Form 8-K EAGLE PHARMACEUTICALS, For: Jan 11
Exhibit 99.1

CONFIDENTIAL AND INTERNAL © 2022 Eagle Pharmaceuticals, Inc. All rights reserved. 1 1 Company Overview January 2022

CONFIDENTIAL AND INTERNAL © 2022 Eagle Pharmaceuticals, Inc. All rights reserved. 2 Forward - Looking Statements This presentation contains “forward - looking statements” within the meaning of the Private Securities Litigation Reform Act of 19 95, as amended, and other securities laws. Forward - looking statements are statements that are not historical facts. Words and phrases such as “anticipated,” “forward,” “will,” “would,” “m ay,” “remain,” “potential,” “prepare,” “expected,” “believe,” “plan,” “near future,” “belief,” “guidance,” and similar expressions are intended to identify forward - looking statements. These statements inc lude, but are not limited to, the Company’s ability to obtain and maintain regulatory approval of its products and product candidates; the Company's clinical development plan for its product can didates, including the number and timing of development initiatives or new indications for the Company’s product candidates and the anticipated development expenses for such development plans; the potential therapeutic and clinical benefits of the Company’s product candidates; the Company’s timing and ability to enroll patients in upcoming clinical trials; the timing, scope or lik eli hood and timing of regulatory filings and approvals from the FDA for the Company’s product candidates, including Landiolol and its fulvestrant product; the timing, progress and success of the Company’s potential launch of any products, including va so pressin and PEMFEXY; the ability of the Company to successfully commercialize its product candidates, including vasopressin and PEMFEXY; the ability of vasopressin to benefit providers and patients as an alternative to Vasostrict ; the period of marketing exclusivity for any of the Company’s products or product candidates, including vasopressin; the pot ent ial market opportunity for any of the Company’s products; the ability of the Company to obtain and maintain coverage and adequate reimbursement for its products; t he success of the Company's collaborations with its strategic partners and the timing and results of these partners’ preclinical studies and clinical trials, including the Company’s colla bor ations with its licensing partners SymBio , Combioxin SA and AOP Orphan Pharmaceuticals GmbH; the future commercial success of its product candidates, if approved, related to such licensing agreeme nts , and anticipated royalty and milestone revenue and potential market opportunity for such product candidates; the ability of the Company’s executive team to execute on the Company’s strat egy and to utilize its cash and other assets to increase shareholder value; expectations regarding the Company’s future growth and its ability to generate significant cash in the future; the Com pan y’s ability to effectively manage and control expenses in line with its budget; the Company's timing and ability to repurchase additional shares of the Company's common stock, if any, under its Sha re Repurchase Program; and the ability of the Company’s product candidates to deliver value to stockholders. All of such statements are subject to certain risks and uncertainties, many of w hic h are difficult to predict and generally beyond the Company’s control, that could cause actual results to differ materially from those expressed in, or implied or projected by, the forward - looking in formation and statements. Such risks and uncertainties include, but are not limited to: the impacts of the ongoing COVID - 19 pandemic, including interruptions or other adverse effects on clinical trial s and delays in regulatory review or further disruption or delay of any pending or future litigation; whether the Company will incur unforeseen expenses or liabilities or other market factors; dela y i n or failure to obtain regulatory approval of the Company's product candidates and successful compliance with FDA, European Medicines Agency and other governmental regulations applicable to pro duc t approvals; whether the Company will successfully implement its development plan for its product candidates, including its fulvestrant product; whether the Company can success ful ly market and commercialize its product candidates; the success of the Company's relationships with its partners; the availability and pricing of third party sourced products and materials; th e o utcome of litigation involving any of its products or that may have an impact on any of its products; successful compliance with the FDA and other governmental regulations applicable to product ap pro vals, manufacturing facilities, products and/or businesses; general economic conditions, including the potential adverse effects of public health issues, including the COVID - 19 pandemic, on econom ic activity and the performance of the financial markets generally; the strength and enforceability of the Company’s intellectual property rights or the rights of third parties; competition fro m o ther pharmaceutical and biotechnology companies and the potential for competition from generic entrants into the market; the risks inherent in drug development and in conducting clinical trials, inc luding that preliminary results from clinical trials are not necessarily predictive of future clinical trial results; and those risks and uncertainties identified in the “Risk Factors” section of th e C ompany’s Annual Report on Form 10 - K for the year ended December 31, 2020 filed with the Securities and Exchange Commission (the “SEC”) on March 5, 2021, as updated by the Company’s Quarterly Re por ts on Form 10 - Q for the quarters ended March 31, 2021, June 30, 2021 and September 30, 2021 filed with the SEC on May 10, 2021, August 9, 2021 and November 9, 2021, respectively, and it s o ther subsequent filings with the SEC. Readers are cautioned not to place undue reliance on the forward - looking statements contained in this presentation, which speak only as of the date h ereof. Except to the extent required by law, the Company undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date hereof.

3 CONFIDENTIAL AND INTERNAL © 2022 Eagle Pharmaceuticals, Inc. All rights reserved. 3 CONFIDENTIAL AND INTERNAL © 2022 Eagle Pharmaceuticals, Inc. All rights reserved. Eagle Overview: A Mainstream Pharmaceutical Company Specializing in Oncology + Acute Care Specialty Pharma Company Pharmaceutical Company Specializing in Acute Care & Oncology Opportunistic 505b2’s Maintain Financial Health Long Duration Assets NCE’s ®

4 CONFIDENTIAL AND INTERNAL © 2022 Eagle Pharmaceuticals, Inc. All rights reserved. Eagle Pharmaceuticals Financial Position, Portfolio & Pipeline *As of 12/31/21 **As of 9/30/21 Share Buybacks $230M or 24%* Net Working Capital of $123.3M** Total Cash and Cash Equivalents $99.7M** No net debt supports opportunistic approach to transactions Bendeka ® Belrapzo ® Ryanodex ® Current Portfolio Vasopressin Pemfexy Œ Upcoming Launches Landiolol CAL02 SM - 88*** Fulvestrant ***Strategic collaboration with Tyme Technologies Product Pipeline Treakisym ® Symbio Japan Strong Financial Position

CONFIDENTIAL AND INTERNAL © 2022 Eagle Pharmaceuticals, Inc. All rights reserved. 5 Near Term Business Highlights » Vasopressin: Shipping to commence on Monday, January 17, 2022, with 180 days of marketing exclusivity. An important product for Eagle, as Vasostrict ® U.S. sales totaled $890 million for the LTM ended September 30, 2021. » PEMFEXY τ : On February 1, 2022, the Company will launch PEMFEXY, a ready - to - use liquid with a unique J - code. Eagle has been building inventory and believes this is a significant opportunity, as the Alimta ® U.S. market totaled $1.2 billion for the LTM ended September 30, 2021. » TREAKISYM: Eagle’s bendamustine franchise continues to grow, with the Japan launch of TREAKISYM ready - to - dilute (“RTD”) formulation. Together with a potential approval of the rapid infusion (“RI”) (50ml) liquid formulation, this could generate approximately $20 million of combined royalty and milestone revenue in 2022. » Fulvestrant: Based on discussions with FDA, Eagle will commence human pilot studies of its fulvestrant product candidate for the treatment of HR+/HER - advanced breast cancer shortly. » Landiolol: Eagle is on track to submit an NDA in the first half of 2022, seeking approval of Landiolol, a novel therapeutic, for the sho rt - term reduction of ventricular rate in patients with supraventricular tachycardia, including atrial fibrillation and atrial flutter . » CAL02: Eagle is preparing to begin clinical trials for CAL02, a novel approach to the treatment of severe bacterial pneumonia, later th is year.

CONFIDENTIAL AND INTERNAL © 2022 Eagle Pharmaceuticals, Inc. All rights reserved. 6 6 CNS/Metabolic Critical Care Pipeline Opportunities Vasopressin

CONFIDENTIAL AND INTERNAL © 2022 Eagle Pharmaceuticals, Inc. All rights reserved. 7 Vasopressin Overview Vasopressin injection is FDA - approved to increase blood pressure in adults with vasodilatory shock (e.g., post - cardiotomy or sepsis) who remain hypotensive despite fluids and catecholamines. Currently Endo/Par markets VASOSTRICT ® (vasopressin) U.S. sales totaled $890 million for the LTM ended September 30, 2021* Eagle is first - to - file an ANDA referencing VASOSTRICT ® for the 20 units per ml presentation Commercial launch on January 17 th 2022 180 - day market exclusivity Successful vasopressin patent trial; Court held Eagle’s proposed vasopressin product does not infringe any of the patents Par asserted against the Company *Source: Endo International plc

CONFIDENTIAL AND INTERNAL © 2022 Eagle Pharmaceuticals, Inc. All rights reserved. 8 8 CNS/Metabolic Critical Care Pipeline Opportunities CAL02 and Landiolol

CONFIDENTIAL AND INTERNAL © 2022 Eagle Pharmaceuticals, Inc. All rights reserved. 9 CAL02 Overview Novel first - in - class antitoxin agent in development for combination use with antibiotics for the treatment of severe pneumonia Proposed injectable treatment for severely infected patients Applying for Qualified Infectious Disease Product Designation under the GAIN Act CAL02 (drug product): Specific mixture of re - engineered empty liposomes solely composed of sphingomyelin and cholesterol capable of capturing and neutralizing a broad spectrum of virulence effectors.

CONFIDENTIAL AND INTERNAL © 2022 Eagle Pharmaceuticals, Inc. All rights reserved. 10 CAL02 – Novel, First - in - Class Antitoxin Agent Mechanism of Action Address the downstream effects of bacterial Virulence Effectors/ Pore Forming Toxins through competitive inhibition » Binds to virulence effector molecules secreted by infecting bacteria, prohibiting host tissue cell binding » Acts as an extracellular “sink” for these toxins » Potential to attenuate pore forming toxin related effects including host tissue damage, immune dysregulation, and inflammation that contribute to increase disease severity Lead Indication Severe Community Acquired Pneumonia » Significant morbidity and mortality despite advances in direct acting antibacterials » Addresses significant medical need and burden on health care systems Differentiated Advantages » Potential to be used as adjuvant therapy with any traditional antibacterial [therapy agnostic] » Potential to be used against any bacteria that produces pore forming toxins [bacteria agnostic] » Potential to carry less risk of antibacterial resistance development Development Program somewhat de - risked for phase of development » FTIH proof of concept study showed tolerability as well as trends toward efficacy » Positive regulatory interactions with FDA and EMA – may be eligible for special designations and review processes » Scalable manufacturing process Anticipate that d evelopment costs through interim results will total approximately $35 million
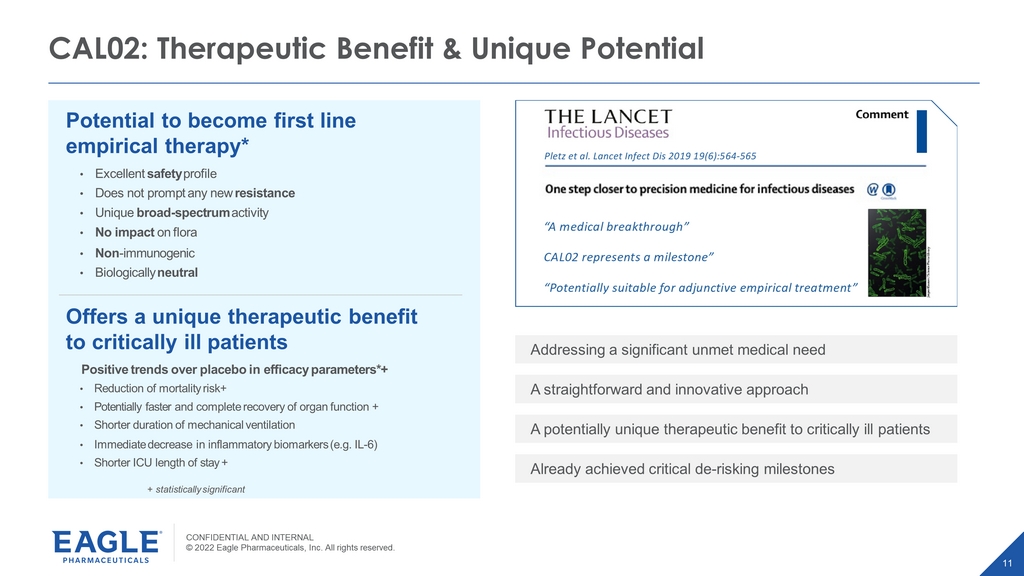
CONFIDENTIAL AND INTERNAL © 2022 Eagle Pharmaceuticals, Inc. All rights reserved. 11 CAL02: Therapeutic Benefit & Unique Potential Potential to become first line empirical therapy* • Excellent safety profile • Does not prompt any new resistance • Unique broad - spectrum activity • No impact on flora • Non - immunogenic • Biologically neutral Offers a unique therapeutic benefit to critically ill patients Positive trends over placebo in efficacy parameters*+ • Reduction of mortality risk+ • Potentially faster and complete recovery of organ function + • Shorter duration of mechanical ventilation • Immediate decrease in inflammatory biomarkers (e.g. IL - 6) • Shorter ICU length of stay + + statistically significant Pletzet al. Lancet Infect Dis 2019 19(6):564-565 “A medical breakthrough” CAL02 represents a milestone” “Potentially suitable for adjunctive empirical treatment” Addressing a significant unmet medical need A straightforward and innovative approach A potentially unique therapeutic benefit to critically ill patients Already achieved critical de - risking milestones

CONFIDENTIAL AND INTERNAL © 2022 Eagle Pharmaceuticals, Inc. All rights reserved. 12 Landiolol : Beta - 1 Adrenergic Blocker; Leading Hospital Emergency Use Product Signed licensing agreement for U.S. commercial rights from AOP Orphan Pharmaceuticals (AOP) in August 2021 Eagle will facilitate regulatory pathway for U.S. approval based on existing data from Japanese and European studies with no additional clinical work expected Approved in Europe for the treatment of non - compensatory sinus tachycardia and tachycardic supraventricular arrhythmias Eagle will support seeking approval of Landiolol for short - term reduction of ventricular rate in patients with supraventricular tachycardia, including atrial fibrillation and atrial flutter in the U.S. Anticipate filing NDA in Q1 2022, with expected ten - month review, based on well - defined feedback from FDA provided during AOP’s Type C meeting Studies for additional indications, including sepsis and other cardioprotective indications, have begun in Europe, with the potential to be pursued in the U.S. Enrollment of study in pediatric patients with supraventricular tachycardia is underway in Europe and will serve as the basis for initial pediatric study plans for a future FDA submission Expect five years of new chemical entity exclusivity

CONFIDENTIAL AND INTERNAL © 2022 Eagle Pharmaceuticals, Inc. All rights reserved. 13 13 CNS/Metabolic Critical Care Pipeline Opportunities RYANODEX ® ®

CONFIDENTIAL AND INTERNAL © 2022 Eagle Pharmaceuticals, Inc. All rights reserved. 14 RYANODEX ® ( dantrolene sodium) injectable suspension Currently indicated for the treatment of malignant hyperthermia (MH) in conjunction with appropriate supportive measures, and for the prevention of MH in patients at high risk July 2014 Approved August 2014 Launched Breakthrough formulation

CONFIDENTIAL AND INTERNAL © 2022 Eagle Pharmaceuticals, Inc. All rights reserved. 15 RYANODEX ® : Building a Successful Franchise Ten U.S. patents issued to date, expiring between 2022 and 2025 New indications under development* Nerve Agent (NA) Exposure* Acute Radiation Syndrome (ARS)* FDA - Approved for Malignant Hyperthermia (MH) Traumatic Brain Injury/ Concussion (TBI)* Alzheimer’s Disease (AD)*

CONFIDENTIAL AND INTERNAL © 2022 Eagle Pharmaceuticals, Inc. All rights reserved. 16 16 Oncology Pipeline Opportunities PEMFEXY Ρ
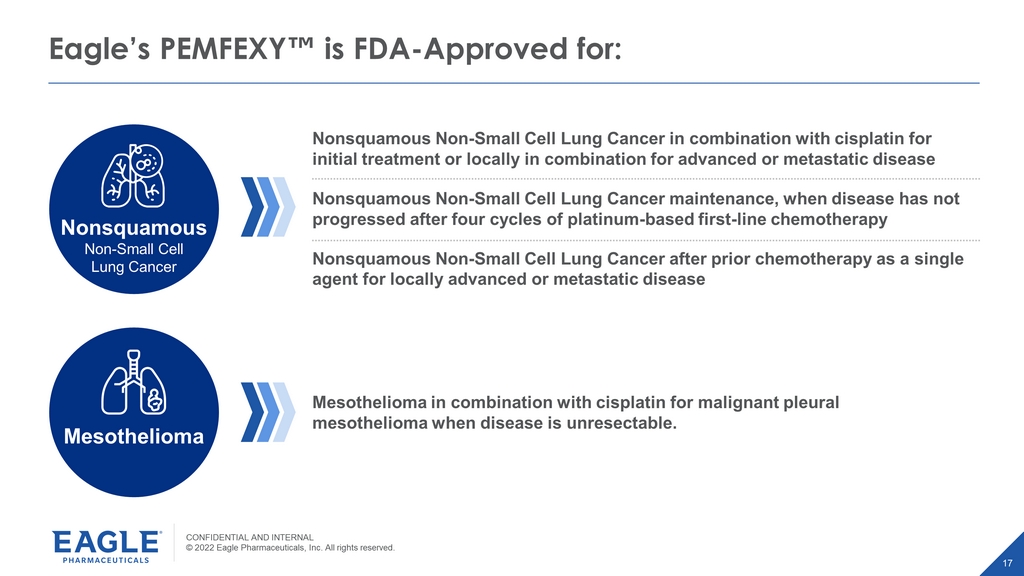
CONFIDENTIAL AND INTERNAL © 2022 Eagle Pharmaceuticals, Inc. All rights reserved. 17 Eagle’s PEMFEXY Œ is FDA - Approved for: Nonsquamous Non - Small Cell Lung Cancer Nonsquamous Non - Small Cell Lung Cancer in combination with cisplatin for initial treatment or locally in combination for advanced or metastatic disease Nonsquamous Non - Small Cell Lung Cancer maintenance, when disease has not progressed after four cycles of platinum - based first - line chemotherapy Nonsquamous Non - Small Cell Lung Cancer after prior chemotherapy as a single agent for locally advanced or metastatic disease Mesothelioma in combination with cisplatin for malignant pleural mesothelioma when disease is unresectable. Mesothelioma

CONFIDENTIAL AND INTERNAL © 2022 Eagle Pharmaceuticals, Inc. All rights reserved. 18 ALIMTA ® (Eli Lilly) - PEMFEXY Œ (Eagle) Currently marketed by Lilly as ALIMTA ® (pemetrexed) 100mg and 500mg powder single dose vials – U.S. market totaled $1.2 billion for the LTM ended September 30, 2021* 1.2B Eagle first to market 505(b)(2) PEMFEXY Œ (pemetrexed) 500mg liquid multi - dose vial – Granted unique J - code by CMS – Launch planned February 1 st 2022 – Generic entrants blocked until May 24, 2022 *Source: Eli Lilly and Company

CONFIDENTIAL AND INTERNAL © 2022 Eagle Pharmaceuticals, Inc. All rights reserved. 19 Eagle’s Differentiated PEMFEXY Œ (pemetrexed): Other pemetrexed formulations are single - dose powder, which require reconstitution Some patients may need 2 - 3 vials; time - consuming for pharmacist/nurse and wastage occurs frequently because they are not multi - dose vials Other pemetrexed Eagle’s formulation is available in a 500mg liquid ready - to - dilute multi - dose vial PEMFEXY Œ eliminates the reconstitution process wastage and helps prevent medication errors. The vial can be reused under refrigeration for 28 days. Eagle’s formulation

CONFIDENTIAL AND INTERNAL © 2022 Eagle Pharmaceuticals, Inc. All rights reserved. 20 20 Oncology Pipeline Opportunities EA - 114 (Fulvestrant)

CONFIDENTIAL AND INTERNAL © 2022 Eagle Pharmaceuticals, Inc. All rights reserved. 21 EA - 114: Our Fulvestrant Product Candidate for HR+/HER2 - Advanced Breast Cancer Impact of Advanced Breast Cancer • Eagle’s 600 - subject PK trial yielded ~18,000 data points, which we mined for insights • For fulvestrant to work, it needs to bind to and block the estrogen receptor (ER) • Not everyone treated with fulvestrant achieves the desired result – a substantial number of women with advanced HR+/HER2 - breast cancer receiving standard treatment experience early disease progression • Currently, low ER inhibition is an important factor resulting in suboptimal treatment, which may lead to faster progression of the disease • Our research suggests Eagle’s product could substantially improve the clinical outcomes for these post - menopausal metastatic breast cancer patients ~75% of breast cancers are HR+ 1 ~30% of patients first diagnosed with early - stage disease eventually develop metastatic disease 2 27% five - year survival for patients in U.S. with metastatic breast cancer 3 1. Keen JC, Davidson NE. The biology of breast carcinoma. Cancer 2003;97:825 – 33. DOI: 10.1002/cncr.11126 2. Zhao H, et al. Incidence and prognostic factors of patients with synchronous liver metastases upon initial diagnosis of br eas t cancer: a population - based study. Dove Press. 27 September 2018. DOI https://doi.org/10.2147/CMAR.S178395. 3. Howlader N, et al (eds). SEER Cancer Statistics Review, 1975 - 2016, National Cancer Institute, Bethesda, MD, https://seer.canc er. gov/csr/1975_2016/, based on November 2018 SEER data submission, posted to the SEER website, April 2019. An Unmet Need

CONFIDENTIAL AND INTERNAL © 2022 Eagle Pharmaceuticals, Inc. All rights reserved. 22 Existing Product Partnerships SM - 88 • In 2020 Eagle and TYME entered into a share purchase agreement and a co - promotion agreement for SM - 88 • SM - 88 is a novel investigational agent in a Phase II/III trial for pancreatic cancer • For SM - 88 Eagle shall earn 15% of U.S. net sales and will be responsible for 25% of the promotional effort • Tyme may buy out Eagle’s rights at any time under the co - promotion agreement for $200mm • SymBio received approval of TREAKISYM Ready - To - Dilute (“RTD”) bendamustine formulation and launched in January 2021 • SymBio is currently conducting a clinical trial for a rapid infusion bendamustine product and pursuing additional indications • Eagle earns tiered royalties on net sales of licensed products and $20 - $25mm from combined royalty and milestone revenue in 2022

CONFIDENTIAL AND INTERNAL © 2022 Eagle Pharmaceuticals, Inc. All rights reserved. 23 23 CONFIDENTIAL AND INTERNAL © 2022 Eagle Pharmaceuticals, Inc. All rights reserved. Inflection Points

CONFIDENTIAL AND INTERNAL © 2022 Eagle Pharmaceuticals, Inc. All rights reserved. 24 Eagle Pharmaceuticals Summary Well positioned to capitalize on near - term opportunities Mainstream pharmaceutical company with over 40 representatives calling into Hospital & Oncologists Commercial infrastructure in place and positioned to take on additional assets Strong financial position supports opportunistic approach to transactions Commercial Expansion Financial Position Experienced Organization

CONFIDENTIAL AND INTERNAL © 2022 Eagle Pharmaceuticals, Inc. All rights reserved. 25 CONFIDENTIAL AND INTERNAL © 2022 Eagle Pharmaceuticals, Inc. All rights reserved. Thank You!
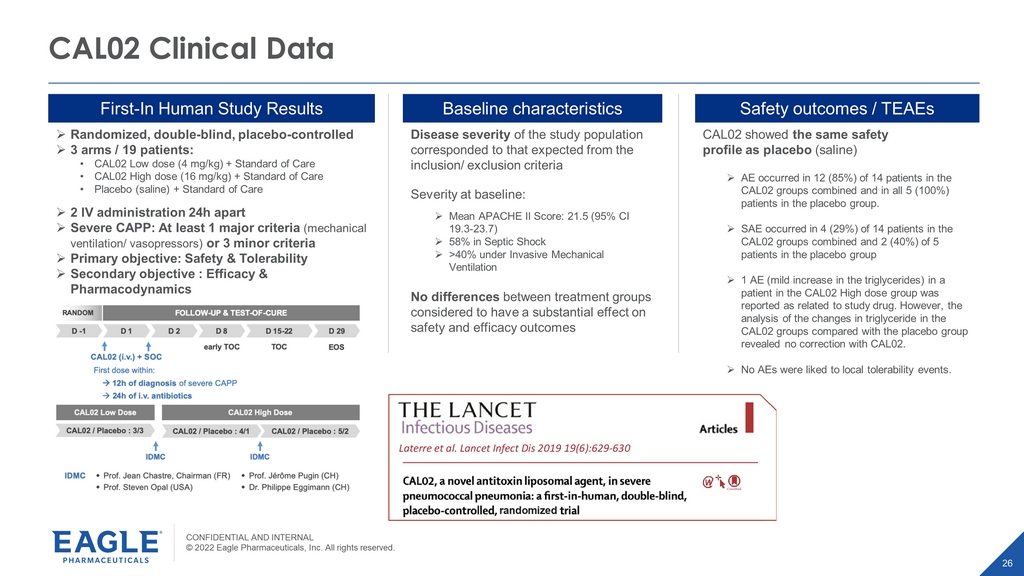
CONFIDENTIAL AND INTERNAL © 2022 Eagle Pharmaceuticals, Inc. All rights reserved. 26 CAL02 Clinical Data First - In Human Study Results » Randomized, double - blind, placebo - controlled » 3 arms / 19 patients: • CAL02 Low dose (4 mg/kg) + Standard of Care • CAL02 High dose (16 mg/kg) + Standard of Care • Placebo (saline) + Standard of Care » 2 IV administration 24h apart » Severe CAPP: At least 1 major criteria (mechanical ventilation/ vasopressors) or 3 minor criteria » Primary objective: Safety & Tolerability » Secondary objective : Efficacy & Pharmacodynamics Disease severity of the study population corresponded to that expected from the inclusion/ exclusion criteria Severity at baseline: » Mean APACHE II Score: 21.5 (95% CI 19.3 - 23.7) » 58% in Septic Shock » >40% under Invasive Mechanical Ventilation No differences between treatment groups considered to have a substantial effect on safety and efficacy outcomes CAL02 showed the same safety profile as placebo (saline) » AE occurred in 12 (85%) of 14 patients in the CAL02 groups combined and in all 5 (100%) patients in the placebo group. » SAE occurred in 4 (29%) of 14 patients in the CAL02 groups combined and 2 (40%) of 5 patients in the placebo group » 1 AE (mild increase in the triglycerides) in a patient in the CAL02 High dose group was reported as related to study drug. However, the analysis of the changes in triglyceride in the CAL02 groups compared with the placebo group revealed no correction with CAL02. » No AEs were liked to local tolerability events. Baseline characteristics Safety outcomes / TEAEs Laterre et al. Lancet Infect Dis 201919(6):629-630 randomized
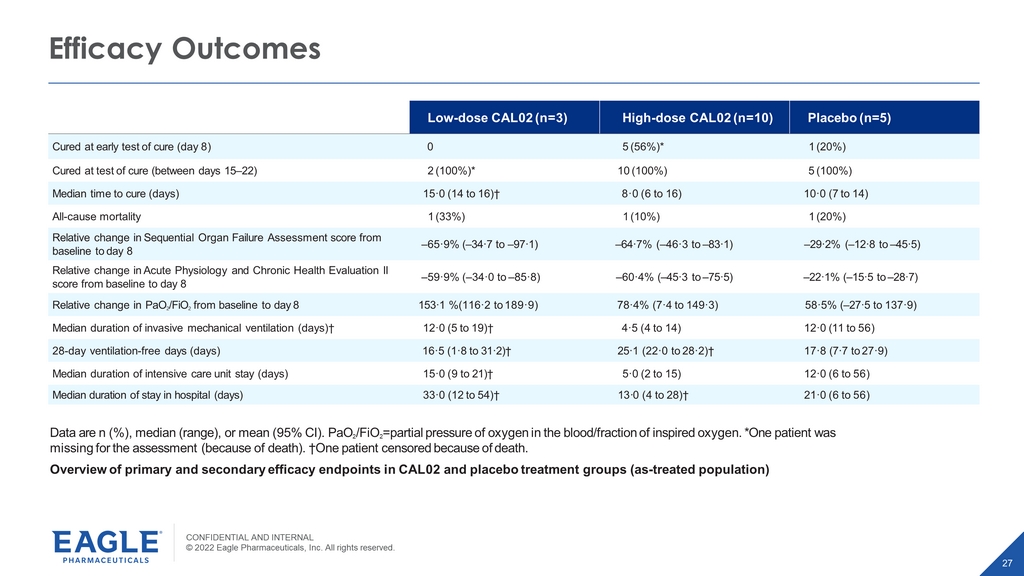
CONFIDENTIAL AND INTERNAL © 2022 Eagle Pharmaceuticals, Inc. All rights reserved. 27 Efficacy Outcomes Low - dose CAL02 (n=3) High - dose CAL02 (n=10) Placebo (n=5) Cured at early test of cure (day 8) 0 5 (56%)* 1 (20%) Cured at test of cure (between days 15 – 22) 2 (100%)* 10 (100%) 5 (100%) Median time to cure (days) 15·0 (14 to 16)† 8·0 (6 to 16) 10·0 (7 to 14) All - cause mortality 1 (33%) 1 (10%) 1 (20%) Relative change in Sequential Organ Failure Assessment score from baseline to day 8 – 65·9% ( – 34·7 to – 97·1) – 64·7% ( – 46·3 to – 83·1) – 29·2% ( – 12·8 to – 45·5) Relative change in Acute Physiology and Chronic Health Evaluation II score from baseline to day 8 – 59·9% ( – 34·0 to – 85·8) – 60·4% ( – 45·3 to – 75·5) – 22·1% ( – 15·5 to – 28·7) Relative change in PaO 2 /FiO 2 from baseline to day 8 153·1 %(116·2 to 189·9) 78·4% (7·4 to 149·3) 58·5% ( – 27·5 to 137·9) Median duration of invasive mechanical ventilation (days)† 12·0 (5 to 19)† 4·5 (4 to 14) 12·0 (11 to 56) 28 - day ventilation - free days (days) 16·5 (1·8 to 31·2)† 25·1 (22·0 to 28·2)† 17·8 (7·7 to 27·9) Median duration of intensive care unit stay (days) 15·0 (9 to 21)† 5·0 (2 to 15) 12·0 (6 to 56) Median duration of stay in hospital (days) 33·0 (12 to 54)† 13 · 0 (4 to 28)† 21·0 (6 to 56) Data are n (%), median (range), or mean (95% CI). Pa O 2 /FiO 2 =partial pressure of o xygen in the blood/fraction of inspired o xygen. *One patient was missing for the assessment (because of death). †One patient censored because of death. Overview of primary and secondary e ffi cacy endpoints in CAL02 and placebo treatment groups (as - treated population)
CONFIDENTIAL AND INTERNAL © 2022 Eagle Pharmaceuticals, Inc. All rights reserved. 28 CAL02 Competitive Advantages Limitations of current approaches (approved / in development) Limited use » Restrictions imposed by stewardship measures and purchasers, as antibiotics are inevitably linked to the emergence of new resistances • Potentially will not drive resistance; fills a significant medical gap • Offers physicians a new treatment; potential to dramatically improve outcomes • Combines with any treatment (antibacterial agnostic) • May lead to a tremendous economy on cost of care; broad - spectrum (used irrespective of pathogen identification or hemoculture or resistance to antibacterials) • Broad therapeutic impact • Potential for expedited regulatory pathway to approval Limited scope of application » Action dedicated against resistant mechanism » New mechanisms ultimately facing resistance issues » Monoclonal antibodies targeting a single toxin » Agents targeting a downstream specific pathway or cytokine dedicated to target patients already in shock Slow and laborious market penetration » Based on non - inferiority results » Last - resort treatments » Increasingly competitive space CAL02

CONFIDENTIAL AND INTERNAL © 2022 Eagle Pharmaceuticals, Inc. All rights reserved. 29 CAL02 Phase 2 Clinical Development Plan Development Costs through Interim Results 1. IND Filing 2. Start P2B/3 Multicenter Global Study – Part 1 3. P2B/3 Multicenter Global Study – Part 1 4. Interim Analysis Results Deal Signing Milestone $10M Phase I – Drug - Drug Interaction $1M P2B/3 Multicenter Global Study – Part 1 Through Interim Analysis Results $21M Clinical Trial Materials $3M $35M Total = Key Next Steps
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Eagle Pharmaceuticals to Present Additional Data from Phase III Trial Demonstrating Sustained Response of Amisulpride for the Rescue Treatment of Postoperative Nausea and Vomiting (PONV) at the Upcomi
- Odyssey Marine Exploration Addresses NASDAQ Compliance Matters
- Eagle Pharmaceuticals (EGRX) Receives Nasdaq Non-compliance Notice
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share