Form 8-K Nemaura Medical Inc. For: Jul 26
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of
the Securities Exchange Act 1934
Date of Report (Date of earliest event reported): July 26, 2021
NEMAURA MEDICAL INC.
(Exact name of registrant as specified in charter)
Nevada
(State or other jurisdiction of incorporation)
|
001-38355 |
46-5027260 | |
| (Commission File Number) | (IRS Employer Identification No.) |
|
57 West 57th Street Manhattan, NY |
10019 | |
| (Address of principal executive offices) | (Zip Code) | |
| Registrant’s telephone number, including area code: |
+1 (646) 416-8000 | |
|
N/A (Former name or former address, if changed since last report) | ||
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of registrant under any of the following provisions:
[_] Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
[_] Soliciting material pursuant to Rule 14a-12(b) under the Exchange Act (17 CFR 240.14a-12(b))
[_] Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
[_] Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| Common Stock | NMRD | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (17 CFR §230.405) or Rule 12b-2 of the Securities Exchange Act of 1934 (17 CFR §240.12b-2).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| |
Item 7.01. Regulation FD Disclosure.
Beginning on July 26, 2021, management of Nemaura Medical Inc. (the “Company”) will deliver the investor presentation attached hereto as Exhibit 99.1 and incorporated herein by reference.
The information included in this Current Report on Form 8-K, including Exhibit 99.1, shall not be deemed to be "filed" for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the "Exchange Act"), or otherwise subject to the liabilities of that section, nor shall such information be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in such a filing. The information set forth under this Item 7.01 shall not be deemed an admission as to the materiality of any information in this Current Report on Form 8-K that is required to be disclosed solely to satisfy the requirements of Regulation FD.
DISCLOSURE REGARDING FORWARD-LOOKING STATEMENTS
Certain statements contained in this Current Report on Form 8-K, including on Exhibit 99.1 hereto, that are not historical facts constitute forward-looking statements, within the meaning of the Private Securities Litigation Reform Act of 1995. Reliance should not be placed on forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, which may cause actual results, performance, or achievements to differ materially from those expressed or implied. Any forward-looking statement speaks only as of the date made. We undertake no obligation to update any forward-looking statements to reflect events or circumstances after the date on which they are made.
The words "believe," "anticipate," "design," "estimate," "plan," "predict," "seek," "expect," "intend," "may," "could," "should," "potential," "likely," "projects," "continue," "will," and "would" and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements are not guarantees of the future as there are a number of meaningful factors that could cause the Company’s actual results to vary materially from those indicated by such forward-looking statements. These statements are based on certain assumptions made based on experience, expected future developments and other factors the Company believes are appropriate in the circumstances. Factors which could cause actual results to differ from expectations, many of which are beyond the Company’s control, include, but are not limited to, obtaining regulatory approval for our sugarBEAT device, conducting successful clinical trials, executing agreements required to successfully advance the Company's objectives; retaining the management and scientific team to advance the product; overcoming adverse changes in market conditions and the regulatory environment; obtaining and enforcing intellectual property rights; obtaining adequate financing in the future through product licensing, public or private equity or debt financing or otherwise; dealing with general business conditions and competition; and other factors referenced in the Company’s filings with the Securities and Exchange Commission, including in the “Risk Factors” section of the Company’s Annual Report on Form 10-K for the fiscal year ended March 31, 2020, as the same may be updated from time to time. Except as required by law, we do not assume any obligation to update any forward-looking statement. We disclaim any intention or obligation to update or revise any forward-looking statement, whether as a result of new information, future events or otherwise.
| Item 9.01 | Financial Statements and Exhibits. |
|
Exhibit No. |
Description | |
| 99.1 | Investor presentation of the registrant dated July 2021. | |
| |
SIGNATURE
Pursuant to the requirements of the Securities and Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| NEMAURA MEDICAL INC. | |||
| Date: July 26, 2021 | |||
| By: | /s/ Dewan F. H. Chowdhury | ||
| Dewan F. H. Chowdhury | |||
| Chief Executive Officer | |||
Exhibit 99.1

Corporate Presentation July 2021 Nasdaq : NMRD
| |

Forward Looking Statements This presentation includes forward - looking statements that are subject to many risks and uncertainties . These forward - looking statements, such as statements about Nemaura’s short - term and long - term growth strategies, can sometimes be identified by use of terms such as “intend,” “expect,” “plan,” “estimate,” “future,” “strive,” and similar words . These statements involve many risks and uncertainties that may cause actual results to differ from what may be expressed or implied in these statements . These risks are discussed in Nemaura’s filings with the Securities and Exchange Commission (the “Commission”), including the risks identified under the section captioned “Risk Factors” in Nemaura’s Annual Report on Form 10 - K filed with the Commission in June 2019 as the same may be updated from time to time . Nemaura disclaims any obligation to update information contained in these forward - looking statements whether as a result of new information, future events, or otherwise .
| |

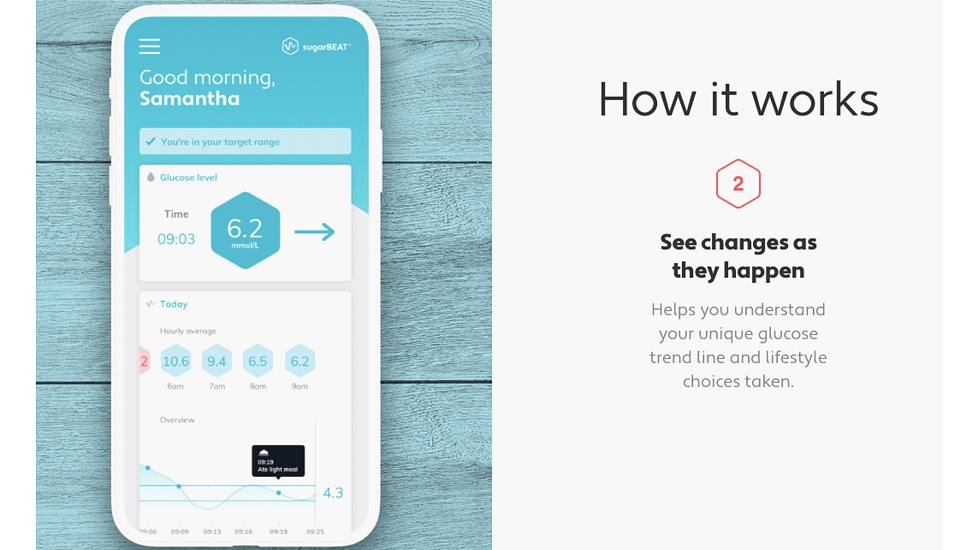
Introduction We developed the world’s first Daily - Wear non - invasive Continuous Glucose Monitor (CGM) – A Class 2b CE approved Medical Device. Launched: sugarBEAT ® and BEAT ® diabetes , supporting Diabetes prevention, management and reversal. Planned Launch in 2021: Mass Market consumer metabolic health App & Glucose Sensor Kit
| |

The Problem… There are over 463 million people living with diabetes worldwide, and over $ 760 Billion was spent in the US alone in 2019 for diabetes related healthcare expenditure 1 . The total addressable market exceeds $ 150 Billion 2 , 3 , 4 . Obesity and Diabetes are two of the major drivers of the chronic disease epidemic
| |

Our Objective: Prevent, Manage, or Reverse Type 2 Diabetes
| |

UNIQUE The world’s first daily wear CGM: no other sensor technology is currently available allowing non - invasive daily use. LIFESTYLE Other competing sensors are worn for 10 - 14 days consecutively. Nemaura’s BEAT® sensors are designed for daily use – any day you choose. Our Unique Solution KNOWLEDGE Glucose sensors based on sugarBEAT provide guidance and insights into the extent of control over sugar levels. ENGAGEMENT A world class digital program [and ecosystem] keeping the user engaged for the long term. OUTCOME We expect the following based on independently published reports using similar programs: improvements in HbA1C, blood cholesterol, blood pressure, and sustainable weight loss. Real results . Sustainable . Affordable.
| |

Our Approach 1. sugarBEAT ® CGM – real time glucose monitoring. 1. BEAT ® diabetes – Digital program for diabetes management and reversal, with intermittent glucose profiling. 1. Mass market consumer metabolic health App and Glucose Sensor Kit, targeting obesity, pre - diabetes and Type 2 diabetes.
| |

Total Addressable Market UK 4.8 million people with diabetes 8 One person diagnosed every 2 minutes Germany 9.5 million have diabetes 9 . 4.5 million of these 9.5 million are undiagnosed and, as a result, may be particularly at risk. U.S. 34.2 million have diabetes 6 88 million people have pre - diabetes 28,000 people diagnosed with diabetes EVERY WEEK in the U.S. alone 7 in a market worth nearly $ 150B
| |

Impact on Healthcare Cost □ Healthcare costs for persons with type 2 diabetes cost approximately 2.5x as much as a person without diabetes. If they experience complications, that number soars even higher 10 □ Employers and healthcare insurers are therefor resorting to programs that will provide long - term sustainable results in stemming the onset of diabetes and, where possible, reversing Type 2 diabetes □ Current programs are cost prohibitive, but Nemaura has both a cost advantage as well as user friendliness from its intermittent use sensors and will focus its efforts on the U.S. and European markets initially, for diabetes prevention, management and potential reversal healthy 1 diabetes with complications $ spent per year $4k diabetes $12k $21k
| |

Product Portfolio sugarBEAT® Non - invasive CGM BEAT ® diabetes Program Consumer Metabolic Health App & Glucose Sensor Kit
| |

CE approved Class IIB Medical Device US FDA PMA approval and launch in the US anticipated by end of 2021
| |

CE approved Class IIB Medical Device US FDA PMA approval and launch in the US anticipated by end of 2021 The world’s first daily wearable Continuous Glucose Monitor that doesn’t use needles. CE Approved Class IIb Medical Device
| |

Core Technology
| |
| |

| |

Why is sugarBEAT ® Different? User does not insert a 1 cm long Filament in the arm using a large needle (like competing invasive sensors) and keep it there for 10 - 15 days . The sensor sits on top of the arm/skin like a band aid . User does not keep the sensor on the skin for 10 - 14 days, the sensor is a daily wear sensor – can be worn during the day or at night, and sensor is thrown away each day . Persons with Obesity, pre - diabetes or Type 2 generally would only need to wear a sensor a few days a month . sugarBEAT® sensor allows flexible wear on demand, something that no other sensor can currently offer . A user pays for only those days of sensor wear they intend, and not 10 - 15 days by default, therefore substantially reducing the monthly cost of sensors, yet providing the user with glucose profile data that acts as a powerful tool to help users manage their health .
| |

sugarBEAT ® and Time in Range A critical advantage of CGM - based systems is the ability to measure the time that glucose levels are in normal range – i . e . , time in range (TIR) . Control over TIR leads to a significant reduction in the onset of complications of diabetes . The same HbA 1 C values can give vastly differing TIR profiles and is inferior to using CGM to measure TIR to prevent the onset of long - term disease complications .
| |

sugarBEAT ® vs. HbA1C 40% high range 40% in range 20% low range 70% high range 25% in range 5% low range 100% in range The same HbA1C value, yet 3 completely different TIR profiles, demonstrating the power of TIR over HbA1C as the new gold standard 11
| |

sugarBEAT ® Testimonials * My Sugar Watch offered me a needle - free blood glucose monitoring solution that’s non - invasive and easy to use. I didn’t even realize I had the My Sugar watch device on my arm as it is so lightweight. It gives me the assurance that my blood sugar reading is accurate, and I have access to my levels on my phone at all times. I was diagnosed with gestational diabetes, and I was informed by my healthcare professional that this may lead to a diagnosis of Type 2 diabetes in the future. Unfortunately, I was diagnosed with Type 2 diabetes after this and I have to manage this diagnosis all by myself and learn to control my blood glucose levels. Using My Sugar Watch has alerted me to changes in my blood glucose levels and helped me understand how these changes make an impact on my body and how I am feeling. To have this information at my fingertips gives me so much control to manage my diabetes. I have been a Type 2 diabetic for 10 years. I sporadically manage my blood sugar with a blood glucose monitoring device. I know that if not controlled or managed effectively I can have real highs and lows and not know when this will happen. I was wearing the My Sugar Watch device and it alerted me to the fact I was about to have a hypo before it happened. This alert enabled me to quickly balance my medication . *UK Licensee users’ feedback
| |

sugarBEAT ® Sales status UK: UK: 200,000 Sensors ordered by licensee following soft launch success Purchase order forecast for (approximately) a further 100,000 sensors per month for the next 2 years, totaling over 2 million sensors. Licensee selling these based on a diabetes management subscription service.
| |

sugarBEAT ® Germany Partnership/Collaboration discussions ongoing Submission for reimbursement made to GBA, the German Regulatory Authority. GBA confirmed they do not need to review this and it is now progressing straight to the National Association of Statutory Health Insurance Funds, thus we expect the process to be a lot quicker. Plan to launch as soon as partnership agreement is signed.
| |

sugarBEAT ® MENA Partnered with TPMENA Registration for Saudi A rabia and UAE in progress Plan to launch on completion of registration
| |

| |

Type 2 Diabetes prevention and management program launched in the U.S.
| |

BEAT ® diabetes – 3 Components 1. Weight loss program originally developed at the Joslin Diabetes Centre – over 12 years of clinical evidence (based on an in - clinic program, subsequently replicated using a virtual program). Sustained long term weight loss achieved without loss of muscle mass 1. proBEAT Ρ Intermittent glucose profiling – using world’s first daily - wearable glucose sensor, developed in - house 2. Coaching: digital 24/7 using app, and specialist 1 to 1 coaching
| |

BEAT ® diabetes – Glucose Profiling Intermittent Glucose Profiling: Benefits 7 - point glucose profiles every 4 weeks. Patients received guidance for diet and exercise adjustments based on SMBG. Outcome: Significant reductions in HbA1c, weight, BMI, systolic BP, diastolic BP, and LDL Cholesterol Kempf et. al., Diabetes Technol Ther (2010)
| |

BEAT ® diabetes Potential Outcome for Payers Healthier Employees = Healthier Business $1,600 $4,700 $6,600 $9,100 Healthy (No conditions) Prediabetes only Hypertension Only Diabetes Only Annual Cost per Employee On average, diabetes costs both employers and insurers over $9,000 per year. Potential savings from prescription medications alone would amount to over $5,000 per year.
| |
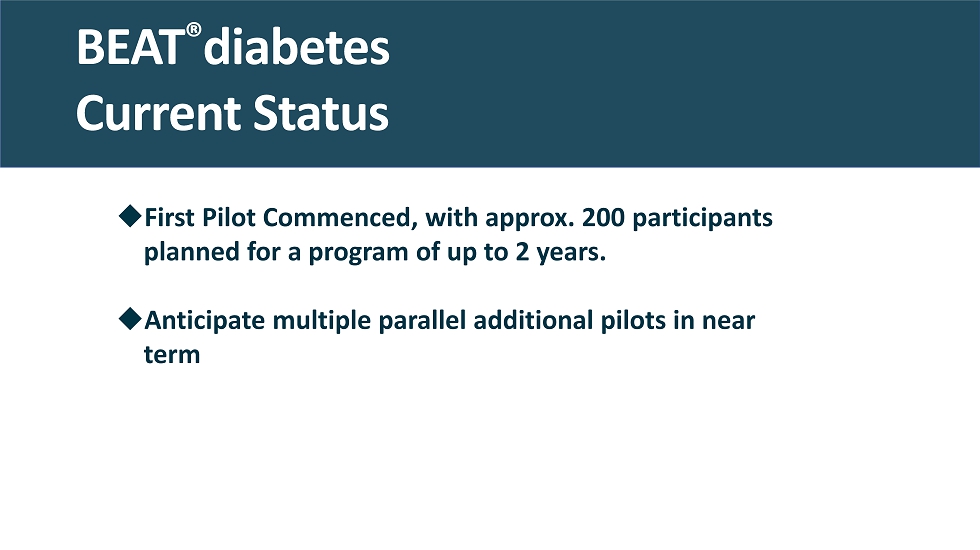
BEAT ® diabetes Current Status First Pilot Commenced, with approx. 200 participants planned for a program of up to 2 years. Anticipate multiple parallel additional pilots in near term
| |

BEAT Metabolic Health Metabolism is life. Can we help 87 MILLION people in the USA from becoming Diabetic using our low cost wearable glucose sensor and digital ecosystem?
| |

Metabolic Health A mass - market consumer product A new program leveraging off the BEAT® wearable sensor platform to address improvements in metabolic health and well - being . Launching in 2021. Applicable to over 80 million people in the US with pre - diabetes as well as general health - conscious individuals, and obesity market.
| |

CONTINUOUS LACTATE MONITORING Assists in threshold maximization in performance athletes Early identification of tissue hypoperfusion or shock for aggressive early resuscitation of critically ill patients to improve the their chances of survival BODY TEMPERATURE MONITORING Gives a more accurate and large data set. For monitoring viral infections and lower limb blood circulation tracking the effectiveness of drugs Wearable temperature sensors market is expected to register a CAGR of 8.3% during the forecast period 2021 - 2026 22 Future Product Opportunities Leveraging the BEAT ® Technology A rich portfolio of additional products to complement existing offering and contribute to increased revenues
| |

ALCOHOL MONITORING Support personal health goals and provide warnings prior to driving. Provide physicians with individual’s drinking habits . Prevention of progression - to - alcohol - related disease DRUG MONITORING Monitoring the impact of drugs and personalized treatment plan for patients. Global therapeutic drug monitoring device market to reach $3.37B by 2024 23 Future Product Opportunities Leveraging the BEAT ® Technology
| |

BIG DATA Predictive analytics based on logic drawn from wearable medical devices using algorithms to seek patterns and structure in data and cluster them into groups or insights. Improving efficiencies per patient’s management of health care. Accuracy of diagnosis and treatment in personal medicine. ARTIFICIAL INTELLIGENCE Empowering users & industry with interpretations of SugarBEAT ® data to enhance treatment & develop personalized therapy The U.S. National Institutes of Health is working with IBM to connect a wide variety of clinical and research datasets to the IBM Watson system. Big Data is projected to have significant applications the healthcare tech space. The market is expected to grow to over $68 Billion by 2024 24 Future Product Opportunities Leveraging the BEAT ® Technology
| |

The Team We are building a world class team So far includes senior level appointments, with experience from companies including: Dexcom, Lifescan , Roche, Abbvie , & Eli Lilly
| |

Intellectual Property □ Nemaura is building an extensive and valuable intellectual property portfolio to position the company to become a global leader in the non - invasive wearable sensor market . □ Nemaura has over 30 patents across several patent families (both approved and pending), and substantial trade secrets, providing a strong IP position . □ Nemaura anticipates filing multiple additional patents over the course of the next 18 months, based on ongoing developments . 1. Sensor related 2. Algorithm and methods of using the CGM data 3. Devices & methods to enhance glucose sensing 4. Methods to enhance glucose sensing 5. Devices and methods to extract glucose The Company has a number of patent families and trade secrets spanning the following:
| |

Future Milestones We expect to report the following over the coming months: - Progress with sales of sugarBEAT ® in multiple territories - Commencement of KOL studies in the UK and USA - Launch of the Metabolic Health App & Sensor Kit - Adoption of BEAT ® diabetes program/pilot studies in the USA - Update on FDA PMA application for sugarBEAT ®
| |

Cash Position As of 26 th July 2021: Strong balance sheet (>$30M, and <$2M quarterly cash burn)
| |

References
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- NHOA: Q1 2024 Trading and Operational update
- ADM Tronics Shareholder Video Conference Replay Available Now for Viewing Online with Update on Vet-Sonotron Veterinary Pain Treatment Technology
- Redfin to Announce First-Quarter 2024 Results on May 7, 2024
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share