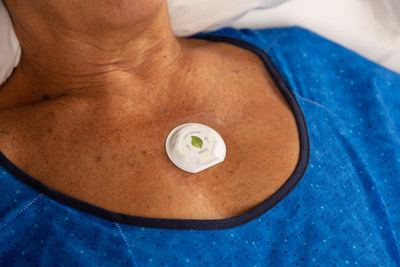
Seven million hours of patient data shows that Smith+Nephew's wireless, wearable LEAF™ Patient Monitoring System helps prevent hospital-acquired pressure injuries
LONDON, April 13, 2021 /PRNewswire/ -- Smith+Nephew (LSE:SN, NYSE: SNN), the global medical technology business, is pleased to announce that its wireless, wearable LEAF Patient Monitoring System has monitored more than 60,000 patients with over 7 million hours of use, helping to reduce the economic and human costs of pressure injuries.
While rates of other hospital-acquired conditions have declined in the US in recent years, hospital acquired pressure injury (HAPI) rates have continued to increase1, leading to an average incremental cost of $21,767 per patient impacted.2 In a randomized clinical trial conducted using the LEAF System, results showed that HAPI odds were reduced by 73%3*, an impact which could help eliminate up to $39 billion in avoidable HAPI costs if expanded nationally.1,2
"We're proud that the LEAF System has reached this major milestone while driving improved patient outcomes and economic savings to healthcare systems. The LEAF System perfectly exemplifies Smith+Nephew's purpose: using technology to take the limits off living, and to help medical professionals do the same. It is an important technology that improves the standard of care and curbs the growth rate of HAPIs," said Simon Fraser, President, Advanced Wound Management, Smith+Nephew.
HAPIs develop when patients are not repositioned with sufficient frequency to prevent prolonged pressure, particularly over bony prominences.4 Studies have shown that average turn adherence using traditional methods is less than 50%5-17 and the subsequent sustained pressure can compress tissue, impair blood flow and lead to localized tissue damage. The LEAF System helps measure turn frequency, turn angle and tissue recovery time to improve patient repositioning. It works by notifying staff when repositioning is needed according to the patient's individualized turn schedule, meeting the new recommendations set in the 2019 International Clinical Practice Guideline for the Prevention and Treatment of Pressure Injuries.18 Facilities that have implemented the LEAF System have been able to increase their turn adherence up to 98%.7
First deployed in U.S. healthcare facilities in 2014, the innovative LEAF System was the first FDA-cleared device to monitor patient orientation and activity in bed-bound, chair-bound, and ambulatory patients. In addition to the recent findings, other studies have shown that the LEAF System helps nurses prioritize patient care, improves unit workflow, and saves hospitals non-reimbursed costs associated with the treatment of pressure injuries, as well as rental bed costs.7,19-22
Smith+Nephew offers additional pressure management products designed to help facilities follow evidence-based protocols and improve clinical practice. In addition to patient repositioning, our portfolio features ALLEVYN™ LIFE Dressings, which are multilayered and uniquely constructed for protecting intact skin as part of a pressure injury prevention protocol, and the SECURA™ range of skin care products, which help maintain skin integrity and hygiene.
To learn more about the LEAF Patient Monitoring System, please visit sn-leaf.com.
*as compared to standard of care
References
1. | National Scorecard on Hospital-Acquired Conditions, Agency for Healthcare Research and Quality (AHRQ). June 2019. |
2. | Wassel CL, Delhougne G, Gayle JA, Dreyfus J, Larson B. (2020). Risk of readmissions, mortality, and hospital-acquired conditions across hospital-acquired pressure injury (HAPI) stages in a US National Hospital Discharge database. Int Wound Journal, DOI; https://doi.org/10.1111/iwj.13482. |
3. | Pickham D, Berte N, Pihulic M, Valdez A, Mayer B, Desai M. Effect of a wearable patient sensor on care delivery for preventing pressure injuries in acutely ill adults: A pragmatic randomized clinical trial (LS-HAPI study). Int J Nurs Stud. 2018;80:12-19. |
4. | Bauer K, Rock K, Nazzal M, Jones O, Weikai Q. Pressure Ulcers in the United States' Inpatient Population From 2008 to 2012: Results of a Retrospective Nationwide Study. Ostomy wound management. 2016;62(11):30-38. |
5. | Bergquist-Beringer S, Dong L, He J, Dunton N. Pressure ulcers and prevention among acute care hospitals in the United States. Jt Comm J Qual Patient Saf. 2013;39(9):404-414. (4) |
6. | Schallom L, Metheny NA, Stewart J, et al. Effect of frequency of manual turning on pneumonia. Am J Crit Care. 2005;14(6):476-478. |
7. | Schutt SC, Tarver C, Pezzani M. Pilot study: Assessing the effect of continual position monitoring technology on compliance with patient turning protocols. Nurs Open. 2017;5(1):21-28. |
8. | Yap T, Kennerly S, Ly K. Pressure ulcer prevention: a pilot study of outcomes and challenges to use of resident monitoring technology in a nursing home. J Wound Ostomy Continence Nurs. 2019;47(3):207-213. |
9. | Voz A, Williams C, Wilson M. Who is turning the patients? A survey study. J Wound Ostomy Continence Nurs. 2011 Jul-Aug;38(4):413-8. |
10. | Gunningberg L. Are patients with or at risk of pressure ulcers allocated appropriate prevention measures? Int. J. of Nursing Practice. 2005; 11: 58–67. |
11. | Bours G, Halfens R, Abu-Saad H, Grol R. Prevalence, prevention and treatment of pressure ulcers: Descriptive study in 89 institutions in the Netherlands. Research in Nursing and Health. 2002; 25: 99-110. |
12. | Goldhill D, Badacsonyi A, Goldhill A, Waldman C. A prospective observational study of ICU patient position and frequency of turning. Anaesthesia. 2008; 63: 509-515. |
13. | Winkelman C, Ling-Chun, C. Manual Turning in Patients Receiving Mechanical Ventilation. Critical Care Nurse 2010; 30 (4): 36-44. |
14. | Krishnagopalan S, Johnson EW, Low LL, Kaufman LJ. Body positioning of intensive care patients: clinical practice versus standards. Crit Care Med. 2002;30(11):2588-2592. |
15. | Wade S. Using Turn Cueing Technology to Reduce HAPIs in LTACH: Pilot Results. Poster presented internally at Centra Specialty Hospital, 2020. |
16. | Rosini L. Leveraging novel technology to decrease hospital-acquired pressure injuries. Poster presented at: American Organization for Nursing Leadership. March 18–21 2020; Nashville, TN, USA. |
17. | Goodridge DM, Sloan JA, LeDoyen YM, McKenzie JA, Knight WE, Gayari M. Risk-assessment scores, prevention strategies, and the incidence of pressure ulcers among the elderly in four Canadian health-care facilities. Can J Nurs Res. 1998;30(2):23-44. |
18. | European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel and Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers/Injuries: Clinical Practice Guideline. Emily Haesler (Ed.). EPUAP/ NPIAP/PPPIA: 2019. |
19. | Parker C, et al. Strive Towards Calnoc Excellence: Adopting Innovation to Improve Bedside Nursing Care. Kaiser Permanente, Redwood City, CA. |
20. | Smith+Nephew 2020.Leveraging novel technology to decrease hospital-acquired pressure injuries. Internal Report. EO.AWM.PCS006.001.v1. |
21. | Gasparini R, Derisma Q, Hannon R. "Turning" to Technology: Reducing Hospital Acquired Pressure Injuries in Critical Care with Visual Turn Cueing. Poster presented at: National Pressure Injury Advisory Panel Annual Conference; March 10- March 12, 2021; Virtual Conference. |
22. | Klaeb M, Krafft K, Walters B, Lowe J, Cooley A. The Influence of Wearable Technology on Nursing Attitudes and Adherence to Patient Turning and Repositioning. Poster presented at: Patient Handling and Mobility Annual Conference; March 5- March 7, 2019; Orlando, Florida, USA. |
About Smith+NephewSmith+Nephew is a portfolio medical technology business that exists to restore people's bodies and their self-belief by using technology to take the limits off living. We call this purpose 'Life Unlimited'. Our 18,000 employees deliver this mission every day, making a difference to patients' lives through the excellence of our product portfolio, and the invention and application of new technologies across our three global franchises of Orthopaedics, Advanced Wound Management and Sports Medicine & ENT.
Founded in Hull, UK, in 1856, we now operate in more than 100 countries, and generated annual sales of $4.6 billion in 2020. Smith+Nephew is a constituent of the FTSE100 (LSE:SN, NYSE: SNN). The terms 'Group' and 'Smith+Nephew' are used to refer to Smith & Nephew plc and its consolidated subsidiaries, unless the context requires otherwise.
For more information about Smith+Nephew, please visit www.smith-nephew.com and follow us on Twitter, LinkedIn, Instagram or Facebook.
To learn more about how we can help you get CLOSER TO ZERO™ pressure injuries, please visit www.closertozero.com.
Forward-looking StatementsThis document may contain forward-looking statements that may or may not prove accurate. For example, statements regarding expected revenue growth and trading margins, market trends and our product pipeline are forward-looking statements. Phrases such as "aim", "plan", "intend", "anticipate", "well-placed", "believe", "estimate", "expect", "target", "consider" and similar expressions are generally intended to identify forward-looking statements. Forward-looking statements involve known and unknown risks, uncertainties and other important factors that could cause actual results to differ materially from what is expressed or implied by the statements. For Smith+Nephew, these factors include: risks related to the impact of COVID-19, such as the depth and longevity of its impact, government actions and other restrictive measures taken in response, material delays and cancellations of elective procedures, reduced procedure capacity at medical facilities, restricted access for sales representatives to medical facilities, or our ability to execute business continuity plans as a result of COVID-19; economic and financial conditions in the markets we serve, especially those affecting health care providers, payers and customers (including, without limitation, as a result of COVID-19); price levels for established and innovative medical devices; developments in medical technology; regulatory approvals, reimbursement decisions or other government actions; product defects or recalls or other problems with quality management systems or failure to comply with related regulations; litigation relating to patent or other claims; legal compliance risks and related investigative, remedial or enforcement actions; disruption to our supply chain or operations or those of our suppliers (including, without limitation, as a result of COVID-19); competition for qualified personnel; strategic actions, including acquisitions and dispositions, our success in performing due diligence, valuing and integrating acquired businesses; disruption that may result from transactions or other changes we make in our business plans or organisation to adapt to market developments; and numerous other matters that affect us or our markets, including those of a political, economic, business, competitive or reputational nature. Please refer to the documents that Smith+Nephew has filed with the U.S. Securities and Exchange Commission under the U.S. Securities Exchange Act of 1934, as amended, including Smith+Nephew's most recent annual report on Form 20-F, for a discussion of certain of these factors. Any forward-looking statement is based on information available to Smith+Nephew as of the date of the statement. All written or oral forward-looking statements attributable to Smith+Nephew are qualified by this caution. Smith+Nephew does not undertake any obligation to update or revise any forward-looking statement to reflect any change in circumstances or in Smith+Nephew's expectations.
™Trademark of Smith+Nephew. Certain marks registered US Patent and Trademark Office.
AWM-AWC-29839 March 2021
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/seven-million-hours-of-patient-data-shows-that-smithnephews-wireless-wearable-leaf-patient-monitoring-system-helps-prevent-hospital-acquired-pressure-injuries-301267586.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/seven-million-hours-of-patient-data-shows-that-smithnephews-wireless-wearable-leaf-patient-monitoring-system-helps-prevent-hospital-acquired-pressure-injuries-301267586.html
SOURCE Smith & Nephew plc
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Mangoceuticals (MGRX) Acquires Global Preventive Care Patent Portfolio
- Toyota Charges Up Investment and Jobs in U.S. Manufacturing
- Kasm Technologies Pioneering Device-as-a-Service Solution with Lenovo
Create E-mail Alert Related Categories
PRNewswire, Press ReleasesRelated Entities
Twitter, FDASign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share