Form 8-K Forma Therapeutics Holdi For: May 26
Exhibit 99.1

Forma Therapeutics Highlights Etavopivat Development Expansion and Introduces New Oncology Program from Research Pipeline at Inaugural Research and Development (R&D) Day
Expanding etavopivat development in 2022 with Phase II trial exploring transfusion burden across sickle cell disease (SCD), thalassemia and myelodysplastic syndromes (MDS)
Phase 1 trial of FT-7051 in mCRPC proceeding with predicted efficacious dose range under evaluation and exploring alternative dosing schedule
FT-3171 (USP1 inhibitor) introduced targeting BRCA mutant tumors with investigational new drug (IND) filing expected in the first half of 2023
Early stage research pipeline focusing on red blood cell (RBC) health and novel mechanisms in rare hematology and targeted oncology indications
Forma well-positioned with $441 million in cash to progress programs in rare hematologic diseases and cancers
WATERTOWN, Mass. – May 26, 2022 – Forma Therapeutics Holdings, Inc. (Nasdaq: FMTX), a clinical-stage biopharmaceutical company focused on sickle cell disease (SCD), prostate cancer and other rare hematologic diseases and cancers, will hold a virtual R&D Day today to provide a comprehensive update on its pipeline and strategic vision.
The R&D event is taking place today at 8:00 a.m. ET. A live webcast will be available in the “News & Investors” section of Forma’s website.
“We are pleased to share insights into Forma’s unique commitment to patients and expansion of etavopivat development into transfusion-dependent populations that have tremendous unmet need across sickle cell disease, thalassemia and MDS,” said Frank Lee, president and chief executive officer of Forma.
“In our early stage research pipeline, we are excited to introduce a USP1 program that targets a novel DNA damage repair pathway relevant to a broad range of tumor types.” said David Cook, chief scientific officer of Forma. “We are also capitalizing on our knowledge of the emerging science of red blood cell health by exploring areas beyond hematologic disease.”
Etavopivat (oral PKR activator) Program in SCD, thalassemia and MDS:
In addition to ongoing enrollment in the Phase II/III Hibiscus Study in SCD, Forma will outline ongoing and anticipated future development plans:
| • | A Phase II trial is enrolling patients in three cohorts: SCD with chronic transfusion, and both transfusion-dependent and non-transfusion-dependent thalassemia. Etavopivat has the potential to address RBC health, hemolytic anemia and/or ineffective erythropoiesis in these populations, leading to reduction of transfusion burden and associated iron overload and improvement of anemia. |
| • | A Phase II trial in lower risk MDS is planned to commence in the second half of 2022. Dr. Michael Savona of Vanderbilt University will review the significant unmet need in lower-risk MDS and the potential for etavopivat to provide a well-tolerated oral treatment that may be able to improve the ability of bone marrow to produce healthier RBCs. |
| • | Analyses from the completed Phase I trial in SCD show benefit in both pain events reported in the trial and vaso-occlusive crises (VOC’s) occurring on treatment. |
| • | Enrollment in the Phase II/III Hibiscus Study in SCD is on track for the first interim analysis (IA1) in late 2022. |
FT-7051 (oral CBP/p300 inhibitor) in prostate cancer:
Forma’s Phase I trial continues to enroll men with metastatic castration-resistant prostate cancer (mCRPC).
| • | As of May 12, 2022, 25 patients have enrolled in the Phase I dose escalation trial, assessing the predicted efficacious exposure range supported by target engagement. |
| • | The trial population is heavily pre-treated, with a high AR-v7 positivity rate and mutation burden. |
| • | Future trial enrollment to include less heavily pre-treated patients and alternative dosing schedule to address adverse events, with updated results expected in the first half of 2023. |
Research Pipeline/RBC Health:
Forma’s ongoing research efforts are focused on novel mechanisms of action in oncology and expansion in red blood cell health and hematologic diseases:
| • | Forma’s research pipeline is led by the USP1 program (FT-3171), targeting a novel DNA damage repair pathway with potential to address multiple tumor types in both poly ADP ribose polymerase inhibitor (PARPi)-sensitive and resistant settings. |
| • | FT-3171 IND filing is expected in the first half of 2023. |
| • | Ongoing pre-clinical research is targeting areas of expansion for RBC health, novel mechanisms that may be complementary to etavopivat in rare hematology, and targeted oncology. |
About Forma Therapeutics
Forma Therapeutics is a clinical-stage biopharmaceutical company focused on the research, development and commercialization of novel therapeutics to transform the lives of patients with rare hematologic diseases and cancers. Our R&D engine combines deep biology insight, chemistry expertise and clinical development capabilities to create drug candidates with differentiated mechanisms of action focused on indications with high unmet need. Our work has generated a broad proprietary portfolio of programs with the potential to provide profound patient benefit. For more information, please visit www.FormaTherapeutics.com or follow us on Twitter @FORMAInc and LinkedIn.
Forward-looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, without limitation, express or implied statements regarding the company’s beliefs and expectations regarding its: business plans and objectives; future plans for etavopivat, FT-7051 and FT-3171, including expectations regarding potential development expansion plans as well as regulatory filings, enrollment, timing, success and data announcements of planned and ongoing clinical and pre-clinical trials; therapeutic potential, clinical benefits, mechanisms of action and safety of our product candidates; plans for the USP1 program (FT-3171) and other research pipeline expansion efforts; upcoming milestones and planned additional trials for the company’s product candidates; growth as a company; upcoming presentations of our R&D programs, including the introduction of a new molecule and related studies; uses and need of capital, expenses and other financial results currently or in the future. The words “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “target” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words.
Any forward-looking statements in this press release are based on management’s current expectations and beliefs and are subject to a number of risks, uncertainties and important factors that may cause actual events or results to differ materially from those expressed or implied by any forward-looking statements contained in this press release, including, without limitation, those risks and uncertainties associated with the following: the impact of the COVID-19 pandemic on the company’s business, operations, supply chain, patient enrollment and retention, clinical trials, strategy, goals and anticipated milestones, as well as global economies and financial markets; the therapeutic potential of our product candidates and the timing and completion of our clinical trials and related data analyses; positive results from a clinical study may not necessarily be predictive of the results of future or ongoing clinical studies; any one or more of our product candidates may not be successfully developed and commercialized; regulatory developments in the United States and foreign countries; our ability to protect and maintain our intellectual property position; and our ability to fund operations; as well as those risks and uncertainties set forth more fully under the caption “Risk Factors” in our most recent annual report on Form 10-Q filed with the United States Securities and Exchange Commission (SEC) and subsequent filings with the SEC. We disclaim any obligation to publicly update or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements. Any forward-looking statements contained in this press release represent our views only as of the date hereof and should not be relied upon as representing our views as of any subsequent date.
Media Contact:
Caitlin Hunt, +1 781-985-5967
Porter Novelli
Investor Contact:
Mario Corso, +1 781-366-5726
Forma Therapeutics
SOURCE: Forma Therapeutics Holdings, Inc.
###

Research and Development Day May 26, 2022 8:00–10:00 a.m. EDT Exhibit 99.2

Legal Disclaimer This Presentation contains forward-looking statements and information of Forma Therapeutics Holdings, Inc. (“we,” “us,” “our,” “Forma” or the “Company”) within the meaning of the Private Securities Litigation Reform Act of 1995 relating to our current and future prospects and our operations and financial results, which are based on currently available information. All statements other than statements of historical facts contained in this Presentation, including express or implied statements regarding our strategy, future financial condition, future operations, projected costs, prospects, plans, objectives of management and expected market growth, are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as ‘‘aim,’’ ‘‘anticipate,’’ ‘‘assume,’’ ‘‘believe,’’ ‘‘contemplate,’’ ‘‘continue,’’ ‘‘could,’’ ‘‘design,’’ ‘‘due,’’ ‘‘estimate,’’ ‘‘expect,’’ ‘‘goal,’’ ‘‘intend,’’ ‘‘may,’’ ‘‘objective,’’ ‘‘plan,’’ ‘‘predict,’’ ‘‘positioned,’’ ‘‘potential,’’ ‘‘seek,’’ ‘‘should,’’ ‘‘target,’’ ‘‘will,’’ ‘‘would’’ and other similar expressions that are predictions of or indicate future events and future trends, or the negative of these terms or other comparable terminology. These forward looking statements include statements about the clinical and preclinical trials of our product candidates etavopivat, FT-7051 and USP1, including expectations regarding timing, success and data announcements of our ongoing and planned pre-clinical and clinical trials; our plans for the USP1 program; therapeutic potential, clinical benefits, mechanisms of action, tolerability and safety of our product candidates; planned regulatory submissions; plans for expanding our pipeline with investigational new drug applications; upcoming milestones and planned additional trials and indications for our product candidates; presentation of additional data at upcoming scientific conferences, and other preclinical data and potential data publications; and development and strategic plans for our business. And product candidates We may not actually achieve the plans, intentions or expectations disclosed in our forward looking statements, and you should not place undue reliance on our forward looking statements. For a discussion of these and other risks and uncertainties, and other important factors, any of which could cause our actual results to differ from those contained in the forward-looking statements, see the section entitled “Risk Factors” in our most recent quarterly report on Form 10-Q filed with the Securities and Exchange Commission, as well as discussions of potential risks, uncertainties, and other important factors in our other filings with the Securities and Exchange Commission. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. We undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, the occurrence of certain events or otherwise. As a result of these risks and others, actual results could vary significantly from those anticipated in this Presentation, and our financial condition and results of operations could be materially adversely affected. This Presentation contains trademarks, trade names and service marks of other companies, which are the property of their respective owners. Certain information contained in this Presentation and statements made orally during this Presentation relate to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While the Company believes these third-party studies, publications, surveys and other data to be reliable as of the date of the Presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, no independent sources has evaluated the reasonableness or accuracy of the Company’s internal estimates or research and no reliance should be made on any information or statements made in this Presentation relating to or based on such internal estimates and research.

Today’s Agenda Opening Remarks Frank Lee, President and Chief Executive Officer Expanding Etavopivat Development Patient perspective Development program overview Phase 1 trial in SCD Unmet need in bone marrow failure syndromes Ify Osunkwo, M.D., Chief Patient Officer Patrick Kelly, M.D., Chief Medical Officer Cameron Trenor, M.D., Executive Director, Clinical Development Michael Savona, M.D., Vanderbilt University Emerging Research and Oncology Research pipeline and RBC health FT-7051 in prostate cancer Introduction to FT-3171, USP1 inhibitor David Cook, Ph.D., Chief Scientific Officer Sylvie Guichard, Ph.D., Program Lead FT-7051, Executive Director Translational Science Maria D. Ribadeneira, Ph.D., Program Lead FT-3171, Executive Director, DMPK and Toxicology Closing Remarks Frank Lee Q&A

Frank Lee President, Chief Executive Officer, Director Opening Remarks

The Science of Giving a Damn
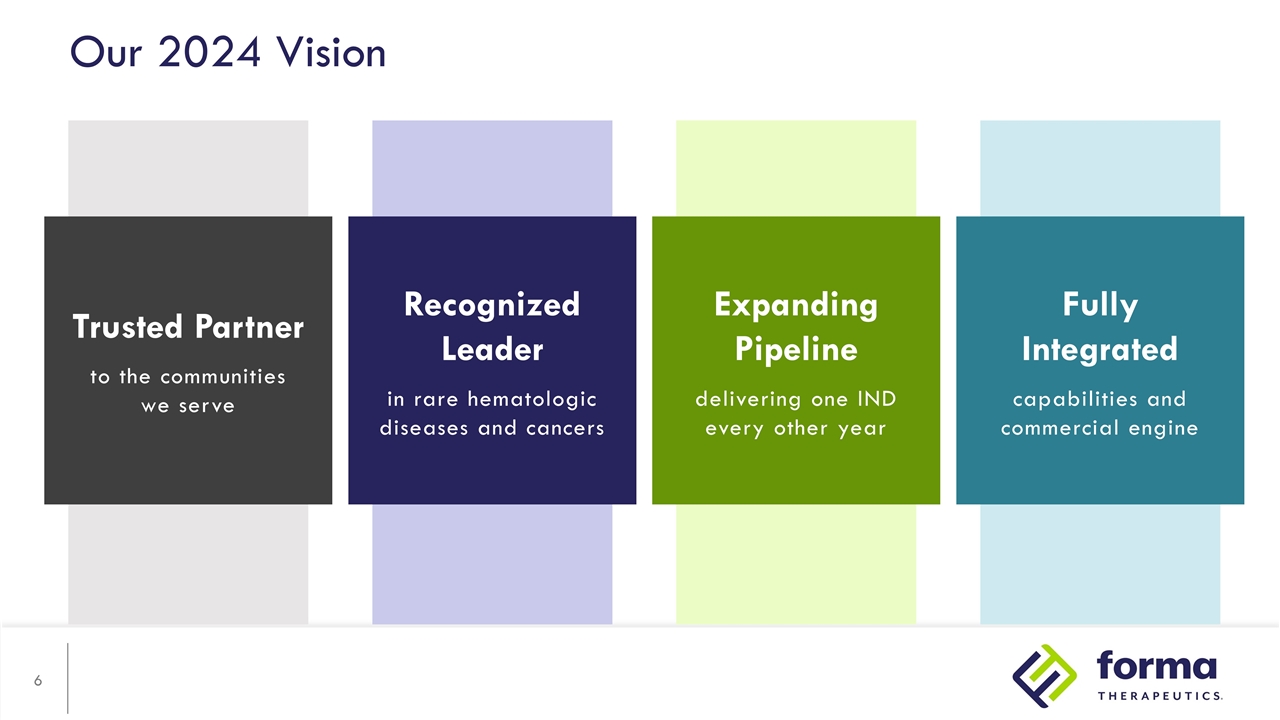
Expanding Pipeline delivering one IND every other year Our 2024 Vision Trusted Partner to the communities we serve Recognized Leader in rare hematologic diseases and cancers Fully Integrated capabilities and commercial engine
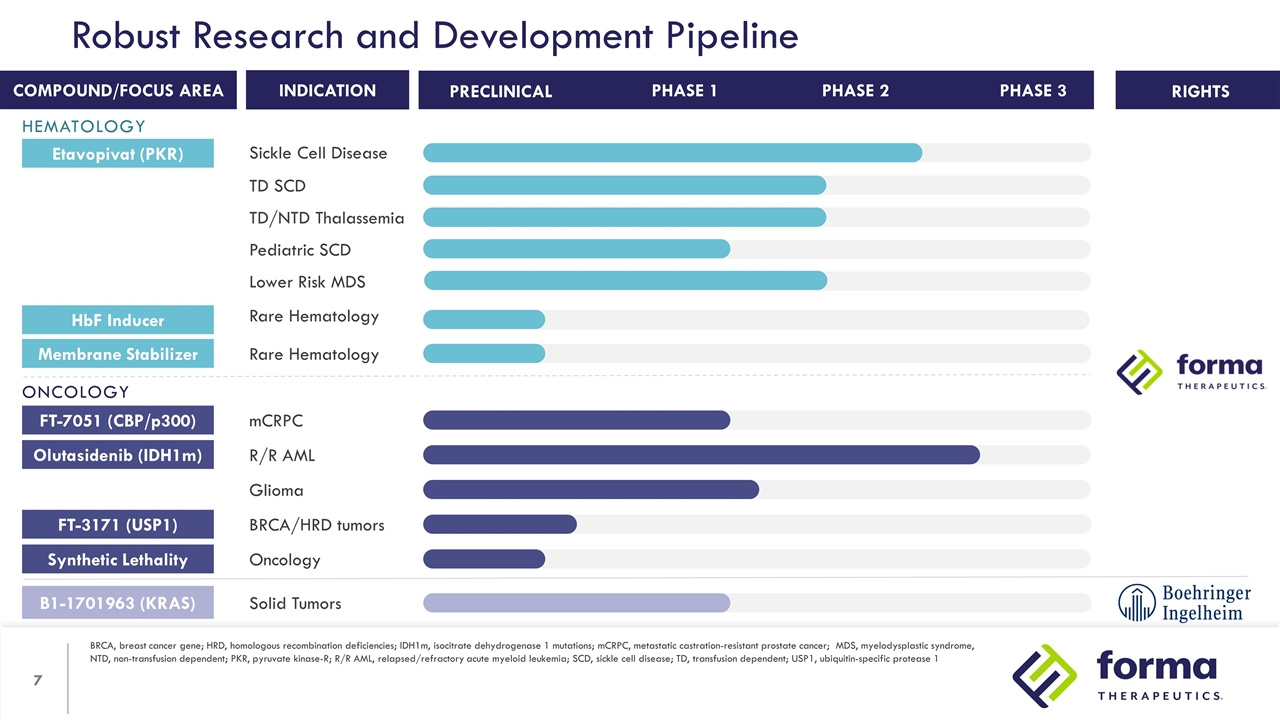
Robust Research and Development Pipeline BRCA, breast cancer gene; HRD, homologous recombination deficiencies; IDH1m, isocitrate dehydrogenase 1 mutations; mCRPC, metastatic castration-resistant prostate cancer; MDS, myelodysplastic syndrome, NTD, non-transfusion dependent; PKR, pyruvate kinase-R; R/R AML, relapsed/refractory acute myeloid leukemia; SCD, sickle cell disease; TD, transfusion dependent; USP1, ubiquitin-specific protease 1 RIGHTS INDICATION COMPOUND/FOCUS AREA PHASE 1 PHASE 2 PRECLINICAL PHASE 3 Etavopivat (PKR) Sickle Cell Disease TD SCD TD/NTD Thalassemia Lower Risk MDS Pediatric SCD HbF Inducer Rare Hematology Membrane Stabilizer Rare Hematology FT-7051 (CBP/p300) mCRPC Olutasidenib (IDH1m) R/R AML Glioma B1-1701963 (KRAS) Solid Tumors FT-3171 (USP1) BRCA/HRD tumors Synthetic Lethality Oncology HEMATOLOGY ONCOLOGY

Etavopivat: Exploring impact on transfusion burden in SCD, Thalassemia and Lower-risk MDS Additional analysis of pain events from Phase 1 study provides further support for potential to demonstrate VOC benefit First look at research pipeline with FT-3171 (USP1) and focus on Red Blood Cell health FT-7051 Trial update: Predicted efficacious dose range under evaluation with new dosing regimen and less heavily pre-treated patients Strong cash position: Actively managing expenses to extend runway Today's Highlights

Ifeyinwa (Ify) Osunkwo, M.D., M.P.H. Senior Vice President, Chief Patient Officer What's It Like To Be A Patient With...


STROKE


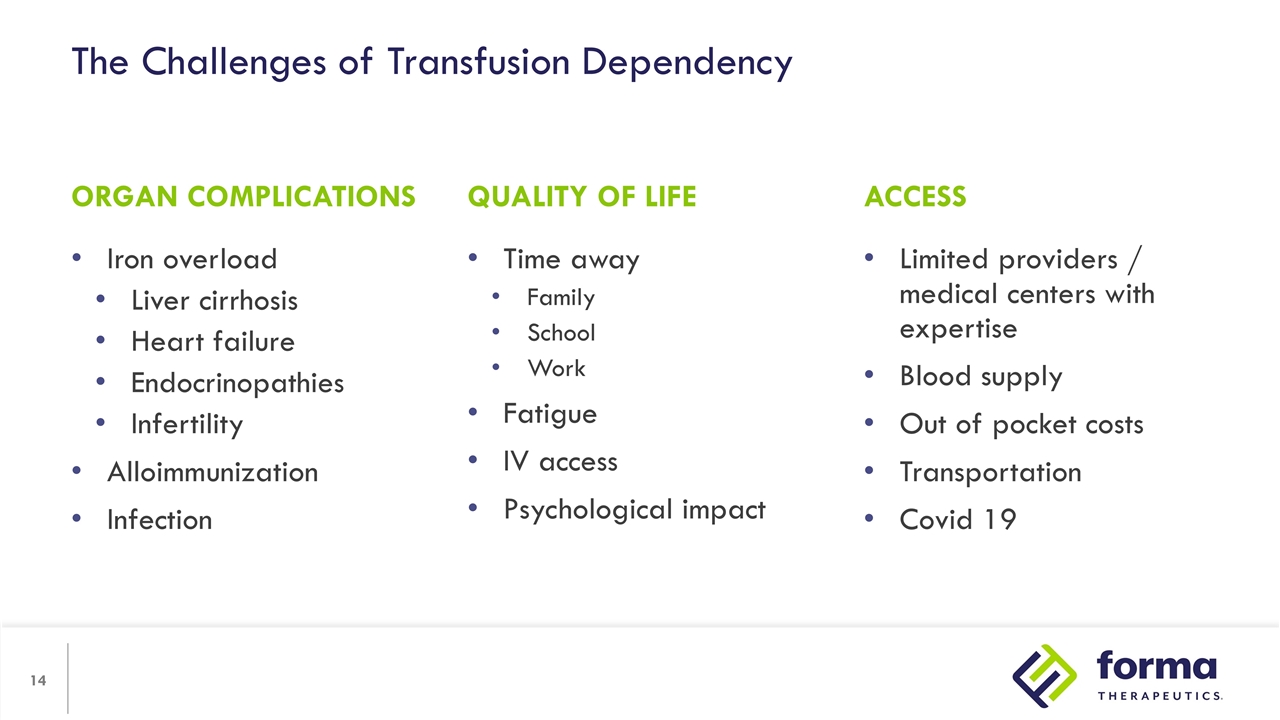
The Challenges of Transfusion Dependency Iron overload Liver cirrhosis Heart failure Endocrinopathies Infertility Alloimmunization Infection Limited providers / medical centers with expertise Blood supply Out of pocket costs Transportation Covid 19 ORGAN COMPLICATIONS ACCESS QUALITY OF LIFE Time away Family School Work Fatigue IV access Psychological impact
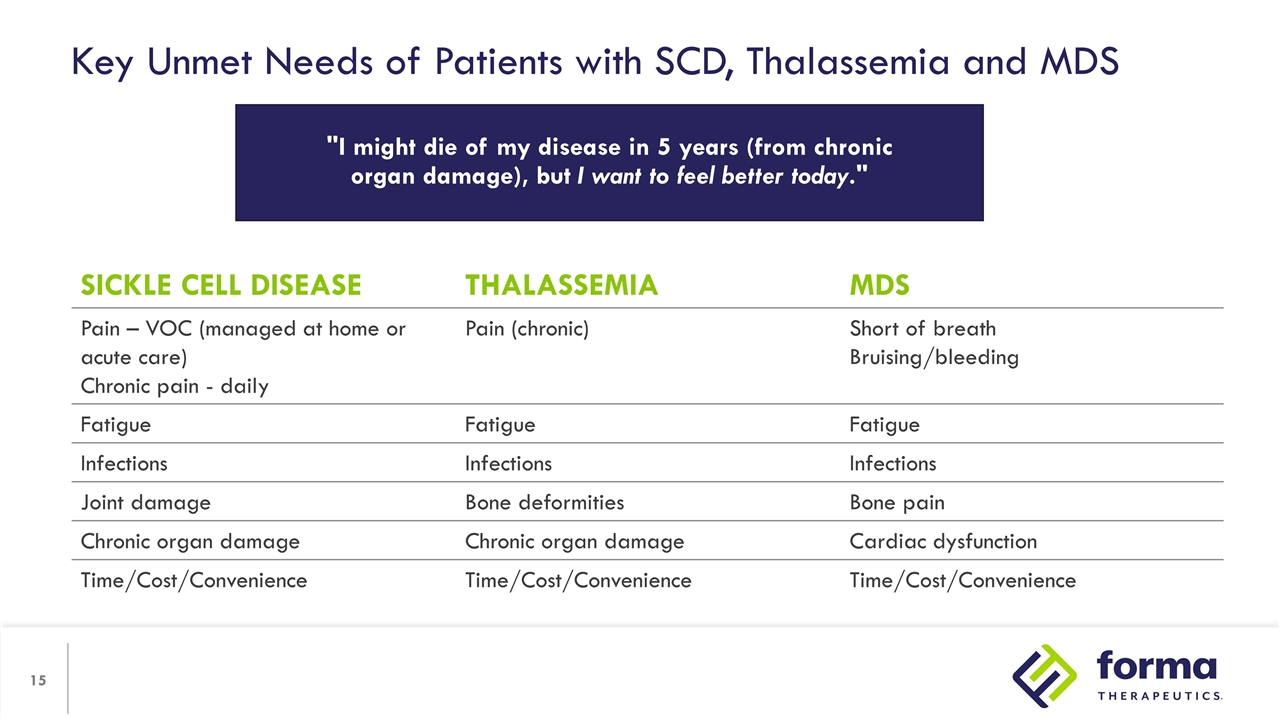
Key Unmet Needs of Patients with SCD, Thalassemia and MDS SICKLE CELL DISEASE THALASSEMIA MDS Pain – VOC (managed at home or acute care) Chronic pain - daily Pain (chronic) Short of breath Bruising/bleeding Fatigue Fatigue Fatigue Infections Infections Infections Joint damage Bone deformities Bone pain Chronic organ damage Chronic organ damage Cardiac dysfunction Time/Cost/Convenience Time/Cost/Convenience Time/Cost/Convenience "I might die of my disease in 5 years (from chronic organ damage), but I want to feel better today."


Joyce and daughter Aaron, 26, living with SCD Etavopivat

Patrick Kelly, M.D. Senior Vice President, Chief Medical Officer Etavopivat Development

Etavopivat Development Highlights Successfully creating a global development program during a global pandemic Phase 1 Study in SCD complete: focus on the dataset supporting etavopivat's potential for VOC reduction Phase 2/3 Hibiscus Study on track for interim analysis 1 (IA1) in late 2022 Phase 2 trials expanding the potential of etavopivat to transfused populations in SCD, thalassemia, MDS
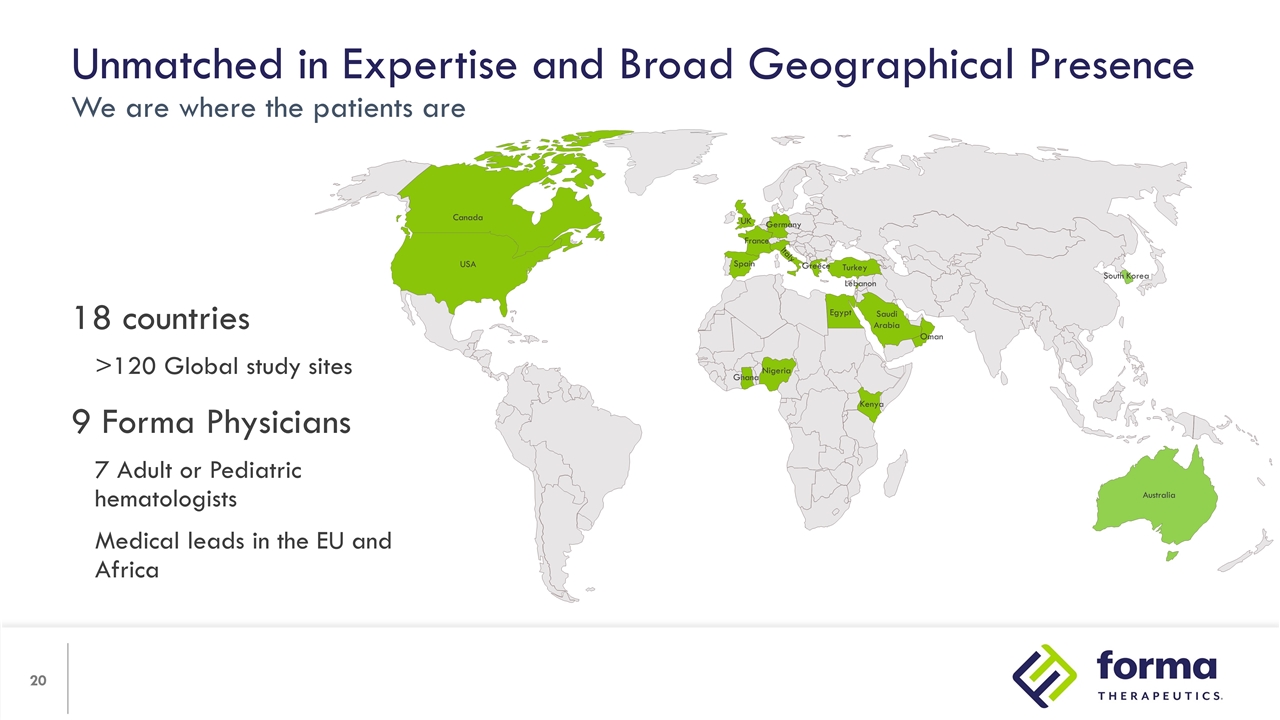
Unmatched in Expertise and Broad Geographical Presence We are where the patients are 18 countries >120 Global study sites 9 Forma Physicians 7 Adult or Pediatric hematologists Medical leads in the EU and Africa Ghana Nigeria Kenya Egypt Saudi Arabia Oman Turkey Greece Germany France Spain Italy UK Lebanon Canada USA Australia South Korea
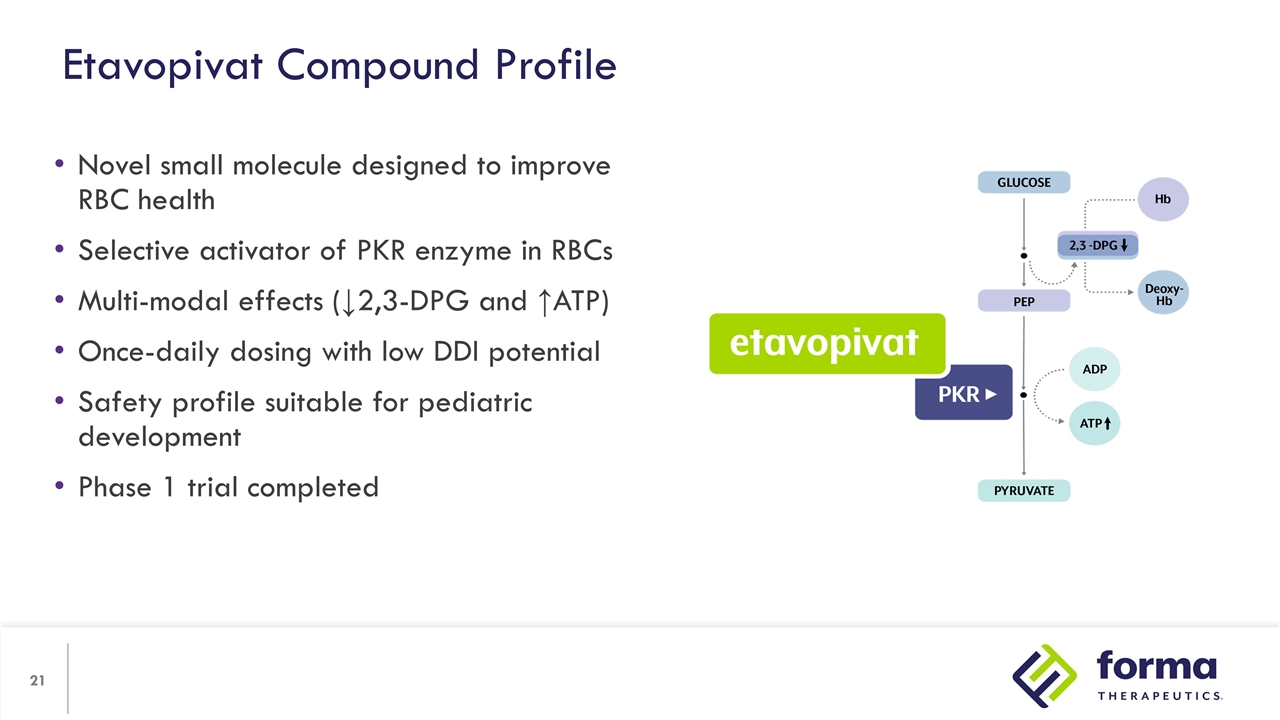
Novel small molecule designed to improve RBC health Selective activator of PKR enzyme in RBCs Multi-modal effects (↓2,3-DPG and ↑ATP) Once-daily dosing with low DDI potential Safety profile suitable for pediatric development Phase 1 trial completed Etavopivat Compound Profile

Cameron Trenor, M.D. Executive Director, Clinical Development Etavopivat Phase 1 Trial in Sickle Cell Disease
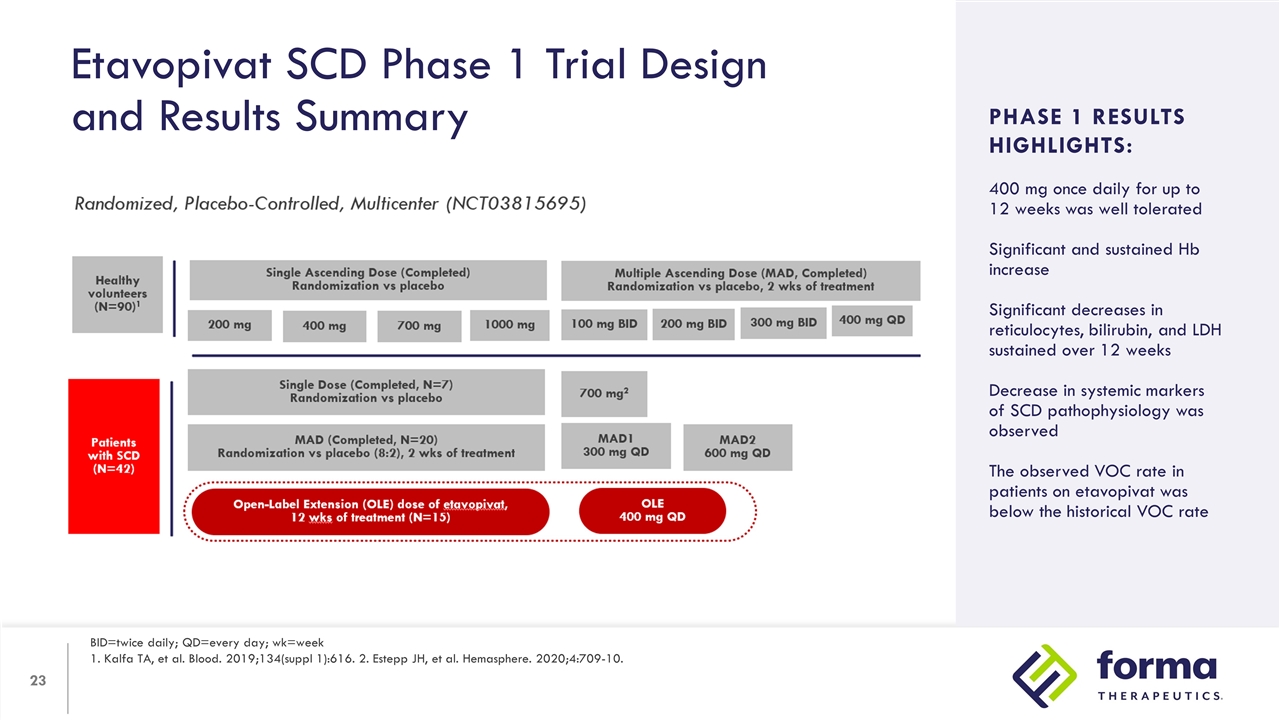
Etavopivat SCD Phase 1 Trial Design and Results Summary BID=twice daily; QD=every day; wk=week 1. Kalfa TA, et al. Blood. 2019;134(suppl 1):616. 2. Estepp JH, et al. Hemasphere. 2020;4:709-10. Study Design: Arms/dosing Objectives Inclusion criteria PHASE 1 RESULTS HIGHLIGHTS: 400 mg once daily for up to 12 weeks was well tolerated Significant and sustained Hb increase Significant decreases in reticulocytes, bilirubin, and LDH sustained over 12 weeks Decrease in systemic markers of SCD pathophysiology was observed The observed VOC rate in patients on etavopivat was below the historical VOC rate
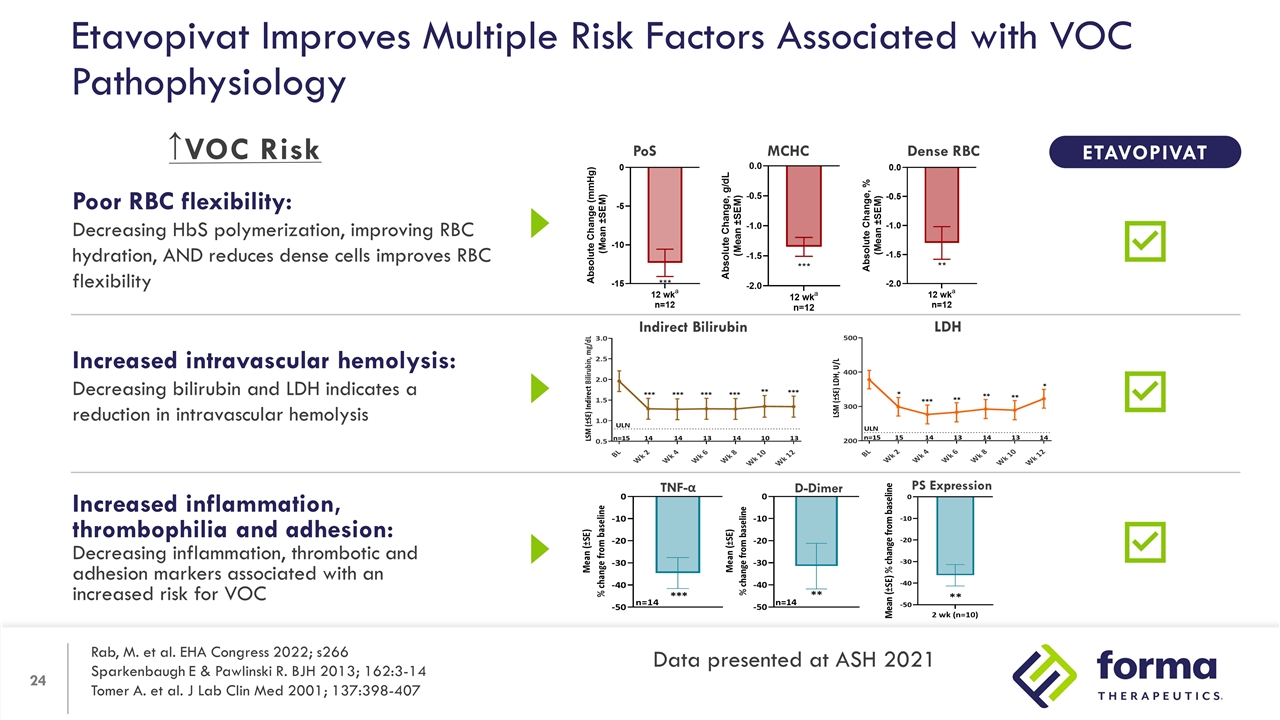
Increased inflammation, thrombophilia and adhesion: Decreasing inflammation, thrombotic and adhesion markers associated with an increased risk for VOC Poor RBC flexibility: Decreasing HbS polymerization, improving RBC hydration, AND reduces dense cells improves RBC flexibility Increased intravascular hemolysis: Decreasing bilirubin and LDH indicates a reduction in intravascular hemolysis Etavopivat Improves Multiple Risk Factors Associated with VOC Pathophysiology TNF-α D-Dimer PS Expression PoS Dense RBC MCHC ETAVOPIVAT Indirect Bilirubin LDH ↑ Rab, M. et al. EHA Congress 2022; s266 Sparkenbaugh E & Pawlinski R. BJH 2013; 162:3-14 Tomer A. et al. J Lab Clin Med 2001; 137:398-407 Data presented at ASH 2021 VOC Risk
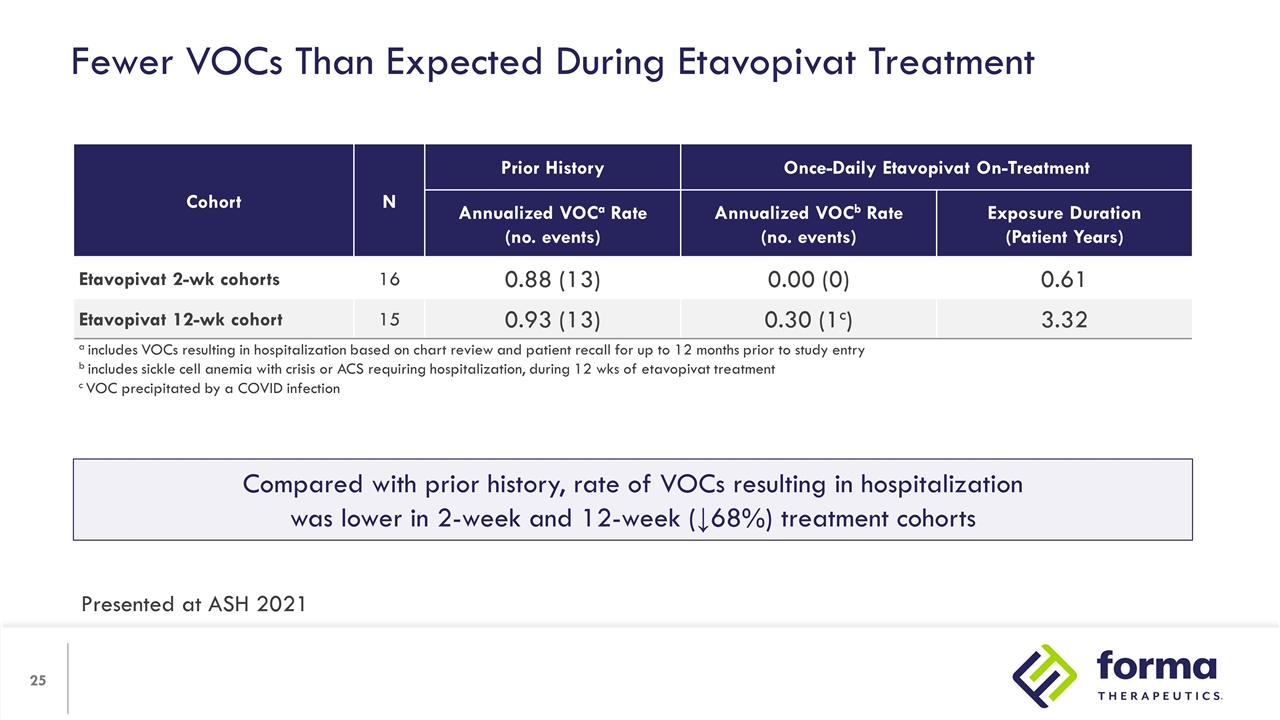
Fewer VOCs Than Expected During Etavopivat Treatment Cohort N Prior History Once-Daily Etavopivat On-Treatment N Annualized VOCa Rate (no. events) Annualized VOCb Rate (no. events) Exposure Duration (Patient Years) Etavopivat 2-wk cohorts 16 0.88 (13) 0.00 (0) 0.61 Etavopivat 12-wk cohort 15 0.93 (13) 0.30 (1c) 3.32 a includes VOCs resulting in hospitalization based on chart review and patient recall for up to 12 months prior to study entry b includes sickle cell anemia with crisis or ACS requiring hospitalization, during 12 wks of etavopivat treatment c VOC precipitated by a COVID infection Compared with prior history, rate of VOCs resulting in hospitalization was lower in 2-week and 12-week (↓68%) treatment cohorts Presented at ASH 2021
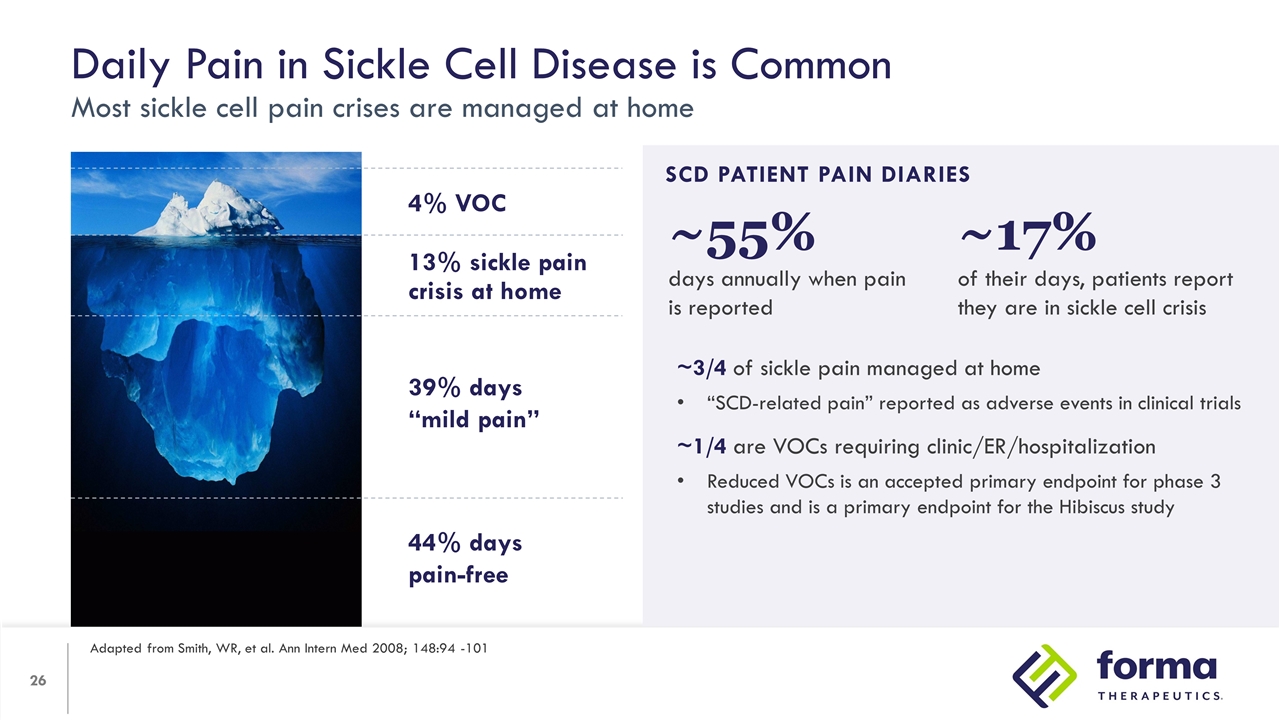
Daily Pain in Sickle Cell Disease is Common Most sickle cell pain crises are managed at home Adapted from Smith, WR, et al. Ann Intern Med 2008; 148:94 -101 39% Days “Mild Pain” SCD PATIENT PAIN DIARIES ~3/4 of sickle pain managed at home “SCD-related pain” reported as adverse events in clinical trials ~1/4 are VOCs requiring clinic/ER/hospitalization Reduced VOCs is an accepted primary endpoint for phase 3 studies and is a primary endpoint for the Hibiscus study 13% sickle pain crisis at home 4% VOC 4% 39% days “mild pain” 44% days pain-free ~17% of their days, patients report they are in sickle cell crisis ~55% days annually when pain is reported
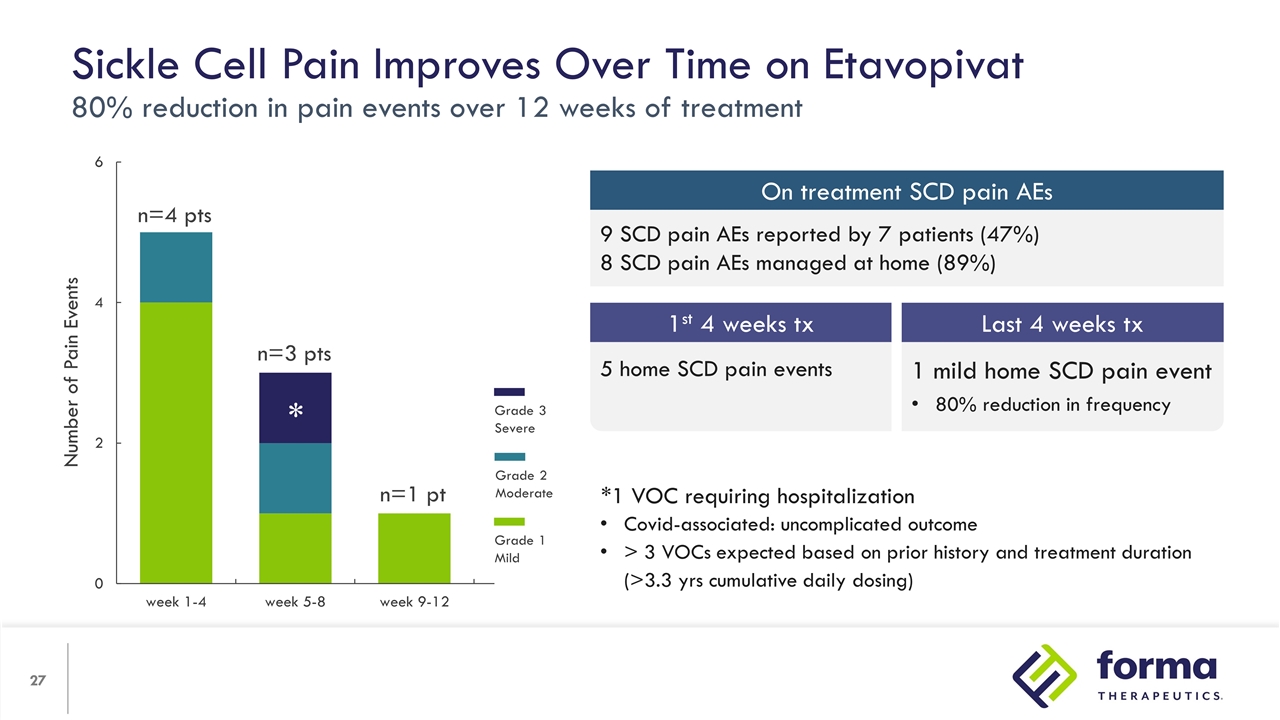
*1 VOC requiring hospitalization Covid-associated: uncomplicated outcome > 3 VOCs expected based on prior history and treatment duration (>3.3 yrs cumulative daily dosing) Sickle Cell Pain Improves Over Time on Etavopivat 80% reduction in pain events over 12 weeks of treatment Number of Pain Events n=4 pts n=3 pts n=1 pt * Grade 3 Severe Grade 2 Moderate Grade 1 Mild 9 SCD pain AEs reported by 7 patients (47%) 8 SCD pain AEs managed at home (89%) On treatment SCD pain AEs 1 mild home SCD pain event 80% reduction in frequency 5 home SCD pain events 1st 4 weeks tx Last 4 weeks tx

Patrick Kelly, M.D. Senior Vice President, Chief Medical Officer Etavopivat Development
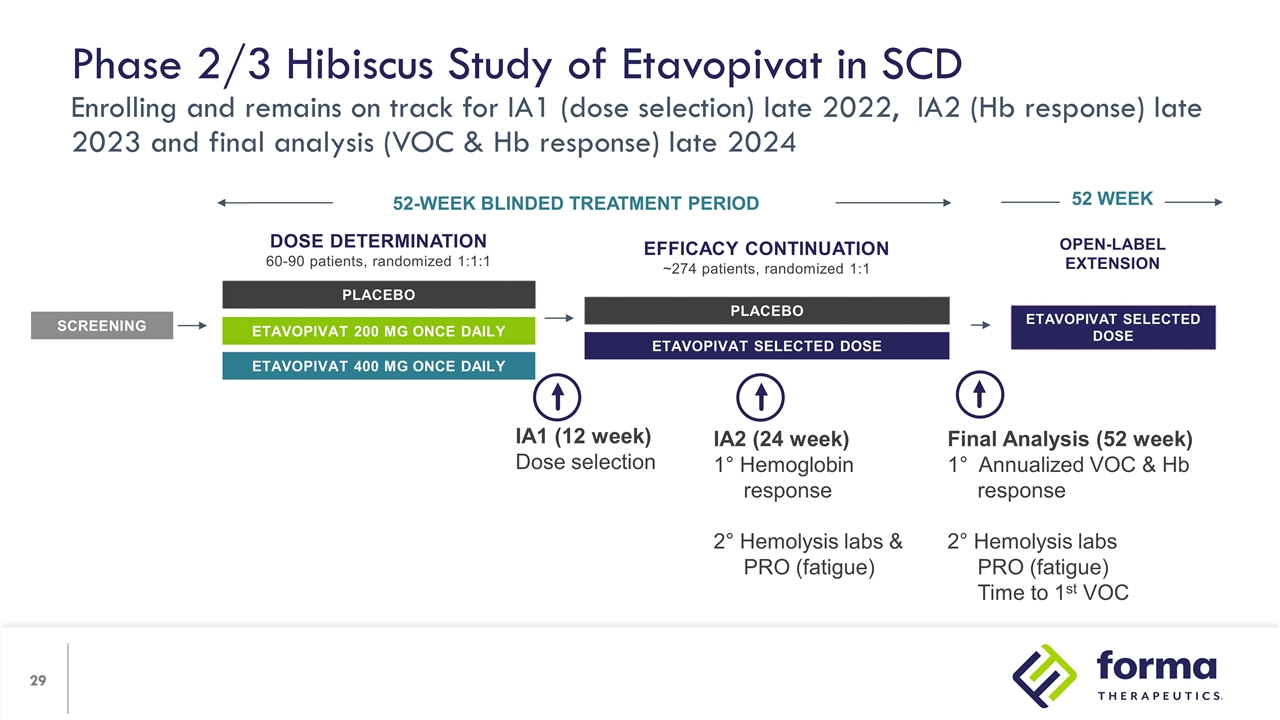
Open-label Extension etavopivat Selected Dose 52 WEEK Phase 2/3 Hibiscus Study of Etavopivat in SCD Enrolling and remains on track for IA1 (dose selection) late 2022, IA2 (Hb response) late 2023 and final analysis (VOC & Hb response) late 2024 Dose Determination 60-90 patients, randomized 1:1:1 PLACEBO etavopivat 200 mg once daily etavopivat 400 mg once daily SCREENING 52-WEEK BLINDED TREATMENT PERIOD Efficacy Continuation ~274 patients, randomized 1:1 PLACEBO etavopivat Selected Dose IA1 (12 week) Dose selection IA2 (24 week) 1° Hemoglobin response 2° Hemolysis labs & PRO (fatigue) Final Analysis (52 week) 1° Annualized VOC & Hb response 2° Hemolysis labs PRO (fatigue) Time to 1st VOC
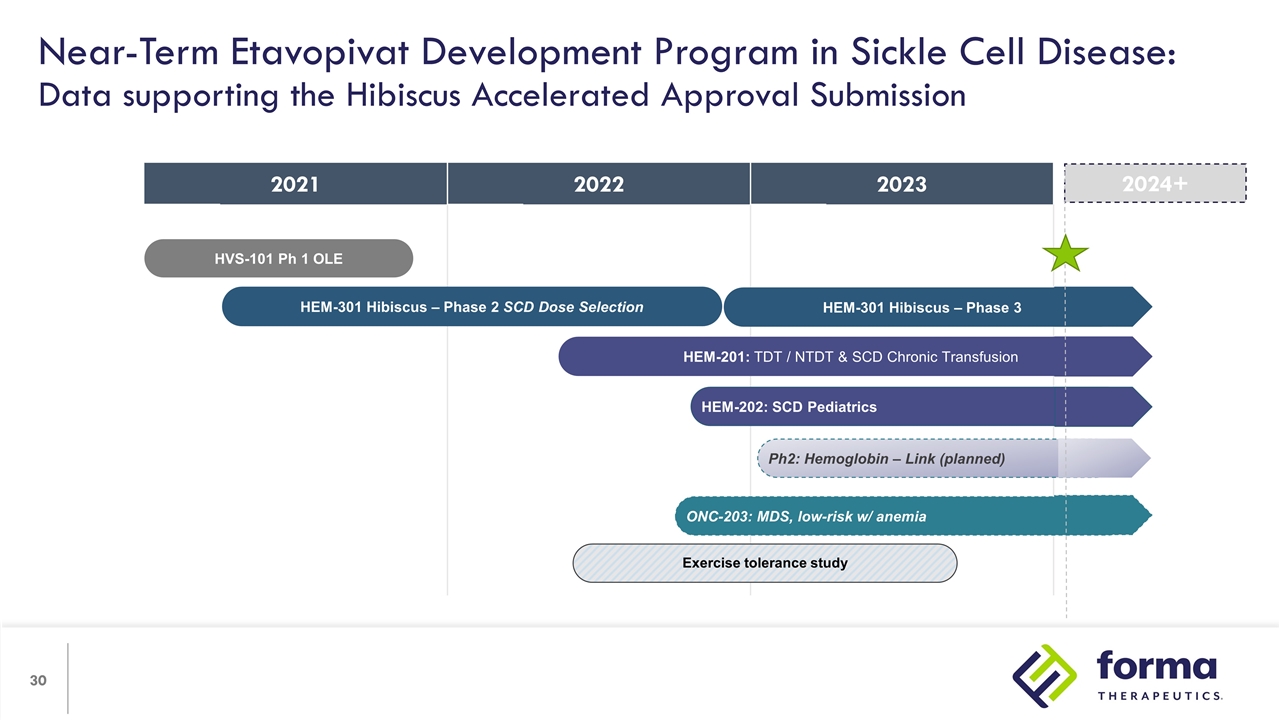
2021 2022 2022 2023 Near-Term Etavopivat Development Program in Sickle Cell Disease: Data supporting the Hibiscus Accelerated Approval Submission HVS-101 Ph 1 OLE HEM-301 Hibiscus – Phase 3 HEM-301 Hibiscus – Phase 2 SCD Dose Selection Exercise tolerance study HEM-201: TDT / NTDT & SCD Chronic Transfusion ONC-203: MDS, low-risk w/ anemia Ph2: Hemoglobin – Link (planned) HEM-202: SCD Pediatrics 2024+
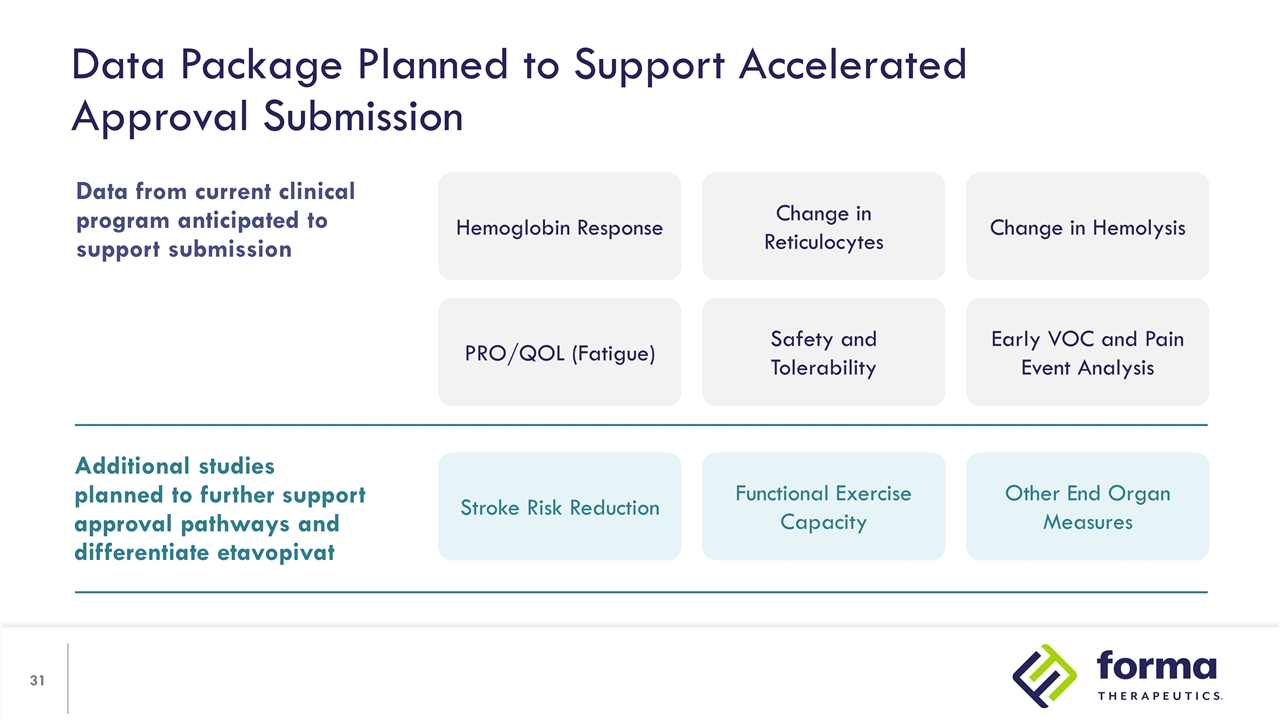
Data Package Planned to Support Accelerated Approval Submission Additional studies planned to further support approval pathways and differentiate etavopivat Stroke Risk Reduction Functional Exercise Capacity Other End Organ Measures Data from current clinical program anticipated to support submission Hemoglobin Response Change in Reticulocytes Change in Hemolysis PRO/QOL (Fatigue) Safety and Tolerability Early VOC and Pain Event Analysis
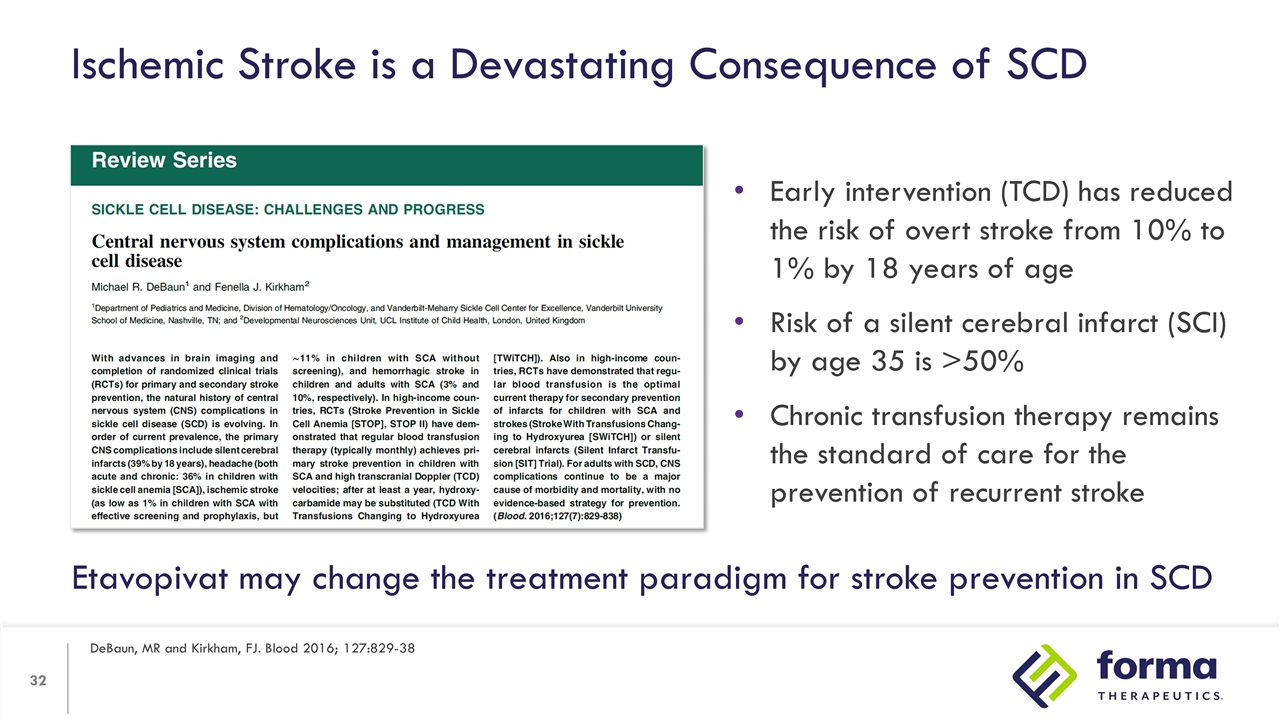
Ischemic Stroke is a Devastating Consequence of SCD DeBaun, MR and Kirkham, FJ. Blood 2016; 127:829-38 Early intervention (TCD) has reduced the risk of overt stroke from 10% to 1% by 18 years of age Risk of a silent cerebral infarct (SCI) by age 35 is >50% Chronic transfusion therapy remains the standard of care for the prevention of recurrent stroke Etavopivat may change the treatment paradigm for stroke prevention in SCD
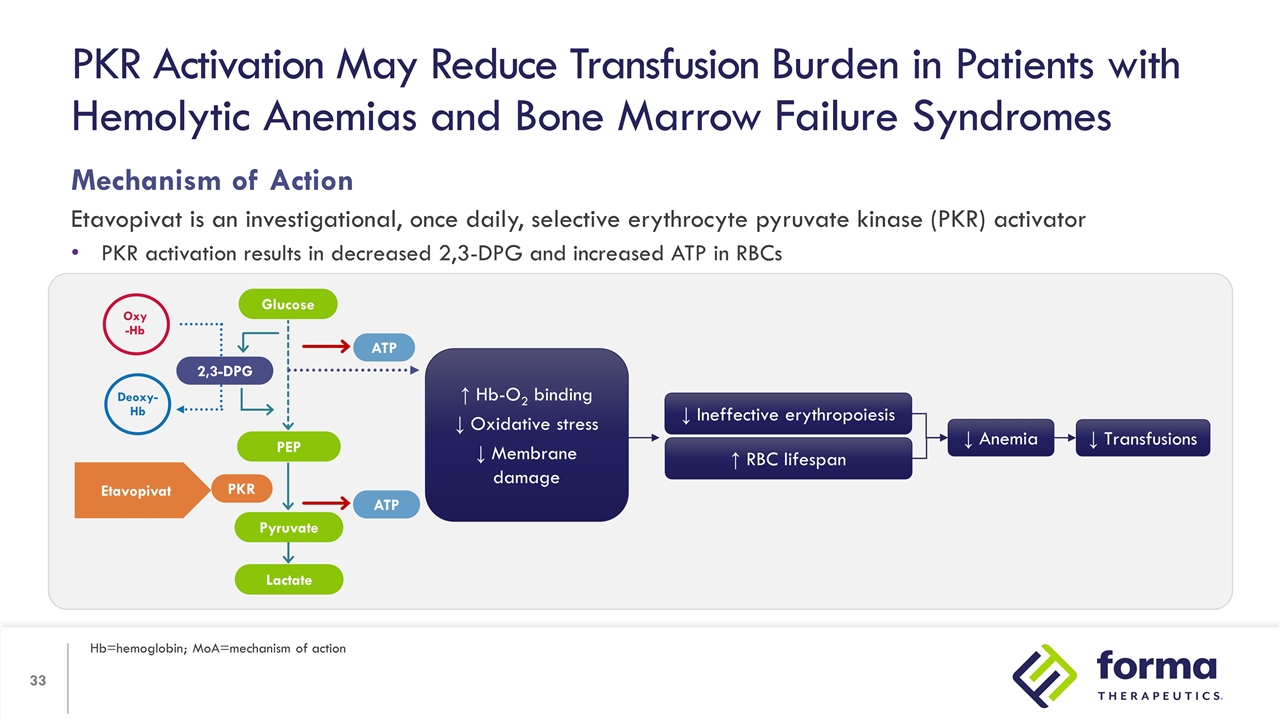
Mechanism of Action Etavopivat is an investigational, once daily, selective erythrocyte pyruvate kinase (PKR) activator PKR activation results in decreased 2,3-DPG and increased ATP in RBCs PKR Activation May Reduce Transfusion Burden in Patients with Hemolytic Anemias and Bone Marrow Failure Syndromes Hb=hemoglobin; MoA=mechanism of action ↑ Hb-O2 binding ↓ Oxidative stress ↓ Membrane damage ↑ RBC lifespan ↓ Ineffective erythropoiesis ↓ Anemia ↓ Transfusions 2,3-DPG PEP Lactate ATP ATP PKR Oxy -Hb Deoxy-Hb Etavopivat Glucose Pyruvate
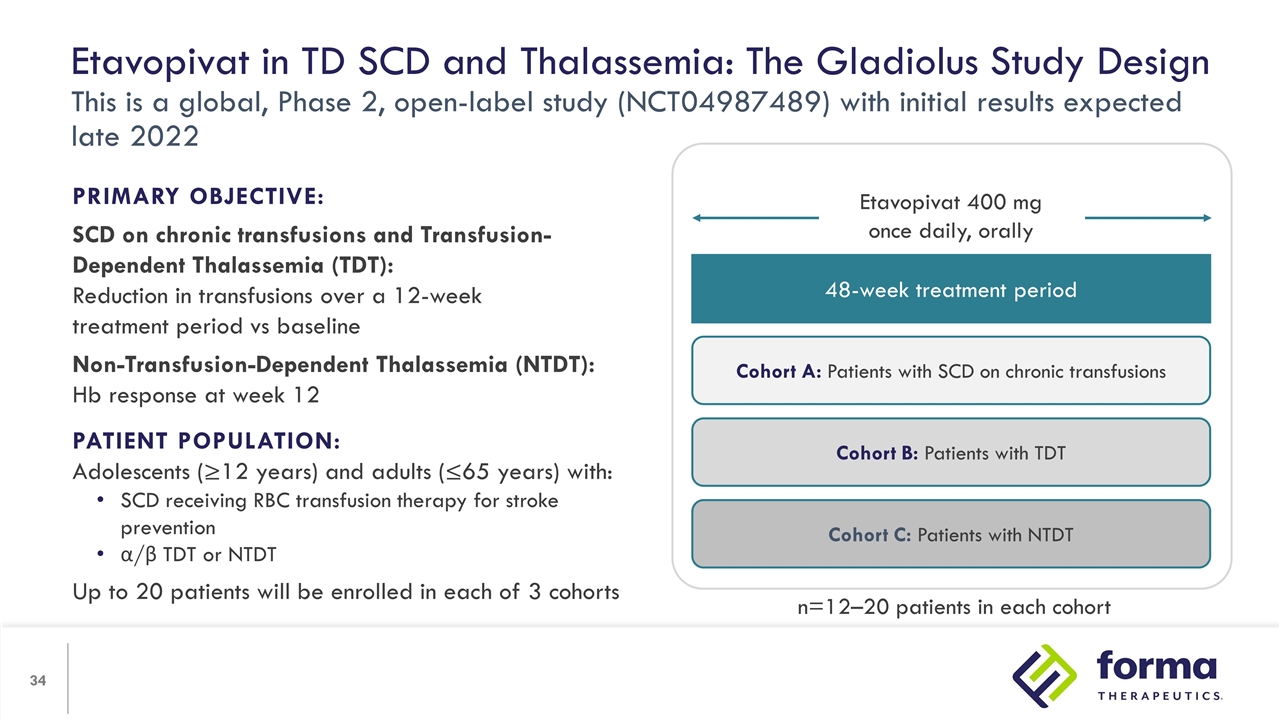
Etavopivat in TD SCD and Thalassemia: The Gladiolus Study Design This is a global, Phase 2, open-label study (NCT04987489) with initial results expected late 2022 PRIMARY OBJECTIVE: SCD on chronic transfusions and Transfusion-Dependent Thalassemia (TDT): Reduction in transfusions over a 12-week treatment period vs baseline Non-Transfusion-Dependent Thalassemia (NTDT): Hb response at week 12 PATIENT POPULATION: Adolescents (≥12 years) and adults (≤65 years) with: SCD receiving RBC transfusion therapy for stroke prevention α/β TDT or NTDT Up to 20 patients will be enrolled in each of 3 cohorts 48-week treatment period Cohort A: Patients with SCD on chronic transfusions Cohort B: Patients with TDT Cohort C: Patients with NTDT Etavopivat 400 mg once daily, orally n=12–20 patients in each cohort
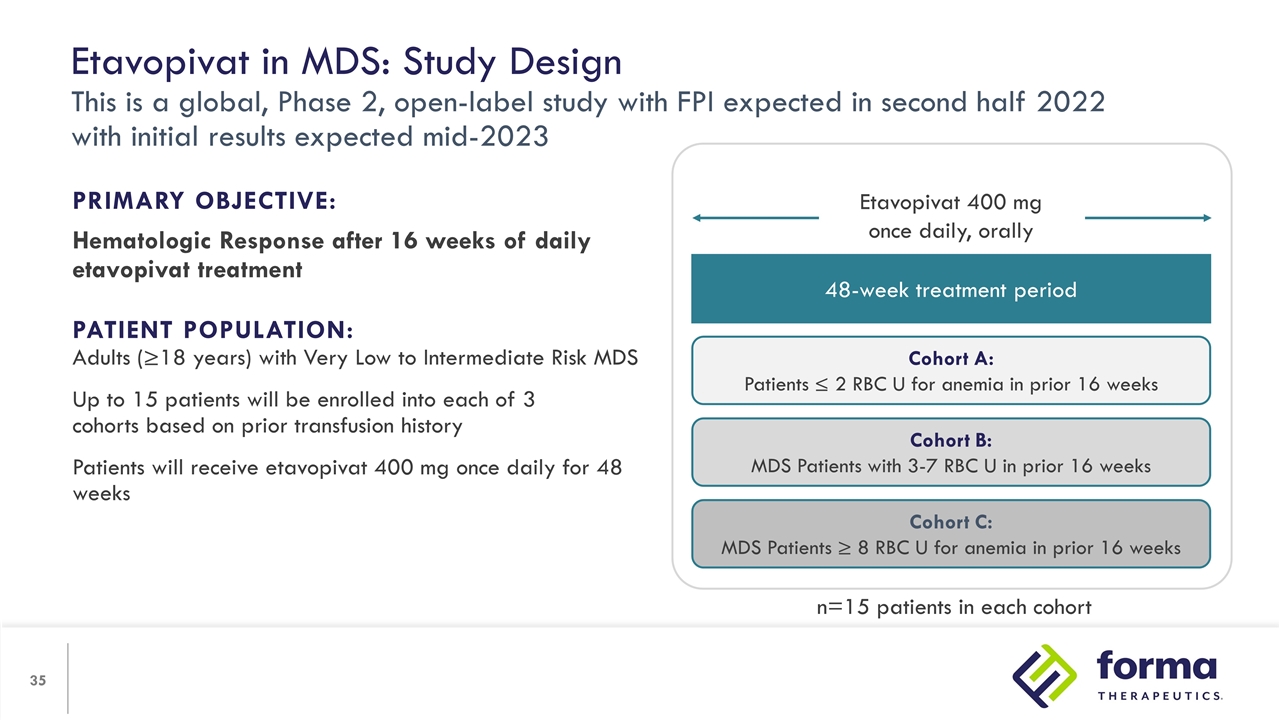
Etavopivat in MDS: Study Design This is a global, Phase 2, open-label study with FPI expected in second half 2022 with initial results expected mid-2023 PRIMARY OBJECTIVE: Hematologic Response after 16 weeks of daily etavopivat treatment PATIENT POPULATION: Adults (≥18 years) with Very Low to Intermediate Risk MDS Up to 15 patients will be enrolled into each of 3 cohorts based on prior transfusion history Patients will receive etavopivat 400 mg once daily for 48 weeks 48-week treatment period Cohort A: Patients ≤ 2 RBC U for anemia in prior 16 weeks Cohort B: MDS Patients with 3-7 RBC U in prior 16 weeks Cohort C: MDS Patients ≥ 8 RBC U for anemia in prior 16 weeks Etavopivat 400 mg once daily, orally n=15 patients in each cohort
Michael Savona, M.D. Chair, MDS/MPN International Working Group; Head, Hematology, Cellular Therapy and Stem Cell Transplantation, Beverly and George Rawlings Directorship in Hematology Research; Professor of Medicine and Cancer Biology, Department of Internal Medicine, Vanderbilt University School of Medicine Unmet Need in Bone Marrow Failure Syndromes: Treating the Functional PK-Deficiency

Disclosures I have the following financial relationships to disclose: Advisory and consulting: Abbvie, Celgene, CTI, Forma Therapeutics, Geron, Karyopharm, Novartis, Ryvu, Taiho, Takeda, TG Therapeutics; Research Support: ALX Oncology, Astex, Incyte, Takeda, TG Therapeutics; Equity: Karyopharm, Ryvu; Data Safety Monitoring Board: Celgene, Sierra Oncology, TG Therapeutics; Licensing Agreement: Boehringer-Ingelheim
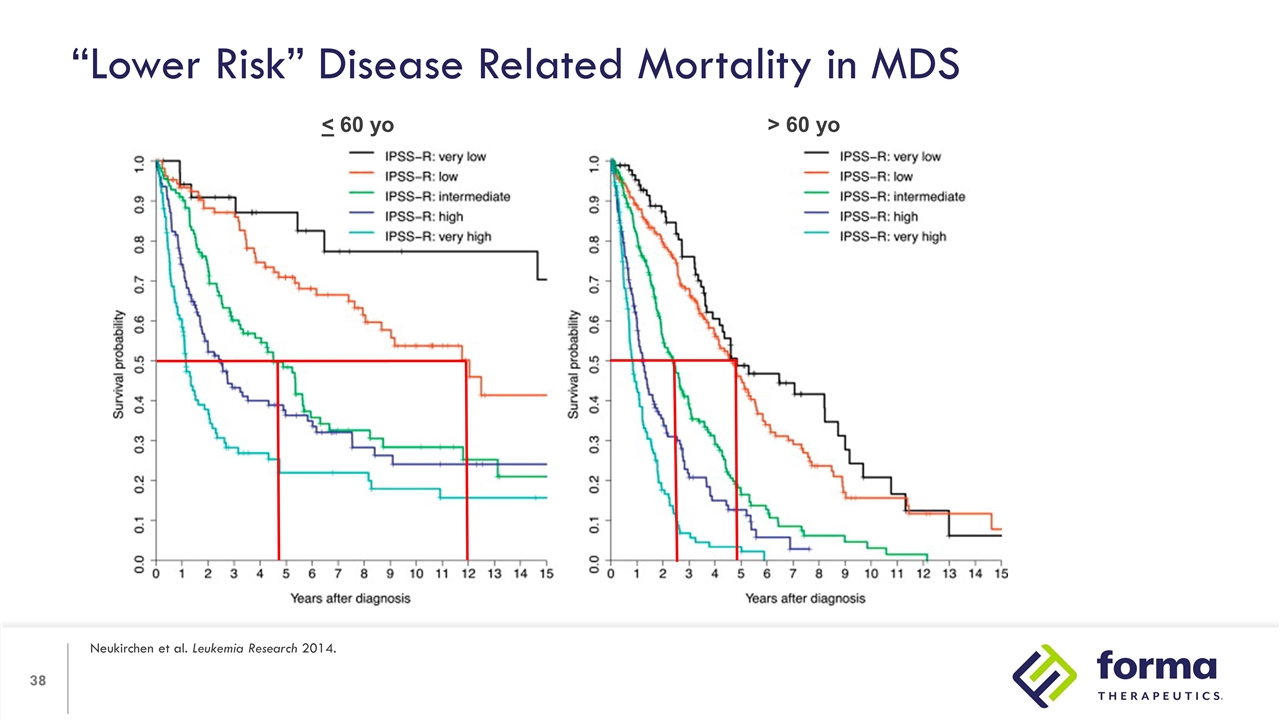
“Lower Risk” Disease Related Mortality in MDS Neukirchen et al. Leukemia Research 2014. < 60 yo > 60 yo
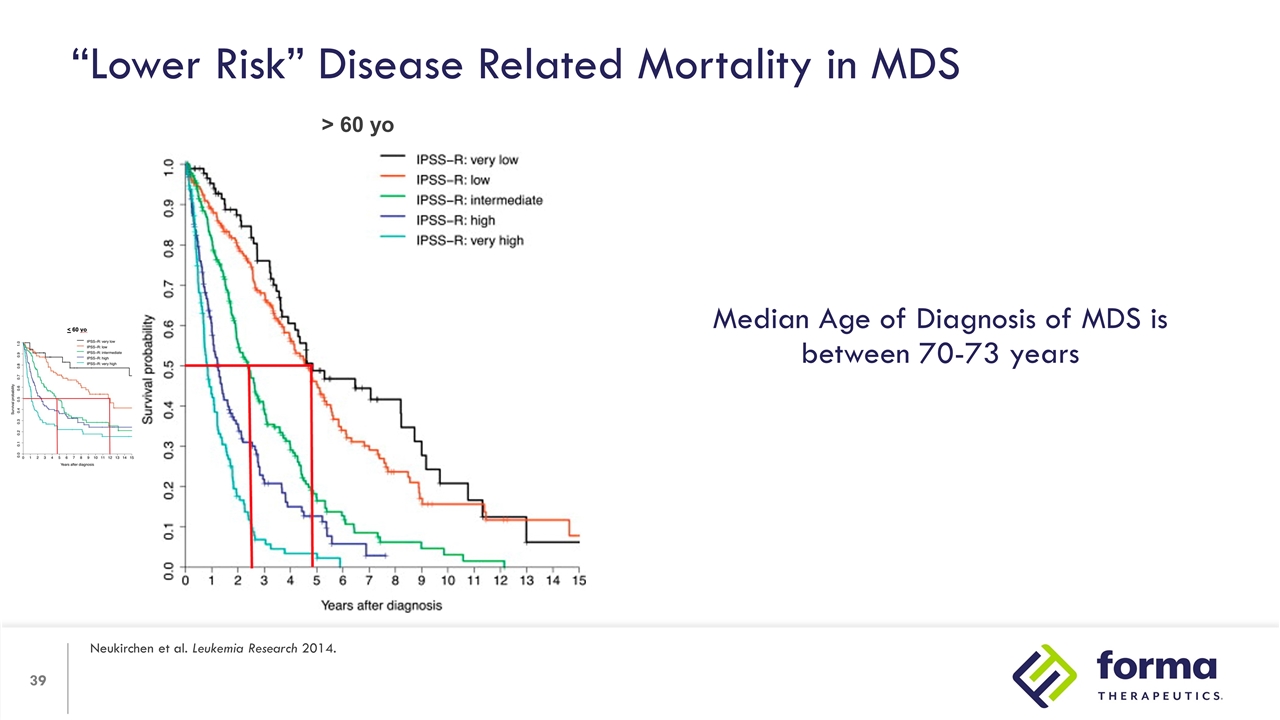
“Lower Risk” Disease Related Mortality in MDS Neukirchen et al. Leukemia Research 2014. > 60 yo Median Age of Diagnosis of MDS is between 70-73 years
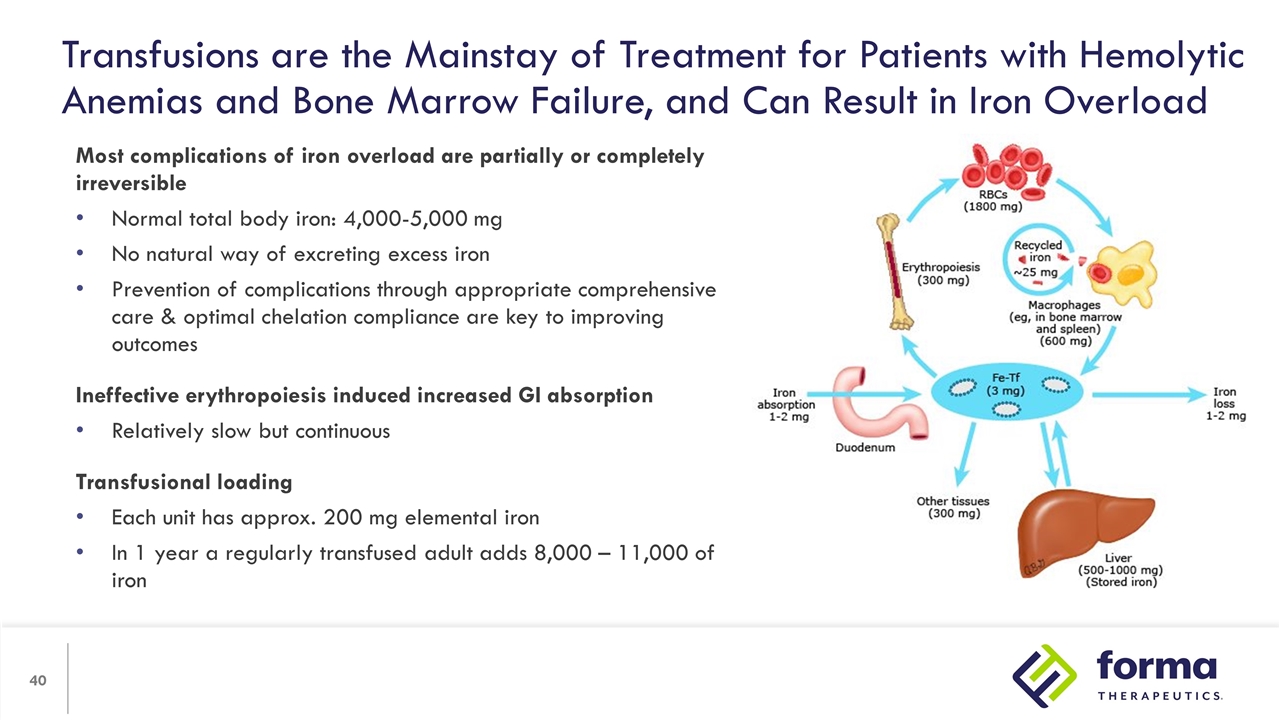
Transfusions are the Mainstay of Treatment for Patients with Hemolytic Anemias and Bone Marrow Failure, and Can Result in Iron Overload Most complications of iron overload are partially or completely irreversible Normal total body iron: 4,000-5,000 mg No natural way of excreting excess iron Prevention of complications through appropriate comprehensive care & optimal chelation compliance are key to improving outcomes Ineffective erythropoiesis induced increased GI absorption Relatively slow but continuous Transfusional loading Each unit has approx. 200 mg elemental iron In 1 year a regularly transfused adult adds 8,000 – 11,000 of iron
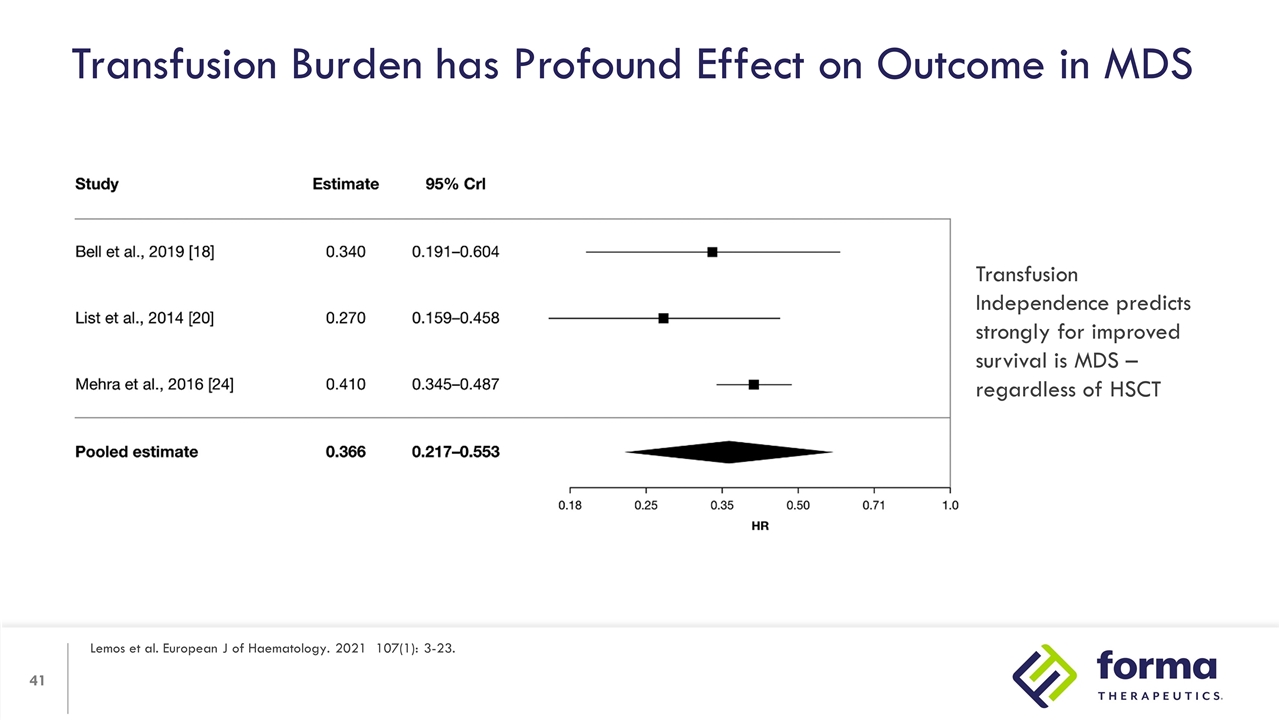
Transfusion Burden has Profound Effect on Outcome in MDS Lemos et al. European J of Haematology. 2021 107(1): 3-23. Transfusion Independence predicts strongly for improved survival is MDS – regardless of HSCT
Recombinant erythropoietin can lead to long term responses in LR-MDS with MDS with isolated anemia epo levels < 500, usually <200 considerable variation in dose and schedule Low thromboembolic risk if given with lower Hgb* Sequencing of ESAs with other therapy for LR-MDS - Unclear Early Erythropoiesis Stimulation in Low Risk MDS Remains Standard of Care *Patients live at Hb level of ~<11g/dL
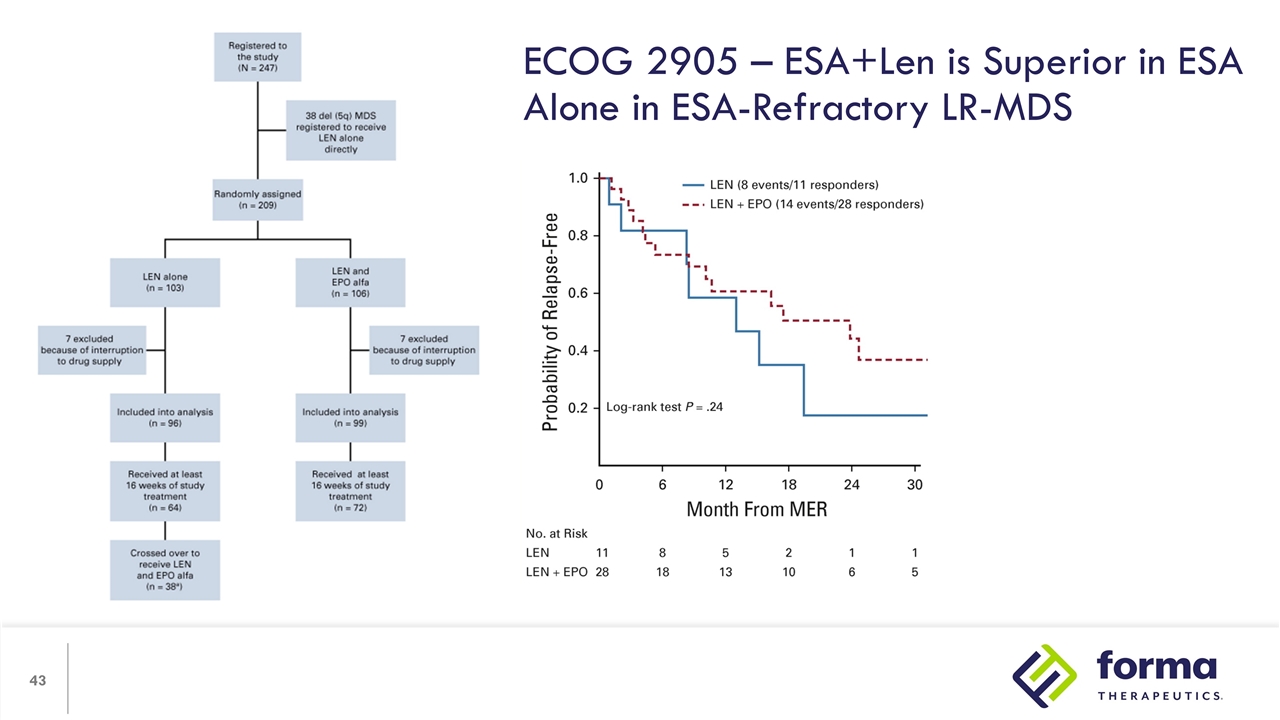
ECOG 2905 – ESA+Len is Superior in ESA Alone in ESA-Refractory LR-MDS
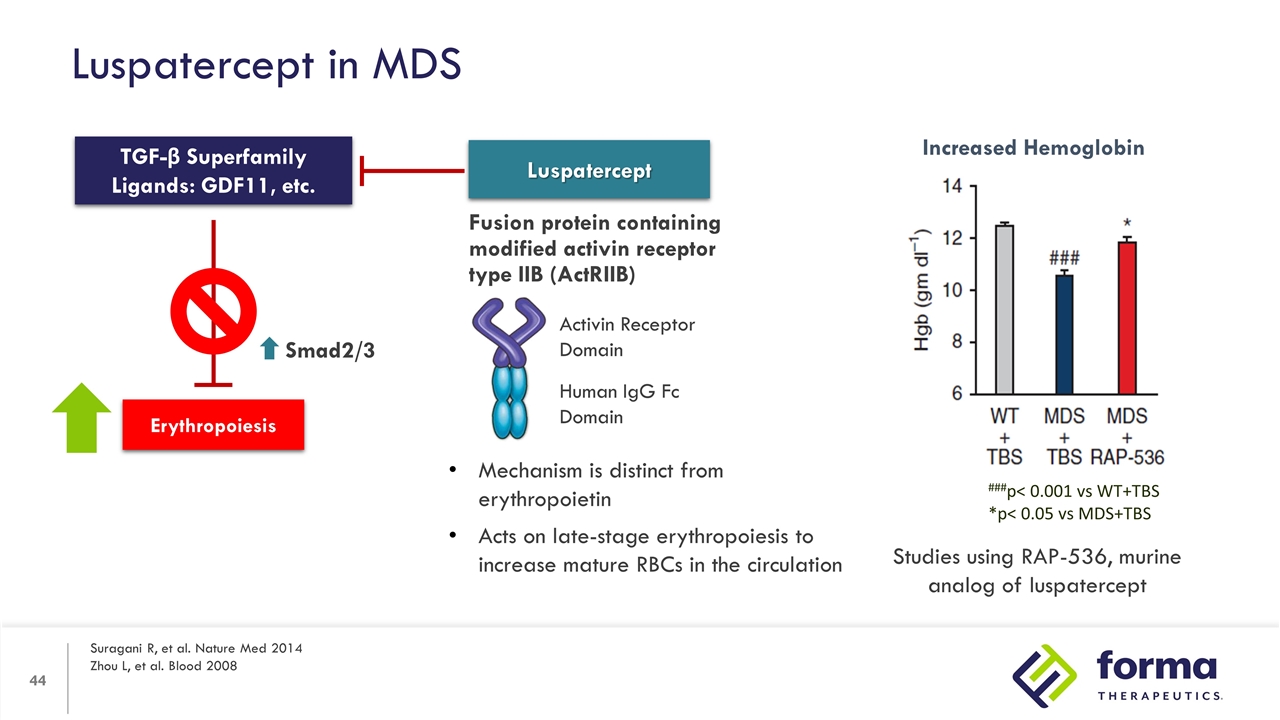
Luspatercept in MDS Suragani R, et al. Nature Med 2014 Zhou L, et al. Blood 2008 Erythropoiesis TGF-β Superfamily Ligands: GDF11, etc. Smad2/3 Luspatercept Fusion protein containing modified activin receptor type IIB (ActRIIB) Activin Receptor Domain Human IgG Fc Domain Mechanism is distinct from erythropoietin Acts on late-stage erythropoiesis to increase mature RBCs in the circulation Increased Hemoglobin ###p< 0.001 vs WT+TBS *p< 0.05 vs MDS+TBS Studies using RAP-536, murine analog of luspatercept
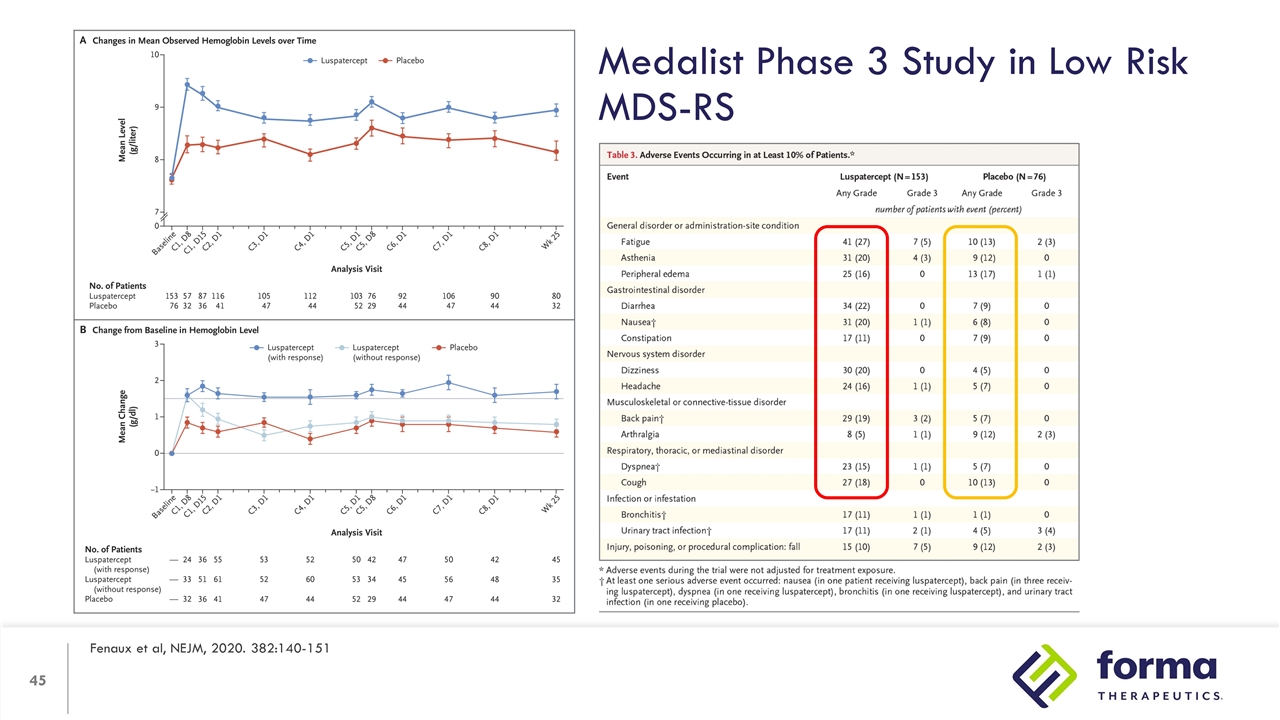
Medalist Phase 3 Study in Low Risk MDS-RS Fenaux et al, NEJM, 2020. 382:140-151

Functional PK-R Deficiency Associated with Anemia of MDS
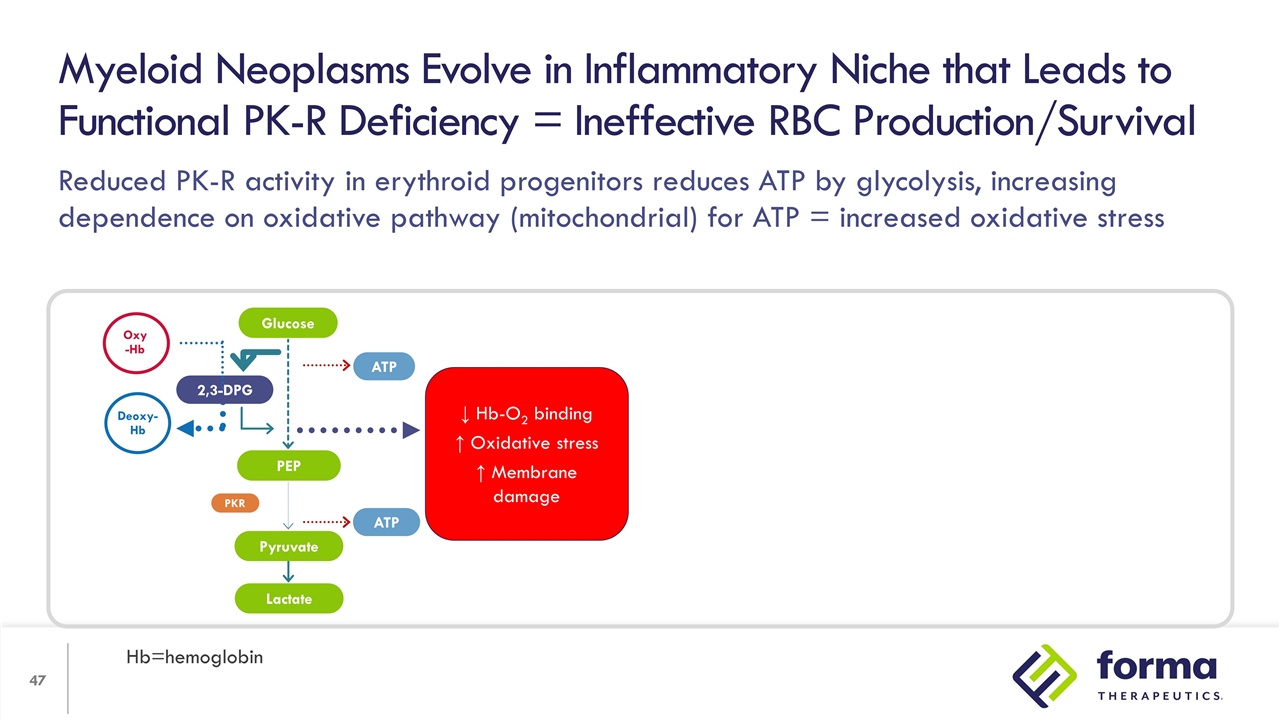
Reduced PK-R activity in erythroid progenitors reduces ATP by glycolysis, increasing dependence on oxidative pathway (mitochondrial) for ATP = increased oxidative stress Myeloid Neoplasms Evolve in Inflammatory Niche that Leads to Functional PK-R Deficiency = Ineffective RBC Production/Survival Hb=hemoglobin 2,3-DPG PEP Lactate ATP ATP PKR Oxy -Hb Deoxy-Hb Glucose Pyruvate ↓ Hb-O2 binding ↑ Oxidative stress ↑ Membrane damage
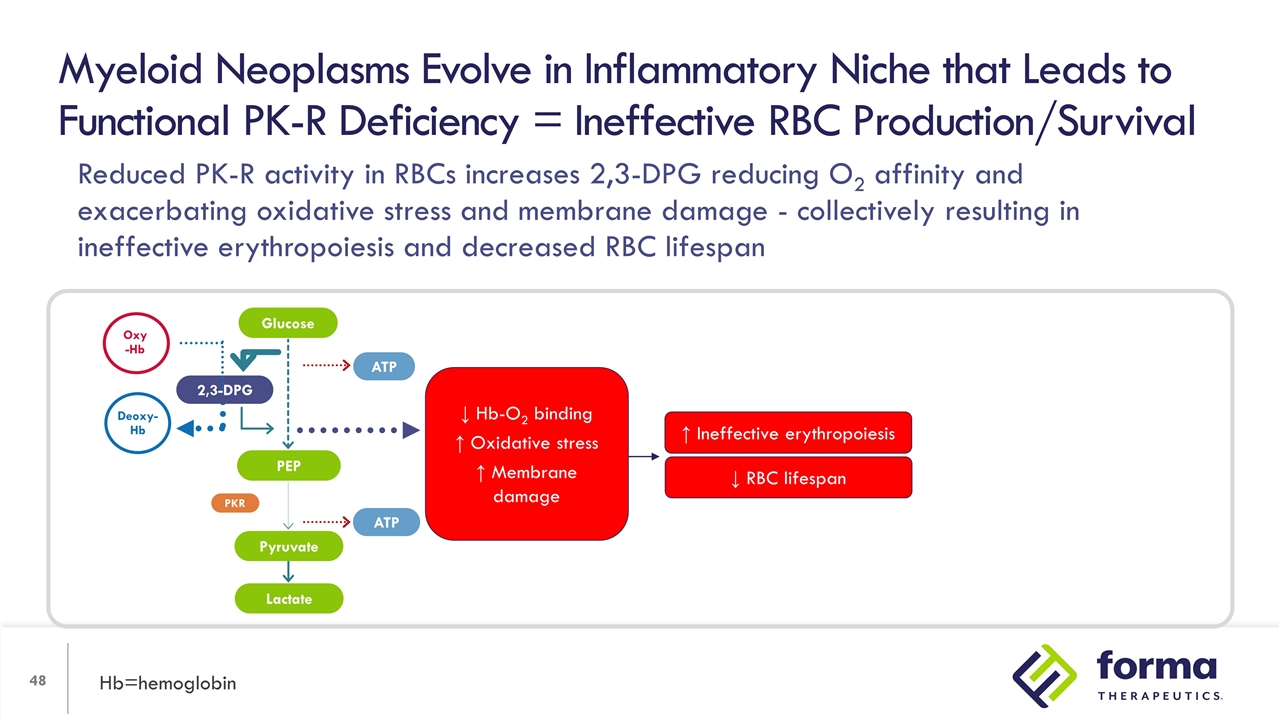
Myeloid Neoplasms Evolve in Inflammatory Niche that Leads to Functional PK-R Deficiency = Ineffective RBC Production/Survival Hb=hemoglobin ↓ RBC lifespan ↑ Ineffective erythropoiesis 2,3-DPG PEP Lactate ATP ATP PKR Oxy -Hb Deoxy-Hb Glucose Pyruvate ↓ Hb-O2 binding ↑ Oxidative stress ↑ Membrane damage Reduced PK-R activity in RBCs increases 2,3-DPG reducing O2 affinity and exacerbating oxidative stress and membrane damage - collectively resulting in ineffective erythropoiesis and decreased RBC lifespan
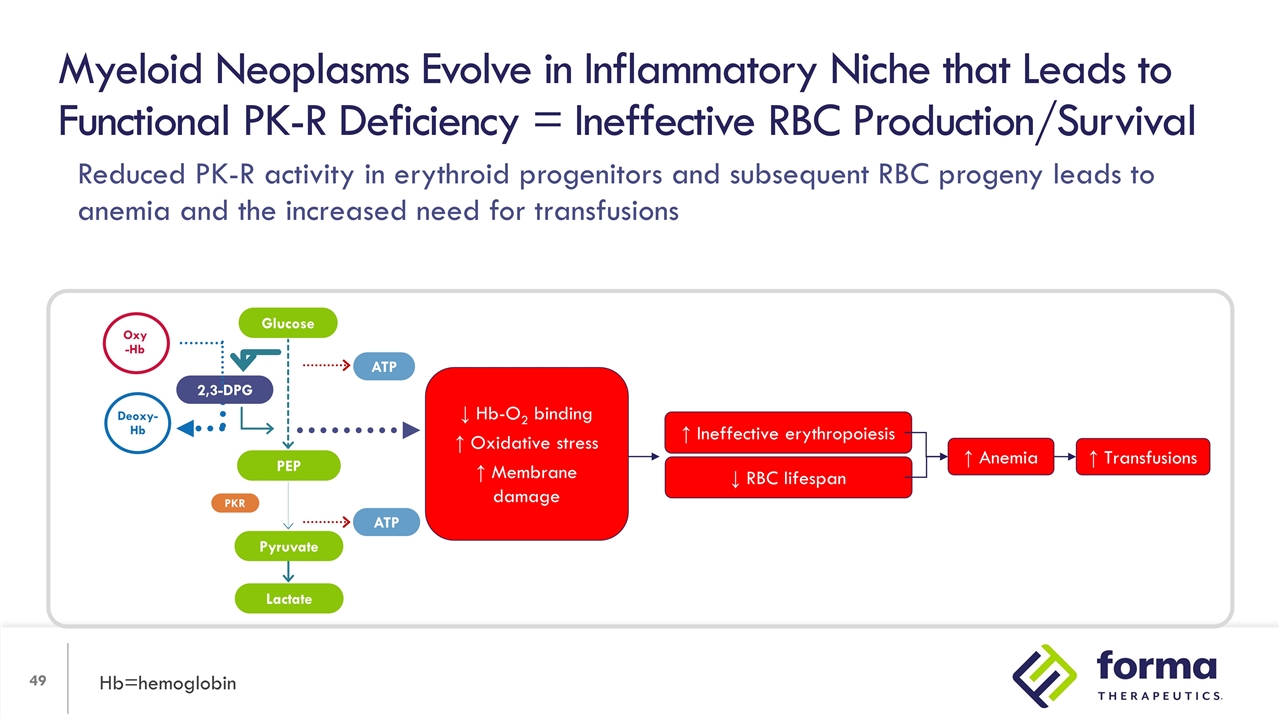
Myeloid Neoplasms Evolve in Inflammatory Niche that Leads to Functional PK-R Deficiency = Ineffective RBC Production/Survival Hb=hemoglobin ↓ RBC lifespan ↑ Ineffective erythropoiesis ↑ Anemia ↑ Transfusions 2,3-DPG PEP Lactate ATP ATP PKR Oxy -Hb Deoxy-Hb Glucose Pyruvate ↓ Hb-O2 binding ↑ Oxidative stress ↑ Membrane damage Reduced PK-R activity in erythroid progenitors and subsequent RBC progeny leads to anemia and the increased need for transfusions
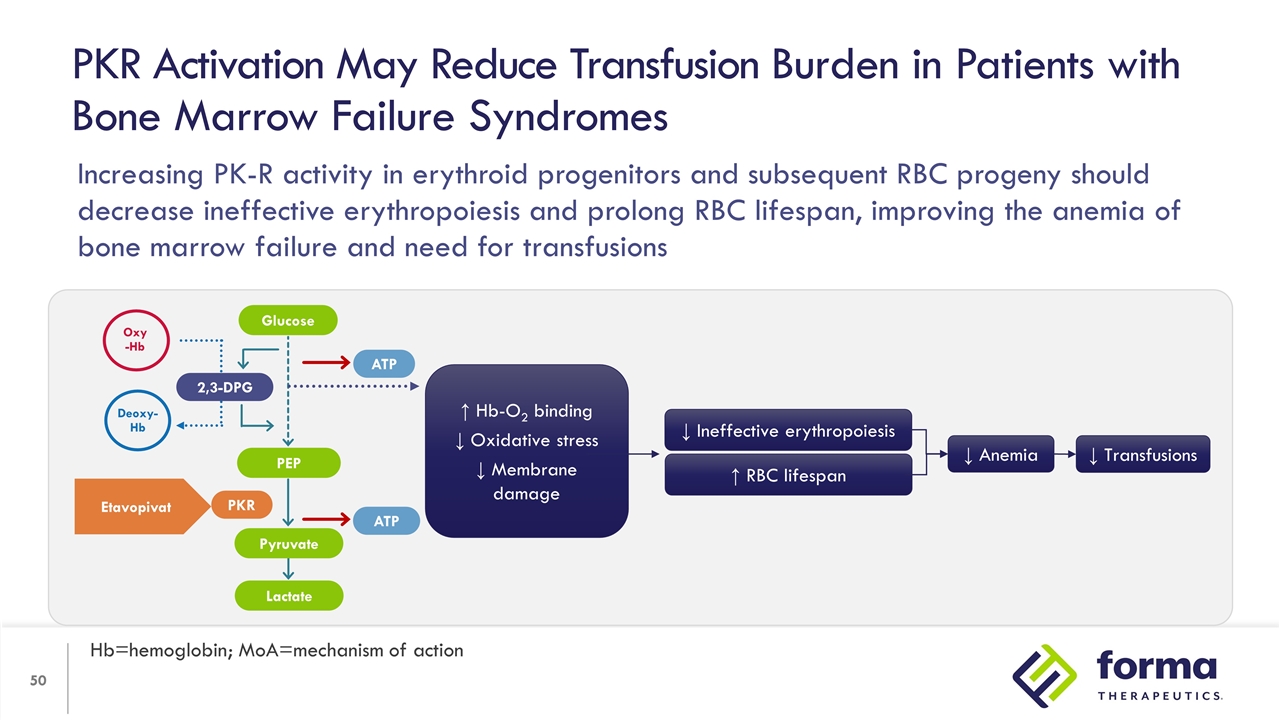
PKR Activation May Reduce Transfusion Burden in Patients with Bone Marrow Failure Syndromes Hb=hemoglobin; MoA=mechanism of action ↑ Hb-O2 binding ↓ Oxidative stress ↓ Membrane damage ↑ RBC lifespan ↓ Ineffective erythropoiesis ↓ Anemia ↓ Transfusions 2,3-DPG PEP Lactate ATP ATP PKR Oxy -Hb Deoxy-Hb Etavopivat Glucose Pyruvate Increasing PK-R activity in erythroid progenitors and subsequent RBC progeny should decrease ineffective erythropoiesis and prolong RBC lifespan, improving the anemia of bone marrow failure and need for transfusions
Clonal Hematopoiesis is a Normal Process of Aging Hematopoietic stem cells (HSCs) are rare and quiescent blood cells (1:10,000 blood cells) yet there are billions of HSC cell divisions in a human lifetime Replication errors are largely repaired, but errors which are not repaired are propagated Propagated errors which provide growth advantages grow become dominant and grow in size While CH in small variant allele fractions are ubiquitous, the term of CH refers to clones present in a substantial number of cells, and repeatedly occur in genes related to epigenetic function, splicing, and DNA damage repair…
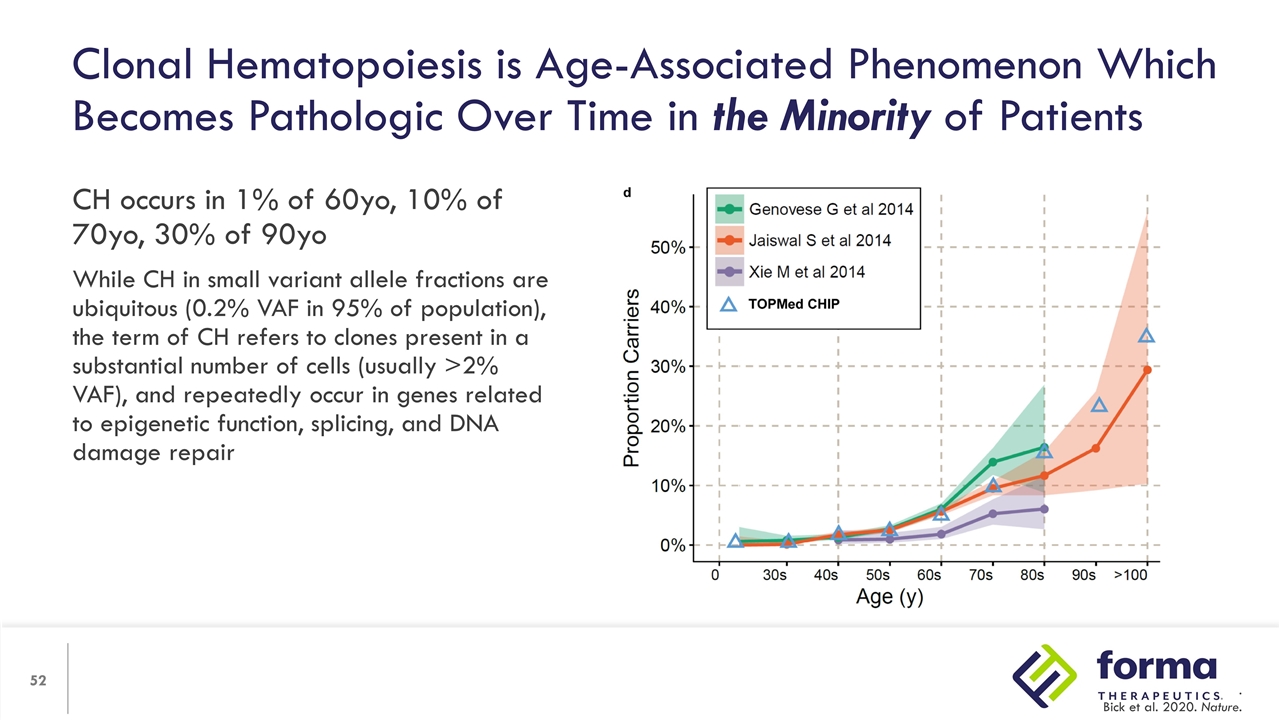
Clonal Hematopoiesis is Age-Associated Phenomenon Which Becomes Pathologic Over Time in the Minority of Patients CH occurs in 1% of 60yo, 10% of 70yo, 30% of 90yo While CH in small variant allele fractions are ubiquitous (0.2% VAF in 95% of population), the term of CH refers to clones present in a substantial number of cells (usually >2% VAF), and repeatedly occur in genes related to epigenetic function, splicing, and DNA damage repair . Bick et al. 2020. Nature.
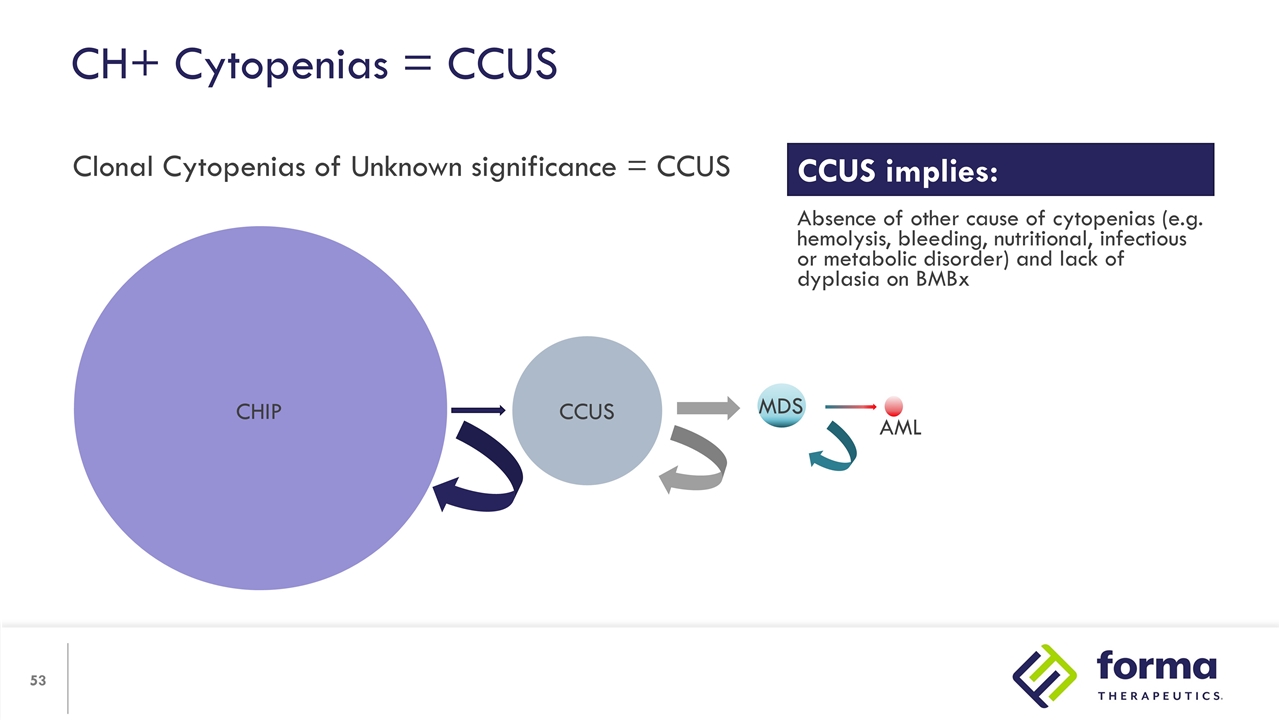
CH+ Cytopenias = CCUS Clonal Cytopenias of Unknown significance = CCUS CHIP CCUS MDS AML CCUS implies: Absence of other cause of cytopenias (e.g. hemolysis, bleeding, nutritional, infectious or metabolic disorder) and lack of dyplasia on BMBx
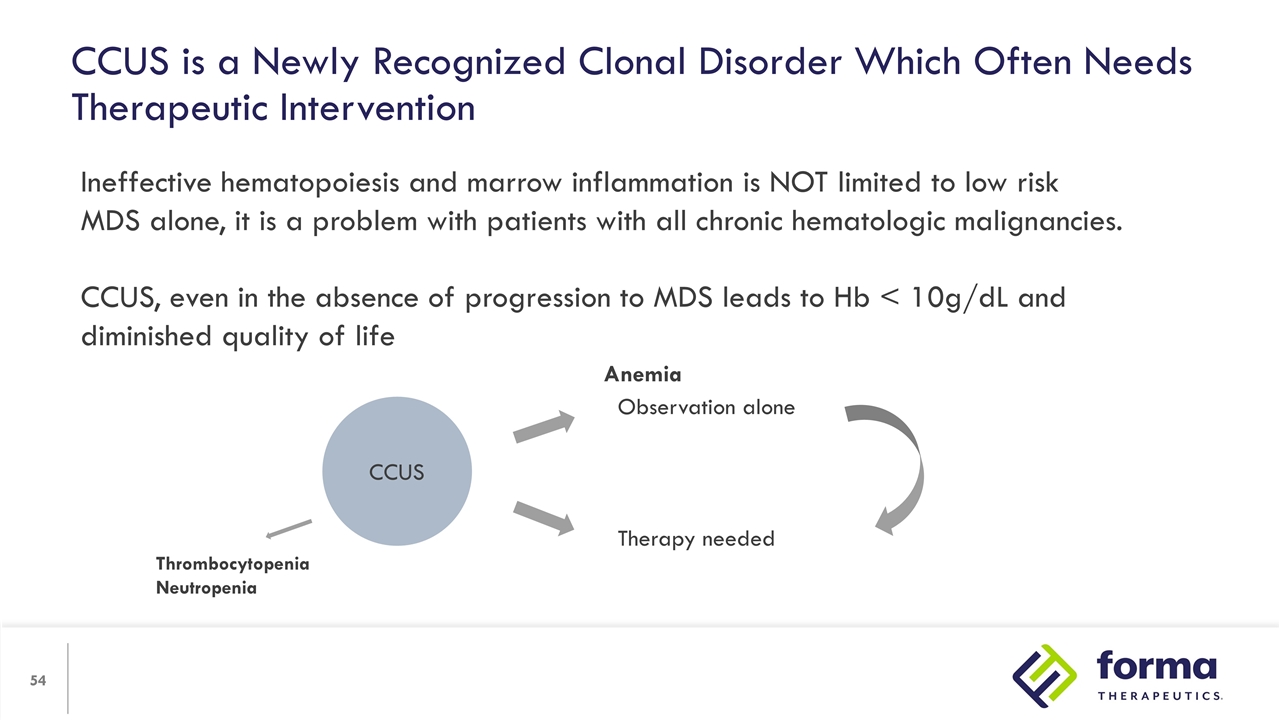
Ineffective hematopoiesis and marrow inflammation is NOT limited to low risk MDS alone, it is a problem with patients with all chronic hematologic malignancies. CCUS, even in the absence of progression to MDS leads to Hb < 10g/dL and diminished quality of life CCUS Observation alone Therapy needed Anemia Thrombocytopenia Neutropenia CCUS is a Newly Recognized Clonal Disorder Which Often Needs Therapeutic Intervention

Lower risk MDS is not low risk and is associated with serious morbidity and higher mortality than realized Iron overload from chronic transfusions is a significant risk factor Current therapy for lower risk MDS is variably effective and is NOT benign Luspatercept provides a new approach specifically to treat anemia in low risk MDS but wanes and is effective in many MDS patients Functional PK-R deficiency has been associated with anemia of bone marrow failure On oral, well-tolerated PK-R activator may provide a significant benefit to patients with LR-MDS and in patients with CCUS QOL due to anemia is an issue in these patients Summary

The patients The MDS and Clonal Hematopoiesis Community Acknowledgments The Biff Ruttenberg Foundation Serodino Family Compact Lovell Family Fellowship Beverly and George Rawlings Directorship in Hematology Research

David N. Cook, Ph.D. Senior Vice President, Chief Scientific Officer Research Pipeline and Red Blood Cell Health
Research Pipeline and RBC Health Highlights Our strategy is to select programs based on scientific promise, patient need and strategic fit with our focus on rare hematology and oncology Our pipeline reflects a rich heritage – with FT-3171, a USP1 inhibitor, now in IND-enabling studies New programs in SCD target mechanisms complementary to etavopivat to create compelling combination approaches We believe there is an underrecognized opportunity in Red Blood Cell Health, both for etavopivat and for novel mechanisms
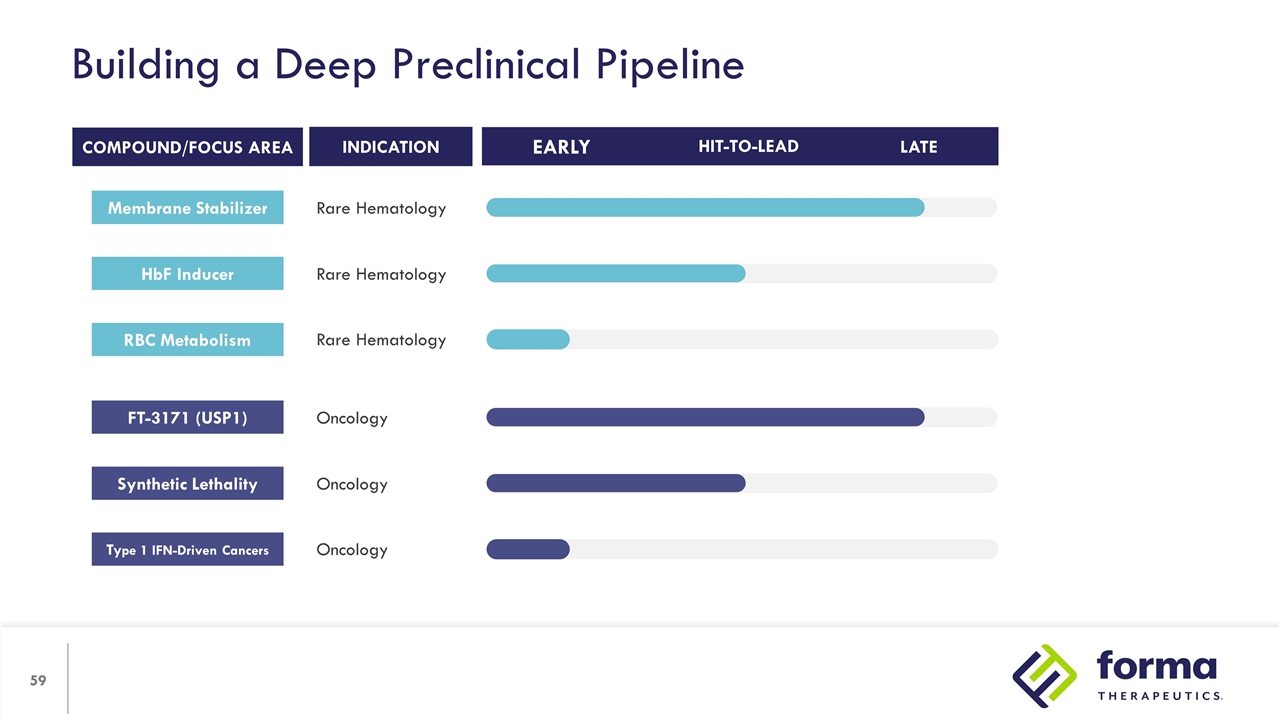
Building a Deep Preclinical Pipeline INDICATION RBC Metabolism Rare Hematology COMPOUND/FOCUS AREA HIT-TO-LEAD LATE EARLY Rare Hematology HbF Inducer Rare Hematology Membrane Stabilizer Type 1 IFN-Driven Cancers Oncology Oncology Synthetic Lethality Oncology FT-3171 (USP1)
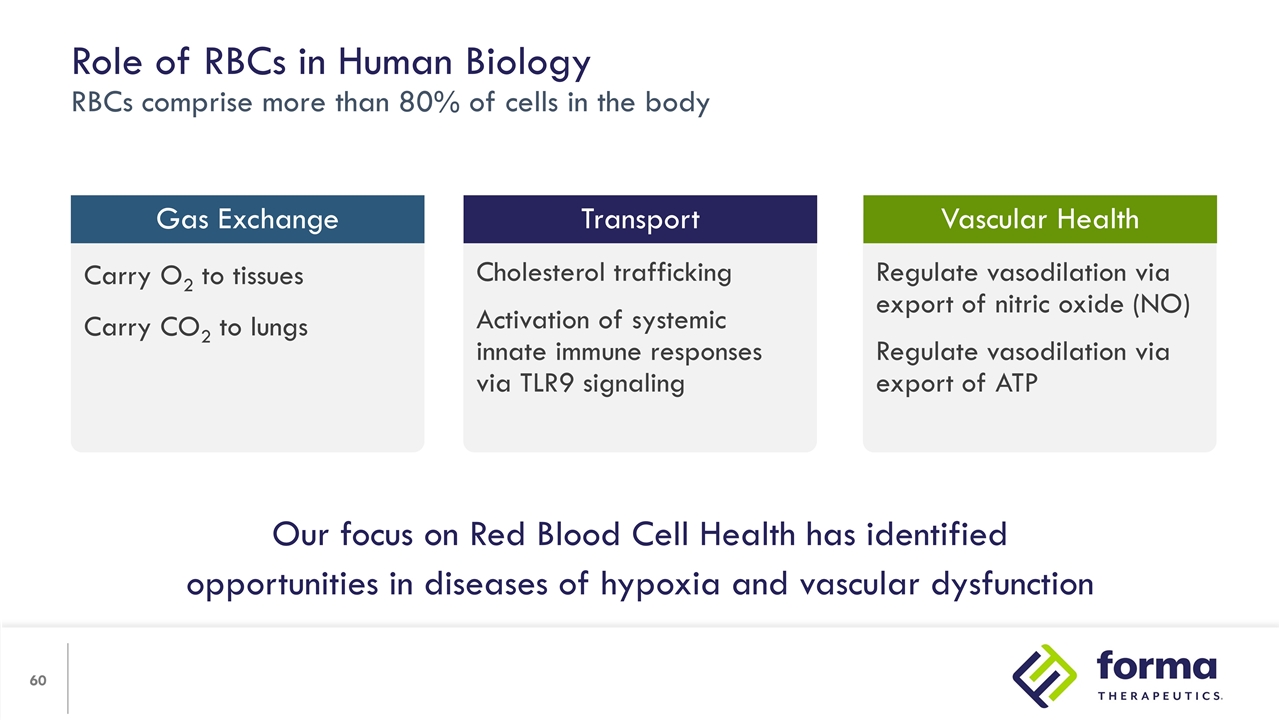
Regulate vasodilation via export of nitric oxide (NO) Regulate vasodilation via export of ATP Cholesterol trafficking Activation of systemic innate immune responses via TLR9 signaling Carry O2 to tissues Carry CO2 to lungs Role of RBCs in Human Biology RBCs comprise more than 80% of cells in the body Gas Exchange Vascular Health Transport Our focus on Red Blood Cell Health has identified opportunities in diseases of hypoxia and vascular dysfunction

We believe there are chronic diseases where improving RBC health will make a substantial improvement in quality of life. Relevant mechanisms include increasing ATP using etavopivat and other intracellular RBC targets that improve function. RBC Health Opportunity
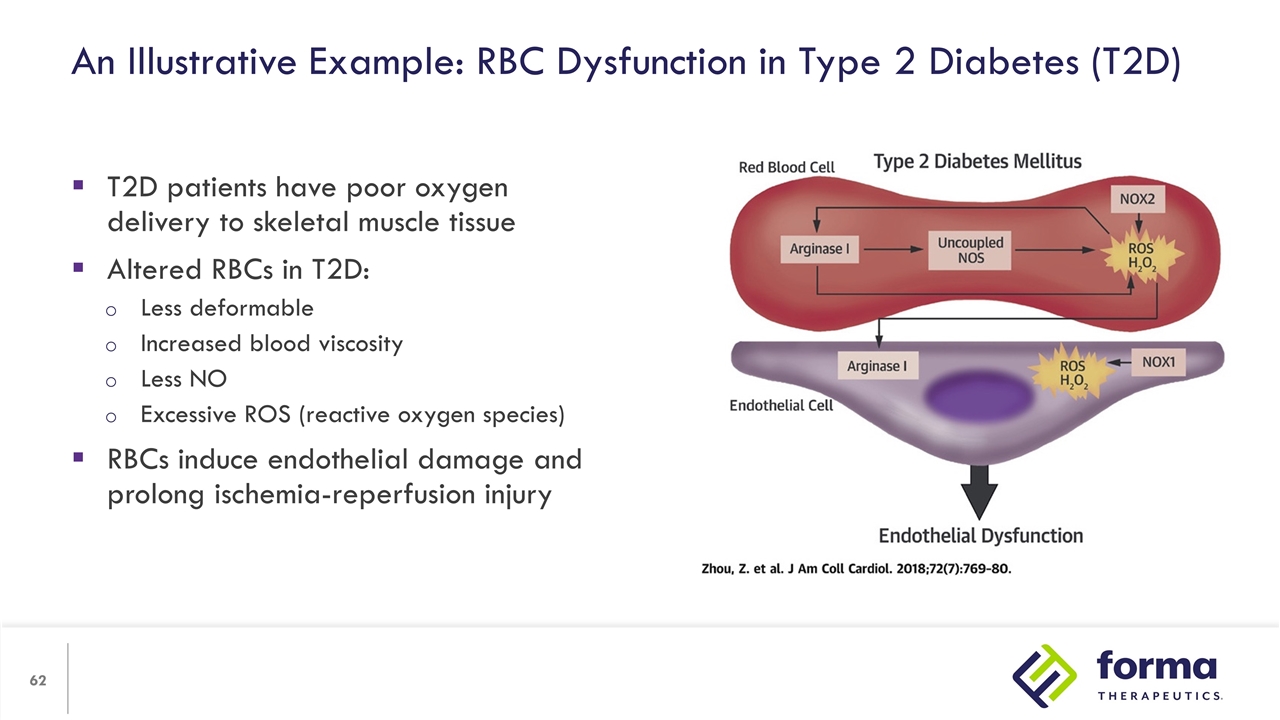
An Illustrative Example: RBC Dysfunction in Type 2 Diabetes (T2D) T2D patients have poor oxygen delivery to skeletal muscle tissue Altered RBCs in T2D: Less deformable Increased blood viscosity Less NO Excessive ROS (reactive oxygen species) RBCs induce endothelial damage and prolong ischemia-reperfusion injury

FT-7051

FT-7051 Program in Metastatic Castration-Resistant Prostate Cancer (mCRPC) Sylvie Guichard, Ph.D. Program Lead FT-7051 Executive Director, Translational Science

FT-7051 Program Highlights 25 patients enrolled as of May 12, 2022 Heavily pre-treated population (lines of therapy, genetics and disease burden) Drug exposures in the predicted efficacious exposure range supported by target engagement biomarkers Alternative schedule of administration based on PK/PD and safety profile to be explored in a less heavily treated patient population
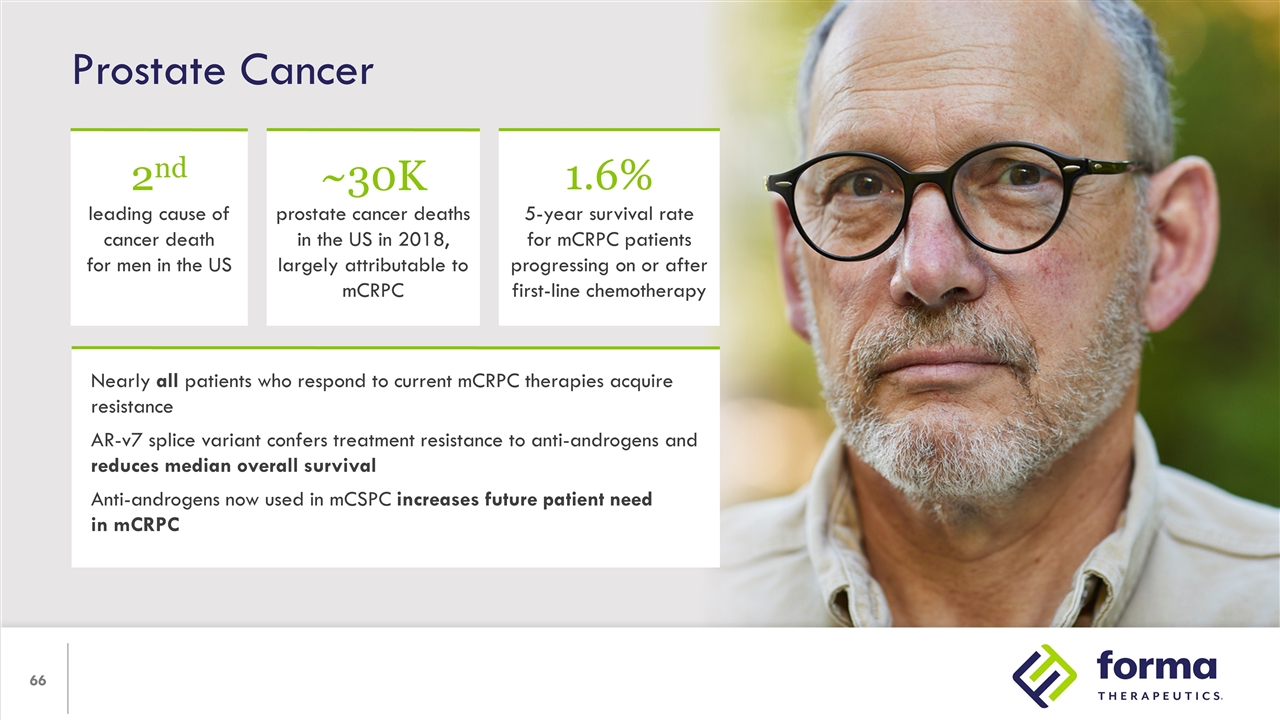
Prostate Cancer Nearly all patients who respond to current mCRPC therapies acquire resistance AR-v7 splice variant confers treatment resistance to anti-androgens and reduces median overall survival Anti-androgens now used in mCSPC increases future patient need in mCRPC 2nd leading cause of cancer death for men in the US ~30K prostate cancer deaths in the US in 2018, largely attributable to mCRPC 1.6% 5-year survival rate for mCRPC patients progressing on or after first-line chemotherapy
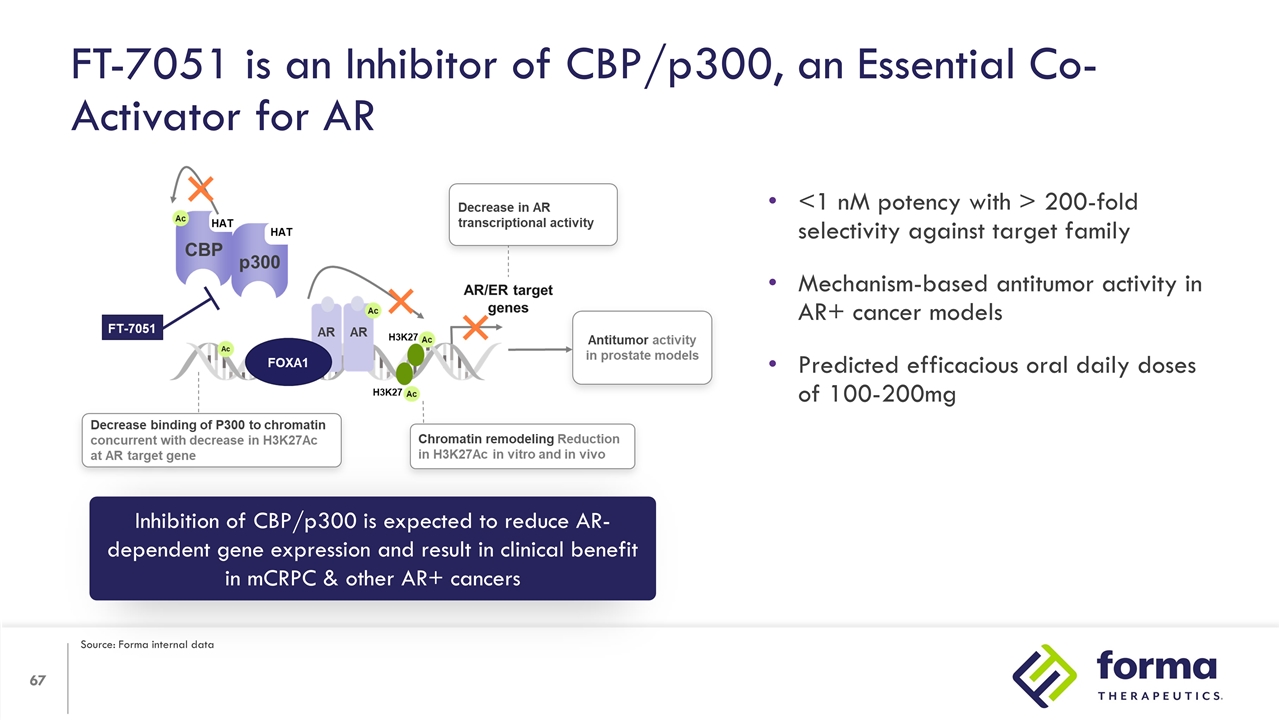
FT-7051 is an Inhibitor of CBP/p300, an Essential Co-Activator for AR Inhibition of CBP/p300 is expected to reduce AR-dependent gene expression and result in clinical benefit in mCRPC & other AR+ cancers <1 nM potency with > 200-fold selectivity against target family Mechanism-based antitumor activity in AR+ cancer models Predicted efficacious oral daily doses of 100-200mg Source: Forma internal data
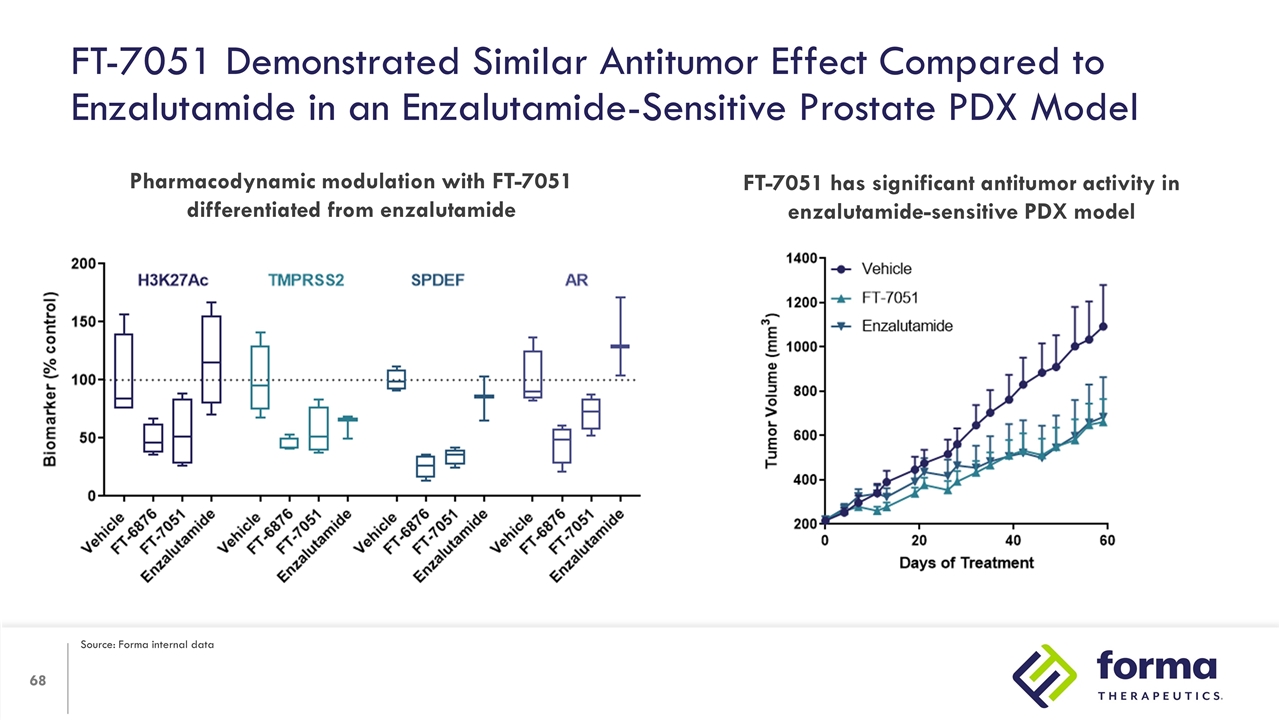
FT-7051 Demonstrated Similar Antitumor Effect Compared to Enzalutamide in an Enzalutamide-Sensitive Prostate PDX Model Pharmacodynamic modulation with FT-7051 differentiated from enzalutamide FT-7051 has significant antitumor activity in enzalutamide-sensitive PDX model Source: Forma internal data
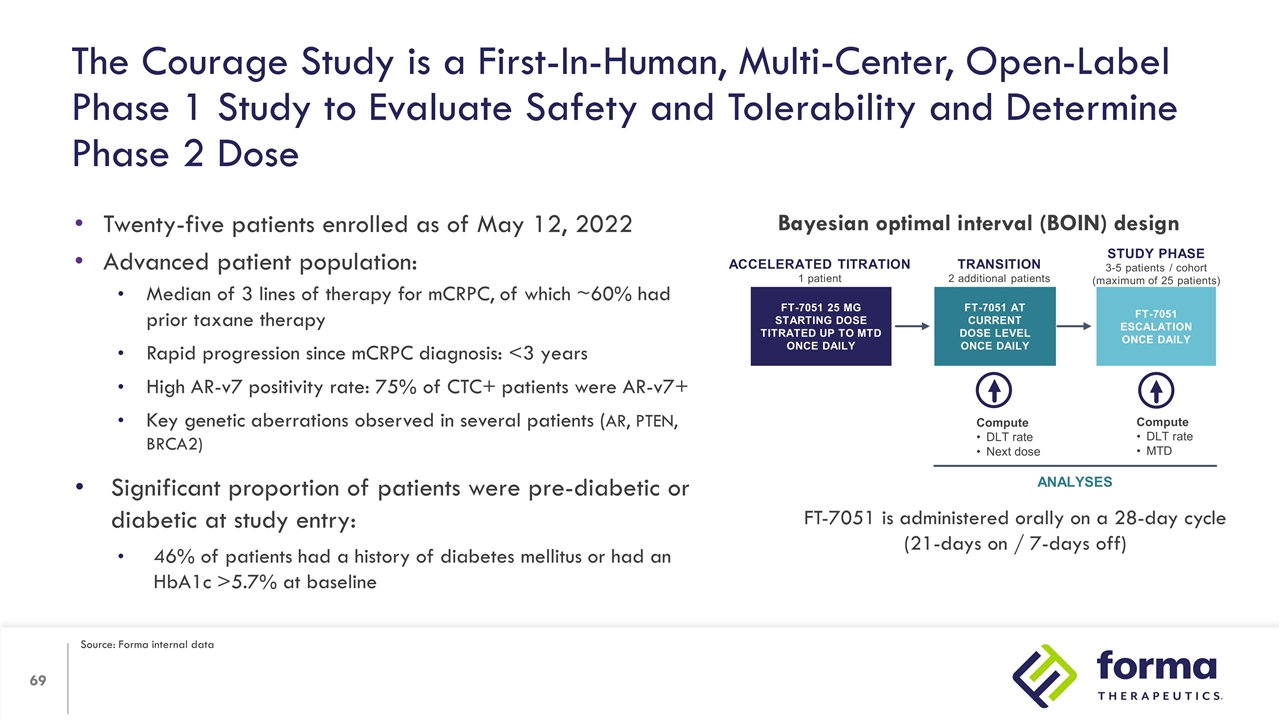
The Courage Study is a First-In-Human, Multi-Center, Open-Label Phase 1 Study to Evaluate Safety and Tolerability and Determine Phase 2 Dose Twenty-five patients enrolled as of May 12, 2022 Advanced patient population: Median of 3 lines of therapy for mCRPC, of which ~60% had prior taxane therapy Rapid progression since mCRPC diagnosis: <3 years High AR-v7 positivity rate: 75% of CTC+ patients were AR-v7+ Key genetic aberrations observed in several patients (AR, PTEN, BRCA2) Significant proportion of patients were pre-diabetic or diabetic at study entry: 46% of patients had a history of diabetes mellitus or had an HbA1c >5.7% at baseline Accelerated titration 1 patient FT-7051 25 mg Starting dose Titrated up to MTD once daily Compute DLT rate Next dose Compute DLT rate MTD ANALYSES TRANSITION 2 additional patients Study phase 3-5 patients / cohort (maximum of 25 patients) FT-7051 ESCALATION Once daily FT-7051 At current dose level Once daily Bayesian optimal interval (BOIN) design FT-7051 is administered orally on a 28-day cycle (21-days on / 7-days off) Source: Forma internal data
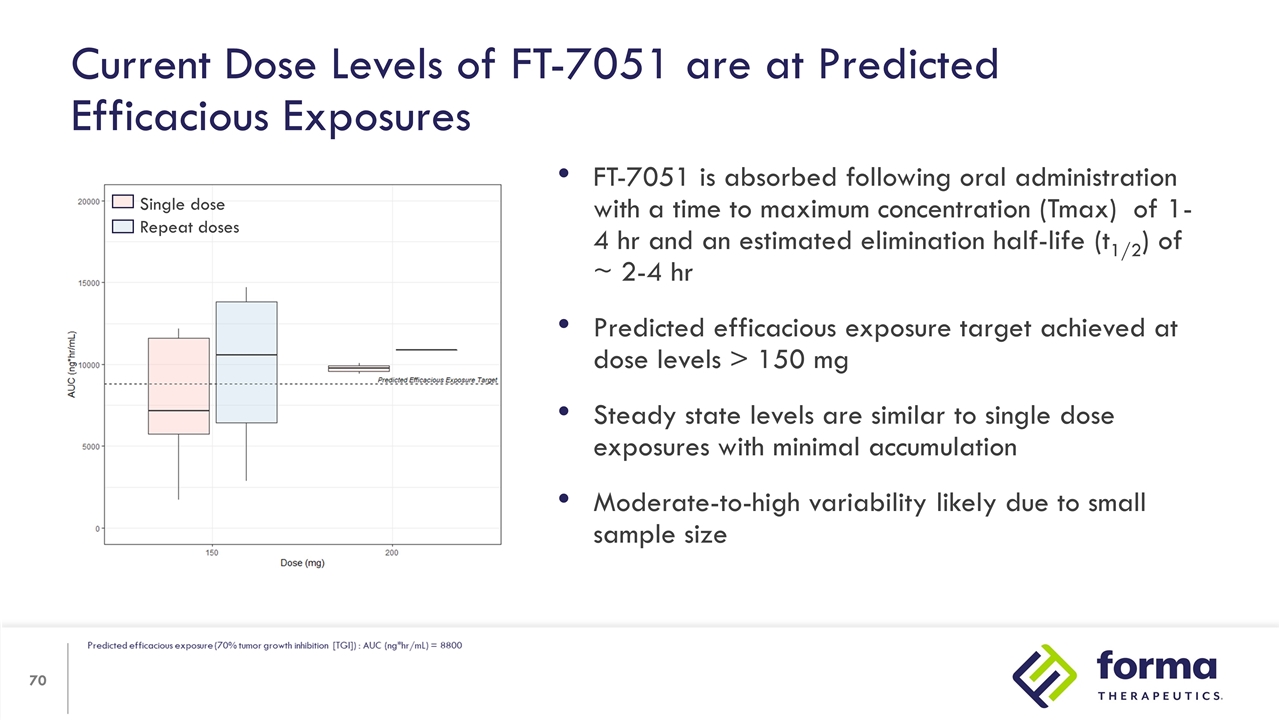
FT-7051 is absorbed following oral administration with a time to maximum concentration (Tmax) of 1- 4 hr and an estimated elimination half-life (t1/2) of ~ 2-4 hr Predicted efficacious exposure target achieved at dose levels > 150 mg Steady state levels are similar to single dose exposures with minimal accumulation Moderate-to-high variability likely due to small sample size Current Dose Levels of FT-7051 are at Predicted Efficacious Exposures Single dose Repeat doses
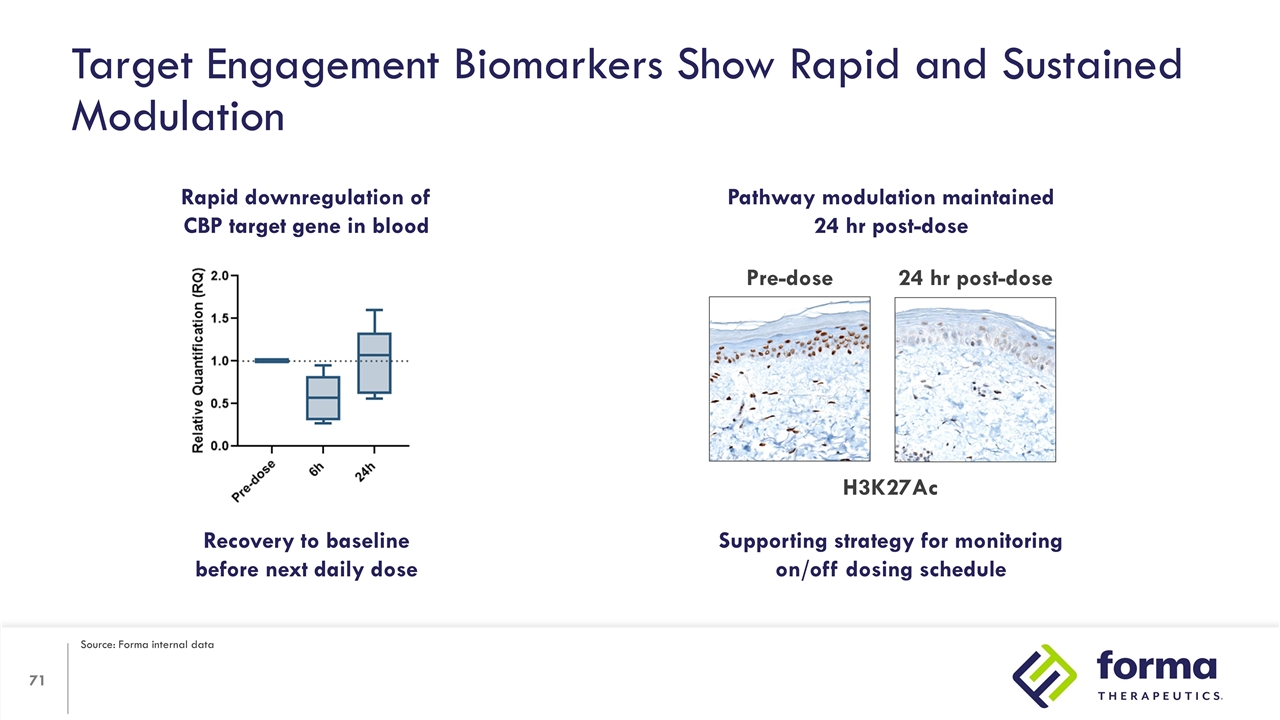
Target Engagement Biomarkers Show Rapid and Sustained Modulation Rapid downregulation of CBP target gene in blood Pathway modulation maintained 24 hr post-dose Pre-dose 24 hr post-dose Recovery to baseline before next daily dose Supporting strategy for monitoring on/off dosing schedule H3K27Ac Source: Forma internal data
On-target toxicity of hyperglycemia observed in patients with glucose intolerance or diabetes Manageable and monitorable with standard treatment Additional AEs such as fatigue and GI events appear cumulative with 3-week on/1-week off schedule Evaluating an alternative dose schedule at the predicted efficacious dose range A less heavily treated population will be enrolled to assess durability of response Safety Data Update

Maria D. Ribadeneira, Ph.D. Program Lead FT-3171 Executive Director DMPK and Toxicology FT-3171 Program USP1 inhibitor for the treatment of advanced solid tumors
Untapped DNA damage repair pathway A differentiated USP1 inhibitor with high potency, exquisite selectivity, and favorable tolerability Once-daily dosing as monotherapy or in combinations including PARPi Evidence of antitumor activity across multiple tumor types First half 2023 IND filing anticipated USP1 Program Highlights USP1: Ubiquitin Specific Protease 1
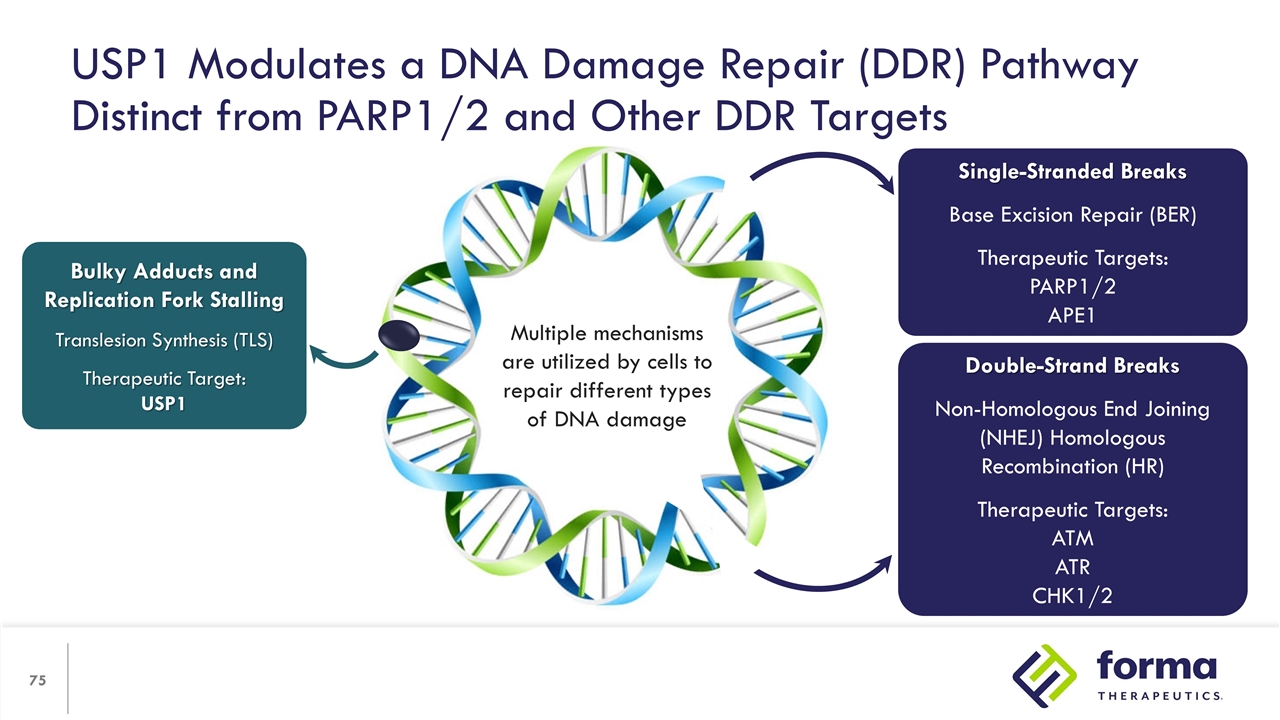
USP1 Modulates a DNA Damage Repair (DDR) Pathway Distinct from PARP1/2 and Other DDR Targets Bulky Adducts and Replication Fork Stalling Translesion Synthesis (TLS) Therapeutic Target: USP1 Single-Stranded Breaks Base Excision Repair (BER) Therapeutic Targets: PARP1/2 APE1 Double-Strand Breaks Non-Homologous End Joining (NHEJ) Homologous Recombination (HR) Therapeutic Targets: ATM ATR CHK1/2 Multiple mechanisms are utilized by cells to repair different types of DNA damage
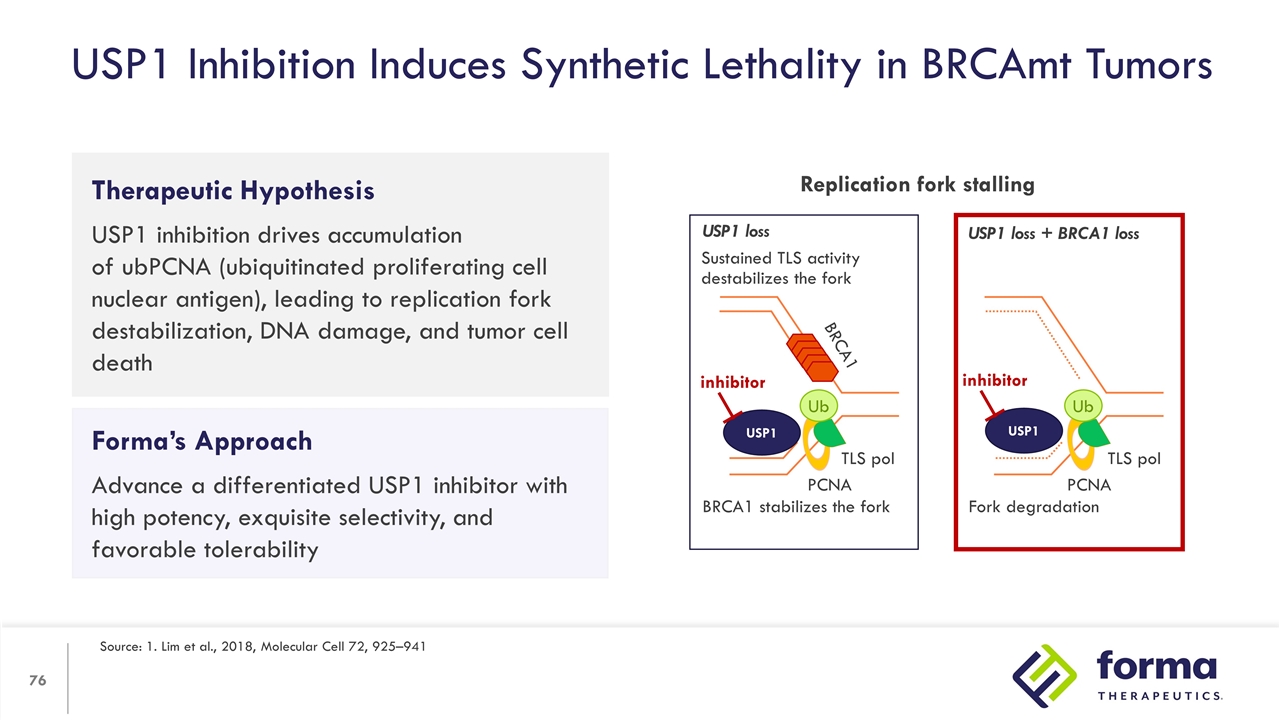
Therapeutic Hypothesis USP1 inhibition drives accumulation of ubPCNA (ubiquitinated proliferating cell nuclear antigen), leading to replication fork destabilization, DNA damage, and tumor cell death USP1 Inhibition Induces Synthetic Lethality in BRCAmt Tumors Source: 1. Lim et al., 2018, Molecular Cell 72, 925–941 Forma’s Approach Advance a differentiated USP1 inhibitor with high potency, exquisite selectivity, and favorable tolerability Replication fork stalling Ub Ub USP1 loss USP1 loss + BRCA1 loss PCNA PCNA TLS pol TLS pol BRCA1 stabilizes the fork Fork degradation Sustained TLS activity destabilizes the fork BRCA1 USP1 inhibitor USP1 inhibitor
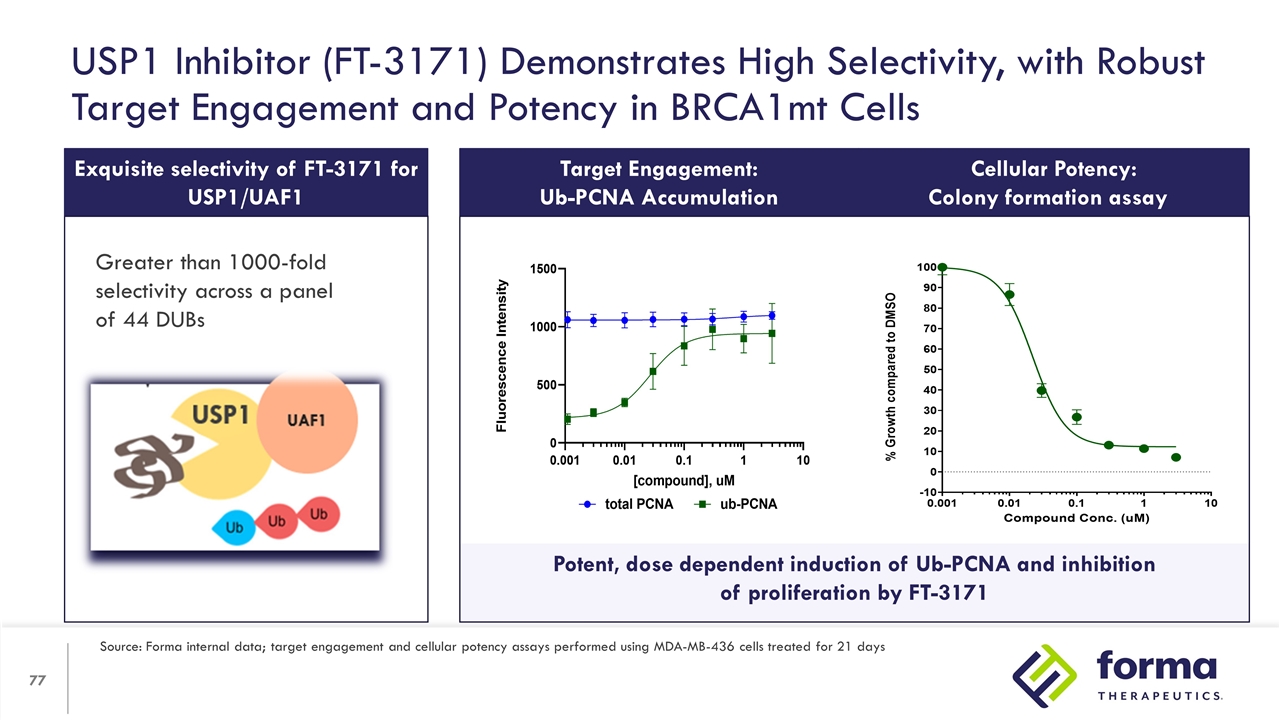
Potent, dose dependent induction of Ub-PCNA and inhibition of proliferation by FT-3171 Exquisite selectivity of FT-3171 for USP1/UAF1 * w/ WDR20 Greater than 1000-fold selectivity across a panel of 44 DUBs USP1 Inhibitor (FT-3171) Demonstrates High Selectivity, with Robust Target Engagement and Potency in BRCA1mt Cells Target Engagement: Ub-PCNA Accumulation Cellular Potency: Colony formation assay Source: Forma internal data; target engagement and cellular potency assays performed using MDA-MB-436 cells treated for 21 days
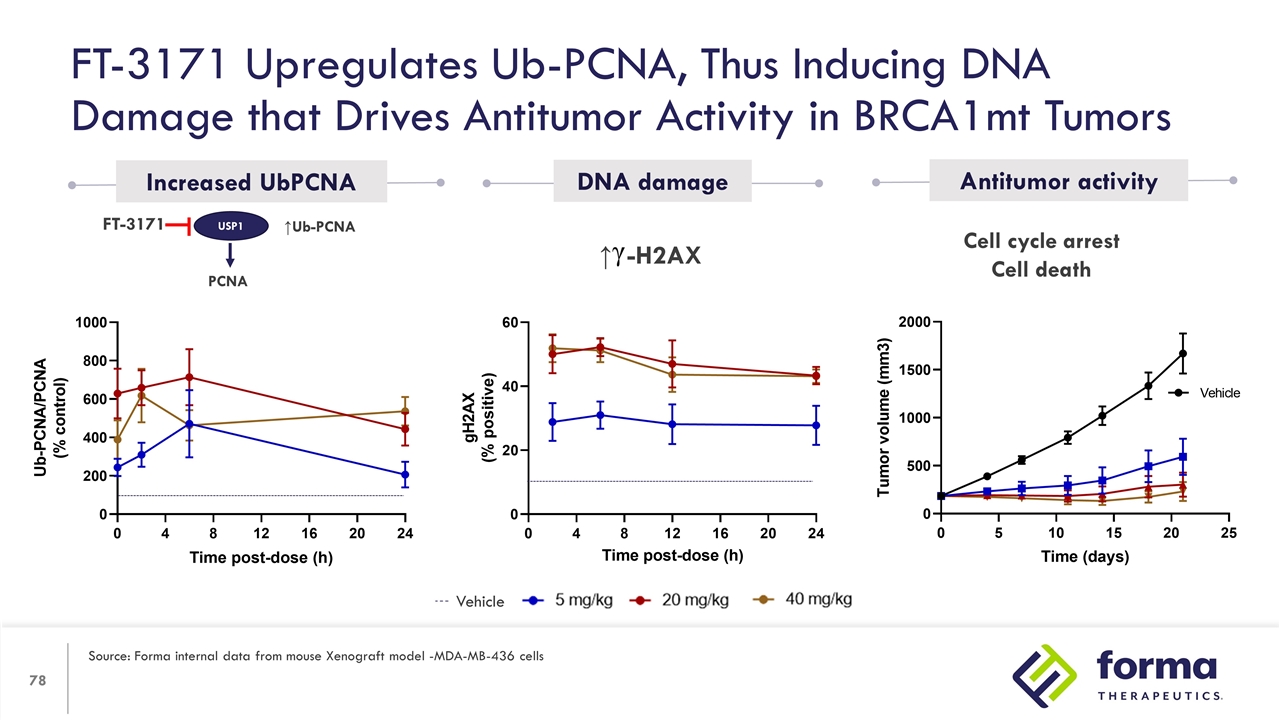
Cell cycle arrest Cell death ↑Ub-PCNA USP1 PCNA FT-3171 Increased UbPCNA DNA damage Antitumor activity FT-3171 Upregulates Ub-PCNA, Thus Inducing DNA Damage that Drives Antitumor Activity in BRCA1mt Tumors Source: Forma internal data from mouse Xenograft model -MDA-MB-436 cells Vehicle ↑ -H2AX
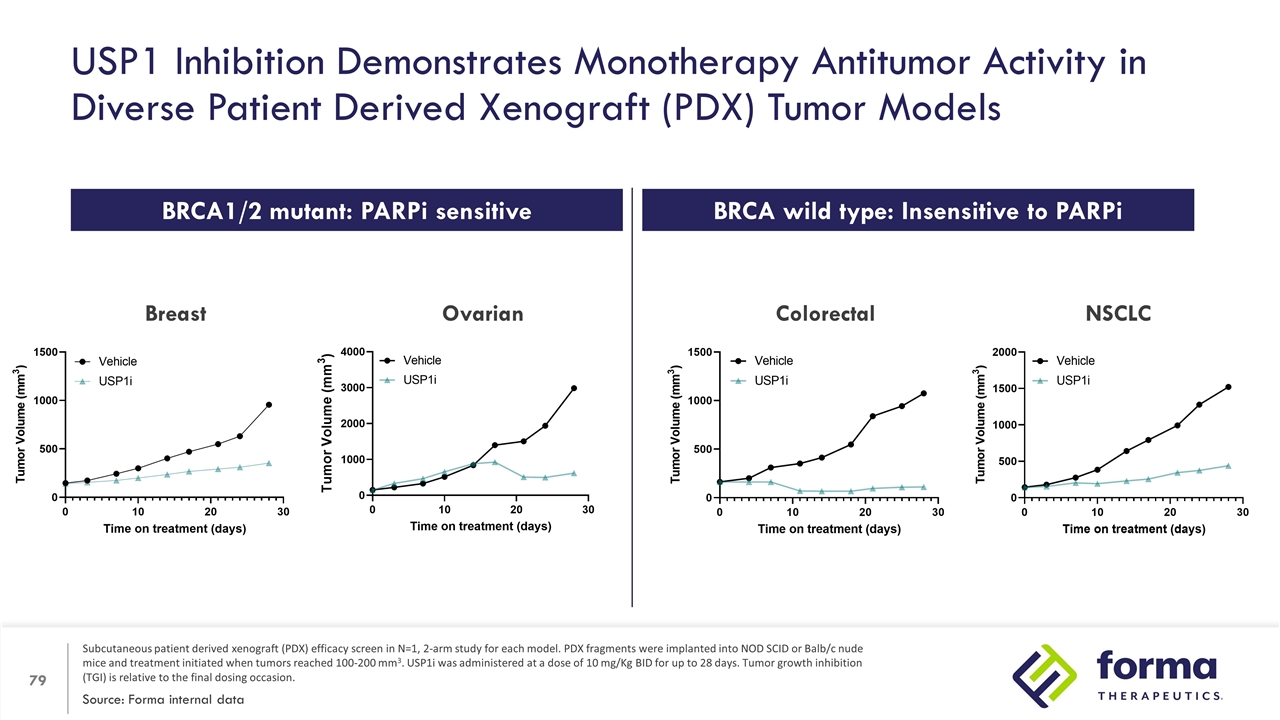
USP1 Inhibition Demonstrates Monotherapy Antitumor Activity in Diverse Patient Derived Xenograft (PDX) Tumor Models Source: Forma internal data Subcutaneous patient derived xenograft (PDX) efficacy screen in N=1, 2-arm study for each model. PDX fragments were implanted into NOD SCID or Balb/c nude mice and treatment initiated when tumors reached 100-200 mm3. USP1i was administered at a dose of 10 mg/Kg BID for up to 28 days. Tumor growth inhibition (TGI) is relative to the final dosing occasion. Breast NSCLC Colorectal BRCA1/2 mutant: PARPi sensitive BRCA wild type: Insensitive to PARPi Ovarian
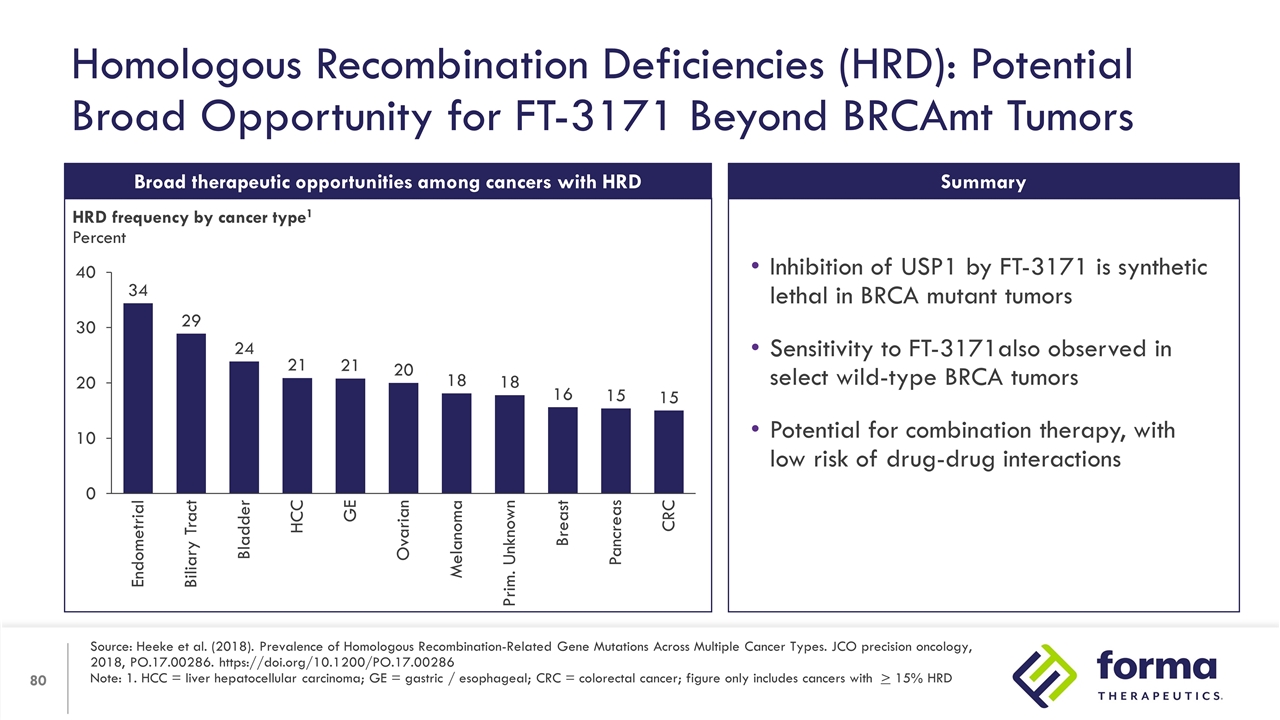
Homologous Recombination Deficiencies (HRD): Potential Broad Opportunity for FT-3171 Beyond BRCAmt Tumors Source: Heeke et al. (2018). Prevalence of Homologous Recombination-Related Gene Mutations Across Multiple Cancer Types. JCO precision oncology, 2018, PO.17.00286. https://doi.org/10.1200/PO.17.00286 Note: 1. HCC = liver hepatocellular carcinoma; GE = gastric / esophageal; CRC = colorectal cancer; figure only includes cancers with > 15% HRD Broad therapeutic opportunities among cancers with HRD HRD frequency by cancer type1 Percent Inhibition of USP1 by FT-3171 is synthetic lethal in BRCA mutant tumors Sensitivity to FT-3171also observed in select wild-type BRCA tumors Potential for combination therapy, with low risk of drug-drug interactions Summary

Frank Lee Chief Executive Officer In Closing…

Etavopivat: Exploring impact on transfusion burden in SCD, Thalassemia and Lower-risk MDS Additional analysis of pain events from Phase 1 study provides further support for potential to demonstrate VOC benefit First look at research pipeline with FT-3171 (USP1) and focus on Red Blood Cell health FT-7051 Trial update: Predicted efficacious dose range under evaluation with new dosing regimen and less heavily pre-treated patients Strong cash position: Actively managing expenses to extend runway Today's Highlights
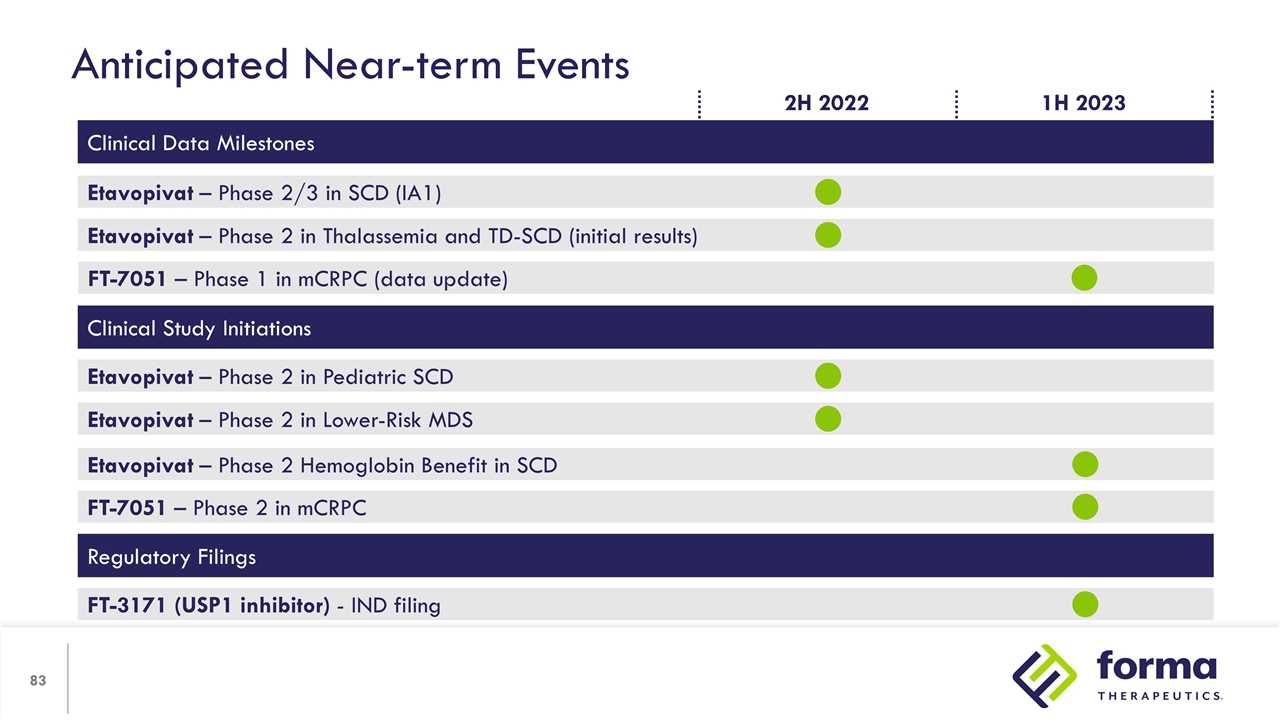
Anticipated Near-term Events Clinical Data Milestones Etavopivat – Phase 2/3 in SCD (IA1) Etavopivat – Phase 2 in Thalassemia and TD-SCD (initial results) FT-7051 – Phase 1 in mCRPC (data update) Clinical Study Initiations Etavopivat – Phase 2 in Lower-Risk MDS Etavopivat – Phase 2 in Pediatric SCD Regulatory Filings FT-3171 (USP1 inhibitor) - IND filing Etavopivat – Phase 2 Hemoglobin Benefit in SCD 1H 2023 2H 2022 FT-7051 – Phase 2 in mCRPC

Rick, living with prostate cancer Q&A

The Science of Giving a Damn
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Behind the Listings: Insights into Echemi Chemical Companies
- High Tech Health International, Inc. Announces Breakthrough to Maximize Health Benefits from Sauna Use
- Nextracker Launches Industry’s First Low Carbon Solar Tracker Solution
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share