Form 8-K SELECTA BIOSCIENCES INC For: Oct 23
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of
the Securities Exchange Act of 1934
Date of report (Date of earliest event reported): October 23, 2018
SELECTA BIOSCIENCES, INC.
(Exact name of registrant as specified in its charter)
Delaware | 001-37798 | 26-1622110 | ||
(State or other jurisdiction of incorporation or organization) | (Commission File Number) | (I.R.S. Employer Identification No.) | ||
480 Arsenal Way
Watertown, MA 02472
(Address of principal executive offices) (Zip Code)
(617) 923-1400
(Registrant’s telephone number, include area code)
N/A
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions
o | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
o | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
o | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
o | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company x
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. x
Item 7.01. Regulation FD Disclosure.
On October 23, 2018, Selecta Biosciences, Inc. (the “Company”) announced new interim data from its ongoing Phase 2 Company-sponsored trial of SEL-212, for the treatment of chronic severe gout, which is assessing single ascending dose safety, pharmacokinetics and pharmacodynamics of SEL-212 in patients with elevated uric acid levels. The full text of the press release issued in connection with the announcement is furnished as Exhibit 99.1 to this Current Report on Form 8-K.
The Company will present the presentation posters ("Presentation Posters") furnished as Exhibits 99.2, 99.3 and 99.4 to this Current Report on Form 8-K, which contains new interim data from patients receiving up to 0.15 mg/kg of SVP-Rapamycin with 0.2 or 0.4 mg/kg of pegadricase from the Phase 2 trial at the 2018 American College of Rheumatology (ACR)/Association for Rheumatology Health Professionals (ARHP) Annual Meeting in Chicago, IL on October 23, 2018.
In connection with the issuance of the press release, the Company is holding a public conference call and webcast on October 23, 2018, at 8:00 a.m. ET, during which the Company will provide the investor presentation attached as Exhibit 99.5 to this Current Report on Form 8-K. The Company undertakes no obligation to update, supplement or amend the materials attached hereto as Exhibit 99.5.
The information furnished under this Item 7.01, including Exhibits 99.1, 99.2, 99.3, 99.4 and 99.5 attached hereto, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such a filing.
Item 8.01. Other Events.
On October 23, 2018, in connection with the presentation and/or distribution of the Presentation Posters, the Company announced new interim data from patients in its Phase 2 trial of SEL-212 receiving five monthly combination doses of SEL-212, consisting of up to 0.15 mg/kg of SVP-Rapamycin in combination with 0.2 or 0.4 mg/kg of pegadricase. In the new cohorts, projections based on the rate of serum uric acid (“SUA”) control for patients who have completed the treatment period suggest that approximately 66% of the evaluable patients may maintain SUA level control below 6 mg/dL throughout five months of therapy with concurrent mitigation of anti-drug antibodies (“ADAs”) against the pegadricase enzyme. However, the Company notes that caution should be exercised in drawing any conclusions from projections of clinical data.
SEL-212 is a monthly combination product candidate being developed as a potential therapy for the sustained control of SUA leading to the removal of urate crystal deposits in patients with chronic severe gout. In the ongoing Phase 2 clinical trial SEL-212 has shown potential reduction of urate deposits in symptomatic gout patients with hyperuricemia as suggested by dual-energy computed tomography (“DECT”). DECT scans of patients enrolled in the Phase 2 clinical trial suggest that a decrease in total urate deposits has occurred progressively over the entire treatment period.
Initiation of urate lowering therapy can increase the incidence of gout flares which can adversely affect patient experience and compliance. Treatment with SVP-Rapamycin, a component of SEL-212, mitigated IL-1β production and neutrophil infiltrates in a monosodium urate-induced model of inflammation in mice. Accordingly, the Company believes that SEL-212 may have potential to reduce gout flares by inhibiting inflammation despite rapid and sustained lowering of SUA.
SEL-212 has been generally well-tolerated in the Phase 2 clinical study.
Forward-Looking Statements Disclaimer
This Current Report on Form 8-K (the “Current Report”) contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this Current Report that do not relate to matters of historical fact should be considered forward-looking statements, including without limitation statements regarding the progress of the Phase 2 clinical trial of SEL-212, whether SEL-212 mitigates immunogenicity and enables sustained control of serum uric acid levels, low rate of gout flares and monthly dosing, the ability of SVP-Rapamycin to mitigate inflammation induced by monosodium urate crystals, the anticipated timing for advancing into Phase 3 (if at all), whether current evaluable SEL-212 patients will be predictive of future evaluable SEL-212 patients, whether projections regarding serum uric acid control for patients who have yet to complete the 20-week study period will be consistent with actual data, whether monthly dosing of SEL-212 leads to significant reduction in uric acid deposits, the potential of SEL-212 to significantly reduce tophi/heavy urate burden and/or rapidly eliminate tissue urate burden, whether SEL-212 has the ability to reduce gout flares frequency initially and over time during SEL-212 therapy, the severity of gout flares experienced by patients receiving SEL-212, and whether SEL-212 will continue to be generally well-tolerated. These forward-looking statements are based on
management’s current expectations. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: the uncertainties inherent in the initiation, completion and cost of clinical trials including their uncertain outcomes; the unproven approach of our SVP technology; undesirable side effects of our product candidates; our reliance on third parties to manufacture our product candidates and to conduct our clinical trials; our inability to maintain our existing or future collaborations or licenses; our inability to protect our proprietary technology and intellectual property; potential delays in regulatory approvals; our dependence on our ability to retain key executives and to attract, retain and motivate qualified personnel; and availability of funding sufficient for our foreseeable and unforeseeable operating expenses and capital expenditure requirements. These and other important factors discussed under the caption “Risk Factors” in our Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission, or SEC, on August 8, 2018, and our other reports filed with the SEC could cause actual results to differ materially from those indicated by the forward-looking statements made in this Current Report. Any such forward-looking statements represent management’s estimates as of the date of this Current Report. While we may elect to update such forward-looking statements at some point in the future, we disclaim any obligation to do so, even if subsequent events cause our views to change. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this Current Report.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits
Exhibit No. | Description | |||
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
SELECTA BIOSCIENCES, INC. | ||
Date: October 23, 2018 | By: | /s/ Werner Cautreels, Ph.D. |
Werner Cautreels, Ph.D. | ||
President and Chief Executive Officer | ||
Exhibit 99.1

Selecta Biosciences Presents New Interim Data from Phase 2 Trial of SEL-212, in Development for Chronic Severe Gout, at ACR 2018
• | Interim analysis indicates serum uric acid (SUA) control has been maintained into months four and five with once monthly combination treatment, SUA control projected to be 66% at end of study period |
• | Patient imaging has shown reduction in tissue urate deposits as measured by Dual Energy Computed Tomography (DECT) during SEL-212 treatment periods (months 1-5) and maintenance of SUA near 0 mg/dL |
• | Low flare rates observed to date in new patient cohorts over treatment period |
• | No new safety signals have been observed in the five combination treatment cohorts |
• | Phase 3 program planned to begin in 2018 with proposed dose regimens |
• | Company to host conference call and live webcast today at 8:00 am ET |
Watertown, Mass., October 23, 2018 - Selecta Biosciences, Inc. (Nasdaq: SELB), a clinical-stage biopharmaceutical company focused on unlocking the full potential of biologic therapies by mitigating unwanted immune responses, today presented new interim Phase 2 data from patients receiving SEL-212, a product candidate in development for the treatment of chronic severe gout designed to lower SUA, at the 2018 American College of Rheumatology (ACR)/Association for Rheumatology Health Professionals (ARHP) Annual Meeting in Chicago, IL.
SEL-212 is a combination product candidate designed to sustain control of SUA levels in patients with chronic severe gout, potentially reducing harmful tissue urate deposits which when left untreated can lead to debilitating gout flares and joint deformity. SEL-212 consists of pegadricase (formerly known as pegsiticase), a pegylated uricase, co-administered with SVP-Rapamycin, designed to mitigate the formation of anti-drug antibodies (ADAs). ADAs develop due to unwanted immune responses to biologic medicines, rendering these therapies less potent, which remains an issue across therapeutic modalities and disease states including chronic severe gout.
The interim data reported today at ACR consist of new cohorts of patients that received five monthly doses of SEL-212, at doses of 0.1 or 0.15 mg/kg of SVP-Rapamycin in combination with 0.2mg/kg of pegadricase. In the new cohorts, projections based on the rate of SUA control for patients who have completed the treatment period suggest that approximately 66% of the evaluable patients may maintain SUA level control below 6 mg/dL throughout five months of therapy with concurrent mitigation of ADAs against the pegadricase enzyme. Final data are still pending for five of these patients. Our projection for these five patients is based on the observation that all other patients in these cohorts that had serum uric acid levels <6 mg/dL at week 12 successfully maintained control of SUA through the entire five-month period. However, caution should be exercised in drawing any conclusions from projections of clinical data. Furthermore, the observed sustained maintenance of SUA near 0 mg/dL has led to rapid reduction in tissue urate deposits as measured by DECT imaging. DECT scans were performed as an exploratory measure to evaluate reduction of tissue urate burden in a subset of patients of the Phase 2 trial.
1
“Today’s reported interim data have met our goal of showing sustained SUA control over the five-month combination period. In addition, SEL-212 provides the added convenience of monthly dosing with a low incidence of flares observed in the Phase 2 clinical trial to date. And importantly, during months four and five of treatment, there have been no new emerging safety findings in the trial,” said Werner Cautreels, Ph.D., President and CEO of Selecta. “The reduction in tissue urate deposits in joints and tissue as shown by our DECT data presented today at ACR represents a potentially important benefit for patients whose disease is not responding to other treatments. With these data now in hand, we believe we are well positioned to execute on our Phase 3 program, which is expected to start later this year.”
Approximately 29% of the patient population treated with SEL-212 in the ongoing Phase 2 trial has experienced gout flares during the first month after treatment with continued reduction of gout flare rates out to month five. 96% of flares have been mild or moderate, and no flares have been reported as a serious adverse event (SAE) nor resulted in discontinuations of the study drug.
SEL-212 has been generally well tolerated at clinically active doses following repeated administrations in the trial. There have been 21 SAEs reported, 11 of which were reported to be not related or unlikely to be related to study drug, nine of which were infusion reactions that were previously reported by the company in June 2018, one of which was an infusion reaction that occurred in the most recent cohorts and one of which was reported to be related to study drug. No infusion reactions have been reported after treatment period two. As far as the Company is aware, all SAEs have been successfully treated without further issues.
Gout is the most common form of inflammatory arthritis with more than 8.3 million patients in the United States having been diagnosed with gout which is caused by high levels of uric acid in the body that accumulate around the joints and other tissues, and can result in flares that cause intense pain. Approximately 160,000 patients in the United States suffer from chronic severe gout, a painful and debilitating condition in which patients are not able to get their SUA levels below 6 mg/dL and therefore have several flares per year and can develop nodular masses of uric acid crystals known as tophi. Elevated SUA levels have been associated with diseases of the heart, vascular system, metabolism, kidney and joints.
Conference Call Reminder
The company will host a conference call via live webcast today at 8:00am ET. The live webcast of the presentation can be accessed via the Investors & Media section of the company’s website,
http://selectabio.com. Individuals may also participate in the live call via telephone by dialing 1-844-845-4170 (domestic) or 1-412-717-9621 (international) and may access a teleconference replay for one week by dialing 1-877-344-7529 (domestic) or 1-412-317-0088 (international) and using confirmation code 10124095.
About Selecta Biosciences, Inc.
Selecta Biosciences, Inc. is a clinical-stage biopharmaceutical company that is focused on unlocking the full potential of biologic therapies by mitigating unwanted immune responses. Selecta plans to combine its tolerogenic Synthetic Vaccine Particles (SVP™) to a range of biologics for rare and serious diseases that require new treatment options. The company’s current proprietary pipeline includes SVP-enabled enzyme, oncology and gene therapeutic candidates. SEL-212, the company’s lead candidate in Phase 2, is being developed to treat severe gout patients and resolve their debilitating symptoms, including flares and gouty arthritis. A Phase 1 trial was initiated for a combination therapeutic candidate consisting of SVP-Rapamycin and LMB-100 (Selecta’s SEL-403 product candidate) for the treatment of patients with malignant pleural or peritoneal mesothelioma. Selecta’s proprietary gene therapy product candidates are bei
2
ng developed for rare inborn errors of metabolism and have the potential to enable repeat administration. We believe the use of SVP also holds potential in the development of vaccines and treatments for allergies and autoimmune diseases. Selecta is based in Watertown, Massachusetts. For more information, please visit http://selectabio.com and follow @SelectaBio on Twitter.
Forward-Looking Statements
Any statements in this press release about the future expectations, plans and prospects of Selecta Biosciences, Inc. (“the company”), including without limitation, statements regarding the progress of the Phase 2 clinical trial of SEL-212, whether SEL-212 mitigates immunogenicity and enables sustained control of serum uric acid levels, low rate of gout flares and monthly dosing, the anticipated timing for advancing into Phase 3 (if at all), whether current evaluable SEL-212 patients will be predictive of future evaluable SEL-212 patients, whether projections regarding serum uric acid control for patients who have yet to complete the 20-week study period will be consistent with actual data, whether 5-monthly combination doses of SEL-212 have the potential to extend serum uric acid control and maintain safety over the entire treatment period, whether monthly dosing of SEL-212 leads to significant reduction in uric acid deposits, projections based on the rate of SUA control for patients who have completed the treatment period, the potential of SEL-212 to significantly reduce tophi/heavy urate burden and/or rapidly eliminate tissue urate burden, whether patients receiving SEL-212 will be able to complete full therapy cycles over 6 months, whether SEL-212 has the ability to reduce gout flares frequency initially and over time during SEL-212 therapy, the severity of gout flares experienced by patients receiving SEL-212, whether SEL-212 will continue to be generally well-tolerated, the company’s commercial plans, the ability of the company’s SVP platform, including SVP-Rapamycin, to mitigate unwanted immunogenicity, unlock the full potential of biologic therapies, enable new therapies and improve the efficacy and safety of existing biologics, the potential of SEL-212 to treat severe gout patients and resolve their debilitating symptoms, the potential of SEL-403 to treat mesothelioma, the potential treatment applications for products utilizing the SVP platform in areas such as enzyme therapy, gene therapy, oncology therapy, vaccines and treatments for allergies and autoimmune diseases, the company’s plan to apply its SVP platform to a range of biologics for rare and serious diseases, the potential of the company’s two gene therapy product candidates to enable repeat administration, the potential of the SVP-Rapamycin platform generally, and other statements containing the words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “hypothesize,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “would,” and similar expressions, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including, but not limited to, the following: the uncertainties inherent in the initiation, completion and cost of clinical trials including their uncertain outcomes, the availability and timing of data from ongoing and future clinical trials and the results of such trials, whether preliminary results from a particular clinical trial will be predictive of the final results of that trial or whether results of early clinical trials will be indicative of the results of later clinical trials, the unproven approach of the company’s SVP technology, potential delays in enrollment of patients, undesirable side effects of the company’s product candidates, its reliance on third parties to manufacture its product candidates and to conduct its clinical trials, the company’s inability to maintain its existing or future collaborations, licenses or contractual relationships, its inability to protect its proprietary technology and intellectual property, potential delays in regulatory approvals, the availability of funding sufficient for its foreseeable and unforeseeable operating expenses and capital expenditure requirements, substantial fluctuation in the price of its common stock, and other important factors discussed in the “Risk Factors” section of the company’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission, or SEC, on August 8, 2018, and in other filings that the company makes with the SEC. In addition, any forward-looking statements included in this press release represent the company’s views only as of the date of its publication and should
3
not be relied upon as representing its views as of any subsequent date. The company specifically disclaims any obligation to update any forward-looking statements included in this press release.
4
Contact Information:
John Leaman, MD
Selecta Biosciences, Inc.
617-231-8081
Sarah McCabe
Stern Investor Relations, Inc.
+1-212-362-1200
5
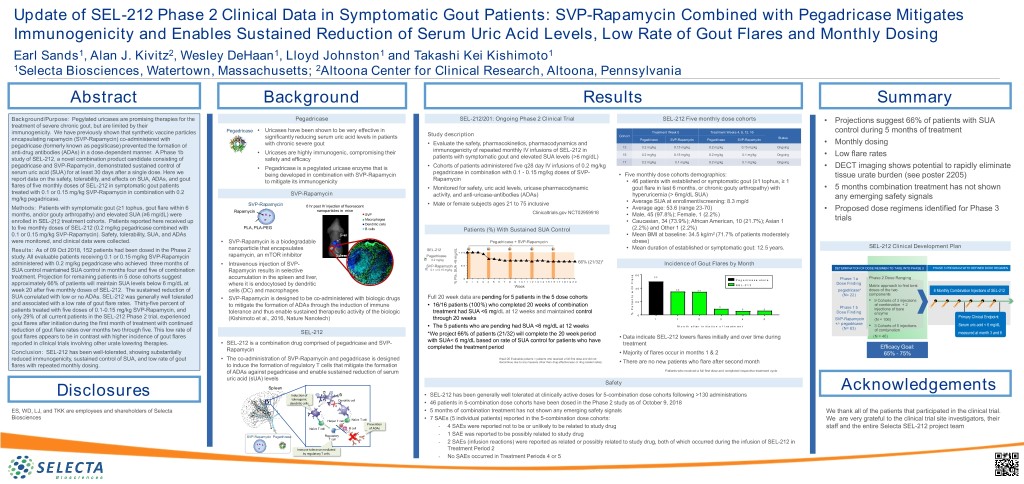
Update of SEL-212 Phase 2 Clinical Data in Symptomatic Gout Patients: SVP-Rapamycin Combined with Pegadricase Mitigates Immunogenicity and Enables Sustained Reduction of Serum Uric Acid Levels, Low Rate of Gout Flares and Monthly Dosing Earl Sands1, Alan J. Kivitz2, Wesley DeHaan1, Lloyd Johnston1 and Takashi Kei Kishimoto1 1Selecta Biosciences, Watertown, Massachusetts; 2Altoona Center for Clinical Research, Altoona, Pennsylvania Abstract Background Results Summary Background/Purpose: Pegylated uricases are promising therapies for the Pegadricase SEL-212/201: Ongoing Phase 2 Clinical Trial SEL-212 Five monthly dose cohorts • Projections suggest 66% of patients with SUA treatment of severe chronic gout, but are limited by their immunogenicity. We have previously shown that synthetic vaccine particles Pegadricase • Uricases have been shown to be very effective in Treatment Week 0 Treatment Weeks 4, 8, 12, 16 control during 5 months of treatment Study description Cohort significantly reducing serum uric acid levels in patients Status encapsulating rapamycin (SVP-Rapamycin) co-administered with Pegadricase SVP-Rapamycin Pegadricase SVP-Rapamycin with chronic severe gout • Evaluate the safety, pharmacokinetics, pharmacodynamics and • Monthly dosing pegadricase (formerly known as pegsiticase) prevented the formation of 13 0.2 mg/kg 0.15 mg/kg 0.2 mg/kg 0.15 mg/kg Ongoing anti-drug antibodies (ADAs) in a dose-dependent manner. A Phase 1b • Uricases are highly immunogenic, compromising their immunogenicity of repeated monthly IV infusions of SEL-212 in 15 0.2 mg/kg 0.15 mg/kg 0.2 mg/kg 0.1 mg/kg Ongoing • Low flare rates study of SEL-212, a novel combination product candidate consisting of safety and efficacy patients with symptomatic gout and elevated SUA levels (>6 mg/dL) 17 0.2 mg/kg 0.1 mg/kg 0.2 mg/kg 0.1 mg/kg Ongoing pegadricase and SVP-Rapamycin, demonstrated sustained control of • Pegadricase is a pegylated uricase enzyme that is • Cohorts of patients administered five q28 day IV infusions of 0.2 mg/kg • DECT imaging shows potential to rapidly eliminate serum uric acid (SUA) for at least 30 days after a single dose. Here we being developed in combination with SVP-Rapamycin pegadricase in combination with 0.1 - 0.15 mg/kg doses of SVP- • Five monthly dose cohorts demographics: tissue urate burden (see poster 2205) report data on the safety, tolerability, and effects on SUA, ADAs, and gout to mitigate its immunogenicity Rapamycin • 46 patients with established or symptomatic gout (≥1 tophus, ≥ 1 flares of five monthly doses of SEL-212 in symptomatic gout patients • Monitored for safety, uric acid levels, uricase pharmacodynamic gout flare in last 6 months, or chronic gouty arthropathy) with • 5 months combination treatment has not shown treated with 0.1 or 0.15 mg/kg SVP-Rapamycin in combination with 0.2 SVP-Rapamycin activity, and anti-uricase-antibodies (ADAs) hyperuricemia (> 6mg/dL SUA) any emerging safety signals mg/kg pegadricase. • Average SUA at enrollment/screening: 8.3 mg/d SVP-Rapamycin • Male or female subjects ages 21 to 75 inclusive Methods: Patients with symptomatic gout (≥1 tophus, gout flare within 6 6 hr post IV injection of fluorescent • Average age: 53.6 (range 23-70) • Proposed dose regimens identified for Phase 3 Rapamycin nanoparticles in mice Clinicaltrials.gov NCT02959918 months, and/or gouty arthropathy) and elevated SUA (≥6 mg/dL) were SVP • Male, 45 (97.8%); Female, 1 (2.2%) enrolled in SEL-212 treatment cohorts. Patients reported here received up Macrophages • Caucasian, 34 (73.9%); African American, 10 (21.7%); Asian 1 trials Dendritic cells to five monthly doses of SEL-212 (0.2 mg/kg pegadricase combined with PLA, PLA-PEG B cells Patients (%) With Sustained SUA Control (2.2%) and Other 1 (2.2%) 0.1 or 0.15 mg/kg SVP-Rapamycin). Safety, tolerability, SUA, and ADAs • Mean BMI at baseline: 34.5 kg/m2 (71.7% of patients moderately were monitored, and clinical data were collected. • SVP-Rapamycin is a biodegradable Pegadricase + SVP-Rapamycin obese) SEL-212 Clinical Development Plan nanoparticle that encapsulates SEL-212 • Mean duration of established or symptomatic gout: 12.5 years. Results: As of 09 Oct 2018, 152 patients had been dosed in the Phase 2 1 0 0 study. All evaluable patients receiving 0.1 or 0.15 mg/kg SVP-Rapamycin rapamycin, an mTOR inhibitor Pegadricase 0.2 mg/kg administered with 0.2 mg/kg pegadricase who achieved three months of 66% (21/32)* • Intravenous injection of SVP- SVP-Rapamycin 5 0 Incidence of Gout Flares by Month DETERMINATION OF DOSE REGIMEN TO TAKE INTO PHASE 3 PHASE 3 PROGRAM WITH DEFINED DOSE REGIMEN SUA control maintained SUA control in months four and five of combination Rapamycin results in selective 0.1 or 0.15 mg/kg e r 6 0 a 5 0 treatment. Projection for remaining patients in 5 dose cohorts suggest accumulation in the spleen and liver, 0 l Phase 2 Dose Ranging P e g a d r i c a s e a l o n e Phase 1 a 0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0 F % mg/dLPts.<6 SUA % Dose Finding approximately 66% of patients will maintain SUA levels below 6 mg/dL at where it is endocytosed by dendritic h S E L - 2 1 2 t Matrix approach to find best Week i 4 0 3 5 3 4 week 20 after five monthly doses of SEL-212. The sustained reduction of w pegadricase* doses of the two 6 Monthly Combination Injections of SEL-212 cells (DC) and macrophages s components t (N= 22) SUA correlated with low or no ADAs. SEL-212 was generally well tolerated • SVP-Rapamycin is designed to be co-administered with biologic drugs Full 20 week data are pending for 5 patients in the 5 dose cohorts n e • 9 Cohorts of 3 injections i 2 0 and associated with a low rate of gout flare rates. Thirty-five percent of t of combination + 2 • 16/16 patients (100%) who completed 20 weeks of combination a to mitigate the formation of ADAs through the induction of immune 9 Phase 1 b P 6 6 injections of bare patients treated with five doses of 0.1-0.15 mg/kg SVP-Rapamycin, and tolerance and thus enable sustained therapeutic activity of the biologic treatment had SUA <6 mg/dL at 12 weeks and maintained control Dose Finding % enzyme only 29% of all current patients in the SEL-212 Phase 2 trial, experienced 0 Primary Clinical Endpoint: (Kishimoto et al., 2016, Nature Nanotech) through 20 weeks 1 1 2 3 4 5 SVP-Rapamycin (N = 106) gout flares after initiation during the first month of treatment with continued +/- pegadricase • The 5 patients who are pending had SUA <6 mg/dL at 12 weeks M o n t h a f t e r i n i t i a t i o n o f t r e a t m e n t • 3 Cohorts of 5 injections Serum uric acid < 6 mg/dL reduction of gout flare rates over months two through five. This low rate of (N= 63) of combination SEL-212 *We project 66% of patients (21/32) will complete the 20 week period measured at month 3 and 6 gout flares appears to be in contrast with higher incidence of gout flares • Data indicate SEL-212 lowers flares initially and over time during (N = 46) with SUA< 6 mg/dL based on rate of SUA control for patients who have reported in clinical trials involving other urate lowering therapies. • SEL-212 is a combination drug comprised of pegadricase and SVP- treatment Rapamycin completed the treatment period Efficacy Goal: Conclusion: SEL-212 has been well-tolerated, showing substantially • Majority of flares occur in months 1 & 2 65% - 75% reduced immunogenicity, sustained control of SUA, and low rate of gout • The co-administration of SVP-Rapamycin and pegadricase is designed Week 20 Evaluable patients = patients who received a full first dose and did not discontinue due to any measure other than drug effectiveness or drug related safety • There are no new patients who flare after second month flares with repeated monthly dosing. to induce the formation of regulatory T cells that mitigate the formation of ADAs against pegadricase and enable sustained reduction of serum Patients who received a full first dose and completed respective treatment cycle uric acid (sUA) levels Safety Spleen Acknowledgements Disclosures Induction of • SEL-212 has been generally well tolerated at clinically active doses for 5-combination dose cohorts following >130 administrations tolerogenic Dendritic cell dendritic cells • 46 patients in 5-combination dose cohorts have been dosed in the Phase 2 study as of October 9, 2018 ES, WD, LJ, and TKK are employees and shareholders of Selecta • 5 months of combination treatment has not shown any emerging safety signals We thank all of the patients that participated in the clinical trial. Biosciences Naïve T cell • 7 SAEs (5 individual patients) reported in the 5-combination dose cohorts: We are very grateful to the clinical trial site investigators, their Mitigation of Helper T cell Prevention - ImmunogenicityNaïve T cell B cell of ADAs 4 SAEs were reported not to be or unlikely to be related to study drug staff and the entire Selecta SEL-212 project team with Tolerogenic - 1 SAE was reported to be possibly related to study drug SVP-Rapamycin Pegadricase Nanoparticles Regulatory T cell - 2 SAEs (infusion reactions) were reported as related or possibly related to study drug, both of which occurred during the infusion of SEL-212 in Immune tolerance mediated Treatment Period 2 by regulatory T cells - No SAEs occurred in Treatment Periods 4 or 5

Initial Phase 2 Clinical Data of SEL-212 in Symptomatic Gout Patients: Measurement of Dissolution of Urate Deposits Associated with Monthly Dosing of a Pegylated Uricase (Pegadricase) with SVP-Rapamycin By Dual Energy Computed Tomography Rehan Azeem1, Earl Sands1, Lloyd Johnston1, Wesley DeHaan1, Alan J. Kivitz2, Takashi Kei Kishimoto1, Justin Park1 and Savvas Nicolaou3, 1Selecta Biosciences, Watertown, Massachusetts; 2Altoona Center for Clinical Research, Altoona, Pennsylvania; 3University of British Columbia, Vancouver, BC, Canada Abstract Background Results Summary Pegadricase Overview of DECT Scans Performed in SEL-212/201 Study Total Urate Change in Patients with at Least One Follow Up Scan Background: Pegylated uricases are therapies for treatment of severe chronic gout, • SEL-212 is a monthly combination product particularly for rapid resolution of tophi. However, uricases are limited by induction of • Uricases have been shown to be very effective in To investigate changes to uric acid deposits, DECT scans were performed Pegadricase Number of anti-drug antibodies (ADAs) that can compromise efficacy and safety. SEL-212 is a Baseline Initial Urate Final Urate Change in candidate being developed as a therapy for significantly reducing serum uric acid levels in patients at the following timepoints as an exploratory measure Patient Study novel combination product candidate consisting of pegadricase (formerly known as SUA Volume Volume urate volume with chronic severe gout - Baseline # Drug pegsiticase) co-administered with synthetic vaccine particles encapsulating (mg/dL) (cm3) (cm3) (%) the sustained control of SUA leading to the • Uricases are highly immunogenic, compromising their - After Treatment Period 3 Infusions rapamycin (SVP-Rapamycin). We report initial Phase 2 data on the effect of the - After Treatment Period 5 or at Early Termination Visit removal of urate crystal deposits in patients intensive lowering of serum uric acid (SUA) levels by SEL-212 on the dissolution of safety and efficacy 1 8.2 5 13.27 0.24 -98.19 Baseline DECT scan was performed in 31 dosed patients, with 19 patients monosodium urate (MSU) crystals in symptomatic gout patients. • Pegadricase is a pegylated uricase enzyme that is 2 6.3 3 0.13 0.24 84.62 with chronic severe gout undergoing a follow-up DECT scan as of 09 Oct 2018 Dual-energy computed tomography (DECT) may be used to differentiate urate being developed in combination with SVP-Rapamycin 3 8.5 2 0.62 0.05 -91.94 crystals from calcium by using specific attenuation characteristics, to diagnose gout. to mitigate its immunogenicity • Demographics for Patients with Follow-Up DECT Scans 4 7.6 5 0.00 0.01 NA* • Average SUA at enrollment/screening: 7.9 mg/dL • SEL-212 has been well-tolerated, and, DECT uses a computer algorithm to produce color-coded images that render uric 5 7.4 5 1.20 0.13 -89.17 • Average age: 55.4 (range 43-71) acid green, cortical bone blue, and trabecular bone purple. In tophaceous gout SVP-Rapamycin 6 8 5 0.39 0.00 -100.00 compared to pegylated uricase alone, has • Male, 17 (89.5%) patients, DECT may be used for serial volumetric quantification of subclinical tophi to 7 6.7 5 0.00 0.04 NA* SVP-Rapamycin 6 hr post IV injection of fluorescent • Caucasian: 52.6%; African American: 47.4% mitigated immunogenicity, reduced flare evaluate response to treatment. 8 8.9 5 0.22 0.06 -72.73 Rapamycin nanoparticles in mice • Mean BMI at baseline: 33.1 kg/m2 (63.2% of patients were moderately SVP 9 7.7 5 0.46 Methods: Patients with symptomatic gout (≥1 tophus, gout flare within 6 months or Macrophages obese) 0.40 -13.04 rates, and enabled repeated monthly gouty arthropathy) and elevated SUA ≥6 mg/dL were treated with monthly doses of Dendritic cells • Mean duration of established or symptomatic gout: 8 years 10 8.9 3 2.27 0.51 -77.53 PLA, PLA-PEG B cells dosing with sustained control of SUA levels pegadricase (0.2 mg/kg or 0.4 mg/kg) alone or in combination with SVP-Rapamycin 11 8.8 5 0.32 0.42 31.25 (0.05 to 0.15 mg/kg). SEL-212 was infused in 28-day cycles x3 doses followed by • SVP-Rapamycin is a Change in Tissue Urate Volume with SEL-212 Treatment 12 11.1 2 0.32 0.03 -90.63 challenge with pegadricase alone on 28-day cycles x2 doses, or in 28-day cycles x5 biodegradable nanoparticle that 13 8.2 5 0.45 0.29 -35.56 • SEL-212 has a significant impact on the combination doses of SVP-Rapamycin and pegadricase. encapsulates rapamycin, an 14 6 5 4.53 0.37 -91.83 Patient 1 reduction of urate deposits in symptomatic To investigate changes in uric acid deposits, DECT scans of hand/wrist, feet/ankles, mTOR inhibitor ) 3 1 0 15 6.4 5 0.48 0.06 -87.50 and knees were performed as an exploratory measure in a subset of patients, during m Patient 14 16^ 6.5 5 0.13 • Intravenous injection of SVP- c 0.05 -61.54 gout patients with hyperuricemia as ( the screening visit, treatment period 3, treatment period 5 or at early termination visit. Rapamycin results in selective e 17^ 9.6 5 0.31 0.00 -100.00 m confirmed by DECT DECT images were analyzed by Arthritis Research Canada (ARC) by two DECT accumulation in the spleen and 18^ 8.6 5 0.17 0.00 -100.00 u l 1 Radiologists utilizing a Syngo Via DECT software package liver, where it is endocytosed by o 19^ 7.5 1 0.00 0.00 NA* V dendritic cells and macrophages • Significant decrease in total urate deposits Results: As of 09 Oct 2018, an initial DECT scan was performed in a subset of 31 e t • Patients with available repeat scans are included in this summary • SVP-Rapamycin is designed to be co-administered with biologic a of 152 dosed patients, with 19 patients with an available follow-up DECT scan. r occurs progressively over the entire U • For the 31 patients with baseline scans, the range of urate drugs to mitigate the formation of ADAs through the induction of 0 . 1 The demographics for patients (N=19) who received a follow-up DECT scan were 43 e volume at baseline was 0.00 to 61.05 cm3 immune tolerance and thus enable sustained therapeutic activity of u treatment period as observed by DECT - 71 years old (mean 55.4 years), male 89.5%, African American 47.4%, and s the biologic (Kishimoto et al, 2016, Nature Nanotech) s • For the 19 patients with repeat scans as of Oct 9 2018, the Caucasian 52.6%. Mean BMI at baseline was 33.1 kg/m2, with 63% of patients i T range of urate volume at baseline was 0.00 to 13.27 cm3 being obese. Mean duration of gout was 8.0 years. 0 . 0 1 • Mean time between the initial and most recent/follow-up DECT SEL-212 The mean SUA at the screening visit was 7.9 mg/dl. Time between the initial and - 4 0 4 8 1 2 1 6 2 0 scan: 121.2 days (ranged from 77 to 163 days) most recent/follow-up DECT scan ranged from 77 - 163 days (mean 121 days), with • SEL-212 is a combination drug candidate comprised of W e e k s P o s t - I n i t i a t i o n o f T r e a t m e n t *NA = Not Applicable, ^Patients in 5 combination dose cohorts a mean change in total urate volume of -1.18 cm3 (range: 0.11 to -13.03 cm3). pegadricase and SVP-Rapamycin Acknowledgements Conclusion: SEL-212 has a significant impact on the reduction of urate deposits in • The co-administration of SVP-Rapamycin and pegadricase is Patient 1 DECT Scan Images Show Significant Reduction of Tissue Urate Burden Patient 14: Before and After Treatment symptomatic gout patients with hyperuricemia as confirmed by DECT. designed to induce the formation of regulatory T cells that mitigate the formation of ADAs against pegadricase and enable Baseline Treatment Period 3 End of Study We thank all of the patients that participated in the clinical trial. We sustained reduction of serum uric acid (SUA) levels are very grateful to the clinical trial site investigators, their staff and ) 3 3 I n i t ia l S c a n the entire Selecta SEL-212 project team Spleen m c F o l lo w - U p S c a n Induction of ( tolerogenic Dendritic cell DECT Imaging e dendritic cells 2 m u • DECT(Dual Energy Computed Tomography) is a sensitive and non-invasive l Naïve T cell o 1,2 Mitigation of Helper T cell V Disclosures diagnostic tool to quantify tissue urate burden Prevention 1 ImmunogenicityNaïve T cell B cell of ADAs 1 e Choi HK et al., Ann Rheum Dis. 2009, 68:1609-12. with Tolerogenic t 2 Regulatory Araujo EG et al, RMD Open. 2015, 1:e000075. SVP-Rapamycin Pegadricase Nanoparticles a T cell r RA, WD, LJ, TKK, JP, and ES are employees and shareholders of U 0 Immune tolerance mediated Selecta Biosciences by regulatory T cells t e e i s l e r k n n W K / / A • Ongoing Phase 2 clinical trial of SEL-212 has demonstrated low d t n o incidence of ADAs resulting in sustained reduction of serum uric a o H F acid (SUA) with monthly dosing (see Abstract 2254)

Mitigation of Inflammation Induced By Monosodium Urate Crystals in Mice By Treatment with SVP-Rapamycin Pallavi Kolte, Robert LaMothe, Joseph Ferrari, Sheldon Leung, Wesley DeHaan, Earl Sands and Takashi Kei Kishimoto Selecta Biosciences, Watertown, Massachusetts Abstract Background Results Background/Purpose: Initiation of urate-lowering therapies is typically Gout Flares Air pouch model of MSU-induced inflammation SVP-Rapamycin inhibits MSU-induced inflammation in air pouch associated with an increase in gout flares due to mobilization of pro-inflammatory urate crystals. SEL-212 is a novel combination product candidate consisting of pegadricase (formerly known as pegsiticase), a pegylated uricase, co- • Acute gout attacks are characterized by a rapid onset of pain in administered with synthetic vaccine particles encapsulating rapamycin (SVP- the affected joint followed by warmth, swelling and pain1 Rapamycin) being developed for the treatment of chronic severe gout. Data from Monosodium • 69% of gout patients describe the pain of an attack as the ongoing open-label Phase 2 multidose study of SEL-212 indicate that SVP- urate crystals Rapamycin mitigates the formation of anti-drug antibodies (ADAs) against “miserable”, 23% of patients compare the pain of a gout attack to pegadricase, enabling monthly dosing and sustained control of serum uric acid shattered glass piercing their skin, 28% to breaking a bone, 34% PBS or SVP-Rapamycin (IV) (SUA) levels in most patients. Despite rapid and sustained reduction of SUA, to a severe burn2 patients treated with SEL-212 experienced a low rate of flares. Here we evaluated in animal studies whether SVP-Rapamycin might have a beneficial • Most people with gout will experience repeated bouts over the IL-1β Neutrophil exudate years * effect on reducing inflammation induced by monosodium urate crystals (MSU) in ) 6 * * * * 1 2 5 0 0 1 . 0 addition to its effects on mitigating the formation of ADAs. www.uptodate.com/contents/treatment-of-gout-flares * * 1 x ( ) 2 2 0 0 s 0 . 8 www.webmd.com/arthritis/news/20100611/gout-survey-offers-peek-at- L l l e Methods: MSU-induced inflammation was investigated in an air pouch model in m / the-pain c g 1 5 0 0 . 6 C57Bl/6 mice. An air pouch was generated on the dorsal aspect of a mouse by + p ( G 6 ß injecting sterile air on d0 and d3. Mice were treated intravenously with placebo or 1 0 0 y 0 . 4 L 1 - + SVP-Rapamycin on d7. MSU crystals were injected in the air pouch on d8 and Effect of Urate Lowering Therapies on Gout Flares L b I 5 0 0 . 2 1 mice were sacrificed 5 hours after MSU injection. Air pouch exudate was 1 0 D 0 . 0 analyzed for cellularity and interleukin-1β (IL-1β) levels as markers of • Dispersion of MSU crystals during the initial phase of deposit C l l o S P S P r o • C57BL/6 mice injected with sterile air subcutaneously on d0 and d3. t B V t r B V inflammation. dissolution exposes the patient to an increased rate of acute n P S n P S o o c C S S flares • MSU crystals injected into air pouch on d6 B Results: Injection of MSU crystals into the air pouch of a mouse has been M S U C h a l l e n g e B P M S U C h a l l e n g e previously shown to induce an acute inflammatory response characterized by P • Increased gout flare can adversely affect patient compliance3 • Sacrifice mice after 6 hours to collect exudate expression of IL-1β and an influx of neutrophils. Intravenous administration of SVP-Rapamycin reduced the generation of IL-1β in the air pouch exudate and • Pegylated uricase therapy, which rapidly debulks tissue uric acid, SVP-Rapamycin but not free rapamycin inhibits MSU-induced IL-1β the number of Ly6G+CD11b+ neutrophils. has been reported to induce gout flares in 75% of patients in the 4 Conclusion: SVP-Rapamycin has been shown to mitigate the formation of first months after initiation of therapy IL-1β Cell infiltrate S e r u m I L - 1 ADAs to biologic therapies by inducing tolerogenic dendritic cells and antigen- 1 5 0 * 3 ) • C57BL/6 mice I.V. treated with SVP- Becker MA et al., Nucleic Acids 2008 27:585-91 l 1 5 8 ) m l specific regulatory T cells. Here we demonstrate that SVP-Rapamycin also 4 / g Sundy et al, JAMA, 306 7:711-720 m Rapamycin or free rapamycin / p 6 ( attenuates inflammatory responses induced by MSU crystals and mediated by 6 1 0 0 e l 1 1 0 0 - 1 m L • Injected I.P. with MSU crystals 16 hours after * innate immune cells. These results may explain why gout patients treated with I / ( 4 g h s l p c pegadricase in combination with SVP-Rapamycin experience a low rate of gout 5 0 l treatment e MSU crystals induce activation of the inflammasome u 5 c o 2 p flares. e v r i i • Serum IL-1β assessed 6 hours after MSU L A 0 0 0 P B S M S U P B S M S U challenge • Monosodium urate (MSU) crystals induce inflammation through e x a a i v T p p a a a o R R activation of NLRP inflammasomes resulting in production of the N N - e P e r V SEL-212 pro-inflammatory cytokine IL-1β F S • SEL-212 is a combination drug candidate comprised of pegadricase (formerly known as pegsiticase) and SVP-Rapamycin Summary Acknowledgements • SVP-Rapamycin is designed to induce the formation of regulatory T cells that mitigate the formation of anti-drug antibodies (ADA) (Kishimoto et al, 2016, Nature Nanotech) • Initiation of urate lowering therapy can increase the incidence of gout We thank Dr. Robert Terkeltaub and Dr. Ru Bryan for • Ongoing Phase 2 clinical trial of SEL-212 has demonstrated low flares which can adversely affect patient experience and compliance helpful discussions incidence of ADAs resulting in sustained reduction of serum uric • Here we show that SVP-Rapamycin treatment can mitigate IL-1β acid (SUA) with monthly dosing (see Abstract 2254) production and neutrophil infiltrates in a monosodium urate-induced • Patients on SEL-212 therapy experienced a low level of gout flares model of inflammation in mice (see Abstract 1294) • An ongoing Phase 2 clinical trial of SEL-212 has demonstrated low 6 0 e 5 0 r incidence of anti-drug antibodies resulting in sustained reduction of a P e g a d r i c a s e a l o n e Disclosures l F S E L - 2 1 2 h serum uric acid (SUA) with monthly dosing (see Abstract 2254) t 4 0 i w 2 9 Ko and Matinon, Nature Rev Rheumatol, 2017, 13:639-647 s t 2 4 • The incidence of gout flares after the initiation of SEL-212 therapy was n The authors are employees and shareholders of Selecta e 2 0 i t 1 2 a lower than anticipated (See Abstract 1294) 9 P Biosciences 3 Potential effect of SVP-Rapamycin on inflammation % 0 • SEL-212 may have the additional benefit of reducing gout flares by 1 1 2 3 4 5 • Rapamycin has been reported to inhibit inflammasome activation5 M o n t h a f t e r i n i t i a t i o n o f t r e a t m e n t inhibiting inflammation despite rapid and sustained lowering of SUA • Here we evaluated the effect of SVP-Rapamycin on inflammation induced by MSU crystals in mice 5Ko et al., 2017, Oncotarget 8:40817-40831

SEL-212 Phase 2 Data Presented at ACR October 23, 2018

Safe Harbor / Disclaimer Any statements in this presentation about the future expectations, plans and prospects of Selecta Biosciences, Inc. (“the company”), including without limitation, statements regarding the progress of the Phase 2 clinical trial of SEL-212 and expectations surrounding End-of-Phase 2 meeting with the FDA, whether SEL-212 mitigates immunogenicity and enables sustained control of serum uric acid levels, low rate of gout flares and monthly dosing, the ability of SVP-Rapamycin to mitigate inflammation induced by monosodium urate crystals, the anticipated timing for advancing into Phase 3 (if at all) and expectations surrounding proposed dose regimens, whether current evaluable SEL-212 patients will be predictive of future evaluable SEL-212 patients, whether projections regarding serum uric acid control for patients who have yet to complete the 20-week study period will be consistent with actual data, whether 5-monthly combination doses of SEL-212 have the potential to extend serum uric acid control and maintain safety over the entire treatment period, whether monthly dosing of SEL-212 leads to significant reduction in uric acid deposits, the potential of SEL-212 to significantly reduce tophi/heavy urate burden and/or rapidly eliminate tissue urate burden, whether patients receiving SEL-212 will be able to complete full therapy cycles over 6 months, whether SEL-212 has the ability to reduce gout flares frequency initially and over time during SEL-212 therapy, the severity of gout flares experienced by patients receiving SEL-212, whether SEL-212 will continue to be generally well-tolerated, the design and timing of a head-to- head trial of SEL-212 and Krystexxa, the company’s commercial plans, the ability of the company’s SVP platform, including SVP-Rapamycin, to mitigate unwanted immunogenicity, unlock the full potential of biologic therapies, enable new therapies and improve the efficacy and safety of existing biologics, the potential of SEL-212 to treat severe gout patients, resolve their debilitating symptoms, and to change the chronic severe gout treatment paradigm, the company’s plan to apply its SVP platform to a range of biologics for rare and serious diseases, and other statements containing the words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “hypothesize,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “would,” and similar expressions, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including, but not limited to, the following: the uncertainties inherent in the initiation, completion and cost of clinical trials including their uncertain outcomes, the availability and timing of data from ongoing and future clinical trials and the results of such trials, whether preliminary results from a particular clinical trial will be predictive of the final results of that trial or whether results of early clinical trials will be indicative of the results of later clinical trials, the unproven approach of the company’s SVP technology, potential delays in enrollment of patients, undesirable side effects of the company’s product candidates, its reliance on third parties to manufacture its product candidates and to conduct its clinical trials, the company’s inability to maintain its existing or future collaborations or licenses, its inability to protect its proprietary technology and intellectual property, potential delays in regulatory approvals, the availability of funding sufficient for its foreseeable and unforeseeable operating expenses and capital expenditure requirements, substantial fluctuation in the price of its common stock, and other important factors discussed in the “Risk Factors” section of the company’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission, or SEC, on August 8, 2018, and in other filings that the company makes with the SEC. In addition, any forward-looking statements included in this presentation represent the company’s views only as of the date of its publication and should not be relied upon as representing its views as of any subsequent date. The company specifically disclaims any obligation to update any forward-looking statements included in this presentation. 2

SEL-212 Executive Summary of Available Phase 2 Data • Evaluable patients who achieved 3-month of SUA control have maintained SUA control in months 4 and 5 of combination treatment • Projection for remaining patients in 5 dose cohorts suggest approximately 66% of evaluable patients could have SUA controlled at week 20 after 5 monthly doses • Based on interim data, 5-monthly SEL-212 combination dose data has resulted in sustained SUA control & favorable tolerability over entire treatment period • SUA Control Observed • Once monthly dosing • Low flare rates • No emerging safety signals • Sustained control of SUA near 0 mg/dL may lead to reduction in uric acid deposits as measured by DECT (Dual Energy Computed Tomography) imaging • Proposed dose regimens for Phase 3 trials • 6 monthly doses of SEL-212 v. Placebo • 0.1 mg/kg of SVP-Rapamycin with 0.2 mg/kg of pegadricase* • 0.15 mg/kg of SVP-Rapamycin with 0.2 mg/kg of pegadricase 3 *Previously called pegsiticase; pegadricase is the new United States Adopted Name (USAN)

SEL-212 Clinical Development Plan DETERMINATION OF DOSE REGIMEN TO TAKE INTO PHASE 3 PHASE 3 PROGRAM WITH DEFINED DOSE REGIMEN Phase 1 a Phase 2 Dose Ranging Dose Finding Matrix approach to find pegadricase* best doses of the two 6 Monthly Combination Injections of SEL-212 (N= 22) components • 9 Cohorts of 3 injections of combination + 2 Phase 1 b injections of bare Dose Finding enzyme Primary Clinical Endpoint: SVP-Rapamycin (N = 106) +/- pegadricase • 3 Cohorts of 5 injections Serum uric acid < 6 mg/dL (N= 63) of combination measured at month 3 and 6 • (N = 46) Efficacy Goal: 65% - 75% Current Stage of SEL-212 Development 4
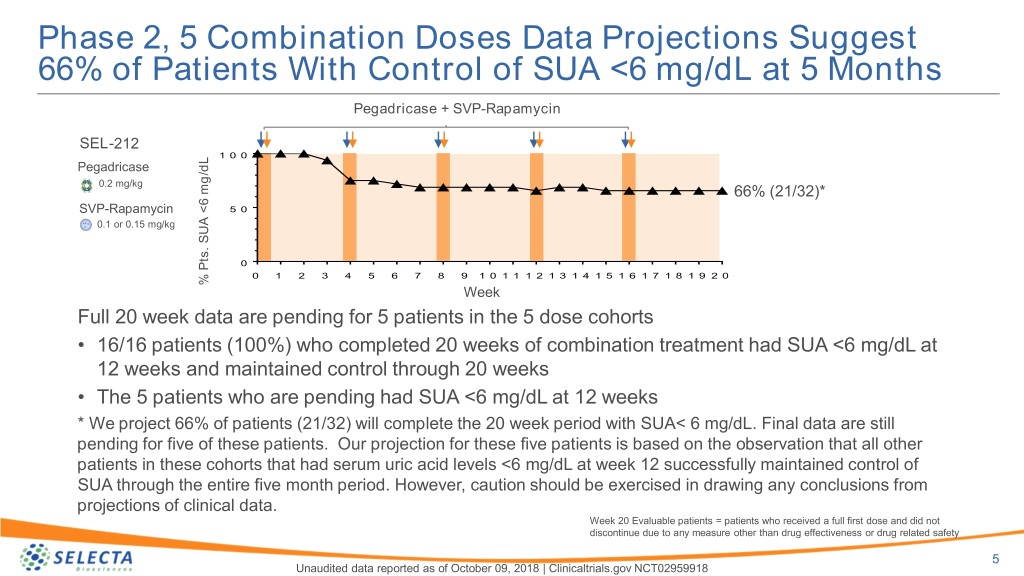
Phase 2, 5 Combination Doses Data Projections Suggest 66% of Patients With Control of SUA <6 mg/dL at 5 Months Pegadricase + SVP-Rapamycin SEL-212 1 0 0 Pegadricase 0.2 mg/kg 66% (21/32)* SVP-Rapamycin 5 0 0.1 or 0.15 mg/kg 0 0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0 % mg/dLPts.<6 SUA % Week Full 20 week data are pending for 5 patients in the 5 dose cohorts • 16/16 patients (100%) who completed 20 weeks of combination treatment had SUA <6 mg/dL at 12 weeks and maintained control through 20 weeks • The 5 patients who are pending had SUA <6 mg/dL at 12 weeks * We project 66% of patients (21/32) will complete the 20 week period with SUA< 6 mg/dL. Final data are still pending for five of these patients. Our projection for these five patients is based on the observation that all other patients in these cohorts that had serum uric acid levels <6 mg/dL at week 12 successfully maintained control of SUA through the entire five month period. However, caution should be exercised in drawing any conclusions from projections of clinical data. Week 20 Evaluable patients = patients who received a full first dose and did not discontinue due to any measure other than drug effectiveness or drug related safety 5 Unaudited data reported as of October 09, 2018 | Clinicaltrials.gov NCT02959918

Cohort 13: 0.15 mg/kg of SVP-Rapamycin + 0.2 mg/kg of pegadricase 0.15 0.15 0.15 0.15 0.15 SVP-Rapamycin Patient 1 0 0.2 0.2 0.2 0.2 0.2 pegadricase1 0 5 8 4 6 1 0 4 1 0 3 2 1 2 0 1 0 1 0 1 0 5 8 4 6 1 0 4 1 0 3 2 2 2 0 1 0 1 0 1 0 5 8 4 6 1 0 4 1 0 3 2 3 2 0 1 0 1 2 1 0 5 1 0 8 1 0 4 6 4 1 0 3 2 4 2 0 1 0 1 0 1 0 5 8 4 6 1 0 4 1 0 3 2 5 2 0 1 0 1 0 1 0 5 8 4 6 1 0 4 1 0 3 2 Anti 6 2 0 1 0 1 0 1 0 5 8 4 6 1 0 Week 20 Evaluable patients = patients who received a full 4 1 0 3 2 7 2 0 1 0 1 0 1 0 5 8 4 first dose and did not discontinue due to any measure 6 1 0 - 4 1 0 3 8 2 2 0 1 0 Uricase Antibody Titer 1 0 1 0 5 other than drug effectiveness or drug related safety 8 4 6 1 0 4 1 0 3 9 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 4 1 0 3 10 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 4 1 0 3 11 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 4 1 0 3 12 2 A 2 0 1 0 1 0 1 0 5 8 1 0 4 6 4 1 0 3 13 2 A 0 1 0 2 1 0 1 0 5 8 1 0 4 6 4 1 0 3 14 2 0 B 1 0 2 1 0 1 0 5 8 4 6 1 0 4 1 0 3 15 2 A Stopping rules met 0 A 1 0 2 1 0 1 0 5 8 4 6 1 0 4 1 0 3 16 2 A 2 0 1 0 1 0 1 0 5 8 4 6 1 0 4 1 0 3 17 2 Serum Uric Acid (mg/dL) Acid Uric Serum A 2 0 1 0 B SAE; infusion reaction 1 0 1 0 5 8 4 6 1 0 4 1 0 3 18 2 A 2 0 1 0 D Withdrawn due to protocol deviation 1 2 1 0 5 1 0 8 1 0 4 6 4 D 1 0 3 2 19 2 0 1 0 1 0 1 0 5 8 4 6 1 0 4 F 1 0 3 2 20 2 0 1 0 1 0 1 0 5 F Withdrawal of consent 8 4 6 1 0 4 F 1 0 3 2 21 2 0 1 0 1 0 1 0 5 8 4 6 1 0 4 F 1 0 3 22 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 G SAE; non-study drug related 4 G 1 0 3 2 23 0 1 0 2 0 1 2 3 4 5 6 7 8 9Week1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0 6 Unaudited data reported as of October 09, 2018 | Clinicaltrials.gov NCT02959918
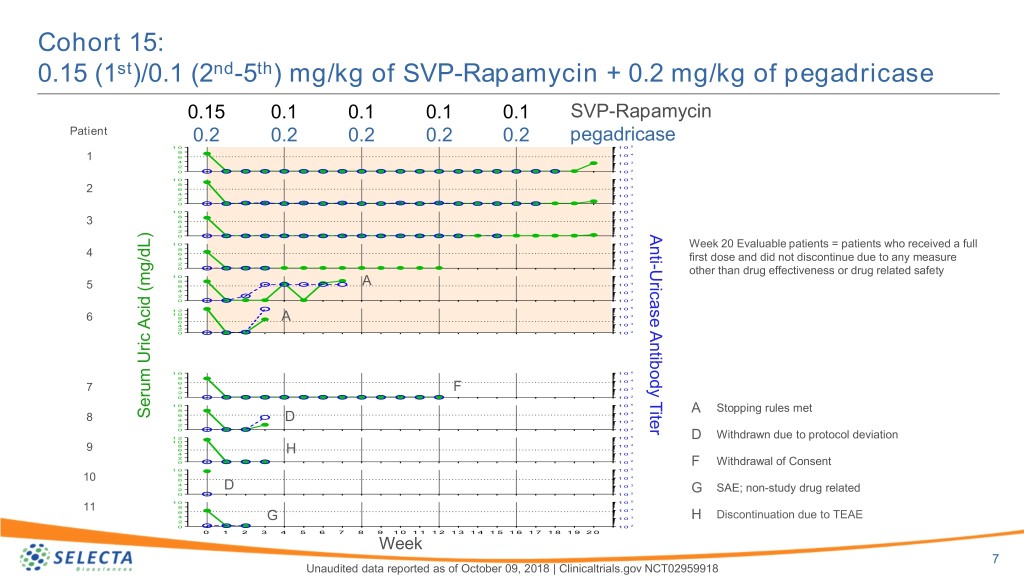
Cohort 15: 0.15 (1st)/0.1 (2nd-5th) mg/kg of SVP-Rapamycin + 0.2 mg/kg of pegadricase 0.15 0.1 0.1 0.1 0.1 SVP-Rapamycin Patient 0.2 0.2 0.2 0.2 0.2 pegadricase 1 0 1 0 5 8 4 1 6 1 0 4 1 0 3 2 0 1 0 2 1 0 1 0 5 8 4 2 6 1 0 4 1 0 3 2 0 1 0 2 1 0 1 0 5 8 4 3 6 1 0 4 1 0 3 2 Anti 0 1 0 2 1 0 1 0 5 Week 20 Evaluable patients = patients who received a full 8 4 4 6 1 0 4 1 0 3 first dose and did not discontinue due to any measure 2 - 2 0 1 0 Uricase Antibody Titer other than drug effectiveness or drug related safety 1 0 1 0 5 8 4 5 6 A 1 0 4 1 0 3 2 0 1 0 2 1 0 5 1 2 1 0 4 6 8 A 1 0 6 3 4 1 0 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 7 4 F 1 0 3 2 0 1 0 2 1 0 1 0 5 8 4 A Stopping rules met 6 1 0 Serum Uric Acid (mg/dL)Acid SerumUric 8 4 D 1 0 3 2 0 1 0 2 1 2 1 0 5 D Withdrawn due to protocol deviation 1 0 8 1 0 4 9 6 4 H 1 0 3 2 0 1 0 2 F Withdrawal of Consent 1 0 1 0 5 8 4 10 6 1 0 4 1 0 3 2 D SAE; non-study drug related 2 G 0 1 0 1 0 1 0 5 8 11 1 0 4 6 4 G 1 0 3 H Discontinuation due to TEAE 2 0 1 0 2 0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0 Week 7 Unaudited data reported as of October 09, 2018 | Clinicaltrials.gov NCT02959918

Cohort 17: 0.1 mg/kg of SVP-Rapamycin + 0.2 mg/kg of pegadricase 0.1 0.1 0.1 0.1 0.1 SVP-Rapamycin Patient 0.2 0.2 0.2 0.2 0.2 pegadricase 1 0 1 0 5 8 4 6 1 0 4 1 0 3 1 2 0 1 0 2 1 0 5 1 0 8 1 0 4 6 4 1 0 3 2 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 4 1 0 3 3 2 0 1 0 2 1 0 1 0 5 Anti 8 4 6 1 0 Week 20 Evaluable patients = patients who received a full 4 4 1 0 3 2 0 1 0 2 first dose and did not discontinue due to any measure 1 0 1 0 5 - 8 Uricase Antibody Titer 4 6 1 0 other than drug effectiveness or drug related safety 5 4 1 0 3 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 6 4 1 0 3 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 7 4 B 1 0 3 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 4 A 3 8 1 0 2 0 1 0 2 A Stopping rules met 1 0 5 Serum Uric Acid (mg/dL) Acid Uric Serum 1 0 8 1 0 4 6 G SAE; infusion reaction 9 4 1 0 3 B 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 10 4 F 1 0 3 E Discontinuation due to infusion reaction 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 E 3 11 4 1 0 2 F Withdrawal of consent 0 1 0 2 1 0 1 0 5 8 4 12 6 1 0 4 E 1 0 3 2 G SAE; non-study drug related 0 1 0 2 0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0 Week 8 Unaudited data reported as of October 09, 2018 | Clinicaltrials.gov NCT02959918

DECT Scans Suggest Potential Reduction of Urate Burden in Phase 2 Study Number of Baseline Initial Urate Final Urate Patient Study Change in urate SUA Volume Volume # Drug volume (%) (mg/dL) (cm3) (cm3) • DECT(Dual Energy Computed Infusions Tomography) has been shown to 1 8.2 5 13.27 0.24 -98.19 be quantitative measure of tissue 2 6.3 3 0.13 0.24 84.62 1,2 3 8.5 2 0.62 0.05 -91.94 urate burden 4 7.6 5 0.00 0.01 NA* • DECT scans were performed as an 5 7.4 5 1.20 0.13 -89.17 6 8 5 0.39 0.00 -100.00 exploratory measure to evaluate 7 6.7 5 0.00 0.04 NA* reduction of tissue urate burden in 8 8.9 5 0.22 0.06 -72.73 a subset of patients: 9 7.7 5 0.46 0.40 -13.04 10 8.9 3 2.27 0.51 -77.53 - at screen 11 8.8 5 0.32 0.42 31.25 12 11.1 2 0.32 0.03 -90.63 - at the end of treatment cycle 3 13 8.2 5 0.45 0.29 -35.56 - at the end of final treatment cycle 14 6 5 4.53 0.37 -91.83 15 6.4 5 0.48 0.06 -87.50 16^ 6.5 5 0.13 0.05 -61.54 17^ 9.6 5 0.31 0.00 -100.00 *NA = Not Applicable, ^Patients in 5 combination dose cohorts 18^ 8.6 5 0.17 0.00 -100.00 19^ 7.5 1 0.00 0.00 NA* 1Choi HK et al., Ann Rheum Dis. 2009, 68:1609-12. 9 2Araujo EG et al, RMD Open. 2015, 1:e000075.

Patient 1 DECT Scan Images Show Reduction of Tissue Urate Burden Baseline Treatment Period 3 End of Study Total urate Total urate Total urate volume volume volume 13.27 cm3 1.83 cm3 0.24 cm3 0.15 mg/kg SVP-Rapa + ) 3 0.2 mg/kg pegadricase 0.2 mg/kg pegadricase ADA Titer 5 1 5 1 0 1 2 4 1 0 9 6 3 1 0 3 2 0 1 0 SUA (mg/dL) SUA 0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0 Tissue urate (cm urate Tissue W e e k 3 A D A A n t i b o d y T i t e r S e r u m U r i c A c i d ( m g / d L ) T i s s u e u r a t e d e p o s i t ( c m ) DECT uses a computer algorithm to produce color-coded images that render uric acid green, cortical bone blue, and trabecular bone purple 10

Observed Reduction in Flare Frequency in 5 Combination Cohorts Has Been Consistent With Total Cohort Flare Data % of Patients in 5 Combination Dose Cohorts by Month e r 6 0 a 5 0 l P e g a d r i c a s e a l o n e F h S E L - 2 1 2 t i 4 0 3 5 w 3 4 s t n e i 2 0 t a 9 P 6 6 % 0 1 1 2 3 4 5 M o n t h a f t e r i n i t i a t i o n o f t r e a t m e n t • SEL-212 has lowered flares initially and over time during treatment • Majority of flares have occurred in months 1 & 2 • There have been no new patients who flare after second month Patients who received a full first dose and completed respective treatment cycle 11 Unaudited data as of October 9, 2018 | Clinicaltrials.gov NCT02959918

Interim Data Continue to Show Reduction in Flare Frequency During SEL-212 Therapy % of Patients from All Cohorts Experiencing Flares by Month Severity of Flares 6 0 8 0 e 5 0 r a 64 l P e g a d r i c a s e a l o n e s F e 6 0 r h 4 0 S E L - 2 1 2 t a i l f w 2 9 f s 4 0 t 2 4 o 32 n e i 2 0 % t a 1 2 2 0 P 9 3 % 4 0 0 1 1 2 3 4 5 M i l d M o d e r a t e S e v e r e M o n t h a f t e r i n i t i a t i o n o f t r e a t m e n t • Data indicate SEL-212 has lowered flares initially and over time during treatment • Majority of flares have occurred in months 1 & 2, and there have been no new patients who flare after second month • 96% of flares have been mild or moderate in severity • No gout flares have been classified as SAEs nor resulted in study drug discontinuations Patients who received a full first dose and completed respective treatment cycle 12 Unaudited data reported as of October 09, 2018 | Clinicaltrials.gov NCT02959918

5 Months Combination Treatment Has Not Shown Any Emerging Safety Signals • SEL-212 has been generally well tolerated at clinically active doses for 5- combination dose cohorts following >130 administrations • 46 patients in 5-combination dose cohorts have been dosed in the Phase 2 study as of October 9, 2018 • 5 months of combination treatment has not shown any emerging safety signals • 7 SAEs (5 individual patients) have been reported in the 5-combination dose cohorts: - 4 SAEs were reported not to be or unlikely to be related to study drug - 1 SAE was reported to be possibly related to study drug - 2 SAEs (infusion reactions) were reported as related or possibly related to study drug, both of which occurred during the infusion of SEL-212 in Treatment Period 2 - No SAEs have occurred in Treatment Periods 4 or 5 13
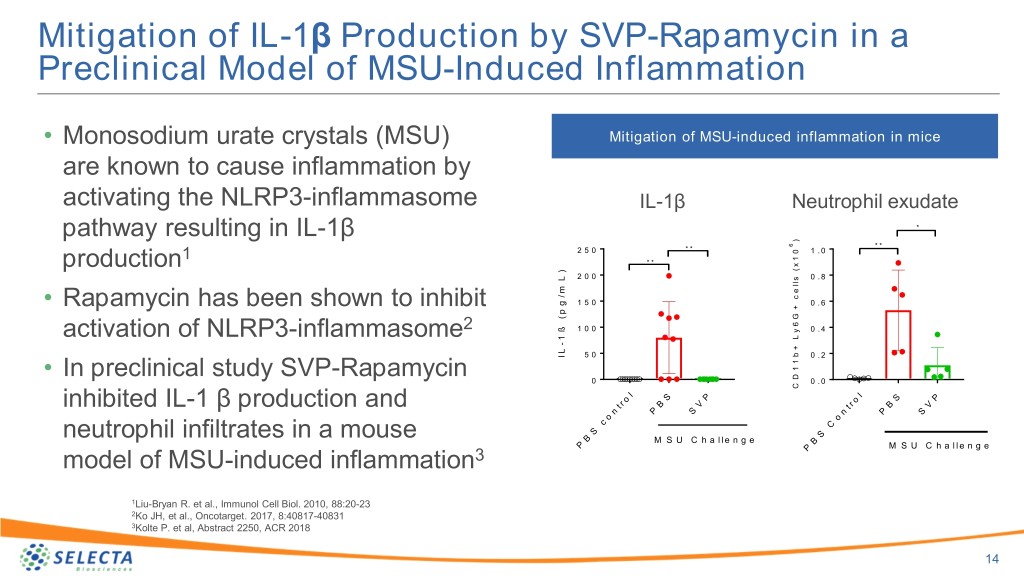
Mitigation of IL-1β Production by SVP-Rapamycin in a Preclinical Model of MSU-Induced Inflammation • Monosodium urate crystals (MSU) Mitigation of MSU-induced inflammation in mice are known to cause inflammation by activating the NLRP3-inflammasome IL-1β Neutrophil exudate pathway resulting in IL-1β * ) * * 6 * * 2 5 0 0 1 . 0 1 1 * * x production ( ) 2 0 0 s 0 . 8 L l l e m / c g 1 5 0 0 . 6 • Rapamycin has been shown to inhibit + p ( G 6 ß 2 1 0 0 y 0 . 4 L activation of NLRP3-inflammasome 1 - + L b I 5 0 0 . 2 1 1 • In preclinical study SVP-Rapamycin D 0 0 . 0 C l l o S P S P r o inhibited IL-1 β production and t B V t r B V n P S n P S o o c C neutrophil infiltrates in a mouse S S B M S U C h a l l e n g e B P M S U C h a l l e n g e model of MSU-induced inflammation3 P 1Liu-Bryan R. et al., Immunol Cell Biol. 2010, 88:20-23 2Ko JH, et al., Oncotarget. 2017, 8:40817-40831 3Kolte P. et al, Abstract 2250, ACR 2018 14

Summary and Next Steps • Projections suggest approximately 66% of patients could have SUA control during 5 months of treatment with monthly dosing of SEL-212 • Low flare rates observed to date • Potential to rapidly eliminate tissue urate burden based on DECT imaging findings • 5 month combination treatment has not shown any emerging safety signals • Proposed dose regimens for Phase 3 trials identified • End of Phase 2 meeting scheduled, and start of Phase 3 planned in 2018 • Head-to-Head trial designed and expected to be conducted in parallel with Phase 3 pivotal trials • Commercial plans accelerated 15

We thank all of the patients that have participated in our clinical program. We are very grateful to the clinical trial site investigators and their staff.

Appendix

SEL-212 Posters Presented at ACR Monday, October 22, 2018; 9:00 AM - 11:00 AM • Initial Phase 2 Clinical Data of SEL-212 in Symptomatic Gout Patients: Monthly Dosing of a Pegylated Uricase (pegadricase*) with SVP-Rapamycin Enables Sustained Reduction of Acute Gout Flares Tuesday, October 23, 2018; 9:00 AM - 11:00 AM • Mitigation of Inflammation Induced By Monosodium Urate Crystals in Mice By Treatment with SVP-Rapamycin • Update of SEL-212 Phase 2 Clinical Data in Symptomatic Gout Patients: SVP-Rapamycin Combined with pegadricase Mitigates Immunogenicity and Enables Sustained Reduction of Serum Uric Acid Levels, Low Rate of Gout Flares and Monthly Dosing • Initial Phase 2 Clinical Data of SEL-212 in Symptomatic Gout Patients: Measurement of Dissolution of Urate Deposits Associated with Monthly Dosing of a Pegylated Uricase (pegadricase) with SVP-Rapamycin By Dual Energy Computed Tomography *Previously called pegsiticase; pegadricase is the new United States Adopted Name (USAN) 18
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- St. Vincent Regional Hospital Verified as Level I Trauma Center by American College of Surgeons
- Publication of the 2023 annual report
- Trial Attorney James P. Lamey Joins Houston-Based PMR Law
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share