Form 8-K PTC THERAPEUTICS, INC. For: Jun 21
| Translarna™(Ataluren)Study 041 Topline Results21stJune 2022 |
| Forward looking statementsThis presentation contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. All statements contained in this presentation, other than statements of historic fact, are forward-looking statements, including statements regarding: the future expectations, plans and prospects for PTC, including with respect to the commercialization of its products and product candidates; PTC's plans for interactions with the EMA and FDA; the clinical utility and potential advantages of Translarna; PTC's strategy, future operations, future financial position, future revenues, projected costs; and the objectives of management. Other forward-looking statements may be identified by the words"guidance", "plan," "anticipate," "believe," "estimate," "expect," "intend," "may," "target," "potential," "will," "would," "could," "should," "continue," and similar expressions.PTC's actual results, performance or achievements could differ materially from those expressed or implied by forward-looking statements it makes as a result of a variety of risks and uncertainties, including those related to: the outcome of pricing, coverage and reimbursement negotiations with third party payors for PTC's products or product candidates that PTC commercializes or may commercialize in the future;PTC's ability to maintain its marketing authorization of Translarnafor the treatment of nmDMDinBrazil,Russia, the European Economic Area (EEA) and other regions, including whether theEuropean Medicines Agency(EMA) determines in future annual renewal cycles that the benefit-risk balance of Translarnaauthorization supports renewal of such authorization; PTC's ability to enroll, fund, complete and timely submit to the EMA the results of Study 041, a randomized, 18-month, placebo-controlled clinical trial of Translarnafor the treatment of nmDMDfollowed by an 18-month open-label extension, which is a specific obligation to continued marketing authorization in the EEA;PTC's ability to utilize results from Study 041to support a marketing approval forTranslarnafor the treatment ofnmDMDintheUnited States; whether investigators agree with PTC's interpretation of the results of clinical trials and the totality of clinical data from our trials in Translarna; significant business effects, including the effects of industry, market, economic, political or regulatory conditions; changes in tax and other laws, regulations, rates andpolicies; the eligible patient base and commercial potential of PTC's products and product candidates; PTC's scientific approach and general development progress; and the factors discussed in the "Risk Factors" section of PTC's most recent Annual Report on Form 10-K, as well as any updates to these risk factors filed from time to time in PTC's other filings with theSEC. You are urged to carefully consider all such factors.As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. There are no guarantees that any product will receive or maintain regulatory approval in any territory, or prove to be commercially successful, including Translarna.The forward-looking statements contained herein represent PTC's views only as of the date of this presentation and PTC does not undertake or plan to update or revise any such forward-looking statements to reflect actual results or changes in plans, prospects, assumptions, estimates or projections, or other circumstances occurring after the date of this presenationexcept as required by law. 2 |
| Agenda 3 Study 041 Topline Results Overview of DMD and TranslarnaSTRIDE Registry123 |
| Overview of DMD and Translarna 4 |
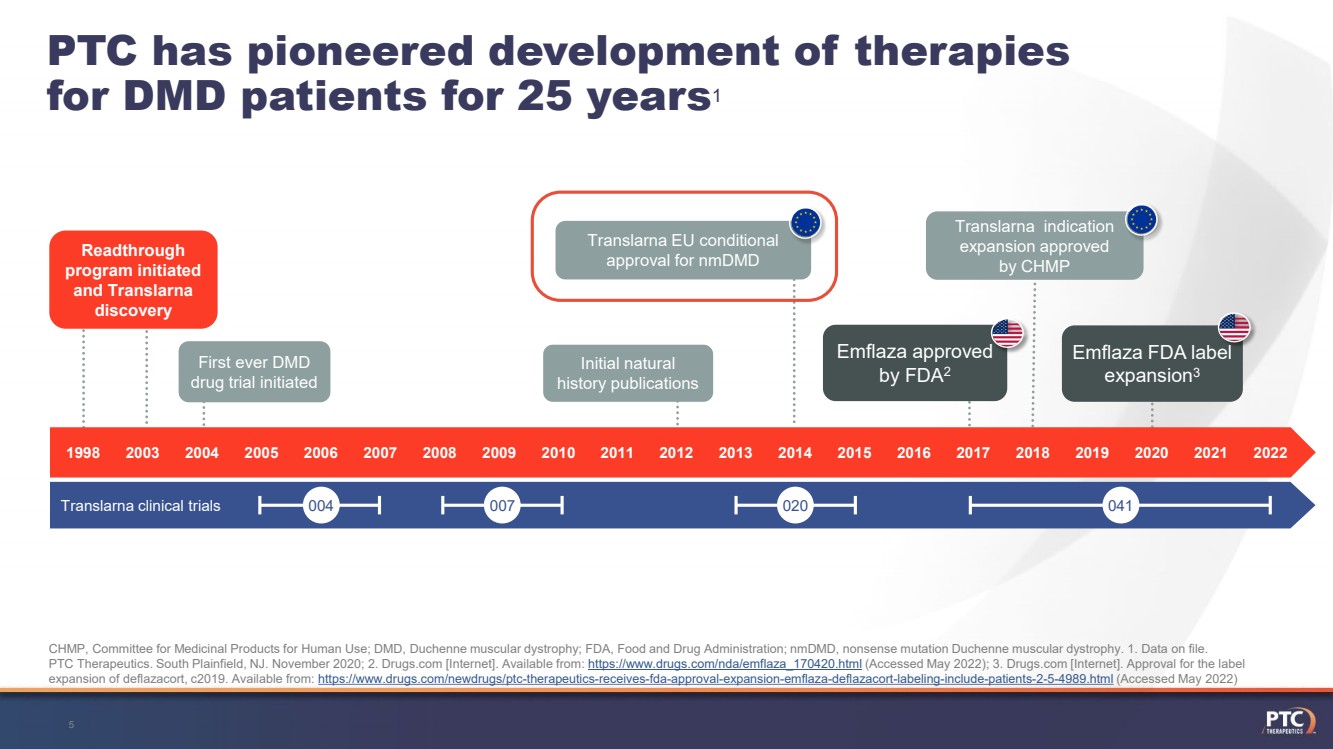
| 5PTC has pioneered development of therapies for DMD patients for 25 years1CHMP, Committee for Medicinal Products for Human Use; DMD, Duchenne muscular dystrophy; FDA, Food and Drug Administration; nmDMD, nonsense mutation Duchenne muscular dystrophy. 1. Data on file. PTC Therapeutics. South Plainfield, NJ. November 2020; 2. Drugs.com[Internet]. Available from: https://www.drugs.com/nda/emflaza_170420.html (Accessed May 2022); 3. Drugs.com[Internet]. Approval for the label expansion of deflazacort, c2019. Available from: https://www.drugs.com/newdrugs/ptc-therapeutics-receives-fda-approval-expansion-emflaza-deflazacort-labeling-include-patients-2-5-4989.html(Accessed May 2022) Readthrough program initiated and Translarnadiscovery TranslarnaEUconditional approval for nmDMD Translarnaindication expansion approved by CHMP Initial natural history publications First ever DMD drug trial initiated Emflaza FDA label expansion3 Emflazaapproved by FDA2 19982003201420182017Translarnaclinicaltrials201220042019202020212022 20132005 20152016201120102008200620092007 004 007 020 041 |
| DMD is a relentlessly progressive, fatal, genetic disorder Debilitating X-linked genetic disorder leading to death in early adulthood1 in every 3,500-5,000 live male births with ~13% caused by nonsense mutations1-7Caused by the lack of dystrophin protein due to mutations that prevent its production 61. van RuitenHJ,etal.ArchDis Child. 2014;99:1074–1077. 2. Bushby K, et al. Lancet Neurol. 2010;9:77‒93. 3. Aartsma-Rus A, et al. J Med Genet. 2016;53(3):145–151. 4. CrisafulliS, et al. OrphanetJ Rare Dis. 2020;15(1):1415. PichavantC, et al. Mol Ther. 2011;19:830–840. 6. Kalman L, et al. J Mol Diagn. 2011;13:167–174. 7. BladenCL, et al. Hum Mutat. 2015;36:395–402 |
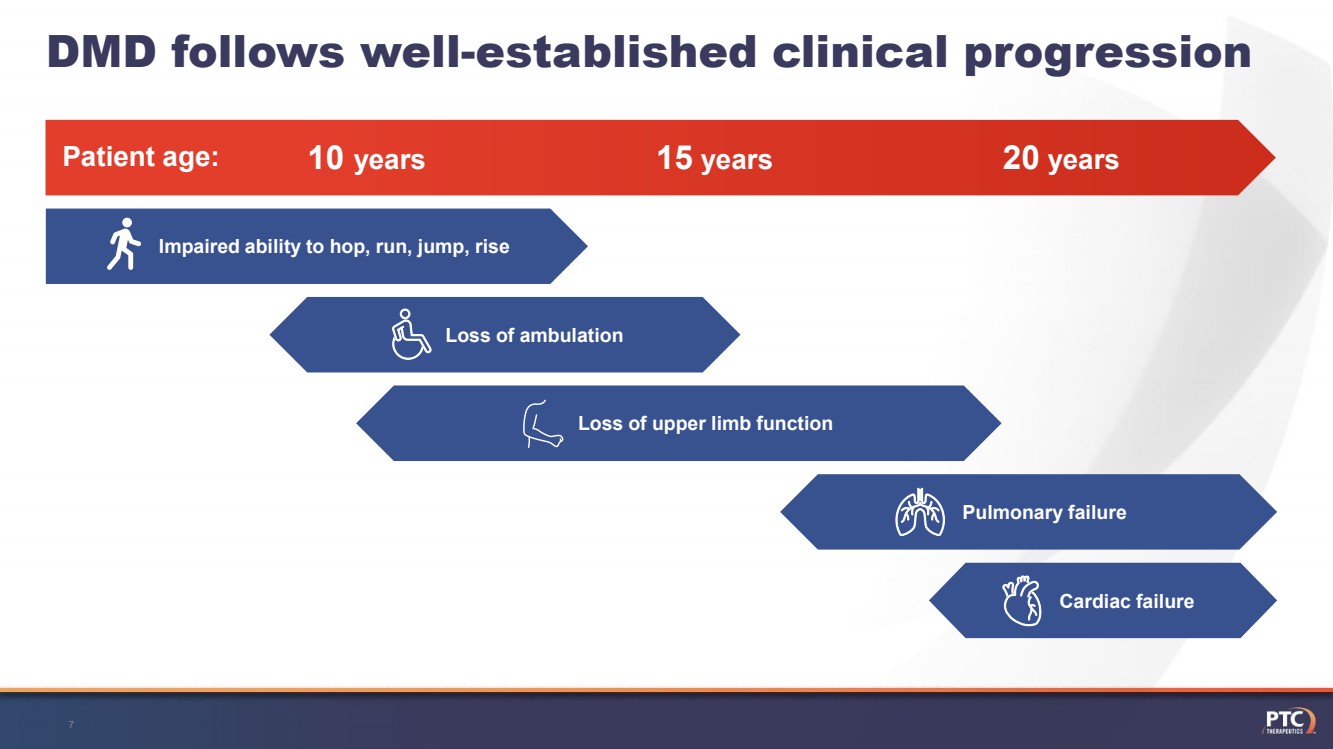
| DMD follows well-established clinical progression7 Patient age:10 years15years20years Loss of ambulation Loss of upper limb function Pulmonary failure Cardiac failure Impaired ability to hop, run, jump, rise |
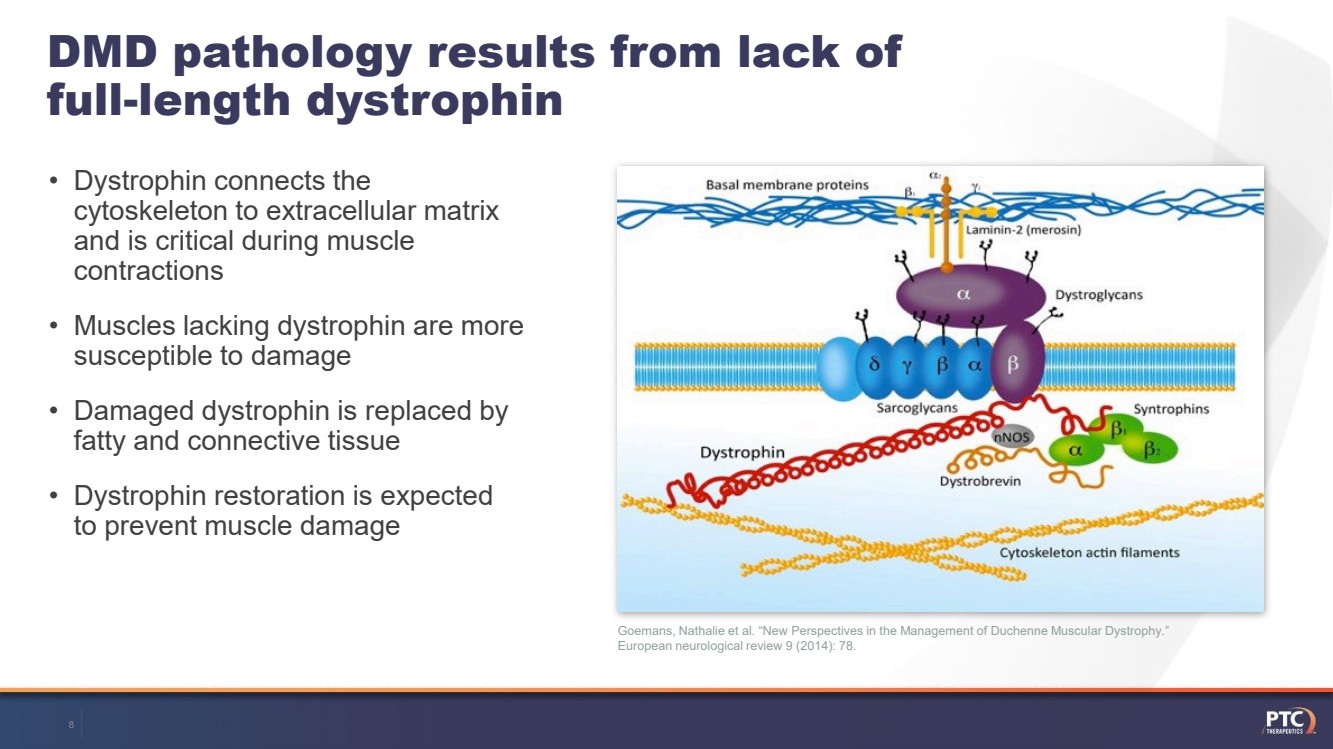
| Goemans, Nathalie et al. “New Perspectives in the Management of Duchenne Muscular Dystrophy.” European neurological review 9 (2014): 78. DMD pathology results from lack of full-length dystrophin•Dystrophin connects the cytoskeleton to extracellular matrix and is critical during muscle contractions•Muscles lacking dystrophin are more susceptible to damage•Damaged dystrophin is replaced by fatty and connective tissue•Dystrophin restoration is expected to prevent muscle damage 8 |
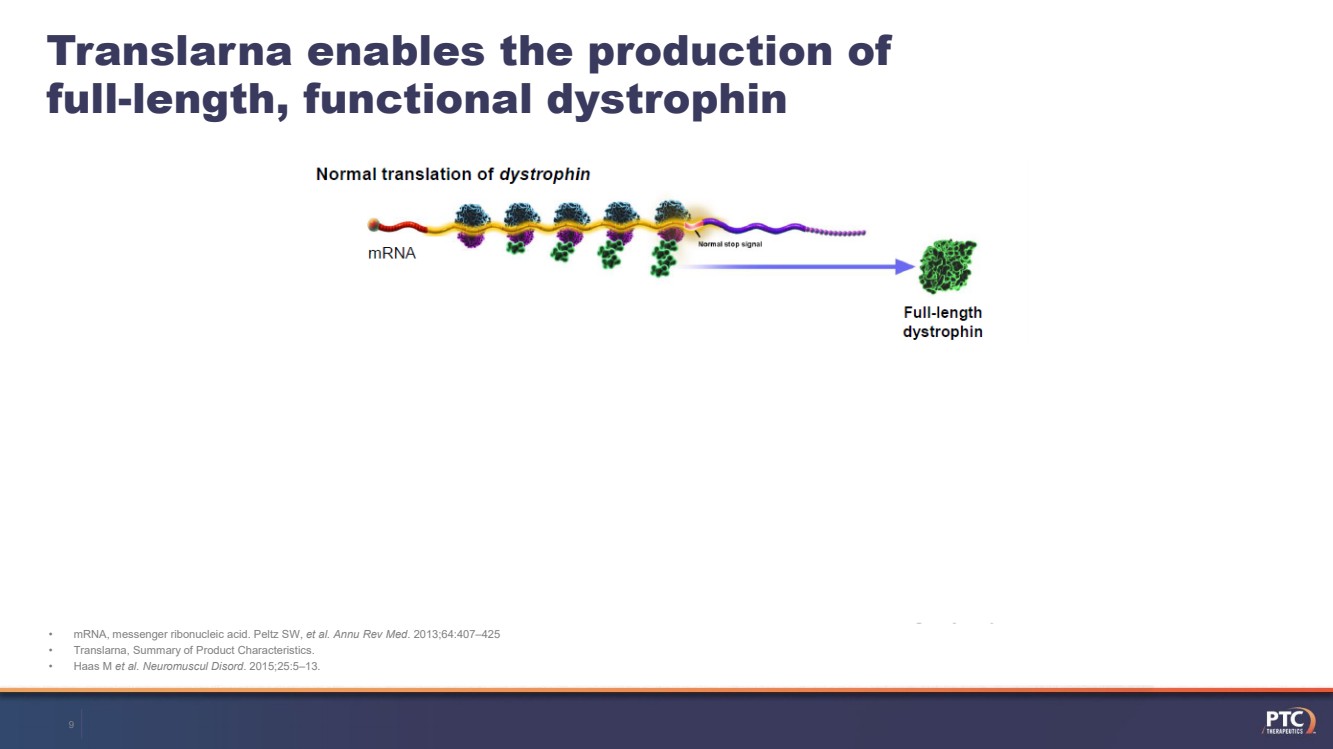
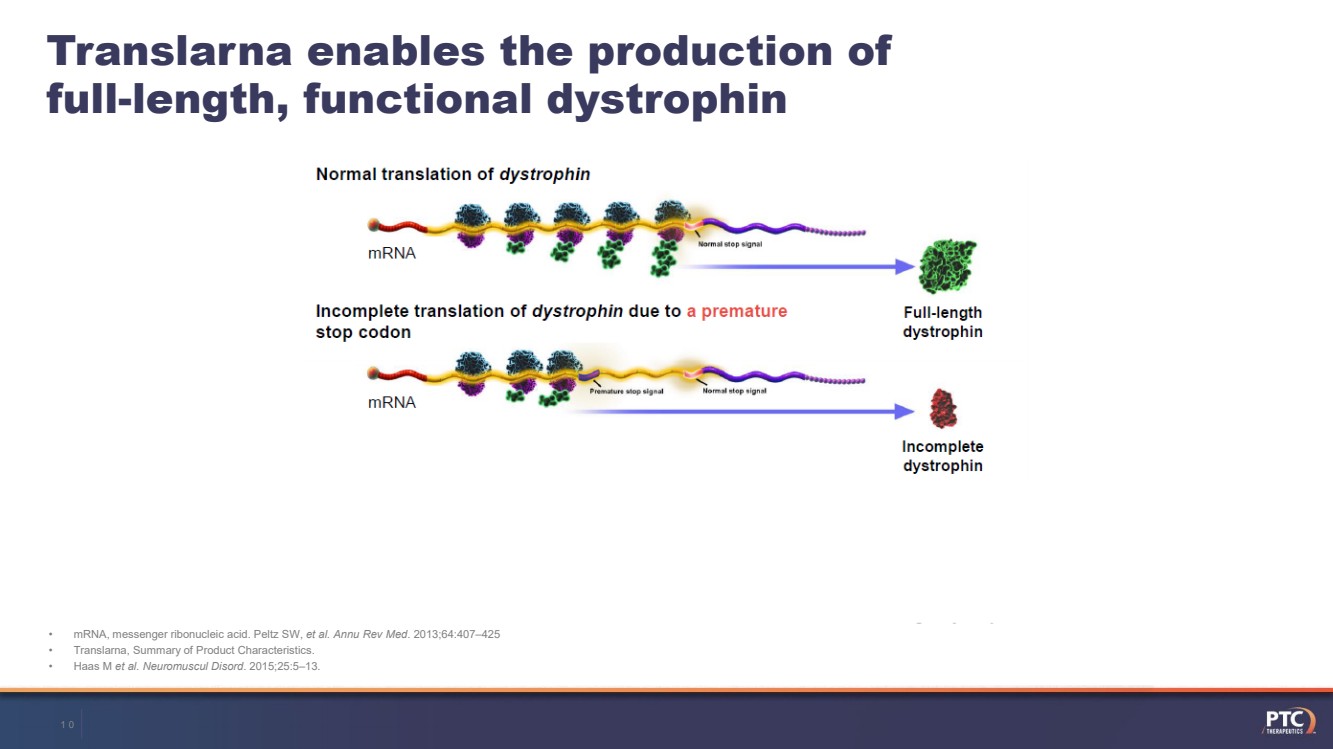
| •mRNA, messenger ribonucleic acid. Peltz SW, et al. AnnuRev Med. 2013;64:407–425•Translarna, Summary of Product Characteristics. •Haas M et al. NeuromusculDisord. 2015;25:5–13.Translarnaenables the production of full-length, functional dystrophin9 |
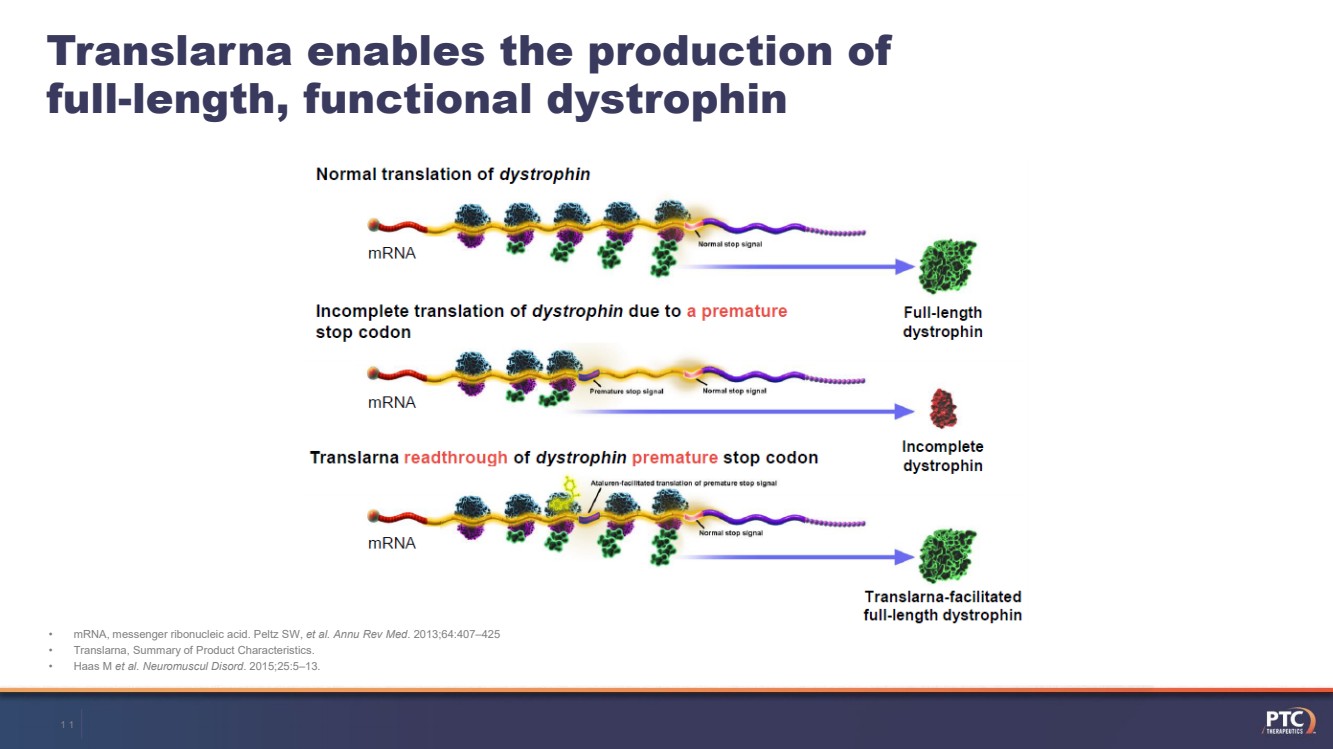
| •mRNA, messenger ribonucleic acid. Peltz SW, et al. Annu Rev Med. 2013;64:407–425•Translarna, Summary of Product Characteristics. •Haas M et al. Neuromuscul Disord. 2015;25:5–13.Translarnaenables the production of full-length, functional dystrophin10 |
| •mRNA, messenger ribonucleic acid. Peltz SW, et al. Annu Rev Med. 2013;64:407–425•Translarna, Summary of Product Characteristics. •Haas M et al. Neuromuscul Disord. 2015;25:5–13.Translarnaenables the production of full-length, functional dystrophin11 |
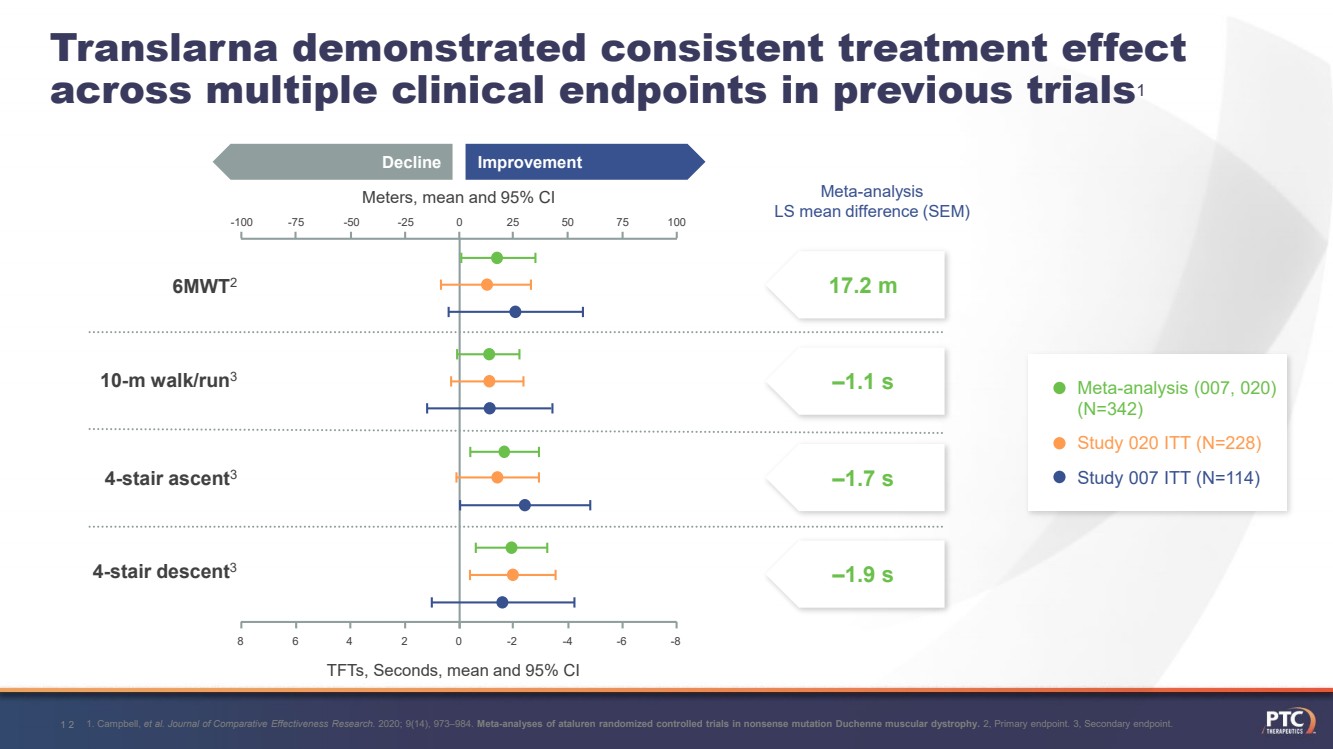
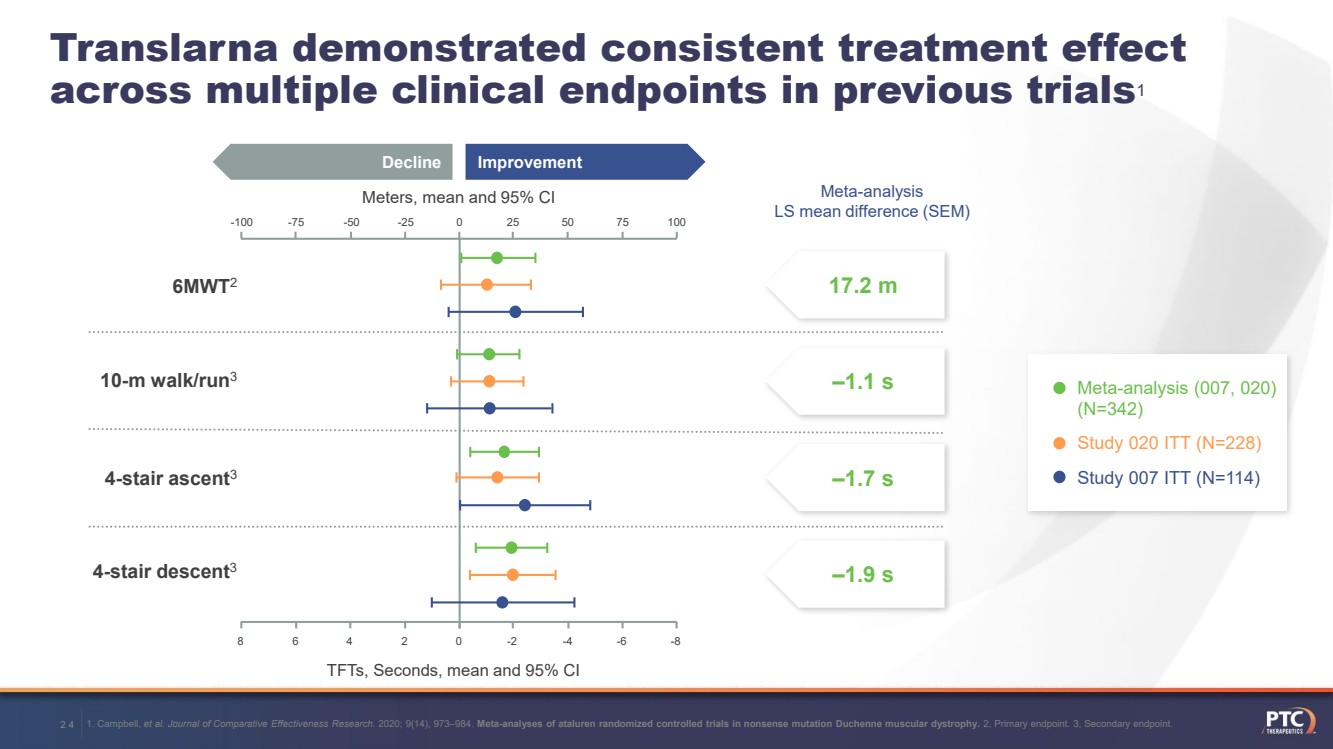
| 1. Campbell, et al. Journal of Comparative Effectiveness Research. 2020; 9(14), 973–984. Meta-analyses of ataluren randomized controlled trials in nonsense mutation Duchenne muscular dystrophy.2, Primary endpoint. 3, Secondary endpoint. 12Translarnademonstrated consistent treatment effect across multiple clinical endpoints in previous trials1 17.2 m ‒1.1 s ‒1.7 s ‒1.9 sMeta-analysis LS mean difference (SEM)Meters, mean and 95% CITFTs, Seconds, mean and 95% CI Improvement Decline 75 100 50 25 0 -25 -50 -75 -100 Meta-analysis (007, 020) (N=342)Study 020 ITT (N=228) Study 007 ITT (N=114) 8 6 4 2 0 -2 -4 -6 -86MWT210-m walk/run34-stair ascent34-stair descent3 |
| STRIDE Registry 13 |
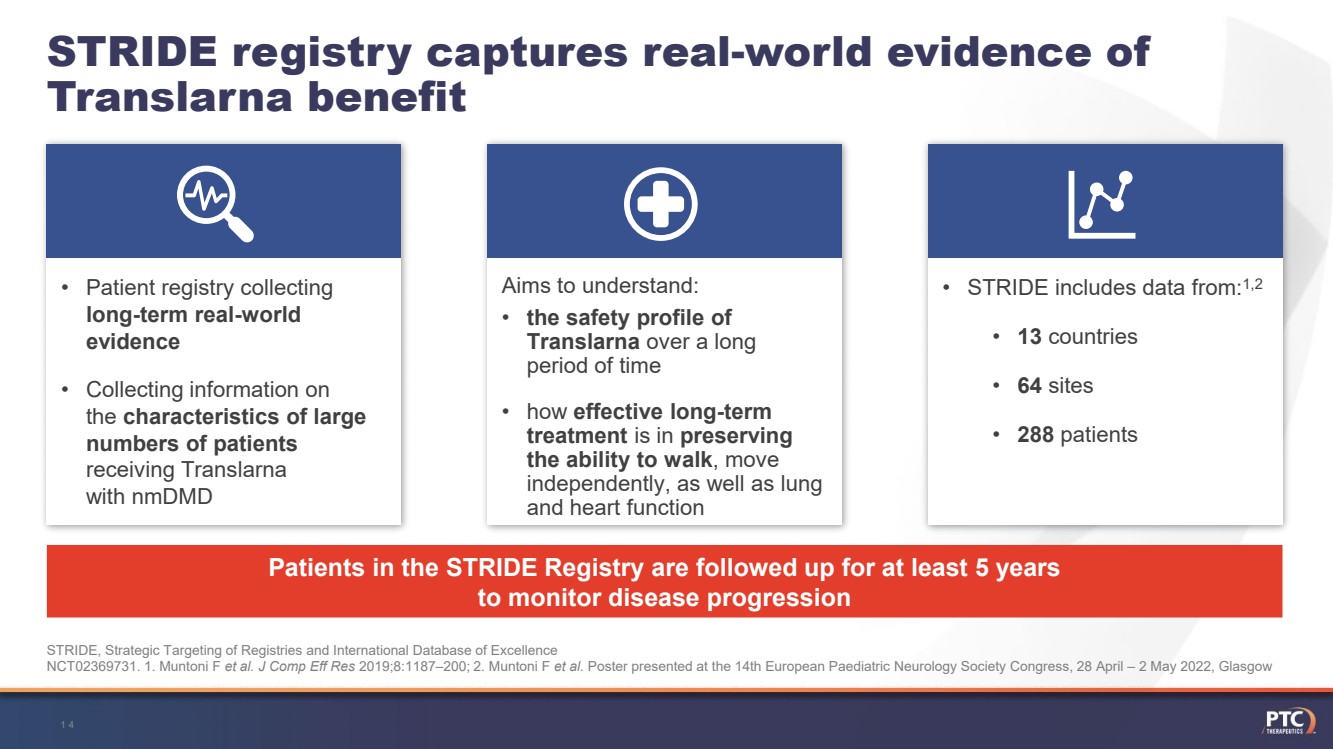
| •STRIDE includes data from:1,2•13countries•64sites•288patients Aims to understand:•the safety profile of Translarnaover a long period of time•how effective long-term treatment is in preservingthe ability to walk, move independently, as well as lung and heart function •Patient registry collecting long-term real-world evidence•Collecting information on the characteristics of large numbers of patients receivingTranslarnawith nmDMD STRIDE registry captures real-world evidence of Translarnabenefit Patients in the STRIDE Registry are followed up for at least 5 yearsto monitor disease progressionSTRIDE, Strategic Targeting of Registries and International Database of ExcellenceNCT02369731. 1. MuntoniF et al. J Comp Eff Res 2019;8:1187–200; 2. MuntoniF et al. Poster presented at the 14th European PaediatricNeurology Society Congress, 28 April –2 May 2022, Glasgow14 |
| 5.4 years longer of being able to walk and move independently1STRIDE registrydemonstratesTranslarnatreatmentsignificantlypreservesambulation CINRG Duchenne Natural HistoryStudySTRIDE Registry 17.9 years old12.5 yearsoldaThemedian (95% CI) ages at loss of ambulation were: 17.9 (14.4, NA) years for STRIDE and 12.5 (11.6, 13.5) years for the CINRG DNHS; p < 0.0001.CINRG DNHS, Cooperative International Neuromuscular Research Group Duchenne Natural History Study; 1. MercuriE et al. Poster presented at the 14th European PaediatricNeurology Society Congress, 28 April –2 May 2022, Glasgow15 |
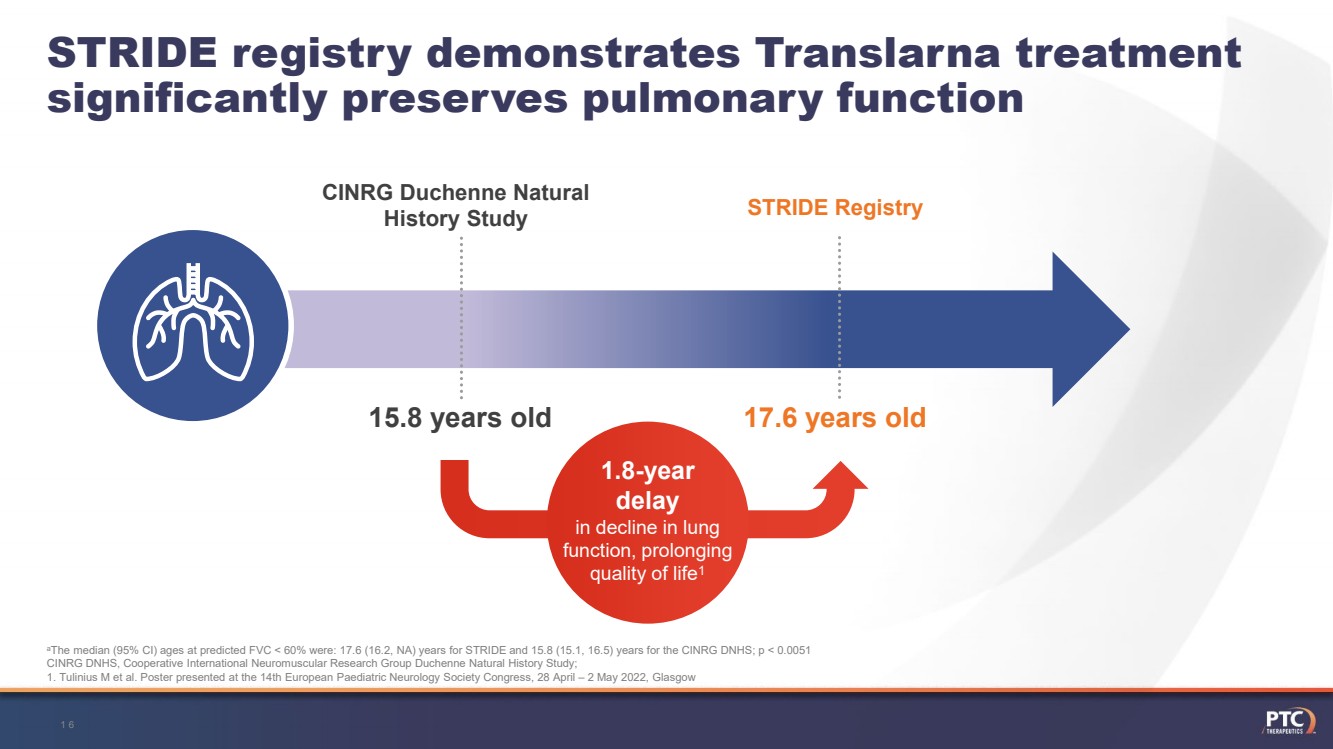
| 1.8-year delay in decline in lung function, prolonging quality of life1 CINRG Duchenne Natural HistoryStudySTRIDE Registry 17.6 years old15.8 yearsoldaThemedian (95% CI) ages at predicted FVC < 60% were: 17.6 (16.2, NA) years for STRIDE and 15.8 (15.1, 16.5) years for the CINRG DNHS; p < 0.0051CINRG DNHS, Cooperative International Neuromuscular Research Group Duchenne Natural History Study; 1. TuliniusM et al. Poster presented at the 14th European Paediatric Neurology Society Congress, 28 April –2 May 2022, Glasgow STRIDE registrydemonstratesTranslarnatreatmentsignificantlypreservespulmonaryfunction16 |
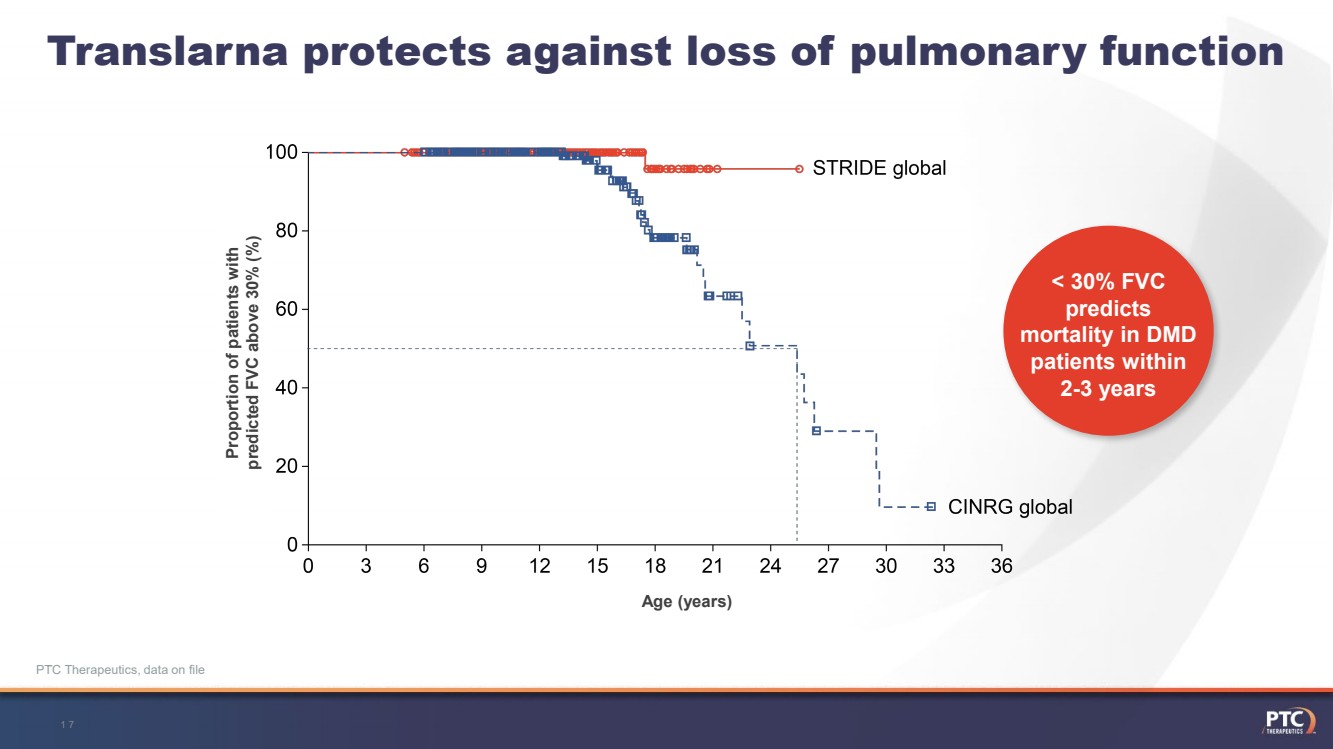
| Translarnaprotects against loss of pulmonary function 17-- < 30% FVC predicts mortality in DMD patients within 2-3 years PTC Therapeutics, data on file Proportion of patients withpredicted FVC above 30% (%) Age (years) |
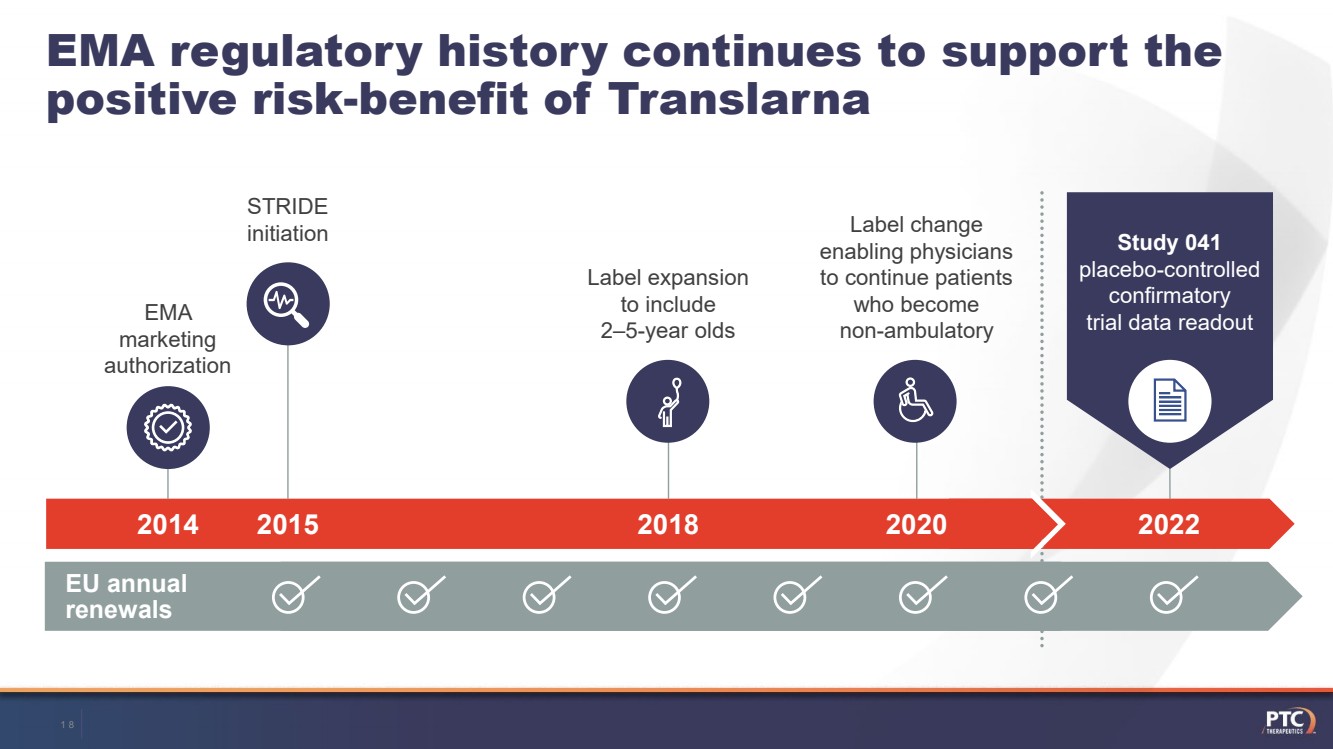
| 2022 Study 041 placebo-controlled confirmatory trial data readout EMA marketingauthorizationLabel expansion to include 2–5-year olds EMA regulatory history continues to support the positive risk-benefit of Translarna Label change enabling physicians to continue patients who become non-ambulatory201420182020 EU annual renewalsSTRIDEinitiation 2015 18 |
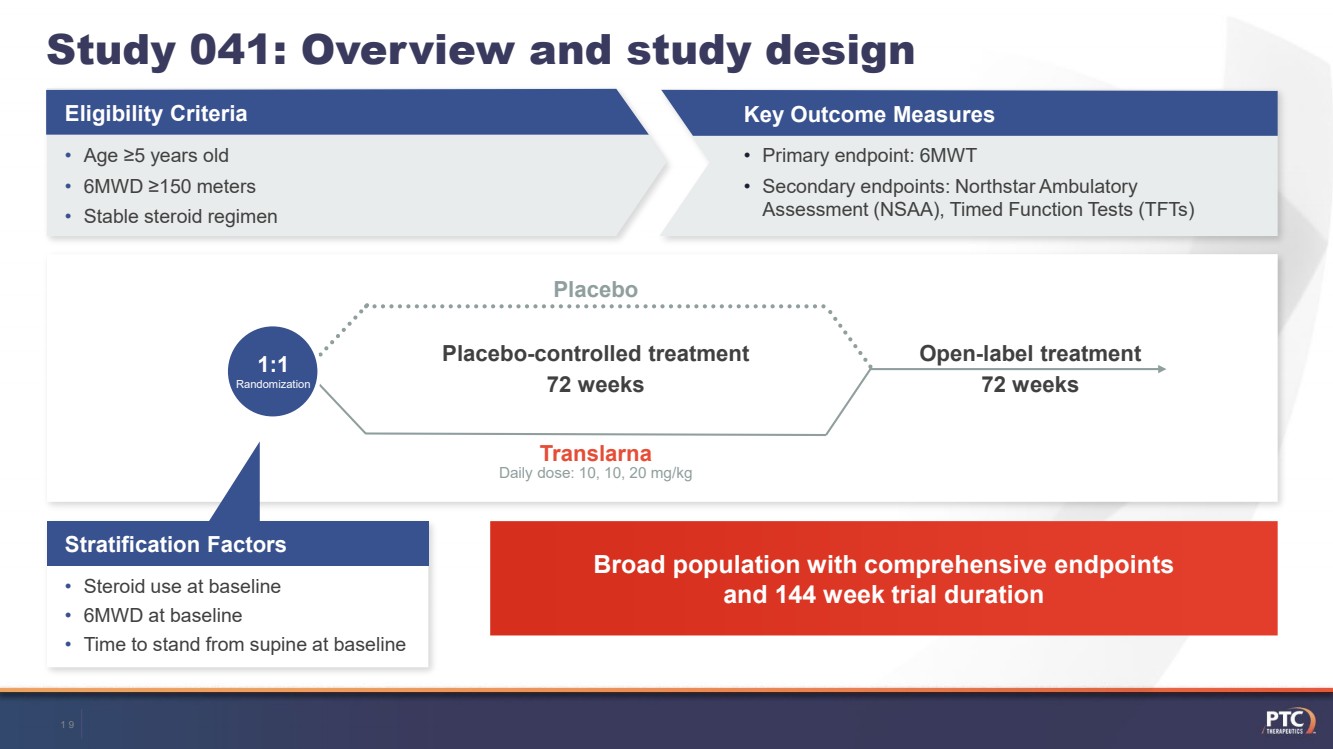
| Study 041: Overview and study design•Age ≥5 years old •6MWD ≥150 meters•Stable steroid regimenEligibility CriteriaKey Outcome Measures•Primary endpoint: 6MWT•Secondary endpoints: NorthstarAmbulatory Assessment (NSAA), Timed Function Tests (TFTs)Open-label treatment72 weeks Placebo 1:1Randomization TranslarnaPlacebo-controlled treatment 72 weeks Broad population with comprehensive endpoints and 144 week trial duration •Steroid use at baseline•6MWD at baseline•Time to stand from supine at baselineStratification Factors Daily dose: 10, 10, 20 mg/kg19 |
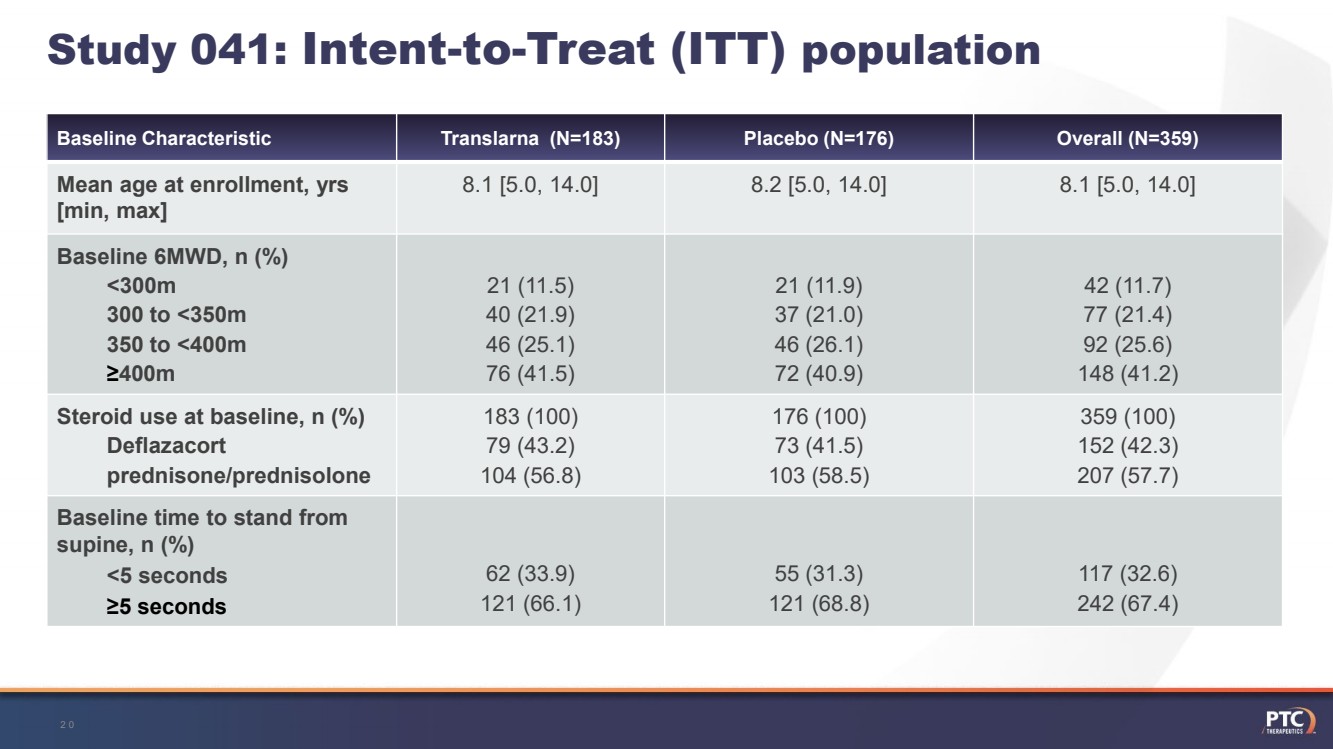
| Study 041: Intent-to-Treat (ITT) population Baseline CharacteristicTranslarna (N=183)Placebo (N=176)Overall (N=359)Mean age at enrollment, yrs[min, max]8.1 [5.0, 14.0]8.2 [5.0, 14.0]8.1 [5.0, 14.0]Baseline 6MWD, n (%)<300m300 to <350m350 to <400m≥400m21 (11.5)40 (21.9)46 (25.1)76 (41.5)21 (11.9)37 (21.0)46 (26.1)72 (40.9)42 (11.7)77 (21.4)92 (25.6)148 (41.2)Steroid use at baseline, n (%)Deflazacortprednisone/prednisolone183 (100)79 (43.2)104 (56.8)176 (100)73 (41.5)103 (58.5)359 (100)152 (42.3) 207 (57.7)Baseline time to stand from supine, n (%)<5 seconds≥5 seconds62 (33.9)121 (66.1)55 (31.3)121 (68.8)117 (32.6)242 (67.4) 20 |
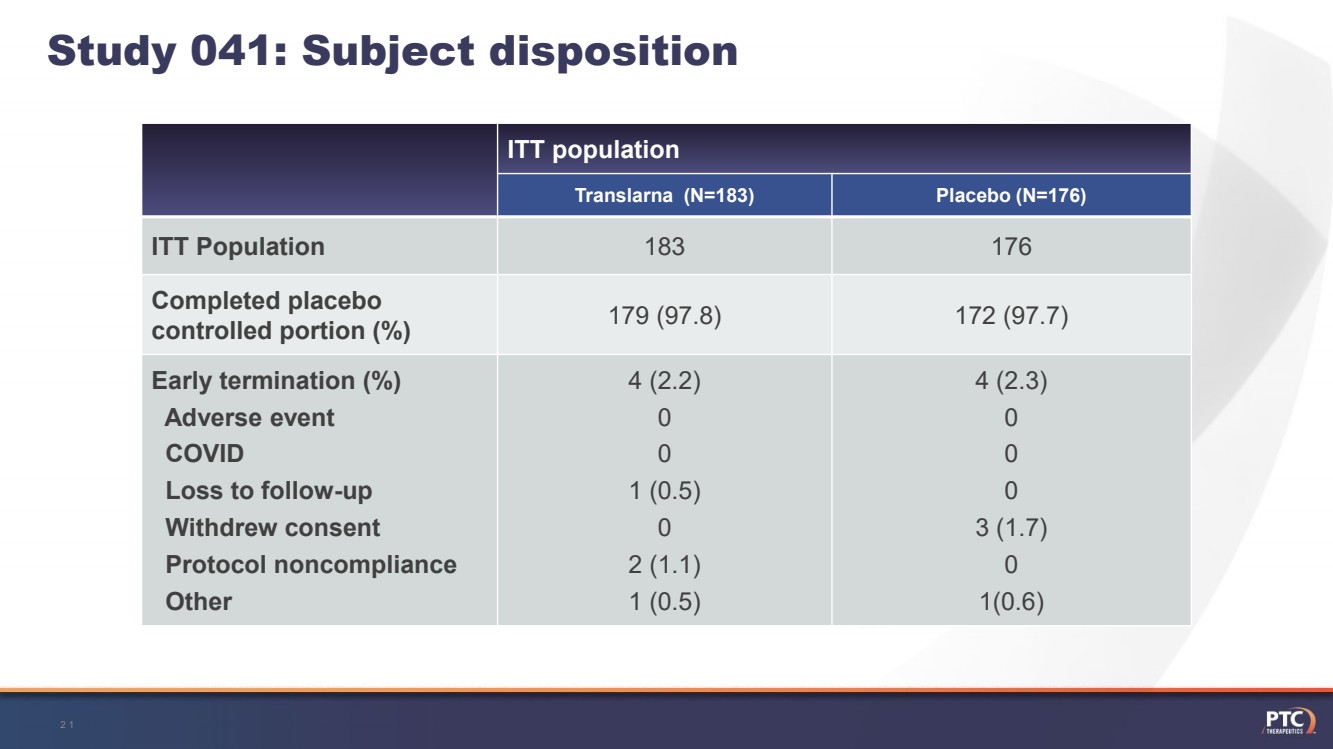
| Study 041: Subject disposition 21 ITT populationTranslarna(N=183)Placebo (N=176)ITT Population183176Completed placebo controlled portion (%)179 (97.8)172 (97.7)Early termination (%)Adverse eventCOVIDLoss to follow-upWithdrew consentProtocol noncomplianceOther4 (2.2)001 (0.5)02 (1.1) 1 (0.5)4 (2.3)0003 (1.7)01(0.6) |
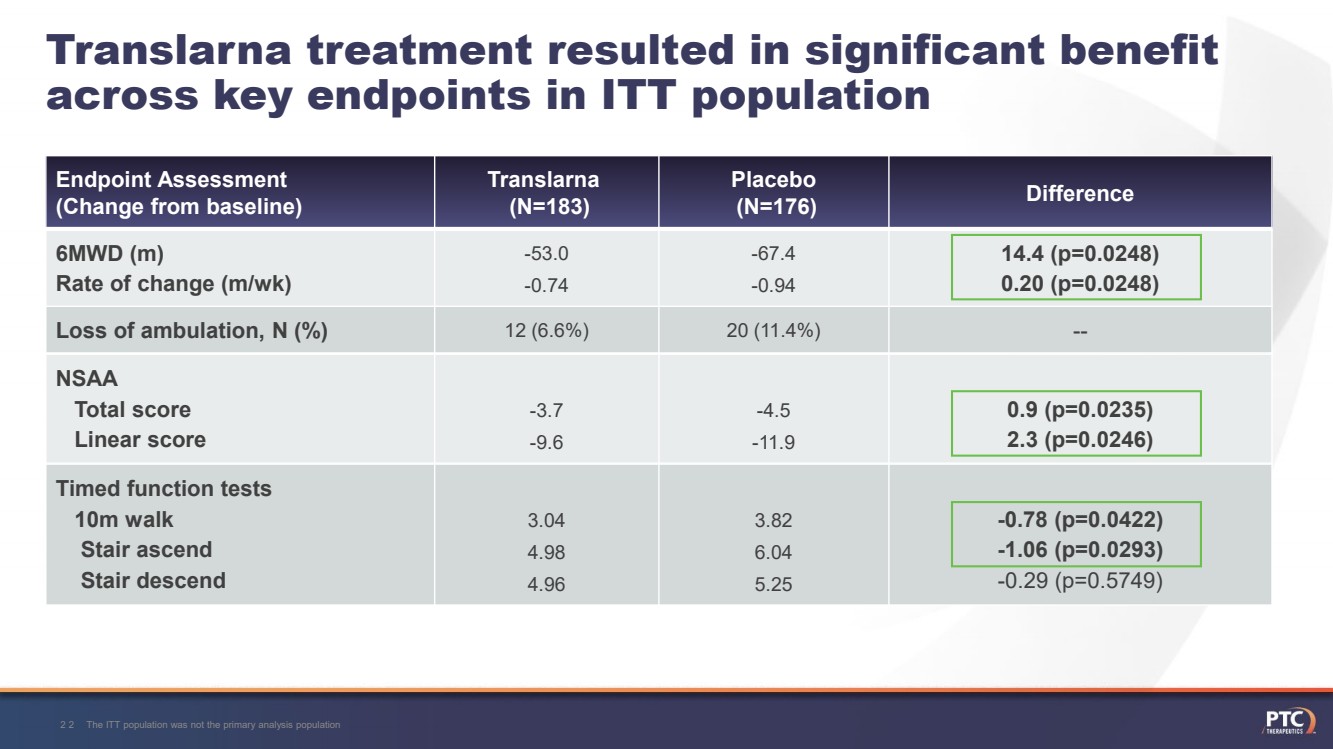
| Translarnatreatment resulted in significant benefit across key endpoints in ITT populationThe ITT population was not the primary analysis population22 Timed function tests10m walkStair ascendStair descend3.044.984.963.826.045.25-0.78 (p=0.0422)-1.06 (p=0.0293)-0.29 (p=0.5749) NSAA Total scoreLinear score-3.7-9.6-4.5-11.90.9 (p=0.0235)2.3 (p=0.0246) Endpoint Assessment(Change from baseline)Translarna(N=183)Placebo(N=176)Difference6MWD (m)Rate of change(m/wk)-53.0-0.74-67.4-0.9414.4 (p=0.0248)0.20 (p=0.0248)Loss of ambulation, N (%)12 (6.6%)20 (11.4%)-- |
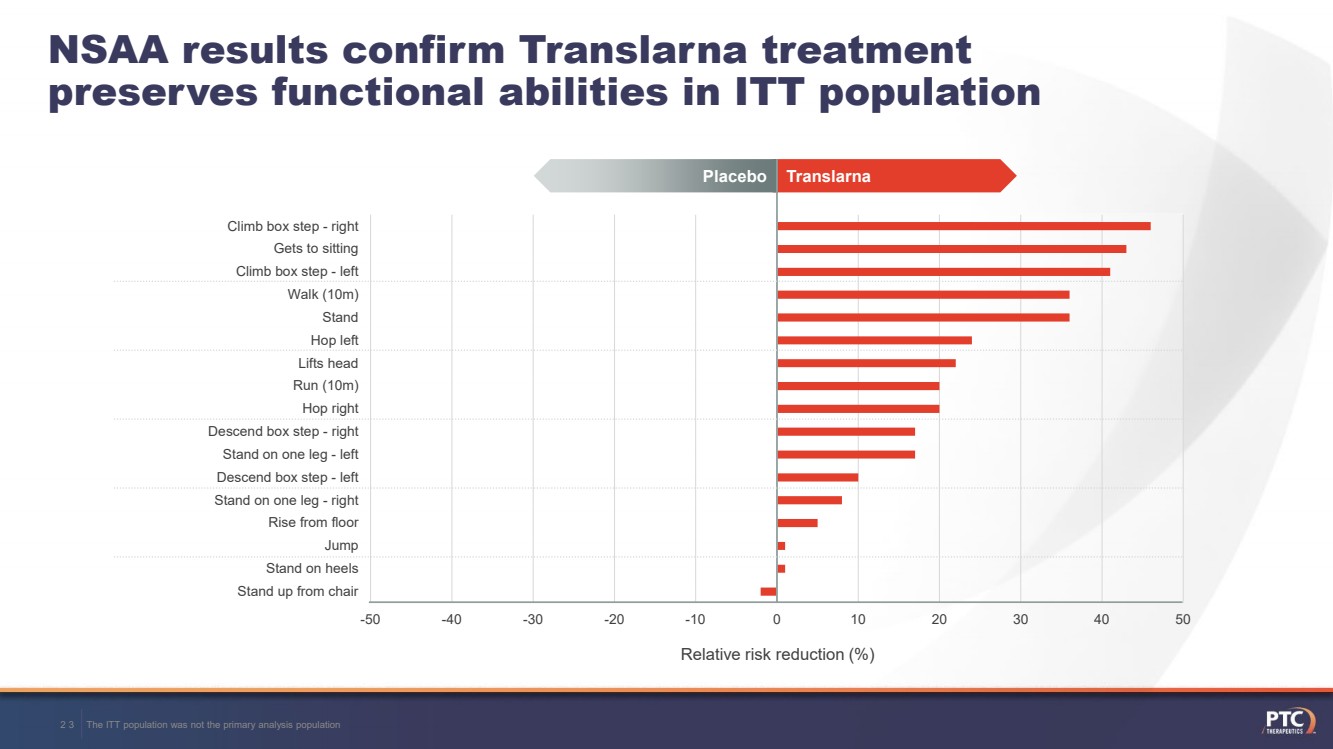
| -50-40-30-20-1001020304050Stand up from chairStand on heelsJumpRise from floorStand on one leg - rightDescend box step - leftStand on one leg - leftDescend box step - rightHop rightRun (10m)Lifts headHop leftStandWalk (10m)Climb box step - leftGets to sittingClimb box step - rightNSAA results confirm Translarnatreatment preserves functional abilities in ITT populationThe ITT population was not the primary analysis population23 Translarna Placebo Relative risk reduction (%) |
| 24Translarnademonstrated consistent treatment effect across multiple clinical endpoints in previous trials1Meta-analysis LS mean difference (SEM)Meters, mean and 95% CITFTs, Seconds, mean and 95% CI Improvement Decline 75 100 50 25 0 -25 -50 -75 -100 8 6 4 2 0 -2 -4 -6 -86MWT210-m walk/run34-stair ascent34-stair descent3 1. Campbell, et al. Journal of Comparative Effectiveness Research. 2020; 9(14), 973–984. Meta-analyses of ataluren randomized controlled trials in nonsense mutation Duchenne muscular dystrophy.2, Primary endpoint. 3, Secondary endpoint. 17.2 m ‒1.1 s ‒1.7 s ‒1.9 s Meta-analysis (007, 020) (N=342)Study 020 ITT (N=228) Study 007 ITT (N=114) |
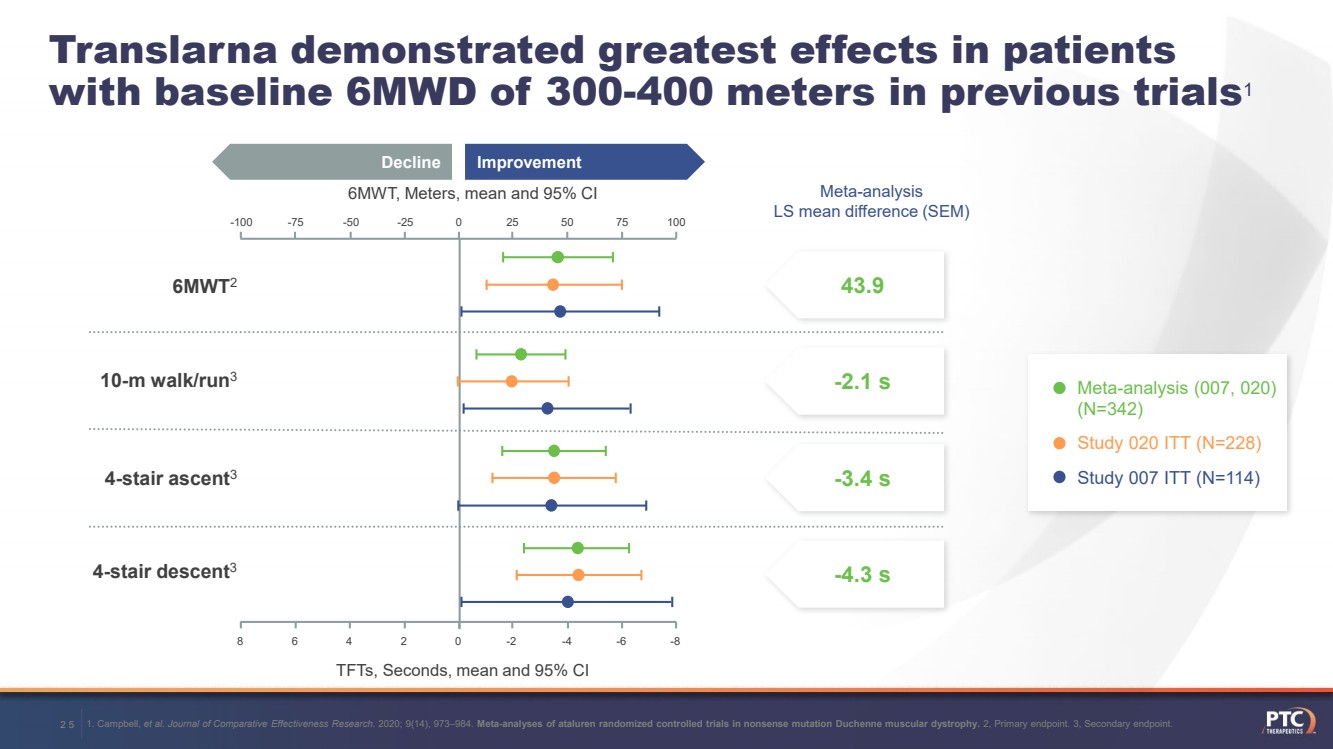
| 25Translarnademonstrated greatest effects in patientswith baseline 6MWD of 300-400metersin previous trials16MWT, Meters, mean and 95% CITFTs, Seconds, mean and 95% CI 43.9 -2.1 s -3.4 s -4.3 sMeta-analysis LS mean difference (SEM) Improvement Decline 75 100 50 25 0 -25 -50 -75 -100 8 6 4 2 0 -2 -4 -6 -86MWT210-m walk/run34-stair ascent34-stair descent3 1. Campbell, et al. Journal of Comparative Effectiveness Research. 2020; 9(14), 973–984. Meta-analyses of ataluren randomized controlled trials in nonsense mutation Duchenne muscular dystrophy.2, Primary endpoint. 3, Secondary endpoint. Meta-analysis (007, 020) (N=342)Study 020 ITT (N=228) Study 007 ITT (N=114) |
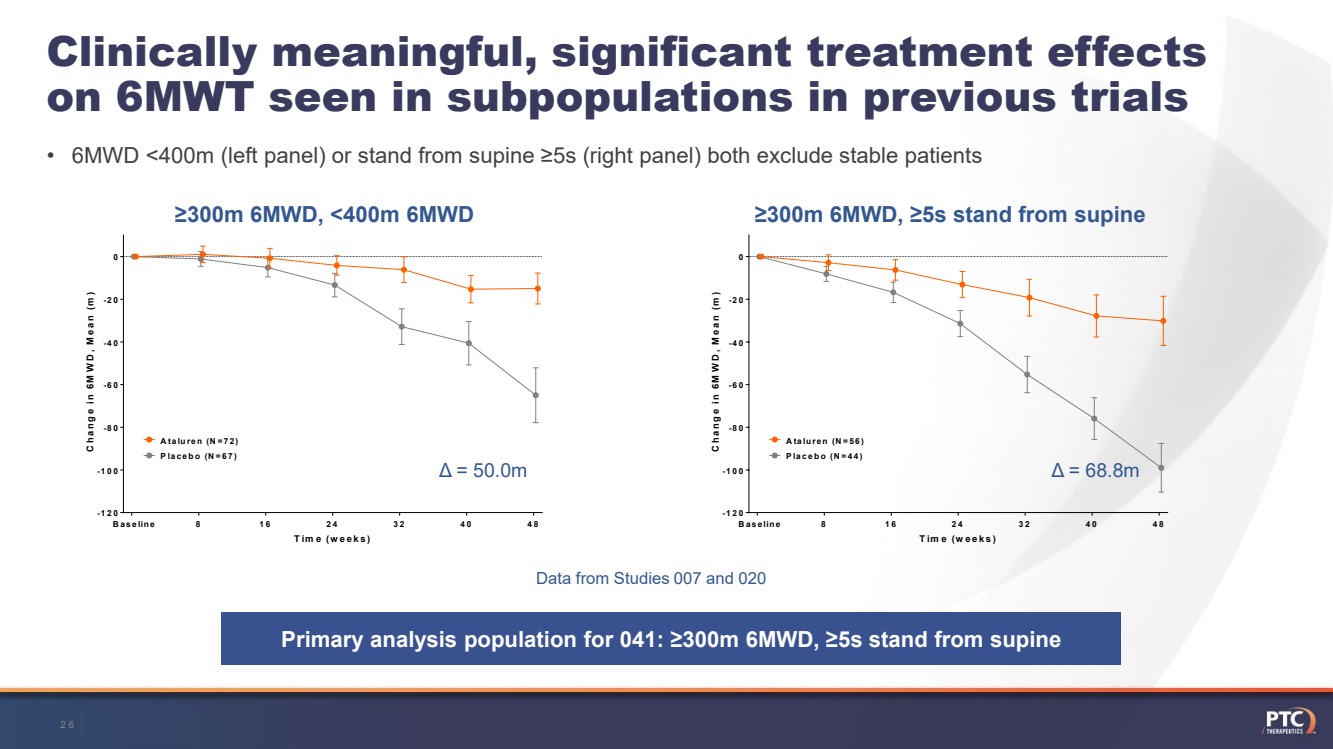
| Clinically meaningful, significant treatment effects on 6MWT seen in subpopulations in previous trials•6MWD <400m (left panel) or stand from supine ≥5s (right panel) both exclude stable patients 81624324048 -120-100-80-60-40 -200 Placebo (N=67) Ataluren (N=72) BaselineTime (weeks)Change in 6MWD, Mean (m) 81624324048 -120-100-80-60-40-200 Placebo (N=44) Ataluren (N=56) BaselineTime (weeks)Change in 6MWD, Mean (m) ≥300m 6MWD, <400m 6MWD≥300m 6MWD, ≥5s stand from supine Primary analysis population for 041: ≥300m 6MWD, ≥5s stand from supineData from Studies 007 and 020Δ= 50.0mΔ= 68.8m26 |
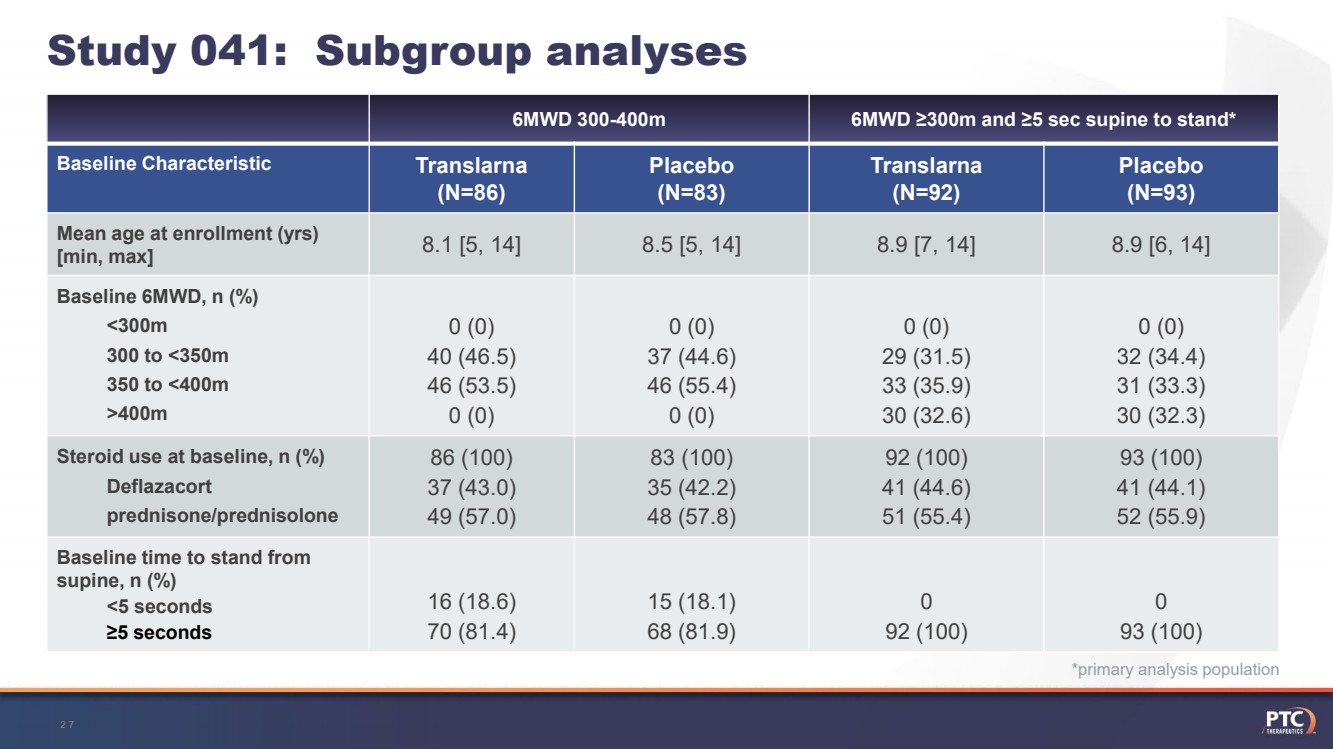
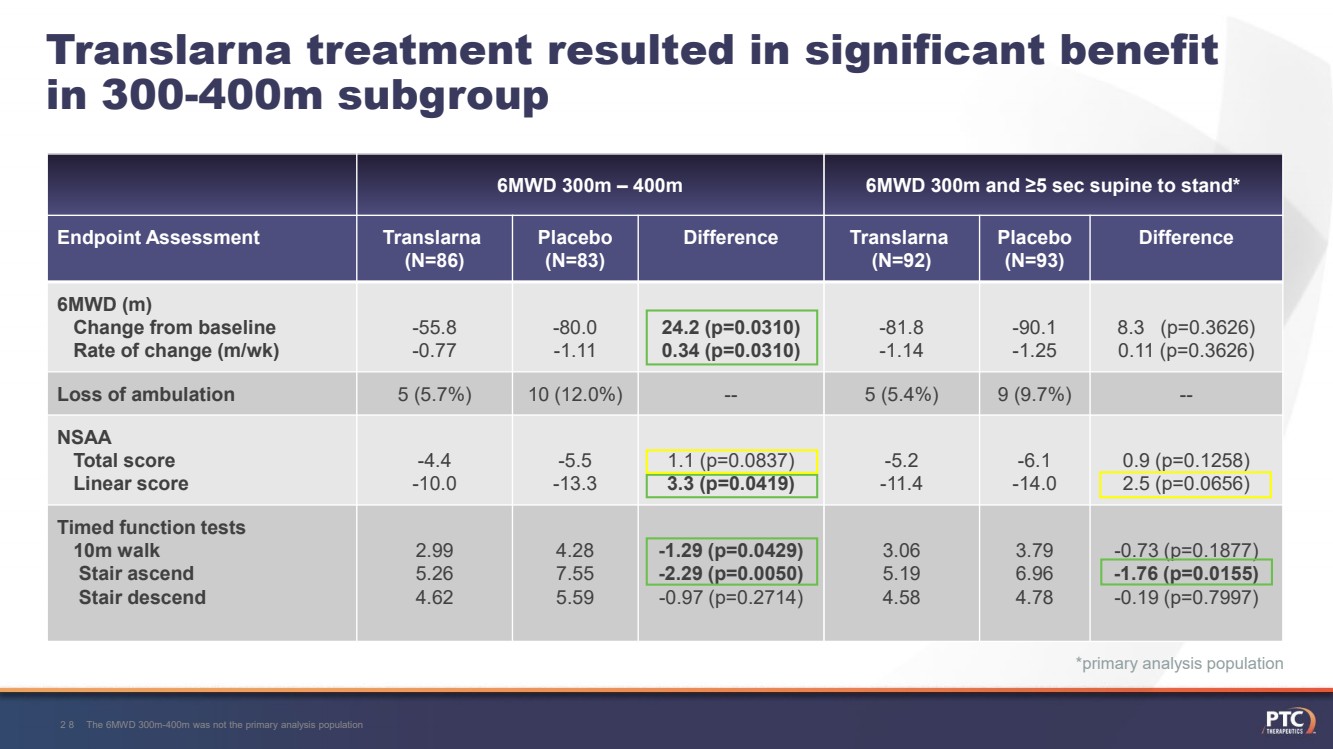
| *primary analysis population 6MWD ≥300m and ≥5 sec supine to stand*Translarna(N=92)Placebo (N=93)8.9 [7, 14]8.9 [6, 14]0 (0)29 (31.5)33 (35.9)30 (32.6)0 (0)32 (34.4)31 (33.3)30 (32.3)92 (100)41 (44.6)51 (55.4)93 (100)41 (44.1) 52 (55.9)092 (100)093 (100)Study 041:Subgroup analyses 27 6MWD 300-400mBaseline CharacteristicTranslarna (N=86)Placebo (N=83)Mean age at enrollment (yrs) [min, max]8.1 [5, 14]8.5 [5, 14]Baseline 6MWD, n (%)<300m300 to <350m350 to <400m >400m0 (0)40 (46.5)46 (53.5)0 (0)0 (0)37 (44.6)46 (55.4)0 (0)Steroid use at baseline, n (%)Deflazacortprednisone/prednisolone86 (100)37 (43.0)49 (57.0)83 (100)35 (42.2)48 (57.8)Baseline time to stand from supine, n (%)<5 seconds≥5 seconds16 (18.6)70 (81.4)15 (18.1)68 (81.9) |
| 6MWD 300m and ≥5 sec supine to stand*Translarna(N=92)Placebo (N=93)Difference -81.8-1.14-90.1 -1.258.3 (p=0.3626)0.11 (p=0.3626)5 (5.4%)9 (9.7%)---5.2-11.4-6.1-14.00.9 (p=0.1258) 2.5 (p=0.0656)3.06 5.19 4.583.79 6.96 4.78-0.73 (p=0.1877)-1.76 (p=0.0155)-0.19 (p=0.7997)*primary analysis population Translarnatreatment resulted in significant benefit in 300-400m subgroup 6MWD 300m –400mEndpoint AssessmentTranslarna(N=86)Placebo (N=83)Difference 6MWD (m)Change from baseline Rate of change(m/wk)-55.8 -0.77-80.0-1.1124.2 (p=0.0310) 0.34 (p=0.0310)Loss of ambulation5 (5.7%)10 (12.0%)--NSAATotal score Linear score-4.4-10.0-5.5-13.31.1 (p=0.0837)3.3 (p=0.0419)Timed function tests10m walkStair ascend Stair descend2.99 5.26 4.624.28 7.55 5.59-1.29 (p=0.0429) -2.29 (p=0.0050)-0.97 (p=0.2714)The 6MWD 300m-400m was not the primary analysis population28 |
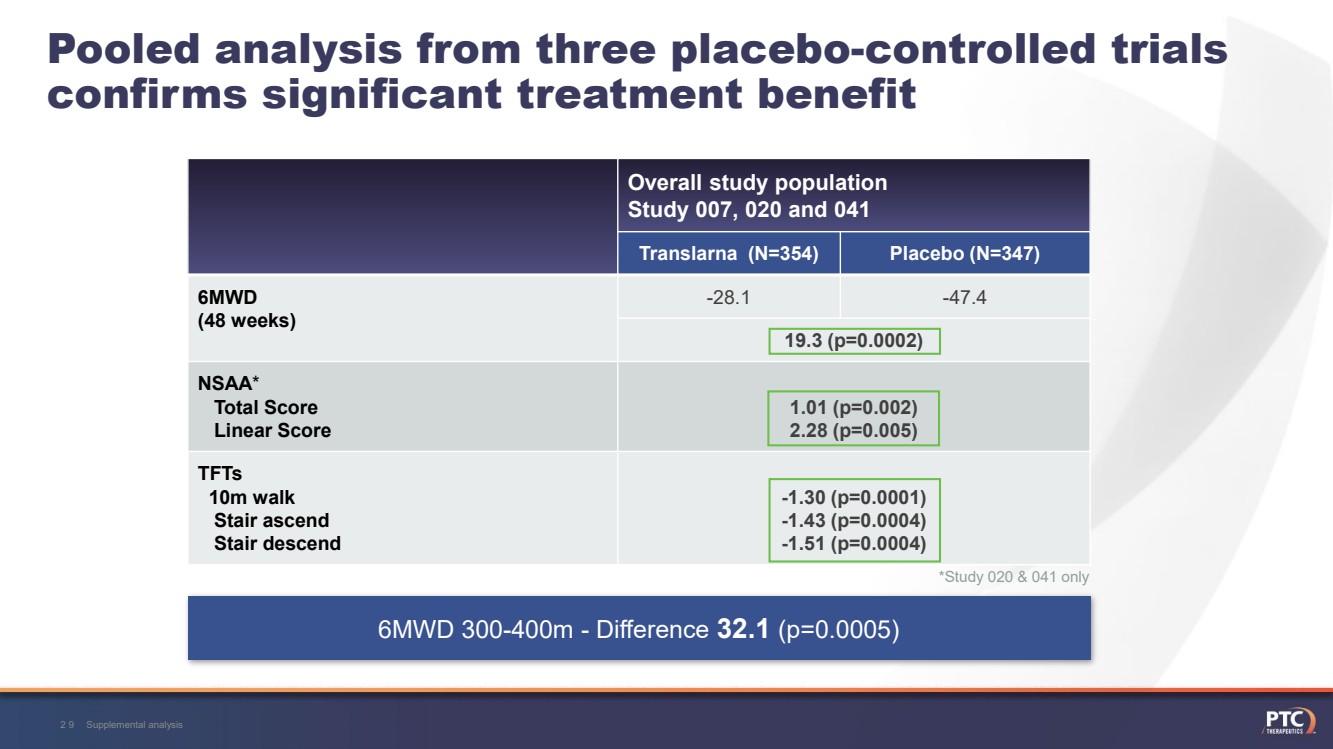
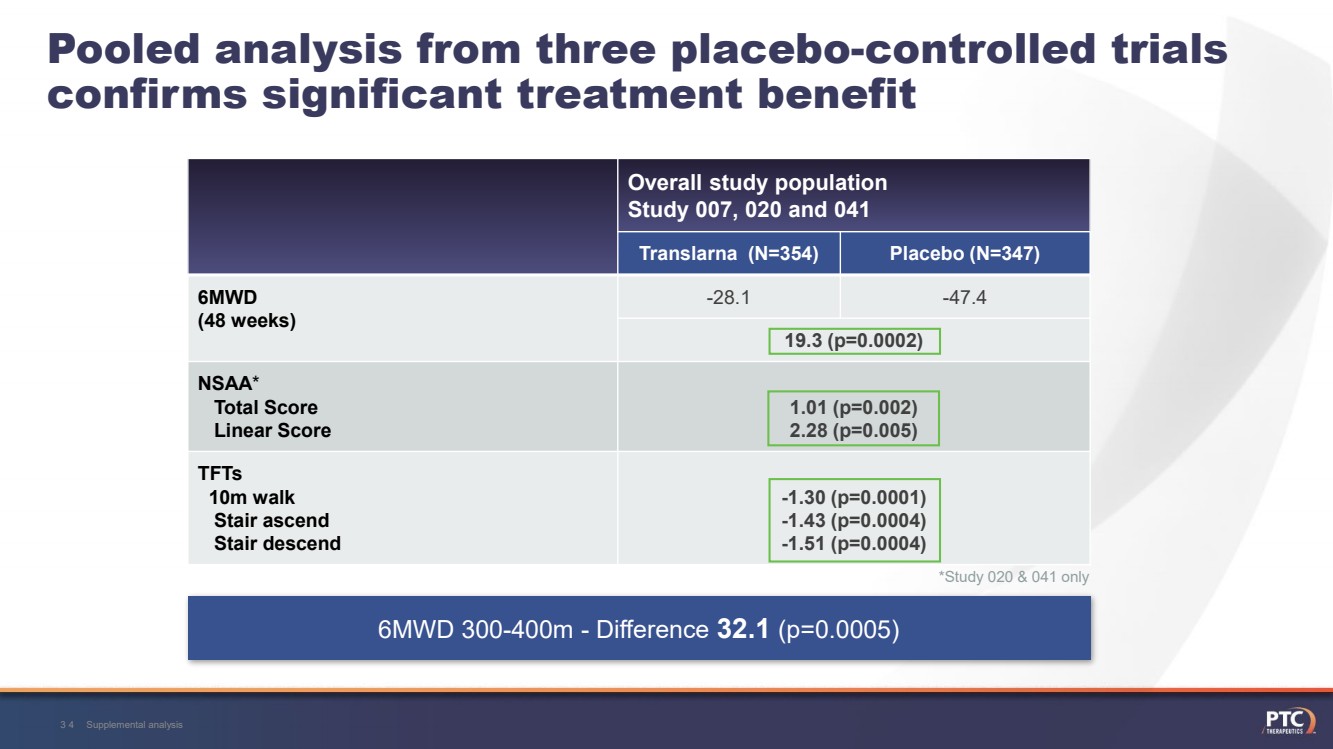
| Pooled analysis from three placebo-controlled trials confirms significant treatment benefit Overall study populationStudy 007, 020 and 041 Translarna (N=354)Placebo (N=347)6MWD (48 weeks)-28.1-47.419.3 (p=0.0002)NSAA*Total Score Linear Score1.01 (p=0.002) 2.28 (p=0.005)TFTs10m walkStair ascend Stair descend -1.30 (p=0.0001) -1.43 (p=0.0004) -1.51 (p=0.0004)Supplemental analysis29 6MWD 300-400m -Difference 32.1 (p=0.0005)*Study 020 & 041 only |
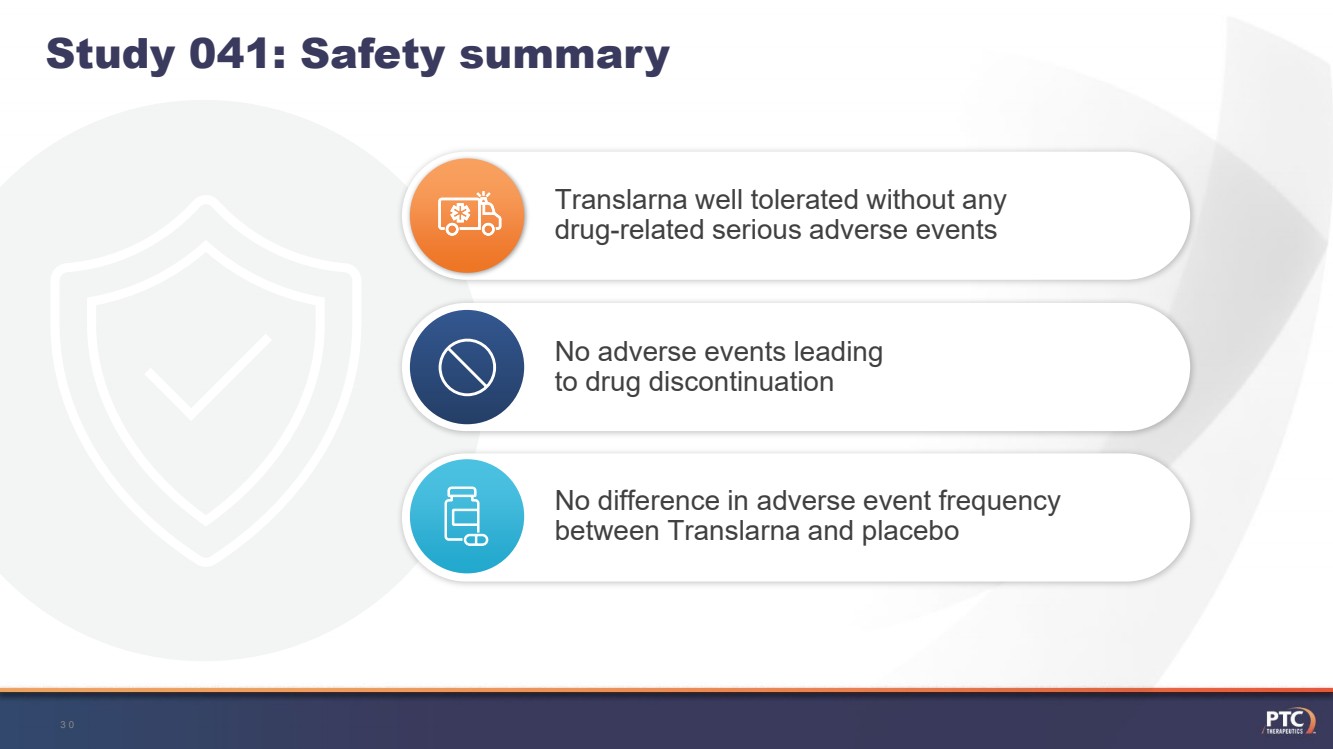
| Study 041: Safety summaryNo difference in adverse event frequency between Translarnaand placebo 30 Translarnawell tolerated without any drug-related serious adverse eventsNo adverse events leading to drug discontinuation |
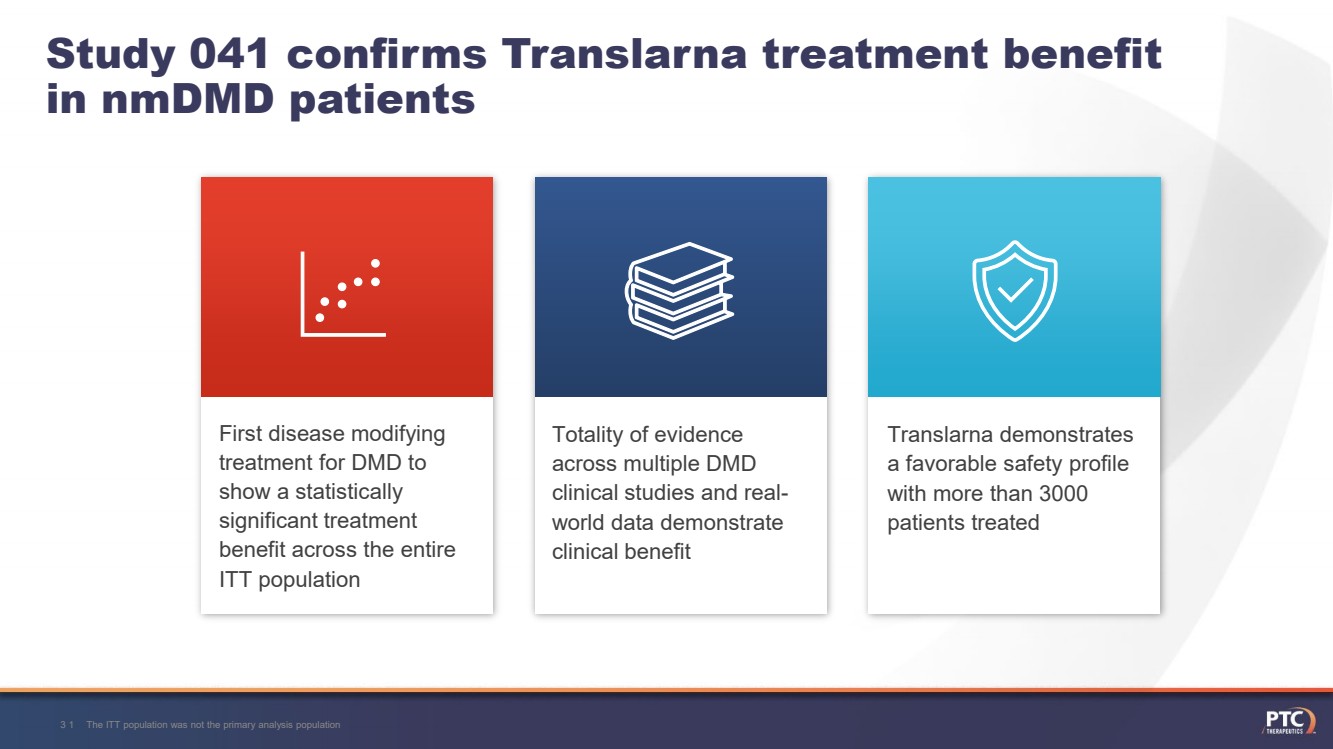
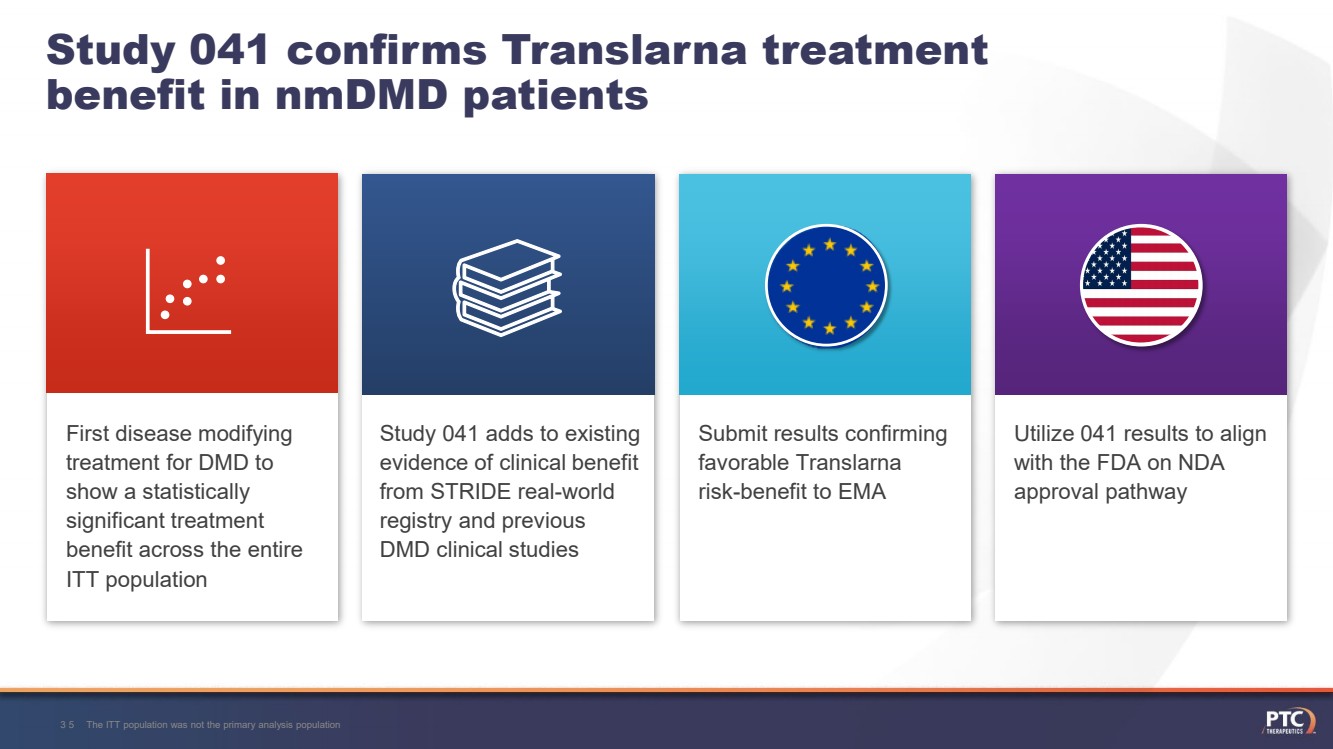
| Study 041 confirms Translarnatreatment benefit in nmDMDpatientsThe ITT population was not the primary analysis population31 Totality of evidence across multiple DMD clinical studies and real-world data demonstrate clinical benefitTranslarnademonstrates a favorable safety profile with more than 3000 patients treated First disease modifying treatment for DMD to show a statistically significant treatment benefit across the entire ITT population |
| Alternative analysis method (ANCOVA) 32 |
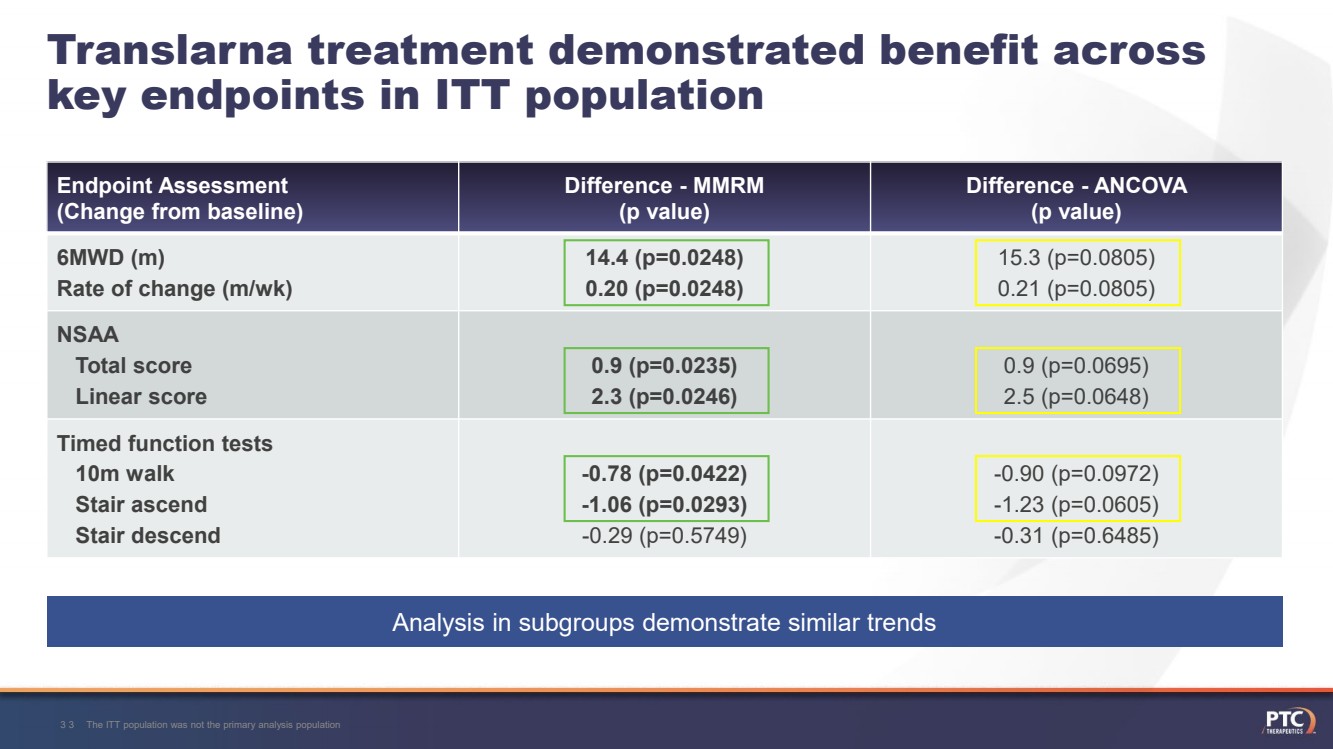
| Translarnatreatment demonstrated benefit across key endpoints in ITT population Endpoint Assessment(Change from baseline)Difference -MMRM (p value)Difference -ANCOVA (p value)6MWD (m)Rate of change (m/wk)14.4 (p=0.0248)0.20 (p=0.0248)15.3 (p=0.0805)0.21 (p=0.0805)NSAATotal score Linear score0.9 (p=0.0235) 2.3 (p=0.0246)0.9 (p=0.0695) 2.5 (p=0.0648)Timed function tests10m walkStair ascendStair descend-0.78 (p=0.0422)-1.06 (p=0.0293)-0.29 (p=0.5749)-0.90 (p=0.0972)-1.23 (p=0.0605)-0.31 (p=0.6485)The ITT population was not the primary analysis population33 Analysis in subgroups demonstrate similar trends |
| Supplemental analysis34Pooled analysis from three placebo-controlled trials confirms significant treatment benefit Overall study populationStudy 007, 020 and 041 Translarna (N=354)Placebo (N=347)6MWD (48 weeks)-28.1-47.419.3 (p=0.0002)NSAA*Total Score Linear Score1.01 (p=0.002) 2.28 (p=0.005)TFTs10m walkStair ascend Stair descend -1.30 (p=0.0001) -1.43 (p=0.0004) -1.51 (p=0.0004) 6MWD 300-400m -Difference 32.1 (p=0.0005)*Study 020 & 041 only |
| First disease modifying treatment for DMD to show a statistically significant treatment benefit across the entire ITT population Study 041 confirms Translarnatreatment benefit in nmDMDpatientsThe ITT population was not the primary analysis population35 Study 041 adds to existing evidence of clinical benefit from STRIDE real-world registry and previous DMD clinical studies Utilize 041 results to align with the FDA on NDA approval pathway Submit results confirming favorable Translarna risk-benefit to EMA |
| Questions? 36 |
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Ecora Resources PLC Announces Transaction in Own Shares
- Vaccinologists Keith Klugman and Shabir Madhi Awarded Sabin’s Prestigious Gold Medal; Infectious Diseases Epidemiologist Nicole Basta Receives Rising Star Award
- Clouds Loom as U.K. Firms Weigh SAP Transition Options
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share