Form 8-K Calithera Biosciences, For: Oct 18
Exhibit 3.1
CALITHERA BIOSCIENCES, INC.
CERTIFICATE OF DESIGNATIONS OF PREFERENCES,
RIGHTS AND LIMITATIONS
OF
SERIES A CONVERTIBLE PREFERRED STOCK
PURSUANT TO SECTION 151 OF THE
DELAWARE GENERAL CORPORATION LAW
The undersigned, Susan Molineaux, does hereby certify that:
1. She is the President and Chief Executive Officer of Calithera Biosciences, Inc., a Delaware corporation (the “Corporation”).
2. The Corporation is authorized to issue ten million (10,000,000) shares of Preferred Stock, $0.0001 par value, none of which have been issued.
TERMS OF PREFERRED STOCK
1. Definitions. For purposes hereof, the following terms shall have the following meanings:
“Additional Shares of Common Stock” shall mean all shares of Common Stock issued by the Corporation after the effective date of the Purchase Agreement, other than (1) the following shares of Common Stock and (2) shares of Common Stock deemed issued pursuant to the following Common Stock Equivalents (clauses (1) and (2), collectively, “Exempted Securities”):
(a) shares of Common Stock or Common Stock Equivalents issued as a dividend or distribution on Preferred Stock;
(b) shares of Common Stock or Common Stock Equivalents issued by reason of a dividend, stock split, split-up or other distribution on shares of Common Stock that is covered by Section 9.1;
(c) shares of Common Stock or Common Stock Equivalent issued to employees or directors of, or consultants or advisors to, the Corporation or any of its subsidiaries pursuant to a plan, agreement or arrangement approved by the Board of Directors of the Corporation;
(d) shares of Common Stock or Common Stock Equivalent issued as acquisition consideration pursuant to the acquisition of another corporation by the Corporation by merger, purchase of substantially all of the assets or other reorganization or to a joint venture agreement, provided that such issuances are approved by the Board of Directors of the Corporation;
(e) shares of Common Stock or Common Stock Equivalent issued to banks, equipment lessors or other financial institutions, or to real property lessors, pursuant to a debt financing, equipment leasing or real property leasing transaction approved by the Board of Directors of the Corporation; or
(f) shares of Common Stock or Common Stock Equivalent issued to suppliers or third party service providers in connection with the provision of goods or services pursuant to transactions approved by the Board of Directors of the Corporation.
“Accounting Cap” means, as of any date, 19.99% of the outstanding Common Stock of the Company on such date.
“Business Day” means on days other than a Saturday or Sunday, on which commercial banks in New York, New York and Tokyo, Japan are open for the general transaction of business.
“Common Stock” means the Corporation’s common stock, par value $0.0001 per share, and stock of any other class of securities into which such securities may hereafter be reclassified or changed.
“Common Stock Equivalents” means any securities of the Corporation which would entitle the holder thereof to acquire at any time Common Stock, including, without limitation, any debt, preferred stock, rights, options, warrants or other instrument that is at any time convertible into or exercisable or exchangeable for, or otherwise entitles the holder thereof to receive, Common Stock.
“Issuance Date” means the date of filing of this Certificate of Designations with the Secretary of State of the State of Delaware.
“Issuance Date Closing Price” means a price (subject to adjustment in the event of a stock split, stock dividend, combination or other proportionate adjustment) that is the lower of (i) the Nasdaq Official Closing Price (as reflected on Nasdaq.com) one (1) Business Day preceding the Issuance Date; and (ii) the average Nasdaq Official Closing Price of the Common Stock (as reflected on Nasdaq.com) for the five trading days immediately preceding the Issuance Date (subject to adjustment in the event of a stock split, stock dividend, combination or other proportionate adjustment).
“Mandatory Pricing Date” means the eighteen (18) month anniversary of the Issuance Date.
“New Securities” means, collectively, equity securities of the Corporation, whether or not currently authorized, as well as rights, options, or warrants to purchase such equity securities, or securities of any type whatsoever that are, or may become, convertible or exchangeable into or exercisable for such equity securities.
“Outside Date” means the five (5) year anniversary of the Issuance Date.
2
“Person” means an individual or corporation, partnership, trust, incorporated or unincorporated association, joint venture, limited liability company, joint stock company, government (or an agency or subdivision thereof) or other entity of any kind.
“Purchase Agreement” means the Preferred Stock Purchase Agreement, dated as of October 18, 2021, by and between the Corporation and the original Holder, as amended, modified or supplemented from time to time in accordance with its terms.
“QF Price” means the weighted-average price per share (subject to adjustment in the event of a stock split, stock dividend, combination or other proportionate adjustment) paid by investors in the Qualified Financing (but will only apply to the number of shares sold not to exceed $40,000,000) of Qualified Financing Stock (subject to adjustment in the event of a stock split, stock dividend, combination or other proportionate adjustment). If the Corporation sells Preferred Stock in the Qualified Financing, then the QF Price for the Preferred Stock shall be equal to the effective price per share of such Preferred Stock on an as-converted to Common Stock basis (subject to adjustment in the event of a stock split, stock dividend, combination or other proportionate adjustment).
“Qualified Financing” means any bona fide transaction or any series of transactions (including sales of Common Stock pursuant to any at-the-market program established by the Company) with the principal purpose of raising capital, pursuant to which the Corporation issues and sells Qualified Financing Stock that results in net proceeds to the Corporation of at least $40,000,000, excluding the conversion of the Series A Preferred Stock.
“Qualified Financing Stock” means the class and series of stock issued by Corporation in the Qualified Financing.
“Requisite Stockholder Approval” means the approval by the holders of Common Stock of the Company for the issuance of shares of Common Stock in excess of the Share Cap in accordance with the rules of The Nasdaq Stock Market LLC.
“Restated Certificate” means the current Amended and Restated Certificate of Incorporation of the Corporation, as may be amended from time to time.
“Share Cap” means a number of shares of Common Stock equal to the product of (i) 0.1999 and (ii) 74,124,484 (subject to adjustment in the event of a stock split, stock dividend, combination or other proportionate adjustment) (for the avoidance of doubt taking into account all prior conversions of Series A Preferred Stock into Common Stock).
“Transfer Agent” means American Stock Transfer & Trust Company, LLC, the current transfer agent of the Corporation, and any successor transfer agent of the Corporation.
2. Designation, Amount and Par Value. The series of Preferred Stock shall be designated as its Series A Convertible Preferred Stock (the “Series A Preferred Stock”), and the number of shares so designated shall be up to one million (1,000,000) (which shall not be subject to increase without the written consent of the holder of the Series A Preferred Stock (the “Holder”)). Each share of Series A Preferred Stock shall have a par value of $0.0001 per share. The “Series A Original Issue Price” shall mean $35.00 per share, subject to appropriate adjustment in the event of any stock dividend, stock split, combination or other similar recapitalization with respect to the Series A Preferred Stock.
3
3. Dividends. Except for stock dividends or distributions for which adjustments are to be made pursuant to Section 9, Holder shall be entitled to receive, and the Corporation shall pay, dividends or distributions on shares of Series A Preferred Stock equal (on an as-if-converted-to-Common Stock basis) to and in the same form as dividends or distributions actually paid on shares of the Common Stock when, as and if such dividends or distributions are paid on shares of the Common Stock. No other dividends or distributions shall be paid on shares of Series A Preferred Stock.
4. Liquidation, Dissolution or Winding Up.
4.1. Preferential Payments to Holder of Series A Preferred Stock. In the event of any voluntary or involuntary liquidation, dissolution or winding up of the Corporation, the Holder of shares of Series A Preferred Stock then outstanding shall be entitled to be paid out of the assets of the Corporation available for distribution to its stockholders, and in the event of a Deemed Liquidation Event (as defined below), the Holder of shares of Series A Preferred Stock then outstanding shall be entitled to be paid out of the consideration payable to stockholders in such Deemed Liquidation Event or out of the Available Proceeds (as defined below), as applicable, before any payment shall be made to the holders of Common Stock by reason of their ownership thereof, an amount per share equal to the greater of (i) the Series A Original Issue Price, plus any dividends declared but unpaid thereon, or (ii) such amount per share as would have been payable had all shares of Series A Preferred Stock been converted into Common Stock pursuant to Section 8.2 immediately prior to such liquidation, dissolution, winding up or Deemed Liquidation Event (the amount payable pursuant to this sentence is hereinafter referred to as the “Series A Liquidation Amount”). If upon any such liquidation, dissolution or winding up of the Corporation or Deemed Liquidation Event, the assets of the Corporation available for distribution to its stockholders shall be insufficient to pay the Holder the full amount to which they shall be entitled under this Section 4.1, the Holder shall share ratably in any distribution of the assets available for distribution in proportion to the respective amounts which would otherwise be payable in respect of the shares held by them upon such distribution if all amounts payable on or with respect to such shares were paid in full.
4.2. Payments to Holders of Common Stock. In the event of any voluntary or involuntary liquidation, dissolution or winding up of the Corporation, after the payment in full of all Series A Liquidation Amount required to be paid to the Holder, the remaining assets of the Corporation available for distribution to its stockholders or, in the case of a Deemed Liquidation Event, the consideration not payable to the Holder pursuant to Section 4.1 or the remaining Available Proceeds, as the case may be, shall be distributed among the holders of shares of Common Stock, pro rata based on the number of shares held by each such holder.
4.3. Deemed Liquidation Events.
4.3.1. Definition.
4
Each of the following events shall be considered a “Deemed Liquidation Event” unless the Holder elects otherwise by written notice sent to the Corporation at least ten (10) days prior to the effective date of any such event:
(a) a merger or consolidation in which
| (i) | the Corporation is a constituent party or |
| (ii) | a subsidiary of the Corporation is a constituent party and the Corporation issues shares of its capital stock pursuant to such merger or consolidation, |
except any such merger or consolidation involving the Corporation or a subsidiary in which the shares of capital stock of the Corporation outstanding immediately prior to such merger or consolidation continue to represent, or are converted into or exchanged for shares of capital stock that represent, immediately following such merger or consolidation, at least a majority, by voting power, of the capital stock of (1) the surviving or resulting corporation; or (2) if the surviving or resulting corporation is a wholly owned subsidiary of another corporation immediately following such merger or consolidation, the parent corporation of such surviving or resulting corporation; or
(b) (1) the sale, lease, transfer, exclusive license or other disposition, in a single transaction or series of related transactions, by the Corporation or any subsidiary of the Corporation of all or substantially all the assets of the Corporation and its subsidiaries taken as a whole, or (2) the sale or disposition (whether by merger, consolidation or otherwise, and whether in a single transaction or a series of related transactions) of one or more subsidiaries of the Corporation if substantially all of the assets of the Corporation and its subsidiaries taken as a whole are held by such subsidiary or subsidiaries, except where such sale, lease, transfer, exclusive license or other disposition is to a wholly owned subsidiary of the Corporation.
4.3.2. Effecting a Deemed Liquidation Event.
(a) The Corporation shall not have the power to effect a Deemed Liquidation Event referred to in Section 4.3.1(a)(i) unless the agreement or plan of merger or consolidation for such transaction (the “Merger Agreement”) provides that the consideration payable to the stockholders of the Corporation in such Deemed Liquidation Event shall be paid to the holders of capital stock of the Corporation in accordance with Section 4.1 and 4.2.
(b) In the event of a Deemed Liquidation Event referred to in Section 4.3.1(a)(ii) or 4.3.1(b), if the Corporation does not effect a dissolution of the Corporation under the General Corporation Law within ninety (90) days after such Deemed Liquidation Event, then (i) the Corporation shall send a written notice to the Holder no later than the ninetieth (90th) day after the Deemed Liquidation Event advising such Holder of its right (and the requirements to be met to secure such right) pursuant to the terms of the following clause; (ii) to require the redemption of such shares of Series A Preferred Stock, and (iii) if the Holder so request in a written instrument delivered to the Corporation not later than one hundred twenty (120) days after such Deemed Liquidation Event, the
5
Corporation shall use the consideration received by the Corporation for such Deemed Liquidation Event (net of any retained liabilities associated with the assets sold or technology licensed, as determined in good faith by the Board of Directors of the Corporation), together with any other assets of the Corporation available for distribution to its stockholders, all to the extent permitted by Delaware law governing distributions to stockholders (the “Available Proceeds”), on the one hundred fiftieth (150th) day after such Deemed Liquidation Event, to redeem all outstanding shares of Series A Preferred Stock at a price per share equal to the Series A Liquidation Amount. Notwithstanding the foregoing, in the event of a redemption pursuant to the preceding sentence, if the Available Proceeds are not sufficient to redeem all outstanding shares of Series A Preferred Stock, the Corporation shall redeem a pro rata portion of the Holder’s shares of Series A Preferred Stock to the fullest extent of such Available Proceeds, based on the respective amounts which would otherwise be payable in respect of the shares to be redeemed if the Available Proceeds were sufficient to redeem all such shares, and shall redeem the remaining shares as soon as it may lawfully do so under Delaware law governing distributions to stockholders. Prior to the distribution or redemption provided for in this Section 4.3.2(b), the Corporation shall not expend or dissipate the consideration received for such Deemed Liquidation Event, except to discharge expenses incurred in connection with such Deemed Liquidation Event or in the ordinary course of business.
5. Amount Deemed Paid or Distributed. The amount deemed paid or distributed to the holders of capital stock of the Corporation upon any such merger, consolidation, sale, transfer, exclusive license, other disposition or redemption shall be the cash or the value of the property, rights or securities to be paid or distributed to such holders pursuant to such Deemed Liquidation Event. The value of such property, rights or securities shall be determined in good faith by the Board of Directors of the Corporation.
6. Voting. On any matter presented to the stockholders of the Corporation for their action or consideration at any meeting of stockholders of the Corporation (or by written consent of stockholders in lieu of meeting), (i) if the Requisite Stockholder Approval has been obtained, the Holder of the Series A Preferred Stock shall be entitled to cast the number of votes equal to the number of whole shares of Common Stock into which the shares of Series A Preferred Stock held by the Holder are convertible pursuant to Section 8 as of the record date for determining stockholders entitled to vote on such matter, and (ii) if the Requisite Stockholder Approval has not been obtained, the Holder of the Series A Preferred Stock shall be entitled to cast up to the Conversion Restriction (as defined below) only that number of votes equal to the number of whole shares of Common Stock into which the shares of Series A Preferred Stock then held by such holders are convertible pursuant to Section 8 as of the record date for determining stockholders entitled to vote on such matter less such number of shares of Common Stock previously converted. In no event, shall the Series A Preferred Stock be entitled to cast votes when combined with the shares of Common Stock then held by the Holder in excess of the Accounting Cap. Except as provided by law or by the provisions of the Restated Certificate, the Holder of shares of Series A Preferred Stock shall vote together with the holders of Common Stock as a single class.
6
7. Series A Preferred Stock Protective Provisions. At any time when shares of Series A Preferred are outstanding, the Corporation shall not, either directly or indirectly by amendment, merger, consolidation or otherwise, do any of the following without (in addition to any other vote required by law or this Certificate of Designations) the written consent or affirmative vote of the Holder given in writing or by vote at a meeting, consenting or voting (as the case may be) separately as a class, and any such act or transaction entered into without such consent or vote shall be null and void ab initio, and of no force or effect:
7.1.1. amend, alter or repeal any provision of this Certificate of Designations or Bylaws of the Corporation in a manner that adversely affects the powers, preferences or rights of the Series A Preferred Stock;
7.1.2. create, or authorize the creation of, or issue or obligate itself to issue shares of, any additional class or series of capital stock unless the same ranks junior to the Series A Preferred Stock with respect to the distribution of assets on the liquidation, dissolution or winding up of the Corporation, the payment of dividends and rights of redemption, or increase the authorized number of shares of Series A Preferred Stock or increase the authorized number of shares of any additional class or series of capital stock of the Corporation unless the same ranks junior to the Series A Preferred Stock with respect to the distribution of assets on the liquidation, dissolution or winding up of the Corporation, the payment of dividends and rights of redemption;
7.1.3. (i) reclassify, alter or amend any existing security of the Corporation that is pari passu with the Series A Preferred Stock in respect of the distribution of assets on the liquidation, dissolution or winding up of the Corporation, the payment of dividends or rights of redemption, if such reclassification, alteration or amendment would render such other security senior to the Series A Preferred Stock in respect of any such right, preference, or privilege or (ii) reclassify, alter or amend any existing security of the Corporation that is junior to the Series A Preferred Stock in respect of the distribution of assets on the liquidation, dissolution or winding up of the Corporation, the payment of dividends or rights of redemption, if such reclassification, alteration or amendment would render such other security senior to or pari passu with the Series A Preferred Stock in respect of any such right, preference or privilege; or
7.1.4. purchase or redeem (or permit any subsidiary to purchase or redeem) or pay or declare any dividend or make any distribution on, any shares of capital stock of the Corporation other than (i) redemptions of or dividends or distributions on the Series A Preferred Stock as expressly authorized herein, (ii) dividends or other distributions payable on the Common Stock solely in the form of additional shares of Common Stock and (iii) repurchases of stock from former employees, officers, directors, consultants or other persons who performed services for the Corporation or any subsidiary in connection with the cessation of such employment or service.
8. Conversion.
8.1. Mandatory, Automatic Conversion.
8.1.1. If a Qualified Financing has not occurred, then on the Mandatory Pricing Date the Series A Preferred Stock will become convertible into that number of shares of Common Stock equal to the Mandatory Pricing Conversion Ratio. The
7
“Mandatory Pricing Conversion Ratio” will be a fraction, the numerator of which is the quotient of $35,000,000 and the lesser of (i) the Issuance Date Closing Price and (ii) the volume weighted-average price (subject to adjustment in the event of a stock split, stock dividend, combination or other proportionate adjustment) of the Corporation’s Common Stock on the thirty (30) trading days prior to the Mandatory Pricing Date (or the next Business Day if the Mandatory Pricing Date is not on a Business Day) and the denominator is the number of shares of Series A Preferred Stock issued on the Issuance Date (subject to adjustment in the event of a stock split, stock dividend, combination or other proportionate adjustment). On the Mandatory Pricing Date, that number of shares of Series A Preferred Stock will automatically convert into the maximum number of shares of Common Stock as is allowed under the Accounting Cap or if lower the Share Cap (if Requisite Stockholder Approval has not been obtained by such date). Any shares of Series A Preferred Stock not so converted shall remain outstanding until the earlier of (A) conversion pursuant to Section 8.6, if the Corporation obtains the Requisite Stockholder Approval, or (B) if the Requisite Stockholder Approval is not approved at a vote by the Corporation’s stockholders, the date that the Corporation and Investor reach written agreement under Section 5(g)(i) of the Purchase Agreement. Once the Share Cap is no longer applicable and after the Mandatory Pricing Date, any remaining shares of Series A Preferred Stock may be converted, at the Mandatory Pricing Conversion Ratio, at the option of the Holder at any time into shares of Common Stock up to the Accounting Cap.
8.1.2. If there is a Qualified Financing before the Mandatory Pricing Date, each share of Series A Preferred Stock will become convertible at the Qualified Financing into the number of shares of Common Stock equal to the Financing Conversion Ratio. The “Financing Conversion Ratio” will be a fraction, the numerator of which is the quotient of $35,000,000 and the lesser of (i) the Issuance Date Closing Price and (ii) the QF Price and the denominator is the number of shares of Series A Preferred Stock issued on the Issuance Date (subject to adjustment in the event of a stock split, stock dividend, combination or other proportionate adjustment). Upon the closing of the Qualified Financing, that number of shares of Series A Preferred Stock will automatically convert into the maximum number of shares of Common Stock as is allowed under the Accounting Cap or if lower the Share Cap (if Requisite Stockholder Approval has not been obtained by such date). Any shares of Series A Preferred Stock not so converted shall remain outstanding until the earlier of (A) conversion pursuant to Section 8.6, if the Corporation obtains the Requisite Stockholder Approval or (B) if the Requisite Stockholder Approval is not approved at a vote by the Corporation’s stockholders, the date that the Corporation and Investor reach written agreement under Section 5(g)(i) of the Purchase Agreement. Once the Share Cap is no longer applicable and after a Qualified Financing has occurred, any remaining shares of Series A Preferred Stock may be converted, at the Financing Conversion Ratio, at the option of the Holder at any time into shares of Common Stock up to the Accounting Cap.
8.1.3. Any conversion pursuant to Section 8.1.1 or 8.1.2 shall occur automatically, except as provided in the last sentence of each such Section, and without any further action by the Holder and whether or not the certificates representing such shares of Series A Preferred Stock are surrendered to the Corporation or its Transfer Agent. Upon the occurrence of such automatic conversion, the Corporation shall provide written notice to the Holder, and the Holder shall, a reasonable time thereafter, surrender the certificates
8
representing such shares at the office of the Corporation or any Transfer Agent for the Series A Preferred Stock. Thereupon, there shall be issued and delivered to such Holder promptly at such office and in its name as shown on the Corporation’s stock records, a certificate or certificates for the number of shares of Common Stock or Preferred Stock into which the shares of Series A Preferred Stock surrendered were convertible on the date on which such automatic conversion occurred. The Corporation shall, or shall cause the Transfer Agent to, send to the registered owner thereof a certificate for any shares of Common Stock issued upon conversion of Series A Preferred Stock.
8.2. Optional Conversion. Prior to the Mandatory Pricing Date and a Qualified Financing, at the written election of Holder in its sole discretion, the shares of Series A Preferred Stock held by Holder may be converted, in whole or in part, into the number of shares of Common Stock at the Optional Conversion Ratio. The “Optional Conversion Ratio” will be a fraction, the numerator of which is the quotient of $35,000,000 and the lesser of (i) the Issuance Date Closing Price and (ii) the volume weighted-average sales price (subject to adjustment in the event of a stock split, stock dividend, combination or other proportionate adjustment) per share of Additional Shares of Common Stock (other than Exempted Securities but including shares of Series A Preferred Stock issued on the Issuance Date) (subject to adjustment in the event of a stock split, stock dividend, combination or other proportionate adjustment), in each case based on the effective price (subject to adjustment in the event of a stock split, stock dividend, combination or other proportionate adjustment) per share on an as-converted to Common Stock basis, sold from the Issuance Date through the date of the written election of Holder (or the next Business Day if not on a Business Day) and the denominator is the number of shares of Series A Preferred Stock issued on the Issuance Date (subject to adjustment in the event of a stock split, stock dividend, combination or other proportionate adjustment). All optional conversions shall be subject to the Share Cap (if Requisite Stockholder Approval has not been obtained as of such date) and the Accounting Cap.
8.3. Fractional Shares. No fractional shares or scrip representing fractional shares shall be issued upon the conversion of the Series A Preferred Stock. As to any fraction of a share which the Holder would otherwise be entitled to purchase upon such conversion, the Corporation shall round up to the next whole share.
8.4. Transfer Taxes and Expenses. The issuance of certificates for shares of the Common Stock on conversion of Series A Preferred Stock shall be made without charge to the Holder for any documentary stamp or similar taxes that may be payable in respect of the issue or delivery of such certificates; provided that the Corporation shall not be required to pay any tax that may be payable in respect of any transfer involved in the issuance and delivery of any such certificate upon conversion in a name other than that of the Holder of such shares of Series A Preferred Stock and the Corporation shall not be required to issue or deliver such certificates unless or until the Person or Persons requesting the issuance thereof shall have paid to the Corporation the amount of such tax or shall have established to the satisfaction of the Corporation that such tax has been paid.
9
8.5. Beneficial Ownership Limitation. Notwithstanding anything else herein to the contrary, prior to the receipt of Requisite Stockholder Approval, the Corporation shall not issue shares of Common Stock, in excess of the Share Cap (the “Conversion Restriction”), and in each case, any shares of Series A Preferred Stock not so converted shall remain outstanding until the earlier of (i) conversion pursuant to Section 8.6, if the Corporation obtains the Requisite Stockholder Approval or (ii) if the Requisite Stockholder Approval is not approved at a vote by the Corporation’s stockholders, the date that the Corporation and Investor reach written agreement under Section 5(g)(ii) of the Purchase Agreement.
8.6. Outside Date. If the Corporation obtains the Requisite Stockholder Approval, then any shares of Series A Preferred Stock that remain outstanding as of the Outside Date shall automatically with no further action of the Holder be converted into Common Stock at the applicable conversion ratio as set forth in Section 8.1.1 and 8.1.2 hereto, in each case subject to the Accounting Cap, and any remaining shares not so converted shall remain outstanding. For clarity, such shares of Series A Preferred Stock will convert into Common Stock at the Mandatory Pricing Conversion Ratio if such shares initially became convertible pursuant to Section 8.1.1 but for the Share Cap and/or Accounting Cap and such shares of Series A Preferred Stock will convert into Common Stock at the Financing Conversion Ratio if such shares initially became convertible pursuant to Section 8.1.2 but for the Share Cap and/or Accounting Cap. On each yearly anniversary thereafter, any shares of Series A Preferred Stock that remain outstanding shall automatically with no further action of the Holder be converted into Common Stock at the applicable conversion ratio as set forth in Section 8.1.1 and 8.1.2 hereto, in each case subject to the Accounting Cap, until such point in time as all shares of Series A Preferred Stock have been converted.
9. Certain Adjustments.
9.1. Calculations. All calculations under this Section 9 shall be made to the nearest cent or the nearest 1/100th of a share, as the case may be. For purposes of this Section 9, the number of shares of Common Stock deemed to be issued and outstanding as of a given date shall be the number of shares of Common Stock (excluding any treasury shares of the Corporation) issued and outstanding.
9.2. Notice to the Holder of Adjustment to Issuance Date Closing Price / Issuance of Additional Shares of Common Stock. Whenever the Issuance Date Closing Price is adjusted, or any shares of Additional Shares of Common Stock have been issued which would lead to a change in the potential Optional Conversion Ratio, the Corporation shall promptly deliver to the Holder a notice setting forth the Issuance Date Closing Price, or the number and terms of any Additional Shares of Common Stock, after such adjustment and setting forth a brief statement of the facts requiring such adjustment.
10. Notices. All notices, instructions and other communications hereunder or in connection herewith shall be in writing, shall be sent to the address of the relevant party set forth in the Purchase Agreement. Any such notice, instruction or communication shall be deemed to have been delivered upon the earlier of actual receipt, or (a) personal delivery to the party to be notified, (b) when sent, if sent by electronic mail or facsimile during normal business hours of the recipient, and if not sent during normal business hours, then on the recipient’s next business day, (c) five (5) days after having been sent by registered or certified mail, return receipt requested, postage prepaid, or (d) one (1) business day after deposit with a nationally recognized overnight courier, freight prepaid, specifying next business day delivery, with written verification of receipt.
10
11. Lost or Mutilated Series A Preferred Stock Certificate. If the Holder’s Series A Preferred Stock certificate shall be mutilated, lost, stolen or destroyed, the Corporation shall execute and deliver, in exchange and substitution for and upon cancellation of a mutilated certificate, or in lieu of or in substitution for a lost, stolen or destroyed certificate, a new certificate for the shares of Series A Preferred Stock so mutilated, lost, stolen or destroyed, but only upon receipt of evidence of such loss, theft or destruction of such certificate, and of the ownership hereof reasonably satisfactory to the Corporation.
12. Waiver. Any waiver by the Corporation or the Holder of a breach of any provision of this Certificate of Designations shall not operate as or be construed to be a waiver of any other breach of such provision or of any breach of any other provision of this Certificate of Designations. The failure of the Corporation or the Holder to insist upon strict adherence to any term of this Certificate of Designations on one or more occasions shall not be considered a waiver or deprive that party of the right thereafter to insist upon strict adherence to that term or any other term of this Certificate of Designations on any other occasion. Any waiver by the Corporation or the Holder must be in writing. Notwithstanding any provision in this Certificate of Designations to the contrary, any provision contained herein and any rights of Holder granted hereunder may be waived as to all shares of Series A Preferred Stock (and the Holder) upon the written consent of Holder.
13. Status of Converted or Redeemed Series A Preferred Stock. Shares of Series A Preferred Stock may only be issued pursuant to the Purchase Agreement. If any shares of Series A Preferred Stock shall be converted, redeemed or reacquired by the Corporation, such shares shall be deemed to be retired and cancelled and shall resume the status of authorized but unissued shares of Preferred Stock and shall no longer be designated as Series A Preferred Stock.
[Remainder of Page Intentionally Left Blank]
11
IN WITNESS WHEREOF, the Corporation has caused this Certificate to be signed this 18th day of October 2021.
| CALITHERA BIOSCIENCES, INC. | ||
| By: | /s/ Susan M. Molineaux | |
| Name: Susan M. Molineaux, Ph.D. | ||
| Title: President and Chief Executive Officer | ||
12
Exhibit 10.1
PREFERRED STOCK PURCHASE AGREEMENT
TABLE OF CONTENTS
| 1. DEFINED TERMS USED IN THIS AGREEMENT |
3 | |||||
| 2. PURCHASE AND SALE OF PREFERRED STOCK |
5 | |||||
| 2.1 |
Sale and Issuance of Preferred Stock | 5 | ||||
| 2.2 |
Closing; Delivery; Adjustments | 6 | ||||
| 3. REPRESENTATION, WARRANTIES AND COVENANTS OF THE COMPANY |
6 | |||||
| 3.1 |
Organization, Good Standing, Corporate Power and Qualification | 6 | ||||
| 3.2 |
Company Capitalization | 6 | ||||
| 3.3 |
Subsidiaries | 7 | ||||
| 3.4 |
Authorization | 7 | ||||
| 3.5 |
Valid Issuance of Shares | 7 | ||||
| 3.6 |
Governmental Consents and Filings | 8 | ||||
| 3.7 |
Litigation | 8 | ||||
| 3.8 |
Compliance with Other Instruments | 8 | ||||
| 3.9 |
Property | 8 | ||||
| 3.10 |
SEC Filings; Financial Statements | 8 | ||||
| 3.11 |
Changes | 10 | ||||
| 3.12 |
Tax Matters | 10 | ||||
| 3.13 |
Employee Benefits Matters | 10 | ||||
| 3.14 |
Labor Matters | 11 | ||||
| 3.15 |
Intellectual Property | 11 | ||||
| 3.16 |
Environmental Matters | 12 | ||||
| 3.17 |
Brokers and Finders | 12 | ||||
| 3.18 |
Insurance | 12 | ||||
| 3.19 |
Anti-Corruption and Anti-Bribery Laws | 12 | ||||
| 3.20 |
Economic Sanctions | 13 | ||||
| 3.21 |
Money Laundering | 13 | ||||
| 3.22 |
Asset Purchase Agreement | 13 | ||||
| 3.23 |
CFIUS | 13 | ||||
| 4. REPRESENTATIONS AND WARRANTIES OF THE INVESTOR |
14 | |||||
| 4.1 |
Authorization | 14 | ||||
| 4.2 |
Purchase Entirely for Own Account | 14 | ||||
| 4.3 |
Disclosure of Information | 14 | ||||
| 4.4 |
Restricted Securities | 14 | ||||
| 4.5 |
Legends | 14 | ||||
| 4.6 |
Accredited Investor | 15 | ||||
1.
| 4.7 |
Foreign Person |
15 | ||||
| 4.8 |
No General Solicitation |
15 | ||||
| 4.9 |
Exculpation |
15 | ||||
| 4.10 |
Residence |
15 | ||||
| 5. INVESTOR RIGHTS AND OBLIGATIONS |
15 | |||||
| 6. RESTRICTIONS ON TRANSFER |
19 | |||||
| 7. REGISTRATION OF CONVERSION SHARES |
20 | |||||
| 8. CONDITIONS TO THE INVESTOR’S OBLIGATIONS |
24 | |||||
| 8.1 |
Representations and Warranties |
24 | ||||
| 8.2 |
Performance |
24 | ||||
| 8.3 |
Asset Purchase Agreement |
24 | ||||
| 8.4 |
Proceedings and Documents |
24 | ||||
| 8.5 |
Qualifications |
24 | ||||
| 8.6 |
Compliance Certificate |
24 | ||||
| 8.7 |
Secretary’s Certificate |
24 | ||||
| 8.8 |
Material Adverse Effect |
24 | ||||
| 8.9 |
Legal Opinion |
25 | ||||
| 8.10 |
Certificate of Designation |
25 | ||||
| 9. CONDITIONS OF THE COMPANY’S OBLIGATIONS AT CLOSING |
25 | |||||
| 9.1 |
Representations and Warranties |
25 | ||||
| 9.2 |
Performance |
25 | ||||
| 9.3 |
Compliance Certificate |
25 | ||||
| 9.4 |
Qualifications |
25 | ||||
| 10. MISCELLANEOUS |
25 | |||||
| 10.1 |
Successors and Assigns |
25 | ||||
| 10.2 |
Governing Law |
25 | ||||
| 10.3 |
Counterparts |
25 | ||||
| 10.4 |
Titles and Subtitles |
25 | ||||
| 10.5 |
Notices |
25 | ||||
| 10.6 |
No Finder’s Fees |
26 | ||||
| 10.7 |
Waivers |
26 | ||||
| 10.8 |
Severability |
26 | ||||
| 10.9 |
Delays or Omissions |
26 | ||||
| 10.10 |
Entire Agreement; Amendment |
26 | ||||
| 10.11 |
Dispute Resolution |
27 | ||||
2.
PREFERRED STOCK PURCHASE AGREEMENT
THIS PREFERRED STOCK PURCHASE AGREEMENT (this “Agreement”), is made as of October 18, 2021 by and between Calithera Biosciences Inc., a Delaware corporation (the “Company”), and Millennium Pharmaceuticals, Inc., a Delaware corporation (the “Investor”).
WHEREAS, the Company and the Investor are entering into that certain Asset Purchase Agreement of even date herewith (the “APA”);
WHEREAS, the obligations of the APA are conditioned upon the execution and delivery of this Agreement, pursuant to which the Company will issue to the Investor, as partial consideration for the transfer of the assets under the APA by the Investor to the Company, a number of shares of its Series A Preferred Stock, par value $0.0001, as provided for herein; and
WHEREAS, the Investor desires the receive, and the Company desires to issue, the Shares (as defined below) on the terms and conditions set forth herein;
NOW, THEREFORE, in consideration of the foregoing recitals and mutual promises, representations, warranties, and covenants hereinafter set forth and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the parties hereby agree as follows:
1. DEFINED TERMS USED IN THIS AGREEMENT. In addition to the terms defined above, the following terms used in this Agreement shall be construed to have the meanings set forth or referenced below.
(a) “Accredited Investor” means an “accredited investor” within the meaning of SEC Rule 501 of Regulation D, as presently in effect.
(b) “Affiliate” means, with respect to a Person, any other Person that, directly or indirectly, through one (1) or more intermediaries, controls, is controlled by or is under common control with such Person. For purposes of this definition, “control” and, with correlative meanings, the terms “controlled by” and “under common control with” means (a) the possession, directly or indirectly, of the power to direct the management or policies of a Person, whether through the ownership of voting securities, by contract relating to voting rights or corporate governance, or otherwise; or (b) the ownership, directly or indirectly, of more than fifty percent (50%) of the voting securities or other ownership interest of a Person (or, with respect to a limited partnership or other similar entity, its general partner or controlling entity). The parties acknowledge that in the case of certain entities organized under the laws of certain countries outside of the United States, the maximum percentage ownership permitted by law for a foreign investor may be less than fifty percent (50%), and that, in such case, such lower percentage shall be substituted in the preceding sentence, provided that such foreign investor has the power to direct the management or policies of such entity.
(c) “Board” means the Board of Directors of the Company.
(d) “Business Day” means on days other than a Saturday or Sunday, on which commercial banks in New York, New York and Tokyo, Japan are open for the general transaction of business.
(e) “Certificate of Designations” means that certain Certificate of Designations filed on the date hereof with the Secretary of State of the State of Delaware, setting forth the rights, preferences and privileges of the Series A Preferred Stock.
(f) “Closing” has the meaning set forth in Section 2.2(a).
3.
(g) “Closing Date” has the meaning set forth in Section 2.2(a).
(h) “Code” means the Internal Revenue Code of 1986, as amended.
(i) “Common Stock” has the meaning set forth in Section 3.2(a)(i).
(j) “Company SEC Reports” has the meaning set forth in Section 3.10(a).
(k) “Contract” shall mean, with respect to any Person, any written or oral agreement, contract, commitment, indenture, note, bond, loan, license, sublicense, lease, sublease, undertaking, statement of work or other arrangement to which such Person is a party or by which any of its properties or assets are subject.
(l) “Conversion Shares” has the meaning set forth in Section 2.1.
(m) “Employee Benefit Plan” shall mean any “employee benefit plan” (as such term is defined in Section 3(3) of ERISA, whether or not subject to ERISA), any severance, employment, incentive or bonus, retention, change in control, deferred compensation, termination pay, profit sharing, retirement, welfare, post-employment welfare, fringe benefit, vacation or paid time off, equity or equity-based or any other plan, policy, program, agreement, contract or arrangement (i) that is sponsored, maintained, contributed to, or required to be contributed to by the Company or under or with respect to which the Company has any current or contingent liability or obligation, or (ii) that provides benefits or compensation to any employee, director, or officer of the Company or any other person performing services for the Company (including any leased employee or individual co-employed by a “professional employer organization”).
(n) “Environmental Law” shall mean all applicable national, supra-national, federal, state, local and foreign laws concerning public health and safety, worker health and safety, pollution or protection of the environment; including without limitation all those relating to the generation, handling, transportation, treatment, storage, disposal, release, exposure to or cleanup of hazardous materials, substances or wastes, including petroleum, asbestos, polychlorinated biphenyls, asbestos, noise or radiation.
(o) “ERISA” shall mean the United States Employee Retirement Income Security Act of 1974, as amended, and the rulings and regulations thereunder.
(p) “Exchange Act” means the Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder.
(q) “Financial Statements” has the meaning set forth in Section 3.10.
(r) “GAAP” means U.S. generally accepted accounting principles.
(s) “Governmental Authority” shall mean any court, agency, authority, department, regulatory body or other instrumentality of any government or country or of any national, federal, state, provincial, regional, county, city or other political subdivision of any such government or country or any supranational organization of which any such country is a member.
(t) “Intellectual Property” shall mean all intellectual property and other similar proprietary rights in any jurisdiction, including such rights in and to: (a) any patent (including all reissues, divisions, continuations, continuations-in-part and extensions thereof), patent application, patent disclosure or other patent right, (b) any trademark, service mark, trade name, business name, brand name, slogan, logo, trade dress and all other indicia of origin together with all goodwill associated therewith, and all registrations, applications for registration, and renewals for any of the foregoing, (c) any copyright, work of authorship (whether or not copyrightable), design, design registration, database rights, and all registrations, applications for registration, and renewals for any of the foregoing (and including in all website content and software), (d) any Internet domain names, and (e) any trade secret, confidential information, know-how and inventions, including processes and formulations.
4.
(u) “Knowledge,” including the phrase “to the Company’s knowledge,” shall mean the actual knowledge (after reasonable inquiry of their direct reports) of the President and Chief Executive Officer, Chief Financial Officer and Chief Medical Officer of the Company.
(v) “Material Adverse Effect” means a material adverse effect on the business, assets (including intangible assets), liabilities, financial condition, property, or results of operations of the Company.
(w) “Person” means any individual, corporation, partnership, trust, limited liability company, association or other entity.
(x) “Preferred Stock” has the meaning set forth in Section 3.2(a)(ii).
(y) “Purchase Price” has the meaning set forth in Section 2.1.
(z) “Restated Certificate” means the current Amended and Restated Certificate of Incorporation of the Company, as amended by the Certificate of Amendment.
(aa) “Tax” or “Taxes” shall mean (a) any federal, state, local, or non-U.S. income, gross receipts, license, payroll, employment, excise, severance, stamp, occupation, premium, windfall profits, environmental (including taxes under Section 59A of the Code), customs duties, capital stock, franchise, profits, withholding, social security (or similar), unemployment, disability, real property, personal property, sales, use, transfer, unclaimed property or escheat (or similar), registration, value added, alternative or add-on minimum, estimated, or other tax of any kind whatsoever, including any interest, penalty, or addition thereto, whether disputed or not, (b) any liability for or in respect of the payment of any amount of a type described in clause (a) of this definition as a result of being a member of an affiliated, combined, consolidated, unitary or other group for tax purposes, or (c) any liability for or in respect of the payment of any amount described in clauses (a) or (b) of this definition as a transferee or successor, by Contract or otherwise.
(bb) “Tax Return” shall mean any return, declaration, report, claim for refund, or information return or statement relating to Taxes, including any schedule or attachment thereto, and including any amendment thereof.
(cc) “SEC” means the U.S. Securities and Exchange Commission.
(dd) “Securities Act” means the Securities Act of 1933, as amended, and the rules and regulations promulgated thereunder.
(ee) “Series A Preferred Stock” has the meaning set forth in Section 3.2(a)(ii).
(ff) “Shares” has the meaning set forth in Section 2.1.
2. PURCHASE AND SALE OF PREFERRED STOCK.
2.1 Sale and Issuance of Preferred Stock. Subject to the terms and conditions of this Agreement, the Investor agrees to receive at the Closing and the Company agrees to sell and issue to the Investor at the Closing 1,000,000 shares of Series A Preferred Stock (the “Shares”), having the preferences, rights and limitations set forth in the form of Certificate of Designations attached hereto as Exhibit A initially at a valuation of $2.04 per equivalent one share of Common Stock, free and clear of all liens, for an aggregate deemed issue price of $35,000,000.00 (the “Purchase Price”), receipt of which is
5.
hereby acknowledged. The Shares are initially convertible at the option of the holder into 17,156,863 shares of Common Stock, subject to the receipt by the Company of the Requisite Stockholder Approval (as defined in the Certificate of Designations) for the issuance of shares of Common Stock in excess of the Share Cap (as defined in the Certificate of Designations) (collectively, the “Conversion Shares”). The Company shall seek the Requisite Stockholder Approval at the next stockholder meeting scheduled after the Effective Date and the Board shall recommend such approval. For so long as any Series A Preferred Stock is outstanding and for one year after all Conversion Shares are issued (and to the extent Investor is subject to a lock-up or is prevented from trading due to exposure to material non-public information it receives as a board observer or under the APA, the foregoing period shall be tolled for additional days equal to the time of the lock-up or time Investor is prevented from trading), the Company agrees not to, either directly or indirectly take any action that would result in the issuance to Investor of, or Investor holding, shares of Common Stock in excess of the Share Cap (if Requisite Stockholder Approval has not been received) or the Accounting Cap (as defined in the Certificate of Designation). This Agreement is being entered into pursuant to Section 5.1 of the APA, and the Investor acknowledges and agrees that, by entering into this Agreement and upon issuance of the Shares to the Investor, the Company has satisfied in full its obligations under Section 5.1 of the APA.
2.2 Closing; Delivery; Adjustments.
(a) The purchase and sale of the Shares shall take place remotely via the exchange of documents and signatures simultaneously with the “Effective Date” (as such term is defined in the APA) or at such other time and place as the Company and the Investor mutually agree upon, orally or in writing (which time and place are designated as the “Closing” and the “Closing Date”). At the Closing, the Company shall issue, and the Investor shall receive the issuance of the Shares.
(b) At the Closing, the Company shall instruct its transfer agent to deliver confirmation of book-entry issuance of the Shares being purchased by the Investor at such Closing.
(c) All numbers of shares and dollar amounts set forth in this Agreement are subject to appropriate adjustment in the event of any stock dividend, stock split, combination or similar recapitalization affecting such shares.
3. REPRESENTATION, WARRANTIES AND COVENANTS OF THE COMPANY. The Company hereby represents and warrants to the Investor that the following representations are true and correct as of the date hereof except as otherwise indicated.
3.1 Organization, Good Standing, Corporate Power and Qualification. The Company is a corporation duly organized, validly existing and in good standing under the laws of the State of Delaware and has all requisite corporate power and authority to carry on its business as presently conducted and as proposed to be conducted. The Company is duly qualified to transact business and is in good standing in each jurisdiction in which the failure to so qualify would have a Material Adverse Effect.
3.2 Company Capitalization.
(a) The authorized and issued capital of the Company consists, as of the date hereof, of:
(i) 200,000,000 shares of Common Stock, $0.0001 par value per share (the “Common Stock”), 74,124,484 shares of which are issued and outstanding. All of the outstanding shares of Common Stock have been duly authorized, are fully paid and nonassessable and were issued in compliance with all applicable federal and state securities laws.
(ii) 10,000,000 shares of Preferred Stock, $0.0001 par value per share (the “Preferred Stock”), of which 1,000,000 shares have been designated Series A Convertible Preferred Stock, $0.0001 par value per share, (the “Series A Preferred Stock”), none of which are issued and outstanding.
6.
(b) Under the Company’s 2010 Equity Incentive Plan (the “2010 Plan”), (i) options to acquire 377,898 shares of Common Stock have been granted and are currently outstanding, and (ii) no shares of Common Stock remain available for future issuance. Under the Company’s 2014 Equity Incentive Plan (the “2014 Plan”), (i) options to acquire 8,070,338 shares of Common Stock have been granted and are currently outstanding, (ii) 1,826,657 restricted stock units have been granted and are currently outstanding, and (iii) 1,479,155 shares of Common Stock remain available for future. Under the Company’s 2018 Inducement Plan (the “2018 Plan”), (i) options to acquire 193,491 shares of Common Stock have been granted and are currently outstanding, (ii) no restricted stock units have been granted and are currently outstanding, and (iii) 806,509 shares of Common Stock remain available for future issuance. 746,190 shares of Common Stock are reserved for future issuance pursuant to the Company’s 2014 Employee Stock Purchase Plan (the “ESPP” and together with the 2010 Plan, the 2014 Plan and the 2018 Plan, the “Plans”).
(c) Except as set forth in the Company SEC Reports filed prior to the Effective Date, other than the shares of Common Stock reserved for issuance under the Plans, there are no outstanding options, rights (including conversion or preemptive rights and rights of first refusal), proxy or shareholder agreements, or agreements of any kind for the purchase or acquisition from the Company of any of its securities, including the Shares. No Person is entitled to preemptive rights, rights of first refusal, rights of participation or similar rights with respect to any securities of the Company, including with respect to the issuance of Shares contemplated hereby. Except as set forth in the Company SEC Reports filed prior to the Effective Date, there are no voting agreements, registration rights agreements or other agreements of any kind between the Company and any other Person relating to the securities of the Company, including the Shares.
(d) All of the issued and outstanding shares of Common Stock have been duly authorized and validly issued and are fully paid and were issued in compliance with all applicable laws concerning the issuance of securities. The Shares have been duly and validly authorized and, when issued pursuant to this Agreement, (i) will be validly issued, and fully paid, (ii) will not be subject to pre-emptive rights, and (iii) shall be free and clear of all liens, except for restrictions on transfer imposed by applicable securities laws.
3.3 Subsidiaries. Except as set forth in the Company SEC Reports, the Company does not currently own or control, directly or indirectly, any interest in any other corporation, partnership, trust, joint venture, limited liability company, association, or other business entity. The Company is not a participant in any joint venture, partnership or similar arrangement.
3.4 Authorization. All corporate action required to be taken by the Company’s Board and stockholders in order to authorize the Company to enter into this Agreement, and to issue the Shares at the Closing and the Common Stock issuable upon conversion of the Shares, has been taken or will be taken prior to the Closing. All action on the part of the officers of the Company necessary for the execution and delivery of this Agreement, the performance of all obligations of the Company under this Agreement to be performed as of the Closing, and the issuance and delivery of the Shares has been taken or will be taken prior to the Closing. This Agreement, when executed and delivered by the Company, shall constitute a valid and legally binding obligation of the Company, enforceable against the Company in accordance with its terms except (a) as limited by applicable bankruptcy, insolvency, reorganization, moratorium, fraudulent conveyance, or other laws of general application relating to or affecting the enforcement of creditors’ rights generally, or (b) as limited by laws relating to the availability of specific performance, injunctive relief, or other equitable remedies.
3.5 Valid Issuance of Shares. The Shares, when issued, sold and delivered in accordance with the terms and for the consideration set forth in this Agreement, will be validly issued, fully paid and nonassessable and free of restrictions on transfer other than restrictions on transfer under this Agreement, applicable state and federal securities laws and liens or encumbrances created by or imposed
7.
by the Investor. Assuming the accuracy of the representations of the Investor in Section 4 of this Agreement and subject to Section 3.6 below, the Shares will be issued in compliance with all applicable federal and state securities laws. The Common Stock issuable upon conversion of the Shares has been duly reserved for issuance, and upon issuance in accordance with the terms of the Restated Certificate, will be validly issued, fully paid and nonassessable and free of restrictions on transfer other than restrictions on transfer under this Agreement, applicable federal and state securities laws and liens or encumbrances created by or imposed by the Investor. Assuming the accuracy of the representations of the Investors in Section 4 of this Agreement and subject to Section 3.6 below, the Common Stock issuable upon conversion of the Shares will be issued in compliance with all applicable federal and state securities laws.
3.6 Governmental Consents and Filings. Assuming the accuracy of the representations made by the Investor in Section 4 of this Agreement, no consent, approval, order or authorization of, or registration, qualification, designation, declaration or filing with, any federal, state or local governmental authority is required on the part of the Company in connection with the consummation of the transactions contemplated by this Agreement, except for filings pursuant to applicable federal or state securities laws, which have been made or will be made in a timely manner.
3.7 Litigation. There is no claim, action, suit, proceeding, arbitration, complaint, charge or, to the Company’s knowledge, investigation pending or, to the Company’s knowledge, currently threatened in writing against the Company or any officer or director of the Company that questions the validity of this Agreement or the right of the Company to enter into it, or to consummate the transactions contemplated by this Agreement or would reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect.
3.8 Compliance with Other Instruments. The Company is not in violation or default (a) of any provisions of its Restated Certificate or the Company’s bylaws (“Bylaws”), (b) of any instrument, judgment, order, writ or decree, (c) under any material agreement, note, indenture, deed of trust, license, lease agreement or mortgage where such violation or default would have a Material Adverse Effect, or (d) to its knowledge, of any provision of federal or state statute, rule or regulation applicable to the Company, the violation of which would have a Material Adverse Effect. The execution, delivery and performance of this Agreement and the consummation of the transactions contemplated by this Agreement will not result in any such violation or be in conflict with or constitute, with or without the passage of time and giving of notice, either (x) a default under any such provision, instrument, judgment, order, writ, decree, material agreement, note, indenture, deed of trust, license, lease agreement or mortgage; or (y) an event which results in the creation of any lien, charge or encumbrance upon any assets of the Company or the suspension, revocation, forfeiture, or nonrenewal of any material permit or license applicable to the Company.
3.9 Property. The property and assets that the Company owns are free and clear of all mortgages, deeds of trust, liens, loans and encumbrances, except for statutory liens for the payment of current taxes that are not yet delinquent and encumbrances and liens that arise in the ordinary course of business and do not materially impair the Company’s ownership or use of such property or assets. With respect to the property and assets it leases, the Company is in compliance with such leases and, to its knowledge, holds a valid leasehold interest free of any liens, claims or encumbrances other than those of the lessors of such property or assets. The Company does not own any real property.
3.10 SEC Filings; Financial Statements.
(a) The Company’s Common Stock is registered pursuant to Section 12(b) of the Exchange Act. The Company has timely and properly filed all forms, schedules, reports, prospectuses, proxy statements and documents required to be filed by the Company with the SEC (the “Company SEC Reports”). The Company’s Common Stock is currently listed on the Nasdaq Global Select Market. The Company is not in violation of the listing requirements of the Nasdaq Stock Market LLC and has no knowledge of any facts that would reasonably lead to delisting or suspension of its common stock from the Nasdaq Stock Market LLC in the foreseeable future. The Company SEC Reports (i) at the time they were filed (or if amended or superseded by a filing prior to the date of this Agreement, then on the date of such
8.
filing) complied in all material respects with the requirements of the Securities Act or the Exchange Act, as the case may be, and the rules and regulations promulgated thereunder, and (ii) did not at the time they were filed (or if amended or superseded by a filing prior to the date of this Agreement, then on the date of such filing) contain any untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary in order to make the statements therein, in light of the circumstances under which they were made, not misleading. The Company makes no representation or warranty whatsoever concerning the Company SEC Reports as of any time other than the time they were filed, amended or superseded.
(b) The Company is in compliance with all requirements of the Sarbanes-Oxley Act of 2002 that are applicable to the Company as of the date hereof, and all applicable rules and regulations promulgated by the SEC thereunder that are effective as of the date hereof. The Company maintains a system of internal accounting controls sufficient to provide reasonable assurance that: (i) transactions are executed in accordance with management’s general or specific authorizations, (ii) transactions are recorded as necessary to permit preparation of financial statements in conformity with GAAP and to maintain asset accountability, (iii) access to assets is permitted only in accordance with management’s general or specific authorization, and (iv) the recorded accountability for assets is compared with the existing assets at reasonable intervals and appropriate action is taken with respect to any differences. The Company has established disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) for the Company and designed such disclosure controls and procedures to provide reasonable assurance that information required to be disclosed by the Company in the reports it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms.
(c) Each of the consolidated financial statements (including, in each case, any related notes thereto) (the “Financial Statements”) contained in the Company SEC Reports has been prepared in accordance with GAAP applied on a consistent basis throughout the period involved (except as may be indicated in the notes thereto) and complied in all material respects with the rules and regulations of the SEC. Each of the Financial Statements fairly presents in all material respects the consolidated financial position of the Company at the respective dates thereof and the consolidated results of its operations and cash flows for the periods indicated, except that the unaudited interim financial statements were or are subject to normal and recurring year-end adjustments which have not had or are not expected to have, individually or in the aggregate, a Material Adverse Effect.
(d) To the extent the Company is not obligated to file Company SEC Reports, and without derogating from any information rights which may otherwise become available at any time to the Investor under the Company’s governing documents, any agreement, or applicable law, for so long as the Investor together with its Affiliates holds at least fifty percent (50%) of the Shares issued hereunder, the Company shall deliver to the Investor:
(i) as soon as available and in any event within forty-five (45) days after the end of each of the first three quarters of each fiscal year of the Company, consolidated balance sheets of the Company and its subsidiaries as of the end of such period, and consolidated statements of income and cash flows of the Company, and its subsidiaries for the period then ended prepared in conformity with generally accepted accounting principles in the United States applied on a consistent basis, except as otherwise noted therein, and subject to the absence of footnotes and to year-end adjustment;
(ii) as soon as available and in any event within one-hundred twenty (120) days after the end of each fiscal year of the Company, a consolidated balance sheet of the Company and its subsidiaries as of the end of such year, and consolidated statements of income and cash flows of the Company and its subsidiaries for the year then ended prepared in conformity with generally accepted accounting principles in the United States applied on a consistent basis, except as otherwise noted therein, together with an auditor’s report thereon of a firm of established national reputation.
9.
3.11 Changes. Except as otherwise disclosed in the Company SEC Reports, since June 30, 2021, there has not been any change in the assets, liabilities, financial condition or operating results of the Company from that reflected in the Financial Statements, except changes in the ordinary course of business that have not caused, in the aggregate, a Material Adverse Effect.
3.12 Tax Matters.
(a) Except as set forth in the Company SEC Reports filed prior to the Effective Date (i) the Company has timely prepared and filed all federal and all other material Tax Returns required to have been filed by each of them with all appropriate Governmental Authorities and timely paid all Taxes shown thereon, (ii) all such Tax Returns are true, correct and complete in all material respects, (iii) all Taxes that the Company is required to withhold or to collect for payment have been duly withheld and collected and paid to the proper Governmental Authority or third party when due, and (iv) no claim has ever been made by a Governmental Authority in a jurisdiction where the Company does not file Tax Returns that the Company is or may be subject to taxation by that jurisdiction;
(b) Except as set forth in the Company SEC Reports filed prior to the Effective Date (i) no federal, state, local, or non-U.S. Tax audits or administrative or judicial Tax proceedings are pending or being conducted with respect to the Company, (ii) the Company has not received from any federal, state, local, or non-U.S. taxing authority any (A) written notice indicating an intent to open an audit or other review related to any material Tax, or (B) written notice of deficiency or proposed adjustment for any material amount of Tax proposed, asserted, or assessed by any taxing authority against the Company;
(c) Except as set forth in the Company SEC Reports filed prior to the Effective Date (i) the Company (A) has not been a member of an affiliated group filing a consolidated federal income Tax Return or (B) does not have any liability for the Taxes of any Person (other than the Company) under U.S. Treas. Reg. § 1.1502-6 (or any similar provision of state, local, or non-U.S. law), as a transferee or successor, by Contract, or otherwise;
(d) The Company has not distributed stock of another Person, or has had its stock distributed by another Person, in a transaction that was purported or intended to be governed in whole or in part by Section 355 or 361 of the Code;
(e) The Company is not and has not been a party to any “listed transaction,” as defined in Section 6707A(c)(2) of the Code and U.S. Treas. Reg. § 1.6011-4(b)(2); and
(f) The Company has never been, nor will it be at the Closing, a United States Real Property Holding Corporation within the meaning of Section 897(c)(2) of the Code during the applicable period specified in Section 897(c)(1)(A)(ii) of the Code.
3.13 Employee Benefits Matters.
(a) Each Employee Benefit Plan (and each related trust, insurance Contract, or fund) has been maintained, funded and administered in accordance with its terms and in compliance with the applicable requirements of applicable law, including ERISA and the Code and other applicable laws, except where the failure to so maintain, fund or administer would reasonably be expected to result in a Material Adverse Effect, and all contributions, distributions, reimbursements and premium payments due with respect to each Employee Benefit Plan have been timely made or properly accrued. Each Employee Benefit Plan that is intended to meet the requirements of a “qualified plan” under Section 401(a) of the Code has received a favorable determination letter (or may rely on a favorable opinion letter) issued by the United States Internal Revenue Service and to the Company’s Knowledge, nothing has occurred that would reasonably be expected to adversely affect the qualification of such Employee Benefit Plan.
(b) The Company does not maintain, sponsor, contribute to, have any obligation to contribute to, or have any current or potential liability or obligation under or with respect to (i) a “defined benefit plan” (as such term is defined in Section 3(35) of ERISA), (ii) a “multiple employer plan” (within the meaning of Section 210 of ERISA or Section 413(c) of the Code), or (iii) a “multiemployer plan”
10.
as defined in Section 3(37) of ERISA, or (iv) a “multiple employer welfare arrangement” (as such term is defined in Section 3(40) of ERISA); no Employee Benefit Plan provides and the Company does not have any current or potential obligation to provide post-termination or post-retirement health, life or other welfare benefits other than as required under Section 4980B of the Code or any similar state law; and the Company does not have any current or potential liability or obligation by reason of at any time being treated as a single employer under Section 414 of the Code with any other Person.
(c) There have been no prohibited transactions (as defined in Section 406 of ERISA or Section 4975 of the Code) and no breach of fiduciary duty (as determined under ERISA) with respect to any Employee Benefit Plan; the Company has, for purposes of each Employee Benefit Plan, correctly classified those individuals performing services for the Company as employees or non-employees; and there do not exist any pending or, to the Company’s Knowledge, threatened claims (other than routine undisputed claims for benefits) or actions, suits, proceedings, arbitrations, complaints or charge with respect to any Employee Benefit Plan.
(d) The transactions contemplated by this Agreement and the APA will not (either alone or in combination with another event) (i) cause the acceleration of vesting in, or payment of, any benefits or compensation under any Employee Benefit Plan, (ii) require the funding of compensation or benefits due to any manager, employee, officer, director, shareholder or other service provider (whether current, former or retired) of the Company or their beneficiaries and, (iii) otherwise accelerate or increase any liability or obligation under any Employee Benefit Plan.
3.14 Labor Matters.
(a) The Company is not a party to nor is bound by any collective bargaining agreement or other Contract or relationship with any union, labor organization, or other collective bargaining representative. There are no strikes, work stoppages or any other material labor disputes against or affecting the Company pending or, to the Company’s Knowledge, threatened, and no such disputes have occurred since June 30, 2021. No union organization or decertification activities are underway or, to the Company’s Knowledge, threatened with respect to employees of the Company and no such activities have occurred since June 30, 2021.
(b) The Company is, and at all times since June 30, 2021 has been, in compliance in all material respects with all applicable laws respecting employment and employment practices, including provisions thereof relating to terms and conditions of employment, wages and hours, overtime, classification of employees and independent contractors, immigration, and the withholding and payment of social security and other employment Taxes, except where the failure to be in such compliance would reasonably be expected to have a Material Adverse Effect.
(c) Since June 30, 2021, the Company has not implemented any plant closing or layoff of employees that could implicate the WARN Act.
3.15 Intellectual Property.
(a) The Company owns all right, title and interest in and to, or has the valid and enforceable right to use pursuant to a written Contract, all Intellectual Property used in the conduct of the business of the Company as currently conducted or proposed to be conducted (collectively, the “Company Intellectual Property”), free and clear of any liens, except where the failure to own or have the right to use would reasonably be expected to have a Material Adverse Effect. To the Company’s Knowledge, the owners of any Intellectual Property licensed to Company have taken necessary actions to maintain and protect such Intellectual Property, except where the failure to have taken such actions would reasonably be expected to have a Material Adverse Effect.
11.
(b) The Company has not infringed, misappropriated, or otherwise violated, or is not currently infringing, misappropriating, or otherwise violating, any Intellectual Property of any other Person and there are no claims, actions, suits, proceedings, arbitrations, complaints or charges pending or, to the Company’s Knowledge, threatened alleging any of the foregoing, including any unsolicited offers for the Company to obtain a license to any Intellectual Property of another Person. To the Company’s Knowledge, no Person is infringing, misappropriating or violating the rights of the Company with respect to any Company Intellectual Property.
(c) The Company has taken commercially reasonable steps to maintain and protect the secrecy and confidentiality of its material confidential information. Without limiting the generality of the foregoing, all current and former employees, contractors and consultants of the Company who have participated in the creation of Intellectual Property have executed and delivered to the Company a valid and enforceable agreement providing for the assignment by such Person to the Company of any such Intellectual Property.
3.16 Environmental Matters. Except as would not reasonably be expected to, individually or in the aggregate, have a Material Adverse Effect: (i) no notice, notification, demand, request for information, citation, summons, complaint or Order has been received within the past three years by, and no claim, action, suit, proceeding, arbitration, complaint or charge is pending or, to the Company’s Knowledge, threatened by, any Person against the Company and no penalty has been assessed against the Company with respect to any matters relating to or arising out of any Environmental Law, and (ii) the Company is, and since June 30, 2021 has been, in compliance with all applicable Environmental Laws, including any consent required by Environmental Laws.
3.17 Brokers and Finders. No Person will have, as a result of the transactions contemplated by this Agreement and the APA, any right, interest or claim against or upon the Company for any commission, fee or other compensation pursuant to any agreement, arrangement or understanding entered into by or on behalf of the Company. The Company agrees to indemnify the Investor for any claims, losses or expenses incurred by the Investor as a result of the representation in this Section 3.17 being untrue.
3.18 Insurance. Except as has not had, and would not reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect, (a) all insurance policies (“Policies”) with respect to the business and assets of the Company are in full force and effect, (b) the Company is not in breach or default, and the Company has not taken any action or failed to take any action that, with notice or the lapse of time, would constitute such a breach or default, or permit termination or modification of any of the Policies, and (c) the Company has not received any written notice of cancellation or threatened cancellation of any of the Policies or of any claim pending regarding the Company under any of such Policies as to which coverage has been questioned, denied or disputed by the underwriters of such Policies. The Company maintains insurance with reputable insurers in such amounts and against such risks as is customary for the industry in which the Company operates and as the management of the Company has in good faith determined to be prudent and appropriate.
3.19 Anti-Corruption and Anti-Bribery Laws. Neither the Company, nor, any of its officers, directors or employees, nor to the Company’s Knowledge its agents, representatives, consultants, or other persons associated with or acting for or on behalf of the Company, has, directly or indirectly, in connection with the operation of their business: (a) made, offered or promised to make or offer any payment, loan or transfer of anything of value, including any reward, advantage or benefit of any kind, to or for the benefit of any government official, candidate for public office, political party or political campaign, for the purpose of (i) influencing any act or decision of such government official, candidate, party or campaign, (ii) inducing such government official, candidate, party or campaign to do or omit to do any act in violation of a lawful duty, (iii) obtaining or retaining business for or with any person, (iv) expediting or securing the performance of official acts of a routine nature, or (v) otherwise securing any improper advantage, in each case, in violation of any applicable anticorruption or anti-bribery law, (b) paid, offered or promised to pay or offer any bribe, payoff, influence payment, kickback, unlawful rebate, or other similar unlawful payment of any nature, (c) made, offered or promised to make or offer any unlawful contributions, gifts, entertainment or other unlawful expenditures, (d) established or maintained any unlawful fund of corporate monies or other properties, (e) created or caused the creation of any false or inaccurate books and records of the
12.
Company related to any of the foregoing, or (f) otherwise violated any provision of the Foreign Corrupt Practices Act of 1977, 15 U.S.C. §§ 78dd-l, et seq., or any other applicable anti-corruption or anti-bribery law. For purposes of this provision, “government official” includes any officer or employee of a government or any department, agency or instrumentality thereof (including wholly or partially owned enterprises or institutions), or of a public international organization, or any person acting in an official capacity for or on behalf of any such government or department, agency or instrumentality, or for or on behalf of any such public international organization.
3.20 Economic Sanctions. None of the Company or its directors, officers, employees or to the Company’s Knowledge its agents (i) is a person with whom transactions are prohibited or limited under any applicable economic sanctions laws, or (ii) within the last five (5) years has done business in or with any Person that is the target of sanctions by the United States. Within the past five (5) years, the Company has not made any voluntary disclosures to applicable Governmental Authorities under applicable economic sanctions laws or applicable export control laws and, to the Knowledge of the Company, the Company has not been the subject of any governmental investigation or inquiry regarding the compliance of the Company with such laws, nor has the Company been assessed any fine or penalty in regard to compliance with such laws.
3.21 Money Laundering. The operations of the Company are and have been conducted at all times in compliance with applicable financial record-keeping and reporting requirements of the Currency and Foreign Transactions Reporting Act of 1970, as amended, applicable money laundering statutes and applicable rules and regulations thereunder (collectively, the “Money Laundering Laws”), and no action, suit or proceeding by or before any court or governmental agency, authority or body or any arbitrator involving the Company with respect to the Money Laundering Laws is pending or, to the Company’s Knowledge, threatened.
3.22 Asset Purchase Agreement. The representations and warranties of the Company contained in Article 6 of the APA are true and correct.
3.23 CFIUS.
(a) The Company does not directly or indirectly engage in the design, fabrication, development, testing, production, or manufacture of Critical Technologies, as defined by Section 1703(a)(6) of the Foreign Investment Risk Review Modernization Act of 2018, Subtitle A of Title XVII of Public Law 115-232 (Aug. 13, 2018) (“Critical Technologies”), (b) it does not collect or store personal health information of U.S. citizens treated with the Company’s products or technology, and (c), with respect to (b), it has no current intention of engaging in such activities in the future. The Company further represents that if its intentions change and it expects to engage in these activities, the Company agrees to cooperate with any CFIUS analysis conducted.
(b) To the extent that the Company engages in or intends to engage in the design, fabrication, development, testing, production, or manufacture of Critical Technologies in the future, whether because of a new categorization of technology by the U.S. government or otherwise, the Company shall immediately provide notice to the Investor. If the Investor in its sole and reasonable discretion requests that the Investor and the Company submit a joint voluntary notice (“JVN”) of the transactions contemplated by this Agreement to the Committee on Foreign Investment in the United States (“CFIUS”), or determines that a mandatory notification to CFIUS is required, the Company shall use reasonable best efforts to cooperate with the Investor to prepare jointly and submit (i) a draft pre-filing notice, (ii) a notification or JVN, and (iii) any other submission or submissions requested by CFIUS in connection with obtaining clearance (“CFIUS Approval”), and to otherwise use reasonable best efforts to finally and successfully obtain CFIUS Approval as promptly as practicable.
13.
4. REPRESENTATIONS AND WARRANTIES OF THE INVESTOR. The Investor hereby represents and warrants to the Company that:
4.1 Authorization. The Investor has full power and authority to enter into this Agreement. This Agreement, when executed and delivered by the Investor, will constitute a valid and legally binding obligation of the Investor, enforceable in accordance with their terms, except as limited by applicable bankruptcy, insolvency, reorganization, moratorium, fraudulent conveyance and any other laws of general application affecting enforcement of creditors’ rights generally, and as limited by laws relating to the availability of specific performance, injunctive relief or other equitable remedies.
4.2 Purchase Entirely for Own Account. The Company is entering into this Agreement with the Investor in reliance upon the Investor’s representation to the Company, which by the Investor’s execution of this Agreement, the Investor hereby confirms that the Shares to be acquired by such Investor will be acquired for investment for the Investor’s own account, not as a nominee or agent, and not with a view to the resale or distribution of any part thereof, and that the Investor has no present intention of selling, granting any participation in, or otherwise distributing the same. By executing this Agreement, the Investor further represents that the Investor does not presently have any contract, undertaking, agreement or arrangement with any Person to sell, transfer or grant participations to such Person or to any third Person, with respect to any of the Shares. The Investor has not been formed for the specific purpose of acquiring the Shares.
4.3 Disclosure of Information. The Investor has had access to all of the Company’s SEC filings that the Investor has requested. The Investor has had an opportunity to discuss the Company’s business, management, financial affairs and the terms and conditions of the offering of the Shares with the Company’s management. The foregoing, however, does not limit or modify the representations and warranties of the Company in Section 3 of this Agreement, or the right of the Investor to rely thereon.
4.4 Restricted Securities. The Investor understands that the Shares have not been, registered under the Securities Act, by reason of a specific exemption from the registration provisions of the Securities Act which depends upon, among other things, the bona fide nature of the investment intent and the accuracy of the Investor’s representations as expressed herein. The Investor understands that the Shares are “restricted securities” under applicable U.S. federal and state securities laws and that, pursuant to these laws, the Investor must hold the Shares indefinitely unless they are registered with the SEC and qualified by state authorities, or an exemption from such registration and qualification requirements is available. The Investor acknowledges that, except as set forth in Section 7 below, the Company does not have any obligation to register or qualify the Shares, or the Common Stock into which it may be converted, for resale. The Investor further acknowledges that if an exemption from registration or qualification is available, it may be conditioned on various requirements including, but not limited to, the time and manner of sale, the holding period for the Shares, and on requirements relating to the Company which are outside of the Investor’s control, and which the Company is not under an obligation, and may not be able, to satisfy.
4.5 Legends. The Investor understands that the Shares will bear the legend set forth in Section 6(b), and any legend required by the securities laws of any state to the extent such laws are applicable to the Shares. The Shares, when issued, shall not bear the restrictive legends set forth in Section 6(b): (i) following a sale of such Shares pursuant to a registration statement covering the resale of such Shares, while such registration statement is effective under the Securities Act, (ii) following any sale of such Shares pursuant to Rule 144 promulgated under the Securities Act (“Rule 144”), (iii) if such Shares are eligible for sale under Rule 144, without the requirement for the Company to be in compliance with the current public information required under Rule 144 as to such Shares and without volume or manner-of-sale restrictions or (iv) if such legend is not required under applicable requirements of the Securities Act (including judicial interpretations and pronouncements issued by the staff of the Commission). The Company agrees that at such time as the restrictive legend set forth in Section 6(b) is no longer required, the Company will (x) no later than five (5) Business Days following the delivery by the Investor to the Company or the Company’s transfer agent of a certificate representing Shares issued with such restrictive legend, deliver or cause to be delivered to the Investor a certificate representing such Shares that is free from such restrictive legend, and (y), in the event that such shares are uncertificated, no later than five (5) Business Days following the delivery of a written request by the Investor to the Company to remove such restrictive legend, remove, or cause to be removed, any such restrictive legend in the Company’s stock records.
14.
4.6 Accredited Investor. The Investor is an Accredited Investor.
4.7 Foreign Person. The Investor is not a foreign person as defined under the Foreign Investment Risk Review Modernization Act of 2018 (FIRRMA) and implementing regulations, 31 CFR part 800 et seq.
4.8 No General Solicitation. Neither the Investor, nor any of its officers, directors, employees, agents, stockholders or partners has either directly or indirectly, including, through a broker or finder (a) engaged in any general solicitation, or (b) published any advertisement in connection with the offer and sale of the Shares.
4.9 Exculpation. The Investor acknowledges that it is not relying upon any Person, other than the Company and its officers and directors, in making its investment or decision to invest in the Company.
4.10 Residence. The office of the Investor in which its principal place of business is identified is the address of the Investor set forth on its signature page hereto.
5. INVESTOR RIGHTS AND OBLIGATIONS.
(a) Exchange Right. If the security issued in the Qualified Financing (as defined in the Certificate of Designations) is not Common Stock, or if the Qualified Financing is achieved through a combination of sales of Common Stock and Preferred Stock, then the Company shall permit the Investor if it so elects for up to thirty (30) days after the Qualified Financing is met, to exchange, for no additional consideration, that portion of the Conversion Shares as the Preferred Stock constitutes of the Qualified Financing at the applicable initial conversion ratio between the Common Stock and such Preferred Stock as set forth in the Qualified Financing documents.
(b) Warrants and Derivative Securities. If in connection with the Qualified Financing, the Company issues warrants or other derivative securities to purchase its capital stock, then the Company will offer to issue to the Investor upon payment of the same consideration as received by the Company from such investors for such warrants or other derivative securities, such number of warrants or other derivative securities in the same form and in the same percentage as issued in the Qualified Financing. For example, if in connection with the Qualified Financing, the Company issues a warrant to purchase one share of Common Stock for each share of Common Stock purchased in the Qualified Financing at a warrant purchase price of $0.01 per warrant, then the Company shall issue the Investor that number of warrants to purchase one share of Common Stock equal to the number of Conversion Shares issued and the Investor will pay the Company the purchase price of $0.01 per warrant.
(c) Market Stand-off Agreement. The Investor agrees that it will not, without the prior written consent of the Company, during the period commencing on the Closing Date and through 180 days thereafter: (i) lend; offer; pledge; sell; contract to sell; sell any option or contract to purchase; purchase any option or contract to sell; grant any option, right, or warrant to purchase; or otherwise transfer or dispose of, directly or indirectly, any of the Shares or Conversion Shares or (ii) enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of the Shares or the Conversion Shares, whether any such transaction described in clause (i) or (ii) above is to be settled by delivery of the Shares, Conversion Shares or other securities, in cash, or otherwise. In addition, during the period commencing on the Closing Date and through eighteen (18) months thereafter, if requested by the managing underwriter or placement agent of securities of the Company, the Investor agrees that it will execute and deliver a lock-up agreement to such party in form and substance reasonably acceptable to the Investor; provided, all the directors and executive officers of the Company have executed and delivered a similar agreement; provided further, that the Investor shall be obligated to execute and deliver a lock-up agreement only once during such period.
15.
(d) Observer Rights. As long as the Investor together with its Affiliates owns not less than fifty percent (50%) of the Shares (including for purposes of this section the Conversion Shares) it is purchasing under this Agreement (as adjusted for stock splits, stock dividends, recapitalizations and the like), the Company shall invite a representative of the Investor, such representative to be reasonably acceptable to the Company, to attend all meetings of the Board, but not its executive sessions or committees of the Board, in a non-voting observer capacity and, in this respect, shall give such representative copies of all notices, minutes, consents, and other materials that it provides to its directors at the same time and in the same manner as provided to such directors related to such meetings, but not its executive sessions or committees of the Board; provided, however, that such representative shall agree to hold in confidence all information so provided; provided further, that such representative reserves the right to refuse to accept such information and to not attend such meeting(s), and provided further, that the Company reserves the right to withhold any information and to exclude such representative from any meeting or portion thereof if access to such information or attendance at such meeting could (i) adversely affect the attorney-client privilege between the Company and its counsel (based upon opinion of counsel), or (ii) result in disclosure of Company trade secrets to the Investor, (iii) create a Conflict of Interest between the Company and the Investor with respect to any pending or proposed transaction or proposed transaction to be discussed or considered at such meeting, as determined in good faith by the Company. As used herein, the term “Conflict of Interest” shall mean the Investor has or may likely have a financial or strategic interest in any pending or proposed transaction involving the Company.
(e) Confidentiality. The Investor agrees that it will keep confidential and will not disclose, divulge, or use for any purpose (other than to monitor or make decisions with respect to its investment in the Company) any confidential information obtained from the Company pursuant to the terms of this Agreement, unless such confidential information (a) is known or becomes known to the public in general (other than as a result of a breach of this Section 5(e) by such Investor), (b) is or has been independently developed or conceived by such Investor without use of the Company’s confidential information, or (c) is or has been made known or disclosed to such Investor by a third party without a breach of any obligation of confidentiality such third party may have to the Company; provided, however, that the Investor may disclose confidential information (i) to its attorneys, accountants, consultants, and other professionals to the extent reasonably necessary to obtain their services in connection with monitoring its investment in the Company; (ii) to any Affiliate, partner, member, stockholder, or wholly-owned subsidiary of such Investor in the ordinary course of business, provided that such Investor informs such Person that such information is confidential and directs such Person to maintain the confidentiality of such information; or (iii) as may otherwise be required by law, regulation, rule, court order or subpoena, provided that such Investor promptly notifies the Company of such disclosure and takes reasonable steps to minimize the extent of any such required disclosure. The Company acknowledges that the Investor may receive certain confidential information disclosed by the Company pursuant to the APA and that such information shall be governed by Article 7 of the APA.
(f) Participation in Future Financing.
(i) Subject to compliance with applicable securities laws, during the period commencing on the Closing Date and as long as the Investor together with its Affiliates owns the Shares it is purchasing under this Agreement, upon (a) any issuance by the Company of un-registered shares of Common Stock or Common Stock Equivalents (a “Private Offering”) or (b) any issuance by the Company of registered shares of Common Stock or Common Stock Equivalents (as defined in the Certificate of Designations) (a “Public Offering” and together with the Private Offering, a “Subsequent Financing”), in each case for cash consideration, indebtedness or a combination thereof, then for a Private Offering the Investor shall have the right to participate, and with respect to a Public Offering the Company shall use commercially reasonable efforts (which must include multiple attempts, on multiple dates, with multiple representatives of the managing underwriter(s), to cause the managing underwriter(s) of the Public Offering, or the placement agent(s) of the Private Offering, to allow the Investor to submit an indication of interest in an amount of the Subsequent Financing up to the Investor’s Pro-Rata Share (as defined below) on the same terms, conditions and price provided for in the Subsequent Financing. For purposes of this Agreement, the Investor’s “Pro-Rata Share” shall be equal to the lesser of (i) number of shares of Common Stock deemed to be beneficially owned by the Investor immediately prior to the closing of the Subsequent
16.
Financing (based upon documentation or written representation reasonably satisfactory to the Company), divided by the total number of shares of Common Stock outstanding (including any shares of Common Stock issuable upon conversion or exercise of outstanding Common Stock Equivalents deemed to be beneficially owned by the Investor and included in the numerator) immediately prior to the closing of the Subsequent Financing or (ii) the number of shares of Common Stock then subject to the Share Cap (as defined in the Certificate of Designations), divided by the total number of shares of Common Stock outstanding (including any shares of Common Stock issuable upon conversion or exercise of outstanding Common Stock Equivalents deemed to be beneficially owned by the Investor and included in the numerator) immediately prior to the closing of the Subsequent Financing.
(ii) At least five (5) trading days prior to the closing of a Public Offering, or at least ten (10) trading days prior to the closing of a Private Offering, as applicable, but in no event sooner than the date that the Company, or the managing underwriter(s) or placement agent(s), as the case may be, solicit indications of interest from potential investors in a Subsequent Financing, the Company shall deliver to the Investor notice of its intention to effect a Subsequent Financing (the “Subsequent Financing Notice”). In the event of a Private Offering, the Subsequent Financing Notice shall be written and describe in reasonable detail the proposed terms of such Subsequent Financing, the amount of proceeds intended to be raised thereunder and the name and contact information of the placement agent(s) for such Private Offering and shall include a term sheet or similar document relating thereto as an attachment. In the event of a Public Offering, the Subsequent Financing Notice shall be verbal and may at the election of the Company upon the advice of counsel be confirmed as delivered in writing. The Investor acknowledges that in no event will the Company be required to deliver any Subsequent Financing Notice which upon the advice of counsel would be required to be filed as a free writing prospectus with the SEC or which could otherwise be deemed a violation of Section 5 of the Securities Act.
(iii) If the Investor desires to participate in such Subsequent Financing, the Investor must provide with respect to a Private Offering a written notice to the Company or with respect to a Public Offering a verbal indication of interest to the managing underwriter(s) by not later than 5:30 p.m. (New York City time) on the second (2nd) trading day after the Investor has received a Subsequent Financing Notice, that the Investor is willing to participate in the Subsequent Financing and stating the amount of the Investor’s elected participation. If the Company or the managing underwriter(s) receive no such notice from the Investor as of such third (3rd) trading day, the Investor shall be deemed to have notified the Company that it does not elect to participate in the Subsequent Financing.
(iv) Notwithstanding anything to the contrary in this Section 5(f) and unless otherwise agreed to by the Investor, in the event the Company determines to abandon a Subsequent Financing, the Company shall cause the managing underwriter(s) or placement agent(s), as the case may be, to confirm such abandonment to the Investor in the same manner and on the same day as such abandonment is communicated to other potential investors. If, by the twentieth (20th) day following delivery of the Subsequent Financing Notice, no public disclosure regarding a transaction with respect to the Subsequent Financing has been made, such Subsequent Financing shall be deemed to have been abandoned and the Investor shall not be in possession of any material, non-public information with respect to the Company, unless the Company advises the Investor that the Subsequent Financing has not been abandoned. The Company understands and confirms that the Investor may rely on this Section 5(f) when effecting transactions in securities of the Company.
(v) Notwithstanding the foregoing, this Section 5(f) shall not apply in respect of an Exempt Issuance. “Exempt Issuance” means the issuance of:
(1) shares of Common Stock or options to employees, consultants, officers or directors of the Company pursuant to any stock or option plan duly adopted for such purpose and in existence on the date of this Agreement as such plan is constituted on the date of this Agreement, by a majority of the non-employee members of the Board of Directors or a majority of the members of a committee of non-employee directors established for such purpose, unless otherwise agreed to by the non-employee members of the Board of Directors,
17.
(2) securities upon the exercise or exchange of or conversion of any Common Stock Equivalents issued and outstanding on the date of this Agreement, provided that such securities have not been amended on or after the date of this Agreement to increase the number of such securities or to decrease the exercise price, exchange price or conversion price of such securities,
(3) securities issued pursuant to acquisitions or strategic transactions approved by a majority of the disinterested directors of the Company, provided that any such issuance shall not include a transaction in which the Company is issuing securities primarily for the purpose of raising capital or to an entity whose primary business is investing in securities, and
(4) an “at-the-market” offering of common stock.
(vi) The Investor further agrees to execute such other documents and agreements as may reasonably be requested of the Investor by the managing underwriter(s) or placement agent(s), as the case may be, in connection with a Subsequent Financing.
(g) Good Faith Negotiation.
(i) If the Company is unable to obtain the Requisite Stockholder Approval as contemplated by the Certificate of Designations, and as a result the Investor is unable to convert all the Shares into Conversion Shares, then the parties shall promptly negotiate in good faith the timing and amount per Share to be paid to compensate the Investor for such inability; provided, however that the Company shall not be required to make any cash payment to the Investor until at least three (3) years after the Closing Date without the Company’s consent (the “Cash Limitation”); provided further that if the Company has closed an equity financing of at least $80.0 million with a volume weighted average price of at least the Issuance Date Closing Price (as defined in the Certificate of Designations) per share then the Cash Limitation shall not apply, and the Company shall instead pay the Investor the Issuance Date Closing Price per share of Series A Preferred Stock that if converted would be above the Share Cap. If the parties are unable to agree in writing within thirty (30) days, Section 5(g)(ii) shall apply.
(ii) If the parties are unable to agree on appropriate compensations to the Investor in writing within thirty (30) days, the parties shall engage in “baseball” style arbitration pursuant to the then-current JAMS Comprehensive Arbitration Rules and Procedures. Each party will submit to the arbitrator a single proposal (subject to the Cash Limitation) for resolution no later than ten (10) days following the arbitration hearing, and the arbitrator shall select one of the proposed resolutions submitted by the parties but for the avoidance of doubt subject to the Cash Limitation.
(h) Standstill. During the period commencing on the Closing Date and through three (3) years thereafter (the “Restricted Period”), without the prior consent of the Company, except as provided for in this Agreement, neither the Investor nor any of Investor’s Affiliates on behalf of the Investor will, in any manner, directly or indirectly:
(i) make, effect, initiate, cause or participate in (A) any acquisition of beneficial ownership (including, but not limited to, beneficial ownership as defined in Rule 13d-3 under the Exchange Act) of any securities of the Company or any debt of the Company or any securities (including derivatives thereof) or debt of any subsidiary or other affiliate of the Company, (B) any acquisition of any assets of the Company or any assets of any subsidiary, division or other affiliate of the Company, (C) any tender offer, exchange offer, merger, business combination, recapitalization, restructuring, liquidation, dissolution or extraordinary transaction involving the Company or any subsidiary or other affiliate of the Company or involving any securities or assets of the Company or any securities or assets of any subsidiary, division or other affiliate of the Company, or (D) any “solicitation” of “proxies” (as those terms are used in the proxy rules of the SEC) or consents with respect to any securities of the Company;
18.
(ii) form, join or participate in a “group” (as defined in the Exchange Act) with respect to the beneficial ownership of any securities of the Company or any subsidiary or division of the Company;
(iii) act in concert with others, to seek to control or influence the management, Board or policies of the Company;
(iv) publicly propose the taking of, any action referred to in clause “(i)”, “(ii)”, or “(iii)” of this Section 5(h);
(v) assist, induce or encourage any other Person to take any action of the type referred to in clause “(i)”, “(ii)”, or “(iii)” of this Section 5(h); or
(vi) enter into any discussions, negotiations, arrangement or agreement with any other Person relating to any of the foregoing.
Nothing in this Agreement will prevent Investor or any of Investor’s Affiliates from communicating with the Chief Executive Officer of the Company or the Board to make a proposal for or to negotiate with the Company in respect of a tender or exchange offer, merger or other business combination, or any other of the transactions described in Section 5(a)(i) involving the Investor and Company so long as such communication is made confidentially. The standstill provisions of this Section 5(h) shall not apply in the event that, without any violation of the standstill provision, (i) a third party unrelated to the Investor shall have entered into a definitive agreement with Company to acquire more than 50% of the outstanding voting securities of the Company or (ii) a third party unrelated to the Investor commences a tender offer for more than 50% of the outstanding voting securities of the Company that the Board publicly recommends; provided, that the standstill provisions of this Section 5(h) shall automatically become applicable again if the third party announces its intent not to proceed with such acquisition or commenced tender offer. The expiration of the Restricted Period will not terminate or otherwise affect any of the other provisions of this Agreement.
6. RESTRICTIONS ON TRANSFER.
(a) The Shares shall not be sold, pledged, or otherwise transferred, and the Company shall not recognize and shall issue stop-transfer instructions to its transfer agent with respect to any such sale, pledge, or transfer, except upon the conditions specified in this Agreement, which conditions are intended to ensure compliance with the provisions of the Securities Act. The Investor will cause any proposed purchaser, pledgee, or transferee of the Shares to agree to take and hold such securities subject to the provisions and upon the conditions specified in this Agreement.
(b) Each certificate, instrument, or book entry representing Shares and any other securities issued in respect of such Shares, including the Conversion Shares, upon any stock split, stock dividend, recapitalization, merger, consolidation, or similar event, shall (unless otherwise permitted by the provisions of Section 6(c)) be notated with a legend substantially in the following form:
THE SECURITIES REPRESENTED HEREBY HAVE BEEN ACQUIRED FOR INVESTMENT AND HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED. SUCH SHARES MAY NOT BE SOLD, PLEDGED, OR TRANSFERRED IN THE ABSENCE OF SUCH REGISTRATION OR A VALID EXEMPTION FROM THE REGISTRATION AND PROSPECTUS DELIVERY REQUIREMENTS OF SAID ACT.
THE SECURITIES REPRESENTED HEREBY MAY BE TRANSFERRED ONLY IN ACCORDANCE WITH THE TERMS OF AN AGREEMENT BETWEEN THE COMPANY AND THE STOCKHOLDER, A COPY OF WHICH IS ON FILE WITH THE SECRETARY OF THE COMPANY.
19.
The Investor consents to the Company making a notation in its records and giving instructions to any transfer agent of the Company’s securities in order to implement the restrictions on transfer set forth in this Section 6.
(c) Before any proposed sale, pledge, or transfer of any Shares, unless there is in effect a registration statement under the Securities Act covering the proposed transaction, the Investor shall give oral notice to the Company of its intention to effect such sale, pledge, or transfer and, if reasonably requested by the Company, cause to be delivered at the Investor’s expense either (i) a written opinion of legal counsel who shall, and whose legal opinion shall, be reasonably satisfactory to the Company, addressed to the Company, to the effect that the proposed transaction may be effected without registration under the Securities Act; (ii) a “no action” letter from the SEC to the effect that the proposed sale, pledge, or transfer of such securities without registration will not result in a recommendation by the staff of the SEC that action be taken with respect thereto; or (iii) any other evidence reasonably satisfactory to counsel to the Company to the effect that the proposed sale, pledge, or transfer of the securities may be effected without registration under the Securities Act, whereupon the Investor shall be entitled to sell, pledge, or transfer such securities in accordance with the terms of the notice given by the Investor to the Company. The Company will not require such a legal opinion or “no action” letter (x) in any transaction in compliance with Rule 144; or (y) in any transaction in which such Holder distributes securities to an Affiliate of such Holder for no consideration; provided that each transferee agrees in writing to be subject to the terms of this Agreement, including Section 5 and Section 6. Each certificate, instrument, or book entry representing the Shares transferred as above provided shall be notated with, except if such transfer is made pursuant to Rule 144, the appropriate restrictive legend set forth in Section 6(b), except that such certificate instrument, or book entry shall not be notated with such restrictive legend if, in the opinion of counsel for the Investor and the Company, such legend is not required in order to establish compliance with any provisions of the Securities Act.
7. REGISTRATION OF CONVERSION SHARES.
(a) The Company agrees that, within fifteen (15) calendar days of the delivery by the Investor of a written request to the Company that the Conversion Shares (or a portion thereof) be registered for resale with the SEC (the “Filing Date”), the Company will file with the SEC (at the Company’s sole cost and expense) a registration statement registering the resale of the Conversion Shares (the “Registration Statement”) and the Company shall use its commercially reasonable efforts to have the Registration Statement declared effective as soon as practicable after the filing thereof (and in any event, no later than thirty (30) calendar days following the Filing Date) (the “Effectiveness Deadline”); provided that the Effectiveness Deadline shall be extended to ninety (90) calendar days after the Filing Date if the Registration Statement is reviewed by, and comments thereto are provided from, the SEC; provided, that if such day falls on a Saturday, Sunday or other day that the SEC is closed for business, the Effectiveness Deadline shall be extended to the next Business Day on which the SEC is open for business. Notwithstanding the foregoing, if the Company is notified (orally or in writing, whichever is earlier) by the SEC that the Registration Statement will not be “reviewed” or subject to further review, the Company shall use its commercially reasonable efforts to have the Registration Statement declared effective within five (5) Business Days of receipt of such notice. Any failure by the Company to file the Registration Statement by the Filing Date or to effect such Registration Statement by the Effectiveness Deadline shall not otherwise relieve the Company of its obligations to file or effect the Registration Statement as set forth above in this Section 7. The Company will use its commercially reasonable efforts to provide a draft of the Registration Statement to the Investor for review (but not comment other than with respect to the accuracy of the information concerning the Investor included therein) at least two (2) Business Days in advance of filing the Registration Statement; provided that, for the avoidance of doubt, in no event shall the Company be required to delay or postpone the filing of such Registration Statement as a result of or in connection with Investor’s review. In no event shall the undersigned be identified as a statutory underwriter in the Registration Statement unless requested by the SEC; provided, that if the SEC requests that the Investor be identified as a statutory underwriter in the Registration Statement, Investor will have the option, in its sole and absolute discretion, to either (i) have the opportunity to withdraw from the Registration Statement upon its prompt written request to the Company, in which case the Company’s obligation to register the Conversion Shares will be deemed satisfied or (ii) be included as such in the Registration Statement.
20.
Notwithstanding the foregoing, if the SEC prevents the Company from including any or all of the shares proposed to be registered under the Registration Statement due to limitations on the use of Rule 415 of the Securities Act for the resale of the Conversion Shares, such Registration Statement shall register for resale such number of Conversion Shares which is equal to the maximum number of Conversion Shares as is permitted by the SEC.
(b) The Company agrees that, except for such times as the Company is permitted hereunder to suspend the use of the prospectus forming part of a Registration Statement, the Company will use commercially reasonable efforts to cause such Registration Statement to remain effective with respect to Investor until the earlier of (i) one (1) year from the issuance of the Conversion Shares, (ii) the date on which all of the Conversion Shares shall have been sold, or (iii) on the first date on which the Investor can sell all of its Conversion Shares under Rule 144 of the Securities Act without limitation as to the manner of sale or the amount of such securities that may be sold. For as long as the Registration Statement shall remain effective pursuant to the immediately preceding sentence, the Company will use commercially reasonable efforts to file all reports, and provide all customary and reasonable cooperation, necessary to enable the undersigned to resell the Conversion Shares pursuant to the Registration Statement or Rule 144 of the Securities Act, as applicable, qualify the Conversion Shares for listing on the applicable stock exchange on which the Company’s shares of common stock are then listed, and update or amend the Registration Statement as necessary to include the Conversion Shares. For as long as the Investor holds the Conversion Shares, the Company will use commercially reasonable efforts to file all reports, and provide all customary and reasonable cooperation, necessary to enable the undersigned to resell the Conversion Shares pursuant to Rule 144 of the Securities Act. The Investor agrees to disclose its beneficial ownership, as determined in accordance with Rule 13d-3 of the Securities Exchange Act of 1934 (as amended, the “Exchange Act”), of Conversion Shares to the Company (or its successor) upon request to assist the Company in making the determination described above. The Company’s obligations to include the Conversion Shares in the Registration Statement are contingent upon the Investor furnishing in writing to the Company such information regarding Investor, the securities of the Company held by Investor and the intended method of disposition of the Conversion Shares as shall be reasonably requested by the Company to effect the registration of the Conversion Shares, and the Investor shall execute such documents for such registration as the Company may reasonably request that are customary of a selling stockholder in similar situations, including providing that the Company shall be entitled to postpone and suspend the effectiveness or use of the Registration Statement during any customary blackout or similar period or as permitted hereunder. In the case of the registration effected by the Company pursuant to this Agreement, the Company shall, upon reasonable request, inform the Investor as to the status of such registration. The Investor shall not be entitled to use the Registration Statement for an underwritten offering of Conversion Shares. The Company shall, at its expense, advise the undersigned within two (2) Business Days:
(A) when a Registration Statement or any amendment thereto has been filed with the SEC and when such Registration Statement or any post-effective amendment thereto has become effective;
(B) of the issuance by the SEC of any stop order suspending the effectiveness of any Registration Statement or the initiation of any proceedings for such purpose;
(C) of the receipt by the Company of any notification with respect to the suspension of the qualification of the Conversion Shares included therein for sale in any jurisdiction or the initiation or threatening of any proceeding for such purpose; and
(D) subject to the provisions in this Agreement, of the occurrence of any event that requires the making of any changes in any Registration Statement or prospectus so that, as of such date, the statements therein are not misleading and do not omit to state a material fact required to be stated therein or necessary to make the statements therein (in the case of a prospectus, in the light of the circumstances under which they were made) not misleading.
21.
Notwithstanding anything to the contrary set forth herein, the Company shall not, when so advising the undersigned of such events, provide the undersigned with any material, non-public information regarding the Company other than to the extent that providing notice to the undersigned of the occurrence of the events listed in (A) through (D) above constitutes material, nonpublic information regarding the Company. The Company shall, at its expense, (1) use its commercially reasonable efforts to obtain the withdrawal of any order suspending the effectiveness of any Registration Statement as promptly as reasonably practicable; (2) upon the occurrence of any event contemplated in Section 7(b)(D), except for such times as the Company is permitted hereunder to suspend, and has suspended, the use of a prospectus forming part of a Registration Statement, the Company shall use its commercially reasonable efforts to as promptly as reasonably practicable prepare a post-effective amendment to such Registration Statement or a supplement to the related prospectus, or file any other required document so that, as thereafter delivered to purchasers of the Conversion Shares included therein, such prospectus will not include any untrue statement of a material fact or omit to state any material fact necessary to make the statements therein, in the light of the circumstances under which they were made, not misleading; and (3) use its commercially reasonable efforts to take all other steps necessary to effect the registration of the Conversion Shares contemplated hereby. Notwithstanding anything to the contrary contained herein, the Company may delay or postpone filing of such Registration Statement, and from time to time require Investor not to sell under the Registration Statement or suspend the use or effectiveness of any such Registration Statement if it determines that in order for the registration statement to not contain a material misstatement or omission, an amendment thereto would be needed, or if such filing or use could materially affect a bona fide business or financing transaction of the Company or would require premature disclosure of information that could materially adversely affect the Company (each such circumstance, a “Suspension Event”); provided, that, (x) the Company shall not so delay filing or so suspend the use of the Registration Statement on more than two (2) occasions for a period of more than sixty (60) consecutive days each or more than a total of one hundred-twenty (120) calendar days, in each case in any three hundred sixty (360) day period and (y) the Company shall use commercially reasonable efforts to make such registration statement available for the sale by the undersigned of such securities as soon as practicable thereafter.
(c) Upon receipt of any written notice from the Company (which notice shall not contain any material non-public information regarding the Company) of the happening of any Suspension Event during the period that the Registration Statement is effective or if as a result of a Suspension Event the Registration Statement or related prospectus contains any untrue statement of a material fact or omits to state any material fact required to be stated therein or necessary to make the statements therein, in light of the circumstances under which they were made (in the case of the prospectus) not misleading, the undersigned agrees that (i) it will immediately discontinue offers and sales of the Shares under the Registration Statement (excluding, for the avoidance of doubt, sales conducted pursuant to Rule 144) until the undersigned receives copies of a supplemental or amended prospectus (which the Company agrees to promptly prepare) that corrects the misstatement(s) or omission(s) referred to above and receives notice that any post-effective amendment has become effective or unless otherwise notified by the Company that it may resume such offers and sales, and (ii) it will maintain the confidentiality of any information included in such written notice delivered by the Company unless otherwise required by law, subpoena or regulatory request or requirement. If so directed by the Company, the undersigned will deliver to the Company or, in the undersigned’s sole discretion destroy, all copies of the prospectus covering the Conversion Shares in the undersigned’s possession; provided, however, that this obligation to deliver or destroy all copies of the prospectus covering the Conversion Shares shall not apply (w) to the extent the undersigned is required to retain a copy of such prospectus (A) in order to comply with applicable legal, regulatory, self-regulatory or professional requirements or (B) in accordance with a bona fide pre-existing document retention policy or (x) to copies stored electronically on archival servers as a result of automatic data back-up.
(d) (i) The Company agrees to indemnify, to the extent permitted by law, the Investor (to the extent a seller under the Registration Statement), its directors, officers and employees, and each person who controls the Investor (within the meaning of the Securities Act) against all losses, claims, damages, liabilities and reasonable and documented out of pocket expenses (including reasonable and documented attorneys’ fees of one law firm (plus the fees of any local counsel)) caused by any untrue or alleged untrue statement of material fact contained in any Registration Statement, prospectus included in
22.
any Registration Statement (“Prospectus”) or preliminary Prospectus or any amendment thereof or supplement thereto or any omission or alleged omission of a material fact required to be stated therein or necessary to make the statements therein (in the case of a Prospectus, in the light of the circumstances under which they were made) not misleading, except insofar as the same are caused by or contained in any information furnished in writing to the Company by or on behalf of the Investor expressly for use therein.
(ii) In connection with any Registration Statement in which Investor is participating, the Investor shall furnish (or cause to be furnished) to the Company in writing such information as the Company reasonably requests for use in connection with any such Registration Statement or Prospectus and, to the extent permitted by law, shall indemnify the Company, its directors, officers and employees, and each person or entity who controls the Company (within the meaning of the Securities Act) against any losses, claims, damages, liabilities and expenses (including, without limitation, reasonable and documented outside attorneys’ fees) resulting from any untrue or alleged untrue statement of material fact contained in, or incorporated by reference in, any Registration Statement, Prospectus or preliminary Prospectus or any amendment thereof or supplement thereto or any omission or alleged omission of a material fact required to be stated therein or necessary to make the statements therein (in the case of a Prospectus, in the light of the circumstances under which they were made) not misleading, but only to the extent that such untrue statement or omission is contained (or not contained in, in the case of an omission) in any information or affidavit so furnished in writing by or on behalf of Investor expressly for use therein; provided, however, that the liability of the Investor shall be limited to the net proceeds received by the Investor from the sale of Conversion Shares giving rise to such indemnification obligation.
(iii) Any person or entity entitled to indemnification herein shall (A) give prompt written notice to the indemnifying party of any claim with respect to which it seeks indemnification (provided that the failure to give prompt notice shall not impair any person’s or entity’s right to indemnification hereunder to the extent such failure has not prejudiced the indemnifying party) and (B) unless in such indemnified party’s reasonable judgment a conflict of interest between such indemnified and indemnifying parties or separate defenses may exist with respect to such claim, permit such indemnifying party to assume the defense of such claim with counsel reasonably satisfactory to the indemnified party. If such defense is assumed, the indemnifying party shall not be subject to any liability for any settlement made by the indemnified party without its consent (but such consent shall not be unreasonably withheld, delayed or conditioned). An indemnifying party who is not entitled to, or elects not to, assume the defense of a claim shall not be obligated to pay the fees and expenses of more than one counsel (plus any local counsel) for all parties indemnified by such indemnifying party with respect to such claim, unless in the reasonable judgment of legal counsel to any indemnified party a conflict of interest exists between such indemnified party and any other of such indemnified parties with respect to such claim. No indemnifying party shall, without the consent of the indemnified party, consent to the entry of any judgment or enter into any settlement which cannot be settled in all respects by the payment of money (and such money is so paid by the indemnifying party pursuant to the terms of such settlement) or which settlement includes a statement or admission of fault and culpability on the part of such indemnified party or which does not include as an unconditional term thereof the giving by the claimant or plaintiff to such indemnified party of a release from all liability in respect to such claim or litigation.
(iv) The indemnification provided for under this Agreement shall remain in full force and effect regardless of any investigation made by or on behalf of the indemnified party or any officer, director or controlling person or entity of such indemnified party and shall survive the transfer of the Conversion Shares.
(v) If the indemnification provided under this Section 7(d) from the indemnifying party is unavailable or insufficient to hold harmless an indemnified party in respect of any losses, claims, damages, liabilities and expenses referred to herein, then the indemnifying party, in lieu of indemnifying the indemnified party, shall contribute to the amount paid or payable by the indemnified party as a result of such losses, claims, damages, liabilities and expenses in such proportion as is appropriate to reflect the relative fault of the indemnifying party and the indemnified party, as well as any other relevant equitable considerations; provided, however, that the liability of the Investor shall be limited to the net proceeds received by such Investor from the sale of Conversion Shares giving rise to such indemnification
23.
obligation. The relative fault of the indemnifying party and indemnified party shall be determined by reference to, among other things, whether any action in question, including any untrue or alleged untrue statement of a material fact or omission or alleged omission to state a material fact, was made by (or not made by, in the case of an omission), or relates to information supplied by (or not supplied by, in the case of an omission), or on behalf of, such indemnifying party or indemnified party, and the indemnifying party’s and indemnified party’s relative intent, knowledge, access to information and opportunity to correct or prevent such action. The amount paid or payable by a party as a result of the losses or other liabilities referred to above shall be deemed to include, subject to the limitations set forth in Sections 7(d)(i), (ii) and (iii) above, any legal or other fees, charges or expenses reasonably incurred by such party in connection with any investigation or proceeding. No person guilty of fraudulent misrepresentation (within the meaning of Section 11(f) of the Securities Act) shall be entitled to contribution pursuant to this Section 7(d)(v) from any person or entity who was not guilty of such fraudulent misrepresentation. Notwithstanding anything to the contrary herein, in no event will any party be liable for consequential, special, exemplary or punitive damages in connection with this Agreement.
8. CONDITIONS TO THE INVESTOR’S OBLIGATIONS. The obligation of the Investor to receive the Shares at the Closing is subject to the fulfillment, on or before the Closing, of each of the conditions set forth below.
8.1 Representations and Warranties. The representations and warranties of the Company contained in Section 3 hereof shall have been true and correct in all respects as of the date hereof.
8.2 Performance. The Company shall have performed and complied, in all material respects, with all covenants, agreements, obligations and conditions contained in this Agreement that are required to be performed or complied with by the Company on or before such Closing.
8.3 Asset Purchase Agreement. The Company shall have simultaneously closed the transactions contemplated by the APA.
8.4 Proceedings and Documents. All corporate and other proceedings in connection with the transactions contemplated at the Closing and all documents incident thereto shall be reasonably satisfactory in form and substance to the Investor, and the Investor (or its counsel) shall have received all such counterpart original and certified or other copies of such documents as reasonably requested.
8.5 Qualifications. All authorizations, approvals or permits, if any, of any governmental authority or regulatory body of the United States or of any state that are required in connection with the lawful issuance and sale of the Shares pursuant to this Agreement shall be obtained and effective as of the Closing.
8.6 Compliance Certificate. The President and Chief Executive Officer of the Company shall deliver to the Investor a certificate certifying that the conditions specified in Section 8.1 and Section 8.2 with respect to the Company have been fulfilled.
8.7 Secretary’s Certificate. The Secretary of the Company shall deliver to the Investor a certificate certifying that (a) attached thereto is a true and complete copy of the Company’s Restated Certificate, including the Certificate of Designations as in effect on the Closing Date, and Bylaws (b) the resolutions of the Board approving this Agreement and the transactions contemplated hereby, and (c) good standing certificates with respect to the Company from the applicable authority(ies) in Delaware and any other jurisdiction in which the Company is qualified to do business, dated within three (3) Business Days of the Closing.
8.8 Material Adverse Effect. No Material Adverse Effect shall have occurred and be continuing.
24.
8.9 Legal Opinion. The Investor shall have received from Cooley LLP, counsel for the Company, an opinion, dated as of the Closing, in a form reasonably satisfactory to the Investor.
8.10 Certificate of Designations. The Company shall have filed the Certificate of Designations with the Secretary of State of the State of Delaware prior to the Closing, which such Certificate of Designations shall continue to be in full force and effect as of the Closing.
9. CONDITIONS OF THE COMPANY’S OBLIGATIONS AT CLOSING. The obligations of the Company to sell Shares to the Investor at the Closing are subject to the fulfillment, on or before the Closing, of each of the following conditions, unless otherwise waived:
9.1 Representations and Warranties. The representations and warranties of the Investor contained in Section 4 shall be true and correct in all respects as of the Closing.
9.2 Performance. The Investor shall have performed and complied with all covenants, agreements, obligations and conditions contained in this Agreement that are required to be performed or complied with by them on or before such Closing.
9.3 Compliance Certificate. An authorized officer of the Investor shall deliver to the Company a certificate certifying that the conditions specified in Section 9.1 and Section 9.2 with respect to the Investor have been fulfilled.
9.4 Qualifications. All authorizations, approvals or permits, if any, of any governmental authority or regulatory body of the United States or of any state that are required in connection with the lawful issuance and sale of the Shares pursuant to this Agreement shall be obtained and effective as of the Closing.
10. MISCELLANEOUS.
10.1 Successors and Assigns. The terms and conditions of this Agreement shall inure to the benefit of and be binding upon the respective successors and assigns of the parties. Nothing in this Agreement, express or implied, is intended to confer upon any party other than the parties hereto or their respective successors and assigns any rights, remedies, obligations or liabilities under or by reason of this Agreement, except as expressly provided in this Agreement.
10.2 Governing Law. This Agreement shall be governed by the internal law of the State of Delaware without regard to principles of conflicts of law.
10.3 Counterparts. This Agreement may be executed in counterparts by a single party, each of which when taken together shall constitute one and the same agreement. Counterparts may be delivered via facsimile, electronic mail (including .pdf or any electronic signature complying with the U.S. federal ESIGN Act of 2000, Uniform Electronic Transactions Act or other applicable law) or other transmission method and any counterpart so delivered shall be deemed to have been duly and validly delivered and be valid and effective for all purposes.
10.4 Titles and Subtitles. The titles and subtitles used in this Agreement are used for convenience only and are not to be considered in construing or interpreting this Agreement.
10.5 Notices. All notices and other communications given or made pursuant to this Agreement shall be in writing and shall be deemed effectively given upon the earlier of actual receipt, or (a) personal delivery to the party to be notified, (b) when sent, if sent by electronic mail or facsimile during normal business hours of the recipient, and if not sent during normal business hours, then on the recipient’s next business day, (c) five (5) days after having been sent by registered or certified mail, return receipt requested, postage prepaid, or (d) one (1) business day after deposit with a nationally recognized overnight courier, freight prepaid, specifying next business day delivery, with written verification of receipt. All
25.
communications shall be sent to the respective parties at their address as set forth on the signature page hereto, or to such e-mail address, facsimile number or address as subsequently modified by written notice given in accordance with this Section 10.5. If notice is given to the Company, a copy shall also be sent to Cooley LLP, 3175 Hanover Street, Palo Alto, CA 94304, Attn: John T. McKenna, Esq., and if notice is given to the Investor, a copy shall also be given to (i) Millennium Pharmaceuticals, Inc., 40 Landsdowne Street Cambridge, MA 02139 Attn: Head of R&D Legal and email to: [email protected]; and (ii) Morgan, Lewis & Bockius LLP, 110 North Wacker Drive, Chicago, IL 60606-1511, Attn: Benjamin H. Pensak.
10.6 No Finder’s Fees. Each party represents that it neither is nor will be obligated for any finder’s fee or commission in connection with this transaction. The Investor agrees to indemnify and to hold harmless the Company from any liability for any commission or compensation in the nature of a finder’s or broker’s fee arising out of this transaction (and the costs and expenses of defending against such liability or asserted liability) for which the Investor or any of its officers, employees or representatives is responsible. The Company agrees to indemnify and hold harmless the Investor from any liability for any commission or compensation in the nature of a finder’s or broker’s fee arising out of this transaction (and the costs and expenses of defending against such liability or asserted liability) for which the Company or any of its officers, employees or representatives is responsible.
10.7 Waivers. A party’s consent to or waiver, express or implied, of the other party’s breach of its obligations hereunder shall not be deemed to be or construed as a consent to or waiver of any other breach of the same or any other obligations of such breaching party. A party’s failure to complain of any act, or failure to act, by the other party, to declare the other party in default, to insist upon the strict performance of any obligation or condition of this Agreement or to exercise any right or remedy consequent upon a breach thereof, no matter how long such failure continues, shall not constitute a waiver by such party of its rights hereunder, of any such breach, or of any other obligation or condition. A party’s consent in any one instance shall not limit or waive the necessity to obtain such party’s consent in any future instance and in any event no consent or waiver shall be effective for any purpose hereunder unless such consent or waiver is in writing and signed by the party granting such consent or waiver.
10.8 Severability. Nothing in this Agreement shall be construed to require the commission of any act contrary to applicable law. If any one or more provisions of this Agreement is held to be invalid, illegal or unenforceable, the affected provisions of this Agreement shall be curtailed and limited only to the extent necessary to bring it within the applicable legal requirements and the validity, legality and enforceability of the remaining provisions of this Agreement shall not in any way be affected or impaired thereby.
10.9 Delays or Omissions. No delay or omission to exercise any right, power or remedy accruing to any party under this Agreement, upon any breach or default of any other party under this Agreement, shall impair any such right, power or remedy of such non-breaching or non-defaulting party nor shall it be construed to be a waiver of any such breach or default, or an acquiescence therein, or of or in any similar breach or default thereafter occurring.
10.10 Entire Agreement; Amendment. This Agreement and the APA (including any Schedules, Exhibits and ancillaries referenced herein and therein), constitute the entire agreement between the Parties as to the subject matter hereof. Except as set forth in this Section 10.10, (a) all prior and contemporaneous negotiations, representations, warranties, agreements, statements, promises and understandings with respect to the subject matter of this Agreement are hereby superseded and merged into, extinguished by and completely expressed by this Agreement and (b) neither party shall be bound by or charged with any written or oral agreements, representations, warranties, statements, promises or understandings not specifically set forth in this Agreement. No amendment, supplement or other modification to any provision of this Agreement shall be binding unless in writing and signed by both parties.
26.
10.11 Dispute Resolution. The parties (a) hereby irrevocably and unconditionally submit to the jurisdiction of the state courts of the state of Delaware and to the jurisdiction of the United States District Court for the District of Delaware for the purpose of any suit, action or other proceeding arising out of or based upon this Agreement, (b) agree not to commence any suit, action or other proceeding arising out of or based upon this Agreement except in the state courts of the state of Delaware or the United States District Court for the District of Delaware, and (c) hereby waive, and agree not to assert, by way of motion, as a defense, or otherwise, in any such suit, action or proceeding, any claim that it is not subject personally to the jurisdiction of the above-named courts, that its property is exempt or immune from attachment or execution, that the suit, action or proceeding is brought in an inconvenient forum, that the venue of the suit, action or proceeding is improper or that this Agreement or the subject matter hereof may not be enforced in or by such court.
WAIVER OF JURY TRIAL: EACH PARTY HEREBY WAIVES ITS RIGHTS TO A JURY TRIAL OF ANY CLAIM OR CAUSE OF ACTION BASED UPON OR ARISING OUT OF THIS AGREEMENT, THE OTHER TRANSACTION DOCUMENTS, THE SECURITIES OR THE SUBJECT MATTER HEREOF OR THEREOF. THE SCOPE OF THIS WAIVER IS INTENDED TO BE ALL-ENCOMPASSING OF ANY AND ALL DISPUTES THAT MAY BE FILED IN ANY COURT AND THAT RELATE TO THE SUBJECT MATTER OF THIS AGREEMENT, INCLUDING, WITHOUT LIMITATION, CONTRACT CLAIMS, TORT CLAIMS (INCLUDING NEGLIGENCE), BREACH OF DUTY CLAIMS, AND ALL OTHER COMMON LAW AND STATUTORY CLAIMS. THIS SECTION HAS BEEN FULLY DISCUSSED BY EACH OF THE PARTIES HERETO AND THESE PROVISIONS WILL NOT BE SUBJECT TO ANY EXCEPTIONS. EACH PARTY HERETO HEREBY FURTHER WARRANTS AND REPRESENTS THAT SUCH PARTY HAS REVIEWED THIS WAIVER WITH ITS LEGAL COUNSEL, AND THAT SUCH PARTY KNOWINGLY AND VOLUNTARILY WAIVES ITS JURY TRIAL RIGHTS FOLLOWING CONSULTATION WITH LEGAL COUNSEL.
[Remainder of Page Intentionally Left Blank]
27.
IN WITNESS WHEREOF, the parties have executed this PREFERRED STOCK PURCHASE AGREEMENT as of the date first written above.
| COMPANY: | ||
| CALITHERA BIOSCIENCES, INC. | ||
| By: | /s/ Susan Molineaux, Ph.D. | |
| Name: | Susan Molineaux, Ph.D. | |
| Title: | President and Chief Executive Officer | |
| 343 Oyster Point Boulevard, Suite 200 | ||
| South San Francisco, CA 94080 | ||
SIGNATURE PAGE TO PREFERRED STOCK PURCHASE AGREEMENT
IN WITNESS WHEREOF, the parties have executed this PREFERRED STOCK PURCHASE AGREEMENT as of the date first written above.
| INVESTOR: | ||
| MILLENNIUM PHARMACEUTICALS, INC. | ||
| By: | /s/ Nenad Grmusa | |
| Name: | Nenad Grmusa | |
| Title: | Head, Center for External Innovation | |
| Address: 40 Landsdowne Street | ||
| Cambridge, MA 02139 United States | ||
SIGNATURE PAGE TO PREFERRED STOCK PURCHASE AGREEMENT
Exhibit A
Certificate of Designations
See Exhibit 3.1 to Current Report on Form8-K, dated October 18, 2021
Exhibit 99.1

Calithera Expands Oncology Pipeline with Acquisition of Two Clinical-Stage Assets from Takeda Pharmaceuticals
— TORC 1/2 inhibitor sapanisertib and SYK inhibitor mivavotinib strengthen Company’s precision oncology pipeline —
— Calithera will initiate Phase 2 clinical trials of sapanisertib and mivavotinib in 2022 —
— Calithera to host webcast and conference call today at 5:30 p.m. ET / 2:30 p.m. PT —
SOUTH SAN FRANCISCO, Calif., October 18, 2021 (GLOBE NEWSWIRE) – Calithera Biosciences, Inc. (Nasdaq: CALA), a clinical-stage, precision oncology biopharmaceutical company, today announced an agreement with Takeda Pharmaceutical Company Limited (“Takeda”) to acquire two clinical-stage compounds, both of which have demonstrated single-agent clinical activity with the greatest potential in biomarker-defined cancer-patient populations. The compounds, sapanisertib (CB-228, formerly TAK-228) and mivavotinib (CB-659, formerly TAK-659), further strengthen Calithera’s pipeline of clinical-stage targeted therapies.
“We believe that these clinical-stage compounds are an excellent complement to our internally-developed pipeline programs, and fit well with our current strategic focus on biomarker-driven therapeutic approaches. We are encouraged by the promising single-agent clinical data that suggest these investigational therapies could help transform treatment for multiple cancer patient populations with high unmet need,” said Susan Molineaux, PhD, president and chief executive officer of Calithera. “Specifically, sapanisertib has the potential to be the first targeted treatment for patients with NRF2-mutated squamous non-small cell lung cancer. We have learned a great deal about the unmet medical need of patients with KEAP1/NRF2 mutations, as well as how to identify and recruit these patients, during the conduct of our KEAPSAKE trial evaluating telaglenastat. This complementary approach in KEAP1/NRF2-mutant squamous NSCLC demonstrates our commitment to these patients and the pathway.
“Additionally, mivavotinib has the potential to be a best-in-class SYK inhibitor in non-Hodgkin’s lymphoma, as well as a first-to-market approach for patients with diffuse large B-cell lymphoma whose tumors harbor MyD88 and/or CD79 mutations.
“We plan to start a clinical trial in squamous NSCLC with sapanisertib and a clinical trial in DLBCL with mivavotinib, both in biomarker specific populations, and generate data in the next 12 to 18 months that will define the clinical development and potential regulatory approval paths for both of these compounds.”
The terms of the transaction include a total upfront cash payment to Takeda of $10 million and $35 million issued to Takeda in Calithera Series A preferred stock. Additionally, Takeda will be eligible to receive from Calithera clinical development, regulatory and sales milestone payments across both programs. Calithera will pay tiered royalties of high single-digits to low teens on future net sales should these candidates achieve regulatory approvals and subsequent commercial availability.
“Collaboration is an important aspect of our R&D strategy and at the center of our efforts to deliver new treatment options to patients. We are confident that Calithera, with their highly capable and experienced team, is the ideal partner to resume the development of sapanisertib and mivavotinib, and to maximize their potential to address underserved patient populations,” said Christopher Arendt, Ph.D., head of Oncology Cell Therapy and Therapeutic Area Unit of Takeda. “We look forward to seeing how these programs advance under Calithera’s leadership.”
Sapanisertib is a dual TORC 1/2 inhibitor that targets a key survival mechanism in KEAP1/NRF2-mutated tumor cells. These mutations are found in a considerable sub-population of patients across multiple solid tumor types. Sapanisertib has demonstrated promising single-agent activity in patients with relapsed/refractory NRF2-mutated squamous non-small cell lung cancer (NSCLC) and exhibits differential anti-tumor activity compared to rapalog inhibitors of TORC1 in NRF2-mutant squamous NSCLC in vivo models. A Phase 2 study planned to begin in the first quarter of 2022 will further evaluate sapanisertib as a monotherapy in patients with squamous NSCLC harboring a NRF2 mutation.
Mivavotinib is a SYK inhibitor that targets the constitutively active BCR pathway in many non-Hodgkin’s lymphoma (NHL) cases as well as the constitutively active inflammatory signaling pathway in MyD88-mutated NHL. In early phase studies, mivavotinib showed promising single-agent responses in relapsed/refractory diffuse large B-cell lymphoma (DLBCL). In addition, recent preclinical studies have shown enhanced SYK activity and sensitivity to SYK inhibition in DLBCL and other NHLs harboring mutations in MyD88 and/or CD79, which comprise a distinct genetic subset of DLBCL known to have poor outcomes with standard-of-care therapy. Accordingly, Calithera plans to initiate a Phase 2 study of mivavotinib in 2022 for the treatment of patients with DLBCL with and without mutations in MyD88 and CD79. Beyond DLBCL, both preclinical and clinical data support expansion across additional NHL subtypes and other hematologic malignancies as part of long-term plans.
More information about sapanisertib and mivavotinib can be found at calithera.com/pipeline.
Webcast and Conference Call Information
Calithera will hold a webcast today, Monday, October 18 at 5:30 p.m. Eastern Time / 2:30 p.m. Pacific Time. To access the link to the webcast, which will be broadcast live in listen-only mode, or the subsequent archived recording, visit the Investors section of the Calithera website at www.calithera.com. Alternatively, the call may be accessed by dialing (855) 783-2599 (domestic) or (631) 485-4877 (international) and referring to conference ID 6946687. The webcast will be recorded and available for replay on Calithera’s website for 30 days.
About Calithera
Calithera Biosciences is a clinical-stage, precision oncology biopharmaceutical company developing targeted therapies to redefine treatment for biomarker-specific patient populations. Driven by a commitment to rigorous science and a passion for improving the lives of people impacted by cancer and other life-threatening diseases, Calithera is advancing a robust pipeline of investigational, small molecule oncology compounds with a biomarker-driven approach that targets genetic vulnerabilities in cancer cells to deliver new therapies for patients suffering from aggressive hematologic and solid tumor cancers for which there are currently limited treatment options.
Calithera is headquartered in South San Francisco, California. For more information about Calithera, please visit www.calithera.com.
Forward Looking Statements
Statements contained in this press release regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as “may,” “will,” “expect,” “anticipate,” “estimate,” “intend,” “poised” and similar expressions (as well as other words or expressions referencing future events, conditions, or circumstances) are intended to identify forward-looking statements. These statements include those related to Calithera’s clinical trials, the timing and enrollment of the KEAPSAKE, sapanisertib Phase 2 and mivavotinib Phase 2 trials, the payment of future royalties, development, regulatory and sales milestone payments to Takeda, the
potential impact and commercialization of sapanisertib for patients with NSCLC and a NRF2/KEAP1 mutation, the potential impact and commercialization of mivavotinib in patients with NHL with and without mutations in MyD88 and CD79. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. The potential product candidates that Calithera develops may not progress through clinical development or receive required regulatory approvals within expected timelines or at all. In addition, clinical trials may not confirm any safety, potency or other product characteristics described or assumed in this press release. Such product candidates may not be beneficial to patients or be successfully commercialized. The failure to meet expectations with respect to any of the foregoing matters may have a negative effect on Calithera’s stock price. Additional information concerning these and other risk factors affecting Calithera’s business can be found in Calithera’s periodic filings with the Securities and Exchange Commission at www.sec.gov. These forward-looking statements are not guarantees of future performance and speak only as of the date hereof, and, except as required by law, Calithera disclaims any obligation to update these forward-looking statements to reflect future events or circumstances.
# # #
CONTACTS:
Stephanie Wong
Chief Financial Officer
(650) 870-1063
Media
Sam Brown, Inc.
Audra Friis
(917) 519-9577
Investors
Burns McClellan
Lee Roth
212.213.0006

Targeting cancer, differently. Susan M. Molineaux, Ph.D. | Founder, President & Chief Executive Officer Exhibit 99.2

Forward-Looking Statements This presentation and the accompanying oral commentary contain “forward‐looking” statements for purposes of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. We may, in some cases, use terms such as “believe,” “will,” “may,” “estimate,” “continue,” “anticipate,” “intend,” “should,” “plan,” “might,” “approximately,” “expect,” “predict,” “could,” “potentially” or the negative of these terms or other words that convey uncertainty of future events or outcomes to identify these forward‐looking statements. All statements other than statements of historical facts contained in this presentation and the accompanying oral commentary are forward‐looking statements, and such forward‐looking statements include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things: plans regarding anticipated clinical trials for our product candidates, including CB-228 (sapanisertib), CB-659 (mivavotinib), CB-839 (telaglenastat), CB-280, INCB001158 and CB-708, the potential safety, efficacy and other benefits of and market opportunity of product candidates, the timing of and our ability to make regulatory filings and obtain and maintain regulatory approvals for our product candidates, statements relating to future royalties and the development, regulatory and sales milestone payments of INCB001158 and CB-708 in connection with our collaborations with Incyte and Antengene, respectively, and of CB-228 and CB-659 in connection with our asset purchase agreement with Takeda, our intellectual property position and cash needs. Forward‐looking statements involve known and unknown risks, uncertainties, assumptions and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward‐looking statements. The potential product candidates that we develop may not progress through clinical development or receive required regulatory approvals within expected timelines or at all. In addition, clinical trials may not confirm any safety, potency or other product characteristics described or assumed in this presentation and the accompanying oral commentary. Such product candidates may not be beneficial to patients or be successfully commercialized. The failure to meet expectations with respect to any of the foregoing matters may have a negative effect on our stock price. We discuss many of these risks in greater detail under the heading "Risk Factors" contained in our Quarterly Report on Form 10‐Q for the quarter ended June 30, 2021, filed with the Securities and Exchange Commission on August 5, 2021. Forward‐looking statements are not guarantees of future performance and our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate may differ materially from the forward‐looking statements contained in this presentation and the accompanying oral commentary. Any forward‐looking statements that we make in this presentation and the accompanying oral commentary speak only as of the date of this presentation. We assume no obligation to update our forward‐looking statements whether as a result of new information, future events or otherwise. No Offer or Solicitation This presentation does not constitute an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction.

is a clinical-stage, precision oncology biopharmaceutical company developing targeted therapies to redefine treatment for biomarker-specific patient populations
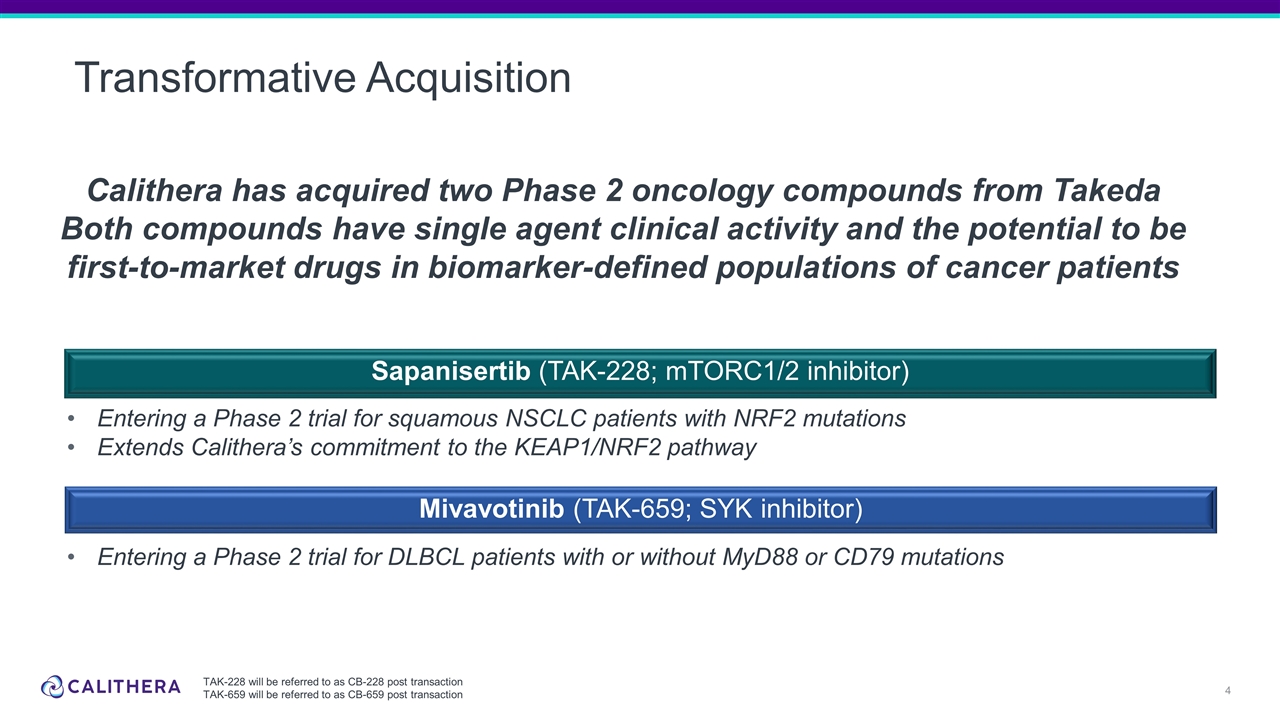
Transformative Acquisition Sapanisertib (TAK-228; mTORC1/2 inhibitor) Calithera has acquired two Phase 2 oncology compounds from Takeda Both compounds have single agent clinical activity and the potential to be first-to-market drugs in biomarker-defined populations of cancer patients Mivavotinib (TAK-659; SYK inhibitor) Entering a Phase 2 trial for squamous NSCLC patients with NRF2 mutations Extends Calithera’s commitment to the KEAP1/NRF2 pathway Entering a Phase 2 trial for DLBCL patients with or without MyD88 or CD79 mutations TAK-228 will be referred to as CB-228 post transaction TAK-659 will be referred to as CB-659 post transaction
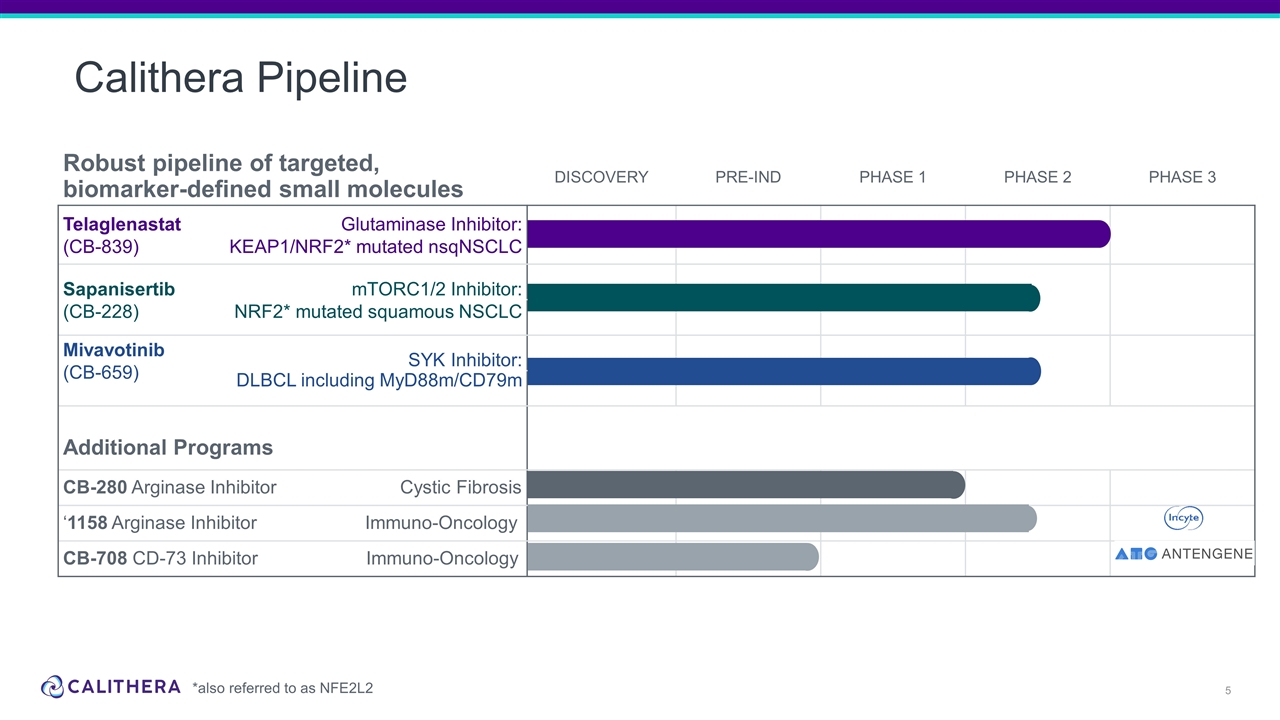
Robust pipeline of targeted, biomarker-defined small molecules DISCOVERY PRE-IND PHASE 1 PHASE 2 PHASE 3 Telaglenastat (CB-839) Glutaminase Inhibitor: KEAP1/NRF2* mutated nsqNSCLC Sapanisertib (CB-228) mTORC1/2 Inhibitor: NRF2* mutated squamous NSCLC Lung KEAP1/NRF2* mutation KEAPSAKE Mivavotinib (CB-659) SYK Inhibitor: DLBCL including MyD88m/CD79m Additional Programs CB-280 Arginase Inhibitor Cystic Fibrosis ‘1158 Arginase Inhibitor Immuno-Oncology CB-708 CD-73 Inhibitor Immuno-Oncology Calithera Pipeline *also referred to as NFE2L2

Telaglenastat and Sapanisertib KEAP1/NRF2 Programs

Telaglenastat Glutaminase Inhibitor in Development for Non-Squamous NSCLC Patients Harboring KEAP1 or NRF2 mutations
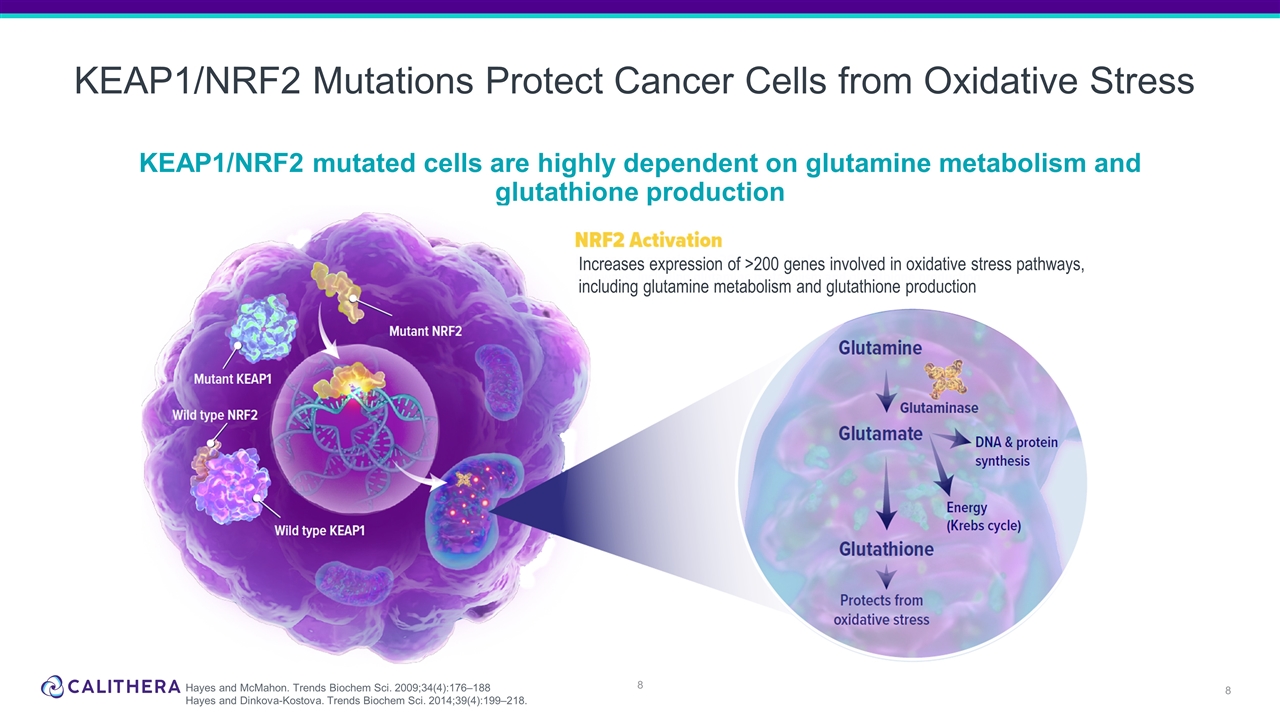
KEAP1/NRF2 Mutations Protect Cancer Cells from Oxidative Stress KEAP1/NRF2 mutated cells are highly dependent on glutamine metabolism and glutathione production Increases expression of >200 genes involved in oxidative stress pathways, including glutamine metabolism and glutathione production Hayes and McMahon. Trends Biochem Sci. 2009;34(4):176–188 Hayes and Dinkova-Kostova. Trends Biochem Sci. 2014;39(4):199–218.
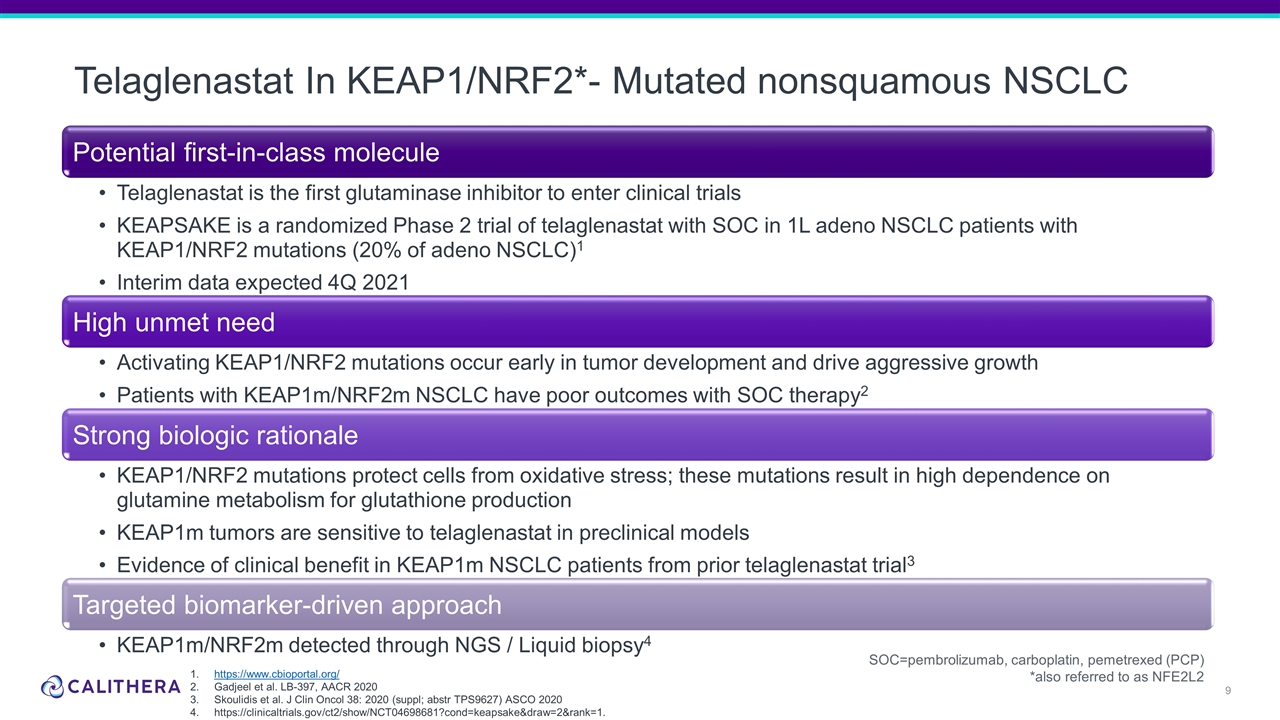
Telaglenastat In KEAP1/NRF2*- Mutated nonsquamous NSCLC SOC=pembrolizumab, carboplatin, pemetrexed (PCP) *also referred to as NFE2L2 https://www.cbioportal.org/ Gadjeel et al. LB-397, AACR 2020 Skoulidis et al. J Clin Oncol 38: 2020 (suppl; abstr TPS9627) ASCO 2020 https://clinicaltrials.gov/ct2/show/NCT04698681?cond=keapsake&draw=2&rank=1. KEAPSAKE is a randomized Phase 2 trial of telaglenastat with SOC in 1L adeno NSCLC patients with KEAP1/NRF2 mutations (20% of adeno NSCLC) 1 Strong biologic rationale KEAP1/NRF2 mutations protect cells from oxidative stress; these mutations result in high dependence on glutamine metabolism for glutathione production Targeted biomarker-driven approach KEAP1m/NRF2m detected through NGS / Liquid biopsy 4 Interim data expected 4Q 2021 Potential first-in-class molecule Telaglenastat is the first glutaminase inhibitor to enter clinical trials High unmet need Activating KEAP1/NRF2 mutations occur early in tumor development and drive aggressive growth Patients with KEAP1m/NRF2m NSCLC have poor outcomes with SOC therapy 2 Evidence of clinical benefit in KEAP1m NSCLC patients from prior telaglenastat trial 3 KEAP1m tumors are sensitive to telaglenastat in preclinical models
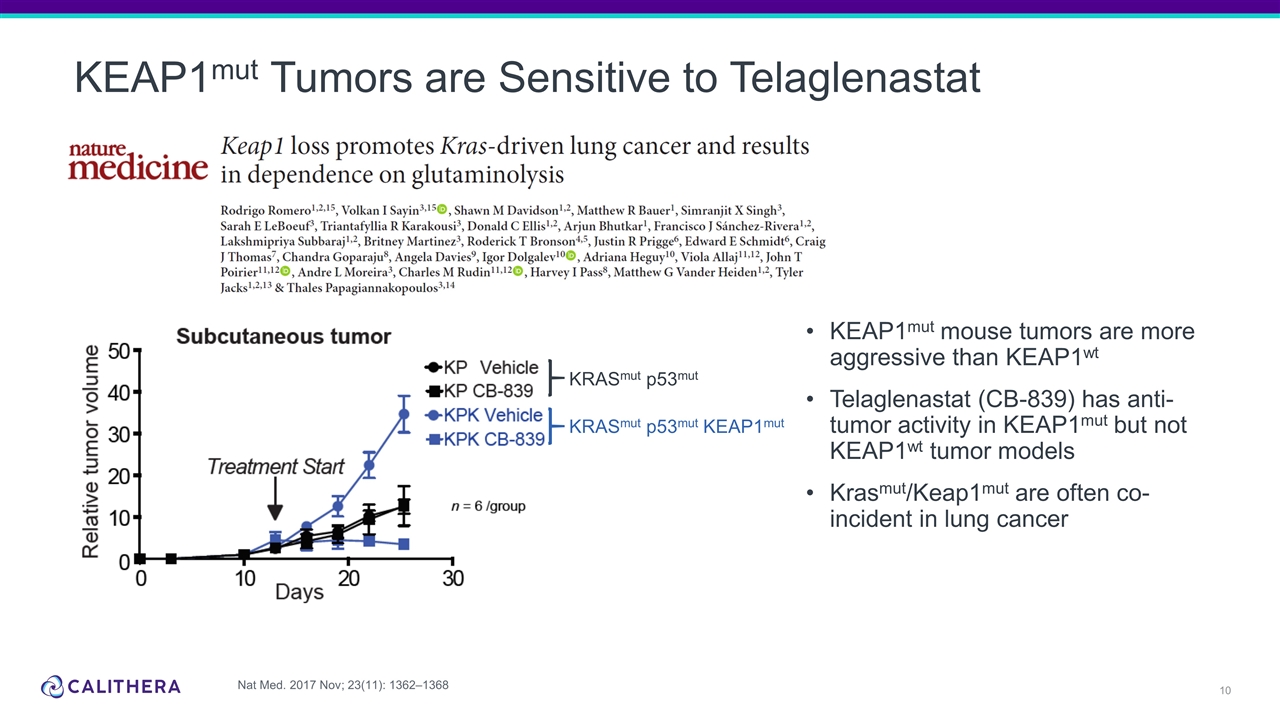
KEAP1mut Tumors are Sensitive to Telaglenastat KRASmut p53mut KRASmut p53mut KEAP1mut KEAP1mut mouse tumors are more aggressive than KEAP1wt Telaglenastat (CB-839) has anti-tumor activity in KEAP1mut but not KEAP1wt tumor models Krasmut/Keap1mut are often co-incident in lung cancer Nat Med. 2017 Nov; 23(11): 1362–1368
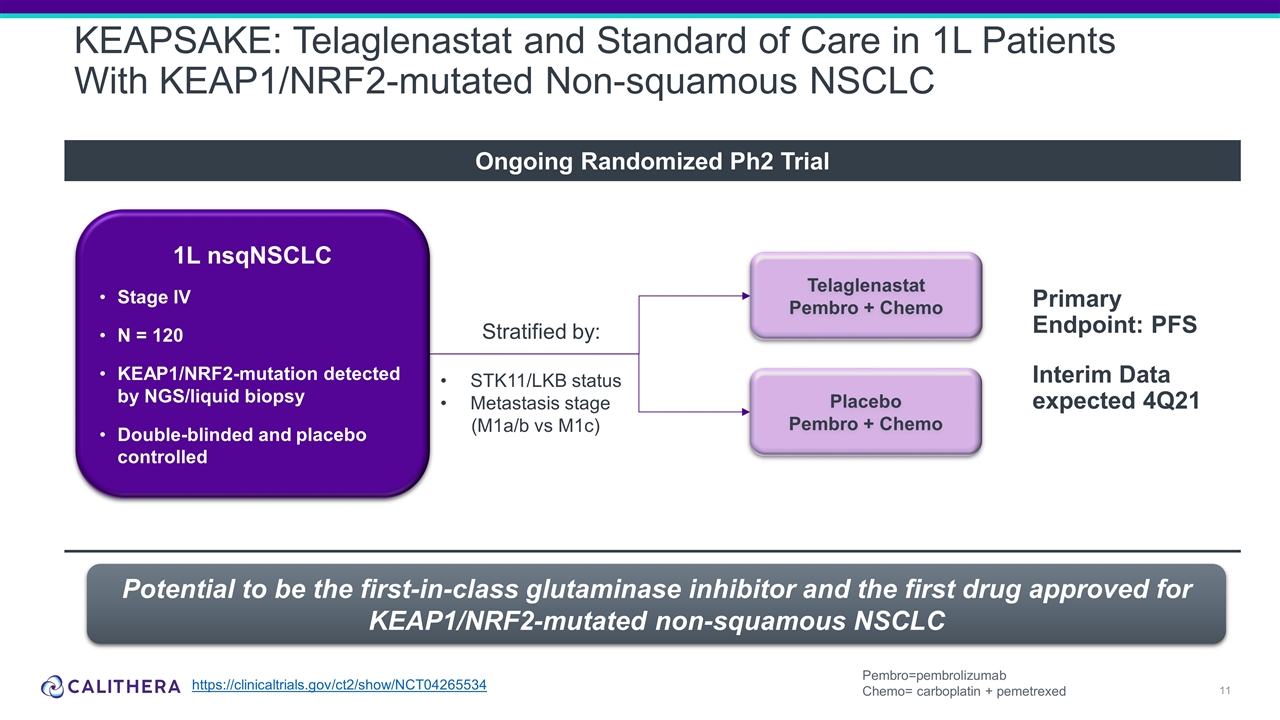
KEAPSAKE: Telaglenastat and Standard of Care in 1L Patients With KEAP1/NRF2-mutated Non-squamous NSCLC Ongoing Randomized Ph2 Trial Placebo Pembro + Chemo 1L nsqNSCLC Stage IV N = 120 KEAP1/NRF2-mutation detected by NGS/liquid biopsy Double-blinded and placebo controlled Telaglenastat Pembro + Chemo Primary Endpoint: PFS Interim Data expected 4Q21 STK11/LKB status Metastasis stage (M1a/b vs M1c) https://clinicaltrials.gov/ct2/show/NCT04265534 Pembro=pembrolizumab Chemo= carboplatin + pemetrexed Stratified by: Potential to be the first-in-class glutaminase inhibitor and the first drug approved for KEAP1/NRF2-mutated non-squamous NSCLC
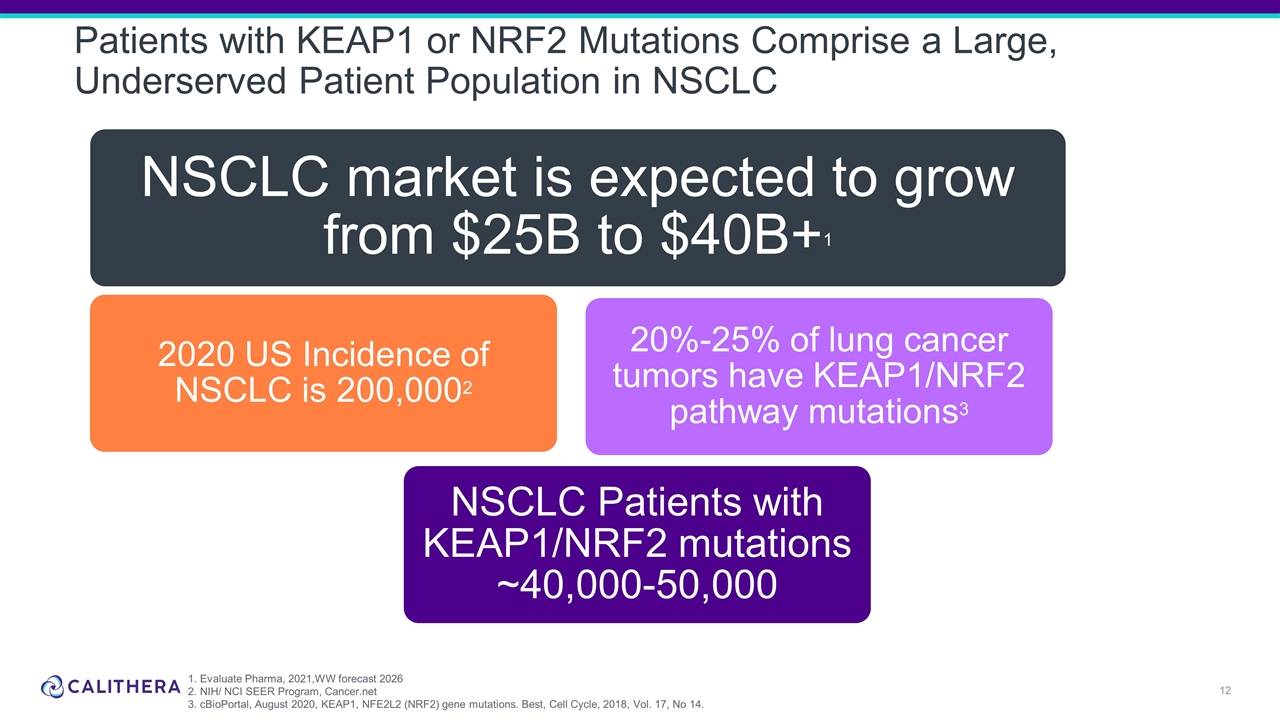
Patients with KEAP1 or NRF2 Mutations Comprise a Large, Underserved Patient Population in NSCLC 1. Evaluate Pharma, 2021,WW forecast 2026 2. NIH/ NCI SEER Program, Cancer.net 3. cBioPortal, August 2020, KEAP1, NFE2L2 (NRF2) gene mutations. Best, Cell Cycle, 2018, Vol. 17, No 14. NSCLC market is expected to grow from $25B to $ 40B+ 1 NSCLC Patients with KEAP1/NRF2 mutations ~40,000-50,000 20%-25% of lung cancer tumors have KEAP1/NRF2 pathway mutations 3 2020 US Incidence of NSCLC is 200,000 2

Sapanisertib mTORC1/2 Inhibitor in Development for Treatment of Squamous NSCLC patients whose tumors harbor NRF2 mutations
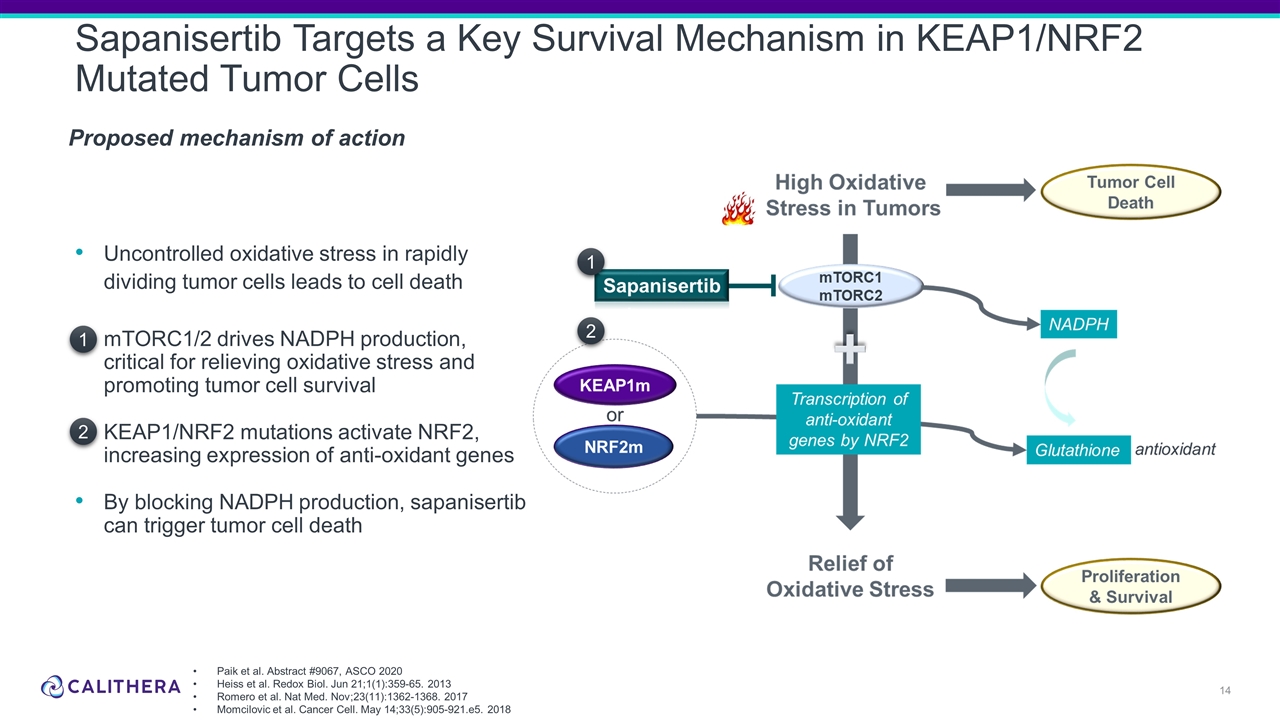
Uncontrolled oxidative stress in rapidly dividing tumor cells leads to cell death mTORC1/2 drives NADPH production, critical for relieving oxidative stress and promoting tumor cell survival KEAP1/NRF2 mutations activate NRF2, increasing expression of anti-oxidant genes By blocking NADPH production, sapanisertib can trigger tumor cell death Sapanisertib Targets a Key Survival Mechanism in KEAP1/NRF2 Mutated Tumor Cells Proposed mechanism of action Paik et al. Abstract #9067, ASCO 2020 Heiss et al. Redox Biol. Jun 21;1(1):359-65. 2013 Romero et al. Nat Med. Nov;23(11):1362-1368. 2017 Momcilovic et al. Cancer Cell. May 14;33(5):905-921.e5. 2018 1 2 1 2
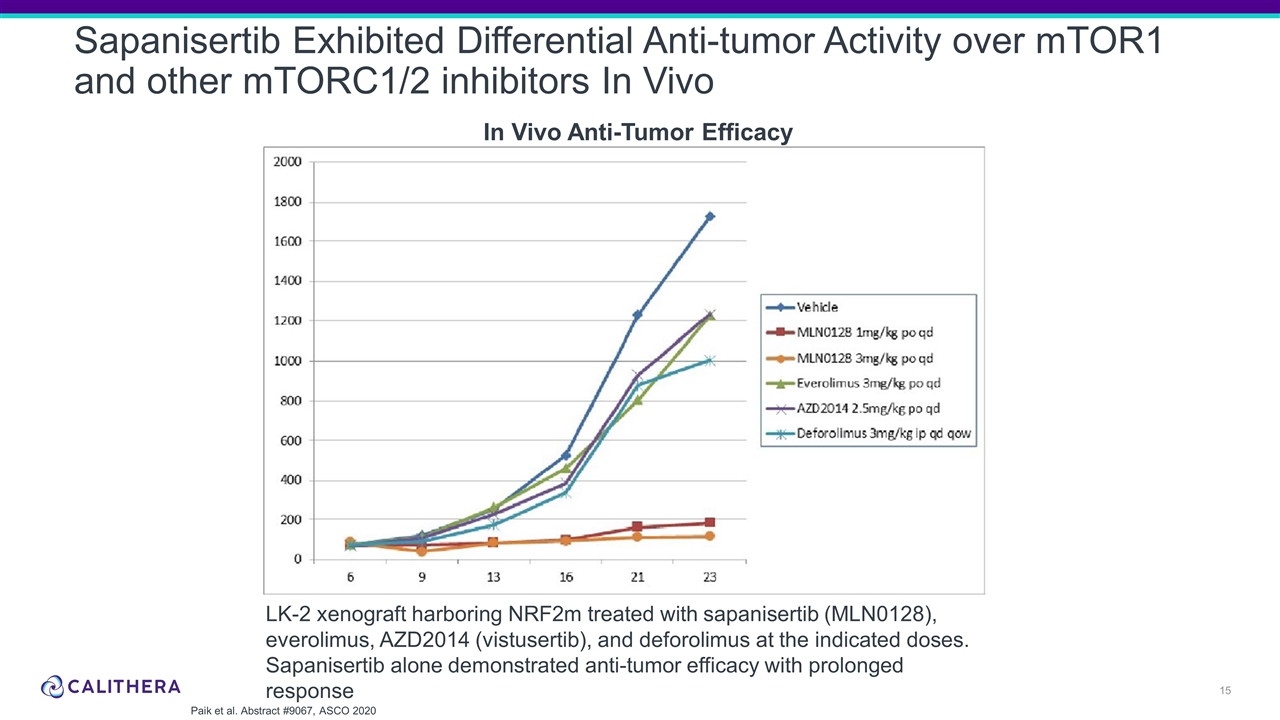
Sapanisertib Exhibited Differential Anti-tumor Activity over mTOR1 and other mTORC1/2 inhibitors In Vivo LK-2 xenograft harboring NRF2m treated with sapanisertib (MLN0128), everolimus, AZD2014 (vistusertib), and deforolimus at the indicated doses. Sapanisertib alone demonstrated anti-tumor efficacy with prolonged response Paik et al. Abstract #9067, ASCO 2020 In Vivo Anti-Tumor Efficacy
Sapanisertib Overview Sapanisertib is an mTORC1/2 inhibitor with single-agent clinical activity in NRF2*m sqNSCLC1 NRF2 mutations occur in ~15% of sqNSCLC patients2 Sapanisertib exhibits differential anti-tumor activity compared to rapalog inhibitors of TORC1 in NRF2-mutant squamous NSCLC in vivo models1 Potential for single agent activity in other tumor types with KEAP1 and NRF2 mutations Sapanisertib has a well-characterized clinical safety profile1,3 IP COM protection through 2036 US/2034 EU assuming full 5-year patent term extension4 *NRF2 is also known as NFE2L2 WT=wild type COM=composition of matter Paik et al.. Abstract #9607, ASCO 2020 Data from TCGA BioPortal https://www.cbioportal.org/ Voss et al. British Journal of Cancer (2020) 123:1590–1598; US 8,476,282 B2 and WO 2010/051043 Fast-to-market strategy in relapsed/refractory squamous NSCLC with potential to be the first targeted treatment for NRF2-mutated cancer

Squamous Non-Small Cell Lung Cancer Treatment Overview >235,000 diagnosed with NSCLC in the US each year, ~25-30% of those are squamous NSCLC1-2 Actionable mutations are only found in 1-5% of squamous NSCLC patients, leaving patients few options after PD1 inhibitors and chemo3,4 The standard-of-care options in R/R sqNSCLC have a mPFS of 3-4.5 mos5,6 NRF2 mutations occur in ~15% of sqNSCLC patients3 NRF2/KEAP1 mutations confer a poorer prognosis than WT for NSCLC patients7 https://seer.cancer.gov/statfacts/html/lungb.html https://www.cancer.gov/types/lung/hp/non-small-cell-lung-treatment-pdq#_359 Data from TCGA BioPortal https://www.cbioportal.org/ NCCN NSCLC guidelines June 2021 Garon et al. Lancet. Aug 23;384(9944):665-73. 2014 Leong et al. J Thorac Oncol. Mar;2(3):230-6. 2007 Rizvi et al. Oral Abstract OA04.07: World Conference on Lung Cancer, 2019 Sapanisertib has the potential to address a substantial underserved patient population
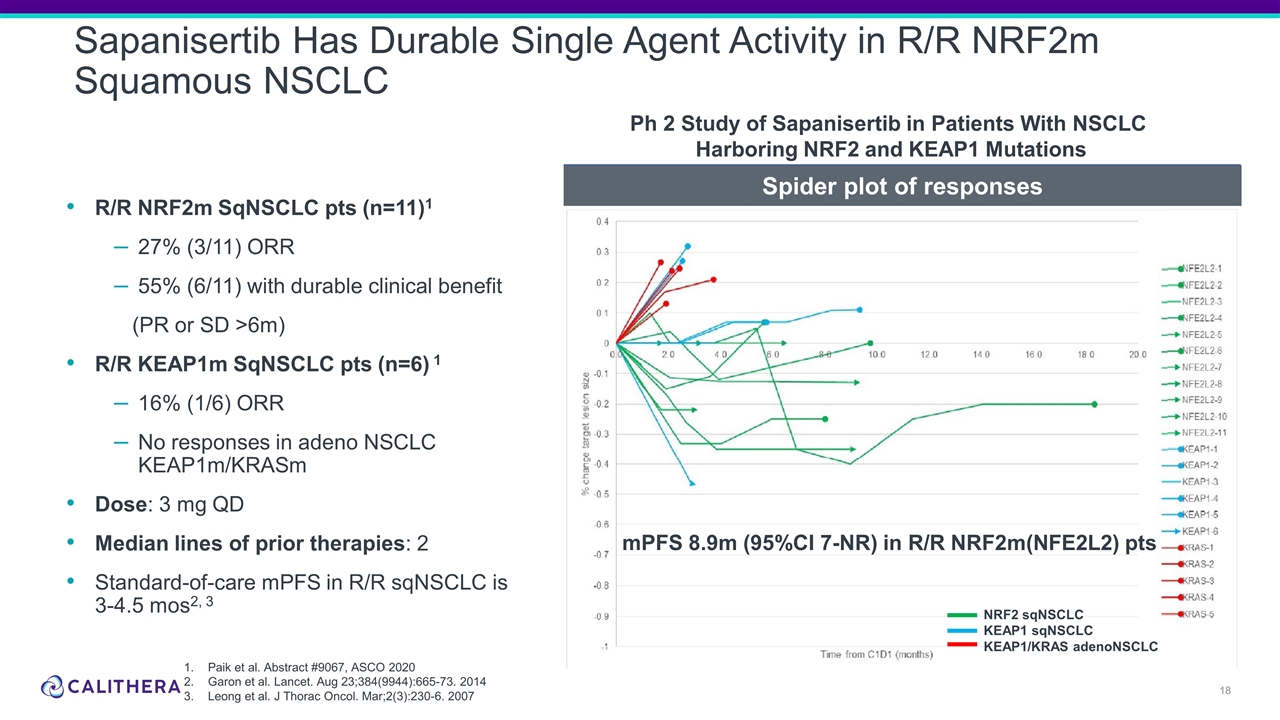
R/R NRF2m SqNSCLC pts (n=11)1 27% (3/11) ORR 55% (6/11) with durable clinical benefit (PR or SD >6m) R/R KEAP1m SqNSCLC pts (n=6) 1 16% (1/6) ORR No responses in adeno NSCLC KEAP1m/KRASm Dose: 3 mg QD Median lines of prior therapies: 2 Standard-of-care mPFS in R/R sqNSCLC is 3-4.5 mos2, 3 Sapanisertib Has Durable Single Agent Activity in R/R NRF2m Squamous NSCLC Paik et al. Abstract #9067, ASCO 2020 Garon et al. Lancet. Aug 23;384(9944):665-73. 2014 Leong et al. J Thorac Oncol. Mar;2(3):230-6. 2007 mPFS 8.9m (95%CI 7-NR) in R/R NRF2m(NFE2L2) pts NRF2 sqNSCLC KEAP1 sqNSCLC KEAP1/KRAS adenoNSCLC Ph 2 Study of Sapanisertib in Patients With NSCLC Harboring NRF2 and KEAP1 Mutations Spider plot of responses
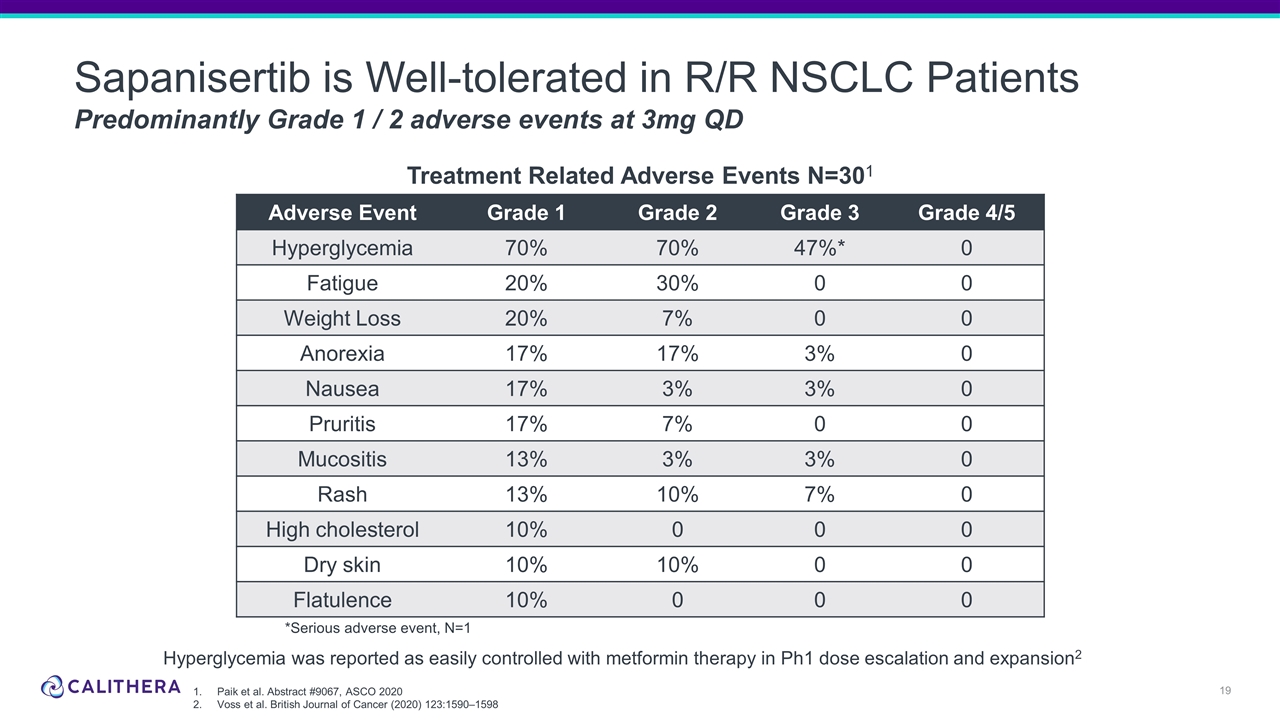
Sapanisertib is Well-tolerated in R/R NSCLC Patients Predominantly Grade 1 / 2 adverse events at 3mg QD Adverse Event Grade 1 Grade 2 Grade 3 Grade 4/5 Hyperglycemia 70% 70% 47%* 0 Fatigue 20% 30% 0 0 Weight Loss 20% 7% 0 0 Anorexia 17% 17% 3% 0 Nausea 17% 3% 3% 0 Pruritis 17% 7% 0 0 Mucositis 13% 3% 3% 0 Rash 13% 10% 7% 0 High cholesterol 10% 0 0 0 Dry skin 10% 10% 0 0 Flatulence 10% 0 0 0 Treatment Related Adverse Events N=301 *Serious adverse event, N=1 Paik et al. Abstract #9067, ASCO 2020 Voss et al. British Journal of Cancer (2020) 123:1590–1598 Hyperglycemia was reported as easily controlled with metformin therapy in Ph1 dose escalation and expansion2
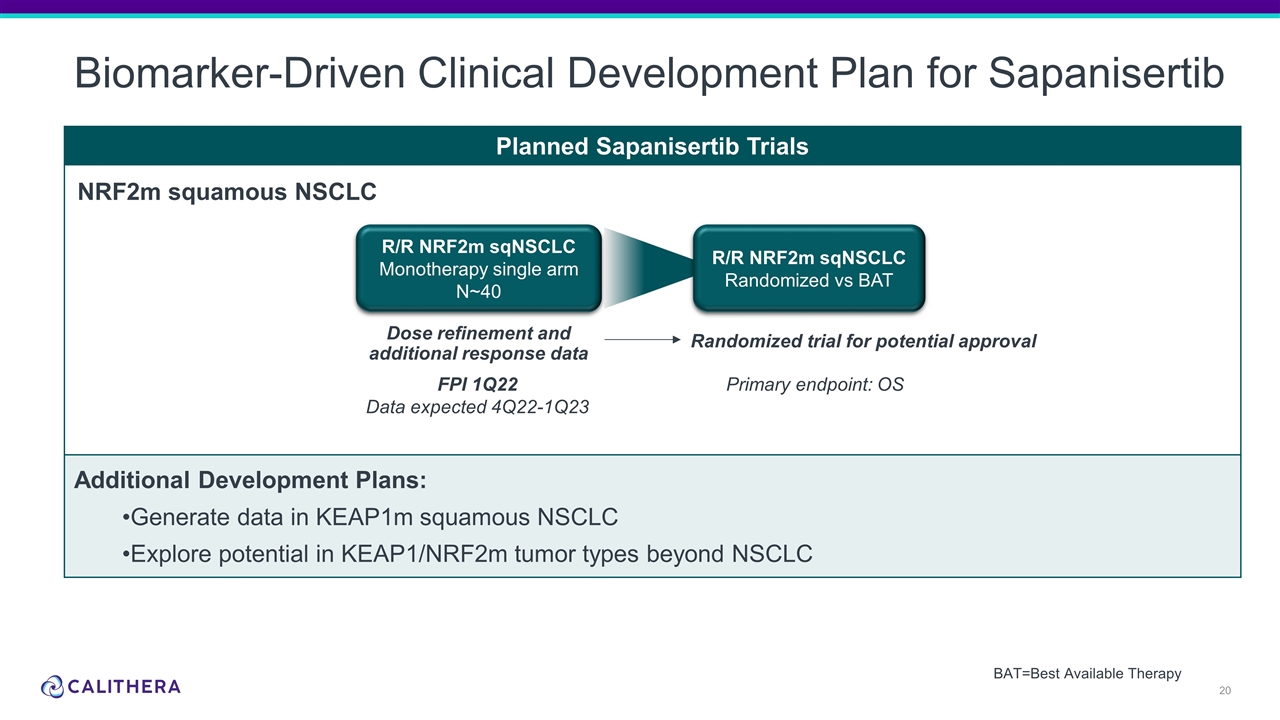
Planned Sapanisertib Trials Additional Development Plans: Generate data in KEAP1m squamous NSCLC Explore potential in KEAP1/NRF2m tumor types beyond NSCLC Biomarker-Driven Clinical Development Plan for Sapanisertib NRF2m squamous NSCLC Primary endpoint: OS R/R NRF2m sqNSCLC Monotherapy single arm N~40 BAT=Best Available Therapy Dose refinement and additional response data Randomized trial for potential approval R/R NRF2m sqNSCLC Randomized vs BAT FPI 1Q22 Data expected 4Q22-1Q23
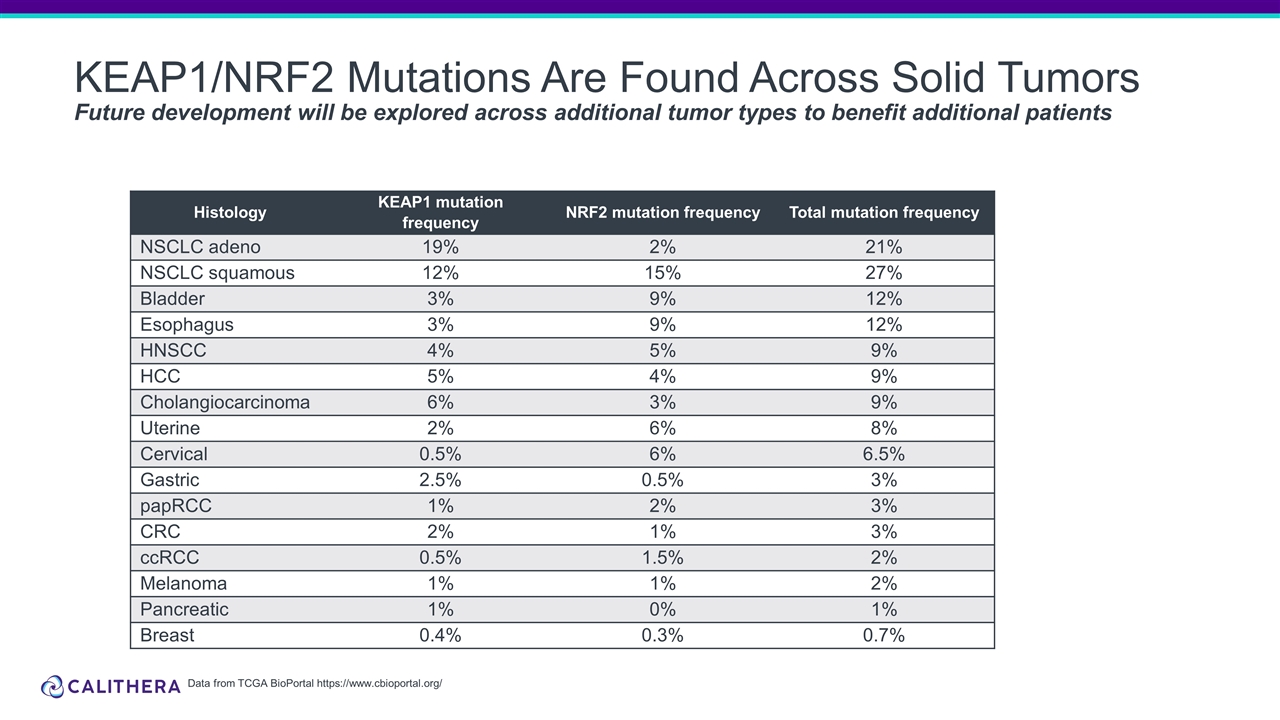
KEAP1/NRF2 Mutations Are Found Across Solid Tumors Future development will be explored across additional tumor types to benefit additional patients Histology KEAP1 mutation frequency NRF2 mutation frequency Total mutation frequency NSCLC adeno 19% 2% 21% NSCLC squamous 12% 15% 27% Bladder 3% 9% 12% Esophagus 3% 9% 12% HNSCC 4% 5% 9% HCC 5% 4% 9% Cholangiocarcinoma 6% 3% 9% Uterine 2% 6% 8% Cervical 0.5% 6% 6.5% Gastric 2.5% 0.5% 3% papRCC 1% 2% 3% CRC 2% 1% 3% ccRCC 0.5% 1.5% 2% Melanoma 1% 1% 2% Pancreatic 1% 0% 1% Breast 0.4% 0.3% 0.7% Data from TCGA BioPortal https://www.cbioportal.org/

Mivavotinib SYK inhibitor for hematologic malignancies

Mivavotinib Overview Mivavotinib is a SYK inhibitor with single-agent clinical activity and best-in-class potential in NHL Recent preclinical data identify NHL with MyD88 or CD79 mutations as particularly vulnerable to SYK inhibition2 Preliminary mivavotinib data suggests early signal in NHL patients with CD79 mutations3 Potential fast-to-market development strategy in DLBCL patients with or without MyD88 and/or CD79 mutations Deep and durable single agent activity in unselected 3L+ DLBCL and other NHLs in completed Phase 1/2 clinical trials1 IP COM protection through 2036 US/2035 EU assuming full 5-year patent term extension4 m=mutation r=rearrangement COM=composition of matter Gordon at al. Clin Cancer Res. Jul 15;26(14):3546-3556. AACR 2020. Munshi et al. Blood Cancer J. Jan 31;10(1):12. 2020 Takeda data on file US 8,440,689 B2 and WO 2011/079051 Potential First-to-Market approach for DLBCL patients with MyD88/CD79 mutations
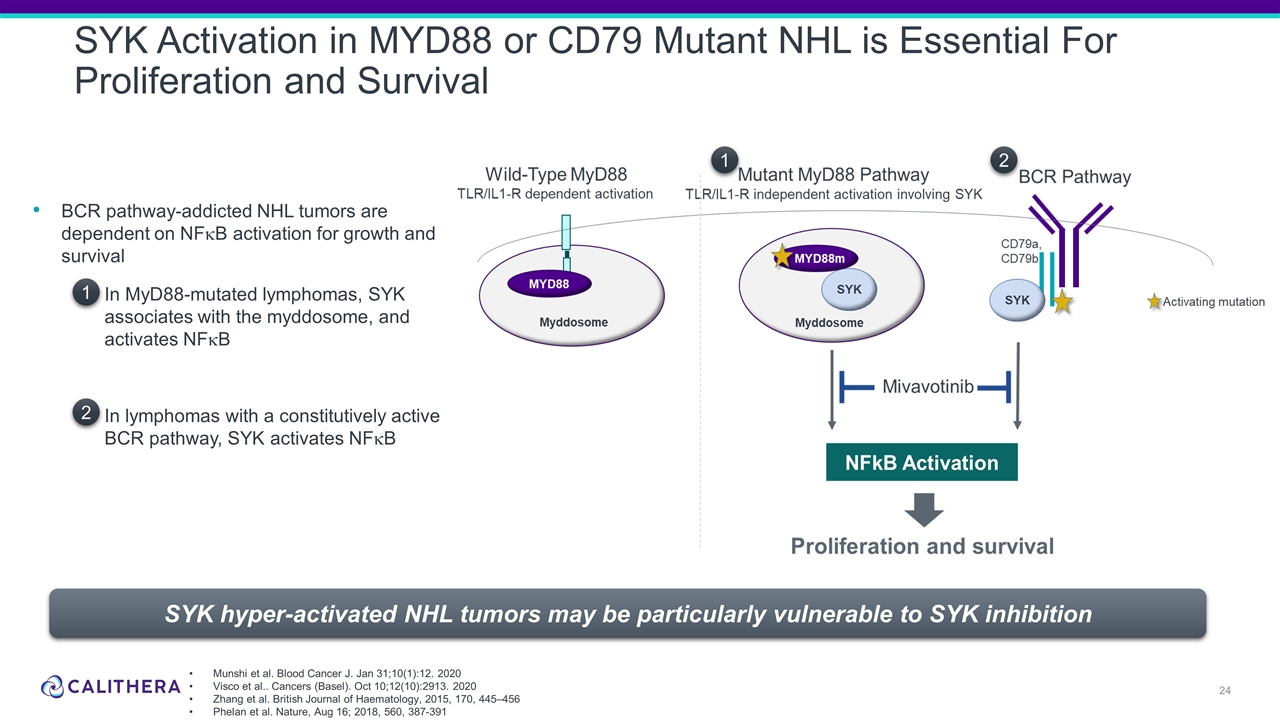
SYK Activation in MYD88 or CD79 Mutant NHL is Essential For Proliferation and Survival BCR pathway-addicted NHL tumors are dependent on NFkB activation for growth and survival In MyD88-mutated lymphomas, SYK associates with the myddosome, and activates NFkB In lymphomas with a constitutively active BCR pathway, SYK activates NFkB 1 2 1 2 SYK hyper-activated NHL tumors may be particularly vulnerable to SYK inhibition Munshi et al. Blood Cancer J. Jan 31;10(1):12. 2020 Visco et al.. Cancers (Basel). Oct 10;12(10):2913. 2020 Zhang et al. British Journal of Haematology, 2015, 170, 445–456 Phelan et al. Nature, Aug 16; 2018, 560, 387-391
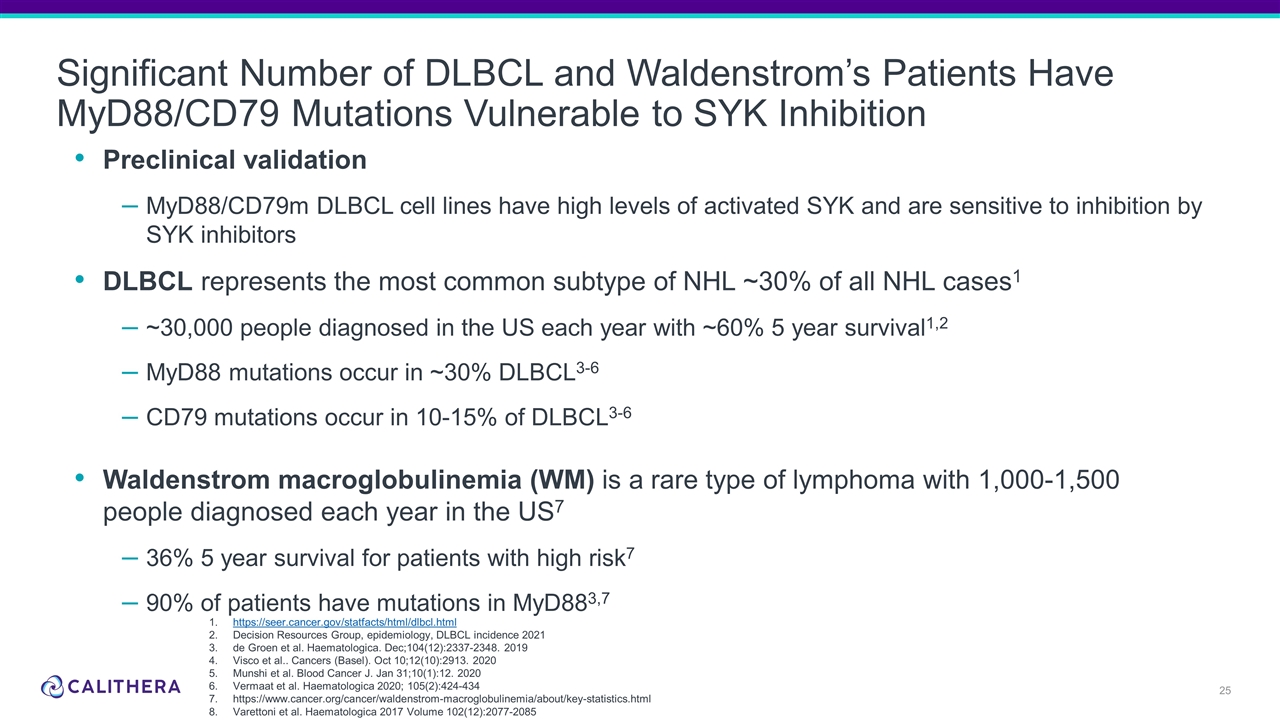
Significant Number of DLBCL and Waldenstrom’s Patients Have MyD88/CD79 Mutations Vulnerable to SYK Inhibition Preclinical validation MyD88/CD79m DLBCL cell lines have high levels of activated SYK and are sensitive to inhibition by SYK inhibitors DLBCL represents the most common subtype of NHL ~30% of all NHL cases1 ~30,000 people diagnosed in the US each year with ~60% 5 year survival1,2 MyD88 mutations occur in ~30% DLBCL3-6 CD79 mutations occur in 10-15% of DLBCL3-6 Waldenstrom macroglobulinemia (WM) is a rare type of lymphoma with 1,000-1,500 people diagnosed each year in the US7 36% 5 year survival for patients with high risk7 90% of patients have mutations in MyD883,7 https://seer.cancer.gov/statfacts/html/dlbcl.html Decision Resources Group, epidemiology, DLBCL incidence 2021 de Groen et al. Haematologica. Dec;104(12):2337-2348. 2019 Visco et al.. Cancers (Basel). Oct 10;12(10):2913. 2020 Munshi et al. Blood Cancer J. Jan 31;10(1):12. 2020 Vermaat et al. Haematologica 2020; 105(2):424-434 https://www.cancer.org/cancer/waldenstrom-macroglobulinemia/about/key-statistics.html Varettoni et al. Haematologica 2017 Volume 102(12):2077-2085
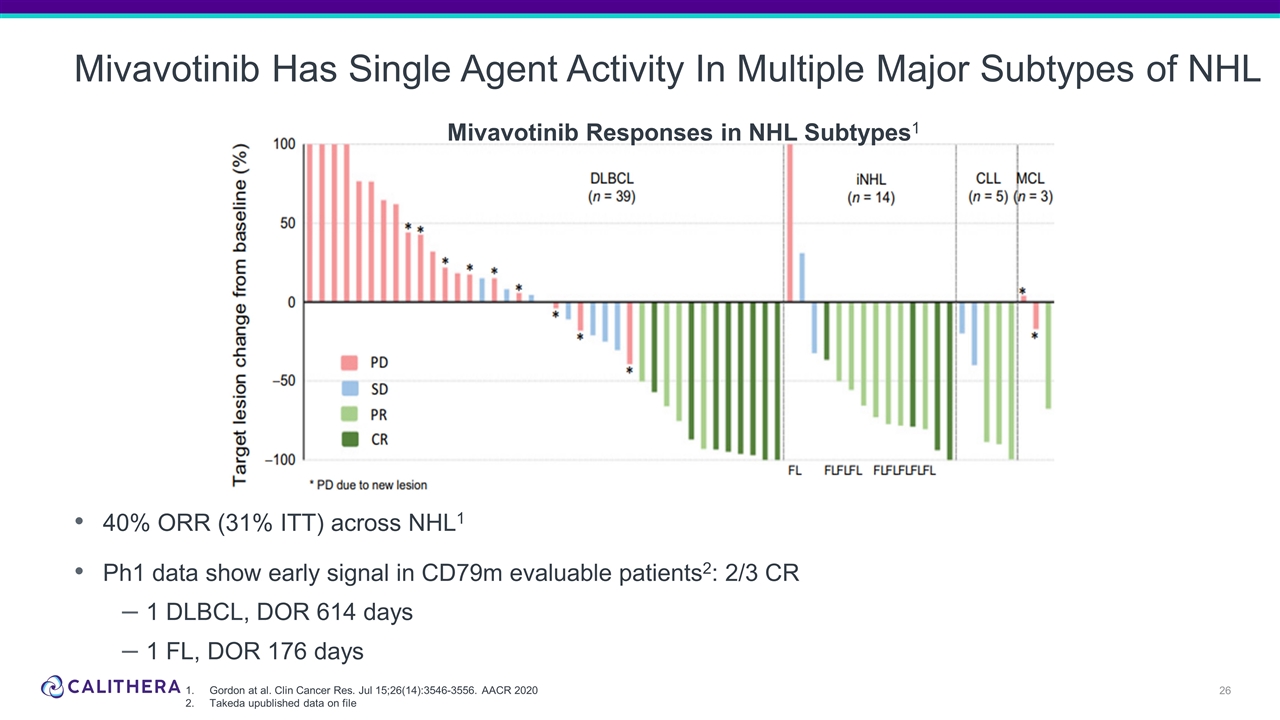
Mivavotinib Has Single Agent Activity In Multiple Major Subtypes of NHL 40% ORR (31% ITT) across NHL1 Ph1 data show early signal in CD79m evaluable patients2: 2/3 CR 1 DLBCL, DOR 614 days 1 FL, DOR 176 days Mivavotinib Responses in NHL Subtypes1 Gordon at al. Clin Cancer Res. Jul 15;26(14):3546-3556. AACR 2020 Takeda upublished data on file
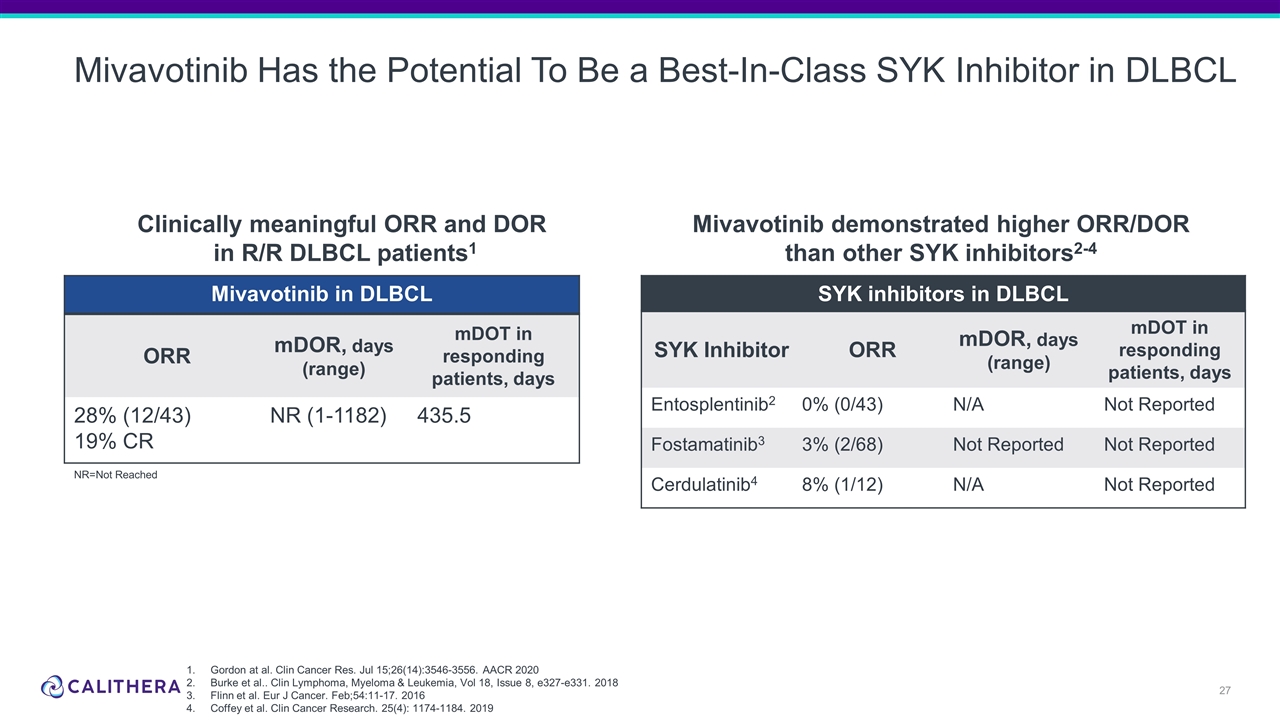
Mivavotinib Has the Potential To Be a Best-In-Class SYK Inhibitor in DLBCL Clinically meaningful ORR and DOR in R/R DLBCL patients1 Mivavotinib in DLBCL ORR mDOR, days (range) mDOT in responding patients, days 28% (12/43) 19% CR NR (1-1182) 435.5 Gordon at al. Clin Cancer Res. Jul 15;26(14):3546-3556. AACR 2020 Burke et al.. Clin Lymphoma, Myeloma & Leukemia, Vol 18, Issue 8, e327-e331. 2018 Flinn et al. Eur J Cancer. Feb;54:11-17. 2016 Coffey et al. Clin Cancer Research. 25(4): 1174-1184. 2019 Mivavotinib demonstrated higher ORR/DOR than other SYK inhibitors2-4 NR=Not Reached SYK inhibitors in DLBCL SYK Inhibitor ORR mDOR, days (range) mDOT in responding patients, days Entosplentinib2 0% (0/43) N/A Not Reported Fostamatinib3 3% (2/68) Not Reported Not Reported Cerdulatinib4 8% (1/12) N/A Not Reported
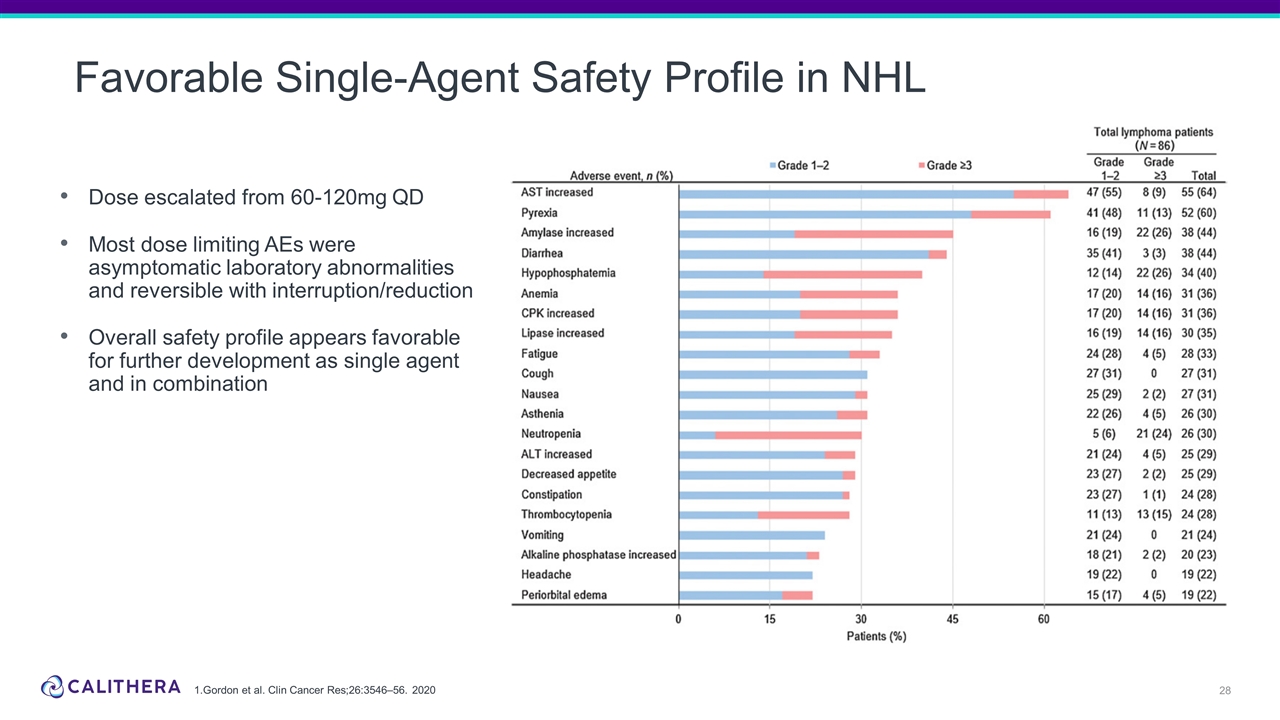
Favorable Single-Agent Safety Profile in NHL Dose escalated from 60-120mg QD Most dose limiting AEs were asymptomatic laboratory abnormalities and reversible with interruption/reduction Overall safety profile appears favorable for further development as single agent and in combination 1.Gordon et al. Clin Cancer Res;26:3546–56. 2020
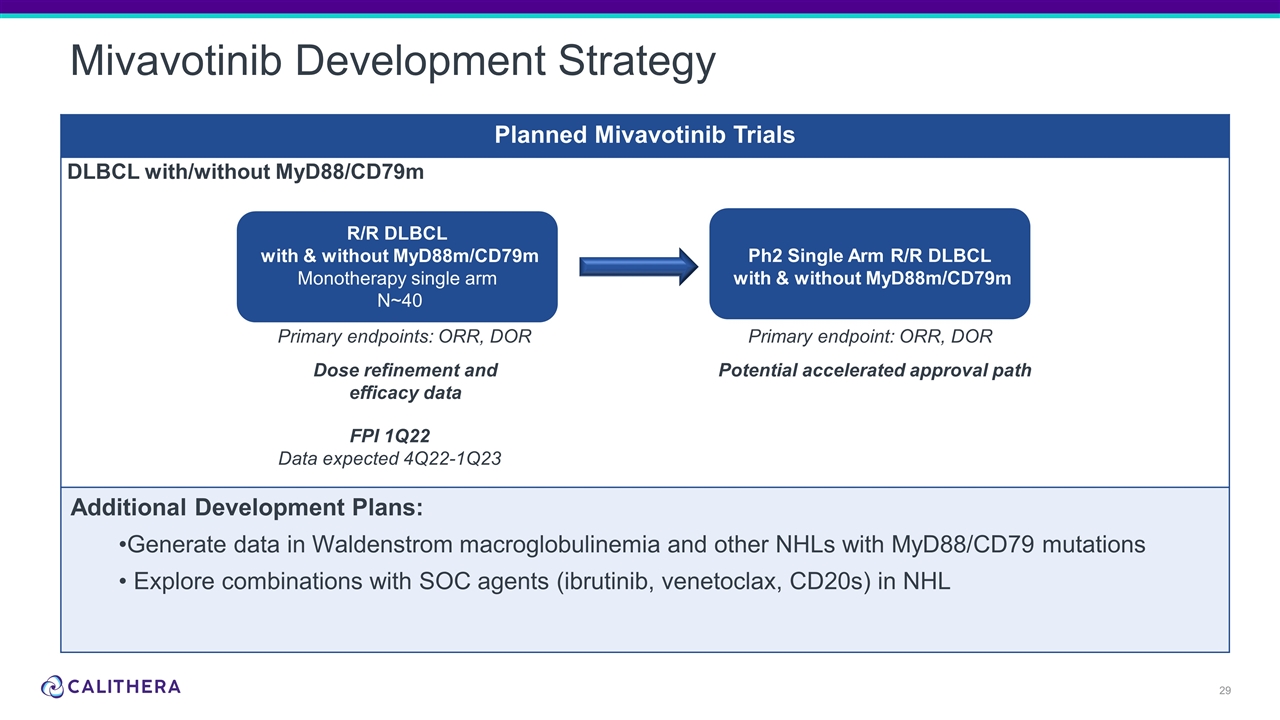
Mivavotinib Development Strategy Planned Mivavotinib Trials Additional Development Plans: Generate data in Waldenstrom macroglobulinemia and other NHLs with MyD88/CD79 mutations Explore combinations with SOC agents (ibrutinib, venetoclax, CD20s) in NHL DLBCL with/without MyD88/CD79m Primary endpoints: ORR, DOR R/R DLBCL with & without MyD88m/CD79m Monotherapy single arm N~40 Potential accelerated approval path FPI 1Q22 Data expected 4Q22-1Q23 Ph2 Single Arm R/R DLBCL with & without MyD88m/CD79m Primary endpoint: ORR, DOR Dose refinement and efficacy data

Arginase Inhibitor CB-280 Cystic Fibrosis, regardless of CFTR genotype
Rationale for Arginase Inhibitors in Cystic Fibrosis Despite recent advances with CFTR modulators, many patients still have impaired lung function Arginase plays a critical role in CF airway disease1, 2, 3 Decreases nitric oxide (NO) production4, increases production of polyamines and proline Inhibition of arginase should increase NO, increasing anti-microbial activity and improving airway function5 Potential for additional benefit when combined with standard of care therapies6 Potential benefit in all CF patients, regardless of CFTR genotype CB-280 is an investigational first-in-class orally-dosed arginase inhibitor in Phase 1b trials supported by a grant from the Cystic Fibrosis Foundation Grasemann et al., Am J Respir Crit Care Med 172: 1523-1528, 2005 Grasemann et al., Respiratory Research 7: 87, 2006 Jaecklin et al., J Appl Physiol 117: 284-288, 2014 Grasemann et al., Eur Respir J 25: 62-68, 2005 Mermis et al, NACF-2020-CX-280-202-TIP-POSTER-08Sep2020 Wu, Mol Pharmacol 96:515-525, 2019
CB-280 Randomized Ph1b Trial In Cystic Fibrosis Ongoing Randomized Double Blind Ph1b Trial in CF Placebo Cystic Fibrosis N = approximately 32 ≥ 18 years old FEV1 40-90% predicted On stable regimen of CF therapies Chronic microbial infection Trial initiated July 2020 CB-280 Dose Escalation Cohorts Primary Endpoint: Safety Secondary Endpoints: PK/PD: arginase inhibition and arginine increase assessed in plasma and sputum Exploratory Endpoints: FENO, sweat chloride, sputum colonization Interim Analysis will be presented at NACFC in November 2021 Randomized 3:1 Potential to benefit all CF patients, regardless of CFTR genotype, as the first-in-class orally dosed arginase inhibitor
Many CF Patients Continue to Have Impaired Lung Function Leading to Long-term Lung Damage, Despite Recent Therapeutic Advances 1. Evaluate Pharma, 2021, WW forecast, 2026 2. Cystic Fibrosis Foundation. www.cff.org CF market size is expected to grow from ~$8B to $11B+ 1 Approximately 1,000 cases are diagnosed in the US each year There are over 70,000 patients living with CF worldwide, with almost half in the US 2

Well Positioned for Success
Interim data read outs in Q4 Data expected 4Q22-1Q23 Calithera Milestones Legacy Clinical Programs New Clinical Programs Robust Discovery Engine Telaglenastat (CB-839): KEAPSAKE interim data expected 4Q 2021 CB-280: Phase 1b interim data at North American Cystic Fibrosis Conference November 2021 Sapanisertib trial in NRF2m squamous R/R NSCLC Mivavotinib trial in R/R DLBCL with and without MyD88m/CD79m Preclinical pipeline of undisclosed synthetic lethality targets with a focus on paralog genes to be announced as lead molecules are identified and IND timelines are in focus
Exhibit 99.3
Risk Factors
Our business involves significant risks, some of which are described below. You should carefully consider the following risk factors, in addition to the other information contained in the reports we file with the Securities and Exchange Commission, or the SEC. The occurrence of any of the events or developments described in the following risk factors could harm our business, financial condition, results of operations, cash flows, the trading price of our common stock and our growth prospects. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also impair our business operations. Our actual results could differ materially from those anticipated in the forward-looking statements as a result of factors that are described below and elsewhere in this Current Report on Form 8-K. The risks relating to our business set forth in our Annual Report on Form 10-K, filed with the SEC, are set forth below and are unchanged substantively as of the date of this filing, except for those risks designated by an asterisk (*).
Summary Risk Factors
Our business is subject to numerous risks and uncertainties, including those highlighted in the section titled “Risk Factors” immediately following this summary. These risks include, among others, the following:
| • | We have incurred significant operating losses since our inception and anticipate that we will continue to incur substantial operating losses for the foreseeable future. We may never achieve or maintain profitability. |
| • | We will need substantial additional funding. If we are unable to raise capital when needed, we would be forced to delay, reduce or eliminate our product development programs or commercialization efforts. |
| • | Our business, operations and clinical development plans and timelines are currently adversely affected by and could be adversely affected in the future by the effects of health epidemics, including the recent COVID-19 pandemic, on the manufacturing, clinical trial and other business activities performed by us or by third parties with whom we conduct business, including our contract manufacturers, Clinical Research Organizations, or CROs, shippers and others. |
| • | Our approach to the discovery and development of product candidates that target tumor metabolism and tumor immunology is unproven and may never lead to marketable products. |
| • | Our drug discovery and development efforts might not generate successful product candidates. |
| • | We may not realize the anticipated benefits from our acquisition of the Takeda assets. |
| • | If clinical trials of our product candidates fail to demonstrate safety and efficacy to the satisfaction of regulatory authorities or do not otherwise produce positive results, we may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of our product candidates. |
| • | If we experience delays or difficulties in enrolling patients in clinical trials, our receipt of necessary regulatory approvals could be delayed or prevented. |
| • | If serious adverse effects or unexpected characteristics of our product candidates are identified during development, we may need to abandon or limit our development of some or all of our product candidates. |
| • | Results of preclinical studies and early clinical trials may not be predictive of results of future clinical trials. |
| • | We face substantial competition, which may result in others discovering, developing or commercializing products before or more successfully than we do. |
| • | Product liability lawsuits against us could cause us to incur substantial liabilities and could limit the commercialization of any product candidates we may develop. |
| • | If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could harm our business. |
| • | We rely on third parties to conduct our clinical trials and some aspects of our research and preclinical testing and manufacture our product candidates, and those third parties may not perform satisfactorily, including failing to meet deadlines for the completion of such trials, research or testing. |
| • | Our arginase inhibitors program in hematology and oncology indications, including INCB001158, is reliant in part on Incyte for the successful development and commercialization in a timely manner. If Incyte does not devote sufficient resources to INCB001158’s development, is unsuccessful in its efforts, or chooses to terminate its agreement with us, our business, operating results and financial condition will be harmed. |
| • | We have in the past and may seek in the future to selectively establish collaborations, and, if we are unable to establish them on commercially reasonable terms, we may have to alter our development and commercialization plans. |
| • | Our internal computer systems, or those used by our Clinical Research Organizations or other contractors or consultants, may fail or suffer security breaches. |
| • | If we are alleged to infringe intellectual property rights of third parties, our business could be harmed. |
| • | We may not be able to protect, or fully exploit, our intellectual property rights throughout the world, which could impair our competitive position. |
| • | Even if we complete the necessary preclinical studies and clinical trials, the marketing approval process is expensive, time-consuming and uncertain and may prevent us from obtaining approvals for the commercialization of some or all of our product candidates. If we or our collaborators are not able to obtain, or if there are delays in obtaining, required regulatory approvals, we will not be able to commercialize, or will be delayed in commercializing, our product candidates, and our ability to generate revenue will be impaired. |
| • | Recently enacted and future legislation may increase the difficulty and cost for us to obtain marketing approval of and commercialize our product candidates and affect the prices we may obtain. |
| • | Our future success depends on our ability to retain our senior management team and to attract, retain and motivate qualified personnel. |
| • | The holders of our Series A preferred stock have liquidation and other rights that are senior to the rights of the holders of shares of our common stock. |
| • | We may be required to issue a significant number of additional shares of common stock for no additional consideration to the holders of our Series A preferred stock pursuant to certain price-based anti-dilution provisions. |
| • | We cannot take certain actions without the consent of a majority of the holders of the Series A preferred stock. |
| • | We may be required to make significant cash payments to the holders of Series A preferred stock if we do not receive requisite stockholder approval to allow the conversion of the Series A preferred stock to common stock. |
| • | We have granted Millennium registration rights with respect to the shares of common stock into which our Series A preferred stock is convertible. If these additional shares are sold, or it is perceived that they will be sold, the market price of our common stock could decline. |
| • | The trading price of our common stock is likely to be volatile, and purchasers of our common stock could incur substantial losses. |
| • | Concentration of ownership of our capital stock may prevent new investors from influencing significant corporate decisions. |
| • | If we are unable to maintain proper and effective internal controls over financial reporting, the accuracy and timeliness of our financial reporting and the market price of our common stock may be adversely affected. |
| • | If we are unable to adequately address these and other risks we face, our business, financial condition, operating results and prospects may be adversely affected. |
2
Risk Factors
Risks Related to Our Financial Position and Need For Additional Capital
We have incurred significant operating losses since our inception and anticipate that we will continue to incur substantial operating losses for the foreseeable future. We may never achieve or maintain profitability.*
Since our inception, we have incurred significant operating losses. Our net loss was $90.1 million and $34.7 million for the year ended December 31, 2020, and the six months ended June 30, 2021, respectively. As of June 30, 2021, we had an accumulated deficit of $410.9 million. To date, we have financed our operations through sales of our capital stock and payments from the Incyte Collaboration Agreement. We have devoted substantially all of our financial resources and efforts to research and development. We expect that it may be many years, if ever, before we receive regulatory approval and have a product candidate ready for commercialization. We expect to continue to incur significant expenses and increasing operating losses for the foreseeable future. Our net losses may fluctuate significantly from quarter to quarter and year to year. We anticipate that our expenses will increase substantially if and as we:
| • | advance further into clinical trials for our existing clinical product candidates, telaglenastat, sapanisertib, mivavotinib and CB-280; |
| • | continue the preclinical development of our research programs and advance candidates into clinical trials; |
| • | identify additional product candidates and advance them into preclinical development; |
| • | pursue regulatory approval of product candidates; |
| • | seek marketing approvals for our product candidates that successfully complete clinical trials; |
| • | establish a sales, marketing and distribution infrastructure to commercialize any product candidates for which we obtain marketing approval; |
| • | become obligated to make milestone payments pursuant to the APA; |
| • | maintain, expand and protect our intellectual property portfolio; |
| • | hire additional clinical, commercial, regulatory and scientific personnel; |
| • | add operational, financial and management information systems and personnel, including personnel to support product development and commercialization; |
| • | acquire or in-license other product candidates and technologies; and |
| • | operate as a public company. |
We have never generated any revenue from product sales and may never be profitable. To become and remain profitable, we and/or our collaborators must develop and eventually commercialize one or more products with significant market potential. This will require us to be successful in a range of challenging activities, including completing preclinical studies and clinical trials of our product candidates, obtaining marketing approval for these product candidates, manufacturing, marketing and selling those product candidates for which we may obtain marketing approval, and satisfying any post-marketing requirements. We may never succeed in these activities and, even if we do, may never generate revenue that is significant or large enough to achieve profitability. Our failure to become and remain profitable would decrease the value of the company and could impair our ability to raise capital, maintain our research and development efforts, expand our business or continue our operations. A decline in the value of our company could also cause you to lose all or part of your investment.
We will need substantial additional funding. If we are unable to raise capital when needed, we would be forced to delay, reduce or eliminate our product development programs or commercialization efforts.
We expect our expenses to increase in connection with our ongoing activities, particularly as we continue the research and development of, continue and initiate clinical trials of, potentially prepare for commercial launch of, and seek marketing approval for our product candidates, specifically telaglenastat, sapanisertib and mivavotinib, and as we become obligated to make milestone payments pursuant to our outstanding license and asset purchase agreements. In addition, if we obtain marketing approval for any of our product candidates, we expect to incur significant commercialization expenses related to product sales, marketing, manufacturing and distribution of the approved product.
Our future capital requirements will depend on many factors, including:
| • | the scope, progress, results and costs of drug discovery, clinical development, laboratory testing and clinical trials for our product candidates, in particular telaglenastat, sapanisertib, mivavotinib and CB-280; |
| • | the costs, timing and outcome of any regulatory review of our product candidates, telaglenastat, sapanisertib, mivavotinib and CB-280; |
3
| • | the cost of any other product programs we pursue; |
| • | the costs and timing of commercialization activities, including manufacturing, marketing, sales and distribution, for any product candidates that receive, or that we anticipate may receive, marketing approval; |
| • | the costs of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending intellectual property-related claims; |
| • | milestone payments pursuant to our outstanding license and asset purchase agreements; |
| • | achieving the total remaining potential development, regulatory and commercialization milestones set forth in the Incyte Collaboration Agreement; |
| • | our obligations to redeem shares of Series A preferred stock; |
| • | our ability to establish and maintain collaborations on favorable terms, if at all; and |
| • | the extent to which we acquire or in-license other product candidates and technologies. |
Identifying potential product candidates and conducting preclinical studies and clinical trials are time consuming, expensive and uncertain processes that take years to complete, and we may never generate the necessary data or results required to obtain marketing approval and achieve product sales for any of our current or future product candidates. In addition, our product candidates, if approved, may not achieve commercial success. Our commercial revenue, if any, will be derived from sales of products that may not be commercially available for many years, if at all.
We do not have any material committed external source of funds or other support for our development efforts other than the Incyte Collaboration Agreement for the development and commercialization of small molecule arginase inhibitors in hematology and oncology indications, including INCB001158, which agreement is terminable by Incyte for convenience or following our uncured breach. If the Incyte Collaboration Agreement is terminated, we would need to obtain substantial additional sources of funding to develop INCB001158 as currently contemplated. If such additional funding is not available on favorable terms or at all, we may need to delay or reduce the scope of our INCB001158 development program or dedicate resources allocated to other programs to fund INCB001158. We may also need to grant rights in the United States, as well as outside the United States, to INCB001158 to one or more partners.
Accordingly, we will need substantial additional funding in connection with our continuing operations and to achieve our goals. We expect that our existing cash, cash equivalents, and investments will be sufficient to enable us to meet our current operating plan for at least the next 12 months. However, our existing cash, cash equivalents and investments may prove to be insufficient for these activities. If we are unable to raise capital when needed or on attractive terms, we would be forced to delay, reduce or eliminate our research and development programs or future commercialization efforts. Adequate additional financing may not be available to us on acceptable terms, or at all. In addition, we may seek additional financing due to favorable market conditions or strategic considerations, even if we believe we have sufficient funds for our operating plans.
Raising additional capital may cause dilution to our stockholders, restrict our operations or require us to relinquish rights to our technologies or product candidates.
Until such time, if ever, as we can generate substantial product revenue, we expect to finance our cash needs through a combination of equity and debt financings, as well as entering into new collaborations, strategic alliances and licensing arrangements. We do not have any committed external source of funds, other than our collaborations, which are limited in scope and duration. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a common stockholder. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends, and may be secured by all or a portion of our assets. If we raise funds by entering into new collaborations, strategic alliances or licensing arrangements in the future with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or to grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings or through collaborations, strategic alliances or licensing arrangements when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
4
Our short operating history may make it difficult for you to evaluate the success of our business to date and to assess our future viability.
We were founded in March 2010 and our operations to date have been limited to organizing and staffing our company, business planning, raising capital, developing our technology, identifying potential product candidates, undertaking preclinical studies and commencing Phase 1 and 2 clinical trials of our product candidates. CB-280, telaglenastat, sapanisertib, mivavotinib are currently being or will be evaluated by us in Phase 1 and Phase 2 clinical trials, respectively. All of our other programs are in research and preclinical development. We have not yet demonstrated our ability to successfully complete any clinical trials, including large-scale, pivotal clinical trials required for regulatory approval of our product candidates, obtain marketing approvals, manufacture a commercial scale product, or arrange for a third party to do so on our behalf, or conduct sales and marketing activities necessary for successful commercialization. Typically, it takes many years to develop one new product from the time it is discovered to when it is commercially available. Consequently, any predictions made about our future success or viability may not be as accurate as they could be if we had a longer operating history or if we had product candidates in advanced clinical trials.
In addition, we may encounter unforeseen expenses, difficulties, complications, delays and other known and unknown factors that may alter or delay our plans. If a product candidate is approved, we will need to transition from a company with a research and development focus to a company capable of supporting successful commercial activities. We may not be successful in any step in such a transition.
Our ability to use our net operating loss carryforwards and certain other tax attributes may be limited.
Sections 382 and 383 place a limitation on the amount of taxable income which can be offset by carryforward tax attributes, such as net operating losses or tax credits, after a change in control. Generally, after a change in control, a loss corporation cannot deduct carryforward tax attributes in excess of the limitation prescribed by Section 382 and 383. Therefore, certain of the Company’s carryforward tax attributes may be subject to an annual limitation regarding their utilization against taxable income in future periods. As a result of the Company’s IPO in 2014, the Company triggered an “ownership change” as defined in Internal Revenue Code Section 382 and related provisions. Additionally, due to stock acquired by investors and reported under Section 13(g), the Company believes that an “ownership change” occurred during 2018, as well. Subsequent ownership changes since 2018 may subject the Company to annual limitations of its net operating loss and credit carryforwards. Such annual limitation could result in the expiration of the net operating loss and credit carryforwards before utilization.
Furthermore, our ability to use our net operating losses and other tax attributes to offset potential future taxable income and related income taxes that would otherwise be due is dependent upon our generation of future taxable income before the expiration dates of the net operating losses, and we cannot predict with certainty when, or whether, we will generate sufficient taxable income to use all of our net operating losses. Federal net operating losses generated prior to 2018 will continue to be governed by the net operating loss tax rules as they existed prior to the adoption of the Tax Cuts and Jobs Act of 2017, or Tax Act, which means that generally they will expire 20 years after they were generated if not used prior thereto. Under the Tax Act, as modified by the Coronavirus Aid, Relief, and Economic Security Act, or CARES Act, signed into law on March 27, 2020, federal net operating losses incurred in 2018 and in future years may be carried forward indefinitely, but the deductibility of such federal net operating losses will be limited to 80% of current year taxable income for taxable years beginning after December 31, 2020.
Our effective tax rate may fluctuate, and tax authorities may disagree with our positions and conclusions regarding certain tax positions, resulting in unanticipated costs, taxes or non-realization of expected benefits.
Our effective tax rate may be different than experienced in the past due to numerous factors, including passage of the newly enacted federal income tax law, changes in the mix of our profitability between jurisdictions in which we are or may become subject to tax, the results of examinations and audits of our tax filings, our inability to secure or sustain acceptable agreements with tax authorities, changes in accounting for income taxes and changes in tax laws. Any of these factors could cause us to experience an effective tax rate significantly different from previous periods or our current expectations and may result in tax obligations in excess of amounts accrued in our financial statements.
A tax authority may disagree with tax positions that we have taken, which could result in increased tax liabilities. For example, a tax authority could assert that we are subject to tax in a jurisdiction where we believe we have not established a taxable nexus, often referred to as a “permanent establishment” under international tax treaties, and such an assertion, if successful, could increase our expected tax liability in one or more jurisdictions. A tax authority may take the position that material income tax liabilities, interest and penalties are payable by us, in which case, we expect that we might contest such assessment. Contesting such an assessment may be lengthy and costly and if we were unsuccessful in disputing the assessment, the implications could increase our anticipated effective tax rate, where applicable.
5
Risks Related to Drug Discovery, Development and Commercialization
Our business, operations and clinical development plans and timelines are currently adversely affected by and could be adversely affected in the future by the effects of health epidemics, including the recent COVID-19 pandemic, on the manufacturing, clinical trial and other business activities performed by us or by third parties with whom we conduct business, including our contract manufacturers, Clinical Research Organizations, or CROs, shippers and others.
Our business could be adversely affected in the future by health epidemics wherever we have clinical trial sites or other business operations. In addition, health epidemics could cause significant disruption in the operations of third-party manufacturers, CROs and other third parties upon whom we rely. For example, in December 2019, a novel strain of coronavirus, SARS-CoV-2, causing a disease referred to as COVID-19, was reported to have surfaced in Wuhan, China. Since then, COVID-19 has spread to multiple countries worldwide, including the United States. In March 2020, the World Health Organization declared the COVID-19 outbreak a pandemic, and the U.S. government imposed travel restrictions on travel between the United States, Europe and certain other countries. Further, the President of the United States declared the COVID-19 pandemic a national emergency and invoked powers under the Stafford Act, the legislation that directs federal emergency disaster response, and under the Defense Production Act, the legislation that facilitates the production of goods and services necessary for national security and for other purposes. Similarly, the State of California declared a state of emergency related to the spread of COVID-19, and the Governor of California and other health officials in California have announced aggressive orders, health directives and recommendations to reduce the spread of the disease. On March 16, 2020, the Health Officer of San Mateo County, the county in which our headquarters is located, issued a “Shelter in Place” Order requiring, among other things, the closure of all non-essential businesses. Further, the Governor of California issued an executive order directing that all non-essential businesses close their physical operations and implement work-from-home schedules, effective as of March 19, 2020. We have implemented work-from-home policies for all employees. The effects of the executive order and our work-from-home policies may continue to negatively impact productivity, disrupt our business and delay our clinical programs and timelines, the magnitude of which will depend, in part, on the length and severity of the restrictions and other limitations on our ability to conduct our business in the ordinary course. For example, the CANTATA trial was fully enrolled in October 2019, and we previously advised that we planned to report top-line efficacy and safety data from the trial in the late third quarter or fourth quarter of 2020. In light of delays associated with COVID-19, top-line data was announced in early first quarter 2021. These and similar, and perhaps more severe, disruptions in our operations could negatively impact our business, operating results and financial condition.
We depend on a worldwide supply chain to manufacture products used in our preclinical studies and clinical trials. Quarantines, shelter-in-place and similar government orders, or the expectation that such orders, shutdowns or other restrictions could occur, whether related to COVID-19 or other infectious diseases, could impact personnel at third-party manufacturing facilities in the United States and other countries, or the availability or cost of materials, which could disrupt our supply chain.
If our relationships with our suppliers or other vendors are terminated or scaled back as a result of the COVID-19 pandemic or other health epidemics, we may not be able to enter into arrangements with alternative suppliers or vendors or do so on commercially reasonable terms or in a timely manner. Switching or adding additional suppliers or vendors involves substantial cost and requires management time and focus. In addition, there is a natural transition period when a new supplier or vendor commences work. As a result, delays may occur, which could adversely impact our ability to meet our desired clinical development and any future commercialization timelines. Although we carefully manage our relationships with our suppliers and vendors, there can be no assurance that we will not encounter challenges or delays in the future or that these delays or challenges will not harm our business.
In addition, our preclinical studies and clinical trials have been and may continue to be affected by the COVID-19 pandemic. Clinical site initiation, patient enrollment and activities that require visits to clinical sites, including data monitoring, have been and may continue to be delayed due to prioritization of hospital resources toward the COVID-19 pandemic or concerns among patients about participating in clinical trials during a pandemic. Some patients may have difficulty following certain aspects of clinical trial protocols if quarantines impede patient movement or interrupt healthcare services. Similarly, if we are unable to successfully recruit and retain patients and principal investigators and site staff who, as healthcare providers, may have heightened exposure to COVID-19 or experience additional restrictions by their institutions, city, or state our clinical trial operations could be adversely impacted.
The spread of COVID-19, which has caused a broad impact globally, may materially affect us economically. While the potential economic impact brought by, and the duration of, COVID-19 may be difficult to assess or predict, a widespread pandemic could result in significant disruption of global financial markets, reducing our ability to access capital, which could in the future negatively affect our liquidity. In addition, a recession or market correction resulting from the spread of COVID-19 could materially affect our business and the value of our common stock.
The global pandemic of COVID-19 continues to evolve rapidly. The ultimate impact of the COVID-19 pandemic or a similar health epidemic is highly uncertain and subject to change. We do not yet know the full extent of potential delays or impacts on our business, our clinical trials, healthcare systems or the global economy as a whole. However, these effects could have a material impact on our operations, and we continue to monitor the COVID-19 situation closely.
6
We may not realize the anticipated benefits from our acquisition of the Takeda assets.
On October 18, 2021, we acquired and licensed from Millennium Pharmaceuticals, Inc., or Millennium, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited, or Takeda, certain technology, intellectual property and other assets related to Takeda’s small molecule programs TAK-228 and TAK-659, or the Takeda Programs, including certain patents and know-how solely related to the Takeda Programs and necessary for the exploitation of products containing the TAK-228 and TAK-659 compounds, as well as specified regulatory materials, agreements, materials and inventory related to the Takeda Programs. This transaction may require us to incur non-recurring and other charges, increase our near and long-term expenditures, pose significant integration challenges, require additional expertise, result in dilution to our existing stakeholders and disrupt our management and business, which could harm our operations and financial results. Under the agreement with Millennium, we are required to pursue commercially reasonable efforts to develop, and subsequently to commercialize, at least one TAK-228 product and one TAK-659 product in each of the United States, Japan and certain European countries. If we fail to properly exercise such efforts to develop and commercialize the Takeda Programs as specified in the asset purchase agreement, we may be subject to various claims by Millennium and parties affiliated with Millennium. In addition, the development of the Takeda Programs and the other products and technologies acquired or licensed may not be successful or they may require significantly greater resources and investments than originally anticipated. Conversely, the liabilities assumed in the transaction could be greater than originally anticipated. As a result, the anticipated benefits of the acquisition may not be realized fully within the expected timeframe or at all or may take longer to realize or cost more than expected, which could harm our business, financial condition, results of operations and growth prospects.
Further, while we seek to mitigate risks and liabilities of the acquisition and in-licensing transaction, and other potential acquisitions and in-licensing transactions, through, among other things, due diligence, there may be risks and liabilities that such due diligence efforts fail to discover, that are not disclosed to us, or that we inadequately assess. Any failure in identifying and managing these risks, liabilities and uncertainties effectively, could harm our business, results of operations and financial condition.
Our approach to the discovery and development of product candidates that target tumor metabolism and tumor immunology is unproven and may never lead to marketable products.
Our scientific approach focuses on using our understanding of cellular metabolic pathways and the role of glutaminase in these pathways, as well as the role of arginase in the anti-tumor immune response, to identify molecules that are potentially promising as therapies for cancer indications. Any product candidates we develop may not effectively modulate metabolic or immunology pathways. The scientific evidence to support the feasibility of developing product candidates based on inhibiting tumor metabolism or impacting the anti-tumor immune response are both preliminary and limited. Although preclinical studies suggest that inhibiting glutaminase can suppress the growth of certain cancer cells, to date no company has translated this mechanism into a drug that has received marketing approval. Even if we are able to develop a product candidate in preclinical studies, we may not succeed in demonstrating the safety and efficacy of the product candidate in human clinical trials. Our expertise in cellular metabolic pathways, the role of glutaminase in these pathways, and the role of arginase in the anti-tumor immune response may not result in the discovery and development of commercially viable products to treat cancer.
Our drug discovery and development efforts might not generate successful product candidates.
We have invested a significant portion of our efforts and financial resources in the identification or asset acquisition of our most advanced product candidates, telaglenastat, sapanisertib, mivavotinib, INCB001158 and CB-280, which are being or will be evaluated in Phase 1 and Phase 2 clinical trials. We have entered into the Incyte Collaboration Agreement for the development and commercialization of INCB001158. Pursuant to the agreement, we and Incyte collaborated on the development of the licensed products for hematology and oncology indications, including INCB001158. Effective September 30, 2020, we have opted out of our co-development obligations and as a result, Incyte will solely develop INCB001158 or any other licensed products. All of our other programs are in research and preclinical development. Telaglenastat and INCB001158 will be developed for use in combination with other approved therapies, and as such, we will be dependent upon the continued marketing availability of the drugs that are used in combination with them. As a result, the timing and costs of the regulatory paths we will follow and marketing approvals remain uncertain. Our ability to generate product revenue, which may not occur for many years, if ever, will depend heavily on the successful development and eventual commercialization of telaglenastat, sapanisertib, mivavotinib and INCB001158. The success of telaglenastat, sapanisertib, mivavotinib, INCB001158 and any other product candidates we may develop will depend on many factors, including the following:
| • | successful enrollment in, and completion of, clinical trials; |
| • | demonstrating safety and efficacy; |
| • | receipt of marketing approvals from applicable regulatory authorities; |
| • | establishing commercial manufacturing capabilities or making arrangements with third-party manufacturers; |
7
| • | obtaining and maintaining patent and trade secret protection and non-patent exclusivity for our product candidates; |
| • | developing a sales and marketing organization or outsourcing these functions to third parties; |
| • | launching commercial sales of the product candidates, if and when approved, whether alone or selectively in collaboration with others; |
| • | developing and commercializing telaglenastat, sapanisertib, mivavotinib and small molecule arginase inhibitors, including INCB001158; |
| • | acceptance of the product candidates, if and when approved, by patients, the medical community and third-party payors; |
| • | effectively competing with other therapies; |
| • | a continued acceptable safety profile of the products following approval; |
| • | enforcing and defending intellectual property rights and claims; and |
| • | other legal, regulatory, compliance, privacy, and fraud and abuse matters. |
If we do not accomplish one or more of these goals in a timely manner, or at all, we could experience significant delays or an inability to successfully commercialize our product candidates, which would harm our business.
We may not be successful in our efforts to identify or discover potential product candidates for clinical development.
Our drug discovery efforts may not be successful in identifying compounds that are useful in treating cancer. Our research programs may initially show promise in identifying potential product candidates, yet fail to yield product candidates for clinical development for a number of reasons. In particular, our research methodology used may not be successful in identifying compounds with sufficient potency or bioavailability to be potential product candidates. In addition, our potential product candidates may, on further study, be shown to have harmful side effects or other negative characteristics.
Research programs to identify new product candidates require substantial technical, financial and human resources. We may choose to focus our efforts and resources on potential product candidates that ultimately prove to be unsuccessful. If we are unable to identify suitable compounds for preclinical and clinical development, we will not be able to generate product revenue, which would harm our financial position and adversely impact our stock price.
If clinical trials of our product candidates fail to demonstrate safety and efficacy to the satisfaction of regulatory authorities or do not otherwise produce positive results, we may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of our product candidates.
Before obtaining marketing approval from regulatory authorities for the sale of our product candidates, we must complete preclinical development and then conduct extensive clinical trials to demonstrate the safety and efficacy of our product candidates in humans. Clinical testing is expensive, difficult to design and implement, can take many years to complete and is uncertain as to outcome. A failure of one or more clinical trials could occur at any stage of testing. The outcome of preclinical testing and early clinical trials may not be predictive of the success of later clinical trials, and interim results of a particular clinical trial do not necessarily predict final results of that trial. For example, our CANTATA trial of telaglenastat in RCC did not meet the primary endpoint of PFS despite earlier encouraging results in this indication in a Phase 1b trial.
Moreover, preclinical and clinical data are often susceptible to multiple interpretations and analyses. Many companies that have believed their product candidates performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain marketing approval of their products.
We may experience numerous unforeseen events during, or as a result of, preclinical testing or clinical trials that could delay or prevent our ability to receive marketing approval or commercialize our product candidates, including that:
| • | regulators or institutional review boards may not authorize us or our investigators to commence a clinical trial or conduct a clinical trial at a prospective trial site; |
| • | we may have delays in reaching or fail to reach agreement on acceptable clinical trial contracts or clinical trial protocols with prospective trial sites; |
| • | clinical trials of our product candidates may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional clinical trials or abandon product development programs; |
8
| • | the number of patients required for clinical trials of our product candidates may be larger than we anticipate; enrollment in these clinical trials may be slower than we anticipate, or participants may drop out of these clinical trials at a higher rate than we anticipate; |
| • | our third-party contractors may fail to comply with regulatory requirements or meet their contractual obligations to us in a timely manner, or at all; |
| • | regulators or institutional review boards may require that we or our investigators suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements or a finding that the participants are being exposed to unacceptable health risks; |
| • | the cost of clinical trials of our product candidates may be greater than we anticipate; and |
| • | the supply or quality of our product candidates or other materials necessary to conduct clinical trials of our product candidates may be insufficient or inadequate. |
If we are required to conduct additional clinical trials or other testing of our product candidates beyond those that we currently contemplate, if we are unable to successfully complete clinical trials of our product candidates or other testing, if the results of these trials or tests are not positive or are only modestly positive or if there are safety concerns, we may:
| • | be delayed in obtaining marketing approval for our product candidates; |
| • | not obtain marketing approval at all; |
| • | obtain approval for indications or patient populations that are not as broad as intended or desired; |
| • | obtain approval with labeling that includes significant use or distribution restrictions or safety warnings, including boxed warnings; |
| • | be subject to additional post-marketing testing requirements; or |
| • | have the product removed from the market after obtaining marketing approval. |
Product development costs will also increase if we experience delays in testing or in receiving marketing approvals. We do not know whether any clinical trials will begin as planned, will need to be restructured or will be completed on schedule, or at all. Significant clinical trial delays also could shorten any periods during which we may have the exclusive right to commercialize our product candidates, could allow our competitors to bring products to market before we do, and could impair our ability to successfully commercialize our product candidates, any of which may harm our business and results of operations.
If we experience delays or difficulties in enrolling patients in clinical trials, our receipt of necessary regulatory approvals could be delayed or prevented.
We may not be able to initiate or continue clinical trials for our product candidates if we are unable to identify and enroll a sufficient number of eligible patients to participate in these trials as required by the U.S. Food and Drug Administration, or the FDA, or analogous regulatory authorities outside the United States. In addition, some of our competitors may have ongoing clinical trials for product candidates that would treat the same indications as our product candidates, and patients who would otherwise be eligible for our clinical trials may instead enroll in clinical trials of our competitors’ product candidates. Patient enrollment is also affected by other factors, including:
| • | severity of the disease under investigation; |
| • | availability and efficacy of approved medications for the disease under investigation; |
| • | eligibility criteria for the trial in question; |
| • | perceived risks and benefits of the product candidate under study; |
| • | efforts to facilitate timely enrollment in clinical trials; |
| • | patient referral practices of health care professionals; |
| • | the ability to monitor patients adequately during and after treatment; and |
| • | proximity and availability of clinical trial sites for prospective patients. |
Our inability to enroll a sufficient number of patients for our clinical trials would result in significant delays or may require us to abandon one or more clinical trials altogether. Enrollment delays in our clinical trials may result in increased development costs for our product candidates, which would cause the value of our company to decline and limit our ability to obtain additional financing.
9
If serious adverse effects or unexpected characteristics of our product candidates are identified during development, we may need to abandon or limit our development of some or all of our product candidates.
We or our collaborators are currently evaluating telaglenastat, sapanisertib, mivavotinib, INCB001158 and CB-280 in Phase 1 and Phase 2 clinical trials. All our other programs are in research and preclinical development and their risk of failure is high. It is impossible to predict when or if any of our product candidates will prove effective or safe in humans or will receive marketing approval. Adverse events or undesirable side effects caused by, or other unexpected properties of, our product candidates could cause us, any current or future collaborators, an institutional review board or regulatory authorities to interrupt, delay or halt clinical trials of one or more of our product candidates and could result in a more restrictive label, or the delay or denial of marketing approval by the FDA or comparable foreign regulatory authorities. If adverse effects were to arise in patients being treated with any of our product candidates, it could require us to halt, delay or interrupt clinical trials of such product candidate or adversely affect our ability to obtain requisite approvals to advance the development and commercialization of such product candidate. If our product candidates are associated with undesirable side effects or have characteristics that are unexpected, we may need to abandon their development or limit development to certain uses or subpopulations in which the undesirable side effects or other characteristics are less prevalent, less severe or more acceptable from a risk-benefit perspective. Many agents that initially showed promise in early stage testing for treating cancer or other diseases have later been found to cause side effects that prevented further development of the agent.
We are in early clinical trials with telaglenastat and INCB001158 and we have seen several adverse events, or AEs, deemed possibly or probably related to study drug in each of those programs. For example, in our evaluation of telaglenastat with nivolumab, during the dose escalation of the combination therapy, there was one report of dose limiting Grade 3 ALT increase. We have treated an insufficient number of patients to fully assess the safety of telaglenastat and INCB001158 and, as these trials progress, we may experience frequent or severe adverse events. Our ongoing and planned trials for telaglenastat, sapanisertib, mivavotinib and CB-280 and Incyte’s ongoing and planned trials for INCB001158 may fail due to safety issues, and we may need to abandon development of product candidates from these programs. Our other research programs may fail due to preclinical or clinical safety issues, causing us to abandon or delay the development of a product candidate from these programs.
Results of preclinical studies and early clinical trials may not be predictive of results of future clinical trials.
The outcome of preclinical studies and early clinical trials may not be predictive of the success of later clinical trials, and interim results of clinical trials do not necessarily predict success in future clinical trials. Many companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in late-stage clinical trials after achieving positive results in earlier development, and we could face similar setbacks. The design of a clinical trial can determine whether its results will support approval of a product and flaws in the design of a clinical trial may not become apparent until the clinical trial is well advanced. We may experience delays in designing and executing clinical trials to support marketing approval. In addition, preclinical and clinical data are often susceptible to varying interpretations and analyses. Many companies that believed their product candidates performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain marketing approval for the product candidates. Even if we, or our current and future collaborators, believe that the results of clinical trials for our product candidates warrant marketing approval, the FDA or comparable foreign regulatory authorities may disagree and may not grant marketing approval of our product candidates.
In some instances, there can be significant variability in safety or efficacy results between different clinical trials of the same product candidate due to numerous factors, including changes in trial procedures set forth in protocols, differences in the size and type of the patient populations, changes in and adherence to the dosing regimen and other clinical trial protocols and the rate of dropout among clinical trial participants. If we fail to receive positive results in clinical trials of our product candidates, the development timeline and regulatory approval and commercialization prospects for our most advanced product candidates, and, correspondingly, our business and financial prospects would be negatively impacted.
We may expend our limited resources to pursue a particular product candidate or indication and fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater likelihood of success.
We have limited financial and managerial resources. As a result, we may forego or delay pursuit of opportunities with other product candidates or for other indications that later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or profitable market opportunities. Our spending on current and future research and development programs and product candidates for specific indications may not yield any commercially viable products. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through collaboration, licensing or other royalty arrangements, including our agreement with Incyte, in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such product candidate. In addition, under our agreement with Incyte, Incyte has the right to commercialize INCB001158 in hematology and oncology indications. If Incyte does not successfully commercialize INCB001158, we may be unable to realize the full value from our collaboration with Incyte.
10
Even if any of our product candidates receives marketing approval, we or others may later discover that the product is less effective than previously believed or causes undesirable side effects that were not previously identified, which could compromise our ability, or that of any future collaborators, to market the product.
Clinical trials of our product candidates are conducted in carefully defined sets of patients who have agreed to enter into clinical trials. Consequently, it is possible that our clinical trials, or those of any future collaborator, may indicate an apparent positive effect of a product candidate that is greater than the actual positive effect, if any, or alternatively fail to identify undesirable side effects. If, following approval of a product candidate, we, or others, discover that the product is less effective than previously believed or causes undesirable side effects that were not previously identified, any of the following adverse events could occur:
| • | regulatory authorities may withdraw their approval of the product or seize the product; |
| • | we, or any future collaborators, may be required to recall the product, change the way the product is administered or conduct additional clinical trials; |
| • | additional restrictions may be imposed on the marketing of, or the manufacturing processes for, the particular product; |
| • | regulatory authorities may require the addition of labeling statements, such as a “black box” warning or a contraindication; |
| • | we, or any future collaborators, may be required to create a Medication Guide outlining the risks of the previously unidentified side effects for distribution to patients; |
| • | we, or any future collaborators, could be sued and held liable for harm caused to patients; |
| • | the product may become less competitive; and |
| • | our reputation may suffer. |
Even if any of our product candidates receive marketing approval, they may fail to achieve the degree of market acceptance by health care professionals, patients, third party payors and others in the medical community necessary for commercial success.
If any of our product candidates receive marketing approval, they may nonetheless fail to gain sufficient market acceptance by health care professionals, patients, third party payors and others in the medical community for us to achieve commercial success. For example, current cancer treatments like chemotherapy and radiation therapy for certain diseases and conditions are well established in the medical community, and doctors may continue to rely on these treatments. If our product candidates do not achieve an adequate level of acceptance, we may not generate significant product revenue to become profitable. The degree of market acceptance of our product candidates, if approved for commercial sale, will depend on a number of factors, including:
| • | the efficacy and potential advantages compared to alternative treatments; |
| • | our ability to offer any approved products for sale at competitive prices; |
| • | convenience and ease of administration compared to alternative treatments; |
| • | the willingness of the target patient population to try new therapies and of health care professionals to prescribe these therapies; |
| • | the strength of marketing and distribution support; |
| • | third-party coverage and sufficient reimbursement; and |
| • | the prevalence and severity of any side effects. |
If, in the future, we are unable to establish adequate sales and marketing capabilities or to selectively enter into agreements with third parties to sell and market our product candidates, we may not be successful in commercializing our product candidates if and when they are approved.
We do not have a sales and marketing infrastructure to support any future commercialization efforts. To achieve commercial success for any approved product for which we retain sales and marketing responsibilities, we must either develop a robust sales and marketing organization and/or outsource these functions to other third parties. For our small molecule arginase inhibitors in hematology and oncology indications, including INCB001158, we will be dependent on Incyte’s sales and marketing infrastructure to effectively commercialize these products. In the future, we may choose to build a focused sales and marketing infrastructure to sell some of our product candidates, if and when they are approved, excluding INCB001158.
There are risks involved both with establishing our own sales and marketing capabilities and with entering into arrangements with third parties to perform these services. For example, recruiting and training a sales force is expensive and time consuming and could delay any product launch. If the commercial launch of a product candidate for which we recruit a sales force and establish marketing capabilities is delayed or does not occur for any reason, we would have prematurely or unnecessarily incurred these commercialization expenses. This may be costly, and our investment would be lost if we cannot retain or reposition our sales and marketing personnel.
11
Factors that may inhibit our efforts to commercialize our product candidates on our own include:
| • | our inability to recruit and retain adequate numbers of effective sales and marketing personnel; |
| • | the inability of sales personnel to obtain access to health care professionals or persuade adequate numbers of health care professionals to prescribe any future products; and |
| • | unforeseen costs and expenses associated with creating an independent sales and marketing organization. |
If we enter into arrangements with third parties to perform sales, marketing and distribution services, our product revenue or the profitability of these product revenue to us may be lower than if we were to market and sell any products that we develop ourselves. In addition, we may not be successful in entering into arrangements with third parties to sell and market our product candidates or may be unable to do so on terms that are favorable to us. We may have little control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market our products effectively. If we do not establish sales and marketing capabilities successfully, either on our own or in collaboration with third parties, we will not be successful in commercializing our product candidates.
We face substantial competition, which may result in others discovering, developing or commercializing products before or more successfully than we do.
The development and commercialization of new drug products is highly competitive. Research and discoveries by others may result in breakthroughs which may render our products obsolete even before they generate any revenue. We face competition with respect to our current product candidates, and will face competition with respect to any product candidates that we may seek to develop or commercialize in the future, from major pharmaceutical companies, specialty pharmaceutical companies and biotechnology companies worldwide. There are a number of large pharmaceutical and biotechnology companies that currently market and sell products or are pursuing the development of products for the treatment of the cancer indications for which we are focusing our product development efforts. Some of these competitive products and therapies are based on scientific approaches that are the same as or similar to our approach and others are based on entirely different approaches. Potential competitors also include academic institutions, government agencies and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and commercialization.
We are developing our product candidates for the treatment of various cancers. There are a variety of available drug therapies marketed for cancer. In many cases, these drugs are administered in combination to enhance efficacy. Some of the currently approved drug therapies are branded and subject to patent protection, and others are available on a generic basis. Many of these approved drugs are well-established therapies and are widely accepted by health care professionals, patients and third-party payors. Insurers and other third-party payors may also encourage the use of generic products. We expect that if our product candidates are approved, they will be priced at a significant premium over competitive generic products. This may make it difficult for us to achieve our business strategy of using our product candidates in combination with existing therapies or replacing existing therapies with our product candidates.
There are also a number of product candidates in preclinical and clinical development by third parties to treat cancer by targeting cellular metabolism. Our principal competitors in the fields of tumor immunology, tumor metabolism, and/or other product candidates in development for advanced cancer treatment include Agios Pharmaceuticals, Inc., Arcus Biosciences, Inc., AstraZeneca plc, Boehringer Ingelheim GmbH, Bayer Pharma AG, Bristol-Myers Squibb Company, Celgene Corporation, Corvus Pharmaceuticals, Inc., Dracen Pharmaceuticals, Inc., Eisai Co., Ltd., Eli Lilly and Company, GlaxoSmithKline plc, Incyte Corporation, iTeos Therapeutics SA, Merck & Co., Merck KGaA, Nektar Therapeutics, Novartis International AG, Pfizer Inc, Roche Holdings AG and its subsidiary Genentech, Inc., and Takeda Pharmaceutical Company Limited.
Our primary competitors or product candidates in clinical development in either NRF2-mutated cancers, or with similar mechanism to an mTORC1/2 inhibitor are Antengene Corporation, Celcuity, Inc., Dracen Pharmaceuticals, Inc.
Our primary competitors or product candidates in clinical development for biomarker-defined diffuse large B-cell lymphoma or with a similar mechanism to a SYK inhibitor are Alexion Pharmaceuticals, Inc., Curis, Inc., Genentech, Inc., HutchMed (China) Limited, Karyopharm Therapeutics, MorphoSys AG.
12
Our primary competitors in the field of Cystic Fibrosis include AbbVie, Inc., Beyond Air Inc., Corbus Pharmaceuticals Holdings, Inc., Novartis AG, Novoteris, LLC, Proteostatis Therapeutics, Inc., Translate Bio, Inc., and Vertex Pharmaceuticals, Inc.
Our competitors may develop products that are more effective, safer, more convenient or less costly than any that we are developing or that would render our product candidates obsolete or non-competitive. In addition, our competitors may discover biomarkers that more efficiently measure metabolic pathways than our methods, which may give them a competitive advantage in developing potential products. Our competitors may also obtain marketing approval from the FDA or other regulatory authorities for their products sooner than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market. Many of our competitors have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller and other early stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties may compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
Even if we are able to commercialize any product candidates, these products may become subject to unfavorable pricing regulations, third-party coverage and reimbursement practices or healthcare reform initiatives, which would harm our business.
The regulations that govern marketing approvals, pricing and reimbursement for new drugs vary widely from country to country. In the United States, new and future legislation may significantly change the approval requirements in ways that could involve additional costs and cause delays in obtaining approvals. Some countries require approval of the sale price of a drug before it can be marketed. In many countries, the pricing review period begins after marketing or product-licensing approval is granted. In some foreign markets, prescription pharmaceutical pricing remains subject to continuing governmental control even after initial marketing approval is granted. As a result, we might obtain marketing approval for a drug in a particular country, but then be subject to price regulations that delay its commercial launch, possibly for lengthy time periods, and negatively impact the revenue we are able to generate from the sale of the drug in that country. Adverse pricing limitations may hinder our ability to commercialize and generate revenue from one or more product candidates, even if our product candidates obtain marketing approval.
Our ability to commercialize any product candidates successfully also will depend in part on the extent to which coverage and adequate reimbursement for these products and related treatments will be available from government health programs, private health insurers and other organizations. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which medications they will pay for and establish reimbursement levels. A significant trend in the U.S. healthcare industry and elsewhere is cost containment. Government authorities and third-party payors have attempted to control costs by limiting coverage and the amount of payment for particular medications. Increasingly, third-party payors are requiring that drug companies provide them with predetermined discounts from list prices and are challenging the prices charged for medical products. Coverage may not be available for any product that we commercialize and, if coverage is available, the level of reimbursement may not be sufficient. Reimbursement may impact the demand for, or the price of, any product candidate for which we obtain marketing approval. If reimbursement is not available or is available only to limited levels, we may not be able to successfully commercialize any product candidate for which we obtain marketing approval.
There may be significant delays in obtaining reimbursement for newly approved products, and coverage may be more limited than the purposes for which the product is approved by the FDA or similar regulatory authorities outside the United States. Moreover, eligibility for coverage does not imply that any product will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution. Interim reimbursement levels for new drugs, if applicable, may also not be sufficient to cover our costs and may not be made permanent. Coverage and reimbursement rates may vary according to the use of the drug and the medical circumstances under which it is used, may be based on reimbursement levels already set for lower cost products or procedures or may be incorporated into existing payments for other services. Net prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the United States. Third-party payors often rely upon Medicare coverage policies and payment limitations in setting their own reimbursement policies. Our inability to promptly obtain coverage and profitable payment rates from both government-funded programs and private payors for any approved products that we develop could have a material adverse effect on our operating results, our ability to raise capital needed to commercialize our approved products and our overall financial condition.
In addition, there has been heightened governmental scrutiny of pharmaceutical pricing practices in light of the rising cost of prescription drugs and biologics. Such scrutiny has resulted in several recent congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products. We expect additional healthcare reform initiatives to be adopted in the future, particularly in light of the new presidential administration. We continue to monitor and evaluate the potential impact of these legislative actions and their effect on our business and operations.
13
Product liability lawsuits against us could cause us to incur substantial liabilities and could limit the commercialization of any product candidates we may develop.
We face an inherent risk of product liability exposure related to the testing of our product candidates in human clinical trials and will face an even greater risk if we commercially sell any products that we may develop after approval. If we cannot successfully defend ourselves against claims that our product candidates caused injuries, we could incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
| • | decreased demand for any product candidates that we may develop; |
| • | injury to our reputation and significant negative media attention; |
| • | withdrawal of clinical trial participants; |
| • | significant costs to defend any related litigation; |
| • | substantial monetary awards to trial participants or patients; |
| • | loss of revenue; and |
| • | the inability to commercialize any products we may develop. |
Although we maintain product liability insurance coverage in the amount of up to $10.0 million per claim and in the aggregate, it may not be adequate to cover all liabilities that we may incur. We anticipate that we will need to increase our insurance coverage as we continue clinical trials and if we successfully commercialize any products. Insurance coverage is increasingly expensive. We may not be able to maintain insurance coverage at a reasonable cost or in an amount adequate to satisfy any liability that may arise.
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could harm our business.
We are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. Our operations involve the use of hazardous and flammable materials, including chemicals and biological and radioactive materials. Our operations also produce hazardous waste products. We generally contract with third parties for the disposal of these materials and wastes. We cannot eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from our use of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties.
Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees in our workplace, including those resulting from the use of hazardous materials, this insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological, chemical, hazardous or radioactive materials.
In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or production efforts. Failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
Risks Related to Our Dependence on Third Parties
We rely on third parties to conduct our clinical trials and some aspects of our research and preclinical testing and manufacture our product candidates, and those third parties may not perform satisfactorily, including failing to meet deadlines for the completion of such trials, research or testing.
We currently rely and expect to continue to rely on third parties, such as our collaborators, contract research organizations, clinical data management organizations, medical institutions and clinical investigators, to conduct our clinical trials and to conduct some aspects of our research and preclinical testing. Any of these third parties may terminate their engagements with us at any time. If these third parties do not successfully carry out their contractual duties, meet expected deadlines or conduct our clinical trials in accordance with regulatory requirements or our stated protocols, we will not be able to obtain, or may be delayed in obtaining, marketing approvals for our product candidates and will not be able to, or may be delayed in our efforts to, successfully commercialize our product candidates. Furthermore, these third parties may also have relationships with other entities, some of which may be our competitors. If we need to enter into alternative arrangements, it would delay our product development activities.
14
Our reliance on these third parties for research and development activities, including our reliance on Millennium and Takeda for prior preclinical and clinical research and development activities relating to the Programs, will reduce our control over these activities but will not relieve us of our responsibilities. For example, we will remain responsible for ensuring that each of our clinical trials is conducted in accordance with the general investigational plan and protocols for the trial, and that all clinical trial activities conducted by our contract research organizations follow applicable laws and regulations, and are conducted in an ethical and compliant manner. Moreover, the FDA requires us to comply with standards, commonly referred to as Good Clinical Practices, for conducting, recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of trial participants are protected. We also are required to register ongoing clinical trials and post the results of completed clinical trials on a government sponsored database, available at www.clinicaltrials.gov, within certain timeframes. Failure by us, or any of the third parties working on our behalf, to do the above can result in fines, adverse publicity and civil and criminal sanctions.
We do not have any manufacturing facilities. We currently rely, and expect to continue to rely, on third party manufacturers for the manufacture of our product candidates for preclinical studies and clinical trials and for commercial supply of any of these product candidates for which we obtain marketing approval. To date, we have obtained or plan to obtain materials for telaglenastat, sapanisertib, mivavotinib, INCB001158 and CB-280 for our current and planned clinical trials from third-party manufacturers. We have engaged third party manufacturers to obtain the active ingredient for telaglenastat, INCB001158 and CB-280 for pre-clinical testing and clinical trials. We do not have a long-term supply agreement with any third-party manufacturers, and we purchase our required drug supply on a purchase order basis.
We may be unable to establish agreements with third-party manufacturers or to do so on acceptable terms. Even if we are able to establish agreements with third-party manufacturers, reliance on third-party manufacturers entails additional risks, including:
| • | reliance on the third party for legal and regulatory compliance and quality assurance; |
| • | the possible breach of the manufacturing agreement by the third party; and |
| • | the possible termination or nonrenewal of the agreement by the third party at a time that is costly or inconvenient for us. |
Third-party manufacturers may not be able to comply with current U.S. Good Manufacturing Practice requirements, or cGMPs, or similar legal and regulatory requirements outside the United States. Our failure, or the failure of our third-party manufacturers, to comply with applicable regulations could result in sanctions being imposed on us, including fines, injunctions, civil penalties, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of product candidates, operating restrictions and criminal prosecutions, any of which could adversely affect supplies of our product candidates and harm our business and results of operations.
Any product that we may develop may compete with other product candidates and products for access to these manufacturing facilities. There are a limited number of manufacturers that operate under cGMPs and that might be capable of manufacturing for us.
Any performance failure on the part of our existing or future manufacturers could delay clinical development or marketing approval. We do not currently have arrangements in place for redundant supply for bulk drug substances. If any one of our current contract manufacturers cannot perform as agreed, we may be required to replace that manufacturer. Although we believe that there are several potential alternative manufacturers who could manufacture our product candidates, we may incur added costs and delays in identifying and qualifying any such replacement.
Our current and anticipated future dependence upon others for the manufacture of our product candidates or products may adversely affect our future profit margins and our ability to commercialize any product candidates that receive marketing approval on a timely and competitive basis.
We also currently rely, and expect to continue to rely, on third parties to store and distribute drug supplies for our clinical trials. Any performance failure on the part of these third parties could delay clinical development or marketing approval of our product candidates or commercialization of our drugs, producing additional losses and depriving us of potential revenue. Although we believe that there are several potential alternative third parties who could store and distribute drug supplies for our clinical trials, we may incur added costs and delays in identifying and qualifying any such replacement.
Our arginase inhibitors program in hematology and oncology indications, including INCB001158, is reliant in part on Incyte for the successful development and commercialization in a timely manner. If Incyte does not devote sufficient resources to INCB001158’s development, is unsuccessful in its efforts, or chooses to terminate its agreement with us, our business, operating results and financial condition will be harmed.
15
In January 2017, we and Incyte Corporation entered into the Incyte Collaboration Agreement. Pursuant to the Incyte Collaboration Agreement, we granted Incyte an exclusive, worldwide license to develop and commercialize small molecule arginase inhibitors, including INCB001158, for hematology and oncology indications. We retained rights to certain arginase inhibitors that are not part of the collaboration for specific orphan indications outside of hematology and oncology, including cystic fibrosis. Pursuant to the Incyte Collaboration Agreement, we and Incyte collaborated on, and co-funded the development of, the licensed products for hematology and oncology indications, including INCB001158, with Incyte bearing 70% and Calithera bearing 30% of global development costs. Effective September 30, 2020, we opted out of our co-development obligations and as a result, Incyte will pay all costs and solely develop INCB001158 or any other licensed products.
The Incyte collaboration may not be clinically or commercially successful due to a number of important factors, including the following:
| • | Subject to the terms of our collaboration agreement, including diligence obligations, although Incyte has certain obligations to use commercially reasonable efforts to develop and commercialize INCB001158, Incyte has discretion in determining the efforts and resources that it will apply to its partnership with us. The timing and amount of any development milestones, and downstream commercial milestones and royalties that we may receive under such partnership will depend on, among other things, the efforts, allocation of resources and successful development and commercialization of INCB001158; |
| • | Incyte may select a dose for INCB001158 that does not have a favorable benefit/risk profile; |
| • | Incyte may terminate its partnership with us without cause and for circumstances outside of our control, which could make it difficult for us to attract new strategic partners or adversely affect how we are perceived in scientific and financial communities; and |
| • | Incyte may develop or commercialize INCB001158 in a way that exposes us to potential litigation that could jeopardize or invalidate our intellectual property rights or expose us to potential liability. |
In April 2020, we filed a complaint against Incyte in Superior Court of California, San Francisco County, asserting claims for breach of contract arising out of Incyte’s failure to pay two milestone payments we believe are due under the Incyte Collaboration Agreement. On September 14, 2021, we entered into a Settlement Agreement and Release with Incyte, or the Settlement Agreement. Pursuant to the Settlement Agreement, which resolves all claims in the complaint without any admission of liability or fault, Incyte will pay us a negotiated settlement amount and the parties have exchanged mutual releases. Concurrently, the parties also filed a dismissal of the action in the Superior Court of California.
If we were to terminate our agreement with Incyte due to Incyte’s breach, or if Incyte were to terminate the agreement without cause, there could be a delay in the return of our rights to INCB001158 and the development and commercialization of INCB001158 would be delayed, curtailed or terminated because we may not have sufficient financial resources or capabilities to continue development and commercialization on our own.
Incyte may enter into one or more transactions with third parties, including a merger, consolidation, reorganization, sale of substantial assets, sale of substantial stock or other change in control, which could divert the attention of its management and adversely affect Incyte’s ability to retain and motivate key personnel who are important to the continued development of the small molecule arginase inhibitor program. In addition, the third party to any such transaction could reprioritize Incyte’s development programs which could delay the development of our programs or cause Incyte to terminate the agreement.
We have in the past and may seek in the future to selectively establish collaborations, and, if we are unable to establish them on commercially reasonable terms, we may have to alter our development and commercialization plans.
Our drug development programs and the potential commercialization of our product candidates will require substantial additional cash to fund expenses. In addition to our collaboration with Incyte, for some of our product candidates, we may decide to collaborate with additional pharmaceutical and biotechnology companies for the development and potential commercialization of those product candidates.
We may also be restricted under existing license agreements from engaging in research and development activities or entering into future agreements on certain terms with potential collaborators. For example, pursuant to our license agreement with Symbioscience, we have agreed not to develop any other arginase inhibitors for use in human healthcare outside of the scope of that agreement. In addition, under our agreement with Incyte, we are not allowed to develop any retained arginase inhibitors (small molecule arginase inhibitors, other than INCB001158, retained by us for research and development in non-hematology/oncology indications) for any indication except specific orphan indications outside of hematology and oncology.
16
We face significant competition in seeking appropriate collaborators. Whether we reach a definitive agreement for a collaboration will depend, among other things, upon our assessment of the collaborator’s resources and expertise, the terms and conditions of the proposed collaboration and the proposed collaborator’s evaluation of a number of factors. Those factors may include the design or results of clinical trials, the likelihood of approval by the FDA or similar regulatory authorities outside the United States, the potential market for the subject product candidate, the costs and complexities of manufacturing and delivering such product candidate to patients, the potential of competing products, the existence of uncertainty with respect to our ownership of technology, which can exist if there is a challenge to such ownership without regard to the merits of the challenge and industry and market conditions generally. The collaborator may also consider alternative product candidates for similar indications that may be available to collaborate on and whether such a collaboration could be more attractive than the one with us for our product candidate.
Collaborations are complex and time-consuming to negotiate and document. In addition, there have been a significant number of recent business combinations among large pharmaceutical companies that have resulted in a reduced number of potential future collaborators.
If we decide to collaborate with any other third parties in connection with any of our development programs or product candidates, we may not be able to negotiate collaborations on a timely basis, on acceptable terms, or at all. If we are unable to do so, we may have to curtail the development program or the product candidate for which we are seeking to collaborate, reduce or delay its development program or one or more of our other development programs, delay its potential commercialization or reduce the scope of any sales or marketing activities, or increase our expenditures and undertake development or commercialization activities at our own expense. If we elect to increase our expenditures to fund development or commercialization activities on our own, we may need to obtain additional capital, which may not be available to us on acceptable terms or at all. If we do not have sufficient funds, we may not be able to further develop our product candidates or bring them to market and generate product revenue.
To the extent we enter into any other collaborations, we may depend on such collaborations for the development and commercialization of our product candidates. If those collaborations are not successful, we may not be able to capitalize on the market potential of our product candidates.
We may selectively seek additional third-party collaborators for the development and commercialization of our product candidates. Our current and any future collaborators for any collaboration arrangements include large and mid-size pharmaceutical companies, regional and national pharmaceutical companies and biotechnology companies. Pursuant to these arrangements and any potential future arrangements, we will have limited control over the amount and timing of resources that our collaborators dedicate to the development or commercialization of our product candidates. Our ability to generate revenue from these arrangements will depend on our collaborators’ abilities to successfully perform the functions assigned to them in these arrangements.
Collaborations involving our product candidates, including our collaboration with Incyte, pose many risks to us, including that:
| • | Collaborators have significant discretion in determining the efforts and resources that they will apply to these collaborations; |
| • | Collaborators may not pursue development and commercialization of our product candidates or may elect not to continue or renew development or commercialization programs based on clinical trial results, changes in the collaborator’s strategic focus or available funding, or external factors such as an acquisition that diverts resources or creates competing priorities; |
| • | Collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial or abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product candidate for clinical testing; |
| • | Collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our product candidates or products if the collaborators believe that competitive products are more likely to be successfully developed or can be commercialized under terms that are more economically attractive than ours; |
| • | A collaborator with marketing and distribution rights to one or more product candidates or products may not commit sufficient resources to the marketing and distribution of such drugs; |
| • | Collaborators may not properly maintain or defend our intellectual property rights or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our proprietary information or expose us to potential litigation; |
| • | Disputes may arise between the collaborators and us, for example our prior claims against Incyte, that result in the delay or termination of the research, development or commercialization of our product candidates or products, or that result in costly litigation or arbitration that diverts management attention and resources; |
| • | We may lose certain valuable rights under circumstances identified in our collaborations if we undergo a change of control; |
17
| • | Collaborations may be terminated and, if terminated, may result in a need for additional capital to pursue further development or commercialization of the applicable product candidates; and |
| • | Collaboration agreements may not lead to development or commercialization of product candidates in the most efficient manner or at all. If a future collaborator of ours were to be involved in a business combination, the continued pursuit and emphasis on our product development or commercialization program under such collaboration could be delayed, diminished or terminated. |
We have in-licensed a portfolio of arginase inhibitors as part of our efforts to develop product candidates for the arginase program, and we are substantially dependent on this in-license for that program. We have acquired sapanisertib and mivavotinib from Millennium. As part of that acquisition from Millennium, Millennium assigned to us certain patents and know-how solely related to sapanisertib and mivavotinib. Millennium also granted us a license under certain other intellectual property necessary for the exploitation of such products. To the extent any such licenses are terminated, our business may be harmed.
Our internal computer systems, or those used by our Clinical Research Organizations or other contractors or consultants, may fail or suffer security breaches.
Despite the implementation of security measures, our internal computer systems, and those of our Clinical Research Organizations and other third parties on which we rely, are vulnerable to damage from computer viruses and unauthorized access, malware, natural disasters, fire, terrorism, war and telecommunication, electrical failures, cyber-attacks or cyber-intrusions over the Internet, attachments to emails, persons inside our organization, or persons with access to systems inside our organization. The risk of a security breach or disruption, particularly through cyber-attacks or cyber intrusion, including by computer hackers, foreign governments, and cyber terrorists, has generally increased as the number, intensity and sophistication of attempted attacks and intrusions from around the world have increased. While we have not experienced any such material system failure or security breach to our knowledge to date, if such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our development programs and our business operations. For example, the loss of clinical trial data from completed, ongoing or future clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. Likewise, we rely on third parties for the manufacture of our product candidates and to conduct clinical trials, and similar events relating to their computer systems could also have a material adverse effect on our business. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development and commercialization of our future product candidates could be delayed.
Risks Related to Our Intellectual Property
Recent laws and rulings by U.S. courts make it difficult to predict how patents will be issued or enforced in our industry.
Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may have a significant impact on our ability to protect our technology and enforce our intellectual property rights. There have been numerous recent changes to the patent laws and to the rules of the United States Patent and Trademark Office, or the USPTO, which may have a significant impact on our ability to protect our technology and enforce our intellectual property rights. For example, the Leahy-Smith America Invents Act, which was signed into law in 2011, includes a transition from a “first-to-invent” system to a “first-to-file” system, and changes the way issued patents are challenged. Certain changes, such as the institution of inter partes review proceedings, came into effect on September 16, 2012. Substantive changes to patent law associated with the America Invents Act may affect our ability to obtain patents, and, if obtained, to enforce or defend them in litigation or post-grant proceedings, all of which could harm our business.
Furthermore, the patent positions of companies engaged in the development and commercialization of biologics and pharmaceuticals are particularly uncertain. Two cases involving diagnostic method claims and “gene patents” have recently been decided by the Supreme Court. On March 20, 2012, the Supreme Court issued a decision in Mayo Collaborative Services v. Prometheus Laboratories, Inc., or Prometheus, a case involving patent claims directed to measuring a metabolic product in a patient to optimize a drug dosage amount for the patient. According to the Supreme Court, the addition of well-understood, routine or conventional activity such as “administering” or “determining” steps was not enough to transform an otherwise patent ineligible natural phenomenon into patent eligible subject matter. On July 3, 2012, the USPTO issued guidance indicating that process claims directed to a law of nature, a natural phenomenon or an abstract idea that do not include additional elements or steps that integrate the natural principle into the claimed invention such that the natural principle is practically applied and the claim amounts to significantly more than the natural principle itself should be rejected as directed to non-statutory subject matter. On June 13, 2013, the Supreme Court issued its decision in Association for Molecular Pathology v. Myriad Genetics, Inc., or Myriad, a case involving patent claims held by Myriad Genetics, Inc. relating to the breast cancer susceptibility genes BRCA1 and BRCA2. Myriad held that isolated segments of naturally occurring DNA, such as the DNA constituting the BRCA1 and BRCA2 genes, is not patent eligible subject matter, but that complementary DNA, which is an artificial construct that may be created from RNA transcripts of genes, may be patent eligible.
18
We cannot assure you that our efforts to seek patent protection for our technology and products will not be negatively impacted by the decisions described above, rulings in other cases or changes in guidance or procedures issued by the USPTO. We cannot fully predict what impact the Supreme Court’s decisions in Prometheus and Myriad may have on the ability of life science companies to obtain or enforce patents relating to their products and technologies in the future.
Moreover, although the Supreme Court has held in Myriad that isolated segments of naturally occurring DNA are not patent-eligible subject matter, certain third parties could allege that activities that we may undertake infringe other gene-related patent claims, and we may deem it necessary to defend ourselves against these claims by asserting non-infringement and/or invalidity positions, or pay to obtain a license to these claims. In any of the foregoing or in other situations involving third-party intellectual property rights, if we are unsuccessful in defending against claims of patent infringement, we could be forced to pay damages or be subjected to an injunction that would prevent us from utilizing the patented subject matter. Such outcomes could harm our business.
If we are alleged to infringe intellectual property rights of third parties, our business could be harmed.
Our research, development and commercialization activities may be alleged to infringe patents, trademarks or other intellectual property rights owned by other parties. Certain of our competitors and other companies in the industry have substantial patent portfolios and may attempt to use patent litigation as a means to obtain a competitive advantage. We may be a target for such litigation. Even if our pending patent applications issue, they may relate to our competitors’ activities and may therefore not deter litigation against us. The risks of being involved in such litigation may also increase as we become more visible as a public company and move into new markets and applications for our product candidates. There may also be patents and patent applications that are relevant to our technologies or product candidates that are unknown to us. For example, certain relevant patent applications may have been filed but not published. If such patents exist, or if a patent issues on any of such patent applications, that patent could be asserted against us. Third parties could bring claims against us that would cause us to incur substantial expenses and, if the claims against us are successful, could cause us to pay substantial damages, including treble damages and attorneys’ fees for willful infringement. The defense of such a suit could also divert the attention of our management and technical personnel. Further, if an intellectual property infringement suit were brought against us, we could be forced to stop or delay research, development or sales of the product that is the subject of the suit.
As a result of infringement claims, or to avoid potential claims, we may choose or be compelled to seek intellectual property licenses from third parties. These licenses may not be available on acceptable terms, or at all. Even if we are able to obtain a license, the license would likely obligate us to pay license fees or royalties or both, and the rights granted to us likely would be nonexclusive, which would mean that our competitors also could obtain licenses to the same intellectual property. Ultimately, we could be prevented from commercializing a product candidate and/or technology or be forced to cease some aspect of our business operations if, as a result of actual or threatened infringement claims, we are unable to enter into licenses of the relevant intellectual property on acceptable terms. Further, if we attempt to modify a product candidate and/or technology or to develop alternative methods or products in response to infringement claims or to avoid potential claims, we could incur substantial costs, encounter delays in product introductions or interruptions in sales.
We may become involved in other lawsuits to protect or enforce our patents or other intellectual property, which could be expensive and time-consuming, and an unfavorable outcome could harm our business.
In addition to the possibility of litigation relating to infringement claims asserted against us, we may become a party to other patent litigation and other proceedings, including inter partes review proceedings, post-grant review proceedings, derivation proceedings declared by the USPTO and similar proceedings in foreign countries, regarding intellectual property rights with respect to our current or future technologies or product candidates or products. The cost to us of any patent litigation or other proceeding, even if resolved in our favor, could be substantial. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their substantially greater financial resources. Patent litigation and other proceedings may also absorb significant management time. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could impair our ability to compete in the marketplace.
Competitors may infringe or otherwise violate our intellectual property, including patents that may issue to or be licensed by us. As a result, we may be required to file claims in an effort to stop third-party infringement or unauthorized use. Any such claims could provoke these parties to assert counterclaims against us, including claims alleging that we infringe their patents or other intellectual property rights. This can be expensive, particularly for a company of our size, and time-consuming, and even if we are successful, any award of monetary damages or other remedy we may receive may not be commercially valuable. In addition, in an infringement proceeding, a court may decide that our asserted intellectual property is not valid or is unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our intellectual property does not cover its technology. An adverse determination in any litigation or defense proceedings could put our intellectual property at risk of being invalidated or interpreted narrowly and could put our patent applications at risk of not issuing.
19
If the breadth or strength of our patent or other intellectual property rights is compromised or threatened, it could allow third parties to commercialize our technology or products or result in our inability to commercialize our technology and products without infringing third-party intellectual property rights. Further, third parties may be dissuaded from collaborating with us.
Interference or derivation proceedings brought by the USPTO or its foreign counterparts may be necessary to determine the priority of inventions with respect to our patent applications, and we may also become involved in other proceedings, such as re-examination proceedings, before the USPTO or its foreign counterparts. Due to the substantial competition in the pharmaceutical space, the number of such proceedings may increase. This could delay the prosecution of our pending patent applications or impact the validity and enforceability of any future patents that we may obtain. In addition, any such litigation, submission or proceeding may be resolved adversely to us and, even if successful, may result in substantial costs and distraction to our management.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. Moreover, intellectual property law relating to the fields in which we operate is still evolving and, consequently, patent and other intellectual property positions in our industry are subject to change and are often uncertain. We may not prevail in any of these suits or other efforts to protect our technology, and the damages or other remedies awarded, if any, may not be commercially valuable. During the course of this type of litigation, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, the market price for our common stock could be significantly harmed.
We may not be able to protect, or fully exploit, our intellectual property rights throughout the world, which could impair our competitive position.
Filing, prosecuting, defending and enforcing patents on all of our technologies, product candidates and products throughout the world would be prohibitively expensive. As a result, we seek to protect our proprietary position by filing patent applications in the United States and in select foreign jurisdictions and cannot guarantee that we will obtain the patent protection necessary to protect our competitive position in all major markets. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export infringing products to territories where we may obtain patent protection but where enforcement is not as strong as that in the United States. These products may compete with our current and future products in jurisdictions where we do not have any issued patents, and our patent claims or other intellectual property rights may not be effective or sufficient to prevent them from so competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, which could make it difficult for us to stop the infringement of our patents or the marketing of competing products in violation of our proprietary rights generally. The legal systems of certain countries make it difficult or impossible to obtain patent protection for pharmaceutical products and services. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and could divert our efforts and attention from other aspects of our business.
Even if we do secure patents in foreign jurisdictions, the legal systems in certain of those countries might require us, as examples, to do business through an entity that is partially owned by a local investor, or to grant license rights to local partners in a manner not required by the jurisdictions in which we currently operate. Requirements such as the foregoing could limit our ability to fully exploit and in the future monetize our product candidates and patents, as well as placing potential additional difficulties on our enforcement efforts in those jurisdictions.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position could be harmed.
In addition to seeking patents for some of our technologies and product candidates, we also rely on trade secrets, including unpatented know-how, technology and other proprietary information, to maintain our competitive position. We seek to protect these trade secrets, in part, by entering into non-disclosure and confidentiality agreements with parties who have access to them, such as our employees, corporate collaborators, outside scientific collaborators, contract manufacturers, consultants, advisors and other third parties. We also enter into confidentiality and invention assignment agreements with our employees and consultants that obligate them to assign to us any inventions developed in the course of their work for us. However, we cannot guarantee that we have executed these agreements with each party that may have or have had access to our trade secrets or that the agreements we have executed will provide adequate protection. Despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. As a result, we may be forced to bring claims against third parties, or defend claims that they bring against us, to determine ownership of what we regard as our intellectual property. Monitoring unauthorized disclosure is difficult and we do not know whether the procedures we have followed to prevent such disclosure have been or will be adequate. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside the United States may be less willing or unwilling to protect trade secrets. If any of the technology or information that we protect as trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to, or independently developed by, a competitor, our competitive position would be harmed.
20
If our trademarks and trade names are not adequately protected, we may not be able to build name recognition in our markets of interest, and our business may be harmed.
Our trademarks or trade names may be challenged, infringed, circumvented, declared generic or determined to be infringing on other marks. As a means to enforce our trademark rights and prevent infringement, we may be required to file trademark claims against third parties or initiate trademark opposition proceedings. This can be expensive and time-consuming, particularly for a company of our size. In addition, in an infringement proceeding, a court may decide that a trademark of ours is not valid or is unenforceable, or may refuse to stop the other party from using the trademark at issue. We may not be able to protect our rights to these and other trademarks and trade names which we need to build name recognition by potential partners or customers in our markets of interest. We do not currently have any registered trademarks in the United States. Any trademark applications in the United States and in other foreign jurisdictions where we may file may not be allowed or may subsequently be opposed. In addition, other companies in the biopharmaceutical space may be using trademarks that are similar to ours and may in the future allege that our use of the trademark infringes or otherwise violates their trademarks. Over the long term, if we are unable to establish name recognition based on our trademarks and trade names, then we may not be able to compete effectively and our business may be harmed.
Third parties may assert ownership or commercial rights to inventions we develop.
Third parties may in the future make claims challenging the inventorship or ownership of our intellectual property. We have written agreements with collaborators that provide for the ownership of intellectual property arising from our collaborations. In some instances, there may not be adequate written provisions to address clearly the resolution of intellectual property rights that may arise from a collaboration. If we cannot successfully negotiate sufficient ownership and commercial rights to the inventions that result from our collaborations, or if disputes otherwise arise with respect to the intellectual property developed in the course of a collaboration, we may be limited in our ability to capitalize on the market potential of these inventions.
In addition, we may face claims by third parties that our agreements with employees, contractors or consultants obligating them to assign intellectual property to us are ineffective or are in conflict with prior or competing contractual obligations of assignment, which could result in ownership disputes regarding intellectual property we have developed or will develop and interfere with our ability to capture the commercial value of such inventions. Litigation may be necessary to resolve an ownership dispute, and if we are not successful, we may be precluded from using certain intellectual property, or may lose our exclusive rights in that intellectual property. Either outcome could have an adverse impact on our business.
Risks Related to Regulatory Approval of Our Product Candidates and Other Legal Compliance Matters
Even if we complete the necessary preclinical studies and clinical trials, the marketing approval process is expensive, time-consuming and uncertain and may prevent us from obtaining approvals for the commercialization of some or all of our product candidates. If we or our collaborators are not able to obtain, or if there are delays in obtaining, required regulatory approvals, we will not be able to commercialize, or will be delayed in commercializing, our product candidates, and our ability to generate revenue will be impaired.
Our product candidates and the activities associated with their development and commercialization, including their design, testing, manufacture, safety, efficacy, recordkeeping, labeling, storage, approval, advertising, promotion, sale and distribution, are subject to comprehensive regulation by the FDA and other regulatory agencies in the United States and by comparable authorities in other countries. Failure to obtain marketing approval for a product candidate will prevent us from commercializing the product candidate. We have not received approval to market any of our product candidates from regulatory authorities in any jurisdiction. We have only limited experience in filing and supporting the applications necessary to gain marketing approvals and expect to rely on third-party contract research organizations to assist us in this process. Securing regulatory approval requires the submission of extensive preclinical and clinical data and supporting information to the various regulatory authorities for each therapeutic indication to establish the product candidate’s safety and efficacy. Securing regulatory approval also requires the submission of information about the product manufacturing process to, and inspection of manufacturing facilities by, the relevant regulatory authority. Our product candidates may not be effective, may be only moderately effective or may prove to have undesirable or unintended side effects, toxicities or other characteristics that may preclude our obtaining marketing approval or prevent or limit commercial use.
The process of obtaining marketing approvals, both in the United States and elsewhere, is expensive, may take many years and can vary substantially based upon a variety of factors, including the type, complexity and novelty of the product candidates involved. We cannot assure you that we will ever obtain any marketing approvals in any jurisdiction. Changes in marketing approval policies during the development period, changes in or the enactment of additional statutes or regulations or changes in regulatory review for each submitted product application may cause delays in the approval or rejection of an application. The FDA and comparable authorities in other countries have substantial discretion in the approval process and may refuse to accept any application or may decide that our data is insufficient for approval and require additional preclinical or other studies, and clinical trials. In addition, varying interpretations of the data obtained from preclinical testing and clinical trials could delay, limit or prevent marketing approval of a product candidate. Additionally, any marketing approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render the approved product not commercially viable.
21
Any product candidate for which we obtain marketing approval could be subject to marketing restrictions or withdrawal from the market, and we may be subject to penalties if we fail to comply with regulatory requirements or if we experience unanticipated problems with our products.
Any product candidate for which we obtain marketing approval, along with the manufacturing processes, post-approval clinical data, labeling, advertising and promotional activities for such product, will be subject to continual requirements of and review by the FDA and other regulatory authorities. These requirements include submissions of safety and other post-marketing information and reports, registration and listing requirements, cGMP requirements, quality assurance and corresponding maintenance of records and documents and requirements regarding the distribution of samples to health care professionals and recordkeeping. Even if marketing approval of a product candidate is granted, the approval may be subject to limitations on the indicated uses for which the product may be marketed or to the conditions of approval, or contain requirements for costly post-marketing testing and surveillance to monitor the safety or efficacy of the medicine. The FDA closely regulates the post approval marketing and promotion of drugs to ensure that they are marketed only for the approved indications and in accordance with the provisions of the approved labeling. The FDA imposes stringent restrictions on manufacturers’ communications regarding off-label use and if we do not market our products for their approved indications, we may be subject to enforcement action for off-label marketing. Physicians, on the other hand, may prescribe products for off-label uses. Although the FDA and other regulatory agencies do not regulate a physician’s choice of drug treatment made in the physician’s independent medical judgment, they do restrict promotional communications from companies or their sales force with respect to off-label uses of products for which marketing clearance has not been issued. Companies may only share truthful and not misleading information that is otherwise consistent with a product’s FDA approved labeling.
In addition, later discovery of previously unknown problems with our products, manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may result in, among other things:
| • | restrictions on such products, manufacturers or manufacturing processes; |
| • | restrictions on the labeling, marketing, distribution or use of a product; |
| • | requirements to conduct post-approval clinical trials; |
| • | warning or untitled letters; |
| • | withdrawal of the products from the market; |
| • | refusal to approve pending applications or supplements to approved applications that we submit; |
| • | recall of products; |
| • | fines, restitution or disgorgement of profits or revenue; |
| • | suspension or withdrawal of marketing approvals; |
| • | refusal to permit the import or export of our products; |
| • | product seizure; and |
| • | injunctions or the imposition of civil or criminal penalties. |
Our relationships with healthcare providers, customers and third-party payors will be subject to applicable anti-kickback, fraud and abuse and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings.
Healthcare providers, customers and third-party payors play a primary role in the recommendation and prescription of any product candidates for which we obtain marketing approval. Our current and future arrangements with healthcare providers, customers and third-party payors may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we research, as well as market, sell and distribute our medicines for which we obtain marketing approval. Restrictions under applicable federal and state healthcare laws and regulations include the following:
| • | the federal healthcare anti-kickback statute prohibits, among other things, persons and entities from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under federal and state healthcare programs such as Medicare and Medicaid; |
| • | the federal false claims laws, including the False Claims Act, which can be enforced through civil whistleblower or qui tam actions, impose criminal and civil penalties against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; |
22
| • | the federal Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act, imposes criminal and civil liability for, among other things, executing a scheme to defraud any healthcare benefit program and also imposes obligations, including mandatory contractual terms, on certain covered healthcare providers, health plans, and healthcare clearinghouses and their respective business associates that perform services for them that involve the use, or disclosure of, individually identifiable health information as well as their covered subcontractors with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
| • | the federal false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services; |
| • | the Physician Payments Sunshine Act requires certain manufacturers of drugs, devices, biologics and medical supplies to report to the Centers for Medicare & Medicaid Services, or CMS, an agency within the Department of Health and Human Services, or HHS, information related to payments and other transfers of value to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals, as well ownership and investment interests held by physicians and their immediate family members. Beginning in 2022, applicable manufacturers also will be required to report information regarding payments and other transfers of value provided during the previous year to physician assistants, nurse practitioners, clinical nurse specialists, certified registered nurse anesthetists, anesthesiologist assistants, and certified nurse midwives during the previous year; and |
| • | analogous state laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers. Some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring drug manufacturers to report information related to payments to physicians and other health care providers, marketing expenditures and/or drug pricing, and other state and local laws require the registration of pharmaceutical sales representatives. |
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, possible exclusion from government funded healthcare programs, such as Medicare and Medicaid, disgorgement, imprisonment, integrity oversight and reporting obligations to resolve allegations of non-compliance with these laws, and the curtailment or restructuring of our operations. If any of the health care professionals or other providers or entities with whom we expect to do business are found to be not in compliance with applicable laws, they may be subject to significant criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.
Recently enacted and future legislation may increase the difficulty and cost for us to obtain marketing approval of and commercialize our product candidates and affect the prices we may obtain. *
In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities and affect our ability to profitably sell any product candidates for which we obtain marketing approval.
Additionally, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, or collectively the PPACA, enacted in 2010, made a number of substantial changes in the way healthcare is financed by both governmental and private insurers. There have been executive, judicial, and Congressional challenges to certain aspects of the PPACA. While Congress has not passed comprehensive repeal legislation, several bills affecting the implementation of certain taxes under PPACA have been signed into law. The Tax Act included a provision which repealed, effective January 1, 2019, the tax-based shared responsibility payment imposed by PPACA on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate”. On June 17, 2021, the U.S. Supreme Court dismissed a challenge on procedural grounds that argued the PPACA is unconstitutional in its entirety because the “individual mandate” was repealed by Congress. Thus, the PPACA will remain in effect in its current form. Further, prior to the U.S. Supreme Court ruling, on January 28, 2021, President Biden issued an executive order that initiated a special enrollment period for purposes of obtaining health insurance coverage through the PPACA marketplace, which began on February 15, 2021 and will remain open through August 15, 2021. The executive order also instructed certain governmental agencies to review and reconsider their existing policies and rules that limit access to healthcare, including among others, reexamining Medicaid demonstration projects and waiver programs that include work requirements, and policies that create unnecessary barriers to obtaining access to health insurance coverage through Medicaid or the PPACA. It is possible that the PPACA will be subject to judicial or Congressional challenges in the future. It is unclear how such challenges and the healthcare reform measures of the Biden administration will impact the PPACA. We continue to evaluate the potential impact of PPACA and its possible repeal or replacement on our business.
23
Policy changes, including potential modification or repeal of all or parts of the PPACA or the implementation of new health care legislation could result in significant changes to the health care system, which may prevent us from being able to generate revenue, attain profitability or commercialize our drugs. We expect that additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand or lower pricing for our product candidates, or additional pricing pressures.
Further, there has been heightened governmental scrutiny of pharmaceutical pricing practices in light of the rising cost of prescription drugs and biologics. Such scrutiny has resulted in several recent congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products. At the federal level, the Trump administration used several means to propose or implement drug pricing reform, including through federal budget proposals, executive orders and policy initiatives. For example, on July 24, 2020 and September 13, 2020, the Trump administration announced several executive orders related to prescription drug pricing that seek to implement several of the administration’s proposals. As a result, the FDA released a final rule on September 24, 2020, effective November 30, 2020, providing guidance for states to build and submit importation plans for drugs from Canada. Further, on November 20, 2020, HHS finalized a regulation removing safe harbor protection for price reductions from pharmaceutical manufacturers to plan sponsors under Medicare Part D, either directly or through pharmacy benefit managers, unless the price reduction is required by law. The implementation of the rule has been delayed by the Biden administration from January 1, 2022 to January 1, 2023 in response to ongoing litigation. The rule also creates a new safe harbor for price reductions reflected at the point-of-sale, as well as a new safe harbor for certain fixed fee arrangements between pharmacy benefit managers and manufacturers, the implementation of which have also been delayed until January 1, 2023. On November 20, 2020, CMS issued an interim final rule implementing the Trump administration’s Most Favored Nation, or MFN, executive order, which would tie Medicare Part B payments for certain physician-administered drugs to the lowest price paid in other economically advanced countries, effective January 1, 2021. On December 28, 2020, the U.S. District Court for the Northern District of California issued a nationwide preliminary injunction against implementation of the interim final rule. On January 13, 2021, in a separate lawsuit brought by industry groups in the U.S. District of Maryland, the government defendants entered a joint motion to stay litigation on the condition that the government would not appeal the preliminary injunction granted in the U.S. District Court for the Northern District of California and that performance for any final regulation stemming from the MFN model interim final rule shall not commence earlier than sixty (60) days after publication of that regulation in the Federal Register. Based on a recent executive order, the Biden administration expressed its intent to pursue certain policy initiatives to reduce drug prices. In addition, there have been and continue to be similar initiatives at the state level to reduce drug costs.
In addition, other legislative changes have been proposed and adopted since the PPACA was enacted. On August 2, 2011, the Budget Control Act of 2011 was signed into law, which includes reductions to Medicare payments to providers of 2% per fiscal year, which went into effect on April 1, 2013 and, due to subsequent legislative amendments to the statute will remain in effect through 2030 unless additional Congressional action is taken. However, COVID-19 relief legislation suspended the 2% Medicare sequester from May 1, 2020 through December 31, 2021. It is possible that additional governmental action will be taken in response to the COVID-19 pandemic. We expect that healthcare reform measures may be adopted in the future, particularly in light of the new presidential administration, which could have a material adverse effect on our industry generally and on our ability to maintain or increase sales of any of our product candidates that we successfully commercialize.
Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We cannot be sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of our product candidates, if any, may be. In addition, increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing testing and other requirements.
Risks Related to Employee Matters and Managing Growth
Our future success depends on our ability to retain our senior management team and to attract, retain and motivate qualified personnel.
We are highly dependent upon our senior management team, as well as the other principal members of our research and development teams. All of our executive officers are employed “at will,” meaning we or they may terminate the employment relationship at any time. We do not maintain “key person” insurance for any of our executives or other employees. The loss of the services of any of these persons could impede the achievement of our research, development and commercialization objectives.
24
Recruiting and retaining qualified scientific, clinical, manufacturing and sales and marketing personnel will also be critical to our success. We may not be able to attract and retain these personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. We also experience competition for the hiring of scientific and clinical personnel from universities and research institutions. In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research and development and commercialization strategy. Our consultants and advisors may be employed by employers other than us and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us.
We may need to expand our operations, and may encounter difficulties in managing our growth, which could disrupt our business.
In the future, we may need to expand the scope of our operations, particularly in the areas of drug development, regulatory affairs and sales and marketing. To manage our future growth, we may need to implement and improve our managerial, operational and financial systems, expand our facilities and continue to recruit and train additional qualified personnel. We may not be able to effectively manage an expansion of our operations or recruit and train additional qualified personnel. Moreover, an expansion of our operations may lead to significant costs and may divert our management and business development resources. For example, our facilities expenses may increase, or decrease, which will vary depending on the time and terms of any facility lease or sublease we may enter into from time to time. Any inability to manage growth could delay the execution of our business plans or disrupt our operations.
We may engage in acquisitions that could disrupt our business, cause dilution to our stockholders or reduce our financial resources.
In the future, we may enter into transactions to acquire other businesses, products or technologies. Because we have not made any acquisitions to date, our ability to do so successfully is unproven. If we do identify suitable candidates, we may not be able to make such acquisitions on favorable terms, or at all. Any acquisitions we make may fail to strengthen our competitive position, and these transactions may be viewed negatively by customers or investors. We may decide to incur debt in connection with an acquisition or issue our common stock or other equity securities to the stockholders of the acquired company, which would reduce the percentage ownership of our existing stockholders. We could incur losses resulting from undiscovered liabilities of the acquired business that are not covered by the indemnification we may obtain from the seller. In addition, we may not be able to successfully integrate the acquired personnel, technologies and operations into our existing business in an effective, timely and non-disruptive manner. Acquisitions may also divert management attention from day-to-day responsibilities, increase our expenses and reduce our cash available for operations and other uses. We cannot predict the number, timing or size of future acquisitions or the effect that any such transactions might have on our operating results.
International expansion of our business exposes us to business, regulatory, political, operational, financial and economic risks associated with doing business in various jurisdictions globally.
Our business strategy incorporates international expansion, including establishing and maintaining relationships with service providers, distributors and manufacturers globally. Doing business internationally involves a number of risks, including:
| • | multiple, conflicting and changing laws and regulations such as tax laws, export and import restrictions, employment laws, anti-bribery and anti-corruption laws, regulatory requirements and other governmental approvals, permits and licenses; |
| • | failure by us or our distributors to obtain appropriate licenses or regulatory approvals for the sale or use of our product candidates, if approved, in various countries; |
| • | difficulties in managing foreign operations; |
| • | complexities of foreign reimbursement regimes and price controls; |
| • | financial risks, such as difficulty enforcing contracts exposure to foreign currency exchange rate fluctuations; |
| • | reduced protection for intellectual property rights; |
| • | reduced protection of contractual rights in the event of bankruptcy or insolvency of the other contracting party; |
| • | natural disasters, political and economic instability, including wars, terrorism and political unrest, outbreak of disease, boycotts, curtailment of trade and other business restrictions; |
| • | difficulties in complying with changes in laws, regulations and costs affecting our foreign operations, including our United Kingdom, or UK, operations potentially affected by the UK exiting the European Union, or EU; |
25
| • | failure to comply with foreign laws, regulations, standards and regulatory guidance governing the collection, use, disclosure, retention, security and transfer of personal data, including the European Union General Data Privacy Regulation, or GDPR, which introduces strict requirements for processing personal data of individuals within the European Union; and |
| • | failure to comply with the United Kingdom Bribery Act 2010, or UK Bribery Act, and similar antibribery and anticorruption laws in other jurisdictions, and the Foreign Corrupt Practices Act, including its books and records provisions and its anti-bribery provisions, including by failing to maintain accurate information and control over sales and distributors’ activities. |
The UK’s withdrawal from the EU, commonly referred to as Brexit, may have a negative effect on global economic conditions, financial markets and our business.
Following the result of a referendum in 2016, the United Kingdom left the European Union on January 31, 2020, commonly referred to as Brexit. Pursuant to the formal withdrawal arrangements agreed between the United Kingdom and the European Union, the United Kingdom will be subject to the Transition Period until December 31, 2020 during which European Union rules will continue to apply. Negotiations between the United Kingdom and the European Union are expected to continue in relation to the customs and trading relationship between the United Kingdom and the European Union following the expiry of the Transition Period.
The lack of clarity over which EU laws and regulations will continue to be implemented in the United Kingdom after the Transition Period (including financial laws and regulations, tax and free trade agreements, intellectual property rights, data protection laws, supply chain logistics, environmental, health and safety laws and regulations, immigration laws and employment laws) may negatively impact foreign direct investment in the United Kingdom, increase costs, depress economic activity and restrict access to capital. The uncertainty concerning the United Kingdom’s legal, political and economic relationship with the European Union after the Transition Period may be a source of instability in the international markets, create significant currency fluctuations, and/or otherwise adversely affect trading agreements or similar cross-border co-operation arrangements (whether economic, tax, fiscal, legal, regulatory or otherwise).
These developments, or the perception that any of them could occur, have had, and may continue to have, a significant adverse effect on global economic conditions and the stability of global financial markets, and could significantly reduce global market liquidity and limit the ability of key market participants to operate in certain financial markets. In particular, it could also lead to a period of considerable uncertainty in relation to the United Kingdom’s financial and banking markets, as well as on the regulatory process in Europe. Asset valuations, currency exchange rates and credit ratings may also be subject to increased market volatility.
If the United Kingdom and the European Union are unable to negotiate acceptable withdrawal terms or if other EU Member States pursue withdrawal, barrier-free access between the United Kingdom and other EU Member States or among the European Economic Area, or EEA, overall could be diminished or eliminated. The long-term effects of Brexit will depend on any agreements (or lack thereof) between the United Kingdom and the European Union and, in particular, any arrangements for the United Kingdom to retain access to EU markets after the Transition Period.
26
Such a withdrawal from the European Union is unprecedented, and it is unclear how the United Kingdom’s access to the European single market for goods, capital, services and labor within the European Union, or single market, and the wider commercial, legal and regulatory environment, will impact us.
Risks Related to Our Common Stock
The holders of our Series A preferred stock have liquidation and other rights that are senior to the rights of the holders of shares of our common stock.*
In the event of a merger, acquisition, liquidation, dissolution, or winding up of Calithera whether voluntary or involuntary, the holders of our Series A preferred stock will be entitled to have set apart for them, or to be paid, out of our assets available for distribution to stockholders after provision for payment of all of our debts and liabilities in accordance with the Delaware General Corporation Law, before any distribution or payment is made with respect to any shares of junior securities, including shares of our common stock, an amount per share equal to the greater of (i) $35.00, being the issuance price per share of Series A preferred stock, and (ii) such amount as would have been payable on the number of shares of common stock into which the shares of Series A preferred stock could have been converted immediately prior to such event. If applicable, this preference would reduce the amount of our assets, if any, available to distribute to holders of our common stock.
We may be required to issue a significant number of additional shares of common stock for no additional consideration to the holders of our Series A preferred stock pursuant to certain price-based anti-dilution provisions.*
We may be required to issue a significant number of shares of common stock for no additional consideration to the holders of our Series A preferred stock, subject to certain beneficial ownership limitations described in the certificate of designations defining the rights of the holders of the Series A preferred stock. The terms of the Series A preferred stock provide that such shares will automatically convert into common stock on the earlier of: (i) the 18-month anniversary of the date of issuance, or the Mandatory Pricing Date, into 17,156,863 shares of common stock, subject to adjustment into additional shares of common stock if the volume weighted-average price of our common stock for the thirty trading days prior to the Mandatory Pricing Date is lower than $2.04 per share, and (ii) a qualified financing that results in net proceeds to us of at least $40.0 million, excluding any conversion of the Series A preferred stock, subject to adjustment into additional shares of common stock if the weighted-average price per paid by investors in such qualified financing is lower than $2.04 per share. The holders of Series A preferred stock also have the option, at any time prior to the Mandatory Pricing Date or such qualified financing to convert the Series A preferred stock into shares of common stock, subject to adjustment into additional shares of common stock if the volume weighted-average sales price of certain shares of common stock are sold from the issuance date of the Series A preferred stock through the date of the written election at an effective price less than $2.04 per share.
Stockholders will incur dilution of their percentage ownership interest in our common stock to the extent we issue additional shares of common stock to the holders of the Series A preferred stock. Any issuance or potential issuance of additional shares of common stock could adversely affect our stock price, make it more difficult for us to raise capital on favorable terms, or at all, and harm our business, results of operations and financial condition
We cannot take certain actions without the consent of the holders of Series A preferred stock.*
Certain matters require the approval of the Series A preferred stock, voting as a separate class, including to:
| • | amend our organizational documents in a way that has an adverse effect on the Series A preferred stock; |
| • | create or authorize the creation of any new security, or reclassify or amend any existing security, that are senior to, or equal in priority with, the Series A preferred stock, including any shares of Series A preferred stock, with respect to the distribution of assets on the liquidation, dissolution or winding up of Calithera, the payment of dividends and rights of redemption; or |
| • | purchase or redeem, or pay or declare, any dividend or make any distribution on, any shares of our capital stock, subject to certain exceptions. |
The interests of Millennium, the sole holder of our Series A preferred stock and those of the holders of common stock may be inconsistent, which may result in our inability to obtain the consent of the holders of Series A preferred stock to matters that may be in the best interests of the common stockholders.
27
We may be required to make significant cash payments to the holders of Series A preferred stock if we do not receive requisite stockholder approval to allow the conversion of the Series A preferred stock to common stock, which may limit our working capital liquidity.*
As part of our acquisition of the Takeda Programs, if we are unable to obtain stockholder approval in accordance with the rules of The Nasdaq Stock Market LLC for the conversion of all of the shares of Series A preferred stock to common stock, and as a result Millennium is unable to convert any portion of the Series A preferred stock to common stock, we will be obligated under our purchase agreement with Millennium to negotiate in good faith as to the timing and form of consideration to compensate Millennium for such inability to convert. We are unable to estimate the actual amount or form of consideration we will be required to make at this time but we may become obligated to make significant cash payments to Millennium after three years following the purchase agreement date, which could limit our working capital liquidity. In addition, we may not have sufficient funds available to meet our obligations to Millennium, which may result in Millennium pursuing remedies under the purchase agreement that could adversely affect our operations.
We have granted Millennium registration rights with respect to the shares of common stock into which our Series A preferred stock is convertible. If these additional shares are sold, or it is perceived that they will be sold, the market price of our common stock could decline.
Millennium has the right, subject to some conditions, to require us to file a registration statement covering the resale of the shares of common stock issuable upon conversion of the Series A preferred stock. If we were to register the resale of these shares, they could be freely sold in the public market without limitation. If these additional shares are sold, or if it is perceived that they will be sold, in the public market, the market price of our common stock could decline.
The trading price of our common stock is likely to be volatile, and purchasers of our common stock could incur substantial losses.
Our stock price has fluctuated in the past and is likely to be volatile in the future. The stock market in general and the market for biotechnology companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. As a result of this volatility, investors may experience losses on their investment in our common stock. The market price for our common stock may be influenced by many factors, including:
| • | the success of competitive products or technologies; |
| • | regulatory actions with respect to our product candidates or our competitors’ product and product candidates; |
| • | announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures, collaborations or capital commitments; |
| • | results of clinical trials of our product candidates or those of our competitors; |
| • | regulatory or legal developments in the United States and other countries; |
| • | developments or disputes concerning patent applications, issued patents or other proprietary rights; |
| • | the recruitment or departure of key personnel; |
| • | actual and anticipated fluctuations in our quarterly operating results; |
| • | the level of expenses related to any of our product candidates or clinical development programs; |
| • | the results of our efforts to in-license or acquire additional products or product candidates; |
| • | actual or anticipated changes in estimates as to financial results, development timelines or recommendations by securities analysts; |
| • | uncertainties regarding the magnitude and duration of impacts we are experiencing due to COVID-19; |
| • | variations in our financial results or those of companies that are perceived to be similar to us; |
| • | fluctuations in the valuation of companies perceived by investors to be comparable to us; |
| • | inconsistent trading volume levels of our shares; |
| • | announcement or expectation of additional financing efforts; |
| • | sales of our common stock by us, our insiders or our other stockholders; |
| • | changes in the structure of healthcare payment systems; |
| • | market conditions in the pharmaceutical and biotechnology sectors; |
| • | general economic, industry and market conditions; and |
| • | the other factors described in this “Risk Factors” section. |
In addition, in the past, stockholders have initiated class action lawsuits against companies following periods of volatility in the market prices of these companies’ stock. Such litigation, if instituted against us, could cause us to incur substantial costs and divert management’s attention and resources.
28
Concentration of ownership of our capital stock may prevent new investors from influencing significant corporate decisions.*
Our executive officers, directors and current beneficial owners of 5% or more of our common stock, in the aggregate, beneficially own a significant percentage of our outstanding common stock. These persons, acting together, will be able to significantly influence all matters requiring stockholder approval, including the election and removal of directors and any merger or other significant corporate transactions. The interests of this group of stockholders may not coincide with the interests of other stockholders.
Takeda, through its affiliate Millennium, beneficially owns a significant percentage of our total outstanding capital stock. Takeda will be able to significantly influence all matters requiring stockholder approval, including the election and removal of directors and any merger or other significant corporate transactions. The interests of Takeda may not coincide with the interests of other stockholders.
If securities or industry analysts do not publish research, or publish unfavorable research, about our business, our stock price and trading volume could decline.
The trading market for our common stock will depend in part on the research and reports that securities or industry analysts publish about us or our business, our market and our competitors. We do not have any control over these analysts. If one or more of the analysts who cover us downgrade our shares or change their opinion of our shares, our share price would likely decline. If one or more of these analysts cease coverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which could cause our share price or trading volume to decline.
We have and will incur costs and demands upon management as a result of complying with the laws and regulations affecting public companies in the United States, which may harm our operating results.
As a public company listed in the United States, we have and will continue to incur significant additional legal, accounting and other expenses. In addition, changing laws, regulations and standards relating to corporate governance and public disclosure, including regulations implemented by the Securities and Exchange Commission, or SEC, and the Nasdaq Global Select Market, may increase legal and financial compliance costs and make some activities more time-consuming. These laws, regulations and standards are subject to varying interpretations, and as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. We intend to invest resources to comply with evolving laws, regulations and standards, and this investment may result in increased general and administrative expenses and a diversion of management’s time and attention from revenue-generating activities to compliance activities. If, notwithstanding our efforts to comply with new laws, regulations and standards, we fail to comply, regulatory authorities may initiate legal proceedings against us, and our business may be harmed.
Further, failure to comply with these laws, regulations and standards might also make it more difficult for us to obtain certain types of insurance, including director and officer liability insurance, and we might be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. The impact of these events could also make it more difficult for us to attract and retain qualified persons to serve on our Board of Directors, on committees of our Board of Directors or as members of senior management.
We do not anticipate paying any cash dividends on our common stock so any returns will be limited to changes in the value of our common stock.
We have never declared or paid cash dividends on our common stock. We currently intend to retain our future earnings, if any, to fund the development and growth of our business. In addition, the terms of any existing or future credit facility may restrict our ability to pay dividends. Any return to stockholders will therefore be limited to the increase, if any, of our stock price.
If we are unable to maintain proper and effective internal controls over financial reporting, the accuracy and timeliness of our financial reporting and the market price of our common stock may be adversely affected.
Effective internal controls are necessary for us to provide reliable financial reports and to protect from fraudulent, illegal or unauthorized transactions. If we cannot provide effective controls and reliable financial reports, our business and operating results could be harmed. We have in the past discovered, and may in the future discover, areas of our internal controls that need improvement. We are required, pursuant to Section 404 of the Sarbanes-Oxley Act, to furnish a report by management on the effectiveness of our internal control over financial reporting. In the future, our independent registered public accounting firm may also need to attest to the effectiveness of our internal control over financial reporting.
If material weaknesses or control deficiencies occur in the future, we are unable to comply with the requirements of Section 404 in a timely manner, we are unable to assert that our internal control over financial reporting is effective, or if our independent registered public accounting firm is unable to express an opinion as to the effectiveness of our internal control over financial reporting, we may be unable to report our financial results accurately on a timely basis, which could cause our reported financial results to be materially misstated and result in the loss of investor confidence and cause the market price of our common stock to decline.
29
Provisions in our corporate charter documents and under Delaware law may prevent or frustrate attempts by our stockholders to change our management or hinder efforts to acquire a controlling interest in us, and the market price of our common stock may be lower as a result.
There are provisions in our certificate of incorporation and bylaws that may make it difficult for a third party to acquire, or attempt to acquire, control of our company, even if a change in control was considered favorable by our stockholders.
Our charter documents also contain other provisions that could have an anti-takeover effect, such as:
| • | establishing a classified Board of Directors so that not all members of our Board of Directors are elected at one time; |
| • | permitting the Board of Directors to establish the number of directors and fill any vacancies and newly created directorships; |
| • | providing that directors may only be removed for cause; |
| • | prohibits cumulative voting for directors; |
| • | requiring super-majority voting to amend some provisions in our certificate of incorporation and bylaws; |
| • | authorizing the issuance of “blank check” preferred stock that our Board of Directors could use to implement a stockholder rights plan; |
| • | eliminating the ability of stockholders to call special meetings of stockholders; and |
| • | prohibiting stockholder action by written consent, which requires all stockholder actions to be taken at a meeting of our stockholders. |
Moreover, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which prohibit a person who owns 15% or more of our outstanding voting stock from merging or combining with us for a period of three years after the date of the transaction in which the person acquired in excess of 15% of our outstanding voting stock, unless the merger or combination is approved in a prescribed manner. Any provision in our certificate of incorporation or our bylaws or Delaware law that has the effect of delaying or deterring a change in control could limit the opportunity for our stockholders to receive a premium for their shares of our common stock, and could also affect the price that some investors are willing to pay for our common stock.
Our amended and restated certificate of incorporation designates the Court of Chancery of the State of Delaware and our amended and restated bylaws designate the federal district courts of the United States of America as the exclusive forums for substantially all disputes between us and our stockholders, which will restrict our stockholders’ ability to choose the judicial forum for disputes with us or our directors, officers, or employees.
Our amended and restated certificate of incorporation provides that the Court of Chancery of the State of Delaware is the exclusive forum for any derivative action or proceeding brought on our behalf; any action asserting a breach of fiduciary duty; any action asserting a claim against us arising pursuant to the Delaware General Corporation Law, our amended and restated certificate of incorporation or our bylaws; or any action asserting a claim against us that is governed by the internal affairs doctrine.
The provisions would not apply to suits brought to enforce a duty or liability created by the Exchange Act or any other claim for which the U.S. federal courts have exclusive jurisdiction. Furthermore, Section 22 of the Securities Act creates concurrent jurisdiction for federal and state courts over all such Securities Act actions. Accordingly, both state and federal courts have jurisdiction to entertain such claims. Our stockholders cannot waive compliance with the federal securities laws and the rules and regulations thereunder. To prevent having to litigate claims in multiple jurisdictions and the threat of inconsistent or contrary rulings by different courts, among other considerations, our amended and restated bylaws provide that, unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States of America will be the exclusive forum for resolving any complaint asserting a cause of action arising under the Securities Act. While the Delaware courts have determined that such choice of forum provisions are facially valid, a stockholder may nevertheless seek to bring a claim in a venue other than those designated in the exclusive forum provisions. In such instance, we would expect to vigorously assert the validity and enforceability of the exclusive forum provisions of our amended and restated certificate of incorporation. This may require significant additional costs associated with resolving such action in other jurisdictions and there can be no assurance that the provisions will be enforced by a court in those other jurisdictions.
30
These exclusive choice of forum provisions may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers, or other employees, which may discourage lawsuits against us and our directors, officers, and other employees. If a court were to find such exclusive-forum provisions to be inapplicable or unenforceable in an action, we may incur further significant additional costs associated with resolving the dispute in other jurisdictions, all of which could harm our business.
31
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Get Up To 15% Flat Discount on Video Promotion Website on VideoIpsum
- Form 8.5 (EPT/RI) - Accrol Group Holdings Plc
- VistaJet’s summer of imaginative journeys
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share