Form 8-K PTC THERAPEUTICS, INC. For: Jan 27
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): January 27, 2018
PTC THERAPEUTICS, INC.
(Exact Name of Company as Specified in Charter)
Delaware | 001-35969 | 04-3416587 | ||
(State or Other Jurisdiction of Incorporation) | (Commission File Number) | (IRS Employer Identification No.) | ||
100 Corporate Court | ||
South Plainfield, NJ | 07080 | |
(Address of Principal Executive Offices) | (Zip Code) | |
Company’s telephone number, including area code: (908) 222-7000
Not applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
o Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
o Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
o Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
o Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company o
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. o
Item 7.01. Regulation FD Disclosure.
On January 27, 2018, PTC Therapeutics, Inc. (the “Company”) issued a press release (the “press release”) announcing the presentation of preliminary data from Part 1 of the FIREFISH trial in Type 1 SMA infants at the International Scientific Congress on Spinal Muscular Atrophy in Krakow, Poland. The full text of the press release issued in connection with the announcement is furnished as Exhibit 99.1 to this Current Report on Form 8-K (this “Report”). A copy of the presentation is also attached to this Report as Exhibit 99.2 and is incorporated by reference into this Item 7.01. Additionally, copies of three posters that were presented at the conference, 1) Mercuri et al. (L. Servais as presenting author), "Updated pharmacodynamic and safety data from SUNFISH Part 1, a study evaluating the oral SMN2 splicing modifier RG7916 in patients with Type 2 or 3 spinal muscular atrophy" (the “SUNFISH poster”), 2) Chiriboga et al. (D. Kraus as presenting author) "Preliminary Evidence for Pharmacodynamics Effects of RG7916 in JEWELFISH, a Study in Patients with Spinal Muscular Atrophy who Previously Participated in a Study with Another SMN2-Splicing Targeting Therapy" (the “JEWELFISH poster”), and 3) Poirier et al. (L. Mueller as presenting author), "Relationship Between Central and Peripheral SMN Protein Increase Upon Treatment with RO7034067 (RG7916)" (the “SMN protein poster”), are attached to this Report as Exhibits 99.3, 99.4 and 99.5, respectively, and are incorporated by reference into this Item 7.01.
The presentation was authored and given by Dr. Giovanni Baranello from the Fondazione Istituto Neurologico Carlo Besta in Milan, Italy, who is a third-party investigator in the trial, and was neither prepared nor presented by or on behalf of the Company. The SUNFISH poster was authored by Dr. Eugenio Mercuri from the Paediatric Neurology and Nemo Center at Catholic University and Policlinico Gemelli in Rome, Italy, who is a third-party investigator in the trial. The JEWELFISH poster was authored by Dr. Claudia A. Chiriboga from the Department of Neurology at Columbia University Medical Center in New York, NY, who is a third-party investigator in the trial. The SMN protein poster was authored by Dr. Agnes Poirier from the Roche Innovation Center in Basel, Switzerland. The SUNFISH poster and the JEWELFISH poster were neither prepared nor presented by or on behalf of the Company. The Company is providing the presentation and the posters as a convenience for investors for informational purposes.
The information in this Current Report on Form 8-K, including Exhibits 99.1, 99.2, 99.3, 99.4 and 99.5 shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such a filing.
Forward Looking Statements: All statements, other than those of historical fact, contained in this Current Report on Form 8-K, including those contained in Exhibits 99.1, 99.2, 99.3, 99.4 and 99.5, are forward-looking statements, including regarding any advancement of the joint development program in SMA with PTC, Roche, and SMAF, in particular as related to the timing of enrollment, completion and evaluation of the Phase 2 clinical studies of RG7916 in SMA patients and the period during which the results of the studies will become available; the clinical utility and potential advantages of RG7916, including its potential to impact every aspect of the disease. The Company's actual results, performance or achievements could differ materially from those expressed or implied by forward-looking statements it makes as a result of a variety of risks and uncertainties, including those related to the initiation, enrollment, conduct and availability of data from either the SUNFISH or FIREFISH studies and the outcome of such studies; and the factors discussed in the “Risk Factors” section of the Company's most recent Quarterly Report on Form 10-Q as well as any updates to these risk factors filed from time to time in the Company's other filings with the SEC. You are urged to carefully consider all such factors. The forward-looking statements contained herein represent the Company's views only as of the date of this Current Report on Form 8-K and the Company does not undertake or plan to update or revise any such forward-looking statements to reflect actual results or changes in plans, prospects, assumptions, estimates or projections, or other circumstances occurring after the date of this Current Report on Form 8-K except as required by law.
Item 9.01. | Financial Statements and Exhibits. |
(d) Exhibits
Exhibit No. | Description | |
99.1 | ||
99.2 | ||
99.3 | ||
99.4 | ||
99.5 | ||
Signature
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this Report to be signed on its behalf by the undersigned hereunto duly authorized.
PTC Therapeutics, Inc. | ||
Date: January 29, 2018 | By: | /s/ Mark E. Boulding |
Name: | Mark E. Boulding | |
Title: | Executive Vice President and Chief Legal Officer | |

Preliminary Data from FIREFISH trial in Type 1 SMA Infants Presented at the International Scientific Congress on Spinal Muscular Atrophy
SOUTH PLAINFIELD, N.J., Jan. 27, 2018 – PTC Therapeutics, Inc. (NASDAQ: PTCT) today announced the presentation of early interim data from Part 1, the dose-finding portion of the FIREFISH study. FIREFISH is a two-part seamless, open-label, multicenter study to investigate the safety and efficacy of RG7916 in infants and babies with Type 1 SMA. RG7916 has been safe and well tolerated at all doses and there have been no drug-related safety findings leading to withdrawal. In addition, data on the ability to swallow and requirements for tracheostomy or permanent ventilation, together with overall survival were also presented. Previously published natural history data indicate that in a comparable historic cohort the median age of event-free survival for SMA Type 1 infants to be between 8 and 10.5 months1,2. The presentation was given by Dr. Giovanni Baranello, Fondazione Istituto Neurologico Carlo Besta in Milan, Italy, at the International Scientific Congress on spinal muscular atrophy in Kraków, Poland, and will be made available via a link under the investor relations section of the PTC website. (www.ptcbio.com/smaeurope)
“It is exciting to show for the first time that an oral small molecule demonstrates early signs of clinical benefit,” stated Stuart W. Peltz, Ph.D., Chief Executive Officer of PTC Therapeutics. “We believe a drug that distributes to both the CNS and peripheral tissues can provide an important benefit to children suffering from this devastating, fatal disease. We anticipate that the trial will transition to the pivotal portion in the coming months.”
FIREFISH (NCT02913482) is a multi-center, open-label, seamless pivotal study evaluating the safety and efficacy of RG7916 in babies aged 1–7 months at enrollment with Type 1 SMA and two SMN2 gene copies. The exploratory Part 1 (n=8–24) is assessing the safety, tolerability, pharmacokinetics and pharmacodynamics of RG7916 at different dose levels. In Part 1, patients receive RG7916 for at least
4 weeks (or 2 weeks after steady-state is achieved) of daily administration; patients then enter an extension phase with RG7916. The confirmatory Part 2 (n=40) will assess the safety and efficacy of RG7916 at the dose level selected from Part 1 over 24 months. The primary endpoint for Part 2 is the proportion of infants sitting without support for 5 seconds, assessed by the Gross Motor Scale of the BSID-III, after 12 months of treatment.
4 weeks (or 2 weeks after steady-state is achieved) of daily administration; patients then enter an extension phase with RG7916. The confirmatory Part 2 (n=40) will assess the safety and efficacy of RG7916 at the dose level selected from Part 1 over 24 months. The primary endpoint for Part 2 is the proportion of infants sitting without support for 5 seconds, assessed by the Gross Motor Scale of the BSID-III, after 12 months of treatment.
“This early interim analysis of survival and the delay to milestone events that are hallmarks of the progression of SMA in infants are promising,” stated Dr. Giovanni Baranello, Fondazione Istituto Neurologico Carlo Besta in Milan, Italy. “These data, in conjunction with earlier poster presentations at the Congress from the trials in patients with type 2/3 SMA, are supportive of safety and sustained increase in SMN protein levels. Clinical trials in SMA patients require a globalization of clinical research; particularly, it is essential for a coordinated multidisciplinary team to support the babies and their families and to ensure the best standard of care to promote the higher benefit to affected children from potential new treatments.”
In addition to the oral presentation, three posters were on display during the Congress: updated pharmacodynamic and safety data from SUNFISH part 1, preliminary evidence for pharmacodynamic effects of RG7916 in JEWELFISH, and preclinical data demonstrating the relationship between central and peripheral SMN protein increase upon treatment with RG7916. The data presented demonstrate systemic and dose-dependent increase of SMN protein levels. The data from mice and other species suggest that SMN protein level increases seen in the blood of patients following RG7916 treatment reflect SMN protein level increases in the CNS, muscle and other key issues affected in SMA. In addition, RG7916 has been safe and well tolerated at all doses and there have been no drug-related safety findings leading to withdrawal.
The U.S. Food and Drug Administration granted orphan drug designation to RG7916 for the treatment of patients with SMA. RG7916 directly targets the underlying molecular deficiency of SMA by modulating SMN2 splicing to increase expression of full-length SMN2 mRNA from the SMN2 gene. An interim analysis from the first part of SUNFISH of the five cohorts treated with RG7916 for 28 days or longer demonstrated an exposure-dependent increase in SMN protein. SMA is characterized by reduced levels of SMN protein, motor neuron loss, and muscle atrophy. To date, RG7916 remains well-tolerated in patients at all doses and there have been no drug-related safety findings leading to withdrawal.
The SMA program was initially developed by PTC Therapeutics in partnership with the SMA Foundation in 2006 to accelerate the development of a treatment for SMA. In November 2011, Roche gained an exclusive worldwide license to the PTC/SMA Foundation SMN2 alternative splicing program. The development of these compounds is being executed by Roche and overseen by a joint steering committee with members from PTC, Roche, and the SMA Foundation.
1 Kolb S, et al. Amer Neuro Assoc 2017; 883-891
2 Finkel R, et al. Amer Acad Neur 2014; 810-817
About Spinal Muscular Atrophy (SMA)
Spinal muscular atrophy (SMA) is a genetic neuromuscular disorder that is the leading genetic cause of mortality in infants and toddlers caused by a missing or defective survival of motor neuron 1 (SMN1) gene, which results in reduced levels of SMN protein. The homologous SMN2 gene is predominantly spliced to a truncated mRNA, and only produces small amounts of functional SMN protein. Insufficient levels of SMN protein are responsible for the loss of motor neurons within the spinal cord leading to
Spinal muscular atrophy (SMA) is a genetic neuromuscular disorder that is the leading genetic cause of mortality in infants and toddlers caused by a missing or defective survival of motor neuron 1 (SMN1) gene, which results in reduced levels of SMN protein. The homologous SMN2 gene is predominantly spliced to a truncated mRNA, and only produces small amounts of functional SMN protein. Insufficient levels of SMN protein are responsible for the loss of motor neurons within the spinal cord leading to
muscle atrophy and death in its most severe form. It is estimated that this devastating disease affects 1 in every 11,000 children born.
About the SMA Clinical Trials
FIREFISH: An open-label, two-part clinical trial. Part 1 is a dose escalation study in at least 8 infants for a minimum of 4 weeks. The primary objective of Part 1 is to assess the safety profile of RG7916 in infants and determine the dose for Part 2. Part 2 is a single-arm study in approximately 40 infants with Type 1 SMA for 24 months, followed by an open-label extension.
FIREFISH: An open-label, two-part clinical trial. Part 1 is a dose escalation study in at least 8 infants for a minimum of 4 weeks. The primary objective of Part 1 is to assess the safety profile of RG7916 in infants and determine the dose for Part 2. Part 2 is a single-arm study in approximately 40 infants with Type 1 SMA for 24 months, followed by an open-label extension.
SUNFISH: A double‐blind, two‐part, placebo‐controlled trial. Part 1 enrolled patients with Type 2 or 3 SMA to evaluate safety, tolerability, and PK/PD of several RG7916 dose levels. The pivotal SUNFISH Part 2, in non‐ambulant patients with Type 2 or 3 SMA, will evaluate safety and efficacy of the RG7916 dose level selected from Part 1.
JEWELFISH: An exploratory, open-label study to establish the safety and tolerability of RG7916 in people who have previously participated in a study with another therapy targeting SMN2 splicing.
About PTC Therapeutics
PTC is a global biopharmaceutical company focused on the discovery, development, and commercialization of novel medicines using our expertise in RNA biology. PTC's internally discovered pipeline addresses multiple therapeutic areas, including rare disorders and oncology. PTC has discovered all of its compounds currently under development using its proprietary technologies. Since its founding 20 years ago, PTC's mission has focused on developing treatments to fundamentally change the lives of patients living with rare genetic disorders. The company was founded in 1998 and is headquartered in South Plainfield, New Jersey. For more information on the company, please visit our website www.ptcbio.com.
For More Information:
Investors:
Emily Hill
+ 1 (908) 912-9327
Media:
Jane Baj
+1 (908) 912-9167
Forward Looking Statements:
All statements, other than those of historical fact, contained in this press release, are forward-looking statements, including statements regarding: any advancement of the joint development program in SMA with PTC, Roche, and SMAF, in particular as related to the timing of enrollment, completion and evaluation of the Phase 2 clinical studies of RG7916 in SMA patients and the period during which the
results of the studies will become available; the clinical utility and potential advantages of RG7916, including its potential to impact every aspect of the disease; the timing and outcome of PTC's regulatory strategy and process; PTC's strategy, future expectations, plans and prospects, future operations, future financial position, future revenues or projected costs; and the objectives of management. Other forward-looking statements may be identified by the words "potential," "will," "promise," "expect," "plan," "target," "anticipate," "believe," "estimate," "intend," "may," "project," "possible," "would," "could," "should," "continue," and similar expressions.
PTC's actual results, performance or achievements could differ materially from those expressed or implied by forward-looking statements it makes as a result of a variety of risks and uncertainties, including those related to: the initiation, enrollment, conduct and availability of data from either the SUNFISH or FIREFISH studies and the outcome of such studies; events during, or as a result of, these studies that could delay or prevent further development of RG7916, including future actions or activities under the SMA joint development program; our expectations for regulatory approvals; PTC's scientific approach and general development progress; and the factors discussed in the "Risk Factors" section of PTC's most recent Quarterly Report on Form 10-Q as well as any updates to these risk factors filed from time to time in PTC's other filings with the SEC. You are urged to carefully consider all such factors.
As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products, including with respect to PTC's joint development program in SMA with Roche and the SMAF. There are no guarantees that any product candidate under the joint development program will receive regulatory approval in any territory or prove to be commercially successful.
The forward-looking statements contained herein represent PTC's views only as of the date of this press release and PTC does not undertake or plan to update or revise any such forward-looking statements to reflect actual results or changes in plans, prospects, assumptions, estimates or projections, or other circumstances occurring after the date of this press release except as required by law.

© 2018 F. Hoffmann-La Roche Ltd. All rights reserved.
FIREFISH, A MULTI-CENTER, OPEN-LABEL TRIAL TO INVESTIGATE THE
SAFETY AND EFFICACY OF RG7916 IN BABIES WITH TYPE 1 SMA:
STUDY UPDATE AND REAL-LIFE EXPERIENCE OF STUDY
IMPLEMENTATION
G Baranello1, J Day2, A Klein3, E Mercuri4, L Servais5, N Deconinck6,
R Masson1, H Kletzl7, C Czech7, M Gerber7, Y Cleary7, F Lee7, K Gelblin7,
S Nave7, K Gorni7 and O Khwaja7
1. Carlo Besta Neurological Research Institute Foundation, Developmental Neurology Unit, Milan, Italy; 2. Department of Neurology,
Stanford University, Palo Alto, CA, USA; 3. University Children’s Hospital Basel, Basel, Switzerland; Inselspital, Bern, Switzerland; 4.
Paediatric Neurology and Nemo Center, Catholic University and Policlinico Gemelli, Rome, Italy; 5. Institute of Myology, Paris, France;
Reference Center for Neuromuscular Disease, Centre Hospitalier Régional de La Citadelle, Liège, Belgium; 6. Queen Fabiola Children's
University Hospital and Université Libre de Bruxelles, Brussels, Belgium; Neuromuscular Reference Center UZ Ghent; Ghent, Belgium; 7.
Roche Pharmaceutical Research and Early Development, Roche Innovation Center, Basel, Switzerland.

© 2018 F. Hoffmann-La Roche Ltd. All rights reserved.
Disclosures
GB is PI in the following clinical trials in SMA: BP39055 and BP39056 (Roche); CLMI070X2201 (Novartis); AVXS-101-CL-302 (Avexis); SMA-
001 (Catalyst); PI in the following clinical trials in Duchenne Muscular Dystrophy: FOR_DMD; DSC/14/2357/48 (Italfarmaco); VBP15-004
(Reveragen); PTC124-GD-025o_DMD (PTC Therapeutics).
JD reports grants from: AMO Pharmaceuticals; aTyr; AveXis; Biogen; Bristol Meyers Squibb; Cytokinetics; Ionis Pharmaceuticals; Roche
Pharmaceuticals; Sanofi-Genzyme; and Sarepta Therapeutics. He has served as a consultant for: AMO Pharmaceuticals; AveXis; Biogen;
Cytokinetics; Ionis Pharmaceuticals; Roche Pharmaceuticals; Pfizer; Sarepta Therapeutics; Santhera Pharmaceuticals. He has patents
licensed to Athena Diagnostics for genetic testing of myotonic dystrophy type 2 (US patent 7442782) and spinocerebellar ataxia type 5 (US
patent 7527931).
AK has received speaker and consulting fees from Biogen, PTC, Roche and Santhera and is PI for F. Hoffmann-La Roche and Santhera
studies.
EM is a consultant for F. Hoffmann-La Roche, AveXis, IONIS and Biogen, and PI for Biogen/IONIS and F. Hoffmann-La Roche studies.
LS is a PI of SMA studies for Roche, Biogen, and Avexis. He has attended SAB of Biogen and Avexis and received consultancy fees from
BiogenND.
RM has no disclosures to report.
HK, CC, MG, YC, FL, KGelblin, SN, KGorni and OK are current employees of F. Hoffmann-La Roche.
RG7916 is an investigational medicine and benefit/risk profile has not been fully established.
The information presented is early interim data.

© 2018 F. Hoffmann-La Roche Ltd. All rights reserved.
Introduction
• SMA is a severe, progressive neuromuscular disease leading to loss of motor function and
reduced life expectancy1
• Increasing evidence suggests that SMA may be a multi-system disorder, where cells and tissues
throughout the body, including motor neurons may be selectively vulnerable to low SMN protein
levels3,4
• RG7916 is an orally administered, centrally and peripherally distributed SMN2 pre-mRNA splicing
modifier that increases SMN protein levels
• Preclinical data show similar RG7916 concentrations in blood, brain, and muscle tissue (Poster P48, A. Poirier et al.)
• Similar SMN protein increase in brain and muscle in SMA mouse models following RG7916 administration (Poster P48)
• Proof-of-mechanism of oral SMN2 splicing modifiers was previously established in preclinical
models5 and in Type 2 and 3 SMA patients with RG79166 (Poster P46, E. Mercuri et al.)
• The FIREFISH study aims to assess the safety and efficacy of RG7916 in babies with Type 1
SMA. This study is sponsored by F. Hoffmann-La Roche Ltd.
SMA, spinal muscular atrophy; SMN, survival of motor neuron.
1. Mercuri E, et al. Lancet Neurol 2012;11:443–452; 2. Acsadi G, et al. J Neurosci Res 2009;12:2748–2756;
3. Singh RN, et al. Biochim Biophys Acta 2017;1860:299–315; 4.Hamilton G and Gillingwater TH. Trends Mol Med 2013;19:40–50;
5. Naryshkin N, et al. Science 2014; 345:688–693; 6. Clinicaltrials.gov NCT02633709 Accessed December 2017

© 2018 F. Hoffmann-La Roche Ltd. All rights reserved.
RG7916 mechanism of action
SMN1
6 8 7 DNA
Pre-mRNA
mRNA
Functional SMN protein
6 8 7
6 8 7
SMN2
6 8 7
6 8 7 6 8 7
6 8 7 6 8
Functional SMN protein Unstable SMN protein
rapidly degraded
RG7916
Pinard E, et al. J Med Chem; 2017;60:4444–4457.
RG7916 modifies SMN2 splicing to produce functional SMN
protein in central and peripheral compartments

© 2018 F. Hoffmann-La Roche Ltd. All rights reserved.
*Comprises minimum two dose-ranging cohorts; Patients to be enrolled in a stepwise fashion based on PK findings to minimize exposure.
https://clinicaltrials.gov/ct2/show/NCT02913482, accessed Jan 2018.
FIREFISH: Study overview
FIREFISH
Type 1 SMA
1–7 months old
Part 2:
Confirmatory
Open-label
Dose level selected
from Part 1
n=40
Active
treatment
RG7916
12 months 24 months
Extension
Part 1*: Dose-
finding
Several RG7916 dose
levels
n=8–24
RG7916 dose
selected for
Part 2
Continued
treatment
RG7916
Open-Label
Extension
RG7916
dose level 1
RG7916
dose level 2
Key
inclusion
criteria
• Genetic confirmation of homozygous deletion or compound heterozygosity predictive of loss of function of SMN1
• Clinical history, signs or symptoms attributable to SMA type 1 after 28 days but prior to 3 months
• Adequate nutrition at time of enrolment and willing to consider tube if required
• Two SMN2 gene copies (confirmed by central testing during screening)
Key
exclusion
criteria
• Concomitant or previous participation in a SMN2 targeting or gene therapy study
• Invasive ventilation or tracheostomy
• Awake non-invasive ventilation or with awake hypoxemia (SaO2 < 95%) with or without ventilator support
• Hospitalization for pulmonary event within the last 2 months, or planned at the time of screening
• Recent history (< 1 year) of ophthalmological disease
Detailed study information: clinicaltrials.gov/ct2/show/NCT02913482 www.roche-sma-clinicaltrials.com
© 2018 F. Hoffmann-La Roche Ltd. All rights reserved.
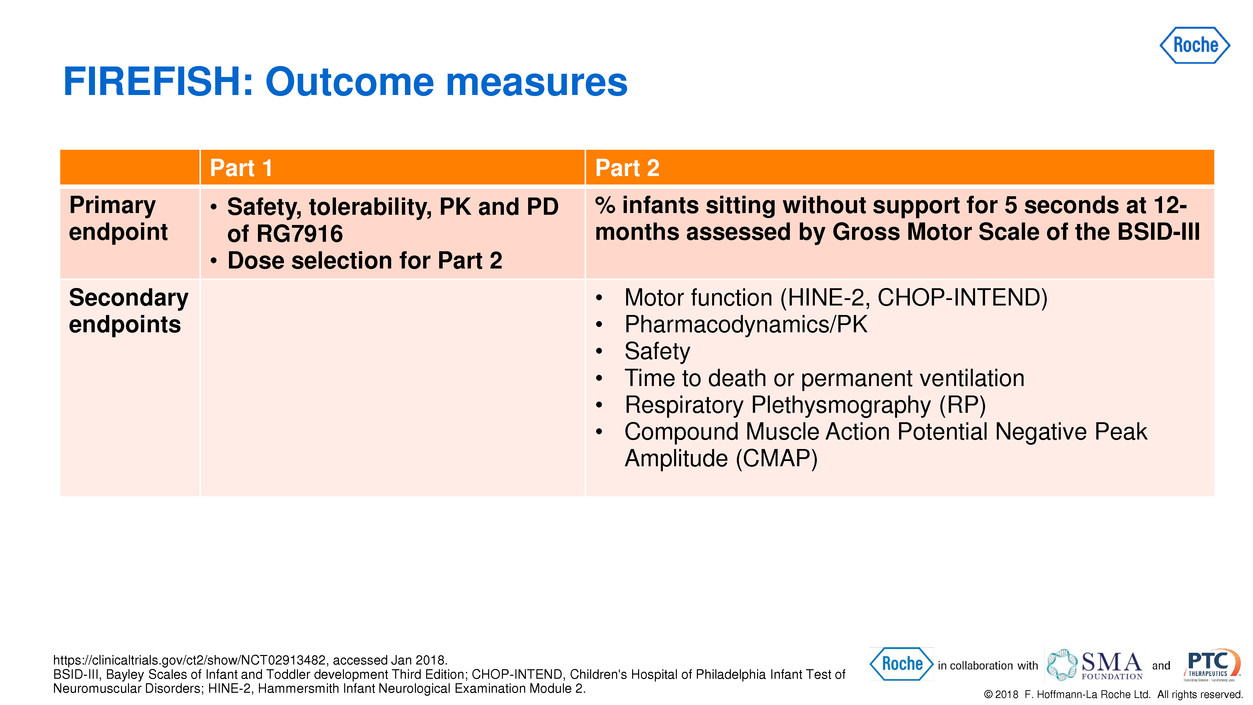
Part 1 Part 2
Primary
endpoint
• Safety, tolerability, PK and PD
of RG7916
• Dose selection for Part 2
% infants sitting without support for 5 seconds at 12-
months assessed by Gross Motor Scale of the BSID-III
Secondary
endpoints
• Motor function (HINE-2, CHOP-INTEND)
• Pharmacodynamics/PK
• Safety
• Time to death or permanent ventilation
• Respiratory Plethysmography (RP)
• Compound Muscle Action Potential Negative Peak
Amplitude (CMAP)
FIREFISH: Outcome measures
https://clinicaltrials.gov/ct2/show/NCT02913482, accessed Jan 2018.
BSID-III, Bayley Scales of Infant and Toddler development Third Edition; CHOP-INTEND, Children's Hospital of Philadelphia Infant Test of
Neuromuscular Disorders; HINE-2, Hammersmith Infant Neurological Examination Module 2.

© 2018 F. Hoffmann-La Roche Ltd. All rights reserved.
Patient demographics and baseline characteristics from
the first 13 patients and study status
IQR=interquartile range.
Data current as of December 7, 2017.
RG7916 is an investigational medicine and benefit/risk profile has not yet been fully established. The information presented is from
early interim analysis.
All Treatments
(N=13)
Age at first dose (months)
Median (IQR) 6.9 (6.3–6.9)
Gender
Female, n (%) 10 (76.9)
Weight at baseline (g)
Median (IQR) 6720 (5650–7600)
Age at diagnosis
(months)
Median (IQR) 3.5 (2.1–4.6)
• Part 1 (dose-finding) screening ongoing with
a total of 16 patients enrolled*
• 10 active sites: Italy, France, USA, Belgium,
Switzerland, Turkey
• Data presented here are from the first 13
patients recruited
• Part 2 expected to start Q1 2018
*Status Jan 5, 2018

© 2018 F. Hoffmann-La Roche Ltd. All rights reserved.
Age of patients and duration of exposure to treatment
Study duration is measured from start date of first dose to date of data extraction. Data current as of December 7, 2017.
RG7916 is an investigational medicine and benefit/risk profile has not yet been fully established. The information presented is from
early interim analysis.
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20
Death
Death
Age (months)
Before first dose
After first dose

© 2018 F. Hoffmann-La Roche Ltd. All rights reserved.
No patient has lost the ability to swallow
Data current as of December 7, 2017.
RG7916 is an investigational medicine and benefit/risk profile has not yet been fully established. The information presented is from early
interim analysis.
Visit All Treatments
Baseline N=13
Able to swallow, n 12
Unable to swallow, n 1
Week 8 N=9
Able to swallow, n 8
Unable to swallow, n 1
Week 17 N=4
Able to swallow, n 4
Unable to swallow, n 0
Week 26 N=4
Able to swallow, n 4
Unable to swallow, n 0
Week 35 N=3
Able to swallow, n 3
Unable to swallow, n 0
Summary of clinical outcomes
No patients have required
tracheostomy or
permanent ventilation*
*Permanent ventilation defined as ≥ 16 hours of assisted
ventilation per day for more than 2 weeks or continuous
intubation ≥ 30 days

© 2018 F. Hoffmann-La Roche Ltd. All rights reserved.
Summary of safety outcomes
• Overall 10 (77%) out of 13 patients experienced at least one adverse event. Most events were
mild in intensity and resolved despite ongoing treatment
• Adverse events reported in more than one patient were pyrexia (n=5), upper respiratory tract
infection (n=3), diarrhea (n=2), vomiting (n=2), erythema (n=2)
• Serious adverse events were reported in four patients: respiratory tract infection viral,
pneumonia & neutropenia, acute respiratory failure and hypoxia
• Ophthalmological monitoring conducted every 2 months did not show any evidence of the retinal
toxicity seen in preclinical monkey studies in any patient exposed to RG7916
• Fatal events were reported in two patients:
• Respiratory tract infection viral with fatal outcome on study Day 21
• Cardiac arrest and respiratory arrest with fatal outcome on study Day 236*
*Event reported after cut off date November 15, therefore not included in serious adverse events count.
Reference: interim safety summary BP39056 part 1 dated 08/01/2018

© 2018 F. Hoffmann-La Roche Ltd. All rights reserved.
• Working with patients with Type 1 SMA presents several challenges, mainly
related to the age and the severity of the disease
• Challenges can affect the infants, family, and staff involved in the studies
• As SMA (and especially Type 1) is a rare and devastating disease, we are
facing a GLOBALIZATION of clinical research
• Families in countries where competing trials and therapies (eg, nusinersen) are not available
may ask to be recruited to studies or to access therapies
• This may accelerate recruitment and development of new treatments
• Give access to potential treatment to a higher number of patients
Tips from real-life experience during the implementation
of the FIREFISH study
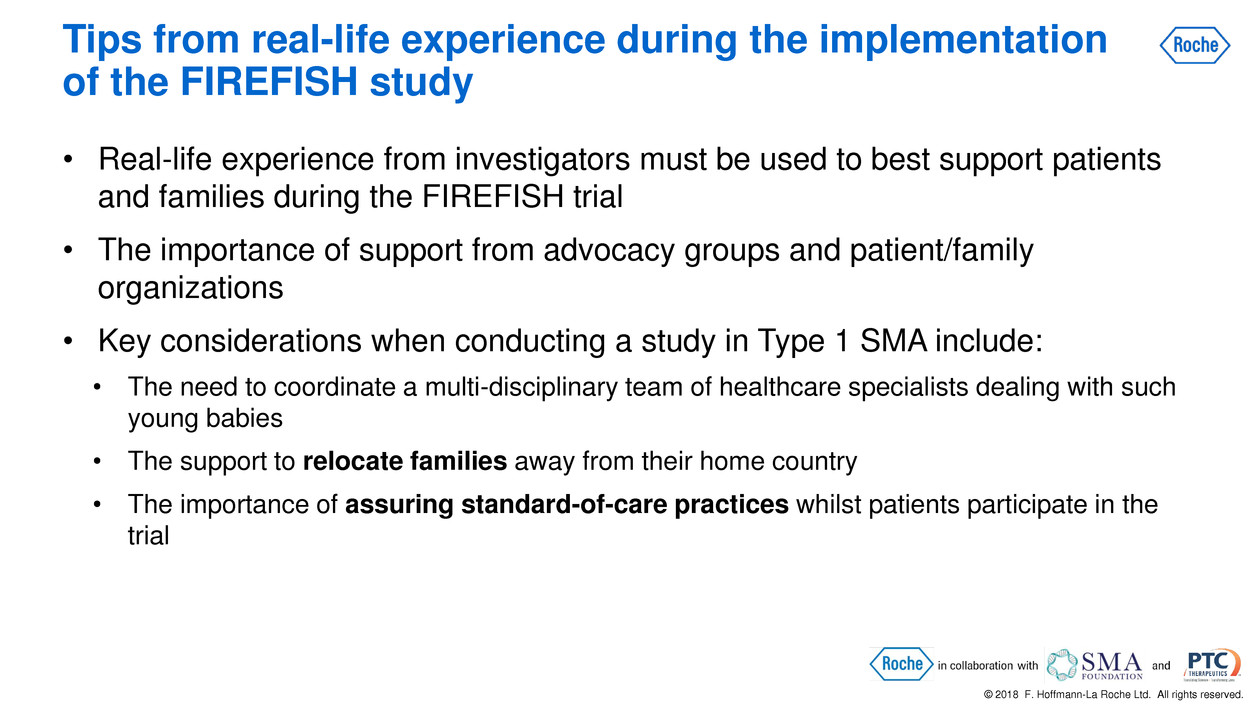
© 2018 F. Hoffmann-La Roche Ltd. All rights reserved.
• Real-life experience from investigators must be used to best support patients
and families during the FIREFISH trial
• The importance of support from advocacy groups and patient/family
organizations
• Key considerations when conducting a study in Type 1 SMA include:
• The need to coordinate a multi-disciplinary team of healthcare specialists dealing with such
young babies
• The support to relocate families away from their home country
• The importance of assuring standard-of-care practices whilst patients participate in the
trial
Tips from real-life experience during the implementation
of the FIREFISH study

© 2018 F. Hoffmann-La Roche Ltd. All rights reserved.
• Families in countries where competing trials and therapies (eg, nusinersen) are
not available may ask to be recruited to studies or to access therapies
• Resource limitations and cultural differences
• Relocation is important for the safety of the child and for the good conduct of
the study
Family relocation

© 2018 F. Hoffmann-La Roche Ltd. All rights reserved.
SMA standard of care implementation has increased survival and
improved quality of life of children and their families
Increased survival
Improved quality of life
Rehabilitation
Orthopedic
Care/
Orthosis
Psycho-social
support
Nutritional care
(growth/
under-nutrition)
Pulmonary care
Respiratory
support
Gastro-intestinal
management
Intensive Care/
Emergency
Patient with SMA
&
family
Bone health
Other organ
involvement

© 2018 F. Hoffmann-La Roche Ltd. All rights reserved.
• A proactive and anticipatory approach is essential to modify the course of the
disease
• Changing phenotype should be paralleled and supported by the implementation
of standard of care (eg, postural control or standing frame as long as the child
reaches new motor milestones, etc)
The application of SoC remains essential despite the emerging therapies
• Need of consistency of management within the study (involving different sites in
different countries)
• Need for training and dissemination of experience among sites and countries
SoC, standard of care.
Clinical trials involving relocation should ensure
multidisciplinary management

© 2018 F. Hoffmann-La Roche Ltd. All rights reserved.
Conclusions
• To date, RG7916 has been safe and well tolerated at all doses and there have been no
drug-related safety findings leading to withdrawal in any SMA patients exposed to
RG7916
• Ophthalmologic monitoring did not show any evidence of the retinal toxicity seen in preclinical
monkey studies in any patient exposed to RG7916
• Early interim clinical data reported:
No patient lost the ability to swallow
No patient has required tracheostomy or reached permanent ventilation
• Important considerations in conducting such clinical studies:
Co-ordination of a multi-disciplinary team
Family relocation
Application of standard-of-care practices
• Study updates will continue to be communicated at congresses in 2018
• Part 2 of the study is expected to start in Q1 2018

© 2018 F. Hoffmann-La Roche Ltd. All rights reserved.
We thank all the patients who participate in these studies and their families.
We thank our collaborators PTC Therapeutics and SMA Foundation.
We thank the FIREFISH, SUNFISH, and JEWELFISH investigators and trial staff.
Acknowledgments


Presented at the International Scientific Congress on Spinal Muscular Atrophy, Krakow, Poland, 25 – 27 January 2018.
Updated pharmacodynamic and safety data from SUNFISH Part 1,
a study evaluating the oral SMN2 splicing modifier RG7916 in
patients with Type 2 or 3 spinal muscular atrophy
Eugenio Mercuri1, Giovanni Baranello2, Janbernd Kirschner3, Laurent Servais4, Nathalie Goemans5, Maria Carmela Pera1, Anne Marquet6, Gillian Armstrong7,
Heidemarie Kletzl6, Marianne Gerber6, Christian Czech6, Yumi Cleary6, Margaret Chan8, Sangeeta Jethwa6, Stephane Nave6, Ksenija Gorni6 and Omar Khwaja6
1Paediatric Neurology and Nemo Center, Catholic University and Policlinico Gemelli, Rome, Italy; 2Carlo Besta Neurological Research Institute Foundation,
Developmental Neurology Unit, Milan, Italy; 3Department of Neuropediatrics and Muscle Disorders, Medical Center-University of Freiburg, Freiburg, Germany;
4Institute of Myology, Paris, France; Reference Center for Neuromuscular Disease, Centre Hospitalier Régional de La Citadelle, Liège, Belgium;
5Neuromuscular Reference Centre, Department of Paediatrics and Child Neurology, University Hospitals Leuven, Belgium; DVC St Jozef Antwerp, Antwerp, Belgium;
6Roche Pharmaceutical Research and Early Development, Roche Innovation Center Basel, Switzerland; 7Roche Products Ltd, Welwyn Garden City, United Kingdom;
8Roche Pharmaceutical Research and Early Development, Roche Innovation Center New York, New York, NY, USA
Background
• Spinal muscular atrophy (SMA) is a severe, progressive neuromuscular disease leading to loss
of motor function and reduced life expectancy.1
• SMA is caused by mutation or deletion of the survival of motor neuron 1 (SMN1) gene;
a second SMN gene, SMN2, only produces low levels of functional SMN protein.2
• SMA has traditionally been described as a disease of lower motor neurons; however, cells
and tissues throughout the body may be vulnerable to reduced levels of SMN protein, and
increasing evidence suggests that SMA is a multi-system disorder.3,4
• RG7916 is an orally administered, centrally and peripherally distributed SMN2 pre-mRNA
splicing modifier that increases SMN protein levels.
• The SUNFISH study aims to assess the safety and efficacy of RG7916 in people with
Type 2 or 3 SMA.
• Here, we report data from Part 1 of the SUNFISH study.
Methods
• SUNFISH study is currently recruiting participants (NCT02908685).
• Study design: SUNFISH is a multicenter, randomized, placebo-controlled, operationally
seamless, Phase 2 study evaluating the efficacy and safety of RG7916 in patients with Type 2 or
Type 3 SMA (Figure 1 and Table 1).
— Part 1. Exploratory: dose-finding; blinded RG7916 or placebo (2:1) in patients with Type 2
or ambulatory and non-ambulatory Type 3 SMA. First patient dosed October 20th 2016.
— Part 2. Confirmatory: efficacy and safety at the selected dose from Part 1; blinded RG7916
or placebo (2:1) for 12 months followed by open-label RG7916 until commercial availability,
in patients with Type 2 or non-ambulatory Type 3 SMA. First patient dosed October 11th 2017.
Figure 1: SUNFISH study design
*Part 1 of SUNFISH comprises 2 age groups (2–11 and 12–25 years); for each age group a minimum of two doses will be tested.
Table 1: SUNFISH study overview; Type 2 or 3 SMA, 2–25 years
Part 1 (n=51) Part 2 (n=168)
Inclusion/exclusion criteria
Key inclusion
criteria
• Confirmed genetic
diagnosis of SMA*
• Confirmed genetic diagnosis of SMA*
• Non-ambulant
• Able to sit independently and can raise hand
to mouth
Key exclusion
criteria
• Previous participation in an SMN2-targeting study or
gene therapy study
• Planned (within 18 months) or previous (<1 year prior) surgery for
scoliosis or hip fixation
Endpoints
Primary
endpoints
• Safety, tolerability, PK
and PD of RG7916
• Dose selection for Part 2
• Change from baseline in MFM32 at
Month 12
Selected
secondary
endpoints
Motor function at 12 months
• HFMSE, RULM, stabilization or improvement
in MFM32, MFM domain scores
Respiratory function at 12 months
• SNIP, MIP†, MEP†, FEV1
†, FVC† and PCF†
PK/PD
• SMN2 mRNA and SMN protein in blood
• Cmax, Ctrough and AUC of RG7916
QoL at 12 months
• SMAIS‡
Safety
*5q-autosomal recessive SMA. †Patients aged 6–25 years only. ‡In patients aged ≥12 years only.
Conclusions
• To date, RG7916 has been safe and well tolerated at all doses, and there have been no drug-
related safety findings leading to withdrawal in any SMA patients exposed to RG7916.
• Analysis of the SUNFISH Part 1 cohorts treated with RG7916 showed dose-dependent
increases in the SMN2 FL/SMN2∆7 mRNA ratio.
• RG7916 treatment resulted in increased SMN protein up to a median of 2.5 fold in patients with SMA.
• These safety, tolerability and PK/PD data were sufficient to inform the selection of a RG7916
dose level predicted to lead to clinically efficacious levels of SMN protein; this dose level will
be assessed in Part 2 of SUNFISH in children and young adults with SMA.
Abbreviations
AUC, area under curve; Cmax, maximum plasma concentration; Ctrough, trough plasma concentration; FEV1, forced expiratory volume in 1
second; FL, full-length; FVC, forced vital capacity; HFMSE, Hammersmith Functional Motor Scale Expanded; MEP, maximum expiratory
pressure; MFM, Motor Function Measure; MIP, maximum inspiratory pressure; PCF, peak cough flow; PD, pharmacodynamic; PK,
pharmacokinetic; QoL, Quality of Life; RULM, Revised Upper Limb Module; SMA, spinal muscular atrophy; SMAIS, Spinal Muscular
Atrophy Independence Scale; SMN, survival of motor neuron; SNIP, sniff nasal inspiratory pressure.
Acknowledgments
We would like to thank the patients and their families who participated in this study, as well as the investigators and trial staff involved in the
SUNFISH study. We thank Tobias Bergauer, Anna-Katharina Wiegers and Johann Karl of the Roche Biomarker Team for their contributions
to this study. We thank Dr. Riccardo Masson of the Carlo Besta Neurological Research Institute Foundation, Developmental Neurology Unit,
Milan, Italy for his support of this study. We would also like to thank our collaborators at PTC Therapeutics and the SMA Foundation. The
study was sponsored by F. Hoffmann-La Roche AG, Basel, Switzerland. Writing and editorial assistance was provided by MediTech Media, UK.
Results
• To date, RG7916 has been safe and well tolerated at all doses assessed.
• There have been no deaths and no drug-related safety findings leading to withdrawal
• Bi-monthly ophthalmological monitoring did not show any evidence of retinal toxicity seen
in preclinical monkey studies.
• Adverse events were mostly mild, resolved despite ongoing treatment and were reflective of the
underlying disease.
• RG7916 produced a dose-dependent increase in SMN2 FL mRNA and a concomitant decrease
in SMN2Δ7 mRNA (Figure 2).
• In patients with SMA, SMN protein was increased in a dose-dependent manner up to a median
of 2.5 fold (Figure 3).
• Increases in SMN protein were sustained up to 250 days (Figure 3).
Table 2: Adverse events occurring in ≥2 patients in at least 1 dose group, Day 1 to 84
by starting dose
Aged
2–25 years
Aged
2–11 years
Aged
12–25 years
Placebo
n=16
Dose 1
n=7
Dose 2
n=7
Dose 3
n=7
Dose 1
n=7
Dose 2
n=7
Patients with at least one event, n
Pyrexia 2 0 1 1 2 1
Oropharyngeal pain 2 0 0 1 1 2
Vomiting 1 2 0 0 0 1
Upper respiratory tract infection 0 0 1 2 0 0
Fatigue 0 0 0 1 2 0
Figure 2: SMN2 mRNA levels versus plasma concentrations of RG7916. Time-matched
concentration of RG7916 versus (A) the ratio of SMN2 FL mRNA to SMN2Δ7 mRNA, (B)
SMN2 FL mRNA, or (C) SMN2Δ7 mRNA is plotted.
0
0
2
4
20 40 60 80 100 120 140 160
6
8
10
12
14
16
18
20
0.0
0.5
1.0
1.5
2.0
2.5
3.0
3.5
4.0
0.0
0.2
0.4
0.6
0.8
1.2
1.0
1.4
1.6
1.8
2.0
A B CSMN2 FL/∆7 ratio
Placebo
12–25 years, dose 1
12–25 years, dose 2
2–11 years, dose 1
2–11 years, dose 2
2–11 years, dose 3
2–11 years, dose 4
0 20 40 60 80
Time-matched plasma RG7916 concentration (ng/mL)
100 120 140 160
SMN2 F
L
ratio (ratio from baseline
)
SMN2 FL
0 20 40 60 80 100 120 140 160
SMN∆7
SMN2 FL
/∆
7
ratio (ratio from baseline
)
SM
N
∆
7
ratio (ratio from baseline
)
Figure 3: SMN protein levels. (A) Concentration of SMN protein in blood by dose.
Ratio of SMN protein change from baseline in blood is plotted by dose in (B) and
median values (range) are displayed in (C).
Day
0
2
3
4
5
6
7
50 100
SMN protein (ng/mL
)
A B
150 200 250
Day
0
0
1
2
3
4
50 100
SMN protei
n
(ratio from baseline
)
150 200 250
2–11 years, dose 4
2–11 years, dose 1
2–11 years, dose 2
2–11 years, dose 3
12–25 years, dose 1*
Placebo
12–25 years, dose 2
C Median [range] SMN protein in blood (n=51 patients), ratio at Day 28 versus baseline
2–11 years 12–25 years
Placebo Dose 1 Dose 2 Dose 3 Dose 4 Dose 1 Dose 2
SMN protein ratio at Day 28
versus baseline; median [range]
0.958
[0.714–1.38]
1.09
[0.813–1.47]
1.51
[0.97–2.30]
1.67
[1.20–1.87]
1.96
[1.17–2.50]
2.25
[1.43–2.52]
2.51
[1.49–3.51]
*Not all samples available for all time points.
References
1. Mercuri E, et al. Lancet Neurol 2012;11:443–452.
2. Acsadi G, et al. J Neurosci Res 2009;12:2748–2756.
3. Singh RN, et al. Biochim Biophys Acta 2017;1860:299–315.
4. Hamilton G and Gillingwater TH. Trends Mol Med 2013;19:40–50.
12 months 24 months
Placebo to
active switch
Extension
Open-label Extension
at pivotal dose
Placebo
Placebo
RG7916
RG7916 dose level 1SUNFISHType 2 or 3 SMA
Part 1: Dose-finding*
RG7916:placebo, 2:1
n=36–72
Part 2: Confirmatory
Dose level selected
from Part 1
RG7916:placebo, 2:1
n=168
Placebo
RG7916 dose level 2
RG7916 dose
selected for Part 2

Preliminary evidence for pharmacodynamic effects of
RG7916 in JEWELFISH, a study in patients with spinal
muscular atrophy who previously participated in a study
with another SMN2-splicing targeting therapy
C.A. Chiriboga1, E. Mercuri2, D. Fischer3, D. Kraus4, M. Alexander5, G. Armstrong6, H. Kletzl4, M. Gerber4, Y. Cleary4,
K. Gelblin4, T. Bergauer4, K. Gorni4 and O. Khwaja4
1Department of Neurology, Columbia University Medical Center, New York, NY, USA; 2Paediatric Neurology and Nemo Center, Catholic University and Policlinico Gemelli, Rome, Italy;
3Department of Neurology, University of Basel Hospital, Basel, Switzerland; 4Roche Pharmaceutical Research and Early Development, Roche Innovation Center, Basel, Switzerland;
5Roche Pharmaceutical Research and Early Development, Roche Innovation Center New York, New York, NY, USA; 6Roche Products Ltd, Welwyn Garden City, UK
Table 1: JEWELFISH study overview
JEWELFISH
Type 2 or 3 SMA
Key
inclusion
criteria
• Confirmed diagnosis of 5q-autosomal recessive SMA
• Previous participation in a study with an antisense oligonucleotide targeting
SMN2 splicing or an SMN2 splicing modifier other than RG7916
• Any number of SMN2 gene copies allowed
Key
exclusion
criteria
• Concomitant participation in any investigational drug or device study
• Participation in any investigational drug or device study, other than an antisense
oligonucleotide targeting SMN2 splicing or SMN2 splicing modifier study, within
90 days of screening or 5 half-lives of the drug, whichever is longer
• History of gene or cell therapy
• Recently initiated treatment (>6 months prior to enrollment) with oral salbutamol
or another beta 2-adrenergic agonist taken orally
• Recent history (>1 year) of ophthalmologic disease
Primary
endpoints
Safety
PK: Mean plasma concentration, Cmax, AUC and Ctrough of RG7916 and metabolites
Secondary
endpoints
PD: SMN mRNA and protein levels in blood
Figure 1: SMN2 FL/SMNΔ7 mRNA ratio over time
Figure 2: SMN protein concentration over time
Figure 3: The SMN protein ratio over time
Background
• Spinal muscular atrophy (SMA) is a rare hereditary neuromuscular disease caused by
loss of function of the survival of motor neuron 1 (SMN1) gene.1
• SMA is characterized by progressive degeneration of spinal cord α-motor neurons,
leading to muscle weakness and atrophy.2
• While SMN1 produces full-length (FL) SMN protein, a second gene, SMN2, produces
only low levels of functional SMN protein.1
• RG7916 is an orally available, centrally and peripherally distributed investigational small
molecule designed to modify the splicing of the SMN2 pre-mRNA, resulting in increased
production of SMN2 FL mRNA, and subsequently SMN protein.3
• Although SMA has traditionally been viewed as a disease of motor neurons, increasing
evidence indicates that SMA is a multi-system or whole-body disorder.4 Therapies that
increase SMN protein levels systemically may have the potential to have broader
therapeutic benefit than those targeting the motor neurons alone.
• JEWELFISH is an exploratory, open-label study (NCT03032172) to establish the safety and
tolerability of RG7916 in people who have previously participated in a study with another
therapy targeting SMN2 splicing.5
Study design
• JEWELFISH is a multicenter, open-label study primarily evaluating the safety and
tolerability of once-daily oral administration of RG7916 in patients aged 12–60 years
with Type 2 or 3 SMA who have previously participated in a study with therapy targeting
SMN2 splicing (Table 1).
— This includes patients previously enrolled in the MOONFISH study with RG7800 and
those previously enrolled in studies with nusinersen.
— Planned enrollment is 24 patients, to include 16 previous MOONFISH patients and
8 previous nusinersen study patients.
• JEWELFISH will also investigate the pharmacodynamics (PD) and pharmacokinetics (PK)
of RG7916 treatment in non-naïve patients.
• As planned in the study protocol, a Safety Monitoring Committee reviews all safety
information from all JEWELFISH participants.
Results
• Data from 3 patients with up to 4 weeks’ exposure are shown.
• To date, no drug-related adverse events leading to study discontinuation have been
observed in JEWELFISH, and no stopping rules have been met.
• Preliminary PD data from 3 JEWELFISH patients show a rapid increase
in the SMN2 FL/SMNΔ7 mRNA ratio, with an approximately 2-fold increase at 4 hours
after treatment onset. The SMN2 FL/SMNΔ7 mRNA ratio increased up to 4-fold from
baseline over 4 weeks of RG7916 treatment (Figure 1).
• SMN protein analysis indicated an increase in SMN concentration over 4 weeks of
RG7916 treatment (Figure 2), with an up to 4-fold increase in the SMN ratio compared
with baseline in 1 patient and up to 2-fold increase in the other 2 patients (Figure 3).
Conclusions
• All 3 patients showed increases in SMN2 FL/SMNΔ7 mRNA ratio and SMN protein
increases of up to 4-fold.
• The JEWELFISH protocol will be amended to the dose level selected for the pivotal
Part 2 of SUNFISH6.
• Safety and PK/PD data from SUNFISH Part 1 informed the selection of a pivotal RG7916
dose level; see Mercuri E et al. SUNFISH poster.
• To date, RG7916 has been safe and well tolerated at all doses and there have been no
drug-related safety findings leading to withdrawal in any SMA patients exposed to RG7916.
Acknowledgments
We would like to thank the patients and their families for participation in these studies. This study is
funded by F. Hoffmann La-Roche. The authors thank Paul Grimsey, Yumi Cleary and Marianne Gerber of
Roche Pharmaceutical Research and Early Development, Roche Innovation Center, Basel, Switzerland for
data analysis support. We would also like to thank our collaborators at PTC Therapeutics and the SMA
Foundation. The authors thank Craig O'Hare, PhD, of MediTech Media UK for providing medical
writing support, which was funded by F. Hoffmann La-Roche Basel, Switzerland, in accordance with
Good Publication Practice (GPP3) guidelines.
Abbreviations
AUC, area under curve; Cmax, maximum observed plasma concentration; Ctrough, trough plasma concentration;
FL = full-length; PD, pharmacodynamics; PK, pharmacokinetics; SMA, spinal muscular atrophy; SMN, survival
of motor neuron.
References
1. Tisdale S, et al. J Neurosci. 2015;35(23):8691-8700.
2. Mercuri E, et al. Lancet Neurol. 2012;11(5):443-452.
3. Naryshkin NA, et al. Science. 2014;345(6197):688-693.
4. Hamilton G & Gillingwater TH. Trends Mol Med. 2013;19(1):40-50.
5. ClinicalTrials.gov. NCT03032172. Updated 2017. Accessed January, 2017.
6. ClinicalTrials.gov. NCT02908685. Updated 2017. Accessed January, 2017.
1
0 1 2 3 4
2
3
4
mRNA
SMN2 FL/SMNΔ
7
(fold increase from baseline
)
Time (weeks)
Patient 1
Patient 2
Patient 3
Baseline
2
0 1 2 3 4
6
4
SMN protein (ng/mL
)
Time (weeks)
Patient 1
Patient 2
Patient 3
1
0 1 2 3 4
2
3
4
S
MN protein rati
o
(fold increase from baseline
)
Time (weeks)
Patient 1
Patient 2
Patient 3
Baseline
Presented at the International Scientific Congress on Spinal Muscular Atrophy, Krakow, Poland, 25–27 January 2018

Relationship between central and peripheral SMN protein increase
upon treatment with RO7034067 (RG7916)
Agnès Poirier1, Marla Weetall2, Hasane Ratni1, Katja Heinig1, Nikolai Naryshkin2, Sergey Paushkin3, Lutz Mueller1
1 Pharmaceutical Sciences, Roche Pharma Research and Early Development, Roche Innovation Center, Basel, Switzerland
2 PTC Therapeutics, South Plainfield, NJ, USA
3 SMA Foundation, New York, NY, USA
Figure 1: RO7034067 tissue vs. plasma concentration
RO7034067 plasma concentration vs. concentration in brain (Figure 1A; n=190), muscle (Figure 1B; n=44) and CSF (Figure 1C; n=35) of mice, rats
and monkeys following single or repeat PO or IP administration.
Conclusions
• RO7034067 distributes well into the CNS, CSF and peripheral tissues, including muscle,
blood and brain, in mice, rats and monkeys following single or repeat oral or IP dosing.
• Oral administration of RO7034067 increased SMN protein levels to a similar magnitude
in the brain and muscle of C/C and Δ7 mouse models of SMA.
• In addition, oral administration of a structurally similar SMN2 splicing modifier,
RO6885247, resulted in parallel increases in SMN protein levels in the blood, brain, and
muscle of C/C-allele mice.
• The data from mice and other species suggest that SMN protein level increases seen
in the blood of patients following RO7034067 (RG7916) treatment reflect SMN protein
level increases in CNS, muscle and other key tissues affected in SMA.
Abbreviations
• AAALAC, Association for Assessment and Accreditation of Laboratory Animal Care International; CNS, central
nervous system; CSF, cerebral spinal fluid; IP, intraperitoneal injection; PO, per oral; MRD1, multi-drug resistance
gene; SMA, spinal muscular atrophy; SMN, survival motor neuron.
References
1. Lunn MR and Wang CH. Lancet 2008; 371:2120–2133.
2. Hamilton G and Gillingwater TH. Trends Mol Med. 2013; 19:40–50.
3. Feng Z et al. Hum Mol Genet. 2016; 25:964-75.
4. Sivaramakrishnan M et al. Nat Commun. 2017; 8:1476,017-01559-4.
Acknowledgements
• We would like to thank the Jackson Laboratory for providing the FVB.129(B6) Smn1tm5(Smn1/SMN2)Mrph/J
strain and FVB.Cg-Tg(SMN2*delta7)4299Ahmb Tg(SMN2)89Ahmb strain; and the Charles River Laboratories
for providing the Wistar Hannover Crl:WI (Han) strain. We would also like to thank our collaborators at
PTC Therapeutics and the SMA Foundation. The study was sponsored by F. Hoffmann-La Roche AG, Basel,
Switzerland. Writing and editorial assistance was provided by Vassia Schiza, PhD, of MediTech Media, UK and
was funded by F. Hoffmann La-Roche Basel, Switzerland, in accordance with Good Publication Practice
(GGP3) guidelines. All animal experiments were carried out in AAALAC-certified facilities, and protocols were
approved by the Institutional Animal Care and Use Committee.
Background
• Spinal muscular atrophy (SMA) is caused by deletion and/or mutation of the Survival
Motor Neuron 1 (SMN1) gene, resulting in insufficient levels of the SMN protein, which is
critical to the survival of motor neurons.1
• Increasing evidence suggests SMN depletion directly affects cells and tissues in both the
CNS and the periphery, suggesting that SMA may be a multi-system disorder.2
• RO7034067 (RG7916) is an orally administered, centrally and peripherally distributed
SMN2 pre-mRNA splicing modifier that increases SMN protein levels.3-4
— RO7034067 is under investigation in the SUNFISH (Type 2/3 SMA), FIREFISH (Type 1
SMA) and JEWELFISH (non-naïve Type 2/3 SMA) clinical studies.
• RO7034067 was designed to penetrate the CNS (avoiding transporter protein
interaction which would restrict this) and peripheral tissues on oral administration
• We assessed the tissue and CSF distribution of orally administered RO7034067 and
effects on SMN protein levels in the CNS and peripheral tissues of preclinical models;
this will inform the significance of increases in blood SMN protein levels seen in
patients receiving RO7034067.
Methods
• RO7034067 tissue and CSF (to serve as surrogate for free concentration in the brain) distribution
were assessed in adult and juvenile, wildtype FvB mice, Brown Norway BN/Crl rats,
Wistar Hannover Crl:WI (Han) rats and cynomolgus monkeys (Macaca fascicularis).
• C/C-allele mouse model of mild SMA was derived from FVB.129(B6)-Smn1tm5(Smn1/
SMN2)Mrph/J strain and dosed from ~7 weeks old. The Δ7 mice was derived from
FVB.Cg-Tg(SMN2*delta7)4299Ahmb Tg(SMN2)89Ahmb strain and dosed from
post-natal Day 3.
• Levels of RO7034067 were quantified using liquid chromatography−tandem mass
spectrometry (LC-MS/MS) on triple quadrupole mass spectrometers.
• SMN protein levels were quantified using Homogeneous Time Resolved Fluorescence
(Human SMN Assay Kit Cisbio) as previously described, and normalised to total protein
concentrations. Fold increase was calculated vs vehicle.
Results
• Single or repeated, daily oral (PO) or intraperitoneal (IP) administration of RO7034067
for up to 39 weeks showed similar total drug levels in brain, muscle and CSF of mice
(n=90), rats (n=148) and monkeys (n=24) (Figure 1).
— An excellent correlation was observed between plasma levels of RO7034067 and
levels in brain, muscle and CSF over a wide range of concentrations.
• Following oral administration for 7 days in cynomolgus monkeys, RO7034067
distributed to key tissues and organs including the CNS and muscle that could be
affected in SMA (Figure 2).
— Total plasma concentrations (794 ng/ml) were very similar to those in brain (783
ng/g) and muscle (668 ng/g), whereas CSF drug levels (53.5 ng/ml) were within the
range of free compound in plasma (119 ng/ml).
• After oral administration of RO7034067 to adult C/C-allele mice (n=4 per dose; 1, 3
or 10 mg/kg/day for 10 days), and Δ7 mice (n=6 or 7 per dose; 0.1, 0.3, 1 or 3 mg/
kg/day for 7 days), the SMN protein increase from baseline was parallel in brain and
muscle (Figure 3).
• Following oral administration of a structurally similar SMN2 splicing modifier,
RO6885247 (RG7800) in C/C-allele mice, comparable fold increases in SMN protein
levels in blood, muscle and brain were observed in (Figure 4).
Figure 2. RO7034067 tissue distribution in cynomolgus monkeys
RO7034067 concentration in plasma (ng/ml), CSF (ng/ml) and tissues (ng/g) of cynomolgus monkeys (n=2) at Day 7 following daily PO dosing
(3mg/kg/day for 7 days).
RO7034067 (ng/ml or ng/g)
10000100010010
Plasma
AUC0–24h@day6 = 8740 ng/h/ml
Monkey 1
Monkey 2Muscle
Brain
Heart
Bone marrow
Spleen
Kidney
Liver
CSF
Free plasma
Figure 4. SMN protein fold increase from baseline in blood, brain and muscle of adult
C/C-allele mice after oral administration of RO6885247
Fold increase from baseline in SMN protein levels in blood vs. brain (Figure 4A; n=74) and fold increase in SMN protein levels in brain, muscle and blood over
time (Figure 4B, n=5 per time point) in adult C/C-allele mice following a single or repeat doses of RO6885247 (10 mg/kg/day) PO for up to 30 days.
0
0 1 2 3 4
2
1
3
4
SMN protein
bloo
d
(fold inc
)
SMN protein brain (fold inc)
0
0 5 10 2015 25 30
2
Brain
Muscle
Blood
1
3
4
SMN protei
n
(fold increase
)
Time (days)
1
1 10 100 1000
100
10
1000
Monkeys
Rats
Mice
3-fold
identity line
n = 190
Brain (ng/g
)
1
1 10 100 1000
100
10
1000
n =44
B
Plasma (ng/ml)
A
n = 35
C
)Plasma (ng/ml)
1
1 10 100
100
10
CSF (ng/ml
)
Free Plasma (ng/ml)
Muscle (ng/g
)
Figure 3. SMN protein levels fold increase from baseline in brain vs. muscle of SMA
mouse models following oral administration of RO7034067
SMN protein levels fold increase from baseline in brain vs. muscle in adult C/C-allele mice (n=4 per dose) dosed 1, 3 or 10 mg/kg/day PO for 10 days,
and in Δ7 mice (n=6 or 7 per dose) dosed 0.1, 0.3, 1 or 3 mg/kg/day PO from post-natal 3 through 9.
0
0 1 2 3 4
2
1
3
4
SMN protein brain (fold inc)
SMN protein
muscl
e (fold inc
)
Presented at the International Scientific Congress on Spinal Muscular Atrophy, Krakow, Poland, 25–27 January 2018
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- PTC Therapeutics Reports Inducement Grants Under Nasdaq Listing Rule 5635(c)(4)
- Blackwell 3D Launches New Website, Eyes Project Development
- Global CIOs geared up to scale AI but organizations aren’t as ready
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share