Form 8-K Synthetic Biologics, For: May 16
Exhibit 99.1

Oncolytic Adenovirus With Hyaluronidase Activity That Evades Neutralizing Antibodies And Allows Re - administration: VCN - 11 Ana Mato - Berciano , Maria V. Maliandi , Sara Morgado , Marti Farrera - Sal, Paz Moreno, Rafael Moreno, Luis A. Rojas, Gabriel Capellà , Miriam Bazan - Peregrino , Manel Cascallo , and Ramon Alemany Virotherapy Group

Disclosures R. Alemany is advisor and owns stock of VCN Biosciences / Synthetic Biologics

VCN - 01 and VCN - 11 oncolytic adenoviruses Fiber Shaft RGDK Hexon ABD VCN - 01 VCN - 11 Ad5 wild type | Reduces liver tropism Fiber Shaft KKTK Host albumin | Enables replication in normal cells | Facilitates liver sequestration | Replication restricted to tumor cells (Rb - E2F defective) Degrades tumor stroma | Binds host albumin to protect against neutralizing antibodies Amplified replication in tumor cells (high levels of E2F) | L5 L3 E1a - Δ 24 415p SA pA K PH20 LITR RITR L1 L2 L4 MLP L5 L3 E1a LITR RITR L1 L2 L4 MLP L5 L3 E1a - Δ 24 415p SA pA K PH20 LITR RITR L1 L2 L4 MLP Fiber Shaft RGDK Lower hepatic toxicity Allows fractionated dosing to improve half life and tumor targeting Induces less NAbs Shielding from NAbs allows readministration

In vivo: VCN - 11 Infection causes hyaluronan degradation Melanoma SKmel28 tumors IT administration VCN - 11 expresses hyaluronidase In vitro:

VCN - 11 binds albumin

Virus in liver at 48 h post admin. IV P B S V C N - 0 1 ( 1 X ) V C N - 1 1 ( 1 X ) 0 200 400 600 800 1000 1500 2000 2500 3000 VCN-01 vs VCN-11 single IV A L T ( I U / L ) *** P B S V C N - 1 1 ( 1 X ) V C N - 1 1 ( 3 X ) V C N - 1 1 ( 6 X ) 0 200 400 600 800 1000 1500 2000 2500 3000 VCN-11 single IV dose escalation A L T ( I U / L ) ** Athymic nude mice bearing tumors VCN - 11 reduced hepatic toxicity compared to VCN - 01 1X Dose = 4E10 vp / mouse i.v.

Reduced VCN - 11 toxicity allows dose fractionation Increased t 1/2 for the second VCN - 11 dose (mouse model) 3 X S i n g l e 3 X ( 1 X @ 0 h + 2 X @ 4 h ) 3 X ( D 1 , D 2 , D 3 ) 0 10 20 30 40 t 1 / 2 ( m i n ) * * Dose fractionation slightly increases hepatic toxicity 5min 16min 14min 3X Dose = 1.2E11 vp / mouse i.v.

Dose fractionation increases VCN - 11 bioavailability and improves tumor targeting Fiber mRNA in tumor (accumulated dose) PH20 mRNA in tumor (availability of the post - dose) • Pre - dose with an oncolytic virus (ICOVIR15KABD) not expressing PH20 @ 1X = 4E+10vp/animal to block liver kupffer cells • Post - dose with VCN - 11 that expresses PH20
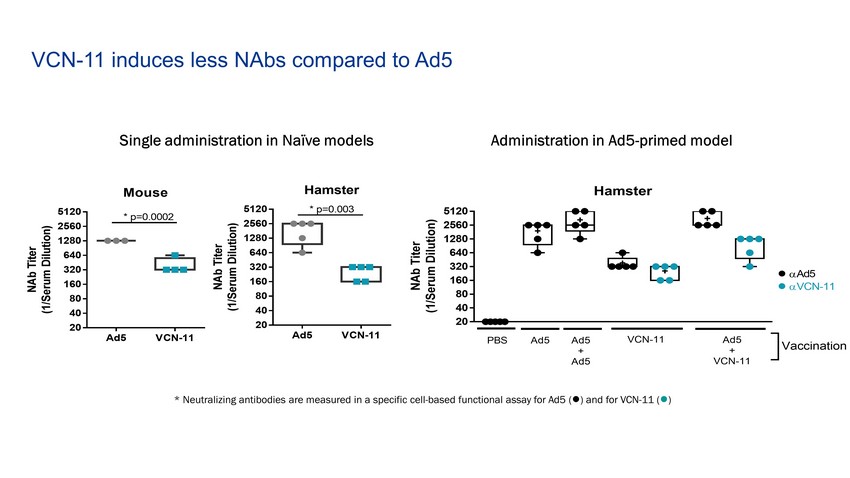
Single administration in Naïve models 20 40 80 160 320 640 1280 2560 5120 Hamster N A b T i t e r ( 1 / S e r u m D i l u t i o n ) Ad5 VCN-11 Vaccination PBS Ad5 Ad5 + Ad5 VCN-11 Ad5 + VCN-11 Ad5 VCN-11 20 40 80 160 320 640 1280 2560 5120 Mouse N A b T i t e r ( 1 / S e r u m D i l u t i o n ) * p=0.0002 Ad5 VCN-11 20 40 80 160 320 640 1280 2560 5120 Hamster N A b T i t e r ( 1 / S e r u m D i l u t i o n ) * p=0.003 Administration in Ad5 - primed model * Neutralizing antibodies are measured in a specific cell - based functional assay for Ad5 ( ● ) and for VCN - 11 ( ● ) VCN - 11 induces less NAbs compared to Ad5
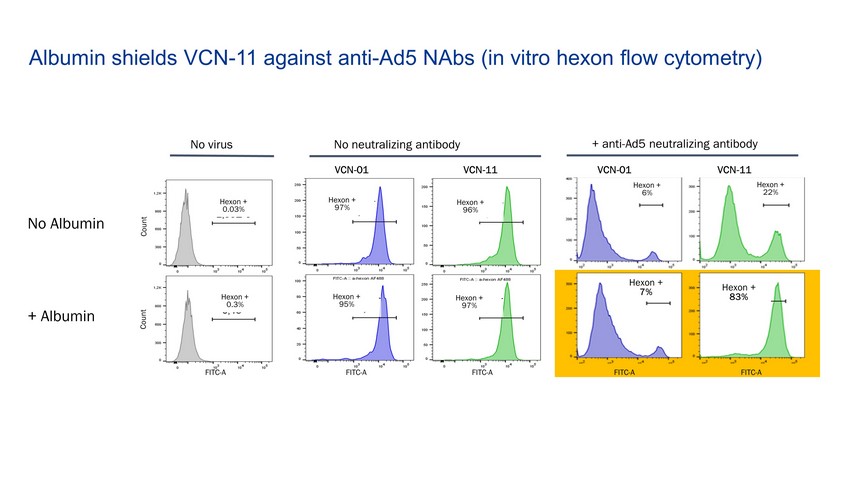
Albumin shields VCN - 11 against anti - Ad5 NAbs (in vitro hexon flow cytometry ) VCN - 01 VCN - 11 VCN - 01 VCN - 11 No neutralizing antibody + anti - Ad5 neutralizing antibody No virus Count Count FITC - A FITC - A FITC - A FITC - A FITC - A Hexon + 6% Hexon + 22% Hexon + 83% Hexon + 7% Hexon + 0.3% Hexon + 0.03% Hexon + 97% Hexon + 95% Hexon + 97% Hexon + 96% No Albumin + Albumin

Tumor cells injection Immunization Ad5 (IP) Immunization (IV) Ad5 (IV) Day 1 Day 8 Day 15 Serum collection C57BL/6 mice immunocompetent Athymic nuce mice immunodeficient Passive immunization Second admin (IV) VCN - 01 or VCN - 11 Day - 1 Day 0 0.0001 0.001 0.01 0.1 1 10 F o l d C h a n g e V G w i t h N A b s / V G w i t h o u t N A b s 1 / 4 0 1 / 1 2 8 0 1 / 4 0 1 / 5 1 2 0 N o N A b s N o N A b s NAbs Titer 1 / 1 2 8 0 * * VCN-01 VCN-11 Albumin shields VCN - 11 against anti - Ad5 NAbs in vivo Viral genomes in tumors

0 10 20 30 40 50 60 70 80 90 0 250 500 750 1000 1250 1500 1750 Days T u m o u r g r o w t h ( % ) PBS VCN-01 * p<0.05 vs PBS VCN-11 * * * * SK-MEL-28 VCN - 11 shows antitumor activity in the presence of anti - Ad5 NAbs
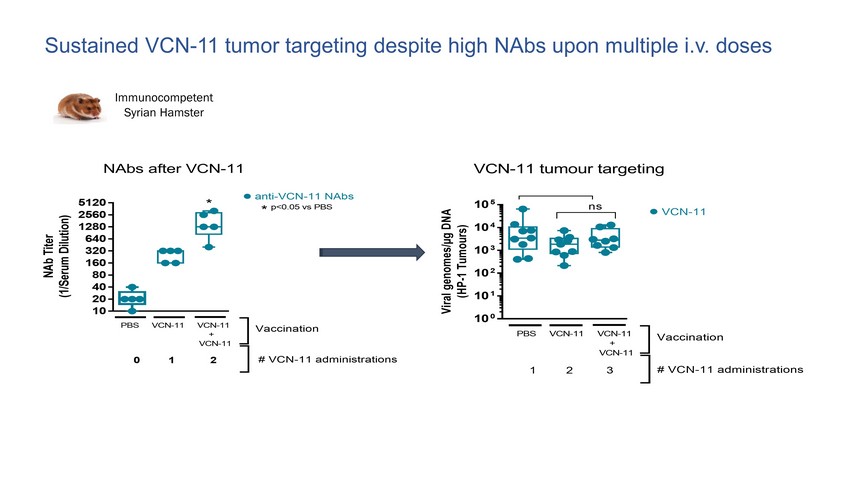
10 0 10 1 10 2 10 3 10 4 10 5 V i r a l g e n o m e s / µ g D N A ( H P - 1 T u m o u r s ) PBS VCN-11 VCN-11 + VCN-11 Vaccination ns VCN-11 # VCN-11 administrations 1 2 3 VCN-11 tumour targeting 10 20 40 80 160 320 640 1280 2560 5120 N A b T i t e r ( 1 / S e r u m D i l u t i o n ) anti-VCN-11 NAbs * * p<0.05 vs PBS PBS VCN-11 VCN-11 + VCN-11 Vaccination NAbs after VCN-11 # VCN-11 administrations 0 1 2 Immunocompetent Syrian Hamster Sustained VCN - 11 tumor targeting despite high NAbs upon multiple i.v. doses

VCN - 11 antitumor activity in the presence of antiAd5+antiVCN - 11 NAbs Non - immunized animals 0 5 10 15 20 25 30 0 200 400 600 800 1000 Pre-immunized animals PBS VCN-01 (NAbs 1/320) VCN-11 (NAbs 1/320) Days T u m o u r v o l u m e ( m m 3 ) *p<0.05 vs PBS *
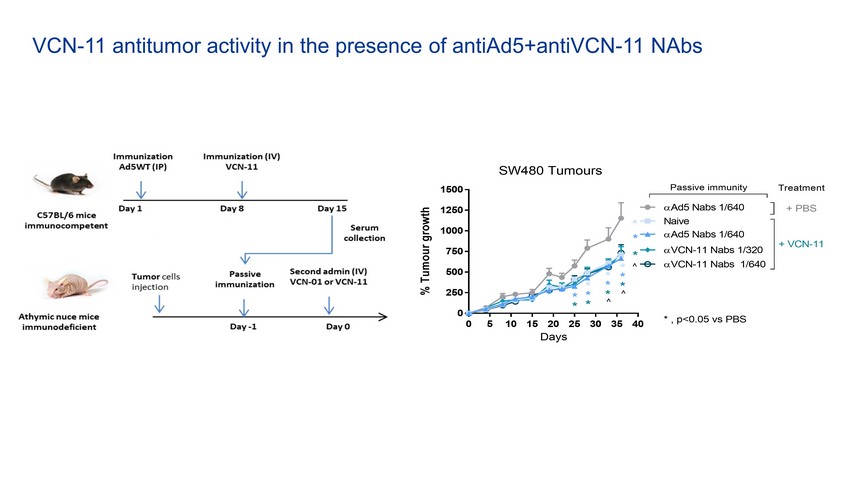
0 5 10 15 20 25 30 35 40 0 250 500 750 1000 1250 1500 Days % T u m o u r g r o w t h VCN-11 Nabs 1/320 VCN-11 Nabs 1/640 Ad5 Nabs 1/640 SW480 Tumours Naive Ad5 Nabs 1/640 + VCN-11 + PBS Passive immunity Treatment ^ * * * * * ^ * * * ^ * * * * * * * , p<0.05 vs PBS VCN - 11 antitumor activity in the presence of antiAd5+antiVCN - 11 NAbs

• VCN - 11 expresses functional hyaluronidase . • Reduced hepatic toxicity of the ABD - modified virus allows to “fractionate” VCN - 11 administration which improves circulating half - life and tumor targeting . • VCN - 11 administration results in lower levels of neutralizing antibodies . • VCN - 11 reaches tumors and is effective after systemic administration in the presence of NAbs • VCN - 11 ´ s albumin shield from NAbs allows effective readministration . Conclusions

This project has been funded by VCN Biosciences, a wholly owned subsidiary of Synthetic Biologics: Ana Mato Maria V. Maliandi Sheila Connelly Michael Kaleko Frank Tufaro Manel Cascalló Miriam Bazan - Peregrino Sara Morgado Martí Farrera - Sal Rafael Moreno Paz Moreno Luís Rojas Gabriel Capellà PID2020 - 119692RB - C21
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Tidal Wave Auto Spa Celebrates Grand Openings in Woodstock, IL, and Waynesboro, VA, With Free Washes
- Initiative Equity Partners acquired 16% equity in ArtIn Energy ramping up expansion in North America
- Uforia unites artists, communities nationwide to support St. Jude Children’s Research Hospital this Dia del Niño
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share