Form 6-K Sol-Gel Technologies For: Aug 04
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
Report of Foreign Private Issuer
Pursuant to Rule 13a-16 or 15d-16
Under the Securities Exchange Act of 1934
For the month of August 2021
Commission File Number 001-38367
SOL-GEL TECHNOLOGIES LTD.
(Translation of registrant’s name into English)
7 Golda Meir Street
Ness Ziona 7403650, Israel
(Address of principal executive offices)
Indicate by check mark whether the registrant files or will file annual reports under cover Form 20-F or
Form 40-F.
Form 20-F ☒ Form 40-F ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule
101(b)(1): ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule
101(b)(7): ☐
INFORMATION CONTAINED IN THIS REPORT ON FORM 6-K
Sol-Gel Technologies Ltd. (the “Company”) is posting on its website a corporate presentation.
Attached hereto and incorporated by reference in this Report on Form 6-K is the following exhibit:
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report
to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
SOL-GEL TECHNOLOGIES LTD.
|
||
|
|
|
|
|
|
Date: August 4, 2021
|
By:
|
/s/ Gilad Mamlok
|
|
|
|
|
Gilad Mamlok
|
|
|
|
|
Chief Financial Officer
|
|
Exhibit 99.1

NASDAQ: SLGL August 2021

FORWARD-LOOKING STATEMENTS This presentation contains forward-looking statements within the
meaning of the Private Securities Litigation Reform Act of 1995. All statements other than statements of historical facts are forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “may,”
“will,” “should,” “expect,” “plan,” “anticipate,” “could,” “future,” “outlook,” “intend,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “potential,” “continue,” or the negative of these terms or other similar
expressions, although not all forward-looking statements contain these words. The forward-looking statements in this presentation relate to, among other things, statements regarding the Food and Drug Administration (FDA) approval and
commercial launch of EPSOLAY®, commercial launch of TWYNEO, the strategic partnership with Galderma, progress on our innovative earlier stage programs, anticipated timing of the initiation of clinical trials for SGT-510, the intellectual
property protection that would be provided by patents for SGT-210 and our tapinarof drug product, the timing of the launch of our tapinarof drug product, the future markets for various skin diseases, the timing of a test and a second POC
study of erlotinib, projected profit margins, our expectations regarding our liquidity and ability to fund operational and capital expenditure requirements. These statements are neither promises nor guarantees, but involve known and unknown
risks, uncertainties, and other important factors that may cause our actual results, performance, or achievements to be materially different from any future results, performance, or achievements expressed or implied by the forward-looking
statement, including but not limited to the following: risks relating to the timing of the PDUFA action date for EPSOLAY®, the timing of FDA approval, if any, of EPSOLAY; the risk that we will not receive all of the anticipated benefits of
the strategic partnership with Galderma, the risk of a delay in the clinical trials for SGT-510; the risk that we don’t progress on our innovative earlier stage programs, the risk that patents for SGT-210 and our tapinarof drug product will
not provide the anticipated intellectual property protection; the risk of a delay in the launch of our tapinarof drug product; the risk that our estimate of the markets for psoriasis, atopic dermatitis and for hyperkeratotic skin diseases
are inaccurate; the risk that our tapinarof drug product will not be the only other player besides the brand for a number of years; the risk of a delay in the timing of a test of erlotinib with a much higher concentrations in an animal
model and the risk of a delay of a second POC study on PPK patients; the fact that we have and expect to continue to incur significant losses; our need for additional funding, which may not be available; our ability to complete the
development of, and obtain marketing approval for, our product candidates; our ability to obtain and maintain regulatory approvals for our product candidates in our target markets and the possibility of adverse regulatory or legal actions
relating to our product candidates even if regulatory approval is obtained; our ability to commercialize and launch our product candidates at all or on a timely basis; our ability to obtain and maintain adequate protection of our
intellectual property; our ability to manufacture our product candidates in commercial quantities, at an adequate quality or at an acceptable cost; acceptance of our product candidates by healthcare professionals and patients; the
possibility that we may face third-party claims of intellectual property infringement; the timing and results of clinical trials that we may conduct or that our competitors and others may conduct relating to our or their products; delays in
the launch of product candidates and generic drugs; intense competition in our industry; potential product liability claims; potential adverse federal, state, and local government regulation in the United States, Europe, or Israel; the
impact of pandemics, such as COVID-19 (coronavirus); and loss or retirement of key executives and research scientists. These and other important factors discussed in the Company's Annual Report on Form 20-F filed with the Securities and
Exchange Commission (“SEC”) on March 4, 2021, and in our other reports filed with the SEC, could cause actual results to differ materially from those indicated by the forward-looking statements made in this presentation. Any such
forward-looking statements represent management’s estimates as of the date of this presentation. While we may elect to update such forward-looking statements at some point in the future, unless required by applicable law, we disclaim any
obligation to do so, even if subsequent events cause our views to change. Thus, one should not assume that our silence over time means that actual events are bearing out as expressed or implied in such forward-looking statements. These
forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this presentation. This presentation contains trademarks, trade names, and service marks of other companies, which are
the property of their respective owners. We do not intend our use or display of other parties' trademarks, trade names, or service marks to imply, and such use or display should not be construed to imply, a relationship with, or endorsement
or sponsorship of us by, these other parties.

ROFLUMILAST (SGT-510)Our innovative investigational topical formulation of
roflumilast (SGT-510) was found to be more effective than roflumilast cream, 0.3%, that was formulated by Sol-Gel according to conventional methods of cream formulation, in a human xenograft psoriasis animal model IPO$86.3M raised in
February 2018 PERRIGO PARTNERSHIPTwelve 50/50 gross profit-sharing collaborations TAPINAROF (SGT-310)We are currently developing an innovative investigational formulation of tapinarof (SGT-310) aiming to offer product
formulation innovations and increased affordability for patients compared to the brand expected to be launched ERLOTINIB (SGT-210)Our proof-of-concept study for erlotinib gel (SGT-210) in palmoplantar keratoderma patients was completed and
indicated a possible modest improvement. We plan to investigate higher concentrations of erlotinib GALDERMA PARTNERSHIP5-year license, with option to regain brands. EPSOLAY PDUFA goal date was set for April 26, 2021 (awaiting
FDA’s pre-approval inspection). TWYNEO FDA Approved July 26, 2021 Our Pipeline OUR DERMATOLOGY COMPANY OVERVIEW
$8 million upfront payment received. Additional regulatory milestone payments of up to $7
millionTiered double-digit royalties (mid- to high-teen percentage) of net salesUp to an additional $9 million in sales milestone paymentsOption to regain commercialization rights 5 years following first sale at no cost to
Sol-Gel STRATEGIC PARTNERSHIP WITH GLOBAL LEADER GALDERMA U.S. COMMERCIAL PARTNERSHIP FOR EPSOLAY® AND TWYNEO® Cash-flow positive deal supporting high-value development pipeline

Aiming to provide effective and tolerable topical therapies to achieve local action THE SCIENCE
BEHIND OUR PROPRIETARY TECHNOLOGY

Silica-based shell wraps the BPO crystal and is intended to serve as a barrier between the BPO
crystals and the skin CRYO-SEM PICTURE After application onto skin, BPO slowly migrates through the shell resulting in a continuous flow of BPO for up to 24 hours ENERGY-DISPERSIVE X-RAY SPECTROSCOPY MAPPING ENCAPSULATED BENZOYL
PEROXIDE (E-BPO) ENCAPSULATION IS DESIGNED TO ALLOW FOR CONTINUOUS FLOW
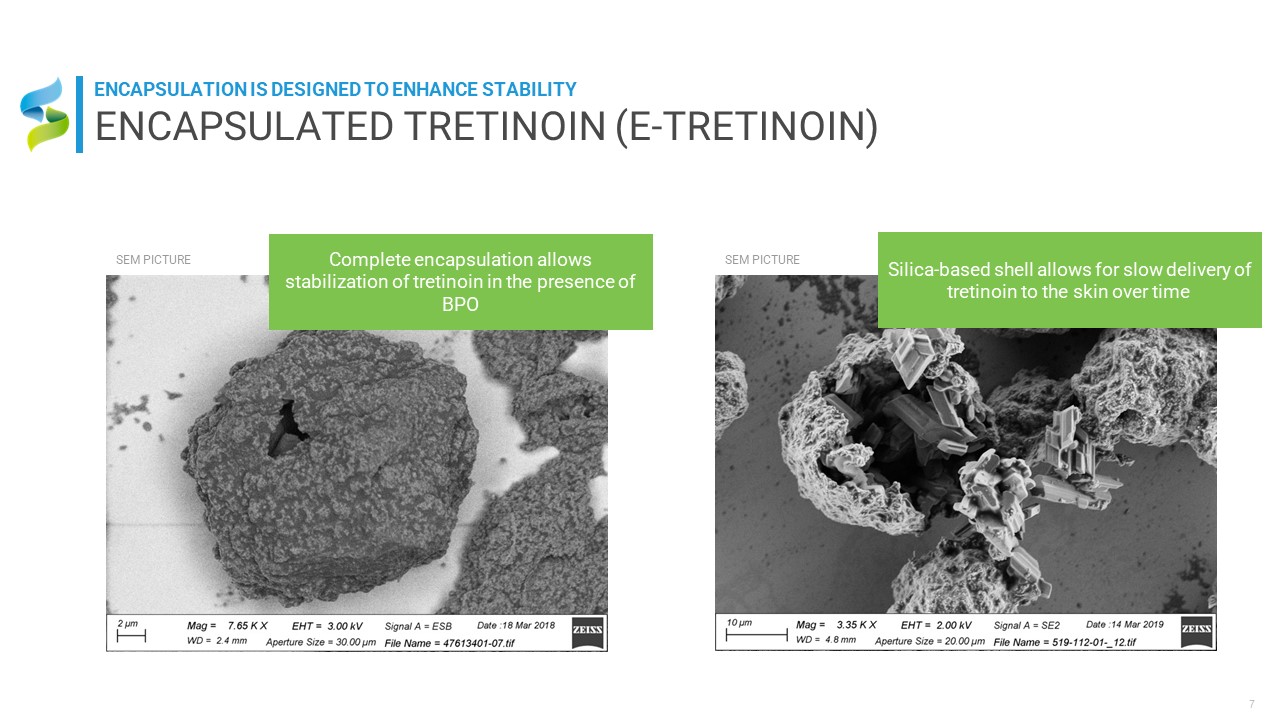
Complete encapsulation allows stabilization of tretinoin in the presence of BPO SEM
PICTURE Silica-based shell allows for slow delivery of tretinoin to the skin over time SEM PICTURE ENCAPSULATED TRETINOIN (E-TRETINOIN) ENCAPSULATION IS DESIGNED TO ENHANCE STABILITY

TWYNEO: FIRST PROPRIETARY DRUG APPROVAL U.S. COMMERCIAL PARTNERSHIP FOR EPSOLAY® AND TWYNEO®
WITH GALDERMA

MULTIFACTORIAL DISEASE REQUIRING POWERFUL COMBINATION TREATMENTS Acne VulgarisA
multifactorial disease of the pilosebaceous unit, involving abnormalities in sebum production, follicular epithelial desquamation, bacterial proliferation, and inflammation How is it Treated?Topical BPO, retinoids (such as tretinoin,
adapalene), antibiotics, and their combinationsOral Isotretinoin and antibiotics Current Treatment ShortfallsInsufficient efficacy negatively affects self-esteemSystemic side effectsContributes to antibiotic resistance UNMET NEED IN ACNE
VULGARIS LONG-TERM SAFETY STUDY

(Tretinoin and Benzoyl Peroxide) Cream, 0.1%/3% TWYNEO®: OUR FIRST BRANDED PRODUCT
APPROVAL Indication: for the treatment of acne vulgaris in adults and pediatric patients nine years of age and olderFirst acne treatment that contains a fixed‑dose combination of tretinoin and benzoyl peroxide, which are separately
encapsulated in silica using Sol‑Gel’s proprietary microencapsulation technology. Tretinoin and benzoyl peroxide are widely prescribed as separate treatments for acne vulgaris; however, these products have not been available for
simultaneous use in a fixed dose combination until the availability of TWYNEO.TWYNEO is protected until 2038 by granted patents and until 2041 by a pending patent application

* Individual results vary BASELINE “Severe”; 29 inflamed lesions31 non-inflamed lesions; 1
nodule WEEK 12 “Moderate”; 9 inflamed lesions5 non-inflamed lesions; No nodules IMPROVEMENT IN SEVERE PATIENT SUBJECT 507-003 || 18 YEARS OLD | FEMALE | WHITE | NOT HISPANIC OR LATINO*

Papulopustular RosaceaChronic, inflammatory condition that primarily affects the face and is
often characterized by flushing, redness, inflamed bumps, and pustules How is it Treated?Topical antimicrobials (metronidazole, clindamycin)Topical anti-mite (ivermectin)Systemic antibiotics (minocycline, doxycycline) Current Treatment
ShortfallsInsufficient efficacy resulting in poor adherenceSystemic side effectsContributing to antibiotic resistance UNMET NEED IN PAPULOPUSTULAR ROSACEA CHRONIC CONDITION WITH POOR ADHERENCE TO CURRENT TREATMENTS THE CHALLENGE

Encapsulation was designed to allow the BPO to slowly migrate from the microcapsules to help
reduce irritationPDUFA goal date was set for April 26, 2021. Awaiting FDA’s pre-approval inspectionPotential to be the first single-active BPO approved by the FDA as a prescription drug product Benzoyl Peroxide Cream, 5% SOL-GEL
SOLUTION*EPSOLAY® * EPSOLAY is investigational. Safety and efficacy have not been established

Two Parallel, Multi-Center, Double-Blinded, Randomized, Vehicle-Controlled Studies, 2:1 Ratio,
QD EPSOLAY® PHASE III STUDIES

P<0.001 P<0.001 Change from Baseline in Inflammatory Lesion Count Success in IGA Week
12Success in IGA (ITT) Week 12Inflammatory Lesion Count Change from Baseline (ITT) Study 54-01 Study 54-02 SUCCESS IN PRIMARY ENDPOINTS PHASE III RESULTS P<0.001 P<0.001 Study 54-01 Study 54-02

Success in IGA Week 2Exploratory Endpoint (ITT) Week 8Secondary Endpoint
(ITT) P<0.009 P<0.017 Study 54-01 Study 54-02 P<0.001 P<0.009 Study 54-01 Study 54-02 P<0.001 P<0.006 Study 54-01 Study 54-02 Week 4Secondary Endpoint (ITT) IMPROVEMENT AS OF WEEK 2 SUCCESS IN IGA

Week 2Exploratory Endpoint (ITT) Week 4Secondary Endpoint (ITT) Week 8Secondary Endpoint
(ITT) Change from Baseline in Inflammatory Lesion Count P<0.001 P<0.001 Study 54-01 Study 54-02 P<0.001 P<0.001 Study 54-01 Study 54-02 P<0.001 P<0.001 Study 54-01 Study 54-02 IMPROVEMENT AS OF WEEK
2 REDUCTION OF LESIONS
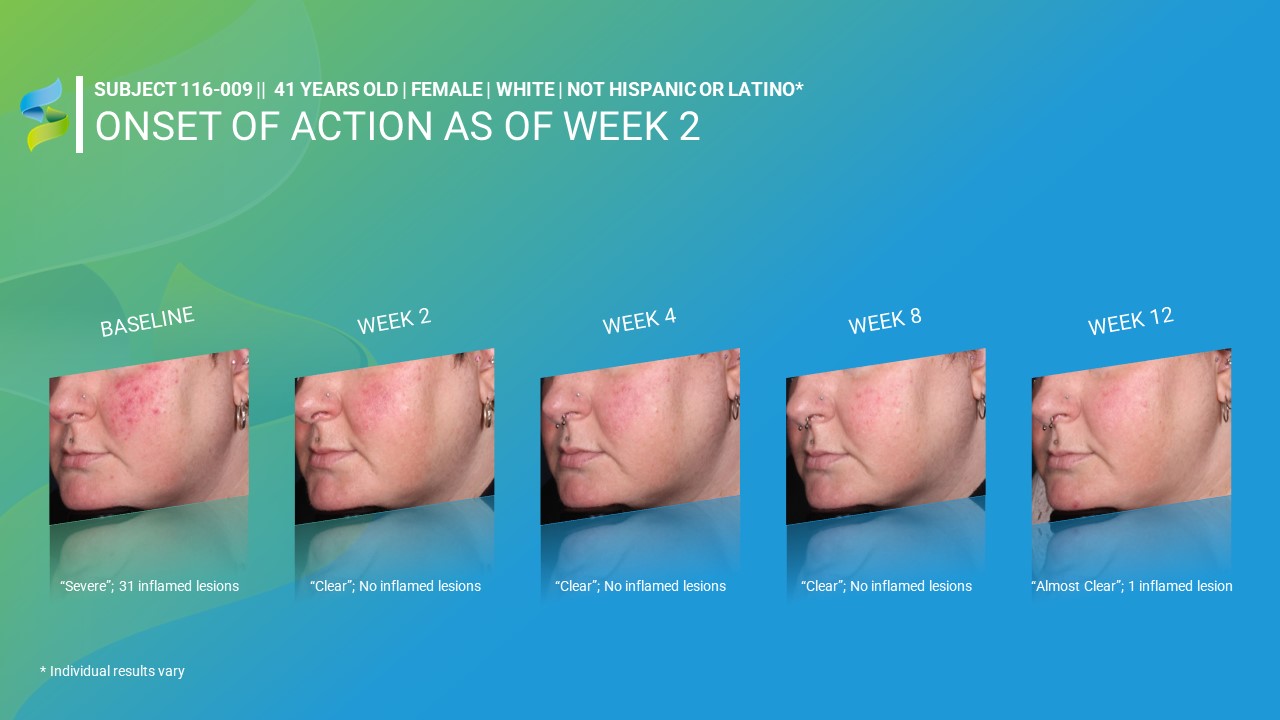
* Individual results vary BASELINE “Severe”; 31 inflamed lesions WEEK 2 “Clear”; No inflamed
lesions WEEK 4 “Clear”; No inflamed lesions WEEK 12 “Almost Clear”; 1 inflamed lesion WEEK 8 “Clear”; No inflamed lesions ONSET OF ACTION AS OF WEEK 2 SUBJECT 116-009 || 41 YEARS OLD | FEMALE | WHITE | NOT HISPANIC OR LATINO*

Phase III Studies Followed by 40 Weeks Long-Term Safety Study Extension Percentage of
Subjects IMPROVEMENT IN IGA* LONG-TERM SAFETY STUDY * This study was not designed for efficacy; however, efficacy was evaluated. Interpret results with caution

BROAD LONG-TERM INTELLECUAL PROPERTYESTATE TWYNEO is protected until 2038 by granted patents
and until 2041 by a pending patent application EPSOLAY is protected until 2040 by granted patents and until 2041 by a pendingpatent application 25 patent applications for erlotinib, tapinarof and roflumilast in various skin
conditions (as of February 26, 2021)
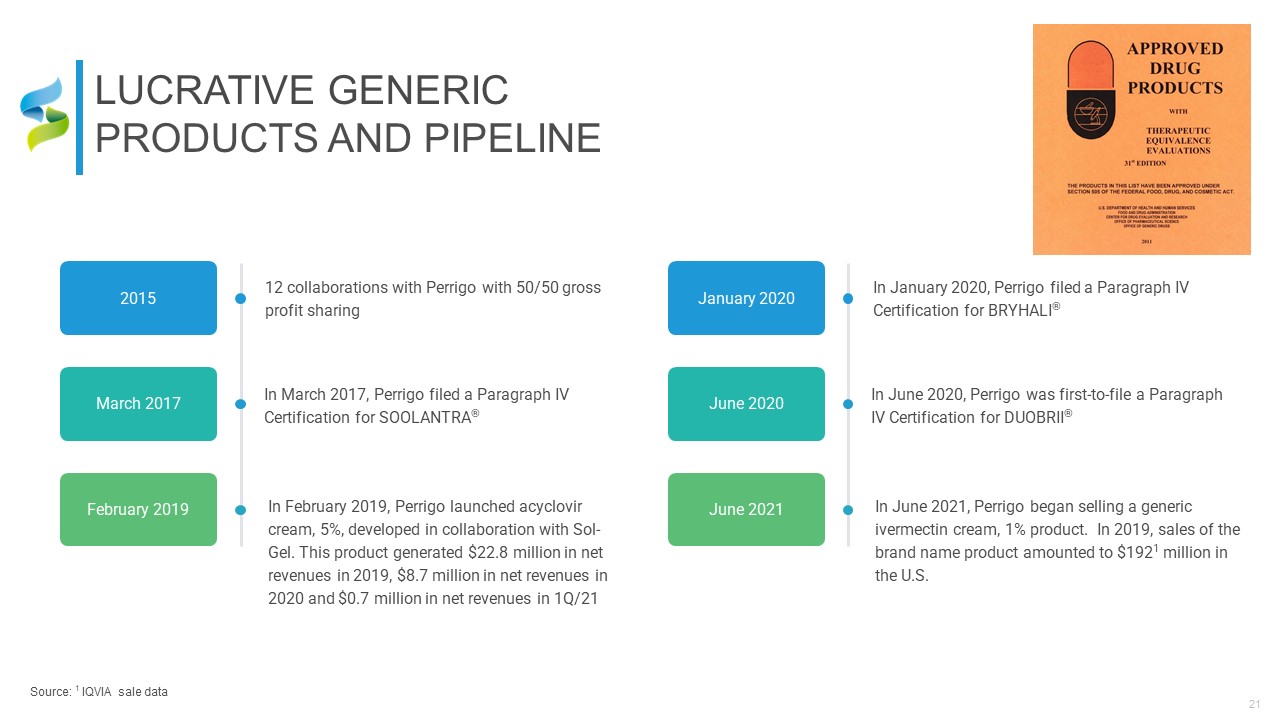
LUCRATIVE GENERIC PRODUCTS AND PIPELINE Source: 1 IQVIA sale data 12 collaborations
with Perrigo with 50/50 gross profit sharing 2015 In March 2017, Perrigo filed a Paragraph IV Certification for SOOLANTRA® March 2017 In February 2019, Perrigo launched acyclovir cream, 5%, developed in collaboration with Sol-Gel.
This product generated $22.8 million in net revenues in 2019, $8.7 million in net revenues in 2020 and $0.7 million in net revenues in 1Q/21 February 2019 13:00 - 14:00 In January 2020, Perrigo filed a Paragraph IV
Certification for BRYHALI® January 2020 In June 2020, Perrigo was first-to-file a Paragraph IV Certification for DUOBRII® June 2020 In June 2021, Perrigo began selling a generic ivermectin cream, 1% product. In 2019, sales of the
brand name product amounted to $1921 million in the U.S. June 2021 13:00 - 14:00

Our innovative investigational topical formulation of roflumilast (SGT-510) was found to be more
effective than roflumilast cream, 0.3%, that was formulated by Sol-Gel according to conventional methods of cream formulation, in a human xenograft psoriasis animal model We are currently developing an innovative investigational
formulation of tapinarof (SGT-310) aiming to offer product formulation innovations and increased affordability for patients compared to the brand expected to be launched Our proof-of-concept study for erlotinib gel (SGT-210) in
palmoplantar keratoderma patients was completed and indicated a possible modest improvement. We plan to investigate higher concentrations of erlotinib PIPELINE PROGRESS RECENT UPDATES

Pipeline focused on large and attractive categories and two active moieties that already demonstrated
positive Phase 3 results 4/26/21 PDUFA EPSOLAY ®(benzoyl peroxide) Cream, 5%Papulopustular Rosacea Galderma Partnership – U.S. research pre-clinical phase II phase III filed SGT-210 (erlotinib)Palmoplantar
Keratoderma research non-clinical phase II phase III filed INNOVATIVE PIPELINE OF TOPICAL SKIN MEDICATIONS REVENUE STREAM FROM PARTNERSHIPS WILL SUPPORT DEVELOPMENT OF OUR INNOVATIVE PIPELINE TWYNEO® (tretinoin and
benzoyl peroxide) cream, 0.1%3%Acne Vulgaris Galderma Partnership – U.S. research pre-clinical phase II phase III filed Approved07/26/21 SGT-310 (tapinarof)Psoriasis & Other Dermatologic
Indications research non-clinical phase II phase III filed SGT-510 (roflumilast)Psoriasis & Other Dermatologic Indications research non-clinical phase II phase III filed

> Gross proceeds of $86.3 million raised in IPO on February 5, 2018 > Gross
proceeds of $11.5 and $23 million raised in public follow-on offerings on August 12, 2019 and February 13, 2020, respectively > Additional $5 million investment by controlling shareholder in April 2020 > 23,029,953 Ordinary Shares
as of June 30, 2021 > $8.7 million net revenues from generic products in 2020 and $1.6 million net revenues from generic products in 1H/21 > $38.9 million in cash and investments as of June 30, 2021 > Based on Galderma’s
upfront and milestone payments, we expect that our cash resources will enable funding of operational and capital expenditure requirements into the first quarter of 2023 (assuming timely approval of EPSOLAY in 2021) FINANCIAL
PROFILE RECENT UPDATES

NASDAQ: SLGL
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Amerant Reports First Quarter 2024 Results
- Leading Industry Publication: Black & Veatch Remains Among Global Critical Infrastructure Leaders as Sustainability, Decarbonization Solutions Drive Growth
- Pivotree to Release First Quarter 2024 Financial Results
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share