MATEON ANNOUNCES POSITIVE INTERIM RESULTS FROM ARTI-19 CLINICAL TRIAL EVALUATING COVID-19 THERAPEUTIC
Get Alerts MATN Hot Sheet
Join SI Premium – FREE
AGOURA HILLS, California, Jan. 13, 2021 (GLOBE NEWSWIRE) -- Mateon Therapeutics, Inc. (OTCQB: MATN) (“Mateon”), a leading developer of TGF-β therapeutics for oncology and COVID-19, reported positive interim results from its ARTI-19 clinical trial evaluating ARTIVedaTM against COVID-19.
“These interim results suggest that ARTIVeda is a well-tolerated and effective treatment for COVID-19 that produces a faster recovery time when compared to Standard of Care alone,” said Dr. Vuong Trieu, Chairman and CEO of Mateon.
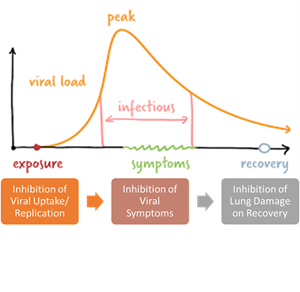
ARTIVeda is Mateon’s lead Ayurvedic drug against COVID-19 in India and is being developed by Mateon in partnership with Windlas Biotech Private Limited (India). ARTI-19 India is being conducted by Windlas as part of Mateon’s global effort to deploy ARTIVeda across India, Africa, and Latin America. These interim results are based on 60 randomized patients (out of 114 randomized to date) across 3 sites in India:
- No adverse events were reported that required discontinuation of treatment
- A majority of the 60 patients who received ARTIVeda plus Standard of Care (SOC) recovered faster than those who received SOC alone, with SOC defined as treatment with Remdesivir, Ivermectin, Dexamethasone, Heparin, as well as Paracetamol, B complex, Vitamin-C, and Zinc.
- The median time to asymptomatic WHO scale of 1 was 5 days for ARTIVeda plus SOC as compared to 14 days for SOC alone. The differences were statistically significant meaning unlikely to happen by chance.
- The trend was more pronounced with higher initial disease status. Log rank statistics: WHO-scale 2,3,4: p= 0.0369 /RR = 1.476 (0.8957-2.433), WHO-scale 3,4: p= 0.026/ RR = 1.581 (0.9094-2.747), WHO-scale 4: p= 0.0043/ RR = 2.038 (0.9961-4.168). RR = rate ratio for recovery.
The ARTI-19 India trial is slated to complete enrollment of 120 randomized patients by end of January, 2021, with final data available 6-8 weeks thereafter. Upon completion of the trial, it is Mateon’s objective to file for Emergency Use Authorization (EUA) with regulatory authorities around the world, including India, the United States, and the United Kingdom; discussions regarding EUA with several of these authorities have commenced.
ARTIVeda combines Mateon’s TGF beta platform and ethnobiology drug knowledge for rapid development and deployment of the drug product against the COVID-19 pandemic. More detailed information about the trial, including more comprehensive data, will be available in a forthcoming report. For more information about the trial, visit CTRI at https://ctri.icmr.org.in/ using identifier: CTRI/2020/09/028044.
Windlas is a prominent global CDMO (contract development and manufacturing organization) that also manufactures, markets and distributes its own branded products (allopathic, nutraceutical and AYUSH formulations) through its “affordable generics platform” spanning over 950 wholesalers across India. Windlas products are sold in markets across the globe in India, USA, Sri Lanka, Vietnam, Thailand, Myanmar, and Africa.
Saran Saund, Chief Business Officer and GM of the AI division of Mateon, commented, “Now that we have clinical support for the efficacy of ARTIVedaTM against COVID-19, we will look to deploying the drug across multiple countries in an effort to stop this pandemic.”
Pease view this 5-minute interview on CGTN at this link to see how AI driven drug discovery is helping the deployment of ARTIVeda: https://www.youtube.com/watch?v=AQDfqeqiZqs&feature=youtu.be
Dr. Suhas Kshirsagar, Director of the Ayurvedic Healing and Integrative Wellness Clinic and advisor to Mateon on ARTIVeda commented, “This positive interim analysis of ARTI-19 further supports our integration of Ayurvedic healing and modern clinical sciences for rapid response to emerging pandemic. I look forward to working with the team at Mateon and Windlas for the successful deployment of this drug. The product, ARTIVedaTM, is a formulated plant extract of the indigenous plant Artemisia, known in Sanskrit texts as Damanaka. ARTIVedaTM is the first Ayurvedic drug against COVID-19 through TGF-β inhibition.”
Dr. Wanjun Chen, Chief of the Mucosal Immunology Section at NIDCR (USA) and advisor to Mateon on this trial commented, “The clinical effectiveness of ARTIVedaTM - a known TGF-beta inhibitor- against COVID-19, validated our concept reported previously by Chen et al., 2020/ Uckun et al. 2020. As it stands ARTIVedaTM with its inhibition of TGF-beta has the potential of treating COVID-19 through inhibition of myriads of pathological responses to the TGF-beta surge due to COVID-19.”
About ARTI-19 India
The ARTI-19 trial is registered under the Clinical Trials Registry India (CTRI) with three active sites and additional sites to be added as the trial progresses and expands. ARTI-19 trial registration information can be found at: CTRI/2020/09/028044. Phase IV study to evaluate the safety and efficacy of ARTIVedaTM on COVID-19 subjects as Interventional. http://ctri.nic.in/Clinicaltrials/advsearch.php. Site specific information is: 1) Government Medical College & Government General Hospital, Srikakulam, ANDHRA PRADESH. 2) Rajarshi Chhatrapati Shahu Maharaj Government Medical College and Chhatrapati Pramila Raje Hospital, MAHARASHTRA. And 3) Seven Star Hospital, MAHARASHTRA. This trial will compare the efficacy of oral doses with standard-of-care (SOC) versus SOC alone. Oral administration of Artemisia absinthium Powder 500mg capsule/day for 5 days with SOC per cycle with the option to repeat as needed until disease is resolved or subject is discharged, up to a total of consecutive 3 cycles (“5 days treatment, 5 days off"). SOC is standard-of-care as per Clinical Management Protocol: COVID-19, Government of India Ministry of Health and Family Welfare Directorate General of Health Services (EMR Division). Safety is defined as: 1) Adverse events (AEs) during the study and 2) Serious adverse events (SAEs) during the study. Efficacy is defined as: 1) Relief in the sign and symptoms of COVID-19 as per WHO Clinical Progression Scale and 2) Relief in the sign and symptoms of COVID-19 per the Duration of Symptoms.
About ARTIVedaTM
The product, ARTIVedaTM, is a formulated plant extract of the indigenous plant Artemisia, known in Sanskrit texts as Damanaka. ARTIVedaTM is the first Ayurvedic drug against COVID-19 through TGF-β inhibition. ARTIVedaTM is expected to be effective through the entire infection cycle. The active component of ARTIVedaTM has been identified as artemisinin. Through proprietary GMP quality extraction and manufacturing processes, the Artemisia extract was rendered active against SARS-CoV-2 with robust Safety Index (SI) greater than 100 (ratio of nonspecific cell kill versus viral kill). Other extracts have SI
About Mateon Therapeutics
Mateon was created by the recent reverse merger with Oncotelic, which became a wholly owned subsidiary of Mateon, thereby creating an immuno-oncology company dedicated to the development of first in class RNA therapeutics as well as small molecule drugs against cancer and infectious diseases. OT-101, the lead immuno-oncology drug candidate of Mateon/Oncotelic, is a first-in-class anti-TGF-βRNA therapeutic that exhibited single agent activity in some relapsed/refractory cancer patients in clinical trial settings. OT-101 also has activity against SARS-CoV-2. Mateon/Oncotelic is seeking to leverage its deep expertise in oncology drug development to improve treatment outcomes and survival of cancer patients with a special emphasis on rare pediatric cancers. Mateon has rare pediatric designation for DIPG (OT-101), melanoma (CA4P), and AML (OXi4503). For more information, please visit www.oncotelic.com and www.mateon.com.
Mateon's Cautionary Note on Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical facts, included in this communication regarding strategy, future operations, future financial position, prospects, plans and objectives of management are forward-looking statements. Words such as “may”, “expect”, “anticipate” “hope”, “vision”, “optimism”, “design”, “exciting”, “promising”, “will”, “conviction”, "estimate," "intend," "believe", “quest for a cure of cancer”, “innovation-driven”, “paradigm-shift”, “high scientific merit”, “impact potential” and similar expressions are intended to identify forward-looking statements. Forward-looking statements contained in this press release include, but are not limited to, statements about future plans, the progress, timing, clinical development, scope and success of future clinical trials, the reporting of clinical data for the company’s product candidates and the potential use of the company’s product candidates to treat various cancer indications. Each of these forward-looking statements involves risks and uncertainties and actual results may differ materially from these forward-looking statements. Many factors may cause differences between current expectations and actual results, including unexpected safety or efficacy data observed during preclinical or clinical studies, clinical trial site activation or enrollment rates that are lower than expected, changes in expected or existing competition, changes in the regulatory environment, failure of collaborators to support or advance collaborations or product candidates and unexpected litigation or other disputes. These risks are not exhaustive, the company faces known and unknown risks, including the risk factors described in the company’s annual report on Form 10-K filed with the SEC on April 10, 2019 and in the company’s other periodic filings. Forward-looking statements are based on expectations and assumptions as of the date of this press release. Except as required by law, the company does not assume any obligation to update forward-looking statements contained herein to reflect any change in expectations, whether as a result of new information future events, or otherwise.
Contact Information: For Mateon Therapeutics, Inc.: Amit Shah [email protected]
Attachment
Source: Mateon Therapeutics, Inc.Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Fisker (FSR) files form 10-K, sees more job cuts, reiterates going concern doubts
- Siebert Financial Corp (SIEB) Receives Nasdaq Non-compliance Notice
- Scilex Holding (SCLX) Prices 15M Share Offering at $1/sh
Create E-mail Alert Related Categories
Globe Newswire, Press ReleasesRelated Entities
Definitive AgreementSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share