Form 8-K Blueprint Medicines Corp For: Nov 27
Exhibit 99.1
 |
 |
Blueprint Medicines to Expand Precision Therapy
Leadership in Lung Cancer with Acquisition
of Lengo Therapeutics
-- Adds LNG-451, a highly selective brain-penetrant precision therapy targeting EGFR exon 20 insertion mutations, to Blueprint Medicines’ lung cancer pipeline
-- Lengo Therapeutics on track to submit IND to FDA for LNG-451 by the end of 2021
-- Lengo Therapeutics to be acquired for $250 million in cash plus $215 million in future potential payments based on the achievement of certain approval and sales-based milestones
-- Blueprint Medicines to host investor conference call and webcast today at 8:30 a.m. ET
CAMBRIDGE, Mass. and San Diego, Calif., Nov. 29, 2021 /PRNewswire/ -- Blueprint Medicines Corporation (NASDAQ: BPMC) today announced that the company has entered into a definitive agreement under which it will acquire Lengo Therapeutics, a privately held precision oncology company, for $250 million in cash plus up to $215 million in additional potential payments based on the achievement of certain regulatory approval and sales-based milestones.
The acquisition includes Lengo Therapeutics’ lead compound LNG-451, a potential best-in-class oral precision therapy in development for the treatment of non-small cell lung cancer (NSCLC) in patients with EGFR exon 20 insertion mutations. Preclinical data show LNG-451 potently inhibits all common EGFR exon 20 insertion variants with marked selectivity over wild-type EGFR and off-target kinases. In addition, LNG-451 is highly brain-penetrant and has demonstrated compelling activity in a preclinical intracranial disease model.
Based on these and other preclinical data, Lengo Therapeutics anticipates it will submit an investigational new drug (IND) application for LNG-451 to the U.S. Food and Drug Administration (FDA) in December 2021.
“Our acquisition of Lengo Therapeutics deepens our commitment to advancing precision oncology therapies and specifically expands our opportunity to transform treatment for patients with EGFR-driven lung cancer,” said Jeff Albers, Chief Executive Officer of Blueprint Medicines. “The Lengo team has done tremendous work in designing a highly selective therapeutic candidate tailored to the needs of patients with EGFR exon 20 lung cancer, including features with the potential to enable treatment or prevention of brain metastases. With our integrated precision therapy research, development and commercial capabilities, Blueprint Medicines is perfectly positioned to carry forward this compound into the clinic and deliver on our goal to meaningfully advance care for NSCLC patients with EGFR exon 20 insertion mutations.”
“With a proven track record of developing and delivering precision therapies for patients with significant medical needs and a compelling lung cancer portfolio, Blueprint Medicines is unique in its abilities to quickly progress LNG-451,” said Enoch Kariuki, Chief Executive Officer of Lengo Therapeutics. “From our inception, the Lengo Therapeutics team has focused on generating best-in-class compound profiles, prioritizing those with brain penetration along with high potency and selectivity, like LNG-451. I am incredibly proud of the team for getting us to this point and excited to see the programs continue under Blueprint Medicines’ leadership.”
With the addition of LNG-451, Blueprint Medicines will have three investigational compounds, each with best-in-class potential, that cover the majority of all activating mutations in EGFR, the second most common oncogenic driver in NSCLC.1 Approximately 12 percent of activating EGFR mutations are exon 20 insertions and significant medical need remains for patients harboring these mutations, including new treatment options with improved tolerability, combinability and enhanced brain penetration to treat or prevent brain metastases.1
The acquisition also brings additional undisclosed preclinical precision oncology programs and research tools, including a catalog of covalent, highly brain penetrant kinase inhibitors that Blueprint Medicines plans to add to its proprietary compound library to further enable future drug discovery efforts.
Blueprint Medicines anticipates the acquisition will close in the fourth quarter of 2021, subject to certain conditions, including the expiration of the waiting period under the Hart-Scott-Rodino Antitrust Improvements Act and other customary conditions.
Goldman Sachs & Co. LLC is acting as financial advisor to Blueprint Medicines and Goodwin Procter LLP is acting as its legal counsel. Centerview Partners LLC is acting as financial advisor to Lengo Therapeutics and Cooley LLP is acting as its legal counsel.
Conference Call Information
Blueprint Medicines will host a live webcast beginning at 8:30 a.m. ET today to discuss the planned acquisition of Lengo Therapeutics. To access the live call, please dial 844-200-6205 (domestic) or 929-526-1599 (international) and refer to conference ID 936793. A webcast of the conference call will be available under "Events and Presentations" in the Investors & Media section of Blueprint Medicines' website at http://ir.blueprintmedicines.com. The archived webcast will be available on Blueprint Medicines' website approximately two hours after the conference call and will be available for 90 days following the call.
About Blueprint Medicines’ EGFR Development Program
Derived from Blueprint Medicines’ proprietary research platform, BLU-945 and BLU-701 are investigational next-generation EGFR non-covalent tyrosine kinase inhibitors. Both treatments are specifically designed to provide comprehensive coverage of the most common activating and on-target resistance mutations, spare wild-type EGFR and other kinases to limit off-target toxicities and enable a range of combination strategies, and treat or prevent central nervous system metastases. BLU-945 is currently being evaluated in the Phase 1/2 SYMPHONY trial in patients with previously treated EGFR-driven NSCLC (NCT04862780). In addition, Blueprint Medicines plans to initiate a Phase 1/2 trial of BLU-701 in the fourth quarter of 2021.
About Lengo Therapeutics
Lengo Therapeutics is a biopharmaceutical company committed to developing novel, small molecule precision therapeutics that target driver mutations in oncology. Lengo Therapeutics’ team is comprised of scientists and industry leaders with extensive expertise in kinase biology, covalent drug-target technology, and oncology drug development. The company’s initial focus is on developing inhibitors of protein kinases with mutations known as EGFR exon 20 insertions which are associated with poor prognoses in non-small cell lung cancer and other solid tumors. Lengo Therapeutics is based in San Diego and is backed by Frazier Healthcare Partners and Velosity Capital.
About Blueprint Medicines
Blueprint Medicines is a global precision therapy company that invents life-changing therapies for people with cancer and hematologic disorders. Applying an approach that is both precise and agile, we create medicines that selectively target genetic drivers, with the goal of staying one step ahead across stages of disease. Since 2011, we have leveraged our research platform, including expertise in molecular targeting and world-class drug design capabilities, to rapidly and reproducibly translate science into a broad pipeline of precision therapies. Today, we are delivering approved medicines directly to patients in the United States and Europe, and we are globally advancing multiple programs for genomically defined cancers, systemic mastocytosis, and cancer immunotherapy. For more information, visit www.BlueprintMedicines.com and follow us on Twitter (@BlueprintMeds) and LinkedIn.
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, without limitation, express or implied statements regarding plans, strategies, timelines and expectations for Blueprint Medicines’ current or future approved drugs and drug candidates, including timelines for marketing applications and approvals, the initiation of clinical trials or the results of ongoing and planned clinical trials; Blueprint Medicines’ plans, strategies and timelines to nominate development candidates; plans and timelines for additional marketing applications for avapritinib and pralsetinib and, if approved, commercializing avapritinib and pralsetinib in additional geographies or for additional indications; the potential benefits of any of Blueprint Medicines’ current or future approved drugs or drug candidates in treating patients; the potential benefits of Blueprint Medicines’ collaborations; timelines and expectations for the proposed acquisition (including future performance and revenue); and Blueprint Medicines’ strategy, goals and anticipated financial performance, milestones, business plans and focus. The words “aim,” “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “target” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Any forward-looking statements in this press release are based on management’s current expectations and beliefs and are subject to a number of risks, uncertainties and important factors that may cause actual events or results to differ materially from those expressed or implied by any forward-looking statements contained in this press release, including, without limitation, risks and uncertainties related to the impact of the COVID-19 pandemic to Blueprint Medicines’ business, operations, strategy, goals and anticipated milestones, including Blueprint Medicines’ ongoing and planned research and discovery activities, ability to conduct ongoing and planned clinical trials, clinical supply of current or future drug candidates, commercial supply of current or future approved products, and launching, marketing and selling current or future approved products; Blueprint Medicines’ ability and plans in continuing to establish and expand a commercial infrastructure, and successfully launching, marketing and selling current or future approved products; Blueprint Medicines’ ability to successfully expand the approved indications for AYVAKIT/AYVAKYT and GAVRETO or obtain marketing approval for AYVAKIT/AYVAKYT in additional geographies in the future; the delay of any current or planned clinical trials or the development of Blueprint Medicines’ current or future drug candidates; Blueprint Medicines’ advancement of multiple early-stage efforts; Blueprint Medicines’ ability to successfully demonstrate the safety and efficacy of its drug candidates and gain approval of its drug candidates on a timely basis, if at all; the preclinical and clinical results for Blueprint Medicines’ drug candidates, which may not support further development of such drug candidates; actions of regulatory agencies, which may affect the initiation, timing and progress of clinical trials; Blueprint Medicines’ ability to obtain, maintain and enforce patent and other intellectual property protection for AYVAKIT/AYVAKYT, GAVRETO or any drug candidates it is developing; Blueprint Medicines’ ability to develop and commercialize companion diagnostic tests for AYVAKIT/AYVAKYT, GAVRETO or any of its current and future drug candidates; Blueprint Medicines’ ability to complete the proposed acquisition in a timely manner or at all; the occurrence of any event, change or other circumstances that could give rise to the termination of the proposed acquisition; and the success of Blueprint Medicines’ current and future collaborations, partnerships or licensing arrangements. These and other risks and uncertainties are described in greater detail in the section entitled “Risk Factors” in Blueprint Medicines’ filings with the Securities and Exchange Commission (SEC), including Blueprint Medicines’ most recent Annual Report on Form 10-K, as supplemented by its most recent Quarterly Report on Form 10-Q and any other filings that Blueprint Medicines has made or may make with the SEC in the future. Any forward-looking statements contained in this press release represent Blueprint Medicines’ views only as of the date hereof and should not be relied upon as representing its views as of any subsequent date. Except as required by law, Blueprint Medicines explicitly disclaims any obligation to update any forward-looking statements.
Blueprint Medicines Contacts
Media:
Sarah Mena Guerrero
+1 (617) 714-6684
Investor Relations:
Kristin Hodous
+1 (617) 714-6674
Lengo Therapeutics Contact
Media:
Kimberly Ha, KKH Advisors
+1 (917) 291-5744
1 Riess JW, Gandara DR, Frampton GM, et al. “Diverse EGFR Exon 20 Insertions and Co-Occurring Molecular Alterations Identified by Comprehensive Genomic Profiling of NSCLC”. J Thorac Oncol. 2018;13(10):1560-1568. doi:10.1016/j.jtho.2018.06.019
Exhibit 99.2

Celebrating our first decade of innovation in precision medicine Expanding our transformative lung cancer pipeline NOVEMEBER 29, 2021 Blueprint Medicines investor call to discuss planned acquisition of Lengo Therapeutics © 2021 Blueprint Medicines Corporation

Forward - looking statements 2 This presentation contains forward - looking statements as defined in the Private Securities Litigation Reform Act of 1995 , as amended . The words "aim," "may," "will," "could," "would," "should," "expect," "plan," "anticipate," "intend," "believe," "estimate," "predict," "project," "potential," "continue," "target" and similar expressions are intended to identify forward - looking statements, although not all forward - looking statements contain these identifying words . In this presentation, forward - looking statements include, without limitation, express or implied statements regarding plans, strategies, timelines and expectations for the current or future approved drugs and drug candidates of Blueprint Medicines Corporation (the “Company”), including timelines for marketing applications and approvals, the initiation of clinical trials or the results of ongoing and planned clinical trials ; the Company’s plans, strategies and timelines to nominate development candidates ; plans and timelines for additional marketing applications for avapritinib and pralsetinib and, if approved, commercializing avapritinib and pralsetinib in additional geographies or for additional indications ; the potential benefits of any of the Company’s current or future approved drugs or drug candidates in treating patients ; timelines and expectations for the proposed acquisition (including future performance and revenue) ; and ௗ the Company’s ௗ financial performance, strategy, goals and anticipated milestones, business plans and focus . The Company has based these forward - looking statements on management's current expectations, assumptions, estimates and projections . If such expectations, assumptions, estimates and projections do not fully materialize or prove incorrect, the events or circumstances referred to in the forward - looking statements may not occur . While the Company believes these expectations, assumptions, estimates and projections are reasonable, such forward - looking statements are only predictions and involve known and unknown risks, uncertainties and other important factors, many of which are beyond the Company's control and may cause actual results, performance or achievements to differ materially from those expressed or implied by any forward - looking statements . These risks and uncertainties include, without limitation, risks and uncertainties related to the impact of the COVID - 19 pandemic to the Company's business, operations, strategy, goals and anticipated milestones, including the Company's ongoing and planned research and discovery activities, ability to conduct ongoing and planned clinical trials, clinical supply of current or future drug candidates, commercial supply of current or future approved drugs, and launching, marketing and selling current or future approved drugs ; the Company’s ability and plans in establishing a commercial infrastructure, and successfully launching, marketing and selling current or future approved products ; the Company’s ability to successfully expand the approved indications for AYVAKIT Œ /AYVAKYT® ( avapritinib ) and GAVRETO Œ ( pralsetinib ) or obtain marketing approval for AYVAKIT/AYVAKYT in additional geographies in the future ; the delay of any current or planned clinical trials or the development of the Company's drug candidates or the licensed drug candidate ; the Company's advancement of multiple early - stage efforts ; the Company's ability to successfully demonstrate the efficacy and safety of its drug candidates and gain approval of its drug candidates on a timely basis, if at all ; the preclinical and clinical results for the Company's drug candidates, which may not support further development of such drug candidates ; actions or decisions of regulatory agencies or authorities, which may affect the initiation, timing and progress of clinical trials or marketing applications ; the Company's ability to obtain, maintain and enforce patent and other intellectual property protection for AYVAKIT/AYVAKYT, GAVRETO or any drug candidates it is developing ; the Company's ability to develop and commercialize companion diagnostic tests for any of the Company's current or future approved drugs or drug candidates ; the Company's ability to complete the proposed acquisition in a timely manner or at all ; the occurrence of any event, change or other circumstances that could give rise to the termination of the proposed acquisition ; and the success of the Company's current and future collaborations, partnerships and licenses . These and other risks and uncertainties are described in greater detail under "Risk Factors" in the Company's filings with the Securities and Exchange Commission ("SEC"), including its most recent Annual Report on Form 10 - K, as supplemented by its most recent Quarterly Report on Form 10 - Q, and any other filings it has made or may make with the SEC in the future . The Company cannot guarantee future results, outcomes, levels of activity, performance, developments, or achievements, and there can be no assurance that its expectations, intentions, anticipations, beliefs, or projections will result or be achieved or accomplished . The forward - looking statements in this presentation are made only as of the date hereof, and except as required by law, the Company undertakes no obligation to update any forward - looking statements contained in this presentation as a result of new information, future events or otherwise . Accordingly, readers are cautioned not to place undue reliance on these forward - looking statements . This presentation also contains estimates, projections and other statistical data made by independent parties and by the Company relating to market size and growth and other data about the Company's industry . These data involve a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates . In addition, projections, assumptions and estimates of the Company's future performance and the future performance of the markets in which the Company operates are necessarily subject to a high degree of uncertainty and risk . Blueprint Medicines, AYVAKIT, AYVAKYT, GAVRETO and associated logos are trademarks of Blueprint Medicines Corporation.

Blueprint Medicines to acquire Lengo Therapeutics IND, investigational new drug application. 3 LEADER IN PRECISION THERAPY LNG - 451 & RESEARCH PORTFOLIO Expands our transformative pipeline for patients with non - small cell lung cancer Adds potential best - in - class, IND - ready EGFR exon 20 precision therapy Leverages our integrated precision therapy business to maximize patient impact $250M purchase price plus $215M in potential regulatory approval and sales - based milestone payments
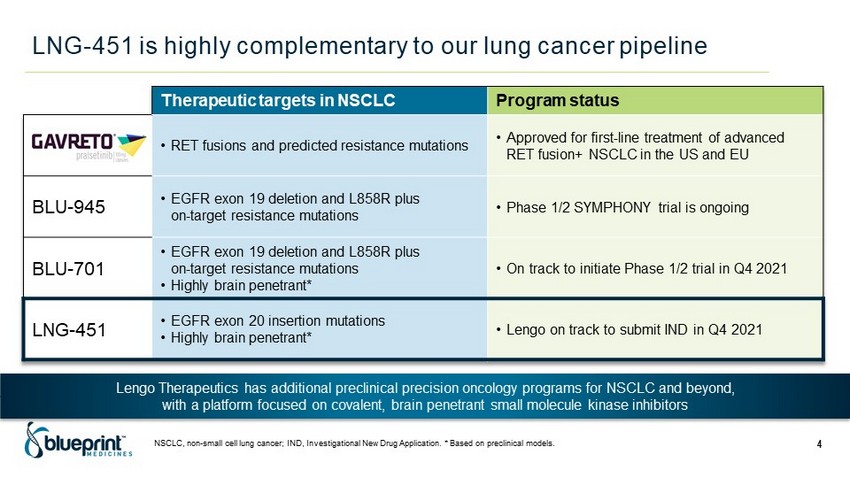
LNG - 451 is highly complementary to our lung cancer pipeline NSCLC, non - small cell lung cancer; IND, Investigational New Drug Application. * Based on preclinical models. 4 Therapeutic targets in NSCLC Program status • RET fusions and predicted resistance mutations • Approved for first - line treatment of advanced RET fusion+ NSCLC in the US and EU BLU - 945 • EGFR exon 19 deletion and L858R plus on - target resistance mutations • Phase 1/2 SYMPHONY trial is ongoing BLU - 701 • EGFR exon 19 deletion and L858R plus on - target resistance mutations • Highly brain penetrant* • On track to initiate Phase 1/2 trial in Q4 2021 LNG - 451 • EGFR exon 20 insertion mutations • Highly brain penetrant* • Lengo on track to submit IND in Q4 2021 Lengo Therapeutics has additional preclinical precision oncology programs for NSCLC and beyond, with a platform focused on covalent, brain penetrant small molecule kinase inhibitors

We have expanded our opportunity to transform EGFR - driven lung cancer 1 Datamonitor , SEER, Incidence and Prevalence Database, GBD 2019, WHO, IARC, RKI, Cancer Research UK, Siegel 2015 CA Cancer J Res, Sonoda 2019 Cancer Manag Res. 2 Riess 2018 J Thorac Oncol. CNS, central nervous system; Del, deletion; Ins, insertion. 5 22% 15% 45% 45% 0% 20% 40% 60% US EU Japan China Our pipeline will address >90% of activating EGFR mutations 2 47% 32% 12% 9% Exon 19 del L858R Exon 20 ins Other rare Significant need exists in patients with exon 20 insertions Estimated EGFR mutation rates in NSCLC adenocarcinoma • All approved and investigational therapies have important safety, efficacy and/or CNS limitations • Opportunities: Better selectivity Improve tolerability including cardiovascular safety Enhanced brain penetration Treat or prevent brain metastases Oral administration Enable patient convenience Frequency of activating mutations in patients with EGFR - driven NSCLC in a recent U.S. multi - center study EGFR is the second most common oncogenic driver in NSCLC 1

LNG - 451 has best - in - class potential Internal company data on file. 6 Potent inhibition of all common EGFR exon 20 insertion variants Highly selective over wild - type EGFR and off - target kinases Oral administration with well - characterized preclinical pharmacology Brain penetrant with robust activity in a preclinical intracranial model Preclinical data show LNG - 451 achieves all target profile features, with potential to translate into improved safety and efficacy, including in patients with brain metastases
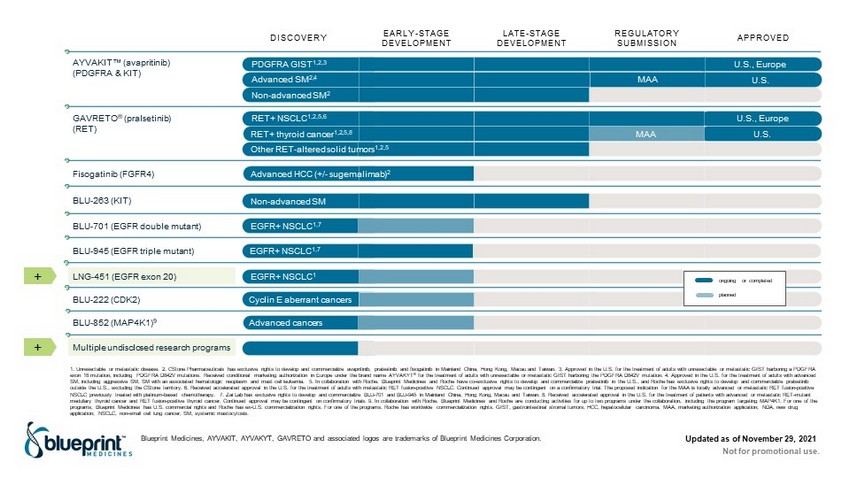
+ Updated as of November 29, 2021 Not for promotional use. Blueprint Medicines, AYVAKIT, AYVAKYT, GAVRETO and associated logos are trademarks of Blueprint Medicines Corporation. 1. Unresectable or metastatic disease. 2. CStone Pharmaceuticals has exclusive rights to develop and commercialize avapritinib , pralsetinib and fisogatinib in Mainland China, Hong Kong, Macau and Taiwan. 3. Approved in the U.S. for the treatment of adults with unresectable or meta st atic GIST harboring a PDGFRA exon 18 mutation, including PDGFRA D842V mutations. Received conditional marketing authorization in Europe under the brand name AYVAKYT ® for the treatment of adults with unresectable or metastatic GIST harboring the PDGFRA D842V mutation. 4 . Approved in the U.S. for the treatment of adults with advanced SM, including aggressive SM, SM with an associated hematologic neoplasm and mast cell leukemia. 5. In collaboration with Roche. Blueprint Medicines and Roche have co - exclusive rights to develop and commercialize pralsetinib in the U.S., and Roche has exclusive rights to develop and commercialize pralsetinib outside the U.S., excluding the CStone territory. 6. Received accelerated approval in the U.S. for the treatment of adults with metastatic RET fusion - positive NSCLC. Continued approval may be contingent on a confirmatory trial. The proposed indication for the MAA is locally advanced or metastatic RET fusion - positive NSCLC previously treated with platinum - based chemotherapy. 7. Zai Lab has exclusive rights to develop and commercialize BLU - 701 and BLU - 945 in Mainland China, Hong Kong, Macau and Taiwan. 8. Received accelerated approval in the U.S. for the treatment of p atients with advanced or metastatic RET - mutant medullary thyroid cancer and RET fusion - positive thyroid cancer. Continued approval may be contingent on confirmatory trials. 9. In collaboration with Roche. Blueprint Medicines and Roche are conducting activities for up to two pr ogr ams under the collaboration, including the program targeting MAP4K1. For one of the programs, Blueprint Medicines has U.S. commercial rights and Roche has ex - U.S. commercialization rights. For one of the programs , Roche has worldwide commercialization rights . GIST, gastrointestinal stromal tumors; HCC, hepatocellular carcinoma; MAA, marketing authorization application; NDA, new dr ug application; NSCLC, non - small cell lung cancer; SM, systemic mastocytosis . DISCOVERY EARLY - STAGE DEVELOPMENT LATE - STAGE DEVELOPMENT REGULATORY SUBMISSION APPROVED U.S., Europe PDGFRA GIST 1,2,3 Non - advanced SM 2 AYVAKIT Œ ( avapritinib ) (PDGFRA & KIT) Fisogatinib (FGFR4) EGFR+ NSCLC 1,7 BLU - 701 (EGFR double mutant) EGFR+ NSCLC 1 LNG - 451 (EGFR exon 20) Cyclin E aberrant cancers BLU - 222 (CDK2) Multiple undisclosed research programs Non - advanced SM BLU - 263 (KIT) Advanced HCC (+/ - sugemalimab ) 2 GAVRETO ® ( pralsetinib ) (RET) Other RET - altered solid tumors 1,2,5 Advanced SM 2,4 RET+ thyroid cancer 1,2,5,8 U.S. MAA RET+ NSCLC 1,2,5,6 U.S., Europe Advanced cancers BLU - 852 (MAP4K1) 9 ongoing or completed planned U.S. MAA EGFR+ NSCLC 1,7 BLU - 945 (EGFR triple mutant) +

Thank you
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Blueprint Medicines to Host Webcast and Conference Call Highlighting Emerging Opportunities for Mast Cell-Targeted Therapeutics
- Stonegate Initiates Coverage on BFF Bank S.P.A (BFF)
- Integrated. Strategic. Partners. (ISP) Announces New Senior Leadership
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share