Form DEFA14A CytoDyn Inc.
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A
Proxy Statement Pursuant to Section 14(a) of
the Securities Exchange Act of 1934
Filed by the Registrant ☒Filed by a Party other than the Registrant ☐
Check the appropriate box:
☐ | Preliminary Proxy Statement |
☐ | Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) |
☐ | Definitive Proxy Statement |
☒ | Definitive Additional Materials |
☐ | Soliciting Material Pursuant to §240.14a-12 |
CytoDyn Inc.
(Name of Registrant as Specified In Its Charter)
(Name of Person(s) Filing Proxy Statement, if other than the Registrant)
Payment of Filing Fee (Check the appropriate box):
☒ | No fee required. |
☐ | Fee paid previously with preliminary materials. |
☐Fee computed on table in exhibit required by Item 25(b) per Exchange Act Rules 14a6(i)(1) and 0-11.
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 8-K
Current Report
Pursuant to Section 13 or 15(d)
of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): December 07, 2022 (December 07, 2022)
CytoDyn Inc.
(Exact name of registrant as specified in its charter)
Delaware | 000-49908 | 83-1887078 |
(State or other jurisdiction of incorporation or organization) | (Commission File Number) | (I.R.S. Employer Identification No.) |
1111 Main Street, Suite 660
Vancouver, Washington 98660
(Address of principal executive offices, including zip code)
(360) 980-8524
(Registrant’s telephone number, including area code)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
| Trading |
| Name of each exchange |
None | | None | | None |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
On December 7, 2022, CytoDyn Inc. (the “Company”) made a presentation during its R&D Update. The presentation has been posted on the Company’s website and a copy of the presentation is furnished as Exhibit 99.1 to this report and incorporated herein by reference.
Item 9.01 Financial Statements And Exhibits.
(d) Exhibits.
99.1 | Investor Presentation dated December 7, 2022** |
| |
Exhibit 104 | The cover page from this Current Report on Form 8-K formatted in Inline XBRL. |
** Furnished, not filed.
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| CYTODYN INC. | |
| | |
Date: December 7, 2022 | By | /s/ Antonio Migliarese |
| | Antonio Migliarese |
| | Chief Financial Officer |
Exhibit 99.1
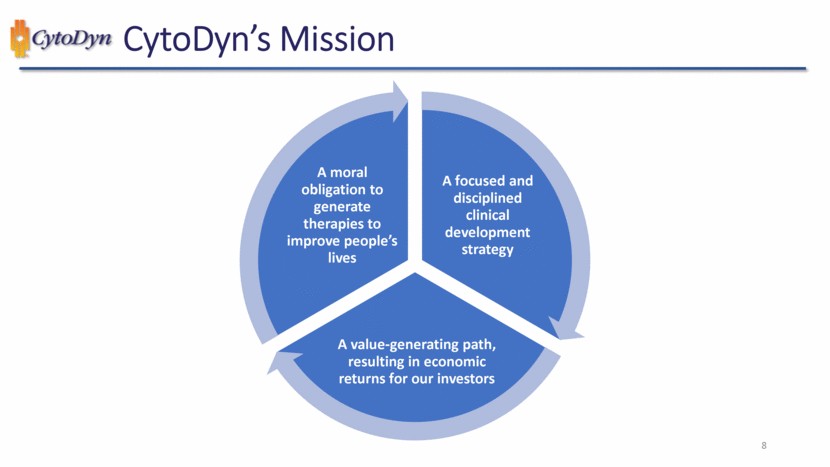
| CytoDyn – R&D Investor 1 Update December 7, 2022 |
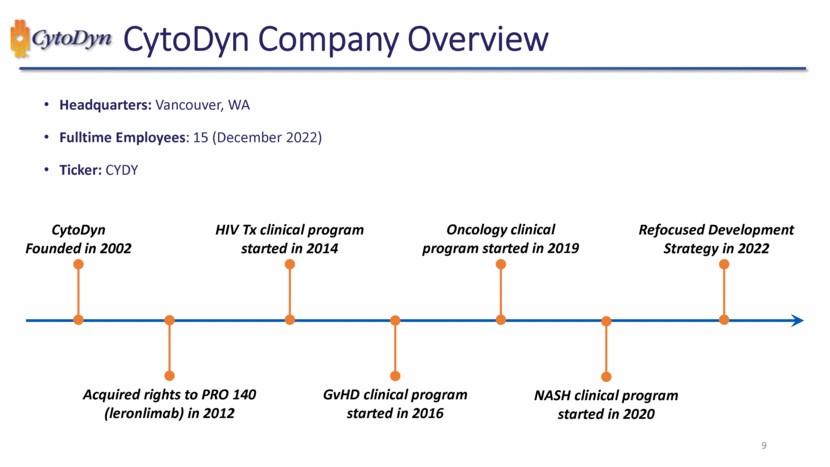
| CytoDyn Company Overview 9 Headquarters: Vancouver, WA Fulltime Employees: 15 (December 2022) Ticker: CYDY CytoDyn Founded in 2002 HIV Tx clinical program started in 2014 Oncology clinical program started in 2019 Refocused Development Strategy in 2022 Acquired rights to PRO 140 (leronlimab) in 2012 GvHD clinical program started in 2016 NASH clinical program started in 2020 9 |
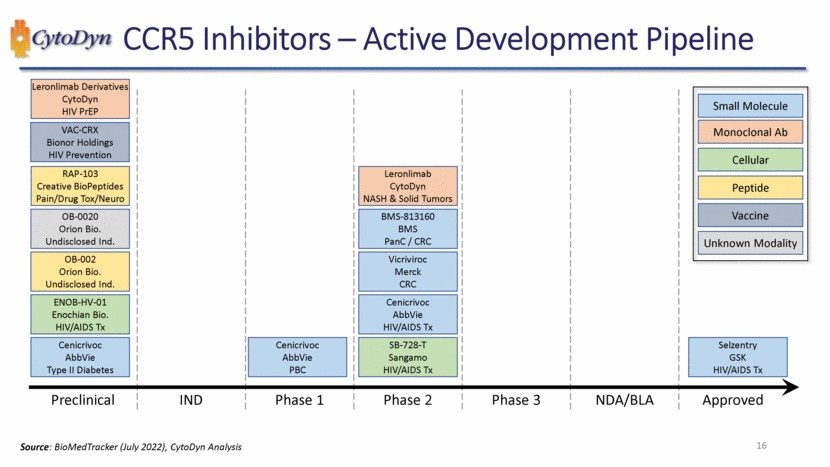
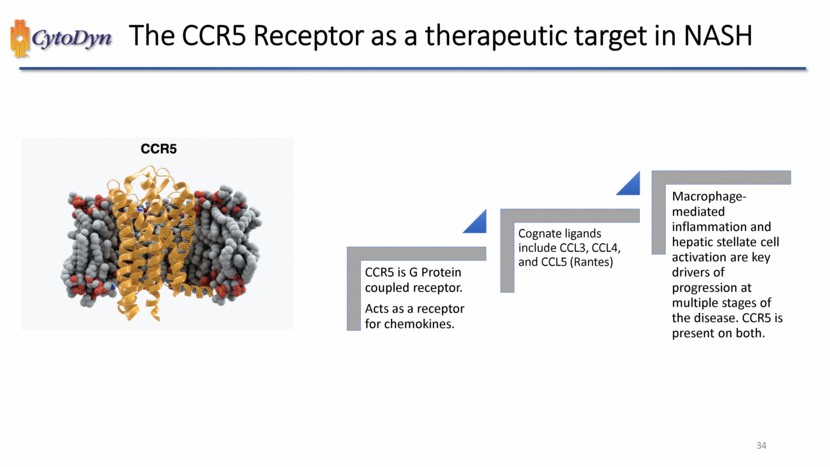
| CCR5 & Leronlimab 12 |
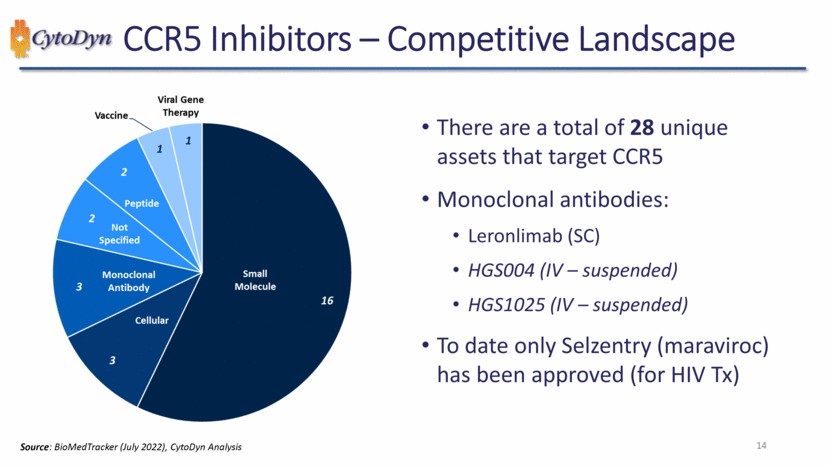
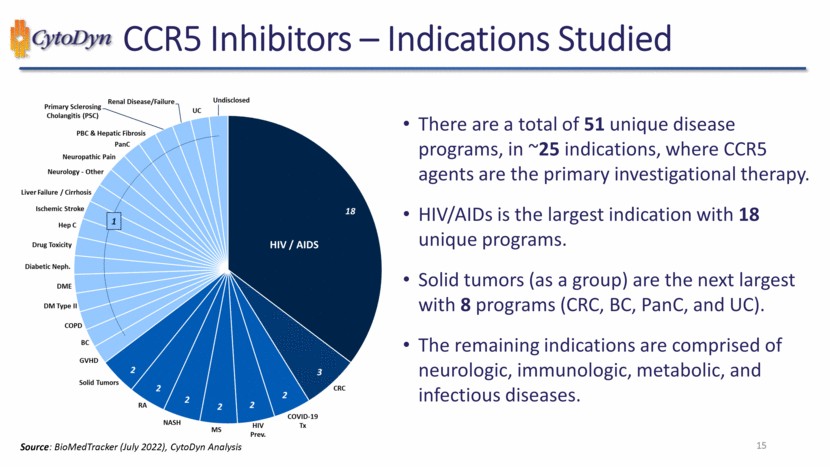
| CCR5 Inhibitors – Indications Studied 1 2 2 2 2 2 2 3 18 Undisclosed Renal Disease/Failure Primary Sclerosing cholangitis (PSC) PBC & Hepatic Fibrosis PanC Neuropathic Pain Neurology – Other Liver Failure / Cirrhosis Ischemic Stroke Hep C Drug Toxicity Diabetic Neph. DME DM Type II COPD BC GVHD Solid Tumors RA NASH MS HIV Prev. COVID-19 Tx CRC HIV / AIDS Source: DataMonitor (July 2022), CytoDyn Analysis 15 |
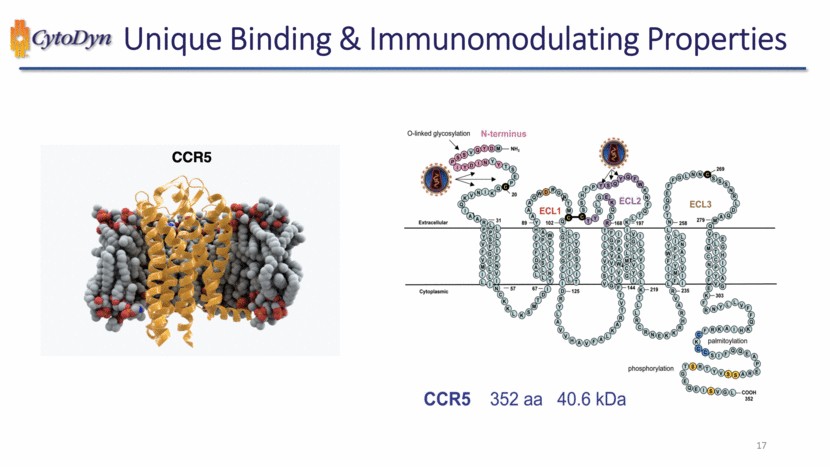
| Unique Binding & Immunomodulating Properties CCR5 CCR5 352 aa 40.6 kDa O-linked glycosylation N-terminus Extracellular NH2 ECL1 ECL2 ECL3 Cytoplasmic phosphorylation palmitoylation 20 31 54 67 102 125 168 144 219 258 235 269 279 303 COOH 352 17 |
| Future Development 21 |
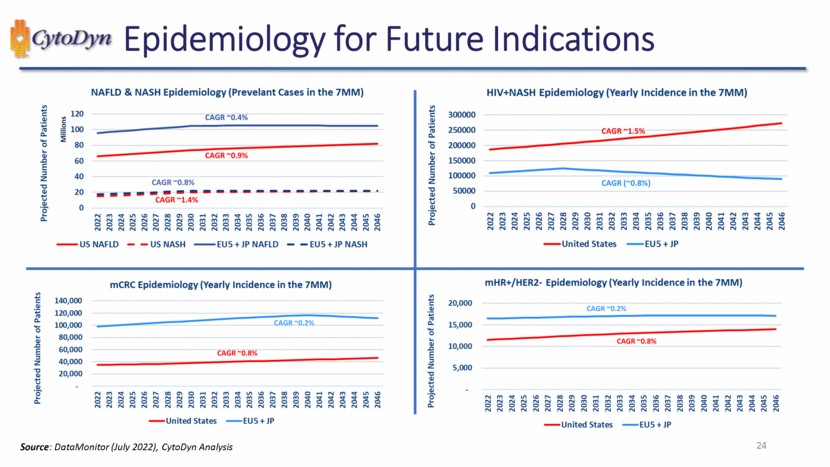
| Epidemiology for Future Indications NAFLD & NASH Epidemiology (Prevelant Cases in the 7MM) Projected Number of Patients Millions 120 100 80 60 40 20 0 CAGR ~0.4% CAGR ~0.9% CAGR ~0.8% CAGR ~1.4% 2022 2023 2024 2025 2026 2027 2028 2029 2030 2031 2032 2033 2034 2035 2036 2037 2038 2039 2040 2041 2042 2043 2044 2045 2046 US NAFLD US NASH EU5 + JP NAFLD EU5 + JP NASH Projected Number of Patients 300000 250000 200000 150000 100000 50000 0 HIV+NASH Epidemiology (Yearly Incidence in the 7MM) CAGR ~1.5% CAGR (~0.8%) 2022 2023 2024 2025 2026 2027 2028 2029 2030 2031 2032 2033 2034 2035 2036 2037 2038 2039 2040 2041 2042 2043 2044 2045 2046 United States EU5 + JP mCRC Epidemiology (Yearly Incidence in the 7MM) Projected Number of Patients 140,000 120,000 100,000 80,000 60,000 40,000 20,000 – CAGR ~0.2% CAGR ~0.8% 2022 2023 2024 2025 2026 2027 2028 2029 2030 2031 2032 2033 2034 2035 2036 2037 2038 2039 2040 2041 2042 2043 2044 2045 2046 United States EU5 + JP mHR+/HER2- Epidemiology (Yearly Incidence in the 7MM) Projected Number of Patients 20,000 15,000 10,000 5,000 – CAGR ~0.2% CAGR ~0.8% 2022 2023 2024 2025 2026 2027 2028 2029 2030 2031 2032 2033 2034 2035 2036 2037 2038 2039 2040 2041 2042 2043 2044 2045 2046 United States EU5 + JP Source: DataMonitor (July 2022), CytoDyn Analysis 24 |
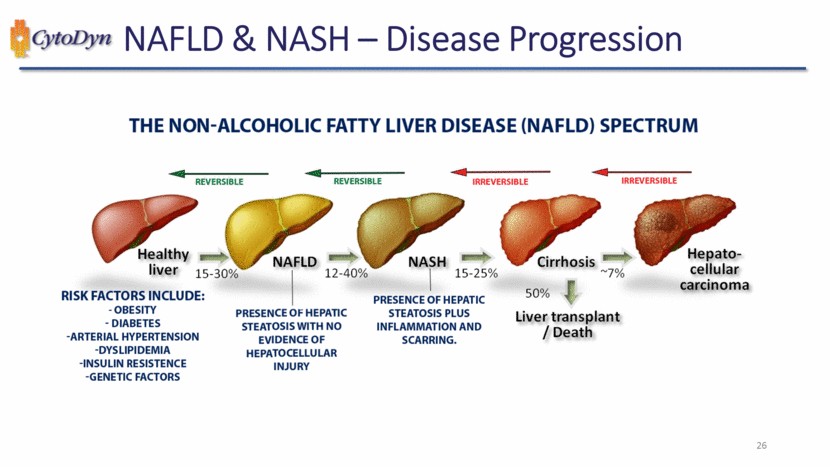
| NAFLD & NASH – Disease Progression THE NON-ALCOHOLIC FATTY LIVER DISEASE (NAFLD) SPECTRUM REVERSIBLE REVERSIBLE IRREVERSIBLE IRREVERSIBLE Healthy liver 15-30% NAFLD 12-40% NASH 15-25% Cirrhosis ~7% Hepato-cellular carcinoma 50% Liver transplant / Death RISK FACTORS INCLUDE: - OBESITY – DIABETES – ARTERIAL HYPERTENSION – DYSLIPIDEMIA -INSULIN RESISTENCE -GENETIC FACTORS PRESENCE OF HEPATIC STEATOSIS WITH NO EVIDENCE OF HEPATOCELLULAR INJURY PRESENCE OF HEPATIC STEATOSIS PLUS INFLAMMATION AND SCARRING. |
| Metastatic CRC – Background and Treatment 28 CRC typically develops through the proliferation of mucosal epithelial cells of the gastrointestinal (GI) wall, eventually forming a polyp or adenoma. As with many other cancers of the GI tract, the vast majority (>95%) are adenocarcinomas. CRC stands as the second deadliest and third most diagnosed form of cancer worldwide The most commonly prescribed targeted therapies in front-line mCRC are angiogenesis inhibitors, particularly bevacizumab, which is available for most tumor types and alongside most chemotherapy combinations. Other drugs, such as Erbitux and Braftovi, are limited by gene expression, whereas PD-1 inhibitors Keytruda, Opdivo, and most recently Jemperli may be used in MSI-H tumors (~10-15% of mCRC). However, these PD-1 inhibitors have failed to show success in micro satellite stable (MSS) tumors to date |
| Breast Cancer HR+/HER2- – Disease Background 29 Hormone receptor-positive (HR+) is the most common breast cancer subtype, with approximately 70% of breast cancers presenting with overexpression of estrogen receptors, progesterone receptors, or both. Overexpression of the hormone receptors allows estrogen and progesterone to drive tumor growth and proliferation. Therefore, endocrine therapy remains the standard treatment for advanced patients with HR+/human epidermal growth factor receptor 2-negative (HER2-) breast cancer. Worldwide, breast cancer is the most common cancer in terms of new cases and the fifth-leading cause of cancer-related death regardless of gender. In the US, breast cancer is the most common cancer in women and the fourth-leading cause of cancer-related death regardless of gender, behind lung cancer, colorectal cancer, and pancreatic cancer. |
| NASH & Leronlimab 30 Mazen Noureddin, M.D., M.H.Sc. Director of the Houston Liver Institute |
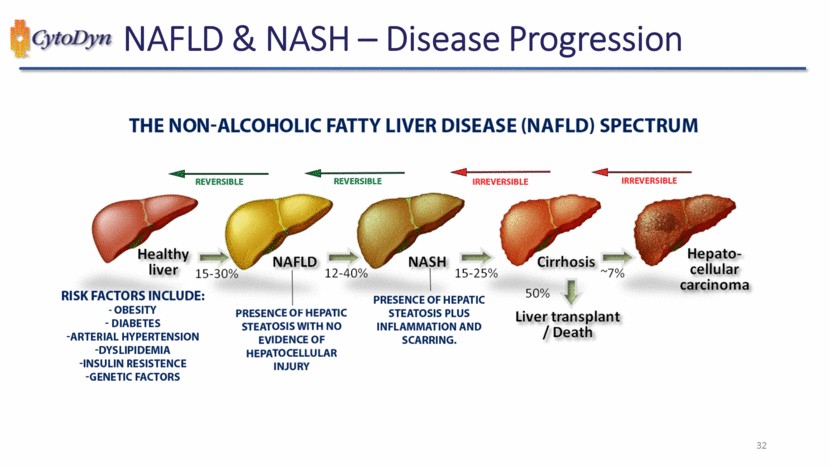
| CytoDyn NAFLD & NASH – Disease Progression THE NON-ALCOHOLIC FATTY LIVER DISEASE (NAFLD) SPECTRUM REVERSIBLE REVERSIBLE IRREVERSIBLE IRREVERSIBLE Healthy liver 15-30% NAFLD 12-40% NASH 15-25% Cirrhosis ~7% Hepato-cellular carcinoma RISK FACTORS INCLUDE: -OBESITY -DIABETES -ARTERIAL HYPERTENSION -DYSLIPIDEMIA -INSULIN RESISTENCE -GENETIC FACTORS PRESENCE OF HEPATIC STEATOSIS PLUS INFLAMMATION AND SCARRING. 50% Liver transplant / Death 32 |
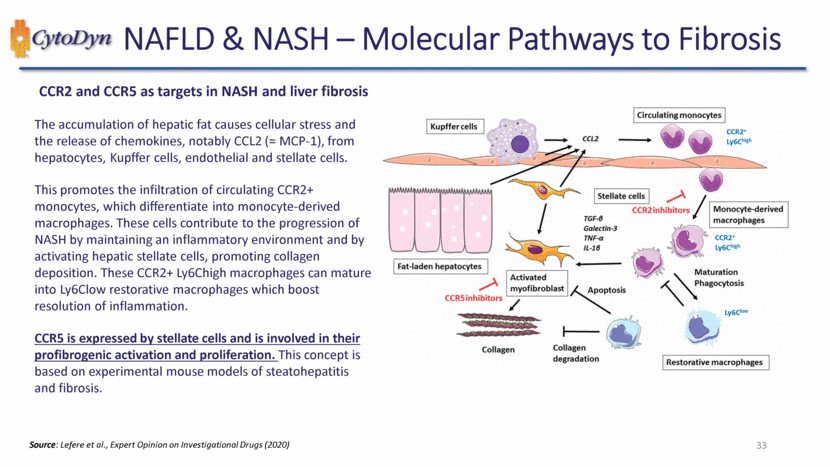
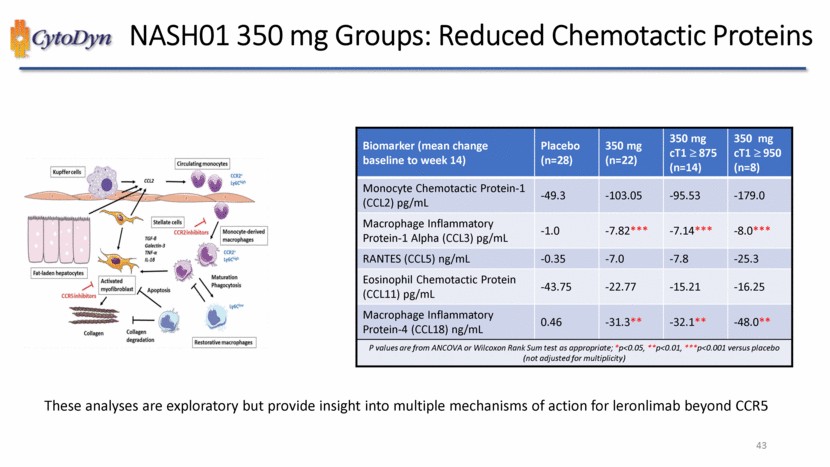
| NAFLD & NASH – Molecular Pathways to Fibrosis CCR2 and CCR5 as targets in NASH and liver fibrosis The accumulation of hepatic fat causes cellular stress and the release of chemokines, notably CCL2 (= MCP-1), from hepatocytes, Kupffer cells, endothelial and stellate cells. This promotes the infiltration of circulating CCR2+ monocytes, which differentiate into monocyte-derived macrophages. These cells contribute to the progression of NASH by maintaining an inflammatory environment and by activating hepatic stellate cells, promoting collagen deposition. These CCR2+ Ly6Chigh macrophages can mature into Ly6Clow restorative macrophages which boost resolution of inflammation. CCR5 is expressed by stellate cells and is involved in their profibrogenic activation and proliferation. This concept is based on experimental mouse models of steatohepatitis and fibrosis. Kupffer cells CCL2 Circulating monocytes CCR2’LY6C high Fat laden hepatocytes TGF- Galectin-3 TNF- IL-1 Stellate cells CCR2inhibitors Monocyte-derived macrophages CCR2+Ly6Chigh CCR5inhibitors Activated myofibroblast Apoptosis Maturation Phagocytosis Collagen Collagen degradation Ly6Clow Restorative macrophages Source: Lefere et al., Expert Opinion on Investigational Drugs (2020) 33 |
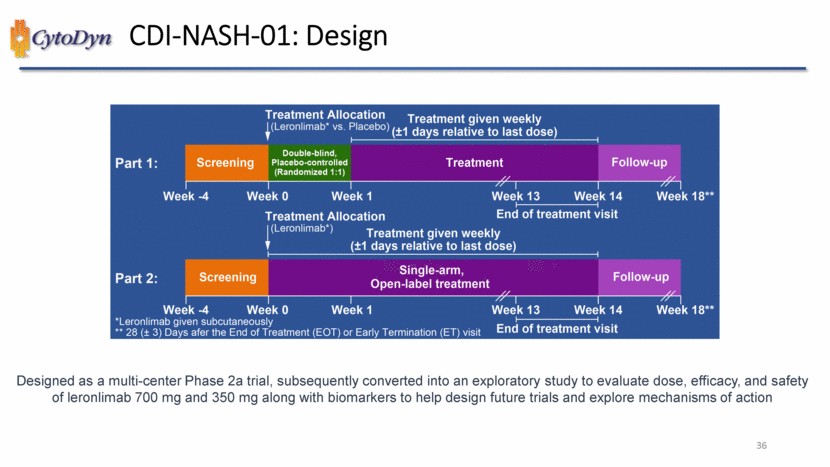
| CDI-NASH-01: Design Treatment Allocation (Leronlimab* vs. Placebo) Treatment given weekly (+-1 days relative to last dose Part1 Screening Double blind, Placebo-controlled (Randomized 1:1) Treatment Follow-up Week -4 Week 0 Week 1 Week 13 Week 14 Week 18** Treatment Allocation End of treatment visit (Leronlimab*) Treatment given weekly (+-1 days relative to last dose) Part2:Screening Single-arm, Open-label treatment Follow-up Week -4 Week 0 Week 1 Week 13 Week 14 End of Treatment visit Week 18** *Leronlimab given subcutaneously ** 28 (+- 3) Days after the End of Treatment (EOT) or Early Termination (ET) visit Designed as a multi-center Phase 2a trial, subsequently converted into an exploratory study to evaluate dose, efficacy, and safety of leronlimab 700 mg and 350 mg along with biomarkers to help design future trials and explore mechanisms of action 36 |
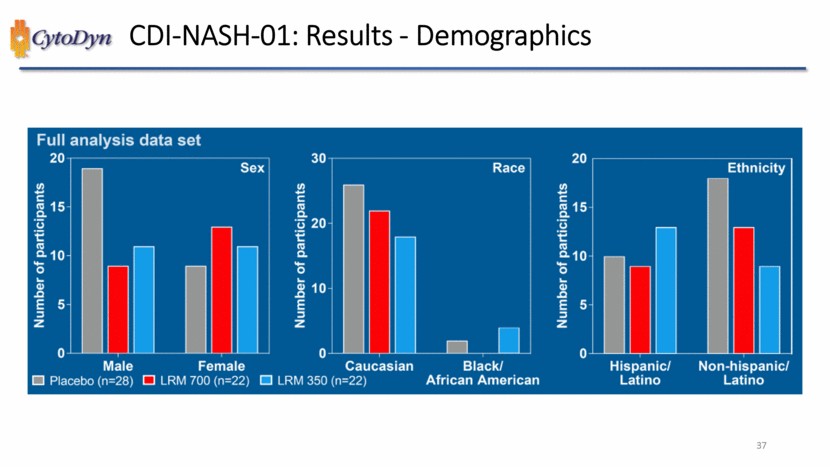
| CDI-NASH-01: Results – Demographics Full analysis data set Number of participants Sex Male Female Number of participants Race Caucasian Black Number of participants Ethnicity Hispanic/ Latino Non-hispanic/ Latino Placebo (n=28) LRM 700 (n=22) LRM 350 (n=22) African American 37 |
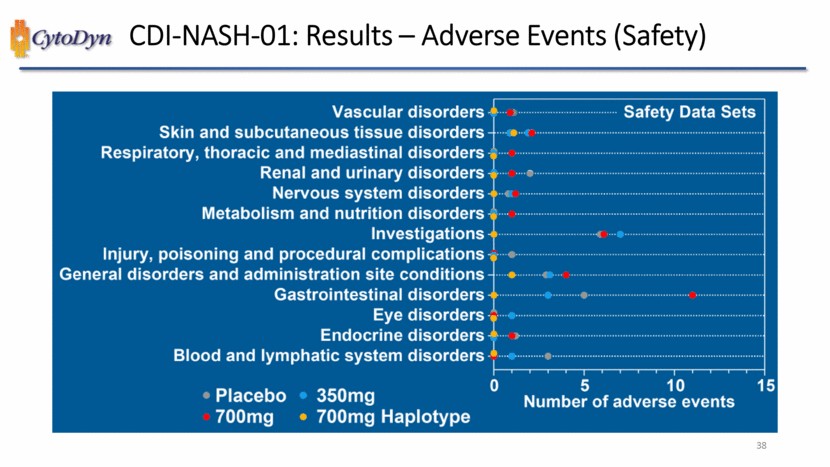
| CDI-NASH-01: Results – Adverse Events (Safety) Vascular disorders Skin and subcutaneous tissue disorders Respiratory, thoracis and mediastinal disorders Renal and urinary disorders Nervous system disorders Metabolism and nutrition disorders Investigations Injury, poisoning and procedural complications General disorders and administration site conditions Gastrointestinal disorders Eye disorders Endocrine disorders Blood and Iymphatic system disorders Safety Data Sets Number of adverse events Placebo 350mg 700mg 700mg Haplotype 38 |
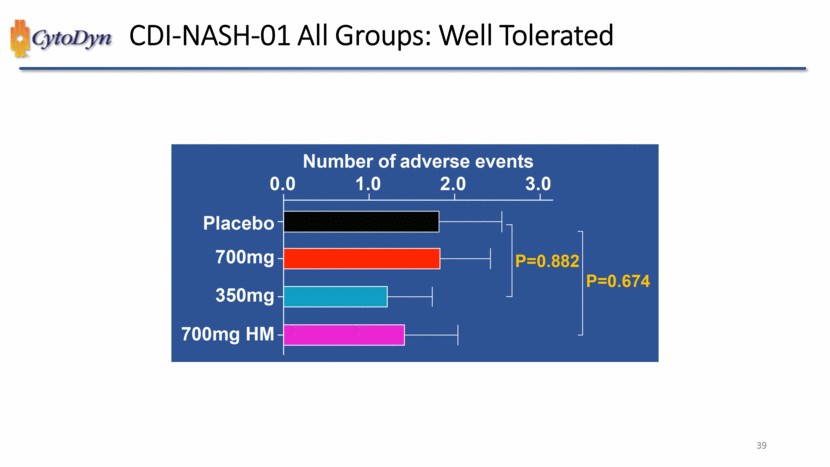
| CDI-NASH-01 All Groups: Well Tolerated Number of adverse events 0.0 1.0 2.0 3.0 Placebo 700mg 350mg 700mg HM P=0.882 P=0.674 39 |
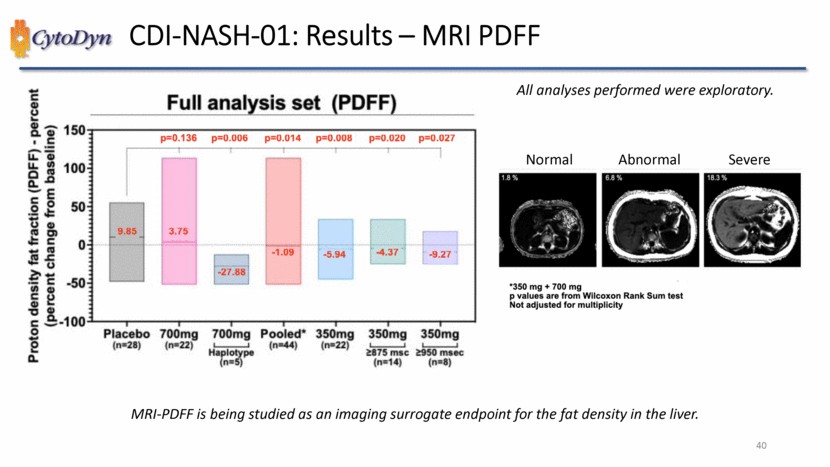
| CDI-NASH-01: Results – MRI PDFF Proton density fat fraction (PDFF) – percent (percent change from baseline) Full analysis set (PDFF) -100 -50 0 50 100 150 p=0.136 p=0.006 p=0.014 p=0.008 p=0.020 p=0.027 9.85 3.75 -27.88 -1.09 -5.94 -4.37 -9.27 Placebo (n=28) 700mg (n=22) 700mg Haplotye (n=5) Pooled*(n=44) 350mg (n=22) 350mg >875 msc (n=14) 350mg >950 msec (n=8) All analyses performed were exploratory. Normal Abnormal Severe *350mg+700mg p values are from Willcoxon Rank Sum text Not adjusted for multiplicity MRI-PDFF is being studied as an imaging surrogate endpoint for the fat density in the liver.40 |
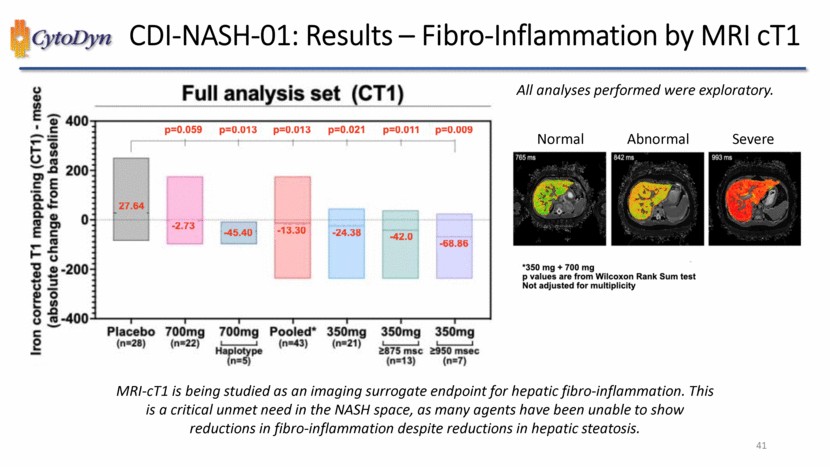
| CDI-NASH-01: Results – Fibro-Inflammation by MRI cT1 Iron corrected T1 mapping (CT1) – msec (absolute change from baseline) Full analysis set (CT1) -400 -200 0 200 400 p=0.059 p=0.013 p=0.013 p=0.021 p=0.011 p=0.009 -27.64 -2.73 -45.40 -13.30 -24.38 -42.0 -68 86 Placebo (n=28) 700mg (n=22) 700mg Haplotye (n=5) Pooled*(n=43) 350mg (n=21) 350mg >875 msc (n=13) 350mg >950 msec (n=9) All analyses performed were exploratory. Normal Abnormal Severe *350mg+700mg p values are from Willcoxon Rank Sum text Not adjusted for multiplicity MRI-cT1 is being studied as an imaging surrogate endpoint for hepatic fibro-inflammation. This is a critical unmet need in the NASH space, as many agents have been unable to show reductions in fibro-inflammation despite reductions in hepatic steatosis.41 |
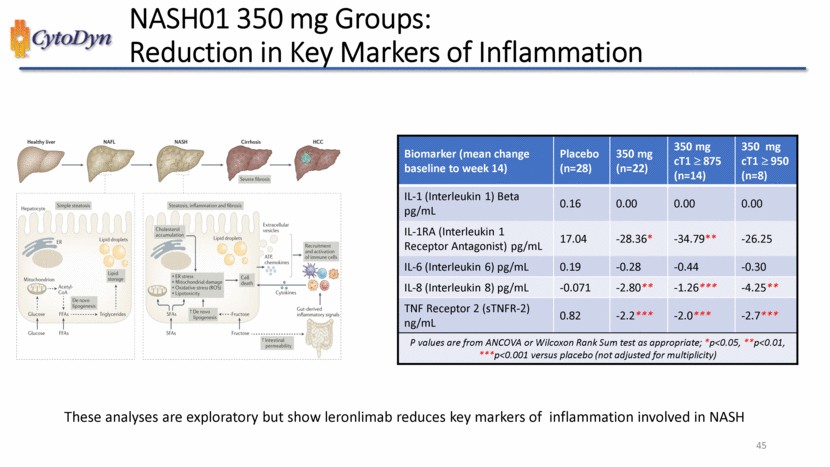
| NASH01 350 mg Groups: Reduced Chemotactic Proteins Biomarker (mean change baseline to week 14) Placebo (n=28) 350 mg (n=22) 350 mg cT1 875 (n=14) 350 mg cT1 950 (n=8) IL-1 (Interleukin 1) Beta pg/mL 0.16 0.00 0.00 0.00 IL-1RA (Interleukin 1 Receptor Antagonist) pg/mL 17.04 -28.36* -34.79** -26.25 IL-6 (Interleukin 6) pg/mL 0.19 -0.28 -0.44 -0.30 IL-8 (Interleukin 8) pg/mL 0.19 -0.28 -0.44 -0.30 IL-8 (Interleukin 8) pg/mL -0.071 -2.80** -1.26*** -4.25** TNF Receptor 2 (sTNFR-2) ng/mL 0.82 -2.2*** -2.0*** -2.7*** P values are from ANCOVA or Wilcoxon Rank Sum test as appropriate; *p<0.05, **p<0.01, ***p<0.001 versus placebo (not adjusted for multiplicity) These analyses are exploratory but show leronlimabreduces key markers of inflammation involved in NASH 45 |
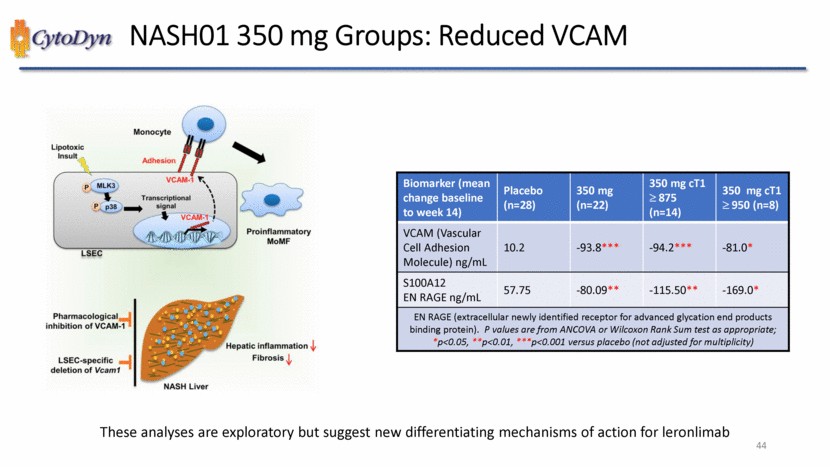
| CytoDyn NASH01 350 mg Groups: Reduced VCAM Biomarker (mean change baseline to week 14) Placebo (n=28) 350 mg (n=22) 350 mg cT1 875 (n=14) 350 mg cT1 950 (n=8) VCAM (Vascular Cell Adhesion Molecule) ng/mL 10.2 -93.8*** -94.2*** -81.0* S100A12 EN RAGE ng/mL 57.75 -80.09** -115.50** -169.0* EN RAGE (extracellular newly identified receptor for advanced glycation end products binding protein). P values are from ANCOVA or Wilcoxon Rank Sum test as appropriate; *p<0.05, **p<0.01, ***p<0.001 versus placebo (not adjusted for multiplicity) These analyses are exploratory but suggest new differentiating mechanisms of action for leronlimab 44 |
| Biomarker (mean change baseline to week 14) Placebo (n=28) 350 mg (n=22) 350 mg cT1 875 (n=14) 350 mg cT1 950 (n=8) IL-1 (Interleukin 1) Beta pg/mL 0.16 0.00 0.00 0.00 IL-1RA (Interleukin 1 Receptor Antagonist) pg/mL 17.04 -28.36* -34.79** -26.25 IL-6 (Interleukin 6) pg/mL 0.19 -0.28 -0.44 -0.30 IL-8 (Interleukin 8) pg/mL 0.19 -0.28 -0.44 -0.30 IL-8 (Interleukin 8) pg/mL -0.071 -2.80** -1.26*** -4.25** TNF Receptor 2 (sTNFR-2) ng/mL 0.82 -2.2*** -2.0*** -2.7*** P values are from ANCOVA or Wilcoxon Rank Sum test as appropriate; *p<0.05, **p<0.01, ***p<0.001 versus placebo (not adjusted for multiplicity) These analyses are exploratory but show leronlimabreduces key markers of inflammation involved in NASH 45 |
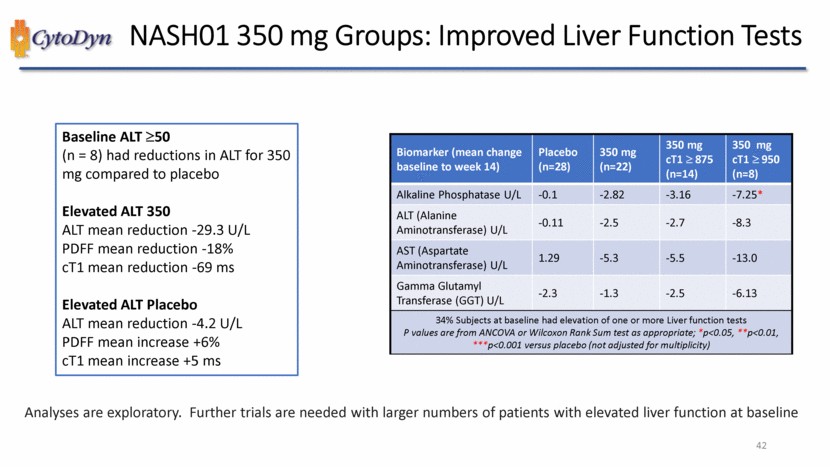
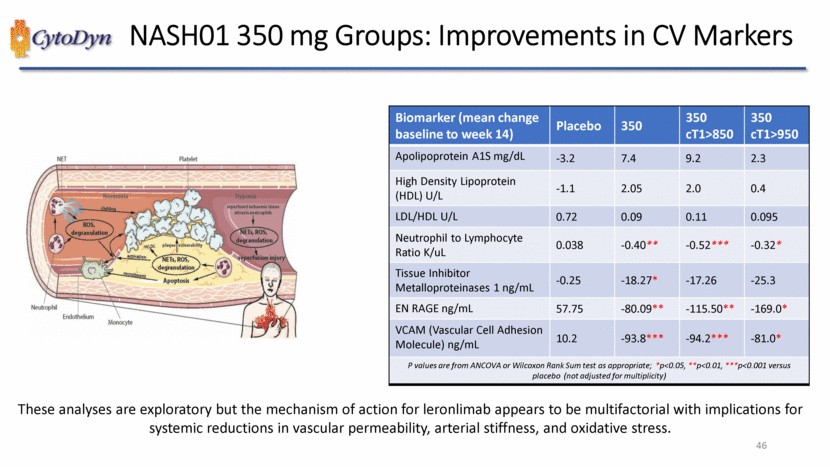
| NASH01 350 mg Groups: Improvements in CV Markers Biomarker (mean change baseline to week 14) Placebo 350 350 cT1>850 350 cT1>950 Apolipoprotein A1S mg/dL -3.2 7.4 9.2 2.3 High Density Lipoprotein (HDL) U/L -1.1 2.05 2.0 0.4 LDL/HDL U/L 0.72 0.09 0.11 0.095 Neutrophil to Lymphocyte Ratio K/uL 0.038 -0.40** -0.52*** -0.32* Tissue Inhibitor Metalloproteinases 1 ng/mL -0.25 -18.27* -17.26 -25.3 EN RAGE ng/mL 57.75 -80.09** -115.50** -169.0* VCAM (Vascular Cell Adhesion Molecule) ng/mL 10.2 -93.8*** -94.2*** -81.0* P values are from ANCOVA or Wilcoxon Rank Sum test as appropriate; *p<0.05, **p<0.01, ***p<0.001 versus placebo (not adjusted for multiplicity) These analyses are exploratory but the mechanism of action for leronlimabappears to be multifactorial with implications for systemic reductions in vascular permeability, arterial stiffness, and oxidative stress. 46 |
| CDI-NASH-01: Conclusions All analyses performed were exploratory Treatment with leronlimab was generally well tolerated in both Part 1 and Part 2 Part 1: Leronlimab 700 mg did not reduce mean change in PDFF and cT1 from baseline to week 14 vs. PBO Part 2: Leronlimab 350 mg significantly reduced mean change in PDFF and cT1 from baseline to week 14 vs. PBO Despite increased fibro-inflammation, in patients with moderate and severe cT1 values at baseline, leronlimab 350 mg still showed significantly reduced cT1 from baseline to week 14 vs. placebo Part 1 & Part 2 pooled Pooled 350 mg + 700 mg group also had significant reductions in PDFF and cT1 vs. placebo Although small sample size (n=5) CCR5 haplotype analysis is suggestive that specific haplotypes may be better suited for the 700 mg dose of leronlimab Results warrant additional follow-up in a larger trial 47 |
| Leronlimab in Oncology – Clinical Data 4 8 |
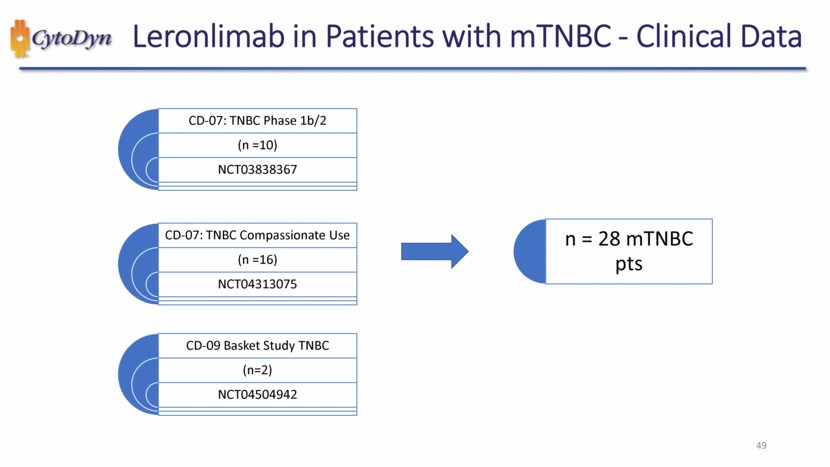
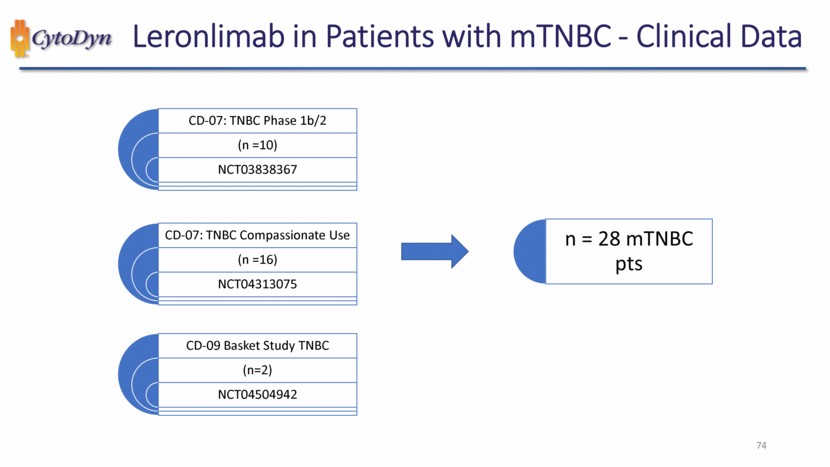
| Leronlimab in Patients with mTNBC - Clinical Data CD-07: TNBC Phase 1b/2 (n =10) NCT03838367 CD-07: TNBC Compassionate Use (n =16) NCT04313075 CD-09 Basket Study TNBC (n=2) NCT04504942 n = 28 mTNBC pts 49 |
| mTNBC Patient Overview Data was available and pooled from three sources (n = 28 patients): All patients were listed as mTNBC n=16 were from Compassionate Use n=10 were from mTNBC trial n=2 were from Basket trial Patients received ≥1 dose of Leronlimab (range 1 - 33 doses) Doses ranged from 350mg - 700mg 4 patients had dose escalation per study protocol Average age was 52 (range 33 - 75) Data was as reported by August 15, 2021 Standard of Care and Sacituzumab Govitecan used as references Standard of Care (SOC) and Sacituzumab Govitecan (SG) were not evaluated directly in these studies SOC and SG reference lines are for hypothetical reference only from historical controls 50 |
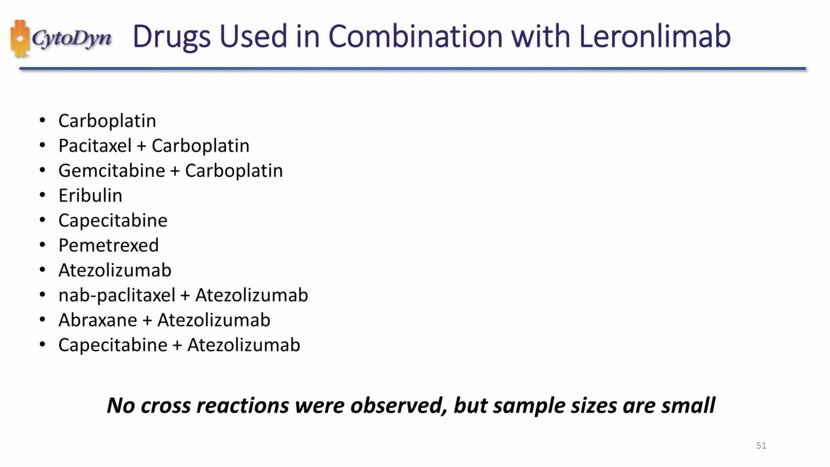
| Drugs Used in Combination with Leronlimab Carboplatin Pacitaxel + Carboplatin Gemcitabine + Carboplatin Eribulin Capecitabine Pemetrexed Atezolizumab nab-paclitaxel + Atezolizumab Abraxane + Atezolizumab Capecitabine + Atezolizumab No cross reactions were observed, but sample sizes are small 51 |
| |
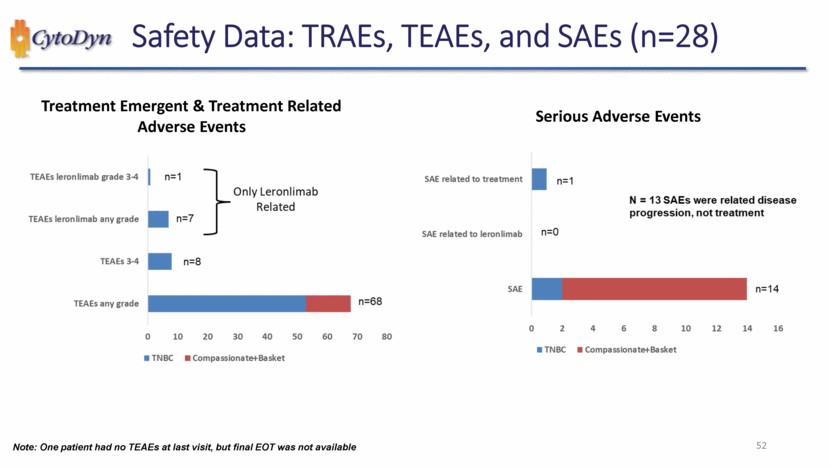
| Safety Data: TRAEs, TEAEs, and SAEs (n=28) Treatment Emergent & Treatment Related Adverse Events TEAEs leronlimab grade 3-4 TEAEs leronlimab any grade TEAEs 3-4 TEAEs any grade only Leronlimab Related TNBC Compassionate+Basket n=1 n=7 n=8 n=68 0 10 20 30 40 50 60 70 80 Serious Adverse Events SAE related to treatment SAE related to leronlimab SAE N-13 SAEs were related disease progression, not treatment TNBC Compassionate+Basket n=1 n=0 n=14 0 2 4 6 8 10 12 14 16 Note: One patient had no TEAEs at last visit, but final EOT was not available 52 |
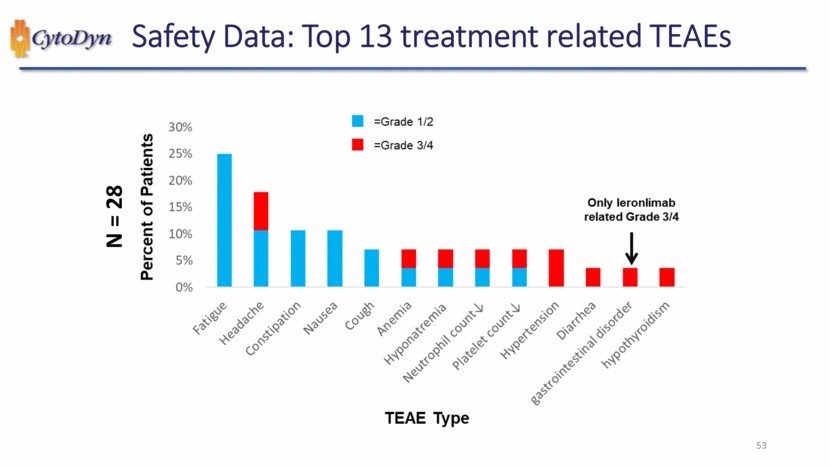
| Safety Data: Top 13 treatment related TEAEs N=28 Percent of Patients =Grade 1/2 , =Grade 3/4 0% 5% 10% 15% 20% 25% 30% Fatigue Headache Constipation Nausea Cough Anemia Hyponatremia Neutrophil count Platelet count Hypertension Diarrhea gastrointestinal disorder hypothyroidism Only leronlimab related Grade 3/4 TEAE Type 53 |
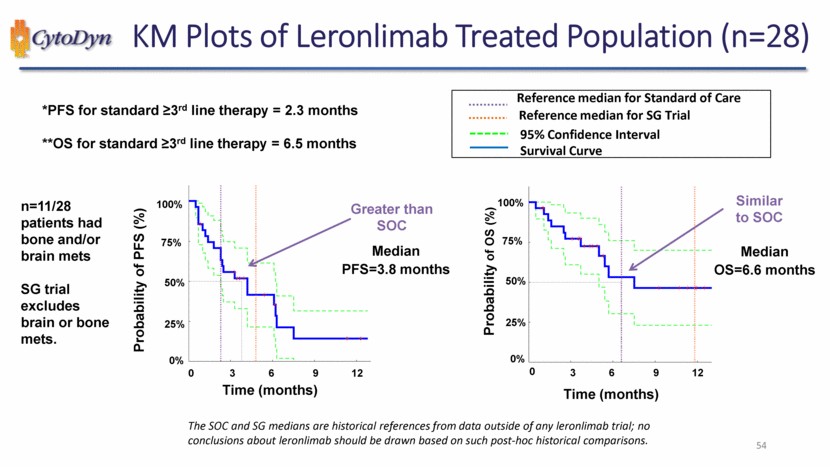
| KM Plots of Leronlimab Treated Population (n=28) *PFS for standard ≥3rd line therapy = 2.3 months **OS for standard ≥3rd line therapy = 6.5 months n=11/28 patients had bone and/or brain mets SG trial excludes brain or bone mets. Probability of PFS (%) Time (months) Greater than SOC Median PFS=3.8 months 0% 25% 50% 75% 100% 0 3 6 9 12 Reference median for Standard of Care Reference median for SG Trial 95% Confidence Interval Survival Curve Probability of OS (%) Time (months) Similar to SOC Median OS=6.6 months 0% 25% 50% 75% 100% 0 3 6 9 12 The SOC and SG medians are historical references from data outside of any leronlimab trial; no conclusions about leronlimab should be drawn based on such post-hoc historical comparisons. 54 |
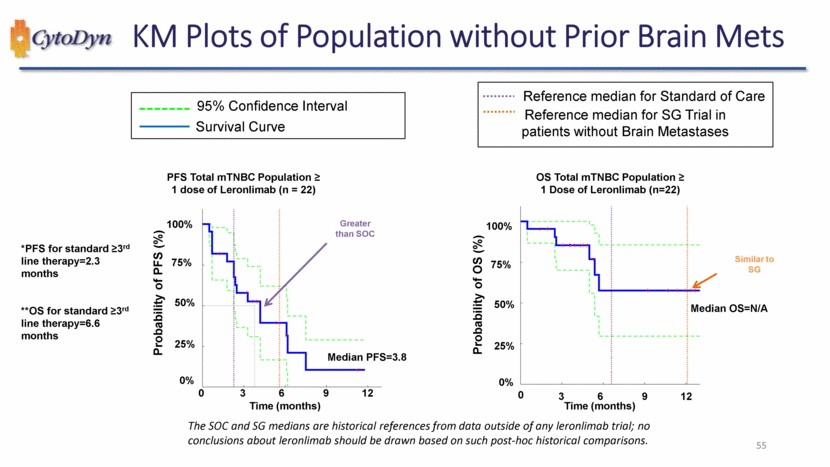
| KM Plots of Population without Prior Brain Mets 95% Confidence Interval Survival Curve PFS Total mTNBC Population ≥ 1 dose of Leronlimab (n = 22) *PFS for standard ≥3rd line therapy=2.3 months**OS for standard ≥3rd line therapy=6.6 months Probability of PFS (%) Time (months) Greater than SOC Median PFS=3.8 0% 25% 50% 75% 100% 0 3 6 9 12 Reference median for Standard of Care Reference median for SG Trial in patients without Brain Metastases OS Total mTNBC Population ≥ 1 Dose of Leronlimab (n=22) Probability of OS (%) Time (months) Similar to SG Median OS=N/A 0% 25% 50% 75% 100% 0 3 6 9 12 The SOC and SG medians are historical references from data outside of any leronlimab trial; no conclusions about leronlimab should be drawn based on such post-hoc historical comparisons. 55 |
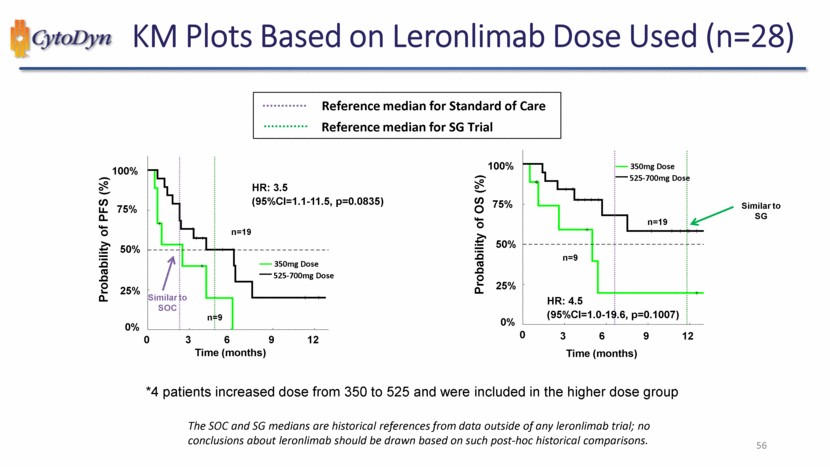
| KM Plots Based on Leronlimab Dose Used (n=28) Reference median for Standard of Care Reference median for SG Trial Probability of PFS (%) HR: 3.5 (95%CI=1.1-11.5, p=0.0835) Similar to SOC Time (months) n=19 350mg Dose 525-700mg Dose Time (months) 0% 25% 50% 75% 100% 0 3 6 9 12 Probability of OS (%) 350mg Dose 525-700mg Dose Similar to SG n=19 n=9 HR: 4.5 (95%CI=1.0-19.6, p=0.1007) Time (months) 0% 25% 50% 75% 100% 0 3 6 9 12 *4 patients increased dose from 350 to 525 and were included in the higher dose group The SOC and SG medians are historical references from data outside of any leronlimab trial; no conclusions about leronlimab should be drawn based on such post-hoc historical comparisons. 56 |
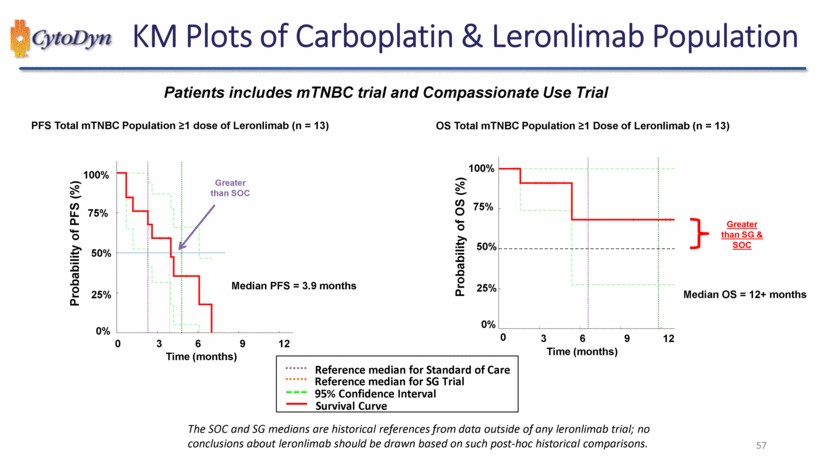
| KM Plots of Carboplatin & Leronlimab Population Patients includes mTNBC trial and Compassionate Use Trial PFS Total mTNBC Population ≥1 dose of Leronlimab (n = 13) Probability of PFS (%) Greater than SOC Median PFS = 3.9 months Time (months) 0% 25% 50% 75% 100% 0 3 6 9 12 OS Total mTNBC Population ≥1 Dose of Leronlimab (n = 13) Probability of OS (%) Greater than SG & SOC Median OS = 12+ months Time (months) 0% 25% 50% 75% 100% 0 3 6 9 12 Reference median for Standard of Care Reference median for SG Trial 95% Confidence Interval Survival Curve The SOC and SG medians are historical references from data outside of any leronlimab trial; no conclusions about leronlimab should be drawn based on such post-hoc historical comparisons. 57 |
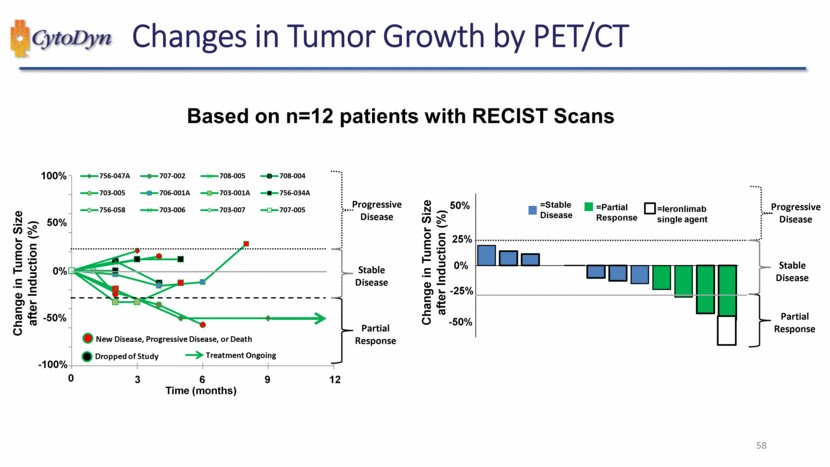
| Changes in Tumor Growth by PET/CT Based on n=12 patients with RECIST Scans Change in Tumor Size after Induction (%) New Disease, Progressive Disease, or Death Dropped of Study Treatment Ongoing Time (months) Progressive Disease Stable Disease Partial Response 756-047A 707-002 708-005 708-004 703-005 706-001A 703-001A 756-034A 756-058 703-006 703-007 707-005 -100% -50% 0% 50% 100% 0 3 6 9 12 Change in Tumor Size after Induction (%) =Stable Disease =Partial Response =leronlimab single agent Progressive Disease Stable Disease Partial Response -50% -20% 0% 25% 50% 57 |
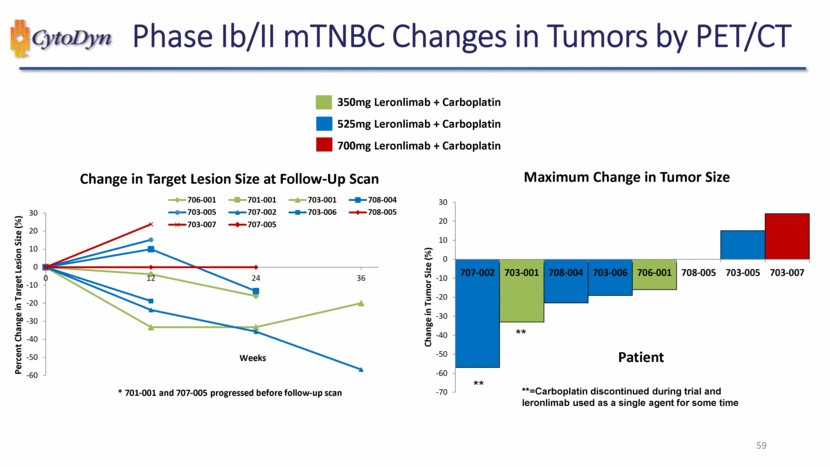
| Phase Ib/II mTNBC Changes in Tumors by PET/CT 350mg Leronlimab + Carboplatin 525mg Leronlimab + Carboplatin 700mg Leronlimab + Carboplatin Change in Target Lesion Size at Follow-Up Scan Percent Change in Target Lesion Size (%) * 701-001 and 707-005 progressed before follow-up scan Weeks 12 24 36 706-001 701-001 703-001 708-004 703-005 707-002 703-006 708-005 703-007 707-005 -60 -50 -40 -30 -20 -10 0 10 20 30 Maximum Change in Tumor Size Change in Tumor Size (%) **=Carboplatin discontinued during trial and leronlimab used as a single agent for some time ** 707-002 703-001 708-004 703-006 706-001 708-005 703-005 703-007 Patient -70 -60 -50 -40 -30 -20 -10 0 10 20 30 59 |
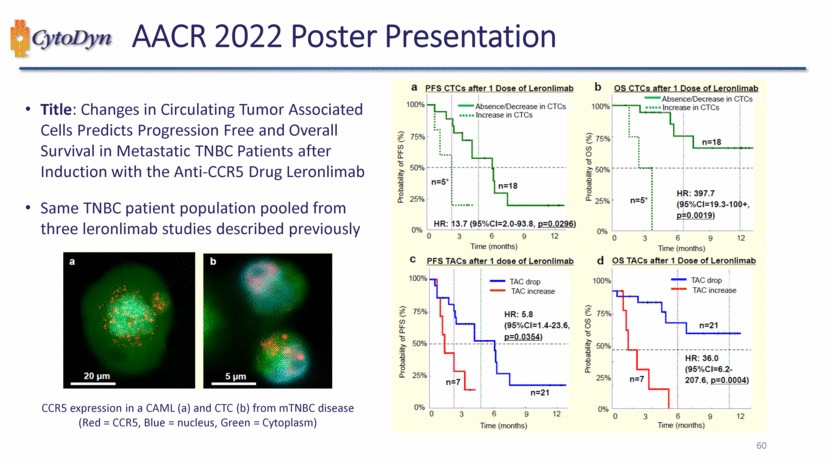
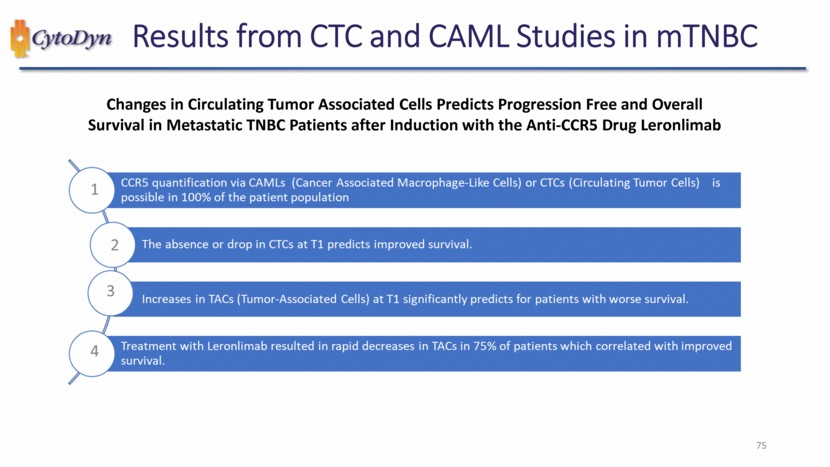
| Title: Changes in Circulating Tumor Associated Cells Predicts Progression Free and Overall Survival in Metastatic TNBC Patients after Induction with the Anti-CCR5 Drug Leronlimab Same TNBC patient population pooled from three leronlimab studies described previously CCR5 expression in a CAML (a) and CTC (b) from mTNBC disease (Red = CCR5, Blue = nucleus, Green = Cytoplasm) 60 |
| 61 Leronlimab in Patients with CRC - Clinical Data CD-09 Basket Study CRC (n=6) NCT04504942 n = 6 CRC pts |
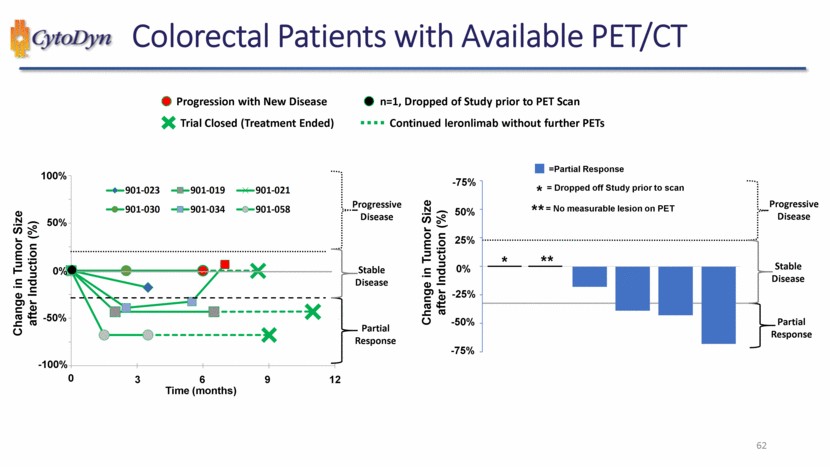
| 62 Colorectal Patients with Available PET/CT Progressive Disease -25% 25% 50% 0% -50% Change in Tumor Size after Induction (%) Partial Response Progressive Disease Stable Disease -75% -75% ** * 0 0% 100% Change in Tumor Size after Induction (%) 50% Time (months) 3 6 9 12 -100% -50% Partial Response Progressive Disease Stable Disease =Partial Response = No measurable lesion on PET * = Dropped off Study prior to scan ** Trial Closed (Treatment Ended) n=1, Dropped of Study prior to PET Scan Progression with New Disease Continued leronlimab without further PETs 901-023 901-019 901-021 901-030 901-034 901-058 |
| Microenvironment and IO in Cancer Therapy 63 Stefan Glück MD PhD FRCPC Professor em. of Medicine Oncology Hematology Immunology Biotech |
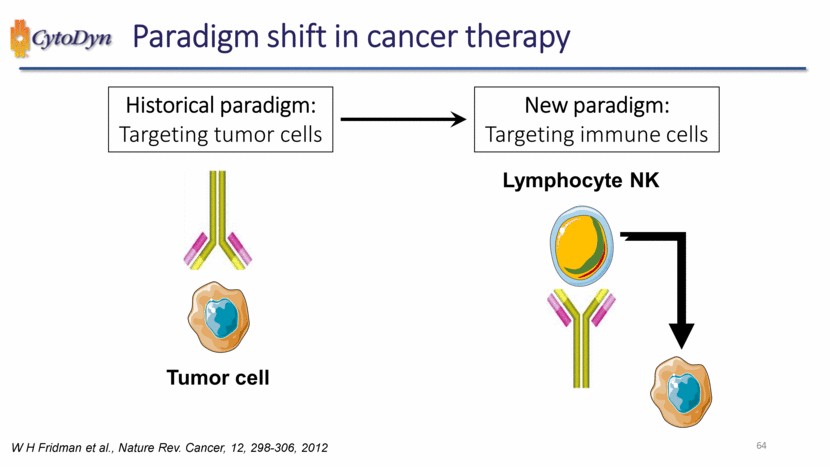
| 64 Paradigm shift in cancer therapy Tumor cell Historical paradigm: Targeting tumor cells Lymphocyte NK New paradigm: Targeting immune cells W H Fridman et al., Nature Rev. Cancer, 12, 298-306, 2012 |
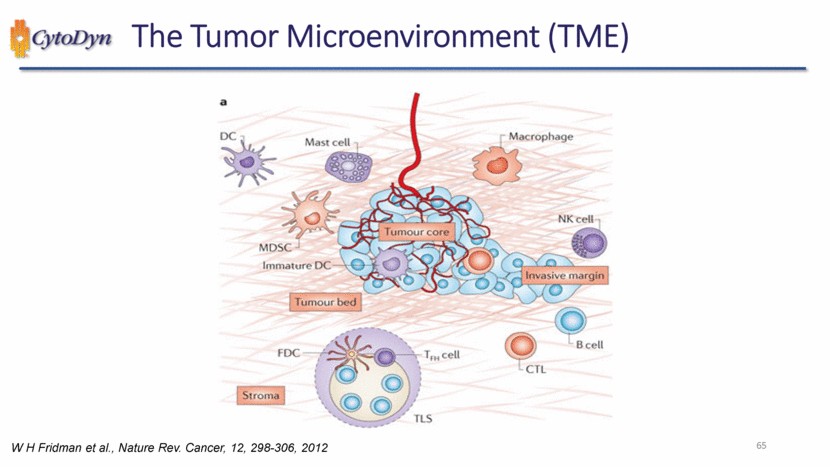
| The Tumor Microenvironment (TME) DC Mast cell Macrophage MDSC Tumour core NK cell Immature DC Invasive margin Tumour bed FDC T cell CTL B cell Stroma TLS W H Fridman et al., Nature Rev. Cancer, 12, 298-306, 2012 65 |
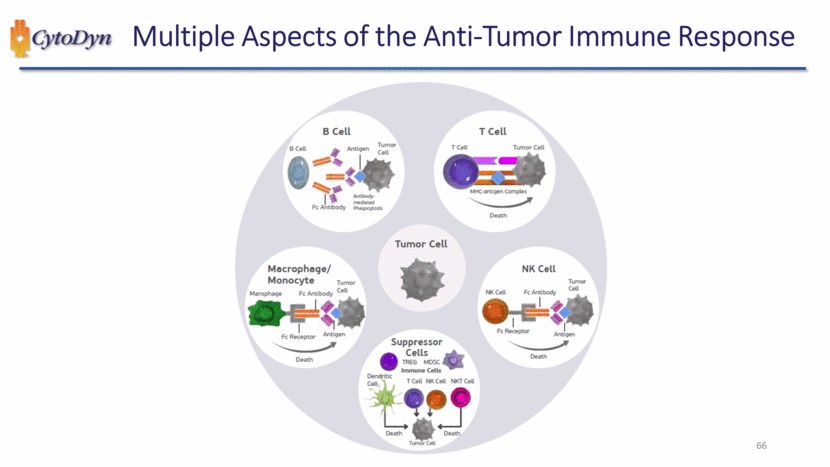
| Multiple Aspects of the Anti-Tumor Immune Response B cell B cell Antigen Tumor cell Fc Antibody Antibody medlated Phagocytosls T cell T cell Tumor Cell MHC-antigen Complex Death Tumor cell Macrophage Monocyte Marophage FC Antibody Tumor Cell FC Receptor Antigen Death Suppressor Cells TREG MDSC Immune Cells Dendritic Cell T Cell NK Cell NKT Cell Death Tumor Cell Death NK Cell NK Cell FC Antibody Tumor Cell FC ReceptorAntigen Death |
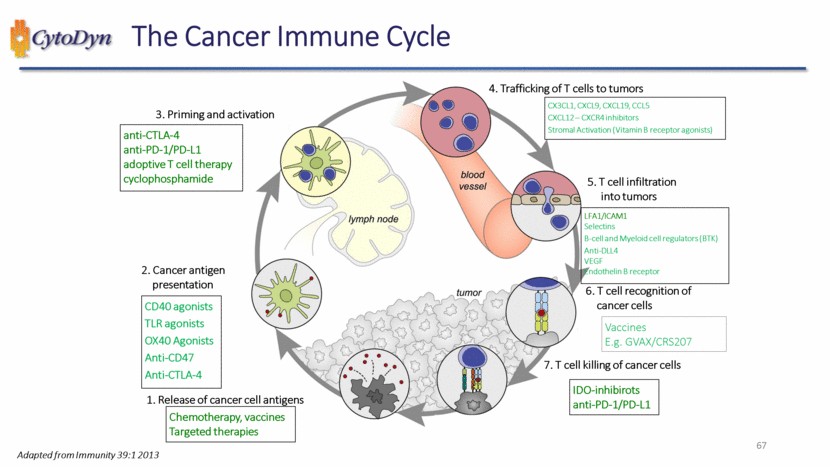
| 67 The Cancer Immune Cycle Chemotherapy, vaccines Targeted therapies CD40 agonists TLR agonists OX40 Agonists Anti-CD47 Anti-CTLA-4 anti-CTLA-4 anti-PD-1/PD-L1 adoptive T cell therapy cyclophosphamide IDO-inhibirots anti-PD-1/PD-L1 3. Priming and activation 4. Trafficking of T cells to tumors 5. T cell infiltration into tumors 6. T cell recognition of cancer cells 7. T cell killing of cancer cells 1. Release of cancer cell antigens 2. Cancer antigen presentation CX3CL1, CXCL9, CXCL19, CCL5 CXCL12 – CXCR4 inhibitors Stromal Activation (Vitamin B receptor agonists) LFA1/ICAM1 Selectins B-cell and Myeloid cell regulators (BTK) Anti-DLL4 VEGF Endothelin B receptor Vaccines E.g. GVAX/CRS207 Adapted from Immunity 39:1 2013 |
| The Nobel Prize in Physiology or Medicine 2018 Illustrations: Niklas Elmehed James P. Allison Tasuku Honjo “for their discovery of cancer therapy by inhibition of negative immune regulation” THE NOBEL ASSEMBLY AT KAROLINSKA INSTITUTET 68 |
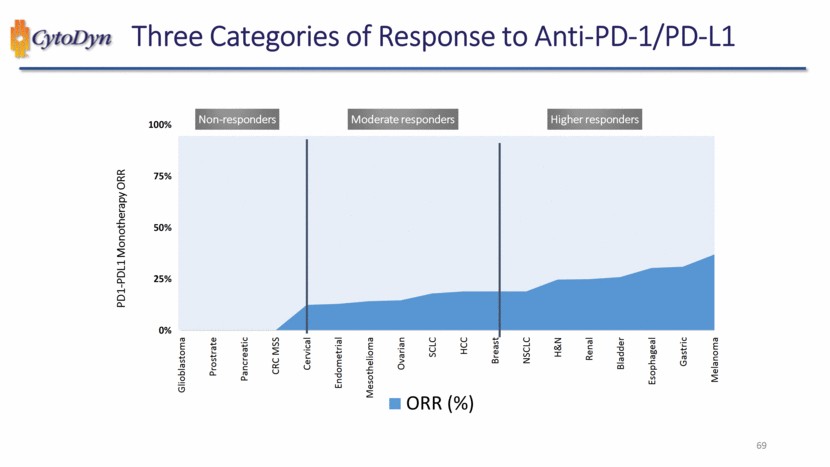
| Three Categories of Response to Anti-PD-1/PD-L1 Non-responders Moderate responders Higher responders PD1-PDL1 Monotherapy ORR Glioblastoma Prostrate Pancreatic CRC MSS Cervical Endometrial Mesothelioma Ovarian SCLC HCC Breast NSCLC H&N Renal Bladder Esophageal Gastric Melanoma ORR (%) 69 |
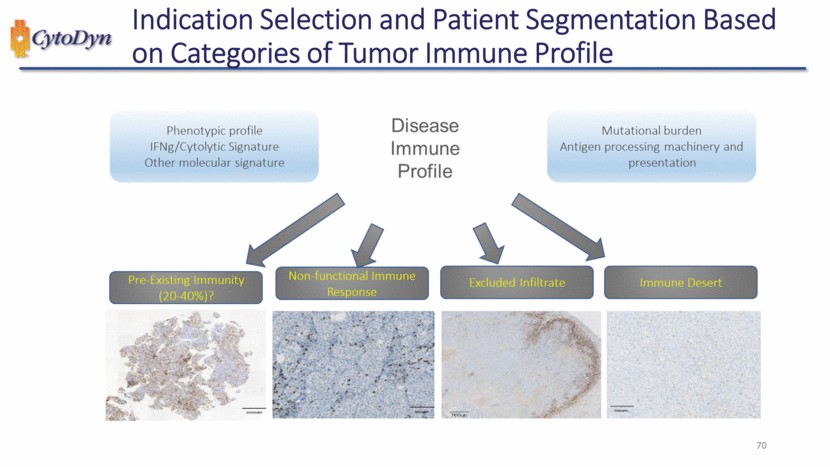
| Indication Selection and Patient Segmentation Based on Categories of Tumor Immune Profile Phenotypic profile IFNg/Cytolytic Signature Other molecular signature Disease Immune Profile Mutational burden Antigen processing machinery and presentation Pre-Existing Immunity (20-40%)? Non-functional Immune Response Excluded Infiltrate Immune Desert 70 |
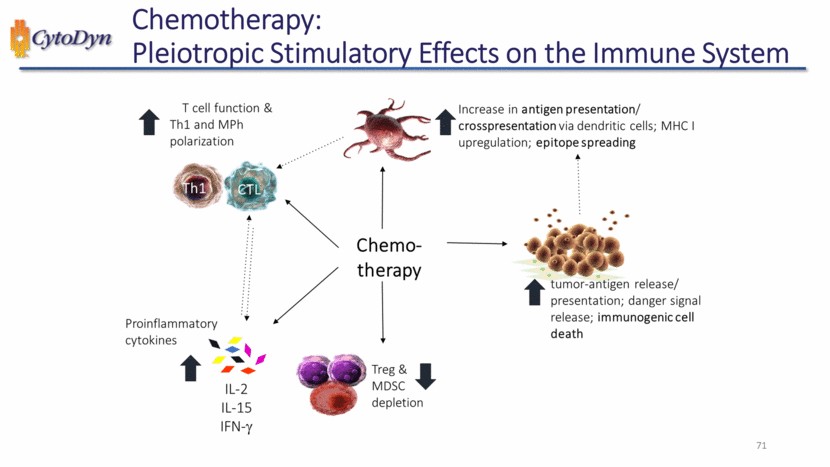
| Chemotherapy: Pleiotropic Stimulatory Effects on the Immune System T cell function & Th1 and MPh polarization Increase in antigen presentation/crosspresentation via dendritic cells; MHC I upregulation; epitope spreading Chemo therapy Proinflammatory cytokines IL-2 IL-15 IFN-y Treg&MDSC depletion tumor -antigen release/presentation; danger signal release; immunogenic cell death 71 |
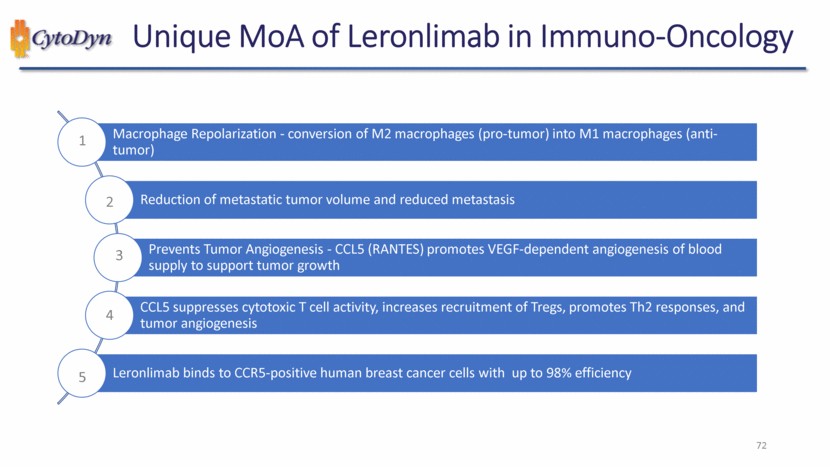
| 72 Unique MoA of Leronlimab in Immuno-Oncology 1 2 3 4 5 Macrophage Repolarization - conversion of M2 macrophages (pro-tumor) into M1 macrophages (anti-tumor) Reduction of metastatic tumor volume and reduced metastasis Prevents Tumor Angiogenesis - CCL5 (RANTES) promotes VEGF-dependent angiogenesis of blood supply to support tumor growth CCL5 suppresses cytotoxic T cell activity, increases recruitment of Tregs, promotes Th2 responses, and tumor angiogenesis Leronlimab binds to CCR5-positive human breast cancer cells with up to 98% efficiency |
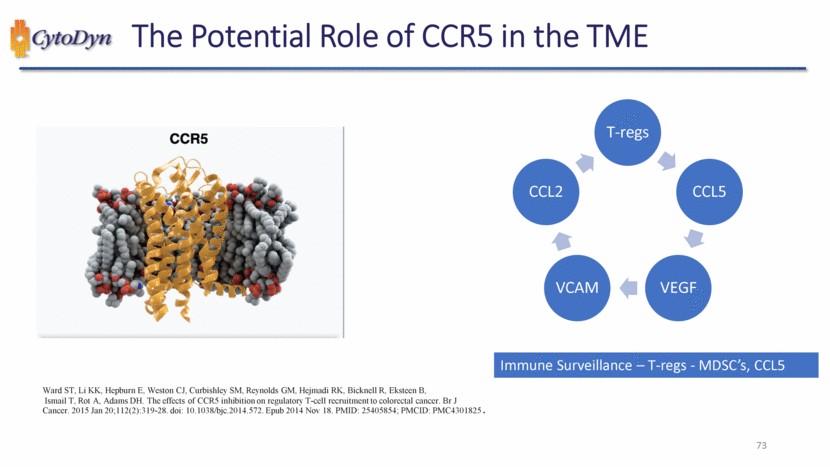
| 73 The Potential Role of CCR5 in the TME Ward ST, Li KK, Hepburn E, Weston CJ, Curbishley SM, Reynolds GM, Hejmadi RK, Bicknell R, Eksteen B, Ismail T, Rot A, Adams DH. The effects of CCR5 inhibition on regulatory T-cell recruitment to colorectal cancer. Br J Cancer. 2015 Jan 20;112(2):319-28. doi: 10.1038/bjc.2014.572. Epub 2014 Nov 18. PMID: 25405854; PMCID: PMC4301825. Immune Surveillance – T-regs - MDSC’s, CCL5 T-regs CCL5 VEGF VCAM CCL2 |
| Leronlimab in Patients with mTNBC - Clinical Data 74 CD-09 Basket Study TNBC (n=2) NCT04504942 CD-07: TNBC Compassionate Use (n =16) NCT04313075 n = 28 mTNBC pts CD-07: TNBC Phase 1b/2 (n =10) NCT03838367 |
| Results from CTC and CAML Studies in mTNBC Changes in Circulating Tumor Associated Cells Predicts Progression Free and Overall Survival in Metastatic TNBC Patients after Induction with the Anti-CCR5 Drug Leronlimab 75 |
| Results from CD-07: TNBC Phase 1b/2 A phase 1b/2 study of leronlimab combined with carboplatin in patients with CCR5+ metastatic Triple-Negative Breast Cancer (mTNBC) 76 |
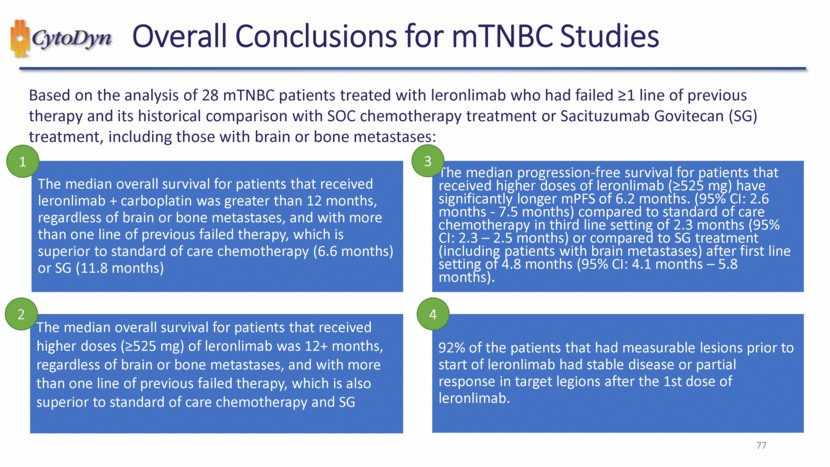
| 77 Overall Conclusions for mTNBC Studies Based on the analysis of 28 mTNBC patients treated with leronlimab who had failed ≥1 line of previous therapy and its historical comparison with SOC chemotherapy treatment or Sacituzumab Govitecan (SG) treatment, including those with brain or bone metastases: The median overall survival for patients that received leronlimab + carboplatin was greater than 12 months, regardless of brain or bone metastases, and with more than one line of previous failed therapy, which is superior to standard of care chemotherapy (6.6 months) or SG (11.8 months) The median overall survival for patients that received higher doses (≥525 mg) of leronlimab was 12+ months, regardless of brain or bone metastases, and with more than one line of previous failed therapy, which is also superior to standard of care chemotherapy and SG The median progression-free survival for patients that received higher doses of leronlimab (≥525 mg) have significantly longer mPFS of 6.2 months. (95% CI: 2.6 months - 7.5 months) compared to standard of care chemotherapy in third line setting of 2.3 months (95% CI: 2.3 – 2.5 months) or compared to SG treatment (including patients with brain metastases) after first line setting of 4.8 months (95% CI: 4.1 months – 5.8 months). 92% of the patients that had measurable lesions prior to start of leronlimab had stable disease or partial response in target legions after the 1st dose of leronlimab. 1 2 3 4 |
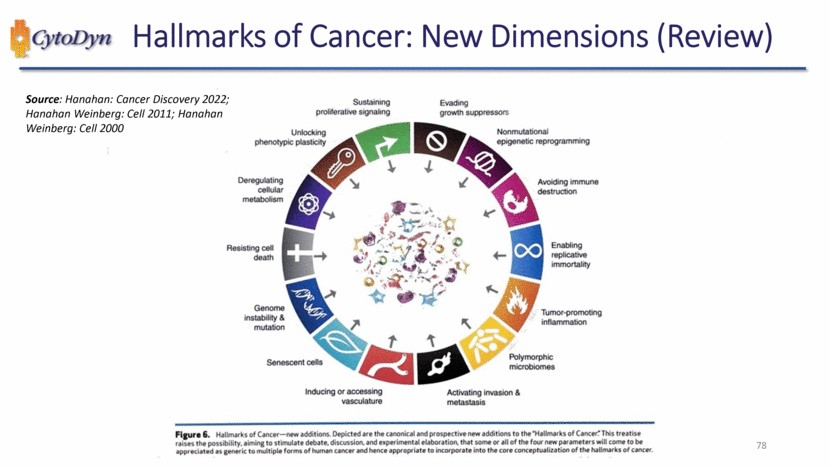
| Sustaining proliferative signaling Evading growth suppressors Nonmutational epigenetic reprogramming Avoiding immune destruction Enabling replicative immortality Tumor-promoting inflammation polymorphic microbiomes Activating invasion & metastasis Inducing or accessing vasculature Senescent cells Genome instability & mutation Resisting cell death Deregulating cellular metabolism Unlocking phenotypic plasticity Figure 6. Hallmarks of Cancer — new additions. Depicted are the canonical and prospective new additions to the “Hallmarks of Cancer.” This treatise raises the possibility, aiming to stimulate debate, discussion, and experimental elaboration, that some or all of the four new parameters will come to be appreciated as generic to multiple forms of human cancer and hence appropriate to incorporate into the core conceptualization of the hallmarks of cancer. |
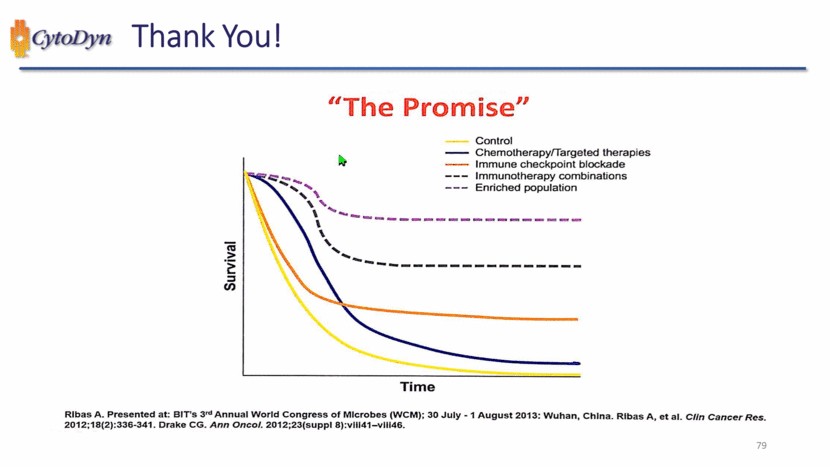
| “The Promise” Control Chemotherapy/Targeted therapies Immune checkpoint blockade Immunotherapy combinations Enriched population Survival Time Rlbas A. Presented at: BIT’s 3rd Annual World Congress of Microbes (WCM); 30 Julty – 1 August 2013: Wuhan, China, Rlbas A, et al. Clin Cancer Res. 2012;18(2):336-341. Drake CG. Ann Oncol. 2012,23(suppl 8):vII41-vIII46 |
| CCR5 in HIV Prevention and Cure 80 Jonah B. Sacha, Ph.D. Oregon Health & Science University |
| CCRS is Critical in Sexual Transmission of HIV CytoDyn LETTERS TO NATURE Resistance to HIV-1infection In caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene MichelSamson ·,Frederick Ubert , Benjamin J. Ooranzt, Joseph Ruckert, Corinne Uesnardt, Claire-Michele Farber§, Sentob Saragosti 1 .Claudine Lapoumeroulie , Opinion I Published: 01 April 2006 Selective transmission of CCRS-utilizing HIV-1: the ·gatekeeper· problem Leonid Margolis & Robin Shattock resolved? Jacqueline Cognaux#,Christine Forceille#, Gaetan Muyldermans#, Chris Verhofstede#, Guy Burtonboy#, MichelGeorges .. .Tsuneo lmai*·, ShaliniRanatt, Yanji Yitt, Robert J.Smythtt, Ronald G.Collmantt,Robert W.Domst, Gilbert Vassart H & Marc Parmentier• REPORTS I VOLUME 86. ISSUE 3. P367-377. AUGUST 09. 1996 Genetic Restriction of HIV-1 Infection and Progression to AIDS by a Deletion Allele of the CKR5 Structural Gene Michael Dean*, Mary Carrington*, Cheryl Winkler, Gavin A. Huttley, Michael W.Smith, Rand o Allikmets, James J. Goedert,-· istance of tion · xton • Sunny Choe • ... Heidi Stuhlmann • R p • n L • Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Franci! 81 andau iel R Natha ichar d A Kou William A Pa Rong Liu Homozygous De fe ct in HIV-1 Coreceptor Acco unts f or Res Some Multipl y-E xposed Individuals to HIV-1 Infec ARTI CLE Cell t' nature reviews microbiology |
| CCR5 in HIV Cure Patient No More Timothy Brown-a.k.a. “the Berlin Patient”-is the Man Who Once Had HIV. “Berlin patient” Timothy Brown HIV remission 13 years 82 |
| CCR5 in HIV Cure Patient No More Timothy Brown-a.k.a. “the Berlin Patient”-is the Man Who Once Had HIV. “Berlin patient” Timothy Brown HIV remission 13 years “London patient” Adam Castillejo HIV remission >4 years 83 |
| CCRS in HIV Cure cytoDyn Patient No More Timothy Brown-a.k.a. "the Bertin Patient" is the Man Who Once Had HIV. a International Maternal Pediatric Adolescent AIDS Clinical Trials Network 84 IMPAACT P1107 Team Presents First Known Case of Woman with HIV Remission |
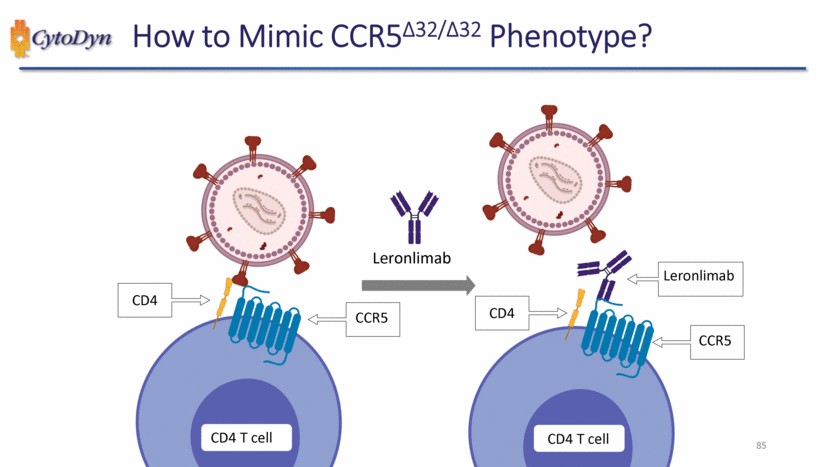
| 85 How to Mimic CCR5Δ32/Δ32 Phenotype? CD4 T cell CD4 CCR5 CD4 T cell CD4 CCR5 Leronlimab Leronlimab |
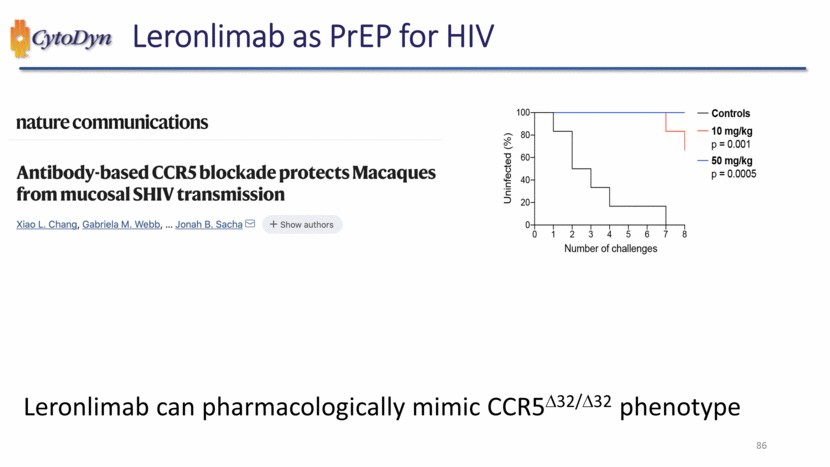
| Leronlimab as PrEP for HIV nature communications Antibody-based CCR5 blockade protects Macaques from mucosal SHIV transmission Xiao L. Chang, Gabriela M. Webb, … Jonah B. Sacha + Show authors Uninfected (%) Number of challenges Controls 10 mg/kg p = 0.001 50 mg/kg p = 0.0005 Leronlimab can pharmacologically mimic CCR5D32/D32 phenotype 86 |
| Long-Acting Injectables for PrEP Science Logn-Acting Integrase Inhibitor Protects Macaques from Intrarectal Simian/Human Immunodeficiency Virus Chasity D. Andrews, 1 William R. Spreen, 2 Hiroshi Mohri,1 Lee Moss,2 Susan Ford,2 Agegnehu Gettie,1 Kasi Russell-Lodrigue,3 Rudolf P. Bohm,3 Cecilia Cheng-Mayer,1 Zhi Hong,2 Martin Markowitz,1 David D. Ho1* FDA NEWS RELEASE FDA Approves First Injectable Treatment for HIV Pre-Exposure Prevention Drug Given Every Two Months Rather Than Daily Pill is Important Tool in Effort to End the HIV Epidemic 87 |
| Long-Acting Injectables for PrEP Science Logn-Acting Integrase Inhibitor Protects Macaques from Intrarectal Simian/Human Immunodeficiency Virus Chasity D. Andrews, 1 William R. Spreen, 2 Hiroshi Mohri,1 Lee Moss,2 Susan Ford,2 Agegnehu Gettie,1 Kasi Russell-Lodrigue,3 Rudolf P. Bohm,3 Cecilia Cheng-Mayer,1 Zhi Hong,2 Martin Markowitz,1 David D. Ho1* FDA NEWS RELEASE FDA Approves First Injectable Treatment for HIV Pre-Exposure Prevention Drug Given Every Two Months Rather Than Daily Pill is Important Tool in Effort to End the HIV Epidemic Leronlimab “monkey-ize” + LS mutation Long acting Leronlimab 88 |
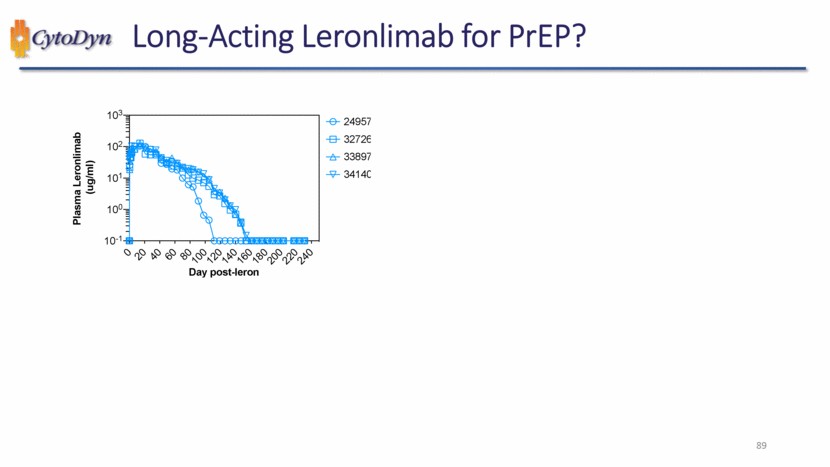
| Long-Acting Leronlimab for PrEP? Plasma Leronlimab (ug/ml) Day post-leron 24957 32726 33897 34140 89 |
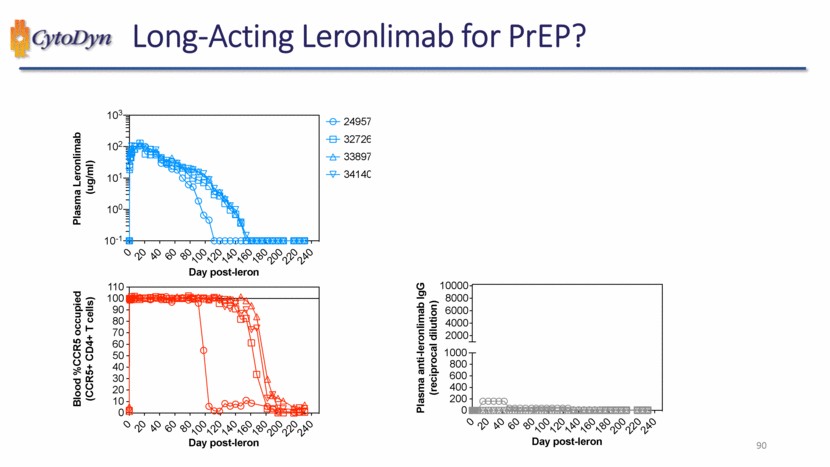
| Long-Acting Leronlimab for PrEP? Plasma Leronlimab (ug/ml) Day post-leron 24957 32726 33897 34140 Blood %CCR5 occupied (CCR5+ CD4+ T cells) Day post-leron Plasma anti-leronlimab IgG (reciprocal dilution) Day post-leron 90 |
| CCR5 in HIV Cure Patient No More Timothy Brown – a.k.a. “the Berlin Patient” – is the Man Who Once Had HIV. International Maternal Pediatric Adolescent AIDS Clinical Trials Network IMPAACT P1107 Team Presents First Known Case of a Woman with HIV Remission 91 |
| CCR5 in HIV Cure AAV Vectors Advance the Frontiers of Gene Therapy Technological developments and therapeutic applications of third-generation AAV vectors. 92 |
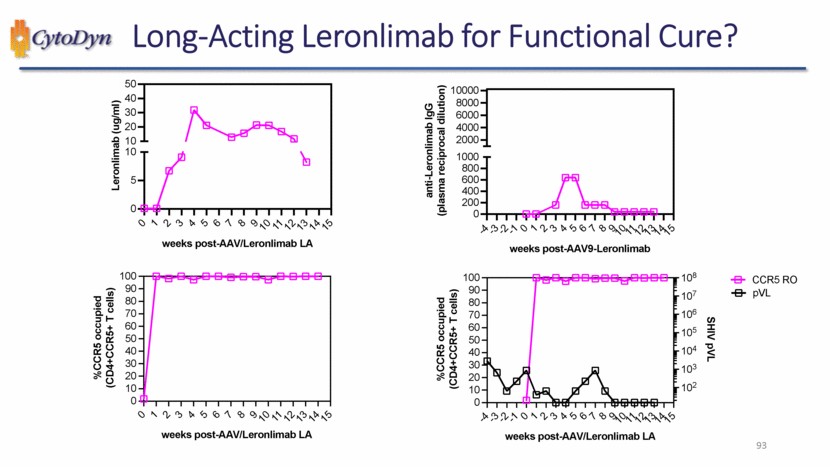
| Long-Acting Leronlimab for Functional Cure? Leronlimab (ug/ml) weeks post-AAV/Leronlimab LA %CCR5 occupied (CD4+CCR5+ T cells) weeks post-AAV/Leronlimab LA anti-Leronlimab IgG (plasma reciprocal dilution) weeks post-AAV9-Leronlimab %CCR5 occupied (CD4+CCR5+ T cells) weeks post-AAV/Leronlimab LA SHIV pVL CCR5 RO pVL 93 |
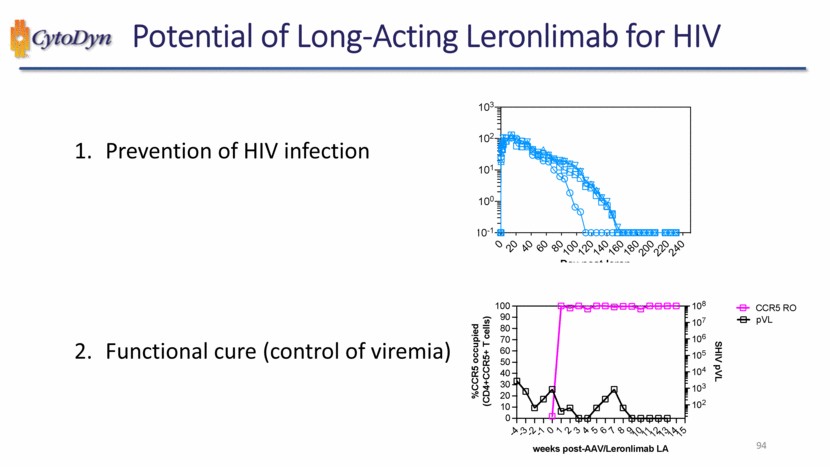
| Potential of Long-Acting Leronlimab for HIV 1. Prevention of HIV infection 2. Functional cure (control of viremia) %CCR5 occupied (CD4+CCR5+ T cells) weeks post-AAV/Leronlimab LA SHIV pVL CCR5 RO pVL 94 |
| Closing Remarks 95 |
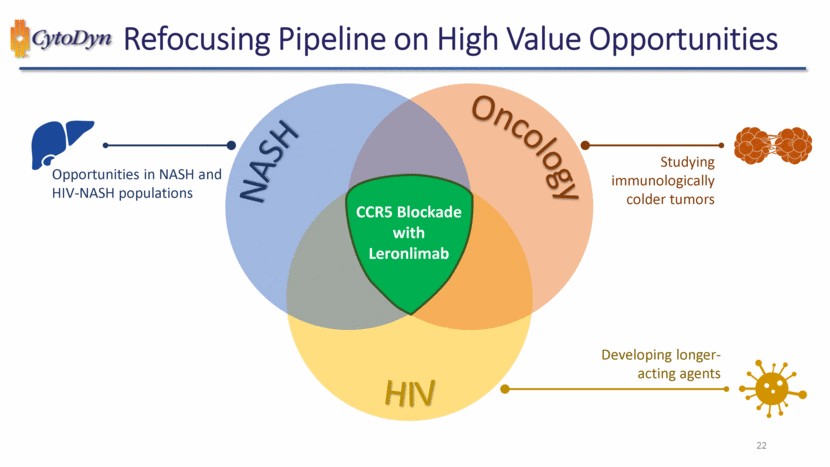
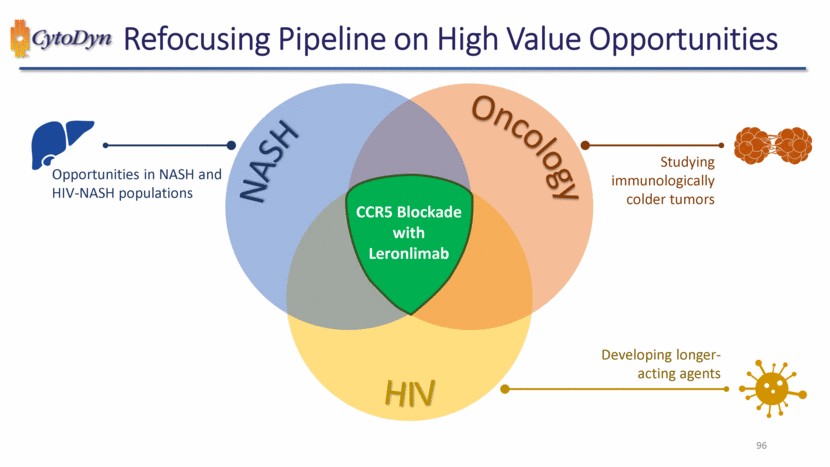
| Refocusing Pipeline on High Value Opportunities 96 NASH Oncology HIV CCR5 Blockade with Leronlimab Studying immunologically colder tumors Developing longer-acting agents Opportunities in NASH and HIV-NASH populations |
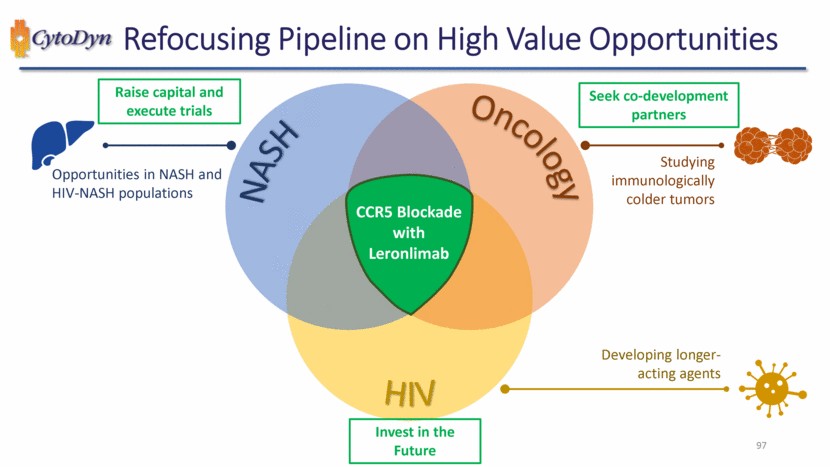
| Refocusing Pipeline on High Value Opportunities 97 NASH Oncology HIV CCR5 Blockade with Leronlimab Studying immunologically colder tumors Developing longer-acting agents Opportunities in NASH and HIV-NASH populations Raise capital and execute trials Invest in the Future Seek co-development partners |
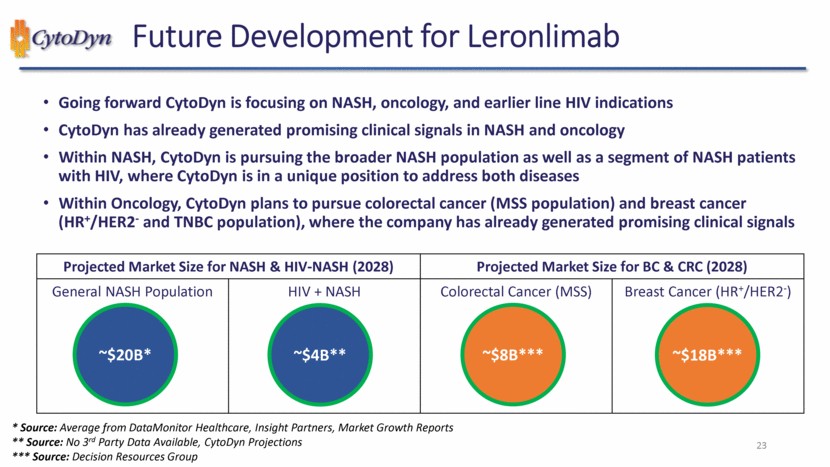
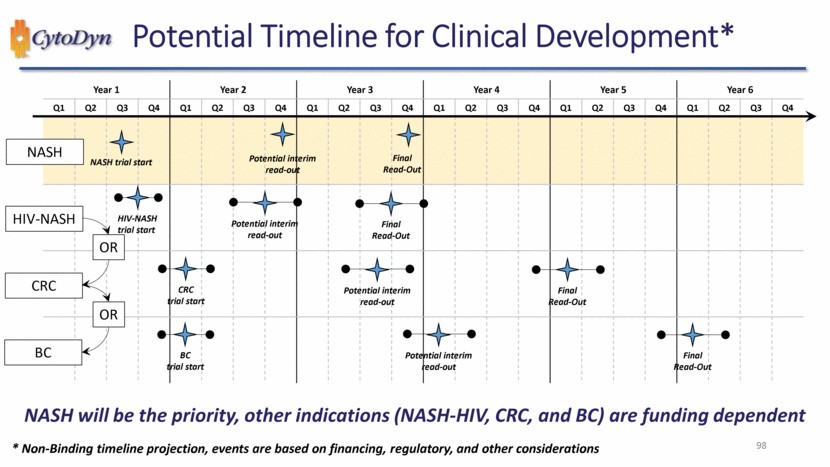
| NASH will be the priority, other indications (NASH-HIV, CRC, and BC) are funding dependent 98 Potential Timeline for Clinical Development* Year 1 Year 2 Year 3 Year 4 Year 5 Year 6 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 NASH trial start * Non-Binding timeline projection, events are based on financing, regulatory, and other considerations Potential interim read-out Final Read-Out HIV-NASH trial start Potential interim read-out Final Read-Out OR CRC trial start BC trial start NASH HIV-NASH CRC BC Potential interim read-out Final Read-Out Potential interim read-out Final Read-Out OR |
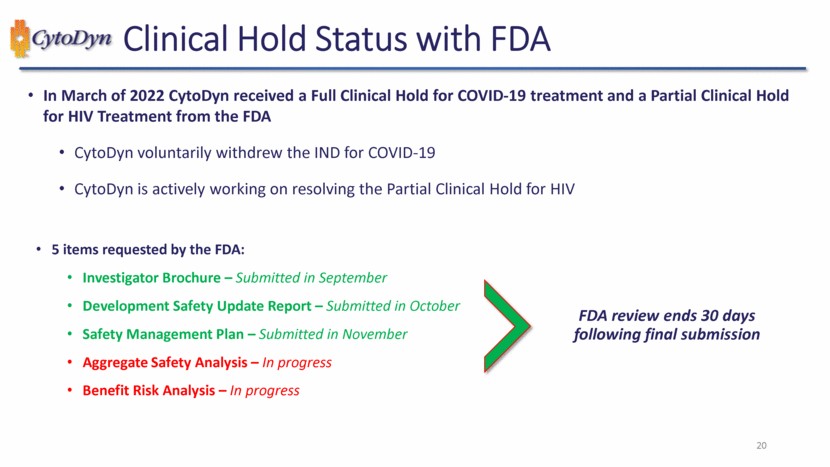
| 99 What We Expect in 2023 Lifting of the Partial Clinical Hold in HIV Financing to fund operations and achieve value inflection Initiating a NASH trial Invest in and advance a long-acting CCR5 molecule Continued contributions in medical meetings and peer-reviewed publications Continuing to reshape of our team and capabilities to meet our goals Corporate Rebranding |
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- HMN FINANCIAL, INC. ANNOUNCES FIRST QUARTER RESULTS
- Alaska Energy Metals Files Amended NI 43-101 Technical Report for the Eureka Deposit, Nikolai Nickel Project, Alaska, USA
- VINCI PARTNERS TO ANNOUNCE FIRST QUARTER 2024 RESULTS AND HOST WEBCAST AFTER MARKET CLOSE ON THURSDAY, MAY 09, 2024
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share