Form 10-Q PERRIGO Co plc For: Mar 31
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
_______________________________________________
FORM 10-Q
_______________________________________________
[X] | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
OR
[ ] | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission file number 001-36353
_______________________________________________
Perrigo Company plc
(Exact name of registrant as specified in its charter)
_______________________________________________
Ireland | Not Applicable | |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) | |
Treasury Building, Lower Grand Canal Street, Dublin 2, Ireland | - | |
(Address of principal executive offices) | (Zip Code) | |
(Registrant’s telephone number, including area code)
Not Applicable
(Former name, former address and former fiscal year, if changed since last report)
________________________________________
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such report), and (2) has been subject to such filing requirements for the past 90 days. YES [X] NO [ ]
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). YES [X] NO [ ]
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See definitions of “large accelerated filer”, “accelerated filer”, “smaller reporting company”, and "emerging growth company" in Rule 12b-2 of the Exchange Act.
Large accelerated filer | [X] | Accelerated filer | [ ] | Non-accelerated filer | [ ] | (Do not check if smaller reporting company) | ||||
Smaller reporting company | [ ] | Emerging growth company | [ ] | |||||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. | [ ] | |||||||||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). [ ] YES [X] NO
As of May 4, 2018, there were 138,462,112 ordinary shares outstanding.
PERRIGO COMPANY PLC
FORM 10-Q
INDEX
PAGE NUMBER | ||
PART I. FINANCIAL INFORMATION | ||
1 | ||
2 | ||
3 | ||
4 | ||
5 | ||
6 | ||
7 | ||
8 | ||
9 | ||
10 | ||
11 | ||
12 | ||
13 | ||
14 | ||
15 | ||
16 | ||
PART II. OTHER INFORMATION | ||
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Certain statements in this report are “forward-looking statements” within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and are subject to the safe harbor created thereby. These statements relate to future events or our future financial performance and involve known and unknown risks, uncertainties and other factors that may cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from those expressed or implied by any forward-looking statements. In particular, statements about our expectations, beliefs, plans, objectives, assumptions, future events or future performance contained in this report, including certain statements contained in “Management’s Discussion and Analysis of Financial Condition and Results of Operations” are forward-looking statements. In some cases, forward-looking statements can be identified by terminology such as “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” "forecast," “predict,” “potential” or the negative of those terms or other comparable terminology.
We have based these forward-looking statements on our current expectations, assumptions, estimates and projections. While we believe these expectations, assumptions, estimates and projections are reasonable, such forward-looking statements are only predictions and involve known and unknown risks and uncertainties, many of which are beyond our control, including: the timing, amount and cost of any share repurchases; future impairment charges; the success of management transition; customer acceptance of new products; competition from other industry participants, some of whom have greater marketing resources or larger market shares in certain product categories than we do; pricing pressures from customers and consumers; potential third-party claims and litigation, including litigation relating to our restatement of previously-filed financial information; potential impacts of ongoing or future government investigations and regulatory initiatives; resolution of uncertain tax positions; the impact of U.S. tax reform legislation and healthcare policy; general economic conditions; fluctuations in currency exchange rates and interest rates; the consummation of announced acquisitions or dispositions and the success of such transactions, and our ability to realize the desired benefits thereof; and our ability to execute and achieve the desired benefits of announced cost-reduction efforts and other initiatives. In addition, we may identify new, or be unable to remediate previously identified, material weaknesses in our internal control over financial reporting. Furthermore, we may incur additional tax liabilities in respect of 2016 and prior years or be found to have breached certain provisions of Irish company law in connection with our restatement of our previously filed financial statements, which may result in additional expenses and penalties. These and other important factors, including those discussed in our Form 10-K for the year ended December 31, 2017 and in this report under “Risk Factors” and in any subsequent filings with the United States Securities and Exchange Commission, may cause actual results, performance or achievements to differ materially from those expressed or implied by these forward-looking statements. The forward-looking statements in this report are made only as of the date hereof, and unless otherwise required by applicable securities laws, we disclaim any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise.
TRADEMARKS, TRADE NAMES AND SERVICE MARKS
This report contains trademarks, trade names and service marks that are the property of Perrigo Company plc, as well as, for informational purposes, trademarks, trade names, and service marks that are the property of other organizations. Solely for convenience, certain trademarks, trade names, and service marks referred to in this report appear without the ®, ™ and SM symbols, but those references are not intended to indicate that we or the applicable owner, as the case may be, will not assert, to the fullest extent under applicable law, our or their rights to such trademarks, trade names, and service marks.
1
Perrigo Company plc - Item 1
PART I. FINANCIAL INFORMATION
ITEM 1. FINANCIAL STATEMENTS (UNAUDITED)
PERRIGO COMPANY PLC
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(in millions, except per share amounts)
(unaudited)
Three Months Ended | |||||||
March 31, 2018 | April 1, 2017 | ||||||
Net sales | $ | 1,217.0 | $ | 1,194.0 | |||
Cost of sales | 724.3 | 729.6 | |||||
Gross profit | 492.7 | 464.4 | |||||
Operating expenses | |||||||
Distribution | 24.7 | 21.1 | |||||
Research and development | 38.4 | 39.8 | |||||
Selling | 161.3 | 155.0 | |||||
Administration | 107.6 | 105.4 | |||||
Impairment charges | — | 12.2 | |||||
Restructuring | 1.5 | 38.7 | |||||
Other operating loss (income) | 2.9 | (36.3 | ) | ||||
Total operating expenses | 336.4 | 335.9 | |||||
Operating income | 156.3 | 128.5 | |||||
Change in financial assets | 9.6 | (17.1 | ) | ||||
Interest expense, net | 31.4 | 53.3 | |||||
Other expense (income), net | 4.3 | (3.5 | ) | ||||
Loss on extinguishment of debt | 0.5 | — | |||||
Income before income taxes | 110.5 | 95.8 | |||||
Income tax expense | 29.7 | 24.2 | |||||
Net income | $ | 80.8 | $ | 71.6 | |||
Earnings per share | |||||||
Basic | $ | 0.57 | $ | 0.50 | |||
Diluted | $ | 0.57 | $ | 0.50 | |||
Weighted-average shares outstanding | |||||||
Basic | 140.8 | 143.4 | |||||
Diluted | 141.4 | 143.6 | |||||
Dividends declared per share | $ | 0.19 | $ | 0.16 | |||
See accompanying Notes to the Condensed Consolidated Financial Statements
2
Perrigo Company plc - Item 1
PERRIGO COMPANY PLC
CONDENSED CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
(in millions)
(unaudited)
Three Months Ended | |||||||
March 31, 2018 | April 1, 2017 | ||||||
Net income | $ | 80.8 | $ | 71.6 | |||
Other comprehensive income: | |||||||
Foreign currency translation adjustments | 73.0 | 65.4 | |||||
Change in fair value of derivative financial instruments, net of tax | (0.6 | ) | 1.6 | ||||
Change in fair value of investment securities, net of tax | — | (11.4 | ) | ||||
Change in post-retirement and pension liability, net of tax | (0.2 | ) | (0.1 | ) | |||
Other comprehensive income, net of tax | 72.2 | 55.5 | |||||
Comprehensive income | $ | 153.0 | $ | 127.1 | |||
See accompanying Notes to the Condensed Consolidated Financial Statements
3
Perrigo Company plc - Item 1
PERRIGO COMPANY PLC
CONDENSED CONSOLIDATED BALANCE SHEETS
(in millions, except per share amounts)
(unaudited)
March 31, 2018 | December 31, 2017 | ||||||
Assets | |||||||
Cash and cash equivalents | $ | 687.3 | $ | 678.7 | |||
Accounts receivable, net of allowance for doubtful accounts of $6.5 million and $6.2 million, respectively | 1,123.4 | 1,130.8 | |||||
Inventories | 843.8 | 806.9 | |||||
Prepaid expenses and other current assets | 246.2 | 203.2 | |||||
Total current assets | 2,900.7 | 2,819.6 | |||||
Property, plant and equipment, net | 829.3 | 833.1 | |||||
Goodwill and other indefinite-lived intangible assets | 4,300.8 | 4,265.7 | |||||
Other intangible assets, net | 3,259.1 | 3,290.5 | |||||
Non-current deferred income taxes | 19.6 | 10.4 | |||||
Other non-current assets | 330.1 | 409.5 | |||||
Total non-current assets | 8,738.9 | 8,809.2 | |||||
Total assets | $ | 11,639.6 | $ | 11,628.8 | |||
Liabilities and Shareholders’ Equity | |||||||
Accounts payable | $ | 512.2 | $ | 450.2 | |||
Payroll and related taxes | 113.0 | 148.8 | |||||
Accrued customer programs | 438.3 | 419.7 | |||||
Accrued liabilities | 205.3 | 230.8 | |||||
Accrued income taxes | 65.7 | 116.1 | |||||
Current indebtedness | 58.0 | 70.4 | |||||
Total current liabilities | 1,392.5 | 1,436.0 | |||||
Long-term debt, less current portion | 3,280.6 | 3,270.8 | |||||
Non-current deferred income taxes | 332.0 | 321.9 | |||||
Other non-current liabilities | 428.9 | 429.5 | |||||
Total non-current liabilities | 4,041.5 | 4,022.2 | |||||
Total liabilities | 5,434.0 | 5,458.2 | |||||
Commitments and contingencies - Note 14 | |||||||
Shareholders’ equity | |||||||
Controlling interest: | |||||||
Preferred shares, $0.0001 par value per share, 10 shares authorized | — | — | |||||
Ordinary shares, €0.001 par value per share, 10,000 shares authorized | 7,769.5 | 7,892.9 | |||||
Accumulated other comprehensive income | 324.3 | 253.1 | |||||
Retained earnings (accumulated deficit) | (1,888.4 | ) | (1,975.5 | ) | |||
Total controlling interest | 6,205.4 | 6,170.5 | |||||
Noncontrolling interest | 0.2 | 0.1 | |||||
Total shareholders’ equity | 6,205.6 | 6,170.6 | |||||
Total liabilities and shareholders' equity | $ | 11,639.6 | $ | 11,628.8 | |||
Supplemental Disclosures of Balance Sheet Information | |||||||
Ordinary shares, issued and outstanding | 139.7 | 140.8 | |||||
See accompanying Notes to the Condensed Consolidated Financial Statements
4
Perrigo Company plc - Item 1
PERRIGO COMPANY PLC
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(in millions)
(unaudited)
Three Months Ended | |||||||
March 31, 2018 | April 1, 2017 | ||||||
Cash Flows From (For) Operating Activities | |||||||
Net income | $ | 80.8 | $ | 71.6 | |||
Adjustments to derive cash flows | |||||||
Depreciation and amortization | 109.5 | 109.4 | |||||
Share-based compensation | 12.7 | 6.1 | |||||
Impairment charges | — | 12.2 | |||||
Change in financial assets | 9.6 | (17.1 | ) | ||||
Loss on extinguishment of debt | 0.5 | — | |||||
Restructuring charges | 1.5 | 38.7 | |||||
Deferred income taxes | (7.2 | ) | (46.0 | ) | |||
Amortization of debt premium | (2.1 | ) | (6.4 | ) | |||
Other non-cash adjustments, net | 12.1 | (1.1 | ) | ||||
Subtotal | 217.4 | 167.4 | |||||
Increase (decrease) in cash due to: | |||||||
Accounts receivable | 2.6 | 50.1 | |||||
Inventories | (43.7 | ) | 0.5 | ||||
Accounts payable | 57.5 | 2.5 | |||||
Payroll and related taxes | (38.9 | ) | (10.1 | ) | |||
Accrued customer programs | 17.3 | (32.7 | ) | ||||
Accrued liabilities | (24.0 | ) | 2.3 | ||||
Accrued income taxes | 6.4 | 41.4 | |||||
Other, net | (22.2 | ) | (26.9 | ) | |||
Subtotal | (45.0 | ) | 27.1 | ||||
Net cash from operating activities | 172.4 | 194.5 | |||||
Cash Flows From (For) Investing Activities | |||||||
Proceeds from royalty rights | 10.0 | 85.3 | |||||
Additions to property, plant and equipment | (13.4 | ) | (22.0 | ) | |||
Net proceeds from sale of business and other assets | 1.3 | 25.3 | |||||
Proceeds from sale of the Tysabri® financial asset | — | 2,200.0 | |||||
Other investing, net | — | (0.8 | ) | ||||
Net cash from (for) investing activities | (2.1 | ) | 2,287.8 | ||||
Cash Flows From (For) Financing Activities | |||||||
Issuances of long-term debt | 431.0 | — | |||||
Payments on long-term debt | (444.5 | ) | (13.6 | ) | |||
Borrowings (repayments) of revolving credit agreements and other financing, net | (6.2 | ) | 0.3 | ||||
Deferred financing fees | (2.4 | ) | (0.4 | ) | |||
Repurchase of ordinary shares | (108.1 | ) | — | ||||
Cash dividends | (26.7 | ) | (23.0 | ) | |||
Other financing, net | (5.7 | ) | (0.5 | ) | |||
Net cash (for) financing activities | (162.6 | ) | (37.2 | ) | |||
Effect of exchange rate changes on cash and cash equivalents | 0.9 | 10.4 | |||||
Net increase (decrease) in cash and cash equivalents | 8.6 | 2,455.5 | |||||
Cash and cash equivalents, beginning of period | 678.7 | 622.3 | |||||
Cash and cash equivalents, end of period | $ | 687.3 | $ | 3,077.8 | |||
See accompanying Notes to the Condensed Consolidated Financial Statements
5
Perrigo Company plc - Item 1
Note 1
NOTE 1 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
General Information
The Company
Perrigo Company plc was incorporated under the laws of Ireland on June 28, 2013 and became the successor registrant of Perrigo Company, a Michigan corporation, on December 18, 2013 in connection with the acquisition of Elan Corporation, plc ("Elan"). Unless the context requires otherwise, the terms "Perrigo," the "Company," "we," "our," "us," and similar pronouns used herein refer to Perrigo Company plc, its subsidiaries, and all predecessors of Perrigo Company plc and its subsidiaries.
We are a leading global healthcare company, delivering value to our customers and consumers by providing Quality Affordable Healthcare Products®. Founded in 1887 as a packager of home remedies, we have built a unique business model that is best described as the convergence of a fast-moving consumer goods company, a high-quality pharmaceutical manufacturing organization and a world-class supply chain network. We believe we are one of the world's largest manufacturers of over-the-counter (“OTC”) healthcare products and suppliers of infant formulas for the store brand market. We are a leading provider of branded OTC products throughout Europe, and also a leading producer of generic pharmaceutical topical products such as creams, lotions, gels, and nasal sprays ("extended topical") prescription drugs. We are headquartered in Ireland, and sell our products primarily in North America and Europe, as well as in other markets, including Australia, Israel and China.
Basis of Presentation
The accompanying unaudited Condensed Consolidated Financial Statements have been prepared in accordance with U.S. generally accepted accounting principles ("GAAP") for interim financial information and with the instructions to Article 10 of Regulation S-X. Accordingly, they do not include all of the information and footnotes required by GAAP for complete financial statements. The unaudited Condensed Consolidated Financial Statements should be read in conjunction with the Consolidated Financial Statements and footnotes included in our Annual Report on Form 10-K for the year ended December 31, 2017. In the opinion of management, all adjustments (consisting of normal recurring accruals and other adjustments) considered necessary for a fair presentation of the unaudited Condensed Consolidated Financial Statements have been included and include our accounts and the accounts of all majority-owned subsidiaries. All intercompany transactions and balances have been eliminated in consolidation.
Recent Accounting Standard Pronouncements
Below are recent Accounting Standard Updates ("ASU") that we are still assessing to determine the effect on our Condensed Consolidated Financial Statements. We do not believe that any other recently issued accounting standards could have a material effect on our Condensed Consolidated Financial Statements. As new accounting pronouncements are issued, we will adopt those that are applicable under the circumstances.
6
Perrigo Company plc - Item 1
Note 1
Recently Issued Accounting Standards Not Yet Adopted | ||||||
Standard | Description | Effective Date | Effect on the Financial Statements or Other Significant Matters | |||
Leases | This guidance was issued to increase transparency and comparability among organizations by requiring recognition of lease assets and lease liabilities on the balance sheet and disclosure of key information about leasing arrangements. For leases with a term of 12 months or less, lessees are permitted to make an election to not recognize right-of-use assets and lease liabilities. The guidance is required to be adopted using the modified retrospective approach. Early adoption is permitted. | January 1, 2019 | We have begun to prepare a full inventory of our portfolio of leases and once complete, we will begin to assess and quantify the likely impact on our Consolidated Financial Statements. In addition, we are in the design phase of our lease integration tool. | |||
Derivatives and Hedging | This update was issued to enable entities to better portray the economics of their risk management activities in the financial statements and enhance the transparency and understandability of hedge results. In addition, the amendments simplify the application of hedge accounting in certain situations. Under the new rule, the entity’s ability to hedge non-financial and financial risk components is expanded. The guidance eliminates the requirement to separately measure and report hedge ineffectiveness and also eases certain documentation and assessment requirements. Early adoption is permitted. | January 1, 2019 | We are currently evaluating the implications of adoption on our Consolidated Financial Statements. | |||
Income Statement - Reporting Comprehensive Income (Topic 220): Reclassification of Certain Tax Effects from Accumulated Other Comprehensive Income | This guidance permits tax effects stranded in accumulated other comprehensive income as a result of tax reform to be reclassified to retained earnings. This reclassification is optional and will require additional disclosure regarding whether reclassification is elected or not. | January 1, 2019 | We are currently evaluating the implications of adoption on our Consolidated Financial Statements. | |||
Measurement of Credit Losses on Financial Instruments | This guidance changes the impairment model for most financial assets and certain other instruments, replacing the current "incurred loss" approach with an "expected loss" credit impairment model, which will apply to most financial assets measured at amortized cost and certain other instruments, including trade and other receivables, loans, held-to-maturity debt securities, and off-balance sheet credit exposures such as letters of credit. Early adoption is permitted. | January 1, 2020 | We are currently evaluating the new standard for potential impacts on our receivables, and other financial instruments. | |||
Intangibles - Goodwill and Other Simplifying the Test for Goodwill | The objective of this update is to reduce the cost and complexity of subsequent goodwill accounting by simplifying the impairment test by removing the Step 2 requirement to perform a hypothetical purchase price allocation when the carrying value of a reporting unit exceeds its fair value. If a reporting unit’s carrying value exceeds its fair value, an entity would record an impairment charge based on that difference, limited to the amount of goodwill attributed to that reporting unit. The proposal would not change the guidance on completing Step 1 of the goodwill impairment test. The proposed guidance would be applied prospectively. Early adoption is permitted. | January 1, 2020 | Upon adoption, this guidance eliminates the requirement to calculate the implied fair value of goodwill to measure a goodwill impairment. After adoption, a Step 1 failure will result in an immediate impairment charge based on the carrying value of the reporting unit. There is no immediate adoption impact on the Consolidated Financial Statements as the standard will be adopted prospectively. | |||
7
Perrigo Company plc - Item 1
Note 2
NOTE 2 – REVENUE RECOGNITION
We adopted ASU 2014-09 Revenue from Contracts with Customers and its related amendments (collectively, "ASC 606"), as required, on January 1, 2018 using the modified retrospective method for all contracts not completed as of the adoption date. The reported results for the periods in 2018 reflect the application of ASC 606 while the results for the comparable reporting periods in 2017 were prepared under the guidance of Revenue Recognition ("ASC 605"). The adoption of ASC 606 represents a change in accounting principle that will more closely align revenue recognition with the transfer of control of our products and will provide enhanced disclosures to understand the nature, amount, timing, and uncertainty of revenues and cash flows arising from contracts with customers. In accordance with ASC 606, revenue is recognized when a customer obtains control of promised products. The amount of revenue recognized reflects the consideration to which we expect to be entitled to receive in exchange for these products.
Product Revenue
Revenues from product sales are recognized when or as the customer obtains control of our products.
We generally recognize product revenues for our contract performance obligations at a point in time, typically upon shipment or delivery of the product to the customer. For point in time customers for which control transfers on delivery to the customer due to free on board destination terms (“FOB”), an adjustment is recorded to defer revenue recognition over an estimate of days until control transfers at the point of delivery. Where we recognize revenues at a point in time, the transfer of title is the primary indicator that control has transferred. In other limited instances, primarily relating to those contracts that relate to contract manufacturing performed for our customers and certain store branded products, control transfers as the product is manufactured. Control is deemed to transfer over time for these contracts as the product does not have an alternative use and we have a contractual right to payment for performance completed to date. Revenue for contract manufacturing contracts is recognized over the transfer period using an input method that measures progress towards completion of the performance obligation as costs are incurred. For store branded product revenues recognized over time, an output method is used to recognize revenue when production of a unit is completed, because product customization occurs when the product is packaged as a finished good under the store brand label of the customer.
Net product sales include estimates of variable consideration for which accruals and allowances are established. Variable consideration for product sales consists primarily of chargebacks, rebates, sales returns, shelf stock allowances, administrative fees and other incentive programs. Certain of these accruals and allowances are recorded in the balance sheet as current liabilities and others are recorded as a reduction in accounts receivable. Where appropriate, these estimates take into consideration a range of possible outcomes in which relevant factors such as historical experience, current contractual and statutory requirements, specific known market events and trends, industry data and forecasted customer buying and payment patterns are either probability weighted to derive an estimate of expected value or the estimate reflects the single most likely outcome. Overall, these reserves reflect the best estimates of the amount of consideration to which we are entitled based on the terms of the contract. Actual amounts of consideration ultimately received may differ from our estimates. If actual results in the future vary from the estimates, these estimates are adjusted, which would affect revenue and earnings in the period such variances become known.
Other Revenue Policies
We receive payments from our customers based on billing schedules established in each contract. Amounts are recorded as accounts receivable when our right to consideration is unconditional. In most cases, the timing of the unconditional right to payment aligns with shipment or delivery of the product and the recognition of revenue; however, for those customers where revenue is recognized at a time prior to shipment or delivery due to over-time revenue recognition, a contract asset is recorded and is reclassified to an accounts receivable when it becomes unconditional under the contract upon shipment or delivery to the customer.
We do not assess whether a contract has a significant financing component if the expectation at contract inception is such that the period between payment by the customer and the transfer of the promised products to the customer will be one year or less, which is the case with substantially all customers.
8
Perrigo Company plc - Item 1
Note 2
Taxes collected from customers relating to product sales and remitted to governmental authorities are excluded from revenues.
Shipping and handling costs billed to customers are included in net sales. Conversely, shipping and handling expenses we incur are included in cost of sales.
Disaggregation of Revenue
We generated net sales in the following geographic locations(1) (in millions):
Three Months Ended | |||
March 31, 2018 | |||
U.S. | $ | 786.4 | |
Europe(2) | 361.9 | ||
All other countries(3) | 68.7 | ||
$ | 1,217.0 | ||
(1) The net sales by geography is derived from the location of the entity that sells to a third party.
(2) Includes Ireland net sales of $5.4 million.
(3) Includes net sales generated primarily in Israel, Mexico, Australia, and Canada.
The following is a summary of our net sales by category (in millions):
Three Months Ended | |||
March 31, 2018 | |||
CHCA | |||
Cough/Cold/Allergy/Sinus(1) | $ | 141.5 | |
Infant Nutritionals | 103.4 | ||
Analgesics(1) | 93.7 | ||
Gastrointestinal(1) | 92.2 | ||
Smoking Cessation | 65.9 | ||
Animal Health | 26.3 | ||
Vitamins, Minerals and Dietary Supplements(1) | 3.0 | ||
Other CHCA(1),(2) | 75.6 | ||
Total CHCA | 601.6 | ||
CHCI | |||
Cough, Cold, and Allergy | 98.7 | ||
Lifestyle | 89.7 | ||
Personal Care and Derma-Therapeutics | 75.6 | ||
Natural Health and Vitamins, Minerals and Dietary Supplements | 33.2 | ||
Anti-Parasite | 28.1 | ||
Other CHCI(3) | 76.1 | ||
Total CHCI | 401.4 | ||
Total RX | 214.0 | ||
Total net sales | $ | 1,217.0 | |
(1) Includes net sales from our OTC contract manufacturing business.
(2) | Consists primarily of branded OTC, diagnostic products and other miscellaneous or otherwise uncategorized product lines and markets, none of which is greater than 10% of the segment net sales. |
(3) | Consists primarily of liquid licensed products, diagnostic products and other miscellaneous or otherwise uncategorized product lines and markets, none of which is greater than 10% of the segment net sales. |
9
Perrigo Company plc - Item 1
Note 2
While the majority of revenue is recognized at a point in time, certain of our product revenues are recognized on an over time basis. Predominately, over time customer contracts exist in contract manufacturing arrangements which occur in both the Consumer Healthcare Americas ("CHCA") and Consumer Healthcare International ("CHCI") segments. Contract manufacturing revenues were $69.4 million for the three months ended March 31, 2018.
We also recognized a portion of the store brand OTC product revenues in the CHCA segment on an over time basis; however, the timing between over time and point in time revenue recognition for store brand contracts is not significant due to the short time period between the customization of the product and shipment or delivery.
Contract Balances
The following table provides information about contract assets from contracts with customers (in millions):
Balance Sheet Location | January 1, 2018 | March 31, 2018 | |||||||
Short-term contract assets | Prepaid expenses and other current assets | $ | 20.5 | $ | 26.1 | ||||
We had no asset impairment charges related to contract assets in the three months ended March 31, 2018.
Impact on financial statements
Condensed Consolidated Statement of Operations
Net sales and Cost of sales were higher in the three months ended March 31, 2018 as a result of adopting ASC 606 due to net sales from contract manufacturing and certain OTC product sales being recognized on an over time basis as the performance obligation was satisfied, compared to the previous revenue recognition under ASC 605, which would have occurred when the product was shipped or delivered. This has resulted in the recognition of a contract asset.
Three Months Ended | |||||||||||
March 31, 2018 | |||||||||||
(in millions, except per share amounts, unaudited) | As reported | Adjustments | Before adoption of ASC 606 | ||||||||
Net sales | $ | 1,217.0 | $ | (5.6 | ) | $ | 1,211.4 | ||||
Cost of sales | 724.3 | (3.1 | ) | 721.2 | |||||||
Gross profit | 492.7 | (2.5 | ) | 490.2 | |||||||
Operating income | 156.3 | (2.5 | ) | 153.8 | |||||||
Net income | $ | 80.8 | $ | (2.5 | ) | $ | 78.3 | ||||
Earnings per share | |||||||||||
Basic | $ | 0.57 | $ | (0.02 | ) | $ | 0.55 | ||||
Diluted | $ | 0.57 | $ | (0.02 | ) | $ | 0.55 | ||||
Condensed Consolidated Statement of Comprehensive Income
Three Months Ended | |||||||||||
March 31, 2018 | |||||||||||
(in millions, unaudited) | As reported | Adjustments | Before adoption of ASC 606 | ||||||||
Net income | $ | 80.8 | $ | (2.5 | ) | $ | 78.3 | ||||
Comprehensive income | $ | 153.0 | $ | (2.5 | ) | $ | 150.5 | ||||
10
Perrigo Company plc - Item 1
Note 2
Condensed Consolidated Balance Sheet
Three Months Ended | |||||||||||
March 31, 2018 | |||||||||||
(in millions, unaudited) | As reported | Adjustments | Before adoption of ASC 606 | ||||||||
Assets | |||||||||||
Inventories | $ | 843.8 | $ | 17.9 | $ | 861.7 | |||||
Prepaid expenses and other current assets | 246.2 | (26.1 | ) | 220.1 | |||||||
Total current assets | 2,900.7 | (8.2 | ) | 2,892.5 | |||||||
Total assets | $ | 11,639.6 | $ | (8.2 | ) | $ | 11,631.4 | ||||
Liabilities and Shareholders’ Equity | |||||||||||
Other non-current liabilities | $ | 428.9 | $ | (0.3 | ) | $ | 428.6 | ||||
Total non-current liabilities | 4,041.5 | (0.3 | ) | 4,041.2 | |||||||
Total liabilities | 5,434.0 | (0.3 | ) | 5,433.7 | |||||||
Shareholders’ equity | |||||||||||
Controlling interest: | |||||||||||
Retained earnings (accumulated deficit) | (1,888.4 | ) | (7.9 | ) | (1,896.3 | ) | |||||
Total controlling interest | 6,205.4 | (7.9 | ) | 6,197.5 | |||||||
Total shareholders’ equity | 6,205.6 | (7.9 | ) | 6,197.7 | |||||||
Total liabilities and shareholders' equity | $ | 11,639.6 | $ | (8.2 | ) | $ | 11,631.4 | ||||
Condensed Consolidated Statement of Cash Flows
Three Months Ended | |||||||||||
March 31, 2018 | |||||||||||
(in millions, unaudited) | As reported | Adjustments | Before adoption of ASC 606 | ||||||||
Cash Flows From (For) Operating Activities | |||||||||||
Net income | $ | 80.8 | $ | (2.5 | ) | $ | 78.3 | ||||
Increase (decrease) in cash due to: | |||||||||||
Inventories | (43.7 | ) | (3.1 | ) | (46.8 | ) | |||||
Other, net | (22.2 | ) | 5.6 | (16.6 | ) | ||||||
Subtotal | (45.0 | ) | 2.5 | (42.5 | ) | ||||||
Net cash from operating activities | $ | 172.4 | $ | — | $ | 172.4 | |||||
NOTE 3 – DIVESTITURES
Prior Year Divestitures
On January 3, 2017, we sold certain Abbreviated New Drug Applications ("ANDAs") for $15.0 million to a third party, which was recorded as a gain in Other operating loss (income) on the Condensed Consolidated Statements of Operations in our Prescription Pharmaceuticals ("RX") segment.
On February 1, 2017, we completed the sale of the animal health pet treats plant fixed assets within our CHCA segment, which were previously classified as held-for sale. We received $7.7 million in proceeds, which resulted in an immaterial loss.
11
Perrigo Company plc - Item 1
Note 4
NOTE 4 – GOODWILL AND OTHER INTANGIBLE ASSETS
Goodwill
Changes in the carrying amount of goodwill, by reportable segment, were as follows (in millions):
Reporting Segments: | December 31, 2017 | Currency translation adjustments | March 31, 2018 | |||||||||
CHCA | $ | 1,847.4 | $ | 1.7 | $ | 1,849.1 | ||||||
CHCI | 1,205.7 | 32.7 | 1,238.4 | |||||||||
RX | 1,122.3 | (0.3 | ) | 1,122.0 | ||||||||
Total goodwill | $ | 4,175.4 | $ | 34.1 | $ | 4,209.5 | ||||||
Intangible Assets
Other intangible assets and related accumulated amortization consisted of the following (in millions):
March 31, 2018 | December 31, 2017 | ||||||||||||||
Gross | Accumulated Amortization | Gross | Accumulated Amortization | ||||||||||||
Definite-lived intangibles: | |||||||||||||||
Distribution and license agreements and supply agreements | $ | 312.3 | $ | 180.2 | $ | 311.2 | $ | 169.8 | |||||||
Developed product technology, formulations, and product rights | 1,362.8 | 629.5 | 1,358.4 | 598.7 | |||||||||||
Customer relationships and distribution networks | 1,675.0 | 500.8 | 1,642.0 | 460.6 | |||||||||||
Trademarks, trade names, and brands | 1,367.5 | 149.9 | 1,335.4 | 129.5 | |||||||||||
Non-compete agreements | 14.8 | 12.9 | 14.7 | 12.6 | |||||||||||
Total definite-lived intangibles | $ | 4,732.4 | $ | 1,473.3 | $ | 4,661.7 | $ | 1,371.2 | |||||||
Indefinite-lived intangibles: | |||||||||||||||
Trademarks, trade names, and brands | $ | 52.8 | $ | — | $ | 52.1 | $ | — | |||||||
In-process research and development | 38.5 | — | 38.2 | — | |||||||||||
Total indefinite-lived intangibles | 91.3 | — | 90.3 | — | |||||||||||
Total other intangible assets | $ | 4,823.7 | $ | 1,473.3 | $ | 4,752.0 | $ | 1,371.2 | |||||||
Certain intangible assets are denominated in currencies other than the U.S. dollar; therefore, their gross and accumulated amortization balances are subject to foreign currency movements.
We recorded amortization expense of $87.2 million and $85.5 million for the three months ended March 31, 2018 and April 1, 2017, respectively.
We recorded an impairment charge of $12.2 million on certain In Process Research and Development ("IPR&D") assets during the three months ended April 1, 2017 due to changes in the projected development and regulatory timelines for various projects. We also recorded a decrease in the contingent consideration liability associated with certain IPR&D assets in Other operating loss (income) on the Condensed Consolidated Statements of Operations (refer to Note 7).
NOTE 5 - ACCOUNTS RECEIVABLE FACTORING
We have accounts receivable factoring arrangements with non-related third-party financial institutions (the “Factors”). Pursuant to the terms of the arrangements, we sell to the Factors certain of our accounts receivable balances on a non-recourse basis for credit approved accounts. An administrative fee per invoice is charged on the gross amount of accounts receivables assigned to the Factors, and interest is calculated at the applicable EUR
12
Perrigo Company plc - Item 1
Note 4
LIBOR rate plus a spread. The total amount factored on a non-recourse basis and excluded from accounts receivable was $22.5 million and $27.5 million at March 31, 2018 and December 31, 2017, respectively.
NOTE 6 – INVENTORIES
Major components of inventory were as follows (in millions):
March 31, 2018 | December 31, 2017 | ||||||
Finished goods | $ | 467.4 | $ | 454.3 | |||
Work in process | 161.1 | 152.8 | |||||
Raw materials | 215.3 | 199.8 | |||||
Total inventories | $ | 843.8 | $ | 806.9 | |||
NOTE 7 – FAIR VALUE MEASUREMENTS
Fair value is the price that would be received upon sale of an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The following fair value hierarchy is used in selecting inputs, with the highest priority given to Level 1, as these are the most transparent or reliable.
Level 1: | Quoted prices for identical instruments in active markets. |
Level 2: | Quoted prices for similar instruments in active markets; quoted prices for identical or similar instruments in markets that are not active; and model-derived valuations in which all significant inputs are observable in active markets. |
Level 3: | Valuations derived from valuation techniques in which one or more significant inputs are not observable. |
The following table summarizes the valuation of our financial instruments carried at fair value and measured at fair value on a recurring and non-recurring basis by the above pricing categories (in millions):
Fair Value | ||||||||||
Fair Value Hierarchy | March 31, 2018 | December 31, 2017 | ||||||||
Measured at fair value on a recurring basis: | ||||||||||
Assets: | ||||||||||
Investment securities | Level 1 | $ | 13.8 | $ | 17.0 | |||||
Foreign currency forward contracts | Level 2 | $ | 5.0 | $ | 6.3 | |||||
Funds associated with Israeli severance liability | Level 2 | 15.0 | 16.3 | |||||||
Total level 2 assets | $ | 20.0 | $ | 22.6 | ||||||
Royalty Pharma contingent milestone payments | Level 3 | $ | 124.9 | $ | 134.5 | |||||
Liabilities: | ||||||||||
Foreign currency forward contracts | Level 2 | $ | 3.8 | $ | 3.8 | |||||
Contingent consideration | Level 3 | $ | 18.1 | $ | 22.0 | |||||
Measured at fair value on a non-recurring basis: | ||||||||||
Assets: | ||||||||||
Definite-lived intangible assets(1) | Level 3 | $ | — | $ | 11.5 | |||||
(1) | As of December 31, 2017, definite-lived intangible assets with a carrying amount of $31.2 million were written down to a fair value of $11.5 million. |
13
Perrigo Company plc - Item 1
Note 7
There were no transfers among Level 1, 2, and 3 during the three months ended March 31, 2018 or the year ended December 31, 2017. Our policy regarding the recording of transfers between levels is to record any such transfers at the end of the reporting period (refer to Note 8 for information on our investment securities and Note 9 for a discussion of derivatives).
Foreign Currency Forward Contracts
The fair value of foreign currency forward contracts is determined using a market approach, which utilizes values for comparable derivative instruments.
Funds Associated with Israel Severance Liability
Israeli labor laws and agreements require us to pay benefits to employees dismissed or retiring under certain circumstances. Severance pay is calculated on the basis of the most recent employee salary levels and the length of employee service. Our Israeli subsidiaries also provide retirement bonuses to certain managerial employees. We make regular deposits to retirement funds and purchase insurance policies to partially fund these liabilities. The funds are determined using prices for recently traded financial instruments with similar underlying terms, as well as directly or indirectly observable inputs, such as interest rates and yield curves, that are observable at commonly quoted intervals.
Financial Assets
On March 27, 2017, we announced the completed divestment of our Tysabri® financial asset to Royalty Pharma for up to $2.85 billion, consisting of $2.2 billion in cash and $250.0 million and $400.0 million in milestone payments if the royalties on global net sales of Tysabri® that are received by Royalty Pharma meet specific thresholds in 2018 and 2020, respectively. As a result of this transaction, we transferred the entire financial asset to Royalty Pharma and recorded a $17.1 million gain during the three months ended April 1, 2017. We elected to account for the contingent milestone payments using the fair value option method, and these were recorded at an estimated fair value of $184.5 million as of April 1, 2017. We chose the fair value option as we believe it will help investors understand the potential future cash flows we may receive associated with the two contingent milestones.
Royalty Pharma Contingent Milestone Payments
We valued our contingent milestone payments from Royalty Pharma using a modified Black-Scholes Option Pricing Model ("BSOPM"). Key inputs in the BSOPM are the estimated volatility and rate of return of royalties on global net sales of Tysabri® that are received by Royalty Pharma until the contingent milestones are resolved. Volatility and the estimated fair value of the milestones have a positive relationship such that higher volatility translates to a higher estimated fair value of the contingent milestone payments. In the valuation of contingent milestone payments performed, we assumed volatility of 30.0% and a rate of return of 8.08% as of March 31, 2018. We assess volatility and rate of return inputs quarterly by analyzing certain market volatility benchmarks and the risk associated with Royalty Pharma achieving the underlying projected royalties. During the three months ended March 31, 2018, the fair value of the Royalty Pharma contingent milestone payments decreased $9.6 million. The decrease was primarily attributed to projected global net sales of Tysabri® continuing to fall below the threshold required for payment of the $250.0 million milestone payment for 2018. Global net sales of Tysabri® are being impacted by competition, namely the launch of Ocrevus® in the U.S. and European markets in 2017 and 2018, respectively.
Payment of the contingent milestone payments is dependent on actual global net sales of Tysabri® in 2018 and 2020. Of the $124.9 million of estimated fair value contingent milestone payments as of March 31, 2018, $68.2 million and $56.7 million relates to the 2018 and 2020 contingent milestone payments, respectively. If Tysabri® global net sales do not meet the prescribed threshold in 2018, we will write off the $68.2 million asset as an expense to Change in financial assets on the Condensed Consolidated Statement of Operations. If the prescribed threshold is exceeded, we will write up the asset to $250.0 million and recognize income of $181.8 million in Change in financial assets on the Condensed Consolidated Statement of Operations. If Tysabri® global net sales do not meet the prescribed threshold in 2020, we will write off the $56.7 million asset as an expense to Change in financial assets on the Condensed Consolidated Statement of Operations. If the prescribed threshold is exceeded,
14
Perrigo Company plc - Item 1
Note 7
we will write up the asset to $400.0 million and recognize income of $343.8 million in Change in financial assets on the Condensed Consolidated Statement of Operations.
Global Tysabri® net sales need to exceed $1.85 billion and $1.95 billion in 2018 and 2020, respectively, in order for Royalty Pharma to receive the level of royalties needed to trigger the milestone payments owed to us.
The table below presents a reconciliation for the Royalty Pharma contingent milestone payments measured at fair value on a recurring basis using significant unobservable inputs (Level 3) (in millions). Change in fair value in the table was recorded in Change in financial assets on the Condensed Consolidated Statements of Operations.
Three Months Ended | |||
March 31, 2018 | |||
Royalty Pharma Contingent Milestone Payments | |||
Beginning balance | $ | 134.5 | |
Change in fair value | (9.6 | ) | |
Ending balance | $ | 124.9 | |
Contingent Consideration
Contingent consideration represents milestone payment obligations obtained through product acquisitions, which are valued using estimates based on probability-weighted outcomes, sensitivity analysis, and discount rates reflective of the risk involved. The estimates are updated quarterly and the liabilities are adjusted to fair value depending on a number of assumptions, including the competitive landscape and regulatory approvals that may impact the future sales of a product. We reduced a contingent consideration liability associated with certain IPR&D assets (refer to Note 4) and recorded a corresponding gain of $16.5 million during the three months ended April 1, 2017, this gain was partially offset by net realized losses of $2.1 million. The liability decrease relates to a reduction of the probability of achievement assumptions and anticipated cash flows.
The table below presents a reconciliation for liabilities measured at fair value on a recurring basis using significant unobservable inputs (Level 3) (in millions). Net realized losses in the table were recorded in Other expense (income), net on the Condensed Consolidated Statements of Operations.
Three Months Ended | |||||||
March 31, 2018 | April 1, 2017 | ||||||
Contingent Consideration | |||||||
Beginning balance | $ | 22.0 | $ | 69.9 | |||
Net realized (gains) losses | 0.4 | (14.4 | ) | ||||
Currency translation adjustments | 0.1 | (0.1 | ) | ||||
Settlements | (4.4 | ) | (3.4 | ) | |||
Ending balance | $ | 18.1 | $ | 52.0 | |||
Non-recurring Fair Value Measurements
The non-recurring fair values represent only those assets whose carrying values were adjusted to fair value during the reporting period.
15
Perrigo Company plc - Item 1
Note 7
Definite-Lived Intangible Assets
When assessing our definite-lived assets for impairment, we utilize either a multi-period excess earnings method ("MPEEM") or a relief from royalty method to determine the fair value of the asset and use the forecasts that are consistent with those used in the reporting unit analysis. Below is a summary of the various metrics used in our valuations:
Year Ended | |
December 31, 2017 | |
Lumara Branded Intangible | |
5-year average growth rate | (4.1)% |
Discount rate | 13.5% |
Valuation method | MPEEM |
Fixed Rate Long-term Debt
Our fixed rate long-term debt consisted of public bonds, a private placement note, and a retail bond as follows:
Fair Value Hierarchy | March 31, 2018 | December 31, 2017 | |||||||
(in billions) | |||||||||
Public Bonds | Level 1 | ||||||||
Carrying Value (excluding discount) | $ | 2.6 | $ | 2.6 | |||||
Fair value | $ | 2.6 | $ | 2.7 | |||||
(in millions) | |||||||||
Retail bond and private placement note | Level 2 | ||||||||
Carrying value (excluding premium) | $ | 314.3 | $ | 306.0 | |||||
Fair value | $ | 348.0 | $ | 342.1 | |||||
The fair values of our public bonds for all periods were based on quoted market prices. The fair values of our retail bond and private placement note for all periods were based on interest rates offered for borrowings of a similar nature and remaining maturities.
The carrying amounts of our other financial instruments, consisting of cash and cash equivalents, accounts receivable, accounts payable, short-term debt and variable rate long-term debt, approximate their fair value.
16
Perrigo Company plc - Item 1
Note 8
NOTE 8 – INVESTMENTS
The following table summarizes our equity security measurement category, balance sheet location, and balances (in millions):
Measurement Category | Balance Sheet Location | March 31, 2018 | December 31, 2017(2) | |||||||
Fair value method | Prepaid expenses and other current assets | $ | 13.8 | $ | 17.0 | |||||
Fair value method(1) | Other non-current assets | $ | 5.1 | $ | 6.3 | |||||
Equity method | Other non-current assets | $ | 4.7 | $ | 4.9 | |||||
(1) The March 31, 2018 equity securities are measured at fair value using the Net Asset Value practical expedient.
(2) The December 31, 2017 balances presented reflect historical recognition and measurement investment categories existing prior to the adoption of ASU 2016-01, which include available for sale and cost method securities.
The following table summarizes our equity security expense (income) recognized in earnings (in millions):
Three Months Ended | ||||||||||
Measurement Category | Income Statement Location | March 31, 2018 | April 1, 2017 | |||||||
Fair value method | Other expense (income), net | $ | 4.4 | $ | — | |||||
Equity method | Other expense (income), net | $ | 0.2 | $ | (0.1 | ) | ||||
On January 1, 2018, as a result of the adoption of ASU 2016-01, we made a $1.4 million cumulative-effect adjustment to Retained earnings (accumulated deficit) that consisted of net unrealized losses on previously classified available for sale securities from Other comprehensive income ("OCI").
NOTE 9 – DERIVATIVE INSTRUMENTS AND HEDGING ACTIVITIES
We enter into certain derivative financial instruments, when available on a cost-effective basis, to mitigate our risk associated with changes in interest rates and foreign currency exchange rates as follows:
Interest rate risk management - We are exposed to the impact of interest rate changes through our cash investments and borrowings. We utilize a variety of strategies to manage the impact of changes in interest rates, including using a mix of debt maturities along with both fixed-rate and variable-rate debt. In addition, we may enter into treasury-lock agreements and interest rate swap agreements on certain investing and borrowing transactions to manage our exposure to interest rate changes and our overall cost of borrowing.
Foreign currency exchange risk management - We conduct business in several major currencies other than the U.S. dollar and are subject to risks associated with changing foreign exchange rates. Our objective is to reduce cash flow volatility associated with foreign exchange rate changes on a consolidated basis to allow management to focus its attention on business operations. Accordingly, we enter into various contracts that change in value as foreign exchange rates change to protect the value of existing foreign currency assets and liabilities, commitments, and anticipated foreign currency sales and expenses.
All derivative instruments are managed on a consolidated basis to efficiently net exposures and thus take advantage of any natural offsets. Gains and losses related to the derivative instruments are expected to be offset largely by gains and losses on the original underlying asset or liability. We do not use derivative financial instruments for speculative purposes.
All of our designated derivatives were classified as cash flow hedges as of March 31, 2018 and December 31, 2017. Designated derivatives meet hedge accounting criteria, which means the fair value of the hedge is recorded in Shareholders’ equity as a component of OCI, net of tax. The deferred gains and losses are recognized in income in the period in which the hedged item affects earnings. Any ineffective portion of the change in fair value of the derivative is immediately recognized in earnings. All of our designated derivatives are assessed for hedge effectiveness quarterly.
17
Perrigo Company plc - Item 1
Note 9
We also have economic non-designated derivatives that do not meet hedge accounting criteria. These derivative instruments are adjusted to current market value at the end of each period through earnings. Gains or losses on these instruments are offset substantially by the remeasurement adjustment on the hedged item.
Interest Rate Swaps
Interest rate swap agreements are contracts to exchange floating rate for fixed rate payments (or vice versa) over the life of the agreement without the exchange of the underlying notional amounts. The notional amounts of the interest rate swap agreements are used to measure interest to be paid or received and do not represent the amount of exposure to credit loss. The differential paid or received on the interest rate swap agreements is recognized as an adjustment to Interest expense, net.
Foreign Currency Derivatives
We enter into foreign currency forward contracts, both designated and non-designated, in order to manage the impact of foreign exchange fluctuations on expected future purchases and related payables denominated in a foreign currency, as well as to hedge the impact of foreign exchange fluctuations on expected future sales and related receivables denominated in a foreign currency. Both types of forward contracts have a maximum maturity date of 18 months. The total notional amount for these contracts was $583.3 million and $592.3 million as of March 31, 2018 and December 31, 2017, respectively.
Effects of Derivatives on the Financial Statements
The below tables indicate the effects of all derivative instruments on the Condensed Consolidated Financial Statements. All amounts exclude income tax effects and are presented in millions.
The balance sheet location and gross fair value of our outstanding derivative instruments were as follows:
Asset Derivatives | |||||||||
Balance Sheet Location | Fair Value | ||||||||
March 31, 2018 | December 31, 2017 | ||||||||
Designated derivatives: | |||||||||
Foreign currency forward contracts | Other current assets | $ | 3.4 | $ | 4.1 | ||||
Non-designated derivatives: | |||||||||
Foreign currency forward contracts | Other current assets | $ | 1.6 | $ | 2.2 | ||||
Liability Derivatives | |||||||||
Balance Sheet Location | Fair Value | ||||||||
March 31, 2018 | December 31, 2017 | ||||||||
Designated derivatives: | |||||||||
Foreign currency forward contracts | Accrued liabilities | $ | 2.2 | $ | 1.4 | ||||
Non-designated derivatives: | |||||||||
Foreign currency forward contracts | Accrued liabilities | $ | 1.6 | $ | 2.4 | ||||
The gains (losses) recorded in OCI for the effective portion of our designated cash flow hedges were as follows:
Amount of Gain/(Loss) Recorded in OCI (Effective Portion) | ||||||||
Three Months Ended | ||||||||
Designated Cash Flow Hedges | March 31, 2018 | April 1, 2017 | ||||||
Foreign currency forward contracts | $ | (0.2 | ) | $ | 2.5 | |||
18
Perrigo Company plc - Item 1
Note 9
The gains (losses) reclassified from Accumulated other comprehensive income ("AOCI") into earnings for the effective portion of our designated cash flow hedges were as follows:
Amount of Gain/(Loss) Reclassified from AOCI into Earnings (Effective Portion) | ||||||||||
Three Months Ended | ||||||||||
Designated Cash Flow Hedges | Income Statement Location | March 31, 2018 | April 1, 2017 | |||||||
Interest rate swap agreements | Interest expense, net | $ | (0.4 | ) | $ | (0.6 | ) | |||
Foreign currency forward contracts | Net sales | (0.1 | ) | 0.2 | ||||||
Cost of sales | 2.3 | 0.7 | ||||||||
Interest expense, net | (1.0 | ) | (0.6 | ) | ||||||
Other expense (income), net | (0.4 | ) | (0.5 | ) | ||||||
Total | $ | 0.4 | $ | (0.8 | ) | |||||
The net of tax amount expected to be reclassified out of AOCI into earnings during the next 12 months is a $1.8 million loss.
The gains (losses) recognized in earnings for the ineffective portion of our designated cash flow hedges were as follows:
Amount of Gain/(Loss) Recognized in Earnings (Ineffective Portion) | ||||||||||
Three Months Ended | ||||||||||
Designated Cash Flow Hedges | Income Statement Location | March 31, 2018 | April 1, 2017 | |||||||
Foreign currency forward contracts | Other expense (income), net | $ | — | $ | 0.9 | |||||
The effects of our non-designated derivatives on the Condensed Consolidated Statements of Operations were as follows:
Amount of Gain/(Loss) Recognized against Earnings | ||||||||||
Three Months Ended | ||||||||||
Non-Designated Derivatives | Income Statement Location | March 31, 2018 | April 1, 2017 | |||||||
Foreign currency forward contracts | Other expense (income), net | $ | 3.5 | $ | (8.9 | ) | ||||
Interest expense, net | (0.9 | ) | (0.4 | ) | ||||||
Total | $ | 2.6 | $ | (9.3 | ) | |||||
19
Perrigo Company plc - Item 1
Note 10
NOTE 10 – INDEBTEDNESS
Total borrowings outstanding are summarized as follows (in millions):
March 31, 2018 | December 31, 2017 | ||||||||||
Term loans | |||||||||||
2018 Term loan due March 8, 2020(1) | $ | 417.9 | $ | — | |||||||
2014 Term loan due December 5, 2019(1) | — | 420.0 | |||||||||
Total term loans | 417.9 | 420.0 | |||||||||
Notes and Bonds | |||||||||||
Coupon | Due | ||||||||||
5.000% | March 23, 2019(1) | 147.9 | 144.0 | ||||||||
3.500% | March 15, 2021 | 280.4 | 280.4 | ||||||||
3.500% | December 15, 2021 | 309.6 | 309.6 | ||||||||
5.105% | July 19, 2023(1) | 166.4 | 162.0 | ||||||||
4.000% | November 15, 2023 | 215.6 | 215.6 | ||||||||
3.900% | December 15, 2024 | 700.0 | 700.0 | ||||||||
4.375% | March 15, 2026 | 700.0 | 700.0 | ||||||||
5.300% | November 15, 2043 | 90.5 | 90.5 | ||||||||
4.900% | December 15, 2044 | 303.9 | 303.9 | ||||||||
Total notes and bonds | 2,914.3 | 2,906.0 | |||||||||
Other financing | 5.3 | 11.7 | |||||||||
Unamortized premium (discount), net | 20.1 | 21.4 | |||||||||
Deferred financing fees | (19.0 | ) | (17.9 | ) | |||||||
Total borrowings outstanding | 3,338.6 | 3,341.2 | |||||||||
Current indebtedness | (58.0 | ) | (70.4 | ) | |||||||
Total long-term debt less current portion | $ | 3,280.6 | $ | 3,270.8 | |||||||
(1) | Debt denominated in euros subject to fluctuations in the euro-to-U.S. dollar exchange rate. |
We are in compliance with all covenants under our debt agreements as of March 31, 2018.
Revolving Credit Agreements
On December 5, 2014, Perrigo Finance entered into a $600.0 million revolving credit agreement, which increased to $1.0 billion on March 30, 2015 (the "2014 Revolver"). On March 8, 2018, we terminated the 2014 Revolver and entered into a $1.0 billion revolving credit agreement maturing on March 8, 2023 (the "2018 Revolver"). There were no borrowings outstanding under the 2018 Revolver or 2014 Revolver as of March 31, 2018 or December 31, 2017, respectively.
Term Loans
On December 5, 2014, Perrigo Finance entered into a term loan agreement consisting of a €500.0 million ($614.3 million) tranche, maturing December 5, 2019. On March 8, 2018, we refinanced the €350.0 million outstanding under the term loan with the proceeds of a new €350.0 million ($431.0 million) term loan, maturing March 8, 2020. In addition, as a result of the refinancing during the three months ended March 31, 2018, we recorded a loss of $0.5 million, consisting of the write-off of deferred financing fees in Loss on extinguishment of debt.
20
Perrigo Company plc - Item 1
Note 10
Other Financing
Overdraft Facilities
We have overdraft facilities available that we use to support our cash management operations. We report any balances outstanding in the above table under "Other financing". The balance outstanding under the overdraft facilities was $1.2 million and $6.9 million at March 31, 2018 and December 31, 2017, respectively.
NOTE 11 – EARNINGS PER SHARE AND SHAREHOLDERS' EQUITY
Earnings per Share
A reconciliation of the numerators and denominators used in the basic and diluted earnings per share ("EPS") calculation is as follows (in millions):
Three Months Ended | |||||||
March 31, 2018 | April 1, 2017 | ||||||
Numerator: | |||||||
Net income | $ | 80.8 | $ | 71.6 | |||
Denominator: | |||||||
Weighted average shares outstanding for basic EPS | 140.8 | 143.4 | |||||
Dilutive effect of share-based awards | 0.6 | 0.2 | |||||
Weighted average shares outstanding for diluted EPS | 141.4 | 143.6 | |||||
Anti-dilutive share-based awards excluded from computation of diluted EPS | 0.7 | 1.0 | |||||
Shareholders' Equity
Shares
We issued shares related to the exercise and vesting of share-based compensation as follows:
Three Months Ended | ||||
March 31, 2018 | April 1, 2017 | |||
53,000 | 14,400 | |||
Share Repurchases
In October 2015, the Board of Directors approved a three-year share repurchase plan of up to $2.0 billion. During the three months ended March 31, 2018, we repurchased 1.3 million ordinary shares at an average repurchase price of $81.92 per share, for a total of $108.1 million. We did not repurchase any shares under the share repurchase plan during the three months ended April 1, 2017.
21
Perrigo Company plc - Item 1
Note 13
NOTE 12 – ACCUMULATED OTHER COMPREHENSIVE INCOME (LOSS)
Changes in our AOCI balances, net of tax were as follows (in millions):
Foreign currency translation adjustments | Fair value of derivative financial instruments, net of tax | Fair value of investment securities, net of tax | Post-retirement and pension liability adjustments, net of tax | Total AOCI | |||||||||||||||
Balance at December 31, 2017 | $ | 260.6 | $ | (9.8 | ) | $ | 1.0 | $ | 1.3 | $ | 253.1 | ||||||||
ASU 2016-01 adoption impact | — | — | (1.0 | ) | — | (1.0 | ) | ||||||||||||
Balance at December 31, 2017 after adoption impact | $ | 260.6 | $ | (9.8 | ) | $ | — | $ | 1.3 | $ | 252.1 | ||||||||
OCI before reclassifications | 73.0 | (0.1 | ) | — | (0.2 | ) | 72.7 | ||||||||||||
Amounts reclassified from AOCI | — | (0.5 | ) | — | — | (0.5 | ) | ||||||||||||
Other comprehensive income | $ | 73.0 | $ | (0.6 | ) | $ | — | $ | (0.2 | ) | $ | 72.2 | |||||||
Balance at March 31, 2018 | $ | 333.6 | $ | (10.4 | ) | $ | — | $ | 1.1 | $ | 324.3 | ||||||||
NOTE 13 – INCOME TAXES
The effective tax rates were as follows:
Three Months Ended | ||||
March 31, 2018 | April 1, 2017 | |||
26.9 | % | 25.3 | % | |
The effective tax rate for the three months ended March 31, 2018 increased in comparison to the prior year period due primarily to additional valuation allowances recorded against deferred tax assets partially offset by discrete tax benefits.
Our tax rate is subject to adjustment over the balance of the fiscal year due to, among other things: the jurisdictions in which our profits are determined to be earned and taxed; changes in the valuation of our deferred tax assets and liabilities; adjustments to estimated taxes upon finalization of various tax returns; adjustments based on differing interpretations of the applicable transfer pricing standards; changes in available tax credits, grants and other incentives; changes in stock-based compensation expense; changes in tax laws or the interpretation of such tax laws (for example, proposals for fundamental U.S. international tax reform); changes in U.S. GAAP; and expiration of or the inability to renew tax rulings or tax holiday incentives.
We file income tax returns in numerous jurisdictions and are therefore subject to audits by tax authorities. Our primary income tax jurisdictions are Ireland, the United States, Israel, Belgium, France, and the United Kingdom.
On August 15, 2017, we filed a complaint in the U.S. District Court for the Western District of Michigan to recover $163.6 million of Federal income tax, penalties, and interest assessed and collected by the Internal Revenue Service (“IRS”), plus statutory interest thereon from the dates of payment, for the fiscal years ended June 27, 2009, June 26, 2010, June 25, 2011, and June 30, 2012 (the “2009 tax year,” “2010 tax year,” “2011 tax year,” and “2012 tax year,” respectively). The IRS audits of those years culminated in the issuances of two statutory notices of deficiency: (1) on August 27, 2014 for the 2009 and 2010 tax years and (2) on April 20, 2017 for the 2011 and 2012 tax years. The statutory notices of deficiency both included un-agreed income adjustments related principally to transfer pricing adjustments regarding the purchase, distribution, and sale of store-brand OTC pharmaceutical products in the United States. In addition, the statutory notice of deficiency for the 2011 and 2012 tax years included the capitalization of certain expenses that were deducted when paid or incurred in defending against certain patent infringement lawsuits. We fully paid the assessed amounts of tax, interest, and penalties set forth in the statutory notices and filed timely claims for refund on June 11, 2015 and June 7, 2017 for the 2009-2010 tax years and 2011-2012 tax years, respectively. Our claims for refund were disallowed by certified letters dated August 18, 2015 and July 11, 2017, for the 2009-2010 tax years and 2011-2012 tax years, respectively. The
22
Perrigo Company plc - Item 1
Note 13
complaint was timely, based upon the refund claim denials, and seeks refunds of tax, interest, and penalties of $37.2 million for the 2009 tax year, $61.5 million for the 2010 tax year, $40.2 million for the 2011 tax year, and $24.7 million for the 2012 tax year. The amounts sought in the complaint for the 2009 and 2010 tax years were recorded as deferred charges in Other non-current assets on our balance sheet during the three months ended March 28, 2015, and the amounts sought in the complaint for the 2011 and 2012 tax years were recorded as deferred charges in Other non-current assets on our balance sheet during the three months ended July 1, 2017.
On December 22, 2016, we received a notice of proposed adjustment for the IRS audit of Athena Neurosciences, Inc. (“Athena”), a subsidiary of Elan acquired in 1996, for the years ended December 31, 2011, December 31, 2012, and December 31, 2013. Perrigo acquired Elan in December 2013. This proposed adjustment relates to the deductibility of litigation costs. We disagree with the IRS’s position asserted in the notice of proposed adjustment and intend to contest it.
On July 11, 2017, we received a draft notice of proposed adjustment associated with transfer pricing positions for the IRS audit of Athena for the years ended December 31, 2011, December 31, 2012, and December 31, 2013. Athena was the originator of the patents associated with Tysabri® prior to the acquisition of Athena by Elan in 1996. In response to the draft notice of proposed adjustment, we provided the IRS with substantial additional documentation supporting our position. The amount of adjustments that may be asserted by the IRS in the final notice of proposed adjustment cannot be quantified at this time; however, based on the draft notice received, the amount to be assessed may be material. We disagree with the IRS’s position as asserted in the draft notice of proposed adjustment and intend to contest it.
We have ongoing audits in multiple other jurisdictions the resolution of which remains uncertain. These jurisdictions include, but are not limited to, the U.S., Israel, Ireland and other jurisdictions in Europe. In addition to the matters discussed above, the IRS is currently auditing our fiscal years ended June 29, 2013, June 28, 2014, and June 27, 2015 (which covers the period of the Elan transaction). The Israel Tax Authority is currently auditing our fiscal years ended June 29, 2013 and June 28, 2014. The Ireland Tax Authority is currently auditing our years ended December 31, 2012 and December 31, 2013.
Tax Law Changes
On December 22, 2017, the U.S. enacted the Tax Cuts and Jobs Act (“U.S. Tax Act”). The U.S. Tax Act includes a number of significant changes to existing U.S. tax laws that impact us. These changes include a corporate income tax rate reduction from 35% to 21% and the elimination or reduction of certain U.S. deductions and credits including limitations on the U.S. deductibility of interest expense and executive compensation. The U.S. Tax Act also transitions the U.S. taxation of international earnings from a worldwide system to a modified territorial system. These changes are effective beginning in 2018. The U.S. Tax Act also includes a one-time mandatory deemed repatriation tax on accumulated U.S. owned foreign corporations’ previously untaxed foreign earnings (“Transition Toll Tax”). The Transition Toll Tax may be paid over an eight-year period, starting in 2018, and will not accrue interest.
On December 22, 2017, Staff Accounting Bulletin No. 118 ("SAB 118") was issued to address the application of the U.S. GAAP ASC 740 income tax accounting for tax law changes enacted during 2017, in situations when a registrant does not have the necessary information available, prepared, or analyzed (including computations) in reasonable detail to complete the accounting for certain income tax effects of the U.S. Tax Act. In accordance with SAB 118, for the year ended December 31, 2017, we recorded an income tax benefit of $2.4 million in connection with the remeasurement of certain deferred tax assets and liabilities and also recorded a $17.5 million increase of current tax expense in connection with the Transition Toll Tax on cumulative U.S. owned foreign earnings of $1.2 billion. The tax impacts represent provisional amounts and are a reasonable estimate. The IRS issued additional guidance related to the U.S. Tax Act during the quarter ended March 31, 2018, which resulted in no changes to the provisional estimates recorded at December 31, 2017. Further work is necessary to perform additional analysis of historical foreign earnings and U.S. cumulative temporary differences, as well as potential correlative adjustments. Any subsequent adjustment to these amounts will be recorded to current tax expense when the analysis is complete in 2018.
The U.S. Tax Act subjects a U.S. shareholder to tax on global intangible low-taxed income ("GILTI") earned by certain foreign subsidiaries. The FASB Staff Q&A, Topic 740, No. 5, Accounting for Global Intangible Low-Taxed
23
Perrigo Company plc - Item 1
Note 13
Income states that an entity can make an accounting policy election to either recognize deferred taxes for temporary basis differences expected to reverse as GILTI in future years or provide for the tax expense related to GILTI in the year the tax is incurred. Given the complexity of the GILTI provisions, we are still evaluating the effects of the GILTI provisions and have not yet determined our accounting policy. At March 31, 2018, we made a reasonable estimate of the tax effect of a GILTI inclusion for 2018, which is not material for the quarter. We also estimate that we will not be subject to the base erosion anti-avoidance tax in 2018 and will not record tax benefits for deductions related to foreign-derived intangible income.
On December 22, 2017, the Belgian Parliament approved Belgian tax reform legislation (“Belgium Tax Act”), which was signed by the Belgian King and enacted on December 25, 2017. The Belgium Tax Act provides for a reduction to the corporate income tax rate from 34% to 30%, for 2018 and 2019, as well as a reduced corporate income tax rate of 25% for 2020 and beyond. The Belgium Tax Act also increased the participation exemption on dividend distributions to Belgium entities from 95% to 100%. The Belgium Tax Act also introduces Belgium tax consolidation and other anti-tax avoidance directives. For the year ended December 31, 2017, we recorded additional income tax expense of $24.1 million for the remeasurement of certain deferred tax assets and additional income tax benefit of $33.2 million for the remeasurement of certain deferred tax liabilities as a result of the Belgium Tax Act.
NOTE 14 – COMMITMENTS AND CONTINGENCIES
In view of the inherent difficulties of predicting the outcome of various types of legal proceedings, we cannot determine the ultimate resolution of the matters described below. We establish reserves for litigation and regulatory matters when losses associated with the claims become probable and the amounts can be reasonably estimated. The actual costs of resolving legal matters may be substantially higher or lower than the amounts reserved for those matters. For matters where the likelihood or extent of a loss is not probable or cannot be reasonably estimated as of March 31, 2018, we have not recorded a loss reserve. If certain of these matters are determined against us, there could be a material adverse effect on our financial condition, results of operations, or cash flows. We currently believe we have valid defenses to the claims in these lawsuits and intend to defend these lawsuits vigorously regardless of whether or not we have a loss reserve. Other than what is disclosed below, we do not expect the outcome of the litigation matters to which we are currently subject to, individually or in the aggregate, have a material adverse effect on our financial condition, results of operations, or cash flows.
Antitrust Violations
We were named as a counterclaim co-defendant in the lawsuit Fera Pharmaceuticals, LLC v. Akorn, Inc., et al. in the Southern District of New York, in which Akorn, Inc. (“Akorn”) alleged tortious interference and antitrust violations against us and Fera Pharmaceuticals, LLC (“Fera”). Trial was set for February 2018 in the Southern District of New York. This litigation arose out of our acquisition of bacitracin ophthalmic ointment from Fera in 2013. Akorn asserted claims under Sections 1 and 2 of the Sherman Antitrust Act alleging that we and Fera conspired to monopolize, attempted to monopolize, and did unlawfully monopolize the market for sterile bacitracin ophthalmic ointment in the United States through the use of an exclusive agreement with a supplier of sterile bacitracin active pharmaceutical ingredient. The parties have executed a written settlement of all claims and the case has been dismissed.
Price-Fixing Lawsuits
We have been named as a co-defendant with other manufacturers in a number of class actions alleging that we and other manufacturers of the same product engaged in anti-competitive behavior to fix or raise the prices of certain drugs starting, in some instances, as early as June 2013. The products in question are Clobetasol, Desonide, and Econazole. These complaints, along with complaints filed against other companies alleging price fixing with respect to more than two dozen other drugs, have been consolidated for pretrial proceedings as part of a case captioned In re Generic Pharmaceuticals Pricing Antitrust Litigation, MDL No. 2724 in the U.S. District Court for the Eastern District of Pennsylvania. Pursuant to the court’s schedule staging various cases in phases, we have moved to dismiss the complaints relating to Clobetasol and Econazole. We have also recently been named a defendant along with 31 other manufacturers in a complaint filed by three supermarket chains alleging that defendants conspired to fix prices of all generic pharmaceutical products starting in 2013. A schedule for responses
24
Perrigo Company plc - Item 1
Note 14
to this complaint will be determined after decisions are rendered on the pending motions to dismiss the class cases. At this stage, we cannot reasonably predict the outcome of the liability, if any, associated with these claims.
Securities Litigation
In the United States
On May 18, 2016, a shareholder filed a securities case against us and our former CEO, Joseph Papa, in the U.S. District Court for the District of New Jersey (Roofers’ Pension Fund v. Papa, et al.). The plaintiff purported to represent a class of shareholders for the period from April 21, 2015 through May 11, 2016, inclusive. The original complaint alleged violations of Securities Exchange Act sections 10(b) (and Rule 10b‑5) and 14(e) against both defendants and 20(a) control person liability against Mr. Papa. In general, the allegations concerned the actions taken by us and the former executive to defend against the unsolicited takeover bid by Mylan in the period from April 21, 2015 through November 13, 2015. The plaintiff also alleged that the defendants provided inadequate disclosure concerning alleged integration problems related to the Omega acquisition in the period from April 21, 2015 through May 11, 2016. On July 19, 2016, a different shareholder filed a securities class action against us and our former CEO, Joseph Papa, also in the District of New Jersey (Wilson v. Papa, et al.). The plaintiff purported to represent a class of persons who sold put options on our shares between April 21, 2015 and May 11, 2016. In general, the allegations and the claims were the same as those made in the original complaint filed in the Roofers' Pension Fund case described above. On December 8, 2016, the court consolidated Roofers' Pension Fund case and the Wilson case under the Roofers' Pension Fund case number. In February 2017, the court selected the lead plaintiffs for the consolidated case and the lead counsel to the putative class. In March 2017, the court entered a scheduling order.
On June 21, 2017, the court-appointed lead plaintiffs filed an amended complaint that superseded the original complaints in the Roofers’ Pension Fund case and the Wilson case. The lead plaintiffs seek to represent a class of shareholders for the period April 21, 2015 through May 3, 2017, and the amended complaint identifies three subclasses - shareholders who purchased shares during the period on the U.S. exchanges; shareholders who purchased shares during the period on the Tel Aviv exchange; and shareholders who owned shares on the final day of the Mylan tender offer November 13, 2015. The amended complaint names as defendants us and 11 current or former directors and officers of Perrigo (Mses. Judy Brown, Laurie Brlas, Jacqualyn Fouse, Ellen Hoffing, and Messrs. Joe Papa, Marc Coucke, Gary Cohen, Michael Jandernoa, Gerald Kunkle, Herman Morris, and Donal O’Connor). The amended complaint alleges violations of Securities Exchange Act sections 10(b) (and Rule 10b‑5) and 14(e) against all defendants and 20(a) control person liability against the 11 individuals. In general, the allegations concern the actions taken by us and the former executives to defend against the unsolicited takeover bid by Mylan in the period from April 21, 2015 through November 13, 2015 and the allegedly inadequate disclosure throughout the entire class period related to purported integration problems related to the Omega acquisition, alleges incorrect reporting of organic growth at the Company and at Omega, alleges price fixing activities with respect to six generic prescription pharmaceuticals, and alleges improper accounting for the Tysabri® royalty stream. The amended complaint does not include an estimate of damages. In August 2017, the defendants filed motions to dismiss the amended complaint. The plaintiffs filed their opposition in October 2017. The defendants filed replies in support of the motions to dismiss in November 2017. The court has not indicated whether there will be oral argument of the motions or whether the court will decide the motions on the papers. We intend to defend the lawsuit vigorously.
On November 1, 2017, Carmignac Gestion, S.A., filed a securities lawsuit against us and three individuals (former Chairman and CEO Joseph Papa, former CFO Judy Brown, and former Executive Vice President and Board member Marc Coucke). This lawsuit is not a securities class action. The case is styled Carmignac Gestion, S.A. v. Perrigo Company plc, et al., and was filed in the U.S. District Court for the District of New Jersey. The complaint asserts claims under Securities Exchange Act sections 10(b) (and Rule 10b-5), 14(e), and 18 against all defendants as well as 20(a) control person liability against the individual defendants. In general, the plaintiff’s allegations focus on events during the period from April 2015 through April 2016. Plaintiff contends that the defendants provided inadequate disclosure throughout the period concerning the valuation and integration of Omega, the financial guidance provided by us during that period, our reporting about the generic prescription pharmaceutical business and its prospects, and the activities surrounding the efforts to defeat the Mylan tender offer during 2015. Many of the allegations in this case overlap with the allegations of the June 2017 amended complaint in the Roofers’ Pension Fund case described above. The plaintiff does not provide an estimate of damages. We intend to defend
25
Perrigo Company plc - Item 1
Note 14
the lawsuit vigorously. The parties jointly requested that the court stay this case pending the outcome of a ruling on the motions to dismiss filed in the Roofers' Pension Fund case (discussed above), and the court granted the stay motion.
On January 16, 2018, Manning & Napier Advisors, LLC filed a securities lawsuit against us and three individuals (former Chairman and CEO Joseph Papa, former CFO Judy Brown, and former Executive Vice President and Board member Marc Coucke). This lawsuit is not a securities class action. The case is styled Manning & Napier Advisors, LLC v. Perrigo Company plc, et al., and was filed in the U.S. District Court for the District of New Jersey. The complaint asserts claims under Securities Exchange Act sections 10(b) (and Rule 10b-5) and 18 against all defendants as well as 20(a) control person liability against the individual defendants. In general, the plaintiff’s allegations focus on events during the period from April 2015 through May 2017. Plaintiff contends that the defendants provided inadequate disclosure at various times during the period concerning valuation and integration of Omega, the financial guidance provided by us during that period, alleged price fixing activities with respect to six generic prescription pharmaceuticals, and alleged improper accounting for the Tysabri® financial asset. Many of the allegations in this case overlap with the allegations of the June 2017 amended complaint in the Roofers' Pension Fund case described above. The plaintiff does not provide an estimate of damages. We intend to defend the lawsuit vigorously. The parties jointly requested that the court stay this case pending the outcome of a ruling on the motion to dismiss filed in the Roofers' Pension Fund case (discussed above), and the court granted the stay motion.
On January 26, 2018, two different plaintiff groups (the Mason Capital group and the Pentwater group) each filed a lawsuit against us and the same individuals who are defendants in the amended complaint in the securities class action case described above (Roofers’ Pension Fund case). The same law firm represents these two plaintiff groups, and the two complaints are substantially similar. These two cases are not securities class actions. One case is styled Mason Capital L.P., et al. v. Perrigo Company plc, et al., and was filed in the U.S. District Court for the District of New Jersey. The other case is styled Pentwater Equity Opportunities Master Fund Ltd., et al. v. Perrigo Company plc, et al., and also was filed in the U.S. District Court for the District of New Jersey. Both cases are assigned to the same federal judge that is hearing the class action case and the other individual cases described above (Carmignac and Manning & Napier). Each complaint asserts claims under Securities Exchange Act sections 14(e) (related to tender offer disclosures) against all defendants as well as 20(a) control person liability against the individual defendants. In general, the plaintiff’s allegations describe events during the period from April 2015 through May 2017. Plaintiff contends that the defendants provided inadequate disclosure during the tender offer period in 2015 and point to disclosures at various times during the period concerning valuation and integration of Omega, the financial guidance provided by us during that period, alleged price fixing activities with respect to six generic prescription pharmaceuticals, and alleged improper accounting for the Tysabri® financial asset. Many of the factual allegations in these two cases overlap with the allegations of the June 2017 amended complaint in the Roofers' Pension Fund case described above and the allegations in the Carmignac case described above. The plaintiff does not provide an estimate of damages. The parties to each case jointly requested that the court stay each case pending the outcome of a ruling on the motions to dismiss filed in the Roofers’ Pension Fund case (discussed above). The court granted the stay motion in each case. We intend to defend both lawsuits vigorously.
On February 13, 2018, a group of plaintiff investors affiliated with Harel Insurance Investments & Financial Services, Ltd. filed a lawsuit against us and the same individuals who are defendants in the amended complaint in the securities class action case described above (Roofers’ Pension Fund case). This lawsuit is not a securities class action. The new complaint is substantially similar to the amended complaint in the Roofers' Pension Fund case. The relevant period in the new complaint stretches from February 2014 to May 2, 2017. The complaint adds as defendants two individuals who served on our Board prior to 2016. The case is styled Harel Insurance Company, Ltd., et al. v. Perrigo Company plc, et al., and was filed in the U.S. District Court for the District of New Jersey and is assigned to the same federal judge that is hearing the class action cases and the four other individual cases described above (Carmignac, Manning & Napier, Mason Capital, and Pentwater). The Harel Insurance Company complaint asserts claims under Securities Exchange Act section 10(b) (and related SEC Rule 10b‑5) and section 14(e) (related to tender offer disclosures) against all defendants as well as 20(a) control person liability against the individual defendants. The complaint also asserts claims based on Israeli securities laws. In general, the plaintiffs' allegations describe events during the period from February 2014 through May 2017. Plaintiffs contend that the defendants provided inadequate disclosure during the tender offer events in 2015 and point to disclosures at various times during the period concerning valuation and integration of Omega, the financial guidance provided by us during that period, alleged price fixing activities with respect to six generic prescription pharmaceuticals, and
26
Perrigo Company plc - Item 1
Note 14
alleged improper accounting for the Tysabri® financial asset from February 2014 until the withdrawal of past financial statements in April 2017. Many of the factual allegations in this case overlap with the allegations of the June 2017 amended complaint in the Roofers' Pension Fund case described above and the allegations in the four opt out cases also described above. The plaintiffs do not provide an estimate of damages. We intend to defend the lawsuit vigorously. The parties jointly requested that the court stay this case pending the outcome of a ruling on the motions to dismiss filed in the Roofers’ Pension Fund case (discussed above), which was granted by the court.
On February 16, 2018, First Manhattan Company filed a securities lawsuit against us and three individuals (former Chairman and CEO Joseph Papa, former CFO Judy Brown, and former Executive Vice President and Board member Marc Coucke). This lawsuit is not a securities class action. The case is styled First Manhattan Co. v. Perrigo Company plc, et al., and was filed in the U.S. District Court for the District of New Jersey. The case was assigned to the same judge hearing the class action case and the five other opt out cases. The complaint asserts claims under Securities Exchange Act sections 10(b) (and Rule 10b-5), 14(e), and 18 against all defendants as well as 20(a) control person liability against the individual defendants. In general, the plaintiff’s allegations focus on events during the period from April 2015 through May 2017. Plaintiff contends that the defendants provided inadequate disclosure at various times during the period concerning valuation and integration of Omega, the financial guidance provided by us during that period, alleged price fixing activities with respect to six generic prescription pharmaceuticals, and alleged improper accounting for the Tysabri® financial asset. This lawsuit was filed by the same law firm that filed the Manning & Napier Advisors case and the Carmignac case described above and generally makes the same factual assertions as in the Manning & Napier Advisors case. Many of the allegations in this case overlap with the allegations of the June 2017 amended complaint in the Roofers' Pension Fund case described above. The plaintiff does not provide an estimate of damages. On April 20, 2018, the plaintiff filed an amended complaint that did not materially change the factual allegations of the original complaint. We intend to defend the lawsuit vigorously. The parties jointly requested that the court stay this case pending the outcome of a ruling on the motions to dismiss filed in the Roofers’ Pension Fund case (discussed above). The court granted the stay motion.
On April 20, 2018, a group of plaintiff investors affiliated with TIAA-CREF filed a lawsuit against us and the same individuals who are the defendants in the Harel Insurance case complaint. This lawsuit is not a securities class action. The law firm representing the plaintiffs in the Harel Insurance case also represents the TIAA-CREF plaintiff entities in this case, and the new complaint is substantially similar to the Harel Insurance complaint. The relevant period in the new complaint is August 14, 2014 to May 2, 2017 inclusive. The case is styled TIAA-CREF Investment Management, LLC., et al. v. Perrigo Company plc, et al., and was filed in the U.S. District Court for the District of New Jersey and is assigned to the same federal judge that is hearing the class action case and the six other individual cases described above (Carmignac, Manning & Napier, Mason Capital, Pentwater, Harel Insurance, and First Manhattan). The TIAA-CREF Investment Management complaint asserts claims under Securities Exchange Act section 10(b) (and related SEC Rule l0b-5), section 14(e) (related to tender offer disclosures) against all defendants as well as section 20(a) control person liability against the individual defendants. In general, plaintiffs' allegations describe events during the period from August 2014 through May 2017. Plaintiffs contend that the defendants provided inadequate disclosure during the tender offer events in 2015 and point to disclosures at various times during the period concerning valuation and integration of Omega, the financial guidance provided by us during that period, alleged price fixing activities with respect to six generic prescription pharmaceuticals, and alleged improper accounting for the Tysabri® financial asset from August 2014 until the withdrawal of past financial statements in April 2017. Many of the factual allegations in this case also overlap with the allegations of the June 2017 amended complaint in the Roofers' Pension Fund case described above. The plaintiffs do not provide an estimate of damages. We intend to defend the lawsuit vigorously. The parties jointly requested that the court stay this case pending the outcome of a ruling on the motions to dismiss filed in the Roofers' Pension Fund case (discussed above). The court granted the stay motion.
In Israel
Because our shares are traded on the Tel Aviv exchange under a dual trading arrangement, we are potentially subject to securities litigation in Israel. Three cases were filed; two were voluntarily dismissed and one was stayed. We are consulting Israeli counsel about our response to these allegations and we intend to defend these cases vigorously.
27
Perrigo Company plc - Item 1
Note 14
On May 22, 2016, shareholders filed a securities class action against us and five individual defendants: Our former CEO Mr. Papa, our former Executive Vice President and General Manager of the BCH segment Marc Coucke, our then Chief Executive Officer John Hendrickson, our former Board member Gary Kunkle, Jr., and our Board member Laurie Brlas alleging violations of Israeli law in the District Court of Tel Aviv-Jaffa (Schweiger et al. v. Perrigo Company plc, et al.). On June 15, 2016, we filed a motion to stay the case pending the outcome of the securities class action pending in the New Jersey Federal Court. The plaintiffs did not oppose the motion. The Israeli court granted the motion on the same day, and the Schweiger action was stayed. In October 2017, the Schweiger plaintiffs dismissed their claims without prejudice because of the pendency of another class action case filed in Israel (see discussion below of the Israel Elec. Corp. Employees’ Educ. Fund case). The court approved the voluntary dismissal.
On March 29, 2017, plaintiff Eyal Keinan commenced an action in the District Court of Tel Aviv-Jaffa asserting securities claims against two defendants: Perrigo and its auditor Ernst & Young LLP ("EY"). The case is styled Keinan v. Perrigo Company plc, et al. The action sought certification of a class of purchasers of Perrigo shares on the Israeli exchange beginning February 6, 2014. The proposed closing date for the class was not clear from the complaint though it appeared to extend into 2017. In general, the plaintiff asserted that we improperly accounted for our stream of royalty income from two drugs: Tysabri® and Prialt. The court filings contended that the alleged improper accounting caused the audited financial results for Perrigo to be incorrect for the six month period ended December 31, 2015, and the years ended June 27, 2015 and June 28, 2014 and the other financial data released by us over those years and 2016 to also be inaccurate. The plaintiff maintained that the defendants are liable under Israeli securities law or, in the alternative, under U.S. securities law. The plaintiff indicated an initial, preliminary class damages estimate of 686.0 million NIS (approximately $192.0 million at 1 NIS = $0.28 cent). In January 2018, the Keinan plaintiff announced its intention to dismiss his claims because of the pendency of another class action case filed in Israel (see discussion below of the Israel Elec. Corp. Employees’ Educ. Fund case). The court granted the dismissal on February 11, 2018.
On June 28, 2017, a plaintiff filed a complaint in Tel Aviv District Court styled Israel Elec. Corp. Employees’ Educ. Fund v. Perrigo Company plc, et al. The lead plaintiff seeks to represent a class of shareholders who purchased Perrigo stock on the Tel Aviv exchange during the period April 24, 2015 through May 3, 2017 and also a claim for those that owned shares on the final day of the Mylan tender offer (November 13, 2015). The amended complaint names as defendants the Company, EY (the Company’s auditor), and 11 current or former directors and officers of Perrigo (Mses. Judy Brown, Laurie Brlas, Jacqualyn Fouse, Ellen Hoffing, and Messrs. Joe Papa, Marc Coucke, Gary Cohen, Michael Jandernoa, Gerald Kunkle, Herman Morris, and Donal O’Connor). The complaint alleges violations under U.S. securities laws of Securities Exchange Act sections 10(b) (and Rule 10b‑5) and 14(e) against all defendants and 20(a) control person liability against the 11 individuals or, in the alternative, under Israeli securities laws. In general, the allegations concern the actions taken by us and our former executives to defend against the unsolicited takeover bid by Mylan in the period from April 21, 2015 through November 13, 2015 and the allegedly inadequate disclosure concerning purported integration problems related to the Omega acquisition, alleges incorrect reporting of organic growth at the Company, alleges price fixing activities with respect to six generic prescription pharmaceuticals, and alleges improper accounting for the Tysabri® royalty stream. The plaintiff indicates an initial, preliminary class damages estimate of 2.7 billion NIS (approximately $760.0 million at 1 NIS = $0.28 cent). We intend to defend the lawsuit vigorously.
On July 12, 2017, the plaintiff in the Israel Elec. Corp. Employees’ Educ. Fund v. Perrigo Company plc, et al. case filed a motion to have all three cases pending in Israel either consolidated or the other two cases dismissed so that the Israel Elec. Corp. Educ. Fund plaintiff can proceed as the sole plaintiff. In October 2017, the Schweiger plaintiffs (see description above) voluntarily dismissed their securities class action without prejudice as part of their response to the motion filed by the Israel Elec. Corp. Educ. Fund plaintiff. A variety of other procedural motions were also pending having to do with the timing of any response by defendants. The court held an initial conference on November 9, 2017 to address the motion filed by the Israel Elec. Corp. Educ. Fund plaintiff. Subsequently, the competing class plaintiffs held discussions and informed the court in January 2018 that they had reached an agreement among themselves such that the Education Fund case will continue and the Keinan plaintiff dismissed its case. The court approved this outcome. At the request of the parties, the court has stayed the Education Fund case pending the final adjudication of the class action case in D.N.J. (the Roofers’ Pension Fund case described above under Securities Litigation In the United States). The court approved the stay.
28
Perrigo Company plc - Item 1
Note 14
Eltroxin
During October and November 2011, nine applications to certify a class action lawsuit were filed in various courts in Israel related to Eltroxin, a prescription thyroid medication manufactured by a third party and distributed in Israel by our subsidiary, Perrigo Israel Agencies Ltd. The respondents included our subsidiaries, Perrigo Israel Pharmaceuticals Ltd. and/or Perrigo Israel Agencies Ltd., the manufacturers of the product, and various healthcare providers who provide healthcare services as part of the compulsory healthcare system in Israel.
One of the applications was dismissed and the remaining eight applications were consolidated into one application. The applications arose from the 2011 launch of a reformulated version of Eltroxin in Israel. The consolidated application generally alleges that the respondents (a) failed to timely inform patients, pharmacists and physicians about the change in the formulation; and (b) failed to inform physicians about the need to monitor patients taking the new formulation in order to confirm patients were receiving the appropriate dose of the drug. As a result, claimants allege they incurred the following damages: (a) purchases of product that otherwise would not have been made by patients had they been aware of the reformulation; (b) adverse events to some patients resulting from an imbalance of thyroid functions that could have been avoided; and (c) harm resulting from the patients' lack of informed consent prior to the use of the reformulation.
Several hearings on whether or not to certify the consolidated application took place in December 2013 and January 2014. On May 17, 2015, the District Court certified the motion against Perrigo Israel Agencies Ltd. and dismissed it against the remaining respondents, including Perrigo Israel Pharmaceuticals Ltd.
On June 16, 2015, we submitted a motion for permission to appeal the decision to certify to the Israeli Supreme Court together with a motion to stay the proceedings of the class action until the motion for permission to appeal is adjudicated. We have filed our statement of defense to the underlying proceedings. The underlying proceedings have been stayed pending the outcome of the mediation process and, if necessary, a decision on the motion to appeal.
On November 14, 2017 the parties submitted the agreed settlement agreement to the approval of the Supreme Court, which referred the approval back to the District Court. During three hearings that took place on November 29, 2017, December 13, 2017 and January 11, 2018 the District Court opined that it would approve the settlement agreement subject to certain amendments to be proposed by the Court (which would not impact the monetary settlement reached) and set a hearing for January 30, 2018 to discuss and finalize the proposed changes. Meanwhile, the Court ordered the settlement to be (1) provided to the Attorney General for review (standard procedure); and (2) published in the written media (newspapers), to enable the class members to submit any objections or “opt-out” to the proposed settlement by February 15, 2018.
On February 21, 2018, the District Court held a hearing to, among others, review objections received from class members who had notified the District Court of their desire to opt out of the settlement. In addition, a representative of the Israeli Attorney General’s office notified the District Court that, based upon their preliminary examination of the settlement, they intend to object to the settlement in its current form. The District Court recommended that the parties continue to discuss and minimize objections to the settlement and scheduled another hearing for May 13, 2018.
The District Court Justice was appointed as a Supreme Court Justice and ordered to move the case to a different panel. In an effort to reach a decision before the appointment, an additional hearing was held on March 12, 2018 in which the court urged the parties to try and exhaust their negotiations to the fullest and provide an update by May 13, 2018. In addition, the Court ordered the Attorney General to submit its opinion to the settlement agreement by May 30, 2018.
29
Perrigo Company plc - Item 1
Note 14
Tysabri® Product Liability Lawsuits
We and our collaborator Biogen are co-defendants in product liability lawsuits arising out of the occurrence of Progressive Multifocal Leukoencephalopathy, a serious brain infection, and serious adverse events, including deaths, which occurred in patients taking Tysabri®. Each co-defendant would be responsible for 50% of losses and expenses arising out of any Tysabri® product liability claims. During calendar year 2016, one case in the U.S. was settled and two others were dismissed with prejudice. In 2017, seven other cases were dismissed with prejudice. While we intend to vigorously defend the remaining lawsuits, management cannot predict how these cases will be resolved. Adverse results in one or more of these lawsuits could result in substantial judgments against us.
Claim Arising from the Omega Acquisition
On December 16, 2016, we and Perrigo Ireland 2 brought an arbitral claim ("Claim") against Alychlo NV ("Alychlo") and Holdco I BE NV ("Holdco") (together the Sellers) in accordance with clause 26.2 of the Share Purchase Agreement dated November 6, 2014 ("SPA") and the rules of the Belgian Centre for Arbitration and Mediation ("CEPANI"). Our Claim relates to the accuracy and completeness of information about Omega provided by the Sellers as part of the sale process, the withholding of information by the Sellers during that process and breaches of Sellers’ warranties. We are seeking monetary damages from the Sellers. The Sellers served their respective responses to the Claim on February 20, 2017. In its response, Alychlo has asserted a counterclaim for monetary damages contending that we breached the duty of good faith in performing the SPA. There can be no assurance that our Claim will be successful, and Sellers deny liability for the Claim. We deny that Alychlo is entitled to any relief (including monetary relief) under the counterclaim. The arbitration proceedings are confidential as required by the SPA and the rules of the CEPANI.
NOTE 15 – RESTRUCTURING CHARGES
We periodically take action to reduce redundant expenses and improve operating efficiencies. The following reflects our restructuring activity (in millions):
Three Months Ended | |||||||
March 31, 2018 | April 1, 2017 | ||||||
Beginning balance | $ | 21.4 | $ | 19.7 | |||
Additional charges | 1.5 | 38.7 | |||||
Payments | (10.8 | ) | (7.1 | ) | |||
Non-cash adjustments | 0.3 | 0.2 | |||||
Ending balance | $ | 12.4 | $ | 51.5 | |||
Restructuring activity includes severance, lease exit costs, and asset impairments. The charges incurred during the three months ended March 31, 2018 were primarily associated with continued costs from actions we took to streamline our organization as announced on February 21, 2017. During the three months ended April 1, 2017, $23.7 million of restructuring expenses were recorded in the CHCA segment. There were no other material restructuring programs that significantly impacted any other reportable segments for the three months ended March 31, 2018 or April 1, 2017. All charges are recorded in Restructuring expense on the Condensed Consolidated financial statements. The remaining $8.5 million liability for employee severance benefits will be paid within the next year, while the remaining $3.9 million liability for lease exit costs will be paid over the remaining terms of the applicable leases.
NOTE 16 – SEGMENT INFORMATION
Our reporting segments are as follows:
• | Consumer Healthcare Americas, comprises our U.S., Mexico and Canada consumer healthcare business (OTC, contract, infant formula and animal health categories). |
30
Perrigo Company plc - Item 1
Note 16
• | Consumer Healthcare International, comprises our branded consumer healthcare business primarily in Europe and our consumer focused businesses in the U.K., Australia, and Israel. This segment also includes our U.K. liquid licensed products business. |
• | Prescription Pharmaceuticals, comprises our U.S. Prescription Pharmaceuticals business. |
We also had a legacy operating segment, Other, which contained our Active Pharmaceuticals ("API") business, which we divested (refer to Note 7). Following the divestitures, there were no substantial assets or operations left in the segment. Our segments reflect the way in which our management makes operating decisions, allocates resources and manages the growth and profitability of the Company.
The below tables show select financial measures by reporting segment (in millions):
Total Assets | ||||||||
March 31, 2018 | December 31, 2017 | |||||||
CHCA | $ | 3,699.0 | $ | 3,786.8 | ||||
CHCI | 5,174.2 | 5,029.0 | ||||||
RX | 2,766.4 | 2,813.0 | ||||||
Total | $ | 11,639.6 | $ | 11,628.8 | ||||
Three Months Ended | |||||||||||||||||||||||
March 31, 2018 | April 1, 2017 | ||||||||||||||||||||||
Net Sales | Operating Income (Loss) | Intangible Asset Amortization | Net Sales | Operating Income (Loss) | Intangible Asset Amortization | ||||||||||||||||||
CHCA | $ | 601.6 | $ | 113.1 | $ | 15.2 | $ | 582.8 | $ | 75.0 | $ | 17.1 | |||||||||||
CHCI | 401.4 | 14.9 | 51.4 | 374.9 | 0.2 | 45.7 | |||||||||||||||||
RX | 214.0 | 61.9 | 20.6 | 217.4 | 88.2 | 22.3 | |||||||||||||||||
Other | — | — | — | 18.9 | 5.7 | 0.4 | |||||||||||||||||
Unallocated | — | (33.6 | ) | — | — | (40.6 | ) | — | |||||||||||||||
Total | $ | 1,217.0 | $ | 156.3 | $ | 87.2 | $ | 1,194.0 | $ | 128.5 | $ | 85.5 | |||||||||||
ITEM 2. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
EXECUTIVE OVERVIEW
This Management’s Discussion and Analysis of Financial Condition and Results of Operations should be read in conjunction with the financial statements included in this Form 10-Q and our Form 10-K for the year ended December 31, 2017 (the “2017 Form 10-K”). These historical financial statements may not be indicative of our future performance. This discussion contains a number of forward-looking statements, all of which are based on our current expectations and could be affected by the uncertainties and risks referred to under “Risk Factors” in Item 1A of our 2017 Form 10-K and Part II. Item 1A of this Form 10-Q.
Perrigo Company plc was incorporated under the laws of Ireland on June 28, 2013 and became the successor registrant of Perrigo Company, a Michigan corporation, on December 18, 2013 in connection with the acquisition of Elan Corporation, plc ("Elan"). Unless the context requires otherwise, the terms "Perrigo," the "Company," "we," "our," "us," and similar pronouns used herein refer to Perrigo Company plc, its subsidiaries, and all predecessors of Perrigo Company plc and its subsidiaries.
We are a leading global healthcare company, delivering value to our customers and consumers by providing Quality Affordable Healthcare Products®. Founded in 1887 as a packager of home remedies, we have built a unique business model that is best described as the convergence of a fast-moving consumer goods company, a high-quality pharmaceutical manufacturing organization and a world-class supply chain network. We believe we are one of the world's largest manufacturers of over-the-counter (“OTC”) healthcare products and suppliers of infant formulas for the store brand market. We are a leading provider of branded OTC products throughout Europe, and also a leading producer of generic pharmaceutical topical products such as creams, lotions,
31
Perrigo Company plc - Item 2
Executive Overview
gels, and nasal sprays ("extended topical") prescription drugs. We are headquartered in Ireland, and sell our products primarily in North America and Europe, as well as in other markets, including Australia, Israel and China.
Our reporting segments are as follows:
• | Consumer Healthcare Americas ("CHCA"), comprises our U.S., Mexico and Canada consumer healthcare business (OTC, contract, infant formula and animal health categories). |
• | Consumer Healthcare International ("CHCI"), comprises our branded consumer healthcare business primarily in Europe and our consumer focused businesses in the U.K., Australia, and Israel. This segment also includes our U.K. liquid licensed products business. |
• | Prescription Pharmaceuticals ("RX"), comprises our U.S. Prescription Pharmaceuticals business. |
Our segments reflect the way in which our management makes operating decisions, allocates resources and manages the growth and profitability of the Company. For results by segment, see "Segment Results" below and Item 1. Note 16.
2018 Highlights
• | We adopted ASU 2014-09 Revenue from Contracts with Customers and its related amendments (collectively, "ASC 606") on January 1, 2018 using the modified retrospective method. The adoption of ASC 606 represents a change in accounting principle that will more closely align revenue recognition with the transfer of control of products to our customers and will provide financial statement readers with enhanced disclosures (refer to Item 1. Note 2). |
• | We adopted ASU 2016-01 Financial Instruments - Recognition and Measurement of Financial Assets and Liabilities effective January 1, 2018 (refer to Item 1. Note 8 and Note 12). |
• | We repurchased $108.1 million worth of shares as part of our authorized share repurchase plan (refer to Item 1. Note 11). |
RESULTS OF OPERATIONS
CONSOLIDATED
Consolidated Results
Three Months Ended | |||||||
($ in millions) | April 1, 2017 | March 31, 2018 | |||||
Net sales | $ | 1,194.0 | $ | 1,217.0 | |||
Gross profit | $ | 464.4 | $ | 492.7 | |||
Gross profit % | 38.9 | % | 40.5 | % | |||
Operating expenses | $ | 335.9 | $ | 336.4 | |||
Operating expenses % | 28.1 | % | 27.6 | % | |||
Operating income | $ | 128.5 | $ | 156.3 | |||
Operating income % | 10.8 | % | 12.8 | % | |||
Change in financial assets | $ | (17.1 | ) | $ | 9.6 | ||
Interest expense, net | $ | 53.3 | $ | 31.4 | |||
Other expense (income), net | $ | (3.5 | ) | $ | 4.3 | ||
Loss on extinguishment of debt | $ | — | $ | 0.5 | |||
Income tax expense | $ | 24.2 | $ | 29.7 | |||
Net income | $ | 71.6 | $ | 80.8 | |||
32
Perrigo Company plc - Item 2
Consolidated
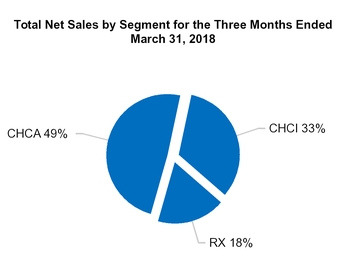
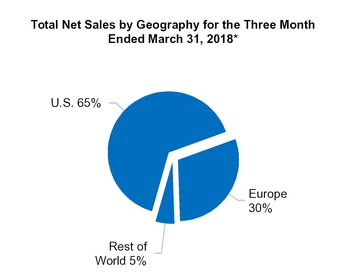
* Total net sales by geography is derived from the location of the entity that sells to a third party.
The $23.0 million increase in consolidated net sales for the three months ended March 31, 2018 as compared to the prior year period was due primarily to new product sales of $41.2 million and favorable foreign currency translation of $44.5 million, partially offset by the absence of sales attributable to the exited Russian business, prior year distribution phase out initiatives of $21.7 million in the CHCI segment, the absence of Active Pharmaceuticals Ingredient ("API") business sales of $18.9 million in the former Other segment, and discontinued products of $8.3 million.
The $27.8 million increase in consolidated operating income for the three months ended March 31, 2018 as compared to the prior year period was due primarily to increased sales volumes, operational efficiencies, and lower operating expenses; partially offset by pricing pressures in certain categories across the organization.
Further details and analysis of our financial results for the three months ended March 31, 2018 are described below by reporting segment and line item. Refer to the "Unallocated Expenses," "Interest, Other and Change in financial assets (Consolidated)," and "Income Taxes (Consolidated)" sections below for discussions related to our expenses.
CONSUMER HEALTHCARE AMERICAS
Segment Results
Three Month Comparison
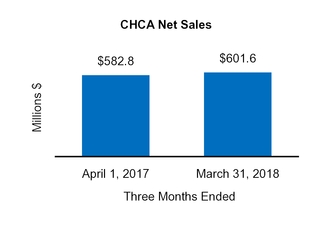
Three Months Ended | |||||||
($ in millions) | April 1, 2017 | March 31, 2018 | |||||
Net sales | $ | 582.8 | $ | 601.6 | |||
Gross profit | $ | 188.4 | $ | 200.4 | |||
Gross profit % | 32.3 | % | 33.3 | % | |||
Operating income | $ | 75.0 | $ | 113.1 | |||
Operating income % | 12.9 | % | 18.8 | % | |||
33
Perrigo Company plc - Item 2
CHCA
Three Months Ended March 31, 2018 vs. Three Months Ended April 1, 2017
Net sales increased $18.8 million, or 3%, over the prior year period due to:
• | New product sales of $11.2 million related primarily to the launches of esomeprazole magnesium (store brand equivalent to Nexium® 24HR capsules) and infant formula products; and |
• | A net increase in sales of existing products of $8.4 million due primarily to: |
• | Higher sale volumes in our infant nutritional, analgesics, and cough/cold/allergy categories; partially offset by |
• | Lower sales in our animal health business; and |
• | Ongoing pricing pressures in the segment, which we expect to continue for the foreseeable future; |
• | Favorable foreign currency translation movement of $1.1 million; partially offset by |
• | The absence of sales of discontinued products of $1.9 million. |
Operating income increased $38.1 million, or 51%, as a result of:
• | An increase of $12.0 million in gross profit due to: |
• | Higher volumes in certain categories, as discussed above; and |
• | Positive contributions from supply chain efficiencies; offset partially by |
• | Pricing pressures in the segment, as discussed above. |
• | A decrease of $26.1 million in operating expenses due to: |
• | Decreased restructuring expense of $23.3 million related to the cost reduction initiatives taken in the prior year period (refer to Item 1. Note 15); |
• | Decreased Research and Development ("R&D") expenses of $1.4 million due to timing of clinical trials; and |
• | Decreased contingent consideration adjustments of $1.5 million (refer to Item 1. Note 7). |
• | Gross profit as a percentage of net sales was 100 bps higher due primarily to improved product mix; partially offset by pricing pressures, as discussed above. |
• | Operating income as a percentage of net sales was 590 bps higher due primarily to decreased restructuring expense and improved product mix, each as discussed above. |
Segment Results
Three Month Comparison
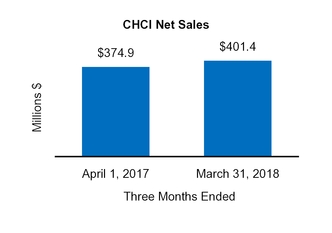
Three Months Ended | |||||||
($ in millions) | April 1, 2017 | March 31, 2018 | |||||
Net sales | $ | 374.9 | $ | 401.4 | |||
Gross profit | $ | 169.5 | $ | 194.6 | |||
Gross profit % | 45.2 | % | 48.5 | % | |||
Operating income | $ | 0.2 | $ | 14.9 | |||
Operating income % | 0.1 | % | 3.7 | % | |||
34
Perrigo Company plc - Item 2
CHCI
Three Months Ended March 31, 2018 vs. Three Months Ended April 1, 2017
Net sales increased $26.5 million, or 7%, over the prior year period due primarily to:
• | Favorable foreign currency translation movement of $43.3 million; |
• | New product sales of $20.3 million; partially offset by |
• | The absence of $21.7 million in sales attributable to the exited Russian business and prior year distribution phase out initiatives; |
• | A net decrease in sales of existing products of $9.7 million due primarily to lower sales in the cough/cold/allergy, personal care, and analgesics categories; and |
• | The absence of sales of discontinued products of $5.7 million. |
Operating income increased $14.7 million, as a result of:
• | An increase of $25.1 million in gross profit due primarily to: |
• | Favorable foreign currency translation movement; and |
• | Improved product mix for sales of existing products. |
• | An increase of $10.4 million in operating expenses due primarily to: |
• | An increase of $11.3 million in selling and administrative expenses due primarily to unfavorable foreign currency translation; and |
• | An increase in distribution costs of $2.3 million due primarily to unfavorable foreign currency translation; offset partially by |
• | Decreased restructuring expense of $2.3 million related to the cost reduction initiatives taken in the prior year (refer to Item 1. Note 15); and |
• | The absence of a $1.1 million impairment charge on certain In-process Research and Development ("IPR&D") assets. |
• | Gross profit as a percentage of net sales was 330 bps higher due primarily to improved product mix, as described above. |
• | Operating income as a percentage of net sales was 360 bps higher due primarily to an increase in gross profit. |
• | Management continues to implement its previously disclosed strategy for brand prioritization, sales force restructuring, and manufacturing in-sourcing, which is expected to reduce selling costs, improve operating margins and focus on higher value OTC products. |
Recent Trends and Developments
• | We continue to experience a significant year-over-year reduction in pricing in our RX segment due to competitive pressures. This softness in pricing is attributable to various factors, including increased focus from customers to capture supply chain productivity savings, competition in specific products, and consolidation of certain customers. We expect this softness to continue to impact the segment for the foreseeable future, and we are forecasting a high single digit pricing decline in this segment for the year ending December 31, 2018. |
• | We are continuing our previously announced portfolio review process, which includes the ongoing comprehensive internal evaluation of the RX segment's market position, growth opportunities, and interdependencies with our manufacturing and shared service operations to determine if strategic alternatives should be explored. |
35
Perrigo Company plc - Item 2
RX
Segment Results
Three Month Comparison
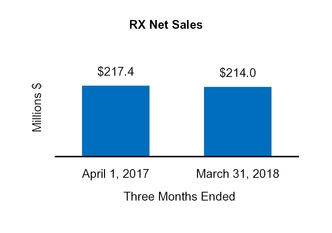
Three Months Ended | |||||||
($ in millions) | April 1, 2017 | March 31, 2018 | |||||
Net sales | $ | 217.4 | $ | 214.0 | |||
Gross profit | $ | 96.3 | $ | 97.8 | |||
Gross profit % | 44.3 | % | 45.7 | % | |||
Operating income | $ | 88.2 | $ | 61.9 | |||
Operating income % | 40.5 | % | 28.9 | % | |||
Three Months Ended March 31, 2018 vs. Three Months Ended April 1, 2017
Net sales decreased $3.4 million, or 2%, due to:
• | New product sales of $9.7 million due primarily to sales of esomeprazole and testosterone 2% topical (generic equivalent to Axiron®); more than offset by |
• | Decreased sales of existing products of $12.4 million due primarily to pricing pressure across the portfolio, which were partially offset by favorable volume and product mix; and |
• | The absence of sales of discontinued products of $0.7 million. |
Segment operating income decreased $26.3 million, or 30%, as a result of:
• | An increase of $1.5 million in gross profit due primarily to favorable volumes and product mix, as discussed above. |
• | An increase of $27.8 million in operating expenses due primarily to: |
• | The absence of the following: |
• | Gain on the sale of certain Abbreviated New Drug Applications ("ANDAs") of $21.8 million (refer to Item 1. Note 3); |
• | Gain related to contingent consideration of $16.2 million (refer to Item 1. Note 7); and |
• | An impairment charge related to certain IPR&D assets of $11.1 million (refer to Item 1. Note 4); |
• | Expenses related to acquisition charges and certain adjustments to contingent consideration of $4.1 million (refer to Item 1. Note 7); partially offset by |
• | Decreased restructuring expense of $5.3 million due to the cost reduction initiatives taken in the prior year (refer to Item 1. Note 15). |
• | Gross profit as a percentage of net sales was 140 bps higher due primarily to favorable product mix; offset partially by pricing pressure across the portfolio, each as discussed above. |
• | Operating income as a percentage of net sales was 1,160 bps lower due primarily to the absence of gains on certain ANDA sales and contingent consideration in the prior year period; offset partially by the absence of impairment charges related to certain IPR&D assets in the prior year period. |
36
Perrigo Company plc - Item 2
Other
OTHER
We had a legacy operating segment, Other, which contained our API businesses, which we divested in 2017 (refer to Item 1. Note 7). Following the divestitures, there were no substantial assets or operations left in the segment. During the three months ended April 1, 2017, the Other segment had $18.9 million of net sales and $5.7 million of operating income.
Unallocated Expenses
Unallocated expenses are comprised of certain corporate services not allocated to our reporting segments and are recorded above Operating income on the Condensed Consolidated Statements of Operations. Unallocated expenses were as follows (in millions):
Three Months Ended | ||||||
April 1, 2017 | March 31, 2018 | |||||
$ | 40.6 | $ | 33.5 | |||
Effective January 1, 2017, due to the sale of the Tysabri® financial asset, all legal expenses associated with the former Specialty Sciences segment were moved to unallocated expenses.
The decrease of $7.1 million in unallocated expenses during the three months ended March 31, 2018 compared to the prior year period was due to a decrease of $5.3 million of administrative expenses driven primarily by decreased legal and consulting fees, and a $6.3 million decrease in restructuring expense related to the cost reduction initiatives taken in the prior year, partially offset by an increase in share-based compensation expense of $4.5 million driven by the absence of prior year adjustments related to the departure of certain executives.
Interest, Other and Change in Financial Assets (Consolidated)
Three Months Ended | |||||||
($ in millions) | April 1, 2017 | March 31, 2018 | |||||
Change in financial assets | $ | (17.1 | ) | $ | 9.6 | ||
Interest expense, net | $ | 53.3 | $ | 31.4 | |||
Other expense (income), net | $ | (3.5 | ) | $ | 4.3 | ||
Loss on extinguishment of debt | $ | — | $ | 0.5 | |||
Change in Financial Assets
On March 27, 2017, we announced the completed divestment of our Tysabri® financial asset to Royalty Pharma for up to $2.85 billion, consisting of $2.2 billion in cash and $250.0 million and $400.0 million in milestone payments if the royalties on global net sales of Tysabri® that are received by Royalty Pharma meet specific thresholds in 2018 and 2020, respectively. As a result of this transaction, we transferred the entire financial asset to Royalty Pharma and recorded a $17.1 million gain during the three months ended April 1, 2017. We elected to account for the contingent milestone payments using the fair value option method.
During the three months ended March 31, 2018, the fair value of the Royalty Pharma contingent milestone payments decreased $9.6 million. The decrease was primarily attributed to projected global net sales of Tysabri® continuing to fall below the threshold required for payment of the $250.0 million milestone payment for 2018. Global net sales of Tysabri® are being impacted by competition, namely the launch of Ocrevus® in the U.S. and European markets in 2017 and 2018, respectively (refer to Item 1. Note 7).
Interest Expense, Net
Interest expense, net was $31.4 million during the three months ended March 31, 2018 compared to $53.3 million for the three months ended April 1, 2017. The $21.9 million decrease was the result of the debt
37
Perrigo Company plc - Item 2
Unallocated, Interest, Other, and Taxes
repayments made during the year ended December 31, 2017 (refer to the "Borrowings and Capital Resources" section below and Item 1. Note 10).
Other (Income) Expense, Net
Other expense (income), net was $4.3 million expense for the three months ended March 31, 2018, compared to $3.5 million income for the three months ended April 1, 2017. The $7.8 million change was due primarily to a $4.4 loss on equity securities, $1.8 million of unfavorable changes in revaluation of monetary assets and liabilities held in foreign currencies, and the absence of a $1.6 million gain recorded on the sale of certain investment securities in the prior year period.
Income Taxes (Consolidated)
The effective tax rates were as follows:
Three Months Ended | ||||
April 1, 2017 | March 31, 2018 | |||
25.3 | % | 26.9 | % | |
The effective tax rate for the three months ended March 31, 2018 increased in comparison to the prior year period due primarily to additional valuation allowances recorded against deferred tax assets partially offset by discrete tax benefits.
Our tax rate is subject to adjustment over the balance of the fiscal year due to, among other things: the jurisdictions in which our profits are determined to be earned and taxed; changes in the valuation of our deferred tax assets and liabilities; adjustments to estimated taxes upon finalization of various tax returns; adjustments based on differing interpretations of the applicable transfer pricing standards; changes in available tax credits, grants and other incentives; changes in stock-based compensation expense; changes in tax laws or the interpretation of such tax laws (for example, proposals for fundamental U.S. international tax reform); changes in U.S. GAAP; and expiration of or the inability to renew tax rulings or tax holiday incentives.
On August 15, 2017, we filed a complaint in the U.S. District Court for the Western District of Michigan to recover $163.6 million of Federal income tax, penalties, and interest assessed and collected by the Internal Revenue Service (“IRS”), plus statutory interest thereon from the dates of payment, for the fiscal years ended June 27, 2009, June 26, 2010, June 25, 2011, and June 30, 2012 (the “2009 tax year,” “2010 tax year,” “2011 tax year,” and “2012 tax year,” respectively). The IRS audits of those years culminated in the issuances of two statutory notices of deficiency: (1) on August 27, 2014 for the 2009 and 2010 tax years and (2) on April 20, 2017 for the 2011 and 2012 tax years. The statutory notices of deficiency both included un-agreed income adjustments related principally to transfer pricing adjustments regarding the purchase, distribution, and sale of store-brand OTC pharmaceutical products in the United States. In addition, the statutory notice of deficiency for the 2011 and 2012 tax years included the capitalization of certain expenses that were deducted when paid or incurred in defending against certain patent infringement lawsuits. We fully paid the assessed amounts of tax, interest, and penalties set forth in the statutory notices and filed timely claims for refund on June 11, 2015 and June 7, 2017 for the 2009-2010 tax years and 2011-2012 tax years, respectively. Our claims for refund were disallowed by certified letters dated August 18, 2015 and July 11, 2017, for the 2009-2010 tax years and 2011-2012 tax years, respectively. The complaint was timely, based upon the refund claim denials, and seeks refunds of tax, interest, and penalties of $37.2 million for the 2009 tax year, $61.5 million for the 2010 tax year, $40.2 million for the 2011 tax year, and $24.7 million for the 2012 tax year. The amounts sought in the complaint for the 2009 and 2010 tax years were recorded as deferred charges in Other non-current assets on our balance sheet during the three months ended March 28, 2015, and the amounts sought in the complaint for the 2011 and 2012 tax years were recorded as deferred charges in Other non-current assets on our balance sheet during the three months ended July 1, 2017.
On December 22, 2016, we received a notice of proposed adjustment for the IRS audit of Athena Neurosciences, Inc. (“Athena”), a subsidiary of Elan acquired in 1996, for the years ended December 31, 2011, December 31, 2012, and December 31, 2013. Perrigo acquired Elan in December 2013. This proposed adjustment
38
Perrigo Company plc - Item 2
Unallocated, Interest, Other, and Taxes
relates to the deductibility of litigation costs. We disagree with the IRS’s position asserted in the notice of proposed adjustment and intend to contest it.
On July 11, 2017, we received a draft notice of proposed adjustment associated with transfer pricing positions for the IRS audit of Athena for the years ended December 31, 2011, December 31, 2012, and December 31, 2013. Athena was the originator of the patents associated with Tysabri® prior to the acquisition of Athena by Elan in 1996. In response to the draft notice of proposed adjustment, we provided the IRS with substantial additional documentation supporting our position. The amount of adjustments that may be asserted by the IRS in the final notice of proposed adjustment cannot be quantified at this time; however, based on the draft notice received, the amount to be assessed may be material. We disagree with the IRS’s position as asserted in the draft notice of proposed adjustment and intend to contest it.
We have ongoing audits in multiple other jurisdictions the resolution of which remains uncertain. These jurisdictions include, but are not limited to, the U.S., Israel, Ireland and other jurisdictions in Europe. In addition to the matters discussed above, the IRS is currently auditing our fiscal years ended June 29, 2013, June 28, 2014, and June 27, 2015 (which covers the period of the Elan transaction). The Israel Tax Authority is currently auditing our fiscal years ended June 29, 2013 and June 28, 2014. The Ireland Tax Authority is currently auditing our years ended December 31, 2012 and December 31, 2013.
For the year ended December 31, 2017, statutory income tax rate changes in the U.S. and Belgium impacted the effective tax rate with a reduction to U.S. income tax expense of $2.4 million and increased Belgium income tax expense by $24.1 million. For the three months ended March 31, 2018, there were no material adjustments to these amounts.
Critical Accounting Policies
The determination of certain amounts in our financial statements requires the use of estimates. These estimates are based upon our historical experiences combined with management’s understanding of current facts and circumstances. Although the estimates are considered reasonable based on the currently available information, actual results could differ from the estimates we have used. There have been no material changes to the critical accounting policies disclosed in our 2017 Form 10-K other than revenue recognition policies that we updated upon adoption of ASC 606 (refer to Item 1. Note 2).
FINANCIAL CONDITION, LIQUIDITY, AND CAPITAL RESOURCES
Cash and Cash Equivalents
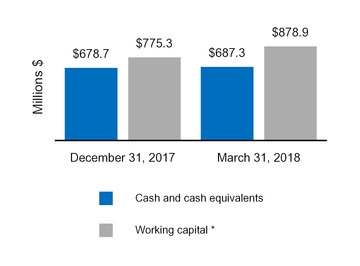
* Working capital represents current assets less current liabilities, excluding cash and cash equivalents, and current indebtedness.
Cash, cash equivalents, cash flows from operations, and borrowings available under our credit facilities are expected to be sufficient to finance the known and/or foreseeable liquidity and capital expenditures. Although our lenders have made commitments to make funds available to us in a timely fashion under our revolving credit
39
Perrigo Company plc - Item 2
Financial Condition, Liquidity and Capital Resources
agreements and overdraft facilities, if economic conditions worsen or new information becomes publicly available impacting the institutions’ credit rating or capital ratios, these lenders may be unable or unwilling to lend money pursuant to our existing credit facilities.
Operating Activities
Three Months Ended | |||||||||||
($ in millions) | April 1, 2017 | March 31, 2018 | Increase/(Decrease) | ||||||||
Cash Flows From (For) Operating Activities | |||||||||||
Net income | $ | 71.6 | $ | 80.8 | $ | 9.2 | |||||
Non-cash adjustments | 95.8 | 136.6 | 40.8 | ||||||||
Subtotal | 167.4 | 217.4 | 50.0 | ||||||||
Increase (decrease) in cash due to: | |||||||||||
Accounts receivable | 50.1 | 2.6 | (47.5 | ) | |||||||
Inventories | 0.5 | (43.7 | ) | (44.2 | ) | ||||||
Accounts payable | 2.5 | 57.5 | 55.0 | ||||||||
Payroll and related taxes | (10.1 | ) | (38.9 | ) | (28.8 | ) | |||||
Accrued customer programs | (32.7 | ) | 17.3 | 50.0 | |||||||
Accrued liabilities | 2.3 | (24.0 | ) | (26.3 | ) | ||||||
Accrued income taxes | 41.4 | 6.4 | (35.0 | ) | |||||||
Other, net | (26.9 | ) | (22.2 | ) | 4.7 | ||||||
Subtotal | $ | 27.1 | $ | (45.0 | ) | $ | (72.1 | ) | |||
Net cash from operating activities | $ | 194.5 | $ | 172.4 | $ | (22.1 | ) | ||||
We generated $172.4 million of cash from operating activities during the three months ended March 31, 2018, a $22.1 million decrease over the prior year period, due to the following:
• | Changes in accounts payable due primarily to timing of payments, mix of payment terms, and an increase in inventory; |
• | Increased net earnings after adjustments for items such as deferred income taxes, impairment charges, restructuring charges, changes in our financial assets, loss on extinguishment of debt, and depreciation and amortization; and |
• | Changes in accrued customer-related programs due primarily to new product launches, which resulted in higher customer related-accruals, pricing dynamics in the RX segment, and timing of rebate payments; more than offset by |
• | Changes in accounts receivable due primarily to timing of sales and receipt of payments, and the discontinuation of our Belgium accounts receivable factoring program; |
• | Changes in inventory due primarily to increased purchasing volumes related to a strategic build-up of inventories and insourcing initiatives in our CHCI segment; |
• | Changes in accrued income taxes due primarily to Federal tax obligation payments made in the current year period, offset by expected tax refunds (refer to Item 1. Note 13); |
• | Changes in payroll and related taxes due primarily to timing of annual management and employee bonus payouts; and |
• | Changes in accrued liabilities due primarily to legal and professional accruals, interest payable related to our debt holdings (refer to Item 1. Note 10), and fair market value adjustments related to contingent consideration (refer to Item 1. Note 7). |
40
Perrigo Company plc - Item 2
Financial Condition, Liquidity and Capital Resources
Investing Activities
Three Months Ended | |||||||||||
($ in millions) | April 1, 2017 | March 31, 2018 | Increase/(Decrease) | ||||||||
Cash Flows From (For) Investing Activities | |||||||||||
Proceeds from royalty rights | $ | 85.3 | $ | 10.0 | $ | (75.3 | ) | ||||
Additions to property, plant and equipment | (22.0 | ) | (13.4 | ) | 8.6 | ||||||
Net proceeds from sale of business and other assets | 25.3 | 1.3 | (24.0 | ) | |||||||
Proceeds from sale of the Tysabri® financial asset | 2,200.0 | — | (2,200.0 | ) | |||||||
Other investing, net | (0.8 | ) | — | 0.8 | |||||||
Net cash from (for) investing activities | $ | 2,287.8 | $ | (2.1 | ) | $ | (2,289.9 | ) | |||
Cash used for investing activities totaled $2.1 million for the three months ended March 31, 2018, compared to cash generated of $2.3 billion in the prior year period. In the current year period, cash used for investing was due primarily to capital expenditures of $13.4 million, as discussed below, offset partially by $10.0 million of proceeds from royalty rights. The prior year inflow was due primarily to the completed divestment of our Tysabri® financial asset to Royalty Pharma, for which we received $2.2 billion in cash at closing.
Cash used for capital expenditures totaled $13.4 million during the three months ended March 31, 2018 compared to $22.0 million in the prior year period. The decrease in cash used for capital expenditures was due primarily to the decrease in the number of projects and timing of significant projects in the current year compared to the prior year period.
Financing Activities
Three Months Ended | |||||||||||
($ in millions) | April 1, 2017 | March 31, 2018 | Increase/(Decrease) | ||||||||
Cash Flows From (For) Financing Activities | |||||||||||
Issuances of long-term debt | $ | — | $ | 431.0 | $ | 431.0 | |||||
Payments on long-term debt | (13.6 | ) | (444.5 | ) | (430.9 | ) | |||||
Borrowings (repayments) of revolving credit agreements and other financing, net | 0.3 | (6.2 | ) | (6.5 | ) | ||||||
Deferred financing fees | (0.4 | ) | (2.4 | ) | (2.0 | ) | |||||
Repurchase of ordinary shares | — | (108.1 | ) | (108.1 | ) | ||||||
Cash dividends | (23.0 | ) | (26.7 | ) | (3.7 | ) | |||||
Other financing, net | (0.5 | ) | (5.7 | ) | (5.2 | ) | |||||
Net cash (for) financing activities | $ | (37.2 | ) | $ | (162.6 | ) | $ | (125.4 | ) | ||
Cash used for financing activities totaled $162.6 million for the three months ended March 31, 2018, compared to $37.2 million used for the comparable prior year period. In the current year period, cash used for financing included $444.5 million of repayments on long-term debt offset by $431.0 million of debt issuance related to our 2018 Term loan due March 8, 2020, $108.1 million in share repurchases, as discussed below, and $26.7 million paid in dividends. The prior year outflow was due primarily to cash used for financing including $13.6 million of repayments on long-term debt, as well as $23.0 million paid in dividends (refer to "Borrowings and Capital Resources" below and Item 1. Note 10).
The declaration and payment of dividends, if any, is subject to the discretion of our Board of Directors and will depend on our earnings, financial condition, availability of distributable reserves, capital and surplus requirements, and other factors our Board of Directors may consider relevant.
41
Perrigo Company plc - Item 2
Financial Condition, Liquidity and Capital Resources
In October 2015, the Board of Directors approved a three-year share repurchase plan of up to $2.0 billion (the "2015 Authorization"). During the three months ended March 31, 2018, we repurchased 1.3 million ordinary shares at an average repurchase price of $81.92 per share, for a total of $108.1 million. We did not repurchase any shares under the share repurchase plan during the three months ended April 1, 2017.
Borrowings and Capital Resources
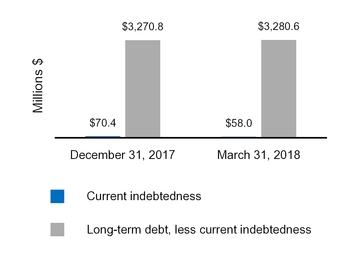
Overdraft Facilities
We have overdraft facilities available that we use to support our cash management operations. The balance outstanding under the overdraft facilities was $1.2 million and $6.9 million at March 31, 2018 and December 31, 2017, respectively.
Accounts Receivable Factoring
We have accounts receivable factoring arrangements with non-related third-party financial institutions (the “Factors”). Pursuant to the terms of the arrangements, we sell to the Factors certain of our accounts receivable balances on a non-recourse basis for credit approved accounts. An administrative fee per invoice is charged on the gross amount of accounts receivables assigned to the Factors, and interest is calculated at the applicable EUR LIBOR rate plus a spread. The total amount factored on a non-recourse basis and excluded from accounts receivable was $22.5 million and $27.5 million at March 31, 2018 and December 31, 2017, respectively.
Revolving Credit Agreements
On December 5, 2014, Perrigo Finance entered into a $600.0 million revolving credit agreement, which increased to $1.0 billion on March 30, 2015 (the "2014 Revolver"). On March 8, 2018, we terminated the 2014 Revolver and entered into a $1.0 billion revolving credit agreement (the "2018 Revolver"). There were no borrowings outstanding under the 2018 Revolver or 2014 Revolver as of March 31, 2018 or December 31, 2017, respectively.
Term Loans and Notes
We had $2.9 billion and $2.9 billion outstanding under our notes and bonds, and $417.9 million and $420.0 million outstanding under our term loan as of March 31, 2018 and December 31, 2017, respectively.
On December 5, 2014, Perrigo Finance entered into a term loan agreement consisting of a €500.0 million ($614.3 million) tranche, maturing December 5, 2019. On March 8, 2018, we refinanced the €350.0 million
42
Perrigo Company plc - Item 2
Financial Condition, Liquidity and Capital Resources
outstanding under the term loan with the proceeds of a new €350.0 million ($431.0 million) term loan, maturing March 8, 2020.
We are in compliance with all covenants under our debt agreements as of March 31, 2018 (refer to Item 1. Note 10 for more information on all of the above debt facilities).
Credit Ratings
Our credit ratings on March 31, 2018 were Baa3 (stable) and BBB- (stable) by Moody's Investors Service and Standard and Poor's Rating Services, respectively.
Credit rating agencies review their ratings periodically and, therefore, the credit rating assigned to us by each agency may be subject to revision at any time. Accordingly, we are not able to predict whether current credit ratings will remain as disclosed above. Factors that can affect our credit ratings include changes in operating performance, the economic environment, our financial position, and changes in business strategy. If changes in our credit ratings were to occur, they could impact, among other things, future borrowing costs, access to capital markets, and vendor financing terms.
Contractual Obligations and Commitments
There were no material changes in contractual obligations as of March 31, 2018 from those provided in our
2017 Form 10-K.
ITEM 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
There have been no material changes to our quantitative or qualitative disclosures found in Item 7A, "Quantitative and Qualitative Disclosures about Market Risk," of our 2017 Form 10-K.
ITEM 4. CONTROLS AND PROCEDURES
Conclusion Regarding the Effectiveness of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rule 13a-15(e) or 15d-15(e) of the Exchange Act) as of March 31, 2018. Based upon that evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that our disclosure controls and procedures were not effective as of March 31, 2018 because of the material weakness in our internal control over financial reporting described below.
All systems of internal control, no matter how well designed, have inherent limitations. Therefore, even
those systems deemed to be effective can provide only reasonable assurance with respect to financial statement
preparation and presentation. A material weakness is a deficiency, or combination of deficiencies, in internal control
over financial reporting, such that there is a reasonable possibility that a material misstatement of a company’s
annual or interim consolidated financial statements will not be prevented or detected on a timely basis.
Evaluation of the Effectiveness of Internal Control over Financial Reporting
Our management assessed the effectiveness of our internal control over financial reporting as of March 31, 2018. The framework used in carrying out our evaluation was the 2013 Internal Control - Integrated Framework published by the Committee of Sponsoring Organizations ("COSO") of the Treadway Commission. In evaluating our information technology controls, we also used components of the framework contained in the Control Objectives for Information and related Technology, which was developed by the Information Systems Audit and Control Association’s IT Governance Institute, as a complement to the COSO internal control framework.
Management has concluded that our internal control over financial reporting was ineffective as of March 31, 2018. The results of management’s assessment have been reviewed with our Audit Committee.
43
Perrigo Company plc - Item 4
Controls and Procedures
Income Taxes
The material weakness over the income tax process that was identified during our fiscal year ended December 31, 2017 was not remediated during the three months ended March 31, 2018, and we determined that we did not design or maintain effective controls over our income tax accounting process. Accordingly, there is a reasonable possibility that a material misstatement will not be prevented or detected on a timely basis.
Remediation Plan
We are committed to remediating the control deficiencies that gave rise to the material weakness described above. Management is responsible for implementing changes and improvements to internal control over financial reporting and for remediating the control deficiencies that gave rise to the material weakness.
With oversight from the Audit Committee, we have taken significant steps to remediate our internal control deficiencies in income taxes by redesigning our controls, many of which operated for the first time at December 31, 2017. Our efforts have consisted primarily of strengthening our tax organization and designing a suite of controls related to the components of our income tax process, including valuation allowances, uncertain tax positions and non-routine events and transactions, to enhance our management review controls over income taxes. Because many of our controls operated for the first time at December 31, 2017, we have not had a sufficient period of time to demonstrate operating effectiveness.
Some of the key remediation actions taken include:
• | Reviewing our income tax processes and controls and enhancing the overall design and procedures performed in calculating our income tax provision on an interim and annual basis |
• | Significantly strengthening our tax capabilities through a combination of key new hires and providing additional resources |
• | Re-designing our management review controls and enhancing the precision of review around the key income tax areas |
To complete the remediation, we plan, with oversight from the Audit Committee, to continue to:
• | Evaluate the sufficiency of our income tax resources and personnel to determine whether additional enhancements are needed |
• | Evaluate whether further enhancements are needed to the design of our income tax procedures and controls |
• | Demonstrate consistent operating effectiveness of our management review controls over income taxes over a number of quarterly periods |
We expect to implement the remaining remediation actions in 2018. Until the remediation actions are fully implemented and the operational effectiveness of related internal controls is validated through testing, the material weakness described above will continue to exist.
We are committed to achieving and maintaining a strong internal control environment and believe the remediation measures will strengthen our internal control over financial reporting and remediate the material weakness identified.
Changes in Internal Control over Financial Reporting
Other than as described above under "Remediation Plan," there have been no changes in our internal control over financial reporting during the three months ended March 31, 2018 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
44
Perrigo Company plc - Part II - Other Information
PART II. OTHER INFORMATION
ITEM 1. LEGAL PROCEEDINGS
Refer to Part I, Item 1. Note 14 of the Notes to the Condensed Consolidated Financial Statements.
ITEM 1A. RISK FACTORS
Our Annual Report on Form 10-K for the year ended December 31, 2017 includes a detailed discussion of our risk factors. At the time of this filing, there have been no material changes to the risk factors that were included in the Form 10-K.
ITEM 2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS
Share repurchase activity during the three months ended March 31, 2018 was as follows:
Total Number of Shares Purchased | Average Price Paid per Share | Total Number of Shares Purchased as Part of Publicly Announced Plans | Value of Shares Available for Purchase(1) | ||||||||||
January 1 - January 31, 2018 | — | $ | — | — | $ | 1.30 | billion | ||||||
February 1 - February 28, 2018 | — | $ | — | — | $ | 1.30 | billion | ||||||
March 1 - March 31, 2018 | 1,319,841 | $ | 81.92 | 1,319,841 | |||||||||
Total | 1,319,841 | $ | 1.20 | billion | |||||||||
(1) The remaining $1.20 billion in the table represents the amount available to be repurchased under our 2015 Authorization as of March 31, 2018.
ITEM 5. OTHER INFORMATION
At the Company’s Annual General Meeting of Shareholders held on May 4, 2018, the Company’s shareholders voted on the following matters:
1. | Election of eleven directors of the Company: |
Nominee | For | Against | Abstain | Broker Non-Votes | ||||||||||||||||
Bradley A. Alford | 113,054,869 | 2,020,823 | 205,840 | 5,392,317 | ||||||||||||||||
Laurie Brlas | 113,545,554 | 1,522,841 | 213,137 | 5,392,317 | ||||||||||||||||
Rolf A. Classon | 113,702,949 | 1,380,203 | 198,381 | 5,392,316 | ||||||||||||||||
Gary M. Cohen | 111,809,782 | 3,139,772 | 331,979 | 5,392,316 | ||||||||||||||||
Adriana Karaboutis | 112,649,079 | 2,484,681 | 147,773 | 5,392,316 | ||||||||||||||||
Jeffrey B. Kindler | 109,778,418 | 5,357,638 | 145,476 | 5,392,317 | ||||||||||||||||
Donal O’Connor | 113,633,212 | 1,499,560 | 148,759 | 5,392,318 | ||||||||||||||||
Geoffrey M. Parker | 113,971,828 | 1,160,926 | 148,779 | 5,392,316 | ||||||||||||||||
Uwe F. Roehrhoff | 112,856,212 | 2,279,156 | 146,165 | 5,392,316 | ||||||||||||||||
Theodore R. Samuels | 113,132,420 | 2,001,258 | 147,853 | 5,392,318 | ||||||||||||||||
Jeffrey C. Smith | 111,107,682 | 4,026,684 | 147,167 | 5,392,316 | ||||||||||||||||
2. | Ratification of the appointment of Ernst & Young LLP as the Company’s independent auditor for the year ending December 31, 2018 and authorization of the Board of Directors, acting through the Audit Committee, to fix the remuneration of the auditor: |
For | Against | Abstain | Broker Non-Votes | |||
117,698,303 | 829,729 | 1,805,376 | 0 | |||
45
Perrigo Company plc - Item 5
Other Information
3. | Advisory vote to approve the Company’s executive compensation: |
For | Against | Abstain | Broker Non-Votes | |||
104,731,659 | 8,891,947 | 1,657,946 | 5,392,297 | |||
4. | Renewal of the Board of Directors’ authority to issue shares under Irish law: |
For | Against | Abstain | Broker Non-Votes | |||
115,550,322 | 4,857,825 | 265,702 | 0 | |||
5. | Renewal of the Board of Directors’ authority to opt-out of statutory pre-emption rights under Irish law: |
For | Against | Abstain | Broker Non-Votes | |||
115,068,951 | 5,327,980 | 276,891 | 0 | |||
46
Perrigo Company plc - Item 6
Exhibits
ITEM 6. EXHIBITS
Exhibit Number | Description | |
3.1 | ||
3.2 | ||
10.1 | ||
10.2 | ||
10.3 | ||
10.4 | ||
10.5 | ||
31.1 | ||
31.2 | ||
32 | ||
101.INS | XBRL Instance Document. | |
101.SCH | XBRL Taxonomy Extension Schema Document. | |
101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document. | |
101.DEF | XBRL Taxonomy Extension Definition Linkbase Document. | |
101.LAB | XBRL Taxonomy Extension Label Linkbase Document. | |
101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document. | |
47
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
PERRIGO COMPANY PLC | |||
(Registrant) | |||
Date: | May 8, 2018 | /s/ Uwe F. Roehrhoff | |
Uwe F. Roehrhoff | |||
Chief Executive Officer and President | |||
(Principal Executive Officer) | |||
Date: | May 8, 2018 | /s/ Ronald L. Winowiecki | |
Ronald L. Winowiecki | |||
Chief Financial Officer | |||
(Principal Accounting and Financial Officer) | |||
48
Exhibit 10.1
EMPLOYMENT AGREEMENT
THIS EMPLOYMENT AGREEMENT (this “Agreement”) is made and entered into, as of May 7, 2018 (the “Effective Date”) by and between Perrigo Management Company, a Michigan corporation (the “Company”), and Uwe Röhrhoff (“Executive”).
WHEREAS, the Executive is currently employed as the Chief Executive Officer of Perrigo Pharma International DAC (“Pharma”) pursuant to an employment agreement between Executive and Pharma dated as of January 15, 2018 (the “Irish Agreement”);
WHEREAS, the Irish Agreement provides that upon the occurrence of a US Immigration Event (as defined in the Irish Agreement), Executive will commence employment with the Company;
WHEREAS, as contemplated in the Irish Agreement, the Company and Executive desire to enter into this Agreement to set forth the terms of Executive’s service to the Company.
NOW, THEREFORE, in consideration of the foregoing, the mutual promises contained herein and other good and valuable consideration, the receipt and sufficiency of which are acknowledged, the parties agree as follows:
1.Employment Period. The Company agrees to employ Executive, and Executive agrees to serve the Company and its Affiliates (as defined below), subject to the terms and conditions of this Agreement, for the period commencing on the Effective Date and ending on the third anniversary of the Effective Date (the “Employment Period”). Thereafter, unless previously terminated, the Employment Period shall be automatically extended for consecutive periods of one year unless either party provides written notice to the other party that the Employment Period will not be extended (a “Non-Renewal”) in accordance with Section 11(b) (a “Notice of NonRenewal”) not less than 180 days prior to the end of the Employment Period as then in effect. Notwithstanding the foregoing, (a) upon a “Change in Control” (as defined in the Perrigo Company plc Change in Control Severance Policy for U.S. Employees, as amended and restated effective of February 6, 2017 (the “CiC Policy”)), the Employment Period shall automatically be extended until the second anniversary of the date such Change in Control is consummated (unless the Employment Period would otherwise expire after such date); and (b) the Employment Period shall immediately terminate upon any termination of Executive’s employment with the Company and its subsidiaries pursuant to Section 4. For purposes of this Agreement, the term “Affiliate” means an entity controlled by, controlling or under common control with Perrigo Company plc, a public limited company incorporated in Ireland (the Company’s ultimate parent entity) (“Parent”) or the Company (for the avoidance of doubt, the Company is an Affiliate of Parent and vice versa).
2. Position and Duties; Location; Standard of Services.
1
(a) Position and Duties. During the Employment Period, Executive shall serve as Chief Executive Officer and President of the Company and shall perform customary and appropriate duties as may be reasonably assigned to Executive from time to time by the Board of
Directors of Parent (the “Board”). In connection with Executive’s employment by and service with the Company, as of the Effective Date, Executive shall (i) hold the titles of Chief Executive Officer and President of Parent, and (ii) serve as a director of the Board (subject to the provisions of Parent’s Memorandum and Articles of Association), in each case, without any additional compensation in respect thereof or the creation of an employment relationship with Parent. Executive shall have such responsibilities, power and authority as those normally associated with such positions in public companies of a similar stature. Executive shall report solely and directly to the Board.
(b) Location. During the Employment Period, Executive’s principal place of employment shall be the Company’s executive offices in Allegan, Michigan, subject to reasonable business travel at the Company’s request; provided that the parties acknowledge that Executive will spend significant time in the Company’s offices in Ireland.
(c) Standard of Services. During the Employment Period, Executive agrees to devote Executive’s full business attention and time to the business and affairs of the Company and its Affiliates and to use Executive’s reasonable best efforts to perform faithfully and efficiently such responsibilities. Within 60 days after the Effective Date, Executive shall resign from all boards of directors of for-profit entities on which he sits. During the Employment Period, Executive may deliver lectures, fulfill speaking engagements, teach at educational institutions, manage personal investments and, subject to the prior written approval of the Board (or a committee thereof), which approval shall not be unreasonably withheld, serve on civic, charitable or other not-for-profit or for-profit boards or committees (collectively, the “Other Activities”), in each case, so long as such Other Activities do not materially interfere with the performance of Executive’s responsibilities in accordance with this Agreement and Executive complies with applicable provisions of any codes of business conduct and ethics of the Company and its Affiliates, as in effect from time to time. For avoidance of doubt, Executive’s engagement in the Other Activities in accordance with this Section 2(c) shall not be deemed a violation of the foregoing requirement that Executive shall devote his full business attention and time to the business and affairs of the Company and its Affiliates and use his reasonable best efforts to perform faithfully and efficiently such responsibilities.
3. Compensation and Employee Benefits.
(a) Annual Base Salary. During the Employment Period, Executive shall receive an annual base salary (the “Annual Base Salary”) of $1,000,000 payable in accordance with the Company’s regular payroll practices, but no less frequently than monthly. The Annual Base Salary shall be reviewed periodically by the Board or an appropriate committee thereof (the Board or such committee, the “Committee”) for possible increase, as determined in the sole and absolute discretion of the Committee, pursuant to the normal performance review policies for senior executives of the Company. Notwithstanding the above, the Committee may decrease the Annual Base Salary in a proportion (not greater than 5%) that generally applies to other senior executives of the Company in connection with an across-the-board senior executive salary decrease as a result of adverse business conditions, so long as such reduction ceases upon the cessation of such adverse business
2
conditions; provided, that the foregoing ability to decrease the Annual Base Salary shall cease to apply upon a Change in Control. The term “Annual Base Salary” as used in this Agreement shall refer to the Annual Base Salary as it may be so adjusted from time to time.
(b) Annual Bonus. During the Employment Period, Executive shall have the opportunity to earn, for each fiscal year of the Company, an annual bonus (the “Annual Bonus”) pursuant to the terms of the management incentive bonus plan (the “MIB”) in which the Company’s senior executives participate, as in effect from time to time. Executive’s target Annual Bonus opportunity shall be no less than 125% of the Annual Base Salary (the “Target Annual Bonus”). The actual amount of the Annual Bonus may range from 0% to 200% of the Target Annual Bonus, as determined by the Committee on the same basis as determinations made with respect to other senior executives of the Company, based on the achievement of pre-established performance goals and its evaluation of Executive’s performance (together, the “Bonus Performance Metrics”); provided, that Executive will be eligible to receive an Annual Bonus equal to no less than 50% of the Target Annual Bonus in the event that the minimum Bonus Performance Metrics for the applicable fiscal year are achieved. Each Annual Bonus that Executive earns pursuant to the applicable Bonus Performance Metrics shall be paid to Executive at the same time as the Company otherwise pays annual bonuses to senior executives of the Company for the applicable fiscal year. Except as provided in Section 5(a), (b), (c), or (d), as applicable, the Annual Bonus shall be subject to Executive being employed on the date of payment of the Annual Bonus for the applicable year. The Annual Bonus shall not be considered an acquired right of Executive, even if it is paid on a repeated basis.
(c) Long-Term Incentive Awards. Executive will be eligible to participate in Parent’s 2013 Long-Term Incentive Plan, as amended (the “2013 LTIP”), on terms and conditions as determined in the sole and absolute discretion of the Committee. During the Employment Period, Executive shall also be eligible to participate in other long-term cash and equity incentive plans, practices, policies, and programs applicable generally to other senior executives of the Company, as determined by the Committee in its sole and absolute discretion.
(d) Other Employee Benefit Plans; Perquisites; Vacation. During the Employment Period, Executive shall be entitled to the perquisites, and to participate in the employee benefit plans, practices, policies and programs, in each case, as in effect from time to time, and that are generally applicable to other senior executives of the Company (including, but not limited to, retirement, deferred compensation and health and welfare benefits) on the same terms as are applicable to other senior executives of the Company. In addition, during the Employment Period, Executive shall be eligible for four weeks of vacation per calendar year, or such greater amount of time as is determined in accordance with the Company’s vacation policy as in effect from time to time, and in all cases subject to the terms of such policy.
(e) Relocation Policy. Executive shall be entitled to receive the benefits and/or reimbursements for relocation costs related to Executive’s relocation to the Allegan, Michigan area, in each case, payable pursuant to the Company’s relocation policy. In addition, in the event that Executive’s employment is terminated by the Company without Cause or by Executive for Good Reason, in each case within two years after the Effective Date, the Company shall reimburse Executive for any loss on the sale of his residence in the Allegan, Michigan area in an amount equal
3
to the excess (if any) of Executive’s purchase price for such residence over the greater of (i) the Appraised Value (as defined below), and (ii) the actual sale price of Executive’s residence, provided that (x) the sale of such residence occurs within 18 months after the termination of employment (the “Resale Protection Period”), unless such Resale Protection Period is extended by mutual agreement of the parties to this Agreement, and (y) such actual sale price is not less than the average value of the residence, as determined within 30 days before the sale by two independent appraisers mutually appointed by Executive and the Company (such average value, the “Appraised Value”).
(f) Temporary Housing Expenses. During the Employment Period, Executive shall be reimbursed (i) on a monthly basis for the reasonable costs incurred by Executive in obtaining temporary housing in the vicinity of the Company’s offices in Allegan, Michigan, and (ii) for all reasonable costs incurred by Executive in obtaining hotel accommodations in the vicinity of the Company’s offices in Ireland during periods in which Executive is performing services from such Irish offices, in each case, in accordance with the applicable Company policy.
(g) Business Expenses. During the Employment Period, and in addition to Executive’s reimbursement rights pursuant to Section 3(f), Executive shall be entitled to receive prompt reimbursement for all reasonable business expenses (including travel, entertainment, professional dues and subscriptions) incurred by Executive, in accordance with the Company’s policies as in effect from time to time.
4. Termination of Employment.
(a) Death or Disability. Executive’s employment shall terminate automatically upon Executive’s death during the Employment Period. If the Board determines in good faith that the Disability of Executive has occurred during the Employment Period (pursuant to the definition of Disability set forth below), it may provide Executive with written notice in accordance with Section 11(b) of its intention to terminate Executive’s employment. In such event, Executive’s employment with the Company and its Affiliates shall terminate effective on the 30th day after Executive’s receipt of such notice (the “Disability Effective Date”), provided that, within the 30 days after such receipt, Executive shall not have returned to full-time performance of Executive’s duties. For purposes of this Agreement, “Disability” shall mean the absence of Executive from Executive’s duties with the Company and its Affiliates on a full-time basis for 90 consecutive days, or for 120 days (which need not be consecutive) within a 365 day period, as a result of incapacity due to mental or physical illness. For the avoidance of doubt, during any period when Executive is absent from duties with the Company and its Affiliates as a result of incapacity due to mental or physical illness, but prior to Executive’s termination of employment due to Disability, Executive shall remain an employee of the Company and shall continue to receive the Annual Base Salary and all employee benefits provided to Executive pursuant to this Agreement in accordance with the terms of this Agreement.
(b) Cause. The Company may terminate Executive’s employment during the Employment Period either with or without Cause. For purposes of this Agreement, “Cause” shall mean, as determined in the sole discretion of the Board:
4
(i) The commission by Executive of an act of dishonesty or breach of trust, that is willful and demonstrably and materially injurious to the business, financial condition or reputation of the Company or its Affiliates;
(ii) Executive’s conviction of, or entry of a plea of guilty or nolo contendere with respect to, a felony crime or a crime involving moral turpitude, fraud, forgery, embezzlement or similar conduct;
(iii) Executive’s willful failure to perform, substantially perform Executive’s material duties with the Company or its Affiliates (other than as a result of physical or mental illness, impairment or disability);
(iv) A willful and material breach by Executive of Executive’s obligations under this Agreement, including a material and willful breach of the restrictive covenants and confidentiality provisions set forth in Section 7;
(v) Executive’s engaging in misconduct involving moral turpitude to the extent that his credibility and reputation no longer conform to the standard of senior executives of the Company or its Affiliates; or
(vi) A failure to assist and cooperate with the Company or its Affiliates in connection with the defense or prosecution of any claim that may be made against or by the Company or its Affiliates, or in connection with any ongoing or future investigation or dispute or claim of any kind involving the Company or its Affiliates, including any proceedings before any arbitral, administrative, regulatory, judicial, legislative or other body or agency.
Executive will not be deemed to be discharged for Cause unless and until there is delivered to Executive a copy of a resolution duly adopted by the affirmative vote of not less than a majority of the entire membership of the Board (excluding Executive, if he is then a member of the Board), at a meeting called and duly held for such purpose, finding in good faith that Executive is guilty of the conduct set forth above and specifying the particulars thereof in detail. Following a Change in Control, any such determination by the Board shall be subject to de novo review by a court of law pursuant to the dispute provisions of Section 11(a).
(c) Good Reason. Executive’s employment may be terminated by Executive either with or without Good Reason. For purposes of this Agreement, “Good Reason” shall mean Executive’s voluntary resignation after any of the following actions are taken by the Company or any of its Affiliates without Executive’s written consent:
(i) A material diminution of Executive’s duties or responsibilities, authorities, powers or functions, including ceasing to be Chief Executive Officer of the Company or assignment of duties materially inconsistent with the position of Chief Executive Officer of the Company, other than during an extended absence due to mental or physical illness (as determined in good faith by the Board), so long as any such material diminution ceases upon Executive’s return to work following such extended absence;
5
(ii) A relocation in Executive’s principal place of employment that would result in Executive’s commute from his principal residence increasing by 20 miles or more; or
(iii) Any material breach of this Agreement by the Company, including any material reduction in Executive’s Annual Base Salary (other than as contemplated by Section 3(a)) or Target Annual Bonus.
In order to invoke a termination for Good Reason, Executive shall provide written notice to the Company of the existence of one or more of the conditions described in clauses (i) through (iii) within 90 days following Executive’s knowledge of the initial existence of such condition or conditions, specifying in reasonable detail the conditions constituting Good Reason, and the Company and its Affiliates shall have 30 days following receipt of such written notice (the “Cure Period”) during which they may remedy the condition. In the event that the Company and its Affiliates fail to remedy the condition constituting Good Reason during the applicable Cure Period, Executive’s “separation from service” (within the meaning of Section 409A of the Internal Revenue Code of 1986, as amended (collectively, with the regulations and other guidance promulgated thereunder, the “Code”)) must occur, if at all, within 30 days following such Cure Period in order for such termination as a result of such condition to constitute a termination for Good Reason.
(d) Notice of Termination. Any termination by the Company with or without Cause, or by Executive with or without Good Reason, shall be communicated by Notice of Termination to the other party hereto given in accordance with Section 11(b). For purposes of this Agreement, a “Notice of Termination” means a written notice that (i) indicates the specific termination provision in this Agreement relied upon, (ii) if the termination is by the Company for Cause or by Executive for Good Reason, sets forth in reasonable detail the facts and circumstances claimed to provide a basis for termination of Executive’s employment under the provision so indicated and (iii) specifies the Date of Termination (as defined below), which date shall not be more than 30 days after the delivery of such notice (except in the case of a Notice of Non-Renewal that is deemed a Notice of Termination in accordance with the following sentence, in which case the Date of Termination shall be the date on which the Employment Period expires pursuant to Section 1). For the avoidance of doubt, a Notice of Non-Renewal delivered pursuant to Section 1 will be deemed to be a Notice of Termination and the expiration of the Employment Period following a Non-Renewal will be a termination of Executive’s employment either by the Company without Cause (if the Company delivers the Notice of Non-Renewal), or by Executive without Good Reason (if Executive delivers the Notice of Non-Renewal).
(e) Date of Termination. “Date of Termination” means (i) if Executive’s employment is terminated by the Company with or without Cause, or by Executive with or without Good Reason, the date of receipt of the Notice of Termination or any later date specified therein within 30 days following such notice (except that in the case of a termination by Executive without Good Reason, the Company may in its sole discretion change any such later date to a date of its choosing between the date of such receipt and such later date), (ii) if Executive’s employment is terminated by reason of death or Disability, the Date of Termination shall be the date of death of Executive or the Disability Effective Date, as the case may be, or (iii) if Executive’s employment is terminated by a Non-Renewal, the date on which the Employment Period expires pursuant to Section 1.
6
(f) Resignation from Other Positions. Upon the termination of Executive’s employment for any reason (unless otherwise agreed in writing by the Company and Executive), Executive shall be deemed to have resigned, without any further action by Executive, from any and all officer and director positions that Executive, immediately prior to such termination, (i) held with Parent, the Company or any of its Affiliates and (ii) held with any other entities at the direction of, or solely as a result of Executive’s affiliation with, the Company or any of its Affiliates. If for any reason this Section 4(f) is deemed to be insufficient to effectuate such resignations, then Executive shall, upon Parent’s or the Company’s request, execute any documents or instruments that Parent or the Company may deem necessary or desirable to effectuate such resignations. In addition, Executive hereby designates the Secretary or any Assistant Secretary of Parent or of any Affiliate to execute any such documents or instruments as Executive’s attorney-in fact to effectuate such resignations if execution by the Secretary or any Assistant Secretary of Parent or any Affiliate is deemed by Parent or any Affiliate to be a more expedient means to effectuate such resignation or resignations.
5. Obligations of the Company upon Termination.
(a) Other than for Cause, Death or Disability; Company Non-Renewal; Resignation for Good Reason. If, during the Employment Period, the Company terminates Executive’s employment without Cause (other than due to death or Disability), there is a Non-Renewal as a result of the Company’s delivery of a Notice of Non-Renewal, or Executive terminates employment for Good Reason, in each case, other than upon or within 24 months following the consummation of a Change in Control (in which case Section 5(b) shall apply), then, subject to Section 11(j) and to, in the case of clauses (ii), (iii), (iv), (v), and (vii) below, Executive’s execution within 50 days following the Date of Termination of a release of claims in the form attached as Exhibit A (the “Release”), which Release has become irrevocable in accordance with its terms (the date the Release becomes irrevocable, the “Release Effective Date”), the Company shall pay to Executive the following:
(i) the sum of (A) the portion of the Annual Base Salary due for the period through the Date of Termination to the extent not theretofore paid, (B) any accrued but unpaid vacation and (C) Executive’s business expenses that have not been reimbursed by the Company as of the Date of Termination that were incurred by Executive prior to the Date of Termination in accordance with the applicable policy of the Company (the sum of the amounts described in clauses (A), (B) and (C) shall be hereinafter referred to as the “Accrued Obligations”), which Accrued Obligations shall be paid in a lump sum in cash within 60 days following the Date of Termination;
(ii) any unpaid Annual Bonus earned by Executive in respect of the fiscal year of the Company that was completed on or prior to the Date of Termination (the “Unpaid Annual Bonus”), which Unpaid Annual Bonus shall be paid in a lump sum in cash on the first payroll date following the Release Effective Date (other than any portion of such Unpaid Annual Bonus that was deferred, which portion shall instead be paid in accordance with the applicable deferral arrangement and any election thereunder);
(iii) a prorated Annual Bonus in respect of the fiscal year of the Company in which the Date of Termination occurs, with such amount to equal the product of (A) the amount determined by the Committee based on actual performance for the fiscal year in
7
which the Date of Termination occurs and otherwise on a basis no less favorable than the basis on which annual incentive award determinations are made by the Committee for other senior executives of the Company in respect of such fiscal year, and (B) a fraction, (I) the numerator of which is the number of days in the fiscal year of the Company in which the Date of Termination occurs through the Date of Termination, and (II) the denominator of which is 365 (the “Prorated Annual Bonus”), which Prorated Annual Bonus shall be paid on the date on which the Company otherwise pays annual bonuses to senior executives of the Company for such fiscal year (other than any portion of such Annual Bonus that was deferred, which portion shall instead be paid in accordance with the applicable deferral arrangement and any election thereunder);
(iv) an amount equal to the product of (A) 1.5 multiplied by (B) the sum of (x) the Annual Base Salary and (y) the Target Annual Bonus as in effect for the fiscal year of the Company in which the Date of Termination occurs, which amount shall be payable in a lump sum on the first payroll date following the Release Effective Date;
(v) if Executive elects health care continuation coverage under Section 4980B of the Code or other applicable law (“COBRA”) for himself and/or his eligible covered dependents equivalent to the coverage which they were receiving immediately prior to the Date of Termination, for 18 months following the Date of Termination, or such shorter period determined in accordance with clause (B) of this sentence (the “Continuation Period”), the Company shall pay the full premium cost of such coverage, based on the prevailing rate (the “Prevailing COBRA Rate”) charged by the Company to persons who elect similar health care continuation coverage under COBRA (the “Health Care Benefits”); provided, however, that (A) the Health Care Benefits shall be reported by the Company as taxable income to Executive to the extent reasonably determined by the Company to be necessary to avoid the Health Care Benefits from being considered to have been provided under a discriminatory self-insured medical reimbursement plan pursuant to Section 105(h) of the Code, and (B) the Continuation Period shall cease at such time that Executive is eligible to receive health care benefits under another employer-provided plan (but no repayment of any previously-paid premium shall be required). In addition, the Company shall pay to Executive on the first day of each of the first six months following the expiration of the Continuation Period an amount in cash equal to the Prevailing COBRA Rate for the coverage for Executive and his eligible covered dependents which was in effect immediately prior to the expiration of the Continuation Period; provided, however, that no such payment shall be made following the time that Executive is eligible to receive health care benefits under another employer-provided plan;
(vi) to the extent not theretofore paid or provided, the Company shall timely pay or provide, in accordance with the terms of the applicable plan, program, policy, practice or contract, to Executive any other amounts or benefits required to be paid or provided or that Executive is eligible to receive under any plan, program, policy, practice or contract of the Company through the Date of Termination (such other amounts and benefits shall be hereinafter referred to as the “Other Benefits”); and
8
(vii) for purposes of any equity incentive awards granted to Executive following the effective date of the Irish Agreement (including, for the avoidance of doubt, any equity incentive awards granted to Executive following the Effective Date), that remain outstanding on the Date of Termination (other than the Sign-On RSUs (as defined in the Irish Agreement)), and notwithstanding anything to the contrary in the applicable award agreement, the 2013 LTIP, or any successor or similar plan, such equity incentive awards that would otherwise be scheduled to vest during the 24 months following the Date of Termination shall continue to vest during such 24-month period according to the vesting schedule in effect prior to the Date of Termination, unless the applicable award agreement, the 2013 LTIP, or any successor or similar plan provides for accelerated or continued vesting that is more favorable to Executive than the foregoing, in which case such more favorable provision shall apply in lieu of the foregoing. For the avoidance of doubt, upon Executive’s termination of employment, the Sign-On RSUs shall be treated in accordance with the RSU Agreement (as defined in the Irish Agreement).
For the avoidance of doubt, if applicable, any amount payable pursuant to Section 5(a) shall be determined without regard to any reduction in compensation that resulted in Executive’s termination of employment for Good Reason. If Executive does not execute the Release within 50 days following the Date of Termination, or if Executive revokes the Release, Executive shall be entitled to only the compensation and benefits contemplated by Sections 5(a)(i) and (vi).
(b) Change in Control Termination. If, during the Employment Period, the Company terminates Executive’s employment without Cause (other than due to death or Disability), there is a Non-Renewal as a result of the Company’s delivery of a Notice of Non-Renewal, or Executive terminates employment for Good Reason, in each case, upon or within 24 months following the consummation of a Change in Control, then, subject to Section 11(j) and, in the case of all payments and benefits other than the Accrued Obligations and the Other Benefits, Executive’s execution within 50 days of the Date of Termination, and nonrevocation, of the Release, the Company shall pay to Executive the following:
(i) the Accrued Obligations, the Unpaid Annual Bonus, the Health Care Benefits and the Other Benefits in accordance with the terms of Sections 5(a)(i), (ii), (v), and (vi), respectively;
(ii) a prorated Annual Bonus in respect of the fiscal year of the Company in which the Date of Termination occurs, with such amount to equal the product of (A) the Target Annual Bonus for the fiscal year in which the Date of Termination occurs, and (B) a fraction, (I) the numerator of which is the number of days that have elapsed in the fiscal year of the Company in which the Date of Termination occurs as of the Date of Termination, and (II) the denominator of which is 365 (the “Prorated Target Bonus”), which Prorated Target Bonus shall be paid in a lump sum in cash on the first payroll date following the Release Effective Date (other than any portion of such Annual Bonus that was deferred, which portion shall instead be paid in accordance with the applicable deferral arrangement and any election thereunder);
9
(iii) an amount equal to the product of (A) two multiplied by (B) the sum of (x) the Annual Base Salary and (y) the Target Annual Bonus as in effect for the fiscal year of the Company in which the Date of Termination occurs, payable in a lump sum on the first payroll date following the Release Effective Date;
(iv) a cash payment in an amount equal to six months’ health care premiums at the Prevailing COBRA Rate for Executive and each of his eligible dependents, payable in a lump sum on the first payroll date following the Release Effective Date; and
(v) for purposes of any equity incentive awards granted to Executive following the effective date of the Irish Agreement (including, for the avoidance of doubt, any equity incentive awards granted to Executive following the Effective Date), that remain outstanding on the Date of Termination (other than the Sign-On RSUs), and notwithstanding anything to the contrary in the applicable award agreement, the 2013 LTIP, or any successor or similar plan, such equity incentive awards shall become fully vested (with any equity incentive awards that are subject to performance-based vesting criteria vesting at “target” levels of achievement). For the avoidance of doubt, upon Executive’s termination of employment, the Sign-On RSUs shall be treated in accordance with the RSU Agreement.
For the avoidance of doubt, if applicable, any amount payable pursuant to Section 5(b) shall be determined without regard to any reduction in compensation that resulted in Executive’s termination of employment for Good Reason. If Executive does not execute the Release within 50 days following the Date of Termination, or if Executive revokes the Release, Executive shall be entitled to only the Accrued Obligations and the Other Benefits.
Other than as set forth in this Section 5(a) or 5(b), as applicable, in the event of a termination of Executive’s employment by the Company without Cause (other than due to death or Disability), due to a Non-Renewal as a result of the Company’s delivery of a Notice of Non-Renewal, or by Executive for Good Reason, the Company and its Affiliates shall have no further obligation to Executive under this Agreement.
(c) Death; Disability. If Executive’s employment is terminated by reason of Executive’s death or Disability during the Employment Period, this Agreement shall terminate without further obligations to Executive, other than for payment of the Accrued Obligations, the Unpaid Annual Bonus and the Prorated Annual Bonus and the timely payment or provision of the Other Benefits. The Accrued Obligations, the Unpaid Annual Bonus and the Prorated Annual Bonus shall be paid to Executive’s estate (in the event of death) or Executive or his legal representative (in the event of Disability), as applicable, on the same schedule as contemplated by Sections 5(a)(i)(iii).
(d) Other Termination. If Executive’s employment is terminated during the Employment Period for a reason other than those governed by Section 5(a), (b), and (c) (including, for the avoidance of doubt, due to a Non-Renewal as a result of Executive’s delivery of a Notice of Non-Renewal), this Agreement shall terminate without further obligations to Executive under this Agreement, other than for (i) payment of the Accrued Obligations within 60 days following the Date of Termination and (ii) the timely payment or provision of the Other Benefits.
10
(e) Full Settlement. The payments and benefits provided under this Section 5 shall be in full satisfaction of the obligations of the Company and its Affiliates to Executive under this Agreement or any other plan, agreement, policy or arrangement of the Company and its Affiliates upon his termination of employment, and in no event shall Executive be entitled to severance pay or benefits beyond those specified in this Section 5 (for the avoidance of doubt, including the Perrigo Company plc Executive Committee Severance Policy, as effective June 14, 2017, the Perrigo Company plc U.S. Severance Policy, as amended and restated effective February 6, 2017, and the CiC Policy).
6. No Mitigation. In no event shall Executive be obligated to seek other employment or take any other action by way of mitigation of any amounts payable to Executive under Section 5 and such amounts shall not be reduced whether or not Executive obtains other employment.
7. Restrictive Covenants. In consideration for Executive’s continued employment and the compensation and benefits payable hereunder, Executive agrees to the covenants set forth below.
(a) Nondisclosure of Confidential Information.
(i) The parties agree that, during the course of Executive’s employment with the Company and its Affiliates, Executive has had and will continue to have access to, and has gained and will continue to gain knowledge with respect to, Confidential Information (as defined below). Executive agrees that Executive shall not, without the prior written consent of the Company, during the period of Executive’s employment with the Company and its Affiliates and thereafter for so long as it remains Confidential Information, use or disclose, or knowingly permit any unauthorized Person (as defined in Section 13(d) of the Securities Exchange Act of 1934) to use, disclose or gain access to, any Confidential Information; provided, however, that Executive may disclose Confidential Information (x) as required by law or (y) as ordered by a court, provided that in any event described in the preceding clause (x) or (y), (A) Executive shall promptly notify the Company in writing, and consult with and assist the Company or its Affiliates in seeking a protective order or request for another appropriate remedy, (B) in the event that such protective order or remedy is not obtained, or if the Company waives compliance with the terms of the preceding clause (A), Executive shall disclose only that portion of the Confidential Information that, in the opinion of Executive’s legal counsel, is legally required to be disclosed and shall exercise reasonable best efforts to assure that confidential treatment shall be accorded to such Confidential Information by the receiving Person and (C) to the extent permitted by applicable law, the Company and its Affiliates shall be given an opportunity to review the Confidential Information prior to disclosure thereof.
(ii) Without limiting the foregoing, Executive agrees to keep confidential the existence of, and any information concerning, any dispute between Executive and the Company or any of its Affiliates, except that Executive may disclose information concerning such dispute to the court that is considering such dispute and to Executive’s legal counsel, provided that such counsel agrees not to disclose any such information other than as necessary to the prosecution or defense of such dispute.
11
(iii) For purposes of this Agreement, “Confidential Information” means information, observations and data concerning the business and affairs of the Company or any of its Affiliates, including all business information (whether or not in written form) that relates to the Company or any of its Affiliates, or their directors, officers, employees, customers, suppliers or contractors or any other third parties with respect to which the Company or any of its Affiliates has a business relationship or owes a duty of confidentiality, or their respective businesses or products, and that is not known to the public generally other than as a result of Executive’s breach of this Agreement, including technical information or reports; trade secrets; unwritten knowledge and “know how”; operating instructions; training manuals; customer lists; customer buying records and habits; product sales records and documents; product development, marketing and sales strategies; market surveys; marketing plans; profitability analyses; product cost; long-range plans; information relating to pricing, competitive strategies and new product development, including processes, formulas, designs, drawings, engineering and technology; information relating to any forms of compensation or other personnelrelated information; contracts; and supplier lists. Confidential Information shall not include such information known to Executive prior to Executive’s involvement with the Company or any of its Affiliates or information rightfully obtained from a third party (other than pursuant to a breach by Executive of this Agreement or any other duty of confidentiality).
(iv) Nothing herein shall preclude (i) Executive’s right to communicate, cooperate or file a complaint with any European, U.S., national, federal, state or local governmental or law enforcement branch, agency or entity (collectively, a “Governmental Entity”) with respect to possible violations of any European, U.S., national, federal, state or local law or regulation, or otherwise make disclosures to any Governmental Entity, in each case, that are protected under the whistleblower or similar provisions of any such law or regulation; provided, that in each case such communications and disclosures are consistent with applicable law or (ii) Executive’s right to receive an award from a Governmental Entity for information provided under any whistleblower or similar program.
(b) Inventions and Patents. Executive agrees that all inventions, innovations, improvements, developments, methods, designs, analyses, drawings, reports and all similar or related information that relate to the actual or anticipated business, research and development or existing or future products or services of the Company or its Affiliates, and that are conceived, developed or made by Executive during his employment with the Company or its Affiliates (“Work Product”) belong to the Company and its Affiliates. Executive shall promptly disclose such Work Product to the Company and its Affiliates and perform all actions reasonably requested by the Company or its Affiliates (whether during or after the Employment Period) to establish and confirm such ownership (including assignments, consents, powers of attorney, and other instruments). To the fullest extent permitted by applicable law all intellectual property (including patents, trademarks, and copyrights) which are made, developed or acquired by Executive in the course of Executive’s employment with the Company or its Affiliates will be and remain the absolute property of the Company and its Affiliates, and Executive shall assist the Company and its Affiliates in perfecting and defending their rights to such intellectual property.
12
(c) Noncompetition. During Executive’s employment with the Company and its Affiliates and for the duration of the Restricted Period (as defined in this Section 7(c)), Executive shall not (i) directly or indirectly, without the prior written consent of the Company, engage in or invest as an owner, partner, stockholder, licensor, director, officer, agent or consultant for any Person that conducts a business that is in competition with a business conducted by the Company or any of its Affiliates anywhere in the world; or (ii) accept employment or an engagement for the provision of services in any capacity, including as an employee, director, consultant or advisor, directly or indirectly, with any Person that conducts a business that is in competition with a business conducted by the Company or any of its Affiliates anywhere in the world. For purposes hereof, conducting a business that is in competition with a business conducted by the Company or any of its Affiliates shall include the sale, manufacture, distribution or research and development of any product or service that is similar to a product or service sold, distributed, marketed or being researched or developed (including through a joint venture or investment in another entity) by the Company or any of its Affiliates, including store brand and value brand OTC drug or nutritional products, extended topical generic prescription pharmaceutical products, infant nutrition products and any other product or products that the Company or an Affiliate is marketing or actively planning to market during Executive’s employment with the Company and, with respect to the period after termination of employment, during the oneyear period following the Date of Termination. Notwithstanding the foregoing, (x) nothing in this provision shall prevent Executive from passively owning two percent (2%) or less of the outstanding securities of any class of any company listed on a national securities exchange or quoted on an automated quotation system, and (y) this Section 7(c) will not apply following the Date of Termination in the event that Executive’s employment is terminated by the Company without Cause pursuant to Section 5(a) or due to a Non-Renewal as a result of the Company’s delivery of a Notice of Non-Renewal.
For purposes of this Agreement, the term “Restricted Period” means: (A) in the event Executive’s employment is terminated for any reason and other than upon or within 24 months following the consummation of a Change in Control, the 18-month period immediately following the Date of Termination; and (B) in the event Executive’s employment is terminated for any reason upon or within 24 months following the consummation of a Change in Control, the 24-month period immediately following the Date of Termination.
(d) Nonsolicitation of Clients. During Executive’s employment with the Company and its Affiliates and for the duration of the Restricted Period, Executive shall not, directly or indirectly, alone or in association with any other Person, without the prior written consent of the Company, (i) induce or attempt to induce any client, customer (whether former or current), supplier, licensee, franchisee, joint venture partner or other business relation of the Company or any of its Affiliates (collectively, “Clients”) to cease doing business with the Company or any such Affiliate, (ii) divert all or any portion of a Client’s business to any competitor of the Company or any such Affiliate, or (iii) in any way interfere with the relationship between any Client, on the one hand, and the Company or any such Affiliate, on the other hand.
(e) Nonsolicitation of Service Providers. During Executive’s employment with the Company and its Affiliates and for the duration of the Restricted Period, Executive shall not, directly or indirectly, without the prior written consent of the Company, (i) actively solicit, recruit or hire
13
any Person who is at such time, or who at any time during the 12 month period prior to such solicitation or hiring had been, an employee or consultant of the Company or any of its Affiliates, (ii) solicit or encourage any employee of the Company or any of its Affiliates to leave the employment of the Company or any of its Affiliates or (iii) interfere with the relationship of the Company or any of its Affiliates with any Person or entity who or that is employed by or otherwise engaged to perform services for the Company or any of its Affiliates.
(f) Mutual Nondisparagement. From and following the Effective Date, Executive shall not make, either directly or by or through another Person, any oral or written negative, disparaging or adverse statements or representations of or concerning the Company or its Affiliates, any of their clients or businesses or any of their current or former officers, directors or employees. From and following the Effective Date, the Company shall not make, and shall direct its officers and directors not to make, either directly or by or through another Person, any oral or written negative, disparaging or adverse statements or representations of or concerning Executive. Notwithstanding the foregoing, subject to Section 7(a) in the case of Executive, nothing herein shall prohibit Executive, the Company, or the Company’s officers from (i) disclosing truthful information if legally required (whether by oral questions, interrogatories, requests for information or documents, subpoena, civil investigative demand or similar process), (ii) exercising any legally protected whistleblower rights (including pursuant to Rule 21F under the Securities Exchange Act of 1934), or (iii) in the case of Executive and the Company’s officers, providing honest assessments in the course of performing their employment duties in good faith.
(g) Return of Property. Executive acknowledges that all documents, records, files, lists, equipment, computer, software or other property (including intellectual property) relating to the businesses of the Company or any of its Affiliates, in whatever form (including electronic), and all copies thereof, that have been or are received or created by Executive while an employee of the Company or any of its Affiliates (including Confidential Information) are and shall remain the property of the Company and its Affiliates, and Executive shall immediately return such property to the Company upon the Date of Termination and, in any event, at the Company’s request. Notwithstanding the foregoing, Executive shall be permitted to retain at all times, including after the Date of Termination, copies of documents related to his personal compensation, including this Agreement, and copies of his contacts information and calendar. Executive further agrees that any property situated on the premises of, and owned by, the Company or any of its Affiliates, including disks and other storage media, filing cabinets or other work areas, is subject to inspection by the Company’s personnel at any time with or without notice.
(h) Remedies and Injunctive Relief. Executive acknowledges that a violation by Executive of any of the covenants contained in this Section 7 would cause irreparable damage to the Company and its Affiliates in an amount that would be material but not readily ascertainable, and that any remedy at law (including the payment of damages) would be inadequate. Accordingly, Executive agrees that, notwithstanding any provision of this Agreement to the contrary, in addition to any other damages it is able to show, the Company and its Affiliates shall be entitled (without the necessity of showing economic loss or other actual damage) to injunctive relief (including temporary restraining orders, preliminary injunctions and permanent injunctions), without posting a bond, in any court of competent jurisdiction for any actual or threatened breach of any of the
14
covenants set forth in this Section 7 in addition to any other legal or equitable remedies it may have. In addition, in the event of Executive’s Willful Restrictive Covenant Breach (as defined in this Section 7(h)), the Company and its Affiliates shall be entitled to cease payment of the compensation and benefits contemplated by Section 5 to the extent not previously paid or provided (including ceasing vesting of outstanding equity incentive awards, but excluding the Accrued Obligations and Other Benefits), and to the prompt return by Executive of any portion of such compensation and the value of such benefits previously paid or provided (including forfeiture of any equity incentive awards that vested pursuant to Section 5 or the repayment of the value of any equity incentive awards that vested pursuant to Section 5 that have been exercised or settled, as applicable). For purposes of this Agreement, “Willful Restrictive Covenant Breach” means Executive’s material breach of any of the covenants set forth in this Section 7 which Executive knew, or with due inquiry, should have known, would constitute such a material breach. The preceding sentences of this Section 7(h) shall not be construed as a waiver of the rights that the Company and its Affiliates may have for damages under this Agreement or otherwise, and all such rights shall be unrestricted. The Restriction Period shall be tolled during (and shall be deemed automatically extended by) any period during which Executive is in violation of the provisions of Section 7(c), (d) or (e), as applicable. In the event that a court of competent jurisdiction determines that any provision of this Section 7 is invalid or more restrictive than permitted under the governing law of such jurisdiction, then, only as to enforcement of this Section 7 within the jurisdiction of such court, such provision shall be interpreted and enforced as if it provided for the maximum restriction permitted under such governing law.
(i) Acknowledgements.
(i) Executive acknowledges that the Company and its Affiliates have expended and will continue to expend substantial amounts of time, money and effort to develop business strategies, employee, customer and other relationships and goodwill to build an effective organization. Executive acknowledges that the Company and its Affiliates have a legitimate business interest in and right to protect its Confidential Information, goodwill and employee, customer and other relationships, and that the Company and its Affiliates could be seriously damaged by the disclosure of Confidential Information and the loss or deterioration of its employee, customer and other relationships. Executive further acknowledges that the Company and its Affiliates are entitled to protect and preserve the going concern value of the Company and its Affiliates to the extent permitted by law.
(ii) In light of the foregoing acknowledgments, Executive agrees that the covenants contained in this Agreement are reasonable and properly required for the adequate protection of the businesses and goodwill of the Company and its Affiliates. Executive further acknowledges that, although Executive’s compliance with the covenants contained in this Agreement may prevent Executive from earning a livelihood in a business similar to the business of the Company and its Affiliates, Executive’s experience and capabilities are such that Executive has other opportunities to earn a livelihood and adequate means of support for Executive and Executive’s dependents.
15
(iii) In light of the acknowledgements contained in this Section 7(i), Executive agrees not to challenge or contest the reasonableness, validity or enforceability of any limitations on, and obligations of, him contained in Section 7 of this Agreement.
8. Treatment of Certain Payments.
(a) Anything in this Agreement to the contrary notwithstanding, in the event the Accounting Firm (as defined below) shall determine that receipt of all Payments (as defined below) would subject Executive to the excise tax under Section 4999 of the Code, the Accounting Firm shall determine whether to reduce any of the Payments paid or payable pursuant to the Agreement (the “Agreement Payments”) so that the Parachute Value (as defined below) of all Payments, in the aggregate, equals the Safe Harbor Amount (as defined below). The Agreement Payments shall be so reduced only if the Accounting Firm determines that Executive would have a greater Net AfterTax Receipt (as defined below) of aggregate Payments if the Agreement Payments were so reduced. If the Accounting Firm determines that Executive would not have a greater Net AfterTax Receipt (as defined below) of aggregate Payments if the Agreement Payments were so reduced, Executive shall receive all Agreement Payments to which Executive is entitled hereunder.
(b) If the Accounting Firm determines that aggregate Agreement Payments should be reduced so that the Parachute Value of all Payments, in the aggregate, equals the Safe Harbor Amount, the Company shall promptly give Executive notice to that effect and a copy of the detailed calculation thereof. All determinations made by the Accounting Firm under this Section 8 shall be binding upon the Company and its Affiliates and Executive and shall be made as soon as reasonably practicable and in no event later than 15 days following the Date of Termination. For purposes of reducing the Agreement Payments so that the Parachute Value of all Payments, in the aggregate, equals the Safe Harbor Amount, only amounts payable under this Agreement (and no other Payments) shall be reduced. The reduction of the amounts payable hereunder, if applicable, shall be made by reducing the payments and benefits in the following order: (i) cash payments that may not be valued under Treas. Reg. § 1.280G¬1, Q&A24(c) (“24(c)”), (ii) equity-based payments that may not be valued under 24(c), (iii) cash payments that may be valued under 24(c), (iv) equitybased payments that may be valued under 24(c) and (v) other types of benefits. With respect to each category of the foregoing, such reduction shall occur first with respect to amounts that are not “deferred compensation” within the meaning of Section 409A of the Code and next with respect to payments that are deferred compensation within the meaning of Section 409A of the Code, in each case, beginning with payments or benefits that are to be paid the farthest in time from the determination of the Accounting Firm. All reasonable fees and expenses of the Accounting Firm shall be borne solely by the Company.
(c) To the extent requested by Executive, the Company shall cooperate with Executive in good faith in valuing, and the Accounting Firm shall take into account the value of, services provided or to be provided by Executive (including Executive’s agreeing to refrain from performing services pursuant to a covenant not to compete or similar covenant, before, on or after the date of a change in ownership or control of the Company (within the meaning of Q&A2(b) of the final regulations under Section 280G of the Code), such that payments in respect of such services may be considered reasonable compensation within the meaning of Q&A9 and Q&A40 to Q&A44 of
16
the final regulations under Section 280G of the Code and/or exempt from the definition of the term “parachute payment” within the meaning of Q&A2(a) of the final regulations under Section 280G of the Code in accordance with Q&A5(a) of the final regulations under Section 280G of the Code.
(d) The following terms shall have the following meanings for purposes of this Section 8:
(i) “Accounting Firm” shall mean a nationally recognized certified public accounting firm or other professional organization that is a certified public accounting firm recognized as an expert in determinations and calculations for purposes of Section 280G of the Code that is selected by the Company prior to a Change in Control for purposes of making the applicable determinations hereunder, which firm shall not, without Executive’s consent, be a firm serving as accountant or auditor for the Person effecting the Change in Control.
(ii) “Net AfterTax Receipt” shall mean the present value (as determined in accordance with Sections 280G(b)(2)(A)(ii) and 280G(d)(4) of the Code) of a Payment net of all taxes imposed on Executive with respect thereto under Sections 1 and 4999 of the Code and under applicable state and local laws, determined by applying the highest marginal rate under Section 1 of the Code and under state and local laws which applied to Executive’s taxable income for the immediately preceding taxable year, or such other rate(s) as the Accounting Firm reasonably determines to be likely to apply to Executive in the relevant tax year(s).
(iii) “Parachute Value” of a Payment shall mean the present value as of the date of the change of control for purposes of Section 280G of the Code of the portion of such Payment that constitutes a “parachute payment” under Section 280G(b)(2) of the Code, as determined by the Accounting Firm for purposes of determining whether and to what extent the excise tax under Section 4999 of the Code will apply to such Payment.
(iv) “Payment” shall mean any payment or distribution in the nature of compensation (within the meaning of Section 280G(b)(2) of the Code) to or for the benefit of Executive, whether paid or payable pursuant to the Agreement or otherwise.
(v) “Safe Harbor Amount” shall mean 2.99 times Executive’s “base amount,” within the meaning of Section 280G(b)(3) of the Code.
9. Successors. This Agreement is personal to Executive and without the prior written consent of the Company shall not be assignable by Executive otherwise than by will or the laws of descent and distribution. This Agreement shall inure to the benefit of and be enforceable by Executive’s legal representatives. This Agreement shall inure to the benefit of and be binding upon the Company and their respective successors and assigns. As used in this Agreement, “Parent” and “Company” shall mean Parent and the Company as hereinbefore defined and any successor to their respective businesses and/or assets as aforesaid which assumes and agrees to perform this Agreement by operation of law, or otherwise.
17
10. Indemnification. Parent and the Company shall indemnify Executive and hold Executive harmless to the fullest extent permitted by the laws of the Republic of Ireland and the State of Michigan, respectively, against and in respect of any and all actions, suits, proceedings, claims, demands, judgments, costs, expenses, losses, and damages resulting from Executive’s good faith performance of Executive’s duties and obligations with the Company and its Affiliates. Parent and the Company shall cover Executive under directors’ and officers’ liability insurance both during and, while potential liability exists, after employment or non-employment service as a director or officer, as applicable, in the same amount and to the same extent as Parent and the Company cover their other officers and directors. These obligations shall survive the termination of Executive’s employment with the Company and its Affiliates. If any proceeding is brought or threatened against Executive in respect of which indemnity may be sought against the Company or its Affiliates pursuant to the foregoing, Executive shall notify the Company promptly in writing of the institution of such proceeding and the Company or its Affiliates shall assume the defense thereof and the employment of counsel and payment of all fees and expenses; provided, however, that if a conflict of interest exists between the Company or its applicable Affiliate and Executive such that it is not legally practicable for the Company or its applicable Affiliate to assume Executive’s defense, Executive shall be entitled to retain separate counsel reasonably acceptable to the Company or its applicable Affiliate and the Company or its applicable Affiliate shall assume payment of all reasonable fees and expenses of such counsel.
11. Miscellaneous.
(a) Governing Law and Dispute Resolution. This Agreement shall be governed by and construed in accordance with the laws of the State of Michigan, without reference to principles of conflict of laws. The parties irrevocably submit to the jurisdiction of any state or federal court sitting in or for Allegan or Kent Counties, Michigan with respect to any dispute arising out of or relating to this Agreement or the Release, and each party irrevocably agrees that all claims in respect of such dispute or proceeding shall be heard and determined in such courts. The parties hereby irrevocably waive, to the fullest extent permitted by law, any objection that they may now or hereafter have to the venue of any dispute arising out of or relating to this Agreement or the transactions contemplated hereby brought in such court or any defense of inconvenient forum for the maintenance of such dispute or proceeding. Each party agrees that a judgment in any such dispute may be enforced in other jurisdictions by suit on the judgment or in any other manner provided by law. THE PARTIES HEREBY WAIVE A TRIAL BY JURY IN ANY ACTION, PROCEEDING, CLAIM OR COUNTER CLAIM BROUGHT OR ASSERTED BY EITHER OF THE PARTIES HERETO AGAINST THE OTHER ON ANY MATTERS WHATSOEVER ARISING OUT OF OR IN ANY WAY RELATED TO THIS AGREEMENT. Following a Change in Control, the Company (including any successor to the Company following a Change in Control) shall reimburse Executive for all reasonable legal fees and expenses incurred by Executive in seeking to obtain or enforce any right or benefit provided under this Agreement, provided that Executive substantially prevails on at least one material issue.
(b) Notices. All notices and other communications hereunder shall be in writing and shall be given by hand delivery to the other party or by registered or certified mail, return receipt requested, postage prepaid, addressed as follows:
18
If to Executive: To the most recent address on file with the Company,
with a copy, which shall not constitute notice, to:
McDermott Will & Emery LLP
340 Madison Avenue
New York, NY 10173-1922
Attention: Steven Eckhaus and Ashley McCarthy
If to the Company:
Perrigo Management Company
515 Eastern Avenue
Allegan, Michigan 49010
Attention: General Counsel
Senior Vice President of Global Human Resources
with a copy to Parent:
Perrigo Company plc
Treasury Building
Lower Grand Canal Street
Dublin 2 Ireland
Attention: General Counsel
or to such other address as either party shall have furnished to the other in writing in accordance herewith. Notice and communications shall be effective when actually received by the addressee.
(c) Acknowledgements; Representations. Prior to execution of this Agreement, Executive was advised by the Company of Executive’s right to seek independent advice from an attorney of Executive’s own selection regarding this Agreement. Executive acknowledges that he has entered into this Agreement knowingly and voluntarily and with full knowledge and understanding of the provisions of this Agreement after being given the opportunity to consult with counsel. Executive represents that, in entering into this Agreement, Executive is not relying on any statements or representations made by any of the directors, officers, employees or agents of the Company and its Affiliates that are not expressly set forth herein, and that Executive is relying only upon Executive’s own judgment and any advice provided by Executive’s attorney. Further, Executive represents and warrants that (i) Executive is not subject to any contract, arrangement, policy or understanding, or to any statute, governmental rule or regulation, that in any way limits Executive’s ability to enter into and fully perform Executive’s obligations under this Agreement, (ii) Executive is not otherwise unable to enter into and fully perform Executive’s obligations under this Agreement and (iii) Executive has not engaged in any conduct, the occurrence and/or publicity of which, due to Executive’s affiliation with the Company or any of its Affiliates, is or could be harmful to, or otherwise reflect negatively on, the Company and/or any of its Affiliates. In the event of any breach of any of the representations, in the preceding sentence, the Company may terminate this Agreement and Executive’s employment without any liability owing to Executive.
19
(d) Cooperation. Executive agrees that upon the reasonable request of the Company or its Affiliates following Executive’s termination of employment, Executive shall use reasonable efforts to assist and cooperate with the Company or its Affiliates in connection with the defense or prosecution of any claim that may be made against or by the Company or its Affiliates, or in connection with any ongoing or future investigation or dispute or claim of any kind involving the Company or its Affiliates, including any proceedings before any arbitral, administrative, regulatory, judicial, legislative or other body or agency. Executive will be entitled to reimbursement for any reasonable out-of-pocket expenses (including travel expenses and attorneys’ fees) incurred in connection with providing such assistance and, in the event that Executive provides more than five hours of service collectively to the Company or its Affiliates in any calendar week at the Company’s or its Affiliates’ request pursuant to this Section 11(d), the Company shall compensate Executive at the rate of $480 per hour for such services in excess of five hours during such calendar week.
(e) Invalidity. If any term or provision of this Agreement or the application thereof to any person or circumstance shall to any extent be invalid or unenforceable, the remainder of this Agreement or the application of such term or provision to persons or circumstances other than those to which it is invalid or unenforceable shall not be affected thereby, and each term and provision of this Agreement shall be valid and be enforced to the fullest extent permitted by law.
(f) Survivability. The provisions of this Agreement that by their terms call for performance subsequent to the termination of either Executive’s employment or this Agreement (including the terms of Sections 5, 7 and 10) shall so survive such termination.
(g) Section Headings; Construction. The section headings used in this Agreement are included solely for convenience and shall not affect, or be used in connection with, the interpretation hereof. For purposes of this Agreement, the term “including” shall mean “including, without limitation.”
(h) Counterparts. This Agreement may be executed in several counterparts, each of which shall be deemed to be an original but all of which together shall constitute one and the same instrument.
(i) Tax Withholding. The Company may withhold from any amounts payable under this Agreement such Federal, state, local or foreign taxes as shall be required to be withheld pursuant to any applicable law or regulation.
(j) Section 409A.
(i) General. It is intended that payments and benefits made or provided under this Agreement shall not result in penalty taxes or accelerated taxation pursuant to Section 409A of the Code. Any payments that qualify for the “short-term deferral” exception, the separation pay exception or another exception under Section 409A of the Code shall be paid under the applicable exception. For purposes of the limitations on nonqualified deferred compensation under Section 409A of the Code, each payment of compensation under this Agreement shall be treated as a separate payment of compensation. All payments to be made upon a termination of employment under this Agreement may only be made upon a
20
“separation from service” under Section 409A of the Code to the extent necessary in order to avoid the imposition of penalty taxes on Executive pursuant to Section 409A of the Code. In no event may Executive, directly or indirectly, designate the calendar year of any payment under this Agreement, and to the extent required by Section 409A of the Code, any payment that may be paid in more than one taxable year (depending on the time that Executive executes the Release) shall be paid in the later taxable year.
(ii) Reimbursements and In-Kind Benefits. Notwithstanding anything to the contrary in this Agreement, all reimbursements and in-kind benefits provided under this Agreement that are subject to Section 409A of the Code shall be made in accordance with the requirements of Section 409A of the Code, including, where applicable, the requirement that (A) any reimbursement is for expenses incurred during Executive’s lifetime (or during a shorter period of time specified in this Agreement); (B) the amount of expenses eligible for reimbursement, or in-kind benefits provided, during a calendar year may not affect the expenses eligible for reimbursement, or in-kind benefits to be provided, in any other calendar year; (C) the reimbursement of an eligible expense will be made no later than the last day of the calendar year following the year in which the expense is incurred; and (D) the right to reimbursement or in-kind benefits is not subject to liquidation or exchange for another benefit.
(iii) Delay of Payments. Notwithstanding any other provision of this Agreement to the contrary, if Executive is considered a “specified employee” for purposes of Section 409A of the Code (as determined in accordance with the methodology established by the Company and its Affiliates as in effect on the Termination Date), any payment that constitutes nonqualified deferred compensation within the meaning of Section 409A of the Code that is otherwise due to Executive under this Agreement during the six-month period immediately following Executive’s separation from service (as determined in accordance with Section 409A of the Code) on account of Executive’s separation from service shall be accumulated and paid to Executive on the first business day of the seventh month following his separation from service (the “Delayed Payment Date”), to the extent necessary to prevent the imposition of tax penalties on Executive under Section 409A of the Code. If Executive dies during the postponement period, the amounts and entitlements delayed on account of Section 409A of the Code shall be paid to the personal representative of his estate on the first to occur of the Delayed Payment Date or 30 calendar days after the date of Executive’s death.
(k) Amendments. No provision of this Agreement shall be modified or amended except by an instrument in writing duly executed by the parties hereto. No custom, act, payment, favor or indulgence shall grant any additional right to Executive or be deemed a waiver by the Company of any of Executive’s obligations hereunder or release Executive therefrom or impose any additional obligation upon the Company. No waiver by any party of any breach by the other party of any term or provision hereof shall be deemed to be an assent or waiver by any party to or of any succeeding breach of the same or any other term or provision. This Agreement is personal to and shall not be assignable by any party, but shall inure to the benefit of the parties hereto and their respective heirs, beneficiaries, successors and assigns.
21
(l) Entire Agreement. This Agreement constitutes the entire agreement of the parties hereto in respect of the terms and conditions of Executive’s employment with the Company and its Affiliates, including his severance entitlements, and, as of the Effective Date, supersedes and cancels in their entirety all prior understandings, agreements and commitments, whether written or oral, relating to the terms and conditions of employment between Executive, on the one hand, and the Company or its Affiliates, on the other hand.
[Signature page follows]
22
IN WITNESS WHEREOF, Executive has hereunto set Executive’s hand and the Company, pursuant to the authorization from its board of directors, has caused these presents to be executed in its name on its behalf, all as of the date first above written.
/s/ Uwe Röhrhoff | |
Uwe Röhrhoff | |
PERRIGO MANAGEMENT COMPANY | |
By: | /s/ Todd W. Kingma |
Todd W. Kingma, EVP, General Counsel & | |
Company Secretary | |
[Signature Page to Employment Agreement]
Exhibit A
GENERAL RELEASE OF CLAIMS
THIS GENERAL RELEASE OF CLAIMS (this “Release”) is executed by Uwe Röhrhoff (“Executive”) as of the date set forth on the signature page hereto.
1. General Release and Waiver of Claims.
(a) Release. In consideration of the payments and benefits afforded under the employment agreement, dated as of ___________, by and between Perrigo Management Company, a Michigan corporation (the “Company”) and Executive (the “Employment Agreement”), and after consultation with counsel, Executive and each of Executive’s respective heirs, executors, administrators, representatives, agents, successors and assigns (collectively, the “Releasors”) hereby irrevocably and unconditionally release and forever discharge the Company and its subsidiaries and affiliates (including, but not limited to “Pharma” and “Parent” (as such terms are defined in the Employment Agreement) and each of their respective officers, employees, directors and agents (“Releasees”) from any and all claims, actions, causes of action, rights, judgments, obligations, damages, demands, accountings or liabilities of whatever kind or character (collectively, “Claims”) that the Releasors may have arising out of Executive’s employment relationship with and service as an employee, officer or director of the Company and its subsidiaries and affiliates, and the termination of any such relationship or service, in each case up to and including Executive’s date of termination. Executive acknowledges that the foregoing sentence includes Claims arising under Federal, state or local laws, statutes, orders or regulations that relate to the employment relationship or prohibiting employment discrimination, including Claims under Title VII of the Civil Rights Act of 1964; The Civil Rights Act of 1991; Sections 1981 through 1988 of Title 42 of the United States Code; the Employee Retirement Income Security Act of 1974; the Immigration Reform and Control Act; the Sarbanes Oxley Act of 2002; the Americans with Disabilities Act of 1990; the Family and Medical Leave Act; the Equal Pay Act; the Fair Credit Reporting Act; Occupational Safety and Health Act; state equivalents of the foregoing statutes, including without limitation the Michigan Elliott Larsen Civil Rights Act, the Michigan Persons with Disabilities Civil Rights Act, and the Michigan Whistleblowers’ Protection Act; and any other federal, state or local civil, human rights, bias, whistleblower, discrimination, retaliation, compensation, employment, labor or other local, state or federal law, regulation or ordinance. Notwithstanding anything contained herein to the contrary, this Release specifically excludes and shall not affect: (i) the obligations of the Company or its affiliates set forth in the Employment Agreement and to be performed after the date hereof, including without limitation under in Sections 5, 8 and 10 thereof, or under any other benefit plan, agreement, arrangement or policy of the Company or its affiliates that is applicable to Executive and that, in each case, by its terms, contains obligations that are to be performed after the date hereof by the Company or its affiliates; (ii) any indemnification or similar rights Executive has as a current or former officer, director, employee or agent of the Company or its affiliates, including, without limitation, any and all rights thereto under applicable law, the bylaws or other governance documents or such entities, or any rights with respect to coverage under any directors’ and officers’ insurance policies and/or indemnification agreements; (iii) any Claim the Releasors may have as the holder or beneficial owners of securities of the Company or its affiliates or other rights relating to securities or equity awards in respect of the common stock of the Company or its affiliates; (iv) rights to accrued but unpaid salary, paid time off, vacation or other compensation due through the date of
termination of employment; (v) any unreimbursed business expenses; (vi) benefits or the right to seek benefits under applicable workers’ compensation and/or unemployment compensation statutes; and (vii) any Claims that may arise in the future from events or actions occurring after Executive’s date of termination of employment or that Executive may not by law release through an agreement such as this.
(b) Specific Release of ADEA Claims. In further consideration of the payments and benefits provided to Executive under the Employment Agreement, the Releasors hereby unconditionally release and forever discharge the Releasees from any and all Claims that the Releasors may have as of the date Executive signs this Release arising under the Federal Age Discrimination in Employment Act of 1967, as amended, and the applicable rules and regulations promulgated thereunder (“ADEA”). By signing this Release, Executive hereby acknowledges and confirms the following: (i) Executive was advised by the Company in connection with Executive’s termination of employment to consult with an attorney of Executive’s choice prior to signing this Release and to have such attorney explain to Executive the terms of this Release, including, without limitation, the terms relating to Executive’s release of claims arising under ADEA, and Executive has in fact consulted with an attorney; (ii) Executive was given a period of not fewer than [twenty-one (21)] [forty-five (45)] calendar days to consider the terms of this Release and to consult with an attorney of Executive’s choosing with respect thereto; and (iii) Executive knowingly and voluntarily accepts the terms of this Release. Executive also understands that Executive has seven (7) calendar days following the date on which Executive signs this Release within which to revoke the release contained in this Section 1(b), by providing the Company a written notice of Executive’s revocation of the release and waiver contained in this Section 1(b).
(c) No Assignment. Executive represents and warrants that Executive has not assigned any of the Claims being released under this Release.
2. Proceedings. Executive has not filed, and agrees not to initiate or cause to be initiated on Executive’s behalf, any complaint, charge, claim or proceeding against the Releasees with respect to any Claims released under Section 1(a) or (b) before any local, state or federal agency, court or other body (each, individually, a “Proceeding”), and agrees not to participate voluntarily in any Proceeding involving such Claims; provided, however, and subject to the immediately following sentence, nothing set forth here in intended to or shall interfere with Executive’s right to participate in a Proceeding with any appropriate federal, state, or local government agency enforcing discrimination laws, nor shall this Release prohibit Executive from cooperating with any such agency in its investigation. Executive waives any right Executive may have to benefit in any manner from any relief (whether monetary or otherwise) arising out of any Proceeding involving such Claims. Notwithstanding the foregoing, the term Proceeding shall not include any complaint, charge, claim or proceeding with respect to the obligations of the Company to Executive under the Employment Agreement or in respect of any other matter described in the proviso to Section 1(a), and Executive retains all of Executive’s rights in connection with the same.
3. Severability Clause. In the event any provision or part of this Release is found to be invalid or unenforceable, only that particular provision or part so found, and not the entire Release, will be inoperative.
2
4. No Admission. Nothing contained in this Release will be deemed or construed as an admission of wrongdoing or liability on the part of the Releasees.
5. Governing Law and Venue. All matters affecting this Release, including the validity thereof, are to be governed by, and interpreted and construed in accordance with, the laws of the State of Michigan applicable to contracts executed in and to be performed in that State.
6. Counterparts. This Release may be executed in counterparts and each counterpart will be deemed an original.
7. Notices. All notices, requests, demands or other communications under this Release shall be in writing and shall be deemed to have been duly given when delivered in person or deposited in the United States mail, postage prepaid, by registered or certified mail, return receipt requested, to the party to whom such notice is being given as follows:
As to Executive:
Executive’s last address on the books and records of the Company
Executive’s last address on the books and records of the Company
As to the Company:
Perrigo Management Company
515 Eastern Avenue
Allegan, Michigan 49010
Attention: General Counsel
Senior Vice President of Global Human Resources
Perrigo Management Company
515 Eastern Avenue
Allegan, Michigan 49010
Attention: General Counsel
Senior Vice President of Global Human Resources
With a copy to Parent:
Perrigo Company plc
Treasury Building
Lower Grand Canal Street
Dublin 2 Ireland
Attention: General Counsel
Perrigo Company plc
Treasury Building
Lower Grand Canal Street
Dublin 2 Ireland
Attention: General Counsel
Any party may change his, her or its address or the name of the person to whose attention the notice or other communication shall be directed from time to time by serving notice thereof upon the other party as provided herein.
[Signature page follows]
3
EXECUTIVE ACKNOWLEDGES THAT EXECUTIVE HAS READ THIS RELEASE AND THAT EXECUTIVE FULLY KNOWS, UNDERSTANDS AND APPRECIATES ITS CONTENTS, AND THAT EXECUTIVE HEREBY EXECUTES THE SAME AND MAKES THIS RELEASE AND THE RELEASE PROVIDED FOR HEREIN VOLUNTARILY AND OF EXECUTIVE’S OWN FREE WILL.
IN WITNESS WHEREOF, Executive has executed this Release on the date set forth below.
Uwe Röhrhoff | |
Dated as of: | |
[Signature Page to General Release]
A-4
May 7, 2018
Uwe Röhrhoff
RE: Acknowledgement of Termination of Employment and Employment Agreement with Perrigo Pharma International DAC
Dear Uwe,
As you are aware, you are currently employed as President and Chief Executive Officer of Perrigo Pharma International DAC (“Perrigo Pharma,” and together with its subsidiaries, the “Company”) pursuant to an employment agreement with Perrigo Pharma dated as of January 15, 2018 (the “Irish Employment Agreement”), which Irish Employment Agreement provides that you will be principally located in Dublin, Ireland until you receive authorization to lawfully work and reside in the United States (as defined in the Irish Employment Agreement, the “U.S. Immigration Event”). Specifically, Section 1(b) of the Irish Employment Agreement provides that upon the occurrence of the U.S. Immigration Event, (i) you will immediately commence employment with Perrigo Management Company, a subsidiary of Perrigo Pharma (“Perrigo Management”) as President and Chief Executive Officer, and relocate to Allegan, Michigan within a reasonable period of time thereafter, and (ii) upon commencing employment with Perrigo Management, your employment with Perrigo Pharma and the Irish Employment Agreement will immediately terminate, subject to certain exceptions set forth in the Irish Employment Agreement.
You hereby acknowledge and agree that, as of the date hereof, the U.S. Immigration Event has occurred, and in connection with such, you have commenced employment with Perrigo Management, and that your employment with Perrigo Pharma and the Irish Employment Agreement have terminated. You further acknowledge and agree that, except as set forth in Section 1(b) therein, the provisions of the Irish Employment Agreement are of no further force and effect as of the date hereof. Finally, you acknowledge and agree that in connection with your commencing employment with Perrigo Management, you will execute an employment agreement therewith in the form previously agreed upon between you and Perrigo Management.
This acknowledgement may be executed via PDF or facsimile and in any number of counterparts, each of which shall be deemed and original, but all such counterparts shall together constitute one in the same instrument.
[Signature page follows]
5
We thank you for your contributions to the success of the Company, and we look forward to your continued contributions in your role as President and Chief Executive Officer of Perrigo Management.
Sincerely, | |
Perrigo Pharma International DAC | |
By: | /s/ Todd W. Kingma |
Name: | Todd W. Kingma |
Title: | EVP, General Counsel & |
Company Secretary | |
Acknowledged and agreed:
/s/ Uwe Röhrhoff | |
Uwe Röhrhoff | |
Date: | 5/7/2018 |
6
Exhibit 31.1
CERTIFICATION
I, Uwe F. Roehrhoff, certify that:
1. | I have reviewed this report on Form 10-Q of Perrigo Company plc; |
2. | Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
3. | Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report; |
4. | The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have: |
a. | designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
b. | designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
c. | evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
d. | disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and |
5. | The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors: |
a. | all significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting, which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and |
b. | any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting. |
Date: May 8, 2018
/s/ Uwe F. Roehrhoff |
Uwe F. Roehrhoff |
Chief Executive Officer |
Exhibit 31.2
CERTIFICATION
I, Ronald L. Winowiecki, certify that:
1. | I have reviewed this report on Form 10-Q of Perrigo Company plc; |
2. | Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
3. | Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report; |
4. | The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have: |
a. | designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
b. | designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
c. | evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
d. | disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and |
5. | The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors: |
a. | all significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting, which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and |
b. | any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting. |
Date: May 8, 2018
/s/ Ronald L. Winowiecki |
Ronald L. Winowiecki |
Chief Financial Officer |
Exhibit 32
The following statement is being made to the Securities and Exchange Commission solely for the purposes of Section 906 of the Sarbanes-Oxley Act of 2002 (18 U.S.C. 1350), which carries with it certain criminal penalties in the event of a knowing or willful misrepresentation.
Securities and Exchange Commission
450 Fifth Street NW
Washington, D.C. 20549
Re: | Perrigo Company plc | |
Ladies and Gentlemen:
In accordance with the requirements of Section 906 of the Sarbanes-Oxley Act of 2002 (18 U.S.C. 1350), each of the undersigned hereby certifies that:
(i) | this Quarterly Report on Form 10-Q fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934 (15 U.S.C. 78m or 78o(d)); and |
(ii) | the information contained in this report fairly presents, in all material respects, the financial condition and results of operations of Perrigo Company plc. |
Date: | May 8, 2018 | /s/ Uwe F. Roehrhoff | |
Uwe F. Roehrhoff | |||
Chief Executive Officer and President | |||
(Principal Executive Officer) | |||
Date: | May 8, 2018 | /s/ Ronald L. Winowiecki | |
Ronald L. Winowiecki | |||
Chief Financial Officer | |||
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Perrigo to Release First Quarter 2024 Financial Results on May 7, 2024
- Atomic Minerals to Proceed with Debt Settlement Transaction
- TransBoden: A New Solana Memecoin, Successfully Launches $BODENX in a 100% Token Fair Launch
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share