Form 8-K PDS Biotechnology Corp For: Jun 07
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the
Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): June 7, 2021
PDS BIOTECHNOLOGY CORPORATION
(Exact Name of Registrant as Specified in Charter)
|
Delaware
|
001-37568
|
26-4231384
|
||
|
(State or Other Jurisdiction of Incorporation)
|
(Commission File Number)
|
(I.R.S. Employer Identification No.)
|
25B Vreeland Road, Suite 300, Florham Park, NJ 07932
(Address of Principal Executive Offices, and Zip Code)
(800) 208-3343
Registrant’s Telephone Number, Including Area Code
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the
following provisions (see General Instruction A.2. below):
|
☐
|
Written communication pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
|
|
☐
|
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
|
|
☐
|
Pre-commencement communication pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
|
|
☐
|
Pre-commencement communication pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
|
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class
|
Trading Symbol(s)
|
Name of each exchange on which registered
|
|
Common Stock, par value $0.00033 per share
|
PDSB
|
The Nasdaq Capital Market
|
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (17 CFR §230.405 of this
chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (17 CFR §240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or
revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. Yes ☐ No ☐
|
Item 7.01
|
Regulation FD.
|
On June 7, 2021, PDS Biotechnology Corporation (the “Company”) updated its corporate presentation slide deck. A copy of the slide deck is
furnished as Exhibit 99.1 hereto.
|
Item 8.01
|
Other Events.
|
On June 8, 2021, the Company issued a press release announcing the presentation of interim data from the National Cancer Institute
(NCI)-led Phase 2 trial at the American Society of Clinical Oncology (ASCO) 2021 Annual Meeting. The press release is filed as Exhibit 99.2 hereto and incorporated herein by reference.
On June 8, 2021, the Company held a Post-ASCO Conference
Call Interim Data Review. A copy of the slide deck presented at this Conference call is filed as Exhibit 99.3 hereto and incorporated herein by reference.
|
Item 9.01
|
Financial Statements and Exhibits.
|
(d) Exhibits.
|
Exhibit
Number
|
Description
|
|
|
Corporate Presentation June 7, 2021
|
||
|
Press Release, dated June 8, 2021.
|
||
|
Post-ASCO Conference Call Interim Data Review dated June 8, 2021.
|
Signature
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf
by the undersigned hereunto duly authorized.
|
PDS BIOTECHNOLOGY CORPORATION
|
||
|
Date: June 8, 2021
|
By:
|
/s/ Frank Bedu-Addo, Ph.D.
|
|
Name:
|
Frank Bedu-Addo, Ph.D.
|
|
|
Title:
|
President and Chief Executive Officer
|
|
Exhibit 99.1

CORPORATE OVERVIEW Frank Bedu-Addo Ph.D. President & CEO JUNE 2021

2 Forward-Looking Statements This presentation contains forward-looking statements about PDS
Biotechnology Corporation (“PDSB”), and its businesses, business prospects, strategies and plans, including but not limited to statements regarding anticipated pre-clinical and clinical drug development activities and timelines and market
opportunities. All statements other than statements of historical facts included in this presentation are forward-looking statements. The words “anticipates,” “may,” “can,” “plans,” “believes,” “estimates,” “expects,” “projects,” “intends,”
“likely,” “will,” “should,” “to be,” and any similar expressions or other words of similar meaning are intended to identify those assertions as forward-looking statements. These forward-looking statements involve substantial risks and
uncertainties that could cause actual results to differ materially from those anticipated.Factors that may cause actual results to differ materially from such forward-looking statements include those identified under the caption “Risk Factors”
in the documents filed with the Securities and Exchange Commission (“SEC”) from time to time, including its Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K. You are cautioned not to place undue
reliance on these forward-looking statements, which speak only as of the date of this presentation. Except to the extent required by applicable law or regulation, PDSB undertakes no obligation to update the forward-looking statements included
in this presentation to reflect subsequent events or circumstances.
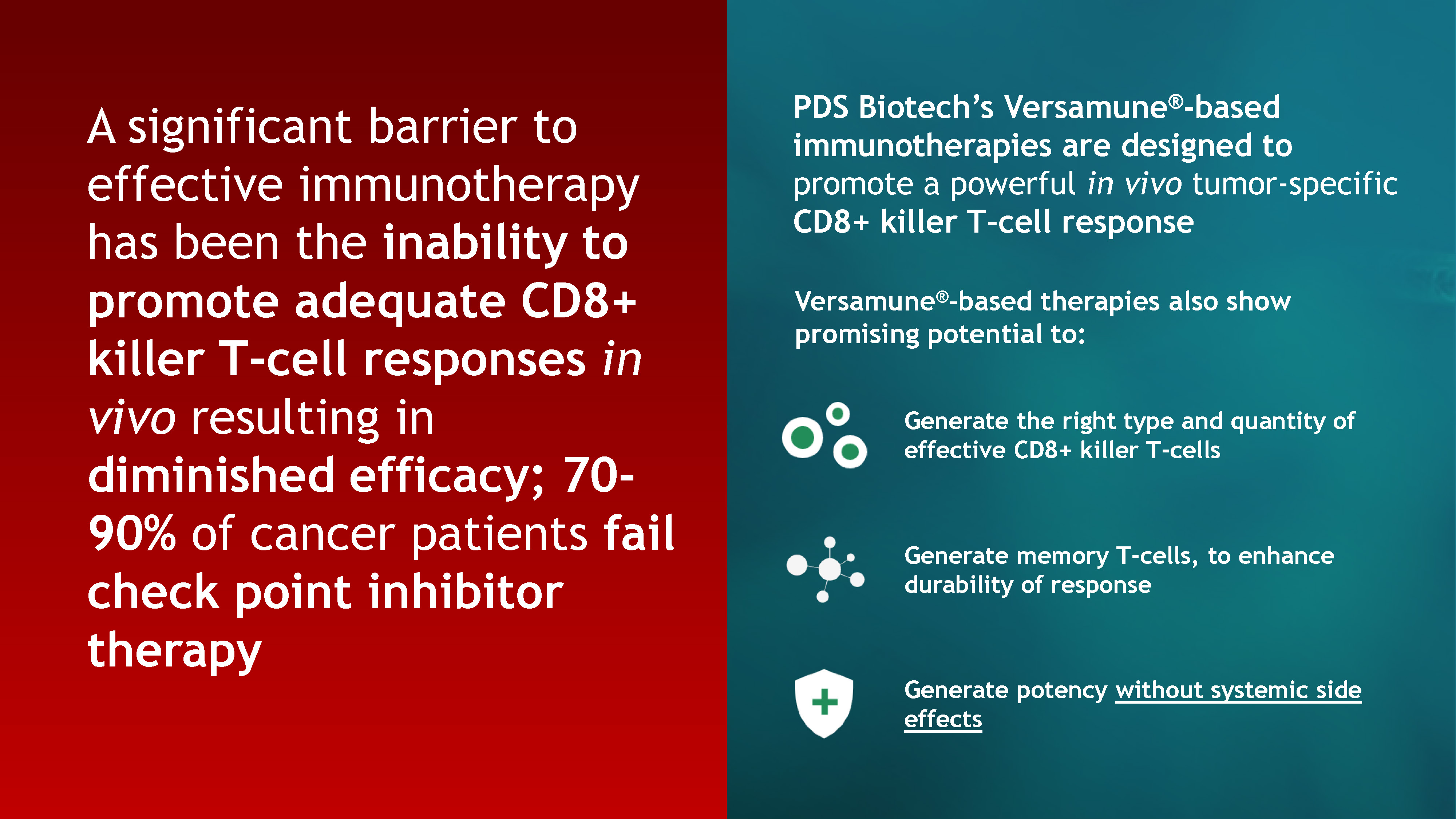
3 PDS Biotech’s Versamune®-based immunotherapies are designed to promote a powerful in vivo
tumor-specific CD8+ killer T-cell response Generate the right type and quantity of effective CD8+ killer T-cells Generate potency without systemic side effects Generate memory T-cells, to enhance durability of response A significant
barrier to effective immunotherapy has been the inability to promote adequate CD8+ killer T-cell responses in vivo resulting in diminished efficacy; 70-90% of cancer patients fail check point inhibitor therapy Versamune®-based therapies also
show promising potential to:
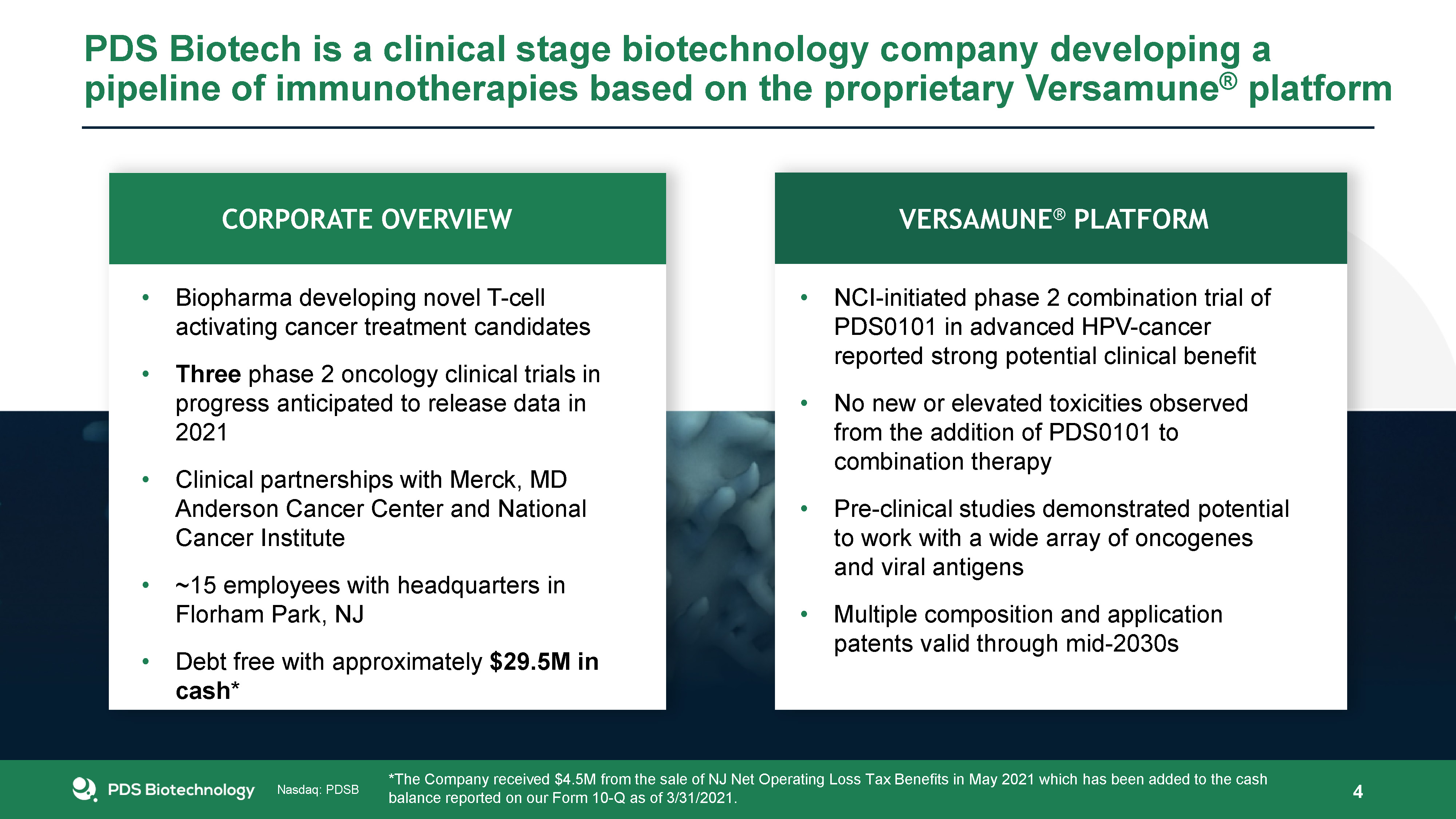
PDS Biotech is a clinical stage biotechnology company developing a pipeline of immunotherapies based on
the proprietary Versamune® platform 4 NCI-initiated phase 2 combination trial of PDS0101 in advanced HPV-cancer reported strong potential clinical benefitNo new or elevated toxicities observed from the addition of PDS0101 to combination
therapyPre-clinical studies demonstrated potential to work with a wide array of oncogenes and viral antigensMultiple composition and application patents valid through mid-2030s Biopharma developing novel T-cell activating cancer treatment
candidates Three phase 2 oncology clinical trials in progress anticipated to release data in 2021Clinical partnerships with Merck, MD Anderson Cancer Center and National Cancer Institute~15 employees with headquarters in Florham Park, NJDebt
free with approximately $29.5M in cash* Pipeline Versamune® Platform Corporate Overview *The Company received $4.5M from the sale of NJ Net Operating Loss Tax Benefits in May 2021 which has been added to the cash balance reported on our
Form 10-Q as of 3/31/2021.
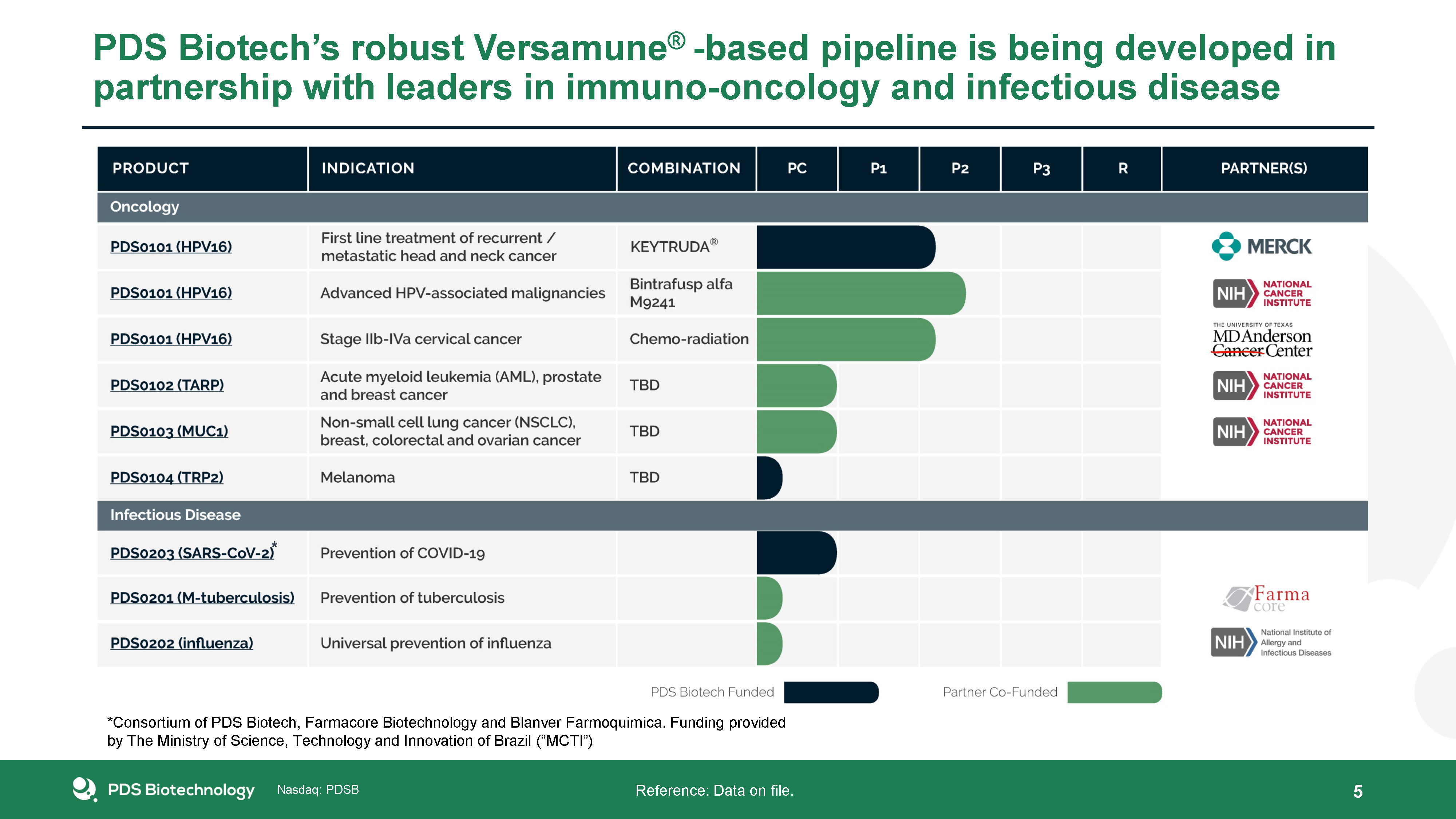
PDS Biotech’s robust Versamune® -based pipeline is being developed in partnership with leaders in
immuno-oncology and infectious disease 5 Reference: Data on file. * *Consortium of PDS Biotech, Farmacore Biotechnology and Blanver Farmoquimica. Funding provided by The Ministry of Science, Technology and Innovation of Brazil (“MCTI”)

PDS Biotech executive team has demonstrated success in the development and commercialization of leading
pharmaceutical products 6 Senior executive experience with management of strategy and execution at both large pharma and biotechsNotable drug development:Abelcet® (Liposome Company/ Elan)PEG-Intron® (Schering-Plough/ Merck) Frank
Bedu-Addo, PhDChief Executive Officer Co-founder>35 years of drug development experience In-depth experience with biotech drug discovery, product development and manufacturing Gregory Conn, PhDChief Scientific Officer >30 years of
translational clinical research experienceFormer Director of Clinical Research at National Cancer Institute Center for Cancer Research (Cancer Vaccine Branch) Lauren V. Wood, MDChief Medical Officer Senior executive experience with over 20
years of experience in high tech companiesIn-depth experience with M&A transactions, capital markets, business development and investor relations Seth Van Voorhees, PhDChief Financial Officer

Introduction to the Versamune® Platform
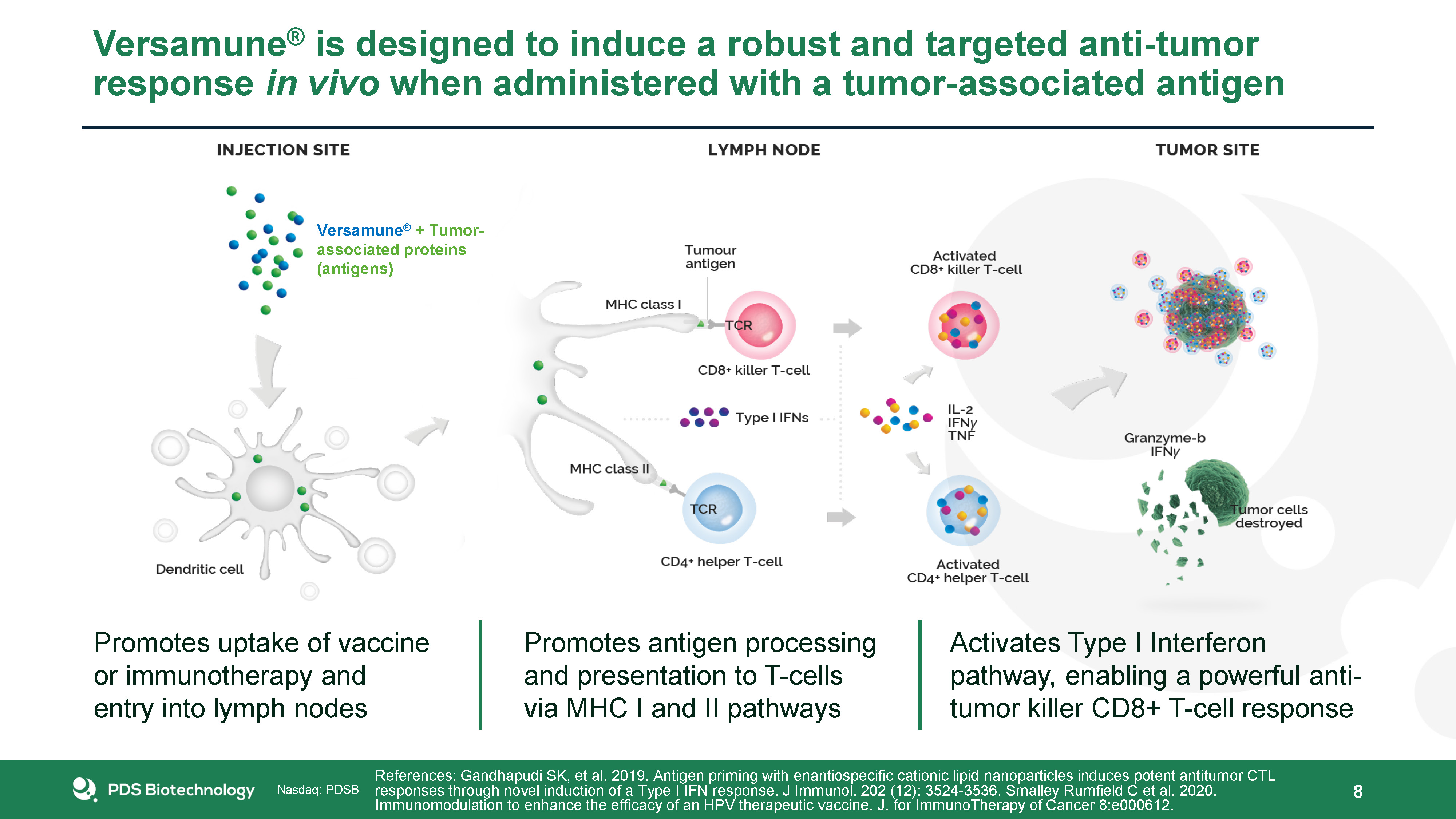
Versamune® is designed to induce a robust and targeted anti-tumor response in vivo when administered with
a tumor-associated antigen 8 References: Gandhapudi SK, et al. 2019. Antigen priming with enantiospecific cationic lipid nanoparticles induces potent antitumor CTL responses through novel induction of a Type I IFN response. J Immunol. 202
(12): 3524-3536. Smalley Rumfield C et al. 2020. Immunomodulation to enhance the efficacy of an HPV therapeutic vaccine. J. for ImmunoTherapy of Cancer 8:e000612. Promotes uptake of vaccine or immunotherapy and entry into lymph
nodes Promotes antigen processing and presentation to T-cells via MHC I and II pathways Activates Type I Interferon pathway, enabling a powerful anti-tumor killer CD8+ T-cell response Versamune® + Tumor-associated proteins (antigens)
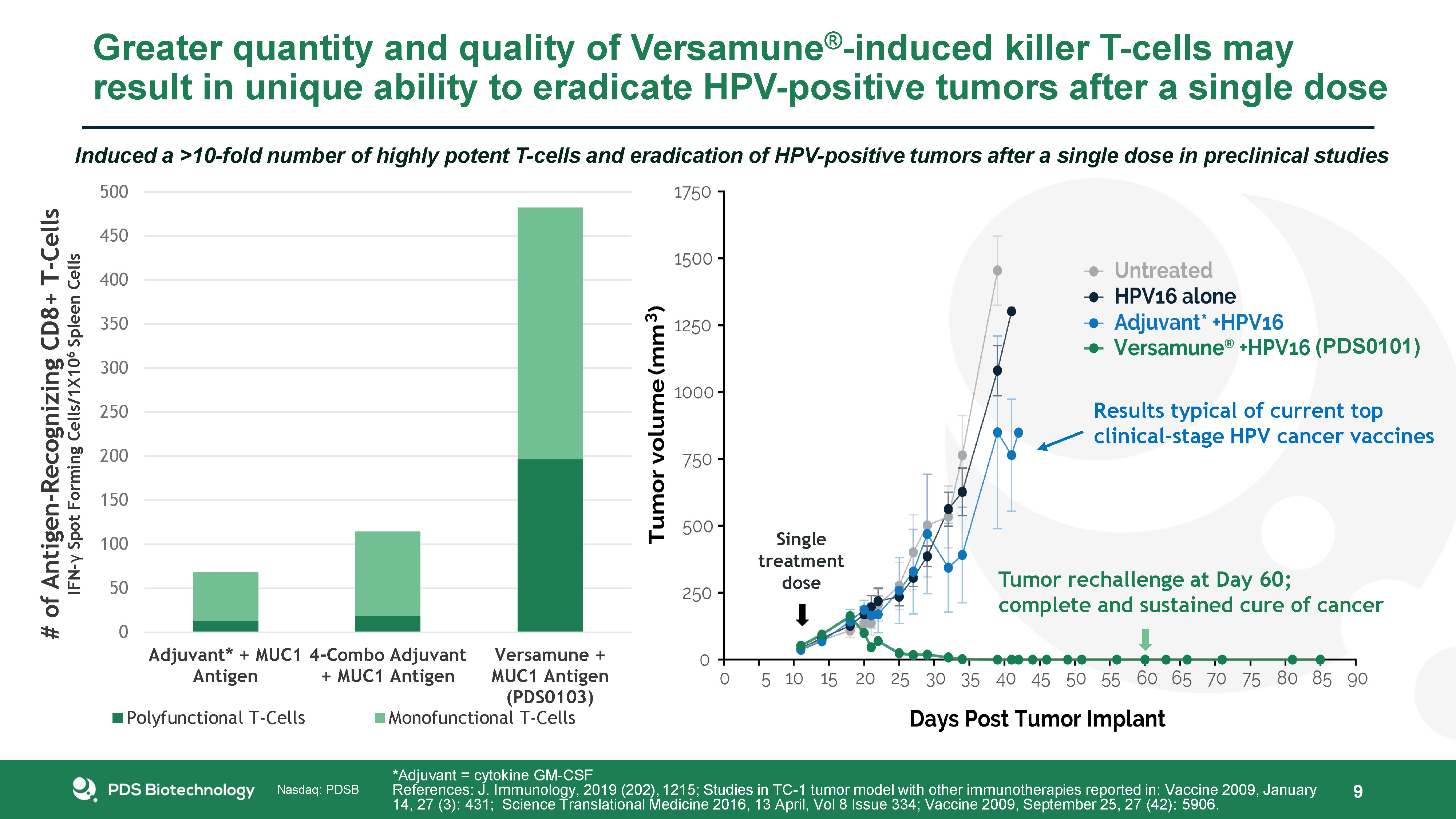
Greater quantity and quality of Versamune®-induced killer T-cells may result in unique ability to
eradicate HPV-positive tumors after a single dose 9 Induced a >10-fold number of highly potent T-cells and eradication of HPV-positive tumors after a single dose in preclinical studies Single treatment dose Results typical of current
topclinical-stage HPV cancer vaccines Tumor rechallenge at Day 60; complete and sustained cure of cancer *Adjuvant = cytokine GM-CSFReferences: J. Immunology, 2019 (202), 1215; Studies in TC-1 tumor model with other immunotherapies
reported in: Vaccine 2009, January 14, 27 (3): 431; Science Translational Medicine 2016, 13 April, Vol 8 Issue 334; Vaccine 2009, September 25, 27 (42): 5906. (PDS0101)

PDS0101 Phase 2 Clinical Development
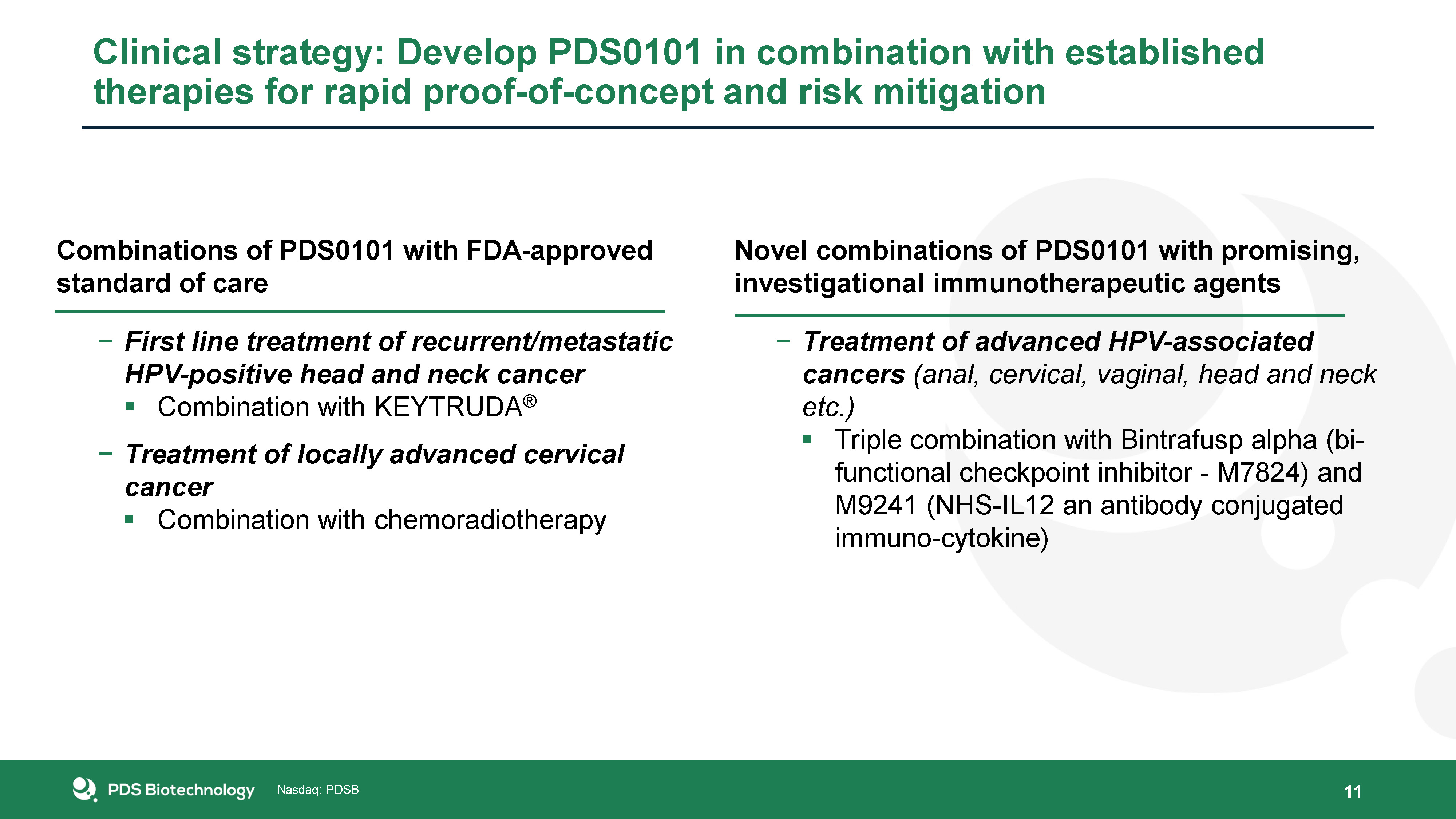
Combinations of PDS0101 with FDA-approved standard of careFirst line treatment of recurrent/metastatic
HPV-positive head and neck cancerCombination with KEYTRUDA®Treatment of locally advanced cervical cancerCombination with chemoradiotherapy 11 Novel combinations of PDS0101 with promising, investigational immunotherapeutic agentsTreatment of
advanced HPV-associated cancers (anal, cervical, vaginal, head and neck etc.)Triple combination with Bintrafusp alpha (bi-functional checkpoint inhibitor - M7824) and M9241 (NHS-IL12 an antibody conjugated immuno-cytokine) Clinical strategy:
Develop PDS0101 in combination with established therapies for rapid proof-of-concept and risk mitigation
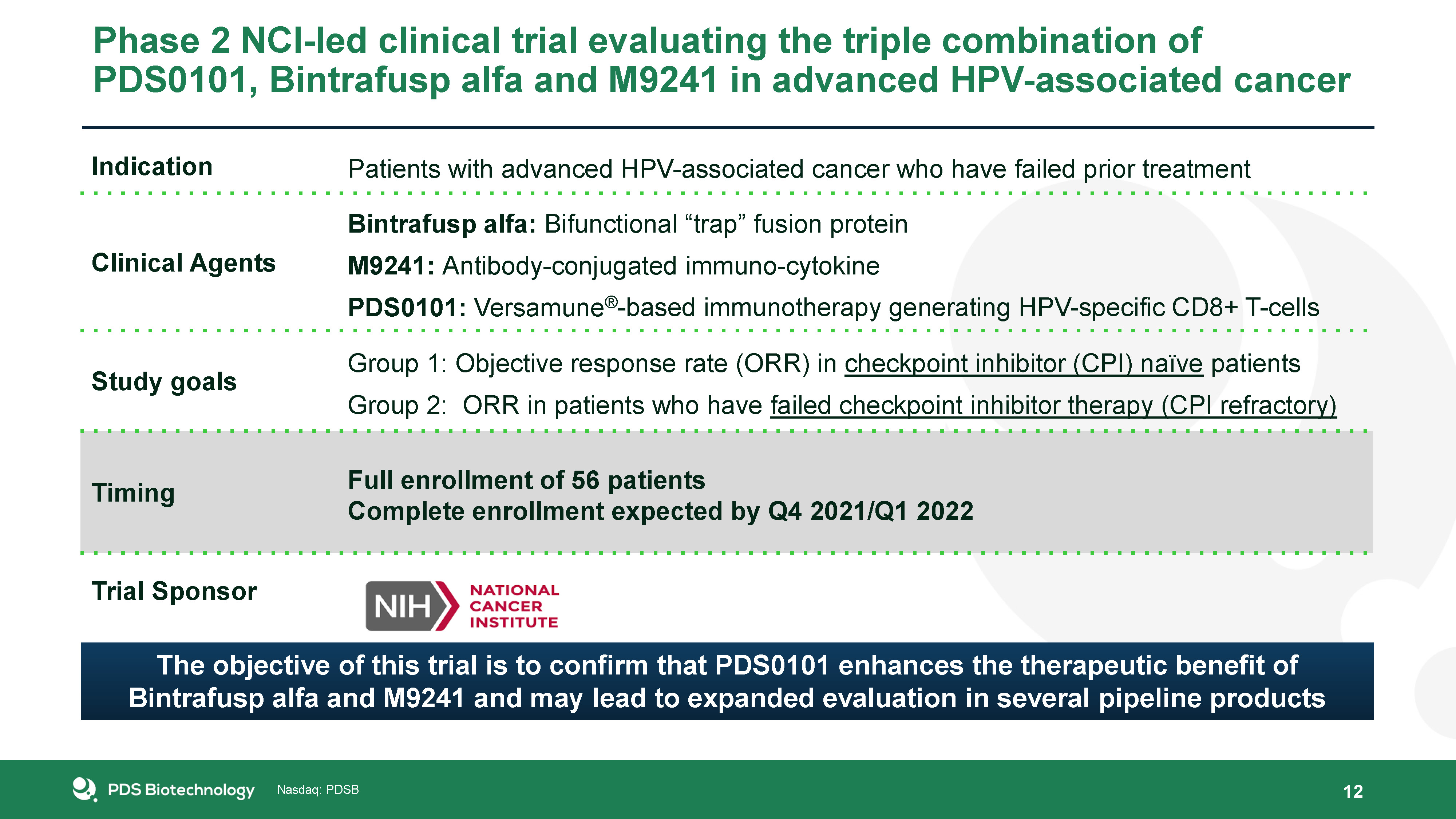
Phase 2 NCI-led clinical trial evaluating the triple combination of PDS0101, Bintrafusp alfa and M9241 in
advanced HPV-associated cancer 12 Indication Patients with advanced HPV-associated cancer who have failed prior treatment Clinical Agents Bintrafusp alfa: Bifunctional “trap” fusion proteinM9241: Antibody-conjugated immuno-cytokinePDS0101:
Versamune®-based immunotherapy generating HPV-specific CD8+ T-cells Study goals Group 1: Objective response rate (ORR) in checkpoint inhibitor (CPI) naïve patientsGroup 2: ORR in patients who have failed checkpoint inhibitor therapy (CPI
refractory) Timing Full enrollment of 56 patientsComplete enrollment expected by Q4 2021/Q1 2022 Trial Sponsor The objective of this trial is to confirm that PDS0101 enhances the therapeutic benefit of Bintrafusp alfa and M9241 and may
lead to expanded evaluation in several pipeline products
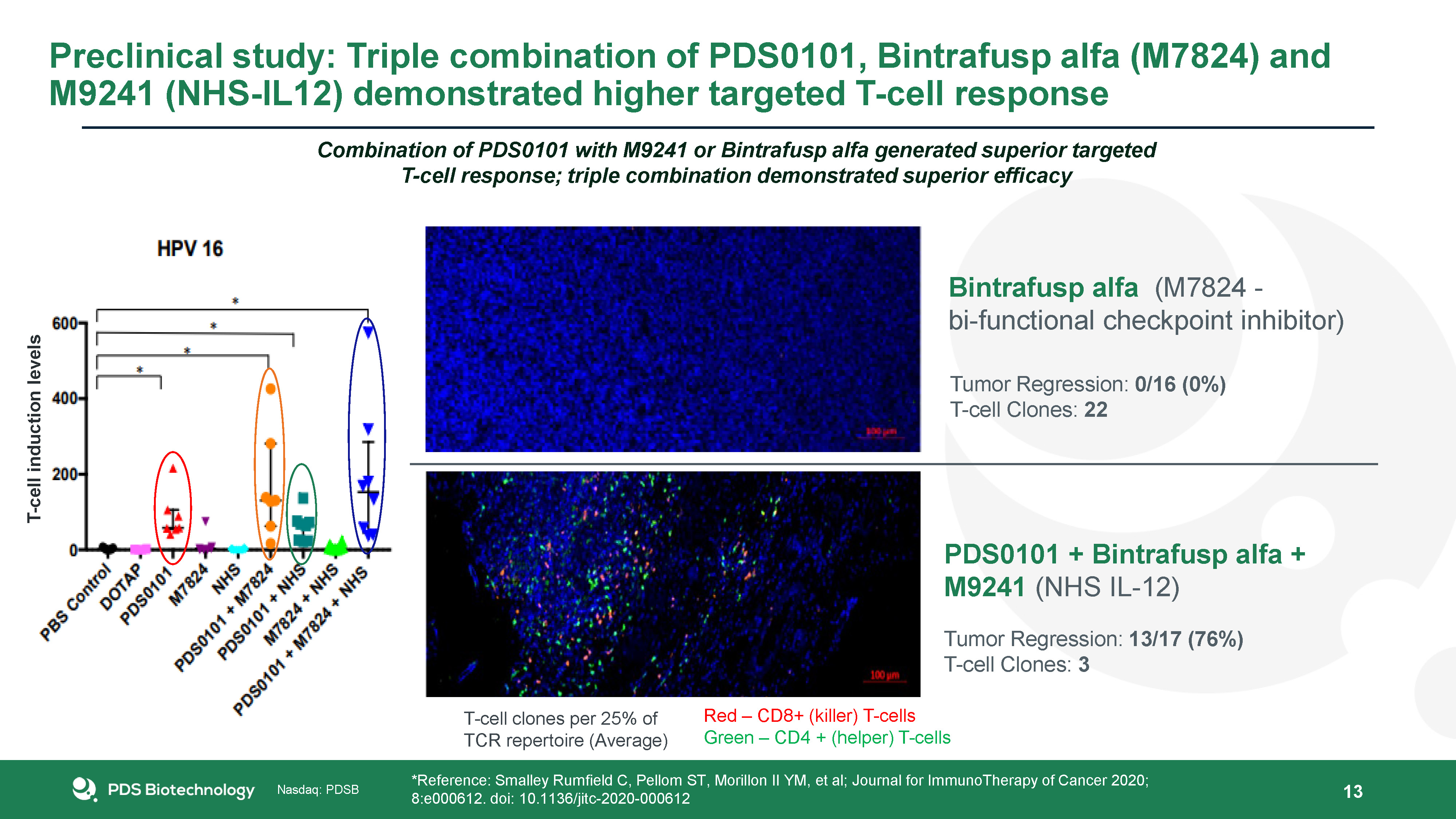
13 Bintrafusp alfa (M7824 - bi-functional checkpoint inhibitor) Tumor Regression: 0/16 (0%)T-cell
Clones: 22 PDS0101 + Bintrafusp alfa + M9241 (NHS IL-12) Tumor Regression: 13/17 (76%)T-cell Clones: 3 *Reference: Smalley Rumfield C, Pellom ST, Morillon II YM, et al; Journal for ImmunoTherapy of Cancer 2020; 8:e000612. doi:
10.1136/jitc-2020-000612 Red – CD8+ (killer) T-cellsGreen – CD4 + (helper) T-cells T-cell clones per 25% of TCR repertoire (Average) Combination of PDS0101 with M9241 or Bintrafusp alfa generated superior targeted T-cell response; triple
combination demonstrated superior efficacy T-cell induction levels Preclinical study: Triple combination of PDS0101, Bintrafusp alfa (M7824) and M9241 (NHS-IL12) demonstrated higher targeted T-cell response
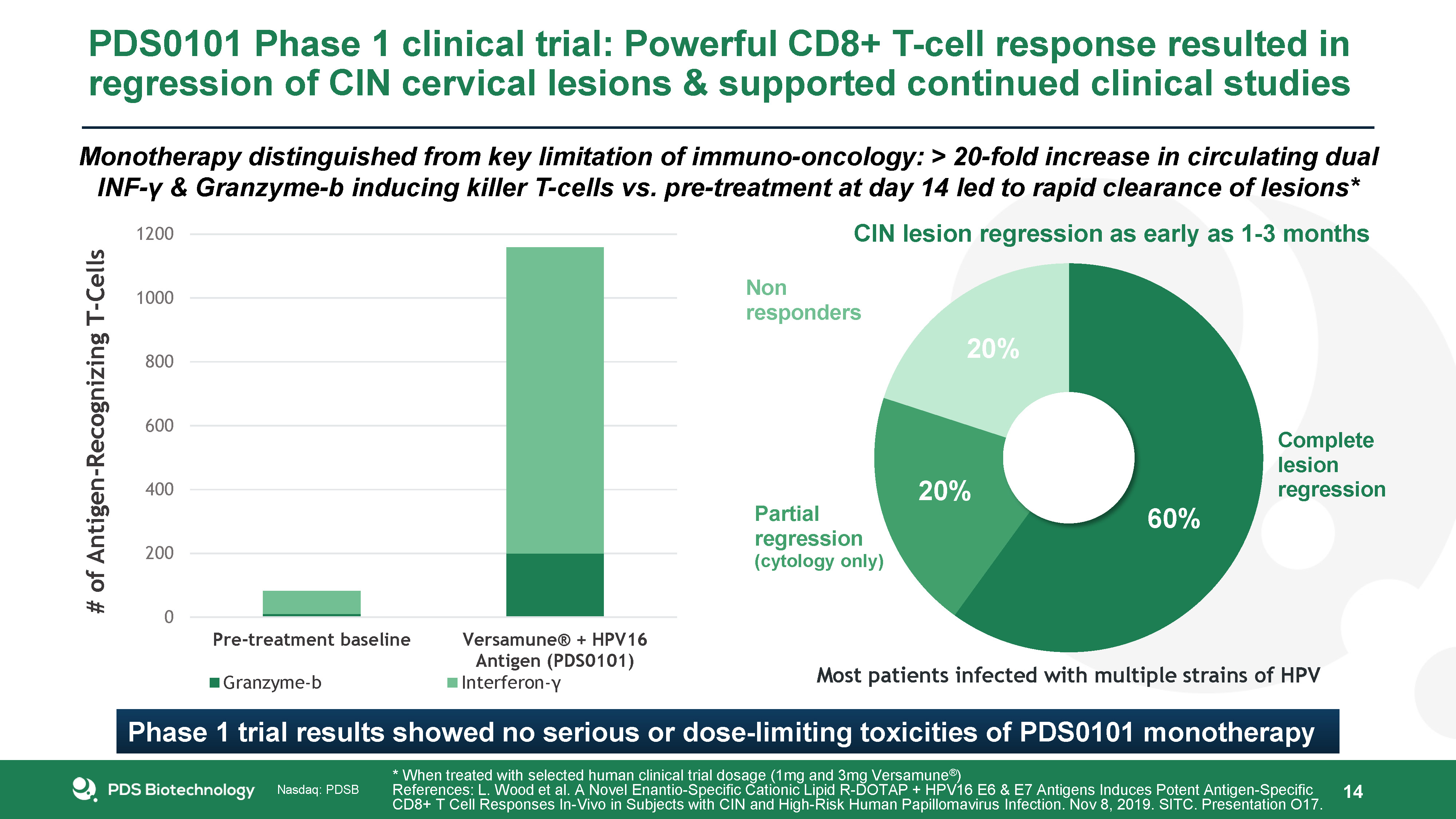
PDS0101 Phase 1 clinical trial: Powerful CD8+ T-cell response resulted in regression of CIN cervical
lesions & supported continued clinical studies 14 * When treated with selected human clinical trial dosage (1mg and 3mg Versamune®)References: L. Wood et al. A Novel Enantio-Specific Cationic Lipid R-DOTAP + HPV16 E6 & E7 Antigens
Induces Potent Antigen-Specific CD8+ T Cell Responses In-Vivo in Subjects with CIN and High-Risk Human Papillomavirus Infection. Nov 8, 2019. SITC. Presentation O17. Most patients infected with multiple strains of HPV CIN lesion regression as
early as 1-3 months 60% 20% 20% Phase 1 trial results showed no serious or dose-limiting toxicities of PDS0101 monotherapy Monotherapy distinguished from key limitation of immuno-oncology: > 20-fold increase in circulating dual INF-γ
& Granzyme-b inducing killer T-cells vs. pre-treatment at day 14 led to rapid clearance of lesions*
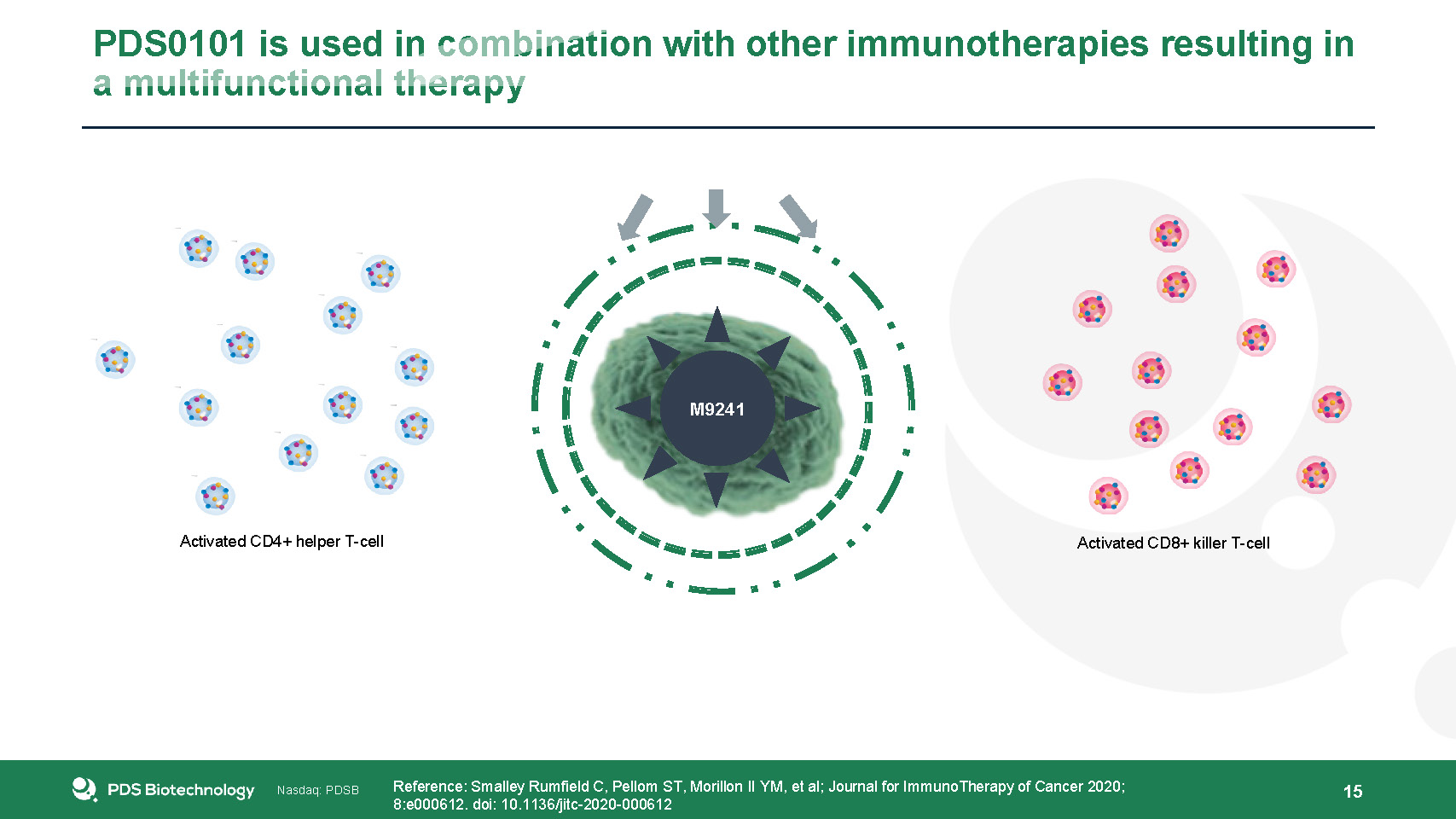
PDS0101 is used in combination with other immunotherapies resulting in a multifunctional
therapy 15 Bintrafusp alfa exposes the tumor to attack and M9241 issues a signal calling T-cells to the tumor Reference: Smalley Rumfield C, Pellom ST, Morillon II YM, et al; Journal for ImmunoTherapy of Cancer 2020; 8:e000612. doi:
10.1136/jitc-2020-000612 Bintrafusp ALFA M9241 PDS0101 induces a powerful, HPV16-targeted CD8+ and CD4+ T-cell response PDS0101 Activated CD8+ killer T-cell Activated CD4+ helper T-cell The triple combination
works in synergy to prompt targeted T-cells to infiltrate and destroy the tumor TUMOR DESTROYED
Objective response rate is measured by RECIST 1.1 and represents at least a 30% reduction in tumor
sizeAdvanced HPV-related cancer that is checkpoint inhibitor naïve:Patients who fail chemotherapy and/or radiation progress to checkpoint inhibitor therapy12-24% ORR with standard of care checkpoint inhibitors30% ORR reported with experimental
monotherapy Bintrafusp alfa is the highest reported to dateAdvanced HPV-related cancer that is checkpoint inhibitor refractory:Few treatment options exist for these patients5-12% ORR reported with checkpoint inhibitors PDS0101 phase 2 triple
combination trial: Evaluated potential for superior preclinical tumor regression in advanced HPV-related cancer 16 A critical limitation of immunotherapy is the inability to induce large numbers of powerful tumor-attacking CD8+ (killer)
T-cells within the body, that can result in tumor reduction or elimination in a significant number of advanced cancer patients Reference: Strauss J, et al. J Immunother Cancer. 2020 Dec;8(2):e001395
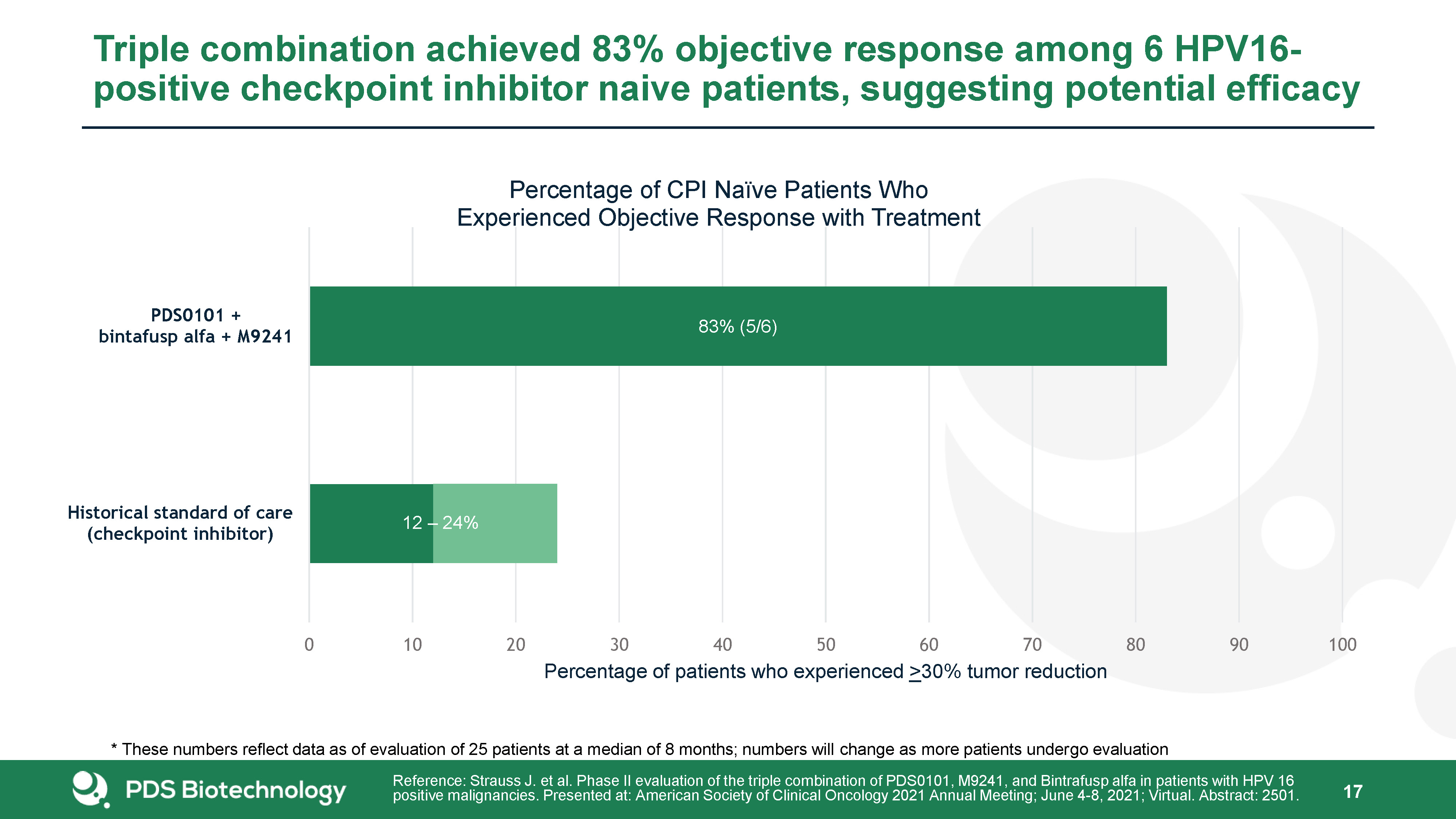
Triple combination achieved 83% objective response among 6 HPV16-positive checkpoint inhibitor naive
patients, suggesting potential efficacy 17 Reference: Strauss J. et al. Phase II evaluation of the triple combination of PDS0101, M9241, and Bintrafusp alfa in patients with HPV 16 positive malignancies. Presented at: American Society of
Clinical Oncology 2021 Annual Meeting; June 4-8, 2021; Virtual. Abstract: 2501. * These numbers reflect data as of evaluation of 25 patients at a median of 8 months; numbers will change as more patients undergo evaluation
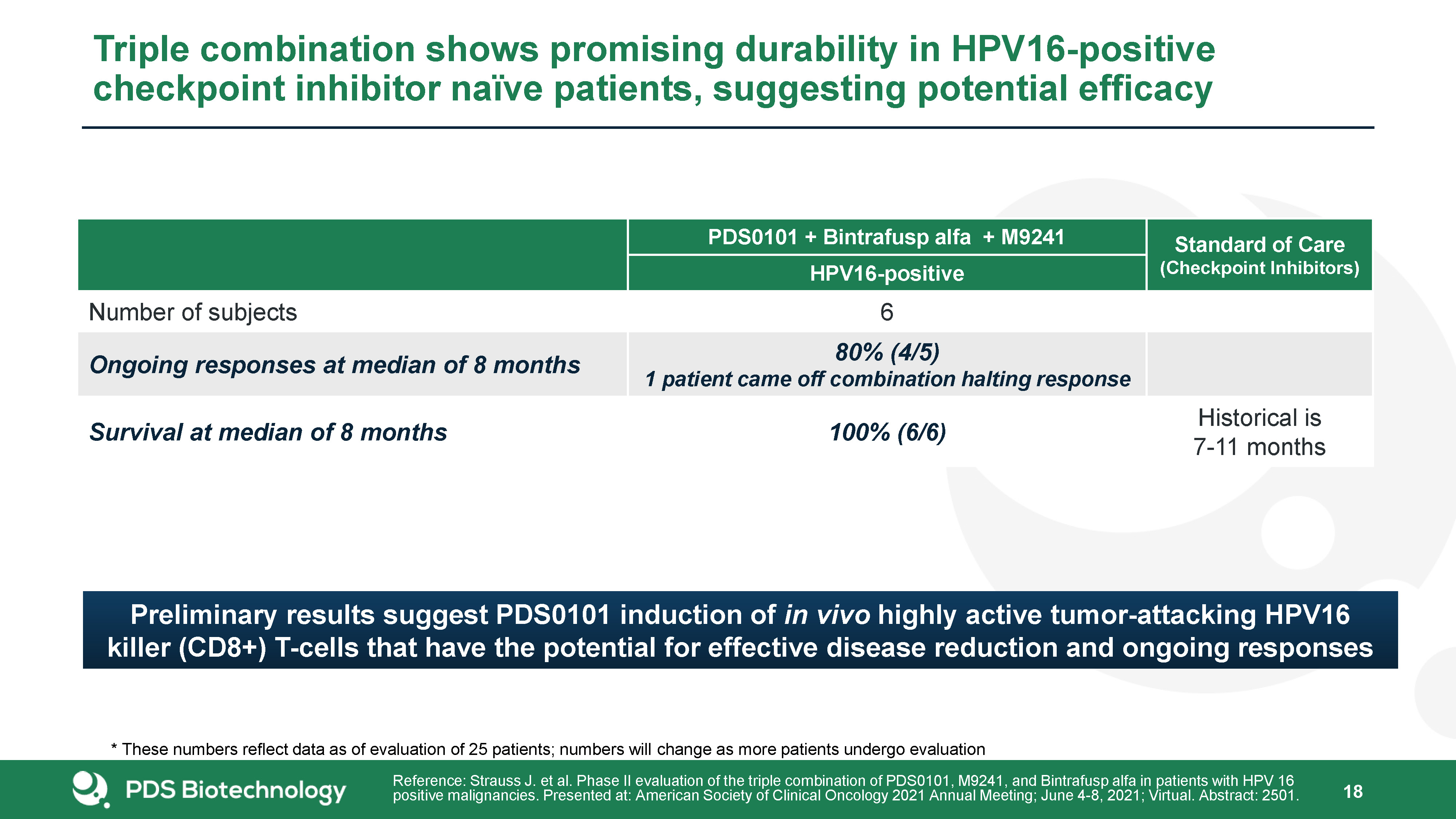
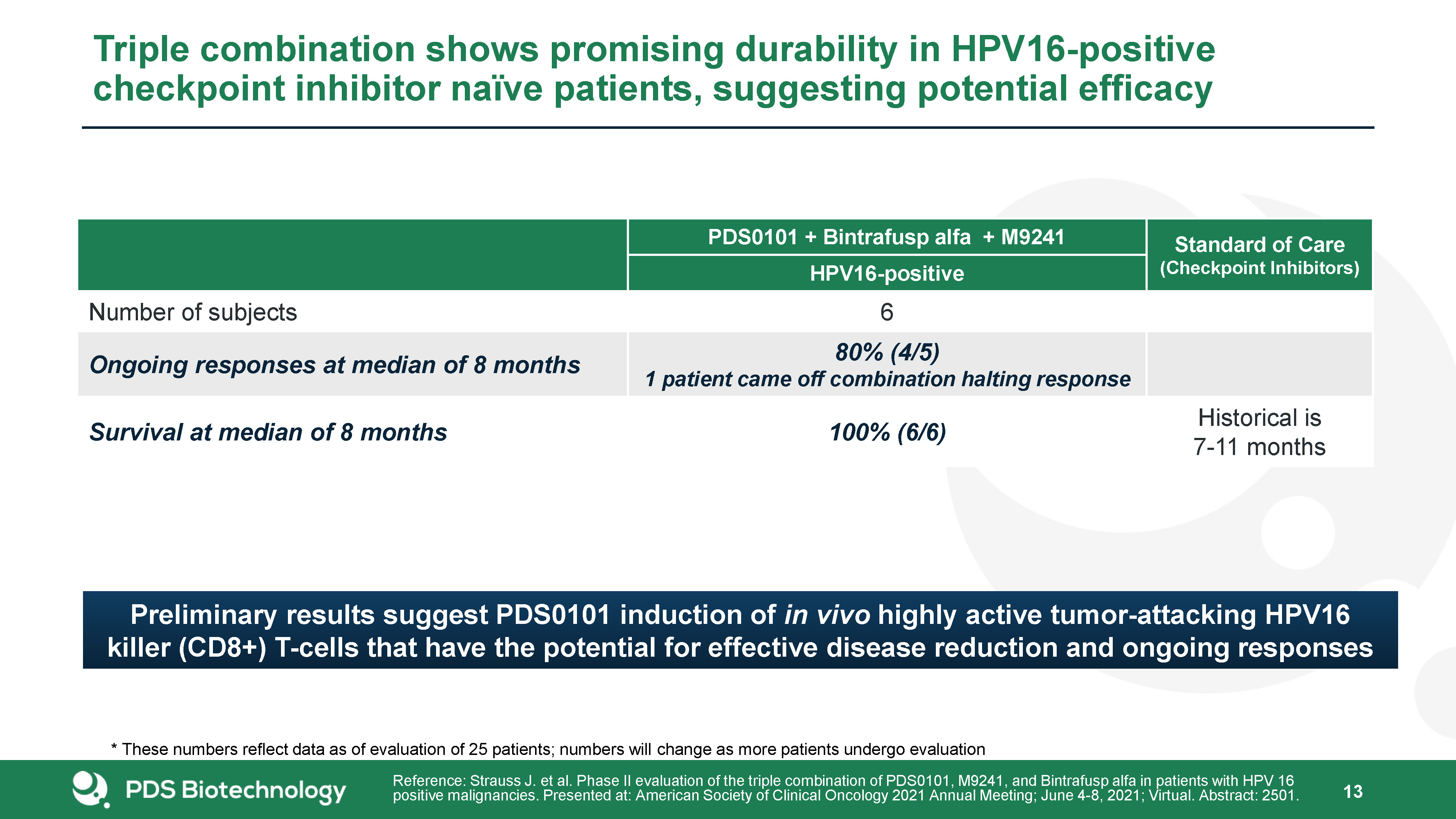
18 Triple combination shows promising durability in HPV16-positive checkpoint inhibitor naïve patients,
suggesting potential efficacy PDS0101 + Bintrafusp alfa + M9241 Standard of Care(Checkpoint Inhibitors) HPV16-positive Number of subjects 6 Ongoing responses at median of 8 months 80% (4/5)1 patient came off combination halting
response Survival at median of 8 months 100% (6/6) Historical is 7-11 months Reference: Strauss J. et al. Phase II evaluation of the triple combination of PDS0101, M9241, and Bintrafusp alfa in patients with HPV 16 positive malignancies.
Presented at: American Society of Clinical Oncology 2021 Annual Meeting; June 4-8, 2021; Virtual. Abstract: 2501. Preliminary results suggest PDS0101 induction of in vivo highly active tumor-attacking HPV16 killer (CD8+) T-cells that have the
potential for effective disease reduction and ongoing responses * These numbers reflect data as of evaluation of 25 patients; numbers will change as more patients undergo evaluation
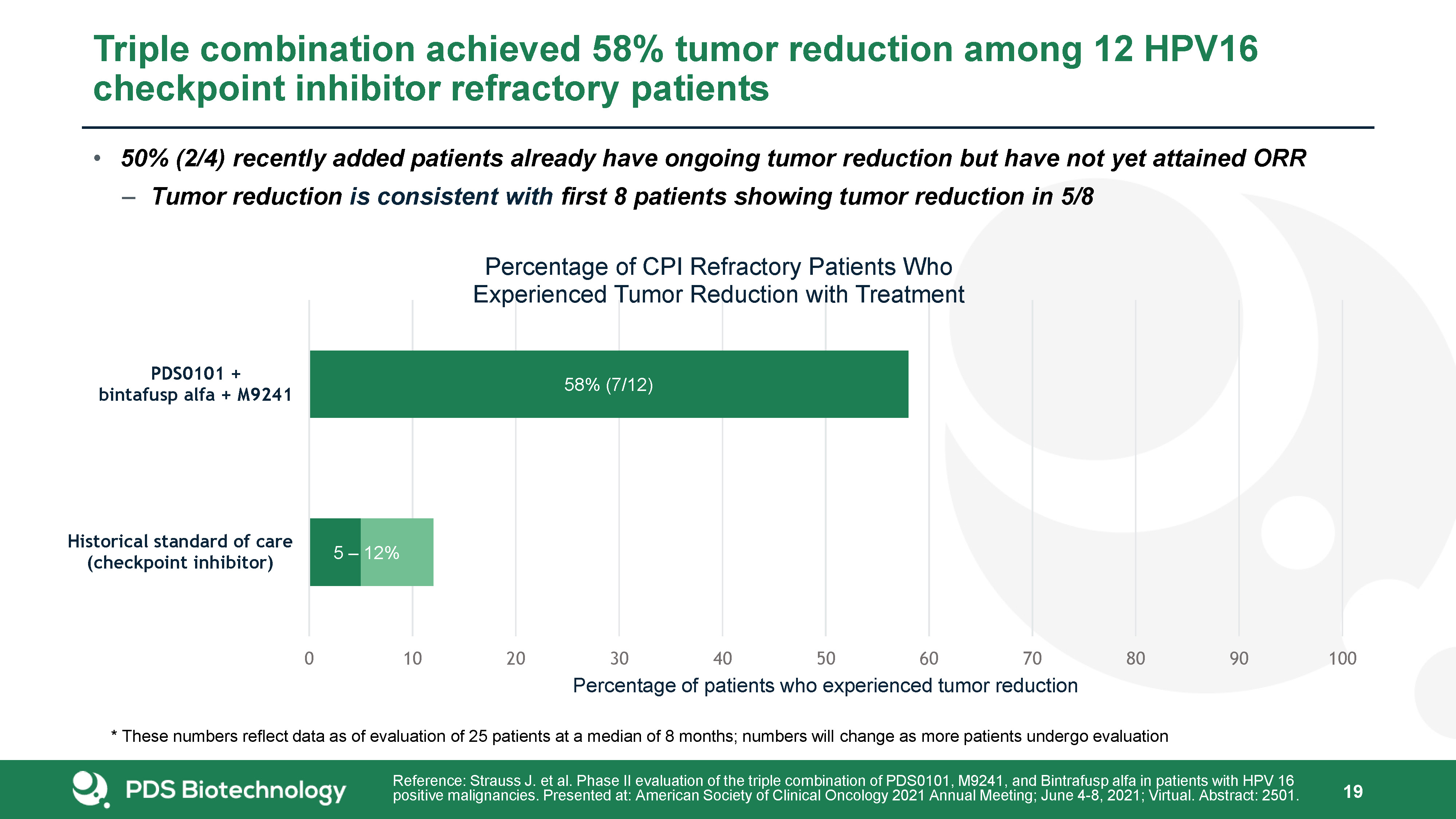
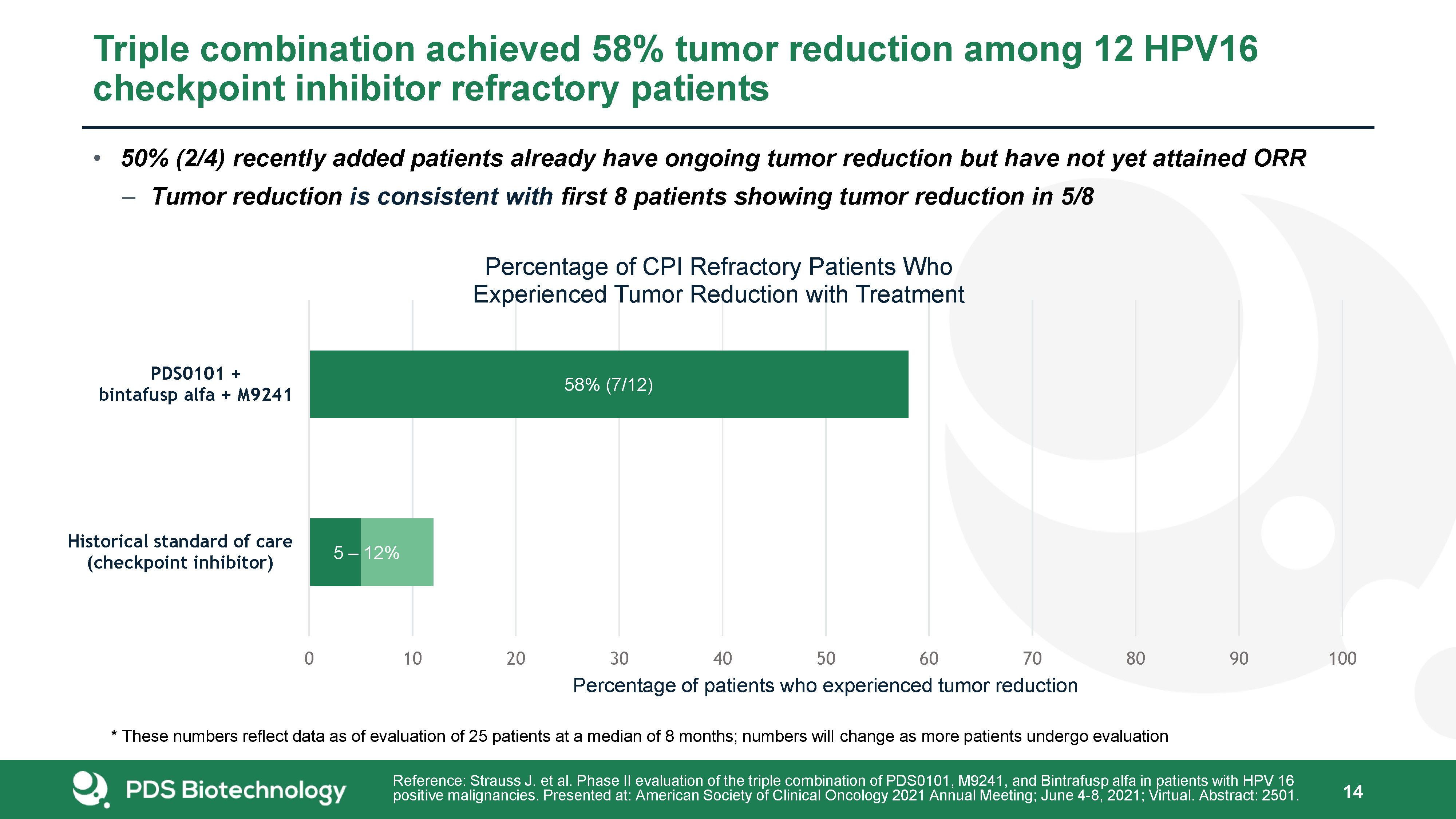
Triple combination achieved 58% tumor reduction among 12 HPV16 checkpoint inhibitor refractory
patients 19 Reference: Strauss J. et al. Phase II evaluation of the triple combination of PDS0101, M9241, and Bintrafusp alfa in patients with HPV 16 positive malignancies. Presented at: American Society of Clinical Oncology 2021 Annual
Meeting; June 4-8, 2021; Virtual. Abstract: 2501. * These numbers reflect data as of evaluation of 25 patients at a median of 8 months; numbers will change as more patients undergo evaluation 50% (2/4) recently added patients already have
ongoing tumor reduction but have not yet attained ORRTumor reduction is consistent with first 8 patients showing tumor reduction in 5/8
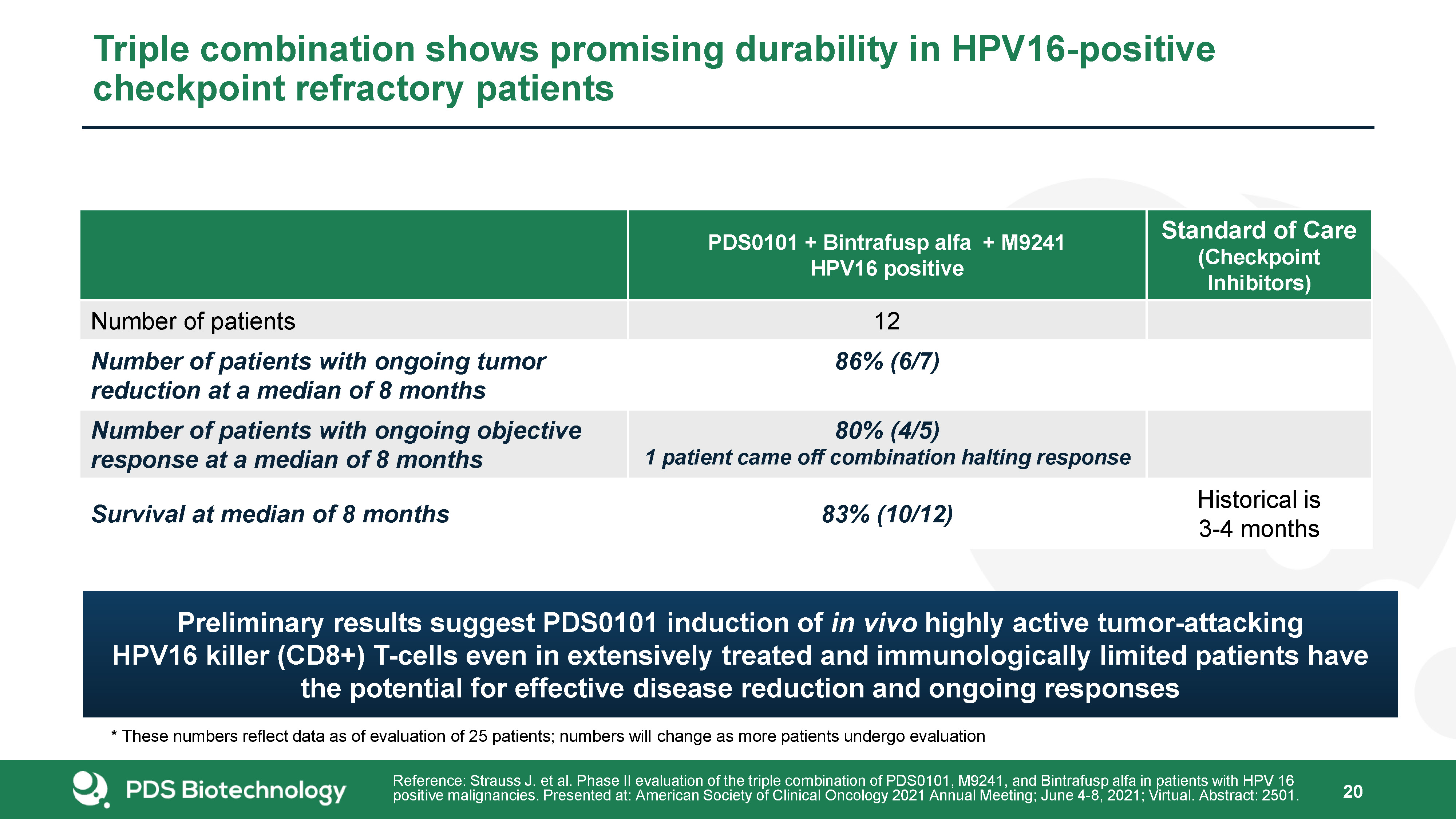
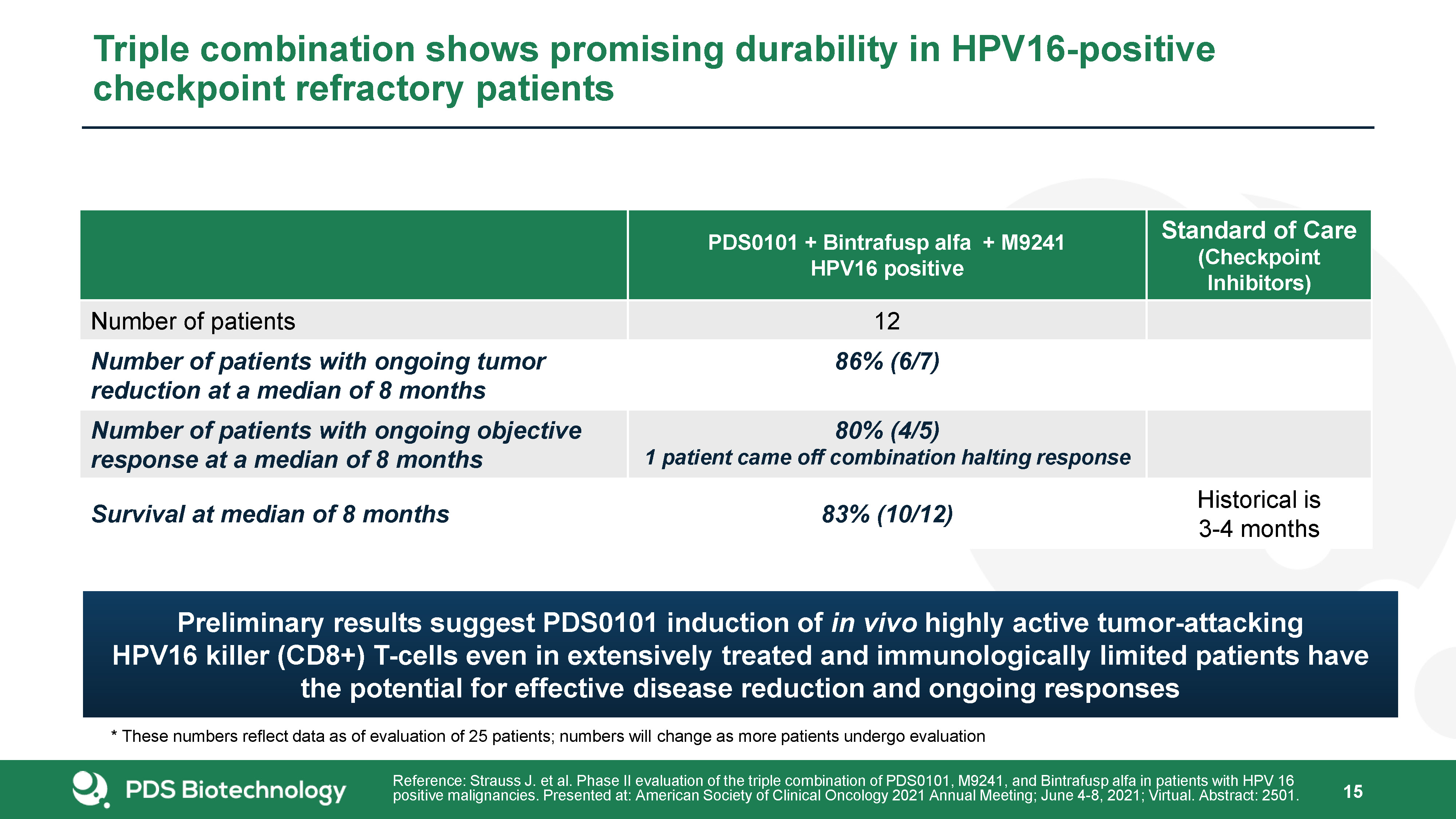
20 Triple combination shows promising durability in HPV16-positive checkpoint refractory
patients PDS0101 + Bintrafusp alfa + M9241HPV16 positive Standard of Care(Checkpoint Inhibitors) Number of patients 12 Number of patients with ongoing tumor reduction at a median of 8 months 86% (6/7) Number of patients with
ongoing objective response at a median of 8 months 80% (4/5)1 patient came off combination halting response Survival at median of 8 months 83% (10/12) Historical is 3-4 months Reference: Strauss J. et al. Phase II evaluation of the
triple combination of PDS0101, M9241, and Bintrafusp alfa in patients with HPV 16 positive malignancies. Presented at: American Society of Clinical Oncology 2021 Annual Meeting; June 4-8, 2021; Virtual. Abstract: 2501. * These numbers reflect
data as of evaluation of 25 patients; numbers will change as more patients undergo evaluation Preliminary results suggest PDS0101 induction of in vivo highly active tumor-attacking HPV16 killer (CD8+) T-cells even in extensively treated and
immunologically limited patients have the potential for effective disease reduction and ongoing responses
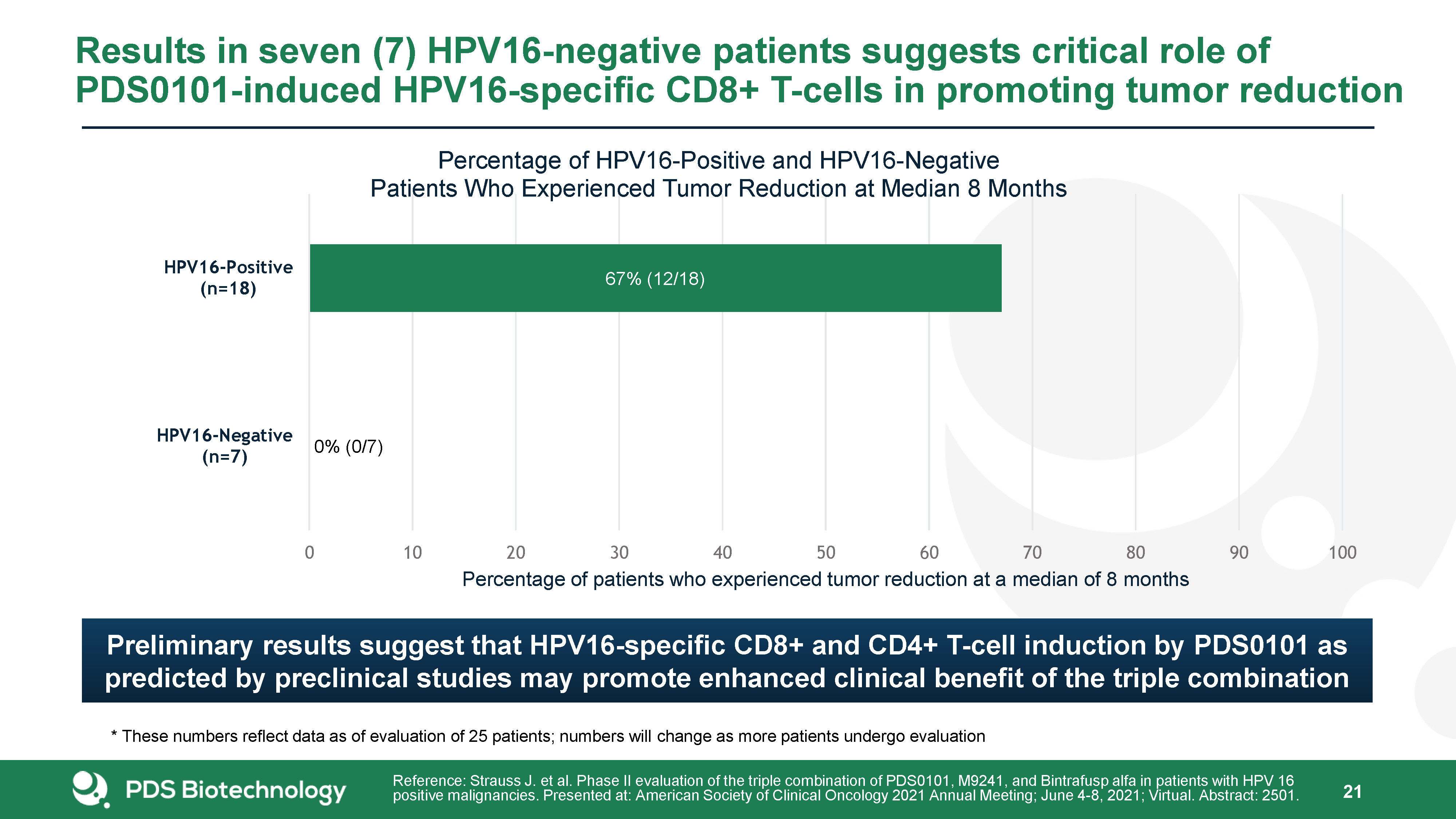
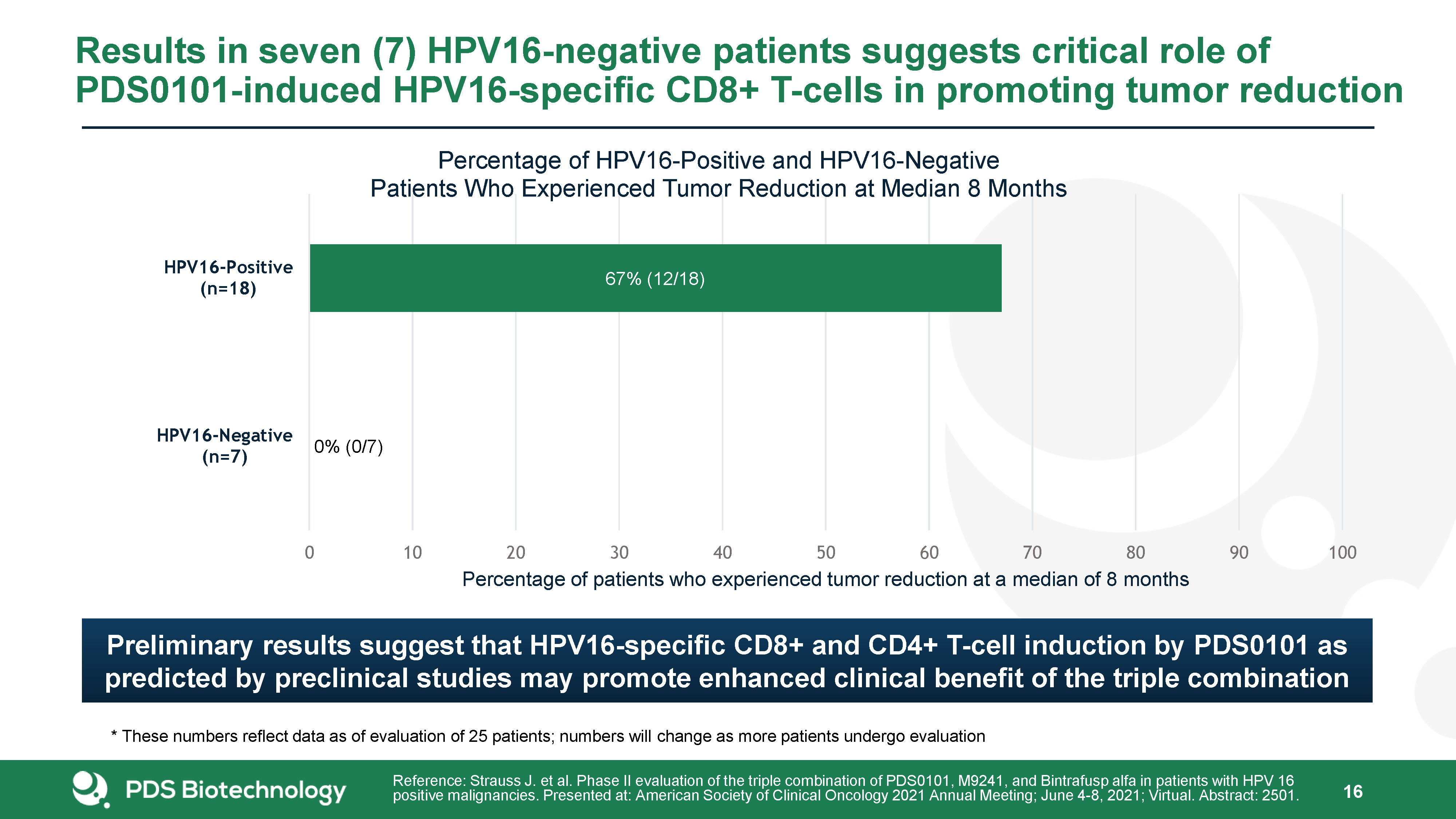
Results in seven (7) HPV16-negative patients suggests critical role of PDS0101-induced HPV16-specific
CD8+ T-cells in promoting tumor reduction 21 Reference: Strauss J. et al. Phase II evaluation of the triple combination of PDS0101, M9241, and Bintrafusp alfa in patients with HPV 16 positive malignancies. Presented at: American Society of
Clinical Oncology 2021 Annual Meeting; June 4-8, 2021; Virtual. Abstract: 2501. * These numbers reflect data as of evaluation of 25 patients; numbers will change as more patients undergo evaluation Preliminary results suggest that
HPV16-specific CD8+ and CD4+ T-cell induction by PDS0101 as predicted by preclinical studies may promote enhanced clinical benefit of the triple combination
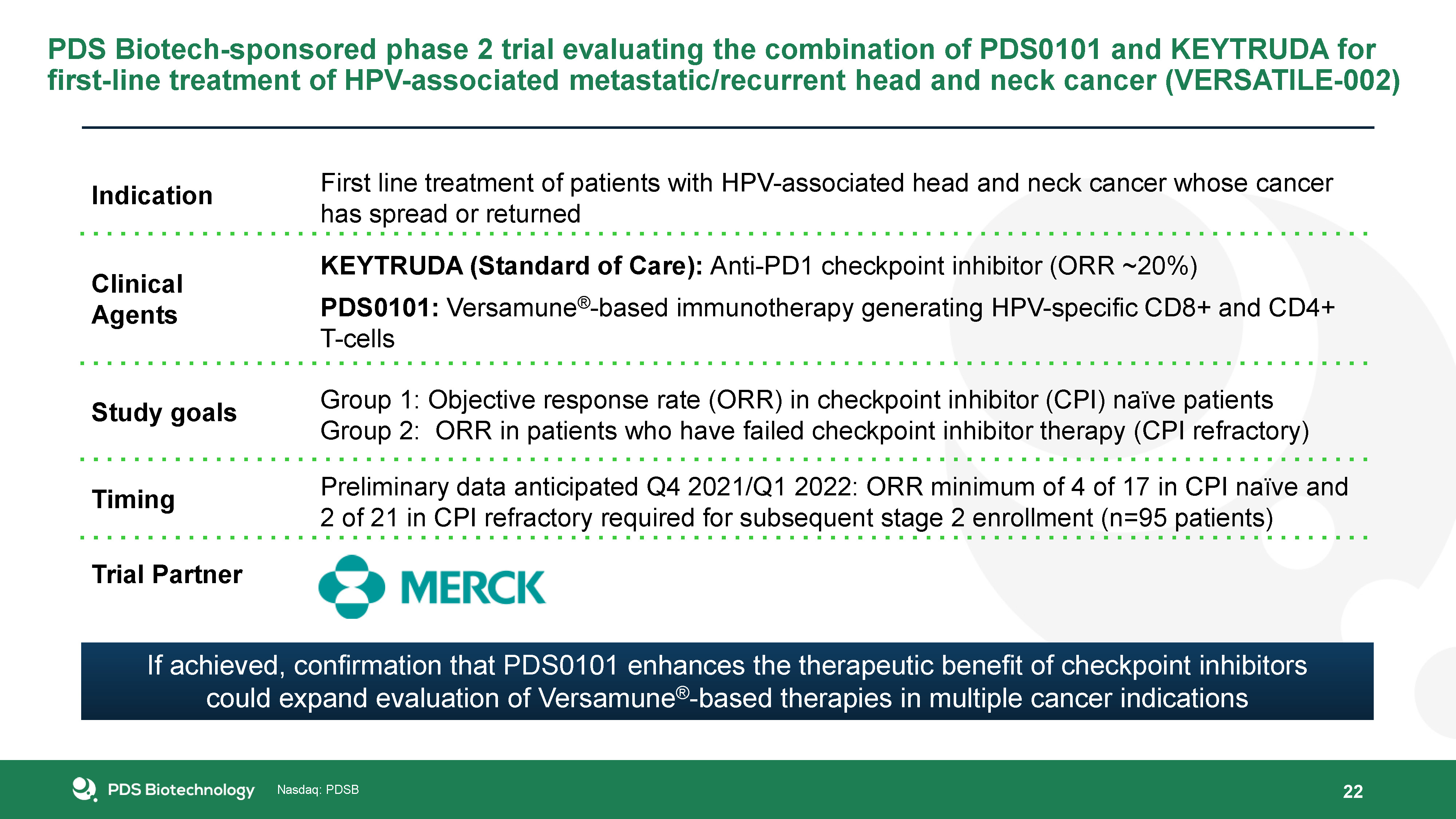
22 PDS Biotech-sponsored phase 2 trial evaluating the combination of PDS0101 and KEYTRUDA for first-line
treatment of HPV-associated metastatic/recurrent head and neck cancer (VERSATILE-002) Indication First line treatment of patients with HPV-associated head and neck cancer whose cancer has spread or returned Clinical Agents KEYTRUDA
(Standard of Care): Anti-PD1 checkpoint inhibitor (ORR ~20%)PDS0101: Versamune®-based immunotherapy generating HPV-specific CD8+ and CD4+ T-cells Study goals Group 1: Objective response rate (ORR) in checkpoint inhibitor (CPI) naïve
patientsGroup 2: ORR in patients who have failed checkpoint inhibitor therapy (CPI refractory) Timing Preliminary data anticipated Q4 2021/Q1 2022: ORR minimum of 4 of 17 in CPI naïve and 2 of 21 in CPI refractory required for subsequent
stage 2 enrollment (n=95 patients) Trial Partner If achieved, confirmation that PDS0101 enhances the therapeutic benefit of checkpoint inhibitors could expand evaluation of Versamune®-based therapies in multiple cancer indications
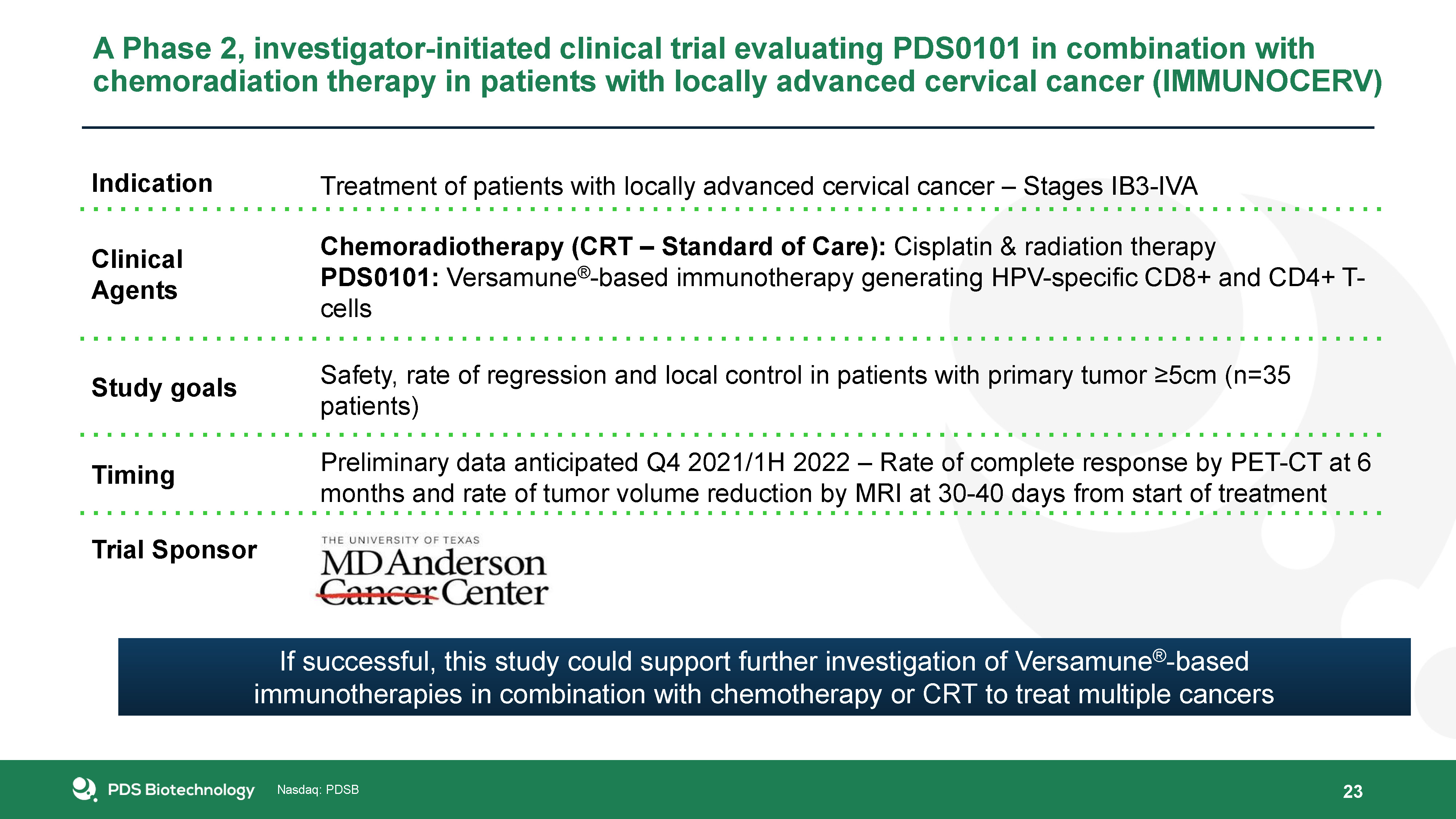
23 A Phase 2, investigator-initiated clinical trial evaluating PDS0101 in combination with
chemoradiation therapy in patients with locally advanced cervical cancer (IMMUNOCERV) Indication Treatment of patients with locally advanced cervical cancer – Stages IB3-IVA Clinical Agents Chemoradiotherapy (CRT – Standard of Care):
Cisplatin & radiation therapyPDS0101: Versamune®-based immunotherapy generating HPV-specific CD8+ and CD4+ T-cells Study goals Safety, rate of regression and local control in patients with primary tumor ≥5cm (n=35
patients) Timing Preliminary data anticipated Q4 2021/1H 2022 – Rate of complete response by PET-CT at 6 months and rate of tumor volume reduction by MRI at 30-40 days from start of treatment Trial Sponsor If successful, this study could
support further investigation of Versamune®-based immunotherapies in combination with chemotherapy or CRT to treat multiple cancers

Studies are designed to demonstrate efficacy and broad applicability of PDS0101 and the Versamune® T-cell
activating platform 24 Potential to enhance anti-cancer efficacy of various cancer treatments: Combinations with checkpoint inhibitors, chemoradiotherapy and novel therapies may further demonstrate Versamune®’s versatility. Broad potential
for additional partnerships: Successful phase 2 studies with PDS0101 could enable a broad pipeline of Versamune®-based oncology products containing various antigens. Potential to treat all types of HPV-cancer: PDS0101 Phase 2 clinical studies
address multiple types of HPV-associated cancers. Potential applications beyond oncology: PDS0203 COVID-19 phase 1/2 trials may demonstrate protection and may induce durable T-cell responses against conserved regions of mutating viruses.

PDS0101 Near-Term Milestones and Market Opportunities
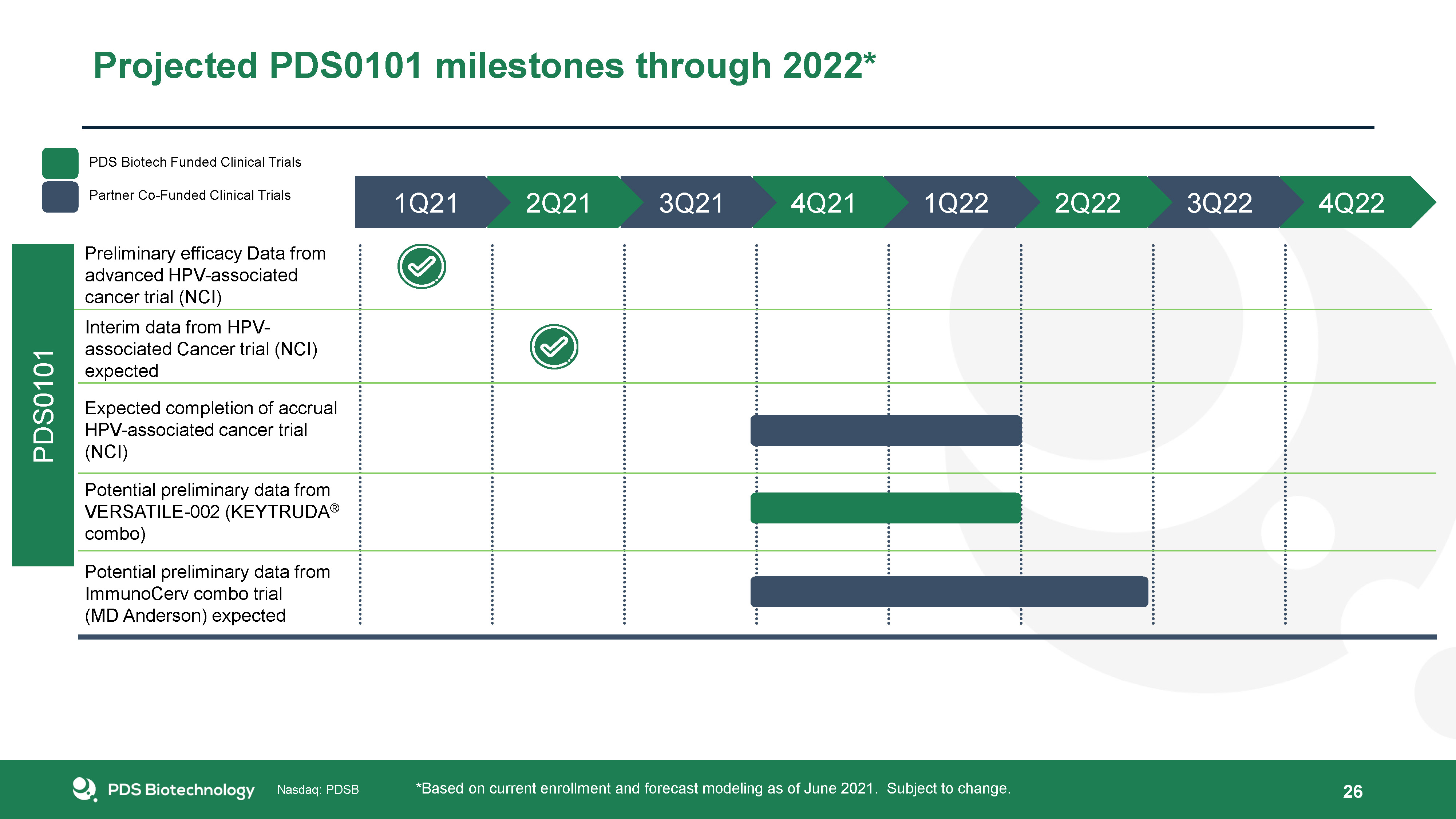
Projected PDS0101 milestones through 2022* *Based on current enrollment and forecast modeling as of June
2021. Subject to change. 4Q22 3Q22 2Q22 1Q22 4Q21 3Q21 2Q21 1Q21 Preliminary efficacy Data from advanced HPV-associated cancer trial (NCI) Interim data from HPV-associated Cancer trial (NCI) expected Potential preliminary data from
ImmunoCerv combo trial (MD Anderson) expected Potential preliminary data from VERSATILE-002 (KEYTRUDA® combo) Expected completion of accrual HPV-associated cancer trial (NCI) PDS0101 26 PDS Biotech Funded Clinical Trials Partner
Co-Funded Clinical Trials
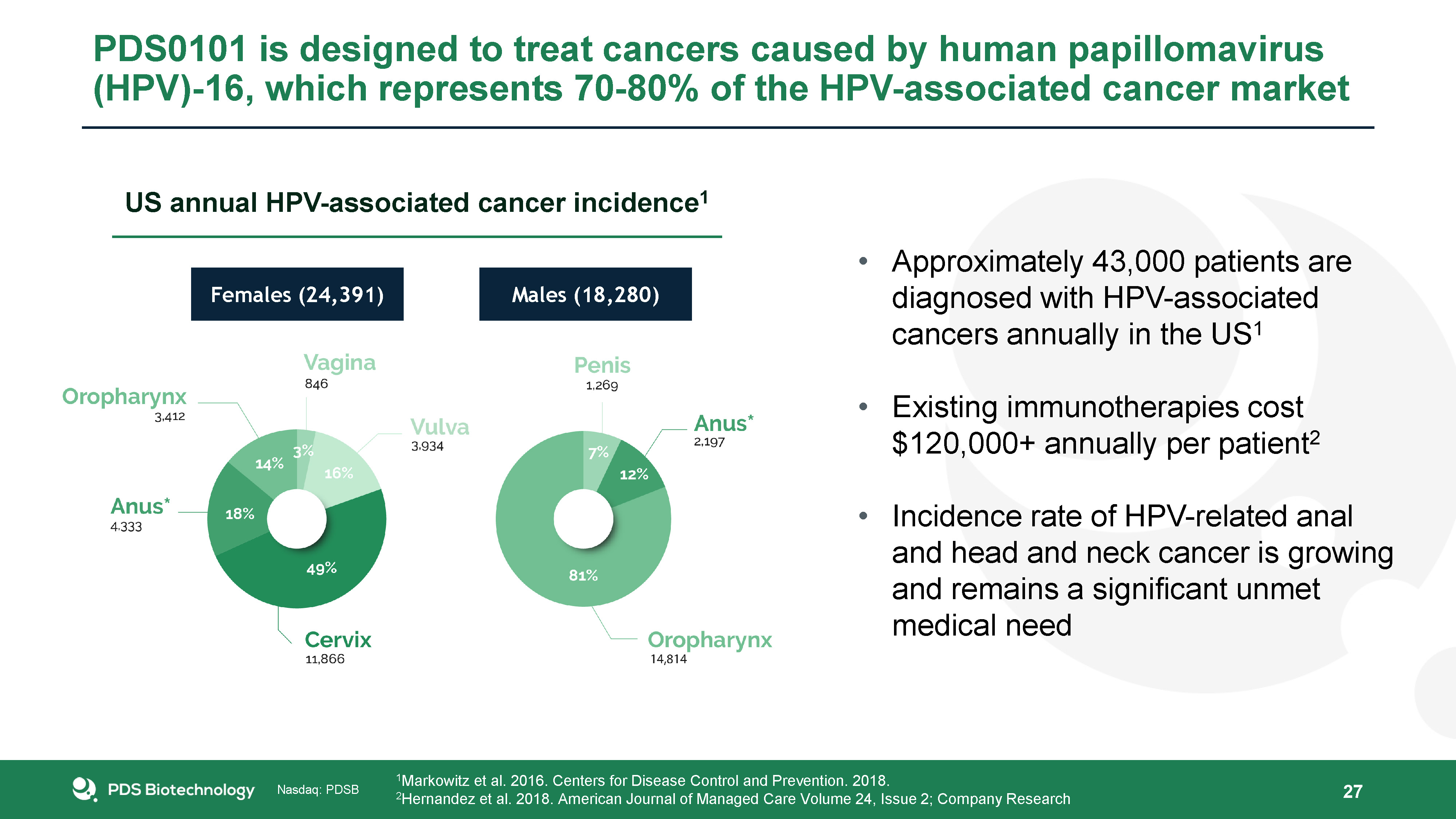
27 Approximately 43,000 patients are diagnosed with HPV-associated cancers annually in the US1Existing
immunotherapies cost $120,000+ annually per patient2Incidence rate of HPV-related anal and head and neck cancer is growing and remains a significant unmet medical need 1Markowitz et al. 2016. Centers for Disease Control and Prevention.
2018.2Hernandez et al. 2018. American Journal of Managed Care Volume 24, Issue 2; Company Research Females (24,391) Males (18,280) US annual HPV-associated cancer incidence1 PDS0101 is designed to treat cancers caused by human
papillomavirus (HPV)-16, which represents 70-80% of the HPV-associated cancer market
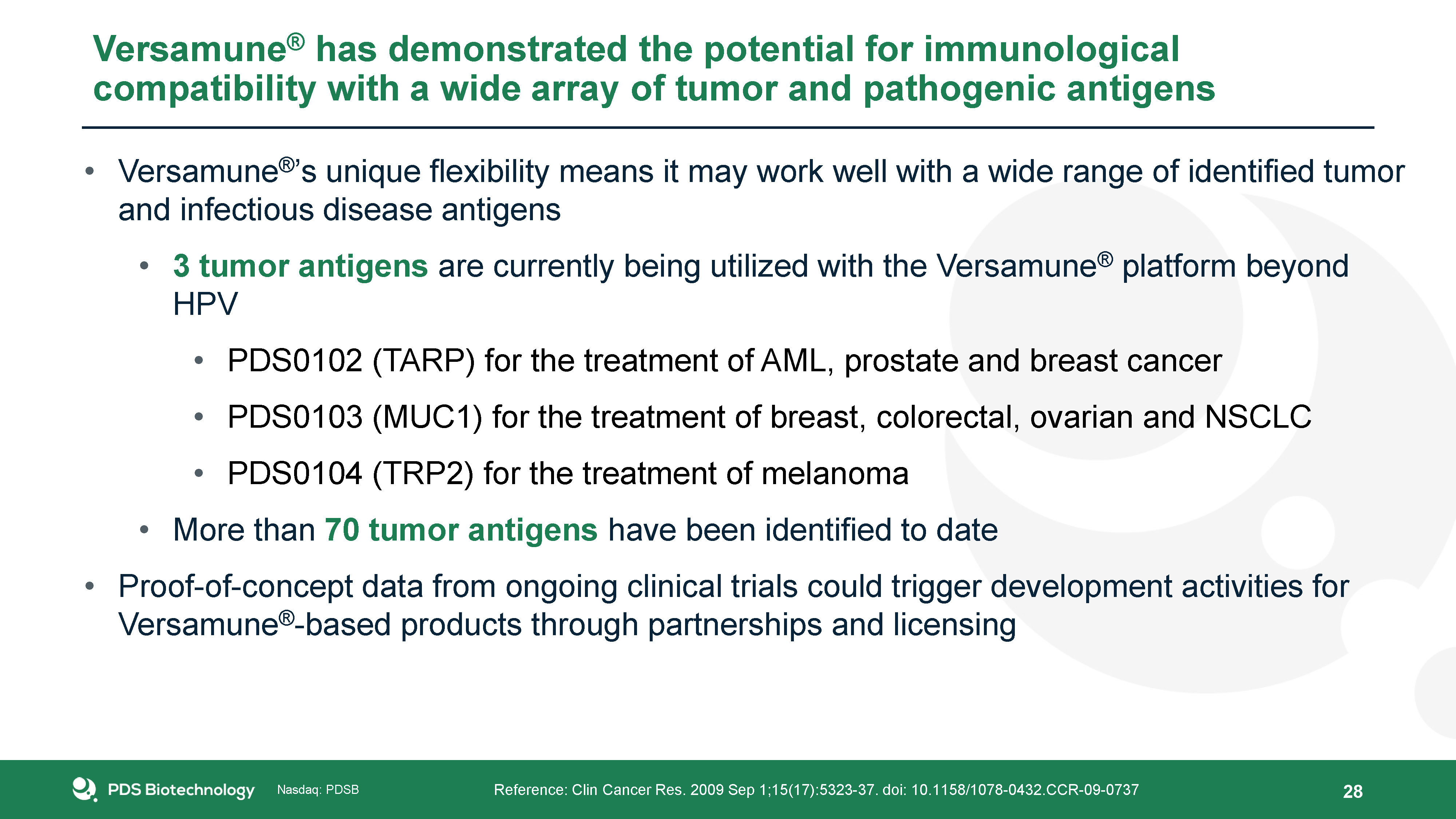
Versamune® has demonstrated the potential for immunological compatibility with a wide array of tumor and
pathogenic antigens Reference: Clin Cancer Res. 2009 Sep 1;15(17):5323-37. doi: 10.1158/1078-0432.CCR-09-0737 Versamune®’s unique flexibility means it may work well with a wide range of identified tumor and infectious disease antigens3 tumor
antigens are currently being utilized with the Versamune® platform beyond HPVPDS0102 (TARP) for the treatment of AML, prostate and breast cancerPDS0103 (MUC1) for the treatment of breast, colorectal, ovarian and NSCLCPDS0104 (TRP2) for the
treatment of melanomaMore than 70 tumor antigens have been identified to dateProof-of-concept data from ongoing clinical trials could trigger development activities for Versamune®-based products through partnerships and licensing 28

PDS Biotech’s robust Versamune® -based oncology pipeline is being developed in partnership with the
leaders in immuno-oncology 29 Reference: Data on file.

Farmacore License Agreement for Versamune® -Based COVID Vaccine
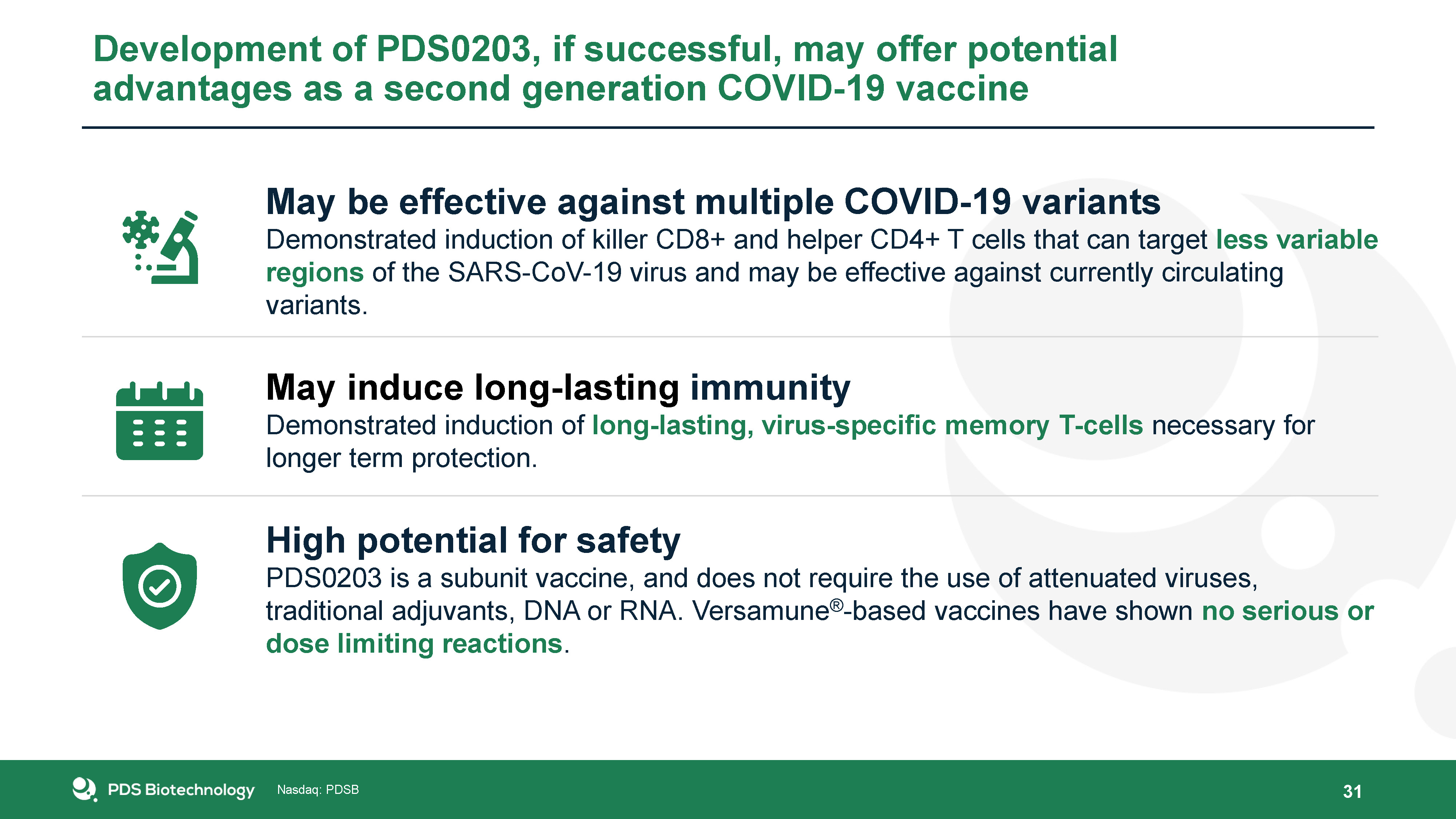
Development of PDS0203, if successful, may offer potential advantages as a second generation COVID-19
vaccine May be effective against multiple COVID-19 variantsDemonstrated induction of killer CD8+ and helper CD4+ T cells that can target less variable regions of the SARS-CoV-19 virus and may be effective against currently circulating
variants.May induce long-lasting immunityDemonstrated induction of long-lasting, virus-specific memory T-cells necessary for longer term protection.High potential for safetyPDS0203 is a subunit vaccine, and does not require the use of
attenuated viruses, traditional adjuvants, DNA or RNA. Versamune®-based vaccines have shown no serious or dose limiting reactions. 31

Consortium has received a commitment of up to ~US$60 million from MCTI, Brazil to support phase 1-3
clinical development and manufacturing scale-upPDS Biotech has licensed Versamune® technology to Farmacore to develop a COVID-19 vaccine candidate Farmacore is responsible for antigen manufacturing and clinical developmentPhase 1/2 study
anticipated to start following Farmacore’s submission of a full data package to and subsequent approval from Anvisa (Brazilian regulatory agency)PDS Biotech is closely monitoring the evolving political situation in Brazil as which may result in
potential challenges to developing a Versamune®-based COVID-19 vaccineIntellectual property protections for a Versamune-based COVID-19 vaccine potentially at riskBrazil’s senate has voted for compulsory licensing for COVID-19 vaccine technology
Legislative process is ongoingFunding release is subject to negotiations among Farmacore, MCTI, and Brazilian authorities PDS0203, if development is successful, could offer another option to address the COVID-19 global health crisis 32

Final Comments
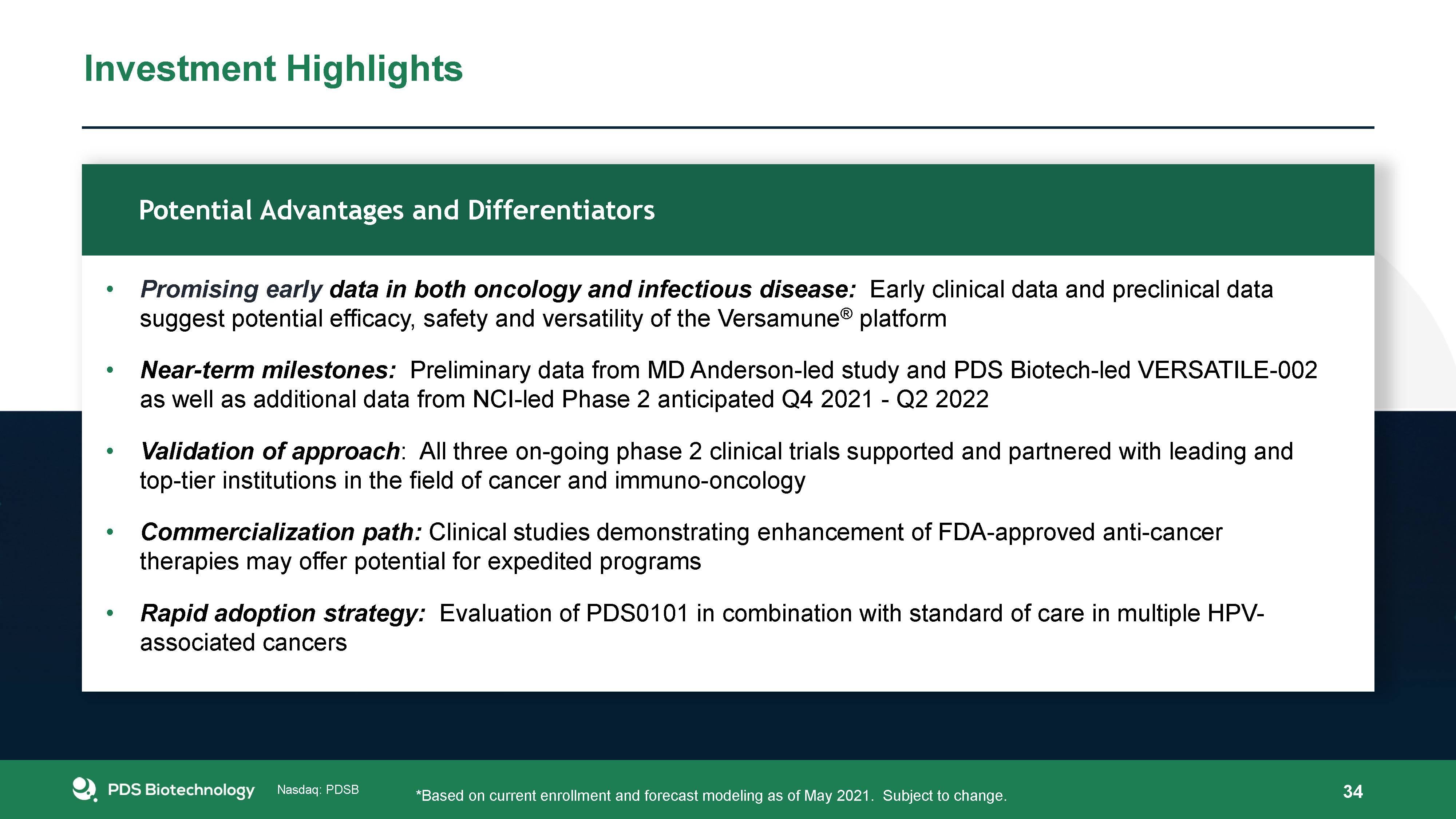
Investment Highlights 34 Promising early data in both oncology and infectious disease: Early clinical
data and preclinical data suggest potential efficacy, safety and versatility of the Versamune® platformNear-term milestones: Preliminary data from MD Anderson-led study and PDS Biotech-led VERSATILE-002 as well as additional data from NCI-led
Phase 2 anticipated Q4 2021 - Q2 2022Validation of approach: All three on-going phase 2 clinical trials supported and partnered with leading and top-tier institutions in the field of cancer and immuno-oncologyCommercialization path: Clinical
studies demonstrating enhancement of FDA-approved anti-cancer therapies may offer potential for expedited programsRapid adoption strategy: Evaluation of PDS0101 in combination with standard of care in multiple HPV-associated
cancers Potential Advantages and Differentiators *Based on current enrollment and forecast modeling as of May 2021. Subject to change.

Exhibit 99.2

PDS Biotech Announces Release of Interim Data for PDS0101 in NCI-Led Phase 2 Clinical Study in Oral Presentation at ASCO 2021 Annual Meeting
Tumor reduction was observed in 83% (5 of 6) of HPV16-positive relapsed or refractory checkpoint inhibitor naïve advanced cancer patients and 58% (7 of 12) of HPV16-positive relapsed or refractory
advanced cancer patients who have also failed checkpoint inhibitor therapy
Florham Park, NJ, June 8, 2021 - PDS Biotechnology Corporation (Nasdaq: PDSB), a clinical-stage immunotherapy company developing novel cancer therapies based on the Company’s proprietary Versamune(R) T-cell
activating technology, today announced the presentation of interim data from the Phase 2 trial led by the National Cancer Institute (NCI), of the National Institutes of Health (NIH), at the American Society of Clinical Oncology (ASCO) 2021 Annual
Meeting.
The Phase 2 trial (NCT04287868) studies PDS0101 (Versamune(R)-HPV16) in combination with two investigational immune-modulating agents: bintrafusp alfa (M7824), a bifunctional “trap” fusion protein targeting TGF-β and
PD-L1, and NHS-IL12 (M9241), a tumor-targeting immunocytokine. PDS0101 is an immunotherapy candidate designed to treat cancers caused by infection with HPV16 (HPV16-positive cancers) by activating the immune system to produce in vivo CD8+ (killer) T-cells to target and kill tumors that are HPV16-positive. Analyses of immune responses and other immune correlates are ongoing.
Highlights from the presentation include the following:
| • |
Data from a total of 25 patients with data available as of the time of presentation submission:
|
| o |
Update on the data previously reported for the original fourteen (14) HPV16-positive patients who were in the subject of the abstract published on May 19th.
|
| o |
An additional seven (7) HPV16-negative patients (patients whose cancer was NOT caused by HPV16 infection) who were not discussed in the abstract.
|
| o |
An additional four (4) HPV16-positive patients who are checkpoint inhibitor refractory whose data became available after the abstract submission.
|
| • |
100% (25/25) of patients enrolled had failed chemotherapy treatment.
|
| • |
96% (24/25) of patients enrolled had failed both chemotherapy and radiation treatment.
|
| • |
56% (14/25) of patients enrolled had failed checkpoint inhibitor therapy (checkpoint inhibitor refractory).
|
| • |
Most types of HPV-related cancers (anal, cervical, head and neck, vaginal and vulvar cancers) were represented among the study subjects.
|
| • |
The following update was provided on the initial six (6) HPV16-positive patients who had NOT been treated with checkpoint inhibitors (checkpoint inhibitor naïve):
|
| o |
83% (5/6) of the patients demonstrated an objective response (tumor reduction >30%). The reported objective response rate with current standard of care checkpoint inhibitor treatment is 12-24%.
|
| o |
100% (6/6) are still alive at 8 months – the historic average (median) survival or life span for this patient population is 7-11 months.
|

| o |
80% (4/5) of patients who had an objective response still have an ongoing response at 8 months.
|
| o |
One (1) patient had a complete response (no evidence of disease).
|
| o |
No new patients had been added to this group by the time of submission.
|
| • |
The following information was provided on the twelve (12) HPV16-positive patients who have also failed treatment with checkpoint inhibitors after failing chemotherapy and radiation treatment (checkpoint inhibitor refractory):
|
| o |
Four patients had recently been added since the abstract. Tumor reduction was observed in 58% (7/12), with an overall objective response rate of 42% (5/12) already achieved; the objective response rate of the current standard of care is
5-12%
|
| o |
One patient in this group had achieved a complete response by the time of reporting
|
| o |
80% (4/5) of patients who had an objective response have an ongoing response at 8 months
|
| o |
83% (10/12) of patients are still alive at 8 months; historic average (median) survival or life span for this patient population is only 3-4 months
|
| • |
PDS0101 is designed to treat patients whose cancer is caused by infection with HPV16. Seven (7) patients had cancer that was not caused by HPV16 (HPV16-negative patients). In this group
|
| o |
0% (0/7) experienced tumor reduction.
|
| o |
80% (4/5) checkpoint inhibitor naïve patients are still alive at 8 months.
|
| o |
0% (0/2) checkpoint inhibitor refractory patients are still alive at 8 months.
|
The NCI Center for Cancer Research’s Laboratory of Tumor Immunology and Biology (LTIB) and Genitourinary Malignancies Branch (GMB) are jointly leading this Phase 2 trial. Bintrafusp alfa is being jointly developed by
Merck KGaA, Darmstadt, Germany, and GlaxoSmithKline; NHS-IL12 is being developed by Merck KGaA, Darmstadt, Germany.
The trial is evaluating the treatment combination in both checkpoint inhibitor naïve and refractory patients with advanced HPV-associated cancers that have progressed or returned after treatment. The vast majority of
these cancers are caused by HPV16 infection. Objective response is measured by radiographic tumor responses according to RECIST 1.1. These reported data provide additional insights following the preclinical studies published by the NCI examining
the potentially complementary mechanisms of action of the three immunotherapies, understood to involve potent in-vivo HPV16-specific killer and helper T-cell induction with effective T-cell tumor
infiltration (PDS0101), blocking of immune checkpoints as well as targeting of TGF-β (Bintrafusp alfa). The preclinical results suggested superior tumor regression.
“The achievement of a 67% tumor reduction among all HPV16-positive cancer patients including both CPI naïve and CPI refractory patients continues to strengthen the evidence supporting continuing clinical
investigation of novel Versamune(R) platform’s potential ability to induce high levels of tumor-specific CD8+ killer T-cells that attack the cancer in which we believe results in a strong synergy with bintrafusp alfa and NHS-IL12. The data provide
early evidence of notable clinical activity, and we saw effective tumor regression in these patients,” commented Dr. Lauren Wood, Chief Medical Officer of PDS Biotech. “The interim data demonstrating that this response was limited only to patients
with HVP16-positive cancer, and also the fact that all responding patients who have stayed on treatment continue to show ongoing responses after a median duration of 8 months solidifies our belief that PDS0101’s ability to generate a robust,
targeted T-cell response may have the potential to significantly improve clinical outcomes for patients with advanced, refractory HPV16-associated cancers who have limited treatment options.”

There are more than 630,000 cases of HPV-associated malignancies including cervical, oropharyngeal and anal cancer worldwide annually. HPV-16 is responsible for most of these cases. About 15-20% of HPV-associated
malignancies respond to PD-(L)1 inhibitors. However, for the overwhelming majority of patients who progress on these immunotherapies there is no effective standard of care therapy.
The company is hosting a conference call tomorrow morning at 8:00 am ET to discuss the data presented at ASCO. Registration for the conference call is now open and a live webcast of the event will be available
online in the investor relations section of the company's website at https://pdsbiotech.com/investors/news-center/events. A replay will be available on the company website for 90 days following the webcast.
For patients interested in enrolling in this clinical study, please call NCI’s toll-free number 1-800-4-Cancer (1-800-422-6237) (TTY: 1-800-332-8615), email [email protected], and/or visit the website:
https://trials.cancer.gov.
About PDS Biotechnology
PDS Biotech is a clinical-stage immunotherapy company developing a growing pipeline of cancer immunotherapies and infectious disease vaccines based on the Company’s proprietary Versamune(R) T-cell activating technology
platform. Our Versamune(R)-based products may overcome the limitations of current immunotherapy by inducing in vivo, large quantities of high-quality, highly potent polyfunctional tumor specific CD4+ helper
and CD8+ killer T-cells. PDS Biotech has developed multiple investigational therapies, based on combinations of Versamune(R) and disease-specific antigens, designed to train the immune system to better recognize diseased cells and effectively attack
and destroy them. Our immuno-oncology product candidates are initially being studied in combination therapy to potentially enhance efficacy without compounding toxicity across a range of cancer types. The company’s lead investigational cancer
immunotherapy product PDS0101 is currently in Phase 2 clinical studies in HPV-associated cancers. To learn more, please visit www.pdsbiotech.com or follow us on Twitter at @PDSBiotech.
About PDS0101
PDS Biotech’s lead candidate, PDS0101, combines the utility of the Versamune(R) platform with targeted antigens in HPV-expressing cancers. In partnership with Merck & Co., PDS Biotech is evaluating a combination
of PDS0101 and KEYTRUDA(R) in a Phase 2 study in first-line treatment of recurrent or metastatic head and neck cancer. PDS Biotech is also conducting two additional Phase 2 studies in advanced HPV-associated cancers and advanced localized cervical
cancer with the NCI and The University of Texas MD Anderson Cancer Center, respectively. The current product targets HPV16-positive cancers, and upon successful proof of concept will be broadened to address cancers caused by other oncogenic
HPV-types.

Forward Looking Statements
This communication contains forward-looking statements (including within the meaning of Section 21E of the United States Securities Exchange Act of 1934, as amended, and Section 27A of the United States Securities Act
of 1933, as amended) concerning PDS Biotechnology Corporation (the “Company”) and other matters. These statements may discuss goals, intentions and expectations as to future plans, trends, events, results of operations or financial condition, or
otherwise, based on current beliefs of the Company’s management, as well as assumptions made by, and information currently available to, management. Forward-looking statements generally include statements that are predictive in nature and depend upon
or refer to future events or conditions, and include words such as “may,” “will,” “should,” “would,” “expect,” “anticipate,” “plan,” “likely,” “believe,” “estimate,” “project,” “intend,” “forecast,” “guidance”, “outlook” and other similar expressions
among others. Forward-looking statements are based on current beliefs and assumptions that are subject to risks and uncertainties and are not guarantees of future performance. Actual results could differ materially from those contained in any
forward-looking statement as a result of various factors, including, without limitation: the Company’s ability to protect its intellectual property rights; the Company’s anticipated capital requirements, including the Company’s anticipated cash
runway and the Company’s current expectations regarding its plans for future equity financings; the Company’s dependence on additional financing to fund its operations and complete the development and commercialization of its product candidates, and
the risks that raising such additional capital may restrict the Company’s operations or require the Company to relinquish rights to the Company’s technologies or product candidates; the Company’s limited operating history in the Company’s current
line of business, which makes it difficult to evaluate the Company’s prospects, the Company’s business plan or the likelihood of the Company’s successful implementation of such business plan; the timing for the Company or its partners to initiate the
planned clinical trials for PDS0101, PDS0203 and other Versamune(R) based products; the future success of such trials; the successful implementation of the Company’s
research and development programs and collaborations, including any collaboration studies concerning PDS0101, PDS0203 and other Versamune(R) based products and the
Company’s interpretation of the results and findings of such programs and collaborations and whether such results are sufficient to support the future success of the Company’s product candidates; the success, timing and cost of the Company’s ongoing
clinical trials and anticipated clinical trials for the Company’s current product candidates, including statements regarding the timing of initiation, pace of enrollment and completion of the trials (including our ability to fully fund our disclosed
clinical trials, which assumes no material changes to our currently projected expenses), futility analyses, presentations at conferences and data reported in an abstract, and receipt of interim results, which are not necessarily indicative of the
final results of the Company’s ongoing clinical trials; any Company statements about its understanding of product candidates mechanisms of action and interpretation of preclinical and early clinical results from its clinical development programs
and any collaboration studies; the acceptance by the market of the Company’s product candidates, if approved; the timing of and the Company’s ability to obtain and maintain U.S. Food and Drug Administration or other regulatory authority approval of,
or other action with respect to, the Company’s product candidates; and other factors, including legislative, regulatory, political and economic developments not within the Company’s control, including unforeseen circumstances or other disruptions to
normal business operations arising from or related to COVID-19. The foregoing review of important factors that could cause actual events to differ from expectations should not be construed as exhaustive and should be read in conjunction with
statements that are included herein and elsewhere, including the risk factors included in the Company’s annual and periodic reports filed with the SEC. The forward-looking statements are made only as of the date of this press release and, except as
required by applicable law, the Company undertakes no obligation to revise or update any forward-looking statement, or to make any other forward-looking statements, whether as a result of new information, future events or otherwise.

Media & Investor Relations Contact:
Deanne Randolph
PDS Biotech
Phone: +1 (908) 517-3613
Email: [email protected]
Rich Cockrell
CG Capital
Phone: +1 (404) 736-3838
Email: [email protected]
Exhibit 99.3

Post-ASCO Conference CallInterim Data Review JUNE 8, 2021

2 Forward-Looking Statements This presentation contains forward-looking statements about PDS
Biotechnology Corporation (“PDSB”), and its businesses, business prospects, strategies and plans, including but not limited to statements regarding anticipated pre-clinical and clinical drug development activities and timelines and market
opportunities. All statements other than statements of historical facts included in this presentation are forward-looking statements. The words “anticipates,” “may,” “can,” “plans,” “believes,” “estimates,” “expects,” “projects,” “intends,”
“likely,” “will,” “should,” “to be,” and any similar expressions or other words of similar meaning are intended to identify those assertions as forward-looking statements. These forward-looking statements involve substantial risks and
uncertainties that could cause actual results to differ materially from those anticipated.Factors that may cause actual results to differ materially from such forward-looking statements include those identified under the caption “Risk Factors”
in the documents filed with the Securities and Exchange Commission (“SEC”) from time to time, including its Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K. You are cautioned not to place undue
reliance on these forward-looking statements, which speak only as of the date of this presentation. Except to the extent required by applicable law or regulation, PDSB undertakes no obligation to update the forward-looking statements included
in this presentation to reflect subsequent events or circumstances.

3 PDS Biotech’s Versamune®-based immunotherapies are designed to promote a powerful in vivo
tumor-specific CD8+ killer T-cell response Generate the right type and quantity of effective CD8+ killer T-cells Generate potency without systemic side effects Generate memory T-cells, to enhance durability of response A significant
barrier to effective immunotherapy has been the inability to promote adequate CD8+ killer T-cell responses in vivo resulting in diminished efficacy; 70-90% of cancer patients fail check point inhibitor therapy Versamune®-based therapies also
show promising potential to:
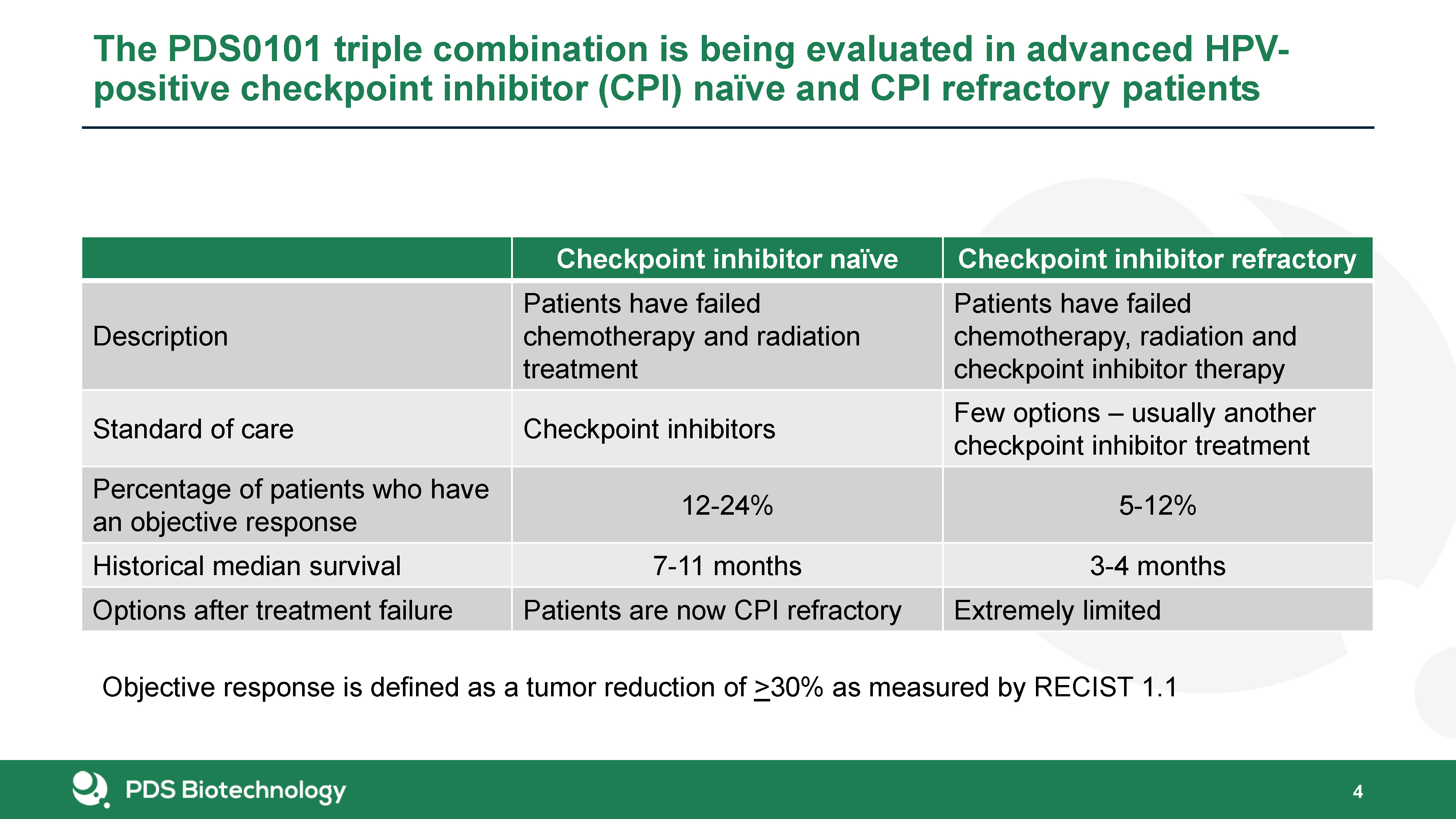
The PDS0101 triple combination is being evaluated in advanced HPV-positive checkpoint inhibitor (CPI)
naïve and CPI refractory patients 4 Checkpoint inhibitor naïve Checkpoint inhibitor refractory Description Patients have failed chemotherapy and radiation treatment Patients have failed chemotherapy, radiation and checkpoint inhibitor
therapy Standard of care Checkpoint inhibitors Few options – usually another checkpoint inhibitor treatment Percentage of patients who have an objective response 12-24% 5-12% Historical median survival 7-11 months 3-4 months Options
after treatment failure Patients are now CPI refractory Extremely limited Objective response is defined as a tumor reduction of >30% as measured by RECIST 1.1
PDS0101 is developed to treat cancers caused by Human papillomavirus (HPV)16 infectionCombines the
Versamune® technology with proprietary modified proteins from the HPV16 viral protein that is expressed by or contained in the HPV16-positive cancersThe interim data suggest that PDS0101 may be effective in promoting the induction of
tumor-attacking HPV16-specific killer T-cells resulting in tumor reductionThe interim data suggest that PDS0101 may be associated with clinically meaningful responses in patients with advanced HPV-associated cancerThe interim data suggest that
the design of immunotherapeutic combinations including polyfunctional CD8+ (killer) and CD4+ (helper) T-cell activating immunotherapies may present the potential for powerful anti-tumor clinical efficacy PDS0101 is designed to efficiently
induce polyfunctional (potent) CD8+ killer T-cells in-vivo that recognize cancers caused by HPV Type 16 5 Reference: Strauss J. et al. Phase II evaluation of the triple combination of PDS0101, M9241, and Bintrafusp alfa in patients with HPV
16 positive malignancies. Presented at: American Society of Clinical Oncology 2021 Annual Meeting; June 4-8, 2021; Virtual. Abstract: 2501.
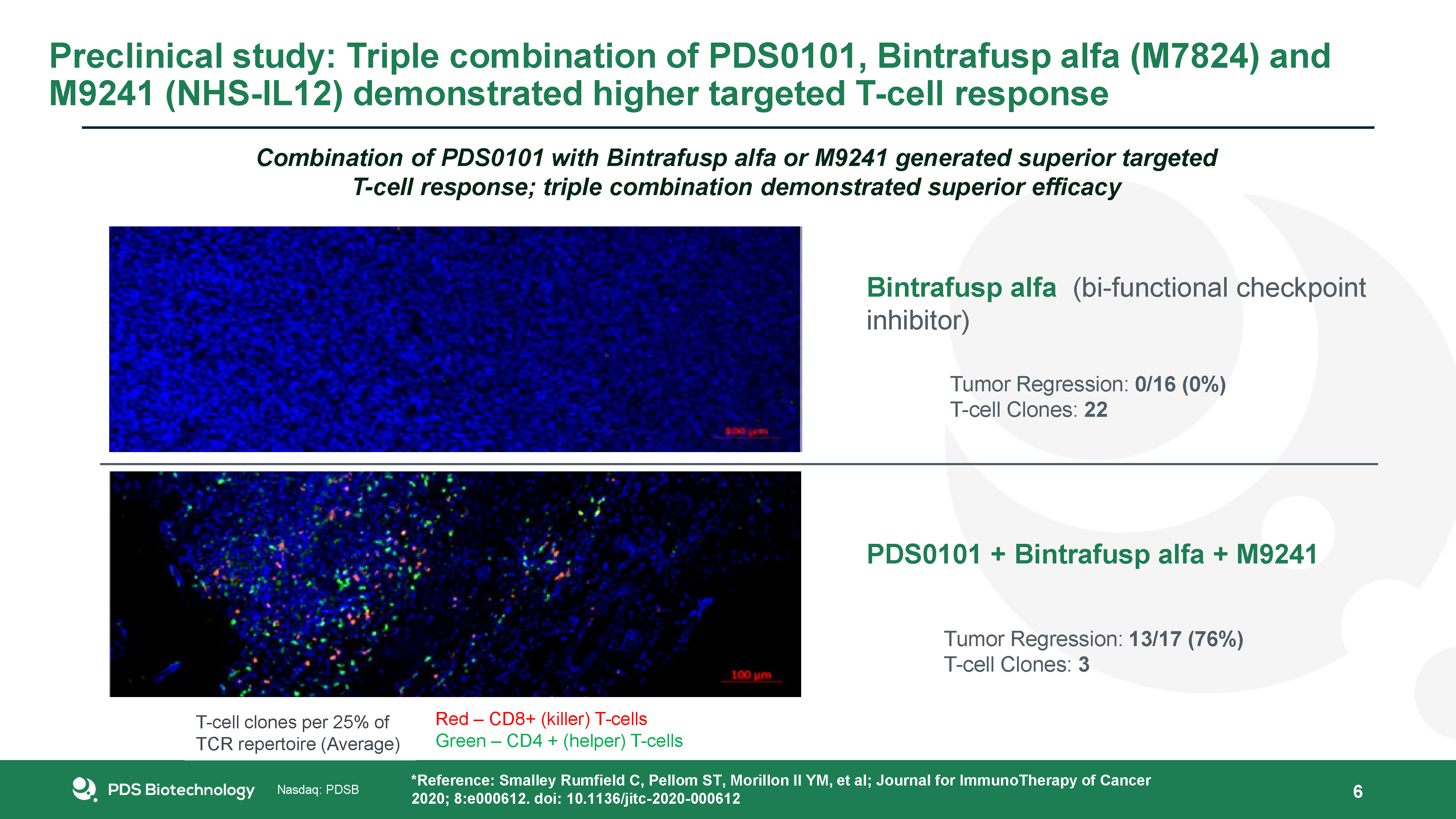
6 Bintrafusp alfa (bi-functional checkpoint inhibitor) Tumor Regression: 0/16 (0%)T-cell Clones:
22 PDS0101 + Bintrafusp alfa + M9241 Tumor Regression: 13/17 (76%)T-cell Clones: 3 *Reference: Smalley Rumfield C, Pellom ST, Morillon II YM, et al; Journal for ImmunoTherapy of Cancer 2020; 8:e000612. doi:
10.1136/jitc-2020-000612 Preclinical study: Triple combination of PDS0101, Bintrafusp alfa (M7824) and M9241 (NHS-IL12) demonstrated higher targeted T-cell response Red – CD8+ (killer) T-cellsGreen – CD4 + (helper) T-cells T-cell clones per
25% of TCR repertoire (Average) Combination of PDS0101 with Bintrafusp alfa or M9241 generated superior targeted T-cell response; triple combination demonstrated superior efficacy
PDS0101 is used in combination with other immunotherapies resulting in a multifunctional
therapy 7 Bintrafusp alfa exposes the tumor to attack and M9241 issues a signal calling T-cells to the tumor Reference: Smalley Rumfield C, Pellom ST, Morillon II YM, et al; Journal for ImmunoTherapy of Cancer 2020; 8:e000612. doi:
10.1136/jitc-2020-000612 Bintrafusp ALFA M9241 PDS0101 induces a powerful, HPV16-targeted CD8+ and CD4+ T-cell response PDS0101 Activated CD8+ killer T-cell Activated CD4+ helper T-cell The triple combination
works in synergy to prompt targeted T-cells to infiltrate and destroy the tumor TUMOR DESTROYED
PHASE 2 TRIAL OF PDS0101 + BINTRAFUSP ALFA + NHS-IL12 INTERIM CLINICAL DATA(L. V. Wood)
Phase 2 NCI-led clinical trial evaluating the triple combination of PDS0101, Bintrafusp alfa and M9241 in
advanced HPV-associated cancer 9 Indication Patients with advanced HPV-associated cancer who have failed prior treatment Clinical Agents Bintrafusp alfa: Bifunctional “trap” fusion proteinM9241: Antibody-conjugated immuno-cytokinePDS0101:
Versamune®-based immunotherapy generating HPV-specific CD8+ T-cells Study goals Group 1: Objective response rate (ORR) in checkpoint inhibitor (CPI) naïve patientsGroup 2: ORR in patients who have failed checkpoint inhibitor therapy (CPI
refractory) Timing Full enrollment of 56 patientsComplete enrollment expected by Q1 2022 Trial Sponsor The objective of this trial is to confirm that PDS0101 enhances the therapeutic benefit of Bintrafusp alfa and M9241 and may lead to
expanded evaluation in several pipeline products
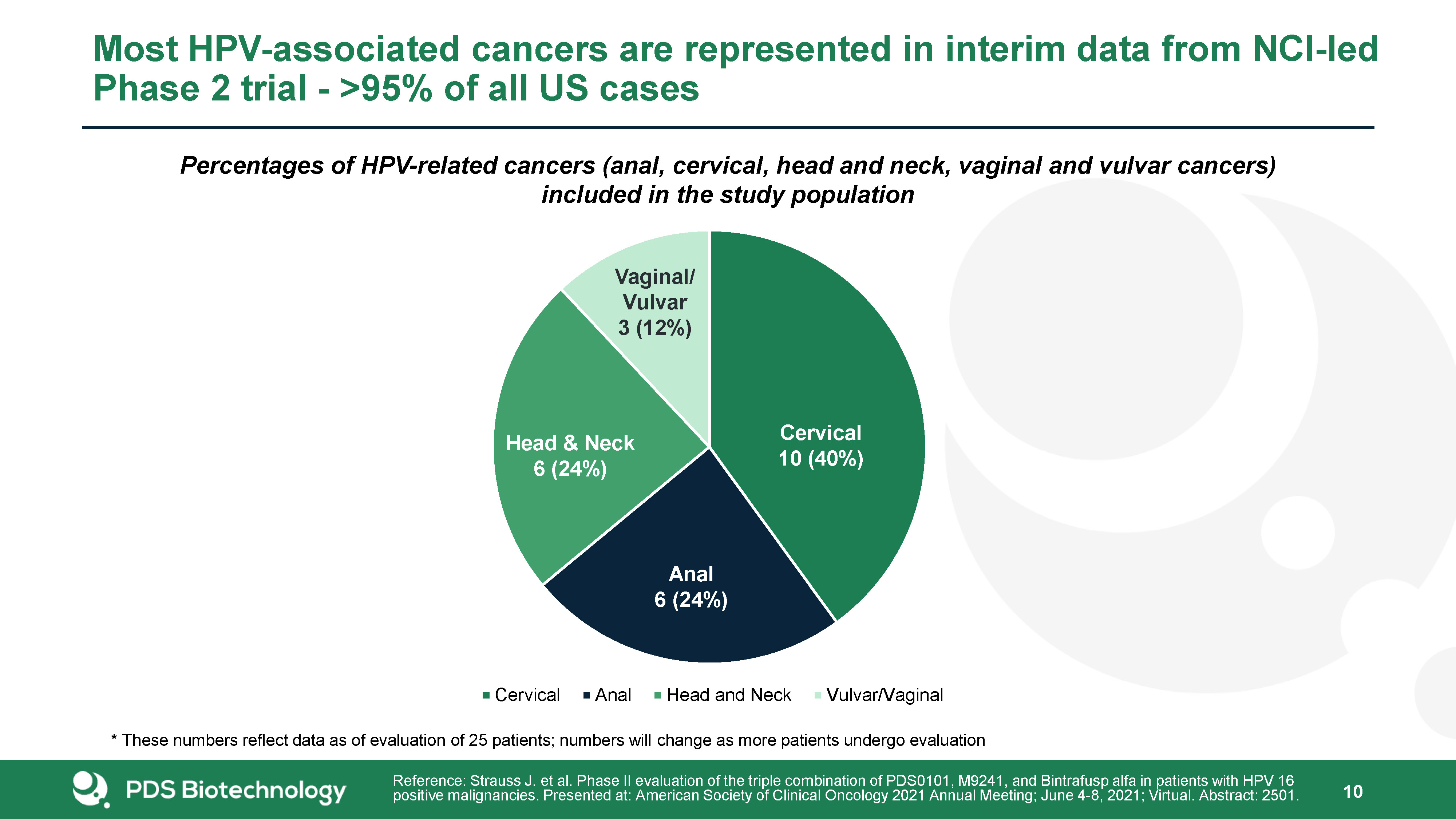
Percentages of HPV-related cancers (anal, cervical, head and neck, vaginal and vulvar cancers) included
in the study population Most HPV-associated cancers are represented in interim data from NCI-led Phase 2 trial - >95% of all US cases 10 Cervical10 (40%) Anal6 (24%) Vaginal/Vulvar3 (12%) Head & Neck6 (24%) Reference: Strauss J.
et al. Phase II evaluation of the triple combination of PDS0101, M9241, and Bintrafusp alfa in patients with HPV 16 positive malignancies. Presented at: American Society of Clinical Oncology 2021 Annual Meeting; June 4-8, 2021; Virtual.
Abstract: 2501. * These numbers reflect data as of evaluation of 25 patients; numbers will change as more patients undergo evaluation
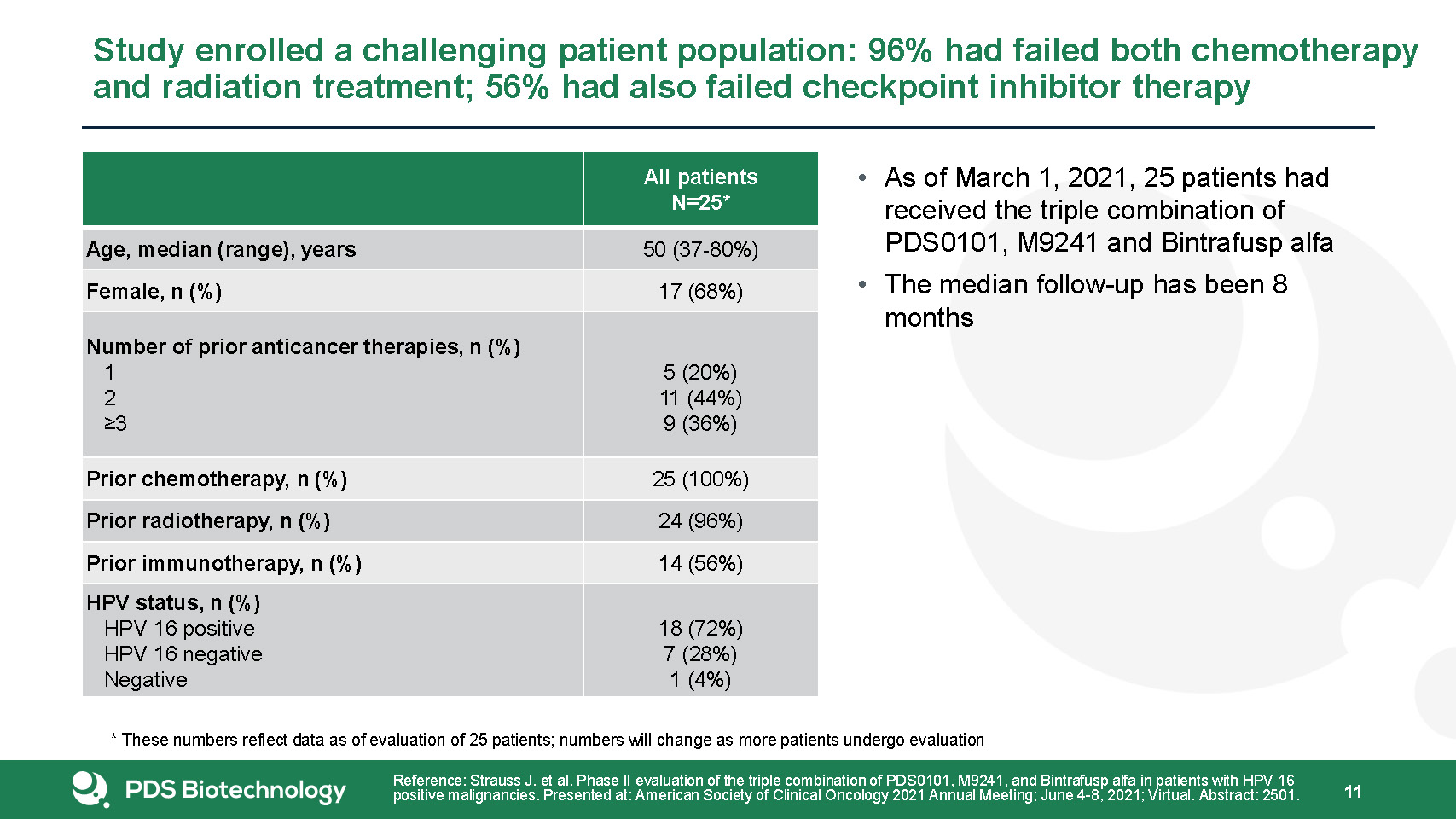
As of March 1, 2021, 25 patients had received the triple combination of PDS0101, M9241 and Bintrafusp
alfa The median follow-up has been 8 months Study enrolled a challenging patient population: 96% had failed both chemotherapy and radiation treatment; 56% had also failed checkpoint inhibitor therapy 11 All patientsN=25* Age, median
(range), years 50 (37-80%) Female, n (%) 17 (68%) Number of prior anticancer therapies, n (%) 1 2 ≥3 5 (20%)11 (44%)9 (36%) Prior chemotherapy, n (%) 25 (100%) Prior radiotherapy, n (%) 24 (96%) Prior immunotherapy, n (%) 14
(56%) HPV status, n (%) HPV 16 positive HPV 16 negative Negative 18 (72%)7 (28%)1 (4%) Reference: Strauss J. et al. Phase II evaluation of the triple combination of PDS0101, M9241, and Bintrafusp alfa in patients with HPV 16 positive
malignancies. Presented at: American Society of Clinical Oncology 2021 Annual Meeting; June 4-8, 2021; Virtual. Abstract: 2501. * These numbers reflect data as of evaluation of 25 patients; numbers will change as more patients undergo
evaluation
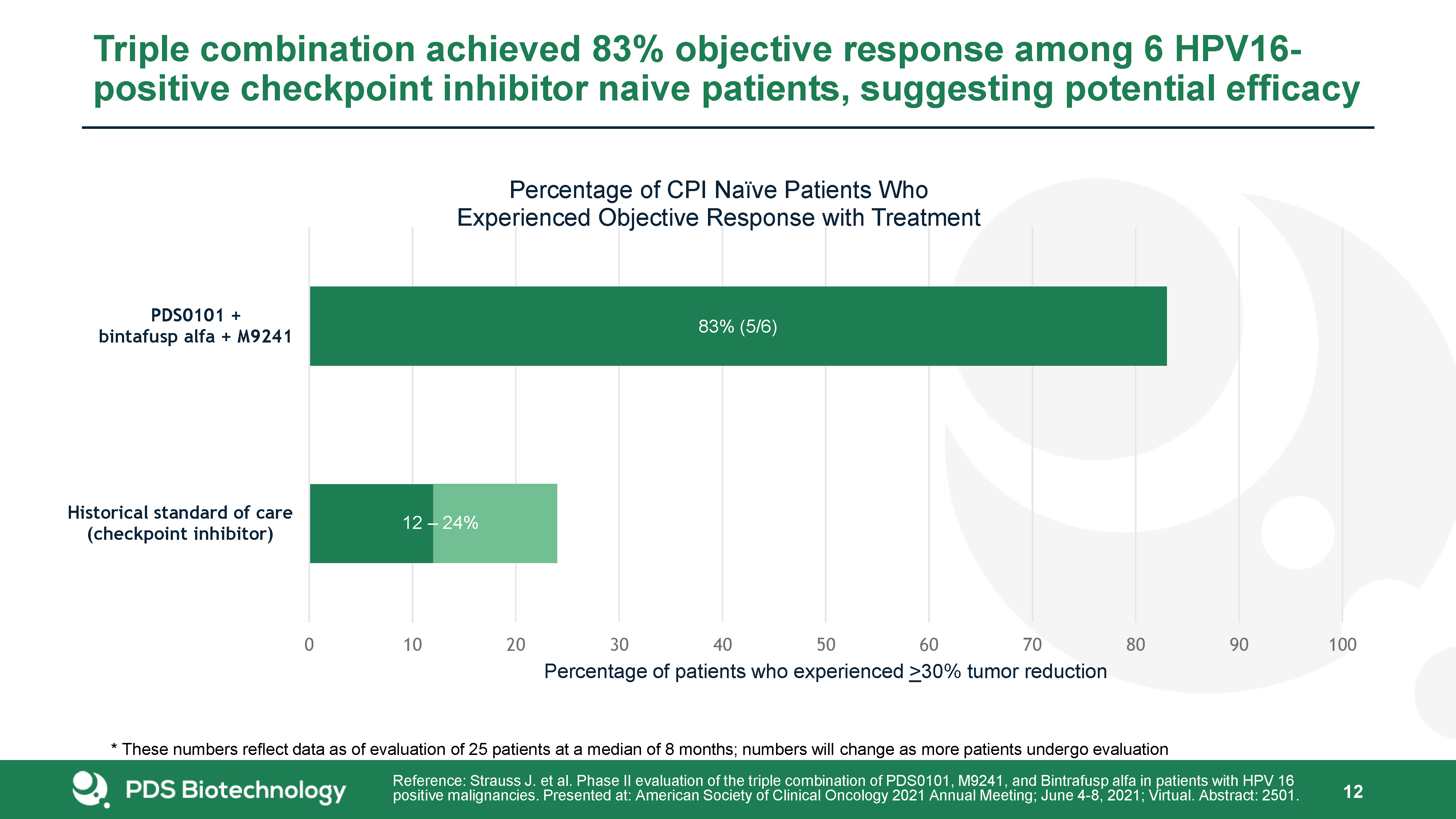
Triple combination achieved 83% objective response among 6 HPV16-positive checkpoint inhibitor naive
patients, suggesting potential efficacy 12 Reference: Strauss J. et al. Phase II evaluation of the triple combination of PDS0101, M9241, and Bintrafusp alfa in patients with HPV 16 positive malignancies. Presented at: American Society of
Clinical Oncology 2021 Annual Meeting; June 4-8, 2021; Virtual. Abstract: 2501. * These numbers reflect data as of evaluation of 25 patients at a median of 8 months; numbers will change as more patients undergo evaluation
13 Triple combination shows promising durability in HPV16-positive checkpoint inhibitor naïve patients,
suggesting potential efficacy PDS0101 + Bintrafusp alfa + M9241 Standard of Care(Checkpoint Inhibitors) HPV16-positive Number of subjects 6 Ongoing responses at median of 8 months 80% (4/5)1 patient came off combination halting
response Survival at median of 8 months 100% (6/6) Historical is 7-11 months Reference: Strauss J. et al. Phase II evaluation of the triple combination of PDS0101, M9241, and Bintrafusp alfa in patients with HPV 16 positive malignancies.
Presented at: American Society of Clinical Oncology 2021 Annual Meeting; June 4-8, 2021; Virtual. Abstract: 2501. Preliminary results suggest PDS0101 induction of in vivo highly active tumor-attacking HPV16 killer (CD8+) T-cells that have the
potential for effective disease reduction and ongoing responses * These numbers reflect data as of evaluation of 25 patients; numbers will change as more patients undergo evaluation
Triple combination achieved 58% tumor reduction among 12 HPV16 checkpoint inhibitor refractory
patients 14 Reference: Strauss J. et al. Phase II evaluation of the triple combination of PDS0101, M9241, and Bintrafusp alfa in patients with HPV 16 positive malignancies. Presented at: American Society of Clinical Oncology 2021 Annual
Meeting; June 4-8, 2021; Virtual. Abstract: 2501. * These numbers reflect data as of evaluation of 25 patients at a median of 8 months; numbers will change as more patients undergo evaluation 50% (2/4) recently added patients already have
ongoing tumor reduction but have not yet attained ORRTumor reduction is consistent with first 8 patients showing tumor reduction in 5/8
15 Triple combination shows promising durability in HPV16-positive checkpoint refractory
patients PDS0101 + Bintrafusp alfa + M9241HPV16 positive Standard of Care(Checkpoint Inhibitors) Number of patients 12 Number of patients with ongoing tumor reduction at a median of 8 months 86% (6/7) Number of patients with
ongoing objective response at a median of 8 months 80% (4/5)1 patient came off combination halting response Survival at median of 8 months 83% (10/12) Historical is 3-4 months Reference: Strauss J. et al. Phase II evaluation of the
triple combination of PDS0101, M9241, and Bintrafusp alfa in patients with HPV 16 positive malignancies. Presented at: American Society of Clinical Oncology 2021 Annual Meeting; June 4-8, 2021; Virtual. Abstract: 2501. * These numbers reflect
data as of evaluation of 25 patients; numbers will change as more patients undergo evaluation Preliminary results suggest PDS0101 induction of in vivo highly active tumor-attacking HPV16 killer (CD8+) T-cells even in extensively treated and
immunologically limited patients have the potential for effective disease reduction and ongoing responses
Results in seven (7) HPV16-negative patients suggests critical role of PDS0101-induced HPV16-specific
CD8+ T-cells in promoting tumor reduction 16 Reference: Strauss J. et al. Phase II evaluation of the triple combination of PDS0101, M9241, and Bintrafusp alfa in patients with HPV 16 positive malignancies. Presented at: American Society of
Clinical Oncology 2021 Annual Meeting; June 4-8, 2021; Virtual. Abstract: 2501. * These numbers reflect data as of evaluation of 25 patients; numbers will change as more patients undergo evaluation Preliminary results suggest that
HPV16-specific CD8+ and CD4+ T-cell induction by PDS0101 as predicted by preclinical studies may promote enhanced clinical benefit of the triple combination
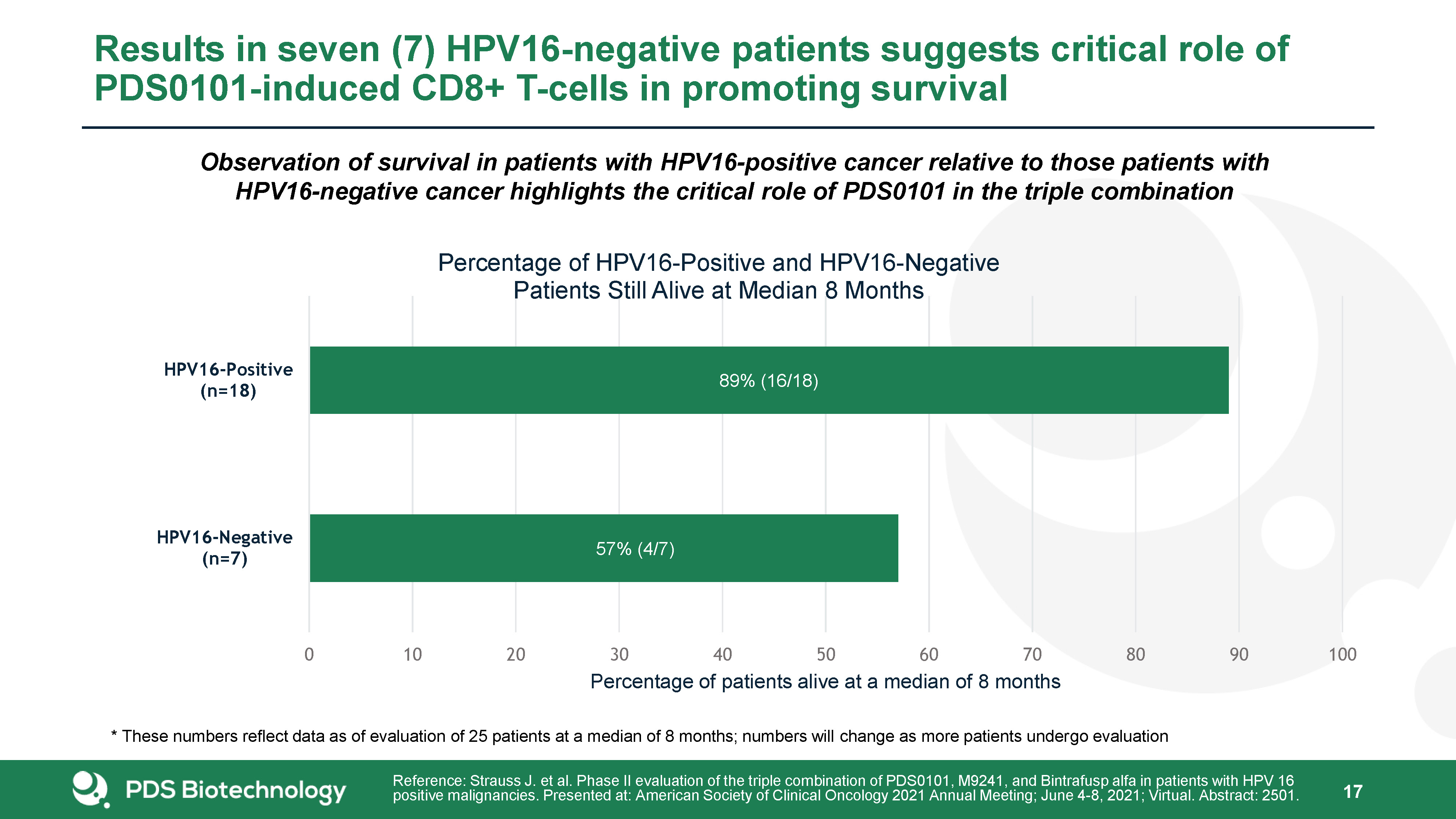
Results in seven (7) HPV16-negative patients suggests critical role of PDS0101-induced CD8+ T-cells in
promoting survival 17 Observation of survival in patients with HPV16-positive cancer relative to those patients with HPV16-negative cancer highlights the critical role of PDS0101 in the triple combination Reference: Strauss J. et al. Phase
II evaluation of the triple combination of PDS0101, M9241, and Bintrafusp alfa in patients with HPV 16 positive malignancies. Presented at: American Society of Clinical Oncology 2021 Annual Meeting; June 4-8, 2021; Virtual. Abstract: 2501. *
These numbers reflect data as of evaluation of 25 patients at a median of 8 months; numbers will change as more patients undergo evaluation
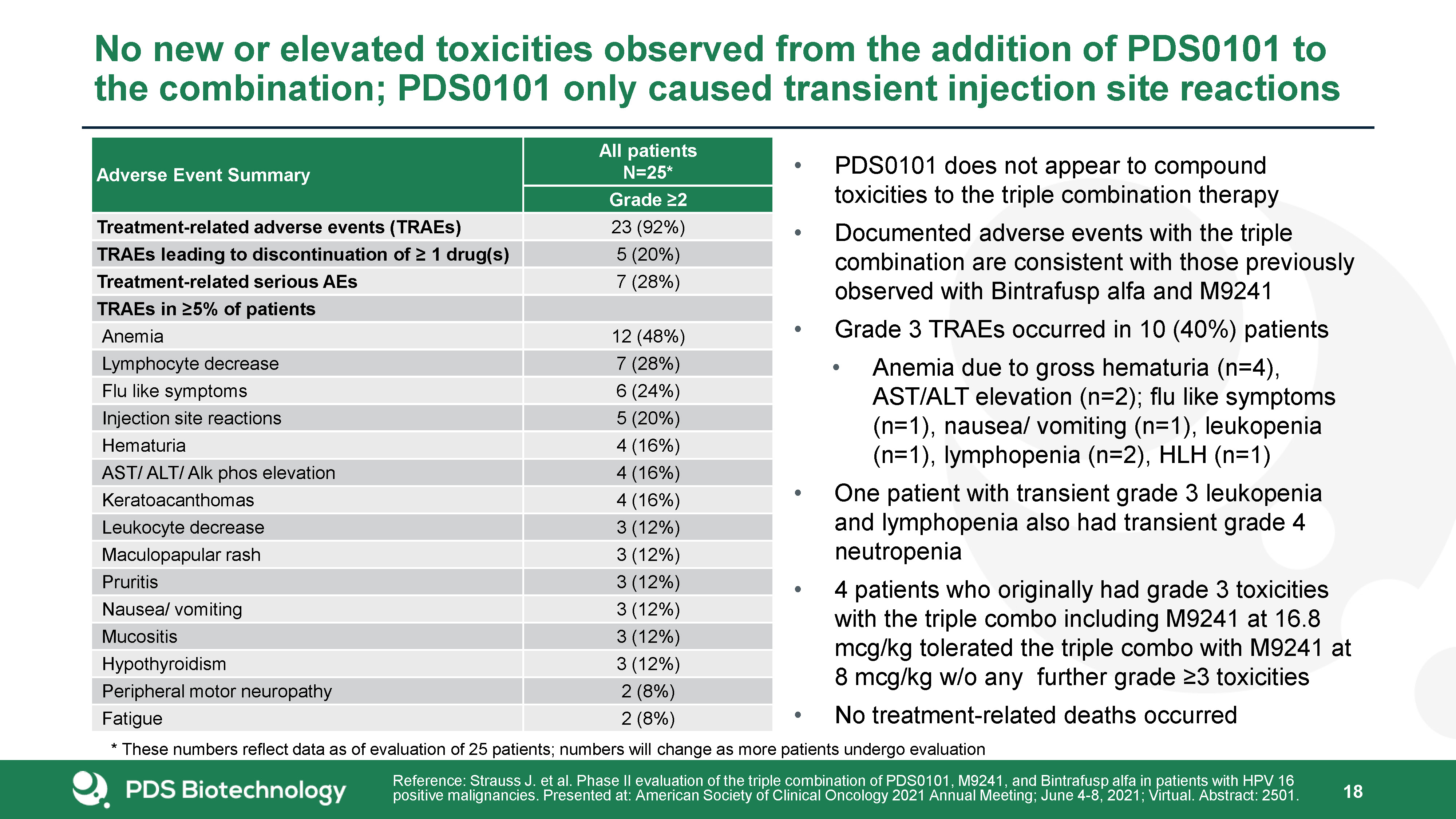
Adverse Event Summary All patientsN=25* Grade ≥2 Treatment-related adverse events (TRAEs) 23
(92%) TRAEs leading to discontinuation of ≥ 1 drug(s) 5 (20%) Treatment-related serious AEs 7 (28%) TRAEs in ≥5% of patients Anemia 12 (48%) Lymphocyte decrease 7 (28%) Flu like symptoms 6 (24%) Injection site reactions 5
(20%) Hematuria 4 (16%) AST/ ALT/ Alk phos elevation 4 (16%) Keratoacanthomas 4 (16%) Leukocyte decrease 3 (12%) Maculopapular rash 3 (12%) Pruritis 3 (12%) Nausea/ vomiting 3 (12%) Mucositis 3 (12%) Hypothyroidism 3
(12%) Peripheral motor neuropathy 2 (8%) Fatigue 2 (8%) PDS0101 does not appear to compound toxicities to the triple combination therapyDocumented adverse events with the triple combination are consistent with those previously observed
with Bintrafusp alfa and M9241Grade 3 TRAEs occurred in 10 (40%) patientsAnemia due to gross hematuria (n=4), AST/ALT elevation (n=2); flu like symptoms (n=1), nausea/ vomiting (n=1), leukopenia (n=1), lymphopenia (n=2), HLH (n=1)One patient
with transient grade 3 leukopenia and lymphopenia also had transient grade 4 neutropenia4 patients who originally had grade 3 toxicities with the triple combo including M9241 at 16.8 mcg/kg tolerated the triple combo with M9241 at 8 mcg/kg w/o
any further grade ≥3 toxicitiesNo treatment-related deaths occurred No new or elevated toxicities observed from the addition of PDS0101 to the combination; PDS0101 only caused transient injection site reactions 18 Reference: Strauss J. et
al. Phase II evaluation of the triple combination of PDS0101, M9241, and Bintrafusp alfa in patients with HPV 16 positive malignancies. Presented at: American Society of Clinical Oncology 2021 Annual Meeting; June 4-8, 2021; Virtual. Abstract:
2501. * These numbers reflect data as of evaluation of 25 patients; numbers will change as more patients undergo evaluation
REVIEW OF KEY FINDINGS ANDIMPLICATIONS FOR PDS BIOTECH(F. Bedu-Addo)
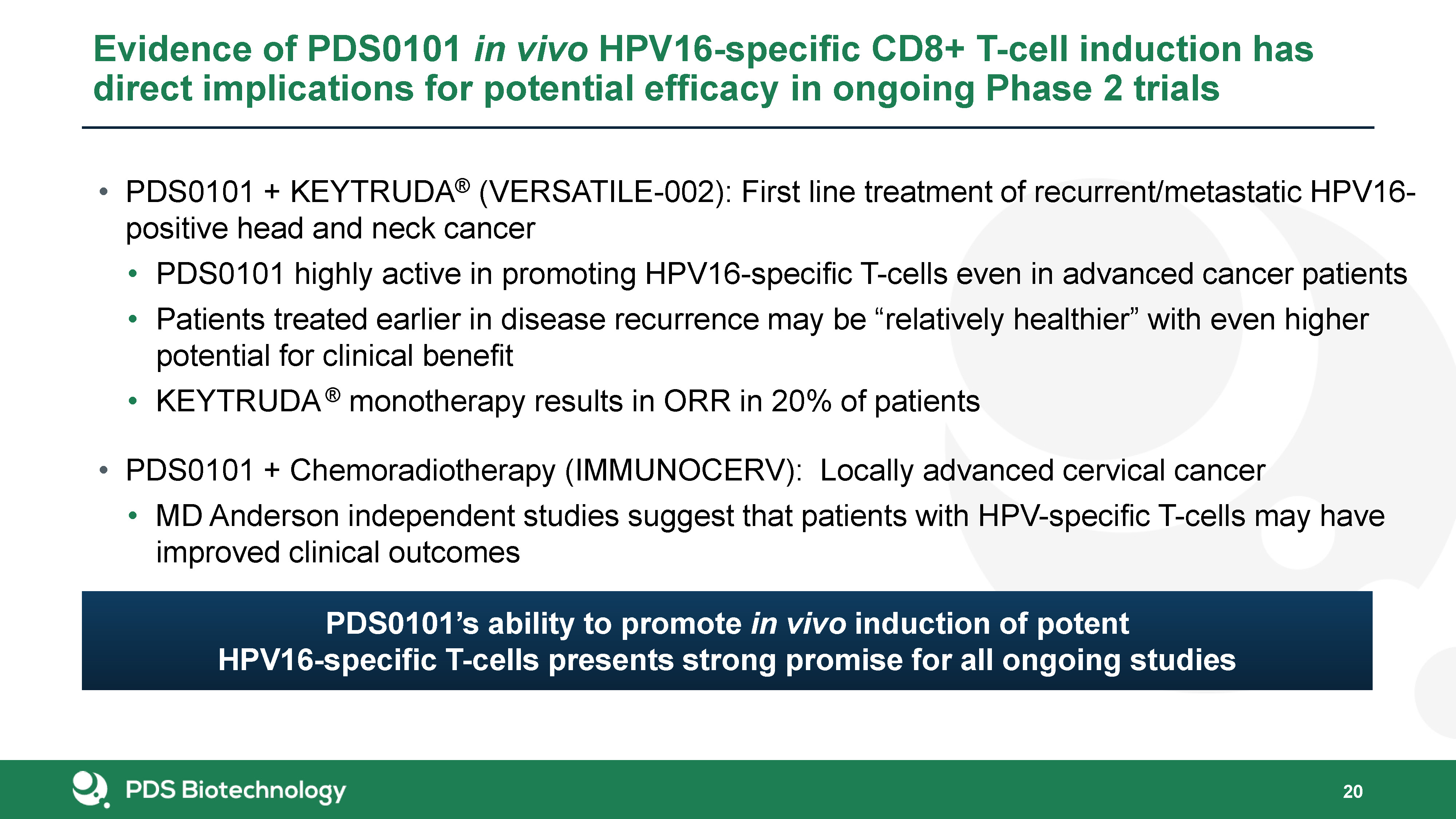
PDS0101 + KEYTRUDA® (VERSATILE-002): First line treatment of recurrent/metastatic HPV16-positive head and
neck cancerPDS0101 highly active in promoting HPV16-specific T-cells even in advanced cancer patientsPatients treated earlier in disease recurrence may be “relatively healthier” with even higher potential for clinical benefitKEYTRUDA ®
monotherapy results in ORR in 20% of patients PDS0101 + Chemoradiotherapy (IMMUNOCERV): Locally advanced cervical cancerMD Anderson independent studies suggest that patients with HPV-specific T-cells may have improved clinical
outcomes Evidence of PDS0101 in vivo HPV16-specific CD8+ T-cell induction has direct implications for potential efficacy in ongoing Phase 2 trials 20 PDS0101’s ability to promote in vivo induction of potent HPV16-specific T-cells presents
strong promise for all ongoing studies
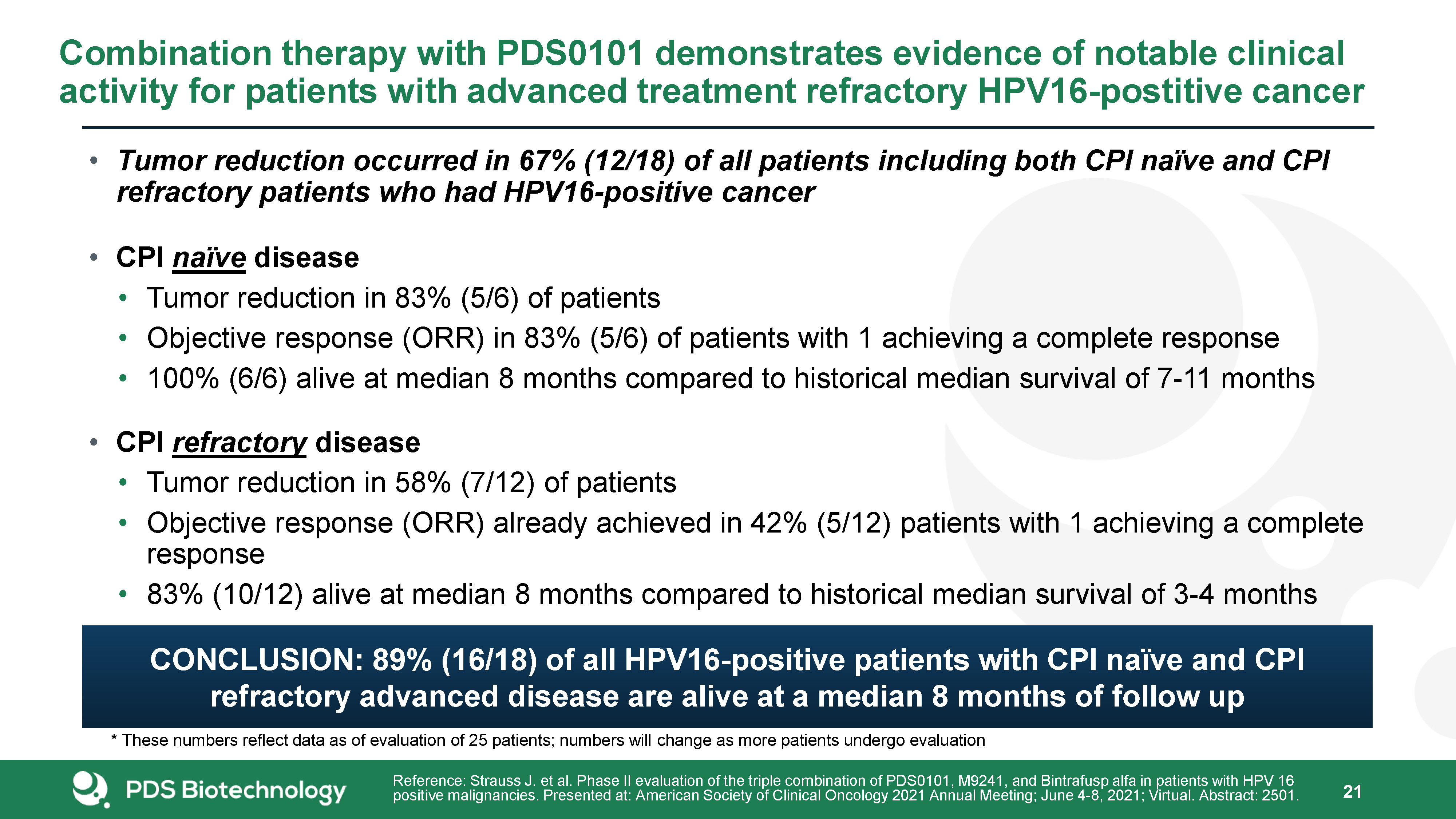
Tumor reduction occurred in 67% (12/18) of all patients including both CPI naïve and CPI refractory
patients who had HPV16-positive cancerCPI naïve diseaseTumor reduction in 83% (5/6) of patientsObjective response (ORR) in 83% (5/6) of patients with 1 achieving a complete response100% (6/6) alive at median 8 months compared to historical
median survival of 7-11 monthsCPI refractory diseaseTumor reduction in 58% (7/12) of patients Objective response (ORR) already achieved in 42% (5/12) patients with 1 achieving a complete response83% (10/12) alive at median 8 months compared to
historical median survival of 3-4 months Combination therapy with PDS0101 demonstrates evidence of notable clinical activity for patients with advanced treatment refractory HPV16-postitive cancer 21 Reference: Strauss J. et al. Phase II
evaluation of the triple combination of PDS0101, M9241, and Bintrafusp alfa in patients with HPV 16 positive malignancies. Presented at: American Society of Clinical Oncology 2021 Annual Meeting; June 4-8, 2021; Virtual. Abstract: 2501. *
These numbers reflect data as of evaluation of 25 patients; numbers will change as more patients undergo evaluation CONCLUSION: 89% (16/18) of all HPV16-positive patients with CPI naïve and CPI refractory advanced disease are alive at a median
8 months of follow up


APPENDIX: 2021 ASCO Presentation
Phase II evaluation of the triple combination of PDS0101, M9241, and Bintrafusp alfa in patients with HPV
16 positive malignancies Julius Strauss1, Charalampos S. Floudas2, Houssein Abdul Sater2, Michell Manu3, Elizabeth Lamping2, Deneise C Francis2, Lisa M Cordes2, Jenn Marte2, Renee N Donahue1, Caroline Jochems1, Jason Redman2, Ravi A Madan2,
Marijo Bilusic2, Fatima Karzai2, Scott Norberg2, Christian S. Hinrichs2, Lauren V Wood4, Frank K Bedu-Addo4, Jeffrey Schlom1, James L Gulley21Laboratory of Tumor Immunology and Biology, NCI; 2Genitourinary Malignancies Branch, NCI; 3Leidos
Biomedical Research, Inc.; 4PDS Biotechnology, Princeton, NJ Abstract No. 2501. Presented at the 2021 ASCO Annual Meeting, June 4–8, 2021; Virtual. Presentation No.
25 J. Strauss Introduction >630,000 new cases of HPV-related cancer (e.g. cervical, oropharyngeal,
anal) reported worldwide annually1PD-1 inhibitors have been evaluated in these tumor types and ORRs have ranged from 13–24%2-8Nivolumab & pembrolizumab are FDA approved for HNSCC; pembrolizumab is approved for PD-L1+ cervical
cancerUnfortunately, the majority of patients who receive these anti PD-1 inhibitors will progress For these patients with checkpoint refractory disease there is no clear effective standard of care therapyHPV infection is also linked to
upregulation of TGF-β signaling9Genome-wide association studies showed that TGF-βR1 is significantly overexpressed in HPV-related cancer10 1. de Martel C, et al. Int J Cancer. 2017;141:664–70; 2. Viens LJ, et al. MMWR Morb Mortal Wkly Rep.; 2.
Bauml J, et al. J Clin Oncol 2017;35:1542–49; 3. Ott PA, et al. Ann Oncol. 2017;28:1036–41; 4. Hollebecque A, et al. J Clin Oncol. 2017;35(Suppl):Abstract 5504; 5. Chung HC, et al. J Clin Oncol. 2018;36(Suppl):Abstract 5522; 6. Ferris RL, et
al. N Engl J Med. 2016;375:1856–67; 7 Mehra R, et al. Br J Cancer. 2018;119:153–59; 8 Morris VK, et al. Lancet Oncol. 2017;18:446–53. 2016;65:661–66; 9. Torres-Poveda K, et al. World J Clin Oncol. 2014;5:753-63; 10. Levovitz C, et al. Cancer
Res. 2014;74:6833-44.

J. Strauss Bintrafusp alfa: a TGF-β and PD-L1 Inhibitor Bintrafusp alfa is an innovativefirst-in-class
bifunctional fusion protein composed of the extracellular domain of the TGF-βRII receptor (a TGF-β “trap”) fused to a human IgG1 mAb blocking PD-L1In a phase 1 study, Bintrafusp alfa was well tolerated and produced durable responses in several
solid tumor types 1-3 1.Strauss J, et al. Clin Cancer Res. 2018;24:1287–95; 2. Paz-Ares L, et al. J Clin Oncol. 2018;36(Suppl):Abstract 9017; 3. Cho BC, et al. Ann Oncol. 2018;29(Suppl):Abstract 1048O.. 26
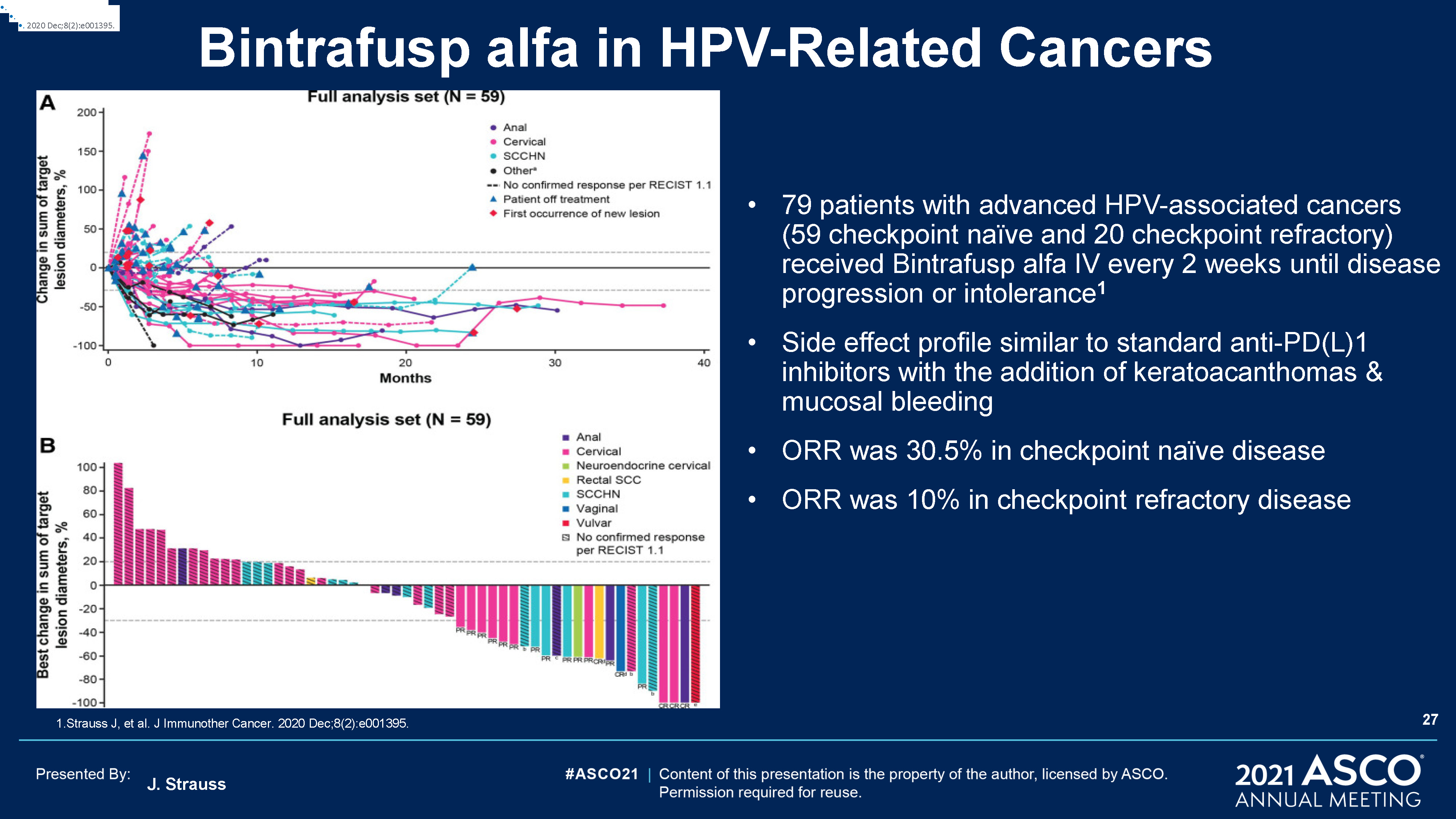
J. Strauss Bintrafusp alfa in HPV-Related Cancers 1.Strauss J, et al. J Immunother Cancer. 2020
Dec;8(2):e001395. . 2020 Dec;8(2):e001395 . 2020 Dec;8(2):e001395 . 2020 Dec;8(2):e001395. 79 patients with advanced HPV-associated cancers (59 checkpoint naïve and 20 checkpoint refractory) received Bintrafusp alfa IV every 2 weeks
until disease progression or intolerance1Side effect profile similar to standard anti-PD(L)1 inhibitors with the addition of keratoacanthomas & mucosal bleeding ORR was 30.5% in checkpoint naïve diseaseORR was 10% in checkpoint refractory
disease 27
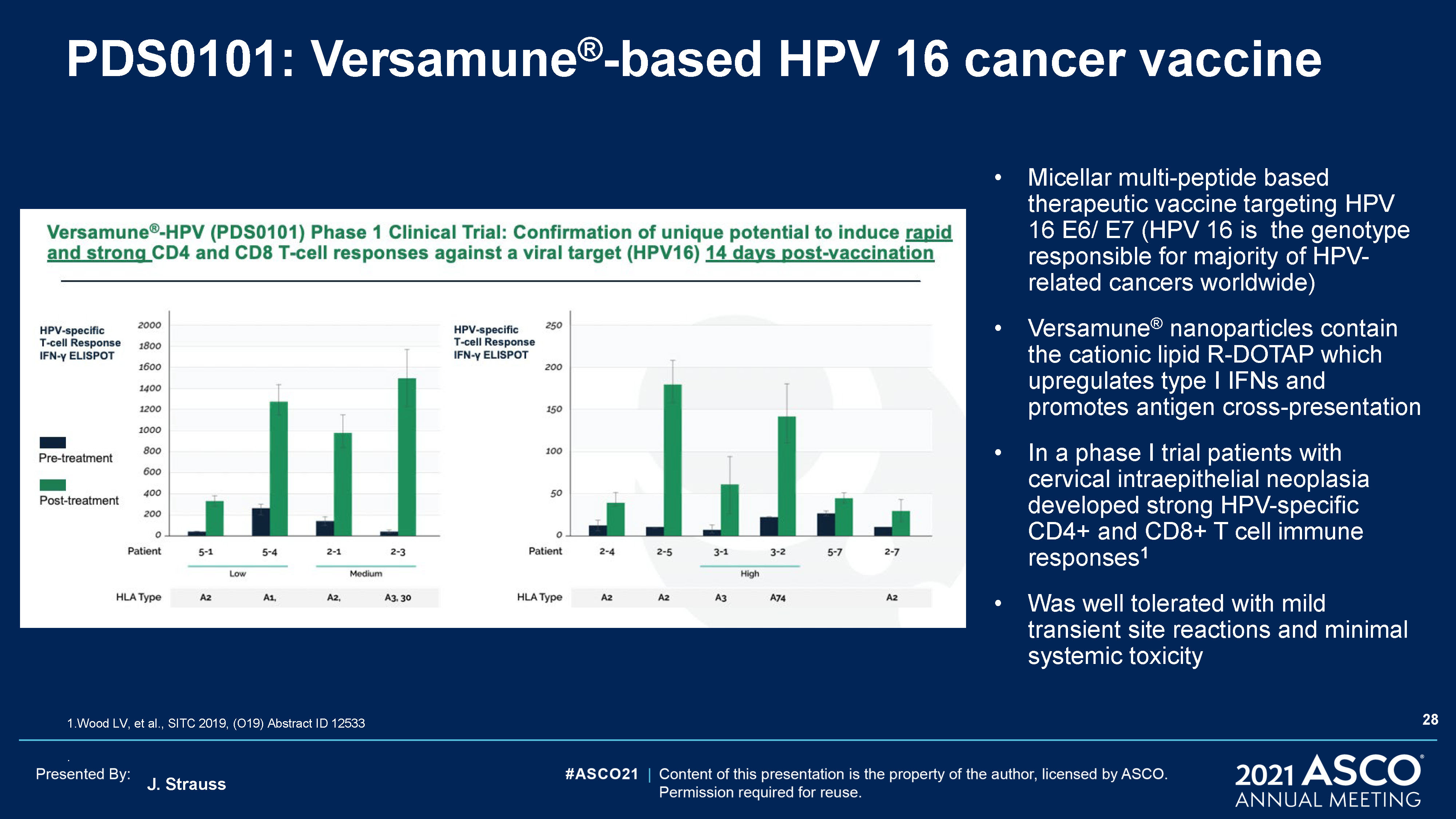
J. Strauss PDS0101: Versamune®-based HPV 16 cancer vaccine Micellar multi-peptide based therapeutic
vaccine targeting HPV 16 E6/ E7 (HPV 16 is the genotype responsible for majority of HPV-related cancers worldwide)Versamune® nanoparticles contain the cationic lipid R-DOTAP which upregulates type I IFNs and promotes antigen cross-presentation
In a phase I trial patients with cervical intraepithelial neoplasia developed strong HPV-specific CD4+ and CD8+ T cell immune responses1 Was well tolerated with mild transient site reactions and minimal systemic toxicity 28 1.Wood LV, et al.,
SITC 2019, (O19) Abstract ID 12533.
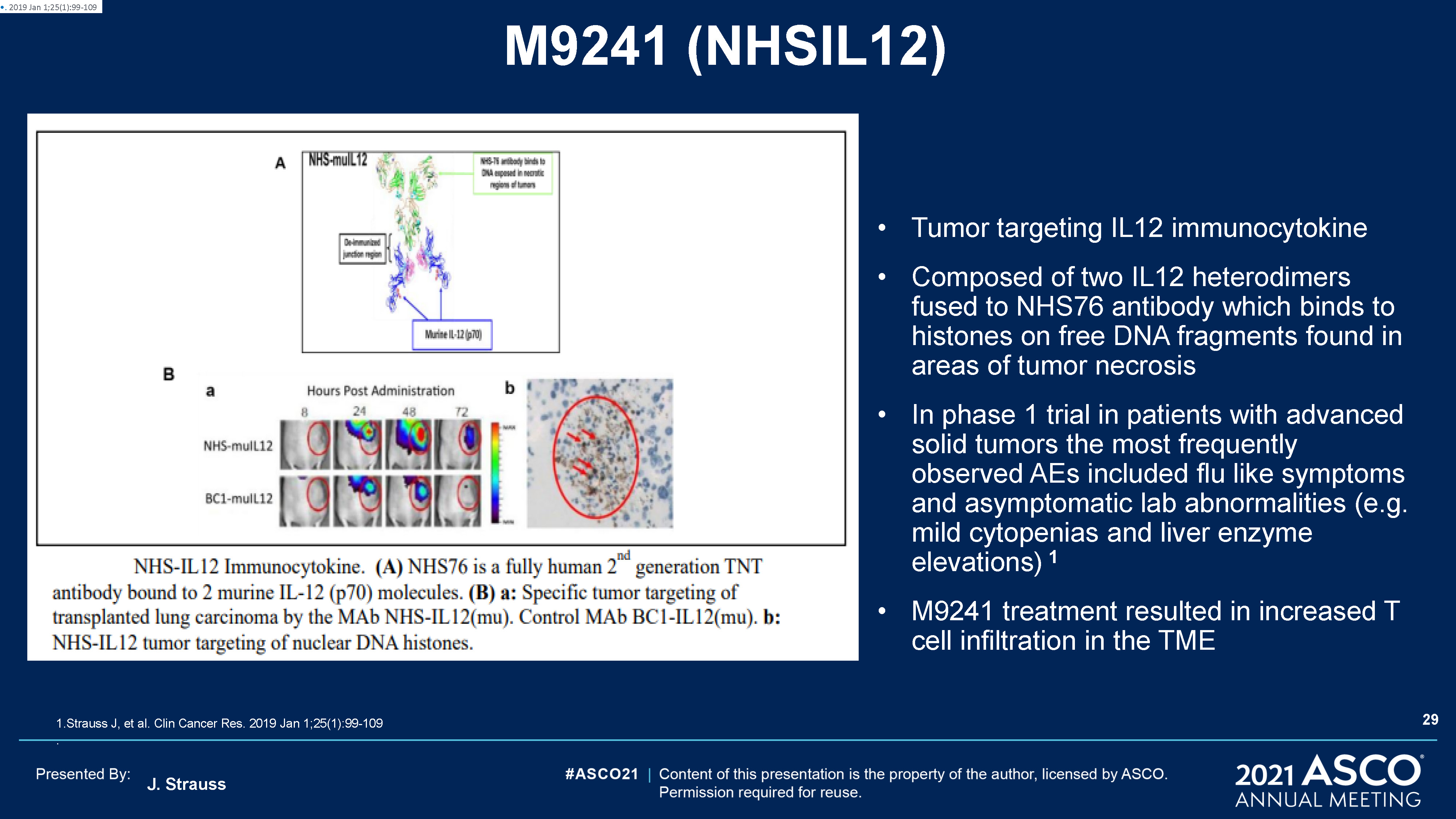
J. Strauss M9241 (NHSIL12) Tumor targeting IL12 immunocytokineComposed of two IL12 heterodimers fused
to NHS76 antibody which binds to histones on free DNA fragments found in areas of tumor necrosisIn phase 1 trial in patients with advanced solid tumors the most frequently observed AEs included flu like symptoms and asymptomatic lab
abnormalities (e.g. mild cytopenias and liver enzyme elevations) 1M9241 treatment resulted in increased T cell infiltration in the TME 1.Strauss J, et al. Clin Cancer Res. 2019 Jan 1;25(1):99-109. . 2019 Jan 1;25(1):99-109 29
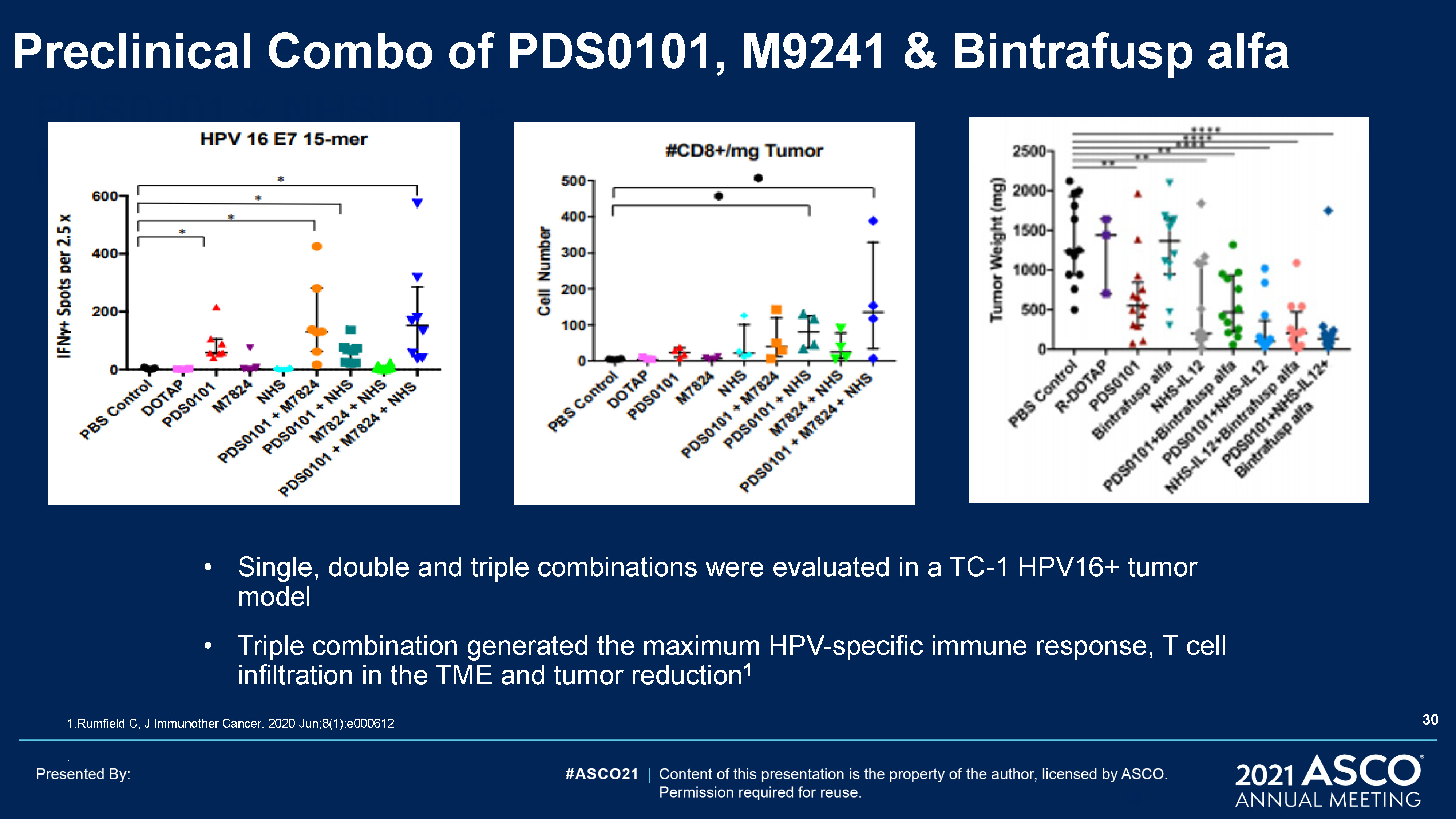
Preclinical Studies with PDS0101 + NHSIL12 + M7824 30 Preclinical Combo of PDS0101, M9241 &
Bintrafusp alfa Single, double and triple combinations were evaluated in a TC-1 HPV16+ tumor modelTriple combination generated the maximum HPV-specific immune response, T cell infiltration in the TME and tumor reduction1 1.Rumfield C, J
Immunother Cancer. 2020 Jun;8(1):e000612. 30
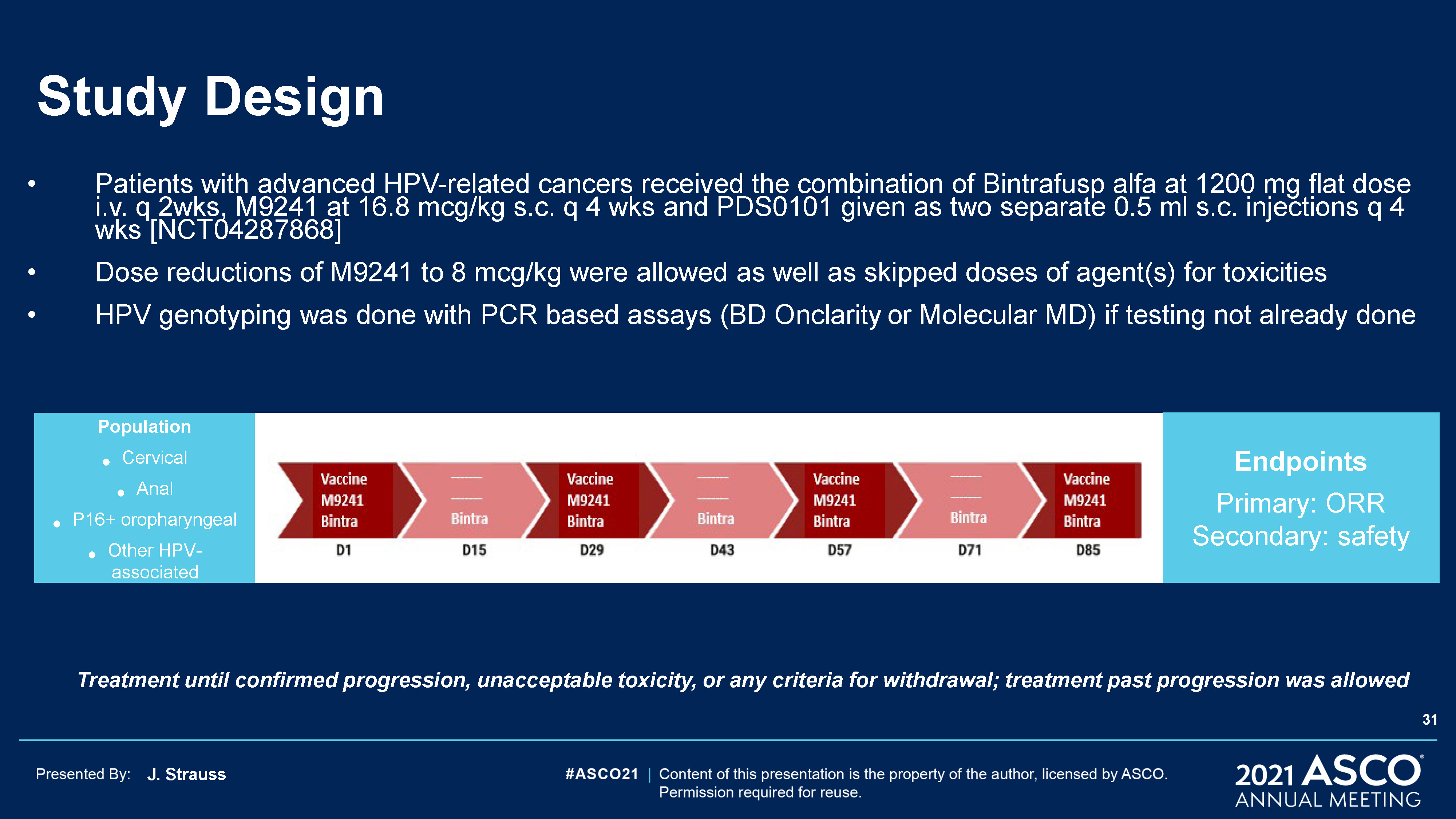
J. Strauss Study Design Patients with advanced HPV-related cancers received the combination of
Bintrafusp alfa at 1200 mg flat dose i.v. q 2wks, M9241 at 16.8 mcg/kg s.c. q 4 wks and PDS0101 given as two separate 0.5 ml s.c. injections q 4 wks [NCT04287868]Dose reductions of M9241 to 8 mcg/kg were allowed as well as skipped doses of
agent(s) for toxicitiesHPV genotyping was done with PCR based assays (BD Onclarity or Molecular MD) if testing not already done PopulationCervicalAnalP16+ oropharyngealOther HPV-associated EndpointsPrimary: ORRSecondary: safety Treatment
until confirmed progression, unacceptable toxicity, or any criteria for withdrawal; treatment past progression was allowed 31
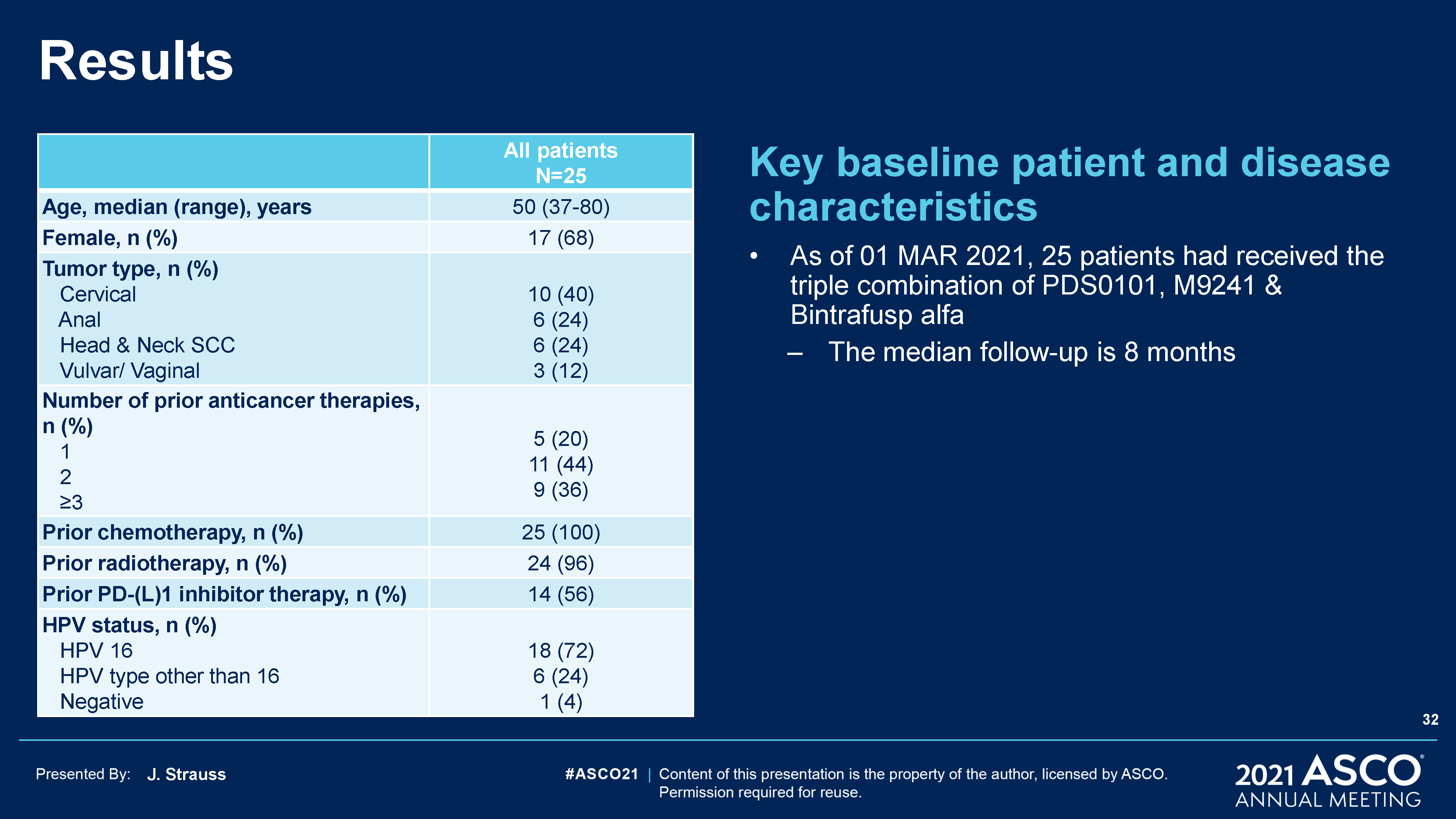
J. Strauss Results Key baseline patient and disease characteristicsAs of 01 MAR 2021, 25 patients had
received the triple combination of PDS0101, M9241 & Bintrafusp alfa The median follow-up is 8 months All patientsN=25 Age, median (range), years 50 (37-80) Female, n (%) 17 (68) Tumor type, n (%) Cervical Anal Head & Neck SCC
Vulvar/ Vaginal 10 (40)6 (24)6 (24)3 (12) Number of prior anticancer therapies, n (%) 1 2 ≥3 5 (20)11 (44)9 (36) Prior chemotherapy, n (%) 25 (100) Prior radiotherapy, n (%) 24 (96) Prior PD-(L)1 inhibitor therapy, n (%) 14 (56) HPV
status, n (%) HPV 16 HPV type other than 16 Negative 18 (72)6 (24)1 (4) 32
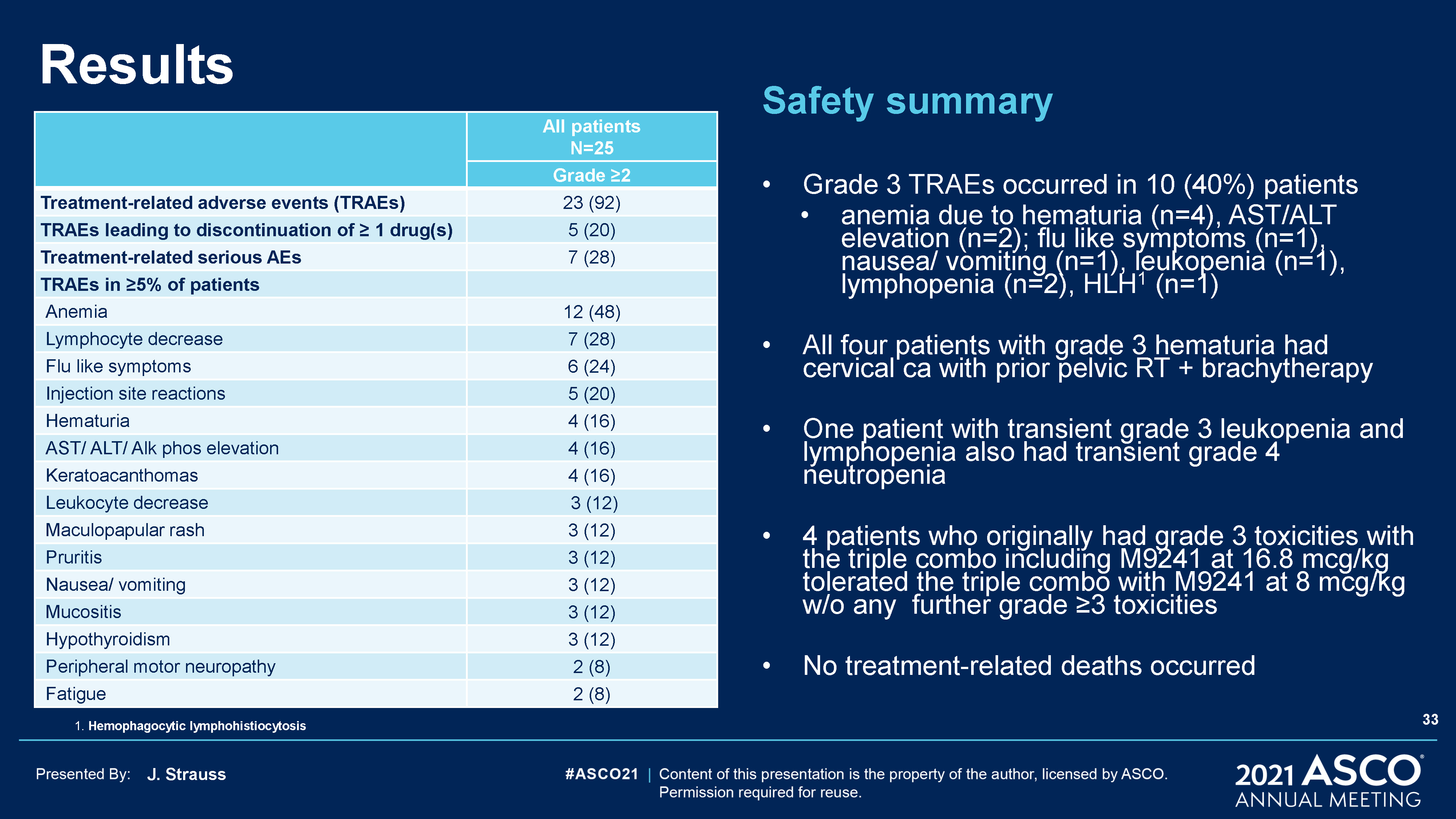
J. Strauss Results Safety summaryGrade 3 TRAEs occurred in 10 (40%) patientsanemia due to hematuria
(n=4), AST/ALT elevation (n=2); flu like symptoms (n=1), nausea/ vomiting (n=1), leukopenia (n=1), lymphopenia (n=2), HLH1 (n=1)All four patients with grade 3 hematuria had cervical ca with prior pelvic RT + brachytherapyOne patient with
transient grade 3 leukopenia and lymphopenia also had transient grade 4 neutropenia4 patients who originally had grade 3 toxicities with the triple combo including M9241 at 16.8 mcg/kg tolerated the triple combo with M9241 at 8 mcg/kg w/o any
further grade ≥3 toxicitiesNo treatment-related deaths occurred All patientsN=25 Grade ≥2 Treatment-related adverse events (TRAEs) 23 (92) TRAEs leading to discontinuation of ≥ 1 drug(s) 5 (20) Treatment-related serious AEs 7
(28) TRAEs in ≥5% of patients Anemia 12 (48) Lymphocyte decrease 7 (28) Flu like symptoms 6 (24) Injection site reactions 5 (20) Hematuria 4 (16) AST/ ALT/ Alk phos elevation 4 (16) Keratoacanthomas 4 (16) Leukocyte
decrease 3 (12) Maculopapular rash 3 (12) Pruritis 3 (12) Nausea/ vomiting 3 (12) Mucositis 3 (12) Hypothyroidism 3 (12) Peripheral motor neuropathy 2 (8) Fatigue 2 (8) 1. Hemophagocytic lymphohistiocytosis 33
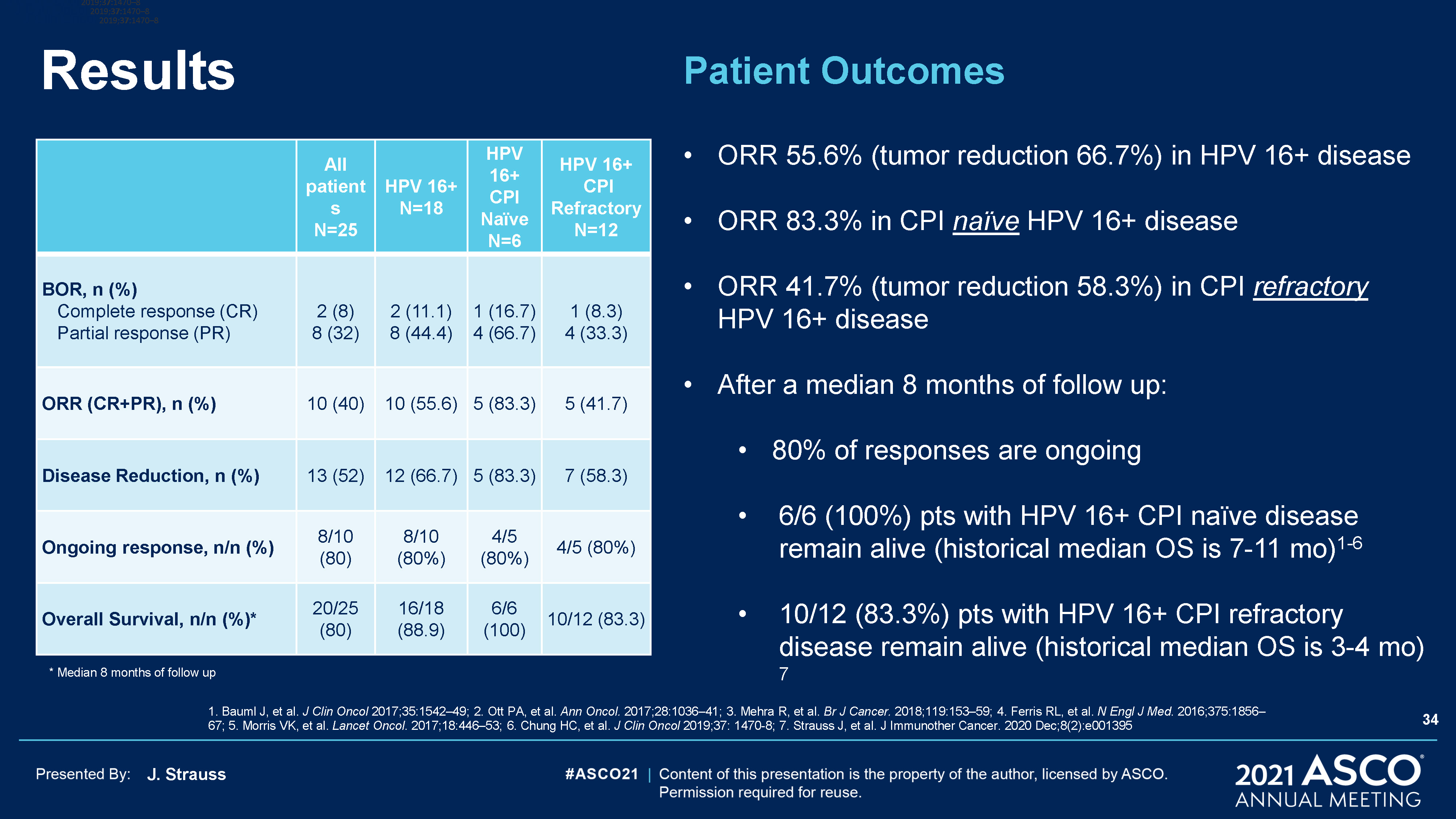
J. Strauss Results All patientsN=25 HPV 16+N=18 HPV 16+ CPI Naïve N=6 HPV 16+ CPI
RefractoryN=12 BOR, n (%) Complete response (CR) Partial response (PR) 2 (8)8 (32) 2 (11.1)8 (44.4) 1 (16.7)4 (66.7) 1 (8.3)4 (33.3) ORR (CR+PR), n (%) 10 (40) 10 (55.6) 5 (83.3) 5 (41.7) Disease Reduction, n (%) 13 (52) 12
(66.7) 5 (83.3) 7 (58.3) Ongoing response, n/n (%) 8/10 (80) 8/10 (80%) 4/5 (80%) 4/5 (80%) Overall Survival, n/n (%)* 20/25 (80) 16/18 (88.9) 6/6 (100) 10/12 (83.3) 1. Bauml J, et al. J Clin Oncol 2017;35:1542–49; 2. Ott PA, et
al. Ann Oncol. 2017;28:1036–41; 3. Mehra R, et al. Br J Cancer. 2018;119:153–59; 4. Ferris RL, et al. N Engl J Med. 2016;375:1856–67; 5. Morris VK, et al. Lancet Oncol. 2017;18:446–53; 6. Chung HC, et al. J Clin Oncol 2019;37: 1470-8; 7.
Strauss J, et al. J Immunother Cancer. 2020 Dec;8(2):e001395 J Clin Oncol 2019;37:1470–8 J Clin Oncol 2019;37:1470–8 J Clin Oncol 2019;37:1470–8 J Clin Oncol 2019;37:1470–8 34 * Median 8 months of follow up Patient OutcomesORR 55.6%
(tumor reduction 66.7%) in HPV 16+ diseaseORR 83.3% in CPI naïve HPV 16+ diseaseORR 41.7% (tumor reduction 58.3%) in CPI refractory HPV 16+ disease After a median 8 months of follow up:80% of responses are ongoing6/6 (100%) pts with HPV 16+ CPI
naïve disease remain alive (historical median OS is 7-11 mo)1-6 10/12 (83.3%) pts with HPV 16+ CPI refractory disease remain alive (historical median OS is 3-4 mo) 7
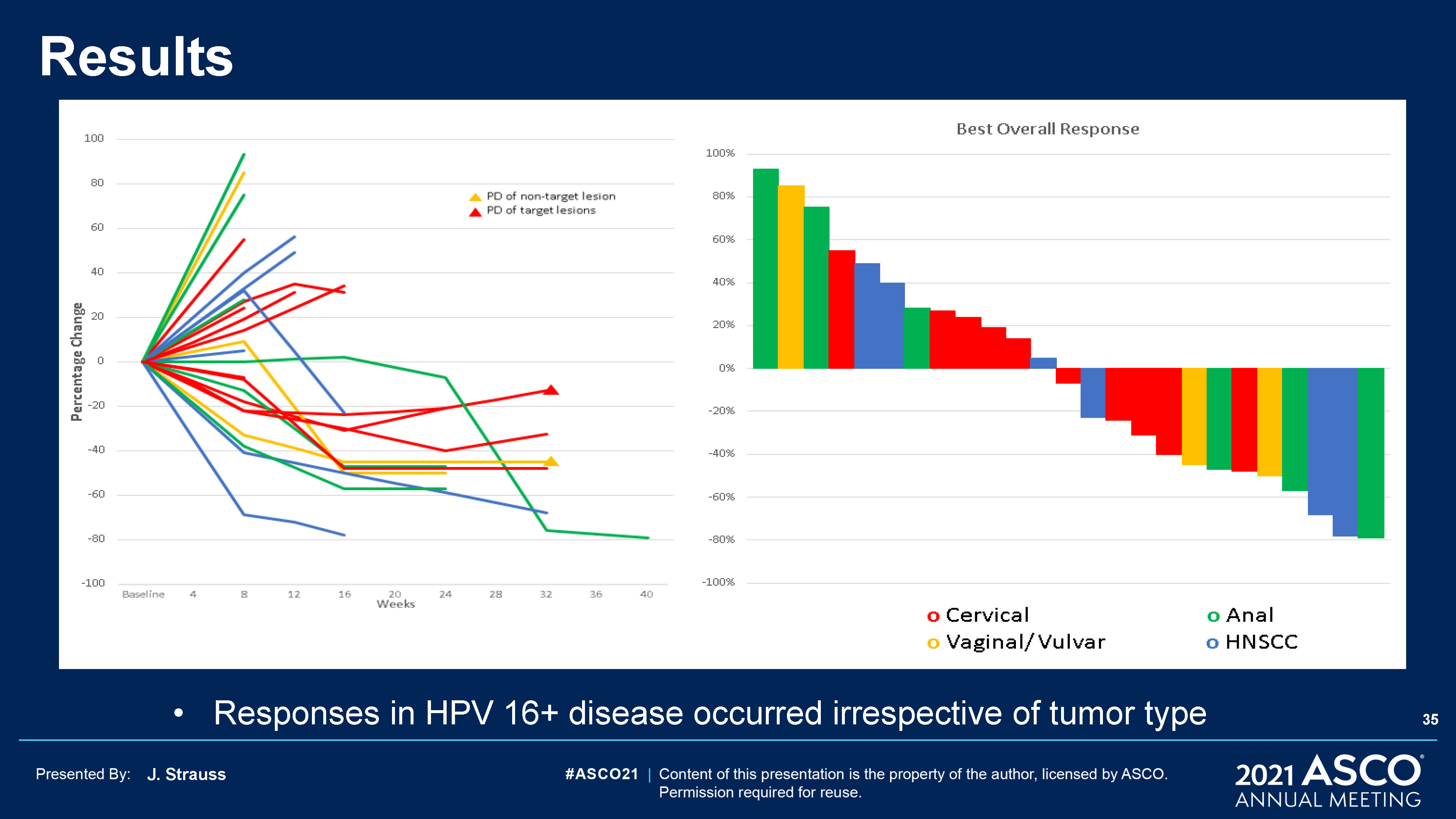
J. Strauss Results Responses in HPV 16+ disease occurred irrespective of tumor type 35
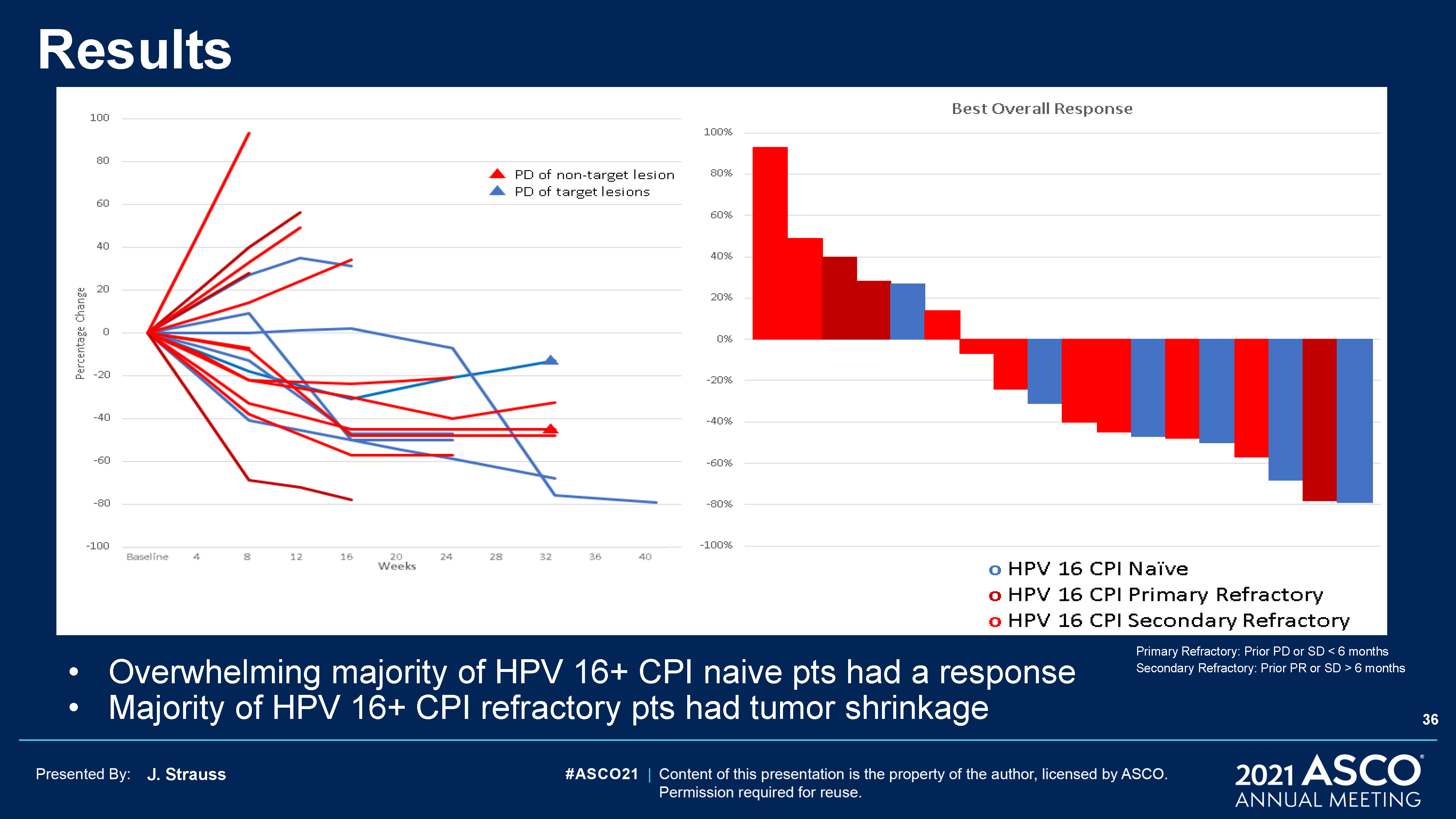
J. Strauss Results Overwhelming majority of HPV 16+ CPI naive pts had a responseMajority of HPV 16+ CPI
refractory pts had tumor shrinkage Primary Refractory: Prior PD or SD < 6 months Secondary Refractory: Prior PR or SD > 6 months 36
J. Strauss Conclusions Triple combination of PDS0101, M9241 and Bintrafusp alfa appears to have a
manageable safety profile along with early evidence of notable clinical activity for pts with advanced HPV 16+ malignanciesClinical activity noted irrespective of tumor type or CPI statusORR was 55.6% (tumor reduction 66.7%) in all pts with
advanced HPV 16+ diseaseORR was 83.3% in patients with CPI naive HPV 16+ disease ORR was 41.7% (tumor reduction 58.3%) in patients with CPI refractory HPV 16+ diseaseAfter a median 8 months of follow up:80% of responses are ongoing6/6 (100%)
pts with HPV 16+ CPI naïve disease remain alive10/12 (83.3%) pts with HPV 16+ CPI refractory disease remain alive Accrual is ongoing to the triple combination [NCT04287868] 37
J. Strauss Acknowledgments The authors would like to thank the patients and their familiesFunding for
the trial was provided by the Center for Cancer Research, National Cancer Institute, and National Institutes of Health Clinical CenterM9241 and Bintrafusp alfa were provided by Merck KGaA, Darmstadt, GermanyPDS0101 was provided by PDS
Biotechnology, Florham Park, NJ Correspondence: Julius Strauss [email protected] 38
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- PDS Biotech to Host Key Opinion Leader Event to Discuss Positive, Updated Data from Phase 2 VERSATILE-002 Clinical Trial with Versamune® HPV in Combination with KEYTRUDA® in Recurrent or Metastatic
- New Spirits and Seltzers Brand, Pink Sand Spirits, Co., Announces a Groundbreaking Partnership with the Global Food Distributor, Sysco, in The Bahamas
- 9-Time GRAMMY® Winner Sheryl Crow and Sens. John Cornyn and Amy Klobuchar to Be Honored at the GRAMMYs on the Hill® Awards
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share