Form 6-K Liminal BioSciences Inc. For: May 24
UNITED STATES SECURITIES
AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16
UNDER THE SECURITIES EXCHANGE ACT OF 1934
For the Month of May 2022
Commission File Number: 001-39131
LIMINAL BIOSCIENCES INC.
(Translation of registrant’s name into English)
440 Armand-Frappier Boulevard, Suite 300
Laval, Québec
H7V 4B4
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F: ☒ Form 20-F ☐ Form 40-F
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): ☐
INCORPORATION BY REFERENCE
This Report on Form 6-K (the “Report”) and Exhibit 99.1 to this Report are hereby expressly incorporated by reference into the registrant’s registration statements on Form F-3 (File nos. 333-251055, 333-245703 and 333-251065) filed with the Securities and Exchange Commission on December 1, 2020, December 2, 2020 and December 2, 2020, respectively, and the registration statement on Form S-8 (File no. 333-235692) filed with the Securities and Exchange Commission on December 23, 2019.
EXHIBIT LIST
Exhibit |
|
Description |
99.1 |
|
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
|
|
Liminal BioSciences Inc. |
||||
|
|
|
|
|||||
Date: May 24, 2022 |
|
|
|
By: |
|
/s/ Bruce Pritchard |
||
|
|
|
|
|
|
Name |
|
Bruce Pritchard |
|
|
|
|
|
|
Title: |
|
Chief Executive Officer |

Aiming to develop Best/First-In-Class Novel Small Molecule Therapeutics for Inflammatory, Metabolic and Fibrotic Diseases Corporate Presentation NASDAQ: LMNL May 2022

Safe Harbour This presentation contains forward-looking statements about Liminal BioSciences’ objectives, strategies and businesses that involve risks and uncertainties. Forward‐looking information includes statements concerning, among other things, advancement of Liminal Biosciences’ product candidates, the outcome of anticipated clinical trials; the analysis of our clinical trial data, the potential development of Liminal Biosciences’ R&D programs, the properties of our lead drug candidate, the timing of initiation or nature of preclinical and clinical trials and potential therapeutic areas. These statements are "forward-looking" because they are based on our current expectations about the markets we operate in and on various estimates and assumptions. Actual events or results may differ materially from those anticipated in these forward-looking statements if known or unknown risks affect our business, or if our estimates or assumptions turn out to be inaccurate. Among the factors that could cause actual results to differ materially from those described or projected herein include, but are not limited to, risks associated with: the Company’s ability to develop, manufacture, and successfully commercialize product candidates, if ever; the impact of the COVID-19 pandemic on the Company’s workforce, business operations, clinical development, regulatory activities and financial and other corporate impacts; the availability of funds and resources to pursue R&D projects, clinical development, manufacturing operations or commercialization activities; the successful and timely initiation or completion of clinical trials; the ability to take advantage of financing opportunities or business opportunities in the pharmaceutical industry; the Company’s ability to resolve the Nasdaq listing deficiency and regain compliance with the Nasdaq Listing Rules; uncertainties associated generally with research and development, clinical trials and related regulatory reviews and approvals; and general changes in economic conditions. You will find a more detailed assessment of these risks, uncertainties and other risks that could cause actual events or results to materially differ from our current expectations in the filings and reports the Company makes with the U.S. Securities and Exchange Commission and Canadian Securities Administrators, including in the Annual Report on Form 20-F for the year ended December 31, 2021, as well as other filings and reports Liminal Biosciences’ may make from time to time. Such risks may be amplified by the ongoing COVID-19 pandemic and any related impacts on Liminal BioSciences’ business and the global economy. As a result, we cannot guarantee that any given forward-looking statement will materialize. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements and estimates, which speak only as of the date hereof. We assume no obligation to update any forward-looking statement contained in this press release even if new information becomes available, as a result of future events or for any other reason, unless required by applicable securities laws and regulations. Copyright notice The information contained in this presentation (including names, images, logos and descriptions portraying Liminal BioSciences’ products and/or services) is the property of Liminal BioSciences Inc., of its divisions and / or of its subsidiaries (“Liminal”) and is protected by copyright, patent and trademark law and / or other intellectual property rights. Neither this presentation nor any part may be reproduced or transmitted in any form or by any means, electronic or mechanical, including printing and photocopying, or by any information storage or retrieval system without prior permission in writing from Liminal. Disclaimer Liminal BioSciences reserves the right to make improvements, corrections and/or changes to this presentation at any time.
Aiming to develop Best/First In Class Novel Small Molecule Therapeutics for Inflammatory, Metabolic and Fibrotic Diseases Liminal Introduction Pipeline is positioned to deliver multiple value inflection milestones in 2022/23 Intellectual Property (IP) all under the control of Liminal Progressing Diverse Pipeline: Fezagepras GPR84 Antagonist OXER1 Antagonist Experienced leadership team committed to excellence, innovation, and scientific rigor in our research and clinical development backed by our data driven philosophy Access to NASDAQ Capital Market: LMNL

LMNL is led by a strong, experienced team with proven track records in the discovery, development, and approval of biopharmaceuticals, all driven to make a difference. Management Team Bruce Pritchard Chief Executive Officer Executive Finance Positions: Prometic Life Sciences Inc., CV Therapeutics Inc., Ardana Biosciences Ltd., Director & Chair of Audit Committee Porton BioPharma, Immediate Past-President ICAS Dr. Jeffrey Smith Strategic Medical Adviser Founder & Managing Director, Alder Biopharmaceuticals Inc. Senior Vice President, Alder Biopharmaceuticals Inc. Dr. Gary Bridger Board Member and Strategic Scientific Adviser Executive Vice President of R&D, Xenon Pharmaceuticals Inc. Senior Vice President of R&D, Genzyme Corporation Nicole Rusaw Interim Chief Financial Officer Chief Financial Officer and Director Klinik Health Ventures Corp. Interim Chief Financial Officer, Nuvo Pharmaceuticals Inc. Chief Financial Officer, Transition Pharmaceuticals Inc. Previous Experience Name & Title

At Liminal, we are focused on elucidating compelling biological mechanisms and plan to advance a pipeline of small molecule therapeutics with best/first-in-class potential across a broad range of significant commercial opportunities. Compelling Biological Mechanisms Data-Driven. Dedicated. We focus on molecules with proprietary IP, comprehensive preclinical evaluation and optimized clinical development. We are pursuing indications with high unmet needs and promising market and partnering/licensing potential. Rigorous Objective Approach With an improved balance sheet, we are focused on value creation for patients, our shareholders, and our employees as we strive to advance multiple assets into clinical development with a cash runway sufficient to achieve these goals. Value Creation Strategy Aimed at Both Best or First in Class Drug Discovery & Development
The conjugation of fezagepras with glutamine shows that fezagepras has the potential to act as a “nitrogen scavenging” drug. Nitrogen scavenger drugs are used in the treatment of conditions characterized by hyperammonaemia such as Urea Cycle Disorders and Hepatic Encephalopathy. Fezagepras The GPR84 receptor is primarily expressed in immune cells in addition to multiple organ systems such as the heart, lung, kidney liver, CNS and GI tract. Its expression is upregulated in response to inflammatory stimuli, therefore in chronic inflammatory metabolic and fibrosis driven disease processes. Antagonising the receptor provides an attractive therapeutic opportunity. GPR84 antagonist OXER-1 is a G protein-coupled receptor (GPCR) that is highly selective for 5-oxo-eicosatetraenioc acid (5-oxo-ETE), a potent human eosinophil chemo-attractant known to be involved in a large number of eosinophilic-driven allergic, inflammatory and proliferative diseases. This provides an opportunity in disease areas where there is still substantial unmet need in eosinophilic driven diseases (EDDs). OXER1 antagonist Pipeline of Novel Small Molecule Therapeutics

Completed a Multiple Ascending Dose (MAD) clinical trial which provided support for the hypothesis that fezagepras has nitrogen scavenging properties. First subject dosed in Phase 1a Single Ascending Dose (SAD) clinical trial designed as a head-to-head comparison with Sodium Phenylbutyrate in May 2022. Fezagepras Status Early-stage Programs H2 2022: Update on the outcome of this additional research and provide guidance on potential new target diseases H2 2022: Nominate preclinical candidate selection for GPR84 2023: Regulatory submission for clinical trial of GPR84 Expected Milestones Pipeline Overview GPR84 Antagonist Program is part of our preclinical program approaching Pre-clinical Candidate (PCC) selection in 2022 OXER1 Antagonist Program is part of our preclinical program approaching PCC selection in 2023

Our Lead Candidate: Fezagepras

In December 2020 the Company initiated a Phase 1 Multiple Ascending Dose (MAD) trial in Fezagepras with a view to proceed with a Phase 2 clinical trial in fibrosis. By mid-2021, based on preliminary PK data from the Phase 1 MAD clinical trial, the Company announced its plan to discontinue the development of fezagepras as a therapeutic agent in Idiopathic Pulmonary Fibrosis (IPF) and hypertriglyceridemia. This conclusion was based on the low plasma concentrations of fezagepras, combined with disproportionate level of metabolites. The PK data demonstrated that the major metabolite of fezagepras was the glutamine conjugate and provided evidence to support the hypothesis that fezagepras had nitrogen scavenging properties. This provides an opportunity for fezagepras’ potential development in diseases associated with high plasma ammonia concentrations. Initiated our Phase 1a Single Ascending Dose study to evaluate the safety, tolerability, and pharmacokinetics of single ascending dose of fezagepras compared to Sodium Phenylbutyrate in healthy subjects in healthy volunteers in May 2022. Fezagepras: A Brief History

Fezagepras: Near-term Clinical Development Plan * Follow on clinical trials subject to result of Phase 1a SAD and relevant regulatory approvals Established baseline safety & PK profile Completed Phase 1 MAD Phase 1a SAD Phase 1a MAD Phase 1b SMAD Timing Study Goal Early Biomarker Data Dose Selection Evidence of Biological Mechanism Safety / PK Up to 2400mg in divided doses Evidence of glutamine conjugation Study Commenced May 2022 Planned Data Readout: Q3 2022 Planned Planned Safety & PK in Biological Mechanism at higher doses than previously administered Safety & PK in Healthy Volunteers at higher doses than previously administered Safety & PK in Disease Participants Relative nitrogen scavenging capability of fezagepras VS sodium phenylbutyrate

Our Early-Stage Candidates

GPR84 antagonist
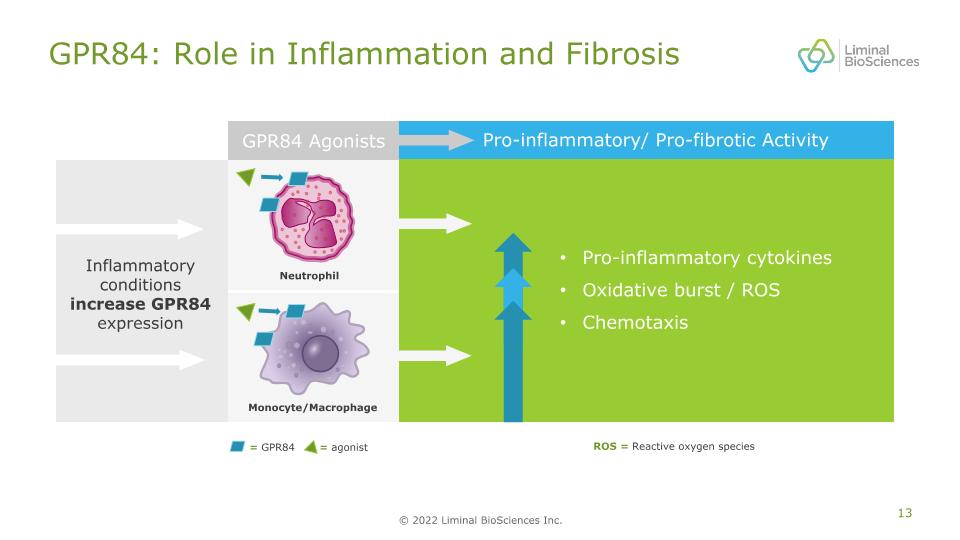
GPR84: Role in Inflammation and Fibrosis = GPR84 = agonist Neutrophil Monocyte/Macrophage ROS = Reactive oxygen species Pro-inflammatory/ Pro-fibrotic Activity GPR84 Agonists Pro-inflammatory cytokines Oxidative burst / ROS Chemotaxis Inflammatory conditions increase GPR84 expression
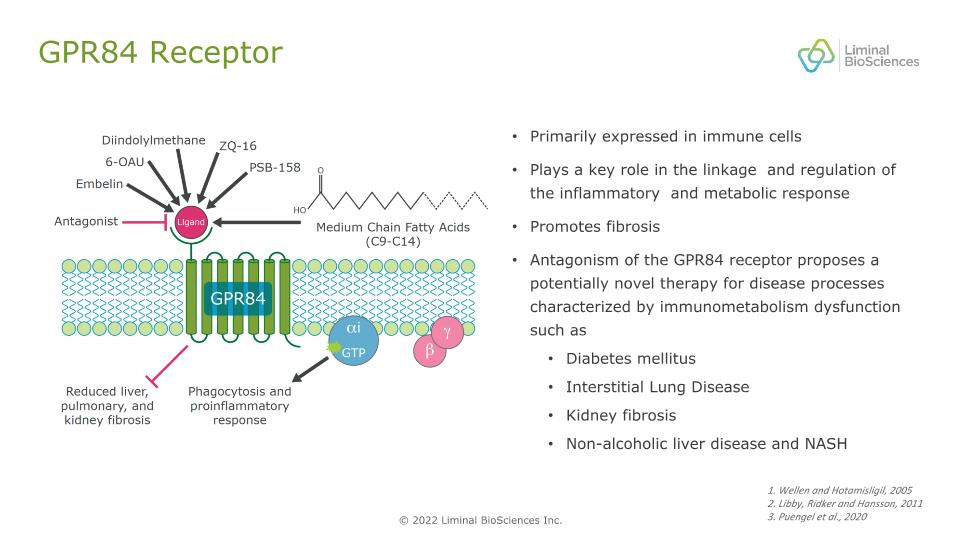
GPR84 Receptor Primarily expressed in immune cells Plays a key role in the linkage and regulation of the inflammatory and metabolic response Promotes fibrosis Antagonism of the GPR84 receptor proposes a potentially novel therapy for disease processes characterized by immunometabolism dysfunction such as Diabetes mellitus Interstitial Lung Disease Kidney fibrosis Non-alcoholic liver disease and NASH 1. Wellen and Hotamisligil, 2005 2. Libby, Ridker and Hansson, 2011 3. Puengel et al., 2020
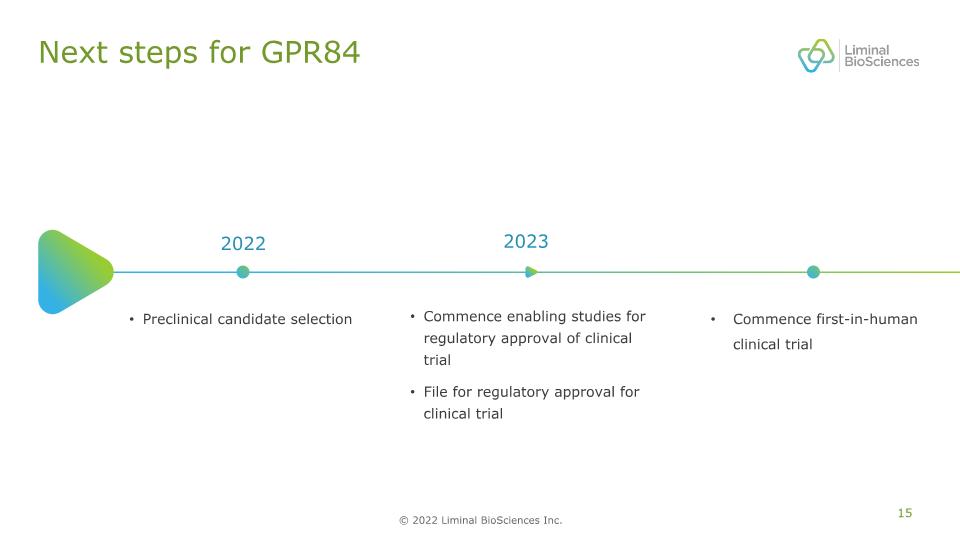
Next steps for GPR84 Commence enabling studies for regulatory approval of clinical trial File for regulatory approval for clinical trial 2023 Commence first-in-human clinical trial 2022 Preclinical candidate selection

OXER1 Antagonist
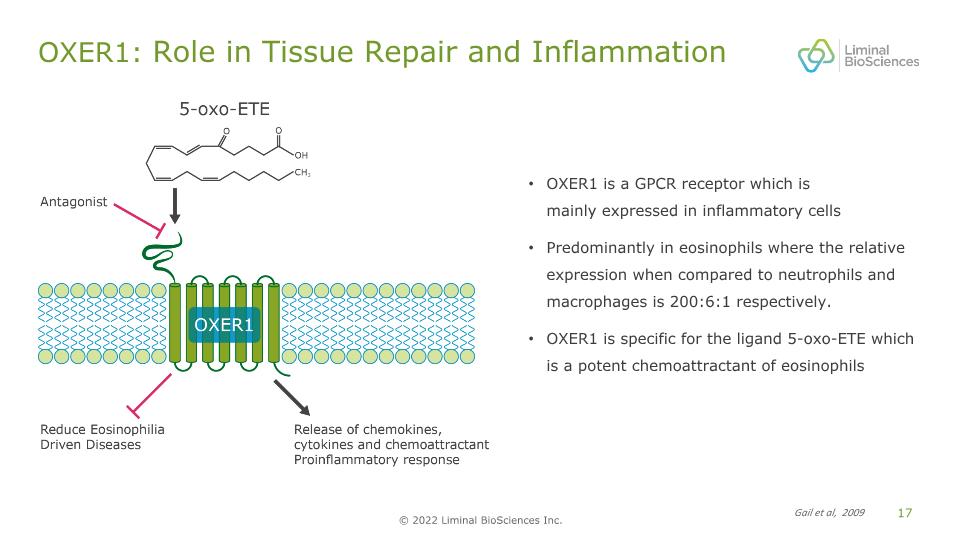
OXER1: Role in Tissue Repair and Inflammation OXER1 is a GPCR receptor which is mainly expressed in inflammatory cells Predominantly in eosinophils where the relative expression when compared to neutrophils and macrophages is 200:6:1 respectively. OXER1 is specific for the ligand 5-oxo-ETE which is a potent chemoattractant of eosinophils Gail et al, 2009
Eosinophils: Both Effector and Immune-Regulator Cells Eosinophils are major effector cells in the immune system Part of the innate immune system; traditionally recognised as the first line of defence against parasitic infections When activated, they release a cocktail of toxic proteins along with cytokines to attract other immune cells These toxic proteins can also damage normal tissue and promote inflammation Eosinophils are also recruited from the blood into the tissues at the sites of inflammation They also play a role in tissue repair and resolution of inflammation Recent research suggests that eosinophils, as one of the ‘first responder’ cell types in tissues like the lung and gut, may also help to regulate the type of immune response that is generated E.g. Aberrantly-activated eosinophils are known to be present in patients with severe asthma and other eosinophil-related diseases In addition to cytotoxic proteins and cytokines, activated eosinophils release lipid mediators that can cause airway smooth muscle contraction and contribute to airway hyper-responsiveness
Eosinophils in Disease Gastrointestinal Diseases Respiratory Diseases Other Diseases Skin Diseases Eosinophils are involved in acute and chronic inflammation and play an important role in a large number of allergic, inflammatory and proliferative diseases. Both eosinophils and mast cells are involved in the pathology of many of these diseases.

Development Rationale & Opportunities in Eosinophil-related Diseases Eosinophil-targeting biology proven with existing, approved, monoclonal antibody drugs Reducing eosinophil levels shown to improve outcomes in severe asthma Blood eosinophil levels offer an easy-to-measure biomarker for early-stage clinical studies Despite existing competitor products in eosinophilic asthma, there is still an opportunity for an effective, small-molecule drug Not many competitor drugs in clinical-stage development for eosinophilic diseases No known competitors identified targeting the 5-oxo-ETE / OXE receptor: its is an entirely novel approach Preclinical candidate nomination expected in 2023.

Expected Milestones Fezagepras Q2 2022: Phase 1a SAD Clinical trial H2 2022: Update on the outcome of this additional research and provide update on determining any potential new target diseases H2 2022: Market update on any further development plans for fezagepras GPR84 2022: Pre-clinical Candidate Selection 2023: Regulatory Submission for Clinical Trial OXER1 2023: Pre-clinical Candidate Selection Business Updates 2022/2023: Continue to actively seek opportunities to monetize non-core assets and to reduce costs Data Driven Execution and Delivery

All figures presented in this section are in Canadian dollars unless otherwise specified. Financed to Deliver on Expected Milestones Cash as of March 31, 2022 $61.2M Cash Runway sufficient to support program-related milestones described on slide 21 Ongoing opportunity to monetise non-core assets Access to NASDAQ Capital Market: LMNL Debt free Company with IP all under the control of Liminal
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- GA-ASI Selected to Build CCA for AFLCMC
- Northfield Bancorp, Inc. Announces First Quarter 2024 Results
- SHAREHOLDER ACTION ALERT: The Schall Law Firm Encourages Investors in Compass Minerals International, Inc. with Losses to Contact the Firm
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share