Form 8-K Taysha Gene Therapies, For: Jun 29
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): June 29, 2021
Taysha Gene Therapies, Inc.
(Exact name of registrant as specified in its Charter)
| Delaware | 001-39536 | 84-3199512 | ||
| (State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
| 3000 Pegasus Park Drive, Suite 1430 Dallas, Texas |
75247 | |
| (Address of Principal Executive Offices) | (Zip Code) |
(214) 612-0000
(Registrant’s Telephone Number, Including Area Code)
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instructions A.2. below):
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered | ||
| Common Stock, $0.00001 par value | TSHA | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 | Regulation FD Disclosure. |
On June 29, 2021, Taysha Gene Therapies, Inc. (the “Company”) will host the second day of a two-day virtual research and development day (the “R&D Day”) for analysts and investors to highlight the Company’s research and development progress, focused on advancement of its early- and late-stage investigational programs. A copy of the presentation, dated June 29, 2021, that the Company intends to use on the second day of the Company’s R&D Day is furnished as Exhibit 99.1 to this Current Report on Form 8-K.
The information in this Item 7.01 of this Current Report on 8-K (including Exhibit 99.1) is furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or subject to the liabilities of that section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended. The information shall not be deemed incorporated by reference into any other filing with the Securities and Exchange Commission made by the Company, whether made before or after today’s date, regardless of any general incorporation language in such filing, except as shall be expressly set forth by specific references in such filing.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits
| Exhibit |
Description | |
| 99.1 | Company presentation, dated June 29, 2021 | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Taysha Gene Therapies, Inc. | ||||||
| Dated: June 29, 2021 | By: | /s/ Kamran Alam | ||||
| Kamran Alam | ||||||
| Chief Financial Officer | ||||||

RESEARCH & DEVELOPMENT DAY DAY 2 – June 29, 2021 | 9:00 AM – 12:00 PM CT Bringing New Cures to Life Exhibit 99.1

Legal disclosure FORWARD LOOKING STATEMENTS This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements regarding our strategy, future operations, future financial position, future revenues, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements are subject to a number of risks, uncertainties and assumptions. Risks regarding our business are described in detail in our Securities and Exchange Commission filings, including in our Annual Report on Form 10-K for the year ended December 31, 2020. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. The forward-looking statements contained in this presentation reflect our current views with respect to future events, and we assume no obligation to update any forward-looking statements except as required by applicable law. This presentation includes statistical and other industry and market data that we obtained from industry publications and research, surveys and studies conducted by third parties as well as our own estimates of potential market opportunities. All of the market data used in this prospectus involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such data. Industry publications and third-party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. Our estimates of the potential market opportunities for our product candidates include several key assumptions based on our industry knowledge, industry publications, third-party research and other surveys, which may be based on a small sample size and may fail to accurately reflect market opportunities. While we believe that our internal assumptions are reasonable, no independent source has verified such assumptions.

Introductions RA Session II President, Founder & CEO

TSHA-104 for SURF1-Associated Leigh Syndrome TSHA-104 SURF1 deficiency Steven Gray, PhD Chief Scientific Advisor, UTSW Gene Therapy Program Suyash Prasad, MBBS, MSc, MRCP, MRCPCH, FFPM Chief Medical Officer and Head of R&D
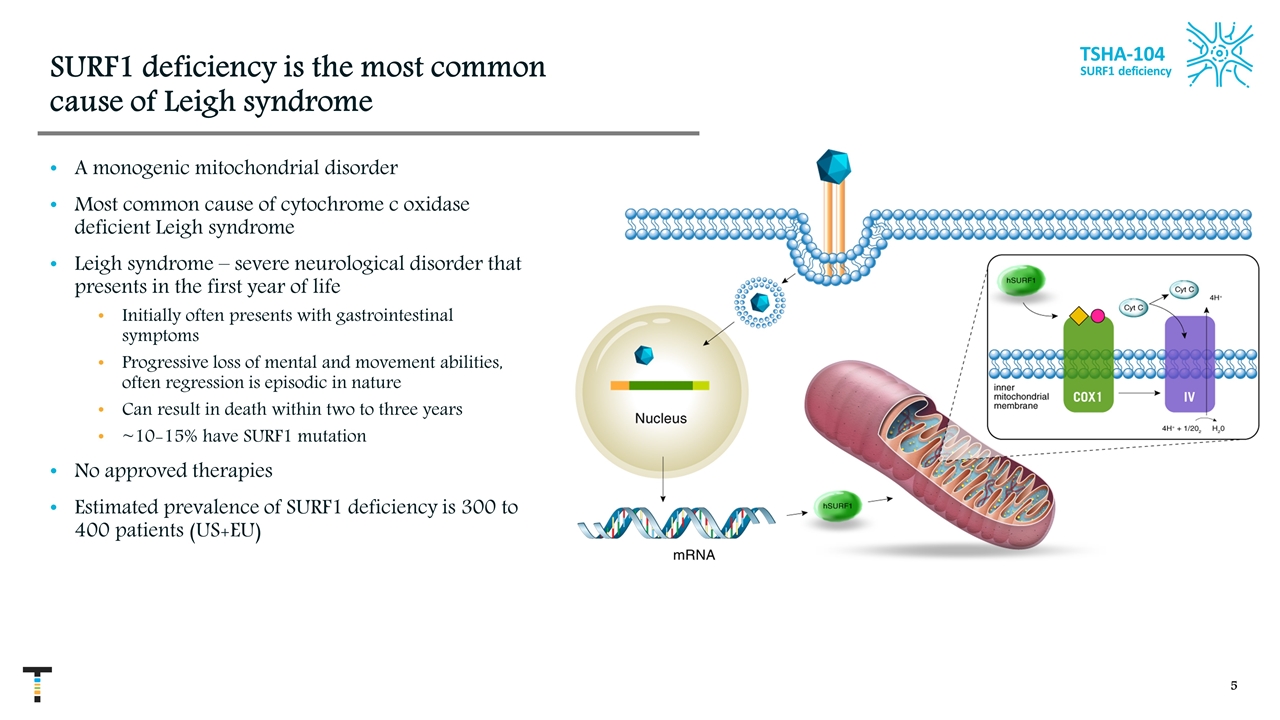
TSHA-104 SURF1 deficiency SURF1 deficiency is the most common cause of Leigh syndrome A monogenic mitochondrial disorder Most common cause of cytochrome c oxidase deficient Leigh syndrome Leigh syndrome – severe neurological disorder that presents in the first year of life Initially often presents with gastrointestinal symptoms Progressive loss of mental and movement abilities, often regression is episodic in nature Can result in death within two to three years ~10-15% have SURF1 mutation No approved therapies Estimated prevalence of SURF1 deficiency is 300 to 400 patients (US+EU)
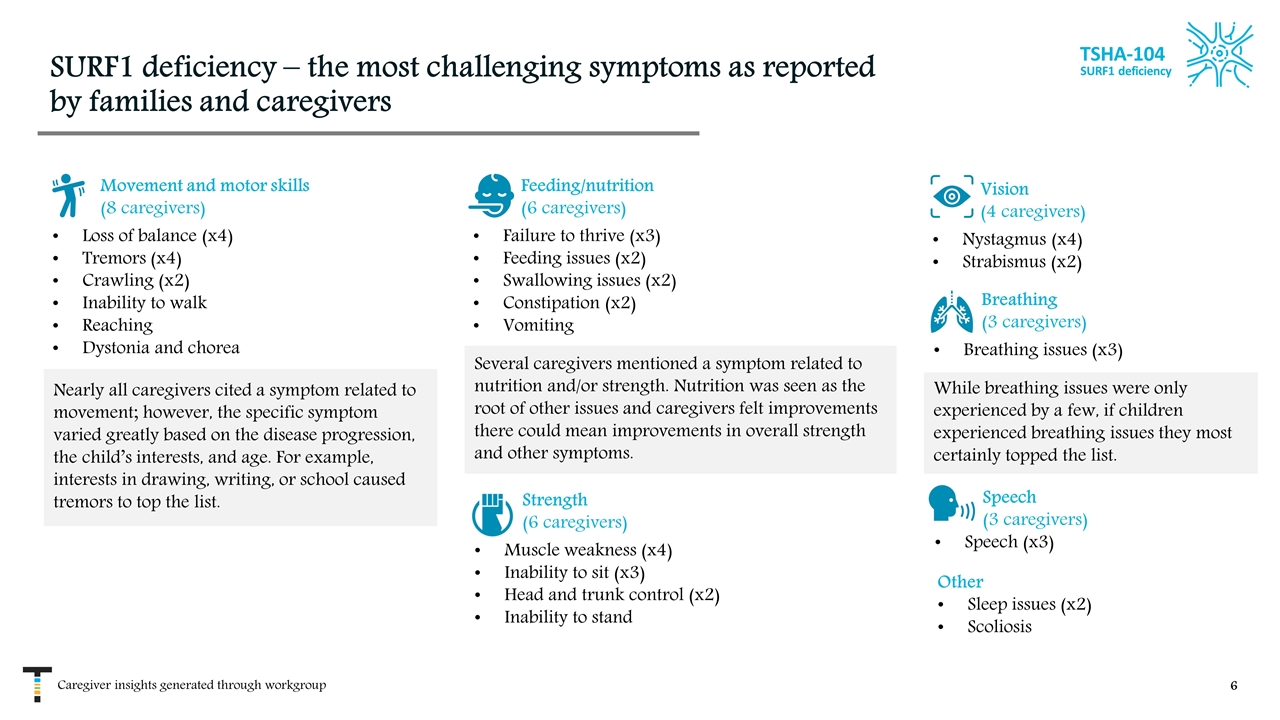
SURF1 deficiency – the most challenging symptoms as reported by families and caregivers Breathing (3 caregivers) Breathing issues (x3) Strength (6 caregivers) Muscle weakness (x4) Inability to sit (x3) Head and trunk control (x2) Inability to stand Movement and motor skills (8 caregivers) Loss of balance (x4) Tremors (x4) Crawling (x2) Inability to walk Reaching Dystonia and chorea Feeding/nutrition (6 caregivers) Failure to thrive (x3) Feeding issues (x2) Swallowing issues (x2) Constipation (x2) Vomiting Vision (4 caregivers) Nystagmus (x4) Strabismus (x2) Speech (3 caregivers) Speech (x3) Other Sleep issues (x2) Scoliosis Nearly all caregivers cited a symptom related to movement; however, the specific symptom varied greatly based on the disease progression, the child’s interests, and age. For example, interests in drawing, writing, or school caused tremors to top the list. Several caregivers mentioned a symptom related to nutrition and/or strength. Nutrition was seen as the root of other issues and caregivers felt improvements there could mean improvements in overall strength and other symptoms. While breathing issues were only experienced by a few, if children experienced breathing issues they most certainly topped the list. Caregiver insights generated through workgroup TSHA-104 SURF1 deficiency
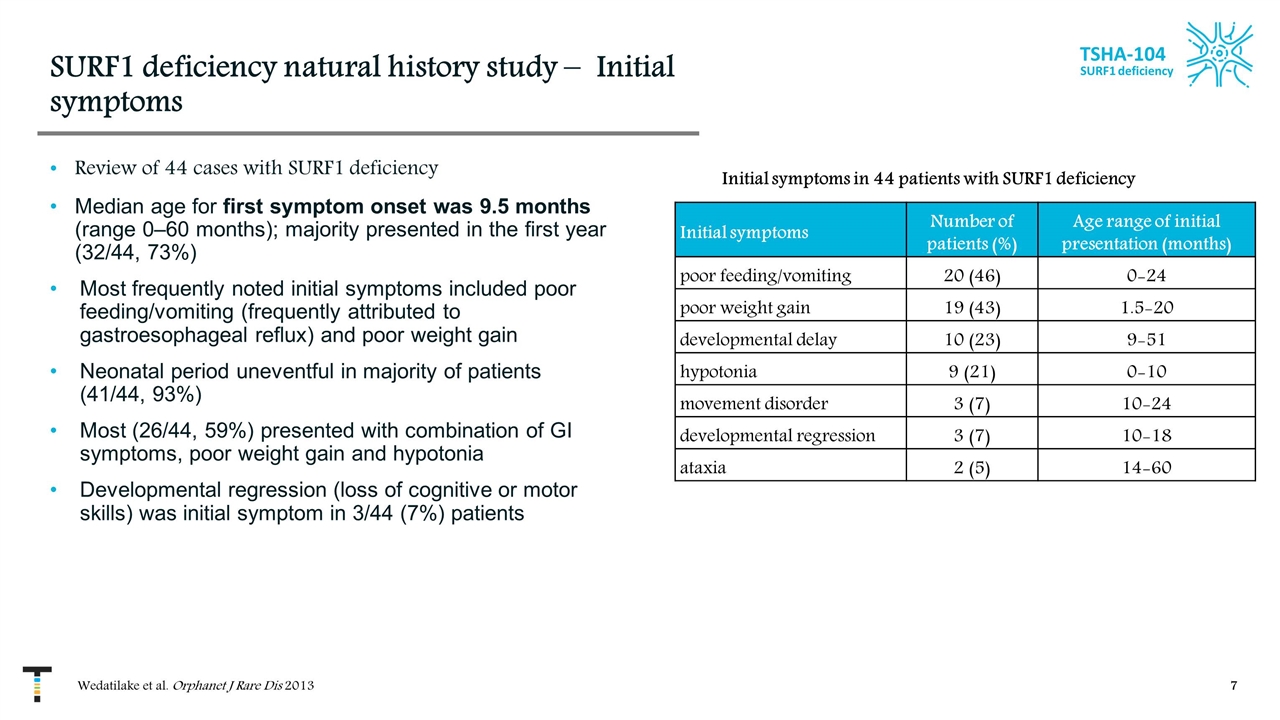
SURF1 deficiency natural history study – Initial symptoms Review of 44 cases with SURF1 deficiency Median age for first symptom onset was 9.5 months (range 0–60 months); majority presented in the first year (32/44, 73%) Most frequently noted initial symptoms included poor feeding/vomiting (frequently attributed to gastroesophageal reflux) and poor weight gain Neonatal period uneventful in majority of patients (41/44, 93%) Most (26/44, 59%) presented with combination of GI symptoms, poor weight gain and hypotonia Developmental regression (loss of cognitive or motor skills) was initial symptom in 3/44 (7%) patients Wedatilake et al. Orphanet J Rare Dis 2013 TSHA-104 SURF1 deficiency Initial symptoms Number of patients (%) Age range of initial presentation (months) poor feeding/vomiting 20 (46) 0-24 poor weight gain 19 (43) 1.5-20 developmental delay 10 (23) 9-51 hypotonia 9 (21) 0-10 movement disorder 3 (7) 10-24 developmental regression 3 (7) 10-18 ataxia 2 (5) 14-60 Initial symptoms in 44 patients with SURF1 deficiency
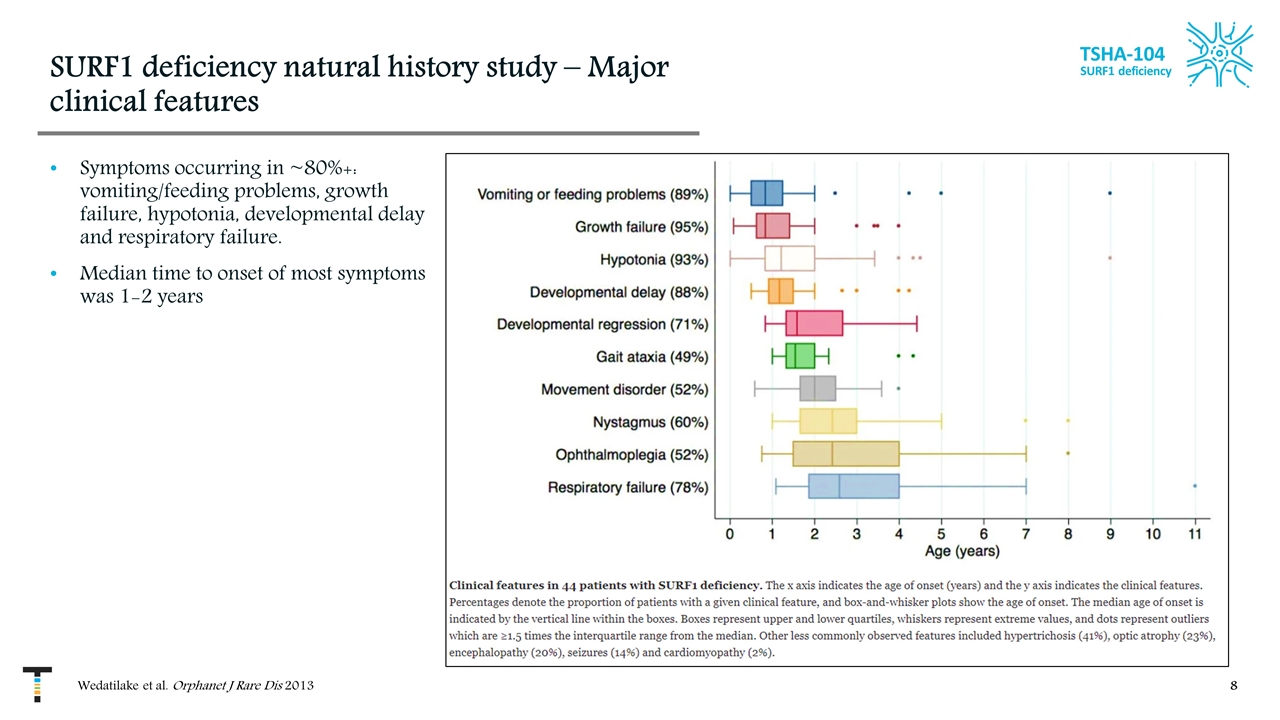
SURF1 deficiency natural history study – Major clinical features Symptoms occurring in ~80%+: vomiting/feeding problems, growth failure, hypotonia, developmental delay and respiratory failure. Median time to onset of most symptoms was 1-2 years TSHA-104 SURF1 deficiency Wedatilake et al. Orphanet J Rare Dis 2013
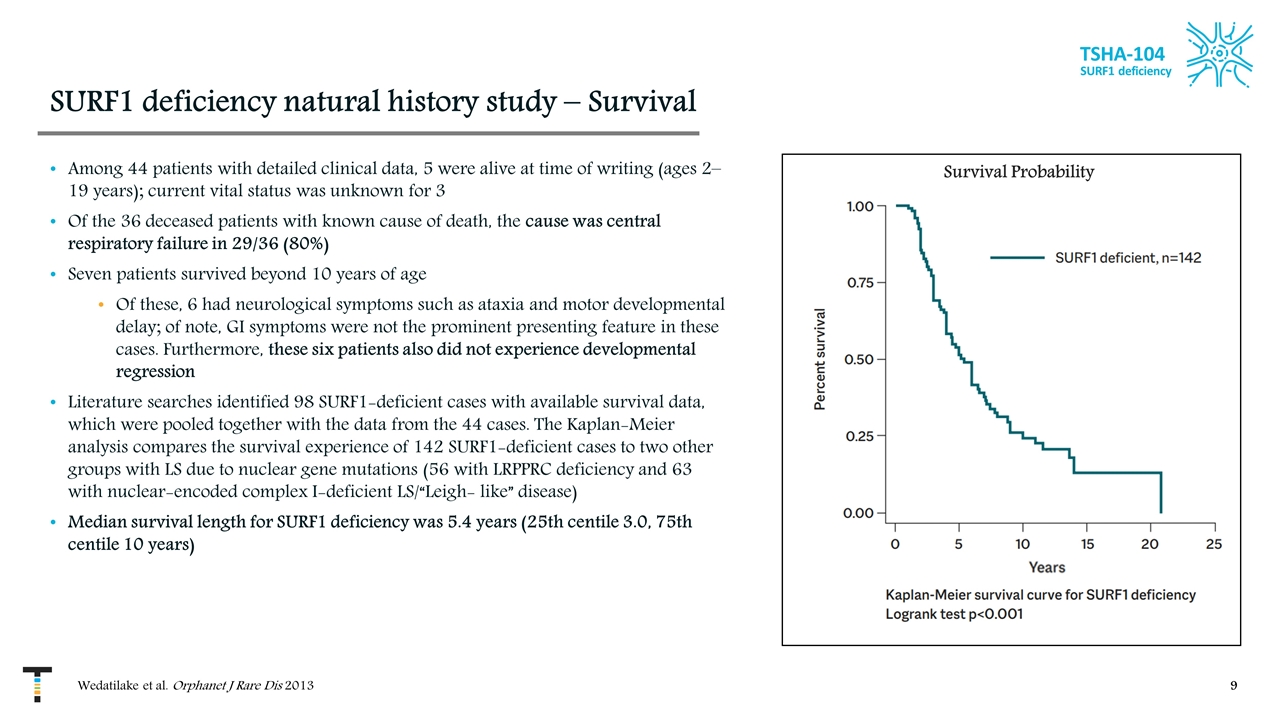
SURF1 deficiency natural history study – Survival Among 44 patients with detailed clinical data, 5 were alive at time of writing (ages 2–19 years); current vital status was unknown for 3 Of the 36 deceased patients with known cause of death, the cause was central respiratory failure in 29/36 (80%) Seven patients survived beyond 10 years of age Of these, 6 had neurological symptoms such as ataxia and motor developmental delay; of note, GI symptoms were not the prominent presenting feature in these cases. Furthermore, these six patients also did not experience developmental regression Literature searches identified 98 SURF1-deficient cases with available survival data, which were pooled together with the data from the 44 cases. The Kaplan-Meier analysis compares the survival experience of 142 SURF1-deficient cases to two other groups with LS due to nuclear gene mutations (56 with LRPPRC deficiency and 63 with nuclear-encoded complex I-deficient LS/“Leigh- like” disease) Median survival length for SURF1 deficiency was 5.4 years (25th centile 3.0, 75th centile 10 years) TSHA-104 SURF1 deficiency Wedatilake et al. Orphanet J Rare Dis 2013 Survival Probability
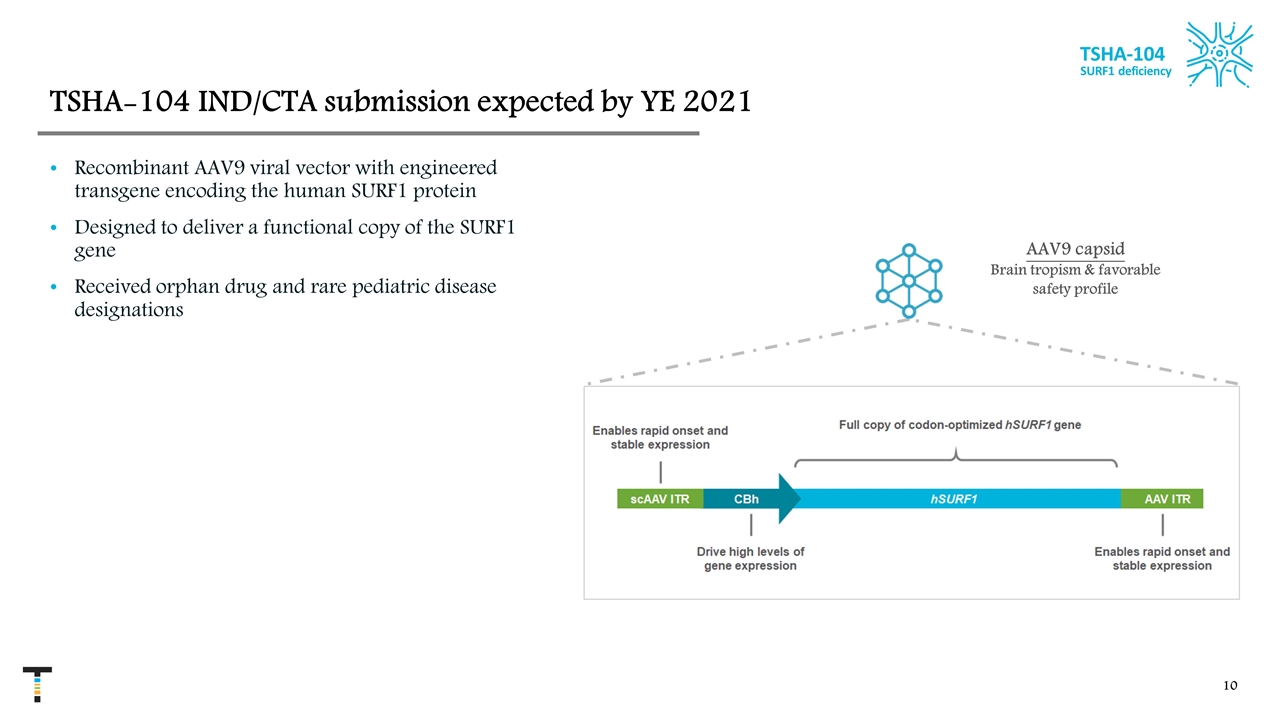
AAV9 capsid Brain tropism & favorable safety profile TSHA-104 IND/CTA submission expected by YE 2021 Recombinant AAV9 viral vector with engineered transgene encoding the human SURF1 protein Designed to deliver a functional copy of the SURF1 gene Received orphan drug and rare pediatric disease designations TSHA-104 SURF1 deficiency

Slight increase in COX1 activity significantly improved clinical phenotype Of 17 samples, 14 were assayed 8 Leigh syndrome & 6 healthy donors (hets) Severely affected individuals had roughly 50% of normal activity Mildly affected individuals had roughly 58% of normal activity A relatively small difference in activity could have significant clinical consequences Other studies measuring COX activity have shown roughly 20% of normal activity for affected individuals Potential reason – “healthy” donors are heterozygote parents of affected children; this and their age may affect their COX activity. In other studies, healthy children were used for reference TSHA-104 SURF1 deficiency Healthy (Het)
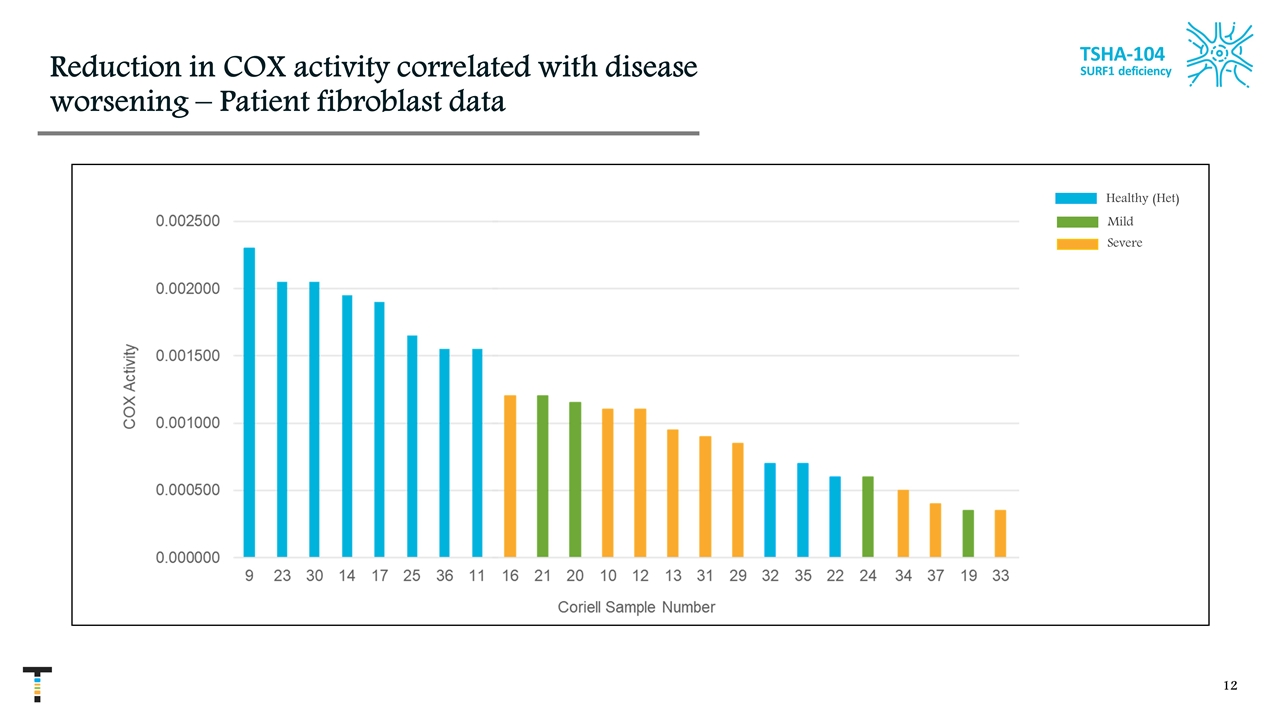
Reduction in COX activity correlated with disease worsening – Patient fibroblast data TSHA-104 SURF1 deficiency Mild Healthy (Het) Severe
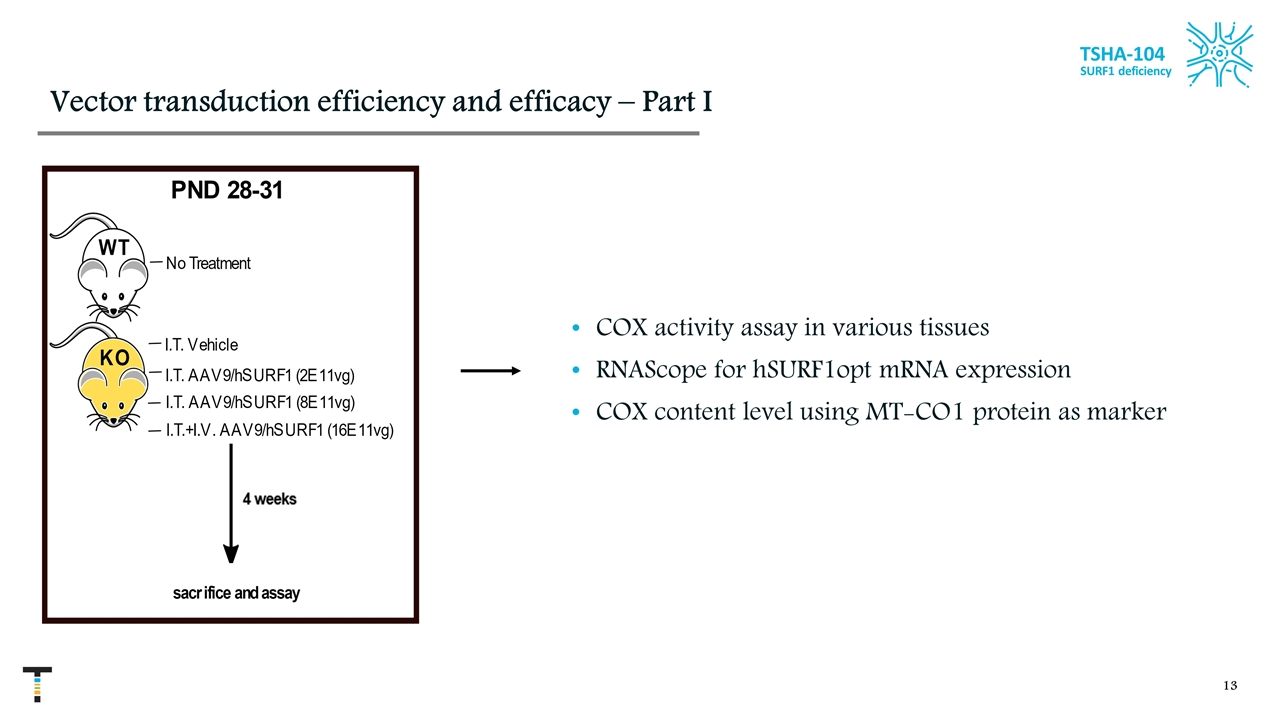
Vector transduction efficiency and efficacy – Part I COX activity assay in various tissues RNAScope for hSURF1opt mRNA expression COX content level using MT-CO1 protein as marker TSHA-104 SURF1 deficiency
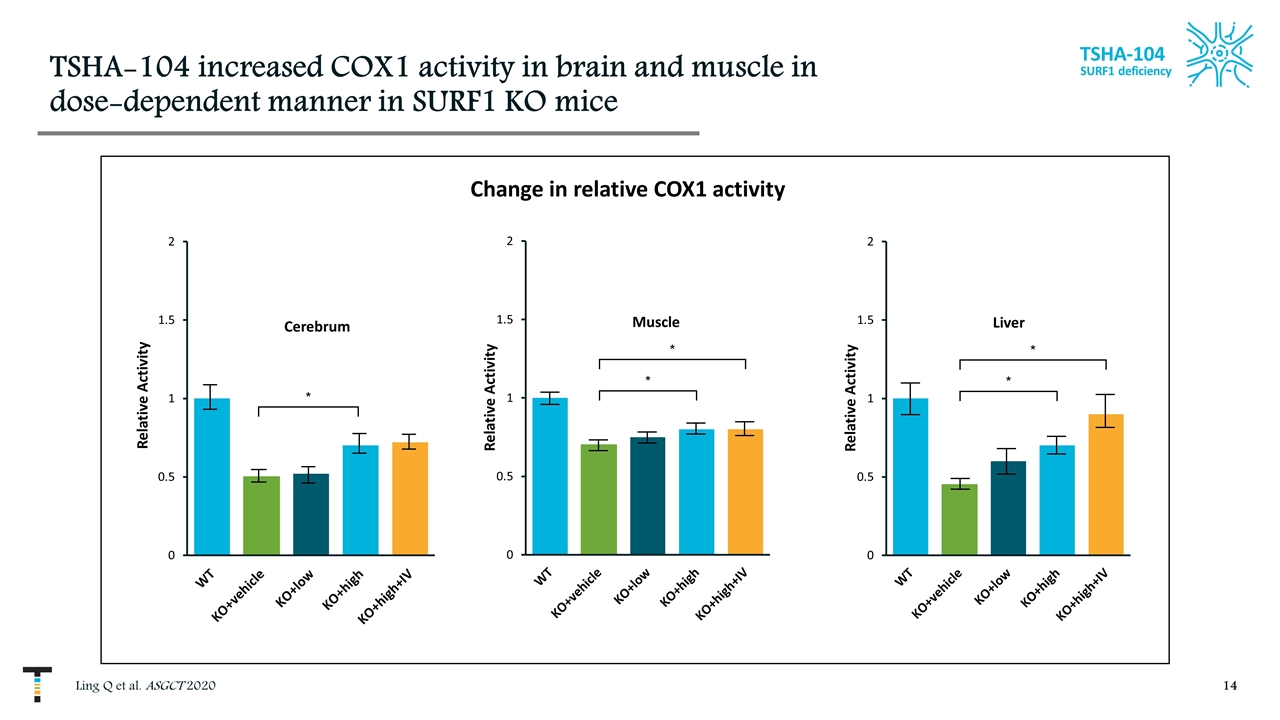
TSHA-104 increased COX1 activity in brain and muscle in dose-dependent manner in SURF1 KO mice Ling Q et al. ASGCT 2020 TSHA-104 SURF1 deficiency * * Relative Activity * Relative Activity Change in relative COX1 activity * * Relative Activity
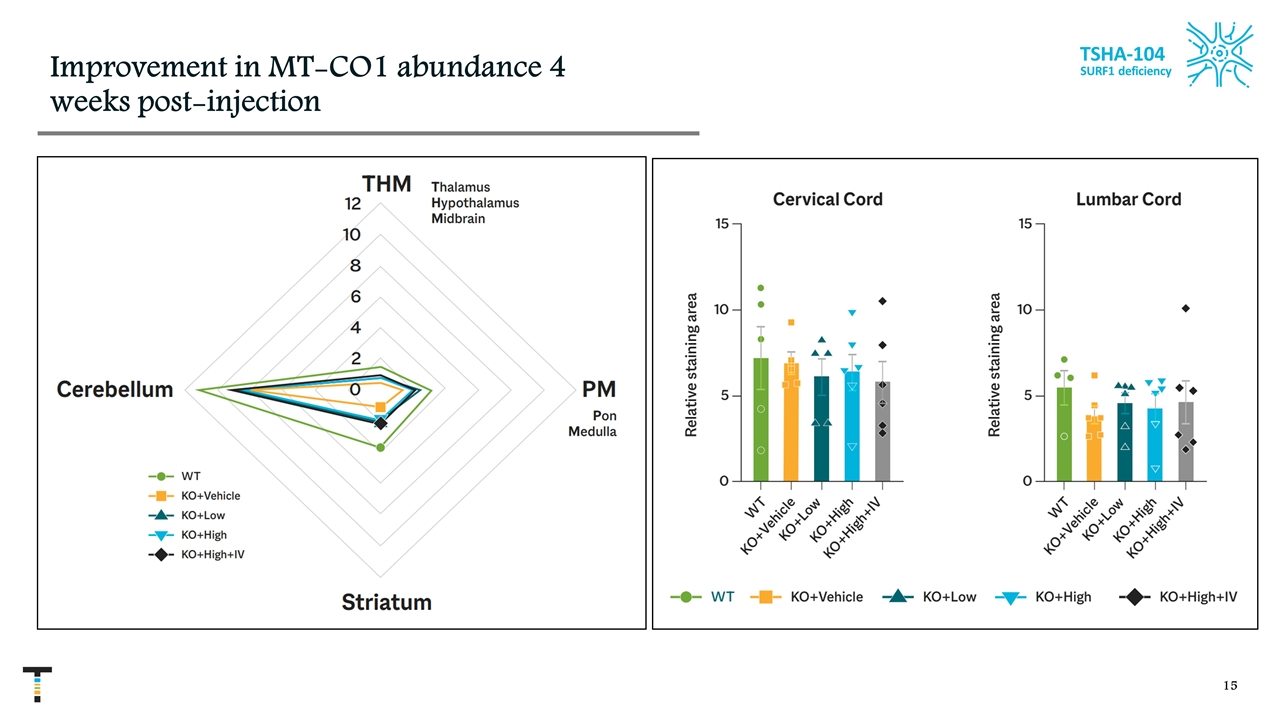
Improvement in MT-CO1 abundance 4 weeks post-injection TSHA-104 SURF1 deficiency
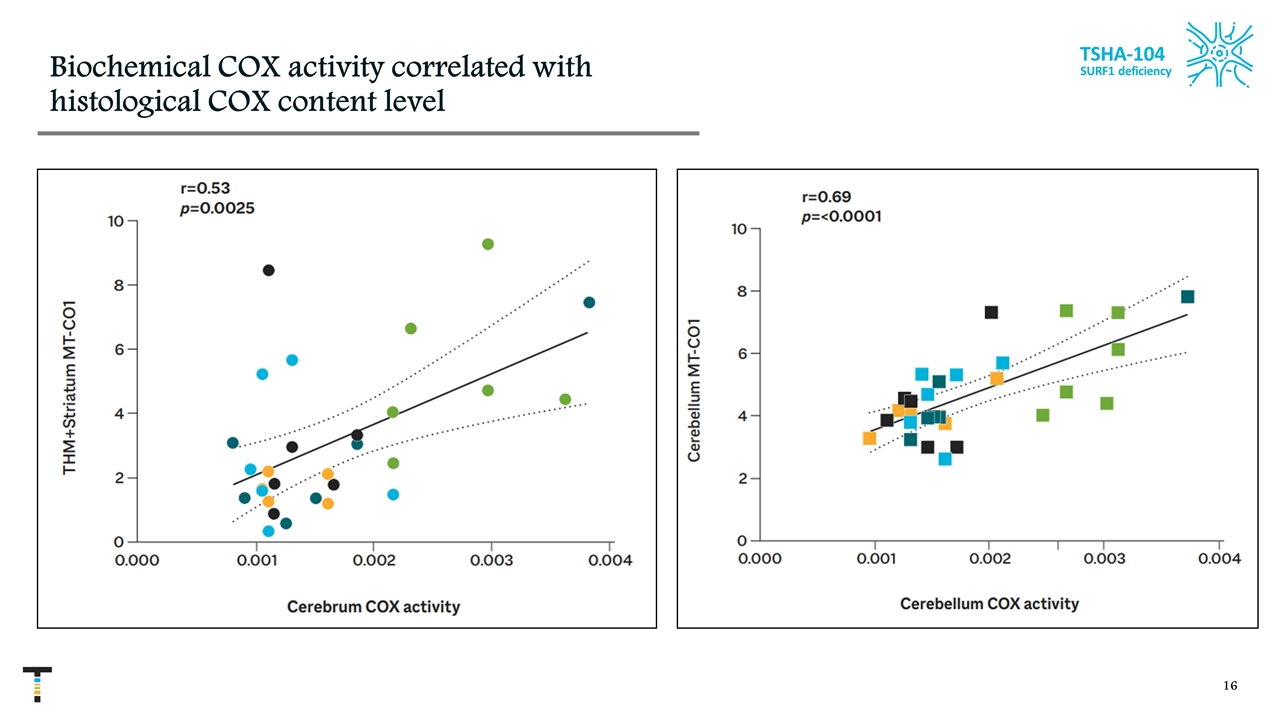
Biochemical COX activity correlated with histological COX content level TSHA-104 SURF1 deficiency
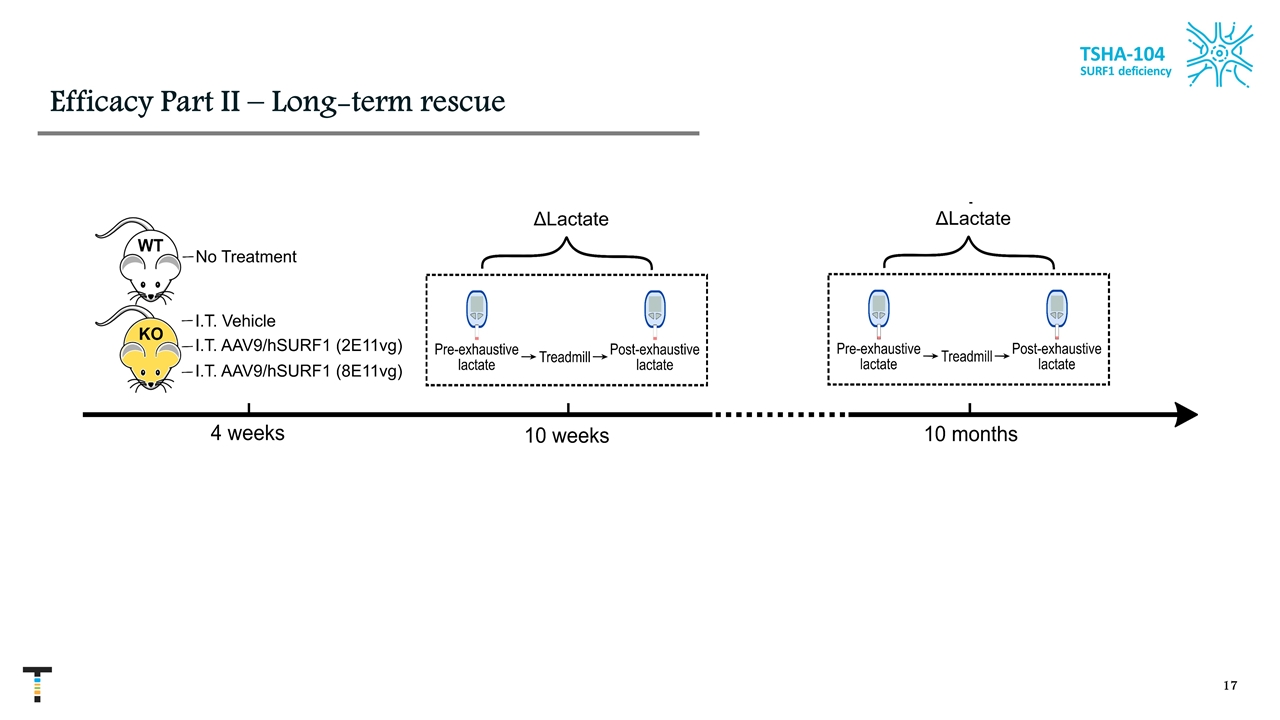
Efficacy Part II – Long-term rescue TSHA-104 SURF1 deficiency
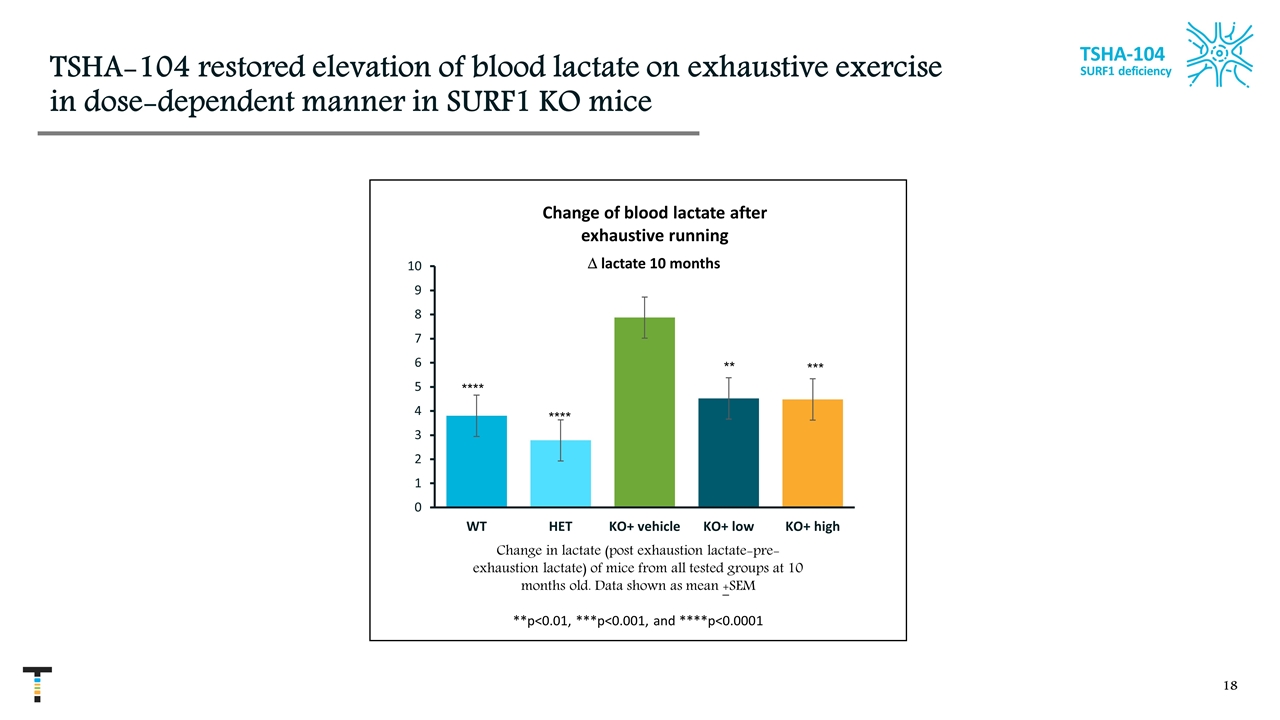
TSHA-104 restored elevation of blood lactate on exhaustive exercise in dose-dependent manner in SURF1 KO mice D lactate 10 months Change in lactate (post exhaustion lactate-pre-exhaustion lactate) of mice from all tested groups at 10 months old. Data shown as mean +SEM **p<0.01, ***p<0.001, and ****p<0.0001 **** **** ** *** TSHA-104 SURF1 deficiency
Non-GLP Toxicology in Mice No significant differences detected in viral vector-treated animals 4 weeks post-dosing No severe damages detected in tissue integrity 12 months post-injection, some mild abnormalities mostly due to aging Summary Efficacy in SURF1 KO Mice Effectively induced mRNA expression of hSURF1opt in various brain regions and spinal cord Diminished exhaustive exercise-induced lactic acidosis 9 months post-dosing Partially restored COX content in varies brain regions Partially restored COX activity in brain, liver and muscle IT administration demonstrated benefit GLP Toxicology in Rats Ongoing IND-enabling toxicology study in normal rats TSHA-104 SURF1 deficiency
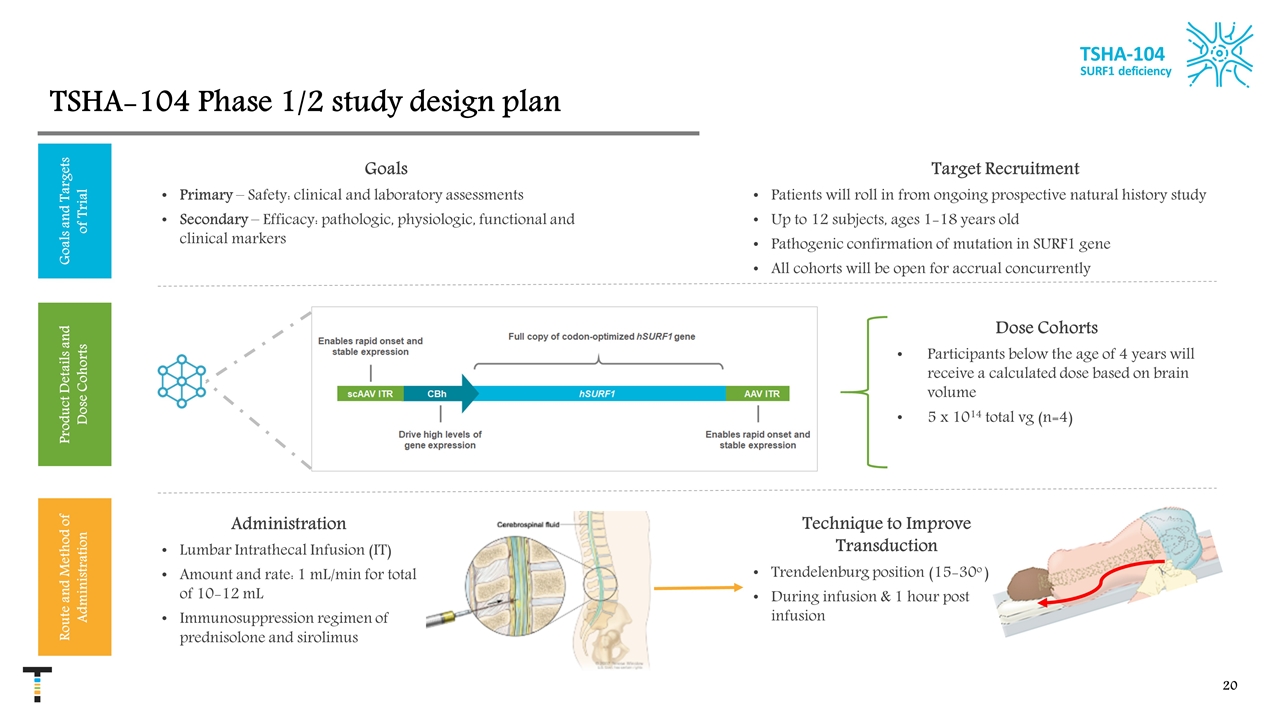
TSHA-104 Phase 1/2 study design plan TSHA-104 SURF1 deficiency Goals and Targets of Trial Product Details and Dose Cohorts Route and Method of Administration Dose Cohorts Participants below the age of 4 years will receive a calculated dose based on brain volume 5 x 1014 total vg (n=4) Goals Primary – Safety: clinical and laboratory assessments Secondary – Efficacy: pathologic, physiologic, functional and clinical markers Target Recruitment Patients will roll in from ongoing prospective natural history study Up to 12 subjects, ages 1-18 years old Pathogenic confirmation of mutation in SURF1 gene All cohorts will be open for accrual concurrently Technique to Improve Transduction Trendelenburg position (15-30o ) During infusion & 1 hour post infusion Administration Lumbar Intrathecal Infusion (IT) Amount and rate: 1 mL/min for total of 10-12 mL Immunosuppression regimen of prednisolone and sirolimus
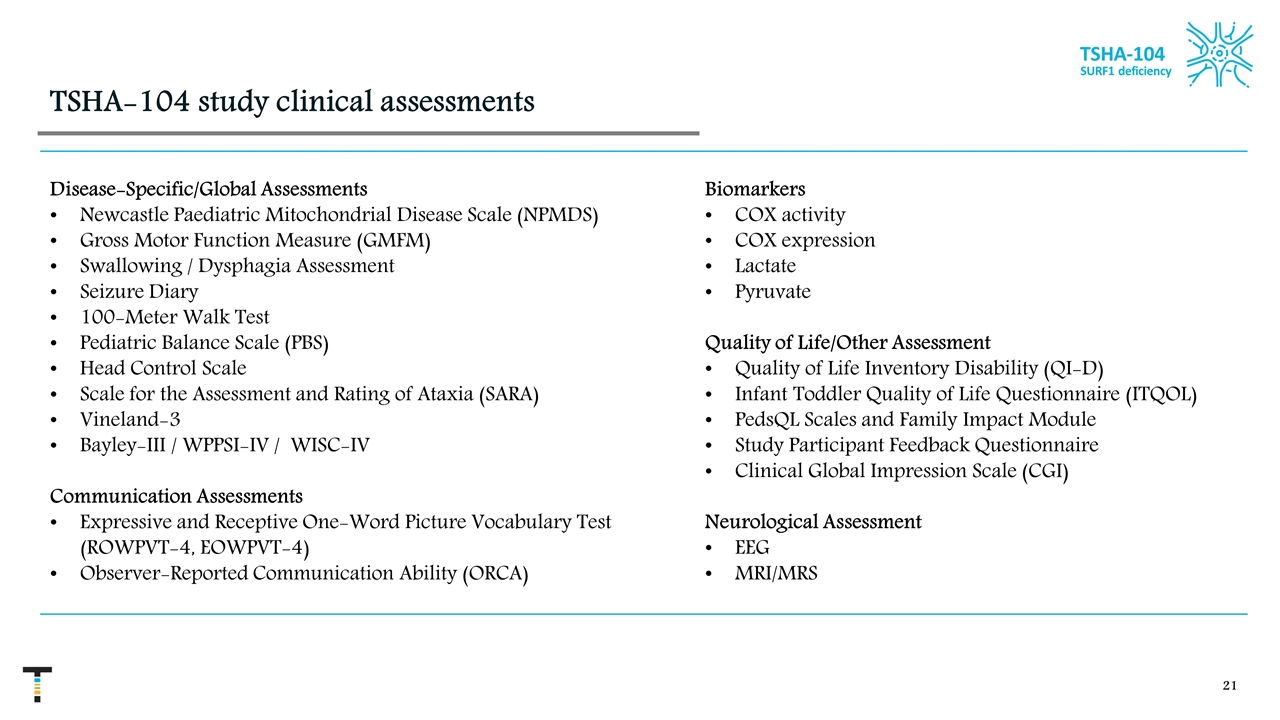
TSHA-104 study clinical assessments Disease-Specific/Global Assessments Newcastle Paediatric Mitochondrial Disease Scale (NPMDS) Gross Motor Function Measure (GMFM) Swallowing / Dysphagia Assessment Seizure Diary 100-Meter Walk Test Pediatric Balance Scale (PBS) Head Control Scale Scale for the Assessment and Rating of Ataxia (SARA) Vineland-3 Bayley-III / WPPSI-IV / WISC-IV Communication Assessments Expressive and Receptive One-Word Picture Vocabulary Test (ROWPVT-4, EOWPVT-4) Observer-Reported Communication Ability (ORCA) Biomarkers COX activity COX expression Lactate Pyruvate Quality of Life/Other Assessment Quality of Life Inventory Disability (QI-D) Infant Toddler Quality of Life Questionnaire (ITQOL) PedsQL Scales and Family Impact Module Study Participant Feedback Questionnaire Clinical Global Impression Scale (CGI) Neurological Assessment EEG MRI/MRS TSHA-104 SURF1 deficiency

Anticipated next steps for TSHA-104 TSHA-104 SURF1 deficiency Complete GMP manufacturing using commercial process Submit IND/CTA in 2H 2021 Initiate Phase 1/2 interventional study by YE 2021 Continue enrollment in natural history study Complete IND-enabling toxicology study

Q & A TSHA-104 SURF1 deficiency

TSHA-105 for SLC13A5 Deficiency TSHA-105 SLC13A5 deficiency Rachel Bailey, PhD UTSW Gene Therapy Program Suyash Prasad, MBBS, MSc, MRCP, MRCPCH, FFPM Chief Medical Officer and Head of R&D
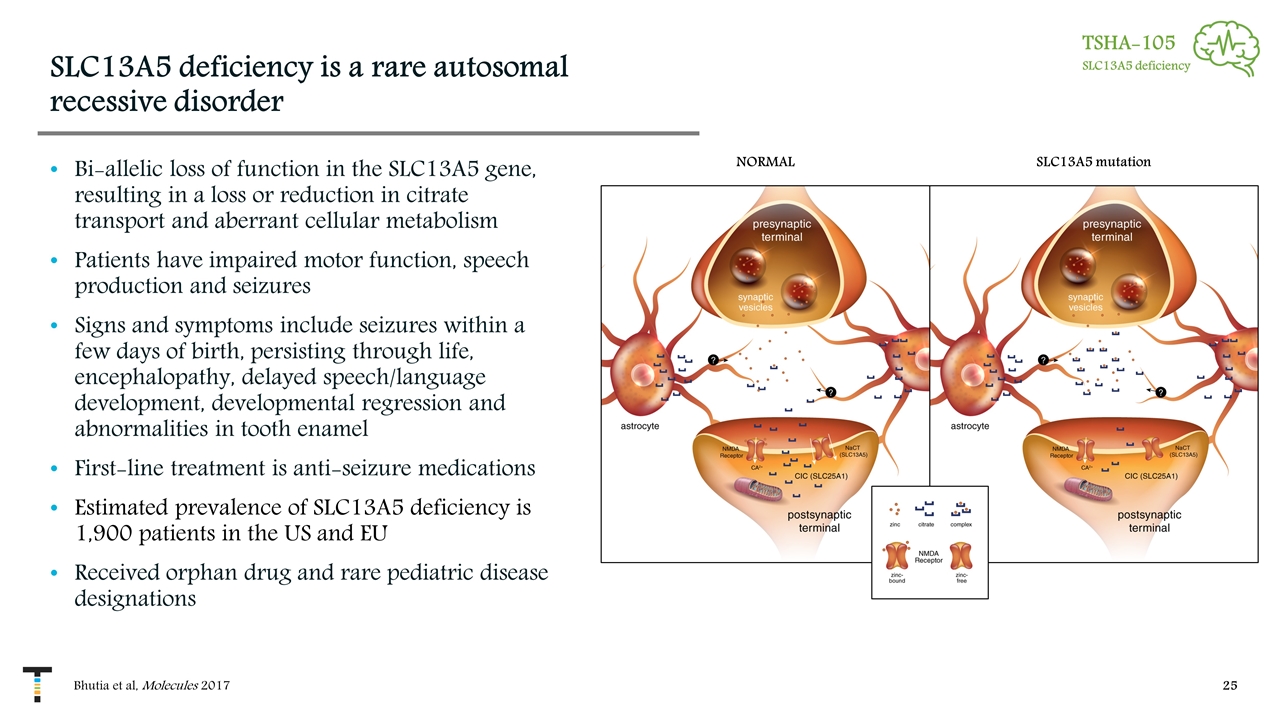
SLC13A5 deficiency is a rare autosomal recessive disorder Bi-allelic loss of function in the SLC13A5 gene, resulting in a loss or reduction in citrate transport and aberrant cellular metabolism Patients have impaired motor function, speech production and seizures Signs and symptoms include seizures within a few days of birth, persisting through life, encephalopathy, delayed speech/language development, developmental regression and abnormalities in tooth enamel First-line treatment is anti-seizure medications Estimated prevalence of SLC13A5 deficiency is 1,900 patients in the US and EU Received orphan drug and rare pediatric disease designations Bhutia et al, Molecules 2017 TSHA-105 SLC13A5 deficiency NORMAL SLC13A5 mutation
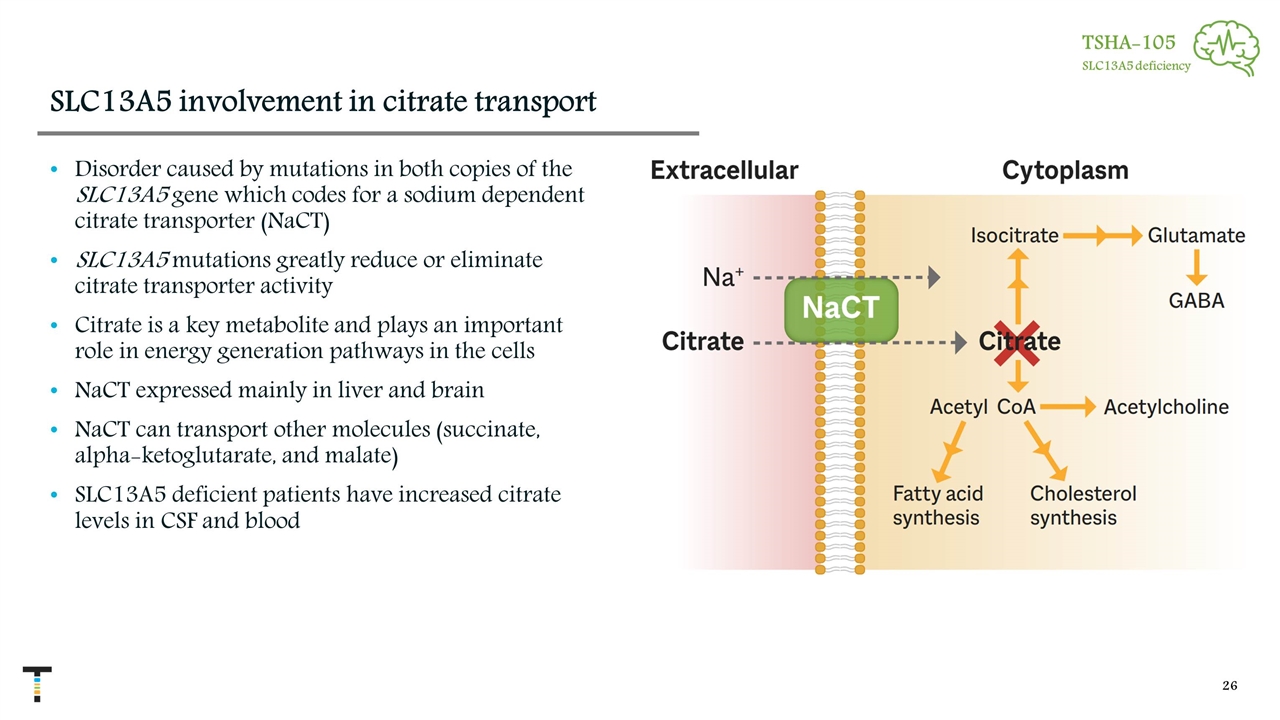
SLC13A5 involvement in citrate transport Disorder caused by mutations in both copies of the SLC13A5 gene which codes for a sodium dependent citrate transporter (NaCT) SLC13A5 mutations greatly reduce or eliminate citrate transporter activity Citrate is a key metabolite and plays an important role in energy generation pathways in the cells NaCT expressed mainly in liver and brain NaCT can transport other molecules (succinate, alpha-ketoglutarate, and malate) SLC13A5 deficient patients have increased citrate levels in CSF and blood TSHA-105 SLC13A5 deficiency
SLC13A5 deficiency results in persistent seizures and developmental delays Seizures Affected children present with seizures within a few days of birth which persist throughout life Multiple seizure types; Anti-epileptic drugs (phenobarbital, valproate, and acetazolamide) have varying success in controlling seizures Refractory to medication Children can succumb to complications of the seizures Movement Disorder Low muscle tone (hypotonia) and a lack of muscle control or coordination of voluntary movements, such as walking or picking up an object (ataxia) Episodes of body stiffening or weakness (minutes to a few hours) Dystonia & chorea TSHA-105 SLC13A5 deficiency Yang et al. Child Neurology Open 2020 Developmental Delay Intellectual disability and global developmental delay Limited to no speech production, but have receptive language Limited and slow motor progress with problems standing or walking independently Biomarkers Nearly all children have abnormal tooth enamel Brain MRIs appear normal or have subtle changes in the white matter EEGs have a relatively well-preserved background for age, even in the face of frequent seizures Elevated citrate levels in blood, urine and CSF No currently approved therapies Patients require constant supervision and care

TSHA-105 currently in IND/CTA-enabling studies Self-complementary AAV9 expressing human SLC13A5 protein under the control of a ubiquitous promoter Delivered intrathecally Currently in IND/CTA-enabling studies TSHA-105 SLC13A5 deficiency AAV9 capsid CNS tropism & favorable safety profile scAAV ITR Codon-optimized hSLC13A5 UsP AAV ITR Drives ubiquitous expression Enables rapid onset and stable expression Full copy of codon-optimized hSLC13A5
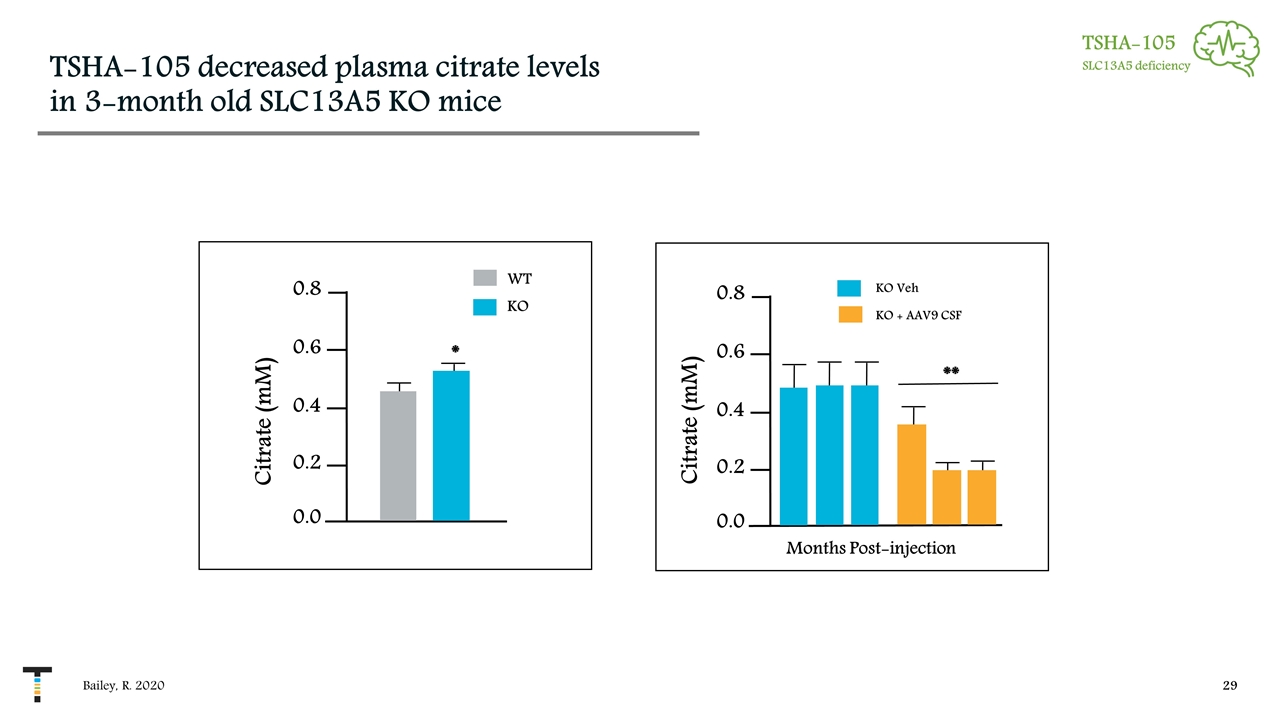
TSHA-105 decreased plasma citrate levels in 3-month old SLC13A5 KO mice Bailey, R. 2020 TSHA-105 SLC13A5 deficiency 0.0 0.6 0.8 0.4 0.2 WT KO Citrate (mM) * KO + AAV9 CSF KO Veh ** Months Post-injection 0.0 0.6 0.8 0.4 0.2 Citrate (mM)
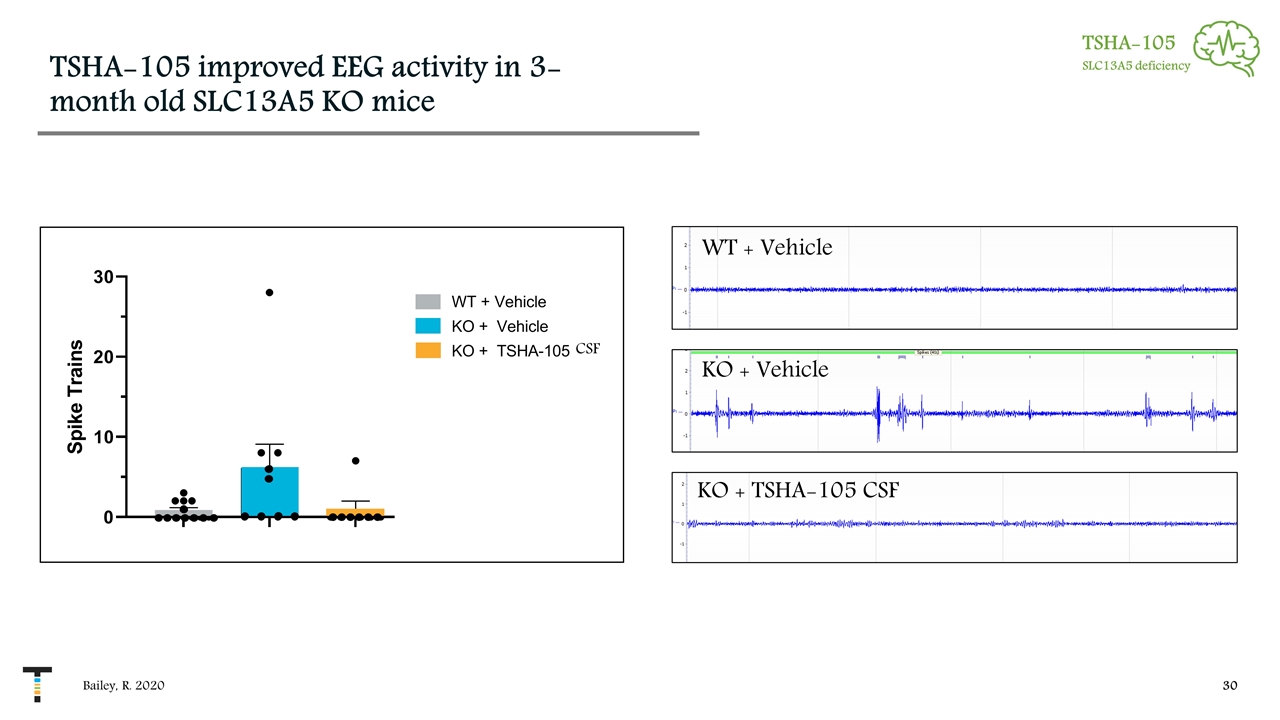
TSHA-105 improved EEG activity in 3-month old SLC13A5 KO mice TSHA-105 SLC13A5 deficiency Bailey, R. 2020 WT + Vehicle KO + Vehicle KO + TSHA-105 CSF CSF Dark Cycle
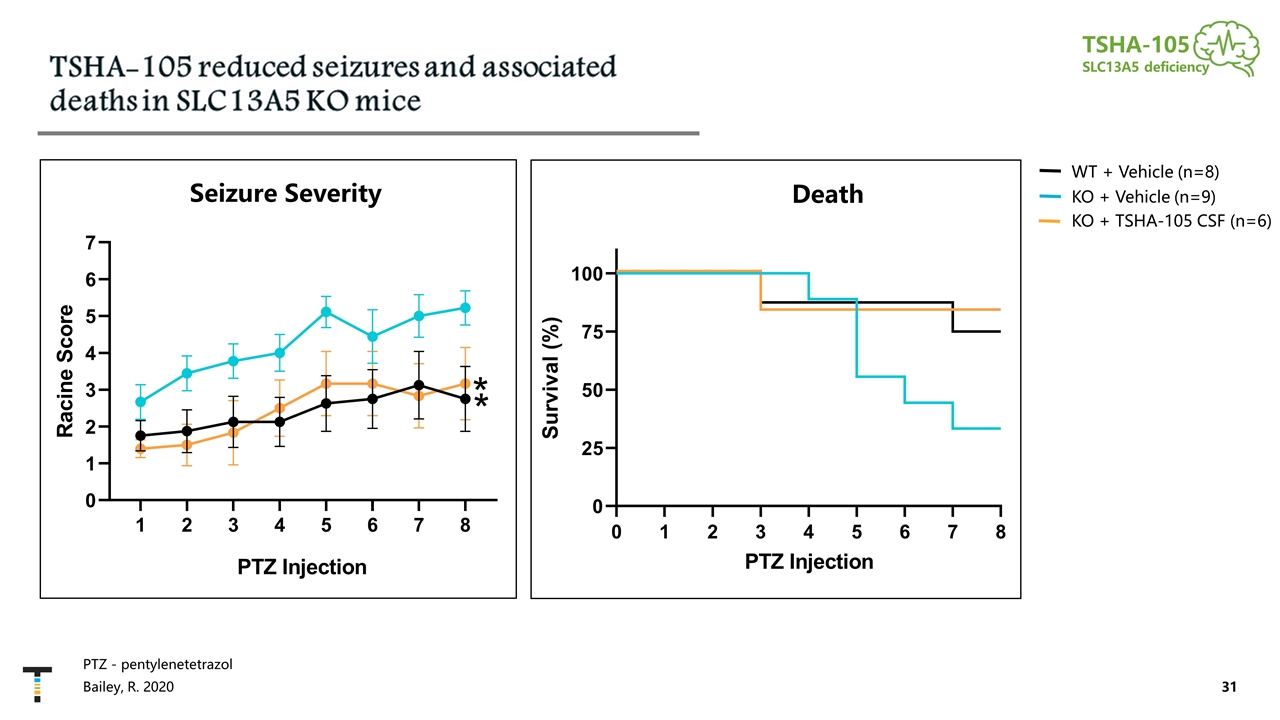
PTZ - pentylenetetrazol TSHA-105 SLC13A5 deficiency Bailey, R. 2020 Seizure Severity Death KO + Vehicle (n=9) KO + TSHA-105 CSF (n=6) WT + Vehicle (n=8)
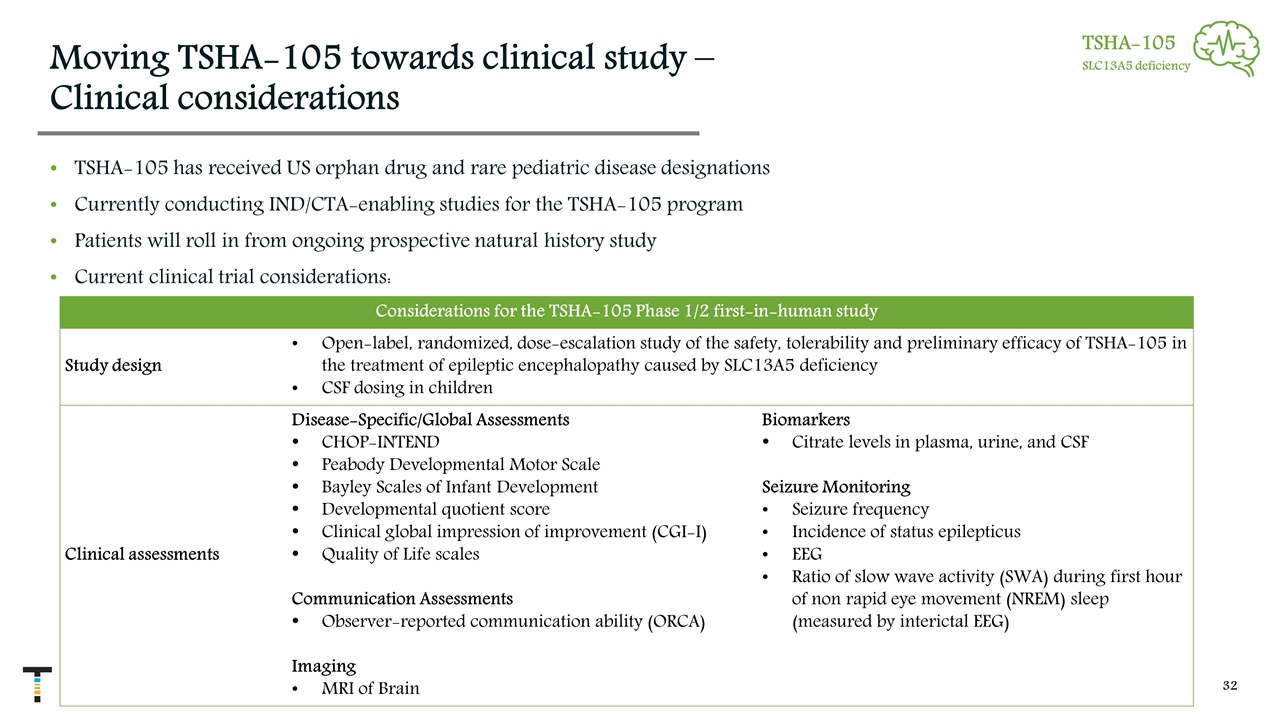
Moving TSHA-105 towards clinical study – Clinical considerations TSHA-105 has received US orphan drug and rare pediatric disease designations Currently conducting IND/CTA-enabling studies for the TSHA-105 program Patients will roll in from ongoing prospective natural history study Current clinical trial considerations: Considerations for the TSHA-105 Phase 1/2 first-in-human study Study design Open-label, randomized, dose-escalation study of the safety, tolerability and preliminary efficacy of TSHA-105 in the treatment of epileptic encephalopathy caused by SLC13A5 deficiency CSF dosing in children Clinical assessments Disease-Specific/Global Assessments CHOP-INTEND Peabody Developmental Motor Scale Bayley Scales of Infant Development Developmental quotient score Clinical global impression of improvement (CGI-I) Quality of Life scales Communication Assessments Observer-reported communication ability (ORCA) Imaging MRI of Brain Biomarkers Citrate levels in plasma, urine, and CSF Seizure Monitoring Seizure frequency Incidence of status epilepticus EEG Ratio of slow wave activity (SWA) during first hour of non rapid eye movement (NREM) sleep (measured by interictal EEG) TSHA-105 SLC13A5 deficiency

Anticipated next steps for TSHA-105 TSHA-105 SLC13A5 deficiency Complete GMP manufacturing using commercial process Pre-IND/CTA meeting anticipated 2H 2021 Continue enrollment in natural history study Complete IND-enabling preclinical work

Q & A TSHA-105 SLC13A5 deficiency

TSHA-103 for SLC6A1 Haploinsufficiency Disorder TSHA-103 SLC6A1 Steven Gray, PhD Chief Scientific Advisor, UTSW Gene Therapy Program Kim Goodspeed, MD UTSW Gene Therapy Program
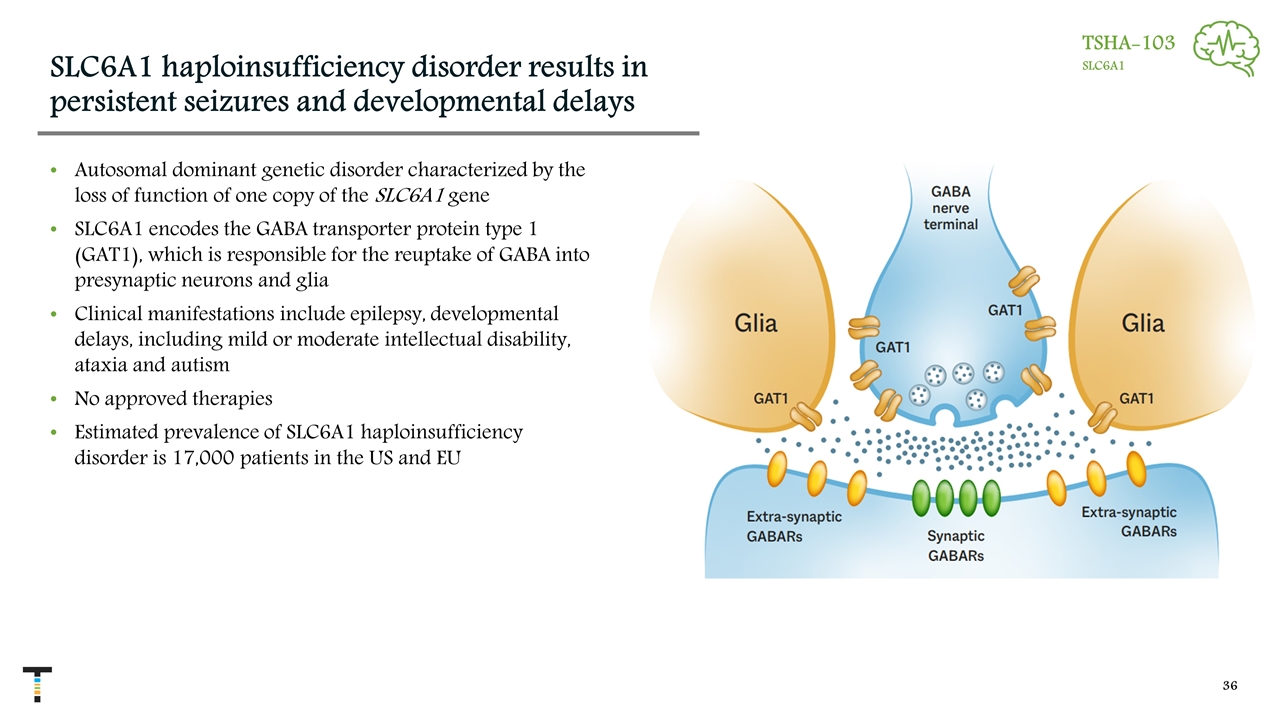
SLC6A1 haploinsufficiency disorder results in persistent seizures and developmental delays Autosomal dominant genetic disorder characterized by the loss of function of one copy of the SLC6A1 gene SLC6A1 encodes the GABA transporter protein type 1 (GAT1), which is responsible for the reuptake of GABA into presynaptic neurons and glia Clinical manifestations include epilepsy, developmental delays, including mild or moderate intellectual disability, ataxia and autism No approved therapies Estimated prevalence of SLC6A1 haploinsufficiency disorder is 17,000 patients in the US and EU TSHA-103 SLC6A1

SLC6A1 Patient Video CONFIDENTIAL
SLC6A1 Patient Video
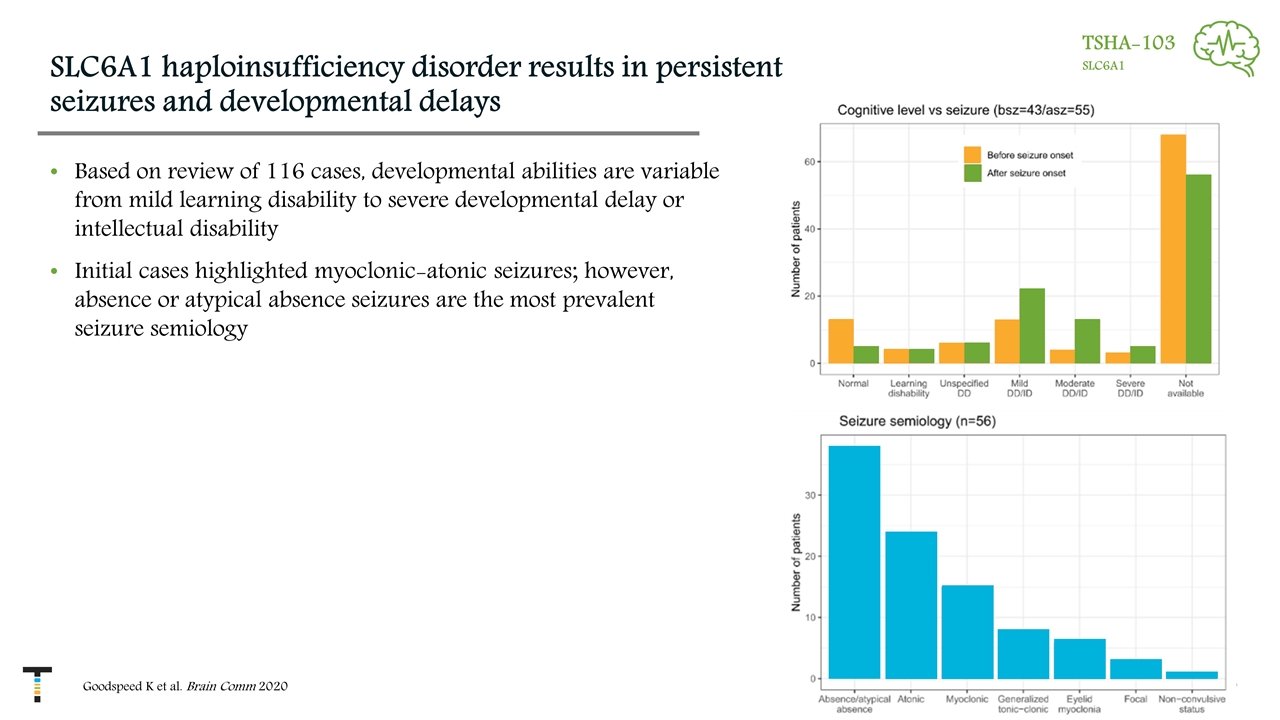
SLC6A1 haploinsufficiency disorder results in persistent seizures and developmental delays Based on review of 116 cases, developmental abilities are variable from mild learning disability to severe developmental delay or intellectual disability Initial cases highlighted myoclonic-atonic seizures; however, absence or atypical absence seizures are the most prevalent seizure semiology Goodspeed K et al. Brain Comm 2020 TSHA-103 SLC6A1
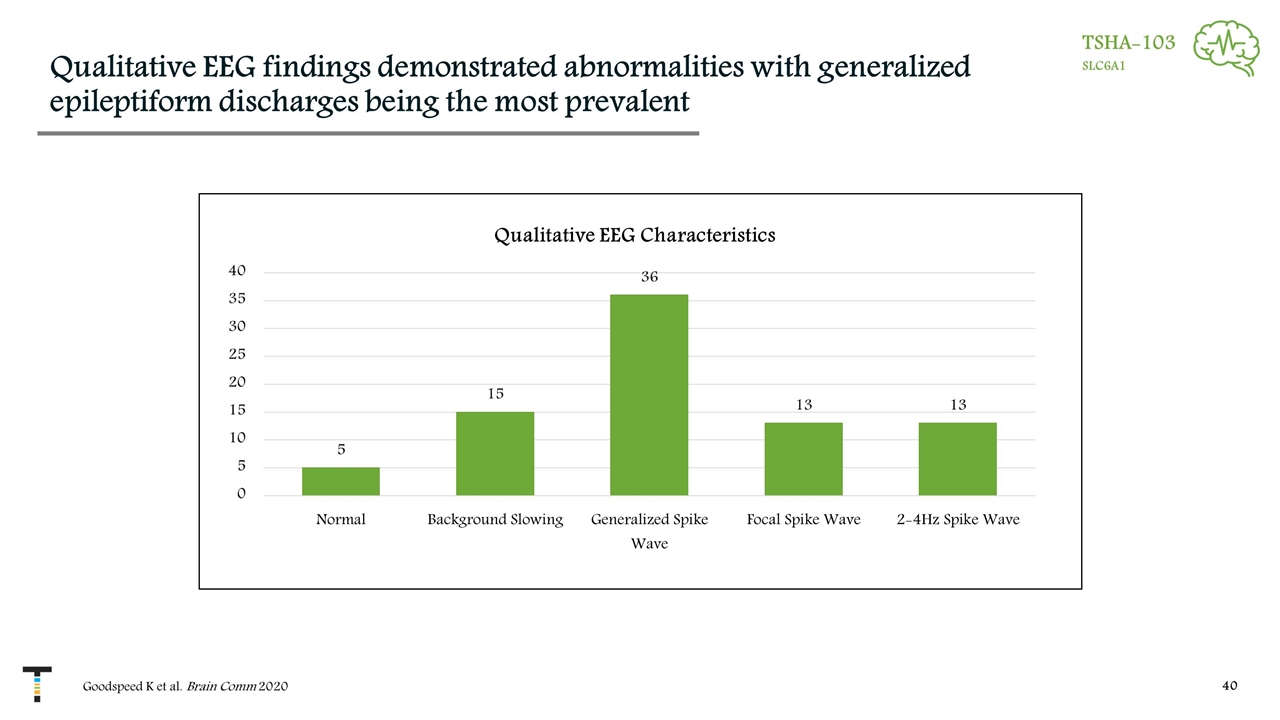
Qualitative EEG findings demonstrated abnormalities with generalized epileptiform discharges being the most prevalent TSHA-103 SLC6A1 Goodspeed K et al. Brain Comm 2020
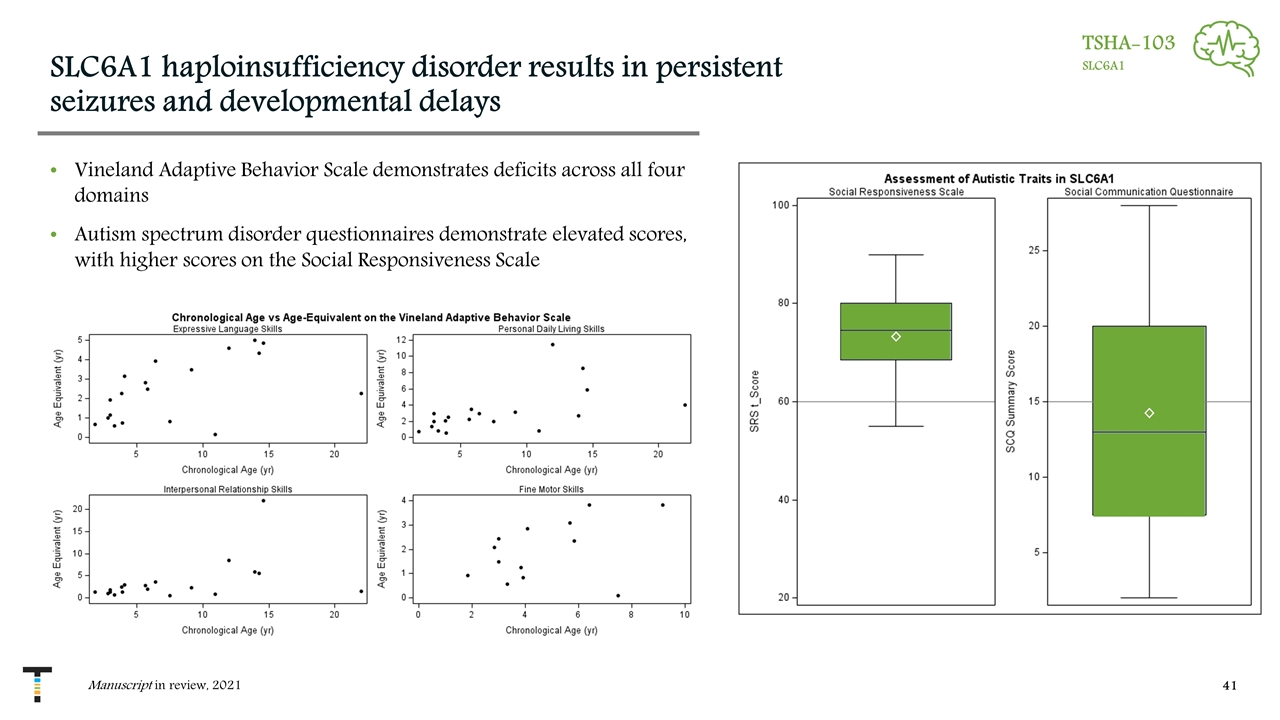
SLC6A1 haploinsufficiency disorder results in persistent seizures and developmental delays Vineland Adaptive Behavior Scale demonstrates deficits across all four domains Autism spectrum disorder questionnaires demonstrate elevated scores, with higher scores on the Social Responsiveness Scale Manuscript in review, 2021 TSHA-103 SLC6A1
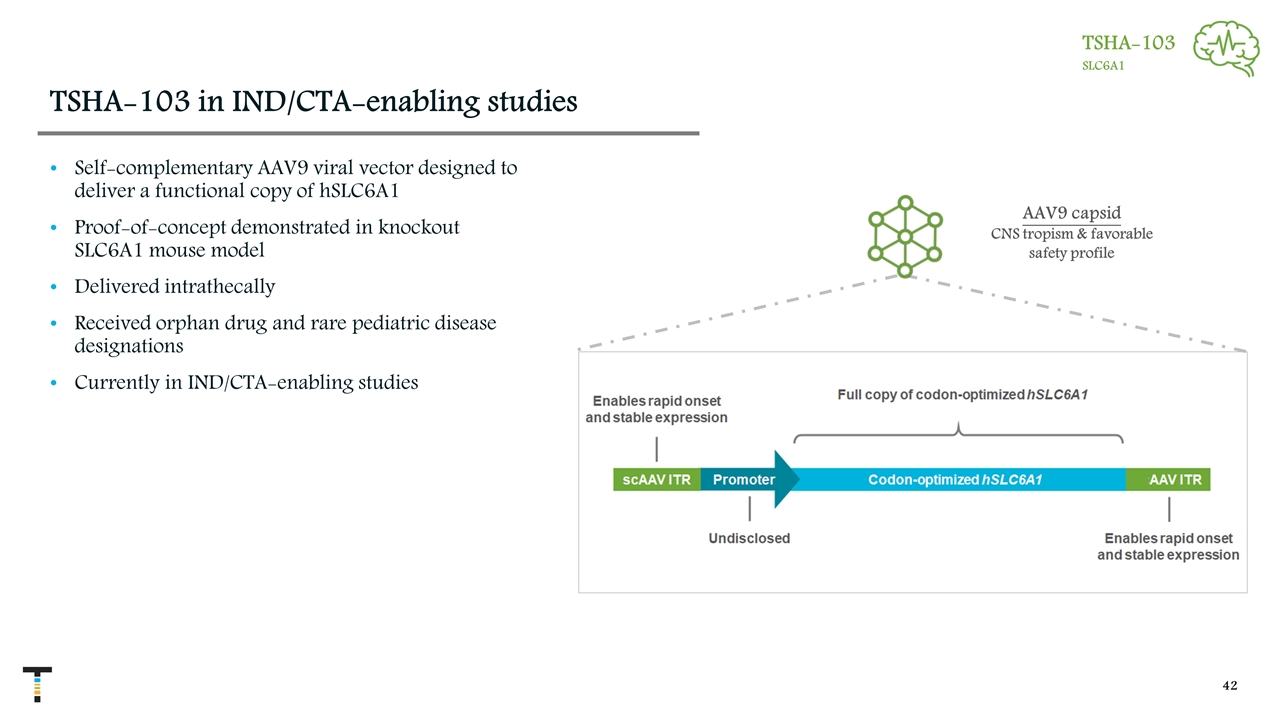
TSHA-103 in IND/CTA-enabling studies Self-complementary AAV9 viral vector designed to deliver a functional copy of hSLC6A1 Proof-of-concept demonstrated in knockout SLC6A1 mouse model Delivered intrathecally Received orphan drug and rare pediatric disease designations Currently in IND/CTA-enabling studies AAV9 capsid CNS tropism & favorable safety profile TSHA-103 SLC6A1
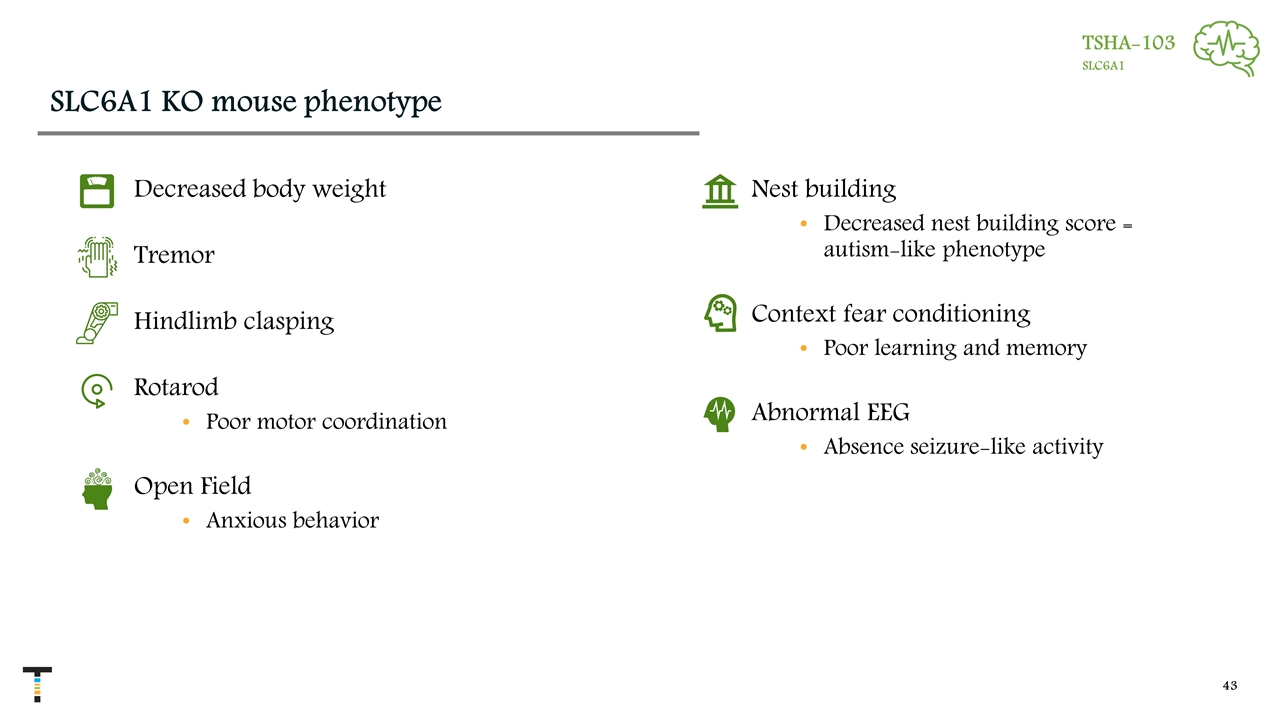
SLC6A1 KO mouse phenotype TSHA-103 SLC6A1 Decreased body weight Tremor Hindlimb clasping Rotarod Poor motor coordination Open Field Anxious behavior Nest building Decreased nest building score = autism-like phenotype Context fear conditioning Poor learning and memory Abnormal EEG Absence seizure-like activity
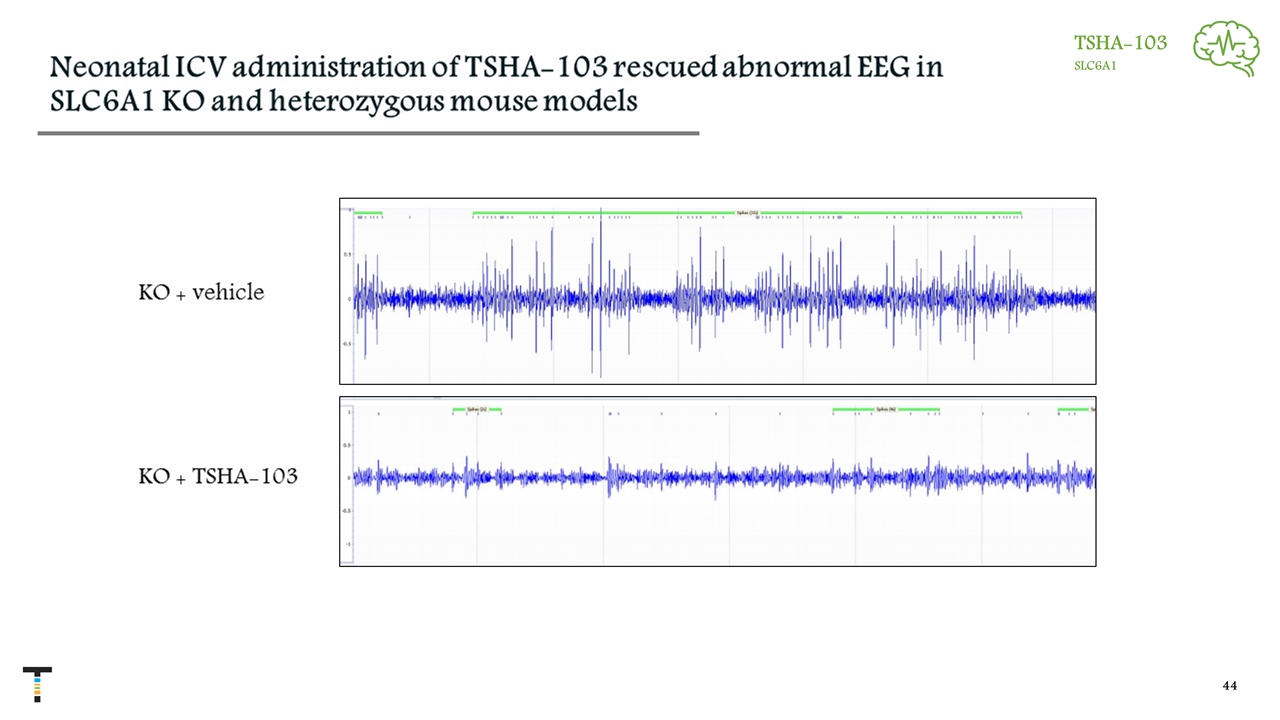
TSHA-103 SLC6A1
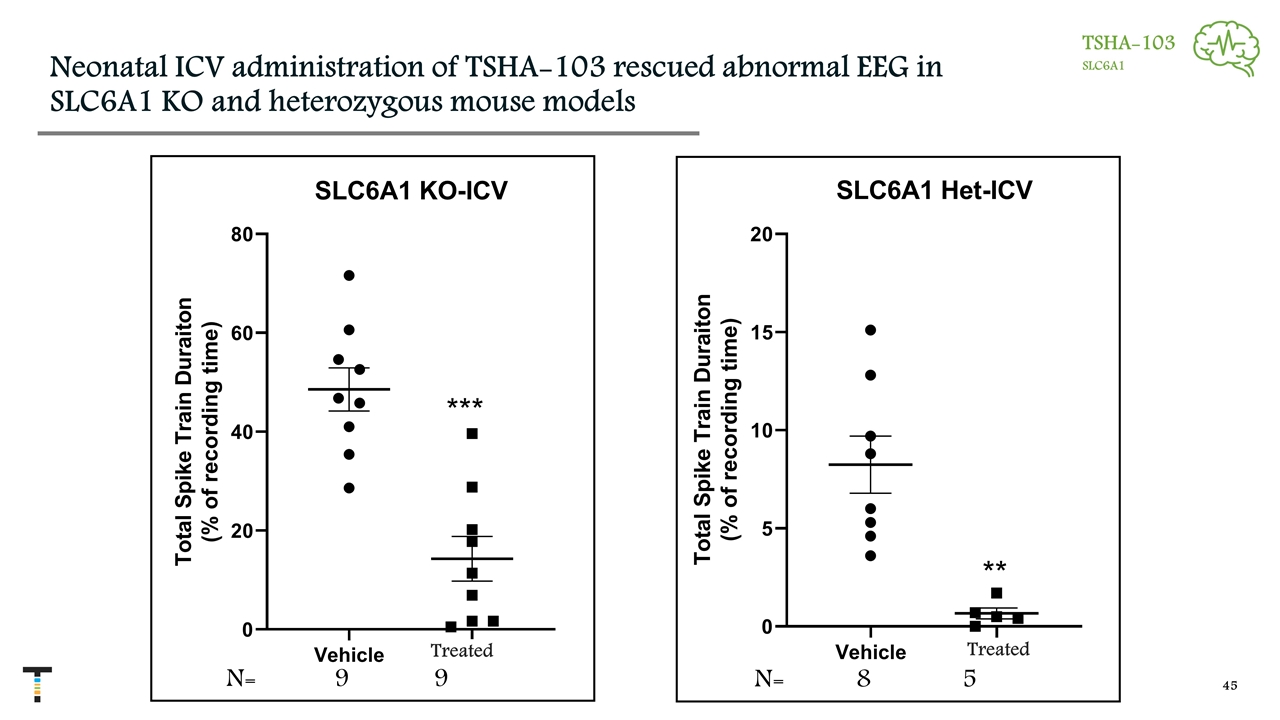
Neonatal ICV administration of TSHA-103 rescued abnormal EEG in SLC6A1 KO and heterozygous mouse models *** N= 9 9 N= 8 5 ** TSHA-103 SLC6A1 Treated Treated
Anticipated next steps for TSHA-103 TSHA-103 SLC6A1 Animal proof-of-concept achieved in KO mouse model Evaluate dose response and age response & finalize dose from pharmacology Natural history study initiated by patient advocacy foundation Interventional trial protocol development underway

Q & A TSHA-103 SLC6A1

TSHA-112 for Adult Polyglucosan Body Disease TSHA-112 APBD Berge Minassian, MD Chief Scientific Advisor, UTSW Gene Therapy Program Suyash Prasad, MBBS, MSc, MRCP, MRCPCH, FFPM Chief Medical Officer and Head of R&D
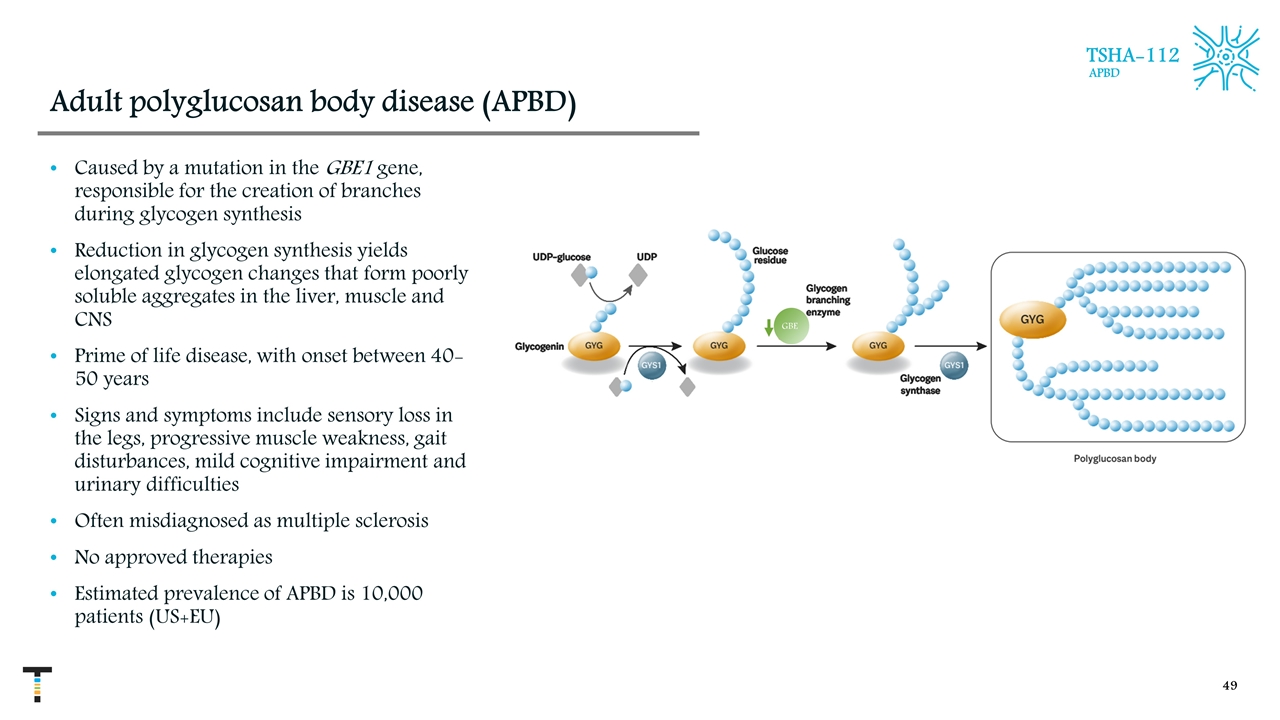
Adult polyglucosan body disease (APBD) Caused by a mutation in the GBE1 gene, responsible for the creation of branches during glycogen synthesis Reduction in glycogen synthesis yields elongated glycogen changes that form poorly soluble aggregates in the liver, muscle and CNS Prime of life disease, with onset between 40-50 years Signs and symptoms include sensory loss in the legs, progressive muscle weakness, gait disturbances, mild cognitive impairment and urinary difficulties Often misdiagnosed as multiple sclerosis No approved therapies Estimated prevalence of APBD is 10,000 patients (US+EU) TSHA-112 APBD GBE
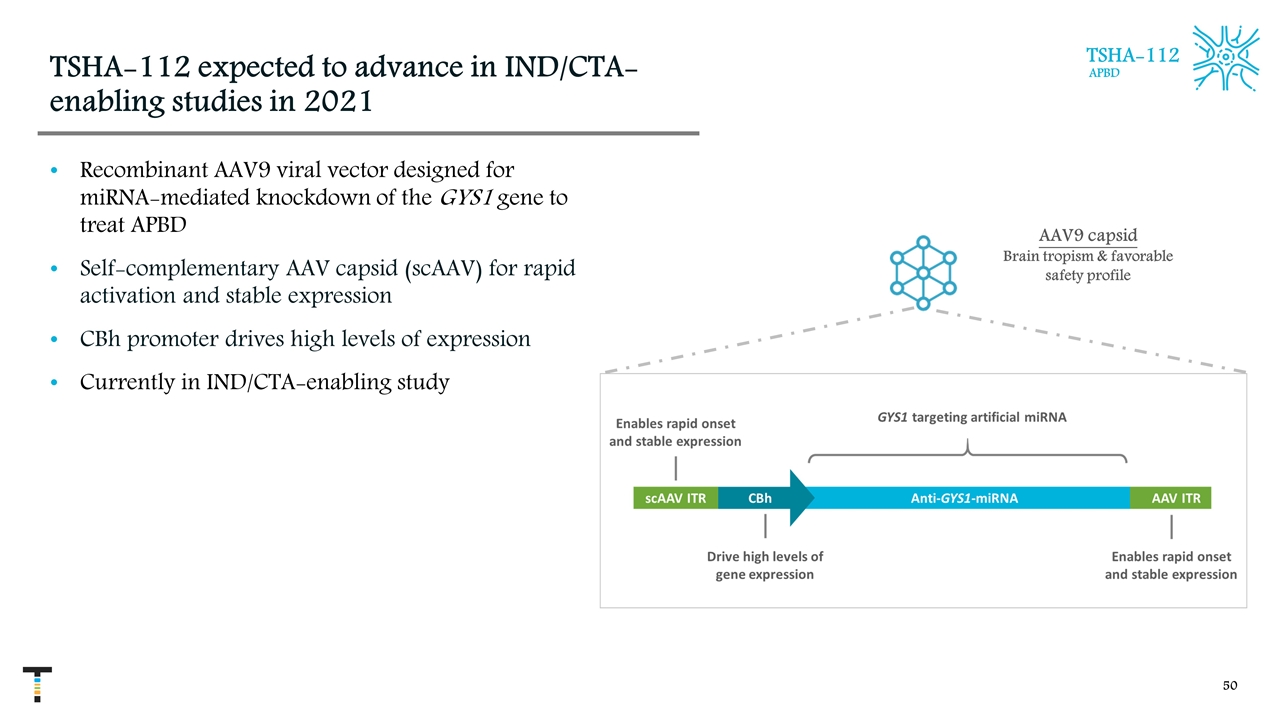
TSHA-112 expected to advance in IND/CTA-enabling studies in 2021 Recombinant AAV9 viral vector designed for miRNA-mediated knockdown of the GYS1 gene to treat APBD Self-complementary AAV capsid (scAAV) for rapid activation and stable expression CBh promoter drives high levels of expression Currently in IND/CTA-enabling study AAV9 capsid Brain tropism & favorable safety profile TSHA-112 APBD
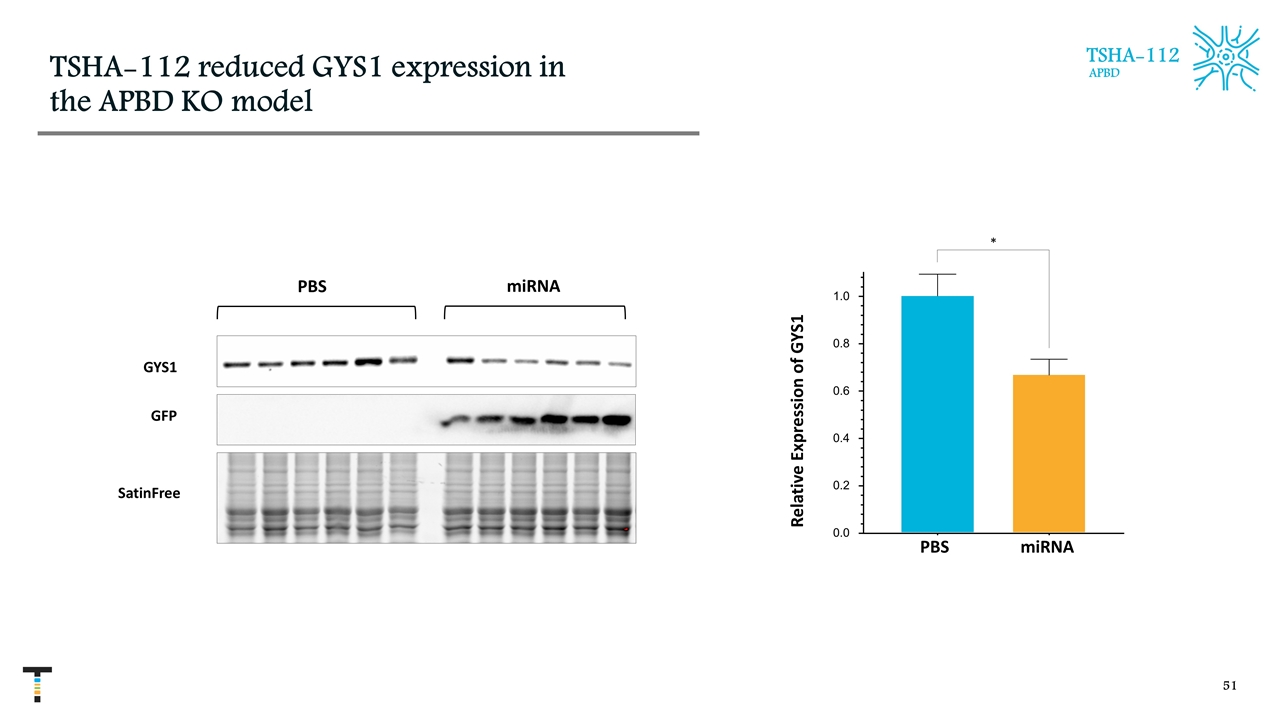
TSHA-112 reduced GYS1 expression in the APBD KO model TSHA-112 APBD PBS miRNA 0.0 0.2 0.4 0.6 0.8 1.0 * Relative Expression of GYS1 PBS miRNA GYS1 GFP SatinFree miRNA PBS
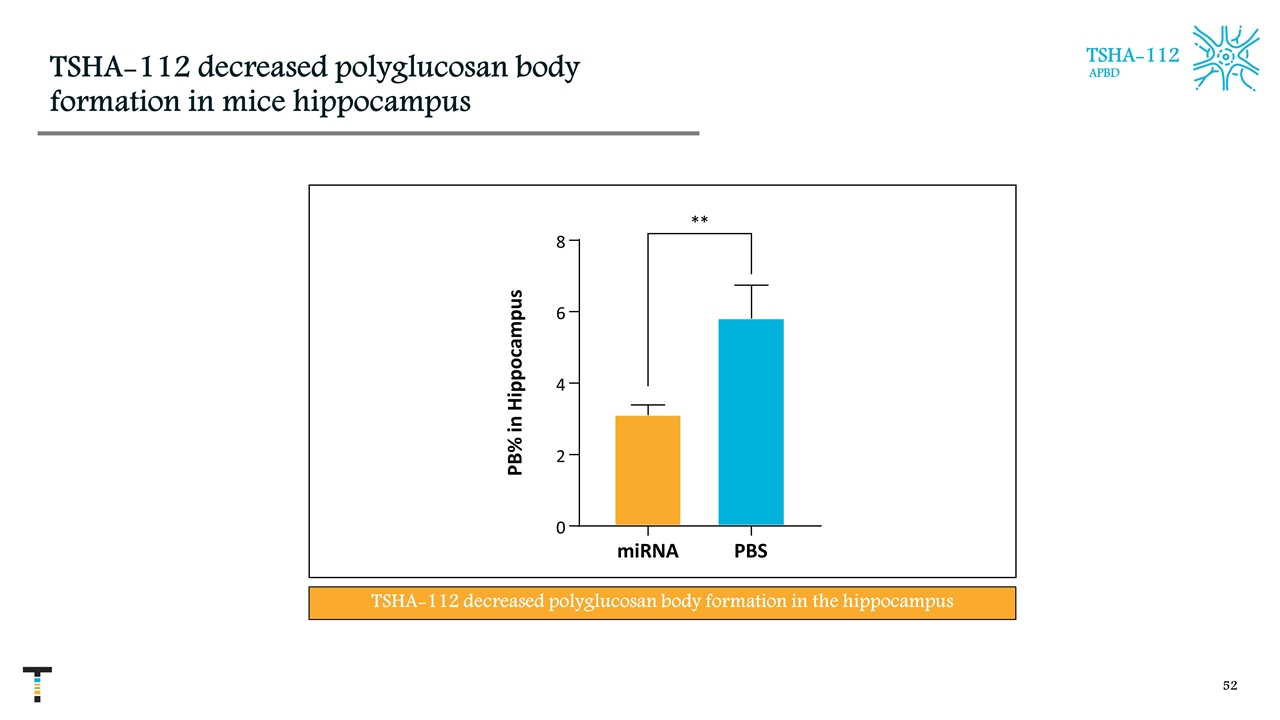
TSHA-112 decreased polyglucosan body formation in mice hippocampus TSHA-112 APBD miRNA PBS 0 2 4 6 8 P B % i n H i p p o c a m p u s ** TSHA-112 decreased polyglucosan body formation in the hippocampus PB% in Hippocampus
Anticipated next steps for TSHA-112 TSHA-112 APBD Animal proof-of-concept achieved in KO mouse model Evaluate dose response and age response & finalize dose from pharmacology Interventional trial protocol development underway

Q & A TSHA-112 APBD

TSHA-111 for Lafora Disease TSHA-111 Lafora disease Suyash Prasad, MBBS, MSc, MRCP, MRCPCH, FFPM Chief Medical Officer and Head of R&D Berge Minassian, MD Chief Scientific Advisor, UTSW Gene Therapy Program
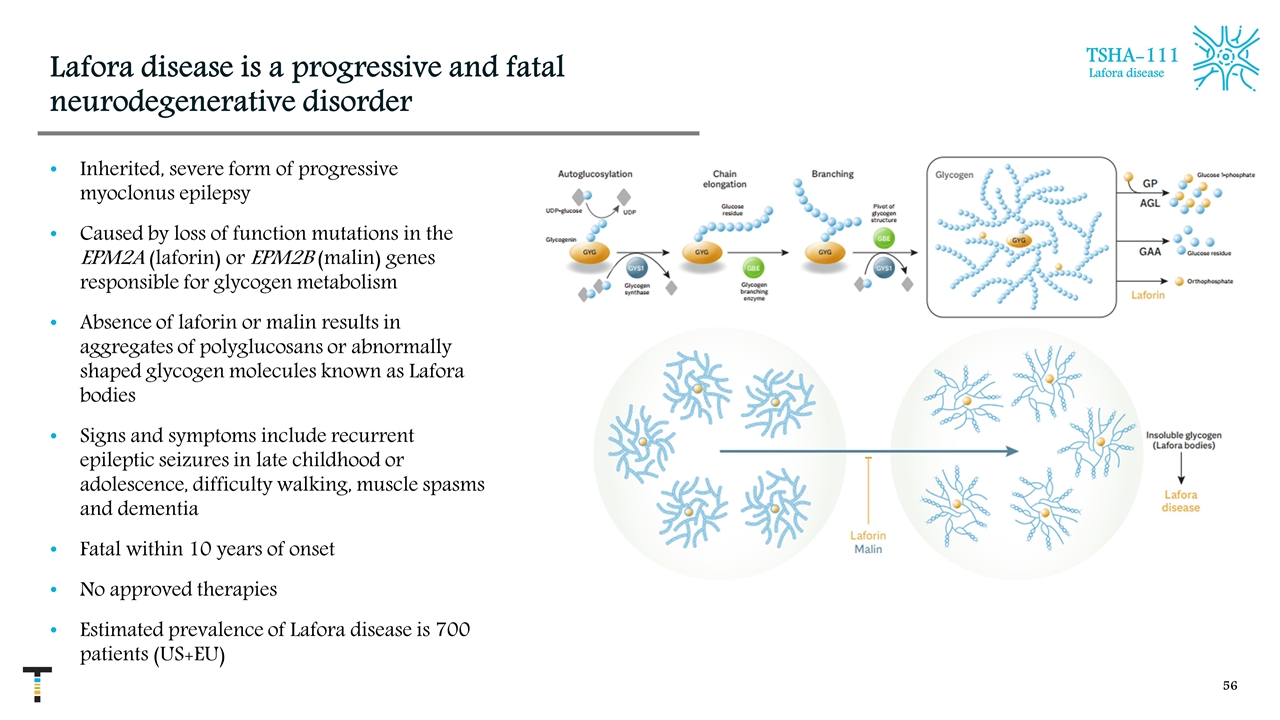
Lafora disease is a progressive and fatal neurodegenerative disorder Inherited, severe form of progressive myoclonus epilepsy Caused by loss of function mutations in the EPM2A (laforin) or EPM2B (malin) genes responsible for glycogen metabolism Absence of laforin or malin results in aggregates of polyglucosans or abnormally shaped glycogen molecules known as Lafora bodies Signs and symptoms include recurrent epileptic seizures in late childhood or adolescence, difficulty walking, muscle spasms and dementia Fatal within 10 years of onset No approved therapies Estimated prevalence of Lafora disease is 700 patients (US+EU) TSHA-111 Lafora disease

Examples of polyglucosan bodies TSHA-112 APBD

TSHA-111 Lafora disease
Chelsea after Lafora disease diagnosis TSHA-111 Lafora disease

Lafora Patient Video
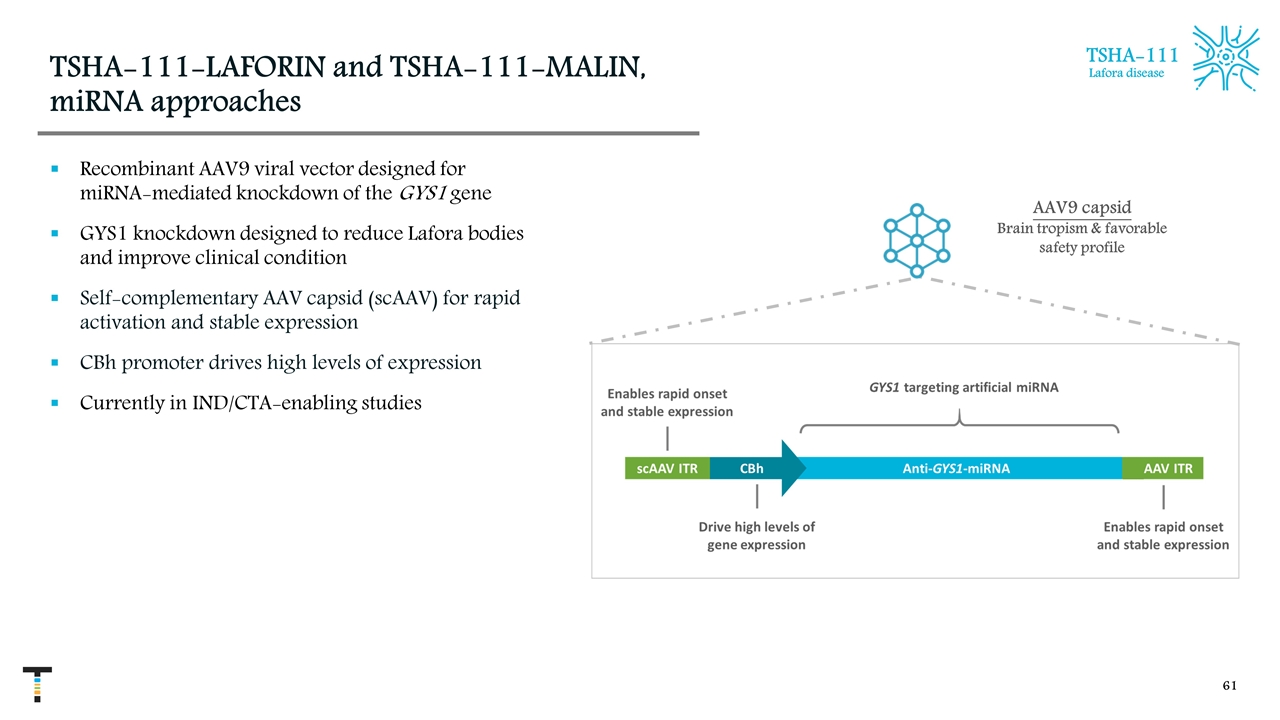
TSHA-111-LAFORIN and TSHA-111-MALIN, miRNA approaches Recombinant AAV9 viral vector designed for miRNA-mediated knockdown of the GYS1 gene GYS1 knockdown designed to reduce Lafora bodies and improve clinical condition Self-complementary AAV capsid (scAAV) for rapid activation and stable expression CBh promoter drives high levels of expression Currently in IND/CTA-enabling studies AAV9 capsid Brain tropism & favorable safety profile TSHA-111 Lafora disease
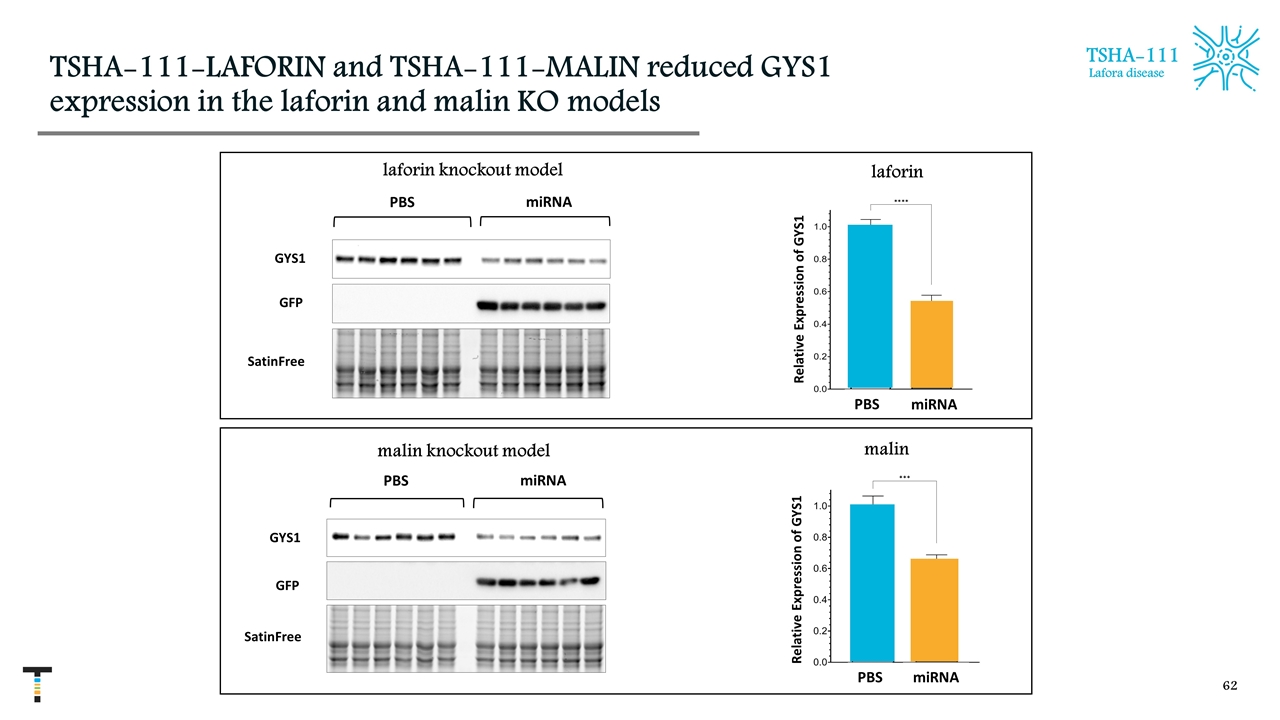
TSHA-111-LAFORIN and TSHA-111-MALIN reduced GYS1 expression in the laforin and malin KO models TSHA-111 Lafora disease malin malin knockout model PBS miRNA laforin knockout model GYS1 GFP SatinFree PBS miRNA laforin GYS1 GFP SatinFree PBS miRNA PBS miRNA Relative Expression of GYS1 Relative Expression of GYS1
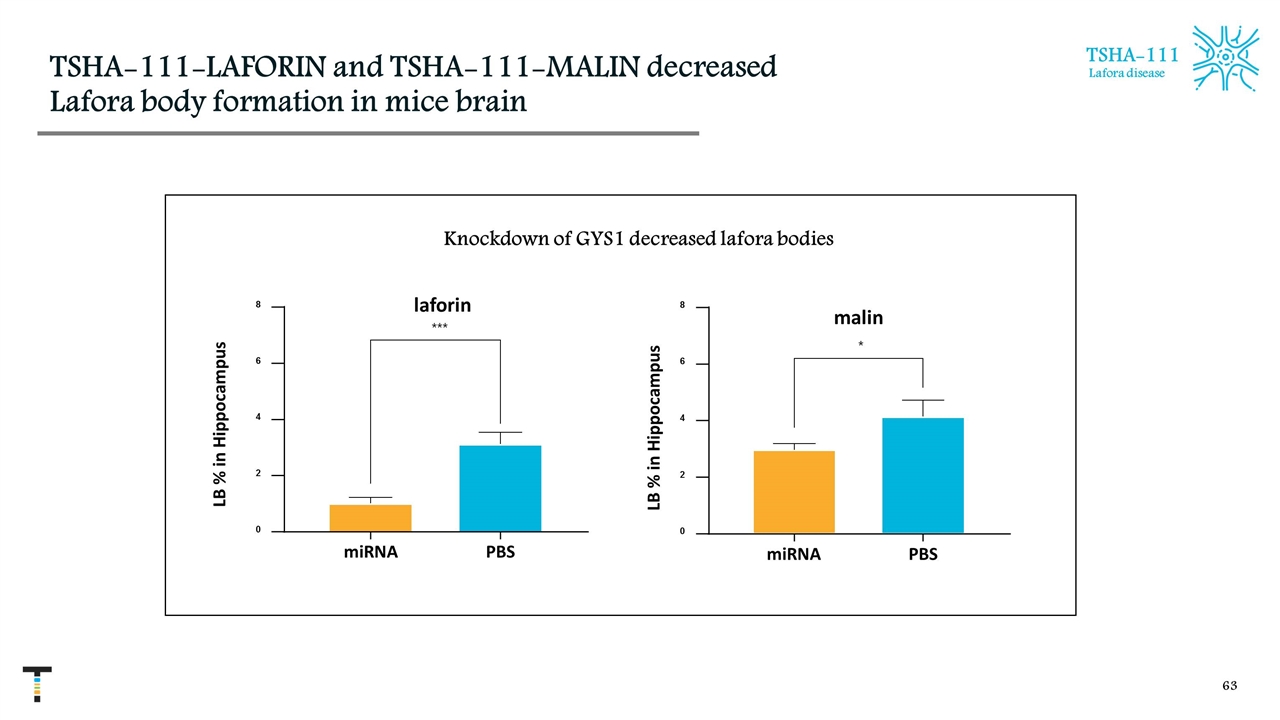
TSHA-111-LAFORIN and TSHA-111-MALIN decreased Lafora body formation in mice brain malin miRNA PBS 0 2 4 6 8 L B i n i p p o c a m p u s * laforin miRNA PBS 0 2 4 6 8 L B % i n H i p p o c a m p u s *** LB % in Hippocampus Knockdown of GYS1 decreased lafora bodies LB % in Hippocampus TSHA-111 Lafora disease
Anticipated next steps for TSHA-111-LAFORIN and TSHA-111-MALIN TSHA-111 Lafora disease Animal proof-of-concept achieved in KO mouse model Evaluate dose response and age response & finalize dose from pharmacology Interventional trial protocol development underway

Q & A TSHA-111 Lafora disease

TSHA-113 for Tauopathies TSHA-113 MAPT Suyash Prasad, MBBS, MSc, MRCP, MRCPCH, FFPM Chief Medical Officer and Head of R&D Rachel Bailey, PhD UTSW Gene Therapy Program
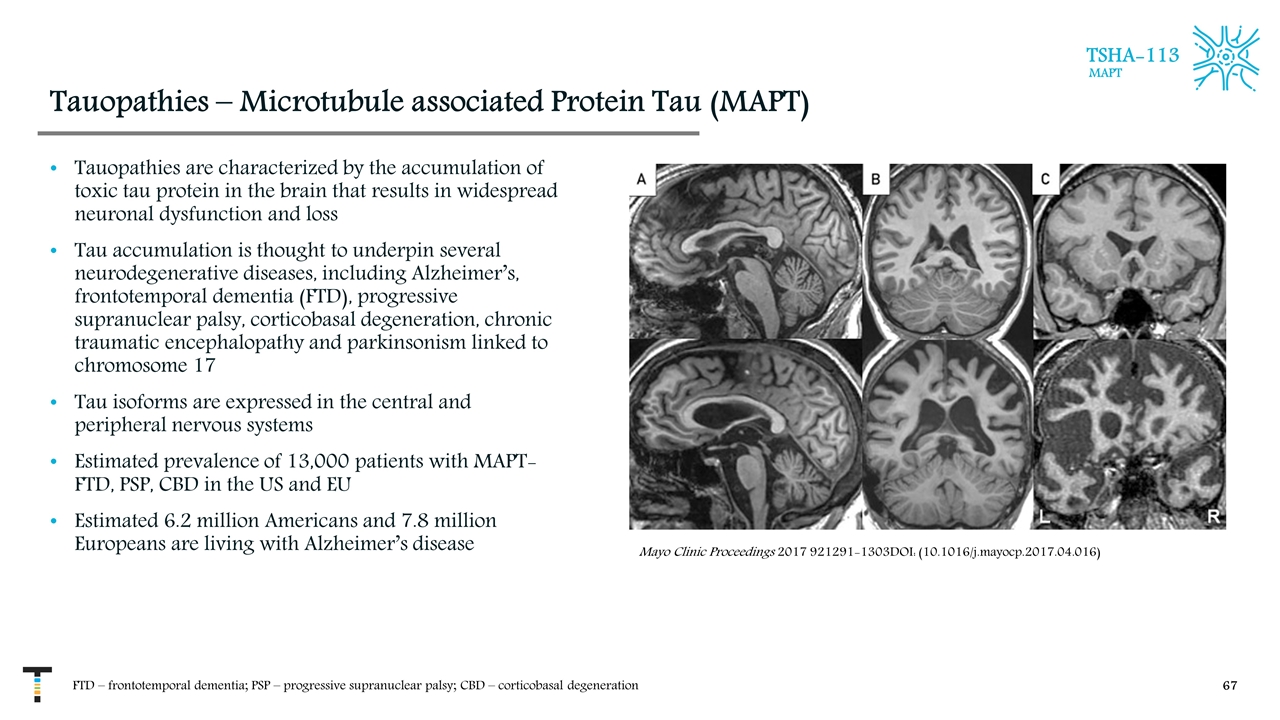
Tauopathies – Microtubule associated Protein Tau (MAPT) Tauopathies are characterized by the accumulation of toxic tau protein in the brain that results in widespread neuronal dysfunction and loss Tau accumulation is thought to underpin several neurodegenerative diseases, including Alzheimer’s, frontotemporal dementia (FTD), progressive supranuclear palsy, corticobasal degeneration, chronic traumatic encephalopathy and parkinsonism linked to chromosome 17 Tau isoforms are expressed in the central and peripheral nervous systems Estimated prevalence of 13,000 patients with MAPT-FTD, PSP, CBD in the US and EU Estimated 6.2 million Americans and 7.8 million Europeans are living with Alzheimer’s disease FTD – frontotemporal dementia; PSP – progressive supranuclear palsy; CBD – corticobasal degeneration TSHA-113 MAPT Mayo Clinic Proceedings 2017 921291-1303DOI: (10.1016/j.mayocp.2017.04.016)
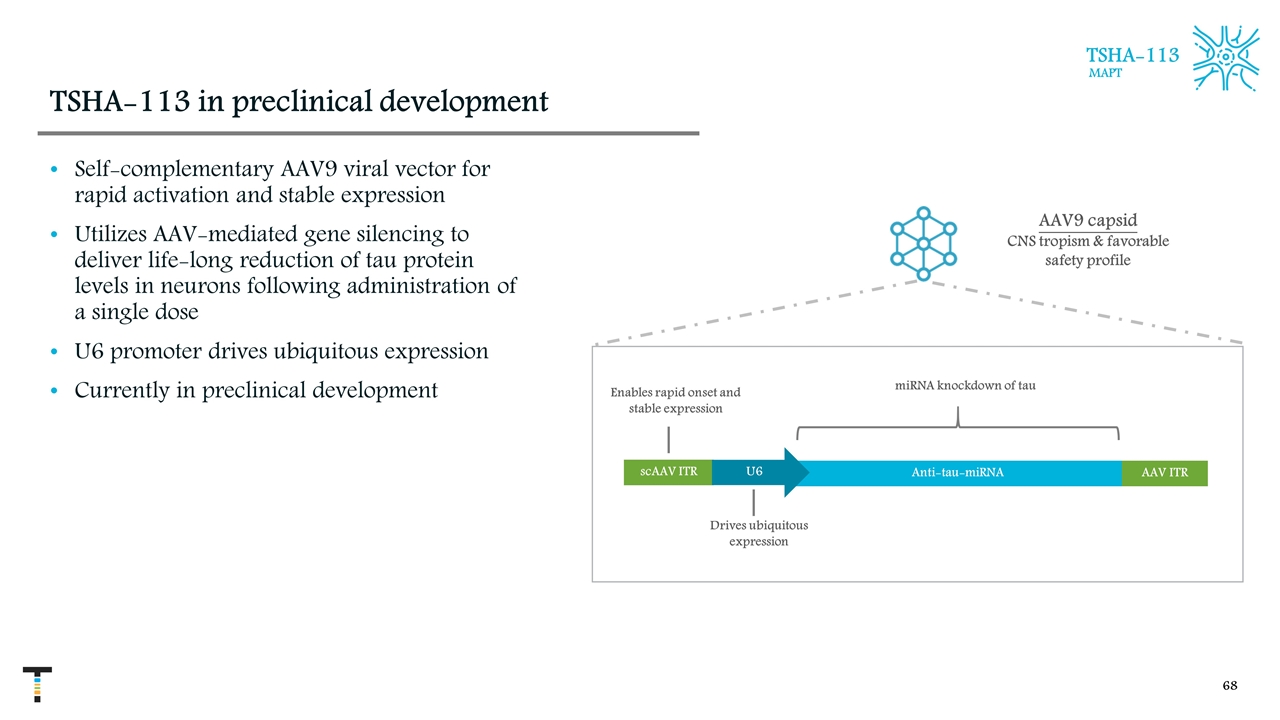
TSHA-113 in preclinical development Self-complementary AAV9 viral vector for rapid activation and stable expression Utilizes AAV-mediated gene silencing to deliver life-long reduction of tau protein levels in neurons following administration of a single dose U6 promoter drives ubiquitous expression Currently in preclinical development AAV9 capsid CNS tropism & favorable safety profile scAAV ITR Anti-tau-miRNA U6 AAV ITR Drives ubiquitous expression Enables rapid onset and stable expression miRNA knockdown of tau TSHA-113 MAPT
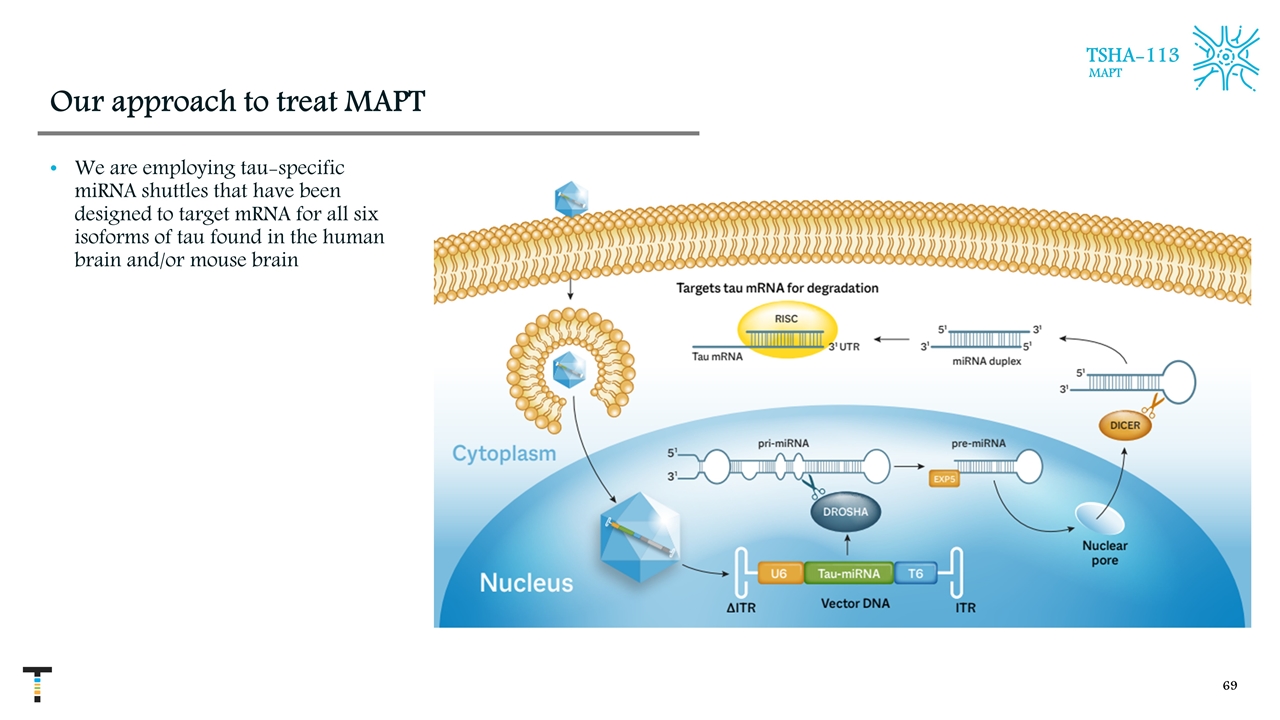
Our approach to treat MAPT We are employing tau-specific miRNA shuttles that have been designed to target mRNA for all six isoforms of tau found in the human brain and/or mouse brain TSHA-113 MAPT
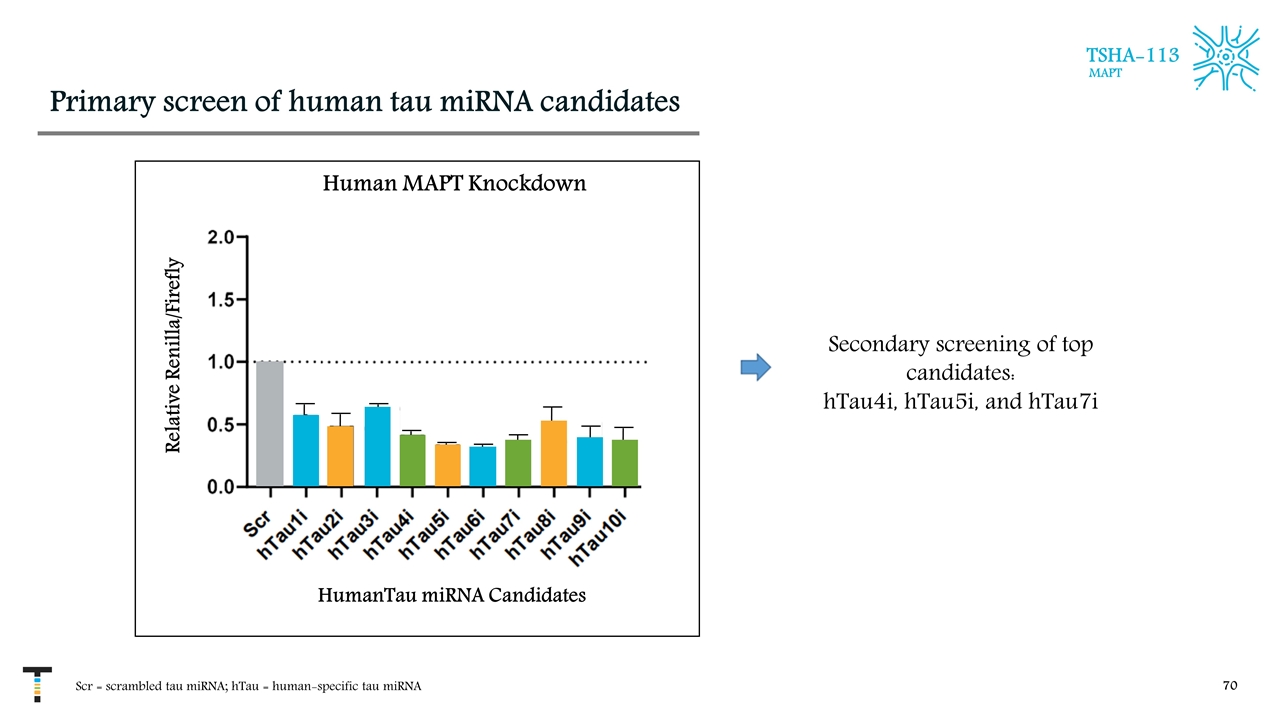
Primary screen of human tau miRNA candidates Scr = scrambled tau miRNA; hTau = human-specific tau miRNA TSHA-113 MAPT Human MAPT Knockdown Relative Renilla/Firefly HumanTau miRNA Candidates Secondary screening of top candidates: hTau4i, hTau5i, and hTau7i
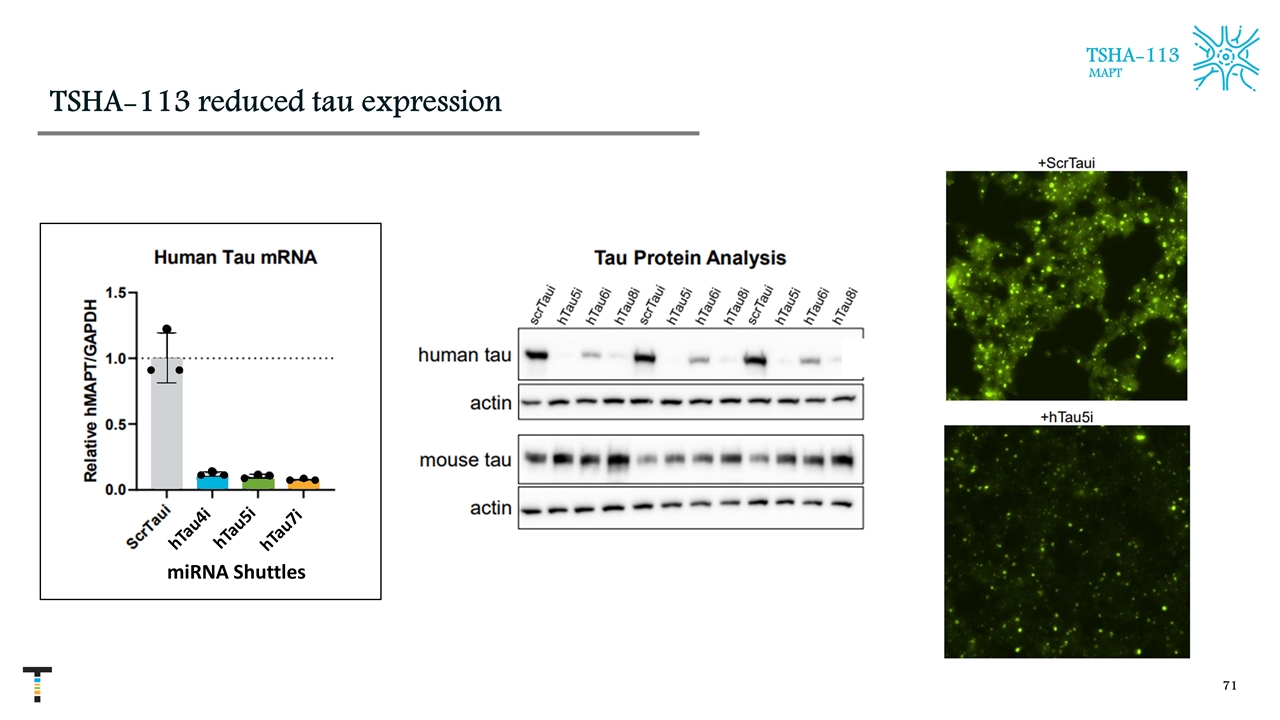
TSHA-113 reduced tau expression TSHA-113 MAPT hTau4i hTau5i miRNA Shuttles hTau7i
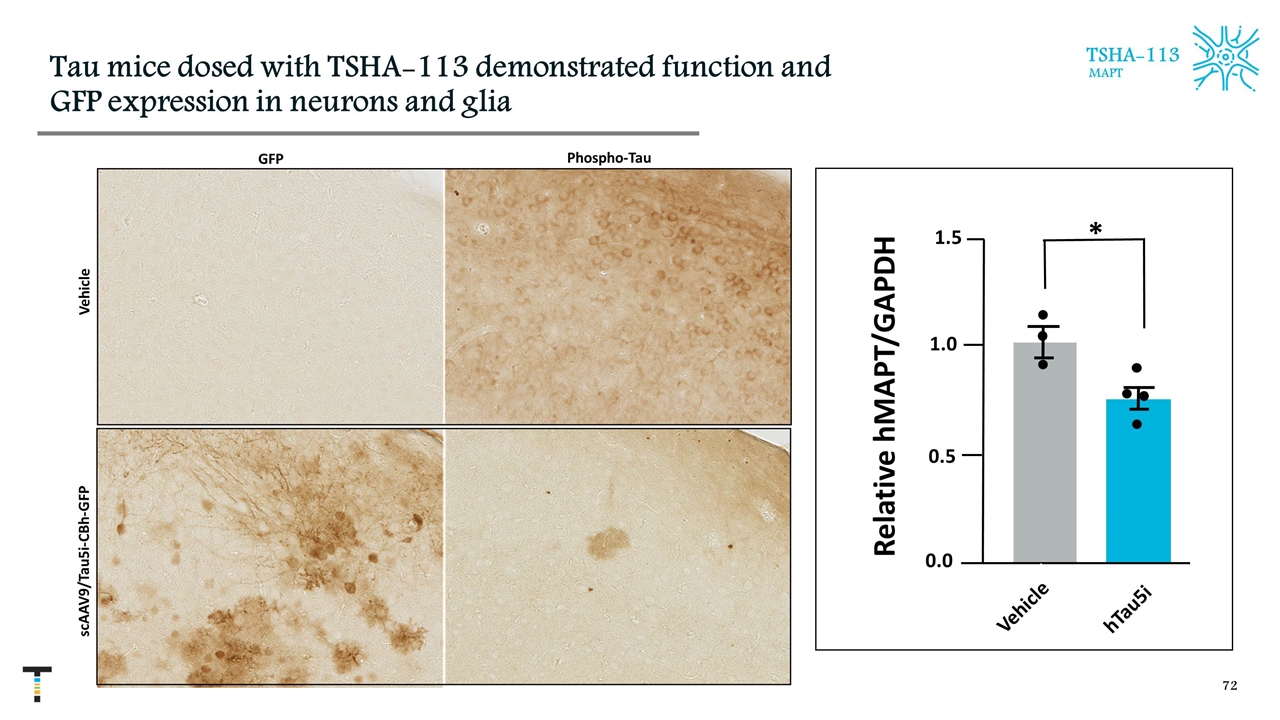
Tau mice dosed with TSHA-113 demonstrated function and GFP expression in neurons and glia TSHA-113 MAPT scAAV9/Tau5i-CBh-GFP Vehicle Relative hMAPT/GAPDH 0.0 0.5 1.0 1.5 * hTau5i Vehicle GFP Phospho-Tau
Anticipated next steps for TSHA-113 TSHA-113 MAPT Animal proof-of-concept achieved in KO mouse model Evaluate dose response and age response & finalize dose from pharmacology Interventional trial protocol development underway

Q & A TSHA-113 MAPT

TSHA-106 for Angelman Syndrome TSHA-106 Angelman Syndrome Suyash Prasad, MBBS, MSc, MRCP, MRCPCH, FFPM Chief Medical Officer and Head of R&D
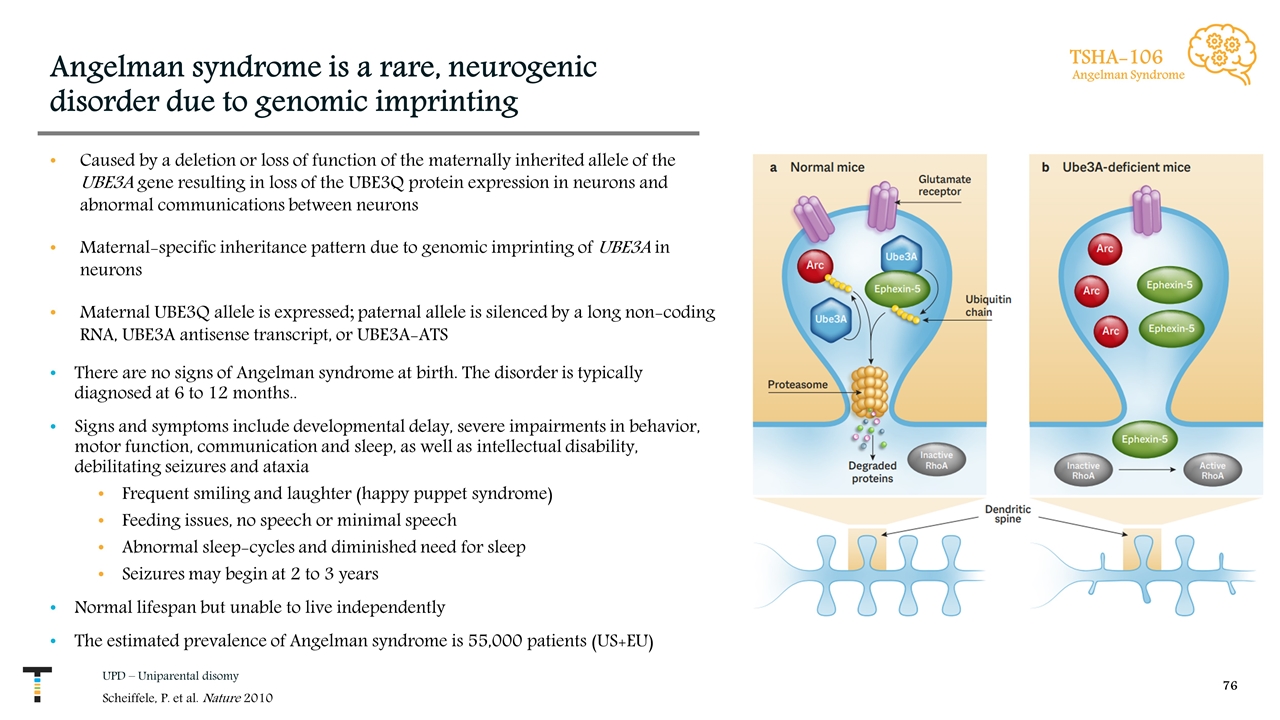
Angelman syndrome is a rare, neurogenic disorder due to genomic imprinting Caused by a deletion or loss of function of the maternally inherited allele of the UBE3A gene resulting in loss of the UBE3Q protein expression in neurons and abnormal communications between neurons Maternal-specific inheritance pattern due to genomic imprinting of UBE3A in neurons Maternal UBE3Q allele is expressed; paternal allele is silenced by a long non-coding RNA, UBE3A antisense transcript, or UBE3A-ATS There are no signs of Angelman syndrome at birth. The disorder is typically diagnosed at 6 to 12 months.. Signs and symptoms include developmental delay, severe impairments in behavior, motor function, communication and sleep, as well as intellectual disability, debilitating seizures and ataxia Frequent smiling and laughter (happy puppet syndrome) Feeding issues, no speech or minimal speech Abnormal sleep-cycles and diminished need for sleep Seizures may begin at 2 to 3 years Normal lifespan but unable to live independently The estimated prevalence of Angelman syndrome is 55,000 patients (US+EU) UPD – Uniparental disomy Scheiffele, P. et al. Nature 2010 TSHA-106 Angelman Syndrome
Current treatment for Angelman syndrome TSHA-106 Angelman Syndrome There is no cure for Angelman syndrome. Current treatment focuses on managing the medical and developmental issues. Anti-seizure medication to control seizures Physical therapy to help with walking and movement problems Communication therapy, which may include sign language and picture communication Behavior therapy to help overcome hyperactivity and a short attention span and to aid in development
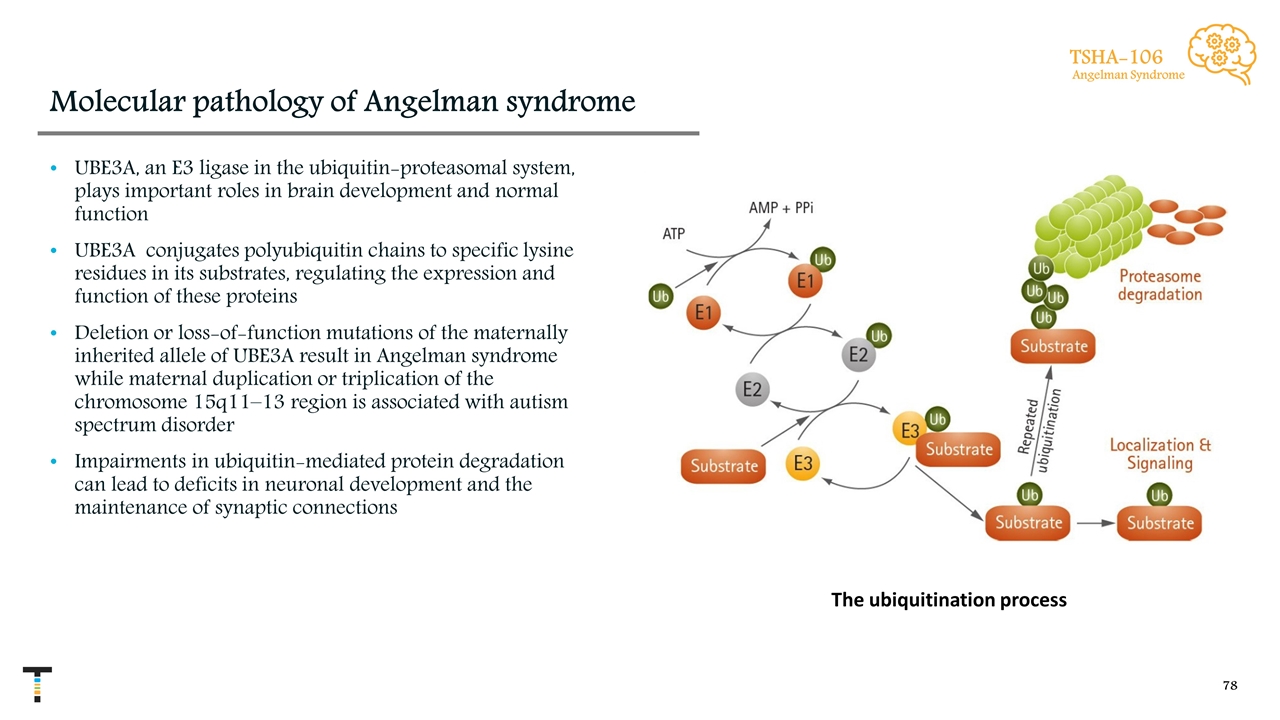
Molecular pathology of Angelman syndrome UBE3A, an E3 ligase in the ubiquitin-proteasomal system, plays important roles in brain development and normal function UBE3A conjugates polyubiquitin chains to specific lysine residues in its substrates, regulating the expression and function of these proteins Deletion or loss-of-function mutations of the maternally inherited allele of UBE3A result in Angelman syndrome while maternal duplication or triplication of the chromosome 15q11–13 region is associated with autism spectrum disorder Impairments in ubiquitin-mediated protein degradation can lead to deficits in neuronal development and the maintenance of synaptic connections The ubiquitination process TSHA-106 Angelman Syndrome
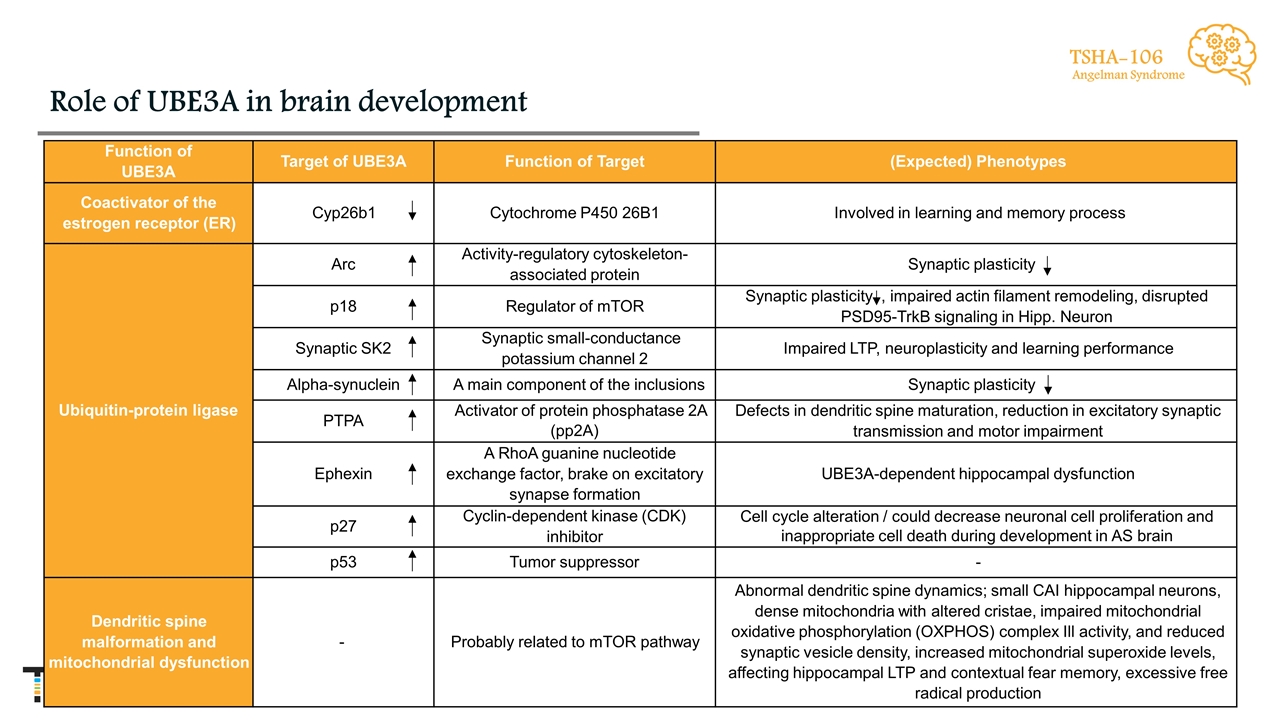
Role of UBE3A in brain development TSHA-106 Angelman Syndrome Function of UBE3A Target of UBE3A Function of Target (Expected) Phenotypes Coactivator of the estrogen receptor (ER) Cyp26b1 Cytochrome P450 26B1 Involved in learning and memory process Ubiquitin-protein ligase Arc Activity-regulatory cytoskeleton-associated protein Synaptic plasticity p18 Regulator of mTOR Synaptic plasticity , impaired actin filament remodeling, disrupted PSD95-TrkB signaling in Hipp. Neuron Synaptic SK2 Synaptic small-conductance potassium channel 2 Impaired LTP, neuroplasticity and learning performance Alpha-synuclein A main component of the inclusions Synaptic plasticity PTPA Activator of protein phosphatase 2A (pp2A) Defects in dendritic spine maturation, reduction in excitatory synaptic transmission and motor impairment Ephexin A RhoA guanine nucleotide exchange factor, brake on excitatory synapse formation UBE3A-dependent hippocampal dysfunction p27 Cyclin-dependent kinase (CDK) inhibitor Cell cycle alteration / could decrease neuronal cell proliferation and inappropriate cell death during development in AS brain p53 Tumor suppressor - Dendritic spine malformation and mitochondrial dysfunction - Probably related to mTOR pathway Abnormal dendritic spine dynamics; small CAI hippocampal neurons, dense mitochondria with altered cristae, impaired mitochondrial oxidative phosphorylation (OXPHOS) complex Ill activity, and reduced synaptic vesicle density, increased mitochondrial superoxide levels, affecting hippocampal LTP and contextual fear memory, excessive free radical production
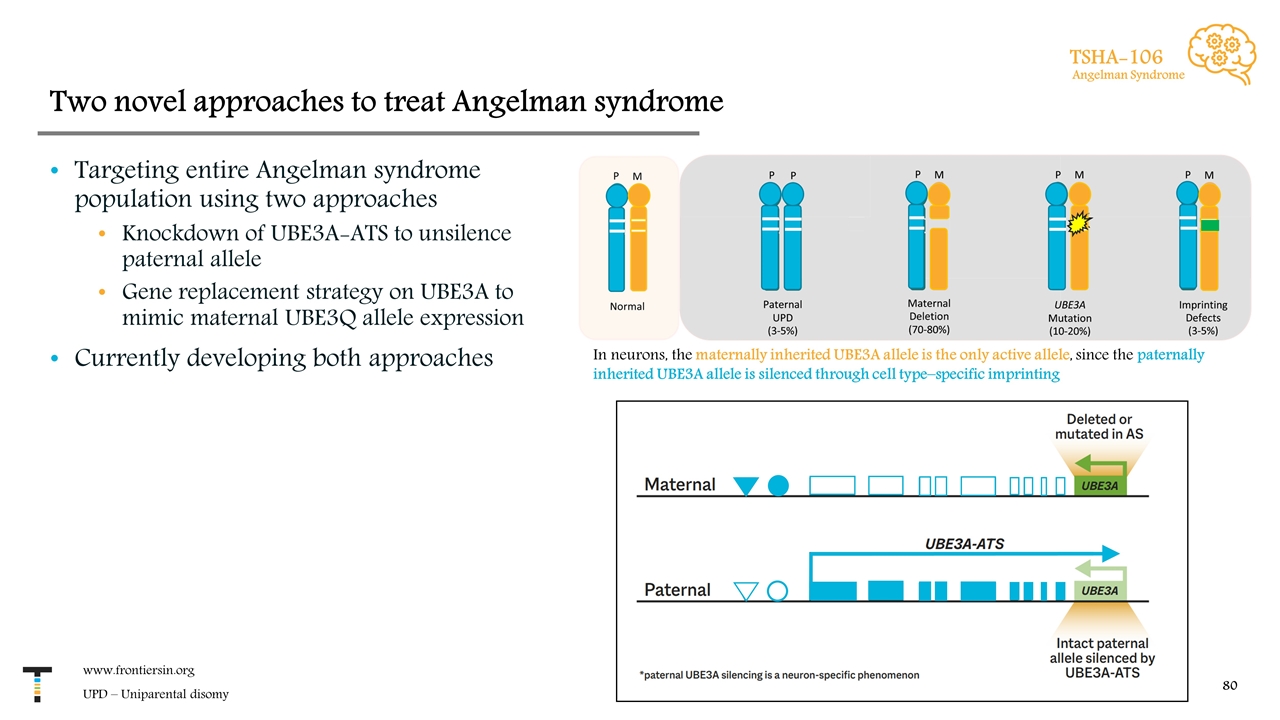
Two novel approaches to treat Angelman syndrome Targeting entire Angelman syndrome population using two approaches Knockdown of UBE3A-ATS to unsilence paternal allele Gene replacement strategy on UBE3A to mimic maternal UBE3Q allele expression Currently developing both approaches www.frontiersin.org UPD – Uniparental disomy TSHA-106 Angelman Syndrome In neurons, the maternally inherited UBE3A allele is the only active allele, since the paternally inherited UBE3A allele is silenced through cell type–specific imprinting
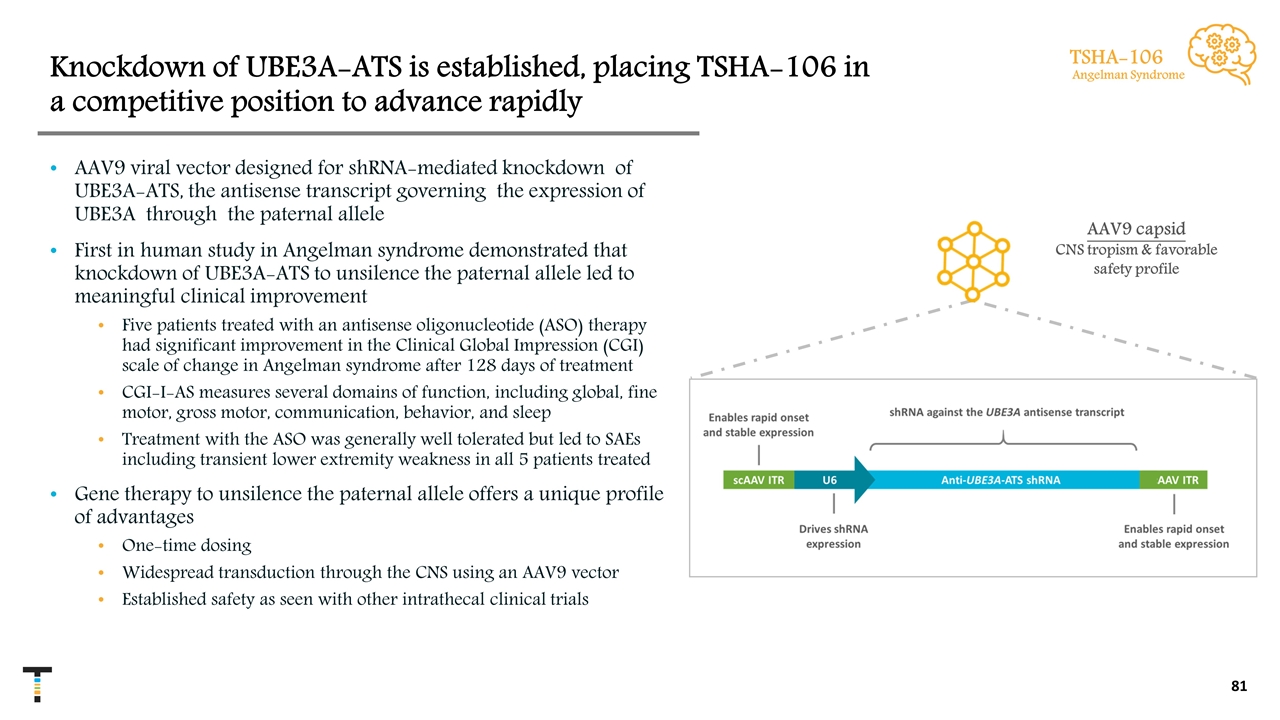
Knockdown of UBE3A-ATS is established, placing TSHA-106 in a competitive position to advance rapidly AAV9 viral vector designed for shRNA-mediated knockdown of UBE3A-ATS, the antisense transcript governing the expression of UBE3A through the paternal allele First in human study in Angelman syndrome demonstrated that knockdown of UBE3A-ATS to unsilence the paternal allele led to meaningful clinical improvement Five patients treated with an antisense oligonucleotide (ASO) therapy had significant improvement in the Clinical Global Impression (CGI) scale of change in Angelman syndrome after 128 days of treatment CGI-I-AS measures several domains of function, including global, fine motor, gross motor, communication, behavior, and sleep Treatment with the ASO was generally well tolerated but led to SAEs including transient lower extremity weakness in all 5 patients treated Gene therapy to unsilence the paternal allele offers a unique profile of advantages One-time dosing Widespread transduction through the CNS using an AAV9 vector Established safety as seen with other intrathecal clinical trials TSHA-106 Angelman Syndrome AAV9 capsid CNS tropism & favorable safety profile

TSHA-106 targets UBE3A-ATS transcript through shRNA knockdown TSHA-106 Angelman Syndrome 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 shRNA Candidates UBE3A-ATS UBE3A 4.0 3.5 3.0 2.5 2.0 1.5 1.0 0.5 0.0 Folds compared to control Testing in neuroblast cell line demonstrated consistent knockdown of UBE3A-ATS and a subsequent increase in UBE3A expression across 26 distinct shRNA candidates
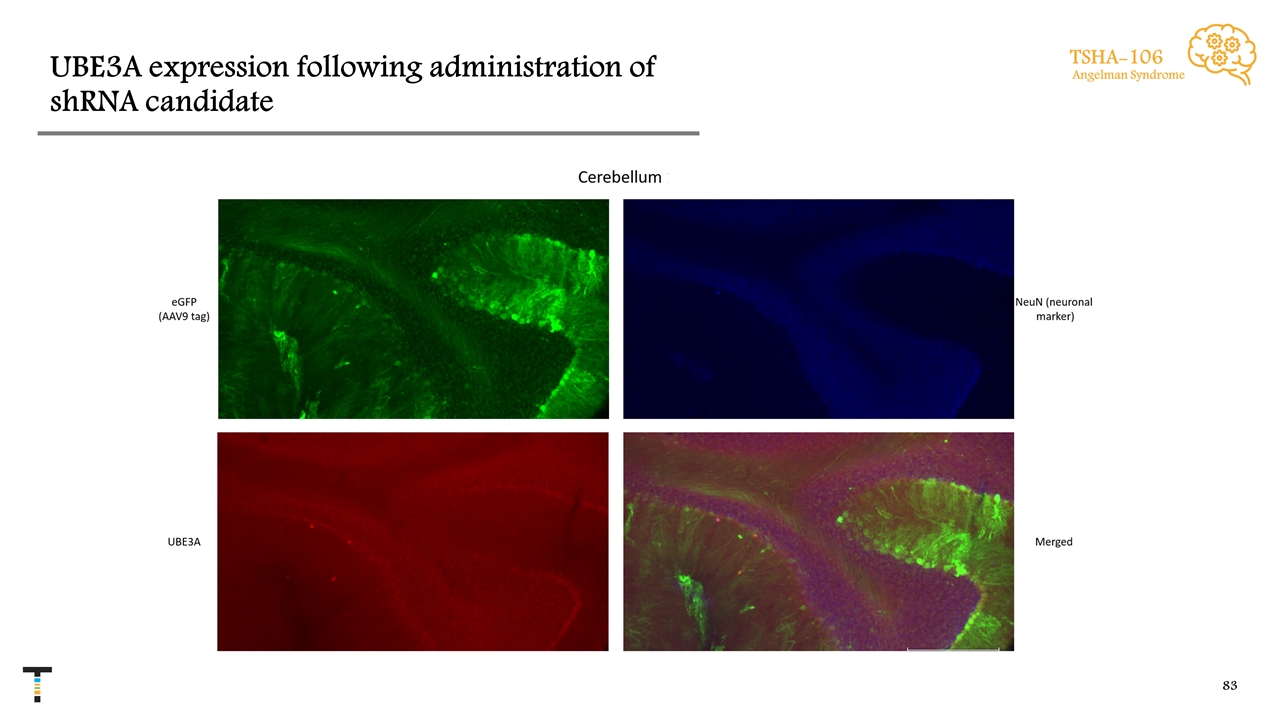
UBE3A expression following administration of shRNA candidate TSHA-106 Angelman Syndrome

Anticipated next steps for TSHA-106 Evaluate dose response and age response & finalize dose from pharmacology Interventional trial protocol development underway Interim expression and safety data from confirmatory NHP studies TSHA-106 Angelman Syndrome

Q & A Angelman Syndrome TSHA-106

Closing Remarks RA Session II President, Founder & CEO
Focused on achieving anticipated near-term milestones in 2021 and building long-term value Gene Replacement GAN clinical program update, including 3.5 x 1014 total vg cohort GM2 gangliosidosis preliminary biomarker data in 2H 2021 CLN1 program to dose first patient in 2021 under open IND 4 open IND/CTAs expected by the end of 2021, including Rett syndrome Initiated construction of internal cGMP facility in 1H 2021 5 additional programs currently in IND-enabling studies Manufacturing Investor Day July 27, 2021 TSHA-118 CLN1 Investor Day August 2021 TSHA-102 Rett syndrome Investor DaySeptember 2021 Numerous value generating catalysts over the next 18 months
Upcoming Investor Mini-Series Gene Replacement Manufacturing Investor Day July 27, 2021 TSHA-118 CLN1 Investor DayAugust 2021 TSHA-102 Rett syndrome Investor DaySeptember 2021
Thank you
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Indivior Announces Q1 2024 Financial Results
- Green Hydrogen Systems and BWSC sign strategic collaboration agreement on green hydrogen projects
- Strategic Review Announcement
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share