Form 20FR12G/A BRIGHT MINDS BIOSCIENCES
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 20-F/A
(Amendment No. 4)
☒ REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) or 12(g) OF THE SECURITIES EXCHANGE ACT OF 1934
OR
☐ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended _______________________
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from _______________ to _______________
OR
☐ SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
Date of event requiring this shell company report _______________
Commission file number ___________
BRIGHT MINDS BIOSCIENCES INC.
(Exact name of Registrant specified in its charter)
Not Applicable
(Translation of Registrant's name into English)
British Columbia, Canada
(Jurisdiction of incorporation or organization)
Suite 1500, 1055 West Georgia Street, PO Box 11117
Vancouver, British Columbia, Canada, V6E 4N7
(Address of principal executive offices)
Ian McDonald; (647) 407-2515; [email protected]
Suite 1500, 1055 West Georgia Street, PO Box 11117
Vancouver, British Columbia, Canada, V6E 4N7
(Name, Telephone, Email and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act.
|
Title of Each Class |
Trading Symbol(s) |
Name of each exchange on which registered |
|
N/A |
N/A |
N/A |
Securities registered or to be registered pursuant to Section 12(g) of the Act.
Common Shares, without par value
(Title of Class)
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act.
None
(Title of Class)
Number of outstanding shares of each of the issuer's classes of capital or common stock as of the close of business of the period covered by the annual report.
Not Applicable
Indicate by check mark if the Registrant is a well-known seasoned issuer as defined in Rule 405 of the Securities Act.
Yes ☐ No ☒
If this report is an annual or transition report, indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Yes ☐ No ☐
Indicate by check mark whether Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes ☐ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Yes ☐ No ☐
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company. See definition of "accelerated filer," "large accelerated filer," and "emerging growth company" in Rule 12b-2 of the Exchange Act.
|
Large Accelerated Filer ☐ |
Accelerated Filer ☐ |
|
Non Accelerated Filer ☒ |
Emerging Growth Company ☒ |
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management's assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
|
U.S. GAAP ☐ |
International Financial Reporting Standards as issued |
Other ☐ |
|
|
by the International Accounting Standards Board ☒ |
|
If "Other" has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow: Item 17 ☐ Item 18 ☐
If this is an annual report, indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b 2 of the Exchange Act):
Yes ☐ No ☐
TABLE OF CONTENTS
Page
GENERAL MATTERS
In this Registration Statement on Form 20-F (this "Registration Statement"), all references to "Bright Minds" are to Bright Minds Biosciences Inc., and all references to the "Company", "our", "us" or "we" refer to Bright Minds Biosciences Inc. and its consolidated subsidiaries, unless the context clearly requires otherwise.
We report under International Financial Reporting Standards ("IFRS"), as issued by the International Accounting Standards Board. Readers should be aware that financial statements prepared in accordance with IFRS may differ in certain respects from financial statements prepared in accordance with U.S. generally accepted accounting principles ("U.S. GAAP").
We present our financial statements in Canadian dollars. All references to "C$" are to Canadian dollars and references to "US$" are to United States dollars. On June 15, 2021, the daily average exchange rate for the conversion of Canadian dollars into U.S. dollars as reported by the Bank of Canada was C$1.00 = US$0.8205. See also Item 3, "Key Information" for more detailed currency and conversion information.
FORWARD LOOKING STATEMENTS
This Registration Statement contains statements and information that, to the extent that they are not historical fact, may constitute "forward-looking information" within the meaning of applicable securities legislation. Forward-looking information may include financial and other projections, as well as statements regarding future plans, objectives or economic performance, or the assumption underlying any of the foregoing. This Registration Statement uses words such as "may", "would", "could", "will", "likely", "except", "anticipate", "believe", "intend", "plan", "forecast", "project", "estimate", "outlook", and other similar expressions to identify forward-looking information. These forward-looking statements include, among other things, statements relating to:
-
the effect of COVID-19 and any other pandemics on the Company's workforce, business, operations and financial condition;
-
the Company's expectations regarding the achievement of clinical and regulatory milestones;
-
the executive compensation of the Company;
-
the composition of the board of directors (the "Board") and management of the Company;
-
the Company's expectations regarding its revenue, expenses and research and development operations;
-
the Company's anticipated cash needs and its needs for additional financing;
-
the Company's intention to grow the business and its operations;
-
expectations with respect to the success of its research and development of serotonergic therapeutics;
-
expectations regarding growth rates, growth plans and strategies of the Company;
-
expectations that the provisional patent applications will be refiled as regular patent applications or as new provisional applications in Q2 of 2021;
-
the Company's strategy with respect to the expansion and protection of its intellectual property;
-
the medical benefits, safety, efficacy, dosing and consumer acceptance of serotonergic therapeutics;
-
the Company's ability to comply with provincial, federal, local and regulatory agencies in the United States, Canada and other jurisdictions in which the Company operates;
-
the Company's competitive position and the regulatory environment in which the Company operates;
-
the Company's expected business objectives for the next 12 months;
-
the Company's plans with respect to the payment of dividends;
-
beliefs and intentions regarding the ownership of material trademarks and domain names used in connection with the design, production, marketing, distribution and sale of the Company's products and services; and
-
the Company's ability to obtain additional funds through the sale of equity or debt commitments.
Forward-looking information is based on the reasonable assumptions, estimates, analysis and opinions of management made in light of its experience and its perception of trends, current conditions and expected developments, as well as other factors that management believes to be relevant and reasonable in the circumstances at the date that such statements are made, but which may prove to be incorrect. The material factors and assumptions used to develop the forward-looking statements contained in this Registration Statement include, without limitation:
-
obtaining the necessary regulatory approvals;
-
that regulatory requirements will be maintained;
-
general business and economic conditions;
-
the duration of COVID-19 and the extent of its economic and social impact;
-
the Company's ability to successfully execute its plans and intentions;
-
the availability of financing on reasonable terms;
-
the Company's ability to attract and retain skilled staff;
-
market competition;
-
the products, services and technology offered by the Company's competitors; and
-
that the Company's current good relationships with the Company's suppliers, service providers and other third parties will be maintained.
Readers are cautioned that the foregoing list is not exhaustive of all factors and assumptions which may have been used. Although, the Company believes that the assumptions underlying these statements are reasonable, they may prove to be incorrect, and the Company cannot assure that actual results will be consistent with these forward-looking statements. Given these risks, uncertainties and assumptions, prospective purchasers of Securities should not place undue reliance on these forward-looking statements. Whether actual results, performance or achievements will conform to the Company's expectations and predictions is subject to a number of known and unknown risks, uncertainties, assumptions and other factors, including those listed under Item 3.D "Risk Factors" which include:
-
limited operating history;
-
the Company's actual financial position and results of operations may differ materially from the expectations of the Company's management;
-
the Company may be required to obtain and maintain certain permits, licenses, and approvals in the jurisdictions where its products or technologies are being researched, developed, or commercialized;
-
the Company may encounter substantial delays or difficulties with its clinical trial;
-
clinical trials are expensive, time consuming and difficult to design and implement;
-
the Company may not be successful in its efforts to identify, license or discover additional product candidates;
-
risks associated with the development of the Company's products which are at early stages of development;
-
there is no assurance that the Company will turn a profit or generate immediate revenues;
-
the continued operation of the Company as a going concern;
-
the Company's intellectual property and licenses thereto;
-
the Company may not achieve timelines for project development set out in this Registration Statement;
-
the Company faces product liability exposure;
-
the Company has international operations, which subject the Company to risks inherent with operations outside of Canada;
-
exchange rate fluctuations between the U.S. dollar and the Canadian dollar;
-
changes to patent laws or the interpretation of patent laws;
-
the risk of patent-related or other litigation;
-
the Company may not be able to enforce its intellectual property rights throughout the world;
-
the lack of product for commercialization;
-
the lack of experience of the Company/management in marketing, selling, and distribution products;
-
the size of the Company's target market is difficult to quantify;
-
potentials for conflicts of interest for the Company's officers and directors;
-
in certain circumstances, the Company's reputation could be damaged;
-
negative operating cash flow;
-
need for additional financing;
-
uncertainty and discretion of use of proceeds;
-
the potential for a material weakness in the Company's internal controls over financial reporting;
-
difficulties with forecasts;
-
market price of Common Shares and volatility; and
-
dilution of Common Shares.
Although management has attempted to identify important factors that could cause actual results to differ materially from those contained in forward-looking statements, there may be other factors that cause results not to be as anticipated, estimated or intended. There is no assurance that forward-looking statements will prove to be accurate, as actual results and future events could differ materially from those anticipated in such forward-looking statements. Accordingly, readers should not place undue reliance on forward-looking statements. These cautionary remarks expressly qualify, in their entirety, all forward-looking statements attributable to the Company or persons acting on the Company's behalf. We do not undertake to update any forward-looking statements to reflect actual results, changes in assumptions or changes in other factors affecting such statements, except as, and to the extent required by, applicable securities laws. You should carefully review the cautionary statements and risk factors contained in this Registration Statement and other documents that the Company may file from time to time with the securities regulators.
PART I
ITEM 1. IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS
A. Directors and Senior Management
The Directors and senior management of our Company as of June 15, 2021 are as follows:
|
Name |
Business Address |
Function |
|
Ian McDonald |
Suite 1500, 1055 West Georgia Street, PO Box 11117, Vancouver, BC, V6E 4N7 |
As the Company's Chief Executive Officer and President Mr. McDonald is responsible for strategic planning and operations, oversight of the Company, as well as managing the Company's relations with its lawyers, regulatory authorities and investor community; as a director, Mr. McDonald participates in management oversight and helps to ensure compliance with the Company's corporate governance policies and standards. |
|
Ryan Cheung |
Suite 1500, 1055 West Georgia Street, PO Box 11117, Vancouver, BC, V6E 4N7 |
As the Company's Chief Financial Officer, Mr. Cheung is responsible for the management and supervision of all of the financial aspects of the Company's business and corporate governance and standards. |
|
Dr. Gideon Shapiro |
Suite 1500, 1055 West Georgia Street, PO Box 11117, Vancouver, BC, V6E 4N7 |
As the Company's Vice President (Discovery), Dr. Shapiro is responsible for the Company's discovery efforts and leading scientific research initiatives. |
|
Alan Kozikowski |
Suite 1500, 1055 West Georgia Street, PO Box 11117, Vancouver, BC, V6E 4N7 |
As our Chief Science Officer, Mr. Kozikowski is responsible for oversight of the Company's scientific research and development programs as well as general scientific strategy. As a director, Mr. Kozikowski participates in management oversight and helps to ensure compliance with the Company's corporate governance policies and standards. |
|
Dr. Revati Shreeniwas |
Suite 1500, 1055 West Georgia Street, PO Box 11117, Vancouver, BC, V6E 4N7 |
As our Chief Medical Officer, Dr. Shreeniwas is responsible for the Company's clinical and translational trial planning and implementation. |
|
Nils Christian Bottler |
Suite 1500, 1055 West Georgia Street, PO Box 11117, Vancouver, BC, V6E 4N7 |
As an independent director, Mr. Bottler supervises management and helps to ensure compliance with the Company's corporate governance policies and standards. |
|
Jeremy Fryzuk |
Suite 1500, 1055 West Georgia Street, PO Box 11117, Vancouver, BC, V6E 4N7 |
As an independent director, Mr. Fryzuk supervises management and helps to ensure compliance with the Company's corporate governance policies and standards. |
|
Dr. Emer Leahy |
Suite 1500, 1055 West Georgia Street, PO Box 11117, Vancouver, BC, V6E 4N7 |
As an independent director, Dr. Leahy supervises management and helps to ensure compliance with the Company's corporate governance policies and standards. |
B. Advisers
Our legal advisers are McMillan LLP, with a business address at Suite 1500, 1055 West Georgia Street, P.O. Box 11117, Vancouver, British Columbia, Canada V6E 4N7.
C. Auditors
Our auditors are DeVisser Gray LLP, with a business address at 401-905 West Pender Street, Vancouver, British Columbia, Canada V6C 1L6, which are members of the Institute of Chartered Accountants of British Columbia and are registered with both the Canadian Public Accountability Board (CPAB) and the U.S. Public Company Accounting Oversight Board (PCAOB). DeVisser Gray LLP has audited our consolidated financial statements for the fiscal year ended September 30, 2020 and the fiscal period from May 31, 2019 (date of incorporation) to September 30, 2019.
ITEM 2. OFFER STATISTICS AND EXPECTED TIMETABLE
Not applicable.
ITEM 3. KEY INFORMATION
A. Selected financial data
The selected historical consolidated financial information set forth below has been derived from our financial statements for the fiscal year ended September 30, 2020 and the fiscal period from May 31, 2019 (date of incorporation) to September 30, 2019 and the six months ended March 31, 2021.
Consolidated Statement of Net Loss
Consolidated Statement of Income (Loss)
|
|
Six months ended March |
Year ended September 30, |
Period ended September |
| (C$) | (C$) | (C$) | |
|
Revenues |
- |
- |
- |
|
Gross Profit |
- |
- |
- |
|
Net Income (Loss) |
(2,250,335) |
(480,377) |
(78,717) |
|
Loss per Share - Basic and Diluted |
(0.35) |
(0.13) |
(0.04) |
Consolidated Statement of Financial Position
|
|
Six months ended March |
Year ended September 30, |
Period ended September |
| (C$) | (C$) | (C$) | |
|
Cash and cash equivalents |
25,450,001 |
799,929 |
79,991 |
|
Current Assets |
25,739,551 |
878,216 |
79,991 |
|
Total Assets |
25,741,551 |
880,216 |
81,991 |
|
Current Liabilities |
409,238 |
150,923 |
36,708 |
|
Total Liabilities |
409,238 |
150,923 |
36,708 |
|
Shareholders' Equity (Deficit) |
25,332,313 |
729,293 |
45,283 |
B. Capitalization and Indebtedness
The table below sets forth our total indebtedness and show the capitalization of the Company as of March 31, 2021. This table should be read in conjunction with our condensed interim consolidated financial statements for the three and six months ended March 31, 2021 and 2020, together with the accompanying notes thereto. See Item 18.
| Total Indebtedness | (C$) | ||
| Equity | |||
| Share Capital | $ | 27,239,872 | |
| Subscriptions receivable | ($33,684 | ) | |
| Subscriptions received | $ | 10,000 | |
| Reserves | $ | 925,554 | |
| Deficit | ($2,809,429 | ) | |
| Total Shareholders' Equity | $ | 25,332,313 | |
| Total capitalization | $ | 25,332,313 |
The Company's authorized capital consists of unlimited number of shares of common stock, without par value (the "Common Shares"). As of June 15, 2021, the Company had 11,834,361 Common Shares issued and outstanding.
C. Reasons for the offer and use of proceeds
Not applicable.
D. Risk Factors
An investment in our securities carries a significant degree of risk. You should carefully consider the following risks, as well as the other information contained in this Registration Statement, including our financial statements and related notes included elsewhere in this Registration Statement, before you decide to purchase our securities. Any one of these risks and uncertainties has the potential to cause material adverse effects on our business, prospects, financial condition and operating results which could cause actual results to differ materially from any forward-looking statements expressed by us and a significant decrease in the value of our common shares. Refer to "Forward-Looking Statements".
We may not be successful in preventing the material adverse effects that any of the following risks and uncertainties may cause. These potential risks and uncertainties may not be a complete list of the risks and uncertainties facing us. There may be additional risks and uncertainties that we are presently unaware of, or presently consider immaterial, that may become material in the future and have a material adverse effect on us. You could lose all or a significant portion of your investment due to any of these risks and uncertainties.
Risks Related to the Business of the Company
We have a limited operating history and have not yet generated any revenues.
We have a very limited history of operations and is considered a start-up company, which makes evaluating our business and future prospects difficult. As such, we are subject to many risks common to such enterprises, including under-capitalization, cash shortages, limitations with respect to personnel, financial and other resources and lack of revenues. There is no assurance that we will be successful in achieving a return on shareholders' investment and the likelihood of our success must be considered in light of our early stage of operations.
Our actual financial position and results of operations may differ materially from the expectations of our management.
Our actual financial position and results of operations may differ materially from our management's expectations. We have experienced some changes in our operating plans and certain delays in our plans. As a result, our revenue, net income and cash flow may differ materially from our expected revenue, net income and cash flow. The process for estimating our revenue, net income and cash flow requires the use of judgment in determining the appropriate assumptions and estimates. These estimates and assumptions may be revised as additional information becomes available and as additional analyses are performed. In addition, the assumptions used in planning may not prove to be accurate, and other factors may materially affect our financial condition or results of operations.
We may be required and have not yet obtained regulatory approvals, licenses, and permits in the jurisdictions where our products or technologies are being researched, developed or commercialized, which failure to obtain such regulatory approvals, licenses and permits will likely have a material adverse effect on our business, financial condition and results of operations.
We, or our service providers, may be required to obtain and maintain certain permits, licenses, and approvals in the jurisdictions where our products or technologies are being researched, developed, or commercialized. We have not obtained regulatory approval for any product candidate and it is possible that none of our existing product candidates or any future product candidates will ever obtain regulatory approval. There can be no assurance that we will be able to obtain or maintain any necessary licenses, permits, or approvals. Any material delay or inability to receive these items is likely to delay and/or inhibit our ability to conduct our business, and would have an adverse effect on its business, financial condition, and results of operations. In particular, we will require approval from the FDA (as defined herein) and equivalent organizations in other countries before any of our products can be marketed. There is no assurance that such approvals will be forthcoming. Furthermore, the exact nature of the studies these regulatory agencies will require is not known and can be changed at any time by the regulatory agencies, increasing the financing risk and potentially increasing the time to market we face, which could adversely affect our business, financial condition or results of operations.
We may encounter substantial delays or difficulties with our clinical trials, which could have a material adverse effect on our financial condition and results of operations.
We may not commercialize, market, promote or sell any product candidate without obtaining marketing approval from the FDA or comparable foreign regulatory authorities, and we may never receive such approvals. It is impossible to predict when or if any of our product candidates will prove effective or safe in humans and will receive regulatory approval. Before obtaining marketing approval from regulatory authorities for the sale of its product candidates, we must complete preclinical development and then conduct extensive clinical trials to demonstrate the safety and efficacy of our product candidates. Clinical testing is expensive, difficult to design and implement, can take many years to complete and is uncertain as to outcome. A failure of one or more clinical trials can occur at any stage of testing. Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses, and many companies that have believed their product candidates performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain marketing approval of their products.
We may experience numerous unforeseen events prior to, during, or as a result of, clinical trials that could delay or prevent our ability to receive marketing approval or commercialize current and any future product candidates, including:
-
delays in reaching a consensus with regulatory authorities on design or implementation of clinical trials;
-
regulators or institutional review boards, or IRBs (as defined herein), may not authorize us or its investigators to commence a clinical trial or conduct a clinical trial at a prospective trial site;
-
delays in reaching agreement on acceptable terms with prospective clinical research organizations and clinical trial sites;
-
the number of patients required for clinical trials of our product candidates may be larger than we anticipate, enrollment in these clinical trials may be slower than we anticipate, patients may drop out of these clinical trials at a higher rate than we anticipate or fail to return for post-treatment follow-up or we may fail to recruit suitable patients to participate in a trial;
-
clinical trials of our product candidates may produce negative or inconclusive results;
-
imposition of a clinical hold by regulatory authorities as a result of a serious adverse event, concerns with a class of product candidates or after an inspection of our clinical trial operations, trial sites or manufacturing facilities;
-
occurrence of serious adverse events associated with the product candidate that are viewed to outweigh its potential benefits;
-
changes in regulatory requirements and guidance that require amending or submitting new clinical protocols; or
-
we may decide, or regulators may require us, to conduct additional clinical trials or abandon product development programs.
Any inability to successfully complete preclinical and clinical development could result in additional costs to us or impair our ability to generate revenue. In addition, if we make manufacturing or formulation changes to our product candidates, we may need to conduct additional testing to bridge our modified product candidate to earlier versions. Clinical trial delays could also shorten any periods during which we may have the exclusive right to commercialize our product candidates, if approved, or allow competitors to bring competing drugs to market before us, which could impair our ability to successfully commercialize our product candidates and may harm our business, financial condition, results of operations and prospects.
Additionally, if the results of our clinical trials are inconclusive or if there are safety concerns or serious adverse events associated with product candidates, we may:
-
be delayed in obtaining marketing approval, if at all;
-
obtain approval for indications or patient populations that are not as broad as intended or desired;
-
obtain approval with labeling that includes significant use or distribution restrictions or safety warnings;
-
be subject to additional post-marketing testing requirements;
-
be required to perform additional clinical trials to support approval or be subject to additional post-marketing testing requirements;
-
be subject to the addition of labeling statements, such as warnings or contraindications;
-
be sued; or
-
experience damage to its reputation.
Our product development costs will also increase if we experience delays in testing or obtaining marketing approvals. We do not know whether any of our preclinical studies or clinical trials will begin as planned, need to be restructured or be completed on schedule, if at all.
Further, we, the FDA or an IRB may suspend our clinical trials at any time if it appears that we or our collaborators are failing to conduct a trial in accordance with regulatory requirements, such as the FDA's current GCP (as defined herein), that we are exposing participants to unacceptable health risks, or if the FDA finds deficiencies in our INDs (as defined herein), or in the conduct of these trials. Therefore, we cannot predict with any certainty the schedule for commencement and completion of future clinical trials. If we experience delays in the commencement or completion of our clinical trials, or if we terminate a clinical trial prior to completion, the commercial prospects of our product candidates could be negatively impacted, and our ability to generate revenues from our product candidates may be delayed.
Clinical trials are expensive, time consuming and difficult to design and implement, which could have a material adverse effect on our business, financial condition or results of operations.
Our product candidates will require clinical testing before we can submit an NDA (as defined herein) for regulatory approval. We cannot predict with any certainty if or when we might submit an NDA for regulatory approval for any of our product candidates or whether any such NDA will be approved by the FDA. Human clinical trials are very expensive and difficult to design and implement, in part because they are subject to rigorous regulatory requirements. For instance, the FDA may not agree with our proposed endpoints for any future clinical trial of our product candidates, which may delay the commencement of our clinical trials. The clinical trial process is also time consuming. Furthermore, failure can occur at any stage, and we could encounter problems that cause us to abandon or repeat clinical trials, which could have a material adverse effect on our business, financial condition or results of operations.
We may not be successful in our efforts to identify, license or discover additional product candidates, which may have a material adverse effect on our business and could potentially cause us to cease operations.
Although a substantial amount of our effort will focus on the continued research and pre-clinical testing, potential approval and commercialization of our existing product candidates, the success of our business also depends in part upon our ability to identify, license or discover additional product candidates. Our research programs or licensing efforts may fail to yield additional product candidates for clinical development for a number of reasons, including but not limited to the following:
- our research or business development methodology or search criteria and process may be unsuccessful in identifying potential product candidates;
-
we may not be able or willing to assemble sufficient resources to acquire or discover additional product candidates;
-
our product candidates may not succeed in pre-clinical or clinical testing;
-
our product candidates may be shown to have harmful side effects or may have other characteristics that may make the products unmarketable or unlikely to receive marketing approval;
-
competitors may develop alternatives that render our product candidates obsolete or less attractive;
-
product candidates we develop may be covered by third parties' patents or other exclusive rights;
-
the market for a product candidate may change during our program so that such a product may become unreasonable to continue to develop;
-
a product candidate may not be capable of being produced in commercial quantities at an acceptable cost, or at all; and
-
a product candidate may not be accepted as safe and effective by patients, the medical community or third-party payors.
If any of these events occurs, we may be forced to abandon our development efforts to identify, license or discover additional product candidates, which would have a material adverse effect on our business and could potentially cause us to cease operations. Research programs to identify new product candidates require substantial technical, financial and human resources. We may focus our efforts and resources on potential programs or product candidates that ultimately prove to be unsuccessful.
There is no assurance that we will turn a profit or generate immediate revenues.
There is no assurance as to whether we will be profitable, earn revenues, or pay dividends. We have incurred and anticipate that we will continue to incur substantial expenses relating to the development and initial operations of our business. The payment and amount of any future dividends will depend upon, among other things, our results of operations, cash flow, financial condition, and operating and capital requirements. There is no assurance that we pay any future dividends, if at all, and, if dividends are paid, there is no assurance with respect to the amount of any such dividends.
We have a going concern risk, which if we are unable to generative positive cash flows and/or obtain additional financing sufficient to fund continued activities and acquisitions, may materially adversely affect our financial condition and results of operations as well as our ability to continue operations.
Our continued operation as a going concern is dependent upon our ability to generate positive cash flows and/or obtain additional financing sufficient to fund continuing activities and acquisitions. While we continue to review our operations in order to identify strategies and tactics to increase revenue streams and financing opportunities, there is no assurance that we will be successful in such efforts; if we are not successful, we may be required to significantly reduce or limit operations, or no longer operate as a going concern. It is also possible that operating expenses could increase in order to grow the business. If we do not start generating and significantly increase revenues to meet these increased operating expenses and/or obtain financing until our revenues meet these operating expenses, our business, financial condition and operating results could be materially adversely affected. We cannot be sure when or if we will ever achieve profitability and, if we do, we may not be able to sustain or increase that profitability.
We may not be able to adequately protect and maintain our intellectual property and licenses, which could result in a material adverse effect to our business, financial condition and results of operations.
Our success will depend in part on our ability to protect and maintain our intellectual property rights and our licenses. No assurance can be given that the license or rights used by us will not be challenged, invalidated, infringed or circumvented, nor that the rights granted thereunder will provide competitive advantages to us. It is not clear whether the pending patent applications will result in the issuance of patents. There is no assurance that we will be able to enter into licensing arrangements, develop or obtain alternative technology in respect of patents issued to third parties that incidentally cover its production processes. Moreover, we could potentially incur substantial legal costs in defending legal actions which allege patent infringement or by instituting patent infringement suits against others. Our commercial success also depends on us not infringing patents or proprietary rights of others and not breaching any license granted to us. There can be no assurance that we will be able to maintain such licenses that we may require to conduct our business or that such licenses have been obtained at a reasonable cost. Furthermore, there can be no assurance that we will be able to remain in compliance with our licenses. Consequently, there may be a risk that such licenses may be withdrawn with no compensation or penalties to us.
Our inability to achieve timelines for publicly disclosed projects may result in material adverse effects on our business, financial condition and results of operations.
Our business is dependent on a number of key inputs and their related costs including raw materials and supplies related to our operations, as well as electricity, water and other utilities. Any significant interruption or negative change in the availability or economics of the supply chain for key inputs could materially impact the business, financial condition operating results, and timelines for our project development. Any inability to secure required supplies and services or to do so on appropriate terms could have a materially adverse impact on the business, financial condition, operating results, and timelines for our project development.
We may need additional capital for future operations and if we are not able to secure any required capital, we may be forced to curtail or discontinue our operations.
It is possible that costs associated with the operating our business will exceed our projections depending on the timing of future operating and capital expenses. Assuming our existing funds sustain our operations for next 12 months, we believe that we may thereafter require additional capital for additional product development, sales and marketing operations, other operating expenses and for general corporate purposes to fund growth in our Company's markets. We do not know how much additional funding we may require. We may therefore be required to seek other sources of financing in the future, which sources (assuming we are able to locate such alternative sources of financing) may be on terms less favorable to us than those of our previous securities offerings. Any additional equity financing may be dilutive to shareholders, and debt financing, if available, may involve restrictive covenants. If additional funds are raised through the issuance of equity securities, the percentage ownership of our shareholders will be reduced, shareholders may experience additional dilution in net book value per share, or such equity securities may have rights, preferences or privileges senior to those of the holders of the Common Shares. If adequate funds are not available on acceptable terms, we may be unable to develop or enhance our products and services, take advantage of future opportunities or respond to competitive pressures, any of which could have a material adverse effect on our business, financial condition and operating results, or we may be forced to curtail or cease our operations.
We may become subject to product liability claims, which could harm our financial condition and liquidity if we are not able to successfully defend or insure against such claims.
The risk of product liability is inherent in the research, development, manufacturing, marketing and use of pharmaceutical products. Product candidates and products that we may commercially market in the future may cause, or may appear to have caused, injury or dangerous drug reactions, and expose us to product liability claims. These claims might be made by patients who use the product, healthcare providers, pharmaceutical companies, corporate collaborators or others selling such products. If our product candidates during clinical trials were to cause adverse side effects, we may be exposed to substantial liabilities. Regardless of the merits or eventual outcome, product liability claims or other claims related to our product candidates may result in:
-
decreased demand for our products due to negative public perception;
-
injury to our reputation;
-
withdrawal of clinical trial participants or difficulties in recruiting new trial participants;
-
initiation of investigations by regulators;
-
costs to defend or settle related litigation;
-
a diversion of management's time and resources;
-
substantial monetary awards to trial participants or patients;
-
product recalls, withdrawals or labeling, marketing or promotional restrictions;
-
loss of revenues from product sales; and
-
the inability to commercialize any of product candidates, if approved.
We intend to obtain clinical trial insurance once a clinical trial is initiated. However, the insurance coverage may not be sufficient to reimburse us for any expenses or losses we may suffer. Insurance coverage is becoming increasingly expensive, and, in the future, we, or any of our collaborators, may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts or at all to protect against losses due to liability. Even if our agreements with any future collaborators entitle us to indemnification against product liability losses, such indemnification may not be available or adequate should any claim arise. Our inability to obtain sufficient product liability insurance at an acceptable cost to protect against product liability claims could prevent or inhibit the commercialization of our product candidates. If a successful product liability claim or a series of claims is brought against us for uninsured liabilities or in excess of insured liabilities, our assets may not be sufficient to cover such claims and our business operations could be impaired.
Should any of the events described above occur, this could have a material adverse effect on our business, financial condition and results of operations.
Health and safety issues related to our products may have a material adverse effect on our business and results of operations.
Health and safety issues related to our products may arise that could lead to litigation or other action against us or to regulation of certain of our product components. We may be required to modify our products and may also be required to pay damages that may reduce our profitability and adversely affect our financial condition. Even if these concerns prove to be baseless, the resulting negative publicity could affect our ability to market certain of our products and, in turn, could harm our business and results from operations.
We have international operations, which subjects us to risks inherent with operations outside of Canada.
We have international operations and may seek to obtain market approvals in foreign markets that we deem could generate significant opportunities. However, even with the cooperation of a commercialization partner, conducting drug development in foreign countries involves inherent risks, including, but not limited to: difficulties in staffing, funding and managing foreign operations; different and unexpected changes in regulatory requirements; export restrictions; tariffs and other trade barriers; different reimbursement systems; economic weaknesses or political instability in particular foreign economies and markets; compliance with tax, employment, immigration and labour laws for employees living or travelling abroad; supply chain and raw materials management; difficulties in protecting, acquiring, enforcing and litigating intellectual property rights; fluctuations in currency exchange rates; and potentially adverse tax consequences.
If we were to experience any of the difficulties listed above, or any other difficulties, our international development activities and our overall financial condition may suffer and cause it to reduce or discontinue our international development and market approval efforts.
Exchange rate fluctuations between the U.S. dollar and the Canadian dollar may negatively affect our earnings and cash flows.
Our functional currency is the Canadian dollar. We may incur expenses in Canadian Dollars and U.S. dollars. As a result, we are exposed to the risks that the Canadian dollar may devalue relative to the U.S. Dollar, or, if the Canadian dollar appreciates relative to the U.S. Dollar, that the inflation rate in Canada may exceed such rate of devaluation of the Canadian dollar, or that the timing of such devaluation may lag behind inflation in Canada. We cannot predict any future trends in the rate of inflation in Canada or the rate of devaluation, if any, of the Canadian dollar against the U.S. Dollar.
If patent laws or the interpretation of patent laws change, our competitors may be able to develop and commercialize our discoveries.
Important legal issues remain to be resolved as to the extent and scope of available patent protection for biopharmaceutical products and processes in Canada and other important markets outside Canada, such as Europe or the United States. As such, litigation or administrative proceedings may be necessary to determine the validity, scope and ownership of certain of ours and others' proprietary rights. Any such litigation or proceeding may result in a significant commitment of resources in the future and could force us to do one or more of the following: cease selling or using any of our future products that incorporate a challenged intellectual property, which would adversely affect our revenue; obtain a license or other rights from the holder of the intellectual property right alleged to have been infringed or otherwise violated, which license may not be available on reasonable terms, if at all; and redesign our future products to avoid infringing or violating the intellectual property rights of third parties, which may be time-consuming or impossible to do. In addition, changes in patent laws in Canada and other countries may result in allowing others to use our discoveries or develop and commercialize our products. We cannot provide assurance that the patents we obtain will afford us significant commercial protection.
We may not be able to enforce our intellectual property rights throughout the world. This risk is exacerbated because we expect that one or more of our product candidates will be manufactured and used in a number of foreign countries.
The laws of foreign countries may not protect intellectual property rights to the same extent as the laws of Canada. Many companies have encountered significant problems in protecting and defending intellectual property rights in certain foreign jurisdictions. This risk is exacerbated for us because we expect that future product candidates could be manufactured, and used in a number of foreign countries.
The legal systems of some countries, particularly developing countries, do not favor the enforcement of patents and other intellectual property protection, especially those relating to life sciences. This could make it difficult to stop the infringement or other misappropriation of our intellectual property rights. For example, several foreign countries have compulsory licensing laws under which a patent owner must grant licenses to third parties. In addition, some countries limit the enforceability of patents against third parties, including government agencies or government contractors. In these countries, patents and trade secrets may provide limited or no benefit.
Most jurisdictions in which we intend to apply for patents have patent protection laws similar to those of Canada, but some of them do not. For example, we may do business in the future in countries that may not provide the same or similar protection as that provided in Canada. Additionally, due to uncertainty in patent protection law, we have not filed applications in many countries where significant markets exist.
Proceedings to enforce patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business. Accordingly, efforts to protect intellectual property rights in such countries may be inadequate. In addition, changes in the law and legal decisions by courts in Canada, the U.S., and foreign countries may affect our ability to obtain adequate protection for our technology and the enforcement of our intellectual property.
The lack of product for commercialization would have a material adverse effect on our business, financial condition and results of operations.
We cannot successfully develop, manufacture and distribute our products, or if we experience difficulties in the development process, such as capacity constraints, quality control problems or other disruptions, we may not be able to develop market-ready commercial products at acceptable costs, which would adversely affect our ability to effectively enter the market. A failure by us to achieve a low-cost structure through economies of scale or improvements in cultivation and manufacturing processes would have a material adverse effect on our commercialization plans and our business, prospects, results of operations and financial condition.
Failure to develop new and innovative products may have a material adverse effect on our business.
Our success will depend, in part, on our ability to develop, introduce and market new and innovative products. If there is a shift in consumer demand, we must meet such demand through new and innovative products or else our business will fail. Our ability to develop, market and produce new products is subject to us having substantial capital. There is no assurance that we will be able to develop new and innovative products or have the capital necessary to develop such products.
The lack of experience of our management in marketing, selling, and distributing products may have a material adverse effect on our business and financial condition.
Our management's lack of experience in marketing, selling, and distributing our products could lead to poor decision-making, which could result in cost-overruns and/or the inability to produce the desired products. Although our management intends to hire experienced and qualified staff, this inexperience could also result in our inability to consummate revenue contracts or any contracts at all. Any combination of the aforementioned may result in the failure of our business and a loss of your investment.
The size of our target market is difficult to quantify, and investors will be reliant on their own estimates on the accuracy of market data.
Because the industry in which we operate is in a nascent stage with uncertain boundaries, there is a lack of information about comparable companies available for potential investors to review in deciding whether to invest in us and, few, if any, established companies whose business model we can follow or upon whose success we can build. Accordingly, investors will have to rely on their own estimates in deciding about whether to invest in us. There can be no assurance that our estimates are accurate or that the market size is sufficiently large for our business to grow as projected, which may negatively impact our financial results.
You may face difficulties in protecting your interests, and your ability to protect your rights through the U.S. federal courts may be limited because we are incorporated under the laws of the Province of British Columbia, a substantial portion of our assets are in Canada and some of our executive officers and directors reside outside the United States
We are organized pursuant to the laws of the Province of British Columbia under the Business Corporations Act (British Columbia) (the "BCBCA"), and our executive offices are located outside of the United States in Vancouver, British Columbia. Two of our four officers, our auditor and all but two directors reside outside the United States. In addition, a substantial portion of their assets and our assets are located outside of the United States. As a result, you may have difficulty serving legal process within the United States upon us or any of these persons. You may also have difficulty enforcing, both in and outside of the United States, judgments you may obtain in U.S. courts against us or these persons in any action, including actions based upon the civil liability provisions of U.S. federal or state securities laws. Furthermore, there is substantial doubt as to the enforceability in Canada against us or against any of our directors, officers and the expert named in this Registration Statement who are not residents of the United States, in original actions or in actions for enforcement of judgments of U.S. courts, of liabilities based solely upon the civil liability provisions of the U.S. federal securities laws. In addition, shareholders in British Columbia companies may not have standing to initiate a shareholder derivative action in U.S. federal courts. As a result, our public shareholders may have more difficulty in protecting their interests through actions against us, our management, our directors or our major shareholders than would shareholders of a corporation incorporated in a jurisdiction in the United States.
We continue to sell shares for cash to fund operations, capital expansion, mergers and acquisitions that will dilute our current shareholders.
There is no guarantee that we will be able to achieve our business objectives. Our continued development will require additional financing. The failure to raise such capital could result in the delay or indefinite postponement of current business objectives or us going out of business. There can be no assurance that additional capital or other types of financing will be available if needed or that, if available, the terms of such financing will be favorable to us.
If additional funds are raised through issuances of equity or convertible debt securities, existing shareholders could suffer significant dilution. Our articles permit the issuance of an unlimited number of Common Shares, and shareholders will have no pre-emptive rights in connection with such further issuance. Our directors have discretion to determine the price and the terms of issue of further issuances. In addition, from time to time, we may enter into transactions to acquire assets or the shares of other companies. These transactions may be financed wholly or partially with debt, which may temporarily increase our debt levels above industry standards. Any debt financing secured in the future could involve restrictive covenants relating to capital raising activities and other financial and operational matters, which may make it more difficult for us to obtain additional capital and to pursue business opportunities, including potential acquisitions. We may require additional financing to fund our operations to the point where it is generating positive cash flows. Negative cash flow may restrict our ability to pursue our business objectives.
If you purchase our Common Shares in an offering, you will experience substantial and immediate dilution, because the price that you pay may be substantially greater than the net tangible book value per share of our Common Shares that you acquire. This dilution is due in large part to the fact that our earlier investors will most likely have paid substantially less than the offering price which you may pay if you purchase our Common Shares.
Our officers and directors may be engaged in a range of business activities resulting in conflicts of interest, which may have a material adverse effect on our operations.
We may be subject to various potential conflicts of interest because some of our officers and directors may be engaged in a range of business activities. In addition, our executive officers and directors may devote time to their outside business interests, so long as such activities do not materially or adversely interfere with their duties to us. In some cases, our executive officers and directors may have fiduciary obligations associated with these business interests that interfere with their ability to devote time to our business and affairs and that could adversely affect our operations. These business interests could require significant time and attention of our executive officers and directors.
In addition, we may become involved in other transactions which conflict with the interests of our directors and officers who may from time to time deal with persons, firms, institutions or companies with which we may be dealing, or which may be seeking investments similar to those desired by us. The interests of these persons could conflict with ours. In addition, from time to time, these persons may be competing with us for available investment opportunities. Conflicts of interest, if any, will be subject to the procedures and remedies provided under applicable laws. In particular, if such a conflict of interest arises at a meeting of our directors, a director who has such a conflict will abstain from voting for or against the approval of such participation or such terms. In accordance with applicable laws, our directors are required to act honestly, in good faith and in our best interests.
In certain circumstances, our reputation could be damaged, which may have a material adverse effect on our financial performance, financial condition, cash flows and growth prospects.
Damage to our reputation can be the result of the actual or perceived occurrence of any number of events, and could include any negative publicity, whether true or not. The increased usage of social media and other web-based tools used to generate, publish and discuss user-generated content and to connect with other users has made it increasingly easier for individuals and groups to communicate and share opinions and views regarding us and our activities, whether true or not. Although we believe that we operate in a manner that is respectful to all stakeholders and that we take care in protecting our image and reputation, we do not ultimately have direct control over how we are perceived by others. Reputation loss may result in decreased investor confidence, increased challenges in developing and maintaining community relations and an impediment to our overall ability to advance our projects, thereby having a material adverse impact on financial performance, financial condition, cash flows and growth prospects.
We have negative operating cash flow.
Our business has incurred losses since its inception. Although we expect to become profitable, there is no guarantee that will happen, and we may never become profitable. We currently have a negative operating cash flow and may continue to have a negative operating cash flow for the foreseeable future. To date, we have not generated any revenues and a large portion of our expenses are fixed, including expenses related to facilities, equipment, contractual commitments and personnel. As a result, we expect our net losses from operations to improve. Our ability to generate additional revenues and potential to become profitable will depend largely on our ability to manufacture and market our products and services. There can be no assurance that any such events will occur or that the Company will ever become profitable. Even if we do achieve profitability, we cannot predict the level of such profitability. If we sustain losses over an extended period of time, we may be unable to continue our business.
Our forward-looking statements may prove to be inaccurate.
Investors should not place undue reliance on forward-looking statements. By their nature, forward-looking statements involve numerous assumptions, known and unknown risks and uncertainties, of both general and specific nature, that could cause actual results to differ materially from those suggested by the forward-looking statements or contribute to the possibility that predictions, forecasts or projections will prove to be materially inaccurate. Additional information on the risks, assumptions and uncertainties can be found in this Registration Statement under the heading "Forward-Looking Statements".
If we have a material weakness in our internal controls over financial reporting, investors could lose confidence in the reliability of our financial statements, which could result in a decrease in the value of its securities.
One or more material weaknesses in our internal controls over financial reporting could occur or be identified in the future. In addition, because of inherent limitations, our internal controls over financial reporting may not prevent or detect misstatements, and any projections of any evaluation of effectiveness of internal controls to future periods are subject to the risk that controls may become inadequate because of changes in conditions or that the degree of compliance with our policies or procedures may deteriorate. If we fail to maintain the adequacy of our internal controls, including any failure or difficulty in implementing required new or improved controls, our business and results of operations could be harmed, we may not be able to provide reasonable assurance as to our financial results or meet our reporting obligations and there could be a material adverse effect on the price of our securities.
Difficulties with forecasts.
We must rely largely on our own market research to forecast sales as detailed forecasts are not generally obtainable from other sources at this early stage of the biotechnology industry dedicated to the discovery of serotonergic therapeutics. A failure in the demand for its products and services to materialize as a result of competition, technological change or other factors could have a material adverse effect on our business, results of operations and financial condition.
COVID-19 may materially and adversely affect our business and financial results.
Our business could be materially and adversely affected by health epidemics in regions where we conduct research and development activities.
In December 2019, a novel strain of COVID-19 was reported in China. Since then, COVID-19 has spread globally. On March 11, 2020, the World Health Organization (WHO) declared the outbreak of COVID-19 as a "pandemic", or a worldwide spread of a new disease. Many countries around the world, including Canada, the United States and most countries in Europe, have imposed quarantines and restrictions on travel and mass gatherings to slow the spread of the virus, and have closed non-essential businesses.
The COVID-19 pandemic and any other health epidemics have the potential to cause significant disruption in the operations of the laboratories upon whom we rely, including laboratories situated in various parts of the United States and Europe. We are reliant on the continued operations of such laboratories. The regulations imposed by governments in response to the COVID-19 pandemic may cause laboratories to operate at limited occupancy rates, which may slow the rate at which research and development activities can be conducted. We may not have control over the protocols adopted in response to the COVID-19 pandemic by such laboratories in response to the regulations imposed by the governments in the regions in which they operate. The effects of such protocols and/or regulations may negatively impact productivity, disrupt our business and delay our research and development timelines, as well as potentially impact our financial condition and result of operations. The magnitude of these potential effects is uncertain and will depend, in part, on the length and severity of the COVID-19 pandemic and the restrictions imposed by governments in response.
To the knowledge of our management as of the date hereof, COVID-19 does not present, at this time, any specific known impacts to us in relation to our timelines, business objectives or disclosed milestones related thereto. We rely on third parties to process and manufacture our products.
Risks Relating to the Common Shares
Our executive officers and directors beneficially own approximately 41% of our common shares.
As of June 15, 2021, our executive officers and directors beneficially own, in the aggregate, approximately 41% of our common shares, which includes shares that our executive officers and directors have the right to acquire pursuant to warrants and stock options which have vested. As a result, they are able to exercise a significant level of control over all matters requiring shareholder approval, including the election of directors, amendments to our Articles and approval of significant corporate transactions. This control could have the effect of delaying or preventing a change of control of our Company or changes in management and will make the approval of certain transactions difficult or impossible without the support of these shareholders.
The continued sale of our equity securities will dilute the ownership percentage of our existing shareholders and may decrease the market price for our common shares.
Our Notice of Articles authorize the issuance of an unlimited number of Common Shares. Our Board of Directors has the authority to issue additional shares of our capital stock to provide additional financing in the future. The issuance of any such Common Shares may result in a reduction of the book value or market price of our outstanding Common Shares. Given our lack of revenues, we will likely have to issue additional equity securities to obtain working capital we require in the future. Our efforts to fund our intended business plans will therefore result in dilution to our existing shareholders. If we do issue any such additional Common Shares, such issuance also will cause a reduction in the proportionate ownership and voting power of all other shareholders. As a result of such dilution, if you acquire Common Shares your proportionate ownership interest and voting power could be decreased. Furthermore, any such issuances could result in a change of control or a reduction in the market price for our Common Shares.
Additionally, we had 1,017,000 stock options and 3,974,204 warrants outstanding as of June 15, 2021. The exercise price of some of these options and warrants is below our current market price, and you could purchase shares in the market at a price in excess of the exercise price of our outstanding warrants or options. If the holders of these options and warrants elect to exercise them, your ownership position will be diluted and the per share value of the Common Shares you have or acquire could be diluted as well. As a result, the market value of our Common Shares could significantly decrease as well.
The market price of our Common Shares may be volatile and may fluctuate in a way that is disproportionate to our operating performance.
Securities of companies with a small market capitalization have experienced substantial volatility in the past, often based on factors unrelated to the companies' financial performance or prospects. These factors include macroeconomic developments in North America and globally, as well as market perceptions of the attractiveness of particular industries. Factors unrelated to the Company's performance that may affect the price of the Common Shares include the following: the extent of analytical coverage available to investors concerning our business may be limited if investment banks with research capabilities do not follow us; lessening in trading volume and general market interest in the Common Shares may affect an investor's ability to trade significant numbers of Common Shares; the size of our public float may limit the ability of some institutions to invest in Common Shares; and a substantial decline in the price of the Common Shares that persists for a significant period of time could cause the Common Shares, if listed on an exchange, to be delisted from such exchange, further reducing market liquidity. As a result of any of these factors, the market price of the Common Shares at any given point in time may not accurately reflect our long-term value. Securities class action litigation often has been brought against companies following periods of volatility in the market price of their securities. We may in the future be the target of similar litigation. Securities litigation could result in substantial costs and damages and divert management's attention and resources.
The market price of the Common Shares is affected by many other variables which are not directly related to our success and are, therefore, not within our control. These include other developments that affect the breadth of the public market for the Common Shares, the release or expiration of lock-up or other transfer restrictions on the Common Shares, and the attractiveness of alternative investments. The effect of these and other factors on the market price of the Common Shares is expected to make the Common Share price volatile in the future, which may result in losses to investors.
Our stock price is expected to be volatile and will be drastically affected by governmental and regulatory regimes and other factors outside of our control. We cannot fully predict the results of our operations expected to take place in the future. The results of these activities will inevitably affect our decisions related to future operations and will likely trigger major changes in the trading price of the Company shares.
We do not intend to pay dividends and there will thus be fewer ways in which you are able to make a gain on your investment.
We have never paid any cash or stock dividends and we do not intend to pay any dividends for the foreseeable future. To the extent that we require additional funding in the future, our funding sources may prohibit the payment of any dividends. Because we do not intend to declare dividends, any gain on your investment will need to result from an appreciation in the price of our Common Shares. There will therefore be fewer ways in which you are able to make a gain on your investment.
FINRA sales practice requirements may limit your ability to buy and sell our Common Shares, which could depress the price of our shares.
Financial Industry Regulation Authority ("FINRA") rules require broker-dealers to have reasonable grounds for believing that an investment is suitable for a customer before recommending that investment to the customer. Prior to recommending speculative low-priced securities to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer's financial status, tax status and investment objectives, among other things. Under interpretations of these rules, FINRA believes that there is a high probability such speculative low-priced securities will not be suitable for at least some customers. Thus, FINRA requirements may make it more difficult for broker-dealers to recommend that their customers buy our Common Shares, which may limit your ability to buy and sell our Common Shares, have an adverse effect on the market for our Common Shares and, thereby, depress their market prices.
Our Common Shares have been thinly traded, and you may be unable to sell at or near ask prices or at all if you need to sell your Common Shares to raise money or otherwise desire to liquidate your shares.
From February 8, 2021, our Common Shares have been trading on the Canadian Securities Exchange where they are "thinly-traded", meaning that the number of persons interested in purchasing our Common Shares at or near bid prices at any given time was relatively small or non-existent. This could be due to a number of factors, including that we are relatively unknown to stock analysts, stock brokers, institutional investors and others in the investment community that generate or influence sales volume, and that even if we came to the attention of such persons, they tend to be risk-averse and might be reluctant to follow an unproven company such as ours or purchase or recommend the purchase of our Common Shares until such time as we became more seasoned. As a consequence, there may be periods of several days or more when trading activity in our common shares is minimal or non-existent, as compared to a seasoned issuer which has a large and steady volume of trading activity that will generally support continuous sales without an adverse effect on share price. Broad or active public trading market for our Common Shares may not develop or be sustained.
We are a foreign private issuer within the meaning of the rules under the Exchange Act, and as such we are exempt from certain provisions applicable to United States domestic public companies.
We are a foreign private issuer within the meaning of the rules under the Exchange Act. As such, we are exempt from certain provisions applicable to United States domestic public companies. For example:
| • | we are not required to provide as many Exchange Act reports, or as frequently, as a domestic public company; | |
| • | for interim reporting we are permitted to comply solely with our home country requirements, which are less rigorous than the rules that apply to domestic public companies; | |
| • | we are not required to provide the same level of disclosure on certain issues, such as executive compensation; | |
| • | we are exempt from provisions of Regulation FD aimed at preventing issuers from making selective disclosures of material information; | |
| • | we are not required to comply with the sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations in respect of a security registered under the Exchange Act; and | |
| • | we are not required to comply with Section 16 of the Exchange Act requiring insiders to file public reports of their share ownership and trading activities and establishing insider liability for profits realized from any "short-swing" trading transaction. |
Our shareholders may not have access to certain information they may deem important and are accustomed to receive from U.S. reporting companies.
As an "emerging growth company" under applicable law, we will be subject to lessened disclosure requirements. Such reduced disclosure may make our common shares less attractive to investors.
For as long as we remain an "emerging growth company", as defined in the JOBS Act, we will elect to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not "emerging growth companies" and including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports, exemptions from the requirements of holding a non-binding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. Because of these lessened regulatory requirements, our shareholders would be left without information or rights available to shareholders of more mature companies. If some investors find our Common Shares less attractive as a result, there may be a less active trading market for such securities and their market prices may be more volatile.
We incur significant costs as a result of being a public company, which costs will grow after we cease to qualify as an "emerging growth company."
We incur significant legal, accounting and other expenses as a public company that we did not incur as a private company. The Sarbanes-Oxley Act, as well as rules subsequently implemented by the SEC and Nasdaq, impose various requirements on the corporate governance practices of public companies. We are an "emerging growth company", as defined in the JOBS Act, and will remain an emerging growth company until the earlier of : (1) the last day of the fiscal year (a) following the fifth anniversary of the date of the first sale of our common equity securities pursuant to an effective registration statement under the U.S. Securities Act, (b) in which we have total annual gross revenue of at least US$1.07 billion or (c) in which we are deemed to be a large accelerated filer, which means the market value of our common shares that is held by non-affiliates exceeds US$700 million as of the prior June 30th; and (2) the date on which we have issued more than US$1.0 billion in non-convertible debt during the prior three-year period. An emerging growth company may take advantage of specified reduced reporting and other requirements that are otherwise applicable generally to public companies. These provisions include exemption from the auditor attestation requirement under Section 404 of the Sarbanes-Oxley Act in the assessment of the emerging growth company's internal control over financial reporting and permission to delay adopting new or revised accounting standards until such time as those standards apply to private companies.
Compliance with these rules and regulations increases our legal and financial compliance costs and makes some corporate activities more time-consuming and costlier. After we are no longer an emerging growth company, we expect to incur significant expenses and devote substantial management effort toward ensuring compliance with the requirements of Section 404 and the other rules and regulations of the SEC. For example, as a public company, we have been required to increase the number of independent directors and adopt policies regarding internal controls and disclosure controls and procedures. We have incurred additional costs in obtaining director and officer liability insurance. In addition, we incur additional costs associated with our public company reporting requirements. It may also be more difficult for us to find qualified persons to serve on our board of directors or as executive officers. We are currently evaluating and monitoring developments with respect to these rules and regulations, and we cannot predict or estimate with any degree of certainty the amount of additional costs we may incur or the timing of such costs.
ITEM 4. INFORMATION ON THE COMPANY
History and development of the Company
Bright Minds was incorporated under the BCBCA on May 31, 2019 under the name "1210954 B.C. Ltd." On March 6, 2020, the Company changed its name under the BCBCA to "Bright Minds Biosciences Inc." The head office of the Company is 1500 - 1055 West Georgia Street, Vancouver, British Columbia, V6E 4N7 and the registered and records office of the Company is 1500 - 1055 West Georgia Street, Vancouver, British Columbia, V6E 4N7.
The Company has three wholly owned subsidiaries, PsilocybinLabs Ltd., a company incorporated under the BCBCA, Bright Minds Biosciences LLC, a limited liability company formed pursuant to the laws of Delaware, and Bright Minds Bioscience Pty. Ltd., a company formed pursuant to the laws of Australia. None of the subsidiaries are a material subsidiary to the Company.
The Common Shares are listed on the CSE under the trading symbol "DRUG". The Company is a reporting issuer in all provinces of Canada (except Quebec). The Company's principal expenditures are focussed on research and development costs relating to the creation of a pipeline of medicines to improve the lives of patients with severe and life-altering diseases. The Company is based in British Columbia, Canada with vendors across North America. The primary source of financing has been through the sale of common shares of the Company.
Additional information related to the Company is available on SEDAR at www.sedar.com and www.brightmindsbio.com. We do not incorporate the contents of our website or of sedar.com into this Registration Statement. Information on our website does not constitute part of this Registration Statement. In addition, the U.S. Securities and Exchange Commission (the "SEC") maintains an internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC which can be viewed as www.sec.gov.
General Description of Business
Overview
Bright Minds is a biotechnology company dedicated to developing therapeutics to improve the lives of patients with severe and life-altering diseases. Bright Minds is creating new chemical entities as targeted therapeutic agents for treatment of disorders where a serotonin (5-HT) receptor (either 5-HT2A, 5-HT2C or 5-HT2A/C) driven mechanism is the underlying pathology. These targeted neurocircuit disorders include neuropsychiatric, neurodegenerative, neuroinflammatory, and pain disorders. Examples of these diseases include 5-HT2C disorders like- tobacco, opiate, and cocaine addiction, binge eating disorder, alcoholism, dementia related psychosis, 5-HT2A receptor disorders like depression, post-traumatic stress disorder (PTSD), and 5-HT2A/C neuropathic pain syndromes including cluster headaches, and chemotherapy induced peripheral neuropathy.
By leveraging the extensive drug discovery experience of the Bright Minds team, the Company continues to create a pipeline of 5-HT agonists with designer drug characteristics. The Bright Minds compounds are also biased agonists designed to have less receptor desensitization and tolerance to drug effects. The Company’s goal is to develop molecules that achieve selectivity for specific 5-HT2A and 5-HT2C receptor subtypes, while avoiding the valvulopathy related to 5-HT2B receptor agonist activity.
Principal Products
Serotonin (5-HT) is the most prominent neurotransmitter in the brain and modulates many functions. Dysfunction of serotonin receptors, transporters, and associated neurocircuits is fundamental to many diseases including epilepsy, pain, and neuropsychiatry. Selective serotonin reuptake inhibitors are widely used in the treatment of depression with a market of $14.3 Billion1. Similarly, other serotoninergic drugs are widely used in the treatment of pain (Triptans in migraine)2, Parkinson's disease related psychosis (Pimavanserin)3, and seizures (Fintepla)4. The Company believes the full potential of serotonin-based therapeutics has not been achieved due to the lack of drugs that are specific to certain serotonin receptor subtypes that are fundamental to disease pathology, without non-specific effects on other serotonin receptors in the body that are associated with cardiac toxicities and have resulted in previous drugs being withdrawn from the market.5

Bright Minds has a portfolio of patented, selective serotonin (5-HT2C and 5-HT2C/A-receptor subtypes) agonists that were identified using high-throughput screening methods in combination with advanced molecular modeling techniques to interrogate the interaction between the drug and its targeted receptors to increase downstream signaling while avoiding off-target effects.
_____________________________________
1 Research and Markets, "Global Antidepressants Market (2020 to 2030) - COVID-19 Implications and Growth" (21 April 2020), online: Intrado GlobeNewswire <https://www.globenewswire.com/news-release/2020/04/21/2019282/0/en/Global-Antidepressants-Market-2020-to-2030-COVID-19-Implications-and-Growth.html>.
2 Samar Nicolas & Diala Nicolas, "Triptans" (26 May 2020), online: National Center for Biotechnology Information <https://www.ncbi.nlm.nih.gov/books/NBK554507/>.
3 Cerner Multum, "Pimavanserin" (5 February 2020), online: Drugs.com <https://www.drugs.com/mtm/pimavanserin.html>.
4 "Fintepla FDA Approval History", online: Drugs.com <https://www.drugs.com/history/fintepla.html>.
5 Joshua D. Hutcheson, et al., "Serotonin Receptors and Heart Valve Disease - it was meant 2B" (2 April 2011), 132(2) Pharmacol Ther 146-157, online: PMC <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3179857/>; Nistala Pallavi, "5-HT2B Receptor-mediated Cardiac Valvulopath" (2018), online (pdf): <https://scholarscompass.vcu.edu/cgi/viewcontent.cgi?article=6777&context=etd>.
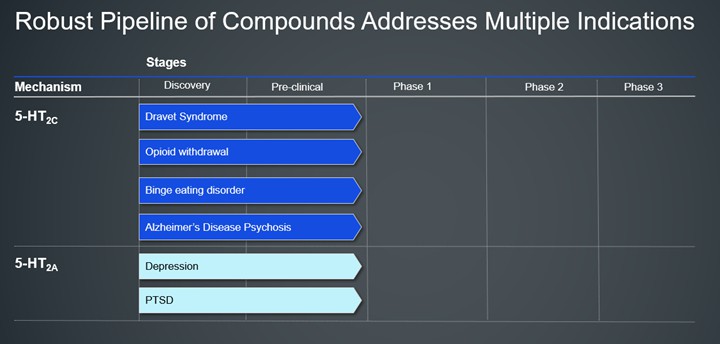
The Company’s lead 5-HT2 subtype selective drug portfolio candidate is a synthetic 5-HT2C receptor agonist without psychedelic effects. The Company expects its 5-HT2A and 5-HT2A/C selective compounds will possess desirable pharmacokinetic and pharmacodynamic effects and a wide effective dose range in the target populations, and could thus be used without the need for close supervision by psychotherapists in the clinic. Bright Minds is partnered with the National Institute of Neurological Disorders and Stroke (“NINDS”), a component of the National Institutes of Health, with respect to the Epilepsy Therapy Screening Program (the “ETSP Program”) which permits the Company to have its product candidates screened and tested by the NINDS for the purposes of determining whether the product candidates are anticonvulsant, antiepileptogenic, or have activity against resilient epilepsy and related disorders. The Company is also partnered with the NINDS with respect to the Pre-clinical Screening Platform for Pain Program (the “PSPP Program”) which permits the Company to have its product candidates screened and tested by the NINDS for the purpose of identifying non-opioid therapeutics for the treatment of pain. The NINDS screens and tests Company’s product candidates in both the ETSP Program and PSPP Program free of charge and the NINDS retains zero financial interest in the Company. Further, the NINDS is not permitted to use the Company’s product candidates tested in either the ETSP Program or PSPP Program for any commercial purposes nor is the NINDS permitted to make any derivative, resynthesize, or make any modification to the Company’s product candidates.
The Company has completed the following preclinical on its product candidates:
|
Program |
Indications |
Study |
Major Objective |
Study Outcome |
|||
|
5-HT2C |
- |
• |
ADMEPK (studies on |
• |
Describe the ADMEPK |
• |
Orally bioavailable |
|
|
|
|
absorption, distribution, |
|
profile of the test |
• |
Brain penetrant in mice, rats |
|
|
|
|
metabolism, and excretion |
|
compounds. Ensure the |
• |
Low plasma protein binding |
|
|
|
|
and pharmacokinetics) |
|
drug-like properties and |
• |
Stable after incubation with |
|
|
|
• |
Mouse, rat, dog, monkey PK |
|
estimating human PK |
|
rat, human and mouse |
|
|
|
• |
Brain binding and plasma |
|
properties |
|
microsomal enzymes |
|
|
|
• |
protein binding |
|
|
• |
Good IVIVC between IV |
|
|
|
Plasma stability, Hepatocyte |
|
|
|
CL and hepatocyte CL in |
|
|
|
|
|
stability, CYP (Cytochrome |
|
|
|
vitro. Moderate to low |
|
|
|
|
P450) inhibition, |
|
|
|
inhibition of major liver |
|
|
|
• |
Permeability in CaCO2 cells |
|
|
|
CYPs. |
|
|
|
Metabolites |
|
|
• |
No hERG inhibition |
|
|
|
|
|
identification/profiling in |
|
|
• |
Not cytotoxic or genotoxic |
|
|
|
|
cross-species |
|
|
• |
Favorable metabolite profile |
|
|
|
|
hepatocyte/plasma, Kinetic |
|
|
||
|
|
|
|
solubility |
|
|
|
|
|
|
- |
Formulation studies: |
• |
Perform preformulation |
• |
Test compound has a good | |
|
|
|
• |
Formulation stability and |
|
study of test compound, |
|
solubility in aqueous media |
|
|
|
|
homogeneity |
|
evaluate stability and |
|
and organic solvents. |
|
|
|
• |
Solubility at different pH |
|
solubility |
• |
The API (active |
|
|
|
|
solutions/solvents |
|
|
|
pharmaceutical ingredient) |
|
|
|
• |
Preformulation studies |
|
|
|
solid was chemically stable |
|
|
Dravet |
• |
Zebrafish and Mouse models |
• |
Define if test compound |
• |
Zebrafish treated with test |
|
|
Syndrome |
|
of Dravet Syndrome |
|
has an efficacy in animal |
|
compound experienced |
|
|
|
|
(Belgium, confidential |
|
models of Dravet |
|
reduced locomotion and |
|
|
|
|
collaboration) |
|
Syndrome |
|
duration of epileptiform, and |
|
|
|
|
|
|
|
|
mice treated with test |
|
|
|
|
|
|
|
|
compound experienced |
|
|
|
|
|
|
|
|
reduced duration of seizures. |
|
|
Epilepsy |
• |
NIH ETSP program |
• |
Test the compound in |
• |
Mice treated with test |
|
|
|
|
(collaboration on epilepsy). |
|
animal seizure models |
|
compound at higher dose |
|
|
|
|
Series of studies. |
|
|
|
experienced no seizures |
| • | Mouse Maximal | when induced at 0,25 and 1 | |||||
| Electroshock (MES),6 Hz | hour. | ||||||
|
|
|
|
Seizure, and Rotarod Motor |
|
|
• |
¾ of Mice treated with |
|
|
|
|
Impairment Assays |
|
|
|
middle dose experienced no |
| seizures at 0,25 hours. | |||||||
|
|
|
|
|
|
|
• |
Mice treated with lower |
| dose experienced no | |||||||
| difference in induced | |||||||
| seizures. | |||||||
|
|
Opioid |
• |
Substance use Disorder in |
• |
Determine the efficacy of |
• |
Rats treated with test |
|
|
withdrawal |
|
rats (Dr. Cunningham lab) |
|
test compound to suppress |
|
compound (in higher doses) |
|
|
(Opioid use |
|
|
|
drug intake in male rats |
|
experienced 65% less |
|
|
disorder, |
|
|
|
trained to stably self- |
|
fentanyl intake in an opioid |
| OUD) | administer fentanyl | use rat model | |||||
|
|
|
|
|
|
|
|
|
|
|
Opioid |
• |
Substance use Disorder in |
• |
Determine the efficacy of |
• |
In process |
|
|
|
rats (Dr. Cunningham lab) |
|
lead compound to reduce |
|
|
|
|
|
|
|
|
fentanyl seeking |
|
|
|
|
|
|
|
|
behaviour |
|
|
|
|
|
|
|
• |
New compound and |
|
|
|
|
|
|
|
|
additional dosages |
|
|
|
|
|
|
|
|
included in new study |
|
|
|
|
|
|
|
|
design |
|
|
|
|
|
Binge |
• |
BED trial in rats (Dr. |
• |
Determine the efficacy of |
• |
Rats treated with test |
|
|
Eating |
|
Cunningham) |
|
test compound to suppress |
|
compound experienced 47% |
|
|
Disorder |
|
|
|
binge eating behavior in |
|
fewer binge eating episodes |
|
(BED) |
male rats | in validated rat model to a | |||||
| similar extent as lorcaserin | |||||||
| (reference) | |||||||
|
|
Depression |
• |
Lead optimization - BRET |
• |
Describe the 5-HT2 |
• |
Design, molecule modeling, |
|
|
and PTSD |
|
(Bioluminescence |
|
profile of the test |
|
and synthesis continue to |
|
|
|
Resonance Energy Transfer) |
|
compounds |
|
identify highly selective/safe |
|
|
|
|
assays (John McCorvy) |
|
|
|
2A agonists |
|
|
5-HT2A |
Depression |
• |
Head Twitch Response trials |
• |
Evaluate head twitch |
• |
Promising 5-HT2A |
|
|
and PTSD |
|
(Halberstadt lab) |
|
response in mice |
|
compounds with minimal 2B |
|
|
To be |
|
|
|
|
|
agonism available |
|
|
determined |
ADMEPK studies: |
• |
Describe the ADMEPK |
• |
Plasma PK profile |
|
|
|
|
• |
Brain binding and plasma |
|
properties in mice. Ensure |
• |
Brain penetrant in mice |
|
|
|
|
protein binding |
|
the drug-like properties |
|
|
|
|
|
• |
Mouse PK: ip PK and brain |
|
|
|
|
|
|
|
|
penetration |
|
|
|
|
|
|
• |
NIH PSPP collaboration |
• |
In vitro opioid & abuse |
• |
The test compound has | |
|
|
|
|
|
|
liability, PK and protein |
|
passed the Tier 1 and is in |
|
|
|
|
|
|
binding studies |
|
process of Tier 2 studies |
|
|
|
|
|
|
|
|
(rotarod test will start in |
|
|
|
|
|
|
|
|
August) |
|
5-Ht2a+2c |
SmartCube |
• |
PsychoGenics Inc. |
• |
To assess the potential of |
• |
Contract and plans mutually |
|
|
|
|
collaboration |
|
compounds to treat |
|
agreed |
|
|
|
|
|
|
psychiatric disorders by |
|
|
|
|
|
|
|
|
comparing their complex |
|
|
|
|
|
|
|
|
behavioral profiles with |
|
|
|
|
|
|
|
|
those from a proprietary |
|
|
|
|
|
|
|
|
reference database at the |
|
|
|
|
|
|
|
|
drug class level |
|
|
Competition
The biotechnology and biopharmaceutical industries, and the neurological subsector, are characterized by rapid evolution of technologies, fierce competition, and strong defense of intellectual property. Any product candidates that we successfully develop and commercialize will have to compete with existing therapies and new therapies that may become available in the future. While we believe that our rational approach to drug design, along with our scientific expertise in the field of serotonergic drugs and central nervous system ("CNS") function, provide us with competitive advantages, a wide variety of institutions, including large biopharmaceutical companies, specialty biotechnology companies, academic research departments, and public and private research institutions, are actively developing potentially competitive products and technologies. Our competitors generally fall within the following categories:
-
Antidepressants and anxiolytics. Alkermes Plc, Allergan Plc, Bristol Myers Squibb Co., Eli Lilly and Co., GlaxoSmithKline Plc, H. Lundbeck, Eli Lilly and Co., Merck & Co. Inc., Pfizer Inc., and Takeda Pharmaceutical Co. Ltd Teva Pharmaceutical Industries Ltd., AstraZeneca, Johnson & Johnson, and others.
-
Treatment of cluster headache. Amgen, Novartis, Teva, Eli Lilly, Lundbeck, Allergan, Generic Drugs.
-
Binge eating disorder. Takeda Pharmaceutical Company Limited, Sunovion Pharmaceuticals Inc., H. Lundbeck A/S, Orexigen Therapeutics, Inc., Novo Nordisk A/S, Eli Lilly and Company, Jazz Pharmaceuticals Inc., and VIVUS Inc.
-
Epilepsy. LivaNova PLC, Johnson & Johnson Services Inc., Eisai Co. Ltd., GlaxoSmithKline PLC, Pfizer Inc., UCB SA, Medtronic PLC, NeuroPace Inc., Novartis AG, GW Pharmaceuticals PLC, and Abbott Laboratories, Zogenix.
- Opioid use disorder. Merck & Co., Inc, Teva Pharmaceutical Industries Ltd, Pfizer Inc, Novartis, Sanofi N.V, Johnson & Johnson Services, F. Hoffmann-La Roche Ltd, Bayer AG, Alkermes.
Patents and Patent Applications
Kozikowski-Roth Patents
The Company has exclusively licensed a family of patents based on PCT/US2011/023535, which is co-owned by the University of Illinois Chicago and the University of North Carolina. This family of licensed patents includes patents granted in Australia (AU Pat No 2011212930), Canada (CA Pat No 2788416), Europe (EU Pat No 2531485), Japan (JP Pat No 5810099), United States (US Pat No 8492591 and US Pat No 8754132). In addition, the Company has exclusively licensed a family of patents based on PCT/US2016/015019, which is solely owned by the University of Illinois Chicago. This family of licensed patents includes patents applied for or granted in China (CN Publication No 107810175), Europe (EU Publication No 3250549), Hong Kong SAR (HK Publication No 1251831), and the United States (US Pat No 10407381). The latest patent to issue is US Pat No 10,407,381 which will expire on January 27, 2036.
These patents were based on the past research completed by Dr. Alan Kozikowski and Dr. Bryan Roth that is documented in United States publication number US20090203750A1 "5-HT2C Receptor Agonists as Anorectic Agents". The invention related to the discovery of novel selective 5-HT2C and 5-HT2C/A agonists that could be used for the treatment of multiple neurological conditions.
On May 26, 2020, the Company entered into an option agreement (the “Roth Kozikowski Agreement”) with the University of Illinois at Chicago (“UIC”) in which UIC granted the Company, in consideration for an option fee, an exclusive option to: (i) evaluate the inventions described in PCT/US2011/023535 and all counterpart patents related thereto and described in PCT/US2016/015019 and all counterpart patents related thereto (collectively, the “Inventions”); and (ii) obtain an exclusive license to the Inventions.
On April 23, 2021, the Company entered into an exclusive license agreement (the “Exclusive License Agreement”) with UIC pursuant to the exercise of its option under the Roth Kozikowski Agreement. Pursuant to the terms and conditions of the Exclusive License Agreement, UIC granted the Company an exclusive license to the Inventions (the “License”). In consideration for the License, the Company (i) paid UIC a signing fee of USD$100,000, less USD$15,000 paid by the Company pursuant to the Roth Kozikowski Agreement; and (ii) issued 63,000 Common Shares at a deemed price of $5.85 per Common Share to the UIC (part of which was received by UIC on behalf of the University of North Carolina at Chapel Hill). Additionally, the Company agreed to pay UIC a royalty on net sales of products derived from the Inventions and a portion of all revenue received by the Company from sublicensees.
The Company may terminate the Exclusive License Agreement at any time on written notice to UIC at least ninety (90) days prior to the termination date specified in the notice. The notice of termination must also include the Company’s reason for such termination. UIC may terminate the Exclusive License Agreement if the Company: (a) fails to pay any amount, or provide any other consideration, or make any report when required, and the Company does not cure such failure within ninety (90) days after receiving notice thereof; (b) is in breach of any provision of the Exclusive License Agreement not covered by (a) and the Company does not cure such failure within forty-five (45) days after receiving notice thereof; (c) is in breach of any obligations that the Company has to UIC under any other agreement between the Company and UIC and the Company does not cure such failure within ninety (90) days after receiving notice thereof, however, should the Company be aware it is unable to remedy such breach within ninety (90) days, the Company shall have the option to provide written notice to UIC after which the Company and UIC shall negotiate in good faith to determine an appropriate extension to said ninety (90) day time frame; (d) makes any materially false report and receives written notice from UIC; (e) to the extent not prohibited by applicable law commences a voluntary case as a debtor under the Bankruptcy Code of the United States or any successor statute (the “Bankruptcy Code”), or if an involuntary case is commenced against the Company under the Bankruptcy Code, or if an order for relief shall be entered in such case, or if the same or any similar circumstance shall occur under the laws of any foreign jurisdiction; and/or (f) takes any action that purports to cause or causes any of the patent rights or technical information subject to the Exclusive License Agreement to be subject to any lien or encumbrance, and such termination shall be upon written notice to the Company.
Filed Patent Applications
Based upon molecular modeling studies in concert with data available from published research articles, the Bright Minds chemistry team designed novel analogs of psilocin that they believed would retain 5-HT2A activity while having no propensity to activate the 5-HT2B receptors. These new chemical entities were thus anticipated to retain the brain re-booting activity of psilocin while showing no propensity to cause valvulopathy issues.
In 2020, Bright Minds filed two provisional patent applications that cover psilocin analogs that have been decorated with functionality appropriate to achieving the goals of maintaining the desired 5-HT2A activity while being devoid of 5-HT2B activity:
|
Patent |
Region |
Title |
Inventors |
Applicant |
Filing Date |
Status as of |
|
62/988,926 |
USA |
Indole Compounds and Methods of Preparation Thereof |
Alan KOZIKOWSKI Gideon SHAPIRO Werner TUECKMANTEL |
Bright Minds Biosciences Inc. |
March 12, 2020 |
Expired |
|
63/017,627 |
USA |
Heterocyclic Compounds and Methods of Preparation Thereof |
Werner TUECKMANTEL Alan KOZIKOWSKI |
Bright Minds Biosciences Inc. |
April 29, 2020 |
Expired |
The Company has now synthesized and tested some of these designed analogs, and found that they possess the desired pharmacological profiles. On March 12, 2021, Bright Minds filed a Patent Cooperation Treaty patent application that claims priority to US 62/988,926. Such Patent Cooperation Treaty patent application has been assigned a serial number of PCT/CA2021/050336.
On April 29, 2021, US 63/017,627 expired without further public disclosure. On May 4, 2021, Bright Minds filed a new United States provisional application that has been assigned a serial number of US 63/184,040. US 63/184,040 includes subject matter that was previously recited in US 63/017,627.
On May 26, 2021, Bright Minds further filed a new United States provisional application that has been assigned a serial number of US 63/193,062. This patent application focuses on substitutions at a particular position on an indole structure.
Bright Minds is currently listed as an applicant in the following three active patent applications:
|
Patent Application |
Region |
Title |
Inventors |
Applicant |
Filing Date |
Status |
|
PCT/CA2021/050336 |
Patent Cooperation Treaty |
Indole Compounds and Methods of Preparation Thereof |
Alan KOZIKOWSKI Gideon SHAPIRO Werner TUECKMANTEL John McCORVY |
Bright Minds Biosciences Inc.; The Medical College of Wisconsin Inc. |
March 12, 2021 |
Pending |
|
63/184,040 |
USA |
Heterocyclic Compounds and Methods of Preparation Thereof |
Werner TUECKMANTEL Alan KOZIKOWSKI |
Bright Minds Biosciences Inc. |
May 4, 2021 |
Pending |
|
63/193,062 |
USA |
Heterocyclic Compounds and Methods of Preparation Thereof |
Alan KOZIKOWSKI Werner TUECKMANTEL John MCCORVY Uros LABAN |
Bright Minds Biosciences Inc.; The Medical College of Wisconsin Inc. |
May 26, 2021 |
Pending |
Trademarks
Bright Minds has applied to register the following trademark applications:
|
Trademark |
Country |
Application Number |
|
BRIGHT MINDS |
Canada |
2,016,213 |
|
BRIGHT MINDS |
United States of America |
90/245,748 |
Web Domains
Bright Minds has use and control over the following domain names: brightmindsbio.com.
Government Regulation
Regulatory Framework
Drug products must be approved by the appropriate governing body before it can be sold in that country or area. The United States Food and Drug Administration (the "FDA") approves products for the United States market and Health Canada approves products for the Canadian market. The European Medicines Agency approves products for the European Union. While the process by which products are approved by the FDA and Health Canada is very similar, each regulatory body has its own unique requirements for a product. In both cases, the development of a product through to approval can be a lengthy process and, in some cases, can take over 10 years. While early studies conducted in one jurisdiction will usually be accepted in the other, further and somewhat modified studies may be required to have a product approved in another jurisdiction.
Canadian Government Regulation
Drug products in Canada are regulated by Health Canada under the Food and Drugs Act and the Food and Drugs Regulations. Health Canada regulates, among other things, research and development activities and the testing, approval, manufacture, quality control, safety, effectiveness, labeling, storage, record keeping, advertising and promotion of any product candidates or commercial products.
For a drug product to be approved in Canada, it must provide sufficient evidence of safety, efficacy and chemical quality based on preclinical investigation and Phase I, II and III clinical trials using approved and compliant manufacturing and clinical sites.
To obtain approval to market a drug in Canada, a sponsor usually requests a pre-submission meeting with the review division of Health Canada responsible for the therapeutic field. If the meeting is granted, the sponsor must submit a Pre-Submission Information package to the Therapeutic Products Directorate ("TPD") to meet with the review division. This process occurs prior to submitting the New Drug Submission ("NDS") application. The purpose of the pre-submission meeting is to review the evidence (non-clinical and clinical research, quality information, indication) that will be submitted in the NDS application.
During the drug development process, the sponsor prepares study reports. Once the sponsor releases the last study required for the submission, the sponsor completes the NDS application and submits it to the TPD. Prior to submitting the NDS and, if applicable, based on the intended use of the product in the identified patient population, the sponsor may submit in advance a request for priority review status.
After submitting the NDS application, the file undergoes a screening process prior to being accepted for review. TPD has 45 calendar days from receipt to complete the screening review process. If granted a priority review, the screening period is reduced to 25 calendar days.
After a comprehensive review of an NDS application, Health Canada will issue a Notice of Compliance ("NOC") if the product is approved or a Notice of Non-Compliance ("NON") if further questions remain. If a NOC is issued, a Drug Identification Number ("DIN") is also issued that is required to be printed on each label of the product, as well as the final version of the Product Monograph that has been agreed to between Health Canada and the sponsor.
The average target time for reaching a first decision on an NDS is 300 calendar days, unless the submission has received a priority review in which case the time is 180 calendar days. Fees are levied for a review of an NDS application.
The regulatory approval process is generally lengthy and expensive, with no guarantee of a positive result. Once approved, Health Canada continues to monitor the product and license holders have obligations related to reporting to Health Canada, record keeping and ensuring continued safety and efficacy of the product. Failure to comply with any of the above applicable regulations, regulatory authorities or other requirements may result in civil or criminal penalties, recall or seizure of products, injunctive relief including partial or total suspension of production, or withdrawal of a product from the market.
United States Government Regulation
In the United States, the FDA regulates drugs under the United States Food, Drug, and Cosmetic Act (the "FDCA"), and its implementing regulations, and biologics under the FDCA and the Public Health Service Act, and its implementing regulations. FDA approval is required before any new unapproved drug or biologic or dosage form, including a new use of a previously approved drug, can be marketed in the United States. In some cases, changes to aspects of an approved drug product also require pre-approval prior to implementation of these changes. Drugs and biologics are also subject to other federal, state, and local statutes and regulations. If Bright Minds fails to comply with applicable FDA or other requirements at any time during the product development process, clinical testing, the approval process or after approval, Bright Minds may become subject to administrative or judicial sanctions. These sanctions could include the FDA's refusal to approve pending applications, license suspension or revocation, withdrawal of an approval, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, civil monetary penalties or criminal prosecution. Any FDA enforcement action could have a material adverse effect on Bright Minds.
The process required by the FDA before drug products may be marketed in the United States generally involves the following:
-
Completion of extensive preclinical laboratory tests and preclinical animal studies, some performed in accordance with the GLP regulations;
-
submission to the FDA of an Investigational New Drug ("IND") Application, which must be reviewed by the FDA and become active before human clinical trials may begin and must be updated annually;
-
approval by an independent review board ("IRB") or ethics committee representing each clinical site before each clinical trial may be initiated;
-
performance of adequate and well-controlled human clinical trials conducted under Good Clinical Practices ("GCP") to establish the safety and efficacy of the product candidate for each proposed indication;
-
preparation of and submission to the FDA of a New Drug Application ("NDA") or Biologics License Application ("BLA") after completion of all pivotal clinical trials;
-
a determination by the FDA within 60 days of its receipt of an NDA or BLA to file the application for review;
-
potential review of the product application by an FDA advisory committee, where appropriate and if applicable;
-
satisfactory completion of an FDA pre-approval inspection of the manufacturing facilities where the proposed product is produced to assess compliance with current GMP ("cGMP");
-
a potential FDA audit of the preclinical research and clinical trial sites that generated the data in support of the NDA or BLA; and
-
FDA review and approval of an NDA or BLA prior to any commercial marketing or sale of the product in the United States.
The preclinical research, clinical testing and approval process require substantial time, effort, and financial resources, and Bright Minds cannot be certain that any approvals for the Company's product candidates will be granted on a timely basis, if at all. An IND is a request for authorization from the FDA to administer an investigational new drug product to humans in clinical trials. The central focus of an IND submission is on the general investigational plan and the protocol(s) for human clinical trials. The IND also includes results of animal studies assessing the toxicology, pharmacokinetics, pharmacology, and pharmacodynamic characteristics of the product; chemistry, manufacturing, and controls information; and any available human data or literature to support the use of the investigational new drug.
An IND must become effective before human clinical trials may begin in the US. An IND will automatically become effective 30 days after receipt by the FDA, unless before that time the FDA raises concerns or questions related to the proposed clinical trials. In such a case, the IND may be placed on clinical hold and the IND sponsor and the FDA must resolve any outstanding concerns or questions before clinical trials can begin. Accordingly, submission of an IND may or may not result in the FDA allowing clinical trials to commence. As drug product programs continue in development, clinical trial protocols, additional preclinical testing results, and manufacturing information is submitted with the IND to facilitate discussions with the FDA and approval of additional clinical trials.
Clinical Trials
Clinical trials involve the administration of the IND to human subjects under the supervision of qualified investigators in accordance with GCPs, which include the requirement that all research subjects provide their informed consent for their participation in any clinical trial. Clinical trials are conducted under protocols detailing, among other things, the objectives of the study, the parameters to be used in monitoring safety, and the efficacy criteria to be evaluated. A protocol for each clinical trial and any subsequent protocol amendments must be submitted to the FDA as part of the IND. Additionally, approval must also be obtained from each clinical trial site's IRB or ethics committee, before the trials may be initiated, and the IRB or ethics committee must monitor the trial until completed. All subjects must provide informed consent prior to participating in the trial. There are also requirements governing the reporting of ongoing clinical trials and clinical trial results to public registries.
The clinical investigation of a drug is generally divided into three or four phases. Although the phases are usually conducted sequentially, they may overlap or be combined.
-
Phase I. The drug is initially introduced into healthy human subjects or, in some cases, patients with the target disease or condition. These studies are designed to evaluate the safety, tolerance, metabolism, pharmacokinetic and pharmacologic actions of the investigational new drug in humans, and the side effects associated with increasing doses.
-
Phase II. The drug is administered to a limited patient population to evaluate safety and optimal dose levels for safety and efficacy, identify possible adverse side effects and safety risks, and preliminarily evaluate efficacy.
-
Phase III. The drug is administered to an expanded patient population, generally at geographically dispersed clinical trial sites to generate sufficient data to statistically evaluate dose levels, clinical effectiveness and safety, to establish the overall benefit-risk relationship of the IND product, and to provide an adequate basis for physician labeling.
-
Phase IV. In some cases, the FDA may conditionally approve an NDA or BLA for a drug product with the sponsor's agreement to conduct additional clinical trials after approval. In other cases, a sponsor may voluntarily conduct additional clinical trials after approval to gain more information about the drug. Such post-approval studies are typically referred to as Phase IV clinical trials.
Clinical trial sponsors must also report to the FDA, within certain timeframes: (i) serious and unexpected adverse reactions, (ii) any clinically important increase in the rate of a serious suspected adverse reaction over that listed in the protocol or investigator's brochure, or (iii) any findings from other studies or animal testing that suggest a significant risk in humans exposed to the product candidate. The FDA, the IRB, the ethics committee or the clinical trial sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects are being exposed to an unacceptable health risk. Additionally, some clinical trials are overseen by an independent group of qualified experts organized by the clinical trial sponsor, known as a data safety monitoring board or committee. This group provides authorization for whether or not a trial may move forward at designated check points based on access to certain data from the trial.
The clinical trial process can take years to complete, and there can be no assurance that the data collected will support FDA approval or licensing of the product. Results from one trial are not necessarily predictive of results from later trials. The Company may also suspend or terminate a clinical trial based on evolving business objectives and/or competitive climate.
Submission of an NDA or BLA to the FDA
Assuming successful completion of all required preclinical studies and clinical testing in accordance with all applicable regulatory requirements, detailed IND product information is submitted to the FDA in the form of an NDA or BLA requesting approval to market the product for one or more indications. Under federal law, the submission of most NDAs and BLAs is subject to an application user fee. Applications for Oppositional Defiant Disorder ("ODD") products are exempted from the NDA and BLA application user fee, unless the application includes an indication for other than a rare disease or condition, and may be exempted from product and establishment user fees under certain conditions. An NDA or BLA must include all relevant data available from pertinent preclinical studies and clinical trials, including negative or ambiguous results as well as positive findings, together with detailed information relating to the product's chemistry, manufacturing, controls, and proposed labeling, among other things. Data comes from company-sponsored clinical trials intended to test the safety and effectiveness of a use of a product, and may also come from several alternative sources, including clinical trials initiated by investigators. To support marketing approval, the data submitted must be sufficient in quality and quantity to establish the safety and effectiveness of the investigational new drug product to the satisfaction of the FDA.
Once an NDA or BLA has been submitted, the FDA's goal is to review the application within ten months after it accepts the application for filing, or, if the application relates to an unmet medical need in a serious or life-threatening indication, six months after the FDA accepts the application for filing. The review process is often significantly extended by the FDA's requests for additional information or clarification. Before approving an NDA or BLA, the FDA typically will inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving an NDA or BLA, the FDA will typically inspect one or more clinical sites to assure compliance with GCP and related regulations.
The FDA is required to refer an NDA or BLA for a novel drug (in which no active ingredient has been approved in any other application) to an advisory committee or explain why such referral was not made. Typically, an advisory committee is a panel of independent experts, including clinicians and other scientific experts, that reviews, evaluates and provides a recommendation as to whether the application should be approved and the conditions thereof. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
The FDA's Decision on an NDA or BLA
After the FDA evaluates the NDA or BLA and conducts inspections of manufacturing facilities where the product will be produced, the FDA will issue either an approval letter or a complete response letter ("Complete Response Letter"). An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications. A Complete Response Letter indicates that the review cycle of the application is complete and the application is not ready for approval. To satisfy deficiencies identified in a Complete Response Letter, additional clinical data and/or an additional Phase III clinical trial(s), and/or other significant, expensive and time-consuming requirements related to clinical trials, preclinical studies or manufacturing may be required for the drug product.
Even if such additional information is submitted, the FDA may ultimately decide that the NDA or BLA does not satisfy the criteria for approval. The FDA could also approve the NDA or BLA with a risk evaluation and mitigation strategy, which could include medication guides, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. The FDA may also conditionally approve a drug product subject to, among other things, changes to proposed labeling, development of adequate controls and specifications, or a commitment to conduct one or more post-market studies or clinical trials. Such post-market testing may include Phase IV clinical trials and surveillance to further assess and monitor the product's safety and effectiveness after commercialization. New government requirements, including those resulting from new legislation, may be established during the review process, or the FDA's policies may change, which could delay or prevent regulatory approval of the Company's products under development.
The Company has numerous options as it relates to contract manufactures of GMP (good manufacturing products) grade active pharmaceutical ingredients and finished products. The Company does not expect to encounter any issues sourcing raw materials nor do we foresee material volatility in raw materials and finished good pricing.
Legal Proceedings
Bright Minds is not involved in, or aware of, any legal or administrative proceedings contemplated or threatened by any governmental authority or any other party that is likely to have a material adverse effect on the Company's business. As of the date of this Registration Statement, no director, officer or affiliate is a party adverse to us in any legal proceeding or has an adverse interest to us in any legal proceeding.
Organizational structure
The Company has three wholly owned subsidiaries, PsilocybinLabs Ltd., a company incorporated under the BCBCA, Bright Minds Biosciences LLC, a limited liability company formed pursuant to the laws of Delaware, and Bright Minds Bioscience Pty. Ltd., a company formed pursuant to the laws of Australia. The Company owns 100% of the voting and dispositive control over each subsidiary. The following chart illustrates, as at the date of this Registration Statement, the Company’s material subsidiaries, including their respective jurisdiction of incorporation and percentage of voting securities in each that are held by the Company either directly or indirectly:

Property, plant and equipment
The Company does not own or lease any real property and it does not own any equipment. The Company's registered office is located in Vancouver, Canada. The nature of the space is immaterial to the Company's operations as physical operating activities related to research and development programs are primarily outsourced to trusted contract research organizations and research institutions, including but not limited to the University of Texas and the Medical College of Wisconsin.
ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS
General
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our unaudited condensed interim consolidated financial statements as at and for the six months ended March 31, 2021 and 2020 and our audited consolidated financial statements as at and for the fiscal year ended September 30, 2020 and the fiscal period from May 31, 2019 (date of incorporation) to September 30, 2019 in each case, together with the notes thereto. Our financial statements included in this Registration Statement were prepared in accordance with International Financial Reporting Standards ("IFRS"), as adopted by the International Accounting Standards Board.
The preparation of financial statements in conformity with these accounting principles requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent liabilities at the financial statement date and reported amounts of revenue and expenses during the reporting period. On an on-going basis we review our estimates and assumptions. The estimates were based on historical experience and other assumptions that we believe to be reasonable under the circumstances. Actual results are likely to differ from those estimates or other forward-looking statements under different assumptions or conditions, but we do not believe such differences will materially affect our financial position or results of operations. Our actual results may differ materially as a result of many factors, including those set forth under the headings entitled "Forward-Looking Statements" and "Risk Factors" herein.
Critical accounting policies, the policies we believe are most important to the presentation of our financial statements and require the most difficult, subjective and complex judgments, are outlined below under the heading "Critical Accounting Policies and Estimates", and have not changed significantly since our founding.
Figures in this Item 5 are in Canadian dollars unless otherwise indicated.
Overview
Bright Minds Biosciences Inc. was incorporated under the BCBCA on May 31, 2019. The Company's objective is to generate income and achieve long term profitable growth through the development of therapeutics to improve the lives of patients with certain severe and life-altering diseases. The head office, and principal address of the Company are located at 1500 - 1055 West Georgia Street, Vancouver, British Columbia, V6E 4N7, Canada.
Additional information related us is available on SEDAR at www.sedar.com and www.brightmindsbio.com. We do not incorporate the contents of our website or of sedar.com into this Registration Statement. Information on our website does not constitute part of this Registration Statement.
Financing
Our ability to continue operations will depend on our continued ability to raise capital on acceptable terms. We incurred losses of $1,583,277 and $2,250,335 for the three and six months ended March 31, 2021 compared to a net loss of $6,142 and $48,137 for the three and six months ended March 31, 2020. We incurred a net loss of $480,377 for the year ended September 30, 2020 compared to $78,717 for the year ended September 30, 2019. We had negative operating cash flows of $1,956,983 for the period ended March 31, 2021, and anticipate negative operating cash flows from operations for the remainder of the fiscal year ended September 30, 2021. Although we had working capital surplus of $25,330,313, including cash of $25,450,001, at March 31, 2021, we anticipate further financings through the sale of our shares. If we are not successful in raising additional capital on terms that are acceptable to us, we may be forced to curtail or cease operations.
Market conditions, trends or events
Our ability to continue operations also depends on market conditions outside of our control. Significant changes in the Canadian and United States drug and health laws may materially and adversely affect our business and prospects. The regulatory approval process is generally lengthy and expensive, with no guarantee of a positive result. Once approved, Health Canada and the FDA would continue to monitor the product and license holders have obligations related to reporting to these agencies, record keeping and ensuring continued safety and efficacy of the product. Failure to comply with any of the above applicable regulations, regulatory authorities or other requirements may result in civil or criminal penalties, recall or seizure of products, injunctive relief including partial or total suspension of production, or withdrawal of a product from the market.
A. Operating Results
Results of Operations for the three months ended March 31, 2021 as compared to the three months ended March 31, 2020
We incurred a net loss of $1,583,277 for the three months ended March 31, 2021 compared to a net loss of $6,142 for the three months ended March 31, 2020. During the previous year, the Company was ramping up operations and experienced limited operating activity. All categories of expense as presented in the three and six months ended March 31, 2021 interim financial statements show an increase in the current year over the prior year reflecting the increase in operating activity. The following explains increases in the certain expense categories for the three months ended March 31, 2021 when compared to the three months ended March 31, 2020:
Consulting fees. Consulting fees were $128,827 for the three months ended March 31, 2021 compared to $Nil for the three months ended March 31, 2020. The increase of $128,827 was the result of hiring consultants to assist in the day to day operations of the business and included $91,426 in share-based compensation expenses.
Foreign exchange. Foreign exchange expenses were $26,965 for the three months ended March 31, 2021 compared to $Nil for the three months ended March 31, 2020. The increase of $26,965 in foreign exchange expense was a result of raising funds in Canadian dollars, and converting these to US dollars to pay US vendors.
Funds processing fees. Funds processing fees were $1,455 for the three months ended March 31, 2021 compared to $Nil for the three months ended March 31, 2020. The increase of $1,455 in funds processing fees was the result of private placement costs not attributable to a reduction of share capital.
Marketing, advertising and investor relations expenses. Marketing, advertising and investor relations expenses were $217,379 for the three months ended March 31, 2021 compared to $Nil for the three months ended March 31, 2020. The increase of $217,379 was the result of increased marketing and awareness of the Company's business to analysts, investors and potential investors.
Office and administrative expenses. Office and administrative expenses were $24,639 for the three months ended March 31, 2021 compared to $169 for the three months ended March 31, 2020. The increase of $24,470 was a result of the Company incurring office related costs.
Professional fees. Professional fees were $141,804 for the three months ended March 31, 2021 compared to $Nil for the three months ended March 31, 2020. The increase of $141,804 in professional fees was the result of services provided to assist the Company's capital raises, prospectus filings, general and corporate legal work.
Regulatory and filing expenses. Regulatory and filing expenses were $62,039 for the three months ended March 31, 2021 compared to $Nil for the three months ended March 31, 2020. The increase of $62,039 in regulatory and filing fees was the result of the listing process and increased share capital activity.
Research and development expenses. Research and development expenses were $980,169 for the three months ended March 31, 2021 compared to $5,973 for the three months ended March 31, 2020. The increase of $974,196 in research and development expenses was the result of increased activity developing the Company’s pipeline of drugs and included $33,815 in share-based compensation expenses.
Results of Operations for the six months ended March 31, 2021 as compared to the six months ended March 31, 2020
We incurred a net loss of $2,250,335 for the six months ended March 31, 2021 compared to a net loss of $48,137 for the six months ended March 31, 2020. During the previous year, the Company was ramping up operations and experienced limited operating activity. All categories of expense as presented in the three and six months ended March 31, 2021 interim financial statements show an increase in the current year over the prior year reflecting the increase in operating activity. The following explains increases in the certain expense categories for the six months ended March 31, 2021 when compared to the six months ended March 31, 2020:
Consulting fees. Consulting fees were $218,375 for the six months ended March 31, 2021 compared to $Nil for the six months ended March 31, 2020. The increase of $218,375 was the result of hiring consultants to assist in the day to day operations of the business and included $177,575 in share-based compensation expenses.
Foreign exchange. Foreign exchange expenses were $59,594 for the six months ended March 31, 2021 compared to $Nil for the six months ended March 31, 2020. The increase of $59,594 in foreign exchange expense was a result of raising funds in Canadian dollars, and converting these to US dollars to pay US vendors.
Funds processing fees. Funds processing fees were $18,665 for the six months ended March 31, 2021 compared to $Nil for the six months ended March 31, 2020. The increase of $18,665 in funds processing fees was the result of private placement costs not attributable to a reduction of share capital.
Marketing, advertising and investor relations expenses. Marketing, advertising and investor relations expenses were $229,377 for the six months ended March 31, 2021 compared to $Nil for the six months ended March 31, 2020. The increase of $229,377 was the result of increased marketing and awareness of the Company's business to analysts, investors and potential investors.
Office and administrative expenses. Office and administrative expenses were $52,034 for the six months ended March 31, 2021 compared to $250 for the six months ended March 31, 2020. The increase of $51,784 was a result of the Company incurring office related costs.
Professional fees. Professional fees were $271,871 for the six months ended March 31, 2021 compared to $Nil for the six months ended March 31, 2020. The increase of $271,871 in professional fees was the result of services provided to assist the Company's capital raises, prospectus filings, general and corporate legal work.
Regulatory and filing expenses. Regulatory and filing expenses were $115,919 for the six months ended March 31, 2021 compared to $Nil for the six months ended March 31, 2020. The increase of $115,919 in regulatory and filing fees was the result of the listing process and increased share capital activity.
Research and development expenses. Research and development expenses were $1,284,500 for the six months ended March 31, 2021 compared to $47,887 for the six months ended March 31, 2020. The increase of $1,236,613 in research and development expenses was the result of increased activity developing the Company’s pipeline of drugs and included $65,679 in share-based compensation expenses.
Results of Operations for the Year ended September 30, 2020 as Compared to the Period from May 31, 2019 (date of inception) to September 30, 2019
We incurred net loss of $480,377 for the year ended September 30, 2020 compared to a net loss of $78,717 for the period ended September 30, 2019. During the period ended September 30, 2019, the Company was ramping up operations and experienced limited operating activity. All categories of expense as presented in the September 30, 2020 audited financial statements show an increase in the current year over the period ended September 30, 2019 reflecting the increase in operating activity. The following explains increases in the certain expense categories for the year ended September 30, 2020 when compared to the period ended September 30, 2019:
Funds processing fees. Funds processing fees were $5,568 for the year ended September 30, 2020 compared to $Nil for the period ended September 30, 2019. The increase of $5,568 in funds processing fees was the result of private placement costs not attributable to a reduction of share capital.
Marketing, advertising and investor relations expenses. Marketing, advertising and investor relations expenses were $9,618 for the year ended September 30, 2020 compared to $Nil for the period ended September 30, 2019. The increase of $9,618 was the result of increased marketing and awareness of the Company's business to analysts, investors and potential investors.
Office expenses. Office and administrative expenses were $637 for the year ended September 30, 2020 compared to $Nil for the period ended September 30, 2019. The increase of $637 was a result of the Company incurring office related costs.
Professional fees. Professional fees were $104,251 for the year ended September 30, 2020 compared to $36,708 for the period ended September 30, 2019. The increase of $67,543 in professional fees was the result of services provided to assist the Company's capital raises, prospectus filings, general and corporate legal work.
Regulatory and filing expenses. Regulatory and filing expenses were $5,451 for the year ended September 30, 2020 compared to $Nil for the period ended September 30, 2019. The increase of $5,451 in regulatory and filing fees was the result of the listing process and increased share capital activity.
Research and development expenses. Research and development expenses were $354,852 for the year ended September 30, 2020 compared to $42,009 for the period ended September 30, 2019. The increase of $312,843 in research and development expenses was the result of increased activity developing the Company’s pipeline of drugs and included $161,300 of share-based compensation expenses.
B. Liquidity and Capital Resources
The Company is a biotechnology company dedicated to developing therapeutics to improve the lives of patients with severe and life‐altering diseases. The Company initially focused on new chemical entities for a variety of pain indications, seizures, and neuropsychiatric disorders. By leveraging the extensive drug discovery experience of the management team, the Company is endeavoring to create a pipeline of best‐in‐class 5‐HT (serotonin) medicines.
The Company's financial success depends in part upon its ability to identify, license or discover additional product candidates. The Company's historical capital needs have been met by the sale of the Company's common shares. Equity funding might not be possible at the times required by the Company. If no funds can be raised, then the Company may require a significant curtailing of operations to ensure its survival or may be required to cease operations.
The financial statements have been prepared on a basis which assumes that the Company will be able to realize its assets and discharge its liabilities in the normal course of business for the foreseeable future. As a result of the above factors, the Company incurred a net loss of $1,583,277 and $2,250,335 for the three and six months ended March 31, 2021. In addition, we incurred a net loss of $480,377 for the year ended September 30, 2020, and had a cash and a working capital surplus of $25,450,001 and $25,330,313, respectively, as at March 31, 2021. In addition, the Company had cash and working capital surplus of $799,929 and $727,293, respectively, as at September 30, 2020. The Company's ability to meet its obligations as they fall due and to continue to operate depends on the continued financial support of its shareholders. In the past the Company has relied on sales of its equity securities to meet its cash requirements. Funding from this or other sources might not be sufficient in the future to continue its operations. Even if the Company is able to obtain new financing, it may not be on commercially reasonable terms or terms that are acceptable to it. Failure to obtain such financing on a timely basis could cause the Company to reduce or terminate its operations.
As of March 31, 2021, the Company had 9,823,361 issued and outstanding shares and 16,722,565 shares outstanding on a fully-diluted basis. The Company began trading on Canadian Stock Exchange on February 8, 2021.
The Company had $25,330,313 of working capital surplus as at March 31, 2021, compared to $727,293 of working capital surplus as at September 30, 2020. The increase in working capital resulted from cash generated from financing activities of $26,610,853 during the six-month period ended March 31, 2021 (March 31, 2020: $91,680) due to the to the issuance of 5,079,820 common shares for net cash proceeds of $26,600,853 (March 31, 2020: $Nil) and an increase in cash used in operating activities of $1,956,983 (March 31, 2021: $97,556) related to the ramp up of operating activity.
Capital Resources
As at March 31, 2021, the Company had cash of $25,450,001 (September 30, 2020: $799,929). The Company continues to pursue additional equity financing although there can be no guarantees given that the Company will be successful in such endeavors.
Critical Accounting Policies and Estimates
The preparation of the Company's financial statements requires management to use estimates and assumptions that affect the reported amounts of assets and liabilities as well as revenue and expenses. These are based on the best information available at the time utilizing generally accepted industry standards
Significant estimates and assumptions
The preparation of the financial statements in conformity with IFRS requires management to make estimates, judgments and assumptions that affect the application of accounting policies and the reported amounts of assets, liabilities, income and expenses. Actual results may differ from these estimates. Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any future periods affected.
Certain of the Company's accounting policies and disclosures require key assumptions concerning the future and other estimates that have a significant risk of causing a material adjustment to the carrying amounts of assets and liabilities or disclosures within the next fiscal year. Where applicable, further information about the assumptions made is disclosed in the notes specific to that asset or liability. The critical accounting estimates and judgments set out below have been applied consistently to all periods presented in these financial statements.
Significant judgements
The preparation of financial statements in accordance with IFRS requires the Company to make judgements, apart from those involving estimates, in applying accounting policies. The most significant judgements in applying the Company's financial statements include:
|
|
• |
the classification of financial instruments; and |
|
|
• |
the calculation of deferred income taxes require judgement in interpreting tax rules and regulations. |
Financial Instruments
Financial instruments are accounted for in accordance with IFRS 9, "Financial Instruments: Classification and Measurement". A financial instrument is any contract that gives rise to a financial asset of one entity and a financial liability or equity instrument of another entity.
Financial assets
(a) Recognition and measurement of financial assets
The Company recognizes a financial asset when it becomes a party to the contractual provisions of the instrument.
(b) Classification of financial assets
The Company classifies financial assets at initial recognition as financial assets: measured at amortized cost, measured at fair value through other comprehensive income ("FVTOCI") or measured at fair value through profit or loss ("FVTPL").
(i) Financial assets measured at amortized cost
A financial asset that meets both of the following conditions is classified as a financial asset measured at amortized cost:
• The Company's business model for such financial assets is to hold the assets in order to collect contractual cash flows.
• The contractual terms of the financial asset give rise on specified dates to cash flows that are solely payments of principal and interest on the amount outstanding.
A financial asset measured at amortized cost is initially recognized at fair value plus transaction costs directly attributable to the asset. After initial recognition, the carrying amount of the financial asset measured at amortized cost is determined using the effective interest method, net of impairment loss, if necessary.
(ii) Financial assets measured at FVTOCI
A financial asset measured at fair value through other comprehensive income is recognized initially at fair value plus transaction costs directly attributable to the asset. After initial recognition, the asset is measured at fair value with changes in fair value included as "financial asset at fair value through other comprehensive income" in other comprehensive income or loss.
(iii) Financial assets measured at FVTPL
A financial asset measured at fair value through profit or loss is initially recognized at fair value with any associated transaction costs being recognized in profit or loss when incurred. Subsequently, the financial asset is re-measured at fair value and a gain or loss is recognized in profit or loss in the reporting period in which it arises.
The Company's cash is classified as subsequently measured at FVTPL.
(c) Derecognition of financial assets
The Company derecognizes a financial asset if the contractual rights to the cash flows from the asset expire, or the Company transfers substantially all the risks and rewards of ownership of the financial asset. Any interests in transferred financial assets that are created or retained by the Company are recognized as a separate asset or liability. Gains and losses on derecognition are generally recognized in the statement of comprehensive loss. However, gains and losses on derecognition of financial assets classified as FVTOCI remain within accumulated other comprehensive income or loss.
Financial liabilities
(a) Recognition and measurement of financial liabilities
The Company recognizes a financial liability when it becomes a party to the contractual provisions of the instrument.
(b) Classification of financial liabilities
(i) Financial liabilities measured at amortized cost
A financial liability measured at amortized cost is initially measured at fair value less transaction costs directly attributable to the issuance of the financial liability. Subsequently, the financial liability is measured at amortized cost using the effective interest method.
The Company's accounts payable and accrued liabilities are classified as subsequently measured at amortized cost.
(ii) Financial liabilities measured at fair value through profit or loss
A financial liability measured at fair value through profit or loss is initially measured at fair value with any associated transaction costs being recognized in profit or loss when incurred. Subsequently, the financial liability is re-measured at fair value and a gain or loss is recognized in profit or loss in the reporting period in which it arises.
(c) Derecognition of financial liabilities
The Company derecognizes a financial liability when the financial liability is discharged, cancelled or expired. Generally, the difference between the carrying amount of the financial liability derecognized and the consideration paid and payable, including any non-cash assets transferred or liabilities assumed, is recognized in the statement of comprehensive loss.
Offsetting financial assets and liabilities
Financial assets and liabilities are offset and the net amount is presented in the statement of financial position only when the Company has a legally enforceable right to offset the recognized amounts and intends either to settle on a net basis, or to realize the asset and settle the liability simultaneously.
Impairment of financial assets
The Company recognizes a loss allowance for expected credit losses on financial assets that are measured at amortized cost.
At each reporting date, the Company measures the loss allowance for the financial asset at an amount equal to the lifetime expected credit losses if the credit risk on the financial asset has increased significantly since initial recognition. If at the reporting date, the financial asset has not increased significantly since initial recognition, the Company measures the loss allowance for the financial asset at an amount equal to the twelve-month expected credit losses. The Company recognizes in the statement of comprehensive income or loss, as an impairment loss (or gain), the amount of expected credit losses (or reversal) that is required to adjust the loss allowance at the reporting date to the amount that is required to be recognized.
Share-based payments
Share-based compensation expense relates to stock options as well as cash and equity settled restricted share units ("RSUs"). The grant date fair values of stock options and equity settled RSUs granted are recognized as an expense, with a corresponding increase in reserves in equity, over the vesting period. The amount recognized as an expense is based on the estimate of the number of awards expected to vest, which is revised if subsequent information indicates that actual forfeitures are likely to differ from the estimate. Upon exercise of stock options, the consideration paid by the holder is included in share capital and the related reserves associated with the stock options exercised is reclassified into share capital. Upon vesting of equity settled RSUs, the related reserves associated with the RSU is reclassified into share capital.
For cash settled RSUs, the fair value of the RSUs is recognized as share-based compensation expense, with a corresponding increase in accrued liabilities over the vesting period. The amount recognized as an expense is based on the estimate of the number of RSUs expected to vest. Cash settled RSUs are measured at their fair value at each reporting period on a mark-to-market basis. Upon vesting of the cash settled RSUs, the liability is reduced by the cash payout.
Share-based payments are included in the Company’s Consolidated Statements of Comprehensive Loss on a functional account basis.
Research and development expenses
Research costs are expensed when incurred. Development costs, including direct material, direct labor and contract service costs, are capitalized as intangible assets when: we can demonstrate that the technical feasibility of a project has been established; when we intend to complete the asset for use or sale and have the ability to do so; when the asset can generate probable future economic benefits; when the technical and financial resources are available to complete the development; and when we can reliably measure the expenditure attributable to the intangible asset during its development. After initial recognition, internally generated intangible assets are recorded at cost less accumulated amortization and accumulated impairment losses. These costs are amortized on a straight-line basis over the estimated useful life. To date the Company did not have any development costs that met the capitalization criteria.
Income taxes
Current income tax:
Current income tax assets and liabilities for the current period are measured at the amount expected to be recovered from or paid to the taxation authorities. The tax rates and tax laws used to compute the amount are those that are enacted or substantively enacted, at the reporting date, in the countries where the Company operates and generates taxable income.
Deferred tax:
Deferred tax is provided on temporary differences at the reporting date between the tax bases of assets and liabilities and their carrying amounts for financial reporting purposes. The carrying amount of deferred tax assets is reviewed at the end of each reporting period and recognized only to the extent that it is probable that sufficient taxable profit will be available to allow all or part of the deferred tax asset to be utilized.
Deferred tax assets and liabilities are measured at the tax rates that are expected to apply to the year when the asset is realized or the liability is settled, based on tax rates (and tax laws) that have been enacted or substantively enacted by the end of the reporting period. Deferred tax assets and deferred tax liabilities are offset if a legally enforceable right exists to set off current tax assets against current income tax liabilities and the deferred taxes relate to the same taxable entity and the same taxation authority.
C. Research and Development, Patents and Licenses, etc.
The following table summarizes the material components of research and development expenditure across its drug portfolio:
| For the three months ended | For the six months ended | |||||||||||
| Drug Portfolio | March 31, 2021 |
March 31, 2020 |
March 31, 2021 |
March 31, 2020 |
||||||||
| $ | $ | $ | $ | |||||||||
| 5-HT2A | 297,866 | 1,991 | 395,890 | 15,962 | ||||||||
| 5-HT2C | 445,487 | 1,991 | 550,554 | 15,962 | ||||||||
| 5-HT2C/A | 203,001 | 1,991 | 272,377 | 15,963 | ||||||||
| TOTAL | 946,354 | 5,973 | 1,218,821 | 47,887 | ||||||||
| Drug Portfolio | For the year ended September 30, 2020 |
For the year ended September 30, 2019 |
||||
| $ | $ | |||||
| 5-HT2A | 64,517 | 14,003 | ||||
| 5-HT2C | 64,517 | 14,003 | ||||
| 5-HT2C/A | 64,518 | 14,003 | ||||
| TOTAL | 193,552 | 42,009 |
During the current and three and six months period ended March 31, 2021, and for the year ended September 30, 2020, the Company ramped up its expenditures across the three drugs in its portfolio over their comparable periods. The Company expects a continued expenditure trend upwards given its stated research objectives.
D. Trend Information
Due to our short operating history, except as noted below, we are not aware of any trends that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that is material to investors.
Potential Impact of the COVID-19 Pandemic
The recent outbreak of the coronavirus, also known as "COVID-19", has spread across the globe and is impacting worldwide economic activity. Conditions surrounding the coronavirus continue to rapidly evolve and government authorities have implemented emergency measures to mitigate the spread of the virus. The outbreak and the related mitigation measures may have an adverse impact on global economic conditions as well as on the Company's business activities. The extent to which the coronavirus may impact the Company's business activities will depend on future developments, such as the duration of the outbreak, travel restrictions, business disruptions, and the effectiveness of actions taken in Canada and other countries to contain and treat the disease. These events are highly uncertain and as such, the Company cannot determine their financial impact at this time.
E. Off-Balance Sheet Arrangements
The Company has no off-balance sheet arrangements nor does it have any transactions, arrangements, obligations (including contingent obligations) or other relationships with any unconsolidated entities or other persons that may have material current or future effect on financial conditions, changes in the financial conditions, results of operations, liquidity, capital expenditures, capital resources or significant components of revenue or expenses.
ITEM 6. DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
A. Directors and Senior Management
|
Name, Province/State and Country of Residence |
Age |
Position |
Director/Officer Since |
|
Ian McDonald, Ontario, Canada |
34 |
President, Chief Executive Officer and Director |
May 31, 2019 |
|
Ryan Cheung , British Columbia, Canada |
42 |
Chief Financial Officer |
May 29, 2020 |
|
Dr. Gideon Shapiro, Florida, USA |
61 |
Vice President (Discovery) |
September 29, 2020 |
|
Dr. Revati Shreeniwas, California, USA |
60 |
Chief Medical Officer |
June 5, 2020 |
|
Nils Christian Bottler(1)(2)(3), Berlin, Germany |
34 |
Director |
September 29, 2020 |
|
Jeremy Fryzuk(1)(2)(3), London, UK |
36 |
Director |
September 29, 2020 |
|
Dr. Alan Kozikowski, Illinois, USA |
71 |
Chief Science Officer and Director |
September 29, 2020 |
|
Dr. Emer Leahy(1)(2)(3, Pennsylvania, USA |
55 |
Director |
June 10, 2021 |
_________________________
Notes
(1) Member of the Audit Committee.
(2) Member of the Nominating and Corporate Governance Committee.
(3) Member of the Compensation Committee.
Business Experience
The following summarizes the occupation and business experience during the past five years or more for our directors and executive officers as of the date of this Registration Statement:
Ian McDonald, President, Chief Executive Officer and Director
Mr. McDonald is an entrepreneur and former investment banker. Prior to joining the Company, Mr. McDonald served on the management team at a TSX-listed gold mining company. In that capacity, Mr. McDonald developed and implemented the corporate strategy as it relates to M&A and capital markets resulting in a $160 million sale within one year. Previously, he worked in a senior role at a Canadian investment bank and in private equity in Vancouver, London and Toronto. Under Mr. McDonald's guidance, clients raised hundreds of millions of dollars in capital. Mr. McDonald has served as a member of the board of directors of several TSX Venture Exchange, Canadian Securities Exchange listed and private companies.
Ryan Cheung, Chief Financial Officer
Mr. Cheung is the founder and managing partner of MCPA Services Inc., Chartered Professional Accountants, in Vancouver, B.C. Leveraging his experience as a former auditor of junior venture and resource companies, Mr. Cheung serves as a director and officer or consultant for public and private companies, providing financial reporting, taxation and strategic guidance.
He has been an active member of the Chartered Professional Accountants of British Columbia (formerly Institute of Chartered Accountants of British Columbia) since January 2008. Mr. Cheung holds a diploma in accounting from the University of British Columbia and a Bachelor of Commerce in international business from the University of Victoria.
Dr. Gideon Shapiro, Vice President (Discovery)
Mr. Shapiro, PhD, trained as an organic chemist at UNC Chapel Hill and UC Berkeley, before going on to do postdoctoral research at the ETH Federal Institute of Technology in Zurich, Switzerland. He began his drug discovery career as a senior medicinal chemist in Central Nervous System department of Sandoz Pharmaceuticals in Basel, Switzerland. Over his 10-year career at Sandoz he advanced to lead the Alzheimer's and Neurodegeneration drug discovery group which was responsible for promoting numerous drug candidates into clinical trials including the marketed Alzheimer's drug Exelon®. After the merger of Sandoz with Ciba-Geigy to form Novartis, he transitioned his career to founding and leading biopharmaceutical ventures. He was co-founder and CEO of EraGen Biosciences, one of the first Novartis Venture Companies. EraGen was acquired by Luminex for its novel marketed DNA chemistry based diagnostic Multicode®-RTx product line co-invented by Dr. Shapiro. Backed by Alliance Technology Ventures out of Atlanta, Georgia, he went on to found Somatocor Pharmaceuticals based on his inventions of peptidomimetic drugs of the peptide hormone somatostatin and marketed drug Sandostatin®.
Over the last 15-years Dr. Shapiro has had a central role in drug discovery, development and corporate partnering efforts at Fidelity venture backed companies. He was Vice President of Chemistry at the Fidelity neuroscience venture EnVivo Pharmaceuticals (subsequently Forum Pharmaceuticals), and played a leadership role in the invention and advancement of a portfolio numerous CNS drugs that entered late stage clinical trials in patients.
Among these the alpha-7 nicotinic agonist drug encenicline (FRM-6124) advanced to Phase III clinical trials in Alzheimer's disease and schizophrenia for cognitive enhancement. Most recently Dr. Shapiro led the discovery and development as Chief Scientific Officer of Rugen which is advancing new small molecule drug therapies for psychiatric diseases. Dr. Shapiro has extensive experience and participated in numerous R&D collaborations and licensing deals with biopharmaceutical and global pharmaceutical industry partners. He has over 100 patents and publications to his name.
Dr. Revati Shreeniwas, Chief Medical Officer
Ms. Shreeniwas, MD, is a physician researcher with 17 years of experience in the pharmaceutical industry passionate about bringing novel therapeutics to patients with an unmet clinical need. She has served as an executive level clinical expert with operational knowledge in leading complex programs for a range of therapeutics (Phase I-IV studies). She has worked on several drugs that have gone on to be approved and are commercially successful, including Tracleer Lexiscan, Natrecor, Rytary, Esbriet, Sunosi and Talzenna. Dr. Shreeniwas has served in senior leadership roles in other privately held companies such as Neuraxon Pharmaceuticals (developing migraine drugs) and has also run a boutique clinical development consulting company providing startup companies with strategic drug development services to help achieve key milestones in a cost effective manner.
Nils Christian Bottler, Director
Mr. Bottler is a venture capitalist currently working at Think.Health Ventures as an associate partner. The company focuses on investment in early-stage start-ups in the fields of digital health and medical device technology. Think.Health supports its portfolio beyond financial investment with knowledge, experience and access to an extensive business network. Mr. Bottler's prior work experience was in the banking industry working mainly on M&A projects as well as on a number of consulting projects in Germany, China, the UK, and the United Arab Emirates. He then moved to digital media and analyzed, developed and executed new business models at the Axel Springer SE in Berlin before taking a deep dive into the German health care market as SVP RHÖN-Innovations and the premier hospital chain RHÖNKLINIKUM AG.
Jeremy Fryzuk, Director
Mr. Fryzuk is a private equity investment professional based in London. He has over 10 years of experience in private equity. He started his career in investment banking in Toronto with BMO Capital Markets. Mr. Fryzuk holds a Bachelor of Commerce with a major in Finance from Dalhousie University in Canada.
Dr. Alan Kozikowski, Director
Dr. Kozikowski, trained at Michigan, Berkeley, and Harvard, began his own career as a professor of organic chemistry at the University of Pittsburgh. Following his interests in the applications of chemistry to biological problems, he moved on to the Mayo Clinic, and then assumed a position at the Georgetown University Medical Center as director of the Drug Discovery Program. After a decade at Georgetown, he led a research group at the University of Illinois at Chicago in the Department of Medicinal Chemistry.
Specifically, Dr. Kozikowski's continued efforts to identify possible treatments for Alzheimer's disease have resulted in the advancement of the natural product huperzine A to the clinic, an acetylcholinesterase inhibitor that has now become an OTC pharmaceutical. He has also developed a new Positron Emission Tomography (PET) imaging agent for use in prostate cancer diagnosis, which is in the clinic. Other novel inventions have been created, with a number of these moving into the clinical realm. A new company, Actuate Therapeutics Inc., has been founded to advance small molecule kinase inhibitors for the treatment of brain cancers. Actuate has raised millions of dollars to advance a GSK-3 inhibitor that he invented to Phase II clinical trials for the treatment of melanoma, breast cancer, and pancreatic cancer. He has experience running biotechnology companies including acting as the CEO of StarWise Therapeutics, focused on developing novel HDAC6 inhibitors for use in Fragile X syndrome, Charcot Marie Tooth disease, and Rett syndrome. Dr. Kozikowski has over 550 publications and more than 100 patents to his name.
Dr. Emer Leahy, Director
Dr. Emer Leahy received her Ph.D. in Neuropharmacology from University College Dublin, Ireland and her MBA from Columbia University. She is CEO of PsychoGenics Inc., a preclinical CNS service company, and CEO of PGI Drug Discovery LLC, a company engaged in psychiatric drug discovery. She holds an Adjunct Associate Professors of Neuroscience position at Mount Sinai School of Medicine. Dr. Leahy has over thirty years of experience in drug discovery, clinical development and business development for pharmaceutical and biotechnology companies, including extensive knowledge of technology assessment, licensing, mergers and acquisitions, and strategic planning. Dr. Leahy served on the Emerging Companies Section Governing Board for the Board of Directors of the Biotechnology Industry Organization (BIO), the Business Review Board for the Alzheimer's Drug Discovery Foundation, and the Scientific Advisory Board of the International Rett Syndrome Foundation. She currently serves on the board of directors of PsychoGenics Inc., the board of directors of Intensity Therapeutics, and the board of trustees of BIONJ.
Family Relationships
There are no family relationships among any of our directors and executive officers.
Term of Office
All appointments of officers are to be made on the terms and conditions and at the remuneration (whether by way of salary, fee, commission, participation in profits or otherwise) that the Board thinks fit and are subject to termination at the pleasure of the Board, and an officer may in addition to such remuneration be entitled to receive, after he or she ceases to hold such office or leaves the employment of the Company, a pension or gratuity. The Board may, from time to time, appoint such officers, if any, as it determines and the Board may, at any time, terminate any such appointment.
Involvement in Certain Legal Proceedings
Except as disclosed below, during the past ten years, none of our directors or executive officers have been the subject of the following events:
1. a petition under the Federal bankruptcy laws or any state insolvency law was filed by or against, or a receiver, fiscal agent or similar officer was appointed by a court for the business or property of such person, or any partnership in which he was a general partner at or within two years before the time of such filing, or any Company or business association of which he was an executive officer at or within two years before the time of such filing;
2. convicted in a criminal proceeding or is a named subject of a pending criminal proceeding (excluding traffic violations and other minor offenses);
3. the subject of any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining him from, or otherwise limiting, the following activities:
(a) acting as a futures commission merchant, introducing broker, commodity trading advisor, commodity pool operator, floor broker, leverage transaction merchant, any other person regulated by the Commodity Futures Trading Commission, or an associated person of any of the foregoing, or as an investment adviser, underwriter, broker or dealer in securities, or as an affiliated person, director or employee of any investment company, bank, savings and loan association or insurance company, or engaging in or continuing any conduct or practice in connection with such activity;
(b) engaging in any type of business practice; or
(c) engaging in any activity in connection with the purchase or sale of any security or commodity or in connection with any violation of Federal or State securities laws or Federal commodities laws;
4. the subject of any order, judgment or decree, not subsequently reversed, suspended or vacated, of any Federal or State authority barring, suspending or otherwise limiting for more than 60 days the right of such person to engage in any activity described in paragraph 3(a) in the preceding paragraph or to be associated with persons engaged in any such activity;
5. was found by a court of competent jurisdiction in a civil action or by the SEC to have violated any Federal or State securities law, and the judgment in such civil action or finding by the SEC has not been subsequently reversed, suspended, or vacated;
6. was found by a court of competent jurisdiction in a civil action or by the Commodity Futures Trading Commission to have violated any Federal commodities law, and the judgment in such civil action or finding by the Commodity Futures Trading Commission has not been subsequently reversed, suspended or vacated;
7. was the subject of, or a party to, any Federal or State judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated, relating to an alleged violation of:
(a) any Federal or State securities or commodities law or regulation;
(b) any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order, or
(c) any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or
8. was the subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Exchange Act (15 U.S.C. 78c(a)(26))), any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act (7 U.S.C. 1(a)(29))), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member.
Mr. Ryan Cheung is currently the CFO of DMG Blockchain Solutions Inc. ("DMG"), a company listed on the TSX Venture Exchange. DMG was issued a failure-to-file cease trade order on February 1, 2019 by the British Columbia Securities Commission (the "BCSC") for failing to file its annual audited financial statements for the year ended September 30, 2018 and the related management's discussion and analysis and certification. This failure-to-file cease trade order was revoked on August 28, 2019.
Mr. Cheung was formerly the CFO, CEO and a director of Xemplar Energy Corp. ("Xemplar"), a company previously listed on the TSX Venture Exchange and currently listed on the NEX board of the TSX Venture Exchange. Xemplar was issued a failure-to-file cease trade order on May 8, 2015 by the BCSC for failing to file its annual audited financial statements for the year ended December 31, 2014 and the related management's discussion and analysis and certification. Xemplar was issued another failure-to-file cease trade order on August 7, 2015 by the Alberta Securities Commission for failing to file its annual audited financial statements for the year ended December 31, 2014 and the related management's discussion and analysis and certification, as well as the interim unaudited financial statements for the period ended March 31, 2015 and the related management's discussion and analysis and certification. Both failure-to-file cease trade orders have not been revoked as of the date of this Registration Statement. Mr. Cheung resigned as CFO on April 30, 2013 and resigned as CEO and director on April 28, 2015
Director Independence
Directors are considered to be independent if they have no direct or indirect material relationship with the Company. A "material relationship" is a relationship which could, in the Board's opinion, be reasonably expected to interfere with the exercise of a director's independent judgment.
The Board facilitates its independent supervision over management of the Company through frequent meetings of the Board at which members of management or non-independent directors are not in attendance and by retaining independent consultants where it deems necessary. Management is delegated the responsibility for meeting defined corporate objectives, implementing approved strategic and operating plans, carrying on the Company's business in the ordinary course, managing cash flow, evaluating new business opportunities, recruiting staff and complying with applicable regulatory requirements. The Board facilitates its independent supervision over management by reviewing and approving long-term strategic, business and capital plans, material contracts and business transactions, and all debt and equity financing transactions. Through its Audit Committee, the Board examines the effectiveness of the Company's internal control processes and management information systems. The Board reviews executive compensation and recommends stock option grants.
The independent members of the Board are Emer Leahy, Nils Bottler and Jeremy Fryzuk. Alan Kozikowski and Ian McDonald are not independent as they are officers of the Company.
Code of Business Conduct and Ethics
The Board has adopted a Code of Business Conduct and Ethics (the "Code of Ethics") that applies to all of our employees and officers, including our principal executive officer, principal financial officer, principal accounting officer or controller or persons performing similar functions. The Code of Ethics meets the requirements for a "code of ethics" within the meaning of that term in Item 16B of Form 20-F. A copy of our Code of Ethics will be provided to any person without charge upon request. All requests for a copy of our Code of Ethics should be directed in writing to the attention of Ian McDonald at [email protected].
B. Compensation
Compensation Discussion and Analysis
This section sets out the objectives of our Company's executive compensation arrangements, our Company's executive compensation philosophy and the application of this philosophy to our Company's executive compensation arrangements. It also provides an analysis of the compensation design, and the decisions that the Board made in fiscal 2020 with respect to its NEOs (as herein defined). Subsequent to end of the 2020 fiscal year, the Company established the Compensation Committee. The Compensation Committee determines compensation for the directors and officers of the Company as well as the procedures for this determination. See "Directors, Senior Management and Employees - Board Practices - Compensation Committee".
Elements of the Compensation Program
The responsibilities relating to executive and director compensation, including reviewing and recommending compensation of the Company's officers and employees and overseeing the Company's base compensation structure and equity-based compensation program is performed by the Board as a whole. The Board also assumes responsibility for reviewing and monitoring the long-range compensation strategy for the Company's senior management. The Board generally reviews the compensation of senior management on an annual basis taking into account compensation paid by other issuers of similar size and activity and the performance of officers generally and in light of the Company's goals and objectives.
The Company is a small biotechnology company with limited resources. The compensation for senior management of the Company is designed to ensure that the level and form of compensation achieves certain objectives, including: (a) attracting and retaining talented, qualified and effective executives; (b) motivating the short and long-term performance of executives; and (c) better aligning the interests of executive officers with those of the Company's shareholders. In the Board's view, paying salaries which are competitive in the markets in which the Company operates is a first step to attracting and retaining talented, qualified and effective executives. Competitive salary information on comparable companies is compiled from a variety of sources, including national and international publications.
The Board determines the compensation for the CEO. The compensation of the Company's executives is determined by the Board after the recommendation of the CEO. In each case, the Board takes into consideration the prior experience of the executive, industry standards, competitive salary information on comparable companies of similar size and stage of development, the degree of responsibility and participation of the executive in the day-to-day affairs of the Company, and the Company's available cash resources.
In the Board's view, to attract and retain qualified and effective executives, the Company must pay base salaries which are reasonable in relation to the level of service expected while remaining competitive in the markets in which the Company operates.
The Board has assessed the Company's compensation plans and programs for its executive officers to ensure alignment with the Company's business plan and to evaluate the potential risks associated with those plans and programs. The Board has concluded that the compensation policies and practices do not create any risks that are reasonably likely to have a material adverse effect on the Company. The Board considers the risks associated with executive compensation and corporate incentive plans when designing and reviewing such plans and programs.
The Company has not adopted a policy restricting its executive officers or directors from purchasing financial instruments that are designated to hedge or offset a decrease in market value of equity securities granted as compensation or held, directly or indirectly, by its executive officers or directors. To the knowledge of the Company, none of the executive officers or directors has purchased such financial instruments.
Philosophy and Objectives
The compensation program for the senior management of the Company is designed within this context with a view that the level and form of compensation achieves certain objectives, including:
-
attracting and retaining qualified executives;
-
motivating the short and long-term performance of these executives; and
-
better aligning their interests with those of the Company's shareholders.
Base Salary or Consulting Fees
In the Board's view, paying base salaries which are reasonable in relation to the level of service expected while remaining competitive in the markets in which the Company operates is a first step to attracting and retaining qualified and effective executives.
Base salary ranges for the executive officers were initially determined upon a review of companies within the biotechnology industry, which were of the same size as the Company, at the same stage of development as the Company and considered comparable to the Company.
In determining the base salary of an executive officer, the Board considers the following factors:
-
the particular responsibilities related to the position;
-
salaries paid by other companies in the biotechnology industry which were similar in size as the Company;
-
the experience level of the executive officer;
-
the amount of time and commitment which the executive officer devotes to the Company; and
-
the executive officer's overall performance and performance in relation to the achievement of corporate milestones and objectives.
Executive Compensation
Except for the grant of incentive share options and restricted share unit awards to the NEOs and any compensation payable pursuant to an executive compensation agreement between the CEO or CFO and the Company, there are no arrangements under which NEOs were compensated by the Company during the two most recently completed financial years for their services in their capacity as NEOs, directors or consultants.
Director Compensation
The directors receive no cash compensation for acting in their capacity as directors of the Company.
Except for the grant to directors of stock options and restricted share unit awards, there are no arrangements under which directors were compensated by the Company during the two most recently completed financial years for their services in their capacity as directors.
Bonus Incentive Compensation
The Company's objective is to achieve certain strategic objectives and milestones. The Board considers executive bonus compensation dependent upon the Company meeting those strategic objectives and milestones and sufficient cash resources being available for the granting of bonuses. The Board approves executive bonus compensation dependent upon compensation levels based on recommendations of the CEO. Such recommendations are generally based on information provided by issuers that are similar in size and scope to the Company's operations.
Equity Participation
The Company believes that encouraging its executives and consultants to become shareholders is the best way of aligning their interests with those of its shareholders. Equity participation is accomplished through the Company's existing stock option plan and its restricted share unit plan. Stock options and RSUs are granted to executives and employees taking into account a number of factors, including the amount and term of options and RSUs previously granted, base salary and bonuses and competitive factors. The amounts and terms of options and RSUs granted are determined by the Compensation and Corporate Governance Committee based on recommendations put forward by the CEO. Prior to the establishment of the Compensation and Corporate Governance Committee, grants of stock options and RSUs were considered and approved by the board of directors of the Company.
Compensation Review Process
Risks Associated with the Company's Compensation Program
The Company's directors have not considered the implications of any risks to the Company associated with decisions regarding the Company's compensation program. The Company intends to formalize its compensation policies and practices and will take into consideration the implications of the risks associated with the Company's compensation program and how it might mitigate those risks.
The Company did not retain a compensation consultant during financial years ending September 30, 2020 and September 30, 2019.
Benefits and Perquisites
Company does not, as of the date of this Registration Statement, offer any benefits or perquisites to its NEOs other than potential grants of incentive stock options and RSUs as otherwise disclosed and discussed herein.
Hedging by Directors or NEOs
The Company has adopted a policy restricting directors, officers and employees of the Company from hedging or monetizing transactions to lock in the value of holdings in securities (whether debt or equity) of the Company. The objective of the policy is to prohibit individuals subject to the policy from (i) directly or indirectly engaging in the hedging against future declines in the market value of any securities of the Company (including through the purchase of financial instruments designed to offset such risk), and (ii) pledging Company securities as collateral for a loan (whether in a margin account or otherwise).
As of the date of this Registration Statement, entitlement to grants of incentive stock options under the Option Plan (as defined herein) and unit awards under the RSU Plan (as defined herein) are the only equity security elements awarded by the Company to its executive officers and directors.
Pension Disclosure
The Company does not have a pension plan that provides for payments or benefits to the NEOs at, following, or in connection with retirement.
Summary Compensation Table
The following table sets forth all compensation paid, payable, awarded, granted, given or otherwise provided, directly or indirectly, by Bright Minds or its subsidiaries, to each of the executive officers set out below (each, an "NEO"), in any capacity, including, for greater certainty, all plan and non‐plan compensation, direct or indirect pay, remuneration, economic or financial award, reward, benefit, gift or perquisite paid, payable, awarded, granted, given or otherwise provided to the NEO for services provided and for services to be provided, directly or indirectly, to Bright Minds or its subsidiaries in the two most recently completed financial years ended September 30, 2020 and September 30, 2019.
|
Named |
Year |
Salary |
Share (C$) |
Option (C$)(1) |
Annual (C$) |
Long-term Plan (C$) |
Pension |
All Other sation (C$) |
Total sation (C$) |
|
Ian McDonald(2) President and CEO |
2020 2019 |
Nil Nil |
Nil Nil |
Nil Nil |
Nil Nil |
Nil Nil |
Nil Nil |
Nil Nil |
Nil Nil |
|
Ryan Cheung(3) CFO |
2020 2019 |
22,575 Nil |
Nil Nil |
Nil Nil |
Nil Nil |
Nil Nil |
Nil Nil |
Nil Nil |
22,575 Nil |
|
Revati |
2020 |
26,777 |
23,300 |
138,000 |
Nil |
Nil |
Nil |
Nil |
$188,077 |
|
Shreeniwas(4) |
|
|
|
|
|
|
|
|
|
|
Chief Medical |
2019 |
Nil |
Nil |
Nil |
Nil |
Nil |
Nil |
Nil |
Nil |
|
Officer |
|
|
|
|
|
|
|
|
|
|
Alan |
2020 |
6,125 |
Nil |
Nil |
Nil |
Nil |
Nil |
Nil |
6,125 |
|
Kozikowski(5) |
|
|
|
|
|
|
|
|
|
|
Chief Science |
2019 |
Nil |
Nil |
Nil |
Nil |
Nil |
Nil |
Nil |
Nil |
|
Officer |
|
|
|
|
|
|
|
|
|
Notes:
(1) Option-based awards represent the fair value of stock options granted in the year under our Stock Option Plan. The fair value of stock options granted is calculated as of the grant date using the Black-Scholes option pricing model. For discussion of the assumptions made in the valuation, refer to Note 6 to our financial statements for our fiscal year ended September 30, 2020.
(2) Mr. McDonald was appointed President and a director of the Company on May 31, 2019 and as CEO on June 5, 2020.
(3) Mr. Cheung was appointed CFO of the Company on May 29, 2020.
(4) Dr. Shreeniwas was engaged as Chief Medical Officer of the Company on June 5, 2020.
(5) Dr. Kozikowski was engaged as Chief Science Officer of the Company on October 29, 2020.
Executive Compensation Agreements
The Company has entered into an Independent Contractor Agreement dated October 29, 2020 between the Company and Dr. Alan Kozikowski engaging the services of Dr. Kozikowski as Chief Science Officer of the Company, with compensation to be determined by the Board of Directors of the Company. The agreement contains standard nondisclosure, noncompetition and non-solicitation provisions and automatically renews on an annual basis unless terminated by either party.
The Company has entered into an Independent Contractor Agreement dated November 17, 2020 between the Company and Dr. Gideon Shapiro engaging the services of Dr. Shapiro as Vice President (Discovery) of the Company, with compensation to be determined by the Board of Directors of the Company. The agreement contains standard nondisclosure, noncompetition and non-solicitation provisions and automatically renews on an annual basis unless terminated by either party.
The Company entered into an Independent Consultant Agreement dated June 5, 2020, between the Company a corporation controlled by Dr. Revati Shreeniwas, pursuant to which Dr. Revati Shreeniwas was engaged to perform services as Chief Medical Officer of the Company. The agreement contains standard nondisclosure, noncompetition and non-solicitation provisions and automatically renews on an annual basis unless terminated by either party.
Other than as set out above, the Company has not entered into any other contract, agreement, plan or arrangement that provides for payments to a NEO or a director at, following or in connection with any termination (whether voluntary, involuntary or constructive), resignation, retirement a change in control of the Company or a change in an NEOs or directors responsibilities).
Securities Authorized For Issuance Under Equity Compensation Plans
The Company has two equity compensation plans: (i) a 10% "rolling" stock option plan and (ii) a 10% "rolling" restricted share unit plan, as described in this Registration Statement. The Company received shareholder approval of the Option Plan and RSU Plan on May 18, 2021.
The following table sets forth details of the Company's equity compensation plan information as at the financial year ended September 30, 2020:
|
Plan Category |
Number of |
Weighted-average |
Number of |
|
Equity compensation plans approved by securityholders |
N/A |
N/A |
N/A |
|
Equity compensation plans not approved by securityholders(2) |
150,000 (Options) 380,000 (RSUs) |
$1.25 (Options/RSUs) |
324,353 (Options) 94,353 (RSUs) |
|
Total |
150,000 (Options) 380,000 (RSUs) |
|
324,353 (Options) 94,353 (RSUs) |
Note:
(1) The figures are presented on a post-Consolidation basis. The Company consolidated the Common Shares on the basis of 2.5 pre-Consolidation share for 1 post-Consolidation share on November 10, 2020.
Stock Option Plan
The Company adopted a 10% rolling stock option plan (the "Option Plan"), which became effective on July 1, 2020. The Option Plan is filed as Exhibit 15.1 hereto.
The principal purpose of the Option Plan is to advance the interests of the Company by encouraging the directors, employees and consultants of the Company and of its subsidiaries or affiliates, if any, by providing them with the opportunity, through options, to acquire Common Shares in the share capital of the Company, thereby increasing their proprietary interest in the Company, encouraging them to remain associated with the Company and furnishing them with additional incentive in their efforts on behalf of the Company in the conduct of its affairs.
The Option Plan provides that the number of Common Shares issuable under the Option Plan, together with all of the Company's other previously established or proposed share compensation arrangements, may not exceed 10% of the total number of the Company's issued and outstanding Common Shares.
The Option Plan is administered by the board of directors of the Company or by a special committee of the directors appointed from time to time by the board of directors of the Company. The maximum term may not exceed ten (10) years from the date of grant.
The following information is intended to be a brief description of the Option Plan and is qualified in its entirety by the full text of the Option Plan. All capitalized words used but not defined have the meanings ascribed to such term in the Option Plan:
-
the maximum number of Options which may be granted to any one holder under the Option Plan within any 12 month period shall be 5% of the number of issued and outstanding Common Shares (unless the Company has obtained disinterested shareholder approval if required by applicable laws);
-
if required by applicable laws, disinterested shareholder approval is required to grant to related persons, within a 12 month period, of a number of Options which, when added to the number of outstanding Options granted to related persons within the previous 12 months, exceed 10% of the issued Common Shares;
-
the expiry date of an Option shall be no later than the tenth anniversary of the grant date of such Option;
-
the maximum number of Options which may be granted to any one consultant within any 12 month period must not exceed 2% of the number of issued and outstanding Common Shares;
-
the maximum number of Options which may be granted within any 12 month period to employees or consultants engaged in investor relations activities must not exceed 2% of the number of issued and outstanding Common Shares and such Options must vest in stages over 12 months with no more than 25% of the Options vesting in any three month period;
-
the exercise price of any Option issued under the Option Plan shall not be less than the Market Value (as defined in the Option Plan) of the Common Shares as of the grant date; and
-
the Board, or any committee to whom the Board delegates, may determine the vesting schedule for any Option.
The foregoing summary of the Option Plan is not complete and is qualified in its entirety by reference to the Option Plan, which is filed as Exhibit 15.1 hereto and is incorporated by reference herein.
Restricted Share Unit Plan and Restricted Share Units
The Company has in place a restricted share unit plan which became effective July 1, 2020 (the "RSU Plan"). A copy of the RSU Plan is filed as Exhibit 15.2 hereto. The RSU Plan was designed to provide certain directors, officers, consultants and other key employees (an "Eligible Person") of the Company and its related entities with the opportunity to acquire restricted share units ("RSUs") of the Company. The acquisition of RSUs allows an Eligible Person to participate in the long-term success of the Company thus promoting the alignment of an Eligible Persons. The following is a summary of the RSU Plan. Capitalized terms used but not defined have the meanings ascribed to them in the RSU Plan.
Nature and Administration of the RSU Plan
All Directors, Officers, Consultants and Employees (as defined in the RSU Plan) of the Company and its related entities ("Eligible Persons") are eligible to participate in the RSU Plan (as "Participants"), and the Company reserves the right to restrict eligibility or otherwise limit the number of persons eligible for participation as Participants in the RSU Plan. Eligibility to participate as a Participant in the RSU Plan does not confer upon any person a right to receive an award of RSUs.
Subject to certain restrictions, the Board or its appointed committee, can, from time to time, award RSUs to Eligible Persons. RSUs will be credited to an account (an "Account") maintained for each Participant on the books of the Company as of the award date. The number of RSUs to be credited to each Participant's account shall be determined at the discretion of the Board and pursuant to the terms of the RSU Plan. RSUs and all other rights, benefits or interests in the RSU Plan are not transferable or assignable otherwise than by will or the laws of descent and distribution, and shall be exercisable during the lifetime of the Participant only by the Participant and after death only by the Participant's legal representative.
Credit for Dividends
A Participant's Account will be credited with additional RSUs (the "Dividend RSUs") as of each dividend payment date in respect of which cash dividends are paid on Common Shares. The number of Dividend RSUs credited to a Participant's Account in connection with the payment of dividends on Common Shares will be based on the actual amount of cash dividends that would have been paid to such Participant had he or she been holding such number of Common Shares equal to the number of RSUs credited to the Participant's Account on the date on which cash dividends are paid on the Common Shares and the market price of the Common Shares on the payment date. Note that the Company is not obligated to pay dividends on Common Shares.
Resignation, Termination, Leave of Absence or Death
Generally, if a Participant's employment or service is terminated, or if the Participant resigns from employment with the Company, then all RSUs held by the Participant (whether vested or unvested) shall terminate automatically upon the termination of the Participant's service or employment.
In the event a Participant is terminated by reason of (i) termination by the Company other than for cause or (ii) the Participant's death, the Participant's unvested RSUs shall vest automatically as of such date. In the event the termination of the Participant's services is by reason of voluntary resignation, only the Participant's unvested RSUs shall terminate automatically as of such date.
Change of Control
In the event of a Change of Control, the Board may, in its discretion, without the necessity or requirement for the agreement or consent of any Participant: (i) accelerate, conditionally or otherwise, on such terms as it sees fit, the vesting date of any RSU; (ii) permit the conditional settlement of any RSU, on such terms as it sees fit; (iii) otherwise amend or modify the terms of the RSU, including for greater certainty permitting Participants to settle any RSU, to assist the Participants to tender the underlying Common Shares to, or participate in, the actual or potential Change of Control Event (as defined in the RSU Plan) or to obtain the advantage of holding the underlying Common Shares during such Change of Control Event; and (iv) terminate, following the successful completion of such Change of Control Event, on such terms as it sees fit, the RSUs not settled prior to the successful completion of such Change of Control Event, including, without limitation, for no payment or other compensation. The determination of the Board in respect of any such Change of Control Event shall for the purposes of this RSU Plan be final, conclusive and binding.
Adjustments
In the event there is a change in the outstanding Common Shares by reason of any stock dividend or split, recapitalization, amalgamation, consolidation, combination or exchange of shares, or other corporate change, the Board shall make, subject to the prior approval of the CSE and NASDAQ where necessary, appropriate substitution or adjustment in (i) the number or kind of Common Shares or other securities reserved for issuance pursuant to the RSU Plan, and (ii) the number and kind of Common Shares or other securities subject to unsettled and outstanding RSUs granted pursuant to the RSU Plan.
Vesting
Each award of RSUs vests on the date(s) specified by the Board on the award date, and is reflected in the applicable RSU agreement certificate.
Limitations under the RSU Plan
The maximum number of Common Shares made available for issuance pursuant to the RSU Plan shall be determined from time to time by the Board, but in any case, shall not exceed 10% of the Common Shares issued and outstanding from time to time, subject to adjustments as provided in the RSU Plan.
Incentive Plan Awards
Outstanding Option-based Awards
There were no Option-based awards outstanding for any NEO as at the year ended September 30, 2020.
Outstanding Share-Based Awards
There were no Share-based awards outstanding for any NEO as at the year ended September 30, 2020.
Incentive Plan Awards - Value Vested or Earned During the Year
The following table sets out the value vested or earned under the Option Plan awards and the RSU Plan awards during the financial year ended September 30, 2020, for each NEO:
|
Name of NEO |
Option-based awards - |
Share-based awards - Value |
Non-equity incentive |
|
Ian McDonald |
Nil |
Nil |
Nil |
|
Ryan Cheung |
Nil |
Nil |
Nil |
Director Compensation
The following table sets forth all compensation for services as a director to the Company during the fiscal years ended September 30, 2020 and 2019 in respect of the directors set out below, which for those directors who are NEOs excludes compensation for services provided as an NEO:
|
Name |
Year |
Salary |
Share- based Awards ($) |
Equity ($) |
All Other ($) |
Total Compensation ($) |
|
Ian McDonald |
2020 2019 |
Nil Nil |
Nil Nil |
Nil Nil |
Nil Nil |
Nil Nil |
|
Nils Christian Bottler |
2020 2019 |
Nil Nil |
Nil Nil |
Nil Nil |
Nil Nil |
Nil Nil |
|
Jeremy Fryzuk |
2020 2019 |
Nil Nil |
Nil Nil |
Nil Nil |
Nil Nil |
Nil Nil |
|
Alan Kozikowski |
2020 2019 |
6,099 Nil |
Nil Nil |
Nil Nil |
Nil Nil |
6,099 Nil |
We reimburse out-of-pocket costs that are incurred by the directors.
Pension Disclosure
The Company does not have a pension plan that provides for payments or benefits to the NEOs at, following, or in connection with retirement.
Termination and Change of Control Benefits
The agreement pursuant to which Dr. Revati Shreeniwas performs services as Chief Medical Officer of the Company may be terminated by the Company on thirty days notice to Dr. Shreeniwas for cause, as defined in the agreement, or on three months' notice for any other reason.
C. Board Practices
Board of Directors
The Articles of the Company provide that the number of directors is set at:
(a) subject to paragraphs (b) and (c), the number of directors that is equal to the number of the Company's first directors;
(b) if the Company is a public company, the greater of three and the number most recently elected by ordinary resolution (whether or not previous notice of the resolution was given); and
(c) if the Company is not a public company, the number most recently elected by ordinary resolution (whether or not previous notice of the resolution was given).
The Board currently consists of five directors. The directors are elected annually at each annual meeting of our Company's shareholders. The Board assesses potential Board candidates to fill perceived needs on the Board for required skills, expertise, independence and other factors.
The Board is responsible for appointing the Company's officers.
The Company has entered into an Independent Contractor Agreement dated October 29, 2020 between the Company and Dr. Alan Kozikowski engaging the services of Dr. Kozikowski as Chief Science Officer of the Company, with compensation to be determined by the Board of Directors of the Company. The agreement contains standard nondisclosure, noncompetition and non-solicitation provisions and automatically renews on an annual basis unless terminated by either party.
Board of Director Committees
The Board currently has four committees, the Audit Committee, The Nominating and Corporate Governance Committee, the Compensation Committee and the Corporate Disclosure Committee.
Audit Committee
The Audit Committee consists of Dr. Emer Leahy, Jeremy Fryzuk and Nils Christian Bottler (Chair). Each member of the Audit Committee satisfies the "independence" requirements of Rule 5605(a)(2) of the Listing Rules of the Nasdaq and meets the independence standards under Rule 10A-3 under the Exchange Act. The Audit Committee consists solely of independent directors that satisfy the Nasdaq and SEC requirements. The Audit Committee oversees the accounting and financial reporting processes and the audits of the financial statements of the Company. The Audit Committee is responsible for, among other things:
-
ensuring, through discussion with management and the external auditors, that the Company's annual and quarterly financial statements (individually and collectively, the "Financial Statements"), as applicable, present fairly in all material respects the financial conditions, results of operations and cash flows of the Company as of and for the periods presented;
-
reviewing and recommending for approval to the Board, the Company's financial statements, accounting policies that affect the financial statements, annual MD&A and associated press release(s);
-
reviewing significant issues affecting financial reports;
-
monitoring the objectivity and credibility of the Company's financial reports;
-
considering the effectiveness of the Company's internal controls over financial reporting and related information technology security and control;
-
reviewing with auditors any issues or concerns related to any internal control systems in the process of the audit;
-
reviewing with management, external auditors and legal counsel any material litigation claims or other contingencies, including tax assessments, and adequacy of financial provisions, that could materially affect financial reporting;
-
overseeing the work of the external auditor engaged for the purpose of preparing or issuing an auditor's report or performing such other audit, review or attest services for the Company, including the resolution of disagreements between management and the external auditor regarding financial reporting; and
-
taking such other actions within the general scope of its responsibilities as the Audit Committee shall deem appropriate or as directed by the Board of Directors.
Nominating and Corporate Governance Committee
On June 13, 2021, 2021, the Board of Directors adopted a new Nominating and Corporate Governance Committee Charter that complies with the requirements of Nasdaq Listing Rule 5605(e)(2), and has established a nominating and corporate governance committee (the "N&CG Committee") which operates under its Nominating and Corporate Governance Committee Charter. The N&CG Committee is currently comprised of Nils Bottler (Chair), Jeremy Fryzuk and Dr. Emer Leahy. The N&CG Committee is responsible for (i) identifying and recommending to the Board, individuals qualified to be nominated for election to the Board; (ii) recommending to the Board, the members and chairperson for each Board committee; and (iii) periodically reviewing and assessing the Company's corporate governance principles contained in the Nominating and Corporate Governance Committee Charter and making recommendations for changes thereto to the Board.
The N&CG Committee is responsible for, among other things:
-
leading the Company's search for individuals qualified to become members of the Board;
-
evaluating and recommending to the Board for nomination candidates for election or re-election as directors;
-
establishing and overseeing appropriate director orientation and continuing education programs;
-
making recommendations to the Board regarding an appropriate organization and structure for the Board of Directors;
-
evaluating the size, composition, membership qualifications, scope of authority, responsibilities, reporting obligations and charters of each committee of the Board;
-
periodically reviewing and assessing the adequacy of the Company's corporate governance principles as contained in the Nominating and Corporate Governance Committee Charter and, should it deem it appropriate, it may develop and recommend to the Board of Directors for adoption of additional corporate governance principles;
-
periodically reviewing the Company's Articles in light of existing corporate governance trends, and shall recommend any proposed changes for adoption by the Board of Directors or submission by the Board of Directors to the Company's shareholders;
-
making recommendations on the structure and logistics of Board of Directors' meetings and may recommend matters for consideration by the Board of Directors;
-
considering, adopting and overseeing all processes for evaluating the performance of the Board of Directors, each committee and individual directors; and
-
annually reviewing and assessing its own performance.
Compensation Committee
On June 13, 2021, the Board of Directors adopted a new Compensation Committee Charter which complies with the requirements of Nasdaq Listing Rule 5605(d)(1) and the Board of Directors has established a Compensation Committee (the "Compensation Committee"). The Compensation Committee is comprised of Nils Bottler (Chair), Dr. Emer Leahy and Jeremy Fryzuk.
The Compensation Committee assists the Board in fulfilling its oversight responsibilities relating to officer and director compensation, succession planning for senior management, development and retention of senior management and such other duties as directed by the Board.
Each of the Compensation Committee members satisfies the "independence" requirements of Rule 5605(a)(2) of the Listing Rules of Nasdaq. The Compensation Committee will be responsible for, among other things:
-
reviewing and approving the Company's compensation guidelines and structure;
-
reviewing and approving on an annual basis the corporate goals and objectives with respect to the CEO of the Company;
-
reviewing and approving on an annual basis the evaluation process and compensation structure for the Company's other officers, including salary, bonus, incentive and equity compensation;
-
reviewing the Company's incentive compensation and other equity-based plans and recommending changes in such plans to the Board as needed.
-
Periodically making recommendations to the Board regarding the compensation of non-management directors, including Board and committee retainers, meeting fees, equity-based compensation and such other forms of compensation and benefits as the Committee may consider appropriate; and
- overseeing the appointment and removal of executive officers, and reviewing and approving for executive officers, including the CEO, any employment, severance or change in control agreements.
Corporate Disclosure Committee
The Company's Corporate Disclosure Committee consists of Nils Bottler (Chair), Dr. Emer Leahy and Jeremy Fryzuk. The Corporate Disclosure Committee oversees the effectiveness of risk management policies, procedures and practices implemented by management of the Company with respect to the Company's disclosure controls and procedures.
D. Employees
As of June 15, 2021, we had no employees, and operated solely through the use of our consultants.
The Company has entered into the following consulting Agreements with the following consultants on the following terms:
-
Scientific Advisory Board Agreement dated June 1, 2020 between the Company and Narayan R. Kissoon, MD, engaging the services of Narayan R. Kissoon as a member of the Company's Scientific Advisory Board and as a consultant to the Company;
-
Scientific Advisory Board Agreement dated July 14, 2020 between the Company and Peter Hendricks, engaging Peter Hendricks as a member of the Company's Scientific Advisory Board and as a consultant to the Company;
-
Scientific Advisory Board Agreement dated April 21, 2021 between the Company and Jianmin Duan, engaging Jiamin Duan as a member of the Company's Scientific Advisory Board and as a consultant to the Company;
-
Consulting agreement dated August 15, 2020 between the Company and Dr. Krista Lanctot, engaging the public relations services of Dr. Lanctot as a consultant;
-
Consulting agreement dated August 15, 2020 between the Company and Werner Tueckmantel, engaging the public relations services of Mr. Tueckmantel as a consultant;
-
Consulting agreement dated August 15, 2020 between the Company and John McCorvy, engaging the public relations services of Mr. McCorvy as a consultant;
-
Consulting agreement dated August 15, 2020 between the Company and Peter Kowey, MD, engaging the public relations services of Dr. Kowey as a consultant;
-
Consulting agreement dated August 15, 2020 between the Company and Arina Zhukova engaging the services of Ms. Zhukova as a consultant;
-
Consulting agreement dated August 15, 2020 between the Company and Laurentiu Nicolae, engaging the services of Mr. Nicolae as a consultant;
-
Consulting agreement dated August 15, 2020 between the Company and Jesse Damsker engaging the services of Mr. Damsker as a consultant;
-
Consulting agreement dated October 9, 2020 between the Company and Dorothy G. Flood, Ph.D., principal of Ms. Flood Consulting LLC, engaging the services of Ms. Flood as a consultant;
-
Consulting Agreement dated January 4, 2021 between the Company and Uroš Laban, engaging the services of Mr. Laban as a consultant;
-
Consulting Agreement dated January 25, 2021 between the Company and John McCall, engaging the services of Mr. McCall as a consultant;
-
Independent contractor agreement dated May 7, 2021 between the Company and Bay Area Clinical Research Consulting LLC, Katherine McDougall, engaging the services of Katherine McDougall as a consultant; and
-
Consulting agreement dated December 22, 2020 between the Company and Toxicology Services, Inc., engaging the services of Dr. Thomas Grizzle. as a consultant.
E. Share Ownership
Shares
The shareholdings of our officers and directors are set out in Item 7 below.
Options
The stock options, exercisable into common shares of the Company, held by our officers and directors are set out in Item 6 B above.
ITEM 7. MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
A. Major Shareholders
Security Ownership of Certain Beneficial Owners and Management
The following table sets forth certain information regarding the beneficial ownership of our common share as of June 15, 2021 by (a) each stockholder who is known to us to own beneficially 5% or more of our outstanding common share; (b) all directors; (c) our executive officers, and (d) all executive officers and directors as a group. Except as otherwise indicated, all persons listed below have (i) sole voting power and investment power with respect to their common shares, except to the extent that authority is shared by spouses under applicable law, and (ii) record and beneficial ownership with respect to their common shares.
|
Name |
Common Shares of |
Percentage of |
|
Directors and Executive Officers: |
|
|
|
Ian McDonald, Chief Executive Officer, President and Director |
895,400 |
7.57% |
|
Ryan Cheung(3), Chief Financial Officer |
25,000 |
0.21% |
|
Alan Kozikowski(4), Chief Science Officer and Director |
2,000,000 |
16.90% |
|
Gideon Shapiro(5), Vice President (Discovery) |
1,496,000 |
12.64% |
|
Revati Shreeniwas(6), Chief Medical Officer |
587,500 |
4.89% |
|
Jeremy Fryzuk(7) , Director |
40,000 |
0.34% |
|
Nils Bottler(8), Director |
20,000 |
0.17% |
|
Emer Leahy, Director |
Nil |
0.00% |
|
Directors and Executive Officers as a Group (8 persons) |
5,041,400 |
41.85% |
|
Other 5% or more Shareholders: |
|
|
|
Sphera Global Healthcare Management LP(9) |
1,079,000 |
9.90% |
|
OrbiMed Advisors LLC(10) |
990,900 |
8.15% |
________________________________
Notes
(*) Less than 1%.
(1) Under Rule 13d-3, a beneficial owner of a security includes any person who, directly or indirectly, through any contract, arrangement, understanding, relationship, or otherwise has or common shares: (i) voting power, which includes the power to vote, or to direct the voting of common shares; and (ii) investment power, which includes the power to dispose or direct the disposition of common shares. Certain shares may be deemed to be beneficially owned by more than one person (if, for example, persons share the power to vote or the power to dispose of the common shares). In addition, shares are deemed to be beneficially owned by a person if the person has the right to acquire the shares (for example, upon exercise of an option) within 60 days of the date as of which the information is provided. In computing the percentage ownership of any person, the amount of shares outstanding is deemed to include the amount of shares beneficially owned by such person (and only such person) by reason of these acquisition rights. As a result, the percentage of outstanding shares of any person as shown in this table does not necessarily reflect the person's actual ownership or voting power with respect to the number of common shares actually outstanding on June 15, 2021.
(2) The percentage is calculated based on 11,834,361 common shares that were outstanding as of June 15, 2021.
(3) Shares beneficially owned consist of stock options to purchase 25,000 Common Shares.
(4) Shares beneficially owned consist of 2,000,000 Common Shares held directly by Mr. Kozikowski.
(5) Shares beneficially owned consist of 1,496,000 Common Shares held directly by Mr. Shapiro.
(6) Shares beneficially owned consist of (i) 400,000 Common Shares held directly by Dr. Shreeniwas, (ii) stock options to purchase 150,000 Common Shares, and (iii) 37,500 restricted share units.
(7) Shares beneficially owned consist of (i) 40,000 Common Shares held directly by Mr. Fryzuk.
(8) Shares beneficially owned consist of (i) 20,000 Common Shares held directly by Mr. Bottler.
(9) Shares beneficially owned consist of 924,500 Common Shares and 462,250 2021 Warrants. Mr. Amit Drach has voting and investment control over the shares held by Sphera Global Healthcare Management LP (“Sphera”). Pursuant to the terms and conditions of a Warrant Exercise Agreement with the Company dated March 9, 2021, Sphera is only permitted to exercise that number of Warrants which will result in Sphera being deemed to own, control or have the power to vote securities of the Company which would represent 9.9% of any class of voting securities of the Company as of the date of the exercise of such Warrants.
(10) Shares beneficially owned consist of 660,600 Common Shares and 330,300 2021 Warrants. Mr. Geoffrey Hsu has voting and investment control over the shares held by OrbiMed Advisors LLC.
The information as to shares beneficially owned, not being within our knowledge, has been furnished by the officers and directors.
As at June 15, 2021, there were 172 holders of record of our common shares.
Transfer Agent
Our Common Shares are recorded in registered form on the books of our transfer agent, Computershare Trust Company located at 3rd Floor, 510 Burrard Street, Vancouver, British Columbia, Canada, V6C 3B9.
B. Related Party Transactions
Related Party Contracts
On June 5, 2020, the Company entered into an independent consultant agreement (the "CMO ICA") whereby the consultant Revati, Inc., a private corporation incorporated in the State of California, USA, was engaged and the consultant's representative, Dr. Revati Shreeniwas, will serve as the Company's Chief Medical Officer, with the services being provided in California. As compensation for performing these services, the consultant or the consultant's representative will participate in the Company's equity incentive plans and will be eligible for cash payments in respect of fees at such time as the Company begins to compensate other C-level personnel in cash and in similar proportion to total compensation (the "fees"). The non-cash portion of the consultant's fees for the first year of the term was in the form of a grant of 150,000 vested stock options and 150,000 RSUs. The services will continue for an initial term of one year unless sooner terminated. The CMO ICA can be terminated by either party giving the other 30 days written notice or by mutual written agreement. At the end of the initial term, the CMO ICA will automatically be extended for additional one-year period(s) unless either party gives the other 30 days written notice. As at December 31, 2020, the Board of Directors have not finalized amounts pertaining to cash payments for fees.
On October 29, 2020, the Company entered into an independent contractor agreement (the "CSO ICA") whereby the contractor, Dr. Alan Kozikowsi, was engaged to serve as the Company's Chief Science Officer on an as-needed basis. The contractor will be compensated for these services as determined by the Board of Directors of the Company. The services will continue for an initial term of one year unless sooner terminated. The CSO ICA can be terminated by the Company providing five working days written notice, the contractor providing three months' written notice or by mutual written agreement. At the end of the initial term, the CSO ICA will automatically be extended for additional one-year period(s) unless the Company provides contractor with 30 days written notice.
ITEM 8. FINANCIAL INFORMATION
A. Consolidated Statements and Other Financial Information
Financial Statements
Our unaudited condensed interim consolidated financial statements as at and for the three and six months ended March 31, 2021 and 2020 and our audited consolidated financial statements as at and for the year ended September 30, 2020, and for the period ended September 30, 2019 are attached hereto and found immediately following the text of this Registration Statement, beginning on page F-1. The financial statements of the Company have been prepared in accordance with IFRS, as issued by the International Accounting Standards Board and are included under Item 18 of this Registration Statement. The financial statements including related notes are accompanied by the report of the Company's independent registered public accounting firm, DeVisser Gray LLP.
Legal Proceedings
As of the date of this Registration Statement, in the opinion of our management, we are not currently a party to any litigation or legal proceedings which are material, either individually or in the aggregate, and, to our knowledge, no legal proceedings of a material nature involving us currently are contemplated by any individuals, entities or governmental authorities.
Dividends
We have not paid any dividends on the Common Shares since incorporation. Management anticipates that the Company will retain all future earnings and other cash resources for the future operation and development of the business. The Company does not intend to declare or pay any cash dividends in the foreseeable future. Payment of any future dividends will be at the Board's discretion, subject to applicable law, after taking into account many factors including our operating results, financial condition and current and anticipated cash needs.
B. Significant Changes
We have not experienced any significant changes since the date of the financial statements included with this Registration Statement except as disclosed in this Registration Statement.
ITEM 9. THE OFFER AND LISTING
A. Offer and Listing
The Common Shares are listed for trading on the CSE under the trading symbol “DRUG”. The Company has applied to have its Common Shares listed on Nasdaq Capital Market under the trading symbol “DRUG”. Such a listing is dependent upon this Registration Statement being becoming effective as well as our meeting all the necessary listing requirements of Nasdaq Capital Market, however, our registration is not conditions on obtaining such listing.
As of the date of this Registration Statement, our authorized capital consisted of an unlimited number of Common Shares without par value. Our issued and outstanding Common Shares are fully paid and non-assessable. We have no other authorized class of equity securities.
Our Common Shares are in registered form and the transfer of our Common Shares is managed by our transfer agent, Computershare Trust Company, 510 Burrard Street, 3rd Floor, Vancouver, British Columbia, V6C 3B9 (Tel: (604) 661-9400). Our transfer agent in the United States is expected to be Computershare Trust Company, N.A., Canton, MA.
For additional details regarding our Common Shares, see Item 10.A, "Share Capital".
B. Plan of Distribution
Not applicable.
C. Markets
See Item 9.A "Offer and Listing Details". The Common Shares trade on the CSE under the symbol "DRUG" and the CUSIP number for the Common Shares is 10919W108.
D. Selling Shareholders
Not applicable.
E. Dilution
Not applicable.
F. Expenses of the Issue
Not applicable.
ITEM 10. ADDITIONAL INFORMATION
A. Share Capital
Our authorized capital consists of an unlimited number of Common Shares without par value. The holders of the Common Shares are entitled to receive notice of and to attend all annual and special meetings of our shareholders and to one vote in respect of each Common Share held at such meetings.
The following table sets forth certain particulars regarding the Common Shares that are issued and outstanding as at June 15, 2021. All Common Shares issued and outstanding are fully paid.
(Dollar Amounts are in Canadian dollars)
|
Number of |
Number of |
Par |
Book value |
|
Unlimited |
11,834,361 (1) |
No par value |
$25,332,313 |
|
Note: (1) The Company consolidated the Common Shares on the basis of 2.5 pre-Consolidation share to 1 post-Consolidation share on November 10, 2020. |
|||
History of Issuances
In the past three years, we have issued and sold the securities described below without registering the securities under the Securities Act. We believe that each of the following issuances was exempt from registration under the Securities Act in reliance on Regulation S promulgated under the Securities Act regarding sales by an issuer in offshore transactions, Regulation D under the Securities Act, Rule 701 under the Securities Act or pursuant to Section 4(a)(2) of the Securities Act regarding transactions not involving a public offering.
2019
On July 30, 2019, the Company completed a distribution of units consisting of one Common Share and one Common Share purchase warrant (each, a "2019 Warrant") at purchase price of $0.02 per unit, for 10,199,000 units and gross aggregate proceeds of $203,980. Each 2019 Warrant is exercisable to purchase one Common Share at an exercise price of $0.05 per Common Share until July 30, 2024.
On August 7, 2019, the Company completed the acquisition of 100,000 common shares in the capital of its subsidiary, Psilocybinlabs Ltd. from Alex Vasilkevich in consideration of the issuance of 100,000 Common Shares in the capital of the Company granted to Mr. Vasilkevich at a deemed price of $0.02 per Common Share.
2020
On September 30, 2020, the Company issued 1,559,847 Common Shares at an issue price of $0.50 per Common Share for gross aggregate proceeds of $779,923.50.
On November 2, 2020, the Company issued 4,072,843 Common Shares at an issue price of $0.50 per Common Share for gross proceeds of $2,036,421.50.
On November 2, 2020 the Company issued 45,750 special warrants (the "Special Warrants") at an issue price of $0.50 per Common Share for gross proceeds of $22,875 (the "Special Warrant Offering").
On November 10, 2020, the Company consolidated (the "Consolidation") its Common Shares on the basis of 2.5:1. Prior to the completion of the Consolidation there were 15,931,691 Common Shares issued and outstanding, and on completion of the Consolidation there were 6,372,679 Common Shares issued and outstanding.
2021
On January 19, 2021, the Company rescinded the issuance of an aggregate of 5,750 Special Warrants initially issued pursuant to the Special Warrant Offering, and provided a full refund of the purchase price of such Special Warrants to the purchasers thereof. The rescission was completed at the request of the BCSC after they conducted a compliance review of the Special Warrant Offering. The BCSC determined that the exemption from prospectus requirements the Company relied on with regard to certain purchasers was non-compliant, and the Company then took action to remedy the issuance of securities to any such purchasers.
On January 13, 2021, the Company issued 14,799 Common Shares in connection with a debt settlement agreement between the Company and a consultant to settle an aggregate amount of $18,500 in debt owed to the consultant by the Company for past services rendered.
On February 3, 2021, the Company issued 16,000 Common Shares upon the deemed exercise of 40,000 Special Warrants as adjusted to give effect to the Consolidation, resulting in 6,403,478 Common Shares issued and outstanding.
On March 17, 2021, the Company issued 3,419,883 units at an issue price of $7.57 per unit for aggregate gross proceeds of $25,888,514.31 (the "2021 Offering"). Each unit consists of one Common Share and one-half of one Common Share purchase warrant of the Company (each whole warrant, a "2021 Warrant"). Each 2021 Warrant is exercisable to acquire one Common Share at an exercise price of $9.46 per Common Share until March 17, 2024, subject to adjustment and acceleration in certain events. If the daily volume weighted average trading price of the Common Shares on the CSE is greater than $13.25 per Common Share for any ten consecutive trading days, the Company will have the right to accelerate the expiry date of the 2021 Warrants to a date that is at least 30 trading days following the date of the Company issuing a press release disclosing such acceleration.
As of June 15 2021, there were a total of 1,709,938 outstanding 2021 Warrants,132,666 compensation warrants, 2,131,600 outstanding 2019 Warrants, 1,017,000 outstanding Options issued under the Option Plan and 380,000 outstanding RSUs under the RSU Plan. In addition, as at June 15, 2021, 166,436 Options remain available for future grant under the Option Plan and 803,436 RSUs remain available for future grant under the RSU Plan.
B. Memorandum and Articles of Association
The following is a summary of the Company's Notice of Articles (the "Notice of Articles") and Articles of Incorporation (the "Articles"). You should read those documents for a complete understanding of the rights and limitations set out therein. The Company number, as assigned by the British Columbia Registry Services, is BC1210954.
Remuneration of Directors
The directors are entitled to the remuneration, if any, for acting as directors as the directors may from time to time determine. If the directors so decide, the remuneration of the directors will be determined by the shareholders. That remuneration may be in addition to any salary or other remuneration paid to a director in such director's capacity as an officer or employee of the Company.
Number of Directors
According to Article 13.1 of the Articles, the number of directors, excluding additional directors appointed under Article 14.8 is set at:
(a) subject to paragraphs (b) and (c), the number of directors that is equal to the number of the Company's first directors;
(b) if the Company is a public company, the greater of three and the number most recently of: (i) the number of directors set by a resolution of the directors (whether or not previous notice of the resolutions was given); and the number of directors in office pursuant to Article 14.4; and
(c) if the Company is not a public company, the most recently set of: (i) the number of directors set by a resolution of the directors (whether previous notice of the resolution was given); and (ii) the number of directors in office pursuant to Article 14.4.
Directors
Directors are elected annually at each annual meeting of the Company's shareholders. The Articles provide that the Board may, between annual meetings or by unanimous resolutions contemplated by Article 10.2, appoint one or more additional directors to serve until the next annual meeting, but the number of additional directors must not at any time exceed:
(a) one-third of the number of first directors, if, at the time of the appointments, one or more of the first directors have not yet completed their first term of office; or
(b) in any other case, one-third of the number of the current directors who were elected or appointed as directors other than under Article 14.8.
The Articles provide that the directors may from time to time, on behalf of the Company, without shareholder approval:
(a) create one or more classes or series of shares or, if none of the shares of a class or series of shares are allotted or issued, eliminate that class or series of shares;
(b) increase, reduce or eliminate the maximum number of shares that we are authorized to issue out of any class or series of shares or establish a maximum number of shares that we are authorized to issue out of any class or series of shares for which no maximum is established;
(c) subdivide or consolidate all or any of its unissued, or fully paid issued, shares;
(d) if the Company is authorized to issue shares of a class of shares with par value: (i) decrease the par value of those shares; or (ii) if none of the shares of that class of shares are allotted or issued, increase the par value of those shares;
(e) change all or any of its unissued, or fully paid issued, shares with par value into shares without par value or any of its unissued shares without par value into shares with par value;
(f) alter the identifying name of any of its shares;
(g) otherwise alter its shares or authorized share structure when required or permitted to do so by the BCBCA where it does not specify by a special resolution;
(h) borrow money in the manner and amount, on the security, from the sources and on the terms and conditions that they consider appropriate;
(i) issue bonds, debentures and other debt obligations either outright or as security for any liability or obligation of the Company or any other person and at such discounts or premiums and on such other terms as the directors consider appropriate;
(j) guarantee the repayment of money by any other person or the performance of any obligation of any other person;
(k) mortgage, charge, whether by way of specific or floating charge, grant a security interest in, or give other security on, the whole or any part of the present and future assets and undertaking of the Company.
The Articles also provide that the Company may by resolution of the directors authorize an alteration to the Notice of Articles to change the Company's name or adopt or change any translation of that name.
The Articles provide that the directors may meet together for the conduct of business, adjourn and otherwise regulate their meetings as they think fit, and meetings of the Board of Directors held at regular intervals may be held at the place and at the time that the Board of Directors may by resolution from time to time determine. Questions arising at any meeting of directors are to be decided by a majority of votes and, in the case of an equality of votes, the chair of the meeting does not have a second or casting vote. A director may participate in a meeting of the directors or of any committee of the directors in person, or by telephone or other communications medium, if all directors participating in the meeting are able to communicate with each other. A director who participates in a meeting in a manner contemplated by such provisions of the Articles is deemed for all purposes of the BCBCA and the Articles to be present at the meeting and to have agreed to participate in that manner.
The Articles provide that the quorum necessary for the transaction of the business of the directors may be set by the directors and, if not so set, is deemed to be a majority of the directors or, if the number of directors is set at one, is deemed to be set at one director, and that director may constitute a meeting.
The Articles provide that a director or senior officer who holds a disclosable interest (as that term is used in the BCBCA) in a contract or transaction in to which the Company has entered or proposes to enter is liable to account to the Company for any profit that accrues to the director or senior officer under or as a result of the contract or transaction only if and to the extent provided in the BCBCA. Additionally, a director who holds a disclosable interest in a contract or transaction into which the Company has entered or proposes to enter is not entitled to vote on any directors' resolution to approve that contract or transaction, unless all the directors have a disclosable interest in that contract or transaction, in which case any or all of those directors may vote on such resolution. A director or senior officer who holds any office or possess any property, right or interest that materially conflicts with that individual's duty or interest as a director or senior officer, must disclose the nature and extent of the conflict as required by the BCBCA.
The Articles do not set out a mandatory retirement age for the Company's directors. The directors are not required to own securities of the Company to serve as directors.
Authorized Capital
The Notice of Articles provide that the Company's authorized capital consists of an unlimited number of common shares, without par value.
Rights, Preferences and Restrictions Attaching to Our Shares
Each Common Share entitles the holder thereof to notice and to attend and to cast one vote for each matter to be decided at a general meeting of the Company. The Common Shares do not have special rights or restrictions attached.
Shareholder Meetings
Part 10 of the Articles regulates the meetings of shareholders. Article 10.1 of the Articles provides that, unless an annual general meeting is deferred or waived in accordance with the BCBCA, an annual general meeting must be held at least once in each calendar year and not more than 15 months after the last annual reference date at such time and place as may be determined by directors.
The notice for meetings of Shareholders is contemplated in Article10.4 of the Articles and provides that the Company must send notice of the date, time and location of any meeting of shareholders, in the manner provided in the Articles or in such other manner, if any, as may be prescribed by ordinary resolution, to each shareholder entitled to attend the meeting, to each director and to the auditor of the Company, unless the Articles otherwise provide, at least 21 days before the meeting. Article 10.5 provides that the directors may set a date as the record date for the purpose of determining shareholders entitled to notice of any meeting of shareholders and entitled to vote at any meeting of shareholders, the record date must not precede the date on which the meeting is to be held by more than two months and at least 21 days before.
Generally, notice of a meeting of the shareholders called for any purpose other than consideration of the financial statements and any reports of the directors or auditor, the setting and changing of the number of directors, the election or appointment of directors and appointment of auditor, the setting of the remuneration of the auditor, shall state the general nature of the special business and if the special business includes considering, approving, ratifying, adopting or authorizing any document or the signing of or giving effect to any document, have attached to it a copy of the document or state that a copy of the document will be available for inspection by shareholders at our records office or such other reasonably accessible location in British Columbia.
The accidental omission to send notice of any meeting to, or the non-receipt of any notice by, a shareholder will not invalidate any proceedings at that meeting. A shareholder may in any manner waive notice of or otherwise consent to a meeting of shareholders.
C. Material Contracts
Except for contracts entered into in the ordinary course of business, the following are our only material agreements (the "Material Contracts"):
-
the escrow agreement dated January 28, 2021 among Bright Minds, Computershare Investor Services Inc. and certain shareholders of the Company;
- the following agreements, referred to under “Directors, Senior Management and Employees – Compensation”
- Independent Contractor Agreement dated October 29, 2020 between the Company and Dr. Alan Kozikowski engaging the services of Dr. Kozikowski as Chief Science Officer of the Company;
- Independent Consultant Agreement dated June 5, 2020, between the Company and Revati, Inc. a corporation controlled by Dr. Revati Shreeniwas, pursuant to which Dr. Revati Shreeniwas was engaged to perform services as Chief Medical Officer of the Company; and
- Independent Contractor Agreement dated November 17, 2020 between the Company and Dr. Gideon Shapiro engaging the services of Dr. Shapiro as Vice President (Discovery) of the Company;
- Independent Contractor Agreement dated October 29, 2020 between the Company and Dr. Alan Kozikowski engaging the services of Dr. Kozikowski as Chief Science Officer of the Company;
-
the following agreements, referred to under "Directors, Senior Management and Employees - Employees"
-
Consulting Agreement with Dr. Krista Lanctot;
-
Consulting Agreement with Werner Tueckmantel;
-
Consulting Agreement with John McCorvy;
-
Consulting Agreement with Peter Kowey;
-
Consulting Agreement with Arina Zhukova;
-
Consulting Agreement with Laurentiu Nicolae;
-
Consulting Agreement with Dorothy G. Flood;
-
Consulting Agreement with Jesse Damsker;
-
Consulting Agreement with Toxicology Services Inc.;
-
Consulting Agreement with Uroš Laban;
-
Consulting Agreement with John McCall;
-
Independent Contractor Agreement with Bay Area Clinical Research Consulting LLC, Katherine McDougall;
-
Scientific Advisory Board Agreement with Narayan R. Kisson, MD;
-
Scientific Advisory Board Agreement with Jianmin Duan; and
-
Scientific Advisory Board Agreement with Peter Hendricks.
-
-
the Services Agreement dated June 22, 2020 between the Company and the Medical College of Wisconsin, Inc. ("MCW") engaging MCW, led by John McCorvy, to provide certain consulting, laboratory and/or research-related services to the Company;
-
the Underwriting Agreement dated February 23, 2021 among the Company, Eight Capital, Stifel Nicolaus Canada Inc., Beacon Securities Limited and Haywood Securities Inc. entered into in connection with the 2021 Offering;
-
the warrant indenture dated March 17, 2021 between the Company and Computershare Trust Company of Canada governing the terms of the 2021 Warrants;
-
the Sponsored Research Agreement dated October 15, 2020 between the Company and the University of Texas Medical Branch at Galveston d/b/a UTMB Health ("UTMB"), engaging the research services of UTMB, led by Dr. Kathryn Cunningham to conduct a research program sponsored by the Company;
-
the First Amendment to the Sponsored Research Agreement between the Company and UTMB dated June 11, 2021;
- the Option Agreement dated May 26, 2020 between the Board of Trustees of the University of Illinois and the Company in respect of the exclusive option to licence patent and related technical information; and
-
the Exclusive License Agreement dated April 23, 2021 between the Board of Trustees of the University of Illinois and the Company in respect of the exclusive license granted to the Company of a patent and related technical information.
D. Limitation on Rights on Non-Canadians
We are incorporated pursuant to the laws of the Province of British Columbia, Canada. There is no law or governmental decree or regulation in Canada that restricts the export or import of capital, or affects the remittance of dividends, interest or other payments to a non-resident holder of common shares, other than withholding tax requirements. Any such remittances to United States residents are generally subject to withholding tax, however no such remittances are likely in the foreseeable future. See "Certain Canadian Federal Income Tax Information For United States Residents" below.
There is no limitation imposed by Canadian law or by the charter or other constituent documents of our Company on the right of a non-resident to hold or vote common shares of our company. However, the Investment Canada Act (Canada) (the "Investment Act") has rules regarding certain acquisitions of shares by non-Canadians, along with other requirements under that legislation.
The following discussion summarizes the principal features of the Investment Act for a "non-Canadian" (as defined under the Investment Act) who proposes to acquire common shares of our Company. The discussion is general only; it is not a substitute for independent legal advice from an investor's own advisor; and it does not anticipate statutory or regulatory amendments.
The Investment Act is a federal statute of broad application regulating the establishment and acquisition of Canadian businesses by non-Canadians, including individuals, governments or agencies thereof, corporations, partnerships, trusts or joint ventures (each an "entity"). Investments by non-Canadians to acquire control over existing Canadian businesses or to establish new ones are either reviewable or notifiable under the Investment Act. If an investment by a non-Canadian to acquire control over an existing Canadian business is reviewable under the Investment Act, the Investment Act generally prohibits implementation of the investment unless, after review, the Minister of Innovation, Science and Economic Development Canada (the "Minister") is satisfied that the investment is likely to be of net benefit to Canada.
A non-Canadian would acquire control of our Company for the purposes of the Investment Act through the acquisition of common shares if the non-Canadian acquired a majority of the common shares of our Company.
Further, the acquisition of less than a majority but one-third or more of the common shares of our Company by a non-Canadian would be presumed to be an acquisition of control of our Company unless it could be established that, on the acquisition, our Company was not controlled in fact by the acquirer through the ownership of common shares.
For a direct acquisition that would result in an acquisition of control of our Company, subject to the exception for "WTO-investors" that are controlled by persons who are nationals or permanent residents of World Trade Organization ("WTO") member nations, a proposed investment generally would be reviewable where the value of the acquired assets is CAD$5 million or more.
For a proposed indirect acquisition by an investor other than a so-called WTO investor that would result in an acquisition of control of our Company through the acquisition of a non-Canadian parent entity, the investment generally would be reviewable where the value of the assets of the entity carrying on the Canadian business, and of all other entities in Canada, the control of which is acquired, directly or indirectly is CAD$50 million or more.
In the case of a direct acquisition by a "WTO investor", the threshold is significantly higher. An investment in common shares of our Company by a WTO investor that is not a state-owned enterprise would be reviewable only if it was an investment to acquire control of the company and the enterprise value of the assets of the company was equal to or greater than a specified amount, which is published by the Minister after its determination for any particular year. For 2021, this amount is CAD$1.043 billion (unless the investor is controlled by persons who are nationals or permanent residents of countries that are party to one of a list of certain free trade agreements, in which case the amount is CAD$1.565 billion for 2021); each January 1, both thresholds are adjusted by a GDP (Gross Domestic Product) based index.
The higher WTO threshold for direct investments and the exemption for indirect investments do not apply where the relevant Canadian business is carrying on a "cultural business". The acquisition of a Canadian business that is a "cultural business" is subject to lower review thresholds under the Investment Act because of the perceived sensitivity of the cultural sector.
In 2009, amendments were enacted to the Investment Act concerning investments that may be considered injurious to national security. If the Minister has reasonable grounds to believe that an investment by a non-Canadian "could be injurious to national security," the Minister may send the non-Canadian a notice indicating that an order for review of the investment may be made. The review of an investment on the grounds of national security may occur whether or not an investment is otherwise subject to review on the basis of net benefit to Canada or otherwise subject to notification under the Investment Act.
Certain transactions, except those to which the national security provisions of the Investment Act may apply, relating to common shares of our Company are exempt from the Investment Act, including:
(a) the acquisition of our common shares by a person in the ordinary course of that person's business as a trader or dealer in securities;
(b) the acquisition of control of our company in connection with the realization of security granted for a loan or other financial assistance and not for a purpose related to the provisions on the Investment Act, if the acquisition is subject to approval under the Bank Act, the Cooperative Credit Associations Act, the Insurance Companies Act or the Trust and Loan Companies Act; and
(c) the acquisition of control of our company by reason of an amalgamation, merger, consolidation or corporate reorganization following which the ultimate direct or indirect control in fact of our company, through the ownership of common shares, remained unchanged.
E. Taxation
Certain Canadian Federal Income Tax Considerations for United States Residents
The following is a summary of certain Canadian federal income tax considerations generally applicable to the holding and disposition of our common shares acquired by a holder who, at all relevant times, (a) for the purposes of the Income Tax Act (Canada) (the "Tax Act") (i) is not resident, or deemed to be resident, in Canada, (ii) deals at arm's length with us and any underwriters that we have recently used, and is not affiliated with us or the underwriters that we have recently used, (iii) holds our common shares as capital property, (iv) does not use or hold the common shares in the course of carrying on, or otherwise in connection with, a business carried on or deemed to be carried on in Canada and (v) is not a "registered non-resident insurer", an "authorized foreign bank" (each as defined in the Tax Act), or other holder of special status or in special circumstances, and (b) for the purposes of the Canada-U.S. Tax Convention (the "Tax Treaty"), is a resident of the United States, has never been a resident of Canada, does not have and has not had, at any time, a permanent establishment or fixed base in Canada, and who qualifies for the full benefits of the Tax Treaty. Holders who meet all the criteria in clauses (a) and (b) above are referred to herein as "U.S. Holders", and this summary only addresses such U.S. Holders.
This summary does not deal with special situations, such as the particular circumstances of traders or dealers, tax exempt entities, insurers or financial institutions, or other holders of special status or in special circumstances. Such holders, and all other holders who do not meet the criteria in clauses (a) and (b) above, should consult their own tax advisors.
This summary is based on the current provisions of the Tax Act, the regulations thereunder in force at the date hereof (the "Regulations"), the current provisions of the Tax Treaty, and our understanding of the administrative and assessing practices of the Canada Revenue Agency published in writing prior to the date hereof. This summary takes into account all specific proposals to amend the Tax Act and Regulations publicly announced by or on behalf of the Minister of Finance (Canada) prior to the date hereof (the "Proposed Amendments") and assumes that any such Proposed Amendments will be enacted in the form proposed. However, such Proposed Amendments might not be enacted in the form proposed, or at all. This summary does not otherwise take into account or anticipate any changes in law or administrative or assessing practices, whether by legislative, governmental or judicial decision or action, nor does it take into account tax laws of any province or territory of Canada or of any other jurisdiction outside Canada, which may differ significantly from those discussed in this summary.
For the purposes of the Tax Act, all amounts relating to the acquisition, holding or disposition of our securities must be expressed in Canadian dollars. Amounts denominated in United States currency generally must be converted into Canadian dollars using the rate of exchange that is acceptable to the Canada Revenue Agency.
This summary is of a general nature only and is not intended to be, nor should it be construed to be, legal or tax advice to any particular U.S. Holder, and no representation with respect to the Canadian federal income tax consequences to any particular U.S. Holder or prospective U.S. Holder is made. This summary is not exhaustive of all Canadian federal income tax considerations. Accordingly, all prospective purchasers (including U.S. Holders as defined above) should consult with their own tax advisors for advice with respect to their own particular circumstances.
Withholding Tax on Dividends
Amounts paid or credited or deemed to be paid or credited as, on account or in lieu of payment of, or in satisfaction of, dividends on our common shares to a U.S. Holder will be subject to Canadian withholding tax. Under the Tax Treaty, the rate of Canadian withholding tax on dividends paid or credited by us to a U.S. Holder that beneficially owns such dividends and substantiates eligibility for the benefits of the Tax Treaty is generally 15% of the gross amount of the dividends (unless the beneficial owner is a company that owns at least 10% of our voting stock at that time, in which case the rate of Canadian withholding tax is generally reduced to 5%).
Dispositions
A U.S. Holder will, in general terms, not be subject to tax under the Tax Act on a capital gain realized on a disposition or deemed disposition of common shares unless the common shares are "taxable Canadian property" to the U.S. Holder for purposes of the Tax Act and the U.S. Holder is not entitled to relief under the Tax Treaty.
Provided the common shares are listed on a "designated stock exchange" as defined in the Tax Act (which currently includes the CSE) at the time of disposition, the common shares generally will not constitute "taxable Canadian property" of a U.S. Holder at that time unless, at any time during the 60 month period immediately preceding the disposition, the following two conditions are met: (i) the U.S. Holder, persons with whom the U.S. Holder did not deal at arm's length, partnerships in which the U.S. Holder or such non-arm's length persons holds a membership interest (either directly or indirectly through one or more partnerships), or the U.S. Holder together with all such persons, owned 25% or more of the issued shares of any class or series of shares of our company; and (ii) more than 50% of the fair market value of the common shares of the company was derived directly or indirectly from one or any combination of real or immovable property situated in Canada, Canadian resource properties (as defined in the Tax Act), timber resource properties (as defined in the Tax Act) or options in respect of, or interests in, or for civil law rights in, property described in any of the foregoing whether or not the property exists. Notwithstanding the foregoing, in certain other circumstances set out in the Tax Act, common shares could also be deemed to be "taxable Canadian property".
U.S. Holders who may hold common shares as "taxable Canadian property" should consult their own tax advisors with respect to the application of Canadian capital gains taxation, any potential relief under the Tax Treaty, and compliance procedures under the Tax Act, none of which is described in this summary.
F. Dividends and Paying Agents
Not Applicable.
G. Statements by Experts
Not Applicable.
H. Documents on Display
Upon the effectiveness of this filing, we will be subject to the informational requirements of the Securities Exchange Act of 1934, as amended, and we will thereafter file reports and other information with the SEC. The SEC maintains a web site that contains reports and other information regarding registrants that file electronically with the SEC at www.sec.gov. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330.
The documents concerning our Company referred to in this Registration Statement may be viewed at our principal executive offices, 1500 - 1055 West Georgia St., Vancouver, BC, V6E 4N7 (Telephone: (647) 407-2515), during normal business hours. Copies of our financial statements and other continuous disclosure documents required under the Securities Act (British Columbia) are available for viewing on SEDAR at www.sedar.com. All of the documents referred to are in English.
I. Subsidiary Information
See Item 4.C, "Organizational Structure", for the Company's active subsidiaries as at the date of this Registration Statement.
ITEM 11. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
The Company is exposed in varying degrees to a variety of financial instrument related risks.
Credit risk
Credit risk is the risk that one party to a financial instrument will fail to discharge an obligation and cause the other party to incur a financial loss. The Company's primary exposure to credit risk is on its cash balance. At March 31, 2021, the Company had cash of C$25,450,001. Of this amount, $11,494 was held in one trust account and the remainder was deposited in a bank account held with a major bank in Canada. Because of the balance on deposit with one bank, there is a concentration of credit risk. This risk is managed by using a major bank that is a high credit quality financial institution as determined by rating agencies. The maximum exposure to credit risk is the carrying amount of the Company's financial instruments. The credit risk is assessed as low.
Foreign exchange risk
Foreign currency risk is the risk that the fair values of future cash flows of a financial instrument will fluctuate because they are denominated in currencies that differ from the respective functional currency. At March 31, 2021, the company had the following foreign currency balances - cash (US$ 122,369) and accounts payable (US$ 237,865 and EURO 3,764) and prepaid (US$ 80,900). The Company is not exposed to significant foreign exchange risk.
Liquidity risk
Liquidity risk arises through the excess of financial obligations over available financial assets due at any point in time. The Company's objective in managing liquidity risk is to maintain sufficient readily available reserves in order to meet its liquidity requirements at any point in time. The Company's main source of funding has been the issuance of equity securities for cash, primarily through private placements. The Company's access to financing is always uncertain. There can be no assurance of continued access to significant equity funding. At March 31, 2021, the company had cash of C$25,450,001 to cover current liabilities of C$409,238.
Capital Management
Management's objective is to manage its capital to ensure that there are adequate capital resources to safeguard the Company's ability to continue as a going concern through the optimization of its capital structure. The capital structure consists of share capital and working capital. In order to achieve this objective, management makes adjustments to it in light of changes in economic conditions and risk characteristics of the underlying assets. To maintain or adjust the capital structure, management may invest its excess cash in interest bearing accounts of Canadian chartered banks and/or raise additional funds externally as needed. The Company is not subject to externally imposed capital requirements. The Company's management of capital did not change during the period ended March 31, 2021.
ITEM 12. DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES
Not applicable.
PART II
ITEM 13. DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES
There have not been any defaults with respect to dividends, arrearages or delinquencies since incorporation on May 31, 2019.
ITEM 14. MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS
Not applicable.
ITEM 15. CONTROLS AND PROCEDURES
Not applicable.
ITEM 16A. AUDIT COMMITTEE FINANCIAL EXPERT
Not applicable.
ITEM 16B. CODE OF ETHICS
Not applicable.
ITEM 16C. PRINCIPAL ACCOUNTANT FEES AND SERVICES
Not applicable.
ITEM 16D. EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES
Not applicable.
ITEM 16E. PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS
Not applicable.
ITEM 16F. CHANGE IN REGISTRANT'S CERTIFYING ACCOUNTANT
Not applicable.
ITEM 16G. CORPORATE GOVERNANCE
The Company is a foreign private issuer and has applied to have its Common Shares listed on Nasdaq Capital Market. Nasdaq Marketplace Rule 5615(a)(3) permits a foreign private issuer to follow its home country practices in lieu of most of the requirements of the 5600 Series, the requirement to disclose third party director and nominee compensation set forth in Rule 5250(b)(3), and the requirement to distribute annual and interim reports set forth in Rule 5250(d) of the Nasdaq Marketplace Rules. In order for the Company, which is applying to have the first U.S. listing of its common shares on Nasdaq, to follow home country practice in lieu of a requirement of Rule 5600 Series, 5250(b)(3), or 5250(d), the Company is required to disclose in this registration statement or on its website each corporate governance requirement that it does not follow and describe the home country practice followed by the Company in lieu of such corporate governance requirement. The Company confirms that except as described below, the Company is in compliance with the Nasdaq corporate governance requirements in all significant respects:
Shareholder Approval Requirements
Nasdaq Marketplace Rule 5635 requires shareholder approval for issuances of common shares, or any securities convertible or exercisable into common shares:
(a) in connection with the acquisition of the stock or assets of another company
(i) where, due to the present or potential issuance of common shares (including shares issued pursuant to an earn-out or similar type of provision, or securities convertible into or exercisable for common shares) other than a public offering for cash:
(A) the common shares constitute or will upon issuance constitute at least 20% of the voting power outstanding before the issuance of the common shares (or, if applicable before the issuance of the securities convertible into or exercisable for common shares); or
(B) the common shares constitute or will upon issuance constitute at least 20% of the number of common shares outstanding before the issuance; or
(ii) if any director, officer or Substantial Shareholder (as defined by Rule 5635(e)(3) of the Nasdaq Marketplace Rules) of the listed company has a 5% or greater interest (or such persons collectively have a 10% or greater interest), directly or indirectly, in the target company or assets to be acquired, or in the consideration to be paid in the transaction or series of related transactions, and the present or potential issuance of common shares, or securities convertible into or exercisable for common shares, could result in an increase of 5% or more in the outstanding common shares or voting power of the listed company;
(b) where the common shares sold (or the number of common shares into which the securities sold are convertible or exercisable), either alone or together with sales by officers, directors or Substantial Shareholders of the listed company, constitute at least
(i) 20% of the outstanding common shares before the issuance, or
(ii) 20% of the voting power of the outstanding common shares before the issuance,
in either case except for (A) public offerings of common shares for cash, and (B) transactions involving the sale, issuance or potential issuance of common shares at a price, or securities convertible or exercisable into common shares with a conversion or exercise price, that is greater than or equal to the lesser of (1) the last closing price immediately preceding the signing of a binding agreement, and (2) the average closing price of the common shares on Nasdaq for the five trading days immediately preceding the signing of the binding agreement; and
(c) where the issuance would result in a change of control of the listed company.
The Company intends to follow the British Columbia corporate and securities laws, which do not require shareholder approval for dilutive events unless the Company were to dispose of all or substantially all of its undertaking. In addition, the Company intends to follow the Canadian Securities Exchange policies which require shareholder approval on the occurrence of a “fundamental change”, defined by the policies of the Canadian Securities Exchange to be an acquisition pursuant to which at least 50% of the issuer’s assets or anticipated revenues will be a result of the acquisition, in combination with a change of control. The determination of a change of control in such context would include the distribution of 100% of the number of equity securities of the issuer outstanding prior to the transaction, or otherwise may be determined through a substantial change of the management or the board of directors of the issuer.
Nasdaq Marketplace Rule 5635(c) also requires shareholder approval of all stock option or purchase plans or other arrangements that provide for equity securities as compensation to officers, directors, employees or consultants, and any material amendments to such plans or arrangements, except for certain plans and arrangements, including:
(a) stock purchase plans available on equal terms to all security holders of the listed company (such as a typical dividend reinvestment plan);
(b) tax qualified, non-discriminatory employee benefit plans (e.g., plans that meet the requirements of section 401(a) or 423 of the Internal Revenue Code) or parallel nonqualified plans, provided that such plans are approved by the listed company's independent compensation committee or a majority of the company's independent directors;
(c) those plans or arrangements allowing employees, directors or service providers to buy such securities on the open market or from the listed company for current fair market value;
(d) grants of options or other equity-based compensation as a material inducement to the grantee's entering into employment with the listed company, provided that such grants are approved by the listed company's independent compensation committee or a majority of the company's independent directors; and
(e) conversions, replacements or adjustments of outstanding options or other equity compensation awards to reflect a merger or acquisition.
The Company intends to follow British Columbia corporate and securities laws, which do not require shareholder approval of equity compensation plans or most discount to market offerings of securities unless otherwise indicated in the Articles of the Company. In addition, the Company intends to follow the Canadian Securities Exchange policies and certain provisions of Canadian securities laws which require limitations on the number of equity compensation securities that can be distributed to persons performing investor relations services to 1% of the issued and outstanding amount of listed securities in a 12-month period, and further limit the number of equity compensation securities that can be distributed to a director, officer or a related entity of the issuer, or an associate thereof (each a “related person”), on a fully diluted basis to not exceed 5% of the outstanding securities of the issuer, or collectively to related persons exceeds 10% of the outstanding securities of the issuer.
Quorum Requirement
NASDAQ Marketplace Rule 5620(c) requires that each company that is not a limited partnership shall provide for a quorum as specified in its by-laws for any meeting of holders of common stock; provided, however, that in no case shall such quorum be less than 33 1/3% of the outstanding shares of the company’s common voting stock. The Company will not follow this NASDAQ Marketplace Rule. Instead, the Company intends to comply with British Columbia corporate and securities laws and its Articles which do not require a quorum of no less than 33 1/3% of the outstanding shares of the Company’s common voting stock and provides that the quorum for the transaction of business at a meeting of shareholders is the quorum established by the Company’s Articles, which is at least one person who is, or who represents by proxy, one or more shareholders who, in the aggregate, hold at least 5% of the issued shares entitled to be voted at the meeting.
Executive Sessions
Under Nasdaq Marketplace Rule 5605(b)(2), a listed company must have regularly scheduled meetings at which only independent directors are present (“executive sessions”). The rule contemplates that executive sessions will occur at least twice a year, and perhaps more frequently, in conjunction with regularly scheduled board meetings. Under applicable Canadian rules, customs and practice, the Company's independent directors are not required to hold executive sessions. However, if the Company’s common shares are listed for trading on Nasdaq, the Company will be subject to certain disclosure requirements prescribed in Canadian Form 58-101F1 - Corporate Governance Disclosure. In particular, the Company must disclose whether the independent directors hold executive sessions and, if such executive sessions are held, how many of these meetings have been held since the beginning of the Company's most recently completed financial year. If the Company does not hold executive sessions, the Company must describe what the Board does to facilitate open and candid discussion among its independent directors.
Proxy Delivery Requirements
Under Nasdaq Marketplace Rule 5620(b), a listed company that is not a limited partnership must solicit proxies and provide proxy statements for all meetings of shareholders, and also provide copies of such proxy solicitation materials to Nasdaq. The Company is a "foreign private issuer" as defined in Rule 3b-4 under the Exchange Act, and the equity securities of the Company are accordingly exempt from the proxy rules set forth in Sections 14(a), 14(b), 14(c) and 14(f) of the Exchange Act. The Company solicits proxies in accordance with applicable rules and regulations in Canada.
Distribution of Annual and Interim Reports
Under Nasdaq Marketplace Rule 5250(d)(1), a listed company (including a limited partnership) shall make available to shareholders an annual report containing audited financial statements of the Company and its subsidiaries (which, for example may be on Form 10-K, 20-F, 40-F or N-CSR) within a reasonable period of time following the filing of the annual report with the SEC. A company may comply with this requirement either:
(a) by mailing the report to the shareholders;
(b) by satisfying the requirements for furnishing an annual report contained in Rule 14a-16 under the Act; or
(c) by posting the annual report to shareholders on or through the company's website (or, in the case of a company that is an investment company that does not maintain its own website, on a website that the company is allowed to use to satisfy the website posting requirement in Rule 16a-3(k) under the Exchange Act), along with a prominent undertaking in the English language to provide shareholders, upon request, a hard copy of the company's annual report free of charge. A company that chooses to satisfy this requirement pursuant to this paragraph (C) must, simultaneous with this posting, issue a press release stating that its annual report has been filed with the SEC (or other regulatory authority). This press release shall also state that the annual report is available on the company's website and include the website address and that shareholders may receive a hard copy free of charge upon request. A company must provide such hard copies within a reasonable period of time following the request.
In addition, under Nasdaq Marketplace Rule 5250(d)(4)(A), each company that is not a limited partnership and is not subject to Rule 13a-13 under the Exchange Act and that is required to file with the SEC, or other regulatory authority, interim reports relating primarily to operations and financial position, shall make available to shareholders reports which reflect the information contained in those interim reports. Such reports shall be made available to shareholders either before or as soon as practicable following filing with the appropriate regulatory authority. If the form of the interim report provided to shareholders differs from that filed with the regulatory authority, the company shall file one copy of the report to shareholders with Nasdaq in addition to the report to the regulatory authority that is filed with Nasdaq pursuant to Rule 5250(c)(1).
The Company intends to comply with Nasdaq Marketplace Rules 5250(d)(1) and 5250(d)(4)(A), however, the Company may not do so or on a consistent basis. Instead, the Company may determine to comply with British Columbia corporate and securities laws which do not require the distribution of annual or interim reports to shareholders but do require the Company to place before the annual general meeting the annual financial statements that the Company is required to with the applicable securities commissions in Canada under the Securities Act (British Columbia) in relation to the most recently completed financial year, file annual and interim financial statements on SEDAR at www.sedar.com, and send annually a request form to the registered holders and beneficial owners of its securities that can be used to request a paper copy of the Company’s annual financial statements and management discussion and analysis for the annual financial statements, and a copy of the Company’s interim financial reports and management discussion and analysis for the interim financial reports free of charge.
ITEM 16H. MINE SAFETY DISCLOSURE
Not applicable.
PART III
ITEM 17. FINANCIAL STATEMENTS
Not applicable.
ITEM 18. FINANCIAL STATEMENTS
The following financial statements and notes thereto are filed with and incorporated herein as part of this Registration Statement:
(a) audited annual consolidated financial statements of the Company for the year ended September 30, 2020 and the period from May 31, 2019 (date of incorporation) to September 30, 2019, including consolidated statements of financial position, consolidated statements of comprehensive loss, consolidated statements of changes in shareholders' equity, consolidated statements of cash flows, and notes to the consolidated financial statements;
(b) unaudited condensed interim consolidated financial statements of the Company for the three and six months ended March 31, 2021 and 2020, including condensed interim consolidated statements of financial position, condensed interim consolidated statements of comprehensive loss, condensed interim consolidated statements of changes in shareholders' equity, condensed interim consolidated statements of cash flows, and notes to the condensed interim consolidated financial statements.
Bright Minds Biosciences Inc.
Consolidated Financial Statements
For the year ended September 30, 2020
and the period from May 31, 2019 (date of incorporation) to September 30, 2019
(Expressed in Canadian Dollars)

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of Bright Minds Biosciences Inc.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated statements of financial position of Bright Minds Biosciences Inc. ("the Company") as of September 30, 2020 and 2019, and the related consolidated statements of comprehensive loss, changes in shareholders' equity and cash flows for the year ended September 30, 2020 and the period from May 31, 2019 (date of incorporation) to September 30, 2019, and the related notes (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of September 30, 2020 and 2019, and the results of its operations and its cash flows for the year ended September 30, 2020 and the period from May 31, 2019 (date of incorporation) to September 30, 2019, in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance whether the financial statements are free of material misstatement, whether due to fraud or error. The Company is not required to have, nor were we engaged to perform, an audit of internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ DeVisser Gray LLP
CHARTERED PROFESSIONAL ACCOUNTANTS
We have served as the Company's auditor since 2020.
Vancouver, Canada
June 9, 2021
Bright Minds Biosciences Inc.
Consolidated Statements of Financial Position
(Expressed in Canadian dollars)
| September 30, | September 30, | ||||||
| As at | Notes | 2020 | 2019 | ||||
| $ | $ | ||||||
| ASSETS | |||||||
| Current Assets | |||||||
| Cash | 799,929 | 79,991 | |||||
| Prepaids | 11 | 78,287 | - | ||||
| 878,216 | 79,991 | ||||||
| Non- Current Assets | |||||||
| Intangible assets | 4 | 2,000 | 2,000 | ||||
| TOTAL ASSETS | 880,216 | 81,991 | |||||
| LIABILITIES AND SHAREHOLDERS' EQUITY | |||||||
| Current Liabilities | |||||||
| Accounts payable and accrued liabilities | 5 | 150,923 | 36,708 | ||||
| TOTAL LIABILITIES | 150,923 | 36,708 | |||||
| Shareholders' equity | |||||||
| Share capital | 6 | 980,661 | 205,980 | ||||
| Subscriptions receivable | 6 | (1,000 | ) | (81,980 | ) | ||
| Subscriptions received | 6 | 147,426 | - | ||||
| Reserves | 6 | 161,300 | - | ||||
| Deficit | (559,094 | ) | (78,717 | ) | |||
| TOTAL SHAREHOLDERS' EQUITY | 729,293 | 45,283 | |||||
| TOTAL LIABILITIES AND SHAREHOLDERS' EQUITY | 880,216 | 81,991 |
Nature and continuance of operations (Note 1)
Subsequent events (Note 11)
|
Approved on behalf of the Board of Directors: |
|||
|
|
|
|
|
|
"Ian McDonald" |
|
"Alan Kozikowski" |
|
|
Director |
|
Director |
|
The accompanying notes are an integral part of these consolidated financial statements.
Bright Minds Biosciences Inc.
Consolidated Statements of Comprehensive Loss
(Expressed in Canadian dollars)
| For the year ended |
For the period ended |
||||||
| Notes | September 30, 2020 |
September 30, 2019 |
|||||
| $ | $ | ||||||
| EXPENSES | |||||||
| Funds processing fees - private placement | 5,568 | - | |||||
| Marketing, advertising and investor relations | 9,618 | - | |||||
| Office | 637 | - | |||||
| Professional fees | 7 | 104,251 | 36,708 | ||||
| Regulatory and filing | 5,451 | - | |||||
| Research and development | 6, 7 | 354,852 | 42,009 | ||||
| Net loss and comprehensive loss | (480,377 | ) | (78,717 | ) | |||
| Basic and diluted loss per share (*) | (0.13 | ) | (0.04 | ) | |||
| Weighted average number of common shares outstanding -basic and diluted (*) | 3,612,436 | 2,073,240 |
(*) In November 2020, the Company's common shares were consolidated on a 2.5:1 basis (see Note 11). Except for the loss per share amounts, all common shares in these financial statements are presented on a pre-consolidation basis.
The accompanying notes are an integral part of these consolidated financial statements.
Bright Minds Biosciences Inc.
Consolidated Statements of Changes in Shareholders’ Equity
(Expressed in Canadian Dollars)
| Share Capital | |||||||||||||||||||||
| Number of shares |
Share capital |
Subscriptions receivable |
Subscriptions received |
Reserves | Deficit | Total | |||||||||||||||
| $ | $ | $ | $ | $ | $ | ||||||||||||||||
| Balance at May 31, 2019 (date of incorporation) | - | - | - | - | - | - | - | ||||||||||||||
| Incorporator share | 1 | - | - | - | - | - | - | ||||||||||||||
| Private placement | 10,199,000 | 203,980 | (81,980 | ) | - | - | - | 122,000 | |||||||||||||
| Acquisition of intangible assets | 100,000 | 2,000 | - | - | - | - | 2,000 | ||||||||||||||
| Net loss | - | - | - | - | - | (78,717 | ) | (78,717 | ) | ||||||||||||
| Balance as at September 30, 2019 | 10,299,001 | 205,980 | (81,980 | ) | - | - | (78,717 | ) | 45,283 | ||||||||||||
| Private placement | 1,559,847 | 779,924 | 80,980 | 147,426 | - | - | 1,008,330 | ||||||||||||||
| Share issue costs | - | (5,243 | ) | - | - | - | - | (5,243 | ) | ||||||||||||
| Share-based compensation | - | - | - | - | 161,300 | - | 161,300 | ||||||||||||||
| Net loss | - | - | - | - | - | (480,377 | ) | (480,377 | ) | ||||||||||||
| Balance as at September 30, 2020 | 11,858,848 | 980,661 | (1,000 | ) | 147,426 | 161,300 | (559,094 | ) | 729,293 | ||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
Bright Minds Biosciences Inc.
Consolidated Statements of Cash Flows
(Expressed in Canadian Dollars)
| For the year ended |
For the period ended |
|||||
| September 30, 2020 |
September 30, 2019 |
|||||
| $ | $ | |||||
| Operating activities | ||||||
| Net loss for the period | (480,377 | ) | (78,717 | ) | ||
| Non-cash items: | ||||||
| Share-based compensation | 161,300 | - | ||||
| Changes in non-cash operating working capital items: | ||||||
| Prepaids | (78,287 | ) | - | |||
| Accounts payable and accrued liabilities | 108,972 | 36,708 | ||||
| Net cash used in operating activities | (288,392 | ) | (42,009 | ) | ||
| Financing activities | ||||||
| Private placement proceeds | 1,008,330 | 122,000 | ||||
| Net cash from financing activities | 1,008,330 | 122,000 | ||||
| Change in cash | 719,938 | 79,991 | ||||
| Cash, beginning of period | 79,991 | - | ||||
| Cash, end of period | 799,929 | 79,991 | ||||
| Supplementary Information | ||||||
| Acquisition of intangible assets by issuing common shares | - | 2,000 | ||||
| Share issue costs included in accounts payable | 5,243 | - |
The accompanying notes are an integral part of these consolidated financial statements.
|
Bright Minds Biosciences Inc. |
1. NATURE AND CONTINUANCE OF OPERATIONS
Bright Minds Biosciences Inc. (the "Company") was incorporated under the Business Corporations Act of British Columbia on May 31, 2019. The Company's objective is to generate income and achieve long term profitable growth through the development of therapeutics to improve the lives of patients with certain severe and life-altering diseases. The head office, and principal address of the Company are located at 1500 - 1055 West Georgia Street, Vancouver, British Columbia, V6E 4N7, Canada.
On February 8, 2021, the Company started trading on the Canadian Stock Exchange (the "CSE") under the symbol DRUG.
These financial statements have been prepared on a going concern basis which assumes that the Company will be able to realize its assets and discharge its liabilities in the normal course of business for the foreseeable future. As at September 30, 2020, the Company is not able to finance day to day activities through operations and has incurred a loss of $480,377 for the year ended September 30, 2020. The Company has a deficit of $559,094 since inception and negative operating cash flows. The continuing operations of the Company are dependent upon its ability to attain profitable operations and generate funds therefrom. Management intends to finance operating costs with equity financings, loans from directors and companies controlled by directors and/or private placement of common shares. On March 17, 2021, the Company issued 3,419,883 units for aggregate gross proceeds of $25,888,514. See Note 11.
If the Company is unable to continue as a going concern, the net realizable value of its assets may be materially less than the amounts on its statement of financial position.
The coronavirus, also known as "COVID-19", has spread across the globe and is impacting worldwide economic activity. Government authorities have implemented emergency measures to mitigate the spread of the virus. The outbreak and the related mitigation measures have had an adverse impact on global economic conditions as well as on the Company's business activities, specifically related to possible disruptions in the operations of the laboratories upon whom the Company relies, including laboratories situated in various parts of the United States and Europe. The extent to which the coronavirus may impact the Company's business activities will depend on future developments, such as the duration of the outbreak, travel restrictions, business disruptions, and the effectiveness of actions taken in Canada and other countries to contain and treat the disease. These events are highly uncertain and as such, the Company cannot determine their financial impact at this time.
2. STATEMENT OF COMPLIANCE AND BASIS OF PREPARATION
Statement of compliance
These financial statements have been prepared in accordance with the International Financial Reporting Standards ("IFRS") issued by the International Accounting Standards Board ("IASB") and Interpretations of the International Financial Reporting Interpretations Committee ("IFRIC"). The principal accounting policies applied in the preparation of these financial statements are set out below.
These financial statements were approved and authorized for issue by the Board of Directors on June 9, 2021.
Basis of preparation
Depending on the applicable IFRS requirements, the measurement basis used in the preparation of these financial statements is cost, net realizable value, fair value or recoverable amount. These financial statements, except for the statement of cash flows, are based on the accrual basis.
|
Bright Minds Biosciences Inc. |
3. SIGNIFICANT ACCOUNTING POLICIES
Basis of consolidation
These financial statements include the accounts of the Company and its inactive, wholly-owned subsidiary Psilocybinlabs Ltd (see Note 4). A subsidiary is an entity that the Company controls, either directly or indirectly, where control is defined as the power to govern the financial and operating policies of an entity so as to obtain benefits from its activities. The financial results of the Company's subsidiary are included in the financial statements from the date that control commences until the date that control ceases. The accounting policies of the Company's subsidiary have been aligned with the policies adopted by the Company. When the Company ceases to control a subsidiary, the financial statements of that subsidiary are de-consolidated.
Inter-company balances and transactions, and any income and expenses arising from inter-company transactions, have been eliminated in these financial statements.
Critical accounting estimates
The preparation of the financial statements in conformity with IFRS requires management to make estimates, judgments and assumptions that affect the application of accounting policies and the reported amounts of assets, liabilities, income and expenses. Actual results may differ from these estimates. Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any future periods affected.
Certain of the Company's accounting policies and disclosures require key assumptions concerning the future and other estimates that have a significant risk of causing a material adjustment to the carrying amounts of assets and liabilities or disclosures within the next fiscal year. Where applicable, further information about the assumptions made is disclosed in the notes specific to that asset or liability. The critical accounting estimates and judgments set out below have been applied consistently to all periods presented in these financial statements.
Business acquisitions
IFRS 3, Business Combinations requires that acquisition transactions be assessed to determine whether the acquisition is a business combination (by satisfying the three elements - input, process and output - of a business) or an asset acquisition. This assessment process requires management to exercise judgement. If management determines that the acquisition is a business combination, judgement is required in measuring the fair value of the assets acquired, equity instruments issued and liabilities and contingent liabilities incurred or assumed. See Note 4.
Share-based compensation
The fair value of stock options is measured using a Black Scholes option pricing model. Measurement inputs include the common share price on the grant date, the exercise price of the instrument, the expected common share price volatility, the weighted average expected life of the instruments, the expected dividends and the risk-free interest rate. Service and non-market performance conditions are not taken into account in determining fair value. The fair value of equity settled RSUs is measured based on management's best estimate of the Company's share price on the grant date.
The share-based compensation recognized is also determined based on management's grant date estimate of the forfeitures that are expected to occur over the life of the stock options and equity settled RSUs. Cash settled RSUs outstanding are fair valued using a mark-to-market calculation based on the Company's closing common share price at the end of the period. The number of stock options and RSUs that actually vest could differ from the estimated number of awards expected to vest and any differences between the actual and estimated forfeitures are recognized prospectively as they occur.
|
Bright Minds Biosciences Inc. |
3. SIGNIFICANT ACCOUNTING POLICIES (continued)
Foreign currency translation
The functional and reporting currency of the Company and its subsidiary is the Canadian dollar. Transactions in currencies other than the functional currency are recorded at the rates of exchange prevailing on the transaction date. Monetary assets and liabilities that are denominated in foreign currencies are translated at the rates prevailing at each reporting date. Non-monetary assets and liabilities denominated in foreign currencies that are measured at fair value are retranslated to the functional currency at the exchange rate at the date the fair value was determined. Non-monetary items that are measured in terms of historical cost in a foreign currency are not retranslated. Foreign currency translation differences are recognized in profit or loss.
Business combinations
The Company uses the acquisition method to account for business combinations. The Company measures goodwill as the fair value of the consideration transferred, less the net recognized amount (generally fair value) of the identifiable assets acquired and liabilities assumed, all measured as of the acquisition date. When the excess is negative, a gain on acquisition is recognized immediately in net income or loss.
Goodwill is not amortized and is tested for impairment annually. Additionally, goodwill is reviewed at each reporting date to determine if events or changes in circumstances indicate that the asset might be impaired, in which case an impairment test is performed. Goodwill is measured at cost less accumulated impairment losses.
Transaction costs, other than those associated with the issue of debt or equity securities, that the Company incurs in connection with a business combination are expensed as incurred.
Internally generated intangible assets - Research and development expenditure
Intangible assets acquired separately are initially recognized at cost. Expenditure on research activities is recognized as an expense in the period in which it is incurred. An internally-generated intangible asset arising from development (or from the development phase of an internal project) is recognized if, and only if, all of the following have been demonstrated:
- The technical feasibility of completing the intangible asset so that it will be available for use or sale;
- The intention to complete the intangible asset and use or sell it;
- The ability to use or sell the intangible asset;
- How the intangible asset will generate probable future economic benefits;
- The availability of adequate technical, financial and other resources to complete the development and to use or sell the intangible asset; and
- The ability to measure reliably the expenditure attributable to the intangible asset during its development.
The amount initially recognized for internally-generated intangible assets is the sum of the expenditure incurred from the date when the intangible asset first meets the recognition criteria listed above. Where no internally-generated intangible asset can be recognized, development expenditure is recognized in profit or loss in the period in which it is incurred.
Subsequent to initial recognition, internally-generated intangible assets are reported at cost less accumulated amortization and accumulated impairment losses, on the same basis as intangible assets that are acquired separately.
At September 30, 2020 and 2019, the Company has not recognized any internally-generated intangible assets.
|
Bright Minds Biosciences Inc. |
3. SIGNIFICANT ACCOUNTING POLICIES (continued)
Share-based compensation awards
Share-based compensation expense relates to stock options as well as cash and equity settled restricted share units ("RSUs"). The grant date fair values of stock options and equity settled RSUs granted are recognized as an expense, with a corresponding increase in reserves in equity, over the vesting period. The amount recognized as an expense is based on the estimate of the number of awards expected to vest, which is revised if subsequent information indicates that actual forfeitures are likely to differ from the estimate. Upon exercise of stock options, the consideration paid by the holder is included in share capital and the related reserves associated with the stock options exercised is reclassified into share capital. Upon vesting of equity settled RSUs, the related reserves associated with the RSU is reclassified into share capital.
For cash settled RSUs, the fair value of the RSUs is recognized as share-based compensation expense, with a corresponding increase in accrued liabilities over the vesting period. The amount recognized as an expense is based on the estimate of the number of RSUs expected to vest. Cash settled RSUs are measured at their fair value at each reporting period on a mark-to-market basis. Upon vesting of the cash settled RSUs, the liability is reduced by the cash payout.
Provisions
A provision is recognized if, as a result of a past event, the Company has a present legal or constructive obligation that can be estimated reliably, and it is probable that an outflow of economic benefits will be required to settle the obligation. Provisions are determined by discounting the expected future cash flows at a pre-tax rate that reflects current market assessments of the time value of money and the risks specific to the liability. The unwinding of the discount is recognized as a finance cost within net income or loss.
Income taxes
Current income tax:
Current income tax assets and liabilities for the current period are measured at the amount expected to be recovered from or paid to the taxation authorities. The tax rates and tax laws used to compute the amount are those that are enacted or substantively enacted, at the reporting date, in the countries where the Company operates and generates taxable income.
Deferred tax:
Deferred tax is provided on temporary differences at the reporting date between the tax bases of assets and liabilities and their carrying amounts for financial reporting purposes. The carrying amount of deferred tax assets is reviewed at the end of each reporting period and recognized only to the extent that it is probable that sufficient taxable profit will be available to allow all or part of the deferred tax asset to be utilized.
Deferred tax assets and liabilities are measured at the tax rates that are expected to apply to the year when the asset is realized or the liability is settled, based on tax rates (and tax laws) that have been enacted or substantively enacted by the end of the reporting period. Deferred tax assets and deferred tax liabilities are offset if a legally enforceable right exists to set off current tax assets against current income tax liabilities and the deferred taxes relate to the same taxable entity and the same taxation authority.
|
Bright Minds Biosciences Inc. |
3. SIGNIFICANT ACCOUNTING POLICIES (continued)
Loss per share
Basic loss per share is calculated by dividing the loss attributable to common shareholders by the weighted average number of common shares outstanding in the period. The loss attributable to common shareholders equals the reported loss attributable to owners of the Company. Diluted loss per share is calculated using the treasury stock method. Under the treasury stock method, the weighted average number of common shares outstanding for the calculation of diluted loss per share assumes that the proceeds to be received on the exercise of dilutive share options and warrants are used to repurchase common shares at the average market price during the period. Because the Company incurred net losses, the effect of dilutive instruments would be anti-dilutive and therefore diluted loss per share equals loss per share.
Share capital
Financial instruments issued by the Company are classified as equity only to the extent that they do not meet the definition of a financial liability or financial asset. The Company's common shares are classified as equity instruments.
Incremental costs directly attributable to the issue of new common shares are recognized as a deduction from equity, net of tax.
Financial instruments
Financial instruments are accounted for in accordance with IFRS 9, "Financial Instruments: Classification and Measurement". A financial instrument is any contract that gives rise to a financial asset of one entity and a financial liability or equity instrument of another entity.
Financial assets
(a) Recognition and measurement of financial assets
The Company recognizes a financial asset when it becomes a party to the contractual provisions of the instrument.
(b) Classification of financial assets
The Company classifies financial assets at initial recognition as financial assets: measured at amortized cost, measured at fair value through other comprehensive income ("FVTOCI") or measured at fair value through profit or loss ("FVTPL").
(i) Financial assets measured at amortized cost
A financial asset that meets both of the following conditions is classified as a financial asset measured at amortized cost:
- The Company's business model for such financial assets is to hold the assets in order to collect contractual cash flows; and
- The contractual terms of the financial asset give rise on specified dates to cash flows that are solely payments of principal and interest on the amount outstanding.
A financial asset measured at amortized cost is initially recognized at fair value plus transaction costs directly attributable to the asset. After initial recognition, the carrying amount of the financial asset measured at amortized cost is determined using the effective interest method, net of impairment loss, if necessary.
|
Bright Minds Biosciences Inc. |
3. SIGNIFICANT ACCOUNTING POLICIES (continued)
Financial instruments (continued)
(ii) Financial assets measured at FVTOCI
A financial asset measured at FVTOCI is recognized initially at fair value plus transaction costs directly attributable to the asset. After initial recognition, the asset is measured at fair value with changes in fair value included as "financial asset at fair value through other comprehensive income" in other comprehensive income or loss.
(iii) Financial assets measured at FVTPL
A financial asset measured at FVTPL is initially recognized at fair value with any associated transaction costs being recognized in profit or loss when incurred. Subsequently, the financial asset is re-measured at fair value and a gain or loss is recognized in profit or loss in the reporting period in which it arises.
The Company's cash is classified as subsequently measured at FVTPL.
(c) Derecognition of financial assets
The Company derecognizes a financial asset if the contractual rights to the cash flows from the asset expire, or the Company transfers substantially all the risks and rewards of ownership of the financial asset. Any interests in transferred financial assets that are created or retained by the Company are recognized as a separate asset or liability. Gains and losses on derecognition are generally recognized in the statement of comprehensive loss. However, gains and losses on derecognition of financial assets classified as FVTOCI remain within accumulated other comprehensive income or loss.
Financial liabilities
(a) Recognition and measurement of financial liabilities
The Company recognizes a financial liability when it becomes a party to the contractual provisions of the instrument.
(b) Classification of financial liabilities
(i) Financial liabilities measured at amortized cost
A financial liability measured at amortized cost is initially measured at fair value less transaction costs directly attributable to the issuance of the financial liability. Subsequently, the financial liability is measured at amortized cost using the effective interest method.
The Company's accounts payable and accrued liabilities are classified as subsequently measured at amortized cost.
(ii) Financial liabilities measured at FVTPL
A financial liability measured at FVTPL is initially measured at fair value with any associated transaction costs being recognized in profit or loss when incurred. Subsequently, the financial liability is re-measured at fair value and a gain or loss is recognized in profit or loss in the reporting period in which it arises.
(c) Derecognition of financial liabilities
The Company derecognizes a financial liability when the financial liability is discharged, cancelled or expired. Generally, the difference between the carrying amount of the financial liability derecognized and the consideration paid and payable, including any non-cash assets transferred or liabilities assumed, is recognized in the statement of comprehensive loss.
|
Bright Minds Biosciences Inc. |
3. SIGNIFICANT ACCOUNTING POLICIES (continued)
Financial instruments (continued)
Offsetting financial assets and liabilities
Financial assets and liabilities are offset and the net amount is presented in the statement of financial position only when the Company has a legally enforceable right to offset the recognized amounts and intends either to settle on a net basis, or to realize the asset and settle the liability simultaneously.
Impairment of financial assets
The Company recognizes a loss allowance for expected credit losses on financial assets that are measured at amortized cost.
At each reporting date, the Company measures the loss allowance for the financial asset at an amount equal to the lifetime expected credit losses if the credit risk on the financial asset has increased significantly since initial recognition. If at the reporting date, the financial asset has not increased significantly since initial recognition, the Company measures the loss allowance for the financial asset at an amount equal to the twelve-month expected credit
losses. The Company recognizes in the statement of comprehensive loss, as an impairment loss (or gain), the amount of expected credit losses (or reversal) that is required to adjust the loss allowance at the reporting date to the amount that is required to be recognized.
Leases
Leases are accounted for in accordance with IFRS 16, "Leases". At inception of a contract, the Company assesses whether a contract is, or contains, a lease. A contract is, or contains, a lease if the contract conveys the right to control the use of an identified asset over a period of time in exchange for consideration. The Company assesses whether the contract involves the use of an identified asset, whether it has the right to obtain substantially all of the economic benefits from the use of the asset during the term of the contract and if it has the right to direct the use of the asset.
As a lessee, the Company recognizes a right-of-use asset and a lease liability at the commencement date of the lease.
Right-of-use asset
The right-of-use asset is initially measured at cost, which is comprised of the initial amount of the lease liability adjusted for any lease payments made and any initial direct costs incurred at or before the commencement date, less any lease incentives received.
The right-of-use asset is subsequently depreciated from the commencement date to the earlier of the end of the lease term, or the end of the useful life of the asset. In addition, the right-of-use asset may be reduced due to impairment losses, if any, and adjusted for certain re-measurements of the lease liability.
Lease liability
A lease liability is initially measured at the present value of the lease payments that are not paid at the commencement date discounted by the interest rate implicit in the lease or, if that rate cannot be readily determined, the incremental borrowing rate. The lease liability is subsequently measured at amortized cost using the effective interest method.
|
Bright Minds Biosciences Inc. |
3. SIGNIFICANT ACCOUNTING POLICIES (continued)
New standards and interpretations not yet adopted
A number of new standards, amendments to standards and interpretations are not yet effective for the year ended September 30, 2020 and have not been applied in preparing these financial statements. The following new standards have not been adopted which may impact the Company in the future:
IFRS 3 - Business combinations
Narrow-scope amendments to IFRS 3 were issued in October 2018 and apply to annual reporting periods beginning on or after January 1, 2020. The amendments clarify the definition of a business, provide guidance in determining whether an acquisition is a business combination or a combination of a group of assets, emphasize that the output of a business is to provide goods and services to customers and provide a supplementary guidance.
IAS 1 - Presentation of financial statements
An amendment to IAS 1 was issued in January 2020 and applies to annual reporting periods beginning on or after January 1, 2023. The amendment clarifies the criterion for classifying a liability as non-current relating to the right to defer settlement of a liability for at least 12 months after the reporting period.
4. SHARE EXCHANGE
Psilocybinlabs Ltd. ("PL") was incorporated under the laws of the province of British Columbia on April 25, 2019, with the incorporator share being held by a company controlled by the CEO of the Company. On May 17, 2019, this share was transferred to the Company. On April 25, 2019, PL entered into a confirmatory assignment and waiver (the "CAW") with an individual, which was amended and restated on May 17, 2019. Pursuant to the amended and restated CAW, this individual assigned all of the right, title and interest, including all other intellectual property rights (the Rights, as described) to PL. As compensation for the assignment of the Rights, PL issued 100,000 common shares valued at $2,000 to this individual. On August 7, 2019, the Company then purchased the 100,000 common shares of PL by issuing 100,000 common shares of the Company valued at $2,000.
The Company has recorded the reacquisition of PL as an asset acquisition as follows:
| Purchase Price Consideration: | |||
| $ | |||
| Common shares issued | 2,000 | ||
| Assets acquired: | |||
| Intangible assets | 2,000 |
5. ACCOUNTS PAYABLE AND ACCRUED LIABILITIES
| September 30, 2020 |
September 30, 2019 |
|||||
| $ | $ | |||||
| Accounts payable | 138,423 | 36,708 | ||||
| Accrued liabilities | 12,500 | - | ||||
| Total accounts payable and accrued liabilities | 150,923 | 36,708 |
|
Bright Minds Biosciences Inc. |
6. SHARE CAPITAL
Authorized share capital
Unlimited number of common shares without par value.
Issued share capital for the year ended September 30, 2020
On September 30, 2020, the Company closed the first tranche of a non-brokered private placement financing through the issuance of 1,559,847 common shares at a price $0.50 per common share for gross proceeds of $779,924.
Issued share capital from May 31, 2019 (date of incorporation) to September 30, 2019
On July 30, 2019, the Company closed a non-brokered private placement financing through the issuance of 10,199,000 units at a price of $0.02 per unit for gross proceeds of $203,980. Of this amount, $80,980 was received subsequent to year end. Each unit comprises one common share, and one share purchase warrant exercisable at $0.05 per share until July 31, 2024.
See Note 4 for common shares issued on August 7, 2019.
Share subscriptions received/receivable
During the fiscal year ended September 30, 2020, the Company received $147,426 in subscriptions for 294,852 common shares relating to a private placement that closed on November 2, 2020. See Note 11.
On July 31, 2019, 4,049,000 common shares were issued for which the gross proceeds of $80,980 were received on October 7, 2019 and September 28, 2020.
Stock options
The Company's stock option plan provides for stock options to be issued to directors, officers, employees and consultants of the Company, its subsidiaries and any personal holding company of such individuals so that they may participate in the growth and development of the Company. Subject to the specific provisions of the stock option plan, eligibility, vesting period, terms of the options and the number of options granted are to be determined by the Board of Directors at the time of grant. The stock option plan allows the Board of Directors to issue up to 10% of the Company's outstanding common shares as stock options.
On July 23, 2020, the Company granted 375,000 options to the Company's Chief Medical Officer.
The following table summarizes the movements in the Company's outstanding stock options:
| Number of options | Weighted average exercise price |
|||||
| Balance at May 31, 2019 | - | - | ||||
| Granted | - | - | ||||
| Balance at September 30, 2019 | - | - | ||||
| Granted | 375,000 | $ | 0.50 | |||
| Balance at September 30, 2020 | 375,000 | $ | 0.50 |
The continuity of share purchase options is as follows:
| Expiry Date | Exercise Price | September 30, 2019 |
Granted | Exercised | Expired/ Cancelled |
September 30, 2020 |
||||||||||||
| July 23, 2025 | $ | 0.50 | - | 375,000 | - | - | 375,000 | |||||||||||
| Exercisable | - | 375,000 | - | - | 375,000 | |||||||||||||
| Weighted average exercise price | $ | - | $ | 0.50 | $ | - | $ | - | $ | 0.50 | ||||||||
|
Bright Minds Biosciences Inc. |
6. SHARE CAPITAL (continued)
Stock options (continued)
The fair vale of these stock options was measured using the Black Scholes option pricing model using the following inputs: i) exercise price: $0.50; ii) share price: $0.50; iii) term: 5 years; iv) volatility: 100%; v) discount rate: 0.35%; and dividends: nil.
The weighted-average remaining life of the outstanding options was 4.81 years.
Restricted share unit plan
The Company's restricted share unit ("RSU") plan provides RSUs to be issued to directors, officers, employees and consultants of the Company, its subsidiaries and any personal holding company of such individuals so that they may participate in the growth and development of the Company. Subject to the specific provisions of the RSU plan, eligibility, vesting period, terms of the RSUs and the number of RSUs granted are to be determined by the Board of Directors at the time of the grant. The RSU plan allows the Board of Directors to issue common shares of the company as equity settled RSUs, provided that, when combined, the maximum number of common shares reserved for issuance under all share-based compensation arrangements of the Company does not exceed 10% of the Company's outstanding common shares.
On July 23, 2020 and September 18, 2020, the Company issued 375,000 RSUs and 575,000 RSUs, respectively, to the Chief Medical Officer of the Company. These RSUs vest on an annual basis over a period of four years commencing on the first anniversary of the grant date.
The following table summarizes the movements in the Company's outstanding RSUs:
| Equity settled | Cash settled | Total | |||||||
| Balance at May 31, 2019 | - | - | - | ||||||
| Granted | - | - | - | ||||||
| Balance at September 30, 2019 | - | - | - | ||||||
| Granted | 950,000 | - | 950,000 | ||||||
| Vested | - | - | - | ||||||
| Balance at September 30, 2020 | 950,000 | - | 950,000 |
The estimated fair value of the equity settled RSUs granted during the year ended September 30, 2020 was $475,000 (period ended September 30, 2019 - $nil) and will be recognized as an expense over the vesting period of the RSUs.
The accounting fair value of the equity settled RSUs as at the grant date was estimated by management using the following inputs:
| Year ended September 30, 2020 |
Period ended September 30, 2019 |
|||||
| Share price on grant date | $ | 0.50 | $ | - | ||
| Forfeiture rate | 0% | - |
|
Bright Minds Biosciences Inc. |
6. SHARE CAPITAL (continued)
Restricted share unit plan (continued)
Share-based compensation expense recognized in the consolidated statements of comprehensive loss is comprised of the following:
| Year ended September 30, 2020 |
Period ended September 30, 2019 |
|||||
| Stock options | $ | 138,000 | $ | - | ||
| Restricted share units - equity settled grants | 23,300 | - | ||||
| Total equity settled share-based compensation expense | 161,300 | - | ||||
| Restricted share units - cash settled grants | - | - | ||||
| Total share-based compensation expense (1) | $ | 161,300 | $ | - |
(1) Share-based compensation expense is included in ‘Research and development’ in the consolidated statements of comprehensive loss.
Warrants
The following table summarizes the movements in the Company's outstanding warrants:
| Number of warrants | Weighted average exercise price |
|||||
| Balance at May 31, 2019 | - | - | ||||
| Issued | - | - | ||||
| Balance at September 30, 2019 | - | - | ||||
| Issued | 10,199,000 | $ | 0.05 | |||
| Balance at September 30, 2020 | 10,199,000 | $ | 0.05 |
The continuity of share purchase warrants is as follows:
| Expiry Date | Exercise Price | September 30, 2019 | Issued | Exercised | Expired/ Cancelled |
September 30, 2020 | ||||||||||||
| July 30, 2024 | $ | 0.05 | - | 10,199,000 | - | - | 10,199,000 | |||||||||||
| - | 10,199,000 | - | - | 10,199,000 | ||||||||||||||
| Weighted average exercise price | $ | - | $ | 0.05 | $ | - | $ | - | $ | 0.05 | ||||||||
The weighted average remaining life of outstanding warrants was 3.83 years.
On November 2, 2020, the Directors of the Company reduced the exercise price of the outstanding warrants from $0.05 to $0.02 effective July 11, 2020.
7. RELATED PARTY TRANSACTIONS
Related party transactions were recorded at the exchange value, which is the consideration determined and agreed to by the related parties. The Company's related parties include directors, key management and companies controlled by directors and key management.
|
Bright Minds Biosciences Inc. |
7. RELATED PARTY TRANSACTIONS (continued)
Compensation of Key Management Personnel
Key management personnel are those persons that have authority and responsibility for planning, directing and controlling the activities of the Company, directly and indirectly, and by definition include the directors of the Company.
The following table summarizes expenses related to key management personnel:
| Year ended | Period ended | |||||
| September 30, 2020 |
September 30, 2019 |
|||||
| $ | $ | |||||
| Professional fees | 22,575 | - | ||||
| Research and development | 26,777 | - | ||||
| Share-based compensation (1) | 161,300 | - | ||||
| 210,652 | - |
(1) The total fair value of stock options and RSUs granted to key management personnel for the year ended September 30, 2020 was equal to $613,000 (period ended September 30, 2019 - $nil), which is being recognized in profit or loss over the stock option's and RSU's vesting period.
See Notes 4 and 6. See Note 8 for related party contractual obligations.
8. CONTRACTUAL OBLIGATIONS
Option agreement
On May 26, 2020, the Company entered into an option agreement (the "OA") with the University of Illinois at Chicago (the "UIC") whereby the Company obtained the right to evaluate certain of the UIC's technology (as defined) for the purpose of making a decision as to whether to exclusively license the rights to the technology. Pursuant to the OA, the Company paid a US$5,000 ($6,999) non-refundable option fee and was granted an exclusive option to evaluate the technology and obtain an exclusive license to the patents and patent applications (as listed) and a non-exclusive right to use the technology for non-commercial research purposes. The option expires on the earlier of 90 days or the execution of a license agreement between the Company and the UIC. The Company can extend the first option period three additional times for an additional 90 days each with payment of a US$5,000 option extension fee for each 90-day option period. During the option period and any renewals thereof, the Company must pay all out of pocket expenses incurred by the UIC in connection with the preparation, filing, prosecution and maintenance of the patent applications and patents under the patent rights.
On July 6, 2020, the Company paid a US$5,000 ($6,896) option extension fee. As at September 30, 2020, no amounts were payable to the UIC in respect of out of pocket expenses incurred. On April 23, 2021, the Company exercised the option. See Note 11.
Related Party Contracts
On June 5, 2020, the Company entered into an independent consultant agreement (the "ICA") whereby the consultant, a private corporation incorporated in the State of California, USA, was engaged and the consultant's representative will serve as the Company's Chief Medical Officer, with the services being provided in California. As compensation for performing these services, the consultant or the consultant's representative will participate in the Company's equity incentive plans and will be eligible for cash payments in respect of fees at such time as the Company begins to compensate other C-level personnel in cash and in similar proportion to total compensation (the "fees"). The non-cash portion of the consultant's fees for the first year of the term was in the form of a grant of 375,000 vested stock options and 375,000 RSUs (see Note 6). The services will continue for an initial term of one
|
Bright Minds Biosciences Inc. |
8. CONTRACTUAL OBLIGATIONS (continued)
Related Party Contracts (continued)
year unless sooner terminated. The ICA can be terminated by either party giving the other 30 days written notice or by mutual written agreement. At the end of the initial term, the ICA will automatically be extended for additional one-year period(s) unless either party gives the other 30 days written notice. Commencing in March 2021, the consultant began invoicing the Company a monthly fee of US$15,000.
Scientific advisory board agreements
On June 1, 2020 and July 14, 2020, the Company entered into scientific advisory board agreements (the "SABAs") whereby the advisors were retained to serve as members of the Company's scientific advisory board and as consultants to the Company and senior management in the areas of scientific, technical and business advice. As compensation for performing these services, the Company will pay the advisors hourly rates of $150 and $160 per hour. Both advisors have the same hour requirements and restrictions as noted below. The services will continue for initial terms of one year unless sooner terminated. At the end of the initial terms, the SABAs will automatically be extended for an additional one-year period(s) unless either party gives the other 30 days written notice. On November 17, 2020, the Company granted an aggregate of 50,000 share purchase options to the consultants, of which 20,000 were subsequently cancelled on January 21, 2021. The Company also granted an aggregate of 80,000 share purchase options to the consultants on April 28, 2021. See Note 11.
Consulting agreements
The Company has entered into numerous consulting agreements (the "CAs") whereby the consultants were retained to serve as advisors to the Company and senior management in the areas of public relations and content creation and scientific, technical and business advice. As compensation for performing these services, the Company will pay the advisors hourly rates ranging from $150 to $600. The advisors being paid $400 and $600 per hour will reserve at least six full days of services to the Company and such additional days as requested by the Company each annual period, but not to exceed 36 full days of service per year unless otherwise agreed and up to a maximum of 288 hours total per year, unless otherwise agreed. The services will continue for initial terms of one year unless sooner terminated. At the end of the initial terms, the CAs will automatically be extended for an additional one-year period(s) unless either party gives the other 30 days written notice. On November 17, 2020 and April 28, 2021, the Company granted an aggregate of 142,000 and 160,000, respectively, share purchase options to certain of the consultants. See Note 11.
9. FINANCIAL INSTRUMENTS AND CAPITAL MANAGEMENT
The following table summarizes the carrying value of financial assets and liabilities:
| September 30, 2020 |
September 30, 2019 |
|||||
| FVTPL | $ | $ | ||||
| Cash | 799,929 | 79,991 | ||||
| Amortized cost | ||||||
| Accounts payable and accrued liabilities | 150,923 | 36,708 |
Fair value measurement
Financial assets and liabilities that are recognized on the statement of financial position at fair value can be classified in a hierarchy that is based on the significance of the inputs used in making the measurements.
|
Bright Minds Biosciences Inc. |
9. FINANCIAL INSTRUMENTS AND CAPITAL MANAGEMENT (continued)
Fair value measurement (continued)
The levels in the hierarchy are:
Level 1 - quoted prices (unadjusted) in active markets for identical assets or liabilities;
Level 2 - inputs other than quoted prices included within level 1 that are observable for the asset or liability, either directly (that is, as prices) or indirectly (that is, derived from prices); and
Level 3 - inputs for the asset or liability that are not based on observable market data (that is, unobservable inputs).
The Company's cash is classified as Level 1, whereas accounts payable and accrued liabilities are classified as Level 2. As at September 30, 2020, the Company believes that the carrying values of cash and accounts payable and accrued liabilities approximate their fair values because of their nature and relatively short maturity dates or durations.
Financial risk management
The Company is exposed in varying degrees to a variety of financial instrument related risks. The Board of Directors approves and monitors the risk management processes. The type of risk exposure and the way in which such exposure is managed is provided as follows:
Credit risk
Credit risk is the risk that one party to a financial instrument will fail to discharge an obligation and cause the other party to incur a financial loss. The Company's primary exposure to credit risk is on its cash balance. At September 30, 2020, the Company had cash of $799,929. Of this amount, $596,839 was held in two trust accounts and $203,090 was deposited in a bank account held with a major bank in Canada. Because of the balance on deposit with one bank, there is a concentration of credit risk. This risk is managed by using a major bank that is a high credit quality financial institution as determined by rating agencies. The maximum exposure to credit risk is the carrying amount of the Company's financial instruments. The credit risk is assessed as low.
Foreign exchange risk
Foreign exchange risk is the risk that the fair values of future cash flows of a financial instrument will fluctuate because they are denominated in currencies that differ from the respective functional currency. At September 30, 2020, the company had the following foreign currency balances - cash (US$9,994) and accounts payable (US$27,840). The Company is not exposed to significant foreign exchange risk.
Liquidity risk
Liquidity risk arises through the excess of financial obligations over available financial assets due at any point in time. The Company's objective in managing liquidity risk is to maintain sufficient readily available reserves in order to meet its liquidity requirements at any point in time. The Company's main source of funding has been the issuance of equity securities for cash, primarily through private placements. The Company's access to financing is always uncertain. There can be no assurance of continued access to significant equity funding. At September 30, 2020, the company had cash of $799,929 to cover current liabilities of $150,923. On March 17, 2021, the Company issued 3,419,883 units for aggregate gross proceeds of $25,888,514. See Note 11.
Capital Management
Management's objective is to manage its capital to ensure that there are adequate capital resources to safeguard the Company's ability to continue as a going concern through the optimization of its capital structure. The capital structure consists of share capital and working capital. In order to achieve this objective, management makes adjustments to it in light of changes in economic conditions and risk characteristics of the underlying assets. To maintain or adjust capital structure, management may invest its excess cash in interest bearing accounts of Canadian chartered banks and/or raise additional funds externally as needed. The Company is not subject to externally imposed capital requirements. The Company's management of capital did not change during the year ended September 30, 2020.
|
Bright Minds Biosciences Inc. |
10. INCOME TAXES
A reconciliation of the expected income tax recovery to the actual income tax recovery is as follows:
| September 30, 2020 |
September 30, 2019 |
|||||
| $ | $ | |||||
| Net loss | (480,377 | ) | (78,717 | ) | ||
| Statutory tax rate | 27% | 27% | ||||
| Expected income tax recovery | (129,702 | ) | (21,254 | ) | ||
| Deductible and non-deductible items | 42,136 | - | ||||
| Change in deferred tax assets not recognized | 87,566 | 21,254 | ||||
| Total income tax recovery | - | - |
The Company has the following deductible temporary differences for which no deferred tax has been recognized:
| September 30, 2020 |
September 30, 2019 |
|||||
| $ | $ | |||||
| Non-capital losses | 403,000 | 79,000 | ||||
| Share issue costs | 4,000 | - | ||||
| Intangible assets | (2,000 | ) | (2,000 | ) | ||
| Valuation allowance | (405,000 | ) | (77,000 | ) | ||
| Net deferred income tax assets | - | - |
The Company has non-capital losses of approximately $403,000 which may be carried forward and applied against taxable income in future years. These losses, if not utilized, will expire through to 2040. Future tax benefits which may arise as a result of these losses have not been recognized in these financial statements and have been offset by a valuation allowance.
The Company's non-capital loss carry-forwards expire as follows:
|
Year of Origin |
Year of Expiry |
Non-Capital Losses |
|
|
|
$ |
|
2019 |
2039 |
79,000 |
| 2020 | 2040 | 324,000 |
|
|
|
403,000 |
11. SUBSEQUENT EVENTS
Events not disclosed elsewhere in these financial statements are as follows:
On October 9, 2020, the Company entered into a consulting agreement whereby the consultant was retained to serve as an advisor to the Company in the areas of scientific, technical and business advice. As compensation for performing these services, the Company will pay the advisor an hourly rate of US$130. On November 17, 2020, the Company granted 90,000 share purchase options to the consultant.
|
Bright Minds Biosciences Inc. |
11. SUBSEQUENT EVENTS (continued)
In October 2020, the Company entered into subscription agreements for special warrants (the "SWs") whereby the subscribers subscribed for a total of 45,750 SWs at $0.50 per SW. Pursuant to the subscription agreements:
- The SWs will provide that each SW is deemed to be exercised, without payment of any additional consideration and without any further action by the SW holders, for one SW share, subject to adjustment in accordance with the provisions of the SW certificate on the SW exercise date, being the earlier of (i) the third business day after a receipt for a final prospectus qualifying the distribution of the shares issuable on the conversion of the SWs and (ii) four months and one day after the issue date of the SWs (the escrow release conditions). For greater certainty, no SWs may be exercised by the SW holders prior to the SW exercise date;
- The SW certificate will provide that the gross proceeds of the offering due on the closing date (the escrowed proceeds) will be delivered to and held in escrow on behalf of the subscribers by the Company in a segregated, interest bearing account. Upon and subject to the satisfaction of one of the escrow release conditions, the escrowed proceeds and accrued interest will be released to the Company, at which time each SW will be deemed to be exercised for one SW share. The deemed exercise of the SWs is expected to occur prior to the proposed listing of the common shares of the Company on the CSE, with delivery of the subscribers' SW shares taking place after the completion of the proposed listing, such that the subscribers will receive a share certificate or DRS statement representing the subscribers' SW shares once the Company is a public company under the applicable securities laws; and
- The SWs will be governed by the terms and conditions of the SW certificate.
On October 29, 2020, the Company entered into an independent contractor agreement (the "ICA") whereby the contractor was engaged to serve as the Company's Chief Science Officer on an as-needed basis. The contractor will be compensated for these services as determined by the Board of Directors of the Company. The services will continue for an initial term of one year unless sooner terminated. The ICA can be terminated by the Company providing five working days written notice, the contractor providing three months' written notice or by mutual written agreement. At the end of the initial term, the ICA will automatically be extended for additional one-year period(s) unless the Company provides the contractor with 30 days written notice. Commencing in March 2021, the contractor began invoicing the Company a monthly fee of US$15,000.
On November 2, 2020, the Company issued 45,750 SWs at $0.50 per SW for gross proceeds of $22,875, which have been received.
On November 2, 2020, the Company closed the second tranche of a non-brokered private placement financing through the issuance of 4,072,843 common shares at a price $0.50 per common share for gross proceeds of $2,036,422, which have been received.
On November 6, 2020, the Company entered into a sponsored research agreement (the "SRA") with the University of Texas Medical Branch (the "UTMB") whereby the UTMB will conduct a research program on behalf of the Company. Pursuant to the SRA, the agreement is effective as of October 15, 2020 and the research program will be carried out through to and including February 15, 2021. As consideration for UTMB's performance, the Company will pay US$66,764 of which US$55,811 ($74,968) was paid on September 21 and 22, 2020 and is included in prepaids at September 30, 2020. The balance of US$10,953 ($13,985) was paid on February 3, 2021. The research program concluded on February 15, 2021.
On November 10, 2020, the Directors of the Company approved the consolidation of the Company's issued and outstanding common shares on a 2.5:1 basis.
|
Bright Minds Biosciences Inc. |
11. SUBSEQUENT EVENTS (continued)
On November 17, 2020, the Company entered into an ICA whereby the contractor was engaged to serve as the Company's Vice President (Discovery). The contractor will be compensated for these services as determined by the Board of Directors of the Company. The services will continue for an initial term of one year unless sooner terminated. The ICA can be terminated by the Company providing five working days written notice, the contractor providing three months' written notice or by mutual written agreement. At the end of the initial term, the ICA will automatically be extended for additional one-year period(s) unless the Company provides the contractor with 30 days written notice. Commencing in March 2021, the contractor began invoicing the Company a monthly fee of US$15,000.
On November 17, 2020, the Company granted 467,000 options, on a post-consolidated basis, to the Chief Financial Officer of the Company, two directors of the Company and seven consultants. These options have an exercise price of $1.25 per share, expire on November 17, 2025 and vest as follows:
- 25,000 options - 100% on the date of grant;
- 14,000 options - 25% on the Company's listing date, 25% on the first anniversary of the listing date and 50% on the second anniversary of the listing date;
- 4,000 options - 50% on the Company's listing date and 50% on the six-month anniversary of the listing date; and
- 424,000 options - 33% on the first anniversary of the grant date, 33% on the second anniversary of the grant date and 33% on the third anniversary of the grant date.
The fair vale of these stock options ($55,661) was measured using the Black Scholes option pricing model using the following inputs: i) exercise price: $1.25; ii) share price: $1.25; iii) term: 5 years; iv) volatility: 100%; v) discount rate: 0.43%; and dividends: nil. On January 21, 2021, the Company cancelled 20,000 options granted to a consultant in error.
On January 6, 2021, the Company issued 14,799 common shares, on a post-consolidated basis, at a deemed price of $1.25 per share to settle an $18,500 debt owing to a consultant pursuant to a debt settlement agreement entered into by the Company with the consultant.
On January 19, 2021, as a result of a compliance review of the SW offering by the British Columbia Securities Commission, the Company rescinded the issuance of 2,300 SWs and refunded the $2,875 in proceeds received. On February 3, 2021, the $20,000 in escrowed proceeds was released to the Company, the SWs were deemed to be exercised for SW shares and 16,000 common shares of the Company were issued to the SW holders.
On January 28, 2021, the Company entered into an escrow agreement under National Policy 46-201 Escrow for Initial Public Offerings (the "Policy") in connection with the listing of common shares of the Company on the CSE, whereby 2,852,800 common shares of the Company and 1,948,000 share purchase warrants, being an aggregate of 4,800,800 securities, were deposited to be held in escrow. On April 23, 2021, the share purchase warrants were exercised for gross proceeds of $97,400. As the Company is defined as an emerging issuer under the Policy, the escrowed securities will be released as follows:
- 480,080 on the date that the Company's shares are listed on the CSE (February 8, 2021); and
- 720,120 six, 12, 18, 24, 30 and 36 months after the listing date.
|
Bright Minds Biosciences Inc. |
11. SUBSEQUENT EVENTS (continued)
On March 17, 2021, the Company issued 3,419,883 units at a price per unit of $7.57 for aggregate gross proceeds of $25,888,514. Each unit comprised one common share and one-half of one common share purchase warrant of the Company. Each warrant is exercisable to acquire one common share of the Company at an exercise price of $9.46 per warrant until March 17, 2024, subject to adjustment and acceleration in certain events. If the daily volume weighted average trading price of the common shares on the CSE is equal to or greater than $13.25 per common share for any 10 consecutive trading days, the Company shall have the right to accelerate the expiry date of the warrants to a date that is at least 30 trading days following the date of the Company issuing a press release disclosing such acceleration. The underwriters were paid fees for their services in the amount of $916,317 and received compensation warrants entitling them to purchase an aggregate of 132,666 common shares at a price of $7.57 per common share for a period of thirty-six months following closing. The fair value of these compensation warrants ($521,000) was measured using the Black Scholes option pricing model using the following inputs: i) exercise price: $7.57; ii) share price: $6.65; iii) term: 3 years; iv) volatility: 100%; v) discount rate: 0.35%; and dividends: nil.
On April 23, 2021, 1,948,000 share purchase warrants were exercised for $0.05 per share for gross proceeds of $97,400. As at March 31, 2021, the Company received $10,000 of the gross proceeds in advance of the exercise.
On April 23, 2021, the Company entered into an exclusive license agreement with equity (the "LA") with the Board of Trustees of the UIC (the "University") whereby the University granted to the Company, in all fields of use and worldwide, an exclusive, non-transferable license with the right to sublicense under the University's rights in and to the Patent Rights (as defined) and a non-exclusive, non-transferable license with the right to sublicense under the University's rights in and to the Technical Information (as defined) to make, have made, construct, have constructed, use, import, sell, and offer for sale royalty-bearing Product (as defined). As consideration for the grant of license, the Company will pay the following amounts (in USD$) to the University:
- Signing Fee - a signing fee of $100,000 less $15,000 in option fees (CDN$105,502) was paid and 63,000 common shares of the Company were issued to the University on April 29, 2021;
- Net Sales - royalties on Net Sales (as defined) ranging from 3% (under $1 billion) to 4.5% (over $2 billion), with such royalty payments being credited toward the annual minimum for the license year in which the royalty payment accrues;
- Sublicensee Revenues - royalties (as for net sales above) on Sublicensee Revenue (as defined), with such royalty payments being credited toward the annual minimum for the license year in which the royalty payment accrues and 12% on all non-royalty revenue until the Company has raised $7.5 million and then 10% thereafter;
- Annual Minimums - if the total royalties paid to the University for any license year are less than the following annual minimums, the Company must pay the University the amount equal to the shortfall:
- Years 1 and 2 - $nil;
- Year 3 - $5,000;
- Year 4 - $15,000;
- Year 5 - $35,000;
- Year 6 and thereafter - $50,000; and
- After first commercial sale - $250,000 or net sales royalty, whichever is higher.
|
Bright Minds Biosciences Inc. |
11. SUBSEQUENT EVENTS (continued)
- Milestone Payments - milestone payments after the occurrence of the following milestone events:
Prior to any sublicensing agreements, joint ventures or change of control:
- $10,000 upon dosing the first patient in a Phase I trial;
- $50,000 upon dosing the first patient in the first Phase II trial;
- $250,000 upon dosing the first patient in a Phase III trial in the first clinical indication; and
- $2 million upon the first commercial sale of each clinical indication.
After any sublicensing agreements, joint ventures or change of control:
- As above;
- $250,000 upon dosing the first patient in each Phase II trial;
- $500,000 upon dosing the first patient in each Phase III trial; and
- $2 million upon the first commercial sale of each clinical indication.
Unless otherwise agreed to in writing by the University, the Company will reimburse the University for all documented costs and expenses in connection with the Patent Rights, including the preparation, filing, prosecution, maintenance and defense thereof. From time to time, the anticipated costs and expenses may be significant and, upon request, the Company will pay the estimated costs and expenses in advance of such costs and expenses being incurred by the University.
The term of the LA ends on the later of the last to expire of the Patent Rights, expiration of regulatory exclusivity for Product or when the Company provides notice that use of Technical Information has ceased. The University has the right to terminate the LA if the Company fails to make any required payments or is in breach of any provision of the LA. The Company may terminate the LA at any time upon providing at least 90 days written notice to the University. See Note 8.
On April 28, 2021, the Company granted 240,000 options, to three consultants of the Company. These options have an exercise price of $7.60 per share, expire on April 28, 2026 and vest as follows:
- 160,000 options - 25% on the first anniversary of the grant date, 25% on the second anniversary of the grant date, 25% on the third anniversary of the grant date and 25% on the fourth anniversary of the grant date; and
- 80,000 options - 25% on the on the six-month anniversary of the grant date, 25% on the first anniversary of the grant date, 25% on the eighteen-month anniversary of the grant date and 25% on the second anniversary of the grant date.
Bright Minds Biosciences Inc.
Condensed Interim Consolidated Financial Statements
For the three and six months ended March 31, 2021 and 2020
(Expressed in Canadian Dollars)
(Unaudited)
Bright Minds Biosciences Inc.
Condensed Interim Consolidated Statements of Financial Position
(Expressed in Canadian dollars)
(Unaudited)
| March 31, | September 30, | ||||||
| As at | Notes | 2021 | 2020 | ||||
| $ | $ | ||||||
| ASSETS | |||||||
| Current Assets | |||||||
| Cash | 25,450,001 | 799,929 | |||||
| Sales tax receivable | 36,256 | - | |||||
| Prepaids | 7 | 252,804 | 78,287 | ||||
| Due from Bright Minds Biosciences LLC | 490 | - | |||||
| 25,739,551 | 878,216 | ||||||
| Non-Current Assets | |||||||
| Intangible assets | 4 | 2,000 | 2,000 | ||||
| TOTAL ASSETS | 25,741,551 | 880,216 | |||||
| LIABILITIES AND SHAREHOLDERS' EQUITY | |||||||
| Current Liabilities | |||||||
| Accounts payable and accrued liabilities | 5 | 409,238 | 150,923 | ||||
| TOTAL LIABILITIES | 409,238 | 150,923 | |||||
| Shareholders' equity | |||||||
| Share capital | 6 | 27,239,872 | 980,661 | ||||
| Subscriptions receivable | 6 | (33,684 | ) | (1,000 | ) | ||
| Subscriptions received | 6 | 10,000 | 147,426 | ||||
| Reserves | 6 | 925,554 | 161,300 | ||||
| Deficit | (2,809,429 | ) | (559,094 | ) | |||
| TOTAL SHAREHOLDERS' EQUITY | 25,332,313 | 729,293 | |||||
| TOTAL LIABILITIES AND SHAREHOLDERS' EQUITY | 25,741,551 | 880,216 |
Nature of operations and going concern (Note 1)
Subsequent events (Note 10)
|
Approved on behalf of the Board of Directors: |
|||
|
|
|
|
|
|
"Ian McDonald" |
|
"Alan Kozikowski" |
|
|
Director |
|
Director |
|
The accompanying notes are an integral part of these condensed interim consolidated financial statements.
Bright Minds Biosciences Inc.
Condensed Interim Consolidated Statements of Comprehensive Loss
(Expressed in Canadian dollars)
(Unaudited)
| For the three months ended |
For the three months ended |
For the six months ended |
For the six months ended |
||||||||||
| Notes | March 31, 2021 |
March 31, 2020 |
March 31, 2021 |
March 31, 2020 |
|||||||||
| $ | $ | $ | $ | ||||||||||
| EXPENSES | |||||||||||||
| Consulting fees | 6 | 128,827 | - | 218,375 | - | ||||||||
| Foreign exchange | 26,965 | - | 59,594 | - | |||||||||
| Funds processing fees - private placements | 1,455 | - | 18,665 | - | |||||||||
| Marketing, advertising, and investor relations | 217,379 | - | 229,377 | - | |||||||||
| Office and administrative | 24,639 | 169 | 52,034 | 250 | |||||||||
| Professional fees | 7 | 141,804 | - | 271,871 | - | ||||||||
| Regulatory and filing | 62,039 | - | 115,919 | - | |||||||||
| Research and development | 6, 7 | 980,169 | 5,973 | 1,284,500 | 47,887 | ||||||||
| Net loss and comprehensive loss | (1,583,277 | ) | (6,142 | ) | (2,250,335 | ) | (48,137 | ) | |||||
| Basic and diluted loss per share | (0.23 | ) | (0.00 | ) | (0.35 | ) | (0.01 | ) | |||||
| Weighted average number of common shares outstanding -basic and diluted | 6,928,429 | 4,119,600 | 6,352,107 | 4,119,600 |
The accompanying notes are an integral part of these condensed interim consolidated financial statements.
Bright Minds Biosciences Inc.
Condensed Interim Consolidated Statements of Changes in Shareholders’ Equity
(Expressed in Canadian Dollars)
(Unaudited)
| Share Capital | |||||||||||||||||||||
| Number of shares |
Share capital |
Subscriptions receivable |
Subscriptions received |
Reserves | Deficit | Total | |||||||||||||||
| $ | $ | $ | $ | $ | $ | ||||||||||||||||
| Balance as at September 30, 2019 | 4,119,600 | 205,980 | (81,980 | ) | - | - | (78,717 | ) | 45,283 | ||||||||||||
| Share subscriptions received | - | - | 60,000 | - | - | - | 60,000 | ||||||||||||||
| Share subscription received in advance | - | - | - | 31,680 | - | - | 31,680 | ||||||||||||||
| Net loss | - | - | - | - | - | (48,137 | ) | (48,137 | ) | ||||||||||||
| Balance as at March 31, 2020 | 4,119,600 | 205,980 | (21,980 | ) | 31,680 | - | (126,854 | ) | 88,826 | ||||||||||||
| Balance as at September 30, 2020 | 4,743,541 | 980,661 | (1,000 | ) | 147,426 | 161,300 | (559,094 | ) | 729,293 | ||||||||||||
| Private placements | 5,049,021 | 27,924,936 | (32,684 | ) | (147,426 | ) | - | - | 27,744,826 | ||||||||||||
| Finder's fees - cash | - | (916,317 | ) | - | - | - | - | (916,317 | ) | ||||||||||||
| Finder's fees - broker warrants | - | (521,000 | ) | - | - | 521,000 | - | ||||||||||||||
| Share issue costs | - | (266,908 | ) | - | - | - | - | (266,908 | ) | ||||||||||||
| Special warrant conversion | 16,000 | 20,000 | - | - | - | - | 20,000 | ||||||||||||||
| Debt settlement with shares | 14,799 | 18,500 | - | - | - | - | 18,500 | ||||||||||||||
| Warrant exercise funds received in advance | - | - | - | 10,000 | - | - | 10,000 | ||||||||||||||
| Share-based compensation | - | - | - | - | 243,254 | - | 243,254 | ||||||||||||||
| Net loss | - | - | - | - | - | (2,250,335 | ) | (2,250,335 | ) | ||||||||||||
| Balance as at March 31, 2021 | 9,823,361 | 27,239,872 | (33,684 | ) | 10,000 | 925,554 | (2,809,429 | ) | 25,332,313 | ||||||||||||
The accompanying notes are an integral part of these condensed interim consolidated financial statements.
Bright Minds Biosciences Inc.
Condensed Interim Consolidated Statements of Cash Flows
(Expressed in Canadian Dollars)
(Unaudited)
| For the six months ended |
For the six months ended |
|||||
| March 31, 2021 |
March 31, 2020 |
|||||
| $ | $ | |||||
| Operating activities | ||||||
| Net loss for the period | (2,250,335 | ) | (48,137 | ) | ||
| Non-cash items: | ||||||
| Foreign exchange | 3,798 | - | ||||
| Share-based compensation | 243,254 | - | ||||
| Changes in non-cash operating working capital items: | ||||||
| Sales tax receivable | (36,256 | ) | - | |||
| Prepaids | (174,517 | ) | - | |||
| Due from Bright Minds Biosciences LLC | (490 | ) | - | |||
| Accounts payable and accrued liabilities | 257,563 | (49,419 | ) | |||
| Net cash used in operating activities | (1,956,983 | ) | (97,556 | ) | ||
| Financing activities | ||||||
| Private placement proceeds | 27,784,078 | - | ||||
| Finder's fees | (916,317 | ) | - | |||
| Share issuance costs | (266,908 | ) | - | |||
| Warrant exercise funds received in advance | 10,000 | - | ||||
| Private placement proceeds received in advance | - | 91,680 | ||||
| Net cash from financing activities | 26,610,853 | 91,680 | ||||
| Change in cash | 24,653,870 | (5,876 | ) | |||
| Effect of foreign exchange on cash | (3,798 | ) | - | |||
| Cash, beginning of period | 799,929 | 79,991 | ||||
| Cash, end of period | 25,450,001 | 74,115 | ||||
| SUPPLEMENTARY INFORMATION | ||||||
| Debt settled by issuing shares | 18,500 | - | ||||
| Fair value ascribed by brokers' warrants issued | 521,000 | - | ||||
| Share issuance costs included in accounts payable and accrued liabilities | 752 | - |
The accompanying notes are an integral part of these condensed interim consolidated financial statements.
|
Bright Minds Biosciences Inc. |
1. NATURE AND CONTINUANCE OF OPERATIONS
Bright Minds Biosciences Inc. ("the Company") was incorporated under the Business Corporations Act of British Columbia on May 31, 2019. The Company's objective is to generate income and achieve long term profitable growth through the development of therapeutics to improve the lives of patients with certain severe and life-altering diseases. On February 8, 2021, the Company started trading on the Canadian Stock Exchange ("CSE") under the symbol DRUG. The head office, and principal address of the Company are located at 1500 - 1055 West Georgia Street, Vancouver, British Columbia, V6E 4N7, Canada.
These condensed interim consolidated financial statements have been prepared on a going concern basis which assumes that the Company will be able to realize its assets and discharge its liabilities in the normal course of business for the foreseeable future. As at March 31, 2021, the Company is not able to finance day to day activities through operations and has incurred a loss of $2,250,335 for the period ended March 31, 2021. The Company has a deficit of $2,809,429 since inception and negative operating cash flows. As at March 31, 2021, the Company has working capital of $25,330,313 (September 30, 2020 - $727,293). The continuing operations of the Company are dependent upon its ability to attain profitable operations and generate funds therefrom. Management intends to finance operating costs with equity financings, loans from directors and companies controlled by directors and/or private placement of common shares. If the Company is unable to continue as a going concern, the net realizable value of its assets may be materially less than the amounts on its statement of financial position.
The coronavirus, also known as "COVID-19", has spread across the globe and is impacting worldwide economic activity. Government authorities have implemented emergency measures to mitigate the spread of the virus. The outbreak and the related mitigation measures may have an adverse impact on global economic conditions as well as on the Company's business activities specifically related to possible disruptions in the operations of the laboratories upon whom the Company relies, including laboratories situated in various parts of the United States and Europe. The extent to which the coronavirus may impact the Company's business activities will depend on future developments, such as the duration of the outbreak, travel restrictions, business disruptions, and the effectiveness of actions taken in Canada and other countries to contain and treat the disease. These events are highly uncertain and as such, the Company cannot determine their financial impact at this time.
2. STATEMENT OF COMPLIANCE AND BASIS OF PREPARATION
These financial statements were authorized for issue on June 9, 2021 by the directors of the Company.
Statement of compliance
The Company applies International Financial Reporting Standards ("IFRS") as issued by the International Accounting Standards Board ("IASB"). These unaudited condensed interim financial statements have been prepared in accordance with International Accounting Standard 34 - Interim Financial Reporting. Accordingly, they do not include all of the information required for full annual financial statements required by IFRS as issued by the IASB. The policies applied in these unaudited condensed interim consolidated financial statements are based on IFRSs issued and outstanding as of June 9, 2021, the date the Board of Directors approved the statements. The same accounting policies and methods of computation are followed in these unaudited condensed interim consolidated financial statements as compared with the most recent annual financial statements as at and for the year ended September 30, 2020 except as otherwise noted. Any subsequent changes to IFRS that are given effect in the Company's annual financial statements for the year ending September 30, 2021 could result in restatement of these unaudited condensed consolidated interim financial statements.
Basis of preparation
Depending on the applicable IFRS requirements, the measurement basis used in the preparation of these financial statements is cost, net realizable value, fair value or recoverable amount. These financial statements, except for the statement of cash flows, are based on the accrual basis.
|
Bright Minds Biosciences Inc. |
3. SIGNIFICANT ACCOUNTING POLICIES
Basis of consolidation
These financial statements include the accounts of the Company and its inactive, wholly-owned subsidiary Psilocybinlabs Ltd. (see Note 4).
Functional currency
The functional currency of the Company and its subsidiary is the Canadian Dollar.
Please refer to Note 3 of the audited consolidated financials of the Company for the year ended September 30, 2020 for full disclosure of the Significant Accounting Policies.
4. SHARE EXCHANGE
Psilocybinlabs Ltd. ("PL") was incorporated under the laws of the province of British Columbia on April 25, 2019, with the incorporator share being held by a company controlled by the CEO of the Company. On May 17, 2019, this share was transferred to the Company. On April 25, 2019, PL entered into a confirmatory assignment and waiver (the "CAW") with an individual, which was amended and restated on May 17, 2019. Pursuant to the amended and restated CAW, this individual assigned all of the right, title and interest, including all other intellectual property rights (the Rights, as described) to PL. As compensation for the assignment of the Rights, PL issued 100,000 common shares valued at $2,000 to this individual. On August 7, 2019, the Company then purchased the 100,000 common shares of PL by issuing 100,000 common shares of the Company valued at $2,000.
The Company has recorded the reacquisition of PL as an asset acquisition as follows:
| Purchase Price Consideration: | |||
| $ | |||
| Common shares issued | 2,000 | ||
| Assets acquired: | |||
| Intangible assets | 2,000 |
5. ACCOUNTS PAYABLE AND ACCRUED LIABILITIES
| March 31, 2021 |
September 30, 2020 |
|||||
| $ | $ | |||||
| Accounts payable | 409,238 | 138,423 | ||||
| Accrued liabilities | - | 12,500 | ||||
| Total accounts payable and accrued liabilities | 409,238 | 150,923 |
|
Bright Minds Biosciences Inc. |
6. SHARE CAPITAL
Authorized share capital
Unlimited number of common shares without par value.
On November 10, 2020, the Directors of the Company approved the consolidation of the Company's issued and outstanding common shares on a 2.5:1 basis. All common shares, stock options and warrant references in these financial statements reflect the effect of the share consolidation.
Issued share capital for the six months ended March 31, 2021
On November 2, 2020, the Company closed the second tranche of a non-brokered private placement financing through the issuance of 1,629,138 common shares at a price $1.25 per common share for gross proceeds of $2,036,422.
On January 6, 2021, the Company issued 14,799 common shares at a deemed price of $1.25 per share to settle an $18,500 debt owing to a consultant pursuant to a debt settlement agreement entered into by the Company with the consultant.
On March 17, 2021, the Company issued 3,419,883 Units at a price per Unit of $7.57 for aggregate gross proceeds of $25,888,514. Each Unit comprised one common share and one-half of one common share purchase warrant of the Company. Each warrant is exercisable to acquire one common share of the Company at an exercise price of $9.46 per warrant until March 17, 2024, subject to adjustment and acceleration in certain events. If the daily volume weighted average trading price of the common shares on the CSE is equal to or greater than $13.25 per common share for any 10 consecutive trading days, the Company shall have the right to accelerate the expiry date of the warrants to a date that is at least 30 trading days following the date of the Company issuing a press release disclosing such acceleration. The underwriters were paid fees for their services in the amount of $916,317 and received compensation warrants entitling them to purchase an aggregate of 132,666 common shares at a price of $7.57 per common share for a period of thirty-six months following closing. These warrants have an ascribed value of $521,000.
Issued share capital for the year ended September 30, 2020
On September 30, 2020, the Company closed the first tranche of a non-brokered private placement financing through the issuance of 623,941 common shares at a price $1.25 per common share for gross proceeds of $779,924.
Special Warrants and Resulting Share Issuance
In October 2020, the Company entered into subscription agreements for special warrants (the "SWs") whereby the subscribers subscribed for a total of 18,300 SWs at $1.25 per SW, with the SWs providing that each SW is deemed to be exercised, without payment of any additional consideration and without any further action by the SW holders, for one SW share, subject to adjustment in accordance with the provisions of the SW certificate on the SW exercise date.
On November 2, 2020, the Company issued 18,300 SWs for gross proceeds of $22,875. On January 19, 2021, as a result of a compliance review of the SW offering by the British Columbia Securities Commission, the Company rescinded the issuance of 2,300 SWs and refunded the $2,875 in proceeds received. On February 3, 2021, the $20,000 in escrowed proceeds was released to the Company, the SWs were deemed to be exercised for SW shares and 16,000 common shares of the Company were issued to the SW holders.
Share subscriptions received/receivable
During the fiscal year ended September 30, 2020, the Company received $147,426 in subscriptions for 294,852 common shares relating to the private placement that closed on November 2, 2020.
|
Bright Minds Biosciences Inc. |
6. SHARE CAPITAL (continued)
Stock options
The Company's stock option plan provides for stock options to be issued to directors, officers, employees and consultants of the Company, its subsidiaries and any personal holding company of such individuals so that they may participate in the growth and development of the Company. Subject to the specific provisions of the stock option plan, eligibility, vesting period, terms of the options and the number of options granted are to be determined by the Board of Directors at the time of grant. The stock option plan allows the Board of Directors to issue up to 10% of the Company's outstanding common shares as stock options.
Options granted during the three and six months ended March 31, 2021
On November 17, 2020, the Company granted 467,000 options, to the Chief Financial Officer of the Company, two directors of the Company and seven consultants. These options have an exercise price of $1.25 per share, expire on November 17, 2025 and vest as follows:
- 25,000 options - 100% on the date of grant;
- 14,000 options - 25% on the Company's listing date, 25% on the first anniversary of the listing date and 50% on the second anniversary of the listing date;
- 4,000 options - 50% on the Company's listing date and 50% on the six-month anniversary of the listing date; and
- 424,000 options - 33% on the first anniversary of the grant date, 33% on the second anniversary of the grant date and 33% on the third anniversary of the grant date.
The fair vale of these stock options was measured using the Black Scholes option pricing model using the following inputs: i) exercise price: $1.25; ii) share price: $1.25; iii) term: 5 years; iv) volatility: 100%; v) discount rate: 0.43%; and dividends: nil.
Options granted during the year ended September 30, 2020
On July 23, 2020, the Company granted 150,000 options to the Company's Chief Medical Officer. The fair vale of these stock options was measured using the Black Scholes option pricing model using the following inputs: i) exercise price: $1.25; ii) share price: $1.25; iii) term: 5 years; iv) volatility: 100%; v) discount rate: 0.35%; and dividends: nil.
The following table summarizes the movements in the Company's outstanding stock options for the six months ended March 31, 2021 and the year ended September 30, 2020:
| Number of options | Weighted average exercise price |
|||||
| Balance at September 30, 2019 | - | - | ||||
| Granted | 150,000 | $ | 1.25 | |||
| Balance at September 30, 2020 | 150,000 | $ | 1.25 | |||
| Granted | 467,000 | $ | 1.25 | |||
| Cancelled* | (20,000 | ) | $ | 1.25 | ||
| Balance at March 31, 2021 | 597,000 | $ | 1.25 |
* On January 21, 2021, the Company cancelled 20,000 options granted to a consultant in error on November 17, 2020.
As at March 31, 2021, the options have a weighted average remaining life of 4.56 years (September 30, 2020 - 4.81).
|
Bright Minds Biosciences Inc. |
6. SHARE CAPITAL (continued)
The following table summarizes the stock options issued and outstanding:
| Options Outstanding and Exercisable | |||
| Expiry Date |
Number of options |
Exercise price |
Remaining life (Years) |
| July 23, 2025 | 150,000 | $1.25 | 4.32 |
| November 17, 2025 | 447,000 | $1.25 | 4.64 |
Restricted share unit plan
The Company's restricted share unit ("RSU") plan provides RSUs to be issued to directors, officers, employees and consultants of the Company, its subsidiaries and any personal holding company of such individuals so that they may participate in the growth and development of the Company. Subject to the specific provisions of the RSU plan, eligibility, vesting period, terms of the RSUs and the number of RSUs granted are to be determined by the Board of Directors at the time of the grant. The RSU plan allows the Board of Directors to issue common shares of the company as equity settled RSUs, provided that, when combined, the maximum number of common shares reserved for issuance under all share-based compensation arrangements of the Company does not exceed 10% of the Company's outstanding common shares.
On July 23, 2020 and September 18, 2020, the Company issued 150,000 RSUs and 230,000 RSUs, respectively, to the Chief Medical Officer of the Company. These RSUs vest on an annual basis over a period of four years commencing on the first anniversary of the grant date.
The following table summarizes the movements in the Company's outstanding RSUs for the six months ended March 31, 2021 and the year ended September 30, 2020:
| Equity settled | Cash settled | Total | |||||||
| Balance at September 30, 2019 | - | - | - | ||||||
| Granted | 380,000 | - | 380,000 | ||||||
| Vested | - | - | - | ||||||
| Balance at September 30, 2020 and March 31, 2021 | 380,000 | - | 380,000 |
The estimated fair value of the equity settled RSUs granted during the year ended September 30, 2020 was $475,000 and will be recognized as an expense over the vesting period of the RSUs.
The accounting fair value of the equity settled RSUs as at the grant date was estimated by management using the following inputs:
| Year ended September 30, 2020 |
|||
| Share price on grant date | $ | 1.25 | |
| Forfeiture rate | 0% |
|
Bright Minds Biosciences Inc. |
6. SHARE CAPITAL (continued)
Share-based compensation expense recognized in the statements of comprehensive loss is comprised of the following:
| Period ended March 31, 2021 |
Period ended March 31, 2020 |
|||||
| $ | $ | |||||
| Stock options | 119,085 | - | ||||
| Restricted share units - equity settled grants | 124,169 | - | ||||
| Total equity settled share-based compensation expense | 243,254 | - | ||||
| Restricted share units - cash settled grants | - | - | ||||
| Total share-based compensation expense | 243,2541 | - |
____________________
1 Share-based compensation is included in consulting expense and research and development expense on the Consolidated Statements of Comprehensive Loss.
Warrants
The following table summarizes the movements in the Company's outstanding warrants for the six months ended March 31, 2021 and the year ended September 30, 2020:
| Number of warrants | Weighted average exercise price |
|||||
| Balance at September 30, 2019 | - | $ | - | |||
| Issued | 4,079,600 | 0.05 | ||||
| Balance at September 30, 2020 | 4,079,600 | 0.05 | ||||
| Issued | 1,709,938 | 9.46 | ||||
| Issued - broker | 132,666 | 7.57 | ||||
| Balance at March 31, 2021 | 5,922,204 | $ | 2.94 |
On November 2, 2020, the Directors of the Company reduced the exercise price of the outstanding warrants from $0.125 to $0.05 effective July 11, 2020.
On March 17, 2021, the Company granted 132,666 compensation warrants to underwriters. The fair value of these share purchase warrants of $521,000 was measured using the Black Scholes option pricing model using the following inputs: i) exercise price: $7.57; ii) share price: $6.65; iii) term: 3 years; iv) volatility: 100%; v) discount rate: 0.35%; and dividends: nil. The fair value of these broker warrants was recorded as a reduction against share capital.
As at March 31, 2021, the warrants have a weighted average remaining life of 3.22 (September 30, 2020 - 3.83) years.
The following table summarizes the warrants issued and outstanding:
|
|
Warrants Outstanding |
|
|
Expiry date |
Number of warrants |
Exercise price |
|
July 30, 2024 |
4,079,600 |
$ 0.05 |
|
March 17, 2024 |
1,709,938 |
$ 9.57 |
|
March 17, 2024 |
132,666 |
$ 7.57 |
|
Bright Minds Biosciences Inc. |
7. RELATED PARTY TRANSACTIONS
Related party transactions were recorded at the exchange value, which is the consideration determined and agreed to by the related parties. The Company's related parties include directors, key management and companies controlled by directors and key management.
Compensation of Key Management Personnel
Key management personnel are those persons that have authority and responsibility for planning, directing and controlling the activities of the Company, directly and indirectly, and by definition include the directors of the Company.
As at March 31, 2021, $63,579 (September 30, 2020 - $Nil) was payable to officers of the Company for fees and expenses incurred on behalf of the Company. In addition, $50,110 (September 30, 2020 - $Nil) advanced to an officer of the Company was included in prepaids.
The following table summarizes expenses related to key management personnel:
| Period ended | Period ended | |||||
| March 31, 2021 |
March 31, 2020 |
|||||
| $ | $ | |||||
| Professional fees | 39,800 | - | ||||
| Research and development | 88,978 | - | ||||
| Share-based compensation included in research and development | 56,298 | - | ||||
| 185,076 | - |
See Note 8 for related party contractual obligations.
8. CONTRACTUAL OBLIGATIONS
Option agreement
On May 26, 2020, the Company entered into an option agreement (the "OA") with the University of Illinois at Chicago (the "UIC") whereby the Company obtained the right to evaluate certain of the UIC's technology (as defined) for the purpose of making a decision as to whether to exclusively license the rights to the technology. Pursuant to the OA, the Company paid a US$5,000 ($6,999) non-refundable option fee and was granted an exclusive option to evaluate the technology and obtain an exclusive license to the patents and patent applications (as listed) and a non-exclusive right to use the technology for non-commercial research purposes. The option expires on the earlier of 90 days or the execution of a license agreement between the Company and the UIC. The company can extend the first option period three additional times for an additional 90 days each with payment of a US$5,000 option extension fee for each 90-day option period. During the option period and any renewals thereof, the Company must pay all out of pocket expenses incurred by the UIC in connection with the preparation, filing, prosecution and maintenance of the patent applications and patents under the patent rights.
On April 23, 2021, the Company entered into the License Agreement with the UIC pursuant to the exercise of its OA. Pursuant to the terms and conditions of the License Agreement, the UIC granted the Company to exclusive licenses for payment of: A signing fee of USD$100,000, less USD$15,000 paid by the Company pursuant to the Option Agreement; and (ii) 63,000 commons shares at a deemed price of $5.85 per Common Share to the UIC on April 28, 2021.
See Note 10.
|
Bright Minds Biosciences Inc. |
8. CONTRACTUAL OBLIGATIONS (continued)
Related Party Contracts
On June 5, 2020, the Company entered into an independent consultant agreement (the "ICA") whereby the consultant, a private corporation incorporated in the State of California, USA, was engaged and the consultant's representative will serve as the Company's Chief Medical Officer, with the services being provided in California. As compensation for performing these services, the consultant or the consultant's representative will participate in the Company's equity incentive plans and will be eligible for cash payments in respect of fees at such time as the Company begins to compensate other C-level personnel in cash and in similar proportion to total compensation (the "fees"). The non-cash portion of the consultant's fees for the first year of the term was in the form of a grant of 150,000 vested stock options and 150,000 RSUs (see Note 6). The services will continue for an initial term of one year unless sooner terminated. The ICA can be terminated by either party giving the other 30 days written notice or by mutual written agreement. At the end of the initial term, the ICA will automatically be extended for additional one-year period(s) unless either party gives the other 30 days written notice. As at March 31, 2021, the Board of Directors authorized a monthly fee of $15,000 USD.
On October 29, 2020, the Company entered into an independent contractor agreement (the "ICA") whereby the contractor was engaged to serve as the Company's Chief Science Officer on an as-needed basis. The contractor will be compensated for these services as determined by the Board of Directors of the Company. The services will continue for an initial term of one year unless sooner terminated. The ICA can be terminated by the Company providing five working days written notice, the contractor providing three months' written notice or by mutual written agreement. At the end of the initial term, the ICA will automatically be extended for additional one-year period(s) unless the Company provides the contractor with 30 days written notice. As at March 31, 2021, the Board of Directors authorized a monthly fee of $15,000 USD.
Scientific advisory board agreements
On June 1, 2020, July 14, 2020 and April 12, 2021, the Company entered into scientific advisory board agreements (the "SABAs") whereby the advisors were retained to serve as members of the Company's scientific advisory board and as consultants to the Company and senior management in the areas of scientific, technical and business advice. As compensation for performing these services, the Company will pay the advisors hourly rates of $150 and $160 per hour. The Company also granted 130,000 share purchase options to the advisors as part of the Company's November 17, 2020 and April 28, 2021 grant of options of which 20,000 options were cancelled on January 21, 2021 (Note 6 and 10). The advisors have the same hour requirements and restrictions as noted below. The services will continue for initial terms of one year unless sooner terminated. At the end of the initial terms, the SABAs will automatically be extended for an additional one-year period(s) unless either party gives the other 30 days written notice.
Consulting agreements
The Company has entered into numerous consulting agreements (the "CAs") whereby the consultants were retained to serve as advisors to the Company and senior management in the areas of public relations and content creation and scientific, technical and business advice. As compensation for performing these services, the Company will pay the advisors hourly rates of $150, $300, $400 and $600. The Company also granted 302,000 share purchase options to six advisors as part of the Company's November 17, 2020 and April 28, 2021 grant of options (Note 6 and 10). The advisors being paid $400 and $600 per hour will reserve at least six full days of services to the Company and such additional days as requested by the Company each annual period, but not to exceed 36 full days of service per year unless otherwise agreed and up to a maximum of 288 hours total per year, unless otherwise agreed. The services will continue for initial terms of one year unless sooner terminated. At the end of the initial terms, the CAs will automatically be extended for an additional one-year period(s) unless either party gives the other 30 days written notice.
On October 9, 2020, the Company entered into a consulting agreement whereby the consultant was retained to serve as an advisor to the Company in the areas of scientific, technical and business advice. As compensation for performing these services, the Company will pay the advisor an hourly rate of US$130. The Company granted 90,000 stock options to the consultant on November 17, 2020 (Note 6).
|
Bright Minds Biosciences Inc. |
8. CONTRACTUAL OBLIGATIONS (continued)
On November 6, 2020, the Company entered into a sponsored research agreement (the "SRA") with the University of Texas Medical Branch (the "UTMB") whereby the UTMB conducted a research program on behalf of the Company. Pursuant to the SRA, the agreement is effective as of October 15, 2020 and the research program was carried out through to its conclusion on February 15, 2021. As consideration for UTMB's performance, the Company paid US$66,764 which was recorded in research and development costs.
On November 17, 2020, the Company entered into an ICA whereby the contractor was engaged to serve as the Company's Vice President (Discovery). The contractor will be compensated for these services as determined by the Board of Directors of the Company. The services will continue for an initial term of one year unless sooner terminated. The ICA can be terminated by the Company providing five working days written notice, the contractor providing three months' written notice or by mutual written agreement. At the end of the initial term, the ICA will automatically be extended for additional one-year period(s) unless the Company provides the contractor with 30 days written notice. As at March 31, 2021, the Board of Directors approved a fee of $15,000 USD per month billed on a month-to-month basis.
9. FINANCIAL INSTRUMENTS AND CAPITAL MANAGEMENT
The following table summarizes the carrying value of financial assets and liabilities:
| March 31, 2021 |
September 30, 2020 |
|||||
| FVTPL | $ | $ | ||||
| Cash | 25,450,001 | 799,929 | ||||
| Amortized cost | ||||||
| Accounts payable and accrued liabilities | 409,238 | 150,923 |
Fair value measurement
Financial assets and liabilities that are recognized on the statement of financial position at fair value can be classified in a hierarchy that is based on the significance of the inputs used in making the measurements.
The levels in the hierarchy are:
Level 1 - quoted prices (unadjusted) in active markets for identical assets or liabilities;
Level 2 - inputs other than quoted prices included within level 1 that are observable for the asset or liability, either directly (that is, as prices) or indirectly (that is, derived from prices); and
Level 3 - inputs for the asset or liability that are not based on observable market data (that is, unobservable inputs).
The Company's cash is classified as Level 1, whereas accounts payable and accrued liabilities are classified as Level 2. As at March 31, 2021, the Company believes that the carrying values of cash and accounts payable and accrued liabilities approximate their fair values because of their nature and relatively short maturity dates or durations.
Financial risk management
The Company is exposed in varying degrees to a variety of financial instrument related risks. The Board of Directors approves and monitors the risk management processes. The type of risk exposure and the way in which such exposure is managed is provided as follows:
|
Bright Minds Biosciences Inc. |
9. FINANCIAL INSTRUMENTS AND CAPITAL MANAGEMENT (continued)
Credit risk
Credit risk is the risk that one party to a financial instrument will fail to discharge an obligation and cause the other party to incur a financial loss. The Company's primary exposure to credit risk is on its cash balance. At March 31, 2021, the Company had cash of $25,450,001. Of this amount, $11,494 was held in one trust account and the remainder was deposited in a bank account held with a major bank in Canada. Because of the balance on deposit with one bank, there is a concentration of credit risk. This risk is managed by using a major bank that is a high credit quality financial institution as determined by rating agencies. The maximum exposure to credit risk is the carrying amount of the Company's financial instruments. The credit risk is assessed as low.
Foreign exchange risk
Foreign currency risk is the risk that the fair values of future cash flows of a financial instrument will fluctuate because they are denominated in currencies that differ from the respective functional currency. At March 31, 2021, the company had the following foreign currency balances - cash (US$ 122,369) and accounts payable (US$ 237,865 and EURO 3,764) and prepaid (US$ 80,900). The Company is not exposed to significant foreign exchange risk.
Liquidity risk
Liquidity risk arises through the excess of financial obligations over available financial assets due at any point in time. The Company's objective in managing liquidity risk is to maintain sufficient readily available reserves in order to meet its liquidity requirements at any point in time. The Company's main source of funding has been the issuance of equity securities for cash, primarily through private placements. The Company's access to financing is always uncertain. There can be no assurance of continued access to significant equity funding. At March 31, 2021, the company had cash of $25,450,001 to cover current liabilities of $409,238.
Capital Management
Management's objective is to manage its capital to ensure that there are adequate capital resources to safeguard the Company's ability to continue as a going concern through the optimization of its capital structure. The capital structure consists of share capital and working capital. In order to achieve this objective, management makes adjustments to it in light of changes in economic conditions and risk characteristics of the underlying assets. To maintain or adjust the capital structure, management may invest its excess cash in interest bearing accounts of Canadian chartered banks and/or raise additional funds externally as needed. The Company is not subject to externally imposed capital requirements. The Company's management of capital did not change during the period ended March 31, 2021.
10. SUBSEQUENT EVENTS
Events not disclosed elsewhere in these financial statements are as follows:
On April 23, 2021, 1,948,000 share purchase warrants were exercised for $0.05 per share for gross proceeds of $97,400. As at March 31, 2021, the Company received $10,000 of the gross proceeds in advance of the exercise.
On April 23, 2021, the Company entered into the License Agreement with the UIC pursuant to the exercise of its OA. Pursuant to the terms and conditions of the License Agreement, the UIC granted the Company to exclusive licenses for payment of: A signing fee of USD$100,000, less USD$15,000 paid by the Company pursuant to the Option Agreement; and (ii) 63,000 commons shares at a deemed price of $5.85 per Common Share to the UIC on April 28, 2021.
|
Bright Minds Biosciences Inc. |
10. SUBSEQUENT EVENTS (continued)
On April 23, 2021, the Company entered into an exclusive license agreement with equity (the "LA") with the Board of Trustees of the UIC (the "University") whereby the University granted to the Company, in all fields of use and worldwide, an exclusive, non-transferable license with the right to sublicense under the University's rights in and to the Patent Rights (as defined) and a non-exclusive, non-transferable license with the right to sublicense under the University's rights in and to the Technical Information (as defined) to make, have made, construct, have constructed, use, import, sell, and offer for sale royalty-bearing Product (as defined). As consideration for the grant of license, the Company will pay the following amounts (in USD$) to the University:
- Signing Fee - a signing fee of $100,000 less $15,000 in option fees was paid and 63,000 common shares of the Company were issued to the University;
- Net Sales - royalties on Net Sales (as defined) ranging from 3% (under $1 billion) to 4.5% (over $2 billion), with such royalty payments being credited toward the annual minimum for the license year in which the royalty payment accrues;
- Sublicensee Revenues - royalties (as for net sales above) on Sublicensee Revenue (as defined), with such royalty payments being credited toward the annual minimum for the license year in which the royalty payment accrues and 12% on all non-royalty revenue until the Company has raised $7.5 million and then 10% thereafter;
- Annual Minimums - if the total royalties paid to the University for any license year are less than the following annual minimums, the Company must pay the University the amount equal to the shortfall:
- Years 1 and 2 - $nil;
- Year 3 - $5,000;
- Year 4 - $15,000;
- Year 5 - $35,000;
- Year 6 and thereafter - $50,000; and
- After first commercial sale - $250,000 or net sales royalty, whichever is higher.
- Milestone Payments - milestone payments after the occurrence of the following milestone events:
Prior to any sublicensing agreements, joint ventures or change of control:
- $10,000 upon dosing the first patient in a Phase I trial;
- $50,000 upon dosing the first patient in the first Phase II trial;
- $250,000 upon dosing the first patient in a Phase III trial in the first clinical indication; and
- $2 million upon the first commercial sale of each clinical indication.
After any sublicensing agreements, joint ventures or change of control:
- As above;
- $250,000 upon dosing the first patient in each Phase II trial;
- $500,000 upon dosing the first patient in each Phase III trial; and
- $2 million upon the first commercial sale of each clinical indication.
Unless otherwise agreed to in writing by the University, the Company will reimburse the University for all documented costs and expenses in connection with the Patent Rights, including the preparation, filing, prosecution, maintenance and defense thereof. From time to time, the anticipated costs and expenses may be significant and, upon request, the Company will pay the estimated costs and expenses in advance of such costs and expenses being incurred by the University.
|
Bright Minds Biosciences Inc. |
10. SUBSEQUENT EVENTS (continued)
The term of the LA ends on the later of the last to expire of the Patent Rights, expiration of regulatory exclusivity for Product or when the Company provides notice that use of Technical Information has ceased. The University has the right to terminate the LA if the Company fails to make any required payments or is in breach of any provision of the LA. The Company may terminate the LA at any time upon providing at least 90 days written notice to the University.
See Note 8.
On April 28, 2021, the Company granted 240,000 options, to three consultants of the Company. These options have an exercise price of $7.60 per share, and expire on April 28, 2026 and vest as follows:
- 160,000 options - 25% on the first anniversary of the grant date, 25% on the second anniversary of the grant date, 25% on the third anniversary of the grant date and 25% on the fourth anniversary of the grant date;
- 80,000 options - 25% on the on the six-month anniversary of the grant date, 25% on the first anniversary of the grant date, 25% on the eighteen-month anniversary of the grant date and 25% on the second anniversary of the grant date.
ITEM 19. EXHIBITS
Exhibits Required by Form 20-F
The following exhibits are filed as part of this Registration Statement on Form 20-F:
Notes:
+ Portions of this exhibit have been omitted.
* Previously filed.
SIGNATURES
The Registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this Registration Statement on its behalf.
| BRIGHT MINDS BIOSCIENCES INC. | ||
| Dated: September 16, 2021 | By: | /s/ Ian McDonald |
|
Ian McDonald |
||
| Chief Executive Officer | ||
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Rotoplas: First Quarter 2024 Results
- Report on Financial Results for the Year Ended December 31, 2023
- SBA Announces 2024 Growth Accelerator Fund Competition Stage One Winners, Up to $3 Million in Prizes Awarded
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share