Form 8-K Clarus Therapeutics Hold For: Sep 20
Exhibit 99.1

1 CORPORATE PRESENTATION SEPTEMBER 2021 ANDROGEN AND METABOLIC THERAPIES FOR BETTER HEALTH

2 DISCLAIMER Certain statements in this presentation constitute “forward - looking statements” for purposes of the federal securities laws. The words “anticipate,” “believe,” “contemplate,” “continue,” “could,” “estimate,” “expect,” “intends,” “may,” “might,” “plan,” “possible,” “potential,” “predic t,” “project,” “should,” “will,” “would” and similar expressions may identify forward - looking statements, but the absence of these words does not mean that a statement is not forwar d - looking. These statements are based on our management’s beliefs and assumptions and on information currently available to management. Forward - looking statemen ts include all statements other than statements of historical fact contained in this presentation, including information concerning our current and fut ure financial performance, business plans and objectives, including but not limited to the expansion of commercialization of JATENZO®, our pipeline, continued growth and expansion, including collaboration with third parties and expected outcomes, and our ability to deliver value to patients and stockholders . In addition, any statements that refer to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumptions, are forward - looking statemen ts. These forward - looking statements are based on current expectations and beliefs concerning future developments and their potential effects. There ca n b e no assurance that future developments affecting us will be those that we have anticipated. These forward - looking statements involve a number of risks, un certainties (some of which are beyond our control) or other assumptions that may cause actual results or performance to be materially different from those e xpr essed or implied by these forward - looking statements. These risks and uncertainties include, but are not limited to, risks associated with our financial positi on, risks associated with our indebtedness, our dependence on JATENZO ®, and risks associated with our industry, along with those other factors described under the heading “Risk Factors” in the proxy statement/prospectus filed with the Securities and Exchange Commission (the “SEC”) on July 23, 2021, and those that are inclu ded in any of our future filings with the SEC. Should one or more of these risks or uncertainties materialize, or should any of our assumptions prove incorrect, ac tua l results may vary in material respects from those projected in these forward - looking statements. Some of these risks and uncertainties may in the future be amplified b y the COVID - 19 pandemic and there may be additional risks that we consider immaterial, or which are unknown. It is not possible to predict or identify all such ri sks. Accordingly, undue reliance should not be placed upon the forward - looking statements. Our forward - looking statements only speak as of the date they are made, and w e do not undertake any obligation to update or revise any forward - looking statements, whether as a result of new information, future events or otherwise, except a s may be required under applicable securities laws. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data ob tai ned from third - party sources and our own internal estimates and research. While we believe these third - party sources to be reliable as of the date of this presentati on, we have not independently verified, and make no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third - party sources. In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while we believe our own internal research is reliable, such research has not been verified by any inde pen dent source.

3 3 1 2 6 3 4 Clarus markets JATENZO ® , an FDA approved, first - in - class oral testosterone (T) replacement therapy (“TRT”) with strong potential to capture & grow market share. Each JATENZO market share point in the U.S. is expected to yield ~$33MM in annual net sales. With adequate support , we believe JATENZO can achieve low double - digit market share. Experienced pharma executive team and a commercial organization with a track record of building TRT brand leadership. Clarus is exploring out - licensing opportunities for JATENZO in attractive pharma markets outside the U. S. (e.g., EU, Asia, Middle East) to secure non - dilutive revenue. Clarus owns a worldwide patent estate for JATENZO with protection until at least 2030. Currently, JATENZO has 5 Orange Book listed patents. INVESTMENT THESIS 5 Clarus recently in - licensed two products with ‘orphan drug’ potential (and beyond) to build its R&D product pipeline. Broader business development activities are planned.

4 ROBERT DUDLEY, PH.D President & CEO • Over 30 years experience in TRT field • Clarus co - founder • Extensive R&D experience and FDA regulatory experience • Co - inventor and developer of AndroGel 1% • Co - inventor of JATENZO • Track record of success with public company • Abbott, Solvay, Unimed • Urologist devoted to Men’s Health issues • Extensive experience with TRT • Focus in MH diagnostics and therapeutics • Wide network of urology thought leaders • Launched two novel urologic diagnostics • Abbott/ AbbVie, OPKO, Genomic Health JAY NEWMARK, MD, MBA Chief Medical Officer • Tech transfer of 200+ drugs and biologics • Directed manufacturing site & process design • Launched multiple drugs, biologics, & vaccines • Grew R&D engineering outsourcing • Pharmacia, Wyeth, Cardinal Health, Catalent JAMES HOLLOWAY SVP, Manufacturing & Supply CLARUS LEADERSHIP TEAM CLARUS BOARD OF DIRECTORS KIMBERLY MURPHY Chair JOHN AMORY, MD, MPH, MSc Director ELIZABETH A. CERMAK Director & Chair, Nominating & Corporate Governance Committees ROBERT DUDLEY, PH.D. Founder, President, CEO JOSEPH HERNANDEZ Director MARK A. PRYGOCKI, SR. Director & Chair, Audit Committee ALEX ZISSON Director & Chair, Compensation Committee • Over 20 years of successful global financial leadership • Experienced in capital markets, private and public offerings (IPO), secondary financings • Established relationships with institutional funds, financial analysts and investment bankers • Medicis , Novan , Sienna RIC PETERSON Chief Financial Officer • 17 years experience in TRT market; • Extensive finance experience • Clarus co - founder • Over 30 years in start - up life sciences; • Raised over $250M in debt & equity • Aksys, PWC, Ernst & Young STEVEN BOURNE Chief Administrative Officer FRANK JAEGER, MA, MBA Chief Commercial Officer • 20+ years commercial background with significant TRT experience • Launched AndroGel 1.62% and grew to $1.3B • Successful co - commercialization experience • Responsible for multiple launches over career • AbbVie, Abbott, Solvay

5 THE JATENZO ® OPPORTUNITY

6 6 U. S. TESTOSTERONE (T) MARKET IS LARGE & GROWING T DEFICIENCY IN MEN (HYPOGONADISM) STILL HAS AREAS OF UNMET NEED Diagnosed Population Opportunity to educate men about the signs and symptoms of hypogonadism due to certain medical conditions References: U.S. Census (2019); Int J of Clin Pract (2006); J Clin Endo Metabolism (2007); Symphony Healthcare (2014); IMS Health (2015); StrataMark survey results ~ 8MM TRx/year ~ $33MM Net JATENZO Sales / Share Point Affected Population Opportunity to educate men who have been either diagnosed or previously treated about the benefits of JATENZO® Treated Population Opportunity to switch on - label treated patients from injectables and gel forms of TRT and start previously untreated hypogonadal men on JATENZO® ~20 MM ~6 MM ~2.2 MM Male Hypogonadism Prevalence Diagnosed Patients Currently Treated Patients

7 7 Current Non - oral Treatments Pose Administration Challenges The Cycle of Discontinuation More than 95,000 men change TRTs at least once per year* • Injection pain • Procedures • Mess, drying time • Transference to partner/kids • Skin irritation • Gum irritation/disorders • Nasal irritation IMPORTANCE OF AN ORAL T OPTION SIGNIFICANT UNMET NEEDS AND CHALLENGES WITH NON - ORAL TREATMENTS * Data from Symphony PTD Rx claims from July 2019 to August 2020. Included approved claims only; rejections and reversals not included.

8 8 THE JATENZO OPPORTUNITY FIRST ORAL T OF ITS KIND APPROVED BY FDA – A LONG - AWAITED ADVANC E! CONVENIENT • Easy - to - swallow soft gel taken BID with food (twice daily) • Dose adjustable, if necessary EFFECTIVE • 87% of men achieved T levels in normal range • Restored T levels to mid - normal range SAFE • Safety profile consistent with TRT class • No liver toxicity -- JATENZO bypasses first - pass hepatic metabolism * In JATENZO Phase III Clinical Trials * *

9 9 JATENZO PRODUCT DIFFERENTIATORS A SAFE ORAL TRT WITHOUT LIVER TOXICITY THAT ADDRESSES COMMON CON CERNS SEEN WITH INJECTIONS AND GELS LACK OF A SAFE AND EFFECTIVE ORAL TESTOSTERONE INJECTABLE T PRODUCTS CAN BE PAINFUL AND CARRY SIGNIFICANT RISKS TOPICAL T PRODUCTS ARE MESSY AND CARRY RISK OF TRANSFERENCE 95% OF ALL TRT PRESCRIPTIONS WRITTEN 76% Of patients believe their needs are not very well met by currently available TRTs* * On behalf of Clarus Therapeutics, Inc., Harris Poll conducted online survey between May 6 – June 5, 2020.

10 10 Increased Bone Mineral Density Improved Body Composition Improved Psychosexual Symptoms Increased Free (Active) Testosterone References: Swerdloff, RS and Dudley, RE. Ther Adv Ur o l 2020; 12: 1 - 16. Swerdloff, RS, et al. J Clin Endocrinol Metab 2020:105: 1 - 17

11 Adverse Reactions ≥ 2% in 4 - Month Pivotal Trial of JATENZO 1 JATENZO WAS SHOWN TO BE SAFE AND WELL - TOLERATED JATENZO SAFETY PROFILE CONSISTENT WITH TRT CLASS References: 1 Swerdloff, RS, et al. J Clin Endocrinol Metab 2020:105: 1 - 17; 2 Swerdloff, RS and Dudley, RE. Ther Adv Ur o l 2020; 12: 1 - 16; (see also: www.JATENZO.com) OVERALL (N=166) Headache 8 (4.8%) Hematocrit Increased 8 (4.8%) Hypertension 6 (3.6%) HDL Cholesterol Decreased 5 (3.0%) Nausea 4 (2.4%) In Phase 3 Trials, JATENZO was not a ssociated with 2 : • Liver toxicity • Prostate disease • Adverse changes in CV risk biomarkers [ hs - CRP, LpPLA 2 , Lp (a)] • Clinically significant increases in LDL (‘bad’) cholesterol or triglycerides

12 MAXIMIZING JATENZO’S COMMERCIAL POTENTIAL

13 TRT MARKET IS LARGE & GROWING HEALTHCARE PROVIDERS CONTINUE TO ACTIVELY DIAGNOSE AND TREAT HYP OGONADAL PATIENTS 13 • Market Volume • High volume of ~8MM TRxs annually • Growth Rate • 7.5% 5 - year CAGR* • Valuable to Clarus • Payers reimburse branded agents • Each JATENZO share point is ~ $33MM in net sales * Data Sources: Symphony Metys Data Q3, 2016 through the end of Q2, 2021 0 500,000 1,000,000 1,500,000 2,000,000 2,500,000 Quarterly TRT Total Market TRx Volume

14 JATENZO IS POISED TO ACCELERATE GROWTH THE COMMERCIAL FOUNDATION HAS BEEN ESTABLISHED 14 55 Territories 62% Coverage of TRT Market Endo, URO, and PCP Top Deciles 5 National Account Managers Commercial, Part D, Medicaid Health Care Provider (HCP ) Education; Patient Advertising Data Source: Symphony Health PrescriberSource data February 2021

15 JATENZO HAS COMPETITIVE & AFFORDABLE COVERAGE JATENZO IS COVERED ACROSS ALL PAYER CHANNELS 15 Data Sources: Competitive data using MMIT, (as of 8/26/2021) Payer Coverage Across ALL payer channels* * Payer Channels include Commercial, Medicare Part D, Medicaid, and Health Exchanges 90% 64% 61% 68% 57% 70% 61% 71% 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% Androderm AndroGel Fortesta Natesto Testim Xyosted JATENZO Current JATENZO 2021 Target Lives Covered

16 DTC ADVERTISING WILL BE A KEY GROWTH DRIVER BY COMMUNICATING TO CONSUMERS, WE BELIEVE PATIENTS WILL DRIVE JA TENZO CONVERSATIONS WITH HCP s ) 16 Patient Awareness Patient Demand

17 EXPANDED SALES FORCE REACH INCREASES HCP AWARENESS … AND WHEN COMBINED WITH DTC ADVERTISING, FOSTERS APPROPRIATE DIA LOG BETWEEN PATIENTS AND HCP s 17 Current 55 Territories Future Planned Expansion

18 PATIENT ‘HUB’ CREATES KEY POINT OF PATIENT CONTACT TO FACILITATE EFFECTIVE JATENZO DISTRIBUTION HUB ASSISTS WITH DRUG BENEFITS, PRIOR AUTHORIZATIONS, JATENZO R x DELIVERY, CO - PAY SUPPORT, AND PATIENT EDUCATION 18 Supports patient j ourney … … all the way to product fulfillment

19 FOCUS ON PATIENT DESIGNED TO DRIVE JATENZO DEMAND 19 Telemedicine Find - a - Doc Targeted HCPs Clarus Expanded Sales Force Increases “Reach and Frequency” Direct Ship Patient Patient Easily Reorders JATENZO Patient HUB Service Patient Sees JATENZO DTC Ad Patient Visits JATENZO Website

20 GROWTH OPPORTUNITIES

21 3 - PRONGED APPROACH TO GROWING STRONG BUSINESS 21 JATENZO LIFE - CYCLE MANAGEMENT AND BUSINESS DEVELOPMENT PLANNED T O GROW BUISNESS JATENZO LIFE - CYCLE MANGEMENT ESTABLISH R & D PIPELINE BUSINESS DEVELOPMENT Hypogonadism ~8 MM Prescriptions/ yr F - T - M Transgender 700K Male Patients Chronic Kidney Disease >2 MM Hypogonadal Men T + A (CLAR - 121) - Inflammatory breast disease (potential Orphan Drug) - Adjunctive therapy in ER+/AR+ breast cancer CoQ 10 + Caspofungin (CLAR - 222) - Primary & secondary CoQ 10 deficiencies (potential Orphan Drug)

22 • Painful, debilitating inflammation of breast tissue – predominantly in women • No effective pharmaceutical intervention; often results in disfiguring surgery • CLAR - 121 mechanism - of - action is to activate AR - dependent anti - inflammatory mechanism in breast tissue • History of CLAR - 121 clinical use in Australia expected to support rapid path to Phase 2 clinical study • Estimated U. S. cases of ~150,000 women/year; potential orphan disease with large market opportunity TESTOSTERONE (T) + ANSTROZOLE (A) -- CLAR - 121 A PROPRIETARY T + A PRODUCT FOR ANDROGEN DEPENDENT INFLAMMATORY BREAST DISEASE AND ER+/AR+ BREAST CANCER • Androgen receptor suppresses ER+/AR+ breast cancer 1 - ~80% of all ER+ breast cancers in U.S. are AR+ • CLAR - 121 mechanism - of - action is AR activation and suppression of estradiol binding to ER to suppress tumor growth • Target population for CLAR - 121 is ~ 180,000 patients/year; potential orphan disease with large market opportunity https://www.eurekalert.org/pub_releases/2021 - 01/uoa - ndi011421.php 1 Hickey, TE, Selth , LA, Chia, KM, et al. The androgen receptor is a tumor suppressor in estrogen receptor – positive breast cancer. Nature Med. 2 021; 27: 310 - 320. INFLAMMATORY PERIDUCTAL MASTITIS ESTROGEN RECEPTOR (ER+) / ANDROGEN RECEPTOR (AR+) BREAST CANCER

23 CoQ 10 + CASPOFUNGIN FOR CoQ 10 DEFICIENCIES 1 Wang, Y. and Hekimi, S. Micellization of coenzyme Q by the fungicide caspofungin allows for safe intravenous administration t o reach extreme supraphysiological concentrations . Redox Biol 2020; 36: 101680.; Figures from: Stefely JA and Pagliarini DJ. Biochemistry of mitochondrial Coenzyme Q biosynthesis. Trends Biochem Sci. 2017, 42:824 - 843. 2 Ng, YS and Turnbull, DM. Mitochorndiral disease: genetics and management. J. Neurol. 263: 179 - 191. 3 https://my.clevelandclinic.org/health/diseases/15612 - mitochondrial - diseases#:~:text=How%20common%20are%20mitochondrial%20disease s,born%20with%20a%20mitochondrial%20disease. POTENTIAL TREATMENT FOR PRIMARY AND SECONDARY UBIQUINONE DEFICIE NCY; MITOCHONDRIAL DISEASES • Technology in - licensed from McGill University - Worldwide rights; upfront, milestone & royalties - US / PCT patent applications pending • CoQ 10 is natural coenzyme vital for mitochondrial function; caspofungin is antifungal drug approved for adult and pediatric use; large safety margin • Extensive preclinical proof - of - concept data generated by McGill 1 • Orphan Drug candidate for certain CoQ 10 deficiencies; • Broader market opportunities for less severe genetic CoQ 10 deficiencies - Genetic mitochondrial diseases afflict > 1 in 5000 adults and ~1000+ children per year in U.S. 2, 3

24 R & D PROGRAMS & MILESTONES 24 R & D Program Description / Remark Clinical Testing Phase Projected Initiation JATENZO®: CKD Treatment of hypogonadal men with chronic kidney disease / labeling expansion Phase 4 2H 2021 Once - Daily Oral TU Once - daily oral TU product to treat male hypogonadism / JATENZO life - cycle management Phase 2 1H 2022 JATENZO: Male Transgender T therapy for female - to - male transgender individuals / labeling expansion Phase 4 1H 2022 CLAR - 121 (T + A) In - licensed from HavaH Therapeutics (Australia) for treatment of inflammatory breast disease (PDM) and as adjunctive therapy in ER+/AR+ breast cancer Phase 2 (PDM) 1H 2022 CLAR - 122 (CoQ 10 + CF) In - licensed from McGill University (Canada) for treatment of primary and secondary forms of CoQ 10 deficiency and related mitochondrial dysfunction Phase 1 2H 2022

25 INTELLECTUAL PROPERTY & EXCLUSIVITY PATHWAYS • Robust JATENZO IP Estate: • Clear Pathway to Issued IP and Market Exclusivity for T+A (CLAR - 121) and CoQ 10 + CF (CLAR - 222): - Patent applications pending in U.S. and major non - U.S. pharma markets - Potential for 5 - Year Hatch - Waxman market exclusivity on approval - Potential for 7 - Year Orphan Drug market exclusivity No pending patent infringement or interference actions against Clarus. (Prior claims by Lipocine settled favorably for Clarus) Clarus owns full rights to patents and applications for JATENZO (U.S. and main ex - U.S. pharma markets) Five Orange Book - listed patents with expiry in 2029 (1) and 2030 (4) - requires Paragraph IV filing for generics (None filed to date) Potential for 6 - month period of exclusivity to be added to all existing patents on completion of FDA - acceptable pediatric study

26 FINANCIAL HIGHLIGHTS
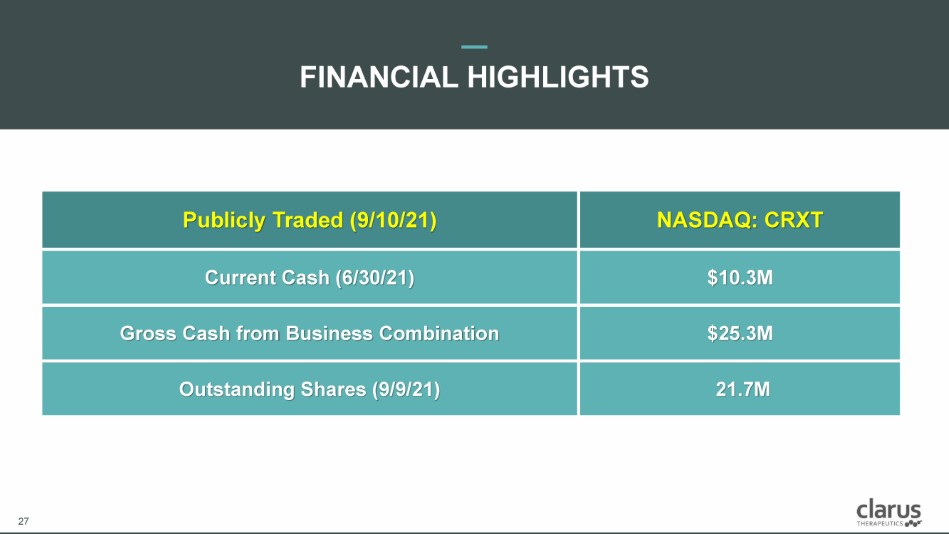
27 FINANCIAL HIGHLIGHTS 27 Publicly Traded (9/10/21) NASDAQ: CRXT Current Cash (6/30/21) $10.3M Gross Cash from Business Combination $25.3M Outstanding Shares (9/9/21) 21.7M

Clarus Therapeutics Confidential & Proprietary Information: Internal Use Only – Do Not Copy or Distribute / Contains Draft Propo sals Subject to Internal Review and Change 28 2 8 » Experienced team with track record of success developing and commercializing innovative therapies in our target markets » Clarus sells JATENZO , an FDA approved, first - in - class oral testosterone (T) replacement therapy (“TRT”) with strong potential to capture & grow market share KEY TAKEAWAYS » We are developing an R & D pipeline with solid commercial potential that is focused on androgen and metabolic therapies for unmet medical needs of men and women » Our ongoing business development activities are aimed at identifying assets that strategically align with our expanding portfolio and/or commercial call points
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Mercer International Inc. Announces Conference Call for First Quarter 2024 Results
- XWELL, Inc. Reports Fiscal Year 2023 Results
- Heliogen Extends Limited Duration Stockholders Rights Plan
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share