Form 8-K Brooklyn ImmunoTherapeut For: Sep 20
Exhibit 99.1

September 20, 2021 mRNA Engineered Cell & Cytokine Medicines A platform company in cell,
gene-editing & cytokine therapies

Disclaimer This presentation is intended to provide summary information about the business of Brooklyn
ImmunoTherapeutics, Inc. (“BTX”). The information in this presentation is in no respects complete, comprehensive or exhaustive, and it should be read in conjunction with BTX’s public filings with the Securities and Exchange Commission,
including information set forth in those filings under “Risk Factors” and similar headings. Forward-Looking Statements. Certain statements presented below on pages 3, 4, 7, 8 and 20 are forward-looking statements for purposes of the safe
harbor provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements are any statements that are not statements of historical fact and may be identified by terminology such as “expect,” “plan,” “potential,”
“project” or “will” or other similar words. Forward-looking statements are based on current beliefs and assumptions that are subject to risks and uncertainties and are not guarantees of future performance. Actual results may vary significantly
from BTX’s expectations based on a number of risks and uncertainties, including but not limited to the following: (i) the evolution of BTX’s business model into a platform company focused on cellular, gene editing and cytokine programs; (ii)
BTX’s ability to successfully, cost effectively and efficiently develop its technology and products; (iii) BTX’s ability to successfully commence clinical trials of any products on a timely basis or at all; (iv) BTX’s ability to successfully
fund and manage the growth of its development activities; (v) BTX’s ability to obtain regulatory approvals of its products for commercialization; and (vi) uncertainties related to the impact of the COVID-19 pandemic on the business and
financial condition of BTX, including on the timing and cost of its clinical trials. BTX cannot guarantee any future results, levels of activity, performance or achievements. The industry in which BTX operates is subject to a high degree of
uncertainty and risk due to variety of factors, including those described in BTX’s public filings with the Securities and Exchange Commission, including its Current Report on Form 8-K filed with the Securities and Exchange Commission on May 11,
2021 and its Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2021 for a more complete discussion of these factors and other risks, particularly under the heading “Risk Factors.” BTX expressly disclaims any obligation to
update forward-looking statements after the date of this presentation. 2

BTX: World’s Only mRNA Cell-Engineering Platform What is mRNA cell-engineering? Messenger RNA (mRNA) is
a special class of molecules that contain the instructions that determine how cells function. BTX’s platform uses synthetic mRNA with minimized immune response to engineer cells to treat disease by repairing disease-causing mutations and
directing the formation of stem cells.BTX is developing a platform of gene-editing and cell therapies based on exclusively in-licensed mRNA technologySynthetic mRNA allows gene editing without provoking an immune responsemRNA therapeutics are
safe and highly effective in patientsFast to market – mRNA products have proven accelerated entry into clinical development Low cost of goods sold – mRNA products avoid complex and costly viral-vector manufacturingCan target any geneNo limit to
the number or complexity of cellular modifications that can be madeEnables high-potency mRNA and precision cell therapiesEngineered cells are fully rejuvenated with greater expansion allowing more consistent treatments BTX’s in-licensed vehicle
safely delivers mRNA to cells inside and outside the body 3

BTX: Leveraging In-licensed Patent Portfolio to Advance Medicine BTX has an exclusive license from
Factor Bioscience to a portfolio of granted patents around mRNA-based cell engineering that will provide BTX with a competitive advantageBTX’s major platform components:mRNA Cell Reprogramming (25 patents, extensive cellular data)mRNA Gene
Editing (15 patents, extensive cellular data)NoveSlice™ Gene-Editing Protein (15 patents, extensive cellular data)ToRNAdo™ mRNA Delivery Vehicle (4 patents, extensive cell and animal data) 4 NoveSlice™ and ToRNAdo™ are trademarks of Factor
Bioscience Limited. 2022 2023 Reverse Merger with NTN Buzztime (NYSE American: BTX) 2021 Established R&D Center in Cambridge, MA In-Licensed mRNA Patents Acquired Novellus Therapeutics IND-Enabling Pre-Clinical Studies IND –
Indication 1 (est.)

Brooklyn’s Licensed mRNA-Based and LNP Technologies 5 Cell reprogramming Gene editing mRNA and LNP
are toolsto make engineered cell medicine Nucleic acid delivery mRNA and LNP are the drugas in vivo gene-editing medicine

Fields of Medicine Addressable with BTX Technology 6

ToRNAdo™ Delivery Offers Superior Results 7 Vector toxicity

An Investment in BTX Today Offers Future Value Breadth and depth of BTX technology offer numerous
opportunities for successful clinical resultsBTX in-licensed patent portfolio offers potential for licensing and royalty revenue in coming yearsBTX technology platform offers predictable path to clinical development, with initial clinical
testing of cellular products expected within 2 yearsBTX patent portfolio offers range of clinical applications at low cost and low riskBTX legacy cytokine portfolio and completed Phase 2b trial offer additional valueOpportunity for partnering
in the Ph3 registration studyOpportunity for advancing another Ph2 study in a different oncologic indication 8
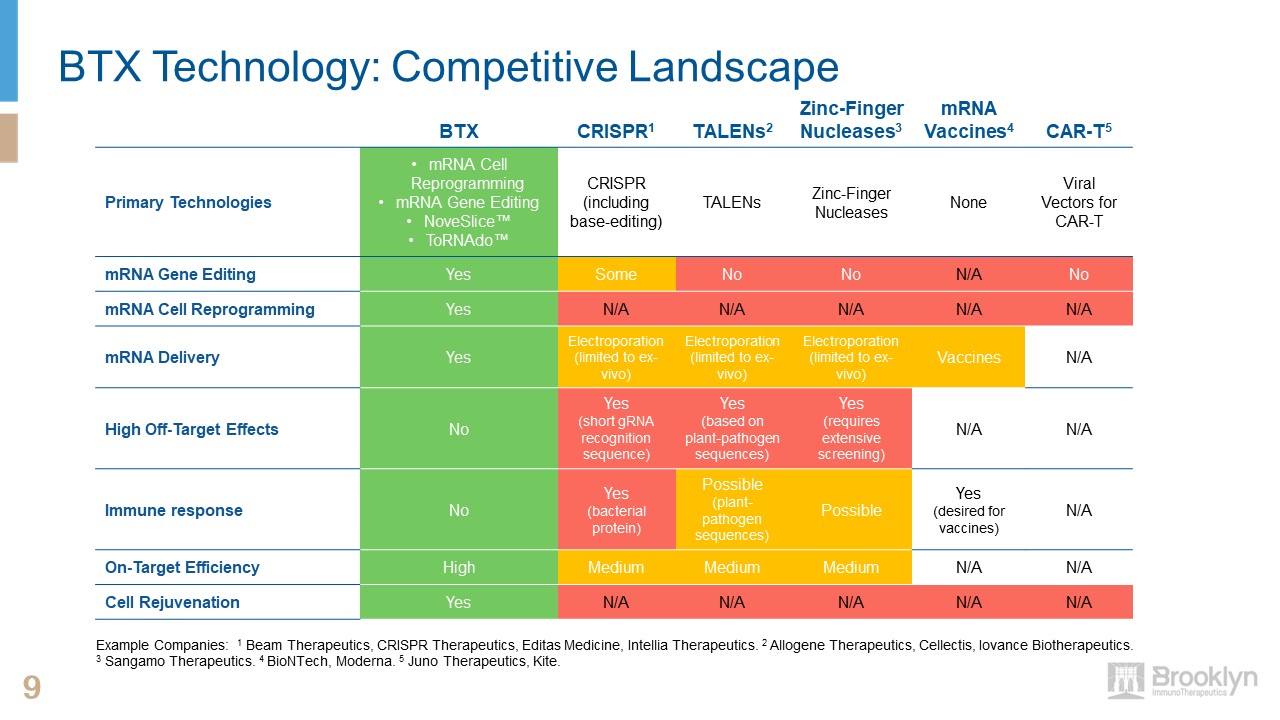
9 9 BTX CRISPR1 TALENs2 Zinc-Finger Nucleases3 mRNA Vaccines4 CAR-T5 Primary Technologies mRNA
Cell ReprogrammingmRNA Gene EditingNoveSlice™ToRNAdo™ CRISPR(including base-editing) TALENs Zinc-Finger Nucleases None Viral Vectors for CAR-T mRNA Gene Editing Yes Some No No N/A No mRNA Cell
Reprogramming Yes N/A N/A N/A N/A N/A mRNA Delivery Yes Electroporation (limited to ex-vivo) Electroporation (limited to ex-vivo) Electroporation (limited to ex-vivo) Vaccines N/A High Off-Target Effects No Yes(short gRNA
recognition sequence) Yes(based on plant-pathogen sequences) Yes(requires extensive screening) N/A N/A Immune response No Yes(bacterial protein) Possible(plant- pathogen sequences) Possible Yes(desired for vaccines) N/A On-Target
Efficiency High Medium Medium Medium N/A N/A Cell Rejuvenation Yes N/A N/A N/A N/A N/A BTX Technology: Competitive Landscape Example Companies: 1 Beam Therapeutics, CRISPR Therapeutics, Editas Medicine, Intellia Therapeutics. 2
Allogene Therapeutics, Cellectis, Iovance Biotherapeutics. 3 Sangamo Therapeutics. 4 BioNTech, Moderna. 5 Juno Therapeutics, Kite.

First Product Tier is a Stem Cell Product Platform Mesenchymal Stem Cells (MSC)Multipotent stem cells
capable of expansion and differentiation into different kinds of tissuesSources of trophic factors modulating the immune system and inducing repair of tissuesProduct development leveraging extensive adult-derived MSC precedentsLong history of
clinical use and strong safety track recordInconsistent or insufficient efficacy has plagued field, due product inconsistency or poor choice of clinical indicationWell-described and long-practiced manufacturingMultiple capable CDMO options
existA single Drug Product can be used across multiple and varied indicationsExploits homing/migration and anti-inflammatory properties of MSC 10

The iPSC Technology Addresses Issues with Adult Sources 11 iPSC-derived MSC advantagesAmenable to more
scaled manufacturing due longer proliferative life-spanOne of the easiest iPSC-derived cell products to manufactureMore consistent characteristics and improved therapeutic properties relative to adult sourcesCan gene edit the iPSC to program
additional properties into iMSC and broaden therapeutic use
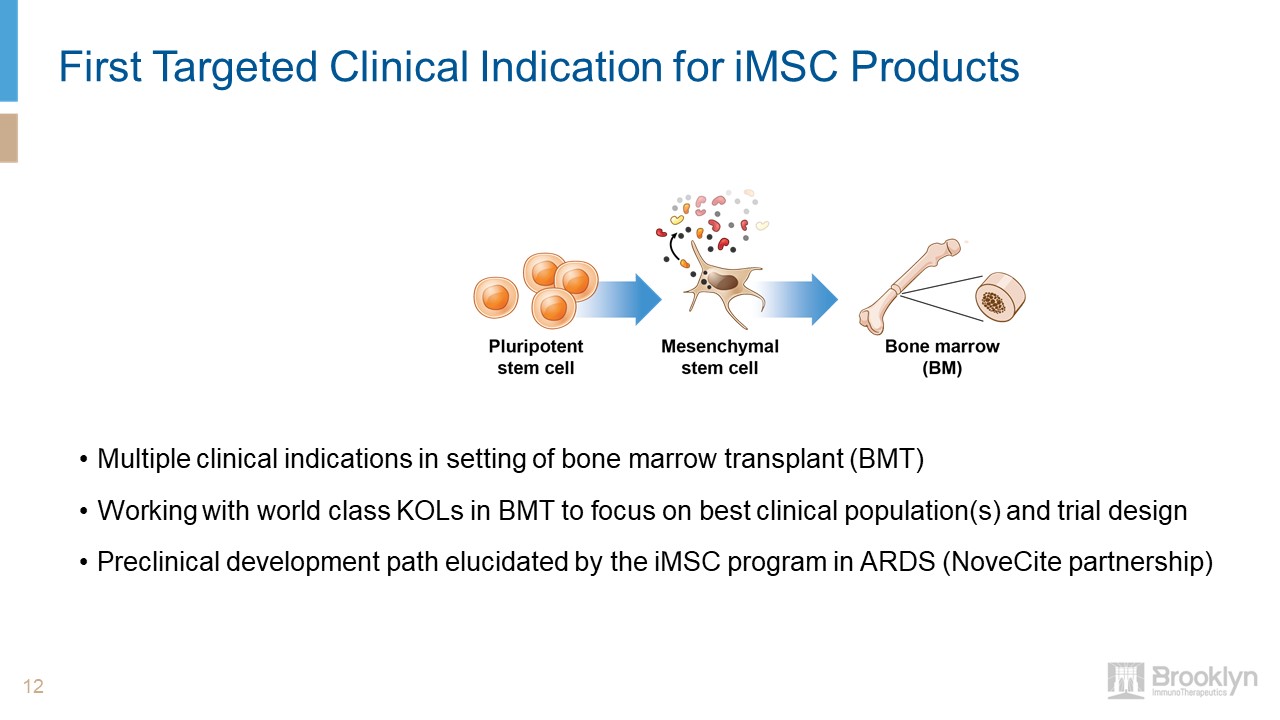
First Targeted Clinical Indication for iMSC Products 12 Multiple clinical indications in setting of
bone marrow transplant (BMT)Working with world class KOLs in BMT to focus on best clinical population(s) and trial designPreclinical development path elucidated by the iMSC program in ARDS (NoveCite partnership)

Developing Gene-edited Versions of iMSC Products Initially Targeting Poorly Addressed Solid
Tumors 13 Rationale for gene-edited iMSC in oncologyHarnessing MSC homing to tumors to locally deliver immune stimulating proteinsAddition of specific cytokines counters the pro-tumor effect that sometimes observed (i.e. anti-inflammatory
properties of MSC )Rationale for IL-7 and IL-15 (members of IL-2 family)IL-7/IL-15 used in vitro to enhance CAR-T tumor activity in vivo. Unlike IL-2, they will not induce proliferation of TregsLocal delivery to tumor sites should reduce
toxicities associated with systemic administrationTargeting solid tumor clinical indications of high unmet need

Second Product Tier is a Genetic Medicine Product Platform Proprietary lipid nanoparticle for nucleic
acid deliveryProperties can be tuned to access different cell types and target tissuesCan deliver RNA or DNA; facilitates gene correction approachesDelivery of mRNA encoding for proprietary site-specific nucleaseNuclease can target any gene
through design of protein binding domainsHigh specificity to target genomic siteAchieves high level but transient expression of nuclease, enhancing safety 14

Developing In Vivo Gene-editing Products Addressing Rare Disease Indications (Orphan Designation) Direct
gene editing in the liver, brain or eye for monogenic disordersAbility to knock-out or correct the target geneInitial gene targets includeTTR for Familial Transthyretin Amyloidosis (ATTR)ABCA4 for Stargardt Disease (monogenic form of macular
degeneration) 15 ToRNAdo™ is a trademark of Factor Bioscience Inc.

Third Product Tier is a Personalized Cell Therapy Platform Licensed technology is the safest, most
efficient, and fastest method for iPSC derivation Safe: Non-integrating method using synthetic mRNA to produce reprogramming factorsEfficient: Uses LNP for repeated in vitro delivery with low toxicityEfficient: Can combine reprogramming and
gene editing in single step derivationFast: Reprogramming and iPSC colony formation within 2 weeksThe safety, reliability and speed enable autologous iPSC programsEfficiency of reprogramming permits low quantity of cells from biopsy and
simultaneous correction of gene defects Can quickly produce multiple iPSC clones per patientAbsence of genome integration facilitates screening to identify and characterize a safe clone 16
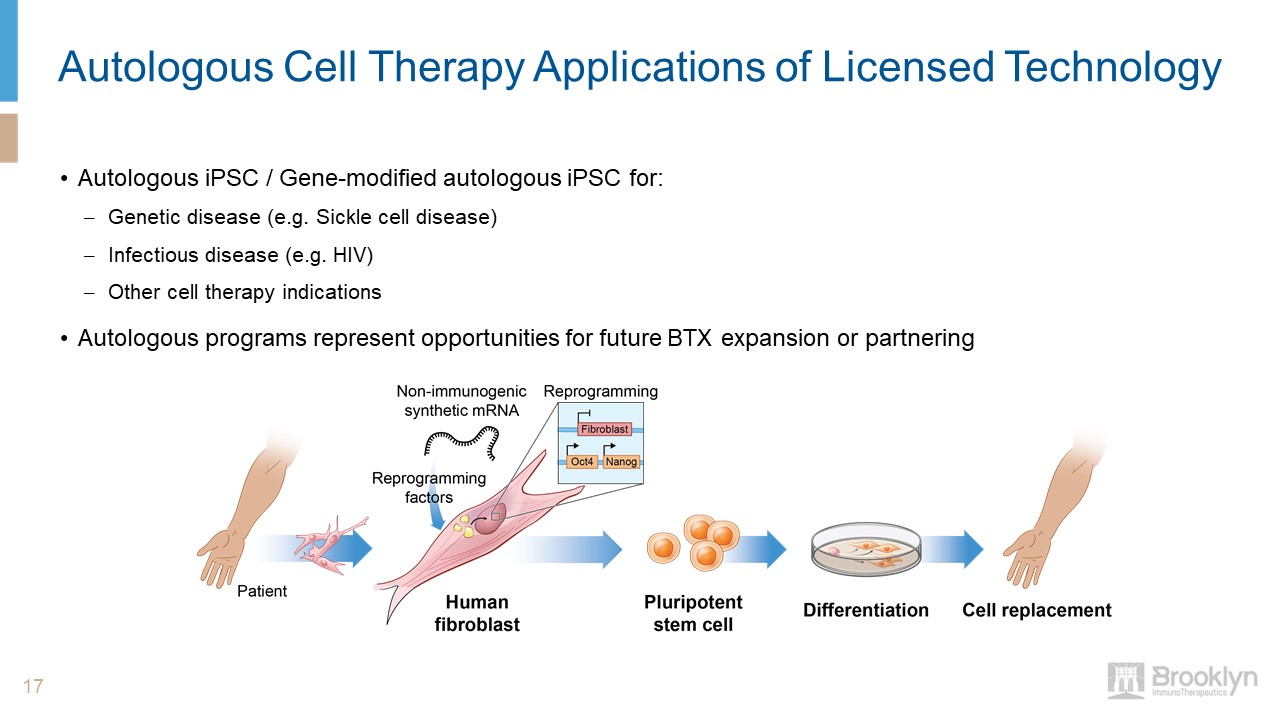
Autologous Cell Therapy Applications of Licensed Technology Autologous iPSC / Gene-modified autologous
iPSC for: Genetic disease (e.g. Sickle cell disease)Infectious disease (e.g. HIV)Other cell therapy indicationsAutologous programs represent opportunities for future BTX expansion or partnering 17

BTX Cytokine, Cell Therapy, and Gene Editing
Pipeline 18 Indication Approach Discovery Preclinical IND-enabling Early clinical Late clinical Comments IRX-2: human cell-derived mixed cytokine Head & Neck Cancer SubQ injections Phase 2b Various
IST SubQ injections Phase 1/2 iMSC: iPSC-derived mesenchymal stem cells ARDS I.V. injection NoveCite program BMT failure I.V. injection TBD(inflammation, autoimmunity) I.V.
injection TBD(inflammation, autoimmunity) other than I.V. Solid tumors Gene-edited(IL-7 + IL-15) In vivo gene editing Transthyretin Amyloidosis I.V. or CNS Stargardt
Disease Retinal injection

IRX-2 Mixed Cytokine Product Invigorates the Immune Response to Tumors Multiple cytokines in mixture can
act locally and potentially in systemic fashionHuman cell-derived source addresses toxicity observed with other IL-2 cancer therapiesCurrently in Phase 2b for Neoadjuvant Head and Neck Cancer, data readout in 1H-2022 19 Cold tumor Hot
tumor Cytokine mixture attractsimmune cells

BTX Most Advanced Asset: IRX-2 Human-Derived Cytokines Phase 2 Company Sponsored Study in 1 IST
Indication targeted to begin in 2022Phase 3 Study in Neoadjuvant Head and Neck Cancer targeted to begin in 2023 20 Renal Cell CancerLiver CancerHead and Neck CancerGastrointestinal Cancer Currently in Phase 2b for Neoadjuvant Head and Neck
Cancer Final data readout expected in 1H2022 Additional Investigator Sponsored Trials (ISTs) in: Future Planned Studies: Strong IP and Patent Position Cervical/Vulvar Interstitial NeoplasiaTriple Negative Breast CancerEarly Stage
Breast Cancer

September 20, 2021 A platform company in cell, gene-editing & cytokine therapies mRNA Engineered
Cell & Cytokine Medicines
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- ASE Technology Holding Co., Ltd. Reports Its Unaudited Consolidated Financial Results for the First Quarter of 2024
- LBank Exchange Will List Armonia Meta Chain (AMAX) on April 28, 2024
- New CPS Protection Platform: TXOne Networks Presents SageOne in Hannover
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share