations in the U.S. were found to be in violation of money laundering legislation or otherwise, such transactions may be viewed as proceeds of crime under one or more of the statutes noted above or any other applicable legislation. This could restrict or otherwise jeopardize the ability of the Resulting Issuer to declare or pay dividends, effect other distributions or subsequently repatriate such funds back to Canada. Furthermore, while the Resulting Issuer has no current intention to declare or pay dividends on its Common Shares in the foreseeable future, in the event that a determination was made that the Resulting Issuer’s proceeds from operations (or any future operations or investments in the U.S.) could reasonably be shown to constitute proceeds of crime, the Resulting Issuer may decide or be required to suspend declaring or paying dividends without advance notice and for an indefinite period of time.
Risk of Civil Asset Forfeiture
Because the cannabis industry remains illegal under U.S. federal law, any property owned by participants in the cannabis industry which are either used in the course of conducting such business, or are the proceeds of such business, could be subject to seizure by law enforcement and subsequent civil asset forfeiture. Even if the owner of the property were never charged with a crime, the property in question could still be seized and subject to an administrative proceeding by which, with minimal due process, it could be subject to forfeiture.
Investments in the United States May be Subject to Heightened Scrutiny
For the reasons set forth above, the Resulting Issuer’s existing operations in the U.S., and any future operations or investments, may become the subject of heightened scrutiny by regulators, stock exchanges and other authorities in Canada. As a result, the Resulting Issuer may be subject to significant direct and indirect interaction with public officials. There can be no assurance that this heightened scrutiny will not in turn lead to the imposition of certain restrictions on the Resulting Issuer’s ability to operate or invest in the U.S. or any other jurisdiction, in addition to those described herein.
-110-
Government policy changes or public opinion may also result in a significant influence over the regulation of the marijuana industry in Canada, the U.S. or elsewhere. A negative shift in the public’s perception of medical marijuana in the U.S. or any other applicable jurisdiction could affect future legislation or regulation. Among other things, such a shift could cause state jurisdictions to abandon initiatives or proposals to legalize medical marijuana, thereby limiting the number of new state jurisdictions into which the Resulting Issuer could expand. Any inability to fully implement the Resulting Issuer’s expansion strategy may have a material adverse effect on the Resulting Issuer’s business, financial condition and results of operations.
Constraints on Marketing Products
The development of the Resulting Issuer’s business and operating results may be hindered by applicable restrictions on sales and marketing activities imposed by government regulatory bodies. The regulatory environment in the United States limits the Resulting Issuer’s ability to compete for market share in a manner similar to other industries. If the Resulting Issuer is unable to effectively market its products and compete for market share, or if the costs of compliance with government legislation and regulation cannot be absorbed through increased selling prices for its products, the Resulting Issuer’s sales and operating results could be adversely affected.
Settlements of Trades
On February 8, 2018, following discussions with the Canadian Securities Administrators and recognized Canadian securities exchanges, the TMX Group announced the signing of a Memorandum of Understanding (“MOU”) with Aequitas NEO Exchange Inc., the CSE, the Toronto Stock Exchange, and the Toronto Stock Venture Exchange. The MOU outlines the parties’ understanding of Canada’s regulatory framework applicable to the rules, procedures, and regulatory oversight of the exchanges and CDS Clearing and Depository Services Inc. (“CDS”) as it relates to issuers with cannabis-related activities in the United States. The MOU confirms, with respect to the clearing of listed securities, that CDS relies on the exchanges to review the conduct of listed issuers. As a result, there is no CDS ban on the clearing of securities of issuers with cannabis-related activities in the United States. However, there can be no guarantee that this approach to regulation will continue in the future. If such a ban were to be implemented at a time when the common shares are listed on a stock exchange, it would have a material adverse effect on the ability of holders of common shares to make and settle trades. In particular, the common shares would become highly illiquid until an alternative was implemented, investors would have no ability to effect a trade of the common shares through the facilities of the applicable stock exchange.
Access to Banks
The Resulting Issuer may have difficulty accessing the service of banks, which may make it difficult for it to operate.
Since the use of marijuana is illegal under U.S. federal law, and in light of concerns in the banking industry regarding money laundering and other federal financial crime related to marijuana, U.S. banks have been reluctant to accept deposit funds from businesses involved with the marijuana industry. Consequently, businesses involved in the marijuana industry often have difficulty finding a bank willing to accept their business. Likewise, marijuana businesses have limited, if any, access to credit card processing services. As a result, marijuana businesses in the U.S. are largely cash-based. This complicates the implementation of financial controls and increases security issues. The inability to open or maintain bank accounts or take credit cards may make it difficult for us to operate our contemplated medical marijuana businesses.
Legality of contracts
Because the Resulting Issuer’s contracts involve cannabis and other activities that are not legal under U.S. federal law and in some jurisdictions, the Resulting Issuer may face difficulties in enforcing its contracts in U.S. federal and certain state courts.
-111-
Unfavorable Tax Treatment of Cannabis Businesses
Under Section 280E (“Section 280E”) of the United States Internal Revenue Code of 1986 as amended (the “U.S. Tax Code”), “no deduction or credit shall be allowed for any amount paid or incurred during the taxable year in carrying on any trade or business if such trade or business (or the activities which comprise such trade or business) consists of trafficking in controlled substances (within the meaning of schedule I and II of the Controlled Substances Act) which is prohibited by Federal law or the law of any State in which such trade or business is conducted.” This provision has been applied by the U.S. Internal Revenue Service to cannabis operations, prohibiting them from deducting expenses directly associated with the sale of cannabis. Section 280E therefore has a significant impact on the retail side of cannabis, but a lesser impact on cultivation and manufacturing operations. A result of Section 280E is that an otherwise profitable business may, in fact, operate at a loss, after taking into account its U.S. income tax expenses. There are currently several pending cases before various administrative and federal courts challenging these restrictions. There can be no guarantee that any of these challenges will result in any favorable interpretation of Section 280E for cannabis businesses.
United States Tax Classification of the Resulting Issuer
The Resulting Issuer, which is and will continue to be a Canadian corporation as of the date of this Listing Statement, is also expected to be classified for U.S. federal income tax purposes as a United States corporation under Section 7874 of the Code. Section 7874 of the U.S. Tax Code, contains rules that can cause a non-United States corporation to be taxed as a United States corporation for United States federal income tax purposes. Under section 7874 of the U.S. Tax Code, a corporation created or organized outside the United States. (i.e., a non-United States corporation) will nevertheless be treated as a United States corporation for United States federal income tax purposes (such treatment is referred to as an inversion) if each of the following three conditions are met: (i) the non-United States corporation acquires, directly or indirectly, or is treated as acquiring under applicable United States Treasury Regulations, substantially all of the assets held, directly or indirectly, by a United States corporation or United States trade or business, (ii) after the acquisition, the former stockholders of the acquired United States corporation hold at least 80% (by vote or value) of the shares of the non-United States corporation by reason of holding shares of the United States acquired corporation, trade or business, and (iii) after the acquisition, the non-United States corporation’s expanded affiliated group does not have substantial business activities in the non- United States corporation’s country of organization or incorporation when compared to the expanded affiliated group’s total business activities.
The Resulting Issuer intends to be treated as a United States corporation for United States federal income tax purposes under section 7874 of the U.S. Tax Code and is expected to be subject to United States federal income tax on its worldwide income. However, for Canadian tax purposes, the Resulting Issuer is expected, regardless of any application of section 7874 of the U.S. Tax Code, to be treated as a Canadian resident company (as defined in the Income Tax Act (Canada) (the “ITA”) for Canadian income tax purposes. As a result, the Resulting Issuer will be subject to taxation both in Canada and the United States which could have a material adverse effect on its financial condition and results of operations. The Resulting Issuer may not qualify for certain U.S.-Canada income tax treaty benefits, which could have a material adverse effect on its financial condition and results of operations.
It is unlikely that the Resulting Issuer will pay any dividends on the Subordinate Voting Shares in the foreseeable future. However, dividends received by shareholders who are residents of Canada for purpose of the ITA will be subject to U.S. withholding tax. Any such dividends may not qualify for a reduced rate of withholding tax under the Canada-United States tax treaty. In addition, a foreign tax credit or a deduction in respect of foreign taxes may not be available.
Dividends received by U.S. shareholders will not be subject to U.S. withholding tax but will be subject to Canadian withholding tax. Dividends paid by the Resulting Issuer will be characterized as U.S. source income for purposes of the foreign tax credit rules under the U.S. Tax Code. Accordingly, U.S. shareholders generally will not be able to claim a credit for any Canadian tax withheld unless, depending on the circumstances, they have an excess foreign tax credit limitation due to other foreign source income that is subject to a low or zero rate of foreign tax.
Dividends received by shareholders that are neither Canadian nor U.S. shareholders will be subject to U.S. withholding tax and will also be subject to Canadian withholding tax. These dividends may not qualify for a reduced rate of U.S. withholding tax under any income tax treaty otherwise applicable to a shareholder of the Resulting Issuer, subject to examination of the relevant treaty.
-112-
Because the common shares will be treated as shares of a U.S. domestic corporation, the U.S. gift, estate and generation-skipping transfer tax rules generally apply to a non-U.S. shareholder of common shares.
EACH SHAREHOLDER SHOULD SEEK TAX ADVICE, BASED ON SUCH SHAREHOLDER’S PARTICULAR CIRCUMSTANCES, FROM AN INDEPENDENT TAX ADVISOR.
Consumer Acceptance of Marijuana
We are dependent on the popularity of consumer acceptance of Cresco product lines.
The Resulting Issuers ability to generate revenue and be successful in the implementation of the Corporation’s business plan is dependent on consumer acceptance and demand of Cresco products. Acceptance of Cresco products will depend on several factors, including availability, cost, ease of use, familiarity of use, convenience, effectiveness, safety, and reliability. If these customers do not accept Cresco products, or if such products fail to meet customers’ needs and expectations adequately, our ability to continue generating revenues could be reduced.
A drop in the retail price of medical marijuana products may negatively impact the business.
The demand for Cresco products depends in part on the price of commercially-grown marijuana. Fluctuations in economic and market conditions that impact the prices of commercially-grown marijuana, such as increases in the supply of such marijuana and the decrease in the price of products using commercially-grown marijuana, could cause the demand for marijuana products to decline, which would have a negative impact on our business.
Security Risks
As cash businesses, the premises of the marijuana dispensaries are a target for theft. While the Resulting Issuer has implemented security measures and continues to monitor and improve its security measures, its cultivation, processing and dispensary facilities could be subject to break-ins, robberies and other breaches in security. In the event of robbery or theft, the loss of cannabis plants, cannabis oils, cannabis flowers and cultivation and processing equipment could have a material adverse impact on the business, financial condition and results of operation of the Resulting Issuer.
As the Resulting Issuer’s business involves the movement and transfer of cash which is collected from dispensaries and used to purchase trim, accessories etc. or deposited into its bank, there is a risk of theft or robbery during the transport of cash. The Resulting Issuer has engaged a security firm to provide armed guards and security in the transport and movement of large amounts of cash. Sales representatives sometimes transport cash and/or products and each sales representative has a panic button in their vehicle and, if requested, may be escorted by armed guards. While the Resulting Issuer has taken robust steps to prevent theft or robbery of cash during transport, there can be no assurance that there will not be a security breach during the transport and the movement of cash involving the theft of product or cash.
Risk of Litigation
If the Resulting Issuer incurs substantial liability from litigation, complaints, or enforcement actions, its financial condition, business and results of operation could suffer.
The Resulting Issuer’s participation in the medical marijuana industry may lead to litigation, formal or informal complaints, enforcement actions, and inquiries by various U.S. federal, State, or local governmental authorities against the Resulting Issuer or its subsidiaries. Litigation, complaints, and enforcement actions involving the Resulting Issuer or its subsidiaries could consume considerable amounts of financial and other corporate resources, which could have a negative impact on our sales, revenue, profitability, and growth prospects. The Resulting Issuer’s subsidiaries are presently engaged in the distribution of marijuana, however, neither the Resulting Issuer nor its subsidiaries are currently, subject to any litigation, complaint or enforcement action regarding marijuana brought by any U.S. federal, state, or local governmental authority with respect to the business.
-113-
From time-to-time in the normal course of business operations, the Resulting Issuer may become subject to litigation that may result in liability material to its financial statements as a whole or may negatively affect its operating results if changes to its business operations are required. The cost to defend such litigation may be significant and may require a diversion of resources. There also may be adverse publicity associated with litigation that could negatively affect customer perception of the business, regardless of whether the allegations are valid or whether the Resulting Issuer is ultimately found liable. Insurance may not be available at all or in sufficient amounts to cover any liabilities with respect to these or other matters. A judgment or other liability in excess of the Resulting Issuer’s insurance coverage for any claims could adversely affect its business and the results of operations.
Risks Inherent in an Agricultural Business
The Resulting Issuer’s business involves the growing of medical and adult-use marijuana, an agricultural product. Such business will be subject to the risks inherent in the agricultural business, such as insects, plant diseases and similar agricultural risks. Although the Resulting Issuer expects that any such growing will be completed indoors under climate controlled conditions, there can be no assurance that natural elements will not have a material adverse effect on any such future production.
Reliance on License
The Resulting Issuer’s ability to cultivate, store, produce and distribute medical and adult-use marijuana products in Illinois, Pennsylvania, Ohio, Nevada, Arizona, California and New York is dependent on maintaining its licenses in good standing with each applicable State regulator. Failure to comply with the requirements of any of its licenses or any failure to maintain any of the licenses would have a material adverse impact on the business, financial condition and operating results of the Resulting Issuer. The Resulting Issuer’s (or its subsidiaries) licences related to its ability to cultivate, store, produce and distribute medical and adult-use marijuana products (as applicable) in Illinois, Pennsylvania, Ohio, Nevada, Arizona, California and New York are currently in good standing and the Resulting Issuer remains fully compliant with the respective associated state laws and regulations.
Product Liability
As a distributor of products designed to be ingested by humans, the Resulting Issuer faces an inherent risk of exposure to product liability claims, regulatory action and litigation if its products are alleged to have caused significant loss or injury. In addition, the sale of the Resulting Issuer’s products involves the risk of injury to consumers due to tampering by unauthorized third parties or product contamination. Previously unknown adverse reactions resulting from human consumption of the Resulting Issuer’s products alone or in combination with other medications or substances could occur. The Resulting Issuer may be subject to various product liability claims, including, among others, that the Cresco’s products caused injury or illness, include inadequate instructions for use or include inadequate warnings concerning possible side effects or interactions with other substances.
A product liability claim or regulatory action against the Resulting Issuer could result in increased costs, could adversely affect the Resulting Issuer’s reputation with its clients and consumers generally, and could have a material adverse effect on our results of operations and financial condition of the Resulting Issuer. Although the Resulting Issuer has secured product liability insurance, and strictly enforces a quality standard within the operations, there can be no assurances that the Resulting Issuer will be able to maintain its product liability insurance on acceptable terms or with adequate coverage against potential liabilities. This scenario could prevent or inhibit the commercialization of the Resulting Issuer’s potential products. To date, there have been no product related issues.
Product Recalls
Manufacturers and distributors of products are sometimes subject to the recall or return of their products for a variety of reasons, including product defects, such as contamination, unintended harmful side effects or interactions with other substances, packaging safety and inadequate or inaccurate labeling disclosure. If any of the Resulting Issuer’s products
-114-
are recalled due to an alleged product defect or for any other reason, the Resulting Issuer could be required to incur the unexpected expense of the recall and any legal proceedings that might arise in connection with the recall. The Resulting Issuer may lose a significant amount of sales and may not be able to replace those sales at an acceptable margin or at all. In addition, a product recall may require significant management attention. Although the Resulting Issuer has detailed procedures in place for testing finished products, there can be no assurance that any quality, potency or contamination problems will be detected in time to avoid unforeseen product recalls, regulatory action or lawsuits. Additionally, if one of the Resulting Issuer’s significant brands were subject to recall, the image of that brand and the Resulting Issuer as its owner could be harmed. A recall for any of the foregoing reasons could lead to decreased demand for the Resulting Issuer’s products and could have a material adverse effect on the results of operations and financial condition of the Resulting Issuer. Additionally, product recalls may lead to increased scrutiny of the Resulting Issuer’s operations by the FDA or other regulatory agencies, requiring further management attention and potential legal fees and other expenses.
Regulatory or Agency proceedings, Investigations and Audits
The Resulting Issuer’s business requires compliance with many laws and regulations. Failure to comply with these laws and regulations could subject the Resulting Issuer to regulatory or agency proceedings or investigations and could also lead to damage awards, fines and penalties. The Resulting Issuer may become involved in a number of government or agency proceedings, investigations and audits. The outcome of any regulatory or agency proceedings, investigations, audits, and other contingencies could harm the Resulting Issuer’s reputation, require the Resulting Issuer to take, or refrain from taking, actions that could harm its operations or require the Resulting Issuer to pay substantial amounts of money, harming its financial condition. There can be no assurance that any pending or future regulatory or agency proceedings, investigations and audits will not result in substantial costs or a diversion of management’s attention and resources or have a material adverse impact on the Resulting Issuer’s business, financial condition and results of operation.
Newly Established Legal Regime
The Resulting Issuer business activities will rely on newly established and/or developing laws and regulations in the states in which it operates. These laws and regulations are rapidly evolving and subject to change with minimal notice. Regulatory changes may adversely affect the Resulting Issuer’s profitability or cause it to cease operations entirely. The cannabis industry may come under the scrutiny or further scrutiny by the FDA, Securities and Exchange Commission, the Department of Justice, the Financial Industry Regulatory Advisory or other U.S. federal or applicable state or nongovernmental regulatory authorities or self-regulatory organizations that supervise or regulate the production, distribution, sale or use of cannabis for medical or nonmedical purposes in the United States. It is impossible to determine the extent of the impact of any new laws, regulations or initiatives that may be proposed, or whether any proposals will become law. The regulatory uncertainty surrounding the industry may adversely affect the business and operations of the Resulting Issuer, including without limitation, the costs to remain compliant with applicable laws and the impairment of its business or the ability to raise additional capital.
General Economic Risks
The Resulting Issuer’s operations could be affected by the economic context should the unemployment level, interest rates or inflation reach levels that influence consumer trends and spending and, consequently, impact the Resulting Issuer’s sales and profitability.
| 18. | Promoters |
No person or company has been within the two years immediately preceding the date of this Listing Statement, a promoter of the Resulting Issuer.
| 19. | Legal Proceedings |
Other than a civil dispute whereby Cresco, as plaintiff, is seeking damages for breach of contract, to the Resulting Issuer’s knowledge, there are no legal proceedings or regulatory actions material to the Resulting Issuer to which it is a party, or has been a party to, or of which any of its property is or was the subject matter of, and no such proceedings or actions are known by the Resulting Issuer to be contemplated.
-115-
There have been no penalties or sanctions imposed against the Resulting Issuer by a court or regulatory authority, and the Resulting Issuer has not entered into any settlement agreements before any court relating to provincial or territorial securities legislation or with any securities regulatory authority, in the three years prior to the date of this Listing Statement.
| 20. | Interest of Management and Others in Material Transactions |
Other than as disclosed below and elsewhere in this Listing Statement no director, executive officer or unitholder or shareholder that beneficially owns, or controls or directs, directly or indirectly, more than 10% of the issued Common Units or more than 10% of the voting securities of the Resulting Issuer, or any of their respective associates or affiliates, has any material interest, direct or indirect, in any transaction within the three years before the date of this Listing Statement which has materially affected or is reasonably expected to materially affect the Resulting Issuer or a subsidiary of the Resulting Issuer.
| 21. | Auditors, Transfer Agents and Registrars |
The auditor of the Resulting Issuer is MNP LLP and the transfer agent and registrar for the Subordinate Voting Shares is Odyssey Trust Company at its principal offices in Calgary, Alberta.
| 22. | Material Contracts |
During the course of the two years prior to the date of the Listing Statement, Cresco has entered into the following material contracts, other than contracts entered into in the ordinary course of business:
| • | Support Agreement entered into by and among the Resulting Issuer, Cresco Corp and Cresco; |
| • | Tax Receivable Agreement entered into by and among Cresco Corp, Cresco, the Cresco Members and the Cresco LTIP Unitholders; |
| • | Letter Agreement between Randsburg and Cresco regarding the Business Combination; |
| • | Agency Agreement among the Agents, Cresco and Cresco Acquisition relating to the SR Offering; |
| • | Subscription Receipt Agreement among the Escrow Agent, Cresco Acquisition, Cresco and Randsburg relating to the SR Offering; and |
| • | A&R LLC Agreement |
23. Interest of Experts
No person or corporation whose profession or business gives authority to a statement made by the person or corporation and who is named as having prepared or certified a part of this Listing Statement or as having prepared or certified a report or valuation described or included in this Listing Statement holds any beneficial interest, direct or indirect, in any securities or property of Cresco or of an Associate or Affiliate of Cresco and no such person is expected to be elected, appointed or employed as a director, senior officer or employee of Cresco or of an Associate or Affiliate of Cresco and no such person is a promoter of Cresco or an Associate or Affiliate of Cresco. UHY McGovern Hurley LLP is independent of Cresco in accordance with the rules of professional conduct of the Institute of Chartered Professional Accountants of Ontario. FGMK, LLC, is independent of Cresco in accordance with the rules of professional conduct of the Institute of Chartered Professional Accountants of Illinois.
-116-
24. Other Material Facts
CERTAIN UNITED STATES FEDERAL INCOME TAX CONSIDERATIONS
The following discussion is a summary of certain material U.S. federal income tax considerations for U.S. Holders and Non-U.S. Holders (each as defined below) relating to the ownership and disposition of Subordinate Voting Shares. This summary is general in nature and does not discuss all aspects of U.S. federal income taxation that may be relevant to a holder of Subordinate Voting Shares in light of its particular circumstances. In addition, this summary does not address the U.S. federal alternative minimum tax, the Medicare tax on net investment income, U.S. federal estate and gift taxes, U.S. state and local taxes or foreign taxes. This summary deals only with Subordinate Voting Shares held as capital assets within the meaning of Section 1221 of the Code (generally, property held for investment), and does not address tax considerations applicable to any holder of Subordinate Voting Shares that may be subject to special treatment under the United States federal income tax laws, including:
| • | a bank or other financial institution; |
| • | a tax-exempt or governmental organization; |
| • | a retirement plan or other tax-deferred account (other than with respect to US Holders in the 401(k) Plan),; |
| • | a partnership, an S corporation or other entity treated as a partnership or pass-through (or an investor therein); |
| • | an insurance company; |
| • | a mutual fund, regulated investment company or real estate investment trust; |
| • | a person that purchases or sells Subordinate Voting Shares as part of a wash sale for tax purposes; |
| • | a dealer or broker in stocks and securities, or currencies; |
| • | a trader in securities that elects mark-to-market treatment; |
| • | a holder of Subordinate Voting Shares subject to the alternative minimum tax provisions of the Code; |
| • | a holder of Subordinate Voting Shares that received Subordinate Voting Shares through the exercise of an employee stock option, through a tax qualified retirement plan or otherwise as compensation; |
| • | a person that owns (or is deemed to own) 5% or more of the outstanding Subordinate Voting Shares; |
| • | a U.S. Holder whose functional currency is not the U.S. dollar; |
| • | a person that holds Subordinate Voting Shares as part of a hedge, straddle, constructive sale, conversion or other integrated transaction; |
| • | “controlled foreign corporations”; |
| • | “passive foreign investment companies”; or |
| • | a U.S. expatriate. |
This summary is based on the Code, Treasury Regulations promulgated under the Code, and rulings and judicial decisions, all as in effect as of the date hereof, and all of which are subject to change or differing interpretations at any time, possibly with retroactive effect. We have not sought, and do not intend to seek, any ruling from the IRS with respect to the statements made and the conclusions reached in the following summary, and no assurance can be given that the IRS will agree with the views expressed herein, or that a court will not sustain any challenge by the IRS in the event of litigation.
If a partnership (including any entity or arrangement treated as a partnership for U.S. federal income tax purposes) holds Subordinate Voting Shares, the tax treatment of a partner in the partnership generally will depend upon the status of the partner and the activities of the partnership. A partner in a partnership should consult its own tax advisors regarding the tax consequences of acquiring, holding and disposing of Subordinate Voting Shares.
THIS DISCUSSION IS INTENDED ONLY AS A GENERAL SUMMARY OF CERTAIN MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES TO A HOLDER OF SHARES. WE URGE BENEFICIAL OWNERS OF SHARES TO CONSULT THEIR TAX ADVISORS WITH RESPECT TO THE SPECIFIC TAX CONSEQUENCES OF THE OFFER OR THE MERGER IN LIGHT OF THEIR PARTICULAR CIRCUMSTANCES.
-117-
U.S. Holders.
A “U.S. Holder” of Subordinate Voting Shares means a holder that is for U.S. federal income tax purposes:
| • | An individual citizen or resident of the U.S.; |
| • | A corporation (or other entity taxable as a corporation) created or organized in or under the laws of the U.S. or any state thereof or the District of Columbia; |
| • | An estate the income of which is subject to U.S. federal income taxation regardless of its source; or |
| • | A trust if it: (1) is subject to the primary supervision of a court within the U.S. and one or more U.S. persons have the authority to control all substantial decisions of the trust; or (2) has a valid election in effect under applicable Treasury Regulations to be treated as a U.S. person. |
With respect to the first bullet point above, an individual is generally treated as a resident of the U.S. in any calendar year for U.S. federal income tax purposes if the individual either (i) is the holder of a green card, generally during any point of such year, or (ii) is present in the U.S. for at least 31 days in that calendar year, and for an aggregate of at least 183 days during the three-year period ending on the last day of the current calendar year. For purposes of the 183-day calculation (often referred to as the Substantial Presence Test), all of the days present in the U.S. during the current year, one-third of the days present in the U.S. during the immediately preceding year, and one-sixth of the days present in the second preceding year are counted. Residents are generally treated for U.S. federal income tax purposes as if they were U.S. citizens.
Non-U.S. Holders.
A “Non-U.S. Holder” is a beneficial owner of Subordinate Voting Shares (other than an entity or arrangement classified as a partnership for U.S. federal income tax purposes) that is not a U.S. Holder.
Tax Classification as a U.S. Domestic Corporation
As a result of the Business Combination, pursuant to Section 7874(b) of the Code and the Treasury Regulations promulgated thereunder, notwithstanding that the Resulting Issuer is organized under the provisions of the BCBCA, solely for U.S. federal income tax purposes, it is anticipated that the Resulting Issuer will be treated as a U.S. domestic corporation.
The Resulting Issuer anticipates that it will experience a number of significant and complicated U.S. federal income tax consequences as a result of being treated as a U.S. domestic corporation for U.S. federal income tax purposes, and this summary does not attempt to describe all such U.S. federal income tax consequences. Section 7874 of the Code and the Treasury Regulations promulgated thereunder do not address all the possible tax consequences that arise from the Resulting Issuer being treated as a U.S. domestic corporation for U.S. federal income tax purposes. Accordingly, there may be additional or unforeseen U.S. federal income tax consequences to the Resulting Issuer that are not discussed in this summary.
Generally, the Resulting Issuer will be subject to U.S. federal income tax on its worldwide taxable income (regardless of whether such income is “U.S. source” or “foreign source”) and will be required to file a U.S. federal income tax return annually with the IRS. The Resulting Issuer anticipates that it will also be subject to tax in Canada. It is unclear whether the Resulting Issuer will be entitled to foreign tax credits under the Code and how the foreign tax credit rules will operate in certain circumstances, as a result of the Resulting Issuer being treated as a U.S. domestic corporation for U.S. federal income tax purposes and the taxation of the Resulting Issuer also in Canada. Accordingly, it is possible that the Resulting Issuer will be subject to double taxation with respect to all or part of its taxable income. It is anticipated that such U.S. and Canadian tax treatment will continue indefinitely and that the Subordinate Voting Shares will be treated indefinitely as shares in a U.S. domestic corporation for U.S. federal income tax purposes, notwithstanding future transfers. The remainder of this summary assumes that the Resulting Issuer will be treated as a U.S. domestic corporation for U.S. federal income tax purposes.
-118-
Tax Considerations for U.S. Holders
Distributions
Distributions of cash or property with respect to Subordinate Voting Shares will constitute dividends for U.S. federal income tax purposes to the extent paid from the Resulting Issuer’s current or accumulated earnings and profits, as determined under U.S. federal income tax principles. Dividends will generally be taxable to a non-corporate U.S. Holder at the preferential rates applicable to long-term capital gains, provided that such holder meets certain holding period and other requirements. Distributions in excess thereof will generally be treated first, as a return of capital and be applied against, and reduce, a U.S. Holder’s adjusted tax basis in its Subordinate Voting Shares, but not below zero, and thereafter be treated as capital gain and treated as described under “– Sale or Other Taxable Disposition” below. Dividends received by corporate U.S. Holders may be eligible for a dividends received deduction, subject to certain restrictions relating to, among others, the corporate U.S. Holder’s taxable income, holding period and debt financing.
Sale or Other Taxable Disposition
Upon the sale or other taxable disposition of Subordinate Voting Shares, a U.S. Holder will generally recognize capital gain or loss equal to the difference between (i) the amount realized by such U.S. Holder in connection with such sale or other taxable disposition, and (ii) such U.S. Holder’s adjusted tax basis in such stock. Such capital gain or loss will generally be long-term capital gain or loss if the U.S. Holder’s holding period respecting such stock is more than twelve months. U.S. Holders who are individuals are eligible for preferential rates of taxation respecting their long-term capital gains. Deductions for capital losses are subject to limitations.
Foreign Tax Credit Limitations
Because it is anticipated that the Resulting Issuer will be subject to tax both as a U.S. domestic corporation and as a Canadian corporation, a U.S. Holder may pay, through withholding, Canadian tax, as well as U.S. federal income tax, with respect to dividends paid on Subordinate Voting Shares. For U.S. federal income tax purposes, a U.S. Holder generally may elect for any taxable year to receive either a credit or a deduction for foreign income taxes paid by the holder during the year. Complex limitations apply to the foreign tax credit, including a general limitation that the credit cannot exceed the proportionate share of a taxpayer’s U.S. federal income tax that the taxpayer’s foreign source taxable income bears to the taxpayer’s worldwide taxable income. In applying this limitation, items of income and deduction must be classified, under complex rules, as either foreign source or U.S. source. The status of the Resulting Issuer as a U.S. domestic corporation for U.S. federal income tax purposes will cause dividends paid by the Resulting Issuer to be treated as U.S. source rather than foreign source for this purpose. As a result, a foreign tax credit may be unavailable to U.S. Holders for any Canadian tax paid on dividends received from the Resulting Issuer. Similarly, to the extent a sale or disposition of the Subordinate Voting Shares by a U.S. Holder results in Canadian tax payable by the U.S. Holder (for example, in the event the Subordinate Voting Shares constitute taxable Canadian property within the meaning of the ITA), a U.S. foreign tax credit may be unavailable to the U.S. Holder for such Canadian tax. In each case, however, the U.S. Holder should be able to take a deduction for the U.S. Holder’s Canadian tax paid, provided that the U.S. Holder has not elected to credit other foreign taxes during the same taxable year. The foreign tax credit rules are complex, and each U.S. Holder should consult its own tax advisors regarding these rules.
Foreign Currency
The amount of any distribution paid to a U.S. Holder in foreign currency, or the amount of proceeds paid in foreign currency on the sale, exchange or other taxable disposition of Subordinate Voting Shares, generally will be equal to the U.S. dollar value of such foreign currency based on the exchange rate applicable on the date of receipt (regardless of whether such foreign currency is converted into U.S. dollars at that time). A U.S. Holder will have a basis in the foreign currency equal to its U.S. dollar value on the date of receipt. Any U.S. Holder who converts or otherwise disposes of the foreign currency after the date of receipt may have a foreign currency exchange gain or loss that would be treated as ordinary income or loss, and generally will be U.S. source income or loss for foreign tax credit purposes. Different rules apply to U.S. Holders who use the accrual method of tax accounting. Each U.S. Holder should consult its own U.S. tax advisors regarding the U.S. federal income tax consequences of receiving, owning, and disposing of foreign currency.
-119-
Information Reporting and Backup Withholding
U.S. backup withholding (currently 24%) is imposed upon certain payments to persons that fail (or are unable) to furnish the information required pursuant to U.S. information reporting requirements. Distributions to U.S. Holders will generally be exempt from backup withholding, provided the U.S. Holder meets applicable certification requirements, including providing a U.S. taxpayer identification number on a properly completed IRS Form W-9, or otherwise establishes an exemption. The Resulting Issuer must report annually to the IRS and to each U.S. Holder the amount of distributions and dividends paid to that U.S. Holder and the proceeds from the sale or other disposition of Subordinate Voting Shares, unless such U.S. Holder is an exempt recipient.
Backup withholding does not represent an additional tax. Any amounts withheld from a payment to a U.S. Holder under the backup withholding rules will generally be allowed as a credit against such U.S. Holder’s U.S. federal income tax liability, and may entitle such U.S. Holder to a refund, provided the required information and returns are timely furnished by such U.S. Holder to the IRS.
Tax Considerations for Non-U.S. Holders
Distributions
Distributions of cash or property on Subordinate Voting Shares will constitute U.S. source dividends for U.S. federal income tax purposes to the extent paid from the Resulting Issuer’s current or accumulated earnings and profits, as determined under U.S. federal income tax principles. Distributions in excess thereof will first constitute a return of capital and be applied against and reduce a Non-U.S. Holder’s adjusted tax basis in its Subordinate Voting Shares, but not below zero, and thereafter be treated as capital gain and will be treated as described under “Sale or Other Taxable Disposition” below.
Subject to the discussions under “Information Reporting and Backup Withholding” and under “FATCA” below, any dividend paid to a Non-U.S. Holder of Subordinate Voting Shares generally will be subject to U.S. federal withholding tax at a rate of 30%, or such lower rate as may be specified under an applicable income tax treaty, unless the dividend is effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the U.S.. In order to receive a reduced treaty rate, a Non-U.S. Holder must provide its financial intermediary with an IRS Form W-8BEN or IRS Form W-8BEN-E, as applicable (or an appropriate successor form), properly certifying such holder’s eligibility for the reduced rate. If a Non-U.S. Holder holds Subordinate Voting Shares through a financial institution or other agent acting on the Non-U.S. Holder’s behalf, the Non-U.S. Holder will be required to provide appropriate documentation to such agent, and the Non-U.S. Holder’s agent will then be required to provide such (or a similar) certification to us, either directly or through other intermediaries. A Non-U.S. Holder that does not timely furnish the required certification, but that qualifies for a reduced treaty rate, generally may apply for and obtain a refund of any excess amounts withheld by timely filing an appropriate claim for refund with the IRS. Non-U.S. Holders should consult their own tax advisors regarding their entitlement to benefits under any applicable income tax treaty.
Dividends paid to a Non-U.S. Holder that are effectively connected with the Non-U.S. Holder’s conduct of a trade or business in the U.S. (or, if required by an applicable income tax treaty, are attributable to a U.S. permanent establishment, or fixed base, of the Non-U.S. Holder) generally will be exempt from the withholding tax described above and instead will be subject to U.S. federal income tax on a net income basis at regular graduated U.S. federal income tax rates applicable to U.S. Holders. In such case, the Resulting Issuer will not have to withhold U.S. federal tax so long as the Non-U.S. Holder timely complies with the applicable certification and disclosure requirements. In order to obtain this exemption from withholding tax, a Non-U.S. Holder must provide its financial intermediary with an IRS Form W-8ECI properly certifying its eligibility for such exemption. Any such effectively connected dividends received by a corporate Non-U.S. Holder may be subject to an additional “branch profits tax” at a rate of 30% (or such lower rate as may be specified by an applicable income tax treaty), as adjusted for certain items. Non-U.S. Holders should consult their own tax advisors regarding any applicable tax treaties that may provide for different rules.
-120-
Sale or Other Taxable Disposition
Subject to the discussions under “Information Reporting and Backup Withholding” and under “FATCA” below, any gain realized on the sale or other disposition of Subordinate Voting Shares by a Non-U.S. Holder generally will not be subject to U.S. federal income tax unless:
| • | the gain is effectively connected with the Non-U.S. Holder’s conduct of a trade or business in the U.S. (or, if required by an applicable income tax treaty, is attributable to a U.S. permanent establishment, or fixed base, of the Non-U.S. Holder); |
| • | the Non-U.S. Holder is an individual who is present in the United States for 183 days or more in the taxable year of disposition, and certain other conditions are met; or |
| • | the rules of the Foreign Investment in Real Property Tax Act of 1980 (“FIRPTA”) apply to treat the gain as effectively connected with a U.S. trade or business. |
A Non-U.S. Holder who has gain that is described in the first bullet point immediately above generally will be subject to U.S. federal income tax on the gain derived from the sale or other disposition pursuant to regular graduated U.S. federal income tax rates in the same manner as if it were a U.S. Holder. In addition, a corporate Non-U.S. Holder described in the first bullet point immediately above may be subject to the branch profits tax equal to 30% of its effectively connected earnings and profits (or at such lower rate as may be specified by an applicable income tax treaty), as adjusted for certain items.
A Non-U.S. Holder who meets the requirements described in the second bullet point immediately above will be subject to a flat 30% tax (or a lower tax rate specified by an applicable tax treaty) on the gain derived from the sale or other disposition, which gain may be offset by certain U.S. source capital losses (even though the individual is not considered a resident of the U.S.), provided the Non-U.S. Holder has timely filed U.S. federal income tax returns with respect to such losses.
With respect to the third bullet point above, pursuant to FIRPTA, in general, a Non-U.S. Holder is subject to U.S. federal income tax in the same manner as a U.S. Holder on any gain realized on the sale or other disposition of a “U.S. real property interest” (“USRPI”). For purposes of these rules, a USRPI generally includes stock in a U.S. corporation if such corporation’s interests in U.S. real property constitute 50% or more, by value, of the sum of the U.S. corporation’s (i) assets used in a trade or business, (ii) U.S. real property interests, and (iii) interests in real property outside of the U.S. A U.S. corporation whose interests in U.S. real property constitute 50% or more, by value, of the sum of such assets is commonly referred to as a U.S. real property holding corporation (“USRPHC”). If Subordinate Voting Shares are treated as regularly traded on an established securities market (within the meaning of Section 897(c)(3) of the Code), FIRPTA generally will not apply to a disposition of Subordinate Voting Shares by a Non-U.S. Holder that owns directly (or is deemed to own pursuant to attribution rules) 5% or less of the Subordinate Voting Shares at any time during the relevant period, in which case such gain will be subject to U.S. federal income tax at rates generally applicable to U.S. Holders, except that the branch profits tax will not apply. The Resulting Issuer believes that it is not, and has not been, a USRPHC at any time during the relevant five-year period.
Information Reporting and Backup Withholding
With respect to distributions and dividends on Subordinate Voting Shares, the Resulting Issuer must report annually to the IRS and to each Non-U.S. Holder the amount of distributions and dividends paid to such Non-U.S. Holder and any tax withheld with respect to such distributions and dividends, regardless of whether withholding was required with respect thereto. Copies of the information returns reporting such dividends and distributions and withholding also may be made available to the tax authorities in the country in which the Non-U.S. Holder resides or is established under the provisions of an applicable income tax treaty, tax information exchange agreement or other arrangement. A Non-U.S. Holder will be subject to backup withholding for dividends and distributions paid to such Non-U.S. Holder unless either (i) such Non-U.S. Holder certifies under penalty of perjury that it is not a U.S. person (as defined in the Code), which certification is generally satisfied by providing a properly executed IRS Form W-8BEN, IRS Form W-8BEN-E, or IRS Form W-8ECI (or appropriate successor form), and the payor does not have actual knowledge or reason to know that such holder is a U.S. person, or (ii) such Non-U.S. Holder otherwise establishes an exemption.
-121-
With respect to sales or other dispositions of Subordinate Voting Shares, information reporting and, depending on the circumstances, backup withholding will apply to the proceeds of a sale or other disposition of Subordinate Voting Shares within the U.S. or conducted through certain U.S.-related financial intermediaries, unless either (i) such Non-U.S. Holder certifies under penalty of perjury that it is not a U.S. person (as defined in the Code), which certification is generally satisfied by providing a properly executed IRS Form W-8BEN, IRS Form W-8BEN-E, or IRS Form W-8ECI (or appropriate successor form), and the payor does not have actual knowledge or reason to know that such holder is a U.S. person, or (ii) such Non-U.S. Holder otherwise establishes an exemption.
Whether with respect to distributions and dividends, or the sale or other disposition of Subordinate Voting Shares, backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules may be allowed as a refund or a credit against a Non-U.S. Holder’s U.S. federal income tax liability, if any, provided the required information is timely furnished to the IRS.
FATCA
Withholding taxes may be imposed pursuant to FATCA (Sections 1471 through 1474 of the Code) on certain types of payments made to non-U.S. financial institutions and certain other non-U.S. entities. Specifically, except as discussed below, a 30% withholding tax may be imposed on dividends on, or gross proceeds from the sale or other disposition (including certain distributions treated as a sale or other disposition) of, Subordinate Voting Shares paid to a “foreign financial institution” or a “non-financial foreign entity” (each as defined in the Code).
Such 30% FATCA withholding will not apply to a foreign financial institution if such institution undertakes certain diligence and reporting obligations, or otherwise qualifies for an exemption from these rules. The diligence and reporting obligations include, among others, entering into an agreement with the U.S. Department of Treasury pursuant to which the foreign financial institution must (i) undertake to identify accounts held by certain “specified United States persons” or “United States-owned foreign entities” (each as defined in the Code), (ii) annually report certain information about such accounts, and (iii) withhold 30% on certain payments to non-compliant foreign financial institutions and certain other account holders. Foreign financial institutions located in jurisdictions that have an intergovernmental agreement with the U.S. governing FATCA may be subject to different rules.
The 30% FATCA withholding will not apply to a non-financial foreign entity which either certifies that it does not have any “substantial United States owners” (as defined in the Code), furnishes identifying information regarding each substantial United States owner, or otherwise qualifies for an exemption from these rules.
Under the applicable Treasury Regulations and administrative guidance, withholding under FATCA (i) generally applies currently to payments of dividends on Subordinate Voting Shares, and (ii) will apply to payments of gross proceeds from the sale or other disposition of such stock (including certain distributions treated as a sale or other disposition) on or after January 1, 2019.
United States Tax Classification of the Resulting Issuer
The Resulting Issuer, which is and will continue to be a Canadian corporation as of the date of this Listing Statement, is also expected to be classified for U.S. federal income tax purposes as a United States corporation under Section 7874 of the Code. Under Section 7874 of the Code, a corporation created or organized outside the United States. (i.e., a non-United States corporation) will nevertheless be treated as a United States corporation for United States federal income tax purposes (such treatment is referred to as an inversion if each of the following three conditions are met (i) the non-United States corporation acquires, directly or indirectly, or is treated as acquiring under applicable United States Treasury Regulations, substantially all of the assets held, directly or indirectly, by a United States corporation or United States trade or business, (ii) after the acquisition, the former owners of the acquired United States corporation or business hold at least 80% (by vote or value) of the shares of the non-United States corporation by reason of their ownership of the United States acquired corporation, trade or business, and (iii) after the acquisition, the non-United States corporation’s expanded affiliated group does not have substantial business activities in the non-United States corporation’s country of organization or incorporation when compared to the expanded affiliated group’s total business.
-122-
The Resulting Issuer expects and intends to be treated as a United States corporation for United States federal income tax purposes under Section 7874 of the U.S. Tax Code and is expected to be subject to United States federal income tax on its worldwide income indefinitely. However, for Canadian tax purposes, the Resulting Issuer is expected, regardless of any application of Section 7874 of the Code, to be treated as a Canadian resident company (as defined in the ITA for Canadian income tax purposes.
The Resulting Issuer will be subject to taxation both in Canada and the United States and also may not qualify for certain U.S.-Canada income tax treaty benefits, which could have a material adverse effect on its financial condition and results of operations.
It is unlikely that the Resulting Issuer will pay any dividends on the common shares in the foreseeable future. However, dividends received by shareholders who are residents of Canada for purpose of the ITA will be subject to U.S. withholding tax. Any such dividends may not qualify for a reduced rate of withholding tax under the Canada-United States tax treaty. In addition, a foreign tax credit or a deduction in respect of foreign taxes may not be available.
As described above, Dividends received by U.S. Holders will not be subject to U.S. withholding tax but will be subject to Canadian withholding tax. Dividends paid by the Resulting Issuer will be characterized as U.S. source income for purposes of the foreign tax credit rules under the U.S. Tax Code. Accordingly, U.S. Holders generally will not be able to claim a credit for any Canadian tax withheld unless, depending on the circumstances, they have an excess foreign tax credit limitation due to other foreign source income that is subject to a low or zero rate of foreign tax.
Dividends received by shareholders that are neither Canadian nor U.S. shareholders will be subject to U.S. withholding tax and will also be subject to Canadian withholding tax. These dividends may not qualify for a reduced rate of U.S. withholding tax under any income tax treaty otherwise applicable to a shareholder of the Resulting Issuer, subject to examination of the relevant treaty.
Because Subordinate Voting Shares will be treated as shares of a U.S. domestic corporation, the U.S. gift, estate and generation-skipping transfer tax rules generally apply to a non-U.S. shareholder of common shares.
THE FOREGOING SUMMARY IS NOT INTENDED TO CONSTITUTE A COMPLETE DESCRIPTION OF ALL TAX CONSEQUENCES THAT MAY BE RELEVANT TO PARTICULAR HOLDERS OF SUBORDINATE VOTING SHARES AND IS NOT TAX OR LEGAL ADVICE. HOLDERS OF SUBORDINATE VOTING SHARES SHOULD CONSULT THEIR OWN TAX ADVISORS AS TO THE PARTICULAR TAX CONSEQUENCES TO THEM (INCLUDING THE APPLICATION AND EFFECT OF ANY STATE, LOCAL, NON-U.S. INCOME AND OTHER TAX LAWS) OF ACQUIRING, HOLDING AND DISPOSING OF SUBORDINATE VOTING SHARES.
25. Financial Statements
Please see attached the following financial statements:
| • | Audited annual consolidated financial statements for Cresco as of and for the years ended December 31, 2017 and 2016, and related notes thereto attached hereto as Schedule “A”. |
| • | Unaudited consolidated financial statements for Cresco as of and for the three and six months ended June 30, 2018 and 2017, and related notes thereto attached hereto as Schedule “B”. |
| • | Audited annual financial statements for Randsburg as of and for the years ended January 31, 2018 and 2017, and related notes thereto attached hereto as Schedule “D”. |
| • | Condensed interim financial statements (restated) of Randsburg as at and for the three and six month periods ended July 31, 2018 and 2017, and related notes thereto attached hereto as Schedule “E”. |
| • | Consolidated pro forma financial statements of the Resulting Issuer and related notes thereto attached hereto as Schedule “F”. |
-123-
SCHEDULE “A”
AUDITED ANNUAL CONSOLIDATED FINANCIAL STATEMENTS FOR CRESCO AS OF AND FOR
THE YEARS ENDED DECEMBER 31, 2017 AND 2016
(See attached.)
Cresco Labs, LLC
CONSOLIDATED FINANCIAL STATEMENTS
AS OF AND FOR THE YEARS ENDED
DECEMBER 31, 2017 AND 2016
(Expressed in United States Dollars)
Cresco Labs, LLC
INDEX TO FINANCIAL STATEMENTS
| Page | ||||
| INDEPENDENT AUDITOR’S REPORT |
1 | |||
| FINANCIAL STATEMENTS: |
||||
| Consolidated Statements of Financial Position |
2 | |||
| Consolidated Statements of Operations |
3 | |||
| Consolidated Statements of Changes in Members’ Equity |
4 | |||
| Consolidated Statements of Cash Flows |
5 | |||
| Notes to the Consolidated Financial Statements |
6 - 27 | |||
CRESCO LABS, LLC
MANAGEMENT’S RESPONSIBILITY FOR FINANCIAL REPORTING
To the Members of Cresco Labs, LLC:
The accompanying consolidated financial statements in this annual report were prepared by management of Cresco Labs, LLC (“the Company”), and were reviewed and approved by the Board of Directors of Cresco Labs, LLC.
Management is responsible for the consolidated financial statements and believes that they fairly present the
Company’s consolidated financial condition and results of consolidated operations in conformity with International Financial Reporting Standards. Management has included in the Company’s consolidated financial statements amounts based on estimates and judgments that it believes are reasonable, under the circumstances.
To discharge its responsibilities for financial reporting and safeguarding of assets, management believes that it has established appropriate systems of internal accounting control which provide reasonable assurance that the financial records are reliable and form a proper basis for the timely and accurate preparation of consolidated financial statements. Consistent with the concept of reasonable assurance, the Company recognizes that the relative cost of maintaining these controls should not exceed their expected benefits. Management further assures the quality of the financial records through careful selection and training of personnel and through the adoption and communication of financial and other relevant policies.
These consolidated financial statements have been audited by the Company’s auditor, FGMK LLC, and their report is represented herein.
| Charlie Bachtell |
Ken Amann | |
| Chief Executive Officer | Chief Financial Officer |
November 9, 2018

INDEPENDENT AUDITOR’S REPORT
To the Board of Directors of
Cresco Labs, LLC
We have audited the accompanying consolidated financial statements of Cresco Labs, LLC, which comprise the consolidated statements of financial position as of December 31, 2017 and 2016, and the consolidated statements of operations, changes in members’ equity, and cash flows for the years then ended, and notes to the consolidated financial statements, including a summary of significant accounting policies.
Management’s Responsibility for the Consolidated Financial Statements
Management is responsible for the preparation and fair presentation of these consolidated financial statements in accordance with International Financial Reporting Standards, and for such internal control as management determines is necessary to enable the preparation of consolidated financial statements that are free from material misstatement, whether due to fraud or error.
Auditor’s Responsibility
Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We conducted our audits in accordance with Canadian generally accepted auditing standards. Those standards require that we comply with ethical requirements and plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free from material misstatement.
An audit involves performing procedures to obtain audit evidence about the amounts and disclosures in the consolidated financial statements. The procedures selected depend on the auditor’s judgment, including the assessment of the risks of material misstatement of the consolidated financial statements, whether due to fraud or error. In making those risk assessments, the auditor considers internal control relevant to the entity’s preparation and fair presentation of the consolidated financial statements in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the entity’s internal control. An audit also includes evaluating the appropriateness of accounting policies used and the reasonableness of accounting estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements.
We believe that the audit evidence we have obtained in our audits is sufficient and appropriate to provide a basis for our audit opinion.
Opinion
In our opinion, these consolidated financial statements present fairly, in all material respects, the financial position of Cresco Labs, LLC, as at December 31, 2017 and 2016 and its financial performance and its cash flows for the years then ended in accordance with International Financial Reporting Standards.
November 9, 2018
Bannockburn, Illinois

CRESCO LABS, LLC
Consolidated Statements of Financial Position
As of December 31, 2017 and 2016
(Amounts Expressed in United States Dollars)
| December 31, | ||||||||||||
| 2017 | 2016 | |||||||||||
| ASSETS | ||||||||||||
| Current Assets: |
||||||||||||
| Cash and Cash Equivalents |
Note 2(f) | $ | 27,043,219 | $ | 1,300,464 | |||||||
| Accounts Receivable |
Note 2(g) | 1,010,620 | 442,649 | |||||||||
| Biological Assets |
Note 3 | 2,636,654 | 1,147,480 | |||||||||
| Inventory, Net |
Note 4 | 3,191,109 | 2,042,948 | |||||||||
| Due from Related Parties |
Note 15 | — | 1,205,111 | |||||||||
| Other Current Assets |
183,515 | 110,555 | ||||||||||
|
|
|
|
|
|||||||||
| Total Current Assets |
34,065,117 | 6,249,207 | ||||||||||
| Property and Equipment, Net |
Note 6 | 4,973,447 | 1,133,032 | |||||||||
| Intangible Assets |
Note 7 | 247,083 | 102,083 | |||||||||
| Due from Related Party |
Note 15 | — | 1,113,500 | |||||||||
| Investments |
Note 5 | 989,611 | 1,594,000 | |||||||||
| Security Deposits - Related Parties |
Note 14 & 15 | 1,342,049 | 1,324,585 | |||||||||
|
|
|
|
|
|||||||||
| TOTAL ASSETS |
$ | 41,617,307 | $ | 11,516,407 | ||||||||
|
|
|
|
|
|||||||||
| LIABILITIES AND MEMBERS’ EQUITY | ||||||||||||
| Liabilities: |
||||||||||||
| Current Liabilities: |
||||||||||||
| Accounts Payable and Other Accrued Expenses |
Note 8 | $ | 2,640,582 | $ | 940,826 | |||||||
| Subscription Deposits Refundable |
Note 9 | 399,800 | — | |||||||||
| Due to Related Party |
Note 9 | 725,000 | — | |||||||||
| Notes Payable - Related Parties |
Note 10 | 328,125 | — | |||||||||
|
|
|
|
|
|||||||||
| Total Current Liabilities |
4,093,507 | 940,826 | ||||||||||
| Long-Term Liabilities: |
||||||||||||
| Deferred Rent |
Note 14 | 1,587,230 | 809,273 | |||||||||
| Notes Payable - Related Parties |
Note 10 | — | 562,500 | |||||||||
|
|
|
|
|
|||||||||
| Total Long-Term Liabilities |
1,587,230 | 1,371,773 | ||||||||||
|
|
|
|
|
|||||||||
| TOTAL LIABILITIES |
5,680,737 | 2,312,599 | ||||||||||
| TOTAL MEMBERS’ EQUITY |
Note 11 | 35,936,570 | 9,203,808 | |||||||||
|
|
|
|
|
|||||||||
| TOTAL LIABILITIES AND MEMBERS’ EQUITY |
$ | 41,617,307 | $ | 11,516,407 | ||||||||
|
|
|
|
|
|||||||||
Nature of Operations (Note 1)
Commitments and Contingencies (Note 14)
Subsequent Events (Note 18)
Approved and authorized for issue on behalf of the Members on November 9, 2018:
| Charlie Bachtell |
Ken Amann | |
| Chief Executive Officer | Chief Financial Officer |
See accompanying notes to consolidated financial statements.
2
CRESCO LABS, LLC
Consolidated Statements of Operations
For the Years Ended December 31, 2017 and 2016
(Amounts Expressed in United States Dollars)
| For the Year Ended December 31, |
||||||||||||
| 2017 | 2016 | |||||||||||
| Revenue |
$ | 10,982,313 | $ | 3,390,437 | ||||||||
| Excise Taxes |
(672,310 | ) | (233,205 | ) | ||||||||
|
|
|
|
|
|||||||||
| Net Revenue |
10,310,003 | 3,157,232 | ||||||||||
| Inventory Production Costs Expensed to Cost of Sales |
11,320,233 | 7,912,252 | ||||||||||
|
|
|
|
|
|||||||||
| Gross Loss Before Unrealized Gain on Changes in Fair Value of Biological Assets |
(1,010,230 | ) | (4,755,020 | ) | ||||||||
| Unrealized Gain on Changes in Fair Value of Biological Assets |
Note 3 | 1,489,174 | 594,579 | |||||||||
|
|
|
|
|
|||||||||
| Gross Profit (Loss) After Unrealized Gain on Changes in Fair Value of Biological Assets |
478,944 | (4,160,441 | ) | |||||||||
|
|
|
|
|
|||||||||
| Expenses: |
||||||||||||
| Selling, General and Administrative |
Note 13 | 4,567,746 | 3,594,439 | |||||||||
| Depreciation |
38,759 | 22,736 | ||||||||||
|
|
|
|
|
|||||||||
| Total Expenses |
4,606,505 | 3,617,175 | ||||||||||
|
|
|
|
|
|||||||||
| Loss from Operations |
(4,127,561 | ) | (7,777,616 | ) | ||||||||
|
|
|
|
|
|||||||||
| Other Income: |
||||||||||||
| Interest Income, Net |
46,110 | 53,213 | ||||||||||
| Other Income, Net |
93,928 | — | ||||||||||
|
|
|
|
|
|||||||||
| Total Other Income, Net |
140,038 | 53,213 | ||||||||||
|
|
|
|
|
|||||||||
| Loss |
(3,987,523 | ) | (7,724,403 | ) | ||||||||
| Loss Attributable to Non-Controlling Interest |
(812,170 | ) | — | |||||||||
|
|
|
|
|
|||||||||
| Net Loss Attributable to Controlling Interest |
$ | (3,175,353 | ) | $ | (7,724,403 | ) | ||||||
|
|
|
|
|
|||||||||
See accompanying notes to consolidated financial statements.
3
CRESCO LABS, LLC
Consolidated Statements of Changes in Members’ Equity
For the Years Ended December 31, 2017 and 2016
(Amounts Expressed in United States Dollars)
| Class A Units | Class B Units |
Class C Units |
Class D Units |
Class E Units |
Founders Units |
Share Capital |
Accumulated Deficit |
Subscription Receivable |
Non- Controlling Interest |
Total Members’ Equity |
||||||||||||||||||||||||||||||||||
| BALANCE AS OF JANUARY 1, 2016 |
93,000,000 | 14,055,556 | 15,420,000 | 250,000 | — | 33,000,000 | $ | 20,294,444 | $ | (4,935,504 | ) | $ | — | $ | — | $ | 15,358,940 | |||||||||||||||||||||||||||
| Issuance of Units Cash |
— | — | 850,000 | — | — | — | 1,200,000 | — | — | — | 1,200,000 | |||||||||||||||||||||||||||||||||
| Issuance of Class D for Share Compensation |
— | — | — | 1,853,750 | — | — | 125,000 | — | — | — | 125,000 | |||||||||||||||||||||||||||||||||
| Share Compensation - Equity Allocation Plan |
— | — | — | — | — | — | 244,271 | — | — | — | 244,271 | |||||||||||||||||||||||||||||||||
| Net Loss |
— | — | — | — | — | — | — | (7,724,403 | ) | — | — | (7,724,403 | ) | |||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| BALANCE AS OF DECEMBER 31, 2016 |
93,000,000 | 14,055,556 | 16,270,000 | 2,103,750 | — | 33,000,000 | $ | 21,863,715 | $ | (12,659,907 | ) | $ | — | $ | — | $ | 9,203,808 | |||||||||||||||||||||||||||
| Issuance of Units Cash |
— | — | 500,000 | — | 14,006,523 | — | 15,908,609 | — | (260,000 | ) | 15,070,597 | 30,719,206 | ||||||||||||||||||||||||||||||||
| Share Compensation - Equity Allocation Plan |
— | — | — | — | — | — | 1,079 | — | — | — | 1,079 | |||||||||||||||||||||||||||||||||
| Net Loss |
— | — | — | — | — | — | — | (3,175,353 | ) | — | (812,170 | ) | (3,987,523 | ) | ||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| BALANCE AS OF DECEMBER 31, 2017 |
93,000,000 | 14,055,556 | 16,770,000 | 2,103,750 | 14,006,523 | 33,000,000 | $ | 37,773,403 | $ | (15,835,260 | ) | $ | (260,000 | ) | $ | 14,258,427 | $ | 35,936,570 | ||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
See accompanying notes to consolidated financial statements.
4
CRESCO LABS, LLC
Consolidated Statements of Cash Flows
For the Years Ended December 31, 2017 and 2016
(Amounts Expressed in United States Dollars)
| For the Year Ended December 31, |
||||||||
| 2017 | 2016 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: |
||||||||
| Net Loss |
$ | (3,987,523 | ) | $ | (7,724,403 | ) | ||
| Adjustments to Reconcile Net Loss to Net Cash Used in Operating Activities: |
||||||||
| Depreciation and amortization |
645,652 | 521,064 | ||||||
| Allowance for Uncollectible Accounts |
— | 20,000 | ||||||
| Compensation Expense Recognized Under Equity Allocation Plan |
1,079 | 244,271 | ||||||
| Compensation Expense Under Equity Grants |
— | 125,000 | ||||||
| Loss from Sale of Property and Equipment |
11,111 | — | ||||||
| Unrealized Gain on Investment |
(27,111 | ) | — | |||||
| Unrealized Gain on Changes in Fair Value of Biological Assets |
(1,489,174 | ) | (594,579 | ) | ||||
| Changes in Operating Assets and Liabilities: |
||||||||
| Accounts Receivable |
(567,971 | ) | (462,649 | ) | ||||
| Inventory |
(1,148,161 | ) | (2,042,881 | ) | ||||
| Other Current Assets |
(72,960 | ) | 29,242 | |||||
| Security Deposits |
(17,464 | ) | (1,324,585 | ) | ||||
| Intangible Assets |
(590,000 | ) | (300,000 | ) | ||||
| Accounts Payable |
1,379,196 | (199,762 | ) | |||||
| Accrued Expenses |
320,560 | 504,180 | ||||||
| Redemption Payable |
— | (100,000 | ) | |||||
| Investment payable |
1,124,800 | — | ||||||
| Deferred Rent |
777,957 | 738,441 | ||||||
|
|
|
|
|
|||||
| NET CASH USED IN OPERATING ACTIVITIES |
(3,640,009 | ) | (10,566,661 | ) | ||||
|
|
|
|
|
|||||
| CASH FLOWS FROM INVESTING ACTIVITIES: |
||||||||
| Purchases of Property and Equipment |
(4,057,067 | ) | (102,632 | ) | ||||
| Cash Received from Sale of Property and Equipment |
4,889 | — | ||||||
| Net Change in Due From Related Parties |
2,318,611 | 816,576 | ||||||
| Purchases of Investments |
(67,500 | ) | (94,000 | ) | ||||
| Proceeds from Sale of Investments |
699,000 | — | ||||||
|
|
|
|
|
|||||
| NET CASH (USED IN) PROVIDED BY INVESTING ACTIVITIES |
(1,102,067 | ) | 619,944 | |||||
|
|
|
|
|
|||||
| CASH FLOWS FROM FINANCING ACTIVITIES: |
||||||||
| Capital Contributions from Members |
30,719,206 | 850,000 | ||||||
| Cash Received from Issuance of Notes Payable |
— | 562,500 | ||||||
| Principal Repayments of Notes Payable |
(234,375 | ) | — | |||||
| Net Change in Subscriptions Receivable |
— | 60,000 | ||||||
|
|
|
|
|
|||||
| NET CASH PROVIDED BY FINANCING ACTIVITIES |
30,484,831 | 1,472,500 | ||||||
|
|
|
|
|
|||||
| NET INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS |
25,742,755 | (8,474,217 | ) | |||||
| Cash and Cash Equivalents, Beginning of Year |
1,300,464 | 9,774,681 | ||||||
|
|
|
|
|
|||||
| CASH AND CASH EQUIVALENTS, END OF YEAR |
$ | 27,043,219 | $ | 1,300,464 | ||||
|
|
|
|
|
|||||
| CASH PAID DURING THE YEAR: |
||||||||
| Interest |
$ | 60,484 | $ | 56 | ||||
|
|
|
|
|
|||||
See accompanying notes to consolidated financial statements.
5
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Years Ended December 31, 2017 and 2016
(Amounts Expressed in United States Dollars)
| 1. | NATURE OF OPERATIONS |
Cresco Labs, LLC, an Illinois limited liability company (“Cresco” or the “Company”), is licensed to cultivate manufacture and sell medical cannabis and medical cannabis products. The Company operates in Illinois, Pennsylvania, and Ohio, pursuant to the Illinois Compassionate Use of Medical Cannabis Pilot Program Act, the Pennsylvania Compassionate Use of Medical Cannabis Act, and the Ohio Medical Marijuana Control Program, respectively (collectively, the “Acts”).
During 2016, the Company created two wholly-owned subsidiaries, JDRC Managed Services, LLC (“JDRC”) and Cresco Edibles, LLC (“Cresco Edibles”). Cresco Edibles holds a 75% interest in an operating company, TSC Cresco, LLC (“TSC”).
During 2017, the Company created a majority owned subsidiary, Cresco Labs PA, LLC (“Cresco PA”), which holds a 45% interest in Cresco Yeltrah (“Yeltrah”) and JDRCB Ohio, LLC (“JDRCBO”) which holds a 54% interest in Cresco Labs OH, LLC (“Cresco Ohio”).
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
| (a) | Basis of Preparation |
The accompanying consolidated financial statements of the Company have been prepared in accordance with International Financial Reporting Standards (“IFRS”) as issued by the International Accounting Standards Board (“IASB”) and interpretations of the IFRS Interpretations Committee (“IFRIC”) in effect for the years ended December 31, 2017 and 2016.
These consolidated financial statements were approved and authorized for issue by the Board of Directors of the Company on November 9, 2018.
| (b) | Basis of Measurement |
These accompanying consolidated financial statements have been prepared on the going concern basis, under the historical cost convention, except for biological assets, which are measured at fair value; inventory, which is recorded at the lower of cost or net realizable value; and certain investments, which are recorded at fair value. Historical cost is generally based upon the fair value of the consideration given in exchange for assets.
| (c) | Classification of Expenses |
The expenses within the accompanying statements of operations are presented by function. See Note 13 for details of expenses by nature.
| (d) | Functional Currency |
The Company and its affiliates’ functional currency, as determined by management, is the United States (“U.S.”) dollar. These accompanying consolidated financial statements are presented in U.S. dollars.
6
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Years Ended December 31, 2017 and 2016
(Amounts Expressed in United States Dollars)
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued) |
| (e) | Basis of Consolidation |
These accompanying consolidated financial statements include the accounts of the Company and subsidiaries, over which the Company has control, which are fully consolidated from the date control commences until the date control ceases. Control exists when the Company is exposed, or has rights, to variable returns from its involvement with the entity and has the ability to affect those returns through its power over the entity. In assessing control, potential voting rights that are currently exercisable are taken into account. Non-controlling interest in the equity of consolidated subsidiaries are shown separately in the statement of operations and in the accompanying statements of changes in members equity. All intercompany balances and transactions are eliminated on consolidation. The information below list of the Company’s subsidiaries that are consolidated in these accompanying financial statements and the ownership interest held.
| • | Cresco Labs PA, LLC (“Cresco PA”), majority owned, which holds a 45% interest in an operating company, Cresco Yeltrah, LLC (“Yeltrah”), nets total Yeltrah ownership of 31%. Yeltrah is determined to be controlled by the Company as the Company has management and board control, the power to affect the relevant activities of Yeltrah, and the power to affect its exposure to its variable returns in Yeltrah. Accordingly, Yeltrah and the results of its operations are included in the accompanying consolidated financial statements. |
| • | JDRCB Ohio, LLC (“JDRCBO”), wholly-owned, which holds a 32% interest in an operating company, Cresco Labs, OH, LLC (“Cresco Ohio”). Cresco Ohio is determined to be controlled by the Company as the Company has management and board control, the power to affect the relevant activities of Cresco Ohio, and the power to affect its exposure to its variable returns in Cresco Ohio. Accordingly, Cresco Ohio and the results of its operations are included in the accompanying consolidated financial statements. |
| • | JDRC Managed Services, LLC (“JDRC”), wholly-owned. |
| • | Cresco Edibles, wholly-owned, which holds a 75% interest in TSC Cresco, LLC (“TSC”). TSC, is determined to be controlled by the Company as the Company has management and board control, the power to affect the relevant activities of TSC, and the power to affect its exposure to its variable returns in TSC. Accordingly, TSC and the results of its operations are included in the accompanying consolidated financial statements. |
| • | Cresco Joliet, LLC (“Cresco Joliet”), of which the Company owns 94%. |
| • | Cresco Kankakee, LLC (“Cresco Kankakee”), of which the Company owns 94%. |
| • | Cresco Lincoln, LLC (“Cresco Lincoln”), of which the Company owns 94%. |
| (f) | Cash and Cash Equivalents |
Cash and cash equivalents include cash deposits in financial institutions and other deposits that are readily convertible into cash.
| (g) | Accounts Receivable |
Accounts receivables are classified as loans and receivable financial assets. Accounts receivables are recognized initially at fair value and subsequently measured at amortized cost, less any provisions for impairment. When an accounts receivable is uncollectible, it is written off against the provision.Subsequent recoveries of amounts previously written off are credited to the consolidated statements of operations.
7
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Years Ended December 31, 2017 and 2016
(Amounts Expressed in United States Dollars)
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued) |
| (h) | Inventory |
Inventory is valued at the lower of cost and net realizable value. Cost is determined using the weighted-average method. Net realizable value is determined as the estimated selling price in the ordinary course of business less estimated costs to sell. Packaging and supplies are initially valued at cost.
| • | Seed and Genomics: Actual cost is used to value raw materials such as treatment chemicals and packaging as well as goods in process. Costs for substantially all finished goods are valued at weighted-average actual cost. Weighted average actual costs includes growing and harvesting costs, plant conditioning and packaging costs, and overhead costs. |
| • | Agricultural Productivity: Actual costs is used to value raw materials and supplies. Standard cost, which approximates the actual cost, is used to value finished goods and goods in process. Standard cost includes direct labor and raw materials and manufacturing overhead based on normal capacity. |
As of December 31, 2017 and 2016, the Company did not have material goods in process.
The Company reviews inventory for obsolete, redundant and slow-moving goods and any such inventory is written-down to net realizable value. As of December 31, 2017, the Company recorded an inventory reserve of $123,390. As of December 31, 2016, the Company determined that no reserve was necessary.
| (i) | Property and Equipment |
Property and equipment is stated at cost, net of accumulated depreciation. Depreciation is calculated using the straight-line method over the useful life of the asset. The assets’ residual values, useful lives and methods of depreciation are reviewed at each financial year-end and adjusted prospectively if appropriate. An item of equipment is derecognized upon disposal or when no future economic benefits are expected from its use. Any gain or loss arising on derecognition of the asset (calculated as the difference between the net disposal proceeds and the carrying value of the asset) is included in the accompanying consolidated statements of operations in the year the asset is derecognized.
| Category |
Depreciation Method |
Estimated Useful Life | ||
| Leashold Improvements |
Amortized Over the Lesser of the Life of the Lease or Estimated Useful Life of the Improvement | 8 - 15 Years | ||
| Machinery and Equipment |
Over the Estimated Useful Life of the Asset | 5 - 20 Years | ||
| Furniture and Fixtures |
Over the Estimated Useful Life of the Asset | 7 Years | ||
| Vehicles | Over the Estimated Useful Life of the Asset | 5 Years | ||
| Website and Software |
Over the Estimated Useful Life of the Asset | 3 Years | ||
| Computer Equipment |
Over the Estimated Useful Life of the Asset | 3 - 5 Years | ||
Repairs and maintenance that do not improve efficiency or extend economic life are charged to expense as incurred.
8
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Years Ended December 31, 2017 and 2016
(Amounts Expressed in United States Dollars)
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued) |
| (j) | Intangible Assets |
Intangible assets, separately acquired, are initially recorded at cost. Intangible assets acquired in a business combination are measured at fair value at the acquisition date. Intangible assets that have definite lives are amortized and the amortization is recorded on a straight-line basis over their estimated useful lives, which do not exceed the contractual period, if any. Intangible assets that have indefinite useful lives are not subject to amortization and are tested annually for impairment, or more frequently if events or changes in circumstances indicate that they might be impaired. The estimated useful lives, residual values and amortization methods are reviewed at each year-end, and any changes in estimates are accounted for prospectively. For the years ended December 31, 2017 and 2016, the Company did not recognize any impairment losses.
| (k) | Income Taxes |
The Company is a limited liability company that has elected to be treated as a partnership for U.S. federal income tax purposes. Under federal law, the taxable income or loss of a limited liability company is allocated to its members. Accordingly, no provision has been made for federal income taxes. The Company is responsible for certain other state taxes.
| (l) | Revenue Recognition |
Revenue is recognized at the fair value of consideration received or receivable. Revenue from the sale of goods is recognized when all the following conditions have been satisfied, which are generally met once the following criteria are met:
| • | The Company has transferred the significant risks and rewards of ownership of the goods to the purchaser; |
| • | The Company retains neither continuing managerial involvement to the degree usually associated with ownership nor effective control over the goods sold; |
| • | The amount of revenue can be measured reliably; |
| • | It is probable that the economic benefits associated with the transaction will flow to the entity; and |
| • | The costs incurred or to be incurred in respect of the transaction can be measured reliably. |
| (m) | Unit-Based Compensation |
The Company measures equity settled unit-based payments based on their fair value at the grant date and recognizes compensation expense over the vesting period based on the Company’s estimate of equity instruments that will eventually vest. Expected forfeitures are estimated at the date of grant and subsequently adjusted if further information indicates actual forfeitures may vary from the original estimate. The impact of the revision of the original estimate is recognized in profit or loss such that the cumulative expense reflects the revised estimate. For unit-based payments granted to non-employees the compensation expense is measured at the fair value of the good and services received except where the fair value cannot be estimated in which case it is measured at the fair value of the equity instruments granted. The fair value of unit-based compensation to non-employees is periodically re-measured until counterparty performance is complete, and any change therein is recognized over the period and in the same manner as if the Company had paid cash instead of paying with or using equity instruments.
9
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Years Ended December 31, 2017 and 2016
(Amounts Expressed in United States Dollars)
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued) |
| (n) | Financial Instruments |
A financial instrument is any contract that gives rise to a financial asset of one entity and a financial liability or equity instrument of another entity. Financial assets and financial liabilities are recognized in the accompanying consolidated statement of financial position at the time the Company becomes a party to the contractual provisions of the financial instrument.
| (i) | Initial Measurement of Financial Assets and Financial Liabilities |
Financial assets and liabilities are recognized at fair value upon initial recognition plus any directly attributable transaction costs when not subsequently measured at fair value through profit or loss.
| (ii) | Subsequent Measurement |
Measurement in subsequent periods is dependent on the classification of the financial instrument. The Company classifies its financial instruments in the following categories: at fair value through profit or loss, loans and receivables, held to maturity, available for sale, and other financial liabilities.
| (iii) | Impairment of Financial Assets |
A financial asset not carried at Fair Value Through Profit or Loss is reviewed at each reporting date to determine whether there is any indication of impairment. A financial asset is impaired if objective evidence indicates that a loss event has occurred after the initial recognition of the asset, and that the loss event had a negative effect on the estimated future cash flows of that asset that can be estimated reliably. As of December 31, 2017 and 2016, the Company did not recognize any impairment losses.
An impairment loss in respect of a financial asset measured at amortized cost is calculated as the difference between its carrying amount and the present value of the estimated future cash flows discounted at the assets’ original effective interest rate. Losses are recognized in profit or loss with a corresponding reduction in the financial asset. When a subsequent event causes the amount of impairment loss to decrease, the decrease in impairment loss is reversed through the consolidated statements of operations.
Financial Assets
| (i) | Cash is comprised of deposits held in financial institutions. |
| (ii) | Loans and receivables are non-derivative financial assets with fixed or determinable payments that are not quoted in an active market. Such assets include the Company’s accounts receivable and due from related parties, which are recognized initially at fair value and subsequently on an amortized cost basis using the effective interest method, less any impairment losses. They are included in current assets, except for maturities greater than 12 months after the end of the reporting period, which are classified as non-current assets. |
10
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Years Ended December 31, 2017 and 2016
(Amounts Expressed in United States Dollars)
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued) |
Financial Liabilities
Other financial liabilities include the Company’s accounts payable and accrued liabilities and due from related parties. The effective interest method is used to calculate the amortized cost of a financial liability and allocates interest income over the corresponding period. The effective interest rate is the rate that is used to discount estimated future cash receipts or payments over the expected life of the financial asset or liability.
| (o) | Significant Accounting Judgments, Estimates, and Assumptions |
The preparation of the Company’s accompanying consolidated financial statements requires management to make judgments, estimates and assumptions that affect the application of policies and reported amounts of assets and liabilities, and revenue and expenses. Actual results may differ from these estimates. The estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimate is revised if the revision affects only that period or in the period of the revision and future periods if the review affects both current and future periods.
Significant judgments, estimates, and assumptions that have the most significant effect on the amounts recognized in the accompanying consolidated financial statements are described below.
| (i) | Biological assets and inventory |
In calculating the value of the biological assets and inventory, management is required to make a number of estimates, including estimating the stage of growth of the cannabis up to the point of harvest, harvesting costs, selling costs, average or expected selling prices and list prices, expected yields for the cannabis plants, and oil conversion factors. In calculating final inventory values, management compares the inventory cost to estimated net realizable value. Further information on estimates used in determining the fair value of biological assets is contained in Note 3.
| (ii) | Estimated Useful Lives and Depreciation of Property and Equipment |
Depreciation of property and equipment is dependent upon estimates of useful lives which are determined through the exercise of judgment. The assessment of any impairment of these assets is dependent upon estimates of recoverable amounts that take into account factors such as economic and market conditions and the useful lives of assets.
| (iii) | Unit-Based Compensation |
In calculating the unit-based compensation expense, key estimates such as the rate of forfeiture of options granted, the expected life of the option, the volatility of the Company’s stock price and the risk-free interest rate are used. To calculate the unit-based compensation expense related to key employee performance milestones associated with the terms of an acquisition, the Company must estimate the number of units that will be earned and when they will be issued based on estimated discounted probabilities.
11
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Years Ended December 31, 2017 and 2016
(Amounts Expressed in United States Dollars)
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued) |
| (p) | Recent Accounting Pronouncements |
The following IFRS standards have been recently issued by the IASB. Pronouncements that are not applicable or where it has been determined do not have a significant impact to the Company have been excluded herein.
| (i) | IFRS 7, Financial Instruments: Disclosure (“IFRS 7”) |
IFRS 7 was amended to require additional disclosures on transition from IAS 39 to IFRS 9. IFRS 7 is effective on adoption of IFRS 9, which is effective for annual periods commencing on or after January 1, 2018.
| (ii) | IFRS 9, Financial Instruments (“IFRS 9”) |
In July 2014, the IASB issued the final version of IFRS 9, which reflects all phases of the financial instruments project and replaces IAS 39 and all previous versions of IFRS 9. The standard introduces new requirements for classification and measurement, impairment, and hedge accounting. IFRS 9 is effective for annual periods beginning on or after January 1, 2018, with early application permitted. The Company does not expect significant impact on its consolidated financial statements from the adoption of this new standard.
| (iii) | IFRS 15, Revenue from Contracts with Customers (“IFRS 15”) |
The IASB replaced IAS 18, Revenue, in its entirety with IFRS 15. The standard contains a single model that applies to contracts with customers and two approaches to recognizing revenue: at a point in time or over time. The model features a contract-based five-step analysis of transactions to determine whether, how much and when revenue is recognized. New estimates and judgmental thresholds have been introduced, which may affect the amount and/or timing of revenue recognized. IFRS 15 is effective for annual periods beginning on or after January 1, 2018, with early application permitted. The Company does not expect significant impact on its consolidated financial statements from the adoption of this new standard.
| (iv) | IFRS 16, Leases (“IFRS 16”) |
In January 2016, the IASB issued IFRS 16, which will replace IAS 17, Leases. This standard introduces a single lessee accounting model and requires a lessee to recognize assets and liabilities for all leases with a term of more than twelve months unless the underlying asset is of low value. A lessee is required to recognize a right-of-use asset representing its right to use the underlying asset and a lease liability representing its obligation to make lease payments. The standard will be effective for annual periods beginning on or after January 1, 2019, with earlier application permitted for entities that apply IFRS 15 at or before the date of initial adoption of IFRS 16. The Company expects significant impacts on its consolidated financial statements from the adoption of this new standard. The Company leases the majority of its cultivation facilities and dispensaries and anticipates the consolidated statement of financial position to increase by the amount determined in Note 14.
12
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Years Ended December 31, 2017 and 2016
(Amounts Expressed in United States Dollars)
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued) |
| (v) | IFRS 2, Share-based Payment (“IFRS 2”) |
In June 2016, the IASB issued amendments to IFRS 2, Share-based Payment in relation to the classification and measurement of share-based payment transactions. The amendment provided guidance introduces accounting requirements for cash-settled share-based payments that follows the same approach as used for equity-settled share-based payments. On such modifications, the original liability recognized in respect of the cash-settled share-based payment is derecognized and the equity-settled share-based payment is recognized at the modification date fair value to the extent services have been rendered up to the modification date. Any difference between the carrying amount of the liability as at the modification date and the amount recognized in equity at the same date would be recognized in profit and loss immediately. The amendments are effective for annual periods beginning on or after January 1, 2018. Earlier application is permitted. The amendments are to be applied prospectively. However, retrospective application is allowed if this is possible without the use of hindsight. The Company does not expect significant impact on its consolidated financial statements from the adoption of this new standard.
| (vi) | IAS 28, Long-term Interests (“IAS 28”) |
In October 2017, the IASB amended IAS 28, Long-term Interests in Associates and Joint Ventures. The amendments were added to clarify that an entity applies IFRS 9 ‘Financial Instruments’ to long-term interests in an associate or joint venture that form part of the net investment in the associate or joint venture but to which the equity method is not applied. The standard which will be effective for annual periods beginning on or after January 1, 2019, with earlier adoption permitted. The extent of the impact of adoption of the standard has not yet been determined.
| 3. | BIOLOGICAL ASSETS |
The Company’s biological assets consist of cannabis plants. The Company expenses all direct and indirect costs in the period they are incurred related to the biological transformation of the biological assets between the point of initial recognition and the point of harvest. The expense policy provides better visibility to revenues and cost of goods sold and the results of the biological transformation. The Company then measures the biological assets at fair value less cost to sell up to the point of harvest. Any subsequent post-harvest costs are capitalized to inventory to the extent that cost is less than net realizable value. The net unrealized gains or losses arising from changes in fair value less cost to sell during the year are included in the results of operations of the related year.
13
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Years Ended December 31, 2017 and 2016
(Amounts Expressed in United States Dollars)
| 3. | BIOLOGICAL ASSETS (Continued) |
The Company’s biological assets consists of seeds and cannabis plants. A reconciliation of the beginning and ending balances of biological assets for the year ended December 31, 2017 and 2016 are as follows:
| December 31, | ||||||||
| 2017 | 2016 | |||||||
| Balance at Beginning of Year |
$ | 1,147,480 | $ | 552,901 | ||||
| Purchases |
2,669,969 | 1,201,161 | ||||||
| Expenditures |
7,674,781 | 3,000,150 | ||||||
| Changes in Fair Value of Biological Assets |
1,489,174 | 594,579 | ||||||
| Transferred to Inventory Upon Harvest |
(10,344,750 | ) | (4,201,311 | ) | ||||
|
|
|
|
|
|||||
| Balance at End of Year |
$ | 2,636,654 | $ | 1,147,480 | ||||
|
|
|
|
|
|||||
On average the growing time for a full harvest approximates 17 weeks. As listed below, key estimates are involved in the valuation process of the cannabis plants. The Company’s estimates are subject to changes that could result in future gains or losses of biological assets. Changes in estimates could result from volatility of sales prices, changes in yields, and variability of the costs necessary to complete the harvest. Prior to harvest, all production costs are expensed.
The fair value of biological assets is considered a Level 3 categorization in the IFRS fair value hierarchy. The significant estimates and inputs used to assess the fair value of biological assets include the following assumptions:
| (a) | The selling prices, which are based on average market prices in the locations the Company operates in during the year ended December 31, 2017 and 2016; |
| (b) | the cost to complete the cannabis production process post-harvest, and the cost to sell; |
| (c) | the stage of plant growth; and |
| (d) | expected yields from each cannabis plant. |
A plant typically produces approximately 230 grams. The Company has quantified the sensitivity of the inputs in relation to the biological assets for the year ended December 31, 2017 and 2016 and expects that a 5% increase or decrease in the selling price per gram would increase or decrease the fair value of biological assets by approximately $188,000. A 5% increase or decrease in the estimated yield per cannabis plant would result in an increase or decrease in the fair value of biological assets of approximately $132,000. Additionally, an increase or decrease of 5% in the costs of production would increase or decrease the fair value of biological assets by approximately $56,000.
| 4. | INVENTORY |
At December 31, 2017 and 2016, inventory totaled $3,191,109 and $2,042,948, respectively, and are comprised primarily of finished goods cannabis and cannabis-related products.
14
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Years Ended December 31, 2017 and 2016
(Amounts Expressed in United States Dollars)
| 5. | INVESTMENTS |
The Company determines how to account for investments based on the level of control it has over the investment. If control is determined, then the investment should be consolidated. The Company’s investments that have been controlled and consolidated are further described in Note 2(e). Investments in which the Company has significant influence, but no control, are considered investments in associates. Significant influence is the power to participate in the financial and operating policy decisions of the investee, but without control or joint control over those policies. Investments in associates are accounted for using the equity method of accounting. Interests in associates accounted for using the equity method are initially recognized at cost. The carrying value is then adjusted for the Company’s share of comprehensive income (loss) and distributions from the investee. The carrying value of associates is assessed for impairment at each balance sheet date. As of the year ended December 31, 2017 and 2016, the Company had no investments in associates. Investments that are not controlled or the Company does not have significant influence are first recognized at cost. At each reporting period, changes from the initial cost and fair value are recognized through profit and loss. As of December 31, 2017 and 2016, the Company’s investments are investments at fair value.
The Company has investments in the following entities: CHP Fresco, LLC (“CHP Fresco”), a real estate holding entity, DDD Digital, LLC (“DDD”), an entity offering a technology platform for the cannabis industry, MassRoots, Inc. (“MassRoots”), a technology company for the cannabis industry, and 420 Capital Management, LLC (“420 Capital”), a cannabis investment company. These investments are accounted for at fair market value as the Company has determined that they do not have significant influence over the investee. The following is a description of the investments held at December 31, 2017 and 2016:
| December 31, | ||||||||
| 2017 | 2016 | |||||||
| CHP Fresco |
$ | 801,000 | $ | 1,500,000 | ||||
| DDD |
— | 94,000 | ||||||
| MassRoots |
121,111 | — | ||||||
| 420 Capital |
67,500 | — | ||||||
|
|
|
|
|
|||||
| Total Investments |
$ | 989,611 | $ | 1,594,000 | ||||
|
|
|
|
|
|||||
During the year ended December 31, 2017, the Company invested $67,500 in 420 Capital and sold a portion of its investment in CHP Fresco at cost for $699,000. During the year ended December 31, 2016, the Company purchased interest in DDD for $94,000. In 2017, DDD was acquired by MassRoots, an outside third party, which is publicly traded. The Company received their investment’s worth of Mass Roots shares during the acquisition.
15
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Years Ended December 31, 2017 and 2016
(Amounts Expressed in United States Dollars)
| 6. | PROPERTY AND EQUIPMENT |
As of December 31, 2017 and 2016, property and equipment consist of:
| December 31, | ||||||||
| 2017 | 2016 | |||||||
| Leasehold improvements |
$ | 206,019 | $ | 156,381 | ||||
| Machinery and equipment |
1,047,251 | 699,676 | ||||||
| Furniture and fixtures |
290,528 | 165,966 | ||||||
| Vehicles |
81,717 | 58,311 | ||||||
| Website and software |
63,660 | 72,469 | ||||||
| Computer equipment |
576,070 | 148,848 | ||||||
| Construction in progress |
3,081,143 | 3,670 | ||||||
|
|
|
|
|
|||||
| Total Property and Equipment, Gross |
5,346,388 | 1,305,321 | ||||||
| Less: Accumulated Depreciation |
(372,941 | ) | (172,289 | ) | ||||
|
|
|
|
|
|||||
| Property and Equipment, Net |
$ | 4,973,447 | $ | 1,133,032 | ||||
|
|
|
|
|
|||||
A reconciliation of the beginning and ending balances of property and equipment for the year ended December 31, 2017 is as follows:
| Property and Equipment, Gross |
Accumulated Depreciation |
Property and Equipment, Net |
||||||||||
| Balance as of January 1, 2017 |
$ | 1,305,321 | $ | (172,289 | ) | $ | 1,133,032 | |||||
| Additions |
$ | 4,061,955 | $ | — | $ | 4,061,955 | ||||||
| Dispositions |
$ | (20,888 | ) | $ | 4,888 | $ | (16,000 | ) | ||||
| Depreciation |
$ | — | $ | (205,540 | ) | $ | (205,540 | ) | ||||
|
|
|
|
|
|
|
|||||||
| Balance as of December 31, 2017 |
$ | 5,346,388 | $ | (372,941 | ) | $ | 4,973,447 | |||||
|
|
|
|
|
|
|
|||||||
A reconciliation of the beginning and ending balances of property and equipment for the year ended December 31, 2016 is as follows:
| Property and Equipment, Gross |
Accumulated Depreciation |
Property and Equipment, Net |
||||||||||
| Balance as of January 1, 2016 |
$ | 1,204,563 | $ | (19,766 | ) | $ | 1,184,797 | |||||
| Additions |
102,632 | — | 102,632 | |||||||||
| Dispositions |
(1,874 | ) | 1,874 | — | ||||||||
| Depreciation |
— | (154,397 | ) | (154,397 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Balance as of December 31, 2016 |
$ | 1,305,321 | $ | (172,289 | ) | $ | 1,133,032 | |||||
|
|
|
|
|
|
|
|||||||
Depreciation expense of $200,652 and $154,397 was recorded for the years ended December 31, 2017 and 2016, respectively, of which $166,783 and $131,661, respectively, are included in cost of sales.
16
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Years Ended December 31, 2017 and 2016
(Amounts Expressed in United States Dollars)
| 7. | INTANGIBLE ASSETS |
Permits are issued by various state governments within the U.S. and grant the Company the right to construct and operate medical cannabis cultivation centers and dispensaries within each respective state. Permits are capitalized and amortized on a straight line-basis over the term of the permit. The term of the permits is one year from the date of issuance and requires yearly renewal.
As of December 31, 2017 and 2016, intangible assets consist of:
| December 31, | ||||||||
| 2017 | 2016 | |||||||
| Permit Application Costs |
$ | 1,565,000 | $ | 975,000 | ||||
| Less: Accumulated Amortization |
(1,317,917 | ) | (872,917 | ) | ||||
|
|
|
|
|
|||||
| Intangible Assets, Net |
$ | 247,083 | $ | 102,083 | ||||
|
|
|
|
|
|||||
A reconciliation of the beginning and ending balances of intangible assets for the year ended December 31, 2017 is as follows:
| Intangible Assets, Gross |
Accumulated Amortization |
Intangible Assets, Net |
||||||||||
| Balance as of January 1, 2017 |
$ | 975,000 | $ | (872,917 | ) | $ | 102,083 | |||||
| Additions |
590,000 | — | 590,000 | |||||||||
| Amortization |
— | (445,000 | ) | (445,000 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Balance as of December 31, 2017 |
$ | 1,565,000 | $ | (1,317,917 | ) | $ | 247,083 | |||||
|
|
|
|
|
|
|
|||||||
A reconciliation of the beginning and ending balances of intangible assets for the year ended December 31, 2016 is as follows:
| Intangible Assets, Gross |
Accumulated Amortization |
Intangible Assets, Net |
||||||||||
| Balance as of January 1, 2016 |
$ | 675,000 | $ | (506,250 | ) | $ | 168,750 | |||||
| Additions |
300,000 | — | 300,000 | |||||||||
| Amortization |
— | (366,667 | ) | (366,667 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Balance as of December 31, 2016 |
$ | 975,000 | $ | (872,917 | ) | $ | 102,083 | |||||
|
|
|
|
|
|
|
|||||||
Amortization expense of $445,000 and $366,667 was recorded for the years ended December 31, 2017 and 2016, respectively, of which $400,000 and $366,667, respectively, are included in cost of sales. For the year ended December 31, 2017, an additional $45,000 of amortization is included in selling, general and administrative.
17
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Years Ended December 31, 2017 and 2016
(Amounts Expressed in United States Dollars)
| 8. | ACCOUNTS PAYABLE AND OTHER ACCRUED EXPENSES |
As of December 31, 2017 and 2016, accounts payable and other accrued expenses were comprised of the following:
| December 31, | ||||||||
| 2017 | 2016 | |||||||
| Accounts payable |
$ | 1,715,577 | $ | 336,381 | ||||
| Accrued expenses |
444,061 | 189,526 | ||||||
| Property taxes payable |
361,952 | 339,225 | ||||||
| Payroll liabilities |
79,307 | 66,817 | ||||||
| Licensing fee payable |
36,903 | — | ||||||
| Other |
2,782 | 8,877 | ||||||
|
|
|
|
|
|||||
| Total Accrued Liabilities |
$ | 2,640,582 | $ | 940,826 | ||||
|
|
|
|
|
|||||
| 9. | SUBSCRIPTION DEPOSITS REFUNDABLE AND DUE TO RELATED PARTY |
Subscription deposits refundable represents capital contributions received by the Company prior to successfully obtaining a license to produce and/or sell cannabis in a new state market. The Company received $399,800 in subscription deposits refundable as of December 31, 2017. The Company was unsuccessful in securing a license for this opportunity and refunded the subscriptions in 2018. There were no refundable subscriptions for the year ended December 31, 2016.
During 2017, the Company received subscriptions for expansion in to the Pennsylvania medical cannabis market. Upon successful awarding of licenses, the Company was responsible for funding these subscriptions to Yeltrah. The Company held $725,000 in contributions as of December 31, 2017, which were remitted in 2018.
| 10. | NOTES PAYABLE - RELATED PARTIES |
On December 30, 2016, the Company entered into Promissory Note Agreements (“Promissory Notes”) with its founders in the aggregate amount of $562,500. The terms of the Promissory Notes include maturity on the first anniversary of issuance with the Company having an election to extend the maturity date for up to two successive 12-month periods, but in no event beyond the third anniversary date of the Promissory Note Agreement. Additionally, the Promissory Notes accrue interest at an annual rate of 10%. The Company elected to extend the maturity date for one 12-month period. As of December 31, 2017 and 2016, the amount outstanding under these agreements was $328,125 and $562,500, respectively.
18
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Years Ended December 31, 2017 and 2016
(Amounts Expressed in United States Dollars)
| 11. | MEMBERS’ EQUITY |
The Company is authorized to have six classes of units (the “Units”), designated as Founder’s Units (“Founder’s Units”), Class A Units (“A Units”), Class B Units (“B Units”), Class C Units (“C Units”), Class D Units (“D Units”), and Class E Units (“E Units”). Under the Company’s Operating Agreement (the “Agreement”), the Founder’s Units, A Units, B Units, C Units, and E Units shall be identical in all respects except that C Units are non-voting except that to the extent the Founder’s Units represent not less than fifteen percent of all outstanding Units, the Founder’s Units shall, as a class, have voting rights equal to the greater of the actual voting rights of the Founder’s Units and fifty percent plus one vote of the aggregate voting rights of the Company’s outstanding units. If the Founder’s Units represent less than fifteen percent of the outstanding Units, the Founder’s Units, A Units, B Units, and E Units will vote as a single class, with each Unit representing one vote.
D Units are issued pursuant to a Profits Interest Plan, which is defined as any profits interest award plan of the Company, as amended, modified, supplemented, or replaced from time to time. D Units are awarded to individuals at the current fair value of Company and have no voting rights. The Company did not issue any Class D units for the year ended December 31, 2017. During the year ended December 31, 2016, the Company issued Class D Units totaling 1,853,750.
During the year ended December 31, 2017, the Company raised equity under Series E. A total of 14,006,523 Class E units were issued for capital contributions totaling $15,908,609. All amounts which are determined by the Board to be available for distribution shall be distributed as follows:
| • | First, distributions will be made 87.5% to the A Unit holders in proportion to their respective number of A Units and 12.5% to the B Unit holders in proportion to their respective number of B Units until the Capital Contributions of the A Units have been returned to the Members holding A Units. |
| • | Thereafter, to all Members in proportion to their respective number of Units, provided, however, that no distributions with respect to D Units will be made if such distribution causes an Adjusted Capital Account Deficit to any Class D Member. |
| 12. | EQUITY ALLOCATION PLAN |
The Company has an Equity Allocation Plan (the “Plan”) for key employees and service providers. Under the Plan, units issued have no voting rights and vest proportionately over periods ranging from six months to four years from the issuance date.
19
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Years Ended December 31, 2017 and 2016
(Amounts Expressed in United States Dollars)
| 12. | EQUITY ALLOCATION PLAN (Continued) |
The calculated value of each unit award granted is estimated on the date of grant using a valuation method. The expected term of the common units is derived from the output of the potential payout model and represent the period that units are expected to vest and begin participating in net income and distributions. A summary of the non-vested activity under the Plan as of December 31, 2017 and 2016, and the changes during the years then ended is as follows:
| Non-Vested Units |
Weighted- Average Grant Date Fair Value |
|||||||
| Outstanding - January 1, 2016 |
202,061 | $ | 0.12 | |||||
| Granted |
76,639 | $ | 0.12 | |||||
| Vested |
(29,024 | ) | $ | 0.12 | ||||
|
|
|
|
|
|||||
| Outstanding - December 31, 2016 |
249,676 | $ | 0.12 | |||||
| Granted |
1,376,342 | $ | 0.12 | |||||
| Forfeited |
(74,267 | ) | $ | 0.12 | ||||
| Vested |
(519,292 | ) | 0 | |||||
|
|
|
|
|
|||||
| Outstanding - December 31, 2017 |
1,032,459 | $ | 0.12 | |||||
|
|
|
|
|
|||||
The Company’s units had a fair value of $0.12 at the time of the grants during the years ended December 31,
2017 and 2016 based on Level 3 inputs including, the volatility of the unit price, the expected life of the unit, dividend yield, and interest rates. The Company recorded compensation expense in the amount of $1,079 and $244,271 for the years ended December 31, 2017 and 2016, respectively.
| 13. | SELLING, GENERAL, AND ADMINISTRATIVE EXPENSES |
For the year ended December 31, 2017 and 2016, selling, general and administrative expenses were comprised of the following:
| December 31, | ||||||||||||
| 2017 | 2016 | |||||||||||
| Salaries and Related |
Note 15 | $ | 1,464,954 | $ | 1,105,224 | |||||||
| Consulting and Professional Fee |
1,048,829 | 1,114,679 | ||||||||||
| Travel and Entertainment |
255,606 | 216,281 | ||||||||||
| Rent |
159,708 | 84,123 | ||||||||||
| Office |
147,167 | 135,666 | ||||||||||
| Selling and Marketing |
454,817 | 463,913 | ||||||||||
| Other |
1,036,665 | 474,553 | ||||||||||
|
|
|
|
|
|||||||||
| $4,567,746 | $3,594,439 | |||||||||||
|
|
|
|
|
|||||||||
20
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Years Ended December 31, 2017 and 2016
(Amounts Expressed in United States Dollars)
| 14. | COMMITMENTS AND CONTINGENCIES |
| (a) | Leased Assets |
A lease of property and equipment is classified as a capital lease if it transfers substantially all the risks and rewards incidental to ownership to the Company. A lease of property and equipment is classified as an operating lease whenever the terms of the lease do not transfer substantially all the risks and rewards of ownership to the lessee. Lease payments are recognized as an expense on a straight-line basis over the lease term, except where another systematic basis is more representative of the time pattern in which the economic benefits are consumed.
| (b) | Office and Operating Leases |
The Company leases its Chicago, Illinois headquarters under a non-cancelable sublease agreement with an affiliated entity, which expires in July 2019 (this sublease agreement is pursuant to a master lease agreement). Rent expense was approximately $160,000 and $84,000 for the years ended December 31, 2017 and 2016, respectively, which is included in selling, general and administrative expenses in the accompanying consolidated statements of operations.
The Company leases its cultivation facilities in Joliet, Lincoln, and Kankakee, Illinois from an affiliated entity. The commencement dates of the non-cancelable leases are determined based upon a Substantial Completion Date, as defined in the lease agreements, or six months after the Illinois Department of Agriculture awards the license. The Joliet lease commenced in December 2015, the Lincoln lease commenced in February 2016, and the Kankakee lease commenced in April 2016. The terms of these lease agreements are fifteen years from the commencement date. Rent expense for these facilities was approximately $4,317,000 and $3,849,000 for the years ended December 31, 2017 and 2016, respectively, which is included in cost of goods sold in the accompanying consolidated statements of operations. For financial reporting purposes, rent expense has been recorded on a straight-line basis over the terms of the leases resulting in deferred rent liability of approximately $1,587,000 and $809,000 as of December 31, 2017 and 2016, respectively.
The Company leases its cultivation facility in Brookville, Pennsylvania. The non-cancelable lease commenced on June 30,2017, upon the announcement of a successful license application, and terms after 60 months. Rent expense was approximately $138,000 for the year ended December 31,2017 with approximately $60,000 attributed to deferred rent liability. The Company leases dispensary locations in Butler, Pennsylvania and Pittsburgh, Pennsylvania with 60-month terms and the option to extend. Rent expense was approximately $72,000 for the year ended December 31, 2017 with approximately $14,000 in deferred rent liability.
21
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Years Ended December 31, 2017 and 2016
(Amounts Expressed in United States Dollars)
| 14. | COMMITMENTS AND CONTINGENCIES (Continued) |
Future minimum lease payments under non-cancelable operating leases having an initial or remaining term of more than one year are as follows:
| Year Ending December 31 |
Scheduled Payments |
|||
| 2018 |
$ | 3,942,436 | ||
| 2019 |
3,740,618 | |||
| 2020 |
3,805,790 | |||
| 2021 |
3,918,776 | |||
| 2022 |
4,035,151 | |||
| 2023 - 2031 |
36,811,610 | |||
|
|
|
|||
| Total Future Minimum Lease Payments |
$ | 56,254,381 | ||
|
|
|
|||
In addition to the future minimum rentals disclosed above, the Company is responsible for real estate taxes and comment operating expenses incurred by the building or facility in which it leases space. The Company was also required to pay to the affiliate a security deposit. The balance of the security deposit paid to the affiliate was approximately $1,325,000 as of both December 31, 2017 and 2016.
| (c) | Contingencies |
The Company’s operations are subject to a variety of local, state, and federal regulation. Failure to comply with one or more of those regulations could result in fines, restrictions on its operations, or losses of permits that could result in the Company ceasing operations. While management of the Company believes that the Company is in compliance with applicable local, state, and federal regulation as of December 31, 2017, medical cannabis regulations continue to evolve and are subject to differing interpretations. As a result, the Company may be subject to regulatory fines, penalties, or restrictions in the future.
| (d) | Claims and Litigation |
From time to time, the Company may be involved in litigation relating to claims arising out of operations in the normal course of business. As of December 31, 2017, there were no pending or threatened lawsuits that could reasonably be expected to have a material effect on the results of the Company’s operations. There are also no proceedings in which any of the Company’s directors, officers or affiliates is an adverse party or has a material interest adverse to the Company’s interest.
| 15. | RELATED PARTY TRANSACTIONS |
The Company has a note payable due to its founders, see Note 10. Additionally, the Company leases its offices and cultivation facilities from affiliated entities, see Note 14.
As of December 31, 2016, the Company has amounts due from certain related parties for advances the Company provided for the development and cultivation of facilities. The balance due to the Company at December 31, 2016 was $2,318,611. These amounts were paid in full during the year ended December 31, 2017. As of December 31, 2017, no further amounts were due from these related parties.
Included in salaries expenses for the years ended December 31, 2017 and 2016 is approximately $656,000 and $350,000, respectively, related to the compensation of key management personnel; $172,000 of this balance was included as a liability as of December 31, 2017. There was no liability as of December 31, 2016.
22
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Years Ended December 31, 2017 and 2016
(Amounts Expressed in United States Dollars)
| 16. | FINANCIAL INSTRUMENTS AND FINANCIAL RISK MANAGEMENT |
Financial Instruments
The Company’s financial instruments consist of cash and cash equivalents, accounts receivables, due from related parties, accounts payables and other accrued expenses, subscription deposits refundable, due to related party, and notes payable – related parties. The carrying values of these financial instruments approximate their fair values as of December 31, 2017 and 2016 due to their nature and relatively short maturity date.
Financial instruments recorded at fair value are classified using a fair value hierarchy that reflects the significance of the inputs to fair value measurements. The three levels of hierarchy are:
Level 1 – Unadjusted quoted prices in active markets for identical assets or liabilities;
Level 2 – Inputs other than quoted prices that are observable for the asset or liability, either directly or indirectly; and
Level 3 – Inputs for the asset or liability that are not based on observable market data.
There have been no transfers between fair value levels valuing these assets during the year.
The following table summarizes the Company’s financial instruments as of December 31, 2017:
| Loans and Receivables |
Other Financial Liabilities |
Total | ||||||||||
| Financial Assets: |
||||||||||||
| Cash and Cash Equivalents |
$ | 27,043,219 | $ | — | $ | 27,043,219 | ||||||
| Accounts Receivable |
$ | 1,010,620 | $ | — | $ | 1,010,620 | ||||||
| Due From Related Parties |
$ | — | $ | — | $ | — | ||||||
| Financial Liabilities: |
||||||||||||
| Accounts Payable and Other Accrued Expenses |
$ | — | $ | 2,640,582 | $ | 2,640,582 | ||||||
| Subscription Deposits Refundable |
$ | — | $ | 399,800 | $ | 399,800 | ||||||
| Due to Related Party |
$ | — | $ | 725,000 | $ | 725,000 | ||||||
| Notes Payable - Related Parties |
$ | — | $ | 328,125 | $ | 328,125 | ||||||
The following table summarizes the Company’s financial instruments as of December 31, 2016:
| Loans and Receivables |
Other Financial Liabilities |
Total | ||||||||||
| Financial Assets: |
||||||||||||
| Cash and Cash Equivalents |
$ | 1,300,464 | $ | — | $ | 1,300,464 | ||||||
| Accounts Receivable |
$ | 442,649 | $ | — | $ | 442,649 | ||||||
| Due From Related Parties |
$ | 1,205,111 | $ | — | $ | 1,205,111 | ||||||
| Financial Liabilities: |
||||||||||||
| Accounts Payable and Other Accrued Expenses |
$ | — | $ | 940,826 | $ | 940,826 | ||||||
| Subscription Deposits Refundable |
$ | — | $ | — | $ | — | ||||||
| Due to Related Party |
$ | — | $ | — | $ | — | ||||||
| Notes Payable - Related Parties |
$ | — | $ | 562,500 | $ | 562,500 | ||||||
23
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Years Ended December 31, 2017 and 2016
(Amounts Expressed in United States Dollars)
| 16. | FINANCIAL INSTRUMENTS AND FINANCIAL RISK MANAGEMENT (Continued) |
Financial Risk Management
The Company is exposed in varying degrees to a variety of financial instrument related risks. The Board mitigates these risks by assessing, monitoring and approving the Company’s risk management processes:
| (a) | Credit and Banking Risk |
Credit risk is the risk of a potential loss to the Company if a customer or third party to a financial instrument fails to meet its contractual obligations. The maximum credit exposure at December 31, 2017 and 2016 is the carrying amount of cash. The Company does not have significant credit risk with respect to its customers. Although all cash is placed with major U.S. financial institutions, there has been no change in the U.S. federal banking laws related to the deposit and holding of funds derived from activities related to the cannabis industry. Given that U.S. federal law provides that the production and possession of cannabis is illegal, there is a strong argument that banks cannot accept for deposit funds from business involved with the cannabis industry.
| (b) | Asset Forfeiture Risk |
Because the cannabis industry remains illegal under U.S. federal law, any property owned by participants in the cannabis industry which are either used in the course of conducting such business, or are the proceeds of such business, could be subject to seizure by law enforcement and subsequent civil asset forfeiture. Even if the owner of the property were never charged with a crime, the property in question could still be seized and subject to an administrative proceeding by which, with minimal due process, it could be subject to forfeiture.
| (c) | Liquidity Risk |
Liquidity risk is the risk that the Company will not be able to meet its financial obligations associated with financial liabilities. The Company manages liquidity risk through the management of its capital structure. The Company’s approach to managing liquidity is to ensure that it will have sufficient liquidity to settle obligations and liabilities when due.
In addition to the commitments outlined in Note 10 and Note 14, the Company has the following contractual obligations as of December 31, 2017:
| < 1 Year | 1 to 3 Years |
3 to 5 Years |
Total | |||||||||||||
| Accounts Payable and Other Accrued Expenses |
$ | 2,640,582 | $ | — | $ | — | $ | 2,640,582 | ||||||||
| Subscription Deposits Refundable |
$ | 399,800 | $ | — | $ | — | $ | 399,800 | ||||||||
| Due to Related Party |
$ | 725,000 | $ | — | $ | — | $ | 725,000 | ||||||||
| Notes Payable - Related Parties |
$ | 328,125 | $ | — | $ | — | $ | 328,125 | ||||||||
24
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Years Ended December 31, 2017 and 2016
(Amounts Expressed in United States Dollars)
| 16. | FINANCIAL INSTRUMENTS AND FINANCIAL RISK MANAGEMENT (Continued) |
In addition to the commitments outlined in Note 10 and Note 14, the Company has the following contractual obligations as of December 31, 2016:
| < 1 Year | 1 to 3 Years |
3 to 5 Years |
Total | |||||||||||||
| Accounts Payable and Other Accrued Expenses |
$ | 940,826 | $ | — | $ | — | $ | 940,826 | ||||||||
| Subscription Deposits Refundable |
$ | — | $ | — | $ | — | $ | — | ||||||||
| Due to Related Party |
$ | — | $ | — | $ | — | $ | — | ||||||||
| Notes Payable - Related Parties |
$ | — | $ | 562,500 | $ | — | $ | 562,500 | ||||||||
| (d) | Market Risk |
(i) Currency Risk
The Company had no exposure to foreign currency transaction or translation risk for the periods presented in these consolidated financial statements.
(ii) Interest Rate Risk
Interest rate risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in market interest rates. The Company’s financial debts have fixed rates of interest and therefore expose the Company to a limited interest rate fair value risk.
(iii) Price Risk
Price risk is the risk of variability in fair value due to movements in equity or market prices.
(iv) Tax Risk
Tax risk is the risk of changes in the tax environment that would have a material adverse effect on the Company’s business, results of operations, and financial condition. Currently, state licensed marijuana businesses are assessed a comparatively high effective federal tax rate due to section 280E which bars businesses from deducting all expenses except their cost of goods sold (COGS) when calculating federal tax liability. Any negative increase in additional tax measures may have a further adverse effect on the operations of the Company, while any decrease in tax pressures will be beneficial to future operations.
(v) Regulatory Risk
Regulatory risk pertains to the risk that the Company’s business objectives are contingent, in part, upon the compliance of regulatory requirements. Due to the nature of the industry, the company recognizes that regulatory requirements are more stringent and punitive in nature. Any delays in obtaining, or failure to obtain regulatory approvals can significantly delay operational and product development and can have a material adverse effect on the Company’s business, results of operation, and financial condition.
25
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Years Ended December 31, 2017 and 2016
(Amounts Expressed in United States Dollars)
| 16. | FINANCIAL INSTRUMENTS AND FINANCIAL RISK MANAGEMENT (Continued) |
The Company is cognizant of the advent of regulatory changes occurring in the cannabis industry on the city, state, and national levels. Although regulatory outlook on the cannabis industry has been moving in a positive trend, the Company is aware of the effect of unforeseen regulatory changes can have on the goals and operations of the business as a whole.
| 17. | CAPITAL MANAGEMENT |
The Company’s objective is to maximize sufficient capital base so as to maintain investor, creditor and customer confidence, future development of its business strategy and provide the ability to continue as a going concern. Management defines capital as the Company’s shareholders’ equity. The Board of Directors does not establish quantitative return on capital criteria for management; but rather promotes year over year growth. The Company currently has not paid any dividends to its shareholders.
As of December 31, 2017, and 2016 total managed capital was comprised of members’ equity of approximately $35,936,570 and $9,203,808, respectively.
There were no changes in the Company’s approach to capital management during the year.
| 18. | SUBSEQUENT EVENTS |
The Company has evaluated subsequent events through November 9, 2018, which is the date on which these financial statements were available to be issued.
On January 19, 2018, the Company, through its newly created subsidiary, Cresco Labs Phoenix, LLC, entered into the Unit Purchase and Sale Agreement to invest approximately $2,640,000 in exchange for approximately 66% interest in Phoenix Farms Partners, LLC.
On January 26, 2018, the Company, through its newly created subsidiary, Cresco Labs Nevada, LLC, entered into a Unit Purchase and Sale Agreement with Lighthouse Strategies, LLC, to acquire 25% of the issued and outstanding units for $5,500,000 in cash consideration and 500,000 common membership units in Cresco Labs, LLC.
On April 24, 2018, the Company, through its newly created subsidiary, Cresco Labs TINAD,LLC, entered into a Unit Purchase and Sale Agreement with TINAD, LLC, to acquire 35.46% of the issued and outstanding units for $801,000.
On May 31, 2018, the Company executed an office lease agreement effective for 5,747 sq.ft. commercial premises in the building located at 520 West Erie Street, Chicago, Illinois, 60654. The monthly base rent is $11,494 and the term expires on July 31, 2019.
On June 1, 2018, the Company executed a commercial lease agreement for 4,615 sq.ft. located at 180 Main Street, Wintersville, OH, 43953. The monthly base rent is $3,999.37 and the term expires on May 31, 2023 with three consecutive five year extension options.
On June 7, 2018, the Company, though its newly created subsidiary, Cresco Labs San Luis Obispo, LLC, entered into a Purchase and Sale Agreement with SLO Cultivation, Inc. (“SLO”). Under the terms of the agreement, the Company with acquire a sixty percent interest in SLO for $1,500,000 in consideration to pursue certain cannabis-related licenses in the state of California.
26
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Years Ended December 31, 2017 and 2016
(Amounts Expressed in United States Dollars)
| 18. | SUBSEQUENT EVENTS (Continued) |
On June 25, 2018 the Company entered into a Transaction Agreement pursuant to which Cresco agreed to loan SPAZ REI $1,200,000 under the terms and conditions of the Loan Document which provides the Company the ability to convert the note into a twenty percent equity position.
On September 14, 2018, the Company issued 26,666,667 of new Class F units and closed its capital raise with $100,000,000 in new investment.
On October 1, 2018, the Company entered into a Purchase and Sale agreement with FloraMedex, LLC, an Illinois Limited Liability Company to acquire 100% of the issued and outstanding units of FloraMedex in exchange for $10,000,000 in cash consideration.
On October 5, 2018, the Company entered in a binding Memorandum of Understanding with Hope Heal Health, Inc., a Massachusetts corporation to acquire 100% of the shares and interest in exchange for $27,500,000.
On October 24, 2018, the Company entered a Plan of Merger Agreement with Gloucester Street Capital, LLC (“GSC”), a New York limited liability company to acquire 100% of the issued and outstanding units of GSC in exchange for $32,500,000 cash consideration and 13,466,667 Class F units in the Company of which 5,333,333 is contingent consideration.
On October 24, 2018, the Company entered a Membership Interest Purchase Agreement with Arizona Facilities Supply, LLC (“AFS”) an Arizona limited liability company to acquire 100% of the outstanding membership interests of AFS. Total consideration for purchase is $25,300,000, whereas $22,300,000 is in exchange for acquisition of AFS, but excluding its subsidiary, AFS Maryland, and $3,000,000 in consideration of the indirect acquisition of AFS Maryland.
During October 2018, the Company announced completion of a private placement of Class F units for gross proceeds of US$100 million (U.S. dollar) and a proposed reverse takeover transaction of Randsburg International Gold Corp., a Canadian publicly listed company (“Randsburg”). Under the transaction, Randsburg will be required to change its name to Cresco Labs, Inc., consolidate its outstanding common shares, such that the shareholders of Randsburg retain an aggregate of C$2.2 million (Canadian dollar) in resulting issuer shares, and replace all directors and officers of Randsburg, and create a new class of non-participating super voting shares issued to certain principals of Cresco. A vote for Randsburg shareholders has been scheduled for November 14, 2018 to approve the proposed transaction.
27
SCHEDULE “B”
UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS FOR CRESCO AS OF AND FOR THE
THREE AND SIX MONTHS ENDED JUNE 30, 2018 AND 2017
(See attached.)
Cresco Labs, LLC
CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
FOR THE THREE AND SIX MONTHS ENDED
JUNE 30, 2018 AND 2017
(Expressed in United States Dollars)
| Page | ||||
| FINANCIAL STATEMENTS: |
||||
| Unaudited Consolidated Statements of Financial Position |
2 | |||
| Unaudited Consolidated Statements of Operations |
3 | |||
| Unaudited Consolidated Statements of Changes in Members’ Equity |
4 | |||
| Unaudited Consolidated Statements of Cash Flows |
5 | |||
1
CRESCO LABS, LLC
Unaudited Consolidated Statements of Financial Position
(Amounts Expressed in United States Dollars)
| June 30, 2018 |
December 31, 2017 |
|||||||||||
| (Unaudited) | (Audited) | |||||||||||
| ASSETS |
||||||||||||
| Current Assets: |
||||||||||||
| Cash and Cash Equivalents |
Note 2 | (f) | $ | 31,699,077 | $ | 27,043,219 | ||||||
| Accounts Receivable |
Note 2 | (g) | 2,301,138 | 1,010,620 | ||||||||
| Biological Assets |
Note 3 | 4,807,223 | 2,636,654 | |||||||||
| Inventory, Net |
Note 4 | 6,043,506 | 3,191,109 | |||||||||
| Other Current Assets |
768,659 | 183,515 | ||||||||||
|
|
|
|
|
|||||||||
| Total Current Assets |
45,619,603 | 34,065,117 | ||||||||||
| Property and Equipment, Net |
Note 6 | 16,779,572 | 4,973,447 | |||||||||
| Intangible Assets |
Note 7 | 3,406,140 | 247,083 | |||||||||
| Investments |
Note 5 | 7,305,668 | 989,611 | |||||||||
| Security Deposits - Related Parties |
Note 15 & 16 | 1,354,459 | 1,342,049 | |||||||||
|
|
|
|
|
|||||||||
| TOTAL ASSETS |
$ | 74,465,442 | $ | 41,617,307 | ||||||||
|
|
|
|
|
|||||||||
| LIABILITIES AND MEMBERS’ EQUITY |
||||||||||||
| Liabilities: |
||||||||||||
| Current Liabilities: |
||||||||||||
| Accounts Payable and Other Accrued Expenses |
Note 8 | $ | 3,659,875 | $ | 2,640,582 | |||||||
| Subscription Deposits Refundable |
Note 9 | 12,500 | 399,800 | |||||||||
| Notes Payable - Related Parties |
Note 10 | 187,500 | 328,125 | |||||||||
| Due to Related Parties |
Note 9 | 1,500,000 | 725,000 | |||||||||
|
|
|
|
|
|||||||||
| Total Current Liabilities |
5,359,875 | 4,093,507 | ||||||||||
| Long-Term Liabilities: |
||||||||||||
| Deferred Rent |
Note 15 | 1,894,076 | 1,587,230 | |||||||||
|
|
|
|
|
|||||||||
| Total Long-Term Liabilities |
1,894,076 | 1,587,230 | ||||||||||
| TOTAL LIABILITIES |
7,253,951 | 5,680,737 | ||||||||||
|
|
|
|
|
|||||||||
| TOTAL MEMBERS’ EQUITY |
Note 11 | 67,211,491 | 35,936,570 | |||||||||
|
|
|
|
|
|||||||||
| TOTAL LIABILITIES AND MEMBERS’ EQUITY |
$ | 74,465,442 | $ | 41,617,307 | ||||||||
|
|
|
|
|
|||||||||
Nature of Operations (Note 1)
Commitments and Contingencies (Note 15)
Subsequent Events (Note 19)
Approved and authorized for issue on behalf of the Members on November 9, 2018:
| Charlie Bachtell |
Ken Amann | |||
| Chief Executive Officer |
Chief Financial Officer |
See accompanying notes to unaudited consolidated financial statements.
2
CRESCO LABS, LLC
Unaudited Consolidated Statements of Operations
For the Three and Six Month Periods Ended June 30, 2018
(Amounts Expressed in United States Dollars)
| For the Three Months Ended June 30, |
For the Six Months Ended June 30, |
|||||||||||||||||||
| 2018 | 2017 | 2018 | 2017 | |||||||||||||||||
| Revenue |
$ | 8,473,297 | $ | 2,668,683 | $ | 13,566,187 | $ | 4,730,401 | ||||||||||||
| Excise Taxes |
396,200 | 183,539 | 686,051 | 325,348 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net Revenue |
8,077,097 | 2,485,144 | 12,880,136 | 4,405,053 | ||||||||||||||||
| Inventory Production Costs Expensed to Cost of Sales |
4,569,802 | 2,806,935 | 8,346,496 | 4,386,620 | ||||||||||||||||
| Gross Profit (Loss) Before Unrealized Gain on Changes in Fair Value of Biological Assets |
3,507,295 | (321,791 | ) | 4,533,639 | 18,433 | |||||||||||||||
| Unrealized Gain on Changes in Fair Value of Biological Assets |
Note 3 | 761,279 | 623,161 | 2,170,569 | 667,439 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Gross Profit After Unrealized Gain on Changes in Fair Value of Biological Assets |
4,268,574 | 301,370 | 6,704,208 | 685,872 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Expenses: |
||||||||||||||||||||
| Selling, General and Administration |
Note 14 | 2,234,825 | 610,928 | 3,705,360 | 1,382,166 | |||||||||||||||
| Depreciation |
50,230 | 9,847 | 89,780 | 19,248 | ||||||||||||||||
| Advertising and Marketing |
344,017 | 99,183 | 647,345 | 141,098 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total Expenses |
2,629,072 | 719,958 | 4,442,485 | 1,542,512 | ||||||||||||||||
| Gain (Loss) from Operations |
1,639,502 | (418,588 | ) | 2,261,723 | (856,640 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Other Income (Expense): |
||||||||||||||||||||
| Interest Income (Expense), Net |
(8,088 | ) | 10,431 | (17,331 | ) | (16,115 | ) | |||||||||||||
| Other Income (Expense), Net |
29,748 | (16,095 | ) | 17,130 | (48,449 | ) | ||||||||||||||
| Shares of Income from Investment in Associate |
Note 5 | 320,824 | — | 320,824 | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total Other Income (Loss), Net |
342,484 | (5,664 | ) | 320,623 | (64,564 | ) | ||||||||||||||
| Net Income (Loss) |
1,981,986 | (424,252 | ) | 2,582,346 | (921,204 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net (Income) Attributable to Non-Controlling Interests |
(558,824 | ) | — | (600,859 | ) | — | ||||||||||||||
| Net Income (Loss) Attributable to Controlling Interest |
$ | 1,423,162 | $ | (424,252 | ) | $ | 1,981,487 | $ | (921,204 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
See accompanying notes to unaudited consolidated financial statements.
3
CRESCO LABS, LLC
Unaudited Consolidated Statements of Changes in Members’ Equity
For the Year Ended December 31, 2017 and Six Month Period Ended June 30, 2018
(Amounts Expressed in United States Dollars)
| Class A Units |
Class B Units |
Class C Units |
Class D Units |
Class E Units |
Class F | Founders Units |
Share Capital |
Accumulated Deficit |
Subscription Receivable |
Noncontrolling interest |
Total | |||||||||||||||||||||||||||||||||||||
| BALANCE AS OF JANUARY 1, 2017 |
93,000,000 | 14,055,556 | 16,270,000 | 2,103,750 | — | — | 33,000,000 | $ | 21,863,715 | $ | (12,659,907 | ) | $ | — | $ | — | $ | 9,203,808 | ||||||||||||||||||||||||||||||
| Issuance of Units Cash |
— | — | 500,000 | — | 14,006,523 | — | — | 15,908,609 | — | (260,000 | ) | 15,070,597 | 30,719,206 | |||||||||||||||||||||||||||||||||||
| Share Compensation - Equity Allocation Plan |
— | — | — | — | — | — | — | 1,079 | — | — | — | 1,079 | ||||||||||||||||||||||||||||||||||||
| Net Loss |
— | — | — | — | — | — | — | — | (3,175,353 | ) | — | (812,170 | ) | (3,987,523 | ) | |||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| BALANCE AS OF DECEMBER 31, 2017 |
93,000,000 | 14,055,556 | 16,770,000 | 2,103,750 | 14,006,523 | — | 33,000,000 | $ | 37,773,403 | $ | (15,835,260 | ) | $ | (260,000 | ) | $ | 14,258,427 | $ | 35,936,570 | |||||||||||||||||||||||||||||
| Issuance of Units Cash |
— | — | 125,000 | — | — | 14,993,328 | — | 24,441,170 | — | — | 2,604,536 | 27,045,706 | ||||||||||||||||||||||||||||||||||||
| Acquisition of Non-Controlling Interest |
— | — | — | — | — | — | — | — | — | — | 597,324 | 597,324 | ||||||||||||||||||||||||||||||||||||
| Issuance of Class D for Share Compensation |
— | — | — | 2,617,333 | — | — | — | 157,123 | — | — | — | 157,123 | ||||||||||||||||||||||||||||||||||||
| Share Compensation - Equity Allocation Plan |
— | — | — | — | — | — | — | 64,522 | — | — | — | 64,522 | ||||||||||||||||||||||||||||||||||||
| Issuance of Shares for an Interest in an Associate |
— | — | — | — | 500,000 | — | — | 567,900 | — | — | — | 567,900 | ||||||||||||||||||||||||||||||||||||
| Cash received from Subscription Receivable |
— | — | — | — | — | — | — | — | — | 260,000 | — | 260,000 | ||||||||||||||||||||||||||||||||||||
| Net Income |
— | — | — | — | — | — | — | — | 1,981,487 | — | 600,859 | 2,582,346 | ||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| BALANCE AS OF JUNE 30, 2018 (UNAUDITED) |
93,000,000 | 14,055,556 | 16,895,000 | 4,721,083 | 14,506,523 | 14,993,328 | 33,000,000 | $ | 63,004,118 | $ | (13,853,773 | ) | $ | — | $ | 18,061,146 | $ | 67,211,491 | ||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
See accompanying notes to unaudited consolidated financial statements.
4
CRESCO LABS, LLC
Unaudited Consolidated Statements of Cash Flows
For the Six Month Periods Ended June 30, 2018 and 2017
(Amounts Expressed in United States Dollars)
| For the Six Months Ended June 30, |
||||||||
| 2018 | 2017 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: |
||||||||
| Net Income |
$ | 2,582,346 | $ | (921,204 | ) | |||
| Adjustments to Reconcile Net Income to Net Cash (Used in) Provided by Operating Activities: |
||||||||
| Depreciation and Amortization |
679,185 | 238,135 | ||||||
| Issuance of Class D for Share Compensation |
157,123 | — | ||||||
| Share Compensation - Equity Allocation Plan |
64,522 | 540 | ||||||
| Income from Associate |
(320,824 | ) | — | |||||
| Unrealized Loss on Investments at Fair Market Value |
72,667 | — | ||||||
| Unrealized Gain on Changes in Fair Value of Biological Assets |
(2,170,569 | ) | (667,439 | ) | ||||
| Changes in Operating Assets and Liabilities: |
— | — | ||||||
| Receivables |
(832,899 | ) | (370,662 | ) | ||||
| Inventory |
(2,779,692 | ) | (1,188,666 | ) | ||||
| Prepaid Expenses and Other Current Assets |
(584,084 | ) | (101,260 | ) | ||||
| Other Assets |
(12,410 | ) | — | |||||
| Due from Related Parties |
— | 1,661,276 | ||||||
| Accounts Payable and Other Accrued Expenses |
833,827 | 447,489 | ||||||
| Deferred Rent |
306,846 | 355,738 | ||||||
| Other Current Liabilities |
(387,300 | ) | 113,697 | |||||
| Due to Related Paries |
(725,000 | ) | — | |||||
|
|
|
|
|
|||||
| NET CASH USED IN OPERATING ACTIVITIES |
(3,116,262 | ) | (432,356 | ) | ||||
|
|
|
|
|
|||||
| CASH FLOWS FROM INVESTING ACTIVITIES: |
||||||||
| Purchases of Property and Equipment |
(11,918,351 | ) | (101,477 | ) | ||||
| Purchase of Investments |
(5,500,000 | ) | — | |||||
| Cash Paid for Acquisitions, Net of Cash Acquired |
(1,629,613 | ) | — | |||||
| Sale of Investments |
— | 699,000 | ||||||
| Purchase of Intangibles |
(345,000 | ) | (300,000 | ) | ||||
|
|
|
|
|
|||||
| NET CASH (USED IN) PROVIDED BY INVESTING ACTIVITIES |
(19,392,964 | ) | 297,523 | |||||
|
|
|
|
|
|||||
| CASH FLOWS FROM FINANCING ACTIVITIES: |
||||||||
| Capital Contributions from Members |
27,045,706 | 1,785,758 | ||||||
| Loans from Shareholders |
— | 46,875 | ||||||
| Principal Repayments of Notes Payable |
(140,624 | ) | — | |||||
| Cash received from Subscription Receivable |
260,000 | — | ||||||
| Loan from Related Parties |
— | — | ||||||
|
|
|
|
|
|||||
| NET CASH PROVIDED BY FINANCING ACTIVITIES |
27,165,082 | 1,832,633 | ||||||
|
|
|
|
|
|||||
| NET INCREASE IN CASH |
4,655,857 | 1,697,801 | ||||||
| Cash and Cash Equivalents, Beginning of Period |
27,043,219 | 1,300,464 | ||||||
|
|
|
|
|
|||||
| CASH AND CASH EQUIVALENTS, END OF PERIOD |
$ | 31,699,076 | $ | 2,998,265 | ||||
|
|
|
|
|
|||||
See accompanying notes to unaudited consolidated financial statements.
5
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Six Months Ended June 30, 2018
(Amounts Expressed in United States Dollars)
| 1. | NATURE OF OPERATIONS |
Cresco Labs, LLC, an Illinois limited liability company (“Cresco” or the “Company”), is licensed to cultivate manufacture and sell medical cannabis and medical cannabis products. The Company operates in Illinois, Pennsylvania, and Ohio, pursuant to the Illinois Compassionate Use of Medical Cannabis Pilot Program Act, the Pennsylvania Compassionate Use of Medical Cannabis Act, and the Ohio Medical Marijuana Control Program, respectively (collectively, the “Acts”).
During 2016, the Company created two wholly-owned subsidiaries, JDRC Managed Services, LLC (“JDRC”) and Cresco Edibles, LLC (“Cresco Edibles”). Cresco Edibles holds a 75% interest in an operating company, TSC Cresco, LLC (“TSC”).
During 2017, the Company created a majority owned subsidiary, Cresco Labs PA, LLC (“Cresco PA”), which holds a 45% interest in Cresco Yeltrah (“Yeltrah”) and JDRCB Ohio, LLC (“JDRCBO”) which holds a 54% interest in Cresco Labs OH, LLC (“Cresco Ohio”).
During 2018, the Company created Cresco Labs of California, LLC which holds a 60% interest in San Luis Obispo Cultivation (“SLO”) and created Cresco Labs Phoenix, LLC which holds a 66% interest in Phoenix Farms Partners, LLC (“Phoenix”).
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
| (a) | Basis of Preparation |
The accompanying consolidated financial statements of the Company have been prepared in accordance with International Financial Reporting Standards (“IFRS”) as issued by the International Accounting Standards Board (“IASB”) and interpretations of the IFRS Interpretations Committee (“IFRIC”) in effect for the periods ended June 30, 2018 and 2017.
These accompanying consolidated financial statements were approved and authorized for issue by the Board of Directors of the Company on November 9, 2018.
| (b) | Basis of Measurement |
These accompanying consolidated financial statements have been prepared on the going concern basis, under the historical cost convention, except for biological assets, which are measured at fair value; inventory, which is recorded at the lower of cost or next realizable value; and certain investments, which are recorded at fair value. Historical cost is generally based upon the fair value of the consideration given in exchange for assets.
| (c) | Classification of Expenses |
The expenses within the accompanying statements of operations are presented by function. See Note 14 for details of expenses by nature.
| (d) | Functional Currency |
The Company and its affiliates’ functional currency, as determined by management, is the United States (“U.S.”) dollar. These accompanying consolidated financial statements are presented in U.S. dollars.
6
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Six Months Ended June 30, 2018
(Amounts Expressed in United States Dollars)
| (e) | Basis of Consolidation |
These accompanying consolidated financial statements include the accounts of the Company and subsidiaries, over which the Company has control, which are fully consolidated from the date control commences until the date control ceases. Control exists when the Company is exposed, or has rights, to variable returns from its involvement with the entity and has the ability to affect those returns through its power over the entity. In assessing control, potential voting rights that are currently exercisable are taken into account. Non-controlling interest in the equity of consolidated subsidiaries are shown separately in the statement of operations and in the accompanying statements of changes in members equity. All intercompany balances and transactions are eliminated on consolidation. The information below list of the Company’s subsidiaries that are consolidated in these accompanying financial statements and the ownership interest held.
| • | Cresco Labs PA, LLC (“Cresco PA”), majority owned, which holds a 45% interest in an operating company, Cresco Yeltrah, LLC (“Yeltrah”), nets total Yeltrah ownership of 31%. Yeltrah is determined to be controlled by the Company as the Company has management and board control, the power to affect the relevant activities of Yeltrah, and the power to affect its exposure to its variable returns in Yeltrah. Accordingly, Yeltrah and the results of its operations are included in the accompanying consolidated financial statements. |
| • | JDRCB Ohio, LLC (“JDRCBO”), wholly-owned, which holds a 54% interest in an operating company, Cresco Labs, OH, LLC (“Cresco Ohio”). Cresco Ohio is determined to be controlled by the Company as the Company has management and board control, the power to affect the relevant activities of Cresco Ohio, and the power to affect its exposure to its variable returns in Cresco Ohio. Accordingly, Cresco Ohio and the results of its operations are included in the accompanying consolidated financial statements. |
| • | JDRC Managed Services, LLC (“JDRC”), wholly-owned. |
| • | Cresco Edibles, wholly-owned, which holds a 75% interest in TSC Cresco, LLC (“TSC”). TSC, is determined to be controlled by the Company as the Company has management and board control, the power to affect the relevant activities of TSC, and the power to affect its exposure to its variable returns in TSC. Accordingly, TSC and the results of its operations are included in the accompanying consolidated financial statements. |
| • | Cresco Labs Phoenix, LLC, wholly-owned, which holds a 66% interest in Phoenix Farms Partners, LLC (“Phoenix”). Phoenix is determined to be controlled by the Company as the Company has management and board control, the power to affect the relevant activities of Phoenix, and the power to affect its exposure to its variable returns in Phoenix. Accordingly, Phoenix and the results of its operations are included in the accompanying consolidated financial statements. |
| • | Cresco Labs of California, LLC, wholly-owned, which holds a 60% interest in SLO Cultivation. (“SLO”). SLO, is determined to be controlled by the Company as the Company has management and board control, the power to affect the relevant activities of SLO, and the power to affect its exposure to its variable returns in SLO. Accordingly, SLO and the results of its operations are included in the accompanying consolidated financial statements. |
| • | Cresco Joliet, LLC (“Cresco Joliet”), of which the Company owns 94%. |
| • | Cresco Kankakee, LLC (“Cresco Kankakee”), of which the Company owns 94%. |
| • | Cresco Lincoln, LLC (“Cresco Lincoln”), of which the Company owns 94%. |
7
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Six Months Ended June 30, 2018
(Amounts Expressed in United States Dollars)
| (f) | Cash and Cash Equivalents |
Cash and cash equivalents include cash deposits in financial institutions and other deposits that are readily convertible into cash, generally with an original maturity of three months or less.
| (g) | Accounts Receivable |
Accounts receivable are classified as loans and receivable financial assets. Accounts receivable are recognized initially at fair value and subsequently measured at amortized cost, less any provisions for impairment. When an accounts receivable is uncollectible, it is written off against the provision. Subsequent recoveries of amounts previously written off are credited to the consolidated statements of operations.
| (h) | Inventory |
Inventory is valued at the lower of cost and net realizable value. Cost is determined using the weighted-average method. Net realizable value is determined as the estimated selling price in the ordinary course of business less estimated costs to sell. Packaging and supplies are initially valued at cost.
| • | Seed and Genomics: Actual cost is used to value raw materials such as treatment chemicals and packaging as well as goods in process. Costs for substantially all finished goods are valued at weighted-average actual cost. Weighted average actual costs includes growing and harvesting costs, plant conditioning and packaging costs, and overhead costs. |
| • | Agricultural Productivity: Actual cost is used to value raw materials and supplies. Standard cost, which approximates the actual cost, is used to value finished goods and goods in process. Standard cost includes direct labor and raw materials and manufacturing overhead based on normal capacity. |
As of June 30, 2018 and December 31, 2017 the Company did not have material goods in process.
The Company reviews inventory for obsolete, redundant and slow-moving goods and any such inventory is written-down to net realizable value. As of June 30, 2018 and December 31, 2017, the Company recorded an inventory reserve of $173,481 and $123,390, respectively.
| (i) | Property and Equipment |
Property and equipment is stated at cost, net of accumulated depreciation. Depreciation is calculated using the straight-line method over the useful life of the asset. The assets’ residual values, useful lives and methods of depreciation are reviewed at each financial year-end and adjusted prospectively if appropriate. An item of equipment is derecognized upon disposal or when no future economic benefits are expected from its use. Any gain or loss arising on derecognition of the asset (calculated as the difference between the net disposal proceeds and the carrying value of the asset) is included in the accompanying consolidated statements of operations in the year the asset is derecognized.
8
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Six Months Ended June 30, 2018
(Amounts Expressed in United States Dollars)
| Category |
Depreciation Method |
Estimated Useful Life | ||
| Leashold Improvements | Amortized Over the Lesser of the Life of the Lease or Estimated Useful Life of the Improvement | 8 - 15 Years | ||
| Machinery and Equipment | Over the Estimated Useful Life of the Asset | 5 - 20 Years | ||
| Furniture and Fixtures | Over the Estimated Useful Life of the Asset | 7 Years | ||
| Vehicles | Over the Estimated Useful Life of the Asset | 5 Years | ||
| Website and Software | Over the Estimated Useful Life of the Asset | 3 Years | ||
| Computer Equipment | Over the Estimated Useful Life of the Asset | 3 - 5 Years | ||
Repairs and maintenance that do not improve efficiency or extend economic life are charged to expense as incurred.
| (j) | Intangible Assets |
Intangible assets are initially recorded at cost, less accumulated amortization and impairment losses, if any. Intangible assets acquired in a business combination are measured at fair value at the acquisition date. Amortization is recorded on a straight-line basis over their estimated useful lives, which do not exceed the contractual period, if any. Intangible assets that have indefinite useful lives are not subject to amortization and are tested annually for impairment, or more frequently if events or changes in circumstances indicate that they might be impaired. The estimated useful lives, residual values and amortization methods are reviewed at each year-end, and any changes in estimates are accounted for prospectively. For the periods ended June 30, 2018 and 2017, the Company did not recognize any impairment losses.
| (k) | Goodwill |
Goodwill represents the excess of the purchase price paid for the acquisition of a business over the fair value of the net tangible and intangible assets acquired. Goodwill is allocated to the cash-generating unit (“CGU”) or CGUs which are expected to benefit from the synergies of the combination. The Company has determined that the goodwill associated with all acquisitions belong to the medical cannabis segment.
Goodwill that has an indefinite useful life is not subject to amortization and is tested annually for impairment, or more frequently if events or changes in circumstances indicate that they might be impaired. Other assets are tested for impairment whenever events or changes in circumstances indicate that the carrying amount may not be recoverable.
Impairment is determined for goodwill by assessing if the carrying value of a CGU, including the allocated goodwill, exceeds its recoverable amount determined as the greater of the estimated fair value less costs to sell and the value in use. Impairment losses recognized in respect of a CGU are first allocated to the carrying value of goodwill and any excess is allocated to the carrying amount of assets in the CGU. Any goodwill impairment loss is recognized in the Consolidated Statement of Operations in the period in which the impairment is identified. Impairment losses on goodwill are not subsequently reversed. The Company’s most recent goodwill impairment test during the second quarter did not result in the recognition of any impairment losses.
9
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Six Months Ended June 30, 2018
(Amounts Expressed in United States Dollars)
| (l) | Income Taxes |
The Company is a limited liability company that has elected to be treated as a partnership for U.S. federal income tax purposes. Under federal law, the taxable income or loss of a limited liability company is allocated to its members. Accordingly, no provision has been made for federal income taxes. The Company is responsible for certain other state taxes.
| (m) | Revenue Recognition |
Revenue is recognized at the fair value of consideration received or receivable. Revenue from the sale of goods is recognized when all the following conditions have been satisfied, which are generally met once the following are met:
| • | The Company has transferred the significant risks and rewards of ownership of the goods to the purchaser; |
| • | The Company retains neither continuing managerial involvement to the degree usually associated with ownership nor effective control over the goods sold; |
| • | The amount of revenue can be measured reliably; |
| • | It is probable that the economic benefits associated with the transaction will flow to the entity; and |
| • | The costs incurred or to be incurred in respect of the transaction can be measured reliably. |
| (n) | Unit-Based Compensation |
The Company measures equity settled unit-based payments based on their fair value at the grant date and recognizes compensation expense over the vesting period based on the Company’s estimate of equity instruments that will eventually vest. Expected forfeitures are estimated at the date of grant and subsequently adjusted if further information indicates actual forfeitures may vary from the original estimate. The impact of the revision of the original estimate is recognized in profit or loss such that the cumulative expense reflects the revised estimate. For unit-based payments granted to non-employees the compensation expense is measured at the fair value of the good and services received except where the fair value cannot be estimated in which case it is measured at the fair value of the equity instruments granted. The fair value of unit-based compensation to non-employees is periodically re-measured until counterparty performance is complete, and any change therein is recognized over the period and in the same manner as if the Company had paid cash instead of paying with or using equity instruments.
| (o) | Financial Instruments |
A financial instrument is any contract that gives rise to a financial asset of one entity and a financial liability or equity instrument of another entity. Financial assets and financial liabilities are recognized in the accompanying consolidated statement of financial position at the time the Company becomes a party to the contractual provisions of the financial instrument.
| (i) | Initial Measurement of Financial Assets and Financial Liabilities |
Financial assets and liabilities are recognized at fair value upon initial recognition plus any directly attributable transaction costs when not subsequently measured at fair value through profit or loss.
10
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Six Months Ended June 30, 2018
(Amounts Expressed in United States Dollars)
| (ii) | Subsequent Measurement |
Measurement in subsequent periods is dependent on the classification of the financial instrument. The Company classifies its financial instruments in the following categories: at fair value through profit or loss, loans and receivables, held to maturity, available for sale, and other financial liabilities.
| (iii) | Impairment of Financial Assets |
A financial asset not carried at Fair Value Through Profit or Loss is reviewed at each reporting date to determine whether there is any indication of impairment. A financial asset is impaired if objective evidence indicates that a loss event has occurred after the initial recognition of the asset, and that the loss event had a negative effect on the estimated future cash flows of that asset that can be estimated reliably. As of June 30, 2018 and December 31, 2017, the Company did not recognize any impairment losses.
An impairment loss in respect of a financial asset measured at amortized cost is calculated as the difference between its carrying amount and the present value of the estimated future cash flows discounted at the assets’ original effective interest rate. Losses are recognized in profit or loss with a corresponding reduction in the financial asset. When a subsequent event causes the amount of impairment loss to decrease, the decrease in impairment loss is reversed through the consolidated statements of operations.
Financial Assets
| (i) | Cash is comprised of deposits held in financial institutions. |
| (ii) | Loans and receivables are non-derivative financial assets with fixed or determinable payments that are not quoted in an active market. Such assets include the Company’s accounts receivable and due from related parties, which are recognized initially at fair value and subsequently on an amortized cost basis using the effective interest method, less any impairment losses. They are included in current assets, except for maturities greater than 12 months after the end of the reporting period, which are classified as non-current assets. |
| Financial | Liabilities |
| (i) | Other financial liabilities include the Company’s accounts payable and accrued liabilities and due from related parties. The effective interest method is used to calculate the amortized cost of a financial liability and allocates interest income over the corresponding period. The effective interest rate is the rate that is used to discount estimated future cash receipts or payments over the expected life of the financial asset or liability. |
| (p) | Significant Accounting Judgments, Estimates, and Assumptions |
The preparation of the Company’s accompanying consolidated financial statements requires management to make judgments, estimates and assumptions that affect the application of policies and reported amounts of assets and liabilities, and revenue and expenses. Actual results may differ from these estimates. The estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimate is revised if the revision affects only that period or in the period of the revision and future periods if the review affects both current and future periods.
11
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Six Months Ended June 30, 2018
(Amounts Expressed in United States Dollars)
Significant judgments, estimates, and assumptions that have the most significant effect on the amounts recognized in the accompanying consolidated financial statements are described below.
| (i) | Biological assets and inventory |
In calculating the value of the biological assets and inventory, management is required to make a number of estimates, including estimating the stage of growth of the cannabis up to the point of harvest, harvesting costs, selling costs, average or expected selling prices and list prices, expected yields for the cannabis plants, and oil conversion factors. In calculating final inventory values, management compares the inventory cost to estimated net realizable value. Further information on estimates used in determining the fair value of biological assets is contained in Note 3.
| (ii) | Estimated Useful Lives and Depreciation of Property and Equipment |
Depreciation of property and equipment is dependent upon estimates of useful lives which are determined through the exercise of judgment. The assessment of any impairment of these assets is dependent upon estimates of recoverable amounts that take into account factors such as economic and market conditions and the useful lives of assets.
| (iii) | Estimated Useful Lives and Amortization of Intangible Assets |
Amortization of intangible assets is recorded on a straight-line basis over their estimated useful lives, which do not exceed the contractual period, if any. Intangible assets that have indefinite useful lives are not subject to amortization and are tested annually for impairment, or more frequently if events or changes in circumstances indicate that they might be impaired.
| (iv) | Unit-Based Compensation |
In calculating the unit-based compensation expense, key estimates such as the rate of forfeiture of options granted, the expected life of the option, the volatility of the Company’s stock price and the risk-free interest rate are used. To calculate the unit-based compensation expense related to key employee performance milestones associated with the terms of an acquisition, the Company must estimate the number of units that will be earned and when they will be issued based on estimated discounted probabilities.
| (q) | Recent Accounting Pronouncements |
The following IFRS standards have been recently issued by the IASB. Pronouncements that are not applicable or where it has been determined do not have a significant impact to the Company have been excluded herein.
| (i) | IFRS 7, Financial Instruments: Disclosure (“IFRS 7”) |
IFRS 7 was amended to require additional disclosures on transition from IAS 39 to IFRS 9. IFRS 7 is effective on adoption of IFRS 9, which is effective for annual periods commencing on or after January 1, 2018.
12
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Six Months Ended June 30, 2018
(Amounts Expressed in United States Dollars)
| (ii) | IFRS 9, Financial Instruments (“IFRS 9”) |
In July 2014, the IASB issued the final version of IFRS 9, which reflects all phases of the financial instruments project and replaces IAS 39 and all previous versions of IFRS 9. The standard introduces new requirements for classification and measurement, impairment, and hedge accounting. IFRS 9 is effective for annual periods beginning on or after January 1, 2018, with early application permitted. The adoption of this new standard has had no significant impacts on the Company’s accompanying consolidated financial statements.
| (iii) | IFRS 15, Revenue from Contracts with Customers (“IFRS 15”) |
The IASB replaced IAS 18, Revenue, in its entirety with IFRS 15. The standard contains a single model that applies to contracts with customers and two approaches to recognizing revenue: at a point in time or over time. The model features a contract-based five-step analysis of transactions to determine whether, how much and when revenue is recognized. New estimates and judgmental thresholds have been introduced, which may affect the amount and/or timing of revenue recognized. IFRS 15 is effective for annual periods beginning on or after January 1, 2018, with early application permitted. The adoption of this new standard has had no significant impacts on the Company’s accompanying consolidated financial statements.
| (iv) | IFRS 16, Leases (“IFRS 16”) |
In January 2016, the IASB issued IFRS 16, which will replace IAS 17, Leases. This standard introduces a single lessee accounting model and requires a lessee to recognize assets and liabilities for all leases with a term of more than twelve months unless the underlying asset is of low value. A lessee is required to recognize a right-of-use asset representing its right to use the underlying asset and a lease liability representing its obligation to make lease payments. The standard will be effective for annual periods beginning on or after January 1, 2019, with earlier application permitted for entities that apply IFRS 15 at or before the date of initial adoption of IFRS 16. The Company expects significant impacts on its financial statements from the adoption of this new standard. The Company leases the majority of its cultivation facilities and dispensaries and anticipates the statement of financial position to increase by the amount determined in Note 15.
| (v) | IFRS 2, Share-based Payment (“IFRS 2”) |
In June 2016, the IASB issued amendments to IFRS 2, Share-based Payment in relation to the classification and measurement of share-based payment transactions. The amendment provided guidance introduces accounting requirements for cash-settled share-based payments that follows the same approach as used for equity-settled share-based payments. On such modifications, the original liability recognized in respect of the cash-settled share-based payment is derecognized and the equity-settled share-based payment is recognized at the modification date fair value to the extent services have been rendered up to the modification date. Any difference between the carrying amount of the liability as at the modification date and the amount recognized in equity at the same date would be recognized in profit and loss immediately. The amendments are effective for annual periods beginning on or after January 1, 2018. Earlier application is permitted. The amendments are to be applied prospectively. However, retrospective application if allowed if this is possible without the use of hindsight. The adoption of this new standard has had no significant impacts on the Company’s accompanying consolidated financial statements.
13
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Six Months Ended June 30, 2018
(Amounts Expressed in United States Dollars)
| (vi) | IAS 28, Long-term Interests (“IAS 28”) |
In October 2017, the IASB amended IAS 28, Long-term Interests in Associates and Joint Ventures. The amendments were added to clarify that an entity applies IFRS 9 ‘Financial Instruments’ to long-term interests in an associate or joint venture that form part of the net investment in the associate or joint venture but to which the equity method is not applied. The standard which will be effective for annual periods beginning on or after January 1, 2019, with earlier adoption permitted. The Company does not expect significant impact on its consolidated financial statements from the adoption of this new standard.
| 3. | BIOLOGICAL ASSETS |
The Company’s biological assets consist of cannabis plants. The Company expenses all direct and indirect costs in the period they are incurred related to the biological transformation of the biological assets between the point of initial recognition and the point of harvest. The expense policy provides better visibility to revenues and cost of sales and the results of the biological transformation. The Company then measures the biological assets at fair value less cost to sell up to the point of harvest. Any subsequent post-harvest costs are capitalized to inventory to the extent that cost is less than net realizable value. The net unrealized gains or losses arising from changes in fair value less cost to sell during the year are included in the results of operations of the related year.
The Company’s biological assets consists of seeds and cannabis plants. A reconciliation of the beginning and ending balances of biological assets for the periods ended June 30, 2018 and December 31, 2017 are as follows:
| 06/30/2018 | 12/31/2017 | |||||||
| Balance at Beginning of Period |
$ | 2,636,654 | $ | 1,147,480 | ||||
| Purchases |
1,485,937 | 2,669,969 | ||||||
| Expenditures |
6,860,559 | 7,674,781 | ||||||
| Changes in Fair Value of Biological Assets |
2,170,569 | 1,489,174 | ||||||
| Transferred to Inventory Upon Harvest |
(8,346,496 | ) | (10,344,750 | ) | ||||
|
|
|
|
|
|||||
| Balance at End of Period |
$ | 4,807,223 | $ | 2,636,654 | ||||
|
|
|
|
|
|||||
On average the growing time for a full harvest approximates 17 weeks. As listed below, key estimates are involved in the valuation process of the cannabis plants. The Company’s estimates are subject to changes that could result in future gains or losses of biological assets. Changes in estimates could result from volatility of sales prices, changes in yields, and variability of the costs necessary to complete the harvest. Prior to harvest, all production costs are expensed.
The fair value of biological assets is considered a Level 3 categorization in the IFRS fair value hierarchy. The significant estimates and inputs used to assess the fair value of biological assets include the following assumptions:
| (a) | The selling prices, which are based on average market prices in the locations in which the Company operates in during the six months ended June 30, 2018 and 2017; |
| (b) | the cost to complete the cannabis production process post-harvest, and the cost to sell; |
| (c) | the stage of plant growth; and |
| (d) | expected yields from each cannabis plant. |
14
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Six Months Ended June 30, 2018
(Amounts Expressed in United States Dollars)
A plant typically produces approximately a total of 220 grams. The Company has quantified the sensitivity of the inputs in relation to the biological assets for the six months ended June 30, 2018 and year ended December 31, 2017 and expects that a 5% increase or decrease in the selling price per gram would increase or decrease the fair value of biological assets by approximately $227,000. A 5% increase or decrease in the estimated yield per cannabis plant would result in an increase or decrease in the fair value of biological assets of approximately $240,000. Additionally, an increase or decrease of 5% in the costs of production would increase or decrease the fair value of biological assets by approximately $66,500.
| 4. | INVENTORY |
As of June 30, 2018 and December 31, 2017, inventory totaled $6,043,506 and $3,191,109, respectively, and are comprised primarily of finished goods cannabis and cannabis-related products.
| 5. | INVESTMENTS |
The Company determines how to account for investments based on the level of control it has over the investment. If control is determined, then the investment should be consolidated. The Company’s investments that have been controlled and consolidated are further described in Note 2(e). Investments in which the Company has significant influence, but no control, are considered investments in associates. Significant influence is the power to participate in the financial and operating policy decisions of the investee, but without control or joint control over those policies. Investments in associates are accounted for using the equity method of accounting. Interests in associates accounted for using the equity method are initially recognized at cost. The carrying value is then adjusted for the Company’s share of comprehensive income (loss) and distributions from the investee. The carrying value of associates is assessed for impairment at each balance sheet date. As of December 31, 2017, the Company had no investments in associates. Investments that are not controlled, or the Company does not have significant influence, are first recognized at cost. At each reporting period, changes from the initial cost and fair value are recognized through profit and loss. As of June 30, 2018, the Company has both investments at fair value and investments in associates.
The following is a detailed discussion of the Company’s types of investments held:
| (a) | Investments at Fair Value |
The Company has investments in three entities: CHP Fresco, LLC (“CHP Fresco”), a real estate holding entity, MassRoots, Inc. (“MassRoots”), a publicly traded cannabis industry, and 420 Capital Management, LLC (“420 Capital”), a cannabis investment Company. CHP Fresco, MassRoots, and 420 Capital investments are accounted for at fair market value as the Company has determined that they do not have significant influence over the investee. The following is a description of the investments held at June 30, 2018 and December 31, 2017:
| June 30, 2018 |
December 31, 2017 |
|||||||
| CHP Fresco |
$ | 801,000 | $ | 801,000 | ||||
| MassRoots |
48,444 | 121,111 | ||||||
| 420 Capital Management |
67,500 | 67,500 | ||||||
|
|
|
|
|
|||||
| Total Investments |
$ | 916,944 | $ | 989,611 | ||||
|
|
|
|
|
|||||
15
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Six Months Ended June 30, 2018
(Amounts Expressed in United States Dollars)
| (b) | Investments in Associates |
During the period ended June 30, 2018, the Company purchased a 25 percent interest in Lighthouse Strategies, LLC (“Lighthouse”). Lighthouse owns and operates a cultivation facility in Nevada. As part of the purchase of interest, the Company paid approximately $5,500,000 in cash and issued 500,000 series E member units of the Company, valued at approximately $568,000. Management determined through its percentage ownership along with factors such as board and management representation on Lighthouse, the Company is able to significantly influence, but not control the entity. Accordingly, the investment in Lighthouse is classified as an investment in an associate. The following table below describes the balance of the investment as of June 30, 2018 and December 31, 2017.
| June 30, 2018 |
December 31, 2017 |
|||||||
| Lighthouse |
$ | 6,388,724 | — | |||||
|
|
|
|
|
|||||
| Total Investments |
$ | 6,388,724 | $ | — | ||||
|
|
|
|
|
|||||
In addition to the above, the Company recognized its pro-rata portion of Lighthouse’s net income during the year. The amount recognized as income from an investment in associate was approximately $320,000 for both the three and six months ended June 30, 2018.
| 6. | PROPERTY AND EQUIPMENT |
As of June 30, 2018 and December 31, 2017 property and equipment consist of:
| June 30, 2018 |
December 31, 2017 |
|||||||
| Furniture and Fixtures |
$ | 1,378,726 | $ | 290,528 | ||||
| Manufacturing Equipment |
2,042,557 | 1,047,251 | ||||||
| Leasehold Improvements |
6,186,084 | 206,019 | ||||||
| Construction In Progress |
7,121,129 | 3,081,143 | ||||||
| Automobiles |
153,803 | 81,717 | ||||||
| Computer Software & Hardware |
709,867 | 639,730 | ||||||
|
|
|
|
|
|||||
| Total Property and Equipment, Gross |
17,592,166 | 5,346,388 | ||||||
| Less: Accumulated Depreciation |
(812,594 | ) | (372,941 | ) | ||||
|
|
|
|
|
|||||
| Property and Equipment, Net |
$ | 16,779,572 | $ | 4,973,447 | ||||
|
|
|
|
|
|||||
16
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Six Months Ended June 30, 2018
(Amounts Expressed in United States Dollars)
A reconciliation of the beginning and ending balances of property and equipment for the six months ended June 30, 2018 is as follows:
| Property and Equipment, Gross |
Accumulated Depreciation |
Property and Equipment, Net |
||||||||||
| Balance as of January 1, 2018 |
$ | 5,346,388 | $ | (372,941 | ) | $ | 4,973,447 | |||||
| Additions |
12,245,778 | — | 12,245,778 | |||||||||
| Depreciation |
— | (439,653 | ) | (439,653 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Balance as of June 30, 2018 |
$ | 17,592,166 | $ | (812,594 | ) | $ | 16,779,572 | |||||
|
|
|
|
|
|
|
|||||||
A reconciliation of the beginning and ending balances of property and equipment for the year ended December 31, 2017 is as follows:
| Property and Equipment, Gross |
Accumulated Depreciation |
Property and Equipment, Net |
||||||||||
| Balance as of January 1, 2017 |
$ | 1,305,321 | $ | (172,289 | ) | $ | 1,133,032 | |||||
| Additions |
$ | 4,061,955 | $ | — | $ | 4,061,955 | ||||||
| Dispositions |
$ | (20,888 | ) | $ | 4,888 | $ | (16,000 | ) | ||||
| Depreciation |
$ | — | $ | (205,540 | ) | $ | (205,540 | ) | ||||
|
|
|
|
|
|
|
|||||||
| Balance as of December 31, 2017 |
$ | 5,346,388 | $ | (372,941 | ) | $ | 4,973,447 | |||||
|
|
|
|
|
|
|
|||||||
Depreciation expense of $221,000 and $45,000 was recorded for the three months ended June 30, 2018 and 2017, respectively, of which $193,000 and $35,000, respectively, was included in cost of sales. Depreciation expense of $384,000 and $88,000 was recorded for the six months ended June 30, 2018 and 2017, respectively, of which $339,000 and $69,000, respectively, are included in cost of sales.
| 7. | INTANGIBLE ASSETS |
Permits are issued by various state governments within the U.S. and grant the Company the right to construct and operate medical cannabis cultivation centers and dispensaries within each respective state. Permits are capitalized and amortized on a straight line-basis over the term of the permit. The term of the permits is one year from the date of issuance and requires yearly renewal.
As of June 30, 2018 and December 31, 2017, intangible assets consist of:
| June 30, 2018 |
December 31, 2017 |
|||||||
| Intangible Assets Acquired |
$ | 3,109,057 | $ | — | ||||
| Permit Application Costs |
1,910,000 | 1,565,000 | ||||||
| Less: Accumulated Amortization |
(1,612,917 | ) | (1,317,917 | ) | ||||
|
|
|
|
|
|||||
| Intangible Assets, Net |
$ | 3,406,140 | $ | 247,083 | ||||
|
|
|
|
|
|||||
17
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Six Months Ended June 30, 2018
(Amounts Expressed in United States Dollars)
A reconciliation of the beginning and ending balances of intangible assets for the six months ended June 30, 2018 is as follows:
| Intangible Assets, Gross |
Accumulated Amortization |
Intangible Assets, Net |
||||||||||
| Balance as of January 1, 2018 |
$ | 1,565,000 | $ | (1,317,917 | ) | $ | 247,083 | |||||
| Additions |
3,454,057 | — | 3,454,057 | |||||||||
| Amortization |
— | (295,000 | ) | (295,000 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Balance as of June 30, 2018 |
$ | 5,019,057 | $ | (1,612,917 | ) | $ | 3,406,140 | |||||
|
|
|
|
|
|
|
|||||||
A reconciliation of the beginning and ending balances of intangible assets for the year ended December 31, 2017 is as follows:
| Intangible Assets, Gross |
Accumulated Amortization |
Intangible Assets, Net |
||||||||||
| Balance as of January 1, 2017 |
$ | 975,000 | $ | (872,917 | ) | $ | 102,083 | |||||
| Additions |
590,000 | — | 590,000 | |||||||||
| Amortization |
— | (445,000 | ) | (445,000 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Balance as of December 31, 2017 |
$ | 1,565,000 | $ | (1,317,917 | ) | $ | 247,083 | |||||
|
|
|
|
|
|
|
|||||||
Amortization expense of $147,500 and $75,000 was recorded for the three months ended June 30, 2018 and 2017 of which $125,000 and $75,000, respectively, was included in cost of sales. Amortization expense of $295,000 and $150,000 was recorded for the six months ended June 30, 2018 and 2017 of which $250,000 and $150,000, respectively, was included in cost of sales. For the three and six months ended June 30, 2018, an additional $22,500 and 45,000 of amortization is included in selling, general, and administrative expense, respectively.
| 8. | ACCOUNTS PAYABLE AND OTHER ACCRUED EXPENSES |
As of June 30, 2018 and December 31, 2017, accrued expenses were comprised of the following:
| June 30, 2018 |
December 31, 2017 |
|||||||
| Accounts Payable |
$ | 2,804,087 | $ | 1,715,577 | ||||
| Accrued Expenses |
355,117 | 444,061 | ||||||
| Property Taxes Payable |
422,638 | 361,952 | ||||||
| Payroll Liabilities |
10,408 | 79,307 | ||||||
| Licensing Fee Payable |
67,625 | 36,903 | ||||||
| Other |
— | 2,782 | ||||||
|
|
|
|
|
|||||
| Total Accrued Liabilities |
$ | 3,659,875 | $ | 2,640,582 | ||||
|
|
|
|
|
|||||
18
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Six Months Ended June 30, 2018
(Amounts Expressed in United States Dollars)
| 9. | SUBSCRIPTION DEPOSITS REFUNDABLE AND DUE TO RELATED PARTY |
Subscription deposits refundable represents capital contributions received by the Company prior to successfully obtaining a license to produce and/or sell cannabis in a new state market. The Company had $12,500 and $399,800 in subscription deposits refundable as of June 30, 2018 and December 31, 2017, respectively. During 2018, the Company was unsuccessful in securing a license for this opportunity and refunded the subscriptions.
During 2017, the Company received subscriptions for expansion in to the Pennsylvania medical cannabis market. Upon successful awarding of licenses, the Company was responsible for funding these subscriptions to Yeltrah. The Company held $725,000 due to related party as of December 31, 2017, which were remitted in 2018. As of June 30, 2018, $1,500,000 was due to related party related to the acquisition of SLO.
| 10. | NOTES PAYABLE – RELATED PARTIES |
On December 30, 2016, the Company entered into Promissory Note Agreements (“Promissory Notes”) with its founders in the aggregate amount of $562,500. The terms of the Promissory Notes include maturity on the first anniversary of issuance with the Company having an election to extend the maturity date for up to two successive 12-month periods, but in no event beyond the third anniversary date of the Promissory Note Agreement. Additionally, the Promissory Notes accrue interest at an annual rate of 10%. The Company elected to extend the maturity date for two 12-month periods. As of June 30, 2018 and December 31, 2017, the amount outstanding under these agreements was $187,500 and $328,125, respectively.
| 11. | MEMBERS’ EQUITY |
The Company is authorized to have six classes of units (the “Units”), designated as Founder’s Units (“Founder’s Units”), Class A Units (“A Units”), Class B Units (“B Units”), Class C Units (“C Units”), Class D Units (“D Units”), Class E Units (“E Units”), and Class F Units (“F Units”). Under the Company’s Operating Agreement (the “Agreement”), the Founder’s Units, A Units, B Units, C Units, E Units, and F Units shall be identical in all respects except that C Units are non-voting except that to the extent the Founder’s Units represent not less than fifteen percent of all outstanding Units, the Founder’s Units shall, as a class, have voting rights equal to the greater of the actual voting rights of the Founder’s Units and fifty percent plus one vote of the aggregate voting rights of the Company’s outstanding units. If the Founder’s Units represent less than fifteen percent of the outstanding Units, the Founder’s Units, A Units, B Units, E Units, and F Units will vote as a single class, with each Unit representing one vote.
D Units are issued pursuant to a Profits Interest Plan, which is defined as any profits interest award plan of the Company, as amended, modified, supplemented, or replaced from time to time. D Units are awarded to individuals at the current fair value of Company and have no voting rights.
During October 2018, the Company announced completion of a private placement of Class F units for gross proceeds of US$100 million (U.S. dollar) and a proposed reverse takeover transaction of Randsburg International Gold Corp., a Canadian publicly listed company (“Randsburg”). Under the transaction, Randsburg will be required to change its name to Cresco Labs, Inc. consolidate its outstanding common shares, such that the shareholders of Randsburg retain an aggregate of C$2.2 million (Canadian dollar) in resulting issuer shares, and replace all directors and officers of Randsburg, and create a new class of non-participating super voting shares issued to certain principals of Cresco. A vote for Randsburg shareholders has been scheduled for November 14, 2018 to approve the proposed transaction.
19
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Six Months Ended June 30, 2018
(Amounts Expressed in United States Dollars)
| 12. | EQUITY ALLOCATION PLAN |
The Company has an Equity Allocation Plan (the “Plan”) for key employees and service providers. Under the Plan, units issued have no voting rights and vest proportionately over periods ranging from six months to four years from the issuance date.
The calculated value of each unit award granted is estimated on the date of grant using a valuation method, see Note 2. The expected term of the common units is derived from the output of the potential payout model and represent the period that units are expected to vest and begin participating in net income and distributions. A summary of the non-vested activity under the Plan as of June 30, 2018 and December 31, 2017, and the changes during the years then ended is as follows:
| Non-Vested Units |
Weighted- Average Grant Date Fair Value |
|||||||
| Outstanding - January 1, 2017 |
249,676 | $ | 0.12 | |||||
| Granted |
1,376,342 | $ | 0.12 | |||||
| Forfeited |
(74,267 | ) | $ | 0.12 | ||||
| Vested |
(519,292 | ) | $ | 0.12 | ||||
|
|
|
|
|
|||||
| Outstanding - December 31, 2017 |
1,032,459 | $ | 0.12 | |||||
| Granted |
3,540,000 | $ | 0.72 | |||||
| Forfeited |
(100,753 | ) | $ | 0.08 | ||||
| Exercised |
(64,247 | ) | $ | 0.12 | ||||
| Vested |
(977,708 | ) | $ | 0.20 | ||||
|
|
|
|
|
|||||
| Outstanding - June 30, 2018 |
3,429,751 | $ | 0.14 | |||||
|
|
|
|
|
|||||
The Company’s units had a weighted average fair value of $0.14 at the time of the grants as of June 30, 2018 and $0.12 as of December 31, 2017 based on Level 3 inputs including, the volatility of the unit price, the expected life of the unit, dividend yield, and interest rates. The Company recorded compensation expense of approximately $22,000 and $300 for the three months ended June 30, 2018 and 2017, respectively, and $42,000 and $500 for the six months ended June 30, 2018 and 2017, respectively.
| 13. | ACQUISITIONS AND MERGERS |
| (a) | Accounting Policy |
| (i) | Business Combinations |
A business combination is a transaction or event in which an acquirer obtains control of one or more businesses and is accounted for using the acquisition method. The total consideration paid for the acquisition is the aggregate of the fair values of assets given, liabilities incurred or assumed, and equity instruments issued in exchange for control of the acquiree at the acquisition date. The acquisition date is the date where the Company obtains control of the acquiree. The identifiable assets acquired and liabilities assumed are recognized at their acquisition date fair values, except for deferred taxes and share-based payment awards where IFRS provides exceptions to recording the amounts at fair value. Acquisition costs are expensed to profit or loss. See Note 2 for the Company’s policy for measurement of contingent consideration.
20
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Six Months Ended June 30, 2018
(Amounts Expressed in United States Dollars)
Noncontrolling interest in the acquiree, if any, is recognized either at fair value or at the noncontrolling interest’s proportionate share of the acquiree’s net assets, determined on an acquisition-by-acquisition basis. For each acquisition, the excess of total consideration over the fair value of previously held equity interest prior to obtaining control, and the noncontrolling interest in the acquiree over the fair value of the identifiable net assets acquired, is recorded as goodwill.
Certain fair values may be estimated at the acquisition date pending confirmation or completion of the valuation process. Where provisional values are used in accounting for a business combination, they may be adjusted retrospectively in subsequent periods. The measurement period is the period from the acquisition date to the date complete information about facts and circumstances that existed as of the acquisition date is received. However, the measurement period does not exceed one year from the acquisition date.
| (ii) | Asset Acquisitions |
Acquisitions that do not meet the definition of a business combination are accounted for as an asset acquisition. Consideration paid for an asset acquisition is allocated to the individual identifiable assets acquired and liabilities assumed based on their relative fair values. Asset acquisitions do not give rise to goodwill.
| (b) | Significant Judgements |
Classification of an acquisition as a business combination or an asset acquisition depends on whether the assets acquired constitute a business, which can be a complex judgment. Whether an acquisition is classified as a business combination or asset acquisition can have a significant impact on the entries made on and after acquisition.
In determining the fair value of all identifiable assets, liabilities and contingent liabilities acquired, the most significant estimates relate to contingent consideration and intangible assets. Management exercises judgement in estimating the probability and timing of when earn-outs are expected to be achieved which is used as the basis for estimating fair value. For any intangible asset identified, depending on the type of intangible asset and the complexity of determining its fair value, an independent valuation expert or management may develop the fair value, using appropriate valuation techniques, which are generally based on a forecast of the total expected future net cash flows. The evaluations are linked closely to the assumptions made by management regarding the future performance of these assets and any changes in the discount rate applied.
| (c) | Business Combinations |
| (i) | Phoenix |
On January 19, 2018, the Company, through its wholly owned subsidiary, Cresco Labs Phoenix, LLC, purchased a controlling interest in Phoenix Farms Partners, LLC (“Phoenix”). The acquisition was accounted for in accordance with IFRS 3, “Business Combinations” (“IFRS 3”). Phoenix owns and operates a retail dispensary in Champaign, Illinois.
21
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Six Months Ended June 30, 2018
(Amounts Expressed in United States Dollars)
The following table summarizes the consideration paid for the acquisition:
| Cash Paid |
$ | 2,640,611 | ||
|
|
|
|||
| Total Consideration |
$ | 2,640,611 | ||
|
|
|
The purchase price allocation for the acquisition, as set forth in the table below, reflects various fair value estimates and analyses which are subject to change within the measurement period. The primary areas of the purchase price allocation that are subject to change relate to the fair values of certain tangible assets, the valuation of intangible assets acquired, and residual goodwill. The Company expects to continue to obtain information to assist in determining the fair value of the net assets acquired at the acquisition date during the measurement period. Measurement period adjustments that the Company determines to be material will be applied retrospectively to the period of acquisition in the Company’s consolidated financial statements and, depending on the nature of the adjustments, other periods subsequent to the period of acquisition could also be affected. The Company expects to finalize the accounting for the acquisition by December 31, 2018.
The following table summarizes the preliminary accounting estimates of the acquisition with a purchase price of $2,640,611:
| Cash |
$ | 625,637 | ||
| Accounts Receivable |
17,643 | |||
| Inventory |
72,705 | |||
| Prepaid Expenses |
1,061 | |||
| Property and Equipment |
271,959 | |||
| Accounts Payable |
(179,534 | ) | ||
| Non-Controlling Interest |
(269,555 | ) | ||
| Intangible Assets |
2,100,695 | |||
|
|
|
|||
| Preliminary Accounting Estimates of Net Assets Acquired |
$ | 2,640,611 | ||
|
|
|
The Company incurred no acquisition related costs in the periods presented.
For the period ended June 30, 2018, Phoenix accounted for approximately $115,000 in contributed net income since January 19, 2018. This amount included revenues of approximately $684,000.
| (ii) | SLO |
On June 7, 2018, the Company, through its wholly owned subsidiary, Cresco Labs San Luis Obispo, LLC, a controlling interest in SLO, LLC (SLO). The acquisition was accounted for in accordance with IFRS 3, “Business Combinations” (“IFRS 3”). SLO owns and operates cultivation facility in San Luis Obispo, California. The Consideration is to be paid over time as defined in the agreement.
The following table summarizes the consideration for the acquisition:
| Cash Payable |
$ | 1,500,000 | ||
|
|
|
|||
| Total Consideration |
$ | 1,500,000 | ||
|
|
|
22
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Six Months Ended June 30, 2018
(Amounts Expressed in United States Dollars)
The purchase price allocation for the acquisition, as set forth in the table below, reflects various fair value estimates and analyses which are subject to change within the measurement period. The primary areas of the purchase price allocation that are subject to change relate to the fair values of certain tangible assets, the valuation of intangible assets acquired, and residual goodwill. The Company expects to continue to obtain information to assist in determining the fair value of the net assets acquired at the acquisition date during the measurement period. Measurement period adjustments that the Company determines to be material will be applied retrospectively to the period of acquisition in the Company’s consolidated financial statements and, depending on the nature of the adjustments, other periods subsequent to the period of acquisition could also be affected. The Company expects to finalize the accounting for the acquisition by June 30, 2019.
The following table summarizes the preliminary accounting estimates of the acquisition with a purchase price of $1,500,000:
| Cash |
$ | 385,362 | ||
| Accounts Receivable |
439,978 | |||
| Accrued Expenses |
(5,932 | ) | ||
| Non-Controlling Interest |
(327,769 | ) | ||
| Intangible Assets |
1,008,361 | |||
|
|
|
|||
| Preliminary Accounting Estimates of Net Assets Acquired |
$ | 1,500,000 | ||
|
|
|
The Company incurred no acquisition related costs in the periods presented.
For the period ended June 30, 2018, SLO accounted for approximately $124,000 in contributed net income since June 7, 2018. This amount included revenues of approximately $198,000.
| 14. | SELLING, GENERAL, AND ADMINISTRATIVE EXPENSES |
For the period endings ending June 30, 2018 and 2017, selling, general and administrative expenses were comprised of the following:
| Three Months Ended June 30, |
Six Months Ended June 30, |
|||||||||||||||||||
| 2018 | 2017 | 2018 | 2017 | |||||||||||||||||
| Salaries and Related |
Note 16 | $ | 951,882 | $ | 309,418 | $ | 1,728,431 | $ | 575,855 | |||||||||||
| Consulting and Professional Fees |
403,417 | 143,053 | 681,471 | 298,181 | ||||||||||||||||
| Travel and Entertainment |
156,437 | 30,105 | 270,722 | 45,655 | ||||||||||||||||
| Rent |
137,365 | 21,031 | 225,109 | 42,062 | ||||||||||||||||
| Office |
87,077 | 15,876 | 206,436 | 44,830 | ||||||||||||||||
| Other |
498,647 | 91,445 | 593,191 | 375,583 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at End of Year |
$ | 2,234,825 | $ | 610,928 | $ | 3,705,360 | $ | 1,382,166 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
23
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Six Months Ended June 30, 2018
(Amounts Expressed in United States Dollars)
| 15. | COMMITMENTS AND CONTINGENCIES |
| (a) | Leased Assets |
A lease of property and equipment is classified as a capital lease if it transfers substantially all the risks and rewards incidental to ownership to the Company. A lease of property and equipment is classified as an operating lease whenever the terms of the lease do not transfer substantially all the risks and rewards of ownership to the lessee. Lease payments are recognized as an expense on a straight-line basis over the lease term, except where another systematic basis is more representative of the time pattern in which the economic benefits are consumed.
| (b) | Office and Operating Leases |
The Company leases its Chicago, Illinois headquarters under a non-cancelable sublease agreement with an affiliated entity, which expires in July 2019 (this sublease agreement is pursuant to a master lease agreement). Total rent expense was approximately $1,151,000 and $1,065,000 for the three months ended June 30, 2018 and 2017, respectively, and $2,297,000 and $2,128,000 for the six months ended June 30, 2018 and 2017, respectively, which is included in cost of sales and selling, general and administrative expenses in the accompanying consolidated statements of operations.
The Company leases its cultivation facilities in Joliet, Lincoln, and Kankakee, Illinois from an affiliated entity. The commencement dates of the non-cancelable leases are determined based upon a Substantial Completion Date, as defined in the lease agreements, or six months after the Illinois Department of Agriculture awards the license. The Joliet lease commenced in December 2015, the Lincoln lease commenced in February 2016, and the Kankakee lease commenced in April 2016. The terms of these lease agreements are fifteen years from the commencement date. Rent expense for these facilities was approximately $1,047,000 and $1,044,000 for the three months ended June 30, 2018 and 2017, respectively, and $2,093,000 and $2,086,000 for the six months ended June 30, 2018 and 2017, respectively, which is included in cost of sales in the accompanying consolidated statements of operations. For financial reporting purposes, rent expense has been recorded on a straight-line basis over the terms of the leases resulting in deferred rent liability of approximately $1,894,000 and $1,587,000 as of June 30, 2018 and December 31, 2017, respectively.
The Company leases its cultivation facility in Brookville, Pennsylvania. The non-cancelable lease commenced on June 30, 2017, upon the announcement of a successful license application, and terms after 60 months. Rent expense was approximately $40,000 and $0 for the three months ended June 30, 2018 and 2017, respectively, and $81,000 and $0 for the six months ended June 30, 2018 and 2017, respectively. The Company leases dispensary locations in Butler, Pennsylvania and Pittsburgh, Pennsylvania with 60-month terms and the option to extend. Rent expense was approximately $42,000 and $0 for the three months ended June 30, 2018 and 2017, respectively, and $79,000 and $0 for the six months ended June 30, 2018 and 2017, respectively, with approximately $9,000 and $14,000 in deferred rent liability as of June 30, 2018 and December 31, 2017, respectively.
In addition, the Company is responsible for real estate taxes and comment operating expenses incurred by the building or facility in which it leases space. The Company was also required to pay to the affiliate a security deposit. The balance of the security deposit paid to the affiliate was approximately $1,325,000 as of June 30, 2018 and December 31, 2017.
24
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Six Months Ended June 30, 2018
(Amounts Expressed in United States Dollars)
| (c) | Contingencies |
The Company’s operations are subject to a variety of local, state, and federal regulation. Failure to comply with one or more of those regulations could result in fines, restrictions on its operations, or losses of permits that could result in the Company ceasing operations. While management of the Company believes that the Company is in compliance with applicable local, state, and federal regulation as of June 30, 2018, medical cannabis regulations continue to evolve and are subject to differing interpretations. As a result, the Company may be subject to regulatory fines, penalties, or restrictions in the future.
| (d) | Claims and Litigation |
From time to time, the Company may be involved in litigation relating to claims arising out of operations in the normal course of business. As of June 30, 2018, there were no pending or threatened lawsuits that could reasonably be expected to have a material effect on the results of the Company’s operations. There are also no proceedings in which any of the Company’s directors, officers or affiliates is an adverse party or has a material interest adverse to the Company’s interest.
| 16. | RELATED PARTY TRANSACTIONS |
The Company has a note payable due to its founders, see Note 10. Additionally, the Company leases its offices and cultivation facilities from affiliated entities, see Note 15.
Included in salaries expenses for the three months ended June 30, 2018 and 2017 is approximately $112,000 and $69,000, respectively, and for the six months ended June 30, 2018 and 2017 is approximately $223,000 and $138,000, respectively, related to the compensation of key management personnel; with a balance of $0 and $172,000 included as a liability as of June 30, 2018 and December 31, 2017, respectively.
| 17. | FINANCIAL INSTRUMENTS AND FINANCIAL RISK MANAGEMENT |
Financial Instruments
The Company’s financial instruments consist of cash and cash equivalents, accounts receivables, due from related parties, accounts payables and other accrued expenses, subscription deposits refundable, due to related party, and notes payable – related parties. The carrying values of these financial instruments approximate their fair values as of June 30, 2018 and December 31, 2017 due to their nature and relatively short maturity date.
Financial instruments recorded at fair value are classified using a fair value hierarchy that reflects the significance of the inputs to fair value measurements. The three levels of hierarchy are:
Level 1 – Unadjusted quoted prices in active markets for identical assets or liabilities;
Level 2 – Inputs other than quoted prices that are observable for the asset or liability, either directly or indirectly; and
Level 3 – Inputs for the asset or liability that are not based on observable market data.
There have been no transfers between fair value levels valuing these assets during the year.
25
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Six Months Ended June 30, 2018
(Amounts Expressed in United States Dollars)
The following table summarizes the Company’s financial instruments as of June 30, 2018:
| Loans and Receivables |
Other Financial Liabilities |
Total | ||||||||||
| Financial Assets: |
||||||||||||
| Cash and Cash Equivelents |
$ | 31,699,077 | $ | — | $ | 31,699,077 | ||||||
| Accounts Receivable |
$ | 2,301,138 | $ | — | $ | 2,301,138 | ||||||
| Financial Liabilities: |
||||||||||||
| Accounts Payable and Accrued Expenses |
$ | — | $ | 3,659,875 | $ | 3,659,875 | ||||||
| Subscription Deposits Refundable |
$ | — | $ | 12,500 | $ | 12,500 | ||||||
| Notes Payable - Related Parties |
$ | — | $ | 187,500 | $ | 187,500 | ||||||
| Due to Related Party |
$ | — | $ | 1,500,000 | $ | 1,500,000 | ||||||
The following table summarizes the Company’s financial instruments as of December 31, 2017:
| Loans and Receivables |
Other Financial Liabilities |
Total | ||||||||||
| Financial Assets: |
||||||||||||
| Cash |
$ | 27,043,219 | $ | — | $ | 27,043,219 | ||||||
| Accounts Receivable |
$ | 1,010,620 | $ | — | $ | 1,010,620 | ||||||
| Financial Liabilities: |
||||||||||||
| Accounts Payable and Accrued Expenses |
$ | — | $ | 2,640,582 | $ | 2,640,582 | ||||||
| Subscription Deposits Refundable |
$ | — | $ | 399,800 | $ | 399,800 | ||||||
| Notes Payable - Related Parties |
$ | — | $ | 328,125 | $ | 328,125 | ||||||
| Due to Related Party |
$ | — | $ | 725,000 | $ | 725,000 | ||||||
Financial Risk Management
The Company is exposed in varying degrees to a variety of financial instrument related risks. The Board mitigates these risks by assessing, monitoring and approving the Company’s risk management processes:
| (a) | Credit and Banking Risk |
Credit risk is the risk of a potential loss to the Company if a customer or third party to a financial instrument fails to meet its contractual obligations. The maximum credit exposure at June 30, 2018 and December 31, 2017 is the carrying amount of cash. The Company does not have significant credit risk with respect to its customers. Although all cash is placed with major U.S. financial institutions, there has been no change in the U.S. federal banking laws related to the deposit and holding of funds derived from activities related to the cannabis industry. Given that U.S. federal law provides that the production and possession of cannabis is illegal, there is a strong argument that banks cannot accept for deposit funds from business involved with the cannabis industry.
| (b) | Asset Forfeiture Risk |
Because the cannabis industry remains illegal under U.S. federal law, any property owned by participants in the cannabis industry which are either used in the course of conducting such business, or are the proceeds of such business, could be subject to seizure by law enforcement and subsequent civil
26
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Six Months Ended June 30, 2018
(Amounts Expressed in United States Dollars)
asset forfeiture. Even if the owner of the property were never charged with a crime, the property in question could still be seized and subject to an administrative proceeding by which, with minimal due process, it could be subject to forfeiture.
| (c) | Liquidity Risk |
Liquidity risk is the risk that the Company will not be able to meet its financial obligations associated with financial liabilities. The Company manages liquidity risk through the management of its capital structure. The Company’s approach to managing liquidity is to ensure that it will have sufficient liquidity to settle obligations and liabilities when due.
In addition to the commitments outlined in Note 10 and Note 15, the Company has certain other financial liabilities recorded on its unaudited consolidated balance sheets. As of both June 30, 2018 and December 31, 2017, these liabilities are classified as current and are expected to be paid within one year or less.
| (d) | Market Risk |
| (i) | Currency Risk |
The Company had no exposure to foreign currency transaction or translation risk for the periods presented in these unaudited consolidated financial statements.
| (ii) | Interest Rate Risk |
Interest rate risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in market interest rates. The Company’s financial debts have fixed rates of interest and therefore expose the Company to a limited interest rate fair value risk.
| (iii) | Price Risk |
Price risk is the risk of variability in fair value due to movements in equity or market prices.
| (iv) | Tax Risk |
Tax risk is the risk of changes in the tax environment that would have a material adverse effect on the Company’s business, results of operations, and financial condition. Currently, state licensed marijuana businesses are assessed a comparatively high effective federal tax rate due to section 280E which bars businesses from deducting all expenses except their cost of goods sold (COGS) when calculating federal tax liability. Any negative increase in additional tax measures may have a further adverse effect on the operations of the Company, while any decrease in tax pressures will be beneficial to future operations.
| (v) | Regulatory Risk |
Regulatory risk pertains to the risk that the Company’s business objectives are contingent, in part, upon the compliance of regulatory requirements. Due to the nature of the industry, the company recognizes that regulatory requirements are more stringent and punitive in nature. Any delays in obtaining, or failure to obtain regulatory approvals can significantly delay operational and product development and can have a material adverse effect on the Company’s business, results of operation, and financial condition.
27
CRESCO LABS, LLC
Notes to the Consolidated Financial Statements
For the Six Months Ended June 30, 2018
(Amounts Expressed in United States Dollars)
The Company is cognizant of the advent of regulatory changes occurring in the cannabis industry on the city, state, and national levels. Although regulatory outlook on the cannabis industry has been moving in a positive trend, the Company is aware of the effect of unforeseen regulatory changes can have on the goals and operations of the business as a whole.
| 18. | CAPITAL MANAGEMENT |
The Company’s objective is to maximize sufficient capital base so as to maintain investor, creditor, and customer confidence, future development of its business strategy and provide the ability to continue as a going concern. Management defines capital as the Company’s shareholders’ equity. The Board of Directors does not establish quantitative return on capital criteria for management; but rather promotes year-over-year growth. The Company currently has not paid any dividends to its shareholders.
As of June 30, 2018 and December 31, 2017 total managed capital was comprised of members’ equity of approximately $67,211,491 and $35,936,570, respectively.
There were no changes in the Company’s approach to capital management during the year.
| 19. | SUBSEQUENT EVENTS |
The Company has evaluated subsequent events through November 9, 2018, which is the date on which these financial statements were available to be issued.
On September 14, 2018, the Company issued 26,666,667 of new Class F units and closed its capital raise with $100,000,000 in new investment.
On October 1, 2018, the Company entered into a Purchase and Sale agreement with FloraMedex, LLC, an Illinois limited liability company to acquire 100% of the issued and outstanding units of FloraMedex in exchange for $10,000,000 in cash consideration.
On October 5, 2018, the Company entered a binding Memorandum of Understanding with Hope Heal Health, Inc., a Massachusetts corporation to acquire 100% of the shares and interest in exchange for $27,500,000.
On October 24, 2018, the Company entered a Plan of Merger Agreement with Gloucester Street Capital, LLC (“GSC”), a New York limited liability company to acquire 100% of the issued and outstanding units of GSC in exchange for $32,500,000 cash consideration and 13,466,667 Class F units in the Company of which 5,333,333 is contingent consideration.
On October 24, 2018, the Company entered a Membership Interest Purchase Agreement with Arizona Facilities Supply, LLC (“AFS”) an Arizona limited liability company to acquire 100% of the outstanding membership interests of AFS. Total consideration for purchase is $25,300,000, whereas $22,300,000 is in exchange for acquisition of AFS, but excluding its subsidiary, AFS Maryland, and $3,000,000 in consideration of the indirect acquisition of AFS Maryland.
During October 2018, the Company announced completion of a private placement of Class F units for gross proceeds of US$100 million (U.S. dollar) and a proposed reverse takeover transaction of Randsburg International Gold Corp., a Canadian publicly listed company (“Randsburg”). Under the transaction, Randsburg will be required to change its name to Cresco Labs, Inc., consolidate its outstanding common shares, such that the shareholders of Randsburg retain an aggregate of C$2.2 million (Canadian dollar) in resulting issuer shares, and replace all directors and officers of Randsburg, and create a new class of non-participating super voting shares issued to certain principals of Cresco. A vote for Randsburg shareholders has been scheduled for November 14, 2018 to approve the proposed transaction.
28
SCHEDULE “C”
CRESCO’S MANAGEMENT DISCUSSION & ANALYSIS
(See attached.)
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS FOR THE YEAR ENDED DECEMBER 31, 2017 AND THE THREE AND SIX MONTHS ENDED JUNE 30, 2018
This management discussion and analysis (“MD&A”) of the financial condition and results of operations of Cresco Labs, LLC (the “Company” or “Cresco”) is for the year ended December 30, 2017 and for the three and six months ended June 30, 2018. It is supplemental to, and should be read in conjunction with, the Company’s audited combined financial statements and the accompanying notes for the year ended December 31, 2017 and the Company’s unaudited interim combined financial statements and the accompanying notes for the three and six months ended June 30, 2018. The Company’s financial statements are prepared in accordance with International Financial Reporting Standards (“IFRS”). Financial information presented in this MD&A is presented in United States dollars (“$” or “US$”), unless otherwise indicated.
This MD&A has been prepared by reference to the MD&A disclosure requirements established under National Instrument 51-102–Continuous Disclosure Obligations of the Canadian Securities Administrators.
This MD&A contains certain “forward-looking statements” and certain “forward-looking information” as defined under applicable United States securities laws and Canadian securities laws. Please refer to the discussion of forward-looking statements and information set out under the heading “Cautionary Note Regarding Forward Looking Information”, located at the beginning of this Listing Statement. As a result of many factors, the Company’s actual results may differ materially from those anticipated in these forward-looking statements and information.
OVERVIEW OF THE COMPANY
Cresco is an Illinois limited liability company which is licensed to cultivate manufacture and sell medical cannabis and medical cannabis products. The Company operates in Illinois, Pennsylvania, Ohio, California, Nevada and Arizona.
Cresco is comprised of the following companies:
| • | Cresco Labs PA, LLC (“Cresco PA”), wholly-owned, which holds a 98% interest in an operating company, Cresco Yeltrah, LLC (“Yeltrah”). |
| • | JDRCB Ohio, LLC (“JDRCBO”), wholly-owned, which holds a 98% interest in an operating company, Cresco Labs, OH, LLC (“Cresco Ohio”). |
| • | JDRC Managed Services, LLC (“JDRC”), wholly-owned. |
| • | Cresco Edibles, wholly-owned, which holds a 75% interest in an operating company, TSC Cresco, LLC (“TSC”). |
| • | Cresco Joliet, LLC (“Cresco Joliet”), of which the Company owns 100%. |
| • | Cresco Kankakee, LLC (“Cresco Kankakee”), of which the Company owns 100%. |
| • | Cresco Lincoln, LLC (“Cresco Lincoln”), of which the Company owns 100%. |
| • | Cresco Labs California, LLC (“California”), wholly-owned, which holds an 80% interest in an operating company, SLO Cultivation, Inc (“SLO”) |
| • | Cresco Labs TINAD, LLC wholly-owned, which holds a 35% interest is an operating entity, TINAD, LLC (“TINAD”) |
| • | Cresco Labs Phoenix, LLC wholly-owned, which holds an 90% interest in an operating company, Phoenix Farms, LLC (“Phoenix”). |
| • | Cresco Labs Nevada, LLC wholly-owned, which holds a 25% interest in an operating company, Lighthouse Strategies, LLC (“Phoenix”). |
| • | Cresco Labs Arizona, LLC wholly-owned, which holds a 100% interest in an operating company, Arizona Facilities Supply, LLC and which holds a 100% interest in an operating company, Encanto Green Cross Dispensary, LLC, (collectively, “Arizona”). |
Cresco is primarily to engage in the business of cultivating medical grade cannabis, manufacturing medical products derived from cannabis cultivation and the distribution of such products to medical or adult use consumers in legalized cannabis markets. Cresco exists to provide high-quality and consistent cannabis-based products to consumers. Cresco’s business focuses on regulatory compliance while working to develop condition-specific strains of cannabis and non-invasive delivery methods (alternatives to smoke inhalation) to provide controlled-dosage medicinal cannabis relief to qualified patients and consumers in legalized cannabis markets. It currently operates three medical cannabis cultivation and manufacturing centers in Illinois, three dispensary locations in Illinois, one medical cannabis cultivation and manufacturing center in Pennsylvania, two dispensary locations in Pennsylvania (with a third location pending) and was awarded one medical cannabis cultivation license in Ohio for which the facility is currently under construction and one dispensary license in Ohio. In Illinois, Cresco’s three applications received the highest, second highest and third highest scores, respectively of all applications reviewed by the State of Illinois. In Pennsylvania, Cresco was awarded the highest score during the application process and had the second highest overall score, making it one of only five cultivators that was also awarded a dispensary license which allows for up to three dispensaries. In Ohio, Cresco received the seventh highest overall score.
The States We Operate In, Their Legal Framework and How It Affects Our Business
Illinois Operations
The Compassionate Use of Medical Cannabis Pilot Program Act, which allows individuals diagnosed with a debilitating medical condition access to medical marijuana, became effective January 1, 2014 and is extended through July 1, 2020. There are over 41 qualifying conditions as part of the medical program, including epilepsy, traumatic brain injury, and post-traumatic stress disorder (“PTSD”).
Illinois’ retail market size for 2017 was over $85 million, representing an over 140% year-over- year increase. In the first three calendar months of 2018, recorded state-wide sales are already over 1/3 of the total market size for all of 2017. The first quarter net revenues of 2018 represent an approximate 14% sequential increase over the fourth quarter of 2017.
In March 2018, Cook County voters (which is by far and large the most populous county in the state, encompassing all of Chicagoland metro area) responded positively for state-wide recreational legalization with a 63% majority. Although the vote was non-binding, the voting leverage of Cook County, which encompasses more than 130 municipalities, is anticipated to play a significant role in the November 2018 gubernatorial elections for which numerous candidates have outwardly pledged their support for cannabis legislation.
Cresco currently operates three (3) medical cannabis cultivation and manufacturing centers in Illinois and an ownership interest in five (5) dispensary locations in Illinois. Licenses were awarded based on merit in a highly competitive application process to applicants who demonstrated strong operational expertise and financial backing. To date, Cresco has established a 25%+ wholesale market share in Illinois.
Cresco is also spearheading clinical trials in collaboration with the Northwestern University Feinberg School of Medicine, the University of Illinois College of Pharmacy and the UIC/NIH Center for Botanical Dietary Supplements Research to formulate a Phase 1 trial related to the bioavailability of topical cannabinoid applications and the efficacy of such application for diabetic neuropathic pain.
- 2 -
Cresco is collaborating with biopharmaceutical scientists and the University of Illinois at Chicago College of Pharmacy to develop standards and methods for the accurate testing of cannabinoids and other molecular attendants contained in raw cannabis and cannabis derivative products. Such efforts will result in the most developed, thorough and accurate analytical methodologies developed related to cannabis to date.
Cresco is completing experimental trials with senior faculty at the University of Illinois School of Agriculture using two naturally occurring compounds, applied to the root zone of cannabis plants with the goal of increasing potency and disease resistance. Data from the experiments will be statistically analyzed to determine any significant effect resulting from the compound addition with the intent of publication.
Cresco is licensed to operate in the state of Illinois as a medical cultivator, and medical product manufacturer. Phoenix Farms, LLC, a subsidiary of Cresco (“Phoenix Farms”), is licensed to operate a retail dispensary in the state of Illinois. Under applicable laws, the licenses permit Cresco and Phoenix Farms to collectively, cultivate, manufacture, process, package, sell, and purchase marijuana pursuant to the terms of the licenses, which are issued by the Department of Agriculture and the Department of Financial and Professional Regulation under the provisions of the Illinois Revised Statutes 410 ILCS 130. All licenses are, as of the date hereof, active with the State of Illinois. There are two categories of licenses in Illinois: (i) cultivation/processing; and (ii) dispensary. The licenses are independently issued for each approved activity.
All cultivation/processing establishments must register with Illinois Department of Agriculture. All dispensaries must register with the Illinois Department of Financial and Professional Regulation. If applications contain all required information and after vetting by officers, establishments are issued a medical marijuana establishment registration certificate. Registration certificates are valid for a period of one year and are subject to annual renewals after required fees are paid and the business remains in good standing. Renewal requests are typically communicated through email from the Department of Agriculture or Illinois Department of Financial and Professional Regulation and include a renewal form.
The retail dispensary license held by Phoenix Farms permits it to purchase marijuana and marijuana products from cultivation/processing facilities, and allows the sale of marijuana and marijuana products to registered patients.
The three medical cultivation licenses held by Cresco permit it to acquire, possess, cultivate, manufacture/process into edible medical marijuana products and/or medical marijuana-infused products, deliver, transfer, have tested, transport, supply or sell marijuana and related supplies to medical marijuana dispensaries.
Pennsylvania Operations
The Pennsylvania medical marijuana program was signed into law on April 17, 2016 under Act 16 and provided access to state residents with one of 17 qualifying conditions, including epilepsy, chronic pain, and PTSD. The state, which consists of over 12 million U.S. citizens and qualifies as the fifth largest population in the US, operates as a high-barrier market with very limited market participation. The state originally awarded only 12 licenses to cultivate/process and 27 licenses to operate retail dispensaries (which entitled holders to up to three medical dispensary locations). Out of the hundreds of applicants in each license category, Cresco Yeltrah, LLC (“Cresco Yeltrah”), a subsidiary of Cresco, was awarded one (1) medical cannabis cultivation and manufacturing center in Pennsylvania, two (2) dispensary locations in Pennsylvania and there is a third location pending. Cresco Yeltrah has established a 30% market share in Pennsylvania.
Retail sales opened in February 2018 to a limited number of retail locations across the state. Cresco Yeltrah, on February 15, was the first cultivator/processor to release product into Pennsylvania market (approximately 6 weeks ahead of any other producer) and its dispensary was the first to sell product to patients in the state.
On March 22, 2018, it was announced that the final phase of the Pennsylvania medical marijuana program would initiate its rollout, which will include 13 additional cultivation/processing licenses and 23 additional dispensary licenses. The application period ran from April 2018 through May 17, 2018. Cresco Yeltrah submitted additional dispensary applications and anticipates that the Pennsylvania Department of Health will announce the license awards in the fourth quarter of 2018.
- 3 -
In the introductory months of the program, Pennsylvania’s medical marijuana dispensaries experienced supply shortages and were unable to keep up with statewide demand. It was announced on April 17, 2018 that dry flower would be included in the regulations as an approved product form for sale and consumption (in addition to the already approved forms of concentrates, pills, and tinctures). Simultaneously, it was announced that the list of qualifying conditions would expand from 17 to 21, including additions of cancer remission therapy and opioid-addiction therapy.
Cresco Yeltrah is licensed to operate in the Commonwealth of Pennsylvania as a medical cannabis cultivator/processor and to operate three (3) medical cannabis dispensaries. Under applicable laws, the licenses permit Cresco Yeltrah to cultivate, manufacture, process, package, sell, and purchase medical marijuana pursuant to the terms of the licenses, which are issued by the Pennsylvania Department of Health under the provisions of Medical Marijuana Act (35 P.S. § § 10231.101— 10231.2110) and Chapters 1141, 1151 and 1161 of the Pennsylvania regulations. All licenses are, as of the date hereof, active with the Commonwealth of Pennsylvania. There are two categories of licenses in Pennsylvania: (i) cultivation/processing; and (ii) dispensary. The licenses are independently issued for each approved activity for use at Cresco Yeltrah facilities in Pennsylvania.
All cultivation/processing establishments must register with Pennsylvania Department of Health. All dispensaries must register with the Pennsylvania Department of Health. Registration certificates are valid for a period of one year and are subject to annual renewals after required fees are paid and the business remains in good standing. Specifically, for licenses that Cresco Yeltrah currently holds have each undergone one renewal.
The retail dispensary licenses permit Cresco Yeltrah to purchase marijuana and marijuana products from cultivation/processing facilities, and allows the sale of marijuana and marijuana products to registered patients.
The medical cultivation licenses permit Cresco Yeltrah to acquire, possess, cultivate, manufacture/process into edible medical marijuana products and/or medical marijuana-infused products, deliver, transfer, have tested, transport, supply or sell marijuana and related supplies to medical marijuana dispensaries.
Ohio Operations
House Bill 523, effective on September 8, 2016, legalized medical marijuana in Ohio. The Ohio Medical Marijuana Control Program (“MMCP”) allows people with certain medical conditions, upon the recommendation of an Ohio-licensed physician certified by the State Medical Board, to purchase and use medical marijuana. House Bill 523 required that the framework for the MMCP will be in place no later than September 2018. This timeframe allowed for a deliberate process to ensure the safety of the public and to promote access to a safe product. Due to construction delays and lawsuits, as of the end of September 2018 medical marijuana is not yet sold in Ohio. It is speculated that January 2019 is new target date for medical marijuana sales to begin in Ohio.
The three following state government agencies are responsible for the operation of Ohio’s Medical Marijuana Control Program: (1) the Ohio Department of Commerce is responsible for overseeing medical marijuana cultivators, processors and testing laboratories; (2) the State of Ohio Board of Pharmacy is responsible for overseeing medical marijuana retail dispensaries, the registration of medical marijuana patients and caregivers, the approval of new forms of medical marijuana and coordinating the Medical Marijuana Advisory Committee; and (3) the State Medical Board of Ohio is responsible for certifying physicians to recommend medical marijuana and may add to the list of qualifying conditions for which medical marijuana can be recommended. Qualifying medical conditions for medical marijuana include: HIV/AIDS, Lou Gehrig’s disease, Alzheimer’s disease, Cancer, Chronic traumatic encephalopathy, Crohn’s disease, epilepsy or other seizure disorder, fibromyalgia, glaucoma, hepatitis C, inflammatory bowel disease, multiple sclerosis (MS), pain (either chronic, severe, or intractable), Parkinson’s disease, post-traumatic stress disorder (PTSD), sickle cell anemia, spinal cord disease or injury, Tourette’s syndrome, traumatic brain injury, ulcerative colitis. In order for a patient to be eligible to obtain medical marijuana, a physician must make the diagnosis of one of these conditions.
Several forms of medical marijuana are legal in Ohio, these include: inhalation of marijuana through a vaporizer (not direct smoking), oils, Tinctures, plant material, edibles, patches and any other forms approved by the State Board of Pharmacy.
- 4 -
On June 4, 2018, the State of Ohio Board of Pharmacy awarded 56 medical marijuana provisional dispensary licenses. The licenses were awarded after an extensive review of 376 submitted dispensary applications.
Provisional licensees are authorized to begin the process of establishing a dispensary in accordance with the representations in their applications and the rules adopted by the State of Ohio Board of Pharmacy. Per rule, all provisional license holders have a maximum of six months to demonstrate compliance with the dispensary operational requirements to obtain a certificate of operation. Compliance will be determined through an inspection by a Board of Medical Marijuana Compliance Agent. Once a dispensary is awarded a certificate of operation, it can begin selling medical marijuana to Ohio patients and caregivers in accordance with Ohio laws and rules.
By rule, the State of Ohio Board of Pharmacy is limited to issuing up to 60 dispensary licenses across the state, but will have the authority to increase the number of licenses after September 8, 2018. To date, no announcement has been made if the number of licenses will be increased. Per the program rules, the Board will consider, on at least a biennial basis, whether enough medical marijuana dispensaries exist, considering the state population, the number of patients seeking to use medical marijuana, and the geographic distribution of dispensary sites.
Cresco Labs Ohio, LLC (“Cresco Ohio”), a subsidiary of Cresco, was awarded one provisional dispensary license. Cresco Ohio will take all steps needed to meet the six-month operational deadline at its dispensary location.
Cresco Ohio applied for and on November 30, 2017 received one provisional cultivation license. Cresco Ohio’s cultivation facility is a hybrid greenhouse structure located in Yellow Springs, Ohio.
A holder of a provisional cultivation license is prohibited from operating as a licensed cultivator and performing any cultivation or production activities, including the procurement of seeds, seedlings, or other starting plant material until a Certificate of Operation is issued by the Ohio Department of Commerce. This provisional license serves as authorization from the Ohio Department of Commerce for Cresco Ohio to begin the construction or modification of the facility and to secure any other applicable permits needed from local jurisdictions in order to receive a Certificate of Operation. Pursuant to Ohio Administrative Code s. 3796:2-1-06(B), a provisional license holder has nine (9) months to obtain a Certificate of Operation. On September 14, 2018, Cresco Ohio received its Certificate of Operation.
The dispensary license, once fully approved, will permit Cresco Ohio to purchase marijuana and marijuana products from cultivation/processing facilities, and allows the sale of marijuana and marijuana products to registered patients.
The medical cultivation licenses permit will permit Cresco Ohio to acquire, possess, cultivate, manufacture/process into medical marijuana products, deliver, transfer, have tested, transport, supply or sell marijuana and related supplies to medical marijuana dispensaries.
California Operations
In 1996, California was the first state to legalize medical marijuana through Proposition 215, the Compassionate Use Act of 1996 (“CUA”). This legalized the use, possession and cultivation of medical marijuana by patients with a physician recommendation for treatment of cancer, anorexia, AIDS, chronic pain, spasticity, glaucoma, arthritis, migraine, or any other illness for which marijuana provides relief.
In 2003, Senate Bill 420 was signed into law establishing an optional identification card system for medical marijuana patients.
In September 2015, the California legislature passed three bills collectively known as the “Medical Cannabis Regulation and Safety Act” (“MCRSA”). The MCRSA established a licensing and regulatory framework for medical marijuana businesses in California. The system created multiple license types for dispensaries, infused products manufacturers, cultivation facilities, testing laboratories, transportation companies, and distributors. Edible infused product manufacturers would require either volatile solvent or non-volatile solvent manufacturing licenses depending on their specific extraction methodology. Multiple agencies would oversee different aspects of the program and businesses would require a state license and local approval to operate. However, in November 2016, voters in California overwhelmingly passed Proposition 64, the “Adult Use of Marijuana Act” (“AUMA”) creating an adult-use marijuana program for adult-use 21 years of age or older. AUMA had some conflicting provisions with MCRSA,
- 5 -
so in June 2017, the California State Legislature passed Senate Bill No. 94, known as Medicinal and Adult-Use Cannabis Regulation and Safety Act (“MAUCRSA”), which amalgamates MCRSA and AUMA to provide a set of regulations to govern medical and adult-use licensing regime for cannabis businesses in the State of California. The four agencies that regulate marijuana at the state level are BCC, California Department of Food and Agriculture, California Department of Public Health, and California Department of Tax and Fee Administration.
In order to legally operate a medical or adult-use cannabis business in California, the operator must have both a local and state license. This requires license holders to operate in cities with marijuana licensing programs. Therefore, cities in California are allowed to determine the number of licenses they will issue to marijuana operators or can choose to outright ban marijuana.
MAUCRSA went into effect on January 1, 2018.
On June 7, 2018 Cresco acquired a 60% ownership interest in SLO Cultivation Inc. (“SLO”) a marijuana cultivation operation in operation in the cities of Carpinteria (Santa Barbara County) and San Luis Obispo (San Luis Obispo County) California. On September 27, 2018, Cresco acquired a further 20% ownership interest to bring the total ownership to 80%. The cultivation facility has a capacity of up to 600,000 square feet of greenhouse production space.
SLO through its wholly-owned subsidiaries (the “Cal Subsidiaries”) are licensed to operate as medical and adult-use cultivator and processor under applicable California and local jurisdictional law (the “California License”). The California License permits the Cal Subsidiaries to cultivate and process medical and adult-use cannabis in the State of California pursuant to the terms of the California License issued by the BCC under the provision of the MAUCRSA and California Assembly Bill No. 133. In California, licenses are independently issued for each approved activity for use.
California state and local licenses are renewed annually. Each year, licensees are required to submit a renewal application per guidelines published by BCC. While renewals are annual, there is no ultimate expiry after which no renewals are permitted. Additionally, in respect of the renewal process, provided that the requisite renewal fees are paid, the renewal application is submitted in a timely manner, and there are no material violations noted against the applicable license, SLO would expect to receive the applicable renewed license in the ordinary course of business. While SLO’s compliance controls have been developed to mitigate the risk of any material violations of a license arising, there is no assurance that the licenses will be renewed in the future in a timely manner. Any unexpected delays or costs associated with the licensing renewal process could impede the ongoing or planned operations of the Resulting Issuer and have a material adverse effect on its business, financial condition, results of operations or prospects.
SLO will be applying for and has been granted licenses permitting it to cultivate, manufacture, distribute and retail medical (and in some instances, adult use) cannabis and cannabis-related products:
Mendota (Fresno County)
| • | SLO has been issued a temporary license for Type 7 (Manufacturing 2 – Volatile), Adult Use & Medical (“A&M”). |
| • | SLO has submitted an application for a temporary Type 11 (Distribution), A&M. |
Willow Road (SLO County)
| • | SLO has submitted an application for a Type 3B (Cultivation; Mixed-Light; Medium), Type M (medical) only. Cresco has also initiated a license application for a non-store front Type 9 Retailer. |
Carpinteria (SB County)
| • | SLO has submitted 38 applications for Type 2B, Tier 1 (Cultivation; Specialty Mixed-Light; Small, less than 25 watts per light (Tier 1)), Medical only for the 9-acre Parcel (3889 Foothill Road, Carpinteria CA; APN 005-310-024). |
- 6 -
| • | Another three (3) license applications are pending for the Concrete Building (5134 Foothill Road, Carpinteria, CA; APN 004-004-037): |
| • | Type 11 Distributor (however, Santa Barbara County requires 10% of the material distributed from a premises to be grown on site); |
| • | Type 2B, Tier 1 (Small Cultivation, Specialty Mixed Light, applied for in conjunction with our Type 11 distribution license); and |
| • | Processor Type (“a cultivation site that conducts only trimming, drying, curing, grading or packaging of cannabis and nonmanufactured cannabis products.”) |
Nevada Operations
Medical marijuana use was legalized in Nevada by a ballot initiative in 2000. In November 2016, voters in Nevada passed an adult use marijuana measure to allow for the sale of recreational marijuana in the state. The first dispensaries to sell adult use marijuana began sales in July 2017. The Nevada Department of Taxation (“DOT”) is the regulatory agency overseeing the medical and adult use cannabis programs. Similar to California, cities and counties in Nevada are allowed to determine the number of local marijuana licenses they will issue.
Cresco via a Unit Purchase and Sales Agreement with Lighthouse Strategies Inc. (“Lighthouse”) and Cresco Labs Nevada, LLC, acquired a 25% ownership interest in Paradise Wellness Center, LLC (“Paradise Wellness”) d/b/a Las Vegas Releaf and Silver State Wellness, LLC (“Silver State”), entities licensed to operate in the state of Nevada. And received state and local approvals to effectuate the transfer of ownership interest.
Lighthouse is licensed to operate in the state of Nevada as a cultivator, product manufacturer and a retail dispensary. Under applicable laws, the licenses permit Lighthouse to cultivate, manufacture, process, package, sell, and purchase marijuana pursuant to the terms of the licenses, which are issued by the DOT under the provisions of Nevada Revised Statutes section 453A. All Nevada licenses are, as of the date hereof, active with the State of Nevada. All licenses are independently issued for each approved activity for use at the Lighthouse facilities and retail locations in Nevada.
In the state of Nevada, only cannabis that is grown/produced in the state by a licensed establishment may be sold in the state. Lighthouse is vertically integrated and has the capabilities to cultivate, harvest, process and sell/dispense/deliver cannabis and cannabis products. The state also allows Lighthouse to make wholesale purchase of cannabis from another licensed entity within the state.
The retail dispensary licenses and registration certificate permit Lighthouse to purchase marijuana from cultivation facilities, marijuana and marijuana products from product manufacturing facilities and marijuana from other retail stores, and allows the sale of marijuana and marijuana products to consumers.
The medical cultivation licenses permit Lighthouse to acquire, possess, cultivate, deliver, transfer, have tested, transport, supply or sell marijuana and related supplies to medical marijuana dispensaries, facilities for the production of edible medical marijuana products and/or medical marijuana-infused products, or other medical marijuana cultivation facilities.
The medical product-manufacturing license permits Lighthouse to acquire, possess, manufacture, deliver, transfer, transport, supply, or sell edible marijuana products or marijuana infused products to other medical marijuana production facilities or medical marijuana dispensaries. Lighthouse intends to apply for additional dispensary licenses as they become available.
All marijuana establishments must register with DOT. If applications contain all required information and after vetting by officers, establishments are issued a medical marijuana establishment registration certificate. In a local governmental jurisdiction that issues business licenses, the issuance by DOT of a medical marijuana establishment registration certificate is considered provisional until the local government has issued a business license for operation and the establishment is in compliance with all applicable local governmental ordinances. Final registration certificates are valid for a period of one year and are subject to annual renewals after required fees are paid and the business
- 7 -
remains in good standing. Renewal requests are typically communicated through email from DOT and include a renewal form. The renewal periods serve as an update for DOT on the licensee’s status toward active licensure. It is important to note provisional licenses do not permit the operation of any commercial or medical cannabis activity. Only after a provisional licensee has gone through necessary state and local inspections, if applicable, and has received a final registration certificate from DOT may an entity engage in cannabis business operation.
Arizona Operations
In 2010, Arizona passed Ballot Proposition 203, which amended Title 36 to the Arizona Revised Statutes. This amendment added Chapter 28.1, titled the Arizona Medical Marijuana Act. (the “AMMA”). The AMMA is codified in Arizona Revised Statutes (‘‘ARS’’) § 36-2801 et. seq. The AMMA also appointed the Arizona Department of Health Services (the “ADHS”) as the regulator for the program and authorized ADHS to promulgate, adopt and enforce regulations for the AMMA. These ADHS Regulations are embodied in the Arizona Administrative Code (‘‘AAC’’) Title 9 Chapter 17 (the ‘‘Rules’’). ARS § 36-2801(11) defines a ‘‘nonprofit medical cannabis dispensary’’ as a not-for-profit entity that acquires, possesses, cultivates, manufactures, delivers, transfers, transports, supplies, sells or dispenses cannabis or related supplies and educational materials to cardholders (a ‘‘Dispensary’’).
The ADHS has established the Arizona Department of Health Services Medical Marijuana Program (‘‘MMJ Program’’), which includes a vertically integrated license, meaning if allocated a Medical Marijuana Dispensary Registration Certificate (‘‘Dispensary License’’), entities are authorized to dispense and cultivate medical cannabis. Each Dispensary License allows the holding entity to operate one on-site cultivation facility, and one off-site cultivation facility which can be located anywhere within the State of Arizona. An entity holding a Dispensary License is required to file an application to renew with the ADHS on an annual basis, which must also include audited annual financial statements. While a Dispensary License may not be sold, transferred or otherwise conveyed, Dispensary License holders typically contract with third parties to provide various services related to the ongoing operation, maintenance and governance of its dispensary and/or cultivation facility so long as such contracts do not violate the requirements of the AMMA or the MMJ Program.
The ADHS had until April 2012 to establish a registration application system for patients and nonprofit marijuana dispensaries, as well as a web-based verification platform for use by law officials and dispensaries to verify a patient’s status as such. It also specified patients’ rights, qualifying medical conditions, and allowed out-of-state medical marijuana patients to maintain their patient status (though not to purchase cannabis).
On December 6, 2012, Arizona’s first licensed medical marijuana dispensary opened in Glendale.
In order to qualify to use medical marijuana under the AMMA, a patient is required to have a “debilitating medical condition. Valid medical conditions include: HIV, cancer, glaucoma, immune deficiency syndrome, hepatitis C, chron’s disease, agitation of Alzheimer’s disease, ALS, cachexia/wasting syndrome, muscle spasms, nausea, seizures, severe and chronic pain or another chronic or debilitating condition.
In order for an applicant to receive a Dispensary Registration Certificate (a ‘‘Certificate’’) they must: (i) fill out an application on the form proscribed by ADHS, (ii) submit the applying entity’s articles of incorporation and by-laws, (iii) submit fingerprints for each principal officer or board member of the applicant for a background check to exclude felonies, (iv) submit a business plan and policies and procedures for inventory control, security, patient education, and patient recordkeeping that are consistent with the AMMA and the Rules to ensure that the Dispensary will operate in compliance and (v) designate an Arizona licensed physician as the Medical Director for the Dispensary. Certificates are renewed annually so long as the Dispensary is in good standing with ADHS and pays the renewal fee and submits an independent third-party financial audit.
Once an applicant has been issued a Certificate, they are allowed to establish one physical retail dispensary location, one cultivation location which is co-located at the dispensary’s retail site (if allowed by local zoning) and one additional off-site cultivation location. None of these sites can be operational, however, until the Dispensary receives an approval to operate from ADHS for the applicable site. This approval to operate requires: (i) an application on the ADHS form, (ii) demonstration of compliance with local zoning regulations, (iii) a site plan and floor plan for the applicable property, and (iv) an in-person inspection by ADHS of the applicable location to ensure compliance with the Rules and consistency with the Dispensary’s applicable policies and procedures.
- 8 -
Cresco has obtained a 100% ownership interest in Arizona Facilities Supply, LLC and Encanto Green Cross Dispensary, LLC, collectively, a vertically integrated cultivation, processing, and dispensary operation in Arizona.
The licenses in Arizona are renewed annually. Before expiry, licensees are required to submit a renewal application. While renewals are granted annually, there is no ultimate expiry after which no renewals are permitted. Additionally, in respect of the renewal process, provided that the requisite renewal fees are paid, the renewal application is submitted in a timely manner, and there are no material violations noted against the applicable license, Cresco would expect to receive the applicable renewed license in the ordinary course of business. While Cresco’s compliance controls have been developed to mitigate the risk of any material violations of a license arising, there is no assurance that Arizona Cannabis’ licenses will be renewed in the future in a timely manner. Any unexpected delays or costs associated with the licensing renewal process could impede the ongoing or planned operations of Arizona Cannabis have a material adverse effect on the Resulting Issuer’s business, financial condition, results of operations or prospects.
Any Dispensary facility (both retail and cultivation) must abide by the following security requirements: (i) ensure that access to the facilities is limited to authorized agents of the Dispensary (“Dispensary Agents”) who are in possession of a Dispensary Agent identification card, and (ii) equip the facility with: (a) intrusion alarms and surveillance equipment, (b) exterior and interior lighting to facilitate surveillance, (c) at least one 19-inch monitor for surveillance and a video capable of printing a high resolution still image, (d) high resolution video cameras at all points of sale, entrances, exits, and limited access areas, both in and around the building, (e) 30 days’ video storage, (f) failure notifications and battery backups for the security system and (g) panic buttons inside each building.
Dispensaries may transport medical cannabis between their own sites or between their sites and another Dispensary’s site and must comply with the following Rules: (i) prior to transportation, the Dispensary Agent must complete a trip plan showing: (a) the name of the Dispensary Agent in charge of transporting the cannabis, (b) the date and start time of the trip, (c) a description of the cannabis, cannabis plants, or cannabis paraphernalia being transported; and (d) the anticipated route of transportation, (ii) during transport the Dispensary Agent shall: (a) carry a copy of the trip plan at all times, (b) use a vehicle with no medical cannabis identification, (c) carry a cell phone, and (d) ensure that no cannabis is visible, and (iii) Dispensaries must maintain trip plan records.
ADHS may inspect a facility at any time upon five (5) days’ notice to the Dispensary. However, if someone has alleged that the Dispensary is not in compliance with the AMMA or the Rules, ADHS may conduct an unannounced inspection. ADHS will provide written notice to the Dispensary of any violations found during any inspection and the Dispensary then has 20 working days to take corrective action and notify ADHS.
ADHS must revoke a Certificate if a Dispensary: (i) operates before obtaining approval to operate a dispensary from the ADHS, (ii) dispenses, delivers, or otherwise transfers cannabis to an entity other than another dispensary with a valid dispensary registration certificate issued by the ADHS, a qualifying patient with a valid registry identification card, or a designated caregiver with a valid registry identification card, (iii) acquires usable cannabis or mature cannabis plants from any entity other than another dispensary with a valid dispensary registration certificate issued by the ADHS, a qualifying patient with a valid registry identification card, or a designated caregiver with a valid registry identification card, or (iv) if a principal officer or board member has been convicted of an excluded felony offense.
Furthermore, ADHS may revoke a Certificate if a Dispensary does not: (i) comply with the requirements of the AMMA or the Rules, (ii) implement the policies and procedures or comply with the statements provided to the ADHS with the dispensary’s application.
Components of Our Results of Operations
Revenue
We derive the majority of our revenue from wholesale of cannabis product to dispensary locations represents approximately 65% of our revenue. Our revenue from retail dispensary locations represents the remaining 35%.
- 9 -
Gross Profit
Gross profit is our revenue less cost of goods sold. Cost of goods sold includes the direct costs attributable to the production of the products sold in a company. Cost of goods sold are comprised of the following:
| • | Direct Labor Costs: These expenses include all salaries, benefits, and taxes for all employees at the facility. |
| • | Direct Supplies: The total direct material cost for maintenance of the plants, the supplies and nutrients, and the production expenses and equipment used to process medical marijuana. |
| • | Facility Expenses: The facility expense for the cultivation operations is the cost for the facility, utilities, property taxes, maintenance, and costs associated with monitoring the security systems. |
| • | Other Operating Expenses: These expenses include all costs associated with the facility itself including: insurance, community outreach programs, professional services, uniforms, employee training programs, tracking and inventory management systems, product testing, distribution, business development, back office expenses related to accounting, finance, human resources, and information technology and license renewal fees. |
Cannabis costs are affected by various state regulations that limits the sourcing and procurement of cannabis product, which may create fluctuations in gross profit over comparative periods as the regulatory environment changes.
Selling, General and Administrative Expenses (“SG&A”)
SG&A expenses consist of mainly salary and benefit cost of executive and back office staff, professional fees such as legal and accounting, travel and entertainment, and office rent expense.
Selling costs generally correlate to revenue. As a percentage of sales, we expect selling costs to decrease slightly as our business continues to grow. The decrease is expected to be driven primarily by efficiencies associated with scaling the business.
For the three and six months ended June 30, 2018 and year ended 2017, selling, general and administrative expenses were comprised of the following:
| Three Months Ended June 30, |
Six Months Ended June 30, |
Year Ended December 31, |
||||||||||||||||||
| 2018 | 2017 | 2018 | 2017 | 2017 | ||||||||||||||||
| Salaries and Related |
$ | 951,882 | $ | 309,418 | $ | 1,728,431 | $ | 575,855 | $ | 1,464,954 | ||||||||||
| Consulting and Professional Fees |
403,417 | 143,053 | 681,470 | 298,181 | 1,048,829 | |||||||||||||||
| Travel and Entertainment |
156,437 | 30,105 | 270,722 | 45,655 | 255,606 | |||||||||||||||
| Rent |
137,365 | 21,031 | 225,109 | 42,062 | 159,708 | |||||||||||||||
| Office |
87,077 | 15,876 | 206,435 | 44,830 | 147,167 | |||||||||||||||
| Selling and Marketing |
344,017 | 99,183 | 647,346 | 141,098 | 454,817 | |||||||||||||||
| Other |
498,647 | 91,445 | 593,191 | 375,583 | 1,036,665 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance at End of Year |
$ | 2,578,842 | $ | 710,111 | $ | 4,352,706 | $ | 1,523,264 | $ | 4,567,746 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
Income Taxes
The Company is a limited liability company that has elected to be treated as a partnership for federal income tax purposes. Under federal law, the taxable income or loss of a limited liability company is allocated to its members. Accordingly, no provision has been made for federal income taxes. The Company is responsible for certain other state taxes.
- 10 -
SELECTED FINANCIAL INFORMATION
The Company reports results of operations of its affiliates from the date that control commences, either through the purchase of the business or control through a management agreement. The following selected financial information includes only the results of operations after the Company established control of its affiliates. Accordingly, the information included below may not be representative of the results of operations if such affiliates had included their results of operations for the entire reporting period.
The following table sets forth selected combined financial information for the periods indicated that was derived from our audited combined financial statements and unaudited interim combined financial statements and the respective accompanying notes prepared in accordance with IFRS.
The selected combined financial information set out below may not be indicative of the Company’s future performance:
| As of and for the | ||||||||||||||||||||||||
| Three Months Ended June 30, | Six Months Ended June 30, | Year Ended December 31, | ||||||||||||||||||||||
| 2018 | 2017 | 2018 | 2017 | 2017 | 2016 | |||||||||||||||||||
| Net Revenue |
$ | 8,077,097 | $ | 2,485,144 | $ | 12,880,136 | $ | 4,405,053 | $ | 10,310,003 | $ | 3,157,232 | ||||||||||||
| Cost of Goods Sold |
$ | 4,569,802 | $ | 2,806,935 | $ | 8,346,496 | $ | 4,386,620 | $ | 11,320,233 | $ | 7,912,252 | ||||||||||||
| Gross Profit |
$ | 4,268,574 | $ | 301,370 | $ | 6,704,208 | $ | 685,872 | $ | 478,944 | ($ | 4,160,441 | ) | |||||||||||
| Total Expenses |
$ | 2,629,072 | $ | 719,958 | $ | 4,442,485 | $ | 1,542,512 | $ | 4,606,505 | $ | 3,617,175 | ||||||||||||
| Net Income (Loss) Attributed to Controlling Interest |
$ | 1,423,162 | ($ | 424,252 | ) | $ | 1,981,487 | ($ | 921,204 | ) | ($ | 3,175,353 | ) | ($ | 7,724,403 | ) | ||||||||
| Total Assets |
$ | 74,465,442 | $ | 74,465,442 | $ | 41,617,307 | $ | 11,516,407 | ||||||||||||||||
| Long Term Debt |
$ | — | $ | — | $ | — | $ | 562,500 | ||||||||||||||||
Three Months Ended June 30, 2018 Compared to Three Months Ended June 30, 2017
Net Revenue
Revenue for the three months ended June 30, 2018 was $8,077,097, an increase of $5,591,953, or 225.0%, compared to revenue of $2,485,144 for the three months ended June 30, 2017. The increase in revenue was driven by an 85% increase in the number of patients in the Illinois market of which our market share increased from 24.3% in 2017 to 25.3% in 2018 leading to an increase in revenue of $1,850,191. The launch of our Pennsylvania market in February 2018 also led to incremental revenue of $3,347,759.
Cost of Goods Sold and Gross Profit
Cost of goods sold for the three months ended June 30, 2018 was $4,569,802, an increase of $1,762,867 compared to a cost of goods sold of $2,806,935 for the three months ended June 30, 2017. Gross profit for the three months ended June 30, 2018 was $4,268,574, representing a gross margin of 53%, compared with gross profit of $301,370 for the three months ended June 30, 2017. The increases in cost of goods sold and gross profit were due to the increase of revenue during the respective time periods and increased cost efficiencies gained from the expansion of our cultivation facilities. Retail sales were also introduced in 2018 contributing to the higher profit margins.
- 11 -
Total Expenses
Total expenses for the three months ended June 30, 2018 were $2,629,072, an increase of $1,909,114, or 265%, compared to total expenses of $719,958 for the three months ended June 30, 2017. The increase in total expenses was attributable to a significant increase in employee headcount and additional administrative expenses necessary for our expansion in to new markets during this three month period.
Total Other Income (Expense)
Total other income for the three months ended June 30, 2018 was $342,484, an increase of $348,148 compared to total other expense of $5,664 for the three months ended June 30, 2017. The increase in total other expense was due to our share of income from investment in associates. During the three months ended June 30, 2018, other income increased by $320,824 for our pro-rata share of Lighthouse’s net income.
Provision for Income Taxes
The Company is a limited liability company that has elected to be treated as a partnership for federal income tax purposes. Under federal law, the taxable income or loss of a limited liability company is allocated to its members. Accordingly, no provision has been made for federal income taxes. The Company is responsible for certain other state taxes.
Net Income (Loss)
Net income attributable to controlling interest for the three months ended June 30, 2018 was $1,423,162, an increase of $1,847,414, or 435% compared to a net loss of $424,252 for the three months ended June 30, 2017. The increase in net income was driven by the inclusion of retail dispensary sales and increased cost efficiencies gained from the expansion of both Illinois and Pennsylvania cultivation facilities.
Six Months Ended June 30, 2018 Compared to Six Months Ended June 30, 2017
Net Revenue
Revenue for the six months ended June 30, 2018 was $12,880,136, an increase of $8,475,083, or 192%, compared to revenue of $4,405,053 for the six months ended June 30, 2017. The increase in revenue was driven by cultivation and dispensary sales in Pennsylvania market adding incremental revenue of $4,473,300.
Cost of Goods Sold and Gross Profit
Cost of goods sold for the six months ended June 30, 2018 was $8,346,496, an increase of $3,959,876 compared to $4,386,620 for the six months ended June 30, 2017. Gross profit for the six months ended June 30, 2018 was $6,704,208, representing a gross margin of 52.1%, compared with gross profit of $685,872 for the six months ended June 30, 2017. The increases in cost of goods sold and gross profit were mainly due to the launch of the cultivation and dispensary operation in the Pennsylvania market.
Total Expenses
Total expenses for the six months ended June 30, 2018 were $4,442,485, an increase of $2,899,973 compared to total expenses of $1,542,512, or 188%, for the six months ended June 30, 2017, which represents 34.5% of revenue for the six months ended June 30, 2018 compared to 35.0% of revenue for the six months ended June 30, 2017. The increase in total expenses was attributable to the launch of the cultivation and dispensary operation in the Pennsylvania market and the expansion of the Joliet cultivation facility in Illinois.
- 12 -
Total Other Income (Expense)
Total other income for the six months ended June 30, 2018 was $320,623, an increase of $385,187 compared to total other expense of $64,564 for the six months ended June 30, 2017.
Provision for Income Taxes
The Company is a limited liability company that has elected to be treated as a partnership for federal income tax purposes. Under federal law, the taxable income or loss of a limited liability company is allocated to its members. Accordingly, no provision has been made for federal income taxes. The Company is responsible for certain other state taxes.
Net Income (Loss)
Net income attributable to controlling interest for the six months ended June 30, 2018 was $1,981,487, an increase of $2,902,691, or 315.1% compared to a net loss of $921,204 for the six months ended June 30, 2017. The increase in net income resulted from higher revenues in the Illinois market driven by an 85% increase in the number of patients in the Illinois market of which our market share increased from 24.3% in 2017 to 25.3% in 2018 leading to an increase in revenue of $1,850,191. The launch of our Pennsylvania market in February 2018 also led to incremental revenue of $3,347,759
Year Ended December 31, 2017 Compared to Year Ended December 31, 2016
Net Revenue
Revenue for the year ended December 31, 2017 was $10,310,003, an increase of $7,152,771, or 226.6%, compared to revenue of $3,157,232 for the year ended December 30, 2016. The increase in revenue was driven by the full year impact of operations in Illinois 2017, an increase in patient count of 103% from prior year, and an increase in market share from 15.3% to 23.5%.
Cost of Goods Sold and Gross Profit (Loss)
Cost of goods sold for the year ended December 31, 2017 was $11,320,233, an increase of $3,407,981 compared with cost of goods sold of $7,912,252 for the year ended December 31, 2016. Gross profit for the year ended December 31, 2017 was $478,944, representing a gross margin of 4.6%, compared with gross loss of $4,160,441 for the year ended December 31, 2016. The increases in cost of goods sold and gross profit were due to the expansion of the Illinois program which led to higher revenues, cost of good sold, and gross profit.
Total Expenses
Total expenses for the year ended December 31, 2017 were $4,606,505, an increase of $989,330, or 27.3%, compared to total expenses of $3,617,175 for the year ended December 31, 2016, which represents 44.7% of revenue for the year ended December 31, 2017 compared to 114.6% of revenue for the year ended December 31, 2016. The increase in total expenses was attributable to higher Selling, General and Administrative Costs of $973,307 mainly driven from higher salary, professional fees, and travel expenses.
Total Other Income (Expense)
Total other income for the year ended December 31, 2017 was $140,038, an increase of $86,825 compared to total other expense of $53,213 for the year ended December 31, 2016.
Provision for Income Taxes
The Company is a limited liability company that has elected to be treated as a partnership for federal income tax purposes. Under federal law, the taxable income or loss of a limited liability company is allocated to its members. Accordingly, no provision has been made for federal income taxes. The Company is responsible for certain other state taxes.
- 13 -
Net Loss
Net loss attributable to controlling interest for the year ended December 31, 2017 was $3,175,353, a decrease of $4,549,050, or 58.9%, compared to a net loss of $7,724,403 for the year ended December 31, 2016. The decrease in net loss was driven by higher revenue of $7,152,771, or 226.6%, associated with an increase in patient count of 103% from prior year, and an increase in market share from 15.3% to 23.5%.
LIQUIDITY AND CAPITAL RESOURCES
Overview
As of December 31, 2017, we had $27,043,219 of cash and cash equivalents, $0 of restricted cash and $29,971,610 of working capital (current assets minus current liabilities), compared with $1,300,464 of cash and cash equivalents, $0 of restricted cash and $5,308,381 of working capital as of December 31, 2016. The increase of $24,663,229 in our working capital was primarily due to capital contributions from members of $30,719,206, partially offset by a net loss from operating activities of $3,987,523 and an increase in the purchase of property and equipment of $3,954,435.
We expect that our cash on hand and cash flows from operations, along with private and/or public financing, will be adequate to meet our capital requirements and operational needs for the next 12 months.
Cash Flows
Cash Used in Operating Activities
Net cash used in operating activities was $3,116,260 for the six months ended June 30, 2018, an increase of $2,683,904 compared to $432,356 for the six months ended June 30, 2017. The increase in net cash used in operating activities was primarily due to higher working capital requirements, partially offset by higher net income.
Net cash used in operating activities was $3,640,009 for the year ended December 31, 2017, a decrease of $6,926,652 compared to $10,566,661 for the year ended December 31, 2016. The decrease in net cash used in operating activities was primarily due to reduction in operating loss from prior year of $3,736,880.
Cash Flow from Investing Activities
Net cash used in investing activities was $19,392,964 for the six months ended June 30, 2018, an increase of $19,690,487 compared to $297,523 provided by investing for the six months ended June 30, 2017. The increase in net cash used in investing activities was primarily due an increase in the purchase of property and equipment of $11,816,874, and purchases of investments and acquisitions of businesses in the current period of $5,500,000 and $1,629,613, respectively.
Net cash used in investing activities was $1,102,067 for the year ended December 31, 2017, an increase of $1,722,011 compared to $619,944 provided by investing for the year ended December 31, 2016. The increase in net cash used in investing activities was primarily due an increase in the purchase of property and equipment of $3,954,435, partially offset by an increase in the net change due from related parties of $1,502,035.
Cash Flow from Financing Activities
Net cash provided by financing activities was $27,165,082 for the six months ended June 30, 2018, an increase of $25,332,449 compared to $1,832,633 for the six months ended June 30, 2017. The increase in net cash provided by financing activities was primarily due to an increase in capital contributions from members of $25,259,948.
- 14 -
Net cash provided by financing activities was $30,484,831 for the year ended December 31, 2017, an increase of $29,012,331 compared to $1,472,500 for the year ended December 31, 2016. The increase in net cash provided by financing activities was primarily due to capital contributions from members of $30,719,206.
CONTRACTUAL OBLIGATIONS
As of the six months ended June 30, 2018, and in the normal course of business, the Company has the following obligations to make future payments, representing contracts and other commitments that are known and committed.
The Company leases certain business facilities from third parties under operating lease agreements that specify minimum rentals. The Company leases its Chicago, Illinois headquarters under a non-cancelable sublease agreement with an affiliated entity, which expires in July 2019 (this sublease agreement is pursuant to a master lease agreement). Rent expense was approximately $44,000 and $160,000 for the six months ended June 30, 2018 and year ended December 31, 2017, respectively, which is included in selling, general and administrative expenses in the accompanying consolidated statements of operations.
The Company leases its cultivation facilities in Joliet, Lincoln, and Kankakee, Illinois from an affiliated entity. The commencement dates of the non-cancelable leases are determined based upon a Substantial Completion Date, as defined in the lease agreements, or six months after the Illinois Department of Agriculture awards the license. The Joliet lease commenced in December 2015, the Lincoln lease commenced in February 2016, and the Kankakee lease commenced in April 2016. The terms of these lease agreements are fifteen years from the commencement date. Rent expense for these facilities was approximately $2,093,000 and $4,317,000 for the six months ended June 30, 2018 and year ended December 31, 2017, respectively, which is included in cost of goods sold in the accompanying consolidated statements of operations. For financial reporting purposes, rent expense has been recorded on a straight-line basis over the terms of the leases resulting in deferred rent of approximately $1,894,000 and $1,587,000 as of June 30, 2018 and December 31, 2017, respectively.
The Company leases its cultivation facility in Brookville, Pennsylvania. The non-cancelable lease commenced on June 30,2017, upon the announcement of a successful license application, and terms after 60 months. Rent expense was approximately $81,000 and $138,000 as of June 30, 2018 and December 31,2017, respectively. The Company leases dispensary locations in Butler, Pennsylvania and Pittsburgh, Pennsylvania with 60-month terms and the option to extend. Rent expense was approximately $79,000 and $72,000 as of June 30,2018 and December 31, 2017, respectively, with approximately $9,000 and $14,000 in deferred rent liability.
Future minimum lease payments under non-cancelable operating leases having an initial or remaining term of more than one year are as follows:
| Year Ending December 31, 2017 |
Scheduled Payments |
|||
| 2018 |
$ | 3,942,436 | ||
| 2019 |
$ | 3,740,618 | ||
| 2020 |
$ | 3,805,790 | ||
| 2021 |
$ | 3,918,776 | ||
| 2022 |
$ | 4,035,151 | ||
| 2023-2031 |
$ | 36,811,610 | ||
|
|
|
|||
| Total Future Minimum Lease Payments |
$ | 56,254,381 | ||
|
|
|
|||
In addition to the future minimum rentals disclosed above, the Company is responsible for real estate taxes and comment operating expenses incurred by the building or facility in which it leases space. The Company was also required to pay to the affiliate a security deposit. The balance of the security deposit paid to the affiliate was approximately $1,325,000 as of June 30, 2018 and December 31, 2017 and 2016.
- 15 -
OFF-BALANCE SHEET ARRANGEMENTS AND PROPOSED TRANSACTIONS
The Company has no material undisclosed off-balance sheet arrangements or proposed transactions that have, or are reasonably likely to have, a current or future effect on its results of operations, financial condition, revenues or expenses, liquidity, capital expenditures or capital resources that are material to investors.
RELATED PARTY TRANSACTIONS
Management Fees and Services
The Company incurred the following transactions with related parties during the six months ended June 30, 2018 and year ended December 31, 2017:
| Six Months Ended June 30, | Year Ended December 30, | |||||||||||
| 2018 | 2017 | 2017 | ||||||||||
| Promissory Note |
$ | 187,500 | $ | 609,375 | $ | 328,125 | ||||||
| Agreements with founders |
||||||||||||
| Office and Operating Leases |
$ | 4,228,000 | $ | 3,293,000 | $ | 6,064,000 | ||||||
|
|
|
|
|
|
|
|||||||
| $ | 4,415,500 | $ | 3,902,375 | $ | 6,392,125 | |||||||
|
|
|
|
|
|
|
|||||||
Related Party Balances
As of December 31, 2017 and June 30, 2018, amounts due to/from related parties were as follows:
| June 30, 2018 | December 31, 2017 | |||||||
| Amounts Due from Related Parties |
$ | 0 | $ | 0 | ||||
| Amounts Due to Related Parties |
$ | 1,500,000 | $ | 725,000 | ||||
|
|
|
|
|
|||||
| Net Amount Due to Related Parties |
$ | 1,500,000 | $ | 725,000 | ||||
|
|
|
|
|
|||||
CHANGES IN OR ADOPTION OF ACCOUNTING POLICIES
There were no new standards effective July 1, 2016 that had an impact on the Company’s combined financial statements. The following IFRS standards have been recently issued by the IASB. The Company is assessing the impact of these new standards on future combined financial statements. Pronouncements that are not applicable or where it has been determined do not have a significant impact to the Company have been excluded herein.
IFRS 2, Share-based Payment
In June 2016, the IASB issued amendments to IFRS 2, Share-based Payment in relation to the classification and measurement of share-based payment transactions. The amendment provided guidance introduces accounting requirements for cash-settled share-based payments that follows the same approach as used for equity-settled share-based payments. On such modifications, the original liability recognized in respect of the cash-settled share-based payment is derecognized and the equity-settled share-based payment is recognized at the modification date fair value to the extent services have been rendered up to the modification date. Any difference between the carrying amount of the liability as at the modification date and the amount recognized in equity at the same date would be recognized in profit and loss immediately. The amendments are effective for annual periods beginning on or after January 1, 2018. Earlier application is permitted. The amendments are to be applied prospectively. However, retrospective application if allowed if this is possible without the use of hindsight. The adoption of this new standard has had no significant impacts on the Company’s accompanying consolidated financial statements.
- 16 -
IFRS 7, Financial instruments: Disclosure
IFRS 7, Financial instruments: Disclosure was amended to require additional disclosures on transition from IAS 39 to IFRS 9. IFRS 7 is effective on adoption of IFRS 9, which is effective for annual periods commencing on or after January 1, 2018.
IFRS 9, Financial Instruments
In July 2014, the IASB issued the final version of IFRS 9, Financial Instruments, which reflects all phases of the financial instruments project and replaces IAS 39, Financial Instruments: Recognition and Measurement, and all previous versions of IFRS 9. The standard introduces new requirements for classification and measurement, impairment, and hedge accounting. IFRS 9 is effective for annual periods beginning on or after January 1, 2018, with early application permitted. The adoption of this new standard has had no significant impacts on the Company’s accompanying consolidated financial statements.
IFRS 15, Revenue from Contracts with Customers
The IASB replaced IAS 18, Revenue, in its entirety with IFRS 15, Revenue from Contracts with Customers. The standard contains a single model that applies to contracts with customers and two approaches to recognizing revenue: at a point in time or over time. The model features a contract-based five-step analysis of transactions to determine whether, how much and when revenue is recognized. New estimates and judgmental thresholds have been introduced, which may affect the amount and/or timing of revenue recognized. IFRS 15 is effective for annual periods beginning on or after January 1, 2018, with early application permitted. The adoption of this new standard has had no significant impacts on the Company’s accompanying consolidated financial statements.
IFRS 16, Leases
In January 2016, the IASB issued IFRS 16, Leases, which will replace IAS 17, Leases. This standard introduces a single lessee accounting model and requires a lessee to recognize assets and liabilities for all leases with a term of more than twelve months unless the underlying asset is of low value. A lessee is required to recognize a right-of-use asset representing its right to use the underlying asset and a lease liability representing its obligation to make lease payments. The standard will be effective for annual periods beginning on or after January 1, 2019, with earlier application permitted for entities that apply IFRS 15, Revenue from Contracts with Customers, at or before the date of initial adoption of IFRS 16. The Company expects significant impacts on its financial statements from the adoption of this new standard. The Company leases the majority of its cultivation facilities and dispensaries and anticipates the statement of financial position to increase by the amount determined in Contractual Obligations.
IAS 28, Long-term Interests
In October 2017, the IASB amended IAS 28, Long-term Interests in Associates and Joint Ventures. The amendments were added to clarify that an entity applies IFRS 9 ‘Financial Instruments’ to long-term interests in an associate or joint venture that form part of the net investment in the associate or joint venture but to which the equity method is not applied. The standard which will be effective for annual periods beginning on or after January 1, 2019, with earlier adoption permitted. The Company does not expect significant impact on its financial statements from the adoption of this new standard.
CRITICAL ACCOUNTING ESTIMATES
The preparation of the Company’s accompanying consolidated financial statements requires management to make judgments, estimates and assumptions that affect the application of policies and reported amounts of assets and liabilities, and revenue and expenses. Actual results may differ from these estimates. The estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimate is revised if the revision affects only that period or in the period of the revision and future periods if the review affects both current and future periods.
- 17 -
Significant judgments, estimates, and assumptions that have the most significant effect on the amounts recognized in the financial statements are described below.
Biological Assets and Inventory
In calculating the value of the biological assets and inventory, management is required to make a number of estimates, including estimating the stage of growth of the cannabis up to the point of harvest, harvesting costs, selling costs, average or expected selling prices and list prices, expected yields for the cannabis plants, and oil conversion factors. In calculating final inventory values, management compares the inventory cost to estimated net realizable value.
Estimated Useful Lives and Depreciation of Property and Equipment
Depreciation of property and equipment is dependent upon estimates of useful lives which are determined through the exercise of judgment. The assessment of any impairment of these assets is dependent upon estimates of recoverable amounts that take into account factors such as economic and market conditions and the useful lives of assets.
Estimated Useful Lives and Amortization of Intangible Assets
Amortization of intangible assets is recorded on a straight-line basis over their estimated useful lives, which do not exceed the contractual period, if any. Intangible assets that have indefinite useful lives are not subject to amortization and are tested annually for impairment, or more frequently if events or changes in circumstances indicate that they might be impaired.
Business Combinations
In a business combination, all identifiable assets, liabilities and contingent liabilities acquired are recorded at their fair values. One of the most significant estimates relates to the determination of the fair value of these assets and liabilities. Contingent consideration is measured at its acquisition-date fair value and included as part of the consideration transferred in a business combination. Contingent consideration that is classified as equity is not remeasured at subsequent reporting dates and its subsequent settlement is accounted for within equity. Contingent consideration that is classified as an asset or a liability is remeasured at subsequent reporting dates in accordance with International Standards on Auditing (“IAS”) 39, Financial Instruments: Recognition and Measurement, or IAS 37, Provisions, Contingent Liabilities and Contingent Assets, as appropriate, with the corresponding gain or loss being recognized in profit or loss. For any intangible asset identified, depending on the type of intangible asset and the complexity of determining its fair value, an independent valuation expert or management may develop the fair value, using appropriate valuation techniques, which are generally based on a forecast of the total expected future net cash flows. The evaluations are linked closely to the assumptions made by management regarding the future performance of the assets concerned and any changes in the discount rate applied.
Certain fair values may be estimated at the acquisition date pending confirmation or completion of the valuation process. Where provisional values are used in accounting for a business combination, they may be adjusted retrospectively in subsequent periods. However, the measurement period will last for one year from the acquisition date.
Unit-Based Compensation
In calculating the unit-based compensation expense, key estimates such as the rate of forfeiture of options granted, the expected life of the option, the volatility of the Company’s stock price and the risk-free interest rate are used. To calculate the unit-based compensation expense related to key employee performance milestones associated with the terms of an acquisition, the Company must estimate the number of units that will be earned and when they will be issued based on estimated discounted probabilities.
- 18 -
Goodwill Impairment
Goodwill is tested for impairment annually and whenever events or changes in circumstances indicate that the carrying amount of goodwill has been impaired. In order to determine if the value of goodwill has been impaired, the cash-generating unit to which goodwill has been allocated must be valued using present value techniques. When applying this valuation technique, the Company relies on a number of factors, including historical results, business plans, forecasts and market data. Changes in the conditions for these judgments and estimates can significantly affect the assessed value of goodwill.
FINANCIAL INSTRUMENTS AND FINANCIAL RISK MANAGEMENT
The Company’s financial instruments consist of cash and cash equivalents, accounts receivables, due from related parties, accounts payables and other accrued expenses, subscription deposits refundable, due to related party, and notes payable – related parties. The carrying values of these financial instruments approximate their fair values as of June 30, 2018 and December 31, 2017 due to their nature and relatively short maturity date. Financial instruments recorded at fair value are classified using a fair value hierarchy that reflects the significance of the inputs to fair value measurements. The three levels of hierarchy are:
| Level 1 – | Unadjusted quoted prices in active markets for identical assets or liabilities; | |
| Level 2 – | Inputs other than quoted prices that are observable for the asset or liability, either directly or indirectly; and | |
| Level 3 – | Inputs for the asset or liability that are not based on observable market data. There have been no transfers between fair value levels valuing these assets during the year. | |
The following table summarizes the Company’s financial instruments as of June 30, 2018:
| Loans and Receivables |
Other Financial Liabilities |
Total | ||||||||||
| Financial Assets: |
||||||||||||
| Cash and Cash Equivelents |
$ | 31,699,077 | $ | — | $ | 31,699,077 | ||||||
| Accounts Receivable |
$ | 2,301,138 | $ | — | $ | 2,301,138 | ||||||
| Financial Liabilities: |
||||||||||||
| Accounts Payable and Accrued Expenses |
$ | — | $ | 3,659,875 | $ | 3,659,875 | ||||||
| Subscription Deposits Refundable |
$ | — | $ | 12,500 | $ | 12,500 | ||||||
| Notes Payable - Related Parties |
$ | — | $ | 187,500 | $ | 187,500 | ||||||
| Due to Related Party |
$ | — | $ | 1,500,000 | $ | 1,500,000 | ||||||
- 19 -
The following table summarizes the Company’s financial instruments as of December 31, 2017:
| Loans and Receivables |
Other Financial Liabilities |
Total | ||||||||||
| Financial Assets: |
||||||||||||
| Cash |
$ | 27,043,219 | $ | — | $ | 27,043,219 | ||||||
| Accounts Receivable |
$ | 1,010,620 | $ | — | $ | 1,010,620 | ||||||
| Financial Liabilities: |
||||||||||||
| Accounts Payable and Accrued Expenses |
$ | — | $ | 2,640,582 | $ | 2,640,582 | ||||||
| Subscription Deposits Refundable |
$ | — | $ | 399,800 | $ | 399,800 | ||||||
| Notes Payable - Related Parties |
$ | — | $ | 328,125 | $ | 328,125 | ||||||
| Due to Related Party |
$ | — | $ | 725,000 | $ | 725,000 | ||||||
Financial Risk Management
The Company is exposed in varying degrees to a variety of financial instrument related risks. The Board mitigates these risks by assessing, monitoring and approving the Company’s risk management processes:
Credit and Banking Risk
Credit risk is the risk of a potential loss to the Company if a customer or third party to a financial instrument fails to meet its contractual obligations. The maximum credit exposure at June 30, 2018 and December 31, 2017 is the carrying amount of cash. The Company does not have significant credit risk with respect to its customers. Although all cash is placed with major U.S. financial institutions, there has been no change in the U.S. federal banking laws related to the deposit and holding of funds derived from activities related to the cannabis industry. Given that U.S. federal law provides that the production and possession of cannabis is illegal, there is a strong argument that banks cannot accept for deposit funds from business involved with the cannabis industry.
Asset Forfeiture Risk
Due to the cannabis industry remaining illegal under U.S. federal law, any property owned by participants in the cannabis industry which are either used in the course of conducting such business, or are the proceeds of such business, could be subject to seizure by law enforcement and subsequent civil asset forfeiture. Even if the owner of the property were never charged with a crime, the property in question could still be seized and subject to an administrative proceeding by which, with minimal due process, it could be subject to forfeiture.
Liquidity Risk
Liquidity risk is the risk that the Company will not be able to meet its financial obligations associated with financial liabilities. The Company manages liquidity risk through the management of its capital structure. The Company’s approach to managing liquidity is to ensure that it will have sufficient liquidity to settle obligations and liabilities when due.
The Company has certain other financial liabilities recorded on its unaudited consolidated balance sheets. As of both June 30, 2018 and December 31, 2017, these liabilities are classified as current and are expected to be paid within one year or less.
- 20 -
Market Risk
Currency Risk
The Company had no exposure to foreign currency transaction or translation risk for the periods presented.
Interest Rate Risk
Interest rate risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in market interest rates. The Company’s financial debts have fixed rates of interest and therefore expose the Company to a limited interest rate fair value risk.
Price Risk
Price risk is the risk of variability in fair value due to movements in equity or market prices.
Tax Risk
Tax risk is the risk of changes in the tax environment that would have a material adverse effect on the Company’s business, results of operations, and financial condition. Currently, state licensed marijuana businesses are assessed a comparatively high effective federal tax rate due to section 280E which bars businesses from deducting all expenses except their cost of goods sold (COGS) when calculating federal tax liability. Any negative increase in additional tax measures may have a further adverse effect on the operations of the Company, while any decrease in tax pressures will be beneficial to future operations.
Regulatory Risk
Regulatory risk pertains to the risk that the Company’s business objectives are contingent, in part, upon the compliance of regulatory requirements. Due to the nature of the industry, the company recognizes that regulatory requirements are more stringent and punitive in nature. Any delays in obtaining, or failure to obtain regulatory approvals can significantly delay operational and product development and can have a material adverse effect on the Company’s business, results of operation, and financial condition.
The Company is cognizant of the advent of regulatory changes occurring in the cannabis industry on the city, state, and national levels. Although regulatory outlook on the cannabis industry has been moving in a positive trend, the Company is aware of the effect of unforeseen regulatory changes can have on the goals and operations of the business as a whole.
- 21 -
SCHEDULE “D”
AUDITED ANNUAL FINANCIAL STATEMENTS FOR RANDSBURG AS OF AND FOR THE YEARS ENDED JANUARY 31, 2018 AND 2017
(See attached.)
RANDSBURG INTERNATIONAL GOLD CORP.
FINANCIAL STATEMENTS
(Expressed in Canadian dollars)
JANUARY 31, 2018 and 2017
MANAGEMENT’S RESPONSIBILITY FOR FINANCIAL REPORTING
The accompanying financial statements of Randsburg International Gold Corp. (the “Company”) are the responsibility of management and the Board of Directors.
The financial statements have been prepared by management, on behalf of the Board of Directors, in accordance with the accounting policies disclosed in the notes to the financial statements. Where necessary, management has made informed judgments and estimates in accounting for transactions which were not complete at the balance sheets date. In the opinion of management, the financial statements have been prepared within acceptable limits of materiality and are in accordance with International Financial Reporting Standards using accounting policies consistent with International Financial Reporting Standards appropriate in the circumstances.
Management has established systems of internal control over the financial reporting process, which are designed to provide reasonable assurance that relevant and reliable financial information is produced.
The Board of Directors is responsible for reviewing and approving the financial statements together with other financial information of the Company and for ensuring that management fulfills its financial reporting responsibilities. An Audit Committee assists the Board of Directors in fulfilling this responsibility. The Audit Committee meets with management to review the financial reporting process and the financial statements together with other financial information of the Company. The Audit Committee reports its findings to the Board of Directors for its consideration in approving the financial statements together with other financial information of the Company for issuance to the shareholders.
Management recognizes its responsibility for conducting the Company’s affairs in compliance with established financial standards, and applicable laws and regulations, and for maintaining proper standards of conduct for its activities.
| (signed) “Michael Opara” Michael Opara Chief Executive Officer, President and Director |
(signed) “Matthew Chodorowicz” Matthew Chodorowicz Chief Financial Officer and Director |
|||||
| Toronto, Canada May 31, 2018 |
||||||

| 251 Consumer Road, Suite 800 Toronto, Ontario M2J 4R3 Canada
Tel 416-496-1234 Fax 416-496-0125 Email [email protected] Web www.uhymh.com |
INDEPENDENT AUDITOR’S REPORT
To the Shareholders of Randsburg International Gold Corp.
We have audited the accompanying financial statements of Randsburg International Gold Corp., which comprise the statements of financial position as at January 31, 2018 and 2017, and the statements of operations and comprehensive loss, statements of changes in equity (deficiency) and statements of cash flows for the years then ended, and a summary of significant accounting policies and other explanatory information.
Management’s Responsibility for the Financial Statements
Management is responsible for the preparation and fair presentation of these financial statements in accordance with International Financial Reporting Standards and for such internal control as management determines is necessary to enable the preparation of financial statements that are free from material misstatement, whether due to fraud or error.
Auditor’s Responsibility
Our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits in accordance with Canadian generally accepted auditing standards. Those standards require that we comply with ethical requirements and plan and perform the audit to obtain reasonable assurance about whether the financial statements are free from material misstatement.
An audit involves performing procedures to obtain audit evidence about the amounts and disclosures in the financial statements. The procedures selected depend on the auditor’s judgment, including the assessment of the risks of material misstatement of the financial statements, whether due to fraud or error. In making those risk assessments, the auditor considers internal control relevant to the entity’s preparation and fair presentation of the financial statements in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the entity’s internal control. An audit also includes evaluating the appropriateness of accounting policies used and the reasonableness of accounting estimates made by management, as well as evaluating the overall presentation of the financial statements.
We believe that the audit evidence we have obtained is sufficient and appropriate to provide a basis for our audit opinion.
Opinion
In our opinion, the financial statements present fairly, in all material respects, the financial position of Randsburg International Gold Corp. as at January 31, 2018 and 2017, and its financial performance and its cash flows for the years then ended in accordance with International Financial Reporting Standards.
Emphasis of Matter
Without qualifying our opinion, we draw attention to Note 1 in the financial statements which indicates that Randsburg International Gold Corp. had continuing losses during the year ended January 31, 2018, and a working capital deficiency as at January 31, 2018. Randsburg International Gold Corp. also disposed of its mineral property interest during the year ended January 31, 2018. These conditions along with other matters set forth in Note 1 indicate the existence of material uncertainties which cast significant doubt about the ability of Randsburg International Gold Corp. to continue as a going concern.
| UHY McGovern Hurley LLP |
|
| Chartered Professional Accountants Licensed Public Accountants |
Toronto, Canada
May 31, 2018
A member of UHY International, a network of independent accounting and consulting firms
RANDSBURG INTERNATIONAL GOLD CORP.
Statements of Financial Position
(Expressed in Canadian Dollars)
| As at January 31, 2018 |
As at January 31, 2017 |
|||||||
| ASSETS |
||||||||
| Current Assets |
||||||||
| Cash and cash equivalents |
$ | — | $ | — | ||||
| Mineral property interest held for resale (Note 5) |
— | 96,200 | ||||||
| HST receivable |
2,268 | 3,064 | ||||||
|
|
|
|
|
|||||
| Total Current Assets |
2,268 | 99,264 | ||||||
|
|
|
|
|
|||||
| Total Assets |
$ | 2,268 | $ | 99,264 | ||||
|
|
|
|
|
|||||
| LIABILITIES AND SHAREHOLDERS’ (DEFICIENCY) EQUITY |
||||||||
| Current Liabilities |
||||||||
| Accounts payable and accrued liabilities |
$ | 308,720 | $ | 311,183 | ||||
| Liability related to flow-through financing (note 11) |
87,520 | 79,492 | ||||||
| Due to related parties (Note 7) |
918,661 | 915,919 | ||||||
|
|
|
|
|
|||||
| Total Current Liabilities |
1,314,901 | 1,306,594 | ||||||
|
|
|
|
|
|||||
| Shareholders’ (Deficiency) Equity |
||||||||
| Share capital (Note 6) |
13,628,895 | 13,628,895 | ||||||
| Contributed surplus |
3,566,604 | 3,566,604 | ||||||
|
|
|
|
|
|||||
| 17,195,499 | 17,195,499 | |||||||
| Deficit |
(18,508,132 | ) | (18,402,829 | ) | ||||
|
|
|
|
|
|||||
| Total (Deficiency) Equity |
(1,312,633 | ) | (1,207,330 | ) | ||||
|
|
|
|
|
|||||
| Total Liabilities and Shareholders’ (Deficiency) Equity |
$ | 2,268 | $ | 99,264 | ||||
|
|
|
|
|
|||||
Nature of operations and going concern (Note 1)
Commitments and contingencies (Notes 7 and 11)
Approved on behalf of the Board:
“Michael Opara” Director
“Matthew Chodorowicz” Director
The accompanying notes to the financial statements are an integral part of these statements.
RANDSBURG INTERNATIONAL GOLD CORP.
Statements of Operations and Comprehensive Loss
(Expressed in Canadian Dollars)
| Year Ended January 31, 2018 |
Year Ended January 31, 2017 |
|||||||
| Amortization |
$ | — | $ | 19 | ||||
| Consulting |
12,752 | 12,150 | ||||||
| Interest and bank charges (Note 7(b)) |
51,324 | 40,375 | ||||||
| Listing and transfer agent fees |
9,325 | 11,535 | ||||||
| Management fees (Note 7(a)) |
18,000 | 18,000 | ||||||
| Office and miscellaneous |
1,481 | 2,074 | ||||||
| Professional fees |
13,240 | 12,384 | ||||||
|
|
|
|
|
|||||
| Total operating expenses |
106,122 | 96,537 | ||||||
|
|
|
|
|
|||||
| Loss before other items |
(106,122 | ) | (96,537 | ) | ||||
| Cost recoveries |
819 | 12,095 | ||||||
| Write down of mineral property interest |
— | (56,769 | ) | |||||
| Write off of equipment |
— | (358 | ) | |||||
|
|
|
|
|
|||||
| Net (loss) and comprehensive (loss) for the year |
$ | (105,303 | ) | $ | (141,569 | ) | ||
|
|
|
|
|
|||||
| Basic and diluted (loss) per share |
$ | (0.004 | ) | $ | (0.005 | ) | ||
|
|
|
|
|
|||||
| Weighted average number of common shares outstanding – basic and diluted |
28,273,939 | 28,273,939 | ||||||
|
|
|
|
|
|||||
The accompanying notes to the financial statements are an integral part of these statements.
RANDSBURG INTERNATIONAL GOLD CORP.
Statements of Changes in Equity (Deficiency)
(Expressed in Canadian Dollars)
| Number of Shares |
Common Shares |
Contributed Surplus |
Deficit | Total Equity (Deficiency) |
||||||||||||||||
| Balance, January 31, 2016 |
28,273,939 | $ | 13,628,895 | $ | 3,566,604 | $ | (18,261,260 | ) | $ | (1,065,761 | ) | |||||||||
| Loss for year |
— | — | — | (141,569 | ) | (141,569 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance, January 31, 2017 |
28,273,939 | 13,628,895 | 3,566,604 | (18,402,829 | ) | (1,207,330 | ) | |||||||||||||
| Loss for the year |
— | — | — | (105,303 | ) | (105,303 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance, January 31, 2018 |
28,273,939 | $ | 13,628,895 | $ | 3,566,604 | $ | (18,508,132 | ) | $ | (1,312,633 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
The accompanying notes to the financial statements are an integral part of these statements.
RANDSBURG INTERNATIONAL GOLD CORP.
Statements of Cash Flows
(Expressed in Canadian Dollars)
| Year Ended January 31, 2018 |
Year Ended January 31, 2017 |
|||||||
| CASH FLOWS FROM OPERATING ACTIVITIES |
||||||||
| Net (loss) for the year |
$ | (105,303 | ) | $ | (141,569 | ) | ||
| Amortization |
— | 19 | ||||||
| Write off of equipment |
— | 358 | ||||||
| Write down of mineral property interest |
— | 56,769 | ||||||
| Interest on related party loans and advances |
39,650 | 40,375 | ||||||
|
|
|
|
|
|||||
| (65,653 | ) | (44,048 | ) | |||||
| Changes in non-cash operating working capital: |
||||||||
| Decrease (increase) in HST receivable |
796 | (345 | ) | |||||
| Decrease in mineral property interest held for resale |
96,200 | — | ||||||
| (Decrease) increase in accounts payable and accrued liabilities |
(2,463 | ) | 19,378 | |||||
| Increase (decrease) in liability related to flow-through financing |
8,028 | (4,897 | ) | |||||
|
|
|
|
|
|||||
| Net cash used in operating activities |
36,908 | (29,912 | ) | |||||
|
|
|
|
|
|||||
| CASH FLOWS FROM FINANCING ACTIVITIES |
||||||||
| (Decrease) Increase in due to related parties |
(36,908 | ) | 29,912 | |||||
|
|
|
|
|
|||||
| Changes in cash and cash equivalents during the year |
— | — | ||||||
| Cash and cash equivalents, beginning of year |
— | — | ||||||
|
|
|
|
|
|||||
| Cash and cash equivalents, end of year |
$ | Nil | $ | Nil | ||||
|
|
|
|
|
|||||
| Cash paid for interest |
$ | Nil | $ | Nil | ||||
|
|
|
|
|
|||||
| Cash paid for income taxes |
$ | Nil | $ | Nil | ||||
|
|
|
|
|
|||||
| Supplemental Information |
||||||||
| Securities received for mineral property interest held for resale |
$ | 96,200 | $ | Nil | ||||
|
|
|
|
|
|||||
| Securities disposed of in settlement of amounts due to related parties |
$ | 96,200 | $ | Nil | ||||
|
|
|
|
|
|||||
The accompanying notes to the financial statements are an integral part of these statements
RANDSBURG INTERNATIONAL GOLD CORP.
Notes to Financial Statements
(Expressed in Canadian Dollars)
January 31, 2018 and 2017
| 1. | NATURE OF OPERATIONS AND GOING CONCERN |
Randsburg International Gold Corp. (the “Company”) was incorporated under the laws of British Columbia on July 6, 1990.
The Company is listed on the NEX, having the symbol RGZ.H. The address of the Company’s corporate office and principal place of business is 44 Victoria Street, Suite 1060 Toronto, Ontario, M5C 1Y2 Canada.
The financial statements of the Company for the years ended January 31, 2018 and 2017 were reviewed by and authorized for issue by the Board of Directors on May 31, 2018.
The Company’s principal business activity is the acquisition and exploration of mineral property interests in Canada. The Company is considered to be in the exploration stage and substantially all of the Company’s efforts are devoted to financing and exploring its property interests. The Company has not determined whether its properties contain ore reserves which are economically recoverable. The recovery of the amounts shown for mineral property interests is dependent upon the existence of economically recoverable ore reserves, the ability of the Company to obtain necessary financing to complete the exploration and development of its properties, and upon future profitable production or proceeds from the disposal of its properties.
Although the Company has taken steps to verify title to the properties on which it is conducting exploration in which it has an interest, in accordance with industry standards for the current stage of exploration. These procedures do not guarantee the Company’s title. Property title may be subject to unregistered prior agreements, non-compliance with regulatory and environmental requirements, social requirements or aboriginal land claims. The Company’s assets may also be subject to increases in taxes and royalties, renegotiation of contracts, political uncertainty and currency exchange fluctuations and restrictions.
The Company’s exploration and evaluation activities are subject to laws and regulations governing the protection of the environment. These laws and regulations are continually changing and generally becoming more restrictive. The Company believes its activities are materially in compliance with all applicable laws and regulations. The Company has made, and expects to make in the future, expenditures to comply with such laws and regulations. During 2018, the Company disposed of its mineral property interest.
These financial statements have been prepared in accordance with International Financial Reporting Standards (“IFRS”), applicable to a going concern. Accordingly, they do not give effect to adjustments that would be necessary should the Company be unable to continue as a going concern and therefore be required to realize on its assets and settle its liabilities and commitments in other than the normal course of business and at amounts different from those in the accompanying financial statements.
The Company has incurred operating losses over the last several years, earns no operating revenues and has a working capital deficiency of $1,312,633 (January 31, 2017 - $1,207,330). While the Company has been successful in obtaining its required financing in the past, through additional equity and non – arm’s length loans, there is no assurance that such financing will continue to be available or be available on favorable terms. The Company’s ability to continue as a going concern is uncertain and is dependent upon the generation of profits, obtaining additional financing or maintaining continued support from its shareholders and creditors. In the event that additional financial support is not received or operating profits are not generated, the carrying values of the Company’s assets may be adversely affected. These conditions represent material uncertainties which cast significant doubt about the Company’s ability to continue as a going concern.
RANDSBURG INTERNATIONAL GOLD CORP.
Notes to Financial Statements
(Expressed in Canadian Dollars)
January 31, 2018 and 2017
| 2. | BASIS OF PREPARATION AND STATEMENT OF COMPLIANCE |
Basis of presentation
These financial statements have been prepared on a historical cost basis except for certain financial instruments that have been measured at fair value. In addition, these financial statements have been prepared using the accrual basis of accounting except for cash flow information.
Statement of compliance
The accompanying financial statements have been prepared in accordance with International Financial Reporting Standards (“IFRS”) issued by the International Accounting Standards Board (“IASB”) and interpretations of the International Financial Reporting Interpretations Committee (“IFRIC”), effective for the Company’s reporting for the year ended January 31, 2018.
| 3. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
The accounting policies set out below have been applied consistently to all periods presented in these financial statements.
| 3.1 | Share based payments |
The Company grants stock options to acquire common shares of the Company to directors, officers, employees and consultants. An individual is classified as an employee when the individual is an employee for legal or tax purposes, or provides services similar to those performed by an employee.
The fair value of stock options is measured on the date of the grant, using the Black-Scholes pricing model, and is recognized over the vesting period. Consideration paid for the shares on the exercise of the stock options is credited to capital stock.
In situations where equity instruments are issued to non-employees and some or all of the goods and services received by the entity as consideration cannot be specifically identified, they are measured at fair value of the share-based payment. Otherwise, share-based payments are measured at the fair value of goods and services received.
| 3.2 | Mineral property interests |
The Company records its mineral property interests, including wholly-owned mining properties, undivided interests in mining properties and deferred exploration costs, at cost less certain recoveries. Exploration costs are capitalized on the basis of specific mining property or areas of geological interest until the mining assets to which they relate are placed into production, sold or are allowed to lapse. General exploration costs not related to specific mining assets are expensed in the statement of operations as incurred.
The recoverability of the amounts recorded as mineral property interests is dependent upon the discovery of economically recoverable reserves, the ability of the Company to obtain the financing needed to complete development, and future profitable production or proceeds from disposal of these assets. The amounts shown for mineral property interests are not necessarily indicative of present or future values.
RANDSBURG INTERNATIONAL GOLD CORP.
Notes to Financial Statements
(Expressed in Canadian Dollars)
January 31, 2018 and 2017
| 3.3 | Impairment of assets |
Mineral property interests are tested for impairment when events or changes in circumstances indicate that the carrying amount may be impaired. Common indicators of impairment in the mineral resource industry may include: the period for which the entity has the right to explore in the specific area has expired during the period or will expire in the near future, and is not expected to be renewed, substantive expenditure on further exploration and evaluation of mineral resources in s specific area is neither budgeted nor planned, or have not led to the discovery of commercially viable quantities of mineral resources and the entity has decided to discontinue such activities in the specific area; or sufficient data exists to indicate that, although a development in a specific area is likely to proceed, the carrying amount of the exploration and evaluation asset is unlikely to be recovered in full from successful development or by sale.
When an indication of impairment loss or a reversal of an impairment loss exists, the recoverable amount of the individual asset must be estimated. If it is not possible to estimate the recoverable amount of the individual asset, the recoverable amount of the cash generating unit to which the asset belongs must be determined. Identifying the cash generating units requires considerable management judgment. In testing an individual asset or cash generating unit for impairment and identifying a reversal or impairment losses, management estimates the recoverable amount of the asset or the cash-generating unit. This requires management to make several assumptions as to future events or circumstances. These assumptions and estimates are subject to change if new information becomes available. Actual results with respect to impairment losses or reversals of impairment losses could differ in such a situation and significant adjustments to the Company’s assets and losses may occur during a subsequent period.
| 3.4 | Income taxes |
Income tax on profits or loss for the periods presented comprises current and deferred tax. Income tax is recognized in profit or loss except to the extent that it relates to items recognized directly in equity, in which case it is recognized in equity. Current tax expense is the expected tax payable on the taxable income for the year, using tax rates, enacted or substantively enacted at period end, adjusted for amendments to tax payable with regards to previous years.
Deferred tax is recognized in respect of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for taxation purposes. Deferred tax is not recognized for the following temporary differences: goodwill not deductible for tax purposes; the initial recognition of assets or liabilities that affect neither accounting or taxable loss and differences relating to investments in subsidiaries to the extent that they will probably not reverse in the foreseeable future. The amount of deferred tax provided is based on the expected manner of realization or settlement of the carrying amount of the asset and liabilities, using the tax rates enacted or substantially enacted at the date of the statement of financial position.
A deferred tax asset is recognized only to the extent that it is probable that future taxable profits will be available against which the asset can be utilized.
Deferred tax assets and liabilities are offset when there is a legally enforceable right to set off current tax assets against current tax liabilities and when they relate to income taxes levied by the same taxation authority and the Company intends to settle its current tax assets and liabilities on a net basis.
RANDSBURG INTERNATIONAL GOLD CORP.
Notes to Financial Statements
(Expressed in Canadian Dollars)
January 31, 2018 and 2017
| 3.5 | Basic and diluted (loss) per share |
The Company presents basic (loss) per share data for its common shares, calculated by dividing the (loss) attributable to common shareholders of the Company by the weighted average number of common shares outstanding during the period. Diluted loss per share is calculated whereby the weighted average number of common shares outstanding used for the calculation of diluted loss per share assumes that the proceeds to be received on the exercise of dilutive stock options and warrants are used to repurchase common shares at the average market price during the period. Basic and diluted (loss) per share are the same for the years’ ended January 31, 2018 and 2017.
| 3.6 | Cash and cash equivalents |
Cash and cash equivalents comprise cash and term deposits with original maturity dates of less than three months. As at January 31, 2018, the Company had cash and cash equivalents of $nil (2017 - $nil).
| 3.7 | Provisions and contingent liabilities |
Provisions are recognized when the Company has a present obligation (legal or constructive) that has arisen as a result of a past event and it is probable that a future outflow of resources will be required to settle the obligation, provided that a reliable estimate can be made of the amount of the obligation.
Provisions are measured at the present value of the expenditures expected to be required to settle the obligation using a pre-tax rate that reflects current market assessments of the time value of money and the risk specific to the obligation. The increase in the provision due to the passage of time is recognized as interest expense.
Onerous Contracts:
A provision for onerous contracts would be recognized when the expected benefits to be derived by the Company from a contract are lower than the unavoidable cost of meeting its obligations under the contract. The provision would be measured at the present value of the lower of the expected cost of terminating the contract and the expected net cost of continuing with the contract. Before a provision is established, the Company would recognize any impairment loss on the assets associated with the contract.
RANDSBURG INTERNATIONAL GOLD CORP.
Notes to Financial Statements
(Expressed in Canadian Dollars)
January 31, 2018 and 2017
| 3.8 | Decommissioning, restoration and similar liabilities |
The Company recognizes liabilities for statutory, contractual, constructive or legal obligations including those associated with the reclamation of mineral properties and equipment, when those obligations result from the acquisition, construction, development or normal operation of the asset. Initially, a liability for an asset retirement obligation is recognized at its fair value in the period in which it is incurred. Upon initial recognition of the liability, the corresponding asset retirement obligation is added to the carrying amount of the related asset and the cost is amortized as an expense over the economic life of the asset using either the unit-of-production or the straight-line method as appropriate. Following the initial recognition of the asset retirement obligation, the carrying amount of the liability is increased for the passage of time and adjusted for changes to the current market-based discount rate, amount or timing of the underlying cash flows needed to settle the obligation.
| 3.9 | Equity |
Share capital represents the amount received on the issuance of shares, less issue costs.
Reserve for share-based payments includes charges related to share-based payments until the exercise of options issued as compensation and it also includes warrants granted until the exercise of these warrants.
Deficit includes all current and prior period losses, except for other comprehensive losses that are included in accumulated other comprehensive income or loss.
Canadian income tax legislation permits an enterprise to issue securities referred to as flow-through shares, whereby the investor can claim the tax deductions arising from the renunciation of the related resource expenditures. The Company accounts for flow-through shares whereby the premium paid for the flow-through shares in excess of the market value of the shares without the flow-through feature at the time of issue is credited to other liabilities and as a reduction of deferred tax expense when the obligation is fulfilled, at the time the eligible expenditures are incurred and there is intension to renounce.
The Company has indemnified the subscribers of flow-through shares for tax rated amounts that may become due if the Company does not complete its required expenditures on eligible expenditures, or if the taxation authorities deem expenditures to not be eligible. Certain expenditure requirements were not met in 2007. In consideration of the passage of time, management does not believe any claims are forthcoming and has not recorded a provision related to the flow-through share indemnity. See also Note 11.
| 3.10 | Financial instruments |
Financial assets:
All financial assets are recognized and derecognized on the trade date where the purchase or sale of a financial asset is under a contract whose terms require delivery of the financial asset within the time frame established by the market concerned, and are initially measured at fair value, plus transaction costs, except for those financial assets classified as at fair value through profit or loss which are initially measured at fair value.
Measurement in subsequent periods depends on the classification of the financial instrument.
RANDSBURG INTERNATIONAL GOLD CORP.
Notes to Financial Statements
(Expressed in Canadian Dollars)
January 31, 2018 and 2017
| 3.10 | Financial instruments |
Financial assets: (continued)
Financial assets are classified into the following categories: financial assets ‘at fair value through profit or loss’ (“FVTPL”), “held-to-maturity investments”, “available-for-sale” and “loans and receivables”. The classification depends on the nature and purpose of the financial assets and is determined at the time of initial recognition
i) Financial assets at fair value through profit or loss (FVTPL)
Financial assets are classified as FVTPL when acquired principally for the purpose of trading, if so designated by management (fair value option), or if they are derivative assets that are not part of an effective and designated hedging relationship. Financial assets classified as FVTPL are measured at fair value, with changes recognized in the statements of loss.
As at January 31, 2018 and 2017, the Company does not currently hold any derivative instruments or apply hedge accounting.
ii) Available-for-sale financial assets
Financial assets are classified as available-for-sale when so designated by management. Financial assets classified as available-for-sale are measured at fair value, with changes recognized in other comprehensive income.
iii) Loans and receivables
Loans and receivables are non-derivative financial assets that have fixed or determinable payments and are not quoted in an active market. Subsequent to initial recognition, loans and receivables are carried at amortized cost using the effective interest method.
Financial liabilities
Financial liabilities are classified as either financial liabilities at “FVTPL” or “other financial liabilities”.
Other financial liabilities:
Other financial liabilities are financial liabilities that are not classified as FVTPL and are initially measured at fair value, net of transaction costs. Subsequent to initial recognition, other financial liabilities that are not subject to hedge accounting, are measured at amortized cost using the effective interest method.
The effective interest method is a method of calculating the amortized cost of an instrument and of allocating interest income over the relevant period. The effective interest rate is the rate that exactly discounts estimated future cash receipts (including all fees on points paid or received that form an integral part of the effective interest rate, transaction costs and other premiums or discounts) through the expected life of the debt instrument to the net carrying amount on initial recognition.
RANDSBURG INTERNATIONAL GOLD CORP.
Notes to Financial Statements
(Expressed in Canadian Dollars)
January 31, 2018 and 2017
| 3.10 | Financial instruments |
Financial liabilities: (continued)
De-recognition of financial liabilities:
The Company derecognizes financial liabilities when the obligations are discharged, cancelled or expire. The Company’s financial instruments consist of the following:
| Financial assets: | Classification: | |
| Cash and cash equivalents | FVTPL | |
| Marketable securities | FVTPL | |
| Receivables | Loans and receivables | |
| Financial liabilities: | Classification: | |
| Accounts payable and accrued liabilities | Other financial liabilities | |
| Due to related parties | Other financial liabilities | |
Impairment of financial assets:
Financial assets are assessed for indicators of impairment at the end of each reporting period. Financial assets are impaired when there is objective evidence that, as a result of one or more events that occurred after the initial recognition of the financial assets, the estimated future cash flows of the investments have been negatively impacted. Evidence of impairment could include: significant financial difficulty of the issuer or counterparty; or default or delinquency in interest or principal payments; or the likelihood that the borrower will enter bankruptcy or financial reorganization.
If, in a subsequent period, the amount of the impairment loss decreases and the decrease can be related objectively to an event occurring after the impairment was recognized, the previously recognized impairment loss is reversed through profit or loss to the extent that the carrying amount of the investment at the date the impairment is reversed does not exceed what the amortized cost would have been had the impairment not been recognized.
Financial instruments recorded at fair value:
Financial instruments recorded at fair value on the statements of financial position are classified using a fair value hierarchy that reflects the significance of the inputs used in making the measurements. The fair value hierarchy has the following levels: Level 1 - valuation based on quoted prices (unadjusted) in active markets for identical assets or liabilities; Level 2 - valuation techniques based on inputs other than quoted prices included in Level 1 that are observable for the asset or liability, either directly (i.e. as prices) or indirectly (i.e. derived from prices); and Level 3 - valuation techniques using inputs for the asset or liability that are not based on observable market data (unobservable inputs). During the year ended January 31, 2018 the only financial assets or liability measured at fair value is the Company’s cash and cash equivalents.
Cash and cash equivalents and marketable securities are considered Level 1 for purposes of the fair value hierarchy.
The fair value of cash and cash equivalents, HST receivable, accounts payable and accrued liabilities and due to related parties approximate their respective carrying values due to their short term nature.
RANDSBURG INTERNATIONAL GOLD CORP.
Notes to Financial Statements
(Expressed in Canadian Dollars)
January 31, 2018 and 2017
| 3.11 | Accounting estimates and critical judgments |
The preparation of financial statements requires management to make estimates, assumptions and judgments about future events. These estimates and judgments are constantly challenged. They are based on past experience and other factors, particularly, forecasts of future events that are reasonable in the circumstances. The actual results are likely to differ from the estimates, assumptions and judgments made by management, and may not equal estimated results.
The following paragraphs describes the most critical management estimates and assumptions in the recognition of assets, liabilities and expenses and the most critical management judgment’s in applying accounting policies:
Impairment of assets
An impairment loss is recognized when the carrying amount of an asset is not recoverable and exceeds its fair value. Management reviews on a regular basis the impairment assessment of its mineral property interests without a recovery test. (Note 3.3)
Share-based payments
The estimation of share-based payment costs require the selection of an appropriate valuation model and consideration as to the inputs necessary for the valuation model chosen. The Company has made estimates as to the volatility of its own share price, the probable life of options, the time of exercise of those options and expected extinguishments. The valuation model used by the Company is Black-Scholes.
Income, value added, withholding and other taxes
The Company is subject to income, value added, withholding and other taxes. Significant judgment is required in determining the Company’s provisions for taxes. There are many transactions and calculations for which the ultimate tax determination is uncertain during the ordinary course of business. The Company recognizes liabilities for anticipated tax audit issues based on estimates of whether additional taxes will be due. The determination of the Company’s income, value added, withholding and other tax liabilities requires interpretation of complex laws and regulations. The Company’s interpretation of taxation law as applied to transactions and activities may not coincide with the interpretation of the tax authorities. All tax related filings are subject to government audit and potential reassessment subsequent to the financial statement reporting period. Where the final tax outcome of these matters is different from the amounts that were initially recorded, such differences will impact the tax related accruals and deferred income tax provisions in the period in which such determination is made.
Contingencies (See Note 11).
| 3.12 | Future accounting policies |
Certain pronouncements were issued by the IASB or the IFRIC that are mandatory for accounting periods on or after February 1, 2018 or later periods. Many are not applicable or do not have a significant impact to the Company and have been excluded. The following have not yet been adopted and are being evaluated to determine their impact on the Company.
IFRS 9 – Financial Instruments (“IFRS 9”) was issued by the IASB in November 2009 with additions in October 2010 and May 2013 and will replace IAS 39 Financial Instruments: Recognition and Measurement (“IAS 39”). IFRS 9 uses a single approach to determine whether a financial asset is measured at amortized cost or fair value, replacing the multiple rules in IAS 39. The approach in IFRS 9 is based on how an entity manages its financial instruments in the 2context of its business model and the contractual cash flow characteristics of the financial assets. Most of the
RANDSBURG INTERNATIONAL GOLD CORP.
Notes to Financial Statements
(Expressed in Canadian Dollars)
January 31, 2018 and 2017
| 3.12 | Future accounting policies (continued): |
requirements in IAS 39 for classification and measurement of financial liabilities were carried forward unchanged to IFRS 9, except that an entity choosing to measure a financial liability at fair value will present the portion of any change in its fair value due to changes in the entity’s own credit risk in other comprehensive income, rather than within profit or loss. The new standard also requires a single impairment method to be used, replacing the multiple impairment methods in IAS 39. IFRS 9 is effective for annual periods beginning on or after January 1, 2018.
IFRS 2 – Share-based Payment (“IFRS 2”) was amended by the IASB in June 2016 to clarify the accounting for cash-settled share-based payment transactions that include a performance condition, the classification of share-based payment transactions with net settlement features and the accounting for modifications of share-based payment transactions from cash-settled to equity-settled. The amendments are effective for annual periods beginning on or after January 1, 2018.
IFRIC 23 – Uncertainty Over Income Tax Treatments (“IFRIC 23”) was issued in June 2017 and clarifies the accounting for uncertainties in income taxes. The interpretation committee concluded that an entity shall consider whether it is probable that a taxation authority will accept an uncertain tax treatment. If an entity concludes it is probable that the taxation authority will accept an uncertain tax treatment, then the entity shall determine taxable profit (tax loss), tax bases, unused tax losses and credits or tax rates consistently with the tax treatment used or planned to be used in its income tax filings. If an entity concludes it is not probable that the taxation authority will accept an uncertain tax treatment, the entity shall reflect the effect of uncertainty in determining the related taxable profit (tax loss), tax bases, unused tax losses and credits or tax rates. IFRIC 23 is effective for annual periods beginning on or after January 1, 2019. Earlier adoption is permitted.
| 3.13 | New accounting policies: |
During 2018, the Company adopted a number of new IFRS standards, interpretations, amendments and improvements of existing standards. These included IAS 7 and IAS 12. These new standards and changes did not have any material impact on the Company’s financial statements.
RANDSBURG INTERNATIONAL GOLD CORP.
Notes to Financial Statements
(Expressed in Canadian Dollars)
January 31, 2018 and 2017
| 4. | CAPITAL MANAGEMENT |
The Company manages its capital with the following objectives:
| • | to ensure sufficient financial flexibility to achieve the ongoing business objective including funding of future growth opportunities, and pursuit of accretive acquisitions; and |
| • | to maximize shareholder return through enhancing the share value. |
The Company manages its capital structure and makes adjustments to it, based on the funds available to the Company, in order to support the acquisition and exploration of mineral properties. The Board of Directors does not establish quantitative return on capital criteria for management, but rather relies on the expertise of the Company’s management to sustain future development of the business. The Company defines capital that it manages as share capital, contributed surplus and deficit.
The Company is in the exploration stage; as such the Company has relied on the equity markets to fund its activities. The Company will continue to assess new sources of financing available and to manage its expenditures to reflect current financial resources in the interest of sustaining long term viability.
Management reviews its capital management approach on an ongoing basis and believes that this approach, given the relative size of the Company, is reasonable.
The Company’s capital management objectives, policies and processes have remained unchanged during the years ended January 31, 2018 and 2017. The Company is not subject to externally imposed capital requirements.
RANDSBURG INTERNATIONAL GOLD CORP.
Notes to Financial Statements
(Expressed in Canadian Dollars)
January 31, 2018 and 2017
| 5. | MINERAL PROPERTY INTERESTS |
| Balance January 31, 2018 |
Balance January 31, 2017 |
|||||||
| Flett and Angus Township |
||||||||
| Acquisition costs |
$ | — | $ | 117,000 | ||||
| Exploration costs |
— | 35,969 | ||||||
| Provision for write down |
— | (56,769 | ) | |||||
| Book Value |
$ | — | $ | 96,200 | ||||
| Total Costs |
$ | — | $ | — | ||||
Flett & Angus Townships, Northern Ontario: The Company held a 20% interest in certain claims located in the Flett and Angus Townships that was subject to a 3% NSR that could be purchased by the Company for $1,500,000. The Company sold its remaining interest in the property during the year ended January 31, 2018 and wrote the carrying value down to its estimated recoverable amount based on the proceeds received during the year ended January 31, 2018.
On January 25, 2017, the Company entered into an agreement with Prophecy Development Corporation to sell its remaining 20% interest in certain mining claims in its Flett & Angus Township Property. The consideration received consisted of 20,000 common shares of Prophecy Development Corporation with a fair market value of $96,200 based on the quoted market price of the shares on the closing date. The shares received were disposed of in settlement of certain amounts due to related parties on the same date as the closing date.
RANDSBURG INTERNATIONAL GOLD CORP.
Notes to Financial Statements
(Expressed in Canadian dollars)
January 31, 2018 and 2017
| 6. | SHARE CAPITAL |
| Authorized: |
||||||||
| Unlimited common shares, without par value |
||||||||
| January 31, 2018 |
January 31, 2017 |
|||||||
| Issued: |
||||||||
| Common shares - 28,273,939 (2017 – 28,273,939) |
$ | 13,628,895 | $ | 13,628,895 | ||||
|
|
|
|
|
|||||
Stock options
The Company has a stock option plan under which it is authorized to grant options to executive officers and directors, employees and consultants enabling them to acquire up to 10% of the issued and outstanding common stock of the Company. Under the plan, the exercise price of each option equals the market price of the Company’s stock, less an applicable discount, as calculated on the date of grant. The options can be granted for a maximum term of 5 years and vest at the discretion of the board of directors. No stock options were outstanding at January 31, 2018 and 2017.
RANDSBURG INTERNATIONAL GOLD CORP.
Notes to Financial Statements
(Expressed in Canadian Dollars)
January 31, 2018 and 2017
| 7. | RELATED PARTY TRANSACTIONS |
Related parties include Board of Directors, close family members, key management personnel, employees and others who exercise significant influence over the reporting entity.
During the year ended January 31, 2018, the Company entered into the following transactions with related parties not disclosed elsewhere in the financial statements:
a) Incurred management fees totaling $18,000 (2017 - $18,000) to two directors of the Company.
b) Incurred interest of $39,650 (2017 - $40,375) to a director and a person related to the president of the Company.
c) The Company is subject to a loan due from Essex Oil Ltd. (“Essex”) in the amount of approximately $85,000 plus interest at 10% per annum. A director and officer of the Company is a director of Essex. The outstanding balance of the loan receivable plus all accrued interest as at January 31, 2018 and 2017 has been fully allowed for.
d) The Company sold a bridge to a person related to the President of the Company for proceeds of $10,000 which approximates its fair market value in 2017.
The balances due to related parties as at January 31, 2018 and 2017 are summarized below:
| January 31, 2018 | January 31, 2017 | |||||||
| Advances from a director of $30,026 (2017 - $30,026) that bears interest at an annual rate of 12%,
is unsecured, and has no fixed terms of repayment. The total includes accrued interest of $75,907; |
$ | 105,933 | $ | 94,269 | ||||
| Advances net of repayment from a person related to the President of the Company that bears interest at an annual rate of 12% and have no fixed terms of repayment. The advances are secured by a General Security Agreement. The total includes accrued interest of $141,927; (2017 - $114,827) |
261,821 | 263,621 | ||||||
| Advances due to the President and a company controlled by the President that are unsecured, non-interest bearing and have no fixed terms of repayment. |
416,669 | 422,996 | ||||||
| Advances due to a director that are unsecured, non-interest bearing and have no fixed terms of repayment. |
112,086 | 112,881 | ||||||
| Advances due to a former director that are unsecured, non-interest bearing and have no fixed terms of repayment. |
22,152 | 22,152 | ||||||
|
|
|
|
|
|||||
| $ | 918,661 | $ | 915,919 | |||||
|
|
|
|
|
|||||
RANDSBURG INTERNATIONAL GOLD CORP.
Notes to Financial Statements
(Expressed in Canadian dollars)
January 31, 2018 and 2017
| 8. | FINANCIAL INSTRUMENTS AND RISK MANAGEMENT |
The Company’s activities expose it to a variety of financial risks: credit risk, liquidity risk and market risk (including interest rate, foreign currency risk and commodity and equity price risk). Risk management is carried out by the Company’s management team under policies approved by the Board of Directors. The Board of Directors also provides regular guidance for overall risk management. There were no changes to credit risk, liquidity risk or market risk for the fiscal years ended January 31, 2018 and 2017.
(i) Credit risk
Credit risk is the risk of loss associated with a counterparty’s inability to fulfill its payment obligations. The Company’s credit risk is primarily attributable to cash and cash equivalents and HST receivable. Cash and cash equivalents is held with select major Canadian chartered banks and the amount of receivables are due from Government of Canada, from which management believes the risk of loss to be minimal.
(ii) Liquidity risk
Liquidity risk is the risk that the Company will not have sufficient cash resources to meet its financial obligations as they come due. The Company’s liquidity and operating results may be adversely affected if its access to the capital market is hindered, whether as a result of a downturn in stock market conditions generally or matters specific to the Company. The Company generates cash flow primarily from its financing activities. The Company prepares annual capital expenditure budgets, which are monitored and updated as required. In addition, the Company requires authorization for expenditures on projects to assist with the management of capital. The Company’s financial liabilities comprise accounts payable and other liabilities, and liability related to flow-through financing, which both are due within 12 months, and amounts due to related parties with no fixed terms of repayment that is due on demand. Certain of these amounts bear interest at an annual rate of 12% (see note 7).
(iii) Market risk
Market risk is the risk of loss that may arise from changes in market factors such as interest rates, foreign exchange rates and commodity and equity prices.
(a) Interest rate risk
The Company currently has short-term debts that bear interest at a fixed annual rate of 12% and as such, the fluctuation of market interest rate has limited impact on these balances.
(b) Foreign currency risk
The Company’s functional and reporting currency is the Canadian dollar and the Company only holds cash balances in Canadian dollars, which is not subject to foreign exchange risk.
(c) Commodity and equity price risk
The Company is exposed to price risk with respect to commodity prices. Commodity price risk is defined as the potential adverse impact on earnings and economic value due to commodity price movements and volatilities. The Company closely monitors commodity prices, as they relate to precious and base metals and other minerals, and the stock market to determine the appropriate course of action to be taken by the Company.
RANDSBURG INTERNATIONAL GOLD CORP.
Notes to Financial Statements
(Expressed in Canadian dollars)
January 31, 2018 and 2017
| 8. | FINANCIAL INSTRUMENTS AND RISK MANAGEMENT (CONTINUED) |
Commodity price risk could adversely affect the Company. In particular, the Company’s future profitability and viability of its development properties depends upon the world market price of precious and base metals and other minerals. Precious and base metals and other mineral prices have fluctuated widely in recent years. There is no assurance that, even if commercial quantities of precious and base metals and other minerals are produced in the future, a profitable market will exist for them. As of January 31, 2018, the Company was not a precious mineral, base metals and other minerals producer. Even so, commodity price risk may affect the completion of future equity transactions such as equity offerings. This may also affect the Company’s liquidity and its ability to meet its ongoing obligations.
| 9. | INCOME TAXES |
Major items causing the Company’s effective income tax rate to differ from the combined Canadian federal and provincial statutory rate of 26.5% (2017 - 26.5%) were as follows:
| 2018 | 2017 | |||||||
| (Loss) before income taxes |
$ | (105,303 | ) | $ | (141,569 | ) | ||
|
|
|
|
|
|||||
| Expected income tax (recovery) based on statutory rate |
$ | (27,905 | ) | $ | (37,516 | ) | ||
| Unrecognized benefit of non-capital losses |
27,905 | 37,516 | ||||||
|
|
|
|
|
|||||
| Net income tax expense (recovery) |
$ | — | $ | — | ||||
|
|
|
|
|
|||||
Deferred income tax assets have not been recognized in respect of the following deductible temporary differences:
| 2018 | 2017 | |||||||
| Unrecognized deferred tax assets |
||||||||
| Non-capital loss carry forward |
$ | 2,656,000 | $ | 2,551,000 | ||||
| Equipment |
241,000 | 241,000 | ||||||
| Mineral property interests |
5,142,000 | 5,046,000 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 8,039,000 | $ | 7,838,000 | ||||
|
|
|
|
|
|||||
Deferred tax assets have not been recognized in respect of these items because it is not probable that future taxable profit will be available against which the Company can use the benefits.
The tax losses expire from 2026 to 2038. The other temporary differences do not expire under current legislation.
RANDSBURG INTERNATIONAL GOLD CORP.
Notes to Financial Statements
(Expressed in Canadian dollars)
January 31, 2018 and 2017
| 10. | SEGMENTED INFORMATION |
The Company’s one reportable operating segment is the acquisition and exploration of mineral resource properties.
| 11. | COMMITMENTS AND CONTINGENT LIABILITIES |
In the ordinary course of business activities, the Company is a party in certain litigation and other claim. Management believes that the resolution of such litigation and claim will not have a material effect on the financial position of the Company.
The Company has an outstanding amount with Canada Revenue Agency (CRA) totaling $87,520 at January 31, 2018 (2017 - $79,492) for which CRA is actively requesting payment. The amount is fully recorded in these financial statements.
Flow-through share indemnity. See note 3.9.
SCHEDULE “E”
CONDENSED INTERIM FINANCIAL STATEMENTS (RESTATED) OF RANDSBURG AS AT JULY 31,
2018 AND FOR THE THREE AND
SIX-MONTH PERIODS ENDED JULY 31, 2018 AND 2017
(See attached.)
RANDSBURG INTERNATIONAL GOLD CORP.
Condensed Interim Financial Statements
(Expressed in Canadian dollars)
JULY 31, 2018
MANAGEMENT’S RESPONSIBILITY FOR FINANCIAL REPORTING
The accompanying condensed interim financial statements of Randsburg International Gold Corp. (the “Company”) are the responsibility of management and the Board of Directors.
The financial statements have been prepared by management, on behalf of the Board of Directors, in accordance with the accounting policies disclosed in the notes to the financial statements. Where necessary, management has made informed judgments and estimates in accounting for transactions which were not complete at the balance sheets date. In the opinion of management, the financial statements have been prepared within acceptable limits of materiality and are in accordance with International Financial Reporting Standards using accounting policies consistent with International Financial Reporting Standards appropriate in the circumstances.
Management has established systems of internal control over the financial reporting process, which are designed to provide reasonable assurance that relevant and reliable financial information is produced.
The Board of Directors is responsible for reviewing and approving the condensed interim financial statements together with other financial information of the Company and for ensuring that management fulfills its financial reporting responsibilities. An Audit Committee assists the Board of Directors in fulfilling this responsibility. The Audit Committee meets with management to review the financial reporting process and the financial statements together with other financial information of the Company. The Audit Committee reports its findings to the Board of Directors for its consideration in approving the financial statements together with other financial information of the Company for issuance to the shareholders.
Management recognizes its responsibility for conducting the Company’s affairs in compliance with established financial standards, and applicable laws and regulations, and for maintaining proper standards of conduct for its activities.
| (Signed) “Michael Lerner” | (Signed)“Balu Gopalakrishnan” | |
| Michael Lerner | Balu Gopalakrishnan | |
| Chief Executive Officer, President | Chief Financial Officer and Director | |
| and Director |
Toronto, Canada
September 27, 2018
NOTICE TO READER
The accompanying unaudited condensed interim financial statements of the Company have been prepared by and are the responsibility of management. The unaudited condensed interim financial statements have not been reviewed by the Company’s auditors.
RANDSBURG INTERNATIONAL GOLD CORP.
Condensed Interim Statements of Financial Position
(Expressed in Canadian Dollars)
| As at July 31, 2018 |
As at January 31, 2018 |
|||||||
| ASSETS |
||||||||
| Current Assets |
||||||||
| Accounts receivable |
$ | 850 | $ | 2,268 | ||||
|
|
|
|
|
|||||
| Total Current Assets |
850 | 2,268 | ||||||
|
|
|
|
|
|||||
| Total Assets |
$ | 850 | $ | 2,268 | ||||
|
|
|
|
|
|||||
| LIABILITIES AND |
||||||||
| SHAREHOLDERS’ (DEFICIENCY) EQUITY |
||||||||
| Current Liabilities |
||||||||
| Accounts payable and accrued liabilities |
$ | 30,220 | $ | 308,720 | ||||
| Liability related to flow-through financing |
86,571 | 87,520 | ||||||
| Loans and advances (Note 7) |
1,040,401 | — | ||||||
| Due to related parties (Note 6) |
33,147 | 918,661 | ||||||
|
|
|
|
|
|||||
| Total Current Liabilities |
1,190,339 | 1,314,901 | ||||||
|
|
|
|
|
|||||
| Shareholders’ (Deficiency) Equity |
||||||||
| Share capital (Note 5) |
13,628,895 | 13,628,895 | ||||||
| Contributed surplus |
3,566,604 | 3,566,604 | ||||||
|
|
|
|
|
|||||
| 17,195,499 | 17,195,499 | |||||||
| Deficit |
(18,384,988 | ) | (18,508,132 | ) | ||||
|
|
|
|
|
|||||
| Total Equity |
(1,189,489 | ) | (1,312,633 | ) | ||||
|
|
|
|
|
|||||
| Total Liabilities and Shareholders’ Equity |
$ | 850 | $ | 2,268 | ||||
|
|
|
|
|
|||||
| Nature of operations and going concern (Note 1) |
||||||||
| Subsequent events (Note 8) |
||||||||
| Commitments and Contingent Liabilities (Note 9) |
||||||||
| Approved on behalf of the Board: |
||||||||
| “Michael Lerner” Director |
||||||||
| “Balu Gopalakrishnan” Director |
||||||||
The accompanying notes to financial statements are an integral part of these statements.
RANDSBURG INTERNATIONAL GOLD CORP.
Condensed Interim Statements of Operations and Comprehensive Loss
(Expressed in Canadian Dollars)
| Three Months Ended July 31, 2018 |
Three Months Ended July 31, 2017 |
|||||||
| Operating expenses |
||||||||
| Consulting |
$ | 2,500 | $ | 5,252 | ||||
| Interest and bank charges |
7,187 | 8,620 | ||||||
| Listing and transfer agent fees |
239 | 2,209 | ||||||
| Management fees (Note 6(a)) |
3,000 | 4,500 | ||||||
| Office and miscellaneous |
1,649 | 50 | ||||||
|
|
|
|
|
|||||
| Total operating expenses |
14,575 | 20,631 | ||||||
|
|
|
|
|
|||||
| Loss before other items |
(14,575 | ) | (20,631 | ) | ||||
| Gain on debt settlement |
14,968 | — | ||||||
| Write off of debt |
56,124 | — | ||||||
| Cost recoveries |
93,310 | — | ||||||
|
|
|
|
|
|||||
| Net Income (Loss) and Comprehensive Loss for the Period |
149,827 | (20,631 | ) | |||||
| Deficit, beginning of Period |
(18,534,815 | ) | (18,420,752 | ) | ||||
|
|
|
|
|
|||||
| Deficit, end of Period |
$ | (18,384,988 | ) | $ | (18,441,383 | ) | ||
|
|
|
|
|
|||||
| Basic and diluted income (loss) per common share |
$ | (0.000 | ) | $ | (0.000 | ) | ||
|
|
|
|
|
|||||
| Weighted average number of common shares outstanding |
28,273,939 | 28,273,939 | ||||||
|
|
|
|
|
|||||
The accompanying notes to the financial statements are an integral part of these statements.
RANDSBURG INTERNATIONAL GOLD CORP.
Condensed Interim Statements of Operations and Comprehensive Loss
(Expressed in Canadian Dollars)
| Six Months Ended July 31, 2018 |
Six Months Ended July 31, 2017 |
|||||||
| Operating expenses |
||||||||
| Consulting |
$ | 7,500 | $ | 7,752 | ||||
| Interest and bank charges |
18,642 | 17,015 | ||||||
| Legal fees |
— | 1,000 | ||||||
| Listing and transfer agent fees |
5,968 | 3,737 | ||||||
| Management fees (Note 6(a)) |
7,500 | 9,000 | ||||||
| Office and miscellaneous |
1,648 | 50 | ||||||
|
|
|
|
|
|||||
| Total operating expenses |
41,258 | 38,554 | ||||||
|
|
|
|
|
|||||
| Loss before other items |
(41,258 | ) | (38,554 | ) | ||||
| Gain on debt settlement |
14,968 | — | ||||||
| Write off of debt |
56,124 | — | ||||||
| Cost recoveries |
93,310 | — | ||||||
|
|
|
|
|
|||||
| Net Income (Loss) and Comprehensive Income (Loss) for the Period |
123,144 | (38,554 | ) | |||||
| Deficit, beginning of Year |
(18,508,132 | ) | (18,402,829 | ) | ||||
|
|
|
|
|
|||||
| Deficit, end of Period |
$ | (18,384,988 | ) | $ | (18,441,383 | ) | ||
|
|
|
|
|
|||||
| Basic and diluted income (loss) per common share |
$ | (0.000 | ) | $ | (0.000 | ) | ||
|
|
|
|
|
|||||
| Weighted average number of common shares outstanding |
28,273,939 | 28,273,939 | ||||||
|
|
|
|
|
|||||
The accompanying notes to the financial statements are an integral part of these statements.
RANDSBURG INTERNATIONAL GOLD CORP.
Condensed Interim Statements of Changes in Equity
(Expressed in Canadian Dollars)
| Number of Shares |
Common Shares |
Contributed Surplus |
Deficit | Total Equity (Deficiency) |
||||||||||||||||
| Balance, January31, 2017 |
28,273,939 | $ | 13,628,895 | $ | 3,566,604 | $ | (18,402,829 | ) | $ | (1,207,330 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Transaction in period: |
||||||||||||||||||||
| Loss for the period |
— | — | — | ( 38,554 | ) | ( 38,554 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance, July 31 2017 |
28,273,939 | 13,628,895 | 3,566,604 | (18,441,383 | ) | (1,245,844 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Transactions in period: |
||||||||||||||||||||
| Loss for period |
— | — | — | ( 66,749 | ) | ( 66,749 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance, January 31, 2018 |
28,273,939 | 13,628,895 | 3,566,604 | (18,508,132 | ) | (1,312,633 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Transactions in period: |
||||||||||||||||||||
| Ner Income for the Period |
— | — | — | 123,144 | 123,144 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance, July 31, 2018 |
28,273,939 | $ | 13,628,895 | $ | 3,566,604 | $ | (18,384,988 | ) | $ | (1,189,489 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
The accompanying notes to the financial statements are an integral part of these statements.
RANDSBURG INTERNATIONAL GOLD CORP.
Condensed Interim Statements of Cash Flows
(Expressed in Canadian Dollars)
| Six Months Ended July 31, 2018 |
Six Months Ended July 31, 2017 |
|||||||
| CASH FLOWS FROM OPERATING ACTIVITIES |
||||||||
| Net Income (Loss) for the period |
$ | 123,144 | $ | (17,923 | ) | |||
| Interest on related party loans and advances |
17,322 | 8,395 | ||||||
|
|
|
|
|
|||||
| 140,466 | (9,528 | ) | ||||||
| Changes in non-cash operating working capital: |
||||||||
| (Increase) decrease in receivable |
1,419 | 2,734 | ||||||
| (Increase) decrease in prepaid expenses |
— | — | ||||||
| Increase (decrease) in accounts payable and accrued liabilities |
(278,499 | ) | 2,532 | |||||
| Increase in loans and advances |
1,040,401 | — | ||||||
| Increase in liability for flow-through financing |
(949 | ) | (3,063 | ) | ||||
| Increase(decrease) in due to related parties |
(902,838 | ) | (88,875 | ) | ||||
|
|
|
|
|
|||||
| Net cash used in operating activities |
— | (96,200 | ) | |||||
|
|
|
|
|
|||||
| CASH FLOWS FROM INVESTING ACTIVITIES |
||||||||
| Decrease in mineral property interests held for resale |
— | 96,200 | ||||||
|
|
|
|
|
|||||
| Net cash provided by investing activities |
— | 96,200 | ||||||
|
|
|
|
|
|||||
| Changes in cash and cash equivalents during the period |
— | — | ||||||
| Cash and cash equivalents; beginning of year |
— | — | ||||||
|
|
|
|
|
|||||
| Cash and cash equivalents (bank indebtedness); end of period |
$ | — | $ | — | ||||
|
|
|
|
|
|||||
| Cash paid for interest |
$ | Nil | $ | Nil | ||||
|
|
|
|
|
|||||
| Cash paid for income taxes |
$ | Nil | $ | Nil | ||||
|
|
|
|
|
|||||
The accompanying notes to the financial statements are an integral part of these statements.
RANDSBURG INTERNATIONAL GOLD CORP.
Notes to Condensed Interim Financial Statements
(Expressed in Canadian Dollars)
July 31, 2018
| 1. | NATURE OF OPERATIONS AND GOING CONCERN |
Randsburg International Gold Corp. (the “Company”) was incorporated under the laws of British Columbia on July 6, 1990.
The Company is listed on the NEX, having the symbol RGZ.H. The address of the Company’s corporate office and principal place of business is 120 Adelaide Street West, Suite 2105, Toronto, Ontario, M5H 1T1 Canada.
The financial statements of Randsburg International Gold Corp. for the three and six month period ended July 31, 2018 and 2017 were reviewed by and authorized for issue by the Board of Directors on September 27, 2018.
The Company’s principal business activity is the acquisition and exploration of mineral property interests in Canada. The Company is considered to be in the exploration stage and substantially all of the Company’s efforts are devoted to financing and developing these property interests. The Company has not determined whether its properties contain ore reserves which are economically recoverable. The recovery of the amounts shown for mining properties is dependent upon the existence of economically recoverable ore reserves, the ability of the Company to obtain necessary financing to complete the exploration and development of its properties, and upon future profitable production or proceeds from the disposal of its properties.
These financial statements have been prepared in accordance with International Financial Reporting Standards (“IFRS”), applicable to a going concern. Accordingly, they do not give effect to adjustments that would be necessary should the Company be unable to continue as a going concern and therefore be required to realize on its assets and settle its liabilities and commitments in other than the normal course of business and at amounts different from those in the accompanying financial statements.
The Company has incurred operating losses over the last several years, earns no revenues and has a working capital deficiency of $1,189,489 - as at July 31, 2018 (January 31, 2018 - $1,312,633). While the Company has been successful in obtaining its required financing in the past, through additional equity and non – arm’s length loans, there is no assurance that such financing will be available or be available on favorable terms. The Company’s ability to continue as a going concern is uncertain and is dependent upon the generation of profits from mineral properties, obtaining additional financing or maintaining continued support from its shareholders and creditors. In the event that additional financial support is not received or operating profits are not generated, the carrying values of the Company’s assets may be adversely affected.
Although the Company has taken steps to verify title to the properties on which it is conducting exploration in which it has an interest, in accordance with industry standards for the current stage of exploration. These procedures do not guarantee the Company’s title. Property title may be subject to unregistered prior agreements, non- compliance with regulatory requirements or aboriginal land claims. The Company’s assets may also be subject to increases in taxes and royalties, renegotiation of contracts, political uncertainty and current exchange fluctuations and restrictions.
The Company’s exploration and evaluation activities are subject to laws and regulations governing the protection of the environment. These laws and regulations are continually changing and generally becoming more restrictive. The Company believes its activities are materially in compliance with all applicable laws and regulations. The Company has made, and expects to make in the future, expenditures to comply with such laws and regulations. During 2018, the Company disposed of its mineral property interest.
RANDSBURG INTERNATIONAL GOLD CORP.
Notes to Condensed Interim Financial Statements
(Expressed in Canadian Dollars)
July 31, 2018
| 1. | NATURE OF OPERATIONS AND GOING CONCERN (continued) |
These financial statements have been prepared in accordance with International Financial Reporting Standards (“IFRS”), applicable to a going concern. Accordingly, they do not give effect to adjustments that would be necessary should the Company be unable to continue as a going concern and therefore be required to realize on its assets and settle its liabilities and commitments in other than the normal course of business and at amounts different from those in the accompanying financial statements.
The Company has incurred operating losses over the last several years, earns no operating revenues and has a working capital deficiency of $1,189,489 (January 31, 2018 - $1,312,633). While the Company has been successful in obtaining its required financing in the past, through additional equity and non – arm’s length loans, there is no assurance that such financing will continue to be available or be available on favorable terms. The Company’s ability to continue as a going concern is uncertain and is dependent upon the generation of profits, obtaining additional financing or maintaining continued support from its shareholders and creditors. In the event that additional financial support is not received or operating profits are not generated, the carrying values of the Company’s assets may be adversely affected. These conditions represent material uncertainties which cast significant doubt about the Company’s ability to continue as a going concern.
| 2. | BASIS OF PREPARATION AND STATEMENT OF COMPLIANCE |
Statement of compliance
The Company applies International Financial Reporting Standards (“IFRS”), as issued by the International Accounting Standards Board (“ISAB”). These condensed consolidated interim financial statements have been prepared in accordance with International Accounting Standard 34, Interim Financial reporting. Accordingly, they do not include all of the information required for full annual financial statements required by IFRS as issued by IASB and interpretations issued by IFRIC.
The policies applied in these condensed interim financial statements are based on IFRSs issued and outstanding as of September 27, 2018, the date the Board of Directors approved the statements. The same accounting policies and methods of computation are followed in these condensed interim financial statements as compared with the most recent annual financial statements as at and for the year ended January 31, 2018. Any subsequent changes to IFRS that are given effect in the Company’s annual financial statements for the year ended January 31, 2018 could result in restatement of these condensed interim financial statements.
New and future accounting changes The Company has adopted the following amendments effective January 1, 2018
| (i) | IFRS 9 – Financial Instruments |
Classification and measurement applies to classification and measurement of financial assets and liabilities as defined in IAS 39. This amendment is effective for annual periods beginning on or after January 1, 2018 with early adoption permitted. The adoption of this standard had no material impact on the financial statements.
| (ii) | IFRS 15 – Revenue from Contracts with Customers. |
(“IFRS 15”) was issued in May 2014 when the IASB and the Financial Accounting Standards Board (“FASB”) completed its joint project to clarify the principles for recognizing revenue and to develop a common revenue standard for IFRS and US GAAP. As a result of the joint project, the IASB issued IFRS15 to establish principles to address the nature, amount, timing and uncertainty of revenue and cash flows arising from an entity’s contracts with customers. IFRS 15 is effective for annual periods beginning on or after January 1, 2018. The adoption of this standard had no material impact on the financial statements.
RANDSBURG INTERNATIONAL GOLD CORP.
Notes to Condensed Interim Financial Statements
(Expressed in Canadian Dollars)
July 31, 2018
| 2. | BASIS OF PREPARATION AND STATEMENT OF COMPLIANCE (continued) |
| (iii) | IFRS 2 – Share based payments. |
In June 2016, the IASB issued amendments to IFRS 2, which clarify how to classify and measure certain type of share-based payment transactions.
These amendments are effective for annual periods beginning on or after January 1, 2018 and can be applied prospectively. The adoption of this standard had no material impact on the financial statements. The following has not yet been adopted and are being evaluated to determine its impact on the Company.
| (iv) | IFRS 16 – Leases (“IFRS 16”) was issued by the IASB on January 13, 2016, and will replace IAS 17, Leases. IFRS 16 will bring most leases on-balance sheet for lessees under a single model, eliminating the distinction between operating and finance leases. Lessor accounting however, remains largely unchanged and the distinction between operating and finance leases is retained. IFRS 16 is effective for annual reporting periods beginning on or after January 1, 2019. |
Basis of presentation
These financial statements have been prepared on a historical cost basis except for certain financial instruments that have been measured at fair value. In addition, these financial statements have been prepared using the accrual basis of accounting, except for cash flow information.
The accounting policies set are the same as are disclosed in the annual audited financial statements for the year ended January 31, 2018 (See Note 3) to these financial statements which have been filed on (“SEDAR; the “System for Electronic Document and Retrieval”) for the disclosure of public company documents in Canada.
The accounting policies have been applied consistently to all periods presented in these financial statements.
| 3. | CAPITAL MANAGEMENT |
The Company manages its capital with the following objectives:
| • | to ensure sufficient financial flexibility to achieve the ongoing business objective including funding of future growth opportunities, and pursuit of accretive acquisitions; and |
| • | to maximize shareholder return through enhancing the share value. |
The Company manages its capital structure and makes adjustments to it, based on the funds available to the Company, in order to support the acquisition and exploration of mineral properties. The Board of Directors does not establish quantitative return on capital criteria for management, but rather relies on the expertise of the Company’s management to sustain future development of the business. The Company defines capital that it manages as share capital and cash.
RANDSBURG INTERNATIONAL GOLD CORP.
Notes to Condensed Interim Financial Statements
(Expressed in Canadian Dollars)
July 31, 2018
The Company is in the exploration stage; as such the Company has relied on the equity markets to fund its activities. The Company will continue to assess new sources of financing available and to manage its expenditures to reflect current financial resources in the interest of sustaining long term viability.
Management reviews its capital management approach on an ongoing basis and believes that this approach, given the relative size of the Company, is reasonable.
The Company is not subject to any external capital requirements.
| 4. | MINERAL PROPERTY INTERESTS |
| Balance July 31, 2018 |
Balance January 31, 2018 |
Balance January 31, 2017 |
||||||||||
| Flett and Angus Township |
||||||||||||
| Acquisition costs |
$ | — | $ | — | $ | 117,000 | ||||||
| Exploration costs |
— | — | 35,969 | |||||||||
| — | — | (56,769 | ) | |||||||||
| Book Value |
$ | — | $ | — | $ | 96,200 | ||||||
| Total Costs |
$ | — | $ | — | $ | 96,200 | ||||||
On January 25, 2017 the Company entered into an agreement with Prophecy Development Corporation to sell its remaining 20% interest in certain mining claims in its Flett & Angus Township Property. The consideration was received on February 10, 2017 and consisted 20,000 common shares of Prophecy Development Corporation with a fair market value of $96,200 based on the quoted market price of the shares. The shares received were disposed of in settlement of certain amounts due to related parties on the same date as the closing date.
Title to mining property interests
Although the Company has taken steps to verify the title to mineral properties in which it has an interest, in accordance with industry standards for the current stage of exploration of such properties, these procedures do not guarantee the Company’s title. Property title may be subject to unregistered prior agreements or transfers and title may be affected by undetected defects.
| 5. | SHARE CAPITAL |
| Authorized: |
||||||||
| Unlimited Common shares, without par value |
||||||||
| July 31, 2018 |
January 31, 2018 |
|||||||
| Issued: |
||||||||
| Common shares - 28,273,939 |
$ | 13,628,895 | $ | 13,628,895 | ||||
|
|
|
|
|
|||||
RANDSBURG INTERNATIONAL GOLD CORP.
Notes to Condensed Interim Financial Statements
(Expressed in Canadian Dollars)
July 31, 2018
| 5. | SHARE CAPITAL (continued): |
Stock options
The Company has a stock option plan under which it is authorized to grant options to executive officers and directors, employees and consultants enabling them to acquire up to 10% of the issued and outstanding common stock of the Company. Under the plan, the exercise price of each option equals the market price of the Company’s stock, less an applicable discount, as calculated on the date of grant. The options can be granted for a maximum term of 5 years and vest at the discretion of the board of directors.
As at July 31, 2018 there were no stock options outstanding.
| 6. | RELATED PARTY TRANSACTIONS |
During the three-month ended July 31, 2018, the Company entered into the following transactions with related parties not disclosed elsewhere in the financial statements: These transactions were in the normal course of operations and are measured at the exchange amount, which is the amount of consideration established and agreed to by the related parties.
| a) | Accrued management fees totaling $4,500 (January 31, 2018 - $18,000) to two directors of the Company. |
| b) | Accrued interest of $ 11,454 (2018 - $39,650) to a director and a person related to the president of the Company. |
| c) | The Company is subject to a loan due from Essex Oil Ltd. (“Essex”) in the amount of approximately $85,000 plus interest at 10% per annum. A director and officer of the Company is a director of Essex. The outstanding balance of the loan receivable plus all accrued interest as at January 31, 2018 and 2017 has been fully allowed for. |
| d) | On July 3, 2018 related party debt with a face value of $870,241 was sold to unrelated parties. |
RANDSBURG INTERNATIONAL GOLD CORP.
Notes to Condensed Interim Financial Statements
(Expressed in Canadian Dollars)
July 31, 2018
| 6. | RELATED PARTY TRANSACTIONS (continued): |
The balances due to related parties as at July 31, 2018 and January 31, 2018 are summarized below:
| July 31, 2018 |
January 31, 2018 |
|||||||
| Advances from a former director, George Van Voorhis III of $nil (January 31, 2018 - $30,026), that bears interest at an annual rate of 12%, are unsecured, and have no fixed terms of repayment. The total includes accrued interest of $nil (January 31, 2018 - $75,907). |
$ | — | $ | 105,933 | ||||
| Advances net of repayment from Elena Opara, a person related to the former president of the Company that bears interest at an annual rate of 12% and have no fixed terms of repayment. The advances are secured by a General Security Agreement. The total includes accrued interest of $14,188 (January 31, 2018 - $141,927). |
26,268 | 261,821 | ||||||
| Advances due to a former director, William Quan that are unsecured, non-interest bearing and have no fixed terms of repayment. |
— | 22,152 | ||||||
| Advances due to a former director, Matthew Chodorowicz that are unsecured, non-interest bearing and have no fixed terms of repayment. |
— | 112,086 | ||||||
| Amounts due to a former President and a company controlled by the president and director, Michael Opara that are unsecured, non-interest bearing and have no fixed terms of repayment. |
6,879 | 416,669 | ||||||
|
|
|
|
|
|||||
| $ | 33,147 | $ | 918,661 | |||||
|
|
|
|
|
|||||
| 7. | LOANS AND ADVANCES |
Loans and advances are non-interest bearing and have no terms of repayment.
| 8. | SUBSEQUENT EVENTS |
There are no subsequent events to report up to and including September 27, 2018.
| 9. | COMMITMENTS AND CONTINGENT LIABILITIES |
In the ordinary course of business activities, the Company is a party in certain litigation and other claim. Management believes that the resolution of such litigation and claim will not have a material effect on the financial position of the Company.
The Company has an outstanding amount with Canada Revenue Agency (CRA) totaling $86,571 at July 31, 2018 (January 31, 2018 - $87,520) for which CRA is actively requesting payment. The amount is fully recorded in these financial statements.
SCHEDULE “F”
CONSOLIDATED PRO FORMA FINANCIAL STATEMENTS OF THE RESULTING ISSUER
(See attached.)
CRESCO LABS INC.
UNAUDITED PRO FORMA CONSOLIDATED STATEMENT OF FINANCIAL POSITION
(Expressed in US dollars)
| as at |
Randsburg International Gold Corp. July 31, 2018 |
Cresco Labs LLC June 30, 2018 |
Note | Pro Forma Adjustments |
Cresco Labs Inc. Pro Forma Consolidation |
|||||||||||||
| Assets |
||||||||||||||||||
| Current assets |
||||||||||||||||||
| Cash and cash equivalents |
— | 31,699,077 | 3(b) | 250,037 | 174,013,440 | |||||||||||||
| 3(d) | (100,000 | ) | ||||||||||||||||
| 3(i) | 100,000,000 | |||||||||||||||||
| 3(n) | 82,553,954 | |||||||||||||||||
| 4 | (40,389,628 | ) | ||||||||||||||||
| Accounts receivable |
654 | 2,301,138 | 4 | 90,128 | 2,391,920 | |||||||||||||
| Biological assets |
— | 4,807,223 | — | 4,807,223 | ||||||||||||||
| Inventory |
— | 6,043,506 | 4 | — | 6,043,506 | |||||||||||||
| Other current assets |
— | 768,658 | 4 | 439,650 | 1,208,308 | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||
| 654 | 45,619,602 | 142,844,141 | 188,464,397 | |||||||||||||||
| Property and equipment |
— | 16,779,572 | 4 | 2,497,234 | 19,276,806 | |||||||||||||
| Intangible assets and goodwill |
— | 3,406,140 | 4 | 147,777,772 | 151,183,912 | |||||||||||||
| Investments |
— | 7,305,668 | 4 | 6,528,540 | 13,834,208 | |||||||||||||
| Security deposits |
— | 1,354,460 | 4 | — | 1,354,460 | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||
| 654 | 74,465,442 | 299,647,687 | 374,113,783 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||
| Liabilities |
||||||||||||||||||
| Current liabilities |
||||||||||||||||||
| Accounts payable |
23,250 | 3,659,875 | 4 | 10,000 | 3,693,125 | |||||||||||||
| Liability related to flow-through financing |
66,603 | — | 3(f) | (66,603 | ) | — | ||||||||||||
| Warrant liability |
— | — | 4 | 7,420,000 | 7,420,000 | |||||||||||||
| Loans and advances |
800,425 | — | 3(a) | (800,425 | ) | — | ||||||||||||
| Subscription receipts |
— | 12,500 | — | 12,500 | ||||||||||||||
| Notes payable |
— | 187,500 | 187,500 | |||||||||||||||
| Due to related parties |
25,501 | 1,500,000 | 4 | 10,000 | 1,535,501 | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||
| 915,779 | 5,359,875 | 6,572,972 | 12,848,626 | |||||||||||||||
| Deferred rent |
— | 1,894,076 | — | 1,894,076 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||
| 915,779 | 7,253,951 | 6,572,972 | 14,742,702 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||
| Shareholders’ Equity |
||||||||||||||||||
| Capital stock |
10,485,298 | 63,004,118 | 3(a) | 800,425 | 497,824,253 | |||||||||||||
| 3(b) | 250,037 | |||||||||||||||||
| 3(c) | (11,535,760 | ) | ||||||||||||||||
| 3(e) | 1,692,555 | |||||||||||||||||
| 3(g) | 141,938,930 | |||||||||||||||||
| 3(i) | 100,000,000 | |||||||||||||||||
| 3(n) | 81,684,954 | |||||||||||||||||
| 4 | 109,503,696 | |||||||||||||||||
| Reserves |
2,743,943 | — | 3(c) | (2,743,943 | ) | 38,970,000 | ||||||||||||
| 3(e) | 171,000 | |||||||||||||||||
| 3(n) | 869,000 | |||||||||||||||||
| 3(o) | 37,930,000 | |||||||||||||||||
| Deficit |
(14,144,366 | ) | (13,853,773 | ) | 3(c) | 14,144,366 | (179,680,815 | ) | ||||||||||
| 3(e) | (1,761,615 | ) | ||||||||||||||||
| 3(g) | (126,135,427 | ) | ||||||||||||||||
| 3(o) | (37,930,000 | ) | ||||||||||||||||
| Non-controlling interest |
— | 18,061,146 | 3(g) | (15,803,503 | ) | 2,257,643 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||
| (915,125 | ) | 67,211,491 | 293,074,715 | 359,371,081 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||
| 654 | 74,465,442 | 299,647,687 | 374,113,783 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||
CRESCO LABS INC.
NOTES TO THE UNAUDITED PRO FORMA CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in US dollars unless otherwise stated)
(Unaudited)
| 1. | Basis of presentation |
On October 10, 2018, Randsburg International Gold Corp. (“Randsburg”) and Cresco Labs LLC (“Cresco”) announced that they have entered into a binding letter agreement (the “Letter Agreement”) to affect a business combination that will result in a reverse-takeover of Randsburg by Cresco (the “Transaction”).
These unaudited pro forma consolidated financial statements have been prepared to give effect to the proposed Transaction between Randsburg and Cresco. The resulting issuer will comprise Randsburg as the parent (which will be renamed “Cresco Labs Inc.”) and Cresco as its wholly owned subsidiary. For accounting purposes, the reverse-takeover will be presented as an issuance of shares by Cresco to acquire 100% of the issued and outstanding shares of Randsburg.
The pro forma financial statements have been prepared from information derived from, and should be read in conjunction with, the following historical financial information which was prepared in accordance with International Financial Reporting Standards (“IFRS”):
| a. | For the unaudited pro forma consolidated statement of financial position (giving effect to the Transaction as if it had occurred on June 30, 2018): |
| i. | the unaudited condensed interim consolidated statement of financial position of Randsburg International Gold Corp. as at July 31, 2018; |
| ii. | the unaudited condensed interim consolidated statement of financial position of Cresco Labs LLC as at June 30, 2018. |
Pro forma consolidated statements of operations and comprehensive income have not been prepared as the resulting information contained therein would not be substantially different from the financial statements of Cresco.
These unaudited pro forma consolidated financial statements have been compiled using the significant accounting policies as set out in the audited consolidated financial statements of Cresco for the year ended December 31, 2017 which are incorporated by reference into this document. Management has reclassified certain line items from the Randsburg financial statements in an attempt to conform to the presentation of the Cresco financial statements. It is management’s opinion that these unaudited pro forma consolidated financial statements include all adjustments necessary for the fair presentation of the transactions described in Note 2 in accordance with International Financial Reporting Standards (“IFRS”), as issued by the International Accounting Standards Board (“IASB”).
These unaudited pro forma consolidated financial statements should be read in conjunction with the consolidated financial statements and notes thereto of Cresco described above. These unaudited pro forma consolidated financial statements are not intended to reflect the results of operations or the financial position of the Cresco which would have actually resulted had the proposed transactions been affected on the dates indicated. Further, the unaudited pro forma financial information is not necessarily indicative of the results of operations that may be obtained in the future. The pro forma adjustments and allocations of the purchase price for Randsburg are based in part on estimates of the fair value of the assets acquired and liabilities assumed. The final purchase price allocation will be completed after asset and liability valuations are finalized. The final valuation will be based on the actual net tangible and intangible assets of Randsburg that exist as of the date of the completion of the acquisition.
| 2. | Transaction |
The Transaction will be treated for accounting purposes as an asset acquisition of Randsburg by Cresco. In consideration for the acquisition of Randsburg, Cresco will issue 0.001231 subordinate voting shares (“SVS”) of Cresco for each outstanding common share of Randsburg totaling approximately 258,824 SVS to shareholders of Randsburg. The final distribution of securities will be based on the quoted market price and the actual number of Randsburg issued and outstanding shares, options, and warrants on the actual date of closing.
Each Randsburg stock option or share purchase warrant which gives the holder the right to acquire shares in the common stock of Randsburg when presented for execution will be exchanged for a warrant or stock option which will give the holder the right to acquire SVS of Cresco on the same basis as the exchange of Randsburg common shares for Cresco SVS. These options and warrants have been included in the purchase consideration at their fair value of approximately $nil and $171,000, respectively, based on the Black-Scholes pricing model.
For the purpose of determining the value of the purchase consideration, the total number of outstanding shares, options, and warrants have been derived from the latest published financial statements of Randsburg as at June 30, 2018 and adjusted for any known or assumed transactions (see Note 3). The value of the purchase consideration for accounting purposes will differ from the amount assumed in the unaudited pro forma consolidated financial statement information for changes in the value and number of outstanding shares, options, and warrants as of the transaction closing date.
The fair value of the net assets of Randsburg to be acquired will ultimately be determined during the fourth quarter of 2018. Therefore, it is likely that the fair values of assets and liabilities acquired will vary from those shown below and the differences may be material.
CRESCO LABS INC.
NOTES TO THE UNAUDITED PRO FORMA CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in US dollars unless otherwise stated)
(Unaudited)
| 2. | Transaction (continued) |
The preliminary purchase price allocation has been assumed as follows:
| Fair value of 258,824 SVS to be issued ($6.54 per share) |
$ | 1,692,555 | ||
| Fair value of 53,325 share purchase warrants issued |
171,000 | |||
| Transaction costs (Note 3(d)) |
100,000 | |||
|
|
|
|||
| Total purchase price |
$ | 1,963,555 | ||
|
|
|
|||
| Cash and cash equivalents |
$ | 250,037 | ||
| Accounts receivable |
654 | |||
| Accounts payable |
(23,250 | ) | ||
| Due to related parties |
(25,501 | ) | ||
|
|
|
|||
| Net assets acquired |
(201,940 | ) | ||
| Listing costs expensed |
1,761,615 | |||
|
|
|
|||
| Total purchase price |
$ | 1,963,555 | ||
|
|
|
| 3. | Pro forma assumptions and adjustments |
These unaudited pro forma consolidated financial statements incorporate the following pro forma assumptions:
| (a) | On October 12, 2018, Randsburg issued 138,720,173 common shares to extinguish $800,425 (CAD $1,040,041) of loans and advances. |
| (b) | On October 12, 2018, Randsburg issued 43,333,333 units for $250,037 (CAD $325,000). |
| (c) | Equity balances of Randsburg are eliminated. |
| (d) | Acquisition costs of $100,000 have been allocated to the acquired assets and liabilities on a pro forma basis as described in Note 2. |
| (e) | 258,824 subordinate voting shares (“SVS”) of Cresco valued at $1,692,555 (CAD $2,200,000) and 53,325 SVS purchase warrants valued at $171,000 are assumed to be issued to the shareholders of Randsburg to acquire the net assets of Randsburg. The fair value of the warrants was calculated using the Black Scholes option pricing model and the following weighted average assumptions: share price - $6.54; exercise price – $4.69; expected life – 1 year; expected volatility – 100%; expected dividend yield – 0%; risk free interest rate – 1.25%. The excess of the purchase consideration issued to Randsburg shareholders over carrying values of Randsburg’s net assets has been expensed representing the value paid by Cresco to acquire Randsburg’s listing status as a public company which does not meet the recognition criteria of an asset. |
| (f) | Liability related to flow-through financing is not recognized in the purchase price allocations as it does not represent an actual financial liability; accordingly, it is eliminated in the pro forma consolidated statement of financial position. |
| (g) | Prior to the Transaction, Cresco will increase the proportionate ownership of several of its subsidiaries. Cresco will issue 21,705,135 units valued at $141,938,930 ($6.54 per share) and will extinguish $15,803,503 of non-controlling interest. |
| (h) | Prior to the transaction, Cresco issued 500,000 Class F units pursuant to the terms of its investment agreement for a 25% interest in Lighthouse Strategies LLC (“Nevada”). The value of the units had been recognized as at June 30, 2018 but their formal issuance was pending final regulatory approval. |
| (i) | Cresco closed an equity financing for 27,066,124 Class F units for total proceeds of $100,000,000 ($3.69 per share). |
| (j) | Cresco will acquire a cannabis license in the state of New York. See Note 4 (i). |
| (k) | Cresco will affect a share reorganization converting the 253,409,416 units outstanding into 8,990,819 subordinate voting shares (“SVS”), 500,000 super voting shares (“MVS”) which carry 2,000 votes per share, 503,640 proportionate voting shares each of which are convertible into 200 SVS and bear proportional voting rights, and 143,690,687 redeemable shares which are the legal capital of Cresco LLC and are redeemable into one (1) SVS per redeemable share. |
| (l) | Cresco plans to close an acquisition of MedMar. See Note 4 (ii). |
| (m) | Cresco plans to close an acquisition of PDI. See Note 4 (iii). |
CRESCO LABS INC.
NOTES TO THE UNAUDITED PRO FORMA CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in US dollars unless otherwise stated)
(Unaudited)
| 3. | Pro forma assumptions and adjustments (continued) |
| (n) | Cresco will complete a concurrent financing for 12,624,054 SVS and total proceeds of $82,553,954 ($6.54 per share), and issue 343,745 broker warrants from subscription receipt financing valued at $869,000. The fair value of the warrants was calculated using the Black Scholes option pricing model and the following weighted average assumptions: share price – $6.54; exercise price – $6.54; expected life – 1 year; expected volatility – 100%; expected dividend yield – 0%; risk free interest rate – 1.25%. |
| (o) | Cresco issued 13,010,000 stock options valued at $37,930,000. The fair value of the options was calculated using the Black Scholes option pricing model and the following weighted average assumptions: share price – $3.75; exercise price – $2.05; expected life – 4 years; expected volatility – 100%; expected dividend yield – 0%; risk free interest rate – 1.25%. |
| (p) | For presentation in this pro forma consolidated statement of financial position, all transactions and balances denominated in Canadian dollars, including the statement of financial position of Randsburg as at June 30, 2018, have been translated to US dollars at a rate of 1.29981 CAD per USD, the rate prevailing on October 10, 2018, the date of the announcement of the Transaction. |
| (q) | The expected future tax rate of Cresco Labs Inc. is 26.5%. |
| 4. | Acquisitions and investments |
| NY Acquisition (i) |
MedMar Acquisition (ii) |
Total | ||||||||||
| Cash |
32,500,000 | 7,231,022 | 39,731,022 | |||||||||
| PVS |
88,064,152 | 19,750,439 | 107,814,591 | |||||||||
| Warrants |
7,420,000 | — | 7,420,000 | |||||||||
|
|
|
|
|
|
|
|||||||
| 127,984,152 | 26,981,461 | 154,965,613 | ||||||||||
| Cash and cash equivalents |
4,195,529 | (14,700 | ) | 4,180,829 | ||||||||
| Accounts receivable |
46,128 | 44,000 | 90,128 | |||||||||
| Inventory |
— | — | — | |||||||||
| Other current assets |
206,050 | 233,600 | 439,650 | |||||||||
| PP&E |
2,491,734 | 5,500 | 2,497,234 | |||||||||
| Security deposits |
— | — | ||||||||||
| Accounts payable |
(10,000 | ) | — | (10,000 | ) | |||||||
| Due to related parties |
(10,000 | ) | — | (10,000 | ) | |||||||
| Licenses and other intangibles |
121,064,711 | 26,713,061 | 147,777,772 | |||||||||
|
|
|
|
|
|
|
|||||||
| 127,984,152 | 26,981,461 | 154,965,613 | ||||||||||
| (i) | Cresco plans to acquire a cannabis license in New York State through the acquisition of Valley Agriceuticals, a New York limited liability company, by paying $32,500,000 cash and issuing 13,466,667 Class F Units valued using the closing price on the date of the transaction (subsequently converted into redeemable shares – Note 3(k)) and 4,000,000 share purchase warrants (the “NY Acquisition”). The fair value of the warrants was calculated using the Black Scholes option pricing model and the following assumptions: share price - $3.75; exercise price – $4.28; expected life – 2 years; annualized volatility – 100%; dividend yield – 0%; discount rate – 1.25%. The exercise price of the warrants is subject to adjustment based on, among other items, the number of shares of the Company outstanding on closing of the Transaction. Accordingly, the warrants will be classified as a derivative liability at fair value through profit or loss until such time as the exercise price is fixed or the warrants are exercised or expired. |
| (ii) | Cresco plans to close an acquisition of MedMar Inc. and its subsidiaries (collectively “MedMar”) by paying $7,231,022 in cash and issuing 14,912.29 PVS. The PVS have been valued using the closing price on the date of the Transaction and adjusted for the 200:1 conversion ratio of PVS into SVS. The number of PVS to be issued will be determined by dividing $15,598,249 by 80% of the Transaction price adjusted for the 200:1 conversion ratio. Note: amounts in tables include another immaterial license purchase transaction of approximately $200,000. |
| (iii) | Cresco plans to close on its investment in PDI and its subsidiaries (collectively “PDI”) by paying $4,839,435 cash and issuing 1,291.47 PVS. The PVS have been valued using the closing price on the date of the transaction and adjusted for the 200:1 conversion ratio of PVS into SVS. The number of PVS to be issued will be determined by dividing $1,350,878 by 80% of the Transaction price adjusted for the 200:1 conversion ratio. |
CRESCO LABS INC.
NOTES TO THE UNAUDITED PRO FORMA CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in US dollars unless otherwise stated)
(Unaudited)
| 5. | Pro forma share capital |
Pro forma share capital as at June 30, 2018 has been determined as follows:
| Cresco Labs LLC Units |
Common Shares | Subordinate Voting Shares (SVS) |
Super Voting Shares (MVS) |
Proportionate Voting Shares (PVS) |
Redeemable Shares | Share Capital ($) | ||||||||||||||||||||||||||
| Cresco issued and outstanding June 30, 2018 |
190,671,490 | — | — | — | — | — | 63,004,118 | |||||||||||||||||||||||||
| Randsburg issued and outstanding July 31, 2018 |
— | 28,273,939 | — | — | — | — | 10,485,298 | |||||||||||||||||||||||||
| Randsburg shares issued for debt |
3(a | ) | — | 138,720,173 | — | — | — | — | 800,425 | |||||||||||||||||||||||
| Randsburg offering |
3(b | ) | — | 43,333,333 | — | — | — | — | 250,037 | |||||||||||||||||||||||
| Elimination of Randsburg equity |
3(c | ) | — | (210,327,445 | ) | — | — | — | — | (11,535,760 | ) | |||||||||||||||||||||
| Deemed issuance of shares to acquire Randsburg |
3(e | ) | — | — | 258,824 | — | — | — | 1,692,555 | |||||||||||||||||||||||
| Acquisitions of non controlling interest |
3(g | ) | 21,705,135 | — | — | — | — | — | 141,938,930 | |||||||||||||||||||||||
| Investment in Nevada |
3(h | ) | 500,000 | — | — | — | — | — | — | |||||||||||||||||||||||
| Class F Equity Financing |
3(i | ) | 27,066,124 | — | — | — | — | — | 100,000,000 | |||||||||||||||||||||||
| NY Acquisition |
3(j | ) | 13,466,667 | — | — | — | — | — | 88,064,152 | |||||||||||||||||||||||
| Reorganization of equity into SVS, MVS, PVS, and Redeemable shares |
3(k | ) | (253,409,416 | ) | — | 8,990,819 | 500,000 | 503,640 | 143,690,687 | — | ||||||||||||||||||||||
| MedMar Acquisition (20% discount) |
3(l | ) | — | — | — | — | 15,101 | — | 19,750,439 | |||||||||||||||||||||||
| PDI Acquisition (20% discount) |
3(m | ) | — | — | — | — | 1,291 | — | 1,689,105 | |||||||||||||||||||||||
| Concurrent financing |
3(n | ) | — | — | 12,624,054 | — | — | — | 81,684,954 | |||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| — | — | 21,873,697 | 500,000 | 520,032.10 | 143,690,687 | 497,824,253 | ||||||||||||||||||||||||||
Exhibit 5.1
INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM’S CONSENT
We consent to the incorporation by reference in this Registration Statement on Form F-10 of Cresco Labs Inc. of our report dated April 28, 2020 included in Form 40-F as filed on January 13, 2021, with respect to our audit of the consolidated financial statements of Cresco Labs Inc. and its subsidiaries as of December 31, 2019 and for the year ended December 31, 2019. Our report on the consolidated financial statements refers to a change in the method of accounting related to the adoption of IFRS 16, Leases, effective January 1, 2019.
/s/ MARCUM LLP
Marcum LLP
Chicago, IL
February 26, 2021
Exhibit 7.1
CRESCO LABS INC.
INDENTURE
Dated as of , 20
[ ]
Trustee
Senior Debt Securities
TABLE OF CONTENTS
| Page | ||||||
| ARTICLE I DEFINITIONS AND INCORPORATION BY REFERENCE | 1 | |||||
| Section 1.1 |
Definitions |
1 | ||||
| Section 1.2 |
Other Definitions |
4 | ||||
| Section 1.3 |
Incorporation by Reference of Trust Indenture Act |
5 | ||||
| Section 1.4 |
Rules of Construction |
5 | ||||
| ARTICLE II THE SECURITIES | 6 | |||||
| Section 2.1 |
Issuable in Series |
6 | ||||
| Section 2.2 |
Establishment of Terms of Series of Securities |
6 | ||||
| Section 2.3 |
Denominations; Provision for Payment |
8 | ||||
| Section 2.4 |
Execution and Authentication |
8 | ||||
| Section 2.5 |
Registrar and Paying Agent |
9 | ||||
| Section 2.6 |
Paying Agent to Hold Money in Trust |
10 | ||||
| Section 2.7 |
Securityholder Lists |
10 | ||||
| Section 2.8 |
Transfer and Exchange |
10 | ||||
| Section 2.9 |
Mutilated, Destroyed, Lost and Stolen Securities |
11 | ||||
| Section 2.10 |
Outstanding Securities |
12 | ||||
| Section 2.11 |
Treasury Securities |
12 | ||||
| Section 2.12 |
Temporary Securities |
13 | ||||
| Section 2.13 |
Cancellation |
13 | ||||
| Section 2.14 |
Defaulted Interest |
13 | ||||
| Section 2.15 |
Global Securities |
13 | ||||
| Section 2.16 |
CUSIP Numbers |
14 | ||||
| ARTICLE III REDEMPTION | 15 | |||||
| Section 3.1 |
Notice to Trustee |
15 | ||||
| Section 3.2 |
Selection of Securities to be Redeemed |
15 | ||||
| Section 3.3 |
Notice of Redemption |
15 | ||||
| Section 3.4 |
Effect of Notice of Redemption |
16 | ||||
| Section 3.5 |
Deposit of Redemption Price |
17 | ||||
| Section 3.6 |
Securities Redeemed in Part |
17 | ||||
| ARTICLE IV COVENANTS | 17 | |||||
| Section 4.1 |
Payment of Principal and Interest |
17 | ||||
| Section 4.2 |
Reports by Company |
17 | ||||
| Section 4.3 |
Compliance Certificate |
18 | ||||
| Section 4.4 |
Stay, Extension and Usury Laws |
18 | ||||
| Section 4.5 |
Corporate Existence |
18 | ||||
-i-
TABLE OF CONTENTS
(continued)
| Page | ||||||
| ARTICLE V SUCCESSORS | 19 | |||||
| Section 5.1 |
Consolidation, Merger and Sale of Assets |
19 | ||||
| Section 5.2 |
Successor Person Substituted |
19 | ||||
| ARTICLE VI DEFAULTS AND REMEDIES | 19 | |||||
| Section 6.1 |
Events of Default |
19 | ||||
| Section 6.2 |
Acceleration of Maturity; Rescission and Annulment |
21 | ||||
| Section 6.3 |
Collection of Indebtedness and Suits for Enforcement by Trustee |
21 | ||||
| Section 6.4 |
Trustee May File Proofs of Claim |
22 | ||||
| Section 6.5 |
Trustee May Enforce Claims Without Possession of Securities |
23 | ||||
| Section 6.6 |
Application of Money Collected |
23 | ||||
| Section 6.7 |
Limitation on Suits |
23 | ||||
| Section 6.8 |
Unconditional Right of Holders to Receive Principal and Interest |
24 | ||||
| Section 6.9 |
Restoration of Rights and Remedies |
24 | ||||
| Section 6.10 |
Rights and Remedies Cumulative |
24 | ||||
| Section 6.11 |
Delay or Omission Not Waiver |
25 | ||||
| Section 6.12 |
Control by Holders |
25 | ||||
| Section 6.13 |
Waiver of Past Defaults |
25 | ||||
| Section 6.14 |
Undertaking for Costs |
26 | ||||
| ARTICLE VII TRUSTEE | 26 | |||||
| Section 7.1 |
Duties of Trustee |
26 | ||||
| Section 7.2 |
Rights of Trustee |
27 | ||||
| Section 7.3 |
Individual Rights of Trustee |
29 | ||||
| Section 7.4 |
Trustee’s Disclaimer |
29 | ||||
| Section 7.5 |
Notice of Defaults |
29 | ||||
| Section 7.6 |
Reports by Trustee to Holders |
29 | ||||
| Section 7.7 |
Compensation and Indemnity |
29 | ||||
| Section 7.8 |
Replacement of Trustee |
30 | ||||
| Section 7.9 |
Successor Trustee by Merger, Etc |
31 | ||||
| Section 7.10 |
Eligibility; Disqualification |
31 | ||||
| Section 7.11 |
Preferential Collection of Claims Against Company |
31 | ||||
| ARTICLE VIII SATISFACTION AND DISCHARGE; DEFEASANCE | 32 | |||||
| Section 8.1 |
Satisfaction and Discharge of Indenture |
32 | ||||
| Section 8.2 |
Application of Trust Funds; Indemnification |
33 | ||||
| Section 8.3 |
Legal Defeasance of Securities of any Series |
33 | ||||
| Section 8.4 |
Covenant Defeasance |
35 | ||||
-ii-
TABLE OF CONTENTS
(continued)
| Page | ||||||
| Section 8.5 |
Repayment to Company |
36 | ||||
| Section 8.6 |
Reinstatement |
36 | ||||
| ARTICLE IX AMENDMENTS AND WAIVERS | 36 | |||||
| Section 9.1 |
Without Consent of Holders |
36 | ||||
| Section 9.2 |
With Consent of Holders |
37 | ||||
| Section 9.3 |
Limitations |
38 | ||||
| Section 9.4 |
Compliance with Trust Indenture Act |
38 | ||||
| Section 9.5 |
Revocation and Effect of Consents |
38 | ||||
| Section 9.6 |
Notation on or Exchange of Securities |
39 | ||||
| Section 9.7 |
Trustee Protected |
39 | ||||
| ARTICLE X MISCELLANEOUS | 39 | |||||
| Section 10.1 |
Trust Indenture Act Controls |
39 | ||||
| Section 10.2 |
Notices |
39 | ||||
| Section 10.3 |
Communication by Holders with Other Holders |
40 | ||||
| Section 10.4 |
Certificate and Opinion as to Conditions Precedent |
40 | ||||
| Section 10.5 |
Statements Required in Certificate or Opinion |
41 | ||||
| Section 10.6 |
Rules by Trustee and Agents |
41 | ||||
| Section 10.7 |
Legal Holidays |
41 | ||||
| Section 10.8 |
No Recourse Against Others |
41 | ||||
| Section 10.9 |
Counterparts |
42 | ||||
| Section 10.10 |
Governing Law; Jury Trial Waiver |
42 | ||||
| Section 10.11 |
No Adverse Interpretation of Other Agreements |
42 | ||||
| Section 10.12 |
Successors |
42 | ||||
| Section 10.13 |
Severability |
42 | ||||
| Section 10.14 |
Table of Contents, Headings, Etc |
42 | ||||
| Section 10.15 |
Securities in a Foreign Currency |
43 | ||||
| Section 10.16 |
Judgment Currency |
43 | ||||
| Section 10.17 |
Force Majeure |
44 | ||||
| ARTICLE XI SINKING FUNDS | 44 | |||||
| Section 11.1 |
Applicability of Article |
44 | ||||
| Section 11.2 |
Satisfaction of Sinking Fund Payments with Securities |
44 | ||||
| Section 11.3 |
Redemption of Securities for Sinking Fund |
45 | ||||
-iii-
CRESCO LABS INC.
Reconciliation and tie between Trust Indenture Act of 1939 and Indenture
dated as of ____________, 20
| § 310(a)(1) | 7.10 | |
| (a)(2) | 7.10 | |
| (a)(3) | Not Applicable | |
| (a)(4) | Not Applicable | |
| (a)(5) | 7.10 | |
| (b) | 7.10 | |
| § 311(a) | 7.11 | |
| (b) | 7.11 | |
| § 312(a) | 2.7 | |
| (b) | 10.3 | |
| (c) | 10.3 | |
| § 313(a) | 7.6 | |
| (b)(1) | 7.6 | |
| (b)(2) | 7.6 | |
| (c)(1) | 7.6 | |
| (d) | 7.6 | |
| § 314(a) | 4.2, 10.5 | |
| (b) | Not Applicable | |
| (c)(1) | 10.4 | |
| (c)(2) | 10.4 | |
| (c)(3) | Not Applicable | |
| (d) | Not Applicable | |
| (e) | 10.5 | |
| (f) | Not Applicable | |
| § 315(a) | 7.1 | |
| (b) | 7.5 | |
| (c) | 7.1 | |
| (d) | 7.1 | |
-iv-
| (e) | 6.14 | |
| § 316(a) | 2.11 | |
| (a)(1)(A) | 6.12 | |
| (a)(1)(B) | 6.13 | |
| (b) | 6.8 | |
| § 317(a)(1) | 6.3 | |
| (a)(2) | 6.4 | |
| (b) | 2.6 | |
| § 318(a) | 10.1 | |
Note: This reconciliation and tie shall not, for any purpose, be deemed to be part of the Indenture.
v
Indenture dated as of __________, 20__, between CRESCO LABS INC., a corporation organized under the laws of British Columbia, Canada (“Company”), and _______________, as trustee (“Trustee”).
Each party agrees as follows for the benefit of the other party and for the equal and ratable benefit of the Holders of the Securities issued under this Indenture.
ARTICLE I
DEFINITIONS AND INCORPORATION BY REFERENCE
Section 1.1 Definitions.
“Affiliate” of any specified person means any other person directly or indirectly controlling or controlled by or under common control with such specified person. For the purposes of this definition, “control” (including, with correlative meanings, the terms “controlled by” and “under common control with”), as used with respect to any person, shall mean the possession, directly or indirectly, of the power to direct or cause the direction of the management or policies of such person, whether through the ownership of voting securities or by agreement or otherwise.
“Agent” means any Registrar or Paying Agent.
“Board of Directors” means the board of directors of the Company or any duly authorized committee thereof.
“Board Resolution” means a copy of a resolution certified by the Secretary or an Assistant Secretary of the Company to have been adopted by the Board of Directors or pursuant to authorization by the Board of Directors and to be in full force and effect on the date of the certification and delivered to the Trustee.
“Business Day” means, for a particular Series, any day except a Saturday, Sunday or any day, including a legal holiday, on which banking institutions are authorized or required by law, regulation or executive order to close in The City of New York (or in connection with any payment, the place of payment).
“Capital Stock” of any person means any and all shares, interests, participations, rights or other equivalents (however designated) of the equity of such person.
“Certificated Securities” means definitive Securities in registered non-global certificated form.
“Company” means the party named as such above until a successor, which duly assumes the obligations under this Indenture, replaces it and thereafter means the successor.
“Company Order” means a written order signed in the name of the Company by an Officer.
“Corporate Trust Office” means the office of the Trustee at which at any particular time its corporate trust business related to this Indenture shall be principally administered, which office at the date hereof is located at __________________,; Attention: _______________, or such other address as the Trustee may designate from time to time by notice to the Holders and the Company, or the corporate trust office of any successor Trustee at which this Indenture shall be administered (or such other address as a successor Trustee may designate from time to time by notice to the Holders of the Company).
“Default” means any event which is, or after notice or passage of time or both would be, an Event of Default.
“Depositary” means, with respect to the Securities of any Series issuable or issued in whole or in part in the form of one or more Global Securities, the person designated as Depositary for such Series by the Company, which Depositary shall be a clearing agency registered under the Exchange Act; and if at any time there is more than one such person, “Depositary” as used with respect to the Securities of any Series shall mean the Depositary with respect to the Securities of such Series.
“Discount Security” means any Security that provides for an amount less than the stated principal amount thereof to be due and payable upon declaration of acceleration of the maturity thereof pursuant to Section 6.2.
“Dollars” and “$” means the currency of The United States of America.
“Exchange Act” means the Securities Exchange Act of 1934, as amended.
“Foreign Currency” means any currency or currency unit issued by a government other than the government of The United States of America.
“Foreign Government Obligations” means, with respect to Securities of any Series that are denominated in a Foreign Currency, direct obligations of, or obligations guaranteed by, the government that issued or caused to be issued such currency for the payment of which obligations its full faith and credit is pledged and which are not callable or redeemable at the option of the issuer thereof.
“GAAP” means accounting principles generally accepted in The United States of America set forth in the opinions and pronouncements of the Accounting Principles Board of the American Institute of Certified Public Accountants and statements and pronouncements of the Financial Accounting Standards Board or in such other statements by such other entity as have been approved by a significant segment of the accounting profession, which are in effect as of the date of determination.
“Global Security” or “Global Securities” means a Security or Securities, as the case may be, in the form established pursuant to Section 2.2 evidencing all or part of a Series of Securities, issued to the Depositary for such Series or its nominee, and registered in the name of such Depositary or nominee.
2
“Holder” or “Securityholder” means a person in whose name a Security is registered on the books of the Registrar.
“Indenture” means this Indenture as amended or supplemented from time to time and shall include the form and terms of particular Series of Securities established as contemplated hereunder.
“interest” means, with respect to any Security, any interest on such Security, and with respect to any Discount Security which by its terms bears interest only after Maturity, interest payable after Maturity.
“Maturity,” when used with respect to any Security, means the date on which the principal of such Security becomes due and payable as therein or herein provided, whether at the Stated Maturity or by declaration of acceleration, call for redemption or otherwise.
“Officer” means the Chairman of the Board of Directors, the Chief Executive Officer, the Chief Financial Officer, the President, any Senior Vice President or Vice President, the Treasurer, Assistant Treasurer, Secretary or Assistant Secretary of the Company.
“Officer’s Certificate” means a certificate signed by any Officer (or any person designated in writing by an Officer of the Company as authorized to execute and deliver Officer’s Certificates) and delivered to the Trustee.
“Opinion of Counsel” means a written opinion of legal counsel. The counsel may be an employee of or counsel to the Company. Opinions of Counsel required to be delivered under this Indenture may have qualifications customary for opinions of the type required.
“person” means any individual, corporation, company, voluntary association, partnership, trust, joint venture, limited liability company, unincorporated organization or government or any agency, instrumentality or political subdivision thereof.
“principal” of a Security means the principal of the Security plus, when appropriate, the premium, if any, on the Security.
“Responsible Officer” means any officer of the Trustee in its Corporate Trust Office having direct responsibility for administration of this Indenture and also means, with respect to a particular corporate trust matter, any other officer to whom any corporate trust matter is referred because of his or her knowledge of and familiarity with a particular subject and who shall have direct responsibility for the administration of this Indenture.
“SEC” means the Securities and Exchange Commission.
“Securities” means the debentures, notes or other debt instruments of the Company of any Series authenticated and delivered under this Indenture.
“Series” or “Series of Securities” means each series of debentures, notes or other debt instruments of the Company created pursuant to Sections 2.1 and 2.2 hereof.
3
“Stated Maturity” when used with respect to any Security, means the date specified in such Security as the fixed date on which the principal of such Security is due and payable.
“Subsidiary” means, with respect to any person, any corporation, partnership, joint venture, limited liability company or other business entity of which a majority of the outstanding shares of Capital Stock or other interests having the power to vote in the election of directors, managers or trustees thereof is at the time directly or indirectly owned or controlled by such person or one or more of the other Subsidiaries of such person, or a combination thereof.
“TIA” means the Trust Indenture Act of 1939 (15 U.S. Code §§ 77aaa-77bbbb) as in effect on the date of this Indenture; provided, however, that in the event the Trust Indenture Act of 1939 is amended after such date, “TIA” means, to the extent required by any such amendment, the Trust Indenture Act as so amended.
“Trustee” means the person named as the “Trustee” in the first paragraph of this instrument until a successor Trustee shall have become such pursuant to the applicable provisions of this Indenture, and thereafter “Trustee” shall mean or include each person who is then a Trustee hereunder, and if at any time there is more than one such person, “Trustee” as used with respect to the Securities of any Series shall mean the Trustee with respect to Securities of that Series.
“United States” or “U.S.” means The United States of America (including the states thereof and the District of Columbia), its territories and possessions and other areas subject to its jurisdiction.
“U.S. Government Obligations” means securities which are direct obligations of, or guaranteed by, The United States of America for the payment of which its full faith and credit is pledged and which are not callable or redeemable at the option of the issuer thereof, and shall also include a depository receipt issued by a bank or trust company as custodian with respect to any such U.S. Government Obligation or a specific payment of interest on or principal of any such U.S. Government Obligation held by such custodian for the account of the holder of a depository receipt, provided that (except as required by law) such custodian is not authorized to make any deduction from the amount payable to the holder of such depository receipt from any amount received by the custodian in respect of the U.S. Government Obligation evidenced by such depository receipt.
Section 1.2 Other Definitions.
| TERM | DEFINED IN SECTION | |||
| “Bankruptcy Law” |
6.1 | |||
| “Custodian” |
6.1 | |||
| “Event of Default” |
6.1 | |||
| “Judgment Currency” |
10.16 | |||
| “Legal Holiday” |
10.7 | |||
4
| “mandatory sinking fund payment” |
11.1 | |||
| “optional sinking fund payment” |
11.1 | |||
| “Paying Agent” |
2.5 | |||
| “Registrar” |
2.5 | |||
| “Required Currency” |
10.16 | |||
| “successor person” |
5.1 |
Section 1.3 Incorporation by Reference of Trust Indenture Act.
Whenever this Indenture refers to a provision of the TIA, the provision is incorporated by reference in and made a part of this Indenture. The following TIA terms used in this Indenture have the following meanings:
“Commission” means the SEC.
“indenture securities” means the Securities.
“indenture security holder” means a Securityholder.
“indenture to be qualified” means this Indenture.
“indenture trustee” or “institutional trustee” means the Trustee.
“obligor” on the indenture securities means the Company and any successor obligor upon the Securities.
All other terms used in this Indenture that are defined by the TIA, defined by TIA reference to another statute or defined by SEC rule under the TIA and not otherwise defined herein are used herein as so defined.
Section 1.4 Rules of Construction.
Unless the context otherwise requires:
(a) a term has the meaning assigned to it;
(b) an accounting term not otherwise defined has the meaning assigned to it in accordance with GAAP;
(c) “or” is not exclusive;
(d) words in the singular include the plural, and in the plural include the singular; and
(e) provisions apply to successive events and transactions.
5
ARTICLE II
THE SECURITIES
Section 2.1 Issuable in Series.
The aggregate principal amount of Securities that may be authenticated and delivered under this Indenture is unlimited. The Securities may be issued in one or more Series. All Securities of a Series shall be identical except as may be set forth or determined in the manner provided in a Board Resolution, supplemental indenture hereto or Officer’s Certificate establishing the terms of such Series. In the case of Securities of a Series to be issued from time to time, the Board Resolution, Officer’s Certificate or supplemental indenture establishing the terms thereof may provide for the method by which specified terms (such as interest rate, maturity date, record date or date from which interest shall accrue) are to be determined. Securities may differ between Series in respect of any matters, provided that all Series of Securities shall be equally and ratably entitled to the benefits of this Indenture.
Section 2.2 Establishment of Terms of Series of Securities.
At or prior to the issuance of any Securities within a Series, the following shall be established (as to the Series generally, in the case of Subsection 2.2.1 and either as to such Securities within the Series or as to the Series generally in the case of Subsections 2.2.2 through 2.2.23) by or pursuant to a Board Resolution, and set forth or determined in the manner provided in a Board Resolution, supplemental indenture hereto or Officer’s Certificate:
2.2.1 the title (which shall distinguish the Securities of that particular Series from the Securities of any other Series) of the Series;
2.2.2 the price or prices (expressed as a percentage of the principal amount thereof) at which the Securities of the Series will be issued;
2.2.3 any limit upon the aggregate principal amount of the Securities of the Series which may be authenticated and delivered under this Indenture (except for Securities authenticated and delivered upon registration of transfer of, or in exchange for, or in lieu of, other Securities of the Series pursuant to Section 2.8, 2.9, 2.12, 3.6 or 9.6);
2.2.4 the date or dates on which the principal of the Securities of the Series is payable;
2.2.5 the rate or rates (which may be fixed or variable) per annum or, if applicable, the method used to determine such rate or rates (including, but not limited to, any commodity, commodity index, stock exchange index or financial index) at which the Securities of the Series shall bear interest, if any, the date or dates from which such interest, if any, shall accrue, the date or dates on which such interest, if any, shall commence and be payable and any regular record date for the interest payable on any interest payment date;
2.2.6 the place or places where the principal of and interest, if any, on the Securities of the Series shall be payable, where the Securities of such Series may be surrendered for registration of transfer or exchange and where notices and demands to or upon the Company in respect of the Securities of such Series and this Indenture may be delivered, and the method of such payment, if by wire transfer, mail or other means;
6
2.2.7 if applicable, the period or periods within which, the price or prices at which and the terms and conditions upon which the Securities of the Series must be redeemed or may be redeemed, in whole or in part, at the option of the Company;
2.2.8 the obligation, if any, of the Company to redeem or purchase the Securities of the Series pursuant to any sinking fund or analogous provisions or at the option of a Holder thereof and the period or periods within which, the price or prices at which and the terms and conditions upon which Securities of the Series shall be redeemed or purchased, in whole or in part, pursuant to such obligation;
2.2.9 the dates, if any, on which and the price or prices at which the Securities of the Series will be repurchased by the Company at the option of the Holders thereof and other detailed terms and provisions of such repurchase obligations;
2.2.10 if other than denominations of $1,000 and integral multiples of $1,000 in excess thereof, the denominations in which the Securities of the Series shall be issuable;
2.2.11 the forms of the Securities of the Series and whether the Securities will be issuable as Global Securities;
2.2.12 if other than the principal amount thereof, the portion of the principal amount of the Securities of the Series that shall be payable upon declaration of acceleration of the maturity thereof pursuant to Section 6.2;
2.2.13 the currency of denomination of the Securities of the Series, which may be Dollars or any Foreign Currency, and if such currency of denomination is a composite currency, the agency or organization, if any, responsible for overseeing such composite currency;
2.2.14 the designation of the currency, currencies or currency units in which payment of the principal of and interest, if any, on the Securities of the Series will be made;
2.2.15 if payments of principal of or interest, if any, on the Securities of the Series are to be made in one or more currencies or currency units other than that or those in which such Securities are denominated, the manner in which the exchange rate with respect to such payments will be determined;
2.2.16 the manner in which the amounts of payment of principal of or interest, if any, on the Securities of the Series will be determined, if such amounts may be determined by reference to an index based on a currency or currencies or by reference to a commodity, commodity index, stock exchange index or financial index;
2.2.17 the provisions, if any, relating to any security provided for the Securities of the Series;
7
2.2.18 any addition to, deletion of or change in the Events of Default which applies to any Securities of the Series and any change in the right of the Trustee or the requisite Holders of such Securities to declare the principal amount thereof due and payable pursuant to Section 6.2;
2.2.19 any addition to, deletion of or change in the covenants set forth in Articles IV or V which applies to Securities of the Series;
2.2.20 any Depositaries, trustees, interest rate calculation agents, exchange rate calculation agents or other agents with respect to Securities of such Series if other than those appointed herein;
2.2.21 the provisions, if any, relating to conversion or exchange of any Securities of such Series, including if applicable, the conversion or exchange price, the conversion or exchange period, the securities or other property into which the Securities will be convertible, provisions as to whether conversion or exchange will be mandatory, at the option of the Holders thereof or at the option of the Company, the events requiring an adjustment of the conversion price or exchange price and provisions affecting conversion or exchange if such Series of Securities are redeemed;
2.2.22 whether any of the Company’s direct or indirect Subsidiaries will guarantee the Securities of that Series, including the terms of subordination, if any, of such guarantees; and
2.2.23 any other terms of the Series (which may supplement, modify or delete any provision of this Indenture insofar as it applies to such Series), including any terms that may be required under applicable law or regulations or advisable in connection with the marketing of Securities of that Series.
All Securities of any one Series need not be issued at the same time and may be issued from time to time, consistent with the terms of this Indenture, if so provided by or pursuant to the Board Resolution, supplemental indenture hereto or Officer’s Certificate referred to above.
Section 2.3 Denominations; Provision for Payment.
The Securities of any Series shall be issuable, except as otherwise provided with respect to Securities of any Series pursuant to Section 2.2, as registered Securities in the denominations of one thousand Dollars ($1,000) or any integral multiples of $1,000 in excess thereof. Unless otherwise provided with respect to Securities of any Series pursuant to Section 2.2, the principal of and the interest on the Securities of any Series, if any, thereon, shall by payable in Dollars at the Corporate Trust Office of the Trustee. Unless otherwise specified pursuant to Section 2.2 with respect to any Securities of any Series, interest on the Securities of any Series shall be computed on the basis of a 360-day year consisting of twelve 30-day months.
Section 2.4 Execution and Authentication.
Two Officers shall sign the Securities for the Company by manual or facsimile signature.
8
If an Officer whose signature is on a Security no longer holds that office at the time the Security is authenticated, the Security shall nevertheless be valid.
A Security shall not be valid until authenticated by the manual signature of the Trustee or an authenticating agent. The signature shall be conclusive evidence that the Security has been authenticated under this Indenture.
The Trustee shall at any time, and from time to time, authenticate Securities for original issue in the principal amount provided in the Board Resolution, supplemental indenture hereto or Officer’s Certificate, upon receipt by the Trustee of a Company Order. Each Security shall be dated the date of its authentication.
The aggregate principal amount of Securities of any Series outstanding at any time may not exceed any limit upon the maximum principal amount for such Series set forth in the Board Resolution, supplemental indenture hereto or Officer’s Certificate delivered pursuant to Section 2.2, except as provided in Section 2.9.
Prior to the issuance of Securities of any Series, the Trustee shall have received and (subject to Section 7.1) shall be fully protected in conclusively relying on: (a) the Board Resolution, supplemental indenture hereto or Officer’s Certificate delivered pursuant to Section 2.2 establishing the form of the Securities of that Series or of Securities within that Series and the terms of the Securities of that Series or of Securities within that Series, (b) an Officer’s Certificate complying with Section 9.7 (with respect to the execution of supplemental indentures) and Section 10.4, and (c) an Opinion of Counsel complying with Section 9.7 (with respect to the execution of supplemental indentures) and Section 10.4.
The Trustee shall have the right, but not the obligation, to decline to authenticate and deliver any Securities of such Series: (a) if the Trustee, being advised by counsel, determines that such action may not be taken lawfully; or (b) if the Trustee in good faith determines that such action would expose the Trustee to personal liability.
The Trustee may appoint an authenticating agent acceptable to the Company to authenticate Securities. An authenticating agent may authenticate Securities whenever the Trustee may do so. Each reference in this Indenture to authentication by the Trustee includes authentication by such agent. An authenticating agent has the same rights as an Agent to deal with the Company or an Affiliate of the Company.
Section 2.5 Registrar and Paying Agent.
The Company shall maintain, with respect to each Series of Securities, at the place or places specified with respect to such Series, an office or agency where Securities of such Series may be presented or surrendered for payment (“Paying Agent”) and where Securities of such Series may be surrendered for registration of transfer or exchange (“Registrar”). The Registrar shall keep a register with respect to each Series of Securities and to their transfer and exchange. The Company will give prompt written notice to the Trustee of the name and address, and any change in the name or address, of each Registrar or Paying Agent. If at any time the Company shall fail to maintain any such required Registrar or Paying Agent or shall fail to furnish the Trustee with the name and address thereof, such presentations and surrenders may be made or served at the Corporate Trust Office of the Trustee, and the Company hereby appoints the Trustee as its agent to receive all such presentations and surrenders.
9
The Company may also from time to time designate one or more co-registrars or additional paying agents and may from time to time rescind such designations; provided, however, that no such designation or rescission shall in any manner relieve the Company of its obligations to maintain a Registrar or Paying Agent in each place so specified for Securities of any Series for such purposes. The Company will give prompt written notice to the Trustee of any such designation or rescission and of any change in the name or address of any such co-registrar or additional paying agent. The term “Registrar” includes any co-registrar; and the term “Paying Agent” includes any additional paying agent. The Company or any of its Affiliates may serve as Registrar or Paying Agent.
The Company hereby appoints the Trustee as the initial Registrar and Paying Agent for each Series unless another Registrar or Paying Agent, as the case may be, is appointed prior to the time Securities of that Series are first issued.
Section 2.6 Paying Agent to Hold Money in Trust.
The Company shall require each Paying Agent other than the Trustee to agree in writing that the Paying Agent will hold in trust, for the benefit of Securityholders of any Series of Securities, or the Trustee, all money held by the Paying Agent for the payment of principal of or interest on the Securities of that Series, and will notify the Trustee in writing of any default by the Company in making any such payment. While any such default continues, the Trustee may require a Paying Agent to pay all money held by it to the Trustee. The Company at any time may require a Paying Agent to pay all money held by it to the Trustee. Upon payment over to the Trustee, the Paying Agent (if other than the Company or a Subsidiary of the Company) shall have no further liability for the money. If the Company or a Subsidiary of the Company acts as Paying Agent, it shall segregate and hold in a separate trust fund for the benefit of Securityholders of any Series of Securities all money held by it as Paying Agent. Upon any bankruptcy, reorganization or similar proceeding with respect to the Company, the Trustee shall serve as Paying Agent for the Securities.
Section 2.7 Securityholder Lists.
The Trustee shall preserve in as current a form as is reasonably practicable the most recent list available to it of the names and addresses of Securityholders of each Series of Securities and shall otherwise comply with TIA § 312(a). If the Trustee is not the Registrar, the Company shall furnish to the Trustee at least ten days before each interest payment date and at such other times as the Trustee may request in writing a list, in such form and as of such date as the Trustee may reasonably require, of the names and addresses of Securityholders of each Series of Securities.
Section 2.8 Transfer and Exchange.
Where Securities of a Series are presented to the Registrar or a co-registrar with a request to register a transfer or to exchange them for an equal principal amount of Securities of the same Series, the Registrar shall register the transfer or make the exchange if its requirements for such
10
transactions are met. To permit registrations of transfers and exchanges, the Trustee shall authenticate Securities at the Registrar’s request. No service charge shall be made for any registration of transfer or exchange (except as otherwise expressly permitted herein), but the Company may require payment of a sum sufficient to cover any transfer tax or similar governmental charge payable in connection therewith (other than any such transfer tax or similar governmental charge payable upon exchanges pursuant to Sections 2.12, 3.6 or 9.6).
Neither the Company nor the Registrar shall be required (a) to issue, register the transfer of, or exchange Securities of any Series for the period beginning at the opening of business fifteen days immediately preceding the mailing of a notice of redemption of Securities of that Series selected for redemption and ending at the close of business on the day of such mailing, or (b) to register the transfer of or exchange Securities of any Series selected, called or being called for redemption as a whole or the portion being redeemed of any such Securities selected, called or being called for redemption in part.
Section 2.9 Mutilated, Destroyed, Lost and Stolen Securities.
If any mutilated Security is surrendered to the Trustee, the Company shall execute and the Trustee shall authenticate and deliver in exchange therefor a new Security of the same Series and of like tenor and principal amount and bearing a number not contemporaneously outstanding.
If there shall be delivered to the Company and the Trustee (a) evidence to their satisfaction of the destruction, loss or theft of any Security and (b) such security or indemnity bond as may be required by each of them to hold itself and any of its agents harmless, then, in the absence of written notice to the Company or the Trustee that such Security has been acquired by a bona fide purchaser, the Company shall execute and upon receipt of a Company Order the Trustee shall authenticate and make available for delivery, in lieu of any such destroyed, lost or stolen Security, a new Security of the same Series and of like tenor and principal amount and bearing a number not contemporaneously outstanding.
In case any such mutilated, destroyed, lost or stolen Security has become or is about to become due and payable, the Company in its discretion may, instead of issuing a new Security, pay such Security.
Upon the issuance of any new Security under this Section 2.9, the Company may require the payment of a sum sufficient to cover any tax or other governmental charge that may be imposed in relation thereto and any other expenses (including the fees and expenses of the Trustee) connected therewith.
Every new Security of any Series issued pursuant to this Section 2.9 in lieu of any destroyed, lost or stolen Security shall constitute an original additional contractual obligation of the Company, whether or not the destroyed, lost or stolen Security shall be at any time enforceable by anyone, and shall be entitled to all the benefits of this Indenture equally and proportionately with any and all other Securities of that Series duly issued hereunder.
11
The provisions of this Section 2.9 are exclusive and shall preclude (to the extent lawful) all other rights and remedies with respect to the replacement or payment of mutilated, destroyed, lost or stolen Securities.
Section 2.10 Outstanding Securities.
The Securities outstanding at any time are all the Securities authenticated by the Trustee except for those canceled by the Registrar and those described in this Section 2.10 as not outstanding.
If a Security is replaced pursuant to Section 2.9, it ceases to be outstanding unless the Trustee receives proof satisfactory to it that the replaced Security is held by a bona fide purchaser.
If the Paying Agent (other than the Company, a Subsidiary of the Company or an Affiliate of the Company) holds on the Maturity of Securities of a Series money sufficient to pay such Securities payable on that date, then on and after that date such Securities of the Series cease to be outstanding and interest on them ceases to accrue.
The Company may purchase or otherwise acquire the Securities, whether by open market purchases, negotiated transactions or otherwise. A Security does not cease to be outstanding because the Company or an Affiliate of the Company holds the Security.
In determining whether the Holders of the requisite principal amount of outstanding Securities have given any request, demand, authorization, direction, notice, consent or waiver hereunder, the principal amount of a Discount Security that shall be deemed to be outstanding for such purposes shall be the amount of the principal thereof that would be due and payable as of the date of such determination upon a declaration of acceleration of the Maturity thereof pursuant to Section 6.2.
Section 2.11 Treasury Securities.
In determining whether the Holders of the required principal amount of Securities of a Series have concurred in any request, demand, authorization, direction, notice, consent or waiver, Securities of a Series owned by the Company or any Affiliate of the Company shall be disregarded, except that for the purposes of determining whether the Trustee shall be protected in conclusively relying on any such request, demand, authorization, direction, notice, consent or waiver, only Securities of a Series that a Responsible Officer of the Trustee actually knows are so owned shall be so disregarded. Securities so owned which have been pledged in good faith shall not be disregarded if the pledgee establishes to the satisfaction of the Trustee the pledgee’s right to deliver any such request, demand, authorization, direction, notice, consent or waiver with respect to the Securities and that the pledgee is not the Company or any other obligor upon the Securities or any Affiliate of the Company or of such other obligor.
12
Section 2.12 Temporary Securities.
Until definitive Securities are ready for delivery, the Company may prepare and the Trustee shall authenticate temporary Securities upon a Company Order. Temporary Securities shall be substantially in the form of definitive Securities but may have variations that the Company considers appropriate for temporary Securities. Without unreasonable delay, the Company shall prepare and the Trustee upon receipt of a Company Order shall authenticate definitive Securities of the same Series and date of maturity in exchange for temporary Securities. Until so exchanged, temporary Securities shall have the same rights under this Indenture as the definitive Securities.
Section 2.13 Cancellation.
The Company at any time may deliver Securities to the Trustee for cancellation. The Registrar and the Paying Agent, if not the Trustee, shall forward to the Trustee any Securities surrendered to them for registration of transfer, exchange or payment. The Trustee shall cancel all Securities surrendered for transfer, exchange, payment, replacement, conversion or cancellation and shall dispose of such canceled Securities (subject to the record retention requirement of the Exchange Act and the Trustee) in accordance with its customary procedures and deliver a certificate of such cancellation to the Company upon written request of the Company. The Company may not issue new Securities to replace Securities that it has paid or delivered to the Trustee for cancellation.
Section 2.14 Defaulted Interest.
If the Company defaults in a payment of interest on a Series of Securities, it may pay the defaulted interest, plus, to the extent permitted by law, any interest payable on the defaulted interest, to the persons who are Securityholders of the Series on a subsequent special record date. The Company shall fix the record date and payment date. At least 10 days before the special record date, the Company shall mail to the Trustee and to each Securityholder of the Series a notice that states the special record date, the payment date and the amount of interest to be paid. The Company may pay defaulted interest in any other lawful manner.
Section 2.15 Global Securities.
2.15.1 Terms of Securities. A Board Resolution, a supplemental indenture hereto or an Officer’s Certificate shall establish whether the Securities of a Series shall be issued in whole or in part in the form of one or more Global Securities and the Depositary for such Global Security or Securities.
2.15.2 Transfer and Exchange. Notwithstanding any provisions to the contrary contained in Section 2.8 of this Indenture and in addition thereto, any Global Security shall be exchangeable pursuant to Section 2.8 of this Indenture for Securities registered in the names of Holders other than the Depositary for such Security or its nominee only if (a) such Depositary notifies the Company that it is unwilling or unable to continue as Depositary for such Global Security or if at any time such Depositary ceases to be a clearing agency registered under the Exchange Act, and, in either case, the Company fails to appoint a successor Depositary registered as a clearing agency under the Exchange Act within 90 days of such event or (b) the Company determines in its sole discretion not to have such Securities represented by one or more Global Securities and executes and delivers to the Trustee an Officer’s Certificate to the effect that such Global Security shall be so exchangeable. Any Global Security that is exchangeable pursuant to the preceding sentence shall be exchangeable for Securities registered in such names as the Depositary shall direct in writing in an aggregate principal amount equal to the principal amount of the Global Security with like tenor and terms.
13
Except as provided in this Section 2.15.2, a Global Security may not be transferred except as a whole by the Depositary with respect to such Global Security to a nominee of such Depositary, by a nominee of such Depositary to such Depositary or another nominee of such Depositary or by the Depositary or any such nominee to a successor Depositary or a nominee of such a successor Depositary.
2.15.3 Legend. Any Global Security issued hereunder shall bear a legend in substantially the following form:
“THIS SECURITY IS A GLOBAL SECURITY WITHIN THE MEANING OF THE INDENTURE HEREINAFTER REFERRED TO AND IS REGISTERED IN THE NAME OF THE DEPOSITARY OR A NOMINEE OF THE DEPOSITARY. THIS SECURITY IS EXCHANGEABLE FOR SECURITIES REGISTERED IN THE NAME OF A PERSON OTHER THAN THE DEPOSITARY OR ITS NOMINEE ONLY IN THE LIMITED CIRCUMSTANCES DESCRIBED IN THE INDENTURE, AND MAY NOT BE TRANSFERRED EXCEPT AS A WHOLE BY THE DEPOSITARY TO A NOMINEE OF THE DEPOSITARY, BY A NOMINEE OF THE DEPOSITARY TO THE DEPOSITARY OR ANOTHER NOMINEE OF THE DEPOSITARY, OR BY THE DEPOSITARY OR ANY SUCH NOMINEE TO A SUCCESSOR DEPOSITARY OR A NOMINEE OF SUCH A SUCCESSOR DEPOSITARY.”
2.15.4 Acts of Holders. The Depositary, as a Holder, may appoint agents and otherwise authorize participants to give or take any request, demand, authorization, direction, notice, consent, waiver or other action which a Holder is entitled to give or take under this Indenture.
2.15.5 Payments. Notwithstanding the other provisions of this Indenture, unless otherwise specified as contemplated by Section 2.2, payment of the principal of and interest, if any, on any Global Security shall be made to the Holder thereof, which in the case of a Depositary therefor will be made in accordance with its applicable procedures.
2.15.6 Consents, Declaration and Directions. The Company, the Trustee and any Agent shall treat a person as the Holder of such principal amount of outstanding Securities of such Series represented by a Global Security as shall be specified in a written statement of the Depositary or by the applicable procedures of such Depositary with respect to such Global Security, for purposes of obtaining any consents, declarations, waivers or directions required to be given by the Holders pursuant to this Indenture.
Section 2.16 CUSIP Numbers.
The Company in issuing the Securities may use “CUSIP” numbers (if then generally in use), and, if so, the Trustee shall use “CUSIP” numbers in notices of redemption as a convenience to Holders; provided that any such notice may state that no representation is made as to the correctness of such numbers either as printed on the Securities or as contained in any
14
notice of a redemption and that reliance may be placed only on the other elements of identification printed on the Securities, and any such redemption shall not be affected by any defect in or omission of such numbers. The Trustee shall have no liability for any defect in the “CUSIP” numbers as they appear on any Security, notice or elsewhere. The Company will promptly notify the Trustee in writing of any change in the “CUSIP” numbers.
ARTICLE III
REDEMPTION
Section 3.1 Notice to Trustee.
The Company may, with respect to any Series of Securities, reserve the right to redeem and pay the Series of Securities or may covenant to redeem and pay the Series of Securities or any part thereof prior to the Stated Maturity thereof at such time and on such terms as provided for in such Securities. If a Series of Securities is redeemable and the Company wants or is obligated to redeem prior to the Stated Maturity thereof all or part of the Series of Securities pursuant to the terms of such Securities, it shall notify the Trustee in writing of the redemption date and the principal amount of Series of Securities to be redeemed. The Company shall give the notice to the Trustee at least 45 days before the redemption date, unless a shorter period is satisfactory to the Trustee.
Section 3.2 Selection of Securities to be Redeemed.
Unless otherwise indicated for a particular Series by a Board Resolution, a supplemental indenture hereto or an Officer’s Certificate, if less than all the Securities of a Series are to be redeemed, the Trustee shall select the Securities of the Series to be redeemed in any manner that the Trustee deems fair and appropriate, including selecting by lot or other method, unless otherwise required by law or applicable stock exchange requirements, subject, in the case of Global Securities, to the applicable rules and procedures of the Depositary; provided that the unredeemed portion of the principal amount of any Security shall be in an authorized denomination (which shall not be less than the minimum authorized denomination) for such Security. The Trustee shall make the selection from Securities of the Series outstanding not previously called for redemption. Provisions of this Indenture that apply to Securities of a Series called for redemption also apply to portions of Securities of that Series called for redemption.
Section 3.3 Notice of Redemption.
Unless otherwise indicated for a particular Series by Board Resolution, a supplemental indenture hereto or an Officer’s Certificate, at least 30 days but not more than 60 days before a redemption date, the Company shall mail a notice of redemption by first-class mail to each Holder whose Securities are to be redeemed.
The notice shall identify the Securities of the Series to be redeemed and shall state:
(a) the redemption date;
(b) the redemption price and the amount of accrued interest, if any, to be paid;
15
(c) the name and address of the Paying Agent and, if applicable, the conversion Agent;
(d) for convertible Securities, the conversion price;
(e) if any Global Security is being redeemed in part, the portion of the principal amount of such Global Security to be redeemed and that, after the redemption date upon surrender of such Global Security, the principal amount thereof will be decreased by the portion thereof redeemed pursuant thereto;
(f) if any Certificated Security is being redeemed in part, the portion of the principal amount of such Security to be redeemed, and that, after the redemption date, upon surrender of such Security, a new Certificated Security in principal amount equal to the unredeemed portion thereof will be issued in the name of the Holder thereof upon cancellation of the original Certificated Security;
(g) that Securities of the Series (or portion thereof) called for redemption must be surrendered to the Paying Agent to collect the redemption price;
(h) that interest on Securities of the Series called for redemption ceases to accrue on and after the redemption date unless the Company defaults in the deposit of the redemption price;
(i) the CUSIP number, if any, and state that no representation is made as to the correctness or accuracy of the CUSIP number, if any, listed in the SEC’s notice or printed on the Securities; and
(j) any other information as may be required by the terms of the particular Series or the Securities of a Series being redeemed.
At the Company’s request, the Trustee shall give the notice of redemption in the Company’s name and at its expense, provided, however, that the Company has delivered to the Trustee, at least 15 days (unless a shorter time shall be acceptable to the Trustee) prior to the notice date, an Officer’s Certificate requesting that the Trustee give such notice and setting forth the information to be stated in such notice.
Section 3.4 Effect of Notice of Redemption.
Once notice of redemption is mailed as provided in Section 3.3, Securities of a Series called for redemption become due and payable on the redemption date and at the redemption price. Except as otherwise provided in the supplemental indenture, Board Resolution or Officer’s Certificate for a Series, a notice of redemption may not be conditional. Upon surrender to the Paying Agent, such Securities shall be paid at the redemption price plus accrued interest to the redemption date other than Securities or portions of Securities called for redemption which have been delivered by the Company to the Registrar for cancellation. The Paying Agent shall return to the Company any money not required for that purpose.
16
Unless the Company shall default in the payment of Securities (and accrued interest) called for redemption, interest on such Securities shall cease to accrue after the redemption date. Convertible Securities called for redemption shall cease to be convertible after the close of business on the Business Day immediately preceding the redemption date, unless the Company shall default in the payment of such Securities on the redemption date, in which event the Securities shall remain convertible until paid (together with accrued interest).
Failure to give notice of redemption, or any defect in such notice to the Holder of any Security of a Series designated for redemption, in whole or in part, shall not affect the sufficiency of any notice of redemption with respect to the Holder of any other Security of such Series.
Section 3.5 Deposit of Redemption Price.
On or before 10:00 a.m., New York City time, on the redemption date, the Company shall deposit with the Paying Agent money sufficient to pay the redemption price of and accrued interest, if any, on all Securities to be redeemed on that date.
Section 3.6 Securities Redeemed in Part.
Upon surrender of a Certificated Security that is redeemed in part, the Trustee shall authenticate for the Holder a new Certificated Security of the same Series and the same maturity equal in principal amount to the unredeemed portion of the Security surrendered and concurrently cancel the surrendered Certificated Security.
ARTICLE IV
COVENANTS
Section 4.1 Payment of Principal and Interest.
The Company covenants and agrees for the benefit of the Holders of each Series of Securities that it will duly and punctually pay the principal of and interest, if any, on the Securities of that Series in accordance with the terms of such Securities and this Indenture. On or before 10:00 a.m., New York City time, on the applicable payment date, the Company shall deposit with the Paying Agent money sufficient to pay the principal of and interest, if any, on the Securities of each Series in accordance with the terms of such Securities and this Indenture. Principal and interest shall be considered paid on the date due if the Paying Agent holds in accordance with this Indenture on that date money sufficient to pay all principal and interest then due and the Paying Agent is not prohibited from paying such money to the Holders on such date pursuant to the terms of this Indenture.
Section 4.2 Reports by Company.
(a) As long as any Securities are outstanding, the Company shall file with the Trustee, and transmit to the Holders, such information, documents and other reports, and such summaries thereof, as may be required pursuant to TIA § 314(a). All reports, information and documents referred to in this Section 4.2 will be deemed to be filed with the Trustee and transmitted to the Holders at the time such reports, information or documents are publicly filed with the SEC via the SEC’s EDGAR filing system (or any successor system), it being understood that the Trustee shall have no responsibility whatsoever to determine if such filings have been made.
17
(b) Delivery of reports, information and documents to the Trustee under this Section 4.2 are for informational purposes only and shall not constitute a representation or warranty as to the accuracy or completeness of the reports, information and documents. The Trustee’s receipt of the foregoing shall not constitute constructive notice of any information contained therein or determinable from information contained therein, including the Company’s compliance with any of its covenants hereunder (as to which the Trustee is entitled to rely exclusively on Officer’s Certificates).
Section 4.3 Compliance Certificate.
To the extent any Securities of a Series are outstanding, the Company shall deliver to the Trustee, within 120 days after the end of each fiscal year of the Company, an Officer’s Certificate (which need not contain the statements provided for in Section 10.4) from its principal executive officer, principal financial officer or principal accounting officer stating that a review of the activities of the Company and its Subsidiaries during the preceding fiscal year has been made under the supervision of the signing Officer with a view to determining whether the Company has kept, observed, performed and fulfilled its obligations under this Indenture, and further stating, as to such Officer signing such certificate, that to his or her knowledge the Company is not in default in the performance or observance of any of the terms, provisions and conditions hereof (or, if a Default or Event of Default shall have occurred, describing all such Defaults or Events of Default of which the Officer has knowledge). Such Officer’s Certificate need not include a reference to any non-compliance that has been fully cured prior to the date as of which such certificate speaks.
Section 4.4 Stay, Extension and Usury Laws.
The Company covenants (to the extent that it may lawfully do so) that it will not at any time insist upon, plead, or in any manner whatsoever claim or take the benefit or advantage of, any stay, extension or usury law wherever enacted, now or at any time hereafter in force, which may affect the covenants or the performance of this Indenture or the Securities; and the Company (to the extent it may lawfully do so) hereby expressly waives all benefit or advantage of any such law and covenants that it will not, by resort to any such law, hinder, delay or impede the execution of any power herein granted to the Trustee, but will suffer and permit the execution of every such power as though no such law has been enacted.
Section 4.5 Corporate Existence.
Subject to Article V, the Company will do or cause to be done all things necessary to preserve and keep in full force and effect its corporate existence and rights (charter and statutory); provided, however, that the Company shall not be required to preserve any such right if the Board of Directors shall determine that the preservation thereof is no longer desirable in the conduct of the business of the Company and its Subsidiaries taken as a whole and that the loss thereof is not adverse in any material respect to the Holders.
18
ARTICLE V
SUCCESSORS
Section 5.1 Consolidation, Merger and Sale of Assets.
The Company may not consolidate with or merge with or into, sell, convey, transfer or dispose of all or substantially all of its assets to any other person (a “successor person”), whether in one transaction or a series of related transactions, unless:
(a) (i) the Company is the surviving corporation or (ii) the successor person (if other than the Company) (A) is a corporation, limited liability corporation, partnership or trust organized under the laws of the United States; and (B) expressly assumes, by an indenture supplemental hereto, the Company’s obligations on the Securities and under this Indenture; and
(b) immediately after giving effect to the transaction, no Default or Event of Default shall have happened and be continuing.
The Company shall deliver to the Trustee prior to the consummation of the proposed transaction an Officer’s Certificate to the foregoing effect and an Opinion of Counsel stating that the proposed transaction and any supplemental indenture comply with Section 5.1 of this Indenture.
Notwithstanding the above, any Subsidiary of the Company may consolidate with, merge into or transfer all or part of its properties to the Company. Neither an Officer’s Certificate nor an Opinion of Counsel shall be required to be delivered in connection therewith.
Section 5.2 Successor Person Substituted.
Upon any consolidation or merger, or any sale, conveyance, transfer, or lease of all or substantially all of the assets of the Company and its Subsidiaries in accordance with Section 5.1, the successor person formed by such consolidation or into or with which the Company is merged or to which such sale, conveyance, transfer, or lease is made shall succeed to, and be substituted for, and may exercise every right and power of, the Company under this Indenture and the Securities with the same effect as if such successor person has been named as the Company herein; and, thereafter, the predecessor Company, in the case of a sale, conveyance or transfer (other than a lease), shall be released from all obligations and covenants under this Indenture and the Securities.
ARTICLE VI
DEFAULTS AND REMEDIES
Section 6.1 Events of Default.
“Event of Default,” wherever used herein with respect to Securities of any Series, means any one of the following events, unless in the establishing Board Resolution, supplemental indenture or Officer’s Certificate, it is provided that such Series shall not have the benefit of said Event of Default:
19
(a) failure to pay any interest on any Security of that Series when it becomes due and payable, and continuance of such default for a period of 30 days (unless the entire amount of such payment is deposited by the Company with the Trustee or with a Paying Agent prior to 10:00 a.m., New York City time, on the 30th day of such period);
(b) failure to pay principal of any Security of that Series at its Maturity;
(c) default in the performance or breach of any covenant of the Company in this Indenture (other than defaults pursuant to sub-clauses (a) through (c) above or defaults related to a covenant that has been included in this Indenture solely for the benefit of a Series of Securities other than that Series), which default continues uncured for a period of 90 days after there has been given, by registered or certified mail, to the Company by the Trustee or to the Company and the Trustee by the Holders of at least 25% in principal amount of the outstanding Securities of that Series a written notice specifying such default or breach and requiring it to be remedied and stating that such notice is a “Notice of Default” hereunder;
(d) the Company pursuant to or within the meaning of any Bankruptcy Law:
(i) commences a voluntary case,
(ii) consents to the entry of an order for relief against it in an involuntary case,
(iii) consents to the appointment of a Custodian of it or for all or substantially all of its property, or
(iv) makes a general assignment for the benefit of its creditors;
(e) a court of competent jurisdiction enters an order or decree under any Bankruptcy Law that:
(i) is for relief against the Company in an involuntary case,
(ii) appoints a Custodian of the Company or for all or substantially all of its property, or
(iii) orders the liquidation of the Company, and the order or decree remains unstayed and in effect for 60 days; or
(f) any other Event of Default provided with respect to Securities of that Series, which is specified in a Board Resolution, a supplemental indenture hereto or an Officer’s Certificate, in accordance with Section 2.2.18.
The term “Bankruptcy Law” means title 11, U.S. Code or any similar federal or state law for the relief of debtors. The term “Custodian” means any receiver, trustee, assignee, liquidator or similar official under any Bankruptcy Law.
20
A Default under one Series of Securities issued under this Indenture will not necessarily be a default under another Series of Securities under this Indenture.
The Company will, so long as any of the Securities are outstanding, deliver to the Trustee, within 30 days of becoming aware of any Default or Event of Default, an Officer’s Certificate specifying such Default or Event of Default and what action the Company is taking or proposes to take with respect thereto.
Section 6.2 Acceleration of Maturity; Rescission and Annulment.
If an Event of Default with respect to Securities of any Series at the time outstanding occurs and is continuing (other than an Event of Default referred to in Section 6.1(d) or (e)) then in every such case the Trustee or the Holders of not less than 25% in principal amount of the outstanding Securities of that Series may declare the principal amount (or, if any Securities of that Series are Discount Securities, such portion of the principal amount as may be specified in the terms of such Securities) of and accrued and unpaid interest, if any, on all of the Securities of that Series to be due and payable immediately, by a notice in writing to the Company (and to the Trustee if given by Holders), and upon any such declaration such principal amount (or specified amount) and accrued and unpaid interest, if any, shall become immediately due and payable. If an Event of Default specified in Section 6.1(d) or (e) shall occur, the principal amount (or specified amount) of and accrued and unpaid interest, if any, on all outstanding Securities shall ipso facto become and be immediately due and payable without any declaration or other act on the part of the Trustee or any Holder.
At any time after such a declaration of acceleration with respect to any Series has been made and before a judgment or decree for payment of the money due has been obtained by the Trustee as hereinafter in this Article provided, the Holders of a majority in principal amount of the outstanding Securities of that Series, by written notice to the Company and the Trustee, may rescind and annul such declaration and its consequences if all Events of Default with respect to Securities of that Series, other than the non-payment of the principal and interest, if any, of Securities of that Series which have become due solely by such declaration of acceleration, have been cured or waived as provided in Section 6.13.
No such rescission shall affect any subsequent Default.
Section 6.3 Collection of Indebtedness and Suits for Enforcement by Trustee.
The Company covenants that if
(a) default is made in the payment of any interest on any Security when such interest becomes due and payable and such default continues for a period of 30 days, or
(b) default is made in the payment of principal of any Security at the Maturity thereof, or
(c) default is made in the deposit of any sinking fund payment, if any, when and as due by the terms of a Security,
21
then, the Company will, upon demand of the Trustee, pay to it, for the benefit of the Holders of such Securities, the whole amount then due and payable on such Securities for principal and interest and, to the extent that payment of such interest shall be legally enforceable, interest on any overdue principal and any overdue interest at the rate or rates prescribed therefor in such Securities, and, in addition thereto, such further amount as shall be sufficient to cover the costs and expenses of collection, including the reasonable compensation, expenses, disbursements and advances of the Trustee, its agents and counsel.
If the Company fails to pay such amounts forthwith upon such demand, the Trustee, in its own name and as trustee of an express trust, may institute a judicial proceeding for the collection of the sums so due and unpaid, may prosecute such proceeding to judgment or final decree and may enforce the same against the Company or any other obligor upon such Securities and collect the moneys adjudged or deemed to be payable in the manner provided by law out of the property of the Company or any other obligor upon such Securities, wherever situated.
If an Event of Default with respect to any Securities of any Series occurs and is continuing, the Trustee may in its discretion proceed to protect and enforce its rights and the rights of the Holders of Securities of such Series by such appropriate judicial proceedings as the Trustee shall deem most effectual to protect and enforce any such rights, whether for the specific enforcement of any covenant or agreement in this Indenture or in aid of the exercise of any power granted herein, or to enforce any other proper remedy.
Section 6.4 Trustee May File Proofs of Claim.
In case of the pendency of any receivership, insolvency, liquidation, bankruptcy, reorganization, arrangement, adjustment, composition or other judicial proceeding relating to the Company or any other obligor upon the Securities or the property of the Company or of such other obligor or their creditors, the Trustee (irrespective of whether the principal of the Securities shall then be due and payable as therein expressed or by declaration or otherwise and irrespective of whether the Trustee shall have made any demand on the Company for the payment of overdue principal or interest) shall be entitled and empowered, by intervention in such proceeding or otherwise,
(a) to file and prove a claim for the whole amount of principal and interest owing and unpaid in respect of the Securities and to file such other papers or documents as may be necessary or advisable in order to have the claims of the Trustee (including any claim for the reasonable compensation, expenses, disbursements and advances of the Trustee, its agents and counsel) and of the Holders allowed in such judicial proceeding, and
(b) to collect and receive any moneys or other property payable or deliverable on any such claims and to distribute the same,
and any custodian, receiver, assignee, trustee, liquidator, sequestrator or other similar official in any such judicial proceeding is hereby authorized by each Holder to make such payments to the Trustee and, in the event that the Trustee shall consent to the making of such payments directly to the Holders, to pay to the Trustee any amount due it for the reasonable compensation, expenses, disbursements and advances of the Trustee, its agents and counsel, and any other amounts due the Trustee under Section 7.7.
22
Nothing herein contained shall be deemed to authorize the Trustee to authorize or consent to or accept or adopt on behalf of any Holder any plan of reorganization, arrangement, adjustment or composition affecting the Securities or the rights of any Holder thereof or to authorize the Trustee to vote in respect of the claim of any Holder in any such proceeding.
Section 6.5 Trustee May Enforce Claims Without Possession of Securities.
All rights of action and claims under this Indenture or the Securities may be prosecuted and enforced by the Trustee without the possession of any of the Securities or the production thereof in any proceeding relating thereto, and any such proceeding instituted by the Trustee shall be brought in its own name as trustee of an express trust, and any recovery of judgment shall, after provision for the payment of the reasonable compensation, expenses, disbursements and advances of the Trustee, its agents and counsel, be for the ratable benefit of the Holders of the Securities in respect of which such judgment has been recovered.
Section 6.6 Application of Money Collected.
Any money or property collected by the Trustee pursuant to this Article shall be applied in the following order, at the date or dates fixed by the Trustee and, in case of the distribution of such money or property on account of principal or interest, upon presentation of the Securities and the notation thereon of the payment if only partially paid and upon surrender thereof if fully paid:
First: To the payment of all amounts due to the Trustee under this Indenture; and
Second: To the payment of the amounts then due and unpaid for principal of and interest on the Securities in respect of which or for the benefit of which such money has been collected, ratably, without preference or priority of any kind, according to the amounts due and payable on such Securities for principal and interest, respectively; and
Third: To the Company.
Section 6.7 Limitation on Suits.
No Holder of any Security of any Series shall have any right to institute any proceeding, judicial or otherwise, with respect to this Indenture, or for the appointment of a receiver or trustee, or for any other remedy hereunder, unless:
(a) such Holder has previously given written notice to the Trustee of a continuing Event of Default with respect to the Securities of that Series;
(b) the Holders of not less than 25% in principal amount of the outstanding Securities of that Series have made written request to the Trustee to institute proceedings in respect of such Event of Default in its own name as Trustee hereunder;
23
(c) such Holder or Holders have offered to the Trustee indemnity or security satisfactory to the Trustee against the costs, expenses and liabilities which might be incurred by the Trustee in compliance with such request;
(d) the Trustee has failed to institute any such proceeding for 60 days after its receipt of such notice, request and offer of indemnity; and
(e) no direction inconsistent with such written request has been given to the Trustee during such 60-day period by the Holders of a majority in principal amount of the outstanding Securities of that Series;
it being understood, intended and expressly covenanted by the Holder of every Security with every other Holder and the Trustee that no one or more of such Holders shall have any right in any manner whatever by virtue of, or by availing of, any provision of this Indenture to affect, disturb or prejudice the rights of any other of such Holders, or to obtain or to seek to obtain priority or preference over any other of such Holders or to enforce any right under this Indenture, except in the manner herein provided and for the equal and ratable benefit of all such Holders of the applicable Series; provided, however, that the Trustee does not have an affirmative duty to ascertain whether or not such actions or forbearances are unduly prejudicial to such Holders.
Section 6.8 Unconditional Right of Holders to Receive Principal and Interest.
Notwithstanding any other provision in this Indenture, the Holder of any Security has the right, which is absolute and unconditional, to receive payment of the principal of and interest, if any, on such Security on the Maturity of such Security, including the Stated Maturity expressed in such Security (or, in the case of redemption, on the redemption date) and to institute suit for the enforcement of any such payment, and such rights shall not be impaired without the consent of such Holder.
Section 6.9 Restoration of Rights and Remedies.
If the Trustee or any Holder has instituted any proceeding to enforce any right or remedy under this Indenture and such proceeding has been discontinued or abandoned for any reason, or has been determined adversely to the Trustee or to such Holder, then and in every such case, subject to any determination in such proceeding, the Company, the Trustee and the Holders shall be restored severally and respectively to their former positions hereunder and thereafter all rights and remedies of the Trustee and the Holders shall continue as though no such proceeding had been instituted.
Section 6.10 Rights and Remedies Cumulative.
Except as otherwise provided with respect to the replacement or payment of mutilated, destroyed, lost or stolen Securities in Section 2.9, no right or remedy herein conferred upon or reserved to the Trustee or to the Holders is intended to be exclusive of any other right or remedy, and every right and remedy shall, to the extent permitted by law, be cumulative and in addition to every other right and remedy given hereunder or now or hereafter existing at law or in equity or otherwise. The assertion or employment of any right or remedy hereunder, or otherwise, shall not, to the extent permitted by law, prevent the concurrent assertion or employment of any other appropriate right or remedy.
24
Section 6.11 Delay or Omission Not Waiver.
No delay or omission of the Trustee or of any Holder of any Securities to exercise any right or remedy accruing upon any Event of Default shall impair any such right or remedy or constitute a waiver of any such Event of Default or an acquiescence therein. Every right and remedy given by this Article or by law to the Trustee or to the Holders may be exercised from time to time, and as often as may be deemed expedient, by the Trustee or by the Holders, as the case may be.
Section 6.12 Control by Holders.
The Holders of a majority in principal amount of the outstanding Securities of any Series shall have the right to direct the time, method and place of conducting any proceeding for any remedy available to the Trustee, or exercising any trust or power conferred on the Trustee, with respect to the Securities of such Series, provided that:
(a) such direction shall not be in conflict with any rule of law or with this Indenture;
(b) the Trustee may take any other action deemed proper by the Trustee which is not inconsistent with such direction;
(c) subject to the provisions of Section 7.1, the Trustee shall have the right to decline to follow any such direction if the Trustee in good faith shall, by a Responsible Officer of the Trustee, determine that the proceeding so directed would involve the Trustee in personal liability; and
(d) prior to taking any action as directed under this Section 6.12, the Trustee shall be entitled to indemnity satisfactory to it against the costs, expenses and liabilities which might be incurred by it in compliance with such request or direction.
Section 6.13 Waiver of Past Defaults.
The Holders of not less than a majority in principal amount of the outstanding Securities of any Series may on behalf of the Holders of all the Securities of such Series waive any past Default hereunder with respect to such Series and its consequences, except a Default in the payment of the principal of or interest on any Security of such Series (provided, however, that the Holders of a majority in principal amount of the outstanding Securities of any Series may rescind an acceleration and its consequences, including any related payment default that resulted from such acceleration). Upon any such waiver, such Default shall cease to exist, and any Event of Default arising therefrom shall be deemed to have been cured, for every purpose of this Indenture; but no such waiver shall extend to any subsequent or other Default.
25
Section 6.14 Undertaking for Costs.
All parties to this Indenture agree, and each Holder of any Security by his acceptance thereof shall be deemed to have agreed, that any court may in its discretion require, in any suit for the enforcement of any right or remedy under this Indenture, or in any suit against the Trustee for any action taken, suffered or omitted by it as Trustee, the filing by any party litigant in such suit of an undertaking to pay the costs of such suit, and that such court may in its discretion assess reasonable costs, including reasonable attorneys’ fees and expenses, against any party litigant in such suit, having due regard to the merits and good faith of the claims or defenses made by such party litigant; but the provisions of this Section 6.14 shall not apply to any suit instituted by the Company, to any suit instituted by the Trustee, to any suit instituted by any Holder, or group of Holders, holding in the aggregate more than 10% in principal amount of the outstanding Securities of any Series, or to any suit instituted by any Holder for the enforcement of the payment of the principal of or interest on any Security on or after the Maturity of such Security, including the Stated Maturity expressed in such Security (or, in the case of redemption, on the redemption date).
ARTICLE VII
TRUSTEE
Section 7.1 Duties of Trustee.
(a) If an Event of Default has occurred and is continuing, the Trustee shall exercise the rights and powers vested in it by this Indenture and use the same degree of care and skill in their exercise as a prudent person would exercise or use under the circumstances in the conduct of such person’s own affairs.
(b) Except during the continuance of an Event of Default:
(i) The Trustee need perform only those duties that are specifically set forth in this Indenture and no others.
(ii) In the absence of bad faith on its part, the Trustee may conclusively rely, as to the truth of the statements and the correctness of the opinions expressed therein, upon Officer’s Certificates or Opinions of Counsel furnished to the Trustee and conforming to the requirements of this Indenture; however, in the case of any such Officer’s Certificates or Opinions of Counsel which by any provisions hereof are specifically required to be furnished to the Trustee, the Trustee shall examine such Officer’s Certificates and Opinions of Counsel to determine whether or not they conform to the requirements of this Indenture (but need not confirm or investigate the accuracy of mathematical calculations or other facts stated therein).
(c) The Trustee may not be relieved from liability for its own negligent action, its own negligent failure to act or its own willful misconduct, except that:
(i) This sub-clause (c) does not limit the effect of sub-clause (b) of this Section 7.1.
26
(ii) The Trustee shall not be liable for any error of judgment made in good faith by a Responsible Officer, unless it is proved that the Trustee was negligent in ascertaining the pertinent facts.
(iii) The Trustee shall not be liable with respect to any action taken, suffered or omitted to be taken by it with respect to Securities of any Series in good faith in accordance with the direction of the Holders of a majority in principal amount of the outstanding Securities of such Series relating to the time, method and place of conducting any proceeding for any remedy available to the Trustee, or exercising any trust or power conferred upon the Trustee, under this Indenture with respect to the Securities of such Series in accordance with Section 6.12.
(d) Every provision of this Indenture that in any way relates to the Trustee is subject to sub-clauses (a), (b) and (c) of this Section 7.1.
(e) The Trustee may refuse to perform any duty or exercise any right or power unless it receives indemnity satisfactory to it against the costs, expenses and liabilities which might be incurred by it in performing such duty or exercising such right or power.
(f) The Trustee shall not be liable for interest on any money received by it. Money held in trust by the Trustee need not be segregated from other funds except to the extent required by law.
(g) No provision of this Indenture shall require the Trustee to risk its own funds or otherwise incur any financial liability in the performance of any of its duties, or in the exercise of any of its rights or powers, if adequate indemnity against such risk is not assured to the Trustee in its satisfaction.
(h) The Paying Agent, the Registrar and any authenticating agent shall be entitled to the protections and immunities as are set forth in sub-clauses (e), (f) and (g) of this Section 7.1 and in Section 7.2, each with respect to the Trustee.
Section 7.2 Rights of Trustee.
(a) The Trustee may conclusively rely on and shall be protected in acting or refraining from acting upon any document (whether in its original or facsimile form) believed by it to be genuine and to have been signed or presented by the proper person. The Trustee need not investigate any fact or matter stated in the document.
(b) Before the Trustee acts or refrains from acting, it shall be entitled to receive an Officer’s Certificate or an Opinion of Counsel or both. The Trustee shall not be liable for any action it takes or omits to take in good faith in conclusive reliance on such Officer’s Certificate or Opinion of Counsel.
(c) The Trustee may act through agents and shall not be responsible for the misconduct or negligence of any agent appointed with due care. No Depositary shall be deemed an agent of the Trustee and the Trustee shall not be responsible for any act or omission by any Depositary.
27
(d) The Trustee shall not be liable for any action it takes or omits to take in good faith which it believes to be authorized or within its rights or powers, provided that the Trustee’s conduct does not constitute willful misconduct or negligence.
(e) The Trustee may consult with counsel of its selection and the advice of such counsel or any Opinion of Counsel shall be full and complete authorization and protection in respect of any action taken, suffered or omitted by it hereunder in good faith and in reliance thereon.
(f) The Trustee shall be under no obligation to exercise any of the rights or powers vested in it by this Indenture at the request or direction of any of the Holders of Securities unless such Holders shall have offered to the Trustee security or indemnity reasonably satisfactory to it against the costs, expenses and liabilities which might be incurred by it in compliance with such request or direction.
(g) The Trustee shall not be bound to make any investigation into the facts or matters stated in any resolution, certificate, statement, instrument, opinion, report, notice, request, direction, consent, order, bond, debenture, note, other evidence of indebtedness or other paper or document, but the Trustee, in its discretion, may make such further inquiry or investigation into such facts or matters as it may see fit and shall incur no liability or additional liability of any kind by reason of such inquiry or investigation.
(h) The Trustee shall not be deemed to have notice of any Default or Event of Default unless a Responsible Officer of the Trustee has actual knowledge thereof or unless written notice of any event which is in fact such a default is received by a Responsible Officer of the Trustee at the Corporate Trust Office of the Trustee, and such notice references the Securities generally or the Securities of a particular Series and this Indenture.
(i) In no event shall the Trustee be liable to any person for special, punitive, indirect, consequential or incidental loss or damage of any kind whatsoever (including but not limited to lost profits), even if the Trustee has been advised of the likelihood of such loss or damage.
(j) The permissive right of the Trustee to take the actions permitted by this Indenture shall not be construed as an obligation or duty to do so.
(k) The rights, privileges, protections, immunities and benefits given to the Trustee, including, without limitation, its right to be indemnified, are extended to, and shall be enforceable by, the Trustee in each of its capacities hereunder, and each agent, custodian and other Person employed to act hereunder.
(l) The Trustee shall not be required to give any bond or surety in respect of the performance of its powers and duties hereunder.
(m) The Trustee may request that the Company deliver a certificate setting forth the names of individuals and/or titles of officers authorized at such time to take specified actions pursuant to this Indenture.
28
Section 7.3 Individual Rights of Trustee.
The Trustee in its individual or any other capacity may become the owner or pledgee of Securities and may otherwise deal with the Company or an Affiliate of the Company with the same rights it would have if it were not Trustee. Any Agent may do the same with like rights. However, the Trustee is also subject to Sections 7.10 and 7.11 hereof.
Section 7.4 Trustee’s Disclaimer.
The Trustee makes no representation as to the validity or adequacy of this Indenture or the Securities, it shall not be accountable for the Company’s use of the proceeds from the Securities, and it shall not be responsible for any statement in the Securities other than its authentication.
Section 7.5 Notice of Defaults.
If a Default or Event of Default occurs and is continuing with respect to the Securities of any Series and if it is actually known to a Responsible Officer of the Trustee, the Trustee shall mail to each Securityholder of the Securities of that Series notice of a Default or Event of Default within 90 days after it occurs or, if later, after a Responsible Officer of the Trustee has knowledge of such Default or Event of Default. Except in the case of a Default or Event of Default in payment of principal of or interest on any Security of any Series, the Trustee may withhold the notice if and so long as it in good faith determines that withholding the notice is in the interests of Securityholders of that Series.
Section 7.6 Reports by Trustee to Holders.
Within 60 days after each anniversary of the date of this Indenture, the Trustee shall transmit by mail to all Securityholders, as their names and addresses appear on the register kept by the Registrar, a brief report dated as of such reporting date, in accordance with, and to the extent required under, TIA § 313.
A copy of each report at the time of its mailing to Securityholders of any Series shall be filed with the SEC and each national securities exchange on which the Securities of that Series are listed. The Company shall promptly notify the Trustee in writing when Securities of any Series are listed on any national securities exchange or of any delisting thereof.
Section 7.7 Compensation and Indemnity.
The Company shall pay to the Trustee from time to time compensation for its services as the Company and the Trustee shall from time to time agree upon in writing. The Trustee’s compensation shall not be limited by any law on compensation of a trustee of an express trust. The Company shall reimburse the Trustee upon request for all reasonable out of pocket expenses incurred by it. Such expenses shall include the reasonable compensation and expenses of the Trustee’s agents and counsel.
29
The Company shall indemnify each of the Trustee and any predecessor Trustee against any cost, expense, claim (whether asserted by the Company, a Holder or any other person) or liability (including the cost of defending itself), including taxes (other than taxes based upon, measured by or determined by the income of the Trustee), incurred by it except as set forth in the next paragraph in the performance of its duties under this Indenture as Trustee or Agent. The Trustee shall notify the Company promptly of any claim for which it may seek indemnity. Failure by the Trustee to so notify the Company shall not relieve the Company of its obligations hereunder, unless and to the extent that the Company is materially prejudiced thereby. The Company shall defend the claim and the Trustee shall cooperate in the defense. The Trustee may have one separate counsel and the Company shall pay the reasonable fees and expenses of such counsel. The Company need not pay for any settlement made without its consent, which consent will not be unreasonably withheld. This indemnification shall apply to officers, directors, employees, shareholders and agents of the Trustee.
The Company need not reimburse any expense or indemnify against any loss or liability incurred by the Trustee or by any officer, director, employee or shareholder of the Trustee through willful misconduct or negligence.
To secure the Company’s payment obligations in this Section 7.7, the Trustee shall have a lien prior to the Securities of any Series on all money or property held or collected by the Trustee, except that held in trust to pay principal of and interest on particular Securities of that Series.
When the Trustee incurs expenses or renders services after an Event of Default specified in Section 6.1(f) or (g) occurs, the expenses and the compensation for the services are intended to constitute expenses of administration under any Bankruptcy Law.
The provisions of this Section 7.7 shall survive the termination of this Indenture or the resignation or removal of the Trustee.
Section 7.8 Replacement of Trustee.
A resignation or removal of the Trustee and appointment of a successor Trustee shall become effective only upon the successor Trustee’s acceptance of appointment as provided in this Section 7.8.
The Trustee may resign at any time with respect to the Securities of one or more Series by so notifying the Company at least 30 days prior to the date of the proposed resignation. The Holders of a majority in principal amount of the Securities of any Series may remove the Trustee with respect to that Series by so notifying the Trustee and the Company in writing. The Company may remove the Trustee with respect to Securities of one or more Series if:
(a) the Trustee fails to comply with Section 7.10;
(b) the Trustee is adjudged a bankrupt or an insolvent or an order for relief is entered with respect to the Trustee under any Bankruptcy Law;
(c) a Custodian or public officer takes charge of the Trustee or its property; or
(d) the Trustee becomes incapable of acting.
30
If the Trustee resigns or is removed or if a vacancy exists in the office of Trustee for any reason, the Company shall promptly appoint a successor Trustee. Within one year after the successor Trustee takes office, the Holders of a majority in principal amount of the then outstanding Securities may appoint a successor Trustee to replace the successor Trustee appointed by the Company.
If a successor Trustee with respect to the Securities of any one or more Series does not take office within 30 days after the retiring Trustee resigns or is removed, the retiring Trustee, the Company or the Holders of at least a majority in principal amount of the Securities of the applicable Series may petition any court of competent jurisdiction for the appointment of a successor Trustee at the expense of the Company.
A successor Trustee shall deliver a written acceptance of its appointment to the retiring Trustee and to the Company. Immediately after that, the retiring Trustee shall transfer all property held by it as Trustee to the successor Trustee subject to the lien provided for in Section 7.7, the resignation or removal of the retiring Trustee shall become effective, and the successor Trustee shall have all the rights, powers and duties of the Trustee with respect to each Series of Securities for which it is acting as Trustee under this Indenture. A successor Trustee shall mail a notice of its succession to each Securityholder of each such Series. Notwithstanding replacement of the Trustee pursuant to this Section 7.8, the Company’s obligations under Section 7.7 hereof shall continue for the benefit of the retiring Trustee with respect to expenses and liabilities incurred by it for actions taken or omitted to be taken in accordance with its rights, powers and duties under this Indenture prior to such replacement.
Section 7.9 Successor Trustee by Merger, Etc.
If the Trustee consolidates with, merges or converts into, or transfers all or substantially all of its corporate trust business to, another corporation, the successor corporation without any further act shall be the successor Trustee, if such successor corporation is eligible and qualified under Section 7.10.
Section 7.10 Eligibility; Disqualification.
This Indenture shall always have a Trustee who satisfies the requirements of TIA § 310(a)(1), (2) and (5). The Trustee shall always have a combined capital and surplus of at least $25,000,000 as set forth in its most recent published annual report of condition. The Trustee shall comply with TIA § 310(b).
Section 7.11 Preferential Collection of Claims Against Company.
The Trustee is subject to TIA § 311(a), excluding any creditor relationship listed in TIA § 311(b). A Trustee who has resigned or been removed shall be subject to TIA § 311(a) to the extent indicated.
31
ARTICLE VIII
SATISFACTION AND DISCHARGE; DEFEASANCE
Section 8.1 Satisfaction and Discharge of Indenture.
This Indenture shall upon Company Order cease to be of further effect (except as hereinafter provided in this Section 8.1), and the Trustee, at the expense of the Company, shall execute instruments acknowledging satisfaction and discharge of this Indenture, when
(a) either
(i) all Securities theretofore authenticated and delivered (other than Securities that have been destroyed, lost or stolen and that have been replaced or paid as provided in Section 2.9) have been delivered to the Trustee for cancellation; or
(ii) all such Securities not theretofore delivered to the Trustee for cancellation:
(1) have become due and payable, or
(2) will become due and payable at their Stated Maturity within one year, or
(3) have been called for redemption or are to be called for redemption within one year under arrangements satisfactory to the Trustee for the giving of notice of redemption by the Trustee in the name, and at the expense, of the Company;
and the Company, in the case of (1), (2) or (3) above, has irrevocably deposited or caused to be deposited with the Trustee as trust funds in trust an amount of money or U.S. Government Obligations sufficient for the purpose of paying and discharging the entire indebtedness on such Securities not theretofore delivered to the Trustee for cancellation, for principal and interest to the date of such deposit (in the case of Securities which have become due and payable on or prior to the date of such deposit) or to the Stated Maturity or redemption date, as the case may be;
(b) the Company has paid or caused to be paid all other sums payable hereunder by the Company; and
(c) the Company has delivered to the Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that all conditions precedent herein provided for relating to the satisfaction and discharge of this Indenture have been complied with.
Notwithstanding the satisfaction and discharge of this Indenture, the obligations of the Company to the Trustee under Section 7.7, and, if money shall have been deposited with the Trustee pursuant to sub-clause (a) of this Section 8.1, the provisions of Sections 2.5, 2.8, 2.9, 8.2 and 8.5 shall survive.
32
Section 8.2 Application of Trust Funds; Indemnification.
(a) Subject to the provisions of Section 8.5, all money or U.S. Government Obligations deposited with the Trustee pursuant to Section 8.1, all money and U.S. Government Obligations or Foreign Government Obligations deposited with the Trustee pursuant to Section 8.3 or 8.4 and all money received by the Trustee in respect of U.S. Government Obligations or Foreign Government Obligations deposited with the Trustee pursuant to Section 8.3 or 8.4, shall be held in trust and applied by it, in accordance with the provisions of the Securities and this Indenture, to the payment, either directly or through any Paying Agent (including the Company acting as its own Paying Agent) as the Trustee may determine, to the persons entitled thereto, of the principal and interest for whose payment such money has been deposited with or received by the Trustee or to make mandatory sinking fund payments or analogous payments as contemplated by Sections 8.3 or 8.4.
(b) The Company shall pay and shall indemnify the Trustee against any tax, fee or other charge imposed on or assessed against U.S. Government Obligations or Foreign Government Obligations deposited pursuant to Sections 8.3 or 8.4 or the interest and principal received in respect of such obligations other than any payable by or on behalf of Holders.
(c) The Trustee shall deliver or pay to the Company from time to time upon Company Order any U.S. Government Obligations or Foreign Government Obligations or money held by it as provided in Sections 8.3 or 8.4 which, in the opinion of a nationally recognized firm of independent certified public accountants or investment bank expressed in a written certification thereof delivered to the Trustee, are then in excess of the amount thereof which then would have been required to be deposited for the purpose for which such U.S. Government Obligations or Foreign Government Obligations or money were deposited or received. This provision shall not authorize the sale by the Trustee of any U.S. Government Obligations or Foreign Government Obligations held under this Indenture.
Section 8.3 Legal Defeasance of Securities of any Series.
Unless this Section 8.3 is otherwise specified, pursuant to Section 2.2, to be inapplicable to Securities of any Series, the Company shall be deemed to have paid and discharged the entire indebtedness on all the outstanding Securities of any Series on the 91st day after the date of the deposit referred to in sub-clause (d) hereof, and the provisions of this Indenture, as it relates to such outstanding Securities of such Series, shall no longer be in effect (and the Trustee, at the expense of the Company, shall, upon receipt of a Company Order, execute instruments acknowledging the same), except as to:
(a) the rights of Holders of Securities of such Series to receive, from the trust funds described in sub-clause (d) hereof, (i) payment of the principal of and each installment of principal of and interest on the outstanding Securities of such Series on the Maturity of such principal or installment of principal or interest and (ii) the benefit of any mandatory sinking fund payments applicable to the Securities of such Series on the day on which such payments are due and payable in accordance with the terms of this Indenture and the Securities of such Series;
(b) the provisions of Sections 2.5, 2.8, 2.9, 8.2, 8.3 and 8.5; and
33
(c) the rights, powers, trust and immunities of the Trustee hereunder and the Company’s obligations in connection therewith;
provided that, the following conditions shall have been satisfied:
(d) the Company shall have deposited or caused to be irrevocably deposited (except as provided in Section 8.2(c)) with the Trustee as trust funds in trust for the purpose of making the following payments, specifically pledged as security for and dedicated solely to the benefit of the Holders of such Securities: (i) in the case of Securities of such Series denominated in Dollars, cash in Dollars and/or U.S. Government Obligations, or (ii) in the case of Securities of such Series denominated in a Foreign Currency (other than a composite currency), money and/or Foreign Government Obligations, which through the payment of interest and principal in respect thereof in accordance with their terms (and without reinvestment), will provide, not later than one day before the due date of any payment of money, an amount in cash, sufficient, in the opinion of a nationally recognized firm of independent public accountants or investment bank expressed in a written certification thereof delivered to the Trustee, to pay and discharge each installment of principal of and interest, if any, on and any mandatory sinking fund payments in respect of all the Securities of such Series on the dates such installments of interest or principal and such sinking fund payments are due;
(e) such deposit will not result in a breach or violation of, or constitute a default under, this Indenture or any other agreement or instrument to which the Company is a party or by which it is bound;
(f) no Default or Event of Default with respect to the Securities of such Series shall have occurred and be continuing on the date of such deposit or during the period ending on the 91st day after such date;
(g) the Company shall have delivered to the Trustee an Officer’s Certificate and an Opinion of Counsel to the effect that (i) the Company has received from, or there has been published by, the Internal Revenue Service a ruling, or (ii) since the date of execution of this Indenture, there has been a change in the applicable U.S. federal income tax law, in either case to the effect that, and based thereon such Opinion of Counsel shall confirm that, the Holders of the Securities of such Series will not recognize income, gain or loss for U.S. federal income tax purposes as a result of such deposit, defeasance and discharge and will be subject to U.S. federal income tax on the same amount and in the same manner and at the same times as would have been the case if such deposit, defeasance and discharge had not occurred;
(h) the Company shall have delivered to the Trustee an Officer’s Certificate stating that the deposit was not made by the Company with the intent of defeating, hindering, delaying or defrauding any other creditors of the Company; and
(i) the Company shall have delivered to the Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that all conditions precedent provided for relating to the defeasance contemplated by this Section 8.3 have been complied with.
34
Section 8.4 Covenant Defeasance.
Unless this Section 8.4 is otherwise specified pursuant to Section 2.2 to be inapplicable to Securities of any Series, the Company may omit to comply with respect to the Securities of any Series with any term, provision or condition set forth under Sections 4.2 and 4.3, 4.4 and 5.1 as well as any additional covenants specified in a supplemental indenture for such Series of Securities or a Board Resolution or an Officer’s Certificate delivered pursuant to Section 2.2 (and the failure to comply with any such covenants shall not constitute a Default or Event of Default with respect to such Series under Section 6.1) and the occurrence of any event specified in a supplemental indenture for such Series of Securities or a Board Resolution or an Officer’s Certificate delivered pursuant to Section 2.2.18 and designated as an Event of Default shall not constitute a Default or Event of Default hereunder, with respect to the Securities of such Series, provided that the following conditions shall have been satisfied:
(a) With reference to this Section 8.4, the Company has deposited or caused to be irrevocably deposited (except as provided in Section 8.2(c)) with the Trustee as trust funds in trust for the purpose of making the following payments specifically pledged as security for, and dedicated solely to, the benefit of the Holders of such Securities: (i) in the case of Securities of such Series denominated in Dollars, cash in Dollars and/or U.S. Government Obligations, or (ii) in the case of Securities of such Series denominated in a Foreign Currency (other than a composite currency), money and/or Foreign Government Obligations, which through the payment of interest and principal in respect thereof in accordance with their terms (and without reinvestment), will provide, not later than one day before the due date of any payment of money, an amount in cash, sufficient, in the opinion of a nationally recognized firm of independent certified public accountants or investment bank expressed in a written certification thereof delivered to the Trustee, to pay and discharge each installment of principal of and interest, if any, on and any mandatory sinking fund payments in respect of the Securities of such Series on the dates such installments of interest or principal and such sinking fund payments are due;
(b) Such deposit will not result in a breach or violation of, or constitute a default under, this Indenture or any other agreement or instrument to which the Company is a party or by which it is bound;
(c) No Default or Event of Default with respect to the Securities of such Series shall have occurred and be continuing on the date of such deposit;
(d) The Company shall have delivered to the Trustee an Opinion of Counsel to the effect that Holders of the Securities of such Series will not recognize income, gain or loss for U.S. federal income tax purposes as a result of such deposit and covenant defeasance and will be subject to U.S. federal income tax on the same amounts, in the same manner and at the same times as would have been the case if such deposit and covenant defeasance had not occurred;
(e) The Company shall have delivered to the Trustee an Officer’s Certificate stating the deposit was not made by the Company with the intent of defeating, hindering, delaying or defrauding any other creditors of the Company; and
(f) The Company shall have delivered to the Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that all conditions precedent herein provided for relating to the covenant defeasance contemplated by this Section 8.4 have been complied with.
35
Section 8.5 Repayment to Company.
Subject to applicable abandoned property law, the Trustee and the Paying Agent shall pay to the Company upon request any money held by them for the payment of principal or interest that remains unclaimed for two years after such principal or interest has become due and payable. After that, Securityholders entitled to the money must look to the Company for payment as general creditors unless an applicable abandoned property law designates another person.
Section 8.6 Reinstatement.
If the Trustee or the Paying Agent is unable to apply any money deposited with respect to Securities of any Series in accordance with Section 8.1 by reason of any legal proceeding or by reason of any order or judgment of any court or governmental authority enjoining, restraining or otherwise prohibiting such application, the obligations of the Company under this Indenture with respect to the Securities of such Series and under the Securities of such Series shall be revived and reinstated as though no deposit had occurred pursuant to Section 8.1 until such time as the Trustee or the Paying Agent is permitted to apply all such money in accordance with Section 8.1; provided, however, that if the Company has made any payment of principal of or interest on any Securities because of the reinstatement of its obligations, the Company shall be subrogated to the rights of the Holders of such Securities to receive such payment from the money or U.S. Government Obligations held by the Trustee or Paying Agent after payment in full to the Holders.
ARTICLE IX
AMENDMENTS AND WAIVERS
Section 9.1 Without Consent of Holders.
The Company and the Trustee may amend or supplement this Indenture or the Securities of one or more Series without the consent of any Securityholder:
(a) to add guarantees with respect to any Series of Securities or secure any Series of Securities;
(b) to surrender any of the Company’s rights or powers under this Indenture;
(c) to add covenants or Events of Default for the benefit of the Securityholders of any Series of Securities;
(d) to comply with the applicable rules or procedures of the Depositary;
(e) to cure any ambiguity, defect or inconsistency, as described in the Officer’s Certificate delivered pursuant to Section 10.4;
(f) to comply with Article V;
36
(g) to provide for uncertificated Securities in addition to or in place of certificated Securities;
(h) to make any change that does not materially adversely affect the rights of any Securityholder;
(i) to provide for the issuance of and establish the form and terms and conditions of Securities of any Series as permitted by this Indenture;
(j) to evidence and provide for the acceptance of appointment hereunder by a successor Trustee with respect to the Securities of one or more Series and to add to or change any of the provisions of this Indenture as shall be necessary to provide for or facilitate the administration of the trusts hereunder by more than one Trustee;
(k) to comply with requirements of the SEC in order to effect or maintain the qualification of this Indenture under the TIA;
(l) to comply with the rules or regulations of any securities exchange or automated quotation system on which any of the Securities may be listed or traded; and
(m) to change or eliminate any of the provisions of this Indenture, provided that any such change or elimination shall not be effective with respect to any outstanding Securities of any Series created prior to the execution of such supplemental indenture which is entitled to the benefit of such provision.
Section 9.2 With Consent of Holders.
The Company and the Trustee may enter into a supplemental indenture with the written consent of the Holders of at least a majority in principal amount of the outstanding Securities of each Series affected by such supplemental indenture (including consents obtained in connection with a tender offer or exchange offer for the Securities of such Series), for the purpose of adding any provisions to or changing in any manner or eliminating any of the provisions of this Indenture or of any supplemental indenture or of modifying in any manner the rights of the Securityholders of each such Series. Except as provided in Section 6.13, the Holders of at least a majority in principal amount of the outstanding Securities of any Series by written notice to the Trustee (including consents obtained in connection with a tender offer or exchange offer for the Securities of such Series) may waive compliance by the Company with any provision of this Indenture or the Securities with respect to such Series.
It shall not be necessary for the consent of the Holders of Securities under this Section 9.2 to approve the particular form of any proposed supplemental indenture or waiver, but it shall be sufficient if such consent approves the substance thereof. After a supplemental indenture or waiver under this Section 9.2 becomes effective, the Company shall mail to the Holders of Securities affected thereby, a notice briefly describing the supplemental indenture or waiver. Any failure by the Company to mail or publish such notice, or any defect therein, shall not, however, in any way impair or affect the validity of any such supplemental indenture or waiver.
37
Section 9.3 Limitations.
Without the consent of each Securityholder affected, an amendment or waiver may not:
(a) reduce the principal amount of Securities whose Holders must consent to an amendment, supplement or waiver;
(b) reduce the rate of or extend the time for payment of interest (including default interest) on any Security or that Series;
(c) reduce the principal of, or change the Stated Maturity of, any Security or reduce the amount of, or postpone the date fixed for, the payment of any sinking fund or analogous obligation;
(d) reduce the principal amount of Discount Securities payable upon acceleration of the maturity thereof;
(e) waive a Default or Event of Default in the payment of the principal of or interest, if any, on any Security (except a rescission of acceleration of the Securities of any Series by the Holders of at least a majority in principal amount of the then outstanding Securities of such Series and a waiver of the payment default that resulted from such acceleration);
(f) make the principal of or interest, if any, on any Security payable in any currency other than that stated in the Security;
(g) make any change in Sections 6.8 or 6.13 or this Section 9.3; or
(h) waive a redemption payment with respect to any Security.
Section 9.4 Compliance with Trust Indenture Act.
Every amendment to this Indenture or the Securities of one or more Series shall be set forth in a supplemental indenture hereto that complies with the TIA as then in effect.
Section 9.5 Revocation and Effect of Consents.
Until an amendment is set forth in a supplemental indenture or a waiver becomes effective, a consent to it by a Holder of a Security is a continuing consent by the Holder and every subsequent Holder of a Security or portion of a Security that evidences the same debt as the consenting Holder’s Security, even if notation of the consent is not made on any Security.
Any amendment or waiver once effective shall bind every Securityholder of each Series affected by such amendment or waiver unless it is of the type described in any of sub-clauses (a) through (h) of Section 9.3. In that case, the amendment or waiver shall bind each Holder of a Security who has consented to it and every subsequent Holder of a Security or portion of a Security that evidences the same debt as the consenting Holder’s Security.
38
The Company may, but shall not be obligated to, fix a record date for the purpose of determining the Holders entitled to give their consent or take any other action described above or required or permitted to be taken pursuant to this Indenture. If a record date is fixed, then notwithstanding the immediately preceding paragraph, those Persons who were Holders at such record date (or their duly designated proxies), and only those persons, shall be entitled to give such consent or to revoke any consent previously given or take any such action, whether or not such Persons continue to be Holders after such record date. No such consent shall be valid or effective for more than 120 days after such record date.
Section 9.6 Notation on or Exchange of Securities.
The Company or the Trustee may place an appropriate notation about an amendment or waiver on any Security of any Series thereafter authenticated. The Company in exchange for Securities of that Series may issue and the Trustee shall authenticate upon request new Securities of that Series that reflect the amendment or waiver.
Section 9.7 Trustee Protected.
In executing, or accepting the additional trusts created by, any supplemental indenture permitted by this Article or the modifications thereby of the trusts created by this Indenture, the Trustee shall receive, and (subject to Section 7.1) shall be fully protected in conclusively relying upon, an Officer’s Certificate or an Opinion of Counsel or both complying with Section 10.4 and stating that the supplemental indenture is the legal, valid and binding obligation of the Company, enforceable against the Company in accordance with its terms, subject to customary exceptions. The Trustee shall sign all supplemental indentures upon delivery of such an Officer’s Certificate or Opinion of Counsel or both, except that the Trustee need not sign any supplemental indenture that, in its sole discretion, adversely affects its rights.
ARTICLE X
MISCELLANEOUS
Section 10.1 Trust Indenture Act Controls.
If any provision of this Indenture limits, qualifies, or conflicts with another provision which is required or deemed to be included in this Indenture by the TIA, such required or deemed provision shall control.
Section 10.2 Notices.
Any request, demand, notice or communication by the Company or the Trustee to the other, or by a Holder to the Company or the Trustee, is duly given if in writing and delivered in person or mailed by first-class mail:
39
if to the Company:
Cresco Labs Inc.
if to the Trustee:
Attention:
The Company or the Trustee by notice to the other may designate additional or different addresses for subsequent notices or communications.
Any notice or communication to a Securityholder shall be mailed by first-class mail to his address shown on the register kept by the Registrar. Failure to mail a notice or communication to a Securityholder of any Series or any defect in it shall not affect its sufficiency with respect to other Securityholders of that or any other Series.
If a notice or communication is mailed or published in the manner provided above, within the time prescribed, it is duly given, whether or not the Securityholder receives it.
If the Company mails a notice or communication to Securityholders, it shall mail a copy to the Trustee and each Agent at the same time.
Notwithstanding any other provision of this Indenture or any Security, where this Indenture or any Security provides for notice of any event (including any notice of redemption) to a Holder of a Global Security (whether by mail or otherwise), such notice shall be sufficiently given to the Depositary for such Security (or its designee) pursuant to the customary procedures of such Depositary.
Section 10.3 Communication by Holders with Other Holders.
Securityholders of any Series may communicate pursuant to TIA § 312(b) with other Securityholders of that Series or any other Series with respect to their rights under this Indenture or the Securities of that Series or all Series. The Company, the Trustee, the Registrar and anyone else shall have the protection of TIA § 312(c).
Section 10.4 Certificate and Opinion as to Conditions Precedent.
Upon any request or application by the Company to the Trustee to take any action under this Indenture, the Company shall furnish to the Trustee:
40
(a) an Officer’s Certificate stating that, in the opinion of the signers, all conditions precedent, if any, provided for in this Indenture relating to the proposed action have been complied with; and
(b) an Opinion of Counsel stating that, in the opinion of such counsel, all such conditions precedent have been complied with.
Section 10.5 Statements Required in Certificate or Opinion.
Each certificate or opinion with respect to compliance with a condition or covenant provided for in this Indenture (other than a certificate provided pursuant to TIA § 314(a)(4)) shall comply with the provisions of TIA § 314(e) and shall include:
(a) a statement that the person making such certificate or opinion has read such covenant or condition;
(b) a brief statement as to the nature and scope of the examination or investigation upon which the statements or opinions contained in such certificate or opinion are based;
(c) a statement that, in the opinion of such person, he has made such examination or investigation as is necessary to enable him to express an informed opinion as to whether or not such covenant or condition has been complied with; and
(d) a statement as to whether or not, in the opinion of such person, such condition or covenant has been complied with.
Section 10.6 Rules by Trustee and Agents.
The Trustee may make reasonable rules for action by or a meeting of Securityholders of one or more Series. Any Agent may make reasonable rules and set reasonable requirements for its functions.
Section 10.7 Legal Holidays.
Unless otherwise provided by Board Resolution, Officer’s Certificate or supplemental indenture hereto for a particular Series, a “Legal Holiday” is any day that is not a Business Day. If a payment date is a Legal Holiday at a place of payment, payment may be made at that place on the next succeeding day that is not a Legal Holiday, and no interest shall accrue for the intervening period.
Section 10.8 No Recourse Against Others.
A director, officer, employee or stockholder (past or present), as such, of the Company shall not have any liability for any obligations of the Company under the Securities or this Indenture or for any claim based on, in respect of or by reason of such obligations or their creation. Each Securityholder by accepting a Security waives and releases all such liability. The waiver and release are part of the consideration for the issue of the Securities.
41
Section 10.9 Counterparts.
This Indenture may be executed in any number of counterparts and by the parties hereto in separate counterparts, each of which when so executed shall be deemed to be an original and all of which taken together shall constitute one and the same agreement. The exchange of copies of this Indenture and of signature pages by facsimile or PDF transmission shall constitute effective execution and delivery of this Indenture as to the parties hereto and may be used in lieu of the original Indenture for all purposes. Signatures of the parties hereto transmitted by facsimile or PDF shall be deemed to be their original signatures for all purposes.
Section 10.10 Governing Law; Jury Trial Waiver.
THIS INDENTURE AND THE SECURITIES, INCLUDING ANY CLAIM OR CONTROVERSY ARISING OUT OF OR RELATING TO THIS INDENTURE OR THE SECURITIES, SHALL BE GOVERNED BY THE LAWS OF THE STATE OF NEW YORK (WITHOUT REGARD TO THE CONFLICTS OF LAWS PROVISIONS THEREOF OTHER THAN SECTION 5-1401 OF THE GENERAL OBLIGATIONS LAW).
EACH OF THE COMPANY AND THE TRUSTEE HEREBY IRREVOCABLY WAIVES, TO THE FULLEST EXTENT PERMITTED BY APPLICABLE LAW, ANY AND ALL RIGHT TO TRIAL BY JURY IN ANY LEGAL PROCEEDING ARISING OUT OF OR RELATING TO THIS INDENTURE, THE SECURITIES OR THE TRANSACTION CONTEMPLATED HEREBY.
Section 10.11 No Adverse Interpretation of Other Agreements.
This Indenture may not be used to interpret another indenture, loan or debt agreement of the Company or a Subsidiary of the Company. Any such indenture, loan or debt agreement may not be used to interpret this Indenture.
Section 10.12 Successors.
All agreements of the Company in this Indenture and the Securities shall bind its successor. All agreements of the Trustee in this Indenture shall bind its successor.
Section 10.13 Severability.
In case any provision in this Indenture or in the Securities shall be invalid, illegal or unenforceable, the validity, legality and enforceability of the remaining provisions shall not in any way be affected or impaired thereby.
Section 10.14 Table of Contents, Headings, Etc.
The Table of Contents, Cross Reference Table, and headings of the Articles and Sections of this Indenture have been inserted for convenience of reference only, are not to be considered a part hereof, and shall in no way modify or restrict any of the terms or provisions hereof.
42
Section 10.15 Securities in a Foreign Currency.
Unless otherwise specified in a Board Resolution, a supplemental indenture hereto or an Officer’s Certificate delivered pursuant to Section 2.2 of this Indenture with respect to a particular Series of Securities, whenever for purposes of this Indenture any action may be taken by the Holders of a specified percentage in aggregate principal amount of Securities of all Series or all Series affected by a particular action at the time outstanding and, at such time, there are outstanding Securities of any Series which are denominated in more than one currency, then the principal amount of Securities of such Series which shall be deemed to be outstanding for the purpose of taking such action shall be determined by converting any such other currency into a currency that is designated upon issuance of any particular Series of Securities. Unless otherwise specified in a Board Resolution, a supplemental indenture hereto or an Officer’s Certificate delivered pursuant to Section 2.2 of this Indenture with respect to a particular Series of Securities, such conversion shall be at the spot rate for the purchase of the designated currency as published in The Financial Times in the “Currency Rates” section (or, if The Financial Times is no longer published, or if such information is no longer available in The Financial Times, such source as may be selected in good faith by the Company) on any date of determination. The provisions of this paragraph shall apply in determining the equivalent principal amount in respect of Securities of a Series denominated in currency other than Dollars in connection with any action taken by Holders of Securities pursuant to the terms of this Indenture.
All decisions and determinations provided for in the preceding paragraph shall, in the absence of manifest error, to the extent permitted by law, be conclusive for all purposes and irrevocably binding upon the Trustee and all Holders.
Section 10.16 Judgment Currency.
The Company agrees, to the fullest extent that it may effectively do so under applicable law, that (a) if for the purpose of obtaining judgment in any court it is necessary to convert the sum due in respect of the principal of or interest or other amount on the Securities of any Series (the “Required Currency”) into a currency in which a judgment will be rendered (the “Judgment Currency”), the rate of exchange used shall be the rate at which in accordance with normal banking procedures the Trustee could purchase in The City of New York the Required Currency with the Judgment Currency on the day on which final unappealable judgment is entered, unless such day is not a Business Day, then the rate of exchange used shall be the rate at which in accordance with normal banking procedures the Trustee could purchase in The City of New York the Required Currency with the Judgment Currency on the Business Day preceding the day on which final unappealable judgment is entered and (b) its obligations under this Indenture to make payments in the Required Currency (i) shall not be discharged or satisfied by any tender, or any recovery pursuant to any judgment (whether or not entered in accordance with subsection (a)), in any currency other than the Required Currency, except to the extent that such tender or recovery shall result in the actual receipt, by the payee, of the full amount of the Required Currency expressed to be payable in respect of such payments, (ii) shall be enforceable as an alternative or additional cause of action for the purpose of recovering in the Required Currency the amount, if any, by which such actual receipt shall fall short of the full amount of the Required Currency so expressed to be payable, and (iii) shall not be affected by judgment being obtained for any other sum due under this Indenture.
43
Section 10.17 Force Majeure.
In no event shall the Trustee be responsible or liable for any failure or delay in the performance of its obligations hereunder arising out of or caused by, directly or indirectly, forces beyond its control, including, without limitation, strikes, work stoppages, accidents, acts of war or terrorism, civil or military disturbances, nuclear or natural catastrophes or acts of God, and interruptions, loss or malfunctions of utilities, communications or computer (software and hardware) services, it being understood that the Trustee shall use reasonable best efforts which are consistent with accepted practices in the banking industry to resume performance as soon as practicable under the circumstances.
Section 10.18 U.S.A. Patriot Act.
The parties hereto acknowledge that in accordance with Section 326 of the U.S.A. Patriot Act, the Trustee, like all financial institutions and in order to help fight the funding of terrorism and money laundering, is required to obtain, verify, and record information that identifies each person or legal entity that establishes a relationship or opens an account with the Trustee. The parties to this Indenture agree that they will provide the Trustee with such information as it may request in order for the Trustee to satisfy the requirements of the U.S.A. Patriot Act.
ARTICLE XI
SINKING FUNDS
Section 11.1 Applicability of Article.
The provisions of this Article shall be applicable to any sinking fund for the retirement of the Securities of a Series if so provided by the terms of such Securities pursuant to Section 2.2 and except as otherwise permitted or required by any form of Security of such Series issued pursuant to this Indenture.
The minimum amount of any sinking fund payment provided for by the terms of the Securities of any Series is herein referred to as a “mandatory sinking fund payment” and any other amount provided for by the terms of Securities of such Series is herein referred to as an “optional sinking fund payment.” If provided for by the terms of Securities of any Series, the cash amount of any sinking fund payment may be subject to reduction as provided in Section 11.2. Each sinking fund payment shall be applied to the redemption of Securities of any Series as provided for by the terms of the Securities of such Series.
Section 11.2 Satisfaction of Sinking Fund Payments with Securities.
The Company may, in satisfaction of all or any part of any sinking fund payment with respect to the Securities of any Series to be made pursuant to the terms of such Securities (a) deliver outstanding Securities of such Series to which such sinking fund payment is applicable (other than any of such Securities previously called for mandatory sinking fund redemption) and (b) apply as credit Securities of such Series to which such sinking fund payment is applicable and which have been repurchased by the Company or redeemed either at the election of the Company pursuant to the terms of the Securities of such Series (except pursuant to any mandatory sinking fund) or through the application of permitted optional sinking fund payments
44
or other optional redemptions pursuant to the terms of such Securities, provided that such Securities have not been previously so credited. Such Securities shall be received by the Trustee, together with an Officer’s Certificate with respect thereto, not later than 15 days prior to the date on which the Trustee begins the process of selecting Securities for redemption, and shall be credited for such purpose by the Trustee at the price specified in such Securities for redemption through operation of the sinking fund and the amount of such sinking fund payment shall be reduced accordingly. If as a result of the delivery or credit of Securities in lieu of cash payments pursuant to this Section 11.2, the principal amount of Securities of such Series to be redeemed in order to exhaust the aforesaid cash payment shall be less than $100,000, the Trustee need not call Securities of such Series for redemption, except upon receipt of a Company Order that such action be taken, and such cash payment shall be held by the Trustee or a Paying Agent and applied to the next succeeding sinking fund payment, provided, however, that the Trustee or such Paying Agent shall from time to time upon receipt of a Company Order pay over and deliver to the Company any cash payment so being held by the Trustee or such Paying Agent upon delivery by the Company to the Trustee of Securities of that Series purchased by the Company having an unpaid principal amount equal to the cash payment required to be released to the Company.
Section 11.3 Redemption of Securities for Sinking Fund.
Not less than 45 days (unless otherwise indicated in the Board Resolution, supplemental indenture hereto or Officer’s Certificate in respect of a particular Series of Securities) prior to each sinking fund payment date for any Series of Securities, the Company will deliver to the Trustee an Officer’s Certificate specifying the amount of the next ensuing mandatory sinking fund payment for that Series pursuant to the terms of that Series, the portion thereof, if any, which is to be satisfied by payment of cash and the portion thereof, if any, which is to be satisfied by delivering and crediting of Securities of that Series pursuant to Section 11.2, and the optional amount, if any, to be added in cash to the next ensuing mandatory sinking fund payment, and the Company shall thereupon be obligated to pay the amount therein specified. Not less than 30 days (unless otherwise indicated in the Board Resolution, Officer’s Certificate or supplemental indenture in respect of a particular Series of Securities) before each such sinking fund payment date the Trustee shall select the Securities to be redeemed upon such sinking fund payment date in the manner specified in Section 3.2 and cause notice of the redemption thereof to be given in the name of and at the expense of the Company in the manner provided in Section 3.3. Such notice having been duly given, the redemption of such Securities shall be made upon the terms and in the manner stated in Sections 3.4, 3.5 and 3.6.
[Signature page follows]
45
IN WITNESS WHEREOF, the parties hereto have caused this Indenture to be duly executed as of the day and year first above written.
CRESCO LABS INC., as Issuer
| By: | ||||
| Name: | ||||
| Its: | ||||
| , as Trustee | ||||
| By: | ||||
| Name: | ||||
| Its: | ||||
46
Exhibit 7.2
CRESCO LABS INC.
INDENTURE
Dated as of , 20
[ ]
Trustee
Subordinated Debt Securities
TABLE OF CONTENTS
| Page | ||||||
| ARTICLE I DEFINITIONS AND INCORPORATION BY REFERENCE | 1 | |||||
| Section 1.1 |
Definitions |
1 | ||||
| Section 1.2 |
Other Definitions |
4 | ||||
| Section 1.3 |
Incorporation by Reference of Trust Indenture Act |
5 | ||||
| Section 1.4 |
Rules of Construction |
5 | ||||
| ARTICLE II THE SECURITIES | 6 | |||||
| Section 2.1 |
Issuable in Series |
6 | ||||
| Section 2.2 |
Establishment of Terms of Series of Securities |
6 | ||||
| Section 2.3 |
Denominations; Provision for Payment |
8 | ||||
| Section 2.4 |
Execution and Authentication |
9 | ||||
| Section 2.5 |
Registrar and Paying Agent |
9 | ||||
| Section 2.6 |
Paying Agent to Hold Money in Trust |
10 | ||||
| Section 2.7 |
Securityholder Lists |
10 | ||||
| Section 2.8 |
Transfer and Exchange |
11 | ||||
| Section 2.9 |
Mutilated, Destroyed, Lost and Stolen Securities |
11 | ||||
| Section 2.10 |
Outstanding Securities |
12 | ||||
| Section 2.11 |
Treasury Securities |
12 | ||||
| Section 2.12 |
Temporary Securities |
13 | ||||
| Section 2.13 |
Cancellation |
13 | ||||
| Section 2.14 |
Defaulted Interest |
13 | ||||
| Section 2.15 |
Global Securities |
13 | ||||
| Section 2.16 |
CUSIP Numbers |
15 | ||||
| ARTICLE III REDEMPTION | 15 | |||||
| Section 3.1 |
Notice to Trustee |
15 | ||||
| Section 3.2 |
Selection of Securities to be Redeemed |
15 | ||||
| Section 3.3 |
Notice of Redemption |
15 | ||||
| Section 3.4 |
Effect of Notice of Redemption |
16 | ||||
| Section 3.5 |
Deposit of Redemption Price |
17 | ||||
| Section 3.6 |
Securities Redeemed in Part |
17 | ||||
| ARTICLE IV COVENANTS | 17 | |||||
| Section 4.1 |
Payment of Principal and Interest |
17 | ||||
| Section 4.2 |
Reports by Company |
18 | ||||
| Section 4.3 |
Compliance Certificate |
18 | ||||
| Section 4.4 |
Stay, Extension and Usury Laws |
18 | ||||
| Section 4.5 |
Corporate Existence |
19 | ||||
| ARTICLE V SUCCESSORS | 19 | |||||
| Section 5.1 |
Consolidation, Merger and Sale of Assets |
19 | ||||
-i-
TABLE OF CONTENTS
(continued)
| Page | ||||||
| Section 5.2 |
Successor Person Substituted |
19 | ||||
| ARTICLE VI DEFAULTS AND REMEDIES | 20 | |||||
| Section 6.1 |
Events of Default |
20 | ||||
| Section 6.2 |
Acceleration of Maturity; Rescission and Annulment |
21 | ||||
| Section 6.3 |
Collection of Indebtedness and Suits for Enforcement by Trustee |
22 | ||||
| Section 6.4 |
Trustee May File Proofs of Claim |
22 | ||||
| Section 6.5 |
Trustee May Enforce Claims Without Possession of Securities |
23 | ||||
| Section 6.6 |
Application of Money Collected |
23 | ||||
| Section 6.7 |
Limitation on Suits |
24 | ||||
| Section 6.8 |
Unconditional Right of Holders to Receive Principal and Interest |
24 | ||||
| Section 6.9 |
Restoration of Rights and Remedies |
24 | ||||
| Section 6.10 |
Rights and Remedies Cumulative |
25 | ||||
| Section 6.11 |
Delay or Omission Not Waiver |
25 | ||||
| Section 6.12 |
Control by Holders |
25 | ||||
| Section 6.13 |
Waiver of Past Defaults |
26 | ||||
| Section 6.14 |
Undertaking for Costs |
26 | ||||
| ARTICLE VII TRUSTEE | 26 | |||||
| Section 7.1 |
Duties of Trustee |
26 | ||||
| Section 7.2 |
Rights of Trustee |
28 | ||||
| Section 7.3 |
Individual Rights of Trustee |
29 | ||||
| Section 7.4 |
Trustee’s Disclaimer |
29 | ||||
| Section 7.5 |
Notice of Defaults |
29 | ||||
| Section 7.6 |
Reports by Trustee to Holders |
29 | ||||
| Section 7.7 |
Compensation and Indemnity |
30 | ||||
| Section 7.8 |
Replacement of Trustee |
30 | ||||
| Section 7.9 |
Successor Trustee by Merger, Etc |
31 | ||||
| Section 7.10 |
Eligibility; Disqualification |
31 | ||||
| Section 7.11 |
Preferential Collection of Claims Against Company |
32 | ||||
| ARTICLE VIII SATISFACTION AND DISCHARGE; DEFEASANCE | 32 | |||||
| Section 8.1 |
Satisfaction and Discharge of Indenture |
32 | ||||
| Section 8.2 |
Application of Trust Funds; Indemnification |
33 | ||||
| Section 8.3 |
Legal Defeasance of Securities of any Series |
33 | ||||
| Section 8.4 |
Covenant Defeasance |
35 | ||||
| Section 8.5 |
Repayment to Company |
36 | ||||
| Section 8.6 |
Reinstatement |
36 | ||||
-ii-
TABLE OF CONTENTS
(continued)
| Page | ||||||
| ARTICLE IX AMENDMENTS AND WAIVERS | 36 | |||||
| Section 9.1 |
Without Consent of Holders |
36 | ||||
| Section 9.2 |
With Consent of Holders |
37 | ||||
| Section 9.3 |
Limitations |
38 | ||||
| Section 9.4 |
Compliance with Trust Indenture Act |
38 | ||||
| Section 9.5 |
Revocation and Effect of Consents |
38 | ||||
| Section 9.6 |
Notation on or Exchange of Securities |
39 | ||||
| Section 9.7 |
Trustee Protected |
39 | ||||
| ARTICLE X MISCELLANEOUS | 39 | |||||
| Section 10.1 |
Trust Indenture Act Controls |
39 | ||||
| Section 10.2 |
Notices |
40 | ||||
| Section 10.3 |
Communication by Holders with Other Holders |
40 | ||||
| Section 10.4 |
Certificate and Opinion as to Conditions Precedent |
41 | ||||
| Section 10.5 |
Statements Required in Certificate or Opinion |
41 | ||||
| Section 10.6 |
Rules by Trustee and Agents |
41 | ||||
| Section 10.7 |
Legal Holidays |
41 | ||||
| Section 10.8 |
No Recourse Against Others |
42 | ||||
| Section 10.9 |
Counterparts |
42 | ||||
| Section 10.10 |
Governing Law; Jury Trial Waiver |
42 | ||||
| Section 10.11 |
No Adverse Interpretation of Other Agreements |
42 | ||||
| Section 10.12 |
Successors |
42 | ||||
| Section 10.13 |
Severability |
43 | ||||
| Section 10.14 |
Table of Contents, Headings, Etc |
43 | ||||
| Section 10.15 |
Securities in a Foreign Currency |
43 | ||||
| Section 10.16 |
Judgment Currency |
43 | ||||
| Section 10.17 |
Force Majeure |
44 | ||||
| Section 10.18 |
U.S.A. Patriot Act |
44 | ||||
| ARTICLE XI SINKING FUNDS | 44 | |||||
| Section 11.1 |
Applicability of Article |
44 | ||||
| Section 11.2 |
Satisfaction of Sinking Fund Payments with Securities |
45 | ||||
| Section 11.3 |
Redemption of Securities for Sinking Fund |
45 | ||||
| ARTICLE XII SUBORDINATION OF SECURITIES | 46 | |||||
| Section 12.1 |
Subordination of Terms |
46 | ||||
-iii-
CRESCO LABS INC.
Reconciliation and tie between Trust Indenture Act of 1939 and
Indenture, dated as of , 20
| § 310(a)(1) | 7.10 | |
| (a)(2) | 7.10 | |
| (a)(3) | Not Applicable | |
| (a)(4) | Not Applicable | |
| (a)(5) | 7.10 | |
| (b) | 7.10 | |
| § 311(a) | 7.11 | |
| (b) | 7.11 | |
| § 312(a) | 2.7 | |
| (b) | 10.3 | |
| (c) | 10.3 | |
| § 313(a) | 7.6 | |
| (b)(1) | 7.6 | |
| (b)(2) | 7.6 | |
| (c)(1) | 7.6 | |
| (d) | 7.6 | |
| § 314(a) | 4.2, 10.5 | |
| (b) | Not Applicable | |
| (c)(1) | 10.4 | |
| (c)(2) | 10.4 | |
| (c)(3) | Not Applicable | |
| (d) | Not Applicable | |
| (e) | 10.5 | |
| (f) | Not Applicable | |
| § 315(a) | 7.1 | |
| (b) | 7.5 | |
| (c) | 7.1 | |
| (d) | 7.1 | |
| (e) | 6.14 | |
-iv-
| § 316(a) | 2.11 | |
| (a)(1)(A) | 6.12 | |
| (a)(1)(B) | 6.13 | |
| (b) | 6.8 | |
| § 317(a)(1) | 6.3 | |
| (a)(2) | 6.4 | |
| (b) | 2.6 | |
| § 318(a) | 10.1 | |
Note: This reconciliation and tie shall not, for any purpose, be deemed to be part of the Indenture.
-v-
Indenture dated as of , 20 , between CRESCO LABS INC., a corporation organized under the laws of British Columbia, Canada (“Company”), and , as trustee (“Trustee”).
Each party agrees as follows for the benefit of the other party and for the equal and ratable benefit of the Holders of the Securities issued under this Indenture.
ARTICLE I
DEFINITIONS AND INCORPORATION BY REFERENCE
Section 1.1 Definitions.
“Affiliate” of any specified person means any other person directly or indirectly controlling or controlled by or under common control with such specified person. For the purposes of this definition, “control” (including, with correlative meanings, the terms “controlled by” and “under common control with”), as used with respect to any person, shall mean the possession, directly or indirectly, of the power to direct or cause the direction of the management or policies of such person, whether through the ownership of voting securities or by agreement or otherwise.
“Agent” means any Registrar or Paying Agent.
“Board of Directors” means the board of directors of the Company or any duly authorized committee thereof.
“Board Resolution” means a copy of a resolution certified by the Secretary or an Assistant Secretary of the Company to have been adopted by the Board of Directors or pursuant to authorization by the Board of Directors and to be in full force and effect on the date of the certification and delivered to the Trustee.
“Business Day” means, for a particular Series, any day except a Saturday, Sunday or any day, including a legal holiday, on which banking institutions are authorized or required by law, regulation or executive order to close in The City of New York (or in connection with any payment, the place of payment).
“Capital Stock” of any person means any and all shares, interests, participations, rights or other equivalents (however designated) of the equity of such person.
“Certificated Securities” means definitive Securities in registered non-global certificated form.
“Company” means the party named as such above until a successor, which duly assumes the obligations under this Indenture, replaces it and thereafter means the successor.
“Company Order” means a written order signed in the name of the Company by an Officer.
- 1 -
“Corporate Trust Office” means the office of the Trustee at which at any particular time its corporate trust business related to this Indenture shall be principally administered, which office at the date hereof is located at , Attention: , or such other address as the Trustee may designate from time to time by notice to the Holders and the Company, or the corporate trust office of any successor Trustee at which this Indenture shall be administered (or such other address as a successor Trustee may designate from time to time by notice to the Holders of the Company).
“Default” means any event which is, or after notice or passage of time or both would be, an Event of Default.
“Depositary” means, with respect to the Securities of any Series issuable or issued in whole or in part in the form of one or more Global Securities, the person designated as Depositary for such Series by the Company, which Depositary shall be a clearing agency registered under the Exchange Act; and if at any time there is more than one such person, “Depositary” as used with respect to the Securities of any Series shall mean the Depositary with respect to the Securities of such Series.
“Discount Security” means any Security that provides for an amount less than the stated principal amount thereof to be due and payable upon declaration of acceleration of the maturity thereof pursuant to Section 6.2.
“Dollars” and “$” means the currency of The United States of America.
“Exchange Act” means the Securities Exchange Act of 1934, as amended.
“Foreign Currency” means any currency or currency unit issued by a government other than the government of The United States of America.
“Foreign Government Obligations” means, with respect to Securities of any Series that are denominated in a Foreign Currency, direct obligations of, or obligations guaranteed by, the government that issued or caused to be issued such currency for the payment of which obligations its full faith and credit is pledged and which are not callable or redeemable at the option of the issuer thereof.
“GAAP” means accounting principles generally accepted in The United States of America set forth in the opinions and pronouncements of the Accounting Principles Board of the American Institute of Certified Public Accountants and statements and pronouncements of the Financial Accounting Standards Board or in such other statements by such other entity as have been approved by a significant segment of the accounting profession, which are in effect as of the date of determination.
“Global Security” or “Global Securities” means a Security or Securities, as the case may be, in the form established pursuant to Section 2.2 evidencing all or part of a Series of Securities, issued to the Depositary for such Series or its nominee, and registered in the name of such Depositary or nominee.
- 2 -
“Holder” or “Securityholder” means a person in whose name a Security is registered on the books of the Registrar.
“Indenture” means this Indenture as amended or supplemented from time to time and shall include the form and terms of particular Series of Securities established as contemplated hereunder.
“interest” means, with respect to any Security, any interest on such Security, and with respect to any Discount Security which by its terms bears interest only after Maturity, interest payable after Maturity.
“Maturity,” when used with respect to any Security, means the date on which the principal of such Security becomes due and payable as therein or herein provided, whether at the Stated Maturity or by declaration of acceleration, call for redemption or otherwise.
“Officer” means the Chairman of the Board of Directors, the Chief Executive Officer, the Chief Financial Officer, the President, any Senior Vice President or Vice President, the Treasurer, Assistant Treasurer, Secretary or Assistant Secretary of the Company.
“Officer’s Certificate” means a certificate signed by any Officer (or any person designated in writing by an Officer of the Company as authorized to execute and deliver Officer’s Certificates) and delivered to the Trustee.
“Opinion of Counsel” means a written opinion of legal counsel. The counsel may be an employee of or counsel to the Company. Opinions of Counsel required to be delivered under this Indenture may have qualifications customary for opinions of the type required.
“person” means any individual, corporation, company, voluntary association, partnership, trust, joint venture, limited liability company, unincorporated organization or government or any agency, instrumentality or political subdivision thereof.
“principal” of a Security means the principal of the Security plus, when appropriate, the premium, if any, on the Security.
“Responsible Officer” means any officer of the Trustee in its Corporate Trust Office having direct responsibility for administration of this Indenture and also means, with respect to a particular corporate trust matter, any other officer to whom any corporate trust matter is referred because of his or her knowledge of and familiarity with a particular subject and who shall have direct responsibility for the administration of this Indenture.
“SEC” means the Securities and Exchange Commission.
“Securities” means the subordinated debentures, notes or other debt instruments of the Company of any Series authenticated and delivered under this Indenture.
“Series” or “Series of Securities” means each series of debentures, notes or other debt instruments of the Company created pursuant to Sections 2.1 and 2.2 hereof.
- 3 -
“Stated Maturity” when used with respect to any Security, means the date specified in such Security as the fixed date on which the principal of such Security is due and payable.
“Subsidiary” means, with respect to any person, any corporation, partnership, joint venture, limited liability company or other business entity of which a majority of the outstanding shares of Capital Stock or other interests having the power to vote in the election of directors, managers or trustees thereof is at the time directly or indirectly owned or controlled by such person or one or more of the other Subsidiaries of such person, or a combination thereof.
“TIA” means the Trust Indenture Act of 1939 (15 U.S. Code §§ 77aaa-77bbbb) as in effect on the date of this Indenture; provided, however, that in the event the Trust Indenture Act of 1939 is amended after such date, “TIA” means, to the extent required by any such amendment, the Trust Indenture Act as so amended.
“Trustee” means the person named as the “Trustee” in the first paragraph of this instrument until a successor Trustee shall have become such pursuant to the applicable provisions of this Indenture, and thereafter “Trustee” shall mean or include each person who is then a Trustee hereunder, and if at any time there is more than one such person, “Trustee” as used with respect to the Securities of any Series shall mean the Trustee with respect to Securities of that Series.
“United States” or “U.S.” means The United States of America (including the states thereof and the District of Columbia), its territories and possessions and other areas subject to its jurisdiction.
“U.S. Government Obligations” means securities which are direct obligations of, or guaranteed by, The United States of America for the payment of which its full faith and credit is pledged and which are not callable or redeemable at the option of the issuer thereof, and shall also include a depository receipt issued by a bank or trust company as custodian with respect to any such U.S. Government Obligation or a specific payment of interest on or principal of any such U.S. Government Obligation held by such custodian for the account of the holder of a depository receipt, provided that (except as required by law) such custodian is not authorized to make any deduction from the amount payable to the holder of such depository receipt from any amount received by the custodian in respect of the U.S. Government Obligation evidenced by such depository receipt.
Section 1.2 Other Definitions.
| TERM | DEFINED IN SECTION | |||
| “Bankruptcy Law” |
6.1 | |||
| “Custodian” |
6.1 | |||
| “Event of Default” |
6.1 | |||
| “Judgment Currency” |
10.16 | |||
| “Legal Holiday” |
10.7 | |||
- 4 -
| “mandatory sinking fund payment” |
11.1 | |||
| “optional sinking fund payment” |
11.1 | |||
| “Paying Agent” |
2.5 | |||
| “Registrar” |
2.5 | |||
| “Required Currency” |
10.16 | |||
| “successor person” |
5.1 |
Section 1.3 Incorporation by Reference of Trust Indenture Act.
Whenever this Indenture refers to a provision of the TIA, the provision is incorporated by reference in and made a part of this Indenture. The following TIA terms used in this Indenture have the following meanings:
“Commission” means the SEC.
“indenture securities” means the Securities.
“indenture security holder” means a Securityholder.
“indenture to be qualified” means this Indenture.
“indenture trustee” or “institutional trustee” means the Trustee.
“obligor” on the indenture securities means the Company and any successor obligor upon the Securities.
All other terms used in this Indenture that are defined by the TIA, defined by TIA reference to another statute or defined by SEC rule under the TIA and not otherwise defined herein are used herein as so defined.
Section 1.4 Rules of Construction.
Unless the context otherwise requires:
(a) a term has the meaning assigned to it;
(b) an accounting term not otherwise defined has the meaning assigned to it in accordance with GAAP;
(c) “or” is not exclusive;
(d) words in the singular include the plural, and in the plural include the singular; and
(e) provisions apply to successive events and transactions.
- 5 -
ARTICLE II
THE SECURITIES
Section 2.1 Issuable in Series.
The aggregate principal amount of Securities that may be authenticated and delivered under this Indenture is unlimited. The Securities may be issued in one or more Series. All Securities of a Series shall be identical except as may be set forth or determined in the manner provided in a Board Resolution, supplemental indenture hereto or Officer’s Certificate establishing the terms of such Series. In the case of Securities of a Series to be issued from time to time, the Board Resolution, Officer’s Certificate or supplemental indenture establishing the terms thereof may provide for the method by which specified terms (such as interest rate, maturity date, record date or date from which interest shall accrue) are to be determined. Securities may differ between Series in respect of any matters, provided that all Series of Securities shall be equally and ratably entitled to the benefits of this Indenture.
Section 2.2 Establishment of Terms of Series of Securities.
At or prior to the issuance of any Securities within a Series, the following shall be established (as to the Series generally, in the case of Subsection 2.2.1 and either as to such Securities within the Series or as to the Series generally in the case of Subsections 2.2.2 through 2.2.24) by or pursuant to a Board Resolution, and set forth or determined in the manner provided in a Board Resolution, supplemental indenture hereto or Officer’s Certificate:
2.2.1 the title (which shall distinguish the Securities of that particular Series from the Securities of any other Series) of the Series;
2.2.2 the price or prices (expressed as a percentage of the principal amount thereof) at which the Securities of the Series will be issued;
2.2.3 any limit upon the aggregate principal amount of the Securities of the Series which may be authenticated and delivered under this Indenture (except for Securities authenticated and delivered upon registration of transfer of, or in exchange for, or in lieu of, other Securities of the Series pursuant to Section 2.8, 2.9, 2.12, 3.6 or 9.6);
2.2.4 the date or dates on which the principal of the Securities of the Series is payable;
2.2.5 the rate or rates (which may be fixed or variable) per annum or, if applicable, the method used to determine such rate or rates (including, but not limited to, any commodity, commodity index, stock exchange index or financial index) at which the Securities of the Series shall bear interest, if any, the date or dates from which such interest, if any, shall accrue, the date or dates on which such interest, if any, shall commence and be payable and any regular record date for the interest payable on any interest payment date;
2.2.6 the place or places where the principal of and interest, if any, on the Securities of the Series shall be payable, where the Securities of such Series may be surrendered for registration of transfer or exchange and where notices and demands to or upon the Company in respect of the Securities of such Series and this Indenture may be delivered, and the method of such payment, if by wire transfer, mail or other means;
- 6 -
2.2.7 if applicable, the period or periods within which, the price or prices at which and the terms and conditions upon which the Securities of the Series must be redeemed or may be redeemed, in whole or in part, at the option of the Company;
2.2.8 the obligation, if any, of the Company to redeem or purchase the Securities of the Series pursuant to any sinking fund or analogous provisions or at the option of a Holder thereof and the period or periods within which, the price or prices at which and the terms and conditions upon which Securities of the Series shall be redeemed or purchased, in whole or in part, pursuant to such obligation;
2.2.9 the dates, if any, on which and the price or prices at which the Securities of the Series will be repurchased by the Company at the option of the Holders thereof and other detailed terms and provisions of such repurchase obligations;
2.2.10 if other than denominations of $1,000 and integral multiples of $1,000 in excess thereof, the denominations in which the Securities of the Series shall be issuable;
2.2.11 the forms of the Securities of the Series and whether the Securities will be issuable as Global Securities;
2.2.12 if other than the principal amount thereof, the portion of the principal amount of the Securities of the Series that shall be payable upon declaration of acceleration of the maturity thereof pursuant to Section 6.2;
2.2.13 the currency of denomination of the Securities of the Series, which may be Dollars or any Foreign Currency, and if such currency of denomination is a composite currency, the agency or organization, if any, responsible for overseeing such composite currency;
2.2.14 the designation of the currency, currencies or currency units in which payment of the principal of and interest, if any, on the Securities of the Series will be made;
2.2.15 if payments of principal of or interest, if any, on the Securities of the Series are to be made in one or more currencies or currency units other than that or those in which such Securities are denominated, the manner in which the exchange rate with respect to such payments will be determined;
2.2.16 the manner in which the amounts of payment of principal of or interest, if any, on the Securities of the Series will be determined, if such amounts may be determined by reference to an index based on a currency or currencies or by reference to a commodity, commodity index, stock exchange index or financial index;
2.2.17 the provisions, if any, relating to any security provided for the Securities of the Series;
- 7 -
2.2.18 any addition to, deletion of or change in the Events of Default which applies to any Securities of the Series and any change in the right of the Trustee or the requisite Holders of such Securities to declare the principal amount thereof due and payable pursuant to Section 6.2;
2.2.19 any addition to, deletion of or change in the covenants set forth in Articles IV or V which applies to Securities of the Series;
2.2.20 any Depositaries, trustees, interest rate calculation agents, exchange rate calculation agents or other agents with respect to Securities of such Series if other than those appointed herein;
2.2.21 the provisions, if any, relating to conversion or exchange of any Securities of such Series, including if applicable, the conversion or exchange price, the conversion or exchange period, the securities or other property into which the Securities will be convertible, provisions as to whether conversion or exchange will be mandatory, at the option of the Holders thereof or at the option of the Company, the events requiring an adjustment of the conversion price or exchange price and provisions affecting conversion or exchange if such Series of Securities are redeemed;
2.2.22 whether any of the Company’s direct or indirect Subsidiaries will guarantee the Securities of that Series, including the terms of subordination, if any, of such guarantees;
2.2.23 the subordination terms of the Securities of the Series; and
2.2.24 any other terms of the Series (which may supplement, modify or delete any provision of this Indenture insofar as it applies to such Series), including any terms that may be required under applicable law or regulations or advisable in connection with the marketing of Securities of that Series.
All Securities of any one Series need not be issued at the same time and may be issued from time to time, consistent with the terms of this Indenture, if so provided by or pursuant to the Board Resolution, supplemental indenture hereto or Officer’s Certificate referred to above.
Section 2.3 Denominations; Provision for Payment.
The Securities of any Series shall be issuable, except as otherwise provided with respect to Securities of any Series pursuant to Section 2.2, as registered Securities in the denominations of one thousand Dollars ($1,000) or any integral multiples of $1,000 in excess thereof. Unless otherwise provided with respect to Securities of any Series pursuant to Section 2.2, the principal of and the interest on the Securities of any Series, if any, thereon, shall by payable in Dollars at the Corporate Trust Office of the Trustee. Unless otherwise specified pursuant to Section 2.2 with respect to any Securities of any Series, interest on the Securities of any Series shall be computed on the basis of a 360-day year consisting of twelve 30-day months.
- 8 -
Section 2.4 Execution and Authentication.
Two Officers shall sign the Securities for the Company by manual or facsimile signature.
If an Officer whose signature is on a Security no longer holds that office at the time the Security is authenticated, the Security shall nevertheless be valid.
A Security shall not be valid until authenticated by the manual signature of the Trustee or an authenticating agent. The signature shall be conclusive evidence that the Security has been authenticated under this Indenture.
The Trustee shall at any time, and from time to time, authenticate Securities for original issue in the principal amount provided in the Board Resolution, supplemental indenture hereto or Officer’s Certificate, upon receipt by the Trustee of a Company Order. Each Security shall be dated the date of its authentication.
The aggregate principal amount of Securities of any Series outstanding at any time may not exceed any limit upon the maximum principal amount for such Series set forth in the Board Resolution, supplemental indenture hereto or Officer’s Certificate delivered pursuant to Section 2.2, except as provided in Section 2.9.
Prior to the issuance of Securities of any Series, the Trustee shall have received and (subject to Section 7.1) shall be fully protected in conclusively relying on: (a) the Board Resolution, supplemental indenture hereto or Officer’s Certificate delivered pursuant to Section 2.2 establishing the form of the Securities of that Series or of Securities within that Series and the terms of the Securities of that Series or of Securities within that Series, (b) an Officer’s Certificate complying with Section 9.7 (with respect to the execution of supplemental indentures) and Section 10.4, and (c) an Opinion of Counsel complying with Section 9.7 (with respect to the execution of supplemental indentures) and Section 10.4.
The Trustee shall have the right, but not the obligation, to decline to authenticate and deliver any Securities of such Series: (a) if the Trustee, being advised by counsel, determines that such action may not be taken lawfully; or (b) if the Trustee in good faith determines that such action would expose the Trustee to personal liability.
The Trustee may appoint an authenticating agent acceptable to the Company to authenticate Securities. An authenticating agent may authenticate Securities whenever the Trustee may do so. Each reference in this Indenture to authentication by the Trustee includes authentication by such agent. An authenticating agent has the same rights as an Agent to deal with the Company or an Affiliate of the Company.
Section 2.5 Registrar and Paying Agent.
The Company shall maintain, with respect to each Series of Securities, at the place or places specified with respect to such Series, an office or agency where Securities of such Series may be presented or surrendered for payment (“Paying Agent”) and where Securities of such Series may be surrendered for registration of transfer or exchange (“Registrar”). The Registrar shall keep a register with respect to each Series of Securities and to their transfer and exchange.
- 9 -
The Company will give prompt written notice to the Trustee of the name and address, and any change in the name or address, of each Registrar or Paying Agent. If at any time the Company shall fail to maintain any such required Registrar or Paying Agent or shall fail to furnish the Trustee with the name and address thereof, such presentations and surrenders may be made or served at the Corporate Trust Office of the Trustee, and the Company hereby appoints the Trustee as its agent to receive all such presentations and surrenders.
The Company may also from time to time designate one or more co-registrars or additional paying agents and may from time to time rescind such designations; provided, however, that no such designation or rescission shall in any manner relieve the Company of its obligations to maintain a Registrar or Paying Agent in each place so specified for Securities of any Series for such purposes. The Company will give prompt written notice to the Trustee of any such designation or rescission and of any change in the name or address of any such co-registrar or additional paying agent. The term “Registrar” includes any co-registrar; and the term “Paying Agent” includes any additional paying agent. The Company or any of its Affiliates may serve as Registrar or Paying Agent.
The Company hereby appoints the Trustee as the initial Registrar and Paying Agent for each Series unless another Registrar or Paying Agent, as the case may be, is appointed prior to the time Securities of that Series are first issued.
Section 2.6 Paying Agent to Hold Money in Trust.
The Company shall require each Paying Agent other than the Trustee to agree in writing that the Paying Agent will hold in trust, for the benefit of Securityholders of any Series of Securities, or the Trustee, all money held by the Paying Agent for the payment of principal of or interest on the Securities of that Series, and will notify the Trustee in writing of any default by the Company in making any such payment. While any such default continues, the Trustee may require a Paying Agent to pay all money held by it to the Trustee. The Company at any time may require a Paying Agent to pay all money held by it to the Trustee. Upon payment over to the Trustee, the Paying Agent (if other than the Company or a Subsidiary of the Company) shall have no further liability for the money. If the Company or a Subsidiary of the Company acts as Paying Agent, it shall segregate and hold in a separate trust fund for the benefit of Securityholders of any Series of Securities all money held by it as Paying Agent. Upon any bankruptcy, reorganization or similar proceeding with respect to the Company, the Trustee shall serve as Paying Agent for the Securities.
Section 2.7 Securityholder Lists.
The Trustee shall preserve in as current a form as is reasonably practicable the most recent list available to it of the names and addresses of Securityholders of each Series of Securities and shall otherwise comply with TIA § 312(a). If the Trustee is not the Registrar, the Company shall furnish to the Trustee at least ten days before each interest payment date and at such other times as the Trustee may request in writing a list, in such form and as of such date as the Trustee may reasonably require, of the names and addresses of Securityholders of each Series of Securities.
- 10 -
Section 2.8 Transfer and Exchange.
Where Securities of a Series are presented to the Registrar or a co-registrar with a request to register a transfer or to exchange them for an equal principal amount of Securities of the same Series, the Registrar shall register the transfer or make the exchange if its requirements for such transactions are met. To permit registrations of transfers and exchanges, the Trustee shall authenticate Securities at the Registrar’s request. No service charge shall be made for any registration of transfer or exchange (except as otherwise expressly permitted herein), but the Company may require payment of a sum sufficient to cover any transfer tax or similar governmental charge payable in connection therewith (other than any such transfer tax or similar governmental charge payable upon exchanges pursuant to Sections 2.12, 3.6 or 9.6).
Neither the Company nor the Registrar shall be required (a) to issue, register the transfer of, or exchange Securities of any Series for the period beginning at the opening of business fifteen days immediately preceding the mailing of a notice of redemption of Securities of that Series selected for redemption and ending at the close of business on the day of such mailing, or (b) to register the transfer of or exchange Securities of any Series selected, called or being called for redemption as a whole or the portion being redeemed of any such Securities selected, called or being called for redemption in part.
Section 2.9 Mutilated, Destroyed, Lost and Stolen Securities.
If any mutilated Security is surrendered to the Trustee, the Company shall execute and the Trustee shall authenticate and deliver in exchange therefor a new Security of the same Series and of like tenor and principal amount and bearing a number not contemporaneously outstanding.
If there shall be delivered to the Company and the Trustee (a) evidence to their satisfaction of the destruction, loss or theft of any Security and (b) such security or indemnity bond as may be required by each of them to hold itself and any of its agents harmless, then, in the absence of written notice to the Company or the Trustee that such Security has been acquired by a bona fide purchaser, the Company shall execute and upon receipt of a Company Order the Trustee shall authenticate and make available for delivery, in lieu of any such destroyed, lost or stolen Security, a new Security of the same Series and of like tenor and principal amount and bearing a number not contemporaneously outstanding.
In case any such mutilated, destroyed, lost or stolen Security has become or is about to become due and payable, the Company in its discretion may, instead of issuing a new Security, pay such Security.
Upon the issuance of any new Security under this Section 2.9, the Company may require the payment of a sum sufficient to cover any tax or other governmental charge that may be imposed in relation thereto and any other expenses (including the fees and expenses of the Trustee) connected therewith.
Every new Security of any Series issued pursuant to this Section 2.9 in lieu of any destroyed, lost or stolen Security shall constitute an original additional contractual obligation of the Company, whether or not the destroyed, lost or stolen Security shall be at any time
- 11 -
enforceable by anyone, and shall be entitled to all the benefits of this Indenture equally and proportionately with any and all other Securities of that Series duly issued hereunder.
The provisions of this Section 2.9 are exclusive and shall preclude (to the extent lawful) all other rights and remedies with respect to the replacement or payment of mutilated, destroyed, lost or stolen Securities.
Section 2.10 Outstanding Securities.
The Securities outstanding at any time are all the Securities authenticated by the Trustee except for those canceled by the Registrar and those described in this Section 2.10 as not outstanding.
If a Security is replaced pursuant to Section 2.9, it ceases to be outstanding unless the Trustee receives proof satisfactory to it that the replaced Security is held by a bona fide purchaser.
If the Paying Agent (other than the Company, a Subsidiary of the Company or an Affiliate of the Company) holds on the Maturity of Securities of a Series money sufficient to pay such Securities payable on that date, then on and after that date such Securities of the Series cease to be outstanding and interest on them ceases to accrue.
The Company may purchase or otherwise acquire the Securities, whether by open market purchases, negotiated transactions or otherwise. A Security does not cease to be outstanding because the Company or an Affiliate of the Company holds the Security.
In determining whether the Holders of the requisite principal amount of outstanding Securities have given any request, demand, authorization, direction, notice, consent or waiver hereunder, the principal amount of a Discount Security that shall be deemed to be outstanding for such purposes shall be the amount of the principal thereof that would be due and payable as of the date of such determination upon a declaration of acceleration of the Maturity thereof pursuant to Section 6.2.
Section 2.11 Treasury Securities.
In determining whether the Holders of the required principal amount of Securities of a Series have concurred in any request, demand, authorization, direction, notice, consent or waiver, Securities of a Series owned by the Company or any Affiliate of the Company shall be disregarded, except that for the purposes of determining whether the Trustee shall be protected in conclusively relying on any such request, demand, authorization, direction, notice, consent or waiver, only Securities of a Series that a Responsible Officer of the Trustee actually knows are so owned shall be so disregarded. Securities so owned which have been pledged in good faith shall not be disregarded if the pledgee establishes to the satisfaction of the Trustee the pledgee’s right to deliver any such request, demand, authorization, direction, notice, consent or waiver with respect to the Securities and that the pledgee is not the Company or any other obligor upon the Securities or any Affiliate of the Company or of such other obligor.
- 12 -
Section 2.12 Temporary Securities.
Until definitive Securities are ready for delivery, the Company may prepare and the Trustee shall authenticate temporary Securities upon a Company Order. Temporary Securities shall be substantially in the form of definitive Securities but may have variations that the Company considers appropriate for temporary Securities. Without unreasonable delay, the Company shall prepare and the Trustee upon receipt of a Company Order shall authenticate definitive Securities of the same Series and date of maturity in exchange for temporary Securities. Until so exchanged, temporary Securities shall have the same rights under this Indenture as the definitive Securities.
Section 2.13 Cancellation.
The Company at any time may deliver Securities to the Trustee for cancellation. The Registrar and the Paying Agent, if not the Trustee, shall forward to the Trustee any Securities surrendered to them for registration of transfer, exchange or payment. The Trustee shall cancel all Securities surrendered for transfer, exchange, payment, replacement, conversion or cancellation and shall dispose of such canceled Securities (subject to the record retention requirement of the Exchange Act and the Trustee) in accordance with its customary procedures and deliver a certificate of such cancellation to the Company upon written request of the Company. The Company may not issue new Securities to replace Securities that it has paid or delivered to the Trustee for cancellation.
Section 2.14 Defaulted Interest.
If the Company defaults in a payment of interest on a Series of Securities, it may pay the defaulted interest, plus, to the extent permitted by law, any interest payable on the defaulted interest, to the persons who are Securityholders of the Series on a subsequent special record date. The Company shall fix the record date and payment date. At least 10 days before the special record date, the Company shall mail to the Trustee and to each Securityholder of the Series a notice that states the special record date, the payment date and the amount of interest to be paid. The Company may pay defaulted interest in any other lawful manner.
Section 2.15 Global Securities.
2.15.1 Terms of Securities. A Board Resolution, a supplemental indenture hereto or an Officer’s Certificate shall establish whether the Securities of a Series shall be issued in whole or in part in the form of one or more Global Securities and the Depositary for such Global Security or Securities.
2.15.2 Transfer and Exchange. Notwithstanding any provisions to the contrary contained in Section 2.8 of this Indenture and in addition thereto, any Global Security shall be exchangeable pursuant to Section 2.8 of this Indenture for Securities registered in the names of Holders other than the Depositary for such Security or its nominee only if (a) such Depositary notifies the Company that it is unwilling or unable to continue as Depositary for such Global Security or if at any time such Depositary ceases to be a clearing agency registered under the Exchange Act, and, in either case, the Company fails to appoint a successor Depositary registered as a clearing agency under the Exchange Act within 90 days of such event or (b) the
- 13 -
Company determines in its sole discretion not to have such Securities represented by one or more Global Securities and executes and delivers to the Trustee an Officer’s Certificate to the effect that such Global Security shall be so exchangeable. Any Global Security that is exchangeable pursuant to the preceding sentence shall be exchangeable for Securities registered in such names as the Depositary shall direct in writing in an aggregate principal amount equal to the principal amount of the Global Security with like tenor and terms.
Except as provided in this Section 2.15.2, a Global Security may not be transferred except as a whole by the Depositary with respect to such Global Security to a nominee of such Depositary, by a nominee of such Depositary to such Depositary or another nominee of such Depositary or by the Depositary or any such nominee to a successor Depositary or a nominee of such a successor Depositary.
2.15.3 Legend. Any Global Security issued hereunder shall bear a legend in substantially the following form:
“THIS SECURITY IS A GLOBAL SECURITY WITHIN THE MEANING OF THE INDENTURE HEREINAFTER REFERRED TO AND IS REGISTERED IN THE NAME OF THE DEPOSITARY OR A NOMINEE OF THE DEPOSITARY. THIS SECURITY IS EXCHANGEABLE FOR SECURITIES REGISTERED IN THE NAME OF A PERSON OTHER THAN THE DEPOSITARY OR ITS NOMINEE ONLY IN THE LIMITED CIRCUMSTANCES DESCRIBED IN THE INDENTURE, AND MAY NOT BE TRANSFERRED EXCEPT AS A WHOLE BY THE DEPOSITARY TO A NOMINEE OF THE DEPOSITARY, BY A NOMINEE OF THE DEPOSITARY TO THE DEPOSITARY OR ANOTHER NOMINEE OF THE DEPOSITARY, OR BY THE DEPOSITARY OR ANY SUCH NOMINEE TO A SUCCESSOR DEPOSITARY OR A NOMINEE OF SUCH A SUCCESSOR DEPOSITARY.”
2.15.4 Acts of Holders. The Depositary, as a Holder, may appoint agents and otherwise authorize participants to give or take any request, demand, authorization, direction, notice, consent, waiver or other action which a Holder is entitled to give or take under this Indenture.
2.15.5 Payments. Notwithstanding the other provisions of this Indenture, unless otherwise specified as contemplated by Section 2.2, payment of the principal of and interest, if any, on any Global Security shall be made to the Holder thereof, which in the case of a Depositary therefor will be made in accordance with its applicable procedures.
2.15.6 Consents, Declaration and Directions. The Company, the Trustee and any Agent shall treat a person as the Holder of such principal amount of outstanding Securities of such Series represented by a Global Security as shall be specified in a written statement of the Depositary or by the applicable procedures of such Depositary with respect to such Global Security, for purposes of obtaining any consents, declarations, waivers or directions required to be given by the Holders pursuant to this Indenture.
- 14 -
Section 2.16 CUSIP Numbers.
The Company in issuing the Securities may use “CUSIP” numbers (if then generally in use), and, if so, the Trustee shall use “CUSIP” numbers in notices of redemption as a convenience to Holders; provided that any such notice may state that no representation is made as to the correctness of such numbers either as printed on the Securities or as contained in any notice of a redemption and that reliance may be placed only on the other elements of identification printed on the Securities, and any such redemption shall not be affected by any defect in or omission of such numbers. The Trustee shall have no liability for any defect in the “CUSIP” numbers as they appear on any Security, notice or elsewhere. The Company will promptly notify the Trustee in writing of any change in the “CUSIP” numbers.
ARTICLE III
REDEMPTION
Section 3.1 Notice to Trustee.
The Company may, with respect to any Series of Securities, reserve the right to redeem and pay the Series of Securities or may covenant to redeem and pay the Series of Securities or any part thereof prior to the Stated Maturity thereof at such time and on such terms as provided for in such Securities. If a Series of Securities is redeemable and the Company wants or is obligated to redeem prior to the Stated Maturity thereof all or part of the Series of Securities pursuant to the terms of such Securities, it shall notify the Trustee in writing of the redemption date and the principal amount of Series of Securities to be redeemed. The Company shall give the notice to the Trustee at least 45 days before the redemption date, unless a shorter period is satisfactory to the Trustee.
Section 3.2 Selection of Securities to be Redeemed.
Unless otherwise indicated for a particular Series by a Board Resolution, a supplemental indenture hereto or an Officer’s Certificate, if less than all the Securities of a Series are to be redeemed, the Trustee shall select the Securities of the Series to be redeemed in any manner that the Trustee deems fair and appropriate, including selecting by lot or other method, unless otherwise required by law or applicable stock exchange requirements, subject, in the case of Global Securities, to the applicable rules and procedures of the Depositary; provided that the unredeemed portion of the principal amount of any Security shall be in an authorized denomination (which shall not be less than the minimum authorized denomination) for such Security. The Trustee shall make the selection from Securities of the Series outstanding not previously called for redemption. Provisions of this Indenture that apply to Securities of a Series called for redemption also apply to portions of Securities of that Series called for redemption.
Section 3.3 Notice of Redemption.
Unless otherwise indicated for a particular Series by Board Resolution, a supplemental indenture hereto or an Officer’s Certificate, at least 30 days but not more than 60 days before a redemption date, the Company shall mail a notice of redemption by first-class mail to each Holder whose Securities are to be redeemed.
- 15 -
The notice shall identify the Securities of the Series to be redeemed and shall state:
(a) the redemption date;
(b) the redemption price and the amount of accrued interest, if any, to be paid;
(c) the name and address of the Paying Agent and, if applicable, the conversion Agent;
(d) for convertible Securities, the conversion price;
(e) if any Global Security is being redeemed in part, the portion of the principal amount of such Global Security to be redeemed and that, after the redemption date upon surrender of such Global Security, the principal amount thereof will be decreased by the portion thereof redeemed pursuant thereto;
(f) if any Certificated Security is being redeemed in part, the portion of the principal amount of such Security to be redeemed, and that, after the redemption date, upon surrender of such Security, a new Certificated Security in principal amount equal to the unredeemed portion thereof will be issued in the name of the Holder thereof upon cancellation of the original Certificated Security;
(g) that Securities of the Series (or portion thereof) called for redemption must be surrendered to the Paying Agent to collect the redemption price;
(h) that interest on Securities of the Series called for redemption ceases to accrue on and after the redemption date unless the Company defaults in the deposit of the redemption price;
(i) the CUSIP number, if any, and state that no representation is made as to the correctness or accuracy of the CUSIP number, if any, listed in the SEC’s notice or printed on the Securities; and
(j) any other information as may be required by the terms of the particular Series or the Securities of a Series being redeemed.
At the Company’s request, the Trustee shall give the notice of redemption in the Company’s name and at its expense, provided, however, that the Company has delivered to the Trustee, at least 15 days (unless a shorter time shall be acceptable to the Trustee) prior to the notice date, an Officer’s Certificate requesting that the Trustee give such notice and setting forth the information to be stated in such notice.
Section 3.4 Effect of Notice of Redemption.
Once notice of redemption is mailed as provided in Section 3.3, Securities of a Series called for redemption become due and payable on the redemption date and at the redemption price. Except as otherwise provided in the supplemental indenture, Board Resolution or Officer’s Certificate for a Series, a notice of redemption may not be conditional. Upon surrender to the
- 16 -
Paying Agent, such Securities shall be paid at the redemption price plus accrued interest to the redemption date other than Securities or portions of Securities called for redemption which have been delivered by the Company to the Registrar for cancellation. The Paying Agent shall return to the Company any money not required for that purpose.
Unless the Company shall default in the payment of Securities (and accrued interest) called for redemption, interest on such Securities shall cease to accrue after the redemption date. Convertible Securities called for redemption shall cease to be convertible after the close of business on the Business Day immediately preceding the redemption date, unless the Company shall default in the payment of such Securities on the redemption date, in which event the Securities shall remain convertible until paid (together with accrued interest).
Failure to give notice of redemption, or any defect in such notice to the Holder of any Security of a Series designated for redemption, in whole or in part, shall not affect the sufficiency of any notice of redemption with respect to the Holder of any other Security of such Series.
Section 3.5 Deposit of Redemption Price.
On or before 10:00 a.m., New York City time, on the redemption date, the Company shall deposit with the Paying Agent money sufficient to pay the redemption price of and accrued interest, if any, on all Securities to be redeemed on that date.
Section 3.6 Securities Redeemed in Part.
Upon surrender of a Certificated Security that is redeemed in part, the Trustee shall authenticate for the Holder a new Certificated Security of the same Series and the same maturity equal in principal amount to the unredeemed portion of the Security surrendered and concurrently cancel the surrendered Certificated Security.
ARTICLE IV
COVENANTS
Section 4.1 Payment of Principal and Interest.
The Company covenants and agrees for the benefit of the Holders of each Series of Securities that it will duly and punctually pay the principal of and interest, if any, on the Securities of that Series in accordance with the terms of such Securities and this Indenture. On or before 10:00 a.m., New York City time, on the applicable payment date, the Company shall deposit with the Paying Agent money sufficient to pay the principal of and interest, if any, on the Securities of each Series in accordance with the terms of such Securities and this Indenture. Principal and interest shall be considered paid on the date due if the Paying Agent holds in accordance with this Indenture on that date money sufficient to pay all principal and interest then due and the Paying Agent is not prohibited from paying such money to the Holders on such date pursuant to the terms of this Indenture.
- 17 -
Section 4.2 Reports by Company.
(a) As long as any Securities are outstanding, the Company shall file with the Trustee, and transmit to the Holders, such information, documents and other reports, and such summaries thereof, as may be required pursuant to TIA § 314(a). All reports, information and documents referred to in this Section 4.2 will be deemed to be filed with the Trustee and transmitted to the Holders at the time such reports, information or documents are publicly filed with the SEC via the SEC’s EDGAR filing system (or any successor system), it being understood that the Trustee shall have no responsibility whatsoever to determine if such filings have been made.
(b) Delivery of reports, information and documents to the Trustee under this Section 4.2 are for informational purposes only and shall not constitute a representation or warranty as to the accuracy or completeness of the reports, information and documents. The Trustee’s receipt of the foregoing shall not constitute constructive notice of any information contained therein or determinable from information contained therein, including the Company’s compliance with any of its covenants hereunder (as to which the Trustee is entitled to rely exclusively on Officer’s Certificates).
Section 4.3 Compliance Certificate.
To the extent any Securities of a Series are outstanding, the Company shall deliver to the Trustee, within 120 days after the end of each fiscal year of the Company, an Officer’s Certificate (which need not contain the statements provided for in Section 10.4) from its principal executive officer, principal financial officer or principal accounting officer stating that a review of the activities of the Company and its Subsidiaries during the preceding fiscal year has been made under the supervision of the signing Officer with a view to determining whether the Company has kept, observed, performed and fulfilled its obligations under this Indenture, and further stating, as to such Officer signing such certificate, that to his or her knowledge the Company is not in default in the performance or observance of any of the terms, provisions and conditions hereof (or, if a Default or Event of Default shall have occurred, describing all such Defaults or Events of Default of which the Officer has knowledge). Such Officer’s Certificate need not include a reference to any non-compliance that has been fully cured prior to the date as of which such certificate speaks.
Section 4.4 Stay, Extension and Usury Laws.
The Company covenants (to the extent that it may lawfully do so) that it will not at any time insist upon, plead, or in any manner whatsoever claim or take the benefit or advantage of, any stay, extension or usury law wherever enacted, now or at any time hereafter in force, which may affect the covenants or the performance of this Indenture or the Securities; and the Company (to the extent it may lawfully do so) hereby expressly waives all benefit or advantage of any such law and covenants that it will not, by resort to any such law, hinder, delay or impede the execution of any power herein granted to the Trustee, but will suffer and permit the execution of every such power as though no such law has been enacted.
- 18 -
Section 4.5 Corporate Existence.
Subject to Article V, the Company will do or cause to be done all things necessary to preserve and keep in full force and effect its corporate existence and rights (charter and statutory); provided, however, that the Company shall not be required to preserve any such right if the Board of Directors shall determine that the preservation thereof is no longer desirable in the conduct of the business of the Company and its Subsidiaries taken as a whole and that the loss thereof is not adverse in any material respect to the Holders.
ARTICLE V
SUCCESSORS
Section 5.1 Consolidation, Merger and Sale of Assets.
The Company may not consolidate with or merge with or into, sell, convey, transfer or dispose of all or substantially all of its assets to any other person (a “successor person”), whether in one transaction or a series of related transactions, unless:
(a) (i) the Company is the surviving corporation or (ii) the successor person (if other than the Company) (A) is a corporation, limited liability corporation, partnership or trust organized under the laws of the United States; and (B) expressly assumes, by an indenture supplemental hereto, the Company’s obligations on the Securities and under this Indenture; and
(b) immediately after giving effect to the transaction, no Default or Event of Default shall have happened and be continuing.
The Company shall deliver to the Trustee prior to the consummation of the proposed transaction an Officer’s Certificate to the foregoing effect and an Opinion of Counsel stating that the proposed transaction and any supplemental indenture comply with Section 5.1 of this Indenture.
Notwithstanding the above, any Subsidiary of the Company may consolidate with, merge into or transfer all or part of its properties to the Company. Neither an Officer’s Certificate nor an Opinion of Counsel shall be required to be delivered in connection therewith.
Section 5.2 Successor Person Substituted.
Upon any consolidation or merger, or any sale, conveyance, transfer, or lease of all or substantially all of the assets of the Company and its Subsidiaries in accordance with Section 5.1, the successor person formed by such consolidation or into or with which the Company is merged or to which such sale, conveyance, transfer, or lease is made shall succeed to, and be substituted for, and may exercise every right and power of, the Company under this Indenture and the Securities with the same effect as if such successor person has been named as the Company herein; and, thereafter, the predecessor Company, in the case of a sale, conveyance or transfer (other than a lease), shall be released from all obligations and covenants under this Indenture and the Securities.
- 19 -
ARTICLE VI
DEFAULTS AND REMEDIES
Section 6.1 Events of Default.
“Event of Default,” wherever used herein with respect to Securities of any Series, means any one of the following events, unless in the establishing Board Resolution, supplemental indenture or Officer’s Certificate, it is provided that such Series shall not have the benefit of said Event of Default:
(a) failure to pay any interest on any Security of that Series when it becomes due and payable, and continuance of such default for a period of 30 days (unless the entire amount of such payment is deposited by the Company with the Trustee or with a Paying Agent prior to 10:00 a.m., New York City time, on the 30th day of such period);
(b) failure to pay principal of any Security of that Series at its Maturity;
(c) default in the performance or breach of any covenant of the Company in this Indenture (other than defaults pursuant to sub-clauses (a) through (c) above or defaults related to a covenant that has been included in this Indenture solely for the benefit of a Series of Securities other than that Series), which default continues uncured for a period of 90 days after there has been given, by registered or certified mail, to the Company by the Trustee or to the Company and the Trustee by the Holders of at least 25% in principal amount of the outstanding Securities of that Series a written notice specifying such default or breach and requiring it to be remedied and stating that such notice is a “Notice of Default” hereunder;
(d) the Company pursuant to or within the meaning of any Bankruptcy Law:
(i) commences a voluntary case,
(ii) consents to the entry of an order for relief against it in an involuntary case,
(iii) consents to the appointment of a Custodian of it or for all or substantially all of its property, or
(iv) makes a general assignment for the benefit of its creditors;
(e) a court of competent jurisdiction enters an order or decree under any Bankruptcy Law that:
(i) is for relief against the Company in an involuntary case,
(ii) appoints a Custodian of the Company or for all or substantially all of its property, or
(iii) orders the liquidation of the Company, and the order or decree remains unstayed and in effect for 60 days; or
- 20 -
(f) any other Event of Default provided with respect to Securities of that Series, which is specified in a Board Resolution, a supplemental indenture hereto or an Officer’s Certificate, in accordance with Section 2.2.18.
The term “Bankruptcy Law” means title 11, U.S. Code or any similar federal or state law for the relief of debtors. The term “Custodian” means any receiver, trustee, assignee, liquidator or similar official under any Bankruptcy Law.
A Default under one Series of Securities issued under this Indenture will not necessarily be a default under another Series of Securities under this Indenture.
The Company will, so long as any of the Securities are outstanding, deliver to the Trustee, within 30 days of becoming aware of any Default or Event of Default, an Officer’s Certificate specifying such Default or Event of Default and what action the Company is taking or proposes to take with respect thereto.
Section 6.2 Acceleration of Maturity; Rescission and Annulment.
If an Event of Default with respect to Securities of any Series at the time outstanding occurs and is continuing (other than an Event of Default referred to in Section 6.1(d) or (e)) then in every such case the Trustee or the Holders of not less than 25% in principal amount of the outstanding Securities of that Series may declare the principal amount (or, if any Securities of that Series are Discount Securities, such portion of the principal amount as may be specified in the terms of such Securities) of and accrued and unpaid interest, if any, on all of the Securities of that Series to be due and payable immediately, by a notice in writing to the Company (and to the Trustee if given by Holders), and upon any such declaration such principal amount (or specified amount) and accrued and unpaid interest, if any, shall become immediately due and payable. If an Event of Default specified in Section 6.1(d) or (e) shall occur, the principal amount (or specified amount) of and accrued and unpaid interest, if any, on all outstanding Securities shall ipso facto become and be immediately due and payable without any declaration or other act on the part of the Trustee or any Holder.
At any time after such a declaration of acceleration with respect to any Series has been made and before a judgment or decree for payment of the money due has been obtained by the Trustee as hereinafter in this Article provided, the Holders of a majority in principal amount of the outstanding Securities of that Series, by written notice to the Company and the Trustee, may rescind and annul such declaration and its consequences if all Events of Default with respect to Securities of that Series, other than the non-payment of the principal and interest, if any, of Securities of that Series which have become due solely by such declaration of acceleration, have been cured or waived as provided in Section 6.13.
No such rescission shall affect any subsequent Default.
- 21 -
Section 6.3 Collection of Indebtedness and Suits for Enforcement by Trustee.
The Company covenants that if
(a) default is made in the payment of any interest on any Security when such interest becomes due and payable and such default continues for a period of 30 days, or
(b) default is made in the payment of principal of any Security at the Maturity thereof, or
(c) default is made in the deposit of any sinking fund payment, if any, when and as due by the terms of a Security,
then, the Company will, upon demand of the Trustee, pay to it, for the benefit of the Holders of such Securities, the whole amount then due and payable on such Securities for principal and interest and, to the extent that payment of such interest shall be legally enforceable, interest on any overdue principal and any overdue interest at the rate or rates prescribed therefor in such Securities, and, in addition thereto, such further amount as shall be sufficient to cover the costs and expenses of collection, including the reasonable compensation, expenses, disbursements and advances of the Trustee, its agents and counsel.
If the Company fails to pay such amounts forthwith upon such demand, the Trustee, in its own name and as trustee of an express trust, may institute a judicial proceeding for the collection of the sums so due and unpaid, may prosecute such proceeding to judgment or final decree and may enforce the same against the Company or any other obligor upon such Securities and collect the moneys adjudged or deemed to be payable in the manner provided by law out of the property of the Company or any other obligor upon such Securities, wherever situated.
If an Event of Default with respect to any Securities of any Series occurs and is continuing, the Trustee may in its discretion proceed to protect and enforce its rights and the rights of the Holders of Securities of such Series by such appropriate judicial proceedings as the Trustee shall deem most effectual to protect and enforce any such rights, whether for the specific enforcement of any covenant or agreement in this Indenture or in aid of the exercise of any power granted herein, or to enforce any other proper remedy.
Section 6.4 Trustee May File Proofs of Claim.
In case of the pendency of any receivership, insolvency, liquidation, bankruptcy, reorganization, arrangement, adjustment, composition or other judicial proceeding relating to the Company or any other obligor upon the Securities or the property of the Company or of such other obligor or their creditors, the Trustee (irrespective of whether the principal of the Securities shall then be due and payable as therein expressed or by declaration or otherwise and irrespective of whether the Trustee shall have made any demand on the Company for the payment of overdue principal or interest) shall be entitled and empowered, by intervention in such proceeding or otherwise,
(a) to file and prove a claim for the whole amount of principal and interest owing and unpaid in respect of the Securities and to file such other papers or documents as may be necessary or advisable in order to have the claims of the Trustee (including any claim for the reasonable compensation, expenses, disbursements and advances of the Trustee, its agents and counsel) and of the Holders allowed in such judicial proceeding, and
- 22 -
(b) to collect and receive any moneys or other property payable or deliverable on any such claims and to distribute the same,
and any custodian, receiver, assignee, trustee, liquidator, sequestrator or other similar official in any such judicial proceeding is hereby authorized by each Holder to make such payments to the Trustee and, in the event that the Trustee shall consent to the making of such payments directly to the Holders, to pay to the Trustee any amount due it for the reasonable compensation, expenses, disbursements and advances of the Trustee, its agents and counsel, and any other amounts due the Trustee under Section 7.7.
Nothing herein contained shall be deemed to authorize the Trustee to authorize or consent to or accept or adopt on behalf of any Holder any plan of reorganization, arrangement, adjustment or composition affecting the Securities or the rights of any Holder thereof or to authorize the Trustee to vote in respect of the claim of any Holder in any such proceeding.
Section 6.5 Trustee May Enforce Claims Without Possession of Securities.
All rights of action and claims under this Indenture or the Securities may be prosecuted and enforced by the Trustee without the possession of any of the Securities or the production thereof in any proceeding relating thereto, and any such proceeding instituted by the Trustee shall be brought in its own name as trustee of an express trust, and any recovery of judgment shall, after provision for the payment of the reasonable compensation, expenses, disbursements and advances of the Trustee, its agents and counsel, be for the ratable benefit of the Holders of the Securities in respect of which such judgment has been recovered.
Section 6.6 Application of Money Collected.
Any money or property collected by the Trustee pursuant to this Article shall be applied in the following order, at the date or dates fixed by the Trustee and, in case of the distribution of such money or property on account of principal or interest, upon presentation of the Securities and the notation thereon of the payment if only partially paid and upon surrender thereof if fully paid:
First: To the payment of all amounts due to the Trustee under this Indenture; and
Second: To the payment of all indebtedness of the Company to which such Series of Securities is subordinated to the extent required by Article 12 of this Indenture; and
Third: To the payment of the amounts then due and unpaid for principal of and interest on the Securities in respect of which or for the benefit of which such money has been collected, ratably, without preference or priority of any kind, according to the amounts due and payable on such Securities for principal and interest, respectively; and
Fourth: To the Company.
- 23 -
Section 6.7 Limitation on Suits.
No Holder of any Security of any Series shall have any right to institute any proceeding, judicial or otherwise, with respect to this Indenture, or for the appointment of a receiver or trustee, or for any other remedy hereunder, unless:
(a) such Holder has previously given written notice to the Trustee of a continuing Event of Default with respect to the Securities of that Series;
(b) the Holders of not less than 25% in principal amount of the outstanding Securities of that Series have made written request to the Trustee to institute proceedings in respect of such Event of Default in its own name as Trustee hereunder;
(c) such Holder or Holders have offered to the Trustee indemnity or security satisfactory to the Trustee against the costs, expenses and liabilities which might be incurred by the Trustee in compliance with such request;
(d) the Trustee has failed to institute any such proceeding for 60 days after its receipt of such notice, request and offer of indemnity; and
(e) no direction inconsistent with such written request has been given to the Trustee during such 60-day period by the Holders of a majority in principal amount of the outstanding Securities of that Series;
it being understood, intended and expressly covenanted by the Holder of every Security with every other Holder and the Trustee that no one or more of such Holders shall have any right in any manner whatever by virtue of, or by availing of, any provision of this Indenture to affect, disturb or prejudice the rights of any other of such Holders, or to obtain or to seek to obtain priority or preference over any other of such Holders or to enforce any right under this Indenture, except in the manner herein provided and for the equal and ratable benefit of all such Holders of the applicable Series; provided, however, that the Trustee does not have an affirmative duty to ascertain whether or not such actions or forbearances are unduly prejudicial to such Holders.
Section 6.8 Unconditional Right of Holders to Receive Principal and Interest.
Notwithstanding any other provision in this Indenture, the Holder of any Security has the right, which is absolute and unconditional, to receive payment of the principal of and interest, if any, on such Security on the Maturity of such Security, including the Stated Maturity expressed in such Security (or, in the case of redemption, on the redemption date) and to institute suit for the enforcement of any such payment, and such rights shall not be impaired without the consent of such Holder.
Section 6.9 Restoration of Rights and Remedies.
If the Trustee or any Holder has instituted any proceeding to enforce any right or remedy under this Indenture and such proceeding has been discontinued or abandoned for any reason, or has been determined adversely to the Trustee or to such Holder, then and in every such case, subject to any determination in such proceeding, the Company, the Trustee and the Holders shall be restored severally and respectively to their former positions hereunder and thereafter all rights and remedies of the Trustee and the Holders shall continue as though no such proceeding had been instituted.
- 24 -
Section 6.10 Rights and Remedies Cumulative.
Except as otherwise provided with respect to the replacement or payment of mutilated, destroyed, lost or stolen Securities in Section 2.9, no right or remedy herein conferred upon or reserved to the Trustee or to the Holders is intended to be exclusive of any other right or remedy, and every right and remedy shall, to the extent permitted by law, be cumulative and in addition to every other right and remedy given hereunder or now or hereafter existing at law or in equity or otherwise. The assertion or employment of any right or remedy hereunder, or otherwise, shall not, to the extent permitted by law, prevent the concurrent assertion or employment of any other appropriate right or remedy.
Section 6.11 Delay or Omission Not Waiver.
No delay or omission of the Trustee or of any Holder of any Securities to exercise any right or remedy accruing upon any Event of Default shall impair any such right or remedy or constitute a waiver of any such Event of Default or an acquiescence therein. Every right and remedy given by this Article or by law to the Trustee or to the Holders may be exercised from time to time, and as often as may be deemed expedient, by the Trustee or by the Holders, as the case may be.
Section 6.12 Control by Holders.
The Holders of a majority in principal amount of the outstanding Securities of any Series shall have the right to direct the time, method and place of conducting any proceeding for any remedy available to the Trustee, or exercising any trust or power conferred on the Trustee, with respect to the Securities of such Series, provided that:
(a) such direction shall not be in conflict with any rule of law or with this Indenture;
(b) the Trustee may take any other action deemed proper by the Trustee which is not inconsistent with such direction;
(c) subject to the provisions of Section 7.1, the Trustee shall have the right to decline to follow any such direction if the Trustee in good faith shall, by a Responsible Officer of the Trustee, determine that the proceeding so directed would involve the Trustee in personal liability; and
(d) prior to taking any action as directed under this Section 6.12, the Trustee shall be entitled to indemnity satisfactory to it against the costs, expenses and liabilities which might be incurred by it in compliance with such request or direction.
- 25 -
Section 6.13 Waiver of Past Defaults.
The Holders of not less than a majority in principal amount of the outstanding Securities of any Series may on behalf of the Holders of all the Securities of such Series waive any past Default hereunder with respect to such Series and its consequences, except a Default in the payment of the principal of or interest on any Security of such Series (provided, however, that the Holders of a majority in principal amount of the outstanding Securities of any Series may rescind an acceleration and its consequences, including any related payment default that resulted from such acceleration). Upon any such waiver, such Default shall cease to exist, and any Event of Default arising therefrom shall be deemed to have been cured, for every purpose of this Indenture; but no such waiver shall extend to any subsequent or other Default.
Section 6.14 Undertaking for Costs.
All parties to this Indenture agree, and each Holder of any Security by his acceptance thereof shall be deemed to have agreed, that any court may in its discretion require, in any suit for the enforcement of any right or remedy under this Indenture, or in any suit against the Trustee for any action taken, suffered or omitted by it as Trustee, the filing by any party litigant in such suit of an undertaking to pay the costs of such suit, and that such court may in its discretion assess reasonable costs, including reasonable attorneys’ fees and expenses, against any party litigant in such suit, having due regard to the merits and good faith of the claims or defenses made by such party litigant; but the provisions of this Section 6.14 shall not apply to any suit instituted by the Company, to any suit instituted by the Trustee, to any suit instituted by any Holder, or group of Holders, holding in the aggregate more than 10% in principal amount of the outstanding Securities of any Series, or to any suit instituted by any Holder for the enforcement of the payment of the principal of or interest on any Security on or after the Maturity of such Security, including the Stated Maturity expressed in such Security (or, in the case of redemption, on the redemption date).
ARTICLE VII
TRUSTEE
Section 7.1 Duties of Trustee.
(a) If an Event of Default has occurred and is continuing, the Trustee shall exercise the rights and powers vested in it by this Indenture and use the same degree of care and skill in their exercise as a prudent person would exercise or use under the circumstances in the conduct of such person’s own affairs.
(b) Except during the continuance of an Event of Default:
(i) The Trustee need perform only those duties that are specifically set forth in this Indenture and no others.
(ii) In the absence of bad faith on its part, the Trustee may conclusively rely, as to the truth of the statements and the correctness of the opinions expressed therein, upon Officer’s Certificates or Opinions of Counsel furnished to the Trustee and conforming to the requirements of this Indenture; however, in the case of any such Officer’s
- 26 -
Certificates or Opinions of Counsel which by any provisions hereof are specifically required to be furnished to the Trustee, the Trustee shall examine such Officer’s Certificates and Opinions of Counsel to determine whether or not they conform to the requirements of this Indenture (but need not confirm or investigate the accuracy of mathematical calculations or other facts stated therein).
(c) The Trustee may not be relieved from liability for its own negligent action, its own negligent failure to act or its own willful misconduct, except that:
(i) This sub-clause (c) does not limit the effect of sub-clause (b) of this Section 7.1.
(ii) The Trustee shall not be liable for any error of judgment made in good faith by a Responsible Officer, unless it is proved that the Trustee was negligent in ascertaining the pertinent facts.
(iii) The Trustee shall not be liable with respect to any action taken, suffered or omitted to be taken by it with respect to Securities of any Series in good faith in accordance with the direction of the Holders of a majority in principal amount of the outstanding Securities of such Series relating to the time, method and place of conducting any proceeding for any remedy available to the Trustee, or exercising any trust or power conferred upon the Trustee, under this Indenture with respect to the Securities of such Series in accordance with Section 6.12.
(d) Every provision of this Indenture that in any way relates to the Trustee is subject to sub-clauses (a), (b) and (c) of this Section 7.1.
(e) The Trustee may refuse to perform any duty or exercise any right or power unless it receives indemnity satisfactory to it against the costs, expenses and liabilities which might be incurred by it in performing such duty or exercising such right or power.
(f) The Trustee shall not be liable for interest on any money received by it. Money held in trust by the Trustee need not be segregated from other funds except to the extent required by law.
(g) No provision of this Indenture shall require the Trustee to risk its own funds or otherwise incur any financial liability in the performance of any of its duties, or in the exercise of any of its rights or powers, if adequate indemnity against such risk is not assured to the Trustee in its satisfaction.
(h) The Paying Agent, the Registrar and any authenticating agent shall be entitled to the protections and immunities as are set forth in sub-clauses (e), (f) and (g) of this Section 7.1 and in Section 7.2, each with respect to the Trustee.
- 27 -
Section 7.2 Rights of Trustee.
(a) The Trustee may conclusively rely on and shall be protected in acting or refraining from acting upon any document (whether in its original or facsimile form) believed by it to be genuine and to have been signed or presented by the proper person. The Trustee need not investigate any fact or matter stated in the document.
(b) Before the Trustee acts or refrains from acting, it shall be entitled to receive an Officer’s Certificate or an Opinion of Counsel or both. The Trustee shall not be liable for any action it takes or omits to take in good faith in conclusive reliance on such Officer’s Certificate or Opinion of Counsel.
(c) The Trustee may act through agents and shall not be responsible for the misconduct or negligence of any agent appointed with due care. No Depositary shall be deemed an agent of the Trustee and the Trustee shall not be responsible for any act or omission by any Depositary.
(d) The Trustee shall not be liable for any action it takes or omits to take in good faith which it believes to be authorized or within its rights or powers, provided that the Trustee’s conduct does not constitute willful misconduct or negligence.
(e) The Trustee may consult with counsel of its selection and the advice of such counsel or any Opinion of Counsel shall be full and complete authorization and protection in respect of any action taken, suffered or omitted by it hereunder in good faith and in reliance thereon.
(f) The Trustee shall be under no obligation to exercise any of the rights or powers vested in it by this Indenture at the request or direction of any of the Holders of Securities unless such Holders shall have offered to the Trustee security or indemnity reasonably satisfactory to it against the costs, expenses and liabilities which might be incurred by it in compliance with such request or direction.
(g) The Trustee shall not be bound to make any investigation into the facts or matters stated in any resolution, certificate, statement, instrument, opinion, report, notice, request, direction, consent, order, bond, debenture, note, other evidence of indebtedness or other paper or document, but the Trustee, in its discretion, may make such further inquiry or investigation into such facts or matters as it may see fit and shall incur no liability or additional liability of any kind by reason of such inquiry or investigation.
(h) The Trustee shall not be deemed to have notice of any Default or Event of Default unless a Responsible Officer of the Trustee has actual knowledge thereof or unless written notice of any event which is in fact such a default is received by a Responsible Officer of the Trustee at the Corporate Trust Office of the Trustee, and such notice references the Securities generally or the Securities of a particular Series and this Indenture.
(i) In no event shall the Trustee be liable to any person for special, punitive, indirect, consequential or incidental loss or damage of any kind whatsoever (including but not limited to lost profits), even if the Trustee has been advised of the likelihood of such loss or damage.
(j) The permissive right of the Trustee to take the actions permitted by this Indenture shall not be construed as an obligation or duty to do so.
- 28 -
(k) The rights, privileges, protections, immunities and benefits given to the Trustee, including, without limitation, its right to be indemnified, are extended to, and shall be enforceable by, the Trustee in each of its capacities hereunder, and each agent, custodian and other Person employed to act hereunder.
(l) The Trustee shall not be required to give any bond or surety in respect of the performance of its powers and duties hereunder.
(m) The Trustee may request that the Company deliver a certificate setting forth the names of individuals and/or titles of officers authorized at such time to take specified actions pursuant to this Indenture.
Section 7.3 Individual Rights of Trustee.
The Trustee in its individual or any other capacity may become the owner or pledgee of Securities and may otherwise deal with the Company or an Affiliate of the Company with the same rights it would have if it were not Trustee. Any Agent may do the same with like rights. However, the Trustee is also subject to Sections 7.10 and 7.11 hereof.
Section 7.4 Trustee’s Disclaimer.
The Trustee makes no representation as to the validity or adequacy of this Indenture or the Securities, it shall not be accountable for the Company’s use of the proceeds from the Securities, and it shall not be responsible for any statement in the Securities other than its authentication.
Section 7.5 Notice of Defaults.
If a Default or Event of Default occurs and is continuing with respect to the Securities of any Series and if it is actually known to a Responsible Officer of the Trustee, the Trustee shall mail to each Securityholder of the Securities of that Series notice of a Default or Event of Default within 90 days after it occurs or, if later, after a Responsible Officer of the Trustee has knowledge of such Default or Event of Default. Except in the case of a Default or Event of Default in payment of principal of or interest on any Security of any Series, the Trustee may withhold the notice if and so long as it in good faith determines that withholding the notice is in the interests of Securityholders of that Series.
Section 7.6 Reports by Trustee to Holders.
Within 60 days after each anniversary of the date of this Indenture, the Trustee shall transmit by mail to all Securityholders, as their names and addresses appear on the register kept by the Registrar, a brief report dated as of such reporting date, in accordance with, and to the extent required under, TIA § 313.
A copy of each report at the time of its mailing to Securityholders of any Series shall be filed with the SEC and each national securities exchange on which the Securities of that Series are listed. The Company shall promptly notify the Trustee in writing when Securities of any Series are listed on any national securities exchange or of any delisting thereof.
- 29 -
Section 7.7 Compensation and Indemnity.
The Company shall pay to the Trustee from time to time compensation for its services as the Company and the Trustee shall from time to time agree upon in writing. The Trustee’s compensation shall not be limited by any law on compensation of a trustee of an express trust. The Company shall reimburse the Trustee upon request for all reasonable out of pocket expenses incurred by it. Such expenses shall include the reasonable compensation and expenses of the Trustee’s agents and counsel.
The Company shall indemnify each of the Trustee and any predecessor Trustee against any cost, expense, claim (whether asserted by the Company, a Holder or any other person) or liability (including the cost of defending itself), including taxes (other than taxes based upon, measured by or determined by the income of the Trustee), incurred by it except as set forth in the next paragraph in the performance of its duties under this Indenture as Trustee or Agent. The Trustee shall notify the Company promptly of any claim for which it may seek indemnity. Failure by the Trustee to so notify the Company shall not relieve the Company of its obligations hereunder, unless and to the extent that the Company is materially prejudiced thereby. The Company shall defend the claim and the Trustee shall cooperate in the defense. The Trustee may have one separate counsel and the Company shall pay the reasonable fees and expenses of such counsel. The Company need not pay for any settlement made without its consent, which consent will not be unreasonably withheld. This indemnification shall apply to officers, directors, employees, shareholders and agents of the Trustee.
The Company need not reimburse any expense or indemnify against any loss or liability incurred by the Trustee or by any officer, director, employee or shareholder of the Trustee through willful misconduct or negligence.
To secure the Company’s payment obligations in this Section 7.7, the Trustee shall have a lien prior to the Securities of any Series on all money or property held or collected by the Trustee, except that held in trust to pay principal of and interest on particular Securities of that Series.
When the Trustee incurs expenses or renders services after an Event of Default specified in Section 6.1(f) or (g) occurs, the expenses and the compensation for the services are intended to constitute expenses of administration under any Bankruptcy Law.
The provisions of this Section 7.7 shall survive the termination of this Indenture or the resignation or removal of the Trustee.
Section 7.8 Replacement of Trustee.
A resignation or removal of the Trustee and appointment of a successor Trustee shall become effective only upon the successor Trustee’s acceptance of appointment as provided in this Section 7.8.
The Trustee may resign at any time with respect to the Securities of one or more Series by so notifying the Company at least 30 days prior to the date of the proposed resignation. The Holders of a majority in principal amount of the Securities of any Series may remove the Trustee with respect to that Series by so notifying the Trustee and the Company in writing. The Company may remove the Trustee with respect to Securities of one or more Series if:
- 30 -
(a) the Trustee fails to comply with Section 7.10;
(b) the Trustee is adjudged a bankrupt or an insolvent or an order for relief is entered with respect to the Trustee under any Bankruptcy Law;
(c) a Custodian or public officer takes charge of the Trustee or its property; or
(d) the Trustee becomes incapable of acting.
If the Trustee resigns or is removed or if a vacancy exists in the office of Trustee for any reason, the Company shall promptly appoint a successor Trustee. Within one year after the successor Trustee takes office, the Holders of a majority in principal amount of the then outstanding Securities may appoint a successor Trustee to replace the successor Trustee appointed by the Company.
If a successor Trustee with respect to the Securities of any one or more Series does not take office within 30 days after the retiring Trustee resigns or is removed, the retiring Trustee, the Company or the Holders of at least a majority in principal amount of the Securities of the applicable Series may petition any court of competent jurisdiction for the appointment of a successor Trustee at the expense of the Company.
A successor Trustee shall deliver a written acceptance of its appointment to the retiring Trustee and to the Company. Immediately after that, the retiring Trustee shall transfer all property held by it as Trustee to the successor Trustee subject to the lien provided for in Section 7.7, the resignation or removal of the retiring Trustee shall become effective, and the successor Trustee shall have all the rights, powers and duties of the Trustee with respect to each Series of Securities for which it is acting as Trustee under this Indenture. A successor Trustee shall mail a notice of its succession to each Securityholder of each such Series. Notwithstanding replacement of the Trustee pursuant to this Section 7.8, the Company’s obligations under Section 7.7 hereof shall continue for the benefit of the retiring Trustee with respect to expenses and liabilities incurred by it for actions taken or omitted to be taken in accordance with its rights, powers and duties under this Indenture prior to such replacement.
Section 7.9 Successor Trustee by Merger, Etc.
If the Trustee consolidates with, merges or converts into, or transfers all or substantially all of its corporate trust business to, another corporation, the successor corporation without any further act shall be the successor Trustee, if such successor corporation is eligible and qualified under Section 7.10.
Section 7.10 Eligibility; Disqualification.
This Indenture shall always have a Trustee who satisfies the requirements of TIA § 310(a)(1), (2) and (5). The Trustee shall always have a combined capital and surplus of at least $25,000,000 as set forth in its most recent published annual report of condition. The Trustee shall comply with TIA § 310(b).
- 31 -
Section 7.11 Preferential Collection of Claims Against Company.
The Trustee is subject to TIA § 311(a), excluding any creditor relationship listed in TIA § 311(b). A Trustee who has resigned or been removed shall be subject to TIA § 311(a) to the extent indicated.
ARTICLE VIII
SATISFACTION AND DISCHARGE; DEFEASANCE
Section 8.1 Satisfaction and Discharge of Indenture.
This Indenture shall upon Company Order cease to be of further effect (except as hereinafter provided in this Section 8.1), and the Trustee, at the expense of the Company, shall execute instruments acknowledging satisfaction and discharge of this Indenture, when
(a) either
(i) all Securities theretofore authenticated and delivered (other than Securities that have been destroyed, lost or stolen and that have been replaced or paid as provided in Section 2.9) have been delivered to the Trustee for cancellation; or
(ii) all such Securities not theretofore delivered to the Trustee for cancellation:
(1) have become due and payable, or
(2) will become due and payable at their Stated Maturity within one year, or
(3) have been called for redemption or are to be called for redemption within one year under arrangements satisfactory to the Trustee for the giving of notice of redemption by the Trustee in the name, and at the expense, of the Company;
and the Company, in the case of (1), (2) or (3) above, has irrevocably deposited or caused to be deposited with the Trustee as trust funds in trust an amount of money or U.S. Government Obligations sufficient for the purpose of paying and discharging the entire indebtedness on such Securities not theretofore delivered to the Trustee for cancellation, for principal and interest to the date of such deposit (in the case of Securities which have become due and payable on or prior to the date of such deposit) or to the Stated Maturity or redemption date, as the case may be;
(b) the Company has paid or caused to be paid all other sums payable hereunder by the Company; and
- 32 -
(c) the Company has delivered to the Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that all conditions precedent herein provided for relating to the satisfaction and discharge of this Indenture have been complied with.
Notwithstanding the satisfaction and discharge of this Indenture, the obligations of the Company to the Trustee under Section 7.7, and, if money shall have been deposited with the Trustee pursuant to sub-clause (a) of this Section 8.1, the provisions of Sections 2.5, 2.8, 2.9, 8.2 and 8.5 shall survive.
Section 8.2 Application of Trust Funds; Indemnification.
(a) Subject to the provisions of Section 8.5, all money or U.S. Government Obligations deposited with the Trustee pursuant to Section 8.1, all money and U.S. Government Obligations or Foreign Government Obligations deposited with the Trustee pursuant to Section 8.3 or 8.4 and all money received by the Trustee in respect of U.S. Government Obligations or Foreign Government Obligations deposited with the Trustee pursuant to Section 8.3 or 8.4, shall be held in trust and applied by it, in accordance with the provisions of the Securities and this Indenture, to the payment, either directly or through any Paying Agent (including the Company acting as its own Paying Agent) as the Trustee may determine, to the persons entitled thereto, of the principal and interest for whose payment such money has been deposited with or received by the Trustee or to make mandatory sinking fund payments or analogous payments as contemplated by Sections 8.3 or 8.4.
(b) The Company shall pay and shall indemnify the Trustee against any tax, fee or other charge imposed on or assessed against U.S. Government Obligations or Foreign Government Obligations deposited pursuant to Sections 8.3 or 8.4 or the interest and principal received in respect of such obligations other than any payable by or on behalf of Holders.
(c) The Trustee shall deliver or pay to the Company from time to time upon Company Order any U.S. Government Obligations or Foreign Government Obligations or money held by it as provided in Sections 8.3 or 8.4 which, in the opinion of a nationally recognized firm of independent certified public accountants or investment bank expressed in a written certification thereof delivered to the Trustee, are then in excess of the amount thereof which then would have been required to be deposited for the purpose for which such U.S. Government Obligations or Foreign Government Obligations or money were deposited or received. This provision shall not authorize the sale by the Trustee of any U.S. Government Obligations or Foreign Government Obligations held under this Indenture.
Section 8.3 Legal Defeasance of Securities of any Series.
Unless this Section 8.3 is otherwise specified, pursuant to Section 2.2, to be inapplicable to Securities of any Series, the Company shall be deemed to have paid and discharged the entire indebtedness on all the outstanding Securities of any Series on the 91st day after the date of the deposit referred to in sub-clause (d) hereof, and the provisions of this Indenture, as it relates to such outstanding Securities of such Series, shall no longer be in effect (and the Trustee, at the expense of the Company, shall, upon receipt of a Company Order, execute instruments acknowledging the same), except as to:
- 33 -
(a) the rights of Holders of Securities of such Series to receive, from the trust funds described in sub-clause (d) hereof, (i) payment of the principal of and each installment of principal of and interest on the outstanding Securities of such Series on the Maturity of such principal or installment of principal or interest and (ii) the benefit of any mandatory sinking fund payments applicable to the Securities of such Series on the day on which such payments are due and payable in accordance with the terms of this Indenture and the Securities of such Series;
(b) the provisions of Sections 2.5, 2.8, 2.9, 8.2, 8.3 and 8.5; and
(c) the rights, powers, trust and immunities of the Trustee hereunder and the Company’s obligations in connection therewith;
provided that, the following conditions shall have been satisfied:
(d) the Company shall have deposited or caused to be irrevocably deposited (except as provided in Section 8.2(c)) with the Trustee as trust funds in trust for the purpose of making the following payments, specifically pledged as security for and dedicated solely to the benefit of the Holders of such Securities: (i) in the case of Securities of such Series denominated in Dollars, cash in Dollars and/or U.S. Government Obligations, or (ii) in the case of Securities of such Series denominated in a Foreign Currency (other than a composite currency), money and/or Foreign Government Obligations, which through the payment of interest and principal in respect thereof in accordance with their terms (and without reinvestment), will provide, not later than one day before the due date of any payment of money, an amount in cash, sufficient, in the opinion of a nationally recognized firm of independent public accountants or investment bank expressed in a written certification thereof delivered to the Trustee, to pay and discharge each installment of principal of and interest, if any, on and any mandatory sinking fund payments in respect of all the Securities of such Series on the dates such installments of interest or principal and such sinking fund payments are due;
(e) such deposit will not result in a breach or violation of, or constitute a default under, this Indenture or any other agreement or instrument to which the Company is a party or by which it is bound;
(f) no Default or Event of Default with respect to the Securities of such Series shall have occurred and be continuing on the date of such deposit or during the period ending on the 91st day after such date;
(g) the Company shall have delivered to the Trustee an Officer’s Certificate and an Opinion of Counsel to the effect that (i) the Company has received from, or there has been published by, the Internal Revenue Service a ruling, or (ii) since the date of execution of this Indenture, there has been a change in the applicable U.S. federal income tax law, in either case to the effect that, and based thereon such Opinion of Counsel shall confirm that, the Holders of the Securities of such Series will not recognize income, gain or loss for U.S. federal income tax purposes as a result of such deposit, defeasance and discharge and will be subject to U.S. federal income tax on the same amount and in the same manner and at the same times as would have been the case if such deposit, defeasance and discharge had not occurred;
- 34 -
(h) the Company shall have delivered to the Trustee an Officer’s Certificate stating that the deposit was not made by the Company with the intent of defeating, hindering, delaying or defrauding any other creditors of the Company; and
(i) the Company shall have delivered to the Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that all conditions precedent provided for relating to the defeasance contemplated by this Section 8.3 have been complied with.
Section 8.4 Covenant Defeasance.
Unless this Section 8.4 is otherwise specified pursuant to Section 2.2 to be inapplicable to Securities of any Series, the Company may omit to comply with respect to the Securities of any Series with any term, provision or condition set forth under Sections 4.2 and 4.3, 4.4 and 5.1 as well as any additional covenants specified in a supplemental indenture for such Series of Securities or a Board Resolution or an Officer’s Certificate delivered pursuant to Section 2.2 (and the failure to comply with any such covenants shall not constitute a Default or Event of Default with respect to such Series under Section 6.1) and the occurrence of any event specified in a supplemental indenture for such Series of Securities or a Board Resolution or an Officer’s Certificate delivered pursuant to Section 2.2.18 and designated as an Event of Default shall not constitute a Default or Event of Default hereunder, with respect to the Securities of such Series, provided that the following conditions shall have been satisfied:
(a) With reference to this Section 8.4, the Company has deposited or caused to be irrevocably deposited (except as provided in Section 8.2(c)) with the Trustee as trust funds in trust for the purpose of making the following payments specifically pledged as security for, and dedicated solely to, the benefit of the Holders of such Securities: (i) in the case of Securities of such Series denominated in Dollars, cash in Dollars and/or U.S. Government Obligations, or (ii) in the case of Securities of such Series denominated in a Foreign Currency (other than a composite currency), money and/or Foreign Government Obligations, which through the payment of interest and principal in respect thereof in accordance with their terms (and without reinvestment), will provide, not later than one day before the due date of any payment of money, an amount in cash, sufficient, in the opinion of a nationally recognized firm of independent certified public accountants or investment bank expressed in a written certification thereof delivered to the Trustee, to pay and discharge each installment of principal of and interest, if any, on and any mandatory sinking fund payments in respect of the Securities of such Series on the dates such installments of interest or principal and such sinking fund payments are due;
(b) Such deposit will not result in a breach or violation of, or constitute a default under, this Indenture or any other agreement or instrument to which the Company is a party or by which it is bound;
(c) No Default or Event of Default with respect to the Securities of such Series shall have occurred and be continuing on the date of such deposit;
(d) The Company shall have delivered to the Trustee an Opinion of Counsel to the effect that Holders of the Securities of such Series will not recognize income, gain or loss for U.S. federal income tax purposes as a result of such deposit and covenant defeasance and will be subject to U.S. federal income tax on the same amounts, in the same manner and at the same times as would have been the case if such deposit and covenant defeasance had not occurred;
- 35 -
(e) The Company shall have delivered to the Trustee an Officer’s Certificate stating the deposit was not made by the Company with the intent of defeating, hindering, delaying or defrauding any other creditors of the Company; and
(f) The Company shall have delivered to the Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that all conditions precedent herein provided for relating to the covenant defeasance contemplated by this Section 8.4 have been complied with.
Section 8.5 Repayment to Company.
Subject to applicable abandoned property law, the Trustee and the Paying Agent shall pay to the Company upon request any money held by them for the payment of principal or interest that remains unclaimed for two years after such principal or interest has become due and payable. After that, Securityholders entitled to the money must look to the Company for payment as general creditors unless an applicable abandoned property law designates another person.
Section 8.6 Reinstatement.
If the Trustee or the Paying Agent is unable to apply any money deposited with respect to Securities of any Series in accordance with Section 8.1 by reason of any legal proceeding or by reason of any order or judgment of any court or governmental authority enjoining, restraining or otherwise prohibiting such application, the obligations of the Company under this Indenture with respect to the Securities of such Series and under the Securities of such Series shall be revived and reinstated as though no deposit had occurred pursuant to Section 8.1 until such time as the Trustee or the Paying Agent is permitted to apply all such money in accordance with Section 8.1; provided, however, that if the Company has made any payment of principal of or interest on any Securities because of the reinstatement of its obligations, the Company shall be subrogated to the rights of the Holders of such Securities to receive such payment from the money or U.S. Government Obligations held by the Trustee or Paying Agent after payment in full to the Holders.
ARTICLE IX
AMENDMENTS AND WAIVERS
Section 9.1 Without Consent of Holders.
The Company and the Trustee may amend or supplement this Indenture or the Securities of one or more Series without the consent of any Securityholder:
(a) to add guarantees with respect to any Series of Securities or secure any Series of Securities;
(b) to surrender any of the Company’s rights or powers under this Indenture;
- 36 -
(c) to add covenants or Events of Default for the benefit of the Securityholders of any Series of Securities;
(d) to comply with the applicable rules or procedures of the Depositary;
(e) to cure any ambiguity, defect or inconsistency, as described in the Officer’s Certificate delivered pursuant to Section 10.4;
(f) to comply with Article V;
(g) to provide for uncertificated Securities in addition to or in place of certificated Securities;
(h) to make any change that does not materially adversely affect the rights of any Securityholder;
(i) to provide for the issuance of and establish the form and terms and conditions of Securities of any Series as permitted by this Indenture;
(j) to evidence and provide for the acceptance of appointment hereunder by a successor Trustee with respect to the Securities of one or more Series and to add to or change any of the provisions of this Indenture as shall be necessary to provide for or facilitate the administration of the trusts hereunder by more than one Trustee;
(k) to comply with requirements of the SEC in order to effect or maintain the qualification of this Indenture under the TIA;
(l) to comply with the rules or regulations of any securities exchange or automated quotation system on which any of the Securities may be listed or traded; and
(m) to change or eliminate any of the provisions of this Indenture, provided that any such change or elimination shall not be effective with respect to any outstanding Securities of any Series created prior to the execution of such supplemental indenture which is entitled to the benefit of such provision.
Section 9.2 With Consent of Holders.
The Company and the Trustee may enter into a supplemental indenture with the written consent of the Holders of at least a majority in principal amount of the outstanding Securities of each Series affected by such supplemental indenture (including consents obtained in connection with a tender offer or exchange offer for the Securities of such Series), for the purpose of adding any provisions to or changing in any manner or eliminating any of the provisions of this Indenture or of any supplemental indenture or of modifying in any manner the rights of the Securityholders of each such Series. Except as provided in Section 6.13, the Holders of at least a majority in principal amount of the outstanding Securities of any Series by written notice to the Trustee (including consents obtained in connection with a tender offer or exchange offer for the Securities of such Series) may waive compliance by the Company with any provision of this Indenture or the Securities with respect to such Series.
- 37 -
It shall not be necessary for the consent of the Holders of Securities under this Section 9.2 to approve the particular form of any proposed supplemental indenture or waiver, but it shall be sufficient if such consent approves the substance thereof. After a supplemental indenture or waiver under this Section 9.2 becomes effective, the Company shall mail to the Holders of Securities affected thereby, a notice briefly describing the supplemental indenture or waiver. Any failure by the Company to mail or publish such notice, or any defect therein, shall not, however, in any way impair or affect the validity of any such supplemental indenture or waiver.
Section 9.3 Limitations.
Without the consent of each Securityholder affected, an amendment or waiver may not:
(a) reduce the principal amount of Securities whose Holders must consent to an amendment, supplement or waiver;
(b) reduce the rate of or extend the time for payment of interest (including default interest) on any Security or that Series;
(c) reduce the principal of, or change the Stated Maturity of, any Security or reduce the amount of, or postpone the date fixed for, the payment of any sinking fund or analogous obligation;
(d) reduce the principal amount of Discount Securities payable upon acceleration of the maturity thereof;
(e) waive a Default or Event of Default in the payment of the principal of or interest, if any, on any Security (except a rescission of acceleration of the Securities of any Series by the Holders of at least a majority in principal amount of the then outstanding Securities of such Series and a waiver of the payment default that resulted from such acceleration);
(f) make the principal of or interest, if any, on any Security payable in any currency other than that stated in the Security;
(g) make any change in Sections 6.8 or 6.13 or this Section 9.3; or
(h) waive a redemption payment with respect to any Security.
Section 9.4 Compliance with Trust Indenture Act.
Every amendment to this Indenture or the Securities of one or more Series shall be set forth in a supplemental indenture hereto that complies with the TIA as then in effect.
Section 9.5 Revocation and Effect of Consents.
Until an amendment is set forth in a supplemental indenture or a waiver becomes effective, a consent to it by a Holder of a Security is a continuing consent by the Holder and every subsequent Holder of a Security or portion of a Security that evidences the same debt as the consenting Holder’s Security, even if notation of the consent is not made on any Security.
- 38 -
Any amendment or waiver once effective shall bind every Securityholder of each Series affected by such amendment or waiver unless it is of the type described in any of sub-clauses (a) through (h) of Section 9.3. In that case, the amendment or waiver shall bind each Holder of a Security who has consented to it and every subsequent Holder of a Security or portion of a Security that evidences the same debt as the consenting Holder’s Security.
The Company may, but shall not be obligated to, fix a record date for the purpose of determining the Holders entitled to give their consent or take any other action described above or required or permitted to be taken pursuant to this Indenture. If a record date is fixed, then notwithstanding the immediately preceding paragraph, those Persons who were Holders at such record date (or their duly designated proxies), and only those persons, shall be entitled to give such consent or to revoke any consent previously given or take any such action, whether or not such Persons continue to be Holders after such record date. No such consent shall be valid or effective for more than 120 days after such record date.
Section 9.6 Notation on or Exchange of Securities.
The Company or the Trustee may place an appropriate notation about an amendment or waiver on any Security of any Series thereafter authenticated. The Company in exchange for Securities of that Series may issue and the Trustee shall authenticate upon request new Securities of that Series that reflect the amendment or waiver.
Section 9.7 Trustee Protected.
In executing, or accepting the additional trusts created by, any supplemental indenture permitted by this Article or the modifications thereby of the trusts created by this Indenture, the Trustee shall receive, and (subject to Section 7.1) shall be fully protected in conclusively relying upon, an Officer’s Certificate or an Opinion of Counsel or both complying with Section 10.4 and stating that the supplemental indenture is the legal, valid and binding obligation of the Company, enforceable against the Company in accordance with its terms, subject to customary exceptions. The Trustee shall sign all supplemental indentures upon delivery of such an Officer’s Certificate or Opinion of Counsel or both, except that the Trustee need not sign any supplemental indenture that, in its sole discretion, adversely affects its rights.
ARTICLE X
MISCELLANEOUS
Section 10.1 Trust Indenture Act Controls.
If any provision of this Indenture limits, qualifies, or conflicts with another provision which is required or deemed to be included in this Indenture by the TIA, such required or deemed provision shall control.
- 39 -
Section 10.2 Notices.
Any request, demand, notice or communication by the Company or the Trustee to the other, or by a Holder to the Company or the Trustee, is duly given if in writing and delivered in person or mailed by first-class mail:
if to the Company:
Cresco Labs, Inc.
if to the Trustee:
Attention:
The Company or the Trustee by notice to the other may designate additional or different addresses for subsequent notices or communications.
Any notice or communication to a Securityholder shall be mailed by first-class mail to his address shown on the register kept by the Registrar. Failure to mail a notice or communication to a Securityholder of any Series or any defect in it shall not affect its sufficiency with respect to other Securityholders of that or any other Series.
If a notice or communication is mailed or published in the manner provided above, within the time prescribed, it is duly given, whether or not the Securityholder receives it.
If the Company mails a notice or communication to Securityholders, it shall mail a copy to the Trustee and each Agent at the same time.
Notwithstanding any other provision of this Indenture or any Security, where this Indenture or any Security provides for notice of any event (including any notice of redemption) to a Holder of a Global Security (whether by mail or otherwise), such notice shall be sufficiently given to the Depositary for such Security (or its designee) pursuant to the customary procedures of such Depositary.
Section 10.3 Communication by Holders with Other Holders.
Securityholders of any Series may communicate pursuant to TIA § 312(b) with other Securityholders of that Series or any other Series with respect to their rights under this Indenture or the Securities of that Series or all Series. The Company, the Trustee, the Registrar and anyone else shall have the protection of TIA § 312(c).
- 40 -
Section 10.4 Certificate and Opinion as to Conditions Precedent.
Upon any request or application by the Company to the Trustee to take any action under this Indenture, the Company shall furnish to the Trustee:
(a) an Officer’s Certificate stating that, in the opinion of the signers, all conditions precedent, if any, provided for in this Indenture relating to the proposed action have been complied with; and
(b) an Opinion of Counsel stating that, in the opinion of such counsel, all such conditions precedent have been complied with.
Section 10.5 Statements Required in Certificate or Opinion.
Each certificate or opinion with respect to compliance with a condition or covenant provided for in this Indenture (other than a certificate provided pursuant to TIA § 314(a)(4)) shall comply with the provisions of TIA § 314(e) and shall include:
(a) a statement that the person making such certificate or opinion has read such covenant or condition;
(b) a brief statement as to the nature and scope of the examination or investigation upon which the statements or opinions contained in such certificate or opinion are based;
(c) a statement that, in the opinion of such person, he has made such examination or investigation as is necessary to enable him to express an informed opinion as to whether or not such covenant or condition has been complied with; and
(d) a statement as to whether or not, in the opinion of such person, such condition or covenant has been complied with.
Section 10.6 Rules by Trustee and Agents.
The Trustee may make reasonable rules for action by or a meeting of Securityholders of one or more Series. Any Agent may make reasonable rules and set reasonable requirements for its functions.
Section 10.7 Legal Holidays.
Unless otherwise provided by Board Resolution, Officer’s Certificate or supplemental indenture hereto for a particular Series, a “Legal Holiday” is any day that is not a Business Day. If a payment date is a Legal Holiday at a place of payment, payment may be made at that place on the next succeeding day that is not a Legal Holiday, and no interest shall accrue for the intervening period.
- 41 -
Section 10.8 No Recourse Against Others.
A director, officer, employee or stockholder (past or present), as such, of the Company shall not have any liability for any obligations of the Company under the Securities or this Indenture or for any claim based on, in respect of or by reason of such obligations or their creation. Each Securityholder by accepting a Security waives and releases all such liability. The waiver and release are part of the consideration for the issue of the Securities.
Section 10.9 Counterparts.
This Indenture may be executed in any number of counterparts and by the parties hereto in separate counterparts, each of which when so executed shall be deemed to be an original and all of which taken together shall constitute one and the same agreement. The exchange of copies of this Indenture and of signature pages by facsimile or PDF transmission shall constitute effective execution and delivery of this Indenture as to the parties hereto and may be used in lieu of the original Indenture for all purposes. Signatures of the parties hereto transmitted by facsimile or PDF shall be deemed to be their original signatures for all purposes.
Section 10.10 Governing Law; Jury Trial Waiver.
THIS INDENTURE AND THE SECURITIES, INCLUDING ANY CLAIM OR CONTROVERSY ARISING OUT OF OR RELATING TO THIS INDENTURE OR THE SECURITIES, SHALL BE GOVERNED BY THE LAWS OF THE STATE OF NEW YORK (WITHOUT REGARD TO THE CONFLICTS OF LAWS PROVISIONS THEREOF OTHER THAN SECTION 5-1401 OF THE GENERAL OBLIGATIONS LAW).
EACH OF THE COMPANY AND THE TRUSTEE HEREBY IRREVOCABLY WAIVES, TO THE FULLEST EXTENT PERMITTED BY APPLICABLE LAW, ANY AND ALL RIGHT TO TRIAL BY JURY IN ANY LEGAL PROCEEDING ARISING OUT OF OR RELATING TO THIS INDENTURE, THE SECURITIES OR THE TRANSACTION CONTEMPLATED HEREBY.
Section 10.11 No Adverse Interpretation of Other Agreements.
This Indenture may not be used to interpret another indenture, loan or debt agreement of the Company or a Subsidiary of the Company. Any such indenture, loan or debt agreement may not be used to interpret this Indenture.
Section 10.12 Successors.
All agreements of the Company in this Indenture and the Securities shall bind its successor. All agreements of the Trustee in this Indenture shall bind its successor.
- 42 -
Section 10.13 Severability.
In case any provision in this Indenture or in the Securities shall be invalid, illegal or unenforceable, the validity, legality and enforceability of the remaining provisions shall not in any way be affected or impaired thereby.
Section 10.14 Table of Contents, Headings, Etc.
The Table of Contents, Cross Reference Table, and headings of the Articles and Sections of this Indenture have been inserted for convenience of reference only, are not to be considered a part hereof, and shall in no way modify or restrict any of the terms or provisions hereof.
Section 10.15 Securities in a Foreign Currency.
Unless otherwise specified in a Board Resolution, a supplemental indenture hereto or an Officer’s Certificate delivered pursuant to Section 2.2 of this Indenture with respect to a particular Series of Securities, whenever for purposes of this Indenture any action may be taken by the Holders of a specified percentage in aggregate principal amount of Securities of all Series or all Series affected by a particular action at the time outstanding and, at such time, there are outstanding Securities of any Series which are denominated in more than one currency, then the principal amount of Securities of such Series which shall be deemed to be outstanding for the purpose of taking such action shall be determined by converting any such other currency into a currency that is designated upon issuance of any particular Series of Securities. Unless otherwise specified in a Board Resolution, a supplemental indenture hereto or an Officer’s Certificate delivered pursuant to Section 2.2 of this Indenture with respect to a particular Series of Securities, such conversion shall be at the spot rate for the purchase of the designated currency as published in The Financial Times in the “Currency Rates” section (or, if The Financial Times is no longer published, or if such information is no longer available in The Financial Times, such source as may be selected in good faith by the Company) on any date of determination. The provisions of this paragraph shall apply in determining the equivalent principal amount in respect of Securities of a Series denominated in currency other than Dollars in connection with any action taken by Holders of Securities pursuant to the terms of this Indenture.
All decisions and determinations provided for in the preceding paragraph shall, in the absence of manifest error, to the extent permitted by law, be conclusive for all purposes and irrevocably binding upon the Trustee and all Holders.
Section 10.16 Judgment Currency.
The Company agrees, to the fullest extent that it may effectively do so under applicable law, that (a) if for the purpose of obtaining judgment in any court it is necessary to convert the sum due in respect of the principal of or interest or other amount on the Securities of any Series (the “Required Currency”) into a currency in which a judgment will be rendered (the “Judgment Currency”), the rate of exchange used shall be the rate at which in accordance with normal banking procedures the Trustee could purchase in The City of New York the Required Currency with the Judgment Currency on the day on which final unappealable judgment is entered, unless such day is not a Business Day, then the rate of exchange used shall be the rate at which in accordance with normal banking procedures the Trustee could purchase in The City of New
- 43 -
York the Required Currency with the Judgment Currency on the Business Day preceding the day on which final unappealable judgment is entered and (b) its obligations under this Indenture to make payments in the Required Currency (i) shall not be discharged or satisfied by any tender, or any recovery pursuant to any judgment (whether or not entered in accordance with subsection (a)), in any currency other than the Required Currency, except to the extent that such tender or recovery shall result in the actual receipt, by the payee, of the full amount of the Required Currency expressed to be payable in respect of such payments, (ii) shall be enforceable as an alternative or additional cause of action for the purpose of recovering in the Required Currency the amount, if any, by which such actual receipt shall fall short of the full amount of the Required Currency so expressed to be payable, and (iii) shall not be affected by judgment being obtained for any other sum due under this Indenture.
Section 10.17 Force Majeure.
In no event shall the Trustee be responsible or liable for any failure or delay in the performance of its obligations hereunder arising out of or caused by, directly or indirectly, forces beyond its control, including, without limitation, strikes, work stoppages, accidents, acts of war or terrorism, civil or military disturbances, nuclear or natural catastrophes or acts of God, and interruptions, loss or malfunctions of utilities, communications or computer (software and hardware) services, it being understood that the Trustee shall use reasonable best efforts which are consistent with accepted practices in the banking industry to resume performance as soon as practicable under the circumstances.
Section 10.18 U.S.A. Patriot Act.
The parties hereto acknowledge that in accordance with Section 326 of the U.S.A. Patriot Act, the Trustee, like all financial institutions and in order to help fight the funding of terrorism and money laundering, is required to obtain, verify, and record information that identifies each person or legal entity that establishes a relationship or opens an account with the Trustee. The parties to this Indenture agree that they will provide the Trustee with such information as it may request in order for the Trustee to satisfy the requirements of the U.S.A. Patriot Act.
ARTICLE XI
SINKING FUNDS
Section 11.1 Applicability of Article.
The provisions of this Article shall be applicable to any sinking fund for the retirement of the Securities of a Series if so provided by the terms of such Securities pursuant to Section 2.2 and except as otherwise permitted or required by any form of Security of such Series issued pursuant to this Indenture.
The minimum amount of any sinking fund payment provided for by the terms of the Securities of any Series is herein referred to as a “mandatory sinking fund payment” and any other amount provided for by the terms of Securities of such Series is herein referred to as an “optional sinking fund payment.” If provided for by the terms of Securities of any Series, the cash amount of any sinking fund payment may be subject to reduction as provided in Section 11.2. Each sinking fund payment shall be applied to the redemption of Securities of any Series as provided for by the terms of the Securities of such Series.
- 44 -
Section 11.2 Satisfaction of Sinking Fund Payments with Securities.
The Company may, in satisfaction of all or any part of any sinking fund payment with respect to the Securities of any Series to be made pursuant to the terms of such Securities (a) deliver outstanding Securities of such Series to which such sinking fund payment is applicable (other than any of such Securities previously called for mandatory sinking fund redemption) and (b) apply as credit Securities of such Series to which such sinking fund payment is applicable and which have been repurchased by the Company or redeemed either at the election of the Company pursuant to the terms of the Securities of such Series (except pursuant to any mandatory sinking fund) or through the application of permitted optional sinking fund payments or other optional redemptions pursuant to the terms of such Securities, provided that such Securities have not been previously so credited. Such Securities shall be received by the Trustee, together with an Officer’s Certificate with respect thereto, not later than 15 days prior to the date on which the Trustee begins the process of selecting Securities for redemption, and shall be credited for such purpose by the Trustee at the price specified in such Securities for redemption through operation of the sinking fund and the amount of such sinking fund payment shall be reduced accordingly. If as a result of the delivery or credit of Securities in lieu of cash payments pursuant to this Section 11.2, the principal amount of Securities of such Series to be redeemed in order to exhaust the aforesaid cash payment shall be less than $100,000, the Trustee need not call Securities of such Series for redemption, except upon receipt of a Company Order that such action be taken, and such cash payment shall be held by the Trustee or a Paying Agent and applied to the next succeeding sinking fund payment, provided, however, that the Trustee or such Paying Agent shall from time to time upon receipt of a Company Order pay over and deliver to the Company any cash payment so being held by the Trustee or such Paying Agent upon delivery by the Company to the Trustee of Securities of that Series purchased by the Company having an unpaid principal amount equal to the cash payment required to be released to the Company.
Section 11.3 Redemption of Securities for Sinking Fund.
Not less than 45 days (unless otherwise indicated in the Board Resolution, supplemental indenture hereto or Officer’s Certificate in respect of a particular Series of Securities) prior to each sinking fund payment date for any Series of Securities, the Company will deliver to the Trustee an Officer’s Certificate specifying the amount of the next ensuing mandatory sinking fund payment for that Series pursuant to the terms of that Series, the portion thereof, if any, which is to be satisfied by payment of cash and the portion thereof, if any, which is to be satisfied by delivering and crediting of Securities of that Series pursuant to Section 11.2, and the optional amount, if any, to be added in cash to the next ensuing mandatory sinking fund payment, and the Company shall thereupon be obligated to pay the amount therein specified. Not less than 30 days (unless otherwise indicated in the Board Resolution, Officer’s Certificate or supplemental indenture in respect of a particular Series of Securities) before each such sinking fund payment date the Trustee shall select the Securities to be redeemed upon such sinking fund payment date in the manner specified in Section 3.2 and cause notice of the redemption thereof to be given in the name of and at the expense of the Company in the manner provided in Section 3.3. Such notice having been duly given, the redemption of such Securities shall be made upon the terms and in the manner stated in Sections 3.4, 3.5 and 3.6.
- 45 -
ARTICLE XII
SUBORDINATION OF SECURITIES
Section 12.1 Subordination of Terms.
The payment by the Company of the principal of, premium, if any, and interest on any Series of Securities issued under this Indenture shall be subordinated to the extent set forth in a Board Resolution, supplemental indenture hereto or Officer’s Certificate relating to such Series of Securities.
[Signature page follows]
- 46 -
IN WITNESS WHEREOF, the parties hereto have caused this Indenture to be duly executed as of the day and year first above written.
| CRESCO LABS INC., as Issuer | ||
| By: | ||
| Name: | ||
| Its: | ||
| , as Trustee | ||
| By: | ||
| Name: | ||
| Its: | ||
- 47 -
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- European stocks mixed; Euro banking sector, Meta Platforms in the spotlight
- Meta shares dip on softer Q2 revenue guidance, elevated AI spending plans
- Oil prices dip as Iran-Israel fears cool, economic jitters persist
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share