Form 8-K Corindus Vascular Roboti For: Sep 05
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
Date of Report (Date of earliest event reported): September 5, 2018
| CORINDUS VASCULAR ROBOTICS, INC. |
(Exact Name of Registrant as Specified in its Charter)
| Delaware | 001-37406 | 30-0687898 | ||
|
(State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
309 Waverley Oaks Road, Suite 105 Waltham, MA 02452 |
| (Address of Principal Executive Office) (Zip Code) |
Registrant’s telephone number, including area code: (508) 653-3335
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2 below):
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230-405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 8.01 | Other Events. |
Attached hereto as Exhibit 99.1 and incorporated by reference herein is the September 2018 Wells Fargo Securities Healthcare Conference presentation of Corindus Vascular Robotics, Inc.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits
|
Exhibit
|
Description |
|
| 99.1 | Presentation of Corindus Vascular Robotics, Inc. dated September 2018. | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| Date: September 5, 2018 | CORINDUS VASCULAR ROBOTICS, INC. | |
| By: | /s/ David W. Long | |
| David W. Long | ||
| Chief Financial Officer | ||
Corindus Vascular Robotics 8-K
Exhibit 99.1

Precision Vascular Robotics Corindus Vascular Robotics (CVRS) September 2018

Forward Looking Statements This presentation contains “forward - looking statements” (as such term is defined in Section 27 A of the Securities Act of 1933 , as amended, and Section 21 E of the Securities Exchange Act of 1934 , as amended), and information relating to the company, that are based on the current beliefs of, and assumptions made by our management and the information currently available to our management . Forward - looking statements relate to expectations concerning matters that are not historical facts . Words such as "anticipate," "believe," "estimate," "expect," "intend," "plan," "predict," "opinion," "will" and similar expressions and their variants, are intended to identify forward - looking statements . These forward - looking statements include, but are not limited to statements related to our expected business, products and adoption of robotic medical procedures, including AI/automation, telestenting and remote procedures, and expanding our technology platform for use in the neurovascular market, results of operations, future financial condition, ability to increase our revenues, and similar matters . These forward - looking statements should be considered in light of various important factors, including, without limitation, our ability to expand our technology platform and achieve the advances necessary for telestenting and remote procedures, including in humans ; our ability to expand our technology platform for use in other segments of the vascular intervention market, including neurointerventional and other more complex cardiac interventions, obtaining necessary regulatory approvals for the use on humans and marketing of our products in the United States and in other countries, the rate of adoption of our CorPath System and the rate of use of our cassettes ; risks associated with market acceptance, including pricing and reimbursement ; our ability to enforce our intellectual property rights ; our need for additional funds to support our operations ; our ability to manage expenses and cash flow ; factors relating to engineering, regulatory, manufacturing, sales and customer service challenges ; potential safety and regulatory issues that could slow or suspend our sales ; the effect of credit, financial and economic conditions on capital spending by our potential customers ; the impact of global and regional economic and credit market conditions on health care spending ; health care reform legislation in the United States and its impact on hospital spending, reimbursement and fees which will be levied on certain medical device revenues, decreases in hospital admissions and actions by payers to limit or manage surgical procedures timing and success of product development and market acceptance of developed products, procedure counts ; regulatory approvals, clearances and restrictions ; guidelines and recommendations in the health care and patient communities, intellectual property positions and litigation, competition in the medical device industry and in the specific markets of surgery in which we operate, the inability to meet demand for products, the results of legal proceedings to which we are or may become a party, product liability and other litigation claims, adverse publicity regarding our company and safety of our products and the adequacy of training ; our ability to expand in foreign markets ; and other risk factors . Readers are cautioned not to place undue reliance on these forward - looking statements, which are based on current expectation and are subject to risks, uncertainties ; and assumptions that are difficult to predict, including those risk factors described in the Company’s Annual Report on Form 10 - K for the fiscal year ended on December 31 , 2017 . Our actual results may differ materially and adversely from those expressed in any forward - looking statements . We undertake no obligation to publicly update or release any revisions to these forward - looking statements except as required by law . 2
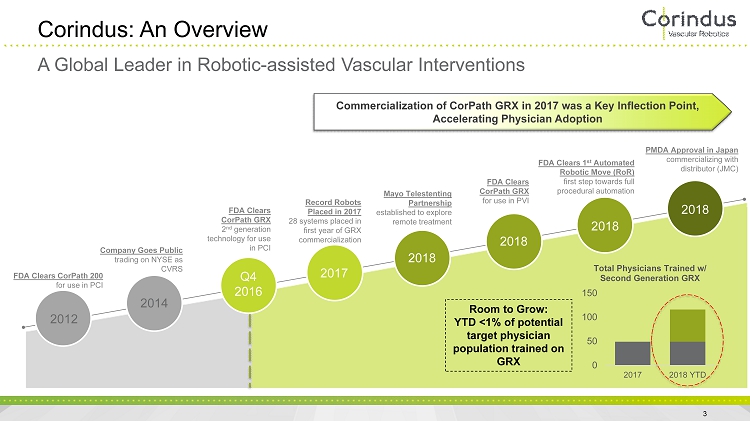
3 Corindus: An Overview FDA Clears CorPath GRX 2 nd generation technology for use in PCI Company Goes Public trading on NYSE as CVRS FDA Clears CorPath 200 for use in PCI Mayo Telestenting Partnership established to explore remote treatment FDA Clears 1 st Automated Robotic Move (RoR) first step towards full procedural automation 2012 2014 Q4 2016 2017 2018 2018 2018 Record Robots Placed in 2017 28 systems placed in first year of GRX commercialization PMDA Approval in Japan commercializing with distributor (JMC) 0 50 100 150 2017 2018 YTD Total Physicians Trained w/ Second Generation GRX Room to Grow: YTD <1% of potential target physician population trained on GRX Commercialization of CorPath GRX in 2017 was a Key Inflection Point, Accelerating Physician Adoption A Global Leader in Robotic - assisted Vascular Interventions 2018 FDA Clears CorPath GRX for use in PVI

• CorPath GRX cleared in late 2016 • Launched in 2017 • ~2,000 robotic CorPath GRX cassettes sold globally PCI Corindus’ Robotic Technology 4 1 CorPath Systems are not indicated for use in neuro interventions. • Ongoing clinical trials • Steering committee established in 2018 • Potential disruptor to stroke therapy NEURO 1 PERIPHERAL • CorPath GRX cleared in 2018 Potential to be First Disruptive Treatment Option in Vascular Medicine in 40+ years Evolution of Robot Capabilities Corindus ’ Goal is to be the First Robotics System for All Major Vascular Markets

Adding Value in PCI & Peripheral Across Key Stakeholders 5 Paradigm Shift in Place Will Drive Adoption 1 Smilowitz N, et al. Robotic - Enhanced PCI Compared to the Traditional Manual Approach. J Invasive Cardiol , 2014;26(7):318 - 321. 2 Campbell PT, et al. The Impact of Precise Robotic Lesion Length Measurement on Stent Length Selection: Ramification for stent s avings. Cardiovasc Revasc Med. 2015;piii:S1553 - 8389. • Grow Patient Volume – 1/mo. • Safe Work Environment • Patient Satisfaction • Telerobotics Expansion • Reduces Burnout • Extends Career • Attracts Physician Talent • Extends Physician Reach • Less Radiation Exposure 1 • More Precise Treatment 2 • Faster & More Comfortable Recovery • Treated Locally Hospital Physician Patient
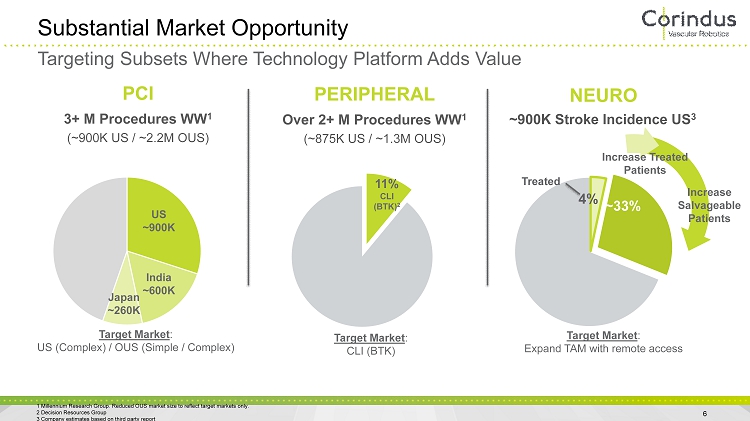
~900K Stroke Incidence US 3 NEURO ~33% 4% Treated Substantial Market Opportunity 6 3+ M Procedures WW 1 (~900K US / ~2.2M OUS) PCI Over 2+ M Procedures WW 1 (~875K US / ~1.3M OUS) PERIPHERAL 11% CLI (BTK) Target Market : Expand TAM with remote access Target Market : US (Complex) / OUS (Simple / Complex) Target Market : CLI (BTK) 11% CLI (BTK) 2 Increase Salvageable Patients 1 Millennium Research Group. Reduced OUS market size to reflect target markets only. 2 Decision Resources Group 3 Company estimates based on third party report Increase Treated Patients Targeting Subsets Where Technology Platform Adds Value US ~900K India ~600K Japan ~260K
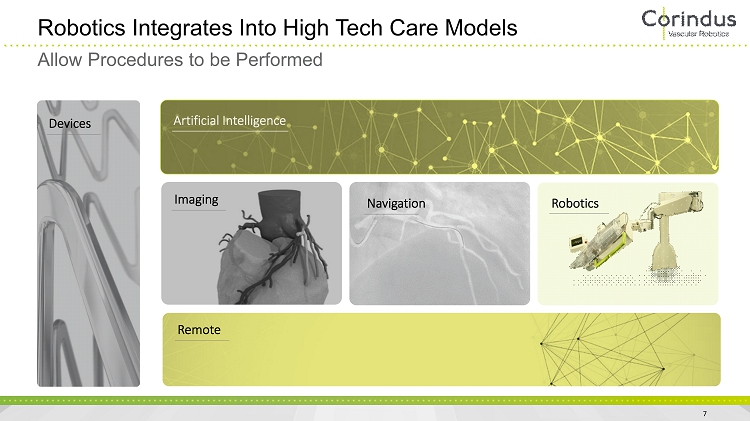
Robotics Integrates Into High Tech Care Models 7 Allow Procedures to be Performed Devices Robotics Imaging Artificial Intelligence Navigation Remote

Procedural Automation 8 • High percentage of procedure time is dedicated to wire manipulation • Varying skill levels among operators • Create algorithms based on techniques of highly skilled operators • Reduced procedure time may positively impact patient outcomes Problem Strategy New automated movements aimed at reducing navigation time, increasing success of lesion crossing Continued development of technIQ™ Series Madder, R. et al. Impact of a Novel Advanced Robotic Wiring Algorithm on Time to Wire a Coronary Artery Bifurcation in a Porc ine Model. Journal of the American College of Cardiology Oct 2017, 70 (18 Supplement) B223; DOI: 10.1016/j.jacc.2017.09.712

Addressing Unmet Needs • Access to treatment in remote/rural locations • Aging patient population • Shortage of skilled specialists • Emergent procedures: T ime to treatment is critical • Increase access to care & reduce time to treatment • Expand capable facility footprint • Enable tele - proctoring Problem Strategy Realizing remote capabilities 9 • Initiated preclinical studies with the Mayo Clinic • Rapid technology development & end user testing • Remote porcine test case from 100+ miles away Key Accomplishments Dr. Ryan Madder conducting first successful telestent procedure in porcine model from Ludington, MI

Neurovascular Steering Committee / Milestones 10 Aquilla S. Turk, D.O. Medical University of South Carolina Ricardo A. Hanel , M.D. PhD Baptist Health System Tudor G. Jovin , M.D. University of Pittsburgh Medical Center J. Mocco , M.D. Mt. Sinai Hospital Vitor Mendes Pereria , M.D. Toronto Western Hospital Adnan Siddiqui, M.D. Toshiba Stroke & Vascular Research Center Satoshi Tateshima , M.D. Ronald Reagan UCLA Medical Center Raymond D. Turner, M.D. Medical University of South Carolina Raul G. Nogueria , M.D. Grady Memorial Hospital CMO, Neuro Steering Committee Steering Committee Formed Multiple Neuro Animal Lab Studies KOL Neuro Summit CorPath GRX FDA Submission 1 Product Optimization for Remote Stroke Underway 1 Expected timing 2018 2019 GRX Neuro Product Launch 1 Neuroendovascular Remote Robotic Development 1
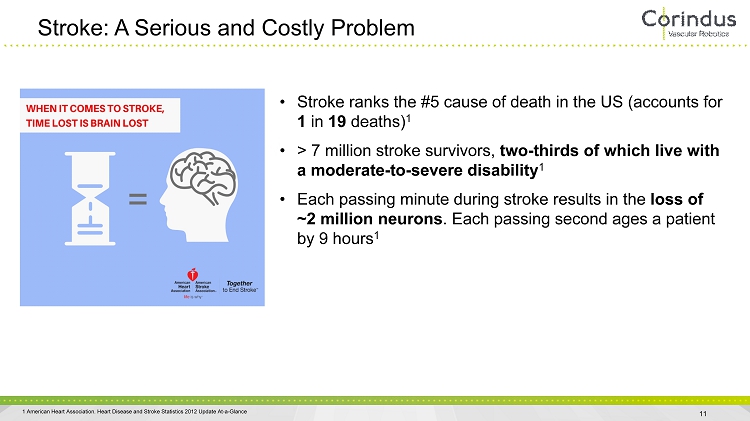
Stroke: A Serious and Costly Problem 11 • Stroke ranks the #5 cause of death in the US (accounts for 1 in 19 deaths) 1 • > 7 million stroke survivors, two - thirds of which live with a moderate - to - severe disability 1 • Each passing minute during stroke results in the loss of ~2 million neurons . Each passing second ages a patient by 9 hours 1 1 American Heart Association. Heart Disease and Stroke Statistics 2012 Update At - a - Glance

Remote Access Capabilities May Significantly Increase TAM 12 Ischemic Stroke ~775K Large Vessel Occlusion ~335K Salvageable Tissue ~160K Patients Treated ~35K US Incidence 1 ~900K Stroke • Time to treatment is key in the stroke market • Current treatment paradigm requires treatment within a 24 hour window • Lack of proximity to facilities and limited number of specialists leads to a meaningful % of patients not being treated CVRS’ Remote Access Capabilities has Opportunity to Significantly Expand Treatable Patients: TIME IS BRAIN Opportunity to increase patients treated with remote access and expand market further with next generation robot 1 Company estimates based on third party report.

9 16 23 33 37 41 0 5 10 15 20 25 30 35 40 45 Q1 2017 Q2 2017 Q3 2017 Q4 2017 Q1 2018 Q2 2018 Financials Snapshot 13 Revenue $ 0.8 M $ 2.3 M $ 2.4 M $ 4.2 M $ 1.5 M $ 1.7 M 0 0.5 1 1.5 2 2.5 3 3.5 4 4.5 Q1 2017 Q2 2017 Q3 2017 Q4 2017 Q1 2018 Q2 2018 ~Cash balance of $34.3M, Debt of $11.6M, 188.9M shares outstanding and 1M preferred (convertible to 20.2M common shares) as of June 30, 2018 GRX Installed Base Sites that upgraded from a CorPath 200 to a CorPath GRX have, on average, nearly doubled the percentage of PCI procedures performed robotically 284 303 370 455 353 390 0 50 100 150 200 250 300 350 400 450 500 Q1 2017 Q2 2017 Q3 2017 Q4 2017 Q1 2018 Q2 2018 Cassette Sales

Clinical Catalysts: Next 12 - 24 Months 14 Key Expected Milestones for Clinical Development Remote • Live remote PCI at TCT 2018 • First - in - human completed by year - end 2018 in India • Planned US clinical trials Neuro • FDA submission for neuro indication by year - end 2018 or early 2019 • Partnership for commercialization and co - development • FDA clearance and launch of CorPath GRX for neuro in first half 2019
Corindus Summary / Key Investment Thesis 15 • Significant progress in technology development and clinical expansion is creating a burning platform for hospitals to build a robotic program • AI / automation and remote access have the potential to add significant value to hospitals, patients, and doctors in the interventionalist market • Remote capabilities have the ability to increase TAM of the stroke market with opportunity to further expand the addressable market post launch of the next generation robot • Strong intellectual property portfolio / first mover advantage Corindus is a Leading Vascular Robotics Company with Opportunities in Coronary, Peripheral, and Neurovascular Markets
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- TransAlta Joins Other Water Licence Holders and the Alberta Government to collaborate on flow management on the Bow River System
- Wipro Announces Results for the Quarter and Year ended March 31, 2024
- Emergia Inc. Announces Refreshed Board of Directors and Appointment of Faraj Nakhleh as Chairman
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share