Form 6-K NeuroSense Therapeutics For: Jul 05
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
Report of Foreign Private Issuer Pursuant to Rule 13a-16 or 15d-16
Under the Securities Exchange Act of 1934
For the Month of July 2022
Commission File Number: 001-41084
NeuroSense Therapeutics Ltd.
(Translation of registrant’s name into English)
11
HaMenofim Street, Building B
Herzliya 4672562 Israel
+972-9-799-6183
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): ☐
NeuroSense Therapeutics Ltd. (the “Company”) has made available a presentation about its business (the “Presentation”), a copy of which is furnished herewith as Exhibit 99.1 to this Report on Form 6-K. Please see additional slides inserted (23-25) and updates on slide 26. The furnishing of the Presentation is not an admission as to the materiality of any information therein. The information contained in the Presentation is summary information that should be considered in the context of the Company’s filings with the Ssecurities and Exchange Commission and other public announcements the Company may make by press release or otherwise from time to time.
Exhibit Index
| Exhibit No. | Description | |
| 99.1 | Presentation, dated July 2022 |
1
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| NeuroSense Therapeutics Ltd. | ||
| Date: July 5, 2022 | By: | /s/ Alon Ben-Noon |
| Alon Ben-Noon | ||
| Chief Executive Officer | ||
2
Exhibit 99.1
1 Corporate Presentation July 2022 Nasdaq: NRSN

2 Disclaimer This presentation and oral statements made regarding the subject of this presentation contain "forward - looking statements" withi n the meaning of the U.S. Private Securities Litigation Reform Act of 1995 that involve substantial risks and uncertainties. All statements co nta ined in this presentation other than statements of historical facts, including our business strategy and plans and objectives for future operations, in clu ding our financial performance, are forward looking statements. The words " anticipate"," believe," "continue," "estimate," "expect," "intend," "ma y," "will" and similar expressions are intended to identify forward looking statements. We have based these forward looking statements largely on ou r c urrent expectations and projections about future events and trends that we believe may affect our financial condition, results of op era tions, business strategy, short term and long term business operations and objectives, and financial needs. Forward looking statements made in this presentation include statements about the market for therapeutics targeting neurodege ner ative diseases and its opportunities for our product candidates; our expectations regarding our competitive advantages; the planned developm ent timeline of our product candidates; and characterizations of the pre - clinical and clinical trial results of our product candidates. Forward look ing statements are subject to a number of risks and uncertainties and represent our views as of the date of the presentation. The future events and trends discussed in this presentation may not occur and actual results could differ materially and adversely from those anticipated or implied in th e forward looking statements. You should not rely on these statements as representing our views in the future. More information about the risks an d uncertainties affecting the Company is contained under the heading "Risk Factors" in the Annual Report on Form 20 - F filed with the Securities and Exchange Commission on April 14, 2022. We undertake no obligation or duty to update information contained in these forward looking sta tem ents, whether as a result of new information, future events or otherwise. Trademarks in this presentation are the property of their respective owners and used for informational and educational purpos es only.
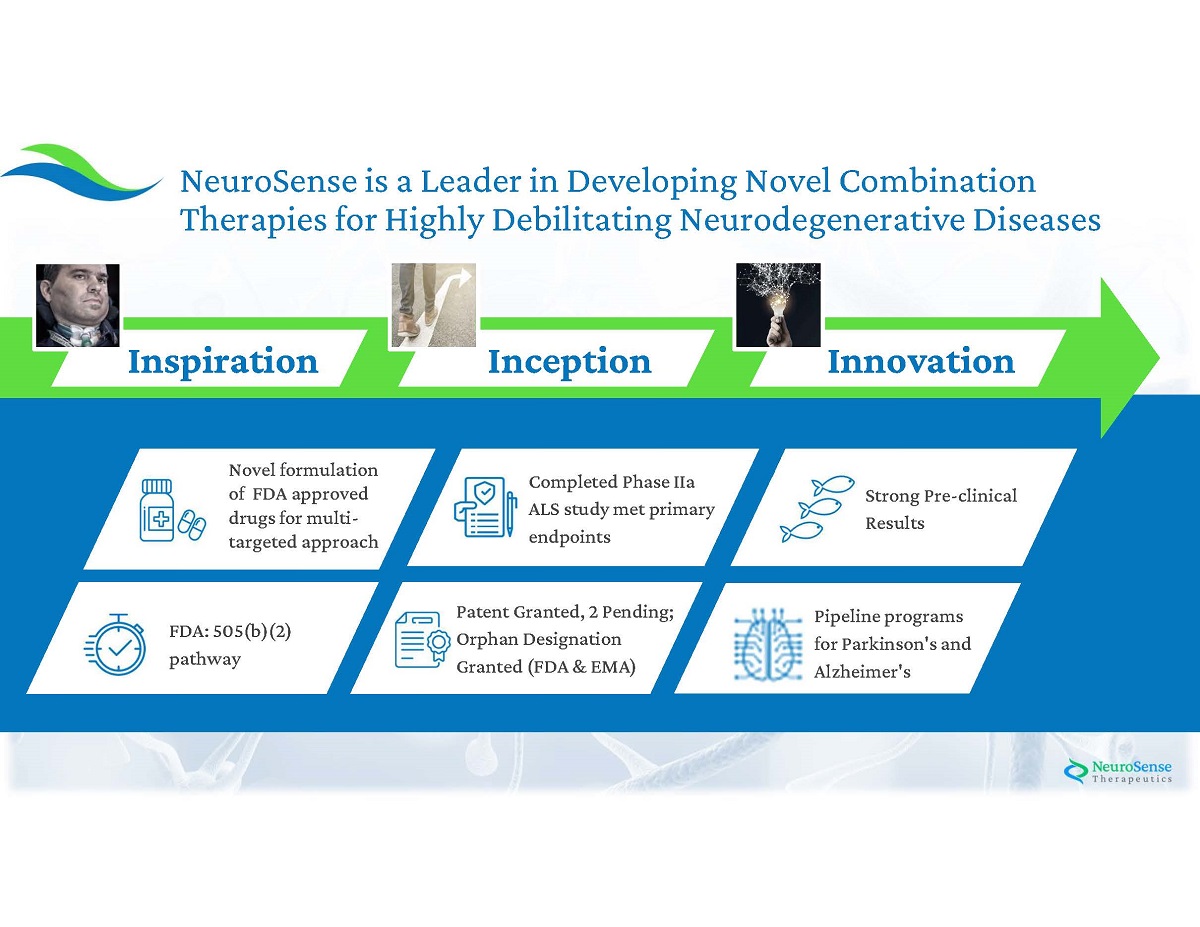
3 Strong Pre - clinical Results Completed Phase IIa ALS study met primary endpoints Patent Granted, 2 Pending; Orphan Designation Granted (FDA & EMA) Novel formulation of FDA approved drugs for multi - targeted approach FDA: 505 (b)( 2 ) pathway Pipeline programs for Parkinson's and Alzheimer's NeuroSense is a Leader in Developing Novel Combination Therapies for Highly Debilitating Neurodegenerative Diseases Inspiration In cep tion In novation
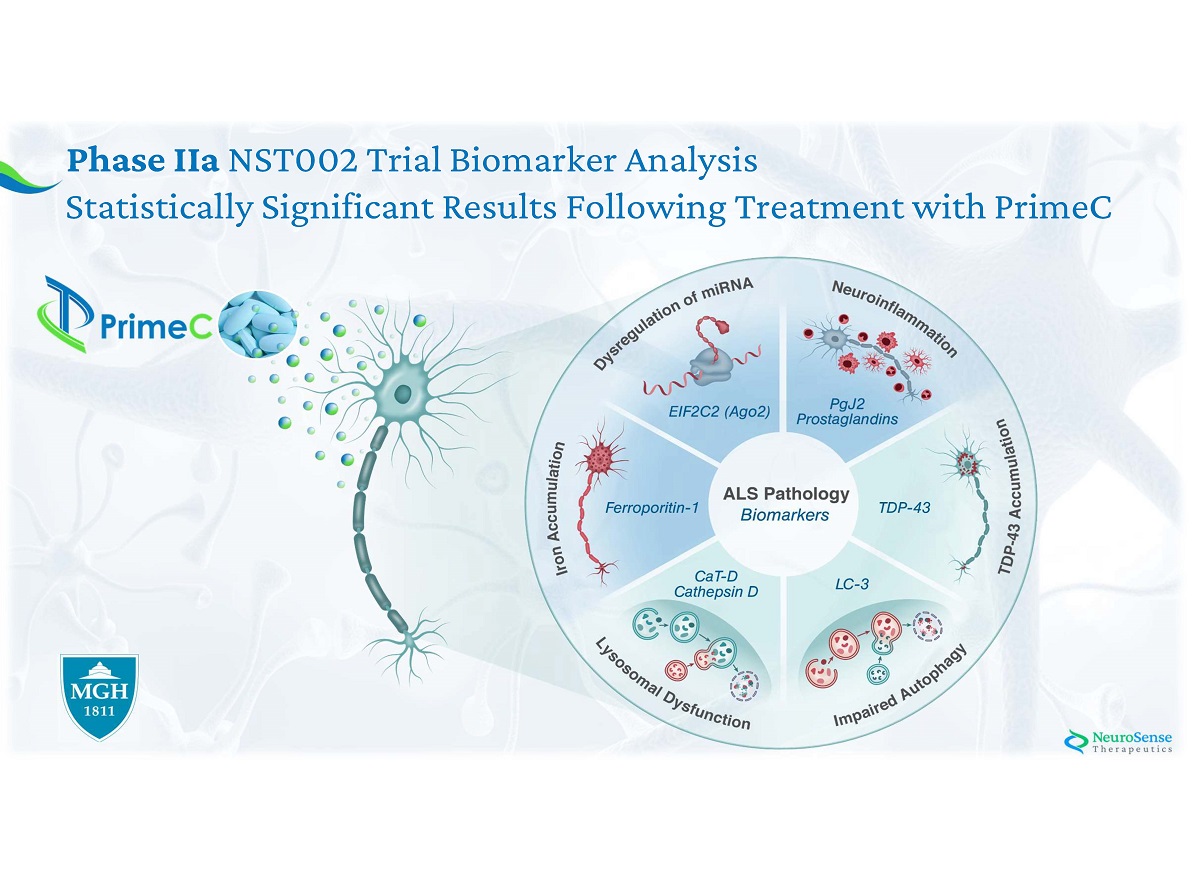
Phase IIa NST002 Trial Biomarker Analysis Statistically Significant Results Following Treatment with PrimeC
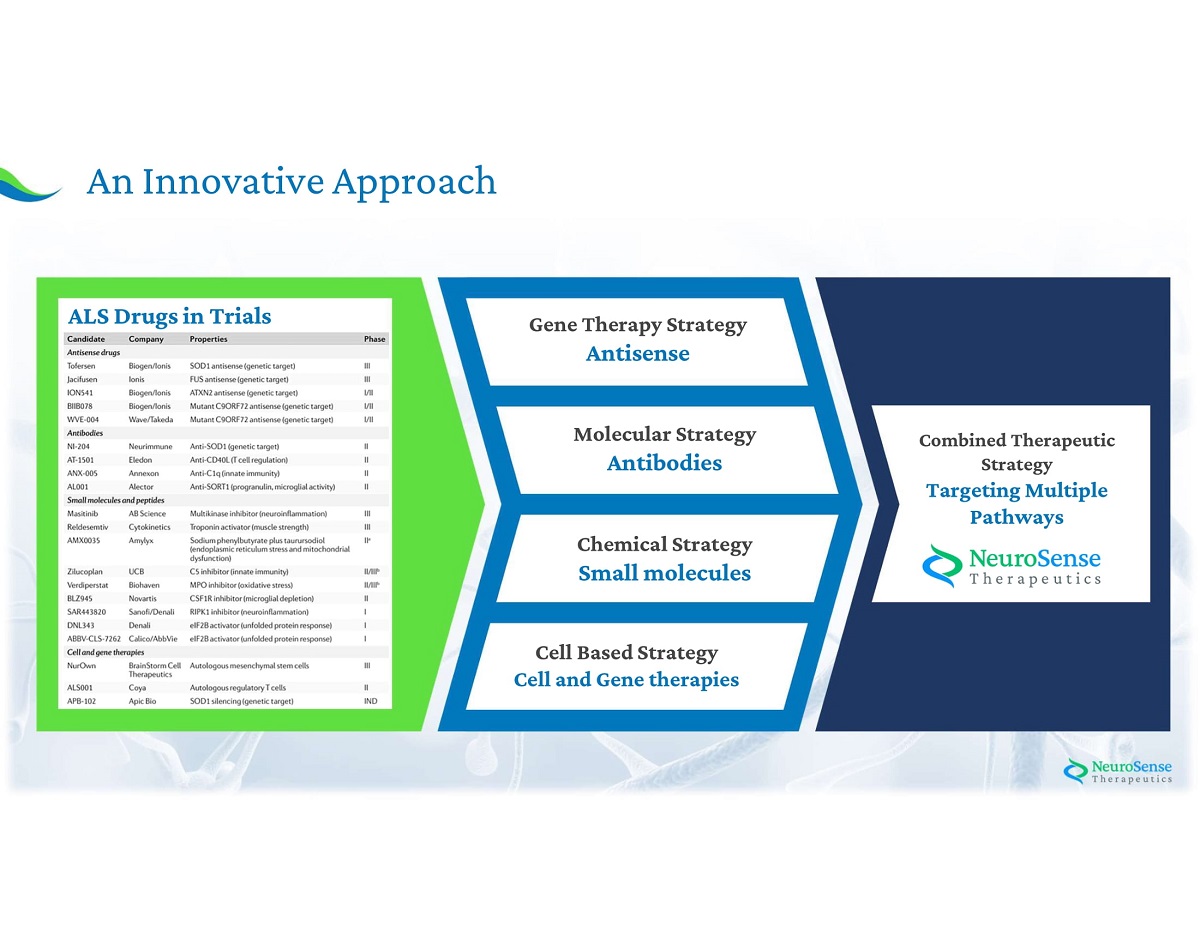
5 An Innovative Approach Gene Therapy Strategy Antisense Molecular Strategy Antibodies Chemical Strategy Small molecules Cell Based Strategy Cell and Gene therapies Combined Therapeutic Strategy Targeting Multiple Pathways ALS Drugs in Trials

6 Experienced Leadership = Head of Scientific Program Shiran Zimri, PhD CMO Ferenc Tracik, MD CEO, Board Member Alon Ben - Noon Chairman of the Board Mark Leuchtenberger CFO Or Eisenberg Niva Russek - Blum, PhD VP Discovery & IP Generator VP BD Nedira Salzman Head of ALS Program Avital Pushett

7 Scientific Advisory Board • Senior VP at the Barrow Neurological Institute • Chair of the Department of Neurology Prof. Jeremy Shefner (Chair) Dr. Jinsy Andrews • Associate Professor of Neurology, Division of Neuromuscular Medicine, Columbia University • Director of Neuromuscular Clinical Trials Prof. Merit Cudkowicz • Chief of Neurology at Mass General and Director, Sean M. Healey & AMG Center for ALS • Professor of Neurology at Harvard Medical School Dr. Jeffery Rosenfeld • Professor of Neurology and Associate Chairman of Neurology at Loma Linda University School of Medicine • Medical Director of Center for Restorative Neurology at Loma Linda University Prof. Orla Hardiman • Head of Academic Unit of Neurology at Trinity College Dublin and Consultant Neurologist at Beaumont • Co - Chair of the European Consortium to Cure ALS and Chair of the Scientific Committee of ENCALS
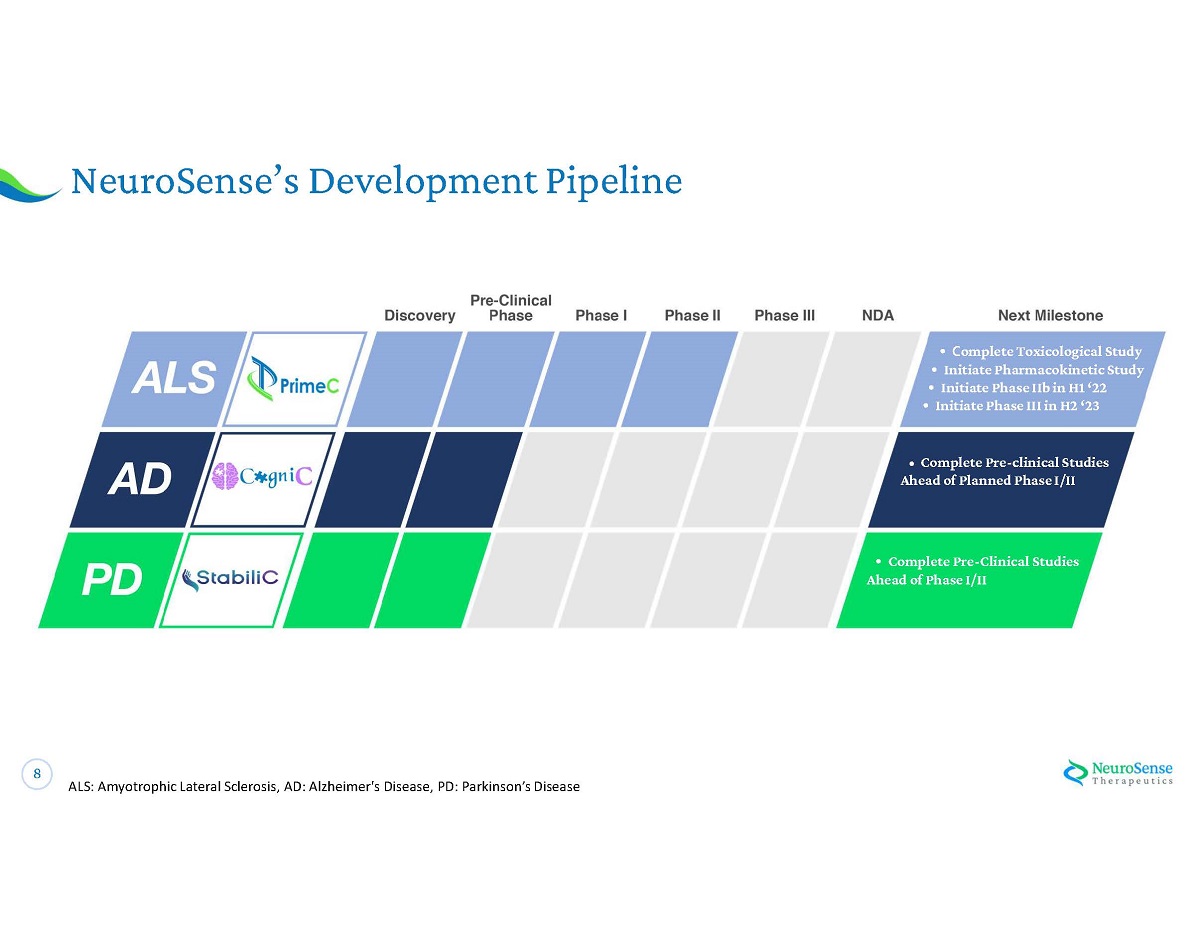
8 NeuroSense ’ s Development Pipeline ALS: Amyotrophic Lateral Sclerosis, AD: Alzheimer's Disease, PD: Parkinson ’ s Disease • C omplete Toxicological Study • Initiate Pharmacokinetic Study • Initiate Phase IIb in H 1 ‘ 22 • Initiate Phase III in H 2 ‘ 23 • Complete Pre - clinical Studies Ahead of Planned Phase I/II • Complete Pre - Clinical Studies Ahead of Phase I/II

9 ALS in Numbers = A nnual burden in the US alone = ~ 2 4 % Growth in Patients by 2040 in the US and EU = > 80 , 000 ALS Patients in NeuroSense ’ s planned target market = + 5 , 000 P eople are diagnosed with ALS each y ear (US) = ALS is an incurable neurodegenerative disease causing complete paralysis and ultimately death within 2 - 5 years from diagnosis >$ 1 B 1 2 2 3 1. ALS - Amyotrophic Lateral Sclerosis, Johns Hopkins Medicine 2. Projected increase in amyotrophic lateral sclerosis from 2015 to 2040 , Nature Communications, 2016 3. Cost of illness for neuromuscular diseases in the United States, Muscle & Nerve, 2013

10 The People Behind The Numbers Oct. 2016

11 Current Treatments Show Limited Efficacy = Current Treatments Phase 3 Clinical trials in the past 15 years have failed, we believe, due mainly to: A Combined Therapeutic Strategy Targeting Multiple Pathways A NEW APPROACH WAS NEEDED Single Target - Aiming for only one target in complex diseases with multiple mechanisms Two FDA approved drugs, Riluzole and Edaravone , both known to have mild effect on prolonging lifespan or improving patients ’ quality of life and independence

12 NeuroSense's Lead Candidate: PrimeC Two Compounds - One Potentially Powerful Outcome NeuroSense ’ s Flagship Treatment A novel formulation , consisting of specific doses of two FDA - approved drugs, Ciprofloxacin & Celecoxib, designed to work synergistically on more than one target : • Regulating microRNA synthesis • Affecting iron accumulation • Reducing neuroinflammation = 1 . Management estimate = $ 3.2 B Annual market potential 1
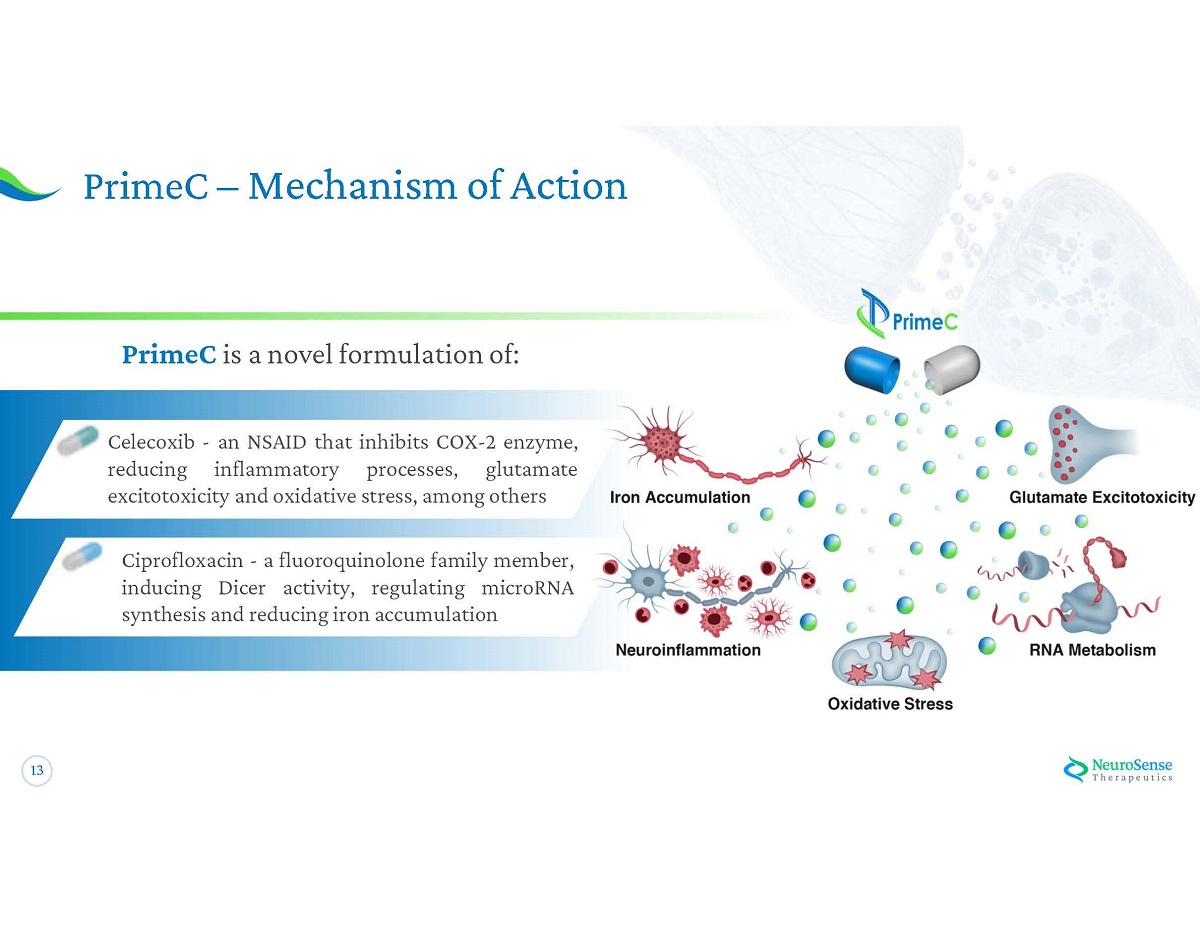
13 PrimeC – Mechanism of Action PrimeC is a novel formulation of : Celecoxib - an NSAID that inhibits COX - 2 enzyme, reducing inflammatory processes, glutamate excitotoxicity and oxidative stress, among others Ciprofloxacin - a fluoroquinolone family member, inducing Dicer activity, regulating microRNA synthesis and reducing iron accumulation

14 Pre - Clinical Results in a Key Animal Model PrimeC improved locomotor and cellular deficits of ALS zebrafish models, indicating a neuroprotective effect PrimeC treatment: x Improved motor performance of both SOD 1 and TDP - 43 , widely accepted models, by 84 % and 110 % respectively x Recovered impaired motor neurons morphology x Recovered abnormal neuromuscular junction structure x Preserved the ramified morphology of microglia cells 1 . Efficacy of Ciprofloxacin/Celecoxib combination in zebrafish models of amyotrophic lateral sclerosis, Annals of Clinical an d T ranslational Neurology, 2020 . 1
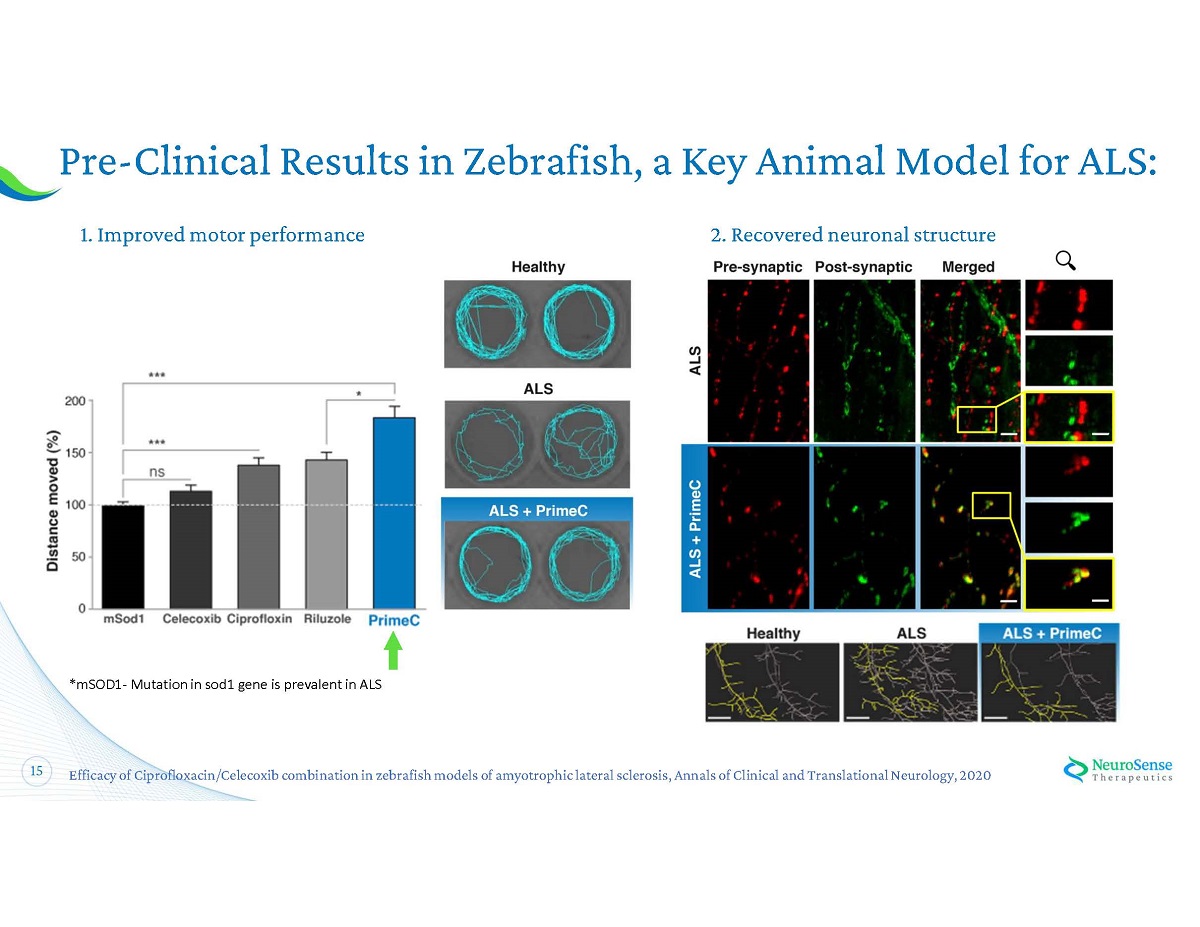
15 Pre - Clinical Results in Zebrafish, a Key Animal Model for ALS: 1 . Improved motor performance 2 . Recovered neuronal structure Efficacy of Ciprofloxacin/Celecoxib combination in zebrafish models of amyotrophic lateral sclerosis, Annals of Clinical and Tra nslational Neurology, 2020 *mSOD 1 - Mutation in sod 1 gene is prevalent in ALS

16 Phase 2 a Trial Design Prof. Vivian Drory NST 002 : Tel Aviv Sourasky Medical Center Phase IIa Safety Blood test, electrocardiogram ( ECG), urea, vital signs, adverse events ( AE) Efficacy ALSFRS - R - Revised ALS Functional Rating Scale – 0 - 48 FVC - Forced Vital Capacity Biomarkers Examination of key elements for ALS diagnosis as well as PrimeC mechanism of action NST 002 15 patients , 12 months dosing, clinic visit every 3 months, phone visit every 1.5 months

17 * P ooled R esource O pen - A ccess ALS C linical T rials Database All patients completing the trial have opted to continue into an extension study with PrimeC Exploratory Endpoint: PrimeC vs. PRO - ACT* (matched) The trial was conducted with an intermediate formulation and dose PrimeC Reduced the Rate of Decline

18 Phase IIa Clinical Trial: PrimeC intermediate formulation x Reduced Functional and Respiratory Deterioration x Significant changes in ALS - related biomarkers x Well Tolerated, No Drug Related SAEs Primary endpoint met, exploratory endpoints showed positive results with PrimeC
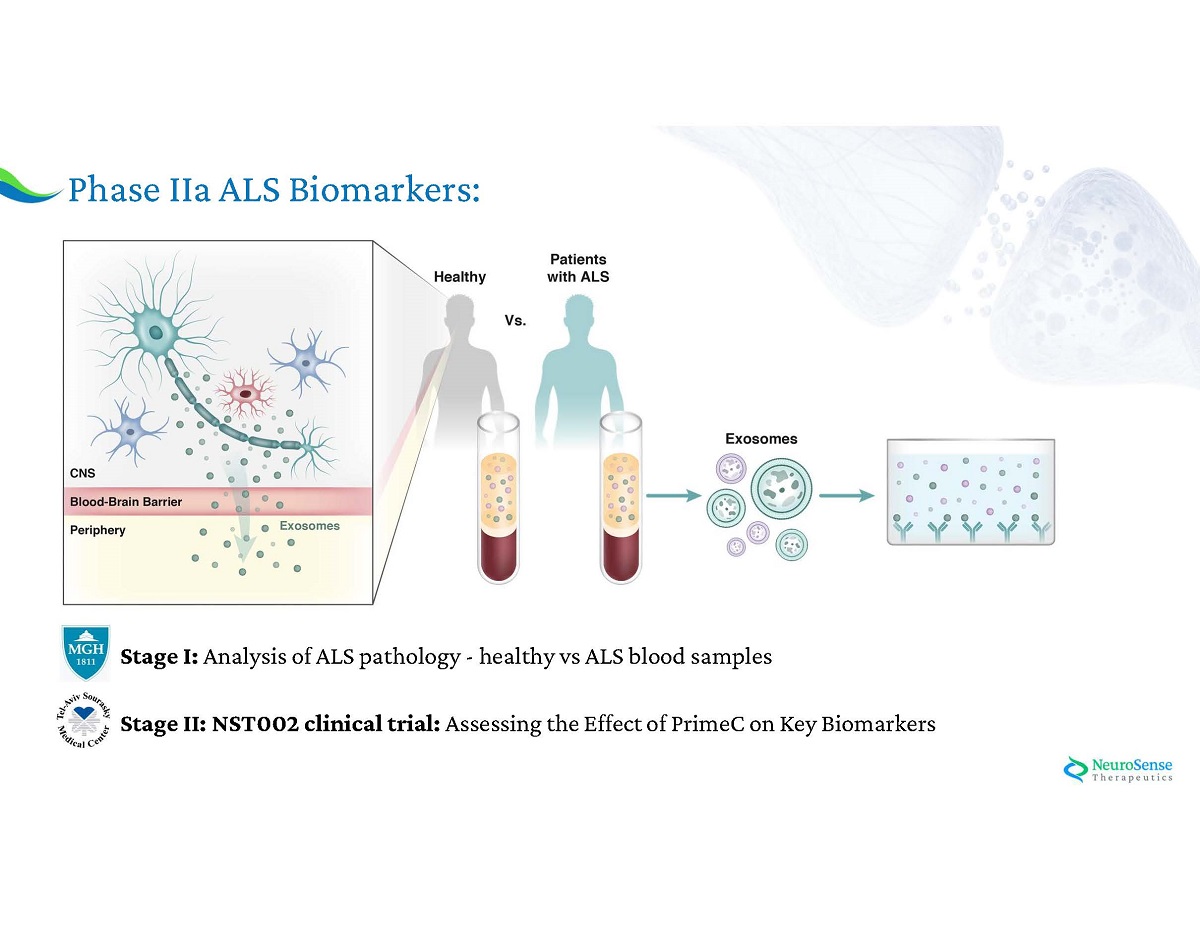
Phase IIa ALS Biomarkers: Stage I : Analysis of ALS pathology - healthy vs ALS blood samples Stage II : NST 002 clinical trial : Assessing the Effect of PrimeC on Key Biomarkers
Phase IIa NST 002 Trial Biomarker Analysis Statistically Significant Results Following Treatment with PrimeC PrimeC ’ s effect on Biomarkers may lead to: 1 . Patient Stratification 2 . Precision medicine In collaboration with Massachusetts General Hospital
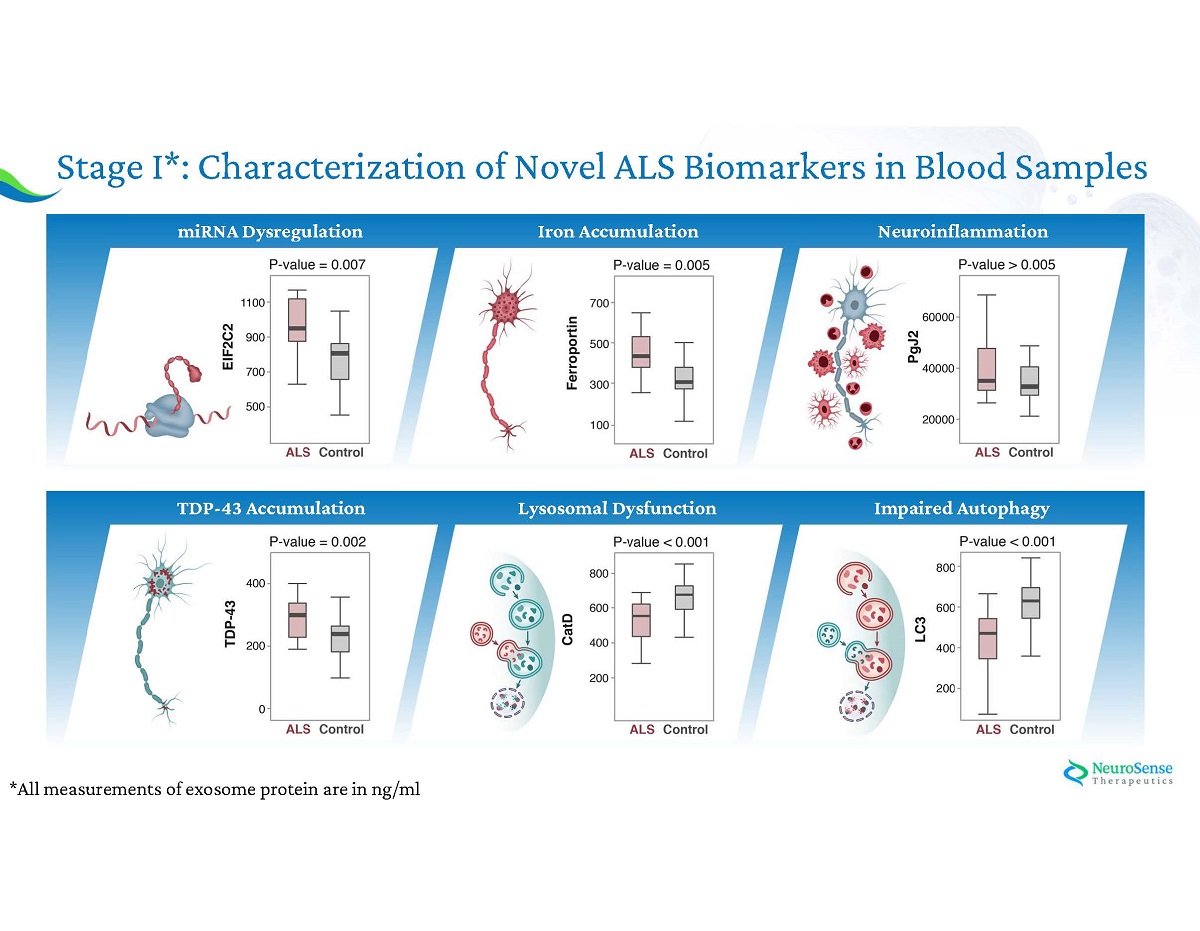
*All measurements of exosome protein are in ng/ml Stage I*: Characterization of Novel ALS Biomarkers in Blood Samples Neuroinflammation Impaired Autophagy Lysosomal Dysfunction Iron Accumulation TDP - 43 Accumulation miRNA Dysregulation
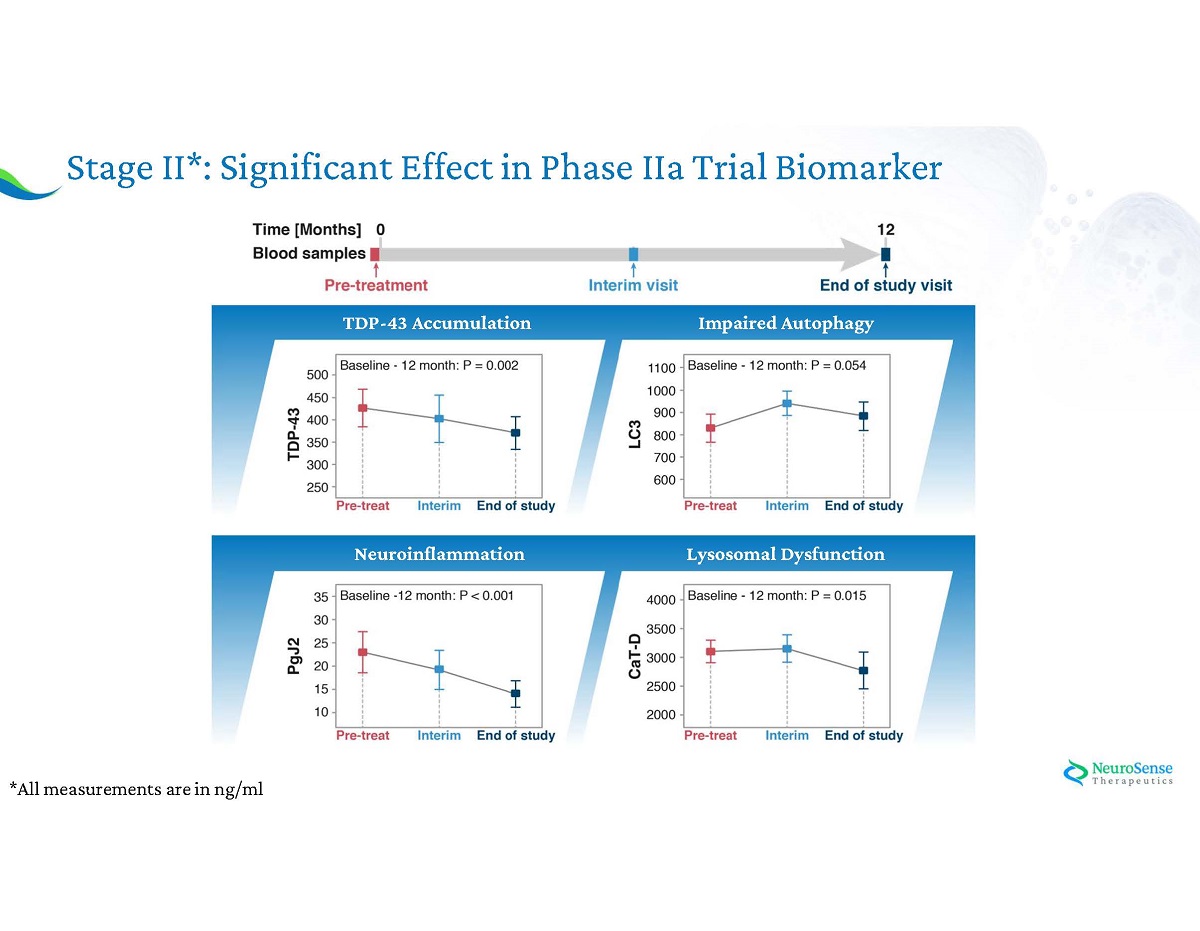
Stage II*: Significant Effect in Phase IIa Trial Biomarker *All measurements are in ng/ml Neuroinflammation Lysosomal Dysfunction Neuroinflammation Impaired Autophagy TDP - 43 Accumulation Lysosomal Dysfunction
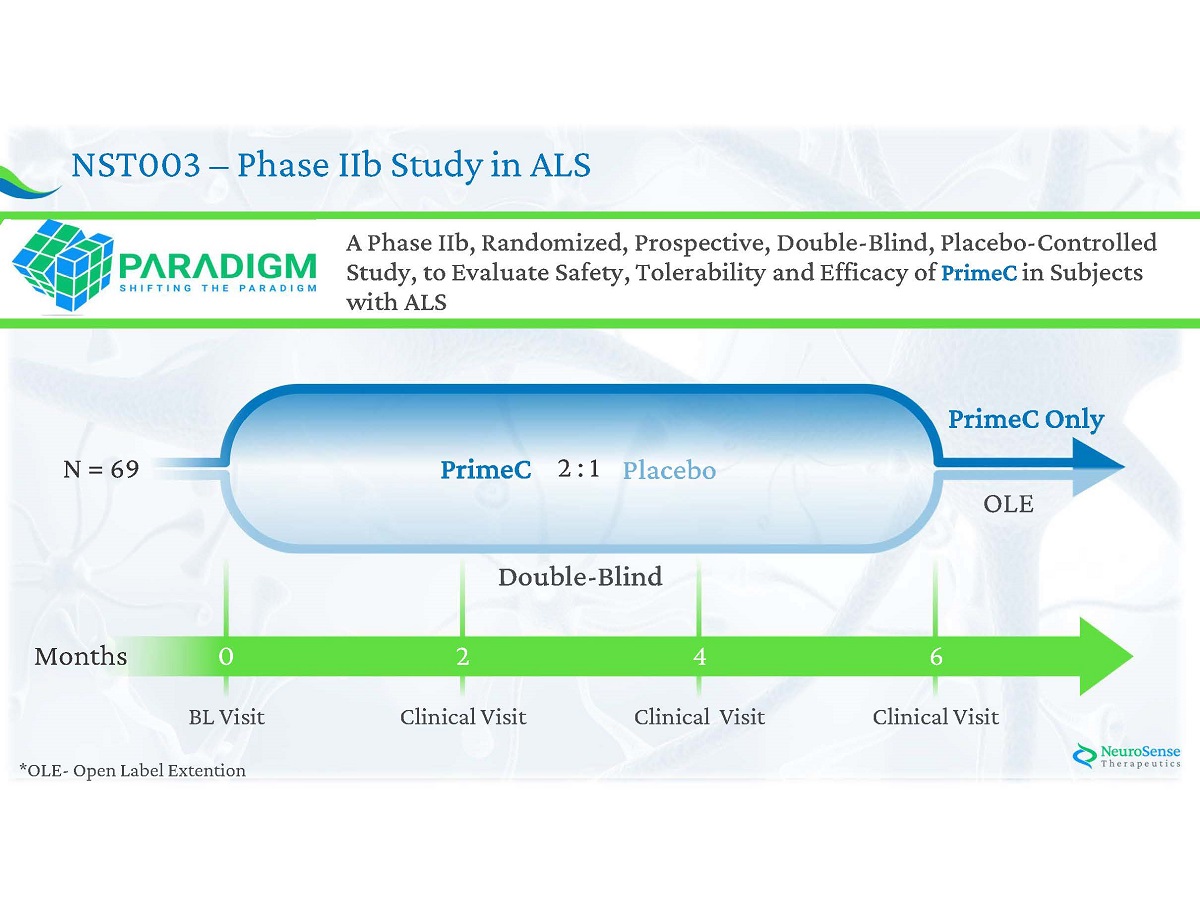
NST 003 – Phase II b Study in ALS Clinical Visit N = 69 2 : 1 0 Months 6 2 4 Clinical Visit Clinical Visit BL Visit OLE A Phase IIb, Randomized, Prospective, Double - Blind, Placebo - Controlled Study, to Evaluate Safety, Tolerability and Efficacy of PrimeC in Subjects with ALS PrimeC PrimeC Only Placebo *OLE - Open Label Extention Double - Blind
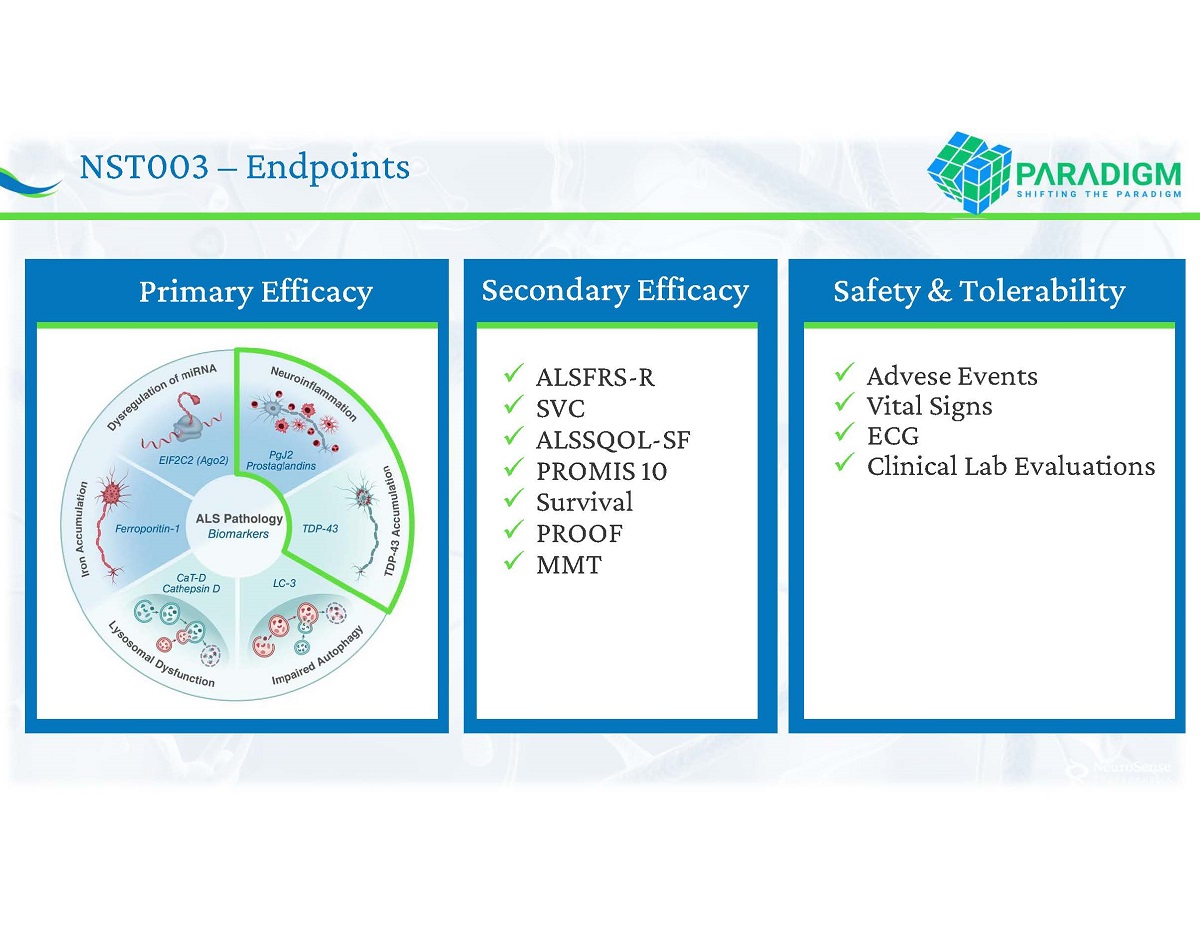
NST 003 – Endpoints Safety & Tolerability Primary Efficacy Secondary Efficacy x ALSFRS - R x SVC x ALSSQOL - SF x PROMIS 10 x Survival x PROOF x MMT x Advese Events x Vital Signs x ECG x Clinical Lab Evaluations
U S Italy Israel
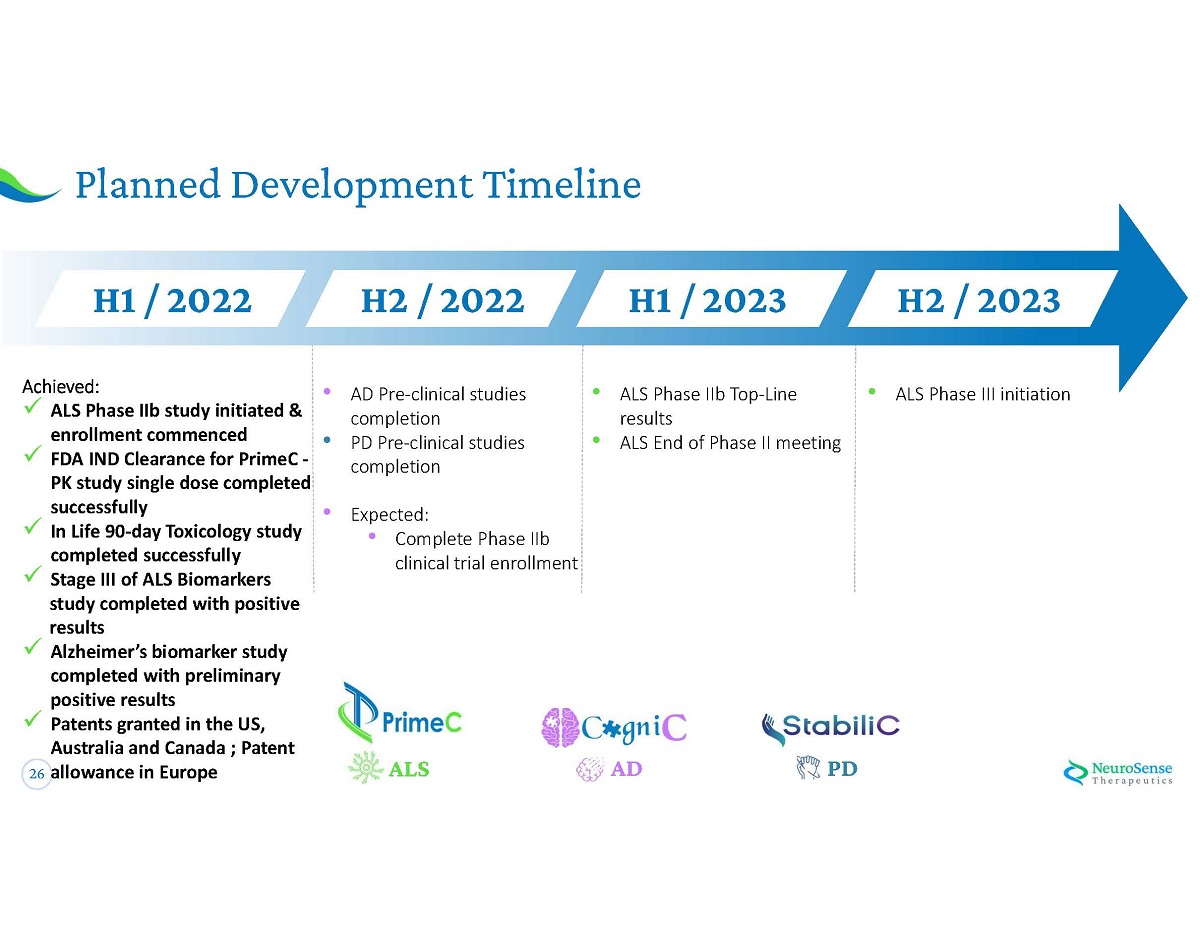
26 Planned Development Timeline • AD Pre - clinical studies completion • PD Pre - clinical studies completion • Expected: • Complete Phase IIb clinical trial enrollment • ALS Phase IIb Top - Line results • ALS End of Phase II meeting • ALS Phase III initiation H 1 / 2022 H 2 / 2022 H 1 / 2023 H 2 / 2023 Achieved: x ALS Phase IIb study initiated & enrollment commenced x FDA IND Clearance for PrimeC - PK study single dose completed successfully x In Life 90 - day Toxicology study completed successfully x Stage III of ALS Biomarkers study completed with positive results x Alzheimer ’ s biomarker study completed with preliminary positive results x Patents granted in the US, Australia and Canada ; Patent allowance in Europe AD PD ALS
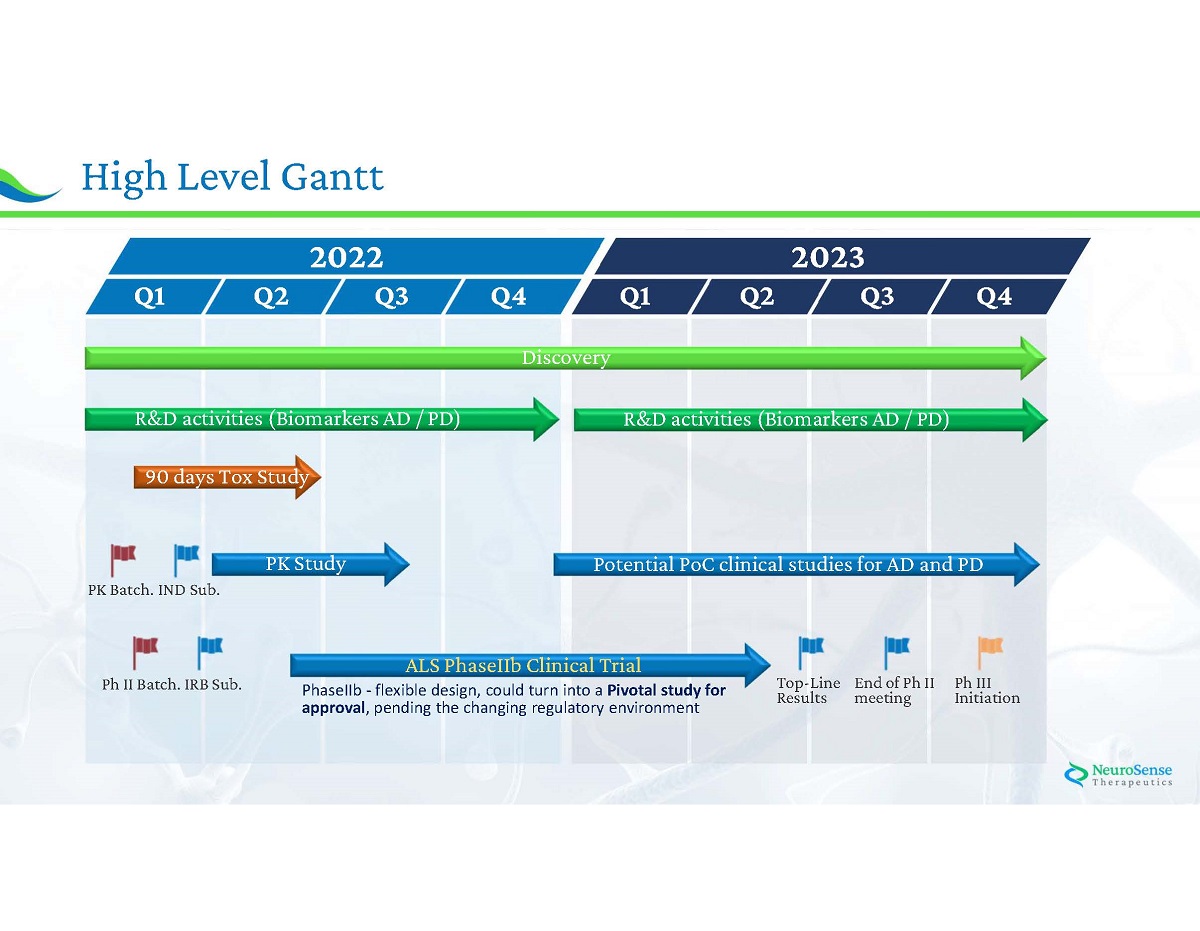
27 High Level Gantt 2022 2023 Q 1 Q 2 Q 3 Q 4 Q 1 Q 2 Q 3 Q 4 Discovery R&D activities (Biomarkers AD / PD) 90 days Tox Study PK Study Potential PoC clinical studies for AD and PD ALS PhaseIIb Clinical Trial IND Sub. PK Batch. IRB Sub. Ph II Batch. Top - Line Results End of Ph II meeting Ph III Initiation PhaseIIb - flexible design, could turn into a Pivotal study for approval , pending the changing regulatory environment R&D activities (Biomarkers AD / PD)
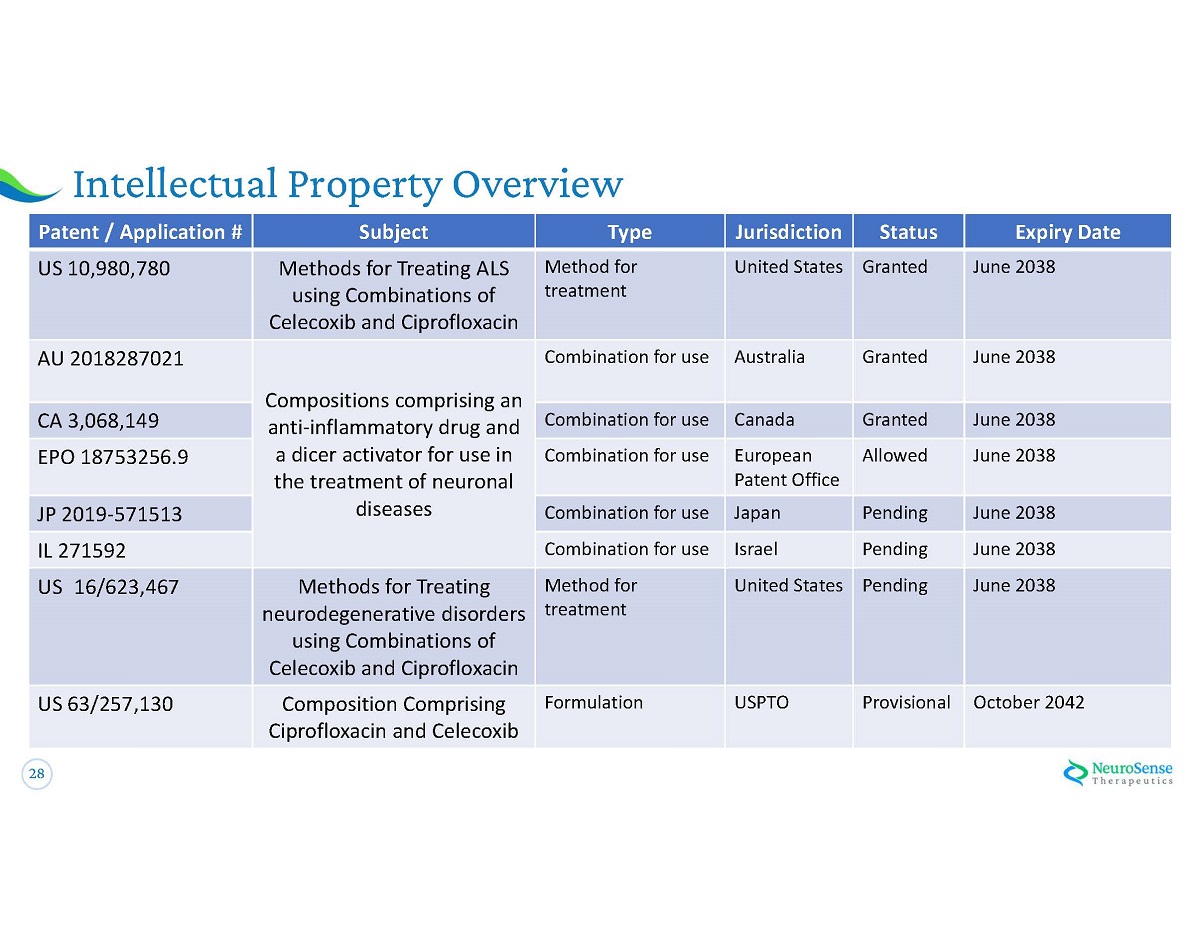
28 Intellectual Property Overview Patent / Application # Subject Type Jurisdiction Status Expiry Date US 10,980,780 Methods for Treating ALS using Combinations of Celecoxib and Ciprofloxacin Method for treatment United States Granted June 2038 AU 2018287021 Compositions comprising an anti - inflammatory drug and a dicer activator for use in the treatment of neuronal diseases Combination for use Australia Granted June 2038 CA 3,068,149 Combination for use Canada Granted June 2038 EPO 18753256.9 Combination for use European Patent Office Allowed June 2038 JP 2019 - 571513 Combination for use Japan Pending June 2038 IL 271592 Combination for use Israel Pending June 2038 US 16 / 623,467 Methods for Treating neurodegenerative disorders using Combinations of Celecoxib and Ciprofloxacin Method for treatment United States Pending June 2038 US 63/257,130 Composition Comprising Ciprofloxacin and Celecoxib Formulation USPTO Provisional October 2042

29 NRSN – Highlights Summary x Novel formulation addresses multiple targets in a synergistic manner x Promising Phase IIa efficacy results x Statistically significant biomarker data which correlates to a meaningful clinical effect x Patents granted and additional IP coverage (valid until 2038 ) x Funded beyond the expected completion of Phase IIb ALS study x Strong pipeline with short, mid and long term developments in big market indications

30 Fun Facts: Management Team of NeuroSense Board Members are Women of Board members are US based with vast experience in Biotech public companies 66 % 50 % ʕ 70 % of NeuroSense team are Women

31 THANK YOU! For more information: www.NeuroSense - tx.com info@NeuroSense - tx.com
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- NeuroSense and Genetika+ Initiate Precision Medicine Collaboration Beginning with Ongoing Phase 2 Clinical Trial in Alzheimer's Disease
- NeuroSense Presents Positive Data Validating Phase 2b Topline Readout During Emerging Science Presentation at the American Academy of Neurology Annual Meeting
- Report on Financial Results for the Year Ended December 31, 2023
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share