Form 6-K GH Research PLC For: Mar 04
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER PURSUANT TO RULE 13a-16 OR 15d-16 UNDER THE SECURITIES EXCHANGE ACT OF 1934
For the month of March, 2022.
Commission File Number: 001-40530
GH Research PLC
(Exact name of registrant as specified in its charter)
28 Baggot Street Lower
Dublin 2
D02 NX43
Ireland
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F:
|
Form 20-F
|
☒ |
Form 40-F
|
☐ |
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): ☐
GH Research PLC (the “Company”) will be participating in the Cowen 42nd Annual Health Care Conference starting on March 7, 2022. On March 4, 2022, the Company made available an updated investor presentation on its website to be used for
presentation at the conference. A copy of the investor presentation is attached hereto as Exhibit 99.1.
The fact that this presentation is being made available and furnished herewith should not be deemed an admission as to the materiality of any information contained in the materials. The information contained in the presentation is being provided
as of March 4, 2022 and the Company does not undertake any obligation to update the presentation in the future or to update forward-looking statements to reflect subsequent actual results.
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
GH Research PLC
|
||
|
Date: March 4, 2022
|
||
|
By:
|
/s/ Julie Ryan
|
|
|
Name:
|
Julie Ryan
|
|
|
Title:
|
Vice President, Finance
|
|
EXHIBIT INDEX
|
Exhibit No.
|
Description
|
|
Corporate Presentation for March 2022
|
Exhibit 99.1

1 Corporate PresentationGH Research PLC (NASDAQ: GHRS) GH Research March 2022 2022© GH Research
PLC

Disclaimer Regarding Forward-Looking Statements This presentation has been prepared by GH Research PLC
(“GH Research”) for informational purposes only and not for any other purpose. Nothing contained in this presentation is, or should be construed as, a recommendation, promise or representation by the presenter or GH Research or any director,
employee, agent, or adviser of GH Research. This presentation does not purport to be all-inclusive or to contain all of the information you may desire.This presentation does not constitute an offer to sell or the solicitation of an offer to
buy securities, nor shall there be any sale of these securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or
jurisdiction. 2 This presentation contains forward-looking statements, all of which are qualified in their entirety by this cautionary statement. Many of the forward-looking statements contained herein can be identified by the use of
forward-looking words such as “may”, “anticipate”, “believe”, “could’, “expect”, “should”, “plan”, “intend”, “estimate”, “will”, “potential” and “ongoing”, among others, although not all forward-looking statements contain these identifying
words.Any statements contained herein that do not describe historical facts are forward-looking statements that are based on management’s expectations and are subject to certain factors, risks and uncertainties that may cause actual results,
outcomes, timing and performance to differ materially from those expressed or implied by such statements. These factors, risks and uncertainties include, but are not limited to: the costs and uncertainties associated with GH Research’s
research and development efforts; the inherent uncertainties associated with the conduct, timing and results of nonclinical and clinical studies of GH Research’s product candidates; GH Research’s ability to obtain, maintain, enforce and
defend issued patents; the adequacy of GH Research’s capital resources and availability of additional funding; and other factors, risks and uncertainties described in GH Research’s filings with the U.S. Securities and Exchange Commission.
Except as otherwise noted, these forward-looking statements speak only as of the date of this presentation, and GH Research undertakes no obligation to update or revise any of such statements to reflect events or circumstances occurring after
this presentation. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond GH Research’s control, you should not rely on these
forward-looking statements as predictions of future events. The events and circumstances reflected in any such forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the
forward-looking statements. GH Research cautions you not to place undue reliance on the forward-looking statements contained in this presentation 2022© GH Research PLC

SeekingUltra-Rapid, Durable Remissionsin Depression 3 2022© GH Research PLC

Pipeline 4 2022© GH Research PLC Stage of
Development PROGRAMS INDICATION PRECLINICAL PHASE 1 PHASE 2a PHASE 2b PHASE 3 Milestone GH0015-MeO-DMT for inhalation administration Treatment-Resistant Depression (TRD) Initiate Phase 2b trial in
TRD Psychiatric Disorder* Initiate Phase 2a trial in undisclosed psychiatric disorder Psychiatric Disorder* Initiate Phase 2a trial in undisclosed psychiatric disorder GH002 / GH0035-MeO-DMT for injection /
intranasal administration Psychiatric or Neurological Disorder Complete preclinical development Complete *In light of our completed Phase 1 clinical trial of GH001 in healthy volunteers and our completed Phase 1/2 trial
in TRD, we plan to request clearance from European regulatory authorities to begin Phase 2a clinical trials in patients with two additional undisclosed psychiatric disorders

... Remission Rates in TRD < 15% Established Therapies are Slow-Acting ~33% no remission despite
4 treatment steps Adapted from Trivedi et al., Am J Psychiatry 2006 and Rush et al., Am J Psychiatry 2006 Average time to remission is ~6 weeks (STAR*D study, Remission Rate Over Time, Treatment Step 1 = Citalopram) The Problem for
Patients with Depression (STAR*D study, Remission Rates Treatment Steps 1 to 4) 5 2 or more prior therapies = Treatment-Resistant Depression (TRD) 2022© GH Research PLC

Large and Open Depression Market EU and US 6 First Line MDD Second Line MDD Treatment-Resistant
Depression (TRD) Patients cycle through ineffective therapies for TRD Diagnosed: ~48MTreated (pharmacotherapy ± psychotherapy): ~24M Non-response to first line: ~13M Non-response to two prior lines: ~9M Company estimates based
on: https://www.nimh.nih.gov/health/statistics/major-depression.shtml; Wittchen et al., The size and burden of mental disorders and other disorders of the brain in Europe 2010, European Neuropsychopharmacology (2011); Rush et al., Acute and
Longer-Term Outcomes in Depressed Outpatients Requiring One or Several Treatment Steps: A STAR*D Report, Am J Psychiatry 2006MDD, Major Depressive Disorder 2022© GH Research PLC
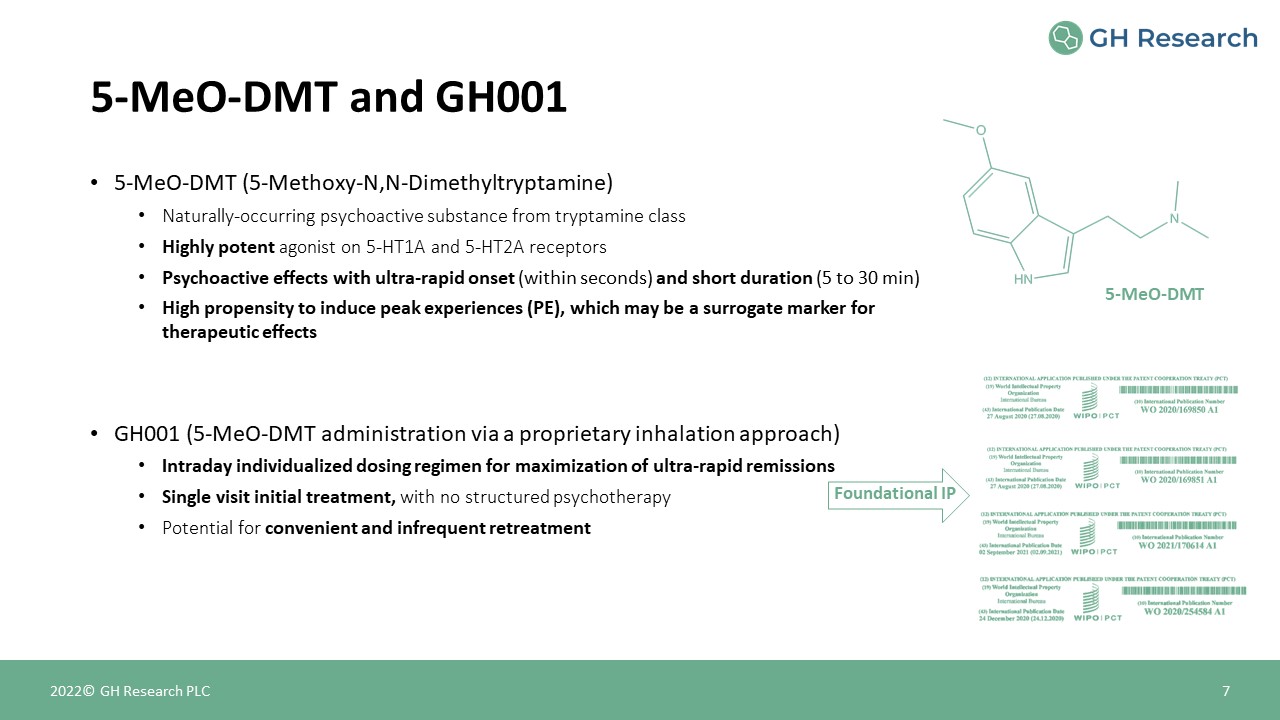
5-MeO-DMT (5-Methoxy-N,N-Dimethyltryptamine)Naturally-occurring psychoactive substance from tryptamine
classHighly potent agonist on 5-HT1A and 5-HT2A receptorsPsychoactive effects with ultra-rapid onset (within seconds) and short duration (5 to 30 min)High propensity to induce peak experiences (PE), which may be a surrogate marker for
therapeutic effectsGH001 (5-MeO-DMT administration via a proprietary inhalation approach)Intraday individualized dosing regimen for maximization of ultra-rapid remissions Single visit initial treatment, with no structured psychotherapy
Potential for convenient and infrequent retreatment 5-MeO-DMT and GH001 7 2022© GH Research PLC 5-MeO-DMT Foundational IP

GH001 Single Dose: GH001 Individualized Dosing Regimen (IDR): More Chances for
Remission MADRS score MADRS score MADRS score MADRS score Dose 1 Dose 1 Dose 2 Dose 3 Dose 2 Dose 1 No remission Remission Remission Remission Remission Hypothetical Patient 1 Hypothetical Patient
2 Hypothetical Patient 3 Hypothetical Patient 1 Hypothetical Patient 2 No remission No remission No remission Dose 1 GH001 – Individualized Dosing Regimen (IDR) Designed to Achieve Ultra-Rapid and Durable Remissions 8 2022© GH
Research PLC MADRS score Dose 1

Phase 1 Trial in Healthy Volunteers GH001-HV-101(Completed) 9 Clinicaltrials.gov ID
NCT04640831 2022© GH Research PLC

GH001Administration Day 1 Day 7 GH001 2 mg (n=4) GH001 6 mg (n=6) GH001 12 mg (n=4) GH001
18 mg (n=4) HV(n=18) Part A (Single Dose) Part B (IDR) Primary Endpoint:Safety until day 7Peak Experience Scale (PE Scale)1 HV(n=4) Primary Endpoint:Safety until day 7Peak Experience Scale (PE Scale)1 GH001 IDR6, 12, 18 mg to achieve
PE(up to 3 doses, 3h interval) Key Assessments SafetyPE ScaleCognitive function Safety SafetyCognitive function 1The PE Scale averages answers scored by the subject by marking a visual analogue scale between 0 and 100 for the
following three questions: 1. How intense was the experience; 2. To what extent did you lose control; 3. How profound (i.e., deep and significant) was the experience? Design of Phase 1 Trial in Healthy Volunteers (GH001-HV-101) 10 2022© GH
Research PLC PE, Peak ExperienceIDR, Individualized Dosing Regimen
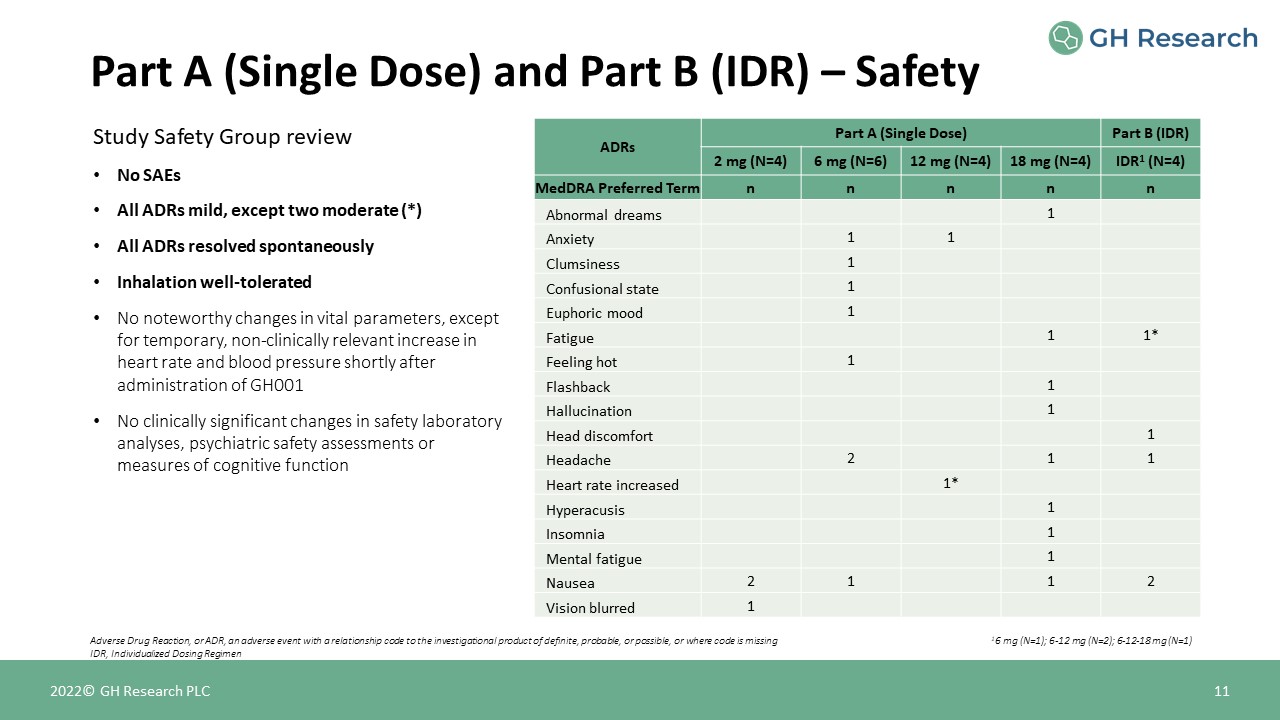
Study Safety Group reviewNo SAEsAll ADRs mild, except two moderate (*)All ADRs resolved
spontaneouslyInhalation well-toleratedNo noteworthy changes in vital parameters, except for temporary, non-clinically relevant increase in heart rate and blood pressure shortly after administration of GH001No clinically significant changes in
safety laboratory analyses, psychiatric safety assessments or measures of cognitive function ADRs Part A (Single Dose) Part B (IDR) ADRs 2 mg (N=4) 6 mg (N=6) 12 mg (N=4) 18 mg (N=4) IDR1 (N=4) MedDRA Preferred
Term n n n n n Abnormal dreams 1 Anxiety 1 1 Clumsiness 1 Confusional state 1 Euphoric mood 1 Fatigue 1 1* Feeling
hot 1 Flashback 1 Hallucination 1 Head discomfort 1 Headache 2 1 1 Heart rate increased 1* Hyperacusis 1 Insomnia 1 Mental
fatigue 1 Nausea 2 1 1 2 Vision blurred 1 Part A (Single Dose) and Part B (IDR) – Safety 11 Adverse Drug Reaction, or ADR, an adverse event with a relationship code to the investigational product of
definite, probable, or possible, or where code is missingIDR, Individualized Dosing Regimen 2022© GH Research PLC 16 mg (N=1); 6-12 mg (N=2); 6-12-18 mg (N=1)

Average fordose group PE Scale PEThreshold Part A – Peak Experience (PE) Dose-Effectsand
Inter-Person Variability 12 PE, Peak Experience 2022© GH Research PLC
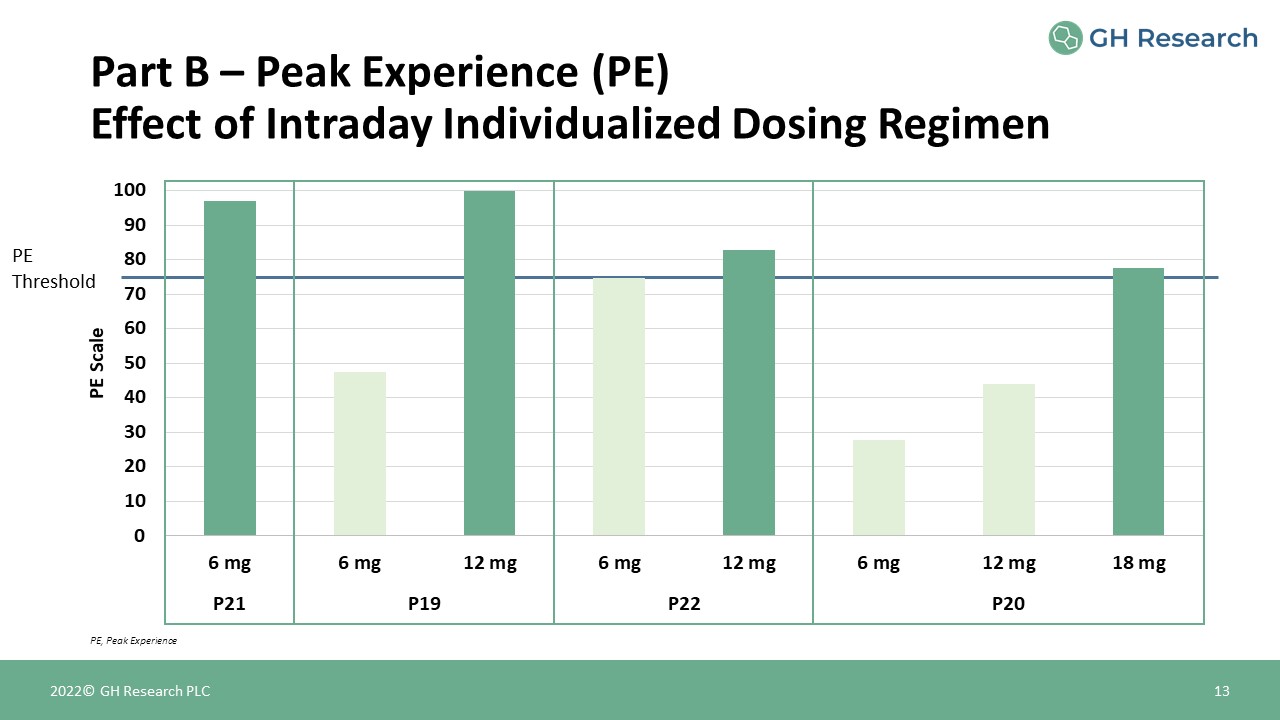
PEThreshold Part B – Peak Experience (PE)Effect of Intraday Individualized Dosing
Regimen 13 PE, Peak Experience 2022© GH Research PLC

Phase 1/2 Trial inTreatment-Resistant DepressionGH001-TRD-102(Completed) 14 Clinicaltrials.gov ID
NCT04698603 2022© GH Research PLC

Key Assessments MADRS 2-hrsPE ScaleSafety MADRS 1-dayCognitive functionSafety MADRS 7-dayCognitive
functionSafety GH001Administration Day 1 Day 7 Design of Phase 1/2 Trial in TRD (GH001-TRD-102) 15 PE, Peak Experience; MADRS, Montgomery-Åsberg Depression Rating ScaleIDR, Individualized Dosing Regimen 1Defined as inadequate
response to at least two adequate courses of pharmacological therapy or one adequate course of pharmacological therapy and at least one adequate course of evidence-based psychotherapy 2022© GH Research PLC Phase 1 (Single Dose) Phase 2
(IDR) GH001 12 mg (n=4) GH001 18 mg (n=4) TRD1(n=8) Primary Endpoint:Safety until day 7 TRD1(n=8) Primary Endpoint:MADRS remission day 7 (MADRS≤10) GH001 IDR6, 12, 18 mg to achieve PE(up to 3 doses, 3h interval)
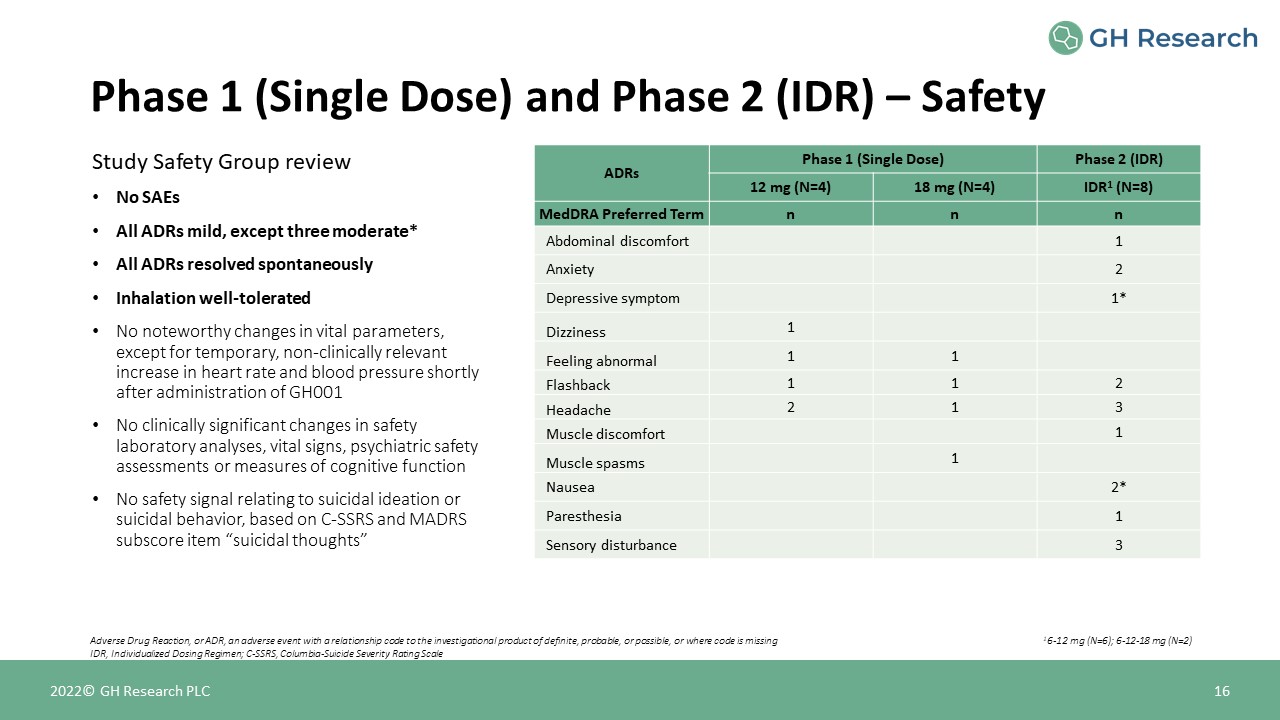
Phase 1 (Single Dose) and Phase 2 (IDR) – Safety 16 Adverse Drug Reaction, or ADR, an adverse event
with a relationship code to the investigational product of definite, probable, or possible, or where code is missingIDR, Individualized Dosing Regimen; C-SSRS, Columbia-Suicide Severity Rating Scale 2022© GH Research PLC Study Safety Group
reviewNo SAEsAll ADRs mild, except three moderate*All ADRs resolved spontaneouslyInhalation well-toleratedNo noteworthy changes in vital parameters, except for temporary, non-clinically relevant increase in heart rate and blood pressure
shortly after administration of GH001No clinically significant changes in safety laboratory analyses, vital signs, psychiatric safety assessments or measures of cognitive functionNo safety signal relating to suicidal ideation or suicidal
behavior, based on C-SSRS and MADRS subscore item “suicidal thoughts” ADRs Phase 1 (Single Dose) Phase 2 (IDR) ADRs 12 mg (N=4) 18 mg (N=4) IDR1 (N=8) MedDRA Preferred Term n n n Abdominal
discomfort 1 Anxiety 2 Depressive symptom 1* Dizziness 1 Feeling abnormal 1 1 Flashback 1 1 2 Headache 2 1 3 Muscle discomfort 1 Muscle
spasms 1 Nausea 2* Paresthesia 1 Sensory disturbance 3 16-12 mg (N=6); 6-12-18 mg (N=2)
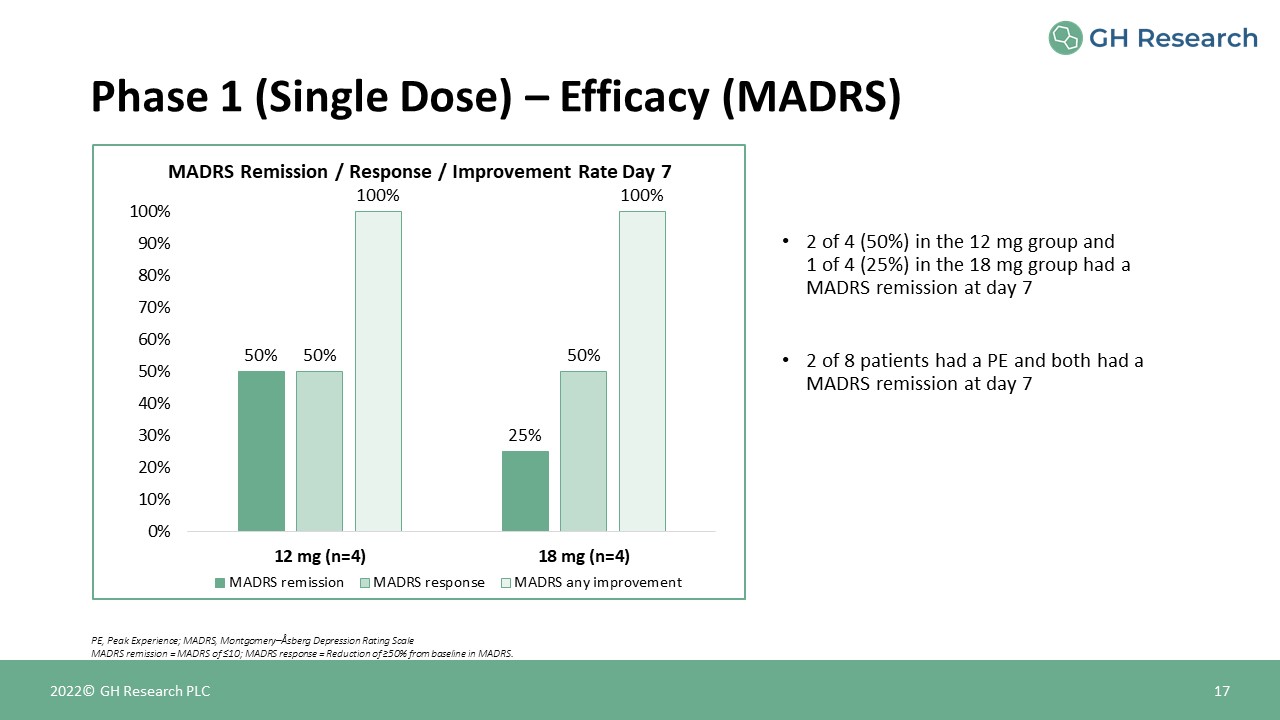
MADRS Remission / Response / Improvement Rate Day 7 Phase 1 (Single Dose) – Efficacy (MADRS) 17 PE,
Peak Experience; MADRS, Montgomery–Åsberg Depression Rating ScaleMADRS remission = MADRS of ≤10; MADRS response = Reduction of ≥50% from baseline in MADRS. 2022© GH Research PLC 2 of 4 (50%) in the 12 mg group and1 of 4 (25%) in the 18 mg
group had a MADRS remission at day 72 of 8 patients had a PE and both had a MADRS remission at day 7

Phase 2 (IDR) – Efficacy (MADRS) 18 2022© GH Research PLC MADRS Remission / Response / Improvement
Rate Day 7 Primary endpoint met: 7 of 8 patients (87.5%) had a MADRS remission at day 7, p<0.00017 of 8 patients had a PE and 6 of those had a MADRS remission at day 7 PE, Peak Experience; MADRS, Montgomery–Åsberg Depression Rating
ScaleMADRS remission = MADRS of ≤10; MADRS response = Reduction of ≥50% from baseline in MADRS.

Phase 2 (IDR) – Efficacy (MADRS Change from Baseline) 2022© GH Research PLC Baseline1 2 hours Day
1 Day 7 GH001 19 p=0.0018 p<0.0001 p<0.0001 Primary endpoint met: 7 of 8 patients (87.5%) had a MADRS remission at day 7, p<0.00017 of 7 remissions from day 1 and5 of 7 remissions from 2 hours 1Baseline mean MADRS=32

MADRS and PE – Observed Improved Outcome in Phase 2 (IDR) vs Phase 1 (Single Dose) Phase 2
(IDR) Phase 1 (Single Dose) 12 mg Phase 1 (Single Dose) 18 mg MADRS Remission Rate Day 7 87.5% (7 of 8) 50% (2 of 4) 25% (1 of 4) Mean MADRS Change Day 7 -24.4 (-76%) -21.0 (-65%) -12.8 (-41%) Rate of PE 87.5% (7 of 8) 50% (2 of
4) 0% (0 of 4) Mean PE Score 90.4 (at final dose) 58.2 59.1 2022© GH Research PLC 20 PE, Peak Experience; MADRS, Montgomery-Åsberg Depression Rating ScaleIDR, Individualized Dosing Regimen

Phase 1 Clinical Pharmacology Trial in Healthy Volunteers GH001-HV-103(Completed) 21 2022© GH
Research PLC

GH001Administration Day 7 GH001 6 mg (n=8+2 placebo) GH001 12 mg (n=8+2 placebo) GH001 18 mg
(n=8+2 placebo) HV(n=30) Single-Dose Part IDR Part HV(n=16) GH001 IDR6, 12, 18 mg to achieve PE(up to 3 doses, 1h interval, n=8) Key Assessments SafetyPharmacokineticsPE ScaleCognitive function SafetyCognitive
function Safety 22 Day 30 GH001 IDR6, 12, 18 mg to achieve PE(up to 3 doses, 2h interval, n=8) Primary Endpoint:Pharmacokinetic profile of 5-MeO-DMT and bufotenine 2022© GH Research PLC Design of Phase 1 Clinical Pharmacology Trial
in Healthy Volunteers (GH001-HV-103) PE, Peak ExperienceIDR, Individualized Dosing Regimen

Safety ReviewNo SAEs All ADRs mildAll ADRs resolved spontaneouslyInhalation well-toleratedNo noteworthy
changes in vital parameters, except for temporary, non-clinically relevant increase in heart rate and blood pressure shortly after administration of GH001No clinically relevant changes in ECG, safety laboratory analyses, peak flow assessment,
and psychiatric safety assessments, including the C-SSRS Single Dose and IDR – Safety 23 Adverse Drug Reaction, or ADR, an adverse event with a relationship code to the investigational product of definite, probable, or possible, or where
code is missingIDR, Individualized Dosing Regimen; C-SSRS, Columbia-Suicide Severity Rating Scale; Pharmacokinetic analyses and analyses of various secondary endpoints are still ongoing. 2022© GH Research PLC ADRs Single-dose
IDR 6 mg (N=8) 12 mg (N=8) 18 mg (N=8) Placebo (N=6) 1h interval (N=8)2 2h interval (N=8)3 MedDRA Preferred Term n n n n n n Abnormal dreams 1 Chest
discomfort 1 Crying 2 2 Dizziness 1 Dry mouth 1 Dyskinesia 1 Fatigue 1 2 1 Headache 3 1 1 1 Hypoesthesia oral 1 Paresthesia
oral 1 Retching 1 Somnolence 1 Tachycardia 2 Tension 1 Tremor 1 26 mg (N=1), 6-12 mg (N=3); 6-12-18 mg (N=4)36-12 mg (N=3); 6-12-18 mg (N=5)
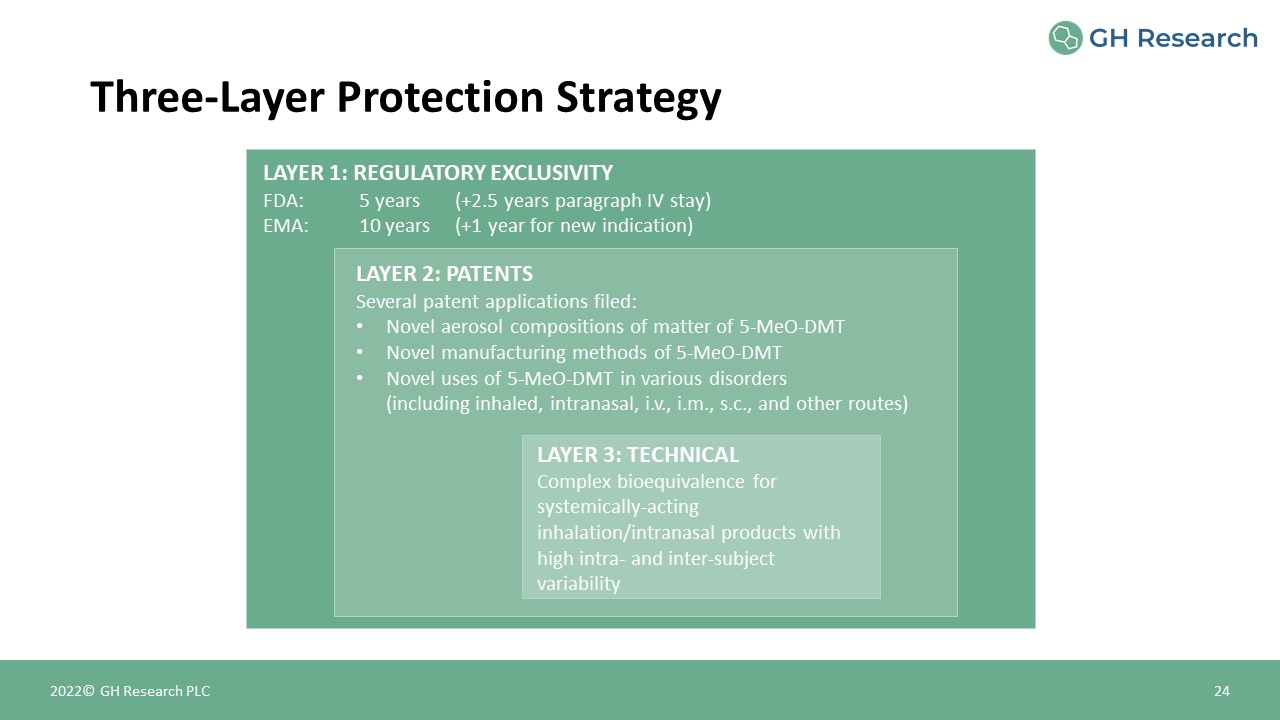
LAYER 1: REGULATORY EXCLUSIVITYFDA: 5 years (+2.5 years paragraph IV stay)EMA: 10 years (+1 year
for new indication) LAYER 3: TECHNICALComplex bioequivalence for systemically-acting inhalation/intranasal products with high intra- and inter-subject variability LAYER 2: PATENTSSeveral patent applications filed: Novel aerosol
compositions of matter of 5-MeO-DMTNovel manufacturing methods of 5-MeO-DMTNovel uses of 5-MeO-DMT in various disorders (including inhaled, intranasal, i.v., i.m., s.c., and other routes) Three-Layer Protection Strategy 24 2022© GH
Research PLC

Board of Directors & Management 25 Florian Schönharting Spike Loy Michael Forer MScChairman of
the Board, Co-founder JDBoard Member BA, LLBBoard Member 2022© GH Research PLC Dermot Hanley Duncan Moore BSC, MBABoard Member MPhil, PhDBoard Member Theis Terwey PD Dr. med. CEO, Co-founder Julie Ryan ACA, MAcc, BCommVP,
Finance Magnus Halle BScManaging Director, Ireland, Co-founder

Scientific Advisors 26 Michael Thase M.D.Professor of Psychiatry,Perelman School of
MedicineUniversity of Pennsylvania Madhukar Trivedi M.D.Professor of Psychiatry,UT Southwestern Medical Center Mark Zimmerman M.D.Professor of Psychiatry and Human Behavior,Brown University Eduard Vieta Prof. Dr. Head, Psychiatry
Unit,Hospital Clínic de Barcelona Michael Bauer Prof. Dr. rer. nat. Dr. med.Chair, Department of Psychiatry and Psychotherapy,Technische Universität Dresden Malek Bajbouj Prof. Dr. med.Head, Center for Affective Neuroscience,Charité,
Berlin Johannes Ramaekers Prof. Dr.Professor, Faculty of Psychologyand Neuroscience of Maastricht University 2022© GH Research PLC

Anticipated Milestones GH001Request a pre-IND meeting with the FDA in Q1 20221 Initiate randomized,
controlled Phase 2b trial in TRDRequest regulatory clearance for two Phase 2a trials in two additional psychiatric disorders in Q1 2022GH002 and GH003Complete preclinical work and initiate Phase 1 trial in Healthy Volunteers 27 2022© GH
Research PLC 1EMA Scientific Advice not considered necessary at this time.

SeekingUltra-Rapid, Durable Remissionsin Depression 28 2022© GH Research PLC
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Exclusive Selections to Prepare for Summer: April Offers of Tineco
- HANDSHAKE SPEAKEASY IN MEXICO CITY NAMED AS THE BEST BAR IN NORTH AMERICA AS RANKING OF NORTH AMERICA'S 50 BEST BARS IS REVEALED AT THIRD ANNUAL AWARDS CEREMONY
- Festi hf.: Presentation of Q1 2024 results
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share