Form DEF 14A FONAR CORP For: May 23
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A INFORMATION
Proxy Statement Pursuant to section 14(a) of the Securities and
Exchange Act of 1934 (Amendment No.)
Filed by the Registrant [X]
Filed by a Party other than the Registrant [ ]
Check the appropriate box:
[ ] Preliminary Proxy Statement
[ ] Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2))
[X] Definitive Proxy Statement
[ ] Definitive Additional Materials
[ ] Soliciting Material Pursuant to §240.14a-12
Fonar Corporation
......................................................................................................................................
(Name of Registrant as Specified In Its Charter)
.....................................................................................................................................
(Name of Person(s) Filing Proxy Statement if other than the Registrant)
Payment of Filing Fee (Check the appropriate box):
[X] No fee required
[ ] Fee computed on table below per Exchange Act Rules 14-6(i) (1) and 0-11.
1) Title of each class of securities to which transaction applies:
N/A
...............................................................................................
2) Aggregate number of securities to which transaction applies:
N/A
..............................................................................................
3) Per unit price or other underlying value of transaction computed pursuant to
Exchange Act Rule 0-11 (Set forth the amount of which the filing fee is
calculated and state how it was determined:)
N/A
..............................................................................................
| Page 1 |
4) Proposed maximum aggregate value of transaction:
N/A
..............................................................................................
5) Total fee paid:
N/A
..............................................................................................
[ ] Fee paid previously with preliminary materials.
[ ] Check box if any part of the fee is offset as provided by Exchange Act Rule 240.0-11 (a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration number, or the Form or Schedule and the date of its filing.
1) Amount Previously Paid:
...........................................................
2) Form, Schedule or Registration Statement No.:
...........................................................
3) Filing Party:
...........................................................
4) Date Filed:
..........................................................
| Page 2 |

FONAR CORPORATION
110 Marcus Drive
Melville, New York 11747
(631) 694-2929
NOTICE OF ANNUAL MEETING OF STOCKHOLDERS
Monday, May 23, 2022
To The Stockholders:
The Annual Meeting of the stockholders of Fonar Corporation will be held at the Double Tree Hotel, Wilmington Downtown, 700 King Street, Wilmington, Delaware 19801 (302-655-0400), on Monday, May 23, 2022, at 10:00 a.m. local time for the following purposes:
1. To elect five Directors to the Board of Directors.
2. To approve, on an advisory basis, the compensation of the Company’s named executive officers.
3. To ratify the selection of Marcum LLP as the Company’s auditors for the fiscal year ending June 30, 2022.
4. To transact such other business as may properly come before the meeting.
Only stockholders of record at the close of business on March 28, 2022 are entitled to notice of, and to vote at, this meeting. A list of such stockholders will be available for examination by any stockholder for any purpose germane to the meeting, during normal business hours, at the principal office of the Company, 110 Marcus Drive, Melville, New York, for a period of ten days prior to the meeting.
Whether or not you expect to attend in person, we urge you to vote your shares at your earliest convenience. You may vote by internet, by phone or by signing, dating, and returning your proxy at your earliest convenience. Voting by internet, telephone or mail will not prevent you from voting your stock at the meeting if you desire to do so, as your proxy is revocable at your option.
BY ORDER OF THE BOARD OF DIRECTORS
/s/ Claudette J.V. Chan
Claudette J.V. Chan, Secretary
| Page 3 |
PROXY STATEMENT
FOR ANNUAL MEETING OF
STOCKHOLDERS TO BE HELD MONDAY, MAY 23, 2022
This proxy statement, which is first being made available to shareholders on or about April 8, 2022 on the internet, is furnished in connection with the solicitation of proxies by the Board of Directors of Fonar Corporation (the “Company”), to be voted at the annual meeting of the stockholders of the Company to be held at 10:00 a.m. on May 23, 2022 and any adjournment(s) thereof for the purposes set forth in the accompanying Notice of Annual Meeting of Stockholders. At the same time a paper notice regarding the availability of proxy materials will be mailed to stockholders. Stockholders who execute proxies retain the right to revoke them at any time prior to the exercise of the powers conferred thereby. The cost of solicitation of proxies will be borne by the Company.
The stockholders will have several options as to how to view the materials and vote their shares.
The Company is posting the Notice of Annual Meeting and Proxy Statement, together with the Annual Report on the internet. You may read the materials online or print out a copy. You will also have the ability to vote online.
In the alternative, you may elect to receive an e-mail or the traditional paper copies of the Notice of Annual Meeting and Proxy Statement, and the Annual Report. There is no charge for receiving e-mail or paper copies, BUT you must request them if you want them. To facilitate timely delivery please make the request as instructed on or before April 30, 2022.
To view the materials and vote on the internet, have the 12 Digit Control Number(s) located on the Notice Regarding the Availability of Proxy Materials available and visit: www.proxyvote.com.
Stockholders may request a copy of the Proxy Materials:
1. By internet - visit www.proxy.com
2. By telephone - 1-800-579-1639
3. By e-mail - [email protected]
| Page 4 |
Only stockholders of record at the close of business on March 28, 2022 will be entitled to vote at the meeting. Shares of Common Stock are entitled to one vote per share, shares of Class B Common Stock are entitled to ten votes per share and shares of Class C Common Stock are entitled to twenty-five votes per share. At the close of business on March 28, 2022, there were issued and outstanding 6,554,210 shares of Common Stock held of record by approximately 993 stockholders, 146 shares of Class B Common Stock held of record by 11 stockholders and 382,513 shares of Class C Common Stock held of record by 3 stockholders. The shares of Class A Nonvoting Preferred Stock, 313,438 shares held of record by approximately 1,154 stockholders at the close of business on March 28, 2022, are not entitled to vote. Except for the shares of Class A Nonvoting Preferred Stock, there are no shares of Preferred Stock issued and outstanding.
Any proxy may be revoked at any time before it is exercised by delivery of a written instrument of revocation or a later dated proxy to the Secretary of the Company at the principal executive office of the Company or, while the meeting is in session, to the Secretary of the meeting, without, however, affecting any vote previously taken. The presence of a stockholder at the meeting will not operate to revoke his proxy. The casting of a ballot by a stockholder who is present at the meeting, however, will revoke his proxy, but only as to the matters on which the ballot is cast and not as to any matters on which he does not cast a ballot or as to matters previously voted upon.
Proxies received by management will be voted at the meeting or any adjournment thereof. EACH PROXY WILL BE VOTED IN ACCORDANCE WITH THE SPECIFICATIONS MADE THEREIN BY THE PERSON GIVING THE PROXY. TO THE EXTENT NO CHOICE IS SPECIFIED, HOWEVER, THE PROXY WILL BE VOTED FOR MANAGEMENT’S PROPOSALS. All of management’s proposals have been unanimously approved by the Board of Directors.
1. ELECTION OF DIRECTORS AND MANAGEMENT INFORMATION
Five directors are to be elected at the annual meeting, to hold office until the next annual meeting of stockholders and until their successors are elected and qualified. It is intended that the accompanying proxy will be voted in favor of the following nominees to serve as directors unless the stockholder indicates to the contrary on the proxy. All of the nominees are currently directors. Management expects that each of the nominees will be available for election.
NOMINEES FOR ELECTION OF DIRECTORS
1. Raymond V. Damadian
2. Claudette J.V. Chan
3. Ronald G. Lehman
4. Richard E. Turk
5. John Collins
| Page 5 |
BIOGRAPHIES FOR DIRECTORS AND OFFICERS
Raymond V. Damadian, M.D. (age 86) has been the Chairman of the Board since its inception in 1978 and Treasurer since February, 2001. Up until February 11, 2016, Dr. Damadian also served as the President and Chief Executive Officer of Fonar. Dr. Damadian was employed by the State University of New York, Downstate Medical Center, New York, as an Associate Professor of Biophysics and Associate Professor of Internal Medicine from 1967 until September 1979. He received an M.D. degree in 1960 from Albert Einstein College of Medicine, New York, and a B.S. degree in mathematics from the University of Wisconsin in 1956. In addition, Dr. Damadian conducted post-graduate work at Harvard University, where he studied extensively in the fields of physics, mathematics and electronics. Dr. Damadian is the author of numerous articles and books on the nuclear magnetic resonance effect in human tissue, which is the theoretical basis for the Fonar MRI scanners. He is a 1988 recipient of the National Medal of Technology. In 1989 he was inducted into the National Inventors Hall of Fame, for his contributions in conceiving and developing the application of magnetic resonance technology to medical applications including whole body scanning and diagnostic imaging. Dr. Damadian is the President, Treasurer and director of Health Management Corporation of America (“HMCA”), a Manager of Imperial Management Services, LLC (“Imperial”) and a Manager of Health Diagnostics Management, LLC (“HDM”) which three entities are subsidiaries of Fonar. Dr. Damadian is the father of Timothy Damadian and brother of Claudette J.V. Chan.
Timothy Damadian (age 57) has been the President and Chief Executive Officer of Fonar since February 11, 2016. From 2010 to 2016 he served as an independent consultant, with a focus on the Company’s MRI facility management business. Timothy Damadian began his career at Fonar in 1985, installing MRI scanners and components for Fonar customers. Over the course of the following 16 years, he held positions of increasing authority, eventually becoming Vice President of Operations. In 1997, Timothy Damadian was appointed President of the newly formed Health Management Corporation of America (HMCA), a wholly-owned subsidiary of Fonar that was formed to manage medical and diagnostic imaging offices. In 2001, Timothy Damadian left Fonar to form Integrity Healthcare Management, Inc., a diagnostic imaging management company that would eventually manage 11 MRI scanning centers in New York and Florida. The company was a success and was sold to Health Diagnostics, LLC in 2007.
Mr. Damadian returned to Fonar as a consultant in 2010 and was appointed as Fonar’s Chief Executive Officer and President on February 11, 2016. He also serves as a Manager of Imperial Management Services, LLC and a Manager of Health Diagnostics Management, LLC, which are subsidiaries of HMCA. Timothy Damadian is the son of Dr. Raymond V. Damadian and nephew of Claudette J.V. Chan.
Luciano B. Bonanni (age 66) has served as Chief Operating Officer (COO) and Executive Vice President (EVP) for Fonar Corporation since June 27, 2016. Prior to his appointment as COO, Mr. Bonanni had served the Company as Vice President since 1989, during which time he oversaw general operations, research and development, manufacturing, service, sales, finance, accounting and regulatory compliance. Prior to 1989, Mr. Bonanni held the title of Vice President of Production and Engineering from the time of Fonar’s initial public offering in 1981. Mr. Bonanni joined the Company as an electrical engineer in 1978. He holds a Bachelor of Electrical Engineering degree from Manhattan College.
| Page 6 |
Claudette J.V. Chan (age 84) has been a Director of Fonar since October 1987 and Secretary of Fonar since January 2008. Mrs. Chan was employed from 1992 through 1997 by Raymond V. Damadian, M.D. MR Scanning Centers Management Company and since 1997 by HMCA, as "site inspector," in which capacity she is responsible for supervising and implementing standard procedures and policies for MRI scanning centers. From 1989 to 1994 Mrs. Chan was employed by St. Matthew's and St. Timothy's Neighborhood Center, Inc., as the director of volunteers in the "Meals on Wheels" program, a program which cares for the elderly. From approximately 1983 to 1989, Mrs. Chan was President of the Claudette Penot Collection, a retail mail-order business specializing in women's apparel and gifts. Mrs. Chan practiced and taught in the field of nursing until 1973, when her son was born. She received a bachelor of science degree in nursing from Cornell University in 1960. Mrs. Chan is the sister of Raymond V. Damadian and aunt of Timothy Damadian.
Ronald G. Lehman (age 45) has been a Director of Fonar since April, 2012, when he was unanimously appointed by the remaining four Directors to fill the vacancy resulting from the death of the former Director Robert Djerejian. From October, 2009 to the present, Mr. Lehman has served as Managing Director of Investment Banking with Bruderman Brothers, LLC, a private New York-based broker-dealer registered with the Securities and Exchange Commission and which is a member of the Financial Industry Regulatory Authority (FINRA) and the Securities Investor Protection Corporation (SIPC). Mr. Lehman directly manages all facets of the firm’s transaction processes, from deal origination, to sourcing capital, to negotiating deal structures, through documentation and closing. The firm provides buy and sell-side advisory, capital raising, and consulting services to lower middle-market companies. Mr. Lehman specializes in advising healthcare services companies and has recently completed several recapitalizations in the industry. He also participates in the firm’s merchant banking investments and oversees many of these assignments. From May, 2008 to October, 2009, Mr. Lehman served as Senior Vice President of Acquisitions at Health Diagnostics, LLC, where he managed the company’s acquisition and corporate finance activities. From March, 2000 to May, 2008, Mr. Lehman worked for various Bruderman entities as a buy and sell-side advisor and as a principal in several private equity transactions. From September, 1998 to March, 2000, Mr. Lehman worked at Deutsche Bank Securities, Inc. and last held the position of Associate in their Global Custody Group. Mr. Lehman graduated from Columbia University with a B.A. in 1998.
Richard E. Turk (age 37) has been a Director of Fonar since June, 2020, when he was appointed to fill the vacancies on the Board of Directors and Audit Committee of the Board of Directors resulting from the death of his predecessor, Robert J. Janoff. Mr. Turk is the Chief Financial Officer of PRISM Vision Group, a private equity-backed, multi-location, outpatient comprehensive eye care practice headquartered in Union, New Jersey. Since joining PRISM in November, 2018, Mr. Turk has helped source, analyze, and complete 12 acquisitions. He spearheaded growth efforts that helped PRISM expand from a single-speciality (retina) provider with 17 locations and 21 physicians to a comprehensive, vertically-integrated, multi-specialty, eye care organization with approximately 90 physicians and more than 50 locations across New Jersey, Pennsylvania, Delaware and Maryland. Prior to his tenure at PRISM, Mr. Turk was employed by Professional Physical Therapy, a private equity-backed outpatient physical and occupational therapy company headquartered in Uniondale, New Jersey with more than 180 locations across New York, New Jersey, Connecticut, Massachusetts and New Hampshire. During his four years at Professional Physical Therapy, Mr. Turk sourced, analyzed, and completed 32 acquisitions comprised of 116 clinics, expanding the company’s services and adding three states. From 2007 to 2014, Mr. Turk was employed by Bruderman Brothers, a broker dealer involved in investment banking, merchant banking, investment advisory, and consulting for lower middle market companies ($10M-$250M of enterprise value) in a variety of industries, including healthcare. Mr. Turk was Vice President of Bruderman Brothers from 2011 to 2014. Mr. Turk graduated from Columbia University with a B.A. in American History in 2007.
| Page 7 |
John Collins (age 41) has been Director of Fonar since November 17, 2021, when he was unanimously appointed by the remaining four Directors to fill the vacancy resulting from the death of his predecessor, Charles N. O’Data. Mr. Collins is a partner at Brownell Partners, PLLC. Mr. Collins first joined the firm in October of 2018, and he was promoted to Partner in January 2020. The practice provides litigation defense for the Insureds of various commercial and personal lines insurance companies. The practice also deals with insurance coverage disputes and provides risk management consulting services. Mr. Collins has successfully defended all manner of liability claims at both the trial and appellate level. Prior to joining Brownell Partners, Mr. Collins was in private practice from 2016 – 2018, working on both contractual and litigation based projects for a variety of corporate and individual clients. Prior to that, he was affiliated with a variety of regional insurance defense firms in the greater New York City area. Mr. Collins graduated magna cum laude from New York Law School in 2012, where he was a John Marshall Harlan Scholar and a staff editor for the New York Law School Law Review. In 2011, Mr. Collins served as a judicial intern at the Southern District of New York, in the chambers of the Honorable Paul A. Crotty. Upon graduation, Mr. Collins completed a Fellowship with the Corporation Counsel of the City of New York.
CORPORATE GOVERNANCE, THE BOARD AND ITS COMMITTEES
All of the nominees are presently directors of the Company. The five nominees will be elected to hold office for the ensuing year or until their respective successors are elected and qualified. Of the five nominees, Messrs. Ronald G. Lehman, Richard E. Turk and John Collins are independent, as defined in the Securities and Exchange Commission Regulations and Nasdaq Market Place Rules. In making such determinations, there were no transactions, relationships or arrangements not disclosed in our SEC filings to be considered by the Board of Directors, in determining whether the director was independent.
BOARD MEETINGS
During the year ended June 30, 2021 the Board of Directors unanimously consented to take action in lieu of a meeting on two occasions, and the audit committee met four times.
The attendance of the Board of Directors at annual meetings is not required. The Chairman of the Board, Dr. Raymond V. Damadian, however, when he attends the annual meeting of stockholders, acts as Chairman of the Meeting.
Dr. Damadian receives no compensation for serving on the Board. The other directors are each paid $20,000 per year in their capacities as directors. This is the sole compensation payable to the directors.
Board Leadership Structure. The current Board Chairman is Dr. Raymond V. Damadian. In addition, although the Company has not selected a lead independent director, Ronald G. Lehman effectively functions as such. The Company believes that the Company’s current leadership structure is appropriate for the Company in the context of the specific circumstances facing the Company. Consideration of the Company’s leadership structure is a continuing process which the Board of Directors and Management of the Company undertake in coordination with each other.
The lead independent director, Ronald G. Lehman, acts as the Chairman of the Audit Committee. As such he plays a leading role in the engagement of auditors and the review of the Company’s financial statements. Under certain circumstances, he will also serve as a contact point for employees.
The Company believes its present leadership structure is successfully meeting the Company’s current needs, including:
| Page 8 |
The Company believes its present leadership structure is successfully meeting the Company’s current needs, including:
● |
Efficient communication between Management and the Board; | |
| ● | Clarity for the Company’s stockholders on corporate leadership and accountability; | |
| ● | The Chairman of the Board having the Company’s strategy, operations and financial conditions; and | |
| ● | Continuity in the Company’s leadership, as the Chairman of the Board, Dr. Raymond V. Damadian, founded the Company in 1978. |
The Company's Board of Directors has an audit committee. There is no standing compensation committee, nominating committee or other committee of the Board.
In accordance with the Nasdaq Marketplace Rules, the Board of Directors adopted a written charter for the audit committee which took effect in June, 2001 and was revised on November 17, 2004. All of the directors on the audit committee are independent.
Stockholders may communicate with directors by writing to them at the Company in accordance with the Company’s corporate governance policies and code of conduct, or in any other manner the particular director may provide. Depending on the sensitivity and timing of a matter raised by a stockholder and the need for disclosure of matters to be made not to just one stockholder, but to the stockholders as a whole, it may not be possible for the director to reply to the stockholder.
The shareholdings of the Company’s Chairman of the Board, Dr. Raymond V. Damadian, have more than 50% of the voting power of the Company’s outstanding stock. Consequently, the Company is a controlled company for purposes of NASDAQ Marketplace Rule 4350(c).
AUDIT COMMITTEE
The Audit Committee, which is comprised solely of independent directors, is governed by a Board approved charter that contains, among other things, the Committee’s membership requirements and responsibilities. The audit committee oversees the Company’s accounting, financial reporting processes, internal controls and audits, and consults with management and the independent public accountants on, among other items, matters related to the annual audit, the published financial statements and the accounting principles applied. As part of its duties, the audit committee appoints, evaluates and retains the Company’s independent public accountants. It also maintains direct responsibility for the compensation, termination and oversight of the Company’s independent public accountants and evaluates the independent public accountants’ qualifications, performance and independence.
Financial Expert on Audit Committee: The Board has determined that Mr. Ronald G. Lehman, is the audit committee financial expert. The Board made a qualitative assessment of Mr. Lehman’s level of knowledge and experience based on a number of factors, including his formal education and experience.
| Page 9 |
Board Oversight of Risk Management. The Company faces risk in many different areas, including business strategy; government regulation; financial condition; health care compliance; product research and development; competition for talent; business vitality; operational efficiency; quality assurance; reputation; intellectual property; and trade secrets, among others. The oversight function is carried out in the quarterly and annual Audit Committee meetings and by communication and meetings with the Company’s Management, which exercises the responsibility for oversight of risk management.
AUDIT COMMITTEE REPORT
The audit committee has (a) reviewed and discussed the audited financial statements with management, (b) discussed with the independent auditors the matters required to be discussed by SAS 61 (Statement on Auditing Standards No. 61) and (c) has received the written disclosures and the letter from the independent accountants required by Independence Standards Board, Standard No. 1 and has discussed with the independent accountants the independent accountant’s independence.
Based on the foregoing review and discussions, the audit committee recommended to the Board of Directors that the audited financial statements be included in the Company’s Annual Report on Form 10-K for the fiscal year ended June 30, 2021.
The members of the audit committee are Messrs. Ronald G. Lehman, Richard E. Turk and John Collins. Messrs. Lehman, Turk and Collins are independent directors, as defined in the Securities and Exchange Commission Regulations and Nasdaq Market Place Rules.
NOMINATING COMMITTEE
The Board of Directors does not believe it requires a separate standing nominating committee because the Board of Directors is relatively small and can make the nominations acting as a whole. The Board does not have a policy with regard to director candidates recommended by stockholders because the absence of such recommendations makes a formal policy unnecessary. Historically, there usually has not been a need for a committee to identify new nominees in the case of the resignation or death of an existing director. The remaining directors evaluate a new nominee based on his integrity, loyalty, competence and experience, and how his background complements that of the remaining directors.
Promoting diversity in the selection of nominees has not yet been considered, but the Board follows a policy of nondiscrimination and equal opportunity.
COMPENSATION COMMITTEE
COMPENSATION COMMITTEE INTERLOCKS AND INSIDER PARTICIPATION
The Board of Directors does not believe it requires a separate standing compensation committee because the management, under the authority of the Chairman of the Board and the Chief Executive Officer, is best equipped to make compensation decisions. The Board reserves the right to change this policy at any time.
Dr. Raymond V. Damadian, who serves as Chairman of the Board, and Timothy Damadian, who serves as Chief Executive Officer and President of the Company, participate in the deliberation and determination of executive officer and director compensation.
| Page 10 |
VOTE REQUIRED AND BOARD RECOMMENDATION
The directors will be elected by the vote of a plurality of the votes represented at the meeting. THE BOARD OF DIRECTORS RECOMMENDS A VOTE FOR ALL OF THE NOMINEES FOR THE DIRECTORS OF THE COMPANY.
INFORMATION REGARDING BENEFICIAL OWNERSHIP OF PRINCIPAL STOCKHOLDERS, DIRECTORS, AND MANAGEMENT
The following table sets forth information regarding the beneficial ownership of the Company's common shares held by holders of at least 5% of the shares of any class, by the nominees for directors, the Company's Chief Executive Officer, and the directors and executive officers as a group as of the close of business on March 18, 2022 unless otherwise noted.
| Name and Address of Beneficial Owner (1) | Shares Beneficially Owned | Percent of Class | |||
Raymond V. Damadian, M.D. c/o Fonar Corporation, Melville, New York Director and Treasurer 5% + Stockholder | |||||
| Common Stock | 121,402 | 1.85 | % | ||
| Class C Stock | 382,447 | 99.98 | % | ||
| Class A Preferred | 19,093 | 6.09 | % | ||
Kayne Anderson Rudnick Investment Management LLC (2) 1800 Avenue of the Stars, 2nd Floor Los Angeles, CA 90067 | |||||
| Common Stock (as of 12/31/2021) | 657,067 | 10.03 | % | ||
Renaissance Technologies LLC Renaissance Technologies Holding Corporation (2) 800 Third Avenue, New York, New York 10022 | |||||
| Common Stock (as of 12/31/2021) | 404,616 | 6.17 | % | ||
Dimensional Fund Advisors LP (2) (3) Building One, 6300 Bee Cave Road Austin, Texas 78746 | |||||
| Common Stock (as of 12/31/2021) | 374,741 | 5.6 | % | ||
Timothy R. Damadian, President and Chief Executive Officer | |||||
| Common Stock | 38,000 | * | |||
| Class A Preferred | 800 | * | |||
| Page 11 |
CONTINUATION
| Name and Address of Beneficial Owner (1) | Shares Beneficially Owned | Percent of Class | |||
| Luciano B. Bonanni | |||||
| Executive Vice President And Chief Operating Officer | |||||
| Common Stock | 49,726 | * | |||
| Class A Preferred | 1,285 | * | |||
|
Claudette Chan, Director and Secretary | |||||
| Common Stock | 106 | * | |||
| Class A Preferred | 32 | ||||
| Ronald G. Lehman, Director | |||||
| Common Stock | 4,330 | * | |||
| Richard E. Turk, Director | |||||
| Common Stock | 0 | * | |||
| John Collins, Director | * | ||||
| Common Stock | 0 | ||||
| All Officers and Directors as a Group (7 persons) | |||||
| Common Stock | 212,706 | 3.25 | % | ||
| Class C Stock | 382,447 | 99.98 | % | ||
| Class A Preferred | 21,210 | 6.77 | % | ||
___________________________
* Less than one percent
___________________________
1. Address provided for each beneficial owner owning more than five percent of the voting securities of Fonar.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
See Item 13, “Certain Relationships and Related Transactions” of the Company’s Annual Report on Form 10-K for the fiscal year ended June 30, 2021 which is specifically incorporated by reference herein. A copy of the Form 10-K is included in the Annual Report to Stockholders which is being sent to the Company’s stockholders with this Proxy Statement.)
The Company believes that each of the related transactions described therein were on terms at least as favorable to the Company as were available from non-affiliated parties.
| Page 12 |
COMPENSATION DISCUSSION AND ANALYSIS OF DIRECTORS AND EXECUTIVE OFFICERS
The compensation of the Company’s executive officers is based on a combination of salary and bonuses based on performance. Decisions concerning compensation are made on a case by case basis and not pursuant to standardized formulas, programs, policies or criteria, except for commissions in the case of sales. The Board of Directors does not have a compensation committee and does not believe such a committee is required, in view of the manner in which compensation matters are handled. Dr. Raymond V. Damadian and Claudette J.V. Chan are executive officers as well as members of the Board of Directors. Dr. Damadian, who also has voting control of the Company and serves as Chairman of the Board, Timothy Damadian, who has served as PEO and President of the Company since February 11, 2016, and Luciano Bonanni the Executive Vice President and Chief Operating Officer of the Company since June 27, 2016, participate in the determination of executive compensation for the Company’s officers.
As noted above, the Company's compensation policy is primarily based upon the practice of pay-for-performance. Section 162(m) of the Internal Revenue Code imposes a limitation on the deductibility of nonperformance-based compensation in excess of $1 million paid to the Principal Executive Officer. No officer of the Company received compensation in excess of $1 million in fiscal 2016 or in any previous fiscal year. The Board currently believes that the Company should be able to continue to manage its executive compensation program for others so as to preserve the related federal income tax deductions.
The Company does not believe that there are any risks arising from its compensation policies and practices for its employees that are likely to have a material adverse effect on the Company.
The Company maintains no pension or deferred compensation plans except for a 401(k) plan.
SUMMARY COMPENSATION TABLE
The following table discloses compensation received for the three years ended June 30, 2019, June 30, 2020 and June 30, 2021 by the Company’s Principal Executive Officer, the Company’s Principal Financial Officer and any officer of the Company receiving at least $100,000 for the year.
| Name and Principal Position | Year | Salary | Cash Bonus | Stock Awards | Total | |||||||||||||||
| Timothy R. Damadian | 2021 | $ | 0 | $ | 155,800 | $ | 0 | $ | 155,800 | |||||||||||
| President, Principal | 2020 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | |||||||||||
| Executive Officer | 2021 | $ | 0 | $ | 155,800 | $ | 0 | $ | 155,800 | |||||||||||
| Raymond V. Damadian | 2021 | $ | 153,098 | $ | 305,800 | $ | 0 | $ | 458,895 | |||||||||||
| Chairman of the Board; | 2020 | $ | 153,095 | $ | 0 | $ | 0 | $ | 153,095 | |||||||||||
| Principal Financial Officer; | 2019 | $ | 153,095 | $ | 305,800 | $ | 0 | $ | 458,895 | |||||||||||
| Director | ||||||||||||||||||||
| Luciano Bonanni | 2021 | $ | 146,038 | $ | 0 | $ | 152,931 | $ | 298,969 | |||||||||||
| Executive Vice President | 2020 | $ | 146,496 | $ | 0 | $ | 152,902 | $ | 299,398 | |||||||||||
| and Chief Operating Officer | 2019 | $ | 145,672 | $ | 0 | $ | 152,740 | $ | 298,412 | |||||||||||
| Page 13 |
No executive officer has a written or unwritten employment agreement with the Company. Salaries, bonuses and discretionary stock and stock option awards comprise the full amount of total compensation. The only exceptions are commissions, based on a percentage of the sales prices, payable to salesmen.
Compensation Pursuant to Stock Options and SAR Grants
No stock options or stock appreciation rights were granted to the Company’s Principal Executive Officer and Principal Financial Officer during fiscal 2020.
Option/SAR Exercises and Year End Values
No options or stock appreciation rights were exercised by the Company’s Chief Executive Officer during fiscal 2020. The Company’s Chief Executive Officer did not hold any unexercised stock options or stock appreciation rights at the end of fiscal 2020.
DIRECTOR COMPENSATION
The following table shows the compensation paid to the Directors for fiscal 2021:
| Name | Fees earned in pad in cash ($) | Stock awards ($) | Option awards ($) | Non-equity incentive plan compen- sation | Nonqualified deferred compen- sation earnings ($) |
All other compen- sation ($) | Total ($) | |||||||||
| (a) | (b) | (c) | (d) | (e) | (f) | (g) | (h) | |||||||||
| A. Claudette J.V. Chan | $ | 20,000 | 0 | 0 | 0 | 0 | 0 | $ | 20,000 | |||||||
| B. Charles N. O'Data (deceased) | $ | 20,000 | 0 | 0 | 0 | 0 | 0 | $ | 20,000 | |||||||
| C. Ronald G. Lehman | $ | 20,000 | 0 | 0 | 0 | 0 | 0 | $ | 20,000 | |||||||
| D. Richard E. Turk | $ | 20,000 | 0 | 0 | 0 | 0 | 0 | $ | 20,000 | |||||||
With the exception of Dr. Damadian who receives no compensation for serving as a director, each director is entitled to receive $20,000 per annum for his or her services as a director of the Company, including service on any committee of the Board of Directors. No other fees are paid to the directors for their services as directors of the Company.
2. ADVISORY VOTE ON COMPENSATION OF THE COMPANY’S NAMED EXECUTIVE OFFICERS
The following proposal provides the Company’s stockholders with an opportunity to vote to approve, on an advisory basis, the compensation of the Company’s named executive officers, as disclosed in this proxy statement. In considering your vote, you may wish to review with care the “Compensation Discussion and Analysis” section, which provides details as to the Company’s compensation policies, procedures and decisions, as well as the Summary Compensation Table and other related compensation tables, notes and narrative disclosures under the executive compensation section of this proxy statement. This vote is not intended to address any specific element of the Company’s executive compensation program, but rather the overall compensation program for the Company’s named executive officers. This vote currently is being taken on an annual basis at the Company’s annual meeting.
| Page 14 |
In accordance with Section 14A of the Securities Exchange Act of 1934, we are asking stockholders to approve the following advisory resolution at the Annual Meeting of Stockholders
RESOLVED, that the stockholders of Fonar Corporation (the “Corporation”) approve, on an advisory basis, the overall compensation of the Corporation’s named executive officers disclosed in the Compensation Discussion and Analysis, Summary Compensation Table and related compensation tables, notes and narrative discussion in this Proxy Statement for the Annual Meeting of Stockholders.
The Board of Directors recommends a vote FOR this resolution because it believes that the policies and practices described in the Compensation Discussion and Analysis are effective in achieving the Company’s goals of rewarding sustained financial and operating performance and leadership excellence and aligning the executives’ long-term interests with those of the stockholders, as well as motivating the executives to remain with the Company for long and productive careers.
This advisory resolution, commonly referred to as a “say-on-pay” resolution, is non-binding on the Board of Directors. Although non-binding, the Board will review and consider the voting results when evaluating our executive compensation program.
3. RATIFICATION OF SELECTION OF AUDITORS
The Board of Directors selected Marcum LLP, as the Company's independent auditors for the fiscal year ending June 30, 2022. The stockholders will be asked to ratify this action by the Board. Marcum LLP were the Company’s auditors for the fiscal years ended June 30, 2019, June 30, 2020 and June 30, 2021.
One or more representatives of Marcum LLP, may be present at the Meeting with the opportunity to make a statement if they desire to do so, and to be available to respond to appropriate questions.
The affirmative vote of shares holding a majority of the votes represented at the meeting is required to ratify the selection of auditors by the Board of Directors. THE BOARD OF DIRECTORS RECOMMENDS A VOTE FOR THE PROPOSAL.
AUDIT FEES
The aggregate fees billed by Marcum LLP for the audit of the Company’s annual financial statements for the fiscal year ended June 30, 2021 and the reviews of the financial statements included in the Company’s Forms 10-Q for the fiscal year ended June 30, 2021 were $345,000.
The aggregate fees billed by Marcum LLP for the audit of the Company’s annual financial statements for the fiscal year ended June 30, 2020, and the reviews of the financial statements included in the Company’s Forms 10-Q for the fiscal year ended June 30, 2020 were $409,000.
All work on the audits in each of the last two fiscal years was performed by full-time permanent employees of Marcum LLP.
AUDIT-RELATED FEES
No fees were billed by Marcum LLP for the fiscal years ended June 30, 2021 and June 30, 2020 for services related to the audit or review of our financial statements that are not included under the caption “AUDIT FEES”.
| Page 15 |
TAX FEES
No fees were billed by Marcum LLP for tax compliance, tax advice or tax planning in the fiscal years ended June 30, 2021 and June 30, 2020.
ALL OTHER FEES
No fees were billed by Marcum LLP for any other services during the fiscal years ended June 30, 2021 and June 30, 2020.
Since January 1, 2003, the audit committee has adopted policies and procedures for pre-approving all non-audit work performed by its auditors. Specifically, the committee must pre-approve the use of the auditors for all such services. The audit committee has pre-approved all non-audit work since that time and in making its determination has considered whether the provision of such services was compatible with the independence of the auditors. Marcum LLP has not provided any services in addition to audit services in fiscal 2021 and 2020.
Our audit committee believes that the provision by Marcum LLP of services in addition to audit services in previous years were compatible with maintaining their independence.
PROPOSALS OF STOCKHOLDERS
Proposals of stockholders intended to be presented at next year’s annual meeting of stockholders must be received by the Company no later than January 19, 2023 to be included in the Company's proxy statement and form of proxy related to that meeting.
SOLICITATION OF PROXIES
The proxy accompanying this proxy statement is solicited by the Board of Directors of the Company. Proxies may be solicited by officers, directors, and regular supervisory and executive employees of the Company, none of whom will receive any additional compensation for their services. Such solicitations may be made personally, or by mail, e-mail, facsimile, telephone, telegraph, or messenger. The Company will pay persons holding shares of stock in their names or in the names of nominees, but not owning such shares beneficially, such as brokerage houses, banks, and other fiduciaries, for the expense of forwarding solicitation materials to their principals. All of the costs of solicitation of proxies will be paid by the Company.
VOTING TABULATION
The election of the Company's directors requires a plurality of the votes represented in person or by proxy at the meeting. The ratification of proposals and the selection of auditors requires the affirmative vote of a majority of the votes represented in person or by proxy at the meeting. Votes cast by proxy or in person at the meeting will be tabulated by the Company.
| Page 16 |
A stockholder who abstains from voting on any or all proposals will be included in the number of shareholders present at the meeting for the purpose of determining the presence of a quorum. Abstentions will not be counted either in favor of or against the election of the nominees or other proposals. Under the rules of the National Association of Securities Dealers, brokers holding stock for the accounts of their clients who have not been given specific voting instructions as to a matter by their clients in certain cases may vote their clients' proxies in their own discretion. Where a proposal requires a majority of the votes present for its passage, an abstention or broker non-vote will have the same effect as a negative vote.
OTHER MATTERS
The Board of Directors does not intend to bring any other business before the meeting, and so far as is known to the Board, no matters are to be brought before the meeting except as specified in the notice of the meeting. However, as to any other business which may properly come before the meeting, it is intended that proxies, in the form enclosed, will be voted in respect thereof in accordance with the judgment of the persons voting such proxies, where the authorization to do so has been granted.
DATED: Melville, New York, April 8, 2022
A COPY OF THE COMPANY'S FORM 10-K REPORT FOR FISCAL YEAR 2021 CONTAINING INFORMATION ON OPERATIONS, FILED WITH THE SECURITIES AND EXCHANGE COMMISSION, IS AVAILABLE UPON REQUEST. PLEASE WRITE TO:
INVESTOR RELATIONS DEPARTMENT
FONAR CORPORATION
110 MARCUS DRIVE
MELVILLE, NEW YORK 11747
| Page 17 |
FONAR CORPORATION
Proxy - Annual Meeting of Stockholders
May 23, 2022, 10:00 AM
THIS PROXY IS SOLICITED ON BEHALF OF THE BOARD OF DIRECTORS
The undersigned, a stockholder of Fonar Corporation (the "Company"), hereby revoking any proxy heretofore given, does hereby appoint Raymond V. Damadian, Luciano Bonanni, Daniel Culver and Ellen Yeske, and each of them, proxies with full power of substitution, for and in the name of the undersigned to attend the Annual Meeting of the Stockholders of the Company to be held at the Double Tree Hotel, Wilmington Downtown, 700 King Street, Wilmington, Delaware on May 23, 2022, at 10:00 a.m., local time, and at any adjournment(s) thereof, and there to vote upon all matters specified in the notice of said meeting, as set forth herein, and upon such other business as may properly and lawfully come before the meeting, all shares of stock of the Company which the undersigned would be entitled to vote if personally present at said meeting.
THIS PROXY WHEN PROPERLY EXECUTED WILL BE VOTED IN THE MANNER DIRECTED HEREIN BY THE UNDERSIGNED STOCKHOLDER. IF NO DIRECTION IS GIVEN, SUCH SHARES WILL BE VOTED FOR ALL PROPOSALS.
The Board of Directors Recommends you vote FOR the following:
No. 1. Election of Directors
| FOR ALL | WITHOLD ALL | FOR ALL EXCEPT | ||||
INSTRUCTION: To withhold authority to vote for any individual nominee(s), mark "FOR ALL EXCEPT" and circle or cross out the name(s) of those nominee(s).
01 - Raymond V. Damadian, 02 - Claudette J. V. Chan, 03 - Ronald G. Lehman,
04 - Richard E. Turk, 05 - John Collins
The Board of Directors recommends you vote for proposals 2, 3 and 4:
No. 2. On an advisory basis, to approve the executive compensation.
| FOR | AGAINST | ABSTAIN | ||||
| Page 18 |
No. 3. To ratify the selection of Marcum LLP as the Company's independent auditors for the fiscal year ended June 30, 2022.
| FOR | AGAINST | ABSTAIN | ||||
No. 4. In their discretion, the Proxies are authorized to vote upon such other business as may properly come before the meeting.
| FOR | AGAINST | ABSTAIN | ||||
__________________________________ _______________________
Signature Date
__________________________________ _______________________
Signature (Joint owners) Date
Please sign exactly as your name(s) appear(s) hereon or on your stock certificate(s). When signing as an attorney, executor, proxy, administrator, trustee, guardian or other fiduciary, please give full title as such. Joint owners should each sign personally. All holders must sign. If a corporation, please sign in full corporate name, by an authorized officer. If a partnership, limited liability company or other entity, please sign in the company’s name by an authorized person, indicating your capacity.
| Page 19 |
ANNUAL REPORT
FONAR CHAIRMAN’S LETTER TO SHAREHOLDERS
MAY 2022
Dear Shareholders,
When the Coronavirus reached America in March of 2020, it had an immediate crushing effect on medical providers, patients, employees, and therefore FONAR Service Revenue and HMCA Management Fees. The crisis left us with a very disappointing fourth quarter (fiscal 2020) and also the urgency for us to recover as quickly as possible. Even though the effects of this relentless pandemic continued to hamper our business, to the credit of our management team and our employees, we have to date prevailed and as of December 31, 2021 FONAR posted 47 consecutive quarters of positive net income and positive income from operations.
FONAR’s primary source of income and growth is attributable to its diagnostic imaging management subsidiary, Health Management Company of America (HMCA).
| Ø | HMCA |
The Birth of HMCA in 1997
FONAR, the original MRI company, introduced the world’s first MRI scanner in 1980 and from there grew solely by selling and servicing its ever-innovative line of MRI products. But eventually, after years of competing with well-heeled corporate giants and struggling with the financial uncertainties that accompanied an erratic MRI equipment sales market, FONAR, in 1997, expanded by forming its diagnostic imaging management subsidiary, Health Management Company of America (HMCA) in order to provide the company with a steady and reliable source of revenue.
Up until that time, FONAR had been working with hundreds of customers for nearly 20 years, most of them independent MRI business owners. As a result of our ongoing relationships with them, we became very familiar with how they managed their businesses, their successes and their failures. We observed which of their methods, practices and strategies worked and which ones didn’t. What we learned from their experiences made FONAR’s expansion into the diagnostic imaging management business a natural one, and so we developed a business plan that would eventually result in HMCA becoming the company’s leading source of revenue and profit.
A New Management Team in 2010
By 2009, annual scan volume at the nine facilities managed by HMCA (six in New York and three in Florida) reached 29,000 with Total Revenues-Net of $31.8 million (fiscal 2010). When my son, Timothy R. Damadian, who is currently President and CEO of FONAR, returned to FONAR in 2010 his first order of business was to assemble a new management team. Each one chosen was trustworthy, had worked with Tim for many years, and had a record of success in managing and growing MRI scanning centers and businesses. Together they launched a growth strategy based upon the ever-growing appeal among patients and their physicians of our FONAR UPRIGHT® Multi-Position™ MRI, also known as the Stand-Up® MRI. From that point on, the company has grown steadily, enjoying remarkable success. Today, HMCA manages 39 MRI scanners: 25 in New York and 14 in Florida. In 2021, annual scan volume at HMCA-managed facilities was 178,000 with Total Revenues-Net of $89.9 million (fiscal 2021).
| Page 20 |
The HMCA Growth Strategy
HMCA’s growth strategy is three-pronged: 1) Grow by increasing scan volume at existing sites by applying proven marketing techniques and providing outstanding service to patients and their physicians. 2) Acquire or establish de novo sites in locations that would enhance or expand our existing networks. To pinpoint a de novo site, we conduct thorough demographic studies, identify the primary major medical insurance carriers in the region, and access the competitive landscape. 3) Install additional MRI scanners at sites in need of reducing patient backlog and/or sites that would benefit by expanding MRI services in their regions. Vertical growth, by adding a second or even a third MRI to a site, is very cost effective. Aside from the expense of the added equipment, the only other major costs are those associated with the additional space needed to house the equipment and a minimal increase in staff to accommodate the increase in patient volume.
The Effect of the COVID-19 Pandemic on HMCA
When COVID-19 arrived in March of 2020, all HMCA-managed sites were considered “essential businesses,” which allowed them to continue to serve patients in need of diagnostic testing. For the safety of patients and employees at the sites and at HMCA headquarters, sanitary policies and procedures were immediately implemented and Personal Protective Equipment (PPE) distributed, while, as expected, scan volume at every HMCA site immediately plummeted. In the last quarter of fiscal 2020, when the Coronavirus first took hold, HMCA’s total scan volume, which depends entirely on referrals from medical providers who were also suffering from the effects of COVID-19, was 42% lower than it was in the corresponding quarter of the previous fiscal year. Our management team responded without delay by shortening business hours, reducing staff by either cutting work hours or furloughing, and where possible, allowing employees to work from their homes in order to maintain productivity. As the result of a devastating fourth quarter, the total scan volume for fiscal 2020 (166,698 scans) was down 9% in comparison to fiscal 2019 (184,028 scans).
HMCA’s recovery began in the early part of Fiscal 2021. Over the course of the year, business hours were expanded to accommodate the slowly rising demand. At the same time and in spite of the pandemic, we persevered to complete four major projects that had previously been scheduled for Fiscal 2021: the establishment of a new site in Pembroke Pines, Florida in July of 2020; the addition of a second MRI scanner in Islandia, New York in October of 2020; the addition of a second MRI scanner in the White Plains, New York site in January of 2021; and the acquisition of an existing MRI facility in Yonkers, New York in March 2021. With the increase in scans attributable to the completion of these projects together with the partial, company-wide return to pre-COVID scan volumes, we finished fiscal 2021 with 178,364 scans, which was 7% more than we completed in fiscal 2020 and just 3% shy of our scan volume in pre-COVID fiscal 2019. We are both grateful and proud to have posted Total Revenue-Net of $89.9 million in fiscal 2021, 5% higher than that of fiscal 2020.
| Page 21 |
Looking Ahead
Unfortunately, illnesses due to COVID-19 or its variants, as well as quarantining requirements and vaccination/masking mandates continue to hamper patient volume and also make it very challenging for HMCA to keep the sites and headquarters properly staffed. Yet, we continue to succeed. HMCA scan volume in the first half of fiscal 2022 was the highest first six months in the history of the company and 9.8% higher than the scan volume in the first half of fiscal 2021. I enthusiastically congratulate our management team and all of our employees for their competence, loyalty, and hard work.
Projects already completed in fiscal 2022 or currently underway: the equipment upgrade of one of the two MRI scanners in the White Plains, New York site was completed in July of 2021; a second MRI scanner was installed in the Pembroke Pines, Florida site in November of 2021; the uptown Manhattan site (77th Street) was relocated to midtown Manhattan (55th Street) in December of 2021; a de novo site in the South Bronx, New York is planned to open by the end of fiscal 2022; and a de novo site in Central Florida is scheduled to open shortly after that.
| Ø | FONAR |
FONAR is the Inventor of MR Scanning™. We introduced the world’s first commercial MRI in 1980, went public in 1981, and have installed over 400 MRIs all over the world. We are located in Melville, New York, where we continue to manufacture, sell, service, and upgrade FONAR scanners. Our signature product is the FONAR UPRIGHT® Multi-Position™ MRI (also known as the STAND-UP® MRI). The success of HMCA is largely attributable to this unique product for two primary reasons:
First, it is the only whole-body MRI that performs Position Imaging™ (pMRI™), which means it scans patients in numerous weight-bearing positions, i.e. standing, sitting, in flexion and extension, as well as the conventional lie-down position. The UPRIGHT® MRI has detected problems that were underestimated or missed entirely on “weightless-only” MRI scanners, particularly scans of the spine. With its ability to “see it all,” the UPRIGHT® MRI provides referring physicians the means to achieve BETTER OUTCOMES FOR THEIR PATIENTS. Most MRI studies are of the spine, and it is widely reported that about 80% of adults experience low back pain at some point in their lifetimes. This explains why the UPRIGHT® MRI has gained traction in the medical community.
Second, a significant portion of the patient population seek to avoid or flatly refuse to have their MRI exams in a claustrophobia-inducing “tunnel” or “tube” MRI, which is typical of most MRIs. The comfort and openness of the UPRIGHT® MRI make it the Patient-Friendly™ MRI. Our scanner is very appealing to patients and also to referring physicians who want to accommodate patients who are fearful of or cannot tolerate confining, conventional MRI scanners. To their relief, the UPRIGHT® MRI allows most patients to be scanned while sitting and watching a wide-screen TV.
FONAR users have, for years, benefitted substantially from these highly-prized features and, since the design of the UPRIGHT® MRI remains patent-protected by FONAR, they will continue to enjoy this competitive advantage in the future.
| Page 22 |
Ongoing Research
MRI has brought a new dimension to MEDICAL TREATMENT, the power to VISUALIZE ANATOMIC DETAIL in the body's VITAL SOFT TISSUES (brain, heart, kidney, liver, spleen, lungs, pancreas, intestines) plus MRI's new power to non-invasively QUANTIFY (e.g. measure T1, T2, diffusion, chemical spectra) the response of these VITAL TISSUES to treatment.
Regarding our research efforts, over the past few years we have been making cinés (movies) of the cerebrospinal fluid (CSF) as it flows up and down the neck and in and around the brain. Thanks to the UPRIGHT® MRI’s ability to scan patients in weight-bearing positions as well as in the recumbent, non-weight-bearing position, we are finding significant postural differences in CSF flow. These differences may provide clues which will enable physicians to find solutions to a variety of unsolved medical problems and the power to quantify the degree to which the impaired CSF flow responsible for the patient’s symptoms has been rectified by the patient’s surgical and non-surgical CCJ (craniocervical junction) treatment. Currently, our research is focused on quantifying CSF flow and the velocity at which it navigates through the neck and head. We’ve been able to use this quantitative CSF data collected from asymptomatic patients to identify the degree to which CSF flow impairment is responsible for the patient’s symptoms and the degree to which the patient’s surgical or non-surgical CCJ treatment has restored the patient’s critical brain and central nervous system’s physiology to normal.
This important research of the imaging and quantifying of cerebrospinal fluid (CSF) flow as it circulates from the brain, down the spine and throughout the brain, I believe, is research that will lead to a new understanding of the role of CSF physiology for the neurodegenerative diseases Multiple Sclerosis, Alzheimer’s, Parkinson’s, Amyotrophic Lateral Sclerosis (ALS) and childhood autism. Although progress has been temporarily slowed by the pandemic in fiscal 2021, the Company will, in the future, continue with this valuable research made possible by the introduction of FONAR’s UPRIGHT® MRI imaging technology.
The Highlight of Fiscal 2021: The 50th Birthday of MRI
March 9, 2021 was a very special day for me as well as for FONAR employees, HMCA employees, FONAR users, FONAR stockholders, Nasdaq, family and friends, as it has been 50 years since my discovery of MRI was published in the scientific journal Science on March 19, 1971. In celebration, FONAR had the honor of ringing the Nasdaq closing bell on March 9, 2021. Due to COVID-19, it was a virtual ceremony that could be viewed via a Nasdaq-provided live-stream link.
| Page 23 |

Excerpts from FONAR’s March 9, 2021 press release follow:
FONAR To Ring The Nasdaq Stock Market Closing Bell, As It Celebrates 50th Birthday of MRI
MELVILLE, NEW YORK, March 9, 2021 - FONAR Corporation (NASDAQ-FONR), The Inventor of MR Scanning™, will ring the Nasdaq Stock Market Closing Bell on March 9, 2021. Nasdaq and FONAR selected this day as it is 50 years since the discovery of MRI by Raymond V. Damadian, M.D., FONAR’s founder and chairman, was published in the widely-read, peer-reviewed scientific journal Science on March 19, 1971.
Dr. Damadian said, “I got my first idea for MRI in 1969, and in 1970 made the discovery that is the basis for the making of every MRI image ever produced and the foundation of the MRI industry. This discovery is that there is a marked difference in the relaxation times of the NMR (MRI) signals between normal and cancerous tissues of the same type, as well as between different types of normal tissues. This discovery, published in the peer-reviewed journal ‘Science,’ marked the beginning of the MRI industry.”
The discovery resulted in the generation of the pronounced pixel contrast that was deficient in traditional medical imaging technology (x-ray, CT: 4%) and was needed for adequate visualization of the body's vital organs and assessment of their well-being. The pixel contrast provided by MRI imaging was raised to 131%. As reported in edubilla.com: “But without this discovery of the profound sensitivity of the relaxation time to different tissue types and malignant tissue THERE WOULD BE NO PICTURE AT ALL.”
| Page 24 |
Prior to this 1971 article in the journal Science, there was no realization that existing NMR test tube technology could be transformed into a scanner of the live human body. Soon scientists around the world began researching the discovery.
Dr. Damadian’s discovery, as reported in Science 1971, soon led to the first patent in MRI.** In 1977, with his two post-doctorate students, Lawrence Minkoff, Ph.D., and Michael Goldsmith. Ph.D., he went on to make the world’s first MRI scanner.
Dr. Minkoff recalled the preparations for the world’s first MRI scan. He said: “Dr. Damadian agreed to be the first to go into a huge magnetic field and have resonating radio frequencies directed at his body and specifically the area around his heart. This was a risk as no one in the world to our knowledge had done this before. So the first time, a cardiologist was on hand to help if there was a problem. Unfortunately, the scan did not work. It was later determined that the coil needed further development. So, finally on the evening of July 2, we were able to get an NMR (MRI) signal from my chest. With this successful achievement we were then able to proceed and make the world’s first MRI image of a live human being.”
Dr. Damadian began FONAR, the world’s first dedicated MRI Company, in 1978, and the Company installed the world’s first commercial MRI in 1980. In 1981 FONAR became a NASDAQ-listed company. The Company, today, is at the Nasdaq Stock Market Closing Bell Ceremony delightedly celebrating the 50th anniversary of the birth of MRI.
* R. Damadian, “Tumor Detection by Nuclear Magnetic Resonance,” Science, Volume 171, pp.1151-1153. This paper was referenced by other Scientist-Authors in the field so frequently that it was recognized as a ‘Citation Classic’ by the Institute for Scientific Information, Philadelphia, PA, whose mission it is to monitor scientific citations.
** U.S. Patent #3,789,832, “Apparatus and Method for Detecting Cancer in Tissue,” (1974) that patented the relaxation differences and their use in medical scanning. It was acknowledged and enforced by the U.S. Supreme Court in 1997. There are currently more than 4,500 patents on MRI issued by the U.S. Patent Office.
| Page 25 |
Conclusion
When COVID-19 reached our shores, many thought it would run its course in a matter of weeks, maybe a couple of months. Now it’s in its third year, and we are still unsure of when it will be gone, if ever. We are grateful that not only have we successfully coped with what many describe as one of the most formidable crises Americans have ever faced, but we have remained profitable through it all. The credit belongs to FONAR’s enduring technology; the skillful management of our diagnostic imaging management subsidiary, HMCA; the competence, loyalty and hard work of our employees; and the loyal support of our stockholders. We long for a full return to a pandemic-free business environment in which we can expect even greater success.
Sincerely,
Raymond V. Damadian
Chairman and Founder
FONAR Corporation
| SECURITIES AND EXCHANGE COMMISSION | ||
| WASHINGTON, D.C. 20549 | ||
| FORM 10-K |
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended June 30, 2021
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES AND EXCHANGE ACT OF 1934
For the transition period from _____________ to _____________
Commission File No. 0-10248

| FONAR CORPORATION | ||
| (Exact name of registrant as specified in its charter) |
| delaware | 11-2464137 | |||
| (State of incorporation) | (IRS Employer Identification Number) | |||
| 110 Marcus Drive, Melville, New York | 11747 | |||
| (Address of principal executive offices) | (Zip Code) |
| (631) 694-2929 | ||
| (Registrant’s telephone number, including area code) | ||
| Securities registered pursuant to Section 12(b) of the Act: | ||
| Common Stock, par value $.0001 per share | ||
| Securities registered pursuant to Section 12(g) of the Act: | ||
| None |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒ .
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒ .
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐ .
Indicate by check mark whether the registrant (1) has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (Section 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
| Page 26 |
Indicate by check mark if disclosure of delinquent filers, pursuant to Item 405 of Regulation S-K, §229.405 of this Chapter, is not contained, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this 10-K or any amendment to the Form 10-K. ☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer”, “accelerated filer and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer ☐ | Accelerated filer ☐ | Non-accelerated filer ☒ |
| Smaller reporting company ☒ | Emerging Growth Company ☐ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒ .
The aggregate market value of the shares of Common Stock held by non-affiliates as of December 31, 2020 based on the closing price of $17.36 per share on such date as reported on the NASDAQ System, was approximately $110 million. The other outstanding classes do not have a readily determinable market value.
As of September 15, 2021, 6,554,210 shares of Common Stock, 146 shares of Class B Common Stock, 382,513 shares of Class C Common Stock and 313,438 shares of Class A Non-voting Preferred Stock of the registrant were outstanding.
| Page 27 |
DOCUMENTS INCORPORATED BY REFERENCE
| FORM 10-K ITEMS | PAGE | |||||
| PART I | Item 1. | Business | 4 | |||
| Item 1A. | Risk Factors | 30 | ||||
| Item 1B. | Unresolved Staff Comments | 33 | ||||
| Item 2. | Properties | 33 | ||||
| Item 3. | Legal Proceedings | 33 | ||||
| Item 4. | Mine Safety Disclosures | 34 | ||||
| PART II | Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters | 34 | |||
| Item 6. | Selected Financial Data | 35 | ||||
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations | 36 | ||||
| Item 8. | Financial Statements | 47 | ||||
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | 90 | ||||
| Item 9A. | Controls and Procedures | 90 | ||||
| Item 9B. | Other Information | 91 | ||||
| PART III | Item 10. | Directors and Executive Officers | 91 | |||
| Item 11. | Executive Compensation | 95 | ||||
| Item 12. | Security Ownership of Certain Beneficial Owners and Management | 98 | ||||
| Item 13. | Certain Relationships and Related Transactions, and Director Independence | 100 | ||||
| Item 14. | Principal Accountant Fees and Services | 101 | ||||
| PART IV | Item 15. | Exhibits | 102 |
| Page 28 |
FONAR CORPORATION AND SUBSIDIARIES
PART I
ITEM 1. BUSINESS
GENERAL
Fonar Corporation, sometimes referred to as the “Company” or “Fonar”, is a Delaware corporation which was incorporated on July 17, 1978. Our address is 110 Marcus Drive, Melville, New York 11747 and our telephone number is 631-694-2929. Fonar also maintains a website at www.fonar.com. Fonar provides copies of its filings with the Securities and Exchange Commission on Forms 10-K, 10-Q and 8-K and amendments to these reports to stockholders on request.
We conduct our business in two segments. Our medical equipment segment is conducted directly through Fonar. Our physician management and diagnostic services segment is conducted through our subsidiary Health Diagnostic Management, LLC (“HMCA”), also called Health Management Company of America. HMCA provides management services, administrative services, billing and collection services, credentialing services, contract negotiations, compliance consulting, purchasing, IT services, hiring, conducting interviews and managing personnel, storage of medical records, office space, equipment, repair, maintenance service, and clerical and other non-medical personnel to medical providers engaged in diagnostic imaging. In addition to acting as a management company, HMCA owns and operates four diagnostic imaging facilities in Florida, where the corporate practice of medicine is permitted.
We restructured the corporate organization of our physician and diagnostic services management segment of our business effective July 1, 2015. Imperial Management Services, LLC (“Imperial”), a subsidiary which owned the assets used in the business of its parent, Health Management Corporation of America (which is wholly-owned by Fonar), transferred those assets to Health Diagnostics Management, LLC (“HDM”), which is another subsidiary of Health Management Corporation of America. As a result, going forward our physician and diagnostic management business will be conducted entirely through HDM, which is operating under the assumed name Health Management Company of America.
Fonar is engaged in the business of designing, manufacturing, selling and servicing magnetic resonance imaging scanners, also referred to as “MRI” or “MR” scanners, which utilize MRI technology for the detection and diagnosis of human disease, abnormalities, other medical conditions and injuries. Fonar’s founders built the first MRI scanner in 1977 and Fonar introduced the first commercial MRI scanner in 1980. Fonar is also the originator of the iron-core non-superconductive and permanent magnet MRI technology.
Fonar’s iron frame technology made Fonar the originator of “open” MRI scanners. We introduced the first “open” MRI in 1980. Since that time we have concentrated on further application of our “open” MRI, introducing most recently the Upright® Multi-Position™” MRI scanner (also referred to as the “Upright®” or “Stand-Up®” MRI scanner) and the Fonar 360™ MRI scanner. The Fonar 360™ MRI is not presently being marketed.
See Note 17 to the Consolidated Financial Statements for separate financial information regarding our medical equipment and physician and diagnostic management services segments.
| Page 29 |
FONAR CORPORATION AND SUBSIDIARIES
FORWARD LOOKING STATEMENTS.
Certain statements made in this Annual Report on Form 10-K are “forward-looking statements”, within the meaning of the Private Securities Litigation Reform Act of 1995, regarding the plans and objectives of Management for future operations. Such statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. These forward-looking statements are based on current expectations that involve numerous risks and uncertainties. Our plans and objectives are based, in part, on assumptions involving the expansion of business. These assumptions involve judgments with respect to, among other things, future economic, competitive and market conditions and future business decisions, all of which are difficult or impossible to predict accurately and many of which are beyond our control. Although we believe that our assumptions underlying the forward-looking statements are reasonable, any of the assumptions could prove inaccurate and, therefore, there can be no assurance that the forward-looking statements included in this Annual Report will prove to be accurate. In light of the significant uncertainties inherent in our forward-looking statements, the inclusion of such information should not be regarded as a representation by us or any other person that our objectives and plans will be achieved.
Among the risks and assumptions which must now be taken into account is the COVID-19 virus, which adds additional uncertainties to future expectations. Although the impact will be negative, the severity, duration and recurrence of new strains of the COVID-19 virus such as the Delta variant adds a new dimension to the difficulties facing our business and the world economy in general.
THE UPRIGHT® MRI SCANNER
The Upright® MRI scanner is the product we are presently promoting. The Upright® MRI (also known as the “Stand-Up® MRI”) is a “whole-body” MRI, meaning it can be used to scan any part of the body. Unlike conventional recumbent MRI scanners, where the patient must lie on his or her back, the Upright® MRI permits MRI scans to be taken in a weight-bearing state. Patients can be scanned while standing, sitting, bending or lying down. This means that an abnormality or injury, such as a slipped disk, may be scanned in a weight-bearing posture, which more often than not is the position in which patients experience pain. An adjustable bed allows patients to stand, sit or lie on their backs, sides or stomachs. The Upright® MRI is by design a non-claustrophobic MRI scanner. We have introduced the name “Upright®” as an alternative to “Stand-Up®” because of the multiplicity of positions in which the patient may be scanned where the patient is not standing.
As of June 30, 2021, HMCA manages a total of 39 MRI scanners. Twenty-five (25) MRI scanners are located in New York and fourteen (14) which are located in Florida. We believe that the utilization of Fonar UPRIGHT® MRI scanning systems has been a significant factor in maintaining the patient volume of the scanning facilities and our ability to cope with the effects of the COVID-19 pandemic. In addition, a new facility managed by the Company has been opened in Pembroke Pines, Florida and a total of four additional MRI scanners were added in New York.
| Page 30 |
FONAR CORPORATION AND SUBSIDIARIES
MEDICAL EQUIPMENT SEGMENT
PRODUCTS
The Fonar Upright® MRI is a weight-bearing whole-body open MRI system which enables positional MRI (pMRI®) applications. Operating at a magnetic field strength of 0.6 Tesla, the scanner is a powerful, diagnostically versatile and cost-effective open MRI that provides a broad range of clinical capabilities and a complete set of imaging protocols. Patients can be scanned standing, bending, sitting, upright at an intermediate angle and in the conventional recumbent position. This multi-positional MRI system accommodates an unrestricted range of motion for flexion, extension, lateral bending, and rotation studies of the cervical (upper) and lumbar (lower) spine. Previously difficult patient scanning positions can be achieved and compared using the system’s MRI-compatible, three-dimensional, motorized patient handling system. The system’s lift and tilt functions deliver the targeted anatomical region to the center of the magnet. True image orientation is assured, regardless of the rotation angle, via computer read-back of the table’s position.
There is considerable evidence that the weight-bearing Upright® MRI provides medical benefits not duplicated by any other MRI scanner because patient positioning plays a critical role in accurately detecting clinically significant pathology.
For instance, the Fonar Upright® technology has demonstrated its key value on patients with the Arnold-Chiari Syndrome, which is believed to affect 200,000 to 500,000 Americans. In this syndrome, brain stem compression and subsequent severe neurological symptoms occur in these patients, when because of weakness in the support tissues within the skull, the brain stem descends and is compressed and entrapped at the base of the skull in the foramen magnum, which is the circular bony opening at the base of the skull where the spinal cord exits the skull. The brain structures “entrapped” in Chiari Syndrome are the lowest lying structures of the brain, the tonsils of the cerebellum. The Chiari Syndrome is therefore alternately named Cerebellar Tonsillar Ectopia (CTE) indicating the displacement (ectopia) of these Cerebellar tonsils in this syndrome. Classic symptoms of the Chiari Syndrome include the “drop attack,” where the patient unexpectedly experiences an explosive rush at the base of the brain which runs down the body to the extremities, causing the patient to collapse in a temporary neuromuscular paralysis. These symptoms subside when the patient is lying down. Conventional lie-down MRI scanners cannot make an adequate evaluation of the pathology since the patient’s pathology is most visible and the symptoms are most acute when the patient is scanned in the upright weight-bearing position.
A publication in the Journal “Brain Injury” (Brain Injury 2010, 24 (7-8) 988-994) of 1,200 neck pain patients reported that the fallen cerebellar tonsils of the brain (CTE) were missed 75% of the time when the patient was scanned only in the recumbent position. It is critical to have an image of the patient in an upright position so that the neurosurgeons can fully evaluate the brain stem and choose the most appropriate surgical approach for an operative repair.
| Page 31 |
FONAR CORPORATION AND SUBSIDIARIES
The study was published by 10 authors from distinguished universities in the United States and around the world. The study reported that Cerebellar Tonsillar Ectopia Herniation (CTE) was missed 75% of the time when the patient was scanned lying down instead of upright. At the current rate of 1,000,000 automobile whiplash injuries in the U.S. per year, 750,000 patients each year would have the pathology responsible for their symptoms go undetected if they were examined solely in a conventional recumbent-only MRI.
The Upright® MRI has also demonstrated its value for patients suffering from scoliosis. Scoliosis patients typically have been subjected to routine x-ray exams for years and must be imaged upright for an adequate evaluation of their scoliosis. Because the patient must be standing for the exam, an x-ray machine has been the only modality that could provide that service. The Upright® MRI is the only MRI scanner that allows the patient to stand during the MRI exam. Fonar has developed a new RF receiver and scanning protocol that for the first time allows scoliosis patients to obtain diagnostic pictures of their spines without the risks of x-rays. A study by the National Cancer Institute (2000) of 5,466 women with scoliosis reported a 70% increase in breast cancer resulting from 24.7 chest x-rays these patients received on average in the course of their scoliosis treatment.
Other important new applications are Upright® imaging of the pelvic floor and abdomen to image prolapses and inguinal hernias. Fonar has also developed the first non-invasive method to image the prostate: the patient simply sits on a flat, seat-like coil.
The Upright® MRI is also the world’s most non-claustrophobic whole-body MRI scanner. Frequently, patients can simply walk into the magnet, stand or sit for their scans and then walk out. The magnet’s front-open and top-open design provides an unprecedented degree of comfort because there is nothing in front of the patient’s face except a large (42”) flat-screen TV that is mounted on the wall. The default position for the bed is a tilt back of six degrees that minimizes patient motion. Special RF receiver coil fixtures, a patient seat, Velcro straps, and transpolar stabilizing bars are also used to keep the patient comfortable and motionless throughout the scanning process.
Full-range-of-motion studies of the joints in multiple directions are possible, an especially useful feature for sports injuries. Full range of motion cines, or movies, of the lumbar spine can also be achieved under full body weight.
The Fonar Upright® MRI operates at a significantly higher magnetic field strength than earlier open MRIs that preceded it, and, therefore, benefits from more of the MRI image-producing signal needed to make high-quality MRI images.
Fonar maximizes image quality through an optimal combination of image signal to noise (S/N) and contrast-to noise (C/N) ratios. Technical improvements incorporated into the scanner design include increased image processing speed, high-S/N Organ Specific(TM) RF receiver coils, high performance front-end electronics featuring high-speed, wide-dynamic-range analog-to-digital conversion and a miniaturized ultra-low-noise pre-amplifier, high-speed automatic tuning, bandwidth-optimized pulse sequences, multi-bandwidth sequences, and off-center FOV imaging capability.
| Page 32 |
FONAR CORPORATION AND SUBSIDIARIES
In addition to the signal-to-noise ratio, however, a major determinant of image quality that must be considered is contrast, the quality that enables reading physicians to clearly distinguish adjacent, and sometimes minute, anatomical structures from their surroundings. This quality is measured by contrast-to-noise ratios (C/N). Unlike S/N, which increases with increasing field strength, relaxometry studies have shown that C/N peaks in the mid-field range and actually falls off precipitously at higher field strengths. The Upright® MRI scanners operate squarely in the optimum C/N range.
FONAR’s scanners are equipped with a variety of software features which enhance versatility and diagnostic capability. For example, SMART™ scanning allows for same-scan customization of multi-slice scans, each slice with its own thickness, resolution, angle and position. This is an important feature for scanning parts of the body that include small-structure sub-regions requiring finer slice parameters. There is also Multi-Angle Oblique™ (MAO) imaging, and oblique imaging.
During fiscal 2021, sales of our Upright® MRI scanners accounted for approximately 0.8% of our total revenues and 10.0% of our medical equipment revenues, as compared to 0.1% of total revenues and 1.0% of medical equipment revenues in fiscal 2020.
FONAR’s principal marketing efforts with respect to its products have been focused on the Upright® MRI, which we believe is a particularly unique product. It is the only MRI scanner which is both open and allows for weight-bearing imaging. We expect to continue our focus on the Upright® MRI in the immediate future.
The materials and components used in the manufacture of our products (circuit boards, computer hardware components, electrical components, steel and plastic) are generally available at competitive prices. We have not had difficulty acquiring such materials.
PRODUCT MARKETING
The principal markets for the Company’s scanners are private diagnostic imaging centers and hospitals.
We use internal personnel and independent manufacturer’s representatives for domestic and foreign markets. None of Fonar’s competitors are entitled or been licensed to make the Fonar Upright® MRI scanner.
Fonar’s Website includes interactive product information for interested customers.
During fiscal 2021 and previously sales were made to foreign customers. CEO Matthias Schulz of Medserena, Fonar’s principal foreign sales representative and distributor, has said, “The large number of requests coming from our physicians in Germany are arising because of the special medical need for FONAR’s unique technology. This is in spite of an intensely active MRI market in Germany, where there are already many conventional lie-down MRIs installed.”
| Page 33 |
FONAR CORPORATION AND SUBSIDIARIES
Fonar’s marketing strategy has been designed to reach key purchasing decision makers with information concerning the Upright® MRI. This has led to many inquiries and to some sales of the Upright® MRI scanner and is intended to increase Fonar’s presence in the medical market. Fonar focuses on four target audiences: neurosurgeons, orthopaedic surgeons, radiologists and physicians in general.
| 1) | Neurosurgeons and Orthopaedic Surgeons: These are the surgeons who can most benefit from the superior diagnostic benefits of the Fonar Upright® MRI with its Multi-Position® MRI diagnostic ability. | |
| 2) | Radiologists: These physicians can now offer a new Multi-Position®, weight-bearing MRI modality to their referring physicians. | |
| 3) | All Physicians: The vast number of doctors who send patients for MRI’s need to be aware of the diagnostic advantages of the Fonar Upright® Multi-Position™. |
Our advertising for Fonar and HMCA re-enforces the unique value provided by Fonar MRI scanners. We have increased internet awareness of our product by driving patient traffic to the Upright® scanning centers we manage via the Fonar website (www.fonar.com) as well as by creating Websites for each HMCA location. These websites give prospective customers of Upright® MRI scanners a view of operating Upright® MRI centers and highlight the benefits of using an Upright® MRI scanner. A complete list of the sites managed by HMCA can be found at HMCA’s website, hmca.com.
Our marketing efforts, however, have been compromised by the COVID-19 panademic and economic challenges felt worldwide as a result.
SERVICE AND UPGRADES FOR MRI SCANNERS
Our customer base of installed scanners has been and will continue to be an additional source of income, independent of direct sales.
Income is generated from the installed base in two principal areas, namely, service and upgrades. Service and maintenance revenues from our external installed base were approximately $7.7 million in fiscal 2021 and $8.2 million in fiscal 2020. Our objective is to maintain service revenues at present levels or better, based on the longevity of the technology, and the refurbishments and upgrades which keep the scanners competitive with the latest techniques.
We also anticipate that our scanners will result in upgrades income in future fiscal years. The potential for upgrades income, originates in the versatility and productivity of the Upright® Imaging technology. New medical uses for MRI technology are constantly being discovered and are anticipated for the Upright® Imaging technology as well. New features can often be added to the scanner by the implementation of little more than versatile new software packages, which when coupled with hardware upgrades can add years of useful life to the scanner.
| Page 34 |
FONAR CORPORATION AND SUBSIDIARIES
RESEARCH AND DEVELOPMENT
During the fiscal year ended June 30, 2021, we incurred expenditures of $1,635,979, none of which were capitalized, on research and development, as compared to $2,025,376, none of which were capitalized, during the fiscal year ended June 30, 2020.
Research and development activities have focused principally on software improvements to the user interface of the MRI scanner. The Windows-based Sympulse™ platform controls all of the functions of the Upright® scanner except those of the versatile, multi-position patient table. Separate, dedicated, motion-control software is used to maneuver the Upright® bed, and development of this software is ongoing as well.
While software improvements to the user interface are important in their own right, significant value is added to the MRI scanner by the modification of existing protocols for examining various parts of the body, and the development of new protocols that utilize new underlying capabilities of the pulse sequence software. Over time, FONAR users have become accustomed to the steady improvement in the recommended clinical protocols that accompany new software releases. More significantly, in recent years we have seen increasing adoption of FONAR-recommended clinical protocols over those developed on site. This is a testament to the superior image quality they produce in attractively short scan times.
The development of clinically practical scan protocols and software depends on close contact between research and development scientists and engineers, and end users. That close contact is facilitated in part by the relationship with HMCA and the scanning centers. In addition to that collaboration, R&D staff have pursued a variety of novel and Upright® MRI-specific research projects. It is anticipated that these will ultimately lead to new applications that are made available to existing customers as upgrade add-ons to their machines. For example, phase-contrast imaging techniques originally developed for angiography have recently been applied to cerebro-spinal fluid (CSF) flow. Analysis of CSF flow in upright and recumbent postures may prove to be of significant value in the evaluation of a variety of disorders.
BACKLOG
Our backlog of unfilled orders at September 15, 2021 was approximately $62,000, as compared to $457,000 at September 15, 2020. It is expected that the existing backlog of orders will be filled within the 2022 fiscal year.
PATENTS AND LICENSE
We currently have numerous patents in effect which relate to the technology and components of our MRI scanners. We believe that these patents, and the know-how we have developed, are material to our business.
| Page 35 |
FONAR CORPORATION AND SUBSIDIARIES
One of our patents, issued in the name of Dr. Damadian and licensed to Fonar, was United States patent No. 3,789,832, Apparatus and Method for Detecting Cancer in Tissue, also referred to in this report as the “1974 Patent”. The 1974 Patent was the first MRI patent issued by the United States Patent Office. The development of our MRI scanners has been based upon the 1974 Patent, and we believe that the 1974 Patent was the first of its kind to utilize MR to scan the human body and to detect cancer. The 1974 Patent was extended beyond its original 17-year term and expired in February, 1992.
We have significantly enhanced our patent position within the industry and now possess a substantial patent portfolio which provides us, under the aegis of United States patent law, “the exclusive right to make, use and sell” many of the scanner features which Fonar pioneered and which are now incorporated in most MRI scanners sold by the industry. As of June 30, 2021, 220 patents had been issued to Fonar, and approximately 11 patents were pending. A number of Fonar’s existing patents specifically relate to protecting Fonar’s position in the Upright MRI market. The patents further enhance Dr. Damadian’s pioneer patent, the 1974 Patent, that initiated the MRI industry and provided the original invention of MRI scanning. The terms of the patents in Fonar’s portfolio extend to various times.
We also have patent cross-licensing agreements with other MRI manufacturers. We have not licensed, however, any technology relating to Upright® MRI scanning.
PRODUCT COMPETITION
MRI SCANNERS
MRI takes advantage of the nuclear magnetic resonance signal elicited from the body’s tissues and the exceptional sensitivity of this signal for detecting disease discovered by Fonar. Much of the serious disease of the body occurs in the soft tissue of vital organs. The maximum contrast available by x-ray with which to discriminate disease is 4%. Brain cancers differ from surrounding healthy brain by only 1.6% while the contrast in the brain by MRI is 25 times greater at 40%. X-ray contrasts among the body’s soft tissues are maximally 4%. Their contrast by MRI is 32.5 times greater (130%).
The soft tissue contrasts with which to distinguish cancers on images by MRI are up to 180%. In the case of cancer these contrasts can be even more marked making cancers readily visible and detectable anywhere in the body. This is because the nuclear resonance signals from the body’s normal soft tissue vital organs, differ so dramatically from each other (e.g. small intestine 257 milliseconds, brain 595 milliseconds). Liver cancer and healthy liver signals differ by 180% for example.
A majority of the MRI scanners in use in hospitals and outpatient facilities and at mobile sites in the United States are based on high field (1.5 - 3.0 Tesla) air core superconducting magnet technology.
| Page 36 |
FONAR CORPORATION AND SUBSIDIARIES
Open MRIs manufactured by Fonar’s competitors, are recumbent-only machines based on Fonar’s original iron-frame vertical magnetic field magnet design. These systems have been manufactured and sold by many of our largest competitors over the years. They generally operate at low field strengths (0.2 - 0.35 Tesla). Their prevalence in the marketplace has led to the perception in the medical community that Open MRIs are useful only for anxious and claustrophobic patients, that the Open MRI’s image quality is poor, and that the scan times are long. Recently our competitors have introduced higher field strength Open MRI products (0.5 – 1.2 Tesla). Significantly better imaging performance (especially at 1.2 T) compared to the low field strength systems, is beginning to change that perception. However, Fonar continues to maintain its competitive advantage at 0.6 Tesla due to our front-open non-claustrophobic configuration in which there is nothing in front of the patient’s face, and our unique ability to scan patients in weight-bearing positions. It is also noteworthy that our horizontal transaxial magnetic field allows the Upright MRI, in contrast to the recumbent-only Open MRIs, to use the same flat planar-style radiofrequency receiver coil as the high-field MRI systems to image the lumbar and thoracic spine.
The Upright MRI uses the same configuration RF receiver coil as a high-field MRI system to image the spine other Open MRIs cannot do this. (This is because of the rule in MRI that the axis of symmetry of the RF receiver coil should be perpendicular to the direction of the main magnetic field). The upright patient sits comfortably with his back against a flat (“planar”) RF receiver coil in our horizontal transaxial magnetic field. In contrast, the vertical magnetic field in the recumbent-only Open MRI precludes the use of this type of receiver coil.
Relative to the high-field systems, the Upright MRI has two major competitive advantages:
Sometimes patient positioning is more consequential than a small increase in the image resolution and decrease in the scan time. As it is critical for physicians to not “miss” anything in the images, they recognize that the position-dependent pathology visualized with the Upright MRI will be invisible (“missed”) if their patients are scanned at a higher field strength.
Image artifacts arising from metal implants such as surgical screws are diminished with the 0.6 Tesla Upright MRI compared to those from the high-field MRIs. It is well known that such artifacts get smaller as the MRI magnet’s field strength is reduced, so the anatomy adjacent to implanted hardware will be less obscured with the Upright MRI. This is particularly valuable for surgeons referring their postoperative patients for diagnostic imaging studies.
Fonar faces competition within the MRI industry from such firms as General Electric Company, Philips N.V., Toshiba Corporation, Hitachi Corporation and Siemens A.G. Most competitors have marketing and financial resources more substantial than those available to us. They have in the past, and may in the future, heavily discount the sales price of their scanners. Such competitors sell both high field air core superconducting MRI scanners and iron frame products. Fonar’s original iron frame design, ultimately imitated by Fonar’s competitors to duplicate Fonar’s origination of “Open” MRI magnets, gave rise to current patent protected Upright® MRI technology with the result that Fonar today is the unique and only supplier of the highest field MRI magnets (0.6 Tesla) that are not superconducting, do not use liquid helium and are not therefore susceptible to severe consequences and downtime cause by a system quench.
| Page 37 |
FONAR CORPORATION AND SUBSIDIARIES
The iron frame, because it controls the magnetic lines of force and places them where wanted and removes them from where not wanted, provides a more versatile magnet design than is possible with air core magnets. Air core magnets contain no iron but consist entirely of turns of current carrying wire.
Fonar expects to be the leader in weight-bearing and positional MRI for providing dynamic visualization of body parts including the spine and extremities.
OTHER IMAGING MODALITIES
Fonar’s MRI scanners also compete with other diagnostic imaging systems, all of which are based upon the ability of energy waves to penetrate human tissue and to be detected by either photographic film or electronic devices for presentation of an image on a display monitor. Three different kinds of energy waves - X-ray, gamma and sound - are used in medical imaging techniques which compete with MRI medical scanning, the first two of which involve exposing the patient to potentially harmful radiation. These other imaging modalities compete with MRI products on the basis of specific applications.
X-rays are the most common energy source used in imaging the body and are employed in three imaging modalities:
| 1. | Conventional X-ray systems, the oldest method of imaging, are typically used to image bones and teeth. The image resolution of adjacent structures that have high contrast, such as bone adjacent to soft tissue, is excellent, while the discrimination between soft tissue organs is poor because of the nearly equivalent penetration of x-rays. |
| 2. | Computerized Tomography, also referred to as “CT”, systems couple computers to x-ray instruments to produce cross-sectional images of particular large organs or areas of the body. The CT scanner addresses the need for images, not available by conventional radiography, that display anatomic relationships spatially. However, CT images are generally limited to the transverse plane and cannot readily be obtained in the two other planes, sagittal and coronal. Improved picture resolution is available at the expense of increased exposure to x-rays from multiple projections. Furthermore, the pictures obtained by this method are computer reconstructions of a series of projections and, once diseased tissue has been detected, CT scanning cannot be focused for more detailed pictorial analysis or obtain a chemical analysis. |
| 3. | Digital radiography systems add computer image processing capability to conventional x-ray systems. Digital radiography can be used in a number of diagnostic procedures which provide continuous imaging of a particular area with enhanced image quality and reduced patient exposure to radiation. |
| 4. | Nuclear medicine systems, which are based upon the detection of gamma radiation generated by radioactive pharmaceuticals introduced into the body, are used to provide information concerning soft tissue and internal body organs and particularly to examine organ function over time. |
| Page 38 |
FONAR CORPORATION AND SUBSIDIARIES
| 5. | Ultrasound systems emit, detect and process high frequency sound waves reflected from organ boundaries and tissue interfaces to generate images of soft tissue and internal body organs. Although the images are substantially less detailed than those obtainable with x-ray methods, ultrasound is generally considered harmless and therefore has found particular use in imaging the pregnant uterus. |
X-ray machines, ultrasound machines, digital radiography systems and nuclear medicine compete with the MRI scanners by offering significantly lower price and space requirements. However, Fonar believes that the utility of the images produced by its MRI scanners is generally superior to the utility of the images produced by those other methodologies.
GOVERNMENT REGULATION
FDA Regulation
The Food and Drug Administration in accordance with Title 21 of the Code of Federal Regulations regulates the manufacturing and marketing of Fonar’s MRI scanners. The regulations can be classified as either pre-market or post-market. The pre-market requirements include obtaining marketing clearance, proper device labeling, establishment registration and device listing. Once the products are on the market, Fonar must comply with post-market surveillance controls. These requirements include the Quality Systems Regulation, or “QSR”, also known as Current Good Manufacturing Practices or CGMPs, and Medical Device Reporting, also referred to as MDR regulations. The QSR is a quality assurance requirement that covers the design, packaging, labeling and manufacturing of a medical device. The MDR regulation is an adverse event-reporting program.
Classes of Products
Under the Medical Device Amendments of 1976 to the Federal Food, Drug and Cosmetic Act, all medical devices are classified by the FDA into one of three classes. A Class I device is subject only to general controls, such as labeling requirements and manufacturing practices; a Class II device must comply with certain performance standards established by the FDA; and a Class III device must obtain pre-market approval from the FDA prior to commercial marketing. Fonar’s products are Class II devices. Class II devices are subject to “General Controls”; General Controls include:
1. Establishment registration of companies which are required to register under 21 CFR Part 807.20, such as manufacturers, distributors, re-packagers and re-labelers.
2. Medical device listing with FDA of devices to be marketed.
3. Manufacturing devices in accordance with the Current Good Manufacturing Practices Quality System Regulation in 21 CFR Part 820.
4. Labeling devices in accordance with labeling regulations in 21 CFR Part 801 or 809.
5. Submission of a Premarket Notification, pursuant to 510(k), before marketing a device.
| Page 39 |
FONAR CORPORATION AND SUBSIDIARIES
In addition to complying with general controls, Class II devices are also subject to special controls. Special controls may include special labeling requirements, guidance documents, mandatory performance standards and post-market surveillance.
On October 3, 2000 Fonar received FDA clearance for the Upright® MRI under the name “Indomitable”.
Premarketing Submission
Each person who wants to market Class I, II and some III devices intended for human use in the U.S. must submit a 510(k) to FDA at least 90 days before marketing unless the device is exempt from 510(k) requirements. A 510(k) is a pre-marketing submission made to FDA to demonstrate that the device to be marketed is as safe and effective, that is, substantially equivalent, SE, to a legally marketed device that is not subject to pre-market approval, PMA. Applicants must compare their 510(k) device to one or more similar devices currently on the U.S. market and make and support their substantial equivalency claims.
The FDA is committed to a 90-day clearance after submission of a 510(k), provided the 510(k) is complete and there is no need to submit additional information or data.
The 510(k) is essentially a brief statement and description of the product. As Fonar’s scanner products are Class II products, there are no pre-market data requirements.
An investigational device exemption, also referred to as IDE, allows the investigational device to be used in a clinical study pending FDA clearance in order to collect safety and effectiveness data required to support the Premarket Approval, also referred to as PMA, application or a Premarket Notification pursuant to 510(k), submission to the FDA. Clinical studies are most often conducted to support a PMA.
For the most part, however, we have not found it necessary to utilize IDE’s. The standard 90 day clearance for our new MRI scanner products classified as Class II products makes the IDE unnecessary, particularly in view of the time and effort involved in compiling the information necessary to support an IDE.
Quality System Regulation
The Quality Management System is applicable to the design, manufacture, administration of installation and servicing of magnetic resonance imaging scanner systems. The FDA has authority to conduct detailed inspections of manufacturing plants, to establish Good Manufacturing Practices which must be followed in the manufacture of medical devices, to require periodic reporting of product defects and to prohibit the exportation of medical devices that do not comply with the law.
| Page 40 |
FONAR CORPORATION AND SUBSIDIARIES
Medical Device Reporting Regulation
Manufacturers must report all MDR reportable events to the FDA. Each manufacturer must review and evaluate all complaints to determine whether the complaint represents an event which is required to be reported to FDA. Section 820.3(b) of the Quality Systems regulation defines a complaint as, “any written, electronic or oral communication that alleges deficiencies related to the identity, quality, durability, reliability, safety, effectiveness, or performance of a device after it is released for distribution.”
A report is required when a manufacturer becomes aware of information that reasonably suggests that one of their marketed devices has or may have caused or contributed to a death, serious injury, or has malfunctioned and that the device or a similar device marketed by the manufacturer would be likely to cause or contribute to a death or serious injury if the malfunction were to recur.
Malfunctions are not reportable if they are not likely to result in a death, serious injury or other significant adverse event experience.
A malfunction which is or can be corrected during routine service or device maintenance still must be reported if the recurrence of the malfunction is likely to cause or contribute to a death or serious injury if it were to recur.
We have established and maintained written procedures for implementation of the MDR regulation. These procedures include internal systems that:
provide for timely and effective identification, communication and evaluation of adverse
events;
provide a standardized review process and procedures for determining whether or not an
event is reportable; and
provide procedures to insure the timely transmission of complete reports.
These procedures also include documentation and record keeping requirements for:information that was evaluated to determine if an event was reportable;
all medical device reports and information submitted to the FDA;
any information that was evaluated during preparation of annual certification reports; and
systems that ensure access to information that facilitates timely follow up and inspection by
FDA.
FDA Enforcement
FDA may take the following actions to enforce the MDR regulation:
| Page 41 |
FONAR CORPORATION AND SUBSIDIARIES
FDA-Initiated or Voluntary Recalls
Recalls are regulatory actions that remove a hazardous, potentially hazardous, or a misbranded product from the marketplace. Recalls are also used to convey additional information to the user concerning the safe use of the product. Either FDA or the manufacturer can initiate recalls.
There are three classifications, i.e., I, II, or III, assigned by the Food and Drug Administration to a particular product recall to indicate the relative degree of health hazard presented by the product being recalled.
Class I
Is a situation in which there is a reasonable probability that the use of, or exposure to, a violative product will cause serious adverse health consequences or death.
Class II
Is a situation in which use of, or exposure to, a violative product may cause temporary or medically reversible adverse health consequences or where the probability of serious adverse health consequences is remote.
Class III
Is a situation in which use of, or exposure to, a violative product is not likely to cause adverse health consequences.
Fonar has initiated six voluntary recalls. Five of the recalls were Class II and one was Class III. The recalls involved making minor corrections to the product in the field. Frequently, corrections which are made at the site of the device are called field corrections as opposed to recalls.
Civil Money Penalties
The FDA, after an appropriate hearing, may impose civil money penalties for violations of the FD&C Act that relate to medical devices. In determining the amount of a civil penalty, FDA will take into account the nature, circumstances, extent, and gravity of the violations, the violator’s ability to pay, the effect on the violator’s ability to continue to do business, and any history of prior violations.
Warning Letters
FDA issues written communications to a firm, indicating that the firm may incur more severe sanctions if the violations described in the letter are not corrected. Warning letters are issued to cause prompt correction of violations that pose a hazard to health or that involve economic deception. The FDA generally issues the letters before pursuing more severe sanctions.
| Page 42 |
FONAR CORPORATION AND SUBSIDIARIES
Seizure
A seizure is a civil court action against a specific quantity of goods which enables the FDA to remove these goods from commercial channels. After seizure, no one may tamper with the goods except by permission of the court. The court usually gives the owner or claimant of the seized merchandise approximately 30 days to decide a course of action. If they take no action, the court will recommend disposal of the goods. If the owner decides to contest the government’s charges, the court will schedule the case for trial. A third option allows the owner of the goods to request permission of the court to bring the goods into compliance with the law. The owner of the goods is required to provide a bond or, security deposit, to assure that they will perform the orders of the court, and the owner must pay for FDA supervision of any activities by the company to bring the goods into compliance.
Citation
A citation is a formal warning to a firm of intent to prosecute the firm if violations of the FD&C Act are not corrected. It provides the firm an opportunity to convince FDA not to prosecute.
Injunction
An injunction is a civil action filed by FDA against an individual or company. Usually, FDA files an injunction to stop a company from continuing to manufacture, package or distribute products that are in violation of the law.
Prosecution
Prosecution is a criminal action filed by FDA against a company or individual charging violation of the law for past practices.
Foreign and Export Regulation
We obtain approvals as necessary in connection with the sales of our products in foreign countries. In some cases, FDA approval has been sufficient for foreign sales as well. Our standard practice has been to require either the distributor or the customer to obtain any such foreign approvals or licenses which may be required.
Legally marketed devices that comply with the requirements of the Food Drug & Cosmetic Act require a Certificate to Foreign Government issued by the FDA for export. Other devices that do not meet the requirements of the FD&C Act but comply with the laws of a foreign government require a Certificate of Exportability issued by the FDA. All products which we sell have FDA clearance and would fall into the first category.
| Page 43 |
FONAR CORPORATION AND SUBSIDIARIES
Foreign governments have differing requirements concerning the import of medical devices into their respective jurisdictions. The European Union, also referred to as EU, has some essential requirements described in the EU’s Medical Device Directive, also referred to as MDD. In order to export to one of these countries, we must meet the essential requirements of the MDD and any additional requirements of the importing country. The essential requirements are similar to some of the requirements mandated by the FDA. In addition the MDD requires that we enlist a Notified Body to examine and assess our documentation, a Technical Construction File, and verify that the product has been manufactured in conformity with the documentation. The notified body must carry out or arrange for the inspections and tests necessary to verify that the product complies with the essential requirements of the MDD, including safety performance and Electromagnetic Compatibility, also referred to as EMC. Also required is a Quality System, ISO-13485, assessment by the Notified Body. We were approved for ISO 13485 certification for its Quality Management System in April, 2003.
We received clearance to sell the Upright® MRI scanners in the EU in May, 2002.
Other countries require that their own testing laboratories perform an evaluation of our devices. This requires that we must bring the foreign agency’s personnel to the USA to perform the evaluation at our expense before exporting.
Some countries, including many in Latin America and Africa, have very few regulatory requirements, beyond FDA clearance.
To date, Fonar has been able to comply with all foreign regulatory requirements applicable to its export sales.
PHYSICIAN AND DIAGNOSTIC SERVICES MANAGEMENT BUSINESS
Health Diagnostics Management, LLC (HDM) is owned by Health Management Corporation of America (70%) and investors (30%). Health Management Corporation of America is owned 100% by Fonar Corporation.
HDM operates under the assumed name “Health Management Company of America” (“HMCA”).
The combined business (HDM and Health Management Corporation of America) will be referred to as “HMCA” for all periods before and after July 1, 2015, unless otherwise indicated.
HMCA provides comprehensive non-medical management services to diagnostic imaging facilities. These services include administrative services, billing and collection services, credentialing services, contract negotiations, compliance consulting, purchasing IT services, hiring, conducting interviews, training, supervision and management of non-medical personnel, storage of medical records, office space, equipment, repair maintenance services, accounting, assistance with compliance matters and the development and implementation of practice growth and marketing strategies.
| Page 44 |
FONAR CORPORATION AND SUBSIDIARIES
As of June 30, 2021, HMCA managed a total of 39 MRI scanners of which twenty-five (25) scanners are located in New York and 14 scanners are located in Florida. For the 2021 fiscal year, the revenues HMCA recognized from the MRI facilities has increased to $80.9 million from $77.2 million in fiscal 2020. Five of the facilities in Florida are owned by HMCA subsidiaries, where the corporate practice of medicine is permitted.
We believe the utilization of FONAR Upright® MRI scanning systems, which are produced under the protection of our patents, accounts for the historically robust patient volume at the scanning facilities and, most recently, our steady recovery from the effects of the COVID-19 pandemic. During fiscal 2021, second MRI scanners were installed at our facilities in Islandia, New York and White Plains, New York and a new facility was installed in Pembroke Pines, Florida. The Company also acquired an existing facility in West Yonkers, NY in March 2021.
HMCA GROWTH STRATEGY
HMCA’s growth strategy focuses on upgrading and expanding the existing facilities it manages and expanding the number of facilities it either owns or manages for its clients, including new sites. In connection with improving the performance of the facilities, we have added high field MRI scanners, extremity scanners and x-ray machines to the Upright® MRI scanners at certain of the sites where such additional diagnostic imaging modalities are expected to produce the greatest return. In addition we plan to install three new facilities in fiscal 2022: one in New York and two in Florida.
PHYSICIAN AND DIAGNOSTIC MANAGEMENT SERVICES
HMCA’s services to the facilities it manages encompass substantially all of their business operations. Each facility is controlled, however, not by HMCA, but by the physician owner, or in the case of the four Florida sites owned by HMCA subsidiaries, by the medical director. All medical services are performed by physicians and other medical personnel under the physician-owner’s supervision. HMCA is the management company and performs services of a non-medical nature. These services include:
1. Offices and Equipment. HMCA identifies, negotiates leases for and/or provides office space and equipment to its clients. This includes technologically sophisticated medical equipment. HMCA also provides improvements to leaseholds, assistance in site selection and advice on improving, updating, expanding and adapting to new technology.
2. Personnel. HMCA staffs all the non-medical positions of its clients with its own employees, eliminating the client’s need to interview, train and manage non-medical employees. HMCA processes the necessary tax, insurance and other documentation relating to employees.
| Page 45 |
FONAR CORPORATION AND SUBSIDIARIES
3. Administrative. HMCA assists in the scheduling of patient appointments, purchasing of office and medical supplies and equipment and handling of reporting, accounting, processing and filing systems. It prepares and files the physician portions of complex applications to enable its clients to participate in managed care programs and to qualify for insurance reimbursement. HMCA assists the clients to implement programs and procedures to ensure full and timely regulatory compliance and appropriate cost reimbursement under no-fault insurance and Workers’ Compensation guidelines, as well as compliance with other applicable governmental requirements and regulations, including HIPAA and other privacy requirements.
4. Billing and Collections. HMCA is responsible for the billing and collection of revenues from third-party payors including those governed by No-Fault and Workers’ Compensation statutes. HMCA is presently using a third party to perform its billing and collection services for its clients’ No-Fault and Workers’ Compensation scanning business.
5. Cost Saving Programs. Based on available volume discounts, HMCA seeks to assist in obtaining favorable pricing for office and medical supplies, medical imaging film, equipment, contrast agents, such as gadolinuim, and magnavist and other inventory for its clients.
6. Diagnostic Imaging and Ancillary Services. HMCA can offer access to diagnostic imaging equipment through diagnostic imaging facilities it manages. The Company is expanding the ancillary services offered in its network to include x-rays, and other MRI equipment such as high-field (1.5 or 3.0 Tesla magnet strength) MRI scanners and extremity MRI scanners.
7. Marketing Strategies. HMCA is responsible for developing and proposing marketing plans for its clients.
8. Expansion Plans. HMCA assists the clients in developing expansion plans including the opening of new or replacement facilities where appropriate.
HMCA’s objective is to free physicians from as many non-medical duties as is practicable, allowing physicians to spend less time on business and administrative matters and more time practicing medicine.
The exceptions to this general model of operation are five of the facilities located in Florida. These Florida facilities are owned by limited liability companies which, as our subsidiaries, conduct their operations directly and bill and collect their fees from the patients and third party payors.
The facilities enter into contracts with third party payors, including managed care companies. None of HMCA’s clients, however, participate in any capitated plans or other risk sharing arrangements. Capitated plans are those HMO programs where the provider is paid a flat monthly fee per patient.
The management fees payable by the facilities to HMCA are flat monthly fees. In fiscal 2020, the aggregate amount of management fees was $4,530,422 per month. In fiscal 2021, the aggregate amount of management fees was $4,897,720 per month.
| Page 46 |
FONAR CORPORATION AND SUBSIDIARIES
Fees under the management agreements are subject to adjustment by mutual agreement on an annual basis.
Dr. Damadian owns three HMCA-managed MRI facilities in Florida. The fees for these three sites in Florida owned by Dr. Damadian are flat monthly fees which are subject to adjustment by mutual agreement on an annual basis. In fiscal 2021, the aggregate monthly amount of management fees payable to HMCA by these sites was $931,561 as compared to $897,745 in fiscal 2020.
The Florida facilities owned by HMCA subsidiaries directly bill their patients or the patients’ insurance carriers. Patient fees net of provision for bad debts were $23,307,389 in fiscal 2021 as compared to $22,495,260 in fiscal 2020.
HMCA contracts with an outside billing company (located in Melville, New York) to perform billing and collection for their clients’ No-Fault and Workers’ Compensation business. The fixed monthly fees were $85,000 for HMCA in fiscal 2020 and part of fiscal 2021. This contract was terminated as of January 1, 2021. The Company also entered into a one year renewable agreement to provide IT services to the billing company for a monthly fee of $23,884.
HMCA MARKETING
HMCA’s marketing strategy is to expand the business and improve the facilities which it manages. HMCA is seeking to increase the number of locations of those facilities where market conditions are promising and to promote growth of our clients’ and Florida subsidiaries’ patient volume and revenue.
DIAGNOSTIC IMAGING FACILITIES
Diagnostic imaging facilities managed by HMCA provide diagnostic imaging services to patients referred by physicians. The facilities are operated in a manner which eliminates the admission and other administrative inconveniences of in-hospital diagnostic imaging services. Imaging services are performed in an outpatient setting by trained medical technologists under the direction of physicians. Following diagnostic procedures, the images are reviewed by the interpreting physicians who prepare reports of these tests and their findings. The vast majority of reports for the New York facilities are transcribed by HMCA personnel and the remainder are outsourced to professional transcription services. Reports for the Florida facilities are outsourced to professional transcription services.
HMCA develops marketing programs and educational programs in an effort to establish and maintain referring physician relationships for our clients and Florida subsidiaries.
Managed care providers are an important factor in the diagnostic imaging industry. To further its position, HMCA is seeking to expand the imaging modalities offered at its managed and owned diagnostic imaging facilities. Three facilities in New York and six facilities in Florida have two or more MRI scanners. One facility in New York and two in Florida also perform X-rays. During fiscal 2020, a second MRI was installed at our Ormond Beach, Florida facility and a new HMCA facility became operational in Pembroke Pines, Florida.
| Page 47 |
FONAR CORPORATION AND SUBSIDIARIES
REIMBURSEMENT
HMCA’s clients receive reimbursements for their services through Medicare, Medicaid, managed care, private commercial insurance, third party administrators, Workers’ Compensation, No-Fault and other insurance.
Medicare
The Medicare program provides reimbursement for hospitalization, physician, diagnostic and certain other services to eligible persons 65 years of age and over and certain other individuals. Providers are paid by the federal government in accordance with regulations promulgated by the Department of Health and Human Services, HSS, and generally accept the payment with nominal deductible and co-insurance amounts required to be paid by the service recipient, as payment in full. Hospital inpatient services are reimbursed under a prospective payment system. Hospitals receive a specific prospective payment for inpatient treatment services based upon the diagnosis of the patient.
Under Medicare’s prospective payment system for hospital outpatient services, or OPPS, a hospital is paid for outpatient services on a rate per service basis that varies according to the ambulatory payment classification group, or APC, to which the service is assigned rather than on a hospital’s costs. Each year the Centers for Medicare and Medicaid Services, or CMS, publishes new APC rates that are determined in accordance with the promulgated methodology.
Services provided in non-hospital based freestanding facilities are paid under the Medicare Physician Fee Schedule, or MPFS. All of HMCA’s clients are presently in this category. The MPFS is updated on an annual basis and sometimes modified more frequently.
We have experienced reimbursement reductions for radiology services provided to Medicare beneficiaries, including reductions pursuant to the Deficit Reduction Act, or DRA.
CMS’ 2010 regulatory changes to the MPFS included a downward adjustment to services primarily involving the technical component rather than the physician work component, by adjusting downward malpractice payments for these services. These adjustments have been phased in over a four year period. For our fiscal year ended June 30, 2021, Medicare revenues represented approximately 3.4% of the revenues for HMCA’s clients and subsidiaries as compared to 3.8% for the fiscal year ended June 30, 2020.
| Page 48 |
FONAR CORPORATION AND SUBSIDIARIES
Medicaid
The Medicaid program is a jointly-funded federal and state program providing coverage for low-income persons. In addition to federally-mandated basic services, the services offered and reimbursement methods vary from state to state. In many states, Medicaid reimbursement is patterned after the Medicare program; however, an increasing number of states have established or are establishing payment methodologies intended to provide healthcare services to Medicaid patients through managed care arrangements. In fiscal 2021, approximately 0.09% of the revenues of HMCA’s clients were attributable to Medicaid, as compared to 0.07% in fiscal 2020. Four of the Florida facilities (those owned by HMCA subsidiaries) do not participate in Medicaid.
Managed Care and Private Insurance.
Health Maintenance Organizations, or HMO’s, Preferred Provider Organizations, or PPOs, and other managed care organizations attempt to control the cost of healthcare services by a variety of measures, including imposing lower payment rates, preauthorization requirements, limiting services and mandating less costly treatment alternatives. Managed care contracting is competitive and reimbursement schedules in many cases can be at or below Medicare reimbursement levels. Some managed care organizations have reduced or otherwise limited, and other managed care organizations may reduce or otherwise limit, reimbursement in response to reductions in government reimbursement. These reductions could have an adverse impact on our financial condition and results of operations. These reductions have been, and any future reductions may be, similar to the reimbursement reductions previously proposed.
HMCA COMPETITION
The physician and diagnostic management services field is highly competitive. A number of large hospitals have acquired medical practices and this trend may continue. HMCA expects that more competition will develop. Many competitors have greater financial and other resources than HMCA.
With respect to the diagnostic imaging facilities managed by HMCA, the outpatient diagnostic imaging industry is highly competitive. Competition focuses primarily on attracting physician referrals at the local market level and increasing referrals through relationships with managed care organizations, as well as emphasizing to potential referral sources the advantages of Upright® MRI scanning. HMCA believes that principal competitors for the diagnostic imaging centers are hospitals and independent or management company-owned imaging centers. Competitive factors include quality and timeliness of test results, ability to develop and maintain relationships with managed care organizations and referring physicians, type and quality of equipment, facility location, convenience of scheduling and availability of patient appointment times. HMCA believes that it will be able to effectively meet the competition in the outpatient diagnostic imaging industry with the Fonar Upright® MRI scanners and strategically placed high field MRI scanners at its facilities.
| Page 49 |
FONAR CORPORATION AND SUBSIDIARIES
GOVERNMENT REGULATION APPLICABLE TO HMCA
FEDERAL REGULATION
The healthcare industry is highly regulated and changes in laws and regulations can be significant. Changes in the law or new interpretation of existing laws can have a material effect on our permissible activities, the relative costs associated with doing business and the amount of reimbursement by government and other third-party payors.
Federal False Claims Act
The federal False Claims Act and, in particular, the False Claims Act’s “qui tam” or “whistleblower” provisions allow a private individual to bring actions in the name of the government alleging that a defendant has made false claims for payment from federal funds. After the individual has initiated the lawsuit the government must decide whether to intervene in the lawsuit and to become the primary prosecutor. If the government declines to join the lawsuit, the individual may choose to pursue the case alone, although the government must be kept apprised of the progress of the lawsuit, and may intervene later. Whether or not the federal government intervenes in the case, it will receive the majority of any recovery.
When an entity is determined to have violated the federal False Claims Act, it must pay three times the actual damages sustained by the government, plus mandatory civil penalties for each separate false claim and the government’s attorneys’ fees. Liability arises when an entity knowingly submits, or causes someone else to submit, a false claim for reimbursement to the federal government. The False Claims Act defines the term “knowingly” broadly, though simple negligence will not give rise to liability under the False Claims Act. Examples of the other actions which may lead to liability under the False Claims Act are set forth below:
Failure to comply with the many technical billing requirements applicable to our Medicare and Medicaid business.
Failure to comply with the prohibition against billing for services ordered or supervised by a physician who is excluded from any federal healthcare program, or the prohibition against employing or contracting with any person or entity excluded from any federal healthcare program.
Failure to comply with the Medicare physician supervision requirements for the services we provide, or the Medicare documentation requirements concerning physician supervision.
| Page 50 |
FONAR CORPORATION AND SUBSIDIARIES
The Fraud Enforcement and Recovery Act of 2009 expanded the scope of the False Claims Act by, among other things, broadening protections for whistleblowers and creating liability for knowingly retaining a government overpayment, acting in deliberate ignorance of a government overpayment or acting in reckless disregard of a government overpayment. The healthcare reform bills in the form of the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010 (collectively, “PPACA”) expanded on changes made by the 2009 Fraud Enforcement and Recovery Act with regard to such “reverse false claims.” Under PPACA, the knowing failure to report and return an overpayment within 60 days of identifying the overpayment or by the date a corresponding cost report is due, whichever is later, constitutes a violation of the False Claims Act. HMCA and its clients have never been sued under the False Claims Act and believe they are in compliance with the law.
Stark Law
Under the federal Self-Referral Law, also referred to as the “Stark Law”, which is applicable to Medicare and Medicaid patients, and the self-referral laws of various States, certain health practitioners, including physicians, chiropractors and podiatrists, are prohibited from referring their patients for the provision of designated health services, including diagnostic imaging and physical therapy services, to any entity with which they or their immediate family members have a financial relationship, unless the referral fits within one of the specific exceptions in the statutes or regulations. The federal government has taken the position that a violation of the federal Stark Law is also a violation of the Federal False Claims Act. Statutory exceptions under the Stark Law include, among others, direct physician services, in-office ancillary services rendered within a group practice, space and equipment rental and services rendered to enrollees of certain prepaid health plans. Some of these exceptions are also available under the State self-referral laws. HMCA believes that it and its clients are in compliance with these laws.
Anti-kickback Regulation
We are subject to federal and state laws which govern financial and other arrangements between healthcare providers. These include the federal anti-kickback statute which, among other things, prohibits the knowing and willful solicitation, offer, payment or receipt of any remuneration, direct or indirect, in cash or in kind, in return for or to induce the referral of patients for items or services covered by Medicare, Medicaid and certain other governmental health programs. Under PPACA, knowledge of the anti-kickback statute or the specific intent to violate the law is not required. Violation of the anti-kickback statute may result in civil or criminal penalties and exclusion from the Medicare, Medicaid and other federal healthcare programs, and according to PPACA, now provides a basis for liability under the False Claims Act. In addition, it is possible that private parties may file “qui tam” actions based on claims resulting from relationships that violate the anti-kickback statute, seeking significant financial rewards. Many states have enacted similar statutes, which are not limited to items and services paid for under Medicare or a federally funded healthcare program. Neither HMCA nor its clients engage in this practice.
| Page 51 |
FONAR CORPORATION AND SUBSIDIARIES
In fiscal 2021, approximately 3.4% of the revenues of HMCA’s clients were attributable to Medicare and 0.09% were attributable to Medicaid. In fiscal 2020, approximately 3.8% of the revenues of HMCA’s clients were attributable to Medicare and 0.07% were attributable to Medicaid.
Deficit Reduction Act (DRA)
On February 8, 2006, the President signed into law the DRA. Effective January 1, 2007, the DRA provides that Medicare reimbursement for the technical component for imaging services (excluding diagnostic and screening mammography) performed in freestanding facilities will be capped. Payment is the lesser of the Medicare Physician Fee Schedule or the Hospital Outpatient Prospective Payment System (OPPS) rates. Implementation of these reimbursement reductions contained in the DRA has had an adverse effect on our business. We have been able to counter this effect by increasing our clients’ scan volumes through our vigorous marketing efforts and reducing our operating expenses.
The DRA also codified the reduction in reimbursement for multiple images on contiguous body parts previously announced by CMS, the agency responsible for administering the Medicare program. In November 2005, CMS announced that it would pay 100% of the technical component of the higher priced imaging procedure and 50% of the technical component of each additional imaging procedure for imaging procedures involving contiguous body parts within a family of codes when performed in the same session. CMS had indicated that it would phase in this 50% rate reduction over two years, so that the reduction was 25% for each additional imaging procedure in 2006 and another 25% reduction in 2007. However, for services furnished on or after July 1, 2010, the PPACA requires the full 50% reduction to be implemented.
Health Insurance Portability and Accountability Act
Congress enacted the Health Insurance Portability and Accountability Act of 1996, or HIPAA, in part, to combat healthcare fraud and to protect the privacy and security of patients’ individually identifiable healthcare information. HIPAA, among other things, amends existing crimes and criminal penalties for Medicare fraud and enacts new federal healthcare fraud crimes, including actions affecting non-governmental healthcare benefit programs by means of false or fraudulent representations in connection with the delivery of healthcare services is subject to a fine or imprisonment, or potentially both. In addition, HIPAA authorizes the imposition of civil money penalties against entities that employ or enter into contracts with excluded Medicare or Medicaid program participants if such entities provide services to federal health program beneficiaries. A finding of liability under HIPAA could have a material adverse effect on our business, financial condition and results of operations.
| Page 52 |
FONAR CORPORATION AND SUBSIDIARIES
Further, HIPAA requires healthcare providers and their business associates to maintain the privacy and security of individually identifiable protected health information (“PHI”). HIPAA imposes federal standards for electronic transactions, for the security of electronic health information and for protecting the privacy of PHI. The Health Information Technology for Economic and Clinical Health Act of 2009 (“HITECH”), signed into law on February 17, 2009, dramatically expanded, among other things, (1) the scope of HIPAA to now apply directly to “business associates,” or independent contractors who receive or obtain PHI in connection with providing a service to a covered entity, (2) substantive security and privacy obligations, including new federal security breach notification requirements to affected individuals, DHHS and prominent media outlets, of certain breaches of unsecured PHI, (3) restrictions on marketing communications and a prohibition on covered entities or business associates from receiving remuneration in exchange for PHI, and (4) the civil and criminal penalties that may be imposed for HIPAA violations, increasing the annual cap in penalties from $25,000 to $1.5 million per occurrence. In 2013 additional legal requirements were adopted to provide further protection for PHI.
In addition, many states have enacted comparable privacy and security statues or regulations that, in some cases, are most stringent than HIPAA requirements. In those cases it may be necessary to modify our operations and procedures to comply with the more stringent state laws, which may entail significant and costly changes for us. We believe that we are in compliance with such state laws and regulations. However, if we fail to comply with applicable state laws and regulations, we could be subject to sanctions.
We believe that we are in compliance with the current HIPAA requirements, as amended by HITECH, together with other legislation and regulations, and comparable state laws, but we anticipate that we may encounter certain costs associated with future compliance. Moreover, we cannot guarantee that enforcement agencies or courts will not make interpretations of the HIPAA standards that are inconsistent with ours, or the interpretations of our contracted radiology practices or their affiliated physicians. A finding of liability under the HIPAA standards may result in significant criminal and civil penalties. Noncompliance also may result in exclusion from participation in government programs, including Medicare and Medicaid. These actions could have a material adverse effect on our business, financial condition, and results of operations.
Civil Money Penalty Law and Other Federal Statutes
The Civil Money Penalty, or CMP, law covers a variety of practices. It provides a means of administrative enforcement of the anti-kickback statute, and prohibits false claims, claims for medically unnecessary services, violations of Medicare participating provider or assignment agreements and other practices. The statute gives the Office of Inspector General of the HHS the power to seek substantial civil fines, exclusion and other sanctions against providers or others who violate the CMP prohibitions.
In addition, in 1996, Congress created a new federal crime: healthcare fraud and false statements relating to healthcare matters. The healthcare fraud statute prohibits knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private payors. A violation of this statute is a felony and may result in fines, imprisonment or exclusion from government sponsored programs such as the Medicare and Medicaid programs.
| Page 53 |
FONAR CORPORATION AND SUBSIDIARIES
Certificates of Need
Some states require hospitals and certain other healthcare facilities and providers to obtain a certificate of need, or CON, or similar regulatory approval prior to establishing certain healthcare operations or services, incurring certain capital projects and/or the acquisition of major medical equipment including MRI and PET/CT systems. We are not operating in any such states.
Patient Protection and Affordable Care Act
On March 23, 2010, President Obama signed into law healthcare reform legislation in the form of PPACA. The implementation of this law has had a significant impact on the healthcare industry. Most of the provisions of PPACA are being phased in over time and can be conceptualized as a broad framework not only to provide health insurance coverage to millions of Americans, but to fundamentally change the delivery of care by bringing together elements of health information technology, evidence-based medicine, chronic disease management, medical “homes,” care collaboration and shared financial risk in a way that will accelerate industry adoption and change. We are unable to predict the full impact of PPACA at this time primarily due to the previous administration’s efforts to repeal and replace the PPACA, or to utilize executive action to modify the Act’s provisions where possible.
State Regulation
In addition to the federal self-referral law and federal Anti-kickback statute, many States, including those in which HMCA and its clients operate, have their own versions of self-referral and anti-kickback laws. These laws are not limited in their applicability, as are the federal laws, to specific programs. HMCA believes that it and its clients are in compliance with these laws.
Various States prohibit business corporations from practicing medicine. Various States, including New York, also prohibit the sharing of professional fees or fee splitting. Consequently, in New York HMCA leases space and equipment to clients and provides clients with a range of non-medical administrative and managerial services for agreed upon fees. Under Florida law a business entity can bill patients and third party payors directly if that entity is properly licensed through AHCA. All of the eight facilities in Florida are licensed healthcare clinics through AHCA.
HMCA’s clients and subsidiaries generate revenue from patients covered by no-fault insurance and workers’ compensation programs. For the fiscal year ended June 30, 2021 approximately 55.5% of our clients’ receipts were from patients covered by no-fault insurance and approximately 9.4% of our client’s receipts were from patients covered by workers’ compensation programs. For the fiscal year ended June 30, 2020, approximately 56.7.% of HMCA’s clients’ receipts were from patients covered by no-fault insurance and approximately 9.11% of HMCA’s clients’ receipts were from patients covered by workers’ compensation programs. The foregoing numbers do not include payments from third party administrators. In the event that changes in these laws alter the fee structures or methods of providing service, or impose additional or different requirements, HMCA could be required to modify its business practices and services in ways that could be more costly to HMCA or in ways that decrease the revenues which HMCA receives from its clients.
| Page 54 |
FONAR CORPORATION AND SUBSIDIARIES
Compliance Program
We maintain a program to monitor compliance with federal and state laws and regulations applicable to the healthcare entities. The compliance program includes the adoption of (i) Standards of Conduct for our employees and affiliates and (ii) a process that specifies how employees, affiliates and others may report regulatory or ethical concerns. We believe that our compliance program meets the relevant standards provided by the Office of Inspector General of the Department of Health and Human Services.
An important part of our compliance program consists of conducting periodic audits of various aspects of our operations and that of the contracted radiology practices. We also assist our clients with educational programs designed to familiarize them with the regulatory requirements and specific elements of our compliance program.
HMCA believes that it and its clients are in compliance with applicable Federal, State and local laws. HMCA does not believe that such laws will have any adverse material effect on its business.
EMPLOYEES
Fonar and HMCA had approximately 495 employees as of September 24, 2021. This total number included employees engaged in production, customer support, research and development, information technology, employees engaged in marketing and sales, billing and collection, legal and compliance matters, as well as transcriptionists, Florida technologists, field service technicians and individuals in various administrative positions. A significant number of employees were employed at the MRI facilities managed or owned by HMCA, primarily in administrative positions.
ITEM 1A. RISK FACTORS
An investment in our securities is subject to various risks, the most significant of which are summarized below.
| 1. | Reduced Reimbursement Rates. Most of our revenues are derived from our scanning center business conducted by HMCA. We are experiencing lower reimbursement rates from Medicare, other government programs and private insurance companies. To date, we have been able to counter the impact of these reductions by increasing our volume of scans notwithstanding the Covid-19 pandemic, and reducing our operating expenses, thereby maintaining profitability in this business segment. There is, however, no assurance that we will be able to continue to do so. |
| Page 55 |
FONAR CORPORATION AND SUBSIDIARIES
| 2. | Demand for MRI Scanners. The reduced reimbursement rates also affects our sales of MRI scanners negatively. With lower revenue projections, prospective customers would demand lower prices for scanners. Although the reduced reimbursements may not affect foreign demand, a lower number of sales in the aggregate could reduce economies of scale and consequently, profit margins. |
| 3. | Manufacturing Competition. Many if not most of our competing scanner manufacturers have significantly greater financial resources, production capacity, and other resources than we do. Such competitors would include General Electric, Siemens, Hitachi and Phillips. Although Fonar is the only company which can manufacture and sell the unique Stand-Up® (Upright®) MRI scanner, potential customers must be convinced that the purchase of a Fonar scanner is their best choice. We believe that with time, that objective will be reached, particularly with customers scanning patients having neck, back, knee and various orthopedic issues who would benefit from being scanned in weight-bearing positions. |
| 4. | Dependence on Referrals. HMCA derives substantially all of its revenue, directly or indirectly, from fees charged for the diagnostic imaging services performed at the facilities. We depend on referrals of patients from unaffiliated physicians and other third parties to the facilities we manage or own for the services we perform. If these physicians and other third parties were to reduce the number of patients they refer or discontinue referring patients, scan volumes could decrease, which would reduce our net revenue and operating margins. |
| 5. | Pressure to Control Healthcare Costs. One of the principal objectives of health maintenance organizations and preferred provider organizations is to control the cost of healthcare services. Healthcare providers participating in managed care plans may be required to refer diagnostic imaging tests to certain providers depending on the plan in which a covered patient is enrolled. In addition, managed care contracting has become very competitive. The expansion of health maintenance organizations, preferred provider organizations and other managed care organizations within New York or Florida could have a negative impact on the utilization and pricing of services performed at the facilities HMCA manages or owns to the extent these organizations exert control over patients’ access to diagnostic imaging services, selections of the provider of such services and reimbursement rates for those services. |
| 6. | Scanning Facility Competition. The market for diagnostic imaging services is highly competitive. The facilities we manage or own compete for patients on the basis of reputation, location and the quality of diagnostic imaging services. Groups of radiologists, established hospitals, clinics and other independent organizations that own and operate imaging equipment are the principal competitors. |
| Page 56 |
FONAR CORPORATION AND SUBSIDIARIES
| 7. | Eligibility Changes to Insurance Programs. Due to potential decreased availability of healthcare through private employers, the number of patients who are uninsured or participate in governmental programs may increase. Healthcare reform legislation will increase the participation of individuals in the Medicaid program in states that elect to participate in the expanded Medicaid coverage. A shift in payor mix from managed care and other private payors to government payors or an increase in the number of uninsured patients may result in a reduction in the rates of reimbursement or an increase in uncollectible receivables or uncompensated care, with a corresponding decrease in net revenue. Policies now being offered under various insurance plans are expected to reduce demand for MRI scans as they become less affordable. Changes in the eligibility requirements for governmental programs such as the Medicaid program and state decisions on whether to participate in the expansion of such programs also could increase the number of patients who participate in such programs and the number of uninsured patients. Even for those patients who remain in private insurance plans, changes to those plans could increase patient financial responsibility, resulting in a greater risk of uncollectible receivables. These factors and events could have a material adverse effect on our business, financial condition, and results of operations. |
| 8. | Possible changes in Florida Insurance Law. In early 2019, two senate bills and one house bill in Florida were introduced, all of them calling for the repeal of PIP and replacing PIP with $25,000 Bodily Injury Coverage and Property Damage Liability Coverage. Another Florida senate bill was introduced that would preserve PIP but dramatically cut reimbursement rates. None of the proposed bills made it onto the 2019 legislative agenda. During Fonar’s fiscal 2021, the Florida house and senate reached an agreement and passed similar legislation. It was, however, vetoed by the Governor. We cannot predict whether such efforts by the Florida legislature will continue or be successful. Currently, drivers and passengers get car damages and PIP, paid for up to $10,000, no matter who is at fault in an accident. Drivers have to pay an additional cost to insurance companies to pay for bodily injuries which covers them if they are at fault. While PIP is required, coverage for bodily injury is not. |
| Over the past several years there have been various bills introduced by a number of Florida legislators to eliminate PIP and instead mandate coverage including some combination of a minimum of bodily injury and a reduced or no amount of medical payments (Medpay coverage). Eliminating PIP would mean that the $10,000 drivers now get paid toward medical costs through their insurers might not be there for them to pay for injured drivers. Importantly, payments would be reduced by approximately 60% due to claims being paid at commercial rates or through legal settlements instead of at the presently prevailing PIP fee schedule. This would negatively impact our seven diagnostic imaging facilities (both those we own and those we manage) with more unpaid bills, lower reimbursement rates and elongated waiting times. To date proponents of these changes have been unsuccessful. |
| Page 57 |
FONAR CORPORATION AND SUBSIDIARIES
| 9. | Federal and state privacy and information security laws. We must comply with numerous federal and state laws and regulations governing the collection, dissemination, access, use, security and privacy of PHI, including HIPAA and its implementing privacy and security regulations, as amended by the federal HITECH Act. If we fail to comply with applicable privacy and security laws, regulations and standards, properly maintain the integrity of our data, protect our proprietary rights to our systems, or defend against cybersecurity attacks, our business, reputation, results of operations, financial position and cash flows could be materially and adversely affected. |
Information security risks have significantly increased because of the proliferation of new technologies, the use of the internet and telecommunications technologies to conduct our operations, and the increased sophistication and activities of organized crime, hackers, terrorists and other external parties, including foreign state agents. Our operations rely on the secure processing, transmission and storage of confidential, proprietary and other information in our computer systems and networks.
| 10. | COVID-19. Although we believe we have taken the proper steps and made a good recovery from the impact of the first wave of the COVID-19 virus, new strains of the disease have developed and future variants may continue to develop. The course and severity of the virus in the following months, and the ultimate economic and medical impact it will have worldwide and at home, have so far eluded more than minimal predictability. |
| 11. | Other changes in Domestic and Worldwide Economic Conditions. We are subject to risk arising from adverse changes in general domestic and global economic conditions, including recession or economic slowdown and disruption of credit markets. Turbulence and uncertainty in the United States and international markets and economies may adversely affect our liquidity, financial condition, revenues, profitability and business operations generally. |
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
Fonar and HMCA currently lease approximately 78,000 square feet of office and plant space at its principal offices in Melville, New York. The term of the lease runs through November, 2026. Management believes that the premises will be adequate for its current needs. HMCA also maintains office space for the Facilities owned by its subsidiaries in Florida and for its clients at the clients’ sites in New York and Florida under leases having various terms. HMCA owns the building for the client’s premises in Tallahassee, Florida. The Company received approval from the Suffolk County IDA on February 29, 2016 of a 50% property tax abatement, valued at $440,000, over a 10 year period commencing January, 2017.
ITEM 3. LEGAL PROCEEDINGS.
There are
no material legal proceedings threatened or pending against the Company.
| Page 58 |
FONAR CORPORATION AND SUBSIDIARIES
ITEM 4. MINE SAFETY DISCLOSURES.
Not
Applicable
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Our Common Stock is traded in the Nasdaq SmallCap market under the National Association of Securities Dealers Automated Quotation System, also referred to as “NASDAQ”, under the symbol FONR. The following table sets forth the high and low trades reported in NASDAQ System for the periods shown.
| Fiscal Quarter | High | Low | ||||||||||
| January - March | 2017 | $ | 20.85 | $ | 17.30 | |||||||
| April - June | 2017 | $ | 29.40 | $ | 17.20 | |||||||
| July - September | 2017 | $ | 31.90 | $ | 25.31 | |||||||
| October - December | 2017 | $ | 33.75 | $ | 21.10 | |||||||
| January - March | 2018 | $ | 29.95 | $ | 22.15 | |||||||
| April - June | 2018 | $ | 30.10 | $ | 25.31 | |||||||
| July - September | 2018 | $ | 28.80 | $ | 23.70 | |||||||
| October - December | 2018 | $ | 25.77 | $ | 19.63 | |||||||
| January - March | 2019 | $ | 23.85 | $ | 20.01 | |||||||
| March - June | 2019 | $ | 23.00 | $ | 18.85 | |||||||
| July - September | 2019 | $ | 25.25 | $ | 20.44 | |||||||
| October - December | 2019 | $ | 20.94 | $ | 19.07 | |||||||
| January - March | 2020 | $ | 20.24 | $ | 11.00 | |||||||
| April - June | 2020 | $ | 25.99 | $ | 13.85 | |||||||
| July - September | 2020 | $ | 26.49 | $ | 20.31 | |||||||
| October- December | 2020 | $ | 22.49 | $ | 16.74 | |||||||
| January- March | 2021 | $ | 20.40 | $ | 17.31 | |||||||
| April- June | 2021 | $ | 19.18 | $ | 16.58 | |||||||
| July- September 24 | 2021 | $ | 18.04 | $ | 16.51 | |||||||
Performance Graph
The following graph compares the annual change in the Company’s cumulative total shareholder return on its Common Stock during a period commencing on June 30, 2017 and ending on June 30, 2021 (as measured by dividing (i) the sum of (A) the cumulative amount of dividends for the measurement period, assuming dividend reinvestment and (B) the difference between the Company’s share price at the end and the beginning of the measurement period; by (ii) the share price at the beginning of the measurement period) with the cumulative total return of each of: (a) the CRSP Composite Total Return Index for Nasdaq (“Nasdaq”); and (b) the CRSP Total Return Index for Nasdaq Healthcare companies (“Nas-Hea.”) during such period, assuming a $100 investment on June 30, 2017. The stock price performance on the graph below is not necessarily indicative of future price performance.
| Page 59 |
FONAR CORPORATION AND SUBSIDIARIES
Relative Dollar Values
| June 30, 2017 | June 29, 2018 | June 28, 2019 | June 30, 2020 | June 30, 2021 | ||||||||||||||||
| Fonar Common Stock | $ | 100.00 | $ | 95.68 | $ | 77.52 | $ | 77.02 | $ | 61.63 | ||||||||||
| NASDAQ | $ | 100.00 | $ | 122.31 | 130.39 | $ | 168.82 | $ | 243.43 | |||||||||||
| NAS-Hea | $ | 100.00 | $ | 110.82 | $ | 113.60 | $ | 140.03 | $ | 175.00 | ||||||||||
FONAR CORPORATION AND SUBSIDIARIES
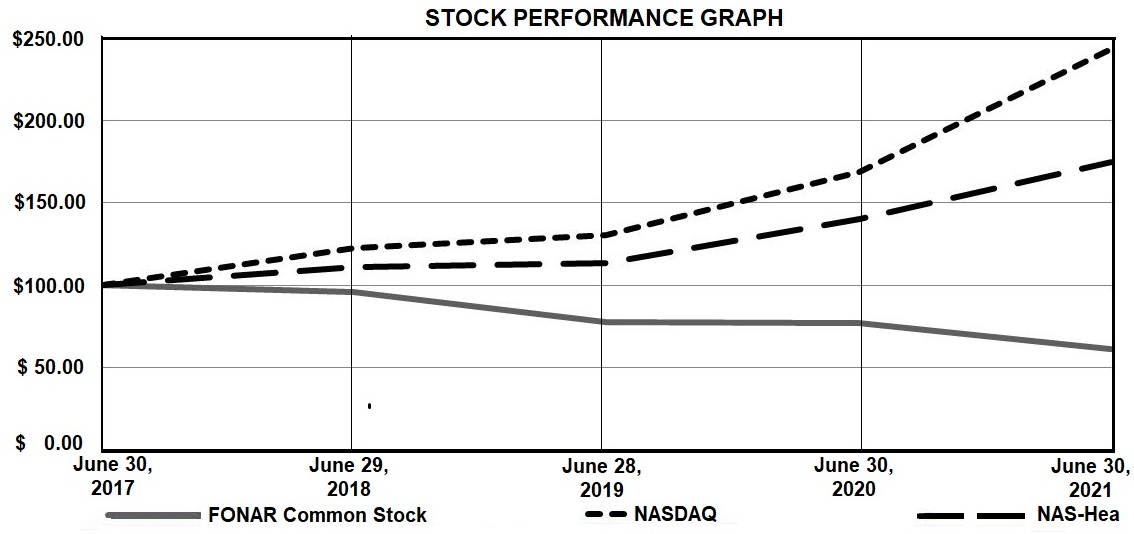
On September 30, 2021, we had approximately 993 stockholders of record of our Common Stock, 12 stockholders of record of our Class B Common Stock, 3 stockholders of record of our Class C Common Stock and 1,155 stockholders of record of our Class A Non-voting Preferred Stock.
At the present time, the only class of our securities for which there is a market is the Common Stock.
We currently have a policy of retaining earnings to finance the development and expansion of our business. We expect to continue this policy for the foreseeable future.
ITEM 6. SELECTED FINANCIAL DATA.
The following selected consolidated financial data has been extracted from our consolidated financial statements for the five years ended June 30, 2021. This consolidated selected financial data should be read in conjunction with our consolidated financial statements and the related notes included in Item 8 of this form.
| Page 60 |
FONAR CORPORATION AND SUBSIDIARIES
| STATEMENT OF OPERATIONS | 2021 | 2020 | 2019 | 2018 | 2017 | |||||||||||||||
| Revenues | $ | 89,929,765 | $ | 85,690,462 | $ | 87,192,887 | $ | 81,515,994 | $ | 78,036,586 | ||||||||||
| Cost of revenues | $ | 46,456,127 | $ | 43,296,825 | $ | 43,984,593 | $ | 41,950,770 | $ | 38,052,425 | ||||||||||
| Research and Development Expenses | $ | 1,635,979 | $ | 2,025,376 | $ | 1,812,347 | $ | 1,755,747 | $ | 1,480,670 | ||||||||||
| Net Income(Loss) | $ | 13,673,811 | $ | 11,704,733 | $ | 20,513,674 | $ | 25,452,185 | $ | 23,678,798 | ||||||||||
| Basic Net Income (Loss)per common share | $ | 1.47 | $ | 1.20 | $ | 2.26 | $ | 3.16 | $ | 2.98 | ||||||||||
| Diluted Net Income (Loss) per common share | $ | 1.45 | $ | 1.18 | $ | 2.22 | $ | 3.10 | $ | 2.92 | ||||||||||
| Basic Weighted average number of shares outstanding | 6,505,283 | 6,443,713 | 6,354,103 | 6,287,510 | 6,161,599 | |||||||||||||||
| Diluted Weighted average number of shares outstanding | 6,632,787 | 6,571,217 | 6,481,607 | 6,415,014 | 6,289,103 | |||||||||||||||
| BALANCE SHEET DATA | ||||||||||||||||||||
| Working capital | $ | 88,534,063 | $ | 77,226,104 | $ | 70,998,783 | $ | 52,497,840 | $ | 39,177,703 | ||||||||||
| Total Assets | $ | 189,506,195 | $ | 180,259,380 | $ | 133,560,210 | $ | 118,310,945 | $ | 98,762,566 | ||||||||||
| Long-term debt and obligations under capital leases | $ | 1,808,685 | $ | 2,116,587 | $ | 273,112 | $ | 306,035 | $ | 336,761 | ||||||||||
| Stockholder’s equity | $ | 135,370,125 | $ | 126,242,616 | $ | 118,112,103 | $ | 102,234,471 | $ | 82,909,953 | ||||||||||
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATION.
INTRODUCTION.
Fonar was formed in 1978 to engage in the business of designing, manufacturing and selling MRI scanners. HMCA, a subsidiary of Fonar, provides management services to diagnostic imaging facilities.
Fonar’s principal MRI product is its Upright® MRI (also called Stand-Up® MRI) scanner. The Upright® MRI allows patients to be scanned for the first time under weight-bearing conditions. The Stand-Up® MRI is the only MRI capable of producing images in the weight-bearing state.
At 0.6 Tesla field strength, the Upright® MRI is among the highest field open MRI scanners in the industry, offering non-claustrophobic MRI together with high-field image quality. Fonar’s open MRI scanners were the first high field strength open MRI scanners in the industry.
| Page 61 |
FONAR CORPORATION AND SUBSIDIARIES
HMCA generates revenues from providing comprehensive management services, including development, administration, accounting, billing and collection services, together with office space, medical equipment, supplies and non-medical personnel to its clients. Revenues are in the form of fees which are earned under contracts with HMCA’s clients except for its three Florida subsidiaries which engage in the practice of medicine, and bill and collect fees from patients, insurers and other third party payors directly.
The most significant adverse impact on on our Company in fiscal 2020 has been the COVID-19 pandemic. Although it had seemed the worst had passed, events have shown a spike in new cases due primarily to the new Delta strain in the viruses. This is by no means a problem confined to our Company, but regardless of our best efforts, our results of operation and financial condition are potentially volatible and severe.
Since March, 2020 the global pandemic of COVID-19 has caused turbulence and uncertainty in the United States and international economies which have adversely affected our profitability and business operations. Generally COVID-19 had caused us to require that much of our workforce work from home and has restricted the ability of our personnel to travel for marketing purposes or to service our customers. The Company experienced a sudden drop in scan volume for a short term period and while we are not back to pre-COVID-19 levels, the scan volume has risen. During the fourth quarter of Fiscal 2020, the Company was able to enact certain measures to allow the Company to survive during the global pandemic and to prevent further losses or additional decreases in scan volume. The Company immediately enacted wide scale furloughs, deferment of up to 50% of management salaries, halted variable compensation plans. Rent deferrals were negotiated with nearly all landlords. Reductions of the salaries of non-controlling interest group members, who were actively involved in the management of the Company, accounted for most of the payroll savings. The Company also received some government stimulus funds from the Paycheck Protection Program (“PPP) and Medicare advances/stimulus payments. Although we are unable to predict if there will be additional consequences on our operations from the continuing global pandemic of COVID-19, the Company believes with the positive cash flows, low debt and cash on hand, it will be able to continue operations going forward. One of the concerns we have are the increased strictness in enforcement of COVID-19 mandates, such as the requirement that employees in healthcare facilities be vaccinated or frequently test. We are in fact facing some of these challenges now. So far we have been able to navigate through these requirements and avoid any significant disruption to our business.
Critical Accounting Policies
Our discussion and analysis of financial condition and results of operations are based on our consolidated financial statements that were prepared in accordance with U.S. generally accepted accounting principles, or GAAP. Management makes estimates and assumptions when preparing financial statements. These estimates and assumptions affect various matters, including:
our reported amounts of assets and liabilities in our consolidated balance sheets at the dates of the financial statements
| Page 62 |
FONAR CORPORATION AND SUBSIDIARIES
our disclosure of contingent assets and liabilities at the dates of the financial statements; and
our reported amounts of net revenue and expenses in our consolidated statements of operations during the reporting periods
These estimates involve judgments with respect to numerous factors that are difficult to predict and are beyond management’s control. As a result, actual amounts could differ materially from these estimates.
The Securities and Exchange Commission defines critical accounting estimates as those that are both most important to the portrayal of a company’s financial condition and results of operations and require management’s most difficult, subjective or complex judgment, often as a result of the need to make estimates about the effect of matters that are inherently uncertain and may change in subsequent periods. In the notes to our consolidated financial statements, we discuss our significant accounting policies.
We believe the following critical accounting policies affect our more significant judgments and estimates used in the preparation of our consolidated financial statements. We recognize revenue and related costs of revenue from sales contracts for our MRI scanners and major upgrades, under the percentage-of-completion method. Under this method, we recognize revenue and related costs of revenue, as each sub-assembly is completed. Amounts received in advance of our commencement of production are recorded as customer advances.
We continuously, qualitatively and quantitatively evaluate the realizability (including both positive and negative evidence) of the net deferred tax assets and assess the valuation allowance periodically. Our evaluation considers the financial condition of the Company and both the business conditions and regulatory environment of the industry. If future taxable income or other factors are not consistent with our expectations, an adjustment to our allowance for net deferred tax assets may be required. For net deferred tax assets we consider estimates of future taxable income, including tax planning strategies, in determining whether our net deferred tax assets are more likely than not to be realized. Our ability to project future taxable income may be significantly affected by our ability to determine the impact of regulatory changes which could adversely affect our future profits. As a result, the benefits of our net operating loss carry forwards could expire before they are utilized.
At June 30, 2020, the net deferred tax asset was valued at $18,809,757. At June 30, 2021, the net deferred tax asset was valued at $15,958,961.
We depreciate our long-lived assets over their estimated economic useful lives with the exception of leasehold improvements where we use the shorter of the assets useful lives or the lease term of the facility for which these assets are associated.
| Page 63 |
FONAR CORPORATION AND SUBSIDIARIES
The Company provides for medical receivables that could become uncollectible by establishing an allowance for doubtful accounts in order to adjust medical receivables to estimated net realizable value. In evaluating the collectability of medical receivables, the Company considers a number of factors, including the age of the account, historical collection experiences, payor type, current economic conditions and other relevant factors. There are various factors that impact collection trends, such as payor mix, changes in the economy, increase burden on copayments to be made by patients with insurance and business practices related to collection efforts. These factors continuously change and can have an impact on collection trends and the estimation process.
We amortize our intangible assets, including patents, and capitalized software development costs, over the shorter of the contractual/legal life or the estimated economic life. Our amortization life for patents and capitalized software development costs is 15 to 17 years and 5 years, respectively. Our amortization of the non-competition agreements entered into with certain individuals in connection with the HDM transaction are depreciated over seven years, and customer relationships are amortized over 20 years.
Goodwill is recorded as a result of business combinations. Management evaluates goodwill, at a minimum, on an annual basis and whenever events and changes in circumstances suggest that the carrying amount may not be recoverable. Impairment of goodwill is tested by comparing the reporting unit’s carrying amount, including goodwill, to the fair value of the reporting unit. The fair value of a reporting unit is estimated using a combination of the income or discounted cash flows approach and the market approach, which uses comparable market data. If the carrying amount of the reporting unit exceeds its fair value, goodwill is considered impaired and a second step is performed to measure the amount of impairment loss, if any. Based on our test for goodwill impairment, we noted no impairment related to goodwill. However, if estimates or the related assumptions change in the future, we may be required to record impairment charges to reduce the carrying amount of goodwill.
We periodically assess the recoverability of long-lived assets, including property and equipment, intangibles and management agreements, when there are indications of potential impairment, based on estimates of undiscounted future cash flows. The amount of impairment is calculated by comparing anticipated discounted future cash flows with the carrying value of the related asset. In performing this analysis, management considers such factors as current results, trends, and future prospects, in addition to other economic factors.
RESULTS OF OPERATIONS. FISCAL 2021 COMPARED TO FISCAL 2020
In fiscal 2021, we recognized net income of $13.7 million on revenues of $89.9 million, as compared to net income of $11.7 million on revenues of $85.7 million for fiscal 2020. This represents an increase in revenues of 4.9%. Patient fee revenue net of contractual allowances increased by 3.6%. Total costs and expenses increased by 1.1%. Our consolidated operating results increased by $3.4 million to an operating income of $17.1 million for fiscal 2021 as compared to operating income of $13.7 million for fiscal 2020.
| Page 64 |
FONAR CORPORATION AND SUBSIDIARIES
Discussion of Operating Results of Medical Equipment Segment
Fiscal 2021 Compared to Fiscal 2020
Revenues attributable to our medical equipment segment increased by 6.8% to $9.0 million in fiscal 2021 from $8.5 million in fiscal 2020, with product sales revenues increasing by 332.9% from $298,000 in fiscal 2020 to $1.3 million in fiscal 2021. Service revenue decreased from $8.2 million in fiscal 2020 to $7.7 million in fiscal 2021.
The Upright® MRI is unique in that it permits MRI scans to be performed on patients upright in the weight-bearing state and in multiple positions that correlate with symptoms.
Product sales to unrelated parties increased by 332.9% in fiscal 2021 from $298,000 in fiscal 2020 to $1.3 million in fiscal 2021. There were no product sales to related parties in fiscal 2021 or 2020.
We believe that one of our principal challenges in achieving greater market penetration is attributable to the better name recognition and larger sales forces of our larger competitors such as General Electric, Siemens, Hitachi, Philips and Toshiba and the ability of some of our competitors to offer attractive financing terms through affiliates, such as G.E. Capital.
In addition, lower reimbursement rates have reduced the demand for our MRI products, resulting in lower sales volumes. As a result of fewer sales, service revenues have decreased since as older scanners are taken out of service, there are fewer new scanners available to sign service contracts.
The operating loss for the medical equipment segment decreased from an operating loss of $6.4 million in fiscal 2020 to an operating loss of $3.4 million in fiscal 2021. The losses are attributable most significantly to the fact that costs increased by a greater amount than revenues. The increase in costs was primarily due to the increase in business activity which resulted in our increased revenues.
We recognized revenues of $733,000 from the sale of our Upright® MRI scanners in fiscal 2021, while in fiscal 2020, we recognized revenues of $96,000 from the sale of Upright® MRI scanners.
Research and development expenses decreased to $1.6 million in fiscal 2021 from $2.0 million in fiscal 2020. Our expenses for fiscal 2021 represented continued research and development of various upgrades for the Upright® MRI scanner. The reason for the decrease in research and development was due mainly to supply chain related delays due to the COVID-19 pandemic.
| Page 65 |
FONAR CORPORATION AND SUBSIDIARIES
Discussion of Operating Results of Physician and Diagnostic Services Management Segment.
Fiscal 2021 Compared to Fiscal 2020
Revenues attributable to the Company’s physician and diagnostic services management segment, HMCA, increased to $80.9 million in fiscal 2021 as compared to $77.2 million in fiscal 2020. The increase in revenues was due to $812,000 of patient fees (net of contractual allowances and discounts less provision for bad debts) from patient and third party payors recognized by five of the facilities in Florida. Also management and other fees increased by $2.9 million due to two additional scanners being installed in existing facilities.
Cost of revenues as a percentage of the related revenues for our physician and diagnostic services management segment increased from $39.8 million or 51.5% of related revenues for the year ended June 30, 2020 to $42.6 million, or 52.7% of related revenues for the year ended June 30, 2021. The revenues increased more than the costs relating to these revenues.
Operating results of this segment increased from operating income of $20.1 million in fiscal 2020 to operating income of $20.5 million in fiscal 2021. We believe that our efforts to expand and improve the operation of our physician and diagnostic services management segment are directly responsible for the profitability of this segment and our company as a whole.
For the fiscal years ended June 30, 2021 and June 30, 2020 12.2% and 11.9%, respectively, of total revenues were derived from contracts with facilities owned by Dr. Raymond V. Damadian, the Chairman of the Board and principal stockholder of Fonar. The agreements with these MRI facilities are for one-year terms which renew automatically on an annual basis, unless terminated. The fees for these sites, which are located in Florida, are flat monthly fees.
Discussion of Certain Consolidated Results of Operations
Fiscal 2021 Compared to Fiscal 2020
Interest and investment income decreased in 2021 compared to 2020. We recognized interest income of $311,931 in 2021 as compared to $502,145 in fiscal 2020, representing a decrease of 37.8%.
Interest expense of $248,665 was recognized in fiscal 2021, as compared to interest expense of $74,321 in fiscal 2020. The increase in interest expense is attributable to an assessment of additional taxes and interest in connection with a state income tax audit.
The 30% noncontrolling interest allocations of $3,466,000 and $3,465,000 for fiscal 2021 and fiscal 2020 respectively, have been calculated by Income from operations, and adding depreciation and amortization net of miscellaneous losses and other income from the Physician and Diagnostic Service Management segment (See Note 17).
| Page 66 |
FONAR CORPORATION AND SUBSIDIARIES
While revenue increased by 4.9% selling, general and administrative expenses decreased by 7.4% to $24.7 million in fiscal 2021 from $26.7 million in fiscal 2020. This increase was almost exclusively due to reserves placed on service contracts and management fees and other receivables resulting from the COVID-19 pandemic. It is too early to know how much of these reserves will be recovered. Also Fonar resolved certain sales tax liabilities during the year and was able to reverse accrued interest and penalties of $602,000 which was recorded under selling, general and administrative expenses.
The compensatory element of stock issuances increased from $0 in fiscal 2020 to $83,277 in fiscal 2021.
Revenue from service and repair fees decreased from $8.2 million in fiscal 2020 to $7.7 million in fiscal 2021.
Continuing our tradition as the originator of MRI, we remain committed to maintaining our position as the leading innovator of the industry through investing in research and development. In fiscal 2021 we continued our investment in the development of various upgrades for the UPRIGHT® MRI, with an investment of $1,635,979 in research and development, none of which was capitalized, as compared to $2,025,376, none of which was capitalized, in fiscal 2020. The research and development expenditures were approximately 18.1% of revenues attributable to our medical equipment segment and 1.8% of total revenues in 2021, and 23.9% of medical equipment segment revenues and 2.4% of total revenues in fiscal 2020. This represented a 19.2% decrease in research and development expenditures in fiscal 2021 as compared to fiscal 2020.
For the physician and diagnostic services management segment, HMCA, revenues increased to $80.9 million in fiscal 2021 as compared to $77.2 million in fiscal 2020. This is primarily attributable to an increase in patient scans resulting from our marketing efforts.
For the fiscal year 2021 the Company recorded an income tax expense of $4.0 million compared with an income tax expense of $2.4 million for 2020. The income tax benefits are attributable to the expected tax benefits associated with the projected realization and utilization of our net operating losses in future periods. The Company has recorded a deferred tax asset of $16.0 million as of June 30, 2021, primarily relating to the tax benefits from the net operating loss carry forwards available to offset future taxable income. The utilization of these tax benefits is dependent on the Company generating future taxable income. Although the Company is expecting to generate taxable income in future periods, they cannot accurately measure the full impact of the adoption of healthcare regulations, including the impact of continuing changes in MRI scanning reimbursement rates, and the severity and the duration of the COVID-19 virus, which could materially impact operations. A partial valuation allowance will be maintained until evidence exists to support that it is no longer needed.
We have been taking steps to improve HMCA revenues by our marketing efforts, which focus on the unique capability of our Upright® MRI scanners to scan patients in different positions. We have also been increasing the number of health insurance plans in which our clients participate. The utilization of these tax benefits is dependent on the Company generating future taxable income and other factors. A partial valuation allowance will be maintained until evidence exists to support that it is no longer needed, (principally related to research and development credits).
| Page 67 |
FONAR CORPORATION AND SUBSIDIARIES
Our management fees are dependent on collection by our clients of fees from reimbursements from Medicare, Medicaid, private insurance, no fault and workers’ compensation carriers, self–pay and other third-party payors. The health care industry is experiencing the effects of the federal and state governments’ trend toward cost containment, as governments and other third-party payors seek to impose lower reimbursement and utilization rates and negotiate reduced payment schedules with providers. The cost-containment measures, consolidated with the increasing influence of managed-care payors and competition for patients, have resulted in reduced rates of reimbursement for services provided by our clients from time to time. Our future revenues and results of operations may be adversely impacted by future reductions in reimbursement rates.
Certain third-party payors have proposed and implemented changes in the methods and rates of reimbursement that have had the effect of substantially decreasing reimbursement for diagnostic imaging services that HMCA’s clients provide. To the extent reimbursement from third-party payors is reduced, it will likely have an adverse impact on the rates they pay us, as they would need to reduce the management fees they pay HMCA to offset such decreased reimbursement rates. Furthermore, many commercial health care insurance arrangements are changing, so that individuals bear greater financial responsibility through high deductible plans, co-insurance and higher co-payments, which may result in patients delaying or foregoing medical procedures. More frequently, however, patients are scanned and we experience difficulty in collecting deductibles and co-payments. We expect that any further changes to the rates or methods of reimbursement for services, which reduce the reimbursement per scan of our clients may partially offset the increases in scan volume we are working to achieve for our clients, and indirectly will result in a decline in our revenues.
On March 23, 2010, President Obama signed into law healthcare reform legislation in the form of the Patient Protection and Affordable Care Act, or PPACA. The ultimate impact of the PPACA is uncertain but to date has reduced our revenues from what they otherwise would have been.
In addition, the use of radiology benefit managers, or RBM’s has increased in recent years. It is common practice for health insurance carriers to contract with RBMs to manage utilization of diagnostic imaging procedures for their insureds. In many cases, this leads to lower utilization of imaging procedures based on a determination of medical necessity. The efficacy of RBMs is still a highly controversial topic. We cannot predict whether the healthcare legislation or the use of RBMs will negatively impact our business, but it is possible that our financial position and results of operations could be negatively affected.
LIQUIDITY AND CAPITAL RESOURCES
Cash, and cash equivalents increased by 20.8% from $36.8 million at June 30, 2020 to $44.5 million at June 30, 2021.
Cash provided by operating activities for fiscal 2021 approximated $19.1 million. Cash provided by operating activities was attributable to the net income of $13.7 million, depreciation and amortization of $4.1 million, deferred income tax expense benefit of $2.9 million which was offset by the increase in accounts, and medical and management fee receivables of $12.1 million.
| Page 68 |
FONAR CORPORATION AND SUBSIDIARIES
Cash used in investing activities for fiscal 2021 approximated $4.8 million. The cash used in investing activities was attributable to purchases of property and equipment of $3.5 million, the purchase of an imaging center for $1.1 million and costs of patents of $164,000.
Cash used in financing activities for fiscal 2021 approximated $6.6 million. The principal uses of cash used in financing activities included the proceeds from loans and capital lease obligations of $63,000, and repayment of borrowings and capital lease obligations of $103,000, and distributions to non-controlling interests of $6.6 million.
Total liabilities increased slightly by 0.2% during fiscal 2021, from approximately $54.0 million at June 30, 2020 to approximately $54.1 million at June 30, 2021.
As at June 30, 2021, our obligations included approximately $877,000 in various state sales taxes, inclusive of penalties and interest. The Company is in the process of negotiating settlements of these obligations.
At June 30, 2021, we had working capital of approximately $88.5 million as compared to working capital of $77.2 million at June 30, 2020, and stockholders’ equity of $135.4 million at June 30, 2021 as compared to stockholders’ equity of $126.2 million at June 30, 2020. For the year ended June 30, 2021, we realized a net income of $13.7 million.
Our principal sources of liquidity are derived from revenues.
Our business plan includes a program for manufacturing and selling our Upright® MRI scanners. In addition, we are enhancing our revenue by participating in the physician and diagnostic services management business through our subsidiary, HMCA and have upgraded the facilities which it manages, most significantly by the replacement of the original MRI scanners with new Upright® MRI scanners. As of June 30, 2021, HMCA manages a total of 39 MRI scanners of which 25 MRI scanners are located in New York and 14 are located in Florida. We have also intensified our marketing activities through the hiring of additional marketers for HMCA’s clients.
Our business plan also calls for a continuing emphasis on providing our customers with enhanced equipment service and maintenance capabilities and delivering state-of-the-art, innovative and high quality equipment upgrades at competitive prices. Fees for on-going service and maintenance from our installed base of scanners were $8.2 million for the year ended June 30, 2020 and $7.7 million for the year ended June 30, 2021.
In order to promote profitability and to reduce demands on our cash and other liquid reserves, we maintain an aggressive program of cost cutting. Previously, these measures included consolidating HMCA’s office space with Fonar’s office space and reducing the size of our workforce, compensation and benefits. We continue to reduce and contain expenses across the board. The cost reductions are intended to enable us to withstand periods of low volumes of MRI scanner sales, by keeping expenditures at levels which can be supported by service revenues and HMCA revenues.
| Page 69 |
FONAR CORPORATION AND SUBSIDIARIES
Current economic credit conditions have contributed to a slower than optimal business environment. As a result our business may suffer, should the credit markets not improve in the near future. The direct impact of these conditions is not fully known.
Revenues from HMCA have been the principal reason for our profitability, and we have so far been able to maintain and increase such revenues by increasing the number of scans being performed by the sites we manage and those we own, notwithstanding reductions in reimbursement rates from third party payors. The likelihood and effect of any subsequent reductions is not fully known.
Capital expenditures for fiscal 2021 approximated $3.7 million. Capitalized patent costs were approximately $164,000. Purchases of property and equipment were approximately $3.5 million.
In July, 2020, we completed the installation of a new MRI facility in Pembroke Pine, Florida.
In March 2021, we acquired a facility by purchase of all of its assets in Yonkers, NY. HMCA will provide the use of the facility and provide non-medical services to Comprehensive MRI of New York, P.C., a medical practice for which we provide equipment, supplies and non-medical services at other facilities as well.
Fonar is committed to making capital expenditures in the 2021 fiscal year, for placing three additional scanners at facilities located in Florida and New York. One of the facilities in Florida will be a new stand-alone facility and another will be the addition of an additional machine in Florida. The location in New York will also be a new stand-alone facility. The current estimated costs of these capital expenditures is approximately $3.0 million.
The Company believes that its business plan has been responsible for the past five consecutive fiscal years of profitability (fiscal 2021, fiscal 2020, fiscal 2019, fiscal 2018 and fiscal 2017) and that its capital resources will be adequate to support operations at current levels through September 30, 2022.
On April 20, 2020, we entered into a $4,917,325 loan agreement with the Paycheck Protection Program (“PPP”) under the CARES Act. Due to an abundance of caution, however, on May 1, 2020, we returned the full amount of the loan, since it became increasingly unclear whether or not a public company would be required to seek other sources of financing.
On June 30, 2020, the Company received loan proceeds in the amount of $700,764 under the Paycheck Protection Program (“PPP”). The PPP, established as part of the Coronavirus Aid, Relief and Economic Security Act (“CARES Act”), provides for loans to qualifying businesses for amount up to 2.5 times of the average monthly payroll expenses of the qualifying business. The Company applied for this additional loan exclusively for the Florida locations during June 2020 due to the fact that the COVID-19 virus was increasing in Florida. The loans and accrued interest are forgivable after 24 weeks as long as the borrower uses the loan proceeds for eligible purposes, including payroll, benefits, rent and utilities, and maintains its payroll levels. The amount of loan forgiveness will be reduced if the borrower terminates employees or reduces salaries during the 24 week period. This loan was forgiven during August 2021.
| Page 70 |
FONAR CORPORATION AND SUBSIDIARIES
During August 2021 the Company renewed their revolving credit agreement. The terms include borrowing limits of up to $10,000,000 and the agreement was extended to August 2022. The interest rate on unpaid principal remains at 4% along with certain financial covenants still applicable.
ITEM 7A. QUALITATIVE AND QUANTITATIVE DISCLOSURES ABOUT MARKET RISK
The Company does not have any investments in marketable securities, foreign currencies, mutual funds, certificates of deposit or other fixed rate instruments. All of our funds are in cash accounts or money market accounts which are liquid.
All of our revenue, expense and capital purchasing activities are transacted in United States dollars.
See Note 11 to the consolidated Financial Statements for information on long-term debt.
| Page 71 |
FONAR CORPORATION AND SUBSIDIARIES
ITEM 8.
FINANCIAL STATEMENTS
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| PAGE. | |
| REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM | 48 |
| CONSOLIDATED BALANCE SHEETS | 51 |
| At June 30, 2021 and 2020 | |
| CONSOLIDATED STATEMENTS OF INCOME | 54 |
| For the Years Ended June 30, 2021 and 2020 | |
| CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY | 56 |
| For the Years Ended June 30, 2021 and 2020 | |
| CONSOLIDATED STATEMENTS OF CASH FLOWS | 58 |
| For the Years Ended June 30, 2021 and 2020 | |
| NOTES TO CONSOLIDATED FINANCIAL STATEMENTS | 59 |
| Page 72 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
FONAR Corporation and Subsidiaries
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of FONAR Corporation and Subsidiaries (the “Company”) as of June 30, 2021 and 2020, the related consolidated statements of income, stockholders’ equity and cash flows for each of the two years in the period ended June 30, 2021, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of June 30, 2021 and 2020, and the results of its operations and its cash flows for each of the two years in the period ended June 30, 2021, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provides a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Patient Accounts Receivable Reserve – Refer to Note 3 to the financial statements
Critical Audit Matter Description
| Page 73 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM (Continued)
Patient accounts receivable is recorded at net realizable value based on the estimated amounts the Company expects to receive from patients and third-party payers. Estimates of contractual allowances under managed care, commercial, and governmental insurance plans are based upon the payment terms specified in the related contractual agreements or as mandated under government payer programs. Management continually reviews the contractual allowance estimation process to consider and incorporate updates to laws and regulations and the frequent changes in managed care contractual terms resulting from contract renegotiations and renewals. Receivables related to uninsured patients and uninsured copayment and deductible amounts for patients who have health insurance coverage may have discounts applied. The Company also records estimated implicit price concessions (based on historical experience) related to accounts to record the accounts receivable at the amount the Company expects to collect from patients and third-party payers. This implied concession requires extensive judgment and subjective assumptions. Implicit price concessions relate primarily to amounts due directly from patients and are based upon management’s assessment of historical write-offs and expected net collections, business and economic conditions, trends in federal, state, and private employer health care coverage, and other collection indicators. Auditing management’s estimate of the price concessions was complex and judgmental due to the significant data inputs and subjective assumptions utilized in determining the net realizable value of accounts receivable.
How the Critical Audit Matter Was Addressed in the Audit
Our audit procedures related to the net realizable value of patient accounts receivable included the following:
- We obtained an understanding, evaluated the design, and tested the operating effectiveness of certain controls that address the risks of material misstatement relating to the measurement of service fee revenue and receivables.
- We tested informational technology general controls around the Company’s billing system and associated database.
- We evaluated management’s methodology and related assumptions, including cash collections, by comparing actual results to management’s historical estimates.
- We tested the underlying data related to the recognition of patient level charges and the subsequent activities, including cash collections and non-cash adjustments.
- We tested the contractual rates set forth by the third-party payers which are input into the Company’s billing system and then billed to patients and/or third-party payers.
- We tested the mathematical accuracy of the estimates applied to period-end accounts receivable.
- We evaluated the appropriateness of the industry, economic, and Company factors that were used in determining the net realizable value of patient accounts receivable.
Management Fee Accounts Receivable Reserve – Refer to Note 3 to the financial statements.
Management fee accounts receivable is related to fees outstanding from the related and non-related professional corporations (“PCs”) under management agreements. Payment of the outstanding fees is dependent on the PCs ability to collect fees from third-party payers and patients because the management fees are collateralized by the PCs accounts receivable. The Company records the management fee accounts receivables net of the estimated implicit price concessions based on the PCs likelihood to collect on the accounts. Implicit price concessions on the PCs are estimated by management in the same manner the patient accounts receivable are analyzed. This implied concession requires extensive judgment and subjective assumptions. Implicit price concessions relate primarily to amounts due directly from patients and are based upon management’s assessment of historical write-offs and expected net collections, business and economic conditions, trends in federal, state, and private employer health care coverage, and other collection indicators. Auditing management’s estimate of the price concessions was complex and judgmental due to the significant data inputs and subjective assumptions utilized in determining the net realizable value of accounts receivable.
| Page 74 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM (Continued)
How the Critical Audit Matter Was Addressed in the Audit
Our audit procedures related to the management fee accounts receivable reserve are consistent with the audit procedures associated with the patient fee accounts receivable reserve. In addition, we traced the management fees to the underlying agreements and the general ledger.
/s/ Marcum llp
Marcum llp
We have served as the Company’s auditor since 1990, such date takes into account the merger of Tabb, Conigliaro, McGann, P.C. (“Tabb”) into another firm in approximately 2001 and the former partners of Tabb joining Marcum LLP in 2002.
New York, New York
October 12, 2021
/s/ Marcum llp
Marcum llp
| Page 75 |
FONAR CORPORATION AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
ASSETS
| June 30, | ||||||||
| 2021 | 2020 | |||||||
| Current Assets: | ||||||||
| Cash and cash equivalents | $ | 44,460,411 | $ | 36,802,342 | ||||
| Short term investments | 32,177 | 31,884 | ||||||
| Accounts receivable – net of allowances for doubtful accounts of $442,270 and $514,561 at June 30, 2021 and 2020, respectively | 4,525,435 | 4,312,999 | ||||||
| Accounts receivable – related party | 11,977 | 5,988 | ||||||
| Medical receivables - net | 17,900,489 | 16,171,782 | ||||||
| Management and other fees receivable – net of allowances for doubtful accounts of $15,786,878 and $11,063,233 at June 30, 2021 and 2020, respectively | 30,947,863 | 27,437,768 | ||||||
| Management and other fees receivable – related party medical practices – net of allowances for doubtful accounts of $4,184,399 and $3,322,055 at June 30, 2021 and 2020, respectively | 7,814,250 | 6,896,482 | ||||||
| Contract assets | — | 152,833 | ||||||
| Inventories | 1,663,419 | 1,648,770 | ||||||
| Income tax receivable | — | 671,185 | ||||||
| Prepaid expenses and other current assets | 1,227,463 | 1,757,499 | ||||||
| Total Current Assets | 108,583,484 | 95,889,532 | ||||||
| Accounts receivable – long term | 2,879,946 | 2,730,071 | ||||||
| Deferred income tax asset | 15,958,961 | 18,809,757 | ||||||
| Property and equipment – net | 21,850,139 | 21,364,034 | ||||||
| Right-of-use-asset – operating leases | 30,133,285 | 31,392,458 | ||||||
| Right-of-use-asset – financing lease | 1,126,990 | 1,325,870 | ||||||
| Goodwill | 4,269,277 | 3,985,397 | ||||||
| Other intangible assets – net | 4,037,599 | 4,109,129 | ||||||
| Other assets | 666,514 | 653,132 | ||||||
| Total Assets | $ | 189,506,195 | $ | 180,259,380 | ||||
See accompanying notes to consolidated financial statements.
| Page 76 |
FONAR CORPORATION AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
LIABILITIES
| June 30, | ||||||||
| 2021 | 2020 | |||||||
| Current Liabilities: | ||||||||
| Current portion of long-term debt and capital leases | $ | 173,206 | $ | 108,379 | ||||
| Accounts payable | 1,866,035 | 1,965,259 | ||||||
| Other current liabilities | 9,162,118 | 8,185,098 | ||||||
| Operating lease liability – current portion | 3,533,656 | 3,370,149 | ||||||
| Financing lease liability – current portion | 202,741 | 74,699 | ||||||
| Unearned revenue on service contracts | 4,365,825 | 4,105,265 | ||||||
| Customer deposits | 731,101 | 854,579 | ||||||
| Contract liabilities | 14,739 | — | ||||||
| Total Current Liabilities | 20,049,421 | 18,663,428 | ||||||
| Long-Term Liabilities: | ||||||||
| Unearned revenue on service contracts | 2,800,522 | 2,655,605 | ||||||
| Deferred income tax liability | 238,316 | 234,106 | ||||||
| Due to related party medical practices | 92,663 | 92,663 | ||||||
| Operating lease liability – net of current portion | 28,975,132 | 30,104,464 | ||||||
| Financing lease liability – net of current portion | 1,048,431 | 1,251,171 | ||||||
| Long-term debt and capital leases, less current portion | 760,254 | 865,416 | ||||||
| Other liabilities | 171,331 | 150,311 | ||||||
| Total Long-Term Liabilities | 34,086,649 | 35,353,736 | ||||||
| Total Liabilities | 54,136,070 | 54,017,164 | ||||||
Commitments, Contingencies and Other Matters
See accompanying notes to consolidated financial statements.
| Page 77 |
FONAR CORPORATION AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
STOCKHOLDERS’ EQUITY
| June 30, | ||||||||
| 2021 | 2020 | |||||||
| Stockholders’ Equity: | ||||||||
| Class A non-voting preferred stock $.0001 par value; 453,000 shares authorized at June 30, 2021 and 2020, 313,438 issued and outstanding at June 30, 2021 and 2020 | $ | 31 | $ | 31 | ||||
| Preferred stock $.001 par value; 567,000 shares authorized at June 30, 2021 and 2020, issued and outstanding – none | — | — | ||||||
| Common stock $.0001 par value; 8,500,000 shares authorized at June 30, 2021 and 2020, 6,565,853 and 6,459,106 issued at June 30, 2021 and 2020, respectively; 6,554,210 and 6,447,463 outstanding at June 30, 2021 and 2020, respectively | 657 | 647 | ||||||
| Class B convertible common stock (10 votes per share) $.0001 par value; 227,000 shares authorized at June 30, 2021 and 2020, 146 issued and outstanding at June 30, 2021 and 2020 | — | — | ||||||
| Class C common stock (25 votes per share) $.0001 par value; 567,000 shares authorized at June 30, 2021 and 2020, 382,513 issued and outstanding at June 30, 2021 and 2020 | 38 | 38 | ||||||
| Paid-in capital in excess of par value | 185,100,976 | 183,076,888 | ||||||
| Accumulated deficit | (46,007,663 | ) | (56,215,251 | ) | ||||
| Treasury stock, at cost – 11,643 shares of common stock at June 30, 2021 and 2020 | (675,390 | ) | (675,390 | ) | ||||
| Total Fonar Corporation’s Stockholders’ Equity | 138,418,649 | 126,186,963 | ||||||
| Noncontrolling interests | (3,048,524 | ) | 55,253 | |||||
| Total Stockholders’ Equity | 135,370,125 | 126,242,216 | ||||||
| Total Liabilities and Stockholders’ Equity | $ | 189,506,195 | $ | 180,259,380 | ||||
See accompanying notes to consolidated financial statements.
| Page 78 |
FONAR CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF INCOME
| For the Years Ended June 30, | ||||||||
| 2021 | 2020 | |||||||
| Revenues | ||||||||
| Patient fee revenue, net of contractual allowances and discounts | $ | 23,307,389 | $ | 22,495,260 | ||||
| Product sales – net | 1,288,483 | 297,613 | ||||||
| Service and repair fees – net | 7,638,608 | 8,055,490 | ||||||
| Service and repair fees – related parties – net | 110,000 | 110,000 | ||||||
| Management and other fees – net | 46,609,449 | 44,565,405 | ||||||
| Management and other fees – related party medical practices – net | 10,975,836 | 10,166,694 | ||||||
| Total Revenues – Net | 89,929,765 | 85,690,462 | ||||||
| Costs and Expenses | ||||||||
| Costs related to product sales | 1,032,676 | 745,375 | ||||||
| Costs related to service and repair fees | 2,740,625 | 2,731,397 | ||||||
| Costs related to service and repair fees – related parties | 39,466 | 37,298 | ||||||
| Costs related to patient fee revenue | 10,917,635 | 10,880,265 | ||||||
| Costs related to management and other fees | 25,384,557 | 22,951,301 | ||||||
| Costs related to management and other fees – related party medical practices | 6,341,168 | 5,951,189 | ||||||
| Research and development | 1,635,979 | 2,025,376 | ||||||
| Selling, general and administrative, inclusive of compensatory element of stock issuances of $83,277 and $0 for the years ended June 30, 2021 and 2020 respectively | 24,740,044 | 26,717,345 | ||||||
| Total Costs and Expenses | 72,832,150 | 72,039,546 | ||||||
| Income from Operations | 17,097,615 | 13,650,916 | ||||||
| Other Income and (Expenses): | ||||||||
| Interest expense | (248,665 | ) | (74,321 | ) | ||||
| Investment income | 311,931 | 502,145 | ||||||
| Other income | 504,450 | 70,771 | ||||||
| Income before provision for income taxes and noncontrolling interests | 17,665,331 | 14,149,511 | ||||||
| Provision for Income Taxes | (3,991,520 | ) | (2,444,778 | ) | ||||
| Net Income | $ | 13,673,811 | $ | 11,704,733 | ||||
| Net Income – Noncontrolling Interests | (3,466,223 | ) | (3,464,528 | ) | ||||
| Net Income – Attributable to FONAR | $ | 10,207,588 | $ | 8,240,205 | ||||
See accompanying notes to consolidated financial statements.
| Page 79 |
FONAR CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF INCOME (Continued)
| 2021 | 2020 | |||||||
| Net Income Available to Common Stockholders | $ | 9,592,134 | $ | 7,735,650 | ||||
| Net Income Available to Class A Non-Voting Preferred Stockholders | $ | 458,710 | $ | 376,055 | ||||
| Net Income Available to Class C Common Stockholders | $ | 156,744 | $ | 128,500 | ||||
| Basic Net Income Per Common Share Available to Common Stockholders | $ | 1.47 | $ | 1.20 | ||||
| Diluted Net Income Per Common Share Available to Common Stockholders | $ | 1.45 | $ | 1.18 | ||||
| Basic and Diluted Income Per Share – Class C Common | $ | 0.41 | $ | 0.34 | ||||
| Weighted Average Basic Shares Outstanding – Common Stockholders | 6,505,283 | 6,443,713 | ||||||
| Weighted Average Diluted Shares Outstanding – Common Stockholders | 6,632,787 | 6,571,217 | ||||||
| Weighted Average Basic and Diluted Shares Outstanding – Class C Common | 382,513 | 382,513 | ||||||
See accompanying notes to consolidated financial statements.
| Page 80 |
FONAR CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
FOR THE YEARS ENDED JUNE 30, 2021 AND 2020
| Class A Non-Voting Preferred | Common Shares | Stock Amount | Class C Common Stock | |||||||||||||
| Balance, July 1, 2019 | $ | 31 | 6,357,482 | $ | 638 | $ | 38 | |||||||||
| Net income | — | — | — | — | ||||||||||||
| Stock issued to employees under stock bonus plans | — | 89,981 | 9 | — | ||||||||||||
| Payments on notes receivable from employee stockholders | — | — | — | — | ||||||||||||
| Distributions to noncontrolling interests | — | — | — | — | ||||||||||||
| Balance, June 30, 2020 | $ | 31 | 6,447,463 | $ | 647 | $ | 38 | |||||||||
| Net income | — | — | — | — | ||||||||||||
| Stock issued to employees under stock bonus plans | — | 106,747 | 10 | — | ||||||||||||
| Distributions to noncontrolling interests | — | — | — | — | ||||||||||||
| Balance, June 30, 2021 | $ | 31 | 6,554,210 | $ | 657 | $ | 38 | |||||||||
| Paid-in Capital in Excess of Par Value | Accumulated Deficit | |||||||
| Balance, July 1, 2019 | $ | 181,086,517 | $ | (64,455,456 | ) | |||
| Net income | — | 8,240,205 | ||||||
| Stock issued to employees under stock bonus plans | 1,990,371 | — | ||||||
| Distributions to noncontrolling interests | — | — | ||||||
| Balance, June 30, 2020 | $ | 183,076,888 | $ | (56,215,251 | ) | |||
| Net income | — | 10,207,588 | ||||||
| Stock issued to employees under stock bonus plans | 2,024,088 | — | ||||||
| Distributions to noncontrolling interests | — | — | ||||||
| Balance, June 30, 2021 | $ | 185,100,976 | $ | (46,007,663 | ) | |||
See accompanying notes to consolidated financial statements.
| Page 81 |
FONAR CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
FOR THE YEARS ENDED JUNE 30, 2021 AND 2020
| Treasury Stock | Noncontrolling Interests | Total | ||||||||||
| Balance, July 1, 2019 | $ | (675,390 | ) | $ | 2,155,725 | $ | 118,112,103 | |||||
| Net income | — | 3,464,528 | 11,704,733 | |||||||||
| Stock issued to employees under stock bonus plans | — | — | 1,990,380 | |||||||||
| Distributions to noncontrolling interests | — | (5,565,000 | ) | (5,565,000 | ) | |||||||
| Balance, June 30, 2020 | $ | (675,390 | ) | $ | 55,253 | $ | 126,242,216 | |||||
| Net income | — | 3,466,223 | 13,673,811 | |||||||||
| Stock issued to employees under stock bonus plans | — | — | 2,024,098 | |||||||||
| Distributions to noncontrolling interests | — | (6,570,000 | ) | (6,570,000 | ) | |||||||
| Balance, June 30, 2021 | $ | (675,390 | ) | $ | (3,048,524 | ) | $ | 135,370,125 | ||||
See accompanying notes to consolidated financial statements.
| Page 82 |
FONAR CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
| For the Years Ended June 30, | ||||||||
| 2021 | 2020 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES | ||||||||
| Net Income | $ | 13,673,811 | $ | 11,704,733 | ||||
| Adjustments to reconcile net income to net cash provided by operating activities: | ||||||||
| Depreciation and amortization | 4,081,687 | 3,908,648 | ||||||
| Provision for bad debts | 5,585,989 | 5,695,420 | ||||||
| Deferred income tax - net | 2,855,006 | 2,118,829 | ||||||
| Income tax receivable | 671,185 | 528,815 | ||||||
| Amortization on right-of-use assets | 1,458,053 | 1,005,560 | ||||||
| Compensatory element of stock issuances | 83,277 | — | ||||||
| Stock issued for costs and expenses | 1,940,821 | 1,990,380 | ||||||
| Abandoned patents | 534 | — | ||||||
| (Increase) decrease in operating assets, net: | ||||||||
| Accounts, medical and management fee receivables | (12,110,859 | ) | (10,797,456 | ) | ||||
| Notes receivable | 46,944 | 28,889 | ||||||
| Contract assets | 152,833 | 372,277 | ||||||
| Inventories | (14,649 | ) | 149,396 | |||||
| Prepaid expenses and other current assets | 526,425 | (133,287 | ) | |||||
| Other assets | (18,087 | ) | (161,350 | ) | ||||
| Increase (decrease) in operating liabilities, net: | ||||||||
| Accounts payable | (99,224 | ) | 104,032 | |||||
| Other current liabilities | 1,382,497 | 3,556,437 | ||||||
| Customer advances | (123,478 | ) | 55,928 | |||||
| Operating lease liabilities | (965,825 | ) | 159,657 | |||||
| Financing lease liabilities | (74,698 | ) | — | |||||
| Contract liabilities | 14,739 | — | ||||||
| Other liabilities | 21,020 | 115,855 | ||||||
| NET CASH PROVIDED BY OPERATING ACTIVITIES | 19,088,001 | 20,402,763 | ||||||
| CASH FLOWS FROM INVESTING ACTIVITIES | ||||||||
| Purchases of property and equipment | (3,533,091 | ) | (7,522,997 | ) | ||||
| Proceeds of Short term investment | (293 | ) | 15,062,932 | |||||
| Purchase of imaging center | (1,122,508 | ) | — | |||||
| Cost of patents | (163,705 | ) | (117,522 | ) | ||||
| NET CASH (USED IN) PROVIDED BY INVESTING ACTIVITIES | (4,819,597 | ) | 7,422,413 | |||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Repayment of borrowings and capital lease obligations | (103,335 | ) | (4,957,936 | ) | ||||
| Proceeds from debt | 63,000 | 5,618,089 | ||||||
| Distributions to noncontrolling interests | (6,570,000 | ) | (5,565,000 | ) | ||||
| NET CASH USED IN FINANCING ACTIVITIES | (6,610,335 | ) | (4,904,847 | ) | ||||
| NET INCREASE IN CASH AND CASH EQUIVALENTS | 7,658,069 | 22,920,329 | ||||||
| CASH AND CASH EQUIVALENTS – BEGINNING OF YEAR | 36,802,342 | 13,882,013 | ||||||
| CASH AND CASH EQUIVALENTS – END OF YEAR | $ | 44,460,411 | $ | 36,802,342 | ||||
See accompanying notes to consolidated financial statements.
| Page 83 |
FONAR CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021 and 2020
NOTE 1 - DESCRIPTION OF BUSINESS AND LIQUIDITY AND CAPITAL RESOURCES
Description of Business
FONAR Corporation (the “Company” or “FONAR”) is a Delaware corporation, which was incorporated on July 17, 1978. FONAR is engaged in the research, development, production and marketing of medical scanning equipment, which uses principles of Magnetic Resonance Imaging (“MRI”) for the detection and diagnosis of human diseases. In addition to deriving revenues from the direct sale of MRI equipment, revenue is also generated from our installed-base of customers through our service and upgrade programs.
FONAR, through its wholly-owned subsidiary Health Management Corporation of America (“HMCA”) provides comprehensive management services to diagnostic imaging facilities. The services provided by the Company include development, administration, leasing of office space, facilities and medical equipment, provision of supplies, staffing and supervision of non-medical personnel, legal services, accounting, billing and collection and the development and implementation of practice growth and marketing strategies.
On July 1, 2015, the Company restructured the corporate organization of the management of diagnostic imaging centers segment of our business. The reorganization was structured to more completely integrate the operations of Health Management Corporation of America and HDM. Imperial contributed all of its assets (which were utilized in the business of Health Management Corporation of America) to HDM and received a 24.2% interest in HDM. Health Management Corporation of America retained a direct ownership interest of 45.8% in HDM, and the original investors in HDM retained a 30.0% ownership interest in the newly expanded HDM. The entire management of diagnostic imaging centers business segment is now being conducted by HDM.
During March 2020 the global pandemic of COVID-19 has caused turbulence and uncertainty in the United States and international markets and economies which has adversely effected our workforce, liquidity, financial conditions, revenues, profitability and business operations. Generally COVID-19 had caused us to require that much of our workforce work from home and has restricted the ability of our personnel to travel for marketing purposes or to service our customers. The Company experienced a sudden drop in scan volume for a short term period and the Company has been steadily recovering to pre-COVID-19 levels. At the end of fiscal year ending June 30, 2020, the Company was able to enact certain decisions to allow the Company to survive during the global pandemic and from further losses or additional decreases in scan volume. The Company also received some government stimulus funds from the Paycheck Protection Program (‘PPP’) program and Medicare advances/stimulus payments. Although we are unable to predict if there will be additional consequences on our operations from the continuing global pandemic of COVID-19 and the new variants, the Company believes with the positive cash flows, low debt and cash on hand, it will be able to continue operations going forward.
| Page 84 |
FONAR CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021 and 2020
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Principles of Consolidation
The consolidated financial statements include the accounts of FONAR Corporation, its majority and wholly-owned subsidiaries and partnerships. The operating activities of subsidiaries are included in the accompanying consolidated statements from the date of acquisition. All significant intercompany accounts and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of the consolidated financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities in the consolidated financial statements and accompanying notes. The most significant estimates relate to receivable allowances, intangible assets, income taxes and related tax asset valuation allowances, useful lives of property and equipment, contingencies, revenue recognition and the assessment of litigation. In addition, healthcare industry reforms and reimbursement practices will continue to impact the Company’s operations and the determination of contractual and other allowance estimates. Actual results could differ from those estimates.
Inventories
Inventories consist of purchased parts, components and supplies, as well as work-in-process, and are stated at the lower of cost, determined on the first-in, first-out method, or market.
Property and Equipment
Property and equipment procured in the normal course of business is stated at cost. Property and equipment purchased in connection with an acquisition is stated at its estimated fair value, generally based on an appraisal. Property and equipment is being depreciated for financial accounting purposes using the straight-line method over their estimated useful lives. Leasehold improvements are being amortized over the shorter of the useful life or the remaining lease term. Upon retirement or other disposition of these assets, the cost and related accumulated depreciation of these assets are removed from the accounts and the resulting gains or losses are reflected in the results of operations. Expenses for maintenance and repairs are charged to operations. Renewals and betterments are capitalized. Maintenance and repair expenses totaled approximately $2,051,000 and $1,870,000 for the years ended June 30, 2021 and 2020 respectively. The estimated useful lives in years are generally as follows:
| Estimated Useful Life in Years for Property and Equipment | |
| Diagnostic equipment | 5–13 |
| Research, development and demonstration equipment | 3-7 |
| Machinery and equipment | 2-7 |
| Furniture and fixtures | 3-9 |
| Leasehold improvements | 3–10 |
| Building | 28 |
| Page 85 |
FONAR CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021 and 2020
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Long-Lived Assets
The Company periodically assesses the recoverability of long-lived assets, including property and equipment and intangibles, other than goodwill, when there are indications of potential impairment, based on estimates of undiscounted future cash flows. The amount of impairment is calculated by comparing anticipated discounted future cash flows with the carrying value of the related asset. In performing this analysis, management considers such factors as current results, trends, and future prospects, in addition to other economic factors.
Other Intangible Assets
1) Patents and Copyrights
Amortization is calculated on the straight-line basis over 15 years.
2) Non-Competition Agreements
The non-competition agreements are being amortized on the straight line basis over the length of the agreement (7 years).
3) Customer Relationships
Amortization is calculated on the straight line basis over 20 years.
Goodwill
Generally accepted accounting principles in the United States require the Company to perform a goodwill impairment test annually and more frequently when negative conditions or a triggering event arises. Impairment of goodwill is tested at the reporting unit level by comparing the reporting unit’s carrying amount, including goodwill to the fair value of the reporting unit. If the carrying amount of the reporting unit exceeds its fair value, goodwill is considered potentially impaired and a second step is performed to measure the amount of impairment loss, if any.
Acquired assets and assumed liabilities
Pursuant to ASC No. 805-10-25, if the initial accounting for a business combination is incomplete by the end of the reporting period in which the combination occurs, but during the allowed measurement period not to exceed one year from the acquisition date, the Company adjusts the provisional amounts recognized at the acquisition date by means of adjusting the amount recognized for goodwill.
| Page 86 |
FONAR CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021 and 2020
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Revenue Recognition
Revenue on sales contracts for scanners, included in “product sales” in the accompanying consolidated statements of operations, is recognized under the percentage-of-completion method in accordance with FASB ASC 606, “Revenue Recognition – Construction-Type and Production-Type Contracts”. The Company manufactures its scanners under specific contracts that provide for progress payments. Production and installation take approximately three to six months.
Revenue on scanner service contracts is recognized on the straight-line method over the related contract period, usually one year.
Revenue from product sales (upgrades and supplies) is recognized upon shipment.
Revenue under management contracts is recognized based upon contractual agreements for management services rendered by the Company primarily under various long-term agreements with various medical providers (the “PCs”). As of June 30, 2021, the Company has twenty two 22 management agreements of which three 3 are with PC’s owned by Raymond V. Damadian, M.D., Chairman of the Board of FONAR (“the Related medical practices”) and nineteen 19 are with PC’s, which are all located in the state of New York (“the New York PC’s”), owned by two unrelated radiologists. The contractual fees for services rendered to the PCs consists of fixed monthly fees per diagnostic imaging facility ranging from approximately $77,000 to $522,000. All fees are re-negotiable at the anniversary of the agreements and each year thereafter. The Company records a provision for bad debts for estimated uncollectible fees, which is reflected in other operating expenses on the Statement of Operations. All fees are re-negotiable at the anniversary of the agreements and each year thereafter.
On July 1, 2018, the Company adopted the new revenue recognition accounting standard issued by the Financial Accounting Standards Board (“FASB”) and codified in the ASC as topic 606 (“ASC 606”). The revenue recognition standard in ASC 606 outlines a single comprehensive model for recognizing revenue as performance obligations, defined in a contract with a customer as goods or services transferred to the customer in exchange for consideration, are satisfied. The standard also requires expanded disclosures regarding the Company’s revenue recognition policies and significant judgments employed in the determination of revenue.
The Company applied the modified retrospective approach to all contracts when adopting ASC 606. As a result, at the adoption of ASC 606 the majority of what was previously classified as the provision for bad debts in the statement of operations is now reflected as implicit price concessions (as defined in ASC 606) and therefore included as a reduction to net operating revenues in 2019. For changes in credit issues not assessed at the date of service, the Company will prospectively recognize those amounts in other operating expenses on the statement of operations.
| Page 87 |
FONAR CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021 and 2020
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Revenue Recognition (Continued)
For periods prior to the adoption of ASC 606, the provision for bad debts has been presented consistent with the previous revenue recognition standards that required it to be presented separately as a component of net operating revenues.
Our revenues generally relate to net patient fees received from various payers and patients themselves under contracts in which our performance obligations are to provide diagnostic services to the patients. Revenues are recorded during the period our obligations to provide diagnostic services are satisfied. Our performance obligations for diagnostic services are generally satisfied over a period of less than one day. The contractual relationships with patients, in most cases, also involve a third-party payer (Medicare, Medicaid, managed care health plans and commercial insurance companies, including plans offered through the health insurance exchanges) and the transaction prices for the services provided are dependent upon the terms provided by (Medicare and Medicaid) or negotiated with (managed care health plans and commercial insurance companies) the third-party payers. The payment arrangements with third-party payers for the services we provide to the related patients typically specify payments at amounts less than our standard charges and generally provide for payments based upon predetermined rates per diagnostic services or discounted fee-for-service rates. Management continually reviews the contractual estimation process to consider and incorporate updates to laws and regulations and the frequent changes in managed care contractual terms resulting from contract renegotiations and renewals.
The Company’s patient fee revenues, net of contractual allowances and discounts less the provision for bad debts for the years ended June 30, 2021 and 2020 are summarized in the following table.
| Patient Fee Revenue - Net | ||||||||
| For the Years Ended June 30 | ||||||||
| 2021 | 2020 | |||||||
| Commercial Insurance/ Managed Care | $ | 4,100,440 | $ | 4,545,987 | ||||
| Medicare/Medicaid | 968,055 | 1,038,288 | ||||||
| Workers’ Compensation/Personal Injury | 15,011,111 | 16,028,737 | ||||||
| Other | 3,227,783 | 882,248 | ||||||
| Net Patient Fee Revenue | $ | 23,307,389 | $ | 22,495,260 | ||||
Research and Development Costs
Research and development costs are charged to expense as incurred. The costs of equipment that are acquired or constructed for research and development activities, and have alternative future uses (either in research and development, marketing or production), are classified as property and equipment and depreciated over their estimated useful lives.
| Page 88 |
FONAR CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021 and 2020
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Advertising Costs
Advertising costs are expensed as incurred. Advertising expense approximated $633,000 and $566,000 and for the years ended June 30, 2021 and 2020, respectively.
Shipping Costs
The Company’s shipping and handling costs are included in revenue from product sales and the related expense included in costs related to product sales is $8,215 and $12,056 for the years ended June 30, 2021 and 2020 respectively.
Income Taxes
Deferred tax assets and liabilities are determined based on the difference between the financial statement carrying amounts and tax basis of assets and liabilities using enacted tax rates in effect in the years in which the differences are expected to reverse.
Customer Advances
Cash advances and progress payments received on sales orders are reflected as customer advances until such time as revenue recognition occurs.
Basic earnings per share (“EPS”) is computed by dividing net income available to common stockholders by the weighted average number of shares of common stock outstanding during the period. In accordance with ASC topic 260-10, “Participating Securities and the Two-Class Method”, the Company used the Two-Class method for calculating basic earnings per share and applied the if converted method in calculating diluted earnings per share for the years ended June 30, 2021 and 2020.
Diluted EPS reflects the potential dilution from the exercise or conversion of all dilutive securities into common stock based on the average market price of common shares outstanding during the period. For the years ended June 30, 2021 and 2020, diluted EPS for common shareholders includes 127,504 shares upon conversion of Class C Common.
| Page 89 |
FONAR CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021 and 2020
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Earnings Per Share (Continued)
| Earnings Per Share | ||||||||||||
| June 30, 2021 | ||||||||||||
| Basic | Total | Common Stock | Class C Common Stock | |||||||||
| Numerator: | ||||||||||||
| Net income available to common stockholders | $ | 10,207,588 | $ | 9,592,134 | $ | 156,744 | ||||||
| Denominator: | ||||||||||||
| Weighted average shares outstanding | 6,505,283 | 6,505,283 | 382,513 | |||||||||
| Basic income per common share | $ | 1.57 | $ | 1.47 | $ | 0.41 | ||||||
| Diluted | ||||||||||||
| Denominator: | ||||||||||||
| Weighted average shares outstanding | 6,505,283 | 382,513 | ||||||||||
| Class C Common Stock | 127,504 | — | ||||||||||
| Total Denominator for diluted earnings per share | 6,632,787 | 382,513 | ||||||||||
| Diluted income per common share | $ | 1.45 | $ | 0.41 | ||||||||
| June 30, 2020 | ||||||||||||
| Basic | Total | Common Stock | Class C Common Stock | |||||||||
| Numerator: | ||||||||||||
| Net income available to common stockholders | $ | 8,240,205 | $ | 7,735,650 | $ | 128,500 | ||||||
| Denominator: | ||||||||||||
| Weighted average shares outstanding | 6,443,713 | 6,443,713 | 382,513 | |||||||||
| Basic income per common share | $ | 1.28 | $ | 1.20 | $ | 0.34 | ||||||
| Diluted | ||||||||||||
| Denominator: | ||||||||||||
| Weighted average shares outstanding | 6,443,713 | 382,513 | ||||||||||
| Class C Common Stock | 127,504 | — | ||||||||||
| Total Denominator for diluted earnings per share | 6,571,217 | 382,513 | ||||||||||
| Diluted income per common share | $ | 1.18 | $ | 0.34 | ||||||||
Cash and Cash Equivalents
Cash and cash equivalents includes cash on hand, cash in banks, investments in certificates of deposit with original maturities of 90 days or less, and money market funds.
| Page 90 |
FONAR CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021 and 2020
NOTE 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Short Term Investments
Short term investments include certificates of deposit with original maturities of greater than 90 days.
Concentration of Credit Risk
Cash: The Company maintains its cash and cash equivalents with various financial institutions, which exceed federally insured limits throughout the year. At June 30, 2021, the Company had cash on deposit of approximately $42,609,000 in excess of federally insured limits of $250,000.
Related Parties: Net revenues from related parties accounted for approximately 12% and 12% of the consolidated net revenues for the years ended June 30, 2021 and 2020, respectively. Net management fee receivables from the related party medical practices accounted for approximately 13% and 13% of the consolidated accounts receivable for the years ended June 30, 2021 and 2020, respectively.
See Note 3 regarding the Company’s concentrations in the healthcare industry.
Fair Value of Financial Instruments
The financial statements include various estimated fair value information at June 30, 2021 and 2020, as required by ASC topic 820, “Disclosures about Fair Value of Financial Instruments”. Such information, which pertains to the Company’s financial instruments, is based on the requirements set forth in that Statement and does not purport to represent the aggregate net fair value to the Company.
The Company has established a three-tier fair value hierarchy, which prioritizes the inputs used in measuring and revaluing fair value. These tiers include, Level 1, defined as observable inputs such as quoted prices in active markets; Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable; and Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions.
The following methods and assumptions were used to estimate the fair value of each class of financial instruments for which it is practicable to estimate that value:
Cash and cash equivalents: The carrying amount approximates fair value because of the short-term maturity of those instruments.
Short term investments: The carrying amount approximates fair value because of the short-term maturity of those instruments. Such amounts include Certificates of Deposits with original maturities greater than 90 days. These securities are classified as Level 1.
Receivable and accounts payable: The carrying amounts approximate fair value because of the short maturity of those instruments.
| Page 91 |
FONAR CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021 and 2020
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Fair Value of Financial Instruments (Continued)
Notes receivable: The carrying amount approximates fair value because the discounted present value of the cash flow generated by the parties approximates the carrying value of the amounts due to the Company.
Long-term debt and notes payable: The carrying amounts of debt and notes payable approximate fair value due to the length of the maturities, the interest rates being tied to market indices and/or due to the interest rates not being significantly different from the current market rates available to the Company.
All of the Company’s financial instruments are held for purposes other than trading.
Recent Accounting Pronouncements
In December 2019, the FASB issued ASU 2019-12 (“ASU 2019-12”), Income Taxes (Topic 740). ASU 2019-12 removes certain exceptions related to the approach for intraperiod tax allocation, the methodology for calculating income taxes in an interim period, and the recognition of deferred tax liabilities for outside basis differences. It also clarifies and simplifies other areas of the standard. ASU 2019-12 was effective beginning in the first quarter of 2021. Certain amendments in this update must be applied on a prospective basis, certain amendments must be applied on a retrospective basis, and certain amendments must be applied on a modified retrospective basis through a cumulative-effect adjustment to retained earnings/(deficit) in the period of adoption. The adoption of this update did not have a material impact on our financial statements.
In January 2017, the FASB issued Accounting Standards Update (“ASU”) 2017-04, Intangibles – Goodwill and Other (Topic 350). The amendments in this update simplify the test for goodwill impairment by eliminating Step 2 from the impairment test, which required the entity to perform procedures to determine the fair value at the impairment testing date of its assets and liabilities following the procedure that would be required in determining fair value of assets acquired and liabilities assumed in a business combination. The amendments in this update are effective for public companies for annual or any interim goodwill impairment tests in fiscal years beginning after December 15, 2019. The Company adopted the Standard on July 1, 2020 and the impact of adopting this guidance had no material impact on our Consolidated Financial Statements.
FASB, the Emerging Issues Task Force and the SEC have issued certain other accounting standards, updates, and regulations as of June 30, 2021 that will become effective in subsequent periods; however, management does not believe that any of those updates would have significantly affected our financial accounting measures or disclosures had they been in effect during 2021 or 2020, and it does not believe that any of those pronouncements will have a significant impact on our consolidated financial statements at the time they become effective.
| Page 92 |
FONAR CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021 and 2020
NOTE 3 – ACCOUNTS RECEIVABLE, MEDICAL RECEIVABLE AND MANAGEMENT AND OTHER FEES RECEIVABLE
Accounts Receivable
Credit risk with respect to the Company’s accounts receivable related to product sales and service and repair fees is limited due to the customer advances received prior to the commencement of work performed and the billing of amounts to customers as sub-assemblies are completed. Service and repair fees are billed on a monthly or quarterly basis and the Company does not continue providing these services if accounts receivable become past due. The Company controls credit risk with respect to accounts receivable from service and repair fees through its credit evaluation process, credit limits, monitoring procedures and reasonably short collection terms. The Company performs ongoing credit authorizations before a product sales contract is entered into or service and repair fees are provided.
Long Term Accounts Receivable
The Company will generate revenue from long-term, non-cancellable contracts to provide service and repair services. Future revenue to be recognized over the following four years at June 30, 2021 is as follows:
| Receivables - Non Current - net | |||||
| 2023 | $ | 1,020,140 | |||
| 2024 | 1,020,140 | ||||
| 2025 | 599,545 | ||||
| 2026 | 160,697 | ||||
| Total | $ | 2,800,522 |
Medical Receivable
Medical receivables are due under fee-for-service contracts from third party payors, such as hospitals, government sponsored healthcare programs, patient’s legal counsel and directly from patients. Substantially all the revenue relates to patients residing in Florida. The carrying amount of the medical receivable is reduced by an allowance that reflects management’s best estimate of the amounts that will not be collected. The Company determines allowances for contractual adjustments and uncollectible accounts based on specific agings, specific payor collection issues that have been identified and based on payor classifications and historical experience at each site.
| Page 93 |
FONAR CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021 and 2020
NOTE 3 – ACCOUNTS RECEIVABLE, MEDICAL RECEIVABLE AND MANAGEMENT AND OTHER FEES RECEIVABLE (Continued)
Management and Other Fees Receivable
The Company’s receivables from the related and non-related professional corporations (“PCs”) substantially consist of fees outstanding under management agreements. Payment of the outstanding fees is dependent on collection by the PCs of fees from third party medical reimbursement organizations, principally insurance companies and health management organizations.
Payment of the management fee receivables from the PC’s may be impaired by the inability of the PC’s to collect in a timely manner their medical fees from the third party payors, particularly insurance carriers covering automobile no-fault and workers compensation claims due to longer payment cycles and rigorous informational requirements and certain other disallowed claims. Approximately 65% and 66%, respectively, of the PCs’ 2021 and 2020 net revenues were derived from no-fault and personal injury protection claims. The Company considers the aging of its accounts receivable in determining the amount of allowance for doubtful accounts. The Company generally takes all legally available steps to collect its receivables. Credit losses associated with the receivables are provided for in the consolidated financial statements and have historically been within management’s expectations.
Net revenues from management and other fees charged to the related party medical practices accounted for approximately 12% and 12%, of the consolidated net revenues for the years ended June 30, 2021 and 2020, respectively.
Tallahassee Magnetic Resonance Imaging, PA, Stand Up MRI of Boca Raton, PA and Stand Up MRI & Diagnostic Center, PA (all related party medical practices) entered into a guaranty agreement, pursuant to which they cross guaranteed all management fees which are payable to the Company, which have arisen under each individual management agreement.
The following table sets forth the number of our facilities for the years ended June 30, 2021 and 2020.
| Total Facilities | ||||||||
| For the Year Ended June 30, | ||||||||
| 2021 | 2020 | |||||||
| Total Facilities Owned or Managed (at Beginning of Year) | 25 | 26 | ||||||
| Facilities Added by: | ||||||||
| Acquisition | 1 | — | ||||||
| Internal development | 1 | — | ||||||
| Managed Facilities Closed | — | (1 | ) | |||||
| Total Facilities Owned or Managed (at End of Year) | 27 | 25 | ||||||
| Page 94 |
FONAR CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021 and 2020
NOTE 4 – CONTRACT ASSETS AND LIABILITIES
Information relating to uncompleted contracts as of June 30, 2021 and 2020 about contract assets and contract (liabilities) is as follows:
| `Costs and Estimated Earnings on Uncompleted Contracts | ||||||||
| As of June 30, | ||||||||
| 2021 | 2020 | |||||||
| Costs incurred on uncompleted contracts | $ | 294,783 | $ | 448,437 | ||||
| Estimated earnings | 567,978 | 309,248 | ||||||
| Costs and estimated earnings on uncompleted contracts | 862,761 | 757,685 | ||||||
| Less: Billings to date | 877,500 | 604,852 | ||||||
| Costs and estimated earnings in excess of billings on uncompleted contracts | $ | (14,739 | ) | $ | 152,833 | |||
NOTE 5 – INVENTORIES
Inventories included in the accompanying consolidated balance sheets consist of:
| Inventories | ||||||||
| As of June 30, | ||||||||
| 2021 | 2020 | |||||||
| Purchased parts, components and supplies | $ | 1,393,329 | $ | 1,544,036 | ||||
| Work-in-process | 270,090 | 104,734 | ||||||
| Inventories | $ | 1,663,419 | $ | 1,648,770 | ||||
NOTE 6 - PROPERTY AND EQUIPMENT
Property and equipment, at cost, less accumulated depreciation and amortization, at June 30, 2021 and 2020, is comprised of:
| Property and Equipment | ||||||||
| As of June 30, | ||||||||
| 2021 | 2020 | |||||||
| Diagnostic equipment | $ | 29,826,829 | $ | 28,434,063 | ||||
| Research, development and demonstration equipment | 6,029,551 | 5,901,961 | ||||||
| Machinery and equipment | 2,069,055 | 2,069,055 | ||||||
| Furniture and fixtures | 3,450,664 | 3,291,666 | ||||||
| Leasehold improvements | 12,961,887 | 10,736,825 | ||||||
| Building | 939,614 | 939,614 | ||||||
| 55,277,600 | 51,373,184 | |||||||
| Less: Accumulated depreciation and amortization | 33,427,461 | 30,009,150 | ||||||
| $ | 21,850,139 | $ | 21,364,034 | |||||
Depreciation and amortization of property and equipment for the years ended June 30, 2021 and 2020 was $3,696,986 and $3,144,580, respectively.
| Page 95 |
FONAR CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021 and 2020
NOTE 7 – OPERATING & FINANCING LEASES
During February 2016, FAS issued ASU 2016-02, Leases (Topic 842). The new standard requires lessees to apply a dual approach, classifying leases as either finance or operating leases based upon the principle of whether or not the lease is effectively a financed purchase by the lessee. This classification will determine whether lease expense is recognized based on an effective interest method or on a straight-line basis over the term of the lease. A lessee is also required to record a right-of-use asset and a lease liability for all leases with a term of greater than 12 months regardless of their classification. Lease with a term of 12 months or less will be accounted for similar to existing guidance for operating leases. The standard was effective for us beginning July 1, 2019. We have elected the optional transition method to apply the standard as of the effective date and therefore, we will not apply the standard to the comparative periods presented in the consolidated financial statements. We have also elected the transition package of thee practical expedients permitted within the standard which eliminates the requirements to reassess prior conclusions about lease identification, lease classification and indirect costs. The adoption of this guidance had a material impact on the Company’s balance sheet by virtue of including the present value of its future operating lease payments as a liability of $33.3 million and related right-to-use lease assets as of July 1, 2019. At the time of adoption of this guidance we had no significant financing leases.
The Company accounts for its various operating leases in accordance with Accounting Standards Codification (‘ASC’) 842 – Lease, as updated by ASU 2016-02. At the inception of a lease, the Company recognizes right-of-use lease assets and related lease liabilities measured at present value of future lease payments on its balance sheet. Lease expense is recognized on a straight-line basis over the term of the lease. Our most common initial term varies in length from 2 to 10 years. Including renewal options negotiated with the landlord, we have a total span of 2 to 16 years at the facilities we lease. The Company reviewed its contracts with vendors and customers, determining that its right-to-use lease assets consisted of only office space operating leases. In determining the right-to-use lease assets and liabilities, the Company did recognize lease extension options which the Company feels would be reasonably exercised. Our incremental borrowing rate (“IBR”) used to discount the stream of operating lease payments is closely related to the interest rates available to the Company. A reconciliation of operating and financing lease payments undiscounted cash flows to lease liabilities recognized as of June 30, 2021 is as follows:
| Reconciliation of operating and financing lease payments | |||||||||
| Year
Ending June 30, | Operating
Lease Payments | Financing Lease Payments | |||||||
| 2022 | $ | 5,077,656 | $ | 244,343 | |||||
| 2023 | 5,172,157 | 244,343 | |||||||
| 2024 | 4,837,900 | 244,343 | |||||||
| 2025 | 4,725,550 | 244,343 | |||||||
| 2026 | 4,285,124 | 244,343 | |||||||
| Thereafter | 16,950,126 | 162,898 | |||||||
| Present value discount | (8,539,725 | ) | (133,441 | ) | |||||
| Total lease liability | $ | 32,508,788 | $ | 1,251,172 | |||||
| Page 96 |
FONAR CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021 and 2020
NOTE 7 – OPERATING & FINANCING LEASES (Continued)
Weighted Average Remaining Lease Term
| Operating leases - years | 9.4 | |||
| Finance lease - years | 5.6 | |||
| Weighted Average Discount Rate | ||||
| Operating leases | 5.5 | % | ||
| Finance lease | 3.6 | % |
The components of lease expense were as follows:
| Components of lease expense | ||||||||
| For Year Ended | ||||||||
| June 30, 2021 | June 30, 2020 | |||||||
| Operating lease cost | $ | 6,145,701 | $ | 5,135,604 | ||||
| Finance lease cost: | ||||||||
| Depreciation of leased equipment | $ | 198,881 | $ | — | ||||
| Interest on lease liabilities | 47,472 | — | ||||||
| Total finance lease cost | $ | 246,353 | $ | — | ||||
Supplemental cash flow information related to leases was as follows:
| Supplemental cash flow information related to leases | ||||||||
| For year ended | ||||||||
| Cash paid for amounts included in the measurement of lease liabilities: | June 30, 2021 | June 30, 2020 | ||||||
| Operating cash flows from operating leases | $ | 4,970,934 | $ | 4,170,977 | ||||
| Financing cash flows from financing leases | $ | 130,038 | $ | |||||
| Right-of-use & equipment assets obtained in exchange for lease obligations: | ||||||||
| Operating leases(1) | $ | 1,531,889 | $ | 34,786,611 | ||||
| Financing leases | $ | — | $ | 1,325,871 | ||||
| (1) | Amounts for the year ended June 30, 2020 include the transition adjustment for the adoption of topic 842 of $31,651,110. |
| Page 97 |
FONAR CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021 and 2020
NOTE 8 - OTHER INTANGIBLE ASSETS
Other intangible assets, net of accumulated amortization, at June 30, 2021 and 2020 are comprised of:
| Other Intagible Assets - Net | ||||||||
| As of June 30, | ||||||||
| 2021 | 2020 | |||||||
| Capitalized software development costs | $ | 7,004,847 | $ | 7,004,847 | ||||
| Patents and copyrights | 5,244,892 | 5,081,721 | ||||||
| Non-competition agreements | 4,150,000 | 4,100,000 | ||||||
| Customer relationships | 3,900,000 | 3,800,000 | ||||||
| 20,299,739 | 19,986,568 | |||||||
| Less: Accumulated amortization | 16,262,140 | 15,877,439 | ||||||
| $ | 4,037,599 | $ | 4,109,129 | |||||
The estimated amortization of other intangible assets for the five years ending June 30, 2026 and thereafter is as follows:
| Schedule Of Other Intangible Assets | |||||||||||||||||
| For
the Years Ending June 30, | Total | Patents and Copyrights | Non-
competition | Customer Relationships | |||||||||||||
| 2022 | $ | 423,209 | $ | 185,709 | $ | 37,500 | $ | 200,000 | |||||||||
| 2023 | 389,168 | 189,168 | — | 200,000 | |||||||||||||
| 2024 | 388,867 | 188,867 | — | 200,000 | |||||||||||||
| 2025 | 383,912 | 183,912 | — | 200,000 | |||||||||||||
| 2026 | 373,220 | 173,220 | — | 200,000 | |||||||||||||
| Thereafter | 2,079,223 | 765,056 | — | 1,314,167 | |||||||||||||
| Other intangible assets - net | $ | 4,037,599 | $ | 1,685,932 | $ | 37,500 | $ | 2,314,167 | |||||||||
The weighted average amortization period for other intangible assets is 11.4 years and they have no expected residual value.
Information related to the above intangible assets for the years ended June 30, 2021 and 2020 is as follows:
| Other Intangible Assets | ||||||||
| As of June 30, | ||||||||
| 2021 | 2020 | |||||||
| Balance – Beginning of Year | $ | 4,109,129 | $ | 4,755,675 | ||||
| Amounts capitalized | 313,705 | 117,522 | ||||||
| Software or patents written off | (534 | ) | — | |||||
| Amortization | (384,701 | ) | (764,068 | ) | ||||
| Balance – End of Year | $ | 4,037,599 | $ | 4,109,129 | ||||
| Page 98 |
FONAR CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021 and 2020
NOTE 8 - OTHER INTANGIBLE ASSETS (Continued)
Amortization of patents and copyrights for the years ended June 30, 2021 and 2020 amounted to $179,701 and $183,592, respectively.
Capitalized software development costs were fully amortized as of June 30, 2018.
Amortization of non-competition agreements for the years ended June 30, 2021 and 2020 amounted to $12,500 and $390,476, respectively.
Amortization of customer relationships for the years ended June 30, 2021 and 2020 amounted to $192,500 and $190,000, respectively.
NOTE 9 - CAPITAL STOCK
Common Stock
Cash dividends payable on the common stock shall, in all cases, be on a per share basis, one hundred twenty percent (120%) of the cash dividend payable on shares of Class B common stock and three hundred sixty percent (360%) of the cash dividend payable on a share of Class C common stock.
Class B Common Stock
Class B common stock is convertible into shares of common stock on a one-for-one basis. Class B common stock has 10 votes per share. There were 146 of such shares outstanding at June 30, 2021 and 2020.
Class C Common Stock
On April 3, 1995, the stockholders ratified a proposal creating a new Class C common stock and authorized the exchange offering of three shares of Class C common stock for each share of the Company’s outstanding Class B common stock. The Class C common stock has 25 votes per share, as compared to 10 votes per share for the Class B common stock and one vote per share for the common stock. The Class C common stock was offered on a three-for-one basis to the holders of the Class B common stock. Although having greater voting power, each share of Class C common stock has only one-third of the rights of a share of Class B common stock to dividends and distributions. Class C common stock is convertible into shares of common stock on a three-for-one basis.
| Page 99 |
FONAR CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021 and 2020
NOTE 9 - CAPITAL STOCK (Continued)
Class A Non-Voting Preferred Stock
On April 3, 1995, the stockholders ratified a proposal consisting of the creation of a new class of Class A non-voting preferred stock with special dividend rights and the declaration of a stock dividend on the Company’s common stock consisting of one share of Class A non-voting preferred stock for every five shares of common stock. The stock dividend was payable to holders of common stock on October 20, 1995. Class A non-voting preferred stock issued pursuant to such stock dividend approximates 313,000 shares.
The Class A non-voting preferred stock is entitled to a special dividend equal to 3-1/4% of first $10 million, 4-1/2% of next $20 million and 5-1/2% on amounts in excess of $30 million of the amount of any cash awards or settlements received by the Company in connection with the enforcement of five of the Company’s patents in its patent lawsuits, less the revised special dividend payable on the common stock with respect to one of the Company’s patents.
The Class A non-voting preferred stock participates on an equal per share basis with the common stock in any dividends declared and ranks equally with the common stock on distribution rights, liquidation rights and other rights and preferences (other than the voting rights).
Stock Bonus Plans
On April 23, 2010, the Board approved the 2010 Stock Bonus Plan. The plan entitles the Company to reserve 2,000,000 shares of common stock. On August 10, 2010, the Company filed Form S-8 to register the 2,000,000 shares. As of June 30, 2021, 450,177 shares of common stock of FONAR were available for future grant under this plan. For the years ended June 30, 2021 and 2020, 106,747 and 89,981 shares were issued respectively, of which $83,277 and $0 were expensed and included in selling, general and administrative expenses for the years ended June 30, 2021 and 2020, respectively.
Options
The Company had stock option plans, which provide for the awarding of incentive and non-qualified stock options to employees, directors and consultants who may contribute to the success of the Company. The options granted vest either immediately or ratably over a period of time from the date of grant, typically three or four years, at a price determined by the Board of Directors or a committee of the Board of Directors, generally the fair value of the Company’s common stock at the date of grant. The options must be exercised within ten years from the date of grant.
| Page 100 |
FONAR CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021 and 2020
NOTE 10 – CONTROLLING AND NONCONTROLLING INTERESTS
On February 13, 2013 the Company entered into an agreement with outside investors to acquire a 50.5% controlling interest in a newly formed limited liability company, Health Diagnostics Management LLC (HDM). According to the February 13, 2013 LLC operating agreement of HDM there are two classes of members; Class A members and one Class B member. The Class A members have an ownership interest of 49.5% of HDM. The Class B member (HMCA) has an ownership of 50.5% of HDM. On all matters on which members may vote every member is entitled to cast the percentage of votes equal to their percentage of ownership interest. Profits and losses on all items of income, gain or loss, deductions or other allocations of the Company will be allocated among the members in the same proportions as their membership interests in the Company bear to all the Class A and Class B membership interests of the Company in the aggregate outstanding. All of the depreciation and amortization of the assets of the Company will be allocated solely to the Class A members, unless and until their interests have been redeemed by the Company in full pursuant to the provisions of the operating agreement. The Company contributed $20,200,000 to HDM and the group of outside investors contributed $19,800,000 for its non-controlling membership interest.
On March 5, 2013 HDM purchased from Health Diagnostics, LLC (“HD”) and certain of its subsidiaries, a business managing twelve (12) Stand-Up MRI Centers and two (2) other scanning centers located in the States of New York and Florida for a total purchase price (including consideration of $1.5 million to outside investors) aggregating $35.9 million. Concurrently with the acquisition, HDM entered into several consulting and non-competition agreements for a consideration of $4.1 million. The acquisition was accounted for using the purchase method in accordance with ASC 805, “Business Combinations”. The Company recognized and measured goodwill as of the acquisition date, as the excess of the fair value of the consideration paid over the fair value of the identified net assets acquired.
On January 8, 2015, the Company purchased 20% of the Class A members ownership interest at a cost of $4,971,094. The Company has a 60.4% ownership interest in HDM after this transaction.
Amount of each class of HDM members’ equity as of June 30, 2021 and 2020
| Class A And B Members' Equity (HDM Acquisition) | ||||||||||||||||
| June 30, 2021 | June 30, 2020 | |||||||||||||||
| Class A Members | Class B Member | Class A Members | Class B Member | |||||||||||||
| Opening Members’ Equity | $ | 55,253 | $ | 39,850,419 | $ | 2,155,725 | $ | 36,543,786 | ||||||||
| Share of Net Income | 3,466,223 | 17,402,961 | 3,464,528 | 16,291,633 | ||||||||||||
| Distributions | (6,570,000 | ) | (15,330,000 | ) | (5,565,000 | ) | (12,985,000 | ) | ||||||||
| Ending Members’ Equity | $ | (3,048,524 | ) | $ | 41,923,380 | $ | 55,253 | $ | 39,850,419 | |||||||
| Page 101 |
FONAR CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021 and 2020
NOTE 11 - LONG-TERM DEBT, NOTES PAYABLE AND CAPITAL LEASES
Long-term debt, notes payable and capital leases consist of the following:
| Long-term debt, notes payable and capital leases | ||||||||
| 2021 | 2020 | |||||||
| Note payable requiring monthly payments of interest at a rate of 7% until May 2009 followed by 240 monthly payments of $4,472 through October 2026. The loan is collateralized by a building with a net book value of $413,330 as of June 30, 2021. | $ | 232,696 | $ | 273,031 | ||||
| Note payable received under the Paycheck Protection Program (‘PPP’) which was established as part of the Coronavirus Aid, Relief and Economic Security Act (“Cares Act’) that provides for loans to qualifying businesses for amounts up to 2.5 times of the average monthly payroll expenses. The loans and accrued interest are forgivable after 24 weeks as long as the proceeds are used for eligible purposes, including payroll, benefits, rent and utilities and maintains certain payroll levels. The unforgiven portion of the PPP loan is payable over 5 five years at an interest rate of 1%, with a deferral of payments for the first six months. The proceeds from the note payable were received on June 30, 2020. This note was forgiven in August 2021. | 700,764 | 700,764 | ||||||
| The revolving credit note was extended to August 2021. The Company can borrow up to $10,000,000 and prepay the loan in whole or part in multiples of $100,000 at any time without penalty. The note bears interest at a rate of 4.0% per annum and is payable monthly. The loan is collateralized by substantially all of the Company’s assets. The loan also contains certain financial covenants that must be met on a periodic basis. The Company still has the ability to draw down on the line. See Note 21. | — | — | ||||||
| 933,460 | 973,795 | |||||||
| Less: Current portion | 173,206 | 108,379 | ||||||
| $ | 760,254 | $ | 865,416 | |||||
| Page 102 |
FONAR CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021 and 2020
NOTE 11 - LONG-TERM DEBT, NOTES PAYABLE AND CAPITAL LEASES (CONTINUED)
The maturities of debt over the next five years and thereafter are as follows:
| Maturities Of Long-Term Debt | |||||
| Years Ending June 30, | |||||
| 2022 | $ | 173,206 | |||
| 2023 | 180,972 | ||||
| 2024 | 183,919 | ||||
| 2025 | 187,155 | ||||
| 2026 | 190,601 | ||||
| Thereafter | 17,607 | ||||
| Long-Term Debt Over Five Years and Thereafter | $ | 933,460 |
NOTE 12 - INCOME TAXES
ASC topic 740 prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a corporate tax return. For those benefits to be recognized, a tax position must be more-likely-than-not to be sustained upon examination by taxing authorities. Differences between tax positions taken or expected to be taken in a tax return and the benefit recognized and measured pursuant to the interpretation are referred to as unrecognized benefits. A liability is recognized (or amount of net operating loss carryforward or amount of tax refundable is reduced) for an unrecognized tax benefit because it represents an enterprise’s potential future obligation to the taxing authority for a tax position that was not recognized as a result of applying the provisions of ASC topic 740. The Company believes there are no uncertain tax positions in prior years tax filings and therefore it has not recorded a liability for unrecognized tax benefits.
In accordance with ASC topic 740, interest costs related to unrecognized tax benefits are required to be calculated (if applicable) and would be classified as “Interest expense, net. Penalties if incurred would be recognized as a component of “Selling, general and administrative” expenses.
The Company files corporate income tax returns in the United States (federal) and in various state and local jurisdictions. In most instances, the Company is no longer subject to federal, state and local income tax examinations by tax authorities for years prior to 2016 for federal and 2015 for state.
The Company has recorded a deferred tax asset of $15,958,961 and a deferred tax liability of $238,316 as of June 30, 2021, primarily relating to its net Federal operating loss carryforwards of approximately $35,574,000 available to offset future taxable income through 2031. In addition the Company has state operating loss carryforwards of approximately $7,094,000 and city operating loss carryforwards of approximately $2,470,000. The net operating losses begin to expire in 2025 for federal tax and state income tax purposes.
| Page 103 |
FONAR CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021 and 2020
NOTE 12 - INCOME TAXES (CONTINUED)
Future ownership changes as determined under Section 382 of the Internal Revenue code could further limit the utilization of net operating loss carryforwards. As of June 30, 2021, no such changes in ownership have occurred.
The ultimate realization of deferred tax assets is dependent on the generation of future taxable income during the periods in which temporary differences become deductible or when such net operating losses can be utilized. The Company considers projected future taxable income, the regulatory environment of the industry, and tax planning strategies in making this assessment. At present, the Company believes that it is more likely than not that the benefits from certain deferred tax asset carryforwards, will not all be fully realized. In recognition of this inherent risk, a valuation allowance was established for the partial value of the deferred tax asset, which principally related to research and development tax credits.
A valuation allowance will be maintained until sufficient positive evidence exists to support the reversal of the remainder of the valuation.
The valuation allowance for deferred tax assets decreased during the year ended June 30, 2021, by approximately $3,547,000. The valuation allowance decreased by approximately $195,000 during the year ended June 30, 2020.
On March 27, 2020 Congress enacted the CARES Act (Coronavirus Aid, Relief and Economic Security Act). The Act provides numerous tax provisions and other stimulus measures, including temporary changes regarding prior and future operation losses, temporary changes to prior and future limitations on interest deductions, temporary suspension of certain payment requirements for the employer portion of Social Security taxes, technical corrections to prior tax legislation for tax depreciation of certain qualified improvement property and enhanced recoverability of AMT tax credits.
At the present time, the only impact of the CARES Act to the Company is allowing a full reimbursement of $1,342,370 of tax credits relating to the alternative minimum tax credits. The Company received the first half payment in June 2020. The balance of alternative minimum tax credits of $671,185 was received in July 2020. Previously, these credits were to be refunded over a 3 year period.
As we continue to monitor tax implications of the CARES Act and other state and federal stimulus tax legislation, we may make adjustments to our estimates and record additional amounts for tax assets and liabilities.
| Page 104 |
FONAR CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021 and 2020
NOTE 12 - INCOME TAXES (CONTINUED)
Components of the provision (benefit) for income taxes are as follows:
| Components Of The Benefit Provision For Income Taxes | ||||||||
| Years Ended June 30 | ||||||||
| Current: | 2021 | 2020 | ||||||
| Federal | $ | $ | (187,255 | ) | ||||
| State | 1,136,514 | 513,204 | ||||||
| Subtotal | 1,136,514 | 325,949 | ||||||
| Deferred: | ||||||||
| Federal deferred taxes | 2,718,046 | 1,953,349 | ||||||
| State deferred taxes | 136,960 | 165,480 | ||||||
| Subtotal | 2,855,006 | 2,118,829 | ||||||
| Provision (Benefit) for Income Taxes - Net | $ | 3,991,520 | $ | 2,444,778 | ||||
A reconciliation of the federal statutory income tax rate to the Company’s effective tax rate as reported is as follows:
| Reconciliation Of Federal Statutory Income Tax Rate To Company's Effective Tax Rate | ||||||||
| Years Ended June 30, | ||||||||
| 2021 | 2020 | |||||||
| Taxes at federal statutory rate | 21.0 | % | 21.0 | % | ||||
| State and local income taxes (benefit), net of federal benefit | 3.3 | % | 4.0 | % | ||||
| Non Controlling interest | (4.9 | )% | (6.1 | )% | ||||
| Expiration of tax credits | 4.6 | % | 0.2 | % | ||||
| Return to provision adjustments | 6.1 | % | — | % | ||||
| Change in the valuation allowance | (20.0 | )% | 0.3 | % | ||||
| Other | 12.5 | % | (2.1 | )% | ||||
| Effective income tax rate | 22.6 | % | 17.3 | % | ||||
As of June 30, 2021, the Company has net operating loss (“NOL”) carryforwards of approximately $35,574,000 that will be available to offset future taxable income. The utilization of certain of the NOLs is limited by separate return limitation year rules pursuant to Section 1502 of the Internal Revenue Code.
The Company has, for federal income tax purposes, research and development tax credits and investments tax credits carryforwards aggregating $3,733,000. However, the realization of these credits may be limited as a result of expiring prior to their utilization. These credits can only be applied after all net operating losses have been used, which expire through 2031. As such, the Company has established a valuation reserve for anticipated unused credits of $890,000.
In addition, for New York State income tax purposes, the Company has tax credit carryforwards aggregating approximately $11,000 which, are accounted for under the flow-through method.
| Page 105 |
FONAR CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021 and 2020
NOTE 12 - INCOME TAXES (CONTINUED)
The Company was also under audit with New York State for income tax and was assessed additional taxes of $423,272 plus interest and penalties. These amounts are accrued for at June 30, 2021 and included in other current liabilities and were paid in August 2021.
Significant components of the Company’s deferred tax assets and liabilities at June 30, 2021 and 2020 are as follows:
| Components Of Company's Deferred Tax Assets And Liabilities | ||||||||
| June 30, | ||||||||
| 2021 | 2020 | |||||||
| Deferred tax assets: | ||||||||
| Allowance for doubtful accounts | $ | 3,827,382 | $ | 3,946,801 | ||||
| Non-deductible accruals | 749,902 | 693,833 | ||||||
| Net operating carryforwards | 8,285,163 | 13,720,637 | ||||||
| Tax credits | 3,732,650 | 4,647,217 | ||||||
| Inventory | 66,316 | 69,940 | ||||||
| Property and equipment and depreciation | 187,632 | 168,371 | ||||||
| Deferred Tax Assets - gross | 16,849,045 | 23,246,799 | ||||||
| Valuation allowance | (890,084 | ) | (4,437,042 | ) | ||||
| Total deferred tax assets | 15,958,961 | 18,809,757 | ||||||
| Intangibles | (238,316 | ) | (234,106 | ) | ||||
| Total deferred tax liabilities | (238,316 | ) | (234,106 | ) | ||||
| Net deferred tax asset | $ | 15,720,645 | $ | 18,575,651 | ||||
NOTE 13 - OTHER CURRENT LIABILITIES
Included in other current liabilities are the following:
| Other Current Liabilities | ||||||||
| June 30, | ||||||||
| 2021 | 2020 | |||||||
| Accrued salaries, commissions and payroll taxes | $ | 5,406,982 | $ | 4,491,941 | ||||
| Litigation accruals | 900,000 | 442,802 | ||||||
| Sales tax payable | 644,623 | 1,353,200 | ||||||
| State income taxes payable | 774,234 | — | ||||||
| Legal and other professional fees | 37,827 | 112,867 | ||||||
| Accounting fees | 127,262 | 120,000 | ||||||
| Self-funded health insurance reserve | 62,548 | 86,504 | ||||||
| Accrued interest and penalty | 493,042 | 877,787 | ||||||
| Other | 715,600 | 699,997 | ||||||
| Other current liabilities | $ | 9,162,118 | $ | 8,185,098 | ||||
| Page 106 |
FONAR CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021 and 2020
NOTE 14 - COMMITMENTS AND CONTINGENCIES
Leases
The Company rents its operating facilities and certain equipment, pursuant to operating lease agreements expiring at various dates through March 2030. The leases for certain facilities contain escalation clauses relating to increases in real property taxes as well as certain maintenance costs.
Rent expense for operating leases approximated $6,146,000 and $5,136,000, for the years ended June 30, 2021 and 2020, respectively.
The Company received approval from the Suffolk County IDA on February 29, 2016 of a 50% property tax abatement, valued at $440,000, over a 10 year period commencing January 2017.
Employee Benefit Plans
The Company has a non-contributory 401(k) Plan (the “401(k) Plan”). The 401(k) Plan covers all non-union employees who are at least 21 years of age with no minimum service requirements. There were $36,799 and $0 employer contributions to the Plan for the years ended June 30, 2021 and 2020.
The stockholders of the Company approved the 2000 Employee Stock Purchase Plan (“ESPP”) at the Company’s annual stockholders’ meeting in April 2000. The ESPP provides for eligible employees to acquire common stock of the Company at a discount, not to exceed 15%. This plan has not been put into effect as of June 30, 2021.
Litigation
In September 2019, The Company was notified by one of its landlords that it was required to vacate the premises within 180 days under the demolition clause in the lease. The Company believes the lease renewal which was not negotiated in good faith since the renewal was negotiated in February 2018. The Company is in the process of relocating to a new location but the original lease provided for penalty payments in the event that the Company had not vacated the leased space. The Company has been making normal rent payments throughout the course of the arbitration proceedings. The Company is estimating the leasehold holdover charges to be approximately $900,000. As of June 30, 2021 this amount has been accrued for under other current liabilities.
In September 2020, the Company entered into a settlement agreement with an unrelated third party for a claim made during March 2018 which was scheduled for arbitration. The settlement was for $1.2 million of which $900,000 was paid by the Company’s insurance on September 15, 2020 with the remaining $315,000 paid by the Company on September 28, 2020.
| Page 107 |
FONAR CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021 and 2020
NOTE 14 - COMMITMENTS AND CONTINGENCIES (Continued)
Other Matters
The Company is subject to other legal proceedings and claims arising from the ordinary course of its business, including personal injury, customer contract and employment claims besides the claim above. In the opinion of management, and with consultation with legal council, the aggregate liability, if any, with respect to such actions, will not have a material adverse effect on the consolidated financial position or results of operations of the Company.
The Company has satisfied most of its delinquencies in filing sales tax returns for certain states, for which the Company has transacted business. The Company has recorded tax obligations of approximately $645,000 plus interest and penalties of approximately $232,000 until the remaining states have been resolved.
The Company maintains a self-funded health insurance program with a stop-loss umbrella policy with a third party insurer to limit the maximum potential liability for individual claims to $110,000 per person and for a maximum potential claim liability based on member enrollment. With respect to this program, the Company considers historical and projected medical utilization data when estimating its health insurance program liability and related expense. As of June 30, 2021 and 2020, the Company had approximately $63,000 and $87,000, respectively, in reserve for its self-funded health insurance programs. The reserves are included in “Other current liabilities” in the consolidated balance sheets.
The Company regularly analyzes its reserves for incurred but not reported claims, and for reported but not paid claims related to its reinsurance and self-funded insurance programs. The Company believes its reserves are adequate. However, significant judgment is involved in assessing these reserves such as assessing historical paid claims, average lags between the claims’ incurred date, reported dates and paid dates, and the frequency and severity of claims. There may be differences between actual settlement amounts and recorded reserves and any resulting adjustments are included in expense once a probable amount is known. There were no significant adjustments recorded in the years covered by this report.
NOTE 15 - SUPPLEMENTAL CASH FLOW INFORMATION
During the years ended June 30, 2021 and 2020 the Company paid $75,178 and $137,200 for interest, respectively.
During the years ended June 30, 2021 and 2020 the Company paid $261,032 and $228,204 for income taxes, respectively.
During the years ended June 30, 2021 and 2020, the Company issued 102,364 and 89,981 shares of common stock for costs and expenses totaling $1,940,821 and $1,990,380, respectively.
During the year ended June 30, 2020 the Company entered into a capital lease for the purchase of equipment in the amount of $1,350,000.
| Page 108 |
FONAR CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021 and 2020
NOTE 15 - SUPPLEMENTAL CASH FLOW INFORMATION (Continued)
During the year, the Company resolved certain sales tax liabilities and was able to reverse accrued interest and penalties in the amount of $602,000 which has been recorded under selling, general and administrative expenses.
NOTE 16 – RELATED PARTY TRANSACTIONS
The CEO and President of the Company is a minority owner of a billing company, which performs billing and collection services with respect to No-Fault and Workers’ Compensation claims of the Company’s clients. The monthly fee charged to the Company was $85,000. The Company terminated this agreement on January 1, 2021. On June 1, 2017, the Company also entered into a one year renewable agreement to provide IT services to the billing company for a monthly fee of $23,884. The agreement was renewed on June 1, 2021 for another year.
Bensonhurst MRI Limited Partnership, in which the CEO and President of the Company holds an interest, is party to an agreement with the Company for the service and maintenance of its Upright MRI Scanner for a price of $110,000 per annum.
NOTE 17 - SEGMENT AND RELATED INFORMATION
The Company provides segment data in accordance with the provisions of ASC topic 280, “Disclosures about Segments of an Enterprise and Related Information”.
The Company operates in two industry segments - manufacturing and the servicing of medical equipment and management of diagnostic imaging centers.
The accounting policies of the segments are the same as those described in the summary of significant accounting policies. All intersegment sales are market-based. The Company evaluates performance based on income or loss from operations.
| Page 109 |
FONAR CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021 and 2020
NOTE 17 - SEGMENT AND RELATED INFORMATION (Continued)
Summarized financial information concerning the Company’s reportable segments is shown in the following table:
| Summarized Segment Financial Information | ||||||||||||
| Manufacturing and Servicing of Medical | Management of Diagnostic Imaging | |||||||||||
| Fiscal 2021: | Equipment | Center | Totals | |||||||||
| Net revenues from external customers | $ | 9,037,091 | $ | 80,892,674 | $ | 89,929,765 | ||||||
| Intersegment net revenues * | $ | 901,250 | $ | — | $ | 901,250 | ||||||
| (Loss) Income from operations | $ | (3,410,189 | ) | $ | 20,507,804 | $ | 17,097,615 | |||||
| Depreciation and amortization | $ | 264,830 | $ | 3,816,857 | $ | 4,081,687 | ||||||
| Compensatory element of stock issuances | $ | 83,277 | $ | — | $ | 83,277 | ||||||
| Total identifiable assets | $ | 24,592,582 | $ | 164,913,613 | $ | 189,506,195 | ||||||
| Capital expenditures | $ | 291,294 | $ | 3,405,502 | $ | 3,696,796 | ||||||
| Fiscal 2020: | ||||||||||||
| Net revenues from external customers | $ | 8,463,103 | $ | 77,227,359 | $ | 85,690,462 | ||||||
| Intersegment net revenues * | $ | 875,000 | $ | — | $ | 875,000 | ||||||
| (Loss) Income from operations | $ | (6,425,411 | ) | $ | 20,076,327 | $ | 13,650,916 | |||||
| Depreciation and amortization | $ | 368,498 | $ | 3,540,150 | $ | 3,908,648 | ||||||
| Compensatory element of stock issuances | $ | — | $ | — | $ | — | ||||||
| Total identifiable assets | $ | 30,492,757 | $ | 149,790,753 | $ | 180,283,510 | ||||||
| Capital expenditures | $ | 2,440,640 | $ | 4,981,773 | $ | 7,422,413 | ||||||
| * | Amounts eliminated in consolidation |
Export Product Sales
The Company’s areas of operations are principally in the United States. The Company had export sales of medical equipment amounting to 69.3% and 20.2% of product sales revenues to third parties for the years ended June 30, 2021 and 2020, respectively.
The foreign product sales, as a percentage of product sales to unrelated parties, were made to customers in the following countries:
| Export Product Sales | ||||||||
| For the Years Ended June 30 | ||||||||
| 2021 | 2020 | |||||||
| Dominican Republic | 67.0 | % | — | % | ||||
| Canada | 0.1 | — | ||||||
| Germany | 2.1 | 20.2 | ||||||
| Puerto Rico | 0.1 | — | ||||||
| 69.3 | % | 20.2 | % | |||||
| Page 110 |
FONAR CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021 and 2020
NOTE 17 - SEGMENT AND RELATED INFORMATION (Continued)
Foreign Service and Repair Fees
The Company’s areas of service and repair are principally in the United States. The Company had foreign revenues of service and repair of medical equipment amounting to 4.5% and 5.6% of consolidated net service and repair fees for the years ended June 30, 2021 and 2020 respectively. Foreign service and repair fees, as a percentage of total service and repair fees, were provided principally to the following countries:
| Foreign Service and Repair Fees | ||||||||
| For the Years Ended June 30, | ||||||||
| 2021 | 2020 | |||||||
| Puerto Rico | 1.5 | % | 1.6 | % | ||||
| Switzerland | 0.3 | 0.3 | ||||||
| Germany | 1.5 | 1.5 | ||||||
| England | 0.6 | 0.7 | ||||||
| Canada | 0.3 | 0.3 | ||||||
| Greece | 0.3 | 0.2 | ||||||
| Australia | — | 1.0 | ||||||
| 4.5 | % | 5.6 | % | |||||
The Company does not have any material assets outside of the United States.
NOTE 18 – ACQUISTION
On March 29, 2021, the Company completed the acquisition of certain assets of Rockland Management Group, located in West Yonkers. The Company used an incremental borrowing rate of 4% to value the right to use asset in connection with the assumed operating lease obligation. We made a preliminary fair value determination of the acquired assets and assumed liabilities as follows:
| Fair value assets and assumed liabilities | ||||
| Property and equipment | $ | 650,000 | ||
| Right to use assets | 434,219 | |||
| Intangible assets | 150,000 | |||
| Security Deposit | 38,628 | |||
| Right to use liability | (434,219 | ) | ||
| Goodwill | 283,880 | |||
| Total purchase consideration | $ | 1,122,508 |
| Page 111 |
FONAR CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021 and 2020
NOTE 18 – ACQUISTION (Continued)
In accordance with ASC 805-10-25-1, Business Combinations – Overall Recognition, the Company recorded the transaction as a business combination. ASC 805-10-25-1 provides the requirements of recording the transaction by applying the acquisition method. The acquisition method requires the Company to determine if the assets and liabilities acquired are a business or not. Under ASC 805-10-25-1, it must be determined if there is a specific acquisition party, acquisition date, identifiable assets acquired and liabilities assumed and you must be able to recognized and measure goodwill or a gain from the purchase. Based upon this guidance, the acquisition had been recorded as a business combination.
The net assets acquired and consideration is as follow:
| Net assets acquired | ||||
| Leasehold Improvements | $ | 550,000 | ||
| Diagnostic Equipment | 100,000 | |||
| Customer Lists | 100,000 | |||
| Covenant Not to Compete | 50,000 | |||
| Security Deposit | 38,628 | |||
| Closing costs - expensed | 3,478 | |||
| Goodwill | 283,880 | |||
| Cash Consideration Paid | $ | 1,125,986 |
The results of operations of Rockland Management Group were diminutive and did not affect the pro forma results of operations.
NOTE 19 – ALLOWANCE FOR DOUBTFUL ACCOUNTS
The following represents a summary of allowance for doubtful accounts for the years ended June 30, 2021 and 2020 respectively:
| Summary of Allowance For Doubtful Accounts | ||||||||||||||||
| Description | Balance
June 30, 2020 | Additions (1) | Deductions | Balance
June 30, 2021 | ||||||||||||
| Accounts receivable | $ | 514,561 | $ | — | $ | 72,291 | $ | 442,270 | ||||||||
| Management and other fees receivable | 11,063,233 | 4,723,645 | — | 15,786,878 | ||||||||||||
| Management and other fees receivable - related medical practices | 3,322,055 | 862,344 | — | 4,184,399 | ||||||||||||
| Notes receivable | 777,354 | — | — | 777,354 | ||||||||||||
| Page 112 |
FONAR CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021 and 2020
NOTE 19 – ALLOWANCE FOR DOUBTFUL ACCOUNTS (Continued)
| Balance | Balance | |||||||||||||||
| Description | June 30, 2019 | Additions | Deductions | June 30, 2020 | ||||||||||||
| Accounts receivable | $ | 190,244 | $ | 380,000 | $ | 55,683 | $ | 514,561 | ||||||||
| Management and other fees receivable | 9,404,944 | 3,526,742 | (1,868,453 | ) | 11,063,233 | |||||||||||
| Management and other fees receivable - related medical practices | 2,310,731 | 1,011,324 | — | 3,322,055 | ||||||||||||
| Notes receivable | — | 777,354 | — | 777,354 | ||||||||||||
| (1) | Included in provision for bad debts. |
NOTE 20 - QUARTERLY FINANCIAL DATA (UNAUDITED)
(000’s omitted, except per share data)
| Quarterly Financial Data | ||||||||||||||||||||
| September 30, 2020 | December 30, 2020 | March 31, 2021 | June 30, 2021 | Total | ||||||||||||||||
| Total Revenues – Net | $ | 20,979 | $ | 21,164 | $ | 23,090 | $ | 24,697 | $ | 89,930 | ||||||||||
| Total Costs and Expenses | 16,829 | 16,182 | 18,968 | 20,853 | 72,832 | |||||||||||||||
| Net Income | 3,251 | 3,928 | 4,299 | 2,196 | 13,674 | |||||||||||||||
| Basic Net Income Per Common Share Available to Common Stockholders | $ | 0.37 | $ | 0.45 | $ | 0.55 | $ | 0.10 | $ | 1.47 | ||||||||||
| Diluted Net Income Per Common Share Available to Common Stockholders | $ | 0.36 | $ | 0.44 | $ | 0.54 | $ | 0.11 | $ | 1.45 | ||||||||||
| September 30, 2019 | December 30, 2019 | March 31, 2020 | June 30, 2020 | Total | ||||||||||||||||
| Total Revenues – Net | $ | 21,747 | $ | 21,451 | $ | 21,686 | $ | 20,806 | $ | 85,690 | ||||||||||
| Total Costs and Expenses | 16,261 | 16,430 | 19,071 | 20,278 | 72,040 | |||||||||||||||
| Net Income | 4,506 | 4,209 | 1,914 | 1,076 | 11,705 | |||||||||||||||
| Basic Net Income Per Common Share Available to Common Stockholders | $ | 0.48 | $ | 0.45 | $ | 0.18 | $ | 0.09 | $ | 1.20 | ||||||||||
| Diluted Net Income Per Common Share Available to Common Stockholders | $ | 0.47 | $ | 0.44 | $ | 0.18 | $ | 0.09 | $ | 1.18 | ||||||||||
| Page 113 |
FONAR CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2021 and 2020
NOTE 21 – SUBSEQUENT EVENTS
The Company evaluates events that have occurred after the balance sheet date, but before the consolidated financial statements are issued.
During August 2021 the Company amended their revolving credit agreement. The agreement was extended to August 2022. The interest rate on borrowings remains at 4% along with certain financial covenants.
The loan of $700,764 received under the Paycheck Protection Program was forgiven in August 2021.
| Page 114 |
FONAR CORPORATION AND SUBSIDIARIES
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE.
There have been no disagreements with our independent registered public accounting firm or other matters requiring disclosure under Regulation S-K, Item 304(b).
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
As of the end of the period covered by this Annual Report on Form 10-K, we performed an evaluation under the supervision of and with the participation of management, including our Principal Executive Officer and our Acting Principal Financial Officer, of the design and effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) or 15d-15(e) under the Securities Exchange Act of 1934 as amended (the “Exchange Act”). Based upon that evaluation, our Principal Executive Officer and Acting Principal Financial Officer concluded, as of the end of the period covered by this Annual Report that our disclosure controls and procedures were effective.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as is defined in the Exchange Act. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of financial statements for external reporting purposes in accordance with GAAP.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
| Page 115 |
FONAR CORPORATION AND SUBSIDIARIES
Our management conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO-2013). Based on this evaluation, our management concluded that our internal control over financial reporting was effective at June 30, 2021.
Based on the COSO criteria, management concluded that our internal controls were effective to prevent material misstatements of the Company’s annual or interim financial statements.
Changes in Internal Controls over Financial Reporting
There have been no changes in our internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act) during the most recent fiscal quarter and year ended June 30, 2021 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. OTHER INFORMATION
None.
PART III
ITEM 10. DIRECTORS AND EXECUTIVE OFFICERS.
Directors serve from the date of their election until the next annual meeting of stockholders and until their successors are elected and qualify. With the exception of Dr. Raymond V. Damadian, who does not receive any fees for serving as a director, each director receives $20,000 per annum for his or her service as a director. Officers serve at the discretion of the Board of Directors.
A majority of our board of directors is composed of independent directors: Charles N. O’Data, Ronald G. Lehman and Richard E. Turk. The outside directors also serve as the members of the audit committee, which is a standing committee of the board of directors having a charter describing its responsibilities. Mr. O’Data has been designated as the audit committee financial expert.. Mr. Lehman would also qualify as an audit committee financial expert, and has acted in Mr. O’Data’s place when Mr. O’Data is unavailable to do so. His relevant experience is described in his biographical information.
| Page 116 |
FONAR CORPORATION AND SUBSIDIARIES
We have adopted a code of ethics applicable to, among other personnel, our principal executive officer, principal financial officer, controllers and persons performing similar functions. The code is designed to deter wrongdoing and to promote: 1. honest and ethical conduct, including the ethical handling of actual or apparent conflicts of interest between personal and professional relationships; 2. full, fair, accurate, timely and understandable disclosure in reports and documents that we file or submit to the Securities and Exchange Commission and in other public communications we make; 3. compliance with applicable governmental laws, rules and regulations; 4. the prompt internal reporting of violations of the code to an appropriate person or persons identified in the code and 5. accountability for adherence to the code. We will provide a copy of the code to any person who requests a copy. A person may request a copy by writing to Fonar Corporation, 110 Marcus Drive, Melville, New York 11747, to the attention of the Legal Department or Investor Relations.
The officers and directors of the Company are set forth below:
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Raymond V. Damadian | 85 | Chairman of the Board of Directors, Director, Principal Financial Officer, Treasurer |
| Timothy R. Damadian | 57 | President, Chief Executive Officer |
| Luciano B. Bonanni | 66 | Executive Vice President and Chief Operating Officer |
| Claudette J.V. Chan | 83 | Director |
| Charles N. O’Data | 85 | Director |
| Ronald J. Lehman | 45 | Director |
| Richard E. Turk | 37 | Director |
Raymond V. Damadian, M.D. has been the Chairman of the Board since its inception in 1978 and Treasurer since February, 2001. Up until February 11, 2016, Dr. Damadian also served as the President and Chief Executive Officer of Fonar. Dr. Damadian was employed by the State University of New York, Downstate Medical Center, New York, as an Associate Professor of Biophysics and Associate Professor of Internal Medicine from 1967 until September 1979. He received an M.D. degree in 1960 from Albert Einstein College of Medicine, New York, and a B.S. degree in mathematics from the University of Wisconsin in 1956. In addition, Dr. Damadian conducted post-graduate work at Harvard University, where he studied extensively in the fields of physics, mathematics and electronics. Dr. Damadian is the author of numerous articles and books on the nuclear magnetic resonance effect in human tissue, which is the theoretical basis for the Fonar MRI scanners. He is a 1988 recipient of the National Medal of Technology. In 1989 he was inducted into the National Inventors Hall of Fame, for his contributions in conceiving and developing the application of magnetic resonance technology to medical applications including whole body scanning and diagnostic imaging. Dr. Damadian is the President, Treasurer and director of Health Management Corporation of America (“HMCA”), and a Manager of Health Diagnostics Management, LLC (“HDM”) which three entities are subsidiaries of Fonar. Raymmond Damadian is the father of Timothy Damadian and the brother of Claudette J.V. Chan.
| Page 117 |
FONAR CORPORATION AND SUBSIDIARIES
Timothy Damadian has been the President and Chief Executive Officer of Fonar since February 11, 2016. From 2010 to 2016 he served as an independent consultant, with a focus on the Company’s MRI facility management business. Timothy Damadian began his career at Fonar in 1985, installing MRI scanners and components for Fonar customers. Over the course of the following 16 years, he held positions of increasing authority, eventually becoming Vice President of Operations. In 1997, Timothy Damadian was appointed President of the newly formed Health Management Corporation of America (HMCA), a wholly-owned subsidiary of Fonar that was formed to manage medical and diagnostic imaging offices. In 2001, Timothy Damadian left Fonar to form Integrity Healthcare Management, Inc., a diagnostic imaging management company that would eventually manage 11 MRI scanning centers in New York and Florida. The company was a success and was sold to Health Diagnostics, LLC in 2007. Mr. Damadian returned to Fonar as a consultant in 2010. He also serves as a Manager of Health Diagnostics Management, LLC, which are subsidiaries of HMCA. Timothy Damadian is the son Dr. Damadian and nephew of Claudette J.V. Chan.
Luciano B. Bonanni has served as Chief Operating Officer (COO) and Executive Vice President (EVP) for Fonar Corporation since June 27, 2016. Prior to his appointment as COO, Mr. Bonanni had served the Company as Vice President since 1989, during which time he oversaw general operations, research and development, manufacturing, service, sales, finance, accounting and regulatory compliance. Prior to 1989, Mr. Bonanni held the title of Vice President of Production and Engineering from the time of Fonar’s initial public offering in 1981. Mr. Bonanni joined the Company as an electrical engineer in 1978. He holds a Bachelor of Electrical Engineering degree from Manhattan College.
Claudette J.V. Chan has been a Director of Fonar since October 1987 and Secretary of Fonar since January 2008. Mrs. Chan was employed from 1992 through 1997 by Raymond V. Damadian, M.D. MR Scanning Centers Management Company and since 1997 by HMCA, as “site inspector,” in which capacity she is responsible for supervising and implementing standard procedures and policies for MRI scanning centers. From 1989 to 1994 Mrs. Chan was employed by St. Matthew’s and St. Timothy’s Neighborhood Center, Inc., as the director of volunteers in the “Meals on Wheels” program, a program which cares for the elderly. From approximately 1983 to 1989, Mrs. Chan was President of the Claudette Penot Collection, a retail mail-order business specializing in women’s apparel and gifts. Mrs. Chan practiced and taught in the field of nursing until 1973, when her son was born. She received a bachelor of science degree in nursing from Cornell University in 1960. Mrs. Chan is the sister of Raymond V. Damadian and aunt of Timothy Damadian.
| Page 118 |
FONAR CORPORATION AND SUBSIDIARIES
Charles N. O’Data has been a Director of Fonar since February 1998. From 1961 to 1997, Mr. O’Data was the Vice President for Development for Geneva College, a liberal arts college located in western Pennsylvania. In that capacity, he acted as the College’s chief investment officer. His responsibilities included management of the College’s endowment fund and fund raising. In July 1997, Mr. O’Data retired from Geneva College after 36 years of service to assume a position of National Sales Executive for SC Johnson Company’s Professional Markets Group, a unit of SC Johnson Wax, and specialized in healthcare and education sales, a position he held until the spring of 1999. In his capacity with SC Johnson he was responsible for sales to the nation’s three largest Group Purchasing Organizations which included some 4,000 hospitals. Mr. O’Data presently acts as an independent financial consultant to various entities. Mr. O’Data served on the board of The Medical Center, Beaver, Pennsylvania, now a part of Heritage Valley Health System, a 500 bed acute care facility, for 26 years, three as its Chair. Mr. O’Data also served on the board of Amerinet, a shared-services and group purchasing organization covering seven states. He founded The Beaver County Foundation, a Community Foundation, in 1992, and serves as its President. Mr. O’Data is listed as a finance associate in the Middle States Association, Commission on Higher Education. The commission is the formal accrediting body for higher education in the eastern region of the country. In this capacity he evaluates the financial aspects of educational organizations. Mr. O’Data is a graduate of Geneva College, where he received a B.S. degree in Economics in 1958.
Ronald G. Lehman has been a Director of Fonar since April, 2012, when he was unanimously appointed by the remaining four Directors to fill the vacancy resulting from the death of former Director Robert Djerejian. From October, 2009 to the present, Mr. Lehman has served as Managing Director of Investment Banking with Bruderman Brothers, LLC, a private New York-based broker-dealer registered with the Securities and Exchange Commission and which is a member of the Financial Industry Regulatory Authority (FINRA) and the Securities Investor Protection Corporation (SIPC). Mr. Lehman directly manages all facets of the firm’s transaction processes, from deal origination, to sourcing capital, to negotiating deal structures, through documentation and closing. The firm provides buy and sell-side advisory, capital raising, and consulting services to lower middle-market companies. Mr. Lehman specializes in advising healthcare services companies and has recently completed several recapitalizations in the industry. He also participates in the firm’s merchant banking investments and oversees many of these assignments. From May, 2008 to October, 2009, Mr. Lehman served as Senior Vice President of Acquisitions at Health Diagnostics, LLC, where he managed the company’s acquisition and corporate finance activities. From March, 2000 to May, 2008, Mr. Lehman worked for various Bruderman entities as a buy and sell-side advisor and as a principal in several private equity transactions. From September, 1998 to March, 2000, Mr. Lehman worked at Deutsche Bank Securities, Inc. and last held the position of Associate in their Global Custody Group. Mr. Lehman graduated from Columbia University with a B.A. in 1998.
| Page 119 |
FONAR CORPORATION AND SUBSIDIARIES
Richard E. Turk has been a Director of Fonar since June, 2020, when he was appointed to fill the vacancies on the Board of Directors and Audit Committee of the Board of Directors resulting from the death of his predecessor, Robert J. Janoff. Mr. Turk is the Chief Development Officer of PRISM Vision Group, a private equity-backed, multi-location, outpatient comprehensive eye care practice headquartered in Union, New Jersey. Since joining PRISM in November, 2018, Mr. Turk has helped source, analyze, and complete 12 acquisitions. He spearheaded growth efforts that helped PRISM expand from a single-speciality (retina) provider with 17 locations and 21 physicians to a comprehensive, vertically-integrated, multi-specialty, eye care organization with approximately 90 physicians and more than 50 locations across New Jersey, Pennsylvania, Delaware and Maryland. Prior to his tenure at PRISM, Mr. Turk was employed by Professional Physical Therapy, a private equity-backed outpatient physical and occupational therapy company headquartered in Uniondale, New Jersey with more than 180 locations across New York, New Jersey, Connecticut, Massachusetts and New Hampshire. During his four years at Professional Physical Therapy, Mr. Turk sourced, analyzed, and completed 32 acquisitions comprised of 116 clinics, expanding the company’s services and adding three states. From 2007 to 2014, Mr. Turk was employed by Bruderman Brothers, a broker dealer involved in investment banking, merchant banking, investment advisory, and consulting for lower middle market companies ($10M-$250M of enterprise value) in a variety of industries, including healthcare. Mr. Turk was Vice President of Bruderman Brothers from 2011 to 2014. Mr. Turk graduated from Columbia University with a B.A. in American History in 2007.
ITEM 11. EXECUTIVE COMPENSATION.
With the exception of the Chief Executive Officer and the Chairman of the Board of Directors, the compensation of the Company’s executive officers is based on a combination of salary and bonuses based on performance. The Chairman of the Board’s compensation consists of a salary. The Chief Executive Officer and the Chairman of the Board have no understandings with the Company with respect to bonuses, options or other incentives; they are not subject to our general policy later discussed.
The Board of Directors does not have a compensation Committee. Dr. Raymond V. Damadian, Chairman of the Board, controls over 50% of the voting power of our capital stock. Dr. Damadian is both an executive officer and a member of the Board of Directors. Dr. Damadian, the Chief Executive Officer and the Chief Operating Officer, participate in the determination of compensation for the Company’s management and other employees.
The Board of Directors has established an audit committee. The members of the committee are Charles N. O’Data, Ronald G. Lehman and Richard E. Turk.
Our compensation policy includes a combination of salary, commissions, bonuses, stock bonuses and stock options, designed to incentivize our employees. There is no universal plan applicable to all of our employees. The fixed and variable components of our employees’ compensation tend to be individualized, based on a combination of the employees’ performance, responsibilities and position, our assessment of how best to motivate a person in such a position and the needs and preferences of the particular employees, as negotiated between employees and their supervisors or management.
| Page 120 |
FONAR CORPORATION AND SUBSIDIARIES
There is set forth in the following Summary Compensation Table the compensation provided by us during fiscal 2021, 2020 and 2019 to our Principal Executive Officer, and our acting Principal Financial Officer. There is set forth in the following Outstanding Equity Awards Table and Director Compensation Table the required information.
I. SUMMARY COMPENSATION TABLE
| Name and All Other Principal Position | Year | Salary ($) | Cash
Bonuses ($) | Stock
Awards ($) | Total Compensation ($) | ||||||||||||||
| (a) | (b) | (c) | (d) | (e) | (f) | ||||||||||||||
| Timothy R. Damadian | 2021 | $ | 0 | $ | 155,800 | $ | 0 | $ | 155,800 | ||||||||||
| President, Principal | 2020 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | ||||||||||
| Executive Officer | 2019 | $ | 0 | $ | 155,800 | $ | 0 | $ | 155,800 | ||||||||||
| Raymond V. Damadian | 2021 | $ | 153,095 | $ | 305,800 | $ | 0 | $ | 458,895 | ||||||||||
| Chairman of the Board, | 2020 | $ | 153,095 | $ | 0 | $ | 0 | $ | 153,095 | ||||||||||
| Treasurer and | 2019 | $ | 153,095 | $ | 305,800 | $ | 0 | $ | 458,895 | ||||||||||
| Principal Financial Officer | |||||||||||||||||||
| Luciano Bonanni | 2021 | $ | 146,038 | $ | 0 | $ | 152,931 | $ | 298,969 | ||||||||||
| Chief Operating Officer and | 2020 | $ | 146,496 | $ | 0 | $ | 152,902 | $ | 299,398 | ||||||||||
| Executive Vice President | 2019 | $ | 145,672 | $ | 0 | $ | 152,740 | $ | 305,565 | ||||||||||
II. OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END
| Name | Number Of Securities Underlying Unexercised Options (#) Exercisable | Option Exercise Price ($) | Option Exercise Expiration Date | |||||||||
| (a) | (b) | (c) | ||||||||||
| Timothy R. Damadian, President and Principal Executive Officer | 0 | 0 | N/A | |||||||||
| Raymond V. Damadian, Chairman of the Board, Treasurer and Principal Financial Officer | 0 | 0 | N/A | |||||||||
| Luciano Bonanni, Chief Operating Officer and Executive Vice President | 0 | 0 | N/A | |||||||||
| Page 121 |
FONAR CORPORATION AND SUBSIDIARIES
III. DIRECTOR COMPENSATION
| Name | Fees Earned or Paid in Cash ($) | Total
($) | ||||||
| Raymond V. Damadian | $ | 0 | $ | 0 | ||||
| Claudette J.V. Chan | $ | 20,000 | $ | 20,000 | ||||
| Robert J. Janoff (deceased) | $ | 20,000 | $ | 20,000 | ||||
| Charles N. O’Data | $ | 20,000 | $ | 20,000 | ||||
| Ronald G. Lehman | $ | 20,000 | $ | 20,000 | ||||
| Richard E. Turk | $ | 20,000 | $ | 20,000 | ||||
EMPLOYEE COMPENSATION PLANS
Fonar’s 2005 Incentive Stock Option Plan, adopted on February 15, 2005, was intended to qualify as an incentive stock option plan under Section 422A of the Internal Revenue code of 1954, as amended. The Plan permits the issuance of stock options covering an aggregate of 80,000 shares of common stock of Fonar. The options issued have an exercise price equal to the fair market value of the underlying stock on the date the option is granted, are non-transferable, are exercisable for a period not exceeding ten years, and expire upon the voluntary termination of employment. The Plan terminated on February 14, 2015.
Fonar adopted its 2010 Stock Bonus Plan, on June 28, 2010. This Plan permits Fonar to issue an aggregate of 2,000,000 shares of common stock of Fonar as bonus or compensation. As of June 30, 2021, 450,177 shares were available for issuance.
| Page 122 |
FONAR CORPORATION AND SUBSIDIARIES
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT.
The following table sets forth the number and percentage of shares of Fonar’s securities held by each director, by each person known by us to own in excess of five percent of Fonar’s voting securities and by all officers and directors as a group as of September 1, 2021.
| Name and Address of Beneficial Owner (1) | Shares Beneficially Owned | Percent of Class | ||||||
| Raymond V. Damadian, M.D. | ||||||||
| c/o Fonar Corporation, Melville, New York | ||||||||
| Director and Treasurer | ||||||||
| 5% + Stockholder | ||||||||
| Common Stock | 121,402 | 1.85 | % | |||||
| Class C Stock | 382,447 | 99.98 | % | |||||
| Class A Preferred | 19,093 | 6.09 | % | |||||
| Kayne Anderson Rudnick | ||||||||
| Investment Management LLC | ||||||||
| 1800 Avenue of the Stars, 2nd Floor | ||||||||
| Los Angeles, CA 90067 | ||||||||
| Common Stock | 788,513 | 12.03 | % | |||||
| Renaissance Technologies LLC | ||||||||
| Renaissance Technologies Holding | ||||||||
| Corporation | ||||||||
| 800 Third Avenue | ||||||||
| New York, New York 10022 | ||||||||
| Common Stock | 489,816 | 7.47 | % | |||||
| Dimensional Fund Advisors LP | ||||||||
| Building One | ||||||||
| 6300 Bee Cave Road | ||||||||
| Austin, Texas 78746 | ||||||||
| Common Stock | 404,018 | 6.16 | % | |||||
| Page 123 |
FONAR CORPORATION AND SUBSIDIARIES
Continued:
| Name and Address of Beneficial Owner (1) | Shares Beneficially Owned | Percent of Class | ||||||
| Timothy R. Damadian, | ||||||||
| President and Chief Executive Officer | ||||||||
| Common Stock | 38,000 | * | ||||||
| Class A Preferred | 800 | * | ||||||
| Luciano B. Bonanni, | ||||||||
| Executive Vice President | ||||||||
| And Chief Operating Officer | ||||||||
| Common Stock | 49,726 | * | ||||||
| Class A Preferred | 1,285 | * | ||||||
| Claudette Chan | ||||||||
| Director and Secretary | ||||||||
| Common Stock | 106 | * | ||||||
| Class A Preferred | 32 | * | ||||||
| Charles N. O’Data | ||||||||
| Director | ||||||||
| Common Stock | 658 | * | ||||||
| Ronald G. Lehman | ||||||||
| Director | ||||||||
| Common Stock | 4,330 | * | ||||||
| Richard E. Turk | ||||||||
| Director | ||||||||
| Common Stock | 0 | * | ||||||
| All Officers and Directors | ||||||||
| as a Group (7 persons) | ||||||||
| Common Stock | 209,892 | 3.20 | % | |||||
| Class C Stock | 382,447 | 99.98 | % | |||||
| Class A Preferred | 21,210 | 6.77 | % | |||||
* Less than one percent
1. Address provided for each beneficial owner owning more than five percent of the voting securities of Fonar.
| Page 124 |
FONAR CORPORATION AND SUBSIDIARIES
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE.
Pursuant to HMCA’s management agreements with its clients, HMCA provides comprehensive non-medical management and administrative services, including billing and collection of accounts, payroll and accounts payable processing, office facilities, supplies and utilities. Under the management agreements, HMCA also provides service for the Fonar Upright® MRI scanners through Fonar. In total, as of September 15, 2021, 22 of our clients had management agreements with HMCA. Five sites in Florida are owned and operated directly by HMCA subsidiaries.
The fees charged under the management agreements are flat fees charged on a monthly basis. These fees ranged from $77,000 to $530,000 per month in fiscal 2021.
Dr. Raymond Damadian, the Chairman of the Board and principal stockholder of the Company, owns three of the imaging facilities in Florida managed by HMCA. The facilities owned by Dr. Damadian in Florida paid HMCA flat rate monthly fees ranging from $258,035 to $347,881 per month during fiscal 2021. These fees are renegotiable on an annual basis.
During the fiscal years ended June 30, 2021 and June 30, 2020, the net revenues received by HMCA from the imaging facilities owned by Dr. Damadian were approximately $11.0 million, and $10.2 million respectively.
Dr. Damadian owns a .75% interest in Health Management Company of America’s Class A membership interests. Dr. Damadian is also a Manager of Health Management Company of America.
Timothy Damadian, the President and Chief Executive Officer of Fonar, is one of the owners of a billing company, which performs billing and collection services for HMCA with respect to No-Fault and Workers’ Compensation claims of HMCA’s clients. The monthly fee charged to HMCA is $85,000. These services were terminated on January 1, 2021.
On June 1, 2017, the Company also entered into a one year renewable agreement to provide IT services to the billing company for a monthly fee of $23,884. Timothy Damadian is also a Manager of Health Management Company of America. The agreement was renewed on June 1, 2020 and June 1, 2021.
Ronald Lehman, a Director of Fonar, holds a .0378% interest in Health Management Company of America’s Class A membership interests.
Claudette J.V. Chan, a Director and the Secretary of Fonar, owns a .0378% interest in Health Management Company of America’s Class A Membership interests.
| Page 125 |
FONAR CORPORATION AND SUBSIDIARIES
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES.
Audit Fees
The aggregate fees billed by Marcum LLP for the audit of our annual consolidated financial statements for the fiscal year ended June 30, 2021 and the reviews of the financial statements included in our Forms 10-Q for the fiscal year ended June 30, 2021 were $345,000.
The aggregate fees billed by Marcum LLP for the audit of our annual financial statements for the fiscal year ended June 30, 2020 and the reviews of the financial statements included in our Forms 10-Q for the fiscal year ended June 30, 2020 were $409,000.
Audit Related Fees
No fees were billed by Marcum LLP for the fiscal years ended June 30, 2021 or June 30, 2020 for services related to the Audit or review of our financial statements that are not included under the caption “Audit Fees”.
No fees were billed by Marcum LLP for the fiscal years ended June 30, 2020 or June 30, 2019 for designing, operating, supervising or implementing any of our financial information systems or any hardware or software systems for our financial information
Tax Fees
No fees were billed by Marcum LLP for tax compliance, tax advice and tax planning in the fiscal year ended June 30, 2021.
No fees billed by Marcum LLP for tax compliance, tax advice and tax planning in the fiscal year ended June 30, 2020.
All Other Fees
No fees were billed by Marcum LLP for any other services during the fiscal years ended June 30, 2021 and June 30, 2020.
Since January 1, 2003, the audit committee has adopted policies and procedures for pre-approving all non-audit work performed by the auditors. Specifically, the committee must pre-approve the use of the auditors for all such services. The audit committee has pre-approved all non-audit work since that time and in making its determination has considered whether the provision of such services was compatible with the independence of the auditors.
Our audit committee believes that the provision by Marcum LLP of services in addition to audit services in previous years were compatible with maintaining their independence.
| Page 126 |
FONAR CORPORATION AND SUBSIDIARIES
PART IV
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES, AND REPORTS ON FORM 8-K.
a) FINANCIAL STATEMENTS AND SCHEDULES
The following consolidated financial statements are included in Part II, Item 8.
Report of Independent Registered Public Accounting Firm
Consolidated Balance Sheets as at June 30, 2021 and 2020.
Consolidated Statements of Income for the Years Ended June 30, 2021 and 2020.
Consolidated Statements of Stockholders’ Equity for the Years Ended June 30, 2021 and 2020.
Consolidated Statements of Cash Flows for the Years Ended June 30, 2021 and 2020 .
Notes to Consolidated Financial Statements.
Information required by schedules called for under Regulation S-X is either not applicable or is included in the consolidated financial statements or notes to the financial statements.
b) REPORTS ON FORM 8-K
c) EXHIBITS
3.1 Certificate of Incorporation, as amended, of the Registrant incorporated by reference to Exhibit 3.1 to the Registrant’s registration statement on Form S-1,Commission File No. 33-13365.
3.5 By-Laws, as amended, of the Registrant incorporated by reference to Exhibit 3.2 to the Registrant’s registration statement on Form S-1, Commission File No. 33-13365.
4.1 Specimen Common Stock Certificate incorporated by reference to Exhibit 4.1 to the Registrant’s registration statement on Form S-1, Commission File No. 33-13365.
4.2 Specimen Class B Common Stock Certificate incorporated by reference to Exhibit 4.2 to the Registrant’s registration statement on Form S-1, Commission File No. 33-13365.
| Page 127 |
FONAR CORPORATION AND SUBSIDIARIES
10.1 License Agreement between the Registrant and Raymond V. Damadian incorporated by reference to Exhibit 10 (e) to Form 10-K for the fiscal year ended June 30, 1983, Commission File No. 0-10248.
| Page 128 |
FONAR CORPORATION AND SUBSIDIARIES
21.1 Subsidiaries of the Registrant. See Exhibits.
23.1 Independent Registered Public Accounting Firms Report. See Exhibits.
31.1 Section 302 Certification. See Exhibits.
32.1 Section 906 Certification. See Exhibits.
SIGNATURES.
Pursuant to the requirements of Section 13 or 15 (d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| FONAR CORPORATION | ||
| Dated: October 12, 2021 | ||
| By: | /s/Timothy R. Damadian | |
| Timothy R. Damadian, President | ||
| and Principal Executive Officer | ||
| By: | /s/Raymond V. Damadian | |
| Raymond V. Damadian, Principal | ||
| Financial Officer, Chairman of | ||
| the Board and Treasurer | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Raymond V. Damadian Raymond V. Damadian | Chairman of the Board of Directors, Director, Principal Financial Officer, Treasurer | October 12, 2021 | ||
| /s/Claudette J.V. Chan | Director | October 12, 2021 | ||
| Claudette J.V. Chan | ||||
| /s/Charles N. O’Data | Director | October 12, 2021 | ||
| Charles N. O’Data | ||||
| /s/Ronald G. Lehman | Director | October 12, 2021 | ||
| Ronald G. Lehman | ||||
| /s/Richard E. Turk | Director | October 12, 2021 | ||
| Richard E. Turk |
| Page 129 |
CORPORATE INFORMATION
Corporate Headquarters
110 Marcus Drive
Melville, NY 11747
(631) 694-2929
Investor Relations
FONAR Corporation
110 Marcus Drive
Melville, NY 11747
(631) 694-2929
Stock Transfer Agency
Computershare
211 Quality Circle, Suite 210
College Station, TX 77845
Shareholder Services Number 800 962 4284
Investor Centre™ portal: www.computershare.com/investor
Auditors
Marcum LLP
New York, New York
Board of Directors
Raymond V. Damadian, M.D.
Chairman of the Board
Claudette J.V. Chan
Director
Ronald G. Lehman
Director
Richard E. Turk
Director
John Collins
Director
Officers
Timothy R. Damadian
President and Chief Executive Officer
Raymond V. Damadian, M.D.
Chairman of the Board and Treasurer
Luciano B. Bonanni
Executive Vice President and Chief Operating Officer
Claudette J.V. Chan
Secretary
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- DWF Labs joins the Klaytn Governance Council
- Share repurchase programme
- NOTICE TO THE ANNUAL GENERAL MEETING OF QPR SOFTWARE PLC
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share