Form 8-K Zynerba Pharmaceuticals, For: May 05
Exhibit 99.1

Zynerba Pharmaceuticals Announces Phase 3 RECONNECT Trial Design for Zygel™ in Fragile X Syndrome
- Confirmatory pivotal trial expected to be initiated in the third quarter of 2021 -
- Zynerba to hold conference call today, May 5, 2021 at 5:30 pm ET -
DEVON, Pa., May 5, 2021 – Zynerba Pharmaceuticals, Inc. (Nasdaq: ZYNE), the leader in innovative pharmaceutically-produced transdermal cannabinoid therapies for rare and near-rare neuropsychiatric disorders, today announced the Company has received guidance from the U.S. Food and Drug Administration (FDA) on a confirmatory Phase 3 trial of Zygel in patients with Fragile X syndrome (FXS). The trial, which will be called RECONNECT (A Randomized, Double-Blind, Placebo-Controlled, Multiple-Center, Efficacy and Safety Study of ZYN002 Administered as a Transdermal Gel to Children and Adolescents with Fragile X Syndrome), is designed to evaluate the efficacy and safety of Zygel in children and adolescents with FXS. The study is planned to confirm the positive results observed in a population of responders in the Company’s CONNECT-FX trial, a randomized, double-blind, placebo-controlled trial that assessed the efficacy and safety of Zygel as a treatment for the behavioral symptoms of FXS previously conducted by the Company. The Company believes that the results, if positive, from RECONNECT will be sufficient to support the submission of a New Drug Application for Zygel in patients with FXS.
“Following productive discussions and alignment with the FDA, we believe we have a clear path forward for Zygel in Fragile X syndrome. We are excited to advance Zygel into the RECONNECT trial, a pivotal, multi-national, confirmatory Phase 3 trial in patients with FXS in the third quarter of 2021,” said Armando Anido, Chairman and Chief Executive Officer of Zynerba. “If the results are positive, Zygel could become the first FDA approved treatment option for the significant unmet medical need that affects patients with FXS and their families.”
The RECONNECT trial will be an 18-week trial which will enroll approximately 200 children and adolescents of which approximately 160 patients will have complete (100%) methylation of their FMR1 gene and approximately 40 patients will have partial methylation of their FMR1 gene. The primary endpoint for the trial will be the change in the Aberrant Behavior Checklist-Community FXS Specific (ABC-CFXS) Social Avoidance subscale in patients who have complete methylation of their FMR1 gene. All patients, including the cohort of partially methylated patients, will be included in a key secondary endpoint analysis.
1

Complete Methylation Results from CONNECT-FX
The Company performed an analysis of the CONNECT-FX population within those patients having complete methylation of their FMR1 gene (n = 137 of 212 in the intent to treat population) to evaluate the effect of Zygel versus placebo. One patient did not have a post-baseline efficacy measure and was therefore not included in the efficacy analysis.
Baseline demographics for patients with complete methylation of the FMR1 gene are shown below. The group is similar to the previously reported full data set of patients and the cohort of patients with ≥90% methylation. The majority of patients were male and the children had a mean age of 9 to 10 years old.
| Placebo | Zygel | Total | ||||
| n | 65 | 72 | 137 | |||
| Age (years) | 9.7 | 9.5 | 9.6 | |||
| Sex – Males | ||||||
| n | 43 | 47 | 90 | |||
| % | 66% | 65% | 66% | |||
| Weight – kg: | ||||||
| Median | 34.5 | 35.7 | 35.2 | |||
| Range – Min, Max | 16.8, 104.7 | 18.6, 87.0 | 16.8, 104.7 | |||
| Baseline psychoactive medications, % | 69% | 56% | 62% |
2

The results in the cohort of patients with complete methylation of the FMR1 gene across the primary and key secondary endpoints that will be used in the RECONNECT trial are summarized below.
| Placebo N=64 | Zygel N=72 | |||||||||||||||||||||||||||
| Baseline Mean | Week
12 Mean Change | Baseline
Mean | Week 12 Mean Change | Treatment Difference** | Odds
Ratio | Treatment P-Value | ||||||||||||||||||||||
| Primary Endpoint: | ||||||||||||||||||||||||||||
| ABC-CFXS Social Avoidance Subscale | 7.25 | -1.84 | 6.88 | -2.92 | -1.08 | 0.027 | * | |||||||||||||||||||||
| Secondary Endpoints: | ||||||||||||||||||||||||||||
| ABC-CFXS Irritability Subscale | 27.84 | -3.98 | 28.89 | -5.83 | -1.85 | 0.220 | ||||||||||||||||||||||
| CGI-I at Week 12 (Any Improvement) | – | 36 | % | – | 50 | % | 1.75 | 0.128 | ||||||||||||||||||||
*Statistically significant vs. placebo
**A negative treatment difference demonstrates that Zygel patients improved versus placebo
“The treatment difference versus placebo and p value on the primary endpoint of improvement in the ABC-CFXS Social Avoidance subscale in patients with complete methylation are consistent with the previously reported findings in patients with at least 90% methylation, despite the fact that the study was not powered to evaluate either of these patient populations.” said Dr. Joseph Palumbo, Chief Medical Officer of Zynerba Pharmaceuticals, Inc. “We believe the CONNECT-FX trial was instrumental in advancing our understanding of the science of Fragile X Syndrome. We look forward to leveraging what we learned as we seek to confirm our findings in the RECONNECT trial.”
Conference call information
Zynerba management will host a live conference call and webcast today at 5:30 pm Eastern Time to discuss the design of the RECONNECT Trial. The call can be accessed by dialing (800) 708-4540 (U.S. and Canada) or (847) 619-6397 (international) and referencing conference ID 50161974. To access the live webcast or the replay, visit the investor page of the Company’s website at http://ir.zynerba.com/. The webcast will be recorded and available on the Company’s website for 30 days.
3

About Zynerba Pharmaceuticals, Inc.
Zynerba Pharmaceuticals is the leader in innovative pharmaceutically-produced transdermal cannabinoid therapies for rare and near-rare neuropsychiatric disorders. We are committed to improving the lives of patients and their families living with severe, chronic health conditions including Fragile X syndrome, autism spectrum disorder, 22q11.2 deletion syndrome, and a heterogeneous group of rare and ultra-rare epilepsies known as developmental and epileptic encephalopathies. Learn more at www.zynerba.com and follow us on Twitter at @ZynerbaPharma.
About Fragile X Syndrome (FXS)
Fragile X syndrome is a rare genetic developmental disability that is the leading known cause of both inherited intellectual disability and autism spectrum disorder, affecting 1 in 3,600 to 4,000 males and 1 in 4,000 to 6,000 females. It is the most common inherited intellectual disability in males and a significant cause of intellectual disability in females, and the leading genetic cause of autism spectrum disorder (ASD). The disorder negatively affects synaptic function, plasticity and neuronal connections, and results in a spectrum of intellectual disabilities and behavioral symptoms, such as social avoidance and irritability. In the US, there are about 71,000 people suffering with FXS, approximately 60% of whom have complete methylation of the FMR1 gene.
FXS is caused by a mutation in FMR1, a gene which modulates a number of systems, including important effects on the endocannabinoid system, and most critically, codes for a protein called FMRP. This protein helps regulate the production of other proteins and plays a role in the development of synapses, which are critical for relaying nerve impulses, and in regulating synaptic plasticity. The FMR1 mutation manifests as multiple repeats of a DNA segment, known as the CGG triplet repeat. In most neurotypical people, the FMR1 gene correctly codes for the FMRP protein. In neurotypical individuals, there are CGG repeats, but these repeats only occur between 5 and 40 times. As a result, FMRP is manufactured at levels that enable control over behaviors like social avoidance and anxiety. In people with full mutation of the Fragile X gene, the CGG segment is repeated more than 200 times and in most cases causes the FMR1 gene to not function. However, the methylation of the FMR1 gene also plays a role in determining functionality of the gene. For patients with complete (100%) methylation, the FMR1 gene is silenced, therefore, no FMRP is produced, and the systems and processes that are expected to be affected by FMRP become dysregulated.
4

Cautionary Note on Forward-Looking Statements
This press release contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. We may, in some cases, use terms such as “predicts,” “believes,” “potential,” “proposed,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. Such statements are subject to numerous important factors, risks and uncertainties that may cause actual events or results to differ materially from the Company’s current expectations. Management’s expectations and, therefore, any forward-looking statements in this press release could also be affected by risks and uncertainties relating to a number of other factors, including the following: the Company’s cash and cash equivalents may not be sufficient to support its operating plan for as long as anticipated; the Company’s expectations, projections and estimates regarding expenses, future revenue, capital requirements, incentive and other tax credit eligibility, collectability and timing, and availability of and the need for additional financing; the Company’s ability to obtain additional funding to support its clinical development programs; the results, cost and timing of the Company’s clinical development programs, including any delays to such clinical trials relating to enrollment or site initiation; clinical results for the Company’s product candidates, including the RECONNECT trial, may not be replicated or continue to occur in additional trials and may not otherwise support further development in a specified indication or at all; the Company’s planned RECONNECT trial may not be determined to be sufficient to support an NDA submission; actions or advice of the U.S. Food and Drug Administration and foreign regulatory agencies may affect the design, initiation, timing, continuation and/or progress of clinical trials or result in the need for additional clinical trials; the Company’s ability to obtain and maintain regulatory approval for its product candidates, and the labeling under any such approval; the Company’s reliance on third parties to assist in conducting pre-clinical and clinical trials for its product candidates; delays, interruptions or failures in the manufacture and supply of the Company’s product candidates the Company’s ability to commercialize its product candidates; the size and growth potential of the markets for the Company’s product candidates, and the Company’s ability to service those markets; the Company’s ability to develop sales and marketing capabilities, whether alone or with potential future collaborators; the rate and degree of market acceptance of the Company’s product candidates; the Company’s expectations regarding its ability to obtain and adequately maintain sufficient intellectual property protection for its product candidates; the timing and outcome of current and future legal proceedings; and the extent to which health epidemics and other outbreaks of communicable diseases, including COVID-19, could disrupt our operations or adversely affect our business and financial conditions. This list is not exhaustive and these and other risks are described in the Company’s periodic reports, including the annual report on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K, filed with or furnished to the Securities and Exchange Commission and available at www.sec.gov. Any forward-looking statements that the Company makes in this press release speak only as of the date of this press release. The Company assumes no obligation to update forward-looking statements whether as a result of new information, future events or otherwise, after the date of this press release.
5

Zynerba Contacts
Peter Vozzo
Westwicke/ICR
Office: 443.213.0505
Cell: 443.377.4767
6
Exhibit 99.2
 |
Corporate Overview May 2021 |
 |
Forward-Looking Statements THE STATEMENTS IN THIS PRESENTATION MAY INCLUDE FORWARD-LOOKING STATEMENTS WITHIN THE MEANING OF THE PRIVATE SECURITIES LITIGATION REFORM ACT OF 1995. THESE STATEMENTS, AMONG OTHER THINGS RELATE TO THE FUTURE OPERATIONS, OPPORTUNITIES OR FINANCIAL PERFORMANCE OF ZYNERBA PHARMACEUTICALS, INC. WE MAY, IN SOME CASES, USE TERMS SUCH AS “PREDICTS,” “BELIEVES,” “POTENTIAL,” “PROPOSED,” “CONTINUE,” “ESTIMATES,” “ANTICIPATES,” “EXPECTS,” “PLANS,” “INTENDS,” “MAY,” “COULD,” “MIGHT,” “WILL,” “SHOULD” OR OTHER WORDS THAT CONVEY UNCERTAINTY OF FUTURE EVENTS OR OUTCOMES TO IDENTIFY THESE FORWARD-LOOKING STATEMENTS. SUCH STATEMENTS ARE SUBJECT TO NUMEROUS IMPORTANT FACTORS, RISKS AND UNCERTAINTIES THAT MAY CAUSE ACTUAL EVENTS OR RESULTS TO DIFFER MATERIALLY FROM THE COMPANY’S CURRENT EXPECTATIONS, INCLUDING THE FOLLOWING: THE COMPANY’S CASH AND CASH EQUIVALENTS MAY NOT BE SUFFICIENT TO SUPPORT ITS OPERATING PLAN FOR AS LONG AS ANTICIPATED; THE RESULTS, COST AND TIMING OF THE COMPANY’S CLINICAL DEVELOPMENT PROGRAMS, INCLUDING ANY DELAYS TO SUCH CLINICAL TRIALS RELATING TO ENROLLMENT OR SITE INITIATION; CLINICAL RESULTS FOR THE COMPANY’S PRODUCT CANDIDATES INCLUDING RECONNECT TRIAL MAY NOT BE REPLICATED OR CONTINUE TO OCCUR IN ADDITIONAL TRIALS AND MAY NOT OTHERWISE SUPPORT FURTHER DEVELOPMENT IN A SPECIFIED INDICATION OR AT ALL; THE COMPANY’S PLANNED RECONNECT TRIAL MAY NOT BE DETERMINED TO BE SUFFICIENT TO SUPPORT AN NDA SUBMISSION; ACTIONS OR ADVICE OF THE U.S. FOOD AND DRUG ADMINISTRATION AND FOREIGN REGULATORY AGENCIES MAY AFFECT THE DESIGN, INITIATION, TIMING, CONTINUATION AND/OR PROGRESS OF CLINICAL TRIALS OR RESULT IN THE NEED FOR ADDITIONAL CLINICAL TRIALS; THE COMPANY’S ABILITY TO OBTAIN AND MAINTAIN REGULATORY APPROVAL FOR ITS PRODUCT CANDIDATES, AND THE LABELING UNDER ANY SUCH APPROVAL; AND THE COMPANY’S EXPECTATIONS REGARDING ITS ABILITY TO OBTAIN AND ADEQUATELY MAINTAIN SUFFICIENT INTELLECTUAL PROPERTY PROTECTION FOR ITS PRODUCT CANDIDATES. THESE AND OTHER RISKS ARE DESCRIBED IN OUR FILINGS WITH THE SECURITIES AND EXCHANGE COMMISSION, AVAILABLE AT WWW.SEC.GOV. ANY FORWARD-LOOKING STATEMENTS THAT THE COMPANY MAKES IN THIS PRESENTATION SPEAK ONLY AS OF THE DATE OF THIS PRESENTATION. THE COMPANY ASSUMES NO OBLIGATION TO UPDATE FORWARD-LOOKING STATEMENTS WHETHER AS A RESULT OF NEW INFORMATION, FUTURE EVENTS OR OTHERWISE, AFTER THE DATE OF THIS P reserved. Zynerba and Zygel are trademarks of Zynerba Pharmaceuticals, Inc. All other trademarks and registered trademarks are property of their respective owners. RESENTATION. © 2020 Zynerba Pharmaceuticals, Inc. All rights2 |
 |
3 A Rare/Near-Rare Neuropsychiatric Company Deep pipeline targeting high unmet medical needs; translating into multi-billion dollar market opportunity with Zygel™ (Cannabidiol Gel) Focused on initiating RECONNECT trial of Zygel in Fragile X syndrome (FXS) in Q3 2021 Continuing to pursue three additional neuropsychiatric indications: 22q11.2 deletion syndrome (22q) – Phase 2 ongoing Autism spectrum disorder (ASD) – Phase 2 complete Developmental and epileptic encephalopathies (DEE) – Phase 2 complete Experienced team with development and commercial expertise in transdermal delivery, orphan diseases, neurology, and psychiatry Cash runway expected to be sufficient to fund operations well into 1H 2024 |
 |
Deep Clinical Pipeline & Zygel Cannabidiol Gel **Orphan Drug designation 4 Indication Preclinical Phase 1 Phase 2 Pivotal Expected Milestones Fragile X Syndrome (FXS)* Initiate confirmatory RECONNECT pivotal trial in Q3 2021 Screening of patients has resumed; timing for top line results TBD when enrollment complete Discuss Phase 2 results and regulatory path forward with FDA in 1H2021 Finalize target syndrome selection in 2021 RECONNECT: P reparing for confir atory trial 22q Deletion Syndrome (22q)** INSPIRE: O going Autism Spectrum Disorder (ASD) Developmental and Epileptic Encephalopathies (DEE) m n BRIGHT: Topline data released BELIEVE: Topline data released *Orphan Drug and Fast Track designation |
 |
Zygel (ZYN002) Cannabidiol Gel Differentiated Transdermal Unique Mechanism Neuropsych Indications of Action First & only patent-protected (2038), permeation-enhanced, pharmaceutically-produced cannabidiol gel Formulation delivers Cannabidiol through the epidermis and into the circulatory system Cannabidiol modulates multiple receptors and mediates numerous pathways, including the endocannabinoid pathway Potential utility in rare / near-rare neuropsychiatric conditions 5 |
 |
FXS is routinely diagnosed by assessing (1) CGG repeats and (2) methylation status *FMR Protein; RNA-binding protein that helps regulate synaptic development and plasticity 6 Fragile X Syndrome (FXS) FXS Rare genetic developmental disability Leading known cause of both inherited intellectual disability and ASD No approved drugs indicated for FXS Symptoms linked to deficiencies in the endocannabinoid system (ECS) Mutation impacts FMR1 gene and causes ECS dysregulation, causing core cognitive, social, and behavioral symptoms of FXS Easily identified mutation manifests as multiple CGG repeats in FMR1 (full mutation = >200 repeats) U.S. patents provide IP protection to 2038 FMR1 gene codes for production of FMRP* which is vital to synapse development Methylation of FMR1 also plays a role in determining functionality of the gene When methylation of FMR1 silences the gene, no FMRP is produced: Systems and processes affected by FMRP become dysregulated ~60% of patients are believed to fall into the completely methylated category ~70K U.S. patients with full mutation FXS |
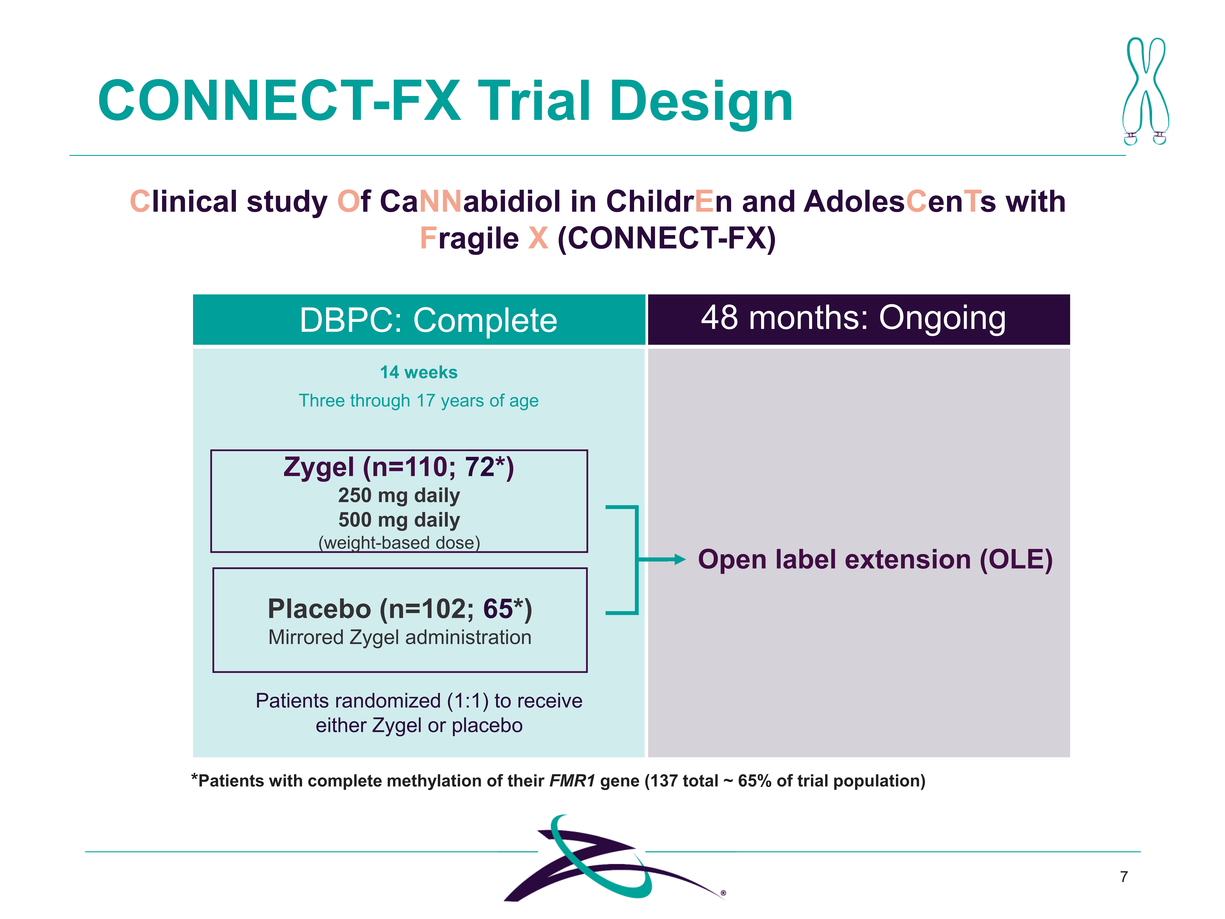 |
*Patients with complete methylation of their FMR1 gene (137 total ~ 65% of trial population) 7 CONNECT-FX Trial Design Clinical study Of CaNNabidiol in ChildrEn and AdolesCenTs with Fragile X (CONNECT-FX) Zygel (n=110; 72*) 250 mg daily 500 mg daily (weight-based dose) Placebo (n=102; 65*) Mirrored Zygel administration DBPC: Complete 14 weeks Three through 17 years of age 48 months: Ongoing Open label extension (OLE) Patients randomized (1:1) to receive either Zygel or placebo |
 |
8 Endpoints and Data Collected Primary endpoint: Change from baseline to end of treatment in ABC-CFXS Social Avoidance subscale Key secondary endpoints: Change from baseline to end of the treatment in ABC-CFXS Irritability subscale score ABC-CFXS Socially Unresponsive/Lethargic subscale score Improvement in Clinical Global Impression (CGI-I) at end of treatment, anchored to FXS behaviors Aligned with FDA’s ‘Voice of the Patient’ Guidance Captured qualitative data on clinical relevance of FXS behaviors |
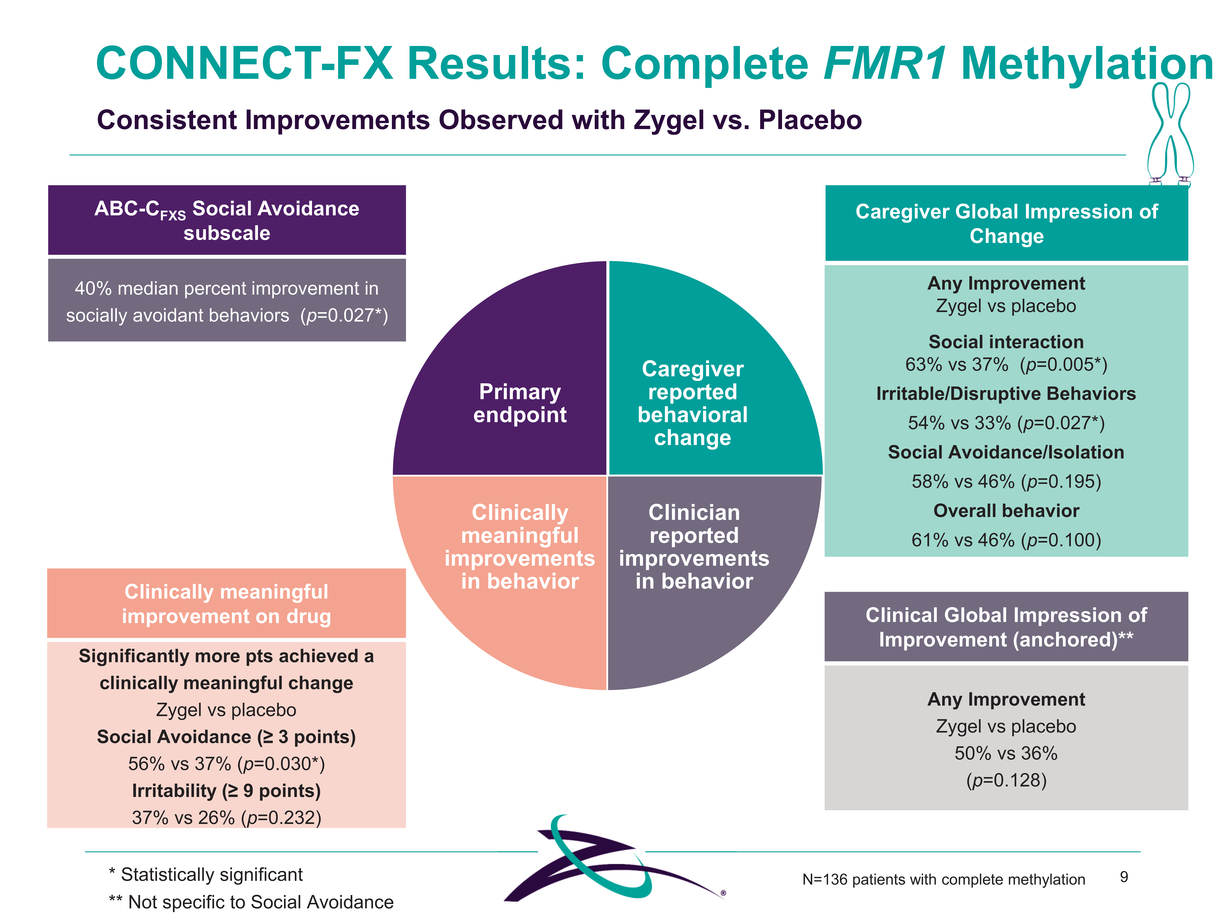 |
CONNECT-FX Results: Complete FMR1 Methylation Consistent Improvements Observed with Zygel vs. Placebo ABC-CFXS Social Avoidance subscale Caregiver Global Impression of Change 40% median percent improvement in socially avoidant behaviors (p=0.027*) Clinically meaningful improvement on drug Significantly more pts achieved a clinically meaningful change Zygel vs placebo Social Avoidance (≥ 3 points) 56% vs 37% (p=0.030*) Irritability (≥ 9 points) 37% vs 26% (p=0.232) Primary endpoint Clinically meaningful improvements in behavior Caregiver reported behavioral change Clinician reported improvements in behavior Any Improvement Zygel vs placebo Social interaction 63% vs 37% (p=0.005*) Irritable/Disruptive Behaviors 54% vs 33% (p=0.027*) Social Avoidance/Isolation 58% vs 46% (p=0.195) Overall behavior 61% vs 46% (p=0.100) Clinical Global Impression of Improvement (anchored)** Any Improvement Zygel vs placebo 50% vs 36% (p=0.128) * Statistically significant ** Not specific to Social Avoidance N=136 patients with complete methylation9 |
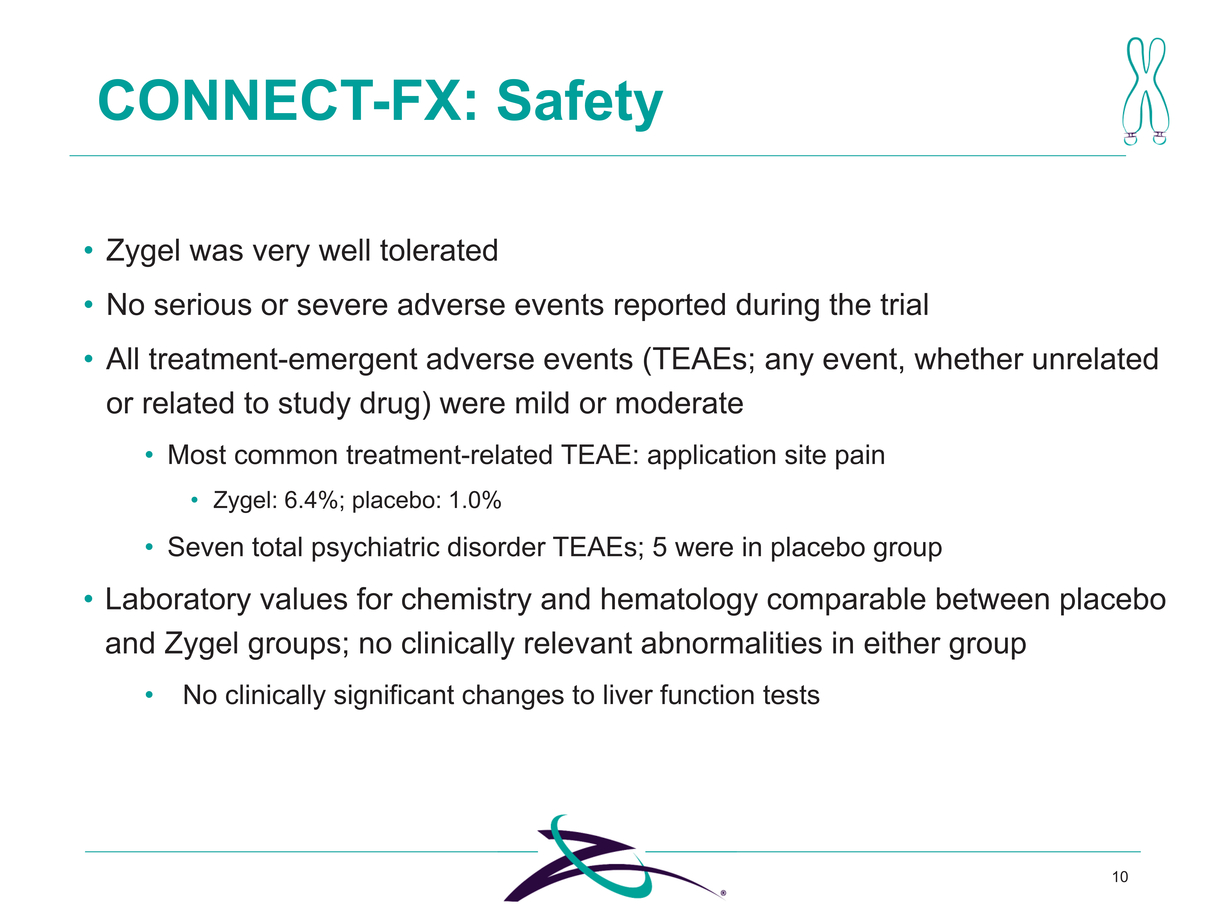 |
10 Zygel was very well tolerated No serious or severe adverse events reported during the trial All treatment-emergent adverse events (TEAEs; any event, whether unrelated or related to study drug) were mild or moderate Most common treatment-related TEAE: application site pain Zygel: 6.4%; placebo: 1.0% Seven total psychiatric disorder TEAEs; 5 were in placebo group Laboratory values for chemistry and hematology comparable between placebo and Zygel groups; no clinically relevant abnormalities in either group No clinically significant changes to liver function tests |
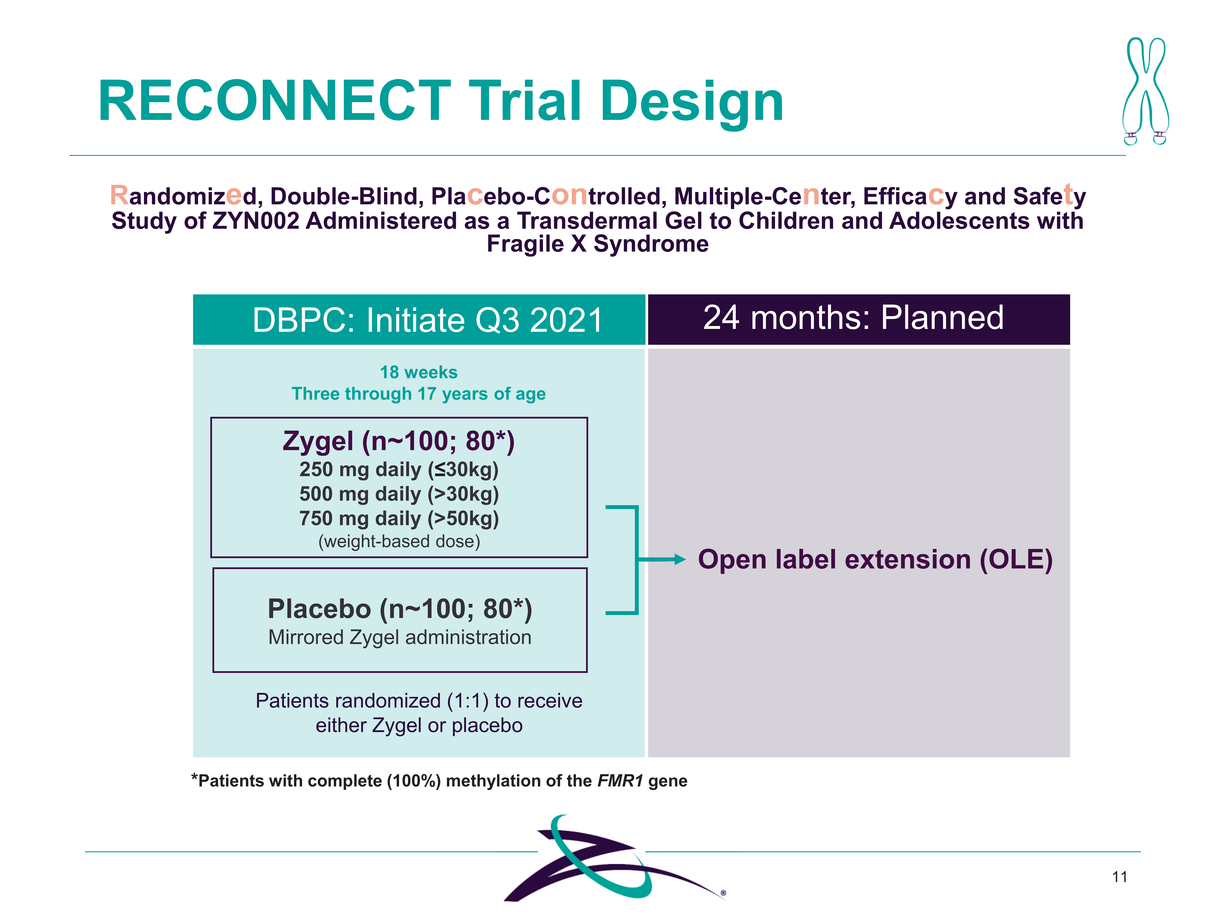 |
*Patients with complete (100%) methylation of the FMR1 gene 11 RECONNECT Trial Design Randomized, Double-Blind, Placebo-Controlled, Multiple-Center, Efficacy and Safety Study of ZYN002 Administered as a Transdermal Gel to Children and Adolescents with Fragile X Syndrome Zygel (n~100; 80*) 250 mg daily (≤30kg) 500 mg daily (>30kg) 750 mg daily (>50kg) (weight-based dose) Placebo (n~100; 80*) Mirrored Zygel administration DBPC: Initiate Q3 2021 18 weeks Three through 17 years of age 24 months: Planned Open label extension (OLE) Patients randomized (1:1) to receive either Zygel or placebo |
 |
and skin diaries 12 RECONNECT Trial Design Primary endpoint will be change vs. placebo in Social Avoidance subscale of ABC-CFXS for children and adolescents who have complete (100%) methylation of the FMR1 gene Partial methylation cohort will have descriptive statistics gathered and they will be combined with full methylation cohort for key secondary analysis 18 week trial design will allow us to determine if the behavioral symptoms improve over time Added a third dosing group of 750 mg for individuals >50kg to maintain appropriate dosing levels for all patients Making trial more patient and family friendly – virtual visits, fewer assessments administered, reducing frequency of lab and ECG tests, providing each family an electronic tablet for recording of assessments |
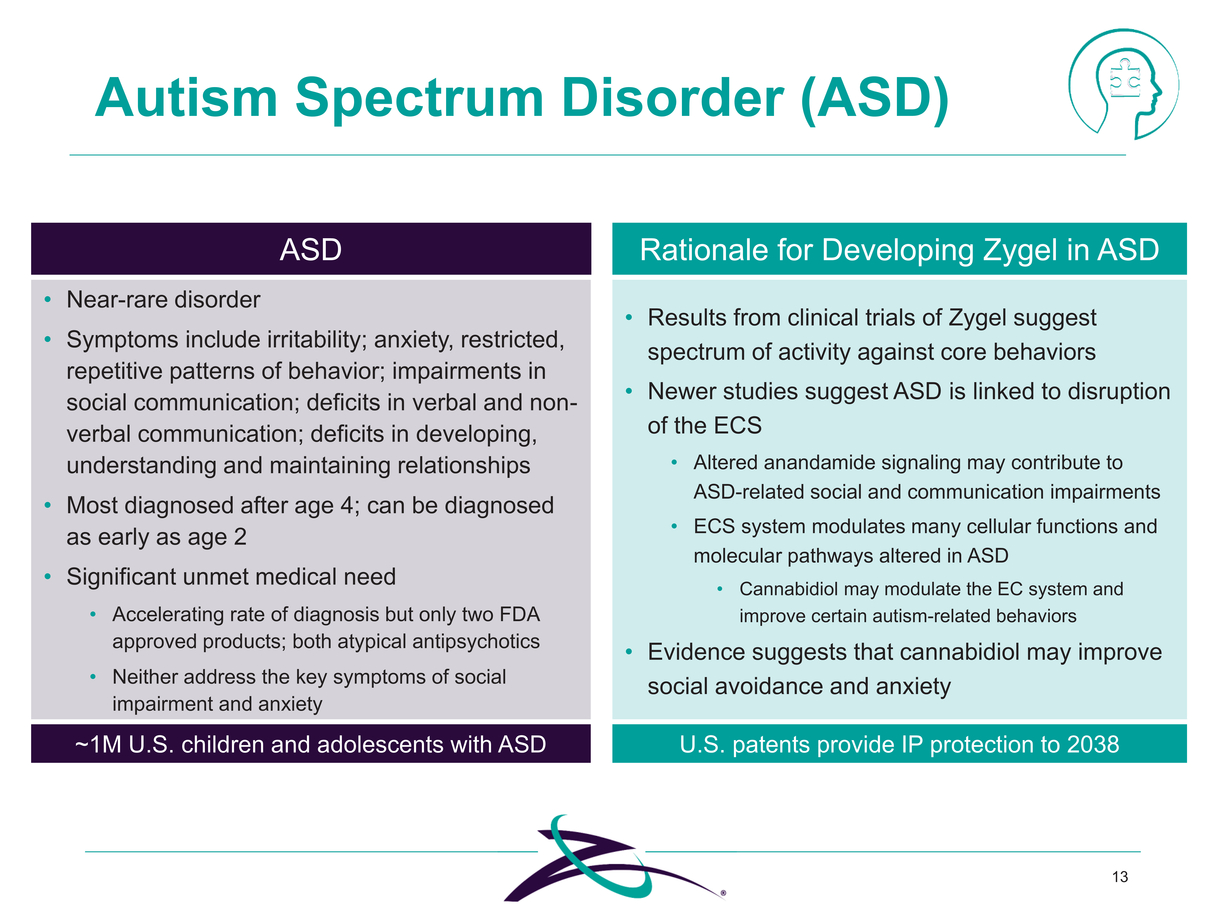 |
Autism Spectrum Disorder (ASD) ASD Results from clinical trials of Zygel suggest spectrum of activity against core behaviors Newer studies suggest ASD is linked to disruption of the ECS Altered anandamide signaling may contribute to ASD-related social and communication impairments ECS system modulates many cellular functions and molecular pathways altered in ASD Cannabidiol may modulate the EC system and improve certain autism-related behaviors Evidence suggests that cannabidiol may improve social avoidance and anxiety 13 Near-rare disorder Symptoms include irritability; anxiety, restricted, repetitive patterns of behavior; impairments in social communication; deficits in verbal and non-verbal communication; deficits in developing, understanding and maintaining relationships Most diagnosed after age 4; can be diagnosed as early as age 2 Significant unmet medical need Accelerating rate of diagnosis but only two FDA approved products; both atypical antipsychotics Neither address the key symptoms of social impairment and anxiety ~1M U.S. children and adolescents with ASD |
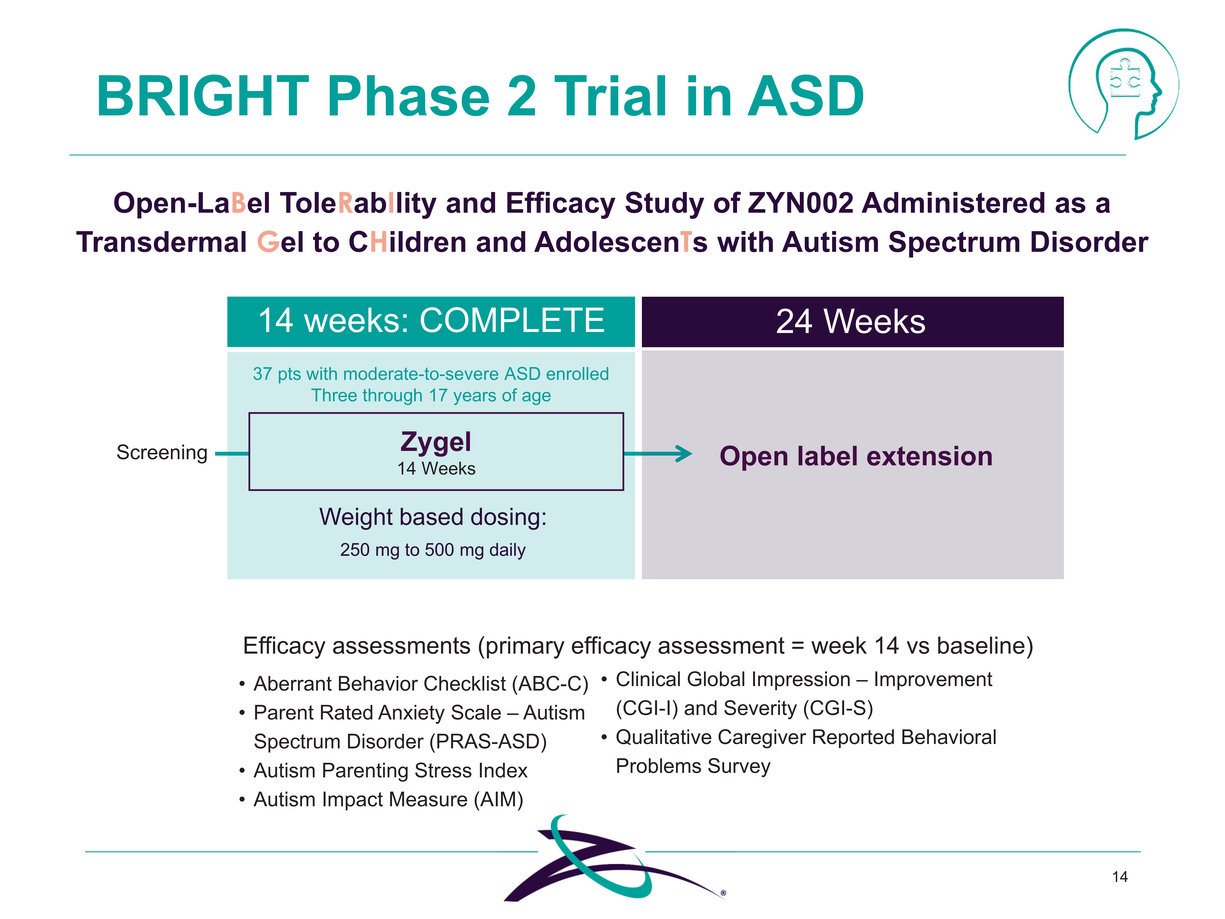 |
BRIGHT Phase 2 Trial in ASD Open-LaBel ToleRabIlity and Efficacy Study of ZYN002 Administered as a Transdermal Gel to CHildren and AdolescenTs with Autism Spectrum Disorder Zygel 14 Weeks 14 weeks: COMPLETE 37 pts with moderate-to-severe ASD enrolled Three through 17 years of age 24 Weeks Open label extension Weight based dosing: 250 mg to 500 mg daily Efficacy assessments (primary efficacy assessment = week 14 vs baseline) Aberrant Behavior Checklist (ABC-C) Parent Rated Anxiety Scale – Autism Spectrum Disorder (PRAS-ASD) Autism Parenting Stress Index Autism Impact Measure (AIM) Clinical Global Impression – Improvement (CGI-I) and Severity (CGI-S) Qualitative Caregiver Reported Behavioral Problems Survey 14 |
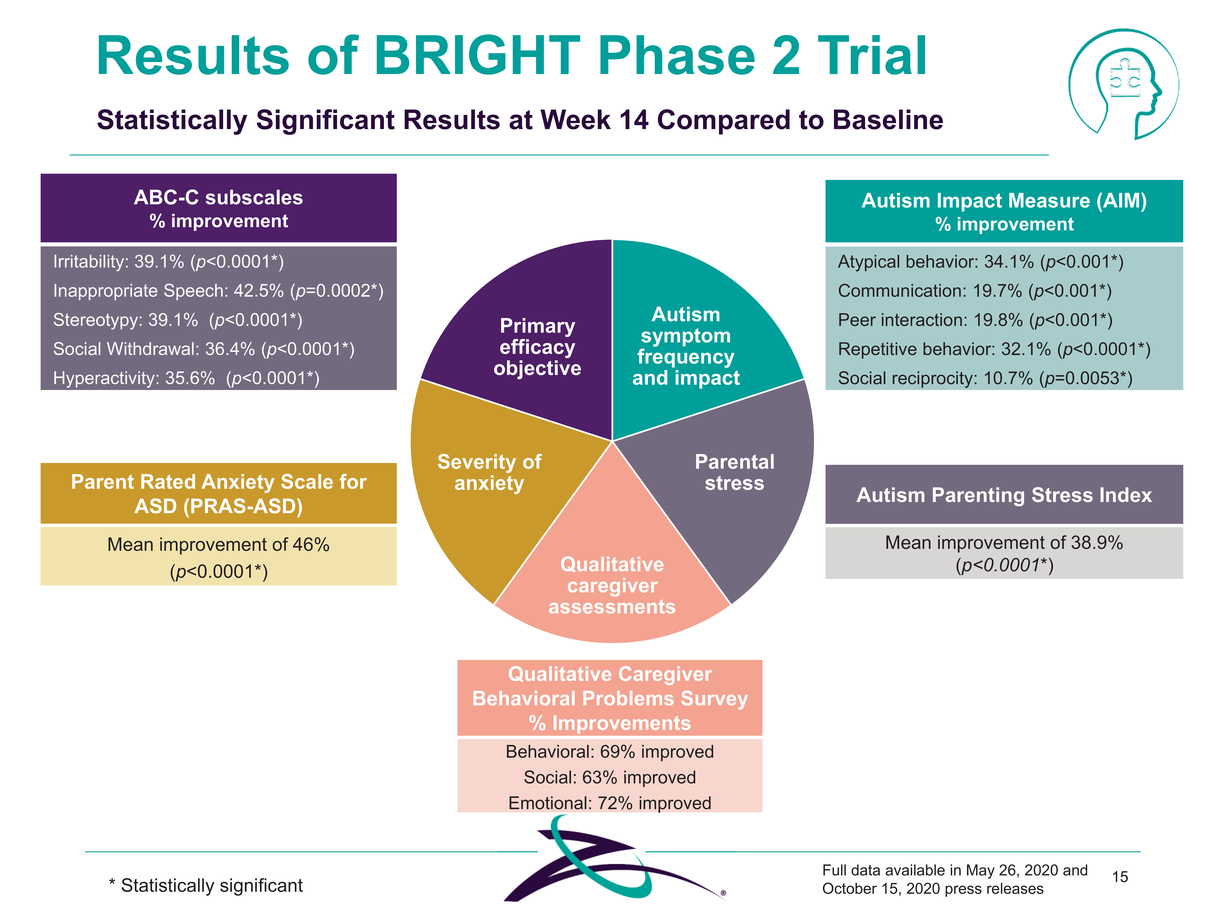 |
Qualitative Caregiver Behavioral Problems Survey % Improvements Behavioral: 69% improved Social: 63% improved Emotional: 72% improved * Statistically significant Full data available in May 26, 2020 and October 15, 2020 press releases 15 ABC-C subscales % improvement Irritability: 39.1% (p<0.0001*) Inappropriate Speech: 42.5% (p=0.0002*) Stereotypy: 39.1% (p<0.0001*) Social Withdrawal: 36.4% (p<0.0001*) Hyperactivity: 35.6% (p<0.0001*) Autism Parenting Stress Index Mean improvement of 38.9% (p<0.0001*) Parent Rated Anxiety Scale for ASD (PRAS-ASD) Mean improvement of 46% (p<0.0001*) Autism Impact Measure (AIM) % improvement Atypical behavior: 34.1% (p<0.001*) Communication: 19.7% (p<0.001*) Peer interaction: 19.8% (p<0.001*) Repetitive behavior: 32.1% (p<0.0001*) Social reciprocity: 10.7% (p=0.0053*) Results of BRIGHT Phase 2 Trial Statistically Significant Results at Week 14 Compared to Baseline Primary efficacy objective Autism symptom frequency and impact Severity of anxiety Parental stress Qualitative caregiver assessments |
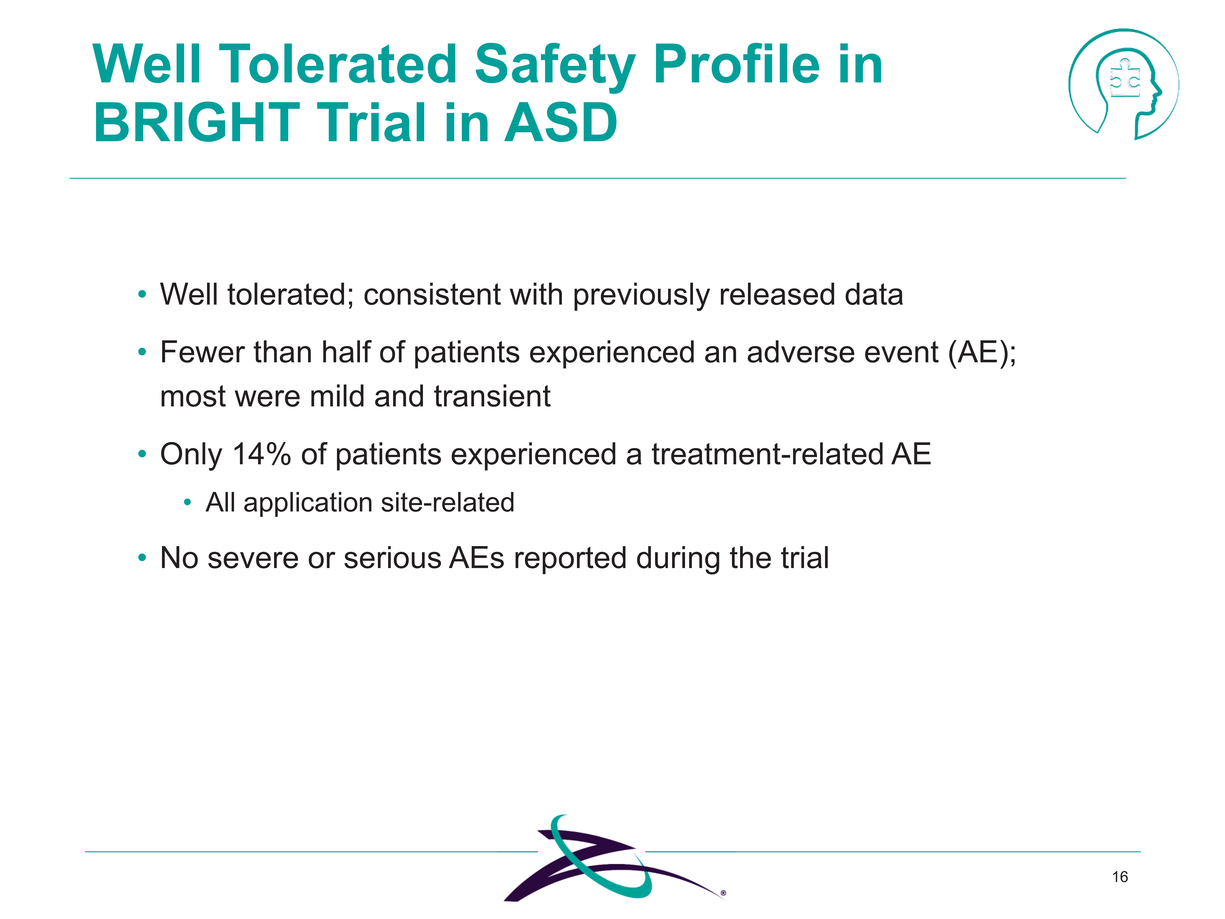 |
16 Well Tolerated Safety Profile in BRIGHT Trial in ASD Well tolerated; consistent with previously released data Fewer than half of patients experienced an adverse event (AE); most were mild and transient Only 14% of patients experienced a treatment-related AE All application site-related No severe or serious AEs reported during the trial |
 |
17 Next Steps in ASD Program In 1H2021, Zynerba intends to discuss data supporting the potential efficacy of Zygel in ASD, including the results of the Phase 2 BRIGHT trial, with the FDA to determine the regulatory path forward Present additional data at future medical meetings |
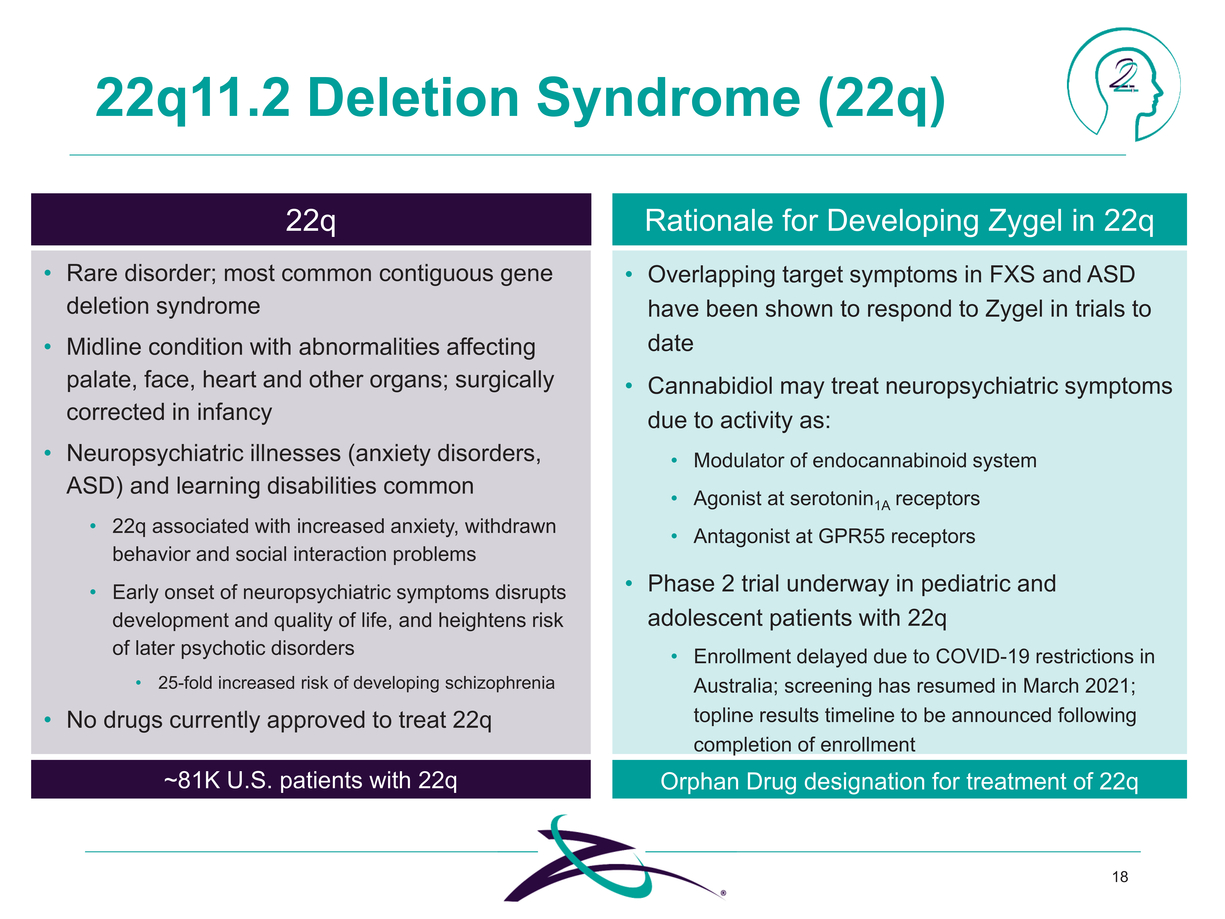 |
22q11.2 Deletion Syndrome (22q) 22q Rare disorder; most common contiguous gene deletion syndrome Midline condition with abnormalities affecting palate, face, heart and other organs; surgically corrected in infancy Neuropsychiatric illnesses (anxiety disorders, ASD) and learning disabilities common 22q associated with increased anxiety, withdrawn behavior and social interaction problems Early onset of neuropsychiatric symptoms disrupts development and quality of life, and heightens risk of later psychotic disorders 25-fold increased risk of developing schizophrenia No drugs currently approved to treat 22q Overlapping target symptoms in FXS and ASD have been shown to respond to Zygel in trials to date Cannabidiol may treat neuropsychiatric symptoms due to activity as: Modulator of endocannabinoid system Agonist at serotonin1A receptors Antagonist at GPR55 receptors Phase 2 trial underway in pediatric and adolescent patients with 22q Enrollment delayed due to COVID-19 restrictions in Australia; screening has resumed in March 2021; topline results timeline to be announced following completion of enrollment ~81K U.S. patients with 22q 18 |
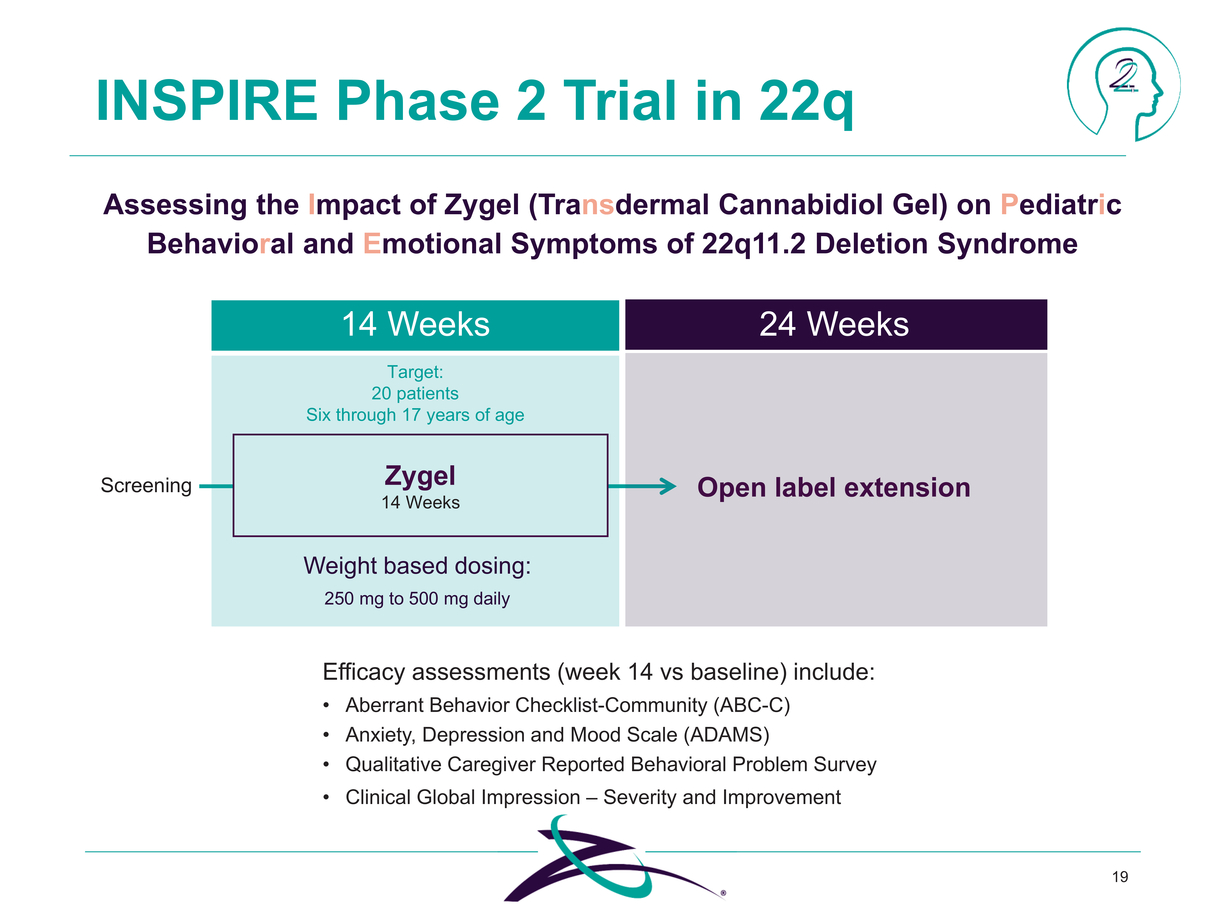 |
Clinical Global Impression – Severity and Improvement 19 INSPIRE Phase 2 Trial in 22q Assessing the Impact of Zygel (Transdermal Cannabidiol Gel) on Pediatric Behavioral and Emotional Symptoms of 22q11.2 Deletion Syndrome Zygel 14 Weeks 14 Weeks Target: 20 patients Six through 17 years of age 24 Weeks Open label extension Weight based dosing: 250 mg to 500 mg daily Efficacy assessments (week 14 vs baseline) include: Aberrant Behavior Checklist-Community (ABC-C) Anxiety, Depression and Mood Scale (ADAMS) Qualitative Caregiver Reported Behavioral Problem Survey |
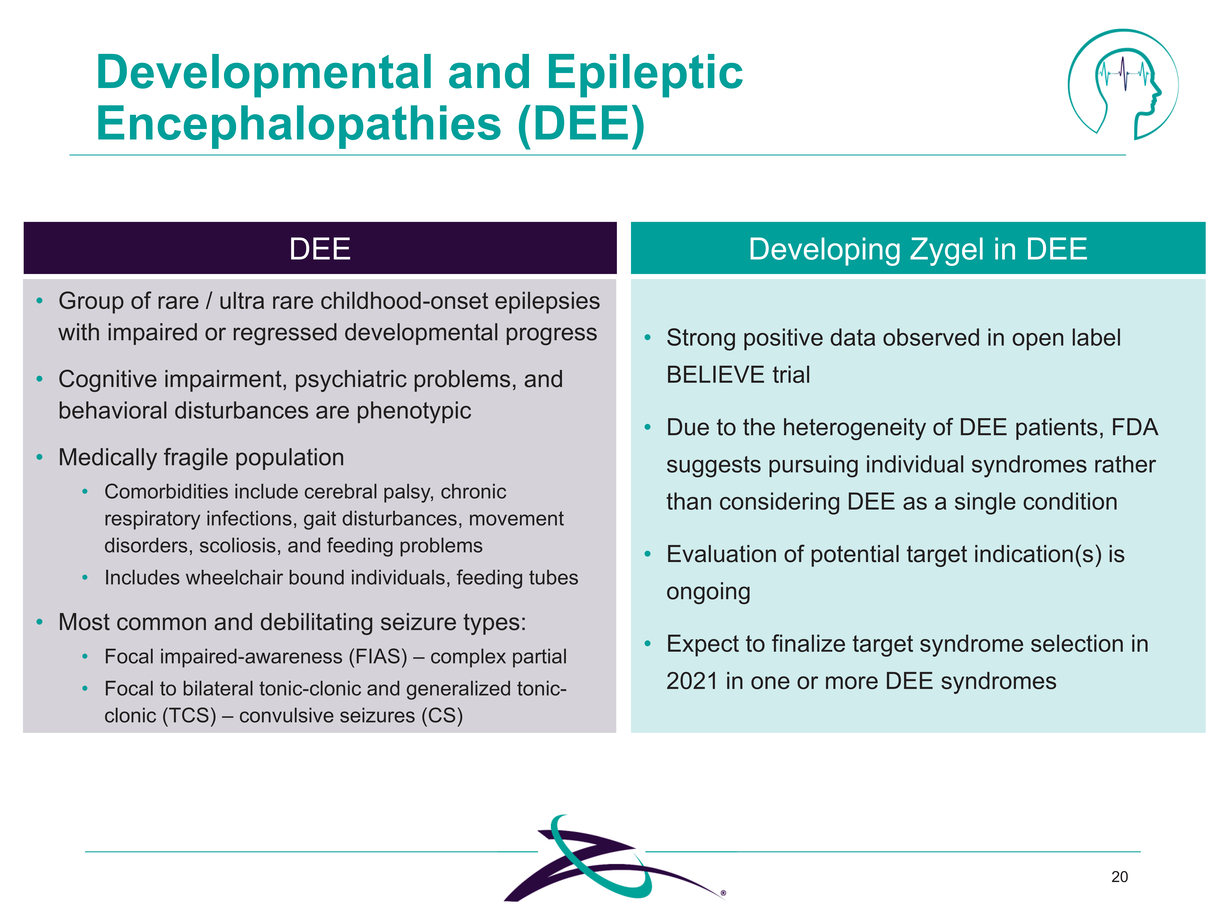 |
20 Developmental and Epileptic Encephalopathies (DEE) DEE Group of rare / ultra rare childhood-onset epilepsies with impaired or regressed developmental progress Cognitive impairment, psychiatric problems, and behavioral disturbances are phenotypic Medically fragile population Comorbidities include cerebral palsy, chronic respiratory infections, gait disturbances, movement disorders, scoliosis, and feeding problems Includes wheelchair bound individuals, feeding tubes Most common and debilitating seizure types: Focal impaired-awareness (FIAS) – complex partial Focal to bilateral tonic-clonic and generalized tonic-clonic (TCS) – convulsive seizures (CS) Strong positive data observed in open label BELIEVE trial Due to the heterogeneity of DEE patients, FDA suggests pursuing individual syndromes rather than considering DEE as a single condition Evaluation of potential target indication(s) is ongoing Expect to finalize target syndrome selection in 2021 in one or more DEE syndromes |
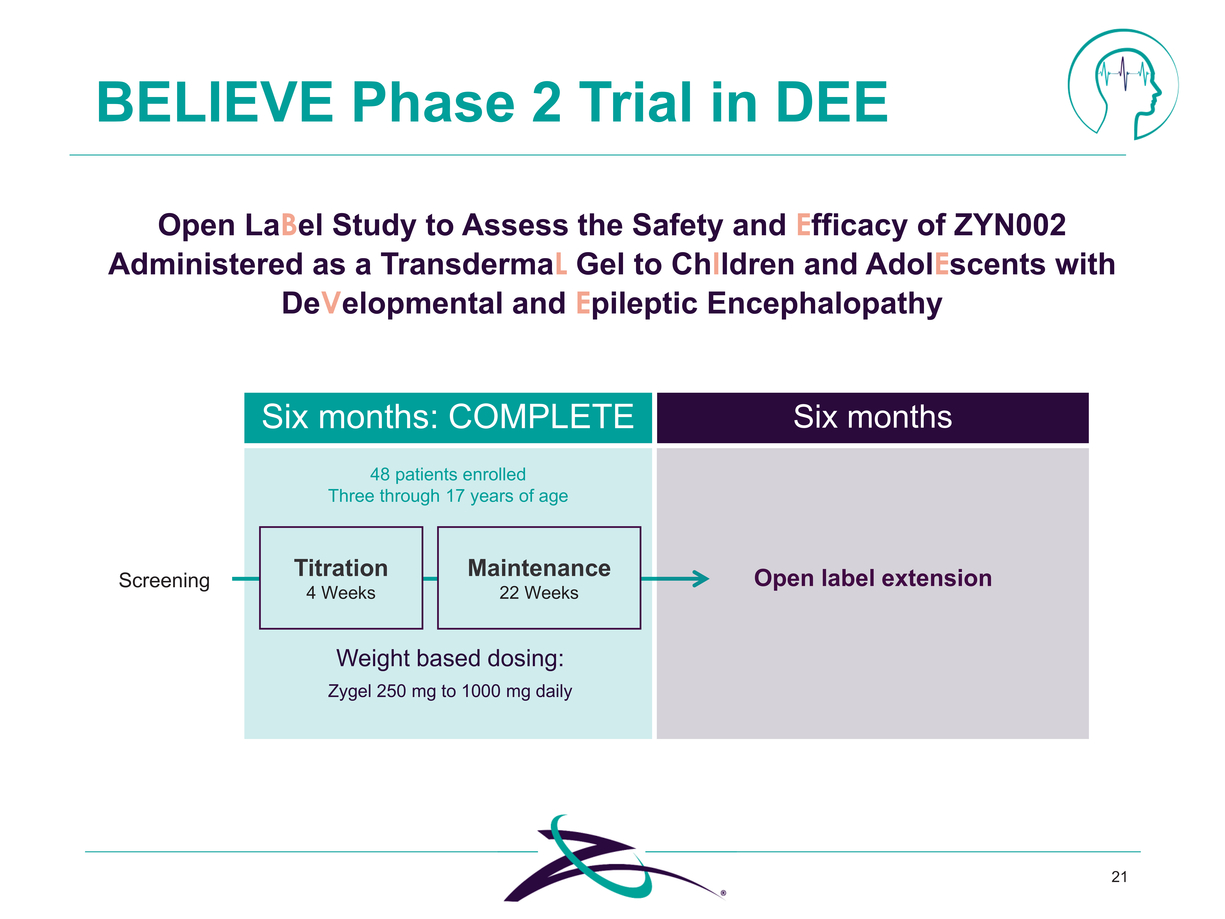 |
21 BELIEVE Phase 2 Trial in DEE Open LaBel Study to Assess the Safety and Efficacy of ZYN002 Administered as a TransdermaL Gel to ChIldren and AdolEscents with DeVelopmental and Epileptic Encephalopathy Maintenance 22 Weeks Titration 4 Weeks Six months: COMPLETE 48 patients enrolled Three through 17 years of age Six months Open label extension Weight based dosing: Zygel 250 mg to 1000 mg daily |
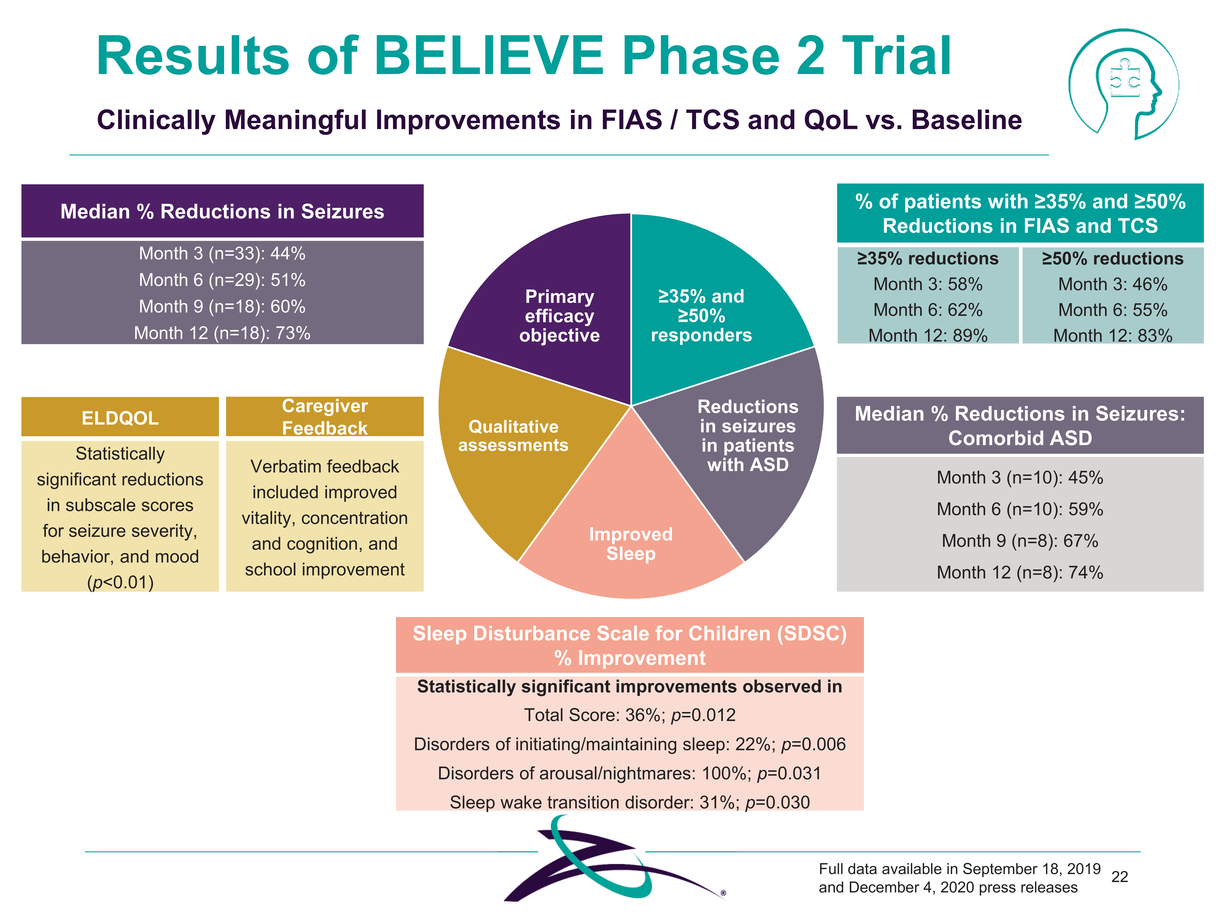 |
Sleep Disturbance Scale for Children (SDSC) % Improvement Statistically significant improvements observed in Total Score: 36%; p=0.012 Disorders of initiating/maintaining sleep: 22%; p=0.006 Disorders of arousal/nightmares: 100%; p=0.031 Sleep wake transition disorder: 31%; p=0.030 Full data available in September 18, 2019 22 and December 4, 2020 press releases Median % Reductions in Seizures Month 3 (n=33): 44% Month 6 (n=29): 51% Month 9 (n=18): 60% Month 12 (n=18): 73% ELDQOL Statistically significant reductions in subscale scores for seizure severity, behavior, and mood (p<0.01) Median % Reductions in Seizures: Comorbid ASD Month 3 (n=10): 45% Month 6 (n=10): 59% Month 9 (n=8): 67% Month 12 (n=8): 74% Results of BELIEVE Phase 2 Trial Clinically Meaningful Improvements in FIAS / TCS and QoL vs. Baseline Primary efficacy objective ≥35% and ≥50% responders Qualitative assessments Reductions in seizures in patients with ASD Improved Sleep % of patients with ≥35% and ≥50% Reductions in FIAS and TCS ≥35% reductions≥50% reductions Month 3: 58%Month 3: 46% Month 6: 62%Month 6: 55% Month 12: 89%Month 12: 83% Caregiver Feedback Verbatim feedback included improved vitality, concentration and cognition, and school improvement |
 |
23 Zygel Well Tolerated over 12 months: No Safety Signal Identified Tolerability profile consistent with the safety database for Zygel Most treatment-emergent adverse events (TEAEs) (any event, whether unrelated or related to study drug) were mild or moderate Two SAEs deemed possibly drug-related (LRTI and status epilepticus) No drug-related clinically significant changes in vital signs, ECGs, or laboratory findings |
 |
24 Clean balance sheet No debt, 41.3 M shares outstanding (as of March 5, 2020) Cash and cash equivalent position of $59.2M as of December 31, 2020 From January 1, 2021 to February 9, 2021 we raised net proceeds of $42.2 million through the sale of 10.2 million shares of equity through our “At The Market” sales agreement Cash runway expected to be sufficient to fund operations and capital requirements well into the first half of 2024 |
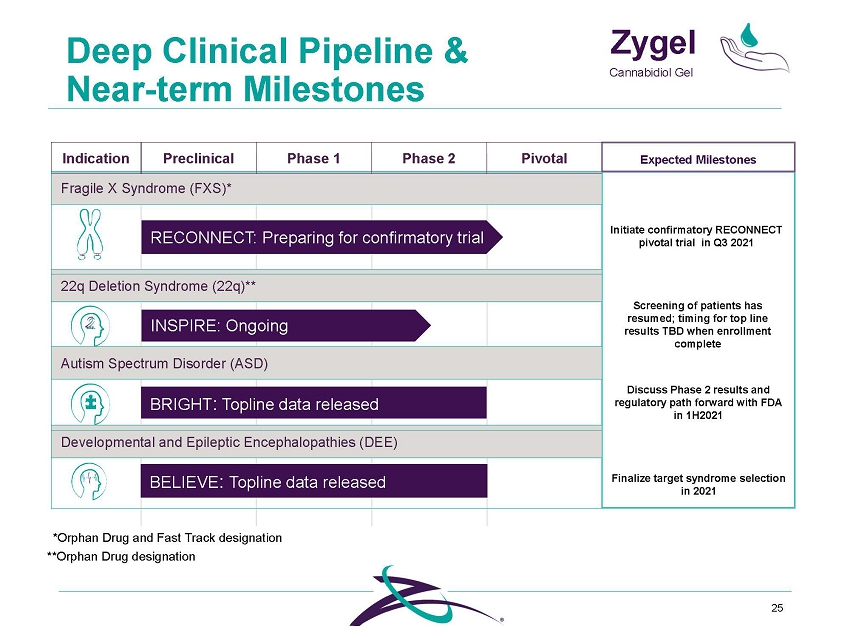 |
Deep Clinical Pipeline & Zygel Cannabidiol Gel **Orphan Drug designation 25 Indication Preclinical Phase 1 Phase 2 Pivotal Expected Milestones Fragile X Syndrome (FXS)* Initiate confirmatory RECONNECT pivotal trial in Q3 2021 Screening of patients has resumed; timing for top line results TBD when enrollment complete Discuss Phase 2 results and regulatory path forward with FDA in 1H2021 Finalize target syndrome selection in 2021 RECONNECT: P reparing for confir atory trial 22q Deletion Syndrome (22q)** INSPIRE: O going Autism Spectrum Disorder (ASD) Developmental and Epileptic Encephalopathies (DEE) m n BRIGHT: Topline data released BELIEVE: Topline data released *Orphan Drug and Fast Track designation |
 |
Corporate Overview May 2021 |
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Northfield Bancorp, Inc. Announces First Quarter 2024 Results
- Pulse Seismic Inc. Reports Q1 2024 Results and Increases Regular Dividend
- SHAREHOLDER ACTION ALERT: The Schall Law Firm Encourages Investors in Compass Minerals International, Inc. with Losses to Contact the Firm
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share