Form 6-K BeyondSpring Inc. For: Aug 05
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16
UNDER THE SECURITIES EXCHANGE ACT OF 1934
For the month of August 2021
Commission File Number: 001-38024
BeyondSpring Inc.
BeyondSpring Inc.
28 Liberty Street, 39th Floor
New York, New York 10005
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): ☐
Note: Regulation S-T Rule 101(b)(1) only permits the submission in paper of a Form 6-K if submitted solely to provide an attached annual report to security holders.
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): ☐
Note: Regulation S-T Rule 101(b)(7) only permits the submission in paper of a Form 6-K if submitted to furnish a report or other document that the registrant foreign private issuer must furnish and make public under the laws of the jurisdiction in
which the registrant is incorporated, domiciled or legally organized (the registrant’s “home country”), or under the rules of the home country exchange on which the registrant’s securities are traded, as long as the report or other document is not a
press release, is not required to be and has not been distributed to the registrant’s security holders, and, if discussing a material event, has already been the subject of a Form 6-K submission or other Commission filing on EDGAR.
BeyondSpring Inc. (the “Company”) has made an investor presentation available on the Company’s website at http://www.beyondspringpharma.com. A copy of the presentation is furnished with this Report of Foreign Private Issuer on Form 6-K as Exhibit
99.1 and is incorporated herein by reference. From time to time, the Company will use this updated presentation in conversations with investors, analysts, and others.
The information in Exhibit 99.1 of this Report of Foreign Private Issuer on Form 6-K is “furnished” only and shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or
otherwise subject to the liabilities of that section, and shall not be incorporated by reference into any registration statement or other document filed under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly
set forth by specific reference in such filing. This Report of Foreign Private Issuer on Form 6-K will not be deemed an admission as to the materiality of any information in this report.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
BeyondSpring Inc.
|
||
|
By:
|
/s/ Lan Huang
|
|
|
Name:
|
Lan Huang
|
|
|
Title:
|
Chairperson and Chief Executive Officer
|
|
|
Date: August 5, 2021
|
||
EXHIBIT INDEX
|
Exhibit No.
|
Exhibit
|
|
Investor presentation of BeyondSpring Inc., dated August 5, 2021.
|
Exhibit 99.1

Plinabulin DUBLIN-3 NSCLC Topline Data August 5 2021 | NASDAQ: BYSI

Disclaimer This presentation has been prepared for informational purposes only. No money or other
consideration is being solicited, and if sent in response, will not be accepted. This presentation shall not constitute an offer to sell, or the solicitation of an offer to buy, any securities, nor shall there be any sale of these securities
in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. The Company is not under any obligation to make an
offering. It may choose to make an offering to some, but not all, of the people who indicate an interest in investing. The information included in any registration statement will be more complete than the information the Company is providing
now, and could differ in important ways.This presentation and any accompanying oral commentary contain forward-looking statements about BeyondSpring Inc. (“BeyondSpring” or the “Company”). Forward- looking statements are based on our
management’s beliefs and assumptions and on information currently available to our management, including those described in the forward-looking statements and risk factors sections of the Company’s 20-F filed on April 30, 2021 and other
filings with the United States Securities and Exchange Commission (SEC).Such statements are subject to known and unknown risks, uncertainties, and other factors that may cause our or our industry’s actual results, levels of activity,
performance, or achievements to be materially different from those anticipated by such statements. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,”
“believes,” “estimates,” “predicts,” “potential,” “intends,” or “continue,” or the negative of these terms or other comparable terminology. Forward-looking statements contained in this presentation include, but are not limited to, (i)
statements regarding the timing of anticipated clinical trials for our product candidates and our research and development programs; (ii) the timing of receipt of clinical data for our product candidates; (iii) our expectations regarding the
potential safety, efficacy, or clinical utility of our product candidates; (iv) the size of patient populations targeted by our product candidates and market adoption of our product candidates by physicians and patients; and (v) the timing or
likelihood of regulatory filings and approvals.Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons why actual results could differ materially from those anticipated
in the forward-looking statements, even if new information becomes available in the future.The market data and certain other statistical information used throughout this presentation are based on independent industry publications,
governmental publications, reports by market research firms or other independent sources. Some data are also based on our good faith estimates. Although we believe these third-party sources are reliable, we have not independently verified the
information attributed to these third-party sources and cannot guarantee its accuracy and completeness. Similarly, our estimates have not been verified by any independent source.By attending this presentation, you acknowledge that you will be
solely responsible for your own assessment of the market and our market position and that you will conduct your own analysis and be solely responsible for forming your own view of the potential future performance of our business.

Plinabulin: First-in-Class, Selective Immunomodulating Microtubule-Binding Agent (SIMBA) - Potent
Antigen Presenting Cell (APC) inducer Note: 1 La Sala et al., 2019 Chem. 2 Kashyap et al., 2019 Cell Reports. 3 Zhang et al., 2005 Mol Cell Biol. 4 Singh et al., 2011 Blood. 5 Suwa et al., 2000 Am J Physiol Heart Circ Physiol; Ghosh et al.,
2018 ACR Annual Conference; Blayney et al., Society of Leukocyte Biology. 6 Asensi et al., 2004 Infection and Immunity. Dendritic cell maturation2,3 GEF-H1 activation2 Rho/ROCK activation1 T cell activation2,3 Kill cancer cells2 Kill
cancer cells4 Increase LSK cells;Accelerate neutrophil maturation5 Neutrophil demargination7 JNK activation2 Microtubules Delay neutrophil apoptosis6 Plinabulin1 Plinabulin Novel Target: Immune Defense Protein GEF-H1

Plinabulin Induces Dendritic Cell Maturation (the most potent APC), a Key Step in Initiating
Anti-cancer Durable Response ❶ + ❷ + ❸ Optimal Immuno-Oncology Response Release Tumor AntigensFor more potent anti-cancer effect ❶ Radiation/Chemotherapy/ Plinabulin Optimize T cell response ❸ Checkpoint Inhibitors ❷
Plinabulin Stimulate maturation of dendritic cells to increase antigen presentation Dendritic cells are the most important antigen-presenting cells 2 1 3

Severely Unmet Medical Need – 2nd/3rd Line NSCLC, EGFR Wild Type 1 Lancet Oncol. 2013
Sep;14(10):981-8. Large patient population with limited treatment optionsEGFR wild type: ~85% western NSCLC and ~70% of Asian NSCLC patients With immunotherapies moved to first line, Docetaxel-based therapies are the mainstay therapyTKIs are
worse than docetaxel1Docetaxel-based Therapies (SOC)Limited efficacy>40% severe neutropenia Efficacy Safety Since nivolumab was approved 6 years ago, no new agent with novel mechanism has been approved in this indication.

Scientific Rationale – Patients with High GEF-H1 Live Longer Patients with High GEF-H1 Immune
Signature Live Longer in Various Cancers1 1 Kashyap et al., 2019 Cell Reports Based on Plinabulin’s Immune MOA, patients with measurable lung lesion were selected prospectively for Dublin-3 Study. Plinabulin Activates GEF-H11

Docetaxel + Plinabulin Compared to Docetaxel + Placebo in Patients With 2nd/3rd line NSCLC, EGFR wild
type (DUBLIN-3) Non-squamous or squamous NSCLCStage IIIb/IVECOG performance status ≤ 2Progression during or after treatment with one or two treatment regimen containing platinumMust have at least one measurable lung lesionPrior checkpoint
inhibitor therapy allowed R Docetaxel + Plinabulin Docetaxel + Placebo Global, Randomized, Single-Blinded (blinding for patients only)Stratified for: Region (Asia/non-Asia), Prior Line, ECOG score60 sites: U.S., China, and
Australia Primary Endpoint: Overall SurvivalSecondary Endpoints: ORR, PFS Percent of patients without severe neutropenia on Day 8 of Cycle 1 Month 24 OS rate, Month 36 OS rate DoR Q-TWiST QoLProportion of patients who received docetaxel
>8 cycles, >10 cycles, and >12 cycles 1:1 ratio

Dublin-3 Phase 3 Topline Data- Significant Improvement in OS, PFS, ORR, 24 M, 36 M OS Rate (Combo vs.
Docetaxel)- Significant Reduction in Grade 4 Neutropenia (Combo vs. Docetaxel) Primary Endpoint Docetaxel (75 mg/m2)N=281 Plinabulin (30 mg/m2) + Docetaxel (75 mg/m2)N=278 OS (months or M) Mean OS, p=0.03OS Log-rank p<0.04; HR =
0.82 Doubling OS rate in 24 M, 36 M, and 10.6% >48 M OS rate – Plinabulin Immune Durable Anti-cancer Benefit Secondary Endpoint- Hierarchy Order DocetaxelN=281 Plinabulin (30 mg/m2) + Docetaxel (75 mg/m2)N=278 ORR (%) P
<0.03 PFS (months or M) P<0.01 Grade 4 neutropenia, cycle 1 Day 8 (%) 27.8% 5.3%; p<0.0001 24 Month OS Rate (%) 12.5% 22.1%; p<0.01 36 Month OS Rate (%) 5.3% 11.7%; p<0.04 48 Month OS Rate (%) -
exploratory 0% 10.6%; p value cannot be calculated

OS Analysis Method Based on Relevance 1. Mean OS p value – Most Relevant for immune agent with more
longer survival patientsAnalysis method stated in Statistical Analysis Plan (SAP); the data will be included in Clinical Study Report (CSR)Mean OS takes into consideration all patients’ overall survival time and censoring time. It is stated
in SAP to use restricted mean survival time (RMST) methods to analyze the mean OS; the expected survival time for the two treatments were compared, restricted to the maximum follow-up time for the study. 2. OS Log-rank p value – Relevant as
it takes into account the whole OS K-M curveAnalysis method stated in SAP; the data will be included in CSROS log-rank p value needs to be p < 0.046, 2-sided test, to meet statistical significance due to adjustment for prespecified interim
looksOS Log rank method is one of the most popular methods of comparing the survival for treatments, which takes the whole follow-up period into account. It is a nonparametric method and has the considerable advantage that it does not require
knowing anything about the shape of the survival curve or the distribution of survival times. 3. OS Hazard Ratio (HR) – Not Relevant for immune agent with more longer survival patients - Per the SAP, HR would not be presented in the CSR if
it fails Cox proportional hazard ratio assumption. Since Plinabulin is an immune agent with treatment effects that vary over different time points, the assumption failed; thus, the HR will not be included in the CSR.Hazard ratio (e.g.,
hazard under active treatment/hazard under control) is often used to characterize the treatment effects for survival data. It is derived under Cox proportional hazard ratio (HR) assumptions. Under this assumption, the HR is the same at all
timepoints (i.e., the treatment effect in terms of improving hazard is the same at all timepoints). The assumption fails to hold if the HR varies over timepoints (i.e., the treatment effects vary over different timepoints). In fact, Cox
proportional hazard ratio assumption often fails for immune anticancer treatments, including plinabulin, as these treatments have greater effects in the later part of the survival curve than in the earlier part. Clearly, in this situation,
the single HR number derived under Cox proportional hazard ratio assumption is not relevant to consider.

Product Profile (Plinabulin + Docetaxel for 2nd/3rd line NSCLC, EGFR wild type) Docetaxel
(Current SOC) Modest survival benefit Severe safety concerns, e.g., CIN Poor Quality of Life Plinabulin - Docetaxel Combination Survival benefit, with longer survival due to GEF-H1 I/O MOA Favorable safety profile, including
significant CIN reduction Improved quality of life Lower Grade 4 AE frequency and a shift to lower grade AENo unexpected AE concerns were identified Next steps: Discuss filing plan with FDA & NMPA in 2021 with potential filing 1H 2022

Plinabulin: potential as the “Cornerstone” Therapy to Add onto Current IO Therapies to Address Severe
Unmet Medical Needs Current Severe Unmet Medical Needs PD-1/PD-L1 resistant patients need later line therapies PD-1 + chemo double efficacy of PD-1,but with CIN risk PD-1 or PD-1+CTLA-4 with high ir-SAE Plinabulin Clinical
Development Plinabulin + I/O + chemo/radiation Plinabulin is developed as a CIN prevention agent (pan cancer, pan chemo) Plinabulin+PD-1+CTLA-4 in SCLC PD-1/PD-L1 non-responsive tumor; Patients who cannot use PD-1/PD-L1 Plinabulin+ I/O +
chemo/radiation Plinabulin + chemo +“Easy-to-use”APC Inducer Potential to greatly expand the addressable market PD-1/PD-L1 Inhibitors - $30 B global annual sales
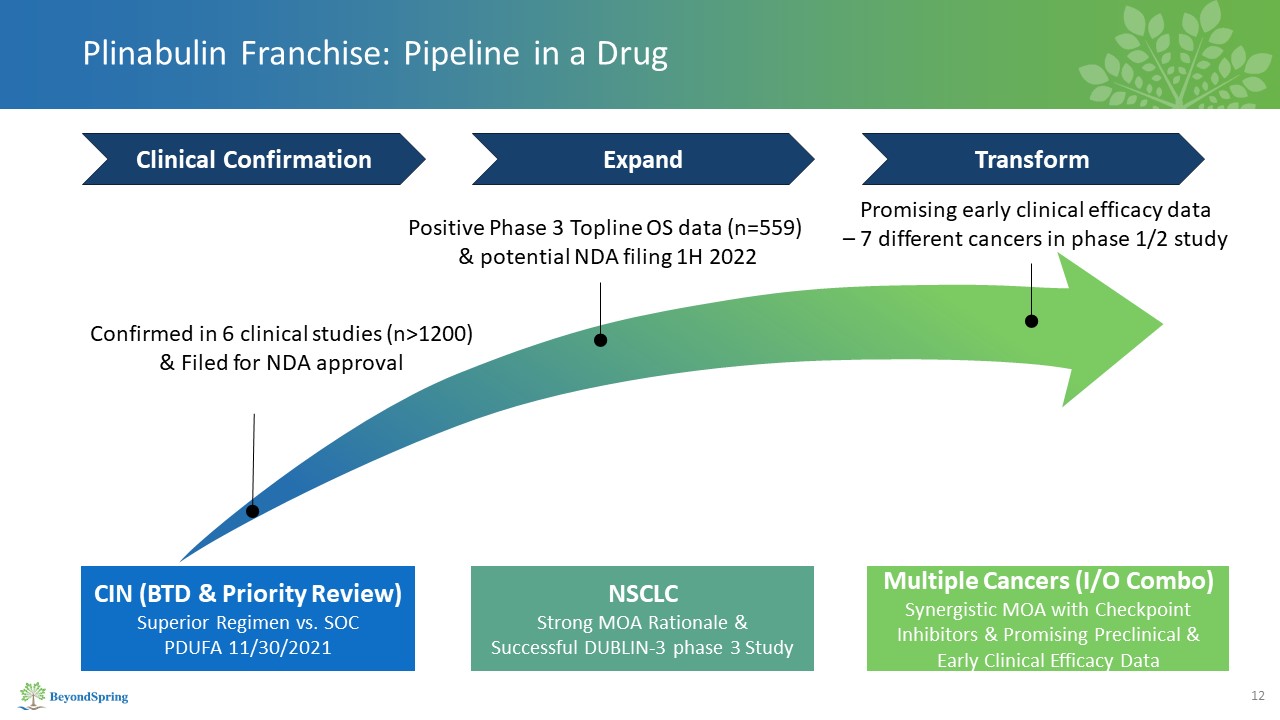
CIN (BTD & Priority Review)Superior Regimen vs. SOCPDUFA 11/30/2021 Multiple Cancers (I/O
Combo)Synergistic MOA with Checkpoint Inhibitors & Promising Preclinical & Early Clinical Efficacy Data NSCLCStrong MOA Rationale & Successful DUBLIN-3 phase 3 Study Promising early clinical efficacy data – 7 different cancers
in phase 1/2 study Positive Phase 3 Topline OS data (n=559) & potential NDA filing 1H 2022 Confirmed in 6 clinical studies (n>1200) & Filed for NDA approval Plinabulin Franchise: Pipeline in a Drug Clinical
Confirmation Expand Transform

www.beyondspringpharma.com
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- C2C Metals Announces Chief Executive Officer
- Altisource Announces First Quarter 2024 Financial Results
- Newmont Reports First Quarter 2024 Results
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share