Form DEF 14A HAEMONETICS CORP For: Jun 17
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
SCHEDULE 14A
Proxy Statement Pursuant to Section 14(a)
of the Securities Exchange Act of 1934
Filed by the Registrant x | Filed by a Party other than the Registrant ☐ | ||||
| Check the appropriate box: | |||||
| ☐ | Preliminary Proxy Statement | ||||
| ☐ | Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) | ||||
| x | Definitive Proxy Statement | ||||
| ☐ | Definitive Additional Materials | ||||
| ☐ | Soliciting Material under §240.14a-12 | ||||

HAEMONETICS CORPORATION
(Name of Registrant as Specified in Its Charter)
| Payment of Filing Fee (Check the appropriate box): | |||||
| x | No fee required. | ||||
| ☐ | Fee paid previously with preliminary materials. | ||||
| ☐ | Fee computed on table in exhibit required by Item 25(b) per Exchange Act Rules 14a-6(i)(1) and 0-11. | ||||
 | ||
| Notice of Annual Meeting of Shareholders and Proxy Statement | ||
| Friday, August 5, 2022 8:00 A.M. Eastern Time | ||


NOTICE OF ANNUAL MEETING OF SHAREHOLDERS
Friday August 5, 2022
8:00 A.M. Eastern Time
8:00 A.M. Eastern Time
125 Summer Street, Boston, Massachusetts 02110
To Our Shareholders:
The 2022 Annual Meeting of Shareholders of Haemonetics Corporation, a Massachusetts corporation (the "Company"), will be held on Friday, August 5, 2022 at 8:00 A.M., Eastern Time, at the offices of the Company, 125 Summer Street, Boston, Massachusetts 02110 for the following purposes:
1 To elect the nine director nominees named in the proxy statement to one-year terms expiring in 2023; | ||
2 To approve, on an advisory basis, the compensation of our named executive officers; | ||
3 To ratify the appointment of Ernst & Young LLP as our independent registered public accounting firm for the fiscal year ending April 1, 2023; and | ||
4 To transact such other business as may properly come before the meeting. | ||
The foregoing items of business are more fully described in the proxy statement accompanying this Notice of Annual Meeting of Shareholders. Additionally, we continue to carefully monitor COVID-19 developments and related guidance issued by Massachusetts regulatory agencies and relevant health organizations. Should we determine that alternative arrangements for the meeting may be advisable or required, such as changing the date, time, location or format of the meeting (including holding a virtual meeting, subject to local law requirements), we will announce our decision by press release and through our filings with the Securities and Exchange Commission and post additional information on our investor relations website at www.haemonetics.com.
We are pleased to continue utilizing the Securities and Exchange Commission rules that allow companies to furnish proxy materials to their shareholders on the Internet. We believe these rules allow us to provide you with the information you need while lowering the costs of delivery and reducing the environmental impact of the meeting. On or about June 20, 2022, we will mail to our shareholders of record as of June 2, 2022, the record date for the meeting, a Shareholder Meeting Notice and Important Notice Regarding the Availability of Proxy Materials containing instructions on how to access our proxy statement and our 2022 Annual Report to Shareholders (unless the shareholder previously requested electronic or paper delivery on an ongoing basis).
Your vote is important. Whether or not you plan to attend the meeting, we encourage you to vote your shares. Accordingly, we request that as soon as possible, you vote via the Internet or, if you have received printed proxy materials, you vote via the Internet, by telephone or by mailing your completed proxy card or voter instruction form.
By Order of the Board of Directors
Michelle L. Basil
Corporate Secretary
Boston, Massachusetts
June 17, 2022
IMPORTANT NOTICE REGARDING THE AVAILABILITY OF PROXY MATERIALS FOR THE ANNUAL MEETING OF SHAREHOLDERS TO BE HELD ON AUGUST 5, 2022: This proxy statement and the Company’s 2022 Annual Report to Shareholders are available at www.envisionreports.com/HAE. | ||
HAEMONETICS CORPORATION | 2022 Proxy Statement 1
TABLE OF CONTENTS
Page Number | |||||
2 HAEMONETICS CORPORATION | 2022 Proxy Statement
PROXY STATEMENT SUMMARY
This summary highlights selected information in this proxy statement (this "Proxy Statement"), which is being furnished in connection with the solicitation of proxies by Haemonetics Corporation for use at the 2022 Annual Meeting of Shareholders. Please review this entire Proxy Statement before voting. References in this Proxy Statement to "Haemonetics," the "Company," "we," "us" or "our" refer to Haemonetics Corporation.
| Voting Roadmap | ||
2022 ANNUAL MEETING OF SHAREHOLDERS
| Date and Time: | Friday, August 5, 2022 at 8:00 A.M., Eastern Time | ||||
| Place: | Haemonetics Corporation 125 Summer Street Boston, MA 02110 | ||||
| Commence Mail Date: | On or about June 20, 2022 | ||||
| Record Date: | June 2, 2022 | ||||
MEETING AGENDA AND VOTING RECOMMENDATIONS
Voting Items | Board Recommendation | For Further Information | |||||||||||||||
| 1 | Election of nine director nominees named in this Proxy Statement for one-year terms expiring at the 2023 Annual Meeting of Shareholders | FOR each director nominee | Page 8 | ||||||||||||||
| 2 | Approval, on an advisory basis, of our named executive officers’ compensation | FOR | Page 18 | ||||||||||||||
| 3 | Ratification of our independent registered public accounting firm for fiscal 2023 | FOR | Page 47 | ||||||||||||||
HOW TO VOTE
ONLINE | BY PHONE | BY MAIL | IN PERSON | |||||||||||||||||
Go to www.envisionreports.com/HAE and enter the 15-digit control number provided on your proxy card or voting instruction form. | If you received a paper copy of your proxy materials by mail, call the number on your proxy card or voting instruction form. You will need the 15-digit control number provided on your proxy card or voting instruction form. | If you received a paper copy of your proxy materials by mail, complete, sign and date the proxy card or voting instruction form and mail it in the accompanying pre-addressed envelope. | See the instructions beginning on page 54 regarding how to attend and vote in person at the meeting. | |||||||||||||||||
HAEMONETICS CORPORATION | 2022 Proxy Statement 3
| Performance Highlights | ||
Haemonetics is a global healthcare company dedicated to providing a suite of innovative medical products and solutions for customers, to help them improve patient care and reduce the cost of healthcare. Our technology addresses important medical markets: blood and plasma component collection, the surgical suite and hospital transfusion services. We view our operations and manage our business in three principal reporting segments: Plasma, Blood Center and Hospital. The Company's common stock trades on the New York Stock Exchange ("NYSE") under the symbol "HAE."
Fiscal 2022 was a challenging year for Haemonetics but we are proud of how our people responded. We continued to strengthen and grow our business despite the continued challenges caused by COVID-19, and we acted with urgency to address the transition of CSL Plasma (“CSL”), one of our largest customers, following its April 2021 notice of intent not to renew its current U.S. supply agreement for the use of Haemonetics PCS2® Plasma Collection System devices and the purchase of plasma disposables that was set to expire in June 2022 (including negotiating an extended transition of the CSL agreement through December 2023, on a non-exclusive basis). Our agility and perseverance through these challenges helped us achieve growth in our Plasma and Hospital businesses and further stabilize our Blood Center business, and we continued to distinguish Haemonetics for the meaningful value we are creating across our markets. As the industry leader, we delivered integrated solutions to help our Plasma customers realize much needed increases in the volume of collections. In the face of unprecedented blood shortages, our Blood Center products helped maximize the impact of donations and attract and retain donors. Hospital, including our new Vascular Closure product line acquired through our March 2021 purchase of Cardiva Medical, Inc. ("Cardiva Medical") continued to exceed expectations and was our fastest growing business in fiscal 2022, helping customers improve patient care and outcomes at less cost. The acquisition of Cardiva Medical was an important step in our transformational growth journey, and we remain focused on further optimizing our portfolio and accelerating growth. Our Operational Excellence Program also proved fundamental to our resilience and ability to quickly address supply chain disruptions and serve all who depend on us. This program will continue to play a critical role in our ongoing transformation, enabling us to sustain our success, become a more agile, efficient and productive company, create lasting cost savings and free up resources to fund growth investments. We look forward to sharing more detail about our long-range plans to deliver value for customers and shareholders at our fiscal 2023 Investor Day.
For further discussion of our fiscal 2022 business highlights and how our fiscal 2022 performance affected our Named Executive Officers' compensation, please see our Compensation Discussion and Analysis beginning on page 21. For additional information on our fiscal 2022 financial results and Haemonetics' COVID-19 response efforts please see our Annual Report on Form 10-K for the fiscal year ended April 2, 2022.
| Governance Highlights | ||
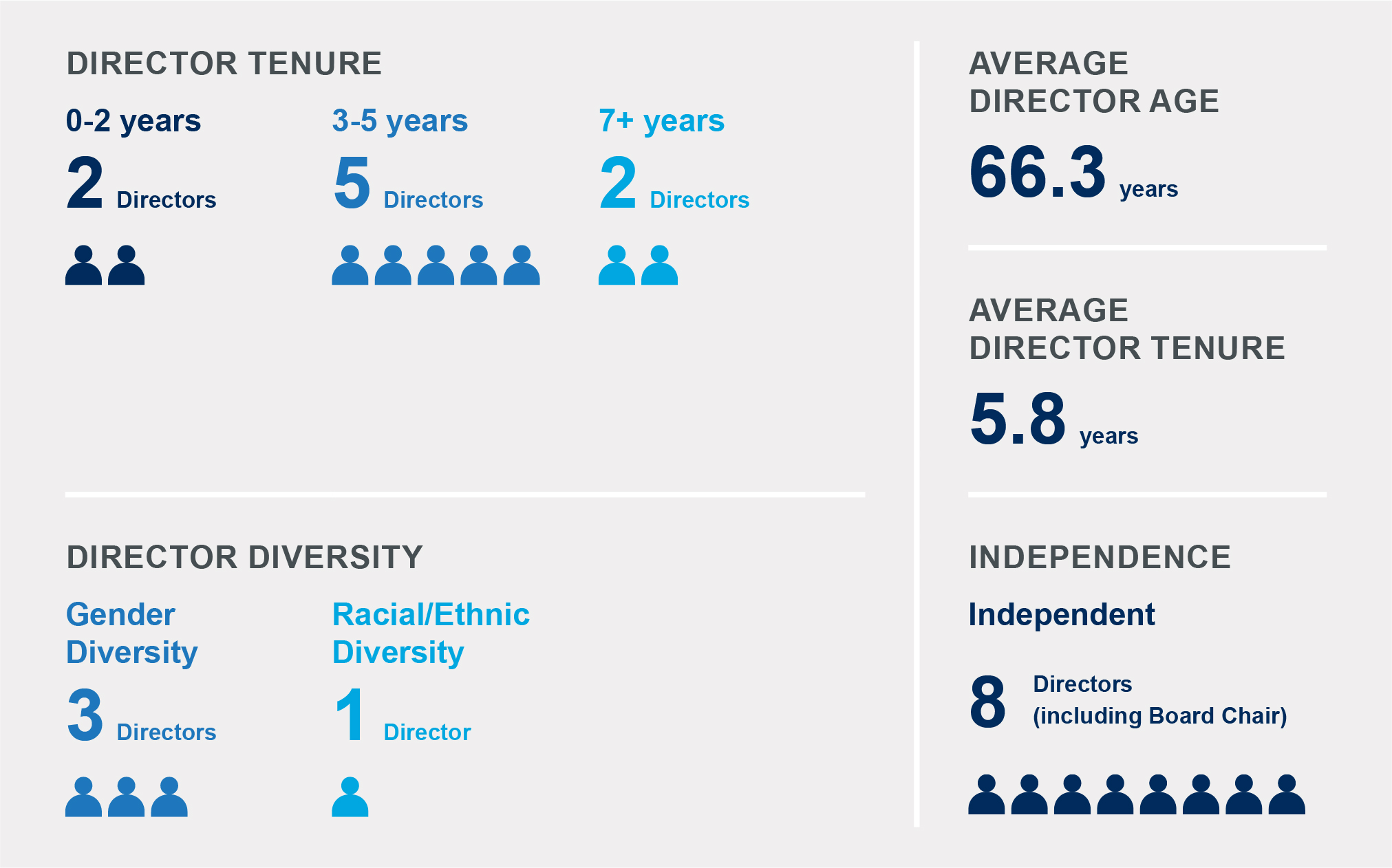
The following "Governance Highlights" are for the nine Board members nominated for re-election at our 2022 Annual Meeting of Shareholders, all of whom (with the exception of Christopher A. Simon, our President and Chief Executive Officer) are independent.
BOARD COMPOSITION SNAPSHOT
4 HAEMONETICS CORPORATION | 2022 Proxy Statement
BOARD MEMBERS
Name and Principal Professional Experience | Age | Director Since | Independent | Committee Membership | ||||||||||
DIRECTOR NOMINEES | ||||||||||||||
Christopher A. Simon President and Chief Executive Officer, Haemonetics | 58 | 2016 | N/A | |||||||||||
Robert E. Abernathy Retired Chairman and Chief Executive Officer, Halyard Health, Inc. | 67 | 2017 | ü | Compensation  Technology | ||||||||||
Catherine M. Burzik Former President and Chief Executive Officer, Kinetic Concepts, Inc. | 71 | 2016 | ü | Audit Technology  | ||||||||||
Michael J. Coyle Former President and Chief Executive Officer, iRhythm Technologies, Inc. | 60 | 2020 | ü | Audit Governance and Compliance | ||||||||||
Charles J. Dockendorff Retired Executive Vice President and Chief Financial Officer, Covidien plc | 67 | 2014 | ü | Audit  Governance and Compliance | ||||||||||
Lloyd E. Johnson Retired Global Managing Director, Finance and Internal Audit, Accenture Corporation | 68 | 2021 | ü | Audit Governance and Compliance | ||||||||||
Mark W. Kroll, Ph.D. Retired Senior Executive Officer, St. Jude Medical, Inc. | 69 | 2006 | ü | Compensation Technology | ||||||||||
Claire Pomeroy, M.D., M.B.A. President, Albert and Mary Lasker Foundation | 67 | 2019 | ü | Compensation Technology | ||||||||||
Ellen M. Zane (Board Chair) CEO Emeritus of Tufts Medical Center | 70 | 2018 | ü | Compensation Governance and Compliance  | ||||||||||
 Committee Chair
Committee ChairINDEPENDENT DIRECTOR QUALIFICATIONS
As discussed below under “Board Composition and the Director Nomination Process” (beginning on page 8), the Governance and Compliance Committee is responsible for reviewing and assessing the appropriate skills, experience and background that should be reflected in the composition of the Board. The experience, expertise and diversity represented by the Board as a collective body allows the Board to lead Haemonetics in a manner that serves its shareholders’ interests appropriately. The Governance and Compliance Committee believes that the independent directors on our Board have an effective mix of experience, qualifications, attributes and skills that are important to our business, which include:
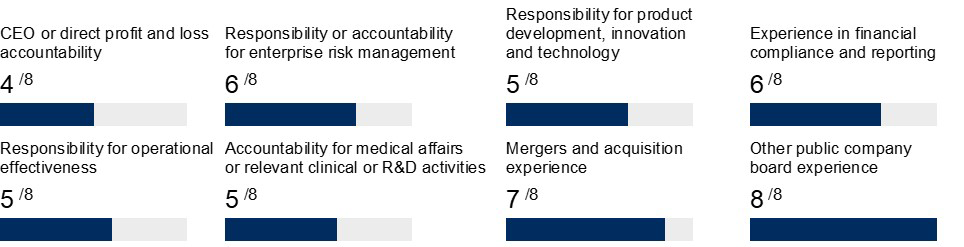
BOARD REFRESHMENT
The Board does not endorse arbitrary term limits for director service, nor does it believe in automatic re-nomination until directors reach the mandatory retirement age. The Board and each Committee of the Board conduct an annual self-evaluation of their performance, which is an important determinant for Board refreshment. Beginning in fiscal 2023, the Board has determined to further enhance its annual self-evaluation process by incorporating an individual Board member peer review component.
HAEMONETICS CORPORATION | 2022 Proxy Statement 5
Under the Company’s Principles of Corporate Governance, directors are required to retire from the Board as of the annual shareholders meeting coincident with or next following his or her 72nd birthday. Upon the recommendation of the Governance and Compliance Committee, the Board may waive this requirement for a director if it deems such waiver to be in the best interests of the Company. During the Company's fiscal 2022 annual shareholder outreach, Board members engaged with shareholders on potential modifications to the age 72 mandatory retirement policy in conjunction with the addition of a peer review component to the annual Board evaluation process, with shareholders expressing either support or deference to the Board's determination. For more information on the Company's' annual shareholder outreach see "Shareholder Outreach" on page 6 below.
| How We Think About Board Refreshment | ||
 | ||
BEST PRACTICES
We are committed to high standards in corporate governance and creating a corporate governance environment that supports the long-term success of our Company. Our governance practices include the following:
BOARD PRACTICES | SHAREHOLDER PRACTICES | |||||||
ü Independent Board Chair and directors (other than CEO) ü Committees consist solely of independent directors ü Annual election of directors (phase out of classified Board complete as of the 2022 Annual Meeting of Shareholders) ü Regular executive sessions of independent directors ü Board oversight of risk management and compliance ü Annual Board/Committee evaluations, including Board member peer review beginning in fiscal 2023 | ü Transparent and active shareholder engagement (outreach to over 52% of shares outstanding in each of last four years) ü Annual say on pay advisory vote, with over 95% approval in each of the last seven years ü Majority voting provisions in Charter and By-Laws ü Shareholder right to call special meetings ü Director resignation policy if a director does not obtain a majority of the votes cast in an uncontested election ü No shareholder rights plan (i.e., a "poison pill") | |||||||
OTHER BEST PRACTICES | ||||||||
ü Maintain strong executive compensation governance and pay practices (see "Strong Governance and Pay Practices" beginning on page 24) | ||||||||
SHAREHOLDER OUTREACH
The Company is committed to transparent and active engagement with its shareholders. On an ongoing basis, members of senior management meet with shareholders to discuss the Company's business fundamentals, performance and long-term outlook. Our Board also proactively engages with shareholders on governance and executive compensation matters and other topics of shareholder interest. During the fall and winter of fiscal 2022, our Board Chair (who also Chairs our Governance and Compliance Committee) and Compensation Committee Chair offered meetings to nine of our largest shareholders that collectively held over 52% of our outstanding shares. Together with our Executive Vice President, General Counsel and Director, Investor Relations, these Board members met in January 2022 with shareholders representing approximately 30% of shares outstanding to discuss, among other things, Haemonetics' corporate strategy and performance, board diversity and refreshment, executive compensation, corporate responsibility and other governance matters. Details of shareholder feedback are discussed throughout this Proxy Statement.
For information on how to contact our Board please see "Communications with the Board of Directors" on page 13.
6 HAEMONETICS CORPORATION | 2022 Proxy Statement
CORPORATE RESPONSIBILITY
"We make it possible. You make it matter." At Haemonetics, our Purpose inspires the important work we do every day to meaningfully advance patient care and drive greater possibilities in healthcare. How we do this work is equally important. Guided by our Purpose, and mindful of our shareholders, customers, employees and other key stakeholders whose trust we value, we are committed to being a good corporate citizen and take responsibility to proactively identify and manage the environmental, social and governance (“ESG”) risks and opportunities that are relevant to our business and those we serve. Please visit the “Corporate Responsibility” page on our website www.haemonetics.com for more information on our ESG-related policies and practices.
We look forward to publishing our first Corporate Responsibility report in fiscal 2023.
| Performance Highlights | ||
ELEMENTS OF TOTAL COMPENSATION
When setting compensation for our Named Executive Officers, or "NEOs," the Compensation Committee focuses on total direct compensation. Total direct compensation includes three major components - base salary, annual short-term incentive compensation and annual long-term incentive compensation - all of which are designed to work together to drive a complementary set of behaviors and outcomes. The following chart illustrates, for fiscal 2022, the target annual compensation mix among the three elements of direct compensation for our Chief Executive Officer and, on average, for our other NEOs.
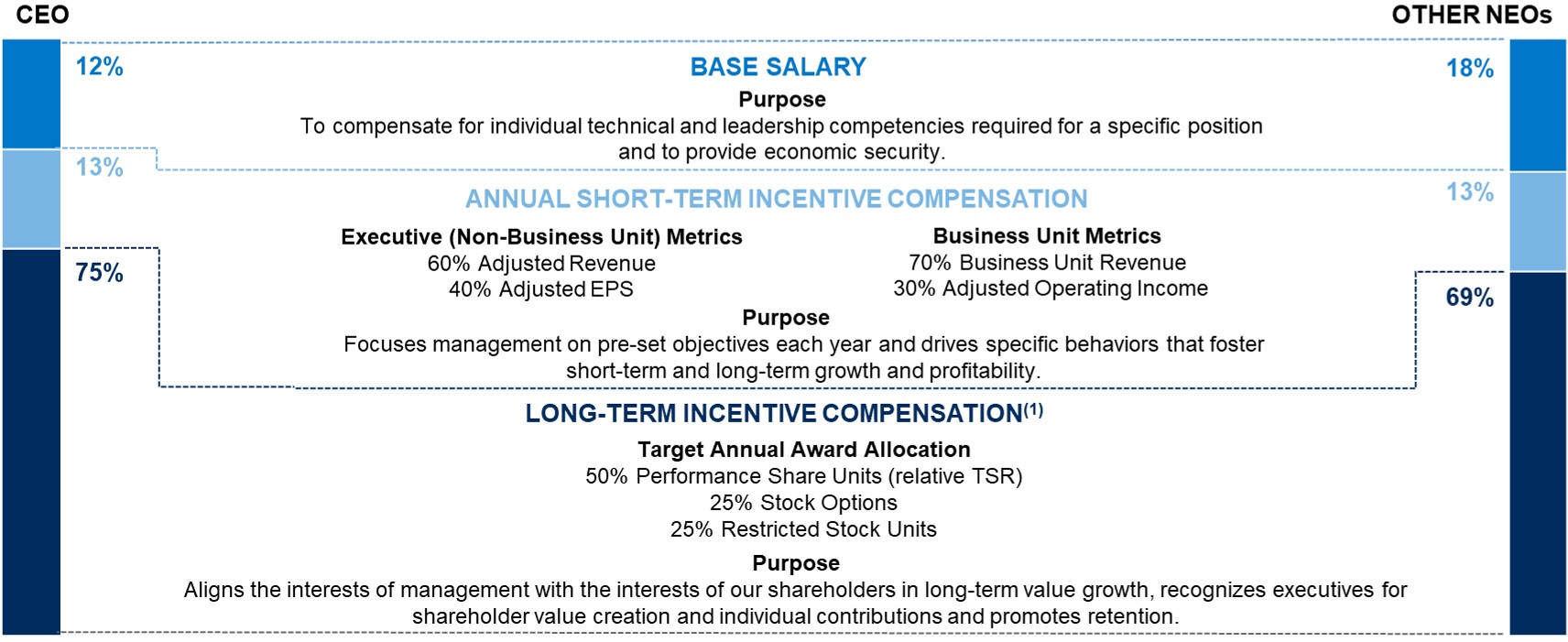
(1)Long-term incentive compensation includes grant value of all fiscal 2022 equity awards to Named Executive Officers. For more information see "Individual Fiscal 2022 Long-Term Incentive Awards" beginning on page 32 of this Proxy Statement.
| Cautionary Note Regarding Forward-Looking Statements | ||
Any statements contained in this Proxy Statement that do not describe historical facts may constitute forward-looking statements. Forward-looking statements in this Proxy Statement may include, without limitation, statements regarding (i) plans and objectives of management for operations of the Company, including plans or objectives related to the development and commercialization of, and regulatory approvals related to, the Company’s products and plans or objectives related to the Operational Excellence Program; (ii) estimates or projections of financial results, financial condition, capital expenditures, capital structure or other financial items; (iii) the impact of the COVID-19 pandemic on the Company’s operations, availability and demand for its products and future financial performance; and (iv) the assumptions underlying or relating to any statement described in points (i), (ii) or (iii) above. Such forward-looking statements are not meant to predict or guarantee actual results, performance, events or circumstances and may not be realized because they are based upon the Company's current projections, plans, objectives, beliefs, expectations, estimates and assumptions and are subject to a number of risks and uncertainties and other influences. Factors that may cause actual results to differ materially from those contemplated by the statements in this Proxy Statement can be found in the Company’s most recent Annual Report on Form 10-K for the fiscal year ended April 2, 2022 under the headings "Cautionary Statement Regarding Forward-Looking Information" and "Risk Factors" and in our other periodic reports filed with the Securities and Exchange Commission ("SEC"). The Company does not undertake to update these forward-looking statements.
This Proxy Statement contains statements regarding individual and Company performance objectives and targets. These objectives and targets are disclosed in the limited context of our compensation plans and programs and should not be understood to be statements of management's future expectations or estimates of future results or other guidance. We specifically caution investors not to apply these statements to other contexts.
HAEMONETICS CORPORATION | 2022 Proxy Statement 7
ITEM 1—ELECTION OF DIRECTORS
Our Board currently has nine members, each of whom is standing for election at our 2022 Annual Meeting of Shareholders (see "Director Nominees" beginning on page 9). If elected, each director will serve for a one-year term expiring at our 2023 Annual Meeting of Shareholders or until his or her successor is duly elected and qualified or until his or her earlier death, resignation or removal. Each nominee has agreed to be named in this Proxy Statement and to serve if elected. We believe that each nominee will be able and willing to serve if elected. However, if any nominee should become unable for any reason or unwilling to serve, proxies may be voted for another person nominated as a substitute by our Board, or our Board may reduce the number of directors.
In 2019, we amended our Charter and By-Laws to phase out our classified Board structure over a three-year period beginning at the 2020 Annual Meeting of Shareholders. As of the 2022 Annual Meeting of Shareholders, our classified Board structure will have been fully phased out and all Haemonetics director nominees with be subject to annual election.
þ | Our Board unanimously recommends that you vote FOR each of the nominees for director named in this Proxy Statement. Directors are elected by a plurality of the votes cast by shareholders entitled to vote at the meeting. Abstentions and broker non-votes will not have any effect on this proposal. Accordingly, the nominees receiving the highest number of “for” votes at the meeting will be elected as directors. However, under a policy adopted by the Board, in an uncontested election, any nominee for director who does not receive the favorable vote of at least a majority of the votes cast with respect to such director is required to tender his or her resignation to the Board, which will consider whether to accept the resignation. This is an uncontested election of directors because the number of nominees for director does not exceed the number of directors to be elected. The persons named in the accompanying proxy will vote all duly submitted proxies FOR the nominees listed below (see "Director Nominees" beginning on page 9) unless instructed otherwise. | ||||
| Haemonetics Board of Directors | ||
BOARD COMPOSITION AND THE DIRECTOR NOMINATION PROCESS
The Governance and Compliance Committee is responsible for reviewing and assessing the appropriate skills, experience and background required for the Board. Because our business operates in regulated healthcare markets around the globe and encompasses research and development, manufacturing and marketing functions that are subject to technological and market changes, the skills, experience and backgrounds that are needed are diverse.
While the priority and emphasis of each factor changes from time to time to take into account the current needs of the Company, the aim is to have a diverse portfolio of talents and backgrounds, including diversity with respect to age, gender, race, ethnicity and experience in aspects of business or technology relevant to the Company’s business. The Governance and Compliance Committee and the Board review and assess the importance of these factors as part of the Board’s annual self-evaluation process, and the Governance and Compliance Committee at least annually reviews and reports to the Board on what skills and characteristics it believes are reflected in the then-current directors and what additional qualifications should be sought in new directors to augment the skills and expertise on the Board. These steps are intended to ensure that the Company continues to create and sustain a Board that can support and effectively oversee the Company’s business.
Although the Board has not adopted any absolute prerequisites for Board service, the Governance and Compliance Committee considers the following minimum criteria when identifying nominees:
Background | Qualifications | ||||
His or her skills, experience and acumen as they relate to the Company's needs and the current state of its markets | His or her independence from the Company and management, as defined under SEC and NYSE rules | ||||
His or her integrity, independence, diversity, experience, leadership and ability to exercise sound judgment | His or her contemporaneous service on other public company boards of directors and related committees | ||||
His or her knowledge of the healthcare sector and the markets in which the Company participates | His or her ability to participate fully in Board activities and represent the Company's stakeholders | ||||
In the case of current directors being considered for re-nomination, the Governance and Compliance Committee will also take into consideration the director’s history of attendance at Board and committee meetings, tenure as a member of the Board and preparation for and participation in such meetings.
8 HAEMONETICS CORPORATION | 2022 Proxy Statement
DIRECTOR NOMINEES
PRESENT TERMS EXPIRING IN 2022 AND PROPOSED TERMS TO EXPIRE IN 2023
 Age: 58 | CHRISTOPHER A. SIMON President and Chief Executive Officer, Haemonetics Corporation | |||||||
Mr. Simon is President and Chief Executive Officer of the Company. He joined Haemonetics in May 2016 and our Board in September 2016. Mr. Simon previously served as a Senior Partner of McKinsey & Company where he led the Global Medical Products Practice. Mr. Simon served as a consultant with McKinsey & Company beginning in 1993 and was the lead partner for McKinsey & Company’s strategy review with Haemonetics that began in October 2015, where he gained invaluable insights into the Company’s business and markets. Prior to his career at McKinsey & Company, Mr. Simon served in commercial roles with Baxter Healthcare Corporation and as a U.S. Army Infantry Officer in Korea with the 1st Ranger Battalion. He also currently serves on the Board of Directors of AdvaMed, a global trade association of companies that develop, produce, manufacture, and market medical technologies. Mr. Simon earned a Bachelor of Science in Economics from the Wharton School at the University of Pennsylvania and an M.B.A. from Harvard Business School. Skills and Qualifications: As President and Chief Executive Officer of the Company, Mr. Simon provides the Board with an intensive understanding of the Company's business, operations and strategy. Mr. Simon also brings to the Board more than 20 years of experience in helping businesses transform and grow. | ||||||||
 Independent Age: 67 Other Public Co. Board Service: •Avient Corp. | ROBERT E. ABERNATHY Retired Chairman and Chief Executive Officer, Halyard Health, Inc. | |||||||
Mr. Abernathy joined our Board in October 2017 and is Chair of the Compensation Committee and a member of the Technology Committee. Mr. Abernathy served as Chairman of the Board of Directors and Chief Executive Officer of Halyard Health Inc., a publicly-traded medical technology company and spin-off from Kimberly-Clark, from October 2014 until his retirement in June 2017 (he continued as Chairman until September 2017). Mr. Abernathy joined Kimberly-Clark, a global personal care products company, in 1982 and held numerous roles of increasing responsibility, including President of Kimberly-Clark’s Global Health Care business, Group President, Developing & Emerging Markets, Managing Director, Kimberly-Clark Australia and President, North Atlantic Consumer Products. Skills and Qualifications: Mr. Abernathy brings to the Board extensive leadership experience in the healthcare industry and in international operations, including in-depth knowledge and insight on the needs of healthcare providers and patients and enterprise risk management matters. | ||||||||
 Independent Age: 71 Other Public Co. Board Service: •Becton, Dickinson and Co. •Orthofix Medical, Inc. (Board Chair) | CATHERINE M. BURZIK Former President and Chief Executive Officer, Kinetic Concepts, Inc. | |||||||
Ms. Burzik joined our Board in October 2016 and is the Chair of the Technology Committee and a member of the Audit Committee. Ms. Burzik is currently President and Chief Executive Officer of CFB Interests, LLC. From 2013 to 2017, Ms. Burzik was also a general partner at Targeted Technology, an early stage venture capital firm focused on medical device, life sciences and biotechnology investments. Ms. Burzik previously served as President and Chief Executive Officer of Kinetic Concepts, Inc., a leading medical device company specializing in the fields of wound care and regenerative medicine, from 2006 until the Company’s sale in 2012. Prior to joining Kinetic Concepts, Inc., Ms. Burzik’s leadership experience included serving as President of Applied Biosystems and holding senior executive positions at Eastman Kodak and Johnson & Johnson, including Chief Executive Officer and President of Kodak Health Imaging Systems and President of Ortho-Clinical Diagnostics, Inc., a Johnson & Johnson company. In 2019, Ms. Burzik received the AdvaMed Lifetime Achievement Award that honors accomplishments of pioneers in the medical technology industry. Skills and Qualifications: Ms. Burzik is a widely respected healthcare industry leader, having successfully led major medical device, diagnostic imaging and life science businesses. Ms. Burzik brings to the Board many years of leadership experience in strategic planning, international operations and financial and enterprise risk management. | ||||||||
HAEMONETICS CORPORATION | 2022 Proxy Statement 9
 Independent Age: 60 | MICHAEL J. COYLE Former President and Chief Executive Officer, iRhythm Technologies, Inc. | |||||||
Mr. Coyle joined our Board in April 2020 and is a member of both the Audit Committee and the Governance and Compliance Committee. Mr. Coyle previously served as the President and Chief Executive Officer of iRhythm Technologies, Inc., a digital healthcare company, from January 2021 to June 2021. From December 2009 to January 2021, Mr. Coyle served as Executive Vice President and Group President, Cardiac and Vascular Group of Medtronic plc (and its predecessor, Medtronic, Inc.), a global medical device company, where he oversaw four of the company’s business divisions. Mr. Coyle previously served as President of the Cardiac Rhythm Management division at St. Jude Medical Inc. from 2001 to 2007 and earlier in his career held numerous leadership positions at St. Jude and Eli Lilly & Company. Mr. Coyle previously served on the boards of iRhythm Technologies, Inc. and of two NASDAQ-listed medical device companies responsible for making catheter-based products. He holds six U.S. patents related to cardiovascular medical device products and technologies. Skills and Qualifications: Mr. Coyle's many years of executive experience in the medical device industry, including building global businesses and bringing technologies to important medical markets, provides the Board with a valuable perspective as the Company pursues growth and advances its innovation agenda. His leadership positions with global medical device companies also brings additional expertise to the Board in strategic planning, enterprise risk management, market development and international operations. | ||||||||
 Independent Age: 67 Other Public Co. Board Service: •Boston Scientific Corporation •Hologic, Inc. •Keysight Technologies, Inc. | CHARLES J. DOCKENDORFF Retired Executive Vice President and Chief Financial Officer, Covidien plc | |||||||
Mr. Dockendorff joined our Board in July 2014 and is Chair of the Audit Committee and a member of the Governance and Compliance Committee. Mr. Dockendorff served as Executive Vice President and Chief Financial Officer of Covidien plc, a global healthcare company, and its predecessor, Tyco Healthcare, from 1995 until his retirement in 2015. Mr. Dockendorff joined the Kendall Healthcare Products Company, the foundation of the Tyco Healthcare business, in 1989 as Controller and was named Vice President and Controller five years later. Prior to joining Kendall/Tyco Healthcare, Mr. Dockendorff was the Chief Financial Officer, Vice President of Finance and Treasurer of Epsco, Inc. and Infrared Industries, Inc. Earlier in his career, Mr. Dockendorff worked as an accountant for Arthur Young & Company (now Ernst & Young LLP) and the General Motors Corporation. Skills and Qualifications: Mr. Dockendorff is a highly-respected healthcare industry leader with extensive experience in finance and corporate management. As a retired Chief Financial Officer of a large global healthcare products company, Mr. Dockendorff brings to the Board many years of leadership experience in accounting and financial management and planning as well as enterprise risk management. | ||||||||
 Independent Age: 68 Other Public Co. Board Service: •Apogee Enterprises, Inc. •Beazer Homes USA, Inc. | LLOYD E. JOHNSON Retired Global Managing Director, Finance and Internal Audit, Accenture Corporation | |||||||
Mr. Johnson joined our Board in August 2021 and is a member of both the Audit Committee and the Governance and Compliance Committee. Mr. Johnson served as Global Managing Director, Finance and Internal Audit at Accenture Corporation from 2004 until his retirement in 2015, where he led the global management consulting company’s audit organization and provided strategic leadership in finance, risk, compliance and governance. Prior to joining Accenture, Mr. Johnson served as Executive Director, M&A and General Auditor for Delphi Automotive PLC, a global automotive technology industry leader. He has also held senior financial leadership positions at Emerson Electric Corporation, Sara Lee Corporation and Shaw Food Services. In addition to his public company board service, Mr. Johnson also serves on the boards of AARP, where he is Second Vice Chair, and the National Association of Corporate Directors (NACD) Carolinas Chapter. He was named one of the "Most Influential Black Corporate Directors" by Savoy in 2017 and has been listed as a "Director to Watch" in 2018 and 2020 by Directors & Boards. Skills and Qualifications: Mr. Johnson has over 35 years of corporate finance leadership experience, mostly at multi-national public companies. He brings to the Board significant expertise and strategic insight in the areas of accounting and financial management, mergers and acquisitions, international operations, business development, corporate governance and enterprise risk management. | ||||||||
10 HAEMONETICS CORPORATION | 2022 Proxy Statement
 Independent Age: 69 Other Public Co. Board Service: •Axon Enterprise, Inc. | MARK W. KROLL, Ph.D. Retired Senior Executive Officer at St. Jude Medical, Inc. | |||||||
Dr. Kroll joined our Board in 2006 and is a member of both the Compensation Committee and the Technology Committee. He currently serves as an Adjunct Full Professor of Biomedical Engineering at the University of Minnesota. From 1995 until his retirement in 2005, Dr. Kroll held a variety of executive leadership positions at St. Jude Medical, Inc., a global medical device company, including as Senior Vice President and Chief Technology Officer for the Cardiac Rhythm Management division and as Vice President of the Tachycardia Business division. Dr. Kroll has more than 25 years of experience with cardiovascular devices and instrumentation and is the named inventor of more than 380 U.S. patents as well as numerous international patents. He is a fellow of the American College of Cardiology, Heart Rhythm Society, Institute of Electronics and Electrical Engineering and the American Institute for Medicine and Biology in Engineering. In 2010, Dr. Kroll was awarded the Career Achievement Award in Biomedical Engineering, among the highest international awards in biomedical engineering. Skills and Qualifications: Dr. Kroll is a well-known pioneer in the field of electrical medical devices and a distinguished technology expert throughout the global medical device industry. He brings to the Board extensive expertise in the areas of medical innovation and technology. | ||||||||
 Independent Age: 67 Other Public Co. Board Service: •Embecta Corp. | CLAIRE POMEROY, M.D., M.B.A. President, Albert and Mary Lasker Foundation | |||||||
Dr. Pomeroy joined our Board in April 2019 and is a member of both the Technology Committee and the Compensation Committee. Since 2013, Dr. Pomeroy has served as the President of the Albert and Mary Lasker Foundation, a private foundation that seeks to improve health by accelerating support for medical research through recognition of research excellence, education and advocacy. Previously, Dr. Pomeroy served as Dean of the UC Davis School of Medicine and Vice Chancellor of the UC Davis Health System. In addition to her public company board service, Dr. Pomeroy also is Chair of the Center for Women in Academic Medicine and Science and a director of the Lasker Foundation, the Sierra Health Foundation, the Science Philanthropy Alliance, the Science Communication Lab and the Morehouse School of Medicine. Dr. Pomeroy previously served on the board of directors of Becton, Dickinson and Company, from which she resigned in March 2022 in connection with joining the board of directors of Embecta Corp., a diabetes care spin-off of Becton, Dickinson and Company. Skills and Qualifications: Dr. Pomeroy is an expert in infectious diseases with broad leadership experience in health system administration, healthcare delivery, medical research and public health. She provides the Board with important perspectives in the areas of global health services, health policy and medical innovation. | ||||||||
 Independent Chair Age: 70 Other Public Co. Board Service: •Azenta, Inc. •Boston Scientific Corporation •Synchrony Financial | ELLEN M. ZANE CEO Emeritus of Tufts Medical Center | |||||||
Ms. Zane joined our Board in January 2018 and serves as the Chair of the Board, Chair of the Governance and Compliance Committee and a member of the Compensation Committee. Ms. Zane is CEO Emeritus of Tufts Medical Center, where she served as President and Chief Executive Officer from 2004 to 2011. Prior to 2004, Ms. Zane served as Network President for Partners Healthcare System, a physician/hospital network sponsored by the Harvard-affiliated Massachusetts General Hospital and Brigham and Women's Hospital. Ms. Zane also previously served as Chief Executive Officer of Quincy Hospital in Quincy, Massachusetts. Ms. Zane has previously served as a director of Century Capital Management, Parexel International Corporation, Lincare Holdings Inc. and Press Ganey Holdings. Ms. Zane previously served on the Company's Board from 2012 to 2016. Ms. Zane was named to the NACD Directorship 100 for 2021, an annual award that recognizes the most influential boardroom leaders each year. Skills and Qualifications: Ms. Zane is a nationally renowned healthcare leader with substantial public company board experience. She brings to the Board extensive functional and leadership expertise in the healthcare industry, including with respect to strategy development, finance, operational effectiveness and enterprise risk management. | ||||||||
HAEMONETICS CORPORATION | 2022 Proxy Statement 11
IDENTIFYING NEW DIRECTORS
The Company’s nomination process for new Board members is as follows:
ASSESS BOARD NEEDS 6 | The Governance and Compliance Committee or other Board member identifies a need to add a new Board member who meets specific criteria or to fill a vacancy on the Board. | |||||||
IDENTIFY CANDIDATES 6 | The Governance and Compliance Committee initiates a search seeking input from Board members and senior management and, if necessary, hires a search firm. The Governance and Compliance Committee also considers recommendations for nominees for directorships submitted by shareholders. | |||||||
EVALUATE POTENTIAL CANDIDATES 6 | An initial list of candidates that will satisfy specific criteria and otherwise qualify for membership on the Board is identified and presented to the Governance and Compliance Committee, which evaluates the candidates. | |||||||
INTERVIEW CANDIDATES 6 | The Board Chair, the Chair of the Governance and Compliance Committee, the Chief Executive Officer and at least one other member of the Governance and Compliance Committee interview top candidates. | |||||||
RECOMMEND CANDIDATES FOR BOARD REVIEW 6 | The Governance and Compliance Committee seeks the entire Board’s endorsement of the final candidate and makes a recommendation to the Board regarding the election of the candidate. | |||||||
NOMINATION AND ELECTION | The final candidate is nominated by the Board for shareholder election or elected by the Board to fill a vacancy. | |||||||
The Governance and Compliance Committee reviews and evaluates all director nominations in the same manner and in accordance with the Company’s By-Laws. Shareholders who wish to submit candidates for consideration as nominees may submit a letter and resume to our Corporate Secretary at the Company’s headquarters located at 125 Summer Street, Boston, Massachusetts 02110.
| Board’s Role and Responsibilities | ||
OVERVIEW
The Board oversees, directs and counsels senior management in conducting the business in the long-term interests of the Company and its shareholders. The Board’s responsibilities include:
•Reviewing and approving the Company’s financial and strategic objectives, operating plans and significant actions, including mergers and acquisitions;
•Overseeing the conduct of the business and compliance with applicable laws and ethical standards;
•Overseeing the processes that maintain the integrity of our financial statements and public disclosures;
•Evaluating and determining the compensation of senior management, including the Chief Executive Officer;
•Overseeing and providing counsel on scientific, innovation and technology activities at the Company; and
•Selecting the Chief Executive Officer, developing succession plans for the position of Chief Executive Officer and supervising senior management succession plans.
THE BOARD’S ROLE IN RISK MANAGEMENT
The Board is responsible for oversight of the Company’s enterprise-wide approach to risk management, while the Company’s management is responsible for managing risk on a day-to-day basis and for bringing to the Board's attention material risks facing the Company. The Board focuses on the quality and scope of the Company’s risk management strategies and considers the most significant areas of risk inherent in the Company’s business strategies and operations and the steps that management is taking to mitigate those risks.
In addition to full Board oversight of the Company’s risk management, Board committees consider discrete categories of risk relating to their respective areas of responsibility. All committees report to the full Board as appropriate, including when a matter rises to the level of a material or enterprise level risk.
Senior management has overall responsibility for the Company’s risk management approach. This responsibility includes identifying, evaluating and addressing potential risks that may exist at the enterprise, strategic, financial, operational, compliance
12 HAEMONETICS CORPORATION | 2022 Proxy Statement
and reporting levels. As discussed above under the heading "Corporate Responsibility" on page 7, the Company is also committed to being a good corporate citizen and take responsibility to proactively identify and manage the ESG risks and opportunities that are relevant to our business and those we serve. The Company’s internal audit function, which reports regularly to the Audit Committee of the Board, serves as the primary monitoring and testing function for compliance with company-wide policies and procedures.
The Company believes that the division of risk management responsibilities described above constitutes an effective program for addressing the risks inherent in the operation of the Company and the achievement of its business objectives.
THE BOARD’S ROLE IN MANAGEMENT SUCCESSION PLANNING
Pursuant to our Principles of Corporate Governance, the Board also plans for succession to the position of Chief Executive Officer as well as certain other senior management positions. To assist the Board, the Chief Executive Officer annually provides the Board with an assessment of senior managers and their potential to succeed him. The Chief Executive Officer also provides the Board with an assessment of persons considered potential successors to certain senior management positions.
COMMUNICATIONS WITH THE BOARD OF DIRECTORS
The Board considers shareholder perspectives, as well as the interests of all stakeholders, when overseeing Company strategy, formulating governance practices and designing compensation programs. Interested parties and shareholders may communicate with the Board, or the non-management directors as a group, or any individual director by sending communications to the attention of our Corporate Secretary, 125 Summer Street, Boston, Massachusetts 02110, who will forward such communications to the appropriate recipients. Communications may also be sent via the Investor Relations page on our website: www.haemonetics.com.
| Board Leadership Structure | ||
STRUCTURE OF THE BOARD OF DIRECTORS
The Board currently has nine members, eight of whom are independent, including the Board Chair. The independent directors are organized into four standing committees: Audit Committee, Compensation Committee, Governance and Compliance Committee and Technology Committee (for more information see "Committees of the Board" beginning on page 14). Ellen M. Zane succeeded Richard J. Meelia as our independent Board Chair on August 6, 2021 following his retirement at our 2021 Annual Meeting of Shareholders. Mr. Meelia had previously served as our independent Board Chair from June 2011 to August 2021. We believe that having separate individuals serving in the roles of Board Chair and Chief Executive Officer is appropriate for the Company at this time in recognition of the different responsibilities of each position and to foster independent leadership of our Board. This structure allows the Chief Executive Officer to focus on the day-to-day leadership of the Company and its operations and the Board Chair to focus on leadership of the Board, while both individuals provide direction and guidance on strategic initiatives.
BOARD INDEPENDENCE
The Board has determined that each director who served during fiscal 2022, with the exception of Mr. Simon, had no material relationship with the Company and is independent within the meaning of the SEC and NYSE director independence standards in effect. In making this determination, the Board considered information provided by the Company and by each director and director nominee with regard to a director’s business and personal activities as they relate to the Company and its management.
EXECUTIVE SESSIONS AND MEETINGS OF THE BOARD
Executive sessions of the independent directors were held during each of the Board’s regular quarterly meetings and at such special meetings of the Board as requested by the independent directors. During fiscal 2022, Mr. Meelia or Ms. Zane, in their capacities as Board Chair, presided over all such executive sessions.
In addition to its regular quarterly meetings, the Board held numerous special meetings during fiscal 2022 to address, among other things, the impacts of COVID-19 on the business. The Board and its committees met as follows during our last fiscal year:
Board of Directors | Audit Committee | Compensation Committee | Governance and Compliance Committee | Technology Committee | |||||||||||||||||||||||||
Regular Meetings | 4 | 4 | 4 | 4 | 4 | ||||||||||||||||||||||||
Special Meetings | 4 | 4 | 1 | 0 | 1 | ||||||||||||||||||||||||
Total Number of Meetings | 8 | 8 | 5 | 4 | 5 | ||||||||||||||||||||||||
In fiscal 2022, each of the directors attended at least 75% of the total number of meetings of the full Board held while he or she was a director and the meetings held by committees of the Board on which he or she served. All directors are strongly encouraged to attend each Annual Meeting of Shareholders. Eight of the directors serving on the Company’s Board at the time of the 2021 Annual Meeting of Shareholders attended the 2021 Annual Meeting of Shareholders held in person at the Company's headquarters.
HAEMONETICS CORPORATION | 2022 Proxy Statement 13
COMMITTEES OF THE BOARD
The Board maintains four standing committees to assist the Board in its various oversight functions: Audit, Compensation, Governance and Compliance and Technology. The Board has determined that all members of our standing committees have no material relationship with the Company and are independent within the meaning of the SEC’s and the NYSE’s director independence standards. The Board has also determined that the service by Charles J. Dockendorff on the audit committees of three other public companies will not impair his ability to effectively serve on our Audit Committee and that our shareholders will benefit from Mr. Dockendorff’s extensive experience as a Chief Financial Officer and audit committee member at multi-national healthcare companies.
AUDIT COMMITTEE | ||||||||
Members | Key Responsibilities | |||||||
Charles J. Dockendorff (Chair) Catherine M. Burzik Michael J. Coyle Lloyd E. Johnson | •Oversee financial reporting and disclosure practices on behalf of the Board, including: - Oversee internal controls and the internal audit function and processes for monitoring compliance by the Company with Company policies - Select, replace and determine the compensation (including pre-approval of all audit and non-audit fees) of the independent registered public accounting firm •Review the scope of the annual audit and its results - Review with the Company’s independent registered public accounting firm •Review various matters relating to financial risk assessments and remediation •Review transactions subject to the Company's Related Party Transactions Policy | |||||||
COMPENSATION COMMITTEE | ||||||||
Members | Key Responsibilities | |||||||
Robert E. Abernathy (Chair) Mark W. Kroll Claire Pomeroy Ellen M. Zane | •Determine total compensation philosophy for executives •Approve peer group and review competitive standing of compensation •Review human capital strategy and practices at least quarterly with management, including talent development, turnover and diversity, equity and inclusion matters •Set competitive short- and long-term compensation elements, benefits and perquisites •Set, and determine achievement of, short- and long-term performance goals •Review and approve Named Executive Officer compensation (Board ratification for CEO) •Oversee employee compensation plans and policies, including performance of an annual risk-assessment of such plans and policies •Recommend changes to Board compensation •Select, replace and determine compensation of independent compensation consultant | |||||||
GOVERNANCE AND COMPLIANCE COMMITTEE | ||||||||
Members | Key Responsibilities | |||||||
Ellen M. Zane (Chair) Michael J. Coyle Charles J. Dockendorff Lloyd E. Johnson | •Consider and make recommendations for CEO role and director nominees •Oversee compliance programs and recommend corporate governance principles •Consider and make recommendations to the Board concerning corporate governance matters, public issues having broad social significance and Company conduct as a responsible corporate citizen •Lead annual Board self-evaluation process •Ensure that directors receive orientation and continuing education as needed | |||||||
TECHNOLOGY COMMITTEE | ||||||||
Members | Key Responsibilities | |||||||
Catherine M. Burzik (Chair) Robert Abernathy Mark W. Kroll Claire Pomeroy | •Review alignment of Company's innovation agenda with strategy and growth objectives •Review overall direction, effectiveness, competitiveness and timing of the Company's research and development programs and pipelines •Review the Company's intellectual property portfolio and related strategies, as well as potentially disruptive technology that could impact the Company and its products •Oversee quality assurance, regulatory affairs and clinical and medical affairs in support of the Company’s new product development and lifecycle management •Review technology aspects of products as they relate to quality, safety and cybersecurity •Receive periodic reports regarding the Company's Scientific Advisory Committee | |||||||
14 HAEMONETICS CORPORATION | 2022 Proxy Statement
AUDIT COMMITTEE FINANCIAL EXPERT
The Board has determined that all Audit Committee members meet the independence and financial literacy requirements of the NYSE for audit committee members. The Board has also determined that each of Messrs. Dockendorff and Johnson and Ms. Burzik qualify as an “audit committee financial expert” as defined under the rules of the SEC.
COMPENSATION COMMITTEE INTERLOCKS AND INSIDER PARTICIPATION
During the fiscal year ended April 2, 2022, the members of the Compensation Committee were Robert E. Abernathy (Chair), Mark W. Kroll, Claire Pomeroy and Ellen M. Zane. During fiscal 2022, no member of the Compensation Committee was an executive officer or employee, or former executive officer or employee, of the Company or any of its subsidiaries. None of our executive officers served as a director or member of the compensation committee of any entity that has one or more executive officers serving on our Board or Compensation Committee.
| Board Policies and Processes | ||
TRANSACTIONS WITH RELATED PERSONS
The Board has adopted a written policy and procedures for approval of transactions between the Company and its directors, director nominees, executive officers, greater than 5% beneficial owners of Haemonetics common stock, and each of their respective immediate family members, where the amount involved in the transaction exceeds or is expected to exceed $120,000 in a single calendar year and the related person has or will have a direct or indirect material interest in the transaction (other than solely as a result of being a director or less than 10% beneficial owner of another entity). The policy, as amended from time to time, provides that the Audit Committee reviews transactions subject to the policy and determines (subject to Board ratification) whether or not to approve those transactions. In addition, in reviewing transactions subject to the policy, the Audit Committee considers, among other factors it deems appropriate:
•The material terms of the transaction, including whether the transaction with the related person is proposed to be, or was, entered into on terms no less favorable to Haemonetics than terms that could have been reached with an unrelated third-party;
•The nature and extent of the related person’s interest in the transaction;
•The approximate dollar value of the amount involved in the transaction;
•The approximate dollar value of the amount of the related person’s interest in the transaction without regard to the amount of any profit or loss;
•Whether the transaction was undertaken in the ordinary course of Haemonetics' business;
•The business purpose of, and the potential benefits to Haemonetics of, the transaction;
•Whether the transaction would impair the independence of a non-employee director;
•Required public disclosure, if any; and
•Any other information regarding the transaction or the related person in the context of the proposed transaction that would be material to investors in light of the circumstances of the particular transaction.
The Company is not aware of any transaction required to be reported under Item 404(a) of Regulation S-K promulgated by the SEC since the beginning of fiscal 2022, nor is the Company aware of any instances during the period in which the foregoing policies and procedures required review, approval or ratification of such transaction but for which such policies and procedures were not followed.
CODE OF CONDUCT, GOVERNANCE PRINCIPLES AND BOARD MATTERS
The Company’s Code of Conduct requires that all of our directors, officers and employees avoid conflicts of interest, comply with all laws and other legal requirements, conduct business in an honest and ethical manner and otherwise act with integrity and in the best interest of the Company. The Company’s Code of Conduct, Principles of Corporate Governance and the charters of the Audit Committee, Compensation Committee, Governance and Compliance Committee and Technology Committee may be viewed on the Investor Relations page on the Company’s website at www.haemonetics.com and printed copies can be obtained by contacting our Corporate Secretary at the Company's headquarters located at 125 Summer Street, Boston, Massachusetts 02110.
HAEMONETICS CORPORATION | 2022 Proxy Statement 15
| Directors’ Compensation | ||
PROCESS FOR DETERMINING DIRECTOR COMPENSATION
We seek to offer our directors compensation that is consistent with other companies of our revenue, industry and operational scope. The Compensation Committee, with input from its independent compensation consultant, is responsible for annually reviewing and recommending to the Board any changes to director compensation.
Directors receive a $55,000 annual retainer with an additional $10,000 meeting retainer which covers attendance at up to eight Board meetings. If the Board meets more than eight times per year, each director receives $2,000 for each additional live meeting and $750 for each additional telephonic meeting. The Board Chair receives an annual retainer of $250,000 in place of the standard Board retainer and meeting fees. Committee Chairs are paid an additional retainer as follows: Audit Committee Chair $20,000; Compensation Committee Chair $15,000; Governance and Compliance Committee and Technology Committee Chairs $10,000. For attendance at Committee meetings, members of the Audit Committee are paid $12,000 for attending up to 12 meetings per year, members of the Compensation Committee are paid $9,000 for up to eight meetings per year, and members of the Governance and Compliance and Technology Committees are paid $6,000 for up to eight meetings per year.
Each non-employee director is eligible to receive an annual equity grant with an approximate value of $180,000. The grant is in the form of restricted stock units which vest on the first anniversary of the date of grant. Directors elected outside of the Annual Meeting of Shareholders receive a prorated annual equity award based on the number of days to be served from their date of election through the first anniversary of the last Annual Meeting of Shareholders.
We reimburse directors for reasonable travel expenses incurred in connection with Board and committee meetings. We also extend coverage to them under our directors’ and officers’ indemnity insurance policies and have entered into our standard form of Indemnification Agreement with each director. We do not provide any other benefits, including retirement benefits or perquisites, to our non-employee directors.
DIRECTOR COMPENSATION TABLE FOR FISCAL YEAR ENDED APRIL 2, 2022
The following table sets forth the compensation paid to our non-employee directors for service on our Board during fiscal 2022. Compensation for Christopher A. Simon, our President and Chief Executive Officer, is set forth in the Summary Compensation Table beginning on page 39. Mr. Simon does not receive any additional compensation for his service as a director.
Name | Fees Earned or Paid in Cash ($) | Stock Awards(1) ($) | Total ($) | |||||||||||||||||
Robert E. Abernathy | $ | 95,000 | $ | 179,943 | $ | 274,943 | ||||||||||||||
Catherine M. Burzik | $ | 93,000 | $ | 179,943 | $ | 272,943 | ||||||||||||||
Michael J. Coyle | $ | 83,000 | $ | 179,943 | $ | 262,943 | ||||||||||||||
Charles J. Dockendorff | $ | 103,000 | $ | 179,943 | $ | 282,943 | ||||||||||||||
Lloyd E. Johnson | $ | 51,392 | $ | 179,943 | $ | 231,335 | ||||||||||||||
Mark W. Kroll | $ | 80,000 | $ | 179,943 | $ | 259,943 | ||||||||||||||
Richard J. Meelia(2) | $ | 86,957 | $ | — | $ | 86,957 | ||||||||||||||
Claire Pomeroy | $ | 80,000 | $ | 179,943 | $ | 259,943 | ||||||||||||||
Ellen M. Zane | $ | 194,346 | $ | 179,943 | $ | 374,289 | ||||||||||||||
(1)Represents the aggregate grant date fair value for annual equity awards of 3,077 restricted stock units, or RSUs, awarded to each such person on August 6, 2021, in each case calculated in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) Topic 718, Compensation - Stock Compensation. The assumptions that we used to calculate these amounts are discussed in Note 17 "Capital Stock" to our financial statements included in our Annual Report on Form 10-K for the fiscal year ended April 2, 2022. See “Director Outstanding Equity Award Table for Fiscal Year Ended April 2, 2022” on page 17 for a description of the number of unvested RSUs and unexercised options held by each director as of the end of fiscal 2022.
(2)Mr. Meelia retired from our Board as of the 2021 Annual Meeting of Shareholders and, therefore, did not receive the annual equity award granted to directors on August 6, 2021.
16 HAEMONETICS CORPORATION | 2022 Proxy Statement
DIRECTOR OUTSTANDING EQUITY AWARD TABLE FOR FISCAL YEAR ENDED APRIL 2, 2022
The following table sets forth the aggregate number of Stock Awards (representing unvested RSUs) held at April 2, 2022 by each non-employee director that served on our Board during fiscal 2022.
| Name | Unvested Stock Awards (RSUs) (#) | ||||
Robert E. Abernathy | 3,077 | ||||
Catherine M. Burzik | 3,077 | ||||
Michael J. Coyle | 3,077 | ||||
Charles J. Dockendorff | 3,077 | ||||
Lloyd E. Johnson | 3,077 | ||||
| Mark W. Kroll | 3,077 | ||||
| Richard J. Meelia | — | ||||
| Claire Pomeroy | 3,077 | ||||
| Ellen M. Zane | 3,077 | ||||
HAEMONETICS CORPORATION | 2022 Proxy Statement 17
ITEM 2—ADVISORY VOTE ON EXECUTIVE COMPENSATION
As described in the Compensation Discussion and Analysis beginning on page 21, our executive compensation programs aim to be competitive with our peers and aligned with our business strategy and corporate objectives. Our compensation philosophy emphasizes a pay for performance culture focused on the long-term interests of our shareholders. We believe that this alignment between executive compensation and shareholder interests will drive corporate performance over time. Additionally, the Company maintains strong governance and pay practices, including meaningful share ownership guidelines for directors and executive officers, clawback policies that apply to short-term cash awards and long-term equity awards, “double trigger” change in control benefits and performance of an annual compensation risk assessment by our Compensation Committee.
Our Compensation Committee continually evaluates the design and direction of our compensation structure. Each year, we take into account the result of the "say-on-pay" vote cast by our shareholders. At our 2021 Annual Meeting of Shareholders, approximately 96.8% of shares voting supported our 2021 executive compensation program. The Compensation Committee is also committed to maintaining an open dialogue with our shareholders throughout the year. In each of the last four years, members of our Board have offered meetings during the fall and winter to large Haemonetics shareholders to discuss governance topics of interest. In fiscal 2022, our Board Chair (who also chairs our Governance and Compliance Committee) and Compensation Committee Chair offered meetings to 9 of our largest shareholders, representing more than 52% of shares outstanding, and met with shareholders representing approximately 30% of shares outstanding in January 2022 to discuss, among other topics, Haemonetics' corporate strategy and performance, board diversity and refreshment, executive compensation, corporate responsibility and other governance matters. Shareholders we met with were complimentary of our executive compensation program overall, and of our senior management team, while asking insightful questions and providing perspective on how they evaluate the program. The feedback from the shareholder outreach efforts was provided to the Governance and Compliance and Compensation Committees and the full Board (for further discussion see "Advisory 'Say on Pay' Vote and Shareholder Outreach" beginning on page 22).
Shareholders are urged to read our Compensation Discussion and Analysis beginning on page 21 and the section entitled “Executive Compensation Tables” beginning on page 39 for additional details about our executive compensation programs, including information about the fiscal 2022 compensation of our Named Executive Officers.
We are asking our shareholders to indicate their support for our Named Executive Officers’ compensation as described in this Proxy Statement. This proposal, commonly known as a "say-on-pay" proposal, gives our shareholders the opportunity to express their views on our Named Executive Officers’ compensation. This vote, as required by Section 14A of the Exchange Act, is not intended to address any specific element of our compensation programs, but rather to address our overall approach to the compensation of our Named Executive Officers described in this Proxy Statement. To that end, we ask our shareholders to vote FOR the following resolution at the meeting:
RESOLVED, that the Company’s shareholders approve, on an advisory basis, the compensation of the Company's named executive officers, as disclosed in the Compensation Discussion and Analysis section, the related executive compensation tables and the related narrative executive compensation disclosures contained in this Proxy Statement.
As an advisory vote, the results of this vote will not be binding on the Board or the Company. However, the Board values the opinions of our shareholders and will consider the outcome of the vote when making future decisions on the compensation of our Named Executive Officers and the Company’s executive compensation principles, policies and procedures.
þ | The Board recommends that shareholders vote, in an advisory manner, FOR the resolution set forth above. Approval of this proposal requires the affirmative vote of a majority of shares present, in person or represented by proxy, and voting on this proposal at the meeting. Abstentions and broker “non-votes” will not have any effect on this proposal. Management proxy holders will vote all duly submitted proxies FOR approval unless instructed otherwise. | ||||
18 HAEMONETICS CORPORATION | 2022 Proxy Statement
| Executive Officers | ||
Executive officers are chosen by and serve at the discretion of the Board. Set forth below are the names and ages of our executive officers, along with certain biographical information, for all but Christopher A. Simon, our President and Chief Executive Officer. For Mr. Simon’s biographical information, please see page 9.
 | Michelle L. Basil, age 50, joined the Company in March 2017 as Executive Vice President, General Counsel. Ms. Basil was previously a Partner and Chair of the Life Sciences Practice Group at Nutter, McClennen & Fish LLP, a Boston-based law firm, where she practiced from September 1997 to March 2017. Ms. Basil focused her practice on corporate and securities law, including mergers and acquisitions, strategic partnerships and corporate governance matters, and represented both public and private companies, principally in the life sciences and medical technology industries. Ms. Basil is a member of the Board of Directors of the Massachusetts Medical Device Industry Council (MassMEDIC). She is admitted to the bar in Massachusetts and holds both a Bachelor of Arts and a Juris Doctor from the University of California at Berkeley. | |||||||||||||
 | James C. D'Arecca, age 51, joined the Company as Executive Vice President, Chief Financial Officer in April 2022. Mr. D'Arecca previously served as Chief Financial Officer of TherapeuticsMD, Inc., a women's healthcare company, from June 2020 to April 2022. Prior to joining TherapeuticsMD, Inc., Mr. D’Arecca served as the Senior Vice President and Chief Accounting Officer of Allergen plc (formerly known as Actavis plc), a global pharmaceutical company, from August 2013 until its merger with AbbVie Inc. in May 2020. Mr. D’Arecca served as Chief Accounting Officer at Bausch & Lomb prior to joining Actavis plc and earlier in his career held finance and business development positions of increasing responsibility at Merck & Co., Inc. and Schering-Plough Corporation. Mr. D’Arecca began his career at PricewaterhouseCoopers LLP from 1992 to 2005, where he had an industry focus on pharmaceuticals, medical devices, and consumer products. Mr. D’Arecca earned a Bachelor of Science in Accounting from Rutgers University and a Master of Business Administration from Columbia University. He is a Certified Public Accountant. | |||||||||||||
 | Anila Lingamneni, age 55, joined the Company as Executive Vice President, Chief Technology Officer in April 2020. Prior to joining the Company, Ms. Lingamneni served as Vice President, Renal R&D at Baxter International from February 2017 to March 2020, where she was responsible for the product portfolio delivering renal therapy solutions to dialysis patients, including devices, software, disposables and fluids. In this role, she led a globally distributed team of engineers and scientists to drive long-term product roadmap definition and therapy innovation and delivered critical product launches. From May 2013 to January 2017, Ms. Lingamneni also served as Vice President, Device Engineering at Baxter International, where she was responsible for all electromechanical devices and software applications for Baxter's medical device portfolio, including infusion systems, compounding systems, renal peritoneal and hemodialysis systems and acute renal therapy systems. Before joining Baxter, Ms. Lingamneni held several roles at General Electric Healthcare, including Chief Technology Officer of the X-Ray Diagnostic Imaging Business Unit. Ms. Lingamneni received a Bachelor of Science in Electrical and Electronics Engineering and a Master of Science in Mathematics from Birla Institute of Technology and Science in India, and she earned a Master of Science degree in Biomedical Engineering from Iowa State University. | |||||||||||||
HAEMONETICS CORPORATION | 2022 Proxy Statement 19
 | Josep L. Llorens, age 60, joined the Company as Senior Vice President, Global Manufacturing and Supply Chain in August 2018. Mr. Llorens possesses over 30 years of experience in leading numerous turnarounds in global health care and consumer businesses across disposables, capital equipment, devices and software. Prior to joining the Company, Mr. Llorens held various operations and supply chain roles of increasing responsibility within the diagnostics and treatment, patient monitoring and cardiac care business of Philips, which he joined in 1992, most recently serving as Senior Vice President, Industrial Strategy and Advanced Manufacturing Engineering Leader for Philips Healthcare Diagnostic Imaging from January 2018 to August 2018. Mr. Llorens received a bachelor’s degree in Business Administration from the University of Barcelona and a master’s degree in Telecommunications Engineering from the Polytechnic University of Catalonia in Barcelona. He also holds an Executive Certificate in Technology, Operations and Value Chain Management from the MIT Sloan School of Management. | |||||||||||||
 | Laurie A. Miller, age 49, was promoted to Senior Vice President, Chief Human Resources Officer of Haemonetics in August 2021. She brings to the Company over 25 years of experience in talent management and organizational development. Ms. Miller joined Haemonetics in 2016 and previously held positions as Vice President, Human Resources and Senior Director, Human Resources and Talent Management, where she helped build the Company’s collaborative, performance-driven culture, direct key employee-focused initiatives and create a strong workplace environment for employees. Prior to joining Haemonetics, Ms. Miller served in positions of increasing responsibility at Iron Mountain Inc., an enterprise information management company, including as Director of Human Resources, from February 2010 to April 2016. Earlier in her career, Ms. Miller held various Human Resources positions of broad and increasing responsibility at Dunkin’ Brands Group Inc., a global franchisor of quick service restaurants, and Shawmut Design and Construction, a construction management firm. Ms. Miller earned a Bachelor of Science in Business Management from Westfield State College and a Master of Science in Management from Emmanuel College. | |||||||||||||
 | Stewart W. Strong, age 55, joined the Company as President, Global Hospital in September 2019. With more than 20 years of experience in interventional cardiology and radiology, general and cardiac surgery and vascular access procedures, Mr. Strong has a robust background developing and leading high performance teams across multiple functions in the medical device space. From February 2017 to September 2019, Mr. Strong served as President and General Manager of the interventional products business at Teleflex Incorporated, a global medical company, where he, among other responsibilities, led the expansion of the company’s interventional cardiology business through organic growth opportunities and strategic acquisitions. Mr. Strong also served as Vice President of North America Sales, Vascular Access and Global Vice President and General Manger, Cardiac Care at Teleflex between December 2013 and February 2017. Before joining Teleflex, Mr. Strong held leadership roles with several medical technology companies, including Vice President of Sales at Vidacare Corporation (prior to its acquisition by Teleflex in 2013) and positions of increasing responsibility at AtriCure, Inc., Medtronic plc’s heart valve division, and Johnson & Johnson’s ethicon endo-surgery division. Mr. Strong holds a Bachelor of Arts from the University of Connecticut. | |||||||||||||
20 HAEMONETICS CORPORATION | 2022 Proxy Statement
| Compensation Discussion and Analysis | ||
For purposes of the following Compensation Discussion and Analysis ("CD&A") and executive compensation disclosures, the individuals listed below are referred to collectively as our "Named Executive Officers." They are our President and Chief Executive Officer ("CEO"), our Executive Vice President, Chief Financial Officer as of the end of fiscal 2022 and our three other most highly compensated executive officers, based on fiscal 2022 compensation.
Named Executive Officer | Title | ||||
Christopher A. Simon | President and Chief Executive Officer | ||||
William P. Burke(1) | Executive Vice President, Chief Financial Officer | ||||
Michelle L. Basil | Executive Vice President, General Counsel | ||||
| Josep L. Llorens | Executive Vice President, Global Manufacturing and Supply Chain | ||||
Stewart W. Strong | President, Global Hospital | ||||
(1)Mr. Burke retired as Chief Financial Officer after the completion of fiscal 2022 and was succeeded by James D'Arecca in April 2022.
EXECUTIVE SUMMARY
The Compensation Committee of our Board (the "Compensation Committee" or the “Committee”) has adopted an integrated executive compensation program that is intended to align our Named Executive Officers’ interests with those of our shareholders and to promote the creation of shareholder value without encouraging excessive or unnecessary risk-taking. The Committee has tied a majority of our Named Executive Officers’ compensation to a number of key performance measures that contribute to or reflect shareholder value and link pay with performance. Specifically, in addition to a base salary, our Named Executive Officers’ total compensation includes annual short-term incentive compensation that is based on the Company’s attainment of objective pre-established financial performance metrics as well as annual long-term incentive compensation consisting of stock options, restricted stock units ("RSUs"), and performance share units ("PSUs") tied to relative total shareholder return. Our executive compensation program plays a significant role in our ability to attract and retain an experienced, successful executive team.
Fiscal 2022 Business Highlights
Fiscal 2022 was a challenging year for Haemonetics, but we are proud of how we responded. We continued to strengthen and grow our business despite the continued challenges caused by the COVID-19 pandemic, including its impacts on the pace of Plasma volume recovery globally, as well as CSL's notification to us in April 2021 of its intent not to renew its current U.S. supply agreement for the use of Haemonetics PCS2 Plasma Collection System devices and the purchase of plasma disposables. Under the guidance of our executive leadership team, the Company demonstrated agility and perseverance in meeting customer needs as we maintained an uninterrupted supply of our products and achieved growth in our Plasma and Hospital business units while further stabilizing our and Blood Center business unit. Our product portfolio continued to evolve in fiscal 2022 as we successfully integrated Cardiva Medical into our Hospital business unit and the resulting Vascular Closure product line generated positive revenue and adjusted operating income results that exceeded our initial expectations. Our Operational Excellence Program, which we expanded in fiscal 2022 to target gross savings of $115 million to $125 million by the end of fiscal 2025, achieved more than half of the program's target gross savings by the end of fiscal 2022 and continued to improve our operating performance, enabling us to respond quickly to supply chain disruptions and helping offset inflationary pressures. We also met critical research and development milestones for our Plasma and Hospital products, including in our newly acquired Vascular Closure business.
As we look to fiscal 2023, Haemonetics is set for robust, transformational growth, propelled by investments in the advancement of our technologies and expansion of our global commercial capabilities. We are proud of our response to the challenges of the COVID-19 pandemic and we recognize there will be more challenges ahead as we plan for the transition of CSL and economic uncertainties relating to the COVID-19 pandemic and geopolitical events. We are committed to taking action, implementing necessary changes and mitigating impacts without compromising the growth of our business. We look forward to sharing more detail about our long-range plans to deliver value at our fiscal 2023 Investor Day.
Financial Highlights
Haemonetics' fiscal 2022 financial results reflect solid underlying performance despite ongoing COVID-19 impacts, including:
REVENUE (GAAP) | ADJUSTED EARNINGS PER SHARE ("EPS") | FREE CASH FLOW BEFORE RESTRUCTURING AND RRC | ADJUSTED OPERATING MARGIN | |||||||||||||||||
$993 million | $2.58 | $117.4 million | 18.8% | |||||||||||||||||
14% reported and 7% organic increase from prior fiscal year | 10% increase from prior fiscal year | 18% increase from prior fiscal year | 100 basis point increase from prior fiscal year | |||||||||||||||||
For further details of our fiscal 2022 financial results, please see our Annual Report on Form 10-K for the fiscal year ended April 2, 2022. Organic revenue growth, adjusted EPS, free cash flow before restructuring and restructuring-related costs ("RRC") and
HAEMONETICS CORPORATION | 2022 Proxy Statement 21
adjusted operating margin are considered “non-GAAP” financial measures under applicable SEC rules. Refer to Appendix A for a description of these measures and a reconciliation to the most directly comparable GAAP (as defined below) financial measures.
Strategic Performance Highlights
The Company had numerous strategic, commercial and operational achievements in fiscal 2022 that aligned with the key value drivers supporting our long-term value creation strategy and through-cycle mindset during the COVID-19 pandemic, including:
| Corporate Strategy |  |  |  | ||||||||
| Value Drivers | 1 Plasma (Business Unit) Market | 3 Mergers and Acquisitions | 5 Operational Excellence | ||||||||
2 Hospital (Business Unit) Market | 4 Innovation Agenda | 6 Resource Allocation | |||||||||
Select Fiscal 2022 Achievements | •Grew year-over-year organic revenue 10% in Plasma and 16% in Hospital, including 20% organic growth in Hemostasis Management product line; (1.0%) decline in Blood Center organic revenue, consistent with stabilizing that business •Vascular Closure product line acquired in March 2021 delivered fiscal 2022 revenue of $94 million and contributed positively to adjusted operating income, significantly outpacing our original expectations | •Completed successful integration of Cardiva Medical and associated Vascular Closure product line into our Hospital business unit, allowing us to shift focus to further penetrating the $2.8 billion interventional cardiology and electrophysiology markets •VASCADE MVP® venous vascular closure system gained the first and only FDA approval for same-day discharge following atrial fibrillation ablation | •Operational Excellence Program delivered approximately $37 million in gross savings in fiscal 2022, freeing up resources to fund additional investments ($71 million in gross savings since inception of plan, slightly ahead of our original forecast) •Grew cash on hand $67 million to $259 million by end of fiscal 2022 to help fund organic and inorganic growth opportunities and other capital allocation priorities | ||||||||
Advisory “Say on Pay” Vote and Shareholder Outreach
The Company holds annual “say on pay” votes and has received over 95% say-on-pay approval from our shareholders for our executive compensation programs in each of the last seven years, including at the 2021 Annual Meeting of Shareholders. While say-on-pay is a key indicator of shareholder feedback, we also are committed to maintaining an open dialogue with our shareholders throughout the year. As discussed elsewhere in this Proxy Statement, our Board Chair (who also Chairs our Governance and Compliance Committee) and Compensation Committee Chair offered meetings to nine of our largest shareholders, representing over 52% of shares outstanding, during the fall and winter of fiscal 2022 and met with shareholders representing approximately 30% of shares outstanding in January 2022 to discuss, among other topics, | APPROXIMATELY 96.8% say on pay approval at 2021 Annual Meeting of Shareholders | ||||
Haemonetics' corporate strategy and performance, board diversity and refreshment, executive compensation, corporate responsibility and other governance matters. Shareholders we met with were complimentary of our executive compensation program overall, and of our senior management team, while asking insightful questions and providing perspective on how they evaluate the program. The feedback from the shareholder outreach efforts was provided to the Governance and Compliance and Compensation Committees as well as the full Board, including with respect to the following compensation matters: | |||||
WHAT WE HEARD | WHAT WE DID | |||||||||||||
•Investors generally not prescriptive on appropriate compensation design to support strategic objectives, but encouraged continued transparency in CD&A linking compensation decision to corporate strategy and long-term shareholder value creation. | •Compensation Committee took all investor feedback into account when reviewing the design of our fiscal 2023 compensation programs and the CD&A. | |||||||||||||
•One investor suggested that the Compensation Committee evaluate adding a financial metric such as ROIC to supplement relative total shareholder return ("rTSR") in future years as the Company pursues additional inorganic growth opportunities. | •Compensation Committee determined to retain rTSR as sole metric for fiscal 2023 PSU awards to drive long-term shareholder return focus, but will evaluate supplemental PSU metrics in future that tie to achievement of long-range goals to be presented at our fiscal 2023 Investor Day. | |||||||||||||
The Compensation Committee will continue to consider the results of shareholder advisory votes and shareholder input on executive compensation when making future decisions relating to our executive compensation program and compensation for Named Executive Officers.
22 HAEMONETICS CORPORATION | 2022 Proxy Statement
Fiscal 2022 Compensation Program Design
In establishing our fiscal 2022 executive compensation program, the Compensation Committee continued to focus on pay for performance and competitive pay, with an emphasis on total direct compensation. As disclosed in our 2021 proxy statement, the Company experienced a substantial decrease in our share price in April 2021 following our announcement that one of our largest customers, CSL, had recently notified us of its intent not to renew its current U.S. supply agreement for the use of Haemonetics PCS2 Plasma Collection System devices and the purchase of plasma disposables that was set to expire in June 2022. We were informed that the decision was based on changes in CSL's internal strategy and was not related to product or service quality. While we did not anticipate CSL's decision would impact fiscal 2022 results and management responded both urgently and deliberately in planning for the CSL transition (including negotiating an extended transition of the CSL agreement through December 2023, on a non-exclusive basis), these events informed the Compensation Committee's fiscal 2022 plan design determinations to ensure continued focus on revenue growth, long-term shareholder value creation and retaining key talent.
The Committee made the following executive pay decisions for our Named Executive Officers in fiscal 2022, consistent with its compensation philosophy and in recognition of ongoing COVID-19 uncertainties, including the impact of new surges and variants on the pace of recovery, and the CSL transition:
•Approved 3% annual salary merit increases for Named Executive Officers, other than our CEO, which the Committee had postponed in fiscal 2021 due to uncertainties related to the onset of the COVID-19 pandemic;
•Structured annual performance bonus opportunities under our fiscal 2022 short-term incentive plan as follows:
◦Capped bonus pool funding at 200% to reward over-achievement against ambitious financial targets, while maintaining wider performance ranges between threshold, target and maximum performance for each metric in recognition of ongoing COVID-19 uncertainties that could materially impact financial results;
◦Retained adjusted revenue and adjusted EPS metrics for corporate (non-business unit) executives, but increased the weight of adjusted revenue from 50% to 60% to align with the Company's strategic focus on revenue growth;
◦Set more individualized performance targets for Stewart Strong based on his role leading our Hospital business unit, with 70% tied to Hospital business unit revenue achievement and 30% tied to overall Company adjusted operating income;
•Increased annual long-term incentive award grant values for select Named Executive Officers based on individual performance and market position, allocated 50% to PSUs, 25% to RSUs and 25% to stock options as in fiscal 2021, and granted special retention awards to certain Named Executive Officers in the form of RSUs that vest over a two-year period to promote retention as the Company urgently responds to the CSL transition and pursues critical business milestones;
•In July 2021, approved select compensation increases for Messrs. Llorens and Strong in conjunction with their receipt of promotions and/or enhanced responsibilities in the first quarter of fiscal 2022, as discussed throughout this CD&A; and
•Retained relative total shareholder return ("rTSR") as the sole performance measure for PSUs to drive emphasis on shareholder return following the April 2021 decrease in our share price.
Fiscal 2022 Compensation Highlights
Our positive fiscal 2022 financial results in a difficult COVID-19 environment, as well as our April 2021 share price decline discussed above, directly affected our Named Executive Officers’ compensation, which was directly aligned with business results, shareholder return and our pay-for-performance philosophy. Most notably:
•The Company's performance against corporate and business unit targets set by the Committee under our fiscal 2022 short-term incentive plan resulted in the following annual bonus pool funding levels for our Named Executive Officers:
◦87.4% of target funding for corporate executives (excluding Mr. Strong), based on 93.8% adjusted revenue and 77.8% adjusted EPS achievement against fiscal 2022 targets, with such metrics weighted 60% and 40%, respectively;
◦169.9% of target funding for Mr. Strong, based on 200% Hospital business unit revenue and 99.7% Company adjusted operating income achievement against fiscal 2022 targets, with such metrics weighted 70% and 30%, respectively;
•No payout under PSU awards previously granted to certain Named Executive Officers in June 2018 and September 2018, all of which were based on the Company's rTSR over a three-year performance period ending in fiscal 2022, evidencing the strong pay for performance culture in our compensation awards.
For further discussion, see "Annual Short-Term Incentive Compensation" beginning on page 28 and "Long-Term Incentive Award Payouts" on page 33.
Target vs. Realizable Named Executive Officer Compensation
To link the financial interests of our executives with those of our shareholders, a significant portion of our Named Executive Officers’ compensation opportunity is “at risk” either because it is subject to performance goals, the fluctuations of our stock price,
HAEMONETICS CORPORATION | 2022 Proxy Statement 23
or both. Set forth below is a diagram that shows for each of fiscal years 2019, 2020 and 2021 the granted target pay and realizable pay for our CEO and, on an aggregate basis, for our other Named Executive Officers (excluding Mr. Strong, who joined the Company during fiscal 2020). As reflected in the diagram below, realizable pay for our CEO and, on an aggregate basis, for our other Named Executive Officers (excluding Mr. Strong) is respectively 45.4% and 62.1% of target pay on average over the three-year period, primarily as a result of the Company’s April 2021 share price decrease following the CSL transition announcement. In particular, options granted during fiscal 2019 through fiscal 2021 were out-of-the-money as of the Company’s last trading day in fiscal 2022 and PSU awards granted during fiscal 2019 and 2020 paid out at 0% upon vesting in June/September 2021 (see “Long-Term Incentive Award Payouts” on page 33) and May 2022, respectively. Additionally, unvested PSU awards granted in fiscal 2021 with performance periods ending in fiscal 2024 would have paid out at 0% based on the Company's rTSR as of the last trading day of fiscal 2022. Thus, the realizable pay analysis demonstrates the strong pay and performance alignment of our executive compensation program.
Target vs. Realizable Compensation (Fiscal 2019 – Fiscal 2021)(1)
CEO (Mr. Simon) | Aggregate Other Named Executive Officers (Ms. Basil and Messrs. Burke and Llorens) | ||||
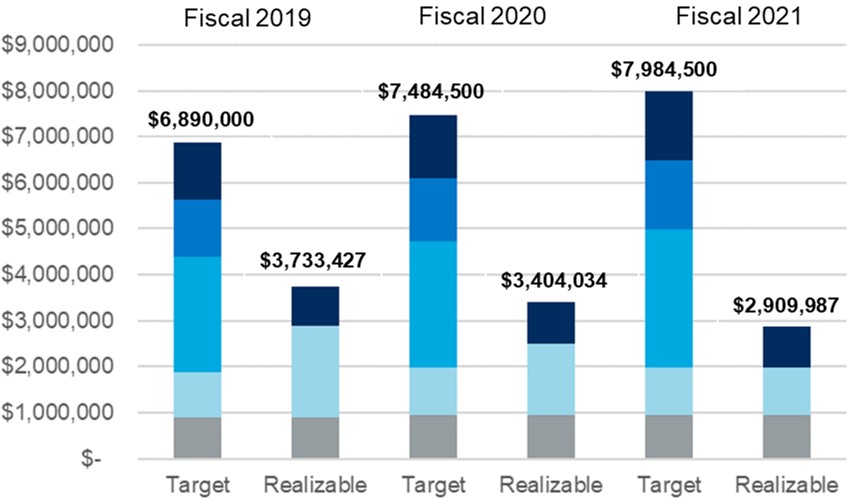 | 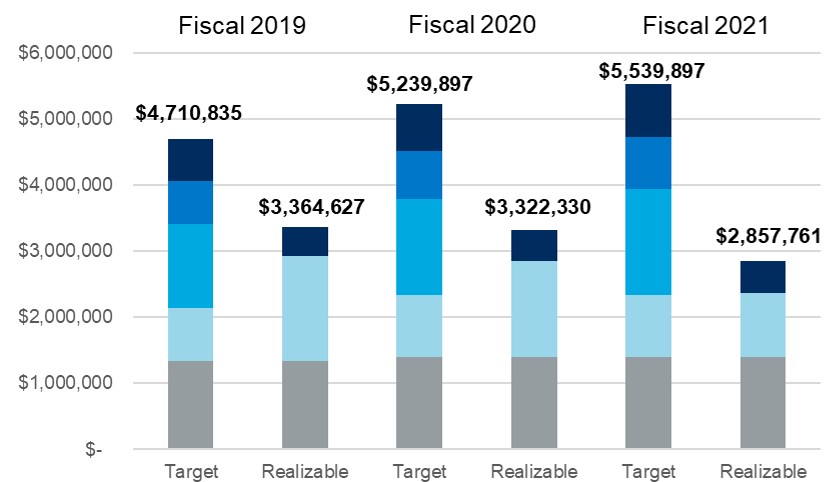 | ||||
 | |||||
(1)For each of our CEO and, on an aggregate basis, our other Named Executive Officers (excluding Mr. Strong), granted target pay reflects the cumulative pay approved by the Compensation Committee in each of fiscal 2019, 2020 and 2021, including base salary, target annual short-term incentive compensation, and target grant value of long-term incentive compensation. Realizable pay represents the cumulative amount of pay that has been realized or may still be realizable from pay granted during the same three fiscal years, including (i) annual base salary earned (on an annualized basis), (ii) actual annual short-term incentive compensation earned in respect of the applicable fiscal year, and (iii) the value of long-term incentive compensation granted during the three-year period as of the end of fiscal 2022. With respect to long-term incentive compensation, realizable pay revalues all equity granted during the period, other than PSUs that have paid out at 0% (or would if they vested as of the end of fiscal 2022), based upon the closing price of $63.85, which was the closing price on the NYSE of our common stock on April 1, 2022 (the last trading day of our common stock in fiscal 2022) to provide an estimate of the realizable value to the executive. This diagram and the total realizable pay reported are intended to provide supplemental information regarding the compensation paid to our executives and should not be viewed as a substitute for the summary compensation tables provided in our proxy statements filed with the SEC. We believe that showing realizable compensation illustrates for shareholders the alignment between pay and performance.
Strong Governance and Pay Practices
We believe that our executive compensation program supports our business strategies and talent management objectives and is consistent with governance best practices that serve our shareholders’ long-term interests.
The following chart lists some of the highlights of our program design and pay practices:
☑ | WHAT WE DO | ☒ | WHAT WE DON’T DO | |||||||||||
•Balanced incentive programs that link pay outcomes to execution of business strategy (i.e., pay for performance), with a significant portion of compensation “at-risk” •Maintain meaningful share ownership guidelines for directors and executive officers •Maintain robust clawback provisions that apply to our short-term cash awards and long-term equity awards •Conduct an annual risk assessment •Consult with an independent compensation consultant •Provide for double-trigger change in control benefits •Regularly scheduled executive sessions without management present | •No gross-up provisions for excise taxes in change-in-control agreements •No hedging or pledging of Haemonetics stock, in accordance with our Securities Trading Policy •No pension or post-employment benefit plans for Named Executive Officers •No repricing of stock options without shareholder approval | |||||||||||||
24 HAEMONETICS CORPORATION | 2022 Proxy Statement
HOW WE DETERMINE EXECUTIVE COMPENSATION
Executive Compensation Philosophy
The key objectives of our executive compensation philosophy are to:
•Attract, retain and motivate exceptional leaders dedicated to the success of the organization;
•Maximize individual and Company performance;
•Display a clear correlation between the cost of compensation and pay for performance; and
•Align the interests of executives with shareholders.
In furtherance of these objectives, the Company has adopted the following practices:
•Offer market competitive compensation opportunities, referencing median total direct compensation while maintaining maximum flexibility to determine individual compensation based on the executive’s responsibilities, experience and performance;
•Pay for performance through a mix of short- and long-term incentive compensation where above-market performance by the Company is rewarded with above-market compensation and underperformance results in lower compensation; and
•Promote ownership in the Company through stock-based compensation and share ownership guidelines.
Participants in the Compensation Setting Process
COMPENSATION COMMITTEE
The Compensation Committee establishes our executive compensation program philosophy, plan design and administration rules and is responsible for reviewing and approving the compensation of our Named Executive Officers (subject to the Board’s ratification of CEO compensation). The Committee is comprised solely of independent, non-employee members of the Board. The Committee works closely with both its independent compensation consultant and management to examine the effectiveness of the Company’s executive compensation program throughout the year. For more information about the Committee and its duties, please see “Committees of the Board—Compensation Committee” beginning on page 14 of this Proxy Statement.
INDEPENDENT COMPENSATION CONSULTANT
The Compensation Committee retained Pearl Meyer & Partners, LLC ("Pearl Meyer") as its fiscal 2022 independent compensation consultant to, among other things, assist it in structuring the Company's fiscal 2022 compensation programs and in its deliberations. Pearl Meyer reported directly and exclusively to the Committee, and neither Pearl Meyer nor any of its respective affiliates provided other services to the Company or management in fiscal 2022. During fiscal 2022, Pearl Meyer provided to the Committee, among other services:
•Expertise-based advice;
•Research and analytical services, including competitive market data benchmarking and analyses, recommendations on peer group composition and compensation recommendations based on the foregoing;
•Assistance regarding the impact of COVID-19 on the Company's performance-based compensation programs, as described in this CD&A;
•Updates on compensation trends and regulatory developments; and
•Attendance and participation in meetings of the Compensation Committee.
Prior to engaging Pearl Meyer for fiscal 2022, the Committee evaluated Pearl Meyer's independence and any potential conflicts of interest under relevant SEC rules and NYSE listing standards and determined that Pearl Meyer satisfied the requirements for independence. The Compensation Committee has sole authority to approve its independent compensation consultant's compensation, determine the scope of its services, evaluate its performance and terminate or continue to retain its independent compensation consultant. The Committee annually reviews its independent compensation consultant engagement, typically at the beginning of each fiscal year.
MANAGEMENT
During fiscal 2022, certain members of management supported the Committee with respect to the following, among other things:
•Recommending performance metrics and goals for Committee review and approval;
•Presenting financial results measured against performance and providing compensation cost analyses;
•Reviewing executive performance and providing leadership competency assessments; and
•Implementing and communicating Committee decisions.
No executive officer participates in the deliberations of the Committee regarding his or her own compensation.
HAEMONETICS CORPORATION | 2022 Proxy Statement 25
Peer Group and Market Data
When reviewing compensation programs for, and setting the compensation of, our Named Executive Officers, the Committee considers the compensation practices of specific peer companies as well as market data from published surveys. With the assistance of its independent compensation consultant, the Committee selects a peer group of companies that are comparable in terms of size and industry and reviews the composition of this peer group at least annually. In setting the compensation of our Named Executive Officers, the Committee, with the assistance of its independent compensation consultant, evaluates each Named Executive Officer’s compensation relative to the median market data for the respective position based on our peer group and industry published surveys. However, the Committee does not strictly tie target compensation to the median of our peer group or survey data, but also considers Company and individual performance, experience and scope of role when determining the appropriate level of compensation for each executive.
PEER GROUP FOR SETTING FISCAL 2022 COMPENSATION
In consultation with Pearl Meyer, the Committee reviewed and updated the Company's peer group in January 2021 for purposes of setting fiscal 2022 compensation. The Committee considered a variety of factors designed to foster year-over-year consistency in peer group composition to the extent possible while recommending a group of companies that, in the aggregate, reflect Haemonetics' profile and position Haemonetics near the median of those companies based on certain size and valuation parameters. In so doing, the Committee, with the assistance of Pearl Meyer, used a filtration methodology that began with a large pool of U.S. publicly-traded companies in the healthcare equipment and supplies or life sciences and tools and services industries. The Committee then considered potential peers based on certain size and valuation metrics (including revenue, market capitalization, market capitalization to revenue ratio and employee count), generally within a range of one-third to three times that of the Company, and applied additional filters to identify profile-appropriate peers with comparable operations to the Company (with products and services in hematology preferred) and/or overlap with proxy advisory firm-identified peers of the Company, reverse peers and peers of peers. Applying this filtration methodology, the Committee updated its fiscal 2022 peer group to more closely approximate, in the aggregate, our increased market capitalization and revenue on a year-over-year basis as of December 2020 by adding Avanos Medical, Inc., a previous Haemonetics peer company, and removing Wright Medical Group N.V. due to its November 2020 acquisition by Stryker Corporation.
The following table sets forth the peer group approved by the Committee for purposes of setting fiscal 2022 compensation, along with the Company’s relative position in the peer group in each of the categories.
Fiscal 2022 Peer Group Composition | |||||||||||||||||||||||||||||
Abiomed, Inc. | Cantel Medical Corp. | Integra LifeSciences | Steris plc | ||||||||||||||||||||||||||
Avanos Medical, Inc.(1) | DexCom, Inc. | Masimo Corporation | West Pharmaceutical Services, Inc. | ||||||||||||||||||||||||||
Bio-Rad Laboratories, Inc. | Globus Medical, Inc. | NuVasive, Inc. | |||||||||||||||||||||||||||
Bio-Techne Corp. | ICU Medical, Inc. | PerkinElmer, Inc. | |||||||||||||||||||||||||||
Bruker Corporation | Insulet Corporation | ResMed Inc. | |||||||||||||||||||||||||||
Peer Group Data(2) | |||||||||||||||||||||||||||||
Revenue | Market Capitalization | Market Capitalization to Revenue Ratio | Employee Count | ||||||||||||||||||||||||||
| Company | (dollars in millions) | ||||||||||||||||||||||||||||
50th Percentile | $1,266 | $14,651 | 6.3 | 4,000 | |||||||||||||||||||||||||
Haemonetics Corporation | $903 | $6,030 | 4.9 | 3,004 | |||||||||||||||||||||||||
Approximate Percentile Rank | 25th | 30th | 45th | 35th | |||||||||||||||||||||||||
(1)Represents an addition to the Company’s peer group for purposes of setting fiscal 2022 compensation. | |||||||||||||||||||||||||||||
(2)Revenue is for the trailing four quarters available as of December 31, 2020 and market capitalization is as of December 31, 2020. | |||||||||||||||||||||||||||||
PEER GROUP FOR SETTING FISCAL 2023 COMPENSATION
In consultation with Pearl Meyer, the Committee reviewed and updated our peer group in January 2022 for purposes of setting our fiscal 2023 compensation. Applying a similar filtration methodology as described above, the Committee updated its fiscal 2023 peer group to more closely approximate, in the aggregate, our decreased market capitalization and revenue on a year-over-year basis as of December 2021 by adding Allscripts Healthcare Solutions, Inc., Azenta, Inc., LivaNova PLC, Merit Medical System, Inc. and Quidel Corporation, five companies that better align with Haemonetics based on key size criteria, and by removing DexCom, Inc., RedMed Inc. and West Pharmacueticals Services, Inc. based on their relatively large size or limited business comparability as well as Steris plc and Cantel Medical Corp. due to their June 2021 business combination and resulting size.
The following table sets forth the peer group approved by the Committee for purposes of setting fiscal 2023 compensation, along with the Company’s relative position in the peer group in each of the categories.
26 HAEMONETICS CORPORATION | 2022 Proxy Statement
Fiscal 2023 Peer Group Composition | |||||||||||||||||||||||||||||
Abiomed, Inc. | Bio-Techne Corp. | Integra LifeSciences | PerkinElmer, Inc. | ||||||||||||||||||||||||||
Allscripts Healthcare Solutions, Inc.(1) | Bruker Corporation | LivaNova PLC(1) | Quidel Corporation(1) | ||||||||||||||||||||||||||
Avanos Medical, Inc. | Globus Medical, Inc. | Masimo Corporation | |||||||||||||||||||||||||||
Azenta, Inc.(1) | ICU Medical, Inc. | Merit Medical Systems, Inc.(1) | |||||||||||||||||||||||||||
Bio-Rad Laboratories, Inc. | Insulet Corporation | NuVasive, Inc. | |||||||||||||||||||||||||||
Peer Group Data(2) | |||||||||||||||||||||||||||||
Revenue | Market Capitalization | Market Capitalization to Revenue Ratio | Employee Count | ||||||||||||||||||||||||||
Company | (dollars in millions) | ||||||||||||||||||||||||||||
50th Percentile | $1,129 | $7,326 | 4.3 | 3,700 | |||||||||||||||||||||||||
Haemonetics Corporation | $934 | $2,711 | 3.9 | 2,708 | |||||||||||||||||||||||||
Approximate Percentile Rank | below 25th | below 25th | 40th | 40th | |||||||||||||||||||||||||
(1)Represents an addition to the Company’s peer group for purposes of setting fiscal 2023 compensation. | |||||||||||||||||||||||||||||
(2)Revenue is for the trailing four quarters available as of December 31, 2021 and market capitalization is as of December 31, 2021. | |||||||||||||||||||||||||||||
Evaluating Executive Performance
The Committee reviews the performance of our Named Executive Officers through our annual performance cycle. No Named Executive Officer participates in the Committee’s discussion regarding their own compensation.
CEO EVALUATION 6 | OTHER NEO EVALUATION 6 | |||||||||||||||||||
With respect to our CEO, our independent Board Chair gathers input from all non-employee directors and completes an assessment of the CEO’s performance. The Board Chair solicits feedback and assesses our CEO based on the Company’s performance, his individual performance and his interactions with directors. Once the Board Chair receives the solicited input, the Board Chair reviews all pertinent information with the Committee, which then evaluates the CEO’s performance in light of the goals and objectives relevant to the CEO’s compensation. | With respect to the other Named Executive Officers, our CEO completes an assessment of each such Named Executive Officer's performance, and proposes compensation adjustments where appropriate. In completing his assessment, the CEO evaluates such officer's (i) achievement of individual objectives and goals; (ii) contributions to the Company’s short- and long-term goals and performance; and (iii) leadership competencies and how such officer achieved the applicable goals. | |||||||||||||||||||
 |  | |||||||||||||||||||
 | The Committee determines, approves and submits to the independent members of the Board for ratification the CEO’s compensation. |  | The Committee reviews and discusses the CEO’s performance assessments and compensation recommendations for each other Named Executive Officer and then approves the compensation payable to each other Named Executive Officer. | |||||||||||||||||
ELEMENTS OF TOTAL COMPENSATION
When setting compensation for our Named Executive Officers, the Committee focuses on total direct compensation. Total direct compensation includes three major components – base salary, annual short-term incentive compensation and annual long-term incentive compensation – all of which are designed to work together to drive a complementary set of behaviors and outcomes.
•Base salary. Base salary is intended to compensate for individual technical and leadership competencies required for such officer’s specific position and to provide economic security.
•Annual Short-Term Incentive Compensation. Annual short-term incentive compensation in the form of a market-competitive, performance-based cash bonus is designed to focus our Named Executive Officers on pre-set objectives each year and drive specific behaviors that foster short-term and long-term growth and profitability.
•Annual Long-Term Incentive Compensation. Annual long-term incentive compensation generally consists of PSUs, stock options and RSUs. Annual long-term incentive compensation is designed to align the interests of Named Executive Officers with the long-term growth interests of our shareholders, reward executives for shareholder value creation, recognize executives for their contributions to the Company and promote retention.
HAEMONETICS CORPORATION | 2022 Proxy Statement 27
In addition to receiving direct compensation, our Named Executive Officers also participate in various employee benefit programs, as described in the “Other Benefits” section of this CD&A beginning on page 33.
The following chart illustrates, for fiscal 2022, the distribution of value among the three elements of direct compensation for our CEO and, on average, for the other Named Executive Officers. For purposes of this illustration, the annual short-term incentive compensation component is based on the target annual short-term incentive opportunities awarded by the Committee for fiscal 2022 short-term incentive awards (for more information see "How the 2022 Bonus Plan Works" beginning on page 28) and the annual long-term incentive compensation component is based on the grant value awarded by the Committee for fiscal 2022 annual long-term incentive awards, together with certain special retention and promotion-based awards, before conversion to the various forms of equity awards (for more information see “Annual Long-Term Incentive Awards" beginning on page 31). As a percentage of total annual compensation, 88% of our CEO’s compensation and, on average, 82% of our other Named Executive Officers’ compensation was variable because it was subject to performance goals, the fluctuations of our stock price, or both.
TARGET ANNUAL COMPENSATION MIX
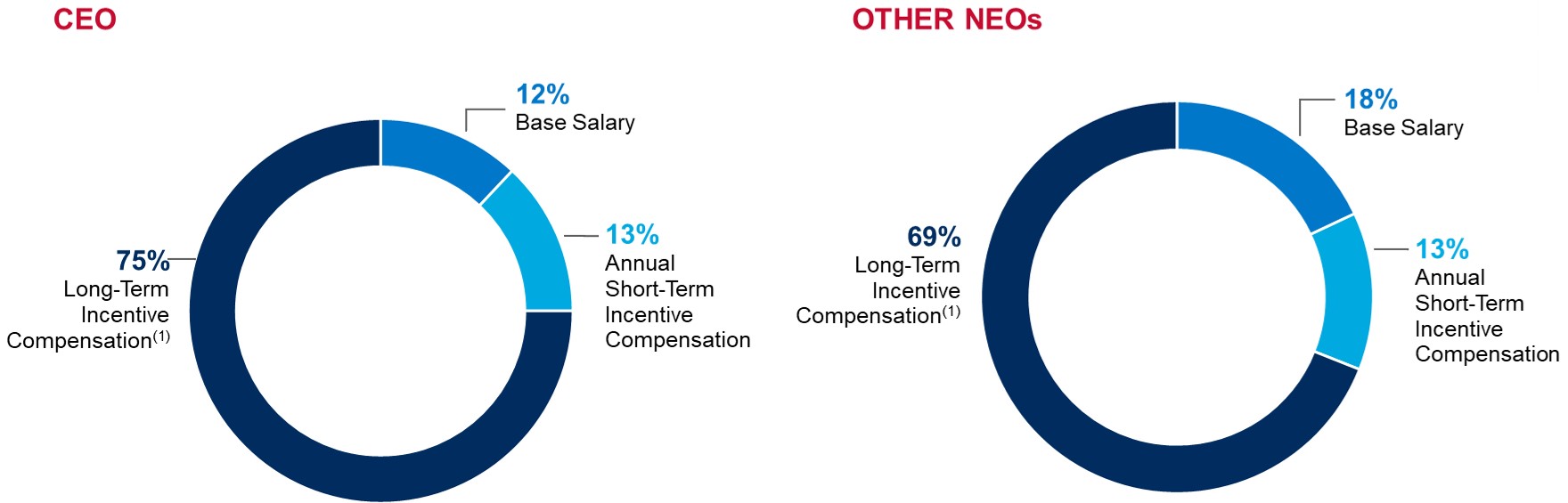
(1)Long-term incentive compensation includes grant value of all fiscal 2022 equity awards to Named Executive Officers. For more information see "Individual Fiscal 2022 Long-Term Incentive Awards" beginning on page 32 of this Proxy Statement.
Base Salary
Base salary, which represents 12% of our CEO’s target total compensation and, on average, 18% of target total compensation for other Named Executive Officers, is paid to provide a fixed component of compensation for the technical and leadership competencies required for the respective position. Target base salary varies based on the field in which each executive operates, the scope of the position, the peer-group comparisons for similarly-situated executives, the experience and qualifications needed for the role, the executive’s performance and an assessment of internal equity among Company executives.
Early in fiscal 2022, the Committee completed the process described above under “Evaluating Executive Performance” (beginning on page 27) and determined appropriate increases to base salaries for our Named Executive Officers. These increases reflected the Committee's assessment of individual performance and contributions as well as its prior determination to postpone fiscal 2021 salary merit increase due to the uncertainties created under the COVID-19 pandemic. In July 2022, the Committee approved a further increase to Josep Llorens' base salary in connection with his promotion to Executive Vice President, Global Manufacturing and Supply Chain. Accordingly, fiscal 2022 base salaries for our Named Executive Officers were as follows:
Named Executive Officer | Fiscal 2021 Base Salary | Fiscal 2022 Base Salary | Percent Increase | ||||||||||||||
Christopher A. Simon | $ | 945,000 | $ | 945,000 | —% | ||||||||||||
William P. Burke | $ | 529,108 | $ | 544,981 | 3% | ||||||||||||
| Michelle L. Basil | $ | 465,271 | $ | 479,230 | 3% | ||||||||||||
| Josep L. Llorens | $ | 405,908 | $ | 450,000 | 10.9%(1) | ||||||||||||
| Stewart W. Strong | $ | 400,000 | $ | 412,000 | 3% | ||||||||||||
(1)Reflects a 3% annual salary merit increase approved by the Committee in early fiscal 2022 and a subsequent salary increase approved by the Committee in July 2022 in connection with Mr. Llorens' promotion to Executive Vice President, Global Manufacturing and Supply Chain.
Annual Short-Term Incentive Compensation
HOW THE 2022 BONUS PLAN WORKS
Annual short-term incentive compensation supports the Committee's pay-for-performance philosophy and aligns individual goals with Company goals. Under our short-term incentive compensation program for fiscal 2022 (the “2022 Bonus Plan”), Named
28 HAEMONETICS CORPORATION | 2022 Proxy Statement
Executive Officers are eligible for a cash award based on the Company’s attainment of pre-established performance goals that the Committee sets based on the Company's annual operating plan. At the beginning of the fiscal year, the Committee, with input from Pearl Meyer, structured the 2022 Bonus Plan as follows:
Fiscal 2022 Performance Goals and Metrics.
•Executive (non-Business Unit) Metrics. For each of our Named Executive Officers, other than Mr. Strong, the Committee tied the 2022 Bonus Plan to achievement of the following two performance goals, with a relative weighting of 60% adjusted revenue to 40% adjusted EPS to align with the Company's strategic focus on revenue growth in a challenging COVID-19 environment while also driving profitability:
Fiscal 2022 Metrics | Weighting | Description | |||||||||
Adjusted Revenue | 60% | Adjusted revenue, which is intended to reflect organic growth, is calculated as revenue determined in accordance with accounting principles generally accepted in the United States ("GAAP") and adjusted to remove the impacts of currency. It may also be adjusted for certain items that affect the comparability of results, including acquisitions or dispositions completed during the fiscal period, other specific large, unusual or nonrecurring items and changes in accounting principles pursuant to GAAP. The Committee selected adjusted revenue as a performance metric because it is a key component of our annual operating plan and is consistent with our strategic objectives to drive accelerated revenue growth. | |||||||||
Adjusted EPS | 40% | Adjusted EPS is calculated as earnings per share determined in accordance with GAAP and adjusted for certain items that affect the comparability of results, including acquisitions or dispositions completed during the fiscal period, other specific large, unusual or nonrecurring items and changes in accounting principles pursuant to GAAP. The Committee selected adjusted EPS as a performance metric because it is a key driver of shareholder return and aligns executives with shareholder value creation. | |||||||||
•Business Unit Metrics. For Mr. Strong, the Committee set more individualized performance goals under the 2022 Bonus Plan that, while aligning with the Company's strategic revenue growth focus and profitability consistent with other executives, recognized Mr. Strong's unique role leading our Hospital business unit's COVID-19 recovery in fiscal 2022:
Fiscal 2022 Metrics | Weighting | Description | |||||||||
Hospital Business Unit Revenue | 70% | Hospital business unit revenue is calculated as revenue determined in accordance with GAAP that is attributable to the Company’s Hospital business unit, based on product-focused and customer-centric alignment and excluding any service/other revenue for which the Hospital business unit leader does not have direct responsibility, adjusted to remove the impacts of currency. It may also be adjusted for certain items that affect the comparability of results, including acquisitions or dispositions completed during the fiscal period, other specific large, unusual or nonrecurring items and changes in accounting principles pursuant to GAAP. The Committee selected this performance metric for Mr. Strong because he has direct responsibility for Hospital revenue growth, including the newly integrated Vascular Closure product line, which is a key element of the Company's strategic revenue growth objectives in fiscal 2022. | |||||||||
Adjusted Operating Income | 30% | Adjusted operating income is calculated as consolidated operating income determined in accordance with GAAP and adjusted to remove the impacts of currency and certain other items that affect the comparability of results, including acquisitions or dispositions completed during the fiscal period, other specific large, unusual or nonrecurring items and changes in accounting principles pursuant to GAAP. The Committee selected adjusted operating income as a performance metric because it is a key driver of shareholder return that Mr. Strong can meaningfully impact through effective leadership and management of Hospital business unit costs and opportunities. | |||||||||
The Compensation Committee set fiscal 2022 growth targets for the 2022 Bonus Plan that significantly exceeded fiscal 2021 performance levels while maintaining wider performance ranges between threshold, target and maximum performance that reflected continued uncertainty regarding the COVID-19 pandemic. The Committee believed these financial performance goals were achievable, but appropriately challenging, based on the ongoing impacts of COVID-19, market climate and internal budgeting and forecasting.
HAEMONETICS CORPORATION | 2022 Proxy Statement 29
Fiscal 2022 Bonus Plan Targets.
The Committee set target payout levels for each Named Executive Officer (expressed as a percentage of base salary) based upon competitive market and internal equity considerations, and it set threshold performance requirements to earn a threshold award (50% of target award) and maximum performance requirements to earn a maximum award (200% of target award). The Committee determined to return the maximum award opportunity to 200% consistent with pre-fiscal 2021 bonus plan design in recognition of a desire to reward outsized performance against an appropriately ambitious fiscal 2022 annual operating plan.
Based upon competitive market and internal equity considerations, the Committee set 2022 Bonus Plan targets for Named Executive Officers, which are expressed as a percentage of base salary, at the same levels as fiscal 2021 bonus plan targets. In July 2022, the Committee increased Messrs. Llorens' and Strong's 2022 Bonus Plan targets in recognition of Mr. Llorens' promotion to Executive Vice President, Global Manufacturing and Supply Chain and Mr. Strong's expanded general management responsibilities in addition to the Hospital business unit (see "Annual Long-Term Incentive Awards" on page 31 for more detail):
Named Executive Officer | Fiscal 2021 Target | Fiscal 2022 Target | ||||||
Christopher A. Simon | 110 | % | 110 | % | ||||
William P. Burke | 70 | % | 70 | % | ||||
Michelle L. Basil | 70 | % | 70 | % | ||||
Josep L. Llorens | 60 | % | 65 | % | ||||
Stewart W. Strong | 65 | % | 70 | % | ||||
Bonuses paid to our Named Executive Officers under the 2022 Bonus Plan are based primarily upon the Company's achievement of specified targets set by the Committee for the performance metrics described above under "Fiscal 2022 Performance Goals and Metrics" and are approved by the Compensation Committee. The compensation of our CEO is also ratified by the Board. The Compensation Committee has authority under the 2022 Bonus Plan to adjust final individual bonuses based on individual performance (within the range of 0 to 200% of target as described above), although any such adjustment is expected to be exercised infrequently. No such adjustments were made in fiscal 2022. After the close of fiscal 2022, the Committee received a report from management regarding Company performance against the pre-established performance goals as well as a recommendation as to an appropriate funding level for the 2022 Bonus Plan pool. The Committee then reviewed the Company’s financial performance against each goal and established the 2022 Bonus Plan pool. After establishing the 2022 Bonus Plan pool funding level, the Committee reviewed the individual performance for all Named Executive Officers and approved final awards.
2022 BONUS PLAN TARGETS AND FUNDING
The following table outlines the threshold, target and maximum financial performance goals for the 2022 Bonus Plan, as well as the results achieved:
Executive (Non Business-Unit) Bonus Metrics
| Metric Weighting | Performance Targets | Achievement | ||||||||||||||||||||||||||||||||||||
Fiscal 2022 Targets(1) | Threshold | Target | Maximum | Full Year Results | Target Award (%) | |||||||||||||||||||||||||||||||||
Adjusted Revenue(2) | 60% | $ | 945.3 | $ | 995.0 | $ | 1,044.8 | $ | 988.9 | 93.8% | ||||||||||||||||||||||||||||
Adjusted EPS(3) | 40% | $ | 2.43 | $ | 2.70 | $ | 2.97 | $ | 2.58 | 77.8% | ||||||||||||||||||||||||||||
Payout Percentage | 50% | 100% | 200% | 87.4% | ||||||||||||||||||||||||||||||||||
(1)All dollar values are in millions except per share data.
(2)For purposes of the 2022 Bonus Plan, adjusted revenue equals fiscal 2022 total Company revenue determined in accordance with GAAP, adjusted to exclude the impacts of currency.
(3)For purposes of the 2022 Bonus Plan, adjusted EPS equals earnings per share determined in accordance with GAAP, adjusted to exclude restructuring and restructuring related costs, deal amortization expenses, asset impairments and PCS2 related charges, costs related to compliance with the European Union Medical Device Regulation and In Vitro Diagnostic Regulation, integration and transaction costs, gains on dispositions, unusual or infrequent and material litigation-related charges and the tax impact of each of these items. Adjusted EPS targets for the 2022 Bonus Plan did not contemplate, and the Company did not engage in, share repurchases during fiscal 2022 and accordingly no adjustment has been made for share repurchases.
Business Unit Bonus Metrics
| Metric Weighting | Performance Targets | Achievement | ||||||||||||||||||||||||||||||||||||
Fiscal 2022 Targets(1) | Threshold | Target | Maximum | Full Year Results | Target Award (%) | |||||||||||||||||||||||||||||||||
Hospital Business Unit Revenue(2) | 70% | $ | 290.3 | $ | 305.6 | $ | 320.9 | $ | 321.6 | 200% | ||||||||||||||||||||||||||||
Adjusted Operating Income(3) | 30% | $ | 151.4 | $ | 168.2 | $ | 185.0 | $ | 168.1 | 99.7% | ||||||||||||||||||||||||||||
Payout Percentage | 50% | 100% | 200% | 169.9% | ||||||||||||||||||||||||||||||||||
30 HAEMONETICS CORPORATION | 2022 Proxy Statement
(1)All dollar values are in millions except per share data.
(2)For purposes of the 2022 Bonus Plan, Hospital business unit revenue equals fiscal 2022 revenue for the Company's Hospital business unit determined in accordance with GAAP, adjusted to exclude the impacts of currency.
(3)For purposes of the 2022 Bonus Plan, Adjusted Operating Income equals the Company's fiscal 2022 consolidated operating income determined in accordance with GAAP, adjusted to exclude the impacts of currency, restructuring and restructuring related costs, deal amortization expenses, asset impairments and PCS2 related charges, costs related to compliance with the European Union Medical Device Regulation and In Vitro Diagnostic Regulation, integration and transaction costs, gains on dispositions and unusual or infrequent and material litigation-related charges.
2022 BONUS PLAN AWARDS AND RESULTS
Based upon the Company’s performance against the established performance goals as described above, the Committee set the overall funding level of the 2022 Bonus Plan pool at (a) 87.4% of target funding for Named Executive Officers, other than Mr. Strong and (b) 169.9% for Mr. Strong. Preliminary individual bonus payouts for Named Executive Officers were then calculated based on this overall funding level applicable to the executive as well as the targeted payout levels for each Named Executive Officer. The Committee then reviewed the performance of each individual during fiscal 2022, taking into consideration the recommendation of the CEO with respect to Named Executive Officers other than the CEO. The Committee then considered adjustments to the preliminary individual bonus payouts in accordance with the 2022 Bonus Plan (as described above) based on each Named Executive Officer's performance during fiscal 2022.
Based on this alignment of performance, total fiscal 2022 short-term incentive payments for our Named Executive Officers are shown below:
Executive | Fiscal 2022 Bonus Target (% Salary) | Fiscal 2022 Bonus Target ($)(1) | Fiscal 2022 Bonus Actual (% Bonus Target) | Fiscal 2022 Bonus Actual ($)(1) | |||||||||||||||||||||||||
Christopher A. Simon | 110 | % | $ | 1,039,500 | 87.4% | $ | 908,523 | ||||||||||||||||||||||
William P. Burke | 70 | % | $ | 381,487 | 87.4% | $ | 333,419 | ||||||||||||||||||||||
Michelle L. Basil | 70 | % | $ | 335,461 | 87.4% | $ | 293,193 | ||||||||||||||||||||||
Josep L. Llorens | 65 | % | $ | 292,500 | 87.4% | $ | 255,645 | ||||||||||||||||||||||
Stewart W. Strong | 70 | % | $ | 288,400 | 169.9% | $ | 489,992 | ||||||||||||||||||||||
(1)Rounded to nearest whole dollar.
Annual Long-Term Incentive Awards
The Committee uses long-term incentive compensation to deliver competitive compensation that recognizes executives for their contributions to the Company and aligns the interests of Named Executive Officers with shareholders by focusing them on long-term growth and share performance. The Committee views long-term incentives as a significant element of total compensation at the executive level and a critical component of the Company’s executive compensation program.
FISCAL 2022 LONG-TERM INCENTIVE AWARD TYPES
Annual Long-Term Incentive Awards
In fiscal 2022, the total award values of annual long-term incentive awards granted to our Named Executive Officers were allocated generally among the following(1):
50% PSUs Performance-based awards vest, if at all, at the end of a three-year performance period based upon satisfaction of performance criteria tied to relative total shareholder return, or "rTSR" (for more information see "How We Establish rTSR Goals" below) |  | 25% Stock Options Four-year ratable vesting period | |||||||||||||||
25% RSUs Four-year ratable vesting period | |||||||||||||||||
(1)Excludes special retention awards (see "Special Retention Awards" beginning on page 32) to select Named Executive Officers and a $250,000 promotion-related grant to Mr. Strong in the form of PSUs (three-year rTSR based) made in July 2021 in connection with his expanded management responsibilities in addition to his role as President, Global Hospital. For more information, see "Individual Fiscal 2022 Long-Term Incentive Awards" below.
HAEMONETICS CORPORATION | 2022 Proxy Statement 31
The Committee considered this allocation of annual long-term incentive awards to be appropriate, as Company performance is reflected in PSUs and stock options (which only have value to the extent the Company’s stock price increases from the stock price on the grant date), while grants of time-based RSUs allow the program to support retention throughout a full business cycle.
HOW WE ESTABLISH rTSR GOALS | |||||||||||||||||
In implementing and setting goals for our PSU awards, the Committee considers market practice as well as our focus on driving shareholder value. For our fiscal 2022 PSU awards, the Committee determined to retain the PSU metric of rTSR (measured over a three-year performance period) because it directly links our Named Executive Officers' compensation to the Company's long-term shareholder value creation objectives, in addition to being well received and supported by our shareholders. The Committee did not make any design changes for fiscal 2022 PSU awards. Achievement under fiscal 2022 PSU awards is tied to the Company's rTSR relative to the components of the S&P MidCap 400 index during the three-year performance period from May 18, 2021 to May 17, 2024. The Company became a member of the S&P MidCap 400 Index in fiscal 2019. Depending on the Company’s rTSR performance, the number of shares that may be earned under these PSU awards ranges from 0% to 200% of target, as follows: | |||||||||||||||||
rTSR | Percentage of Target Shares Earned | ||||||||||||||||
Below 30th percentile | 0% | ||||||||||||||||
30th to 50th percentile (Threshold) | 50% to 99% | ||||||||||||||||
51st to 80th percentile (Target) | 100% to 200% | ||||||||||||||||
80th percentile or higher (Maximum) | Capped at 200% | ||||||||||||||||
| Similar to fiscal 2021 PSU awards, if the Company experiences negative total shareholder return during the performance period, the fiscal 2022 PSU award payout cannot exceed 100% of the target performance level. | |||||||||||||||||
Special Retention Awards
As disclosed in our 2021 proxy statement, the Company experienced a substantial decrease in our share price in April 2021 following our announcement that one of our largest customers, CSL, had recently notified us of its intent not to renew its current U.S. supply agreement for the use of Haemonetics PCS2 Plasma Collection System devices and the purchase of plasma disposables that was set to expire in June 2022. The Company was informed that the decision was based on changes in CSL’s internal strategy and was not related to product or service quality. Accordingly, at the time the Committee granted annual long-term incentive awards in early fiscal 2022, the Committee was focused on retention of the Company’s executive team, and undertook an evaluation of the retentive value of then-outstanding unvested equity awards. The Committee noted that the Company’s key executive talent receive a substantial portion of their target annual compensation in the form of long-term equity awards and had experienced a correspondingly substantial negative impact on their compensation (including 0% achievement of rTSR-based performance goals under fiscal 2019 PSU awards) following the Company’s share price drop during April 2021. Accordingly, the Committee determined to grant special retention awards to certain key executive talent, to be structured as RSUs with a two-year, rather than a four-year, vesting requirement to incentivize recipients to remain with the Company as we continue to navigate a challenging COVID-19 environment and critical milestones, including rollout of a new long-range plan to be announced at our fiscal 2023 Investor Day. The Committee intended that these special retention awards be one-time in nature and does not foresee similar future retention grants absent extraordinary circumstances.
INDIVIDUAL FISCAL 2022 LONG-TERM INCENTIVE AWARDS
When setting annual long-term incentive compensation for Named Executive Officers, the Committee employs the process described in the “Evaluating Executive Performance” section of this CD&A (beginning on page 27). After the Committee establishes a grant value for each Named Executive Officer’s fiscal 2022 annual long-term incentive award, that value is allocated between PSUs, stock options and RSUs (see "Annual Long-Term Incentive Award Types" beginning on page 31), with the exact number of PSUs and RSUs issued based upon the closing price of Company’s common stock on the grant date and the exact number of stock options determined using the applicable Black-Scholes value. With respect to fiscal 2022 special retention awards, which are intended to be one-time in nature, the Compensation Committee determined the size of the special retention awards for applicable Named Executive Officers in its discretion, based on its subjective assessment of individual efforts, criticality and retention considerations.
For Named Executive Officers, other than our CEO, the Committee in early fiscal 2022 approved increases for fiscal 2022 annual long-term incentive awards from fiscal 2021 levels based on individual performance and market position and granted special
32 HAEMONETICS CORPORATION | 2022 Proxy Statement
retention awards. In July 2021, the Committee made an additional $250,000 grant to Mr. Strong in the form of a PSU award (three-year vesting period tied to rTSR) in recognition of his expanded role managing certain global commercial and operational functions beginning in July 2021 in addition to his other functional responsibilities as President of the Hospital business unit. The table below lists the grant value of fiscal 2022 long-term incentive awards granted by the Committee.
Fiscal 2022 Grant Value Awarded(1) | |||||||||||||||||||||||||||||
Named Executive Officer | Fiscal 2021 Grant Value Awarded(1) | Annual Long-Term Incentive Grants | Special Retention Grants | ||||||||||||||||||||||||||
Christopher A. Simon | $ | 6,000,000 | $ | 6,000,000 | $ | 0 | |||||||||||||||||||||||
William P. Burke | $ | 1,300,000 | $ | 1,500,000 | $ | 1,000,000 | |||||||||||||||||||||||
Michelle L. Basil | $ | 1,200,000 | $ | 1,400,000 | $ | 1,000,000 | |||||||||||||||||||||||
Josep L. Llorens | $ | 700,000 | $ | 800,000 | $ | 400,000 | |||||||||||||||||||||||
| Stewart W. Strong | $ | 550,000 | $ | 1,000,000(2) | $ | 375,000 | |||||||||||||||||||||||
(1)The award values in this table reflect the grant values awarded by the Committee while the Summary Compensation Table (beginning on page 39) and the Grants of Plan-Based Awards During Fiscal 2022 table (beginning on page 40) reflect the award values for accounting purposes.
(2)Includes additional $250,000 grant to Mr. Strong in the form of a PSU award tied the Company's rTSR relative to the components of the S&P MidCap 400 index during a three-year performance period (see "How We Establish rTSR Goals").
LONG-TERM INCENTIVE AWARD PAYOUTS
During fiscal 2022, the Committee determined final shares earned under the following PSU award grants made to Messrs. Simon and Burke and Ms. Basil in June 2018 and to Mr. Llorens in September 2018, each with three-year performance periods ending in fiscal 2022:
Performance Targets | Achievement | ||||||||||||||||||||||||||||||||||
Performance Period | Metrics(1) | Metric Weighting | Threshold (50%) | Target (100%) | Maximum (200%) | Results | Results as % of Target | ||||||||||||||||||||||||||||
June 11, 2018 to June 10, 2021 | rTSR | 100% | 41st Percentile | 61st Percentile | 81st Percentile | 11th Percentile | 0% | ||||||||||||||||||||||||||||
| Sept. 4, 2018 to Sept. 3, 2021 | rTSR | 100% | 41st Percentile | 61st Percentile | 81st Percentile | 12th Percentile | 0% | ||||||||||||||||||||||||||||
(1)Based on Company’s rTSR during the performance period relative to performance of the components of the S&P SmallCap 600 and S&P MidCap 400 indices. Results between the 41st to 60th percentile and between the 61st to 80th percentile are interpolated between 50-99% and 100-200% of share payout as a percentage of target award, respectively.
Other Benefits
The Company offers retirement and health and welfare benefits, as well as an employee stock purchase plan, to eligible employees, including Named Executive Officers. The Company offers very limited perquisites to executive officers.
RETIREMENT AND HEALTH AND WELFARE BENEFITS
We maintain a tax-qualified defined contribution 401(k) plan, the Haemonetics Corporation Savings Plus Plan, that is available to all eligible United States employees. As part of our overall compensation offering, our health and welfare benefits are intended to be competitive with peer companies. The health and welfare benefits we provide to our Named Executive Officers are offered to all of our eligible United States-based employees and include medical, dental, prescription drug, vision, life insurance, accidental death and dismemberment, critical illness, accident, short- and long-term disability coverage and the employee assistance program. The Company's long-term disability coverage includes supplemental coverage available for eligible employees, including each of our Named Executive Officers, who earn salaries in excess of amounts covered under our base plan.
EMPLOYEE STOCK PURCHASE PLAN
We maintain a broad-based employee stock purchase plan, the Haemonetics Corporation 2007 Employee Stock Purchase Plan (“ESPP”), which provides eligible employees, including our Named Executive Officers, with the opportunity to purchase Company shares through payroll deductions. We believe that providing an employee stock purchase plan is consistent with our philosophy that compensation should align the interests of employees and shareholders and promote a long-term shareholder perspective. The ESPP is intended to be “qualified” under Internal Revenue Code Section 423 and offers eligible employees the opportunity to purchase Company shares during consecutive offering periods of approximately six months that generally begin with the first of May and November each year. Mr. Simon participated in the ESPP during fiscal 2022.
HAEMONETICS CORPORATION | 2022 Proxy Statement 33
OTHER COMPENSATION AND POLICIES
Employment, Change in Control and Severance Arrangements
Our success in building the sound leadership team in place today was due in large part to our ability to utilize a full complement of compensation tools, including employment, change in control and severance agreements. We believe that together, our employment, change in control and severance agreements, which are guided by our compensation philosophy and governance practices, foster stability within our executive leadership team by helping our executives to better focus their time, attention and capabilities on our business and assist the Company in recruiting and retaining key executives. Details about specific arrangements made with our Named Executive Officers are set forth below.
EMPLOYMENT AGREEMENT WITH CHRISTOPHER A. SIMON
In connection with his appointment as President and Chief Executive Officer in fiscal 2017, Mr. Simon entered into an employment agreement with the Company (the “Employment Agreement”). The Employment Agreement renews automatically each year and provides for a specified annual base salary and a target variable compensation award based on performance, both of which are subject to annual adjustment. Pursuant to the Employment Agreement, Mr. Simon is also eligible to receive annual equity grants under the Company’s long-term incentive compensation plans, as determined by the Compensation Committee. The Employment Agreement also entitles Mr. Simon to participate in all other elements of the Company’s standard employee benefit programs generally available to the Company’s senior executives, to reimbursement for certain housing and travel expenses during his first 18 months of employment (which have since expired), and to reimbursement of up to $20,000 in legal fees in connection with the enforcement of the Employment Agreement.
Additionally, the Employment Agreement provides that Mr. Simon will be entitled to severance benefits under the terms of separate executive severance and change in control agreements between Mr. Simon and the Company. The terms of Mr. Simon’s severance and change in control agreements with the Company are discussed below.
RETENTION AGREEMENT WITH WILLIAM P. BURKE
On November 9, 2021, the Company announced that Mr. Burke had decided to retire from the Company in June 2022. Mr. Burke agreed to remain in his role as Executive Vice President, Chief Financial Officer while the Company conducted a search for a successor and to assist Mr. Simon and the Company's next Chief Financial Officer in the transition of his role thereafter through June 30, 2022 (the "Retention Date"). Mr. Burke formally stepped down as Chief Financial Officer on April 11, 2022 in connection with the appointment of James D'Arecca as the Company's new Executive Vice President, Chief Financial Officer.
In consideration of the important services Mr. Burke agreed to provide during the transition period, the Company and Mr. Burke entered into an agreement dated November 8, 2021 (the “Agreement”) that set forth the terms of Mr. Burke’s transition and retention through the Retention Date, including his continued compensation, a potential retention payment, transition services to be performed, restrictive covenants in favor of the Company and a customary release of claims against the Company. Under the terms of the Agreement, Mr. Burke’s base salary would continue unchanged through the Retention Date and he would remain eligible for a bonus under the Company’s fiscal 2022 short-term incentive plan based on the Company’s actual performance. Additionally, if Mr. Burke does not resign and is not terminated for cause (as defined in his severance agreement with the Company) prior to the Retention Date, he will also receive a retention bonus equal to 50% of his annual base salary. Provided that Mr. Burke remains employed by the Company through the Retention Date and satisfies the other criteria to receive his retention bonus, he will also receive a pro rata bonus under the Company’s fiscal 2023 short-term incentive plan based on the number of days employed by the Company in fiscal 2023. Mr. Burke will not be eligible to receive a fiscal 2023 annual long-term incentive award grant from the Company. The Agreement also specifies that the termination of Mr. Burke's employment on the Retention Date does not give rise to payment under his severance agreement described below.
RETENTION AGREEMENT WITH MICHELLE L. BASIL
On June 16, 2022, the Company announced that Ms. Basil had decided to transition from the Company in October 2022. The Company and Ms. Basil have entered into a retention and transition agreement (the “Retention Agreement”) that provides for Ms. Basil’s continued service as Executive Vice President, General Counsel through October 31, 2022 on her existing employment terms, other than Ms. Basil not being eligible to receive a bonus under the Company’s fiscal 2023 short-term incentive plan as she will no longer be an employee of the Company subsequent to October 31, 2022. The Retention Agreement provides that if Ms. Basil does not resign and is not terminated for cause (as defined in her severance agreement with the Company) prior to October 31, 2022, she will also receive a retention bonus of $500,000, subject to her execution of a customary release of claims and compliance with certain post-employment restrictive covenants contained in her Retention Agreement in favor of the Company. The Retention Agreement also specifies that the termination of Ms. Basil’s employment on October 31, 2022 does not give rise to payment under her severance agreement described below.
34 HAEMONETICS CORPORATION | 2022 Proxy Statement
SEVERANCE AND CHANGE IN CONTROL AGREEMENTS WITH NAMED EXECUTIVE OFFICERS
The Company has entered into severance and change in control agreements with certain of its senior executive officers, including our Named Executive Officers. The purpose of these agreements is to provide financial security if there is a loss of employment in certain circumstances (as discussed in detail below) so the executive can better focus his or her time, attention and capabilities on our business. In consideration for these benefits, each executive agrees to a release of claims and customary confidentiality, non-competition and non-solicitation clauses in favor of the Company contained in the agreements.
Severance Agreements. Each agreement provides that if the executive is terminated for reasons other than death, disability or cause or, solely in the case of Mr. Simon, if he resigns due to constructive termination (as such terms are defined in the severance agreement), the executive is entitled to receive the following benefits:
•An amount equal to (i) for Mr. Simon, two times his base salary payable over a 24-month period and (ii) for our other Named Executive Officers, one times the person's base salary payable over a 12-month period;
•An amount equal to the cost of the Company’s portion of the monthly premium for the executive’s medical and dental insurance coverage for a period of (i) two years for Mr. Simon, payable over a 24-month period, and (ii) one year for our other Named Executive Officers, payable over a 12-month period;
•A pro-rated annual bonus for the year in which the executive was terminated based on the Company’s actual performance during the applicable bonus period and assuming full achievement of any individual performance goals;
•Outplacement services for up to 12 months; and
•If any benefit provided under the agreement is subject to excise taxes under Section 280G of the Internal Revenue Code of 1986, as amended (“Section 280G”), the benefit will either be reduced to the Section 280G cap or paid in full depending on which provides the better after-tax position for the executive.
•Each severance agreement (other than Mr. Simon’s, which continues for the duration of his employment as Chief Executive Officer) has an initial term of three years and will automatically renew for successive one year periods, unless terminated by either party. Severance benefits payable under each agreement will immediately cease if the executive violates certain confidentiality, non-solicitation and non-competition obligations and, in the event of such violation, the executive will be required to repay to the Company any installments of continued base salary or payments for welfare benefits that were previously paid to the executive under the severance agreement. To the extent the executive is entitled to severance benefits under a change in control agreement, the executive will not receive any benefits under the severance agreement.
Double-Trigger Change in Control Agreements. Each agreement provides that if a change in control occurs during the term of the agreement and the executive is terminated for reasons other than death, disability or cause (as defined in the change of control agreement) or terminates his or her employment due to constructive termination (as defined in the change in control agreement) within two years after the occurrence of the change in control (this is known as a "double trigger"), the executive is entitled to receive the following benefits:
•A lump sum cash payment equal to (i) for Mr. Simon, 2.99 times the sum of his base salary plus target annual short-term incentive compensation and/or any other annual cash incentive award opportunity at the time of termination or change in control (whichever is higher), and (ii) for our other Named Executive Officers, two times the sum of their salary plus target annual short-term incentive compensation and/or any other annual cash incentive award opportunity at the time of termination or change in control (whichever is higher);
•a lump sum cash payment equal to (i) for Mr. Simon, thirty-six times the cost of the Company’s portion of the monthly premium for the executive’s medical, dental, life and disability insurance coverage, and (ii) for our other Named Executive Officers, twenty-four times the cost of the Company’s portion of the monthly premium for the executive’s medical, dental, life and disability insurance coverage;
•Outplacement services for up to 12 months;
•Immediate and full vesting of all time-based equity awards and pro rata vesting of performance-based awards, provided that in the event of a conflict between the terms of the change in control agreement and the terms of an individual equity award with respect to vesting of equity awards upon a change in control, the terms of the individual equity award will control if such award provides more favorable vesting terms than the change in control agreement (for a discussion of change in control terms under our equity awards see “Change in Control and Acceleration in Equity Awards” below); and
•If any benefit provided under the agreement is subject to excise taxes under Section 280G, the benefit will either be reduced to the Section 280G cap or paid in full depending on which provides the better after-tax position for the executive.
For purposes of the agreements, a change in control is defined as a person or group acquiring 50% or more of the Company’s stock, the sale of substantially all the assets of the Company to an unrelated person, our “Incumbent Board” ceasing for any reason to constitute a majority of the Board, or the consummation of certain mergers, reorganizations, consolidations and share exchanges. Each change in control agreement (other than Mr. Simon’s, which continues for the duration of his employment as Chief Executive Officer) has an initial term of five years and will automatically renew for successive five year periods, unless terminated by either party. Benefits payable under each agreement will immediately cease if the executive violates certain
HAEMONETICS CORPORATION | 2022 Proxy Statement 35
confidentiality, non-solicitation and non-competition obligations and, in the event of such violation, the executive will be required to repay to the Company any cash amounts that were previously paid to the executive under the agreement.
The amount of the estimated payments and benefits payable to our Named Executive Officers, assuming termination or a change in control of the Company as of the last day of fiscal 2022, is shown in the table on page 44 under the heading “Potential Payments upon Termination or Change in Control.”
CHANGE IN CONTROL AND ACCELERATION IN EQUITY AWARDS
Each option award agreement and RSU award agreement provides that if a change in control occurs and the executive is terminated for reasons other than death, disability or cause (as defined in each agreement) or terminates his or her employment due to constructive termination (as such terms are defined in the agreement) within two years after the occurrence of the change in control (i.e., a double trigger), then all unvested stock options and time-based RSUs will immediately vest. Additionally, option and RSU awards will vest in full upon termination of employment due to the executive's death and, in the case of option awards and RSU awards (other than fiscal 2019 RSU awards), will continue to vest in accordance with the vesting schedule upon a termination of employment due to the executive's disability (as defined in the award agreement).
PSUs provide for “double-trigger” vesting in connection with a change in control. If an executive’s employment is terminated involuntarily without cause or voluntarily for good reason (as defined in the agreement) within two years following a change in control, the requirement for vesting will be met and the executive will be entitled to receive the greater of the amount the executive would receive based on performance achieved as of the change in control date and a pro rata portion of the target award amount based on the period of time elapsed during the performance period. Additionally, upon a termination of employment due to the executive's death, disability or a qualifying retirement (each as defined in the agreement), the executive will remain eligible to receive a pro rata portion of the target award amount at the end of the performance period based on the period of time elapsed during the performance period and achievement of the relevant performance metrics.
Under the terms of our award agreements, a "change in control" would occur upon the acquisition of 50% or more of the Company's shares of common stock, the sale of substantially all Company assets, the turnover of a majority of our then-current Board in a proxy contest, or upon certain mergers, reorganizations, consolidations and share exchanges.
Share Ownership Policy
Our Board believes strongly in aligning the long-term interests of our directors and executives with those of our shareholders. We maintain through our Compensation Committee a share ownership policy requiring that our Board Chair, all other non-employee directors, our CEO and our other Named Executive Officers own shares of Haemonetics common stock at or above prescribed levels based on their role at the Company. Specifically, those individuals must comply with the following ownership requirements (expressed as a multiple of annual retainer or base salary) within five years of their respective election as a director, date of hire as an executive officer, or date of appointment to a new role requiring them to maintain a higher level of stock ownership under the policy:
Organizational Role | Share Ownership Requirement | Compliance Status(1) | ||||||
Non-Employee Directors (Other than Board Chair) | 5X annual cash retainer | Compliant or within 5-year grace period | ||||||
Board Chair | 2X non-employee director dollar threshold | Within 5-year grace period | ||||||
Chief Executive Officer | 5X base salary | Compliant | ||||||
Other Named Executive Officers | 2X base salary | Compliant or within 5-year grace period | ||||||
(1)The Compensation Committee reviews executive and director share ownership compliance at least annually at its October meeting. Compliance status above is measured as of the last day of the Company's fiscal 2022.
The value of shares owned outright, vested “in the money” stock options and unvested RSUs are used in satisfying the share ownership requirements above. The Compensation Committee may temporarily suspend or permanently remove an individual’s share ownership requirement for hardship due to unforeseen and compelling circumstances.
Clawback Policy
To further align the executive compensation program with the interests of shareholders and our culture of ethical behavior, our annual short-term incentive compensation plan and Principles of Corporate Governance include compensation clawbacks that require recoupment of certain short-term and long-term incentive compensation in the form of cash and equity awards paid to an executive officer if, as a result of such executive officer’s misconduct, the Company is required to make an accounting restatement due to material non-compliance with any financial reporting requirement under the securities laws, or if such executive officer violates the Company’s Code of Conduct.
36 HAEMONETICS CORPORATION | 2022 Proxy Statement
RISK ASSESSMENT OF OUR COMPENSATION PROGRAMS
At the Compensation Committee’s direction, representatives of the Company’s human resources department conducted a risk assessment of the Company’s compensation programs and practices during fiscal 2022. This risk assessment consisted of a review of cash and equity compensation provided to Company employees, with a focus on compensation payable to senior executives and incentive compensation plans which provide variable compensation to other Company employees based upon Company and individual performance. The Compensation Committee reviewed the findings of this assessment and agreed with the conclusion that our compensation programs are designed with the appropriate balance of risk and reward in relation to the Company’s overall business strategy and do not create risk that is reasonably likely to have a material adverse effect on the Company. The following characteristics of our compensation programs support this finding:
•Our use of different types of compensation vehicles that provide a balance of short- and long-term incentives with fixed and variable components;
•Our practice of capping non-sales plan awards to limit windfalls;
•Our practice of looking beyond results-oriented performance in assessing the contributions of a particular executive;
•Our share ownership guidelines; and
•Our clawback policy applicable to our incentive plans.
IMPACT OF TAX ACCOUNTING ON COMPENSATION
Deductibility of Compensation
The impact of federal tax laws on our compensation programs is also considered by the Compensation Committee. Section 162(m) of the Internal Revenue Code (the "Code"), as amended, generally limits the deductibility of compensation in excess of $1 million paid to any one “covered employee” (which generally includes the Company’s Named Executive Officers) during any fiscal year. Under the rules in effect before 2018, compensation that qualified as “performance-based” under Section 162(m) was deductible without regard to this $1 million limit. The Tax Cuts and Jobs Act of 2017 repealed the exemption from the Section 162(m) deduction limit for performance-based compensation, effective for taxable years beginning after December 31, 2017. As a result, we expect that compensation paid to our Named Executive Officers in excess of $1 million generally will not be deductible.
Although our compensation programs take into consideration the Section 162(m) rules as a factor, this consideration will not necessarily limit compensation to amounts deductible under Section 162(m). Despite the changes to Section 162(m) the Compensation Committee currently expects to structure the Company’s executive compensation programs such that a significant portion of executive pay is subject to performance goals.
Stock-Based Compensation Expense
The Company began recognizing stock-based compensation expense under Financial Accounting Standards Board ("FASB") Accounting Standards Codification ("ASC") Topic 718 beginning in April 2006. In determining the appropriate fiscal 2022 long-term incentive grant levels, the Company sought to balance its long-term incentive goals with the need to reduce shareholder dilution and manage stock compensation expense.
HAEMONETICS CORPORATION | 2022 Proxy Statement 37
| Compensation Committee Report | ||
The Compensation Committee has reviewed and discussed with management the Compensation Discussion and Analysis contained in this Proxy Statement and, based on such review and discussions, the Compensation Committee recommended to the Board that the Compensation Discussion and Analysis be included in this Proxy Statement and in the Company’s Annual Report on Form 10-K for the fiscal year ended April 2, 2022 for filing with the SEC.
THE COMPENSATION COMMITTEE
Robert E. Abernathy, Chair
Mark W. Kroll
Claire Pomeroy
Ellen M. Zane
38 HAEMONETICS CORPORATION | 2022 Proxy Statement
| Executive Compensation Tables | ||
The following table summarizes the compensation of our Named Executive Officers for each of the last three fiscal years. Mr. Strong became a Named Executive Officer for the first time in fiscal 2022. A description of our compensation policies and practices as well as a description of the components of compensation payable to our Named Executive Officers is included under “Compensation Discussion and Analysis” beginning on page 21.
SUMMARY COMPENSATION TABLE
| Name and Principal Position | Fiscal Year | Salary(1) ($) | Stock Awards(2) ($) | Option Awards(2) ($) | Non-Equity Incentive Plan Compensation(3) ($) | All Other Compensation ($) | Total | ||||||||||||||||||||||||||||||||||
Christopher A. Simon President and Chief Executive Officer | 2022 | $ | 945,000 | $ | 5,319,246 | $ | 1,499,986 | $ | 908,523 | $ | 29,465(4) | $ | 8,702,220 | ||||||||||||||||||||||||||||
| 2021 | $ | 963,173 | $ | 5,096,181 | $ | 1,499,979 | $ | 1,038,461 | $ | 27,600 | $ | 8,625,394 | |||||||||||||||||||||||||||||
| 2020 | $ | 937,211 | $ | 5,493,885 | $ | 1,374,979 | $ | 1,563,408 | $ | 28,721 | $ | 9,398,204 | |||||||||||||||||||||||||||||
William P. Burke Executive Vice President, Chief Financial Officer | 2022 | $ | 543,150 | $ | 2,329,703 | $ | 374,991 | $ | 333,419 | $ | 18,166(5) | $ | 3,599,429 | ||||||||||||||||||||||||||||
| 2021 | $ | 539,283 | $ | 1,104,204 | $ | 324,983 | $ | 370,005 | $ | 16,573 | $ | 2,355,048 | |||||||||||||||||||||||||||||
| 2020 | $ | 525,164 | $ | 1,198,495 | $ | 299,979 | $ | 557,045 | $ | 17,529 | $ | 2,598,212 | |||||||||||||||||||||||||||||
Michelle L. Basil Executive Vice President, General Counsel | 2022 | $ | 477,619 | $ | 2,241,162 | $ | 349,982 | $ | 293,193 | $ | 17,778(6) | $ | 3,379,734 | ||||||||||||||||||||||||||||
| 2021 | $ | 474,204 | $ | 1,019,211 | $ | 299,996 | $ | 325,364 | $ | 16,137 | $ | 2,134,912 | |||||||||||||||||||||||||||||
| 2020 | $ | 461,803 | $ | 1,298,603 | $ | 274,990 | $ | 489,838 | $ | 17,209 | $ | 2,542,443 | |||||||||||||||||||||||||||||
Josep L. Llorens Executive Vice President, Global Manufacturing and Supply Chain | 2022 | $ | 440,794 | $ | 1,109,106 | $ | 199,990 | $ | 255,645 | $ | 22,320(7) | $ | 2,027,855 | ||||||||||||||||||||||||||||
| 2021 | $ | 413,714 | $ | 594,371 | $ | 174,970 | $ | 267,632 | $ | 19,406 | $ | 1,470,093 | |||||||||||||||||||||||||||||
| 2020 | $ | 404,020 | $ | 599,247 | $ | 149,990 | $ | 402,921 | $ | 19,949 | $ | 1,576,127 | |||||||||||||||||||||||||||||
Stewart W. Strong President, Global Hospital | 2022 | $ | 410,615 | $ | 1,374,966 | $ | 187,485 | $ | 489,992 | $ | 17,419(8) | $ | 2,480,477 | ||||||||||||||||||||||||||||
| — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||
| — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||
(1)Salaries for fiscal 2022 listed above differ slightly from the fiscal 2022 base salaries discussed in the CD&A because of fiscal 2022 salary increases approved by the Compensation Committee for each Named Executive Officer that took effect in or after May 2022.
(2)Represents the aggregate grant date fair value for, in the case of Stock Awards, time-based RSUs and performance-based PSUs, and in the case of Option Awards, stock options, in each case granted in the respective fiscal years set forth above and calculated in accordance with FASB ASC Topic 718, Compensation - Stock Compensation. The grant date fair value of RSUs granted under our 2005 Long-Term Incentive Compensation Plan (the "2005 Plan") are calculated using the average of the high and low trading prices of the Company’s common stock on the grant date, and RSU's granted under our 2019 Long-Term Incentive Compensation Plan (the "2019 Plan") are calculated using the closing price of the Company's common stock on the grant date. PSU values were determined based on the expected performance at the time of grant. The Company uses the Monte Carlo model to estimate the probability of satisfying the performance criteria and the resulting fair value of PSU awards with market conditions. If the maximum level of performance is assumed for the PSUs awarded in fiscal 2022, the value of Mr. Simon’s fiscal 2022 PSU awards would be $7,638,586 instead of $3,819,293, Mr. Burke's would be $1,909,538 instead of $954,769, Ms. Basil’s would be $1,782,350 instead of $891,175, Mr. Llorens’ would be $1,018,362 instead of $509,181 and Mr. Strong's would be $1,625,096 instead of $812,548. The grant date fair value of options is estimated using the Black-Scholes option-pricing model based on the average of the high and low stock prices at the grant date for awards under the 2005 Plan, the closing price of the Company's common stock on the grant date for awards under the 2019 Plan, and the weighted average assumptions specific to the underlying options in each case. For a detailed description of the assumptions used to calculate the grant date fair value of our Stock Awards and Option Awards, see Note 17 "Capital Stock" to the Company's consolidated financial statements included in our Annual Report on Form 10-K for the fiscal year ended April 2, 2022. All RSU, PSU and stock option awards granted to Names Executive Officers in fiscal 2022 were made pursuant to the 2019 Plan.
(3)Represents cash payments under our annual short-term incentive compensation plans for fiscal years 2020, 2021 and 2022, as applicable.
(4)Represents (i) the Company's matching contributions under our 401(k) savings plan in the amount of $17,794 and (ii) the Company paid portion of supplemental long-term disability insurance premiums in the amount of $11,671.
(5)Represents (i) the Company's matching contributions under our 401(k) savings plan in the amount of $17,570, (ii) the Company paid portion of supplemental long-term disability insurance premiums, (iii) the Company paid wellness reimbursement available to all U.S. employees, and (iv) commuter benefits under a program available to all Boston-based employees.
(6)Represents (i) the Company's matching contributions under our 401(k) savings plan in the amount of $14,293 and (ii) the Company paid portion of supplemental long-term disability insurance premiums.
(7)Represents (i) the Company's matching contributions under our 401(k) savings plan in the amount of $17,961, (ii) the Company paid portion of supplemental long-term disability insurance premiums and (iii) the Company paid wellness reimbursement available to all U.S. employees.
(8)Represents (i) the Company's matching contributions under our 401(k) savings plan in the amount of $16,262 and (ii) the Company paid portion of supplemental long-term disability insurance premiums.
HAEMONETICS CORPORATION | 2022 Proxy Statement 39
GRANTS OF PLAN-BASED AWARDS DURING FISCAL 2022
The following table summarizes all plan-based award grants to each of the Named Executive Officers during fiscal year 2022.
| Name | Grant Date | Estimated Future Payouts Under Non-Equity incentive Plan Awards(1) | Estimated Future Payouts Under Equity Incentive Plan Awards(2) | All Other Stock Awards: Number of Shares of Stock or Units (#) | All Other Option Awards: Number of Securities Underlying Options(5) (#) | Exercise or Base Price of Option Awards ($/Sh) | Grant Date Fair Value of Stock Awards and Option Awards(6) ($) | |||||||||||||||||||||||||||||||||||||
Threshold ($) | Target ($) | Maximum ($) | Threshold (#) | Target (#) | Maximum (#) | |||||||||||||||||||||||||||||||||||||||
Christopher A. Simon | $ | 519,750 | $1,039,500 | $ | 2,079,000 | |||||||||||||||||||||||||||||||||||||||
| 5/18/2021 | 26,516 | 53,031 | 106,062 | 26,515(3) | 71,733 | $56.57 | $ | 6,819,233 | ||||||||||||||||||||||||||||||||||||
William P. Burke | $ | 190,743 | $381,487 | $ | 762,973 | |||||||||||||||||||||||||||||||||||||||
| 5/18/2021 | 6,629 | 13,257 | 26,514 | 6,628(3) | 17,933 | $56.57 | $ | 1,704,707 | ||||||||||||||||||||||||||||||||||||
| 5/18/2021 | 17,677(4) | $ | 999,988 | |||||||||||||||||||||||||||||||||||||||||
Michelle L. Basil | $ | 167,731 | $335,461 | $ | 670,922 | |||||||||||||||||||||||||||||||||||||||
| 5/18/2021 | 6,187 | 12,374 | 24,748 | 6,187(3) | 16,737 | $56.57 | $ | 1,591,156 | ||||||||||||||||||||||||||||||||||||
| 5/18/2021 | 17,677(4) | $ | 999,988 | |||||||||||||||||||||||||||||||||||||||||
Josep L. Llorens | $ | 146,250 | $292,500 | $ | 585,000 | |||||||||||||||||||||||||||||||||||||||
| 5/18/2021 | 3,535 | 7,070 | 14,140 | 3,535(3) | 9,564 | $56.57 | $ | $909,146 | ||||||||||||||||||||||||||||||||||||
| 5/18/2021 | 7,070(4) | $ | $399,950 | |||||||||||||||||||||||||||||||||||||||||
Stewart W. Strong | $ | 144,200 | $288,400 | $ | 576,800 | |||||||||||||||||||||||||||||||||||||||
| 5/18/2021 | 3,314 | 6,628 | 13,256 | 3,314(3) | 8,966 | $56.57 | $ | 852,307 | ||||||||||||||||||||||||||||||||||||
| 5/18/2021 | 6,628(4) | $ | 374,946 | |||||||||||||||||||||||||||||||||||||||||
| 7/13/2021 | 2,020 | 4,040 | 8,080 | $ | 335,199 | |||||||||||||||||||||||||||||||||||||||
(1)Represents the threshold, target and maximum annual cash incentive awards under our 2022 Bonus Plan. The threshold amount for each Named Executive Officer is 50% of target, as the minimum amount payable (subject to individual performance) if threshold performance is achieved. If the threshold is not achieved, the payment to the Named Executive Officers would be zero. The target amount is based upon achievement of the target performance measures listed in “2022 Bonus Plan Targets and Funding” in the CD&A beginning on page 30. The maximum amount represents 200% of target. The actual amounts earned by each Named Executive Officer are set forth under "2022 Bonus Plan Awards and Results" in the CD&A beginning on page 31.
(2)Represents the threshold, target and maximum award amounts for PSU awards issued to our Named Executive Officers during fiscal 2022. The PSU award amounts will be determined based on the Company’s three-year total shareholder return relative to the components of the S&P MidCap 400 Index. For more information see “Individual Fiscal 2022 Long-Term Incentive Awards” in the CD&A beginning on page 32.
(3)Represents RSUs that vest in annual increments of 25% beginning on the first anniversary of the date of grant.
(4)Represents RSUs that vest in annual increments of 50% beginning on the first anniversary of the date of grant. For more information see “Special Retention Awards” in the CD&A beginning on page 32.
(5)Represents option awards that vest in annual increments of 25% beginning on the first anniversary of the date of grant. The exercise price of option awards equals the closing price of the Company’s common stock on the grant date.
(6)Represents the aggregate grant date fair value for, in the case of Stock Awards, time-based RSUs and performance-based PSUs, and in the case of Options Awards, stock options, in each case calculated in accordance with FASB ASC Topic 718, Compensation - Stock Compensation. For a detailed description of the assumptions used to calculate the grant date fair value of our Stock Awards and Option Awards, see Note 17 “Capital Stock” to the Company's consolidated financial statements included in our Annual Report on Form 10-K for the fiscal year ended April 2, 2022.
40 HAEMONETICS CORPORATION | 2022 Proxy Statement
OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END
The table below reflects all outstanding equity awards made to each of the Named Executive Officers that were outstanding at the end of fiscal 2022. Market or payout values are based upon the closing price of $63.85, which was the closing price on the NYSE of our common stock on April 1, 2022 (the "Closing Price"), the last trading day of our common stock in fiscal 2022.
Option Awards(1) | Stock Awards | |||||||||||||||||||||||||||||||||||||||||||
| Name | Number of Securities Underlying Unexercised Options Exercisable (#) | Number of Securities Underlying Unexercised Options Unexercisable (#) | Option Exercise Price ($) | Option Expiration Date | Number of Shares or Units of Stock That Have Not Vested (#)(2) | Market Value of Shares or Units of Stock That Have Not Vested ($)(2) | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights that Have Not Vested (#)(3) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights that Have Not Vested ($)(3) | ||||||||||||||||||||||||||||||||||||
| Christopher A. Simon | 196,746 | — | (4) | $28.615 | 6/29/2023 | |||||||||||||||||||||||||||||||||||||||
| 104,516 | — | (5) | $41.64 | 6/6/2024 | ||||||||||||||||||||||||||||||||||||||||
| 30,332 | 10,111 | (6) | $93.52 | 6/11/2025 | ||||||||||||||||||||||||||||||||||||||||
| 24,485 | 24,486 | (7) | $98.025 | 5/14/2026 | ||||||||||||||||||||||||||||||||||||||||
| 12,276 | 36,829 | (8) | $103.37 | 5/18/2027 | ||||||||||||||||||||||||||||||||||||||||
| — | 71,733 | (9) | $56.57 | 5/18/2028 | ||||||||||||||||||||||||||||||||||||||||
| 2,841 | (6) | $ | 181,398 | |||||||||||||||||||||||||||||||||||||||||
| 7,014 | (7) | $ | 447,844 | |||||||||||||||||||||||||||||||||||||||||
| 10,883 | (8) | $ | 694,880 | |||||||||||||||||||||||||||||||||||||||||
| 26,515 | (9) | $ | 1,692,983 | |||||||||||||||||||||||||||||||||||||||||
| 28,054 | (3)(7) | $ | 1,791,248 | |||||||||||||||||||||||||||||||||||||||||
| 29,021 | (3)(8) | $ | 1,852,991 | |||||||||||||||||||||||||||||||||||||||||
| 53,031 | (3)(9) | $ | 3,386,029 | |||||||||||||||||||||||||||||||||||||||||
| William P. Burke | 6,958 | 2,320 | (6) | $93.52 | 6/11/2025 | |||||||||||||||||||||||||||||||||||||||
| 5,342 | 5,342 | (7) | $98.025 | 5/14/2026 | ||||||||||||||||||||||||||||||||||||||||
| 2,659 | 7,980 | (8) | $103.37 | 5/18/2027 | ||||||||||||||||||||||||||||||||||||||||
| — | 17,933 | (9) | $56.57 | 5/18/2028 | ||||||||||||||||||||||||||||||||||||||||
| 652 | (6) | $ | 41,630 | |||||||||||||||||||||||||||||||||||||||||
| 1,530 | (7) | $ | 97,691 | |||||||||||||||||||||||||||||||||||||||||
| 2,358 | (8) | $ | 150,558 | |||||||||||||||||||||||||||||||||||||||||
| 6,628 | (9) | $ | 423,198 | |||||||||||||||||||||||||||||||||||||||||
| 17,677 | (9)(13) | $ | 1,128,676 | |||||||||||||||||||||||||||||||||||||||||
| 6,120 | (3)(7) | $ | 390,762 | |||||||||||||||||||||||||||||||||||||||||
| 6,288 | (3)(8) | $ | 401,489 | |||||||||||||||||||||||||||||||||||||||||
| 13,257 | (3)(9) | $ | 846,459 | |||||||||||||||||||||||||||||||||||||||||
| Michelle L. Basil | 14,618 | — | (10) | $38.43 | 3/6/2024 | |||||||||||||||||||||||||||||||||||||||
| 18,444 | — | (5) | $41.64 | 6/6/2024 | ||||||||||||||||||||||||||||||||||||||||
| 7,137 | 2,379 | (6) | $93.52 | 6/11/2025 | ||||||||||||||||||||||||||||||||||||||||
| 4,897 | 4,897 | (7) | $98.025 | 5/14/2026 | ||||||||||||||||||||||||||||||||||||||||
| 2,455 | 7,366 | (8) | $103.37 | 5/18/2027 | ||||||||||||||||||||||||||||||||||||||||
| — | 16,737 | (9) | $56.57 | 5/18/2028 | ||||||||||||||||||||||||||||||||||||||||
| 669 | (6) | $ | 42,716 | |||||||||||||||||||||||||||||||||||||||||
| 1,403 | (7) | $ | 89,582 | |||||||||||||||||||||||||||||||||||||||||
| 811 | (11) | $ | 51,782 | |||||||||||||||||||||||||||||||||||||||||
| 2,177 | (8) | $ | 139,001 | |||||||||||||||||||||||||||||||||||||||||
| 6,187 | (9) | $ | 395,040 | |||||||||||||||||||||||||||||||||||||||||
| 17,677 | (9)(13) | $ | 1,128,676 | |||||||||||||||||||||||||||||||||||||||||
| 5,610 | (3)(7) | $ | 358,199 | |||||||||||||||||||||||||||||||||||||||||
| 5,804 | (3)(8) | $ | 370,585 | |||||||||||||||||||||||||||||||||||||||||
| 12,374 | (3)(9) | $ | 790,080 | |||||||||||||||||||||||||||||||||||||||||
| Josep L. Llorens | 3,535 | 1,179 | (12) | $111.84 | 9/4/2025 | |||||||||||||||||||||||||||||||||||||||
| 2,671 | 2,671 | (7) | $98.025 | 5/14/2026 | ||||||||||||||||||||||||||||||||||||||||
HAEMONETICS CORPORATION | 2022 Proxy Statement 41
Option Awards(1) | Stock Awards | |||||||||||||||||||||||||||||||||||||||||||
| Name | Number of Securities Underlying Unexercised Options Exercisable (#) | Number of Securities Underlying Unexercised Options Unexercisable (#) | Option Exercise Price ($) | Option Expiration Date | Number of Shares or Units of Stock That Have Not Vested (#)(2) | Market Value of Shares or Units of Stock That Have Not Vested ($)(2) | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights that Have Not Vested (#)(3) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights that Have Not Vested ($)(3) | ||||||||||||||||||||||||||||||||||||
| 1,432 | 4,296 | (8) | $103.37 | 5/18/2027 | ||||||||||||||||||||||||||||||||||||||||
| — | 9,564 | (9) | $56.57 | 5/18/2028 | ||||||||||||||||||||||||||||||||||||||||
| 336 | (12) | $ | 21,454 | |||||||||||||||||||||||||||||||||||||||||
| 765 | (7) | $ | 48,845 | |||||||||||||||||||||||||||||||||||||||||
| 1,269 | (8) | $ | 81,026 | |||||||||||||||||||||||||||||||||||||||||
| 3,535 | (9) | $ | 225,710 | |||||||||||||||||||||||||||||||||||||||||
| 7,070 | (9)(13) | $ | 451,420 | |||||||||||||||||||||||||||||||||||||||||
| 3,060 | (3)(7) | $ | 195,381 | |||||||||||||||||||||||||||||||||||||||||
| 3,385 | (3)(8) | $ | 216,132 | |||||||||||||||||||||||||||||||||||||||||
| 7,070 | (3)(9) | $ | 451,420 | |||||||||||||||||||||||||||||||||||||||||
| Stewart W. Strong | 2,179 | 2,179 | (11) | $123.37 | 10/22/2026 | |||||||||||||||||||||||||||||||||||||||
| 1,125 | 3,376 | (8) | $103.37 | 5/18/2027 | ||||||||||||||||||||||||||||||||||||||||
| — | 8,966 | (9) | $56.57 | 5/18/2028 | ||||||||||||||||||||||||||||||||||||||||
| 608 | (11) | $ | 38,821 | |||||||||||||||||||||||||||||||||||||||||
| 998 | (8) | $ | 63,722 | |||||||||||||||||||||||||||||||||||||||||
| 3,314 | (9) | $ | 211,599 | |||||||||||||||||||||||||||||||||||||||||
| 6,628 | (9)(13) | $ | 423,198 | |||||||||||||||||||||||||||||||||||||||||
| 2,431 | (3)(11) | $ | 155,219 | |||||||||||||||||||||||||||||||||||||||||
| 2,660 | (3)(8) | $ | 169,841 | |||||||||||||||||||||||||||||||||||||||||
| 6,628 | (3)(9) | $ | 423,198 | |||||||||||||||||||||||||||||||||||||||||
| 4,040 | (3)(14) | $ | 257,954 | |||||||||||||||||||||||||||||||||||||||||
(1)All stock option awards vest in annual increments of 25% beginning on the first anniversary of the date of grant.
(2)Represents unvested RSUs, the market value for which was determined by multiplying the Closing Price by the number of RSUs. Except where otherwise noted per footnote (13) below, each RSU vests in annual increments of 25% beginning on the first anniversary of the date of grant.
(3)Represents unvested PSUs, the market value for which was determined by multiplying the Closing Price by the number of PSUs assuming that PSU awards would vest at target award amounts. The PSU award amounts will be determined based on the Company's total shareholder return during a three-year performance period relative to a comparison group and vest, if at all, on the last day of the performance period. The last day of the performance period for grants made in May 2019 was May 13, 2022, for grants made in October 2019 is October 21, 2022, for grants made in May 2020 is May 17, 2023, and for grants made in May 2021 is May 17, 2024. The actual number of shares awarded under a PSU may range from 0% to a maximum of 200% of the target award depending upon the Company’s relative total shareholder return.
(4)Date of grant is June 29, 2016.
(5)Date of grant is June 6, 2017.
(6)Date of grant is June 11, 2018.
(7)Date of grant is May 14, 2019.
(8)Date of grant is May 18, 2020.
(9)Date of grant is May 18, 2021.
(10)Date of grant is March 6, 2017.
(11)Date of grant is October 22, 2019.
(12)Date of grant is September 4, 2018.
(13)RSU vests in annual increments of 50% beginning on the first anniversary of the date of grant.
(14)Date of grant is July 13, 2021.
42 HAEMONETICS CORPORATION | 2022 Proxy Statement
OPTION EXERCISES AND STOCK VESTED DURING FISCAL 2022
Option Awards | Stock Awards | ||||||||||||||||||||||
Name | Number of Shares Acquired on Exercise (#) | Value Realized on Exercise(1) ($) | Number of Shares Acquired on Vesting (#) | Value Realized on Vesting(2) ($) | |||||||||||||||||||
Christopher A. Simon | — | $ | — | 16,353 | $ | 919,188 | |||||||||||||||||
William P. Burke | 22,667 | $ | 511,235 | 3,598 | $ | 202,333 | |||||||||||||||||
Michelle L. Basil | — | $ | — | 4,000 | $ | 231,193 | |||||||||||||||||
| Josep L. Llorens | — | $ | — | 1,141 | $ | 66,839 | |||||||||||||||||
Stewart W. Strong | — | $ | — | 636 | $ | 40,593 | |||||||||||||||||
(1)Amounts reflect the difference between the exercise price of the option and the price of the Company’s common stock on the NYSE at the time of exercise.
(2)Amounts reflect the average of the high and low trading price of the Company’s common stock on the NYSE on the vesting date for awards under our 2005 Plan and the closing price of the Company's common stock on the NYSE on the vesting date for awards under our 2019 Plan.
HAEMONETICS CORPORATION | 2022 Proxy Statement 43
POTENTIAL PAYMENTS UPON TERMINATION OR CHANGE IN CONTROL
The following table describes the potential payments upon termination or a change in control for our Named Executive Officers. The material terms of our employment, change of control and severance agreements with our Named Executive Officers and the termination and change in control benefits under our equity awards are discussed above in the CD&A under the sub-heading “Employment, Change in Control and Severance Arrangements” beginning on page 35.
| Name | Cash Severance Payment | Continuation of Benefits | In-the-Money Value of Unvested Equity(1) | Other Benefits(2) | Total | |||||||||||||||||||||||||||
| Christopher A. Simon | ||||||||||||||||||||||||||||||||
Termination by the Company for Cause | $ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||||||||||||||
Voluntary Termination(3) | $ | — | $ | — | $ | 3,869,153 | $ | — | $ | 3,869,153 | ||||||||||||||||||||||
Death of Executive(4) | $ | — | $ | — | $ | 7,408,474 | $ | — | $ | 7,408,474 | ||||||||||||||||||||||
Disability of Executive(5) | $ | — | $ | — | $ | 6,779,232 | $ | — | $ | 6,779,232 | ||||||||||||||||||||||
Involuntary Termination Without Cause or Constructive Termination by Executive (6) | $ | 2,798,523 | $ | — | $ | — | $ | 15,000 | $ | 2,813,523 | ||||||||||||||||||||||
Involuntary Termination (Without Cause) or Termination by Executive for Good Reason following a Change in Control(7) | $ | 5,933,655 | $ | 5,310 | $ | 10,569,588 | $ | 15,000 | $ | 16,523,553 | ||||||||||||||||||||||
| William P. Burke | ||||||||||||||||||||||||||||||||
Termination by the Company for Cause | $ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||||||||||||||
| Voluntary Termination | $ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||||||||||||||
Death of Executive(4) | $ | — | $ | — | $ | 2,846,053 | $ | — | $ | 2,846,053 | ||||||||||||||||||||||
Disability of Executive(5) | $ | — | $ | — | $ | 2,706,732 | $ | — | $ | 2,706,732 | ||||||||||||||||||||||
Involuntary Termination Without Cause(6) | $ | 878,400 | $ | 17,726 | $ | — | $ | 15,000 | $ | 911,126 | ||||||||||||||||||||||
Involuntary Termination Without Cause or Termination by Executive for Good Reason following a Change in Control(7) | $ | 1,852,935 | $ | 38,992 | $ | 3,611,016 | $ | 15,000 | $ | 5,517,943 | ||||||||||||||||||||||
| Michelle L. Basil | ||||||||||||||||||||||||||||||||
Termination by the Company for Cause | $ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||||||||||||||
| Voluntary Termination | $ | — | $ | — | $ | — | $ | $ | — | |||||||||||||||||||||||
Death of Executive(4) | $ | — | $ | — | $ | 2,775,299 | $ | — | $ | 2,775,299 | ||||||||||||||||||||||
Disability of Executive(5) | $ | — | $ | — | $ | 2,643,002 | $ | — | $ | 2,643,002 | ||||||||||||||||||||||
Involuntary Termination Without Cause (6) | $ | 772,423 | $ | 9,363 | $ | — | $ | 15,000 | $ | 796,786 | ||||||||||||||||||||||
Involuntary Termination Without Cause or Termination by Executive for Good Reason following a Change in Control(7) | $ | 1,629,382 | $ | 22,266 | $ | 3,487,507 | $ | 15,000 | $ | 5,154,155 | ||||||||||||||||||||||
| Josep L. Llorens | ||||||||||||||||||||||||||||||||
Termination by the Company for Cause | $ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||||||||||||||
| Voluntary Termination | $ | — | $ | — | $ | — | $ | $ | — | |||||||||||||||||||||||
Death of Executive(4) | $ | — | $ | — | $ | 1,352,787 | $ | — | $ | 1,352,787 | ||||||||||||||||||||||
Disability of Executive(5) | $ | — | $ | — | $ | 1,282,488 | $ | — | $ | 1,282,488 | ||||||||||||||||||||||
Involuntary Termination Without Cause(6) | $ | 705,645 | $ | 17,726 | $ | — | $ | 15,000 | $ | 738,371 | ||||||||||||||||||||||
Involuntary Termination Without Cause or Termination by Executive for Good Reason following a Change in Control(7) | $ | 1,485,000 | $ | 38,992 | $ | 1,761,012 | $ | 15,000 | $ | 3,300,004 | ||||||||||||||||||||||
| Stewart W. Strong | ||||||||||||||||||||||||||||||||
Termination by the Company for Cause | $ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||||||||||||||
| Voluntary Termination | $ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||||||||||||||
Death of Executive(4) | $ | — | $ | — | $ | 1,220,631 | $ | — | $ | 1,220,631 | ||||||||||||||||||||||
Disability of Executive(5) | $ | — | $ | — | $ | 1,220,631 | $ | — | $ | 1,220,631 | ||||||||||||||||||||||
Involuntary Termination Without Cause(6) | $ | 901,992 | $ | 15,524 | $ | — | $ | 15,000 | $ | 932,516 | ||||||||||||||||||||||
Involuntary Termination Without Cause or Termination by Executive for Good Reason following a Change in Control(7) | $ | 1,400,800 | $ | 34,587 | $ | 1,808,824 | $ | 15,000 | $ | 3,259,211 | ||||||||||||||||||||||
(1)Based upon the price of $63.85, which was the closing price on the NYSE of our common stock on April 1, 2022, the last trading day of our common stock in fiscal 2022.
(2)Represents estimated payments for outplacement services pursuant to our executives' change in control and severance agreements.
(3)Employees that voluntarily retire from the Company after age 55 that have completed five consecutive years of service with the Company remain eligible to receive a pro rata portion of the target award amount under their PSU awards at the end of the performance period based on the period of time elapsed during the performance period and achievement of the relevant performance metrics. Mr. Simon was the only Named
44 HAEMONETICS CORPORATION | 2022 Proxy Statement
Executive Officer eligible for this retirement benefit as of April 2, 2022. None of our other Named Executive Officers were retirement eligible as of April 2, 2022 and accordingly would forfeit their PSUs upon voluntary termination.
(4)Payments and benefits are calculated assuming the death of the Named Executive Officer on April 2, 2022. In-the-Money-Value of Unvested Equity includes the value of (a) unvested option awards and RSU awards that accelerate upon the executive's death plus (b) the pro rata value of the target award amount under outstanding PSU awards based on the time elapsed during the performance period through April 2, 2022, assuming the PSU award vests at target award amounts based on the relevant performance metrics. The actual amount payable under these PSU awards can be determined and paid, if at all, only at the end of the performance period and may be more or less than the target performance levels based on the terms of the applicable PSU.
(5)Payments and benefits are calculated assuming the Named Executive Officer’s employment was terminated for a disability as defined under his or her respective equity award agreements. In-the-Money-Value of Unvested Equity includes the value of (a) unvested options and RSU awards that will continue to vest according to their original vesting schedule, and (b) the pro rata value of the target award amount under outstanding PSU awards based on the time elapsed during the performance period through April 2, 2022, assuming the PSU award vests at target award amounts. The actual amount payable under these PSU awards can be determined and paid, if at all, only at the end of the performance period and may be more or less than the target performance levels based on the terms of the applicable PSU.
(6)Payments and benefits calculated assuming the Named Executive Officer’s employment was terminated by the Company without cause or, solely in the case of Mr. Simon, upon Mr. Simon's resignation due to constructive termination (as defined in Mr. Simon's severance agreement), on April 2, 2022 and payable as a lump sum. As previously disclosed, Mr. Burke entered into an agreement dated November 8, 2021 that sets forth the terms of Mr. Burke's transition and retention through June 30, 2022. For more information see "Retention Agreement with William P. Burke" on page 34 of this Proxy Statement. As previously disclosed, Ms. Basil entered into an agreement in June 2022 that sets forth the terms of Ms. Basil’s retention and transition through October 31, 2022. For more information see “Retention Agreement with Michelle L. Basil” on page 34 of this Proxy Statement.
(7)Payments and benefits calculated assuming the Named Executive Officer’s employment was terminated by the Company without cause or by the Named Executive Officer for good reason on April 2, 2022 following a change in control and payable as a lump sum. Additionally, In-the-Money-Value of Unvested Equity assumes that PSU awards would vest at target award amounts. The actual amount payable under these awards can be determined only at the time of the change in control and may be more or less than the target performance levels based on the terms of the applicable PSU.
| CEO Pay Ratio | ||
As required by Item 402(u) of Regulation S-K, we are providing the following information about the relationship of the annual total compensation of our employees and the annual total compensation of our CEO, Mr. Simon. Our CEO pay ratio is a reasonable estimate calculated in a manner consistent with the SEC’s requirements. For fiscal 2022, our median employee annual total compensation was $45,323. Our CEO’s annual total compensation was $8,702,220. Accordingly, the ratio of our CEO’s annual total compensation to the median employee’s annual total compensation for our fiscal 2022 was 192 to 1.
To identify the median of the annual total compensation of all our employees, as well as to determine the annual total compensation of our median employee and our CEO, we used the following methodology, material assumptions, adjustments and estimates:
•We determined that, as of January 3, 2022 (the date chosen for identifying our median employee), our employee population consisted of 2,813 employees worldwide. This number included employees that joined the Company following our acquisition of Cardiva Medical in March 2021.
•We used a consistently applied compensation measure to identify our median employee by comparing the base salary and hourly wages actually paid in calendar year 2021 as reflected in our payroll records. To make them comparable, base salaries and wages for newly hired employees who had worked less than a year were annualized.
•We used all of our worldwide employees, excluding Mr. Simon, in our analysis, and used the currency exchange rate in effect on January 3, 2022 to convert all currencies to U.S. dollars for the comparison. We did not make any cost of living adjustments in identifying the median employee.
•After we identified our median employee, we combined the elements of such employee's compensation for the Company's fiscal year ended April 2, 2022 in accordance with the requirements of Item 402(c)(2)(x) of Regulation S-K.
•With respect to the annual total compensation of our CEO, we used the amount reported in the “Total” column of our fiscal 2022 Summary Compensation Table included in this Proxy Statement.
Because the SEC’s rules for identifying the median compensated employee and calculating the pay ratio based on that employee’s annual total compensation allow companies to adopt a variety of methodologies, to apply certain exclusions, and to make reasonable estimates and assumptions that reflect their compensation practices, the pay ratio reported by other companies in our peer group may not be comparable to the pay ratio reported above, as these other companies may have different employment and compensation practices and may utilize different methodologies, exclusions, estimates and assumptions in calculating their own pay ratios.
HAEMONETICS CORPORATION | 2022 Proxy Statement 45
| Hedging Policy | ||||||||||||||
Our Securities Trading Policy prohibits employees and directors of the Company from engaging in hedging or similarly speculative transactions with respect to the Company’s securities, including, without limitation, same day or short-term trading (i.e., day trading), short sales, and buying, selling or writing puts, calls or other derivatives denominated in Haemonetics securities.
46 HAEMONETICS CORPORATION | 2022 Proxy Statement
ITEM 3—RATIFICATION OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Audit Committee is directly responsible for the appointment, compensation, retention and oversight of the independent registered public accounting firm to audit the Company’s financial statements. The Audit Committee is appointing Ernst & Young LLP as our independent registered public accounting firm for the fiscal year ending April 1, 2023, and the Board is asking shareholders to ratify the selection of Ernst & Young LLP at the meeting. The members of the Audit Committee and the Board believe that the continued retention of Ernst & Young LLP to serve as the Company’s independent registered public accounting firm is in the best interests of the Company’s shareholders.
Representatives of Ernst & Young LLP are expected to be present at the meeting, will have the opportunity to make a statement if they desire to do so and are expected to be available to respond to appropriate questions.
The text of the resolution in respect of Item 3 is as follows:
RESOLVED, that the Company’s shareholders ratify the appointment of Ernst & Young LLP as the Company's independent registered public accounting firm for the fiscal year ending April 1, 2023.
þ | The Board unanimously recommends that you vote FOR the ratification of the appointment of Ernst & Young LLP as the Company’s independent registered public accounting firm for the fiscal year ending April 1, 2023. Approval of this proposal requires the affirmative vote of a majority of shares present, in person or represented by proxy, and voting on this proposal at the meeting. Abstentions and broker "non-votes" will not have any effect on this proposal. Management proxy holders will vote all duly submitted proxies FOR ratification unless instructed otherwise. | ||||
HAEMONETICS CORPORATION | 2022 Proxy Statement 47
| Audit Fees and Services | ||
INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM FEES
The following is a summary of the fees billed to us by Ernst & Young LLP for professional services rendered for the fiscal years ended April 2, 2022 and April 3, 2021:
Fiscal 2022 | Fiscal 2021 | ||||||||||
Audit Fees(1) | $3,031,000 | $4,082,500 | |||||||||
Audit-Related Fees(2) | $10,000 | $75,000 | |||||||||
Tax Fees(3) | $783,000 | $1,000,800 | |||||||||
All Other Fees(4) | $5,000 | $1,005,000 | |||||||||
Total | $3,829,000 | $6,163,300 | |||||||||
(1)Fiscal 2022 and fiscal 2021 audit fees consisted principally of fees billed for professional services rendered in connection with the audit of our consolidated financial statements, the audit of the effectiveness of our internal control over financial reporting, reviews of the interim consolidated financial statements included in our quarterly reports, international statutory audits, regulatory filings and consents and other services related to SEC filings, and accounting consultations which relate to the audited financial statements and are necessary to comply with U.S. generally accepted accounting principles.
(2)Fiscal 2022 and fiscal 2021 audit-related fees consisted principally of fees related to accounting consultations and due diligence procedures for acquisition (including post-closing integration) and divestiture transactions. Fiscal 2021 audit-related fees also included fees related to the audit of the Haemonetics Corporation Savings Plus Plan.
(3)Fiscal 2022 and fiscal 2021 tax fees consisted principally of fees paid for assistance with transfer pricing, tax compliance, reporting and planning. Fiscal 2022 and fiscal 2021 tax fees also included tax planning and due diligence procedures for acquisition and divestiture transactions.
(4)Fiscal 2022 and fiscal 2021 all other fees included aggregate fees billed for the license of technical accounting software. Fiscal 2021 all other fees also included consultation services in connection with the Company's completion of a convertible note financing.
AUDIT COMMITTEE POLICY FOR PRE-APPROVAL OF SERVICES
The Committee’s policy is to pre-approve all audit and permissible non-audit services provided by our independent registered public accounting firm. All audit and non-audit services performed by our independent registered public accounting firm during the fiscal year ended April 2, 2022 were pre-approved in accordance with this policy.
| Audit Committee Report | ||
NATURE OF THE REPORT
The material in this report is not “soliciting material,” is not deemed filed with the SEC and is not to be incorporated by reference in any filing of the Company under the Securities Act of 1933, as amended (the “Securities Act”), or the Securities Exchange Act of 1934, as amended (the “Exchange Act”), whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
AUDIT COMMITTEE REPORT
The Audit Committee (the “Committee”) is currently comprised of four directors, each of whom meets the applicable independence and experience requirements of the SEC and the NYSE, as determined by the Board. The Committee operates under a written charter adopted by the Board.
The primary responsibility of the Committee is to oversee the Company’s financial reporting process on behalf of the Board and to report the results of their activities to the Board regularly. While the Committee has the responsibilities and powers set forth in its charter, it is not the duty of the Committee to plan or conduct audits or to determine that the Company’s consolidated financial statements are complete and accurate and are in accordance with generally accepted accounting principles. Management is responsible for the preparation, presentation and integrity of the Company’s consolidated financial statements and for the appropriateness of the accounting principles and reporting policies that are used by the Company. The independent registered public accounting firm is responsible for auditing the Company’s consolidated financial statements and for reviewing the Company’s unaudited interim consolidated financial statements. In so doing, it is the responsibility of the Committee to maintain free and open communication between the Committee, the independent registered public accounting firm, internal auditors and management of
48 HAEMONETICS CORPORATION | 2022 Proxy Statement
the Company. The Committee is also directly responsible for the appointment, termination and compensation of the independent registered public accounting firm.
The Committee met eight times during fiscal 2022. The meetings were designed, among other things, to facilitate and encourage communication among the Committee, management, the internal audit function and Ernst & Young LLP. The Committee discussed with Ernst & Young LLP, the Company’s independent registered public accounting firm, the overall scope and plans for its audits and the Committee regularly met with Ernst & Young LLP without the presence of management. Ernst & Young LLP has unrestricted access to the Committee.
The Committee reviewed and discussed with management and Ernst & Young LLP the Company’s audited financial statements for the fiscal year ended April 2, 2022. Management represented to the Committee that the Company’s consolidated financial statements were prepared in accordance with generally accepted accounting principles. Discussions about the Company’s audited consolidated financial statements included the independent registered public accounting firm’s judgments about the overall quality of the statements, not just their technical compliance. The Committee focused on the accounting principles used, the reasonableness of significant judgments, management’s evaluation of the effectiveness of the Company’s internal controls and the clarity of disclosures in the Company’s financial statements. The Committee also discussed with Ernst & Young LLP the matters required to be discussed with audit committees under generally accepted auditing standards, including, among other things, the matters required to be discussed by Auditing Standard No. 1301 “Communications with Audit Committees” issued by the Public Company Accounting Oversight Board. Ernst & Young LLP provided the Committee with written disclosures and the letter required by the applicable requirements of the Public Company Accounting Oversight Board, “Independence Discussions with Audit Committees,” as currently in effect, and the Committee discussed with Ernst & Young LLP its independence from our Company.
When considering Ernst & Young LLP’s independence, the Committee considered whether its provision of services to our Company beyond those rendered in connection with its audit of our consolidated financial statements and review of our condensed consolidated financial statements included in our Quarterly Reports on Form 10-Q was compatible with Ernst & Young LLP maintaining their independence.
Based on the Committee’s review and discussion with management and Ernst & Young LLP and the Committee’s review of the representations of management and the report of Ernst & Young LLP to the Committee, the Committee has recommended to the Board that the audited financial statements be included in the Company’s Annual Report on Form 10-K for the fiscal year ended April 2, 2022.
AUDIT COMMITTEE
Charles J. Dockendorff, Chair
Catherine M. Burzik
Michael J. Coyle
Lloyd E. Johnson
Catherine M. Burzik
Michael J. Coyle
Lloyd E. Johnson
HAEMONETICS CORPORATION | 2022 Proxy Statement 49
SHARE OWNERSHIP INFORMATION
| Equity Compensation Plans | ||
The following table sets forth information as of April 2, 2022 with respect to the shares of common stock that may be issued under our three existing equity compensation plans: the 2005 Long-Term Incentive Plan, or 2005 Plan; the 2019 Long-Term Incentive Compensation Plan, or 2019 Plan; and the 2007 Employee Stock Purchase Plan, or ESPP.
Plan Category | Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights (a) | Weighted Average Exercise Price of Outstanding Options, Warrants and Rights (b) | Number of Securities Available for Future Issuance (Excluding Securities Reflected in Column (a) (c) | |||||||||||
Equity Compensation Plans approved by security holders | 1,726,120(1) | $ | 66.87(2) | 5,452,162(3) | ||||||||||
Equity compensation plans not approved by security holders | — | — | — | |||||||||||
Total | 1,726,120 | $ | 66.87 | 5,452,162 | ||||||||||
(1)Includes 386,536 shares issuable upon the vesting of RSUs, 282,946 shares issuable upon the vesting of PSUs and 1,056,638 options to purchase shares of the Company’s common stock. PSUs have been included at their target value. Amounts in columns (a) and (b) include outstanding awards under both our 2005 Plan and 2019 Plan. On July 25, 2019 (the "Effective Date"), the Company's shareholders approved the 2019 Plan, the successor to the 2005 Plan. Upon the Effective Date, no further awards were granted under the 2005 Plan; however, each outstanding award under the 2005 Plan will remain outstanding under that plan and continue to be governed under its terms and any applicable award agreement.
(2)Represents the weighted average exercise price per share of the Company’s non-qualified stock options outstanding at April 2, 2022. The weighted average exercise price does not take into account the shares issuable upon vesting of outstanding RSUs and PSUs, which have no exercise price.
(3)Represents 3,928,160 shares available for future issuance under the 2019 Plan and 1,524,002 shares available for purchase under the ESPP. Issuance of RSUs and PSUs reduce the number of shares available for issuance at a ratio of 2.76 shares to 1 under the 2019 Plan and shares subject to an option reduce the number of shares available at a ratio of 1 to 1 under the 2019 Plan. Additionally, shares of common stock subject to outstanding RSU, PSU and option grants under the 2005 Plan as of the Effective Date that terminate, expire or are canceled, forfeited, exchanged or surrendered on or after the Effective Date, without having been exercised, vested or paid under the 2005 Plan are added to the share reserve under the 2019 Plan at the rates described in the preceding sentence. RSUs and PSUs have reduced the number of securities available for future issuance based on their maximum issuance value of by 1,066,839 and 1,561,861, respectively. During the purchase period ended April 30, 2022, 57,106 shares of common stock of the Company were subject to purchase pursuant to the ESPP.
For a detailed description of the Company’s equity compensation plans and the assumptions used to calculate the number of unvested PSUs see Note 17 “Capital Stock” to the Company's consolidated financial statements included in our Annual Report on Form 10-K for the fiscal year ended April 2, 2022.
50 HAEMONETICS CORPORATION | 2022 Proxy Statement
| Security Ownership of Certain Beneficial Owners, Directors, and Management | ||
The following table sets forth certain information with respect to beneficial ownership of the Company’s common stock by: (i) each person known by the Company to own beneficially more than five percent of the Company’s common stock; (ii) each of the Company’s directors and nominees; (iii) each of the executive officers named in the Summary Compensation Table in this Proxy Statement; and (iv) all directors and executive officers as a group. The information is presented as of June 11, 2022.
Name of Beneficial Owner | Title of Class | Amount and Nature of Beneficial Ownership(1) | Percent of Class(2) | ||||||||
Greater than 5% Beneficial Owners | |||||||||||
BlackRock, Inc.(3) 55 East 52nd Street New York, New York 10055 | Common Stock | 6,692,346 | 13.05% | ||||||||
Wellington Management Group LLP(4) Wellington Group Holdings LLP Wellington Investment Advisors Holdings LLP Wellington Management Company LLP c/o Wellington Management Company LLP 280 Congress Street Boston, MA 02210 | Common Stock | 5,537,538 | 10.79% | ||||||||
Capital Research Global Investors(5) 333 South Hope Street, 55th Floor Los Angeles, CA 90071 | Common Stock | 5,452,006 | 10.63% | ||||||||
The Vanguard Group(6) 100 Vanguard Blvd. Malvern, Pennsylvania 19355 | Common Stock | 4,880,936 | 9.51% | ||||||||
Neuberger Berman Group LLC(7) Neuberger Berman Investment Advisers LLC 1290 Avenue of the Americas New York, NY 10104 | Common Stock | 3,876,521 | 7.56% | ||||||||
Named Executive Officers | |||||||||||
Christopher A. Simon(8) | Common Stock | 579,100 | 1.12% | ||||||||
William P. Burke(8) | Common Stock | 37,710 | * | ||||||||
Michelle L. Basil(8) | Common Stock | 77,409 | * | ||||||||
Josep L. Llorens(8) | Common Stock | 18,039 | * | ||||||||
Stewart W. Strong(8) | Common Stock | 11,230 | * | ||||||||
Non-Employee Directors | |||||||||||
Robert E. Abernathy(8) | Common Stock | 15,125 | * | ||||||||
Catherine M. Burzik(8) | Common Stock | 16,815 | * | ||||||||
Michael J. Coyle(8) | Common Stock | 5,538 | * | ||||||||
Charles J. Dockendorff(8) | Common Stock | 25,400 | * | ||||||||
Lloyd E. Johnson(8) | Common Stock | 3,077 | * | ||||||||
Mark W. Kroll(8) | Common Stock | 21,517 | * | ||||||||
Claire Pomeroy(8) | Common Stock | 7,016 | * | ||||||||
Ellen M. Zane(8) | Common Stock | 9,517 | * | ||||||||
All executive officers and directors as a group (16 persons)(9) | Common Stock | 849,779 | 1.65% | ||||||||
* Less than 1%
(1)The persons named in the table have, to our knowledge, sole voting and investment power with respect to all shares shown as beneficially owned by them, except as noted in the footnotes below.
HAEMONETICS CORPORATION | 2022 Proxy Statement 51
(2)Applicable percentage ownership as of June 11, 2022 is based upon 51,299,384 shares of our common stock outstanding as of the record date. Beneficial ownership is determined in accordance with the rules and regulations of the SEC and includes voting and investment power with respect to shares. Shares of our common stock subject to options currently exercisable or exercisable within 60 days after June 11, 2022 and RSUs and PSUs that vest within 60 days after June 11, 2022 are deemed outstanding for computing the percentage ownership of the person holding such options, RSUs and PSUs, but are not deemed outstanding for computing the percentage ownership of any other person.
(3)Amount and nature of ownership listed is based solely upon information contained in a Schedule 13G/A filed with the SEC by BlackRock, Inc. on January 27, 2022. The Schedule 13G/A indicates that, as of December 31, 2021, BlackRock, Inc. had sole voting power over 6,451,408 shares, sole dispositive power over 6,692,346 shares and shared voting and dispositive power over 0 shares.
(4)Amount and nature of ownership listed is based solely upon information contained in a Schedule 13G/A filed with the SEC by the Wellington Management Group LLP, Wellington Group Holdings LLP, Wellington Investment Advisors Holdings LLP and Wellington Management Company LLP on February 10, 2022. The Schedule 13G/A indicates that, as of January 31, 2022, each reporting person had sole voting power and sole dispositive power over 0 shares, that Wellington Management Group LLP, Wellington Group Holdings LLP and Wellington Investment Advisors Holdings LLP each had shared voting power over 4,965,826 shares and shared dispositive power over 5,537,538 shares, and that Wellington Management Company LLP had shared voting power over 4,842,641 shares and shared dispositive power over 5,292,644 shares.
(5)Amount and nature of ownership listed is based solely upon information contained in a Schedule 13G filed with the SEC by Capital Research Global Investors on April 8, 2022. The Schedule 13G indicates that, as of March 31, 2022, Capital Research Global Investors had sole voting and dispositive power over 5,452,006 shares and shared voting and dispositive power over 0 shares.
(6)Amount and nature of ownership listed is based solely upon information contained in a Schedule 13G/A filed with the SEC by the Vanguard Group on February 10, 2022. The Schedule 13G/A indicates that, as of December 31, 2021, the Vanguard Group had sole voting power over 0 shares, shared voting power over 78,935 shares, sole dispositive power over 4,757,357 shares and shared dispositive power over 123,579 shares.
(7)Amount and nature of ownership listed is based solely upon information contained in a Schedule 13G/A filed with the SEC by Neuberger Berman Group LLC and Neuberger Berman Investment Advisers LLC on February 14, 2022. The Schedule 13G/A indicates that, as of December 31, 2021, each reporting person had sole voting power over 0 shares, shared voting power over 3,730,003 shares, sole dispositive power over 0 shares and shared dispositive power over 3,876,521 shares.
(8)Includes shares that may be acquired upon the exercise of options exercisable within 60 days of June 11, 2022, unvested RSUs vesting within 60 days of June 11, 2022 and, in the case of our Named Executive Officers, unvested PSUs vesting within 60 days of June 11, 2022 as follows:
Name of Beneficial Owner | Stock Options Exercisable Within 60 Days of June 11, 2022 | Unvested RSUs Exercisable Within 60 Days of June 11, 2022 | Unvested PSUs Exercisable Within 60 Days of June 11, 2022 | ||||||||
Christopher Simon | 420,918 | — | — (10) | ||||||||
William P. Burke | 27,093 | — | — (10) | ||||||||
Michelle L. Basil | 59,017 | — | — (10) | ||||||||
Josep L. Llorens | 12,796 | — | — (10) | ||||||||
Stewart W. Strong | 6,670 | — | — | ||||||||
Robert Abernathy | — | 3,077 | N/A | ||||||||
Catherine M. Burzik | — | 3,077 | N/A | ||||||||
Michael J. Coyle | — | 3,077 | N/A | ||||||||
Charles J. Dockendorff | — | 3,077 | N/A | ||||||||
Lloyd E. Johnson | — | 3,077 | N/A | ||||||||
Mark W. Kroll | — | 3,077 | N/A | ||||||||
Claire Pomeroy | — | 3,077 | N/A | ||||||||
Ellen M. Zane | — | 3,077 | N/A | ||||||||
(9)Includes for three Executive Officers not specifically named in the table, an aggregate of 22,286 shares beneficially owned, including 11,508 shares of common stock issuable upon the exercise of options presently exercisable or exercisable within 60 days of June 11, 2022 and 140 RSUs vesting within 60 days of June 11, 2022.
(10)There was no payout under PSUs granted on May 14, 2019 with performance-based objectives tied to the Company's rTSR relative to the components of the S&P MidCap 400 index during the three-year performance period from May 14, 2019 to May 13, 2022.
| Delinquent Section 16(a) Reports | ||
Section 16(a) of the Exchange Act requires the Company’s directors, officers and persons who own more than 10% of the Company’s common stock to file with the SEC reports concerning their ownership of the Company’s common stock and changes in such ownership. To our knowledge, based upon a review of copies of reports filed with the SEC with respect to the fiscal year ended April 2, 2022 and written representations by our directors and officers that no other reports were required with respect to their transactions, during the fiscal year ended April 2, 2022, all reports required to be filed under Section 16(a) by our directors and officers and persons who were beneficial owners of more than 10% of our common stock were timely filed.
52 HAEMONETICS CORPORATION | 2022 Proxy Statement
GENERAL INFORMATION ABOUT THE MEETING AND VOTING
| Why am I receiving these materials? | ||
The Company is making this Proxy Statement, the Company’s 2022 Annual Report to Shareholders and other meeting materials available to you on the Internet or, upon your request, sending printed or electronic versions to you (by mail or email, respectively) because the Board is soliciting your proxy to vote at the 2022 Annual Meeting of Shareholders to be held on August 5, 2022 at 8:00 A.M., Eastern Time, at the Company’s headquarters located at 125 Summer Street, Boston, Massachusetts 02110, and at any adjournment(s) or postponements thereof. You are invited to attend the meeting and are requested to vote on the proposals described in this Proxy Statement.
Under rules adopted by the SEC, we are furnishing proxy materials to our shareholders primarily via the Internet, instead of mailing printed copies of those materials to each shareholder. On or about June 20, 2022, we will mail to our shareholders of record (other than those who previously requested electronic or paper delivery on an ongoing basis) a Shareholder Meeting Notice and Important Notice Regarding the Availability of Proxy Materials (the “Notice”) containing instructions on how to access our proxy materials, including our Proxy Statement and 2022 Annual Report to Shareholders. All shareholders will have the ability to access our proxy materials on the website referred to in the Notice or request a printed set of the proxy materials. Instructions on how to access our proxy materials on the Internet or to request printed versions are provided in the Notice. The Notice also instructs you on how to access your proxy card to vote through the Internet or by telephone. In addition, shareholders may request to receive proxy materials in printed form by mail or electronically by email on an ongoing basis. If you have previously elected to receive our proxy materials electronically, you will continue to receive these materials via email until you elect otherwise.
| What is the purpose of the meeting? | ||
At the 2022 Annual Meeting of Shareholders, shareholders will vote upon matters that are summarized in the formal meeting notice. This Proxy Statement contains important information for you to consider when deciding how to vote on the matters before the meeting. Please read this Proxy Statement, the 2022 Annual Report to Shareholders and the other meeting materials carefully.
| Who can vote? | ||
Our Board has fixed the close of business on June 2, 2022 as the record date. Accordingly, only holders of record of our common stock, $0.01 par value per share, as of the close of business on the record date will be entitled to notice of, and to vote at, the 2022 Annual Meeting of Shareholders or any adjournment(s) or postponement(s) thereof. As of the record date, an aggregate of 51,299,384 shares of our common stock were issued and outstanding, held by 111 holders of record. The holders of our common stock are entitled to one vote per share on any proposal presented at the meeting.
| What items am I voting on? | ||
Shareholders will vote on the following items at the meeting:
1.To elect the nine director nominees named in this Proxy Statement to one-year terms expiring in 2023 (Item 1);
2.To approve, on an advisory basis, the compensation of our Named Executive Officers (Item 2);
3.To ratify the appointment of Ernst & Young LLP as our independent registered public accounting firm for the fiscal year ending April 1, 2023 (Item 3); and
4.To transact such other business as may properly come before the meeting.
HAEMONETICS CORPORATION | 2022 Proxy Statement 53
| What are the recommendations of the Board? | ||
Our Board recommends that you vote your shares:
•FOR each of the nominees for director (Item 1);
•FOR the approval of the advisory vote to approve the compensation of our Named Executive Officers (Item 2); and
•FOR the ratification of the appointment of Ernst & Young LLP as our independent registered public accounting firm for the fiscal year ending April 1, 2023 (Item 3).
| How do I vote my shares? | ||
You may vote in person or by proxy at the 2022 Annual Meeting of Shareholders. Your execution of a proxy will not in any way affect your right to attend the meeting and vote in person.
If you are a shareholder of record (i.e., if you hold shares that are directly registered in your own name), there are four ways to vote:
•Via the Internet. You may vote by proxy via the Internet by following the instructions provided in the Notice.
•By Telephone. If you requested printed copies of proxy materials by mail, you may vote by proxy via telephone by calling the toll free number found on the proxy card.
•By Mail. If you requested printed copies of proxy materials by mail, you may vote by proxy via mail by filling out the proxy card (you must be sure to complete, sign and date the proxy card) and returning it in the envelope provided.
•In Person. You may vote in person at the 2022 Annual Meeting of Shareholders. We will provide you with a ballot when you arrive. Shareholders who plan to attend the meeting must present valid photo identification. Shareholders of record will be verified against an official list available at the registration area. We reserve the right to deny admittance to anyone who cannot show valid identification or sufficient proof of share ownership as of the record date.
If your shares are held in the name of a bank, broker or other holder of record, which is known as being held in “street name,” you will receive separate voting instructions with your proxy materials. If you hold your shares in street name, your ability to vote by Internet or by telephone depends on the voting process of the bank, broker or other holder of record that holds your shares. Although most banks, brokers and other holders of record also offer Internet and telephone voting, availability and specific procedures will depend on their voting arrangements. Please follow their directions carefully. If you want to vote shares that you hold in street name at the meeting, you must request a legal proxy from the bank, broker, or other holder of record that holds your shares and present that proxy, along with valid photo identification and sufficient proof of share ownership as of the record date, at the meeting. We reserve the right to deny admittance to anyone who cannot show valid identification or sufficient proof of share ownership as of the record date.
Even if you plan to attend the 2022 Annual Meeting of Shareholders, we encourage you to vote in advance using one of the voting methods described above so that your vote will be counted if you later decide not to attend the meeting. Directions to the meeting may be obtained by contacting Investor Relations at (781) 848-7100. You may also contact us in writing at the following address: Haemonetics Corporation, 125 Summer Street, Boston, Massachusetts 02110, Attention: Investor Relations.
| Can I change my vote after I have voted? | ||
You may revoke your proxy and change your vote at any time before the final vote at the 2022 Annual Meeting of Shareholders by: (1) filing with our Corporate Secretary a written notice of revocation, (2) executing a later dated proxy relating to the same shares and delivering it to our Corporate Secretary or (3) attending the meeting and voting in person (although attendance at the meeting will not in and of itself constitute a revocation of a proxy). If your shares are held in street name, you should contact your bank, broker or other nominee to revoke your proxy or, if you have obtained a legal proxy from your bank, broker or other nominee giving you the right to vote your shares at the meeting, you may change your vote by attending the meeting and voting in person. Any written notice of revocation or subsequent proxy should be sent to the attention of our Corporate Secretary, Haemonetics Corporation, 125 Summer Street, Boston, Massachusetts, 02110 at or before the final vote at the meeting.
54 HAEMONETICS CORPORATION | 2022 Proxy Statement
| How will I learn if the meeting is changed to a virtual meeting? | ||
We currently intend to hold our 2022 Annual Meeting of Shareholders in person. However, we continue to carefully monitor COVID-19 developments and related guidance issued by Massachusetts regulatory agencies and relevant health organizations. Should we determine that alternative arrangements for the meeting may be advisable or required, such as changing the date, time, location or format of the meeting (including holding a virtual meeting, subject to local law requirements), we will announce our decision by press release and through our filings with the SEC and post additional information on our investor relations website at www.haemonetics.com.
| What vote is required to approve each proposal and how are votes counted? | ||
Election of directors (Item 1) will be determined by a plurality of the votes cast by shareholders entitled to vote at the meeting. Abstentions and broker non-votes will not have any effect on this proposal. Accordingly, the nominees receiving the highest number of “for” votes at the meeting will be elected as directors. However, under a policy adopted by the Board, in an uncontested election, any nominee for director who does not receive the favorable vote of at least a majority of the votes cast with respect to such director is required to tender his or her resignation to the Board, which will consider whether to accept the resignation. This is an uncontested election of directors because the number of nominees for director does not exceed the number of directors to be elected. Accordingly, if a nominee fails to receive the favorable vote of at least a majority of the votes cast with respect to such nominee, then within 90 days after the certification of the election results, the remaining members of our Board shall, through a process managed by the Governance and Compliance Committee and excluding the director nominee in question, determine whether to accept such nominee's resignation. The determination of the Board will be publicly disclosed by the filing of appropriate disclosure with the SEC.
Approval of Item 2 and Item 3 require the affirmative vote of a majority of shares present, in person or represented by proxy, and voting on each such matter at the meeting. Abstentions and broker “non-votes” are included in the number of shares present or represented for purposes of a quorum but are disregarded for purposes of determining whether any of the proposals have been approved.
Banks, brokers or other holders of record may vote shares held for a customer in street name on matters that are considered to be “routine” even if they have not received instructions from their customer. A broker “non-vote” occurs when a bank, broker, or other holder of record has not received voting instructions from a customer and cannot vote the customer’s shares because the matter is not considered routine.
One of the proposals before the meeting this year is deemed a "routine" matter, namely the ratification of the appointment of Ernst & Young LLP as our independent registered public accounting firm for the fiscal year ending April 1, 2023 (Item 3), which means that if your shares are held in street name your bank, broker, or other nominee can vote your shares on that proposal if you do not provide timely instructions for voting your shares. The election of directors (Item 1) and the non-binding advisory vote to approve executive compensation (Item 2) are not considered “routine” matters. As a result, if you do not instruct your bank, broker or nominee how to vote with respect to those matters, your bank, broker or nominee may not vote on those proposals and a broker "non-vote" will occur.
| Where can I find the results of the meeting? | ||
The preliminary voting results will be announced at the 2022 Annual Meeting of Shareholders. The final voting results will be tallied by the inspector of election and published in a Current Report on Form 8-K, which we are required to file with the SEC within four business days following the meeting.
HAEMONETICS CORPORATION | 2022 Proxy Statement 55
| How do I request to receive proxy materials electronically or in hard copy? | ||
The Notice will provide you with instructions regarding the method of delivery for future proxy materials. Choosing to access our proxy materials via the Internet or to receive future proxy materials by email will reduce the impact of our meetings on the environment as well as decrease the cost of printing and mailing documents to you. If you choose to receive future proxy materials by email, you will receive an email next year with instructions containing a link to those materials and a link to the proxy voting site. Your election to receive proxy materials by email will remain in effect until you terminate it. If you hold your stock through a bank, broker or other holder of record and you would like to receive future proxy materials electronically, please refer to the information provided by that entity for instructions on how to elect this option.
If you prefer to receive paper copies of the proxy materials, you can still do so. You may request a paper copy by following the instructions provided in the Notice. The Notice also provides you with instructions on how to request paper copies of the proxy materials on an ongoing basis. There is no charge to receive the materials by mail. You may request printed copies of the materials until one year after the date of the meeting. If you have previously elected to receive printed proxy materials, you will continue to receive these materials in paper format until you elect otherwise.
56 HAEMONETICS CORPORATION | 2022 Proxy Statement
ADDITIONAL INFORMATION
| Solicitation of Proxies | ||
The Company will bear the costs of soliciting proxies, including the reimbursement to record holders of their expenses in forwarding proxy materials to beneficial owners. Directors, officers and regular employees of the Company, without extra compensation, may solicit proxies personally or by mail, telephone, email, fax, telex, telegraph or special letter.
The Company has engaged MacKenzie Partners, Inc. to assist in the solicitation of proxies and provide related advice and informational support, for a services fee, plus customary disbursements, which are not expected to exceed $20,000 in total. The Company will bear these costs.
| Shareholder Proposals for Next Year's Annual Meeting | ||
Pursuant to Rule 14a-8 promulgated under the Exchange Act, some shareholder proposals may be eligible for inclusion in the proxy statement for the Company’s 2023 Annual Meeting of Shareholders. In order for a shareholder proposal to be considered timely for inclusion in the Company’s proxy statement for the 2023 Annual Meeting of Shareholders, the written proposal must be received by the Corporate Secretary at the Company’s office, Haemonetics Corporation, 125 Summer Street, Boston, Massachusetts 02110, no later than February 17, 2023.
Under our By-Laws, if a shareholder wishes to present a proposal or wants to nominate candidates for election as directors at our 2023 Annual Meeting of Shareholders, such shareholder must give written notice to the Corporate Secretary of the Company at the address noted above. The Corporate Secretary must receive such notice not earlier than 120 day prior to the one year anniversary of the date of the 2022 Annual Meeting of Shareholders and not less than 90 days prior to the one year anniversary of the date of the 2022 Annual Meeting of Shareholders; provided, however, that in the event that the 2023 Annual Meeting of Shareholders is called for a date that is advanced by more than 30 days or delayed by more than 60 days after such anniversary date, notice must be received by the Corporate Secretary not earlier than 120 days prior to the 2023 Annual Meeting and not later than the later of 90 days prior to such meeting or the 10th day following the day on which public announcement of the 2023 Annual Meeting was made. Our By-Laws also specify requirements as to the form and content of a shareholder’s notice. The Company will not entertain any proposals or nominations that do not meet those requirements. If the shareholder does not also comply with the requirements of Rule 14a-4(c)(2) under the Exchange Act, the Company may exercise discretionary voting authority under proxies it solicits to vote in accordance with its best judgment on any such shareholder proposal. In addition to satisfying the foregoing requirements under our By-Laws, to comply with the universal proxy rules (effective for annual meetings after August 31, 2022), shareholders who intend to solicit proxies in support of director nominees other than the Company’s nominees must provide notice that sets forth the information required by Rule 14a‐19 under the Exchange Act no later than June 6, 2023.
| Other Matters | ||
Management knows of no matters which may properly be and are likely to be brought before the meeting other than the matters discussed herein. However, if any other matters properly come before the meeting, the proxy holders named in the enclosed proxy card will vote in accordance with their best judgment.
| Incorporation by Reference | ||
To the extent that this proxy statement has been or will be specifically incorporated by reference into any filing made by us under the Securities Act of 1933, as amended, or the Exchange Act, the sections of the proxy statement entitled “Compensation Committee Report” and “Audit Committee Report” shall not be deemed to be so incorporated, unless specifically provided in any such filing.
| Financial Matters and Form 10-K | ||
WE WILL PROVIDE EACH BENEFICIAL OWNER OF OUR SECURITIES WITH A COPY OF OUR ANNUAL REPORT ON FORM 10-K, INCLUDING THE FINANCIAL STATEMENTS AND SCHEDULES THERETO, REQUIRED TO BE FILED WITH THE SECURITIES AND EXCHANGE COMMISSION FOR OUR MOST RECENT FISCAL YEAR, WITHOUT CHARGE, UPON RECEIPT OF A WRITTEN REQUEST FROM SUCH PERSON. SUCH REQUEST SHOULD BE SENT TO INVESTOR RELATIONS, HAEMONETICS CORPORATION, 125 SUMMER STREET, BOSTON, MASSACHUSETTS 02110. ALTERNATIVELY, A BENEFICIAL OWNER MAY ACCESS THE COMPANY’S ANNUAL REPORT ON FORM 10-K ON THE INVESTOR RELATIONS PAGE ON THE COMPANY'S WEBSITE: WWW.HAEMONETICS.COM.
HAEMONETICS CORPORATION | 2022 Proxy Statement 57
| Delivery of Documents to Shareholders Sharing an Address | ||
Some banks, brokers and other nominee record holders may be participating in the practice of “householding” the Notice and, if applicable, hardcopies of the proxy materials. This means that only one copy of the Notice or hardcopy proxy materials may have been sent to multiple shareholders in your household. We will promptly deliver a separate copy of the Notice or hardcopy proxy materials to you upon request if you call or write to Investor Relations, Haemonetics Corporation, 125 Summer Street, Boston, Massachusetts 02110, (781) 848-7100. If you want to receive separate copies of our proxy materials in the future, or if you are receiving multiple copies and would like to receive only one copy for your household, you should contact your bank, broker, or other nominee record holder, or you may contact us at the above address and phone number.
By Order of the Board of Directors
Michelle L. Basil
Corporate Secretary
Boston, Massachusetts
June 17, 2022
June 17, 2022
IMPORTANT NOTICE REGARDING AVAILABILITY OF PROXY MATERIALS FOR THE ANNUAL MEETING OF SHAREHOLDERS TO BE HELD ON AUGUST 5, 2022: This Proxy Statement and the Company’s 2022 Annual Report to Shareholders are available at www.envisionreports.com/HAE. | ||
58 HAEMONETICS CORPORATION | 2022 Proxy Statement
APPENDIX A
| Non-GAAP Financial Reconciliations | ||
The Company reports its financial results in accordance with accounting principles generally accepted in the United States (“GAAP”). This Proxy Statement contains financial measures that are considered “non-GAAP” financial measures under applicable SEC rules and regulations. Management uses non-GAAP measures to monitor the financial performance of the business, make informed business decisions, establish budgets and forecast future results. These non-GAAP financial measures should be considered supplemental to, and not a substitute for, our reported financial results prepared in accordance with U.S. GAAP. In this Proxy Statement, supplemental non-GAAP measures have been provided to assist shareholders in evaluating the performance of the Company’s core operations and provide a baseline for analyzing trends in our underlying businesses. We strongly encourage shareholders to review our financial statements and publicly filed reports in their entirety and not rely on any single financial measure.
When used in this Proxy Statement, organic revenue growth excludes the impact of currency fluctuation, strategic exits of product lines, acquisitions and divestitures and the impact of the 53rd week in fiscal 2021. Adjusted operating income, adjusted net income and adjusted earnings per diluted share exclude restructuring and restructuring related costs, deal amortization expenses, asset impairments, accelerated device depreciation and related costs, costs related to compliance with the European Union Medical Device Regulation and In Vitro Diagnostic Regulation, integration and transaction costs, gains and losses on dispositions, certain tax settlements and unusual or infrequent and material litigation-related charges. Adjusted net income and adjusted earnings per diluted share also exclude the tax impact of these items. Adjusted operating margin equals (i) adjusted operating income divided by (ii) revenue determined in accordance with GAAP. Free cash flow before restructuring and restructuring related costs is defined as cash provided by operating activities less capital expenditures, net of the proceeds from the sale of property, plant and equipment. Because non-GAAP financial measures are not standardized, it may not be possible to compare these financial measures to similarly titled measures used by other companies.
For additional details regarding the reconciliation of the GAAP and non-GAAP financial measures below, see the Company’s Current Report on Form 8-K filed with the SEC on May 10, 2022. This information is also available on the Investor Relations page on the Company’s website at www.haemonetics.com.
HAEMONETICS CORPORATION | 2022 Proxy Statement 59
RECONCILIATION OF REPORTED REVENUE GROWTH TO ORGANIC REVENUE GROWTH FOR FISCAL 2022
(Data in thousands)
Year Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
4/2/2022 | 4/3/2021 | Reported Growth | Currency Impact | Acquisitions and Divestitures(1) | Other Strategic Exits(2) | 53rd Week | Organic Growth | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(unaudited) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Revenues by business unit | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Plasma | $ | 351,347 | $ | 332,236 | 5.8 | % | 0.2 | % | — | % | (2.3) | % | (1.8) | % | 9.7 | % | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Blood Center | 298,512 | 307,452 | (2.9) | % | 1.3 | % | (1.2) | % | — | % | (1.6) | % | (1.4) | % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Hospital(3) | 322,804 | 210,632 | 53.3 | % | 0.6 | % | 37.7 | % | — | % | (1.2) | % | 16.2 | % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Net business unit revenues | $ | 972,663 | $ | 850,320 | 14.4 | % | 0.7 | % | 8.7 | % | (0.8) | % | (1.5) | % | 7.3 | % | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Service | 20,533 | 20,143 | 1.9 | % | 1.6 | % | — | % | — | % | — | % | 0.3 | % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Total Net revenues | $ | 993,196 | $ | 870,463 | 14.1 | % | 0.7 | % | 8.7 | % | (0.9) | % | (1.5) | % | 7.1 | % | ||||||||||||||||||||||||||||||||||||||||||||||||||||
(1)Reflects the impacts in Blood Center of (0.8%) and (0.4%) related to the divestitures of the Company’s U.S. blood donor management software solutions assets and of Inlog Holdings France SAS ("InLog"), respectively. Also reflects the impacts in Hospital of 38.8% related to the acquisition of the Vascular Closure products line from Cardiva Medical, Inc. and of (1.2%) related to the divestiture of InLog.
(2)Reflects adjustments to both fiscal 2022 and 2021 Plasma revenue due to certain strategic exits within the liquid solutions business.
(3)Hospital revenue includes Hemostasis Management revenue of $127.4 million and $107.4 million for fiscal 2022 and 2021, respectively. Hemostasis Management revenue increased 18.6% in fiscal 2022 as compared with fiscal 2021. Hemostasis Management revenue increased 20.3%, on an organic basis, in fiscal 2022 as compared with fiscal 2021. Hospital revenue also includes Vascular Closure revenue of $93.8 million and $7.7 million for fiscal 2022 and fiscal 2021, respectively. Vascular Closure revenue is included in the organic revenue growth rate beginning March 2022.
RECONCILIATION OF FREE CASH FLOW BEFORE RESTRUCTURING AND RESTRUCTURING RELATED COSTS FOR FISCAL 2022 AND FISCAL 2021
(Data in thousands)
Year Ended | ||||||||||||||||||||
4/2/2022 | 4/3/2021 | |||||||||||||||||||
(unaudited) | ||||||||||||||||||||
Free Cash Flow Reconciliation: | ||||||||||||||||||||
| Cash provided by operating activities | $ | 172,263 | $ | 108,805 | ||||||||||||||||
| Capital expenditures, net of proceeds from sale of property, plant and equipment | (94,487) | (35,225) | ||||||||||||||||||
| Free cash flow after restructuring and restructuring related costs | $ | 77,776 | $ | 73,580 | ||||||||||||||||
| Restructuring and restructuring related costs | 50,193 | 32,639 | ||||||||||||||||||
| Tax benefit on restructuring and restructuring related costs | (10,532) | (7,017) | ||||||||||||||||||
| Free cash flow before restructuring and restructuring related costs | $ | 117,437 | $ | 99,202 | ||||||||||||||||
60 HAEMONETICS CORPORATION | 2022 Proxy Statement
RECONCILIATION OF GAAP OPERATING INCOME TO ADJUSTED OPERATING INCOME FOR FISCAL 2022 AND FISCAL 2021
(Data in thousands)
Year Ended | ||||||||||||||||||||
4/2/2022 | 4/3/2021 | |||||||||||||||||||
(unaudited) | ||||||||||||||||||||
| GAAP operating income | $ | 80,750 | $ | 89,747 | ||||||||||||||||
| Deal amortization | 47,414 | 32,830 | ||||||||||||||||||
| Integration and transaction costs | 21,604 | 18,421 | ||||||||||||||||||
| Restructuring and restructuring related costs | 28,824 | 15,661 | ||||||||||||||||||
| Impairment of assets and PCS2 related charges | 5,732 | 25,696 | ||||||||||||||||||
MDR and IVDR costs(1) | 11,033 | 4,130 | ||||||||||||||||||
| Litigation-related charges | 1,368 | 897 | ||||||||||||||||||
| Gain on divestitures and sale of assets | (9,603) | (32,812) | ||||||||||||||||||
| Adjusted operating income | $ | 187,122 | $ | 154,570 | ||||||||||||||||
(1)Refers to European Union Medical Device Regulation (“MDR”) and In Vitro Diagnostic Regulation (“IVDR”) related costs.
RECONCILIATION OF GAAP EARNINGS PER SHARE TO ADJUSTED EARNINGS PER SHARE FOR FISCAL 2022 AND FISCAL 2021
(Data in thousands except per share data)
Year Ended | ||||||||||||||||||||
4/2/2022 | 4/3/2021 | |||||||||||||||||||
(unaudited) | ||||||||||||||||||||
| GAAP net income | $ | 43,375 | $ | 79,469 | ||||||||||||||||
| Deal amortization | 47,414 | 32,830 | ||||||||||||||||||
| Integration and transaction costs | 21,604 | 21,391 | ||||||||||||||||||
| Restructuring and restructuring related costs | 28,824 | 15,661 | ||||||||||||||||||
| Impairment of assets and PCS2 related charges | 5,732 | 25,696 | ||||||||||||||||||
MDR and IVDR costs(1) | 11,033 | 4,130 | ||||||||||||||||||
| Litigation-related charges | 1,368 | 897 | ||||||||||||||||||
| Tax settlement | — | 1,083 | ||||||||||||||||||
| Gain on divestitures and sale of assets | (9,603) | (32,812) | ||||||||||||||||||
| Tax impact associated with adjustments | (17,182) | (27,646) | ||||||||||||||||||
| Adjusted net income | $ | 132,565 | $ | 120,699 | ||||||||||||||||
| GAAP net income per common share | $ | 0.84 | $ | 1.55 | ||||||||||||||||
| Adjusted items after tax per common share assuming dilution | 1.74 | 0.80 | ||||||||||||||||||
| Adjusted net income per common share assuming dilution | $ | 2.58 | $ | 2.35 | ||||||||||||||||
(1)Refers to European Union MDR and IVDR related costs.
HAEMONETICS CORPORATION | 2022 Proxy Statement 61

Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- EU Commission Signs Off on European Solar Charter to Boost Photovoltaic Manufacturing Industry
- Expedia Group Announces Two New Sustainable Travel Programs for Destinations
- Peapack-Gladstone Bank Promotes Kate Sant'Angelo to Senior Vice President, Director of Personal Banking
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share