Form 8-K Arbutus Biopharma Corp For: Aug 05
EXHIBIT 99.1
Arbutus Reports Second Quarter 2021 Financial Results and Provides Corporate Update
New data on AB-729, Arbutus’ proprietary subcutaneously delivered RNAi agent, highlighted in four abstracts at the EASL International Liver Congress™; all AB-729 abstracts selected for best of ILC™
Announced two additional proof-of-concept clinical collaborations to evaluate AB-729 in combination with agents from Vaccitech plc and Antios Therapeutics, Inc.
Announced U.S. Food and Drug Administration (FDA) authorization to proceed with an Investigational New Drug (IND) application in a Phase 2a clinical trial to investigate the safety and anti-viral activity of AB-729 in combination with ongoing nucleos(t)ide analog (NA) therapy and short courses of Peg-IFNα-2a in subjects with chronic HBV
Presentation at EASL of pre-clinical data for AB-836, Arbutus’ proprietary oral capsid inhibitor, suggests the potential for increased efficacy and an enhanced resistance profile relative to previous generation capsid inhibitors
Conference Call and Webcast Scheduled Today at 8:45 AM ET
WARMINSTER, Pa., Aug. 05, 2021 (GLOBE NEWSWIRE) -- Arbutus Biopharma Corporation (Nasdaq: ABUS), a clinical-stage biopharmaceutical company primarily focused on discovering, developing and commercializing a cure for people with chronic hepatitis B virus (HBV) infection, as well as therapies to treat coronaviruses (including COVID-19), today reports its second quarter 2021 financial results and provides a corporate update.
William Collier, President and Chief Executive Officer of Arbutus, stated, “We had a productive second quarter, particularly in advancing our efforts to position AB-729 as a potential cornerstone therapy in future HBV combination regimens. Our recently announced proof-of-concept clinical collaborations with Vaccitech plc and Antios Therapeutics, Inc. to evaluate AB-729 with other agents reflects this objective as does our planned Phase 2a clinical trial to evaluate AB-729 in combination with Peg-IFNα-2a.”
Mr. Collier added, “Looking ahead, we expect a productive second half of 2021 including: additional data from the ongoing Phase 1a/1b clinical trial with AB-729, specifically 90 mg multi-dose data (dosing interval every 12 weeks) in HBV DNA negative subjects and 90 mg multi-dose data (dosing interval every 8 weeks) in HBV DNA positive subjects, initiation of two Phase 2a proof-of-concept clinical trials for AB-729, and initial Phase 1a/1b data from our proprietary oral capsid inhibitor, AB-836.”
Pipeline Update
AB-729
- Arbutus is currently conducting a single- and multi-dose Phase 1a/1b clinical trial to determine the safety, tolerability, pharmacokinetics, and pharmacodynamics of AB-729 in healthy subjects and in subjects with chronic HBV infection. The Company presented three posters and a late breaker oral presentation at the 2021 EASL conference highlighting the most recent data from this clinical trial. AB-729 continues to demonstrate robust mean HBsAg reduction across all doses and dosing intervals with a favorable safety and tolerability profile, followed by a sustained plateau phase:
Mean (range) change in HBsAg with repeat dosing of AB-729:
| Visit | Cohort E AB-729 60 mg Q4W | Cohort F AB-729 60 mg Q8W | Cohort I AB-729 90 mg Q8W | p value between Cohorts |
| Week 16 | -1.44 (-0.71 to -1.95) | -1.39 (-1.61 to -1.08) | -1.63 (-0.89 to -2.44) | p ≥ 0.4 |
| Week 24 | -1.84 (-0.99 to -2.31) | -1.57 (-1.24 to -2.01) | -1.79 (-1.22 to -2.46) | p ≥ 0.2 |
| Week 32 | -1.84 (-0.94 to -2.36) | -1.68 (-1.37 to -2.15) | --- | p = 0.5 |
| Week 40 | -1.84 (-0.88 to -2.47) | -1.78* (-1.40 to -2.14) | --- | p = 0.7 |
| Week 44 | -1.81* (-0.93 to -2.43) | -1.87* (-1.32 to -2.34) [N=6] | --- | p = 0.8 |
| Week 48 | -1.89* (-0.91 to -2.44) | --- | --- | --- |
ⱡ subjects switched to AB-729 60 mg Q12W after Week 20 dose
* Data updated since EASL ILC™ presentation
- The efficacy and safety data for AB-729 derived from up to one year of dosing support our view that 60 mg every 8 weeks is an appropriate dose to move forward in the upcoming Phase 2a clinical trials.
- Additionally, based on 3/5 evaluable subjects, long term dosing with AB-729 demonstrated increased HBV-specific immune responses, providing support for combination therapy including immunomodulatory agents.
- Arbutus expects to provide additional data from ongoing cohorts of the Phase 1a/1b clinical trial in the second half of 2021, including initial data for a 90 mg every 12 weeks cohort in HBV DNA negative subjects and initial data in a 90 mg every 8 weeks cohort in HBV DNA positive subjects.
- In July 2021, Arbutus received authorization from the U.S. Food and Drug Administration to proceed with its Investigational New Drug (IND) application for AB-729 in a Phase 2 proof-of-concept clinical trial to evaluate AB-729 in combination with ongoing NA therapy and short courses of Peg-IFNα-2a in subjects with chronic HBV infection. This clinical trial is expected to initiate in the second half of 2021.
- To further support AB-729 as a potential cornerstone therapeutic in future HBV combination regimens, Arbutus has entered into several clinical collaborations to evaluate AB-729 in combination with other agents:
- Through a collaboration with Assembly Biosciences, Inc. (“Assembly”), subjects are being enrolled in a Phase 2 proof-of-concept clinical trial with a triple combination of AB-729, Assembly’s lead HBV core inhibitor (capsid inhibitor) product candidate, vebicorvir (“VBR”), and nucleos(t)ide analog (“NA”) therapy for the treatment of people with chronic HBV.
- In July 2021, we entered into a clinical collaboration with Vaccitech plc (“Vaccitech”) to evaluate a triple combination of AB-729 with Vaccitech’s proprietary immunotherapeutic, VTP-300, and standard-of-care NA therapy for the treatment of subjects with chronic HBV infection. We expect to file a Clinical Trial Application (CTA) in the second half of 2021 and initiate the clinical trial in early 2022.
- In June 2021, we entered into a clinical collaboration with Antios Therapeutics, Inc. (“Antios”) to evaluate a triple combination of AB-729, Antios’ proprietary active site polymerase inhibitor nucleotide (ASPIN), ATI-2173, and Viread (tenofovir disoproxil fumarate), for the treatment of subjects with chronic HBV infection. This clinical trial is expected to initiate in the second half 2021.
- Through a collaboration with Assembly Biosciences, Inc. (“Assembly”), subjects are being enrolled in a Phase 2 proof-of-concept clinical trial with a triple combination of AB-729, Assembly’s lead HBV core inhibitor (capsid inhibitor) product candidate, vebicorvir (“VBR”), and nucleos(t)ide analog (“NA”) therapy for the treatment of people with chronic HBV.
AB-836: Oral Capsid Inhibitor
- In January 2020, Arbutus selected AB-836, from a novel chemical series, as its next-generation oral capsid inhibitor. At EASL, Arbutus presented pre-clinical data suggesting the potential for increased efficacy and an enhanced resistance profile relative to previous generation capsid inhibitors. Arbutus completed CTA/IND-enabling studies in the fourth quarter of 2020 and initiated a Phase 1a/1b clinical trial for AB-836 in the first quarter of 2021. Initial data from healthy volunteers and HBV subjects from this clinical trial is expected in second half of 2021.
HBV Discovery Programs
- Arbutus’ drug discovery efforts are focused on follow-on compounds for its current HBV pipeline. Arbutus expects to continue to advance its research in its oral PD-L1 inhibitor and RNA-destabilizer programs.
Research Efforts to Combat COVID-19 and Future Coronavirus Outbreaks
- Based on its extensive antiviral drug discovery experience, Arbutus has established an internal research program to identify new small molecule antiviral medicines to treat COVID-19 and future coronavirus outbreaks. This effort, led by Dr. Michael Sofia, Arbutus’ Chief Scientific Officer, is focused on the discovery and development of new molecular entities that address specific viral targets including the nsp12 viral polymerase and the nsp5 viral protease. These targets are essential viral proteins which Arbutus has experience in targeting. Arbutus recently entered into a discovery research and license agreement with X-Chem, Inc. and Proteros biostructures GmbH focused on the discovery of novel inhibitors targeting the SARS-CoV-2 nsp5 main protease (Mpro). The agreement is designed to accelerate the development of pan-coronavirus agents to treat COVID-19 and potential future coronavirus outbreaks.
Financial Results
Cash, Cash Equivalents and Investments
Arbutus had cash, cash equivalents and investments totaling $121.3 million as of June 30, 2021, as compared to $123.3 million as of December 31, 2020. During the six months ended June 30, 2021, Arbutus used $31.9 million in operating activities, which was offset by $30.7 million of net proceeds from the issuance of common shares under Arbutus’s “at-the-market” offering program. The Company believes its cash, cash equivalents and investments of $121.3 million as of June 30, 2021 are sufficient to fund the Company’s operations through the third quarter of 2022.
Net Loss
Net loss attributable to common shares for the three months ended June 30, 2021 was $22.7 million ($0.23 basic and diluted loss per common share) as compared to $17.1 million ($0.25 basic and diluted loss per common share) for the three months ended June 30, 2020. Net loss attributable to common shares for the three months ended June 30, 2021 and 2020 included non-cash expense for the accrual of coupon on the Company’s convertible preferred shares of $3.3 million and $3.0 million, respectively.
Operating Expenses
Research and development expenses were $15.4 million for the three months ended June 30, 2021 compared to $10.5 million in the same period in 2020. The increase in research and development expenses for the three months ended June 30, 2021 versus the same period in 2020 was due primarily to higher expenses for the Company’s clinical development and discovery programs, including activities under our collaboration with Assembly and internal research efforts to treat COVID-19 and future coronavirus outbreaks, both of which initiated in mid-2020. General and administrative expenses were $4.4 million for the three months ended June 30, 2021 compared to $3.6 million for the same period in 2020. This increase was due primarily to increases in non-cash stock-based compensation expense and professional fees.
Outstanding Shares
The Company had approximately 97.7 million common shares issued and outstanding as of June 30, 2021. In addition, the Company had approximately 13.3 million stock options outstanding and 1.164 million convertible preferred shares outstanding, which (including the annual 8.75% coupon) will be mandatorily convertible into approximately 23 million common shares on October 18, 2021.
COVID-19 Impact
In December 2019 an outbreak of a novel strain of coronavirus (COVID-19) was identified in Wuhan, China. This virus continues to spread globally, has been declared a pandemic by the World Health Organization and has spread to nearly every country in the world. The impact of this pandemic has been, and will likely continue to be, extensive in many aspects of society. The pandemic has resulted in and will likely continue to result in significant disruptions to businesses. A number of countries and other jurisdictions around the world have implemented extreme measures to try and slow the spread of the virus. These measures include the closing of businesses and requiring people to stay in their homes, the latter of which raises uncertainty regarding the ability to travel to hospitals in order to participate in clinical trials. Additional measures that have had, and will likely continue to have, a major impact on clinical development, at least in the near-term, include shortages and delays in the supply chain, and prohibitions in certain countries on enrolling subjects in new clinical trials. While we have been able to progress with our clinical and pre-clinical activities to date, it is not possible to predict if the COVID-19 pandemic will materially impact our plans and timelines in the future.
| UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF LOSS | |||||||||||||||
| (in thousands, except share and per share data) | |||||||||||||||
| Three Months Ended June 30, | Six Months Ended June 30, | ||||||||||||||
| 2021 | 2020 | 2021 | 2020 | ||||||||||||
| Revenue | |||||||||||||||
| Collaborations and licenses | $ | 1,185 | $ | 825 | $ | 2,339 | $ | 1,660 | |||||||
| Non-cash royalty revenue | 1,144 | 689 | 2,103 | 1,345 | |||||||||||
| Total Revenue | 2,329 | 1,514 | 4,442 | 3,005 | |||||||||||
| Operating expenses | |||||||||||||||
| Research and development | 15,396 | 10,465 | 28,766 | 20,881 | |||||||||||
| General and administrative | 4,445 | 3,566 | 8,292 | 7,119 | |||||||||||
| Depreciation | 436 | 501 | 879 | 1,001 | |||||||||||
| Change in fair value of contingent consideration | 694 | 116 | 823 | 228 | |||||||||||
| Site consolidation | — | 7 | — | 64 | |||||||||||
| Loss from operations | (18,642 | ) | (13,141 | ) | (34,318 | ) | (26,288 | ) | |||||||
| Other income (loss) | |||||||||||||||
| Interest income | 31 | 200 | 70 | 545 | |||||||||||
| Interest expense | (763 | ) | (1,099 | ) | (1,535 | ) | (2,140 | ) | |||||||
| Foreign exchange gain (loss) | (13 | ) | (47 | ) | 15 | (65 | ) | ||||||||
| Total other loss | (745 | ) | (946 | ) | (1,450 | ) | (1,660 | ) | |||||||
| Net loss | (19,387 | ) | (14,087 | ) | (35,768 | ) | (27,948 | ) | |||||||
| Dividend accretion of convertible preferred shares | (3,266 | ) | (2,995 | ) | $ | (6,478 | ) | $ | (5,973 | ) | |||||
| Net loss attributable to common shares | $ | (22,653 | ) | $ | (17,082 | ) | (42,246 | ) | (33,921 | ) | |||||
| Loss per share | |||||||||||||||
| Basic and diluted | $ | (0.23 | ) | $ | (0.25 | ) | $ | (0.44 | ) | $ | (0.49 | ) | |||
| Weighted average number of common shares | |||||||||||||||
| Basic and diluted | 96,869,805 | 69,604,726 | 95,153,545 | 68,656,566 | |||||||||||
| UNAUDITED CONDENSED CONSOLIDATED BALANCE SHEETS | |||||||
| (in thousands) | |||||||
| June 30, 2021 | December 31, 2020 | ||||||
| Cash, cash equivalents and marketable securities, current | $ | 78,379 | $ | 123,268 | |||
| Accounts receivable and other current assets | 5,087 | 4,436 | |||||
| Total current assets | 83,466 | 127,704 | |||||
| Property and equipment, net of accumulated depreciation | 6,779 | 6,927 | |||||
| Investments in marketable securities, non-current | 42,906 | — | |||||
| Right of use asset | 2,225 | 2,405 | |||||
| Other non-current assets | — | 44 | |||||
| Total assets | $ | 135,376 | $ | 137,080 | |||
| Accounts payable and accrued liabilities | $ | 8,352 | $ | 8,901 | |||
| Liability-classified options | 132 | 250 | |||||
| Lease liability, current | 357 | 390 | |||||
| Total current liabilities | 8,841 | 9,541 | |||||
| Liability related to sale of future royalties | 18,982 | 19,554 | |||||
| Contingent consideration | 4,249 | 3,426 | |||||
| Lease liability, non-current | 2,475 | 2,593 | |||||
| Total stockholders’ equity | 100,829 | 101,966 | |||||
| Total liabilities and stockholders’ equity | $ | 135,376 | $ | 137,080 | |||
| UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOW | |||||||
| (in thousands) | |||||||
| Six Months Ended June 30, | |||||||
| 2021 | 2020 | ||||||
| Net loss | $ | (35,768 | ) | $ | (27,948 | ) | |
| Other non-cash items | 5,005 | 5,114 | |||||
| Changes in working capital | (1,127 | ) | (1,420 | ) | |||
| Net cash used in operating activities | (31,890 | ) | (24,254 | ) | |||
| Net cash (used in) provided by investing activities | (20,526 | ) | 20,970 | ||||
| Net cash provided by financing activities | 31,163 | 17,440 | |||||
| Effect of foreign exchange rate changes on cash and cash equivalents | (44 | ) | (56 | ) | |||
| (Decrease) increase in cash and cash equivalents | (21,297 | ) | 14,100 | ||||
| Cash and cash equivalents, beginning of period | 52,251 | 31,799 | |||||
| Cash and cash equivalents, end of period | 30,954 | 45,899 | |||||
| Investments in marketable securities | 90,331 | 38,089 | |||||
| Cash, cash equivalents and marketable securities, end of period | $ | 121,285 | $ | 83,988 | |||
Conference Call and Webcast Today
Arbutus will hold a conference call and webcast today, Thursday, August 5, 2021 at 8:45 AM Eastern Time to provide a corporate update. You can access a live webcast of the call, which will include presentation slides, through the Investors section of Arbutus’ website at www.arbutusbio.com or directly at Live Webcast. Alternatively, you can dial (866) 393-1607 or (914) 495-8556 and reference conference ID 2719108.
An archived webcast will be available on the Arbutus website after the event. Alternatively, you may access a replay of the conference call by calling (855) 859-2056 or (404) 537-3406, and reference conference ID 2719108.
About AB-729
AB-729 is an RNA interference (RNAi) therapeutic targeted to hepatocytes using Arbutus’ novel covalently conjugated N-acetylgalactosamine (GalNAc) delivery technology that enables subcutaneous delivery. AB-729 inhibits viral replication and reduces all HBV antigens, including hepatitis B surface antigen in preclinical models. Reducing hepatitis B surface antigen is thought to be a key prerequisite to enable reawakening of a patient’s immune system to respond to the virus. Based upon clinical data generated thus far in an ongoing single- and multi-dose Phase 1a/1b clinical trial, AB-729 has demonstrated positive safety and tolerability data and meaningful reductions in hepatitis B surface antigen.
About AB-836
AB-836 is an oral HBV capsid inhibitor. HBV core protein assembles into a capsid structure, which is required for viral replication. The current standard-of-care therapy for HBV, primarily nucleos(t)ide analogues that work by inhibiting the viral polymerase, significantly reduce virus replication, but not completely. Capsid inhibitors inhibit replication by preventing the assembly of functional viral capsids. They also have been shown to inhibit the uncoating step of the viral life cycle thus reducing the formation of new covalently closed circular DNA (cccDNA), the genetic reservoir which the virus uses to replicate itself.
About HBV
Hepatitis B is a potentially life-threatening liver infection caused by HBV. HBV can cause chronic infection which leads to a higher risk of death from cirrhosis and liver cancer. Chronic HBV infection represents a significant unmet medical need. The World Health Organization estimates that over 250 million people worldwide suffer from chronic HBV infection, while other estimates indicate that approximately 2 million people in the United States suffer from chronic HBV infection. Approximately 900,000 people die every year from complications related to chronic HBV infection despite the availability of effective vaccines and current treatment options.
About Arbutus
Arbutus Biopharma Corporation is a publicly traded (Nasdaq: ABUS) biopharmaceutical company primarily focused on discovering, developing and commercializing a cure for people with chronic hepatitis B virus (HBV) infection. The Company is advancing multiple product candidates with distinct mechanisms of action that it believes have the potential to provide a new curative regimen for chronic HBV infection. Arbutus has also initiated a drug discovery and development effort for treating coronaviruses (including COVID-19). For more information, visit www.arbutusbio.com.
Forward-Looking Statements and Information
This press release contains forward-looking statements within the meaning of the Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, and forward-looking information within the meaning of Canadian securities laws (collectively, “forward-looking statements”). Forward-looking statements in this press release include statements about our future development plans for AB-729 and AB-836, including our expectations that in the second half of 2021 we will (a) have additional data from the ongoing Phase 1a/1b clinical trial with AB-729, (b) initiate two Phase 2a proof-of-concept clinical trials for AB-729, and (c) have initial Phase 1a/1b data for AB-836; our expectation to file a CTA in the second half of 2021 and initiate another proof-of-concept clinical trial in early 2022; our expectations and goals for our collaborations with third parties and any potential benefits related thereto; the potential for AB-836 to have increased efficacy and an enhanced resistance profile; our expected cash runway through the third quarter of 2022; and our expectations regarding the impact of the COVID-19 pandemic on our business and clinical trials.
With respect to the forward-looking statements contained in this press release, Arbutus has made numerous assumptions regarding, among other things: the effectiveness and timeliness of preclinical studies and clinical trials, and the usefulness of the data; the timeliness of regulatory approvals; the continued demand for Arbutus’ assets; and the stability of economic and market conditions. While Arbutus considers these assumptions to be reasonable, these assumptions are inherently subject to significant business, economic, competitive, market and social uncertainties and contingencies, including uncertainties and contingencies related to the ongoing COVID-19 pandemic.
Additionally, there are known and unknown risk factors which could cause Arbutus’ actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements contained herein. Known risk factors include, among others: anticipated pre-clinical studies and clinical trials may be more costly or take longer to complete than anticipated, and may never be initiated or completed, or may not generate results that warrant future development of the tested product candidate; Arbutus may elect to change its strategy regarding its product candidates and clinical development activities; Arbutus may not receive the necessary regulatory approvals for the clinical development of Arbutus’ products; economic and market conditions may worsen; Arbutus and its collaborators may never realize the expected benefits of the collaborations; market shifts may require a change in strategic focus; and the ongoing COVID-19 pandemic could significantly disrupt Arbutus’ clinical development programs.
A more complete discussion of the risks and uncertainties facing Arbutus appears in Arbutus’ Annual Report on Form 10-K, Arbutus’ Quarterly Reports on Form 10-Q and Arbutus’ continuous and periodic disclosure filings, which are available at www.sedar.com and at www.sec.gov. All forward-looking statements herein are qualified in their entirety by this cautionary statement, and Arbutus disclaims any obligation to revise or update any such forward-looking statements or to publicly announce the result of any revisions to any of the forward-looking statements contained herein to reflect future results, events or developments, except as required by law.
Contact Information
Investors and Media
William H. Collier
President and CEO
Phone: 267-469-0914
Email: [email protected]
Pam Murphy
Investor Relations Consultant
Phone: 267-469-0914
Email: [email protected]
EXHIBIT 99.2

Corporate Presentation August 2021 NASDAQ: ABUS www.arbutusbio.com

NASDAQ: ABUS www.arbutusbio.com Forward - Looking Statements 2 This presentation contains forward - looking statements within the meaning of the U . S . Private Securities Litigation Reform Act of 1995 and Canadian securities laws . All statements that are not historical facts are hereby identified as forward - looking statements for this purpose and include, among others, statements relating to : the potential market opportunity for HBV ; Arbutus’ ability to meet a significant unmet medical need ; the sufficiency of Arbutus’ cash and cash equivalents to extend through the third quarter of 2022 ; the potential for AB - 729 to be a well - tolerated low dose treatment for HBV with a minimum of injections ; the potential for AB - 836 to be low dose with a greater therapeutic window and to address known capsid resistant variants T 33 N and I 105 T ; the potential for AB - 836 to be once daily dosing ; Arbutus’ expectations regarding the timing and clinical development of Arbutus’ product candidates including its 2021 key clinical objectives with respect to AB - 729 and AB - 836 and its clinical collaborations with Assembly Biosciences, Antios Therapeutics and Vaccitech ; the timeline to a combination cure for HBV ; Arbutus’ coronavirus strategy ; Arbutus’ expectations regarding its technology licensed to Genevant ; and other statements relating to our future operations, future financial performance, future financial condition, prospects or other future events . With respect to the forward - looking statements contained in this presentation, Arbutus has made numerous assumptions regarding, among other things : the timely receipt of expected payments ; the effectiveness and timeliness of preclinical studies and clinical trials, and the usefulness of the data ; the timeliness of regulatory approvals ; the continued demand for Arbutus’ assets ; and the stability of economic and market conditions . While Arbutus considers these assumptions to be reasonable, these assumptions are inherently subject to significant business, economic, competitive, market and social uncertainties and contingencies including uncertainties and contingencies related to the ongoing COVID - 19 pandemic . Forward - looking statements herein involve known and unknown risks, uncertainties and other factors that may cause the actual results, events or developments to be materially different from any future results, events or developments expressed or implied by such forward - looking statements . Such factors include, among others : anticipated pre - clinical and clinical trials may be more costly or take longer to complete than anticipated, and may never be initiated or completed, or may not generate results that warrant future development of the tested drug candidate ; changes in Arbutus’ strategy regarding its product candidates and clinical development activities ; Arbutus may not receive the necessary regulatory approvals for the clinical development of Arbutus' products ; economic and market conditions may worsen ; market shifts may require a change in strategic focus ; the parties may never realize the expected benefits of the collaborations ; and the ongoing COVID - 19 pandemic could significantly disrupt our clinical development programs . A more complete discussion of the risks and uncertainties facing Arbutus appears in Arbutus' Annual Report on Form 10 - K, Quarterly Report on Form 10 - Q and Arbutus' periodic disclosure filings which are available at www . sec . gov and at www . sedar . com . All forward - looking statements herein are qualified in their entirety by this cautionary statement, and Arbutus disclaims any obligation to revise or update any such forward - looking statements or to publicly announce the result of any revisions to any of the forward - looking statements contained herein to reflect future results, events or developments, except as required by law .
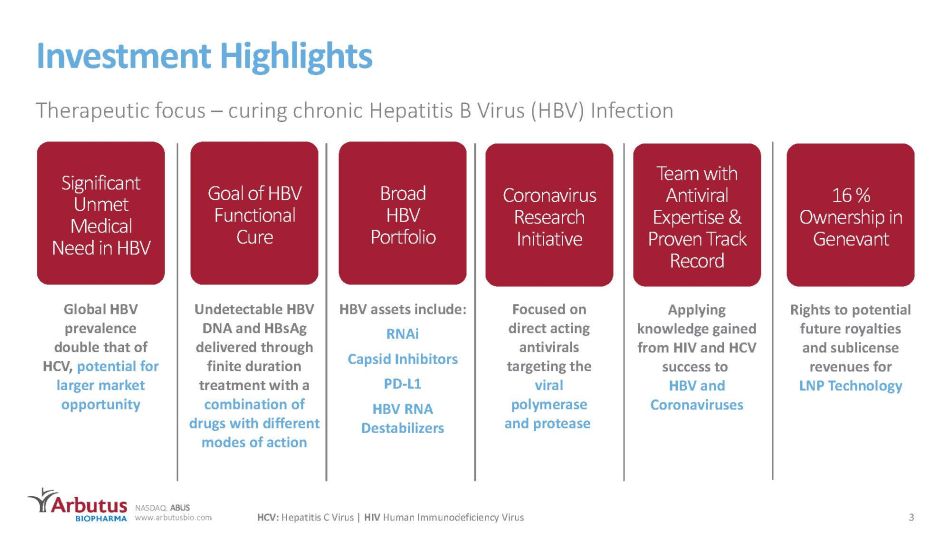
NASDAQ: ABUS www.arbutusbio.com Investment Highlights Therapeutic focus – curing chronic Hepatitis B Virus (HBV) Infection 3 HCV: Hepatitis C Virus | HIV Human Immunodeficiency Virus Significant Unmet Medical Need in HBV Undetectable HBV DNA and HBsAg delivered through finite duration treatment with a combination of drugs with different modes of action Goal of HBV Functional Cure Broad HBV Portfolio HBV assets include: RNAi Capsid Inhibitors PD - L1 HBV RNA Destabilizers Coronavirus Research Initiative Focused on direct acting antivirals targeting the viral polymerase and protease Team with Antiviral Expertise & Proven Track Record Applying knowledge gained from HIV and HCV success to HBV and Coronaviruses Global HBV prevalence double that of HCV, potential for larger market opportunity 16 % Ownership in Genevant Rights to potential future royalties and sublicense revenues for LNP Technology

NASDAQ: ABUS www.arbutusbio.com NASDAQ: ABUS www.arbutusbio.com Proven Leadership Team 4 Successful track records in the discovery, development, and commercialization of multiple antivirals including sofosbuvir, etravirine, rilpivirine , telaprevir and simeprevir William H. Collier Chief Development Officer Chief Financial Officer President and CEO David C. Hastings Gaston Picchio, PhD Michael J. Sofia, PhD Chief Scientific Officer Chief Business Officer Michael J. McElhaugh EVP, General Counsel and Chief Compliance Officer Elizabeth Howard, PhD, JD

NASDAQ: ABUS www.arbutusbio.com Sources : Global Hepatitis Report and Hepatitis B Fact Sheet, WHO (2017) http://www.who.int/mediacentre/factsheets/fs204/en/ Kowdley et al. Hepatology (2012) Prevalence of Chronic Hepatitis B Among Foreign - Born Persons Living in the US by Country of Origin HBV Presents a Significant Unmet Medical Need 5 ~ 900k people die every y ear as a consequence despite the availability of effective vaccines and antivirals. >257M people are chronically infected with HBV, globally. 90M China 15M Europe 2M United States
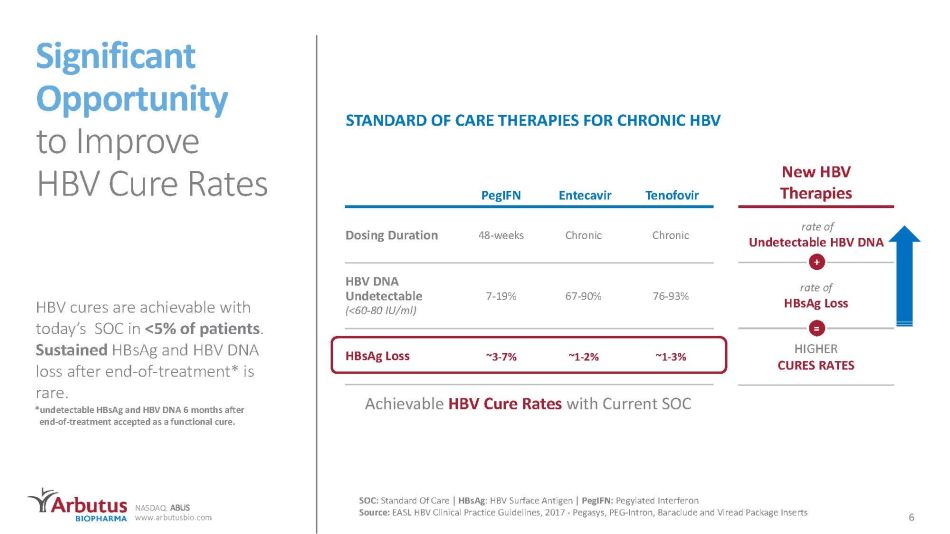
NASDAQ: ABUS www.arbutusbio.com New HBV Therapies rate of Undetectable HBV DNA rate of HBsAg Loss HIGHER CURES RATES SOC: Standard Of Care | HBsAg : HBV Surface Antigen | PegIFN : P egylated Interferon Source: EASL HBV Clinical Practice Guidelines, 2017 - Pegasys, PEG - Intron, Baraclude and Viread Package Inserts Significant Opportunity to Improve HBV Cure Rates PegIFN Entecavir Tenofovir Dosing Duration 48 - weeks Chronic Chronic HBV DNA Undetectable (<60 - 80 IU/ml) 7 - 19% 67 - 90% 76 - 93% HBsAg Loss ~3 - 7% ~1 - 2% ~1 - 3% Achievable HBV Cure Rates with Current SOC + = HBV cures are achievable with today’s SOC in <5% of patients . Sustained HBsAg and HBV DNA loss after end - of - treatment* is rare. 6 * undetectable HBsAg and HBV DNA 6 months after end - of - treatment accepted as a functional cure. STANDARD OF CARE THERAPIES FOR CHRONIC HBV

NASDAQ: ABUS www.arbutusbio.com An HBV curative regimen would substantially increase diagnosis and treatment rates to unlock significant market growth opportunities. diagnosis Compelling Growth Opportunity in the HBV Market 7 SOC: Standard Of Care Source: Global Hepatitis Report and Hepatitis B Fact Sheet, WHO (2017) http://www.who.int/mediacentre/factsheets/fs204/en/ 10.5% Diagnosed 1.8% Treated Low due to sub - optimal SOC cure rate and asymptomatic nature of disease. treatment 27M 257M chronic HBV 4.5M

NASDAQ: ABUS www.arbutusbio.com 3 2 1 2 HBV Lifecycle Illustrates Key Points for Intervention A combination of agents with complementary MOA is needed to cure HBV 1. Nucleoside Analogue 2. Capsid Inhibitor 3. RNAi & RNA Destabilizer NASDAQ: ABUS www.arbutusbio.com MOA: Mechanism of Action

NASDAQ: ABUS www.arbutusbio.com 9 Block Replication ▪ NA ▪ Capsid Inhibitor ▪ RNAi ▪ RNA Destabilizer Reduce cccDNA Pool ▪ Capsid Inhibitor Block HBsAg ▪ RNAi ▪ RNA Destabilizer Immuno - modulation ▪ PD - L1 Inhibitor ▪ Interferon ▪ Therapeutic vaccines Leading to an HBV CURE Keys to Therapeutic Success MOA: Mechanism of Action | NA: Nucleoside Analogue | HBsAg : HBV Surface Antigen Suppress HBV DNA and viral antigens Reawaken host immune response Therapeutic success will require a combination of agents with complementary MOAs Reduce/Suppress Viral DNA 1 Reawaken / Boost Host Immune Response 3 Block HBsAg ▪ RNAi ▪ RNA Destabilizer Reduce/Suppress Viral Antigens 2

NASDAQ: ABUS www.arbutusbio.com Arbutus Pipeline 10 Phase I HBV Lead Optimization CTA/I ND Enabling Healthy Subjects HBV Subjects Phase II HBsAg Reduction RNAi AB - 729 HBV RNA Destabilizers Next Gen HBV DNA Suppression Capsid Inhibitor AB - 836 Immune Reawakening PD - L1 1st gen COVID - 19/Coronaviruses Lead Optimization CTA/I ND Enabling Phase I Phase II Pan - Coronavirus Agent

NASDAQ: ABUS www.arbutusbio.com AB - 729 RNAi Therapeutic 11 Proprietary GalNAc - conjugate delivery technology provides liver targeting and enables subcutaneous dosing Single trigger RNAi agent targeting all HBV transcripts Inhibits HBV replication and lowers all HBV antigens Pan - genotypic activity across HBV genotypes Demonstrated complementarity with capsid inhibitors Actively targets the liver Active against cccDNA derived and integrated HBsAg transcripts Clean profile in long term preclinical safety studies H N HN O O HN O HO HO NH OH O O HO HO NH OH O O HO HO NH OH O O O O O H N O O O O O O O O O N O OHO O Ligand U conjugate "Linker U" Linker GalNAc n Polymerase, Core Ag, eAg , pgRNA sAg sAg HBx
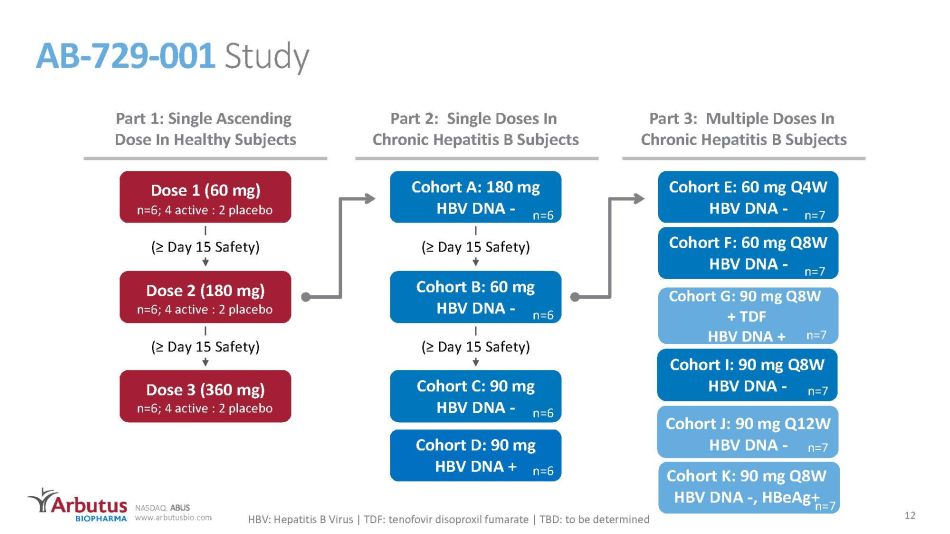
NASDAQ: ABUS www.arbutusbio.com Cohort J: 90 mg Q12W HBV DNA - AB - 729 - 001 Study Part 1: Single Ascending Dose In Healthy Subjects Dose 1 (60 mg) Dose 2 (180 mg) Dose 3 (360 mg) (≥ Day 15 Safety) n=6; 4 active : 2 placebo n=6; 4 active : 2 placebo n=6; 4 active : 2 placebo Cohort A: 180 mg HBV DNA - Cohort B: 60 mg HBV DNA - Cohort C: 90 mg HBV DNA - (≥ Day 15 Safety) (≥ Day 15 Safety) (≥ Day 15 Safety) Cohort D: 90 mg HBV DNA + n=6 n=6 n=6 n=6 Cohort E: 60 mg Q4W HBV DNA - Cohort F: 60 mg Q8W HBV DNA - n=7 n=7 n=7 Part 2: Single Doses In Chronic Hepatitis B Subjects Part 3: Multiple Doses In Chronic Hepatitis B Subjects HBV: Hepatitis B Virus | TDF: tenofovir disoproxil fumarate | TBD: to be determined Cohort I: 90 mg Q8W HBV DNA - n=7 n=7 12 Cohort G: 90 mg Q8W + TDF HBV DNA + Cohort K: 90 mg Q8W HBV DNA - , HBeAg + n=7

NASDAQ: ABUS www.arbutusbio.com Single Doses of AB - 729 Result in Comparable Mean HBsAg Declines at Week 12 Followed by a Sustained Plateau Phase W e e k 1 2 -2.5 -2 -1.5 -1 -0.5 0 0.5 0 4 8 12 16 20 24 28 32 Cohort=A W e e k 1 2 -2.5 -2 -1.5 -1 -0.5 0 0.5 0 4 8 12 16 20 24 28 32 Cohort=C W e e k 1 2 -2.5 -2 -1.5 -1 -0.5 0 0.5 0 4 8 12 16 20 24 28 32 Cohort=B AB - 729 60 mg single dose (N=6) * AB - 729 90 mg single dose (N=6) ** AB - 729 180 mg single dose (N=4) # Week 12 mean (SE): - 0.99 (0.24) Week 12 mean (SE): - 1.23 (0.18) Week 12 mean (SE): - 0.98 (0.43) 3/6 HBsAg <100 IU/mL 1/6 HBsAg <10 IU/mL 5/6 HBsAg <100 IU/mL 1/6 HBsAg <10 IU/mL 0/4 HBsAg <100 IU/mL mean individual *N=5 at Week 10, 14, 18, 22, 28, and 32 **N=4 at Week 14 and 16; N=3 at Weeks 18 – 24 # N=3 after Week 12; nominal visits ± 7 days 13
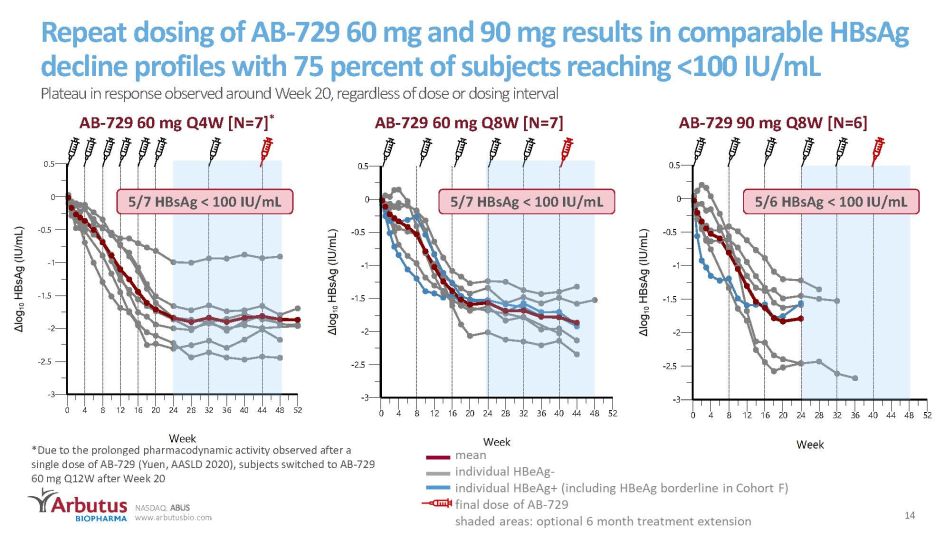
NASDAQ: ABUS www.arbutusbio.com 14 -3 -2.5 -2 -1.5 -1 -0.5 0 0.5 0 4 8 1216202428323640444852 CHG vs NominalWeek 3006-3027 5004-3034 6001-3011 6001-3019 6001-3021 6001-3022 8001-3018 mean Repeat dosing of AB - 729 60 mg and 90 mg results in comparable HBsAg decline profiles with 75 percent of subjects reaching <100 IU/mL Plateau in response observed around Week 20, regardless of dose or dosing interval AB - 729 60 mg Q4W [N=7] * AB - 729 60 mg Q8W [N=7] AB - 729 90 mg Q8W [N=6] -3 -2.5 -2 -1.5 -1 -0.5 0 0.5 0 4 8 1216202428323640444852 CHG vs NominalWeek 6001-3010 8001-3007 8001-3008 8001-3009 8001-3014 8001-3015 8001-3016 mean *Due to the prolonged pharmacodynamic activity observed after a single dose of AB - 729 (Yuen, AASLD 2020), subjects switched to AB - 729 60 mg Q12W after Week 20 5/7 HBsAg < 100 IU/mL 5/7 HBsAg < 100 IU/mL 5/6 HBsAg < 100 IU/mL

NASDAQ: ABUS www.arbutusbio.com There are no significant differences in mean HBsAg response between AB - 729 doses and dosing intervals to date Visit Cohort E AB - 729 60 mg Q4W ⱡ Cohort F AB - 729 60 mg Q8W Cohort I AB - 729 90 mg Q8W p value between Cohorts Week 16 - 1.44 ( - 0.71 to - 1.95) - 1.39 ( - 1.61 to - 1.08) - 1.63 ( - 0.89 to - 2.44) p ≥ 0.4 Week 24 - 1.84 ( - 0.99 to - 2.31) - 1.57 ( - 1.24 to - 2.01) - 1.79 ( - 1.22 to - 2.46) p ≥ 0.2 Week 32 - 1.84 ( - 0.94 to - 2.36) - 1.68 ( - 1.37 to - 2.15) --- p = 0.5 Week 40 - 1.84 ( - 0.88 to - 2.47) - 1.78* ( - 1.40 to - 2.14) --- p = 0.7 Week 44 - 1.81* ( - 0.93 to - 2.43) - 1.87* ( - 1.32 to - 2.34) [N=6] --- p = 0.8 Week 48 - 1.89* ( - 0.91 to - 2.44) --- --- --- Mean (range) ∆HBsAg with repeat dosing of AB - 729 ⱡ subjects switched to AB - 729 60 mg Q12W after Week 20 dose *Data updated since EASL 2021 ILC -2.5 -2 -1.5 -1 -0.5 0 0 8 16 24 32 40 48 CHG vs NominalWeek P3E P3F P3I -2.5 -2 -1.5 -1 -0.5 0 0 8 16 24 32 40 48 Cohort E (N=7) Cohort F (N=7) Cohort I (N=6) 15

NASDAQ: ABUS www.arbutusbio.com AB - 729 90 mg Single Dose Reduces HBsAg and HBV DNA in HBV DNA Positive CHB subjects 16 Figure 1. Individual and mean change from baseline HBsAg following a single dose of AB - 729 90 mg in HBV DNA+ subjects -3 -2 -1 0 1 2 0 4 8 12162024283236404448 Figure 2. Individual and mean change from baseline HBV DNA following a single dose of AB - 729 90 mg in HBV DNA+ subjects -3 -2 -1 0 1 2 0 4 8 12162024283236404448 Week 12 Mean (SE) : - 1.02 (0.13) Week 24 Mean (SE): - 1.05 (0.12) Week 36 Mean (SE): - 0.812 (0.09) Week 44 Mean (SE): - 0.657 (0.10) 3/5 subjects HBsAg < 100 IU/mL at nadir Week 12 Mean (SE) : - 1.53 (0.24) Week 24 Mean (SE): - 0.525 (0.37) Week 36 Mean (SE): - 0.693 (0.07) -3 -2 -1 0 1 2 0 4 8 12162024283236404448 deltaHBsAg vs Nominal… 4001-2059 4001-2066 8001-2047 8001-2061 individual mean -3 -2 -1 0 1 2 0 4 8 12162024283236404448 deltaDNA vs NominalW… 4001-2059 4001-2066 8001-2047 8001-2061 individual mean

NASDAQ: ABUS www.arbutusbio.com AB - 729 Was Safe and Well Tolerated After Single and Repeat Doses ▪ No SAEs or discontinuations due to AEs ▪ No treatment - related Grade 3 or 4 AEs* ▪ No Grade 3 or 4 laboratory abnormalities* ▪ Grade 1 and Grade 2 ALT elevations have decreased with continued treatment ▪ Injection site TEAEs were mild (erythema, pain, pruritis, bruising) or moderate (pain) and transient ▪ No clinically meaningful changes in ECGs or vital signs ▪ All subjects in cohort E and F consented to an additional 6 months of dosing * 1 subject (Cohort A) with rapid decline in HBsAg of ~2.0 log10 IU/mL had an unrelated Gr 2 AE of food poisoning resulting in unrelated transient Grade 3 AEs of ALT/AST elevation (without bilirubin changes) 17

NASDAQ: ABUS www.arbutusbio.com Takeaways ▪ Clinical data supports our view that AB - 729 60 mg every 8 weeks is an appropriate and convenient dose to explore in Phase 2a combination trials ▪ Long - term dosing with AB - 729 resulted in 75 percent of subjects reaching <100 IU/mL of HBsAg, a clinically relevant threshold informing when to stop all therapies ▪ Preliminary data suggest that long - term suppression of HBsAg with AB - 729 results in increased HBV - specific immune response ▪ AB - 729 was safe and well tolerated through 48 weeks of dosing ▪ Based on these findings, we have announced and expect to initiate two proof - of - concept Phase 2a combination trials using AB - 729 as the cornerstone agent in 2H/2021 18

NASDAQ: ABUS www.arbutusbio.com ▪ Accomplished key strategic initiative by announcing three Phase 2a proof - of - concept clinical collaborations to accelerate key combination data read - outs ▪ Assembly Biosciences, Inc. - Phase 2a initiated in the first half of 2021 ▪ Antios Therapeutics, Inc. - collaboration announced in June 2021, clinical trial expected to initiate in the second half of 2021 ▪ Vaccitech plc - collaboration announced in July 2021, clinical trial expected to initiate in early 2022 ▪ In addition to these collaborations, the AB - 729 IND was authorized to initiate a Phase 2a trial in combination with ongoing NA therapy and short courses of Peg - IFNα - 2a in chronic hepatitis B subjects 19 Three Clinical Collaborations Executed and IND Authorized to Leverage AB - 729 in Key Proof - Of - Concept Phase 2a Trials

NASDAQ: ABUS www.arbutusbio.com NA: Nucleoside Analogue | HBeAg : HBV e Antigen AB - 729 Clinical Collaboration Provides accelerated AB - 729 combination proof - of - concept (POC) with Assembly’s capsid inhibitor and a NA 20 Follow Up AB - 729 + vebicorvir + NA AB - 729 + NA vebicorvir + NA Baseline Wk 72 Wk 48 Wk 24 Initiated Phase 2 Clinical Trial Feb 2021 ~60 virologically - suppressed subjects with chronic HBV infection Equal sharing of expertise and costs for this POC open - label trial No financial requirements or restrictions and no business requirements or restrictions

NASDAQ: ABUS www.arbutusbio.com 21 Clinical trial will evaluate the safety, pharmacokinetics, immunogenicity and anti - viral activity of the triple combination of AB - 729, VTP - 300 and an NA compared to the double combinations of AB - 729 with an NA and VTP - 300 with an NA Expected to file CTA in the second half of 2021 and initiate in early 2022 Full rights retained by the Companies of their respective product candidates and all costs will be split equally Assuming positive results parties intend to undertake a larger Phase 2b clinical trial POC Phase 2a clinical trial Evaluating AB - 729 in combination with Vaccitech’s immunotherapeutic, VTP - 300, and a NA AB - 729 Clinical Collaboration

NASDAQ: ABUS www.arbutusbio.com 22 AB - 729 Clinical Collaboration Clinical trial will evaluate AB - 729, ATI - 2173 and a NA in a single cohort in the ongoing Antios Phase 2a ANTT201 clinical trial Expected to initiate in the second half of 2021 Antios will be responsible for the costs and Arbutus will be responsible for supply of AB - 729 Trial cohort will include 10 subjects with chronic HBV assigned 8:2 to active drug or matching placebos; in combination with an NA POC Phase 2a clinical trial AB - 729 in combination with Antios’ proprietary active site polymerase inhibitor nucleotide (ASPIN), ATI - 2173, and a NA

NASDAQ: ABUS www.arbutusbio.com 23 IND Authorized for a Phase 2a POC clinical trial The trial is expected to enroll 40 stably NA - suppressed, HBeAg negative, non - cirrhotic CHB subjects* After a 24 - week dosing period of AB - 729 ( 60 mg every 8 weeks (Q 8 W)), subjects will be randomized into one of 4 groups : ▪ A1: AB - 729 + NA + weekly Peg - IFNα - 2a for 24 weeks (N = 12) ▪ A2: NA + weekly Peg - IFNα - 2a for 24 weeks (N = 12) ▪ B1: AB - 729 + NA + weekly Peg - IFNα - 2a for 12 weeks (N = 8) ▪ B2: NA + weekly Peg - IFNα - 2a for 12 weeks (N = 8) After completion of the assigned Peg - IFNα - 2 a treatment period, all subjects will remain on NA therapy for the initial 24 - week follow up period, and then will discontinue NA treatment if treatment stopping criteria are met Expected to initiate in the third quarter of 2021 AB - 729 in combination with ongoing NA therapy and short courses of Peg - IFNα - in CHB subjects * Pending protocol finalization

NASDAQ: ABUS www.arbutusbio.com Novel chemical series differentiated from AB - 506 and other competitor compounds in the Class II capsid inhibitor space Leverages a novel binding site within the core protein dimer - dimer interface Improved intrinsic potency with EC50 < 10 nM Active against NA resistant variants Potential to address known capsid resistant variants T33N and I105T Provides the potential for low dose and wide therapeutic window Demonstrates high liver concentrations in multiple species Projected to be once daily dosing Pangenotypic Combinable with other MOA agents AB - 836 Capsid Inhibitor In March 2021, received regulatory approval to initiate Phase 1a/1b clinical trial Potential for increased efficacy and enhanced resistance profile relative to earlier generation capsid inhibitors 24

NASDAQ: ABUS www.arbutusbio.com AB - 836: A Next Generation Capsid Inhibitor HBV DNA / 1 o Mechanism cccDNA Formation / 2 o Mechanism Human Serum Shift Compound HepDE19 ; EC 50 μM Ϳ HBV infected PHH ; EC 50 μM Ϳ HBV infected HepG2 - NTCP - C4 ; EC 50 μM) Core I105T Mutation (EC 50 μM ) HBV infected HepG2 - NTCP - C4 ; HBsAg EC 50 μM Ϳ (FC in EC 50 in 40% Human Serum ) AB - ϱϬϲ 0.077 0.032 0.101 1.26 1.430 6x AB - 836 0.010 0.002 0.012 0.118 0.196 2x 3 10 100 0 1 2 3 4 H B V D N A L O G I n h i b i t i o n ( D a y 7 v s V e h i c l e ) (mg/kg QD) AB-836 AB-506 Serum Activity 3 10 100 0 1 2 3 4 H B V D N A L O G I n h i b i t i o n ( D a y 7 v s V e h i c l e ) (mg/kg QD) AB-836 AB-506 Liver Activity in HDI Mouse Model Unique Binding Site HAP: Heteroaryldihydropyrimidine | SBA: Sulfamoylbenzamide I PHH: Primary Human Hepatocytes H A P S B A A B - 5 0 6 25

NASDAQ: ABUS www.arbutusbio.com AB - 836 - 001 Study 26 Dose 6 ≤600mg Dose 4 ≤200mg Dose 3 ≤100mg Dose 2 ≤35mg Dose 1 10mg Dose 5 ≤400mg Cohort A Cohort B Dose 5 FE ≤400mg Dose 7 ≤800mg Part 1: Single Ascending Dose In Healthy Subjects Alternating Cohorts A and B n=8/cohort; 6 active: 2 placebo Part 2: Multiple Ascending Dose in Healthy Subjects Cohort C (≤ 75mg QD) x 10 days N = 10; 8 active: 2 placebo Cohort D (≤200mg QD) x 10 days N = 10; 8 active: 2 placebo Cohort E (≤ 400mg QD) x 10 days N = 10; 8 active: 2 placebo Part 3: Multiple Doses In Chronic Hepatitis B Subjects Cohort F (≤ Cohort C) x 28 days DNA + N = 12; 10 active: 2 placebo Cohort G (≤ Cohort D) x 28 days DNA+ N = 12; 10 active: 2 placebo Cohort H (Dose TBD) x 28 days DNA+ N = 12; 10 active: 2 placebo Cohort I (Dose TBD) + NA x 28 days DNA - N = 12; 10 active: 2 placebo Cohort J (Dose TBD) + TDF x 28 days DNA+ N = 12; 10 active: 2 placebo

NASDAQ: ABUS www.arbutusbio.com Next Gen RNA Destabilizer Program We believe this approach offers potential for an oral HBsAg reducing agent and all oral combination therapy Continuing active research and development of a next generation small molecule Offers a novel mechanism of action to reduce HBsAg and other viral proteins and viral RNA 27
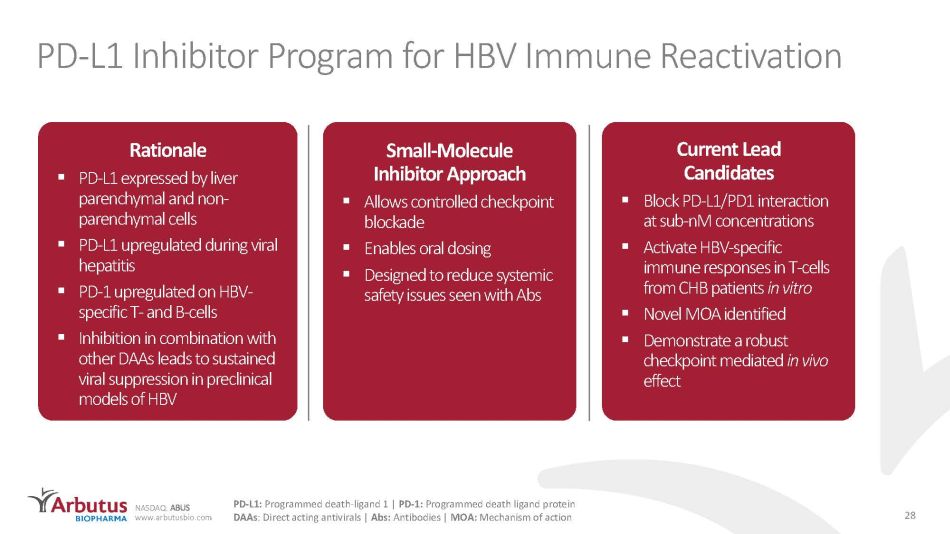
NASDAQ: ABUS www.arbutusbio.com PD - L1 Inhibitor Program for HBV Immune Reactivation Current Lead Candidates ▪ Block PD - L1/PD1 interaction at sub - nM concentrations ▪ Activate HBV - specific immune responses in T - cells from CHB patients in vitro ▪ Novel MOA identified ▪ Demonstrate a robust checkpoint mediated in vivo effect Small - Molecule Inhibitor Approach ▪ Allows controlled checkpoint blockade ▪ Enables oral dosing ▪ Designed to reduce systemic safety issues seen with Abs Rationale ▪ PD - L1 expressed by liver parenchymal and non - parenchymal cells ▪ PD - L1 upregulated during viral hepatitis ▪ PD - 1 upregulated on HBV - specific T - and B - cells ▪ Inhibition in combination with other DAAs leads to sustained viral suppression in preclinical models of HBV 28 PD - L1: Programmed death - ligand 1 | PD - 1: Programmed death ligand protein DAAs : Direct acting antivirals | Abs: Antibodies | MOA: Mechanism of action

NASDAQ: ABUS www.arbutusbio.com Long term commitment Pan - coronavirus focused Small Molecule Direct - Acting Antivirals Directed Effort ▪ nsp12 Viral Polymerase - nucleos (t)ides ▪ nsp5 Main Viral Protease - de novo design X - Chem/ Proteros ▪ Proprietary DEL library screening and structural biology for M PRO inhibitor discovery Coronavirus Strategy Leveraging our proven expertise and capabilities in antiviral drug discovery and development COVID - 19 Virus nsp5 / 3CLpro Viral Polymerase Viral Protease +RNA Virus 31 kb Genome nsp5 protease & nsp12 polymerase essential enzymes for replication 29

NASDAQ: ABUS www.arbutusbio.com 2021 Key Objectives Cash balance of $121.3M as of June 30, 2021, cash runway through 3Q 2022 Objective Anticipated Timing 2021 Additional data from AB - 729 90 mg single - dose in HBV DNA positive subjects 1H Initiate a Phase 2 combination clinical trial to evaluate AB - 729 in combination with Assembly Biosciences’ lead core/capsid inhibitor candidate vebicorvir (VBR) and an NrtI 1H Initiate a Phase 1a/1b clinical trial of AB - 836, our next - generation oral capsid inhibitor 1H Additional data from AB - 729 60 mg multi - dose (4 wk / 8 wk dosing intervals) 1H / 1H Initial data from AB - 729 90 mg multi - dose (8 wk / 12 wk dosing intervals) 1H / 2H Initial data from AB - 729 90 mg multi - dose (8 wk dosing interval) in HBV DNA positive subjects 2H Initiate two Phase 2a combination clinical trials in HBV subjects; both including AB - 729, with one or more approved or investigational agents 2H Initial Phase 1a/1b data for AB - 836 2H 30 x x x x x
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Arbutus to Report First Quarter 2024 Financial Results and Provide Corporate Update
- GenAI Enhancements Highlight the Next Wave of Product Innovation across the Tungsten Automation Portfolio of Solutions
- Allianz Prevents 29% More Fraud and Announces Partnership With Clearspeed
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share