Form 8-K ImmunoGen, Inc. For: Jan 10
Exhibit 99.1
| CONFIDENTIAL1 TARGET A BETTER NOW JP Morgan Healthcare ConferenceJanuary 10-13, 2022NASDAQ: IMGN |
| CONFIDENTIAL2 FORWARD-LOOKING STATEMENTS Thispresentationincludesforward-lookingstatementsregardingImmunoGen’scurrentexpectationsrelatedto:thedesignandpotentialsuccessofImmunoGen’smirvetuximabsoravtansine,IMGN632,IMGC936,andIMGN151preclinicalandclinicalstudiesandregulatorypathways,includingthetimingofinitiatingandreceivingdatafrom,aswellasthelikelihoodofsuccessof,thestudiesfortheseproductcandidates,includingstudiesthatareintendedtosupportregulatoryapprovalofmirvetuximabandIMGN632andthesubmissionoftheCompany'sBLAtotheFDAformirvetuximab;thepotentialofmirvetuximabtobecomeastandardofcareandtransformtheCompanyintoafullyintegratedoncologycompany;thepotentialofmirvetuximabtobecomeacombinationagentofchoice;thepresentationofpreclinicalandclinicaleventsrelatedtotheCompany'sproductcandidates,includingmirvetuximabandIMGN632;thepotentialofIMGN632tobecomeabest-in-classtherapeuticoptionforBPDCNpatientsandaproductmarketedbytheCompany;themarketopportunitiesfortheCompany’sdevelopmentprograms;theoccurrence,timing,andoutcomeofotherpotentialpreclinical,clinical,andregulatoryeventsrelatedtoImmunoGen’sanditscollaborationpartners’programs;theCompany'sbusinessandproductdevelopmentstrategies,includingtheCompany'sexpectedcashrunway;andpotentialfuturecollaborations.VariousfactorscouldcauseImmunoGen’sactualresultstodiffermateriallyfromthosediscussedorimpliedintheforward-lookingstatements,andyouarecautionednottoplaceunduerelianceontheseforward-lookingstatements,whicharecurrentonlyasofthedateofthispresentation.Weundertakenoobligationtoupdateorreviseanyoftheseforward-lookingstatements.Factorsthatcouldcausefutureresultstodiffermateriallyfromsuchexpectationsinclude,butarenotlimitedto:thattop-linedatamaychangeasmorepatientdatabecomeavailableandaresubjecttoauditandverificationprocedures;thedifficultiesinherentinthedevelopmentofnovelbiopharmaceuticals;therisksanduncertaintiesinherentintheCompany’sdevelopmentprograms,includingitspreclinicalandclinicalstudiesandregulatoryprocesses,theirtiming,expense,andresultsaswellasthepossibilitythatstudiesoftheCompany’sdevelopmentprogramsfailtoconfirmthehypothesessuggestedbyexploratoryanalysesorfailtosatisfytherequirementsforapprovalbyoneormoreregulatoryagencies;theCompany’sabilitytofinanciallysupportitsdevelopment programs;additionalmarketresearchandsourcesthatmaycausetheCompany’sexpectationsoffuturemarketopportunitiesforitsdevelopmentprogramstochange;andtherisksanduncertaintiesassociatedwiththescaleanddurationoftheCOVID-19pandemicandresultingimpactonImmunoGen’sindustryandbusiness.Areviewoftheseandotherriskscanbefoundinthe“riskfactors”setforthintheCompany’sAnnualReportonForm10-KfiledwiththeSecuritiesandExchangeCommissiononMarch1,2021,andotherreportsfiledwiththeSecuritiesandExchangeCommissionandavailableatwww.sec.govandonourwebsiteatimmunogen.com.Inaddition,asthereportedcashandcashequivalentsbalanceinthispresentationispreliminary,hasnotbeenauditedandissubjecttochangependingcompletionofourauditedfinancialstatementsfortheyearendedDecember31,2021,itispossiblethatweorourindependentregisteredpublicaccountingfirmmayidentifyitemsthatrequireustomakeadjustmentstothepreliminaryestimatedcashandcashequivalentsbalance,aswellasourexpectedcashrunway,andsuchchangescouldbematerial.AdditionalinformationanddisclosureswouldalsoberequiredforamorecompleteunderstandingofourfinancialpositionandresultsofoperationsasofDecember31,2021. |
| CONFIDENTIAL3 WHY IMMUNOGEN? POISED TO BECOME A FULLY-INTEGRATED ONCOLOGY COMPANYWITH FIRST COMMERCIAL LAUNCH EXPECTED THISYEAR ACCELERATED PATH FOR MIRVETUXIMAB IN PROCPIVOTAL SORAYA STUDY MET PRIMARY ENDPOINTPREPARING BLA SUBMISSION MOVING MIRVETUXIMABINTO BROAD OVARIAN CANCER POPULATIONSPURSUING STUDIES SUPPORTIVEOF LABEL EXPANSION DEFINED PATH FOR IMGN632 FULL APPROVAL IN BPDCNANTICIPATE TOP-LINE BPDCN DATA IN H2 2022ADVANCING AML TRIPLET INNOVATIVE EARLIER STAGE CANDIDATES AND ADVANCED ADC TECHNOLOGYEXPECT IMGN936 PH 1 DATA IN 2022AND IMGN151 FPI IN H1 2022 EXPERIENCED LEADERSHIP AND STRONG CASH POSITION TO SUPPORT COMMERCIAL AND MEDICAL BUILDEXPECTED CASH RUNWAY INTO 2024PROC: platinum-resistant ovarian cancer; BLA: Biologics License Application; BPDCN: blastic plasmacytoid dendritic cell neoplasm; AML: acute myeloid leukemia; ADC: antibody-drug conjugate; PH: phase; FPI: first patient in |
| CONFIDENTIAL4 SIGNIFICANTLY ADVANCEDTHE BUSINESS IN 2021RECENT ACCOMPLISHMENTSMIRVETUXIMAB SORAVTANSINE•Reported positive topline pivotal data from SORAYA •Continued enrollment in MIRASOL•Initiated PICCOLO for patients with FRα-high recurrent platinum-sensitive ovarian cancer•Supported enrollment in mirvetuximab + carboplatin combination ISTs •Presented mature mirvetuximab + bevacizumab combination data in oral session at ASCO 2021•Aligned with FDA on randomized Phase 3 trial for mirvetuximab + bevacizumab in FRα-high platinum sensitive ovarian cancer in the maintenance setting•Advanced collaboration with Huadong Medicine, with first patient enrolled in development program for Greater ChinaIMGN632•Presented initial IMGN632 + venetoclax + azacitidine data in AML in oral session and initial frontline BPDCN data in poster session at ASH 2021 •Continued enrollment in the pivotal CADENZA trial in frontline and R/R BPDCNIMGC936•Presented preclinical data at AACR •Continued dose escalation in Phase 1 studyIMGN151•Submitted IND LEADERSHIP AND FINANCIALS •Appointed Kristen Harrington-Smith as CCO, and Dr. Helen M. Thackray and Tracey L. McCain, Esq. to Board of Directors•Raised gross proceeds of $295.7 million in public offering •~$475M in cash and cash equivalents on hand as of December 31, with runway expected into 20244 FRα: folate receptor alpha; ISTs: investigator-sponsored trials; ASCO: American Society of Clinical Oncology; FDA: US Food and DrugAdministration; AML: acute myeloid leukemia; ASH: American Society of HematologyR/R: relapsed/refractory; BPDCN: blastic plasmacytoid dendritic cell neoplasm; AACR: American Association for Cancer Research; IND: investigational new drug application; CCO: Chief Commercial Officer |
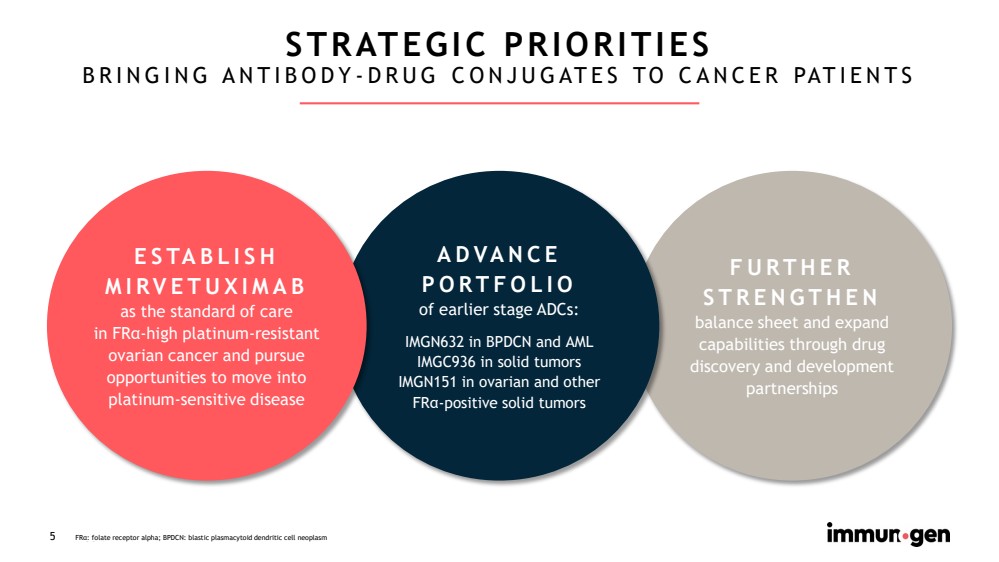
| CONFIDENTIAL5 FURTHER STRENGTHEN balance sheet and expand capabilities through drug discovery and development partnerships ADVANCEPORTFOLIO of earlier stage ADCs: IMGN632 in BPDCN and AMLIMGC936 in solid tumors IMGN151 in ovarian and other FRα-positive solid tumorsSTRATEGIC PRIORITIESBRINGING ANTIBODY-DRUG CONJUGATES TO CANCER PATIENTS ESTABLISH MIRVETUXIMAB as the standard of carein FRα-highplatinum-resistant ovarian cancer and pursue opportunities to move into platinum-sensitive disease FRα: folate receptor alpha; BPDCN: blastic plasmacytoid dendritic cell neoplasm |
| CONFIDENTIAL6 WHAT’S NEXT FOR HER?Someone you know has been diagnosed with ovarian cancer…6 |
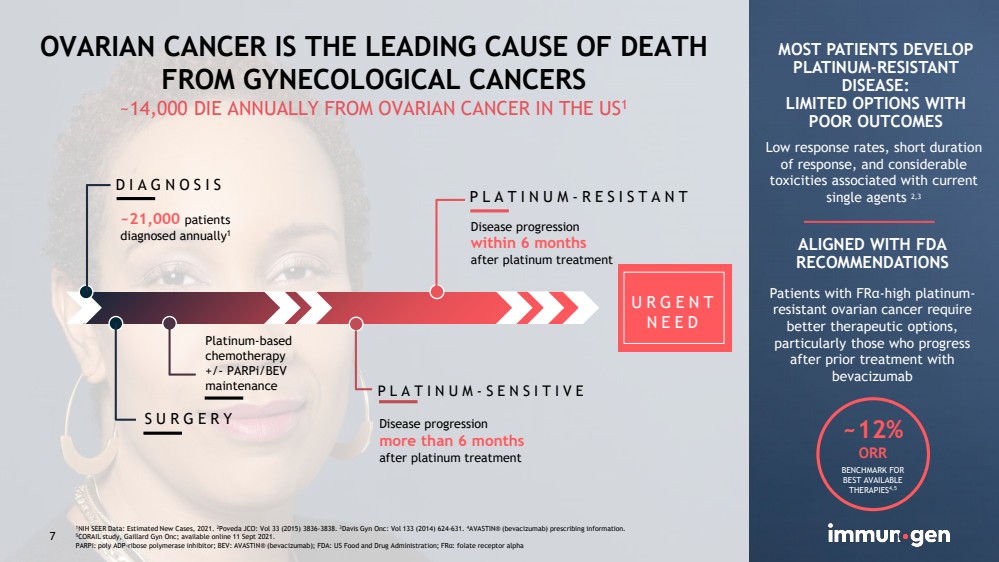
| CONFIDENTIAL7 OVARIAN CANCER IS THE LEADING CAUSE OF DEATHFROM GYNECOLOGICAL CANCERS~14,000 DIE ANNUALLY FROM OVARIAN CANCER IN THE US1 DIAGNOSISSURGERYPLATINUM-SENSITIVEPLATINUM-RESISTANTPlatinum-based chemotherapy+/-PARPi/BEVmaintenance ~21,000 patientsdiagnosed annually1Disease progressionwithin 6 monthsafter platinum treatmentDisease progression more than 6 months afterplatinum treatmentMOST PATIENTS DEVELOP PLATINUM-RESISTANT DISEASE:LIMITED OPTIONS WITH POOR OUTCOMESLow response rates, short duration of response, and considerable toxicities associated with current single agents 2,3 ALIGNED WITH FDARECOMMENDATIONS ~12% ORRBENCHMARK FOR BEST AVAILABLE THERAPIES4,51NIH SEER Data: Estimated New Cases, 2021. 2Poveda JCO: Vol 33 (2015) 3836-3838. 3Davis Gyn Onc: Vol 133 (2014) 624-631. 4AVASTIN® (bevacizumab) prescribing information.5CORAIL study, Gaillard Gyn Onc; available online 11 Sept 2021.PARPi: poly ADP-ribose polymerase inhibitor; BEV: AVASTIN® (bevacizumab); FDA: US Food and Drug Administration; FRα: folate receptor alpha URGENT NEED7 Patients with FRα-high platinum-resistant ovarian cancer require better therapeutic options, particularly those who progress after prior treatment with bevacizumab |
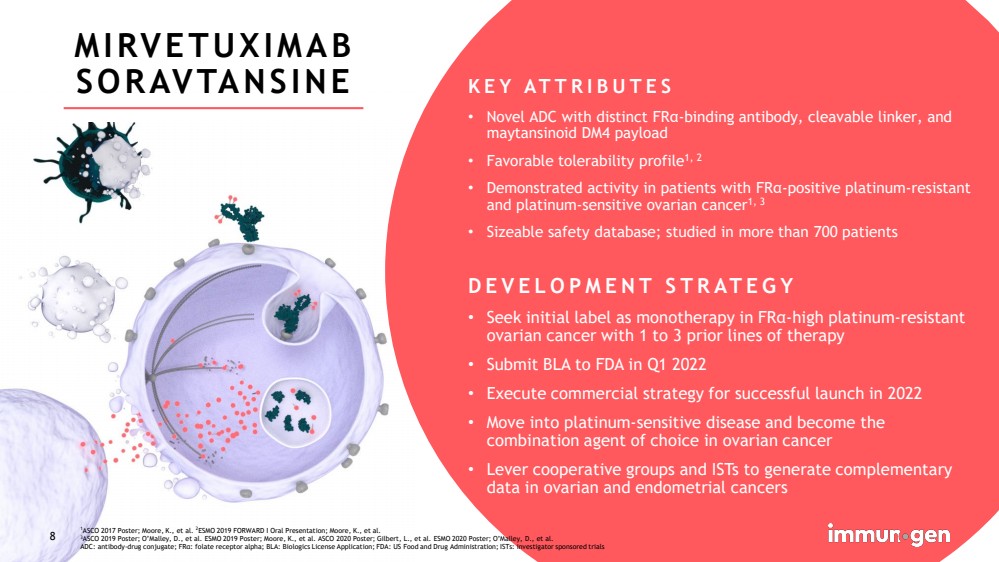
| CONFIDENTIAL8 MIRVETUXIMAB SORAVTANSINE KEY ATTRIBUTES•Novel ADC with distinct FRα-binding antibody, cleavable linker, and maytansinoidDM4 payload•Favorable tolerability profile1, 2•Demonstrated activity in patients with FRα-positive platinum-resistant and platinum-sensitive ovarian cancer1, 3•Sizeable safety database; studied in more than 700 patientsDEVELOPMENT STRATEGY•Seek initial label as monotherapy in FRα-high platinum-resistant ovarian cancer with 1 to 3 prior lines of therapy•Submit BLA to FDA in Q1 2022•Execute commercial strategy for successful launch in 2022•Move into platinum-sensitive disease and become the combination agent of choice in ovarian cancer•Lever cooperative groups and ISTs to generate complementary data in ovarian and endometrial cancers 1ASCO 2017 Poster; Moore, K., et al. 2ESMO 2019 FORWARD I Oral Presentation; Moore, K., et al. 3ASCO 2019 Poster; O’Malley, D., et al. ESMO 2019 Poster; Moore, K., et al. ASCO 2020 Poster; Gilbert, L., et al. ESMO 2020 Poster; O’Malley, D., et al.ADC: antibody-drug conjugate; FRα: folate receptor alpha; BLA: Biologics License Application; FDA: US Food and Drug Administration; ISTs: investigator sponsoredtrials8 |
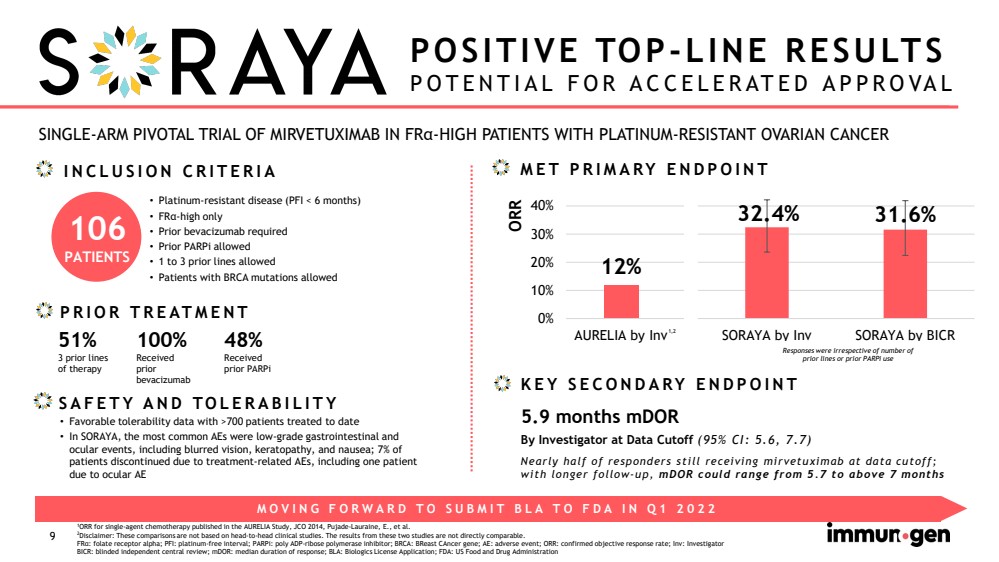
| CONFIDENTIAL9 SINGLE-ARM PIVOTAL TRIAL OF MIRVETUXIMAB IN FRα-HIGH PATIENTS WITH PLATINUM-RESISTANT OVARIAN CANCERPOSITIVE TOP-LINE RESULTSPOTENTIAL FOR ACCELERATED APPROVAL •Platinum-resistant disease (PFI < 6 months)•FRα-high only•Prior bevacizumab required•Prior PARPiallowed•1 to 3 prior lines allowed•Patients with BRCA mutations allowedPRIOR TREATMENT 1ORR for single-agent chemotherapy published in the AURELIA Study, JCO2014, Pujade-Lauraine, E., et al.2Disclaimer: These comparisons are not based on head-to-head clinical studies. The results from these two studies are not directly comparable.FRα: folate receptor alpha; PFI: platinum-free interval; PARPi: poly ADP-ribose polymerase inhibitor; BRCA: BReastCAncergene; AE: adverse event; ORR: confirmed objective response rate; Inv: InvestigatorBICR: blinded independent central review; mDOR: median duration of response; BLA: Biologics License Application; FDA: US Food and Drug AdministrationSAFETY AND TOLERABILITY MOVING FORWARD TO SUBMIT BLA TO FDA IN Q1 2022 INCLUSION CRITERIA Responses were irrespective of number of prior lines or prior PARPi useMET PRIMARY ENDPOINTKEY SECONDARY ENDPOINT ORRBy Investigator at Data Cutoff (95% CI: 5.6, 7.7)Nearly half of responders still receiving mirvetuximab at data cutoff; with longer follow-up, mDORcould range from 5.7 to above 7 months5.9 months mDOR100%Received prior bevacizumab51%3 prior linesof therapy•Favorable tolerability data with >700 patients treated to date •In SORAYA, the most common AEs were low-grade gastrointestinal and ocular events, including blurred vision, keratopathy, and nausea; 7% of patients discontinued due to treatment-related AEs, including one patient due to ocular AE48%Received prior PARPi 106PATIENTS 12%32.4%31.6%0%10%20%30%40%AURELIA by InvSORAYA by InvSORAYA by BICR1,2 32.4%31.6%SORAYA by InvSORAYA by BICR Responses were irrespective of number of prior lines or prior PARPiuse |
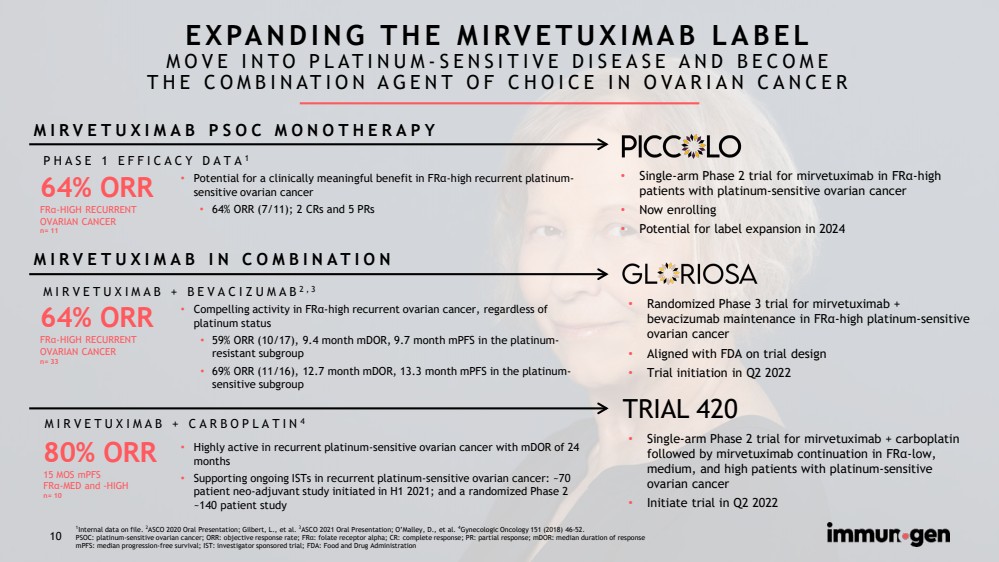
| CONFIDENTIAL10 EXPANDING THE MIRVETUXIMAB LABELMOVE INTO PLATINUM-SENSITIVE DISEASE AND BECOMETHE COMBINATION AGENT OF CHOICE IN OVARIAN CANCER 64% ORRFRα-HIGH RECURRENTOVARIAN CANCERn= 3380% ORR15 MOS mPFSFRα-MED and -HIGHn= 1064% ORRFRα-HIGH RECURRENTOVARIAN CANCERn= 11MIRVETUXIMAB + BEVACIZUMAB2,3MIRVETUXIMAB + CARBOPLATIN4•Compelling activity in FRα-high recurrent ovarian cancer, regardless of platinum status•59% ORR (10/17), 9.4 month mDOR, 9.7 month mPFSin the platinum-resistant subgroup•69% ORR (11/16), 12.7 month mDOR, 13.3 month mPFSin the platinum-sensitive subgroup•Highly active in recurrent platinum-sensitive ovarian cancer with mDORof 24 months•Supporting ongoing ISTs in recurrent platinum-sensitive ovarian cancer: ~70 patient neo-adjuvant study initiated in H1 2021; and a randomized Phase 2 ~140 patient studyPHASE 1 EFFICACY DATA1•Potential for a clinically meaningful benefit in FRα-high recurrent platinum-sensitive ovarian cancer •64% ORR (7/11); 2 CRs and 5 PRsMIRVETUXIMAB IN COMBINATIONMIRVETUXIMAB PSOC MONOTHERAPY •Single-arm Phase 2 trial for mirvetuximab in FRα-high patients with platinum-sensitive ovarian cancer•Now enrolling•Potential for label expansion in 2024•Randomized Phase 3 trial for mirvetuximab + bevacizumab maintenance in FRα-high platinum-sensitive ovarian cancer•Aligned with FDA on trial design•Trial initiation in Q2 2022•Single-arm Phase 2 trial for mirvetuximab + carboplatin followed by mirvetuximab continuation in FRα-low, medium, and high patients with platinum-sensitive ovarian cancer •Initiate trial in Q2 2022 TRIAL 4201Internal data on file. 2ASCO 2020 Oral Presentation; Gilbert, L., et al. 3ASCO 2021 Oral Presentation; O’Malley, D., et al. 4Gynecologic Oncology 151 (2018) 46-52. PSOC: platinum-sensitive ovarian cancer; ORR: objective response rate; FRα: folate receptor alpha; CR: complete response; PR: partial response; mDOR: median duration of response mPFS: median progression-free survival; IST: investigator sponsored trial; FDA: Food and Drug Administration 10 |
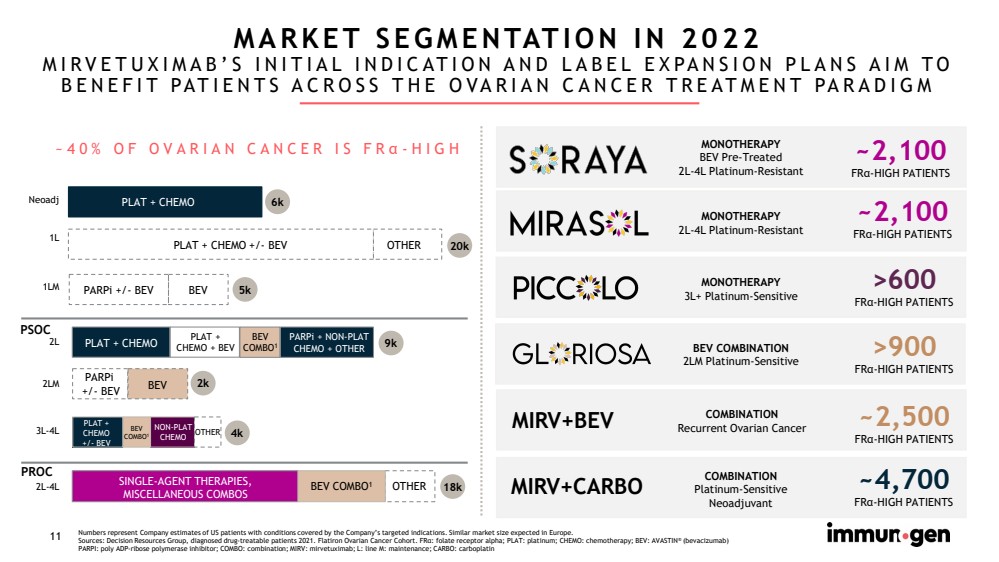
| CONFIDENTIAL11 Numbers represent Company estimates of US patients with conditions covered by the Company’s targeted indications. Similar marketsize expected in Europe.Sources: Decision Resources Group, diagnosed drug-treatable patients 2021. Flatiron Ovarian Cancer Cohort. FRα: folate receptor alpha; PLAT: platinum; CHEMO: chemotherapy; BEV: AVASTIN®(bevacizumab)PARPI: poly ADP-ribose polymerase inhibitor; COMBO: combination; MIRV: mirvetuximab; L: line M: maintenance; CARBO: carboplatin MIRV+CARBO~4,700FRα-HIGH PATIENTS BEV COMBINATION2LM Platinum-Sensitive >900FRα-HIGH PATIENTS>600FRα-HIGH PATIENTS MONOTHERAPY3L+ Platinum-SensitiveMARKET SEGMENTATION IN 2022MIRVETUXIMAB’S INITIAL INDICATION AND LABEL EXPANSION PLANS AIM TO BENEFIT PATIENTS ACROSS THE OVARIAN CANCER TREATMENT PARADIGM ~2,100FRα-HIGH PATIENTSMONOTHERAPY2L-4L Platinum-Resistant2L 9k2L-4L 18k SINGLE-AGENT THERAPIES, MISCELLANEOUS COMBOSPLAT + CHEMO OTHER 1L 20k PLAT + CHEMO +/-BEVOTHERNeoadj 6kPLAT + CHEMO BEV COMBO12LMPARPi +/-BEV 3L-4L 4k NON-PLAT CHEMO PLAT +CHEMO+/-BEV OTHER PARPi+/-BEV 1LM BEV PARPi+/-BEV BEV 5k 2k BEV COMBO1BEV COMBO1 PLAT + CHEMO + BEV PARPi+ NON-PLAT CHEMO + OTHER ~40% OF OVARIAN CANCER IS FRα-HIGHMIRV+BEV~2,500FRα-HIGH PATIENTS MONOTHERAPYBEV Pre-Treated2L-4L Platinum-Resistant~2,100FRα-HIGH PATIENTSCOMBINATIONRecurrent Ovarian CancerPSOCPROCCOMBINATIONPlatinum-SensitiveNeoadjuvant |
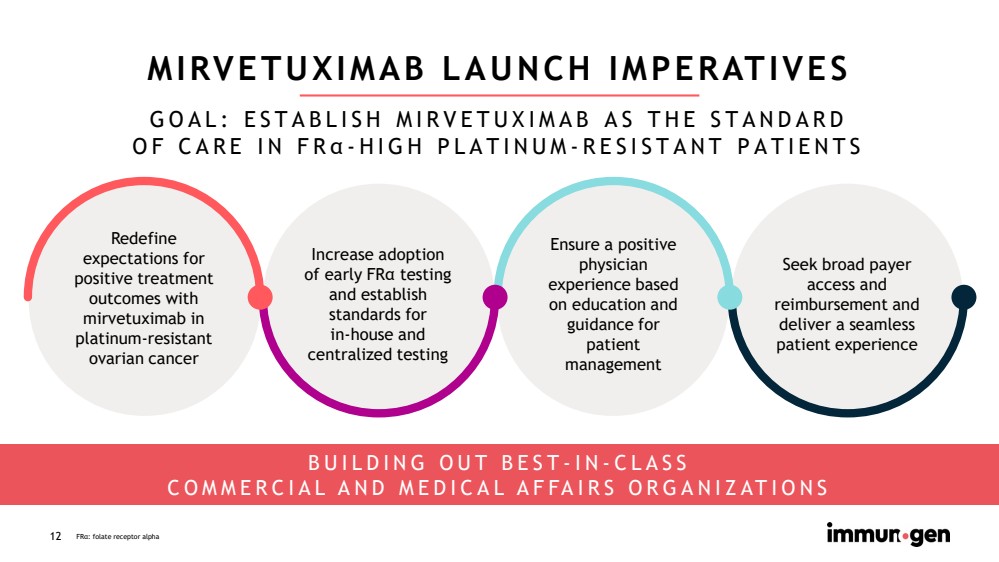
| CONFIDENTIAL12 MIRVETUXIMAB LAUNCH IMPERATIVES Redefineexpectations for positive treatment outcomes with mirvetuximabin platinum-resistant ovarian cancerSeek broad payer access and reimbursement and deliver a seamless patient experienceEnsure a positive physician experience based on education and guidance for patient management BUILDING OUT BEST-IN-CLASSCOMMERCIAL AND MEDICAL AFFAIRS ORGANIZATIONSFRα: folate receptor alpha GOAL: ESTABLISH MIRVETUXIMAB AS THE STANDARDOF CARE IN FRα-HIGH PLATINUM-RESISTANT PATIENTSIncrease adoption of early FRα testing and establish standards forin-house and centralized testing |
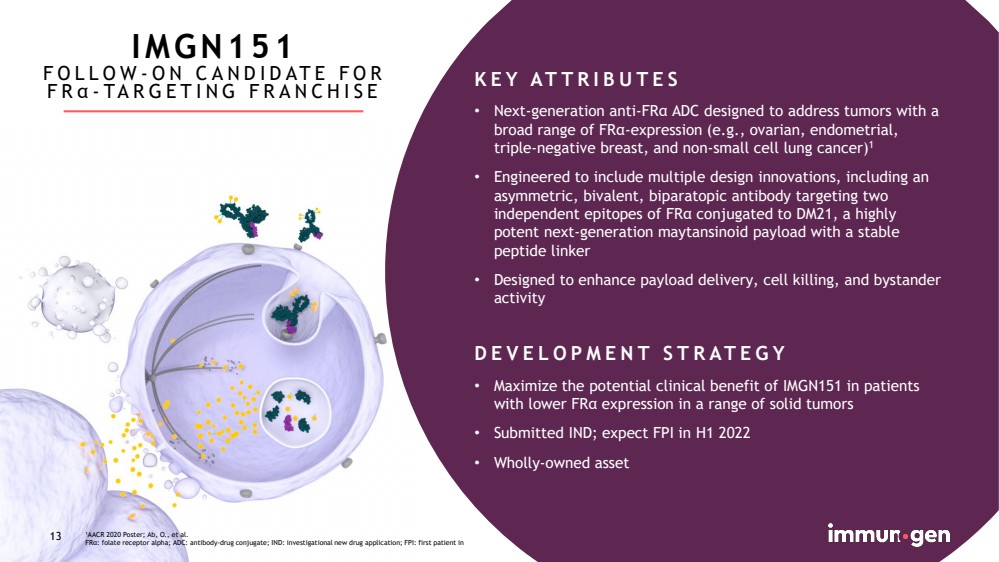
| CONFIDENTIAL13 1AACR 2020 Poster; Ab, O., et al.FRα: folate receptor alpha; ADC: antibody-drug conjugate; IND: investigational new drug application; FPI: first patient in13KEY ATTRIBUTES•Next-generation anti-FRα ADC designed to address tumors with a broad range of FRα-expression (e.g., ovarian, endometrial, triple-negative breast, and non-small cell lung cancer)1•Engineered to include multiple design innovations, including an asymmetric, bivalent, biparatopic antibody targeting two independent epitopes of FRα conjugated to DM21, a highly potent next-generation maytansinoid payload with a stable peptide linker•Designed to enhance payload delivery, cell killing, and bystander activityDEVELOPMENT STRATEGY•Maximize the potential clinical benefit of IMGN151 in patients with lower FRαexpression in a range of solid tumors•Submitted IND; expect FPI in H1 2022•Wholly-owned assetIMGN151FOLLOW-ON CANDIDATE FOR FRα-TARGETING FRANCHISE |
| CONFIDENTIAL14 14 WHAT’S NEXT FOR THEM?Someone you know has been diagnosed with a hematologic malignancy… |
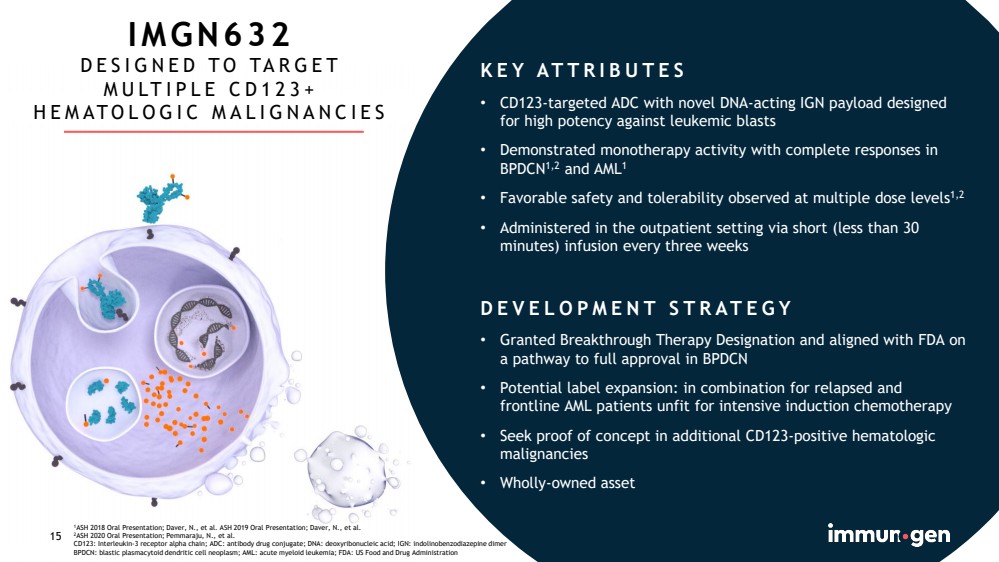
| CONFIDENTIAL15 IMGN632DESIGNED TO TARGET MULTIPLE CD123+ HEMATOLOGIC MALIGNANCIES1ASH 2018 Oral Presentation; Daver, N., et al. ASH 2019 Oral Presentation; Daver, N., et al.2ASH 2020 Oral Presentation; Pemmaraju, N., et al.CD123: Interleukin-3 receptor alpha chain; ADC: antibody drug conjugate; DNA: deoxyribonucleic acid; IGN: indolinobenzodiazepinedimerBPDCN: blastic plasmacytoid dendritic cell neoplasm; AML: acute myeloid leukemia; FDA: US Food and Drug Administration15KEY ATTRIBUTES•CD123-targeted ADC with novel DNA-acting IGN payload designed for high potency against leukemic blasts•Demonstrated monotherapy activity with complete responses in BPDCN1,2and AML1•Favorable safety and tolerability observed at multiple dose levels1,2•Administered in the outpatient setting via short (less than 30 minutes) infusion every three weeksDEVELOPMENT STRATEGY•Granted Breakthrough Therapy Designation and aligned with FDA on a pathway to full approval in BPDCN•Potential label expansion: in combination for relapsed and frontline AML patients unfit for intensive induction chemotherapy•Seek proof of concept in additional CD123-positive hematologic malignancies•Wholly-owned asset |
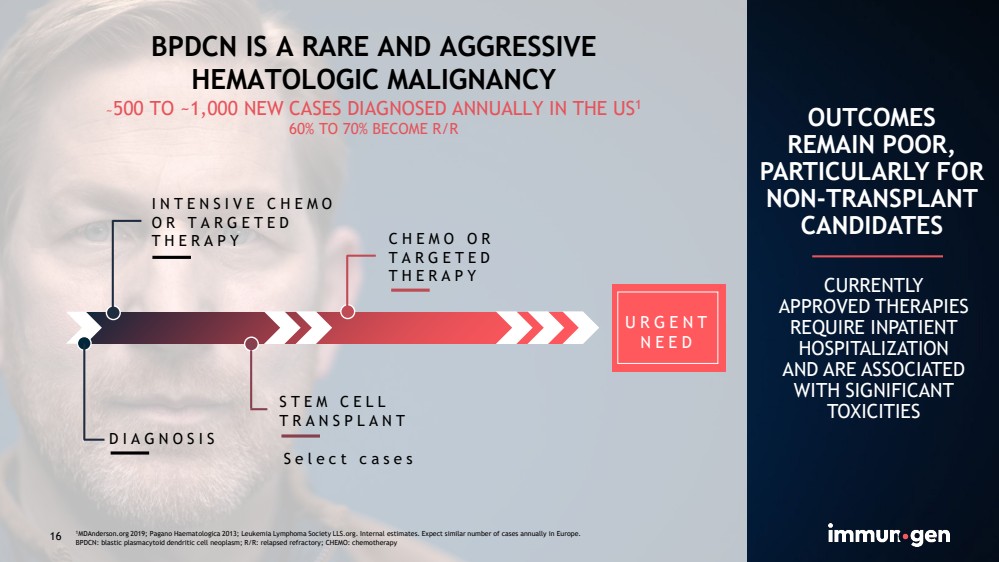
| CONFIDENTIAL16 BPDCN IS A RARE AND AGGRESSIVEHEMATOLOGIC MALIGNANCY~500 TO ~1,000 NEW CASES DIAGNOSED ANNUALLY IN THE US160% TO 70% BECOME R/R INTENSIVE CHEMO OR TARGETED THERAPYDIAGNOSISSTEM CELL TRANSPLANTCHEMO OR TARGETED THERAPY Select casesOUTCOMESREMAIN POOR, PARTICULARLY FOR NON-TRANSPLANT CANDIDATES CURRENTLY APPROVED THERAPIES REQUIRE INPATIENT HOSPITALIZATIONAND ARE ASSOCIATED WITH SIGNIFICANT TOXICITIES1MDAnderson.org 2019; Pagano Haematologica2013; Leukemia Lymphoma Society LLS.org. Internal estimates. Expect similar number of cases annually in Europe.BPDCN: blastic plasmacytoid dendritic cell neoplasm; R/R: relapsed refractory; CHEMO: chemotherapy URGENT NEED 16 |
| CONFIDENTIAL17 IMGN632: ALIGNED WITH FDA ON PATH TO FULL APPROVAL IN BPDCN1ASH 2020 Oral Presentation; Pemmaraju, N., et al. 2ASH 2021 Abstract #1284; Pemmaraju, N., et al.FDA: US Food and Drug Administration; BPDCN: blastic plasmacytoid dendritic cell neoplasm; TEAE: treatment emergent adverse event; R/R: relapsed/refractory; ORR: objective response rate; CR: complete response *CRc: clinical CR = CR criteria EXCEPT limited residual skin disease “marked clearance of all skin lesions from baseline; residual hyperpigmentation or abnormality with BPDCN identified on biopsy (or no biopsy performed)”; CRi: complete remission with incomplete hematologic recovery; PR: partial response; CCR:CR+CRc+CRiFAVORABLE SAFETY PROFILE1•No capillary leak syndrome•No drug-related discontinuations•No drug-related deaths at 30 days•Limited grade ≥3 TEAEsEFFICACY DATA1In all R/R BPDCN patients:•ORR: 29% (8/28, 2 CR, 2 CRc, 1 CRi, 3 PR)•CCR: 18% (5/28)In patients with prior tagraxofuspexposure:•ORR: 31% (4/13, 1 CR, 1CRi, 2 PR)•CCR: 15% (2/13)In frontline BPDCN, 3/3 patients with CRc2COMPELLING PRELIMINARY DATA IN BPDCN801 STUDY: SINGLE-ARM PIVOTAL COHORT IN FRONTLINE BPDCN •Enrolling in the US and EU; up to 20 frontline patients to support label •Top-line data expected H2 2022•Potential to become best-in-class therapeutic option and the Company’s second marketed product in rare oncology 17 |
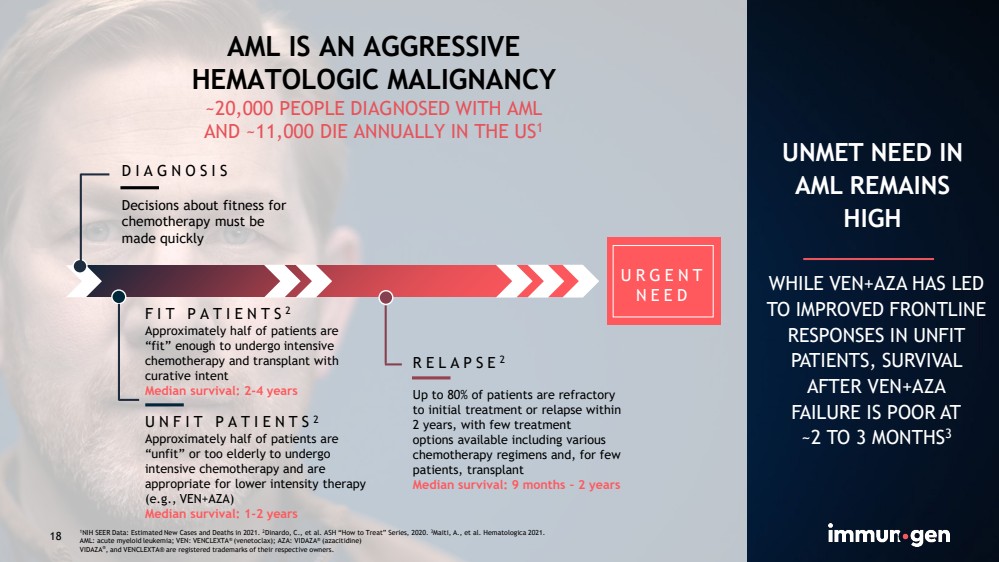
| CONFIDENTIAL18 AML IS AN AGGRESSIVEHEMATOLOGIC MALIGNANCY~20,000 PEOPLE DIAGNOSED WITH AMLAND ~11,000 DIE ANNUALLY IN THE US1 FIT PATIENTS2Approximately half of patients are“fit” enough to undergo intensive chemotherapy and transplant with curative intent Median survival: 2-4 yearsUNFIT PATIENTS2Approximately half of patients are “unfit” or too elderly to undergo intensive chemotherapy and are appropriate for lower intensity therapy (e.g., VEN+AZA)Median survival: 1-2 yearsDIAGNOSISDecisions about fitness for chemotherapy must be made quicklyRELAPSE2Up to 80% of patients are refractoryto initial treatment or relapse within 2 years, with few treatmentoptions available including various chemotherapy regimens and, for few patients, transplant Median survival: 9 months –2 years UNMET NEED IN AML REMAINS HIGH WHILE VEN+AZA HAS LED TO IMPROVED FRONTLINE RESPONSES IN UNFIT PATIENTS, SURVIVAL AFTER VEN+AZAFAILURE IS POOR AT~2TO 3MONTHS3 URGENT NEED 181NIH SEER Data: Estimated New Cases and Deaths in 2021. 2Dinardo, C., et al. ASH “How to Treat” Series, 2020. 3Maiti, A., et al. Hematologica2021.AML: acute myeloid leukemia; VEN: VENCLEXTA®(venetoclax); AZA: VIDAZA®(azacitidine)VIDAZA®, and VENCLEXTA® are registered trademarks of their respective owners. |
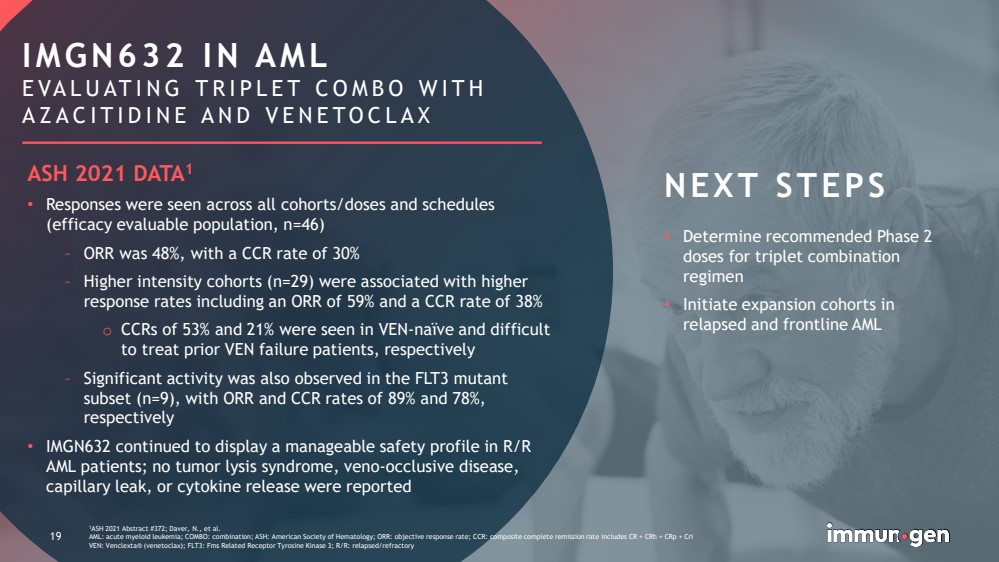
| CONFIDENTIAL19 1ASH 2021 Abstract #372; Daver, N., et al. AML: acute myeloid leukemia; COMBO: combination; ASH: American Society of Hematology; ORR: objective response rate; CCR: composite complete remission rate includes CR + CRh+ CRp+ CriVEN: Venclexta® (venetoclax); FLT3: FmsRelated Receptor Tyrosine Kinase 3; R/R: relapsed/refractory •Determine recommended Phase 2 doses for triplet combination regimen •Initiate expansion cohorts in relapsed and frontline AML 19IMGN632 IN AMLEVALUATING TRIPLET COMBO WITH AZACITIDINE AND VENETOCLAXNEXT STEPSASH 2021 DATA1•Responses were seen across all cohorts/doses and schedules (efficacy evaluable population, n=46)–ORR was 48%, with a CCR rate of 30%–Higher intensity cohorts (n=29) were associated with higher response rates including an ORR of 59% and a CCR rate of 38%oCCRs of 53% and 21% were seen in VEN-naïve and difficult to treat prior VEN failure patients, respectively–Significant activity was also observed in the FLT3 mutant subset (n=9), with ORR and CCR rates of 89% and 78%, respectively•IMGN632 continued to display a manageable safety profile in R/R AML patients; no tumor lysis syndrome, veno-occlusive disease, capillary leak, or cytokine release were reported |
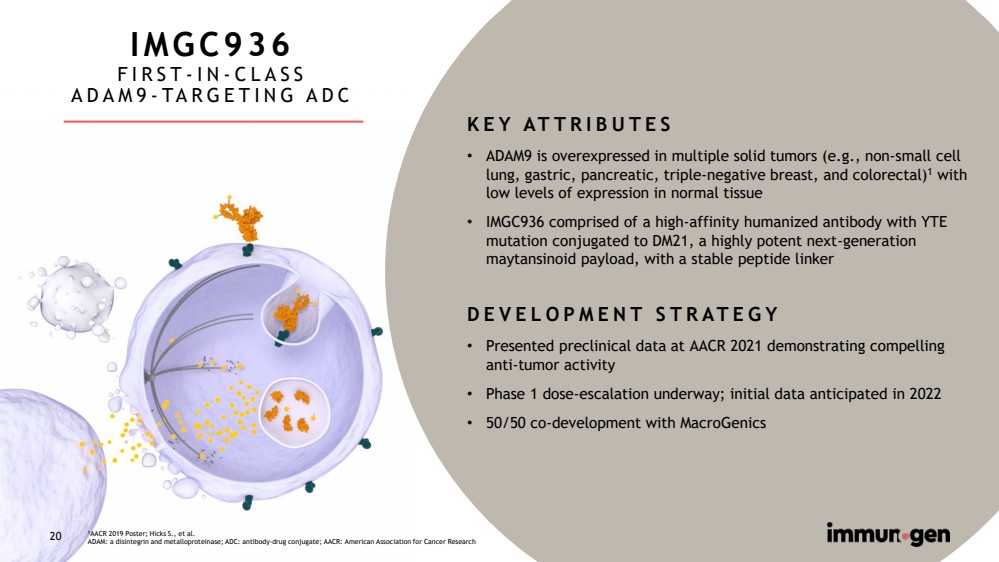
| CONFIDENTIAL20 IMGC936FIRST-IN-CLASSADAM9-TARGETING ADCKEY ATTRIBUTES•ADAM9 is overexpressed in multiple solid tumors (e.g., non-small cell lung, gastric, pancreatic, triple-negative breast, and colorectal)1with low levels of expression in normal tissue•IMGC936 comprised of a high-affinity humanized antibody with YTE mutation conjugated to DM21, a highly potent next-generation maytansinoid payload, with a stable peptide linkerDEVELOPMENT STRATEGY•Presented preclinical data at AACR 2021 demonstrating compelling anti-tumor activity •Phase 1 dose-escalation underway; initial data anticipated in 2022•50/50 co-development with MacroGenics1AACR 2019 Poster; Hicks S., et al.ADAM: a disintegrinand metalloproteinase; ADC: antibody-drug conjugate; AACR: American Association for Cancer Research20 |
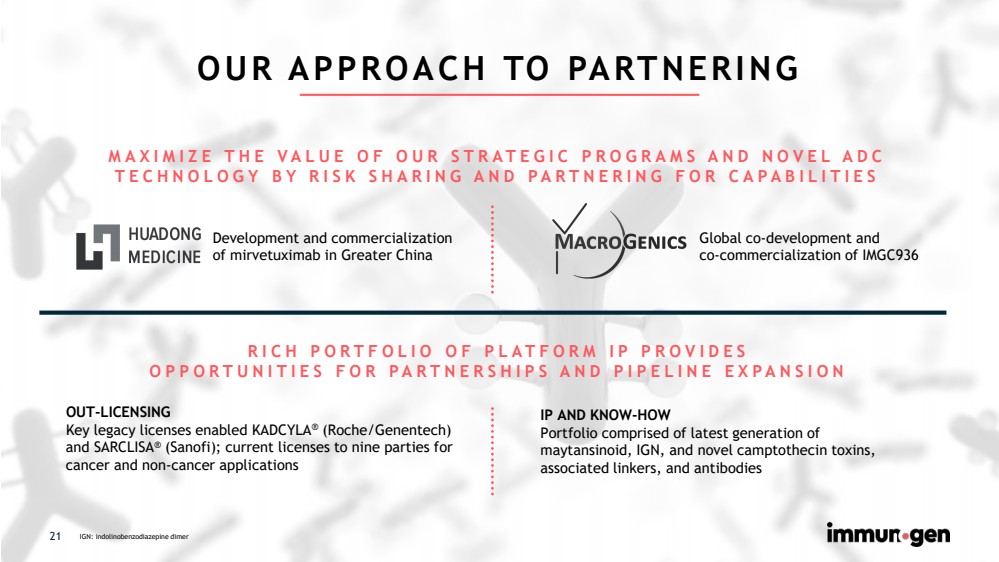
| CONFIDENTIAL21 OUR APPROACH TO PARTNERINGMAXIMIZE THE VALUE OF OUR STRATEGIC PROGRAMS AND NOVEL ADCTECHNOLOGY BY RISK SHARING AND PARTNERING FOR CAPABILITIES IGN: indolinobenzodiazepinedimer Development and commercialization of mirvetuximab in Greater China Global co-development and co-commercialization of IMGC936 OUT-LICENSINGKey legacy licenses enabled KADCYLA®(Roche/Genentech) and SARCLISA®(Sanofi); current licenses to nine parties for cancer and non-cancer applicationsRICH PORTFOLIO OF PLATFORM IP PROVIDES OPPORTUNITIES FOR PARTNERSHIPS AND PIPELINE EXPANSION IP AND KNOW-HOWPortfolio comprised of latest generation of maytansinoid, IGN, and novel camptothecin toxins, associated linkers, and antibodies 21 |
| CONFIDENTIAL22 TARGET A BETTER NOW POSITIVE TOP-LINE DATA GENERATED FOR LEAD MIRVETUXIMAB PROGRAMPLAN TO SUBMIT BLA IN Q1 2022 AND POTENTIAL ACCELERATED APPROVAL IN H2 2022INNOVATIVE EARLIER STAGE CANDIDATES IN SOLID TUMORSIMGC936: FIRST-IN-CLASS ADAM9-TARGETING ADC IN THE CLINIC IMGN151: NEXT-GENERATION FRα-TARGETING ADC BUILDS UPON MIRVETUXIMAB FRANCHISEADVANCING TO BECOME A FULLY-INTEGRATED ONCOLOGY COMPANYPREPARING FOR ANTICIPATED COMMERCIAL LAUNCH IN 2022EXPERIENCED MANAGEMENT TEAM AND STRONG CASH POSITION WITH EXPECTED RUNWAY INTO 2024 PATH TO FULL APPROVAL FOR IMGN632 IN BPDCNEXPECT TOP-LINE DATA IN H2 2022ADVANCING TRIPLET COMBINATION IN AML 22BLA: Biologics License Application; BPDCN: blastic plasmacytoid dendritic cell neoplasm; AML: acute myeloid leukemia; ADAM: a disintegrinand metalloproteinase;ADC: antibody-drug conjugate; FRα: folate receptor alpha |
| CONFIDENTIAL23 Appendix |
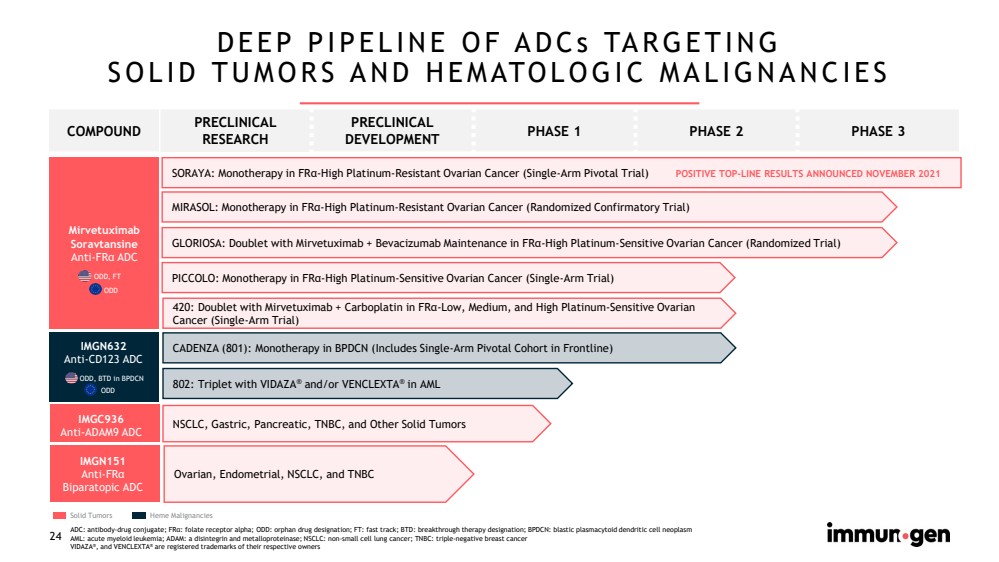
| CONFIDENTIAL24 DEEP PIPELINE OF ADCs TARGETINGSOLID TUMORS AND HEMATOLOGIC MALIGNANCIES ODD, FTODD ODD, BTDin BPDCNODD ADC: antibody-drug conjugate; FRα: folate receptor alpha; ODD: orphan drug designation; FT: fast track; BTD: breakthrough therapy designation; BPDCN: blastic plasmacytoid dendritic cell neoplasmAML: acute myeloid leukemia; ADAM: a disintegrinand metalloproteinase; NSCLC: non-small cell lung cancer; TNBC: triple-negative breast cancerVIDAZA®, and VENCLEXTA®are registered trademarks of their respective ownersSolid Tumors SORAYA: Monotherapy in FRα-High Platinum-Resistant Ovarian Cancer (Single-Arm Pivotal Trial) NSCLC, Gastric, Pancreatic, TNBC, and Other Solid Tumors CADENZA (801): Monotherapy in BPDCN (Includes Single-Arm Pivotal Cohort in Frontline) MIRASOL: Monotherapy in FRα-High Platinum-Resistant Ovarian Cancer (Randomized Confirmatory Trial) PICCOLO: Monotherapy in FRα-High Platinum-Sensitive Ovarian Cancer (Single-Arm Trial) Ovarian, Endometrial, NSCLC, and TNBC 802: Triplet with VIDAZA®and/or VENCLEXTA®in AMLPOSITIVE TOP-LINE RESULTS ANNOUNCED NOVEMBER 2021Heme Malignancies GLORIOSA: Doublet with Mirvetuximab + Bevacizumab Maintenance in FRα-High Platinum-Sensitive Ovarian Cancer (Randomized Trial)MirvetuximabSoravtansineAnti-FRαADCIMGN632Anti-CD123ADCIMGC936Anti-ADAM9ADCIMGN151Anti-FRαBiparatopicADC 420: Doublet with Mirvetuximab + Carboplatin in FRα-Low, Medium, and High Platinum-Sensitive Ovarian Cancer (Single-Arm Trial) COMPOUNDPRECLINICAL RESEARCHPRECLINICAL DEVELOPMENTPHASE 1PHASE 2PHASE 3 |
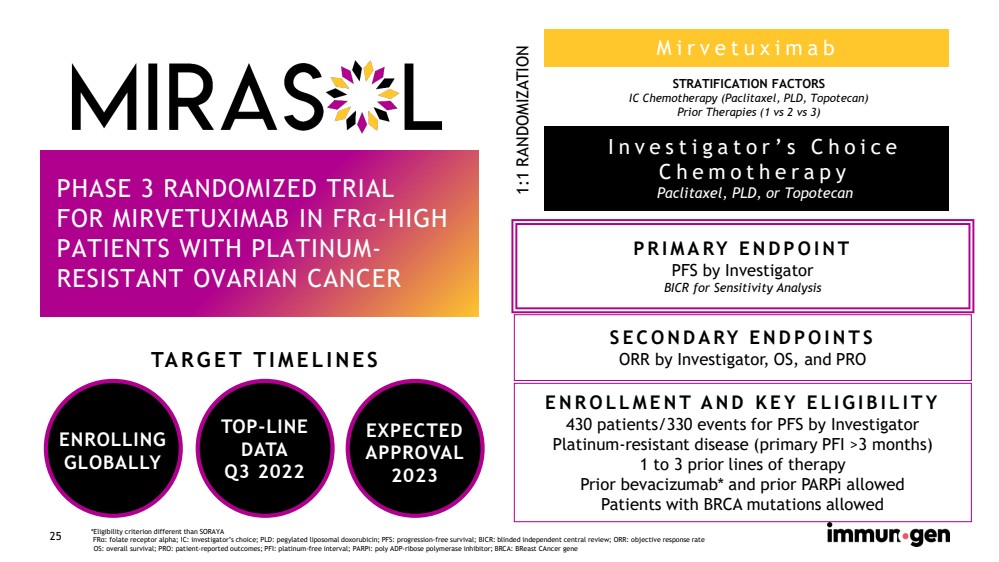
| CONFIDENTIAL25 Mirvetuximab PHASE 3 RANDOMIZED TRIALFOR MIRVETUXIMAB IN FRα-HIGH PATIENTS WITH PLATINUM-RESISTANT OVARIAN CANCER *Eligibility criterion different than SORAYAFRα: folate receptor alpha; IC: investigator’s choice; PLD: pegylated liposomal doxorubicin; PFS: progression-free survival; BICR: blinded independent central review; ORR: objective response rateOS: overall survival; PRO: patient-reported outcomes; PFI: platinum-free interval; PARPi: poly ADP-ribose polymerase inhibitor; BRCA: BReastCAncergene Investigator’s Choice ChemotherapyPaclitaxel, PLD, or TopotecanSTRATIFICATION FACTORSIC Chemotherapy (Paclitaxel, PLD, Topotecan)Prior Therapies (1 vs 2 vs 3)1:1 RANDOMIZATION PRIMARY ENDPOINT PFSby InvestigatorBICRfor Sensitivity Analysis SECONDARY ENDPOINTSORR by Investigator, OS, and PRO ENROLLMENT AND KEY ELIGIBILITY430 patients/330 events for PFSby Investigator Platinum-resistant disease (primary PFI>3 months)1 to 3 prior lines of therapyPrior bevacizumab* and prior PARPi allowedPatients with BRCA mutations allowed ENROLLINGGLOBALLYTARGET TIMELINES TOP-LINEDATAQ3 2022EXPECTEDAPPROVAL2023 |
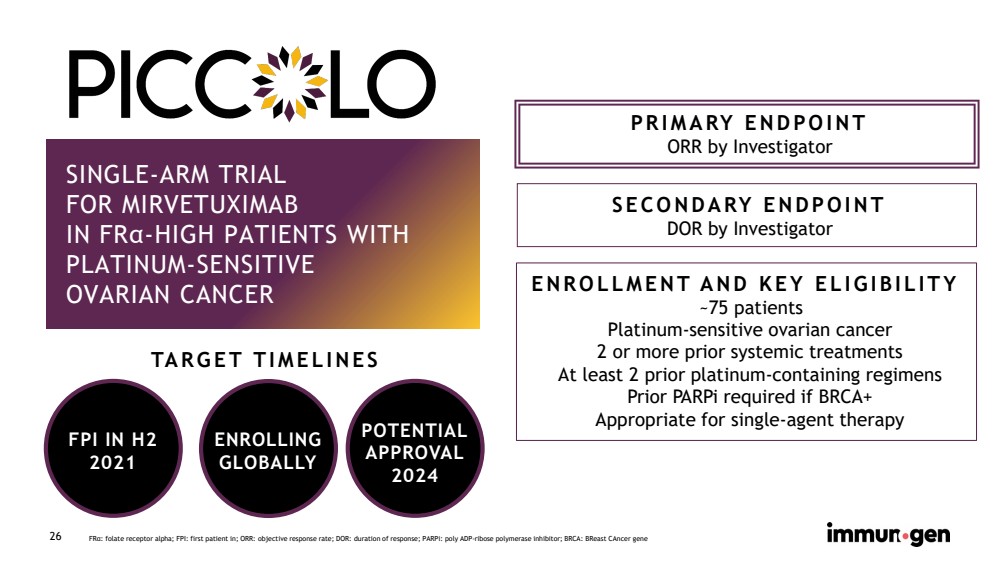
| CONFIDENTIAL26 SINGLE-ARM TRIALFOR MIRVETUXIMABIN FRα-HIGH PATIENTS WITH PLATINUM-SENSITIVEOVARIAN CANCER PRIMARY ENDPOINT ORR by Investigator SECONDARY ENDPOINTDOR by InvestigatorNOW ENROLLING ENROLLMENT AND KEY ELIGIBILITY~75 patients Platinum-sensitive ovarian cancer 2 or more prior systemic treatmentsAt least 2 prior platinum-containing regimensPrior PARPi required if BRCA+Appropriate for single-agent therapy FRα: folate receptor alpha; FPI: first patient in; ORR: objective response rate; DOR: duration of response; PARPi: poly ADP-ribose polymerase inhibitor; BRCA: BReastCAncergene FPI IN H2 2021TARGET TIMELINES POTENTIALAPPROVAL2024 ENROLLINGGLOBALLY |
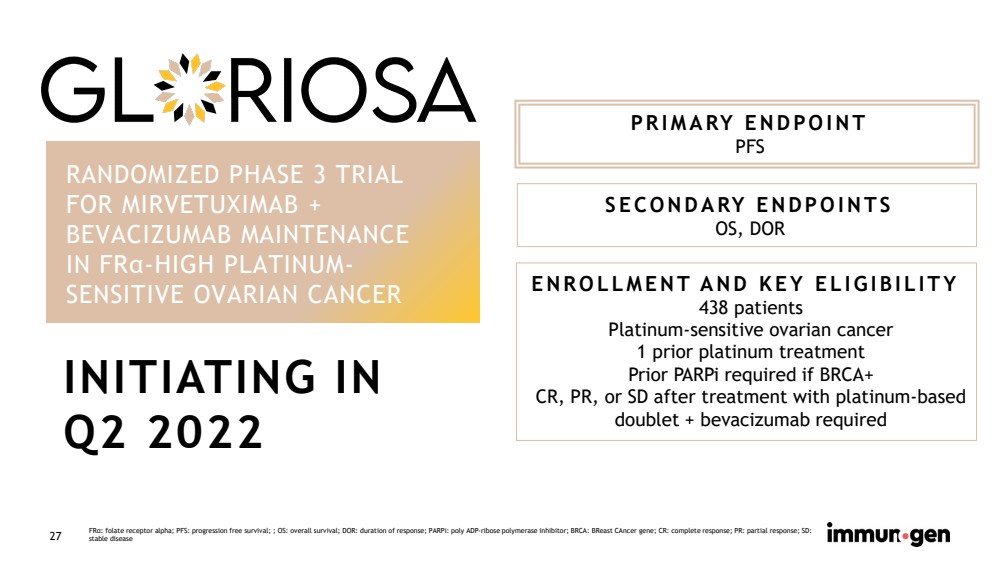
| CONFIDENTIAL27 RANDOMIZED PHASE 3 TRIALFOR MIRVETUXIMAB + BEVACIZUMAB MAINTENANCE IN FRα-HIGH PLATINUM-SENSITIVE OVARIAN CANCER PRIMARY ENDPOINT PFS SECONDARY ENDPOINTSOS, DOR ENROLLMENT AND KEY ELIGIBILITY438 patients Platinum-sensitive ovarian cancer 1 prior platinum treatmentPrior PARPi required if BRCA+CR, PR, or SD after treatment with platinum-based doublet + bevacizumab requiredFRα: folate receptor alpha; PFS: progression free survival; ; OS: overall survival; DOR: duration of response; PARPi: poly ADP-ribose polymerase inhibitor; BRCA: BReastCAncergene; CR: complete response; PR: partial response; SD: stable disease INITIATING IN Q2 2022 |
| CONFIDENTIAL28 SINGLE-ARM PHASE 2 TRIAL OF MIRVETUXIMAB + CARBOPLATIN FOLLOWED BY MIRVETUXIMAB CONTINUATION IN FRα-LOW, MEDIUM, AND HIGH PATIENTS WITH PLATINUM-SENSITIVE OVARIAN CANCER PRIMARY ENDPOINT ORR by Investigator SECONDARY ENDPOINTSDOR, PFS ENROLLMENT AND KEY ELIGIBILITY~110 patients Platinum-sensitive ovarian cancer 1 prior platinum treatmentPrior PARPi required if BRCA+FRα: folate receptor alpha; ORR: overall response rate; DOR: duration of response; PFS: progression free survival; PARPi: poly ADP-ribose polymerase inhibitor; BRCA: BReastCAncergeneINITIATING IN Q2 2022420 STUDY |
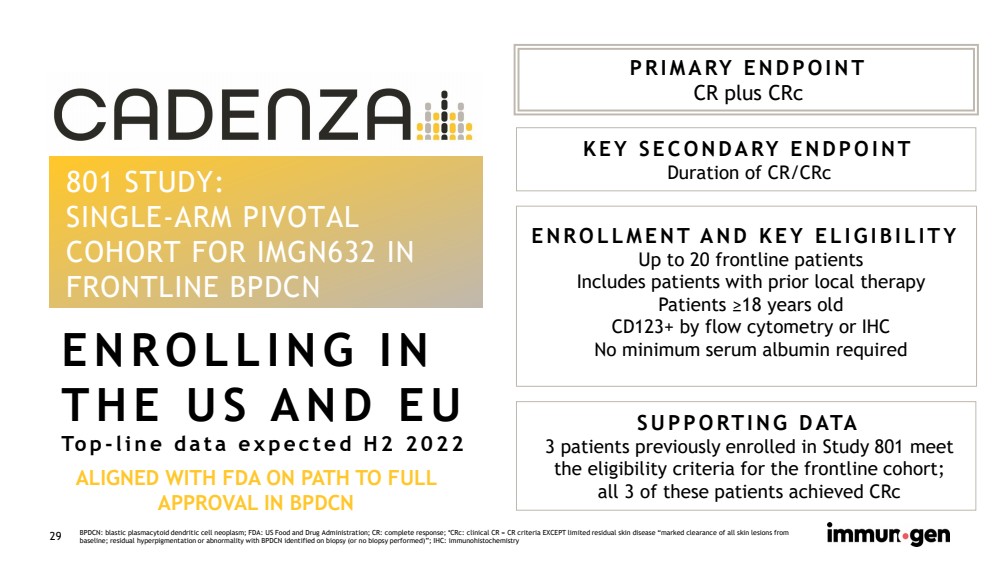
| CONFIDENTIAL29 801 STUDY:SINGLE-ARM PIVOTAL COHORT FOR IMGN632 IN FRONTLINE BPDCN PRIMARY ENDPOINTCR plus CRc KEY SECONDARY ENDPOINTDuration of CR/CRc ENROLLMENT AND KEY ELIGIBILITYUp to 20 frontline patientsIncludes patients with prior local therapy Patients ≥18 years oldCD123+ by flow cytometry or IHCNo minimum serum albumin required SUPPORTING DATA3 patients previously enrolled in Study 801 meet the eligibility criteria for the frontline cohort;all 3 of these patients achieved CRc ENROLLING IN THE US AND EUTop-line data expected H2 2022ALIGNED WITH FDA ON PATH TO FULL APPROVAL IN BPDCN BPDCN: blastic plasmacytoid dendritic cell neoplasm; FDA: US Food and Drug Administration; CR: complete response; *CRc: clinical CR = CR criteria EXCEPT limited residual skin disease “marked clearance of all skin lesions from baseline; residual hyperpigmentation or abnormality with BPDCN identified on biopsy (or no biopsy performed)”; IHC: immunohistochemistry |
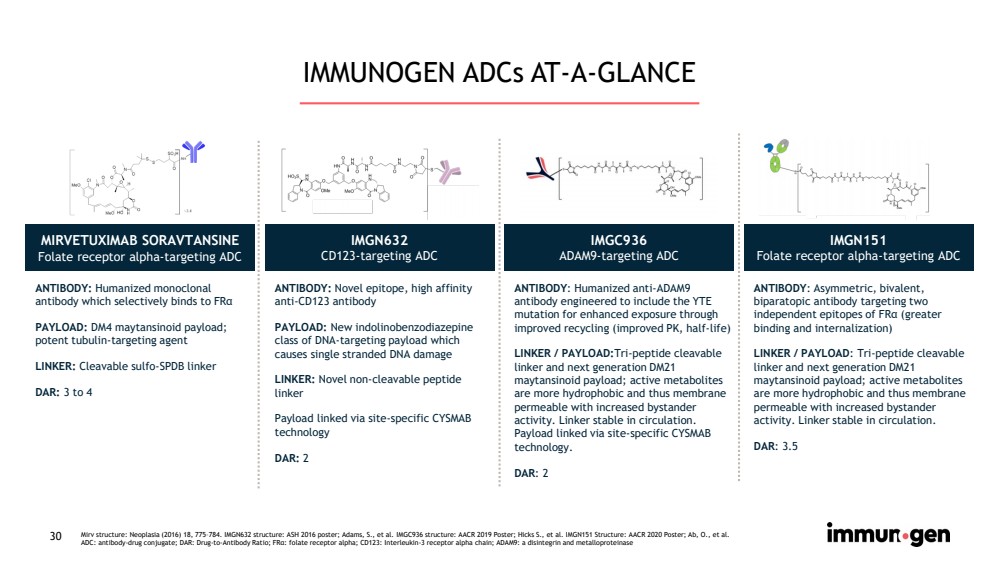
| CONFIDENTIAL30 IMMUNOGEN ADCs AT-A-GLANCE MIRVETUXIMAB SORAVTANSINEFolate receptor alpha-targeting ADC IMGN632CD123-targeting ADC IMGC936ADAM9-targeting ADC IMGN151Folate receptor alpha-targeting ADCANTIBODY: Humanizedmonoclonal antibody which selectively binds to FRαPAYLOAD: DM4 maytansinoid payload; potent tubulin-targeting agentLINKER: Cleavable sulfo-SPDBlinkerDAR: 3 to 4ANTIBODY: Novel epitope, high affinity anti-CD123 antibodyPAYLOAD: Newindolinobenzodiazepineclass of DNA-targeting payload which causes single stranded DNA damageLINKER: Novel non-cleavable peptide linker Payload linked via site-specific CYSMAB technologyDAR: 2ANTIBODY: Humanized anti-ADAM9 antibody engineeredto include the YTE mutation for enhanced exposure through improved recycling (improved PK, half-life)LINKER / PAYLOAD:Tri-peptide cleavable linker and next generation DM21 maytansinoidpayload; active metabolites are more hydrophobic and thus membrane permeable with increased bystander activity. Linker stable in circulation. Payload linked via site-specific CYSMAB technology.DAR: 2ANTIBODY: Asymmetric, bivalent, biparatopicantibody targeting two independent epitopes of FRα(greater binding and internalization)LINKER / PAYLOAD: Tri-peptide cleavable linker and next generation DM21 maytansinoidpayload; active metabolites are more hydrophobic and thus membrane permeable with increased bystander activity. Linker stable in circulation. DAR: 3.5 Mirv structure: Neoplasia (2016) 18, 775–784. IMGN632 structure: ASH 2016 poster; Adams, S., et al. IMGC936 structure: AACR 2019Poster; Hicks S., et al. IMGN151 Structure: AACR 2020 Poster; Ab, O., et al.ADC: antibody-drug conjugate; DAR: Drug-to-Antibody Ratio; FRα: folate receptor alpha; CD123: Interleukin-3 receptor alpha chain; ADAM9: a disintegrinand metalloproteinase |
| CONFIDENTIAL31 |
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- 420 with CNW — Study Enumerates Therapeutic Effects, Quality of Life Benefits of Medical Cannabis
- HCI Group Declares Quarterly Cash Dividend
- TransAlta Corporation Announces Results of the Annual Meeting of Shareholders and Election of all Directors
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share