Form 8-K ROCKWELL MEDICAL, INC. For: May 20
Exhibit 99.1

Rockwell Medical, Inc. Transforming Iron Deficiency Therapy (NASDAQ: RMTI) Corporate Presentation May 2022

Forward - Looking Statements Certain statements in this presentation may constitute "forward - looking statements" within the meaning of federal securities laws, including, but not limited to, Rockwell Medical’s intention to develop FPC for new indications, and maintain concentrate sales . Words such as "may," "might," "will," "should," "believe," "expect," "anticipate," "estimate," "continue," “can,” "could," “would,” “develop,” "plan," "potential," "predict," "forecast," "project," "plan", "intend" or the negatives of these terms, and similar expressions, or statements regarding intent, belief, or current expectations, are forward - looking statements . While Rockwell Medical believes these forward - looking statements are reasonable, undue reliance should not be placed on any such forward - looking statements, which are based on information available to us on the date of this presentation . These forward - looking statements are based upon current estimates and assumptions and are subject to various risks and uncertainties (including, without limitation, those set forth in Rockwell Medical’s SEC filings), many of which are beyond our control and subject to change . Actual results could be materially different . Risks and uncertainties include : our expectations regarding our ability to enter into marketing and other partnership agreements, including amendments to our existing agreements ; the risk that market opportunities are smaller than estimated ; the risk that clinical study designs, timing, results and costs are different than estimated ; the impact of COVID - 19 pandemic (including, applicable federal, state and local orders) on business, clinical development plans and operating results, including our supply chain and dialysis concentrates business ; the challenges inherent in new product development and other indications and therapeutic areas for our products ; the likelihood of success and timing of our international development and commercialization plans, including regulatory filings and clinical trials ; the success and our commercialization of Triferic domestically and internationally ; the expected number of annualized treatments for Triferic Dialysate and Triferic AVNU ; the risk that regulatory authorities delay or fail to approve FPC for new indications ; the risk that Rockwell Medical is not able to seek reimbursement for FPC for new indications ; the risk that FPC is unsafe for new indications ; the risk that our concentrates business does not grow as expected and that we are not able to realize expected reductions in cash burn ; the impact of any further increases in raw material, labor, fuel or other input costs, particularly if we are unable to pass these cost increases along to our customers ; the risk that we are unable to obtain additional financing and raise capital as necessary to fund operations or pursue business opportunities ; expected financial performance, including cash flows, revenues, growth, margins, funding, liquidity and capital resources ; and those risks more fully discussed in the “Risk Factors” section of our Annual Report on Form 10 - K for the year ended December 31 , 2021 , as such description may be amended or updated in any future reports we file with the SEC . Rockwell Medical expressly disclaims any obligation to update our forward - looking statements, except as may be required by law . Triferic® is a registered trademark of Rockwell Medical, Inc . Triferic AVNU is pending with the U . S . Patent and Trademark Office . All other product names, logos, and brands are property of their respective owners in the United States and/or other countries . All company, product and service names used on this website are for identification purposes only . Use of these names, logos, and brands does not imply endorsement 2

Rockwell Medical, Inc. Developing innovative therapies to transform the treatment of iron deficiency 3 x Proprietary Drug • Next generation iron deficiency therapeutic • Applicable to many different patient populations • Two NDA approvals x Significant Opportunity: Home Infusio n Iron Therapy • Phase 2 Trial expected to start in H2 2022 • Multiple Data Readouts anticipated in 2023 x Re - engineered Dialysis Products Business • >$60m annual revenue, updated customer agreement s in 2022 • Expect positive gross margin from dialysis concentrates beginning 2H 2022 x Multiple Near - Term Milestones x Future Pipeline Opportunity: Acute Heart Failure Transforming Iron Deficiency Therapy MT0
Ferric Pyrophosphate Citrate (FPC) Next - generation intravenous iron therapy 4

Iron Deficiency: What problem needs to be solved? 5 Traditional IV iron products require an outpatient clinic visit , associated with rare severe reactions Oral iron is not adequate for many patients with moderate to severe IDA Iron Deficiency Can Result From… 1 Insufficient iron in diet Reduced GI absorption Regular blood loss Inflammation Iron Deficiency Anemia (IDA); a Common Comorbidity in Many Diseases Kidney Disease Heart Failure Cancer Multiple Chronic Gastrointestinal Diseases Chronic Bleeding Disorders ~ 10 million people in the U.S. are iron deficient including ~ 5 million with iron deficiency anemia 2 Inflammation impacts bioavailability of iron and therapeutic efficacy Iron Deficiency Can Lead to Serious Health Complications 1 Moderate to severe iron deficiency and resulting anemia increases risk of… Organ dysfunction Heart conditions I nfection Extended hospital stays Severe Fatigue Depression Treatment Options Have Limitations 1. Warner MJ, Kamran MT. Iron Deficiency Anemia. [Updated 2021 Aug 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan - . 2. Miller et al. Iron Deficiency Anemia: A Common and Curable Disease. Cold Spring Harb Perspect Med 2013;3:a011866.
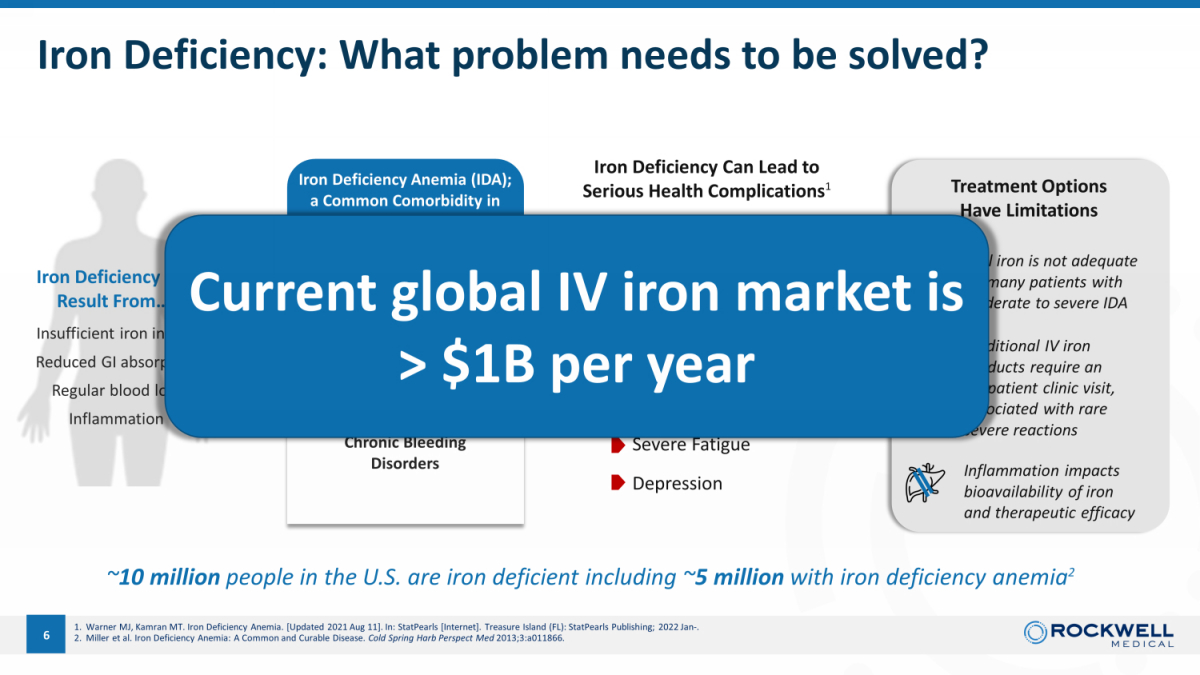
Iron Deficiency: What problem needs to be solved? 6 Traditional IV iron products require an outpatient clinic visit , associated with rare severe reactions Oral iron is not adequate for many patients with moderate to severe IDA Iron Deficiency Can Result From… 1 Insufficient iron in diet Reduced GI absorption Regular blood loss Inflammation Iron Deficiency Anemia (IDA); a Common Comorbidity in Many Diseases Kidney Disease Heart Failure Cancer Multiple Chronic Gastrointestinal Diseases Chronic Bleeding Disorders ~ 10 million people in the U.S. are iron deficient including ~ 5 million with iron deficiency anemia 2 Inflammation impacts bioavailability of iron and therapeutic efficacy Iron Deficiency Can Lead to Serious Health Complications 1 Moderate to severe iron deficiency and resulting anemia increases risk of… Organ dysfunction Heart conditions I nfection Extended hospital stays Severe Fatigue Depression Treatment Options Have Limitations 1. Warner MJ, Kamran MT. Iron Deficiency Anemia. [Updated 2021 Aug 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan - . 2. Miller et al. Iron Deficiency Anemia: A Common and Curable Disease. Cold Spring Harb Perspect Med 2013;3:a011866. Current global IV iron market is > $1B per year

FPC Uniquely Suited to Address Iron Deficiency A true next generation IV iron with established efficacy and safety 7 1. Pratt R, et al. Biometals . 2018;31(6):1081 - 1089 2. Fishbane SN, et al. Ferric pyrophosphate citrate (Triferic Ρ ) administration via the dialysate maintains hemoglobin and iron balance in chronic hemodialysis patients. Nephrol Dial Trans pla nt. 2015;30(12):2019 - 2026. 3. TRIFERIC Prescribing Information. Wixom, MI: Rockwell Medical, Inc.; 2018 FPC d elivers 100% immediately bioavailable iron 1 which is utilized for critical body processes 2 Bypasses the liver, delivers iron directly to circulating transferrin 1 Delivers bioavailable iron even in the presence of inflammation and hepcidin block 3 Adverse events similar to placebo in clinical trials 2,3 Over 1.5 million doses with n o reports of SAEs Replaces iron and maintains hemoglobin 2 Fe 3+ Ferric pyrophosphate citrate (water soluble iron salt) x Proven efficacy Shown to be effective an iron deficiency anemia therapy for hemodialysis patients Priority Development Program: FPC as a Home Infusion Therapy for treatment of iron deficiency anemia x Two NDA approvals x Established safety profile ferric pyrophosphate citrate (FPC) Transforming Iron Deficiency Therapy

FPC for Iron Deficiency Anemia in Home Infusion New FPC product Novel presentation 505(b)(1) FDA approval Unique J - code Differentiated pricing 8

Healthcare is shifting to the home Rapid growth post - pandemic is driven by lower cost of care and higher patient satisfaction 9 Healthcare at home is an attractive alternative. The shift is underway. Home infusion therapy is a critical component. Innovative medicine is needed. • Lower cost of care compared to hospital or clinic • Major QOL benefit for patients • Reduced exposure to infectious risk • COVID - 19 was a catalyst • Payers are creating incentives and reimbursing care at home • Policymakers are funding the transition • Commercial payers are investing in home care programs • Specialized services are providing infused medicines to patients at home vs. in a clinic or hospital • Applicable to a broad range of patient types (GI disorders, oncology, heart failure, others) • Safe and effective medicines suitable for home use are a critical component to enabling home - based care “U.S. Centers for Medicare & Medicaid Services (CMS) authorizes funding for states to expand home and community - based healthcare services and increase access ” Home Healthcare News, March 2022 “Up to $265 billion worth of care services for Medicare fee - for - service and Medicare Advantage beneficiaries could shift to the home by 2025” McKinsey & Company Special Report, February 2022 Transforming Iron Deficiency Therapy
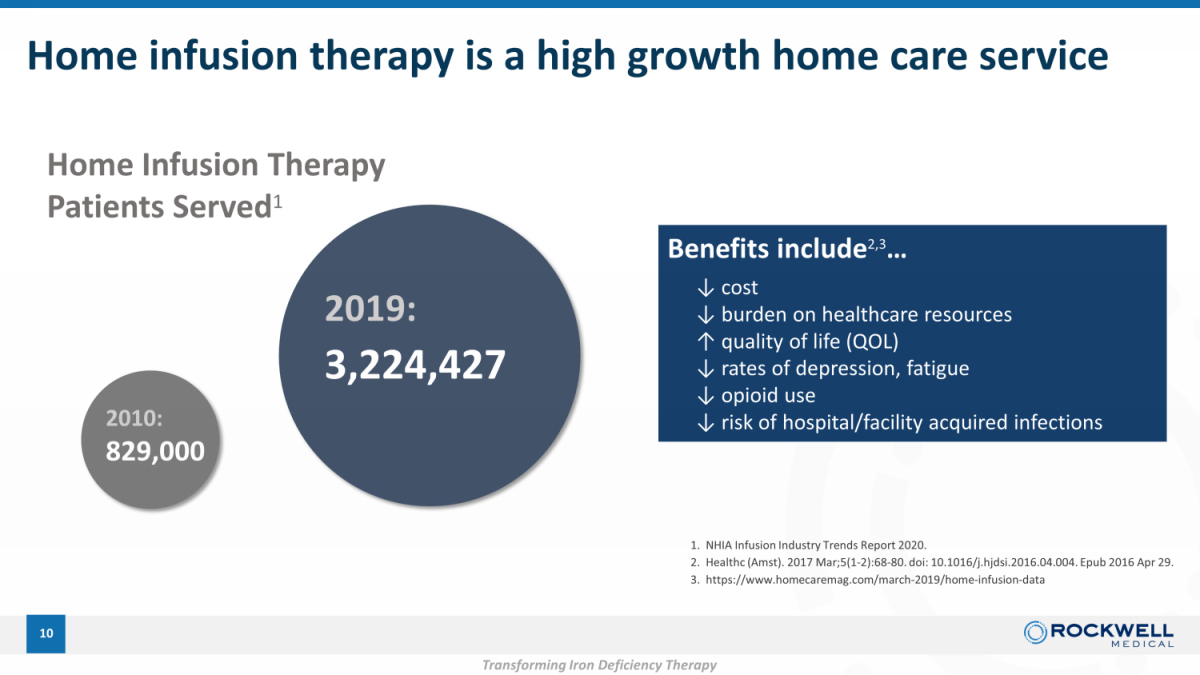
Home infusion therapy is a high growth home care service 10 2010: 829,000 2019: 3,224,427 Home Infusion Therapy Patients Served 1 Benefits include 2,3 … ↓ cost ↓ burden on healthcare resources ↑ quality of life ( QOL) ↓ rates of depression, fatigue ↓ opioid use ↓ risk of hospital/facility acquired infections 1. NHIA Infusion Industry Trends Report 2020. 2. Healthc ( Amst ). 2017 Mar;5(1 - 2):68 - 80. doi : 10.1016/j.hjdsi.2016.04.004. Epub 2016 Apr 29. 3. https://www.homecaremag.com/march - 2019/home - infusion - data Transforming Iron Deficiency Therapy

The home infusion setting presents an opportunity for FPC 11 Iron Deficiency Anemia is a common comorbidity. » Large and growing market » Previously established safety & efficacy » Potential to meaningfully improve lives Presents a significant opportunity for FPC. What is h ome infusion therapy? It is… provision of IV treatments at home. Home infusion therapies allow patients with diseases requiring regular infusions to be treated in the comfort of their own homes and avoid costly and inconvenient visits to hospitals or outpatient infusion clinics. Management of iron deficiency anemia is a broken process . An IV iron product suitable for home infusion is an unmet medical need . For many patients receiving home infusion therapy… Transforming Iron Deficiency Therapy
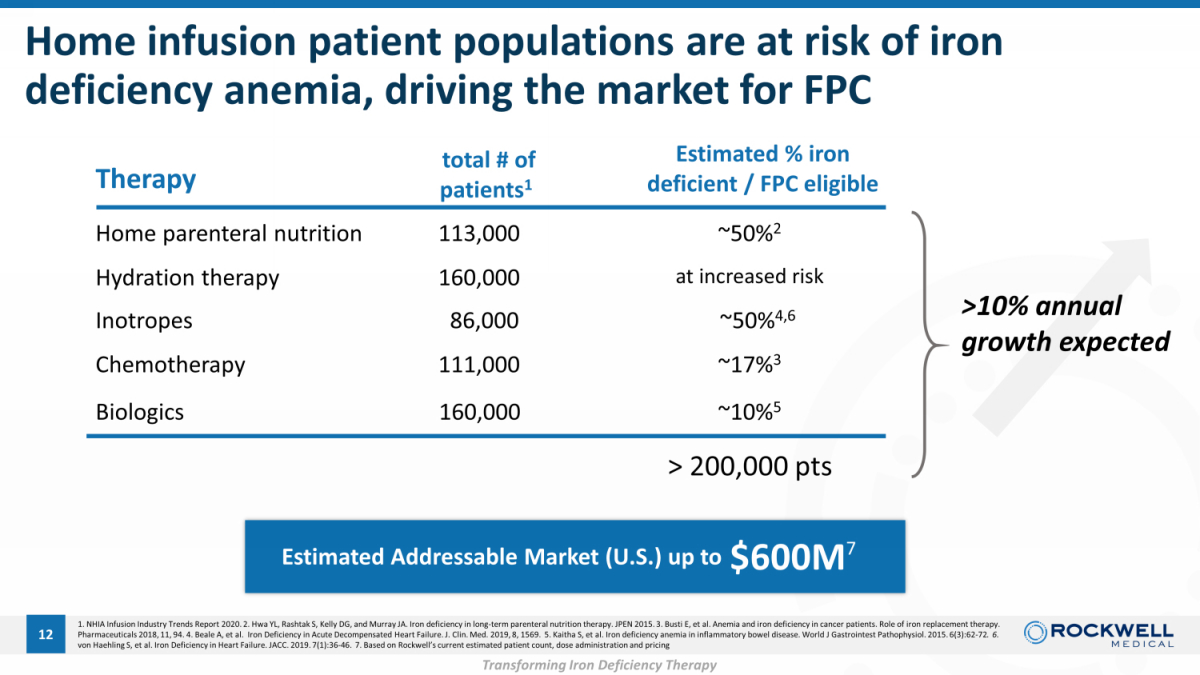
1. NHIA Infusion Industry Trends Report 2020. 2. Hwa YL, Rashtak S, Kelly DG, and Murray JA. Iron deficiency in long - term parenteral nutrition therapy. JPEN 2015. 3. Busti E, et al. Anemia and iron deficiency in cancer patients. Role of iron replacement therapy. Pharmaceuticals 2018, 11, 94. 4. Beale A, et al. Iron Deficiency in Acute Decompensated Heart Failure. J. Clin. Med. 2019, 8, 1569. 5. Kaitha S, et al. Iron deficiency anemia in inflammatory bowel disease. World J Gastrointest Pat hophysiol. 2015. 6(3):62 - 72 . 6. von Haehling S, et al. Iron Deficiency in Heart Failure. JACC. 2019. 7(1):36 - 46 . 7. Based on Rockwell’s current estimated patient count, dose administration and pricing Home parenteral nutrition Hydration therapy Biologics Inotropes Home infusion patient populations are at risk of iron deficiency anemia, driving the market for FPC 12 Therapy total # of patients 1 Estimated % iron deficient / FPC eligible 111,000 160,000 86,000 160,000 ~17% 3 at increased risk ~50% 2 Chemotherapy 113,000 ~50% 4,6 $600M 7 ~10% 5 Est imated Addressable Market (U.S.) up to > 200,000 pts >10% annual growth expected Transforming Iron Deficiency Therapy

Treatment of iron deficiency anemia in home infusion patients is a broken process. 13 A market research survey 1 assessed the current standard of care for management of IDA in home parenteral nutrition patients (HPN) , a subset of the home infusion population. Health care providers say… • Patients regularly monitored for IDA, but no clear consensus on diagnosis or treatment • Most common therapy is IV iron provided in an outpatient clinic • Some concern about giving traditional IV iron in the home setting Patients say… • Awareness of the problem of IDA is very high • Treatment success rates are low • There is a strong preference for an at - home infusion if available vs. a visit to an outpatient clinic 4 of 10 6 of 10 say treatment intervention is not proactive. pharmacists physicians HPN patients are monitored regularly for iron deficiency anemia however, and 4% 56% 9% 16% Low Patient Reported Treatment Success Rates Oral iron (tablets or pills) Traditional IV iron (outpatient) Traditional IV iron (home infusion) Red blood cell transfusion 1. Chole T, Anthony P, DeLegge M. Management of Iron Deficiency Anemia in Home Parenteral Nutrition Patients. Poster present ed at: NHIA Annual Conference; March 2022; Nashville, TN. Inconsistent Treatment Approach Transforming Iron Deficiency Therapy
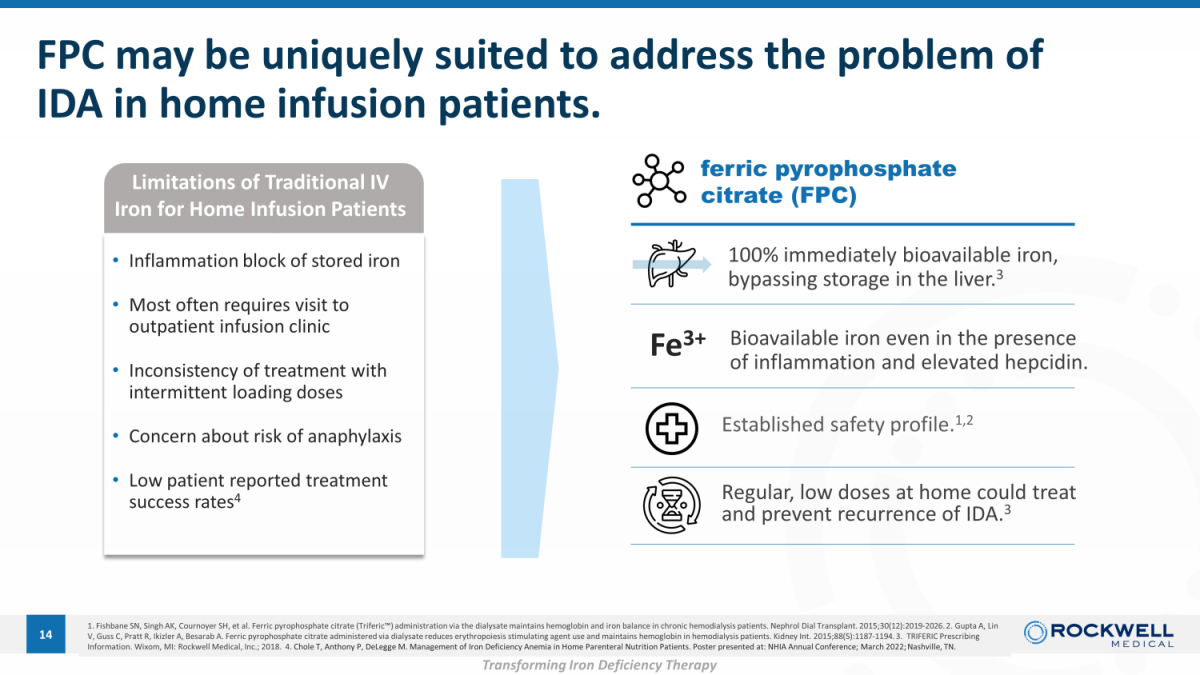
FPC may be uniquely suited to address the problem of IDA in home infusion patients. 14 Bioavailable iron even in the presence of inflammation and elevated hepcidin. Regular, low doses at home could treat and prevent recurrence of IDA. 3 1. Fishbane SN, Singh AK, Cournoyer SH, et al. Ferric pyrophosphate citrate (Triferic Ρ ) administration via the dialysate maintains hemoglobin and iron balance in chronic hemodialysis patients. Nephrol Dial Trans pla nt. 2015;30(12):2019 - 2026. 2. Gupta A, Lin V, Guss C, Pratt R, Ikizler A, Besarab A. Ferric pyrophosphate citrate administered via dialysate reduces erythropoiesis stimulating agent use and maintains hemoglo bi n in hemodialysis patients. Kidney Int. 2015;88(5):1187 - 1194. 3. TRIFERIC Prescribing Information. Wixom, MI: Rockwell Medical, Inc.; 2018. 4. Chole T, Anthony P, DeLegge M. Management of Iron Deficiency Anemia in Home Parenteral Nutrition Patients. Poster presented a t: NHIA Annual Conference; March 2022; Nashville, TN. Established safety profile. 1,2 Limitations of Traditional IV Iron for Home Infusion Patients Fe 3+ ferric pyrophosphate citrate (FPC) 100% immediately bioavailable iron, bypassing storage in the liver. 3 • Inflammation block of stored iron • Most often requires visit to outpatient infusion clinic • Inconsistency of treatment with intermittent loading doses • Concern about risk of anaphylaxis • Low patient reported treatment success rates 4 Transforming Iron Deficiency Therapy
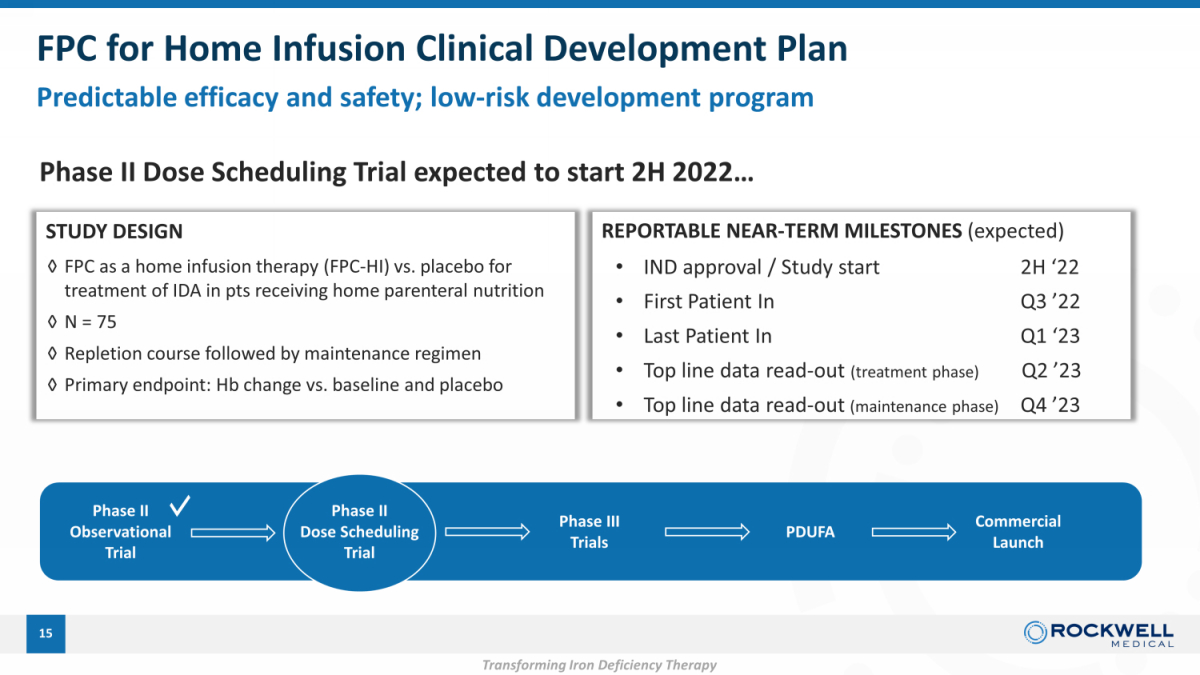
FPC for Home Infusion Clinical Development Plan Predictable efficacy and safety; low - risk development program 15 Phase II Observational Trial Phase III Trials PDUFA Commercial Launch Phase II Dose Scheduling Trial expected to start 2H 2022… ◊ FPC as a home infusion therapy (FPC - HI) vs. placebo for treatment of IDA in pts receiving home parenteral nutrition ◊ N = 75 ◊ Repletion course followed by maintenance regimen ◊ Primary endpoint: Hb change vs. baseline and placebo Phase II Dose Scheduling Trial • IND approval / Study start 2H ‘22 • First Patient In Q3 ’22 • Last Patient In Q1 ‘23 • Top line data read - out (treatment phase) Q2 ’23 • Top line data read - out (maintenance phase) Q4 ’23 STUDY DESIGN REPORTABLE NEAR - TERM MILESTONES (expected) Transforming Iron Deficiency Therapy

Dialysis Market Opportunities

We are an essential supplier in the hemodialysis market. LDO (DaVita) 175k Patients 36% 3 MDOs 58k Patients 10% 492,000 Estimated Total U.S. In - Center Hemodialysis Patients → Current target market is 27% or 132,000 patients LDO (Fresenius) 170,500 LDO (DaVita) 185,400 MDO's 50,200 SDO's / Indep. 86,000 US In - Center Hemodialysis Patients LDO = Large Dialysis Organization MDO = Mid - Sized Dialysis Organization SDO = Small Dialysis Organization 17 United States Renal Data System. 2021 USRDS annual data report: epidemiology of kidney disease in the United States. Bethesda , M D: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2021. Accessed March 7, 2022. https://adr.usrds.org/2021external icon • Large patient population • Patients visit clinics 3x per week year - round for life - saving treatments • Expected growth due to continued increases in the prevalence of end - stage kidney disease

18 Rockwell Dialysis Concentrates #2 >$60 million >15 million DaVitaBaxter Nipro We are an essential supplier in the hemodialysis market.

We are strengthening our dialysis concentrates business 19 Rockwell Dialysis Concentrates » Updated key customer agreements • DaVita, Baxter, Nipro , other » Near - term expectations * … • Revenue growth from $60m to >$75m in 2022 • Cash burn reduction in 2022 by ~$11m (~$15m on an annualized basis) • Expected to generate ~10 - 15% gross margin by end of 2022 * Assumes same volume and product mix as 2021; limited inflation and supply - chain disruptions » Pandemic related cost and supply chain challenges have been addressed » Opportunities exist for growth and improved efficiency

Triferic® and Triferic AVNU Ρ are FDA approved forms of FPC Triferic has a strong value proposition for hemodialysis patients • Delivers 100% bioavailable iron, stabilizing Hb 1,2,3 • Conveniently delivered via dialysate or IV • Demonstrated to reduce overall cost of anemia drugs 4 Established safety and efficacy; growth potential in international markets 20 Full prescribing information available at www.Triferic.com 1.Fishbane SN, et al. Ferric pyrophosphate citrate administration via the dialysate maintains Hb and iron in chronic HD pati ent s. Nephrol Dial Trans. 2015;30(12):2019 - 2026. 2. TRIFERIC Prescribing Information. Wixom, MI: Rockwell Medical, Inc.; 2018. 3. Gupta A, et al. Ferric pyrophosphate citrate administered via dialysate reduces ESA use and ma intains hemoglobin in hemodialysis patients. Kidney Int. 2015;88(5):1187 - 1194. 4. Dellafera L, et al. Institutional Usage of Ferric Pyrophosphate Citrate in Reducing Erythropoiesis - Stimulating Agents. Critical Care Medicine. Jan 2 021:49(1).551 Adoption of Triferic in the U.S. has been limited due to … • Status quo, cost driven market • Consolidated provider base • Bundled CMS reimbursement schema • Established contracts with competitors Triferic pivotal studies and clinical use have established the safety and efficacy of FPC as a drug to treat iron deficiency anemia. We expect royalties from our strategic partnerships in international markets where reimbursement mechanisms may be more supportive of innovative dialysis products. Transforming Iron Deficiency Therapy

Pipeline Opportunity: FPC for Acute Heart Failure Newly formulated FPC product Novel presentation 505(b)(1) FDA approval Unique J - code Differentiated pricing 21

New, Effective Treatments for Acute Heart Failure are Needed 1. Arrigo, M., Jessup, M., Mullens, W. et al. Acute heart failure. Nat Rev Dis Primers 6, 16 (2020). https://doi.org/10.1038/s41572 - 020 - 0151 - 7 . 2. Rizzo, C. Carbonara, R. Ruggieri, R. Iron Deficiency: A New Target for Patients With Heart Failure. Front. Cardiovasc. Med., 10 August 2021 | https://doi.org/10.3389/fcvm.2021.709872 3. Núñez J, Comín - Colet J, Miñana G, Núñez E, Santas E, Mollar A, et al. Iron deficiency and risk of early readmission following a hospitalization for AHF . Eur J Heart Fail. (2016) 18:798 – 802. doi : 10.1002/ejhf.513 22 Acute heart failure is associated with poor outcomes. Therapeutic options are limited. Iron deficiency is one of the most frequent comorbidities. • ID present in up to 50% of outpatients with heart failure 2 • Iron plays an important role in cardiac muscle energetics and mitochondrial energy production 2 • Inflammation in HF can trigger ‘iron trapping’ in the liver making iron unusable despite sufficient iron stores 2 • Absolute ID associated with an increased risk of early readmission after AHF 3 • Studies show improved outcomes with use of IV iron in stable HF patients 3 • Hospital length of stay has remained at ~5 days for decades 2 • 1 in 4 patients are readmitted within 30 days 1 • A limited number of new therapies have been approved for hospitalized AHF patients since the 1990’s 1 • Innovative post - discharge care models have failed to reduce readmission rates 1 Transforming Iron Deficiency Therapy
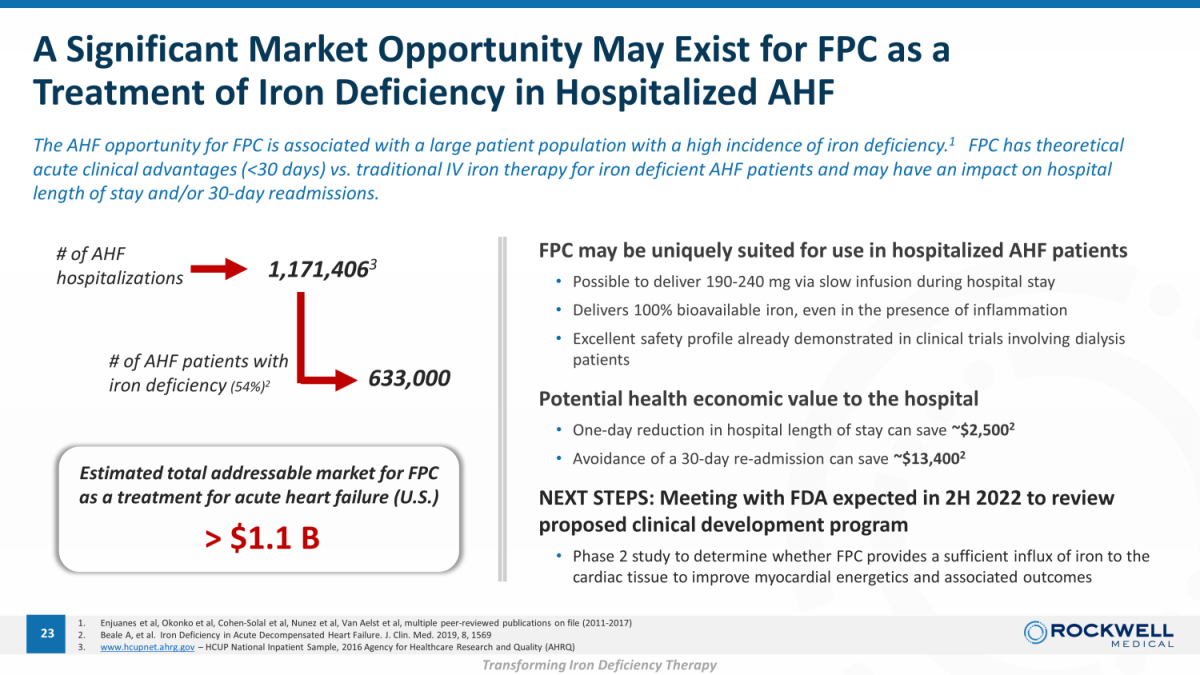
A Significant Market Opportunity May Exist for FPC as a Treatment of Iron Deficiency in Hospitalized AHF 1. Enjuanes et al, Okonko et al, Cohen - Solal et al, Nunez et al, Van Aelst et al, multiple peer - reviewed publications on file (2011 - 2017) 2. Beale A, et al. Iron Deficiency in Acute Decompensated Heart Failure. J. Clin. Med. 2019, 8, 1569 3. www.hcupnet.ahrg.gov – HCUP National Inpatient Sample, 2016 Agency for Healthcare Research and Quality (AHRQ) 23 The AHF opportunity for FPC is associated with a large patient population with a high incidence of iron deficiency. 1 FPC has theoretical acute clinical advantages (<30 days) vs. traditional IV iron therapy for iron deficient AHF patients and may have an impact o n h ospital length of stay and/or 30 - day readmissions. FPC may be uniquely suited for use in hospitalized AHF patients • Possible to deliver 190 - 240 mg via slow infusion during hospital stay • Delivers 100% bioavailable iron, even in the presence of inflammation • Excellent safety profile already demonstrated in clinical trials involving dialysis patients Potential health economic value to the hospital • One - day reduction in hospital length of stay can save ~$2,500 2 • Avoidance of a 30 - day re - admission can save ~$13,400 2 NEXT STEPS: Meeting with FDA expected in 2H 2022 to review proposed clinical development program • Phase 2 study to determine whether FPC provides a sufficient influx of iron to the cardiac tissue to improve myocardial energetics and associated outcomes 1,171,406 3 # of AHF hospitalizations # of AHF patients with iron deficiency (54%) 2 633,000 Estimated total addressable market for FPC as a treatment for acute heart failure (U.S.) > $1.1 B Transforming Iron Deficiency Therapy

Upcoming Milestones

Projected Near - Term Milestones 25 2022 ▪ IND approval / study start FPC for Home Infusion Phase II Study FPC for Acute Heart Failure (pipeline opportunity) ▪ Top - line data readout (treatment phase) ▪ Top - line data readout (maintenance phase) ▪ Updated customer agreements ▪ Positive gross margin ▪ KOR Commercial L aunch ▪ CHN Phase 3 study data readout ▪ IND Phase 3 study start ▪ Pre - IND meeting with FDA 2H 2022 Q2 2022 Q3 2022 2H 2022 Q2 2023 Q2 2022 Q2 2023 Q4 2023 2023 2H 2022 Dialysis Products Dialysis Concentrates Triferic® International Transforming Iron Deficiency Therapy

Rockwell Rockwell Rockwell TBD Jeil Pharma Jeil Pharma SUN Pharma Wanbang Biopharm (Fosun) Drogsan Pharma Advancing Our Portfolio With the Potential to Deliver Near Term Value FPC HI - 01, HI - 02: U.S. FPC HF:01: U.S. Preclinical Phase 1 Phase 2 Phase 3 Submitted Approved Commercial 26 FPC for Iron Deficiency Anemia in Home Infusion FPC for Iron Deficiency in Acute Heart Failure Home Infusion FPC for Hb Maintenance in Dialysis Patients TRIFERIC® (Dialysate) U.S. TRIFERIC® AVNU (I.V.) U.S. TRIFERIC® AVNU (I.V.) Canada TRIFERIC® AVNU (I.V.) Korea TRIFERIC® AVNU (I.V.) India TRIFERIC® (Dialysate) Korea TRIFERIC® AVNU (I.V.) China TRIFERIC® AVNU (I.V.) Turkey Acid and Bicarbonate Concentrates Acute Heart Failure Dialysis Transforming Iron Deficiency Therapy

Rockwell Medical, Inc. Developing innovative therapies to transform the treatment of iron deficiency 27 x Proprietary Drug • Next generation iron deficiency therapeutic • Applicable to many different patient populations • Two NDA approvals x Significant Opportunity: Home Infusio n Iron Therapy • Phase 2 Trial expected to start in H2 2022 • Multiple Data Readouts anticipated in 2023 x Re - engineered Dialysis Products Business • >$60m annual revenue, updated customer agreement s in 2022 • Expect positive gross margin from dialysis concentrates beginning 2H 2022 x Multiple Near - Term Milestones x Future Pipeline Opportunity: Acute Heart Failure Transforming Iron Deficiency Therapy MT0

Thank You
Experienced Management Team Proven track record and expertise in biopharma clinical development and commercialization 29 Management Team Marc Hoffman, M.D. Chief Medical Officer Years: 30+ Michael DeYoung VP Operations Years: 25 + Russell Ellison, M.D. Chief Executive Officer Years: 35+ Russell Skibsted Chief Financial Officer Chief Business Officer Years: 25+ Megan Timmins General Counsel Years: 20+ Timothy Chole Senior VP, Sales and Marketing Years: 25+ Transforming Iron Deficiency Therapy

Balance Sheet Recap and Update • Cash and Cash Equivalents as of March 31, 2022 was $9.9 million. • Working capital as of March 31, 2022 was $5.2 million. • Cash and Cash Equivalents as of April 30, 2022 was $12.5 million. • Working capital as of April 30, 2022 was $10.3 million. 30
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Rockwell Medical (RMTI) Continues to Expand its Distribution Capabilities in Western US
- Quorum Announces Q4 and Year End 2023 Results
- Blackwell 3D Launches New Website, Eyes Project Development
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share