Form 425 Galena Biopharma, Inc. Filed by: Galena Biopharma, Inc.
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): September 13, 2017
Galena Biopharma, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 001-33958 | 20-8099512 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
| 2000 Crow Canyon Place, Suite 380 San Ramon, CA |
94583 | |||
| (Address of principal executive offices) | (Zip Code) | |||
Registrant’s telephone number, including area code: (855) 855-4253
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligations of the registrant under any of the following provisions:
| ☒ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 | Regulation FD Disclosure. |
On September 13, 2017, SELLAS Life Sciences Group Ltd, a Bermuda exempted company (“SELLAS”), gave a poster presentation (abstract #MM-252) at the Annual Meeting of the Society of Hematologic Oncology (SOHO) in Houston, Texas, USA (soho2017.com). The poster and abstract are being furnished as Exhibit 99.1 to this Current Report on Form 8-K. The information in this Item 7.01 and Exhibit 99.1 to this Form 8-K shall not be deemed “filed” for purposes of Section 18 of the Exchange Act or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act or the Exchange Act, except as expressly set forth by specific reference in such a filing.
As previously announced, on August 7, 2017, Galena Biopharma, Inc., a Delaware corporation (“Galena”), SELLAS, Sellas Intermediate Holdings I, Inc., a Delaware corporation and a wholly-owned subsidiary of Galena (“Holdings I”), Sellas Intermediate Holdings II, Inc., a Delaware corporation and a wholly-owned subsidiary of Holdings I (“Holdings II”) and Galena Bermuda Merger Sub, Ltd., a Bermuda exempted company and a wholly-owned subsidiary of Holdings II (“Merger Sub”), entered into an Agreement and Plan of Merger and Reorganization (the “Merger Agreement”), pursuant to which, among other things, subject to the satisfaction or waiver of the conditions set forth in the Merger Agreement, Merger Sub will merge with and into SELLAS, with SELLAS becoming an indirect wholly-owned subsidiary of Galena and the surviving corporation of the merger (the “Merger”).
| Item 8.01 | Other Events. |
Attached hereto and incorporated herein by reference as Exhibit 99.2 is a press release issued by SELLAS on September 14, 2017 entitled “SELLAS’ Galinpepimut-S Induces Specific, Robust and Durable Immune Responses in Patients With High-Risk Multiple Myeloma - Correlated With Clinical Benefit.”
Additional Information about the Proposed Merger between Galena and SELLAS and Where to Find It
In connection with the proposed Merger, Galena and SELLAS intend to file relevant materials with the SEC, including a registration statement on Form S-4 that will contain a proxy statement / prospectus / information statement. Galena and SELLAS will mail the final proxy statement / prospectus / information statement to their respective stockholders. Investors and stockholders of Galena and SELLAS are urged to read these materials when they become available because they will contain important information about Galena, SELLAS and the proposed Merger. The proxy statement / prospectus / information statement and other relevant materials (when they become available), and any other documents filed by Galena with the SEC, may be obtained free of charge at the SEC web site at www.sec.gov. In addition, copies of the documents filed with the SEC by Galena will be available free of charge on the Company’s website at www.galenabiopharma.com (under “Investors” – “Financials”) or by directing a written request to: Galena Biopharma, Inc., 2000 Crow Canyon Place, Suite 380, San Ramon, CA 94583, Attention: Investor Relations or by email to [email protected]. Investors and stockholders are urged to read the proxy statement / prospectus / information statement and the other relevant materials when they become available before making any voting or investment decision with respect to the proposed Merger.
Non-Solicitation
This communication shall not constitute an offer to sell or the solicitation of an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. No offering of securities in connection with the proposed Merger shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933, as amended.
Participants in the Solicitation
Galena and its directors and executive officers and SELLAS and its directors and executive officers may be deemed to be participants in the solicitation of proxies from the stockholders of Galena in connection with the proposed transaction. Information regarding the special interests of these directors and executive officers in the proposed Merger will be included in the proxy statement / prospectus / information statement referred to above. Additional information regarding the directors and executive officers of Galena is also included in the Galena Annual Report on Form 10-K for the year ended December 31, 2016 and the proxy statement for its 2017 Annual Meeting of Stockholders, which was filed with the SEC on April 20, 2017. These documents are available free of charge at the SEC’s website at www.sec.gov, the Investors section of Galena’s website, and from Investor Relations at Galena at the addresses provided above.
Forward-Looking Statements
This Form 8-K contains statements that include the words “expect,” “intend,” “plan,” “believe,” “project,” “estimate,” “may,” “should,” “anticipate,” “will” and similar statements of a future or forward looking nature identify forward-looking statements for purposes of the federal securities laws and otherwise. These forward-looking statements include, without limitation, statements regarding the completion of the proposed Merger. Forward-looking statements are neither historical facts nor assurances of future performance. Actual results could differ materially from those anticipated in such forward-looking statements as a result of various factors, including, without limitation, risks and uncertainties associated with stockholder approval of and the ability to consummate the proposed Merger through the process being conducted by Galena and SELLAS. The forward-looking statements herein are made only as of the date hereof or as of the dates indicated in the forward-looking statements. Galena and SELLAS each disclaim any intent or obligation to update these forward-looking statements to reflect events or circumstances that exist after the date on which they were made. Additional risks and uncertainties relating to Galena and its business can be found under the caption “Risk Factors” and elsewhere in the Company’s SEC filings and reports, including in Galena’s Annual Report on Form 10-K, filed with the SEC on March 15, 2017 and the Quarterly Reports on Form 10-Q, filed with the SEC on May 10, 2017 and August 14, 2017 and in subsequently filed Form 10-Qs.
By filing the information in this Item 8.01 of this Current Report on Form 8-K, Galena makes no admission as to the materiality of any information in this report. The information contained herein is intended to be considered in the context of Galena’s filings with the Securities and Exchange Commission (the “SEC”) and other public announcements that Galena makes, by press release or otherwise, from time to time. Galena undertakes no duty or obligation to publicly update or revise the information contained in this report, although it may do so from time to time as its management believes is appropriate. Any such updating may be made through the filing of other reports or documents with the SEC, through press releases or through other public disclosure.
| Item 9.01 | Financial Statements and Exhibits. |
Exhibit Index
| Exhibit No. |
Description | |
| 99.1 | ||
| 99.2 | ||
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Galena Biopharma, Inc. | ||||||
| Dated: September 14, 2017
|
By: | /s/ Stephen F. Ghiglieri | ||||
| Stephen F. Ghiglieri | ||||||
| Interim Chief Executive Officer & Chief Financial Officer | ||||||
Exhibit 99.1
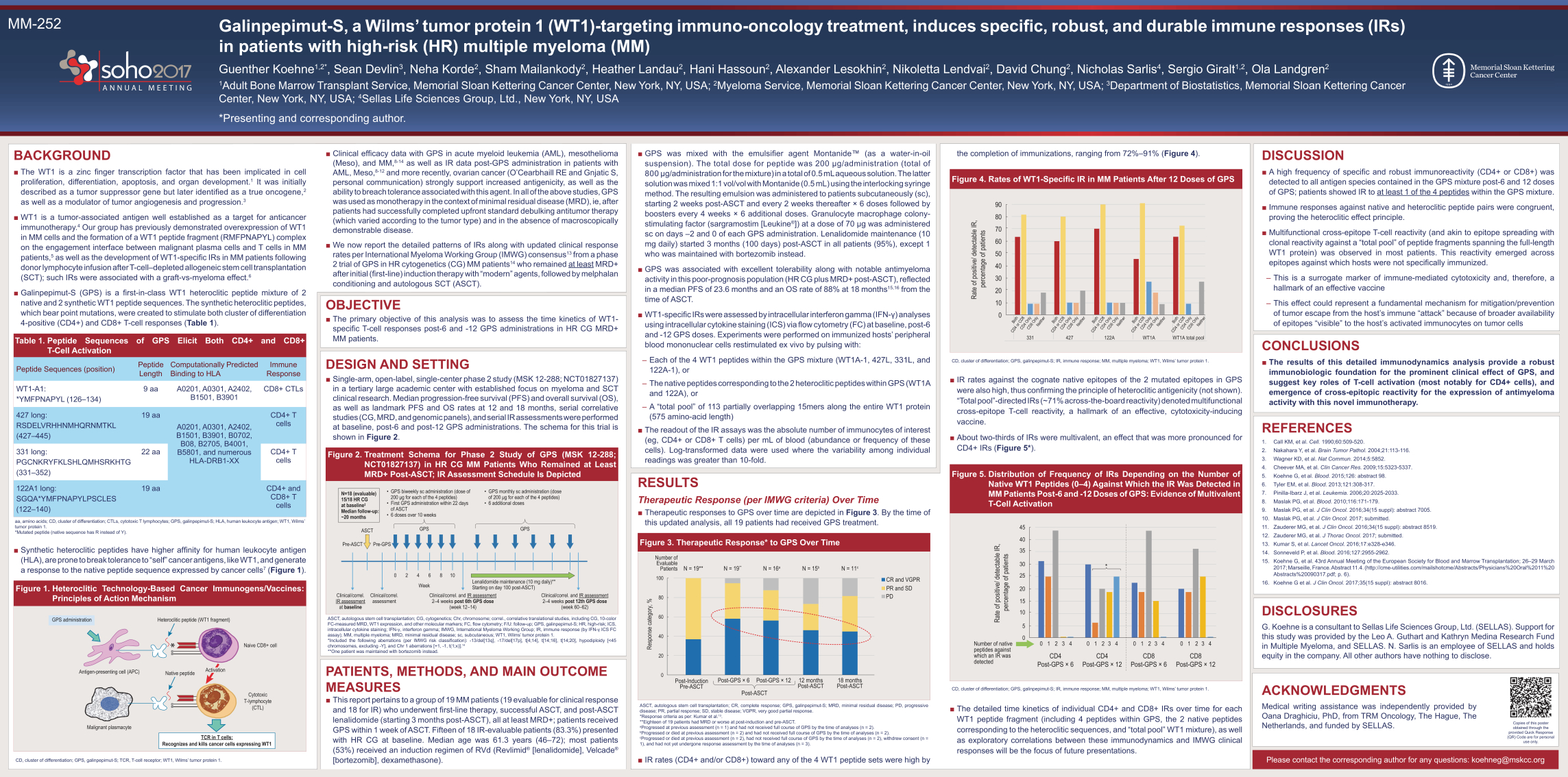
MM-252 Galinpepimut-S, a Wilms’ tumor protein 1 (WT1)-targeting immuno-oncology treatment, induces specific, robust, and durable immune responses (IRs) in patients with high-risk (HR) multiple myeloma (MM) Guenther Koehne1,2*, Sean Devlin3, Neha Korde2, Sham Mailankody2, Heather Landau2, Hani Hassoun2, Alexander Lesokhin2, Nikoletta Lendvai2, David Chung2, Nicholas Sarlis4, Sergio Giralt1,2, Ola Landgren2 1Adult Bone Marrow Transplant Service, Memorial Sloan Kettering Cancer Center, New York, NY, USA; 2Myeloma Service, Memorial Sloan Kettering Cancer Center, New York, NY, USA; 3Department of Biostatistics, Memorial Sloan Kettering Cancer Center, New York, NY, USA; 4Sellas Life Sciences Group, Ltd., New York, NY, USA *Presenting and corresponding author. BACKGROUND The WT1 is a zinc finger transcription factor that has been implicated in cell proliferation, differentiation, apoptosis, and organ development.1 It was initially described as a tumor suppressor gene but later identified as a true oncogene,2 as well as a modulator of tumor angiogenesis and progression.3 WT1 is a tumor-associated antigen well established as a target for anticancer immunotherapy.4 Our group has previously demonstrated overexpression of WT1 in MM cells and the formation of a WT1 peptide fragment (RMFPNAPYL) complex on the engagement interface between malignant plasma cells and T cells in MMpatients,5 as well as the development of WT1-specific IRs in MM patients following donor lymphocyte infusion after T-cell–depleted allogeneic stem cell transplantation (SCT); such IRs were associated with a graft-vs-myeloma effect.6 Galinpepimut-S (GPS) is a first-in-class WT1 heteroclitic peptide mixture of 2 native and 2 synthetic WT1 peptide sequences. The synthetic heteroclitic peptides, which bear point mutations, were created to stimulate both cluster of differentiation 4-positive (CD4+) and CD8+ T-cell responses (Table 1). Table 1. Peptide Sequences of GPS Elicit Both CD4+ and CD8+ T-Cell Activation Peptide Sequences (position) Peptide Length Computationally Predicted Binding to HLA Immune Response WT1-A1: *YMFPNAPYL (126–134) 9 aa A0201, A0301, A2402, B1501, B3901 CD8+ CTLs 427 long: RSDELVRHHNMHQRNMTKL (427–445) 19 aa A0201, A0301, A2402, B1501, B3901, B0702, B08, B2705, B4001, B5801, and numerous HLA-DRB1-XX CD4+ T cells 331 long: PGCNKRYFKLSHLQMHSRKHTG (331–352) 22 aa CD4+ T cells 122A1 long: SGQA*YMFPNAPYLPSCLES (122–140) 19 aa CD4+ and CD8+ T cells aa, amino acids; CD, cluster of differentiation; CTLs, cytotoxic T lymphocytes; GPS, galinpepimut-S; HLA, human leukocyte antigen; WT1, Wilms’ tumor protein 1. *Mutated peptide (native sequence has R instead of Y). Synthetic heteroclitic peptides have higher affinity for human leukocyte antigen (HLA), are prone to break tolerance to “self” cancer antigens, like WT1, and generate a response to the native peptide sequence expressed by cancer cells7 (Figure 1) Figure 1. Heteroclitic Technology-Based Cancer Immunogens/Vaccines: Principles of Action Mechanism Heteroclitic peptide (WT1 fragment) Native peptide Naive CD8+ cell Cytotoxic T-lymphocyte (CTL) Malignant plasmacyte Antigen-presenting cell (APC) GPS administration TCR in T cells: Recognizes and kills cancer cells expressing WT1 Activation CD, cluster of differentiation; GPS, galinpepimut-S; TCR, T-cell receptor; WT1, Wilms’ tumor protein 1. Clinical efficacy data with GPS in acute myeloid leukemia (AML), mesothelioma (Meso), and MM,8-14 as well as IR data post-GPS administration in patients with AML, Meso,8-12 and more recently, ovarian cancer (O’Cearbhaill RE and Gnjatic S, personal communication) strongly support increased antigenicity, as well as the ability to breach tolerance associated with this agent. In all of the above studies, GPS was used as monotherapy in the context of minimal residual disease (MRD), ie, after patients had successfully completed upfront standard debulking antitumor therapy (which varied according to the tumor type) and in the absence of macroscopically demonstrable disease. We now report the detailed patterns of IRs along with updated clinical response rates per International Myeloma Working Group (IMWG) consensus13 from a phase 2 trial of GPS in HR cytogenetics (CG) MM patients14 who remained at least MRD+ after initial (first-line) induction therapy with “modern” agents, followed by melphalan conditioning and autologous SCT (ASCT). OBJECTIVE The primary objective of this analysis was to assess the time kinetics of WT1- specific T-cell responses post-6 and -12 GPS administrations in HR CG MRD+ MM patients. DESIGN AND SETTING Single-arm, open-label, single-center phase 2 study (MSK 12-288; NCT01827137) in a tertiary large academic center with established focus on myeloma and SCT clinical research. Median progression-free survival (PFS) and overall survival (OS), as well as landmark PFS and OS rates at 12 and 18 months, serial correlative studies (CG, MRD, and genomic panels), and serial IR assessments were performed at baseline, post-6 and post-12 GPS administrations. The schema for this trial is shown in Figure 2. Figure 2. Treatment Schema for Phase 2 Study of GPS (MSK 12-288; NCT01827137) in HR CG MM Patients Who Remained at Least MRD+ Post-ASCT; IR Assessment Schedule Is Depicted ASCT Pre-ASCT Pre-GPS 0 2 4 6 8 10 GPS Week Clinical/correl. assessment Clinical/correl. and IR assessment 2–4 weeks post 6th GPS dose (week 12–14) Clinical/correl. and IR assessment 2–4 weeks post 12th GPS dose (week 60–62) Lenalidomide maintenance (10 mg daily)** Starting on day 100 post-ASCT) Clinical/correl. IR assessment at baseline N=18 (evaluable) 15/18 HR CG at baseline Median follow-up: ~20 months GPS GPS biweekly sc administration (dose of 200 g for each of the 4 peptides) First GPS administration within 22 days of ASCT 6 doses over 10 weeks GPS monthly sc administration (dose of 200 g for each of the 4 peptides) 6 additional doses ASCT, autologous stem cell transplantation; CG, cytogenetics; Chr, chromosome; correl., correlative translational studies, including CG, 10-color FC-measured MRD, WT1 expression, and other molecular markers; FC, flow cytometry; F/U: follow-up; GPS, galinpepimut-S; HR, high-risk; ICS, intracellular cytokine staining; IFN-, interferon gamma; IMWG, International Myeloma Working Group; IR, immune response (by IFN- ICS FC assay); MM, multiple myeloma; MRD, minimal residual disease; sc, subcutaneous; WT1, Wilms’ tumor protein 1. *Included the following aberrations (per IMWG risk classification): -13/del[13q], -17/del[17p], t[4;14], t[14;16], t[14;20], hypodiploidy [<45 chromosomes, excluding -Y], and Chr 1 aberrations [+1, -1, t(1;x)].14 **One patient was maintained with bortezomib instead. PATIENTS, METHODS, AND MAIN OUTCOME MEASURES This report pertains to a group of 19 MM patients (19 evaluable for clinical response and 18 for IR) who underwent first-line therapy, successful ASCT, and post-ASCT lenalidomide (starting 3 months post-ASCT), all at least MRD+; patients received GPS within 1 week of ASCT. Fifteen of 18 IR-evaluable patients (83.3%) presented with HR CG at baseline. Median age was 61.3 years (46–72); most patients (53%) received an induction regimen of RVd (Revlimid® [lenalidomide], Velcade® [bortezomib], dexamethasone). GPS was mixed with the emulsifier agent MontanideTM (as a water-in-oil suspension). The total dose for peptide was 200 g/administration (total of 800 g/administration for the mixture) in a total of 0.5 mL aqueous solution. The latter solution was mixed 1:1 vol/vol with Montanide (0.5 mL) using the interlocking syringe method. The resulting emulsion was administered to patients subcutaneously (sc), starting 2 weeks post-ASCT and every 2 weeks thereafter × 6 doses followed by boosters every 4 weeks × 6 additional doses. Granulocyte macrophage colonystimulating factor (sargramostim [Leukine®]) at a dose of 70 g was administered sc on days –2 and 0 of each GPS administration. Lenalidomide maintenance (10 mg daily) started 3 months (100 days) post-ASCT in all patients (95%), except 1 who was maintained with bortezomib instead. GPS was associated with excellent tolerability along with notable antimyeloma activity in this poor-prognosis population (HR CG plus MRD+ post-ASCT), reflected in a median PFS of 23.6 months and an OS rate of 88% at 18 months15,16 from the time of ASCT. WT1-specific IRs were assessed by intracellular interferon gamma (IFN-) analyses using intracellular cytokine staining (ICS) via flow cytometry (FC) at baseline, post-6 and -12 GPS doses. Experiments were performed on immunized hosts’ peripheral blood mononuclear cells restimulated ex vivo by pulsing with: ––Each of the 4 WT1 peptides within the GPS mixture (WT1A-1, 427L, 331L, and 122A-1), or ––The native peptides corresponding to the 2 heteroclitic peptides within GPS (WT1A and 122A), or –A “total pool” of 113 partially overlapping 15mers along the entire WT1 protein (575 amino-acid length) The readout of the IR assays was the absolute number of immunocytes of interest (eg, CD4+ or CD8+ T cells) per mL of blood (abundance or frequency of these cells). Log-transformed data were used where the variability among individual readings was greater than 10-fold. RESULTS Therapeutic Response (per IMWG criteria) Over TimeTherapeutic responses to GPS over time are depicted in Figure 3. By the time of this updated analysis, all 19 patients had received GPS treatment. Figure 3. Therapeutic Response* to GPS Over Time 100 80 60 40 20 Response category, % 0 Post-Induction Pre-ASCT Number of Evaluable Patients Post-GPS × 12 Post-ASCT Post-GPS × 6 12 months Post-ASCT 18 months Post-ASCT N = 19** N = 19** N = 16a N = 15b N = 11c CR and VGPR PR and SD PD
ASCT, autologous stem cell transplantation; CR, complete response; GPS, galinpepimut-S; MRD, minimal residual disease; PD, progressive disease; PR, partial response; SD, stable disease; VGPR, very good partial response. *Response criteria as per: Kumar et al.13. **Eighteen of 19 patients had MRD or worse at post-induction and pre-ASCT. a Progressed at previous assessment (n = 1) and had not received full course of GPS by the time of analyses (n = 2). bProgressed or died at previous assessment (n = 2) and had not received full course of GPS by the time of analyses (n = 2). cProgressed or died at previous assessment (n = 2), had not received full course of GPS by the time of analyses (n = 2), withdrew consent (n = 1), and had not yet undergone response assessment by the time of analyses (n = 3). IR rates (CD4+ and/or CD8+) toward any of the 4 WT1 peptide sets were high by the completion of immunizations, ranging from 72%–91% (Figure 4). Figure 4. Rates of WT1-Specific IR in MM Patients After 12 Doses of GPS 90 40 50 60 70 80 30 20 10 0 Rate of positive/ detectable IR, percentage of patients Both CD4 or CD8 CD4 Only CD8 Only Neither Both CD4 or CD8 CD4 Only CD8 Only Neither Both CD4 or CD8 CD4 Only CD8 Only Neither Both CD4 or CD8 CD4 Only CD8 Only Neither Both CD4 or CD8 CD4 Only CD8 Only Neither 331 427 122A WT1A WT1A total pool CD, cluster of differentiation; GPS, galinpepimut-S; IR, immune response; MM, multiple myeloma; WT1, Wilms’ tumor protein 1. IR rates against the cognate native epitopes of the 2 mutated epitopes in GPS were also high, thus confirming the principle of heteroclitic antigenicity (not shown). “Total pool”-directed IRs (~71% across-the-board reactivity) denoted multifunctional cross-epitope T-cell reactivity, a hallmark of an effective, cytotoxicity-inducing vaccine. About two-thirds of IRs were multivalent, an effect that was more pronounced for CD4+ IRs (Figure 5*). Figure 5. Distribution of Frequency of IRs Depending on the Number of Native WT1 Peptides (0–4) Against Which the IR Was Detected in MM Patients Post-6 and -12 Doses of GPS: Evidence of Multivalent T-Cell Activation 45 20 25 30 35 40 15 10 5 0 0 1 2 CD4 Post-GPS × 6 CD4 Post-GPS × 12 CD8 Post-GPS × 6 CD8 Post-GPS × 12 3 4 0 1 2 3 4 0 1 2 3 4 0 1 2 3 4 * Number of native peptides against which an IR was detected Rate of positive/ detectable IR, percentage of patients CD, cluster of differentiation; GPS, galinpepimut-S; IR, immune response; MM, multiple myeloma; WT1, Wilms’ tumor protein 1. The detailed time kinetics of individual CD4+ and CD8+ IRs over time for each WT1 peptide fragment (including 4 peptides within GPS, the 2 native peptides corresponding to the heteroclitic sequences, and “total pool” WT1 mixture), as well as exploratory correlations between these immunodynamics and IMWG clinical responses will be the focus of future presentations. DISCUSSION A high frequency of specific and robust immunoreactivity (CD4+ or CD8+) was detected to all antigen species contained in the GPS mixture post-6 and 12 doses of GPS; patients showed IR to at least 1 of the 4 peptides within the GPS mixture. Immune responses against native and heteroclitic peptide pairs were congruent, proving the heteroclitic effect principle. Multifunctional cross-epitope T-cell reactivity (and akin to epitope spreading with clonal reactivity against a “total pool” of peptide fragments spanning the full-length WT1 protein) was observed in most patients. This reactivity emerged across epitopes against which hosts were not specifically immunized. ––This is a surrogate marker of immune-mediated cytotoxicity and, therefore, a hallmark of an effective vaccine ––This effect could represent a fundamental mechanism for mitigation/prevention of tumor escape from the host’s immune “attack” because of broader availability of epitopes “visible” to the host’s activated immunocytes on tumor cells CONCLUSIONS The results of this detailed immunodynamics analysis provide a robust immunobiologic foundation for the prominent clinical effect of GPS, and suggest key roles of T-cell activation (most notably for CD4+ cells), and emergence of cross-epitopic reactivity for the expression of antimyeloma activity with this novel immunotherapy. REFERENCES 1. Call KM, et al. Cell. 1990;60:509-520. 2. Nakahara Y, et al. Brain Tumor Pathol. 2004;21:113-116. 3. Wagner KD, et al. Nat Commun. 2014;5:5852. 4. Cheever MA, et al. Clin Cancer Res. 2009;15:5323-5337. 5. Koehne G, et al. Blood. 2015;126: abstract 98. 6. Tyler EM, et al. Blood. 2013;121:308-317. 7. Pinilla-Ibarz J, et al. Leukemia. 2006;20:2025-2033. 8. Maslak PG, et al. Blood. 2010;116:171-179. 9. Maslak PG, et al. J Clin Oncol. 2016;34(15 suppl): abstract 7005. 10. Maslak PG, et al. J Clin Oncol. 2017; submitted. 11. Zauderer MG, et al. J Clin Oncol. 2016;34(15 suppl): abstract 8519. 12. Zauderer MG, et al. J Thorac Oncol. 2017; submitted. 13. Kumar S, et al. Lancet Oncol. 2016;17:e328-e346. 14. Sonneveld P, et al. Blood. 2016;127:2955-2962. 15. Koehne G, et al. 43rd Annual Meeting of the European Society for Blood and Marrow Transplantation; 26–29 March 2017; Marseille, France. Abstract 11.4. (http://cme-utilities.com/mailshotcme/Abstracts/Physicians%20Oral%2011%20 Abstracts%20090317.pdf; p. 6). 16. Koehne G et al. J Clin Oncol. 2017;35(15 suppl): abstract 8016. DISCLOSURES G. Koehne is a consultant to Sellas Life Sciences Group, Ltd. (SELLAS). Support for this study was provided by the Leo A. Guthart and Kathryn Medina Research Fund in Multiple Myeloma, and SELLAS. N. Sarlis is an employee of SELLAS and holds equity in the company. All other authors have nothing to disclose. ACKNOWLEDGMENTS Medical writing assistance was independently provided by Oana Draghiciu, PhD, from TRM Oncology, The Hague, The Netherlands, and funded by SELLAS. Please contact the corresponding author for any questions: [email protected] Copies of this poster obtained through the provided Quick Response (QR) Code are for personal use only.
Galinpepimut-S, a WT1-targeting immuno-Oncology Treatment, Induces Specific, Robust and Durable Immune Responses (IRs) in Patients (Pts) With High-Risk (HR) Multiple Myeloma (MM)
Guenther Koehne1, 2, Sean Devlin3, Neha Korde2, Sham Mailankody2, Heather Landau2, Hani Hassoun4, Alexander Lesokhin2, Nikoletta Lendvai2, David Chung2, Nicholas Sarlis5, Sergio Giralt6, Ola Landgren2.
1Cytotherapy Laboratory, Memorial Sloan Kettering Cancer Center (MSKCC), New York, NY, USA, 2Myeloma Service, Memorial Sloan Kettering Cancer Center (MSKCC), New York, NY, USA, 3Department of Biostatistics, Memorial Sloan Kettering Cancer Center (MSKCC), New York, NY, USA, 4Lymphoma Service, Memorial Sloan Kettering Cancer Center (MSKCC), New York, NY, USA, 5Sellas Life Sciences Group, Ltd., New York, NY, USA, 6Adult Bone Marrow Transplant Service, Memorial Sloan Kettering Cancer Center (MSKCC), New York, NY, USA.
Presenter: undelined
ABSTRACT SUBMITTED TO THE SOHO 2017 MEETING, Houston, TX, Sep. 13-16, 2017
https://soho2017.com/
KEY WORDS: WT1, peptide, high risk, immunotherapy, immune response.
CONTEXT: MM pts with HR cytogenetics (CG) also harboring minimal residual disease (MRD+) after upfront Rx followed by (f/b) ASCT continue to experience poor clinical outcomes, despite the introduction of IMiD maintenance. We have targeted a major MM antigenic target, Wilms Tumor-1 protein (WT1) with a novel immunotherapy.
OBJECTIVE: MM pts immunized with the WT1 heteroclitic peptide mixture galinpepimut-S (GPS) post-ASCT showed an 88% OS at 18 months (mo) and median PFS of 23.6 mo (EBMT & ASCO, 2017). We now report the detailed patterns of corresponding IRs.
DESIGN: Open-label, single-center Phase 2 study. PFS, OS, serial CG, and MRD & IR assessments followed up for a median of 18 mo.
SETTING: Tertiary large oncology center.
PATIENTS OR OTHER PARTICIPANTS: 18 MM pts post-ASCT f/b lenalidomide starting 3 months (mo) post-ASCT (all at least MRD+). 15/18 pts presented with HR-CG.
INTERVENTIONS: GPS was administered with montanide s.c. starting 2 wks post-ASCT and q2 wks thereafter x 6 doses f/b boosters q4 wks x 6 additional doses. GM-CSF was also given. GPS consisted of 4 peptides: WT1A-1(*); 427L (long); 331L, and 122A1L(*). Two of the 4 peptides were mutated (heteroclitic; *) to induce stronger HLA-binding/ reduce tolerance.
MAIN OUTCOMES MEASURES: WT1-specific IRs were assessed by intracellular IFN-g analyses (baseline, post-6 & -12 GPS doses), using PBMC’s pulsed with: each of the 4 WT1 peptides in GPS; or the native peptides corresponding to the 2 heteroclitic ones; or a ‘total pool’ of overlapping 15mers along the entire WT1 protein.
RESULTS: IR rates (CD4 and/or CD8) toward any of the 3 WT1 peptide sets were high by the completion of immunizations, ranging from 72-91%; 2/3’s of IRs were multivalent. IR rates against the cognate native epitopes of the 2 mutated ones in GPS were also high, thus confirming the principle of heteroclitic antigenicity. ‘Total pool’-directed IRs denoted multifunctional cross-epitope T-cell reactivity, a hallmark of an effective, cytotoxicity-inducing vaccine.
CONCLUSIONS: The results of this detailed immunodynamics analysis provide a robust immunobiological foundation for the striking clinical effect of GPS, and suggest key roles of CD4 activation and emergence of cross-epitopic reactivity for antimyeloma activity with this therapy.
Support for this study was provided by Leo A. Guthart and Kathryn Medina Research Fund in Multiple Myeloma, and Sellas Life Sciences Group, Ltd.
Exhibit 99.2
SELLAS’ Galinpepimut-S Induces Specific, Robust and Durable Immune Responses in Patients With High-Risk Multiple Myeloma - Correlated With Clinical Benefit
Heteroclitic peptide immunization principle demonstrated
Durable CD4 and/or CD8 immune responses (IRs) to at least one Wilms tumor 1 (WT1) peptide contained in the galinpepimut-S mixture
Achievement of complete response/very good partial response (CR/VGPR) strongly associated with both frequency and potency of CD4 IR in patients who completed per protocol immunizations
Data presented at the Annual Meeting of SOHO, Houston, TX, USA
HAMILTON, Bermuda, and NEW YORK, NY, September 14, 2017 — SELLAS Life Sciences Group Ltd. (SELLAS), a privately-held, oncology-focused, clinical stage biopharmaceutical company, today reported that its WT1-targeting immuno-oncology (IO) treatment, galinpepimut-S, led to mounting of specific, potent and durable immune responses (IRs) in multiple myeloma (MM) patients. Galinpepimut-S, is SELLAS’ lead product candidate and is currently expected to enter a pivotal, Phase 3 clinical trial in patients with AML and is also in various development phases in multiple myeloma and ovarian cancer, and additional indications are expected as a monotherapy or in combination with other immuno-oncology agents. The data reported in this release pertain to its ongoing Phase 2 study evaluating galinpepimut-S as a treatment in MM.
In the ongoing Phase 2 study, both the rate (frequency) and potency of the durable IRs in MM patients were correlated with clinical benefit accorded by galinpepimut-S, hereby defined as achievement of cCR/VGPR in patients who completed per protocol immunizations (12 doses). These novel data were presented yesterday, September 13, 2017, at the Annual Meeting of the Society of Hematologic Oncology (SOHO) in Houston, Texas, USA (soho2017.com) at a poster session and the relevant abstract (#MM-252) was published in the September 2017 supplemental issue of the journal Clinical Lymphoma, Myeloma & Leukemia. These IR results considerably expand on previous clinical observations of notable anti-myeloma activity of this innovative IO agent after upfront induction, melphalan conditioning and successful autologous stem cell transplantation (ASCT).
SELLAS’ Phase 2 MM study has enrolled a total of 20 patients who are being monitored long-term. A current median progression-free survival (PFS) of 23.6 months post-ASCT was previously reported in a group of 18 patients, all of whom had evidence of at least minimal residual disease (MRD+) post-ASCT, with 15/18 also having high-risk cytogenetics at baseline. This group represents a population at extremely high risk for progression and poor long-term outcomes, even in the face of post-ASCT maintenance therapy with immunomodulatory drugs (IMiD’s).
Rates of IRs (CD4 and/or CD8) toward any of galinpepimut-S’s individual WT1 peptides (two heteroclitic and four native), as well as a pool of 113 partially overlapping 15-mers spanning the entire length of WT1 (‘all pool’ reactivity) were high at the time of completion of per protocol immunizations, ranging from 72-91%. The rates of immunization against the cognate native epitopes (122A and WT1A) of the two heteroclitic peptides (122A1 and WT1A1) within galinpepimut-S were also high, thus demonstrating the principle of heteroclitic peptide antigenicity. Multivalent IRs (i.e., IRs against more than one WT1
peptide) were detected in up to 66% of patients. IR against WT1 ‘all pool’ peptides denoted multifunctional cross-epitope T-cell reactivity - akin to epitope spreading- and is a hallmark of an effective, cytotoxicity-inducing vaccine. Both the frequency and potency of CD4 IRs against the four native WT1 peptides targeted by galinpepimut-S, as well as ‘all pool’ WT1 antigens, showed a strong positive correlation with indices of clinical benefit (rate of CR/VGPR) at the time of completion of per protocol immunizations. Scientific abstracts describing SELLAS’ findings above will be submitted for presentation in upcoming major medical meetings.
“The results of these immunodynamics assays denote a robust immunobiological foundation for the clinical effect of WT1-targeting active immunization with galinpepimut-S, and suggests key roles of both enduring CD4 activation and emergence of cross-epitopic reactivity for antimyeloma activity with this therapy. The data also support our rationale for planning further studies to expand on our experience with this IO agent” said Guenther Koehne, MD, PhD, Principal Investigator on the trial and Attending Physician, Adult Bone Marrow Transplantation Service at Memorial Sloan Kettering Cancer Center , Associate Professor of Medicine, Weill Cornell Medical College.
Dr. Nicholas Sarlis, MD, PhD, Chief Medical Officer of SELLAS, commented: “The correlations between IR and depth of clinical responses in multiple myeloma patients are both intriguing and original, and firmly establish WT1 as a major actionable target for immunotherapy in plasma cell dyscrasias henceforth.”
Dr. Angelos Stergiou, MD, ScD h.c., Vice Chairman and Chief Executive Officer of SELLAS, said: “This new dataset focusing on dissecting the immune mechanisms underlying the marked antimyeloma activity previously documented with galinpepimut-S underscores our steadfast commitment to advance this agent’s clinical development programs, especially in clinical settings of unmet medical need, such as high-risk MRD(+) multiple myeloma after front-line therapy, a space which is currently underserved by standard therapies. These findings further corroborate immune response data from our earlier programs in acute myeloid leukemia (AML) and mesothelioma, where high rates of WT1-specific immunization were seen in conjunction to promising clinical activity signals in these tumor types. Finally, they provide justification for pursuance of future combinatorial approaches with galinpepimut-S, for example with checkpoint inhibitors, especially given the agent’s overall excellent safety profile”.
About SELLAS Life Sciences Group
SELLAS Life Sciences Group Ltd. is a late-stage biopharmaceutical company focused on the development of novel cancer immunotherapies and therapeutics for a broad range of cancer indications. SELLAS’ lead product candidate, galinpepimut-S, is a cancer immunotherapeutic agent licensed from Memorial Sloan Kettering Cancer Center that targets a broad spectrum of hematologic cancers and solid tumor indications. Galinpepimut-S is expected to enter pivotal, Phase 3 clinical trials in patients with AML and is also in various development phases in multiple myeloma and ovarian cancer, and additional indications are expected as a monotherapy or in combination with other immuno-oncology agents. SELLAS recently received orphan drug designations from the U.S. Food & Drug Administration (FDA), as well as the European Medicines Agency, for galinpepimut-S in AML and MPM; as well as fast track designation for AML and MPM from the FDA.
SELLAS was founded in 2012 and is currently headquartered in Hamilton, Bermuda, with additional offices in New York City, NY.
For more information on SELLAS, please visit www.sellaslifesciences.com.
FORWARD-LOOKING STATEMENTS
This press release contains forward-looking statements, including, but not limited to, statements related to the potential of galinpepimut-S as a treatment for multiple myeloma, acute myeloid leukemia, mesothelioma and other cancers, as well as statements regarding SELLAS’ overall research and development plans for galinpepimut-S, including the timing of clinical trials related thereto, and publication of the results in a clinical journal. These forward-looking statements are based on SELLAS’ current plans, objectives, estimates, expectations and intentions, and inherently involve significant risks and uncertainties. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of these risks and uncertainties, which include, without limitation, risks and uncertainties associated with immune-oncology product development and clinical success thereof, the uncertainty of regulatory approval, and other risks and uncertainties affecting SELLAS and its development programs. Other risks and uncertainties of which SELLAS is not currently aware may also affect SELLAS’ forward-looking statements and may cause actual results and the timing of events to differ materially from those anticipated. The forward-looking statements herein are made only as of the date hereof or as of the dates indicated in the forward-looking statements, even if they are subsequently made available by SELLAS on its website or otherwise. SELLAS undertakes no obligation to update or supplement any forward-looking statements to reflect actual results, new information, future events, changes in its expectations or other circumstances that exist after the date as of which the forward-looking statements were made.
Additional Information about the Proposed Merger involving Galena Biopharma, Inc. and SELLAS Life Sciences Group Ltd. and Where to Find It
In connection with the previously disclosed proposed merger involving Galena Biopharma, Inc. (Galena) and SELLAS Life Sciences Group Ltd., Galena and SELLAS intend to file relevant materials with the Securities and Exchange Commission, or the SEC, including a registration statement on Form S-4 that will contain a proxy statement /prospectus / information statement. Galena and SELLAS will mail the final proxy statement / prospectus / information statement to their respective stockholders. Investors and stockholders of Galena and SELLAS are urged to read these materials when they become available because they will contain important information about Galena, SELLAS and the proposed merger.
This communication shall not constitute an offer to sell or the solicitation of an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. No offering of securities in connection with the proposed merger shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933, as amended.
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- AGM Trading Update - April 2024
- Inside information: Meriaura Group's board has conditionally decided to sell Meriaura's minority ownership to Meriaura Invest to rearrange the group's financing
- Q1 2024 results
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share