Form 8-K PhaseBio Pharmaceuticals For: Aug 12
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
___________________________________
FORM 8-K
___________________________________
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): August 12, 2022
___________________________________
(Exact name of registrant as specified in its Charter)
___________________________________
(State or Other Jurisdiction of Incorporation) | (Commission File Number) | (IRS Employer Identification No.) | ||||||
(Address including zip code of principal executive offices)
(610 ) 981-6500
(Registrant’s Telephone Number, Including Area Code)
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
___________________________________
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instructions A.2. below):
Securities registered pursuant to Section 12(b) of the Act.
Title of each class | Trading Symbol(s) | Name of exchange on which registered | ||||||
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
Item 2.02. Results of Operations and Financial Condition.
On August 12, 2022, PhaseBio Pharmaceuticals, Inc. (the “Company”) reported financial results for the second quarter ended June 30, 2022. A copy of this press release is furnished as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated by reference.
The information in this Item 2.02 of this Current Report on Form 8-K, including Exhibit 99.1, is being furnished pursuant to Item 2.02 and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended (the “Securities Act”), or the Exchange Act, except as expressly set forth by specific reference in such filing to this item of this report.
Item 7.01. Regulation FD Disclosure.
On August 12, 2022, the Company updated its corporate presentation for use in meetings with investors, analysts and others. The presentation is available through the Company’s website and a copy is attached as Exhibit 99.2 to this Current Report on Form 8-K.
The information in this Item 7.01 of this Current Report on Form 8-K, including Exhibit 99.2, is being furnished pursuant to Item 7.01 and shall not be deemed “filed” for purposes of Section 18 of the Exchange Act or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act or the Exchange Act, whether made before or after the date hereof, except as expressly set forth by specific reference in such filing to this item of this report.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
| Exhibit No. | Description | |||||||
| 99.1 | ||||||||
| 99.2 | ||||||||
| 104 | Cover page interactive data file (formatted as Inline XBRL). | |||||||
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| PhaseBio Pharmaceuticals, Inc. | ||||||||||||||
| Dated: August 12, 2022 | By: | /s/ John P. Sharp | ||||||||||||
| John P. Sharp | ||||||||||||||
| Chief Financial Officer | ||||||||||||||
Exhibit 99.1

PhaseBio Pharmaceuticals Reports Second Quarter 2022 Financial Results and Recent Business Highlights
Malvern, PA and San Diego, CA, August 12, 2022 — PhaseBio Pharmaceuticals, Inc. (Nasdaq: PHAS), a clinical-stage biopharmaceutical company focused on the development and commercialization of novel therapies for cardiovascular diseases, today reported financial results for the second quarter ended June 30, 2022, and provided an update on corporate activities.
“The second quarter of 2022 marked a period of continued progress for PhaseBio,” said Jonathan Mow, Chief Executive Officer of PhaseBio Pharmaceuticals. “Following a successful meeting with the U.S. Food and Drug Administration (FDA) during our pre-biologics license application (pre-BLA) meeting earlier this year and as previously disclosed, we have been focused on clinical development and regulatory efforts to support a planned BLA submission for our lead program, bentracimab, in the fourth quarter of this year. Additionally, we continue to make progress towards completing initial new drug application (IND) enabling studies for PB6440, our aldosterone synthase inhibitor in development for resistant hypertension. We expect to file our IND for PB6440 in the first half of 2023 and to initiate first-in-human trials in mid-2023.”
Program Highlights
•SFJ Financing and Co-Development Agreement Update: In January 2020, PhaseBio entered into an agreement with SFJ Pharmaceuticals (SFJ Agreement), pursuant to which SFJ provides the company funding to support the global development of bentracimab. Under the agreement, SFJ agreed to pay the company up to $120.0 million to support the clinical development of bentracimab. In addition to $90.0 million of initial funding, the company has elected to receive an additional $30.0 million of funding having met specific, pre-defined clinical development milestones for bentracimab. From the inception of the SFJ Agreement through June 30, 2022, SFJ has provided funding and paid for amounts on the company’s behalf in the aggregate amount of $99.0 million. PhaseBio expects that SFJ will fund or reimburse an additional $21.0 million of clinical trial costs and other expenses.
•PB6440 IND enabling studies continue to advance: In the second quarter of 2022, PhaseBio completed the development and optimization of a robust manufacturing process to support anticipated upcoming proof-of-concept trials, positioning the program for initial GMP manufacturing runs in the fourth quarter of 2022. PB6440 is a highly selective aldosterone synthase inhibitor in development to target treatment resistant hypertension and other indications where elevated aldosterone is known to contribute to disease process, such as uncontrolled hypertension, chronic kidney disease, and heart failure. The drug appears to modulate the renin-angiotensin-aldosterone system, which exhibits a critical role in regulation of systemic blood pressure. According to the American Heart Association, 20% of hypertensive Americans, which potentially represents more than 10 million patients, have not achieved normotensive status despite taking three or more blood pressure drugs; we believe this represents a significant unmet need with a large market potential. PB6440 is undergoing IND-enabling studies, with first human trials targeted for mid-2023.
Quarter Ending June 30, 2022
•Cash and cash equivalents on June 30, 2022, were $7.8 million, compared to $41.8 million at December 31, 2021. The decrease primarily reflects cash used in operating activities.
•Net loss for the quarter was $16.7 million, compared to a net loss of $28.7 million for the prior-year period.
•Research and development expense for the quarter decreased to $20.9 million, as compared to $27.4 million for the same period in 2021. The decrease was primarily attributable to drug manufacturing activity in 2021, study site startup costs for the Phase 2b trial related to bentracimab in 2021, and the voluntary ending of the Phase 2b trial of pemziviptadil in the fourth quarter of 2021, partially offset by an increase in costs related to development of PB6440, and personnel costs and other costs associated with our general research and development efforts.
•General and administrative expense for the quarter increased to $4.6 million, compared to $4.0 million for the prior-year period. The increase was primarily attributable to increases in consulting costs and personnel expenses due to additional headcount.
About Bentracimab (PB2452)
Bentracimab is a novel, recombinant, human monoclonal antibody antigen-binding fragment designed to reverse the antiplatelet activity of ticagrelor in patients who present with uncontrolled bleeding or require surgery. In a Phase 1 clinical trial, bentracimab demonstrated the potential to bring life-saving therapeutic benefit through immediate and sustained reversal of ticagrelor’s antiplatelet activity, mitigating concerns regarding bleeding risks associated with the use of this antiplatelet drug. Data from the Phase 1 clinical trial of bentracimab in healthy volunteers was published in the New England Journal of Medicine in March 2019. In April 2019, bentracimab received Breakthrough Therapy Designation from the FDA. In September 2019, PhaseBio completed a Phase 2a trial in which bentracimab was investigated in healthy, older subjects on dual antiplatelet therapy of ticagrelor and low-dose aspirin. Additionally, the Phase 2a trial investigated a bentracimab regimen for the reversal of supratherapeutic doses of ticagrelor in healthy younger subjects. In November 2021, PhaseBio completed a Phase 2b trial in which bentracimab was investigated in older subjects on dual antiplatelet therapy of ticagrelor and low-dose aspirin, with complete results announced and presented in April 2022. In all active treatment arms in both the Phase 2a and Phase 2b trials, bentracimab achieved immediate and sustained reversal of the antiplatelet effects of ticagrelor and was generally well-tolerated, with only minor adverse events reported. These results are consistent with the results observed in healthy younger subjects treated with ticagrelor in the previously published Phase 1 trial. PhaseBio initiated REVERSE-IT, a pivotal Phase 3 clinical trial of bentracimab, in March 2020 to support a potential Biologics License Application for bentracimab to treat patients with uncontrolled bleeding or requiring surgery. Interim results from the Phase 3 REVERSE-IT trial were presented in November 2021 and subsequently published in NEJM Evidence in December 2021.
About PhaseBio
PhaseBio Pharmaceuticals, Inc. is a clinical-stage biopharmaceutical company focused on the development and commercialization of novel therapies for cardiovascular diseases. The Company’s pipeline includes: bentracimab (PB2452), a novel reversal agent for the antiplatelet therapy ticagrelor; and PB6440, an oral agent for the treatment of resistant hypertension. PhaseBio’s proprietary elastin-like polypeptide technology platform enables the development of therapies with potential for less-frequent dosing and improved pharmacokinetics, and drives both internal and partnership drug-development opportunities.
PhaseBio is located in Malvern, PA, and San Diego, CA. For more information, please visit www.phasebio.com, and follow us on Twitter @PhaseBio and LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as “anticipates,” “believes,” “expects,” “intends,” “potential,” “projects,” “target,” “will,” “would” and “future” or similar expressions are intended to identify forward-looking statements.
Forward-looking statements include statements concerning or implying the conduct or timing of our clinical trials, including enrollment, and our research, development and regulatory plans for our product candidates, the timing of availability or disclosure of data from those clinical trials and the timing of planned regulatory submissions, the potential for these product candidates to receive regulatory approval from the FDA, EMA or equivalent foreign regulatory agencies, and whether, if approved, these product candidates will be successfully distributed, marketed and commercialized, including having sufficient product supply at launch, and our ability to complete post-approval requirements. Forward-looking statements are based on management's current expectations and are subject to various risks and uncertainties that could cause actual results to differ materially and adversely from those expressed or implied by such forward-looking statements. Accordingly, these forward-looking statements do not constitute guarantees of future performance, and you are cautioned not to place undue reliance on these forward-looking statements.
Risks regarding our business are described in detail in our Securities and Exchange Commission filings, including in our Quarterly Report on Form 10-Q for the quarter ended March 31, 2022. These forward-looking statements speak only as of the date hereof, and PhaseBio Pharmaceuticals, Inc. disclaims any obligation to update these statements except as may be required by law.
Investor Contact:
John Sharp
PhaseBio Pharmaceuticals, Inc.
Chief Financial Officer
(610) 981-6506
john.sharp@phasebio.com
Media Contact:
Will Zasadny
Canale Communications, Inc.
(619) 961-8848
will.zasadny@canalecomm.com
PhaseBio Pharmaceuticals, Inc. | ||||||||||||||
Condensed Balance Sheets | ||||||||||||||
(in thousands) | ||||||||||||||
(unaudited) | ||||||||||||||
June 30, 2022 | December 31, 2021 | |||||||||||||
Assets: | ||||||||||||||
Cash and cash equivalents | $ 7,804 | $ 41,800 | ||||||||||||
Prepaid expenses and other assets | 3,760 | 6,984 | ||||||||||||
Property and equipment, net | 9,322 | 10,230 | ||||||||||||
Operating lease right-of-use assets | 1,222 | 1,469 | ||||||||||||
Other assets | 58 | 57 | ||||||||||||
Total assets | $ 22,166 | $ 60,540 | ||||||||||||
Liabilities and stockholders' deficit: | ||||||||||||||
Current portion of long-term debt | $ 4,073 | $ 5,413 | ||||||||||||
Current portion of deferred sublicense revenue | 1,400 | 1,547 | ||||||||||||
Accounts payable, accrued expenses and other current liabilities | 19,206 | 20,923 | ||||||||||||
Long-term debt, net | — | 1,359 | ||||||||||||
Operating lease liabilities, net | 869 | 1,073 | ||||||||||||
Long-term portion of deferred sublicense revenue, net | 7,443 | 7,622 | ||||||||||||
Development derivative liability | 106,573 | 114,843 | ||||||||||||
Other long-term liabilities | — | 794 | ||||||||||||
Total stockholders’ deficit | (117,398) | (93,034) | ||||||||||||
Total liabilities and stockholders' deficit | $ 22,166 | $ 60,540 | ||||||||||||
PhaseBio Pharmaceuticals, Inc. | ||||||||||||||||||||||||||
Condensed Statements of Operations | ||||||||||||||||||||||||||
(in thousands, except share and per share amounts) | ||||||||||||||||||||||||||
(unaudited) | ||||||||||||||||||||||||||
Three Months Ended June 30, | Six Months Ended June 30, | |||||||||||||||||||||||||
2022 | 2021 | 2022 | 2021 | |||||||||||||||||||||||
Revenue: | ||||||||||||||||||||||||||
Sublicense revenue | $ 208 | $ 10,338 | $ 325 | $ 10,338 | ||||||||||||||||||||||
Total revenue | 208 | 10,338 | 325 | 10,338 | ||||||||||||||||||||||
Operating expenses: | ||||||||||||||||||||||||||
Research and development | 20,939 | 27,366 | 35,275 | 49,686 | ||||||||||||||||||||||
General and administrative | 4,581 | 4,025 | 8,590 | 7,352 | ||||||||||||||||||||||
Total operating expenses | 25,520 | 31,391 | 43,865 | 57,038 | ||||||||||||||||||||||
Loss from operations | (25,312) | (21,053) | (43,540) | (46,700) | ||||||||||||||||||||||
Other income (expense) | 8,647 | (6,026) | 15,734 | (7,737) | ||||||||||||||||||||||
Net loss before income taxes | (16,665) | (27,079) | (27,806) | (54,437) | ||||||||||||||||||||||
Provision for income taxes | — | 1,600 | — | 1,600 | ||||||||||||||||||||||
Net loss | $ | (16,665) | $ | (28,679) | $ | (27,806) | $ | (56,037) | ||||||||||||||||||
Net loss per common share, basic and diluted | $ | (0.34) | $ | (0.60) | $ | (0.57) | $ | (1.41) | ||||||||||||||||||
Weighted average common shares outstanding, basic and diluted | 49,182,813 | 47,985,871 | 48,910,437 | 39,680,408 | ||||||||||||||||||||||

Corporate Overview August 2022

This presentation includes forward-looking statements. All statements contained in this presentation other than statements of historical facts, including statements regarding future results of operations and financial position of PhaseBio Pharmaceuticals, Inc. (“we,” “us” or “our”), our business strategy and plans, the preclinical and clinical development of our product candidates and our objectives for future operations, the timing of availability or disclosure of data from those clinical trials and the timing of planned regulatory submissions, the potential for these product candidates to receive regulatory approval from the FDA, EMA, CDE or equivalent foreign regulatory agencies, and whether, if approved, these product candidates will be successfully distributed, marketed and commercialized, are forward-looking statements, although not all forward-looking statements contain these identifying words. The words “anticipate,” “believe,” “continue,” “estimate,” “expect,” “intend,” “may,” “planned”, ‘potential’, “target,” “will,” "would”, and similar expressions are intended to identify forward-looking statements. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy, preclinical and clinical development, short-term and long-term business operations and objectives and financial needs. These forward-looking statements are subject to a number of risks, uncertainties and assumptions. Risks regarding our business are described in detail in our Securities and Exchange Commission filings, including in our Quarterly Report on Form 10-Q for the quarter ended June 30, 2022. Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we accurately assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward- looking statements we may make. In light of these risks, uncertainties and assumptions, the future events and trends discussed in this presentation may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance, achievements or events and circumstances reflected in the forward-looking statements will occur. We are under no duty to update any of these forward-looking statements after the date of this presentation to conform these statements to actual results or revised expectations, except as required by law. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this presentation. Moreover, except as required by law, neither we nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements contained in this presentation. Legal Disclaimer 2

Therapeutic Focus Clinical-stage biopharma company focused on the development and commercialization of novel therapies to treat cardiovascular diseases Product Candidates Bentracimab (PB2452) Novel agent in Phase 3 development for immediate and sustained reversal of ticagrelor, the preferred antiplatelet therapy of the American College of Cardiology, the American Heart Association and the European Society of Cardiology PB6440 Oral aldosterone synthase inhibitor in development for treatment-resistant hypertension and potentially other cardio-renal- metabolic indications, currently in Investigational New Drug application (IND) enabling studies Platform Technology ELP Technology1 • Extends circulating half-life of proteins and peptides, enhances solubility, stability and bioavailability while providing a sustained-release mechanism • Enables product candidates that are straightforward to manufacture and administer Recent Achievements & Upcoming Milestone Targets2 Bentracimab Mid 2021 First 100 patients in REVERSE-IT Phase 3 trial Bentracimab Q4 2022 Planned BLA Submission Bentracimab Q4 2021 Topline results from Phase 2b trial PB6440 1H 2023 Planned IND submission Bentracimab Q4 2021 Topline results from interim analysis of REVERSE-IT PB6440 Mid 2023 Target initiating first-in-human clinical trial Company Overview 31. ELP technology does not apply to bentracimab or PB6440 2. Targeted timelines could be impacted by the continued scope and duration of the COVID-19 pandemic

Program Pre-Clinical Phase 1 Phase 2 Phase 3 Commercial Rights Upcoming Milestone Target2 Bentracimab Reversal of Ticagrelor Antiplatelet Activity Q4 2022 Planned BLA submission PB6440 Resistant Hypertension 1H 2023 Submit Investigational New Drug application (IND) Partnering Opportunities Pemziviptadil Pulmonary Arterial Hypertension (PAH) GLP2-ELP Short Bowel Syndrome CNP-ELP Achondroplasia Early Programs PROPRIETARY LONG-ACTING INJECTABLE RECOMBINANT BIOPOLYMERS (Elastin-like Polypeptides – ELPs) A Clinical Stage, Cardiovascular Focused Biopharmaceutical Company 4 REVERSE-IT1 Phase 3 ongoing Interim completed, targeting to submit BLA in Q4 20222 Phase 2b3 Late research Late research 1. REVERSE-IT: Rapid and SustainEd ReVERSal of TicagrElor – Intervention Trial 2. Targeted timeline could be impacted by the continued scope and duration of the COVID-19 pandemic 3. Phase 2b trial voluntarily stopped early due to COVID-19 impacts on manufacturing, associated drug supply and the rate of enrollment in the study; PhaseBio has elected to stop further development after a strategic review in order to reprioritize resources towards pre-commercialization activities for bentracimab and the advancement of other pipeline programs, including PB6440 for resistant hypertension Pre-Clinical

Corporate

6 Experienced Management Team JONATHAN MOW Chief Executive Officer SUSAN ARNOLD, PhD SVP Technical Operations GLEN BURKHARDT SVP Human Resources KRIS HANSON SVP & General Counsel JOHN LEE, MD, PhD, FACC Chief Medical Officer JOHN SHARP Chief Financial Officer JONATHAN BIRCHALL Chief Commercial Officer Despite the ongoing challenges posed by the ongoing COVID-19 pandemic, 2021 and 2022, to date, have been years of significant progress for PhaseBio. In addition to refining our mission, advancing our pipeline programs and kicking off the REVERSE-IT Phase 3 clinical trial for our lead program, bentracimab, we have evolved our corporate logo and the overall look and feel of our website, drawing inspiration for the PhaseBio brand from our prospective patients, healthcare providers and our people. The new PhaseBio logo is defined by a patient-friendly representation of the heart composed of the letters ‘P’ and ‘B’ from the PhaseBio name. This shows that cardiovascular disease is not just what PhaseBio does – it is who we are. Dedicated to transforming patients’ lives through science and excellence LAUREN RICHARDSON VP Regulatory & Quality

• Sub-license revenue: $0.2M • Operating expense: $25.5M R&D: $20.9M SG&A: $4.6M • Loss from operations: ($25.3M) • Net Loss of ($16.7M) or ($0.34) per share, basic and diluted 49.2M shares used for computing Q2 2022 net loss per share • Cash and cash equivalents as of 06/30/2022: $7.8M • Available SFJ funding as of 06/30/2022: $21.0M Q2 2022 Financial Highlights 7

Bentracimab (PB2452) Reversal Agent for Ticagrelor

Early surgery = Bleed risk P2Y12 Inhibitors: Unmet Need and the Dilemma in Managing Surgical Patients 9 5d - Ticagrelor washout2 Risk of bleeding Risk of thrombosis and procedural delay Bentracimab Later surgery = Thrombosis risk 1. Plavix/clopidogrel Prescribing Information: https://packageinserts.bms.com/pi/pi_plavix.pdf , https://www.ema.europa.eu/en/documents/product - information/plavix -epar-product - information_en.pdf 2. Brilinta/Brilique/ticagrelor Prescribing Information: https://www.azpicentral.com/brilinta/brilinta.pdf#page=1 , https://www.ema.europa.eu/en/documents/product - information/brilique -epar-product - information_en.pdf URGENT SURGERY OR INTERVENTION • Currently oral P2Y12 agents, including ticagrelor, require a 5-day washout prior to surgery1,2 • Urgent surgery often cannot wait 5 days • Higher thrombotic risk during washout • In Phase 1 and Phase 2a studies, bentracimab observed to immediately and sustainably reverse ticagrelor inhibition of platelet activation • Enables immediate surgery MAJOR BLEEDING • Intracranial Haemorrhage (ICH), GI, Trauma • All oral antiplatelet agents have the potential to cause major bleeding, which can be severe or even fatal • Bentracimab designed to immediately and sustainably reverse the antiplatelet effects of ticagrelor Significant unmet need for antiplatelet agent reversal Surgical Dilemma

Bentracimab 10 • Ticagrelor has proven superior efficacy vs. clopidogrel and a unique reversible binding profile within the oral P2Y12 class Clopidogrel and prasugrel both permanently bind to the receptor and cannot be reversed • Bentracimab is the only specific reversal agent in development for ticagrelor for both surgical and active bleed indications Bentracimab clinical data to date have demonstrated both immediate (<5 min) and sustained (~24 hours) reversal of ticagrelor antiplatelet effects • Believe approval would differentiate ticagrelor on safety vs. other oral antiplatelet agents Differentiation would drive increased ticagrelor utilization Bentracimab: Novel Reversal Agent for Brilinta (Ticagrelor) Bentracimab has the potential to eliminate the dilemma of choosing between an increased risk of bleeding and an increased risk of thrombosis or procedural delay in patients requiring surgery

BentracimabBentracimab: Well-Characterized Mechanism of Reversal of Ticagrelor 11 1. 2. 3. 4. 5. ADP binds to P2Y12 receptor causing platelet aggregation Ticagrelor binds to P2Y12, inhibiting ADP-induced platelet aggregation Bentracimab binds to free ticagrelor with very high affinity This is a reversible process with ticagrelor cycling on/off the P2Y12 receptors Bentracimab-ticagrelor binding is preferential to ticagrelor-P2Y12 binding due to 100x higher affinity (Ki 20 pM vs 2nM) As free ticagrelor is eliminated, ADP can again activate the P2Y12 receptor, restoring platelet activity Bentracimab-ticagrelor is cleared from the bloodstream Bentracimab

Clinical

• Received ticagrelor + low-dose aspirin and bentracimab 18 g • Achieved primary endpoint of ticagrelor reversal measured by VerifyNow P2Y12 assay – Statistically significant reduction in % inhibition of PRU within 4 hours; similar extent of reversal to Phase 1 and 2A • Safety profile consistent with Phase 1 and 2A studies – No treatment emergent AEs or SAEs considered related to bentracimab – No thrombotic events observed • Phase 2B results presented during a Late Breaking Featured Clinical Research session at the American College of Cardiology Annual Scientific Session & Expo being held in Washington, D.C., April 2-4, 2022 Immediate and Sustained Ticagrelor Reversal with Bentracimab in Healthy Subjects in Phase 1, 2A, 2B Trials 131. Bhatt DL, Pollack CV, Weitz JI, et al. N Engl J Med. 2019;380:1825-33; NCT03492385; 2. NCT03928353; 3. NCT04122170. • Received ticagrelor + bolus + continuous infusion of bentracimab 18 g • Significant reversal observed 5 minutes after initiation of bentracimab infusion • Duration of reversal was infusion-time dependent, lasting 20–24 hours with a 16-hour infusion • Published in the New England Journal of Medicine • Received ticagrelor + low-dose aspirin and bentracimab 18 g • Ticagrelor reversal consistent with Ph 1 • Well tolerated with minor adverse events • Bentracimab 36 g cohort for supratherapeutic ticagrelor blood levels – Statistically significant reversal achieved within 5 minutes of initiating bentracimab infusion, sustained for 24 hours – Platelet function normalized by 30 minutes following initiation of infusion and remained normal for 24 hours Healthy volunteers 18–50 y Healthy volunteers 50–80 y Healthy volunteers 50–80 y Phase 11 Phase 2a2 Phase 2b3 Bentracimab

bentracimab 14 REVERSE-IT: Bentracimab Pivotal Phase 3 Trial Overview • REVERSE-IT: Rapid and SustainEd ReVERSal of TicagrElor – Intervention Trial • Open-label, single-arm study in patients with uncontrolled major or life-threatening bleeding or who require urgent surgery or invasive procedure • Total of 200 patients targeted for total enrollment First 176 patients to form the basis of accelerated BLA filing in US and MAA in EU Accelerated BLA endpoint is restoration of platelet function based on VerifyNow® PRUTest® platelet function assay • Additional endpoints related to hemostasis captured as part of the primary outcome analysis • US FDA Breakthrough Therapy designation, EMA PRIME designation and Breakthrough designation in China Bentracimab for Ticagrelor Reversal in Patients Undergoing Urgent Surgery • Deepak L. Bhatt, MD, MPH, Charles V. Pollack, Jr., MD, C. David Mazer, MD, • Dominick J. Angiolillo, MD, PhD, Ph. Gabriel Steg, MD, Stefan K. James, MD, PhD, • Jeffrey I. Weitz, MD, Rohit Ramnath, PhD, Susan E. Arnold, PhD, Michael C. Mays, BS, • Bret R. Umstead, MS, Barbara White, MD, Lisa L. Hickey, MS, Lisa K. Jennings, PhD, • Benjamin J. Curry, PhD, John S. Lee MD, PhD, Subodh Verma, MD, PhD, • on Behalf of the REVERSE-IT Investigators Digital journal from the New England Journal of Medicine Group. First issue, January 2022. Article is posted at https://evidence.nejm.org/ REVERSE-IT Interim Results Published in December 2021

bentracimab REVERSE-IT: Baseline Characteristics 15https://evidence.nejm.org/doi/pdf/10.1056/EVIDoa2100047
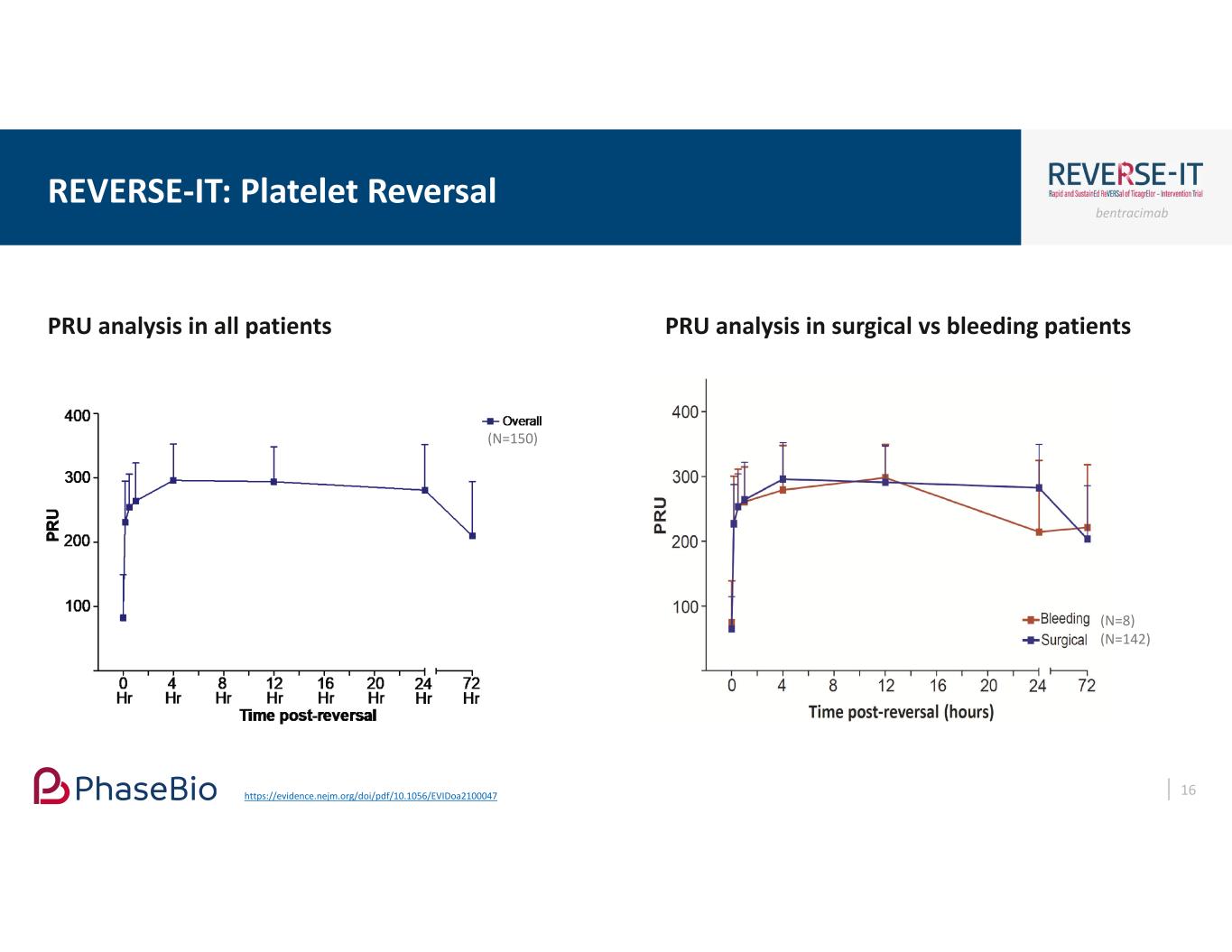
bentracimab PRU analysis in surgical vs bleeding patients REVERSE-IT: Platelet Reversal 16 PRU analysis in all patients (N=150) (N=8) (N=142) https://evidence.nejm.org/doi/pdf/10.1056/EVIDoa2100047

bentracimab REVERSE-IT: Adjudicated Surgical and Bleeding Hemostasis 17 Adjudicated and Investigator-Reported Surgical Outcomes https://evidence.nejm.org/doi/pdf/10.1056/EVIDoa2100047 Adjudicated and Investigator-Reported Bleeding Outcomes

bentracimab P-selectin in surgical and bleeding patients Mean platelet volume in surgical and bleeding patientsEffect of Bentracimab Treatment on P- Selectin and Mean Platelet Volume (MPV). Soluble P-selectin and MPV were measured pre-dose and at multiple timepoints post-initiation of bentracimab treatment to assess for a potentially prothrombotic rebound increase in platelet reactivity post-reversal. Shown are the soluble P-selectin levels in surgical and bleeding patients treated with bentracimab (left). MPV was measured in surgical and bleeding patients treated with bentracimab (right). REVERSE-IT: No Platelet Rebound Activity and No Thrombotic Events Related to Bentracimab 18https://evidence.nejm.org/doi/pdf/10.1056/EVIDoa2100047 Adjudicated Thrombotic Events Occurring Post-Reversal Post-Reversal Thrombotic Events within 30 Days in Reversal Trials 1. https://evidence.nejm.org/doi/pdf/10.1056/EVIDoa2100047 2. Pollack CV Jr, Reilly PA, van Ryn J, Eikelboom JW, Glund S, Bernstein RA, et al. Idarucizumab for dabigatran reversal: Full cohort analysis. N Engl J Med 2017; 377: 431–441. https://www.nejm.org/doi/full/10.1056/nejmoa1707278 3. Connolly SJ, Crowther M, Eikelboom JW. et al; ANNEXA-4 Investigators. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med 2019; 380 (14) 1326-1335 https://www.nejm.org/doi/full/10.1056/NEJMoa1814051#article_citing_articles 1 2 3

Regulatory

Bentracimab Pre-BLA Meeting Summary 20 • Type B pre biologics license application (pre-BLA) meeting held in April 2022 • PhaseBio proposed plans to submit a BLA with data from a total of 25-30 patients with uncontrolled bleeding, together with data from the fully-completed surgical cohort, to support a label with both bleeding and surgical indications • FDA agreed that the plan appeared reasonable, but the final label would be a review issue based on the data submitted • To date, and pending final adjudication, the REVERSE-IT trial has enrolled more than 35 patients taking ticagrelor who experienced uncontrolled bleeding events • FDA noted they would consider separating the indications for possible Accelerated Approval of either uncontrolled bleeding or surgery, if the application was deemed adequate to support approval for only one of the two • For post-marketing requirements, the FDA confirmed prior recommendations: Complete enrollment in the Phase 3 REVERSE-IT trial and submit data from a total of at least 200 patients from this trial Establish a post-approval registry study that will be active ahead of a product launch following a potential Accelerated Approval

Bentracimab Development Program 21 2019 2020 2021 2022 2023 Phase 2B: 50-80 year old volunteers CompletedPhase 2APhase 1 NEJM FDA Breakthrough Therapy FDA EOP1 EMA PRIME Designation N=200, 150 randomized to receive bentracimab N=150, major bleeding + urgent surgery patients Final N=200 PMC Completion of Phase 3Phase 3 REVERSE-IT trial ticagrelor patients Interim analysis completed August 2021 Last patient for interim Targeted timelines could be impacted by the continued scope and duration of the COVID-19 pandemic NEJM= New England Journal of Medicine, EOP1=End-of-Phase 1 Meeting, BLA=Biologics License Application • Phase 1 study published in NEJM (Bhatt DL, Pollack CV, Weitz JI, et al. N Engl J Med. 2019; 380:1825-1833) • Phase 2B study enrollment completed, results presented at ACC April 2022 • Interim analysis of REVERSE-IT Phase 3 trial completed in November 2021, published NEJM Evidence January 2022 • US BLA on track for submission in Q4 2022 following outcome of pre-BLA meeting Breakthrough status in US, PRIME designation in EU and Breakthrough in China (NMPA) Phase 2B Presentation at ACC Bentracimab Targeted BLA Submission Q4 2022 Potential BLA Approval Pre-BLA Meeting with FDA

Bentracimab Opportunity for Accelerated/Conditional Approval 22 Planned Approval Packages Based on Phase 3 - Interim Analysis • Initial BLA/MAA filing package based on a minimum safety requirement of at least 100 Phase 3 patients • US: Accelerated (Based on biomarker) • EU: Conditional (Based on interim analysis) - FDA and EMA agreed the PRU biomarker endpoint at interim likely predictive of clinical benefit in all patients - Pre-BLA Meeting provided opportunity to add uncontrolled bleeding subjects to current data set – US and Europe filing Clinical confirmation of interim biomarker endpoint at Phase 3 Completion – Full Approval • US and EU: Phase 3 completion and registry cohorts • China: Initial submission will include completed Phase 3 study

Commercial

Bentracimab Expect Continued Long-Term Rx Growth of Ticagrelor Bentracimab approval has the potential to drive continued positive momentum • Brilinta/Brilique sales in 2021 were approximately $1.5B Pandemic impact dampened 2020 and 2021 growth • In February 2019, Brilinta Phase 3 THEMIS1 trial met primary endpoint in patients with established coronary artery disease and type-2 diabetes; in May 2020, FDA approved a label update2 for BRILINTA in the US to include the reduction of the risk of a first heart attack or stroke in high-risk patients with coronary artery disease • In January 2020, Brilinta Phase III THALES3 trial met primary endpoint in patients with acute ischemic stroke or patients with high-risk transient ischemic attack; in November 20204, FDA approved a label update for Brilinta in the US to include the reduction of the risk of stroke in patients with acute ischemic stroke or high-risk transient ischemic attack 241. https://www.astrazeneca.com/media -centre/press -releases/2019/brilintas -phase- iii- themis -trial -met -primary -endpoint - in-patients -w ith -established -coronary -artery -disease-and-type -2-diabetes -25022019.html 2. https://www.astrazeneca.com/content/astraz/media -centre/press -releases/2020/brilinta -approved - in- the-us-to-reduce-the-risk-of-a- first -heart -attack -or-stroke- in-high -risk-patients -with -coronary -artery -disease.html 3. https://www.astrazeneca.com/media -centre/press -releases/2020/brilinta -met-primary -endpoint - in-phase- iii- thales -trial - in-stroke-27012020.html 4. https://www.astrazeneca.com/media -centre/press -releases/2020/brilinta -approved - in- the-us- in-stroke.html#:~:text=AstraZeneca's%20 Brilinta%20(ticagrelor)%20has%20been,transient%20ischaemic%20attack%20(TIA) . Ticagrelor Differentiation vs. clopidogrel Now Post Bentracimab Launch Post LOE of ticagrelor Efficacy Safety (no reversal agent) Price (branded vs. generic) (branded vs. generic)

BentracimabUS Patients on P2Y12 Inhibitors: Potential for Significant Long-Term Ticagrelor Patient Growth 25 0.4 3.8 0.2 2.02.2 0.2 Clopidogrel Prasugrel Ticagrelor US Patients on P2Y12 Inhibitors (millions) 2019 2029 (est.) Continued AZ Promotion Bentracimab Launch Brilinta Patent Expiry Potential Drivers of Ticagrelor Volume Growth Iqvia® Analysis of MIDAS Data

Bentracimab Patient Opportunity (2019) Implies $1-2B* Addressable Market 439,1361 ticagrelor pts/year 4.46M1 total P2Y12 pts/year CABG, coronary artery bypass graft 91,833 (21%5) 26 Inpatient Urgent Surgery3 26,452 (6%5) Inpatient Semi-Elective Surgery4, 48,399 (11%5) ICH 1,698GI 6,623 Trauma 3,396 Other 5,264 Urgent/ emergency CABG 11,361 Other 15,091 Inpatient Bleed2 16,982 (4%5) Potential bentracimab patients 1. 2019 P2Y12 class patient count analysis: Iqvia, MIDAS tablet volume 2. Bleeding events are defined as an inpatient admission with a diagnosis code for a bleed 3. Urgent surgery/procedure is defined as an emergency room visit where a surgery/procedure was performed or an inpatient admission with surgery/procedure that had an ambulance service on the same day as admission 4. Semi-elective surgery/procedure is defined as any inpatient surgery/procedure without an ambulance service on the same day 5. Rate data from the IBM MarketScan® Commercial Claims and Encounters, Medicare Supplemental Databases: TIME PERIOD: 2014 THROUGH 2019 * Based on estimated $10,000-$20,000 per dose, ~90k patients with inpatient admissions for bleeding or surgery Bentracimab

Increased Mortality and Health-System Costs Associated with Bleeding from Open Surgical Procedures Highlight Opportunity for Bentracimab 27 *All charges by the hospital for the inpatient episode – includes drugs, devices, professional fees, room and board etc. ‘Bleed’ = Moderate to Severe Bleeding identified via bleeding Dx codes, bleed control procedure codes, or 2+ units of blood visible in the inpatient episode. Additional details available in ‘Key Assumptions & Definitions’ section. Data Source: In-Patient Hospital Admissions in 2019 from Premier Chargemaster Data; Statistical significance testing has not been conducted. Each washout decision today must weigh increasing the patients’ bleeding risk against risk of thrombosis Mortality 7.4% 2.6% 2.9x $50k $27k 1.9x All-cause Hospital Charge*Bleeding Incidence Pre-procedure P2Y12 No P2Y12 5.7% 2.6% 2.2x % O pe n su rg ica l p at ie nt s 1+ Days in ICU % E pi so de s 71% 46% 1.5x Moderate/severe bleeding No bleeding Pre-procedure P2Y12 $ Bentracimab

PB6440 Aldosterone Synthase Inhibitor for Resistant Hypertension

PB6440 PB6440 for Resistant Hypertension • Upwards of 10 million patients in the United States have resistant hypertension and are at risk for serious, costly medical consequences (stroke, heart attack, kidney failure, etc.)1 • Physicians currently prescribe numerous combinations of antihypertensives to lower blood pressure and diminish risk • Blocking aldosterone has been shown to be an effective mechanism for treating resistant hypertension Currently available aldosterone blockers suffer from poor potency and pharmacokinetics (eplerenone) or poor tolerability (spironolactone) and thus are rarely used Recent clinical data from other aldosterone synthase inhibitor (ASI) programs support blocking aldosterone as an effective approach to treating resistant hypertension • Draft guidance from the FDA outlines a streamlined regulatory path for novel drugs to treat resistant hypertension without the need for large outcomes studies2 • Market research indicates that payors aware of high medical costs associated with resistant hypertension 29 Large, growing patient population, coupled with a high unmet need, creates an attractive opportunity for a novel therapy to help patients and care-providers better manage blood pressure 1. the United Sta tes : compa rison of the 2008 a nd 2018 America n hea rt a ssocia tion scientific s ta tements on res is ta nt hypertension. - 2 . to - informa tion/sea rch-fda - -documents /hypertens ion- -s tudies- - trea t-pa tients- - - -

PB6440: A Promising CYP11B2 Inhibitor 30 Characteristics Of PB6440 High in vitro selectivity for CYP11B2 over CYP11B1 High in vitro selectivity for CYP11B2 over drug metabolizing and other steroidal CYPs High oral bioavailability Long half-life in vivo consistent with once-daily dosing Oral PK and selectivity profiles yield a large therapeutic index in vivo Suppression of plasma aldosterone levels >90% in an ACTH-challenge model; no effect on cortisol, DOC or 11-b deoxycortisol levels PB6440 • Potential for an oral, once-daily dosing regimen with a best- in-class profile from a safety and efficacy perspective • Validated mechanism of action positions ASIs as a new class of therapies with the potential to address a significant unmet need • As of Q2 2022, PhaseBio has completed development and optimization of robust manufacturing process for PB6440 to support anticipated upcoming clinical proof-of-concept trials • Potential indications include resistant hypertension and other indications where elevated aldosterone is known to contribute to disease process, such as uncontrolled hypertension, chronic kidney disease, and heart failure • An investigational new drug application (IND) is targeted for submission to the FDA in the first half of 2023, with first-in- human trials planned to initiate in mid-2023

PB6440 CYP11B Potency and Selectivity (IC50, μM) Human Monkey CYP11B2 CYP11B1# Selectivity CYP11B2 CYP11B1 Selectivity PB6440 0.024 4.859 202 0.016 5.802 363 LCI699* 0.0007 0.013 19 0.016 0.059 3.7 PB6440 Is Highly Selective for Aldosterone Synthase (CYP11B2) Selectivity and Potency Demonstrated in Primate Chronic Oral Dosing Model 31 In a primate model, oral PB6440 demonstrated a sustainable reduction in aldosterone without a significant increase in steroids upstream of CYP11B1, suggesting no significant inhibition of CYP11B1 in vivo # Steroid 11 -hydroxylase *Discontinued Novartis compound; active in Phase 2 studies, but blocked cortisol production, likely due to inadequate selectivity

Resistant Hypertension (rHTN)1: Hypertensive patient on three or more antihypertensive medications and still above goal 32 Large market opportunity2 • ~75M hypertensives in the US • ~55M diagnosed and treated • ~10-15M resistant to treatment • Other potential indications include uncontrolled hypertension, kidney disease, heart failure and primary aldosteronism Resistant Hypertension increases risk of serious events • Heart attack +42%; Stroke +67% • Heart failure +97%; Kidney failure +225% Unmet need driving FDA resistant hypertension guidance • FDA identified rHTN as a key area of unmet need in July 2018 Guidance for Industry Blocking aldosterone proven to be the best treatment for rHTN • Aldosterone receptor blocker spironolactone (approved in 1959) demonstrated as most effective treatment for rHTN • Spironolactone is used in <10% of patients due to side effects3 1) Resistant Hypertension (rHTN): on three or more antihypertensive medications and blood pressure still above goal (American Heart Association (AHA) and American College of Cardiology (ACC) 2) U.S. Center for Disease Control and Prevention (CDC); Roberie, 2012 3) Hwang 2017 PB6440
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- AZZ, Inc. (AZZ) Announces Proposed 4M Share Offering
- Centessa Pharmaceuticals (CNTA) Announces Pricing of $100 Million Public Offering of American Depositary Shares
- Siebert Financial Corp (SIEB) Receives Nasdaq Non-compliance Notice
Create E-mail Alert Related Categories
SEC FilingsRelated Entities
S3Sign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share