Form 10-K LEXICON PHARMACEUTICALS, For: Dec 31
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| (Mark One) | ||||||||
| ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||||||||
| For the Fiscal Year Ended | ||||||||
or
| TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |||||
| For the Transition Period from _____________ to _____________ | |||||
Commission File Number: 000-30111
(Exact Name of Registrant as Specified in its Charter)
| (State or Other Jurisdiction of Incorporation or Organization) | (I.R.S. Employer Identification Number) | |||||||||||||
| (Registrant’s Telephone Number, Including Area Code) | ||||||||||||||||||||
| (Address of Principal Executive Offices and Zip Code) | ||||||||||||||||||||
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class | Trading Symbol(s) | Name of Each Exchange on which Registered | ||||||||||||
| | | |||||||||||||
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act of 1933. Yes ☐ No ☑
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Securities Exchange Act of 1934. Yes ☐ No ☑
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days. Yes ☑ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☑ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Securities Exchange Act of 1934. (check one): Large accelerated filer ☐ Accelerated filer ☑ Non-accelerated filer ☐ Smaller reporting company ☐ Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Securities Exchange Act of 1934. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☑
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Securities Exchange Act of 1934). Yes ☐ No ☑
The aggregate market value of voting stock held by non-affiliates of the registrant as of the last day of the registrant’s most recently completed second quarter was approximately $82.0 million, based on the closing price of the common stock on the Nasdaq Global Select Market on June 30, 2020 of $2.00 per share. For purposes of the preceding sentence only, our directors, executive officers and controlling stockholders are assumed to be affiliates. As of March 6, 2021, 144,354,161 shares of common stock were outstanding.
Documents Incorporated by Reference
Lexicon Pharmaceuticals, Inc.
Table of Contents
| Item | ||||||||
| PART I | ||||||||
| 1. | ||||||||
| 1A. | ||||||||
| 1B. | ||||||||
| 2. | ||||||||
| 3. | ||||||||
| 4. | ||||||||
| PART II | ||||||||
| 5. | ||||||||
| 6. | Selected Financial Data | |||||||
| 7. | ||||||||
| 7A. | ||||||||
| 8. | ||||||||
| 9. | ||||||||
| 9A. | ||||||||
| 9B. | ||||||||
| PART III | ||||||||
| 10. | ||||||||
| 11. | ||||||||
| 12. | ||||||||
| 13. | ||||||||
| 14. | ||||||||
| PART IV | ||||||||
| 15. | ||||||||
| 16. | ||||||||
The Lexicon name and logo are registered trademarks of Lexicon Pharmaceuticals, Inc.
_____________________________________________________
In this annual report on Form 10-K, “Lexicon Pharmaceuticals,” “Lexicon,” “the Company,” “we,” “us” and “our” refer to Lexicon Pharmaceuticals, Inc. and its subsidiaries.
_____________________________________________________
Factors Affecting Forward-Looking Statements
This annual report on Form 10-K contains forward-looking statements. These statements relate to future events or our future financial performance. We have attempted to identify forward-looking statements by terminology including “anticipate,” “believe,” “can,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “should” or “will” or the negative of these terms or other comparable terminology. These statements are only predictions and involve known and unknown risks, uncertainties and other factors, including the risks outlined under “Item 1A. Risk Factors,” that may cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements.
Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. We are not under any duty to update any of the forward-looking statements after the date of this annual report on Form 10-K to conform these statements to actual results, unless required by law.
PART I
Item 1. Business
Overview
Lexicon Pharmaceuticals is a biopharmaceutical company with a mission of pioneering medicines that transform patients’ lives. We are devoting most of our resources to the research and development of our most advanced drug candidates:
•We are developing LX9211, an orally-delivered small molecule drug candidate, as a treatment for neuropathic pain. We have reported results from two Phase 1 clinical trials of LX9211 and are now conducting a Phase 2 clinical trial of LX9211 in diabetic peripheral neuropathic pain and a second Phase 2 clinical trial of LX9211 in post-herpetic neuralgia. LX9211 has received Fast Track designation from the U.S. Food and Drug Administration, or FDA, for development in diabetic peripheral neuropathic pain.
•We are developing sotagliflozin, an orally-delivered small molecule drug candidate, as a treatment for heart failure and type 1 diabetes. We have reported positive results from two Phase 3 clinical trials evaluating the effect of sotagliflozin on long-term outcomes related to cardiovascular death and heart failure in approximately 10,500 and 1,200 patients, respectively. We are now working towards an application for regulatory approval to market sotagliflozin for heart failure and seeking a strategic collaboration for the commercialization of sotagliflozin, if approved.
We have reported positive results from three Phase 3 clinical trials evaluating the effect of sotagliflozin on type 1 diabetes in approximately 800, 800 and 1,400 patients, respectively. The FDA issued a complete response letter regarding our application for regulatory approval to market sotagliflozin for type 1 diabetes in the United States and, at our request, has issued a public Notice of Opportunity for Hearing on whether there are grounds for denying approval of our application. Sotagliflozin has been approved in the European Union for use as an adjunct to insulin therapy in the treatment of type 1 diabetes, but has not yet been commercially launched.
•We are conducting preclinical research and development and preparing to conduct clinical development of compounds from a number of additional drug programs originating from our internal drug discovery efforts.
LX9211 originated from our collaborative neuroscience drug discovery efforts with Bristol-Myers Squibb, and sotagliflozin and compounds from a number of additional drug programs originated from our own internal drug discovery efforts. Those efforts were driven by a systematic, target biology-driven approach in which we used gene knockout technologies and an integrated platform of advanced medical technologies to systematically study the physiological and behavioral functions of almost 5,000 genes in mice and assessed the utility of the proteins encoded by the corresponding human genes as potential drug targets. We have identified and validated in living animals, or in vivo, more than 100 targets with promising profiles for drug discovery.
We are working both independently and through collaborations and strategic alliances with third parties to capitalize on our drug target discoveries and drug discovery and development programs. We seek to retain exclusive or co-exclusive rights to the benefits of certain drug discovery and development programs by developing and commercializing drug candidates from those programs internally, particularly in the United States for indications treated by specialist physicians. We seek to collaborate with other pharmaceutical and biotechnology companies with respect to drug discovery or the development and commercialization of certain of our drug candidates, particularly with respect to commercialization in territories outside the United States or commercialization in the United States for indications treated by primary care physicians, or when the collaboration may otherwise provide us with access to expertise and resources that we do not possess internally or are complementary to our own.
Lexicon Pharmaceuticals was incorporated in Delaware in July 1995, commenced operations in September 1995 and was listed on The Nasdaq Global Select Market in April 2000. Our corporate headquarters are located at 8800 Technology Forest Place, The Woodlands, Texas 77381, and our telephone number is (281) 863-3000.
Our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934 are made available free of charge on our corporate website located at www.lexpharma.com as soon as reasonably practicable after the filing of those reports with the Securities and Exchange Commission, or the SEC. Information found on our website should not be considered part of this annual report on Form 10-K. Alternatively, you may access these reports on the SEC’s website at www.sec.gov.
1
Drug Development Programs
We are devoting most of our resources to the research and development of our most advanced drug candidates: LX9211, which we are developing as a treatment for neuropathic pain, and sotagliflozin, which we are developing as a treatment for heart failure and type 1 diabetes. We have also advanced a number of additional compounds into various stages of clinical and preclinical development.
LX9211
LX9211 is an orally-delivered small molecule compound that we are developing as a treatment for neuropathic pain. Our scientists identified the target of LX9211, adapter-associated kinase 1, or AAK1, in our target discovery efforts based on their discovery that mice lacking AAK1 exhibited increased resistance to induced neuropathic pain in preclinical models. LX9211 and another development candidate were discovered by scientists working within our drug discovery alliance with Bristol-Myers Squibb from which we hold exclusive development and commercialization rights. Preclinical studies of LX9211 demonstrated central nervous system penetration and reduction in pain behavior in models of neuropathic pain without affecting opiate pathways. LX9211 has received Fast Track designation from the FDA for development in diabetic peripheral neuropathic pain.
We have completed two Phase 1 clinical trials evaluating the safety, tolerability and pharmacokinetics of LX9211. The first trial enrolled ten cohorts of healthy volunteers in a randomized, double-blind, placebo-controlled, ascending single dose study of daily doses of LX9211. The second trial enrolled five cohorts of healthy volunteers in a randomized, double-blind, placebo-controlled, ascending multiple dose study of daily doses of LX9211, followed by a maintenance dose for 14 days. In both trials, LX9211 demonstrated a safety, tolerability and pharmacokinetics profile identifying the maximum tolerated dose and supportive of once-daily dosing, while exhibiting dose proportional pharmacokinetics. The most common adverse events were headache and dizziness, and there were no drug-related serious adverse events.
We are conducting a Phase 2 clinical trial, RELIEF-DPN-1, evaluating the safety and tolerability of LX9211 and its effects on diabetic peripheral neuropathic pain, or DPN. The trial is expected to enroll approximately 300 patients experiencing DPN in a randomized, double-blind, placebo-controlled study evaluating three treatment groups receiving an initial loading dose of 100mg or 200mg of LX9211 or placebo, followed by once daily doses of 10mg or 20mg of LX9211 or placebo, respectively. The effects of LX9211 will be assessed over an 11-week evaluation period. The primary efficacy endpoint under evaluation is the reduction in an average daily pain score at 6 weeks, with secondary endpoints including the proportion of patients with 30% or greater and 50% or greater reduction in pain intensity at 6 weeks and the proportion of patients discontinuing treatment due to lack of efficacy. Certain patient-reported outcome measures will also be assessed.
We are conducting a second Phase 2 clinical trial, RELIEF-PHN-1, evaluating the safety and tolerability of LX9211 and its effects on post-herpetic neuralgia, or PHN. The trial is expected to enroll approximately 74 patients experiencing PHN in a randomized, double-blind, placebo-controlled study evaluating two treatment groups receiving an initial loading dose of 200mg of LX9211 or placebo, followed by once daily doses of 20mg of LX9211 or placebo, respectively. The effects of LX9211 will be assessed over an 11-week evaluation period. The primary efficacy endpoint under evaluation is the reduction in an average daily pain score at 6 weeks, with secondary endpoints including the proportion of patients with 30% or greater and 50% or greater reduction in pain intensity at 6 weeks and the proportion of patients discontinuing treatment due to lack of efficacy. Certain patient-reported outcome measures will also be assessed.
Sotagliflozin
Sotagliflozin is an orally-delivered small molecule compound that we are developing for the treatment of heart failure and type 1 diabetes. Our scientists identified the targets of sotagliflozin, sodium-glucose cotransporter type 1, or SGLT1, and sodium-glucose cotransporter type 2, or SGLT2, in our target discovery efforts based on their discovery that mice lacking SGLT1, SGLT2 or both exhibited potent anti-diabetic phenotypes across multiple measures of glucose control and metabolism in preclinical models. Preclinical studies of sotagliflozin demonstrated that compounds inhibiting both targets had a favorable preclinical profile relative to compounds selective for SGLT2.
In December 2015, we granted Sanofi-Aventis Deutschland GmbH an exclusive, worldwide (excluding Japan), royalty-bearing right to develop, manufacture and commercialize sotagliflozin. In September 2019, we and Sanofi agreed to terminate our collaboration, pursuant to which we regained and hold exclusive development and commercialization rights to sotagliflozin.
2
Heart Failure.
We have completed two Phase 3 clinical trials evaluating the safety and tolerability of sotagliflozin and its effects on long-term outcomes related to composite primary endpoints of total cardiovascular death, hospitalizations for heart failure and urgent visits for heart failure. Both clinical trials were initiated by Sanofi and transitioned to us in connection with the termination of our collaboration, following which both clinical trials were closed out early beginning in March 2020.
Our SCORED Phase 3 clinical trial enrolled 10,584 patients with type 2 diabetes, chronic kidney disease with an estimated glomerular filtration rate, or eGFR, of 25 to 60 ml per minute per 1.73 m2 of body surface area and risks for cardiovascular disease in a randomized, double-blind, placebo-controlled study of sotagliflozin added to standard of care over a median treatment period of 16 months. Patients were initiated on a 200mg once daily dose of sotagliflozin, which was increased at the discretion of the investigator to 400mg if unacceptable side effects did not occur. The primary efficacy endpoint under evaluation in the study was the total number of events comprised of deaths from cardiovascular causes, hospitalizations for heart failure, and urgent visits for heart failure in patients treated with sotagliflozin compared with placebo. Data from the study showed that treatment with sotagliflozin resulted in a significantly lower total number of cardiovascular deaths, heart failure hospitalizations and urgent visits as compared to placebo, meeting the study’s primary efficacy endpoint. A total of 930 primary endpoint events occurred in the study, with 400 events in the sotagliflozin-treated group and 530 events in the placebo group. There were 5.6 primary endpoint events per 100 patient-years in the sotagliflozin-treated group as compared to 7.5 events per 100 patient-years in the placebo group (HR=0.74; 95% CI=0.63 to 0.88; p<0.001). There were 2.2 events of cardiovascular death per 100 patient-years in the sotagliflozin-treated group as compared to 2.4 events per 100 patient-years in the placebo group (HR=0.90; 95% CI=0.73 to 1.12; p=0.35). The data showed an average reduction in hemoglobin A1c, or A1C, of 0.56% in the sotagliflozin-treated group as compared to a reduction of 0.25% in the placebo group in patients with severe chronic kidney disease, defined as having an eGFR of less than 30 ml per minute per 1.73 m2 of body surface area (p<0.001). In patients with moderate chronic kidney disease, defined as having an eGFR of greater than or equal to 30 ml per minute per 1.73 m2 of body surface area, the data showed an average reduction in A1C of 0.60% in the sotagliflozin-treated group as compared to a reduction of 0.17% in the placebo group (p<0.001). Serious adverse events that led to discontinuation of study drug, as determined by investigators, occurred in 2.1% (n=112) of the patients in the sotagliflozin-treated group and in 1.8% (n=94) of the patients in the placebo group. Based on investigator reported events, the most common adverse events of special interest included urinary tract infections (11.5% on sotagliflozin versus 11.1% on placebo), diarrhea (8.5% on sotagliflozin versus 6.0% on placebo), volume depletion (5.3% on sotagliflozin versus 4.0% on placebo), bone fractures (2.1% on sotagliflozin versus 2.2% on placebo), and genital mycotic infections (2.4% on sotagliflozin versus 0.9% on placebo). Among other adverse events of interest, diabetic ketoacidosis (0.6% on sotagliflozin vs. 0.3% on placebo) was more common in the sotagliflozin-treated group and severe hypoglycemia (1.0% on sotagliflozin vs. 1.0% on placebo) was similar between treatment groups.
The SCORED clinical trial was originally designed with co-primary endpoints, assessed in time-to-event analyses, of the first occurrence of a major adverse cardiovascular, or MACE, event, defined as death from cardiovascular causes, non-fatal myocardial infarction or non-fatal stroke, and the first occurrence of death from cardiovascular causes or hospitalization from heart failure, as determined by independent adjudication. The co-primary endpoints were modified in connection with the early close out of the study to include urgent heart failure visits and reflect the total number of events, as determined by investigators, in order to enhance the statistical power of the comparison. The specification of the primary endpoint based on total events was implemented without any awareness of the study outcomes or study group assignments and without information from an interim analysis. In a time-to-event analysis, applying the original co-primary endpoints based on investigator reported events, the results for both the first occurrence of a MACE event (HR=0.84; 95% CI=0.72 to 0.99; p=0.035) and the first occurrence of death from cardiovascular causes or hospitalization for heart failure (HR=0.78; 95% CI=0.66 to 0.91; p=0.001) were consistent with those of the modified primary endpoint.
Our SOLOIST Phase 3 clinical trial enrolled 1,222 patients with type 2 diabetes who had recently been hospitalized with worsening heart failure in a randomized, double-blind, placebo-controlled study of sotagliflozin initiated either before or within three days of hospital discharge over a median treatment period of nine months. Patients were initiated on a 200mg once daily dose of sotagliflozin, which was increased at the discretion of the investigator to 400mg if unacceptable side effects did not occur. The primary efficacy endpoint under evaluation in the study was the total number of events comprised of deaths from cardiovascular causes, hospitalizations for heart failure, and urgent visits for heart failure in patients treated with sotagliflozin compared with placebo, with dosing initiated either before or within three days of hospital discharge. The first dose of sotagliflozin or placebo was administered prior to hospital discharge in 48.8% of patients and a median of two days following discharge in 51.2% of patients, with the benefits of sotagliflozin being consistent between those prespecified patient subgroups. Data from the study showed that treatment with sotagliflozin resulted in a significantly lower total number of cardiovascular deaths, heart failure hospitalizations and urgent visits as compared to placebo, meeting the study’s primary efficacy endpoint. A total of 600 primary endpoint events occurred in the study, with 245 events in the sotagliflozin-treated group and 355 events in the placebo group. There were 51.0 primary endpoint events per 100 patient-years in the sotagliflozin-treated group as compared to 76.3 events per 100 patient-years in the placebo group (HR=0.67; 95% CI=0.52 to 0.85; p<0.001). There were 10.6 events of cardiovascular death per 100 patient-years in the sotagliflozin-treated group as compared to 12.5
3
events per 100 patient-years in the placebo group (HR=0.84; 95% CI=0.58 to 1.22; p=0.36). Effects on the primary endpoint were consistent among patients suffering from heart failure with reduced ejection fraction, or HFrEF, and heart failure with preserved ejection fraction, or HFpEF. Serious adverse events that led to discontinuation of study drug, as determined by investigators, occurred in 3.0% (n=18) of the patients in the sotagliflozin-treated group and in 2.8% (n=17) of the patients in the placebo group. The most common adverse events of special interest included hypotension (6.0% on sotagliflozin versus 4.6% on placebo), urinary tract infections (4.8% on sotagliflozin versus 5.1% on placebo), acute kidney injury (4.1% on sotagliflozin versus 4.4% on placebo), and diarrhea (6.1% on sotagliflozin versus 3.4% on placebo). Among other adverse events of interest, genital mycotic infections (0.8% on sotagliflozin vs. 0.2% on placebo) were infrequent, severe hypoglycemia (1.5% on sotagliflozin vs. 0.3% on placebo) was more common in the sotagliflozin-treated group and diabetic ketoacidosis (0.3% on sotagliflozin vs. 0.7% on placebo) was similar between treatment groups.
The SOLOIST clinical trial was originally designed with a primary endpoint of the first occurrence of death from cardiovascular causes or hospitalization from heart failure, as determined by independent adjudication. The primary endpoint were modified in connection with the early close out of the study to include urgent heart failure visits and reflect the total number of events, as determined by investigators, in order to enhance the statistical power of the comparison. The specification of the primary endpoint based on total events was implemented without any awareness of the study outcomes or study group assignments and without information from an interim analysis. Applying the original primary endpoint based on investigator reported events, the results for the first occurrence of death from cardiovascular causes or hospitalization for heart failure (HR=0.71; 95% CI=0.57 to 0.89; p=0.003) were consistent with those of the modified primary endpoint.
Results from both the SCORED and SOLOIST clinical trials were published in the New England Journal of Medicine in November 2020. In January 2021, we received feedback from the FDA that the results of the studies can support the submission of an application for regulatory approval for an indication to reduce the risk of cardiovascular death, hospitalization for heart failure, and urgent visits for heart failure in adult patients with type 2 diabetes with either worsening heart failure or additional risk factors for heart failure. We are currently working towards an application for regulatory approval to market sotagliflozin for heart failure and seeking a strategic collaboration for the commercialization of sotagliflozin, if approved.
Type 1 Diabetes.
The FDA issued a complete response letter in March 2019 regarding our application for regulatory approval to market sotagliflozin for type 1 diabetes in the United States and has confirmed that position in denying two appeals of the complete response letter in November 2019 and March 2020. We have requested an opportunity for an administrative hearing on whether there are grounds for denying approval of our application and, in response to such request, the FDA issued a public Notice of Opportunity for Hearing in March 2021.
In April 2019, sotagliflozin was approved in the European Union for use as an adjunct to insulin therapy to improve glycemic control in adults with type 1 diabetes and a body mass index > 27 kg/m2 , who could not achieve adequate glycemic control despite optimal insulin therapy. We have not commercially launched sotagliflozin for the treatment of type 1 diabetes in the European Union or any other region.
We have completed three Phase 3 clinical trials evaluating the safety and tolerability of sotagliflozin and its effects on glycemic parameters associated with type 1 diabetes.
Our pivotal inTandem1 Phase 3 clinical trial enrolled 793 patients with type 1 diabetes in the United States and Canada in a randomized, double-blind, placebo-controlled study of 200mg and 400mg once daily doses of sotagliflozin over a 24-week treatment period, followed by a 28-week extension. Insulin therapy was optimized in patients over a 6-week period prior to dosing. The primary efficacy endpoint under evaluation in the trial was the reduction of A1C versus placebo on optimized insulin treatment at 24 weeks, with secondary endpoints including percentage of patients achieving A1C levels of less than 7% without experiencing an event of severe hypoglycemia or diabetic ketoacidosis, or DKA, change in meal-time, or bolus, insulin use, body weight, fasting plasma glucose and patient-reported assessments. Data from the study showed that patients treated with sotagliflozin experienced statistically significant reductions in A1C from baseline of 0.43% for the 200mg dose (p<0.001) and 0.48% for the 400mg dose (p<0.001), as compared to a reduction of 0.07% on placebo after 24 weeks of treatment, meeting the study’s primary efficacy endpoint at both dose levels. The A1C benefit achieved with sotagliflozin was sustained with statistically significant results over the full 52-week duration of the study for both the 200mg and 400mg doses. Benefits in all secondary efficacy endpoints were observed in both the 200mg and 400mg dose arms compared to placebo, with statistically significant improvements in all secondary efficacy endpoints observed in the 400mg dose arm and in the percentage of patients achieving A1C levels of less than 7% without any severe hypoglycemia or DKA events and weight loss observed in the 200mg dose arm and statistically significant improvements in all secondary efficacy endpoints observed in the 400mg dose arm. Over the full 52-week treatment period, the incidences of treatment-emergent adverse events in the placebo, 200mg and 400mg dose arms were 80.6%, 81.7% and 79.8%, respectively; the incidences of serious adverse events were 7.5%, 10.3% and 11.1%, respectively; and the incidences of discontinuation due to adverse events were 4.1%, 4.9% and 6.5%, respectively. Potential
4
cases of severe hypoglycemia and DKA were reviewed by a blinded adjudication panel, which determined whether such cases met pre-established diagnostic criteria. The number of patients with positively adjudicated severe hypoglycemic events during the full 52-week treatment period was 26 (9.7%), 17 (6.5%) and 17 (6.5%) in the placebo, 200mg and 400mg dose arms, respectively. The number of patients with positively adjudicated DKA events during the full 52-week treatment period was 1 (0.4%), 9 (3.4%) and 11 (4.2%) in the placebo, 200mg and 400mg dose arms, respectively.
Our pivotal inTandem2 Phase 3 clinical trial enrolled 782 patients with type 1 diabetes in Europe and Israel in a randomized, double-blind, placebo-controlled study of 200mg and 400mg once daily doses of sotagliflozin over a 24-week treatment period, followed by a 28-week extension. Insulin therapy was optimized in patients over a 6-week period prior to dosing. As with inTandem1, the primary efficacy endpoint under evaluation in the trial was the reduction of A1C versus placebo on optimized insulin treatment at 24 weeks, with secondary endpoints including percentage of patients achieving A1C levels of less than 7% without experiencing a severe hypoglycemia or DKA event, change in bolus insulin use, body weight, fasting plasma glucose and patient-reported assessments. Data from the study showed that patients treated with sotagliflozin experienced statistically significant reductions in A1C from baseline of 0.39% for the 200mg dose (p<0.001) and 0.37% for the 400mg dose (p<0.001), as compared to a reduction of 0.02% on placebo after 24 weeks of treatment, meeting the study’s primary efficacy endpoint at both dose levels. The A1C benefit achieved with sotagliflozin was sustained with statistically significant results over the full 52-week duration of the study for both the 200mg and 400mg doses. Statistically significant improvements in all secondary efficacy endpoints were observed in both the 200mg and 400mg dose arms compared to placebo. Over the full 52-week treatment period, the incidences of treatment-emergent adverse events in the placebo, 200mg and 400mg dose arms were 61.2%, 68.2% and 68.8%, respectively; the incidences of serious adverse events were 6.6%, 10.0% and 8.0%, respectively; and the incidences of discontinuation due to adverse events were 3.5%, 3.8% and 6.8%, respectively. Potential cases of severe hypoglycemia and DKA were reviewed by a blinded adjudication panel, which determined whether such cases met pre-established diagnostic criteria. The number of patients with positively adjudicated severe hypoglycemic events during the full 52-week treatment period was 13 (5.0%), 13 (5.0%) and 6 (2.3%) in the placebo, 200mg and 400mg dose arms, respectively. The number of patients with positively adjudicated DKA events during the full 52-week treatment period was 0 (0.0%), 6 (2.3%) and 9 (3.4%) in the placebo, 200mg and 400mg dose arms, respectively.
We have additionally reported pooled continuous glucose monitoring, or CGM, data from the inTandem1 and inTandem2 clinical trials. The percentage of time during the initial 24-week treatment period spent inside the target range for CGM glucose (70-180 mg/dL) increased from 52.2% to 57.8% in patients treated with 200mg of sotagliflozin and from 50.7% to 64.1% in patients treated with 400mg of sotagliflozin, with no relevant change observed in patients receiving placebo. The differences from placebo were clinically significant for both the 200mg and 400mg dose groups (p=0.026 and p<0.001, respectively). The increase in time spent in range by both sotagliflozin dose groups was a result of significantly reduced time spent above 180 mg/dL, while the time spent below 70 mg/dL was not increased. These results translate into an additional 1.41 hours and 3.02 hours that a patient would spend within the 70-180 mg/dL target range in a 24-hour period, for the 200mg and 400mg dose groups respectively.
Our inTandem3 Phase 3 clinical trial enrolled 1,405 patients with type 1 diabetes in the United States and Europe in a randomized, double-blind, placebo-controlled study of a 400mg once daily dose of sotagliflozin over a 24-week treatment period. Insulin therapy was not optimized in patients and eligibility criteria included any background insulin therapy. The primary efficacy endpoint under evaluation in the trial was the proportion of patients achieving A1C levels of less than 7% at 24 weeks without experiencing a severe hypoglycemic or DKA event, with secondary endpoints including the change from baseline in A1C, body weight, systolic blood pressure and bolus insulin use. Data from the study showed statistically significant superiority of sotagliflozin (28.6%) compared to placebo (15.2%) in the proportion of patients achieving A1C levels of less than 7% without experiencing a severe hypoglycemic or DKA event (p<0.001), meeting the study’s primary endpoint. Patients treated with sotagliflozin also experienced statistically significant improvements in all secondary efficacy endpoints compared to placebo. The incidences of treatment-emergent adverse events in the placebo and 400mg dose arms were 52.5% and 55.1%, respectively; the incidences of serious adverse events were 3.3% and 6.9%, respectively; and the incidences of discontinuation due to adverse events were 2.3% and 6.3%, respectively. Potential cases of severe hypoglycemia and DKA were reviewed by a blinded adjudication panel, which determined whether such cases met pre-established diagnostic criteria. The number of patients with positively adjudicated severe hypoglycemic events during the 24-week treatment period was 17 (2.4%) and 21 (3.0%) in the placebo and 400mg dose arms, respectively. The number of patients with positively adjudicated DKA events during the 24-week treatment period was 4 (0.6%) and 21 (3.0%) in the placebo and 400mg dose arms, respectively. Results from the inTandem3 trial were published in the New England Journal of Medicine in September 2017.
Additional Drug Discovery and Development Programs
We are conducting preclinical research and development and preparing to conduct clinical development of compounds from a number of additional drug programs originating from our internal drug discovery efforts. Those efforts were driven by a
5
systematic, target biology-driven approach in which we used gene knockout technologies and an integrated platform of advanced medical technologies to systematically study the physiological and behavioral functions of almost 5,000 genes in mice and assessed the utility of the proteins encoded by the corresponding human genes as potential drug targets. We have identified and validated in living animals, or in vivo, more than 100 targets with promising profiles for drug discovery.
Collaborations and Strategic Alliances
We are working both independently and through collaborations and strategic alliances with third parties to capitalize on our drug target discoveries and drug discovery and development programs. Consistent with this approach, we seek to retain exclusive rights to the benefits of certain drug discovery and development programs by developing and commercializing drug candidates from those programs internally, particularly in the United States for indications treated by specialist physicians. We seek to collaborate with other pharmaceutical and biotechnology companies with respect to drug discovery or the development and commercialization of certain of our drug candidates, particularly with respect to commercialization in territories outside the United States or commercialization in the United States for indications treated by primary care physicians, or when the collaboration may provide us with access to expertise and resources that we do not possess internally or are complementary to our own. We also seek to collaborate with other pharmaceutical and biotechnology companies, research institutes and academic institutions to capitalize on our drug target discoveries.
Bristol-Myers Squibb
We established a drug discovery alliance with Bristol-Myers Squibb Company in December 2003 to discover, develop and commercialize small molecule drugs in the neuroscience field. Bristol-Myers Squibb extended the target discovery term of the alliance in May 2006. We initiated the alliance with a number of neuroscience drug discovery programs at various stages of development and used our gene knockout technologies to identify additional drug targets with promise in the neuroscience field. For those targets that were selected for the alliance, we and Bristol-Myers Squibb worked together, on an exclusive basis, to identify, characterize and carry out the preclinical development of small molecule drugs. Bristol-Myers Squibb has the first option to assume full responsibility for clinical development and commercialization of any drugs resulting from the alliance which enter clinical trials, other than LX9211 and additional compounds acting through AAK1, for which we hold exclusive development and commercialization rights under the alliance. We received $86 million in upfront payments and research funding under the agreement during the target discovery portion of the alliance, which expired in October 2009. In addition, we are entitled to receive clinical and regulatory milestone payments ranging, depending on the timing and extent of our efforts in the alliance, up to $76 million for each drug developed by Bristol-Myers Squibb under the alliance. We will also earn royalties on sales of drugs commercialized by Bristol-Myers Squibb under the alliance.
LX9211 and another development compound acting through AAK1 were discovered by scientists working within our alliance with Bristol-Myers Squibb. We have agreed to pay Bristol-Myers Squibb up to $34.5 million in clinical and regulatory milestones for the first indication and up to $16 million in clinical and regulatory milestones for each of the second and third indications, if applicable. We have also agreed to pay single digit royalties on worldwide net sales and up to $40 million in commercial milestones.
Genentech
We established a drug discovery alliance with Genentech, Inc. in December 2002 to discover novel therapeutic proteins and antibody targets. We and Genentech expanded the alliance in November 2005 for the advanced research, development and commercialization of new biotherapeutic drugs. Under the original alliance agreement, we used our target validation technologies to discover the functions of secreted proteins and potential antibody targets identified through Genentech’s internal drug discovery research. In the expanded alliance, we conducted additional, advanced research on a broad subset of those proteins and targets. We have exclusive rights to develop and commercialize biotherapeutic drugs for two of these targets, while Genentech has exclusive rights to develop and commercialize biotherapeutic drugs for the other targets. We retain certain other rights to discoveries made in the alliance, including non-exclusive rights, along with Genentech, for the development and commercialization of small molecule drugs addressing the targets included in the alliance. We received $58 million in upfront payments, research funding and research milestone payments under the agreement during the research collaboration term, which expired in November 2008. In addition, we are entitled to receive clinical and regulatory milestone payments ranging, depending on the extent of our efforts in the alliance, up to $25 million for each drug target for which Genentech develops a biotherapeutic drug under the alliance. We will also earn royalties on sales of biotherapeutic drugs commercialized by Genentech under the alliance. Genentech is entitled to receive milestone payments and royalties on sales of biotherapeutic drugs which we develop or commercialize under the alliance.
6
TerSera
We completed the sale of our XERMELO (telotristat ethyl) product and related assets to TerSera Therapeutics LLC in September 2020. XERMELO originated from our own internal drug discovery and development efforts and we commercially launched XERMELO following regulatory approval in the United States in February 2017 for the treatment of carcinoid syndrome diarrhea. In connection with the sale, TerSera assumed responsibility for the continued development of XERMELO as a treatment for biliary tract cancer, currently in a Phase 2 clinical trial. We are eligible to receive development, regulatory and sales milestone payments from TerSera of up to an aggregate of $65 million for the development and commercialization of XERMELO in patients with biliary tract cancer and mid-teens percentage royalty payments from TerSera on net sales of XERMELO in biliary tract cancer.
Other Collaborations
We have established collaborations with a number of pharmaceutical and biotechnology companies, research institutes and academic institutions under which we have received fees in exchange for generating knockout mice for genes requested by the collaborator, providing phenotypic data with respect to such knockout mice or otherwise granting access to some of our technologies and discoveries. In some cases, we remain eligible to receive milestone or royalty payments on the sale of mice and phenotypic data or on products that our collaborators discover or develop using our technology.
Manufacturing and Product Supply
We do not own or operate manufacturing or distribution facilities or resources for clinical production and distribution of our drug candidates. Instead, we have multiple contractual agreements in place with third-party contract manufacturing organizations, or CMOs, who, on our behalf, manufacture clinical supplies of our drug candidates, and will continue to do so for the foreseeable future. We have selected well-established and reputable global CMOs for our active pharmaceutical ingredient, or API, and drug product manufacturing that have good regulatory standing, large manufacturing capacities, and multiple manufacturing sites within their business footprint. We employ highly skilled personnel with both technical and manufacturing experience to diligently manage the activities at our CMOs. Our quality department audits these suppliers on a periodic basis. We work closely with our third-party manufacturers to ensure compliance with current good manufacturing practices, or cGMP, and other stringent regulatory requirements enforced by the FDA and foreign regulatory agencies in other territories, as applicable.
Raw materials that are used to manufacture our API are sourced from multiple third-party suppliers in Asia and Europe. Third-party API contract manufacturers in Asia and Europe stock sufficient quantities of these materials to ensure they can manufacture adequate API quantities per our requirements for clinical purposes. We store API at third-party facilities, and provide appropriate amounts to third-party drug product contract manufacturers in Asia and North America who then manufacture, package and label our specified quantities of finished goods for our drug candidates. Our third-party contract manufacturers also need to obtain materials such as excipients, components and reagents to manufacture our API and finished drug products.
Within our supply chain, we have established safety stock amounts for both our API and drug products, and store those in multiple locations. The quantities that we store are based on our business needs and take into account scenarios for demand, production lead times, potential supply interruptions and shelf life for our API and drug products. In parallel, for business continuity reasons, we will evaluate the need to establish an additional or backup supplier for our API and drug product, as necessary.
Competition
The biotechnology and pharmaceutical industries are highly competitive and characterized by rapid technological change. We face significant competition in each of the aspects of our business from other pharmaceutical and biotechnology companies, as well as academic research institutions, clinical reference laboratories and governmental agencies that are pursuing research or development activities similar to ours. Many of our competitors have substantially greater research, development and commercialization capabilities and financial, scientific, marketing and human resources than we do. As a result, our competitors may succeed in developing products earlier than we do, obtaining approvals from the FDA or other regulatory agencies for those products more rapidly than we do, developing products that are more effective than those we develop or commercializing products more effectively and profitably than we do. Similarly, our collaborators face similar competition from other competitors who may succeed in developing products more quickly, developing products that are more effective than those developed by our collaborators or commercialize products more effectively and profitably than our collaborators.
7
The competition for our drug candidates includes both marketed products and drug candidates that are being developed by others, including pharmaceutical products that are currently in a more advanced stage of clinical development or commercialization than are our own drug candidates. These competitive marketed products and drug candidates include compounds that employ different mechanisms of action in addressing diseases and conditions for which we are developing our own drug candidates and, in some cases such as sotagliflozin, that employ the same or similar mechanisms of action.
We believe that our ability to successfully compete with these potentially competitive drug candidates and other competitive products currently on the market will depend on, among other things:
•the efficacy, safety and reliability of our products;
•our ability, and the ability of our collaborators, to complete preclinical and clinical development and obtain regulatory approvals for our drug candidates;
•the timing and scope of regulatory approvals of our products;
•our ability, and the ability of our collaborators, to obtain product acceptance by physicians and other health care providers and secure coverage and adequate reimbursement for product use in approved indications;
•our ability, and the ability of our collaborators, to manufacture and sell commercial quantities of our products;
•the skills of our employees and our ability to recruit and retain skilled employees;
•protection of our intellectual property; and
•the availability of substantial capital resources to fund development and commercialization activities.
We expect that our principal competition for sotagliflozin in the treatment of type 1 diabetes would include established insulin therapies, as well as selective SGLT2 inhibitors currently being prescribed off-label, but which may gain regulatory approval, for the treatment of type 1 diabetes in the United States. Such selective SGLT2 inhibitors include dapagliflozin, empagliflozin and canagliflozin, currently marketed for the treatment of type 2 diabetes by AstraZeneca, Boehringer Ingelheim and Eli Lilly, and Janssen (a subsidiary of Johnson & Johnson), respectively. In addition, AstraZeneca has received approval in the European Union for the use of dapagliflozin in type 1 diabetes as an adjunct to insulin in patients with a body mass index of 27 kg/m² or greater, when insulin alone does not provide adequate glycaemic control despite optimal insulin therapy.
We expect that our principal competition for sotagliflozin for the treatment of heart failure would include dapagliflozin and any other selective SGLT2 inhibitors which may gain regulatory approval for the treatment of heart failure, as well as angiotensin-converting enzyme, or ACE, inhibitors and the combination drug sacubitril/valsartan, currently marketed for the treatment of heart failure by Novartis.
We expect that our principal competition for LX9211 for the treatment of diabetic peripheral neuropathic pain, or DPN, would include duloxetine and pregabalin, which are currently marketed for the treatment of DPN by Eli Lilly and Pfizer, respectively. We expect that our principal competition for LX9211 for the treatment of post-herpetic neuralgia, or PHN, would include gabapentin and pregabalin, both currently marketed for the treatment of PHN by Pfizer.
Government Regulation
The development, manufacture and sale of pharmaceutical products are subject to extensive regulation by United States and foreign governmental authorities, including federal, state and local authorities. In the United States, new drugs are subject to regulation under the Federal Food, Drug and Cosmetic Act and the regulations promulgated thereunder, or the FDC Act. The FDA and comparable governmental authorities regulate, among other things, research and development activities and the testing, manufacture, quality control, safety, efficacy, record keeping, reporting, labeling, storage, approval, advertising, promotion, sale, distribution, export and import of pharmaceutical products.
The standard process required by the FDA before a drug candidate may be marketed in the United States generally includes the following:
8
•preclinical laboratory and animal tests performed under current good laboratory practices, or cGLP;
•submission of an IND, which must become effective before human clinical trials may commence;
•adequate and well-controlled human clinical trials to establish the safety and efficacy of the drug candidate for its intended use;
•submission of a New Drug Application, or NDA, for approval of commercial marketing and sale, or of an NDA supplement, or sNDA, for approval of a new indication if the product is already approved for another indication;
•pre-approval inspection of manufacturing facilities and selected clinical investigators for their compliance with cGMP and current good clinical practices, or cGCP;
•if the FDA convenes an advisory committee, satisfactory completion of the advisory committee review; and
•FDA approval of the NDA or sNDA.
This process for the testing and approval of drug candidates requires substantial time, effort and financial resources. Preclinical development of a drug candidate can take from one to several years to complete, with no guarantee that an IND based on those studies will become effective to even permit clinical testing to begin. Before commencing the first clinical trial of a drug candidate in the United States, we must submit an IND to the FDA. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, raises concerns or questions about the conduct of the clinical trial. In such a case, we and the FDA must resolve any outstanding concerns before the clinical trial may begin. Submission of an IND may not result in FDA authorization to commence a clinical trial. A separate submission to the existing IND must be made for each successive clinical trial conducted during product development, and the FDA must grant permission for each clinical trial to start and continue. Further, an independent institutional review board for each medical center proposing to participate in the clinical trial must review and approve the plan for any clinical trial before it commences at that center. Regulatory authorities or an institutional review board or we may suspend a clinical trial at any time on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk.
For purposes of NDA approval, human clinical trials are typically conducted in three sequential phases that may overlap.
•Phase 1 clinical trials are conducted in a limited number of healthy human volunteers or, in some cases, patients, to evaluate the safety, dosage tolerance, absorption, metabolism, distribution and excretion of the drug candidate;
•Phase 2 clinical trials are conducted in groups of patients afflicted with a specified disease or condition to obtain preliminary data regarding efficacy as well as to further evaluate safety and optimize dosing of the drug candidate. Multiple Phase 2 clinical trials may be conducted to obtain information prior to beginning larger and more expensive Phase 3 clinical trials; and
•Phase 3 clinical trials are conducted in larger patient populations at multiple clinical trial sites to obtain statistically significant evidence of the efficacy of the drug candidate for its intended use and to further test for safety in an expanded patient population.
In addition, the FDA may require, or companies may pursue, additional clinical trials after a product is approved. These so-called Phase 4 studies may be made a condition to be satisfied after a drug receives approval. Failure to satisfy such post-marketing commitments can result in FDA enforcement action, up and to including withdrawal of NDA approval. The results of Phase 4 studies can confirm the effectiveness of a drug candidate and can provide important safety information to augment the FDA’s adverse drug reaction reporting system.
After completion of clinical trials, FDA approval of an NDA must be obtained before a new drug may be marketed in the United States. The submission of an NDA requires payment of a substantial user fee to the FDA. An NDA must contain, among other things, information on chemistry, manufacturing controls and potency and purity, non-clinical pharmacology and toxicology, human pharmacokinetics and bioavailability and clinical data. There can be no assurance that the FDA will accept an NDA for filing and, even if accepted for filing, that approval will be granted. The FDA may convene an advisory committee to provide clinical insight on NDA review questions. Although the FDA is not required to follow the recommendations of an advisory committee, the agency typically does so. Among other things, the FDA reviews an NDA to determine whether a product is safe and effective for its intended use and whether the facility in which it is manufactured, processed, packed, or held meets standards designed to assure the product’s continued safety, purity and potency. The FDA may deny approval of an NDA by way of a complete response letter if the applicable regulatory criteria are not satisfied, or it may require additional
9
clinical data or an additional pivotal Phase 3 clinical trial. Even if such data are submitted, the FDA may ultimately decide that the NDA does not satisfy the criteria for approval. An NDA may be approved with significant restrictions on its labeling, marketing and distribution under a Risk Evaluation and Mitigation Strategy or otherwise that could restrict the commercial applications of a product or impose costly procedures in connection with the commercialization or use of the product. Once issued, the FDA may withdraw product approval if ongoing regulatory standards are not met or if safety problems occur after the product reaches the market. In addition, the FDA may require testing and surveillance programs to monitor the effect of approved products which have been commercialized, and the FDA has the power to prevent or limit further marketing of a product based on the results of these post-marketing programs.
In addition to obtaining FDA approval for each product, each drug manufacturing establishment must be inspected and approved by the FDA. All manufacturing establishments are subject to inspections by the FDA and by other federal, state and local agencies and must comply with current Good Manufacturing Practices requirements. Non-compliance with these requirements can result in, among other things, total or partial suspension of production, failure of the government to grant approval for marketing and withdrawal, suspension or revocation of marketing approvals.
Satisfaction of FDA requirements or similar requirements of state, local and foreign regulatory agencies typically takes many years, with the actual time required varying substantially based on, among other things, the nature, novelty and complexity of the drug candidate and of the disease or condition. Government regulation may delay or prevent marketing of drug candidates or new diseases for a considerable period of time and impose costly procedures upon our activities. The FDA or any other regulatory agency may not grant approvals for new indications for our product candidates on a timely basis, if at all. Success in earlier-stage clinical trials does not ensure success in later-stage clinical trials. Targets and pathways identified in vitro may be determined to be less relevant in clinical studies and results in animal model studies may not be predictive of human clinical results. Furthermore, data obtained from clinical activities is not always conclusive and may be susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. Even if a drug candidate receives regulatory approval, the approval may be significantly limited to specific disease states, patient populations and dosages. Further, even after regulatory approval is obtained, later discovery of previously unknown problems with a product may result in restrictions on the product or even complete withdrawal of the product from the market.
Once the FDA approves a product, a manufacturer must provide certain updated safety and efficacy information. Product changes as well as certain changes in a manufacturing process or facility would necessitate additional FDA review and approval. Other post-approval changes may also necessitate further FDA review and approval. Additionally, a manufacturer must meet other requirements including those related to adverse event reporting and record keeping.
Products manufactured or distributed by us pursuant to FDA approvals are subject to continuing regulation by the FDA, including record-keeping requirements and reporting of adverse experiences with the drug. Drug manufacturers and their subcontractors are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP, which impose certain procedural and documentation requirements upon us and our third-party manufacturers.
The FDA closely regulates the marketing and promotion of drugs, including restricting the promotion of uses for which a drug is not approved by the agency. Not only must a company have appropriate substantiation to support claims made about a drug, under the FDA’s current interpretation of relevant laws, a company can make only those claims relating to safety and efficacy that are for indications for which the FDA has approved the drug and are otherwise consistent with the FDA-approved label for the drug. Failure to comply with these requirements can result in adverse publicity, warning letters, corrective advertising and potential civil and criminal penalties. Physicians may, in their independent medical judgment, prescribe legally available drugs for uses that are not described in the product’s labeling and that differ from those tested by us and approved by the FDA. Such off-label uses are common across medical specialties. Physicians may believe that such off-label uses are the best treatment for many patients in varied circumstances. The FDA does not regulate the behavior of physicians in their choice of treatments. The FDA does, however, restrict manufacturers’ communications on the subject of off-label use. Additionally, a significant number of pharmaceutical companies have been the target of inquiries and investigations by various United States federal and state regulatory, investigative, prosecutorial and administrative entities in connection with the promotion of products for off-label uses and other sales practices. These investigations have alleged violations of various United States federal and state laws and regulations, including claims asserting antitrust violations, violations of the FDC Act, false claims laws, the Prescription Drug Marketing Act, anti-kickback laws, and other alleged violations in connection with the promotion of products for unapproved uses, pricing and Medicare and/or Medicaid reimbursement.
The United States Orphan Drug Act is intended to incentivize the development of products for rare diseases or conditions that affect fewer than 200,000 people in the United States. If a drug is being developed for a rare disease or
10
condition, to be eligible for designation as an orphan drug, the FDA must not have previously approved a drug considered the “same drug” for the same orphan indication. If the FDA has previously approved another same drug for the same indication, the sponsor of the subsequent drug would be required to provide a plausible hypotheses of clinical superiority over the previously approved drug to obtain an orphan designation. Upon FDA receipt of orphan drug designation, the sponsor is eligible for tax credits of up to 25% for qualified clinical trial expenses, the ability to apply for annual grant funding and waiver of PDUFA application fee. In addition, upon marketing approval, an orphan-designated drug could be eligible for seven years of market exclusivity for the approved orphan-designated indication. Such orphan drug exclusivity, if awarded, would only block the approval of any drug considered the same drug for the same orphan indication. Moreover, a subsequent same drug could break a previously approved drug’s orphan exclusivity through a demonstration of clinical superiority over the previously approved drug.
The FDA has various programs, including Fast Track, priority review and accelerated approval, which are intended to expedite or simplify the process for developing and reviewing promising drugs, or to provide for the approval of a drug on the basis of a surrogate endpoint. Generally, drugs that are eligible for these programs are those for serious or life-threatening conditions, those with the potential to address unmet medical needs and those that offer meaningful benefits over existing treatments. For example, Fast Track is a process designed to facilitate the development and expedite the review of drugs to treat serious or life-threatening diseases or conditions and fill unmet medical needs. Priority review is designed to give drugs that treat serious conditions and offer major advances in treatment or provide a treatment where no adequate therapy exists an initial review within six months of NDA filing as compared to a standard review time of 10 months from NDA filing. Certain other types of drug applications are also eligible for priority review. Although Fast Track and priority review do not affect the standards for approval, the FDA will attempt to facilitate early and frequent meetings with a sponsor of a Fast Track-designated drug and expedite review of the application for a drug designated for priority review. Accelerated approval provides for an earlier approval for a new drug that is intended to treat a serious or life-threatening disease or condition and that fills an unmet medical need based on a surrogate endpoint. As a condition of approval, the FDA may require that a sponsor of a product candidate receiving accelerated approval perform post-marketing clinical trials to confirm the clinically meaningful outcome as predicted by the surrogate marker trial. In addition to the Fast Track, accelerated approval and priority review programs, the FDA also designates Breakthrough Therapy status to drugs that are intended, alone or in combination with one or more other drugs, to treat a serious or life-threatening disease or condition, and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. Drugs designated as breakthrough therapies are also eligible for accelerated approval. The FDA will seek to ensure the sponsor of a breakthrough therapy product candidate receives intensive guidance on an efficient drug development program, intensive involvement of senior managers and experienced staff on a proactive, collaborative and cross-disciplinary review and rolling review.
Additional programs intended to expedite the development of drug products were included in the 21st Century Cures Act, or the Cures Act. The Cures Act includes various provisions to accelerate the development and delivery of new treatments, such as those intended to expand the types of evidence manufacturers may bring to the FDA to support drug approval, to encourage patient-centered drug development, to liberalize the communication of healthcare economic information to payers, and to create greater transparency with regard to manufacturer expanded access programs. Central to the Cures Act are provisions that enhance and accelerate the FDA’s processes for reviewing and approving new drugs and supplements to approved NDAs, including provisions that:
•require the FDA to establish a program to evaluate the potential use of real world evidence to help support the approval of a new indication for an approved drug and to help support or satisfy post-approval study requirements;
•provide that the FDA may rely upon qualified data summaries to support the approval of a supplemental application with respect to a qualified indication for an already approved drug;
•require the FDA to issue guidance for purposes of assisting sponsors in incorporating complex adaptive and other novel trial designs into proposed clinical protocols and applications for new drugs; and
•require the FDA to establish a process for the qualification of drug development tools for use in supporting or obtaining FDA approval for or investigational use of a drug.
The Cures Act amends Section 114 of the Food and Drug Administration Modernization Act of 1997 to help clarify and facilitate the dissemination of healthcare economic information, including by broadening the definition of healthcare economic information, expressly extending the dissemination of healthcare economic information to payors, and clarifying that healthcare economic information must only relate to an FDA-approved indication rather than directly relate to the indication.
11
Regulation Outside of the United States
In addition to regulations in the United States, we are subject to the regulations of other countries governing clinical trials and the manufacturing, commercial sales and distribution of our products outside of the United States. Whether or not we obtain FDA approval for a product, we must obtain approval by the comparable regulatory authorities of countries outside of the United States before we can commence clinical trials in such countries and approval of the regulators of such countries or economic areas, such as the European Union, before we may market products in those countries or areas. The approval process and requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from place to place, and the time may be longer or shorter than that required for FDA approval.
Under European Union regulatory systems, a company may submit marketing authorization applications, or MAAs, either under a centralized or decentralized procedure. Under the centralized procedure, MAAs are submitted to the European Medicines Agency, or EMA, whose Committee for Medicinal Products for Human Use reviews the application and issues an opinion on it. The opinion is considered by the European Commission which is responsible for deciding applications. If the application is approved, the European Commission grants a single marketing authorization that is valid for all European Union member states as well as Iceland, Liechtenstein and Norway, or the EEA. The national authorization procedures, the decentralized and mutual recognition procedures, as well as national applications, are available for products for which the centralized procedure is not compulsory. The mutual recognition procedure provides for the European Union member states selected by the applicant to mutually recognize a national marketing authorization that has already been granted by the competent authority of another member state, referred to as the Reference Member State, or RMS. The decentralized procedure is used when the product in question has yet to be granted a marketing authorization in any member state. Under this procedure the applicant can select the member state that will act as the RMS. In both the mutual recognition and decentralized procedures, the RMS reviews the application and submits its assessment of the application to the member states where marketing authorizations are being sought, referred to as Concerned Member States or CMS. Within 90 days of receiving the application and assessment report, each CMS must decide whether to recognize the RMS assessment. If a member state does not agree with the assessment and the disputed points cannot be resolved, the matter is eventually referred to the European Commission, whose decision is binding on all member states. If the application is successful, national marketing authorizations will be granted by the competent authorities in each of the member states chosen by the applicant.
Conditional marketing authorizations may be granted for a limited number of medicinal products for human use referenced in European Union law applicable to conditional marketing authorizations where the clinical dataset is not comprehensive, if the risk-benefit balance of the product is positive, it is likely that the applicant will be in a position to provide the required comprehensive clinical trial data, unmet medical needs will be fulfilled and the benefit to public health of the immediate availability on the market of the medicinal product outweighs the risk inherent in the fact that additional data are still required. Specific obligations, such as the completion of ongoing or new studies and obligations relating to the collection of pharmacovigilance data, may be amongst the conditions stipulated in the marketing authorization.
As in the United States, we may apply for designation of a product as an Orphan drug for the treatment of a specific indication in the European Union before the application for marketing authorization is made. In the European Union, orphan designation is available for products in development which are either intended for the diagnosis, prevention or treatment of life-threatening or chronically debilitating conditions affecting not more than 5 in 10,000 persons in the European Union, or intended for the diagnosis, prevention or treatment of a life-threatening, seriously debilitating or serious and chronic condition in the community and when, without incentives, it is unlikely that sales of the drug in the European Union would be sufficient to justify the necessary investment in developing the medicinal product. Additionally, the sponsor of an application for orphan drug designation must establish that there exists no satisfactory authorized method of diagnosis, prevention, or treatment of the condition or even if such treatment exists, the product will be of significant benefit to those affected by that condition.
Orphan drugs in the European Union enjoy economic and marketing benefits, including up to ten years of market exclusivity for the approved indication unless another applicant can show that its product is safer, more effective or otherwise clinically superior to the orphan-designated product. The period of market exclusivity may be reduced to six years if at the end of the fifth year it is established that the criteria for orphan designation are no longer met, including where it is shown that the product is sufficiently profitable not to justify maintenance of market exclusivity.
Healthcare Regulation
Federal and state healthcare laws, including fraud and abuse and health information privacy and security laws, also apply to our business. If we fail to comply with those laws, we could face substantial penalties and our business, results of operations, financial condition and prospects could be adversely affected. The laws that may affect our ability to operate include, but are not limited to: the federal Anti-Kickback Statute, which prohibits. among other things, soliciting, receiving,
12
offering or paying remuneration, directly or indirectly, to induce, or in return for, the purchase or recommendation of an item or service reimbursable under a federal healthcare program, such as the Medicare and Medicaid programs; and federal civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payers that are false or fraudulent. Additionally, we are subject to state law equivalents of each of the above federal laws, which may be broader in scope and apply regardless of whether the payer is a federal healthcare program, and many of which differ from each other in significant ways and may not have the same effect, further complicate compliance efforts.
Numerous federal and state laws, including state security breach notification laws, state health information privacy laws and federal and state consumer protection laws, govern the collection, use and disclosure of personal information. Other countries also have, or are developing, laws governing the collection, use and transmission of personal information. In addition, most healthcare providers who are expected to prescribe our products and from whom we obtain patient health information, are subject to privacy and security requirements under the Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology and Clinical Health Act, or HIPAA. Although we are not directly subject to HIPAA, we could be subject to criminal penalties if we knowingly obtain individually identifiable health information from a HIPAA-covered entity, including healthcare providers, in a manner that is not authorized or permitted by HIPAA. The legislative and regulatory landscape for privacy and data protection continues to evolve, and there has been an increasing amount of focus on privacy and data protection issues with the potential to affect our business, including recently enacted laws in a majority of states requiring security breach notification. These laws could create liability for us or increase our cost of doing business. International laws, such as the EU Data Privacy Directive and Swiss Federal Act on Data Protection, regulate the processing of personal data within the European Union and between countries in the European Union and countries outside of the European Union, including the United States. Failure to provide adequate privacy protections and maintain compliance with safe harbor mechanisms could jeopardize business transactions across borders and result in significant penalties.
In addition, the Patient Protection and Affordable Care Act, as amended by the Health Care Education Reconciliation Act, or the PPACA, created a federal requirement under the federal Open Payments program, that requires certain manufacturers to track and report to the Centers for Medicare and Medicaid Services, or CMS, annually certain payments and other transfers of value provided to physicians and teaching hospitals made in the previous calendar year. In addition, there are also an increasing number of state laws that require manufacturers to make reports to states on pricing and marketing information. These laws may affect our sales, marketing, and other promotional activities by imposing administrative and compliance burdens on us. In addition, given the lack of clarity with respect to these laws and their implementation, our reporting actions could be subject to the penalty provisions of the pertinent state and federal authorities.
For those marketed products which are covered in the United States by the Medicaid program, we have various obligations, including government price reporting and rebate requirements, which generally require products be offered at substantial rebates/discounts to Medicaid and certain purchasers. We are also required to discount such products to authorized users of the Federal Supply Schedule of the General Services Administration, under which additional laws and requirements apply. These programs require submission of pricing data and calculation of discounts and rebates pursuant to complex statutory formulas, as well as the entry into government procurement contracts governed by the Federal Acquisition Regulations, and the guidance governing such calculations is not always clear. Compliance with such requirements can require significant investment in personnel, systems and resources, but failure to properly calculate our prices, or offer required discounts or rebates could subject us to substantial penalties.
Environmental Matters
In addition to the foregoing, our business is subject to regulation under various state and federal environmental and worker safety laws, including the Occupational Safety and Health Act, the Resource Conservation and Recovery Act, the Comprehensive Environmental Response, Compensation and Liability Act and the Toxic Substances Control Act, each as amended from time to time. These and other laws and their implementing regulations govern our manufacture, use, storage, handling, transport and disposal of various biological, chemical, radioactive and other hazardous substances used in our operations and the wastes generated by those activities. We cannot eliminate the risk of accidental contamination or discharge and any resultant injury from these substances. We may face liability for any injury or contamination that results from our use or the use by third parties of those substances, and such liability may exceed our insurance coverage and our total assets. Historically, our environmental and worker safety compliance costs have not had a material adverse effect on our results of operations, but there can be no assurance that such costs will not be material in the future or that such future compliance will not have a material adverse effect on our business and operational results. The trend in environmental and occupational health and safety laws and regulations is to typically place more restrictions and limitations on activities that may adversely affect the environment or expose workers to injury. If existing regulatory requirements or enforcement policies change or new regulatory
13
or enforcement initiatives are developed and implemented in the future, we may be required to make significant, unanticipated capital and operating expenditures.
Patents and Proprietary Rights
We can protect our proprietary rights from unauthorized use by third parties only to the extent that those rights are covered by valid and enforceable patents or are effectively maintained as trade secrets. Accordingly, patents and other proprietary rights are an essential element of our business. We own or exclusively license patents and patent applications throughout the world that claim our products and drug candidates, including:
•issued patents and pending patent applications in Europe, the United States, and other countries throughout the world, including Australia, Brazil, Canada, China, Europe, India, Israel, Japan, Mexico, New Zealand, South Africa, and South Korea, that claim LX9211, pharmaceutical compositions comprising LX9211, and methods of its use; and
•issued patents and pending patent applications in Europe, the United States, and other countries throughout the world, including Australia, Argentina, Brazil, Canada, China, Europe, India, Israel, Japan, Mexico, New Zealand, South Africa, and South Korea, that claim sotagliflozin, crystalline forms of sotagliflozin, pharmaceutical compositions comprising sotagliflozin, and methods of its manufacture and use.
Patents extend for varying periods according to the date of patent filing or grant and the legal term of patents in the various countries where patent protection is obtained. The actual protection afforded by a patent, which can vary from country to country, depends on the type of patent, the scope of its coverage and the availability of legal remedies in the country. We have filed patent applications and hold issued patents covering each of our drug candidates. None of our United States patents that claim one of our drug candidates has a normal expiration date earlier than 2028.
All of our employees, consultants and advisors are required to execute a proprietary information agreement upon the commencement of employment or consultation. In general, the agreement provides that all inventions conceived by the employee or consultant, and all confidential information developed or made known to the individual during the term of the agreement, shall be our exclusive property and shall be kept confidential, with disclosure to third parties allowed only in specified circumstances. We cannot assure you, however, that these agreements will provide useful protection of our proprietary information in the event of unauthorized use or disclosure of such information.
Our patent and intellectual property rights are subject to certain rights and uncertainties. See “Risks Related to Our Intellectual Property” under “Item 1A. Risk Factors.”
Executive Officers
Our executive officers and their ages and positions are listed below.
| Name | Age | Position with the Company | ||||||
| Lonnel Coats | 56 | President and Chief Executive Officer and Director | ||||||
| Alan J. Main, Ph.D. | 67 | Executive Vice President, Innovation & Chemical Sciences | ||||||
| Praveen Tyle, Ph.D. | 60 | Executive Vice President, Research and Development | ||||||
| Jeffrey L. Wade | 56 | Executive Vice President, Corporate and Administrative Affairs and Chief Financial Officer | ||||||
| Brian T. Crum | 48 | Vice President and General Counsel | ||||||
| James F. Tessmer | 61 | Vice President, Finance and Accounting | ||||||
Lonnel Coats has been our president and chief executive officer and a director since July 2014. Mr. Coats previously served in a series of executive leadership positions at Eisai Inc. and Eisai Corporation of North America, where he worked for 18 years before joining our company, most recently as chief executive officer from 2010 to 2014. Prior to joining Eisai, Mr. Coats spent eight years with Janssen Pharmaceuticals, Inc., a division of Johnson & Johnson, where he held a variety of management and sales positions. Mr. Coats serves as a director of Blueprint Medicines Corporation and holds a B.S. from Oakland University.
Alan J. Main, Ph.D. has been our executive vice president of innovation and chemical sciences since September 2020 and previously served in a series of manufacturing and scientific leadership positions since joining our company in 2001. Dr. Main was president and chief executive officer of Coelacanth Corporation, a leader in using proprietary chemistry technologies to rapidly discover new chemical entities for drug development, until our acquisition of Coelacanth in
14
2001. Dr. Main was formerly senior vice president, U.S. Research at Novartis Pharmaceuticals Corporation, where he worked for 20 years before joining Coelacanth. Dr. Main holds a B.S. from the University of Aberdeen, Scotland and a Ph.D. in organic chemistry from the University of Liverpool, England and completed postdoctoral studies at the Woodward Research Institute.
Praveen Tyle, Ph.D. has been our executive vice president of research and development since May 2016. Dr. Tyle was previously a member of the executive management team at Osmotica Pharmaceutical Corp., serving as president and chief executive officer from January 2013 through April 2016 and prior to that as executive vice president and chief scientific officer. Prior to his service at Osmotica, Dr. Tyle held a series of scientific leadership positions within the pharmaceutical industry, including executive vice president and chief science officer for the United States Pharmacopeia, senior vice president and global head of research and development and business development and licensing at Novartis OTC, corporate senior vice president of global research and development and chief scientific officer at Bausch & Lomb Incorporated and vice president and global head of pharmaceutical sciences at Pharmacia Corporation. Dr. Tyle serves as director of Eyegate Pharmaceuticals, Inc. and Orient Europharma Ltd. Dr. Tyle received his B.Pharm. from the Indian Institute of Technology, Banaras Hindu University and his Ph.D. in pharmaceutics and pharmaceutical chemistry from the Ohio State University.
Jeffrey L. Wade has been our executive vice president, corporate and administrative affairs and chief financial officer since February 2015 and previously served in a series of finance and legal leadership positions since joining our company in 1999. Mr. Wade was previously a corporate securities and finance attorney for ten years with the law firm of Andrews & Kurth L.L.P., where he represented companies in the biotechnology, information technology and energy industries. Mr. Wade is a member of the board of directors of the Texas Healthcare and Bioscience Institute. He received his B.A. and J.D. from the University of Texas.
Brian T. Crum has been our vice president and general counsel since May 2010 and previously served in a series of legal leadership positions since joining our company in 2001. Mr. Crum was previously a corporate securities attorney with the law firms of Brobeck, Phleger & Harrison LLP and Andrews & Kurth L.L.P., where he represented companies in the energy and information technology industries. Mr. Crum received his B.B.A. and J.D. from the University of Texas.
James F. Tessmer has been our vice president, finance and accounting since November 2007 and previously served in a series of finance and accounting leadership positions since joining our company in 2001. Mr. Tessmer was previously assistant controller for Mariner Health Network, Inc. and prior to that served in a variety of financial and accounting management positions for HWC Distribution Corp. and American General Corporation. Mr. Tessmer is a certified public accountant and received his B.B.A. from the University of Wisconsin – Milwaukee and his M.B.A. from the University of Houston.
Significant Shareholders
We have valuable relationships with Invus, L.P. and its affiliates, which we collectively refer to as Invus. Invus currently owns approximately 52.3% of the outstanding shares of our common stock.
Human Capital Resources
As of February 28, 2021, we employed 78 persons, of whom 14 hold M.D. or Ph.D. degrees and another 17 hold other advanced degrees. All of our employees are located in the United States. None of our employees are represented by a labor union and we believe that our relationship with our employees is good.
Our company culture is supported by our five core values: innovation, transparency, ownership, respect and integrity. We value a diverse workforce and proudly reflect a company culture developed with a variety of ethnic backgrounds, nationalities, races, religions, military service, sexual preferences and abilities. We are committed to promoting and maintaining an inclusive, high-performing environment where all team members embrace and leverage each other’s talents and backgrounds and nourish innovative thinking in order to achieve their full potential and contribute to our success.
Our most valued resource is the collective talent and time that our employees dedicate to support and advance our mission. Accordingly, we offer our employees a comprehensive compensation and benefits package that is competitive within the industry and make investing in the growth and development of our employees an important priority. Employee development is advanced through talent management, promotions, mentoring, stretch assignments, internships, formal training, speaker series, conferences, continuing education and educational reimbursement.
15
Research and Development Expenses
In 2020, 2019 and 2018, respectively, we incurred expenses of $153.6 million, $91.9 million and $100.2 million in company-sponsored as well as collaborative research and development activities, including $6.4 million, $7.1 million and $6.0 million of stock-based compensation expense in 2020, 2019 and 2018, respectively.
16
Item 1A. Risk Factors
The following risks and uncertainties are important factors that could cause actual results or events to differ materially from those indicated by forward-looking statements. The factors described below are not the only ones we face and additional risks and uncertainties not presently known to us or that we currently deem immaterial also may impair our business operations.
Risk Factor Summary
Below is a summary of the material risks to our business, operations and the investment in our common stock. This summary does not include all of the risks we face and you should carefully review and consider the full discussion of our risk factors below, together with the other information in this annual report on Form 10-K.
Risks Related to Our Business and Industry
•Clinical testing of our drug candidates in humans is an inherently risky and time-consuming process that may fail to demonstrate safety and efficacy, which could result in the delay, limitation or prevention of regulatory approval.
•Our drug candidates are subject to a lengthy and uncertain regulatory process that may not result in the necessary regulatory approvals, which could adversely affect our and our collaborators’ ability to commercialize products.
•We depend on our ability to gain alignment with certain regulatory authorities on our regulatory strategy for sotagliflozin in heart failure. If we fail to effectively gain such alignment, our business will suffer and our stock price will likely decline.
•We are subject to certain healthcare laws, regulation and enforcement; our failure to comply with those laws could have a material adverse effect on our results of operations and financial condition.
•Our competitors may develop products that impair the value of any products that we or our collaborators may develop.
•We face business disruption and related risks resulting from the outbreak of the novel coronavirus, or COVID-19, including delays in the enrollment of ongoing clinical trials and other operational impacts, each of which could have a material adverse effect on our business.
Risks Related to Our Capital Requirements and Financial Results
•We will need additional capital in the future and, if it is unavailable, we will be forced to delay, reduce or eliminate our research and development programs.
•We have a history of net losses, and we expect to continue to incur net losses and may not achieve or maintain profitability.
Risks Related to Our Relationships with Third Parties
•We depend on our ability to establish a strategic collaboration for the commercialization of sotagliflozin in heart failure. If we are unable to establish such a collaboration, sotagliflozin may not be commercialized in heart failure, our business will suffer and our stock price will likely decline.
•We depend on our ability to establish collaborations with pharmaceutical and biotechnology companies for the development and commercialization of our other drug candidates. If we are unable to establish such collaborations, or if pharmaceutical products are not successfully and timely developed and commercialized under such collaborations, our opportunities to generate revenues from our other drug candidates will be greatly reduced.
Risks Related to Our Intellectual Property
•If we are unable to adequately protect our intellectual property, third parties may be able to use our products and technologies, which could adversely affect our ability to compete in the market.
17
Risks Related to Our Employees and Facilities
•The loss of key personnel or the inability to attract and retain additional personnel could impair our ability to operate and expand our operations.
Risks Related to Environmental and Product Liability
•Our business has a substantial risk of product liability and we face potential product liability exposure far in excess of our limited insurance coverage.
Risks Related to Our Common Stock
•Invus, L.P. and its affiliates own a controlling interest in our outstanding common stock and may have interests which conflict with those of our other stockholders.
•Invus has additional rights under its stockholders’ agreement relating to the membership of our board of directors, which provides Invus with substantial influence over significant corporate matters.
Risks Related to Our Business and Industry
Clinical testing of our drug candidates in humans is an inherently risky and time-consuming process that may fail to demonstrate safety and efficacy, which could result in the delay, limitation or prevention of regulatory approval.
In order to obtain regulatory approvals for the commercial sale of any products that we or our collaborators may develop, we or our collaborators are required to complete extensive clinical trials in humans to demonstrate the safety and efficacy of our drug candidates. We or our collaborators may not be able to obtain authority from the FDA, or other equivalent foreign regulatory agencies, to initiate or complete any clinical trials. In addition, we have limited internal resources for making regulatory filings and interacting with regulatory authorities.
Clinical trials are inherently risky and the results from nonclinical testing of a drug candidate that is under development may not be predictive of results that will be obtained in human clinical trials. In addition, the results of early human clinical trials may not be predictive of results that will be obtained in larger-scale, advanced stage clinical trials. A number of companies in the pharmaceutical industry have suffered significant setbacks in advanced clinical trials, even after achieving positive results in earlier trials. Negative or inconclusive results from a nonclinical study or a clinical trial could cause us, our collaborators or the FDA or other equivalent foreign regulatory agencies to terminate a nonclinical study or clinical trial or require that we or our collaborators repeat or modify it. Furthermore, we, one of our collaborators or a regulatory agency with jurisdiction over the trials may suspend clinical trials at any time if the subjects or patients participating in such trials are being exposed to unacceptable health risks or for other reasons.
Any nonclinical or clinical test may fail to produce results satisfactory to the FDA or foreign regulatory authorities. Nonclinical and clinical data can be interpreted in different ways, which could delay, limit or prevent regulatory approval. The FDA or institutional review boards at the medical institutions and healthcare facilities where we or our collaborators sponsor clinical trials may suspend any trial indefinitely if they find deficiencies in the conduct of these trials. Clinical trials must be conducted in accordance with the FDA’s current Good Clinical Practices. The FDA and these institutional review boards have authority to oversee our and our collaborators’ clinical trials, and the FDA may require large numbers of subjects or patients. In addition, we or our collaborators must manufacture, or contract for the manufacture of, the drug candidates that we use in our clinical trials under the FDA’s current Good Manufacturing Practices.
The rate of completion of clinical trials is dependent, in part, upon the rate of enrollment of patients. Patient accrual is a function of many factors, including the size of the patient population, the proximity of patients to clinical sites, the eligibility criteria for the study, the nature of the study, the existence of competitive clinical trials and the availability of alternative treatments. Delays in planned patient enrollment may result in increased costs and prolonged clinical development, which in turn could allow our competitors to bring products to market before we do and impair our ability to commercialize our products or potential products.
We or our collaborators may not be able to successfully complete any clinical trial of a drug candidate within any specified time period. In some cases, we or our collaborators may not be able to complete the trial at all. Moreover, clinical trials may not show our drug candidates to be both safe and effective. Thus, the FDA and other regulatory authorities may not approve any drug candidates that we develop for any indication or may limit the approved indications or impose other conditions.
18
Our drug candidates are subject to a lengthy and uncertain regulatory process that may not result in the necessary regulatory approvals, which could adversely affect our and our collaborators’ ability to commercialize products.
Our drug candidates, as well as the activities associated with their research, development and commercialization, are subject to extensive regulation by the FDA and other regulatory agencies in the United States and by comparable authorities in other countries. Failure to obtain regulatory approval for any drug candidate would prevent us from commercializing that drug candidate. The process of obtaining regulatory approvals is expensive, and often takes many years, if approval is obtained at all, and can vary substantially based upon the type, complexity and novelty of the drug candidates involved. Before a new drug application can be filed with the FDA, the drug candidate must undergo extensive clinical trials, which can take many years and may require substantial expenditures. Any clinical trial may fail to produce results satisfactory to the FDA. For example, the FDA could determine that the design of a clinical trial is inadequate to produce reliable results. Furthermore, prior to approving a new drug, the FDA typically requires that the efficacy of the drug be demonstrated in two double-blind, controlled studies. The regulatory process also requires nonclinical testing, and data obtained from nonclinical and clinical activities are susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. In addition, delays or rejections may be encountered based upon changes in regulatory policy for product approval during the period of product development and regulatory agency review. Changes in regulatory approval policy, regulations or statutes or the process for regulatory review during the development or approval periods of our drug candidates may cause delays in the approval or rejection of an application. Even if the FDA or a comparable authority in another country approves a drug candidate, the approval may impose significant restrictions on the indicated uses, conditions for use, labeling, advertising, promotion, marketing and/or production of such product and may impose ongoing requirements for post-approval studies, including additional research and development and clinical trials. These agencies also may impose various civil or criminal sanctions for failure to comply with regulatory requirements, including withdrawal of product approval.
We depend on our ability to gain alignment with certain regulatory authorities on our regulatory strategy for sotagliflozin in heart failure. If we fail to effectively gain such alignment, our business will suffer and our stock price will likely decline.
We have reported positive results from two Phase 3 clinical trials evaluating the effect of sotagliflozin on long-term outcomes related to cardiovascular death and heart failure in approximately 10,500 and 1,200 patients, respectively. Both clinical trials were initiated by Sanofi and transitioned to us in connection with the termination of our collaboration, following which both clinical trials were closed out early beginning in March 2020. In connection with the early close-out of the studies, the primary endpoints were modified so that, among other things, the events of cardiovascular death, hospitalizations for heart failure and urgent visits for heart failure were determined by clinical investigators rather than by independent adjudication. The FDA has provided feedback that the results of the two clinical trials can support the filing of an application for regulatory approval for an indication to reduce the risk of cardiovascular death, hospitalization for heart failure, and urgent visits for heart failure in patients with type 2 diabetes with either worsening heart failure or additional risk factors for heart failure. However, we cannot offer any assurances that other regulatory authorities, including the European Medicines Agency, will accept the determination of such events without independent adjudication or will not raise other concerns relating to the early close out of the studies. Should such other regulatory authorities require independent adjudication of such events or otherwise object to our strategy of seeking regulatory approval of sotagliflozin in heart failure on the basis of the studies, as closed out early, our business and financial condition could be materially harmed and we may be more heavily dependent on the success of our other drug programs.
The commercial success of any products that we or our collaborators may develop will depend upon the degree of market acceptance among physicians, patients, health care payers and the medical community.
Our or our collaborators’ ability to commercialize any products that we or they may develop will be highly dependent upon the extent to which such products gain market acceptance among physicians, patients, health care payers, such as commercial health insurers, Medicare and Medicaid, and the medical community. If such products do not achieve an adequate level of acceptance, we may not generate adequate product revenues and we may not become profitable. The degree of market acceptance of such products will depend upon a number of factors, including:
•the effectiveness, or perceived effectiveness, of our products in comparison to competing products;
•the existence of any significant side effects, as well as their severity in comparison to any competing products;
•potential advantages or disadvantages in relation to alternative treatments;
•current and future indications for which our products may be approved;
19
•the ability to offer our products for sale at competitive prices;
•relative convenience and ease of administration;
•the strength of marketing and distribution support; and
•sufficient third-party coverage or reimbursement.
If we are unable to reestablish an effective sales force, marketing infrastructure and distribution capabilities, we will not be able to successfully commercialize any products that we or our collaborators may develop.
In order to successfully commercialize any product that we or our collaborators may develop, we or they must establish or maintain an effective commercialization infrastructure supporting such product, including sales force, marketing organization and distribution capabilities. We no longer maintain a commercialization infrastructure following our sale of XERMELO and related assets to TerSera and would need to reestablish such capabilities in order to commercialize any future products. Factors that may hinder efforts to effectively establish, manage and maintain such infrastructure for products that we or our collaborators may develop include:
•inability to recruit, retain and effectively manage adequate numbers of effective sales and marketing personnel;
•inability to maintain relationships with third-party logistics providers, pharmacies, third-party manufacturers and other third parties instrumental in the commercial manufacture and distribution of such products;
•inability to establish or implement internal controls and procedures required in connection with sales of such products;
•inability of sales personnel to obtain access to or convince adequate numbers of physicians to prescribe such products; and
•potential lack of complementary products to be offered by sales personnel, which may put us or our collaborators at a competitive disadvantage relative to companies with more extensive product lines.
If we or our collaborators are unable to sustain our or their sales force, marketing infrastructure and distribution capability for such products, we may not be able to generate any product revenue, may generate increased expenses and may never become profitable.
We or our collaborators will need to expend significant time and resources to train our sales forces to be credible, persuasive and compliant in discussing such products with the physicians treating the patients indicated under the label. We or our collaborators will also need to continue to train our sales forces to ensure that a consistent and appropriate message about such products is being delivered to potential customers. If we or our collaborators are unable to effectively train our sales forces and equip them with effective materials, including medical and sales literature to help them inform and educate potential customers about the benefits and risks of such products and their proper administration, our and their ability to successfully commercialize such products could be diminished, which could have a material adverse effect on our financial condition, stock price and operations.
If we are unable to maintain adequate coverage and reimbursement from third-party payers for any products that we or our collaborators may develop, our revenues and prospects for profitability will suffer.
Our ability to successfully commercialize any products that we or our collaborators may develop is highly dependent on the extent to which coverage and reimbursement for such products are available from third-party payers, including governmental payers, such as Medicare and Medicaid, and private health insurers, including managed care organizations and group purchasing organizations. Many patients are not capable of paying themselves for the products that we or our collaborators may develop, and rely on third-party payers to pay for, or subsidize, their medical needs. If third-party payers do not provide coverage or reimbursement for such products, our revenues and prospects for profitability will suffer. In addition, even if third-party payers provide some coverage or reimbursement for such products, the availability of such coverage or reimbursement for prescription drugs under private health insurance and managed care plans often varies based on the type of contract or plan purchased.
20
In addition, in some foreign countries, particularly the countries in the European Union, the pricing of prescription pharmaceuticals is subject to governmental control. In these countries, price negotiations with governmental authorities can take six to 12 months or longer after the receipt of regulatory marketing approval for a product. To obtain reimbursement and/or pricing approval in some countries, we or our collaborators may be required to conduct a clinical trial that compares the cost effectiveness of our drug candidates or products to other available therapies. The conduct of such a clinical trial could be expensive and result in delays in the commercialization of our drug candidates. Third-party payers are challenging the prices charged for medical products and services, and many third-party payers limit reimbursement for newly approved health care products. In particular, third-party payers may limit the indications for which they will reimburse patients who use any products that we or our collaborators may develop. Cost-control initiatives could decrease prices we or our collaborators might establish for products that may be developed, which would result in lower product revenues to us.
We may not be able to manufacture products that we or our collaborators may develop in commercial quantities, which would impair our ability to commercialize such products.
Our drug candidates have been manufactured in relatively small quantities for nonclinical and clinical trials. If any of these drug candidates are approved by the FDA or other regulatory agencies for commercial sale, we or our collaborators will need to manufacture them in larger quantities. We may not be able to successfully increase the manufacturing capacity, whether in collaboration with third-party manufacturers or on our own, for any approved product in a timely or economic manner, or at all. Significant scale-up of manufacturing may require additional validation studies, which the FDA must review and approve. If we or our collaborators are unable to successfully increase the manufacturing capacity for any such product, the regulatory approval or commercial launch of that product may be delayed or there may be a shortage in supply. Pharmaceutical products typically require precise, high-quality manufacturing. The failure to achieve and maintain these high manufacturing standards, including the incidence of manufacturing errors, could result in patient injury or death, product recalls or withdrawals, delays or failures in product testing or delivery, cost overruns or other problems that could seriously hurt our business.
We and our collaborators are subject to extensive and rigorous ongoing regulation relating to any products that we or our collaborators may develop.
We and our collaborators are subject to extensive and rigorous ongoing domestic and foreign government regulation of, among other things, the research, development, testing, manufacture, labeling, promotion, advertising, distribution and marketing of any products which receive regulatory approvals from the FDA or foreign regulatory authorities. The failure to comply with these requirements or the identification of safety problems during commercial marketing could lead to the need for product marketing restrictions, product withdrawal or recall or other voluntary or regulatory action, which could delay further marketing until the product is brought into compliance. The failure to comply with these requirements may also subject us or our collaborators to stringent penalties.
We are subject to certain healthcare laws, regulation and enforcement; our failure to comply with those laws could have a material adverse effect on our results of operations and financial condition.
We are subject to certain healthcare laws and regulations and enforcement by the federal government and the states in which we conduct our business. The laws that may affect our ability to operate include, without limitation:
•the federal Anti-Kickback Statute, which constrains our marketing practices, educational programs, pricing policies, relationships with healthcare providers or other entities, and other business activities, by prohibiting, among other things, persons and entities from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual for, or the purchase, order or recommendation of, any good or service for which payment may be made under federal healthcare programs such as the Medicare and Medicaid programs;
•federal civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payers that are false or fraudulent;
•federal criminal laws that prohibit executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters;
•state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws which may apply to items or services reimbursed by any third-party payer, including commercial insurers, and state laws governing the
21
privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts;
•the Foreign Corrupt Practices Act, a United States law which regulates certain financial relationships with foreign government officials (which could include, for example, certain medical professionals);
•federal and state consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers;
•state and federal government price reporting laws that require us to calculate and report complex pricing metrics to government programs, where such reported price may be used in the calculation of reimbursement and/or discounts on our marketed drugs (participation in these programs and compliance with the applicable requirements may subject us to potentially significant discounts on our products, increased infrastructure costs, and potentially limit our ability to offer certain marketplace discounts); and
•state and federal marketing expenditure tracking and reporting laws, which generally require certain types of expenditures in the United States to be tracked and reported. Compliance with such requirements may require investment in infrastructure to ensure that tracking is performed properly, and some of these laws result in the public disclosure of various types of payments and relationships, which could potentially have a negative effect on our business and/or increase enforcement scrutiny of our activities.
In addition, certain marketing practices, including off-label promotion, may also violate certain federal and state health regulatory fraud and abuse laws as well as false claims laws, including the civil False Claims Act. Suits filed under the civil False Claims Act, known as “qui tam” actions, can be brought by any individual on behalf of the government and such individuals, commonly known as “whistleblowers,” may share in any amounts paid by the entity to the government in fines or settlement. The filing of qui tam actions has caused a number of pharmaceutical, medical device and other healthcare companies to defend a civil False Claims Act action. When an entity is determined to have violated the civil False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties for each separate false claim.
If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we, or our officers or employees, may be subject to penalties, including administrative civil and criminal penalties, damages, fines, withdrawal of regulatory approval, the curtailment or restructuring of our operations, the exclusion from participation in Medicare, Medicaid and other federal and state healthcare programs, individual imprisonment, contractual damages, reputational harm, diminished profits and future earnings, additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws, any of which could adversely affect our ability to sell our products or operate our business and also adversely affect our financial results. Defending against any such actions can be costly, time-consuming and may require significant financial and personnel resources. Therefore, even if we are successful in defending against any such actions that may be brought against us, our business may be impaired.
Numerous federal and state laws, including state security breach notification laws, state health information privacy laws and federal and state consumer protection laws, govern the collection, use and disclosure of personal information. Other countries also have, or are developing, laws governing the collection, use and transmission of personal information. In addition, most healthcare providers who may be expected to prescribe our products and from whom we may obtain patient health information are subject to privacy and security requirements under the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA. Although we are not directly subject to HIPAA, we could be subject to criminal penalties if we knowingly obtain individually identifiable health information from a HIPAA-covered entity in a manner that is not authorized or permitted by HIPAA. The legislative and regulatory landscape for privacy and data protection continues to evolve, and there has been an increasing amount of focus on privacy and data protection issues with the potential to affect our business, including recently enacted laws in a majority of states requiring security breach notification. These laws could create liability for us or increase our cost of doing business. International laws, such as the EU Data Privacy Directive and Swiss Federal Act on Data Protection, regulate the processing of personal data within Europe and between European countries and the United States. Failure to provide adequate privacy protections and maintain compliance with safe harbor mechanisms could jeopardize business transactions across borders and result in significant penalties.
22
Current healthcare laws and regulations and future legislative or regulatory reforms to the healthcare system may negatively affect our revenues and prospects for profitability.
A primary trend in the United States and some foreign countries is toward reform and cost containment in the health care industry. The United States and some foreign jurisdictions are considering or have enacted a number of legislative and regulatory proposals that may have the effect of reducing the prices that we are able to charge for products we or our collaborators may develop. Healthcare reform measures which may be adopted in the future in the United States and foreign jurisdictions may result in more rigorous coverage criteria and significant downward pressure on the prices drug manufacturers may charge. As a result, our revenues and prospects for profitability could be significantly harmed.
As a result of the overall trend towards cost-effectiveness criteria and managed healthcare in the United States, third-party payers are increasingly attempting to contain healthcare costs by limiting both coverage and the level of reimbursement of new drugs. They may use tiered reimbursement and may adversely affect demand for products we or our collaborators may develop by placing them in an expensive tier. They may also refuse to provide any coverage of uses of approved products for medical indications other than those for which the FDA has granted market approvals. As a result, significant uncertainty exists as to whether and how much third-party payers will reimburse for newly approved drugs, which in turn will put pressure on the pricing of drugs. Further, we do not have experience in ensuring approval by applicable third-party payers outside of the United States for coverage and reimbursement of pharmaceutical products. We also anticipate pricing pressures in connection with the sale of products we or our collaborators may develop due to the increasing influence of health maintenance organizations and additional legislative proposals.
Our competitors may develop products that impair the value of any products that we or our collaborators may develop.
The pharmaceutical and biotechnology industries are highly diversified and are characterized by rapid technological change. We and our collaborators face, and will continue to face, intense competition from biotechnology and pharmaceutical companies, as well as academic research institutions, clinical reference laboratories and government agencies that are pursuing research and development activities similar to ours. In addition, significant delays in the development of our drug candidates could allow our competitors to bring products to market before us, which would impair our or our collaborators’ ability to commercialize our drug candidates. Any products that we or our collaborators develop will compete in highly competitive markets. Further, our competitors may be more effective at using their technologies to develop commercial products. Many of the organizations competing with us have greater capital resources, larger research and development staff and facilities, more experience in obtaining regulatory approvals and more extensive product manufacturing and marketing capabilities. As a result, our competitors may be able to more easily develop products that would render any products that we or our collaborators develop obsolete and noncompetitive. For example, dapagliflozin, empagliflozin and canagliflozin are currently being marketed by AstraZeneca, Boehringer Ingelheim and Eli Lilly, and Janssen (a subsidiary of Johnson & Johnson), respectively, for the treatment of type 2 diabetes and certain other indications, such as heart failure, cardiovascular death, major adverse cardiovascular events and end-stage kidney disease. Each of those products act through SGLT2, one of the targets of sotagliflozin. We expect that our principal competition for sotagliflozin for the treatment of heart failure would include such selective SGLT2 inhibitors which have already received or may gain regulatory approval for the treatment of heart failure, as well as angiotensin-converting enzyme, or ACE, inhibitors and the combination drug sacubitril/valsartan, currently marketed for the treatment of heart failure by Novartis. In addition, there may be drug candidates of which we are not aware at an earlier stage of development that may compete with our drug candidates.
We face business disruption and related risks resulting from the outbreak of the novel coronavirus, or COVID-19, including delays in the enrollment of ongoing clinical trials and other operational impacts, each of which could have a material adverse effect on our business.
Our business has been disrupted and could be materially adversely affected by the COVID-19 pandemic. In response to the ongoing pandemic and resulting state and local restrictions, we implemented work-from-home policies for all employees except certain key essential members involved in business-critical activities. The effects of our work-from-home policies may negatively impact productivity, disrupt our business and delay our ongoing research and development efforts, the magnitude of which will depend, in part, on the length and severity of the pandemic and other limitations on our ability to conduct our business in the ordinary course.
Clinical site initiation and patient enrollment may be delayed due to concerns for patient safety and prioritization of healthcare resources toward the COVID-19 pandemic. Some patients may not be able to comply with clinical trial protocols if quarantines impede patient movement or interrupt healthcare services. Similarly, our ability to recruit and retain patients, principal investigators and site staff (who as healthcare providers may have heightened exposure to COVID-19) may be hindered, which would adversely impact our clinical trial operations. For example, due to the impact of the COVID-19
23
pandemic, we delayed initiation of our Phase 2 clinical trials of LX9211 and enrollment in such trials continue to be negatively affected by the pandemic. In addition, the pandemic could cause significant disruption in the operations of third party manufacturers and research and development organizations upon whom we rely and, as a result, could severely impact our business operations. These and similar, and perhaps more severe, disruptions in our operations due to the COVID-19 pandemic could negatively impact our business, operating results and financial condition.
While the potential economic impact brought by, and the duration of, the COVID-19 pandemic may be difficult to assess or predict, the pandemic has also resulted in disruption of global financial markets. This disruption, if sustained or recurrent, could make it more difficult for us to access capital, which could in the future negatively affect our liquidity. In addition, a recession or market correction resulting from the spread of COVID-19 could materially affect our business and the value of our common stock. The global COVID-19 pandemic continues to rapidly evolve. The ultimate impact of the COVID-19 pandemic or a similar health pandemic or epidemic is highly uncertain and subject to change. We do not yet know the full extent of potential delays or impacts on our business, our clinical trials, healthcare systems or the global economy as a whole. These effects could have a material impact on our operations.
Risks Related to Our Capital Requirements and Financial Results
We will need additional capital in the future and, if it is unavailable, we will be forced to delay, reduce or eliminate our research and development programs. If additional capital is not available on reasonable terms, we will be forced to obtain funds, if at all, by entering into financing agreements on unattractive terms.
As of December 31, 2020, we had $152.3 million in cash, cash equivalents and investments. We anticipate that our existing capital resources and the cash and revenues we expect to derive from collaborations and other sources will enable us to fund our currently planned operations for at least the next 12 months. However, we caution you that we may generate less cash and revenues or incur expenses more rapidly than we currently anticipate. Our currently planned operations for the next twelve months include the continued nonclinical and clinical development of LX9211, sotagliflozin and our other drug candidates.
Although difficult to accurately predict, the amount of our future capital requirements will be substantial and will depend on many factors, including:
•the timing, progress and results of our clinical trials of LX9211, sotagliflozin and our other drug candidates and our ability to obtain necessary regulatory approvals based on such clinical trials;
•our success in establishing new collaborations and licenses, including for the commercialization of sotagliflozin in heart failure;
•the amount and timing of our research, development and commercialization expenditures;
•the effect of competing programs and products, and of technological and market developments; and
•the filing, maintenance, prosecution, defense and enforcement of patent claims and other intellectual property rights.
If our capital resources are insufficient to meet future capital requirements, we will need to raise additional funds to continue our currently planned operations. Our ability to raise additional capital is dependent on a number of factors, including the market demand for our securities, which itself is subject to a number of pharmaceutical development and business risks and uncertainties, as well as uncertainty that we would be able to raise such additional capital at a price or on terms that are favorable to us. If we raise additional capital by issuing equity securities, our then-existing stockholders will experience dilution and the terms of any new equity securities may have preferences over our common stock. We cannot be certain that additional financing, whether debt or equity, will be available in amounts or on terms acceptable to us, if at all. We may be unable to raise sufficient additional capital on reasonable terms, and if so, we will be forced to delay, reduce or eliminate our clinical development programs or commercialization efforts or obtain funds, if at all, by entering into financing agreements on unattractive terms.
We have a history of net losses, and we expect to continue to incur net losses and may not achieve or maintain profitability.
We have incurred aggregate net losses since our inception, including an aggregate net loss of $49.0 million for the three years ended December 31, 2020. As of December 31, 2020, we had an accumulated deficit of $1.4 billion. Because of the numerous risks and uncertainties associated with successfully developing and commercializing drug products, we are unable to predict the extent of any future losses or whether or when we will become profitable, if at all. The size of our net losses will depend, in part, on the rate of decline or growth in our revenues and on the amount of our expenses. We expect to continue to
24
incur significant expenses over the next several years as we expect to make significant investments in the continued nonclinical and clinical development of LX9211, sotagliflozin and our other drug candidates.
We have derived a substantial portion of our revenues from strategic collaborations and other research and development collaborations and technology licenses. Future revenues from our existing collaborations are uncertain because they depend, to a large degree, on the achievement of milestones and payment of royalties we earn from any products developed or commercialized under the collaborations. Our ability to secure future revenue-generating agreements will depend upon our ability to address the needs of our potential future collaborators and licensees, and to negotiate agreements that we believe are in our long-term best interests. We may determine that our interests are better served by retaining rights to our discoveries and advancing our therapeutic programs to a later stage, which could limit our near-term revenues and increase expenses. Because of these and other factors, our operating results have fluctuated in the past and are likely to do so in the future, and we do not believe that period-to-period comparisons of our operating results are a good indication of our future performance.
We expect to spend significant amounts to fund our planned nonclinical and clinical development of LX9211, sotagliflozin and our other drug candidates. As a result, we will need to generate substantial additional revenues to achieve profitability in future periods. Even if we do achieve profitability in future periods, we may not be able to sustain or increase such profitability on a quarterly or annual basis.
Our operating results have fluctuated and likely will continue to fluctuate, and we believe that period-to-period comparisons of our operating results are not a good indication of our future performance.
Our operating results have fluctuated in the past and are likely to fluctuate in the future. A number of factors, many of which we cannot control, could subject our operating results to volatility, including:
•the success of our ongoing nonclinical and clinical development efforts and our ability to obtain regulatory approval of our drug candidates as a result of such efforts;
•the timing and amount of expenses incurred with respect to our nonclinical and clinical development efforts;
•our success in establishing new collaborations and technology licenses, including for the commercialization of sotagliflozin in heart failure, and the timing and financial terms of such arrangements;
•the timing and willingness of our collaborators to commercialize pharmaceutical products that would result in milestone payments and royalties;
•disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our products and technologies; and
•general and industry-specific economic conditions, which may affect our and our collaborators’ research and development expenditures.
Because of these and other factors, including the risks and uncertainties described in this section, our operating results have fluctuated in the past and are likely to do so in the future. Due to the likelihood of fluctuations in our revenues and expenses, we believe that period-to-period comparisons of our operating results are not a good indication of our future performance.
We have indebtedness that may limit cash flow available to invest in the ongoing needs of our business.
We have incurred $11.6 million of indebtedness and we could in the future incur additional indebtedness beyond such amount. Our debt combined with our other financial obligations and contractual commitments could have significant adverse consequences, including:
•requiring us to dedicate a significant portion of cash flow from operations to the payment of interest on, and principal of, our debt, which will reduce the amounts available to fund our research and development efforts and other general corporate purposes;
•increasing our vulnerability to adverse changes in general economic, industry and market conditions; and
25
•limiting our flexibility in planning for, or reacting to, changes in our business and the industry in which we compete.
We intend to satisfy our current and future debt service obligations with our existing cash and cash equivalents and marketable securities and funds from external sources. However, we may not have sufficient funds or may be unable to arrange for additional financing to pay the amounts due under our existing debt. Funds from external sources may not be available on acceptable terms, if at all.
Risks Related to Our Relationships with Third Parties
We depend on our ability to establish a strategic collaboration for the commercialization of sotagliflozin in heart failure. If we are unable to establish such a collaboration, sotagliflozin may not be commercialized in heart failure, our business will suffer and our stock price will likely decline.
Our existing resources and infrastructure are insufficient for the commercialization of sotagliflozin for heart failure. Although we seek to collaborate with another pharmaceutical or biotechnology company or companies under terms which would enable reliance on their resources and infrastructure, in whole or in part, for those commercialization efforts and provide additional funding to support our own activities in support of such efforts, including seeking regulatory approvals, we may be unable to successfully enter into any such collaborative arrangement on reasonable terms, or at all. If we are unable to enter into any such collaborative arrangement, or if we are otherwise unable to raise sufficient additional capital, we will minimally require substantial additional resources to pursue and may elect or be forced to forego pursuit of regulatory approval for and commercialization of sotagliflozin in heart failure.
We depend on our ability to establish collaborations with pharmaceutical and biotechnology companies for the development and commercialization of our other drug candidates. If we are unable to establish such collaborations, or if pharmaceutical products are not successfully and timely developed and commercialized under such collaborations, our opportunities to generate revenues from our other drug candidates will be greatly reduced.
We have derived a substantial majority of our revenues to date from collaborative arrangements with other pharmaceutical and biotechnology companies for the research, development and commercialization of our drug candidates and other research and development collaborations and technology licenses. Future revenues from our existing and future collaborations depend upon the achievement of milestones and payment of royalties we earn from any future products developed under those arrangements. If our relationship terminates with any collaborator, as occurred with our collaboration with Sanofi, our reputation in the business and scientific community may suffer and revenues will be negatively impacted to the extent such losses are not offset by additional collaborations or strategic alliances. If milestones are not achieved or our collaborators are unable to successfully develop and commercialize products from which milestones and royalties are payable, we will not earn the revenues contemplated by those arrangements.
We have limited or no control over the resources that any third party may devote to the development and commercialization of products under our collaborations. Any of our present or future collaborators may not perform their obligations as expected. Our collaborators may breach or terminate their agreements with us or otherwise fail to conduct research, development or commercialization activities successfully or in a timely manner. Further, our collaborators may elect not to develop pharmaceutical products arising out of our arrangements or may not devote sufficient resources to the development, regulatory approval, manufacture, marketing or sale of these products. If any of these events occurs, we may not receive revenue or otherwise realize anticipated benefits from such collaborations, our product development efforts may be delayed and our business, operating results and financial condition could be adversely affected.
Conflicts with our collaborators could jeopardize the success of our collaborative agreements and harm our product development efforts.
We may pursue opportunities in specific disease and therapeutic modality fields that could result in conflicts with our collaborators, if any of our collaborators takes the position that our internal activities overlap with those activities that are exclusive to our collaboration. Moreover, disagreements could arise with our collaborators over rights to our intellectual property or our rights to share in any of the future revenues of compounds or therapeutic approaches developed by our collaborators. Any conflict with or among our collaborators could result in the termination of our collaborative agreements, delay collaborative research or development activities, impair our ability to renew or obtain future collaborative agreements or lead to costly and time consuming litigation. Conflicts with our collaborators could also have a negative impact on our relationship with existing collaborators, materially impairing our business and revenues. Some of our collaborators are also potential competitors or may become competitors in the future. Our collaborators could develop competing products, preclude
26
us from entering into collaborations with their competitors or terminate their agreements with us prematurely. Any of these events could harm our product development efforts.
We rely on third parties to carry out our nonclinical studies and clinical trials, which may harm or delay our research and development efforts.
We rely on clinical research organizations and other third-party contractors to carry out many of our drug development activities, including the performance of nonclinical laboratory and animal tests under the FDA’s current Good Laboratory Practices regulations and the conduct of clinical trials of our drug candidates in accordance with protocols we establish. If these third parties do not successfully carry out their contractual duties or regulatory obligations or meet expected deadlines, our drug development activities may be delayed, suspended or terminated. Such a failure by these third parties could significantly impair our ability to develop and commercialize the affected drug candidates.
We lack the capability to manufacture materials for nonclinical studies, clinical trials and rely on third parties to manufacture our drug candidates, which may harm or delay our research and development efforts.
We currently do not have the manufacturing capabilities or experience necessary to produce materials for nonclinical studies or clinical trials and intend in the future to continue to rely on collaborators and third-party contractors to produce such materials. We will rely on selected manufacturers to deliver materials on a timely basis and to comply with applicable regulatory requirements, including the current Good Manufacturing Practices of the FDA, which relate to manufacturing and quality control activities. These manufacturers may not be able to produce material on a timely basis or manufacture material at the quality level or in the quantity required to meet our development timelines and applicable regulatory requirements. In addition, there are a limited number of manufacturers that operate under the FDA’s current Good Manufacturing Practices and that are capable of producing such materials, and we may experience difficulty finding manufacturers with adequate capacity for our needs. If we are unable to contract for the production of sufficient quantity and quality of materials on acceptable terms, our product development efforts may be delayed. Moreover, noncompliance with the FDA’s current Good Manufacturing Practices can result in, among other things, fines, injunctions, civil and criminal penalties, product recalls or seizures, suspension of production, failure to obtain marketing approval and withdrawal, suspension or revocation of marketing approvals.
Risks Related to Our Intellectual Property
If we are unable to adequately protect our intellectual property, third parties may be able to use our products and technologies, which could adversely affect our ability to compete in the market.
Our commercial success will depend in part upon our ability to obtain patents and maintain adequate protection of the intellectual property related to our products and technologies. The patent positions of biotechnology and pharmaceutical companies, including our patent position, are generally uncertain and involve complex legal and factual questions. We will be able to protect our intellectual property rights from unauthorized use by third parties only to the extent that our products and technologies are covered by valid and enforceable patents or other intellectual property rights, or are effectively maintained as trade secrets or otherwise protected from disclosure by non-disclosure agreements. We will continue to apply for patents covering our products and technologies as, where and when we deem appropriate. However, pending patent applications do not provide protection against competitors because they are not enforceable until they issue as patents. Further, the disclosures contained in our current and future patent applications may not be sufficient to meet statutory requirements for patentability and our applications may fail to result in issued patents. Once issued, patents still may not provide commercially meaningful protection. Our existing patents and any future patents we obtain may not be sufficiently broad to prevent others from developing competing products and technologies. Furthermore, others may independently develop similar or alternative products or technologies or design around our patents. If anyone infringes upon our or our collaborators’ patent rights, enforcing these rights may be difficult, costly and time-consuming and, as a result, it may not be cost-effective or otherwise expedient to pursue litigation to enforce those patent rights. Further, as we customarily assess whether to apply for new patents based on our ongoing research and development activities, this assessment and the filing for additional patent protection may require significant expenditures and therefore may not be commercially practicable.
Our patents and other intellectual property rights may be challenged by third parties and may be invalidated, cancelled or held unenforceable under U.S. or foreign laws, or they may be infringed or misappropriated by third parties. As a result, we may be involved in the defense and enforcement of our patent or other intellectual property rights in a court of law, U.S. Patent and Trademark Office inter partes review or reexamination proceeding, foreign opposition proceeding or related legal and administrative proceeding in the United States and elsewhere. The costs of defending our patents or enforcing our other intellectual property rights, such as trademarks and trade secrets, in post-issuance administrative proceedings and litigation may
27
be substantial and the outcome can be uncertain. An adverse outcome may allow third parties to use our intellectual property without a license and negatively impact our business.
In addition, because patent applications can take many years to issue, third parties may have pending applications, unknown to us, which may later result in issued patents that cover the production, manufacture, commercialization or use of our products and drug candidates. If any such patents are issued to other entities, we may be unable to obtain patent protection for the same or similar discoveries that we make relating to our products and drug candidates. Moreover, we may be blocked from using our drug targets or drug candidates or developing or commercializing our products and other drug candidates, or may be required to obtain a license from a third party that may not be available on reasonable terms, if at all. Further, others may discover uses for our products and technology other than those covered in or claimed by our issued or pending patents, such as other uses for our drug targets and drug candidates, and these other uses may be separately patentable. Even if we have a patent claim on a particular technology or product, the holder of a patent covering the use of a similar technology or product could exclude us from selling a product that is based on the same use of that product.
The laws of some foreign countries do not protect intellectual property rights to the same extent as the laws of the United States, and many companies have encountered significant problems in protecting and defending such rights in foreign jurisdictions. Many countries, including certain countries in Europe, have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties (for example, if the patent owner has failed to “work” the invention in that country or the third party has patented improvements). In addition, many countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of the patent. Compulsory licensing of life-saving drugs is also becoming increasingly popular in developing countries either through direct legislation or international initiatives. Such compulsory licenses could be extended to include some of our products and drug candidates, which could limit our potential revenue opportunities. Moreover, the legal systems of certain countries, particularly certain developing countries, do not favor the aggressive enforcement of patent and other intellectual property protection, which makes it difficult to stop infringement and misappropriation.
We rely on trade secret protection for some of our confidential and proprietary information. We have taken security measures to protect our proprietary information and trade secrets, but these measures may not provide adequate protection. While we seek to protect our proprietary information by entering into confidentiality agreements with employees, collaborators and consultants, we cannot assure you that our proprietary information will not be disclosed, or that we can meaningfully protect our trade secrets. In addition, our competitors may independently develop or duplicate substantially equivalent proprietary information or may otherwise gain access to or misappropriate our trade secrets. For example, publicly available information, such as information in issued patents, published patent applications and scientific literature, can be used by third parties to independently develop technology and we cannot provide assurance that any such independently developed technology will not be equivalent or superior to our proprietary technology.
We rely on registered trademarks to protect our investment in our brand and goodwill. However, competitors may challenge the validity of those trademarks and other brand names in which we have invested or may invest. Such challenges can be expensive and may adversely affect our ability to maintain the goodwill gained in connection with a particular trademark.
We may be involved in patent litigation and other disputes regarding intellectual property rights and may require licenses from third parties for our planned nonclinical and clinical development and commercialization activities. We may not prevail in any such litigation or other dispute or be able to obtain required licenses.
Our products and those of our collaborators, as well as our nonclinical and clinical development efforts, may give rise to claims that they infringe or misappropriate the patents or other intellectual property rights of others. We are aware that other companies and institutions are developing products acting through the same drug targets through which some of our drug candidates currently in clinical development act, have conducted research on many of the same targets that we have identified and have filed patent applications potentially covering drug targets that we have identified and certain therapeutic products addressing such targets. In some cases, patents have issued, and may issue in the future, from these applications. In addition, many companies and institutions have well-established patent portfolios directed to common techniques, methods and means of developing, producing and manufacturing pharmaceutical products. These or other companies or institutions could bring legal actions against us or our collaborators for damages or to stop us or our collaborators from engaging in certain nonclinical or clinical development activities or from manufacturing and marketing therapeutic products that allegedly infringe their patent rights. If any of these actions are successful, in addition to our potential liability for damages, these entities may require us or our collaborators to obtain a license in order to continue engaging in the infringing activities or to manufacture or market the infringing therapeutic products or may force us to terminate such activities or manufacturing and marketing efforts.
28
We may deem it advisable to pursue litigation or other dispute resolution proceedings against others to enforce our patents and intellectual property rights and may be the subject of litigation brought by third parties to enforce their patent and intellectual property rights. In addition, we may become involved in litigation or other dispute resolution proceedings based on intellectual property indemnification undertakings that we have given to certain of our collaborators. Patent and other intellectual property litigation is expensive and requires substantial amounts of management attention. The eventual outcome of any such litigation or dispute resolution proceedings is uncertain and involves substantial risks. If we are sued for infringement or misappropriation and lose, we could be required to pay substantial damages and/or be enjoined from using or selling the allegedly infringing or misappropriation products or technology. The results or costs of any such litigation or dispute resolution proceedings may have an adverse effect on our business, operating results and financial condition.
We believe that there will continue to be significant litigation in our industry regarding patent and other intellectual property rights. We have expended and many of our competitors have expended and are continuing to expend significant amounts of time, money and management resources on intellectual property litigation. If we become involved in future intellectual property litigation, it could consume a substantial portion of our resources and could negatively affect our results of operations.
Data breaches and cyber-attacks could compromise our intellectual property or other sensitive information and cause significant damage to our business, reputational harm and financial loss.
In the ordinary course of our business, we collect, maintain and transmit sensitive data on our networks and systems, including our intellectual property and proprietary or confidential business information (such as research data and personal information) and confidential information with respect to our customers, clinical trial patients and our business partners. We have also outsourced significant elements of our information technology infrastructure and, as a result, third parties may or could have access to our confidential information and personal data. The secure maintenance of this information is critical to our business and reputation. We believe that companies have been increasingly subject to a wide variety of security incidents, cyber-attacks and other attempts to gain unauthorized access and unintentional breaches. These threats can come from a variety of sources, ranging in sophistication from an individual hacker to a state-sponsored attack and motive (including corporate espionage). Cyber threats may be generic, or they may be custom-crafted against our information systems. Our network and storage applications and those of our vendors may be subject to unauthorized access by hackers or information security breaches due to operator error, malfeasance or other system disruptions. It is often difficult to anticipate or immediately detect such incidents and the damage caused by such incidents, particularly for cyber incidents such as advanced persistent threats. These data breaches and any unauthorized access or disclosure of our information or intellectual property could compromise our intellectual property and expose sensitive business information. A data security breach could also lead to public exposure of personal information of our clinical trial patients, customers and others. Cyber-attacks and information security breaches could cause us to incur significant remediation costs, result in product development delays, disrupt key business operations and divert attention of management and key information technology resources. Our network security and data recovery measures and those of our vendors may not be able to detect or prevent every attempted breach and may not permit us to respond effectively to every breach. These incidents could also subject us to liability, expose us to significant expense and cause significant harm to our reputation and business. Reputational harm resulting from a significant cyber incident may cause unquantifiable damage to our established goodwill. Moreover, as cyber incidents continue to evolve, we will likely be required to expend additional resources to enhance our security posture and cybersecurity defenses or to investigate and remediate any vulnerability to or consequences of cyber incidents. Our insurance coverage may not be sufficient to prevent or recover from cyberattacks, including coverage of applicable resulting losses arising from the incident.
Further, each foreign jurisdiction and U.S. state in which we operate may have laws governing how we must respond to a cyber incident that results in the unauthorized access, disclosure, or loss of personal information. Additionally, new laws and regulations governing data privacy and unauthorized disclosure of confidential information, including recent California legislation providing for a private right of action, pose increasingly complex compliance challenges and could potentially elevate our costs over time. As legislation continues to develop and cyber incidents continue to evolve, we will likely be required to expend significant resources to continue to modify or enhance our protective measures to comply with such legislation and to detect, investigate and remediate vulnerabilities to cyber incidents. Any failure by us to comply with such laws and regulations could result in reputational harm, loss of goodwill, penalties, liabilities and/or mandated changes in our business practices.
We may be subject to damages resulting from claims that we, our employees or independent contractors have wrongfully used or disclosed alleged trade secrets of their former employers.
Many of our employees and independent contractors were previously employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. We may be subject to claims that these
29
employees, independent contractors or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation or other dispute resolution proceedings may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation or other dispute resolution proceedings could result in substantial costs and divert management’s attention. If we fail in defending such claims, in addition to paying money claims, we may lose valuable intellectual property rights or personnel. A loss of key research personnel and/or their work product could hamper or prevent our ability to commercialize certain drug candidates, which could severely harm our business.
Risks Related to Our Employees and Facilities
If we are unable to manage our growth, our business, financial condition, results of operations and prospects may be adversely affected.
We may experience growth in the number of our employees and in the scope of our operations. This growth places significant demands on our management, operational and financial resources, and our current and planned personnel, systems, procedures and controls may not be adequate to support our growth. To effectively manage our potential growth, we must continue to improve existing, and implement new, operational and financial systems, procedures and controls and must expand, train and manage our growing employee base, and there can be no assurance that we will effectively manage our growth without experiencing operating inefficiencies or control deficiencies. We may need to increase our medical, clinical, commercial and other personnel, and recruiting and retaining qualified individuals is difficult. If we are unable to manage our growth effectively, or are unsuccessful in recruiting qualified personnel when advisable, our business, financial condition, results of operations and prospects may be adversely affected.
The loss of key personnel or the inability to attract and retain additional personnel could impair our ability to operate and expand our operations.
We are highly dependent upon the principal members of our management, as well as medical and clinical staff, the loss of whose services might adversely impact the achievement of our objectives. Retaining and, where advisable, recruiting qualified medical and clinical personnel are critical to advancing our nonclinical and clinical development programs for LX9211, sotagliflozin and our other drug candidates. Competition is intense for experienced medical and clinical personnel, and we may be unable to retain or recruit such personnel with the expertise or experience necessary to allow us to successfully develop and commercialize our products. Further, all of our employees are employed “at will” and, therefore, may leave our employment at any time.
Our facilities are located near coastal zones, and the occurrence of a hurricane or other disaster could damage our facilities and equipment, which could harm our operations.
Our facilities are located in The Woodlands, Texas and Basking Ridge, New Jersey, and therefore our facilities are vulnerable to damage from hurricanes. We are also vulnerable to damage from other types of disasters, including fire, floods, power loss, communications failures, terrorism and similar events and any insurance we may maintain may not be adequate to cover our losses. If any disaster were to occur, our ability to operate our business at our facilities could be seriously, or potentially completely, impaired.
Risks Related to Environmental and Product Liability
We have used hazardous chemicals and radioactive and biological substances in our business. Any claims relating to improper handling, storage or disposal of these substances could be time consuming and costly.
Our research and development processes have historically involved the controlled use of hazardous substances, including chemicals and radioactive and biological materials, and our operations have produced hazardous waste products. See “Part I, Item 1. Business – Government Regulation – Environmental Matters” for more discussion on these and other environmental matters. Compliance with environmental laws and regulations may be expensive, and current or future environmental regulations may impair our research, development and production efforts.
In addition, our collaborators may use hazardous materials in connection with our collaborative efforts. In the event of a lawsuit or investigation, we could be held responsible for any injury caused to persons or property by exposure to, or release of, these hazardous materials used by these parties. Further, we may be required to indemnify our collaborators against all damages and other liabilities arising out of our development activities or products produced in connection with these collaborations.
30
Our business has a substantial risk of product liability and we face potential product liability exposure far in excess of our limited insurance coverage.
We may be held liable if any product that we or our collaborators develop or commercialize, or any product that is made with the use or incorporation of any of our technologies, causes injury or is found otherwise unsuitable during product testing, manufacturing, marketing or sale. Regardless of merit or eventual outcome, product liability claims could result in decreased demand for our products and product candidates, injury to our reputation, withdrawal of patients from our clinical trials, product recall, substantial monetary awards to third parties and the inability to commercialize any products that we may develop. These claims might be made directly by consumers, health care providers, pharmaceutical companies or others selling or testing our products. We have obtained limited product liability insurance coverage for our clinical trials. However, our insurance may not reimburse us or may not be sufficient to reimburse us for expenses or losses we may suffer. Moreover, if insurance coverage becomes more expensive, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to liability. On occasion, juries have awarded large judgments in class action lawsuits for claims based on drugs that had unanticipated side effects. In addition, the pharmaceutical and biotechnology industries, in general, have been subject to significant medical malpractice litigation. A successful product liability claim or series of claims brought against us could harm our reputation and business.
Risks Related to Our Common Stock
Invus, L.P. and its affiliates own a controlling interest in our outstanding common stock and may have interests which conflict with those of our other stockholders.
Invus, L.P. and its affiliates, which we collectively refer to as Invus, currently own approximately 52.3% of the outstanding shares of our common stock and are thereby able to control the election and removal of our directors and determine our corporate and management policies, including potential mergers or acquisitions, asset sales, the amendment of our articles of incorporation or bylaws and other significant corporate transactions. This concentration of ownership may delay or deter possible changes in control of our company, which may reduce the value of an investment in our common stock. The interests of Invus and its affiliates may not be aligned with the interests of other holders of our common stock.
Invus has additional rights under its stockholders’ agreement relating to the membership of our board of directors, which provides Invus with substantial influence over significant corporate matters.
Under its stockholders’ agreement, Invus has the right to designate a number of directors equal to the percentage of all the outstanding shares of our common stock owned by Invus and its affiliates, rounded up to the nearest whole number of directors. Invus has designated three of the nine current members of our board of directors. While Invus has not presently exercised its director designation rights in full, it may exercise them at any time in the future in its sole discretion. To facilitate the exercise of such rights, we have agreed, upon written request from Invus, to take all necessary steps in accordance with our obligations under the stockholders’ agreement to (1) increase the number of directors to the number specified by Invus (which number shall be no greater than reasonably necessary for the exercise of Invus’ director designation rights under the stockholders’ agreement) and (2) cause the appointment to the newly created directorships of directors so designated by Invus pursuant to its rights under the stockholders’ agreement. Invus also has the right to require proportionate representation of Invus-appointed directors on the audit, compensation and corporate governance committees of our board of directors, subject to certain restrictions. Invus-designated directors currently serve as one of the three members of each of the compensation committee and the corporate governance committee of our board of directors, and no Invus-designated directors currently serve on the audit committee of our board of directors. These rights provide Invus with substantial influence over significant corporate matters and Invus’ interest in those matters may not be aligned with the interests of other holders of our common stock.
Furthermore, in December 2020, we entered into a subscription agreement with an affiliate of Invus and certain other institutional investors. Pursuant to the subscription agreement, we agreed to take all necessary steps to (a) include and recommend the amendment to our certificate of incorporation described below in the proxy statement to be sent to stockholders in advance of our next annual meeting of stockholders (and subsequent meetings of stockholders, if necessary) and (b) solicit from eligible stockholders (including, if necessary, prior to subsequent meetings) proxies in favor of such amendment. The amendment to our certificate of incorporation would grant holders of 20% or more of our issued and outstanding common stock (y) customary preemptive rights and (z) consent rights prior to us taking any of the following actions: (i) creating or issuing any new class or series of shares of capital stock (or securities convertible into or exercisable for shares of capital stock) having rights, preferences or privileges senior to or on parity with the common stock, (ii) subject to certain exceptions, repurchasing, retiring, redeeming or otherwise acquiring any equity securities (or securities convertible into or exchangeable for equity
31
securities) or any subsidiary and (iii) adopting, or proposing to adopt, or maintaining any shareholders’ rights plan, “poison pill” or other similar plan or agreement, unless such stockholder is exempt from such plan or agreement. Because Invus owns a majority of our outstanding common stock, the amendment to our certificate of incorporation will be adopted and Invus will possess such rights if Invus votes to ratify and approve the amendment.
Our stock price may be extremely volatile.
The trading price of our common stock has been highly volatile, and we believe the trading price of our common stock will remain highly volatile and may fluctuate substantially due to factors such as the following, many of which we cannot control:
•results or delays in our or our collaborators’ clinical trials;
•the announcement of FDA approval or non-approval, or delays in the FDA review process, of our or our collaborators’ drug candidates or those of our competitors or actions taken by regulatory agencies with respect to our, our collaborators’ or our competitors’ clinical trials;
•actions taken by regulatory agencies with respect to LX9211, sotagliflozin and our other drug candidates;
•the announcement of new products by our competitors;
•quarterly variations in our or our competitors’ results of operations;
•developments in our relationships with our collaborators, including conflicts, litigation or the termination or modification of our agreements;
•the announcement of an in-licensed drug candidate or strategic acquisition;
•litigation, including intellectual property infringement and misappropriation, and product liability lawsuits, involving us;
•failure to achieve operating results projected by securities analysts;
•changes in earnings estimates or recommendations by securities analysts;
•the satisfaction of outstanding debt obligations or entry into new financing arrangements;
•developments in the biotechnology or pharmaceutical industry;
•sales of large blocks of our common stock or sales of our common stock by our executive officers, directors and significant stockholders;
•departures of key personnel or board members;
•FDA or international regulatory actions;
•third-party coverage and reimbursement policies;
•disposition of any of our drug programs or other technologies; and
•other factors, including general market, economic and political conditions and other factors unrelated to our operating performance or the operating performance of our competitors.
These factors may materially adversely affect the market price of our common stock. In addition, the stock markets in general, and the markets for biotechnology and pharmaceutical stocks in particular, have historically experienced significant volatility that has often been unrelated or disproportionate to the operating performance of particular companies. For example, negative publicity regarding drug pricing and price increases by pharmaceutical companies has negatively impacted, and may continue to negatively impact, the markets for biotechnology and pharmaceutical stocks. Likewise, the broader financial markets could experience significant volatility that could also negatively impact the markets for biotechnology and
32
pharmaceutical stocks. These broad market fluctuations have adversely affected and may in the future adversely affect the trading price of our common stock. Excessive volatility may continue for an extended period of time.
In the past, following periods of volatility in the market price of a company’s securities, securities class action litigation has often been instituted. A securities class action suit against us, such as the securities litigation which is currently pending, could result in substantial costs and divert management’s attention and resources, which could have a material and adverse effect on our business.
We are subject to securities litigation, which is expensive and could divert management attention.
Companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation and we are currently a target of this type of litigation. On January 28, 2019, a purported securities class action complaint captioned Daniel Manopla v. Lexicon Pharmaceuticals, Inc., Lonnel Coats, Jeffrey L. Wade and Pablo Lapuerta, M.D. was filed against us, and certain of our officers in the U.S. District Court for the Southern District of Texas, Houston Division. Our motion to dismiss was granted and the action was dismissed with prejudice by the District Court on August 14, 2020. The lead plaintiffs filed a notice of appeal to the U.S. Court of Appeals for the Fifth Circuit on September 11, 2020 and a brief in support of its appeal on December 17, 2020. We filed a response brief on February 18, 2021. The lawsuit purports to be a class action brought on behalf of purchasers of our securities during the period from March 11, 2016 through January 17, 2019. The complaint alleges that the defendants violated federal securities laws by making materially false and misleading statements and/or omissions concerning data from our Phase 3 clinical trials of sotagliflozin in type 1 diabetes patients and the prospects of FDA approval of sotagliflozin for the treatment of type 1 diabetes. The complaint purports to assert claims for violations of Sections 10(b) and 20(a) of the Securities Exchange Act of 1934 and Rule 10b-5 promulgated thereunder. The complaint seeks, on behalf of the purported class, an unspecified amount of monetary damages, interest, fees and expenses of attorneys and experts, and other relief. This case, and other litigation of this type, could result in substantial costs and diversion of management’s attention and resources, which could adversely impact our business. Any adverse determination in litigation could also subject us to significant liabilities.
Future issuances or sales of our common stock, or the perception that such issuances or sales may occur, may depress our stock price.
A substantial number of shares of our common stock is reserved for issuance upon conversion of notes evidencing our current indebtedness, upon the exercise of stock options and upon vesting of restricted stock units. If we or our stockholders issue or sell substantial amounts of our common stock (including shares issued upon the conversion of notes, exercise of stock options or vesting of restricted stock units) in the public market, or if the market perceives that such sales may occur, the market price of our common stock could fall and it may become more difficult for us to sell equity or equity-related securities in the future at a time and price that we deem appropriate. In addition, any such issuance or sale of our common stock will dilute the ownership interests of existing stockholders and may cause the market price of our common stock to decline.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our stock price and trading volume could decline.
The trading market for our common stock will depend in part on the research and reports that securities or industry analysts publish about us or our business. If one or more of the analysts who cover us downgrade our stock or publish inaccurate or unfavorable research about our business, our stock price would likely decline. If one or more of these analysts cease coverage of our company or fail to publish reports on us regularly, demand for our common stock could decrease, which might cause our stock price and trading volume to decline.
Item 1B. Unresolved Staff Comments
None.
Item 2. Properties
In December 2020, our subsidiary, Lex-Gen Woodlands, L.P., sold our facilities in The Woodlands, Texas for $11.9 million. Concurrent with such sale, we entered into a leaseback agreement with respect to 38,000 square feet of such facilities for a period of up to six months, with monthly gross rent payments of $101,000. In February 2021, we leased a 25,000 square-foot office space in The Woodlands, Texas to which we plan to relocate our corporate offices. The term of the sublease extends from March 1, 2021 through August 31, 2025, and provides for escalating yearly base rent payments starting at $506,000 and increasing to $557,000 in the final year of the lease.
33
In March 2015, our subsidiary, Lexicon Pharmaceuticals (New Jersey), Inc., leased a 25,000 square-foot office space in Basking Ridge, New Jersey. The term of the lease extends from June 1, 2015 through December 31, 2022, and provides for escalating yearly base rent payments starting at $482,000 and increasing to $646,000 in the final year of the lease.
We believe that our facilities are well-maintained, in good operating condition and acceptable for our current operations.
Item 3. Legal Proceedings
On January 28, 2019, a purported securities class action complaint captioned Daniel Manopla v. Lexicon Pharmaceuticals, Inc., Lonnel Coats, Jeffrey L. Wade and Pablo Lapuerta, M.D. was filed against us and certain of our officers in the U.S. District Court for the Southern District of Texas, Houston Division. Our motion to dismiss was granted and the action was dismissed with prejudice by the District Court on August 14, 2020. The lead plaintiffs filed a notice of appeal to the U.S. Court of Appeals for the Fifth Circuit on September 11, 2020 and a brief in support of its appeal on December 17, 2020. We filed a response brief on February 18, 2021 and the lead plaintiffs filed a reply brief on March 11, 2021. The lawsuit purports to be a class action brought on behalf of purchasers of our securities during the period from March 11, 2016 through July 29, 2019. The complaint alleges that the defendants violated federal securities laws by making materially false and misleading statements and/or omissions concerning data from our Phase 3 clinical trials of sotagliflozin in type 1 diabetes patients and the prospects of FDA approval of sotagliflozin for the treatment of type 1 diabetes. The complaint purports to assert claims for violations of Sections 10(b) and 20(a) of the Securities Exchange Act of 1934 and Rule 10b-5 promulgated thereunder. The complaint seeks, on behalf of the purported class, an unspecified amount of monetary damages, interest, fees and expenses of attorneys and experts, and other relief.
On October 16, 2020, we initiated arbitration proceedings against Sanofi seeking to recover damages for breach of contract relating to the Termination and Settlement Agreement and Mutual Releases with Sanofi, dated September 9, 2019. In September 2020, Sanofi withheld approximately $23.2 million from the final $26 million payment due to us under the termination and settlement agreement, offsetting certain third party costs and internal costs incurred by Sanofi and asserted by Sanofi to be payable by us under the terms of the termination and settlement agreement. We dispute that at least a significant portion of such costs are properly reimbursable by us under the terms of the termination and settlement agreement and assert that, in any event, Sanofi was not permitted to withhold any of such costs under the terms of the termination and settlement agreement. We are seeking payment of up to $23.2 million in such disputed costs, together with late interest and attorneys’ fees and costs. Sanofi is seeking a declaratory judgment that we are liable for all disputed costs previously withheld and damages for any additional costs properly reimbursable under the terms of the termination and settlement agreement in excess of those previously withheld, together with late interest and attorneys’ fees.
In addition, we are from time to time party to claims and legal proceedings that arise in the normal course of our business and that we believe will not have, individually or in the aggregate, a material adverse effect on our results of operations, financial condition or liquidity.
Item 4. Mine Safety Disclosures
Not applicable.
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Our common stock is quoted on The Nasdaq Global Select Market under the symbol “LXRX.” As of March 6, 2021, there were approximately 327 holders of record of our common stock.
We have never paid cash dividends on our common stock. We anticipate that we will retain all of our future earnings, if any, for use in the expansion and operation of our business and do not anticipate paying cash dividends in the foreseeable future.
Performance Graph
The following performance graph compares the performance of our common stock to the Nasdaq Composite Index and the Nasdaq Biotechnology Index for the period beginning December 31, 2015 and ending December 31, 2020. The graph assumes that the value of the investment in our common stock and each index was $100 at December 31, 2015, and that all dividends were reinvested. The stock performance shown on the graph below represents historical performance and is not necessarily indicative of future stock price performance.
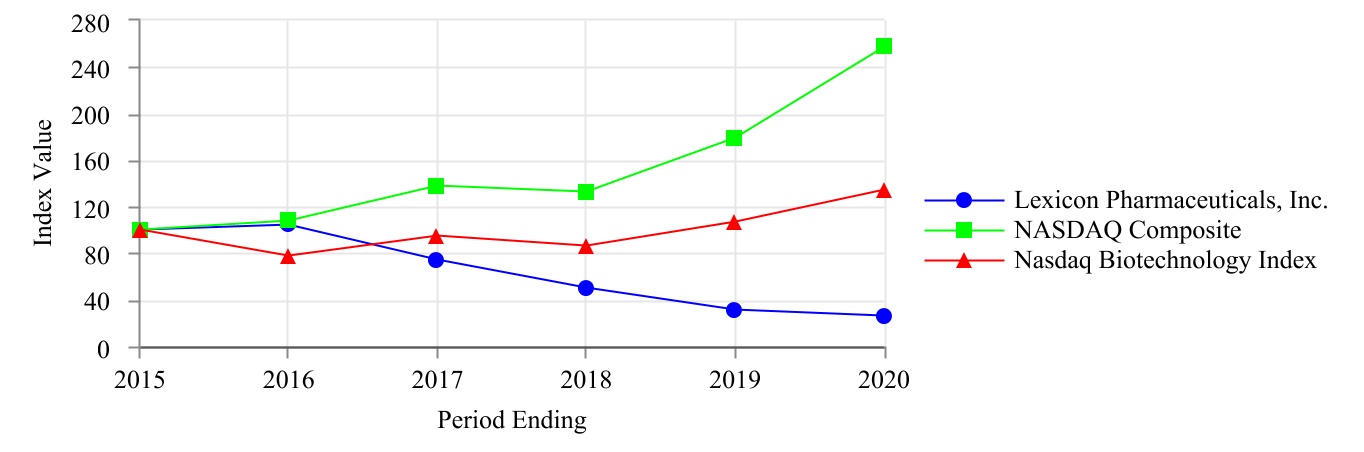
| December 31, | |||||||||||||||||||||||||||||||||||
| 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | ||||||||||||||||||||||||||||||
| Lexicon Pharmaceuticals, Inc. | 100 | 104 | 74 | 50 | 31 | 26 | |||||||||||||||||||||||||||||
| Nasdaq Composite Index | 100 | 108 | 138 | 133 | 179 | 257 | |||||||||||||||||||||||||||||
| Nasdaq Biotechnology Index | 100 | 78 | 95 | 86 | 107 | 134 | |||||||||||||||||||||||||||||
The foregoing stock price performance comparisons shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference by any general statement incorporating by reference this annual report on Form 10-K into any filing under the Securities Act of 1933 or under the Securities Exchange Act of 1934, except to the extent that we specifically incorporate such comparisons by reference.
Item 6. Selected Financial Data
Not applicable.
35
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis should be read with “Selected Financial Data” and our financial statements and notes included elsewhere in this annual report on Form 10-K.
Overview
We are a biopharmaceutical company with a mission of pioneering medicines that transform patients’ lives. We are devoting most of our resources to the research and development of our most advanced drug candidates:
•We are developing LX9211, an orally-delivered small molecule drug candidate, as a treatment for neuropathic pain. We have reported results from two Phase 1 clinical trials of LX9211 and are now conducting a Phase 2 clinical trial of LX9211 in diabetic peripheral neuropathic pain and a second Phase 2 clinical trial of LX9211 in post-herpetic neuralgia. LX9211 has received Fast Track designation from the FDA for development in diabetic peripheral neuropathic pain.
•We are developing sotagliflozin, an orally-delivered small molecule drug candidate, as a treatment for heart failure and type 1 diabetes. We have reported positive results from two Phase 3 clinical trials evaluating the effect of sotagliflozin on long-term outcomes related to cardiovascular death and heart failure in approximately 10,500 and 1,200 patients, respectively. We are now working towards an application for regulatory approval to market sotagliflozin for heart failure and seeking a strategic collaboration for the commercialization of sotagliflozin, if approved.
We have reported positive results from three Phase 3 clinical trials evaluating the effect of sotagliflozin on type 1 diabetes in approximately 800, 800 and 1,400 patients, respectively. The FDA issued a complete response letter regarding our application for regulatory approval to market sotagliflozin for type 1 diabetes in the United States and, at our request, has issued a public Notice of Opportunity for Hearing on whether there are grounds for denying approval of our application. Sotagliflozin has been approved in the European Union for use as an adjunct to insulin therapy in the treatment of type 1 diabetes, but has not yet been commercially launched.
•We are conducting preclinical research and development and preparing to conduct clinical development of compounds from a number of additional drug programs originating from our internal drug discovery efforts.
LX9211 originated from our collaborative neuroscience drug discovery efforts with Bristol-Myers Squibb, and sotagliflozin and compounds from a number of additional drug programs originated from our own internal drug discovery efforts. Those efforts were driven by a systematic, target biology-driven approach in which we used gene knockout technologies and an integrated platform of advanced medical technologies to systematically study the physiological and behavioral functions of almost 5,000 genes in mice and assessed the utility of the proteins encoded by the corresponding human genes as potential drug targets. We have identified and validated in living animals, or in vivo, more than 100 targets with promising profiles for drug discovery.
We are working both independently and through collaborations and strategic alliances with third parties to capitalize on our drug target discoveries and drug discovery and development programs. We seek to retain exclusive or co-exclusive rights to the benefits of certain drug discovery and development programs by developing and commercializing drug candidates from those programs internally, particularly in the United States for indications treated by specialist physicians. We seek to collaborate with other pharmaceutical and biotechnology companies with respect to drug discovery or the development and commercialization of certain of our drug candidates, particularly with respect to commercialization in territories outside the United States or commercialization in the United States for indications treated by primary care physicians, or when the collaboration may otherwise provide us with access to expertise and resources that we do not possess internally or are complementary to our own.
We have derived substantially all of our revenues from strategic collaborations and other research and development collaborations and technology licenses, as well as from commercial sales of our XERMELO product following its commercial launch in February 2017 until our sale of XERMELO and related assets to TerSera in September 2020. To date, we have generated a substantial portion of our revenues from a limited number of sources.
Our operating results and, in particular, our ability to generate additional revenues are dependent on many factors, including the success of our ongoing nonclinical and clinical development efforts and the ability to obtain necessary regulatory approvals of the drug candidates which are the subject of such efforts; TerSera’s ability to successfully develop and commercialize XERMELO for biliary tract cancer and our receipt of milestone payments and royalties from such efforts; our
36
success in establishing new collaborations and licenses, particularly for the commercialization of sotagliflozin for heart failure; and general and industry-specific economic conditions which may affect research and development expenditures.
Future revenues from our sale of XERMELO to TerSera are uncertain because they depend on the achievement of milestones and payment of royalties we earn from TerSera’s development and commercialization of XERMELO in biliary tract cancer. Our ability to secure future revenue-generating agreements will depend upon our ability to address the needs of our potential future collaborators and licensees, and to negotiate agreements that we believe are in our long-term best interests. We may determine, as we have with certain of our drug candidates, that our interests are better served by retaining rights to our discoveries and advancing our therapeutic programs to a later stage, which could limit our near-term revenues and increase expenses. Because of these and other factors, our operating results have fluctuated in the past and are likely to do so in the future, and we do not believe that period-to-period comparisons of our operating results are a good indication of our future performance.
Since our inception, we have incurred significant losses and, as of December 31, 2020, we had an accumulated deficit of $1.4 billion. Our losses have resulted principally from costs incurred in research and development, selling, general and administrative costs associated with our operations, and non-cash stock-based compensation expenses associated with stock options and restricted stock units granted to employees and consultants. Research and development expenses consist primarily of salaries and related personnel costs, external research costs related to our nonclinical and clinical efforts, material costs, facility costs, depreciation on property and equipment, and other expenses related to our drug discovery and development programs. Selling, general and administrative expenses consist primarily of salaries and related expenses for executive, sales and marketing, and administrative personnel, professional fees and other corporate expenses, including information technology, facilities costs and general legal activities. We expect to continue to incur significant research and development costs in connection with the continuing research and development of our drug candidates. As a result, we will need to generate significantly higher revenues to achieve profitability.
Critical Accounting Policies
Revenue Recognition
Product Revenues
Prior to our sale of XERMELO and related assets to TerSera in September 2020, product revenues consisted of commercial sales of XERMELO in the United States and sales of bulk tablets of XERMELO to Ipsen. Product revenues were recognized when the customer obtained control of XERMELO, which occurred upon delivery to the customer. We recognized product revenue net of applicable reserves for variable consideration, including allowances for customer credits, estimated rebates, chargebacks, discounts, returns, distribution service fees, and government rebates, such as Medicare Part D coverage gap reimbursements in the United States, as discussed below. Our estimates were based on the most likely amount method for relevant factors such as current contractual and statutory requirements, industry data and forecasted customer buying and payment patterns. Our net product revenues reflected our best estimates of the amounts of consideration to which we were entitled based on the terms of the respective underlying contracts. Product shipping and handling costs were considered a fulfillment activity when control transferred to our customers and such costs were included in cost of sales.
Customer Credits: Our customers were offered various forms of consideration, including allowances, service fees and prompt payment discounts. We expected that our customers would earn prompt payment discounts. As a result, we deducted the full amount of those discounts from total product sales when revenues were recognized. Service fees were also deducted from product sales as they were earned.
Rebates: Allowances for rebates included mandated discounts under the Medicaid Drug Rebate Program. Rebate amounts were based upon contractual agreements or legal requirements with public sector (e.g., Medicaid) benefit providers. Rebates are amounts owed after the final dispensing of the product to a benefit plan participant and are based upon contractual agreements or legal requirements with public sector benefit providers. The allowance for rebates was based on statutory discount rates and expected utilization. Our estimates for expected utilization of rebates were based on third party market research data and data received from the specialty pharmacies. Rebates were generally invoiced and paid in arrears so that the accrual balance consisted of an estimate of the amount expected to be incurred for the current quarter’s activity, plus an accrual balance for known unpaid rebates from the prior quarter. If actual rebates varied from estimates, we adjusted prior period accruals, which affected revenue in the period of adjustment.
Chargebacks: Chargebacks are discounts that occur when contracted customers purchase directly from a specialty pharmacy or distributor, who acts as a retailer. Contracted customers, which consisted primarily of Public Health Service
37
Institutions, non-profit clinics, and federal government entities purchasing via the Federal Supply Schedule, generally purchase the product at a discounted price. The specialty pharmacy or distributor, in turn, charged back to us the difference between the price paid by the specialty pharmacy or distributor and the discounted price paid to the specialty pharmacy or distributor by the customer. The allowance for chargeback was based on known sales to contracted customers.
Medicare Part D Coverage Gap: The Medicare Part D prescription drug benefit mandates manufacturers to fund a portion of the Medicare Part D insurance coverage gap for prescription drugs sold to eligible patients. Our estimates for the expected Medicare Part D coverage gap were based on data received from the specialty pharmacies and projections based on historical data. Funding of the coverage gap was generally invoiced and paid in arrears so that the accrual balance consisted of an estimate of the amount expected to be incurred for the current quarter’s activity, plus an accrual balance for known prior quarters. If actual funding varied from estimates, we adjusted prior period accruals, which affected revenues in the period of adjustment.
Co-payment assistance: Patients who have commercial insurance and meet certain eligibility requirements may receive co-payment assistance. We accrued a liability for co-payment assistance based on actual program participation and estimates of program redemption using data provided by third-party administrators.
Collaborative Agreements
Revenues under collaborative agreements include both license revenue and contract research revenue. We perform the following five steps in determining the amount of revenue to recognize as we fulfill our performance obligations under each of our agreements: (i) identify the contract(s) with a customer; (ii) identify the performance obligation in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligation in the contract, and (v) recognize revenue when (or as) we satisfy the performance obligation. We apply this five-step model to contracts when it is probable that we will collect the consideration to which we are entitled in exchange for the goods or services we transfer to the customer. At contract inception, we assess the goods or services promised within each contract and determine those that are performance obligations, and assess whether each promised good or service is distinct. We then recognize as revenue the amount of the transaction price that is allocated to the respective performance obligation when (or as) the performance obligation is satisfied. We develop assumptions that require judgment to determine the stand-alone selling price for each performance obligation identified in the contract.
At contract inception, we evaluate whether development milestones are considered probable of being reached and estimate the amount to be included in the transaction price using the most likely amount method. If it is probable that a significant revenue reversal will not occur, the associated development milestone value is included in the transaction price. Development milestones that are not within our control or the control of our licensee, including those requiring regulatory approval, are not considered probable of being achieved until those approvals are received. The transaction price is allocated to each performance obligation on a relative stand-alone selling price basis, for which we recognize revenue when (or as) the performance obligation is satisfied. At the end of each reporting period, we re-evaluate the probability of achievement of the development milestones and any related constraint, and if necessary, adjust our estimates of the overall transaction price. Any such adjustments are recorded on a cumulative catch-up basis, which would affect collaboration revenues in the period of adjustment.
In agreements in which a license to our intellectual property is determined distinct from other performance obligations identified in the agreement, we recognize revenue when the license is transferred to the licensee and the licensee is able to use and benefit from the license.
For agreements that include sales-based royalties, including milestones based on a level of sales, the license is deemed to be the predominant item to which the royalties relate and we recognize revenue at the later of (i) when the related sales occur or (ii) when the performance obligation to which some or all of the royalty has been allocated has been satisfied (or partially satisfied).
We may receive payments from our licensees based on billing schedules established in each contract. Upfront payments and fees are recorded as deferred revenue upon receipt or when due, and may require deferral of revenue recognition to a future period until we perform our obligations under these agreements. Amounts are recorded as accounts receivable when our right to consideration is unconditional.
38
Research and Development Expenses
Research and development expenses consist of costs incurred for research and development activities solely sponsored by us as well as collaborative research and development activities. These costs include direct and research-related overhead expenses and are expensed as incurred. Technology license fees for technologies that are utilized in research and development and have no alternative future use are expensed when incurred.
We are presently devoting most of our resources to the research and development of our most advanced drug candidates:
•LX9211, an orally-delivered small molecule drug candidate, that we are developing as a treatment for neuropathic pain; and
•Sotagliflozin, an orally-delivered small molecule drug candidate that we are developing as a treatment for heart failure and type 1 diabetes.
LX9211 originated from our collaborative neuroscience drug discovery efforts with Bristol-Myers Squibb, and sotagliflozin and compounds from a number of additional drug programs originated from our own internal drug discovery efforts. Those efforts were driven by a systematic, target biology-driven approach in which we used gene knockout technologies and an integrated platform of advanced medical technologies to systematically study the physiological and behavioral functions of almost 5,000 genes in mice and assessed the utility of the proteins encoded by the corresponding human genes as potential drug targets. We have identified and validated in living animals, or in vivo, more than 100 targets with promising profiles for drug discovery.
The drug development process takes many years to complete. The cost and length of time varies due to many factors including the type, complexity and intended use of the drug candidate. We estimate that drug development activities are typically completed over the following periods:
| Phase | Estimated Completion Period | |||||||
| Preclinical development | 1-2 years | |||||||
| Phase 1 clinical trials | 1-2 years | |||||||
| Phase 2 clinical trials | 1-2 years | |||||||
| Phase 3 clinical trials | 2-4 years | |||||||
We expect research and development costs to remain substantial in the future as we continue to fund our nonclinical and clinical development efforts and advance new drug candidates into clinical development. Due to the variability in the length of time necessary for drug development, the uncertainties related to the cost of these activities and ultimate ability to obtain regulatory approval for commercialization, accurate and meaningful estimates of the ultimate costs to bring our drug candidates to market are not available.
We record significant accrued liabilities related to unbilled expenses for products or services that we have received from service providers, specifically related to ongoing nonclinical studies and clinical trials. These costs primarily relate to clinical study management, monitoring, laboratory and analysis costs, drug supplies, toxicology studies and investigator grants. We may have multiple drugs in concurrent nonclinical studies and clinical trials at clinical sites throughout the world. In order to ensure that we have adequately provided for ongoing nonclinical and clinical development costs during the period in which we incur such costs, we maintain accruals to cover these expenses. Substantial portions of our nonclinical studies and clinical trials are performed by third party laboratories, medical centers, contract research organizations and other vendors. For nonclinical studies, we accrue expenses based upon estimated percentage of work completed and the contract milestones remaining. For clinical studies, expenses are accrued based upon the number of patients enrolled and the duration of the study. We monitor patient enrollment, the progress of clinical studies and related activities to the extent possible through internal reviews of data reported to us by the vendors and clinical site visits. Our estimates depend on the timeliness and accuracy of the data provided by our vendors regarding the status of each program and total program spending. We periodically evaluate the estimates to determine if adjustments are necessary or appropriate based on information we receive. Although we use consistent milestones or subject or patient enrollment to drive expense recognition, the assessment of these costs is a subjective process that requires judgment. Upon settlement, these costs may differ materially from the amounts accrued in our consolidated financial statements.
Our estimates of the clinical study costs and costs to transition activities from Sanofi for the development of sotagliflozin for type 2 diabetes, heart failure and chronic kidney disease, as well as the wind down of those activities, were based on estimates of the services to be received and efforts to be expended pursuant to contracts with multiple vendors and the
39
contract research organization that conducted and managed the clinical studies on our behalf. The financial terms of these agreements are subject to negotiation and vary from contract to contract. In accruing the relevant costs, we estimated the time period over which services were to be performed and the level of effort required to complete or wind down each study. Upon completion and settlement, these costs may differ materially from the amounts accrued in our consolidated financial statements.
We record our research and development costs by type or category, rather than by project. Significant categories of costs include personnel, facilities and equipment costs and third-party and other services. In addition, a significant portion of our research and development expenses is not tracked by project as it benefits multiple projects. Consequently, fully-loaded research and development cost summaries by project are not available.
Impairment of Long-Lived Assets
Our long-lived assets include property, plant and equipment, right-of-use assets for leases and goodwill. We regularly review long-lived assets for impairment. The recoverability of long-lived assets, other than goodwill, is measured by comparing the assets carrying amount to the expected undiscounted future cash flows that the asset is expected to generate. Determining whether an impairment has occurred typically requires various estimates and assumptions, including determining which cash flows are directly related to the potentially impaired asset, the useful life over which cash flows will occur, their amount, and the asset’s residual value, if any. We use internal cash flow estimates, quoted market prices when available and independent appraisals as appropriate to determine fair value. We derive the required cash flow estimates from our historical experience and our internal business plans and apply an appropriate discount rate. In 2020, we recognized an impairment loss of $1.6 million to reduce the carrying value of the assets comprising our campus in The Woodlands, Texas, which were sold in December 2020, to an estimated fair value, less estimated selling costs. There were no significant impairments of long-lived assets in 2019 or 2018.
Indefinite-lived intangible assets, which was comprised primarily of in-process research and development, or IPR&D, projects acquired in business combinations which had not reached technological feasibility, is reviewed annually for impairment and whenever events or changes in circumstances indicated that the carrying amount may not have been recoverable. In 2019, we terminated certain research and development activities related to a program for the treatment of irritable bowel syndrome and as a result, recognized $28.6 million of impairment to indefinite-lived intangible assets. There were no impairments to indefinite-lived intangible assets in 2018.
Goodwill is not amortized, but is tested at least annually for impairment at the reporting unit level. We have determined that the reporting unit is the single operating segment disclosed in our current financial statements. Impairment is the condition that exists when the carrying amount of goodwill exceeds its implied fair value. The first step in the impairment process is to determine the fair value of the reporting unit and then compare it to the carrying value, including goodwill. We determined that the market capitalization approach is the most appropriate method of measuring fair value of the reporting unit. Under this approach, fair value is calculated as the average closing price of our common stock for the 30 days preceding the date that the annual impairment test is performed, multiplied by the number of outstanding shares on that date. A control premium, which is representative of premiums paid in the marketplace to acquire a controlling interest in a company, is then added to the market capitalization to determine the fair value of the reporting unit. If the fair value exceeds the carrying value, no further action is required and no impairment loss is recognized. Additional impairment assessments may be performed on an interim basis if we encounter events or changes in circumstances that would indicate that, more likely than not, the carrying value of goodwill has been impaired. There was no impairment of goodwill in 2020, 2019 and 2018.
Recent Accounting Pronouncements
See Note 3, Recent Accounting Pronouncements, of the Notes to Consolidated Financial Statements, for a discussion of the impact of new accounting standards on our consolidated financial statements.
40
Results of Operations – Comparison of Years Ended December 31, 2020, 2019 and 2018
The following discussion and analysis should be read with “Results of Operations” and our financial statements and notes included in our annual report on Form 10-K for the year ended December 31, 2020.
Revenues
Total revenues and dollar and percentage changes as compared to the prior year are as follows (dollar amounts are presented in millions):
| Year Ended December 31, | |||||||||||||||||
| 2020 | 2019 | 2018 | |||||||||||||||
| Total revenues | $ | 24.0 | $ | 322.1 | $ | 63.2 | |||||||||||
| Dollar (decrease) increase | $ | (298.1) | $ | 258.9 | |||||||||||||
| Percentage (decrease) increase | (93) | % | 410 | % | |||||||||||||
Years Ended December 31, 2020 and 2019
•Net product revenues – Net product revenue recognized from the sale of XERMELO in the United States decreased 25% in 2020 to $23.4 million, primarily due to the sale of XERMELO and related assets to TerSera on September 8, 2020. Sales of bulk tablets of XERMELO to Ipsen were zero in 2020 compared to $1.3 million in 2019. Product revenues are recorded net of estimated product returns, pricing discounts including rebates offered pursuant to mandatory federal and state government programs and chargebacks, prompt pay discounts and distribution fees and co-pay assistance. Revenue recognition policies require estimates of the aforementioned sales allowances each period.
•Collaborative agreements – Revenue from collaborative agreements was $289.2 million in 2019. Revenue from collaborative agreements in 2019 included $260 million in revenues recognized from amounts payable by Sanofi pursuant to the termination of our collaboration agreement and recognition of amounts allocated to the performance obligation for development activities of sotagliflozin in the Sanofi collaboration agreement.
•Royalties and other revenue – Revenues from royalties and other revenue increased 9% in 2020 to $0.6 million.
In 2020, two specialty pharmacies, Biologics, Inc. and Diplomat Pharmacy, represented 65% and 22% of revenues, respectively. In 2019, Sanofi represented 89% of revenues and no customers for XERMELO sales represented more than 10% of revenues.
Cost of Sales
Total cost of sales and dollar and percentage changes as compared to the prior year are as follows (dollar amounts are presented in millions):
| Year Ended December 31, | |||||||||||||||||
| 2020 | 2019 | 2018 | |||||||||||||||
| Total cost of sales | $ | 1.9 | $ | 3.2 | $ | 2.5 | |||||||||||
| Dollar (decrease) increase | $ | (1.3) | $ | 0.7 | |||||||||||||
| Percentage (decrease) increase | (40) | % | 30 | % | |||||||||||||
Years Ended December 31, 2020 and 2019
Cost of sales decreased 40% in 2020 to $1.9 million. Cost of sales consists of third-party manufacturing costs, freight and indirect overhead costs associated with sales of XERMELO. Cost of sales in 2020 and 2019 included $1.2 million and $1.8 million, respectively, of amortization of intangible assets related to XERMELO.
41
Research and Development Expenses
Research and development expenses and dollar and percentage changes as compared to the prior year are as follows (dollar amounts are presented in millions):
| Year Ended December 31, | |||||||||||||||||
| 2020 | 2019 | 2018 | |||||||||||||||
| Total research and development expense | $ | 153.6 | $ | 91.9 | $ | 100.2 | |||||||||||
| Dollar increase (decrease) | $ | 61.7 | $ | (8.3) | |||||||||||||
| Percentage increase (decrease) | 67 | % | (8) | % | |||||||||||||
Research and development expenses consist primarily of third-party and other services principally related to nonclinical and clinical development activities, salaries and other personnel-related expenses, facility and equipment costs and stock-based compensation.
Years Ended December 31, 2020 and 2019
•Third-party and other services – Third-party and other services increased 117% in 2020 to $121.2 million, primarily due to increases in external clinical development costs relating to sotagliflozin subsequent to the termination of our collaboration with Sanofi in September 2019. Third-party and other services relate principally to our clinical trial and related development activities, such as nonclinical and clinical studies and contract manufacturing.
•Personnel – Personnel costs decreased 11% in 2020 to $18.4 million, primarily due to lower headcount as a result of the reduction in force of our personnel in September 2020, partially offset by severance costs related to the reduction in force (Refer to Note 4, Asset Sale, of the Notes to Consolidated Financial Statements, for more information). Salaries, bonuses, employee benefits, payroll taxes, recruiting and relocation costs are included in personnel costs.
•Stock-based compensation – Stock-based compensation expense decreased 10% in 2020 to $6.4 million, primarily due to cancellation of unvested share-based awards as a result of the reduction in force of our personnel in September 2020.
•Facilities and equipment – Facilities and equipment costs decreased 6% in 2020 to $2.6 million.
•Other – Other costs were $5.1 million and $5.5 million, respectively, in 2020 and 2019.
Selling, General and Administrative Expenses
Selling, general and administrative expenses and dollar and percentage changes as compared to the prior year are as follows (dollar amounts are presented in millions):
| Year Ended December 31, | |||||||||||||||||
| 2020 | 2019 | 2018 | |||||||||||||||
| Total selling, general and administrative expense | $ | 47.2 | $ | 56.8 | $ | 63.8 | |||||||||||
| Dollar decrease | $ | (9.6) | $ | (6.9) | |||||||||||||
| Percentage decrease | (17) | % | (11) | % | |||||||||||||
Selling, general and administrative expenses consist primarily of personnel costs to support the commercialization of XERMELO and our research and development activities, professional and consulting fees, stock-based compensation expense, and facility and equipment costs.
Years Ended December 31, 2020 and 2019
•Personnel – Personnel costs decreased 12% in 2020 to $24.9 million, primarily due to lower headcount as a result of the reduction in force of our personnel in September 2020, partially offset by severance costs related to the reduction in force (Refer to Note 4, Asset Sale, of the Notes to Consolidated Financial Statements, for more information). Salaries, bonuses, employee benefits, payroll taxes, recruiting and relocation costs are included in personnel costs.
42
•Professional and consulting fees – Professional and consulting fees decreased 25% in 2020 to $9.2 million, primarily due to lower marketing costs and legal fees.
•Stock-based compensation – Stock-based compensation expense decreased 3% in 2020 to $6.9 million, primarily due to cancellation of unvested share-based awards as a result of the reduction in force of our personnel in September 2020.
•Facilities and equipment – Facilities and equipment were $1.7 million and $1.8 million, respectively, in 2020 and 2019.
•Other – Other costs decreased 37% in 2020 to $4.6 million, primarily due to decreases in travel expenses due to the COVID-19 pandemic.
Impairment Losses
An impairment loss of $1.6 million in the year ended December 31, 2020 was recognized as a result of writing down the assets related to our campus in The Woodlands, Texas to the estimated selling price.
An impairment loss of $28.6 million in the year ended December 31, 2019 was recognized to an indefinite-lived intangible asset associated with our 2010 acquisition of Symphony Icon, Inc., due to the decision to terminate research and development activities related to a program for irritable bowel syndrome that was among the assets acquired.
Gain on the sale of XERMELO
A gain of $132.6 million in the year ended December 31, 2020 was recognized in connection with the sale of XERMELO and related assets to TerSera.
Interest Expense and Interest and Other Income, Net
Interest Expense. Interest expense decreased to $14.5 million in 2020 from $20.7 million in 2019, primarily due to the $150 million payoff of the BioPharma term loan in September 2020 and the $75.8 million exchange of convertible notes in September and October 2020.
Interest and Other Income (Expense), Net. Interest and other income, net was $2.8 million and $3.4 million in the years ended December 31, 2020 and 2019, respectively.
Income Tax Benefit
The income tax benefit for the year ended December 31, 2019 was $6.0 million, due to the release of the deferred tax liability related to the impairment of the indefinite-lived intangible asset (see Note 8, Income Taxes of the Notes to Consolidated Financial Statements, for more information). There was no income tax expense or benefit in 2020.
Net Income (Loss) and Net Income (Loss) per Common Share
Net loss was $58.6 million, or a loss of $0.53 per share, in 2020 as compared to net income of $130.1 million, or $1.16 per diluted share in 2019.
Liquidity and Capital Resources
We have financed our operations from inception primarily through sales of common and preferred stock, contract and milestone payments we received under our strategic and other collaborations, target validation, database subscription and technology license agreements, product sales, government grants and contracts, and financing under debt and lease arrangements. We have also financed certain of our research and development activities under our agreements with Symphony Icon, Inc.
As of December 31, 2020, we had $152.3 million in cash, cash equivalents and short-term investments. As of December 31, 2019, we had $271.7 million in cash, cash equivalents and short-term investments. We used cash of $143.0 million in our operations in 2020. This consisted primarily of the net loss for the year of $58.6 million, gain from the sale of
43
XERMELO and related assets to TerSera of $132.6 million and net gain related to debt extinguishments of $1.0 million, partially offset by a net decrease in operating assets net of liabilities of $31.1 million, non-cash charges of $13.3 million related to stock-based compensation expense, $3.9 million related to depreciation and amortization expense, including amortization of debt issuance costs and $1.6 million related to the impairment loss on buildings. Investing activities provided cash of $380.8 million in 2020, primarily due to $209.4 million of net maturities of investments, $160.4 million of proceeds from the sale of XERMELO and $11.0 million of proceeds from the sale of property and equipment. Financing activities used cash of $147.6 million, primarily to repay $216.6 million of debt borrowings and to repurchase $1.0 million of common stock, partially offset by $70.0 million of net proceeds from common stock sales, including $7.0 million of net proceeds from the sale of 3,709,233 shares of common stock on November 17, 2020 under our Open Market Sale AgreementSM with Jefferies LLC. We sold an additional 2,000,000 shares of common stock under the Open Market Sale AgreementSM with Jefferies on January 14, 2021, resulting in an additional $16.4 million in net proceeds. On December 11, 2020, we entered into a subscription agreement with Invus and certain other institutional investors, pursuant to which we issued and sold 20,312,500 shares of our common stock and received net proceeds of $63.0 million.
Other commitments. In April 2019, sotagliflozin was approved in the European Union for use as an adjunct to insulin therapy to improve glycemic control in adults with type 1 diabetes and a body mass index ≥ 27 kg/m2, who could not achieve adequate glycemic control despite optimal insulin therapy. Upon the achievement of certain European regulatory pricing approvals, we will be required to make certain royalty payments, totaling $4.5 million, in three equal annual installments of $1.5 million. In September 2020, we initiated a Phase 2 clinical trial of LX9211 in diabetic peripheral neuropathic pain. As a result of the commencement of the Phase 2 trial, we were required to make a royalty payment of $2.5 million, which was paid in October 2020.
Facilities. In December 2020, our subsidiary, Lex-Gen Woodlands, L.P., sold our facilities in The Woodlands, Texas for $11.9 million. Concurrent with such sale, we entered into a leaseback agreement with respect to 38,000 square feet of such facilities for a period of up to six months, with monthly gross rent payments of $101,000. In February 2021, we leased a 25,000 square-foot office space in The Woodlands, Texas to which we plan to relocate our corporate offices. The term of the sublease extends from March 1, 2021 through August 31, 2025, and provides for escalating yearly base rent payments starting at $506,000 and increasing to $557,000 in the final year of the lease.
In March 2015, our subsidiary, Lexicon Pharmaceuticals (New Jersey), Inc., leased a 25,000 square-foot office space in Basking Ridge, New Jersey. The term of the lease extends from June 1, 2015 through December 31, 2022, and provides for escalating yearly base rent payments starting at $482,000 and increasing to $646,000 in the final year of the lease.
Debt. We have $12.3 million of convertible debt, including $0.6 million of interest, due in December 2021.
Our future capital requirements will be substantial and will depend on many factors, including the success of our ongoing nonclinical and clinical development efforts and the ability to obtain necessary regulatory approvals of the drug candidates which are the subject of such efforts; TerSeras ability to successfully develop and commercialize XERMELO for biliary tract cancer and our receipt of milestone payments and royalties from such efforts; our success in establishing new collaborations and licenses, particularly for the commercialization of sotagliflozin for heart failure; the amount and timing of our research, development and commercialization expenditures; the resources we devote to developing and supporting our products and other factors. Our capital requirements will also be affected by any expenditures we make in connection with license agreements and acquisitions of and investments in complementary technologies and businesses. We expect to continue to devote substantial capital resources to successfully complete our nonclinical and clinical development efforts with respect to LX9211, sotagliflozin and our other drug candidates; and for other general corporate activities. We believe that our current unrestricted cash and investment balances and cash and revenues we expect to derive from strategic and other collaborations and other sources will be sufficient to fund our operations for at least the next 12 months. During or after this period, if cash generated by operations is insufficient to satisfy our liquidity requirements, we will need to sell additional equity or debt securities or obtain additional credit arrangements. Additional financing may not be available on terms acceptable to us or at all. The sale of additional equity or convertible debt securities may result in additional dilution to our stockholders.
From time to time, our board of directors may authorize us to repurchase shares of our common stock, repurchase, in cash or common stock, our outstanding convertible notes, or make a cash payment to holders of our convertible notes to induce conversion pursuant to the terms of the convertible notes, in each case, in privately negotiated transactions, publicly announced programs or otherwise. If and when our board of directors should determine to authorize any such action, it would be on terms and under market conditions that our board of directors determines are in the best interest of us and our stockholders. Any such actions could deplete significant amounts of our cash resources and/or result in additional dilution to our stockholders.
44
Disclosure about Market Risk
We are exposed to limited market and credit risk on our cash equivalents which have maturities of three months or less at the time of purchase. We maintain a short-term investment portfolio which consists of U.S. Treasury bills and corporate debt securities that mature three to 12 months from the time of purchase, which we believe are subject to limited market and credit risk. We currently do not hedge interest rate exposure or hold any derivative financial instruments in our investment portfolio.
We had approximately $152.3 million in cash and cash equivalents and short-term investments as of December 31, 2020. We believe that the working capital available to us will be sufficient to meet our cash requirements for at least the next 12 months. We are not subject to interest rate sensitivity on our outstanding convertible notes as they are subject to a fixed rate of 5.25% per annum. The convertible notes interest is payable in cash semi-annually in arrears and matures in December 2021, unless earlier converted or repurchased in accordance with their terms.
We have operated primarily in the United States and substantially all sales to date have been made in U.S. dollars. Accordingly, we have not had any material exposure to foreign currency rate fluctuations.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
See “Disclosure about Market Risk” under “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations” for quantitative and qualitative disclosures about market risk.
Item 8. Financial Statements and Supplementary Data
The financial statements required by this Item are incorporated under Item 15 in Part IV of this report.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our principal executive officer and principal financial officer, has evaluated the effectiveness of our disclosure controls and procedures (as defined in rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934) as of the end of the period covered by this report. Based on that evaluation, our principal executive officer and principal financial officer have concluded that our disclosure controls and procedures were effective as of December 31, 2020 to ensure that the information required to be disclosed by us in the reports we file under the Securities Exchange Act is gathered, analyzed and disclosed with adequate timeliness, accuracy and completeness.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act).
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2020. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control-Integrated Framework (2013 Framework).
Based on such assessment using those criteria, management concluded that, as of December 31, 2020, our internal control over financial reporting was effective.
Our independent registered public accounting firm, Ernst & Young LLP, has audited the financial statements included in this Annual Report and has issued a report on the effectiveness of our internal control over financial reporting. The report of Ernst & Young LLP is included below.
45
Changes in Internal Control Over Financial Reporting
Subsequent to our evaluation described above, there were no significant changes in internal controls or other factors during the fiscal quarter ended December 31, 2020 that could significantly affect internal controls, including any corrective actions with regard to significant deficiencies and material weaknesses.
46
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and the Board of Directors of Lexicon Pharmaceuticals, Inc.
Opinion on Internal Control Over Financial Reporting
We have audited Lexicon Pharmaceuticals, Inc.’s internal control over financial reporting as of December 31, 2020, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission 2013 framework (the COSO criteria). In our opinion, Lexicon Pharmaceuticals, Inc. (the Company) maintained, in all material respects, effective internal control over financial reporting as of December 31, 2020, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated balance sheets of the Company as of December 31, 2020 and 2019, the related consolidated statements of comprehensive income (loss), stockholders’ equity (deficit), and cash flows for each of the three years in the period ended December 31, 2020, and the related notes and our report dated March 12, 2021 expressed an unqualified opinion thereon.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects.
Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ Ernst & Young LLP
Houston, Texas
March 12, 2021
47
Item 9B. Other Information
None.
PART III
Item 10. Directors, Executive Officers and Corporate Governance
The information required by this Item is hereby incorporated by reference from (a) the information appearing under the captions “Election of Directors,” “Stock Ownership of Certain Beneficial Owners and Management,” “Corporate Governance” and “Executive and Director Compensation” in our definitive proxy statement which involves the election of directors and is to be filed with the SEC pursuant to the Securities Exchange Act of 1934 within 120 days of the end of our fiscal year on December 31, 2020 and (b) the information appearing under Item 1 in Part I of this report.
Item 11. Executive Compensation
The information required by this Item is hereby incorporated by reference from the information appearing under the captions “Corporate Governance” and “Executive and Director Compensation” in our definitive proxy statement which involves the election of directors and is to be filed with the Commission pursuant to the Securities Exchange Act of 1934 within 120 days of the end of our fiscal year on December 31, 2020. Notwithstanding the foregoing, in accordance with the instructions to Item 407(e)(5) of Regulation S-K, the information contained in our proxy statement under the sub-heading “Compensation Committee Report” shall not be deemed to be filed as part of or incorporated by reference into this annual report on Form 10-K.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information required by this Item is hereby incorporated by reference from the information appearing under the captions “Stock Ownership of Certain Beneficial Owners and Management” and “Equity Compensation Plan Information” in our definitive proxy statement which involves the election of directors and is to be filed with the Commission pursuant to the Securities Exchange Act of 1934 within 120 days of the end of our fiscal year on December 31, 2020.
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information required by this Item is hereby incorporated by reference from the information appearing under the captions “Corporate Governance” and “Transactions with Related Persons” in our definitive proxy statement which involves the election of directors and is to be filed with the Commission pursuant to the Securities Exchange Act of 1934 within 120 days of the end of our fiscal year on December 31, 2020.
Item 14. Principal Accounting Fees and Services
The information required by this Item as to the fees we pay our principal accountant is hereby incorporated by reference from the information appearing under the caption “Ratification and Approval of Independent Auditors” in our definitive proxy statement which involves the election of directors and is to be filed with the Commission pursuant to the Securities Exchange Act of 1934 within 120 days of the end of our fiscal year on December 31, 2020.
48
PART IV
Item 15. Exhibits and Financial Statement Schedules
(a)Documents filed as a part of this report:
1.Consolidated Financial Statements
| Page | |||||
2.Financial Statement Schedules
All other financial statement schedules are omitted because they are not applicable or not required, or because the required information is included in the financial statements or notes thereto.
3.Exhibits
| Exhibit No. | Description | ||||||||||
| 3.1 | — | Amended and Restated Certificate of Incorporation (filed as Exhibit 3.1 to the Company’s Current Report on Form 8-K dated April 26, 2012 and incorporated by reference herein). | |||||||||
| 3.2 | — | Certificate of Amendment to Amended and Restated Certificate of Incorporation (filed as Exhibit 3.1 to the Company’s Current Report on Form 8-K dated May 20, 2015 and incorporated by reference herein). | |||||||||
| 3.3 | — | Second Amended and Restated Bylaws (filed as Exhibit 3.2 to the Company’s Current Report on Form 8‑K dated April 26, 2012 and incorporated by reference herein). | |||||||||
| 4.1 | — | Securities Purchase Agreement, dated June 17, 2007, with Invus, L.P. (filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K dated June 17, 2007 and incorporated by reference herein). | |||||||||
| 4.2 | — | Amendment, dated October 7, 2009, to Securities Purchase Agreement, dated June 17, 2007, with Invus, L.P. (filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K dated October 7, 2009 and incorporated by reference herein). | |||||||||
| 4.3 | — | Registration Rights Agreement, dated June 17, 2007, with Invus, L.P. (filed as Exhibit 10.3 to the Company’s Current Report on Form 8-K dated June 17, 2007 and incorporated by reference herein). | |||||||||
| 4.4 | — | Stockholders’ Agreement, dated June 17, 2007, with Invus, L.P. (filed as Exhibit 10.4 to the Company’s Current Report on Form 8-K dated June 17, 2007 and incorporated by reference herein). | |||||||||
| 4.5 | — | Supplement to Transaction Agreements, dated March 15, 2010, with Invus, L.P. and Invus C.V. (filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K dated March 15, 2010 and incorporated by reference herein). | |||||||||
| 4.6 | — | Supplement No. 2 to Transaction Agreements, dated February 23, 2012, with Invus, L.P. and Invus C.V. (filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K dated February 23, 2012 and incorporated by reference herein). | |||||||||
| 4.7 | Supplement No. 3 to the Transaction Agreements, dated December 16, 2020, with Invus, L.P. and Invus C.V. (filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K dated December 16, 2020 and incorporated by reference herein). | ||||||||||
| 4.8 | — | Subscription Agreement, dated December 11, 2020, with Artal International S.C.A., Biotechnology Value Fund, L.P., Biotechnology Value Fund II, L.P., Biotechnology Value Trading Fund OS, L.P. and MSI BVF SPV, L.L.C. (filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K dated December 11, 2020 and incorporated by reference herein). | |||||||||
| 4.9 | — | Indenture related to the 5.25% Convertible Senior Notes due 2021, dated as of November 26, 2014, with Wells Fargo Bank, N.A. (filed as Exhibit 4.1 to the Company’s Current Report on Form 8-K dated November 26, 2014 and incorporated by reference herein). | |||||||||
| 4.10 | — | Form of 5.25% Convertible Senior Notes due 2021 (filed as Exhibit A to Exhibit 4.1 to the Company’s Current Report on Form 8-K dated November 26, 2014 and incorporated by reference herein). | |||||||||
| *4.11 | — | ||||||||||
49
| 10.1 | — | Offer Letter, dated July 1, 2014, with Lonnel Coats, as amended (filed as Exhibit 10.1 to the Company’s Annual Report on Form 10-K for the period ended December 31, 2018 and incorporated by reference herein). | |||||||||
| 10.2 | — | Employment Agreement with Alan Main, Ph.D. (filed as Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the period ended September 30, 2001 and incorporated by reference herein). | |||||||||
| 10.3 | — | Offer Letter, dated March 23, 2016, with Praveen Tyle, Ph.D. (filed as Exhibit 10.4 to the Company’s Annual Report on Form 10-K for the period ended December 31, 2016 and incorporated by reference herein). | |||||||||
| 10.4 | — | Employment Agreement with Jeffrey L. Wade, J.D. (filed as Exhibit 10.3 to the Company’s Registration Statement on Form S-1 (Registration No. 333-96469) and incorporated by reference herein). | |||||||||
| *10.5 | — | Release and Severance Agreement, dated September 30, 2020, with Pablo Lapuerta, M.D. | |||||||||
| *10.6 | — | Release and Severance Agreement, dated September 30, 2020, with Alexander A. Santini | |||||||||
| 10.7 | — | Form of Indemnification Agreement with Officers and Directors (filed as Exhibit 10.7 to the Company’s Registration Statement on Form S-1 (Registration No. 333-96469) and incorporated by reference herein). | |||||||||
| 10.8 | — | Summary of Non-Employee Summary of Non-Employee Director Compensation (filed as Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the period ended March 31, 2019 and incorporated by reference herein). | |||||||||
| 10.9 | — | 2017 Equity Incentive Plan, as amended (filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K dated April 23, 2020 and incorporated by reference herein). | |||||||||
| 10.10 | — | 2017 Non-Employee Directors’ Equity Incentive Plan, as amended (filed as Exhibit 10.9 to the Company's Annual Report on Form 10-K for the period ended December 31, 2018 and incorporated by reference herein). | |||||||||
| 10.11 | — | Form of Stock Option Agreement with Officers under the 2017 Equity Incentive Plan (filed as Exhibit 10.11 to the Company’s Annual Report on Form 10-K for the period ended December 31, 2017 and incorporated by reference herein). | |||||||||
| 10.12 | — | Form of Restricted Stock Unit Agreement with Officers under the 2017 Equity Incentive Plan (filed as Exhibit 10.12 to the Company’s Annual Report on Form 10-K for the period ended December 31, 2017 and incorporated by reference herein). | |||||||||
| 10.13 | — | Form of Notice of Stock Option Grant to Directors under the 2017 Non-Employee Directors’ Equity Incentive Plan (filed as Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q for the period ended March 31, 2019 and incorporated by reference herein). | |||||||||
| 10.14 | — | Form of Notice of Restricted Stock Unit Grant to Directors under the 2017 Non-Employee Directors’ Equity Incentive Plan (filed as Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q for the period ended March 31, 2019 and incorporated by reference herein). | |||||||||
| †10.15 | — | Collaboration and License Agreement, dated November 5, 2015, with Sanofi (filed as Exhibit 10.14 to the Company’s Annual Report on Form 10-K/A for the period ended December 31, 2015 and incorporated by reference herein). | |||||||||
| †10.16 | — | Amendment No. 1, dated July 1, 2017, to Collaboration and License Agreement, dated November 5, 2015, with Sanofi (filed as Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the period ended September 30, 2017 and incorporated by reference herein). | |||||||||
| §10.17 | — | Confidential Termination and Settlement Agreement and Mutual Releases, dated September 9, 2019, with Sanofi-Aventis Deutschland GmbH (filed as Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the period ended September 30, 2019 and incorporated by reference herein). | |||||||||
| 10.18 | — | Collaboration and License Agreement, dated December 17, 2003, with Bristol-Myers Squibb Company (filed as Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q for the period ended March 31, 2015 and incorporated by reference herein). | |||||||||
| 10.19 | — | First Amendment, dated May 30, 2006, to Collaboration and License Agreement, dated December 17, 2003, with Bristol-Myers Squibb Company (filed as Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q for the period ended March 31, 2015 and incorporated by reference herein). | |||||||||
| †10.20 | — | Second Amendment, dated November 2, 2016, to Collaboration and License Agreement, dated December 17, 2003, with Bristol-Myers Squibb Company (filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K dated November 2, 2016 and incorporated by reference herein). | |||||||||
| †10.21 | — | Second Amended and Restated Collaboration and License Agreement, dated November 30, 2005, with Genentech, Inc. (filed as Exhibit 10.22 to the Company’s Annual Report on Form 10-K for the period ended December 31, 2005 and incorporated by reference herein). | |||||||||
| 10.22 | — | Amendment, dated June 8, 2009, to Second Amended and Restated Collaboration and License Agreement, dated November 30, 2005, with Genentech, Inc. (filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K/A dated June 8, 2009 and incorporated by reference herein). | |||||||||
50
| §10.23 | — | Asset Purchase and Sale Agreement, dated July 29, 2020, with TerSera Therapeutics LLC (filed as Exhibit 2.1 to the Company’s Current Report on Form 8-K dated September 8, 2020 and incorporated by reference herein). | |||||||||
| 10.24 | — | First Amendment to Asset Purchase and Sale Agreement, dated August 10, 2020, with TerSera Therapeutics LLC (filed as Exhibit 2.2 to the Company’s Current Report on Form 8-K dated September 8, 2020 and incorporated by reference herein). | |||||||||
| 10.25 | — | Sublease Agreement, dated February 8, 2021, with Repsol Oil & Gas USA, LLC (filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K dated February 8, 2021 and incorporated by reference herein). | |||||||||
| 21.1 | — | Subsidiaries (filed as Exhibit 21.1 to the Company’s Annual Report on Form 10-K for the period ended December 31, 2010 and incorporated by reference herein). | |||||||||
| *23.1 | — | ||||||||||
| *24.1 | — | Power of Attorney (contained in signature page). | |||||||||
| *31.1 | — | ||||||||||
| *31.2 | — | ||||||||||
| *32.1 | — | ||||||||||
| *101.INS | — | XBRL Instance Document. | |||||||||
| *101.SCH | — | XBRL Taxonomy Extension Schema Document. | |||||||||
| *101.CAL | — | XBRL Taxonomy Extension Calculation Linkbase Document. | |||||||||
| *101.DEF | — | XBRL Taxonomy Extension Definition Linkbase Document. | |||||||||
| *101.LAB | — | XBRL Taxonomy Extension Label Linkbase Document. | |||||||||
| *101.PRE | — | XBRL Taxonomy Extension Presentation Linkbase Document. | |||||||||
______________
| * | Filed herewith. | ||||
| † | Confidential treatment has been requested for portions of this exhibit (indicated by “[**]”). The confidential portions of this exhibit have been omitted and filed separately with the Securities and Exchange Commission. | ||||
| § | Certain information (indicated by “[**]”) has been excluded from this exhibit because it is both not material and would likely cause competitive harm to the Company if publicly disclosed. | ||||
Item 16. Form 10-K Summary
Not applicable.
51
Signatures
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| Lexicon Pharmaceuticals, Inc. | |||||||||||
| Date: | March 12, 2021 | By: | /s/ LONNEL COATS | ||||||||
| Lonnel Coats | |||||||||||
| President and Chief Executive Officer | |||||||||||
| Date: | March 12, 2021 | By: | /s/ JEFFREY L. WADE | ||||||||
| Jeffrey L. Wade | |||||||||||
| Executive Vice President, Corporate and Administrative Affairs and Chief Financial Officer | |||||||||||
Power of Attorney
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Lonnel Coats and Jeffrey L. Wade, or either of them, each with the power of substitution, his or her attorney-in-fact, to sign any amendments to this Form 10-K, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, here ratifying and confirming all that each of said attorneys-in-fact, or his or her substitute or substitutes, may do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | |||||||||
| /s/ LONNEL COATS | President, Chief Executive Officer and Director (Principal Executive Officer) | March 12, 2021 | |||||||||
| Lonnel Coats | |||||||||||
| /s/ JEFFREY L. WADE | Executive Vice President, Corporate and Administrative Affairs and Chief Financial Officer (Principal Financial Officer) | March 12, 2021 | |||||||||
| Jeffrey L. Wade | |||||||||||
| /s/ JAMES F. TESSMER | Vice President, Finance and Accounting (Principal Accounting Officer) | March 12, 2021 | |||||||||
| James F. Tessmer | |||||||||||
| /s/ RAYMOND DEBBANE | Chairman of the Board of Directors | March 12, 2021 | |||||||||
| Raymond Debbane | |||||||||||
| /s/ PHILIPPE J. AMOUYAL | Director | March 12, 2021 | |||||||||
| Philippe J. Amouyal | |||||||||||
| /s/ SAMUEL L. BARKER | Director | March 12, 2021 | |||||||||
| Samuel L. Barker, Ph.D. | |||||||||||
| /s/ ROBERT J. LEFKOWITZ | Director | March 12, 2021 | |||||||||
| Robert J. Lefkowitz, M.D. | |||||||||||
| /s/ ALAN S. NIES | Director | March 12, 2021 | |||||||||
| Alan S. Nies, M.D. | |||||||||||
| /s/ FRANK P. PALANTONI | Director | March 12, 2021 | |||||||||
| Frank P. Palantoni | |||||||||||
| /s/ CHRISTOPHER J. SOBECKI | Director | March 12, 2021 | |||||||||
| Christopher J. Sobecki | |||||||||||
| /s/ JUDITH L. SWAIN | Director | March 12, 2021 | |||||||||
| Judith L. Swain, M.D. | |||||||||||
52
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and the Board of Directors of Lexicon Pharmaceuticals, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Lexicon Pharmaceuticals, Inc. (the Company) as of December 31, 2020 and 2019, the related consolidated statements of comprehensive income (loss), stockholders’ equity (deficit), and cash flows for each of the three years in the period ended December 31, 2020, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2020 and 2019, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2020, in conformity with US generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company’s internal control over financial reporting as of December 31, 2020, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 Framework) and our report dated March 12, 2021 expressed an unqualified opinion thereon.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
| Sale of XERMELO product and related assets | ||||||||
| Description of the Matter | As described in Note 4 to the consolidated financial statements, in September 2020, the Company completed the sale of its XERMELO (telotristat ethyl) product and related assets (the “XERMELO sale”) to TerSera Therapeutics LLC. The Company recognized a gain on sale of $132.6 million in loss from operations in the consolidated statements of comprehensive loss for the year ended December 31, 2020. The XERMELO sale did not meet the criteria for reporting as discontinued operations as there was not a strategic shift that has (or will have) a major effect on the Company’s operations. Auditing the XERMELO sale was especially challenging given the nature of the transaction and significant judgment required to determine whether discontinued operations presentation was appropriate, to estimate the variable consideration and to identify the specific assets and liabilities and related financial effects included within the scope of the divestiture given the degree of integration of the XERMELO product and related assets with the Company’s remaining operations. | |||||||
F-1
| How We Addressed the Matter in Our Audit | We obtained an understanding, evaluated the design, and tested the operating effectiveness of controls over the Company’s process for the XERMELO sale. For example, we tested controls over management’s review of the accounting treatment of the XERMELO sale and for calculating the gain on sale. Our audit procedures included, among others, inspecting the transaction agreement, assessing the reasonableness of judgments, testing the accuracy of the gain on sale calculation and evaluating the adequacy of the Company’s disclosures related to the XERMELO sale. For example, we evaluated the appropriateness of the Company’s application of the criteria for reporting of discontinued operations by inspecting management’s supporting documentation, reading of Board of Director meeting minutes and other entity information, and evaluating for contrary evidence based on our understanding of the business. In addition, we tested the Company’s separation of XERMELO’s assets, liabilities and related financial information by inspecting the Company’s accounting records and agreeing it to the terms of the transaction agreement. We also evaluated the appropriateness of the Company’s treatment of variable consideration included in the transaction agreement by inspecting management’s supporting documentation regarding the likelihood of occurrence of the underlying development, regulatory and commercial milestones and evaluating for contrary evidence based on our reading of Board of Director meeting minutes and other entity information, inquiries with management, and our understanding of the business. | |||||||
| Accrued research and development expenses | ||||||||
| Description of the Matter | As described in Note 2 to the consolidated financial statements, the Company records accruals for estimated costs of research and development activities that include contract services for clinical trials. Clinical trial activities performed by third parties are accrued and expensed based upon estimates of the proportion of work completed over the life of the individual clinical trial in accordance with agreements established with contract research organizations (“CROs”), clinical trial sites and other third parties. Estimates are determined by reviewing contracts, vendor agreements, purchase orders, change orders and trial budgets, as well as through discussions with internal clinical personnel and external service providers as to the progress or stage of completion of trials or services and the agreed-upon fee to be paid for such services. Auditing management’s accounting for accrued third-party clinical trial research and development expenses is especially challenging because of the judgment applied by management to determine the progress or stage of completion of the activities under the Company’s research and development agreements and the cost and extent of work performed during the reporting period for services not yet billed by contracted third-party vendors. The testing of the Company’s accrued clinical trial expense models also involves a significant level of effort to test the high volume of data from third parties, which is tracked in spreadsheets. | |||||||
| How We Addressed the Matter in Our Audit | We obtained an understanding, evaluated the design and tested the operating effectiveness of controls over the Company’s accounting for accrued research and development expenses process, including controls over management’s review of clinical trial activity progress in comparison to budgets and invoices received from third parties. Our audit procedures included, among others, testing the accuracy and completeness of the underlying inputs used in management’s analysis to determine costs incurred. We compared expenses incurred to budgeted amounts per executed vendor contracts and to expenses incurred in prior periods and obtained an understanding of the reasons for changes. We inspected the terms and conditions of vendor contracts, change orders and trial budgets, assessed direct fees, pass-through costs and clinical site costs, and clerically tested the cost models to track progress against trial budgets. We evaluated estimated services incurred by third parties by understanding the terms and timeline of significant projects, evaluating management’s estimate of work performed and costs incurred by meeting with members of the Company’s clinical operations team, and obtaining external confirmation of key terms and conditions and other key inputs to the accrual calculation, such as costs incurred and key dates. Further, we inspected invoices received from third parties after the balance sheet date and performed a lookback analysis to evaluate the completeness of accrued research and development expenses. | |||||||
/s/ Ernst & Young LLP
We have served as the Company’s auditor since 2002.
Houston, Texas
March 12, 2021
F-2
Lexicon Pharmaceuticals, Inc.
Consolidated Balance Sheets
(In thousands, except par value)
| As of December 31, | |||||||||||
| 2020 | 2019 | ||||||||||
| Assets | |||||||||||
| Current assets: | |||||||||||
| Cash and cash equivalents | $ | $ | |||||||||
| Short-term investments | |||||||||||
| Accounts receivable, net | |||||||||||
| Inventory | |||||||||||
| Prepaid expenses and other current assets | |||||||||||
| Total current assets | |||||||||||
Property and equipment, net of accumulated depreciation and amortization of $ | |||||||||||
| Goodwill | |||||||||||
| Intangible assets, net | |||||||||||
| Other assets | |||||||||||
| Total assets | $ | $ | |||||||||
| Liabilities and Equity | |||||||||||
| Current liabilities: | |||||||||||
| Accounts payable | $ | $ | |||||||||
| Accrued liabilities | |||||||||||
| Current portion of long-term debt, net of deferred financing costs | |||||||||||
| Total current liabilities | |||||||||||
| Long-term debt, net of deferred financing costs | |||||||||||
| Other long-term liabilities | |||||||||||
| Total liabilities | |||||||||||
| Commitments and contingencies | |||||||||||
| Stockholders' Equity: | |||||||||||
Preferred stock, $ | |||||||||||
Common stock, $ | |||||||||||
| Additional paid-in capital | |||||||||||
| Accumulated deficit | ( | ( | |||||||||
| Accumulated other comprehensive income (loss) | ( | ||||||||||
Treasury stock, at cost, | ( | ( | |||||||||
| Total stockholders' equity | |||||||||||
| Total liabilities and equity | $ | $ | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-3
Lexicon Pharmaceuticals, Inc.
Consolidated Statements of Comprehensive Income (Loss)
(In thousands, except per share amounts)
| Year Ended December 31, | |||||||||||||||||
| 2020 | 2019 | 2018 | |||||||||||||||
| Revenues: | |||||||||||||||||
| Net product revenue | $ | $ | $ | ||||||||||||||
| Collaborative agreements | |||||||||||||||||
| Royalties and other revenue | |||||||||||||||||
| Total revenues | |||||||||||||||||
| Operating expenses: | |||||||||||||||||
Cost of sales (including finite-lived intangible asset amortization) | |||||||||||||||||
Research and development, including stock-based compensation of $ | |||||||||||||||||
Selling, general and administrative, including stock-based compensation of $ | |||||||||||||||||
| Impairment loss on buildings | |||||||||||||||||
| Impairment loss on intangible asset | — | — | |||||||||||||||
| Total operating expenses | |||||||||||||||||
| Other operating income: | |||||||||||||||||
| Gain on sale of XERMELO | — | — | |||||||||||||||
| Income (loss) from operations | ( | ( | |||||||||||||||
| Gain on debt extinguishments, net | — | — | |||||||||||||||
| Interest expense | ( | ( | ( | ||||||||||||||
| Interest and other income, net | |||||||||||||||||
| Net income (loss) before taxes | ( | ( | |||||||||||||||
| Income tax benefit | |||||||||||||||||
| Net income (loss) | $ | ( | $ | $ | ( | ||||||||||||
Net income (loss) per common share, basic | $ | ( | $ | $ | ( | ||||||||||||
Net income (loss) per common share, diluted | $ | ( | $ | $ | ( | ||||||||||||
Shares used in computing net income (loss) per common share, basic | |||||||||||||||||
Shares used in computing net income (loss) per common share, diluted | |||||||||||||||||
Other comprehensive income (loss): | |||||||||||||||||
Unrealized gain (loss) on investments | ( | ||||||||||||||||
Comprehensive income (loss) | $ | ( | $ | $ | ( | ||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
Lexicon Pharmaceuticals, Inc.
Consolidated Statements of Stockholders’ Equity (Deficit)
(In thousands)
| Accumulated | |||||||||||||||||||||||||||||||||||||||||
| Additional | Other | ||||||||||||||||||||||||||||||||||||||||
| Common Stock | Paid-In | Accumulated | Comprehensive | Treasury | |||||||||||||||||||||||||||||||||||||
| Shares | Par Value | Capital | Deficit | Gain (Loss) | Stock | Total | |||||||||||||||||||||||||||||||||||
| Balance at December 31, 2017 | $ | $ | $ | ( | $ | ( | $ | ( | $ | ||||||||||||||||||||||||||||||||
| Cumulative effect of change in accounting principle | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||
| Stock-based compensation | — | ||||||||||||||||||||||||||||||||||||||||
Issuance of common stock under Equity Incentive Plans | |||||||||||||||||||||||||||||||||||||||||
| Repurchase of common stock | — | ( | ( | ||||||||||||||||||||||||||||||||||||||
| Net loss | — | ( | ( | ||||||||||||||||||||||||||||||||||||||
| Unrealized gain on investments | — | ||||||||||||||||||||||||||||||||||||||||
| Balance at December 31, 2018 | ( | ( | ( | ( | |||||||||||||||||||||||||||||||||||||
| Stock-based compensation | — | ||||||||||||||||||||||||||||||||||||||||
Issuance of common stock under Equity Incentive Plans | |||||||||||||||||||||||||||||||||||||||||
| Repurchase of common stock | — | ( | ( | ||||||||||||||||||||||||||||||||||||||
| Net income | — | ||||||||||||||||||||||||||||||||||||||||
Unrealized gain on investments | — | ||||||||||||||||||||||||||||||||||||||||
| Balance at December 31, 2019 | ( | ( | |||||||||||||||||||||||||||||||||||||||
| Stock-based compensation | — | ||||||||||||||||||||||||||||||||||||||||
| Issuance of common stock under an Open Market Sale Agreement, net of issuance fees | |||||||||||||||||||||||||||||||||||||||||
| Issuance of common stock upon private placement, net of issuance fees | |||||||||||||||||||||||||||||||||||||||||
| Issuance of common stock in settlement of convertible notes, net of issuance fees | |||||||||||||||||||||||||||||||||||||||||
Issuance of common stock under Equity Incentive Plans | |||||||||||||||||||||||||||||||||||||||||
| Repurchase of common stock | — | ( | ( | ||||||||||||||||||||||||||||||||||||||
| Net loss | — | ( | ( | ||||||||||||||||||||||||||||||||||||||
| Unrealized loss on investments | — | ( | ( | ||||||||||||||||||||||||||||||||||||||
| Balance at December 31, 2020 | $ | $ | $ | ( | $ | ( | $ | ( | $ | ||||||||||||||||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
Lexicon Pharmaceuticals, Inc.
Consolidated Statements of Cash Flows
(In thousands)
| Year Ended December 31, | |||||||||||||||||
| 2020 | 2019 | 2018 | |||||||||||||||
| Cash flows from operating activities: | |||||||||||||||||
| Net income (loss) | $ | ( | $ | $ | ( | ||||||||||||
Adjustments to reconcile net income (loss) to net cash used in operating activities: | |||||||||||||||||
| Depreciation and amortization | |||||||||||||||||
| Stock-based compensation | |||||||||||||||||
| Amortization of debt issuance costs | |||||||||||||||||
| Deferred tax benefit | ( | ||||||||||||||||
| Gain on sale of XERMELO | ( | ||||||||||||||||
| Impairment loss on building | |||||||||||||||||
| Impairment loss on intangible asset | |||||||||||||||||
| Gain on debt extinguishments, net | ( | ||||||||||||||||
| Gain on disposal of property and equipment | ( | ||||||||||||||||
| Changes in operating assets and liabilities: | |||||||||||||||||
| Decrease (increase) in accounts receivable | ( | ( | |||||||||||||||
| Decrease (increase) in inventory | ( | ||||||||||||||||
| (Increase) decrease in prepaid expenses and other current assets | ( | ( | |||||||||||||||
| Decrease in other assets | |||||||||||||||||
| Increase (decrease) in accounts payable and other liabilities | ( | ( | |||||||||||||||
| Decrease in deferred revenue | ( | ( | |||||||||||||||
| Net cash (used in) provided by operating activities | ( | ( | |||||||||||||||
| Cash flows from investing activities: | |||||||||||||||||
| Purchases of property and equipment | ( | ( | ( | ||||||||||||||
| Proceeds from XERMELO sale | |||||||||||||||||
| Proceeds from disposal of property and equipment | |||||||||||||||||
| Purchases of investments | ( | ( | ( | ||||||||||||||
| Maturities of investments | |||||||||||||||||
| Net cash provided by (used in) investing activities | ( | ||||||||||||||||
| Cash flows from financing activities: | |||||||||||||||||
| Proceeds from issuance of common stock, net of fees | |||||||||||||||||
| Repurchase of common stock | ( | ( | ( | ||||||||||||||
| Proceeds from debt borrowings, net of fees | |||||||||||||||||
| Repayment of debt borrowings | ( | ( | ( | ||||||||||||||
| Net cash used in financing activities | ( | ( | ( | ||||||||||||||
| Net increase (decrease) in cash and cash equivalents | ( | ||||||||||||||||
| Cash and cash equivalents at beginning of year | |||||||||||||||||
| Cash and cash equivalents at end of year | $ | $ | $ | ||||||||||||||
| Supplemental disclosure of cash flow information: | |||||||||||||||||
| Cash paid for interest | $ | $ | $ | ||||||||||||||
| Supplemental disclosure of noncash investing and financing activities: | |||||||||||||||||
| Liabilities assumed by TerSera from the XERMELO sale | $ | $ | — | $ | — | ||||||||||||
| Common stock issued in satisfaction of convertible debt exchanges | $ | $ | — | $ | — | ||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-6
Lexicon Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements
December 31, 2020
1. Organization and Operations
Lexicon Pharmaceuticals, Inc. (“Lexicon” or the “Company”) is a Delaware corporation incorporated on July 7, 1995. Lexicon was organized to discover the functions and pharmaceutical utility of genes and use those gene function discoveries in the discovery and development of pharmaceutical products for the treatment of human disease.
Lexicon has financed its operations from inception primarily through sales of common and preferred stock, contract and milestone payments to it under strategic collaborations and other research and development collaborations, target validation, database subscription and technology license agreements, product sales, government grants and contracts and financing under debt and lease arrangements. The Company’s future success is dependent upon many factors, including, but not limited to, the success of its ongoing nonclinical and clinical development efforts and its ability to obtain necessary regulatory approvals of the drug candidates which are the subject of such efforts; TerSera’s ability to successfully develop and commercialize XERMELO for biliary tract cancer and the Company’s receipt of milestone payments and royalties from such efforts; its success in establishing new collaborations and licenses; the amount and timing of research, development and commercialization expenditures; its resources devoted to developing and supporting its products; general and industry-specific economic conditions which may affect research and development expenditures; and its ability to obtain and enforce patents and other proprietary rights in its discoveries, comply with federal and state regulations, and maintain sufficient capital to fund its activities. As a result of the aforementioned factors and the related uncertainties, there can be no assurance of the Company’s future success.
2. Summary of Significant Accounting Policies
F-7
In 2019, customers in Germany and the United States represented 89 % and 10 %, respectively. In 2018, customers in Germany and the United States represented 53 % and 40 % of revenue, respectively. At December 31, 2020, management believes that the Company has no significant concentrations of credit risk.
Property and Equipment: Property and equipment that is held and used is carried at cost and depreciated using the straight-line method over the estimated useful life of the assets which ranges from to 40 years. Maintenance, repairs and minor replacements are charged to expense as incurred. Leasehold improvements are amortized over the shorter of the estimated useful life or the remaining lease term. Significant renewals and betterments are capitalized.
Accrued liabilities: Accrued liabilities consisted of the following:
| As of December 31, | ||||||||||||||
| 2020 | 2019 | |||||||||||||
| (in thousands) | ||||||||||||||
| Accrued research and development services | $ | $ | ||||||||||||
| Accrued compensation and benefits | ||||||||||||||
| Short term lease liability | ||||||||||||||
| Other | ||||||||||||||
| Accrued liabilities | $ | $ | ||||||||||||
F-8
Revenue Recognition:
Product Revenues
Prior to the Company’s sale of XERELO and related assets to TerSera in September 2020, product revenues consisted of commercial sales of XERMELO in the United States and sales of bulk tablets of XERMELO to Ipsen. Product revenues were recognized when the customer obtains control of the Company’s product, which occurs upon delivery to the customer. The Company recognized product revenue net of applicable reserves for variable consideration, including allowances for customer credits, estimated rebates, chargebacks, discounts, returns, distribution service fees, and government rebates, such as Medicare Part D coverage gap reimbursements in the United States, as discussed below. These estimates were based on the most likely amount method for relevant factors such as current contractual and statutory requirements, industry data and forecasted customer buying and payment patterns. Product shipping and handling costs were considered a fulfillment activity when control transfers to the Company’s customers and such costs were included in cost of sales.
Customer Credits: The Company’s customers were offered various forms of consideration, including allowances, service fees and prompt payment discounts. The Company expected that its customers would earn prompt payment discounts. As a result, the Company deducted the full amount of those discounts from total product sales when revenues were recognized. Service fees were also deducted from product sales as they were earned.
Rebates: Allowances for rebates include mandated discounts under the Medicaid Drug Rebate Program. Rebate amounts are based upon contractual agreements or legal requirements with public sector (e.g., Medicaid) benefit providers. Rebates are amounts owed after the final dispensing of the product to a benefit plan participant and are based upon contractual agreements or legal requirements with public sector benefit providers. The allowance for rebates is based on statutory discount rates and expected utilization. The Company’s estimates for expected utilization of rebates were based on third party market research data and data received from the specialty pharmacies. Rebates were generally invoiced and paid in arrears so that the accrual balance consisted of an estimate of the amount expected to be incurred for the current quarter’s activity, plus an accrual balance for known unpaid rebates from the prior quarter. If actual future rebates varied from estimates, the Company adjusted prior period accruals, which affected revenue in the period of adjustment.
Chargebacks: Chargebacks are discounts that occur when contracted customers purchase directly from a specialty pharmacy or distributor, who acts as a retailer. Contracted customers, which consisted primarily of Public Health Service Institutions, non-profit clinics, and federal government entities purchasing via the Federal Supply Schedule, generally purchase the product at a discounted price. The specialty pharmacy or distributor, in turn, charged back to Lexicon the difference between the price paid by the specialty pharmacy or distributor and the discounted price paid to the specialty pharmacy or distributor by the customer. The allowance for chargeback was based on known sales to contracted customers.
Medicare Part D Coverage Gap: The Medicare Part D prescription drug benefit mandates manufacturers to fund a portion of the Medicare Part D insurance coverage gap for prescription drugs sold to eligible patients. The Company’s estimates for the expected Medicare Part D coverage gap were based on data received from the specialty pharmacies and projections based on historical data. Funding of the coverage gap was generally invoiced and paid in arrears so that the accrual balance consisted of an estimate of the amount expected to be incurred for the current quarter’s activity, plus an accrual balance for known prior quarters. If actual future funding varied from estimates, the Company adjusted prior period accruals, which affected revenues in the period of adjustment.
Co-payment assistance: Patients who have commercial insurance and meet certain eligibility requirements may receive co-payment assistance. The Company accrued a liability for co-payment assistance based on actual program participation and estimates of program redemption using data provided by third-party administrators.
F-9
Revenues under collaborative agreements include both license revenue and contract research revenue. The Company performs the following five steps in determining the amount of revenue to recognize as it fulfills its performance obligations under each of its agreements: (i) identify the contract(s) with a customer; (ii) identify the performance obligation in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligation in the contract, and (v) recognize revenue when (or as) the Company satisfies the performance obligation. The Company applies this five-step model to contracts when it is probable that the Company will collect the consideration it is entitled to in exchange for the goods or services it transfers to the customer. At contract inception, the Company assesses the goods or services promised within each contract and determines those that are performance obligations, and assesses whether each promised good or service is distinct. The Company then recognizes as revenue the amount of the transaction price that is allocated to the respective performance obligation when (or as) the performance obligation is satisfied. The Company develops assumptions that require judgment to determine the stand-alone selling price for each performance obligation identified in the contract.
At contract inception, the Company evaluates whether development milestones are considered probable of being reached and estimates the amount to be included in the transaction price using the most likely amount method. If it is probable that a significant revenue reversal will not occur, the associated development milestone value is included in the transaction price. Development milestones that are not within the control of the Company or the licensee, including those requiring regulatory approval, are not considered probable of being achieved until those approvals are received. The transaction price is allocated to each performance obligation on a relative stand-alone selling price basis, for which the Company recognizes revenue when (or as) the performance obligation is satisfied. At the end of each reporting period, the Company re-evaluates the probability of achievement of the development milestones and any related constraint, and if necessary, adjusts its estimates of the overall transaction price. Any such adjustments are recorded on a cumulative catch-up basis, which would affect collaboration revenues in the period of adjustment.
In agreements in which a license to the Company’s intellectual property is determined distinct from other performance obligations identified in the agreement, the Company recognizes revenue when the license is transferred to the licensee and the licensee is able to use and benefit from the license.
For agreements that include sales-based royalties, including milestones based on a level of sales, the license is deemed to be the predominant item to which the royalties relate and the Company recognizes revenue at the later of (i) when the related sales occur, or (ii) when the performance obligation to which some or all of the royalty has been allocated has been satisfied (or partially satisfied).
The Company may receive payments from its licensees based on billing schedules established in each contract. Upfront payments and fees are recorded as deferred revenue upon receipt or when due, and may require deferral of revenue recognition to a future period until the Company performs its obligations under these agreements. Amounts are recorded as accounts receivable when the Company’s right to consideration is unconditional.
F-10
Stock-Based Compensation: The Company recognizes compensation expense in its statements of comprehensive income (loss) for share-based payments, including stock options and restricted stock units issued to employees, based on their fair values on the date of the grant, with the compensation expense recognized over the period in which an employee is required to provide service in exchange for the stock award. Stock-based compensation expense for awards without performance conditions is recognized on a straight-line basis. Stock-based compensation expense for awards with performance conditions is recognized over the period from the date the performance condition is determined to be probable of occurring through the time the applicable condition is met. As of December 31, 2020, stock-based compensation cost for all outstanding unvested options and restricted stock units was $15.7 million, which is expected to be recognized over a weighted-average period of 1.1 years.
| Expected Volatility | Risk-free Interest Rate | Expected Term | Dividend Rate | ||||||||||||||||||||
| December 31, 2020: | |||||||||||||||||||||||
| Employees | % | ||||||||||||||||||||||
| Officers and non-employee directors | % | ||||||||||||||||||||||
| December 31, 2019: | |||||||||||||||||||||||
| Employees | % | ||||||||||||||||||||||
| Officers and non-employee directors | % | ||||||||||||||||||||||
| December 31, 2018: | |||||||||||||||||||||||
| Employees | % | ||||||||||||||||||||||
| Officers and non-employee directors | % | ||||||||||||||||||||||
Income Taxes: The Company recognizes deferred tax liabilities and assets for the expected future tax consequences of events that have been recognized differently in the financial statements and tax returns. The Company uses the liability method in accounting for income taxes. Under this method, deferred tax liabilities and assets are determined based on the difference between the financial statement carrying amounts and tax bases of liabilities and assets using enacted tax rates and laws in effect in the years in which the differences are expected to reverse. Deferred tax assets are evaluated for realization based on a more-likely-than-not criteria in determining if a valuation allowance should be provided.
The Company maintains a valuation allowance on net operating losses and other deferred tax assets. Accordingly, the Company has not reported any tax benefit relating to the remaining net operating loss carryforwards and income tax credit carryforwards that are available for utilization in future periods. On a periodic basis, the valuation allowance is reassessed on deferred income tax assets, weighing positive and negative evidence to assess the recoverability of the deferred tax assets. In 2020, the Company reassessed the valuation allowance and considered negative evidence, including the cumulative losses over the three years ended December 31, 2020, and positive evidence, including the income during the year ended December 31, 2020 and projections of future income. After assessing both the negative evidence and the positive evidence, the Company concluded that it should continue to maintain the valuation allowance on net operating losses and other deferred tax assets as of December 31, 2020 given the significance of the weight of the negative evidence. Based on recent financial performance and future projections, the Company could record a reversal of all, or a portion of the valuation allowance associated with U.S. deferred tax assets in future periods. However, any such change is subject to actual performance and other considerations that may present positive or negative evidence at the time of the assessment. The total deferred tax asset balance subject to the valuation allowance was approximately $309.0 million at December 31, 2020.
F-11
3. Recent Accounting Pronouncements
In November 2018, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2018-18, Collaborative Arrangements (Topic 808): Clarifying the Interaction between Topic 808 and Topic 606. This targeted amendment to Topic 808 clarifies that certain transactions resulting from a collaborative agreement should be accounted for as revenue under Topic 606 when the collaborative arrangement participant is a customer for a good or service that is a distinct unit-of-account. This amendment is effective for fiscal years, and interim periods within years presented, beginning after December 15, 2019, and should be applied retrospectively to the date of initial application of Topic 606. The Company has applied the provisions of Topic 606 to account for its transactions for collaboration arrangements, including recognition, measurement, presentation and disclosure requirements, and adoption of this ASU did not have a material impact on its consolidated financial statements.
In January 2017, the FASB issued ASU No. 2017-04, Intangibles-Goodwill and Other, which is intended to simplify the subsequent measurement of goodwill. The pronouncement allows an entity, during its annual or interim goodwill impairment evaluation, to compare the fair value of a reporting unit with its carrying amount. An impairment charge is immediately recognized by which the carrying amount exceeds the fair value. This ASU is effective for fiscal years, and interim periods within those years, beginning after December 15, 2019. The adoption of this ASU did not have a material impact on the consolidated financial statements.
4. Asset Sale
In September 2020, the Company completed the sale of its XERMELO (telotristat ethyl) product and related assets (the “XERMELO sale”) to TerSera Therapeutics LLC (“TerSera”) pursuant to an Asset Purchase and Sale Agreement entered into in July 2020. The final consideration paid by TerSera was $160.0 million and the net gain recognized in connection with the XERMELO sale was $132.6 million. The gain is reflected on the consolidated statement of comprehensive income (loss) for the year ended December 31, 2020.
The Company remains eligible to receive development, regulatory and sales milestone payments of up to an aggregate of $65.0 million for the development and commercialization of telotristat ethyl in patients with biliary tract cancer and mid-teens royalty payments on net sales of XERMELO in biliary tract cancer. The Company has determined that these amounts are constrained until the achievement, if any, of specific events. If or when the constraint is determined to be resolved, the Company will re-evaluate the overall gain in connection with the XERMELO sale and recognize an adjustment on a cumulative catch-up basis in the period that the determination is made. Such adjustment would be included in Gain on sale of XERMELO in the consolidated statement of comprehensive income (loss).
The XERMELO sale did not meet the criteria for reporting discontinued operations as there was not a strategic shift that has (or will have) a major effect on the Company’s operations. For the years ended December 31, 2020, 2019 and 2018, the pretax net loss on the condensed consolidated statement of comprehensive income (loss) for the Company’s XERMELO operations is $12.2 million, $15.4 million and $33.7 million, respectively.
As a result of the XERMELO sale, the Company implemented a reduction in force which reduced its workforce by approximately fifty percent. The Company incurred and recognized severance charges of approximate $5.5 million. Of this charge, $2.5 million was recorded in research and development expense and $3.0 million was recorded in selling, general and administrative expense in the accompanying consolidated statement of comprehensive income (loss) for the year ended December 31, 2020.
F-12
5. Cash, Cash Equivalents and Investments
The fair value of cash and cash equivalents and investments held at December 31, 2020 and 2019 are as follows:
| As of December 31, 2020 | |||||||||||||||||||||||
| Amortized Cost | Gross Unrealized Gains | Gross Unrealized Losses | Estimated Fair Value | ||||||||||||||||||||
| (in thousands) | |||||||||||||||||||||||
| Cash and cash equivalents | $ | $ | $ | $ | |||||||||||||||||||
| Securities maturing within one year: | |||||||||||||||||||||||
| Corporate debt securities | ( | ||||||||||||||||||||||
| Total short-term investments | $ | $ | $ | ( | $ | ||||||||||||||||||
| Total cash and cash equivalents and investments | $ | $ | $ | ( | $ | ||||||||||||||||||
| As of December 31, 2019 | |||||||||||||||||||||||
| Amortized Cost | Gross Unrealized Gains | Gross Unrealized Losses | Estimated Fair Value | ||||||||||||||||||||
| (in thousands) | |||||||||||||||||||||||
| Cash and cash equivalents | $ | $ | $ | $ | |||||||||||||||||||
| Securities maturing within one year: | |||||||||||||||||||||||
| U.S. treasury securities | ( | ||||||||||||||||||||||
| Total short-term investments | $ | $ | $ | ( | $ | ||||||||||||||||||
| Total cash and cash equivalents and investments | $ | $ | $ | ( | $ | ||||||||||||||||||
6. Fair Value Measurements
The Company uses various inputs in determining the fair value of its investments and measures these assets on a recurring basis. Assets and liabilities recorded at fair value in the consolidated balance sheets are categorized by the level of objectivity associated with the inputs used to measure their fair value. The following levels are directly related to the amount of subjectivity associated with the inputs to fair valuation of these assets and liabilities:
•Level 1 – quoted prices in active markets for identical assets, which include U.S. treasury securities
•Level 2 – other significant observable inputs (including quoted prices for similar investments, market corroborated inputs, etc.), which include corporate debt securities
•Level 3 – significant unobservable inputs
The inputs or methodology used for valuing securities are not necessarily an indication of the credit risk associated with investing in those securities. The following tables provide the fair value measurements of applicable Company assets and liabilities that are measured at fair value on a recurring basis according to the fair value levels defined above as of December 31, 2020 and 2019.
F-13
| Assets and Liabilities at Fair Value | |||||||||||||||||||||||
| As of December 31, 2020 | |||||||||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | ||||||||||||||||||||
| (in thousands) | |||||||||||||||||||||||
| Assets | |||||||||||||||||||||||
| Cash and cash equivalents | $ | $ | $ | $ | |||||||||||||||||||
| Short-term investments | |||||||||||||||||||||||
| Total cash and cash equivalents and investments | $ | $ | $ | $ | |||||||||||||||||||
| Assets and Liabilities at Fair Value | |||||||||||||||||||||||
| As of December 31, 2019 | |||||||||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | ||||||||||||||||||||
| (in thousands) | |||||||||||||||||||||||
| Assets | |||||||||||||||||||||||
| Cash and cash equivalents | $ | $ | $ | $ | |||||||||||||||||||
| Short-term investments | |||||||||||||||||||||||
| Total cash and cash equivalents and investments | $ | $ | $ | $ | |||||||||||||||||||
The Company did not have any Level 3 assets or liabilities at December 31, 2020 or 2019. Transfers between levels are recognized at the actual date of circumstance that caused the transfer. There were no transfers between Level 1 and Level 2 during the periods presented.
Refer to Note 10, Debt Obligations, for fair value measurements of debt obligations.
7. Property and Equipment
Property and equipment at December 31, 2020 and 2019 are as follows:
| Estimated Useful Lives | As of December 31, | ||||||||||||||||||||||
| In Years | 2020 | 2019 | |||||||||||||||||||||
| (in thousands) | |||||||||||||||||||||||
| Computers and software | $ | $ | |||||||||||||||||||||
| Furniture and fixtures | |||||||||||||||||||||||
| Laboratory equipment | |||||||||||||||||||||||
| Leasehold improvements | |||||||||||||||||||||||
| Buildings | |||||||||||||||||||||||
| Land | — | ||||||||||||||||||||||
| Total property and equipment | |||||||||||||||||||||||
| Less: Accumulated depreciation and amortization | ( | ( | |||||||||||||||||||||
| Net property and equipment | $ | $ | |||||||||||||||||||||
In December 2020, the Company’s subsidiary, Lex-Gen Woodlands, L.P., sold its facilities in The Woodlands, Texas for $11.9 million. The land had a carrying value of $2.7 million and buildings and related assets sold had a net carrying value of $7.9 million. Concurrent with the sale, the Company entered into a leaseback agreement with the purchaser with respect to a portion of such facilities for a period of up to six months.
F-14
8. Income Taxes
The Coronavirus Aid, Relief and Economic Security Act (“CARES Act”) was enacted on March 27, 2020 in the United States to provide emergency assistance to individuals and businesses affected by the COVID-19 pandemic. The CARES Act includes temporary changes to both income and non-income based tax laws. For the year ended December 31, 2020 the impact of the CARES Act was immaterial to the Company’s tax provision. Future regulatory guidance under the CARES Act or additional legislation enacted by Congress in connection with the COVID-19 pandemic could impact our tax provision in future periods.
Lexicon recognizes deferred tax liabilities and assets for the expected future tax consequences of events that have been recognized differently in the financial statements and tax returns. Under this method, deferred tax liabilities and assets are determined based on the difference between the financial statement carrying amounts and tax bases of liabilities and assets using enacted tax rates and laws in effect in the years in which the differences are expected to reverse. Deferred tax assets are evaluated for realization based on a more-likely-than-not criteria in determining if a valuation allowance should be provided.
The components of Lexicon’s deferred tax assets (liabilities) at December 31, 2020 and 2019 are as follows:
| As of December 31, | |||||||||||
| 2020 | 2019 | ||||||||||
| (in thousands) | |||||||||||
| Deferred tax assets: | |||||||||||
| Net operating loss carryforwards | $ | $ | |||||||||
| Research and development tax credits | |||||||||||
| Orphan drug credits | |||||||||||
| Capitalized research and development | |||||||||||
| Stock-based compensation | |||||||||||
| Other | |||||||||||
| Total deferred tax assets | |||||||||||
| Deferred tax liabilities: | |||||||||||
| Deferred tax liability related to acquisition of Symphony Icon | ( | ||||||||||
| Other | ( | ||||||||||
| Total deferred tax liabilities | ( | ||||||||||
| Less: valuation allowance | ( | ( | |||||||||
| Net deferred tax liabilities | $ | $ | |||||||||
F-15
A reconciliation of the statutory tax rate to the effective tax rate for the years ended December 31, 2020, 2019 and 2018 consists of the following:
| Year Ended December 31, | |||||||||||||||||
| 2020 | 2019 | 2018 | |||||||||||||||
| (in thousands) | |||||||||||||||||
Expected income tax expense (benefit) at | $ | ( | $ | $ | ( | ||||||||||||
| State income taxes, net of federal benefit | ( | ( | |||||||||||||||
| Equity compensation | |||||||||||||||||
| Research and development credit | ( | ||||||||||||||||
| Write off of credit carryover due to 382 study | — | — | |||||||||||||||
| Change in valuation allowance | ( | ( | |||||||||||||||
Other (1) | |||||||||||||||||
| Income tax benefit | $ | $ | ( | $ | |||||||||||||
(1) Other is primarily comprised of expiring Research and Development credits for the years ended December 31, 2019 and nondeductible expenses for the years ended December 31, 2020 and 2018.
Section 382 of the Internal Revenue Code of 1986, contains rules that limit the ability of a company that undergoes an “ownership change” to utilize its net operating loss and tax credit carryovers and certain built-in losses recognized in years after the “ownership change.” An “ownership change” is generally defined as any change in ownership of more than 50% of a corporation’s stock over a rolling three-year period by stockholders that own (directly or indirectly) 5% or more of the stock of a corporation, or arising from a new issuance of stock by a corporation. If an ownership change occurs, Section 382 generally imposes an annual limitation on the use of pre-ownership change net operating loss carryovers to offset taxable income earned after the ownership change. The Company completed a Section 382 study and determined that historical ownership changes occurred. The Section 382 annual limitation related to historical ownership changes impacts our ability to utilize our tax attributes. In 2020, the federal deferred tax assets and valuation allowance decreased due to the write-off of attributes that will expire still subject to limitation.
At December 31, 2020, Lexicon had both federal and state NOL carryforwards of approximately $918.1 million and $86.9 million, respectively. In 2020, federal NOLs increased by $38.4 million primarily related to a current year NOL partially offset by $66.9 million decrease in historical NOLs which will expire still subject to Section 382 limitation. The state NOL carryforwards increased due to a legislative change from pre-apportionment to post-apportionment reporting in New Jersey. The federal and state NOL carryforwards will begin to expire in 2024. In 2020, the Company’s R&D tax credit carryforwards decreased by $17.0 million due to Section 382 limitations to approximately $29.3 million which will begin to expire in 2027. Based on the federal tax law limits and the Company’s cumulative loss position, the Company concluded it was appropriate to establish a full valuation allowance for its net deferred tax assets until an appropriate level of profitability is sustained. During the year ended December 31, 2020, the valuation allowance decreased $20.4 million, primarily due to decreases in deferred tax assets associated with attributes subject to IRC 382 limitation and capitalized research and development expenses partially offset by NOLs generated in the current year and the reversal of the deferred tax liability related to the acquisition of Symphony Icon.
Lexicon recorded an income tax benefit of $6.0 million in the year ended December 31, 2019 despite reporting pretax income for the year. The result reflects the impact of the impairment of intangible assets associated with Symphony Icon and the benefit from the utilization of federal NOLs for which a tax benefit had not previously been recognized, partially offset by nondeductible expenses. There were no income tax benefits in the years ended December 31, 2020 and 2018, respectively. As of December 31, 2020 and 2019, the Company did not have any unrecognized tax benefits.
The Company is primarily subject to U.S. federal and New Jersey and Texas state income taxes. The tax years 1999 to current remain open to examination by U.S. federal authorities and 2012 to current remain open to examination by state authorities. The Company’s policy is to recognize interest and penalties related to income tax matters in income tax expense. As of December 31, 2020 and 2019, the Company had no
F-16
9. Goodwill
On July 12, 2001, Lexicon completed the acquisition of Coelacanth Corporation in a merger. Coelacanth, now Lexicon Pharmaceuticals (New Jersey), Inc., formed the core of the Company’s division responsible for small molecule compound discovery. The results of Lexicon Pharmaceuticals (New Jersey), Inc. are included in the Company’s results of operations for the period subsequent to the acquisition. Goodwill associated with the acquisition of $25.8 million, which represents the excess of the $36.0 million purchase price over the fair value of the underlying net identifiable assets, was assigned to the consolidated entity, Lexicon.
On July 30, 2010, Lexicon exercised its Purchase Option and completed the acquisition of Symphony Icon, Inc. Goodwill associated with the acquisition of $18.7 million, which represents the assets recognized in connection with the deferred tax liability acquired and did not result from excess purchase price, was assigned to the consolidated entity, Lexicon.
Goodwill is not subject to amortization, but is tested at least annually for impairment at the reporting unit level, which is the Company’s single operating segment. The Company performed an impairment test of goodwill on its annual impairment assessment date. This test did not result in an impairment of goodwill.
10. Debt Obligations
Convertible Notes. In November 2014, Lexicon completed an offering of $87.5 million in aggregate principal amount of its 5.25 % Convertible Senior Notes due 2021 (the “Convertible Notes”). The conversion feature did not meet the criteria for bifurcation as required by generally accepted accounting principles and the entire principal amount was recorded as long-term debt on the Company’s consolidated balance sheets.
In 2020, the Company entered into separate, privately negotiated exchange agreements to exchange $75.8 million aggregate principal amount of the Convertible Notes for consideration valued at 85 % of the principal amount of the Convertible Notes. In 2020, the Company issued 10,368,956 shares of the Company’s common stock and paid $50.0 million in cash, which included $1.3 million of accrued interest, to exchange such Convertible Notes. The Company recorded the exchanges under the accounting requirements for debt extinguishment of convertible instruments. As a result, a debt extinguishment gain of $9.6 million was recorded and is included in the accompanying consolidated statement of comprehensive income (loss) for the year ended December 31, 2020. As of December 31, 2020, the carrying value of the remaining Convertible Notes was $11.6 million.
The remaining Convertible Notes are governed by an indenture (the “Indenture”), dated as of November 26, 2014, between the Company and Wells Fargo Bank, N.A., as trustee. The Convertible Notes bear interest at a rate of 5.25 % per year, payable semiannually in arrears on June 1 and December 1 of each year, beginning on June 1, 2015. The Convertible Notes mature on December 1, 2021. The Company may not redeem the Convertible Notes prior to the maturity date, and no sinking fund is provided for the Convertible Notes.
Holders of the Convertible Notes may convert their Convertible Notes at their option at any time prior to the close of business on the business day immediately preceding the maturity date. Upon conversion, the Company will deliver for each $1,000 principal amount of converted Convertible Notes a number of shares of its common stock equal to the conversion rate, as described in the Indenture. The conversion rate is initially 118.4553 shares of common stock per $1,000 principal amount of Convertible Notes (equivalent to an initial conversion price of $8.442 per share of common stock). The conversion rate is subject to adjustment in some events but will not be adjusted for any accrued and unpaid interest. In addition, following certain corporate events that occur prior to the maturity date, the Company will increase the conversion rate for a holder who elects to convert its Convertible Notes in connection with such a corporate event in certain circumstances.
If the Company undergoes a fundamental change, holders may require the Company to repurchase for cash all or any portion of their Convertible Notes at a fundamental change repurchase price equal to 100 % of the principal amount of the Convertible Notes to be repurchased, plus accrued and unpaid interest to, but excluding, the fundamental change repurchase date.
In connection with the issuance of the Convertible Notes, the Company incurred $3.4 million of debt issuance costs. The debt issuance costs are amortized as interest expense over the expected life of the Convertible Notes using the effective interest method. The Company determined the expected life of the debt was equal to the -year term of the Convertible Notes. As of December 31, 2020, the balance of unamortized debt issuance costs was $0.1 million, which was adjusted upon execution of the exchange agreements and offsets long-term debt on the consolidated balance sheets.
F-17
The fair value of the remaining Convertible Notes was $9.2 million as of December 31, 2020 and was determined using Level 2 inputs based on the indicative pricing published by certain investment banks or trading levels of the Convertible Notes, which are not listed on any securities exchange or quoted on an inter-dealer automated quotation system.
Mortgage Loan. In August 2018, a wholly owned subsidiary of Lexicon entered into a term loan and security agreement refinancing the previously existing mortgage on its facilities in The Woodlands, Texas (the “Property”). The Company recorded the refinancing as a debt extinguishment, with no recognition of gain or loss on the transaction. The loan agreement provided for a $12.9 million mortgage on the Property and had a -year term with a 10 -year amortization. The mortgage loan bore interest at a rate per annum equal to the greater of (a) the 30-day LIBOR rate plus 5.5 7.5 10.3 11.0 million and was included in current portion of long-term debt.
BioPharma Term Loan. In December 2017, Lexicon entered into a loan agreement with BioPharma Credit PLC and BioPharma Credit Investments IV Sub LP under which $150 million was funded in December 2017 (the “BioPharma Term Loan”). The BioPharma Term Loan was scheduled to mature in December 2022, bore interest at 9 % per year, subject to additional interest if an event of default occurred and was continuing, and was payable quarterly.
The BioPharma Term Loan was subject to mandatory prepayment provisions that required prepayment upon a change of control or receipt of proceeds from certain non-ordinary course transfers of assets. The Company repaid the BioPharma Term Loan in whole, together with required prepayment and make-whole premiums, upon closing of the XERMELO Sale in September 2020. The Company recorded the repayment under the accounting requirements for debt extinguishment and as a result, a loss of $8.6 million was recognized and is included in the accompanying consolidated statement of comprehensive income (loss) for the year ended December 31, 2020.
The Company’s obligations under the BioPharma Term Loan were secured by a first lien security interest in substantially all of the assets of the Company and certain of its subsidiaries, other than its facilities in The Woodlands, Texas. The loan agreement contained certain customary representations and warranties, affirmative and negative covenants and events of default applicable to the Company and certain of its subsidiaries, including among other things, covenants restricting dispositions, fundamental changes in our business, mergers or acquisitions, indebtedness, encumbrances, distributions, investments, transactions with affiliates and subordinated debt. If an event of default occurred and was continuing, all amounts outstanding under the BioPharma Term Loan may have been declared immediately due and payable.
11. Commitments and Contingencies
Operating Lease Obligations: A wholly-owned subsidiary of Lexicon leases office space in Basking Ridge, New Jersey under a lease agreement, the term of which began in June 2015 and terminates in December 2022. As of December 31, 2020, the office space lease right-of-use (ROU) asset had a balance of $1.2 million, which is included in other assets in the consolidated balance sheet, and current and non-current liabilities relating to the ROU asset were $0.6 million and $0.6 million, respectively, which are included in accrued liabilities and other long-term liabilities in the consolidated balance sheet, respectively. The discount rate used to record the office space lease was Lexicon’s estimated borrowing rate of 9 %. Lexicon elected to apply the short-term lease exception to all leases with a term of one year or less.
F-18
The following table reconciles the undiscounted cash flows of the operating lease liability to the recorded lease liability at December 31, 2020:
| (in thousands) | |||||
| 2021 | $ | ||||
| 2022 | |||||
| 2023 | |||||
| 2024 | |||||
| 2025 | |||||
| Thereafter | |||||
| Total undiscounted operating lease liability | |||||
| Less: amount of lease payments representing interest | ( | ||||
| Present value of future lease payments | |||||
| Less: short-term operating lease liability | ( | ||||
| Long-term operating lease liability | $ | ||||
Employment Arrangements: Lexicon has entered into employment arrangements with certain of its corporate officers. Under the arrangements, each officer receives a base salary, subject to adjustment, with an annual discretionary bonus based upon specific objectives to be determined by the compensation committee. The employment arrangements are at-will and some contain non-competition agreements. Some of the arrangements also provide for certain severance payments for either or 12 months and, in some cases, payment of a specified portion of the officer’s bonus target for such year, in the event of a specified termination of the officer’s employment.
Legal Proceedings: On January 28, 2019, a purported securities class action complaint captioned Daniel Manopla v. Lexicon Pharmaceuticals, Inc., Lonnel Coats, Jeffrey L. Wade and Pablo Lapuerta, M.D. was filed against the Company and certain of its officers in the U.S. District Court for the Southern District of Texas, Houston Division. Our motion to dismiss was granted and the action was dismissed with prejudice by the District Court on August 14, 2020. The lead plaintiffs filed a notice of appeal to the U.S. Court of Appeals for the Fifth Circuit on September 11, 2020 and a brief in support of its appeal on December 17, 2020. The Company filed a response brief on February 18, 2021 and the lead plaintiffs filed a reply brief on March 11, 2021. The lawsuit purports to be a class action brought on behalf of purchasers of the Company’s securities during the period from March 11, 2016 through July 29, 2019. The complaint alleges that the defendants violated federal securities laws by making materially false and misleading statements and/or omissions concerning data from its Phase 3 clinical trials of sotagliflozin in type 1 diabetes patients and the prospects of FDA approval of sotagliflozin for the treatment of type 1 diabetes. The complaint purports to assert claims for violations of Sections 10(b) and 20(a) of the Securities Exchange Act of 1934 and Rule 10b-5 promulgated thereunder. The complaint seeks, on behalf of the purported class, an unspecified amount of monetary damages, interest, fees and expenses of attorneys and experts, and other relief.
On October 16, 2020, Lexicon initiated arbitration proceedings against Sanofi-Aventis Deutschland GmbH, or Sanofi, seeking to recover damages for breach of contract relating to the Termination and Settlement Agreement and Mutual Releases with Sanofi, dated September 9, 2019. In September 2020, Sanofi withheld approximately $23.2 million from the final $26 million payment due to the Company under the termination and settlement agreement, offsetting certain third party costs and internal costs incurred by Sanofi and asserted by Sanofi to be payable by the Company under the terms of the termination and settlement agreement. Lexicon disputes that at least a significant portion of such costs are properly reimbursable by the Company under the terms of the termination and settlement agreement and assert that, in any event, Sanofi was not permitted to withhold any of such costs under the terms of the termination and settlement agreement. Lexicon is seeking payment of up to $23.2 million in such disputed costs, together with late interest and attorneys’ fees and costs. Sanofi is seeking a declaratory judgment that Lexicon is liable for all disputed costs previously withheld and damages for any additional costs properly reimbursable under the terms of the termination and settlement agreement in excess of those previously withheld, together with late interest and attorneys’ fees.
In addition, Lexicon is from time to time party to claims and legal proceedings that arise in the normal course of its business and that it believes will not have, individually or in the aggregate, a material adverse effect on its results of operations, financial condition or liquidity.
F-19
12. Equity Incentive Awards
Equity Incentive Plans
2017 Equity Incentive Plan: In September 1995, Lexicon adopted the 1995 Stock Option Plan, which was subsequently amended and renamed the 2017 Equity Incentive Plan (the “Equity Incentive Plan”).
The Equity Incentive Plan provides for the grant of incentive stock options to employees and nonstatutory stock options to employees, directors and consultants of the Company. The plan also permits the grant of stock bonus awards, restricted stock awards, restricted stock unit awards, stock appreciation rights and performance stock awards. Incentive and nonstatutory stock options have an exercise price of 100 % or more of the fair market value of the Company’s common stock on the date of grant. Most stock options granted under the Equity Incentive Plan become vested and exercisable over a period of four years ; however some have been granted with different vesting schedules. Stock options granted under the Equity Incentive Plan have a term of ten years from the date of grant.
The total number of shares of common stock that may be issued pursuant to stock awards under the Equity Incentive Plan shall not exceed in the aggregate 30,000,000 shares at December 31, 2020. In the first quarter of 2020, the Company amended the 2017 Equity Incentive Plan to increase the aggregate number of shares that may be issued under the plan to 30,000,000 shares. As of December 31, 2020, options to purchase 8,026,989 shares and 2,683,875 restricted stock units were outstanding, 1,909,515 shares had been issued upon the exercise of stock options, 3,160,935 shares had been issued pursuant to restricted stock units and 113,940 shares had been issued pursuant to stock bonus awards or restricted stock awards granted under the Equity Incentive Plan.
2017 Non-Employee Directors’ Equity Incentive Plan: In February 2000, Lexicon adopted the 2000 Non-Employee Directors’ Stock Option Plan, which was subsequently amended and renamed the 2017 Non-Employee Directors’ Equity Incentive Plan (the “Directors’ Plan”). Under the Directors’ Plan, non-employee directors may be granted awards under the plan with an aggregate grant date fair value of no more than $500,000 during any calendar year, taken together with any cash fees paid to such non-employee director in compensation for service on Lexicon’s board of directors during such calendar year. Stock options granted under the Directors’ Plan have an exercise price equal to the fair market value of the Company’s common stock on the date of grant and a term of ten years from the date of grant.
The total number of shares of common stock that may be issued pursuant to stock awards under the Directors’ Plan shall not exceed in the aggregate 600,000 shares. As of December 31, 2020, stock options to purchase 369,917 shares were outstanding, none had been issued upon the exercise of stock options, 85,104 restricted stock units were outstanding and 130,936 shares had been issued pursuant to restricted stock and restricted stock unit awards granted under the Directors’ Plan.
Stock Option Activity: The following is a summary of stock option activity under Lexicon’s equity incentive plans:
| 2020 | 2019 | 2018 | ||||||||||||||||||||||||||||||||||||
| (in thousands, except exercise price data) | Options | Weighted Average Exercise Price | Options | Weighted Average Exercise Price | Options | Weighted Average Exercise Price | ||||||||||||||||||||||||||||||||
| Outstanding at beginning of year | $ | $ | $ | |||||||||||||||||||||||||||||||||||
| Granted | ||||||||||||||||||||||||||||||||||||||
| Exercised | ( | |||||||||||||||||||||||||||||||||||||
| Expired | ( | ( | ( | |||||||||||||||||||||||||||||||||||
| Forfeited | ( | ( | ( | |||||||||||||||||||||||||||||||||||
| Outstanding at end of year | ||||||||||||||||||||||||||||||||||||||
| Exercisable at end of year | $ | $ | $ | |||||||||||||||||||||||||||||||||||
The weighted average estimated grant date fair value of stock options granted during the years ended December 31, 2020, 2019 and 2018 were $2.35 , $3.18 and $5.63 , respectively. The total intrinsic value of stock options exercised during the year ended December 31, 2018 was $0.2 million. The weighted average remaining contractual term of stock options outstanding and exercisable was 6.8 and 5.2 years, respectively, as of December 31, 2020. At December 31, 2020, the aggregate intrinsic value of the outstanding stock options was $0.5 million. At December 31, 2020, the intrinsic value of exercisable stock options was zero .
F-20
Stock Bonus and Restricted Stock Unit Activity:
During the year ended December 31, 2020 and 2019, Lexicon granted its non-employee directors 85,104 and 27,728 restricted stock units, respectively, and during the year ended December 31, 2018, granted its non-employee directors 20,512 shares of restricted stock awards. The restricted stock in 2020, 2019 and 2018 had weighted average grant date fair values of $1.86 , $5.67 and $7.80 per share, respectively. Vesting of restricted stock units occurs on the first anniversary of the grant date and vesting of restricted stock awards is immediate.
During the years ended December 31, 2020, 2019 and 2018, Lexicon granted its employees restricted stock units in lieu of or in addition to annual stock option awards. These restricted stock units vest in annual installments. The total fair value of shares vested in 2020, 2019 and 2018 was $3.2 million, $2.9 million and $3.3 million, respectively.
The following is a summary of restricted stock units activity under Lexicon’s stock-based compensation plans for the year ended December 31, 2020:
| Shares | Weighted Average Grant Date Fair Value | |||||||||||||
| (in thousands) | ||||||||||||||
| Outstanding at December 31, 2019 | $ | |||||||||||||
| Granted | ||||||||||||||
| Vested | ( | |||||||||||||
| Forfeited | ( | |||||||||||||
| Outstanding at December 31, 2020 | $ | |||||||||||||
Aggregate Shares Reserved for Issuance
As of December 31, 2020, an aggregate of 11,165,885 shares of common stock were reserved for issuance upon exercise of outstanding stock options and vesting of outstanding restricted stock units and 14,118,789 additional shares were available for future grants under Lexicon’s equity incentive plans. The Company has a policy of using either authorized and unissued shares or treasury shares, including shares acquired by purchase in the open market or in private transactions, to satisfy equity award exercises.
13. Benefit Plan
Lexicon maintains a defined-contribution savings plan under Section 401(k) of the Internal Revenue Code. The plan covers substantially all full-time employees. Participating employees may defer a portion of their pretax earnings, up to the Internal Revenue Service annual contribution limit. Beginning in 2000, the Company was required to match employee contributions according to a specified formula. The matching contributions totaled $0.9 million, $1.2 million and $1.0 million in the years ended December 31, 2020, 2019 and 2018, respectively. Company contributions are vested based on the employee’s years of service, with full vesting after four years of service.
14. Collaboration and License Agreements
Lexicon has derived substantially all of its revenues from drug discovery and development alliances, target validation collaborations for the development and, in some cases, analysis of the physiological effects of genes altered in knockout mice, product sales, government grants and contracts, technology licenses, subscriptions to its databases and compound library sales.
Ipsen. In October 2014, Lexicon entered into a License and Collaboration Agreement, which was subsequently amended in March 2015 (collectively, the “Ipsen Agreement”), with Ipsen for the development and commercialization of XERMELO outside of the United States and Japan (the “Licensed Territory”). The Ipsen Agreement was assigned to TerSera in September 2020 in connection with the XERMELO sale.
Under the Ipsen Agreement, Lexicon granted Ipsen an exclusive, royalty-bearing right and license under its patent rights and know-how to commercialize XERMELO in the Licensed Territory. Ipsen is responsible for using diligent efforts to commercialize XERMELO in the Licensed Territory pursuant to a mutually approved commercialization plan. Subject to certain exceptions, Lexicon was responsible for conducting clinical trials required to obtain regulatory approval for XERMELO
F-21
for carcinoid syndrome in the European Union, including those contemplated by a mutually approved initial development plan, and had the first right to conduct most other clinical trials of XERMELO. Lexicon was responsible for the costs of all clinical trials contemplated by the initial development plan. The costs of additional clinical trials were to be allocated between the parties based on the nature of such clinical trials. Under the Ipsen Agreement, Ipsen paid Lexicon an aggregate of $47.2 million through September 30, 2020, consisting of $24.5 million in upfront payments, a $6.4 million milestone upon the acceptance of the filing submitted by Ipsen to the European Medicines Agency for XERMELO as an adjunct to somatostatin analog therapy for the long-term treatment of carcinoid syndrome, a $5.1 million milestone upon Ipsen’s receipt of approval from the European Commission for the marketing of XERMELO in all member states of the European Union, Norway and Iceland, a $3.8 million milestone upon Ipsen’s first commercial sale in Germany, a $3.8 million milestone upon Ipsen’s first commercial sale in the United Kingdom, a $1.3 million milestone upon Ipsen’s receipt of approval from Health Canada and a $2.3 million milestone upon Ipsen's first commercial sale in Canada. In addition, Lexicon was eligible to receive from Ipsen (a) up to an aggregate of approximately $9.6 million upon the achievement of specified regulatory and commercial launch milestones and (b) up to an aggregate of €72 million upon the achievement of specified sales milestones. Milestone payments that were contingent upon achievement of a substantive milestone were deemed constrained. Lexicon was also entitled to tiered, escalating royalties ranging from low twenties to mid-thirties percentages of net sales of XERMELO in the Licensed Territory, subject to a credit for amounts previously paid to Lexicon by Ipsen for the manufacture and supply of such units of XERMELO. Lexicon and Ipsen had entered into a commercial supply agreement pursuant to which Lexicon supplied Ipsen’s commercial requirements of XERMELO, and Ipsen paid an agreed upon transfer price for such commercial supply.
The Company considered the following as its performance obligations with respect to the revenue recognition of the $24.5 million upfront payment:
•The exclusive license granted to Ipsen to develop and commercialize XERMELO in the Licensed
Territory;
•The development services Lexicon is performing for XERMELO;
•The obligation to participate in committees which govern the development of XERMELO
until commercialization; and
•The obligation to supply commercial supply of XERMELO, under a commercial supply agreement.
The Company determined that the license had stand-alone value because it is an exclusive license that grants Ipsen the right to develop and commercialize XERMELO or to sublicense its rights. In addition, at the time of the agreement, it would have been possible for Ipsen or another third party to conduct clinical trials without assistance from Lexicon. As a result, the Company considered the license and the development services under the Agreement to be separate performance obligations. The Company recognized the portion of the transaction price allocated to the license immediately because Lexicon delivered the license and earned the revenue at the inception of the arrangement. The Company recognized as revenue the amount allocated to the development services and the obligation to participate in committees over the period of time Lexicon performed services, which was completed in 2018.
The Company determined that the commercial supply agreement was a contingent deliverable at the onset. There was inherent uncertainty in obtaining regulatory approval at the time and entry into the commercial supply agreement, thus making the applicability of the commercial supply agreement outside the control of Lexicon and Ipsen. As a result, the Company determined the commercial supply agreement did not meet the definition of a performance obligation that needed to be accounted for at the inception of the arrangement. The Company also determined that there was no significant and incremental discount related to the commercial supply agreement that should have been accounted for at the inception of the arrangement.
The Company determined that the initial transaction price was the $24.5 million upfront payments because they were the only payments that were fixed and determinable at the inception of the arrangement. There was considerable uncertainty at the date of the agreement as to whether Lexicon would earn milestone payments, royalty payments or payments for finished drug product. As such, the Company did not include those payments in the transaction price. The Company allocated the transaction price based on the relative best estimate of selling price of each performance obligation. The Company estimated the selling price of the license deliverable by applying a probability-based income approach utilizing an appropriate discount rate. The significant inputs the Company used to determine the projected income of the license included: estimated future product sales, estimated cost of goods sold, estimated operating expenses, income taxes, and an appropriate discount rate. The Company estimated the selling price of the development services by using internal estimates of the cost to hire third parties to perform the services over the expected period to perform the development. The Company estimated the selling price of the obligation to participate in committees by using internal estimates of the number of internal hours and salary and benefits costs to perform these services.
F-22
As a result of the allocation, the Company recognized $21.2 million of the $24.5 million upfront payment for the license in 2014, and an additional $1.4 million in 2015 upon entering into the amendment. The Company recognized the $1.7 million allocated to the development services deliverable over the estimated period of performance as development occurred, and recognized the $0.1 million allocated to the committee participation deliverable ratably over the period of performance. Milestone payments that were contingent upon the achievement of a substantive milestone were deemed constrained. If or when the constraint was determined to be resolved, the Company re-evaluated the overall transaction price and recognized an adjustment on a cumulative catch-up basis in the period that the adjustment was evaluated. Revenue recognized under the Agreement was $0.3 million, $4.9 million and $4.6 million for the years ended December 31, 2020, 2019 and 2018, respectively. Revenue for each of the years ended December 31, 2020, 2019 and 2018 include $0.3 million of royalties from Ipsen. Revenue for the years ended December 31, 2020, 2019 and 2018 include zero, $1.3 million and $1.6 million, respectively, from sales of bulk tablets of XERMELO to Ipsen.
Sanofi. In November 2015, Lexicon entered into a Collaboration and License Agreement, which was subsequently amended in July 2017 (collectively, the “Sanofi Agreement”), with Sanofi for the worldwide development and commercialization of Lexicon’s diabetes drug candidate sotagliflozin. In December 2016, Sanofi terminated its rights under the Sanofi Agreement with respect to Japan.
Effective as of September 9, 2019 (the “Settlement Date”), Lexicon entered into a Termination and Settlement Agreement and Mutual Releases (the “Termination Agreement”) with Sanofi, pursuant to which the Sanofi Agreement was terminated and associated disputes between Lexicon and Sanofi were settled.
Under the terms of the Termination Agreement, Lexicon regained all rights to sotagliflozin and assumed full responsibility for the worldwide development and commercialization of sotagliflozin in all indications. Sanofi paid Lexicon $208 million in September 2019, $26 million in each of March and September 2020 (less amounts withheld by Sanofi offsetting certain third party costs and internal costs incurred by Sanofi and asserted by Sanofi to be payable by Lexicon under the terms of the Termination Agreement), and neither party will owe additional payments pursuant to the Sanofi Agreement. The parties have cooperated in the transition of responsibility for ongoing clinical studies and other activities, and each party is responsible for its own expenses associated with such transition, subject to certain exceptions. See Note 11, Commitments and Contingencies, for additional information. Beginning in March 2020, Lexicon closed out early the clinical studies related to the Phase 3 development program for sotagliflozin in type 2 diabetes, heart failure and chronic kidney disease.
Under the Sanofi Agreement, Lexicon had granted Sanofi an exclusive, worldwide (excluding Japan), royalty-bearing right and license under its patent rights and know-how to develop, manufacture and commercialize sotagliflozin. Subject to specified exceptions, neither party could (a) perform clinical development activities relating to any other compound which inhibits sodium-glucose cotransporters type 1 or type 2 or (b) commercialize any such compounds in the United States, countries of the European Union and certain other specified countries, in each case during the royalty terms applicable in such countries. Among the specified exceptions was a right Lexicon retained to pursue the development of its development candidate LX2761, with respect to which Lexicon granted Sanofi certain rights of first negotiation specified in the Sanofi Agreement.
Under the Sanofi Agreement, Sanofi paid Lexicon an upfront payment of $300 million. In addition, Lexicon was eligible to receive from Sanofi (a) up to an aggregate of $110 million upon the achievement of four development milestones relating to the results of certain Phase 3 clinical trials of sotagliflozin in type 2 diabetes patients, (b) up to an aggregate of $220 million upon the achievement of four regulatory milestones relating to the first commercial sale following regulatory approval of sotagliflozin for type 1 and type 2 diabetes, respectively, in each of the United States and Europe, of which two milestones representing the substantial majority of such aggregate amount relate to type 2 diabetes and the remaining two milestones relate to type 1 diabetes, (c) $100 million upon the achievement of a milestone based on the results of either of two outcomes studies in type 2 diabetes patients, the completion of which would likely occur after initial regulatory approval of sotagliflozin in type 2 diabetes, and (d) up to an aggregate of $990 million upon the achievement of six commercial milestones that would be achieved upon reaching specified levels of sales. The Company believed that each of the development and regulatory milestones under the Sanofi Agreement was substantive. Due to the uncertainty surrounding the achievement of the future development and regulatory milestones, these payments were deemed constrained and were not recognized as revenue. Commercial milestones would have been accounted for as royalties and recorded as revenue upon achievement of the milestone, assuming all other revenue recognition criteria were met. Lexicon was also entitled to tiered, escalating royalties ranging from low double digit percentages to forty percent of net sales of sotagliflozin, based on indication and territory, with royalties for the higher band of such range attributable to net sales for type 1 diabetes in the United States, and subject in each case to customary royalty reduction provisions.
F-23
Lexicon continued to be responsible for all clinical development activities relating to type 1 diabetes and exercised an exclusive option to co-promote and have a significant role, in collaboration with Sanofi, in the commercialization of sotagliflozin for the treatment of type 1 diabetes in the United States. Under the terms of its co-promotion option, Lexicon would have funded forty percent of the commercialization costs relating to such co-promotion activities. Sanofi was responsible for all clinical development and commercialization of sotagliflozin for the treatment of type 2 diabetes worldwide and would have been solely responsible for the commercialization of sotagliflozin for the treatment of type 1 diabetes outside the United States. Lexicon shared in the funding of a portion of the planned type 2 diabetes development costs over the first years of the collaboration, up to an aggregate of $100 million, which was satisfied in 2018. Sanofi would have booked sales worldwide in all indications.
The parties were responsible for using commercially reasonable efforts to perform their development and commercialization obligations pursuant to mutually approved development and commercialization plans.
The parties’ activities under the Sanofi Agreement were governed by a joint steering committee and certain other governance committees which reflected equal or other appropriate representation from both parties. If the applicable governance committee was not able to make a decision by consensus and the parties were not able to resolve the issue through escalation to specified senior executive officers of the parties, then Sanofi would have final decision-making authority, subject to limitations specified in the Sanofi Agreement.
The Sanofi Agreement would have expired upon the expiration of all applicable royalty terms for all licensed products in all countries. The royalty term for each licensed product in each country was the period commencing on the effective date of the Sanofi Agreement and ending on the latest of expiration of specified patent coverage, expiration of specified regulatory exclusivity and 10 years following the first commercial sale in the applicable country. Either party had the right to terminate the Sanofi Agreement in the event of an uncured material breach by the other party. Prior to completion of the core development activities for type 2 diabetes specified in the development plan, Sanofi had the right to terminate the Sanofi Agreement on a country-by-country and licensed product-by-licensed product basis, in the event of (a) notification of a material safety issue relating to the licensed product or the class of sodium-glucose cotransporters type 1 or type 2 inhibitors resulting in a recommendation or requirement that Lexicon or Sanofi cease development, (b) failure to achieve positive results with respect to certain clinical trial results, (c) the occurrence of specified fundamental adverse events or (d) the exploitation of the licensed product infringing third party intellectual property rights in specified major markets and Sanofi is unable to obtain a license to such third party intellectual property rights.
The Company considered the following as its performance obligations with respect to the revenue recognition of the $300 million upfront payment:
•The exclusive worldwide license granted to Sanofi to develop and commercialize sotagliflozin;
•The development services Lexicon was to perform for sotagliflozin relating to type 1 diabetes; and
•The funding Lexicon was to provide for development relating to type 2 diabetes.
The Company determined that the license had stand-alone value because it was an exclusive license that gave Sanofi the right to develop and commercialize sotagliflozin or to sublicense its rights. In addition, sotagliflozin was then in development and it was possible that Sanofi or another third party could conduct clinical trials without assistance from Lexicon. As a result, the Company considered the license and the development services under the Sanofi Agreement to be separate performance obligations. The Company recognized the portion of the transaction price allocated to the license immediately because Lexicon delivered the license and earned the revenue at the inception of the arrangement. The Company was recognizing as revenue the amount allocated to the development services for type 1 diabetes over the period of time Lexicon performed services, which was expected to be through 2027, and recognized as revenue the obligation to provide funding for development services for type 2 diabetes over the period of time Lexicon provided the funding, which was completed in 2018.
The Company determined that the initial transaction price was the $300 million upfront payment because it was the only payment that was fixed and determinable at the inception of the arrangement. There was considerable uncertainty at the date of the agreement as to whether Lexicon would earn milestone payments or royalty payments. As such, the Company did not include those payments in the allocable consideration. The Company allocated the transaction price based on the relative best estimate of selling price of each performance obligation. The Company estimated the selling price of the license deliverable by applying a probability-based income approach utilizing an appropriate discount rate. The significant inputs the Company used to determine the projected income of the license included: exercising the option to co-promote, estimated future product sales, estimated cost of goods sold, estimated operating expenses, income taxes, and an appropriate discount rate. The Company estimated the selling price of the development services for type 1 diabetes by using internal estimates of the cost to hire third parties to perform the services over the expected period to perform the development. The Company estimated the
F-24
15. Earnings (Loss) Per Share
The following is a summary of Lexicon’s earnings (loss) per share calculations and reconciliations of basic to diluted earnings (loss) per share:
| Year Ended December 31, | |||||||||||||||||
| (In thousands, except per share amounts) | 2020 | 2019 | 2018 | ||||||||||||||
| Numerator: | |||||||||||||||||
| Net income (loss) | $ | ( | $ | $ | ( | ||||||||||||
| Add interest expense on Convertible Notes | |||||||||||||||||
| Adjusted net income (loss) | $ | ( | $ | $ | ( | ||||||||||||
| Denominator: | |||||||||||||||||
| Shares used in computing net income (loss) per common share, basic | |||||||||||||||||
| Add effect of potential dilutive securities | |||||||||||||||||
| Share based awards | |||||||||||||||||
| Convertible Notes | |||||||||||||||||
| Shares used in computing net income (loss) per common share, diluted | |||||||||||||||||
| Net income (loss) per share - basic | $ | ( | $ | $ | ( | ||||||||||||
| Net income (loss) per share - diluted | $ | ( | $ | $ | ( | ||||||||||||
For periods presented with a net loss, the weighted average number of shares outstanding are the same for both basic and diluted net loss per common share. The average number of shares associated with stock options and restricted stock units that were excluded from diluted earnings per share that would potentially dilute earnings per share in the future was 11,113,054 , 8,206,390 and 7,438,134 , respectively, for the years ended December 31, 2020, 2019 and 2018. For periods presented with a net loss, the shares associated with the Convertible Notes are not included in the computation of diluted earnings per share because they are antidilutive.
F-25
16. Other Capital Agreements
Common Stock: In December 2020, Lexicon sold 20,312,500 shares of its common stock at a price of $3.200 per share in a registered direct offering pursuant to an existing shelf registration statement. Sale of the shares resulted in net proceeds of $63.0 million, after deducting underwriting discounts and commissions of $1.8 million and offering expenses of $0.2 million. The investors in the offering were Artal International S.C.A., an affiliate of of Invus, L.P., the Company’s largest stockholder, and BVF Partners L.P. and certain affiliates of BVF Partners L.P. All of the net proceeds of the registered direct offering are reflected as issuance of common stock in the accompanying financial statements.
In October 2020, Lexicon entered into an Open Market Sale AgreementSM (the “sales agreement”) with Jefferies LLC (“Jefferies”) relating to the shares of its common stock. Lexicon may offer and sell common stock having an aggregate sales price of up to $50,000,000 from time to time through Jefferies acting as its sales agent. In November 2020, Lexicon sold 3,709,233 shares of its common stock at a price of $1.992 per share pursuant to the sales agreement, resulting in net proceeds of $7.0 million. The net proceeds from this sale are reflected as issuance of common stock in the accompanying financial statements.
F-26
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Lexicon Pharmaceuticals to Host 2024 Investor Day
- Augment Inc. Raises $227 Million at $977 Million Valuation to Empower Software Teams With AI
- Webster Financial Corporation Declares Common and Preferred Dividends
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share